Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/68/01. The contractual start date was in February 2010. The draft report began editorial review in February 2012 and was accepted for publication in August 2012. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Holmes et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Description of the health problem
Stroke
Stroke is a serious medical condition in which the blood supply to the brain is disrupted, potentially resulting in disability and mortality. The World Health Organization (WHO) defined stroke as rapidly developing clinical signs of focal (sometimes global) disturbance of cerebral function, lasting more than 24 hours or leading to death with no apparent cause other than that of vascular origin. 1 Symptoms of stroke include weakness, numbness, visual loss, speech disturbance and unsteadiness. There are two major types of stroke: ischaemic stroke, which accounts for 85% of strokes, is caused by disrupted blood supply as a result of narrowing or blockage of the circulatory system; haemorrhagic stroke, which accounts for about 15% of strokes, is due to vascular rupture with bleeding into the brain. Brain imaging is required to differentiate between the two types.
Stroke is the second largest cause of death in the UK after heart disease2 and results in > 60,000 deaths each year in the UK. 3 More than 56,000 deaths due to stroke were recorded in England and Wales in 1999, which represents 11% of all deaths recorded that year. 4 Annually in England about 110,000 people have a first or recurrent stroke5 and a further 54,000 individuals have a transient ischaemic attack (TIA). 6
More than 900,000 people in England are living with the effects of stroke, with half of these being dependent on other people for help with everyday activities. 7 Stroke causes a greater range of disabilities than any other condition8 and also causes secondary medical problems including dementia, depression, epilepsy, falls and fractures that place a considerable burden on the economy in England, resulting in estimated annual direct costs to the NHS of £2.8B. 4
Transient ischaemic attack
Transient ischaemic attack has been defined as ‘a transient episode of neurological dysfunction caused by focal brain, spinal cord or retinal ischaemia, without acute infarction’ (p. 2277). 9 In a TIA, symptoms typically subside within a few hours; however, people who have experienced a TIA have a higher risk of stroke, with approximately 20% of TIA patients developing a stroke,10 and therefore patients require prompt medical attention to prevent complications. It has been reported that 10–15% of TIA patients experience a stroke within 3 months,9 with the greatest risk being within the first 72 hours,11 and the risk of a recurrent stroke is 30–43% within 5 years. 4
Risk factors
There are a number of modifiable risk factors, including hypertension, cardiac disease [particularly atrial fibrillation (AF)], diabetes, cigarette smoking, alcohol consumption, hyperlipidaemia and carotid stenosis. 12 Epidemiological research has shown that raised blood pressure is the most important risk factor for ischaemic stroke. 13 The incidence of stroke increases with decreasing socioeconomic conditions. 14 Important non-modifiable risk factors for ischaemic stroke include age, gender, ethnicity and heredity. 12
Age is an important risk factor for ischaemic stroke. The overall incidence by 75–84 years of age is approximately 25 times higher than that at age 45–54 years. 10,15 Ischaemic stroke in adults aged < 45 years is relatively rare, with surveys estimating that about 5% of all cerebral ischaemic infarctions occur in this age group,16 although others studies have indicated this figure to be > 10%. 17
Aetiology
Cerebral embolism may be arterial or cardiac in origin. Cardiac embolism results from thrombus formation in the heart, which then embolises to the intracranial circulation. Cardiac emboli can be of any size but those arising from the cardiac chambers are often large and more likely to cause severe stroke, disability and death.
Cardiac embolism
Estimates of the relative frequency of cardioembolic stroke vary, although cardioembolic stroke has been estimated to result in approximately 20% of ischaemic strokes. 18 There are several potential cardiac sources of embolism but it may be difficult to be certain whether an identified embolic source is the actual cause of stroke, particularly if there are alternative causes such as coexistent large artery disease.
Atrial fibrillation is found in about 15% of all stroke patients19 and is detectable from either clinical examination or electrocardiogram (ECG) monitoring. In the case of patients with cardioembolic stroke, a higher percentage of about 45% are associated with AF. 20
Other potential causes of stroke include left ventricular dysfunction (congestive heart failure), valve disease including prosthetic valves, intracardiac right-to-left shunts [patent foramen ovale (PFO), particularly in conjunction with atrial septum aneurysm] and atheroma of the ascending aorta and the aortic arch. 21 Other conditions that are also considered to be potential sources include sinoatrial disorder, recent acute myocardial infarction, marantic or infective endocarditis, and cardiac tumours. 22
Mitral valve disease is associated with a significant proportion of cardioembolic stroke in young patients and is more common in some populations because of a high prevalence of rheumatic heart disease. 23 The risk of cardioembolic stroke associated with rheumatic heart disease (in the presence or absence of synthetic valve prosthesis) varies considerably (40–70%) among different geographical stroke registries; in Finland, with the virtual disappearance of rheumatic fever, the incidence of rheumatic heart disease is much lower. 24
Diagnosis
Identification of the underlying mechanisms and aetiologies is important so that appropriate therapy can be initiated to decrease the risk of recurrent stroke, although in about one-third of stroke patients no identifiable aetiology is found,25–27 even after complete clinical evaluation. No quantitatively valid clinical criteria exist for the diagnosis of cardioembolic stroke. The diagnosis is based on identifying a potential cardiac source of embolism, eliminating other potential sources of cerebral ischaemia and considering the clinical neurological features for suspected cardioembolic stroke. 18
Abrupt onset of the neurological deficit is not helpful in determining the origin of the stroke as abrupt onset of a maximal neurological deficit occurs in the majority of patients with ischaemic stroke from other causes, such as stroke with a carotid origin. The location of the infarct does not always help to determine causation, even though cardiogenic emboli most commonly lodge in the middle cerebral artery or its branches, as emboli to the vertebrobasilar or anterior cerebral artery can also occur. However, multiple acute brain infarctions in both cerebral hemispheres usually suggest an embolic mechanism, particularly one of aortic or cardiac origin. 28 Other morbidities that can obscure diagnosis are emboli from proximal sources such as the carotid arteries, which may have a similar presentation as those of cardioembolic origin.
Current service provision
No recommendations relating to the use of echocardiography in the assessment of first-episode diagnosed stroke and TIA patients were made within the National Clinical Guideline for Stroke published by the Royal College of Physicians,29 the National Institute for Health and Care Excellence (NICE) acute stroke and TIA guideline30 or the Department of Health National Stroke Strategy. 31 The use of this technology in the management of stroke and TIA patients in the UK appears to be variable (see Chapter 4). The British Society of Echocardiography32 stated that echocardiography was indicated (1) in adults with neurological disease that includes unexplained stroke or TIA without evidence of previous cerebrovascular disease or without significant risk factors for other cause [with the suggestion that saline contrast echocardiography by transthoracic echocardiography (TTE) or transoesophageal echocardiography (TOE) should be used], and (2) in patients for whom a therapeutic decision will depend on the outcome of echocardiography (e.g. anticoagulation). This guidance also stated that echocardiography was not indicated in patients in whom echocardiography would not affect the decision to begin anticoagulation (e.g. patients in AF with a cerebrovascular event and no suspicion of structural heart disease).
Description of technology under assessment
Transthoracic echocardiography is a non-invasive imaging technique that uses sound waves to create a moving picture of the heart. In the UK, a trained sonographer performs the test and interprets the results. An instrument called a transducer that releases high-frequency ultrasound waves is placed between the ribs and the upper abdomen directed towards the heart. The transducer picks up the echoes of ultrasound waves and transmits them as electrical impulses. The echocardiography machine converts these impulses into moving pictures of the heart. Pictures can be two-dimensional or three-dimensional, depending on the part of the heart being evaluated and the type of machine. This technique can provide information about cardiac structure and function, helping to establish the diagnosis and guide therapy. TTE can be performed in fundamental imaging mode (TTEf), which uses the reflected echoes from the same spectral band as that of the emitted pulse, or in second harmonic imaging mode (TTEh), which employs the second harmonic of the emitted frequency band to construct images. The transmission frequency determines the trade-off between penetration depth and spatial resolution. 33
Echocardiography can be performed to identify cardiogenic sources of emboli and has been recommended as a routine test in stroke management. 34 However, the cost-effectiveness of echocardiography in the secondary prevention of stroke is unclear. Some investigators have recommended the use of TTE in all stroke patients35 whereas other evidence suggests the need to perform TOE when no indications for anticoagulation are found with TTE. 36 TOE is used to check the structure and function of the heart. The test requires patients to swallow a probe that is attached to an ultrasound machine. This obtains images of the heart from within the oesophagus, which lies just behind the heart, and can give a clearer view of the heart than normal echocardiography. Procedural risks are low but include transient throat pain, laryngospasm, aspiration, hypotension, hypertension, tachycardia, mucosal bleeding, oesophageal rupture and a rare risk of death. Benzocaine topical spray can cause toxic methaemoglobinaemia.
Chapter 2 Definition of the decision problem
Decision problem
Population
Patients with cardiac pathologies (see Appendix 1) relevant to ischaemic stroke or TIA were included. However, cardiac pathologies that are clinically identifiable without the need for echocardiography, or which are present with symptoms that represent other indications for echocardiography37 such as recent myocardial infarction, dilated cardiomyopathy and infective endocarditis, were excluded.
Echocardiography in newly diagnosed AF patients has been commissioned by the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme as a separate project (reference no. 08/45/01 HTA Technology Assessment Report) and AF is therefore not included in this study.
Intervention (diagnostic index test)
Transthoracic echocardiography is an ultrasound imaging technique utilising beams of ultrasound transmitted at frequencies of 2.5–5 MHz. A transducer is placed on the chest wall, allowing the structures of the heart and velocity of blood flow to be visualised. 38 TTE may be used to determine cardiac sources of stroke or TIA and facilitate treatment and secondary prevention strategies.
The index tests assessed in this review are:
-
TTEf
-
TTEh.
Relevant comparators
The accepted reference standards for the detection of cardiac pathologies are not well defined, and none of the tests, apart from invasive surgical procedures, provides a definitive diagnosis. For the detection of PFO, TOE is often considered the ‘gold standard’ to measure other tests against,21 and this was selected as the reference standard to measure the performance of TTE. Because of the uncertainty of relevant reference standards for other cardiac sources of stroke and TIA, no a priori comparators were stated and all studies were included that compared the diagnostic accuracy of TTE against other commonly available tests.
Outcomes
Patients are classified by the index test (TTE) as being either positive or negative for the cardiac pathology under investigation. The reference standard is also undertaken to identify patients’ true health status. The reference standard is assumed to have 100% sensitivity and specificity; however, subgroup analyses are undertaken whenever possible to test the effect of using different reference standards. Patients fall into one of four groups. When the index test is positive, patients may be true positive (TP), in which case both tests agree that they have a cardiac pathology, or false positive (FP), in which case the index test indicates that they have the cardiac pathology but the reference standard does not. When the index test is negative, patients may be true negative (TN), in which case both tests agree that they are cardiac pathology free, or false negative (FN), in which case the index test incorrectly classifies them as being free of the pathology.
This can be represented in a 2 × 2 table (Table 1). In the clinical setting, FPs can result in patients receiving unnecessary treatment whereas FNs can result in people not receiving the treatment that they require. Sensitivity indicates the effectiveness of the index test in correctly identifying cardiac pathologies. Specificity indicates the effectiveness of the index test in correctly classifying people as cardiac pathology free. Sensitivity and specificity can be calculated as simple percentages. In practice, diagnostic tests often have a high sensitivity at the expense of a low specificity and vice versa. Ideally, a test would have both high sensitivity and high specificity.
| Index test result | Reference standard positive | Reference standard negative |
|---|---|---|
| Index test positive | TP | FP |
| Index test negative | FN | TN |
| Sensitivity = [TP/(TP + FN)] × 100 | Specificity = [TN/(TN + FP)] × 100 |
The majority of included studies used TOE as the reference standard to measure the accuracy of TTE. Other reference tests included ultrafast computerised tomography (CT) for the detection of right and left atrial thrombi and contrast-enhanced magnetic resonance imaging (MRI) and cardiac MRI for the detection of left ventricular thrombus. Additionally, non-imaging tests were used as reference tests, including surgical and cardiac catheterisation to confirm atrial septal defect, and autopsy, aneurysmectomy and indium-111 imaging to confirm left ventricular thrombus. The reference test used for PFO was TOE, but transmitral Doppler (TMD) and transcranial Doppler (TCD) studies were also included (as the reference standard) to measure the accuracy of TOE.
Studies were included only if they reported the numbers of TP, FN, TN and FP results for TTE in comparison to a reference standard test. These values can be used to calculate measures of diagnostic accuracy such as sensitivity and specificity.
Overall aims and objectives of the assessment
The overall aim was to use secondary research methods to determine the most appropriate echocardiographic diagnostic management strategy for first-episode diagnosed stroke and TIA patients in the UK. More specifically, the objectives were to:
-
undertake systematic reviews to determine (1) the prevalence of potential cardiac sources of stroke and TIA and (2) the diagnostic accuracy of echocardiography
-
undertake a survey to describe current practice in the NHS in terms of guidelines and management strategies used by stroke centres
-
evaluate the cost-effectiveness of the addition of TTE to the routine assessment of patients who have had a first-episode diagnosed stroke or TIA in the UK.
Chapter 3 Assessment of prevalence of cardiac sources of stroke and transient ischaemic attack
Methods for reviewing prevalence
A systematic review was undertaken according to the general principles recommended in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. 39
Identification of studies
Search strategy
The search strategy comprised the following elements:
-
searching of electronic databases
-
scrutiny of bibliographies of retrieved papers and previous reviews
-
contact with experts in the field.
Databases
The following databases were searched:
-
MEDLINE (1950 to December 2010)
-
EMBASE (1980 to December 2010)
-
PsycINFO (1806 to December 2010)
-
Web of Science (1899 to December 2010)
-
The Cochrane library (1995 to December 2010)
-
Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1981 to December 2010).
Sensitive keyword strategies using free text and, when available, thesaurus terms using Boolean operators and database-specific syntax were developed to search the electronic databases. Synonyms relating to the condition were combined with search filters aimed at restricting results to prevalence studies and excluding animal studies (used in the searches of MEDLINE, CINAHL, EMBASE and PsycINFO, with an amended version used for Web of Science). Date limits or language restrictions were not used on any database. All resources were searched from inception to December 2010. An example of the MEDLINE search strategy is provided in Appendix 2.
All identified citations from the electronic searches and other resources were imported into and managed using the Reference Manager bibliographic software (version 12.0; Thomson Reuters, Philadelphia, PA, USA).
Titles and abstracts were screened for inclusion by two reviewers. Full-text relevant papers were screened against the inclusion criteria by two reviewers and any disagreements were resolved by consensus.
Inclusion and exclusion criteria
Inclusion criteria
Studies were included if they assessed the prevalence of cardiac sources of embolism in first-episode ischaemic stroke and TIA. Cardiac pathologies that are detectable without the need for echocardiography, for example myocardial infarction, were not included in this evaluation. Inclusion of relevant cardiac pathologies (see Appendix 1) was determined through consultation with clinical experts and with reference to previously published studies. 37,40
Echocardiography in newly diagnosed AF patients has been commissioned by the NIHR HTA Programme as a separate project (reference no. 08/45/01 HTA Technology Assessment Report) and AF is therefore not included in this study.
Exclusion criteria
The following studies were excluded: non-English-language publications, narrative reviews and editorials.
Data extraction strategy
Data were extracted by two reviewers using a standardised data extraction form and cross-checked for accuracy. Discrepancies were resolved by discussion.
Critical appraisal strategy
The diagnosis of specific cardiac sources of stroke is usually unclear and relies on the identification of a potential cardiac source of embolism in the absence of significant cerebrovascular occlusive disease. Patients need to undergo thorough neurological and cardiovascular evaluation including the assessment of clinical findings to distinguish between other potential causes of stroke. Many confounding comorbidities such as AF can coexist in the presence of other cardiac sources of stroke. When several confounding factors are present, establishing the aetiology can be difficult, and often the cause of stroke remains unknown.
The quality of the studies included in the prevalence aspect of this review was not formally evaluated; a consensus decision was taken by the review team based on the data retrieved during the data extraction phase of the review. Many of the included studies were not designed to investigate cardiac sources of embolism to determine prevalence. The data reported were primary risk factor data and methodological detail was limited. Most studies reported cardiac pathologies through routine examination but did not attempt to establish a causal relationship. Hence, it was felt that formal quality appraisal would add little, if any, value to the prevalence review.
Methods of data synthesis
Because of the heterogeneity of the included studies relating to study design, population characteristics, detection methods used and absence of a causal relationship to identify cardiac sources of embolism, a meta-analysis was not undertaken. Instead, the data are tabulated and discussed narratively.
Results
Quantity of research available
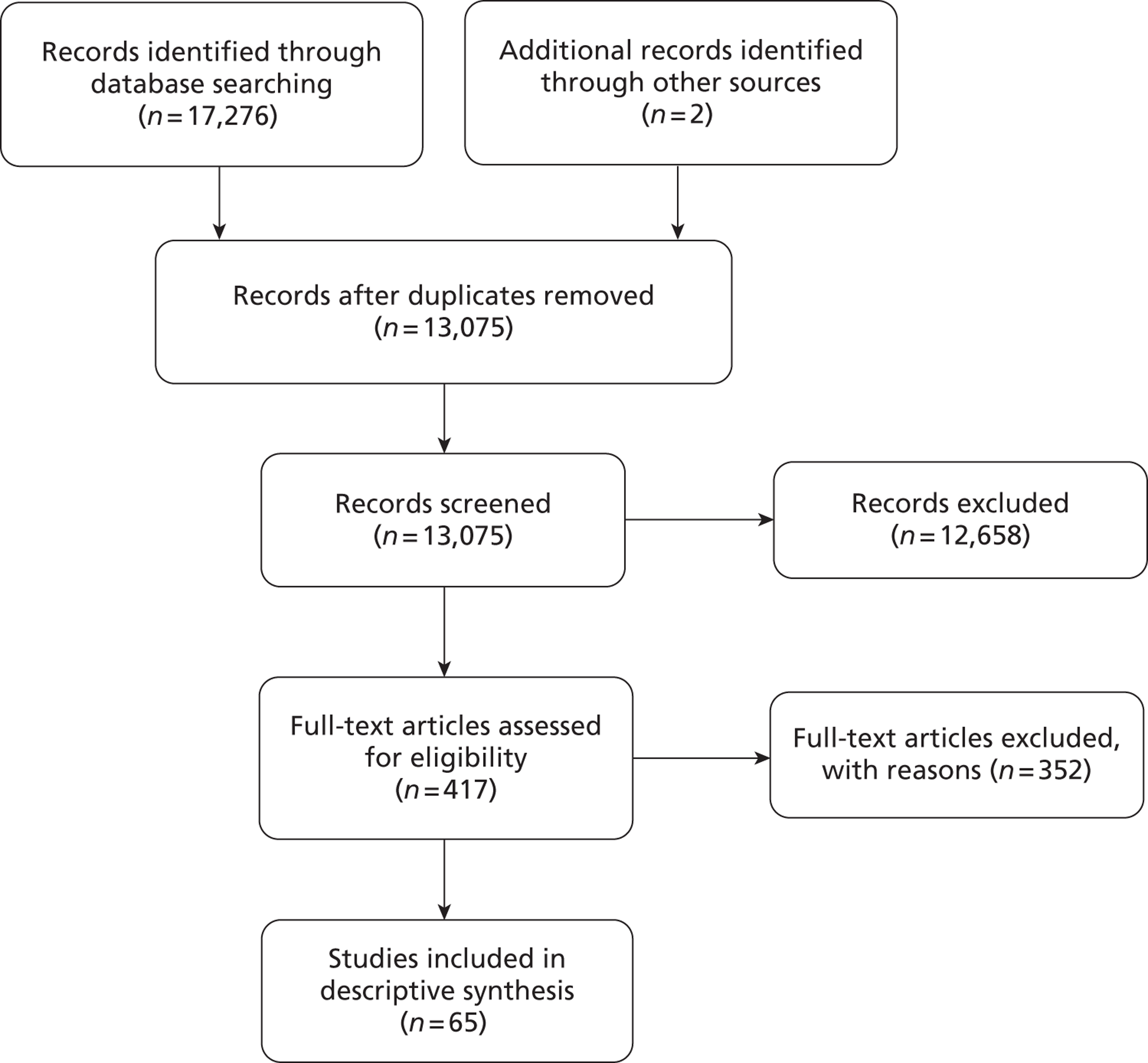
The electronic search identified 17,276 citations. Two further studies were identified from hand searching the reference lists of the included studies (Figure 1). Once duplicates were removed, a further 12,658 studies were excluded at the title/abstract stage and 417 were obtained for examination of the full text. Of these, 352 were excluded because no usable data were reported (see Appendix 3). In total, 65 citations23,26,27,41–102 relating to 65 studies were included in the review.
FIGURE 1.
The PRISMA flow chart of included and excluded studies.
Study characteristics
The cardiac pathologies identified from the included studies, the age range of participants and the diagnostic tests used are reported in Appendix 4. Participants ranged in age from 1 to 94 years. Most studies assessed patients with stroke or TIA who were aged > 40 years.
Most studies reported using a battery of tests, some ancillary, to evaluate potential sources of cardiac emboli. Of these, TOE was the most frequently reported diagnostic tool used to assess cardiac pathologies (45 studies23,26,27,41–82); 38 studies23,26,27,42–45,47,48,50–52,55–65,67–69,71–74,76,77,79,83–87 used TTE during the diagnostic work-up. Only six studies88–93 did not report including a form of electrocardiography during the diagnostic work-up. In total, 27 studies27,42,46,50–52,56–58,60–63,67,68,71,72,79,81–84,88,91,93–95 used MRI; 36 studies23,27,42,46,50,51,55–63,65,67,68,71,72,74,75,81,82,86,88,89,91,93–100 used CT; four studies27,41,43,51 used carotid ultrasonography; 33 studies23,26,42,52,53,56,57,59–61,63,65,67–69,71–74,76,79,82,86,88,91,93–96,98–101 used electrocardiography; five studies42,76,79,91,93 used magnetic resonance angiography (MRA); seven studies23,52,53,65,79,95,96 used 24-hour Holter monitoring; nine studies47,84,94,95,97,99–102 used electrocardiography but did not specify which type; one study96 reported autopsy findings; three studies61,77,88 used Doppler ultrasonography; two studies89,98 used angiography; and 10 studies51,62,67,68,72–74,76,83,93 used TCD ultrasonography. The cardiac pathologies identified, including the prevalence range and median values, are reported in Table 2.
| Cardiac pathology | No. of studies | Prevalence range (%) | Median prevalence (%) | Total population, n | Age (years) |
|---|---|---|---|---|---|
| Atrial septal aneurysm26,41,42,46–49,51,53,54,56–58,63,65,66,68,70,71,74,77–81,85,88,97 | 28 | 0.4–28 | 9.3 | 5560 | 14–93 |
| PFO23,26,27,43–58,62,63,67,68,70–75,77–85,92,94,102 | 39 | 0.25–73 | 17 | 9002 | 2–93 |
| PFO with atrial septal aneurysm26,47,50,63,68,70,75 | 6 | 4.1–24.1 | 10.75 | 1568 | 14–92 |
| PFO with atrial septal defect80,92 | 2 | 3.4–29.6 | 16.5 | 262 | 18–65 |
| Rheumatic valvular disease23,43–45,55,96,97 | 7 | 0.65–26.8 | 4.5 | 1378 | 15–80 |
| Left ventricular thrombus23,27,48,49,61,71,73,77,97 | 9 | 0.2–4.3 | 0.83 | 1892 | 15–93 |
| Atrial septal defect48,53,57,61,68,79,82 | 7 | 0.25–9 | 2.7 | 1011 | 16–90 |
| Left ventricular hypertrophy49,59,69,82,87,97,102 | 7 | 3–42 | 7.7 | 1154 | 16–92 |
| Left atrial thrombus49,57,64,71,76,77 | 5 | 0.9–9 | 1.4 | 1692 | 38–93 |
| Mitral valve regurgitation including mitral valve insufficiency and mitral valve incompetence49,61,63,76,82 | 5 | 1.4–73.2 | 10.3 | 873 | 16–92 |
| SEC left ventricle49 | 1 | 4 | 4 | 523 | 26–92 |
| Unspecified SEC77,80,81 | 3 | 0–3.7 | 1.1 | 740 | 18–91 |
| SEC LA49 | 1 | 15.5 | 15.5 | 523 | 26–92 |
| Aorta SEC49 | 1 | 8.6 | 8.6 | 523 | 26–92 |
| Mitral valve stenosis including mitral valve thickening49,61,64,78,82,87,89,91 | 8 | 0.7–9 | 4.15 | 856 | 16–87 |
| Aortic valve stenosis49,97 | 2 | 0.6–0.65 | 0.625 | 678 | 16–92 |
| Aortic valve calcification including aortic valve sclerosis and aortic valve thickening49,80,82,99 | 4 | 4.5–29.8 | 5.85 | 919 | 16–92 |
| Cardiac tumour26,68,73,77,82,95,98 | 7 | 0–2.0 | 1 | 1389 | 14–81 |
| Valvular vegetations61,82,87 | 3 | 1–9.7 | 1.67 | 178 | 16–81 |
| Mitral valve prolapse26,59,61,63,69,71,77,79,80,82,86,87,89,90,97,100,101 | 17 | 0–31.6 | 3.3 | 1731 | 1–93 |
| Atrial appendage thrombus55 | 1 | 1.1 | 1.1 | 239 | Mean 66 |
| Ventricular hypokinesia55 | 1 | 0.5 | 0.5 | 239 | Mean 66 |
| Mitral annular calcification26,58,77,80,99,100 | 6 | 0.5–9.7 | 1.95 | 1254 | 18–86 |
| Rheumatic heart disease86,90,91,95,98,101 | 6 | 5.1–29.5 | 12.05 | 455 | 15–87 |
| Aortic arch atheroma63 | 1 | 3.4 | 3.4 | 118 | 23–59 |
| Ejection fraction < 35%71 | 1 | 5 | 5 | 121 | 38–93 |
| Ejection fraction < 40%93 | 1 | 16.7 | 16.7 | 6 | 49–75 |
| Left atrial dilatation76 | 1 | 6.8 | 6.8 | 74 | 16–87 |
| Left ventricular dilatation76 | 1 | 5.4 | 5.4 | 74 | 16–87 |
| Left ventricular aneurysm77 | 1 | 1.6 | 1.6 | 441 | No details |
| Aortic aneurysm77 | 1 | 0.2 | 0.2 | 441 | No details |
| Mitral valve strands78 | 1 | 16 | 16 | 318 | 28–87 |
| Intracardiac thrombus76,79,81,92 | 4 | 0–2.7 | 1.9 | 538 | 16–91 |
Discussion
This systematic review summarises the results of 65 studies that have reported the prevalence of potential cardiac sources of stroke and TIA. The multiple sources of potential cardiac pathologies contributing to stroke and TIA reflect the heterogeneous nature of cardioembolic stroke. 37,103
Previous reports have classified cardiac pathologies into major (e.g. left ventricular thrombus, mitral valve stenosis and atrial myxoma) and minor (mitral valve prolapse, mitral annular calcification, aortic stenosis, mitral valve strands, atrial septal aneurysm and PFO) risk factors for stroke. 40 The prevalence rates identified from the included studies for major risk factors ranged from 0% to 9%; for minor risk factors, for which further uncertainty exists around their role in stroke aetiology, the range was wider (0–73%).
Patent foramen ovale was the most frequently reported cardiac pathology, with 39 studies providing data. PFO also exhibited the largest degree of heterogeneity, with prevalence rates ranging from 0.25% to 73%. The study characteristics, however, did not indicate that the heterogeneity was due to differences in the age of patients, tests used or study sample sizes.
Because of the heterogeneous nature of stroke, the diagnosis of cardioembolic stroke or TIA is often uncertain and is reliant on the detection of a potential cardiac source of embolus in the absence of other potential sources of cerebral ischaemia. 103 However, some studies reported the presence of two or more potential sources in one person, which generates further diagnostic uncertainty, although such findings would not necessarily alter the treatment regime.
The studies did not report or indicate that a thorough diagnostic evaluation was undertaken to establish a causal link with stroke; instead, cardiac findings were reported as associated risk factors, and these were often derived using different diagnostic techniques. The systematic review found wide variation in reported rates of cardiac sources of stroke, and this variability is most likely the result of the methodological limitations of the included studies and the heterogeneity of stroke.
Chapter 4 Assessment of diagnostic accuracy
Methods for reviewing diagnostic accuracy
A systematic review was undertaken according to the general principles recommended in the PRISMA statement. 39 Methods used for the analysis and the inclusion criteria were prespecified and documented in the protocol (PROSPERO no. CRD42011001353104).
Identification of studies
Search strategy
The search strategy comprised the following elements:
-
searching of electronic databases
-
scrutiny of bibliographies of retrieved papers and previous reviews
-
contact with experts in the field.
Databases
The following databases were searched:
-
MEDLINE (1950 to September 2011)
-
EMBASE (1980 to September 2011)
-
PsycINFO (1806 to September 2011)
-
Web of Science (1899 to September 2011)
-
The Cochrane Library [including Cochrane Database of Systematic Reviews (CDSR), Cochrane Central Register of Controlled Trials (CENTRAL), Database of Abstracts of Reviews of Effects (DARE), NHS Economic Evaluation Database (NHS EED) and HTA database] (1995 to September 2011)
-
CINAHL (1981 to September 2011).
Sensitive keyword strategies using free text and, when available, thesaurus terms using Boolean operators and database-specific syntax were developed to search the electronic databases. Synonyms relating to the condition (stroke) were combined with terms relating to the technology and a filter was applied aimed at restricting results to diagnostic studies (used in the searches of MEDLINE, CINAHL, EMBASE and PsycINFO, with an amended version used for Web of Science). Date limits or language restrictions were not used on any database. All resources were searched from inception to March 2011. A further update search was performed in September 2011; this included all types of cardiac pathology, irrespective of stroke occurrence. An example of the MEDLINE search strategy is provided in Appendix 5.
All identified citations from the electronic searches and other resources were imported into, and managed using, Reference Manager bibliographic software.
Titles and abstracts were screened for inclusion by two reviewers. Full-text relevant papers were screened against the inclusion criteria by two reviewers and any disagreements were resolved by consensus.
Inclusion and exclusion criteria
Inclusion criteria
Prospective or retrospective studies were included if they assessed the diagnostic accuracy of TTE in patients with cardiac conditions identified as potential sources of stroke or TIA (see Appendix 1). Studies were included only if they reported the numbers of TP, FN, TN and FP results for TTE in comparison to a reference standard test or reported the total number of participants, prevalence (%), sensitivity (%) and specificity (%). Comparators to TTE include other tests that are established reference standards, for example TOE for PFO. When no established reference standard exists for a cardiac condition, studies reporting diagnostic accuracy data between TTE and other tests (e.g. MRI, TMD, TCD, invasive procedures such as surgery) were included.
Exclusion criteria
Non-English-language studies were excluded. Case–control studies (in which the test is evaluated in a group of patients already known to have the outcome and a separate group of patients without the outcome) were excluded.
Data extraction
Data were extracted by two reviewers using a standardised data extraction form and cross-checked for accuracy. Discrepancies were resolved by discussion.
Critical appraisal strategy
Study quality was assessed by one reviewer and checked by a second using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) checklist. 105
Methods of data synthesis
Sensitivity and specificity are presented for each study. Meta-analysis was undertaken to calculate a mean sensitivity and specificity across studies. Sensitivity and specificity are linked so that changing the threshold at which a test is considered positive will tend to increase the sensitivity but decrease the specificity, or vice versa. Forest plots were generated in R statistical software (2011; see www.r-project.org) and summary receiver operating characteristic (SROC) plots were generated within Review Manager software (RevMan 5; see http://ims.cochrane.org/revman).
The diagnostic test data were meta-analysed as follows. A bivariate normal model was used for the logit sensitivities and logit specificities in each study to account for correlation within studies. We let:
The model was completed by giving the uncertain parameters the following prior distributions:
These prior distributions are weakly informative but are slightly more informative than the conventional non-informative prior distribution that is generally used in the analysis of diagnostic test data when there is sufficient data to dominate the prior distributions. The conventional non-informative prior distributions are:
This was done because, in many cases, the model failed to fit with a conventional weak prior distribution as a consequence of (1) some meta-analyses being based on very few studies, (2) several meta-analyses involving a large number of studies with zero counts, mainly for patients classified as being a FP (i.e. control patients) but also for patients classified as being a TP (i.e. patients with the condition) and (3) several meta-analyses including only a small number of patients who actually had the condition.
The consequence of the weakly informative prior distribution for the prior estimate of the between-study standard deviation relative to that based on conventional non-informative prior distributions was to reduce the uncertainty about the prior estimate from 1.5 [95% credible interval (CrI) 0.4 to 32.3] to 0.5 (95% CrI 0.3 to 1.4). This gives more weight to smaller values of the between-study standard deviation whilst acknowledging the possibility of moderate to large heterogeneity between studies a priori.
The consequence of the weakly informative prior distribution had relatively little impact on the prior estimates of the population sensitivities and specificities. The conventional prior distribution is interpreted such that we are uncertain exactly what the population values are but we believe them to be either 0 or 1. In the case of the weakly informative prior distribution we give slightly more weight to other values being plausible.
Data were analysed using freeware WinBUGS software (version 1.4.3; MRC Biostatistics Unit, Cambridge, UK). Convergence was assessed using the Gelman–Rubin convergence statistic. 106 In at least one meta-analysis, convergence occurred after 100,000 iterations and so we used a burn-in of 100,000 for all meta-analyses for consistency. In most meta-analyses there was strong evidence of autocorrelation between successive samples of the Markov chain Monte Carlo (MCMC) method, which indicates that the chains were not mixing well across the posterior distributions. To account for this, the posterior distributions were estimated by generating 20,000 samples after thinning the chains by retaining every 10th iteration of the MCMC chains.
Results of the review of diagnostic accuracy
Results of the search
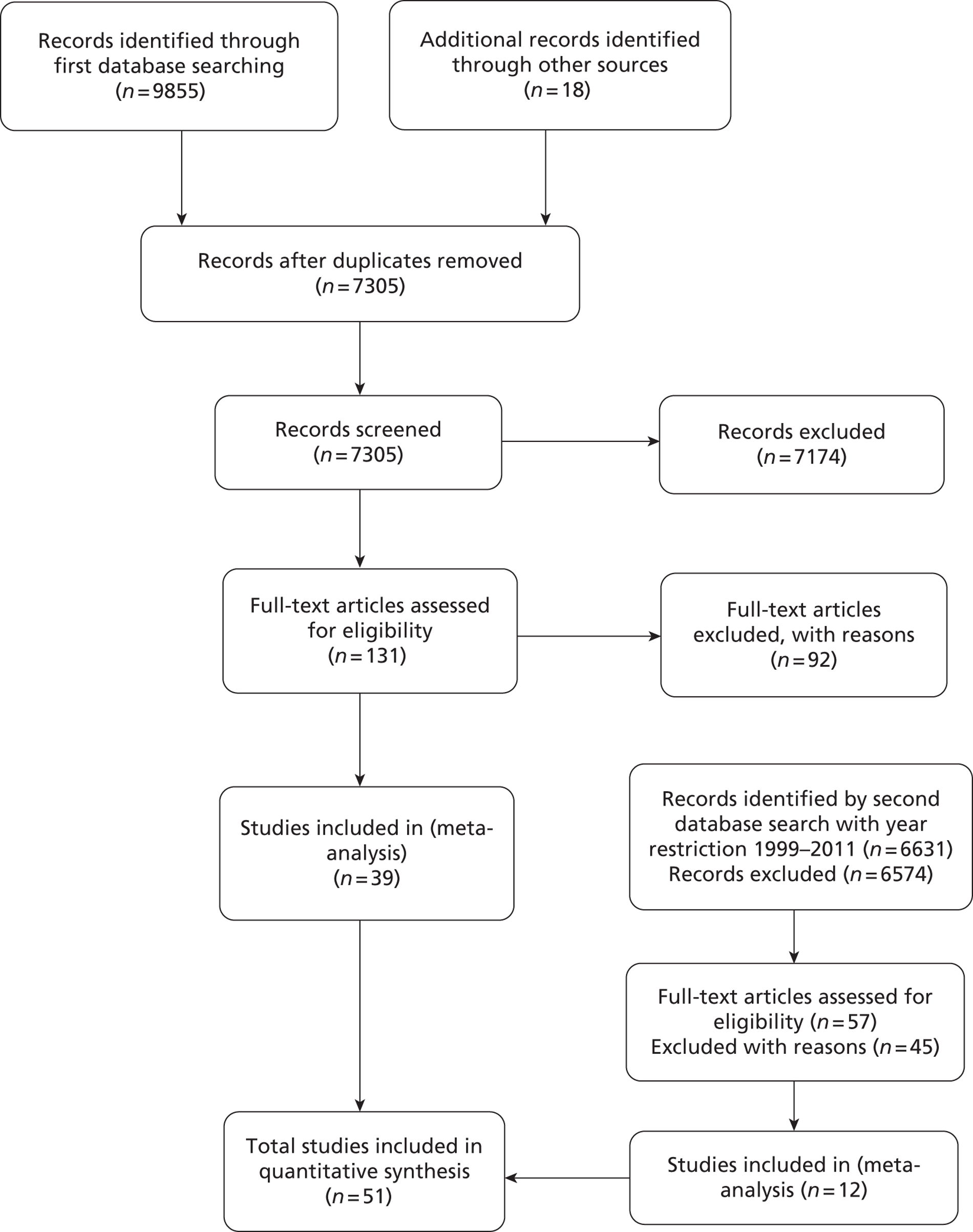
A total of 9855 citations were identified from the initial database search and 18 from other sources such as reference lists; a further 6631 citations were identified using an expanded search phrase but with the year restricted to 1999–2011 (Figure 2). Of these citations, 13,748 were excluded at the title/abstract stage and 188 full-text reports were obtained for inspection. Of these, 137 were excluded and 51 studies61,107–156 were included in the review.
FIGURE 2.
The PRISMA flow chart of included and excluded studies.
Included studies
A summary of the 51 included studies is provided in Table 3. Full details of studies are provided in Appendix 6.
| Study, year | Methods | Participants | Interventions | Outcomes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prospective | Retrospective | Index test results blinded | Reference test results blinded | Representative spectrum of participants | Country | Age (years) | Sex | TTEf | TTEh | Reference standard | PFO | Other outcomes | |
| Akosah 1998107 | • | U/K | U/K | • | USA | 40–85 | M | • | TOE | • | ASD, AAT | ||
| Aschenberg 1986125 | • | U/K | U/K | • | Germany | Mean 51 | M and F | • | TOE | LAAT | |||
| Baur 1982108 | • | U/K | U/K | • | USA | Mean 56 | U/K | • | LV | LVA | |||
| Belkin 2011109 | • | U/K | U/K | • | USA | 19–73 | M and F | • | TOE | • | |||
| Black 1991154 | • | U/K | U/K | • | Australia | 18–90 | M and F | • | TOE | SEC | |||
| Black 1991155 | • | U/K | U/K | • | Australia | 25–86 | M and F | • | TOE | SEC | |||
| Blum 2004145 | • | U/K | U/K | • | Israel | Mean 57 | M and F | • | TOE | • | ASD, LAT | ||
| Chen 1992139 | • | U/K | U/K | • | Taiwan | 17–68 | M and F | • | TOE | • | |||
| Chirillo 2005149 | • | Y | Y | • | Italy | Mean 46 | M and F | • | TOE | Cardiac vegetations | |||
| Clarke 2004143 | • | Y | Y | • | UK | Mean 58 | M and F | • | TOE | • | |||
| Cujec 1991152 | • | U/K | U/K | • | Canada | 18–87 | M and F | • | TOE | • | ASA, LAAT, SEC | ||
| Daniels 2004140 | • | Y | Y | • | Belgium | Mean 63 | M and F | • | TOE | • | |||
| de Bruijn 2006141 | • | Y | Y | • | Holland | U/K | U/K | • | TOE | LAT | |||
| Di Tullio 1993110 | • | Y | Y | • | USA | 63 | M and F | • | TOE | • | ASA | ||
| Fatkin 1996156 | • | U/K | U/K | • | Australia | 38–74 | M and F | • | TOE | LAT, LAAT | |||
| Gonzalez-Alujas 2011146 | • | U/K | U/K | • | Spain | 17–75 | M and F | • | TOE | • | ASA | ||
| Gutiérrez-Chico 2008147 | • | Y | Y | • | Spain | 15–92 | M and F | • | TOE | MVP | |||
| Ha 2000136 | • | U/K | U/K | • | South Korea | Mean 51 | M and F | • | • | TOE | LAT, SEC | ||
| Ha 2001137 | • | U/K | U/K | • | South Korea | 24–89 | U/K | • | • | TOE | • | ||
| Hirata 2008111 | • | Y | Y | • | USA | Mean 57 | M and F | • | TOE | MVP | |||
| Hubail 2011112 | • | U/K | U/K | • | USA | 1.2 to 8.6 | M and F | • | • | TOE | • | ||
| Illien 2002126 | • | U/K | U/K | • | Germany | 57–67 | M and F | • | TOE | LAT | |||
| Jassal 20075 | • | Y | Y | • | Canada | Mean 57, 18–63 | M and F | • | TOE | Cardiac vegetations | |||
| Jax 2010127 | • | U/K | U/K | • | Germany | U/K | U/K | U/K | U/K | TOE | • | ||
| Kerr 2000113 | • | Y | Y | • | USA | 34–76 | M and F | • | • | TOE | • | ||
| Kitayama 1997138 | • | U/K | U/K | • | Japan | Mean 68 | M and F | • | CUCT | LAT | |||
| Kuhl 1999128 | • | U/K | U/K | • | Germany | 20–86 | M and F | • | • | TOE | ASD | ||
| Lee 1991114 | • | Y | Y | • | USA | 20–82 | M and F | • | TOE | • | SEC | ||
| Lembcke 2009129 | • | U/K | U/K | • | Germany | Mean 68 | M and F | U/K | U/K | CC | AVS | ||
| Li 2009135 | • | U/K | U/K | • | China | 43–73 | M and F | U/K | U/K | LV | LVA | ||
| Lipke 2007130 | • | Y | Y | • | Germany | Mean 63 | M and F | • | MRI | LVT | |||
| Madala 2004115 | • | Y | Y | • | USA | 21–88 | M and F | • | TOE | • | |||
| Maffè 2010150 | • | Y | Y | • | Italy | 36–62 | M and F | • | TOE | • | |||
| Mugge 1995131 | • | U/K | U/K | • | Germany | 18–85 | M and F | • | TOE | ASA | |||
| Musolino 200361 | • | U/K | U/K | • | Italy | 17–45 | M and F | • | TOE | • | MVS, MVR, LAAT, ASD, ASA | ||
| Nemec 1991116 | • | U/K | U/K | • | USA | 22–78 | M and F | • | TOE | • | |||
| Neuman 2003117 | • | Y | Y | • | USA | Mean 78 | M and F | • | TOE | Mitral and aortic regurgitation | |||
| Omran 1999132 | • | Y | Y | • | Germany | Mean 54 | M and F | • | TOE | LAAT, SEC | |||
| Pearson 1991118 | • | Y | Y | • | USA | 17–84 | M and F | • | TOE | SEC | |||
| Pop 1990142 | • | U/K | U/K | • | Holland | Mean 60 | M and F | • | TOE | LAAT, SEC | |||
| Roldan 2008119 | • | U/K | U/K | • | USA | Mean 37 | M and F | • | TOE | MVR | |||
| Sallach 2009120 | • | Y | U/K | • | USA | Mean 67 | M and F | • | • | TOE | LAAT | ||
| Shub 1983121 | • | U/K | U/K | • | USA | Mean 31 | M and F | • | CC | ASD | |||
| Siostrzonek 1991134 | • | U/K | U/K | • | Austria | Mean 52 | M and F | • | TOE | • | |||
| Stendel 2000133 | • | Y | Y | • | Germany | Mean 51 | M and F | • | TOE | • | |||
| Stratton 1982122 | • | Y | Y | • | USA | Mean 58 | M and F | • | Autopsy and UPIPI | LVT | |||
| Thanigaraj 2005123 | • | U/K | U/K | • | USA | Mean 45 | M and F | • | TOE | • | ASD | ||
| Trevelyan 2006144 | • | Y | Y | • | UK | Mean 55 | M and F | • | TOE | • | |||
| Vincelj 2001148 | • | U/K | U/K | • | Croatia | Mean 55 | M and F | U/K | U/K | TOE | Atrial myxoma, LAT | ||
| Weinsaft 2011124 | • | • | U/K | U/K | • | USA | Mean 60 | M and F | • | MRI | LVT | ||
| Zito 2009151 | • | Y | Y | • | Italy | Mean 49 | M and F | • | TOE | • | |||
Settings
Eighteen studies107–124 were conducted in the USA; nine125–133 in Germany; one in Austria;134 one in China;135 two in South Korea;136,137 one in Japan;138 one in Taiwan;139 one in Belgium;140 two in Holland;141,142 two in the UK;143,144 one in Israel;145 two in Spain;146,147 one in Croatia;148 four in Italy;61,149–151 two in Canada;152,153 and three in Australia. 154–156 Most studies were undertaken in a hospital/clinical setting, although six studies120,124,131,136,137,151 did not clearly state where the tests were performed. Study size ranged from just 12 participants148 to 400. 154 The mean age of the sample was 56 years.
Reference tests
Two studies108,135 compared TTE with left ventriculography; one study121 compared TTE with surgical and cardiac catheterisation; two studies124,130 compared TTE with cardiac MRI; one study129 compared TTE with cardiac catheterisation; one study138 compared TTE with cardiac ultrafast CT; and one study122compared TTE for assessment of left ventricular thrombus with a combination of procedures: autopsy, aneurysmectomy and unequivocally positive indium-111 platelet imaging.
Eighteen studies112,113,115,119,120,123,124,126,128,136,137,140,143,144,146,149,150,153 compared TTEh with TOE and 19 studies61,107,109,110,114,116–118,125,131–134,139,142,152,154–156 compared TTEf with TOE.
Four studies127,141,145,148 compared TTE with TOE but it was not possible to determine whether TTE was performed in fundamental or second harmonic imaging mode.
Outcome data reported
Patent foramen ovale was reported in 23 studies, 13 using TTEf61,107,109,110,114–116,133,134,137,139,145,152 and 11 using TTEh. 112,113,115,123,137,140,143,144,146,150,151
Four studies61,107,128,145 reported data for atrial septal defect using TTEf and two123,128 reported data for atrial septal defect using TTEh. One study121 reported data for oscium secundum atrial septal defect and ostium primum atrial septal defect using TTEf.
Atrial septal aneurysm data were reported in four studies61,110,131,152 using TTEf and one study146 using TTEh. Two studies61,117 reported data for mitral valve regurgitation using TTEf and one study119 reported data for mitral valve regurgitation using TTEh; eight studies61,107,120,125,132,142,152,156 reported data for left atrial appendage thrombi using fundamental imaging and one study120 used harmonic imaging. Left atrial thrombi data were reported in three studies145,148,156 using TTEf and three studies126,136,141 using TTEh; one study138 reported data for right atrial thrombi using TTEf; three studies122,124,130 reported TTEh and TTEf data for left ventricular thrombi; three studies114,132,142 reported TTEf data for spontaneous echo contrast (SEC); four studies118,152,154,155 reported TTEf data for left atrial SEC and one study136 reported TTEh data for left atrial SEC; one study154 reported data for left ventricular SEC with TTEf; two studies reported TTEf and TTEh data for left ventricular aneurysm;108,135 two studies reported TTEh data for cardiac vegetations149,153 and one study149 reported TTEf data; one study129 reported TTEf data for aortic valve stenosis; one study61 reported TTEf data for mitral valve stenosis; two studies111,147 reported TTEh data for mitral valve prolapse; and one study148 reported TTEf data for atrial myxoma.
Excluded studies
A total of 137 studies were excluded (see Appendix 7). Of these, 72 were excluded because no usable data were reported; six were excluded because concordance could not be established between the test procedures; 12 were excluded because studies did not report relevant cardiac pathologies; 27 were not diagnostic accuracy studies; 15 were not available in the English language; and five did not include a relevant reference standard.
Study quality
A summary of methodological quality across all studies is provided in Figure 3. The methodological quality for each included study is illustrated in Figure 4.
FIGURE 3.
Methodological quality summary: review authors’ judgements about each methodological quality item presented as percentages across all included studies.
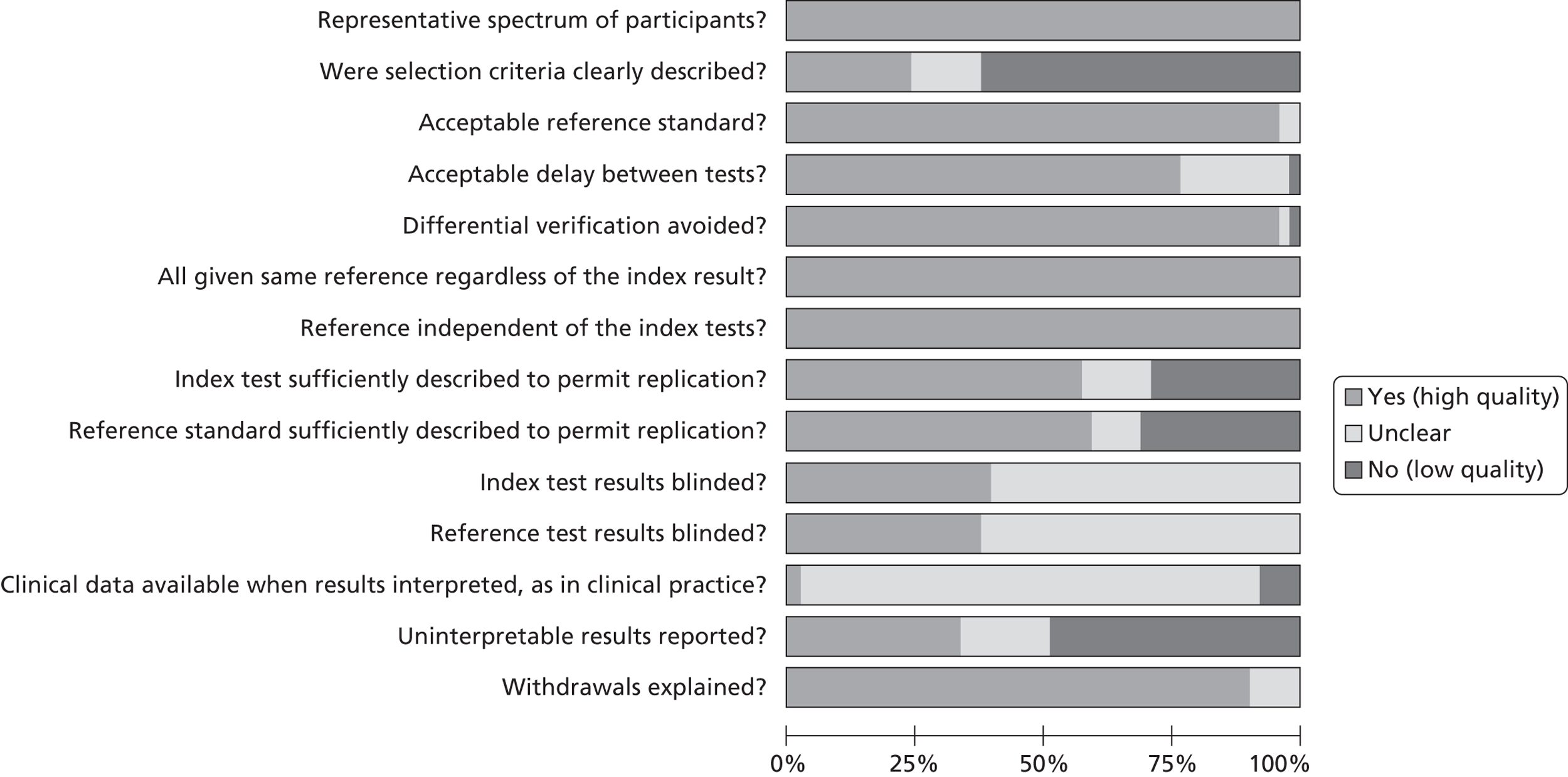
FIGURE 4.
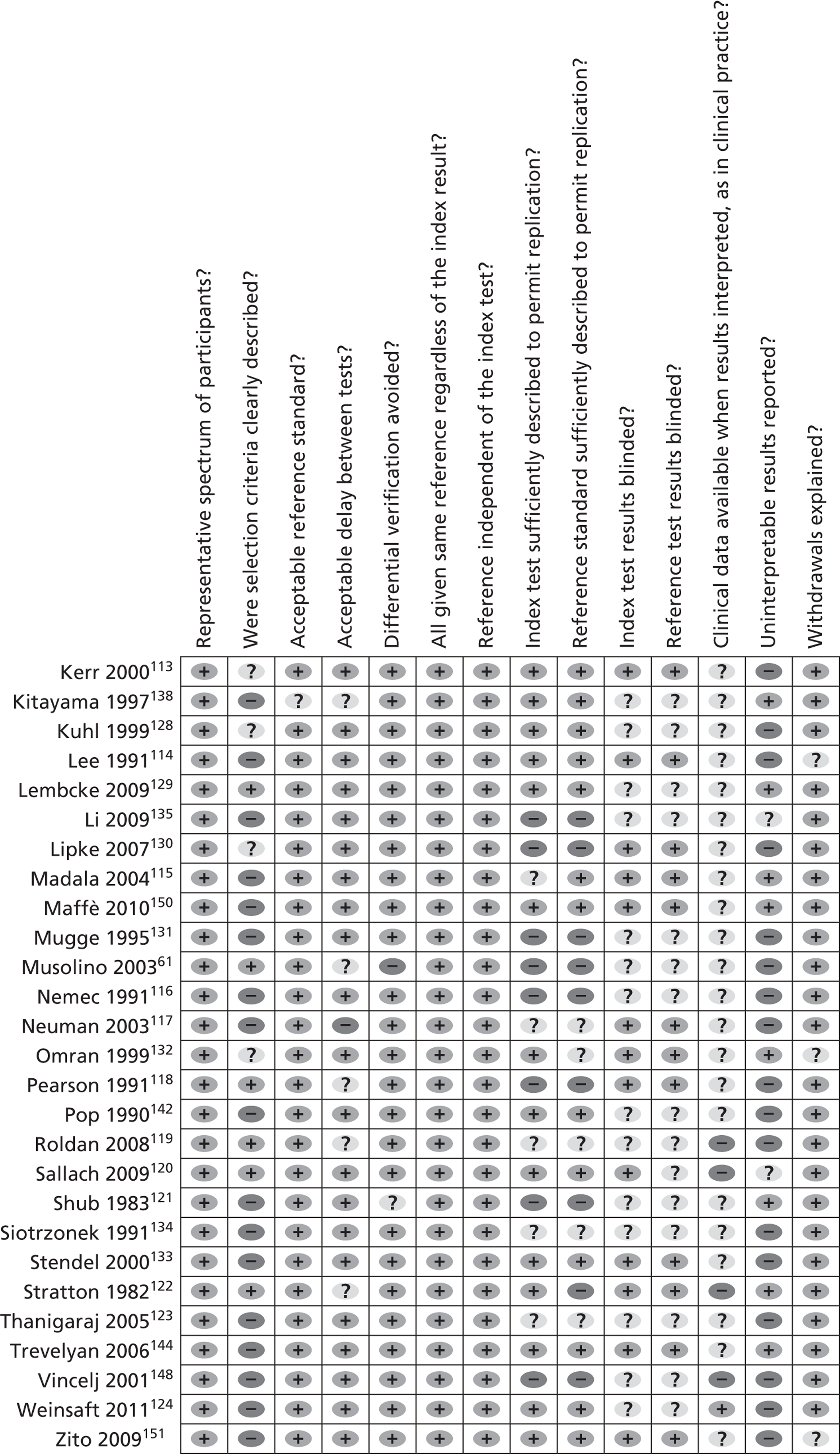
Methodological quality summary: review authors’ judgements about each methodological quality item for each included study.
Spectrum of participants
All 51 studies included patient samples that would be considered representative of the population using the test in practice.
Selection criteria
Most studies (n = 32107–109,111,112,114–117,121,123,124,127,131,133–140,142–144,147,148,150–153,156) did not report how participants were selected for inclusion into the study; 12 studies61,110,118–120,122,125,126,129,145,154,155 provided details on patient selection and seven113,128,130,132,141,146,149 reported only brief details.
Reference standard
Most studies61,107–137,139,140,142–156 used a reference standard that was considered to classify the target condition correctly, and most studies used TOE as the reference standard, that is, the ‘gold standard’. TOE is considered the gold standard for assessing PFO but the test is acknowledged to be imperfect, although it is assumed to be superior to the index test TTE. Other cardiac pathologies used TOE as the reference standard but the literature is less supportive of its status as the reference standard. Four studies compared TTE with invasive procedures including left ventriculography,108,135 surgical procedures and cardiac catheterisation,121 and aneurysmectomy, autopsy and positive indium-111 platelet imaging122 to determine thrombus.
Time between tests
Most studies109–116,120,121,123–125,127–137,139–144,146–153,156 reported the time taken between administering the reference test and the index test, and this was judged to be reasonably short enough to ensure that the target condition did not change. Some studies61,107,108,117,118,119,122,126,138,145,154,155 did not report the time taken between tests for the assessment of PFO, but these studies were not downgraded on quality as PFO will not be affected during the study period.
