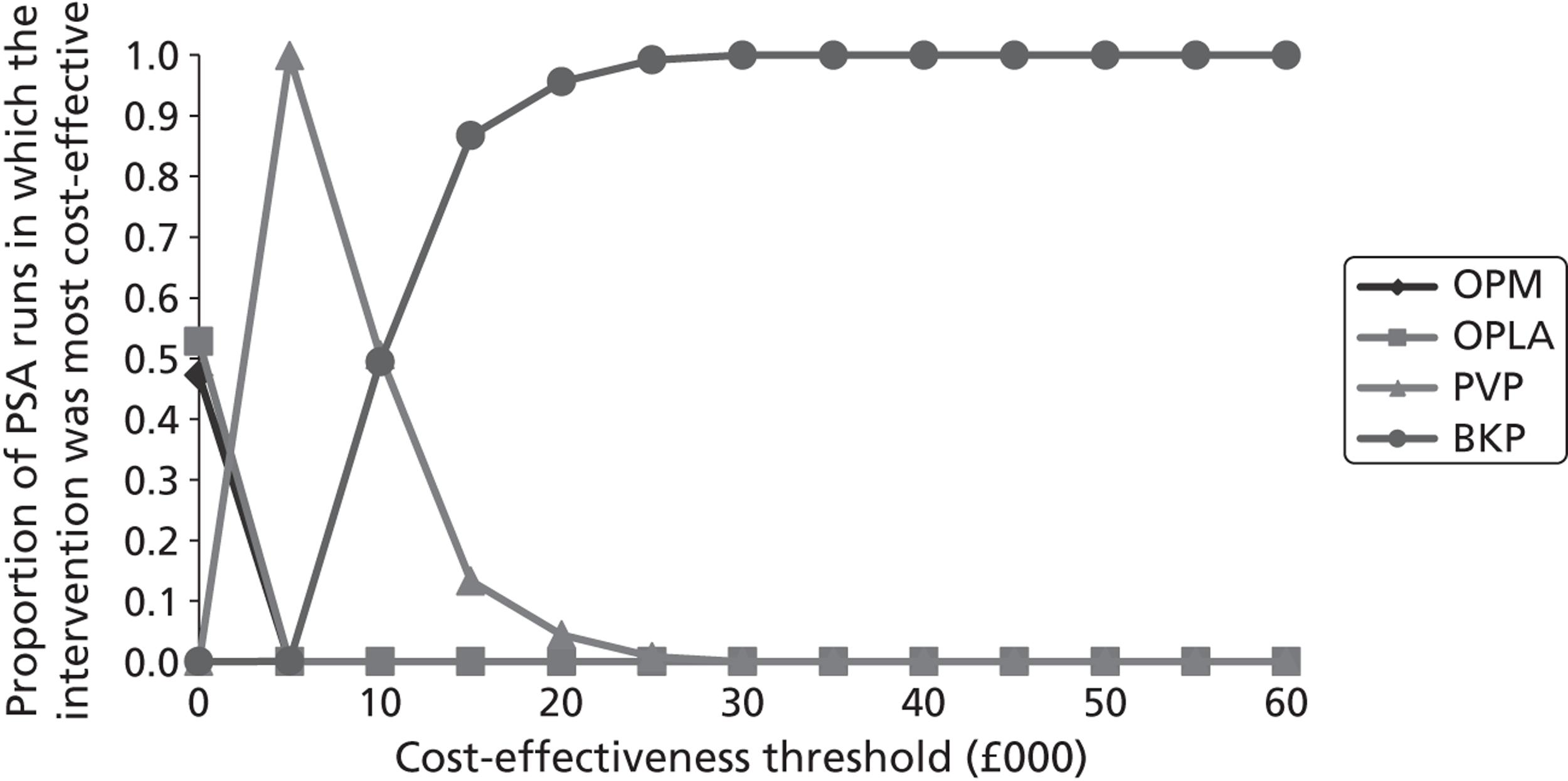
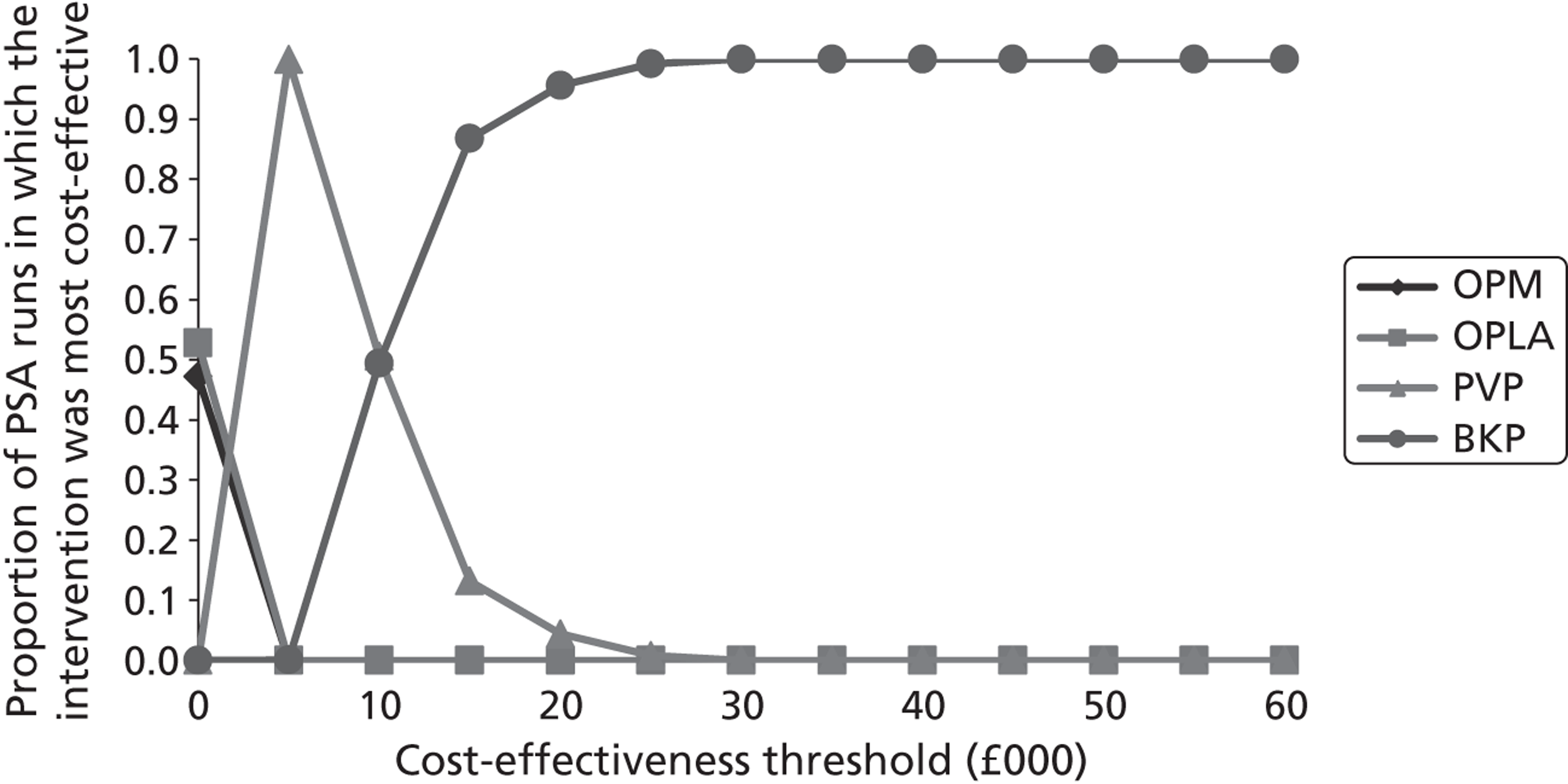
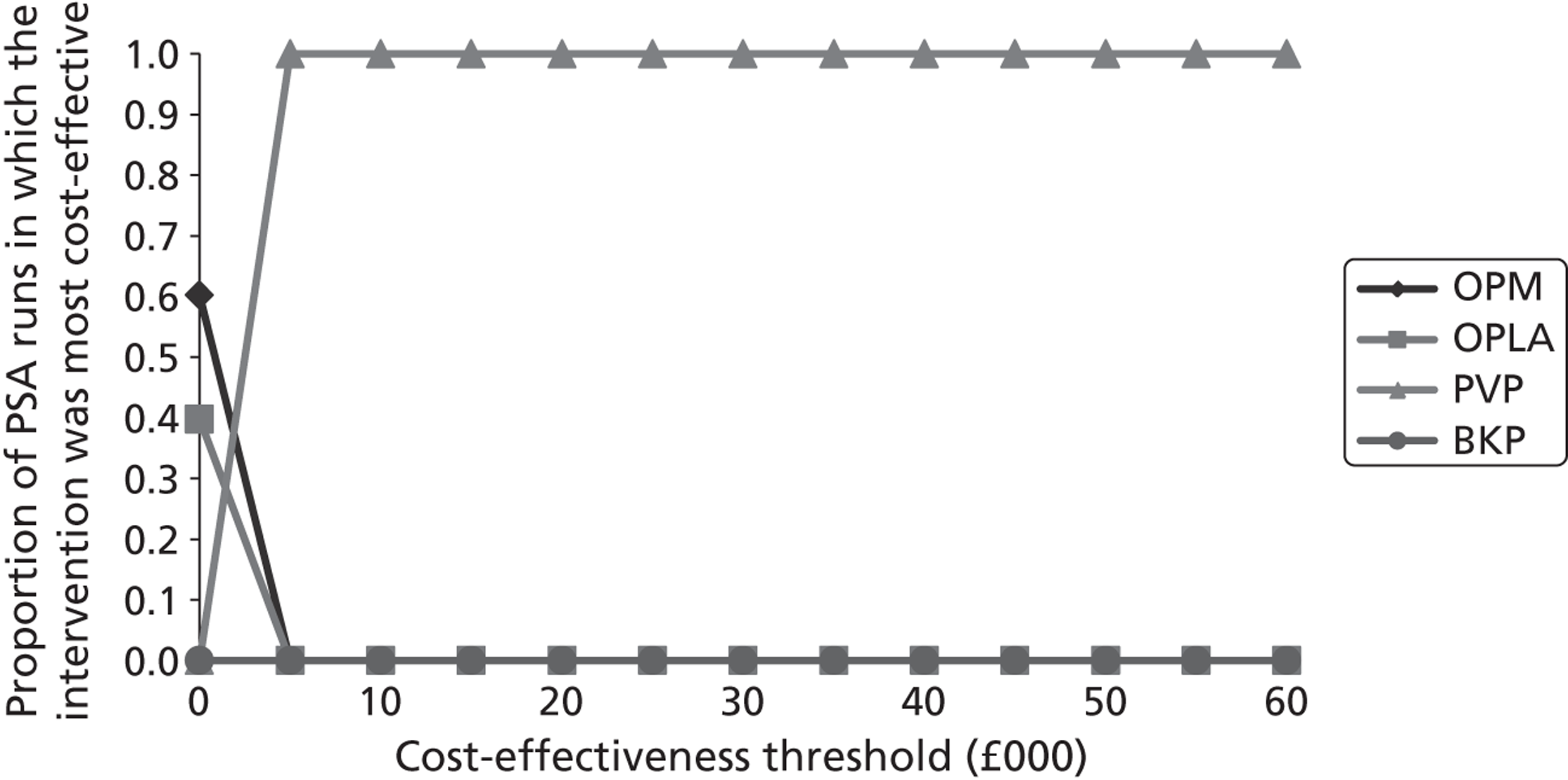
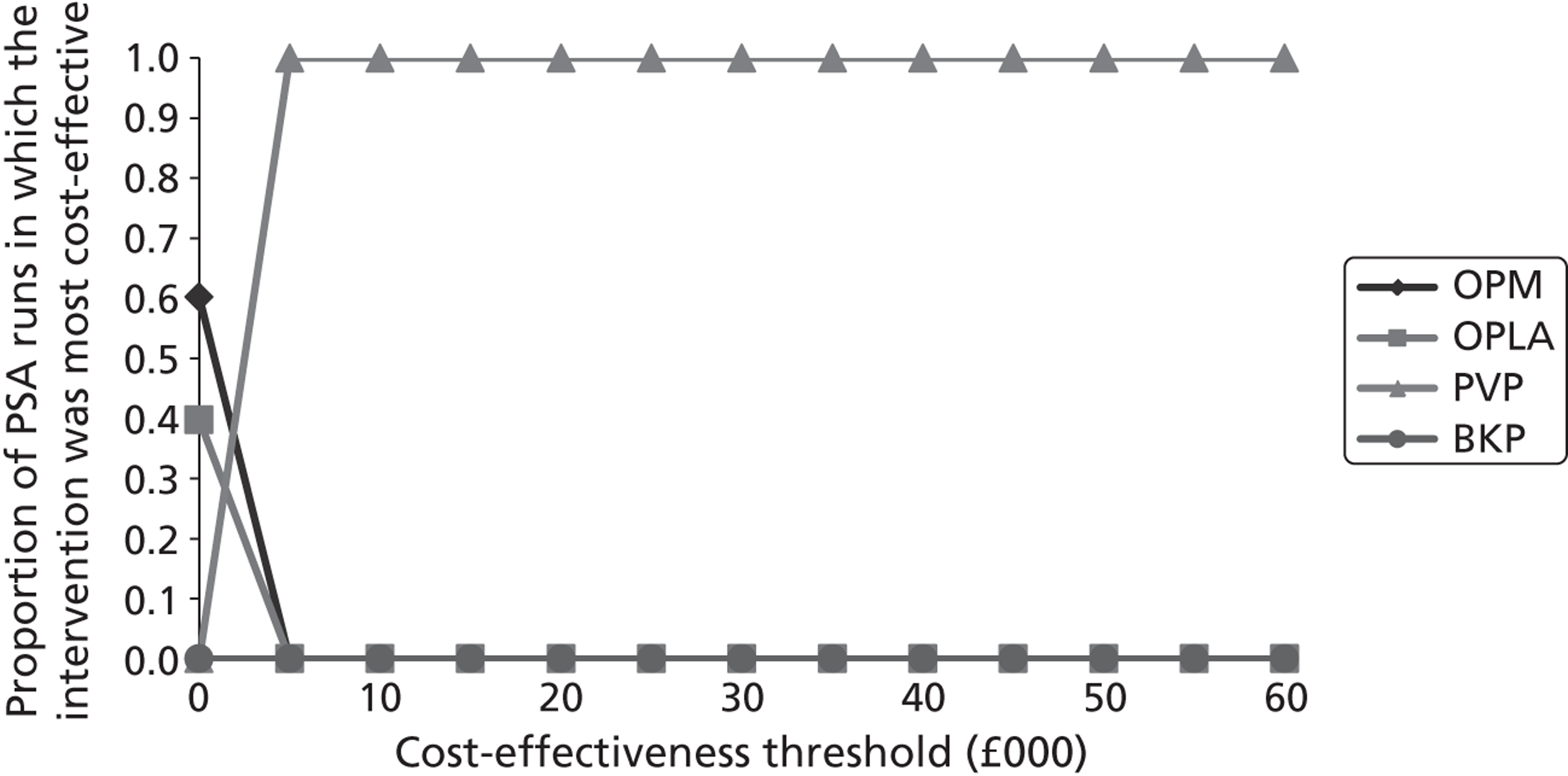
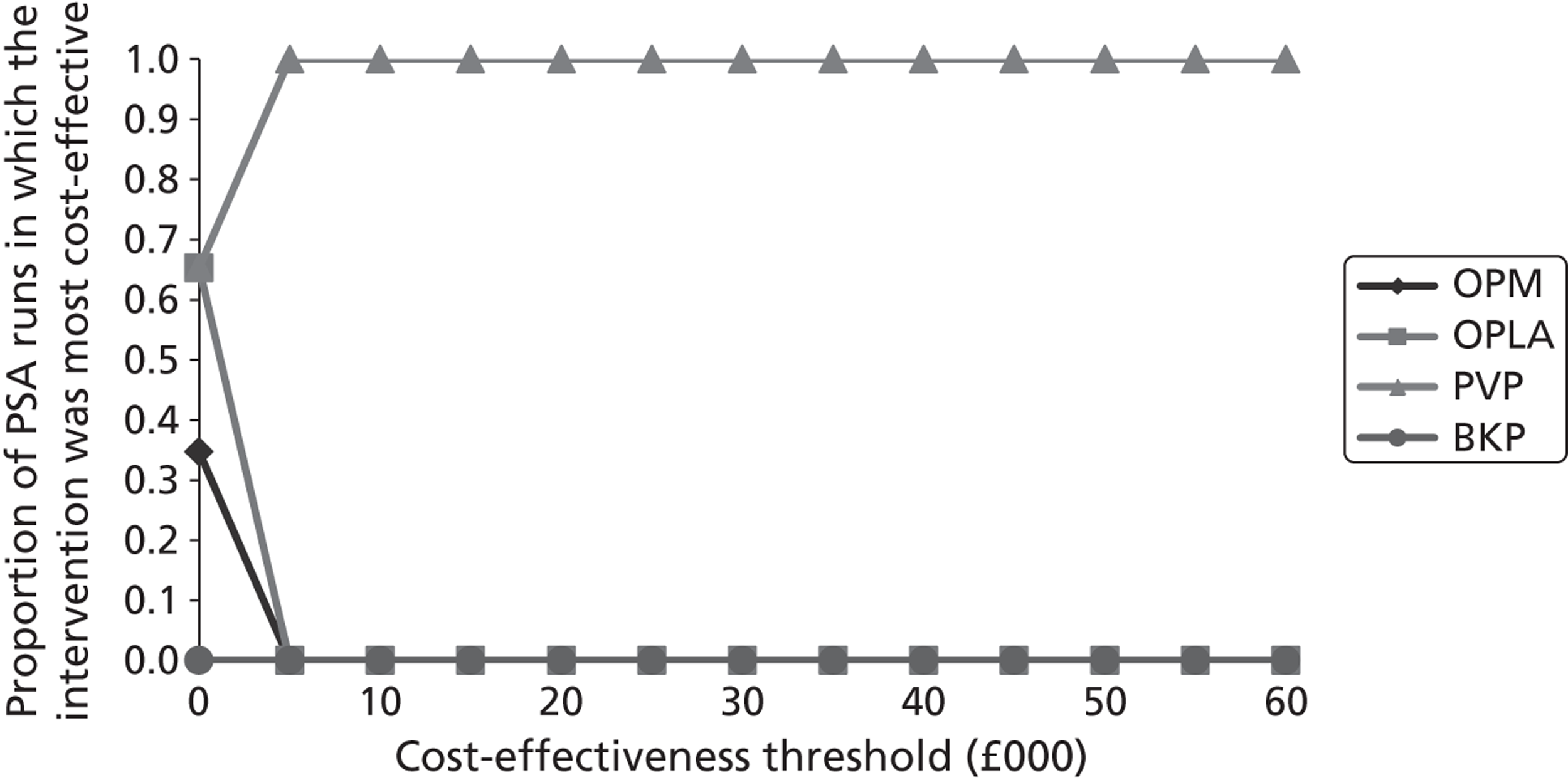
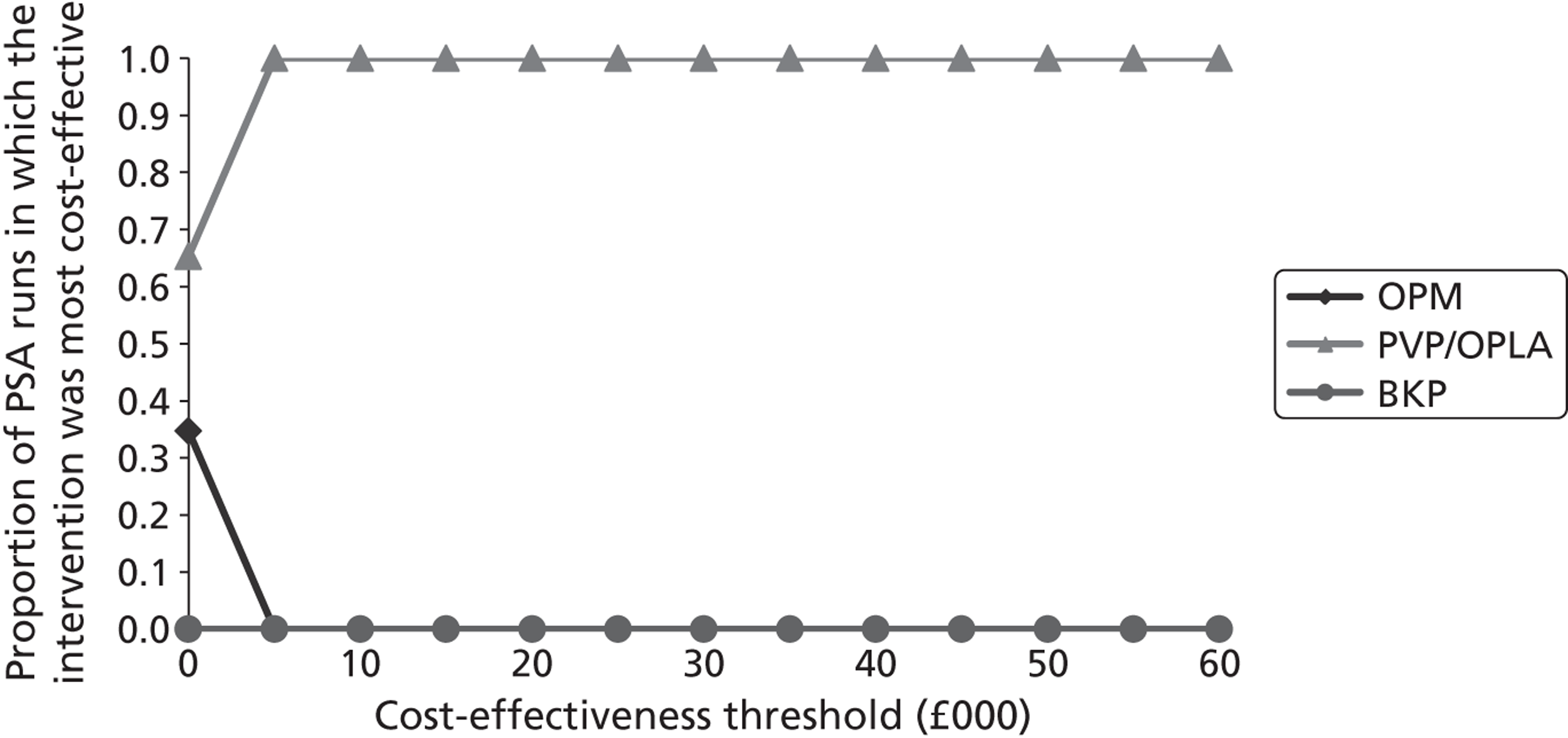
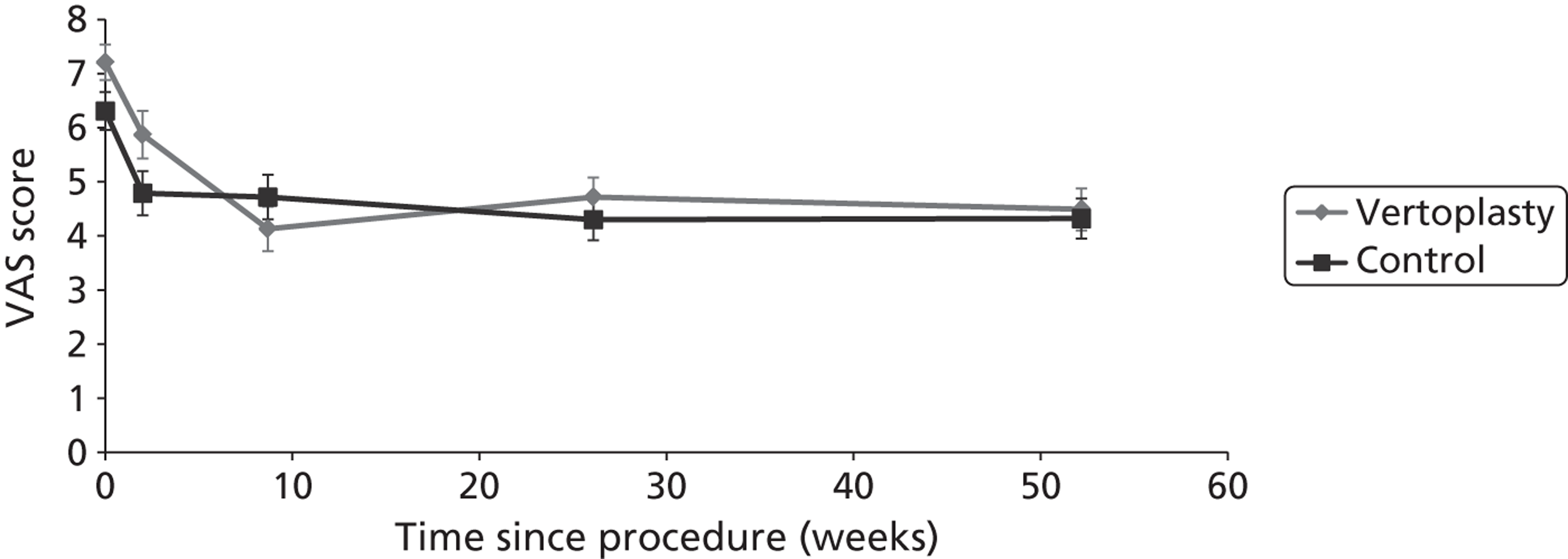
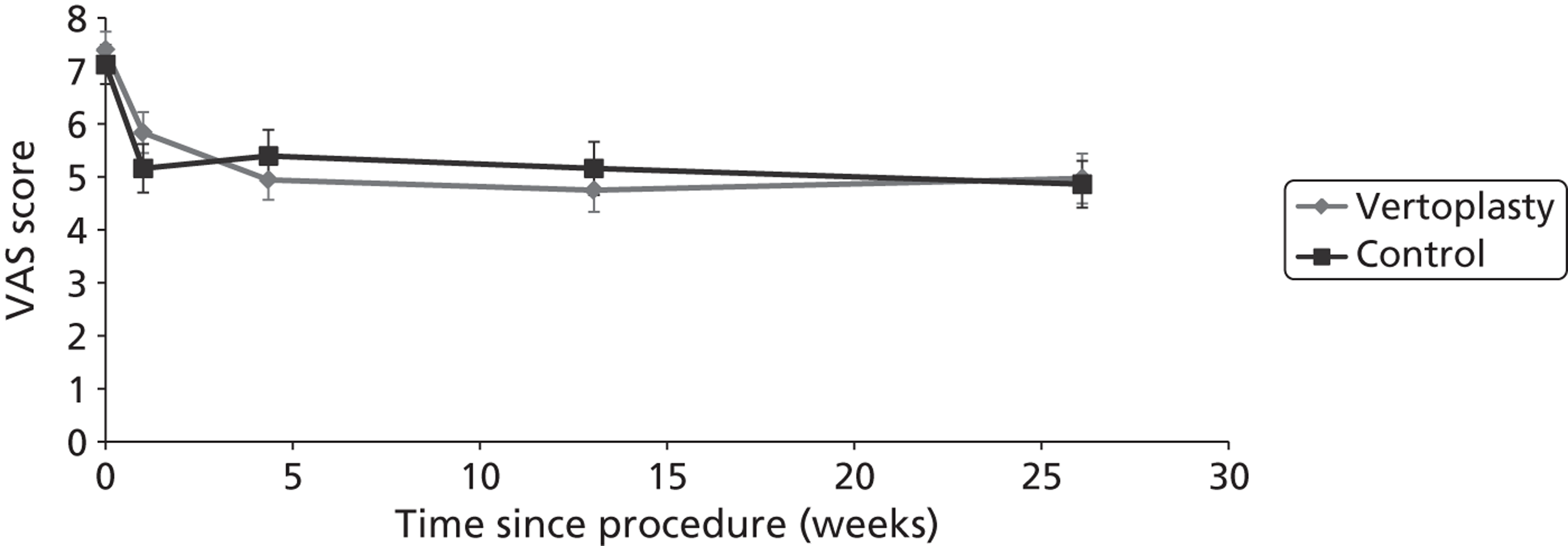
Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 10/122/01. The protocol was agreed in October 2011. The assessment report began editorial review in August 2012 and was accepted for publication in December 2012. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
David Wilson undertakes clinical practice in cement augmentation of the spine in both the NHS and the private sector.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Stevenson et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Description of health problem
Aetiology
Osteoporosis is a systemic skeletal disease characterised by low bone mass and microarchitectural deterioration of bone tissue, with a consequent increase in bone fragility and susceptibility to fracture. 1 A definition of osteoporosis has been developed based on bone mineral density (BMD), as this can be measured with precision and accuracy. This defines osteoporosis in terms of the T-score, which is the number of standard deviations (SDs) by which the individual’s BMD, as measured by dual X-ray absorptiometry (DXA) at the lumbar spine, hip (total hip or femoral neck) and forearm, differs from the average BMD of healthy young women. The BMD osteoporosis threshold proposed for Caucasian women is a T-score of 2.5 SD or more below that average (i.e. a T-score of ≥–2.5); a T-score of between 1 and 2.5 SD below that average (i.e. –1 to –2.5) indicates osteopenia. 2
The clinical significance of osteoporosis lies not in low BMD per se but in the fractures that may occur as a consequence of low BMD: without a fracture, a person suffering from osteoporosis will not suffer morbidity. Fractures are considered to be osteoporotic if they occur in a person with low BMD as a result of little or no trauma – the equivalent of a fall from standing height or lower. 3 Vertebral fractures are among the most common osteoporotic fractures. The risk of such fractures approximately doubles with each SD decrease in lumbar spine BMD. 4 However, as the occurrence of a vertebral compression fracture (VCF), even if asymptomatic, increases the risk of further VCFs by at least fourfold independently of BMD, there appears to be another aspect of bone fragility which is not measured by bone densitometry. 4 Research in women with post-menopausal osteoporosis indicates that, in the absence of antiosteoporotic medication such as bisphosphonates, once a VCF has occurred the risk of a subsequent VCF occurring within 1 year is about 19% [95% confidence interval (CI) 13.6% to 24.8%]. 5 Although about one-quarter of VCFs result from falls, most are associated with routine daily activities such as bending or lifting light objects. 6
In vertebral fracture, or vertebral compression fracture (these terms are used interchangeably within the literature), the vertebra is compressed, leading to a reduction in its height and potentially also to abnormal curvature of the spine (kyphosis). However, there is no universally accepted definition of a VCF. Definitions which depend on a reduction in the height of an individual vertebral body, whether relative or absolute, are restricted in their utility by the need for an earlier image against which to identify the change; they are therefore most commonly used in research studies. The same is true of the most widely accepted definition of VCF, Genant’s semiquantitative method, which classifies changes in vertebral body shape in terms of reductions in overall height and area. 7 In the absence of an earlier image, the reduction in vertebral height may be assessed by comparison with an adjacent undeformed vertebra. VCFs which are identified only on radiographs taken for research, population screening, or other purposes are termed radiographic or morphometric fractures.
Some osteoporotic VCFs are diagnosed clinically, usually when a person presents with back pain and a subsequent radiograph is interpreted as showing a fracture to a vertebral body. However, accurate clinical diagnosis of a new VCF may be confounded by the high prevalence of back pain from other causes, by changes in vertebral morphology which are either longstanding or due to causes other than fracture, or by non-standardised interpretation of spinal radiographs. 8 The evidence from clinical trials in which VCFs are identified radiographically suggests that about two-thirds of VCFs are not brought to clinical attention. 9 This may be because the fractures are associated with no, or only mild, symptoms, or because any symptoms present are attributed to another cause, such as muscle strain. 10 Previously unreported fractures might be identified only when they have caused kyphosis and obvious loss of height. 11 However, kyphosis also occurs in osteoporotic women without VCFs, and in these women it is presumably due to non-skeletal factors such as poor muscle tone and loss of disc height by degenerative spondylosis. 12