Selection bias
The majority of studies107–120,122–156 included the original sample for verification with the reference test. Some studies excluded patients who could not provide a clear image on testing or who did not complete the imaging procedure. Overall, the data suggest a low risk of bias.
Verification and incorporation bias
All studies used the same reference standard regardless of the index test result, and the reference standard was independent of the index test in all studies. The majority of studies108–115,120,124–126,128,129,133,136–139,141–144,146,147,149–152,154,156 provided sufficient details to permit replication of the reference and index tests. However, many studies did not report the procedures used,61,107,110,116,118,121,122,124,127,130,131,135,140,145,148,153,154 with some115,117,119,123,132,134,147,154 reporting only brief details.
Review bias
In about 40% of the studies66,110,111,113–115,117,120,122,130,132,133,140,143,144,147,149–151,153 the results of either the index test or the reference test were interpreted without knowing the findings of the comparator test. Most studies107–109,112–115,117,118,120,122,125–127,130,132,133,136,137,139,141,144–146,150–152,155,156 did not report whether blinding between test results was used, and it is unclear whether the interpretation of the results of the index test may have been influenced by knowledge of the results of the reference standard, although the data do not indicate a greater or lesser diagnostic accuracy when blinding is not known.
Availability of clinical information
The majority of studies61,107–123,125–156 did not state whether patients’ clinical data were available to the investigative team and it is not known whether this influenced the diagnostic test results.
Uninterpretable data reporting
About 30% of included studies110–112,115,121,122,125,129,132,138,140,143,144,146,150,154,155 reported uninterpretable, indeterminate or intermediate test results. Some studies108,120,135–137,141,145,147,149 removed these data from the analysis but most studies61,107,109,113,114,116–119,123,124,126–128,130,133,134,136,139,142,148,151–153,156 did not report how inadequate images were utilised for the diagnostic accuracy test or only briefly reported this information with no clear explanation of how these findings were interpreted.
Analysis of diagnostic accuracy data: transthoracic echocardiography studies
The total number of patients per study is the sum of the TP, FP, FN and TN values. A summary of all outcomes is shown in Appendix 8.
Patent foramen ovale
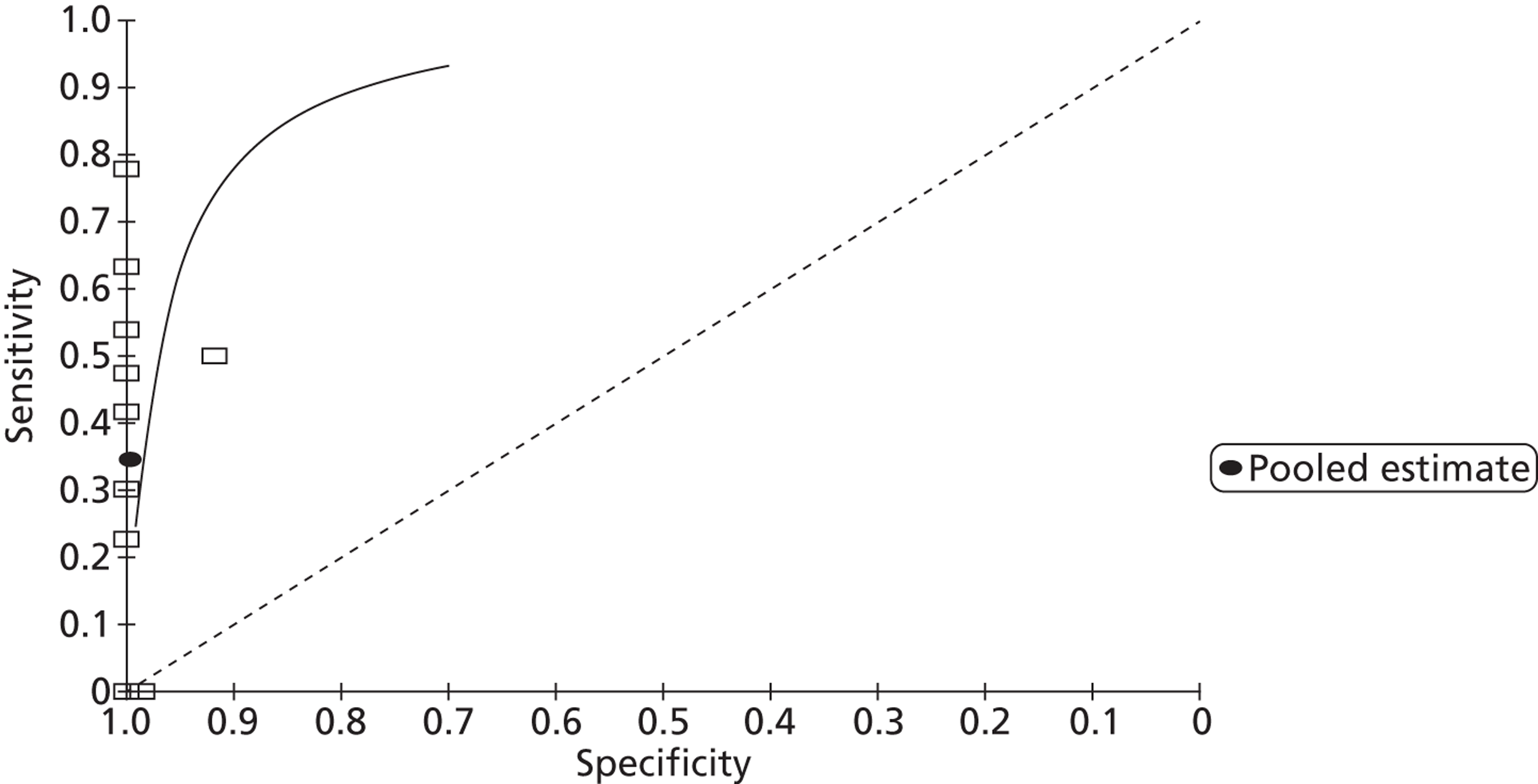
From 13 studies61,107,109,114–116,133,134,137,139,140,145,152 with 905 participants (Figures 5 and 6), the pooled sensitivity of TTE to detect PFO in fundamental imaging mode was 0.34 (95% CrI 0.21 to 0.47) with a pooled specificity of 1.00 (95% CrI 0.99 to 1.00) compared with TOE.
FIGURE 5.
Sensitivity and specificity of TTEf to detect PFO vs. TOE.
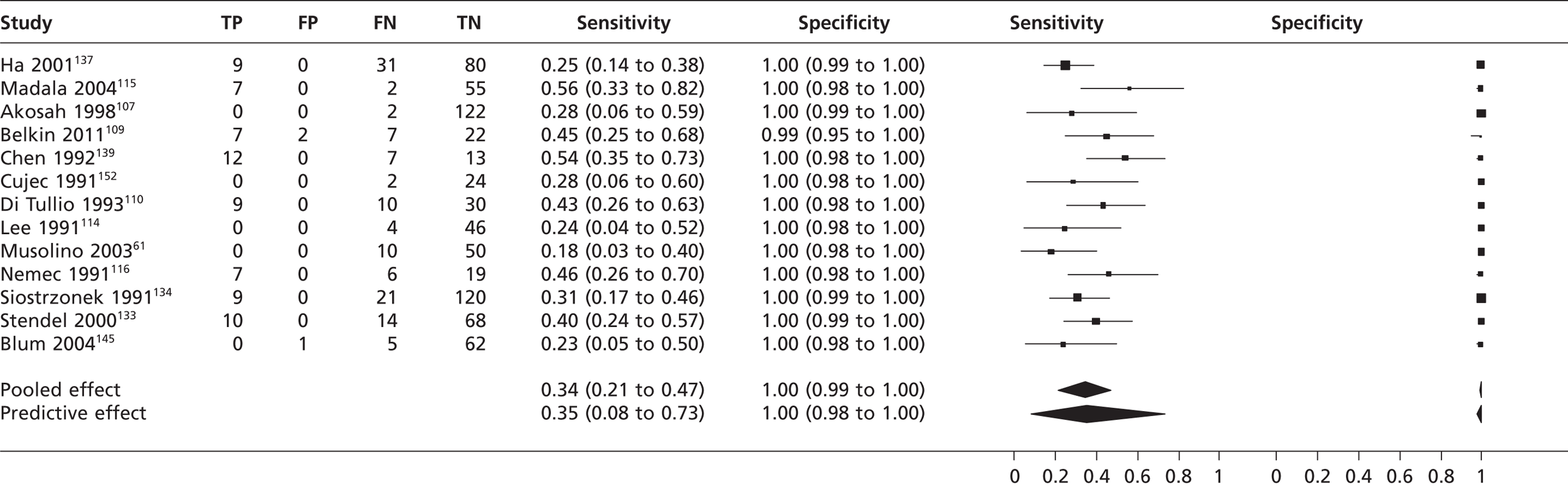
FIGURE 6.
Summary receiver operating characteristic plot of pooled TTEf detection of PFO.
In second harmonic imaging mode (11 studies,112,113,115,123,137,140,143,144,146,150,151n = 1115) the pooled sensitivity of TTE to detect PFO was 0.89 (95% CrI 0.80 to 0.95) with a specificity of 0.99 (95% CrI 0.97 to 1.00) (Figures 7 and 8). In one study,127 frequency mode not specified, the sensitivity of TTE to detect PFO was 0.48 (95% CrI 0.33 to 0.63). Specificity could not be calculated as all patients were positive for PFO.
FIGURE 7.
Sensitivity and specificity of TTEh to detect PFO vs. TOE.
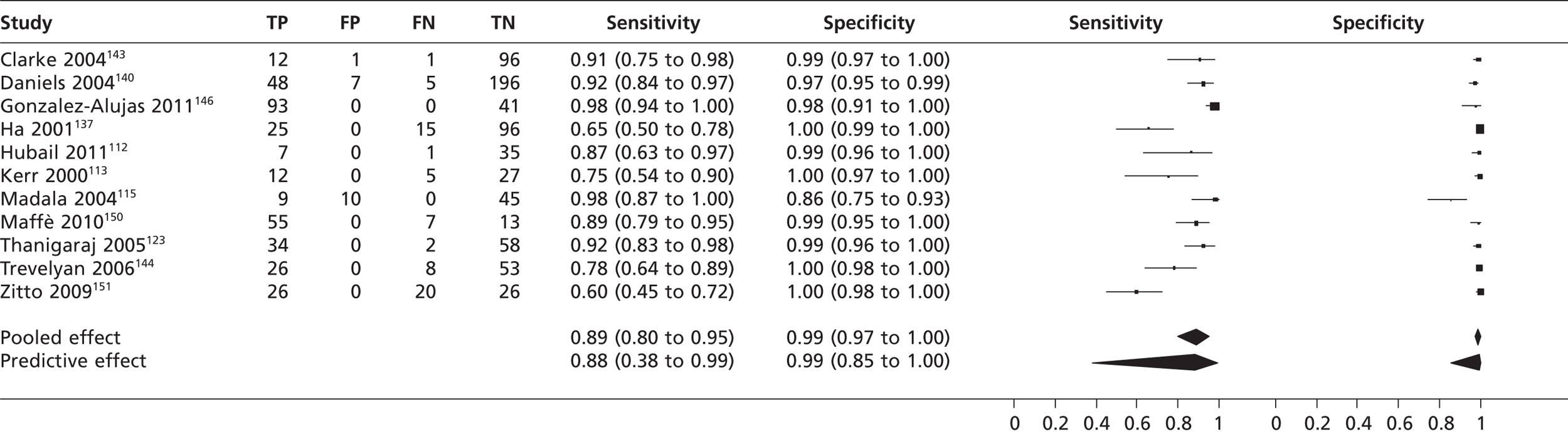
FIGURE 8.
Summary receiver operating characteristic plot of pooled TTEh detection of PFO.
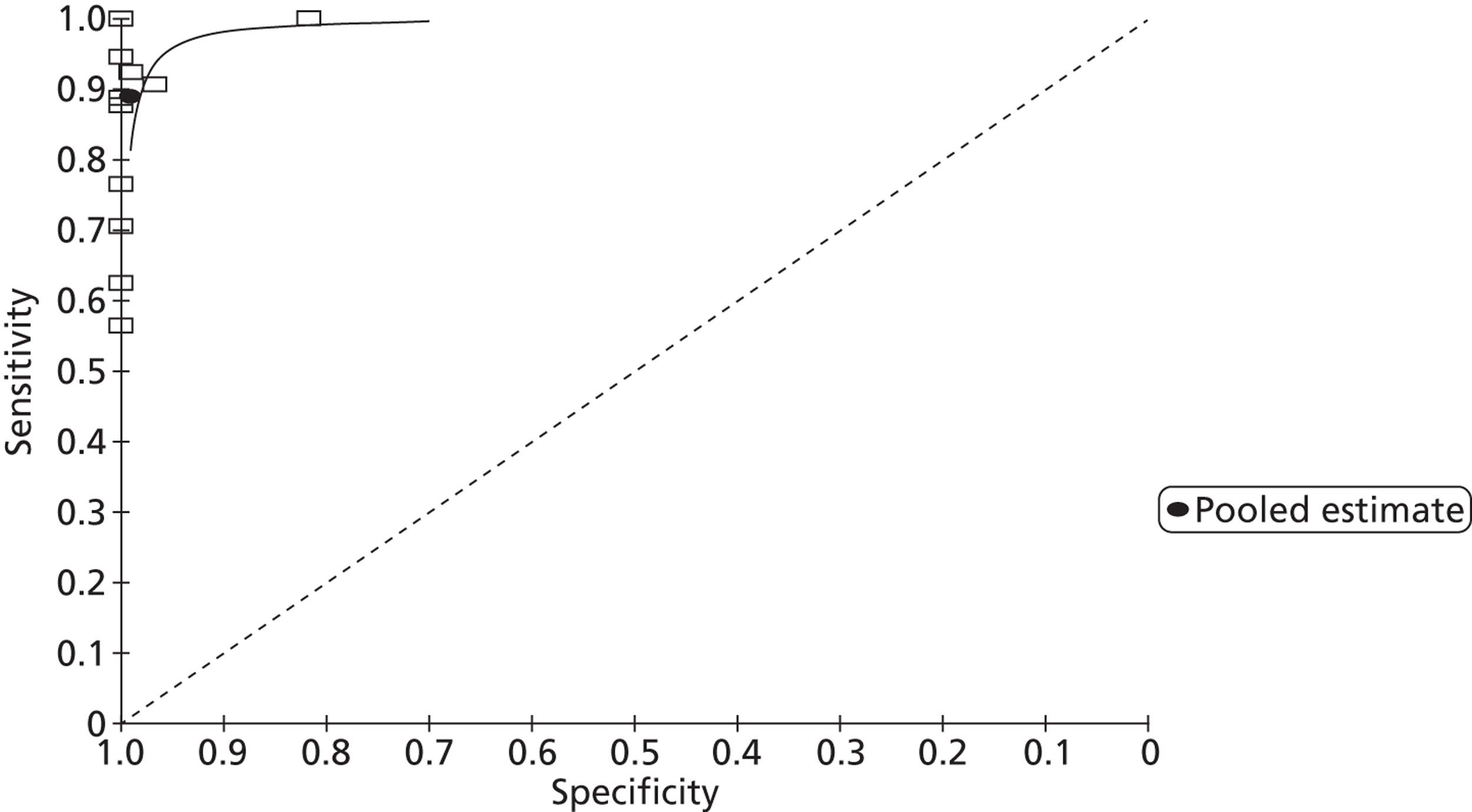
Sensitivity analysis (transoesophageal echocardiography compared with other tests)
In a single study146 (n = 134) comparing the diagnostic accuracy of TOE with that of TCD, the sensitivity of TOE to detect PFO was 0.97 (95% CrI 0.91 to 0.99) with a specificity of 0.98 (95% CrI 0.87 to 1.00). When TOE was compared with TMD113 (n = 44) to detect PFO, the sensitivity of TOE was 0.94 (95% CrI 0.73 to 1.00) with a specificity of 1.00 (95% CrI 0.87 to 1.00).
Atrial thrombi
In Kitayama et al. 138 (n = 70) the sensitivity of TTEf to detect left atrial thrombi was 0.67 (95% CrI 0.22 to 0.96) with a specificity of 1.00 (95% CrI 0.94 to 1.00) compared with ultrafast CT scan.
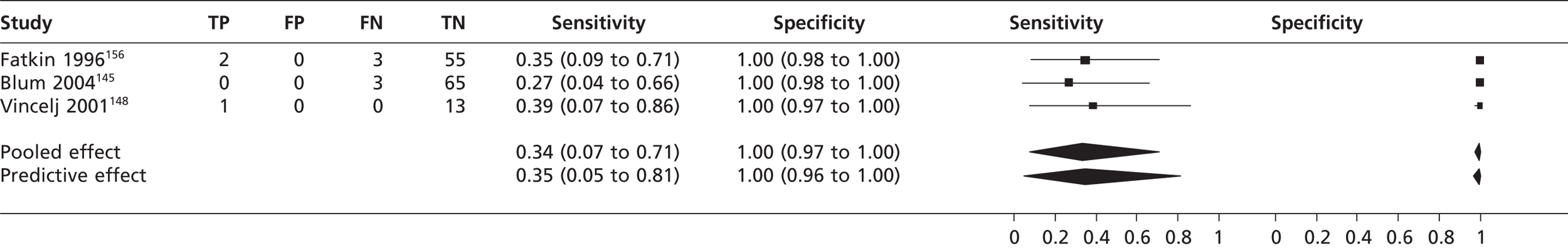
In three studies145,148,156 (n = 142) the pooled sensitivity of TTEf to detect left atrial thrombi was 0.34 (95% CrI 0.07 to 0.71) with a specificity of 1.00 (95% CrI 0.97 to 1.00) compared with TOE (Figure 9).
FIGURE 9.
Sensitivity and specificity of TTEf to detect left atrial thrombi vs. TOE.
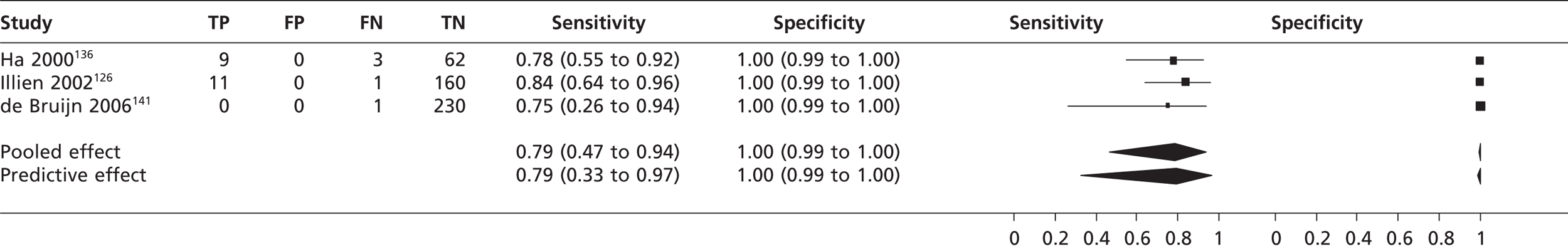
In second harmonic imaging mode the pooled sensitivity of TTE in three studies126,136,141 (n = 477) to detect left atrial thrombi was 0.79 (95% CrI 0.47 to 0.94) with a specificity of 1.00 (95% CrI 0.99 to 1.00) compared with TOE (Figure 10).
FIGURE 10.
Sensitivity and specificity of TTEh to detect left atrial thrombi vs. TOE.
Left ventricular thrombi
In Stratton et al. 122 (n = 78), when TTEf was compared with independent verification of left ventricular thrombi, TTEf had a sensitivity of 0.86 (95% CrI 0.65 to 0.97) with a specificity of 0.95 (95% CrI 0.85 to 0.99). Compared with MRI, the Lipke et al. study130 (n = 34) found that TTEh has a sensitivity to detect left ventricular thrombi of 0.53 (95% CrI 0.27 to 0.79) and a specificity of 0.74 (95% CrI 0.49 to 0.91). In a single study124 (n = 243) using TTE (frequency mode unclear), the sensitivity to detect left ventricular thrombi was 0.33 (95% CrI 0.16 to 0.55) with a specificity of 0.91 (95% CrI 0.86 to 0.94).
Atrial septal defect
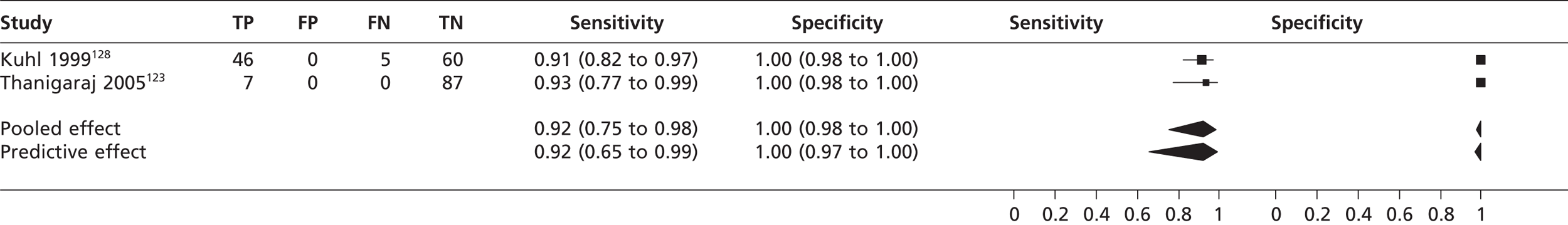
In four studies61,107,128,145 (n = 363) the pooled sensitivity of TTE in fundamental imaging mode to detect atrial septal defect was 0.36 (95% CrI 0.10 to 0.62) with a specificity of 1.00 (95% CrI 0.99 to 1.00) compared with TOE (Figure 11). In two studies123,128 (n = 205) the pooled sensitivity of TTEh to detect atrial septal defect was 0.92 (95% CrI 0.75 to 0.98) with a specificity of 1.00 (95% CrI 0.98 to 1.00) compared with TOE (Figure 12). In Shub et al. 121 the sensitivity of TTEf compared with surgical and cardiac catheterisation for the detection of oscium secundum atrial septal defect was 0.89 (n = 105, 95% CrI 0.81 to 0.94) and for the detection of ostium primum atrial septal defect was 1.00 (95% CrI 0.89 to 1.00); specificity was not estimated as all were positive for atrial septal defect .
FIGURE 11.
Sensitivity and specificity of TTEf to detect atrial septal defect vs. TOE.
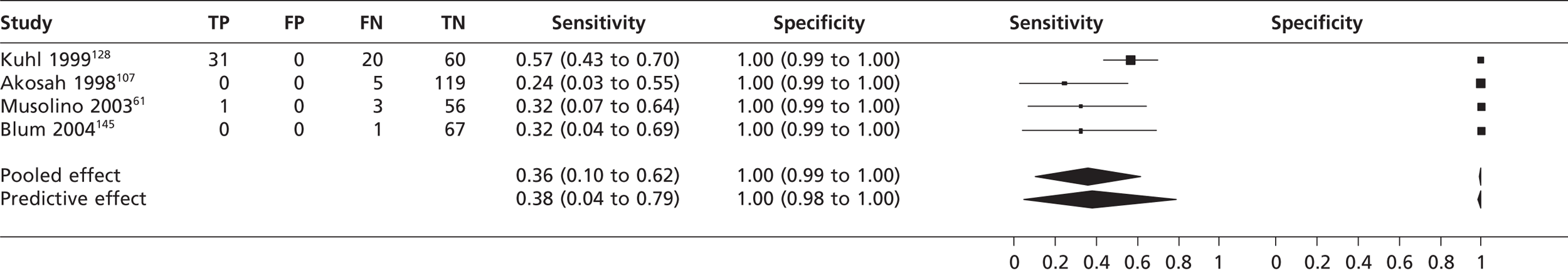
FIGURE 12.
Sensitivity and specificity of TTEh to detect atrial septal defect vs. TOE.
Atrial septal aneurysm
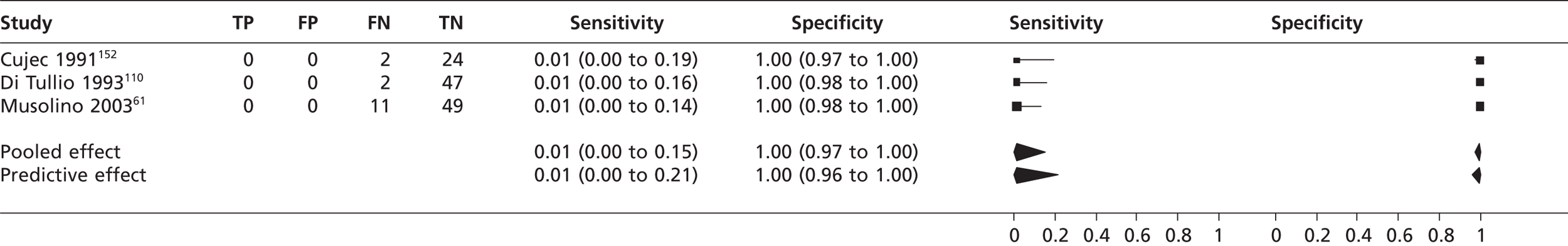
In three studies61,110,152 (n = 135) the pooled sensitivity of TTEf to detect atrial septal aneurysm was 0.01 (95% CrI 0.00 to 0.15) with a pooled specificity of 1.00 (95% CrI 0.97 to 1.00) compared with TOE (Figure 13). In a single study131 the sensitivity of TTEf to detect an atrial septal aneurysm was 53% compared with TOE; specificity was not calculable as all participants had atrial septal aneurysm. In the study by Gonzalez-Alujas et al. 146 (n = 55), TTEh had a sensitivity to detect an atrial septal aneurysm of 0.97 (95% CrI 0.85 to 1.00) with a specificity of 1.00 (95% CrI 0.85 to 1.00) compared with TOE.
FIGURE 13.
Sensitivity and specificity of TTEf to detect atrial septal aneurysm vs. TOE.
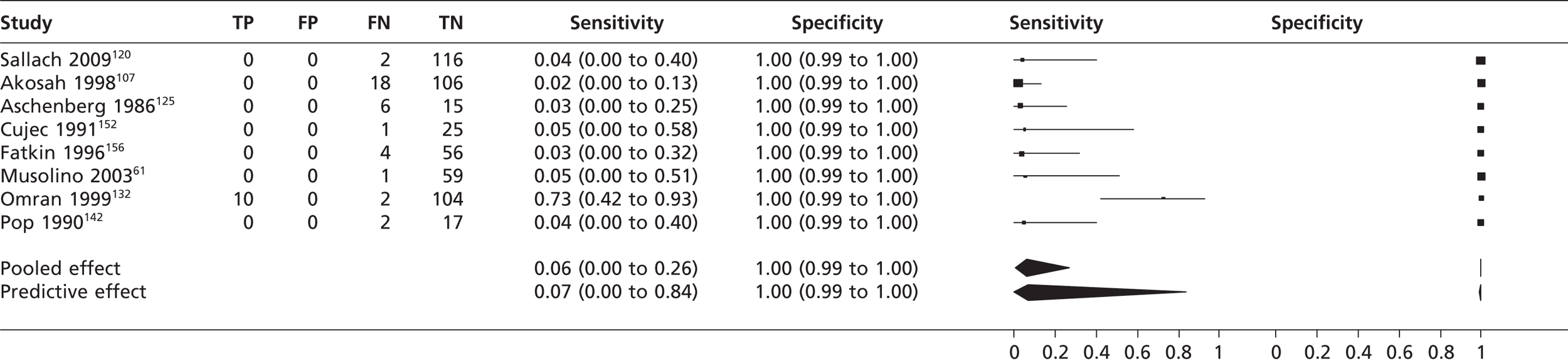
Left atrial appendage thrombi
In eight studies61,107,120,125,132,142,152,156 (n = 544) the pooled sensitivity of TTEf to detect left atrial appendage thrombi was 0.06 (95% CrI 0.00 to 0.26) with a specificity of 1.00 (95% CrI 0.99 to 1.00) compared with TOE (Figure 14). In a single study120 using TTEh (n = 118) the sensitivity to detect left atrial appendage thrombi was 1.00 (95% CrI 0.16 to 1.00) with a specificity of 1.00 (95% CrI 0.97 to 1.00).
FIGURE 14.
Sensitivity and specificity of TTEf to detect left atrial appendage thrombi vs. TOE.
Spontaneous echo contrast
The pooled sensitivity of TTEf to detect SEC from three studies114,132,142 (n = 185) was 0.05 (95% CrI 0.01 to 0.16) with a specificity of 1.00 (95% CrI 0.98 to 1.00) compared with TOE (Figure 15). The pooled sensitivity of TTEf to detect left atrial SEC (four studies,118,152,154,155 n = 605) was 0.00 (95% CrI 0.00 to 0.02) with a specificity of 1.00 (95% CrI 0.99 to 1.00) compared with TOE (Figure 16). In the study by Ha et al. 136 comparing TTEh with TOE (n = 73), the sensitivity to detect left atrial SEC was 0.88 (95% CrI 0.7 to 0.94) with a specificity of 1.00 (95% CrI 0.03 to 1.00). In the study by Black et al. 154 (n = 100), the sensitivity of TTEf to detect left ventricular SEC was 0.00 (95% CrI 0.00 to 0.84) with a specificity of 1.00 (95% CrI 0.96 to 1.00) compared with TOE.
FIGURE 15.
Sensitivity and specificity of TTEf to detect SEC vs. TOE.
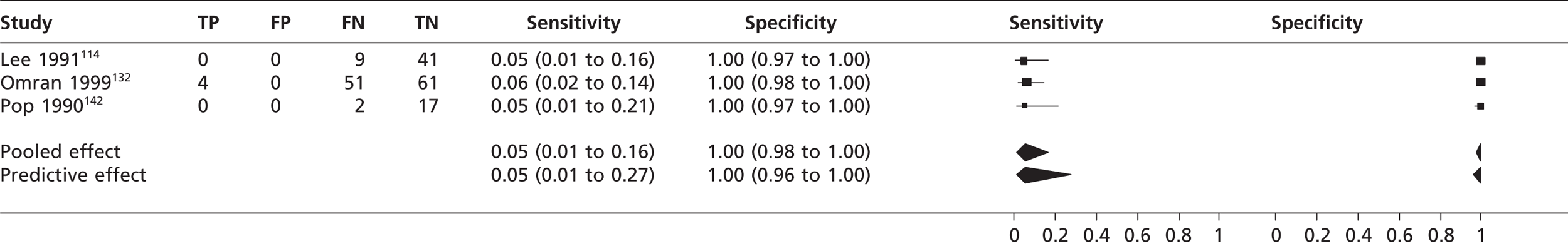
FIGURE 16.
Sensitivity and specificity of TTEf to detect left atrial SEC vs. TOE.
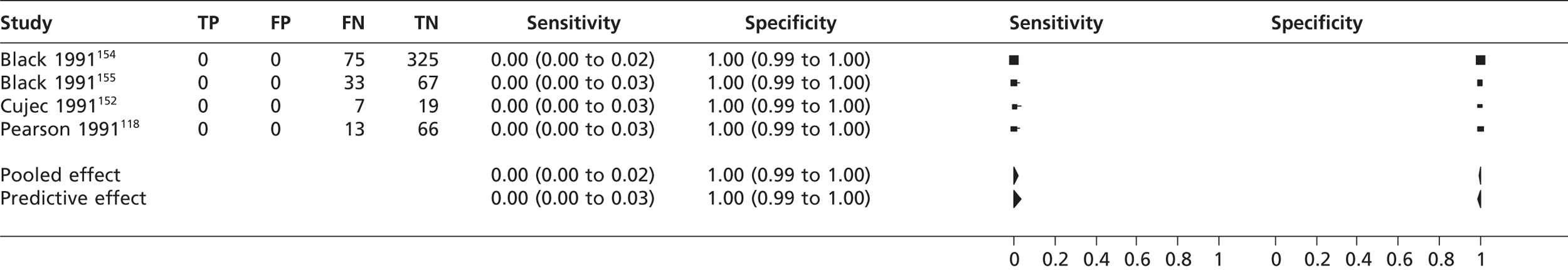
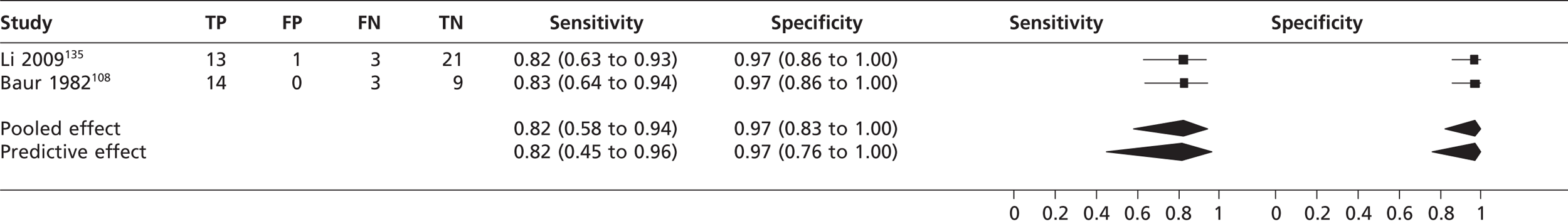
Left ventricular aneurysm
The pooled sensitivity of TTEf to detect left ventricular aneurysm in two studies108,135 (n = 64) was 0.82 (95% CrI 0.58 to 0.94) with a specificity of 0.97 (95% CrI 0.83 to 1.00) compared with left ventriculography (Figure 17).
FIGURE 17.
Sensitivity and specificity of TTEf to detect left ventricular aneurysm vs. left ventriculography.
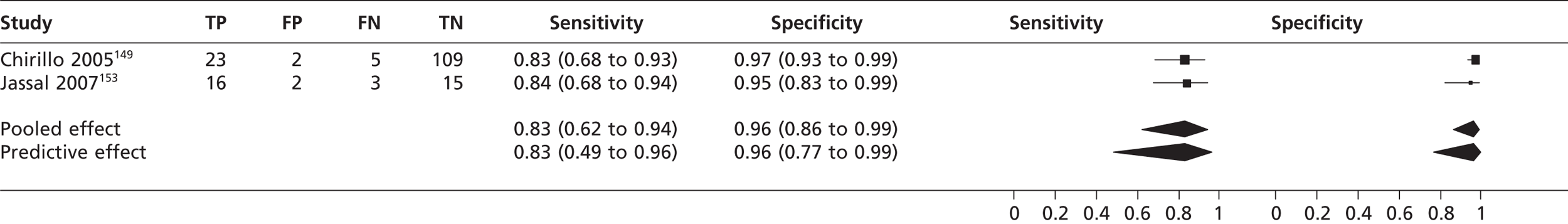
Cardiac vegetations
In two studies149,153 (n = 175) the sensitivity of TTEh to detect cardiac vegetation was 0.83 (95% CrI 0.62 to 0.94) with a specificity of 0.96 (95% CrI 0.86 to 0.99) compared with TOE (Figure 18). In the study by Chirillo et al. 149 (n = 139), the sensitivity of TTEf to detect cardiac vegetations was 0.36 (95% CrI 0.19 to 0.56) with a specificity of 0.80 (95% CrI 0.72 to 0.87) compared with TOE.
FIGURE 18.
Sensitivity and specificity of TTEh to detect cardiac vegetations vs. TOE.
Aortic valve stenosis
From a single study129 (n = 202) the sensitivity of TTEh to detect aortic valve stenosis compared with cardiac catheterisation was 1.00 (95% CrI 0.98 to 1.00) with a specificity of 0.93 (95% CrI 0.81 to 0.99).
Mitral valve regurgitation
In two studies,61,117 (n = 114) the pooled sensitivity of TTEf to detect mitral valve regurgitation was 0.96 (95% CrI 0.77 to 1.00) compared with TOE; specificity could not be calculated as all patients in one study117 were positive for mitral valve regurgitation. The accuracy of TTEh in one study119 (n = 80) for the detection of mitral valve regurgitation was lower than that of TOE, with a sensitivity of 0.57 (95% CrI 0.29 to 0.82) and a specificity of 0.94 (95% CrI 0.85 to 0.98).
Mitral valve stenosis
In the study by Musolino et al. 61 (n = 60), the sensitivity of TTEf to detect mitral valve stenosis was 1.00 (95% CrI 0.16 to 1.00) with a specificity of 1.00 (95% CrI 0.94 to 1.00) compared with TOE.
Mitral valve prolapse
In one study111 (n = 42) the sensitivity of TTEh compared with TOE to detect mitral valve prolapse was 0.93 (95% CrI 0.81 to 0.99). Specificity was not calculable as all participants were positive for mitral valve prolapse. In two studies111,147 using three-dimensional TTEh (n = 83) the pooled sensitivity to detect mitral valve prolapse was 0.97 (95% CrI 0.84 to 1.00) compared with TOE. Specificity could not be calculated, as all patients were positive for mitral valve prolapse.
Atrial myxoma
In one study148 (n = 14) the sensitivity of TTEf to detect atrial myxoma was lower than that of TOE, with a sensitivity of 0.80 (95% CrI 0.44 to 0.97) and a specificity of 1.00 (95% CrI 0.40 to 1.00).
Discussion of clinical effectiveness
Patent foramen ovale
For the diagnostic accuracy of TTEf for the detection of PFO using TOE as the reference standard, the pooled sensitivity was 34% with 100% specificity. The performance of TTEh was superior, with a sensitivity of 89%, but at the expense of specificity, which was 96%. TOE is considered the gold standard for the detection of PFO but its accuracy relies on an adequately performed Valsalva manoeuvre, which is not always possible in immobilised patients, and other studies have found that the sensitivity of TOE was marginally lower when compared with TCD146 and TMD. 113 The poorer performance of TOE in these studies suggests that it is an imperfect gold standard, unless TCD and TMD both gave FP results.
Atrial thrombi
The pooled sensitivity of TTEf to detect left atrial thrombi was 34% based on three studies,145,148,156 although in one study148 sensitivity was 100% based on one participant out of 14 being positive for left atrial thrombi; however, this may be over-representing the sensitivity given the low prevalence within the sample. The sensitivity of TTEh to detect left atrial thrombus was 79%, again based on just three studies,126,136,141 including one study141 that included only one patient positive for left atrial thrombi, which was undetected. Its contribution to the meta-analysis is that it may cause the sensitivity of TTEh to be underestimated. Detection of left atrial thrombus (67%) by TTEf compared with ultrafast CT showed considerable variation in TP detection rates, possibly because of the inclusion of poorly confirmed positive results in the left atrial thrombus group, and these figures may cause the diagnostic accuracy of TTEf to be overestimated. 138
Left ventricular thrombi
The diagnostic accuracy of TTEh to detect left ventricular thrombi was poor (53%) and showed considerable variation in two studies124,130 that used contrast-enhanced cardiac MRI as the reference standard. In another study122 using less advanced technology, TTEf had a sensitivity of 86% to detect left ventricular thrombus compared with positive identification of thrombi by independent verification (autopsy, aneurysmectomy and unequivocally positive indium-111 platelet imaging).
Atrial septal defect
In four studies61,107,128,145 the pooled sensitivity of TTEf to detect atrial septal defect was 36% with 100% specificity. The sensitivities between studies were heterogeneous, which may be because different subtypes of atrial septal defect (ostium secundum, ostium primum, sinus venosus, coronary sinus) were included in the sample populations, although none of the studies stated what type of atrial septal defect was identified. TTEh showed greater sensitivity (92%) to detect atrial septal defect, with 100% specificity, but did not equal the performance of TOE. The single study using surgical and cardiac catheterisation as the gold standard121 found that the sensitivity of TTEf to detect ostium secundum atrial septal defect was 89% and to detect ostium primum atrial septal defect was 100%.
Atrial septal aneurysm
The pooled diagnostic accuracy of TTEf to detect atrial septal aneurysm was 1%. In a single study131 the sensitivity was much higher (53%); however, in this study all 103 participants were positive for atrial septal aneurysm and it is not known whether study personnel were blinded to reference tests results or knowledge of participants’ cardiac condition. Knowing that all participants have atrial septal aneurysm could introduce performance bias, and such variability in sensitivity does not indicate that TTEf is a reliable test to detect atrial septal aneurysm. Only one study146 was included reporting data for the newer TTEh technology and the sensitivity (97%) and specificity (100%) detected are superior to those of the older TTE technology. Only one patient with atrial septal aneurysm was not detected by TTEh, suggesting that its performance is similar to that of TOE.
Left atrial appendage thrombi
The pooled sensitivity of the eight studies61,107,120,125,132,142,152,156 reporting TTEf data for left atrial appendage thrombi was 0.06% with a specificity of 100%. Seven of the studies61,107,120,125,142,152,156 failed to detect a single left atrial appendage thrombus, but one study132 had a detection rate of 83%. It is unclear why there is such inconsistency in the results. When TTEh was used to detect left atrial appendage thrombi the sensitivity and specificity were 100%, although this single study120 had only a small prevalence (2/116) and it is unclear whether this degree of accuracy would be replicated in a larger population.
Spontaneous echo contrast
The diagnostic accuracy of TTEf for detecting cardiac SEC (5%), left atrial SEC (0%) and left ventricular SEC (0%) was poor. TTEh detected more patients with left atrial SEC (sensitivity 88%) but was inferior to TOE, with nine out of 72 cases of left atrial SEC being undetected.
Left ventricular aneurysm
When TTEf was compared with left ventriculography for the detection of left ventricular aneurysm the sensitivity was lower (82%). No data were available to compare TTEf or TTEh against TOE or other routine diagnostic tests.
Aortic valve stenosis
Harmonic TTE had 100% diagnostic accuracy for the detection of aortic valve stenosis but did detect three FPs, resulting in a specificity of 93% compared with cardiac catheterisation. The results are based on only one study129 (n = 202); however, this study included a high proportion of patients (n = 160) with aortic valve stenosis, which decreases the possibility that this is a chance finding.
Cardiac vegetations
The detection of cardiac vegetations with TTEh (83% sensitivity and 96% specificity) was superior to detection with TTEf (36% sensitivity and 80% specificity) compared with TOE, although the use of TTEh would result in an estimated 17% of positive cardiac vegetations cases being undetected.
Mitral valve disorders
Based on two studies61,117 TTEf had an average sensitivity of 96% for the detection of mitral valve regurgitation and identified more patients with mitral valve regurgitation than TTEh (57% sensitivity) using TOE as the reference standard. This reversal in diagnostic accuracy, with the older technology being superior, is probably a reflection of the general heterogeneity in diagnostic accuracy studies and is likely to be a chance finding. For mitral valve stenosis, one study61 found that TTEf was 100% sensitive and specific but only two out of a sample of 60 patients had mitral valve stenosis, which limits the generalisation of this finding. The accuracy of TTEh with three-dimensional imaging for detecting mitral valve prolapse, a minor cardiac risk factor for stroke, was similar to that of TOE (97%). These findings are limited by the small number of studies included.
Atrial myxoma
Only one study148 reported data for atrial myxoma, indicating that TTEf has a lower (80%) sensitivity to detect this cardiac pathology than TOE. No studies using TTEh were identified.
Adverse effects and contraindications
None of the studies reported adverse events. TTE is considered a safe procedure being non-invasive. TOE is also considered a safe procedure although it is dependent on patient willingness and ability to undergo the procedure.
Discussion
The average sensitivity and specificity of TTE in both fundamental imaging mode and harmonic imaging mode were lower than those of the gold standard TOE. Generally, TTEh was superior to TTEf but the greater sensitivity did lead to a decreased specificity. TTEh demonstrated lower sensitivity than reference standards for the detection of cardiac pathologies requiring anticoagulation therapy such as left atrial thrombi and left ventricular thrombi. However, TOE is not suited to the detection of left ventricular apical thrombi, and TTE, although not as accurate as contrast-enhanced MRI, could serve as a screening tool for this pathology. TTEh had good sensitivity and specificity for the detection of left atrial appendage thrombi, albeit based on a small data set. Overall, these findings are limited by the small number of studies and the low prevalence rates within some studies.
Transoesophageal echocardiography demonstrated a greater diagnostic accuracy over a range of cardiac pathologies. However, TOE did not detect all PFO compared with TMD and TCD. Diagnosis of PFO relies on the correct execution of the Valsalva manoeuvre to provoke movement of micro-bubbles across the atrial septum, and this may have reduced the sensitivity of TOE. Most studies used TOE as the reference test to measure the accuracy of TTE and none reported any adverse event data. The differences in the diagnostic accuracy of TTE and TOE were found mainly in their sensitivity to detect cardiac sources of stroke, that is, the probability that the index test (TTE) will be positive in diseased cases; differences in specificity to correctly identify non-diseased cases were less remarkable with most studies reporting a specificity of 1.00.
Although both TTE and TOE are considered safe procedures, TOE is a semi-invasive procedure and requires a fasted patient and more personnel present. TTE is non-invasive, quicker to perform than TOE and needs only one sonographer. However, skeletal structure and tissue may impede test performance of TTE compared with TOE, and TOE is more appropriate for detecting some cardiac pathologies such as left atrial appendage thrombi. Therefore, TTE might be applied primarily to patients with stroke of undetermined aetiology (i.e. patients showing normal results on electrocardiography or carotid ultrasound) and who are candidates for oral anticoagulation, before escalation of further diagnostic tests. With improvements in TTE technology further diagnostic accuracy studies will be needed, and these should conform to the reporting standards of the Standards for Reporting of Diagnostic Accuracy (STARD) initiative157 to ensure that valuable data are accessible.
Chapter 5 Survey of relevant comparators
A survey was conducted with the main aim of gaining knowledge of current UK stroke centre diagnostic protocols to inform the decision as to which diagnostic test should be used as a comparator to TTE. A secondary aim was to gain knowledge of which guidelines are used by stroke centres to investigate and manage stroke or TIA (see Appendix 12 for the survey). The survey was sent by the Royal College of Physicians on our behalf to 170 NHS trusts in England and 15 health boards in Wales, and by NHS National Services of Scotland to 14 health boards in Scotland. The number of responses was 50, 9 and 12 from the English, Welsh and Scottish health authorities respectively. For 43 responders the country of origin is unknown. This represents a 57% response overall. Respondents were given the choice of either completing the survey online via Google Docs or completing the survey in Microsoft Word and returning the file by e-mail. The URL for the Google Docs survey and the Word file were provided in the e-mail sent to stroke units by the Royal College of Physicians and the NHS National Services of Scotland.
There are two questions in the questionnaire. The first asks which diagnostic tests are used in the following circumstances: never, only in young cases, only if all other tests are normal, only if there is strong clinical suggestion of cerebral embolism and in all cases. The second question asks which guidelines are used to investigate and manage stroke or TIA. The diagnostic tests included in the questionnaire were chosen on the advice of our clinical advisors and are 12-lead ECG, Holter monitoring, TOE, TTE, TTE with bubble contrast and ‘other’ tests. Twelve-lead ECG is a transthoracic interpretation of the electrical activity of the heart over a short period of time and is used to detect the underlying pathology of stroke. A Holter monitor is a portable ECG device used to monitor electrical activity of the cardiovascular system over longer periods of time than is possible with a 12-lead ECG.
The responses to the question ‘How often are the following tests used to investigate ischaemic stroke or TIA?’ are provided in Table 4. For 12-lead ECG the 0.88% of centres that use this tool only when there is a strong clinical suggestion of cardioembolism actually represents one centre out of the 114 responders; all other centres use this tool in all cases. Holter monitoring is used by 65% of centres only if there is a strong clinical suggestion of cardioembolism, by 16% of centres only if all other tests are normal and by 14% of centres in all cases. Only 1% of centres never use Holter monitoring. A total of 46% of centres use TOE only in young cases, 35% of centres use TOE only if there is a strong clinical suggestion of cardioembolism, 7% of centres never use TOE and no centres use TOE in all cases. In total, 67% of centres use TTE only if there is a strong clinical suggestion of cardioembolism, 15% of centres use TTE only if all other tests are normal, 9% of centres use TTE only in young cases, 8% of centres use TTE in all cases and 1% of centres never use TTE. A total of 62% of centres use TTE with bubble contrast only in young cases, 21% of centres use it only if there is a strong clinical suggestion of cardioembolism, 13% of centres use this method only if all other tests are normal, 5% of centres never use this method and no centres use this method in all cases.
| Survey response options | 12-lead ECG, n (%) | Holter monitoring, n (%) | TOE, n (%) | TTE, n (%) | TTE with bubble contrast, n (%) |
|---|---|---|---|---|---|
| Never | 0 (0) | 1 (1) | 8 (7) | 1 (1) | 6 (5) |
| Only young cases | 0 (0) | 4 (4) | 52 (46) | 10 (9) | 69 (62) |
| Only if all other tests are normal | 0 (0) | 18 (16) | 14 (12) | 17 (15) | 14 (13) |
| Only if strong clinical suggestion of cerebral embolism | 1 (0.88) | 74 (65) | 39 (35) | 76 (67) | 23 (21) |
| All cases | 113 (99.12) | 16 (14) | 0 (0) | 9 (8) | 0 (0) |
| No response to question | 0 (0) | 1 (1) | 1 (1) | 1 (1) | 2 (2) |
| Total | 114 (100) | 113 (100) | 113 (100) | 113 (100) | 112 (100) |
In England and Wales the Royal College of Physicians guidelines and NICE guidelines are used to investigate stroke or TIA by 44% and 37% of stroke centres respectively. Amended guidelines, internal guidelines and no guidelines are used to investigate stroke or TIA by 10%, 5% and 4% of stroke centres respectively. No centres use ‘other’ guidelines for investigation (Table 5). NICE guidelines and the Royal college of Physicians guidelines are used to manage stroke or TIA by 42% and 40% of stroke centres respectively. Amended guidelines, internal guidelines and no guidelines are used to manage stroke by 10%, 7% and 1% of stroke centres respectively (see Table 5). Stroke centres were asked to provide copies of amended guidelines; unfortunately, however, none were provided and we therefore have no information regarding the amendments.
| Guidelines | Investigate, n (%) | Manage, n (%) |
|---|---|---|
| Internal | 5 (5) | 7 (7) |
| NICE | 38 (37) | 43 (42) |
| Royal College of Physicians | 45 (44) | 41 (40) |
| Other | 0 (0) | 1 (1) |
| Amended for local use | 10 (10) | 10 (10) |
| None | 4 (4) | 1 (1) |
| Total | 102 (100) | 103 (100) |
In Scotland, 75%, 17% and 8% of stroke centres use Scottish Intercollegiate Guidelines Network (SIGN) guidelines, amended guidelines for local use and no guidelines, respectively, to investigate stroke or TIA. No stroke centres use internal, NICE, Royal College of Physicians or other guidelines to investigate stroke or TIA (Table 6). Other guidelines, amended guidelines, internal guidelines, NICE guidelines and no guidelines are used by 45%, 27%, 9%, 9% and 9% of centres, respectively, to manage stroke or TIA. No stroke centres use Royal College of Physicians or SIGN guidelines to manage stroke or TIA (see Table 6). We are unable to explain why 75% of centres use SIGN guidelines to investigate stroke or TIA but none of these centres use these guidelines to manage these conditions.
| Guidelines | Investigate, n (%) | Manage, n (%) |
|---|---|---|
| Internal | 0 (0) | 1 (9) |
| SIGN | 9 (75) | 0 (0) |
| NICE | 0 (0) | 1 (9) |
| Royal College of Physicians | 0 (0) | 0 (0) |
| Other | 0 (0) | 5 (45) |
| Amended for local use | 2 (17) | 3 (27) |
| None | 1 (8) | 1 (9) |
| Total | 12 (100) | 11 (100) |
Discussion of survey results
In the ‘Please state other diagnostic test’ response box, many clinicians took the opportunity to give more details about the decision-making processes that are used to decide which test should be used in which circumstance. A sample of clinicians’ comments can be seen in Appendix 13. It is clear from these responses that protocols are much more complicated and varied than we expected and could not be captured accurately by our questionnaire. To accurately describe current management practice a very sophisticated questionnaire would be required, which may result in poor response rates and yield little useful information. Although the survey distributed had been approved by our clinical advisors, a preferable approach would have been to have convened experts to write guidelines; however, this was beyond the remit of this study. The results of question 1 of our survey should therefore be viewed as a simple overview of the types of protocols used.
Chapter 6 Assessment of cost-effectiveness
This chapter of the report focuses on the health economics of echocardiography diagnostic strategies for the management of ischaemic stroke and TIA. It includes a brief review of existing economic evaluations and a detailed explanation of the methodologies and results of a de novo economic model. The population in the assessment of cost-effectiveness is the same as that defined in Chapter 2 (see Decision problem).
Systematic review of existing cost-effectiveness evidence
The primary objective of this review was to identify and evaluate studies exploring the cost-effectiveness of TTE in the assessment of first-episode diagnosed ischaemic stroke and TIA patients in secondary care. The secondary objective was to evaluate published modelling methodologies to inform our own modelling methodology.
Identification of studies
Electronic databases
Studies were identified by searching the following electronic databases during March 2011:
-
MEDLINE In-Process & Other Non-Indexed Citations (via Ovid SP) (1950 to present)
-
CINAHL (via EBSCOhost) (1981 to present)
-
EMBASE (via Ovid SP) (1980 to present)
-
Web of Science (includes Science Citation Index and Conference Proceedings Citation Index) (via Web of Knowledge) (1899 to present)
-
DARE (via The Cochrane Library) (approximately 1995 to present)
-
NHS EED (via The Cochrane Library) (approximately 1995 to present)
-
PsycINFO (via Ovid SP) (1806 to October 2011 week 4).
Sensitive keyword strategies using free-text and, when available, thesaurus terms using Boolean operators and database-specific syntax were developed to search the electronic databases. Synonyms relating to the condition were combined with a search filter aimed at restricting results to economic and cost-related studies (used in the searches of MEDLINE, CINAHL, EMBASE and PsycINFO, with an amended version used for Web of Science). Date limits or language restrictions were not used on any database. All resources were searched from inception to March 2011. An example of the MEDLINE search strategy is provided in Appendix 9.
All identified citations from the electronic searches and other resources were imported into, and managed using, Reference Manager bibliographic software.
Inclusion and exclusion criteria
Studies were selected for inclusion according to predetermined inclusion and exclusion criteria. Studies were included if they reported the cost-effectiveness of TTE in first-episode diagnosed stroke and TIA patients, and estimated the benefits in terms of life-years gained or quality-adjusted life-years (QALYs). Studies that did not report costs and outcome estimates or that did not report an estimate of cost-effectiveness (e.g. costing studies) were excluded. Studies not published in the English language were also excluded.
One reviewer (AR) independently screened all titles and abstracts. When there was uncertainty in the decision a second reviewer (MH) was used and a consensus was obtained through discussion. Full papers were obtained for any titles/abstracts that were considered relevant or when the title/abstract information was not sufficient to make a decision.
Quality assessment strategy
The quality of the economic evaluation studies that met the inclusion criteria was assessed using an adapted version158 of the Drummond and Jefferson British Medical Journal criteria for economic evaluation159 and the Consensus on Health Economic Criteria (CHEC)-list (see Appendix 11). 160 The use of these checklists ensures a consistent approach to assessing the quality of each economic evaluation.
Results of the cost-effectiveness review
The systematic searches identified 1746 potentially relevant citations. After screening titles and abstracts, two full-text papers37,161 were retrieved and assessed in detail; both of these papers were considered to meet the inclusion criteria for the review. A flow chart describing the process of identifying relevant literature can be found in Appendix 10.
Meenan et al.37
Overview
Meenan et al. 37 developed a decision-analytic Markov model to evaluate the cost-effectiveness of imaging strategies that use TTE and TOE for identifying intracardiac thrombus in new stroke and TIA patients. A systematic review of the evidence was performed to (1) identify the pathologies for which there is evidence of a causal association for stroke or TIA and for which there is evidence that identification of the pathology on echocardiography will change patient management and (2) find data on the sensitivity and specificity of TTE and TOE in detecting intracardiac thrombus. Pathologies that do not represent conditions for which echocardiography is typically used as a screening tool were excluded, as were disorders that may be associated with stroke but which are clinically apparent without echocardiography. In consultation with an expert panel the authors decided that only the identification of left atrial and left ventricular thrombus on echocardiography would alter patient management; all other conditions were excluded.
The model follows for 30 years a cohort of first-episode diagnosed white male stroke patients with a mean starting age of 65 years. Patients diagnosed with either left atrial or left ventricular thrombus received standard medical treatment (SMT) plus warfarin; those without a thrombus received SMT. SMT was assumed to be aspirin alone. The authors did not include other antiplatelet therapies in the model because of a lack of clinical effectiveness evidence for them at the time. Nine testing strategies were evaluated:
-
treat all with SMT
-
treat all with anticoagulation plus SMT (AC; anticoagulation)
-
all receive TTE (all TTE)
-
all receive TOE (all TOE)
-
all with heart disease receive TTE; others receive SMT (cardiac TTE)
-
all with heart disease receive TOE; others receive SMT (cardiac TOE)
-
all receive TTE, negative TTE prompts TOE (TTE sequential)
-
all with heart disease receive TTE, negative TTE prompts TOE (cardiac sequential)
-
all with heart disease receive TTE, negative TTE prompts TOE; all with no heart disease receive TOE (combined sequential).
The only functional difference in the model between patients with heart disease and patients without heart disease was a higher prevalence of intracardiac thrombus in the former.
The states in the Markov model were:
-
TIA
-
minor stroke
-
moderate stroke
-
severe stroke
-
short-term complications
-
long-term complications
-
dead.