Research has shown that women with previously unreported vertebral deformities which are found incidentally during population screening are substantially more likely to have chronic back pain and functional difficulties than women without vertebral deformities. However, women with clinically diagnosed fractures are more likely to report symptoms than those whose fractures are detected only by population screening. 10 Only those patients who present to health care professionals with clinical VCFs and severe pain are likely to be considered for percutaneous vertebroplasty (PVP) or balloon kyphoplasty (BKP). Cummings and Melton4 have suggested that fewer than 10% of radiographically detected fractures – that is to say at most one-third of clinical fractures – are severe enough to require hospital admission. However, patients with fractures of such symptomatic severity are presumably those who are most likely to be offered PVP or BKP.
Osteoporotic VFCs may be due to primary or secondary osteoporosis. Primary osteoporosis is defined as osteoporosis that is not associated with any other illness; it is generally associated with ageing and is particularly common in post-menopausal women. Secondary osteoporosis may be related to certain medical conditions (e.g. hyperthyroidism, malabsorption and extreme dieting) or to prolonged steroid therapy. 13 Most osteoporotic VCFs occur in women with primary post-menopausal osteoporosis. However, because of the increase in chronic steroid use, the incidence of VCFs due to secondary osteoporosis is increasing. 14
The short-term impact of vertebral compression fractures
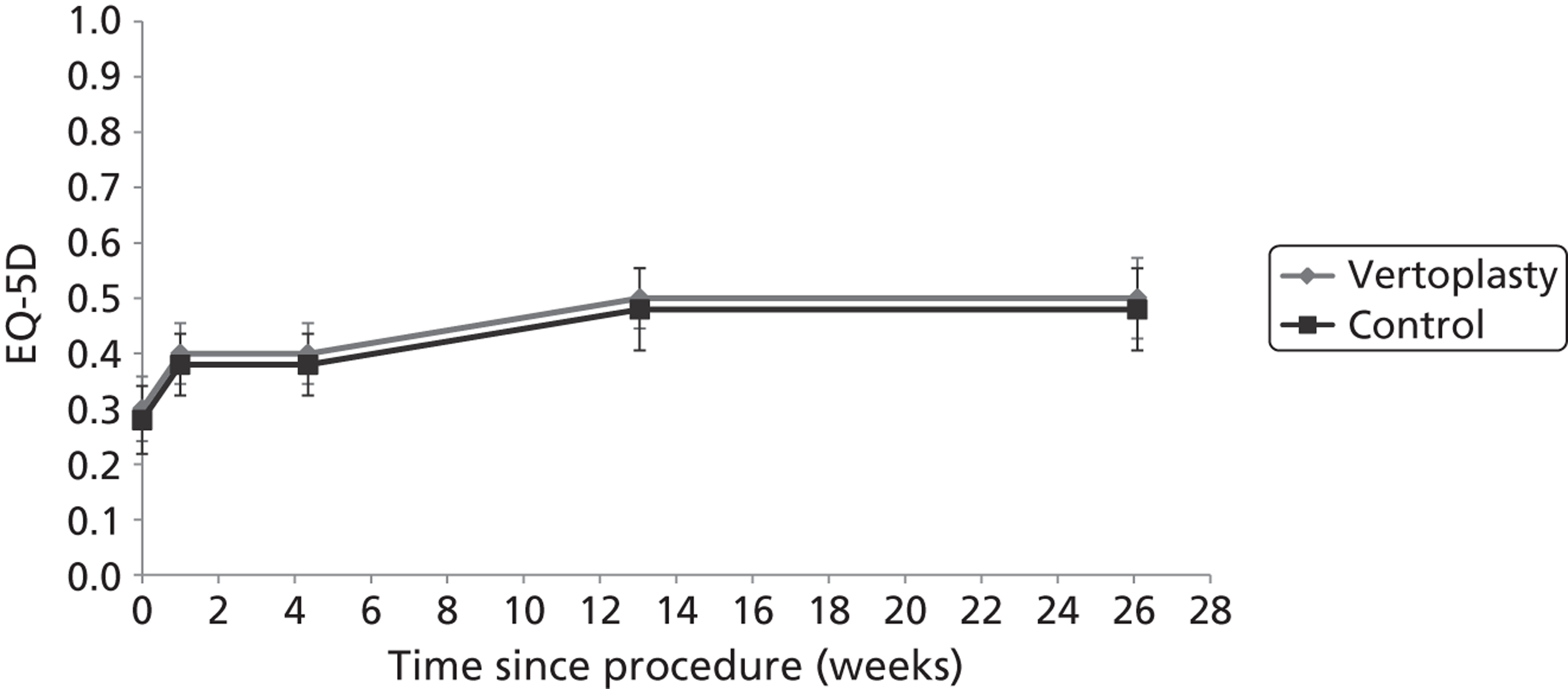
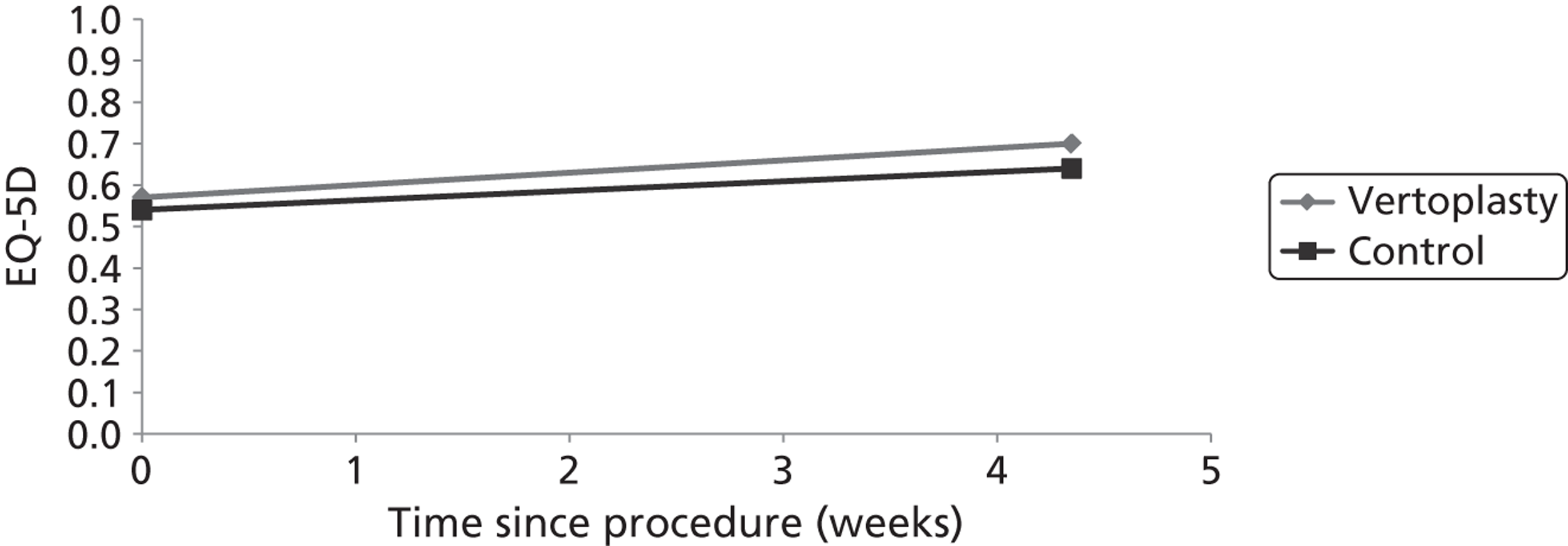
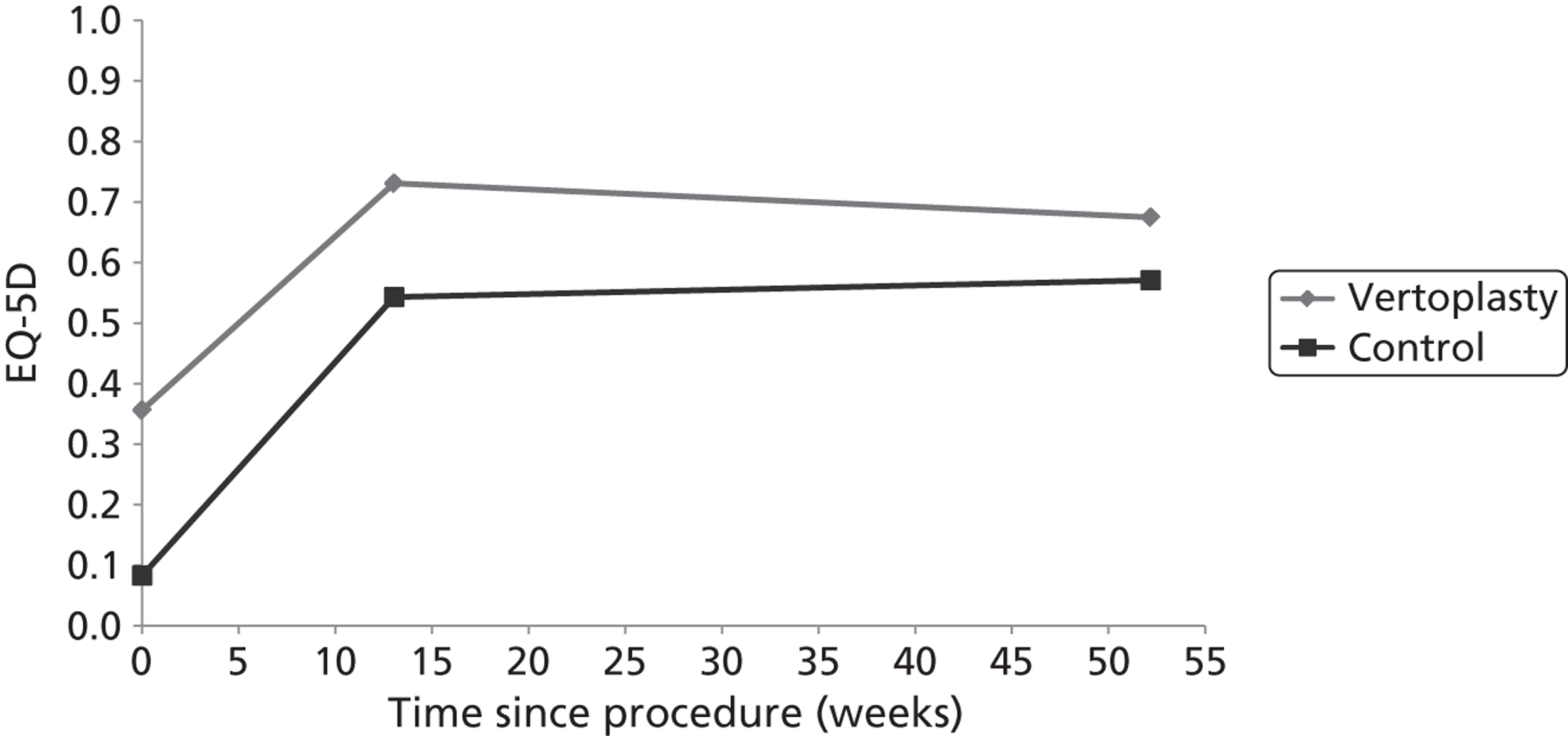
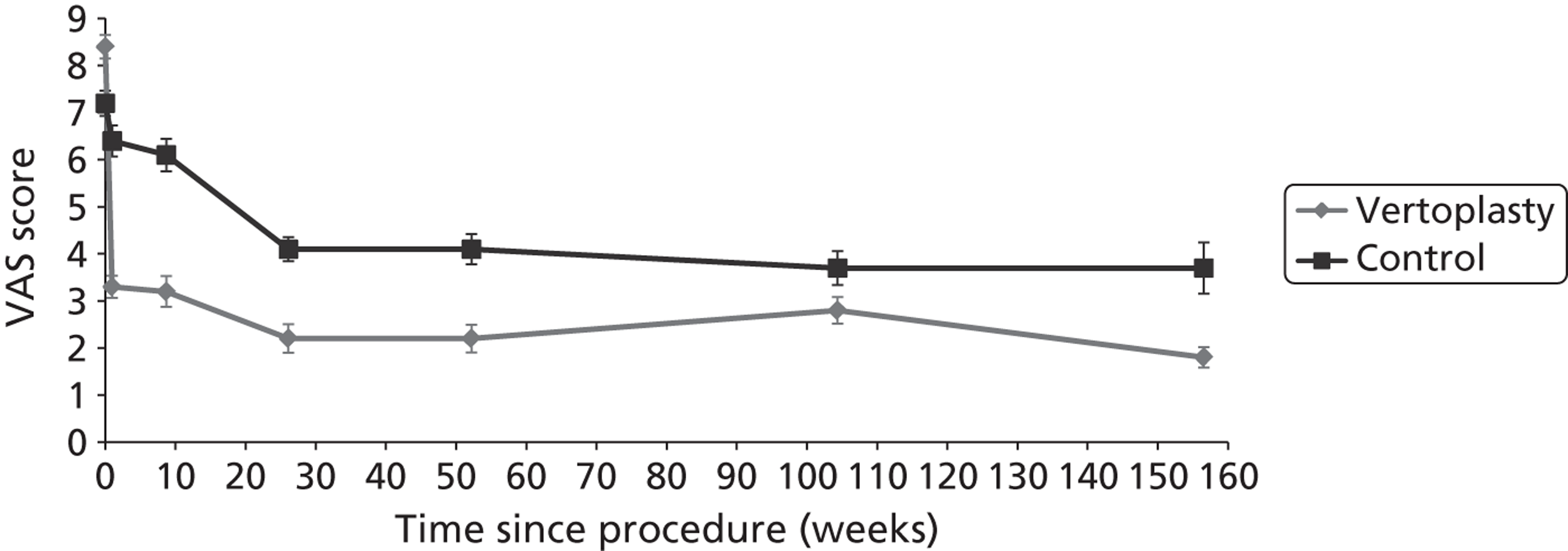
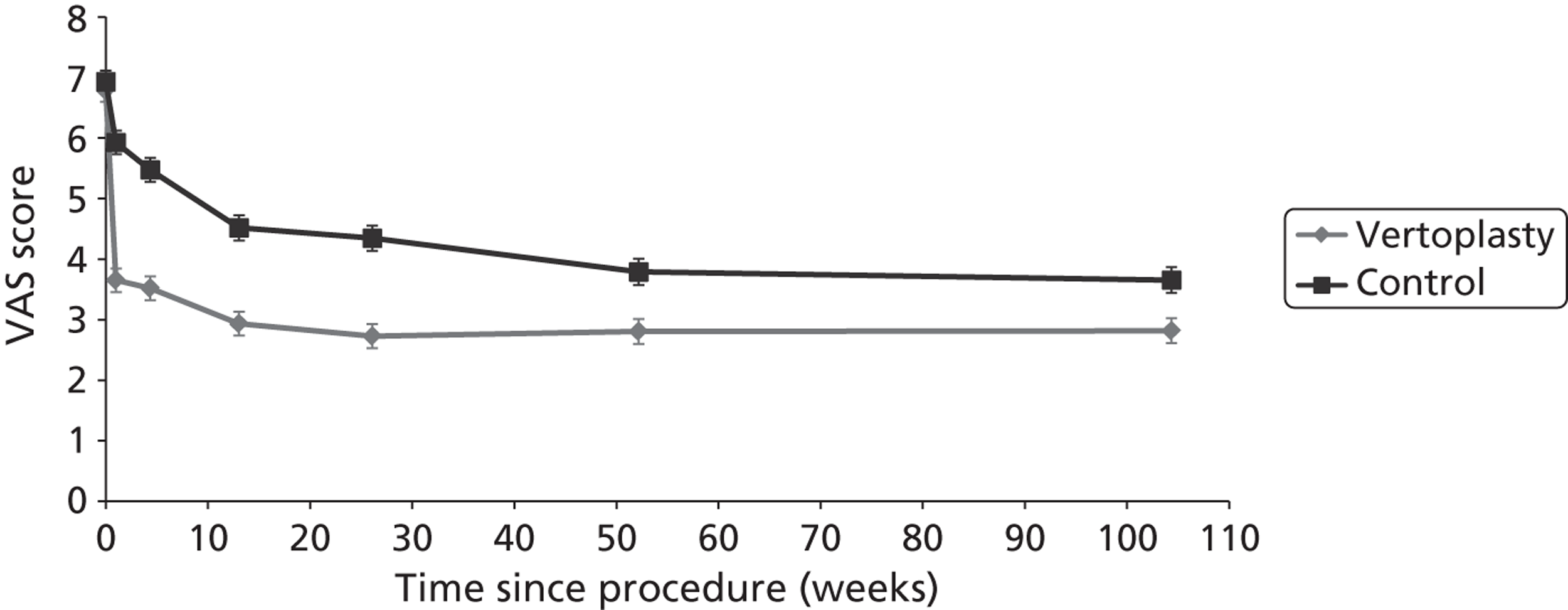
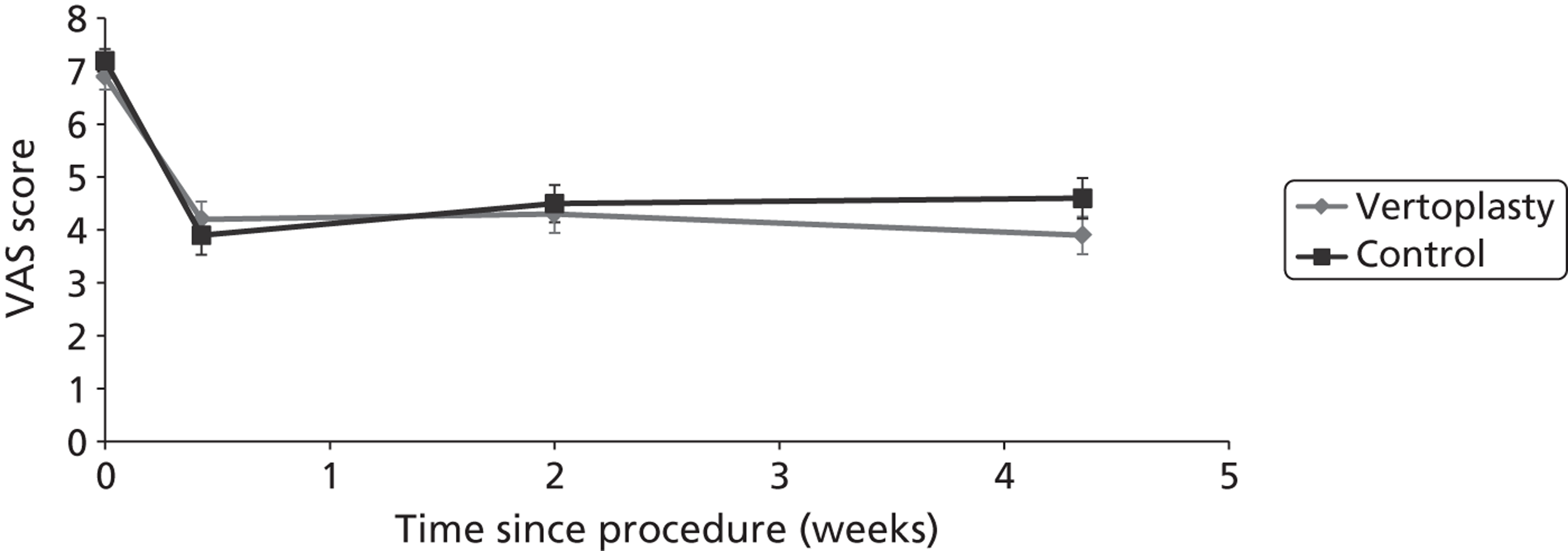
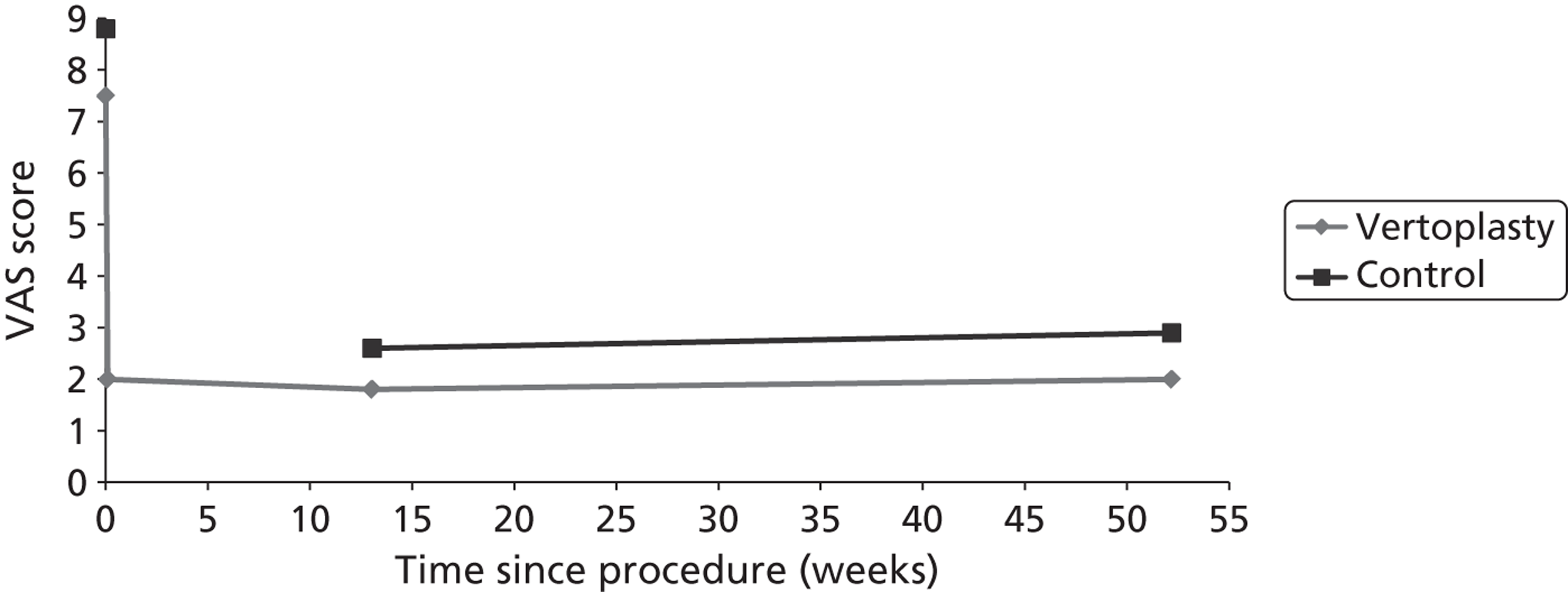
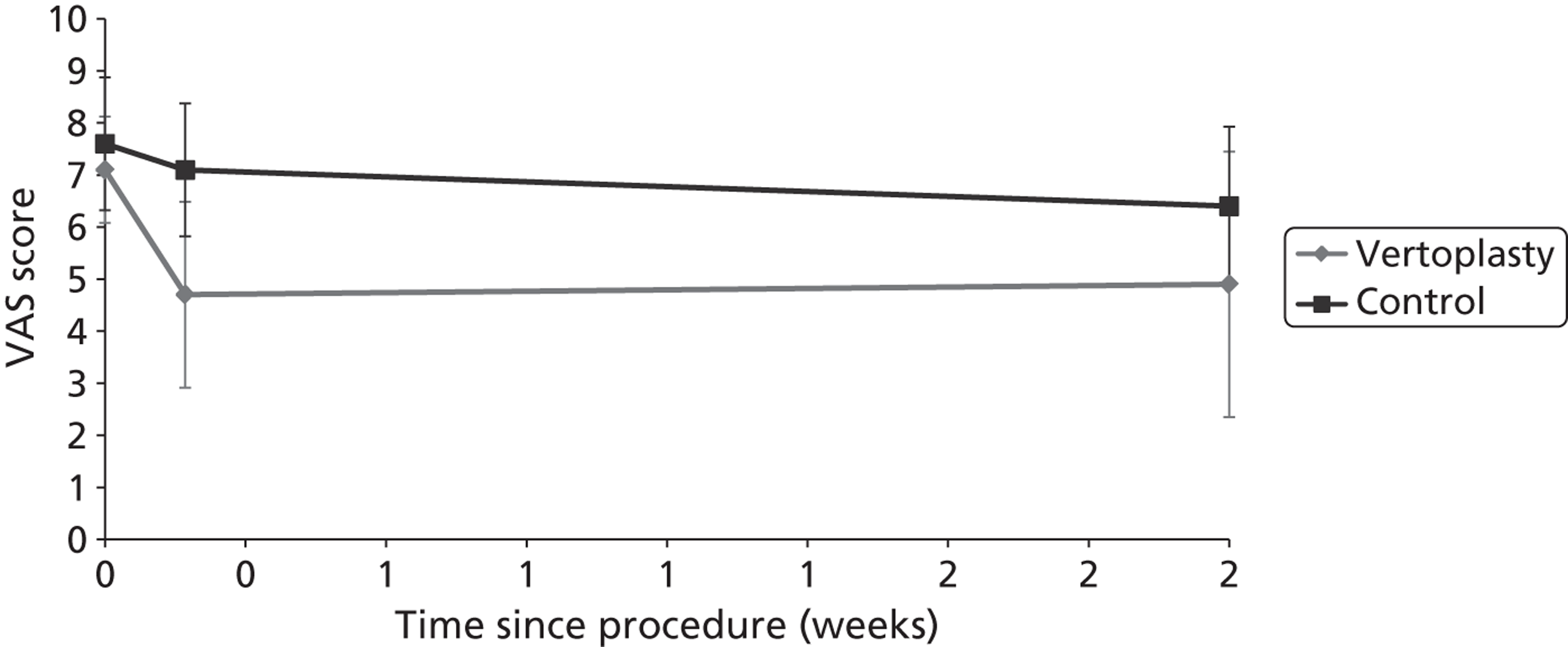
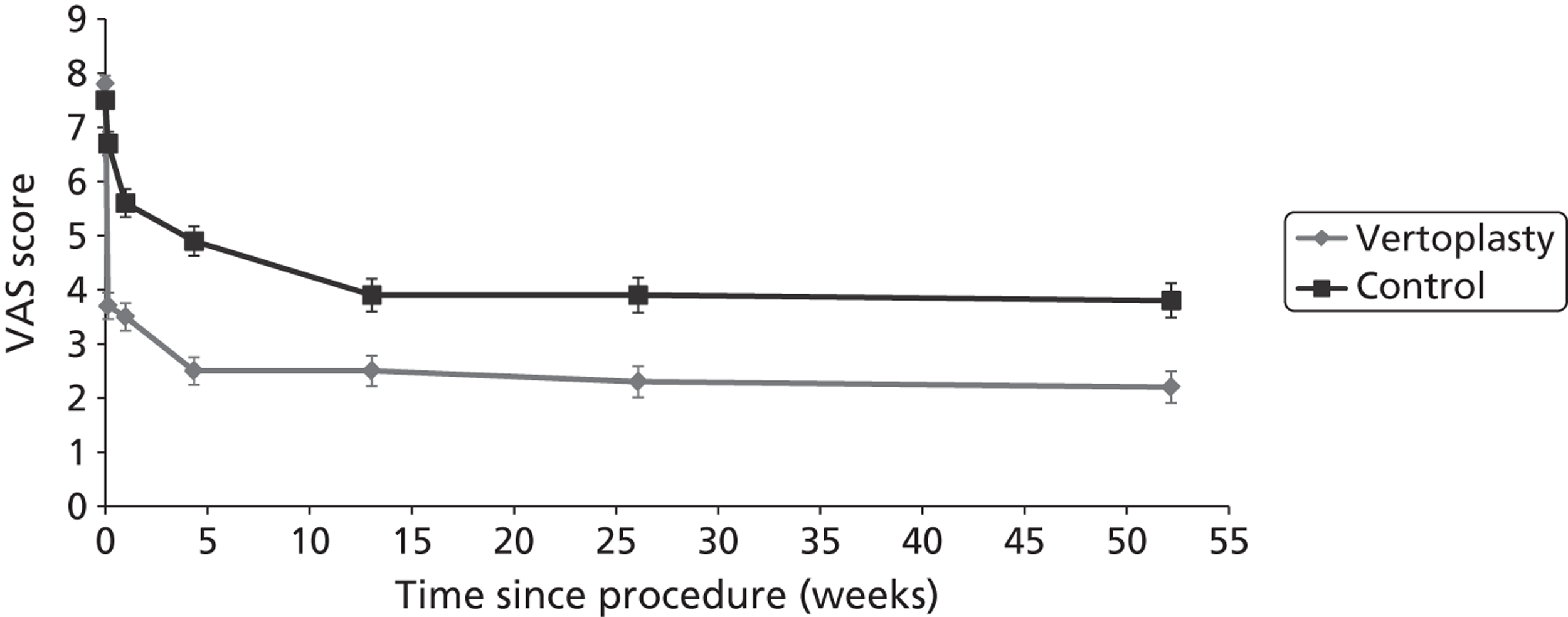
Clinical VCFs can cause considerable acute pain, which may be persistent. This pain is exacerbated by movement and reduced by rest, and may therefore limit mobility;14,15 consequently, patients with particularly severe cases may require hospitalisation. 16 Radiculopathy (pressure on, or other damage to, the nerve root) is not uncommon, and may cause either unilateral or bilateral pain radiating along the affected nerve. 15 Such acute pain is intense at the fracture site and usually lasts 4–6 weeks. This is illustrated by data from the VERTOS II study;17 the study inclusion criteria specified that participants should have had back pain for no more than 6 weeks, and 53% (229/431) of people who initially appeared to be eligible for randomisation became ineligible during the course of the screening process (i.e. in less than 6 weeks from pain onset) because of spontaneous pain relief.
However, in some patients the acute pain associated with a VCF is followed by chronic pain. This often occurs either when one vertebra is particularly severely compressed or when multiple vertebrae are fractured. 18 It may be predominantly caused not by the fracture itself but by strain on muscles and ligaments secondary to kyphosis, and therefore tends not to respond to the management strategies used for acute pain (rest, activity modification, and local and/or systemic analgesics) but may be better addressed through exercise. 10
Investigators have sought means of differentiating patients in whom pain following VCF is likely to resolve relatively quickly from those who are likely to develop chronic pain. Klazen et al. 19 studied conservatively treated patients with a radiographically diagnosed VCF who had had pain for no more than 2 weeks. By 6 months, the mean pain score had decreased significantly (i.e. by 50% or more) from baseline, but no significant decrease was seen between 6 and 23 months; thus, 63% of patients (22/35) had significant pain relief at 6 months, but the proportion had increased only to 69% (25/36) at 23 months. The patients could be divided into two categories: in those with significant pain relief at 23 months, a rapid decline in pain in the first 6 months continued more slowly thereafter, whereas in those without significant pain relief at 23 months, after a small decrease in pain in the