A monthly cycle and a half-cycle correction were used. Transition probabilities varied over time. The only adverse event included for echocardiography was a small mortality risk and a transient quality-of-life (QoL) reduction from undergoing TOE. Adverse events from anticoagulation included gastrointestinal bleeding and intracranial haemorrhage (ICH). Life tables were used to establish baseline mortality rates.
All direct costs related to stroke management were included. Cost estimates were taken from the literature or from Medicare fee schedules. QoL utilities were taken from the Stroke Patient Outcomes Research Team. 162 The utility of a long-term ICH was assumed to be the same as that of a severe stroke and the utility of a short-term ICH was assumed to be equal to that of a minor stroke. Costs and utilities were discounted at an annual rate of 3%.
Both univariate sensitivity analysis and probabilistic sensitivity analysis (PSA) were undertaken. Cost-effectiveness acceptability curves (CEACs) were used to report the PSA results.
In the deterministic analysis the incremental cost-effectiveness ratios (ICERs) for both cardiac TTE and cardiac TOE compared with SMT are in excess of $83,000; all other strategies are dominated by SMT. In the univariate sensitivity analysis, for both cardiac TTE and cardiac TOE compared with SMT, the ICERs were > $59,000, with one exception. When the prevalence of thrombus with heart disease was increased to 0.3 (compared with the baseline value of 0.05), the ICER for cardiac TOE was $33,000. Mean values from the PSA are not reported. The CEAC indicates that SMT is likely to be cost-effective compared with all other strategies at willingness-to-pay (WTP) thresholds of < $58,000. Above this threshold the cardiac TOE strategy was likely to be cost-effective compared with all other strategies. Expected value of perfect information (EVPI) analysis estimated that the EVPI for an individual person is around $100 (threshold stated by the author to be low but the actual threshold is not stated), which equates to $20M on a population basis based on stroke incidence in the USA.
Comments
This appears to be a well-constructed model parameterised by relevant data at the time. This study scored highly on the assessment criteria.
McNamara et al.161
Overview
This study used Markov decision-analysis techniques to evaluate the cost-effectiveness of nine diagnostic strategies in a cohort of 65-year-old patients with first-episode diagnosed stroke. The model cycle was monthly for events including recurrent cerebrovascular accident, ICH, gastrointestinal bleeding and death. The strategies evaluated are:
-
no imaging, treat all
-
no imaging, treat none
-
cardiac history, TTE
-
cardiac history, TTE then TOE if TTE negative
-
no cardiac history, TOE
-
cardiac history, TOE
-
all TTE
-
all TTE then TOE if TTE negative
-
all TOE.
The pathological conditions evaluated in the model are left atrial thrombus, other potential cardiac sources of thrombus, aortic plaque only and no identifiable cardiovascular source of thrombus. SMT appears to be aspirin. Patients with AF were excluded from the model.
All data used in the model were determined using the best available estimates identified from a systematic review of the literature.
Costs in the model included direct medical costs, staff and technical costs and costs due to lost productivity. The model thus takes a societal perspective. Utilities in the model were taken from a study by Solomon et al. 163 Costs and utilities were discounted at an annual rate of 3%.
A univariate sensitivity analysis was undertaken but PSA was not undertaken.
In the base-case results, both the ‘selective TOE’ and the ‘all TOE’ strategies had ICERs that were < $20,000. Strategies that used TTE alone or in sequence with TOE were not found to be cost-effective. In the sensitivity analysis the results were most sensitive to the efficacy of anticoagulation and the rate of ICH with anticoagulation. Of interest is that the results were not sensitive to the sensitivity of TOE.
Comments
This is a moderately well-constructed model with the main criticism being the lack of a PSA analysis. TOE was found to be cost-effective in this model whereas in the model of Meenan et al. 37 it was not. The likely reasons for this discrepancy were outlined in the Meenan et al. 37 study. First, thrombus prevalence was assumed to be 8% in the study by McNamara et al. 160 compared with 2% in the study of Meenan et al. ,37 which could be because the McNamara et al. 161 study used prevalence data that included AF patients. Second, in the base case TOE was assumed to have 100% accuracy in the McNamara et al. 161 study; however, this is unlikely. Third, the stroke recurrence rate was assumed to be 40% in the McNamara et al. 161 study, which is substantially higher than the rate used by Meenan et al. 37 Fourth, all thrombi were assumed to be left atrial and, fifth, the duration of anticoagulation was unspecified in the McNamara et al. 161 study. Finally, the cost of TOE was substantially lower in the McNamara et al. 161 model than in the Meenan et al. 37 model. These assumptions would all favour TOE.
Independent economic assessment
This section details the methods and results of our health economic model, constructed to evaluate the cost-effectiveness of the addition of TTEh to the routine assessment of patients who have had a first-episode diagnosed stroke or TIA in the UK. The project’s clinical advisors provided information that TTEh has superseded TTEf in most hospitals in the UK. For this reason, and also because of the improved diagnostic accuracy of TTEh compared with TTEf, we have considered TTEh as the baseline in this analysis. The strategies evaluated were no test and no treatment; TOE only; TTEh only; TTEh then TOE in patients testing positive on TTEh (TTEh +ve TOE, attempting to identify FPs); and TTEh then TOE in patients testing negative on TTEh (TTEh –ve TOE, attempting to identify FNs). We were unable to include strategies in subgroups of patients in which, for example, TTEh is used first and then TOE is used. Possible subgroups would include young patients with cryptogenic stroke or patients with PFO or atrial septal defect in which greater anatomical/physiological accuracy is needed. This is because of the lack of evidence regarding the rate of recurrent stroke in these subpopulations. The analysis was undertaken to address the lack of any published cost-effectiveness evidence from the perspective of the NHS in the UK. The key aim was to determine the optimal echocardiography management strategy in terms of cost-effectiveness.
Methods
Included and excluded pathologies
The systematic review evaluated the published evidence on the diagnostic accuracy of echocardiography in patients with cardiac pathologies identified to be risk factors for stroke or TIA. The pathologies for which published evidence of diagnostic accuracy was available are:
-
PFO
-
atrial septal defect
-
atrial septal aneurysm
-
mitral valve regurgitation
-
left atrial thrombi
-
left atrial appendage thrombi
-
SEC
-
left atrial SEC
-
mitral valve stenosis
-
left ventricular SEC.
However, for the economic modelling it is important to include only those pathologies for which knowledge of them on echocardiography would alter patient management and for which there is published evidence that treatment of the pathology is effective in preventing further strokes. On the advice of our clinical advisors the only pathologies for which this criterion applies are left atrial and left ventricular thrombi. However, no studies were identified in the systematic review that evaluated the diagnostic accuracy of echocardiography for left ventricular thrombi and therefore only the finding of left atrial thrombi is included in the analysis. The model incorporates an estimate of the prevalence of left atrial thrombus and only these patients receive the benefits, harms and costs of treatment. It should be noted that there is little evidence showing an association between left atrial thrombus (without AF) and stroke; however, we agree with the opinions expressed in the Meenan et al. 37 study that biomedical knowledge suggests that left atrial thrombus is a likely cause of cardioembolic stroke and that there is a general consensus that treatment of intracardiac thrombus with anticoagulants is appropriate and probably reduces the risk of recurrent stroke. The sensitivity of TTEh to detect left atrial thrombus was 79%; this was based on just three studies126,136,141 including one study141 that included only one patient positive for left atrial thrombus, which was undetected. Its contribution to the meta-analysis is that it may cause the sensitivity of TTEh to be underestimated.
The costs and benefits of echocardiography in the management of patients with a first-episode diagnosed stroke or transient ischaemic attack
The main benefits of echocardiography relate to the rapid identification and treatment of patients with left atrial thrombi. The main disadvantage is the risk of bleeding associated with anticoagulation. The direct costs are those of echocardiography, anticoagulation treatment including adverse events (intracranial and gastrointestinal bleeds) and initial stroke or TIA treatment including the CT scan and costs associated with the long-term care of mild, moderate and severe disability due to a stroke. We constructed a model to allow us to analyse the effects of different echocardiography management strategies on these costs and benefits.
The decision-analysis model structure
The modelling was conducted in two stages.
Model 1
This model is an individual patient micro-simulation developed using Simul8 software (version 17; Simul8 Corporation, Boston, MA, USA) to explore the costs and health outcomes associated with echocardiography in the management of TIA/stroke. The analysis was conducted for 100,000 patients aged 45, 55 and 65 years of age when presenting to the emergency department. The model takes a lifetime horizon with mean life expectancy based on UK interim life tables. 164 The analysis did not consider men and women separately. The economic perspective of the model is the NHS in the UK. Costs and health benefits were discounted at an annual rate of 3.5% as recommended by the NICE guide to the methods of technology appraisal. 165 Figure 19 shows the treatment pathways in the model.
FIGURE 19.
Model diagram. IC, intracranial.
The outcomes of this model are the costs and QALYs associated with the following: (1) patients with an intracardiac thrombus and treated (TPs), (2) patients with an intracardiac thrombus and untreated (FNs), (3) patients without an intracardiac thrombus and treated (FPs), (4) patients without an intracardiac thrombus and untreated (TNs).
Patients enter the model with a TIA, a stroke leading to an independent outcome or a stroke leading to a dependent outcome. Patients in the TIA state can have an independent stroke, a dependent stroke or a fatal stroke, or can die from other causes. Patients in the independent stroke state can have a recurrent independent stroke, a dependent stroke or a fatal stroke, or can die of other causes. Patients in the dependent stroke state can have a recurrent dependent stroke, an independent stroke or a fatal stroke or can die of other causes. Patients in the dependent stroke state experiencing an independent stroke incur the cost of initial treatment and remain in the dependent stroke state. We assume that patients in the dependent stroke state already have a poor QoL and this is unaffected by a further independent stroke. Patients receiving anticoagulation treatment can experience a gastrointestinal or an intracranial (IC) bleed. For patients experiencing a bleed event it is assumed that treatment is immediately stopped. Patients with a gastrointestinal bleed incur a cost of treating the bleed and a temporary QALY decrement. Patients with an IC bleed will have an outcome represented by a Glasgow Outcome Score (GOS) ranging from 1 to 5, where GOS 1 = death, GOS 2 = persistent vegetative state (PVS), GOS 3 = severe disability, GOS 4 = moderately disabled and GOS 5 = good recovery. Patients incur appropriate costs and QALYs associated with these outcomes (see Tables 10 and 11).
In the model, the time to the next event determines the pathway that a patient will take. For each of the events described above, the time to the event is sampled and the patient will experience the event that occurs first (see Tables 10 and 11).
All patients are assumed to have an ECG. To model otherwise would require estimates of the diagnostic accuracy of whatever test or clinical decision-making strategy was used and this information is not available.
Model 2
This model was constructed in Microsoft Excel (version 12; Microsoft Corporation, Redmond, WA, USA) to enable the results of the first model (costs and QALYs of TPs, FNs, TPs and TNs) to be combined with the prevalence of left atrial thrombi and the costs and diagnostic accuracy of the different tests to obtain estimates of the costs and QALYs of the five strategies being investigated.
Most studies identified in the assessment of diagnostic accuracy section used TOE as a reference standard to measure the diagnostic accuracy of TTEh, under the assumption that TOE is 100% accurate. In the modelling we have not assumed that TOE is 100% accurate; instead, we have based the diagnostic accuracy of TOE on a study by Meenan et al. 166 As the diagnostic accuracy of TTEh is based on a comparison with TOE, the accuracy of TTEh has been adjusted by multiplying the sensitivity and specificity of TTEh by the sensitivity and specificity of TOE as reported by Meenan et al. 166 This would underestimate the accuracy of TTEh in the case in which the results were discordant between TTEh and TOE and the TTEh diagnosis was actually correct. However, this is expected to introduce little bias.
To model the strategies in which two tests were performed, an estimate of the combined diagnostic accuracy is needed. For example, for the TTEh –ve TOE strategy, using a hypothetical cohort of 1000 patients, the numbers of patients who would be identified as FN, FP, TN and TP were calculated using the prevalence of thrombi and the sensitivity and specificity of each test. Patients who were positive on TTEh were not evaluated further and thus there was a combination of TPs and FPs associated with the test characteristics of TTEh.
Those patients who were diagnosed as negative represented a cohort of TNs and FNs, based on the test characteristics of TTEh. These patients were then assessed with TOE with some patients being reclassified as positives. The final categorisation of patients was used to form an initial estimate of the sensitivity and specificity of the combination of tests. This methodology was repeated for the TTEh +ve TOE strategy. See worked examples below.
TTE –ve transoesophageal echocardiography strategy methodology
Table 7 shows the results of the initial TTEh test. The methodology is shown below:
-
number of patients testing as TP = 1000 × sensitivity (0.7282) × thrombi prevalence (0.0545) = 39.7
-
number of patients testing as TN = 1000 × specificity (0.9685) × 1 – thrombi prevalence (0.9455) = 915.7
-
number of patients testing as FN = 1000 × 1 – sensitivity (0.2718) × thrombi prevalence (0.0545) = 14.8
-
number of patients testing as FP = 1000 × 1 – specificity (0.0315) × 1 – thrombi prevalence (0.9455) = 29.8
| Actual | Test | |
|---|---|---|
| + | – | |
| + | 39.7 | 14.8 |
| – | 29.8 | 915.7 |
Therefore, 14.8 + 915.7 patients are retested and, of these, 14.8 × the sensitivity of TOE (0.93) move to TPs (13.8), leaving 1.0 FN, and 915.7 × (1 – the specificity of TOE) (0.03) move to FPs (27.5), leaving 888.2 TNs.
When considering those who initially tested negative on TTEh, the overall accuracy is as given in Table 8.
| Actual | Test | |
|---|---|---|
| + | – | |
| + | 53.5 | 1.0 |
| – | 57.3 | 888.2 |
The sensitivity of the combined tests (TTEh –ve TOE) is 53.5/(53.5 + 1) = 0.982 and the specificity of the combined tests is 888.2/(888.2 + 57.3) = 0.939.
These estimates, however, indicated that, for the TTEh –ve TOE strategy, the sensitivity of the combined tests would be greater than that of TOE alone. Clinically, however, it is believed that TOE is the more sensitive diagnostic test, with TTEh identifying only a subset of those TP patients diagnosed by TOE. As such, it was not deemed plausible that the combined tests would have a greater sensitivity than TOE alone and therefore the sensitivity of the combined tests was set to the value for TOE alone.
TTE +ve transoesophageal echocardiography strategy methodology
The results of the TTEh +ve TOE strategy in 1000 hypothetical patients are shown in Table 9.
| Actual | Test | |
|---|---|---|
| + | – | |
| + | 36.9 | 17.6 |
| – | 0.9 | 944.6 |
Referring back to Table 7, 39.7 + 29.8 patients are retested and, of these, 39.7 × (1 – sensitivity of TOE) (0.07) move to FN, with the remainder staying as TP, and 29.8 × specificity of TOE (0.97) move to TN, with the remainder staying as FP.
When considering those who initially tested positive on TTEh the overall accuracy is as given in Table 9.
The sensitivity of the combined tests (TTEh +ve TOE) is 36.9/(36.9 + 17.6) = 0.677 and the specificity of the combined tests is 944.6/(944.6 + 0.9) = 0.999.
The effectiveness of TOE and TTEh is based on the meta-analysis of the sensitivity and specificity of these tests described in the clinical systematic review (see Chapter 4, Data analyses of diagnostic accuracy). A weighted average of the costs and QALYs of the four scenarios described above (TP, FP, FN and TN) is estimated based on the prevalence of left atrial thrombus in stroke patients and the sensitivity and specificity of TOE and TTEh.
The methodology above assumes that the tests are not correlated, which we believe to be unlikely. We therefore re-estimated the sensitivity and specificity of the combined tests under the assumption that there was correlation between them. We are unaware of any evidence regarding the degree of correlation and therefore estimated values regarded as sensible. Under these assumptions the sensitivity and specificity of the combined test strategies were estimated at 0.92 and 0.97 respectively. The cost-effectiveness implications of these estimates was evaluated in a sensitivity analysis (see Probabilistic sensitivity analysis results).
Initial and subsequent stroke or transient ischaemic events
The numbers of initial and subsequent TIA or stroke events were taken from a study undertaken for NICE. 167 The proportions of initial strokes that were independent or dependent were taken from a national stroke audit undertaken by the Royal College of Physicians168 and a study by Clark et al. 169 that measured the long-term risks of stroke in patients with a TIA. The proportions of subsequent strokes that were independent, dependent or fatal were also taken from the above studies. 168,169
Stroke recurrence
A literature review was conducted to identify studies that measured the rate of stroke recurrence in patients with a thrombus who were treated and who were untreated. A similar literature review was also conducted by Meenan et al. 37 as part of their cost-effectiveness analysis. Our review failed to identify any further studies than those already identified by Meenan et al. 37 We also reviewed publications by the South London Stroke Register but were unable to find the specific recurrence rates needed for the modelling. The rates of stroke recurrence used for treated and untreated patients are therefore the same as those used by Meenan et al. 37
Anticoagulation complications
The annual rates of fatal and non-fatal gastrointestinal and ICH are taken from Simpson et al. 170 Rates are higher in the first 3 months, which may be due to overprescribing whilst the optimal dose is determined. 170 The GOS outcomes of those surviving are taken from a study by Holmes et al. 171 This study estimated GOS outcomes for patients with an IC bleed requiring surgery that is delayed because the patient is not in hospital when the haemorrhage occurs. It is assumed that this would be the case in our model and it is therefore appropriate to assume delayed treatment. It is assumed that all patients with an ICH require neurosurgery. A gastrointestinal haemorrhage was assumed to be equal to hospitalisation for 2 weeks. 172 During this time patients were assumed to accrue no QALYs but afterwards were assumed to have a normal health-related QoL. The Multi-Society Task Force on Persistent Vegetative State reported the mean length of survival for adults in a PVS (GOS 2) as 3.6 years, which was used in the model. 173
All event rates are shown in Tables 10 and 11.
| Description | Mean value | Distribution | Statistical parameters | Source |
|---|---|---|---|---|
| Initial TIA | 0.208 | Dirichlet | a = 208, b = 792 | NICE167 |
| Initial stroke | 0.792 | Dirichlet | ||
| Independent stroke | 0.42 | Dirichlet | a = 420, b = 580 | ISWP,168 Clark et al.169 |
| Dependent stroke | 0.58 | Dirichlet | ||
| Rate of stroke recurrence or initial stroke following a TIA | ||||
| In patients with an untreated thrombus in year 1 | 0.22 | Beta | a = 1.32, b = 4.68 | Meenan et al.37 |
| In all patients with a thrombus (untreated or treated) after year 1 | 0.03 | Beta | a = 1.17, b = 37.8 | |
| In untreated patients without a thrombus in year 1 | 0.12 | Beta | a = 42.6, b = 311 | |
| In untreated patients without a thrombus after year 1 | 0.03 | Normal | Mean 0.0287, SD 0.0027 | Assumption |
| Effect of treatment on rate of stroke recurrence (relative risk) | ||||
| For patients with a thrombus in year 1 | 0.57 | Beta | a = 2.28, b = 1.72 | Meenan et al.37 |
| For patients without a thrombus in year 1 | 0.76 | Normal | Mean 0.7666, SD 0.0587 | Sandercock et al.174 |
| Patient outcome of recurrent stroke | ||||
| Independent outcome | 0.2333 | Dirichlet | a = 233.33, b = 322.22, c = 444.45 | ISWP,168 Clark169 |
| Dependent outcome | 0.3222 | Dirichlet | ||
| Fatal outcome | 0.4445 | Dirichlet | ||
| Description | Mean value | Distribution | Statistical parameters | Source |
|---|---|---|---|---|
| Probability of anticoagulation-induced haemorrhage | ||||
| In the initial 3 months of treatment | 0.0219 | Normal | Mean 0.0219, SE 0.0015 | Simpson et al.170 |
| Subsequently in patients aged 40–49 years | 0.0060 | Normal | Mean 0.0060, SE 0.0004 | |
| Subsequently in patients aged 50–59 years | 0.0100 | Normal | Mean 0.0100, SE 0.0007 | |
| Subsequently in patients aged 60–69 years | 0.0220 | Normal | Mean 0.0220, SE 0.0015 | |
| Subsequently in patients aged ≥ 70 years | 0.0320 | Normal | Mean 0.0320, SE 0.0021 | |
| Haemorrhages in the first 3 months | ||||
| Non-fatal and non-IC | 0.801 | Dirichlet | a = 10.4, b = 39.4, c = 200.2 | Simpson et al.170 |
| Non-fatal and IC | 0.041 | Dirichlet | ||
| Fatal | 0.158 | Dirichlet | ||
| Haemorrhages after 3 months | ||||
| Non-fatal and gastrointestinal | 0.795 | Dirichlet | a = 22.7, b = 28.4, c = 198.9 | Simpson et al.170 |
| Non-fatal and IC | 0.091 | Dirichlet | ||
| Fatal | 0.114 | Dirichlet | ||
| ICH outcomes | ||||
| GOS 2 | 0.116 | Dirichlet | a = 115.5, b = 140.0, c = 79.3, d = 665.1 | Holmes et al.171 |
| GOS 3 | 0.140 | Dirichlet | ||
| GOS 4 | 0.079 | Dirichlet | ||
| GOS 5 | 0.665 | Dirichlet | ||
| Average patient life expectancy for patients in GOS 2 (years) | 3.59 | Normal | Mean 3.59, SD 0.18 | Holmes et al.171 |
Costs
Costs included in the model are:
-
initial treatment costs for TIA and independent and dependent stroke patients, including emergency room treatment, CT scan and short-term hospitalisation when appropriate
-
long-term cost for patients in the independent stroke state
-
long-term cost for patients in the dependant stroke state
-
costs of warfarin treatment
-
treatment costs for gastrointestinal haemorrhage
-
initial treatment costs for patients with an ICH including emergency room treatment, CT scan and surgery
-
long-term costs of care for patients with moderate disability, with severe disability or who are in a PVS following an ICH.
Initial treatment costs are taken from the Department of Health NHS Reference Costs,175 annual care costs for independent and dependent stroke are taken from the Department of Health Impact Assessment of National Stroke Strategy publication176 and the cost of being in GOS states 2–4 are taken from Holmes et al. 171
The cost of warfarin treatment is taken from Simpson et al. 170 All costs have been inflated to 2009–10 prices using the Hospital and Community Health Services Pay and Prices Index. 177 Costs used in the model with a description of the distributions and statistical parameters used in the PSA, and with Healthcare Resource Group (HRG) codes where applicable, are shown in Table 12.
| Description | Mean cost (£) | Distribution | Statistical parameters (£) | Source (HRG or currency/service code) |
|---|---|---|---|---|
| TIA initial cost | 417 | Normal | SE 11 | Department of Health175 (AA29Z) |
| Independent stroke initial cost | 542 | Normal | SE 15 | Department of Health175 (AA22Z) |
| Independent stroke annual care cost | 3195 | Normal | SD 165 | Department of Health176 |
| Dependent stroke initial cost | 2830 | Normal | SE 62 | Department of Health175 (AA22Z) |
| Dependent stroke annual care cost | 6386 | Normal | SD 325 | Department of Health176 |
| Gastrointestinal haemorrhage initial cost | 1261 | Normal | SE 25 | Department of Health175 (FZ38E) |
| IC procedures except trauma with haemorrhagic cerebrovascular disorders | 8829 | Normal | Department of Health175 (AA17Z) | |
| GOS 2 intensive care cost | 15,469 | Gamma | a = 165, b = 94 | Holmes et al.171 |
| GOS 2 rehabilitation cost | 27,960 | Gamma | a = 250, b = 120 | |
| GOS 2 weekly nursing home cost | 893 | Gamma | a = 159, b = 6 | |
| GOS 3 intensive care cost | 8829 | Normal | SE 633 | Department of Health175 (AA17Z) |
| GOS 3 annual care cost | 33,900 | Gamma | a = 326, b = 104 | Holmes et al.171 |
| GOS 4 intensive care cost | 8829 | Normal | SE 633 | Department of Health176 |
| GOS 4 rehabilitation cost | 17,160 | Gamma | a = 385, b = 45 | Holmes et al.171 |
| Anticoagulation initiation cost | 208 | Fixed | Department of Health175 (324) Simpson et al.170 |
|
| Anticoagulation annual maintenance cost | 439 | Fixed | ||
| TTE | 79.14 | Normal | SE 1.97 | Department of Health175 (RA69Z) |
| TOE | 213 | Normal | SE 1.97 | Department of Health175 (EA45Z) |
| CT scan | 91 | Normal | SE 3.94 | Department of Health175 (RA08Z) |
Quality-of-life utility values
Quality-of-life utility values are taken from a study by Dorman et al. 178 This study reports QoL utility values from the Lothian Stroke Register (LSR) and the International Stroke Trial (IST). The LSR and the IST both used the European Quality of Life-5 Dimensions (EQ-5D) questionnaire to measure QoL in a cohort of stroke patients with outcomes of dependent, independent and recovered. The results are broadly similar; however, as the IST cohort (n = 867) is larger than the LSR cohort (n = 147) we have used the IST QoL utility values in the model.
The mean age in the IST cohort was 69 years. Data from Kind et al. 179 indicate that QoL utility values decrease with age. We would therefore expect the utility values estimated by Dorman et al. 178 to be slightly higher for younger patients. To adjust the Dorman et al. utilities for age, we first divided them by the population norm utility value at age 69 years (0.806) taken from the Kind et al. study179 to obtain an estimate of an age-related multiplier for dependent stroke, independent stroke and TIA (0.38, 0.88, 1.09 respectively). We then multiply the Kind et al. utilities at each age by these multipliers to provide age-related utility values for dependent stroke, independent stroke and TIA. The estimated multiplier for TIA is > 1 and is intuitively wrong as it would result in patients with a TIA having a higher QoL than that of the general population. We have therefore set the multiplier for TIA at 1 under the assumption that a TIA has no impact on QoL. The QoL utility value for patients with a TIA are thus the same as the population norms for a patient’s age and are taken from Kind et al. 179 The QoL utility values used in the model are shown in Table 13.
| Description | Mean value | Distribution | Statistical parameters | Source |
|---|---|---|---|---|
| Gastrointestinal haemorrhage | 0.997 | Uniform | Min. = 0.996, max. = 0.998 | Simpson et al.170 |
| GOS 3 | 0.15 | Beta | a = 5.8, b = 32.7 | Holmes et al.171 |
| GOS 4 | 0.51 | Beta | a = 22.6, b = 21.7 | |
| GOS 5 | 0.88 | Beta | a = 49.8, b = 6.8 |
Model stability
The number of patients in each model run determines the stability of the results for estimating the optimal management strategy. This instability is a result of some events having a rare occurrence and stability can be achieved only by having sufficient numbers of patients to account for these rare events. With 100,000 or more patients the model results became stable and the model was therefore run with this number of patients.
Major assumptions
The following assumptions were made:
-
following their first TIA patients may experience subsequent attacks; however, no data were identified on the rate of TIA recurrence in patients with and without a thrombus, and thus recurrent TIA is not included in the model
-
the rate of stroke recurrence is not dependent on the previous number of strokes sustained
-
patients with an intracardiac thrombus are at a higher risk of experiencing a recurrent stroke in the first year following a stroke than in subsequent years
-
patients may experience any number of recurrent ischaemic strokes resulting in an independent or dependent patient outcome
-
anticoagulant treatment continues for 1 year at which point the patient is re-evaluated and from this point on is considered a new patient
-
anticoagulation treatment is discontinued for those patients experiencing a bleed event
-
the relative risk reduction of recurrent stroke as a result of anticoagulant treatment is constant for as long as the patient receives treatment
-
patients were not receiving anticoagulants or antiplatelet agents at the time of stroke.
Definition of cost-effectiveness terms
A deterministic analysis uses the mean or median value of each parameter in the model and does not take into account the effect of any non-linearities in the model that could affect the ICERs. In PSA each parameter in the model is assigned a distribution that encapsulates the uncertainty within the parameter. For each of the 1000 simulations (of 100,000 patients) each model parameter is randomly sampled from the distribution assigned to it. PSA, unlike deterministic analyses, take non-linearities within the model into consideration and thus the answers are more appropriate. 180
The results are presented as mean and incremental costs and QALYs, ICERs and CEACs. The ICER measures the relative value of two strategies and is calculated as the mean incremental costs divided by the mean incremental benefits. A strategy is dominated when another strategy accrues more QALYs for less cost. Extended dominance occurs when a combination of two alternative strategies can produce the same QALYs as a chosen strategy but at a lower cost. Strategies that are neither dominated nor extendedly dominated constitute the cost-effectiveness frontier and the ICER is reported for these strategies compared with the next least effective strategy.
A CEAC indicates the proportion of times within the PSA that each intervention is the most cost-effective of all scenarios at different WTP levels. 181 Net benefit (NB) is defined as WTP × QALYs – costs.
The WTP threshold is the amount of money that the decision-maker is willing to pay to gain 1 additional QALY. Typical thresholds for decision-making within the UK are considered to be around £20,000–30,000 per QALY. 165
Results
Deterministic results
Table 14 shows the deterministic mean per patient costs and QALYs for the four strategies undertaken at age 45, 55 and 65 years at the index event (stroke or TIA). The costs and QALYs decrease as age increases because of shorter survival times.
| Testing strategy | Age 45 years | Age 55 years | Age 65 years | |||
|---|---|---|---|---|---|---|
| Mean cost (£) | Mean QALYs | Mean cost (£) | Mean QALYs | Mean cost (£) | Mean QALYs | |
| No test | 70,770 | 7.857 | 61,182 | 6.635 | 48,793 | 5.306 |
| TTEh +ve TOE | 70,999 | 7.868 | 61,392 | 6.644 | 48,974 | 5.313 |
| TTEh only | 71,037 | 7.872 | 61,419 | 6.647 | 48,993 | 5.315 |
| TOE only | 71,209 | 7.875 | 61,587 | 6.650 | 49,151 | 5.317 |
| TTEh –ve TOE | 71,317 | 7.878 | 61,685 | 6.652 | 49,243 | 5.318 |
Tables 15–17 show, for each age at the index event, the strategies ordered by ascending effectiveness (QALYs gained). For all ages, at a WTP threshold of £23,000, the optimal strategy is to perform TTEh only.
| Testing strategy | Mean cost (£) | Mean QALYs | Incremental cost (£) | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| No test | 70,770 | 7.857 | |||
| TTEh +ve TOE | 70,999 | 7.868 | Extendedly dominated | ||
| TTEh only | 71,037 | 7.872 | 267 | 0.015 | 17,541 |
| TOE only | 71,209 | 7.875 | Extendedly dominated | ||
| TTEh –ve TOE | 71,317 | 7.878 | 280 | 0.006 | 44,492 |
| Testing strategy | Mean cost (£) | Mean QALYs | Incremental cost (£) | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| No test | 61,182 | 6.635 | |||
| TTEh +ve TOE | 61,392 | 6.644 | Extendedly dominated | ||
| TTEh only | 61,419 | 6.647 | 237 | 0.012 | 19,904 |
| TOE only | 61,587 | 6.650 | Extendedly dominated | ||
| TTEh –ve TOE | 61,685 | 6.652 | 265 | 0.005 | 56,587 |
| Testing strategy | Mean cost (£) | Mean QALYs | Incremental cost (£) | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| No test | 48,793 | 5.306 | |||
| TTEh +ve TOE | 48,974 | 5.313 | Extendedly dominated | ||
| TTEh only | 48,993 | 5.315 | 200 | 0.009 | 22,361 |
| TOE only | 49,151 | 5.317 | Extendedly dominated | ||
| TTEh –ve TOE | 49,243 | 5.318 | 251 | 0.003 | 73,735 |
Univariate sensitivity results
A univariate sensitivity analysis was carried out on the following parameters:
-
rate of stroke recurrence or initial stroke following TIA in patients:
-
with an intracardiac thrombus in year 1
-
with an intracardiac thrombus subsequently
-
without an intracardiac thrombus in year 1
-
without an intracardiac thrombus subsequently
-
-
efficacy of warfarin therapy:
-
relative risk in patients with an intracardiac thrombus
-
relative risk in patients without an intracardiac thrombus
-
-
rate of anticoagulant-induced haemorrhage:
-
in the first 3 months of treatment
-
subsequently in patients aged 40–49 years
-
subsequently in patients aged 50–59 years
-
subsequently in patients aged 60–69 years
-
subsequently in patients aged ≥ 70 years
-
-
the prevalence of left atrial thrombi.
For all of these parameters, altering the parameter to its highest and then lowest value had no effect on the optimal strategy reported in the deterministic results.
Probabilistic sensitivity analysis results
Table 18 shows the mean per patient costs and QALYs from the PSA for the four strategies undertaken at age 45, 55 and 65 years at the index event. As with the deterministic results, the costs and QALYs decrease as age increases because of shorter survival times.
| Testing strategy | Age 45 years | Age 55 years | Age 65 years | |||
|---|---|---|---|---|---|---|
| Mean cost (£) | Mean QALYs | Mean cost (£) | Mean QALYs | Mean cost (£) | Mean QALYs | |
| No test | 71,075 | 7.920 | 61,399 | 6.679 | 48,928 | 5.336 |
| TTEh +ve TOE | 71,295 | 7.929 | 61,599 | 6.686 | 49,106 | 5.342 |
| TTEh only | 71,379 | 7.936 | 61,669 | 6.692 | 49,160 | 5.346 |
| TTEh –ve TOE | 71,574 | 7.937 | 61,861 | 6.693 | 49,350 | 5.346 |
| TOE only | 71,545 | 7.939 | 61,830 | 6.695 | 49,315 | 5.347 |
Tables 19–21 show, for each age at the index event, the strategies ordered by ascending effectiveness (QALYs gained) and report whether they are subject to dominance. When a strategy is not dominated an ICER for each strategy compared with the next least effective treatment on the cost-effectiveness frontier is reported. For all ages, at a WTP threshold of £25,000, the optimal strategy is to perform TTEh only.
| Testing strategy | Mean cost (£) | Mean QALYs | Incremental cost (£) | Incremental QALYs | ICER compared with next least effective treatment on the cost-effectiveness frontier (£) |
|---|---|---|---|---|---|
| No test | 71,075 | 7.920 | |||
| TTEh +ve TOE | 71,295 | 7.929 | Extendedly dominated | ||
| TTEh only | 71,379 | 7.936 | 304 | 0.016 | 18,526 |
| TTEh –ve TOE | 71,574 | 7.937 | Dominated | ||
| TOE only | 71,545 | 7.939 | 166 | 0.003 | 65,490 |
| Testing strategy | Mean cost (£) | Mean QALYs | Incremental cost (£) | Incremental QALYs | ICER compared with next least effective treatment on the cost-effectiveness frontier (£) |
|---|---|---|---|---|---|
| No test | 61,399 | 6.679 | |||
| TTEh +ve TOE | 61,599 | 6.686 | Extendedly dominated | ||
| TTEh only | 61,669 | 6.692 | 270 | 0.0132 | 20,408 |
| TTEh –ve TOE | 61,861 | 6.693 | Dominated | ||
| TOE only | 61,830 | 6.695 | 161 | 0.0021 | 78,109 |
| Testing strategy | Mean cost (£) | Mean QALYs | Incremental cost (£) | Incremental QALYs | ICER compared with next least effective treatment on the cost-effectiveness frontier (£) |
|---|---|---|---|---|---|
| No test | 48,928 | 5.336 | |||
| TTEh +ve TOE | 49,106 | 5.342 | Extendedly dominated | ||
| TTEh only | 49,160 | 5.346 | 232 | 0.009 | 24,648 |
| TTEh –ve TOE | 49,350 | 5.346 | Dominated | ||
| TOE only | 49,315 | 5.347 | 154 | 0.002 | 102,046 |
Figures 20–22 show the CEACs for ages 45, 55 and 65 years. For all ages the TTEh strategy is optimal at a WTP well above the £30,000 mark. The CEACs indicate that there is uncertainty in the results, although that it is only the no test and TTEh strategies that have non-trivial probabilities of being cost-effective in the range of £20,000–30,000 per QALY. As stated above, assuming a cost per QALY threshold of £25,000, TTEh would be the recommended diagnostic strategy.
FIGURE 20.
Cost-effectiveness acceptability curve for age 45 years.
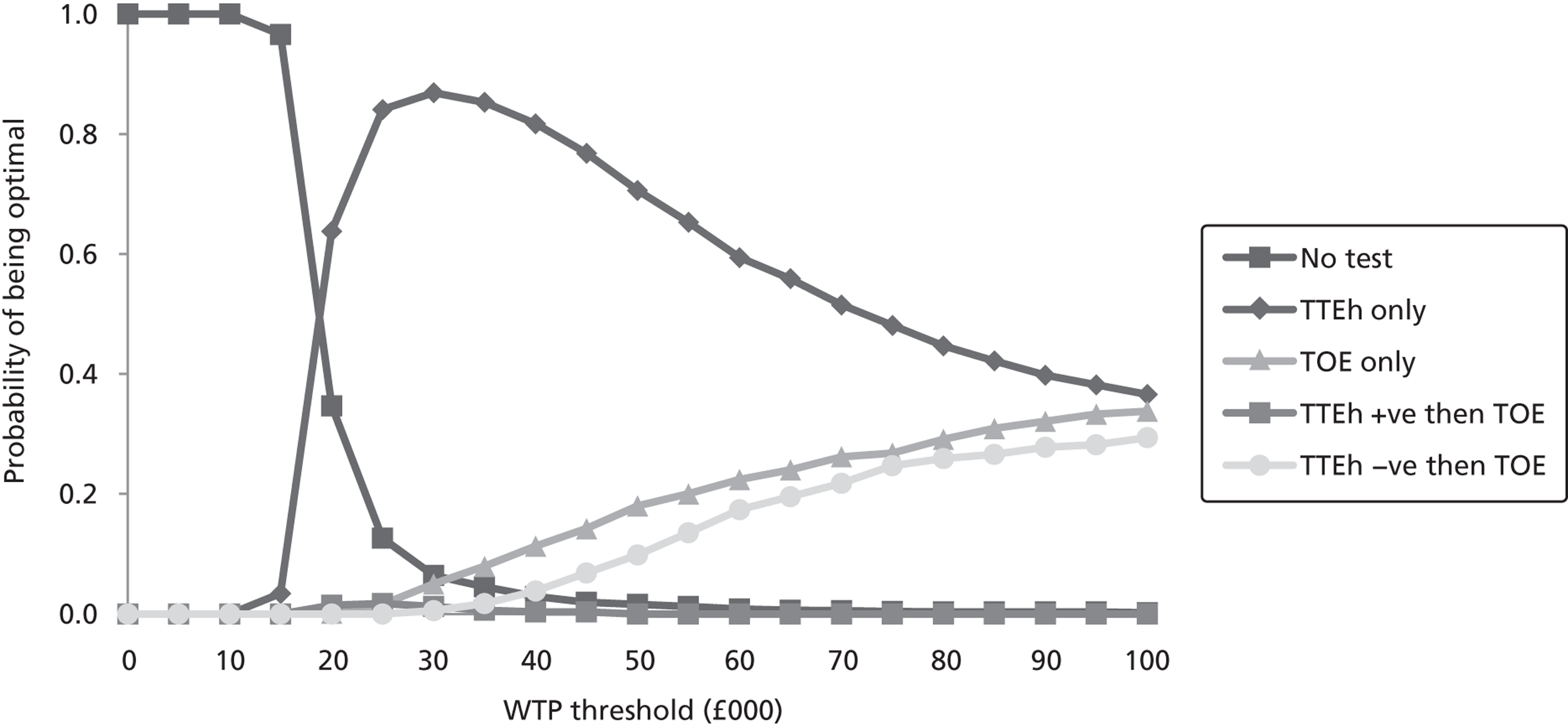
FIGURE 21.
Cost-effectiveness acceptability curve for age 55 years.
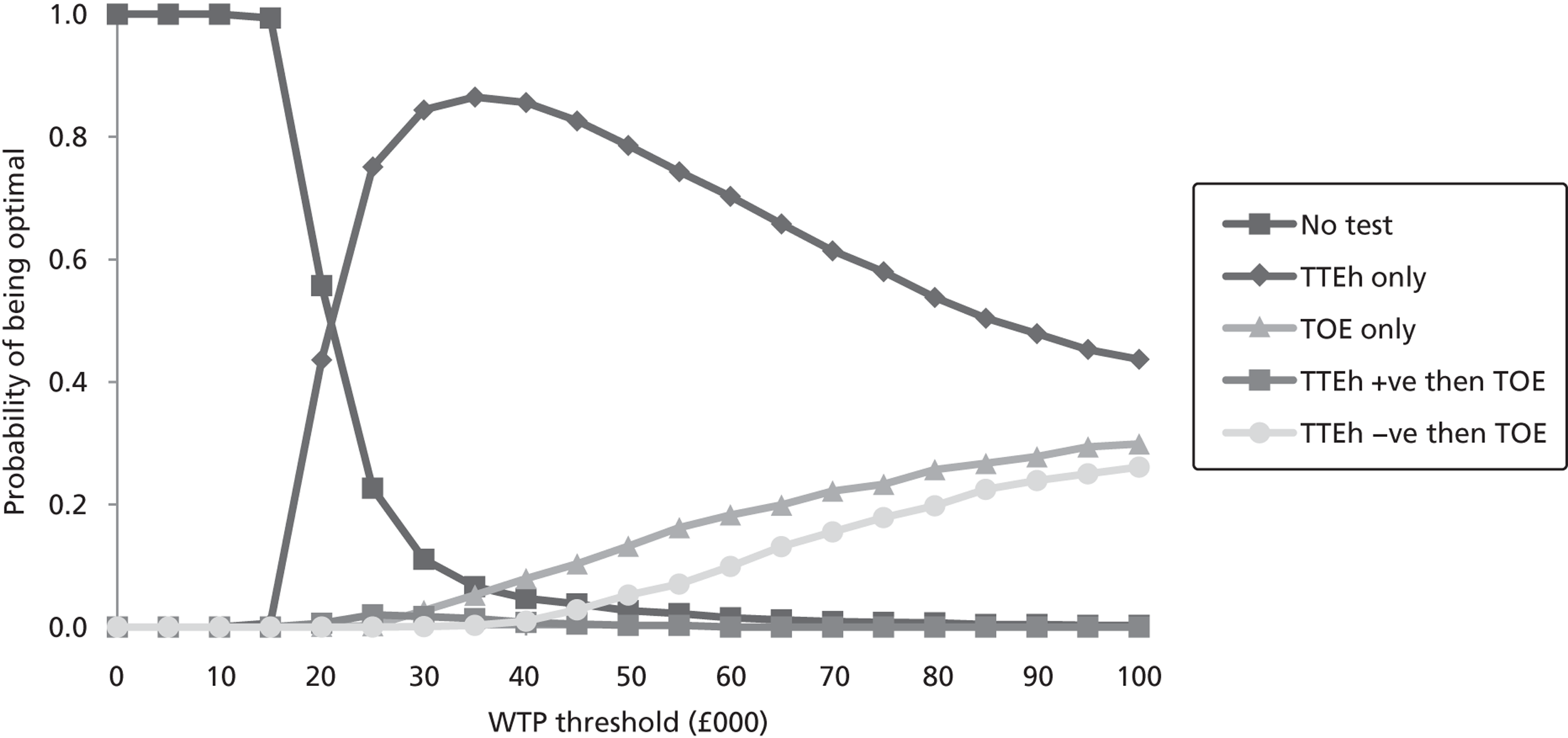
FIGURE 22.
Cost-effectiveness acceptability curve for age 65 years.
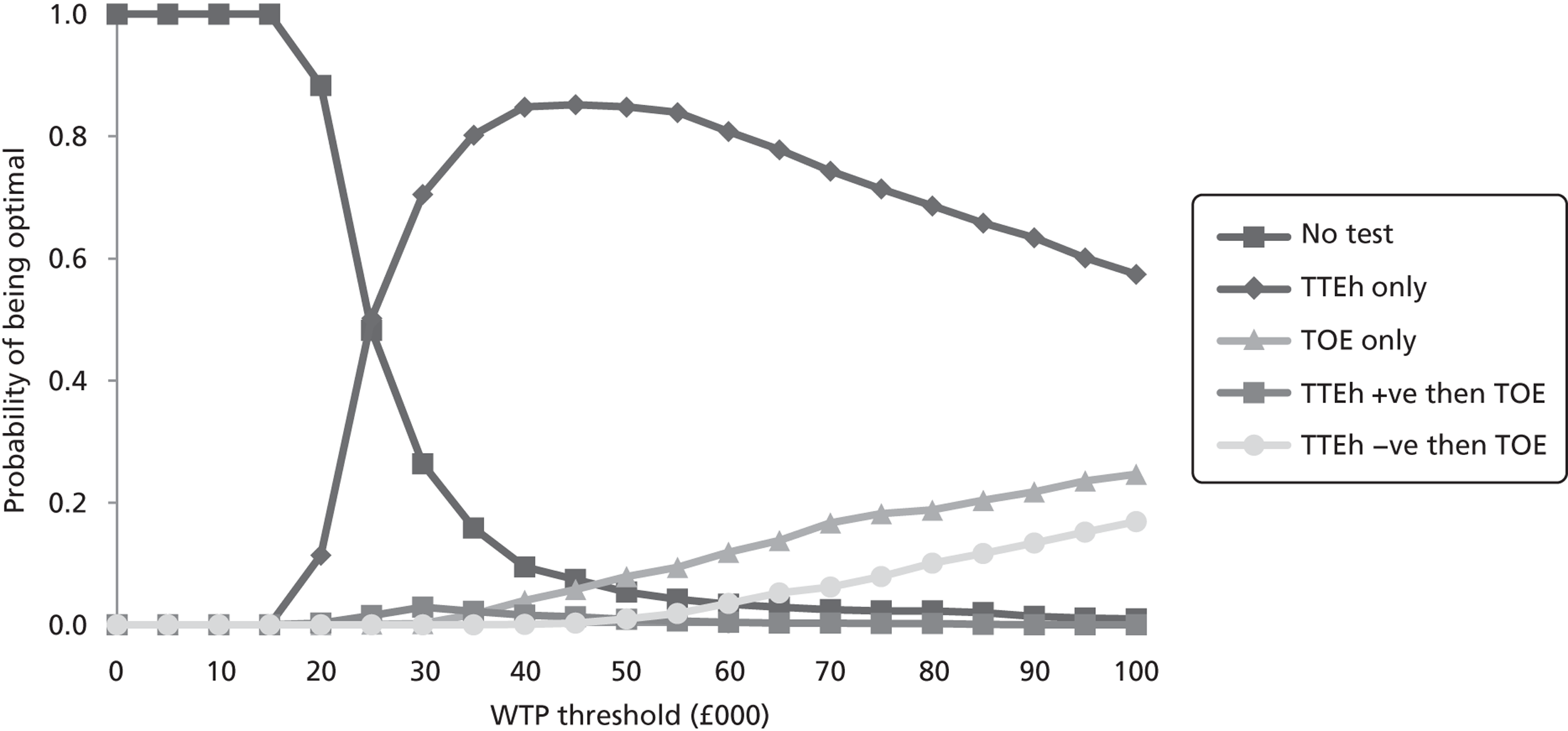
Probabilistic sensitivity analysis assuming correlation between tests when carried out in sequence
The base case assumes that the tests are independent. A sensitivity analysis was carried out under the assumption that the tests are correlated (see The decision-analysis model structure for the methodology). Both the deterministic and probabilistic results were similar to the base-case results and the optimum strategy remained unchanged at a cost per QALY gained threshold of £25,000. The results of the probabilistic analysis are shown in Tables 22–24.
| Testing strategy | Mean cost (£) | Mean QALYs | Incremental cost (£) | Incremental QALYs | ICER compared with next least effective treatment on the cost-effectiveness frontier (£) |
|---|---|---|---|---|---|
| No test | 71,075 | 7.920 | |||
| TTEh +ve TOE | 71,307 | 7.930 | Extendedly dominated | ||
| TTEh –ve TOE | 71,548 | 7.936 | Dominated | ||
| TTEh only | 71,379 | 7.937 | 304 | 0.0164 | 18,525 |
| TOE only | 71,545 | 7.939 | 166 | 0.0025 | 65,489 |
| Testing strategy | Mean cost (£) | Mean QALYs | Incremental cost (£) | Incremental QALYs | ICER compared with next least effective treatment on the cost-effectiveness frontier (£) |
|---|---|---|---|---|---|
| No test | 61,399 | 6.680 | |||
| TTEh +ve TOE | 61,609 | 6.688 | Extendedly dominated | ||
| TTEh –ve TOE | 61,838 | 6.692 | Dominated | ||
| TTEh only | 61,669 | 6.693 | 270 | 0.013 | 20,365 |
| TOE only | 61,830 | 6.695 | 161 | 0.002 | 77,947 |
| Testing strategy | Mean cost (£) | Mean QALYs | Incremental cost (£) | Incremental QALYs | ICER compared with next least effective treatment on the cost-effectiveness frontier (£) |
|---|---|---|---|---|---|
| No test | 48,928 | 5.337 | |||
| TTEh +ve TOE | 49,114 | 5.343 | Extendedly dominated | ||
| TTEh –ve TOE | 49,330 | 5.346 | Dominated | ||
| TTEh only | 49,160 | 5.346 | 232 | 0.009 | 24,648 |
| TOE only | 49,315 | 5.348 | 154 | 0.002 | 102,046 |
Results of the individual patient-level model (model 1)
As described in The decision-analysis model structure, the outcomes of model 1 are the costs and QALYs associated with the following:
-
patients with an intracardiac thrombus and treated (TP)
-
patients with an intracardiac thrombus and untreated (FN)
-
patients without an intracardiac thrombus and treated (FP)
-
patients without an intracardiac thrombus and untreated (TN).
The results of this analysis are presented in Tables 25–27. The results of the economic analysis are dependent on the results of model 1 and it is therefore important that the model 1 results are intuitively correct. We believe that the following comparisons represent intuitive results:
-
Comparing those patients who have a thrombus (TP vs. FN), patients who are treated cost more and have more QALYs. This is intuitively correct as they get both the cost and benefits of treatment.
-
Comparing those patients who do not have a thrombus (FP vs. TN), patients who receive treatment have additional costs and the treatment appears to have a preventative effect as these patients, on average, live a few months longer.
-
Comparing those patients who receive treatment (TP vs. FP), patients without a thrombus live longer and thus gain additional costs.
-
Comparing those patients who do not receive treatment (FN vs. TN), patients without a thrombus live longer and accrue more costs.
| Description | Mean cost (£) | Mean QALYs |
|---|---|---|
| TP, correctly receive treatment | 65,332 | 6.70 |
| FN, incorrectly do not receive treatment | 62,296 | 6.47 |
| FP, incorrectly receive treatment | 72,905 | 8.09 |
| TN, correctly do not receive treatment | 71,587 | 8.00 |
| Description | Mean cost (£) | Mean QALYs |
|---|---|---|
| TP, correctly receive treatment | 57,534 | 5.76 |
| FN, incorrectly do not receive treatment | 55,018 | 5.57 |
| FP, incorrectly receive treatment | 62,921 | 6.82 |
| TN, correctly do not receive treatment | 61,771 | 6.74 |
| Description | Mean cost (£) | Mean QALYs |
|---|---|---|
| TP, correctly receive treatment | 46,838 | 4.70 |
| FN, incorrectly do not receive treatment | 44,894 | 4.56 |
| FP, incorrectly receive treatment | 50,120 | 5.43 |
| TN, correctly do not receive treatment | 49,163 | 5.38 |
Cost-effectiveness of warfarin
This economic analysis allows us to estimate the cost-effectiveness of warfarin compared with no treatment in those patients who have a thrombus. Patients tested as TP have a thrombus and receive treatment whereas patients tested as FN have a thrombus and are not treated. Tables 28–30 show the results of this analysis (based on PSA results). For all ages the ICER is well below accepted thresholds.
| Description | Mean cost (£) | Mean QALYs | Incremental cost (£) | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| TP, correctly receive treatment | 65,332 | 6.70 | |||
| FN, incorrectly do not receive treatment | 62,296 | 6.47 | 3035 | 0.24 | 12,872 |
| Description | Mean cost (£) | Mean QALYs | Incremental cost (£) | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| TP, correctly receive treatment | 57,534 | 5.76 | |||
| FN, incorrectly do not receive treatment | 55,018 | 5.57 | 2515 | 0.19 | 13,171 |
| Description | Mean cost (£) | Mean QALYs | Incremental cost (£) | Incremental QALYs | ICER (£) |
|---|---|---|---|---|---|
| TP, correctly receive treatment | 46,838 | 4.70 | |||
| FN, incorrectly do not receive treatment | 44,894 | 4.56 | 1944 | 0.14 | 13,900 |
It can also be calculated that the use of warfarin in patients without a left atrial thrombus appears cost-effective, with ICERs ranging from £15,000 to £20,000. However, as the QALY gains are lower than those in patients with a thrombus (because of a reduced stroke risk but a constant bleed risk), the clinical community may see this as a less appealing option.
Expected value of perfect information analysis
The EVPI quantifies the economic value of removing uncertainty in a decision model. 182 An estimated 163,000 patients per year suffer a TIA or a stroke. Assuming a 10-year time horizon for the value of further research, the maximum amount of research funding to achieve perfect information is calculated as the EVPI per person × 163,000 × 10.
Table 31 and Figure 23 show the per-person and population EVPI results at WTP thresholds of £20,000 and £30,000.
| WTP threshold | 45 years (£) | 55 years (£) | 65 years (£) |
|---|---|---|---|
| £20,000 | |||
| Per patient | 15 | 18 | 2 |
| Population | 23,873,671 | 28,779,564 | 3,998,567 |
| £30,000 | |||
| Per patient | 7 | 6 | 12 |
| Population | 10,878,722 | 10,462,040 | 19,270,629 |
FIGURE 23.
Per-person and population EVPI results by age.
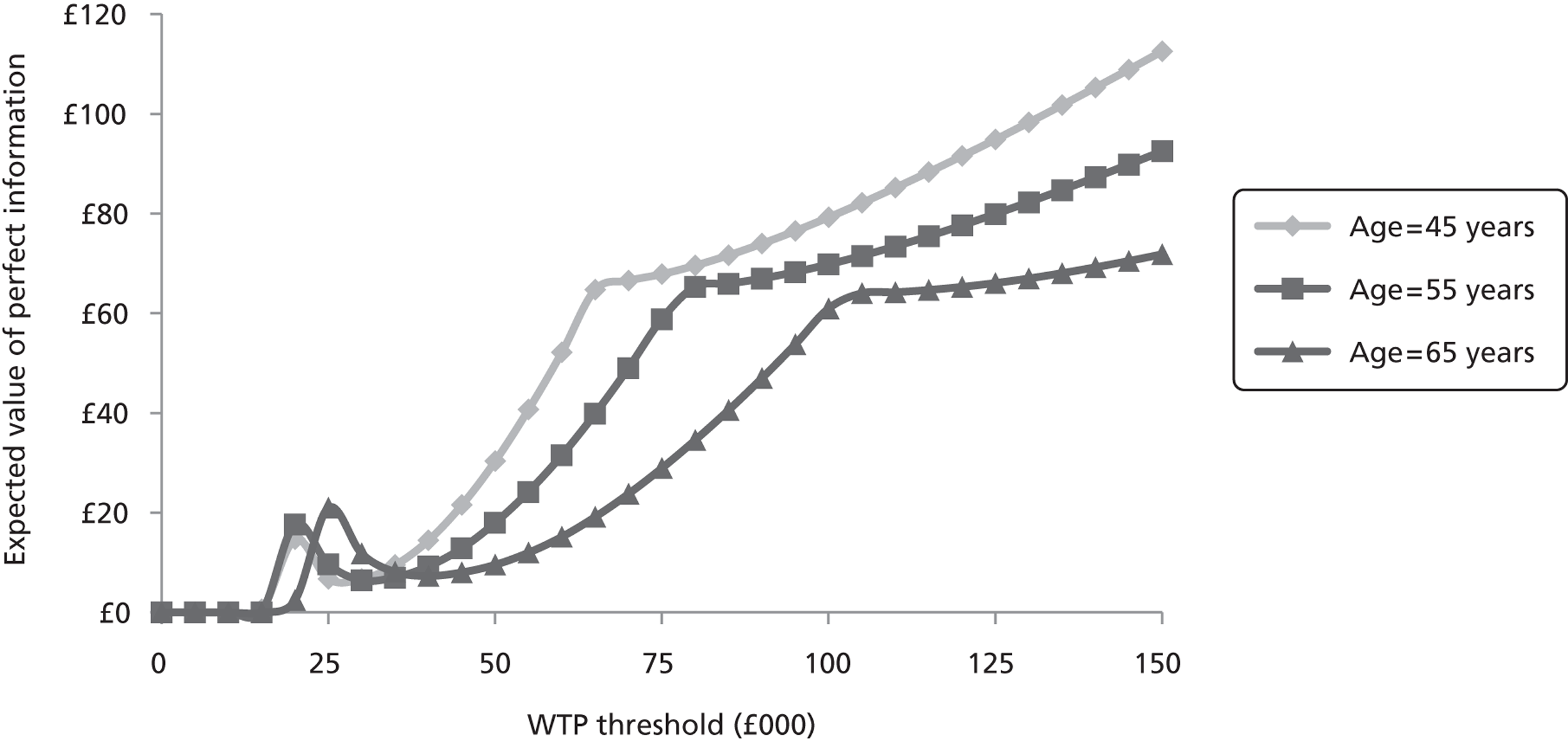
It is seen that, at all ages, the EVPI is large. Further research to reduce the uncertainty may well be a cost-effective use of resources.