first 6 months, there was no further decrease in pain, which might even increase. None of the recorded baseline factors (age, sex, number of VCFs at baseline, conservative therapy frequencies, grade of VCF, or pain medication) predicted significant pain relief at 6 or 23 months, but a high pain score at 6 months predicted no significant pain relief at 23 months [odds ratio (OR) 0.254, 95% CI 0.293 to 0.938, p = 0.030]. However, in a study of osteoporotic post-menopausal women with acute back pain, Lyritis et al. found that those with radiological evidence of a fully collapsed vertebra which was considered responsible for the pain had pain which was severe (9 ± 0.2 on a scale of 0–10) but of short duration (4–8 weeks). By contrast, women with radiological evidence of only a mild fracture, or with no radiological signs of fracture, had on average three attacks of pain, representing gradual fracture progression; thus, the intensity of the pain was less (6 ± 1.8) and the initial attack was of shorter duration, but the time to final resolution was longer (6–20 months). 11
The picture is complicated by the fact that it can be difficult to determine the precise date of occurrence of a VCF. In some cases, following the sudden onset of back pain, conventional radiographs cannot identify a vertebral deformity but scintigraphic imaging may identify a ‘hot spot’ which appears as a typical compression fracture on subsequent radiographs; in other cases, the patient may be identified as having an acute vertebral fracture when the deformity may in fact be seen on earlier radiographs. Moreover, the occurrence of additional episodes of pain associated either with new fractures or with the progression of the original deformity may make it difficult to determine the duration of pain associated with a specific fracture. 10
The longer-term impact of vertebral compression fractures
Patients who have suffered one VCF are not only at risk of developing chronic pain but also at increased risk of suffering another VCF. They are thus also at risk of long-term morbidity caused by the back pain and progressive loss of height and kyphosis associated with multiple fractures, and this in turn may lead to a loss of mobility which will exacerbate the underlying osteoporosis and increase the risk of future fractures. 6
People who have suffered a VCF have higher mortality rates than people of the same age who do not have VCFs. van Staa et al. 20 used data from the General Practice Research Database to compare observed and expected survival in England and Wales in men and women aged 65 and over following vertebral fracture. As these fractures had been recorded in the patients’ medical records, presumably most if not all were clinical rather than radiographic; given the age group being studied, it seems likely that the majority were osteoporotic. A statistically significant excess of mortality was seen in both sexes for up to 5 years following a fracture, but the effect appeared more marked in men than in women ( Table 1 ).
| Men | Women | |||
|---|---|---|---|---|
| Observed | Expected | Observed | Expected | |
| At 3 months | 87.8% | 97.9% | 94.3% | 98.4% |
| At 12 months | 74.3% | 91.8% | 86.5% | 93.6% |
| At 5 years | 42.1% | 64.4% | 56.5% | 69.6% |
It has been suggested that the primary reason for the excess mortality associated with VCFs is the impact on lung function;16 abdominal dysfunction associated with kyphosis may also be a contributory factor. 21 Research has shown that pulmonary function is significantly reduced in patients with primary osteoporosis and vertebral fracture, but not in patients with chronic low back pain without evidence of manifest spinal osteoporosis22 or in healthy control subjects of the same age. 23 A significant association has been found between the number of vertebral fractures and decline in lung function. 24 However, the increased risk of death may also be due, at least in part, to the co-existence of serious underlying diseases in many individuals with VCF. 25 Thus, research carried out in Sweden found that hospitalisation for vertebral fracture (including traumatic fracture) in men and women aged 50 or over was associated with an increase in the relative risk of death compared with the age- and sex-matched population. However, as the risk was particularly high in the younger individuals and decreased with age, it was suggested that this phenomenon might be related to the impact of trauma injuries or other significant comorbidities and secondary causes of osteoporosis. 26 It is also possible that the complications associated with long-term opioid analgesic use, such as respiratory depression, anorexia, and bowel obstruction associated with constipation, contribute to excess mortality.