Discussion of the economic analysis
We have explicitly evaluated the cost-effectiveness of TTEh compared with different diagnostic strategies. A limitation was that, because of the heterogeneous use of diagnostic strategies within the UK, explicit rules on the use of TTEh could not be formulated. We therefore assumed that all patients received TTEh; this would not be the case in practice and is likely to be unfavourable to the cost-effectiveness of TTEh. Furthermore, any incidental benefit that may arise from TTEh scanning, such as identifying thrombi in other locations or identifying those pathologies in which benefit is gained from preventative treatment, would also improve the ICER in the analyses undertaken. Despite these unfavourable biases, TTEh was shown to be cost-effective compared with no testing for all patients, indicating that the conclusion is robust and TTEh should be performed when a clinician deems it appropriate.
However, there will be patients who, in their clinician’s opinion, will require TOE, for example those in whom cardiac interventions are likely, when there is a high suspicion of endocarditis or patients aged < 50 years of age with unexplained cases of stroke. Because of data limitations it was not possible to evaluate the cost-effectiveness of TOE in subsets of such cases. Therefore, no statements could be made regarding the cost-effectiveness of the selective use of TOE when clinicians deem it appropriate.
The results of model 1 appear to suggest that preventative treatment in those patients who do not have a thrombus is beneficial and appears to be cost-effective; however, it may not be clinically appropriate in all circumstances.
Our analysis assumed that the benefits of treatment apply only to patients with a left atrial thrombus and it is reasonable to assume that thrombi in other locations may be identified and treated resulting in further health benefits. It is also reasonable to assume that other pathologies that are believed to be risk factors for thromboembolic events, such as cardiomyopathy, could be identified. For some pathologies that are believed to predispose to thrombi, preventative treatment with anticoagulants is recommended and this may improve the cost-effectiveness of TTEh.
Because of the limitations discussed below, however, the results of the economic analysis should be treated with some caution.
Summary of key results
Cost-effectiveness studies
Two cost-effectiveness studies from the USA were identified. 37,161 TOE was found to be cost-effective in one study161 but not in the other. 37 Both studies found that TTE alone or in combination strategies was not cost-effective. The authors of both studies do not state whether they used TTEf or TTEh imaging techniques.
Strengths and limitations of the analysis
The economic analysis used current best practice to develop the model and followed recommendations produced by NICE. 165 However, economic models are inevitably constrained by the need to make assumptions in developing them and by the limitations of the primary data.
The rate of stroke recurrence is an important parameter in the model. However, we were able to identify only one small study183 in which patients who were identified as having a left atrial thrombus on echocardiography were followed up long term to measure the rate of stroke recurrence.
We were unable to include pathologies other than left atrial thrombus because of a lack of evidence of treatment effect on stroke recurrence in other pathologies.
In this analysis we assumed that all patients received an echocardiogram. This is unlikely to be the case in the clinical setting; however, to model otherwise would require estimates of the diagnostic accuracy of whatever test or clinical decision-making protocol was used in each clinical setting and this information was not available.
Because of these limitations the results of the economic analysis should be treated with some caution.
Chapter 7 Assessment of factors relevant to the NHS and other parties
If a policy of performing TTEh were adopted it has the potential to save the NHS money as the more costly and invasive TOE procedure would be used less often. The potential reduction in the usage of TOE and the consequent impact on the NHS budget are difficult to quantify.
Chapter 8 Discussion
Statement of principal findings
Prevalence
The studies included in the prevalence systematic review report multiple sources of potential cardiac pathologies, reflecting the heterogeneous nature of cardioembolic stroke. 37,103 Because of the heterogeneous nature of stroke, the diagnosis of cardioembolic source of stroke or TIA is often uncertain and reliant on the detection of a potential cardiac source of embolus in the absence of other potential sources of cerebral ischaemia. 103 It is apparent that potential sources of cardioembolic stroke may be absent in patients with stroke or TIA and present in patients without stroke or TIA. Moreover, some studies report the presence of two or more potential sources in one person, which generates further diagnostic uncertainty, but would not necessarily alter the treatment regime. Generally, the studies did not report performing thorough diagnostic evaluations; instead, findings were reported as associated risk factors. Clearly, the value of the reported prevalence data might be regarded as limited when currently it does not appear possible to establish a causal link with any degree of certainty.
The divergent samples used in the included studies produced, in most cases, a prevalence rate with a wide range, making any finding difficult to generalise to larger populations. The prevalence rates identified from the included studies ranged from 0% to 9% for major risk factors and from 0% to 73% for minor risk factors, for which further uncertainty exists. This might be reflective of the heterogeneity relating to the participant populations included in the studies as well as the varying diagnostic methods employed to detect the various sources.
Diagnostic accuracy studies
The average sensitivity and specificity of TTE were lower than those of TOE in both fundamental and harmonic imaging mode. Generally, TTEh was superior to TTEf but the greater sensitivity did lead to a decreased specificity, which increased the number of FNs. TTEh demonstrated lower sensitivity than reference standards for the detection of cardiac pathologies requiring anticoagulation therapy such as left atrial thrombus and left ventricular thrombus. However, TOE is not suited to the detection of left ventricular apical thrombus and TTE, although not as accurate as contrast-enhanced MRI, could serve as a screening tool for this pathology. TTEh indicated good sensitivity and specificity for the detection of left atrial appendage thrombi, although based on a small data set. However, these finding are limited by the small number of studies and the low prevalence rates within some studies.
Transoesophageal echocardiography demonstrated a greater diagnostic accuracy over a range of cardiac pathologies. The difference in the diagnostic accuracy of TTE and TOE was found mainly in their sensitivity to detect cardiac sources of stroke, that is, the probability that the index test (TTE) will be positive in diseased cases; differences in the specificity to correctly identify non-diseased cases was less remarkable, with most studies reporting specificities of 1.00.
Although both TTE and TOE are considered safe procedures, TOE is a semi-invasive procedure and requires a fasted patient and more clinical resources. TTE is non-invasive, quicker to perform than TOE and needs only one sonographer. However, skeletal structure and tissue may impede test performance compared with TOE and TOE is more appropriate for detecting some cardiac pathologies such as left atrial appendage thrombi. Therefore, TTE might be applied primarily to patients with stroke of undetermined aetiology (i.e. patients showing normal results on electrocardiography or carotid ultrasound) and who are candidates for oral anticoagulation, prior to escalation of further diagnostic tests. With improvements in TTE technology further diagnostic accuracy studies will be needed.
Survey of relevant comparators
It is clear from the results of the survey that stroke management is a very complex procedure with protocols appearing to be different in every centre that responded. A more sophisticated questionnaire would be needed to capture the complexity of stroke and TIA management protocols. However, given the variation in protocols used across stroke centres, it is uncertain how informative this would be. To be of real value, the effectiveness of different protocols would need to be assessed in terms of stroke and TIA outcomes. In England and Wales, NICE or Royal College of Physicians guidelines were used by most centres in both the investigation and the management of stroke and TIA. Amended guidelines were used by 10% of centres but no information was provided as to what the amendments were. In Scotland the guidelines issued by the SIGN were used by most centres to investigate stroke and TIA with most centres using ‘other’ guidelines to manage stroke and TIA.
Economic evaluation
Two economic evaluations from the perspective of the US health-care system found that TTE, either alone or in strategies with TOE, was not cost-effective. 37,161 However, neither study reported whether TTEf or TTEH was evaluated. One study found TOE to be cost-effective161 whereas the other did not. 37
Our principal economic finding is that TTEh is a cost-effective use of NHS resources compared with TOE in those cases where clinicians deem it the most appropriate form of testing. We have not evaluated the cost-effectiveness of TOE in those cases where clinicians regard it the most appropriate test.
Our analysis appears to show that warfarin has benefit in a preventative role and that this is cost-effective; however, this may not be clinically relevant for all cases.
Because of the limitations discussed in the following section, the results of the economic analysis should be treated with a certain amount of caution.
Strengths and limitations of the assessment
Clinical evaluation
The prevalence review highlights the difficulty that clinicians face when identifying the cause of cardioembolic stroke with regard to the limitations of the tests, the confounding comorbidities and the inherent mobility of blood clots. The uncertainty surrounding the risk that each cardiac pathology confers on patients is a limiting factor that affects the clinical decision to give anticoagulants when there is risk of haemorrhage.
The diagnostic accuracy review identified > 50 studies and covered a wide range of cardiac pathologies, which enabled comparisons between the older imaging technique of TTEf and the newer technique of TTEh. Good evidence was reported for the diagnostic accuracy of both TTEf and TTEh for the detection of PFO. The value of some outcomes was limited by the small numbers of studies reporting data or because studies included too few participants with a cardiac pathology, leaving a large degree of uncertainty about the underlying diagnostic accuracy.
Survey of relevant comparators
It seems apparent from some of the responses to the questionnaire that the questions asked were not sophisticated enough to capture the complexity of stroke and TIA investigation and management. However, it is also not clear how useful a sufficiently sophisticated questionnaire would be. The results of the survey suggest that an adequately sophisticated survey would produce results that are too heterogeneous to be of value.
Economic evaluation
The economic analysis used current best practice to develop the model and followed recommendations produced by NICE. 165
The model has limitations because of the limited data available for important parameters such as the efficacy of treatment in reducing stroke recurrence. We were unable to include pathologies other than left atrial thrombus because of the lack of evidence of treatment effect on stroke recurrence in other pathologies. It should be noted that the evidence base for the analysis for some of the main parameters in the model was poor and thus the conclusions reached should be treated with a certain amount of caution.
Economic models are inevitably limited by the need to make assumptions, such as the assumption of unlimited stroke recurrence in a single patient and the assumption that all patients receive an echocardiogram. In the case of stroke recurrence it is unlikely that data regarding the relationship between number of strokes and mortality will become available. It is also unlikely, in the clinical setting, that all patients would receive an echocardiogram; however, to model otherwise would require estimates of the diagnostic accuracy of whatever test or clinical decision-making protocol was used and this information was unavailable.
Uncertainties
A number of uncertainties were identified in this report:
-
What are the age-related stroke and TIA recurrence rates in patients with and without those cardiac abnormalities that can be treated?
-
What is the age-related relationship between the number of strokes and patient mortality?
-
What are the age-related rates and outcomes of anticoagulation-induced haemorrhage?
-
What are the long-term costs associated with disability outcomes resulting from stroke and adverse effects of treatment?
-
What are patient outcomes measured by the Barthel Index,184 which can be used to categorise patients into independent, mild, moderate, severe and very severe states?
Research is needed to reduce the uncertainty around the estimates of sensitivity and specificity of TTEh and TOE, singly and in combination, in detecting treatable cardiac abnormalities compared with the gold standard in each pathology.
Uncertainly remains as to the true prevalence rates for cardiac sources of stroke and TIA because of the methodological difficulty of establishing the aetiology of cardiac strokes. Prevalence data were derived mainly from risk factor findings and often patients had several coexisting pathologies, which further increased the uncertainty in these findings.
The above studies would be expensive; however, the results from the EVPI analysis suggest that a maximum of £20M could be spent in removing all uncertainty from the problem.
Chapter 9 Conclusions
Implications for service provision
The implementation of our research findings by the NHS may be money saving but this is difficult to quantify.
Suggested research priorities
The main research priorities suggested by this report are:
-
Long-term UK-based studies measuring the efficacy of treatment for stroke recurrence rates, associated risk factors and patient outcomes. These studies should use the same outcome measure, such as the Barthel Index, to allow data to be combined.
-
Studies measuring the diagnostic accuracy of TTEh and TOE in detecting cardiac abnormalities that respond to treatment. These studies should also include any newer technologies such as crystal and three-dimensional probe designs.
-
To maximise the clinical utility of research findings, diagnostic accuracy studies need to ensure that test procedures and results are fully reported. Investigators conducting diagnostic accuracy studies should ensure that the dissemination of findings conforms to the STARD criteria, and journal editors should include the STARD criteria as a prerequisite for article publication.
-
In the presence of multiple risk factors, establishing the cause of cardioembolic stroke is complex and unlikely to provide an unequivocal answer. Studies attempting to establish the prevalence of cardiac sources of stroke should perform a thorough clinical evaluation to identify all potential risk factors, rule out those that are not relevant and, when possible, grade the findings according to risk.
These research priorities mostly require a large patient cohort and thus substantial funding. When possible, attempts should be made to address multiple objectives in the same study, for example diagnostic test results, stroke recurrence rates, efficacy of treatment, incidence of haemorrhage and patient outcomes.
Any future research to further develop or refine diagnostic strategies may benefit from EVPI analysis using our model to determine whether the benefits of further research justify the costs.
Economic analysis
The economic analysis indicates that, in those cases in which clinicians deem it the most appropriate test, TTEh is a cost-effective use of NHS resources. This analysis has highlighted the need for more research in several areas and until this is carried out the results of the economic evaluation should be treated with a certain amount of caution.
Acknowledgements
We would like to thank the following people for their help with this project: Professor Anthony Rudd, Guy’s & St Thomas’ Hospital, London, and Dr Rick Steeds, University Hospital (Queen Elizabeth) NHS Foundation Trust, Birmingham, for providing clinical expertise and Dr Abdallah Al Mohammad, Northern General Hospital, Sheffield, and Dr Kenneth J Fotherby, New Cross Hospital, Wolverhampton, for peer reviewing the draft report. We would like to thank James Campbell, Stroke Improvement National Audit Programme (SINAP) Manager, Royal College of Physicians, London, for his invaluable help in forwarding our survey to stroke centres in England, Wales and Northern Ireland. Also thanks to Gill Rooney and Andrea Shippam for providing administrative support in preparing and formatting the report.
The views expressed in this report are those of the authors and not necessarily those of the NIHR HTA programme. Any errors are the responsibility of the authors.
Contributions of authors
Mike Holmes (Operational Research Analyst) co-ordinated the review and was responsible for the acquisition of data, analysis and interpretation of data and model construction (for the health economic evaluation and survey of stroke centres) and drafting and revision of the final report.
John Rathbone (Systematic Reviewer) was responsible for the acquisition of data, analysis and interpretation of data (for the systematic reviews and survey of stroke centres), and drafting and revision of the final report.
Chris Littlewood (Systematic Reviewer) was responsible for the acquisition of data, analysis and interpretation of data (for the systematic reviews) and drafting and revision of the final report.
Andrew Rawdin (Cost-Effectiveness Modeller) was responsible for the acquisition of data, analysis and interpretation of data and model construction (for the health economic evaluation) and drafting and revision of the final report.
Matt Stevenson (Reader in Health Economics and Decision Science) oversaw the modelling and reviewed the final report.
John Stevens (Lecturer in Bayesian Statistics) and Jenny Wang (Research Assistant Statistician) provided statistical support and undertook the meta-analyses.
Rachel Archer (Systematic Reviewer) was responsible for the conception and design of the study and sifting the prevalence search results at the title and abstract stage.
Pippa Evans (Information Specialist) was responsible for developing and undertaking the electronic literature searches.
About the School of Health and Related Research
The School of Health and Related Research (ScHARR) is one of the four Schools that comprise the Faculty of Medicine at the University of Sheffield. ScHARR brings together a wide range of medical and health-related disciplines including public health, general practice, mental health, epidemiology, health economics, management sciences, medical statistics, operational research and information science. It includes the Sheffield unit of the Trent Institute for Health Services Research, which is funded by NHS R&D to facilitate high-quality health services research and capacity development.
The ScHARR Technology Assessment Group (ScHARR-TAG) synthesises research on the clinical effectiveness and cost-effectiveness of health-care interventions for the NIHR HTA programme on behalf of a range of policy makers, including NICE. ScHARR-TAG is part of a wider collaboration of a number of units from other regions including Southampton Health Technology Assessment Centre (SHTAC), University of Southampton; Aberdeen Health Technology Assessment Group (Aberdeen HTA Group), University of Aberdeen; Liverpool Reviews & Implementation Group (LRiG), University of Liverpool; Peninsula Technology Assessment Group (PenTAG), University of Exeter; the NHS Centre for Reviews and Dissemination, University of York; Warwick Evidence, University of Warwick; the BMJ Group; and Kleijnen Systematic Reviews.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Hatano S. Experience from a multicentre stroke register: a preliminary report. Bull World Health Organ 1976;54:541-53.
- Murray CJ, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet 1997;349:1436-42. http://dx.doi.org/10.1016/S0140-6736(96)07495-8.
- Allender S, Peto V, Scarborough P, Boxer A, Rayner M. Coronary Heart Disease Statistics. London: British Heart Foundation; 2006.
- Mant J, Wade DT, Winner S, Stevens A, Raftery J, Mant J, et al. Health Care Needs Assessment: The Epidemiologically Based Needs Assessment Review. Oxford: Radcliffe Medical Press; 2004.
- Reducing Brain Damage: Faster Access to Better Stroke Care. National Audit Office Report 2005. London: Department of Health; 2007.
- Giles MF, Rothwell PM. Substantial underestimation of the need for outpatient services for TIA and minor stroke. Age Ageing 2007;36:676-80. http://dx.doi.org/10.1093/ageing/afm088.
- National Institute for Health and Care Excellence . Commissioning a Service for the Diagnosis and Initial Management of Acute Stroke 2010. www.nice.org.uk/usingguidance/commissioningguides/stroke/commissioning.jsp (accessed August 2011).
- Adamson J, Beswick A, Ebrahim S. Stroke and Disability: Reducing Brain Damage: Faster Access to Better Stroke Care 2004. London: National Audit Office; 2005.
- Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke 2009;40:2276-93.
- Rothwell PM, Coull AJ, Silver LE. Population-based study of event-rate, incidence, case fatality and mortality for all acute vascular events in all arterial territories (Oxford Vascular Study). Lancet 2005;366:1773-83. http://dx.doi.org/10.1016/S0140-6736(05)67702-1.
- National Clinical Guidelines for Stroke. London: Royal College of Physicians; 2004.
- Sacco RL. Newer risk factors for stroke. Neurology 2001;57:S31-4. http://dx.doi.org/10.1212/WNL.57.suppl_2.S31.
- Dunbabin DW, Sandercock P. Preventing stroke by the modification of risk factors. Stroke 1990;21:36-9.
- Health Inequalities 1997. London: The Stationery Office; 1997.
- Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. A prospective study of acute cerebrovascular disease in the community: the Oxfordshire Community Stroke Project – 1981–86. 2. Incidence, case fatality rates and overall outcome at one year of cerebral infarction, primary intracerebral and subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry 1990;53:16-22.
- Hart RG, Sherman DG, Miller VT, Easton JD. Diagnosis and management of ischemic stroke. Part II. Selected controversies. Curr Probl Cardiol 1983;8:1-77.
- Bogousslavsky J, Van Melle G, Regli F. The Lausanne Stroke Register: analysis of 1000 consecutive patients with first stroke. Stroke 1988;19:1083-92.
- Schneck MJ, Xu L, Hogan EL. Cardioembolic Stroke 2011. http://emedicine.medscape.com/article/1160370-overview (accessed September 2011).
- Aronow WS, Ahn C, Kronzon I. Prevalence of echocardiographic findings in 554 men and in 1,243 women aged > 60 years in a long-term health care facility. Am J Cardiol 1997;79:379-80. http://dx.doi.org/10.1016/S0002-9149(96)00769-2.
- Arboix A, Miguel M, Scar E, Eroles L, Massons J, Balcells M, et al. Cardiovascular risk factors in patients aged 85 or older with ischemic stroke. Clin Neurol Neurosurg 2006;108:638-43. http://dx.doi.org/10.1016/j.clineuro.2005.10.010.
- Pepi M, Evangelista A, Nihoyannopoulos P, Flachskampf FA, Athanassopoulos G, Colonna P, et al. Recommendations for echocardiography use in the diagnosis and management of cardiac sources of embolism. Eur Assoc Echocardiogr 2010;11:461-76.
- Mohr JP, Albers GW, Amarenco P, Babikian VL, Biller J, Brey RL, et al. American Heart Association Prevention Conference. IV. Prevention and rehabilitation of stroke. Etiology of stroke. Stroke 1997;28:1501-6.
- Ghandehari K, Moud ZI. Incidence and etiology of ischemic stroke in Persian young adults. Acta Neurol Scand 2006;113:121-4. http://dx.doi.org/10.1111/j.1600-0404.2005.00515.x.
- Griffiths D, Sturm J. Epidemiology and etiology of young stroke. Stroke Res Treat 2011. 10.4061/2011/209370.
- Sacco RL, Benjamin EJ, Broderick JP, Dyken M, Easton JD, Feinberg WM, et al. American Heart Association Prevention Conference. IV. Prevention and rehabilitation of stroke. Risk factors. Stroke 1997;28:1507-17.
- Putaala J, Metso AJ, Metso TM, Konkola N, Kraemer Y, Haapaniemi E, et al. Analysis of 1008 consecutive patients aged 15 to 49 with first-ever ischemic stroke: the Helsinki young stroke registry. Stroke 2009;40:1195-203. http://dx.doi.org/10.1161/STROKEAHA.108.529883.
- Varona JF, Guerra JM, Bermejo F, Molina JA, Gomez de la Cámara A. Causes of ischemic stroke in young adults, and evolution of the etiological diagnosis over the long term. Eur Neurol 2007;57:212-18. http://dx.doi.org/10.1159/000099161.
- Saito KMH, Oe H, Miyashita K, Nagatsuka K, Ueno S, Naritomi H. Mechanisms of bihemispheric brain infarctions in the anterior circulation on diffusion-weighted images. Am J Neuroradiol 2005;26:809-14.
- National Clinical Guideline For Stroke: Incorporating the Recommendations from Stroke: National Clinical Guideline for Diagnosis and Initial Management of Acute Stroke and Transient Ischaemic Attack (TIA). London: Royal College of Physicians; 2008.
- Stroke Diagnosis and Initial Management of Acute Stroke and Transient Ischaemic Attack. London: NICE; 2008.
- National Stroke Strategy 2007. London: Department of Health; 2007.
- British Society of Echocardiography . Indications for Echocardiography n.d. www.bsecho.org/indications-for-echocardiography/ (accessed December 2013).
- Van Neer PMJ, Danilouchkine MD, Verweij MD, Libertario D, Voormolen MM, Van Der Steen AFW, et al. Comparison of fundamental, second harmonic, and superharmonic imaging: a simulation study. J Acoust Soc Am 2011;130:3148-57. http://dx.doi.org/10.1121/1.3643815.
- Rem JA, Hachinski VC, Boughner DR, Barnett HJ. Value of cardiac monitoring and echocardiography in TIA and stroke patients. Stroke 1985;16:950-6.
- Abreu T, Mateus S, Correia J. Therapy implications of transthoracic echocardiography in acute ischemic stroke patients. Stroke 2005;37:1565-6.
- De Abreu TT, Mateus S, Carreteiro C, Correla J. Therapeutic implications of transesophageal echocardiography after transthoracic echocardiography on acute stroke patients. Vasc Health Risk Manag 2008;4:1167-72.
- Meenan RT, Saha S, Chou R, Swarztrauber K, Pyle Krages K, O’Keeffe-Rosetti MC, et al. Cost-effectiveness of echocardiography to identify intracardiac thrombus among patients with first stroke or transient ischemic attack. Med Decis Making 2007;27:161-77. http://dx.doi.org/10.1177/0272989X06297388.
- Patient.co.uk . Echocardiography 2011. www.patient.co.uk/doctor/Echocardiography.htm (accessed August 2011).
- Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264-9. http://dx.doi.org/10.7326/0003-4819-151-4-200908180-00135.
- Vahedi K, Amarenco P. Cardiac causes of stroke. Curr Treat Options Neurol 2000;2:305-17.
- Agmon Y, Khandheria B, Meissner I, Gentile F, Whisnant J, Sicks J, et al. Frequency of atrial septal aneurysms in patients with cerebral ischemic events. Circulation 1999;99:1942-4. http://dx.doi.org/10.1161/01.CIR.99.15.1942.
- Arnold M, Halpern M, Meier N, Fischer U, Haefeli T, Kappeler L, et al. Age-dependent differences in demographics, risk factors, co-morbidity, etiology, management, and clinical outcome of acute ischemic stroke. J Neurol 2008;255:1503-7. http://dx.doi.org/10.1007/s00415-008-0949-9.
- Awada A, Al Rajeh S. The Saudi Stroke Data Bank. Analysis of the first 1000 cases. Acta Neurol Scand 1999;100:265-9. http://dx.doi.org/10.1111/j.1600-0404.1999.tb00392.x.
- Barinagarrementeria F, Amaya LE, Cantu C. Causes and mechanisms of cerebellar infarction in young patients. Stroke 1997;28:2400-4. http://dx.doi.org/10.1161/01.STR.28.12.2400.
- Barinagarrementeria F, Gonalez-Duarte A, Miranda L, Cantu C. Cerebral infarction in young women: analysis of 130 cases. Eur Neurol 1998;40:228-33. http://dx.doi.org/10.1159/000007985.
- Belvis R, Santamaria A, Bregas J, Leta RG, Cocho D, Borrell M, et al. Patent foramen ovale and prothrombotic markers in young stroke patients. Blood Coagul Fibrinolysis 2007;18:537-42.
- Cabanes L, Mas JL, Cohen A, Amarenco P, Cabanes PA, Oubary P, et al. Atrial septal aneurysm and patent foramen ovale as risk factors for cryptogenic stroke in patients less than 55 years of age. A study using transesophageal echocardiography. Stroke 1993;24:1865-73. http://dx.doi.org/10.1161/01.STR.24.12.1865.
- Fieschi C, Rasura M, Anzini A, Decastro S, Digianfilippo G, Valesini G, et al. A diagnostic approach to ischemic stroke in young and middle-aged adults. Eur J Neurol 1996;3:324-30. http://dx.doi.org/10.1111/j.1468-1331.1996.tb00225.x.
- Fukujima MM, Tatani SB, Aguiar AS, Ferraz ME, Francisco S, Ferreira LD, et al. Transesophageal echocardiography discloses unexpected cardiac sources of embolus in stroke patients aged more than 45 years. Arq Neuropsiquiatr 2005;63:941-5. http://dx.doi.org/10.1590/S0004-282X2005000600007.
- Handke M, Harloff A, Olschewski M, Hetzel A, Geibel A. Patent foramen ovale and cryptogenic stroke in older patients. N Engl J Med 2007;357:2262-8. http://dx.doi.org/10.1056/NEJMoa071422.
- Homma S, Di Tullio MR, Sacco RL, Mihalatos D, Li Mandri G, Mohr J. Characteristics of patent foramen ovale associated with cryptogenic stroke: a biplane transesophageal echocardiographic study. Stroke 1994;25:582-6. http://dx.doi.org/10.1161/01.STR.25.3.582.
- Kang SY, Kim JS. Anterior cerebral artery infarction: stroke mechanism and clinical-imaging study in 100 patients. Neurology 2008;70:2386-93. http://dx.doi.org/10.1212/01.wnl.0000314686.94007.d0.
- Knebel F, Masuhr F, von Hausen W, Walde T, Dreger H, Raab V, et al. Transesophageal echocardiography in patients with cryptogenic cerebral ischemia. Cardiovasc Ultrasound 2009;7. http://dx.doi.org/10.1186/1476-7120-7-15.
- Lamy C, Giannesini C, Zuber M, Arquizan C, Meder JF, Trystram D, et al. Clinical and imaging findings in cryptogenic stroke patients with and without patent foramen ovale: the PFO-ASA study. Atrial Septal Aneurysm. Stroke 2002;33:706-11. http://dx.doi.org/10.1161/hs0302.104543.
- Lavados PM, Sacks C, Prina L, Escobar A, Tossi C, Araya F, et al. Incidence, case-fatality rate, and prognosis of ischaemic stroke subtypes in a predominantly Hispanic-Mestizo population in Iquique, Chile (PISCIS project): a community-based incidence study. Lancet Neurol 2007;6:140-8. http://dx.doi.org/10.1016/S1474-4422(06)70684-6.
- Lindgren A, Roijer A, Norrving B, Wallin L, Eskilsson J, Johansson BB. Carotid artery and heart disease in subtypes of cerebral infarction. Stroke 1994;25:2356-62. http://dx.doi.org/10.1161/01.STR.25.12.2356.
- Malm J, Kristensen B, Carlberg B, Fagerlund M, Olsson T. Clinical features and prognosis in young adults with infratentorial infarcts. Cerebrovasc Dis 1999;9:282-9. http://dx.doi.org/10.1159/000015979.
- Mattioli AVA. Atrial septal aneurysm as a cardioembolic source in adult patients with stroke and normal carotid arteries. A multicentre study. Eur Heart J 2001;22:261-8. http://dx.doi.org/10.1053/euhj.2001.2293.
- Mochan A, Modi M, Modi G. Stroke in black South African HIV-positive patients: a prospective analysis. Stroke 2003;34:10-5. http://dx.doi.org/10.1161/01.STR.0000043821.35051.FA.
- Mok VC, Fan YH, Lam WW, Hui AC, Wong KS. Small subcortical infarct and intracranial large artery disease in Chinese. J Neurol Sci 2003;216:55-9. http://dx.doi.org/10.1016/S0022-510X(03)00213-2.
- Musolino R, La Spina P, Granata A, Gallitto G, Leggiadro N, Carerj S, et al. Ischaemic stroke in young people: a prospective and long-term follow-up study. Cerebrovasc Dis 2003;15:121-8. http://dx.doi.org/10.1159/000067139.
- Negrão E, Brandi I, Nunes S, Távora D, Nakayama M, Beraldo P. Patent foramen ovale and ischemic stroke in young people: statistical association or causal relation?. Arq Bras Cardiol 2007;88:514-20.
- Nighoghossian N, Perinetti M, Barthelet M, Adeleine P, Trouillas P. Potential cardioembolic sources of stroke in patients less than 60 years of age. Eur Heart J 1996;17:590-4. http://dx.doi.org/10.1093/oxfordjournals.eurheartj.a014913.
- Omran H, Rang B, Schmidt H, Illien S, Schimpf R, Maccarter D, et al. Incidence of left atrial thrombi in patients in sinus rhythm and with a recent neurologic deficit. Am Heart J 2000;140:658-62. http://dx.doi.org/10.1067/mhj.2000.109213.
- Ossemann M, Laloux P, Marchandise B, Jamart J. Association between stroke and atrial septal aneurysm assessed by transesophageal echocardiography in a cardiologic population. Acta Neurol Belg 1995;95:170-7.
- Pearson AC, Nagelhout D, Castello R, Gomez CR, Labovitz AJ. Atrial septal aneurysm and stroke: a transesophageal echocardiographic study. J Am Coll Cardiol 1991;18:1223-9. http://dx.doi.org/10.1016/0735-1097(91)90539-L.
- Pezzini A, Del Zotto E, Magoni M, Costa A, Archetti S, Grassi M, et al. Inherited thrombophilic disorders in young adults with ischemic stroke and patent foramen ovale. Stroke 2003;34:28-33. http://dx.doi.org/10.1161/01.STR.0000046457.54037.CC.
- Rasura M, Spalloni A, Ferrari M, De Castro S, Patella R, Lisi F, et al. A case series of young stroke in Rome. Eur J Neurol 2006;13:146-52. http://dx.doi.org/10.1111/j.1468-1331.2006.01159.x.
- Rauh R, Fischereder M, Spengel FA. Transesophageal echocardiography in patients with focal cerebral ischemia of unknown cause. Stroke 1996;27:691-4. http://dx.doi.org/10.1161/01.STR.27.4.691.
- Rodriguez CJ, Homma S, Sacco RL, Di Tullio MR, Sciacca RR, Mohr JP, et al. Race-ethnic differences in patent foramen ovale, atrial septal aneurysm, and right atrial anatomy among ischemic stroke patients. Stroke 2003;34:2097-102. http://dx.doi.org/10.1161/01.STR.0000085828.67563.42.
- Roijer A, Lindgren A, Algotsson L, Norrving B, Olsson B, Eskilsson J. Cardiac changes in stroke patients and controls evaluated with transoesophageal echocardiography. Scand Cardiovasc J 1997;31:329-37. http://dx.doi.org/10.3109/14017439709075949.
- Roquer J, Campello AR, Gomis M. Sex differences in first-ever acute stroke. Stroke 2003;34:1581-5. http://dx.doi.org/10.1161/01.STR.0000078562.82918.F6.
- Seifert T, Enzinger C, Storch MK, Pichler G, Niederkorn K, Fazekas F. Acute small subcortical infarctions on diffusion weighted MRI: clinical presentation and aetiology. J Neurol Neurosurg Psychiatry 2005;76:1520-4. http://dx.doi.org/10.1136/jnnp.2005.063594.
- Serena J, Segura T, Perez-Ayuso MJ, Bassaganyas J, Molins A, Valos A. The need to quantify right-to-left shunt in acute ischemic stroke: a case–control study. Stroke 1998;29:1322-8.
- Silva MT, Rodrigues R, Tress J, Victer R, Chamiê. Patent foramen ovale in a cohort of young patients with cryptogenic ischemic stroke. Arq Neuropsiquiatr 2005;63:427-9. http://dx.doi.org/10.1590/S0004-282X2005000300012.
- Steinke W, Mangold J, Schwartz A, Hennerici M. Mechanisms of infarction in the superficial posterior cerebral artery territory. J Neurol 1997;244:571-8. http://dx.doi.org/10.1007/s004150050146.
- Strandberg MM. Transoesophageal echocardiography should be considered in patients with ischaemic stroke or transient ischaemic attack. Clin Physiol Funct Imaging 2008;28:156-60. http://dx.doi.org/10.1111/j.1475-097X.2007.00785.x.
- Tice FD, Slivka AP, Walz ET, Orsinelli DA, Pearson AC. Mitral valve strands in patients with focal cerebral ischemia. Stroke 1996;27:1183-6. http://dx.doi.org/10.1161/01.STR.27.7.1183.
- Ueno Y, Kimura K, Iguchi Y, Shibazaki K, Inoue T, Urabe T. Right-to-left shunt and lacunar stroke in patients without hypertension and diabetes. Neurology 2007;68:528-31. http://dx.doi.org/10.1212/01.wnl.0000253197.83777.c7.
- Walpot J, Pasteuning WH, Hoevenaar M, den Braber J, Sorgedrager J, Oostdijk-De RM, et al. Transesophageal echocardiography in patients with cryptogenic stroke: does it alter their management? A 3-year retrospective study in a single non-referral centre. Acta Clin Belg 2006;61:243-8.
- Ward RP, Don CW, Furlong KT, Lang RM. Predictors of long-term mortality in patients with ischemic stroke referred for transesophageal echocardiography. Stroke 2006;37:204-8. http://dx.doi.org/10.1161/01.STR.0000196939.12313.16.
- Zibaeenezhad MJ, Mowla A, Salahi R, Nikseresht AR, Shariat H, Ashjaezadeh N. Cardiac sources of embolic cerebral infarction in transesophageal echocardiography. Ann Saudi Med 2006;26:43-5.
- Benedik MP, Zalatel M, Meglic N, Podnar T. Patent foramen ovale and unexplained ischemic cerebrovascular events in children. Catheter Cardiovasc Interv 2007;70:999-1007. http://dx.doi.org/10.1002/ccd.21305.
- Hoffmann M, Chichkova R, Ziyad M, Malek A. Too much lumping in ischemic stroke – a new classification. Med Sci Monit 2004;10:CR285-7.
- Kristensen B, Malm J, Carlberg B, Stegmayr B, Backman C, Fagerlund M, et al. Epidemiology and etiology of ischemic stroke in young adults aged 18 to 44 years in northern Sweden. Stroke 1997;28:1702-9. http://dx.doi.org/10.1161/01.STR.28.9.1702.
- Siqueira Neto JI, Santos AC, Fabio SR, Sakamoto AC. Cerebral infarction in patients aged 15 to 40 years. Stroke 1996;27:2016-19.
- Sloan MA, Kittner SJ, Feeser BR, Gardner J, Epstein A, Wozniak MA, et al. Illicit drug-associated ischemic stroke in the Baltimore-Washington Young Stroke Study. Neurology 1998;50:1688-93. http://dx.doi.org/10.1212/WNL.50.6.1688.
- Arboix A, Vericat MC, Pujades R, Massons J, Eroles L, Oliveres M. Cardioembolic infarction in the Sagrat Cor-Alianza Hospital of Barcelona Stroke Registry. Acta Neurol Scand 1997;96:407-12. http://dx.doi.org/10.1111/j.1600-0404.1997.tb00307.x.
- Pessin MS, Lathi ES, Cohen MB, Kwan ES, Hedges TR, Caplan LR, et al. Clinical features and mechanism of occipital infarction. Ann Neurol 1987;21:290-9. http://dx.doi.org/10.1002/ana.410210311.
- Noce TR, Fábio SRC, Neto JIS, Carlos dos Santos A, Funayama CAR. Cerebral infarct in children aged zero to fifteen years. Arq Neuro-Psiquiat 2004;62:38-43. http://dx.doi.org/10.1590/S0004-282X2004000100007.
- Skidmore FM, Williams LS, Fradkin KD, Alonso RJ, Biller J. Presentation, etiology, and outcome of stroke in pregnancy and puerperium. J Stroke Cerebrovasc Dis 2001;10:1-10. http://dx.doi.org/10.1053/jscd.2001.20977.
- Williams LS, Garg BP, Cohen M, Fleck JD, Biller J. Subtypes of ischemic stroke in children and young adults. Neurology 1997;49:1541-5. http://dx.doi.org/10.1212/WNL.49.6.1541.
- Wong EH, Pullicino PM, Benedict R. Deep cerebral infarcts extending to the subinsular region. Stroke 2001;32:2272-7. http://dx.doi.org/10.1161/hs1001.096622.
- Bogousslavsky J, Garazi S, Jeanrenaud X, Aebischer N, Van Melle G. Stroke recurrence in patients with patent foramen ovale: the Lausanne Study. Lausanne Stroke with Paradoxal Embolism Study Group. Neurology 1996;46:1301-5.
- Mehndiratta MM, Agarwal P, Sen K, Sharma B. Stroke in young adults: a study from a university hospital in north India. Med Sci Monit 2004;10.
- Bevan H, Sharma K, Bradley W. Stroke in young adults. Stroke 1990;21:382-6. http://dx.doi.org/10.1161/01.STR.21.3.382.
- Lanzino G, Andreoli A, Di Pasquale G, Urbinati S, Limoni P, Serracchioli A. Etiopathogenesis and prognosis of cerebral ischemia in young adults. A survey of 155 treated patients. Acta Neurol Scand 1991;84:321-5.
- Luijckx GJ, Ukachoke C, Limapichat K, Heuts-Van Raak EP, Lodder J. Brain infarct causes under the age of fifty: a comparison between an east-Asian (Thai) and a western (Dutch) hospital series. Clin Neurol Neurosurg 1993;95:199-203. http://dx.doi.org/10.1016/0303-8467(93)90124-Y.
- Sandercock PA, Warlow CP, Jones LN, Starkey IR. Predisposing factors for cerebral infarction: the Oxfordshire community stroke project. BMJ 1989;298:75-80. http://dx.doi.org/10.1136/bmj.298.6666.75.
- Tei H, Uchiyama S, Maruyama S. Capsular infarcts: location, size and etiology of pure motor hemiparesis, sensorimotor stroke and ataxic hemiparesis. Acta Neurol Scand 1993;88:264-8. http://dx.doi.org/10.1111/j.1600-0404.1993.tb04233.x.
- Pun KKW, Wang RYC, Kuang CY. Cardiac embolic cerebrovascular disease in Hong Kong 1979–1982 – A review of 129 consecutive cases. Asian Med J 1984;27:529-36.
- Kasner SE, Lynn MJ, Jackson BP, Pullicino PM, Chimowitz MI. Warfarin Versus Aspirin for Symptomatic Intracranial Disease (WASID) trial investigators . Echocardiography in patients with symptomatic intracranial stenosis. J Stroke Cerebrovasc Dis 2007;16:216-19.
- Pepi M, Evangelista A, Nihoyannopoulos P, Flachskampf FA, Colonna P, Habib G, et al. Recommendations for echocardiography use in the diagnosis and management of cardiac sources of embolism. Eur J Echocardiogr 2010;11:461-76. http://dx.doi.org/10.1093/ejechocard/jeq045.
- University of York, Centre for Reviews and Dissemination . International Prospective Register of Systematic Reviews 2011. www.crd.york.ac.uk/prospero.
- Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3.
- Brooks SP, Gelman A. Alternative methods for monitoring convergence of iterative simulations. J Comput Graph Stat 1998;7:434-55.
- Akosah KOP. Cryptogenic stroke: marked superiority of transesophageal echocardiography and transthoracic echocardiography in identifying intracardiac and intraaortic embolic sources. J Noninvasive Cardiol 1998;2:17-23.
- Baur H, Daniel J, Nelson R. Detection of left ventricular aneurysm on two dimensional echocardiography. Am J Cardiol 1982;50. http://dx.doi.org/10.1016/0002-9149(82)90028-5.
- Belkin RN, Pollack BD, Ruggiero ML, Alas LL, Tatini U. Comparison of transesophageal and transthoracic echocardiography with contrast and color flow Doppler in the detection of patent foramen ovale. Am Heart J 2011;128. http://dx.doi.org/10.1016/0002-8703(94)90626-2.
- Di Tullio MS. Comparison of diagnostic techniques for the detection of a patent foramen ovale in stroke patients. Stroke 1993;24:1020-4.
- Hirata K, Pulerwitz T, Sciacca R, Otsuka R, Oe Y, Fujikura K, et al. Clinical utility of new real time three-dimensional transthoracic echocardiography in assessment of mitral valve prolapse. Echocardiography 2008;25:482-8. http://dx.doi.org/10.1111/j.1540-8175.2008.00630.x.
- Hubail Z, Lemler M, Ramaciotti C, Moore J, Ikemba C. Diagnosing a patent foramen ovale in children: is transesophageal echocardiography necessary?. Stroke 2011;42:98-101. http://dx.doi.org/10.1161/STROKEAHA.110.595876.
- Kerr AJ, Buck T, Chia K, Chow CM, Fox E, Levine RA, et al. Transmitral Doppler: a new transthoracic contrast method for patent foramen ovale detection and quantification. J Am Coll Cardiol 2000;36:1959-66. http://dx.doi.org/10.1016/S0735-1097(00)00951-7.
- Lee RJ, Bartzokis T, Yeoh T, Grogin H, Choi D, Schnittger I. Enhanced detection of intracardiac sources of cerebral emboli by transesophageal echocardiography. Stroke 1991;22:734-9. http://dx.doi.org/10.1161/01.STR.22.6.734.
- Madala D, Zaroff JG, Hourigan L, Foster E. Harmonic imaging improves sensitivity at the expense of specificity in the detection of patent foramen ovale. Echocardiography 2004;21:33-6. http://dx.doi.org/10.1111/j.0742-2822.2004.02168.x.
- Nemec JJ, Marwick TH, Lorig RJ, Davison MB, Chimowitz MI, Litowitz H. Comparison of transcranial Doppler ultrasound and transesophageal contrast echocardiography in the detection of interatrial right-to-left shunts. Am J Cardiol 1991;68:1498-502. http://dx.doi.org/10.1016/0002-9149(91)90285-S.
- Neuman YM, Brasch AV, Kobal S, Khan SS, Mirocha JM, Naqvi TZ, et al. Comparison of transthoracic and intraoperative transesophageal color flow Doppler assessment of mitral and aortic regurgitation. Cardiology 2003;99:145-52. http://dx.doi.org/10.1159/000070671.
- Pearson AC, Labovitz AJ, Tatineni S, Gomez CR. Superiority of transesophageal echocardiography in detecting cardiac source of embolism in patients with cerebral ischemia of uncertain etiology. J Am Coll Cardiol 1991;17:66-72. http://dx.doi.org/10.1016/0735-1097(91)90705-E.
- Roldan CA, Qualls CR, Sopko KS, Sibbitt WL. Transthoracic versus transesophageal echocardiography for detection of Libman–Sacks endocarditis: a randomized controlled study. J Rheumatol 2008;35:224-9.
- Sallach JA, Puwanant S, Drinko JK, Jaffer S, Donal E, Thambidorai SK, et al. Comprehensive left atrial appendage optimization of thrombus using surface echocardiography: the CLOTS multicenter pilot trial. J Am Soc Echocardiogr 2009;22:1165-72. http://dx.doi.org/10.1016/j.echo.2009.05.028.
- Shub C, Dimopoulos I, Seward J, Callahan J, Tancredi R, Schattenberg T, et al. Sensitivity of two-dimensional echocardiography in the direct visualization of atrial septal defect utilizing the subcostal approach: experience with 154 patients. J Am Coll Cardiol 1983;2:127-35. http://dx.doi.org/10.1016/S0735-1097(83)80385-4.
- Stratton J, Lighty G, Pearlman A, Ritchie J. Detection of left ventricular thrombus by two-dimensional echocardiography: sensitivity, specificity, and causes of uncertainty. Circulation 1982;66. http://dx.doi.org/10.1161/01.CIR.66.1.156.
- Thanigaraj S, Valika A, Zajarias A, Lasala J, Perez J. Comparison of transthoracic versus transesophageal echocardiography for detection of right to left atrial shunting using agitated saline contrast. Am J Cardiol 2005;96:1007-10. http://dx.doi.org/10.1016/j.amjcard.2005.05.061.
- Weinsaft JW, Kim HW, Crowley AL, Klem I, Shenoy C, Van Assche L, et al. LV thrombus detection by routine echocardiography: insights into performance characteristics using delayed enhancement CMR. JACC Cardiovasc Imaging 2011;4:702-12.
- Aschenberg W, Schluter M, Kremer P, Schroder E, Siglow V, Bleifield W. Tranesophageal two-dimensional echocardiography for the detection of left atrial appendage thrombus. J Am Coll Cardiol 1986;7:163-6. http://dx.doi.org/10.1016/S0735-1097(86)80275-3.
- Illien S, Becher HL, Luderitz B, Maccarter D, Omran H, Rabahieh R, et al. A Prospective comparison of harmonic transthoracic and transesophageal echocardiography for identifying left atrial thrombi in patients with atrial flutterand/or fibrillation prior to cardioversion. J Clin Basic Cardiol 2002;5:92-9.
- Jax TP. Eur Heart J 2010.
- Kuhl H, Hoffmann R, Merx M, Franke A, Klotzsch C, Lepper W, et al. Transthoracic echocardiography using second harmonic imaging. J Am Coll Cardiol 1999;34:1823-30.
- Lembcke A, Woinke M, Borges AC, Dohmen PM, Lachnitt A, Westermann Y, et al. Grading of aortic valve stenosis at 64-slice spiral computed tomography: comparison with transthoracic echocardiography and calibration against cardiac catheterization. Invest Radiol 2009;44:360-8. http://dx.doi.org/10.1097/RLI.0b013e3181a64d76.
- Lipke C, Katoh M, Franke A, Krombach G, Buecker A, Kuhl HP, et al. The value of non-contrast harmonic transthoracic echocardiography for the detection of left ventricular thrombi in patients with cardiomyopathy: comparison with contrast-enhanced magnetic resonance imaging. Int J Cardiovasc Imaging 2007;23:479-87. http://dx.doi.org/10.1007/s10554-006-9190-8.
- Mugge A, Daniel W, Angermann C, Spes C, Khandheria B, Kronzon I, et al. Atrial septal aneurysm in adult patients: a multicenter study using transthoracic and transoesophageal echocardiography. Circulation 1995;91:2785-92. http://dx.doi.org/10.1161/01.CIR.91.11.2785.
- Omran H, Jung W, Rabahieh R, Wirtz P, Becher H, Illien S, et al. Imaging of thrombi and assessment of left atrial appendage function: a prospective study comparing transthoracic and transoesophageal echocardiography. Heart 1999;81:192-8.
- Stendel R, Gramm HJ, Schroder K, Lober C, Brock M. Transcranial Doppler ultrasonography as a screening technique for detection of a patent foramen ovale before surgery in the sitting position. Anesthesiology 2000;93:971-5. http://dx.doi.org/10.1097/00000542-200010000-00016.
- Siostrzonek P, Zangeneh M, Gossinger H. Comparison of transesophageal and transthoracic echocardiography for the detection of a patent foramen ovale. Am J Cardiol 1991;68:1247-9. http://dx.doi.org/10.1016/0002-9149(91)90206-Z.
- Li XC, Yan CJ, Yao GH, Zhang M, Li JF, Zhang Y, et al. Value of left ventricular regional ejection fraction determined by real-time three-dimensional echocardiography in diagnosis of aneurysm: compared with left ventriculography. Chin Med J 2009;122:2981-4.
- Ha JW, Chung N, Kang SM, Jang KJ, Kim IJ, Rim SJ, et al. Enhanced detection of left atrial spontaneous echo contrast by transthoracic harmonic imaging in mitral stenosis. J Am Soc Echocardiogr 2000;13:849-54. http://dx.doi.org/10.1067/mje.2000.106791.
- Ha J, Shin M, Kang S, Pyun W, Jang K, Byun K, et al. Enhanced detection of right to left shunting through patent foramen ovale by transthoracic echocardiography using harmonic imaging. Am J Cardiol 2001;87:669-71. http://dx.doi.org/10.1016/S0002-9149(00)01455-7.
- Kitayama H, Kiuchi K, Endo T, Hayakawa H. Value of cardiac ultrafast computed tomography for detecting right atrial thrombi in chronic atrial fibrillation. Am J Cardiol 1997;79:1292-5. http://dx.doi.org/10.1016/S0002-9149(97)00107-0.
- Chen W, Kuan P, Lien W, Lin F. Detection of patent foramen ovale by contrast transesophageal echocardiography. Chest 1992;101:1515-20. http://dx.doi.org/10.1378/chest.101.6.1515.
- Daniels C, Weytjens C, Cosyns B, Schoors D, De Sutter J, Paelinck B, et al. Second harmonic transthoracic echocardiography: the new reference screening method for the detection of patent foramen ovale. Eur J Echocardiogr 2004;5:449-52. http://dx.doi.org/10.1016/j.euje.2004.04.004.
- De Bruijn SF, Agema WR, Lammers GJ, Van Der Wall EE, Wolterbeek R, Holman ER, et al. Transesophageal echocardiography is superior to transthoracic echocardiography in management of patients of any age with transient ischemic attack or stroke. Stroke 2006;37:2531-4. http://dx.doi.org/10.1161/01.STR.0000241064.46659.69.
- Pop G, Sutherland GR, Koudstaal PJ, Sit TW, De Jong G, Roelandt JR. Transesophageal echocardiography in the detection of intracardiac embolic sources in patients with transient ischemic attacks. Stroke 1990;21:560-5. http://dx.doi.org/10.1161/01.STR.21.4.560.
- Clarke N, Timperley J, Kelion AD, Banning AP. Transthoracic echocardiography using second harmonic imaging with Valsalva manoeuvre for the detection of right to left shunts. Eur J Echocardiogr 2004;5:176-81. http://dx.doi.org/10.1016/S1525-2167(03)00076-3.
- Trevelyan JS, Steeds RP. Comparison of transthoracic echocardiography with harmonic imaging with transoesophageal echocardiography for the diagnosis of patent foramen ovale. Postgrad Med J 2006;82:613-14. http://dx.doi.org/10.1136/pgmj.2006.045021.
- Blum A, Reisner S, Farbstein Y. Transesophageal echocardiography (TEE) vs. transthoracic echocardiography (TTE) in assessing cardio-vascular sources of emboli in patients with acute ischemic stroke. Med Sci Monit 2004;10:CR521-3.
- Gonzalez-Alujas T, Evangelista A, Santamarina E, Rubiera M, Gomez-Bosch Z, Rodriguez-Palomares JF, et al. Diagnosis and quantification of patent foramen ovale. Which is the reference technique? Simultaneous study with transcranial Doppler, transthoracic and transesophageal echocardiography. Rev Esp Cardiol 2011;64:133-9. http://dx.doi.org/10.1016/j.rec.2010.10.014.
- Gutiérrez-Chico JL, Zamorano Gómez JL, Rodrigo-López JL, Mataix L, Pérez de Isla L, Almería-Valera C, et al. Accuracy of real-time 3-dimensional echocardiography in the assessment of mitral prolapse. Is transesophageal echocardiography still mandatory?. Am Heart J 2008;155:694-8.
- Vincelj J, Sutlic Z, Biocina B, Nikić N, Lajtman Z. Diagnostic accuracy of transesophageal echocardiography for detection of atrial masses. Acta Med Croatica 2001;55:47-51.
- Chirillo F, Pedrocco A, De Leo A, Bruni A, Totis O, Meneghetti P, et al. Impact of harmonic imaging on transthoracic echocardiographic identification of infective endocarditis and its complications. Heart 2005;91:329-33. http://dx.doi.org/10.1136/hrt.2003.031583.
- Maffè S, Dellavesa P, Zenone F, Paino AM, Paffoni P, Perucca A, et al. Transthoracic second harmonic two- and three-dimensional echocardiography for detection of patent foramen ovale. Eur J Echocardiogr 2010;11:57-63.
- Zito C, Dattilo G, Oreto G, Di Bella G, Lamari A, Iudicello R, et al. Patent foramen ovale: comparison among diagnostic strategies in cryptogenic stroke and migraine. Echocardiography 2009;26:495-503. http://dx.doi.org/10.1111/j.1540-8175.2008.00852.x.
- Cujec B, Polasek P, Voll C, Shuaib A. Transesophogeal echocardiography in the detection of potential cardiac source of embolism in stroke patients. Stroke 1991;22:727-33. http://dx.doi.org/10.1161/01.STR.22.6.727.
- Jassal DS, Aminbakhsh A, Fang T, Shaikh N, Embil JM, Mackenzie GS, et al. Diagnostic value of harmonic transthoracic echocardiography in native valve infective endocarditis: comparison with transesophageal echocardiography. Cardiovasc Ultrasound 2007;5. http://dx.doi.org/10.1186/1476-7120-5-20.
- Black I, Hopkins A, Lee L, Walsh W, Jacobson B. Left atrial spontaneous echo contrast: a clinical and echocardiographic analysis. J Am Coll Cardiol 1991;18:398-404. http://dx.doi.org/10.1016/0735-1097(91)90592-W.
- Black I, Hopkins A, Lee L, Jacobson B, Walsh W. Role of tranesophageal echocardiography in evaluation of cardiogenic embolism. Br Heart J 1991;66:302-7. http://dx.doi.org/10.1136/hrt.66.4.302.
- Fatkin D, Scalia G, Jacobs N, Burstow D, Leung D, Walsh W, et al. Accuracy of biplane transesophageal echocardiography in detecting left atrial thrombus. Am J Cardiol 1996;77:321-4. http://dx.doi.org/10.1016/S0002-9149(97)89406-4.
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ 2003;326:41-4. http://dx.doi.org/10.1136/bmj.326.7379.41.
- Drummond M, Sculpher MJ, Torrance GW, O’Brien BJ. Methods for the Economic Evaluation of Health Care Programs. Oxford: Oxford University Press; 2005.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. BMJ 1996;313:275-83. http://dx.doi.org/10.1136/bmj.313.7052.275.
- Evers S, Goossens M, De Vet H, Van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. Int J Technol Assess Health Care 2005;21.
- McNamara RL, Lima JA, Whelton PK, Powe NR. Echocardiographic identification of cardiovascular sources of emboli to guide clinical management of stroke: a cost-effectiveness analysis. Ann Intern Med 1997;127:775-87. http://dx.doi.org/10.7326/0003-4819-127-9-199711010-00001.
- Matchar DB, Samsa GP. Secondary and Tertiary Prevention of Stroke 2000.
- Solomon HA, Click HA, Runo CJ, Schulman KA. Patient preferences for stroke outcomes. Stroke 1994;25:1721-5. http://dx.doi.org/10.1161/01.STR.25.9.1721.
- United Kingdom, Interim Life Tables 1980–82 to 2006–2008. London: ONS; 2008.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2008. www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf (accessed 31 October 2011).
- Meenan RT, Saha S, Chou R, Swarztrauber K, Krages KP, O’Keefee-Rosetti M, et al. Effectiveness and Cost-Effectiveness of Echocardiography and Carotid Imaging in the Management of Stroke 2002.
- National Institute for Health and Care Excellence . Assumptions Used in Estimating a Population Benchmark 2011. www.nice.org.uk/usingguidance/commissioningguides/tia/assumptionstiaservice.jsp (accessed 17 January 2012).
- Intercollegiate Stroke Working Party . National Sentinel Stroke Clinical Audit 2010 Round 7 Public Report for England, Wales and Northern Ireland 2010 n.d. www.rcplondon.ac.uk/sites/default/files/national-sentinel-stroke-audit-2010-public-report-and-appendices_0.pdf (accessed 31 October 2011).
- Clark TG, Murphy MFG, Rothwell PM. Long term risks of stroke, myocardial infarction and vascular death in ‘low risk’ patients with a non-recent transient ischemic attack. J Neurol Neurosurg Pyschiatry 2003;74:577-80.
- Simpson EL, Stevenson MD, Rawdin AC, Papaioannou D. Thrombophilia testing in people with venous thromboembolism: systematic review and cost-effectiveness analysis. Health Technol Assess 2009;13.
- Holmes MW, Goodacre S, Stevenson MD, Pandor A, Pickering H. The cost-effectiveness of diagnostic management strategies for adults with minor head injury. Injury 2012;43:1423-31. http://dx.doi.org/10.1016/j.injury.2011.07.017.
- Goodacre S, Sampson F, Stevenson M, Wailoo A, Sutton A, Thomas S. Measurement of the clinical and cost-effectiveness of non-invasive diagnostic testing strategies for deep vein thrombosis. Health Technol Assess 2006;10.