Similarly, in the USA, a retrospective study was carried out in all residents of Rochester, MN, who had been diagnosed with one or more clinical vertebral fractures between 1985 and 1989; the maximum follow-up appears to have been 5 years, and the mean around 2.4 years. This study found that survival was significantly impaired in the short to medium term in the 276 patients who experienced fracture following mild to moderate trauma (defined as less than or equal to a fall from standing height), and whose fractures were not associated with primary or metastatic cancer or localised bone disease. The most commonly reported causes of death in such patients were cardiovascular diseases (43% – mainly coronary artery disease) and malignancies (18%); the mortality due to coronary artery disease or stroke was not higher than expected, but mortality due to cancer and other causes was elevated. However, relative survival data were presented for all people with clinical vertebral fractures (i.e. including fractures associated with severe trauma or in areas of bone affected by primary or metastatic cancer or localised bone disease). Cooper et al. 27 note that the gradual divergence of observed from expected survival suggests that the impaired survival is unlikely to result from the vertebral fracture per se, but is more likely to be due to an indirect association with comorbid conditions which lead to an increased risk of death, with the fractures simply representing a marker of increased frailty.
In 1991, Browner et al. 28 published data from the Study of Osteoporotic Fractures (SOF) indicating that, in elderly women, osteopenia was associated with an elevated risk of non-trauma mortality, especially deaths from stroke. Subsequently, analysis of data from the FIT trial29 found that, in primarily healthy post-menopausal Caucasian women with osteoporosis or osteopenia, the age-related relative risk of dying following a clinical vertebral fracture was 8.64 (95% CI 4.45 to 16.74). Despite the fact that only 122 women died during the follow-up period of 3 to 4 years (99 before suffering a fracture at any site, and only 11 following a vertebral fracture), the risk was clearly elevated (although the CIs were wide) and remained virtually unchanged when adjusted individually for other factors (hypertension, smoking, physical activity, health status, cardiovascular disease, diabetes and hip BMD). All 11 deaths following a clinical vertebral fracture occurred within 1 year of that fracture. However, the authors note that the elevated risk of death following clinical vertebral fracture may reflect an ascertainment bias, whereby women with more medical conditions and poorer health are more likely to receive a diagnosis of clinical vertebral fracture because they would be under greater medical surveillance. They also note that they were unable to estimate whether a death following a fracture was due to the fracture itself or to an underlying medical condition. Consequently, clinical vertebral fractures may be a marker for increased mortality rather than being independently linked to an increased risk of death. 30
Incidence and/or prevalence
As Cummings and Melton4 have noted, it is difficult to establish the total incidence and prevalence of VCFs both because of the lack of a universally accepted definition of VCF and because a substantial proportion of VCFs do not come to clinical attention. However, although structural deformity associated with VCFs might lead to serious morbidity and mortality, it is currently only symptomatic fractures which come to clinical attention that are candidates for PVP or BKP in the UK, and thus only the incidence of clinically diagnosed fractures is relevant to the current technology assessment. Therefore, we have not evaluated the possibility of the early use of BKP to address sagittal balance.
The prevalence of VCFs varies from country to country, and a number of factors – including environment, genetics, availability of diagnostic tests and willingness of radiologists to report fractures – are likely to play a part. 6 It is therefore important, for the current technology assessment, to identify the incidence of clinically diagnosed VCFs specific to England and Wales. However, as Ström et al. 31 note, data on the incidence of clinical vertebral fractures are not available for the UK. Holroyd et al. 6 have recently estimated that there are 2188 hospital admissions per year in England and Wales for vertebral fractures in patients aged 45 and over. While it is not fully clear what data were used to inform this estimate, the most likely source appears to be the UK General Practice Research Database, which Ström et al. 32 have suggested is likely to incorporate substantial under-reporting of clinical VCFs. Hospital Episode Statistics (HES) data, which relate to hospital admissions and outpatient attendances, appear to provide the most reliable data relating to clinical fractures of sufficient severity to be considered for vertebral augmentation. However, Synthes33 and Medtronic34 have produced incompatible estimates based on HES data:
-
Synthes have estimated, on the basis of 2010–11 HES data, that 20,908 patients per year are diagnosed with osteoporotic VCF in the UK. 33 As the most recent available statistics35 indicate that the population of England and Wales is approximately 89% of that of the UK as a whole, Synthes’ estimate suggests that approximately 18,600 patients in England and Wales are diagnosed with osteoporotic VCF each year; presumably only a proportion of these will then be hospitalised as a result of osteoporotic VCF.
-
Medtronic reported HES data indicating that in 2008–9, 2009–10 and 2010–11, approximately 24,000 patients per year in England and Wales were hospitalised for osteoporotic VCF, while in 2010–11 the total number of patients admitted to hospital for osteoporotic VCFs, vertebral fatigue or collapsed fractures was 27,051. 34
Thus, Medtronic’s estimate appears to be substantially higher than that of Synthes.
In their sponsor submission, Johnson & Johnson36 estimated the number of patients per annum in England and Wales who were hospitalised with debilitating pain from osteoporotic VCFs using data from Dr Foster Intelligence, which routinely collects and analyses data from NHS hospitals in England (http://drfosterintelligence.co.uk). On this basis, 7073 patients a year were identified as potential candidates for vertebral augmentation. This figure appears to apply to England alone, but this is not wholly clear. It is substantially lower than the figures put forward by Synthes and Medtronic; the submission indicates that this is owing to the exclusion of patients with diagnoses other than osteoporosis (e.g. malignancy or trauma),36 thus making it more relevant to the decision problem.
The School of Health and Related Research (ScHARR) model uses data on the incidence of vertebral fractures drawn from a different source, a large-scale prospective Scottish study. 37 The figures from this study were the basis for a clinical effectiveness and cost-effectiveness model which has been used in previous National Institute for Health and Care Excellence (NICE) assessments of osteoporosis interventions. These are UK-specific data and explicitly report vertebral fracture rates rather than relying on estimating these from hip fracture incidence data.
Impact of health problem
Significance for patients in terms of ill health (burden of disease)
The significance for patients of VCF falls into three main categories:
-
pain
-
physical changes and impairment
-
psychosocial decline. 10
These will be discussed in turn below. However, it should be noted that the categories are not entirely independent: pain contributes to physical impairment, and both pain and physical impairment contribute to psychosocial decline. 10
Pain
Vertebral compression fractures are associated with both acute and chronic pain. Acute pain typically lasts for several weeks or months until the fracture heals. It varies widely in severity, and at worst is described as intolerable; however, it may respond to analgesics. By contrast, chronic pain, which can develop when kyphosis causes strain on muscles and ligaments, often does not respond to analgesics, but may respond to exercises which increase the tone and strength of the back muscles. 10,38
In a small case–control study, Lyles et al. 39 found that pain, as measured by the West Haven-Yale Pain Inventory, was significantly worse in women with VCFs than in matched control subjects (p = 0.001).
Physical and functional outcomes
Vertebral compression fractures, and in particular multiple fractures, are associated with decreases in stature and progressive kyphosis which cause loss of lung volume and loss of appetite. 10,24 In the USA, a prospective cohort study of women aged 65 or over found that severe kyphosis was related to pulmonary deaths [hazard ratio (HR) 2.6, 95% CI 1.3 to 5.1]. 40 However, Ettinger et al. 41 found that, in a sample of 610 white women aged 65 to 91, despite greater spinal curvature and height loss, the 10% with the most severe thoracic kyphosis did not report significantly greater back pain or back-related disability, or consider themselves to have poorer health, than the other women.
Vertebral fracture can also lead to a loss of spinal mobility, which causes problems with the activities of daily living. If the fracture is accompanied by acute pain which limits physical activity, this may lead to muscle weakness which may in turn contribute to chronic pain. 10 The rate of decline in BMD also appears to decrease with physical inactivity, and may decrease by as much as 40% during bed rest or post-fracture recovery, thus greatly increasing the risk of subsequent fractures. 10
The preservation of independence in elderly community-living individuals depends substantially on the extent to which they are able to perform everyday activities such as shopping and preparing meals. 42 A number of studies have found an association between symptomatic VCF and problems with such activities. In small studies, Cook et al. 43 found that over 80% of post-menopausal women with a diagnosis of chronic back pain due to osteoporotic VCF reported problems with physical functioning and activities of daily living, while Lyles et al. 39 found that women with two or more confirmed VCFs were significantly more likely than age- and race-matched control subjects with equivalent comorbid conditions to report pain and difficulty in performing functional activities, and to say that their health problems interfered with their daily activities (p = 0.002). Moreover, a population survey of 1010 white community-dwelling Californian women aged 55 and over found that those with clinically diagnosed osteoporotic VCFs were significantly more likely to report difficulty in activities such as lifting, shopping and cooking meals than women without known vertebral fractures [adjusted ORs 3.42 (95% CI 1.23 to 9.50), 5.20 (95% CI 1.61 to 16.78) and 6.93 (95% CI 1.55 to 30.99) respectively]. 44 The SOF,42 a prospective US study of 9704 ambulatory white women aged 65 and over, also found that a history of clinically diagnosed VCF was strongly predictive of impaired function (age-adjusted OR 2.32, 95% CI 1.89 to 2.86). Finally, Ryan45 found that 60% of women with symptomatic VCF attending a specialist bone clinic reported disturbed sleep; there was a significant association between sleep disturbance and the severity of vertebral deformities (p < 0.05). However, Ettinger et al. 46 found that women aged 55 to 75 with moderate to severe vertebral deformities were no more likely to require help at home because of their back than were similar women without vertebral deformities.
Psychosocial outcomes
Ross10 has identified four categories of psychosocial problem associated with osteoporosis. These relate to:
-
quality of life
-
fears, anxiety and depression
-
self-esteem
-
social support and roles.
However, he notes that these categories often overlap.
Quality of life
In people with osteoporotic fracture, quality of life can deteriorate quickly, even when physical function is not drastically affected, if changes in physical appearance, fear of fracture, and impediments to social function cause loss of self-esteem. 10 While most of the relevant research has been performed in post-menopausal women, men with VCFs and primary or secondary osteoporosis attending a UK hospital bone clinic scored much more highly in all six domains of the Nottingham Health Profile than age-matched or elderly male control subjects; the difference was particularly marked for energy, pain and physical mobility. The physical mobility scores indicated greater disability in men with secondary osteoporosis than in those with primary osteoporosis (p < 0.05). 47
Fears, anxiety and depression
Symptomatic VCFs are associated with fears, anxiety and depression, which may relate to fear of future fractures, fear of loss of independence, and a feeling of hopelessness resulting from being told to avoid activities such as bending, twisting and lifting heavy items, without being given advice on how to compensate. 10 Although post-menopausal women with a single VCF retain a good quality of life, once they have more than one fracture their quality of life is adversely affected by high levels of anxiety largely caused by fear of future fractures. 38 Such anxiety often leads to inactivity, which in turn can exacerbate BMD loss and declines in physical fitness, thus increasing the risk of falling. 10,38
In a small case–control study, Lyles et al. 39 found that psychiatric symptoms, as measured by the Hopkins Symptom Checklist Revised (SCL-90-R), were significantly worse in women with VCFs (p = 0.043) than in matched control subjects; however, there was no significant difference in depression as measured by the Beck Depression Inventory (p = 0.129). Cook et al. 43 found that emotional problems were common in post-menopausal women with chronic back pain due to VCF: 82% reported fear of falling, while 66% reported frustration and 53% reported anger. Unfortunately, this study did not include a control group of similar women without chronic back pain due to VCF.
Self-esteem
Vertebral compression fractures may lead to height loss, spinal deformity and abdominal protrusion, which adversely affect self-image and self-confidence, and to functional limitations which may lead to a loss of self-esteem by limiting independence and the ability to participate in social activities. 38 Even relatively mild chronic pain may cause discomfort which discourages participation in social activities that involve sitting or standing for extended periods. Moreover, spinal curvature and height loss may make it difficult or impossible to sit or stand erect, causing problems with conversation and other activities. 10 Cook et al. 43 found that over 50% of post-menopausal women with a diagnosis of chronic back pain due to osteoporotic VCF reported problems with leisure/social activities. However, in a small case–control study, Lyles et al. 39 found that women with VCFs and matched control subjects did not differ significantly in self-esteem as measured by the Rosenberg Self-Esteem Scale (p = 0.731).
Social support and social roles
The pain and physical impairment caused by VCFs can undermine the reciprocity involved in interpersonal relationships by reducing the ability to provide help and support to family and friends, while potentially increasing the need for assistance with activities of daily living and other personal care. If people are obliged to give up work, domestic, recreational or sexual activities because of the limitations on their physical and functional abilities, they may also be deprived of their social roles. 38 The impact may be severe even if the activities in question do not seem to others to be demanding; the inability to stand or sit for extended periods may limit involvement in social events, leading to an inability to fulfil the social roles that form an important source of self-esteem, and thus to a severe reduction in quality of life. 10
Significance for the National Health Service
Osteoporotic VCFs are associated with significant morbidity, mortality and health and social care costs. 40,48 In a large UK-based study, Puffer et al. 49 found that, compared with matched control subjects, women diagnosed with osteoporotic VCFs had significantly more general practitioner (GP) consultations (difference 4.69, 95% CI 4.35 to 5.03, p < 0.001), referrals (difference 0.51, 95% CI 0.45 to 0.58, p < 0.001) and hospital admissions (difference 1.77, 95% CI 1.63 to 1.91, p < 0.001) in the year following diagnosis. The rate of GP consultations, referrals and hospital admissions were also significantly higher in the year prior to diagnosis (all, p < 0.001). Based on these figures, Puffer et al. 49 estimated difference in costs per patient of £1015 and £1598 for pre- and post-diagnosis years respectively. Furthermore, it was found that patients with VCFs had a significantly greater utilisation of pharmacological treatments in the year following diagnosis, with the largest difference being in the prescription of bisphosphonates (difference 52.71%, 95% CI 49.37% to 56.01%, p < 0.001). The total additional cost of pharmacological treatment per patient was estimated to be £97.37 per year. 49
Nevertheless, there are several reasons to interpret these estimates with caution. As noted above, people diagnosed with osteoporotic VCFs are more likely to have significant comorbidities requiring medical care. Hence, it is difficult to establish whether or not additional resource usage arises directly from the VCF. Furthermore, although only 30% of VCFs come to medical attention,50 undiagnosed VCFs are also likely to be associated with greater service use owing to the association of VCFs with excess morbidity and mortality. 51 The limitations VCFs can place on participation and, consequently, on patient well-being are highly significant issues with respect to care provision. 52
Measurement of disease
Osteoporotic VCF is identified by diagnosing both osteoporosis and vertebral fracture. The generally accepted approach to osteoporosis diagnosis is by the measurement of BMD. The presence of osteoporosis is assessed by converting an individual patient’s BMD into a measure known as the T-score, that is to say the number of SDs from healthy young adults matched for ethnicity and sex. A T-score ≤ –2.5 is widely accepted as the diagnostic threshold. 53 A meta-analysis has shown that the predictive value of a 1 SD decrease in bone mass for osteoporotic fractures was roughly similar to that of a 1 SD increase in blood pressure for stroke, and more than a 1 SD increase in serum cholesterol concentration for cardiovascular disease. 54 Methods of assessing BMD include single-photon and X-ray absorptiometry of the forearm and heel, DXA, and dual photon absorptiometry of the lumbar spine, proximal femur, whole body or particular regions thereof, and quantitative computed tomography of the spine or appendicular sites. 2
A number of methods have been proposed for identifying vertebral fractures. A widely used approach is the semiquantitative technique first described by Genant and colleagues,7 which also indicates fracture severity. This approach utilises pre-defined thresholds for fracture severity, based on perceived reductions in vertebral height and area. Hence, vertebral bodies can be classed as normal (grade 0), mildly deformed (grade 1: reduction between 20–25% in anterior, middle, and/or posterior height and a reduction of area of 10–20%), moderately deformed (grade 2: reduction between 25–40% in anterior, middle, and/or posterior height and a reduction of area of 20–40%), and severely deformed (≥ 40% reduction in any height and area). This grading system has demonstrated good to excellent intra- and inter-observer agreement, and similar estimates of incidence to quantitative morphometric measurements of vertebral height loss. 7 Common measures of angular deformity include kyphotic wedge angle, sagittal index and measures of sagittal balance, in particular lateral radiographs measuring the relationship between the C7 and S1 vertebrae (these are discussed in more detail in Chapter 2, Decision problem).
Current service provision
Management of disease
Traditionally, VCFs have been treated with optimal pain management (OPM). Bed rest is often required for 1–2 weeks until the acute pain begins to subside, and therefore hospitalisation may be necessary. 15 Pain relief is generally achieved with oral analgesics: narcotics can be effective for fracture pain, while non-steroidal anti-inflammatory agents (NSAIDs) may relieve pain of inflammation and muscle spasm associated with VCF. 55 Calcitonin (Miacalcic®, Novartis) has also been shown to have a strong analgesic effect on patients with acute osteoporotic VCFs. 56 Patients who develop radicular pain as a result of compression of the nerve root may also require a nerve-root block or epidural injection of steroid and an anaesthetic. If such pain becomes chronic, other medications such as antidepressants, anticonvulsants and alpha-2-agonists may be required. 55 External immobilisation (back bracing or casting) may also be used to reduce pain and promote appropriate posture, although this strategy carries the risk of muscle tone loss. 55 Antiosteoporotic medication should be prescribed to reduce the risk of further vertebral fractures. 15,16
In order to prevent further bone loss, mobilisation should begin as soon as the acute pain begins to subside, and spine extension exercises may be used to strengthen the back muscles. 15 Muscle spasms associated with acute VCFs may be treated with muscle relaxants and heat treatment; massage and physiotherapy may also be required by patients with kyphosis. 57 Patients should also receive walking aids and education about ways to avoid pain in activities of daily living. 55
However, many patients complain of progressive pain and progressive functional limitation and loss of mobility despite conservative management. Thus, 75% of patients (n = 107) who were admitted to a Swedish emergency unit with a painful acute VCF and received conservative treatment reported persistent back pain at 12 months. 58 Moreover, conservative management cannot prevent kyphotic deformity. 59
In theory, open surgery with internal fixation may be performed in patients whose pain does not resolve with conservative management. However, such surgery is rarely undertaken in osteoporotic patients because the poor bone quality reduces the likelihood of achieving good results, while comorbidities in this patient group increase the risks associated with surgery. 60 Consequently, open surgery is generally performed only in patients with neurological deficits61 in whom the balance of risks and benefits differs from that in patients without such deficits.
Optimal pain management is associated with an increased risk of complications of bed rest [e.g. pneumonia, deep-vein thrombosis (DVT) and pulmonary embolism62], side effects of medication, admissions to nursing home and death. 63 Narcotic analgesics may lead to debilitating side effects, in particular cognitive impairment, nausea and constipation, while NSAIDs are associated with gastrointestinal side effects such as nausea, gastritis and ulcers. 55 Injected calcitonin may cause side effects such as nausea and flushing,56 while nasal calcitonin is mainly associated with rhinitis and nasal symptoms. 64 Additional medications which may be used for chronic pain are also associated with a range of side effects. 55 Even in the absence of such severe adverse events, extended bed rest and the use of back bracing or casting may be problematic for many older patients: bed rest may result in loss of bone density and muscle mass, and braces are often poorly tolerated. 19
Current service cost
Medtronic35 reference Ström32 as estimating the cost of treatment of a vertebral fracture in the UK to be approximately 2756 euros in the first year. However, Ström gets this figure from Stevenson et al. 65 Medtronic also reference Swedish data that the total cost is almost as high as the cost of treatment of a hip fracture, with lower initial hospital costs offset by higher community and informal care costs between 12 and 18 months. 66
Synthes state that HES data for the last 12 months (apparently for the UK rather than England and Wales) recorded that 6375 patients (undifferentiated, i.e. not all osteoporotic) who had no surgical intervention (excluding facet injection or analgesia) occupied 78,923 bed days, with an average length of stay of 12.38 days; a further 698 patients received surgical treatment (PVP or BKP with or without stent), with an average length of stay of 7.5 days for PVP and 5.9 days for BKP. 33 In their submission, Medtronic indicated that the average inpatient stay associated with BKP was 5.1 days,34 while Johnson & Johnson identified the average length of stay as 3.24 days for PVP and 4.48 days for BKP,36 with these values provided by Dr Foster Intelligence. The longer lengths of stay identified by Medtronic include patients receiving vertebral augmentation for trauma or malignancy. 34
The assessment group note that a recent review of the cost-effectiveness of vitamin K compared with alendronate used a cost of a vertebral fracture in the first year of £2981. 67 This estimate was based on 2006 costs, which had been inflated by 8% to meet expected 2008 costs.