- Ashwal S, Cranford R, Bernat JL, Celesia G, Coulter D, Eisenberg H, et al. Medical aspects of the persistent vegetative state. N Engl J Med 1994;330:1572-9.
- Sandercock-Peter AG, Counsell C, Kamal AK. Anticoagulants for acute ischaemic stroke. Cochrane Database Syst Rev 2008;4.
- Department of Health . NHS Reference Costs 2009–2010 2010. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_123459 (accessed September 2011).
- Department of Health . Impact Assessment of National Stroke Strategy 2011. www.dh.gov.uk/prod_consum_dh/groups/dh_digitalassets/documents/digitalasset/dh_081054.pdf (accessed January 2012).
- Curtis L. Unit Costs of Health and Social Care 2010. Canterbury: Personal Social Service Research Unit, University of Kent; 2010.
- Dorman P, Denni M, Sandercock P. Are the modified ‘simple questions’ a valid and reliable measure of health related quality of life after stroke?. J Neurol Neurosurg Pyschiatry 2000;69:487-93.
- Kind P, Dolan P, Gudex C, Williams A. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ 1998;316:736-41. http://dx.doi.org/10.1136/bmj.316.7133.736.
- Claxton K, Sculpher M, McCabe C, Briggs A, Akehurst R, Buxton M, et al. Probabilistic sensitivity analysis for NICE technology assessment: not an optional extra. Health Econ 2005;14. http://dx.doi.org/10.1002/hec.985.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10. http://dx.doi.org/10.1002/hec.635.
- Claxton K, Posnett J. An economic approach to clinical trial design and research priority-setting. Health Econ 1996;5:513-24. http://dx.doi.org/10.1002/(SICI)1099-1050(199611)5:6<513::AID-HEC237>3.0.CO;2-9.
- Comess KA, Derook FA, Beach KW, Lytle NJ, Golby AJ, Albers GW. Transesophageal echocardiography and carotid ultrasound in patients with cerebral ischemia: prevalence of findings and recurrent stroke risk. J Am Coll Cardiol 1994;23:1598-603. http://dx.doi.org/10.1016/0735-1097(94)90662-9.
- Wade DT, Collin C. The Barthel ADL Index: a standard measure of physical disability?. Disabil Rehabil 1988;10:64-7. http://dx.doi.org/10.3109/09638288809164105.
- al-Saadon K, Walley VM, Green M, Beanlands DS. Angiographic diagnosis of true and false LV aneurysms after inferior wall myocardial infarction. Cathet Cardiovasc Diagn 1995;3:266-9.
- Helmcke F, Nanda NC, Hsiung MC, Soto B, Adey CK, Goyal RG, et al. Circulation 1987;75:175-83.
- Herzog CA, Bass D, Kane M, Assinger R. Two-dimensional echocardiographic imaging of left atrial appendage thrombi. J Am Coll Cardiol 1984;3:1340-4.
Appendix 1 Cardiac sources of stroke and transient ischaemic attack
| Pathology | Potential cardiac source |
|---|---|
| Chamber defects | Atrial septal defect |
| PFO | |
| Atrial shunt | |
| Atrial/interatrial/intra-atrial septal aneurysm | |
| Hypermobility of atrial septum | |
| Left atrial functional abnormality | |
| Left ventricular aneurysm | |
| Systolic left ventricular dysfunction of ischaemic and non-ischaemic aetiology | |
| Left ventricular ejection fraction < 40% | |
| Cor triatriatum | |
| Valvular defects | Mitral valve stenosis |
| Rheumatic mitral valve disease | |
| Mitral valve regurgitation | |
| Mitral valve prolapse | |
| Aortic valve stenosis | |
| Sclerosis/calcification of the aortic valve | |
| Rheumatic aortic valve disease | |
| Aortic valve regurgitation | |
| Mitral or aortic valve strands | |
| Artificial/prosthetic heart valve complication | |
| Thrombosis | Ventricular or atrial thrombosis |
| Left ventricular/left atrial thrombus | |
| Apical thrombosis | |
| Atrial appendage thrombus | |
| Cardiac masses, endocarditis and vegetation | Cardiac tumour/mass |
| Atrial myxoma | |
| Papillary fibroelastoma | |
| Libman–Sacks endocarditis | |
| Marantic endocarditis | |
| Non-bacterial thrombotic endocarditis | |
| Valvular vegetation | |
| Cardiac enlargement | Dilated left atrium |
| Left atrial enlargement | |
| Dilated left ventricle | |
| Left ventricle hypertrophy | |
| Left ventricular hypertrophic hypertensive disease | |
| Pathologies of the aorta | Aortic aneurysm |
| Dilated proximal aorta | |
| Calcification of the aorta | |
| Aortic dissection | |
| SEC | SEC/’smoke’ |
| Left atrial appendage spontaneous contract | |
| Isolated left atrial ‘smoke’ on echocardiography (no mitral stenosis or AF) | |
| Cardiomyopathy | Cardiomyopathy |
| Dilated cardiomyopathy | |
| Left ventricular non-compaction | |
| Rhythm dysfunction conditions | Atrial flutter |
| Sick sinus syndrome | |
| Others | Regional myocardial dyskinesia |
Appendix 2 MEDLINE search strategy for the systematic review of prevalence studies
-
Stroke/ (38,966)
-
stroke$.mp. (139,568)
-
stroke volume/ (25,577)
-
stroke volume$.mp. (33,208)
-
Cerebrovascular accident.mp. (2654)
-
cerebrovascular event.mp. (478)
-
Cerebrovascular disease.mp. (9488)
-
transient ischaemic event.mp. (4)
-
transient ischaemic attack.mp. (867)
-
vascular accident.mp. (676)
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 (148,787)
-
akinetic left ventricular segment.mp. (3)
-
artificial heart valve complication.mp. (0)
-
atherosclerotic aortic plaques.mp. (13)
-
calcification of the aorta.mp. (140)
-
canal defect$.mp. (264)
-
cardioemboli$.mp. (1386)
-
((atrial or ventricular or cardiac) adj (thromb$ or clot$ or defect$ or patholog$)).mp. (6611)
-
((infective or libman-sacks or marantic or non-bacterial thrombotic) adj endocarditis).mp. (6074)
-
((mitrial valve or aortic valve or valve) adj (sclerosis or stenosis or calcification or disease or regurgitation or prolapse or strands)).mp. (41,648)
-
((ventricular or atrial or apical) adj thromb$).mp. (2384)
-
(aortic adj (aneurysm or arch debris or atheroma or dissection or thrombus)).mp. (36,021)
-
(atrial adj (fibrilation or flutter or myxoma or sept$ or shunt)).mp. (16,689)
-
(cardiac adj (tumour or mass or embol$ or enlargement or mass$ origin$ or source$ or vegetation$)).mp. (2380)
-
cardiogenic.mp. (10,581)
-
Cardiomyopathies/ (18,868)
-
chamber defects.mp. (9)
-
chiari network.mp. (61)
-
congestive heart failure.mp. (28,205)
-
cor triatriatum.mp. (663)
-
coronary artery bypass graft surgery.mp. (3201)
-
dilated left atrium.mp. (82)
-
dilated proximal aorta.mp. (1)
-
Endocarditis/ (4784)
-
false tendon.mp. (70)
-
hypermobility of atrial septum.mp. (0)
-
Hypertrophy, Right Ventricular/ or Hypertrophy, Left Ventricular/ (10,219)
-
lambl’s excrescences.mp. (30)
-
(left atrial adj (appendage functional abnormality or appendage spontaneous contract or band or enlargement or abnormality or septum abnormality)).mp. (562)
-
lipomatous hypertrophy.mp. (152)
-
Myocardial Infarction/co [Complications] (22,786)
-
papillary fibroelastoma.mp. (422)
-
Foramen Ovale, Patent/ (742)
-
pericardial mesothelioma.mp. (165)
-
persistent left superior vena cava.mp. (716)
-
polyarteritis nodosa.mp. (5648)
-
primary systemic amyloidosis.mp. (384)
-
prosthetic heart valve complication.mp. (0)
-
regional myocardial dyskinesia.mp. (2)
-
regional wall motion abnormalit$.mp. (882)
-
sick sinus syndrome.mp. (2807)
-
spontaneous echo contrast.mp. (410)
-
tetralogy of fallot.mp. (8529)
-
Thrombosis/ (49,029)
-
valvular defect$.mp. (219)
-
valvular vegetation.mp. (50)
-
eustachian valve.mp. (164)
-
((atrial or interatrial or interaatrial) adj septal aneurysm).mp. (539)
-
akine$ segments.mp. (143)
-
congenital heart defect$.mp. (4718)
-
dilated left ventricle.mp. (214)
-
dyskine$ segments.mp. (120)
-
(left ventricle$ adj (hypertrophy or hypertension or anuerysm or dysfunction or ejection fraction or noncompaction)).mp. (551)
-
or/12-63 (261,412)
-
exp epidemiologic studies/ (1,296,793)
-
exp epidemiology/ (18,044)
-
epidemiology.tw. (78,229)
-
exp prevalence/ (144,996)
-
prevalence.ti. (65,013)
-
exp incidence/ (142,499)
-
incidence.ti. (61,659)
-
ep.fs. (966,875)
-
or/65-72 (2,048,519)
-
human/ (11,642,629)
-
animal/ (4,756,082)
-
74 not (74 and 75) (10,408,432)
-
11 and 64 and 73 and 76 (6298)
Appendix 3 List of excluded studies (studies all excluded as no relevant data were identified)
1. Stroke prevention in atrial fibrillation and other cardiac sources of embolism. Cerebrovasc Dis 1999;9(Suppl. 4):53–61.
2. Adachi T, Kobayashi S, Yamaguchi S. Frequency and pathogenesis of silent subcortical brain infarction in acute first-ever ischemic stroke. Intern Med 2002;41:103–8.
3. Aizawa YS. Editorial for the manuscript of Zhou and colleagues appearing in this issue: pheochromocytoma and cardioembolic events. Intern Med 2009;48:1571–2.
4. Akhtar N, Kamran SI, Deleu D, D’Souza A, Miyares F, Elsotouhy A, et al. Ischaemic posterior circulation stroke in State of Qatar. Eur J Neurol 2009;16:1004–9.
5. Alam M, Witt N, Nordlander R, Samad BA. Detection of abnormal left ventricular function by Doppler tissue imaging in patients with a first myocardial infarction and showing normal function assessed by conventional echocardiography. Eur J Echocardiogr 2007;8:37–41.
6. Alexandrov AV, Felberg RA, Demchuk AM, Christou I, Burgin WS, Malkoff M, et al. Deterioration following spontaneous improvement: sonographic findings in patients with acutely resolving symptoms of cerebral ischemia. Stroke 2000;31:915–19.
7. Alpar AK, Kutlutürk N, Küçükay F, Ökten S, Arda K. Evaluation of intracranial distribution of cardioembolic infarcts by computed tomography. Turk Klin J Med Sci 2005;25:65–72.
8. Alsheikh-Ali AA, Thaler DE, Kent DM. Patent foramen ovale in cryptogenic stroke: incidental or pathogenic? Stroke 2009;40:2349–55.
9. Alton ME, Pasierski TJ, Orsinelli DA, Eaton GM, Pearson AC. Comparison of transthoracic and transesophageal echocardiography in evaluation of 47 Starr-Edwards prosthetic valves. J Am Coll Cardiol 1992;20:1503–11.
10. Alvarez S, Gil N, Quintana M, Barbera G, Grupo de investigadores del estudio APICA. [Prevalence of asymptomatic peripheral artery disease in patients with non-cardioembolic ischemic stroke.] Neurologia 2009;24:366–72.
11. Alvaro LC, Timiraos J, Sádaba F. [In-hospital stroke: clinical profile and expectations for treatment.] Neurologia 2008;23:4–9.
12. Alzamora MT, Sorribes M, Heras A, Vila N, Vicheto M, Forés, et al. Ischemic stroke incidence in Santa Coloma de Gramenet (ISISCOG), Spain. A community-based study. BMC Neurol 2008;8:5.
13. Amarenco P. Underlying pathology of stroke of unknown cause (cryptogenic stroke). Cerebrovasc Dis 2009;27(Suppl. 1):97–103.
14. Amidon TM, Chou TM, Kee LL, Foster E. Role of echocardiography in primary care medicine – controversies in hypertension, atrial fibrillation, stroke, and endocarditis. West J Med 1996;164:269–75.
15. Amin HA, Aronow WS, Lleva P, McClung JA, Desai H, Gandhi K. Prevalence of transthoracic echocardiographic abnormalities in patients with ischemic stroke, intracerebral hemorrhage, and subarachnoid hemorrhage. Arch Med Sci 2010;6:40–2.
16. An Z, Xing Y, Jin S. Factors influencing ischemic cerebrovascular disease complicated by hyperhomocysteinemia. Neural Regener Res 2008;3:329–32.
17. Anderson DJ, Goldstein LB, Wilkinson WE, Corey GR, Cabell CH, Sanders LL, et al. Stroke location, characterization, severity, and outcome in mitral vs aortic valve endocarditis. Neurology 2003;61:1341–6.
18. Arboix A. Cardioembolic infarction: a renewed topic of interest. Curr Cardiol Rev 2010;6:137.
19. Arboix A, Eroles L, Massons JB, Oliveres M, Pujades R, Targa C, et al. Atrial fibrillation and stroke: clinical presentation of cardioembolic versus atherothrombotic infarction. Int J Cardiol 2000;73:33–42.
20. Arboix A, Miguel M, Císcar E, Eroles L, Massons J, Balcells M, et al. Cardiovascular risk factors in patients aged 85 or older with ischemic stroke. Clin Neurol Neurosurg 2006;108:638–43.
21. Aronow WS, Ahn C, Kronzon I. Risk factors for stroke in the elderly. Prev Cardiol 1999;2:67–74.
22. Aronow WS, Ahn C, Kronzon I. Prevalence of echocardiographic findings in 554 men and in 1,243 women aged > 60 years in a long-term health care facility. Am J Cardiol 1997;79:379–80.
23. Arrigo F, Carerj S, Pizzimenti G. [Role of transesophageal echography in the study of embolism of cardiac origin.] Cardiologia 1993;38(Suppl. 1):301–17.
24. Asil T, Steffenhagen N, Ibrahim M, Demchuk A. Bubble study in patients with massive right-to-left shunt and recurrent stroke. Stroke 2009;40:e505–8.
25. Asimakopoulos GE, Edwards MB, Taylor KM. Aortic valve replacement in patients 80 years of age and older: survival and cause of death based on 1100 cases – collective results from the UK heart valve registry. Circ Cardiovasc Qual Outcomes 1997;96:3403–8.
26. Aszalós Z, Barsi P, Vitrai J, Nagy Z. Risk factors for early death and recurrence in stroke. Orv Hetil 2001;142:715–21.
27. Aszalós Z, Barsi P, Vitrai J, Nagy Z. Hypertension and clusters of risk factors in different stroke subtypes (an analysis of Hungarian patients via Budapest Stroke Data Bank). J Hum Hypertens 2002;16:495–500.
28. Bahou Y, Hamid H, Hadidi A. Ischaemic stroke in Jordan: a 2-year hospital-based study of subtypes and risk factors. East Mediterr Health J 2004;10:138–46.
29. Bahou YH, Hamid H, Raqab MZ. Ischemic stroke in Jordan 2000 to 2002: a two-year, hospital-based study. J Stroke Cerebrovasc Dis 2004;13:81–4.
30. Baker RN, Schwartz WS, Ramseyer JC. Prognosis among survivors of ischemic stroke. Neurology 1968;18:933–41.
31. Bando KK, Kobayashi J, Hirata M, Satoh T, Niwaya K, Tagusari O. Early and late stroke after mitral valve replacement with a mechanical prosthesis: risk factor analysis of a 24-year experience. J Thorac Cardiovasc Surg 2003;126:358–64.
32. Bang OY, Lee PH, Joo SY, Lee JS, Joo IS, Huh K, et al. Frequency and mechanisms of stroke recurrence after cryptogenic stroke. Ann Neurol 2003;54:227–34.
33. Bangalore S, Petre L, Herweg B, Sichrovsky T, Vragel S, Steinberg JS, et al. Cardioversion in patients with left ventricular thrombus is not associated with increased thromboembolic risk. J Am Soc Echocardiogr 2006;19:438–40.
34. Bannan A, Shen R, Silvestry FE, Herrmann HC. Characteristics of adult patients with atrial septal defects presenting with paradoxical embolism. Catheter Cardiovasc Interv 2009;74:1066–9.
35. Barnett HJM. Stroke by cause: some common, some exotic, some controversial. Stroke 2005;36:2523–5.
36. Basnet BK, Manandhar K, Shrestha R, Shrestha S, Thapa M. Electrocardiograph and chest X-ray in prediction of left ventricular systolic dysfunction. J Nepal Med Assoc 2009;48:310–13.
37. Beacock DJW, Watt VB, Oakley GD, Al Mohammad A. Paradoxical embolism with a patent foramen ovale and atrial septal aneurysm. Eur J Echocardiogr 2006;7:171–4.
38. Becker EI, Jung A, ller H, Wegscheider K, Vogel HP, Landgraf H, et al. Cardiogenic embolism as the main cause of ischemic stroke in a city hospital: an interdisciplinary study. Vasa 2001;30:43–52.
39. Bejot Y, Caillier M, Ben SD, Couvreur G, Rouaud O, Osseby GV, et al. Ischaemic stroke subtypes and associated risk factors: a French population based study. J Neurol Neurosurg Psychiatry 2008;79:1344–8.
40. Bejot YO, Osseby GV, Gremeaux V, Durier J, Rouaud O, Moreau T. Changes in risk factors and preventive treatments by stroke subtypes over 20 years: a population-based study. J Neurol Sci 2009;287:84–8.
41. Belkin RN, Hurwitz BJ, Kisslo J Atrial septal aneurysm: association with cerebrovascular and peripheral embolic events. Stroke 1987;18:856–62.
42. Benatru I, Rouaud O, Durier J, Contegal F, Couvreur G, Bejot Y, et al. Stable stroke incidence rates but improved case-fatality in Dijon, France, from 1985 to 2004. Stroke 2006;37:1674–9.
43. Berlit PE, Endemann B, Vetter P. Cerebral ischemia in young adults. Fortschr Neurol Psychiatr 1991;59:322–7.
44. Besson G, Bogousslavsky J, Hommel M, Stauffer JC, Siché JP, et al. Patent foramen ovale in young stroke patients with mitral valve prolapse. Acta Neurol Scand 1994;89:23–6.
45. Bestetti R. Stroke in a hospital-derived cohort of patients with chronic Chagas’ disease. Acta Cardiol 2000;55:33–8.
46. Bhagirath KM, Paulson K, Ahmadie R, Bhalla RS, Robinson D, Jassal DS, et al. Clinical utility of cardiac magnetic resonance imaging in Churg–Strauss syndrome: case report and review of the literature. Rheumatol Int 2009;29:445–9.
47. Bhatt DL, Eagle KA, Ohman EM, Hirsch AT, Goto S, Mahoney EM, et al. Comparative determinants of 4-year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA 2010;304:1350–7.
48. Bikkina M, Levy D, Evans JC, Larson MG, Benjamin EJ, Wolf PA, et al. Left ventricular mass and risk of stroke in an elderly cohort. The Framingham Heart Study. JAMA 1994;272:33–6.
49. Blum AS, Shapira Y, Yeganh S, Rabinkov M. Mitral valve prolapse and thromboembolic events. Isr Med Assoc J 2001;3:282–3.
50. Bogousslavsky J, Regli F. Ischemic stroke in adults younger than 30 years of age. Cause and prognosis. Arch Neurol 1987;44:479–82. [Erratum published in Arch Neurol 1987;44:817.]
51. Boon A, Lodder J, Heuts-van RL, Kessels F. Silent brain infarcts in 755 consecutive patients with a first-ever supratentorial ischemic stroke. Relationship with index-stroke subtype, vascular risk factors, and mortality. Stroke 1994;25:2384–90.
52. Boon A, Lodder J, Cheriex E, Kessels F. Risk of stroke in a cohort of 815 patients with calcification of the aortic valve with or without stenosis. Stroke 1996;27:847–51.
53. Bots ML, Nikitin Y, Salonen JT, Elwood PC, Malyutina S, Freire de CA, et al. Left ventricular hypertrophy and risk of fatal and non-fatal stroke. EUROSTROKE: a collaborative study among research centres in Europe. J Epidemiol Community Health 2002;56(Suppl. 1):i8–13.
54. Broderick JP, Phillips SJ, O’Fallon WM, Frye RL, Whisnant JP. Relationship of cardiac disease to stroke occurrence, recurrence, and mortality. Stroke 1992;23:1250–6.
55. Budillon AM, Nicolini F, Beghi C, Saccani S, De CG, Albertini D, et al. Surgical repair of thoracic aortic aneurysms: results and complications. Acta Biomed Ateneo Parmense 2001;72:33–43.
56. Budzikowski AS. Cardiac sources of cerebral embolism. Cardiol Rev 2002;19:34–6.
57. Burger AJ, Sherman HB, Charlamb MJ. Low incidence of embolic strokes with atrial septal aneurysms: a prospective, long-term study. Am Heart J 2000;139:149–52.
58. Buth J, Harris PL, Hobo R, van Epps R, Cuypers P, Duijm L, et al. Neurologic complications associated with endovascular repair of thoracic aortic pathology: incidence and risk factors. A study from the European Collaborators on Stent/Graft Techniques for Aortic Aneurysm Repair (EUROSTAR) registry. J Vasc Surg 2007;46:1103–10.
59. Buyukoglu B. Atrial septal pathology and cerebral embolism. Sang Thromb Vaiss 1999;11:498.
60. Cabau J, Noël M, Marrero A, Rivest D, Mackey A, Houde C, et al. Atherosclerotic burden findings in young cryptogenic stroke patients with and without a patent foramen ovale. Stroke 2009;40:419–25.
61. Caplan LRH, Hier DB, D’Cruz I. Cerebral embolism in the Michael Reese stroke registry. Stroke 1983;14:530–6.
62. Carod-Artal FJ, Nunes SV, Portugal D, Silva TV, Vargas AP. Ischemic stroke subtypes and thrombophilia in young and elderly Brazilian stroke patients admitted to a rehabilitation hospital. Stroke 2005;36:2012–14.
63. Catapano O, Oldani A, Milandri M, Giondi I, Guidi C, Galletti G, et al. [Evaluation of atrial septum aneurysm with transesophageal echocardiography in cardioembolic cerebral ischemia.] Cardiologia 1992;37:859–64.
64. Chiu EH, Tan TY, Chang KC, Liou CW. Risk factors for ischemic stroke: electrocardiographic findings. Acta Neurol Taiwan 2006;15:232–6.
65. Cho AH, Kwon SU, Kim TW, Lee SJ, Shon YM, Kim BS, et al. High prevalence of unrecognized cerebral infarcts in first-ever stroke patients with cardioembolic sources. Eur J Neurol 2009;16:838–42.
66. Chotmongkol V, Limpawattana P, Chimsuk U. Clinical outcomes of patients with cardiogenic cerebral emboli in Srinagarind Hospital. Southeast Asian J Trop Med Public Health 2006;37:1209–12.
67. Cipriano LE, Steinberg ML, Gazelle GS, Gonzalez RG. Comparing and predicting the costs and outcomes of patients with major and minor stroke using the Boston Acute Stroke Imaging Scale neuroimaging classification system. Am J Neuroradiol 2009;30:703–9.
68. Cohen A, Tzourio C, Chauvel C, Bertrand B, Crassard I, Bernard Y, et al. Mitral valve strands and the risk of ischemic stroke in elderly patients. The French Study of Aortic Plaques in Stroke (FAPS) investigators. Stroke 1997;28:1574–8.
69. Comess KA, DeRook FA, Beach KW, Lytle NJ, Golby AJ, Albers GW, et al. Transesophageal echocardiography and carotid ultrasound in patients with cerebral ischemia: prevalence of findings and recurrent stroke risk. J Am Coll Cardiol 1994;23:1598–603.
70. Contreras AE, Brenna EJ, Salomone OA. Prevalence of patent foramen ovale in patients with cryptogenic stroke or transient ischemic attacks. Rev ArgentCardiologia 2009;77:493–5.
71. Cribier A, Mihout B, Sibille C, Berland J, Champoud O, Layet A, et al. [Cerebral embolism without apparent cause: angiographic study of minor predisposing cardiac anomalies. Prospective study of 64 patients.] Arch Mal Coeur Vaiss 1985;78:407–13.
72. Davenport J, Hart RG. Prosthetic valve endocarditis 1976–1987. Antibiotics, anticoagulation, and stroke. Stroke 1990;21:993–9.
73. Davies RR, Kaple RK, Mandapati D, Gallo A, Botta DM Jr, Elefteriades JA, et al. Natural history of ascending aortic aneurysms in the setting of an unreplaced bicuspid aortic valve. Ann Thorac Surg 2007;83:1338–44.
74. Davis WDH, Hart RG. Cardiogenic stroke in the elderly. Clin Geriatr Med 1991;7:429–42.
75. de Abreu TT, Mateus S, Carreteiro C, Correia J. Therapeutic implications of transesophageal echocardiography after transthoracic echocardiography on acute stroke patients. Vasc Health Risk Manag 2008;4:167–72.
76. de Belder MA, Lovat LB, Tourikis L, Leech G, Camm AJ. Limitations of transoesophageal echocardiography in patients with focal cerebral ischaemic events. Br Heart J 1992;67:297–303.
77. De Castro S, Rasura M, Di Angelantonio E, Beccia M, Passaseo I, Di Lisi F, et al. Distribution of potential cardiac sources of embolism in young and older stroke patients: implications for recurrent vascular events. J Cardiovasc Med (Hagerstown) 2006;7:191–6.
78. Díaz-Guzmán J, Egido-Herrero JA, Fuentes B, Fernández-Pérez C, Gabriel-Sánchez R, Barberà G, et al. Incidence of strokes in Spain: the Iberictus study. Data from the pilot study. Rev Neurol 2009;48:61–5.
79. Di Tullio MR. Patent foramen ovale: echocardiographic detection and clinical relevance in stroke. J Am Soc Echocardiogr 2010;23:144–55.
80. Di Tullio MR, Sacco RL, Savoia MT, Sciacca RR, Homma S. Aortic atheroma morphology and the risk of ischemic stroke in a multiethnic population. Am Heart J 2000;139:329–36.
81. Di Tullio MR, Zwas DR, Sacco RL, Sciacca RR, Homma S. Left ventricular mass and geometry and the risk of ischemic stroke. Stroke 2003;34:2380–4.
82. Di Tullio MR, Sacco RL, Sciacca RR, Jin Z, Homma S. Patent foramen ovale and the risk of ischemic stroke in a multiethnic population. J Am Coll Cardiol 2007;49:797–802.
83. Di Tullio MR, Homma S, Jin Z, Sacco RL. Aortic atherosclerosis, hypercoagulability, and stroke: the APRIS (Aortic Plaque and Risk of Ischemic Stroke) study. J Am Coll Cardiol 2008;52:855–61.
84. Ducrocq X, Lacour JC, Debouverie M, Bracard S, Girard F, Weber M, et al. [Cerebral ischemic accidents in young subjects. A prospective study of 296 patients aged 16 to 45 years.] Rev Neurol (Paris) 1999;155:575–82.
85. Dulli D, D’Alessio DJ, Palta M, Levine RL, Schutta HS. Differentiation of acute cortical and subcortical ischemic stroke by risk factors and clinical examination findings. Neuroepidemiology 1998;17:80–9.
86. Dutta TK, Karas MG, Segal AZ, Kizer JR. Yield of transesophageal echocardiography for nonbacterial thrombotic endocarditis and other cardiac sources of embolism in cancer patients with cerebral ischemia. Am J Cardiol 2006;97:894–8.
87. Eldar R, Zagreba F, Tamir A, Epstein L. Risk factors and causes of stroke in young women in Israel. Int Disabil Stud 1990;12:81–5.
88. Eriksson SE, Olsson JE. Survival and recurrent strokes in patients with different subtypes of stroke: a fourteen-year follow-up study. Cerebrovasc Dis 2001;12:171–80.
89. Estrera ALG, Garami Z, Miller CC, Porat EE, Achouh PE, Dhareshwar J, et al. Acute type A aortic dissection complicated by stroke: can immediate repair be performed safely? J Thorac Cardiovasc Surg 2006;132:1404–8.
90. Fang J, Alderman MH. Trend of stroke hospitalization, United States, 1988–1997. Stroke 2001;32:2221–6.
91. Fann JI, Sarris GE, Miller DC, Mitchell RS, Oyer PE, Stinson EB, et al. Surgical management of acute aortic dissection complicated by stroke. Circ Cardiovasc Qual Outcomes 1989;80:I257–63.
92. Fazlinezhad AA, Azimi S, Azarpazhooh M, Khajedaluee M, Mahdinezhad Kashani M. Patent foramen ovale in young adults with cryptogenic stroke or transient ischemic attack. J Tehran Heart Cent 2009;4:185–8.
93. Feigin VL, Wiebers DO, Nikitin YP, O’Fallon WM, Whisnant JP. Risk factors for ischemic stroke in a Russian community: a population-based case–control study. Stroke 1998;29:34–9.
94. Feurer R, Sadikovic S, Esposito L, Schwarze J, Bockelbrink A, Hemmer B, et al. Lesion patterns in patients with cryptogenic stroke with and without right-to-left-shunt. Eur J Neurol 2009;16:1077–82.
95. Fieschi C, Rasura M, Anzini A, DeCastro S, DiGianfilippo G, Valesini G, et al. A diagnostic approach to ischemic stroke in young and middle-aged adults. Eur J Neurol 1996;3:324–30.
96. Fisher DCF, Fisher EA, Budd JH, Rosen SE, Goldman ME. The incidence of patent foramen ovale in 1,000 consecutive patients: a contrast transesophageal echocardiography study. Chest 1995;107:1504–9.
97. Freed LA, Levy D, Levine RA, Larson MG, Evans JC, Fuller DL, et al. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med 1999;341:1–7.
98. Frey JLJ, Jahnke HK, Bulfinch EW. Differences in stroke between white, Hispanic, and Native American patients: The Barrow Neurological Institute stroke database. Stroke 1998;29:29–33.
99. Fu J-HL. Transcranial Doppler monitoring of cerebral arteries for microemboli in patients with ischemic stroke. Fudan Univ J Med Sci 2002;29:39–41.
100. Fustinoni O. Editorial comment – left ventricular hypertrophy: an unseemly risk factor for stroke? Stroke 2003;34:2385–6.
101. Gandolfo C, Conti M. Stroke in young adults: epidemiology. Neurol Sci 2003;24(Suppl. 1):S1–3.
102. Ghandehari K, Izadi-Mood Z. Khorasan stroke registry: analysis of 1392 stroke patients. Arch Iran Med 2007;10:327–34.
103. Ghosh S, Ghosh AK, Ghosh SK. Patent foramen ovale and atrial septal aneurysm in cryptogenic stroke. Postgrad Med J 2007;83:173–7.
104. Giovannoni G, Fritz VU. Transient ischemic attacks in younger and older patients. A comparative study of 798 patients in South Africa. Stroke 1993;24:947–53.
105. Giroud M, Gras P, Fayolle H, André N, Soichot P, Dumas R. Early seizures after acute stroke: a study of 1,640 cases. Epilepsia 1994;35:959–64.
106. González Hernández A, Fabre Pi O, López Fernández JC, Platero Román M, Cabrera Hidalgo A, Mendoza Grimón MD. [Risk factors, etiology and prognosis in patients older than 80 years old with ischemic stroke.] Rev Esp Geriatr Gerontol 2008;43:366–9.
107. González Hernández A, Fabre Pi O, López Fernández JC, Díaz Nicolás S, Cabrera Hidalgo A [Risk factors, etiology and prognosis in patients with ischemic stroke and diabetes mellitus.] Rev Clin Esp 2008;208:546–50.
108. Grau AJ, Weimar C, Buggle F, Heinrich A, Goertler M, Neumaier S, et al. Risk factors, outcome, and treatment in subtypes of ischemic stroke: the German stroke data bank. Stroke 2001;32:2559–66.
109. Guedes C, Bianchi-Fior P, Cormier B, Barthelemy B, Rat AC, Boissier MC. Cardiac manifestations of rheumatoid arthritis: a case–control transesophageal echocardiography study in 30 patients. Arthritis Rheum 2001;45:129–35.
110. Gupta V, Yesilbursa D, Huang WY, Aggarwal K, Gupta V, Gomez C, et al. Patent foramen ovale in a large population of ischemic stroke patients: diagnosis, age distribution, gender, and race. Echocardiography 2008;25:217–27.
111. Harmsen P, Wilhelmsen L, Jacobsson A. Stroke incidence and mortality rates 1987 to 2006 related to secular trends of cardiovascular risk factors in Gothenburg, Sweden. Stroke 2009;40:2691–7.
112. Hart RG, Foster JW, Luther MF, Kanter MC. Stroke in infective endocarditis. Stroke 1990;21:695–700.
113. Haverich A, Miller DC, Scott WC, Mitchell RS, Oyer PE, Stinson EB. Acute and chronic aortic dissections – determinants of long-term outcome for operative survivors. Circ Cardiovasc Qual Outcomes 1985;72:II22–34.
114. Herman B, Schmitz PI, Leyten AC, Van Luijk JH, Frenken CW, Op De Coul AA, et al. Multivariate logistic analysis of risk factors for stroke in Tilburg, the Netherlands. Am J Epidemiol 1983;118:514–25.
115. Herrschaft H. Cardiac diseases as causes of cerebral signs and syndromes. Fortschr Neurol Psychiatr 1990;58:287–300.
116. Hildick-Smith D, Meier B. Cryptogenic stroke and patent foramen ovale: matched cohorts in observational studies remain matched. Stroke 2009;40:e506–8.
117. Hogue CW, Murphy SF, Schechtman KB, Davila-Roman VG. Risk factors for early or delayed stroke after cardiac surgery. Circ Cardiovasc Qual Outcomes 1999;100:642–7.
118. Homma S, Sacco RL, Di Tullio MR, Sciacca RR, Mohr JP. Atrial anatomy in non-cardioembolic stroke patients: effect of medical therapy. J Am Coll Cardiol 2003;42:1066–72.
119. Hornig CR, Brainin M, Mast H. Cardioembolic stroke: results from three current stroke data banks. Neuroepidemiology 1994;13:318–23.
120. Hughes TAT. Review of ischemic cerebrovascular disease. Cognitive Neuropsych 2004;21.
121. Humphrey RD, Harrison MJ. How often can an embolic stroke be diagnosed clinically? A clinicopathological correlation. Postgrad Med J 1985;61:1039–42.
122. Ionita CC, Xavier AR, Kirmani JF, Dash S, Divani AA, Qureshi AI. What proportion of stroke is not explained by classic risk factors? Prev Cardiol 2005;8:41–6.
123. Jaber WA, Klein AL. How often are atrial septal defects associated with thromboembolism? When should they be looked for? Cleve Clin J Med 2001;68:954–6.
124. Jackson AC, Boughner DR, Barnett HJ. Mitral valve prolapse and cerebral ischemic events in young patients. Neurology 1984;34:784–7.
125. Jackson CA, Hutchison A, Dennis MS, Wardlaw JM, Lindgren A, Norrving B, et al. Differing risk factor profiles of ischemic stroke subtypes: evidence for a distinct lacunar arteriopathy? Stroke 2010;41:624–9.
126. Johansen HL, Wielgosz AT, Nguyen K, Fry RN. Incidence, comorbidity, case fatality and readmission of hospitalized stroke patients in Canada. Can J Cardiol 2006;22:65–71.
127. Joubert J. The MEDUNSA Stroke Data Bank. An analysis of 304 patients seen between 1986 and 1987. S Afr Med J 1991;80:567–70.
128. Kanda N, Yasaka M, Otsubo R, Nagatsuka K, Minematsu K, Yamaguchi T. Right-to-left shunt and atrial septal aneurysm in stroke patients: a contrast transesophageal echocardiographic study. Clin Neurol 1998;38:213–18.
129. Kane WC, Aronson SM. Cardiac disorders predisposing to embolic stroke. Stroke 1970;1:164–72.
130. Karas MG, Francescone S, Segal AZ, Devereux RB, Roman MJ, Liu JE, et al. Relation between mitral annular calcium and complex aortic atheroma in patients with cerebral ischemia referred for transesophageal echocardiography. Am J Cardiol 2007;99:1306–11.
131. Kassem-Moussa H, Mahaffey KW, Graffagnino C, Tasissa G, Sila CA, Simes RJ, et al. Incidence and characteristics of stroke during 90-day follow-up in patients stabilized after an acute coronary syndrome. Am Heart J 2004;148:439–46.
132. Kelly R, Staines A, MacWalter R, Stonebridge P, Tunstall-Pedoe H, Struthers AD. The prevalence of treatable left ventricular systolic dysfunction in patients who present with noncardiac vascular episodes: a case–control study. J Am Coll Cardiol 2002;39:219–24.
133. Kent DM, Thaler DE. Is patent foramen ovale a modifiable risk factor for stroke recurrence? Stroke 2010;41(Suppl. 10):S26–30.
134. Khan GN, Dairywala IT, Liu Z, Li P, Carroll J, Vannan MA, et al. Three-dimensional echocardiography of left atrial appendage thrombus. Echocardiography 2001;18:163–6.
135. Khetarpal V, Mahajan N, Madhavan R, Batra S, Mopala P, Sagar A, et al. Calcific aortic valve and spontaneous embolic stroke: a review of literature. J Neurol Sci 2009;287:32–5.
136. Khoynezhad A. Stroke rate after thoracic endovascular aortic repair may not be equal among various aortic pathologies. Ann Thorac Surg 2008;86:2023.
137. Khoynezhad A, Donayre CE, Bui H, Kopchok GE, Walot I, White RA. Risk factors of neurologic deficit after thoracic aortic endografting. Ann Thorac Surg 2007;83:S882–9.
138. Kichura GM, Castello R. Abnormalities of the interatrial septum as a potential cardiac source of embolism: patent foramen ovale and atrial septal aneurysm. Echocardiography 1993;10:441–9.
139. Kimura K, Kazui S, Minematsu K, Yamaguchi T, Japan Multicenter Stroke Investigators’ Collaboration (J-MUSIC). Hospital-based prospective registration of acute ischemic stroke and transient ischemic attack in Japan. J Stroke Cerebrovasc Dis 2004;13:1–11.
140. Kittner SJ, Sharkness CM, Price TR, Plotnick GD, Dambrosia JM, Wolf PA, et al. Infarcts with a cardiac source of embolism in the NINCDS Stroke Data Bank: historical features. Neurology 1990;40:281–4.
141. Kittner SJ, Sharkness CM, Sloan MA, Price TR, Dambrosia JM, Tuhrim S, et al. Infarcts with a cardiac source of embolism in the NINDS Stroke Data Bank: neurologic examination. Neurology 1992;42:299–302.
142. Knuiman MW, Vu HT. Risk factors for stroke mortality in men and women: the Busselton Study. J Cardiovasc Risk 1996;3:447–52.
143. Kobayashi S. Data bank project for acute stroke patients. Clin Neurol 2002;42:1176–8.
144. Kolominsky-Rabas PL, Weber M, Gefeller O, Neundoerfer B, Heuschmann PU. Epidemiology of ischemic stroke subtypes according to TOAST criteria: incidence, recurrence, and long-term survival in ischemic stroke subtypes: a population-based study. Stroke 2001;32:2735–40.
145. Kraywinkel K, Jauss M, Diener HC, Weimar C. [Patent foramen ovale, atrial septum aneurysm, and stroke. An examination of the status of recent evidence.] Nervenarzt 2005;76:935–42.
146. Laaidi K, Minier D, Osseby GV, Couvreur G, Besancenot JP, Moreau T, et al. [Seasonal variation in strokes incidence and the influence of the meteorological conditions.] Rev Neurol (Paris) 2004;160:321–30.
147. Labovitz A. Transesophageal echocardiography and unexplained cerebral ischemia: a multicenter follow-up study. The STEPS Investigators. Significance of Transesophageal Echocardiography in the Prevention of Recurrent Stroke. Am Heart J 1999;137:1082–7.
148. Lalouschek W, Lang W, llner M, Vienna Stroke Study Group. Current strategies of secondary prevention after a cerebrovascular event: the Vienna stroke registry. Stroke 2001;32:2860–6.
149. Lalouschek W, Schillinger M, Hsieh K, Endler G, Tentschert S, Lang W, et al. Matched case–control study on factor V Leiden and the prothrombin G20210A mutation in patients with ischemic stroke/transient ischemic attack up to the age of 60 years. Stroke 2005;36:1405–9.
150. Laloux P, Galanti L, Jamart J. Lipids in ischemic stroke subtypes. Acta Neurol Belg 2004;104:13–19.
151. Laloux P, Ossemann M, Jamart J. Family history of hypertension is not an independent genetic factor predisposing to ischemic stroke subtypes. Clin Neurol Neurosurg 2007;109:247–9.
152. Lampl Y, Boaz M, Sadeh M. The significance of prestroke aspirin dosage in fatal outcome of acute stroke. Clin Neuropharmacol 2005;28:55–9.
153. Lamy CS. Cerebral vascular pathology during pregnancy and post partum. Rev Neurol (Paris) 1996;152:422–40.
154. Landi G, Cella E, Boccardi E, Musicco M. Lacunar versus non-lacunar infarcts: pathogenetic and prognostic differences. J Neurol Neurosurg Psychiatry 1992;55:441–5.
155. Lazzarino LG, Nicolai A, Poldelmengo P, Toppani D, Valassi F. Risk factors in lacunar strokes. A retrospective study of 52 patients. Acta Neurol 1989;11:265–71.
156. Lee BC, Hwang SH, Jung S, Yu KH, Lee JH, Cho SJ, et al. The Hallym Stroke Registry: a web-based stroke data bank with an analysis of 1,654 consecutive patients with acute stroke. Eur Neurol 2005;54:81–7.
157. Lee BI, Nam HS, Heo JH, Kim DI, Yonsei Stroke Team. Yonsei Stroke Registry: analysis of 1,000 patients with acute cerebral infarctions. Cerebrovasc Dis 2001;12:145–51.
158. Lee E, Kang DW, Kwon SU, Kim JS. Posterior cerebral artery infarction: diffusion-weighted MRI analysis of 205 patients. Cerebrovasc Dis 2009;28:298–305.
159. Lee JH, Han SJ, Yun YH, Choi HC, Jung S, Cho SJ, et al. Posterior circulation ischemic stroke in Korean population. Eur J Neurol 2006;13:742–8.
160. Lee PH, Bang OY, Oh SH, Joo IS, Huh K. Subcortical white matter infarcts: comparison of superficial perforating artery and internal border-zone infarcts using diffusion-weighted magnetic resonance imaging. Stroke 2003;34:2630–5.
161. Lee PH, Oh SH, Bang OY, Joo IS, Huh K. Isolated middle cerebral artery disease: clinical and neuroradiological features depending on the pathogenesis. J Neurol Neurosurg Psychiatry 2004;75:727–32.
162. Lee PH, Bang OY, Hwang EM, Lee JS, Joo US, Mook-Jung I, et al. Circulating beta amyloid protein is elevated in patients with acute ischemic stroke. J Neural Transm 2005;112:1371–9.
163. Lee SJ, Lee KS, Kim YI, An JY, Kim W, Kim JS, et al. Clinical features of patients with a myocardial infarction during acute management of an ischemic stroke. Neurocrit Care 2008;9:332–7.
164. Lee SJ, Kim JS, Lee KS, An JY, Kim W, Kim YI, et al. The leukoaraiosis is more prevalent in the large artery atherosclerosis stroke subtype among Korean patients with ischemic stroke. BMC Neurol 2008;8:31.
165. Lee TH, Hsu WC, Chen CJ, Chen ST. Etiologic study of young ischemic stroke in Taiwan. Stroke 2002;33:1950–5.
166. Leira EC, Adams HP Jr, Rosenthal GE, Torner JC. Baseline NIH stroke scale responses estimate the probability of each particular stroke subtype. Cerebrovasc Dis 2008;26:573–7.
167. Lemesle M, Milan C, Faivre J, Moreau T, Giroud M, Dumas R, et al. Incidence trends of ischemic stroke and transient ischemic attacks in a well-defined French population from 1985 through 1994. Stroke 1999;30:371–7.
168. Lestro Henriques I, Bogousslavsky J, Van Melle G. Predictors of stroke pattern in hypertensive patients. J Neurol Sci 1996;144:142–6.
169. Lethen H, Flachskampf FA, Schneider R, Sliwka U, Köhn G, Noth J, et al. Frequency of deep vein thrombosis in patients with patent foramen ovale and ischemic stroke or transient ischemic attack. Am J Cardiol 1997;80:1066–9.
170. Leung DY, Black IW, Cranney GB, Walsh WF, Grimm RA, Stewart WJ, et al. Selection of patients for transesophageal echocardiography after stroke and systemic embolic events. Role of transthoracic echocardiography. Stroke 1995;26:1820–4.
171. Levine RL, Jones JC, Bee N. Stroke and Parkinson’s disease. Stroke 1992;23:839–42.
172. Leys DL. Cerebral ischemic stroke in young adults. Sang Thromb Vaiss 2004;16:43–50.
173. Liu X, Xu G, Wu W, Zhang R, Yin Q, Zhu W, et al. Subtypes and one-year survival of first-ever stroke in Chinese patients: the Nanjing Stroke Registry. Cerebrovasc Dis 2006;22:130–6.
174. Lloyd-Jones D, Adams RJ, Brown TM, Dai S, De Simone G, Fergusson TB, et al. Executive summary: heart disease and stroke statistics – 2010 update: a report from the American Heart Association. Circ Cardiovasc Qual Outcomes 2010;121:e46–215.
175. Lodder J, Bamford JM, Sandercock PA, Jones LN, Warlow CP. Are hypertension or cardiac embolism likely causes of lacunar infarction? Stroke 1990;21:375–81.
176. Lodder J, Bamford J, Kappelle J, Boiten J. What causes false clinical prediction of small deep infarcts? Stroke 1994;25:86–91.
177. Lodder J, van Raak L, Hilton A, Hardy E, Kessels A, EGASIS Study Group. Diazepam to improve acute stroke outcome: results of the early GABA-Ergic activation study in stroke trial. A randomized double-blind placebo-controlled trial. Cerebrovasc Dis 2006;21:120–7.
178. Loeb C, Gandolfo C, Del Sette M, Conti M, Finocchi C, Calautti C, et al. Asymptomatic cerebral infarctions in patients with ischemic stroke. Eur Neurol 1996;36:343–7.
179. Longstreth WT Jr, Bernick C, Fitzpatrick A, Cushman M, Knepper L, Lima J, et al. Frequency and predictors of stroke death in 5,888 participants in the Cardiovascular Health Study. Neurology 2001;56:368–75.
180. Lopez AEA, Perez PG, Padial LR. Echocardiography in stroke: a pending problem. Med Clin (Barc) 2000;115:347–51.
181. Lovett JK, Coull AJ, Rothwell PM. Early risk of recurrence by subtype of ischemic stroke in population-based incidence studies. Neurology 2004;62:569–73.
182. Lovrencic-Huzjan A, Bosnar M, Huzjan R, Demarin V. Frequency of different risk factors for ischemic stroke. Acta Clin Croatica 1999;38:159–63.
183. Lucas C, Goullard L, Marchau M Jr, Godefroy O, Rondepierre P, Chamas E, et al. Higher prevalence of atrial septal aneurysms in patients with ischemic stroke of unknown cause. Acta Neurol Scand 1994;89:210–13.
184. Lund C, Rygh J, Stensrød B, Sandset PM, Brucher R, Russell D. Cerebral microembolus detection in an unselected acute ischemic stroke population. Cerebrovasc Dis 2000;10:403–8.
185. Makaroun MS, Dillavou ED, Kee ST, Sicard G, Chaikof E, Bavaria J, et al. Endovascular treatment of thoracic aortic aneurysms: results of the phase II multicenter trial of the GORE TAG thoracic endoprosthesis. J Vasc Surg 2005;41:1–9.
186. Marcheselli S. Cavallini A, Tosi P, Quaglini S, Micieli C. Impaired blood pressure increase in acute cardioembolic stroke. J Hypertens 2006;24:1849–56.
187. Markus HS, Khan U, Birns J, Evans A, Kalra L, Rudd AG, et al. Differences in stroke subtypes between black and white patients with stroke: the South London Ethnicity and Stroke Study. Circ Cardiovasc Qual Outcomes 2007;116:2157–64.
188. Marnane M, Duggan CA, Sheehan OC, Merwick A, Hannon N, Curtin D, et al. Stroke subtype classification to mechanism-specific and undetermined categories by TOAST, A-S-C-O, and causative classification system: direct comparison in the North Dublin population stroke study. Stroke 2010;41:1579–86.
189. Marsh EE III, Biller J, Adams HP Jr, Marler JR, Hulbert JR, Love BB, et al. Circadian variation in onset of acute ischemic stroke. Arch Neurol 1990;47:1178–80.
190. Martínez-Avilés P, Barba R, Andújar C, Cantón R, Solís J. [Cerebrovascular accident in young adults. A study of 52 cases.] Rev Neurol 1996;24:443–7.
191. Matenga J, Kitai I, Levy L. Strokes among black people in Harare, Zimbabwe: results of computed tomography and associated risk factors. Br Med J (Clin Res Ed) 1986;292:1649–51.
192. Mathews MS, Sharma J, Snyder KV, Natarajan SK, Siddiqui AH, Hopkins LN, et al. Safety, effectiveness, and practicality of endovascular therapy within the first 3 hours of acute ischemic stroke onset. Neurosurgery 2009;65:860–5.
193. Matias-Guiu J, Alvarez J, Insa R, Moltó JM, Martin R, Codina A, et al. Ischemic stroke in young adults. II. Analysis of risk factors in the etiological subgroups. Acta Neurol Scand 1990;81:314–17.
194. Matsushita T, Kubo M, Yonemoto K, Ninomiya T, Ashikawa K, Liang B, et al. Lack of association between variations of PDE4D and ischemic stroke in the Japanese population. Stroke 2009;40:1245–51.
195. Maulaz AB, Bezerra DC, Bogousslavsky J. Posterior cerebral artery infarction from middle cerebral artery infarction. Arch Neurol 2005;62:938–41.
196. McConnell JP, Cheryk LA, Durocher A, Bruno A, Bang NU, Fleck JD, et al. Urinary 11-dehydro-thromboxane B(2) and coagulation activation markers measured within 24 h of human acute ischemic stroke. Neurosci Lett 2001;313:88–92.
197. McQuillan AM, Eikelboom JW, Hankey GJ, Baker R, Thom J, Staton J, et al. Protein Z in ischemic stroke and its etiologic subtypes. Stroke 2003;34:2415–19.
198. Mesa Rubio DF, Garcíaa D, Crespína M, Leóna C, Toledanoa F, Mazuelosa F, et al. Prevalence of patent foramen ovale in young patients with cryptogenic stroke. Rev Esp Cardiol 2003;56:662–8.
199. Mesa D, Ruiz M, Delgado M, Suárez de LJ, Pan M, Tejero I, et al. Prevalence of patent foramen ovale determined by transesophageal echocardiography in patients with cryptogenic stroke aged 55 years or older. Same as younger patients? Rev Esp Cardiol 2010;63:315–22.
200. Meurer WJ, Sanchez BN, Smith MA, Lisabeth LD, Majersik JJ, Brown DL, et al. Predicting ischaemic stroke subtype from presenting systolic blood pressure: the BASIC Project. J Intern Med 2009;265:388–96.
201. Michel P, Odier C, Rutgers M, Reichhart M, Maeder P, Meuli R, et al. The Acute STroke Registry and Analysis of Lausanne (ASTRAL): design and baseline analysis of an ischemic stroke registry including acute multimodal imaging. Stroke 2010;41:2491–8.
202. Micheli S, Agnelli G, Palmerini F, Caso V, Venti M, Alberti A, et al. Need for extensive diagnostic work-up for patients with lacunar stroke. J Neurol 2008;255:637–42.
203. Milandre L, Brosset C, Gouirand R, Khalil R. Pure cerebellar infarction. Thirty cases. Presse Med 1992;21:1562–5.
204. Milandre L, Brosset C, Khalil R. [Lateral thalamic infarction. 22 cases.] Presse Med 1993;22:1865–9.
205. Milandre L, Brosset C, Habib G, Graziani N, Khalil R. [Cerebral infarction in patients aged 16 to 35 years. Prospective study of 52 cases.] Presse Med 1994;23:1603–8.
206. Modrego PJ, Pina MA, Lerín FJ. The impact of ageing on stroke subtypes, length of stay and mortality: study in the province of Teruel, Spain. Acta Neurol Scand 2003;108:435–42.
207. Modrego PJ, Mainar R, Turull L. Recurrence and survival after first-ever stroke in the area of Bajo Aragon, Spain. A prospective cohort study. J Neurol Sci 2004;224:49–55.
208. Molina CA, Montaner J, Abilleira S, Arenillas JF, Ribó M, Huertas R, et al. Time course of tissue plasminogen activator-induced recanalization in acute cardioembolic stroke: a case–control study. Stroke 2001;32:2821–7.
209. Moncayo J, Devuyst G, Van Melle G, Bogousslavsky J. Coexisting causes of ischemic stroke. Arch Neurol 2000;57:1139–44.
210. Montaner J, Alvarez-Sabín J, Barberá G, Anglés A, Molina C, Abilleira S, et al. [Correlation between the expression of proinflammatory cytokines and matrix metalloproteinases in the acute phase of an ischemic stroke.] Rev Neurol 2001;33:115–18.
211. Morin-Martin M, Gonzalez-Santiago R, Gil-Nunez AC, Vivancos-Mora J. Women and strokes. Hospital epidemiology in Spain. Rev Neurol 2003;37:701–5.
212. Moroney JT, Bagiella E, Paik MC, Sacco RL, Desmond DW, Moroney JT, et al. Risk factors for early recurrence after ischemic stroke: the role of stroke syndrome and subtype. Stroke 1998;29:2118–24.
213. Mounier-Vehier F, Leys D, Rondepierre P, Godefroy O, Pruvo JP. Silent infarcts in patients with ischemic stroke are related to age and size of the left atrium. Stroke 1993;24:1347–51.
214. Munts AG, van Genderen PJ, Dippel DW, van Kooten F, Koudstaal PJ. Coagulation disorders in young adults with acute cerebral ischaemia. J Neurol 1998;245:21–5.
215. Murat Sumer M, Erturk O. Ischemic stroke subtypes: risk factors, functional outcome and recurrence. Neurol Sci 2002;22:449–54.
216. Murtagh B, Smalling RW. Cardioembolic stroke. Curr Atheroscler Rep 2006;8:310–16.
217. Naess H, Hammersvik L, Skeie GO. Aphasia among young patients with ischemic stroke on long-term follow-up. J Stroke Cerebrovasc Dis 2009;18:247–50.
218. Nedeltchev K, der Maur TA, Georgiadis D, Arnold M, Caso V, Mattle HP, et al. Ischaemic stroke in young adults: predictors of outcome and recurrence. J Neurol Neurosurg Psychiatry 2005;76:191–5.
219. Nighoghossian N, Derex L, Perinetti M, Honnorat J, Barthelet M, Loire R, et al. Course of valvular strands in patients with stroke: cooperative study with transesophageal echocardiography. Am Heart J 1998;136:1065–9.
220. Norrving B, Löwenhielm P. Epidemiology of stroke in Lund-Orup, Sweden, 1983–85. Incidence of first stroke and age-related changes in subtypes. Acta Neurol Scand 1988;78:408–13.
221. Nwosu CM, Nwabueze AC, Ikeh VO. Stroke at the prime of life: a study of Nigerian Africans between the ages of 16 and 45 years. East Afr Med J 1992;69:384–90.
222. Ogata T, Kimura K, Minematsu K, Kazui S, Yamaguchi T, Japan Multicenter Stroke Investigators’ Collaboration. Variation in ischemic stroke frequency in Japan by season and by other variables. J Neurol Sci 2004;225:85–9.
223. Ois A, Cuadrado-Godia E, Solano A, Perich-Alsina X, Roquer J. Acute ischemic stroke in anterior choroidal artery territory. J Neurol Sci 2009;281:80–4.
224. Ortiz G, Koch S, Romano JG, Forteza AM, Rabinstein AA. Mechanisms of ischemic stroke in HIV-infected patients. Neurology 2007;68:1257–61.
225. Ory F, Albucher JF, Charlet JP, Guiraud-Chaumeil B, Chollet F. [Risk of recurrent stroke in adults aged under 45 years following a first ischemic stroke: a five-year study of 95 patients.] Rev Neurol (Paris) 2003;159:755–60.
226. Ostfeld AM, Shekelle RB, Tufo HM, Wieland AM, Kilbridge JA, Drori J. Cardiovascular and cerebrovascular disease in an elderly poor urban population. Am J Public Health 1971;61:19–29.
227. Otero Palleiro MM, Barbagelata L. [Etiologic subtypes of ischemic stroke in young adults aged 18 to 45 years: a study of a series of 93 patients.] Rev Clin Esp 2007;207:158–65.
228. Paciaroni M, Silvestrelli G, Caso V, Corea F, Venti M, Milia P, et al. Neurovascular territory involved in different etiological subtypes of ischemic stroke in the Perugia Stroke Registry. Eur J Neurol 2003;10:361–5.
229. Paemelaère JM, Sirinelli A, Dreyfus X, Maillard L, Pottier JM, Raynaud P. [Transesophageal echography and systemic ischemic incidences: 235 cases.] Rev Neurol (Paris) 1996;152:27–31.
230. Paganini-Hill A, Lozano E, Fischberg G, Perez BM, Rajamani K, Ameriso SF, et al. Infection and risk of ischemic stroke: differences among stroke subtypes. Stroke 2003;34:452–7.
231. Paixão LC, Ribeiro AL, Valacio RA, Teixeira AL. Chagas disease: independent risk factor for stroke. Stroke 2009;40:3691–4.
232. Park DC, Nam HS, Lim SR, Lee PH, Heo JH, Lee BI, et al. MRI features of infarcts with potential cardiac source of embolism in the Yonsei Stroke Registry (YSR), Korea. Yonsei Med J 2000;41:431–5.
233. Park HJ, Cho HJ, Kim YD, Lee DW, Choi HY, Kim SM, et al. Comparison of the characteristics for in-hospital and out-of-hospital ischaemic strokes. Eur J Neurol 2009;16:582–8.
234. Peters N, Müller-Schunk S, Freilinger T, Düring M, Pfefferkorn T, Dichgans M. Ischemic stroke of the cortical ‘hand knob’ area: stroke mechanisms and prognosis. J Neurol 2009;256:1146–51.
235. Petty GW, Khandheria BK, Chu CP, Sicks JD, Whisnant JP, Petty GW, et al. Patent foramen ovale in patients with cerebral infarction. A transesophageal echocardiographic study. Arch Neurol 1997;54:819–22.
236. Petty GW, Brown RD Jr, Whisnant JP, Sicks JD, O’Fallon WM, Wiebers DO, et al. Ischemic stroke subtypes: a population-based study of functional outcome, survival, and recurrence. Stroke 2000;31:1062–8.
237. Pinto A, Tuttolomondo A, Di Raimondo D, Fernandez P, Licata G. Risk factors profile and clinical outcome of ischemic stroke patients admitted in a Department of Internal Medicine and classified by TOAST classification. Int Angiol 2006;25:261–7.
238. Pinto A, Tuttolomondo A, Di Raimondo D, Di Sciacca R, Fernandez P, Di Gati M, et al. A case control study between diabetic and non-diabetic subjects with ischemic stroke. Int Angiol 2007;26:26–32.
239. Piper C, Hering D, Langer C, Horstkotte D. Etiology of stroke after mechanical heart valve replacement – results from a ten-year prospective study. J Heart Valve Dis 2008;17:413–17.
240. Pohjasvaara T, Erkinjuntti T, Vataja R, Kaste M. Comparison of stroke features and disability in daily life in patients with ischemic stroke aged 55 to 70 and 71 to 85 years. Stroke 1997;28:729–35.
241. Polychronopoulos P, Gioldasis G, Ellul J, Metallinos IC, Lekka NP, Paschalis C, et al. Family history of stroke in stroke types and subtypes. J Neurol Sci 2002;195:117–22.
242. Pope JM, Canny CL, Bell DA. Cerebral ischemic events associated with endocarditis, retinal vascular disease, and lupus anticoagulant. Am J Med 1991;90:299–309.
243. Poppert H, Sadikovic S, Sander K, Wolf O, Sander D. Embolic signals in unselected stroke patients: prevalence and diagnostic benefit. Stroke 2006;37:2039–43.
244. Pujadas Capmany R, Arboix A, Casañas-Muñoz R, Anguera-Ferrando N. Specific cardiac disorders in 402 consecutive patients with ischaemic cardioembolic stroke. Int J Cardiol 2004;95:129–34.
245. Purroy F, Montaner J, Molina CA, Delgado P, Ribo M, Alvarez S, et al. Patterns and predictors of early risk of recurrence after transient ischemic attack with respect to etiologic subtypes. Stroke 2007;38:3225–9.
246. Rabinstein AA, Chirinos JA, Fernandez FR, Zambrano JP. Is TEE useful in patients with small subcortical strokes? Eur J Neurol 2006;13:522–7.
247. Rasura M, Cao M, Beccia M, Anzini A. [Stroke in the young: a diagnostic protocol.] Ann Ital Med Int 1996;11:8–11.
248. Regli F. Transient ischemic cerebral seizures. Natural course and pathogenesis. Dtsch Med Wochenschr 1971;96:525–30.
249. Rey RC, Lepera SM, Kohler G, Monteverde DA, Sica RE. Cerebral embolism of cardiac origin. Medicina (Firenze) 1992;52:202–6.
250. Riabova VS. [Late sequelae of strokes based on registry data.] Zh Nevropatol Psikhiatr Im S S Korsakova 1986;86:532–6.
251. Robertson SC, Lennarson P, Hasan DM, Traynelis VC. Clinical course and surgical management of massive cerebral infarction. Neurosurgery 2004;55:55–61.
252. Rocha MO, Nunes MC, Ribeiro AL. Morbidity and prognostic factors in chronic chagasic cardiopathy. Mem Inst Oswaldo Cruz 2009;104(Suppl. 1):159–66.
253. Rojas JI, Zurrú MC, Patrucco L, Romano M, Riccio PM, Cristiano E, et al. [Ischemic stroke registry.] Medicina (Firenze) 2006;66:547–51.
254. Rojas JI, Zurrú MC, Romano M, Patrucco L, Cristiano E. Acute ischemic stroke in patients aged 80 or older. Medicina (Firenze) 2007;67:701–4.
255. Rojas JI, Zurrú MC, Romano M, Patrucco L, Falconi M, Cristiano E, et al. Transesophageal echocardiography findings in lacunar stroke. J Stroke Cerebrovasc Dis 2008;17:116–20.
256. Rothrock JF, Lyden PD, Brody ML, Taft-Alvarez B, Kelly N, Mayer J, et al. An analysis of ischemic stroke in an urban southern California population. The University of California, San Diego, Stroke Data Bank. Arch Intern Med 1993;153:619–24.
257. Rouhart F, Zagnoli F, Goas JY, Mocquard Y. [Cerebral ischemic arterial accidents in young adults. 40 cases.] Rev Neurol (Paris) 1993;149:547–53.
258. Rundek T, Hartmann A, Mast H, Chen X, Gan R, Demarin V, et al. Stroke subtype as a predictor of nursing home placement: Northern Manhattan Stroke Study. Acta Clin Croatica 1998;37:175–80.
259. Rus Mancilla C, Mesa Rubio D, Suárez de Lezo Cruz Conde J, Rodríguez Almodovar A, Durán Torralbo C, Delgado Ortega M. [Utility of transesophageal echocardiography in young patients with cryptogenic stroke and low cardiovascular risk.] Med Clin (Barc) 2008;130:241–5.
260. Russmann H, Vingerhoets F, Ghika J, Maeder P, Bogousslavsky J. Acute infarction limited to lenticular nucleus: clinical, etiological, and topographic features. Arch Neurol 2003;60:351–5.
261. Ryglewicz D. Stroke epidemiology in Poland. Acta Clin Croatica Suppl 1998;37:80–3.
262. Ryglewicz D, Baranska-Gieruszczak M, Czlonkowska A, Lechowicz W, Hier DB. Stroke recurrence among 30 days survivors of ischemic stroke in a prospective community-based study. Neurol Res 1997;19:377–9.
263. Sacco RL, Foulkes MA, Mohr JP, Wolf PA, Hier DB, Price TR, et al. Determinants of early recurrence of cerebral infarction. The Stroke Data Bank. Stroke 1989;20:983–9.
264. Sacco SE, Whisnant JP, Broderick JP, Phillips SJ, O’Fallon WM. Epidemiological characteristics of lacunar infarcts in a population. Stroke 1991;22:1236–41.
265. Sacco RL, Kargman DE, Zamanillo MC. Race-ethnic differences in stroke risk factors among hospitalized patients with cerebral infarction: the Northern Manhattan Stroke Study. Neurology 1995;45:659–63.
266. Sacco RL, Kargman DE, Gu Q, Zamanillo MC. Race-ethnicity and determinants of intracranial atherosclerotic cerebral infarction. The Northern Manhattan Stroke Study. Stroke 1995;26:14–20.
267. Sagui E, M’Baye PS, Dubecq C, Ba FK, Niang A, Gning S, et al. Ischemic and hemorrhagic strokes in Dakar, Senegal: a hospital-based study. Stroke 2005;36:1844–7.
268. Salerno SM, Landry FJ, Schick JD, Schoomaker EB. The effect of multiple neuroimaging studies on classification, treatment, and outcome of acute ischemic stroke. Ann Intern Med 1996;124:21–6. [Erratum published in Ann Intern Med 1996;125:428.]
269. Salihovic DO, Smajlovic DM, Sinanovic OI. Reduction of stroke mortality in the Tuzla region, Bosnia and Herzegovina. Neurosciences 2009;14:230–3.
270. Salihovic D, Smajlovic D, Sinanovic O, Kojic B. Sex differences in patients with acute ischemic stroke in Tuzla region, Bosnia and Herzegovina. Bosn J Basic Med Sci 2010;10:116–20.
271. Samiullah S, Humaira M, Hanif G, Ghouri AA, Shaikh K. Etiological patterns of stroke in young patients at a tertiary care hospital. J Pak Med Assoc 2010;60:201–4.
272. Sansoy V, Abbott RD, Jayaweera AR, Kaul S. Low yield of transthoracic echocardiography for cardiac source of embolism. Am J Cardiol 1995;75:166–9.
273. Santonja JM, Vicent V, Pareja A, Láinez JM, Sancho-Rieger J. Cerebrovascular disease in young adults. A study of the evolution of 167 patients. Rev Neurol 1998;26:787–90.
274. Santos-Lasaosa S, Navas I, Mostacero E, López del Val J, Tejero C, Escalza I. [Cardioembolic infarction: clinical course and characteristics.] Rev Neurol 2000;31:1154–8.
275. Saposnik G, Caplan LR. [Ischemia of the vertebrobasilar territory: mechanisms and practical considerations.] Rev Neurol 2001;33:854–64.
276. Saposnik G, Caplan LR, Gonzalez LA, Baird A, Dashe J, Luraschi A, et al. Differences in stroke subtypes among natives and caucasians in Boston and Buenos Aires. Stroke 2000;31:2385–9.
277. Saposnik G, Gonzalez L, Lepera S, Luraschi A, Sica RE, Caplan LR, et al. Southern Buenos Aires stroke project. Acta Neurol Scand 2001;104:130–5.
278. Sawalha AF. Characterization of hospitalized ischemic stroke patients in Palestine. Libyan J Med 2009;4:39–44.
279. Schulz UG, Rothwell PM. Differences in vascular risk factors between etiological subtypes of ischemic stroke: importance of population-based studies. Stroke 2003;34:2050–9.
280. Sciolla R, Ferrari G, Leone M, SINPAC (Società INter-regionale Piemonte e Valle d’Aosta per le Cerebrovasculopatie) Group. Stroke and transient ischaemic attack in 18 neurology departments from two Italian Regions: the SINPAC database. Neurol Sci 2005;26:208–17.
281. Segal OR, Dawson JR, Gupta S. The use of echocardiography for stroke and peripheral embolus: is it time for British/European guidelines? Br J Cardiol 2002;9:287–90.
282. Sempere AP, Duarte J, Cabezas C, Clavería LE. Etiopathogenesis of transient ischemic attacks and minor ischemic strokes: a community-based study in Segovia, Spain. Stroke 1998;29:40–5.
283. Serafini O, Misuraca G, Greco F, Bisignani G, Manes MT, Venneri N, et al. [Prevalence of structural abnormalities of the atrial septum and their association with recent ischemic stroke or transient ischemic attack: echocardiographic evaluation in 18631 patients.] Ital Heart J Suppl 2003;4:39–45.
284. Serafini O, Misuraca G, Siniscalchi A, Manes MT, Meringolo G, Tomaselli C, et al. Prevalence of aneurysm of the interatrial septum in the general population and in patients with a recent episode of cryptogenetic ischemic stroke: a tissue harmonic imaging transthoracic ecocardiography study in 5.631 patients. Monaldi Arch Chest Dis 2006;66:264–9.
285. Serena J, Dávalos MA. Frequency of atrial septal aneurysm in patients with cerebral ischemic events. Circ Cardiovasc Qual Outcomes 2000;102:E27.
286. Sha R-JZ. Etiological factor, risk factor and prognosis in young patients with cerebral infarction and cerebral hemorrhage: comparative analysis among 293 cases. Chin J Clin Rehabil 2004;8:6838.
287. Shahar E, McGovern PG, Sprafka JM, Pankow JS, Doliszny KM, Luepker RV, et al. Improved survival of stroke patients during the 1980s. The Minnesota Stroke Survey. Stroke 1995;26:1–6.
288. Sharifkazemi MB, Aslani A, Zamirian M, Moaref AR. Significance of aortic atheroma in elderly patients with ischemic stroke. A hospital-based study and literature review. Clin Neurol Neurosurg 2007;109:311–16.
289. Silvestrelli G, Corea F, Paciaroni M, Milia P, Palmerini F, Parnetti L, et al. The Perugia hospital-based Stroke Registry: report of the 2nd year. Clin Exp Hypertens 2002;24:485–91.
290. Slowik A, Dziedzic T, Turaj W, Pera J, Glodzik-Sobanska L, Szermer P, et al. A2 alelle of GpIIIa gene is a risk factor for stroke caused by large-vessel disease in males. Stroke 2004;35:1589–93.
291. Soares FA, Monteiro J, Ferreira D, Fonseca TP, Melo TP, Ferro J, et al. [The importance of heart disease in the various types of cerebral vascular disease. A prospective study.] Rev Port Cardiol 1990;9:425–32.
292. Sochurkova D, Moreau T, Lemesle M, Menassa M, Giroud M, Dumas R, et al. Migraine history and migraine-induced stroke in the Dijon stroke registry. Neuroepidemiology 1999;18:85–91.
293. Soler-González R, Muñoz-Torrero JJ, Domínguez F, Oliver J, Díez-Tejedor E. [Analysis of the echocardiographic findings in young patients with cerebral ischemia.] Rev Neurol 1999;29:972–6.
294. Somay G, Topaloglu P, Somay H, Araal O, Usak Halaç G, Bulkan M. Cerebrovascular risk factors and stroke subtypes in different age groups: a hospital-based study. Turk J Med Sci 2006;36:23–9.
295. Somody E, Albucher JF, Delay M, Chollet F, Bonnet JP, Fourcade J, et al. [Value of the study of latent atrial vulnerability in unexplained ischemic cerebrovascular accident of young subjects.] Arch Mal Coeur Vaiss 1996;89:1365–73.
296. Somody E, Delay M, Rouesnel PH, Galley D, Cosnay P, Arquizan C, et al. [Clinical evolution of patients following investigation of atrial vulnerability after a first cerebral ischaemic accident.] Arch Mal Coeur Vaiss 2006;99:221–9.
297. Song YM, Kwon SU, Sung J, Ebrahim S, Smith GD, Sunwoo S, et al. Different risk factor profiles between subtypes of ischemic stroke. A case–control study in Korean men. Eur J Epidemiol 2005;20:605–12.
298. Sorescu D, Turk RJ, Cain M, Lerakis S. Clinical and transthoracic echocardiographic predictors of abnormal transesophageal findings in patients with suspected cardiac source of embolism. Am J Med Sci 2003;326:31–4.
299. Sozio SM, Armstrong PA, Coresh J, Jaar BG, Fink NE, Plantinga LC, et al. Cerebrovascular disease incidence, characteristics, and outcomes in patients initiating dialysis: the choices for healthy outcomes in caring for ESRD (CHOICE) study. Am J Kidney Dis 2009;54:468–77.
300. Spengos K, Vemmos KN, Tsivgoulis G, Synetos A, Zakopoulos N, Zis VP, et al. Seasonal variation of hospital admissions caused by acute stroke in Athens, Greece. J Stroke Cerebrovasc Dis 2003;12:93–6.
301. Spengos K, Tsivgoulis G, Manios E, Synetou M, Vassilopoulou S, Zakopoulos N, et al. Stroke etiology is associated with symptom onset during sleep. Sleep 2005;28:233–8.
302. Spielberg C, Kruck I, von Leitner ER, Helberg C, Schröder R, Seyfert S, et al. [Patients with transient ischemic attacks. Their cardiac status and its prognostic significance.] Dtsch Med Wochenschr 1988;113:592–7.
303. Sprigg N, Gray LJ, Bath PM, Lindenstrøm E, Boysen G, De Deyn PP, et al. Early recovery and functional outcome are related with causal stroke subtype: data from the tinzaparin in acute ischemic stroke trial. J Stroke Cerebrovasc Dis 2007;16:180–4.
304. Stuart-Shor EM, Wellenius GA, DelloIacono DM, Mittleman MA. Gender differences in presenting and prodromal stroke symptoms. Stroke 2009;40:1121–6.
305. Sundar U, Mehetre R. Etiopathogenesis and predictors of in-hospital morbidity and mortality in posterior circulation strokes – a 2 year registry with concordant comparison with anterior circulation strokes. J Assoc Physicians India 2007;55:846–50.
306. Syed NA, Khealani BA, Ali S, Hasan A, Akhtar N, Brohi H, et al. Ischemic stroke subtypes in Pakistan: the Aga Khan University Stroke Data Bank. J Pak Med Assoc 2003;53:584–8.
307. Sylaja PN, Coutts SB, Subramaniam S, Hill MD, Eliasziw M, Demchuk AM, et al. Acute ischemic lesions of varying ages predict risk of ischemic events in stroke/TIA patients. Neurology 2007;68:415–19.
308. Szaflarski JP, Rackley AY, Kleindorfer DO, Khoury J, Woo D, Miller R, et al. Incidence of seizures in the acute phase of stroke: a population-based study. Epilepsia 2008;49:974–81.
309. Szegedi N, May Z, Ováry C, Skopál J, Nagy Z. [Molecular markers of endothelial dysfunction in acute ischemic stroke.] Ideggyogy Sz 2002;55:102–8.
310. Tan KS, Tin Tan C, Churilov L, Mackay M, Donnan GA. Ischaemic stroke in young adults: a comparative study between Malaysia and Australia. Neurol Asia 2010;15:1–9.
311. Tan TY, Tseng MC, Chang KC. Risk factors for first-ever ischemic stroke: a hospital-based case–control study in Kaohsiung, Taiwan. Chang Gung Med J 2004;27:801–7.
312. Tanizaki Y, Kiyohara Y, Kato I, Iwamoto H, Nakayama K, Shinohara N, et al. Incidence and risk factors for subtypes of cerebral infarction in a general population: the Hisayama study. Stroke 2000;31:2616–22.
313. Tarazona B, Ramos W, Arce J, Yarinsueca J, Morales S, Ronceros G, et al. Etiology and risk factors for a first episode of cerebral isquemia in young adults. Neurologia 2010;25:470–7.
314. Tateishi Y, Iguchi Y, Kimura K, Kobayashi K, Shibazaki K, Eguchi K, et al. Right-to-left shunts may be not uncommon cause of TIA in Japan. J Neurol Sci 2009;277:13–16.
315. Tegos TJ, Kalodiki E, Daskalopoulou SS, Nicolaides AN. Stroke: epidemiology, clinical picture, and risk factors – part I of III. Angiology 2000;51:793–808.
316. Telman G, Kouperberg E, Sprecher E, Yarnitsky D. Distribution of etiologies in patients above and below age 45 with first-ever ischemic stroke. Acta Neurol Scand 2008;117:311–16.
317. Timsit SG, Sacco RL, Mohr JP, Foulkes MA, Tatemichi TK, Wolf PA. Early clinical differentiation of cerebral infarction from severe atherosclerotic stenosis and cardioembolism. Stroke 1992;23:486–91.
318. Timsit SG, Sacco RL, Mohr JP, Foulkes MA, Tatemichi TK, Wolf PA, et al. Brain infarction severity differs according to cardiac or arterial embolic source. Neurology 1993;43:728–33.
319. Tipping B, de Villiers L, Wainwright H, Candy S, Bryer A. Stroke in patients with human immunodeficiency virus infection. J Neurol Neurosurg Psychiatry 2007;78:1320–4.
320. Toso V, Carolei A, Gensini GF, Cimminiello C, Micieli G, Toni D, et al. The Stroke in Italy and Related Impact on Outcome (SIRIO) study: design and baseline data. Neurol Sci 2006;27(Suppl. 3):S263–7.
321. Toyoda K, Fujii K, Fujimi S, Kumai Y, Tsuchimochi H, Ibayashi S, et al. Stroke in patients on maintenance hemodialysis: a 22-year single-center study. Am J Kidney Dis 2005;45:1058–66.
322. Uchiyama S. Stroke prevention and prognosis in cardiogenic brain embolism. Nihon Rinsho 1993;51:620–7.
323. Ulrich JN, Hesse B, Schuele S, Vlassak I, Sila CA, Jaber WA, et al. Single-vessel versus multivessel territory acute ischemic stroke: value of transesophageal echocardiography in the differentiation of embolic stroke. J Am Soc Echocardiogr 2006;19:1165–9.
324. Urbach H, Hartmann A, Pohl C, Omran H, Wilhelm K, Flacke S, et al. Local intra-arterial thrombolysis in the carotid territory: does recanalization depend on the thromboembolus type? Neuroradiology 2002;44:695–9.
325. Urbinelli R, Bolard P, Lemesle M, Osseby GV, Thomas V, Boruel D, et al. Stroke patterns in cardio-embolic infarction in a population-based study. Neurol Res 2001;23:309–14.
326. Van Camp GS. Relation between patent foramen ovale and unexplained stroke. Am J Cardiol 1993;71:596–8.
327. van Wijk I, Koudstaal PJ, Kappelle LJ, van Gijn J, Gorter JW, Algra A, et al. Long-term occurrence of death and cardiovascular events in patients with transient ischaemic attack or minor ischaemic stroke: comparison between arterial and cardiac source of the index event. J Neurol Neurosurg Psychiatry 2008;79:895–9.
328. Velho FJ, Dotta F, Scherer L, Bartholomay E, da Silva DA, Fernandes JG, et al. Association between the effect of spontaneous contrast in the thoracic aorta and recent ischemic stroke determined by transesophageal echocardiography. Arq Bras Cardiol 1947;82:52–6.
329. Verdelho A, Pereira JG, Ferro JM. [Multiple vertebro-basilar infarcts and cardio-embolism.] Rev Neurol 1999;28:1027–30.
330. Viana Baptista M, Van Melle G, Bogousslasjy J. Prediction of in-hospital mortality after first-ever stroke: the Lausanne Stroke Registry. J Neurol Sci 1999;166:107–14.
331. Vilalta JL, Arboix A. The Barcelona Stroke Registry. Eur Neurol 1999;41:135–42.
332. Vitebskiy S, Fox K, Hoit BD. Routine transesophageal echocardiography for the evaluation of cerebral emboli in elderly patients. Echocardiography 2005;22:770–4.
333. Voyce SJ, Aurigemma GP, Dahlberg S, Orsinelli D, Pape LA, Sweeney A, et al. A Comparison of 2-dimensional echocardiography vs carotid duplex scanning in older patients with cerebral-ischemia. Arch Intern Med 1992;152:2089–93.
334. Wasay M, Kaul S, Menon B, Venketasubramanian N, Gunaratne P, Khalifa A, et al. Ischemic stroke in young Asian women: risk factors, subtypes and outcome. Cerebrovasc Dis 2010;30:418–22.
335. Whisnant JP, Wiebers DO, O’Fallon WM, Sicks JD, Frye RL, Whisnant JP, et al. A population-based model of risk factors for ischemic stroke: Rochester, Minnesota. Neurology 1996;47:1420–8.
336. Whisnant JP, Brown RD, Petty GW, O’Fallon WM, Sicks JD, Wiebers DO, et al. Comparison of population-based models of risk factors for TIA and ischemic stroke. Neurology 1999;53:532–6.
337. Whisnant JP, Wiebers DO, O’Fallon WM, Sicks JD, Frye RL. Effect of time since onset of risk factors on the occurrence of ischemic stroke. Neurology 2002;58:787–94.
338. Wilmshurst PN. Relation of atrial shunts to migraine in patients with ischemic stroke and peripheral emboli. Am J Cardiol 2006;98:831–3.
339. Woo D, Gebel J, Miller R, Kothari R, Brott T, Khoury J, et al. Incidence rates of first-ever ischemic stroke subtypes among blacks: a population-based study. Stroke 1999;30:2517–22.
340. Wu L, Takahashi K, Kobayashi S, Tian G, Song L, Matui R, et al. [Clinical characteristics of cerebral infarction in China and Japan.] Rinsho Shinkeigaku 2004;44:335–41.
341. Wu L-EL. Liu M, Zhang Y-H, Yang J, Tan C, Zhang S-H, et al. Classification and prognosis of ischemic stroke subtypes according to TOAST criteria: a prospective cohort study. Chin J Neurol 2004;37:292–5.
342. Wu L, Takahashi K, Kobayashi S, Tian G, Song L, Matui R, et al. Clinical characteristics of cerebral infarction in China and Japan. Clin Neurol 2004;44:335–41.
343. Yamamoto H, Bogousslavsky J, Van Melle G. Different predictors of neurological worsening in different causes of stroke. Arch Neurol 1998;55:481–6.
344. Yamamoto Y, Georgiadis AL, Chang HM, Caplan LR. Posterior cerebral artery territory infarcts in the New England Medical Center Posterior Circulation Registry. Arch Neurol 1999;56:824–32.
345. Yamazaki T, Yanaka K, Aoki T, Matsuki T, Ono F, Fukuda T, et al. Usefulness of Doppler echocardiography in patients with ischemic cerebrovascular disease. Neurol Surg 2000;28:233–6.
346. Yang JH, Choi HY, Nam HS, Kim SH, Han SW, Heo JH, et al. Mechanism of infarction involving ipsilateral carotid and posterior cerebral artery territories. Cerebrovasc Dis 2007;24:445–51.
347. Yasaka M, Yamaguchi T, Oita J, Sawada T, Shichiri M, Omae T, et al. Clinical features of recurrent embolization in acute cardioembolic stroke. Stroke 1993;24:1681–5.
348. Yokota C, Minematsu K, Hasegawa Y, Yamaguchi T. Long-term prognosis, by stroke subtypes, after a first-ever stroke: a hospital-based study over a 20-year period. Cerebrovasc Dis 2004;18:111–16.
349. Yonemura K, Kimura K, Hasegawa Y, Yokota C, Minematsu K, Yamaguchi T. Analysis of ischemic stroke in patients aged up to 50 years. Clin Neurol 2000;40:881–6.
350. Yoshida S, Yamamoto T, Yoshioka M, Kuroki S. [Ischemic stroke in childhood.] No Shinkei Geka 1993;21:611–16.
351. Yurekli VAK. Ischemic stroke in young adult. Turk Serebrovaskuler Hastaliklar Dergisi 2005;11:13–18.
352. Zeiler K, Siostrzonek P, Lang W, Gössinger H, Oder W, Ciciyasvilli H, et al. Different risk factor profiles in young and elderly stroke patients with special reference to cardiac disorders. J Clin Epidemiol 1992;45:1383–9.
Appendix 4 Prevalence of cardiac sources of stroke by study
| Study | Age (years) | Cardiac pathologies detected | Tests used |
|---|---|---|---|
| Agmon 199941 | > 45 | ASA 28/355 (7.9%) | TOE, CU |
| Arboix 199788 | Mean 75, range 34–94 | ASA with interatrial shunting 1/231 (0.4%) | DU, MRI, ECG, CT |
| Arnold 200842 | Mean 35.8 | ASA 5/100 (5%) | TTE, TOE, CT, ECG, MRI, MRA |
| Awada 199943 | Range 10–80 | RVD 34/756 (4.5%); PFO 3/756 (0.4%) | TTE, TOE, CU |
| Barinagarrementeria43 | Mean 28, range 11–40 | RVD 29/130 (22.3%); PFO 8/130 (6.2%) | TTE, TOE |
| Barinagarrementeria44 | Mean 30 | RVD 3/37 (8%); PFO 5/37 (13.5%) | TTE, TOE |
| Belvis 200746 | Mean 44.7, range 23–55 | PFO 17/39 (43.5%); ASA 3/39 (7.7%) | TOE, CT, MRI |
| Benedik 200783 | Median 11.5, range 2–17 | PFO 9/18 (50%) | MRI, TCD, ECG, TTE |
| Bevan 199096 | No details | RVD 2/48 (4.2%) | CT, autopsy, ECG, 24-hour HM, EC |
| Bogousslavsky17 | < 60 | PFO 140/340 (41.2%) | ECG, CT, MRI, EC |
| Cabanes 199347 | Mean 40 | ASA 28/100 (28%); PFO 43/100 (43%); ASA and PFO 22/100 (22%) | TTE, TOE, EC |
| Fieschi 199648 | Median 39, range 18–47 | LVT 1/160 (0.6%); PFO 22/160 (13.8%); ASA with or without PFO 14/160 (8.8%); ASD 1/160 (0.6%) | TTE TOE |
| Fukujima 200549 | Mean 63, range 26–92 | LVH 208/523 (39.8%); SEC in left atrium 81/523 (15.5%); SEC in left ventricle 21/523 (4%); SEC in aorta 45/523 (8.6%); LAT 5/523 (0.9%); LVT 3/523 (0.6%); PFO 126/523 (24.1%); MVR 383/523 (73.2%); MVS 4/523 (0.7%); AVC 156/523 (29.8%); interatrial septum aneurysm 38/523 (7.3%); AVS 3/523 (0.6%) | TOE |
| Ghandehari 200623 | Range 15–45 | RVD 18/67 (26.8%); PFO 2/67 (2.9%); LVT 1/67 (1.5%) | CT, ECG, 24-hour HM, TTE, TOE |
| Handke 200750 | Mean ∼ 62, range 20–84 | PFO 77/227 (33.9%); PFO with ASA 33/227 (14.5%) | TTE, TOE, CT, MRI |
| Hoffmann 200484 | 18–49 | PFO 6/133 (4.5%) | MRI, EC, TTE |
| Homma 199451 | No details | PFO 23/74 (31%); ASA 9/74 (12%) | TTE, TOE, CU, CT, MRI, TCD |
| Kang 200852 | Mean 65, range 29–88 | PFO 1/100 (1%) | ECG, TTE, TOE, 24-hour HM, MRI |
| Kasner 2007102 | Mean 63 | PFO 18/264 (7%); LVH 110/264 (42%) | EC |
| Knebel 200953 | Range 18–90 | PFO n = 152 (21.7%); ASD n = 17 (2.4%); ASA n = 51 (7.3%) | ECG, 24-hour HM, TOE |
| Kristensen 199785 | Range 18–44 | PFO 32/97 (33%); ASA 9/100 (9%) | TTE |
| Lamy 200254 | Reported according to absence or presence of PFO: mean 44.5 vs. 40.1 respectively | PFO 267/581 (45.9%); ASA 61/581 (10.5%) | TOE |
| Lanzino 199197 | Range 16–45 | LVH 12/155 (7.7%); MVP 5/155 (3.2%); hypertrophic cardiomyopathy with LVT 1/155 (0.65%); ASA 1/155 (0.65%); AVS 1/155 (0.65%); RMVD 1/155 (0.65%) | CT, two-dimensional EC |
| Lavados 200755 | Mean 66.4 | RVD 3/185 (1.6%); AAT 2/185 (1.1%); PFO 2/185 (1.1%); ventricular hypokinesia 1/185 (0.5%) | CT, TTE, TOE |
| Lindgren 199456 | Mean 73.3 | Severe mitral annulus calcification 49/166 (29.5%); PFO 20/166 (12%); ASA 24/166 (14.5%); calcific aortic stenosis 5/166 (3%) | CT, MRI, ECG, TTE, TOE |
| Luijckx 199398 | Median 39, range 17–50 in the Thai series; median 42, range 15–50 in the Dutch series | Thai series: RHD 13/56 (23%); myxoma cordis 1/56 (1.8%) Dutch series: RHD 1/55 (2%); MVP 3/55 (5.5%) |
CT, ECG, EC, angiography |
| Malm 199957 | Mean 36.9 | LAT 1/24 (4.2%); ASA 1/24 (4.2%); ASD 1/24 (4.2%); PFO 3/24 (12.5%) | CT, MRI, ECG, TTE, TOE |
| Mattioli 200158 | Mean 65.7, range 35–86 | ASA 68/245 (27.7%); PFO 56/245 (22.8%); MAC 24/245 (9.7%); vegetations 3/245 (1.2%) | CT, MRI, TTE, TOE |
| Mehndiratta 200495 | Mean 31.5 | RHD 16/109 (14.7%); atrial myxoma 1/109 (0.9%) | CT, MRI, 24-hour HM, ECG, EC |
| Mochan 200359 | Mean 32.1, range 20–61 | LVH 1/33 (3%); MVP 1/33 (3%) | CT, ECG, TTE, TOE |
| Mok 200360 | Not adequately reported | Nil | CT, MRI, ECG, TTE, TOE |
| Musolino 200361 | Mean 36.4, range 17–45 | Detected by TTE: MVR 6/60 (10%); mitral prosthesis 1/60 (1.67%); MVP 3/60 (5%); aortic valve vegetation 1/60 (1.67%); MVS 2/60 (3.3%); ASD 1/60 (1.67%); LVT 1/60 (1.67%) Detected by TOE (13/60 refused TEE, total = 47): MVR 5/47 (10.6%); mitral prosthesis 1/47 (2.1%); MVP 3/47 (6.4%); aortic valve vegetation 0/47 (0%); mitral stenosis 2/47 (4.3%); ASD 4/47 (8.5%); LVT 2/47 (4.3%); LAT 1/47 (2.1%); ASA 11/47 (23.4%); PFO 10/47 (21.3%); mitral prosthesis thrombus 2/47 (4.3%) |
CT, MRI, ECG, DU, TTE, TOE |
| Negrão 200762 | Mean 33.9 | PFO 47/168 (28%) | CT, MRI, TTE, TOE, TCD |
| Nighoghossian 199663 | Mean 47 | ASA 11/118 (9.3%); PFO 8/118 (6.8%); ASA-PFO 18/118 (15.3%); MVP 9/118 (7.6%); ASA-PFO-MVP 4/118 (3.4%); MV incompetence 4/118 (3.4%); aortic arch atheroma 4/118 (3.4%) | CT, MRI, ECG, TTE, TOE |
| Omran 1999132 | Average 48.6 | LAT 6/583 (1%); mitral stenosis 3/583 (0.5%); MVS 7/583 (1%) | TTE, TOE |
| Ossemann 199565 | Mean 63 | ASA 22/146 (15.1%) | CT, ECG, 24-hour ECG, TTE, TOE |
| Pearson 1991118 | Mean 53 | ASA 20/133 (15%) | TOE |
| Pessin 198789 | Average 58 | Mitral stenosis 1/35 (2.9%); MVP 2/35 (5.7%) | CT, angiography |
| Pezzini 200367 | Mean 34.7 | PFO 36/125 (28.8%) | CT, MRI, TCD, 12-lead ECG, TTE, TOE |
| Pun 1984101 | Mean 54.4, range 20–87 | RHD 38/129 (29.5%); MVP 1/129 (0.8%) | ECG, EC |
| Putaala 200926 | Mean 41.3 | Atrial myxoma 2/198 (1%); PFO 74/198 (37%); PFO-ASA 13/198 (7%); ASA 4/198 (2%); MVP 1/198 (0.5%); MAC 1/198 (0.5%) | ECG, TTE, TOE |
| Rasura 200668 | Mean 36.4, range 14–47 | ASD 1/394 (0.25%); atrial myxoma 2/394 (0.5%); PFO 60/394 (15.2%); ASA 22/394 (5.6%); PFO-ASA 16/394 (4.1%); aortic atheroma 5/394 (1.3%); aortic atheroma + PFO 2/394 (0.5%) | CT, MRI, ECG, TCD, TTE, TOE |
| Rauh 199669 | Median 62, 28–83 | Findings detected by TTE: LVH 11/30 (36.6%); MVP 1/30 (3.3%) Additional findings detected by TOE: PFO 7/30 (23.3%); LAT 3/30 (10%); ASA 2/30 (6.7%) |
ECG, TTE, TOE |
| Noce 200490 | Mean 5.7, range 2 months–15 years | RHD 2/39 (5.1%); MVP 1/39 (2.6%) | Not reported |
| Rodriguez 200370 | Mean 59 | PFO 34%; ASA 11%; PFO-ASA 7% | TOE |
| Roijer 199771 | Mean 70.1, range 38–93 | LAT 11/121 (9%); LVT 1/121 (1%); ejection fraction < 35% 6/121 (5%); LA myxoma 1/121 (1%); ASA 21/121 (17%); PFO 20/121 (17%); MVP 1/121 (1%); annular calcification 26/121 (21%) | CT, MRI, 12-lead ECG, TTE, TOE |
| Roquer 200372 | Mean 71.6 | PFO 4/1581 (0.25%) | CT, MRI, ECG, TCD, TTE, TOE |
| Sandercock 198999 | Not reported | MVS 2/244 (0.8%); aortic sclerosis 11/244 (4.5%); MAC 5/244 (2%); mitral leaflet prolapsed 3/244 (1.2%); aortic stenosis 2/244 (0.8%) | CT, ECG, EC |
| Seifert 200573 | Mean 65.7, range 28–89 | PFO 7/93 (7.5%); LVT 1/93 (1.1%); MV prosthesis 1/93 (1.1%); aortic valve myxoma 1/93 (1.1%) | ECG, TCD, TTE, TOE |
| Serena 199874 | Mean 64.8 | PFO 22/44 (50%); ASA 5/44 (11.4%) | CT, 12-lead ECG, TCD, TTE, TOE |
| Silva 200575 | < 55 | PFO 5/29 (17.2%); PFO-ASA 7/29 (24.1%) | CT, TOE |
| Siqueira 199686 | Range 15–40 | MVP 6/106 (5.7%); RHD 10/106 (9.4%) | CT, ECG, Doppler ECG, TTE |
| Skidmore 200191 | Not adequately reported | RHD with severe MVS 1/16 (6.2%) | CT, ECG, MRI, MRA |
| Sloan 199887 | Mean 36, range 17–44 | MV thickening 1/20 (5%); MVP 3/31 (9.7%); mitral vegetation 3/31 (9.7%); MVP with possible vegetation 1/31 (3.2%); mitral and aortic valve prolapse without vegetation 1/31 (3.2%); aortic and mitral vegetation and aortic regurgitation 1/31 (3.2%); MV nodular thickening 1/31 (3.2%); LVH 2/51 (3.9%) | TTE |
| Steinke 199776 | Mean 65, range 16–87 | LA dilatation 5/74 (6.8%); LV dilatation 4/74 (5.4%); MV insufficiency 1/74 (1.4%); apical/atrial thrombus 2/74 (2.7%) | TCD, MRA, ECG, TTE, TOE |
| Strandberg 200877 | Not reported | LAT 6/441 (1.4%); LVT 1/441 (0.2%); atrial myxoma 0/441; MVS 1/441 (0.2%); MVP 16/441 (3.6%); MAC 7/441 (1.6%); calcified aortic stenosis 5/441 (1.1%); PFO 61/441 (13.8%); SEC 5/441 (1.1%); ASA 18/441 (4.1%); LV aneurysm 7/441 (1.6%); aortic aneurysm 1/441 (0.2%); false tendon 6/441 (1.4%) | TOE, TTE, DU |
| Tei 1993100 | Mean 62.8 | MVP 5/72 (6.9%); MAC 2/72 (2.8%) | CT, 12-lead ECG, two-dimensional EC |
| Tice 199678 | Mean 50, range 28–87 | ASA 5/44 (11.4%); PFO 2/44 (5%); MV thickening 4/44 (9%); MV strands 7/44 (16%) | TOE |
| Ueno 200779 | Mean 67 | PFO 8/11 (73%); ASD 1/11 (9%); large RLS 2/11 (18%); small RLS 7/11 (64%); ASA 2/11 (18%); intracardiac thrombus 0/11, MVP 0/11 | MRI, MRA, 24-hour ECG, TTE, TOE |
| Varona 200727 | Mean 36 | PFO 5/272 (1.8%); LVT 1/272 (0.4%) | CT, MRI, TTE, TOE, extracranial cerebrovascular studies |
| Walpot 200680 | Mean 52.2, range 18–65 | PFO-ASD 16/54 (29.6%); ASA 7/54 (13%); SEC 0/54, AVC 3/54 (5.6%); MAC 1/54 (1.9%); MVP 0/54, aortic sclerosis 3/54 (5.6%) | TOE |
| Ward 200681 | Mean 60.3, range 25–91 | SEC 3.7%; PFO 18.8%; ASA 3.3%; LAT/LVT 2.4%; vegetation/mass/tumour 7.8% | CT, MRI, TOE |
| Williams 199792 | Mean 23.2 | PFO/ASD 7/208 (3.4%); LAT or LVT 3/208 (1.4%) | Not adequately reported |
| Wong 200193 | Range 49–75 | Low ejection fraction (< 40%) 1/6 (16.7%) | CT, MRI, MRA, ECG, TCD |
| Zibaeenezhad 200682 | Mean 50.8, range 16–81 | PFO 9/98 (9.1%); ASD 3/98 (3%); ventricular septal defect 2/98 (2%); interatrial septal aneurysm 2/98 (2%); mitral regurgitation 51/98 (52%); MVP 31/98 (31.6%); MVS 8/98 (8.1%); thick aortic valve 6/98 (6.1%); aortic stenosis 5/98 (5.1%); mass on aortic valve 2/98 (2%); MV vegetation (prosthetic valve) 1/98 (1%); LVH 3/98 (3%) | CT, MRI, ECG, TOE |
Appendix 5 MEDLINE search strategy for the systematic review of diagnostic accuracy studies
-
Stroke/ (39,233)
-
stroke$.mp. (138,756)
-
stroke volume/ (25,237)
-
stroke volume$.mp. (32,752)
-
Cerebrovascular accident.mp. (2591)
-
cerebrovascular event.mp. (471)
-
Cerebrovascular disease.mp. (9405)
-
Ischemic Attack, Transient/ or transient ischemic event.mp. (16,035)
-
transient ischemic attack.mp. (3409)
-
vascular accident.mp. (674)
-
brain emboli$.mp. or Intracranial Embolism/ (2398)
-
cerebral emboli$.mp. (1923)
-
brain infarction.mp. or Brain Infarction/ (3554)
-
cerebral infarction.mp. or Cerebral Infarction/ (21,326)
-
or/1-14 (175,197)
-
Echocardiography.mp. or Echocardiography/ (105,417)
-
transthoracic echocardiography.mp. (4128)
-
Transoesophageal echocardiography.mp. (1369)
-
transesophageal echocardiography.mp. (8092)
-
(echocardiog$ adj (transthorac$ or trans-thorac$ or (trans$ and thorac$))).mp. (427)
-
(echocardiog$ adj (transoesophag$ or trans-oesophag$ or (trans and oesophag$))).mp. (46)
-
(echocardiog$ adj (transesophag$ or trans-esophag$ or (trans and esophag$))).mp. (12,231)
-
24 hour holter.mp. (1157)
-
twenty four hour holter.mp. (111)
-
telemetr$.mp. (9058)
-
secondary prevention.mp. (9681)
-
cardiac imag$.mp. (1883)
-
cardiac magnetic resonance imaging.mp. (1315)
-
cardiac MR.mp. (349)
-
cardiac MRI.mp. (882)
-
carotid ultrasound.mp. (499)
-
carotid doppler.mp. (210)
-
transcranial doppler.mp. (5296)
-
transcranial doppler.mp. (5296)
-
R? Test Evolution.mp. (2)
-
R? Test.mp. (981)
-
reveal device.mp. (1)
-
implantable loop recorder.mp. (154)
-
diagnostic imag$.mp. (29,793)
-
Ultrasonography/ or diagnostic ultrasound.mp. (59,099)
-
ultrasonic diagnosis.mp. (1607)
-
magnetic resonance imaging.mp. or Magnetic Resonance Imaging/ (253,683)
-
or/16-42 (458,448)
-
exp “Sensitivity and Specificity”/ (324,293)
-
sensitivity.tw. (420,807)
-
((pre-test or pretest) adj probability).tw. (940)
-
post-test probability.tw. (261)
-
predictive value$.tw. (51,868)
-
likelihood ratio$.tw. (6225)
-
or/44-49 (671,637)
-
15 and 43 and 50 (3735)
Appendix 6 Description of included studies
| Study | Setting | Age (years) | TTEf/TTEh | TTE details | Reference standard | Reference standard details |
|---|---|---|---|---|---|---|
| Akosah 1998107 | Hunter Holmes McGuire Veterans Affairs Medical Centre, USA | Mean 67, range 40–85 | TTE | TTE was performed using a single and multiplane probe; no further details | TOE | Studies were initially performed using a single-plane probe and later evaluation was performed using a multiplane probe |
| Aschenberg 1986125 | Hospital, Germany | Mean 51 | TTEf | A 2.25-MHz transducer connected to a Diasonics 3400 R phased-array sector scanner was used for the TTE study. The size of the left atrium and ventricle and the morphology and mobility of the mitral valve leaflets were assessed from the standard parasternal and apical views | TOE | TOE was performed with a 3.5-MHz phased-array transducer attached to the tip of a commercial 9-mm gastroscope connected to the same Diasonics sector scanner. With the patient lightly sedated (5–10-mg diazepam), lying supine or slightly upright and monitored by means of a three-lead ECG, the gastroscope was introduced into the oesophagus with the transducer facing anteriorly. After the orientational landmark of the aortic valve had been passed at a distance of 35–40 cm from the patient’s teeth, the left ventricular inflow tract was imaged by means of a 20° left lateral rotation of the gastroscope and further advancement by about 20 mm |
| Baur 1982108 | Hospital, USA | Mean 56 | TTEf | Two-dimensional TTE was performed with a commercially available 80° phased-array sector scanner (Varian 3000). Examination was performed in supine and left lateral decubitus positions with a standard 2.25-MHz transducer. Each patient was examined in the parasternal apical and subxiphoid positions and standard long- and short-axis views as well as four-chamber and two-chamber views | Left ventriculography and coronary angiography | Left ventriculography and coronary angiography were accomplished with the Judkins technique in each case. Additionally, each patient had a complete physical examination and a 12-lead ECG |
| Belkin 2011109 | Hospital, USA | Range 19–73 | TTEf | TTE was performed with the Hewlett-Packard Sonos 500 or 1500 system using a 2.5-MHz transducer in all accessible standard views; directed colour Doppler flow imaging of the interatrial septum was performed in all views in which the structure was visualised | TOE | TOE was performed with a 5-MHz single-plane probe. All patients received topical anaesthesia. The echoscope was inserted into the oesophagus with a patient lying in the left lateral decubitus position. Colour flow imaging of the interatrial septum was performed at multiple depths; microcavitation contrast was performed via an injection of 8–10 ml of agitation saline into a intravenous catheter inserted into the arm. Patients were instructed to perform the Valsalva manoeuvre during all contrast injections |
| Black 1991154 | Hospital, Australia | Mean 59, range 18–90 | TTEf | Patients underwent two-dimensional and Doppler (including colour flow mapping) TTE immediately before TOE with the use of a 2.5-MHz transducer (Hewlett-Packard 77020AC). The left atrial dimension was determined by standard M-mode criteria | TOE | TOE was performed with a 5-MHz single-plane phased-array transducer (Hewlett-Packard 21236A). Intravenous sedation was given to 289 patients (72%) using midazolam and fentanyl. Sixty patients (15%) received antibiotic prophylaxis. The hypopharynx was sprayed with 10% topical lidocaine and the probe introduced using standard techniques |
| Black 1991155 | Hospital, Australia | Mean 60, range 25–86 | TTEf | All patients had initial conventional cross-sectional and Doppler TTE, including colour flow mapping, with 1- to 9-MHz, 2- to 5-MHz and 3- to 5-MHz transducers (Hewlett-Packard 77020A) | TOE | TOE was performed with a standard 5-MHz single-plane phased-array transducer (Hewlett-Packard 21236A). Intravenous sedation (midazolam with fentanyl) was given in 74% of studies |
| Blum 2004145 | Poria Medical Centre, Israel | Mean 57 | TTEf | No details | TOE | No details |
| Chen 1992139 | Hospital, Taiwan | Mean 39, range 17–68 | TTEf | All echocardiographic examinations were performed using an imaging system (Toshiba SSH-65A). A 2.5- or 3.0-MHz phased-array transducer was used for transthoracic examinations, whereas transoesophageal studies were carried out with a 3.75-MHz phased-array transducer fitted to the conventional 10.5-mm endoscope. Routine M-mode and two-dimensional images were assessed by the standard parasternal and apical views. Special attention was paid to the atrial septum using the apical and/or subcostal four-chamber views. Contrast echocardiography was then performed by injecting agitated 5% glucose solution through a 21-gauge winged infusion set into a peripheral vein. At least three injections were given to each patient to obtain optimal contrast effect in the resting state and during the release phase of a Valsalva manoeuvre (phase 3) | TOE | TOE was carried out subsequently to obtain the four-chamber view. After the fossa ovalis of the atrial myocardium septum was visualised, echo contrast was injected both during normal breathing and just before the release of a Valsalva manoeuvre. Shrinkage of the heart observed during the Valsalva manoeuvre indicates the efficiency of this manoeuvre. All studies were interpreted by at least two physicians with full experience of contrast echocardiography who had no previous information about the patient’s status. Colour Doppler imaging was not performed in the present series |
| Chirillo 2005149 | Hospital, Italy | Mean 46 | TTEf and TTEh | All studies were performed with a Sequoia 256 system with a 3-V transducer. Each cardiac valve was examined in detail by M-mode, two-dimensional and Doppler colour flow mapping at minimum depth setting. TTE images were acquired in the fundamental imaging mode at the highest possible transducer frequency that still allowed clear delineation of valve structures. Gain settings were adjusted individually for each patient to visualise valve structures optimally. After all cardiac valves had been examined the transducer was switched into the harmonic mode with a transmitting frequency of 1.75 MHz. The receiver gain was again adjusted individually for each patient to obtain the best visualisation of the cardiac valves | TOE | TOE studies were performed after precordial examination by the same operator. Patients were positioned in the left lateral decubitus position after topical anaesthesia of the pharynx with lidocaine. An omniplane transducer with 5- to 7-MHz transmitting frequency was used. Valves were imaged in all available imaging planes at the highest possible frequency. Each valve was examined by M-mode echocardiography with a 100 cm/s sweep, two-dimensional cross-sectional echocardiography and colour Doppler. As with TTE, four 3-second clips with the best achieved image resolution were acquired for each valve and were stored digitally |
| Clarke 2004143 | Hospital, UK | Mean 58 | TTEh | TTE was performed immediately before TOE. All studies were performed using a Hewlett-Packard Sonos 5500, using a broadband transthoracic transducer capable of second harmonic imaging (Hewlett-Packard S4 with 1.8/3.6 MHz). With harmonic imaging, ultrasound is transmitted at a fundamental frequency (1.8 MHz) and then echoes at the second harmonic frequency are selectively detected (3.6 MHz). Routine images were obtained: parasternal long- and short-axis, apical four-chamber, apical two-chamber and subcostal views using harmonic imaging. Continuous recording was obtained during bubble contrast injections with an apical four-chamber view. Following recordings during normal respiration, this was repeated during a Valsalva manoeuvre | TOE | TOE was performed using 10% topical lignocaine spray for the oropharynx and intravenous sedation (midazolam 3–10 mg). A Hewlett-Packard Sonos 5500 ultrasound machine with an omniplane 5-MHz transoesophageal probe was used in all cases. Patients underwent a complete TOE study including colour flow Doppler of the interatrial septum. The TOE was performed in the left lateral decubitus position |
| Cujec 1991152 | Hospital, Canada | Mean 63, range 18–87 | TTEf | Transthoracic colour Doppler echocardiography was performed on the same day as TOE. Standard parasternal and apical views were obtained using an Aloka 870 imaging system interfaced with a 2.5- or 3.5-MHz transducer. Intravenous saline contrast was not given during TTE | TOE | Transesophageal colour Doppler echocardiography was performed after the patient gave informed consent. A biplane transoesophageal 5-MHz transducer interfaced with the Aloka 870 imaging system was used. A complete biplane transoesophageal examination was performed in all patients. Thirty patients who had an intravenous line inserted for sedation also had saline contrast injected during TOE to exclude an intracardiac shunt |
| Daniels 2004140 | Four clinical centres, Belgium | Mean 63 | TTEh | TOE and TTEh with the consecutive administration of three intravenous contrast injections of agitated saline injections before the release phase of the Valsalva manoeuvre were performed. Semiquantification and timing of contrast passage were assessed during both imaging modalities. A shunt was present if at least one imaging modality showed micro-bubbles appearing in the left atrium. PFO was defined when these bubbles appeared early and arteriovenous pulmonary malformations were suspected if bubbles appeared late after the opacification of the right atrium. Shunts were considered important when bubbles were present in one frame in the left atrium or left ventricle | TOE | See TTE details |
| de Bruijn 2006141 | Hospital, Holland | No details | TTE | TTE was performed in the left lateral decubitus position using a commercially available system (Vingmed system FiVe/Seven, General Electric-Vingmed). Images were obtained using a 3.5-MHz transducer at a depth of 16 cm in the parasternal (standard long- and short-axis images) and apical (standard long-axis and two- and four-chamber images) views. Standard two-dimensional and colour Doppler data, triggered to the QRS complex, were saved in cine loop format. Pulsed- and continuous-wave Doppler data were also stored digitally. Data were analysed using commercial software (Echopac 6.1, General Electric-Vingmed) | TOE | TOE was performed without sedation using a 5.0-MHz multiplane transducer; lidocaine spray was used for local pharyngeal anaesthesia. TOE was performed according to a standardised protocol including adequate visualisation of all cardiac structures with emphasis on both atria, left atrial appendage, interatrial septum, mitral valve apparatus and thoracic aorta; administration of intravenous sterile isotonic saline was used to assess atrial septal defects. Echo contrast with air (ratio 9 : 1) and a subsequent Valsalva manoeuvre was used to evaluate the presence of a PFO. All patients were instructed to perform a Valsalva manoeuvre just before the injection of the contrast and to release on command after arrival of contrast in the right atrium. The Valsalva manoeuvre was considered successful if the interatrial septum in the fossa ovalis region showed a leftward deviation. A moderate to severe shunt secondary to PFO was defined as passage of a cloud of bubbles or intense opacification of the left atrium. All patients underwent TTE directly followed by TOE, which were performed by experienced sonographers according to a predefined protocol |
| Di Tullio 1993110 | Hospital, USA | Mean 63.6 | TTEf | Echocardiography was performed using Hewlett-Packard Sonos 1000 equipment with a 2.5-MHz transducer for transthoracic imaging and a 5.0-MHz biplane transducer for transoesophageal imaging | TOE | See TTE details |
| Fatkin 1996156 | Hospital, Australia | Mean 60, range 38–74 | TTEf | TTE was performed with a Hewlett-Packard Sonos 1000 or Acuson XPlO ultrasonograph using a 2.5- MHz transducer | TOE | TOE was performed using a biplane probe (Hewlett-Packard P21363A or Acuson V510B) in 57 patients and a multiplane probe (Hewlett-Packard 21364A) in three patients. After informed written consent was obtained, fasted patients received topical anaesthesia of the hypopharynx with 10% lidocaine spray and were sedated with midazolam hydrochloride 1–4 mg intravenously (plus fentanyl citrate 50–100 pg and glycopyrrolate 0.2 mg in one centre). In six patients TOE was performed intraoperatively after general anaesthesia but before cardiopulmonary bypass |
| Gonzalez-Alujas 2011146 | Hospital, Spain | Mean 46.4, range 17–75 | TTEh | TTE was performed using the Vivid 7 system (General Electric) fitted with a 4.3-MHz multifrequency probe with harmonic imaging. The apical four-chamber view was used to optimise visualisation of the atria, ventricles and interatrial septum. Three patients had a suboptimal acoustic window but were not excluded from the study. Atrial septal aneurysm was diagnosed when there was a 10-mm midline shift in anatomical M-mode or when the total bidirectional shift was > 15 mm | TOE | TOE with colour Doppler was performed using the same system fitted with a 2.9- to 8-MHz multifrequency probe. Patients were sedated with intravenously administered midazolam at a starting dose of 2 mg followed by 2-mg increments until tolerance was reached. Baseline values were recorded during TTE and once every minute during TOE. An N-550 pulse oximeter (Nellcor) and an automatic M4-I Intellisense blood pressure gauge (Omron) were used |
| Gutiérrez-Chico 2008147 | Hospital, Spain | Mean 59, range 15–92 | TTEh | A complete three-dimensional echocardiographic study was performed including parasternal and apical real-time views of the mitral valve, apical full-volume acquisition plus systematic cropping, and three-dimensional colour views | TOE | TOE was performed with a Philips Sonos 5500 system and Philips T6H probe. Three-dimensional echo was performed with a Philips Sonos 7500 system and X4 matrix probe. Investigators performing TOE were blinded to the three-dimensional results. TOE was performed according to a standard protocol, using the guidelines of the American Society of Echocardiography. A scallop was considered prolapsing according to the criteria defined in this protocol |
| Ha 2000136 | Setting unclear, South Korea | Mean 51 | TTEh and TTEf | Parasternal long-axis and apical four-chamber views were obtained with the use of functional imaging and harmonic imaging sequentially. Harmonic mode denotes that the imaging system is programmed to transmit at one frequency and receive at twice that frequency – its second harmonic. Fundamental mode refers to the standard acquisition and signal processing of b-mode images. Fundamental imaging was performed with the broadband 2- to 4-MHz Sonos 5500 Hewlett-Packard transducer with a fusion setting of 1, 3, or 4 depending on which resulted in the best image quality | TOE | TOE was performed with a 5-MHz phased-array transducer attached to the tip of a commercially available gastroscope (Hewlett-Packard Sonos 5500). The patients who had fasted for at least 4 hours before the examination received mild local pharyngeal anaesthesia immediately before the gastroscope was inserted. TOE was performed in the supine and lateral positions |
| Ha 2001137 | Setting unclear, South Korea | Mean 59, range 24–89 | TTEh | TTE was performed with a Hewlett-Packard Sonos 5500 and broadband (2- to 4-MHz) transducer. After obtaining an optimal apical four-chamber view with good delineation of both atria, the interatrial septum and both ventricles, 10 ml of agitated saline was rapidly injected into a right antecubital vein through an 18-gauge venous cannula. In each patient, TTE with functional imaging and harmonic imaging and agitated saline contrast injection were performed during normal respiration and during the Valsalva manoeuvre. If contrast bubbles reached the right atrium the patient was asked to perform the Valsalva manoeuvre. After the contrast bubbles had completely cleared from the right-sided cardiac chambers the transducer was switched into the harmonic mode, holding the probe in the same position as in the functional imaging mode. Thereafter, contrast injections were repeated. Functional imaging was performed using the broadband (2- to 4-MHz) transducer with a fusion setting of either 1, 3 or 5, depending on which setting resulted in the best image quality. Harmonic imaging was acquired with the same transducer using transmit and receiving frequency settings of 2.1 and 4.2 MHz | TOE | All patients underwent TOE using a 4- to 7-MHz multiplane probe. Patients received local pharyngeal anaesthesia with 10% topical lidocaine and performed the Valsalva manoeuvre before the procedure; its effectiveness was verified by a reduction in ventricular and atrial size and by bulging of the interatrial septum into the left atrium |
| Hirata 2008111 | Hospital, USA | Mean 57 | TTEh | TTE diagnosis of mitral valve prolapse was made by measurement of maximal mitral leaflet superior systolic displacement relative to the line connecting the annular hinge points (displacement > 2 mm). Displacement of the anterior and posterior mitral leaflets was measured in the parasternal and apical long-axis views, which were scanned by tilting the transducer to visualise the medial, middle and lateral scallops of the posterior leaflets. All of the displacements were always confirmed in the other views. Real-time three-dimensional imaging was performed on all mitral valve prolapse patients using a 2.5-MHz (X4) matrix array transducer on the Philips Sonos 7500 ultrasound machine, version 5.1. The X4 transducer provides live RT3D images as well as full-volume acquisition. In live RT3D image mode, the image was displayed as a quadrangular pyramidal image in real time. In the full-volume acquisition mode, four wedges were collected over eight consecutive cardiac cycles during a breath hold with ECG gating. The three-dimensional image volume was obtained in parasternal and apical views using these modes | TOE | All TOE studies were performed using a 5.0-MHz multiplane transducer interfaced with the Sonos 5500 or 7500 ultrasound machine (Philips). Sedation was achieved with the intravenous administration of midazolam and meperidine. The mitral valve was examined using mid-oesophageal four-chamber, commissural, two-chamber, long-axis and transgastric views according to the American Society of Echocardiography criteria. The presence of prolapse was defined as ‘any portion of the mitral valve that moved above the mitral annulus during systole’. The mitral valve was divided into six segments: three anterior leaflet scallops defined as lateral (A1), middle (A2) and medial (A3) and three posterior leaflet scallops defined as lateral (P1), middle (P2) and medial (P3) |