Variation in services and/or uncertainty about best practice
There is no single standard of best practice care provision for people with osteoporotic VCFs because treatment needs can vary substantially according to age, BMD loss, mobility and broader life conditions. Hence, care packages tailored to individual needs have been recommended by a number of authors. 68–70 However, the general aim of rehabilitation is to restore mobility, reduce pain and minimise the incidence of new VCFs. Barriers to adequate treatment in older people with osteoporosis include polypharmacy, comorbidities and cognitive impairment. Therefore, prevention of pain, disability, and functional decline should be pursued with these constraints in mind. 69
Analgesic treatment varies according to pain severity and patient-level contraindications. The need for back-pain relief can typically be met with acetaminophen, and supplementary codeine for breakthrough pain. 71,72 In cases of more severe and persistent pain, narcotic analgesics may be required for satisfactory pain reduction. While short-term use of these drugs is unlikely to lead to adverse events, undesirable side effects, in particular delirium and constipation, tend to be more pronounced in frail older people. 69 NSAIDs are often prescribed to treat low back pain; however, these drugs have been linked to gastrointestinal side effects. Chronic use of NSAIDs is also known to pose a risk of potentially fatal gastroduodenal bleeding. 73
A number of physical approaches to pain relief may also be beneficial for people with osteoporotic VCFs, although their efficacy remains moot. Back bracing is often used to minimise postural flexion and paraspinal muscle spasm, and to facilitate bone healing. 74 While there is moderate evidence that lumbar supports are effective for the treatment of general back pain,75 their effectiveness in osteoporotic VCFs remains poorly understood. 76 Moreover, chronic use of braces may lead to weakening of the paravertebral muscles and increased pain. 77 There is limited evidence that massage and superficial heat and cold therapy reduces general back pain, although evidence is lacking for the effectiveness of either treatment in osteoporotic VCFs specifically. 78,79
Owing to their role in skeletal homeostasis, calcium and vitamin D are widely viewed as the first line in osteoporosis treatment. However, while higher doses of vitamin D may be associated with greater benefits, this effect is yet to be confirmed. 80 Furthermore, a recent meta-analysis found that calcium supplements without co-prescribed vitamin D led to an increased risk of myocardial infarction. 81 Until recently, hormone replacement therapy (HRT) was widely used to treat women with osteoporosis. A meta-analysis of RCTs comparing the risk of VCFs in women treated with HRT compared with placebo found a risk reduction of approximately 33% in the HRT cohort. 82 However, HRT has been linked to a number of adverse events, including a 2.3% increase in the relative risk of breast cancer and an association with venous and pulmonary thromboembolism. 83 While these adverse events are linked only with current or recent use, they nevertheless suggest that HRT should be prescribed with caution. 83
One possibility explored in a recent non-randomised cohort study84 was the use of local anaesthetic and facet joint injection to control VCF-related back pain. Wilson et al. 85 performed facet joint injections under fluoroscopic guidance with lidocaine 1% and bupivacaine 0.5% to anaesthetise the affected area. Approximately one-third of the treated cohort (21 of 61) responded well to the intervention, which led these investigators to hypothesise that facet joint injections may be effective among patients in whom pain does not arise directly from the VCF but from biomechanical effects of the VCF occurring elsewhere in the spine. Anecdotally, the use of this approach prior to more invasive techniques is now widespread in the UK, and indeed is explicitly recommended by some NHS trusts. However, its long-term effectiveness is yet to be assessed.
Relevant national guidelines
The National Institute for Health and Care Excellence has issued Interventional Procedure Guidelines (IPGs) on the use of vertebroplasty and balloon kyphoplasty:
-
NICE IPG 12,86 issued in 2003, states that PVP may be considered for the provision of pain relief in patients with severe painful osteoporosis with loss of height and/or compression fractures of the vertebral body only if their pain is refractory to more conservative treatment. 86
-
NICE IPG 166,87 issued in 2006, states that BKP may be considered in patients with VCFs whose condition is refractory to medical therapy and in whom there is continued vertebral collapse and severe pain.
Both guidelines stipulate that the procedure should only be undertaken:
-
by clinicians trained to an appropriate level of expertise
-
following discussion by a specialist multidisciplinary team which includes a radiologist and a spinal surgeon
-
where there are arrangements for good access to a spinal surgery service.
Description of technologies under assessment
Summary of intervention
Percutaneous vertebroplasty
Percutaneous vertebroplasty is a procedure in which bone cement [such as polymethylmethacrylate (PMMA), glass polymers, hydroxyapatite or calcium compound] is injected into a fractured vertebra with the intention of reducing the pain caused by bone rubbing on bone and strengthening the bone so that it is unlikely to fracture further. 57 PVP is most commonly performed in the thoracic and lumbar vertebrae, and only occasionally in the cervical spine. 14 It is additional, rather than an alternative, to conventional therapy. 57
Percutaneous vertebroplasty is performed under radiological guidance using fluoroscopy. 88,89 It is usually performed using conscious sedation and local anaesthesia of the skin, subcutaneous tissue and the periosteum of the vertebral body into which the needle is to be introduced. 89 Sedation or light general anaesthesia is used in the majority of cases, with decisions being based on patient-level contraindications and anaesthetist preferences. 90 After adequate infiltration of local anaesthetic, a small skin incision is made and a disposable bone biopsy needle or trocar needle is placed centrally in the vertebral body using an image-guided safe access route. This may be done bilaterally through the pedicles, oblique across one pedicle or lateral oblique through the base of the pedicle. Under constant screening, it is advanced through the pedicle into the vertebral body with the aid of a light orthopaedic hammer. 14 An 11- or 13-gauge needle is used. 91 The cement is then injected very slowly, again under constant fluoroscopic screening, and the injection is stopped immediately if the cement begins to spread into a blood vessel or towards the posterior cortical margin. 18 To achieve optimal vertebral filling, two trocars may be used, one on either side of the midline. 90 The procedure may last from 45 minutes to 1 hour, depending on the number of vertebrae being treated. 14 Some centres perform computed tomography (CT) scanning at the end of the procedure to assess the distribution of cement and identify any complications. 14
At the end of the procedure, the patient remains on the operating table until the cement within the vertebral body has set. 16 This usually took about 20 minutes with earlier generations of bone cement. 92 However, setting time has been substantially reduced in recent years. For example, Goto et al. 93 compared the setting time of PMMA with bone cements containing micron-sized titania particles, and found a setting time of 11 minutes in a commercially available PMMA-based cement (Osteobond, Zimmer, Warsaw, IN). Glass-based polymers can set within 2 to 3 minutes. 94 The patient should then be kept in the recumbent position, with monitoring of vital signs and neurological evaluations every 15 minutes for the first hour and then every 30 minutes for the next 2 hours. 16 The initial mobilisation should be supervised by qualified staff. 14
Percutaneous vertebroplasty may be done as a day case if the patient’s general health and social circumstances are appropriate. 90 However, in exceptional cases an overnight stay may be required. 14 Prophylactic intravenous antibiotics may be used both before and after the procedure; some operators limit their use to patients with immunodeficiency. 14 Non-steroidal or steroidal anti-inflammatory drugs may be used for 2–4 days after vertebroplasty to minimise any inflammatory reaction to the heat generated by the polymerisation of the bone cement. 16 This is unlikely to apply to glass polymer-based bone cements, which do not have the same exothermic reaction upon mixing.
A number of bone cements are available for carrying out PVP, and decisions can be based on patient needs and operator preferences. The high-viscosity Confidence Spinal Cement System™ (DePuy Synthes, Indianapolis, IN, USA) is marketed by Johnson & Johnson, and carries an average cost of £1546 per operation (see also section 6.2). Low-viscosity cements are also available to purchase at prices that are lower than that of high-viscosity PMMA cement. The list price for such cements were obtained through NICE, and on clinical advice it was estimated that the costs using lower-viscosity cements, incorporating injection kit, needles cement and assorted consumables, would be in the region of £660, £720 and £780 for one-, two- and three-level procedures respectively. However, our clinical expert estimated that 15% of cases are more complex and would require Cortoss® cement (Stryker, Kalamazoo, MI, USA), collation or thicker cement, while younger patients would need bone-absorbable cement. It was assumed that the added cost of these complex cases would add slightly over £100 to the average cost of an operation resulting in an assumed cost of £800 per low-viscosity cement PVP procedure. Given that the estimate includes a component for using higher-viscosity cement, the price used within the analysis could be equated to a strategy where low-viscosity cement is used within the majority of patients, while higher-viscosity cements are used in a small proportion where the clinician believes that this is appropriate. In addition to the cements themselves, operating equipment, including bone biopsy or special trocar needles and vacuum cement mixing systems, is required.
Percutaneous balloon kyphoplasty
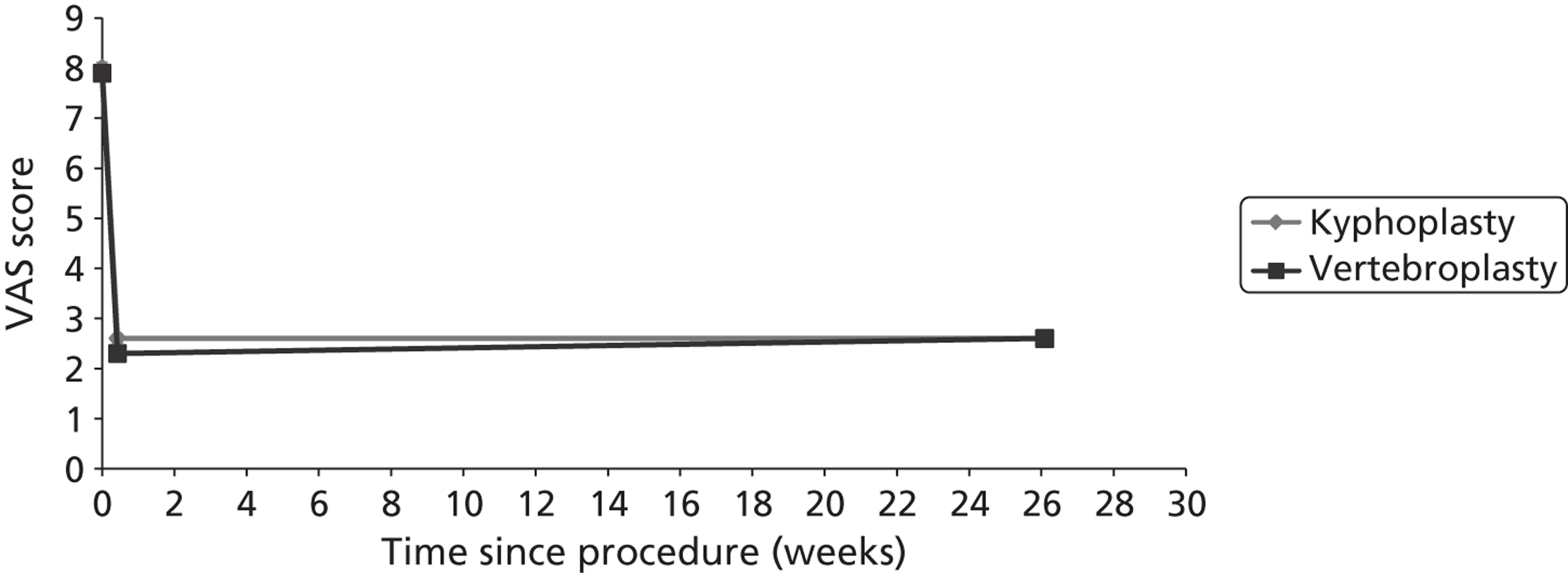
Percutaneous BKP is a variant of PVP in which one or two balloon-like devices (also known as tamps) are inserted bilaterally into the vertebral body. These balloons are slowly inflated until they reach their highest achievable volume, in order to restore vertebral body height (VBH). The balloons are then deflated and removed, leaving a cavity which is filled with bone cement; because of the existence of the cavity, the cement may be injected at a lower pressure than that used for PVP. 35
Sedation is based on practical considerations, such as patient-level contraindications. Some patients receive general anaesthetic and remain in hospital overnight for observation. 91 However, BKP may be done as a day case if the patient’s general health and social circumstances are appropriate. 90
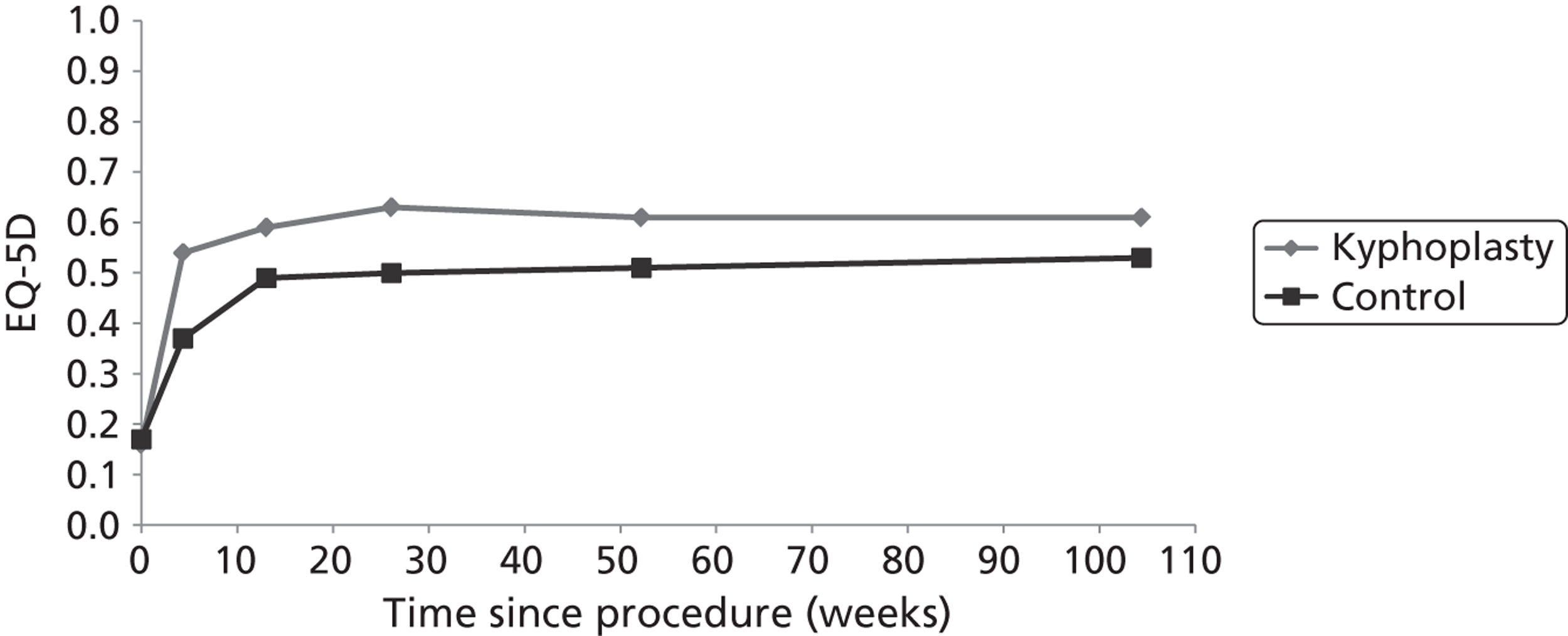
Although there is no apparent reason why BKP should differ from PVP in terms of pain relief, it has some potential additional benefits. Medtronic suggest that the creation of a cavity of known volume into which cement may be injected results in a lower risk of leakage and consequent complications. 34 Furthermore, introduction of the balloon provides the potential for restoring vertebral height and thus correcting deformity. However, neither of these potential benefits has a good evidence base. There is no evidence of a higher complication rate in PVP, as most cement leakages remain asymptomatic. In addition, to be effective in restoring vertebral height and reducing kyphosis, BKP should be performed within a few weeks of fracture; thereafter, although a cavity will still be created within the vertebra, fracture healing is likely to prevent restoration of vertebral height. 95
In the UK, the device required for BKP is marketed by Medtronic as a single-use sterile pack containing two Kyphon® Xpander™ inflatable bone tamps and associated accessories, at a list price of £2600.50. 34 The components of the Medtronic KYPHON BKP kit obtained the CE mark in May 1999, while various components of the Kyphopak – the Osteo Introducers, Balloon, and Bone Fillers – obtained the CE mark in 2001, 2003 and 2002 respectively. 34 The bone cement included in the kit, Kyphon® ActivOs™ Bone Cement with Hydroxyapatite,96 is a PMMA cement to which hydroxyapatite (a calcium compound believed to promote osseointegration) has been added. 97 Kyphon® also produces two alternative cements for use in kyphoplasty: Kyphon® KyphOs FS™ Calcium Phosphate Bone Substitute and Kyphon® HV-R™ Bone Cement (all products are registered to Medtronic, Minneapolis, MN, USA). 96
Balloon kyphoplasty with stenting (stentoplasty)
Balloon kyphoplasty with stenting seeks to overcome a problem inherent in simple PBK, namely that, because of pressure on the vertebra, some of the height restored by the fully inflated balloons may be lost after they are deflated and removed and before the cement is injected. A laboratory comparison of stenting compared with kyphoplasty found that most of the height gained in BKP appeared to be lost after the balloon was deflated. 98 In stentoplasty, a small balloon catheter surrounded by a metal stent is inserted into the vertebral body using a minimally invasive percutaneous approach under radiographic guidance and either local or general anaesthetic. The balloon catheter is then inflated with liquid, under pressure, to create a cavity in which the stent is expanded. The balloon catheter is then deflated and withdrawn but the stent is left in position within the vertebra and maintains the height of the cavity into which high-viscosity PMMA bone cement is then injected. The injected cement hardens within 1 hour, and the patient may then be mobilised. 33
The use of a vertebral body balloon (VBB), an optional site preparation device, is recommended: it enables the operator to identify the feasibility of cavity creation and full expansion of the stents. 33
Synthes market a vertebral body stenting system, which consists of a vertebral body stent catheter, an inflation system, a VBS access kit and an optional VBB catheter. The balloons included in the VBS and VBB catheters are said to be considerably more rigid than current kyphoplasty balloons and therefore less likely to herniate through the fracture. 33
Facet joint injection
Facet joint injections involve the administration of anti-inflammatory steroids and local anaesthetic to facet joints with focal tenderness. They are usually performed on an outpatient basis without sedation. Prior to the procedure, the patient lies in prone position while the operator palpates the back in order to localise the pain. Once identified, the skin and subcutaneous tissue surrounding the affected area are infiltrated with 1% lidocaine. Then, fluoroscopic or CT imaging is used to identify the posterior part of the joint and a 20- or 22-gauge needle is directed vertically into the joint space until bone cartilage is reached. 99 A long-acting steroid such as triamcinolone, methylprednisolone or betamethasone is administered to the joint, along with 0.5% bupivacaine to anaesthetise the area. 100
Although we did not directly assess the clinical effectiveness of facet joint injections for treating painful osteoporotic VCFs, the procedure is noted here because it is emerging as a possible treatment for a subgroup of patients in whom pain and functional impairment arises not from the VCF per se but from the impact of the VCF on other spinous processes. Ryan et al. 101 found that facet joints may be an important site of pain for people with osteoporotic VCFs, and, more recently, a cohort study by Wilson et al. (n = 61 treated patients)84 found that problems with the facet joint may account for back pain in approximately one-third of patients with osteoporotic VCFs. On the basis of their data, Wilson et al. suggested that the apparently high placebo response seen in the ‘sham’ treated cohorts in two recent RCTs102,103 may in fact have been a response to the local anaesthetic by patients whose pain was an indirect consequence of a VCF. Hence, in our cost-effectiveness model, a hypothetical scenario controlling for the potential influence of this patient subgroup has been included.
Bone cement
Percutaneous vertebroplasty and BKP are traditionally performed using PMMA, a low-viscosity acrylic bone cement, to which a radio-opaque substance such as barium, tantalium or tungsten sulphate has been added to facilitate visualisation during the procedure. 14 It is prepared by mixing a liquid component containing the monomer, accelerator and inhibitor with a powder containing the polymer, radio-opacifier and initiator. The heat that is released during the subsequent polymerisation process while the cement is hardening in situ may cause local damage to bone or other tissues. 104 However, in PVP and BKP, such damage may not be entirely detrimental. PMMA appears to have analgesic properties quite apart from those caused by the effect of the stability provided by the cement within the weakened vertebrae. The reason for such analgesic properties remains unclear, but one possibility is that it destroys or damages local nerve endings as a result of both the toxic effects of the free monomers of PMMA and the heat caused by the cement polymerisation. 14 However, we are not aware of any evidence to suggest that cements that do not generate heat are any less effective.
The Food and Drug Administration (FDA)104 states that PMMA is contraindicated in the presence of active or incompletely treated infection at the site where the cement is to be applied. It also notes that hypotensive reactions have been noted between 10 and 165 seconds after its application; as these have lasted from 30 seconds to over 5 minutes, and some have progressed to cardiac arrest, the FDA recommends that patients should be monitored carefully for any changes in blood pressure during and immediately following the application of the cement. Other reported adverse events include pyrexia due to allergy to the cement. In addition, the FDA notes that the heat released while the cement is hardening in situ may damage bone or other tissues surrounding the implant. 105
The FDA also notes that caution is required in preparing and handling PMMA: excessive exposure to the concentrated monomer vapours may produce irritation of the respiratory tract, eyes and possibly the liver; contact lens wearers should not be near or involved in mixing PMMA. 104 However, newer manufacturer kits, such as the PLACOS® bone cement (Zimmer, Hanau, Germany), provide vacuum cement mixing tools to avoid this issue.
The newer composite cement bisphenol-A-glycidyl dimethacrylate (bis-GMA) resin (Cortoss) is more viscous than PMMA and consequently easier to handle. It does not contain the volatile monomers which may be the cause of cardiovascular and respiratory adverse events with PMMA. It is stronger than PMMA, and cures at a lower temperature, reducing the risk of thermal damage and setting more rapidly. It is also inherently opaque, and therefore does not need to be mixed with a toxic radio-opaque material. 14 The bioactive Cerament™ bone analogue cement (Bonesupport, Lund, Sweden) also has radio-opaque properties which obviates this requirement.
Follow-up required
It has been suggested that, following discharge, patients who have undergone PVP should be recalled for evaluation 1 day, 1 week and 1 month after treatment. 92 However, this appears to reflect US practice, and according to our clinical expert (DW) in the UK it would be more normal for a patient to receive a follow-up telephone call at 1 week after discharge and a clinical consultation at 1 month after.
Setting
Percutaneous vertebroplasty and BKP should be performed in a sterile environment which allows fluoroscopic imaging of the thoracolumbar spine. 18 The use of an interventional radiology suite rather than an operating theatre has been recommended because fixed fluoroscopic equipment offers better imaging quality than a mobile C-arm. 16 PVP and BKP should be performed only in hospitals which have adequate neurosurgical backup to deal with potentially serious complications. 14
Equipment required
Magnetic resonance imaging (MRI) equipment is requisite to screen all patients who are considered for PVP or BKP, in order to identify the fracture, assess its age, define its anatomy, assess the posterior vertebral body wall and exclude other causes of back pain. However, CT scanning may be used instead when MRI is unsafe (e.g. in patients with pacemakers). CT equipment is also required if there are any doubts regarding the intactness of the posterior vertebral wall. 16 Fluoroscopic imaging equipment is also required for use during the procedure.
Personnel involved
As stipulated in the NICE guidance,86,87 PVP and BKP should be performed only by clinicians trained to an appropriate level of expertise (for historic reasons, PVP and BKP have most commonly been performed by interventional radiologists). An anaesthetist should preferably be present to monitor sedation even when the procedure is performed under local anaesthesia. 14
Place in the treatment pathway
Percutaneous vertebroplasty and BKP are usually offered as a last resort to people with symptomatic VCFs in whom alternative treatments have not been successful. 89,106 An Australian review has noted that, while people with recent painful VCFs are potential candidates for either PVP or BKP, PVP is not appropriate for people with VCFs which cause symptoms such as pain or breathlessness due to a hunched posture, and who require structural correction for functional kyphotic deformity which is neither congenital nor due to trauma; such patients are potential candidates for either BKP or surgical stabilisation of the fracture with or without fusion of the vertebrae. 57
Criteria for treatment
National Institute for Health and Care Excellence guidance indicates that PVP and BKP should be limited to patients whose pain is refractory to more conservative treatment;86,87 for BKP, there is an additional requirement that they should have continued vertebral collapse and severe pain. 87
Recent guidance from the Cardiovascular and Interventional Radiology Society of Europe (CIRSE) states that PVP is indicated in patients with ‘painful osteoporotic VCF refractory to medical treatment’. It defines failure of medical treatment as ‘minimal or no pain relief with the administration of physician-prescribed analgesics for 3 weeks or achievement of adequate pain relief with only narcotic dosages that induce excessive intolerable sedation, confusion, or constipation’. 16 The CIRSE guidelines further note that PVP may be considered within days of painful fracture if the patient is at high risk of complications resulting from immobility (e.g. thrombophlebitis, DVT, pneumonia or pressure ulcer). 16
Contraindications
The CIRSE guidelines list the following absolute contraindications to PVP:
-
asymptomatic vertebral body compression fracture
-
patient improving on medical treatment
-
osteomyelitis, discitis or active systemic infection
-
uncorrectable coagulopathy
-
allergy to bone cement or opacification agents
-
prophylaxis in osteoporotic patients. 16
Relative contraindications in osteoporotic patients include:
-
radicular pain
-
fracture of the posterior column (which increases the risk of cement leak)
-
spinal canal stenosis
-
lack of surgical backup and monitoring facilities. 16
These contraindications appear to be equally applicable to BKP.
Although neurological symptoms are not an absolute contraindication to PVP or BKP, in patients with such symptoms great care should be taken to avoid cement extravasation as this may exacerbate any pre-existing nerve compression. 90 Thus, prior to PVP/KP, a detailed examination should be performed to detect any neurological compromise and exclude other causes of pain such as degenerative spondylosis. Physical examination is important to accurately localise the symptomatic vertebra, especially in the presence of multiple fractures. 14
Identification of important subgroups
A number of authors have suggested that only patients with acute VCFs (≤ 6 weeks’ duration) are likely to benefit from PVP or BKP. 107,108 However, the clinical experience of rapid and dramatic post-procedural reductions in pain is likely to be confounded by the rapid healing of the fracture itself. This was suggested by the recruitment pattern in the recent VERTOS II trial, in which more than half of initially eligible participants became ineligible prior to enrolment owing to spontaneous pain reduction. 17 Furthermore, Rad and Kallmes109 presented a retrospective analysis of 321 single-level PVP procedures. These were stratified into acute (≤ 6 weeks), subacute (> 6 to ≤ 24 weeks) and chronic (> 24 weeks) fractures, and absolute and proportional pain reductions were compared between the three groups. There was no strong correlation between fracture acuity and pain relief. Hence, vertebral augmentation may be better used to treat people with chronic pain refractory to more conservative measures. 85,110
It has also been suggested that PVP is effective in specifically selected patients with more severe pain. 108 However, the evidence for this claim is unconvincing. Pain is a subjective experience mediated by various psychosocial factors111 and is consequently open to confounding influences and difficult to objectively assess. A strong placebo response due to positive expectations among persons with severe pain could not therefore be ruled out as a cause of apparent effectiveness. Indeed, a recent meta-analysis of individual patient data (IPD) from two operative placebo with local anaesthesia (OPLA)-controlled trials of PVP110 found that post-procedural pain reduction was unrelated to baseline pain severity.
It is possible that vertebral augmentation should be pursued only with those whose pain and functional impairments arise directly from the VCF rather than as indirect consequences thereof. Wilson et al. 84 found that one-third of patients who were eligible for PVP (n = 21 of 61 treated patients) responded favourably to a facet joint injection. It may be that the pain in this group was mediated by overload on the facet joints adjoining the fractured vertebral body, which could therefore be treated successfully with a less invasive procedure.
Current usage in the National Health Service
On the basis of data from Dr Foster Intelligence, Johnson & Johnson have estimated that, between April 2010 and March 2011, 473 vertebroplasties and 225 kyphoplasties were performed for osteoporotic VCF. 36 These figures appear to apply to England alone.
Medtronic reported, on the basis of HES data for 2009/10/11, that 487 patients in England and Wales were treated with PVP and 466 with BKP, for osteoporotic VCF;34 it is not clear whether this is figure relates to the 2-year period or to the average for 1 year.
Synthes note that HES data for 2010–11 indicate that 698 patients in the UK underwent either PVP or BKP with or without stent for osteoporotic VCF. 33
Anticipated costs associated with the intervention
A formal report on the likely costs associated with each analysis is contained in the cost-effectiveness section.
Chapter 2 Definition of the decision problem
Decision problem
The assessment will address the question ‘What is the long-term efficacy, safety and cost-effectiveness of percutaneous vertebroplasty and percutaneous balloon kyphoplasty (with or without vertebral body stenting) as a treatment for osteoporotic vertebral compression fractures?’
Interventions
Percutaneous vertebroplasty or BKP with or without vertebral body stenting, performed under general or local anaesthesia.
Population including subgroups
The relevant population is adults of any age and either sex with painful osteoporotic VCFs. If the evidence permits, consideration will be given to subgroups defined by:
-
time from fracture to treatment
-
presence of fracture-related deformity before treatment
-
receipt of inpatient care before treatment.
People with malignancy-related vertebral fractures and those with neuropathy in the absence of osteoporotic compression fractures are not included the scope of this assessment.
Relevant comparators
The comparators specified in the protocol (see Appendix 1 ) are the interventions themselves and non-invasive management (including no treatment in people who cannot tolerate the relevant active comparator interventions). Injection of local anaesthesia to the affected vertebral body is also considered a relevant comparator, as this has been used as a ‘sham’ intervention in double-blind, OPLA-controlled trials of PVP. Moreover, our clinical advisor (DW) suggested that administration of local anaesthesia with facet joint injection is now routinely offered in the UK as a minimally invasive intervention before patients are considered for vertebral augmentation.
Both the Buchbinder102 and INVEST103 studies used what they describe as a ‘sham’ intervention for the control procedure. The procedure in each of these trials involved infusion of lidocaine 1% into the skin to numb the affected area. The INVEST trial also infiltrated the periosteum of the pedicles with 0.25% bupivacaine. Both trials then mimicked vertebroplasty through physical cues such as pressure to the back and opening of the methacrylate monomer to simulate the smell associated with PMMA preparation. In this review, it was decided that, rather than sham, these procedures would be described as ‘operative placebo with local anaesthesia’ (i.e. OPLA). This term was chosen because of the ongoing debate as to whether or not these procedures actually constitute a sham intervention. A number of authors have argued that the local anaesthetic may have had specific mechanisms of efficacy for long-term pain reduction107,112,113 and, indeed, some empirical evidence is available to support this possibility. 84,114 Conversely, some practitioners have proposed that, owing to the relatively low volumes of cement used in the Buchbinder and INVEST trials, the comparison was effectively placebo versus placebo. 115
Therefore, it was also viewed as important to highlight the possibility of a high placebo response to these interventions, which could be much greater than the response associated with vertebral augmentation. This may be strongly influenced by the elaborate rituals required in any operative procedure. According to Kaptchuk, healing rituals comprise ‘compelling multi-sensory dramas involving evocation, enactment, embodiment and evaluation’. 116 In surgical procedures, these rituals encompass the interventionist’s language and dress, the hospital setting in which they are performed, and the lived experience of being anaesthetised and undergoing the intervention. In short, each dimension of the surgical ritual implies a scientifically derived and culturally sanctioned process designed to move the patient from an ‘ill’ to a ‘well’ state. Such rituals enhance suggestibility and so heighten the probability of a favourable outcome. 117 Consequently, it has been argued that researchers must take these suggestive effects into account, particularly when considering trials that measure subjective outcomes such as pain. 117
There is no gold standard for non-invasive management: the American Academy of Orthopaedic Surgeons considers the strength of the evidence for the various non-invasive treatment options (such as physiotherapy, analgesia and the use of antiosteoporotic agents such as a bisphosphonate or strontium ranelate) to be generally weak to inconclusive, although they provide a recommendation of moderate strength for the short-term use of calcitonin. 118
Outcomes
Primary outcomes
-
Health-related quality of life (HRQoL).
-
Back-specific functional status/mobility.
-
Pain/analgesic use.
-
Vertebral body height and angular deformity.
-
Incidence of new vertebral fractures.
-
Progression of treated fracture.
Secondary outcomes
-
All-cause mortality.
-
Symptomatic and asymptomatic leakage of cement (e.g. into adjacent intervertebral discs).
-
Peri-procedural balloon rupture.
-
Postoperative complications (including infection).
-
Other adverse events.
The majority of the primary outcomes take the form of continuous or quasi-continuous outcomes (e.g. pain measured on a 0–10 scale), whereas the secondary clinical outcomes are binary outcomes (e.g. the number of patients who suffer a given complication). Continuous outcomes can be compared in terms of:
-
the difference between the mean scores in the intervention and control groups at a specified point in time
-
the difference between the change in mean score in each group between two specified points in time (e.g. immediately before and 1 month after treatment).
To ensure that a continuous outcome measures a real difference between the intervention and control groups following the intervention, either the pre-intervention score for that outcome must have been identical in both groups or any pre-intervention differences must be minimised or controlled for through statistical adjustment. For this reason, we have presented continuous outcomes in terms of changes from baseline rather than as results at specified points in time.
While it is easy to determine whether or not any of these differences in outcome are statistically significant, it is less apparent whether or not they are also clinically relevant – in other words, whether or not they represent differences which the patients would recognise as beneficial. For this reason, research has been conducted for some outcome measures to attempt to quantify the smallest change in score that reflects a change in symptom which can be considered clinically relevant: this is termed the minimal clinically important difference (MCID). It should be noted that the proposed MCID values were for individual rather than group changes; in a trial, individual patients may show clinically important improvements even though the between-group difference in means is less than the MCID. 119 Nonetheless, the MCID provides a useful means of assessing whether or not, on average, an intervention is likely to be associated with greater clinical benefit than the control treatment.
Key issues
Research has shown that many patients with acute radiographically diagnosed VCF who receive conservative treatment report a reduction in pain of 50% or more by 6 months. 19 Because of the self-limiting nature of the condition, it is therefore crucial that outcomes are assessed in terms of the difference between the mean changes from baseline in the intervention and control groups in a randomised trial, and not in terms of the mean change from baseline in a single group of patients who have received the intervention.
Overall aims and objectives of assessment
The aim of this review is to systematically evaluate and appraise the clinical effectiveness and cost-effectiveness of PVP and BKP in reducing pain and disability in people with osteoporotic VCFs in England and Wales.
Chapter 3 Assessment of clinical effectiveness
Methods for reviewing clinical effectiveness and safety
A systematic review was undertaken according to the recommendations of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.
Identification of studies
Extensive searches were undertaken with the aim of comprehensive retrieval of studies of clinical effectiveness and cost-effectiveness relating to the research question.
The search strategy comprised the following main elements:
-
searching of electronic databases listed below
-
scrutiny of bibliographies of retrieved papers and previous systematic reviews
-
contact with experts in the field.
Electronic searches
The searches aimed to systematically identify all literature relating to the clinical effectiveness and cost-effectiveness of PVP and BKP as treatments for osteoporotic compression fractures in men and women of all ages. A comprehensive database of relevant published and unpublished articles was constructed using Reference Manager software (Thomson Reuters, New York, NY, USA).
Sources searched
The following electronic databases were searched from inception to 22 November 2011: MEDLINE (Ovid); MEDLINE In Process & Other Non-Indexed Citations; Cumulative Index to Nursing and Allied Health Literature (CINAHL); EMBASE; EconLit; The Cochrane Library including the Cochrane Database of Systematic Reviews, Cochrane Controlled Trials Register (CCTR), Database of Abstracts of Reviews of Effects (DARE), NHS Economic Evaluation Database (NHS EED) and Health Technology Assessment (HTA) databases; and Science Citation Index (SCI).
Search terms
The search strategy was developed in collaboration with the information specialist. Search terms included ‘vertebroplasty’, ‘kyphoplasty’, and a broad variety of related clinical terms (e.g. ‘bone void fill*’, ‘vertebral* and augmentation*) in order to obtain a wide scope. No bibliographic filters were used. Vocabulary around vertebroplasty/kyphoplasty is limited and, therefore, few synonyms were available. The searches were simple with an emphasis on sensitivity, utilising both keywords and MeSH/thesaurus terms where available. The MEDLINE search strategy is provided in Appendix 2 . Search strategies for the other databases are available on request.
Search restrictions
Searches were not restricted by language, publication date or publication type (with exception of removing letters, news, editorials, etc.). Furthermore, they were not restricted by study design, because studies that did not meet the inclusion criteria for the review of clinical effectiveness could have provided relevant information relating to adverse events or been important in identifying further relevant papers and current research.
Scrutiny of bibliographies of retrieved papers and previous systematic reviews
The bibliographies of retrieved papers and the manufacturer’s submission were scrutinised to identify relevant evidence.
Contact with experts in the field
Our clinical expert (DW) in the field was also consulted on whether or not the search had missed any relevant studies. He believed that all of the relevant RCTs had been successfully identified.
Inclusion and exclusion criteria
Inclusion criteria
Population
The population comprised people of any age and either sex with painful osteoporotic VCFs. Studies which also included participants with non-osteoporotic vertebral fractures of other aetiologies (e.g. fractures associated with trauma, myeloma or metastatic cancer) were included if data relating to participants with osteoporotic fractures could be extracted separately, or if the proportion of participants with non-osteoporotic fractures was extremely small.
Intervention(s)
Percutaneous vertebroplasty; BKP with or without vertebral body stenting.
Comparator(s)
The interventions themselves, non-invasive management, OPLA, or no treatment.
Outcomes
The primary outcomes of interest for this appraisal were:
-
HRQoL
-
back-specific functional status/mobility
-
pain/analgesic use
-
vertebral body height and angular deformity
-
progression of treated fracture
-
incidence of new vertebral fractures.
Secondary outcomes were:
-
all-cause mortality
-
symptomatic and asymptomatic leakage of cement (e.g. into adjacent intervertebral discs)
-
peri-procedural balloon rupture
-
postoperative complications (including infection)
-
other adverse events
-
resource utilisation
-
cost utility.
Only studies which reported data relating to at least one of the primary outcomes listed above in relation to the population of interest were eligible for inclusion in the review of clinical effectiveness. This criterion was relaxed for consideration of adverse events, to allow the inclusion of studies which reported data relating to any of the secondary outcomes in the population of interest. However, adverse event data were included only if they related to patients with osteoporotic VCFs because of the possibility that patients with fractures of different aetiology (e.g. malignancy) might be susceptible to more, or different, adverse events.
To facilitate comparison, outcomes measured at or before 3 weeks are grouped together as short-term outcomes, those measured between 1 month and 6 months as medium-term outcomes, and those measured at 12 months or later as long-term outcomes.
Study design
According to the accepted hierarchy of evidence, the review of clinical effectiveness was limited to RCTs, as they provide the most authoritative form of evidence. It was planned that non-randomised studies would be considered if insufficient data were available from RCTs, but this was not necessary.
In reviews of interventions for which beneficial effects are uncertain or contentious, with some possibility of harm, an accompanying review of adverse events can be of substantial importance when deciding whether or not to use the intervention. 120 It is widely recognised that RCTs do not form a good source of evidence for adverse events: they are generally not powered to reliably detect rare adverse events, nor is their follow-up period long enough to permit the detection of adverse events widely separated in time from the original intervention. 121 In addition, their populations are often not wholly typical of the target population; they may be younger and have fewer comorbidities than the general population of patients with the condition of interest. 122 Moreover, RCTs do not always measure all potential side effects. 123 Hence, it was decided to review the literature on adverse events in PVP and BKP to provide additional support for clinical decision-making. Adverse events were addressed using two broad research questions, namely, ‘what adverse events are associated with PVP or BKP in the treatment of osteoporotic VCFs?’ and ‘what is the approximate incidence of adverse events associated with PVP or BKP in the treatment of osteoporotic VCFs?’ Although this broad approach risked an overload of heterogeneous data which could not be easily pooled, it had a twofold advantage: first, it could identify new or previously unrecognised complications, and second, it could provide a more comprehensive overview of potential complications. 120
Two types of evidence were included in the review of safety:
-
Large observational studies (≥ 200 patients), which would allow exploration of the range and incidence of adverse events associated with PVP and BKP. The decision to include only large observational studies was based on a desire to exclude small case series which might display particularly high adverse event rates associated with limited experience of the relevant techniques on the part of the clinician or institution. The decision to set the threshold for inclusion at 200 patients was taken a priori.
-
Individual case reports were used to supplement the RCTs and large observational studies to provide as full a picture as possible of the range of adverse events associated with PVP and BKP. They were therefore used as a source of evidence relating only to adverse events which were not reported in the RCTs or large observational studies. By their nature, individual case reports cannot provide any indication of the incidence of such adverse events.
As with the review of clinical effectiveness, studies which included participants with non-osteoporotic vertebral fractures of other aetiologies (e.g. fractures associated with trauma, myeloma or metastatic cancer) as well as those with osteoporotic VCFs were excluded unless data relating to participants with osteoporotic fractures could be extracted separately. This was because there is some evidence that the type and incidence of adverse events may differ in vertebral fractures of non-osteoporotic origin (e.g. metastatic or traumatic). 124,125
Exclusion criteria
Systematic reviews were excluded from the review of clinical effectiveness and safety, but were retained for discussion and identification of additional relevant primary research studies. Studies which were considered methodologically unsound were excluded from the review, as were the following publication types:
-
animal models
-
pre-clinical and biological studies
-
narrative reviews, editorials and opinions
-
non-randomised studies (except for adverse events)
-
studies published as meeting abstracts only, which reported insufficient methodological details to allow critical appraisal of study quality.
In addition, potentially relevant publications were excluded if they had been superseded by later publications and did not contain any additional useful data: this applied to several conference abstracts.
Study selection
Retrieved studies were selected for inclusion through a two-stage process according to the above inclusion/exclusion criteria. The references identified by the literature searches were assessed for relevance first by title/abstract and then by full text, excluding at each step studies which did not satisfy those criteria; abstract-only publications were retained for full-text review. One reviewer examined titles and abstracts for inclusion, and screening was checked by a second reviewer on 10% of citations. For studies of clinical effectiveness, Cohen’s kappa coefficient (range 0–1) calculated to measure inter-rater reliability was excellent, at 1.0, indicating no discrepancies.
Data extraction strategy
Data were extracted independently by two reviewers using a standardised data extraction form (see Appendix 3 ); discrepancies were resolved by discussion and did not require input from a third reviewer. Where multiple publications relating to the same study were identified, data were extracted and reported as a single study.
Data obtained from the submissions made by the manufacturers have been appraised and commented on where deemed relevant.
Critical appraisal strategy
The methodological quality of each study that met the inclusion criteria for the review of clinical effectiveness was assessed independently by two reviewers, and any discrepancies were resolved by discussion. Where a study was reported in more than one publication, its quality was assessed on the basis of the combined data from all relevant publications.
It was stated in the protocol that quality would be assessed according to criteria based on those proposed by Ploeg et al. 88 for the assessment of studies of PVP (see Appendix 4 ). These criteria were initially adopted because they could be applied to both randomised and non-randomised studies. However, in the event, because sufficient RCTs were identified, it was not necessary to include non-randomised studies, and it was found that the criteria proposed by Ploeg et al. did not discriminate sufficiently between the included RCTs. A new set of criteria was therefore developed; this was based on the criteria proposed by Centre for Reviews and Dissemination and The Cochrane Collaboration for assessing the risk of bias in randomised trials, but also incorporated criteria proposed by Ploeg et al. 88 and Furlan et al. 126 which had particular relevance to the interventions under review. These criteria relate to internal validity, and also to external validity, and precision (for details, see Appendix 5 ). The criterion relating to the blinding of care providers was excluded as such blinding was not possible given the nature of the interventions under review.