| Hubail 2011112 | Hospital, USA | Mean 9.5, range 1.2–8.6 | TTEf and TTEh | Transducer type was chosen to obtain the optimal balance between spatial resolution (higher frequency) and penetration (lower frequency) using anywhere from 3- to 8-MHz transthoracic probes. Harmonics were used if the quality of the images was improved by this modality. Two-dimensional and colour Doppler images were obtained from subcostal, apical and parasternal views on TTE. If no interatrial communication was detected, a contrast study was performed with a 5-ml agitated saline injection in patients < 20 kg and 10 ml in larger patients. If no shunt was detected, the agitated saline injection was repeated with a Valsalva manoeuvre in co-operative patients | TOE | TOE was subsequently performed obtaining two-dimensional colour Doppler images using the TE-V7M probe in patients < 20 kg and the TE-V5Ms probe in larger patients. If required, agitated saline injections with and without the Valsalva manoeuvre |
| Illien 2002126 | Hospital, Germany | Age range 57–67 | TTEh | For TTE a 3.4-MHz transducer was used with a harmonic frequency at 1.7 MHz. All patients were examined in the left lateral, decubitus position. A one-lead ECG was recorded continuously. The M-mode left atrial dimension was measured at end-systole in the parasternal long-axis view and the left ventricular ejection fraction was determined according to the recommendations of the North American Society of Echocardiography | TOE | TOE was performed with a 6.7-MHz multiplane transducer. The oropharynx was anaesthetised with lidocaine spray and a viscous lidocaine solution was used to cover the tip of the transoesophageal probe. When needed, 2.5–5 mg of midazolam was injected for sedation. The probe was placed in the mid-oesophagus behind the left atrium and a transoesophageal four-chamber view was then employed |
| Jassal 2007153 | Hospital, Canada | Mean 57 | TTEh | All studies were performed with a Vivid 7 system (GE Medical Systems). TTEh was performed first using a 1.5- to 1.7-MHz transducer | TOE | All studies were performed with a Vivid 7 system. TOE was performed within 24 hours of TTEh using a 4.5- to 6.2-MHz multiplane transducer in all patients |
| Jax 2010127 | Germany, Hospital | No details | TTE | No details | TOE | No details |
| Kerr 2000115 | Hospital, USA | Mean 59, range 34–76 | TTEf and TTEh | TTE patients were supine in the partial left lateral position using one echocardiographic machine (Agilent Technology Sonos 5500, S4 transducer). For all saline contrast studies 10 ml of saline was agitated with 0.2 ml of air between two 10-ml syringes mounted on a three-way stopcock and injected rapidly through a 20G cannula in the right antecubital vein. Patients were tutored in the performance of the Valsalva manoeuvre and had several trial performances to maximise manoeuvre intensity and timing. The manoeuvre was continued for 5 seconds and release was co-ordinated with opacification of the right atrium | TOE | TOE patients had fasted for 6 hours and received topical pharyngeal anaesthetic (midazolam 1–7 mg and demerol 0–50 mg) for sedation. The TOE examination was performed with an Agilent Technology Sonos 5500 echocardiographic machine at 5.0 MHz with a multiplane transducer. The interatrial septum was carefully studied in multiple planes for evidence of separation of the septum primum from the septum secundum consistent with the diagnosis of a PFO. The same interrogation was performed using colour Doppler with the colour scale reduced to maximise detection of low-velocity flow across the interatrial septum. When a PFO was seen, the plane in which the separation of septum primum and septum secundum was best seen was imaged for at least 10 cardiac cycles and the maximum separation of the limiting orifice was measured. Two saline contrast studies were performed: the first at rest and the second with release of 5 seconds of abdominal compression on complete right atrial opacification |
| Kitayama 1997138 | Hospital, Japan | Mean 68 | TTEf | TTE studies, including M-mode echocardiography, two-dimensional imaging and pulsed and colour Doppler echocardiography, were performed in all 70 patients with use of a Toshiba Sonolayer SSH-140A system with a 2.5- or 3.75-MHz transducer. To detect intracardiac thrombi, two-dimensional echocardiograms were obtained with the transducer in the parasternal, apical and subcostal positions. Thrombus was defined as a mass of echoes in at least two views of the cardiac cavity, seen throughout the cardiac cycle, contiguous with the cardiac wall. The left atrial dimension was measured in the parasternal long-axis view | Cardiac ultrafast CT | Cardiac ultrafast CT was performed using an Imatoron C-100XL system with a matrix size of 512 × 512 cm and a field of view of 30 cm, which resulted in a pixel size of 0.36 mm2. Patients were placed in the supine position on the scanner couch. An intravenous catheter (20 gauge) was inserted into the right antecubital vein for contrast medium injection (Iomeron 350). Contrast medium was administered at a rate of 2.5 ml/second (total dose 80–100 ml) to facilitate endocardial border identification |
| Kuhl 1999128 | Hospital, Germany | Mean 56, range 20–86 | TTEf and TTEh | TTE studies were performed following the TOE study at least 3 minutes after contrast bubbles had disappeared from right heart chambers. There was no change in the patient position between the TOE and TTE studies. For the transthoracic cerebrovascular event study an apical four-chamber view with optimal delineation of both atria, the interatrial septum and both ventricles was selected. TTE images were acquired in the fundamental imaging mode using the highest possible transducer frequency that still allowed clear delineation of the cardiac morphology. After contrast bubbles had completely cleared from the right heart chambers the transducer was switched into the harmonic mode, holding the probe in the same position as in the fundamental imaging mode. Thereafter, contrast injections were repeated. All echocardiographic studies were recorded on S-VHS videotape for offline analysis | TOE | TOE was performed using colour Doppler using a multiplane probe (Omni II, Hewlett-Packard). The patient was positioned in the left lateral decubitus position after topical anaesthesia of the pharynx with lidocaine. All patients were mildly sedated with intravenous administration of 2–3 mg of midazolam; contrast injections were performed in the transoesophageal four-chamber view in the 0° image plane of the transducer. Care was taken to visualise optimally the left atrium, the left ventricle and the interatrial septum. Additional contrast injections were performed in 40–60° image planes and/or 110–130° image planes as needed to demonstrate clearly the site of contrast passage through the interatrial septum |
| Lee 1991114 | Hospital, USA | Mean 63, range 20–82 | TTEf | M-mode and two-dimensional TTE were performed with the patient in the left lateral decubitus position using a 77020A imaging system (Hewlett-Packard) including a 2.5- or 3.5-MHz transducer. Parasternal long- and short-axis views, apical views and subxiphoid views were obtained | TOE | TOE was performed with the patient in the left lateral decubitus position using the Hewlett-Packard 77020A ultrasound imaging system, including the Hewlett-Packard 21362A 5-MHz single-plane transoesophageal transducer. Basal short-axis views, four-chamber views and transgastric short-axis views were obtained. Limb leads were placed on each patient to obtain a simultaneous ECG rhythm strip |
| Lembcke 2009129 | Hospital, Germany | Mean 68.4 (SD 10.4) | TTEh | TTE was performed by a trained cardiologist or cardiac surgeon according to the recommendations of the American Society of Echocardiography. An ultrasound unit with a 2.5-MHz, 128-element, phased-array transducer was used (Vivid 7, General Electric Healthcare) and images were acquired by using standard imaging windows with short breath holds if needed | Cardiac catheterisation | Cardiac catheterisation was performed in a standardised fashion by an expert cardiologist. Using the percutaneous femoral approach, peak-to-mean and mean transvalvular gradients were routinely determined during a pull-back manoeuvre after retrograde crossing of the valve or alternatively by simultaneous measurements with two catheters, one placed trans-septally into the left ventricle and a second placed in the ascending aorta |
| Li 2009135 | Hospital, China | Mean 51, range 43–73 | TTE – no further details | The transthoracic 2-DE examination was carried out using a Sonos 7500 ultrasonographic system (Philips). On two-dimensional echocardiography examination, multiple views, including apical four- and two-chamber views, apical long-axis views and parasternal long-axis and short-axis views, were used to display the left ventricular wall motion. Left ventricular aneurysm was diagnosed if the localised portion of the left ventricular cavity was found to have (1) akinesis or dyskinesis; (2) protrusion outside during the systolic phase; and (3) a wide orifice and continuity in the ventricular wall | Left ventriculography | Left ventriculography is considered the gold standard in the determination of left ventricular aneurysm. An XR Advantx LCV+ Angiographic System (GE Healthcare) was used to perform left ventriculography. According to the methods used by al-Saadon185 and Lee et al.,114 a left ventricular aneurysm is a motion disturbance of the myocardium in which a part of the left ventricular wall shows localised akinesia or dyskinesia during the systolic phase of a cineangiogram |
| Lipke 2007130 | Hospital, Germany | Mean 63 (SD 11) | TTEh | All echocardiographic studies were performed by sonographers with > 7 years’ experience in scanning. All studies were performed on a Vivid 7 (General Electric) echo machine. A standard transducer (M3S) with harmonic capabilities was used. For all studies the Octave mode was applied using frequencies ranging from 1.7 to 2.0 MHz (emit) and from 3.4 to 4.3 MHz (receive) | MRI | Contrast-enhanced MRI was carried out with a 1.5-T scanner (Intera, Philips) using a five-element phased-array cardiac coil and electrocardiographic triggering |
| Madala 2004115 | Hospital, USA | Range 21–88 | TTEh | Agitated saline contrast injection was given intravenously as a 5- to 10-ml bolus injection during TOE, TTEf and TTEh | TOE | During TOE, contrast imaging was carried out in the bicaval view at approximately 90° probe rotation, visualising both the superior and the inferior vena cavae along with the fossa ovalis. All studies were performed with the Agilent Sonos 5500 or Acuson ultrasound system |
| Maffè 2010150 | Hospital, Italy | Mean 49, range 36–62 | TTEh | The TTE studies were performed with a Philips iE33 platform, with a S5–1 transducer (from 5 to 1 MHz) for two-dimensional examination and a X3–1 transducer (from 3 to 1 MHz) for three-dimensional examination. An apical four-chamber view with optimal delineation of both atria, the interatrial septum and both ventricles was selected. Continuous recording was obtained during bubble contrast injections, in basal conditions, and during a Valsalva manoeuvre | TOE | TOE studies were performed with the omniplane MPT7–4 transoesophageal probe of the ATL HD 5000 ultrasound machine. During TOE, the patient was positioned in the left lateral decubitus, using 10% topical lignocaine spray for the oropharynx and eventually intravenous sedation (midazolam 3 mg) |
| Mugge 1995131 | Setting unclear, Germany | Mean 54, range 18–85 | TTEf | No details | TOE | No details |
| Musolino 200361 | Hospital, Italy | Mean 36, range 17–45 | TTEf | TTE studies were carried out using a Vingmed 700 CFM system and since 1993 a Vingmed 800 CFM system | TOE | TOE studies were carried out using a monoplane and since 1995 a multiplane mechanical transducer (Vingmed) |
| Nemec 1991116 | Hospital, USA | Mean 50, range 22–78 | TTEf | Standard TTE and TOE examinations using commercially available machines were performed using two-dimensional, Doppler and colour Doppler evaluations; imaging after contrast injection was performed during normal respiration and during a Valsalva manoeuvre | TOE | See TTE details |
| Neuman 2003117 | Medical centre/hospital, USA | Mean 78 | TTEf | Mitral regurgitation was assessed by colour flow Doppler mapping using the methods of Helmcke et al.186 | TOE | See TTE details |
| Omran 1999132 | Hospital, Germany | Mean 54 | TTEf | TTE was performed with a phased-array 3.3-MHz transducer with 128 elements used. All patients were examined in the left lateral decubitus position. A single-lead ECG was simultaneously recorded. The left atrium was imaged in the standard, parasternal short- and long-axis and apical transducer positions. The left atrial appendage was imaged as described by Herzog et al.187 Digital image processing and storage were used | TOE | TOE was performed with a 5-MHz multiplane transducer. We used topical lignocaine spray and viscous lignocaine solution to anaesthetise the oropharynx before the transoesophageal study |
| Pearson 1991118 | Hospital, USA | Mean 59, range 17–84 | TTEf | All patients underwent TTE and TOE with contrast administration and Doppler colour flow imaging. TTE was performed within 3 days (usually 24 hours after TOE) using several commercially available ultrasound systems | TOE | See TTE details |
| Pop 1990142 | Hospital, Netherlands | Mean 60, range 24–73 | TTEf | TTE was performed with a Toshiba SSH-65A imaging system using 2.5- and 3.75-mHz probes | TOE | TOE was performed systemically and lasted generally for approximately 15 minutes. After introduction, the probe was manipulated until it was located in the stomach and then a series of cross-sectional short axis of the left ventricle views were recorded. The probe was pulled back within the oesophagus until a proper four-chamber view was obtained. In this section attention was focused on the mitral valve and its chordae and the aortic valve and the aortic root were visualised, orienting the probe superiorly |
| Roldan 2008119 | Hospital, USA | Mean ∼37 | TTEh | TTE and TOE were separately videotaped or digitally acquired for offline interpretation. Standard two-dimensional views were obtained at a depth of 8–12 cm for TTE and 4–6 cm for TOE with a narrow sector scan to improve image resolution of the heart valves | TOE | See TTE details |
| Sallach 2009120 | Setting unclear, USA | Mean 67 | TTEf and TTEh | TTE studies were performed using an ATL-Philips 5000 echocardiographic system. The left atrial appendage was first examined using fundamental imaging to assess the left atrial appendage area and the presence of thrombus. Harmonic imaging was then used to evaluate the left atrial appendage area and the presence of thrombus. Harmonic imaging was repeated with a lower mechanical index range of 0.4–0.6 following a single intravenous bolus of Optison | TOE | TOE studies were performed using an ATL-Philips 5000 echocardiographic system |
| Shub 1983121 | Hospital, USA | Mean ∼31, range ∼2 months–74 years | TTEf | Two-dimensional echocardiographic equipment used in the study included commercially available 80° phased-array scanning systems with 2.25- and 3.5-MHz transducers and a mechanical sector scanner with 3- and 5-MHz transducers | Surgical and cardiac catheterisation | No details |
| Siostrzonek 1991134 | Hospital, Austria | Mean 52 | TTEf | TTE and TOE were performed with a Vingmed CFM700 system using a 3.5-MHz and 5-MHz transducer for TTE and TOE respectively. After obtaining optimal visualisation of the atrial septum a bolus of 2–5 ml of a hand-agitated 5.5% solution of oxypolygelatine was injected into a large cubital vein over an in-dwelling 18-gauge cannula; subsequently the appearance of contrast agent in the right atrium was monitored and recorded on videotape. Contrast studies were performed during normal breathing and during Valsalva manoeuvre | TOE | See TTE details |
| Stendel 2000133 | Hospital, Germany | Mean 51, range 25–72 | TTEf | Contrast-enhanced TTE was performed using a 2.5-MHz monoplane electrical transducer and the Ultramark 9 system with the awake patient lying on his or her left side and the upper part of the body elevated by 30°. No sedation was used. The heart was imaged in a four-chamber view. A 10-ml bolus dose of echo-contrast medium was injected into the right cubital vein. The Valsalva manoeuvre was performed 5 seconds after the injection of the echo-contrast medium | TOE | Contrast-enhanced TOE was performed using a 5-MHz monoplane electrical transducer and the Ultramark 9 system with the awake patient lying on his or her left side and the upper part of the body elevated by 30°. Local anaesthesia of the pharynx was performed using lidocaine spray. The ultrasound probe was also prepared with 2% lidocaine gel |
| Stratton 1982122 | Hospital, USA | Mean ∼58 | TTEf | Two-dimensional echocardiography was performed using either a wide-angle, phased-array sector scanner (Toshiba, 45 patients) or a wide-angle, mechanical sector scanner (ATL Laboratories, 33 patients). Parasternal long- and short-axis and apical two- and four-chamber views were obtained using standard transducer positions. In most studies, non-standard views were also obtained using apical and low parasternal echocardiographic windows to examine the apex more thoroughly | Autopsy, aneurysmectomy and unequivocally positive indium-111 platelet imaging | No details |
| Thanigaraj 2005123 | Hospital, USA | Mean 45, range 18–84 | TTEh | TTE was performed using second-harmonic imaging (transmitting frequency 1.8–2 MHz, receiving frequency 3.6–4 MHz). Studies were carried out with the Sonos 5500 (Phillips), Sequoia C256 (Siemens) or Vivid 7 (General Electric) imaging systems | TOE | All TOE studies were performed using a Sequoia multiplanar transducer (Siemens) with fundamental imaging modality (transmitting frequency 3.5–7 MHz). Saline contrast injections and colour Doppler evaluations were performed in the 90° (bicaval) view to document right-to-left atrial shunting |
| Trevelyan 2006144 | Hospital, UK | 55, range 22–80 | TTEh | TTE for the detection of a PFO was carried out using a Hewlett-Packard Sonos 5500 imaging system with second harmonic imaging. Imaging was performed in the apical four-chamber view with injection of 10 ml of agitated saline (9 ml saline, 0.5 ml blood, 0.5 ml air repeatedly agitated through a three-way tap), which achieved opacification of the right heart in all cases | TOE | TOE was performed under local anaesthesia and sedation with midazolam and the procedure repeated as for TTE with the interatrial septum imaged in the 110–130° plane |
| Vincelj 2001148 | Hospital, Croatia | Mean 55.3 | TTE | TTE was performed with a Toshiba SSH 160A imaging system | TOE | TOE was performed either with a Hitachi Ultrasound scanner EUB-555 with a 3.5-MHz biplane transducer, with an ATL 3000 scanner (Universal Diagnostic Solutions) or with a 5000 HDI ultrasound scanner (Philips). Patients were studied in the fasting state after application of topical anaesthesia of the hypopharynx with 10% lidocaine spray and intravenous sedation with diazepam. The oesophageal probe was inserted with patients in the left lateral decubitus position |
| Weinsaft 2011124 | Setting unclear, USA | Mean 60 | TTEh | Two-dimensional TTE ECGs were obtained by experienced sonographers on commercially available equipment (Sonos 5500 or 7500, Philips) with phased- and sector-array transducers. Echoes were acquired in standard parasternal short- and long-axis as well as apical two-, three- and four-chamber imaging planes in accordance with American Society of Echocardiography consensus guidelines | Delayed enhancement cardiac MRI | MRI was undertaken with delayed enhancement using 1.5-T scanners (Siemens Sonata or Avanto) |
| Zito 2009151 | Setting unclear, Italy | Mean 49 | TTEh | A baseline TTE examination was performed with an Aloka ProSound Alpha 10 imaging system using a 3-MHz probe according to standard practice guidelines with the patient in the left lateral position. An apical four-chamber view with optimal visualisation of both the atria, ventricles, and atrial septum was selected and the gain setting was adjusted to analyse the fossa ovalis area | TOE | A TOE study was performed using a Vivid 7 machine (General Electric) with a 5.0-MHz multiplane probe according to a standard protocol including colour flow Doppler data. The atrial septum was analysed from the transverse mid-oesophageal four-chamber view to the longitudinal biatrial–bicaval view. Fourteen patients had a BMI > 30 kg/m2 |
Appendix 7 Diagnostic accuracy excluded studies
| Reason for exclusion | Study |
|---|---|
| No usable data | 1–72 |
| No concordance between groups | 73–78 |
| No relevant cardiac pathologies | 79–90 |
| Not diagnostic accuracy study | 91–117 |
| Not English language | 118–132 |
| No relevant comparator | 133–137 |
References
- Abdulla JS. Evaluation of aortic valve stenosis by cardiac multislice computed tomography compared with echocardiography: a systematic review and meta-analysis. J Heart Valve Dis 2009;18:634-43.
- Ahmad O, Ahmad KE, Dear KB, Harvey I, Hughes A, Lueck CJ, et al. Echocardiography in the detection of cardioembolism in a stroke population. J Clin Neurosci 2010;17:561-5.
- Aune E, Baekkevar M, Rodevand O, Otterstad JE. Reference values for left ventricular volumes with real-time 3-dimensional echocardiography. Scand Cardiovasc J 2010;44:24-30.
- Bacher-Stier C, Muller S, Pachinger O, Strolz S, Erler H, Moncayo R, et al. Thallium-201 gated single-photon emission tomography for the assessment of left ventricular ejection fraction and regional wall motion abnormalities in comparison with two-dimensional echocardiography. Eur J Nucl Med 1999;26:1533-40.
- Basnet BK, Manandhar K, Shrestha R, Shrestha S, Thapa M. Electrocardiograph and chest X-ray in prediction of left ventricular systolic dysfunction. J Nepal Med Assoc 2009;48:310-13.
- Berk F, Isgoren S, Demir H, Kozdag G, Sahin T, Ural D, et al. Assessment of left ventricular function and volumes for patients with dilated cardiomyopathy using gated myocardial perfusion SPECT and comparison with echocardiography. Nucl Med Comm 2005;26:701-10.
- Bernard Y, Meneveau N, Boucher S, Magnin D, Anguenot T, Schiele F, et al. Lack of agreement between left ventricular volumes and ejection fraction determined by two-dimensional echocardiography and contrast cineangiography in postinfarction patients. Echocardiography 2001;18:113-22.
- Boussel L, Cakmak S, Wintermark M, Nighoghossian N, Loffroy R, Coulon P, et al. Ischemic stroke: etiologic work-up with multidetector CT of heart and extra- and intracranial arteries. Radiology 2011;258:206-12.
- Casella F, Rana B, Casazza G, Bhan A, Kapetanakis S, Omigie J, et al. The potential impact of contemporary transthoracic echocardiography on the management of patients with native valve endocarditis: a comparison with transesophageal echocardiography. Echocardiography 2009;26:900-6.
- Cayly M, Kanadasi M, Demir M, Acarturk E. Mitral annular systolic velocity reflects the left atrial appendage function in mitral stenosis. Echocardiography 2006;23:546-52.
- de Abreu TT, Mateus S, Carreteiro C, Correia J. Therapeutic implications of transesophageal echocardiography after transthoracic echocardiography on acute stroke patients. Vasc Health Risk Manag 2008;4:167-72.
- de Belder MA, Lovat LB, Tourikis L, Leech G, Camm AJ. Limitations of transoesophageal echocardiography in patients with focal cerebral ischaemic events. Br Heart J 1992;67:297-303.
- De Castro S, Salandin V, Cartoni D, Valfre C, Salvador L, Magni G, et al. Qualitative and quantitative evaluation of mitral valve morphology by intraoperative volume-rendered three-dimensional echocardiography. J Heart Valve Dis 2002;11:173-80.
- Gabriel LD, Paranon S, Bongard V, Bassil-Eter R, Grosjean-Guitton J, Dulac Y, et al. Quantification of mitral-valve regurgitation in a paediatric population by real-time three-dimensional echocardiography. Arch Cardiovasc Dis 2008;101:697-703.
- Grewal J, Mankad S, Freeman WK, Click RL, Suri RM, Abel MD, et al. Real-time three-dimensional transesophageal echocardiography in the intraoperative assessment of mitral valve disease. J Am Soc Echocardiogr 2009;22:34-41.
- Hind W, Rahmoui H, Keane M, Silvestry F, Sutton M, Ferrari V, et al. Failure of digital echocardiography to accurately diagnose intracardiac shunts. Am Heart J 2008;155:161-5.
- Kerut EK, Truax WD, Borreson TE, VanMeter KW, Given MB, Giles TD. Detection of right to left shunts in decompression sickness in divers. Am J Cardiol 1997;79:377-8.
- La Canna G, Arendar I, Maisano F, Monaco F, Collu E, Benussi S, et al. Real-time three-dimensional transesophageal echocardiography for assessment of mitral valve functional anatomy in patients with prolapse-related regurgitation. Am J Cardiol 2011;107:1365-74.
- Lane C, Dorian P, Ghosh N, Radina M, O’Donnell S, Thorpe K, et al. Limitations in the current screening practice of assessing left ventricular ejection fraction for a primary prophylactic implantable defibrillator in southern Ontario. Can J Cardiol 2010;26:e118-24.
- Lee KS, Appleton CP, Lester SJ, Adam TJ, Hurst RT, Moreno CA, et al. Relation of electrocardiographic criteria for left atrial enlargement to two-dimensional echocardiographic left atrial volume measurements. Am J Cardiol 2007;99:113-18.
- Lembcke A, Borges AC, Dushe S, Dohmen PM, Wiese TH, Rogalla P, et al. Assessment of mitral valve regurgitation at electron-beam CT: comparison with Doppler echocardiography. Radiology 2005;236:47-55.
- Lembcke AT, Thiele H, Lachnitt A, Enzweiler CN, Wagner M, Hein PA, et al. Precision of forty slice spiral computed tomography for quantifying aortic valve stenosis: comparison with echocardiography and validation against cardiac catheterization. Invest Radiol 2008;43:719-28.
- Lembcke A, Kivelitz DE, Borges AC, Lachnitt A, Hein PA, Dohmen PM, et al. Quantification of aortic valve stenosis: head-to-head comparison of 64-slice spiral computed tomography with transesophageal and transthoracic echocardiography and cardiac catheterization. Invest Radiol 2009;44:7-14.
- Lembcke A, Durmus T, Westermann Y, Geigenmueller A, Claus B, Butler C, et al. Assessment of mitral valve stenosis by helical MDCT: comparison with transthoracic Doppler echocardiography and cardiac catheterization. AJR 2011;197:614-22.
- Lesbre JP, Tribouilloy C. Echo-Doppler quantitative assessment of non-ischaemic mitral regurgitation. Eur Heart J 1991;12:10-4.
- Lim TK, Burden L, Janardhanan R, Ping C, Moon J, Pennell D, et al. Improved accuracy of low-power contrast echocardiography for the assessment of left ventricular remodeling compared with unenhanced harmonic echocardiography after acute myocardial infarction: comparison with cardiovascular magnetic resonance imaging. J Am Soc Echocardiogr 2005;18:1203-7.
- Lipiec P, Wejner-Mik P, Krzeminska-Pakula M, Kusmierek J, Plachcinska A, Szuminski R, et al. Gated 99mTc-MIBI single-photon emission computed tomography for the evaluation of left ventricular ejection fraction: comparison with three-dimensional echocardiography. Ann Nucl Med 2006;22:723-6.
- Lucariello RJ, Sun Y, Doganay G, Chiaramida SA. Sensitivity and specificity of left ventricular ejection fraction by echocardiographic automated border detection: comparison with radionuclide ventriculography. Clin Cardiol 1997;20:943-8.
- Maddukuri PV, Vieira ML, DeCastro S, Maron MS, Kuvin JT, Patel AR, et al. What is the best approach for the assessment of left atrial size? Comparison of various unidimensional and two-dimensional parameters with three-dimensional echocardiographically determined left atrial volume. J Am Soc Echocardiogr 2006;19:1026-32.
- Malm S, Sagberg E, Larsson H, Skjaerpe T. Choosing apical long-axis instead of two-chamber view gives more accurate biplane echocardiographic measurements of left ventricular ejection fraction: a comparison with magnetic resonance imaging. J Am Soc Echocardiogr 2005;18:1044-50.
- Malm S, Frigstad S, Sagberg E, Steen PA, Skjarpe T. Real-time simultaneous triplane contrast echocardiography gives rapid, accurate, and reproducible assessment of left ventricular volumes and ejection fraction: a comparison with magnetic resonance imaging. J Am Soc Echocardiogr 2006;19:1494-501.
- Maret E, Brudin L, Lindstrom L, Nylander E, Ohlsson JL, Engvall JE. Computer-assisted determination of left ventricular endocardial borders reduces variability in the echocardiographic assessment of ejection fraction. Cardiovasc Ultrasound 2008;6.
- Marriott KF, Forshaw A, Wright J, Pascoe R. Detection of patent foramen ovale (PFO) using agitated saline contrast imaging: experience with 1162 patients and recommendations for echocardiography. Heart Lung Circ 2011;20:S167-8.
- Marsan NA, Westenberg JJ, Ypenburg C, Delgado V, Van Bomme RJ, Roes SD, et al. Quantification of functional mitral regurgitation by real-time 3D echocardiography: comparison with 3D velocity-encoded cardiac magnetic resonance. JACC Cardiovasc Imaging 2009;2:1245-52.
- Martensson M, Winter R, Cederlund K, Ripsweden J, Mir-Akbari H, Nowak J, et al. Assessment of left ventricular volumes using simplified 3-D echocardiography and computed tomography – a phantom and clinical study. Cardiovasc Ultrasound 2008;6.
- Mehmood F, Vengala S, Nanda N, Dod H, Sinha A, Miller A, et al. Usefulness of live three-dimensional transthoracic echocardiography in the characterization of atrial septal defects in adults. Echocardiography 2004;21:707-13.
- Mele D, Soukhomovskaia O, Pacchioni E, Merli E, Avigni N, Federici L, et al. Improved detection of left ventricular thrombi and spontaneous echocontrast by tissue harmonic imaging in patients with myocardial infarction. J Am Soc Echocardiogr 2006;19:1373-81.
- Mercer-Rosa L, Seliem MA, Fedec A, Rome J, Rychik J, Gaynor JW. Illustration of the additional value of real-time 3-dimensional echocardiography to conventional transthoracic and transesophageal 2-dimensional echocardiography in imaging muscular ventricular septal defects: does this have any impact on individual patient treatment?. J Am Soc Echocardiogr 2006;19:1511-19.
- Monin JL, Dehant P, Roiron C, Monchi M, Tabet JY, Clerc P, et al. Functional assessment of mitral regurgitation by transthoracic echocardiography using standardized imaging planes. J Am Coll Cardiol 2005;46:302-9.
- Monte I, Grasso S, Licciardi S, Badano LP. Head-to-head comparison of real-time three-dimensional transthoracic echocardiography with transthoracic and transesophageal two-dimensional contrast echocardiography for the detection of patent foramen ovale. Eur J Echocardiogr 2010;11:245-9.
- Mor-Avi V, Lang RM. Echocardiographic quantification of left ventricular volume: what can we do better?. J Am Soc Echocardiogr 2008;21:998-1000.
- Muller H, Rangos C, Fleury E, Righetti A, Lerch R, Burri H. Measurement of left ventricular ejection fraction by real time 3D echocardiography in patients with severe systolic dysfunction: comparison with radionuclide angiography. Echocardiography 2010;27:58-63.
- Niakara A, Ouédraogo N, Nébié LV, Kaboré NJ, Megnigbeto CA. Routine electrocardiographic criteria for the diagnosis of left ventricular hypertrophy: performance in Black African. Ann Cardiol Angeiol 2002;51:193-8.
- Nishikage T, Nakai H, Mor-Avi V, Lang RM, Salgo IS, Settlemier SH, et al. Quantitative assessment of left ventricular volume and ejection fraction using two-dimensional speckle tracking echocardiography. Eur J Echocardiogr 2009;10:82-8.
- Nowosielski M, Schocke M, Mayr A, Pedarnig K, Klug G, Kohler A, et al. Comparison of wall thickening and ejection fraction by cardiovascular magnetic resonance and echocardiography in acute myocardial infarction. J Cardiovasc Magn Reson 2009;11.
- Oe H, Hozumi T, Arai K, Matsumura Y, Negishi K, Sugioka K, et al. Comparison of accurate measurement of left ventricular mass in patients with hypertrophied hearts by real-time three-dimensional echocardiography versus magnetic resonance imaging. Am J Cardiol 2005;95:1263-7.
- Pace L, Perrone-Filardi P, Storto G, Della Morte AM, Dellegrottaglie S, Prastaro M, et al. Prediction of improvement in global left ventricular function in patients with chronic coronary artery disease and impaired left ventricular function: rest thallium-201 SPET versus low-dose dobutamine echocardiography. Eur J Nucl Med 2000;27:1740-6.
- Palazzuoli A, Ricci D, Lenzi C, Lenzi J, Palazzuoli V. Transesophageal echocardiography for identifying potential cardiac sources of embolism in patients with stroke. Neurol Sci 2000;21:195-202.
- Pemberton J, Li X, Kenny A, Davies CH, Minette MS, Sahn DJ, et al. Real-time 3-dimensional Doppler echocardiography for the assessment of stroke volume: an in vivo human study compared with standard 2-dimensional echocardiography. J Am Soc Echocardiogr 2005;18:1030-6.
- Qin JX, Jones M, Travaglini A, Song JM, Li J, White RD, et al. The accuracy of left ventricular mass determined by real-time three-dimensional echocardiography in chronic animal and clinical studies: a comparison with postmortem examination and magnetic resonance imaging. J Am Soc Echocardiogr 2005;18:1037-43.
- Rashid H, Exner DV, Mirsky I, Cooper HA, Waclawiw MA, Domanski MJ, et al. Comparison of echocardiography and radionuclide angiography as predictors of mortality in patients with left ventricular dysfunction (studies of left ventricular dysfunction). Am J Cardiol 1999;84:299-303.
- Rauh R, Fischereder M, Spengel FA. Transesophageal echocardiography in patients with focal cerebral ischemia of unknown cause. Stroke 1996;27:691-4.
- Rovai D, Morales MA, Di Bella G, Prediletto R, De NM, Pingitore A, et al. Echocardiography and the clinical diagnosis of left ventricular dysfunction. Acta Cardiol 2008;63:507-13.
- Shahgaldi K, Gudmundsson P, Manouras A, Brodin LA, Winter R. Visually estimated ejection fraction by two dimensional and triplane echocardiography is closely correlated with quantitative ejection fraction by real-time three dimensional echocardiography. Cardiovasc Ultrasound 2009;7.
- Shiran A, Merdler A, Ismir E, Ammar R, Zlotnick AY, Aravot D, et al. Intraoperative transesophageal echocardiography using a quantitative dynamic loading test for the evaluation of ischemic mitral regurgitation. J Am Soc Echocardiogr 2007;20:690-7.
- Singh B, Manoj R, Vikas P, Bhattacharya A, Sharma Y, Mittal BR, et al. Comparison of left ventricular functional parameters measured by gated single photon emission tomography and by two-dimensional echocardiography. Hell J Nucl Med 2006;9:94-8.
- Sobkowicz B, Himle T, Haran T, Wrabec K, Mielecki T. Validation of two-dimensional echocardiography for quantifying left ventricular aneurysm: comparison with magnetic resonance imaging and evaluation during cardiac surgery. Acta Cardiol 2002;57:73-4.
- Strandberg M, Marttila RJ, Helenius H, Hartiala J. Transoesophageal echocardiography in selecting patients for anticoagulation after ischaemic stroke or transient ischaemic attack. J Neurol Neurosurg Psychiatry 2002;73:29-33.
- Sugeng L, Coon P, Weinert L, Jolly N, Lammertin G, Bednarz JE, et al. Use of real-time 3-dimensional transthoracic echocardiography in the evaluation of mitral valve disease. J Am Soc Echocardiogr 2006;19:413-21.
- Suradi H, Byers S, Green-Hess D, Gradus-Pizlo I, Sawada S, Feigenbaum H, et al. Feasibility of using real time ‘Live 3D’ echocardiography to visualize the stenotic aortic valve. Echocardiography 2010;27:1011-20.
- Takeuchi M, Nishikage T, Mor-Avi V, Sugeng L, Weinert L, Nakai H, et al. Measurement of left ventricular mass by real-time three-dimensional echocardiography: validation against magnetic resonance and comparison with two-dimensional and M-mode measurements. J Am Soc Echocardiogr 2008;21:1001-5.
- Tanaka R, Yoshioka K, Niinuma H, Ohsawa S, Okabayashi H, Ehara S. Diagnostic value of cardiac CT in the evaluation of bicuspid aortic stenosis: comparison with echocardiography and operative findings. Am J Roentgenol 2010;195:895-9.
- Tsutsui JM, Maciel RR, Costa JM, Andrade JL, Ramires JF, Mathias W, et al. Hand-carried ultrasound performed at bedside in cardiology inpatient setting – a comparative study with comprehensive echocardiography. Cardiovasc Ultrasound 2004;2.
- Tutar HE, Özçelik N, Atalay S, Derelli E, Ekici F, İmamoğlu A. Clinical and echocardiography correlations in rheumatic fever: evaluation of the diagnostic role of auscultation. Turk Kardiyol Dem Ars 2005;33:460-6.
- van den Bosch AE, Robbers-Visser D, Krenning BJ, Voormolen MM, McGhie JS, Helbing WA, et al. Real-time transthoracic three-dimensional echocardiographic assessment of left ventricular volume and ejection fraction in congenital heart disease. J Am Soc Echocardiogr 2006;19:1-6.
- Walters DL, Sanchez PL, Rodriguez-Alemparte M, Colon-Hernandez PJ, Hourigan LA, Palacios IF, et al. Transthoracic left ventricular puncture for the assessment of patients with aortic and mitral valve prostheses: the Massachusetts General Hospital experience, 1989–2000. Catheter Cardiovasc Interv 2003;58:539-44.
- Yong Y, Wu D, Fernandes V, Kopelen HA, Shimoni S, Nagueh SF, et al. Diagnostic accuracy and cost-effectiveness of contrast echocardiography on evaluation of cardiac function in technically very difficult patients in the intensive care unit. Am J Cardiol 2002;89:711-18.
- Yousry MR, Rickenlund A, Petrini J, Gustafsson T, Liska J, Hamsten A, et al. Transthoracic versus transesophageal echocardiographic determination of aortic valve calcification and valve type. Eur J Echocardiogr 2010;11:ii124-54.
- Yuda S, Inaba Y, Fujii S, Kokubu N, Yoshioka T, Sakurai S, et al. Assessment of left ventricular ejection fraction using long-axis systolic function is independent of image quality: a study of tissue Doppler imaging and m-mode echocardiography. Echocardiography 2006;23:846-52.
- Zuber M, Cuculi F, Oechslin E, Erne P, Jenni R. Is transesophageal echocardiography still necessary to exclude patent foramen ovale?. Scand Cardiovasc J 2008;42:222-5.
- Mor-Avi V, Jenkins C, Kuhl HP, Nesser HJ, Marwick T, Franke A, et al. Real-time 3-dimensional echocardiographic quantification of left ventricular volumes: multicenter study for validation with magnetic resonance imaging and investigation of sources of error. JACC Cardiovasc Imaging 2008;1:413-23.
- Moreira FC, Miglioransa MH, Hartmann IB, Rohde LE. Left atrial appendage assessment by second harmonic transthoracic echocardiography after an acute ischemic neurologic event. J Am Soc Echocardiogr 2005;18:206-12.
- Grossmann G, Wöhrle J, Kochs M, Giesler M, Hombach V, Höher M. Quantification of mitral regurgitation by the proximal flow convergence method – comparison of transthoracic and transesophageal echocardiography. Clin Cardiol 2002;25:517-24.
- Hausmann D, Mugge A, Becht I, Daniel W. Diagnosis of patent foramen ovale by transesophageal echocardiography and association with cerebral and peripheral embolic events. Am J Cardiol 1992;70:668-72.
- Leung DY, Black IW, Cranney GB, Walsh WF, Grimm RA, Stewart WJ, et al. Selection of patients for transesophageal echocardiography after stroke and systemic embolic events. Role of transthoracic echocardiography. Stroke 1995;26:1820-4.
- Seo Y, Maeda H, Ishizu T, Ishimitsu T, Watanabe S, Aonuma K, et al. Peak C-reactive protein concentration correlates with left ventricular thrombus formation diagnosed by contrast echocardiographic left ventricular opacification in patients with a first anterior acute myocardial infarction. Circulat J 2006;70:1290-6.
- Souteyrand G, Motreff P, Lusson JR, Rodriguez R, Geoffroy E, Dauphin C, et al. Comparison of transthoracic echocardiography using second harmonic imaging, transcranial Doppler and transesophageal echocardiography for the detection of patent foramen ovale in stroke patients. Eur J Echocardiogr 2006;7:147-54.
- van Camp G, Franken P, Melis P, Cosyns B, Schoors D, Vanoverschelde J. Comparison of transthoracic echocardiography with second harmonic imaging with transesophageal echocardiography in the detection of right to left shunts. Am J Cardiol 2000;86:1284-7.
- Anderson JL, Horne BD, Pennell DJ. Atrial dimensions in health and left ventricular disease using cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2005;7:671-5.
- Bicudo LS, Tsutsui JM, Shiozaki A, Rochitte CE, Arteaga E, Mady C, et al. Value of real time three-dimensional echocardiography in patients with hypertrophic cardiomyopathy: comparison with two-dimensional echocardiography and magnetic resonance imaging. Echocardiography 2008;25:717-26.
- Bilge AK, Altinkaya E, Ozben B, Pekun F, Adalet K, Yavuz S, et al. Early detection of left ventricular dysfunction with strain imaging in thalassemia patients. Clin Cardiol 2010;33:E29-34.
- Carranza C, Abufhele A, Cartes F, Forero A. Transthoracic versus transesophageal two-dimensional echo Doppler determination of flow velocity in the left atrial appendage. Echocardiography 1997;14:357-62.
- Chu JW, Picard MH, Agnihotri AK, Fitzsimons MG. Diagnosis of congenital unicuspid aortic valve in adult population: the value and limitation of transesophageal echocardiography. Echocardiography 2010;27:1107-12.
- Coletta C, Infusino T, Sciarretta S, Sestili A, Trambaiolo P, Cianfrocca C, et al. Transthoracic Doppler echocardiography for the assessment of left atrial appendage size and blood flow velocity. A multicentre study. J Cardiovasc Med 2008;9:147-52.
- Cosme O, Grodman RS. Estimation of left ventricular systolic function by nonvolumetric echocardiographic analysis in subjects with poor left ventricular visualization: a pilot study. Clin Cardiol 1997;20:247-51.
- De Castro S, Cartoni D, d’Amati G, Beni S, Yao J, Fiorell M, et al. Diagnostic accuracy of transthoracic and multiplane transesophageal echocardiography for valvular perforation in acute infective endocarditis: correlation with anatomic findings. Clin Infect Dis 2000;30:825-6.
- Guenzinger R, Wildhirt SM, Voegele K, Wagner I, Schwaiger M, Bauernschmitt R, et al. Comparison of magnetic resonance imaging and transthoracic echocardiography for the identification of LV mass and volume regression indices 6 months after mitral valve repair. J Cardiac Surg 2008;23:126-32.
- Nguyen VT, Ho JE, Ho CY, Givertz MM, Stevenson LW. Handheld echocardiography offers rapid assessment of clinical volume status. Am Heart J 2008;156:537-42.
- Perez-Avraham G, Kobal SL, Etzion O, Novack V, Wolak T, Liel-Cohen N, et al. Left ventricular geometric abnormality screening in hypertensive patients using a hand-carried ultrasound device. J Clin Hypertens 2010;12:181-6.
- Rivas-Gotz C, Manolios M, Thohan V, Nagueh SF. Impact of left ventricular ejection fraction on estimation of left ventricular filling pressures using tissue Doppler and flow propagation velocity. Am J Cardiol 2003;91:780-4.
- Anwar AM, Soliman OII, Nemes A, Germans T, Krenning BJ, Geleijnse ML, et al. Assessment of mitral annulus size and function by real-time 3-dimensional echocardiography in cardiomyopathy: comparison with magnetic resonance imaging. J Am Soc Echocardiogr 2007;20:941-8.
- Arshad M, Downs MR. What are the best tests to diagnose dissecting thoracic aortic aneurysm?. Evid Based Pract 2009;12:1-2.
- Baman TS, Lange DC, Ilg KJ, Gupta SK, Liu TY, Alguire C, et al. Relationship between burden of premature ventricular complexes and left ventricular function. Heart Rhythm 2010;7:865-9.
- Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. Eur J Echocardiogr 2009;10:1-25.
- Bhagirath KM, Paulson K, Ahmadie R, Bhalla RS, Robinson D, Jassal DS, et al. Clinical utility of cardiac magnetic resonance imaging in Churg–Strauss syndrome: case report and review of the literature. Rheumatol Int 2009;29:445-9.
- Boonyasirinant T, Phankinthongkum R, Komoltri C. Clinical and echocardiographic parameters and score for the left atrial thrombus formation prediction in the patients with mitral stenosis. J Med Assoc Thai 2007;90:9-18.
- Chan MY, Wong HB, Ong HY, Yeo TC. Prognostic value of left atrial size in chronic kidney disease. Eur J Echocardiogr 2008;9:736-40.
- Clark L, Chamberlain SR, Sahakian BJ. Neurocognitive mechanisms in depression: implications for treatment. Ann Rev Neurosci 2009;32:57-74.
- Clergeau MR, Hamon M, Morello R, Saloux E, Viader F, Hamon M, et al. Silent cerebral infarcts in patients with pulmonary embolism and a patent foramen ovale: a prospective diffusion-weighted MRI study. Stroke 2009;40:3758-62.
- Cotter PE, Martin PJ, Belham M. Improved sensitivity of transthoracic contrast echocardiography in the detection of right-to-left shunts. J Am Soc Echocardiogr 2010;23:578-9.
- Dogan A, Kahraman H, Ozturk M, Avsar A. P wave dispersion and left atrial appendage function for predicting recurrence after conversion of atrial fibrillation and relation of p wave dispersion to appendage function. Echocardiography 2004;21:523-30.
- Ellis K, Ziada KM, Vivekananthan D, Latif AA, Shaaraoui M, Martin D, et al. Transthoracic echocardiographic predictors of left atrial appendage thrombus. Am J Cardiol 2006;97:421-5.
- Garg R, Khaja A, Madsen R, Alpert MA, Tejwani L, Aggarwal K, et al. Observer variation in the echocardiographic measurement of maximum atrial septal excursion: a comparison of M-mode with two-dimensional or transesophageal echocardiography. Echocardiography 2009;26:1122-6.
- Homma S. Echocardiography in stroke patients (with emphasis on cryptogenic stroke). Clin Neurol 2006;46:799-804.
- Jesurum JT, Fuller CJ, Renz J, Krabill KA, Spencer MP, Reisman M, et al. Diagnosis of secondary source of right-to-left shunt with balloon occlusion of patent foramen ovale and power M-mode transcranial Doppler. JACC Cardiovasc Interv 2009;2:561-7.
- Khan GN, Dairywala IT, Liu Z, Li P, Carroll J, Vannan MA, et al. Three-dimensional echocardiography of left atrial appendage thrombus. Echocardiography 2001;18:163-6.
- Kini V, Logani S, Ky B, Chirinos JA, Ferrari VA, St John Sutton MG, et al. Transthoracic and transesophageal echocardiography for the indication of suspected infective endocarditis: vegetations, blood cultures and imaging. J Am Soc Echocardiogr 2010;23:396-402.
- Kronzon I, Tunick PA. Review: transthoracic echocardiography and transesophageal echocardiography detect cardiac masses in patients with stroke – commentary on Kapral MK, Silver FL, with the Canadian Task Force on Preventive Health Care. Preventive health care, 1999 update: 2. Echocardiography for the detection of a cardiac source of embolus in patients with stroke. CMAJ 1999;161:989–96. ACP J Club 2000;132.
- Murphy SM, McIntyre D, McAdam B, Moroney JT. Transthoracic echocardiography is not useful in the routine investigation of ischaemic stroke or TIA. Ir Med J 2008;101.
- Pemberton J, Jerosch-Herold M, Li X, Hui L, Silberbach M, Woodward W, et al. Accuracy of real-time, three-dimensional Doppler echocardiography for stroke volume estimation compared with phase-encoded MRI: an in vivo study. Heart 2008;94:1212-13.
- Rahmouni HW, Keane MG, Silvestry FE, Sutton MGSJ, Ferrari VA, Scott CH, et al. Failure of digital echocardiography to accurately diagnose intracardiac shunts. Am Heart J 2008;155:161-5.
- Rost C, Flachskampf FA. Diagnosing left ventricular diastolic dysfunction by echocardiography: Reverend Bayes lends a hand. J Am Soc Echocardiogr 2010;23:162-3.
- Sorescu D, Turk RJ, Cain M, Lerakis S. Clinical and transthoracic echocardiographic predictors of abnormal transesophageal findings in patients with suspected cardiac source of embolism. Am J Med Sci 2003;326:31-4.
- Sporton S, Holdright D. Echocardiography. J R Coll Physicians Lond 1999;33:18-24.
- Stollberger C, Finsterer J. Transoesophageal echocardiography: which stroke patients benefit most from this investigation?. J Neurol Neurosurg Psychiatry 2003;74:283-4.
- Uma N, Chugh SK, Harshwardhan, Goel A, Gopal D. Echocardiography in patients with cerebral infarction. J Assoc Physicians India 1999;47:291-3.
- Yoshikawa J, Owaki T, Kato H, Tanaka K. Ultrasonic diagnosis of ventricular aneurysm. Jpn Heart J 1975;16:394-403.
- Arrigo F, Carerj S, Pizzimenti G. Role of transesophageal echography in the study of embolism of cardiac origin. Cardiologia 1993;38:301-17.
- Balazs E, Pinter KS. Long-term prognostic value of coronary flow reserve in patients without significant left anterior descending coronary artery stenosis: results from the SZEGED Study. Orvosi Hetilap 2010;151:338-43.
- Crepaz R, Pitscheider W, Erlicher A, Knoll P, Braito E. Quantitative evaluation of left ventricular systolic function using bidimensional echocardiography: comparison with cineangiography. G Ital Cardiol 1989;19:393-401.
- Leddet P, Couppié P, De Poli F, Hanssen M. Value of cardiac MRI for intraventricular thrombi’s diagnosis. Ann Cardiol Angeiol 2010;59:285-93.
- Levy M, Bekri N, Pouillart F, Bellorini M, Romano M, Perez T, et al. Comparison of segmental wall motion in electrocardiogram-gated myocardial scintigraphy and transthoracic echocardiography. Arch Mal Coeur Vaiss 2000;93:827-34.
- Mahagney A, Sharif D, Weller B, Abineder E, Sharf B. Diagnosis of cerebral embolism by transesophageal echocardiography. Harefuah 1998;134:256-9.
- Mesa D, Franco M, Suárez de Lezo J, Muñoz J, Rus C, Delgado M, et al. Prevalence of patent foramen ovale in young patients with cryptogenic stroke. Rev Esp Cardiol 2003;56:662-8.
- Meuleman C. Functional assessment of mitral regurgitation by transthoracic echocardiography using standardized imaging planes: diagnostic accuracy and outcome implications. Med Ther Cardio 2005;1:528-9.
- Molins A, Serena J, Genis D, Bassaganyas J, Pérez-Ayuso MJ, Dávalos A. Transcranial Doppler with contrast medium for diagnosing left-to-right shunt in young adults with cerebral infarction. Neurologia 1996;11:205-9.
- Niederle P, Jezek V, Jezková J, Michaljanic A. 2-dimensional echocardiography in the evaluation of right ventricular volume and function. Comparison with echocardiography. Vnitr Lek 1991;37:313-22.
- Oledzka ML. Electrocardiographic signs of left ventricular hypertrophy – is it important in the era of echocardiography?. Fam Med Prim Care Rev 2006;8:722-4.
- Quiles J, García-Fernández MA, Avanzas P, Martínez-Sellés M, Rosas R, Sánchez Hernández A, et al. Comparison of echocardiographic studies made with new portable devices to conventional studies. Rev Esp Cardiol 2003;56:480-6.
- Saygili A, Yildirim SV, Tokel K. Assessment of atrial pathologies in children using transesophageal echocardiography. Anadolu Kardiyol Derg 2004;4:124-9.
- Tei H, Uchiyama S, Koshimizu K, Murakami H, Iwata M. Accuracy of three-step diagnosis in discriminating subtypes of acute ischemic stroke. Rinsho Shinkeigaku 1997;37:21-5.
- Wu H, Zhu W, Xu J. Evaluation of echocardiography for determining left ventricular function. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 1994;16:48-53.
- Chen YZ, Sherrid MV, Dwyer EM. Value of two-dimensional echocardiography in evaluating coronary artery disease: a randomized blinded analysis. J Am Coll Cardiol 1985;5:911-17.
- Corrado G, Massironi L, Torta D, Rigo F, Beretta S, Sansalone D, et al. Contrast transthoracic echocardiography versus transcranial Doppler for patent foramen ovale detection. Int J Cardiol 2011;150:235-7.
- Forissier JF, Charron P, Tezenas du Montcel S, Hagège A, Isnard R, Carrier L, et al. Diagnostic accuracy of a 2D left ventricle hypertrophy score for familial hypertrophic cardiomyopathy. Eur Heart J 2005;26:1882-6.
- Pechacek L, Lazar A, Sonnemaker R, Edelman S, de Castro C, Hall R. Comparison of two-dimensional echocardiography radionuclide ventriculography and cineangiography in detecting surgically documented left ventricular thrombi. Tex Heart Inst J 1984;11:118-27.
- Senior R, Galasko G, Hickman M, Jeetley P, Lahiri A. Community screening for left ventricular hypertrophy in patients with hypertension using hand-held echocardiography. J Am Soc Echocardiogr 2004;17:56-61.