The revised quality assessment tool included some questions that led to subsidiary questions to which the answer could be ‘not applicable’. These subsidiary questions have not been included in the risk of bias tables presented later in this chapter (see Study characteristics).
Methods of data synthesis
Owing to the potential impact of baseline imbalances in the degree of pain and disability reported by patients with osteoporotic VCFs, it is crucial that outcomes which are reported as continuous data (e.g. pain, disability and HRQoL) are assessed in terms of the difference between the mean changes from baseline in the intervention and control groups, and not in terms of the differences between mean scores at any given point in time. Where the original study investigators presented relevant measures of effect in terms of mean changes from baseline, these have been included in the data tables not least because in some cases they also adjusted for stratification variables (e.g. treatment centre). Where adjusted data were not reported, mean between-group differences in change from baseline for continuous outcomes were calculated adjusting for the variance of the within treatment change from baseline, where this was made possible by the data. This method generated CIs but not p-values. For dichotomous outcomes, relative risks, with CIs and p-values, were calculated using The Cochrane Collaboration’s Review Manager software (version 5.1, The Nordic Cochrane Centre, Copenhagen, Denmark) if such data were not reported by the study investigators.
Studies which met the review’s entry criteria were eligible for inclusion in meta-analyses to estimate summary measures of effect if such meta-analysis was appropriate (i.e. if the study populations, intervention and outcomes were comparable). Meta-analysis was carried out using random effects models, using Review Manager. Heterogeneity in the meta-analyses was explored through consideration of the study populations, methods and interventions by visualisation of results and, in statistical terms, by the chi-squared test for homogeneity and the I 2-statistic. However, such meta-analysis was limited to dichotomous outcomes.
The review team did not undertake meta-analyses of data relating to continuous or quasi-continuous outcomes. Such meta-analysis was considered inappropriate because of the existence of a published meta-analysis by Staples et al. 110 of data from the only two double-blind studies of vertebral augmentation (Buchbinder et al. 102 and INVEST103). As this meta-analysis used IPD, it was of a higher quality than could be achieved using published data. There was considered to be too much heterogeneity to justify combining data from all the studies of PVP in a meta-analysis together with published data from the Buchbinder and INVEST studies.
Results
Quantity and quality of research available
Number of studies identified
The electronic literature searches identified 3674 potentially relevant citations. For the review of clinical effectiveness, 3491 of these citations were excluded at the title or abstract stage, leaving 165 which were obtained for examination of the full text.
One hundred and thirty-nine citations were excluded at the full-text stage. A further 17 could not be obtained within the study timescale; as almost all of these appear to have been conference abstracts (for details, see Appendix 6 ), it seems unlikely that potentially valuable information has been missed as a result of their exclusion. Two additional papers were identified from other sources: these related to the studies by Blasco et al. 127 and Rousing et al. 128 Other publications from these studies129–131 had been identified by the electronic searches. Thus, 28 articles relating to a total of nine RCTs were identified and included in the review of clinical effectiveness ( Figure 1 ).
FIGURE 1.
Clinical effectiveness: summary of study selection and exclusion.
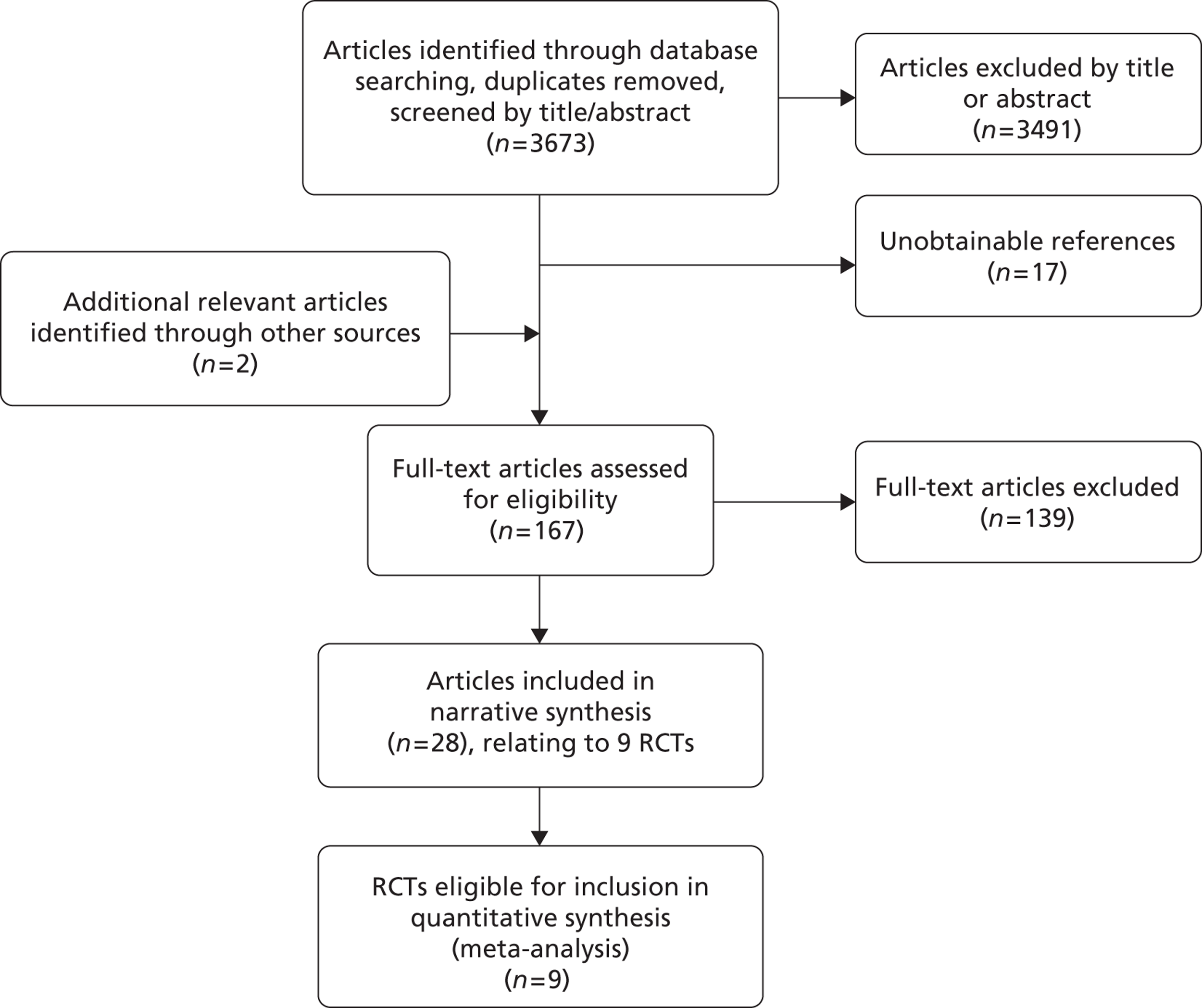
In their systematic review of vertebroplasty for painful osteoporotic VCFs, Muijs et al. 132 identified a further two RCTs which were not identified by the electronic literature searches: a small study by Do et al. 133 and a pilot study by Kallmes et al. ,134 both of which have been published only as conference abstracts. Neither of these studies met the inclusion criteria for the current review. Do et al. 133 randomised 31 patients to either PVP or continued medical therapy, but the latter group received PVP 6 weeks after the PVP group, and only before-and-after data are presented for each group: thus, no comparison was drawn between treated and untreated patients, or indeed between patients in whom PVP was performed sooner or later. Furthermore, baseline data from the control group appear to have been collected immediately before PVP (i.e. 6 weeks later than in the original intervention group), but this is not clear. The study by Kallmes et al. 134 was a pilot study intended to demonstrate the feasibility of enrolling patients into a trial of PVP against OPLA. Only five patients were enrolled; although pain was used as an outcome, it was not quantified, and patients were only said to have gained ‘minimal pain relief’ or ‘complete pain relief’.
Number and type of studies included
A total of nine RCTs met the review inclusion criteria. These compared:
-
percutaneous vertebroplasty with an OPLA (Buchbinder et al. 102 and INVEST103)
-
percutaneous vertebroplasty with optimum pain medication (Farrokhi et al.,135 VERTOS136 and VERTOS II17)
-
percutaneous vertebroplasty with conservative treatment (Blasco et al. 127 and Rousing et al. 128,131)
-
percutaneous balloon kyphoplasty with non-surgical management (FREE137,138)
-
percutaneous balloon kyphoplasty with percutaneous vertebroplasty (Liu et al. 139).
Details of the techniques for vertebral augmentation in these trials are included in Appendix 9 (see Table 100 ). For simplicity, we have used the term optimal pain management (OPM) as a term encompassing conservative treatment and non-surgical management.
The principal source/sources for each study are listed in Table 2 ; a full list of publications relating to each study is included in Appendix 7 .
| Trial name and identifier | Primary report(s) |
|---|---|
| Blasco 2012 (NCT00994032) | Blasco et al. 2012127 |
| Buchbinder 2009 (ACTRN012605000079640) | Buchbinder et al. 2009102 |
| Farrokhi 2011 (IRCT138804252193N1) | Farrokhi et al. 2011135 |
| FREE (NCT00211211) | Wardlaw et al. 2009137 |
| Boonen et al. 2011138 | |
| INVEST (NCT00068822) | Kallmes et al. 2009103 |
| Liu 2010 | Liu et al. 2010139 |
| Rousing 2009 | Rousing et al. 2009128 |
| Rousing et al. 2010131 | |
| VERTOS | Voormolen et al. 2007136 |
| VERTOS II (NCT00232466) | Klazen et al. 201017 |
It should be noted that the FREE study has been included even though, strictly, it does not meet the inclusion criteria because it included four patients with multiple myeloma. However, as these patients formed only 1% of the study population and were evenly distributed between treatment groups, it seemed unreasonable to exclude the study from the review in the absence of any other RCT comparing BKP with non-invasive management.
Number and type of studies excluded
As may be seen in Figure 1 a substantial number of the citations identified by the electronic searches related to studies which were excluded as part of the sifting process because they did not meet the inclusion criteria. Details are therefore given only of those citations which were excluded after a full reading, and then only if they were excluded for a reason other than a simple failure to meet the inclusion and exclusion criteria. Such citations are listed in Appendix 8 together with the reasons for their exclusion.
Ongoing or unpublished trials
Six relevant trials were identified from the National Clinical Trials (NCT) website (http://clinicaltrials.gov/ct2/): NCT00203554 compared PVP with conservative treatment, VERTOS IV compared PVP with OPLA, and the remaining four compared BKP with PVP and, in one case (OSTEO-6), also with conventional treatment ( Table 3 ). One of these trials (NCT00203554) has been completed, but the results have not been released because they are currently submitted to a journal (Leif Sørensen, Aarhus University Hospital, Denmark, 28 February 2012, personal communication). The NCT website previously said that the KAVIAR trial (NCT00323609) was ongoing but not recruiting participants, and had an estimated a primary completion date of August 2011 (access date 21 September 2011); however, the website now says that it has been terminated140 and the Medtronic submission states that the results of a partial analysis are expected in July 2012. 34
Two additional ongoing studies were identified which do not appear to be included in the NCT website:
-
a double-blind study by Longo et al. ,141 identified by the EMBASE search, comparing PVP with conservative treatment
-
STU-SPI-S-06–134–01, identified by the Synthes submission,33 comparing percutaneous stentoplasty with BKP.
Despite the existence of VERTOS II and VERTOS IV, no VERTOS III could be identified.
| Study | Intervention | Comparator(s) | Sponsor | Status |
|---|---|---|---|---|
| NCT00203554142 | Percutaneous vertebroplasty | Conservative treatment of pain | University of Aarhus, Aarhus, Denmark | Completed but unpublished |
| CEEP (NCT00279877)143 | Percutaneous balloon kyphoplasty | Percutaneous vertebroplasty | Mayo Clinic, Rochester, MN | Said to be ongoing, with an estimated primary completion date of August 2011 First results expected March 201234 but not posted on NCT website as of 15 May 2012 |
| KAVIAR trial (NCT00323609)140 | Percutaneous balloon kyphoplasty | Percutaneous vertebroplasty | Medtronic Spine LLC (Sunnyvale, CA, USA) | Terminated but unpublished |
| OSTEO-6 (NCT 00749060)144 | Percutaneous balloon kyphoplasty | Percutaneous vertebroplasty; conventional treatment | Assistance Publique – Hôpitaux de Paris, Paris, France | Estimated completion date of December 2012 |
| OSTEO+6 (NCT00749086)145 | Percutaneous balloon kyphoplasty | Percutaneous vertebroplasty | Assistance Publique – Hôpitaux de Paris, Paris, France | Estimated completion date of December 2012 |
| VERTOS IV (NCT01200277)146 | Percutaneous vertebroplasty | Sham procedure | St. Elisabeth Hospital, Tilburg, Netherlands | Recruiting; data collection ongoing until January 2013 |
| Longo et al. 141 | Percutaneous vertebroplasty | Conservative treatment (defined as 3 weeks’ bed rest wearing a rigid hyperextension suspension brace followed by 2–3 months wearing a Cheneau brace) | None reported | Not clear |
| STU-SPI-S-06–134–0133 | Percutaneous stentoplasty | Percutaneous balloon kyphoplasty | Synthes GmbH (Oberdorf, Switzerland) | Results expected to be available by the end of 2013 |
Study characteristics
A summary of the characteristics of the included studies is provided in Table 4 . Tables providing details of the technical characteristics of the PVP and BKP procedures, and the reporting of clinical outcomes, can be found in Appendix 9 . Baseline demographic data are presented in Table 5 . Only three studies (Farrokhi et al. 135 VERTOS136 and VERTOS II17) defined osteoporosis in terms of BMD; the remainder appeared to assume the presence of osteoporosis from the presence of VCF in the absence of any other known fracture aetiology.
| Study | Country | Recruitment dates | Total numbers randomised | Length of follow-up | Lost to follow-up | Intervention | Comparator | Crossover to intervention permitted | Source of funding |
|---|---|---|---|---|---|---|---|---|---|
| Blasco 2012127 | Spain | April 2006 to January 2010 | 125 | 12 months | 24% | Percutaneous vertebroplasty plus nasal calcitonin for 1 month | Conservative treatment (standardised analgesia; nasal calcitonin for 1 month; rescue therapy with intrathecal infusion if necessary) | Yes | Fundació La Marató de TV3; Spanish Society of Medical Radiology; Catalan Society of Rheumatology |
| Buchbinder 2009102 | Australia | April 2004 to October 2008 | 78 | 2 years. However, data only available for 6 months | 9% | Percutaneous vertebroplasty | Sham procedure without local anaesthesia | No | National Health and Medical Research Council of Australia (284354); Cabrini Education and Research Institute; Cook Australia |
| Farrokhi135 | Islamic Republic of Iran | September 2004 to January 2006 | 82 | 3 years | 7% | Percutaneous vertebroplasty | Optimal medical therapy (suggested baseline analgesia 250 mg paracetamol + codeine and 400 mg ibuprofen, both twice daily; also 1000 mg calcium, 400 IU vitamin D, and 200 IU calcitonin daily; and 70 mg oral alendronate once a week) | Yes after 1 month | Shiraz University of Medical Sciences; Apadana Tajhizgostar Co |
| FREE137 | Multinational (Austria, Belgium, France, Germany, Italy, Netherlands, Sweden, UK) | February 2003 to December 2005 | 300 | 24 months138 | 23% | Balloon kyphoplasty | Non-surgical management according to local practice (also provided to intervention group) | No; patients who wished to cross over were withdrawn from the study | Medtronic Spine LLC |
| INVEST103 | Multinational (USA, UK, Australia) | June 2004 to August 2008 | 131 | Intended to be 12 months.147 However, data only available for 3 months | 5% | Percutaneous vertebroplasty | Sham procedure with local anaesthesia | Yes after 1 month | National Institute of Arthritis and Musculoskeletal and Skin Diseases |
| Liu 2010139 | Taiwan, Province of China | NR | 100 | Minimum of 6 months | NR | Balloon kyphoplasty | Percutaneous vertebroplasty | No | Chung-Shan Medical University Hospital |
| Rousing 2009128 | Denmark | January 2001 to January 2008 | 50 | 12 months | 10% | Percutaneous vertebroplasty | Conservative treatment (pain medication and physiotherapy until discharge, as in intervention group; in addition, bracing also offered) | No | Foundation and Danish government funding |
| VERTOS136 | Belgium and Netherlands | July 2003 to June 2005 | 46 | Intended to be 1 year. However, the study was stopped prematurely at 2 weeks because most control patients asked to cross over to PVP | 26% | Percutaneous vertebroplasty | Optimum pain medication (in ascending order of anaesthesia: paracetamol, NSAIDs or opiate derivatives, according to individual need) | Yes at 2 weeks | NR |
| VERTOS II17 | Belgium and Netherlands | 1 October 2005 to 30 June 2008 | 202 | 12 months | 19% | Percutaneous vertebroplasty | Optimum pain medication (also offered to intervention group); in addition, physiotherapy or bracing also offered | Yes, apparently after 1 week | ZonMw (Dutch organisation for health care research and innovation of care), project number 945–06–351; COOK Medical |
| Study | Mean age in years (intervention/comparator) | Number female (intervention/comparator) | BMD | Method used to assess fracture age | Acceptable duration of fracture pain | Time from estimated fracture onset to intervention (intervention/comparator) | Minimum pain score on 0–10 scale required for inclusion; mean baseline pain score (0–10 scale) (intervention/comparator) | Baseline opioid analgesia use | Fracture aetiology |
|---|---|---|---|---|---|---|---|---|---|
| Blasco 2012127 | 71.33 + 9.95/75.27 ± 8.53 | 47/64 (73%)/50/61 (82%) | Lumbar: –2.48 ± 1.77/–2.80 ± 1.32 Femoral neck: –2.14 ± 0.97/–2.24 ± 0.87 |
Duration of pain; bone marrow oedema seen on MRI or activity on bone scan | < 12 months | Mean duration of back pain (days): 140.3 ± 96.09/143.1 ± 130.33 In weeks: 20.0 ± 13.7/20.4 ± 18.6 |
≥ 4; 7.21 ± 0.33/6.31 ± 0.35 | 47/64 (73%)/31/61 (51%) | Unspecified osteoporosis: 100% |
| Buchbinder 2009102,148 | 74.2 ± 14.0/78.9 ± 9.5 | 31/38 (82%)/31/40 (78%) | NR | Duration of pain; bone marrow oedema, a fracture line, or both seen on MRI (if MRI not feasible, fracture identified by CT and increased uptake compatible with recent vertebral fracture assessed by bone scan) | < 12 months | Median duration of back pain (weeks): 9.0 (3.8–13.0)/9.5 (3.0–17.0) | No minimum requirement specified; 7.4 ± 2.1/7.1 ± 2.3 | 30/38 (79%)/34/40 (85%) | Unspecified osteoporosis: 100% Baseline corticosteroid use: 37% |
| Farrokhi135 | 72 (range 59–90)/74 (range 55–87) | 30/40 (75%)/30/42 (71%) | T-score < –2.5 | Duration of pain; vacuum phenomenon or bone marrow oedema seen on MRI | 4 weeks–12 months | Median duration of back pain (weeks): 27 (4–50)/30 (6–54) | No minimum requirement specified; 8.4 ± 1.6/7.2 ± 1.7 | 30/40 (75%)/30/42 (71%) | Primary osteoporosis: 100% |
| FREE 137 | 72.2 (9.3)/74.1 (9.4) | 115/149 (77%)/117/151 (77%) | NR | Duration of pain; bone marrow oedema seen on MRI | < 3 months | Mean duration of back pain (weeks): 5.6 (4.4)/6.4 (5.2) | ≥ 4; NR | 103/140 (74%)/99/146 (68%) | Primary osteoporosis: 96% Secondary osteoporosis: 3% Multiple myeloma/metastatic: 1% Baseline corticosteroid use: 17% |
| INVEST103,147 | 73.4 ± 9.4/74.3 ± 9.6 | 53/68 (78%)/46/63 (73%) | NR | Duration of pain; if unclear, bone marrow oedema on MRI or increased vertebral-body uptake on bone scanning | < 12 months | Mean duration of back pain (weeks): 6 (IQR 10–36)/20 (IQR 8–38) |
≥ 3; 6.9 ± 2.0/7.2 ± 1.8 | 38/68 (56%)/40/63 (63%) | Unspecified osteoporosis or osteopenia: 100% |
| Liu 2010139 | 72.3 ± 7.6/74.3 ± 6.4 | 39/50 (78%)/38/50 (76%) | NR | NR | NR. PV or BKP said to have been performed within 43 days of injury | Mean duration of back pain (days): 17.0 ± 7.7/15.8 ± 6.7 In weeks: 2.4 (1.1)/2.3 (1.0) |
No minimum requirement specified; 8.0 ± 0.8/7.9 ± 0.7 | NR | Unspecified osteoporosis: 100% |
| Rousing 2009128 | 80 (range 65–96)/80 (range 71–93) | 19/25 (76%)/21/24 (88%) | NR | Duration of pain; if patients had > 1 fracture, fractures were accepted as new if they showed bone marrow oedema on MRI or increased bone turn-over on bone scan | < 8 weeks | Mean fracture age (days): 8.4 (95% CI 3.7 to 13.0)/6.7 (95% CI 2.1 to 11.4) In weeks: 1.2 (0.5–1.9)/1.0 (0.3–1.6) |
No minimum requirement specified; 7.5 (95% CI 6.6 to 8.4)/8.8 (95% CI 8.2 to 9.3) | NR | Unspecified osteoporosis: 100% |
| VERTOS136 | 72 (59–84)/74 (55–88) | 14/18 (78%)/14/16 (88%) | T-score < –2.0 | Duration of pain; bone marrow oedema seen on MRI | 6 weeks–6 months | Mean duration of pain (days) (range): 85 (47–138)/76 (46–141) In weeks: 12.1 (6.7–19.7)/10.9 (6.6–20.1) |
No minimum requirement specified; 7.1 (5–9)/7.6 (5–10) | 6/18 (66%)/5/16 (31%) | Unspecified (implicitly primary) osteoporosis or osteopenia: 100% |
| VERTOS II17 | 75.2 (9.8)/75.4 (8.4) | 70/101 (69%)/70/101 (69%) | T-score ≤ –1 | Duration of pain; bone marrow oedema seen on MRI | ≤ 6 weeks | Mean duration of pain (days): 29.3 ± 17.1/26.8 ± 16.0 In weeks: 4.2 ± 2.4/3.8 ± 2.3 |
≥ 5; 7.8 ± 1.5/7.5 ± 1.6 | 50/95 (53%)/44/92 (48%) | Unspecified osteoporosis or osteopenia: 100% |
Discussion of individual outcome measures included in the review
Health-related quality of life
Generic measures of health status are particularly important in populations with comorbidities, such as the elderly, because disabilities from these comorbidities may influence the patient’s response to treatment. Also, because such measures include mental and social health, they give a more complete picture of the patient’s health than do back-specific instruments. 149 Given the perspective of the research, the measure of most relevance was the EQ-5D. This is detailed first, followed by other HRQoL instruments in alphabetical order.