Appendix 8 Summary of results
| Study | TP | FP | FN | TN | Sensitivity (95% CI) | Specificity (95% CI) |
|---|---|---|---|---|---|---|
| TTE vs. ultrafast CT (left atrial thrombi) | ||||||
| Kitayama 1997138 | 4 | 0 | 2 | 64 | 0.67 (0.22 to 0.96) | 1.00 (0.94 to 1.00) |
| TTE vs. TOE (left atrial thrombi) | ||||||
| Blum 2004145 | 0 | 0 | 3 | 65 | 0.00 (0.00 to 0.71) | 1.00 (0.94 to 1.00) |
| Fatkin 1996156 | 2 | 0 | 3 | 55 | 0.40 (0.05 to 0.85) | 1.00 (0.94 to 1.00) |
| Vincelj 2001148 | 1 | 0 | 0 | 13 | 1.00 (0.03 to 1.00) | 1.00 (0.75 to 1.00) |
| TTEh vs. TOE (left atrial thrombi) | ||||||
| de Bruijn 2006141 | 0 | 0 | 1 | 230 | 0.00 (0.00 to 0.97) | 1.00 (0.98 to 1.00) |
| Ha 2000136 | 9 | 0 | 3 | 62 | 0.75 (0.43 to 0.95) | 1.00 (0.94 to 1.00) |
| Illien 2002126 | 11 | 0 | 1 | 160 | 0.92 (0.62 to 1.00) | 1.00 (0.98 to 1.00) |
| TTE vs. independent verification (left ventricular thrombus) | ||||||
| Stratton 1982122 | 19 | 3 | 3 | 53 | 0.86 (0.65 to 0.97) | 0.95 (0.85 to 0.99) |
| TTEh vs. contrast-enhanced MRI (left ventricular thrombus) | ||||||
| Lipke 2007130 | 8 | 5 | 7 | 14 | 0.53 (0.27 to 0.79) | 0.74 (0.49 to 0.91) |
| TTEh vs. cardiac MRI (left ventricular thrombus) | ||||||
| Weinsaft 2011124 | 8 | 20 | 16 | 199 | 0.33 (0.16 to 0.55) | 0.91 (0.86 to 0.94) |
| TTE vs. TOE (PFO) | ||||||
| Akosah 1998107 | 0 | 0 | 2 | 122 | 0.00 (0.00 to 0.84) | 1.00 (0.97 to 1.00) |
| Belkin 2011109 | 7 | 2 | 7 | 22 | 0.50 (0.23 to 0.77) | 0.92 (0.73 to 0.99) |
| Blum 2004145 | 0 | 1 | 5 | 62 | 0.00 (0.00 to 0.52) | 0.98 (0.91 to 1.00) |
| Chen 1992139 | 12 | 0 | 7 | 13 | 0.63 (0.38 to 0.84) | 1.00 (0.75 to 1.00) |
| Cujec 1991152 | 0 | 0 | 2 | 24 | 0.00 (0.00 to 0.84) | 1.00 (0.86 to 1.00) |
| Di Tullio 1993110 | 9 | 0 | 10 | 30 | 0.47 (0.24 to 0.71) | 1.00 (0.88 to 1.00) |
| Ha 2001137 | 9 | 0 | 31 | 80 | 0.23 (0.11 to 0.38) | 1.00 (0.95 to 1.00) |
| Lee 1991114 | 0 | 0 | 4 | 46 | 0.00 (0.00 to 0.60) | 1.00 (0.92 to 1.00) |
| Madala 2004115 | 7 | 0 | 2 | 55 | 0.78 (0.40 to 0.97) | 1.00 (0.94 to 1.00) |
| Musolino 200361 | 0 | 0 | 10 | 50 | 0.00 (0.00 to 0.31) | 1.00 (0.93 to 1.00) |
| Nemec 1991116 | 7 | 0 | 6 | 19 | 0.54 (0.25 to 0.81) | 1.00 (0.82 to 1.00) |
| Siostrzonek 1991134 | 9 | 0 | 21 | 120 | 0.30 (0.15 to 0.49) | 1.00 (0.97 to 1.00) |
| Stendel 2000133 | 10 | 0 | 14 | 68 | 0.42 (0.22 to 0.63) | 1.00 (0.95 to 1.00) |
| TTEh vs. TOE (PFO) | ||||||
| Clarke 2004143 | 12 | 1 | 1 | 96 | 0.92 (0.64 to 1.00) | 0.99 (0.94 to 1.00) |
| Daniels 2004140 | 48 | 7 | 5 | 196 | 0.91 (0.79 to 0.97) | 0.97 (0.93 to 0.99) |
| Gonzalez-Alujas 2011146 | 93 | 0 | 0 | 41 | 1.00 (0.96 to 1.00) | 1.00 (0.91 to 1.00) |
| Ha 2001137 | 25 | 0 | 15 | 96 | 0.63 (0.46 to 0.77) | 1.00 (0.96 to 1.00) |
| Hubail 2011112 | 7 | 0 | 1 | 35 | 0.88 (0.47 to 1.00) | 1.00 (0.90 to 1.00) |
| Jax 2010127 | 22 | 0 | 24 | 0 | 0.48 (0.33 to 0.63) | Not estimable |
| Kerr 2000113 | 12 | 0 | 5 | 27 | 0.71 (0.44 to 0.90) | 1.00 (0.87 to 1.00) |
| Madala 2004115 | 9 | 10 | 0 | 45 | 1.00 (0.66 to 1.00) | 0.82 (0.69 to 0.91) |
| Maffè 2010150 | 55 | 0 | 7 | 13 | 0.89 (0.78 to 0.95) | 1.00 (0.75 to 1.00) |
| Thanigaraj 2005123 | 34 | 0 | 2 | 58 | 0.94 (0.81 to 0.99) | 1.00 (0.94 to 1.00) |
| Trevelyan 2006144 | 26 | 0 | 8 | 53 | 0.76 (0.59 to 0.89) | 1.00 (0.93 to 1.00) |
| Zito 2009151 | 26 | 0 | 20 | 26 | 0.57 (0.41 to 0.71) | 1.00 (0.87 to 1.00) |
| Sensitivity analysis: TOE vs. TCD (PFO) | ||||||
| Gonzalez-Alujas 2011146 | 90 | 1 | 3 | 40 | 0.97 (0.91 to 0.99) | 0.98 (0.87 to 1.00) |
| Sensitivity analysis: TOE vs. TMD (PFO) | ||||||
| Kerr 2000113 | 17 | 0 | 1 | 26 | 0.94 (0.73 to 1.00) | 1.00 (0.87 to 1.00) |
| TTE vs. TOE (atrial septal defect) | ||||||
| Akosah 1998107 | 0 | 0 | 5 | 119 | 0.00 (0.00 to 0.52) | 1.00 (0.97 to 1.00) |
| Blum 2004145 | 0 | 0 | 1 | 67 | 0.00 (0.00 to 0.97) | 1.00 (0.95 to 1.00) |
| Kuhl 1999128 | 31 | 0 | 20 | 60 | 0.61 (0.46 to 0.74) | 1.00 (0.94 to 1.00) |
| Musolino 200361 | 1 | 0 | 3 | 56 | 0.25 (0.01 to 0.81) | 1.00 (0.94 to 1.00) |
| TTEh vs. TOE (atrial septal defect) | ||||||
| Kuhl 1999128 | 46 | 0 | 5 | 60 | 0.90 (0.79 to 0.97) | 1.00 (0.94 to 1.00) |
| Thanigaraj 2005123 | 7 | 0 | 0 | 87 | 1.00 (0.59 to 1.00) | 1.00 (0.96 to 1.00) |
| TTE vs. surgical + cardiac catheterisation (atrial septal defect – ostium secundum) | ||||||
| Shub 1983121 | 93 | 0 | 12 | 0 | 0.89 (0.81 to 0.94) | Not estimable |
| TTE vs. surgical + cardiac catheterisation (atrial septal defect – ostium primum) | ||||||
| Shub 1983121 | 32 | 0 | 0 | 0 | 1.00 (0.89 to 1.00) | Not estimable |
| TTE vs. TOE (atrial septal aneurysm) | ||||||
| Cujec 1991152 | 0 | 0 | 2 | 24 | 0.00 (0.00 to 0.84) | 1.00 (0.86 to 1.00) |
| Di Tullio 1993110 | 0 | 0 | 2 | 47 | 0.00 (0.00 to 0.84) | 1.00 (0.92 to 1.00) |
| Mugge 1995131 | 103 | 0 | 92 | 0 | 0.53 (0.46 to 0.60) | Not estimable |
| Musolino 200361 | 0 | 0 | 11 | 49 | 0.00 (0.00 to 0.28) | 1.00 (0.93 to 1.00) |
| TTEh vs. TOE (atrial septal aneurysm) | ||||||
| Gonzalez-Alujas 2011146 | 34 | 0 | 1 | 22 | 0.97 (0.85 to 1.00) | 1.00 (0.85 to 1.00) |
| TTE vs. TOE (left atrial appendage thrombi) | ||||||
| Akosah 1998107 | 0 | 0 | 18 | 106 | 0.00 (0.00 to 0.19) | 1.00 (0.97 to 1.00) |
| Aschenberg 1986125 | 0 | 0 | 6 | 15 | 0.00 (0.00 to 0.46) | 1.00 (0.78 to 1.00) |
| Cujec 1991152 | 0 | 0 | 1 | 25 | 0.00 (0.00 to 0.97) | 1.00 (0.86 to 1.00) |
| Fatkin 1996156 | 0 | 0 | 4 | 56 | 0.00 (0.00 to 0.60) | 1.00 (0.94 to 1.00) |
| Musolino 200361 | 0 | 0 | 1 | 59 | 0.00 (0.00 to 0.97) | 1.00 (0.94 to 1.00) |
| Omran 1999132 | 10 | 0 | 2 | 104 | 0.83 (0.52 to 0.98) | 1.00 (0.97 to 1.00) |
| Pop 1990142 | 0 | 0 | 2 | 17 | 0.00 (0.00 to 0.84) | 1.00 (0.80 to 1.00) |
| Sallach 2009120 | 0 | 0 | 2 | 116 | 0.00 (0.00 to 0.84) | 1.00 (0.97 to 1.00) |
| TTEh vs. TOE (left atrial appendage thrombi) | ||||||
| Sallach 2009120 | 2 | 0 | 0 | 116 | 1.00 (0.16 to 1.00) | 1.00 (0.97 to 1.00) |
| TTE vs. TOE (SEC) | ||||||
| Lee 1991114 | 0 | 0 | 9 | 41 | 0.00 (0.00 to 0.34) | 1.00 (0.91 to 1.00) |
| Omran 1999132 | 4 | 0 | 51 | 61 | 0.07 (0.02 to 0.18) | 1.00 (0.94 to 1.00) |
| Pop 1990142 | 0 | 0 | 2 | 17 | 0.00 (0.00 to 0.84) | 1.00 (0.80 to 1.00) |
| TTE vs. TOE (left atrial SEC) | ||||||
| Black 1991154 | 0 | 0 | 75 | 325 | 0.00 (0.00 to 0.05) | 1.00 (0.99 to 1.00) |
| Black 1991155 | 0 | 0 | 33 | 67 | 0.00 (0.00 to 0.11) | 1.00 (0.95 to 1.00) |
| Cujec 1991152 | 0 | 0 | 7 | 19 | 0.00 (0.00 to 0.41) | 1.00 (0.82 to 1.00) |
| Pearson 1991118 | 0 | 0 | 13 | 66 | 0.00 (0.00 to 0.25) | 1.00 (0.95 to 1.00) |
| TTEh vs. TOE (left atrial SEC) | ||||||
| Ha 2000136 | 63 | 0 | 9 | 1 | 0.88 (0.78 to 0.94) | 1.00 (0.03 to 1.00) |
| TTE vs. TOE (left ventricular SEC) | ||||||
| Black 1991155 | 0 | 0 | 2 | 98 | 0.00 (0.00 to 0.84) | 1.00 (0.96 to 1.00) |
| TTE vs. left ventriculography (left ventricular aneurysm) | ||||||
| Baur 1982108 | 14 | 0 | 3 | 9 | 0.82 (0.57 to 0.96) | 1.00 (0.66 to 1.00) |
| Li 2009135 | 13 | 1 | 3 | 21 | 0.81 (0.54 to 0.96) | 0.95 (0.77 to 1.00) |
| TTEh vs. cardiac catheterisation (aortic valve stenosis) | ||||||
| Lembcke 2009129 | 160 | 3 | 0 | 39 | 1.00 (0.98 to 1.00) | 0.93 (0.81 to 0.99) |
| TTEf vs. TOE (cardiac vegetations) | ||||||
| Chirillo 2005149 | 10 | 22 | 18 | 89 | 0.36 (0.19 to 0.56) | 0.80 (0.72 to 0.87) |
| TTEh vs. TOE (cardiac vegetations) | ||||||
| Chirillo 2005149 | 23 | 2 | 5 | 109 | 0.82 (0.63 to 0.94) | 0.98 (0.94 to 1.00) |
| Jassal 20075 | 16 | 2 | 3 | 15 | 0.84 (0.60 to 0.97) | 0.88 (0.64 to 0.99) |
| TTE vs. TOE (mitral valve regurgitation) | ||||||
| Musolino 200361 | 5 | 0 | 0 | 55 | 1.00 (0.48 to 1.00) | 1.00 (0.94 to 1.00) |
| Neuman 2003117 | 51 | 0 | 3 | 0 | 0.94 (0.85 to 0.99) | Not estimable |
| TTEh vs. TOE (mitral valve regurgitation) | ||||||
| Roldan 2008119 | 8 | 4 | 6 | 62 | 0.57 (0.29 to 0.82) | 0.94 (0.85 to 0.98) |
| TTE vs. TOE (mitral valve stenosis) | ||||||
| Musolino 200361 | 2 | 0 | 0 | 58 | 1.00 (0.16 to 1.00) | 1.00 (0.94 to 1.00) |
| TTEh vs. TOE (mitral valve prolapse) | ||||||
| Hirata 2008111 | 39 | 0 | 3 | 0 | 0.93 (0.81 to 0.99) | Not estimable |
| TTEh (three-dimensional) vs. TOE (mitral valve prolapse) | ||||||
| Gutiérrez-Chico 2008147 | 40 | 0 | 1 | 0 | 0.98 (0.87 to 1.00) | Not estimable |
| Hirata 2008111 | 40 | 0 | 2 | 0 | 0.95 (0.84 to 0.99) | Not estimable |
| TTE vs. TOE (atrial myxoma) | ||||||
| Vincelj 2001148 | 8 | 0 | 2 | 4 | 0.80 (0.44 to 0.97) | 1.00 (0.40 to 1.00) |
Appendix 9 Cost-effectiveness review: literature search strategies, a MEDLINE example
Database: Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations and Ovid MEDLINE(R).
Searched: 1948 to present.
-
Stroke/ (39,233)
-
stroke$.mp. (138,756)
-
stroke volume/ (25,237)
-
stroke volume$.mp. (32,752)
-
Cerebrovascular accident.mp. (2591)
-
cerebrovascular event.mp. (471)
-
Cerebrovascular disease.mp. (9405)
-
Ischemic Attack, Transient/ or transient ischemic event.mp. (16,035)
-
transient ischemic attack.mp. (3409)
-
vascular accident.mp. (674)
-
brain emboli$.mp. or Intracranial Embolism/ (2398)
-
cerebral emboli$.mp. (1923)
-
brain infarction.mp. or Brain Infarction/ (3554)
-
cerebral infarction.mp. or Cerebral Infarction/ (21,326)
-
or/1-14 (175,197)
-
Echocardiography.mp. or Echocardiography/ (105,417)
-
transthoracic echocardiography.mp. (4128)
-
Transoesophageal echocardiography.mp. (1369)
-
transesophageal echocardiography.mp. (8092)
-
(echocardiog$ adj (transthorac$ or trans-thorac$ or (trans$ and thorac$))).mp. (427)
-
(echocardiog$ adj (transoesophag$ or trans-oesophag$ or (trans and oesophag$))).mp. (46)
-
(echocardiog$ adj (transesophag$ or trans-esophag$ or (trans and esophag$))).mp. (12,231)
-
24 hour holter.mp. (1157)
-
twenty four hour holter.mp. (111)
-
telemetr$.mp. (9058)
-
secondary prevention.mp. (9681)
-
cardiac imag$.mp. (1883)
-
cardiac magnetic resonance imaging.mp. (1315)
-
cardiac MR.mp. (349)
-
cardiac MRI.mp. (882)
-
carotid ultrasound.mp. (499)
-
carotid doppler.mp. (210)
-
transcranial doppler.mp. (5296)
-
transcranial doppler.mp. (5296)
-
R? Test Evolution.mp. (2)
-
R? Test.mp. (981)
-
reveal device.mp. (1)
-
implantable loop recorder.mp. (154)
-
diagnostic imag$.mp. (29,793)
-
Ultrasonography/ or diagnostic ultrasound.mp. (59,099)
-
ultrasonic diagnosis.mp. (1607)
-
magnetic resonance imaging.mp. or Magnetic Resonance Imaging/ (253,683)
-
or/16-42 (458,448)
-
exp “Sensitivity and Specificity”/ (324,293)
-
sensitivity.tw. (420,807)
-
((pre-test or pretest) adj probability).tw. (940)
-
post-test probability.tw. (261)
-
predictive value$.tw. (51,868)
-
likelihood ratio$.tw. (6225)
-
or/44-49 (671,637)
-
15 and 43 and 50 (3735)
-
from 51 keep 2001-3735 (1735)
-
Economics/ (25,956)
-
“costs and cost analysis”/ (38,507)
-
Cost allocation/ (1887)
-
Cost-benefit analysis/ (49,895)
-
Cost control/ (18,559)
-
Cost savings/ (6894)
-
Cost of illness/ (13,573)
-
Cost sharing/ (1634)
-
“deductibles and coinsurance”/ (1268)
-
Medical savings accounts/ (440)
-
Health care costs/ (20,579)
-
Direct service costs/ (924)
-
Drug costs/ (10,121)
-
Employer health costs/ (1026)
-
Hospital costs/ (6325)
-
Health expenditures/ (11,358)
-
Capital expenditures/ (1889)
-
Value of life/ (5118)
-
exp economics, hospital/ (16,987)
-
exp economics, medical/ (13118)
-
Economics, nursing/ (3833)
-
Economics, pharmaceutical/ (2192)
-
exp “fees and charges”/ (25,020)
-
exp budgets/ (10,802)
-
(low adj cost).mp. (16,143)
-
(high adj cost).mp. (6351)
-
(health?care adj cost$).mp. (2668)
-
(fiscal or funding or financial or finance).tw. (61,105)
-
(cost adj estimate$).mp. (1113)
-
(cost adj variable).mp. (28)
-
(unit adj cost$).mp. (1182)
-
(economic$ or pharmacoeconomic$ or price$ or pricing).tw. (133,214)
-
or/53-84 (378,997)
-
15 and 43 and 85 (331)
Appendix 10 Cost-effectiveness review: Preferred Reporting Items for Systematic Reviews and Meta-Analyses (adapted) flow chart
Appendix 11 Economic evaluation checklist
Drummond et al. adapted criteria158
| Criteria | Meenan et al.37 | McNamara et al.161 |
|---|---|---|
| 1. Was a well-defined question posed in answerable form? | Yes | Yes |
| 2. Was a comprehensive description of the competing alternatives given? | Yes | Unclear |
| 3. Was the effectiveness of the programme or services established? | Yes | Yes |
| 4. Were all the important and relevant costs and consequences for each alternative identified? | Yes | Unclear |
| 5. Were costs and consequences measured accurately in appropriate physical units? | Yes | Unclear |
| 6. Were the cost and consequences valued credibly? | Yes | Unclear |
| 7. Were costs and consequences adjusted for differential timing? | Not applicable | Not applicable |
| 8. Was an incremental analysis of costs and consequences of alternatives performed? | Yes | Yes |
| 9. Was allowance made for uncertainty in the estimates of costs and consequences? | Yes | Unclear |
| 10. Did the presentation and discussion of study results include all issues of concern to users? | Yes | Unclear |
Consensus on Health Economic Criteria (CHEC)-list160
| Criteria | Meenan et al.37 | McNamara et al.161 |
|---|---|---|
| 1. Is the study population clearly described? | Yes | Yes |
| 2. Are competing alternatives clearly described? | Yes | Yes |
| 3. Is a well-defined research question posed in answerable form? | Yes | Yes |
| 4. Is the economic study design appropriate to the stated objective? | Yes | Yes |
| 5. Is the chosen time horizon appropriate to include relevant costs and consequences? | Yes | Yes |
| 6. Is the actual perspective chosen appropriate? | Yes | Yes |
| 7. Are all important and relevant costs for each alternative identified? | Yes | Unclear |
| 8. Are all costs measured appropriately in physical units? | Yes | Unclear |
| 9. Are costs valued appropriately? | Yes | Unclear |
| 10. Are all important and relevant outcomes for each alternative identified? | Yes | Unclear |
| 11. Are all outcomes measured appropriately? | Yes | Yes |
| 12. Are outcomes valued appropriately? | Not applicable | Yes |
| 13. Is an incremental analysis of costs and outcomes of alternatives performed? | Yes | Yes |
| 14. Are all future costs and outcomes discounted appropriately? | Not applicable | Not applicable |
| 15. Are all important variables, whose values are uncertain, appropriately subjected to sensitivity analysis? | Yes | Unclear |
| 16. Do the conclusions follow from the data reported? | Yes | Yes |
| 17. Does the study discuss the generalisability of the results to other settings and patient/client groups? | No | Unclear |
| 18. Does the article indicate that there is no potential conflict of interest of study researcher(s) and funder(s)? | Yes | Unclear |
| 19. Are ethical and distributional issues discussed appropriately? | No | Unclear |
Appendix 12 Stroke survey
1. What guidelines do you use to investigate and manage ischaemic stroke or TIA?
Investigate Manage
-
Internal guidelines [ ] [ ]
-
NICE guidelines [ ] [ ]
-
Royal College of Physicians guidelines [ ] [ ]
-
Other guidelines [ ] [ ]
-
Amended guidelines for local use [ ] [ ]
-
No guidelines [ ] [ ]
When a guideline other than unmodified NICE or Royal College of Physicians is used, please enclose a copy.
2. How often are the following tests used to investigate ischaemic stroke or TIA?
| Never | Only young cases | Only if all other tests normal | Only if strong clinical suggestion of cerebral embolism | All cases | |
|---|---|---|---|---|---|
| 12-lead ECG | |||||
| Holter monitoring | |||||
| Transoesophageal echocardiography (TOE) | |||||
| Transthoracic echocardiography (TTE) | |||||
| TTE with bubble contrast | |||||
| Other (please state) |
Appendix 13 Clinicians’ comments
I am afraid, the questions are all or none type, not very differentiating. As a result, it may lead to skewed results. In our institution, all unexplained cases of young stroke (50 yrs or under) gets a TOE and bubble study, and all stroke 50 or under gets a TTE. Additional tests i.e. CT-angio [computerised tomography angiography], contrast carotid, MR angiogram [magnetic resonance angiography] etc. are done according to clinical indication.
Thrombophilia Screen, Vasculitic Screen, Autoantibodies, Carotid doppler, MRA for carotids and vertebrals, CTA, R on T test [a form of ventricular arrhythmia in which the electrocardiographic tracing shows premature ventricular complexes occurring in early diastole], TCD with bubble contrast, Thrombophilia screen, genetic test for CADASIL [cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy] and Fabry’s disease etc. tcd sometimes used if paradoxical emb [embolism] thought possible.
[I]ts not either/or it is more subtle and often logical decision making depends on many factors. There are also good reasons why a test might be indicated technically but not done as it has no UTILITY for a particular patient. Too often, thoughtless tests are done, then don’t know what to do with the answer!!
[W]e used 7 days holter monitoring for most ischaemic stroke, if the 12 lead ECG showed sinus rhythm to rule out PAF [paroxysmal atrial fibrillation]. It started as a research project, and we may use as routine test, as initial finding shows that up to 12% of patients have PAF [paroxysmal atrial fibrillation] on prolonged monitoring.
[W]e tend to do a bubble contrast TTE and if shunt seen we proceed to TOE.
I found that the questionnaire limited some other options. For example, I do request a transthoracic echo for all young patients and in the case of middle aged and elderly, I arrange an echo for unexplained ischaemic stroke/TIA, and associated co-morbidity.
A Holtoer monitoring is used in unexplained stroke (Ischaemic) for excluding PAF.
Young patients < 50: 1. Antiphsopholipid antibodies 2. Thrombophilia 3. Vasculitis screen 4. Homocystein.
In all young patients, i.e. 50 or less and those aged 50–60 without other significant risk factors, a work up of: ECG, TTE, 24 hour tape, vasculitic and thrombophilia screen are requested. If all these prove normal then consideration is given to TOE being undertaken with patient involvement.
TCD with bubble test. All cases get ECG, the majority get Holter, some get TTE but if we strongly suspect embolic source in young person then we do TCD with bubble and if positive TTE with bubble.
CT angiogram. Other questions don’t allow multiple options, e.g. TTE used to investigate all young cases AND those not young but strong clinical suggestion of cerebral embolism. Carotid imaging (doppler, MRA, CTA – not just young patients), Trans-cranial doppler (not just young patients), Lupus anticoagulant, Anticardiolipin antibodies
Thrombophilia/AIP [acute intermittent porphyria]/Cardiolipin in < 55 years. Dopplers in all anterior circulation events if fit for surgery. MRA with fat suppression in all suspected dissections. CTA in some posterior circulation recurrent events. FAST MRI sequencing in (approx. 40% events) when diagnosis unclear or neurology in doubt/questioned.
TOE
Thrombophilia screen
Vasculitis screen
Screen for Fabry’s disease
MRA or CT angiogram
VRV [ventricular residual volume] if sinus venous thrombosis suspected
Cerebral angiography
All admitted patients have 72 hour cardiac monitoring. Outpatients have 24 hour tapes. If TOE is performed then bubble contrast is considered in all cases by cardiologist.
Appendix 14 Protocol
09/68/01 HTA TAR
Revised Protocol
February 2010
1. Title of the project:
Routine echocardiography in the management of stroke and transient ischemic attack (TIA)
2. Name of TAR team and project ‘lead’
TAR team: ScHARR Technology Appraisal Group, University of Sheffield
Project lead: Rachel Jackson, Research Fellow, ScHARR, University of Sheffield, Regent Court, 30 Regent Street, Sheffield, S1 4DA
Email: R.Jackson@Sheffield.ac.uk, Tel: 0114 222 0793, Fax: 0114 272 4095
Address for correspondence
All correspondence should be sent to the project lead (R.Jackson@Sheffield.ac.uk), the project administrator (Gill Rooney, G.Rooney@Sheffield.ac.uk) and the managing director of ScHARR-TAG (Eva Kaltenthaler, E.Kaltenthaler@Sheffield.ac.uk).
3. Plain English Summary
Stroke is a serious medical condition in which the blood supply to the brain is disrupted, potentially resulting in disability and mortality. The World Health Organisation defined stroke as ‘rapidly developing clinical signs of focal (sometimes global) disturbance of cerebral function, lasting more than 24 hours or leading to death with no apparent cause other than that of vascular origin’ (Hatano, 1976). Symptoms of stroke include numbness, disrupted vision, slurred speech, confusion and headache (Stroke Association, 2009). There are two major types of stroke: ischaemic stroke, in which the blood supply is disrupted due to a narrowing or blockage of the circulatory system; and haemorrhagic stroke, in which blood loss in the brain causes neurological damage. Transient ischaemic attack (TIA) has been defined as ‘a transient episode of neurological dysfunction caused by focal brain, spinal cord or retinal ischaemia, without acute infarction’ (Eastonet al. , 2009). In a transient ischaemic attack, symptoms typically subside within a few hours (Stroke Association, 2009). However, people who have experienced a TIA have a high risk of stroke following the event (Coullet al. , 2004) and therefore should receive prompt medical attention.
It is estimated that approximately 110,000 people experience a stroke and a further 20,000 individuals have a TIA in England each year (National Audit Office, 2005). It has been reported that 10–15% of TIA patients experience a stroke within 3 months (Eastonet al. , 2009). Over 56,000 deaths were attributable to stroke in England and Wales in 1999, representing 11% of total deaths for this period (Mantet al. , 2004). Stroke places a considerable burden on the economy in England, resulting in direct costs to the NHS of £2.8 billion (Mantet al. , 2004).
The identification of the origin of a stroke or TIA can inform treatment and secondary prevention strategies. Embolism of cardiac origin has been estimated to account for approximately 20% of ischaemic strokes (Palacio & Hart, 2002). Imaging technologies such as transthoracic echocardiography (TTE) and transoesophageal echocardiography (TOE) facilitate the detection of potentially-treatable cardiac sources of stroke and TIA. Of the two methods, transthoracic echocardiography is less invasive. Both of these imaging methods are capable of detecting a number of potential cardiac sources of stroke and TIA, including left ventricular/left atrial thrombus (which can be treated by anticoagulation with warfarin), cardiomyopathy (treatable with warfarin or antiplatelet therapy), and patent foramen ovale/atrial septal aneurysm (treatable by anticoagulation, surgical closure, antiplatelet therapy, or by observation) (Yuet al. , 2009).
No recommendations relating to the use of echocardiography in the assessment of first episode diagnosed stroke and TIA patients were made within the national clinical guidelines for stroke published by the Royal College of Physicians (2004), the NICE stroke clinical guideline (NICE, 2008) or the National Stroke Strategy (Department of Health, 2007). The use of this technology in the management of stroke and TIA patients in the UK appears to be variable. The British Society of Echocardiography stated that echocardiography was indicated in adult cases of neurological disease in several instances including: a) unexplained stroke or TIA without evidence of prior cerebrovascular disease or without significant risk factors for other cause (with the suggestion that saline contrast echocardiography by TTE or TOE be used), and b) in patients for whom a therapeutic decision will depend on the outcome of echocardiography (eg. anticoagulation). This guidance also stated that echocardiography was not indicated in patients in whom echocardiography would not affect the decision to begin anticoagulation (eg. patients in atrial fibrillation with cerebrovascular event and no suspicion of structural heart disease).
McNamara et al. (1997) found in their US-specific cost effectiveness analysis that transthoracic echocardiography (either alone or in sequence with transoesophageal echocardiography) was not cost effective compared with transoesophageal echocardiography. The 2007 update of the 2002 Agency for Healthcare Research and Quality (AHRQ) assessment (Meenanet al. , 2007) found that current cost effectiveness evidence was insufficient to justify widespread use of echocardiography in stroke patients in the United States.
The aim of this assessment is to explore the use of transthoracic echocardiography in the assessment of stroke and TIA patients in a UK context.
A related assessment is currently being undertaken by the TAR team in Sheffield entitled ‘Echocardiography in newly diagnosed atrial fibrillation patients’ (08/45/01).
4 Decision problem
4.1 Purpose of assessment
The aim of this assessment is to answer the following research question: What is the clinical and cost effectiveness of the addition of an echocardiogram to the routine assessment of patients who have had a stroke or transient ischaemic attack (TIA) in the UK?
4.2 Clear definition of the intervention
Transthoracic echocardiography (TTE) is an ultrasound imaging technique utilising beams of sound transmitted at frequencies of 2.5–5 MHz. A transducer is placed on the chest, allowing the structures of the heart and velocity of blood flow to be visualised (Patient UK, 2009). TTE may be used to determine cardiac sources of stroke or TIA and facilitate treatment and secondary prevention strategies.
4.3 Place of the intervention in the treatment pathway(s)
The assessment will investigate the effects of undertaking TTE in the routine assessment of all first episode diagnosed stroke and TIA patients in secondary care. Typically, once a stroke has been established as being ischaemic in nature via brain imaging (CT or MRI scanning), further imaging technologies may then be employed to determine the underlying aetiology of the episode and inform patient management. If data are available, the cost effectiveness of performing TTE in specific population subgroups will be determined.
4.4 Relevant comparators
Current UK diagnostic protocol (to be identified by researchers). As data available on current practice within the UK from clinical guidelines and the existing literature are limited, we propose to collect information on current UK diagnostic protocols. Managing staff at stroke units across the UK will be approached and a copy of any current stroke diagnostic protocol(s) will be requested. Clinical advisors to the team will be involved in the identification of an appropriate sample. If necessary, professional bodies may be requested to further advise on recruitment. Following collection of diagnostic protocols, the comparator will then be selected in conjunction with clinical advisors. Comparators may include transoesophageal echocardiography, 24 hour Holter monitoring or cardiac monitoring via telemetry (used alone or in combination with TTE and each other).
4.5 Population and relevant subgroups
Patients who have had an ischaemic stroke or TIA (but have no other indication for a TTE) (NB: Echocardiography in newly diagnosed atrial fibrillation patients is being considered in a separate Health Technology Assessment). If data are available, the effectiveness of performing TTE in specific population subgroups (eg. by age, ethnicity) will be described. Such subgroups are to be defined following the completion of Review 1.
4.6 Key factors to be addressed
The objectives of the review are:
-
To investigate by systematic review the prevalence of cardiac sources of stroke and TIA (limited to those detectable by TTE) (Review 1)
-
To investigate by systematic review the diagnostic accuracy of TTE for these cardiac sources (Review 2)
-
To estimate the potential benefits and harms arising from the alteration of treatment based on results of TTE
-
To estimate the incremental cost effectiveness of providing routine TTE to all first episode diagnosed stroke and TIA patients in secondary care
-
To estimate the incremental cost effectiveness of providing routine TTE to subgroups within the first episode diagnosed stroke and TIA patient population in secondary care (where data are available). Subgroups are to be defined based on the findings of Review 1.
5. Report methods for synthesis of evidence of clinical effectiveness
5.1 Description of reviews
Two systematic evidence reviews (Review 1: Prevalence of cardiac sources of stroke and TIA; Review 2: Diagnostic accuracy of TTE for cardiac sources of stroke and TIA) will be undertaken informed by the general principles recommended in the PRISMA (formerly QUOROM) statement (Moheret al. , 2009).
Review 1: Prevalence of cardiac sources of embolism in stroke and TIA
Prevalence of cardiac sources of embolism in stroke and TIA will be investigated using epidemiological studies. Cardiac sources will be restricted to those identifiable by TTE. These include left ventricular/left atrial thrombus, patent foramen ovale and atrial septal aneurysm (Yuet al. , 2009). It is proposed that conditions that may be associated with cardioembolic stroke such as recent myocardial infarction, dilated cardiomyopathy, infective endocarditis and atrial fibrillation be excluded since they are typically clinically apparent without echocardiography or are present with symptoms that represent other indications for echocardiography (as per Meenanet al. , 2007).
Review 2: Diagnostic accuracy of TTE for cardiac sources of embolism in stroke and TIA
Diagnostic accuracy of TTE will be investigated using studies comparing the identification of cardiac sources of stroke or TIA by TTE with other diagnostic tools. Outcomes relating to screening performance will be described. TTE may be compared against a diagnostic gold standard or alternative imaging method for the detection of cardiac sources of stroke or TIA (eg. transoesophageal echocardiography) within the literature. To inform the economic evaluation, these will need to be synthesised into a consistent evidence base. Studies relating to the prognostic value of TTE (ie. the ability of TTE results to predict subsequent stroke or TIA outcomes) will also be identified. A structured search defined on ad hoc criteria will be undertaken to identify adverse events as a result of the tests under study. Whilst no physical harms appear to be associated with the use of transthoracic echocardiography, there is the potential for the occurrence of adverse events as a result of local anaesthetic or sedation procedures used during the insertion of the transducer probe in transoesophageal echocardiography. Furthermore, patient harms may result as a consequence of diagnostic inaccuracies and resulting inappropriate care.
5.2 Identifying and systematically reviewing clinical effectiveness evidence
Population
The population will be the same for both reviews
Inclusion
First episode diagnosed ischaemic stroke and TIA patients
Interventions
Transthoracic echocardiography (TTE) in the routine assessment of first episode diagnosed stroke and TIA patients in secondary care
Comparators
Current UK diagnostic protocol (to be identified by researchers). Clarification of the care pathway and current UK diagnostic practice is required. As data available on current practice within the UK from clinical guidelines and the existing literature are limited, we propose to collect information on current UK diagnostic protocols. Managing staff at stroke units across the UK will be approached and a copy of any current stroke diagnostic protocol(s) will be requested. Clinical advisors to the team will be involved in the identification of an appropriate sample. If necessary, professional bodies may be requested to further advise on recruitment. Following collection of diagnostic protocols, the comparator will then be selected in conjunction with clinical advisors. Comparators may include transoesophageal echocardiography, 24 hour Holter monitoring or cardiac monitoring via telemetry (used alone or in combination with TTE and each other).
Search strategy
The search strategy for both reviews will comprise the following main elements: searching of electronic databases; contact with experts in the field; scrutiny of bibliographies of retrieved papers. The electronic databases to be searched will include MEDLINE; MEDLINE in Process (for latest publications); EMBASE; Cochrane Database of Systematic Reviews, Cochrane Controlled Trials Register, CINAHL, DARE, NHS EED and HTA databases; NHS EED; NIHR Clinical Research Network Portfolio database, NRR (National Research Register) Archive, Web of Science Proceedings, Science Citation Index; Current Controlled Trials, Clinical Trials.gov, FDA website, EMEA website, and relevant conference proceedings.
The draft search strategy is presented in Appendix 1.
Study selection
In both reviews, citations will be imported into reference management software and screened for inclusion. The following publication types will be excluded: studies which are only published in languages other than English; studies based on animal models; preclinical and biological studies; narrative reviews, editorials, opinions; and reports published as meeting abstracts only (where insufficient methodological details are reported to allow critical appraisal of study quality). Titles and abstracts will be examined for inclusion by one reviewer. Two reviewers will independently make decisions on inclusion of studies at full text stage and any discrepancies resolved by discussion.
Data extraction strategy
In both reviews, data will be extracted independently by one reviewer (with no blinding to authors or journal) using a standardised form and checked by a second reviewer. Discrepancies will be resolved by discussion, with involvement of a third reviewer when necessary.
Quality assessment strategy
Quality assessment will be subject to the types of studies identified but will be undertaken using appropriate and established tools (eg. checklists specifically designed for quality assessment of diagnostic studies such as the QUADAS checklist (QUality Assessment of Diagnostic Accuracy Studies; Whitinget al. , 2003, see Appendix 2). The quality assessment of epidemiological studies is likely to be based on the STROBE statement (Elmet al. , 2007) (see Appendix 2). Quality assessment will be confirmed by a second reviewer.
Methods of analysis/synthesis
Data will be tabulated and discussed in a narrative review. For the review of diagnostic accuracy of TTE in the detection of cardiac sources of stroke or TIA, we will combine data to provide pooled estimates of diagnostic performance where appropriate.
Further information needed
Further clinical data needed for economic modelling will be sought from clinical guidelines and advice from clinical experts. If a large group of data are required, non systematic searches may be undertaken. If studies of prognostic accuracy (ie. the ability of TTE to predict later outcomes in stroke and TIA) are not available, it may be necessary to find data on the risk of later events arising from each clinically important pathology. In considering how each clinically important pathology is treated, details of current NHS practice and data on the benefits and harms of these treatments in the relevant population will be required.
6. Report methods for synthesising evidence of cost effectiveness
6.1 Identifying and systematically reviewing published cost effectiveness studies
The sources detailed in section 5 will be used to identify studies of the cost effectiveness of TTE in the management of first episode diagnosed stroke and TIA patients. An economic search filter will be incorporated into the search strategy to identify relevant studies. Identified economic literature will be critically appraised and quality assessed using the critical appraisal checklist for economic evaluations proposed by Drummondet al. (2005). Existing cost effectiveness analyses will also be used to identify sources of evidence to inform structural modelling assumptions and parameter values for the economic model.
6.2 Development of a health economic model
A de novo economic evaluation of the cost effectiveness of TTE in the assessment of first episode diagnosed stroke and TIA patients in secondary care will be conducted. A model will be developed to identify whether the routine testing of all patients (who do not already have an indication for TTE) would result in more cost effective treatment of patients with stroke and TIA compared with current practice. Cost effectiveness modelling will take account of potential benefits and harms of altered treatment, and (if data allow) will identify any subgroups of patients in whom TTE is most likely to be cost effective.
The primary outcome from the model will be an estimate of the incremental cost per additional quality-adjusted life-year (QALY) gained associated with the use of TTE in the assessment of first episode diagnosed stroke and TIA patients. A lifetime time horizon will be used in order to reflect the chronic effects of stroke and the ongoing risk of further cerebrovascular events and potential mortality. The perspective used will be that of the National Health Services and Personal Social Services. Costs and QALYS will be discounted at 3.5% as recommended in current guidelines (NICE, 2008). Modelling assumptions will be taken from the literature, supplemented by clinical expert opinion where required.
The ScHARR modelling team have published papers using different modelling techniques (such as discrete event simulation (Stevensonet al. , In press a; Stevensonet al. , In press b; Michaelset al. , 2009), transition state modelling (Wardlawet al. , 2009) and meta-modelling (Stevensonet al. , 2004)). The model structure and software used to construct the model will be determined following data collection in order that the most appropriate technique is used for this particular assessment. Clinical experts will be consulted at the conceptual stage to ensure that the structure of the model is appropriate to clinical practice. The model will include estimates of the effects of TTE on the management of different types of stroke and TIA patients, as well as costs of intervention and subsequent downstream costs associated with appropriate and inappropriate care. If data allow, this approach will enable an analysis of whether the cost effectiveness of the use of TTE in the routine assessment of stroke and TIA patients differs between patient groups.
Ideally, health related quality-of-life evidence will be available directly from the review literature. In the absence of such evidence, the mathematical model may use indirect evidence on quality of life from alternative sources. Quality-of-life data will be reviewed and used to generate the quality adjustment weights required for the model. In addition to the reviewed literature, national sources (eg. NHS reference costs (Department of Health), national unit costs (Curtis, 2008), British National Formulary (http://bnf.org)) will be used to estimate resource use and costs for use in the economic model.
It is anticipated that there may be limited evidence for some of the parameters that will be included in the economic model. Therefore, the uncertainty around the parameter estimates will be modelled to take this into account. The uncertainty in the central value for each required parameter will be represented by a distribution, enabling probabilistic sensitivity analysis to be undertaken. This will allow an assessment of the uncertainty to be made. If resources allow, the cost effectiveness of collecting further information will be explicitly explored using Expected Value of Sample Information techniques (Stevenson et al. , In Press; Stevenson & Lloyd-Jones, In Press).
7. Expertise in this TAR team
TAR centre
The School of Health and Related Research (ScHARR) is one of the four Schools that comprise the Faculty of Medicine at the University of Sheffield. ScHARR brings together a wide range of medical and health-related disciplines, including public health, general practice, mental health, epidemiology, health economics, management sciences, medical statistics, operational research, and information science. The ScHARR Technology Assessment Group (ScHARR-TAG) synthesises research on the effectiveness and cost effectiveness of healthcare interventions for the NHS R&D Health Technology Assessment Programme on behalf of a range of policy makers, including the National Institute for Health and Clinical Excellence.
Team members’ contributions
Rachel Jackson (Research Fellow, ScHARR) has experience in systematic reviews of health technologies. She will act as the project lead and lead reviewer on this assessment. She has compiled the study protocol.
Sophie Whyte (Research Associate, ScHARR) has experience in cost-effectiveness analysis. She will undertake the review of cost effectiveness evidence and development of the cost effectiveness model.
Munira Essat (Research Associate, ScHARR) will assist in the systematic reviewing of clinical evidence.
Angie Rees (Information Specialist, ScHARR) is experienced in conducting searches for health technology assessments. She will develop the search strategy and undertake the electronic literature searches.
Matt Stevenson (Senior Research Fellow, ScHARR) assisted in the drafting of the study protocol. He will provide support to the cost effectiveness modelling where appropriate and will oversee the project.
Clinical advisors (including echocardiography and stroke specialists) have been approached by the research team and are to be confirmed.
8. Competing interests of authors
None
9. Timetable/milestones
| Milestone | Date |
|---|---|
| Draft protocol | 30th October 2009 |
| Final protocol | 5th February 2010 |
| Progress report | 29th April 2011 |
| Assessment report | 31st May 2011 |
10. Appendices
Appendix 1: Draft search strategy
Review 1: Prevalence of cardiac sources of stroke and transient ischaemic attack
-
Stroke
-
Cerebrovascular accident
-
Cerebrovascular event
-
Transient ischaemic attack
-
TIA
-
vascular accident.mp.
-
cva.mp.
-
stroke.mp.
-
or/1–8
-
Cardiac source$
-
Cardiac origin$
-
Cardioemboli$
-
Cardiogenic
-
Patent foramen ovale
-
Atrial thromb$/clot$
-
Ventricular thromb$/clot$
-
Cardiac thromb$/clot
-
Cardiac embol$
-
Cardiomyopath$
-
Hypertroph$
-
Atrial sept$
-
Cardiac mass$
-
Cardiac vegetation$
-
Endocarditis
-
or/10–24
-
9 and 25
-
Exp Epidemiologic studies
-
Exp Epidemiology
-
epidemiology.tw
-
Exp Prevalence
-
prevalence.ti
-
Exp Incidence
-
incidence.ti
-
ep.fs
-
or/27–34
-
26 and 35
Review 2: Diagnostic accuracy of TTE for cardiac sources of embolism in stroke and TIA
-
Stroke$
-
Cerebrovascular accident$
-
Cerebrovascular event$
-
Transient ischaemic attack$
-
TIA$
-
vascular accident.mp.
-
cva.mp.
-
stroke.mp.
-
or/1–8
-
Echocardiography
-
Transthoracic echocardiography
-
TTE
-
Transoesophageal echocardiography
-
Transesophageal echocardiography
-
TOE
-
TEE
-
24/Twenty four h$Holter
-
Telemetr$
-
Secondary prevention
-
Cardiac imag$
-
or/10–20
-
Exp sensitivity and specificity
-
Sensitivity.tw
-
Specificity.tw
-
((pre-test ot pretest) adj probability).tw
-
Post-test probability
-
Predictive value$.tw
-
Likelihood ratio$
-
exp diagnosis/
-
di.fs.
-
diagnos$.tw.
-
exp predictive value of tests/
-
value.ti.
-
accuracy.ti.
-
correlat$.ti.
-
or/22–35
-
9 and 21 and 36
Appendix 2: Draft data extraction
Forms are to be adapted from the following tools:
QUADAS (quality assessment of studies of diagnostic accuracy) (Whitinget al., 2003)
Was the spectrum of patients described in the paper and was it chosen adequately?
Were selection criteria described clearly?
Was the method of population recruitment consecutive?
Was the setting of the study relevant?
In light of current technology, was the reference standard chosen appropriate to verify test results?
Was there an abnormally long time period between the performance of the test under evaluation and the confirmation of the diagnosis with the reference standard?
Was the execution of the index test described in sufficient detail to permit replication of the test?
Was the execution of the reference standard described in sufficient detail to permit replication of the test?
Did the whole sample, or a random selection of the sample, receive verification using a reference standard of diagnosis?
Did all patients receive the same reference standard regardless of the index test result?
Were the results of the index test incorporated in the results of the reference standard?
Were the index test results interpreted blind to the results of the reference standard?
Were the reference standard results interpreted blind to the results of the index test?
Was clinical data available when test results were interpreted?
Were uninterpretable/indeterminate/intermediate results reported and included in the results?
Were reasons for drop-out from the study reported?
STROBE (Strengthening the reporting of observational studies in epidemiology) (Elmet al., 2007)
| Title and abstract | 1 |
|
| Introduction | ||
|---|---|---|
| Background/rationale | 2 | Explain the scientific background and rationale for the investigation being reported |
| Objectives | 3 | State specific objectives, including any prespecified hypotheses |
| Methods | ||
| Study design | 4 | Present key elements of study design early in the paper |
| Setting | 5 | Describe the setting, locations, and relevant dates, including periods of recruitment, exposure, follow-up, and data collection |
| Participants | 6 |
|
| Variables | 7 | Clearly define all outcomes, exposures, predictors, potential confounders, and effect modifiers. Give diagnostic criteria, if applicable |
| Data sources/measurement | 8* | For each variable of interest, give sources of data and details of methods of assessment (measurement). Describe comparability of assessment methods if there is more than one group |
| Bias | 9 | Describe any efforts to address potential sources of bias |
| Study size | 10 | Explain how the study size was arrived at |
| Quantitative variables | 11 | Explain how quantitative variables were handled in the analyses. If applicable, describe which groupings were chosen and why |
| Statistical methods | 12 |
|
| Results | ||
| Participants | 13* |
|
| Descriptive data | 14 |
|
| Outcome data | 15 | Cohort study – Report numbers of outcome events or summary measures over time. Case–control study – Report numbers in each exposure category, or summary measures of exposure. Cross-sectional study – Report numbers of outcome events or summary measures |
| Main results | 16 |
|
| Other analyses | 17 | Report other analyses done – eg analyses of subgroups and interactions, and sensitivity analyses |
| Discussion | ||
| Key results | 18 | Summarise key results with reference to study objectives |
| Limitations | 19 | Discuss limitations of the study, taking into account sources of potential bias or imprecision. Discuss both direction and magnitude of any potential bias |
| Interpretation | 20 | Give a cautious overall interpretation of results considering objectives, limitations, multiplicity of analyses, results from similar studies, and other relevant evidence |
| Generalisability | 21 | Discuss the generalisability (external validity) of the study results |
| Other information | ||
| Funding | 22 | Give the source of funding and the role of the funders for the present study and, if applicable, for the original study on which the present article is based |
11. References
- British National Formulary 2009. URL: http://bnf.org.
- British Society of Echocardiography . Clinical Indications for Echocardiography. n.d. URL: http://www.bsecho.org.
- Coull A, Lovett J, Rothwell P. On behalf of the Oxford Vascular Study . Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. British Medical Journal 2004;328:326-8.
- Curtis L. Unit Costs of Health and Social Care 2008.
- National Stroke Strategy. London, UK; 2007.
- NHS reference costs 2007–08. London, UK; 2009.
- Drummond M.F, Sculpher M.J, Torrance G.W, O’Brien B.J., Stoddart G.L. Methods for the economic evaluation of health care programmes. 2005.
- Easton, . Definition and evaluation of transient ischaemic attack: A scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anaesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. Stroke 2009;40:2276-93.
- Elm E. Von, Altman D, Egger M, Pocock S.J, Gotzsche P.C, Vandenbroucke J.P. STROBE Initiative . Strengthening the reporting of observational studied in epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806-8.
- Hatano S. Experience from a multicentre stroke register: a preliminary report. Bulletin of the World Health Organisation 1976;54:541-53.
- Mant J, Wade D.T, Winner S., Stevens A, Raftery J, Mant J., et al. Health care needs assessment: the epidemiologically based needs assessment reviews. Oxford: Radcliffe Medical Press; 2004.
- Meenan R.T, Saha S, Chou R, Swarztrauber K, Pyle Krages K, O’Keeffe-Rosetti M.C, et al. Cost-effectiveness of echocardiography to identify intracardiac thrombus among patients with first stroke or transient ischaemic attack. Medical Decision Making 2007;27:171-7.
- Michaels JA, Campbell B, King B, Palfreyman SJ, Shackley P, Stevenson M. Randomised Controlled Trial and Cost-effectiveness Analysis of Silver-Donating Antimicrobial Dressings for Venous Leg Ulcers: The VULCAN Trial. British Journal of Surgery 2009;96:1147-56.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264-9.
- Reducing brain damage: faster access to better stroke care. London: The Stationery Office; 2005.
- Guide to the methods of technology appraisals. London, UK; 2008.
- NICE clinical guideline 68. London, UK; 2008.
- Palacio S., Hart R.G. Neurological manifestations of cardiogenic embolism: an update. Neurol Clin n.d.;20:179-93.
- Patient UK website . Echocardiography n.d. URL: http://www.patient.co.uk/doctor/Echocardiography.htm (accessed October 2009–10–15).
- National clinical guidelines for stroke. London, UK; 2004.
- Stevenson MD, Oakley J, Chilcott JB. Gaussian process modelling in conjunction with individual patient simulation modelling. A case study describing the calculation of cost-effectiveness ratios for the treatment of osteoporosis. Med Decis Making 2004;24:89-100.
- Stevenson MD, Macdonald FC, Langley J, Hunsche E, Akehurst RL. The cost-effectiveness of bosentan in the UK for patients with pulmonary arterial hypertension of WHO functional class III. Value in Health n.d.
- Stevenson MD, Simpson EL, Rawdin AC. Papaioannou DEA review of discrete event simulation in National Coordinating Centre for Health Technology Assessment funded work and a case study exploring the cost-effectiveness of testing for thrombophilia in patients presenting with an initial idiopathic venous thromboembolism. Journal of Simulation n.d.
- Stevenson MD, Oakley JE, Lloyd Jones M, Brennan A, Compston JE, McCloskey EV, et al. The cost-effectiveness of an RCT to establish whether 5 or 10 years of bisphosphonate treatment is the better duration for women with a prior fracture. Medical Decision Making n.d.
- Stevenson MD, Lloyd Jones M. The cost effectiveness of an RCT comparing alendronate with Vitamin K1. Medical Decision Making n.d.
- Stroke Association . Common Symptoms 2009. URL: http://www.stroke.org.uk/information/what_is_a_stroke/common_symptoms.html (accessed 28/10/09).
- Wardlaw JM, Stevenson M, Chappell F, Rothwell PM, Gillard J, Young G, et al. Carotid artery imaging for secondary stroke prevention: both imaging modality and rapid access to imaging are important. Stroke 2009;40:3511-7.
- Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3.
- Yu E.H, Lungu C, Kanner R.M, Libman R.B. The use of diagnostic tests in patients with acute ischaemic stroke. Journal of Stroke and Cerebrovascular Diseases 2009;18:178-84.
Glossary
- Aortic valve stenosis
- A disease of the heart valves in which the opening of the aortic valve is narrowed.
- Atrial myxoma
- A non-cancerous tumour in the upper left or right side of the heart. It grows on the wall (atrial septum) that separates the two sides of the heart.
- Atrial septal aneurysm
- An abnormally enlarged, bulging and mobile atrial septum. The atrial septum is the membrane that separates the left and the right upper chambers of the heart (the atria).
- Atrial septal defect
- A congenital heart defect in which the wall that separates the upper heart chambers (atria) does not close completely. ‘Congenital’ means that the defect is present at birth.
- Cardiac vegetations
- An abnormal growth of tissue around a valve composed of fibrin, platelets and bacteria.
- False negative
- A patient with a condition who is wrongly diagnosed as not having it.
- False positive
- A patient without a condition who is wrongly diagnosed as having it.
- Left ventricular aneurysm
- Left ventricular aneurysm is due to weakened tissue in the left ventricular wall, which swells into a bubble filled with blood. This in turn may block the passageways leading out of the heart, leading to severely constricted blood flow to the body.
- Mitral valve prolapse
- A valvular heart disease characterised by the displacement of an abnormally thickened mitral valve leaflet into the left atrium during systole.
- Mitral valve regurgitation
- A backflow of blood from the left ventricle to the left atrium of the heart due to mitral insufficiency from incomplete closure of the mitral valve.
- Mitral valve stenosis
- A narrowing of the mitral valve in the heart. This restricts the flow of blood through the valve leading to back pressure that builds up behind the narrowed valve.
- Patent foramen ovale
- A patent foramen ovale is a defect in the septum wall between the two upper (atrial) chambers of the heart. Specifically, the defect is an incomplete closure of the atrial septum that results in the creation of a flap or valve-like opening in the atrial septal wall. A patent foramen ovale is present in everyone before birth but seals shut in about 80% of people.
- Sensitivity
- The effectiveness of a diagnostic test in correctly identifying those with a condition (true positives divided by all those with the condition).
- Specificity
- The effectiveness of a diagnostic test in correctly diagnosing as negative those who do not have a condition (true negatives divided by all those without the condition).
- Spontaneous echo contrast
- Spontaneous echo contrast is a swirling pattern of blood flow, distinct from white noise artefacts, caused by an increased ultrasonic backscatter from aggregation of the cellular components of blood in the conditions of blood stasis or low-velocity blood flow.
- Transoesophageal echocardiogram
- A test using ultrasound waves via a probe passed into the patient’s oesophagus to obtain images of the heart. Ultrasound waves are sent through the probe, which picks up echoes of the sound waves as they bounce off different parts of the heart. These echoes are turned into moving pictures of the heart.
- Transthoracic echocardiogram
- A test using ultrasound waves via a probe passed over the outside of the chest wall to obtain images of the heart. Ultrasound waves are sent through the probe, which picks up echoes of the sound waves as they bounce off different parts of the heart. These echoes are turned into moving pictures of the heart.
- True negative
- A patient without a condition who is correctly diagnosed as not having it.
- True positive
- A patient with a condition who is correctly diagnosed as having it.
List of abbreviations
- AF
- atrial fibrillation
- CEAC
- cost-effectiveness acceptability curve
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CrI
- credible interval
- CT
- computerised tomography
- DARE
- Database of Abstracts of Reviews of Effects
- ECG
- electrocardiogram
- EVPI
- expected value of perfect information
- FN
- false negative
- FP
- false positive
- GOS
- Glasgow Outcome Score
- HRG
- Healthcare Resource Group
- HTA
- Health Technology Assessment
- IC
- intracranial
- ICER
- incremental cost-effectiveness ratio
- ICH
- intracranial haemorrhage
- IST
- International Stroke Trial
- LSR
- Lothian Stroke Register
- MCMC
- Markov chain Monte Carlo
- MRA
- magnetic resonance angiography
- MRI
- magnetic resonance imaging
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- PFO
- patent foramen ovale
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PSA
- probabilistic sensitivity analysis
- PVS
- persistent vegetative state
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- SE
- standard error
- SEC
- spontaneous echo contrast
- SIGN
- Scottish Intercollegiate Guidelines Network
- SMT
- standard medical treatment
- STARD
- Standards for Reporting of Diagnostic Accuracy
- TCD
- transcranial Doppler
- TIA
- transient ischaemic attack
- TMD
- transmitral Doppler
- TN
- true negative
- TOE
- transoesophageal echocardiography
- TP
- true positive
- TTE
- transthoracic echocardiography
- TTEf
- transthoracic echocardiography in fundamental imaging mode
- TTEh
- transthoracic echocardiography in second harmonic imaging mode
- TTEh +ve TOE
- perform TOE on those patients testing positive on TTEh imaging
- TTEh −ve TOE
- perform TOE on those patients testing negative on TTEh imaging
- WTP
- willingness to pay