The EQ-5D has five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/depression) each assessed by a single question on a three-point ordinal scale (no problems, some problems, extreme problems). These are responses are combined and presented as a quasi-continuous outcome on a scale of –0.59 to 1.00, where 0 represents death and 1.00 indicates ‘full health’; negative scores represent health states valued as worse than death. The estimated MCID for people with back pain is 0.08. 150
The Assessment of Quality of Life (AQoL) measure was designed for use in the evaluation of health-care interventions, and is sensitive to changes in the frail elderly. It is a multiattribute quality of life utility instrument, which can be used to provide scores on four dimensions: independent living, relationships, mental health and senses. Alternatively, a single utility score may be computed by combining all of the scales except the illness scale; the score ranges from 1.00 (representing full HRQoL) to –0.04 (representing HRQoL states worse than death), with 0.00 representing death. The MCID appears to be 0.06. 151
The Dallas Pain Questionnaire (DPQ) is a 16-item instrument designed to evaluate the effect of chronic pain on four aspects of life: daily activities, work and leisure activities, anxiety/depression and social interest. Each item is scored on a scale whose extremities are marked 0% and 100%; although this scale appears to be continuous, for each item it is divided into five, six, seven or eight segments, each of which has a score. The item scores for each of the four aspects are added together and multiplied by a constant. They are then reported as a percentage, with lower scores indicating better quality of life. 152 No MCID was identified for the DPQ.
The Mini Mental State Examination (MMSE) is designed to measure cognitive status in adults. It is scored from 0 to 30, with higher scores indicating better cognitive condition. 153
The Short Form questionnaire-36 items (SF-36) is a questionnaire designed specifically to measure self-reported HRQoL; it has been proposed as the most appropriate measure of generic health status for use in people with spinal disorders. 149 It contains 36 questions which measure functional status, well-being and overall health in eight dimensions (physical functioning, role limitations owing to physical health, bodily pain, general health perceptions, vitality, social functioning, role limitations owing to emotional health, and mental health); these eight dimensions may be aggregated to produce summary measures of physical health [the physical component score (PCS)] and mental health [the mental component score (MCS)]. Results are presented on a scale of 0 to 100, with higher scores indicating better health, and may be transformed to take into account population norms. 154 Copay et al. 155 have suggested a MCID of 4.9 points specifically for the PCS, while Angst et al. 156 have suggested a MCID for improvement of 2.0 in the PCS, 7.8 in the bodily pain subscale, and 3.3 in the physical function subscale. Wiebe et al. 157 have suggested a MCID of 3.0 for the PCS and 4.6 for the MCS. No MCID has been identified for the overall SF-36 utility score.
Some of the studies included in this review only used the PCS, ignoring the scales for vitality, social functioning, role limitations owing to emotional health and mental health, thus undermining the value of using a generic measure of quality of life.
In addition to the generic quality of life measures, one measure, the Quality of Life Questionnaire of the European Foundation for Osteoporosis (QUALEFFO), has been developed and validated specifically for use in clinical trials in patients with vertebral osteoporosis. The QUALEFFO-41 has 41 questions relating to five domains: pain, physical function, social function, general health and mental health. 158 The domain scores are presented on a scale of 0 to 100, where 0 corresponds to the best HRQoL and 100 to the worst HRQoL. 159 QUALEFFO-41 has been shown to discriminate better between patients with and without vertebral fractures than the EQ-5D. 159 No MCID is known to have been suggested for the QUALEFFO. There is also a shorter version: the QUALEFFO-31. 160
Back-specific functional status/mobility
Measures of functional status assess the ability to perform specific tasks: this ability may have only a weak relationship with the reported level of pain. 161 However, for many patients, the greatest problem caused by a VCF is the limitation of activity rather than pain per se, and therefore functional status is a more clinically meaningful outcome than pain status. Moreover, it has been argued that the Roland–Morris Disability Questionnaire (RDQ), one of the most commonly used back-specific scales, is more useful than self-reported pain for assessing the impact of vertebral fractures on the patient’s daily life because it is more objective: self-reported pain is influenced by the patient’s perception and tolerance of pain, and relates only loosely to functional limitation. 162 This argument also applies to other measures of disability.
Furlan et al. 126 differentiate between back-specific functional status, as measured by items such as the RDQ, and disability, which can be assessed in terms of factors such as the ability to perform activities of daily living, work absenteeism, etc.; they consider both to be important patient-centred outcomes, along with symptoms (e.g. pain), perception of overall improvement, satisfaction with treatment, and well-being (e.g. quality of life measured with the SF-36, etc.). The studies included in this review use a number of different measures of functional status. These are detailed in alphabetical order, preceding a selection of direct observer-assessed measures of physical function.
The Barthel Index is a 10-item scale designed to evaluate the observer-assessed ability of a patient with a neuromuscular or musculoskeletal disorder to care for him or herself. The items assess independence in key activities of daily living (feeding, transferring from wheelchair to bed and back, grooming, toilet use, bathing, mobility on a level surface, ascending and descending stairs, dressing, faecal continence and urinary continence). It was originally scored from 0 to 100, with lower scores indicating greater disability,163 but may also be scored from 0 to 20, and in either case lower scores indicate greater disability.
A change of 1 point in the 0–20 Barthel Index (5 points in the original 0–100 version) represents a change in level of dependency in any of the key activities, and is therefore likely to be clinically meaningful. 57
The Oswestry Disability Index (ODI) was designed to measure patient-reported disruption to activities of daily living attributed to back pain. It comprises 10 dimensions [pain intensity, personal care, lifting, walking, sitting, standing, sleeping, sex life (if applicable), social life and travelling], each containing six response categories scored from 0 (indicating no disruption) to 5 (the worst state). The score is reported as a percentage of the maximum possible score (i.e. on a scale of 0–100). The weighted mean score for ‘normal’ populations is 10.19. 164
It has been suggested that, for the ODI, the MCID should be 4 points in relation to mean scores between groups but 15 points in patients before and after surgery. 164 More recently, Ostelo et al. 119 have suggested a MCID of 10 points in the transformed (0–100) scale, or 30% improvement from baseline, while Lauridsen et al. 165 suggest a MCID of 11 points in all patients, and 8 points in patients attending hospital back pain clinics.
Both the ODI and the RDQ are easy to use, reliable and valid. Because of floor and ceiling effects, the ODI is recommended for use with patients who are likely to have persistent severe disability, and the RDQ with patients who are likely to have relatively little disability, but for most patient groups both instruments appear to function satisfactorily in groups with severe disability. 164 However, the modified, 23-item version of the RDQ166 has been shown to be more responsive than the ODI in patients with low back pain only, whereas the ODI appeared slightly more responsive in patients with leg pain and/or low back pain. 165 Moreover, self-reported disability measures such as the RDQ have been shown to display only modest correlation with direct measures of physical function. 164
The RDQ was designed to assess physical disability due to low back pain. 164 It assesses self-reported functional status in eight dimensions (physical activities, housework, mobility, dressing, getting help, appetite, irritability and pain) by measuring 24 activity limitations. 167 Scores range from 0 (no disability) to 24 (maximum disability). 164 The original RDQ contained a six-point pain rating scale. However, this has now been excluded with the recommendation that the SF-36 pain scale be used for this purpose. 164 The modified RDQ (RDQ-23) contains 23 questions, some of which differ from those in the original questionnaire; it is scored from 0 to 23. 152
The MCID for the RDQ varies according to the level of disability of the patients, from 1 to 2 points in patients with little disability to 7–8 points in patients reporting high levels of disability, and 5 points in uncategorised patients; Ostelo et al. 119 suggest 5 points, or 30% improvement from baseline. Lauridsen et al. 165 suggest an overall MCID of 5 points, but recommend that 2 points should be used in populations attending hospital back pain clinics. However, Roland and Fairbank164 recommend that, in clinical trials which use the modified, 23-item version of the RDQ, a MCID of 2–3 points should be used for sample size calculations.
The Study of Osteoporotic Fractures-Activities of Daily Living (SOF-ADL) scale consists of six questions relating to activities undertaken in a typical day. The scale ranges from 0 to 18, with higher scores indicating more back-related disability. 153
Direct observer-assessed measures of physical function include:
-
the timed ‘up and go’ test, which assesses functional mobility by measuring the time in seconds required to rise from a standard armchair, walk 3 metres, turn round, return to the chair and sit down again128
-
the tandem test, which assesses balance by measuring the time for which the patient can stand in three different positions168
-
the repeated chair test, which tests muscle power by asking the patient to rise from, and return to, sitting as many times as possible in 30 seconds; higher scores indicate better health status. 153
The included studies also assessed functional status in terms of the use of aids and appliances, days of bed rest or reduced activity, and perceived recovery.
Pain/analgesic use
The recommended measure of global pain severity is the bodily pain subscale of the SF-36, a two-item scale which measures pain intensity on six levels (from none to very severe) and interference with activities on five levels (from not at all to extremely). 149 This measure is recommended not least because of the availability of normative data. 169 An absolute cut-off value has been suggested for the MCID of 3 points overall or 2 points in populations attending hospital back pain clinics. 165
However, many of the included studies used either:
-
a visual analogue scale (VAS), whereby patients mark the point which best represents their pain on a line, usually 10 cm long, whose ends bear labels describing the extremes of pain intensity (e.g. ‘no pain’ and ‘worst imaginable pain’); or
-
a numeric rating scale, whereby patients rate the intensity of their pain on a scale of 0 to 10 (11-point scale) or 0 to 100 (101-point scale), where 0 represents no pain and 10 or 100 the worst possible pain. 169
The VAS, which is formatted without numbers, represents a continuous range of values, while the numeric rating scale is formatted using whole numbers to form a segmented scale. 170
Because it is usually measured in millimetres, and can therefore be regarded as having 101 response categories, a 10 cm VAS is potentially more sensitive than an 11-point numeric rating scale. However, it has been shown to be more difficult to understand than other measures of pain intensity, especially for people at risk of cognitive difficulties (e.g. some elderly individuals, or people taking high doses of opioid analgesics). 169 Moreover, the VAS was developed for assessing chronic pain and is less reliable in the immediate postoperative period, when any single VAS score may have an imprecision of ±20 mm (20%). 171 Numeric rating scales are easier to use, and their sensitivity and validity has also been demonstrated. Both Bolton and Wilkinson172 and Grotle et al. 173 suggest that the numeric rating scale may be more responsive than the VAS. However, unlike the VAS, numeric rating scales have not been demonstrated to have ratio qualities (i.e. a change in pain score from 9.0 to 6.0 cannot be assumed to represent a 33% decrease in perceived pain). 169
Some of the included studies (Blasco et al.,127 Farrokhi et al.,135 Liu et al.,139 Rousing et al. 128 and VERTOS II174) stated that they used a VAS scale; others (Buchbinder, et al. 148 FREE,137 INVEST147 and VERTOS136) specified that they used an 11-point numeric rating scale, although in the FREE137 and VERTOS136 studies this was called a VAS.
As the distribution of scores from VAS and numeric rating scales is not normal, non-parametric statistical analyses are appropriate. 57 Moreover, because initial and subsequent pain ratings on the VAS tend to be correlated, any between-group comparison should compare changes in scores from baseline rather than simply differences in the final post-treatment score. 57
Ostelo et al. 119 have proposed an absolute cut-off value for the MCID of 15 on a 100-unit VAS, with a relative cut-off value a 30% improvement from baseline. However, DeLoach et al. 171 suggest that, because of the imprecision found in the immediate postoperative period, the MCID in that period should be 20 out of 100 units. Ostelo et al. 119 also proposed that, when an 11-point numeric rating scale is used for low back pain, the absolute cut-off value for the MCID should be 2 points, again with a relative cut-off value of a 30% improvement from baseline. However, Copay et al. 155 suggest a MCID of 1.2 points for back pain.
The INVEST study also used a modified version of the Deyo–Patrick pain frequency and bothersomeness scale. 147 The original scale measured the frequency with which patients experience pain, and how pain impacts on their daily life, on a scale of 0 to 6, where 6 indicates the highest impact. 166 The INVEST study used a scale of 0 to 4, with higher scores again indicating more severe pain. 103
While the technology assessment protocol did not specify analgesic use as an outcome, it has been included because some of the included studies used analgesic use as a proxy for pain relief. This may be measured either quantitatively (i.e. amount of analgesia used) or qualitatively (i.e. type of analgesia used). Most of the included studies used a qualitative approach, grouping analgesics into categories such as non-opioid, weak opioid and strong opioid. Doidge et al. 57 note that these distinctions are clinically important because the risk profiles of the different categories of analgesic differ substantially.
Vertebral body height and angular deformity
Vertebral body height may be measured at different parts of the vertebra (posterior, middle and anterior). In addition, restoration of vertebral body height may be reported in four different ways:
-
absolute restoration in millimetres
-
per cent restoration relative to pre-operative height of the fractured vertebra
-
per cent restoration relative to lost vertebral height (based either on a pre-fracture radiograph or on an estimate of the unfractured height of the fractured vertebra)
-
per cent restoration relative to referent vertebral height (the height of the nearest non-fractured vertebra).
McKiernan et al. 175 found substantial variations in the reported magnitude of height restoration when radiographs of the same vertebrae were measured using all four methods. Unless the same referent normative height was included in each radiograph, the comparison of ‘absolute’ values, both between studies and between radiographs in the same study, was unreliable because of the possibility of magnification error. The use of relative data allowed comparison both within and between studies which used the same fixed dimension, provided that the precision error of the measurement was acceptable. However, studies which used different fixed dimensions could not be compared: thus, for instance, the apparent magnitude of height restoration was almost four times greater when anterior vertebral height was measured using method 2 than when using method 4. The choice of fixed dimension may have a differential effect on fractures of varying degrees of severity: in severely compressed vertebrae, the apparent restoration was greater using the pre-operative height (method 2) than the lost vertebral height (method 3), whereas, in very mild fractures, the restoration was greater using method 3 than method 2. 175
McKiernan et al. 175 therefore recommended that reports of vertebral height restoration should:
-
include all index vertebral height dimensions (posterior, middle and anterior)
-
include absolute measurements of all referent vertebral heights
-
be reported relative to a referent normative height (either referent vertebral height or a radio-opaque object of known dimension included in the radiographic field), in order to permit correction for inter-radiographic measurement error
-
take into account the dynamic mobility of some osteoporotic VCFs
-
include the calculated precision error for all measurements. 175
He notes elsewhere that the precision error for older, osteoporotic, populations is in the region of 2% to 5%. 176
None of the included studies appear to meet McKiernan et al. ’s standards.
Doidge et al. 57 note that vertebral height is a surrogate outcome and that, as such, criteria have not been established to determine the clinical importance of any changes.
Angular deformity may be evaluated in terms of the kyphotic angle, the sagittal index or measures of sagittal balance.
The kyphotic angle has been defined as the angle defined by the intersection of a line drawn on a radiograph through the posterior–superior and anterior–superior endplate margins of an individual vertebra and a line drawn through the posterior–inferior and anterior–inferior endplate margins of the same vertebra. McKiernan et al. 177 reported a precision error of 15.6% in the measurement of the kyphotic angle in elderly osteoporotic patients undergoing PVP or BKP. However, McKiernan et al. 177 notes that most studies which report improvements in the kyphotic angle fail to provide an osteoporosis-appropriate precision error, and states that, in the absence of a measured and reported precision error, the statistical significance of any comparison of kyphotic angles is suspect. 176
The sagittal index is a measure of kyphotic segmental deformity at the level of a given mobile segment (i.e. one vertebra and one disc), corrected for the normal sagittal contour at the level of the deformed segment. 178 Taking the baseline sagittal contour into account gives the sagittal index a potential advantage as a diagnostic assessment tool. 179 Jiang et al. 179 found the sagittal index to have acceptable correlation coefficients for both inter- and intra-observer reliability. However, they also noted that these rates of agreement were lower than those of two other methods of kyphotic angle assessment – the Cobb angle and the Gardner angle. It was suggested that the lower level of reliability was observed because the sagittal index includes a smaller area of measurement, thereby maximising differences between measurements. Further potential sources of measurement variability may include radiograph quality, type of fracture, fracture location and the position of the radiographic beam relative to the vertebral level in question. 179
Sagittal spinal balance is a harmonious alignment of the pelvis and spine which allows ease of standing. It can be assessed using lateral radiographs measuring the relationship between the C7 and S1 vertebrae, with greater plumb line deviation of the C7 vertebra representing higher levels of imbalance. Increases in positive sagittal balance have shown strong linear correlations with a variety of health and disability measures. 180
As Doidge et al. 57 note, no criteria have been established to determine the clinical importance of any changes in angular deformity. Moreover, the potential for change in angular deformity is presumably dependent upon the morphology of the vertebral fracture being treated.
Vertebral fractures may be symptomatic or asymptomatic. Symptomatic, or clinical, vertebral fractures cause either sufficient discomfort for the patient to bring them to the attention of a health professional or a measurable loss of height. Their presence can be confirmed by radiographs. However, radiographs can also identify asymptomatic fractures. Some studies undertake routine imaging at follow-up and thus report radiographically identified fractures (also termed radiographic or morphometric), which will include both symptomatic and asymptomatic fractures. However, some studies report only clinical fractures.
None of the various approaches which have been developed to identify radiographic vertebral osteoporotic fractures has been agreed to be the gold standard. The purely qualitative approach, which depends on the visual identification of abnormalities in vertebral shape or height, is a subjective method with poor inter- and intra-rater reliability; however, unlike a purely quantitative method, when performed by an expert it can exclude vertebral abnormalities which are not osteoporotic in origin. 179 More recently, Jiang et al. 179 have developed an algorithm-based qualitative approach which aims to facilitate differentiation between osteoporotic fracture and deformity due to other causes. Quantitative methods are more objective and reproducible than qualitative methods, but may identify non-fracture deformities as fractures, while failing to recognise mild endplate fractures. 179 However, the number of false positives may be reduced if the definition of incident fracture requires a 20% or greater reduction in anterior, central or posterior vertebral height. 181 The semiquantitative method developed by Genant et al. 7,182 grades each vertebra according to the visually apparent degree of reduction in vertebral height and area, irrespective of the type of deformity, but also gives careful attention to changes in vertebral shape, enabling non-fracture deformities to be excluded while endplate fractures which are not associated with a 20% reduction in vertebral height can be identified. 183 The semiquantitative method is more objective and reproducible than the qualitative method, but has better specificity and sensitivity than the quantitative method because it reduces the number of false positives while identifying mild deformities which the quantitative method would exclude. 183 However, some researchers claim that the semiquantitative method can be difficult to apply accurately, and that it overestimates fracture prevalence by failing to differentiate adequately between true fractures and non-fracture deformities. 179,184
Doidge et al. 57 note that the relationship between a vertebral fracture and subsequent vertebral fractures is known to be time-dependent – that is to say, the risk of subsequent fractures reduces over time. It is also likely that, if PVP or BKP affect the risk of subsequent fracture, they will do so in a time-dependent manner. Consequently, risks estimated in populations which differ in terms of either baseline fracture age or length of follow-up are not directly comparable. 57
It has been suggested that vertebrae adjacent to those which have been treated by PVP or BKP may be susceptible to subsequent fractures because the treated vertebrae, being stiffer than those which have not been treated, may transmit increased force to adjacent vertebrae. 185 In this context, the most meaningful outcome measure is the proportion of patients who experience at least one clinically important adjacent fracture. 57 Some studies report only adjacent fractures, while others report all incident fractures whether adjacent or distant; some report the number of patients who suffer fractures, and others only the number of fractures.
There is also potential for confounding of the data in that patients who have had vertebral augmentation and experienced considerable benefit may become more active and be at a greater risk of fracture than more sedentary patients.
Progression of treated fracture is defined in terms of loss of vertebral height. The only study to report this outcome, VERTOS II,186 defines progression as a loss of vertebral height of 4 mm or over, categorising a loss of 4–7 mm as moderate and a loss of over 8 mm as severe. 186
Percutaneous vertebroplasty and BKP have been associated with a range of adverse events. These include:
-
Complications related to insertion of a needle, including infection, venous bleeding187 and damage to neural or other structures. 86
-
Complications related to the leakage of bone cement or the displacement of bone marrow and other material by the cement. Leakage occurs when the cement is not wholly contained by the fractured vertebra but escapes through either the fracture or the track created by the needle. Cement may leak into the paravertebral soft tissues, the spinal canal or neural foramina, the adjacent vertebral disc spaces, or nearby blood vessels. 57 Such leaks may compress the nerve root (causing radiculopathy which may be transient and treatable with NSAIDs or local steroid injections, or, if pain is persistent, may require surgical removal of the cement) or the spinal cord (resulting in myelopathy, and requiring urgent neurosurgical decompression to prevent neurological sequelae including paresis or paralysis). 16,187 They may also result in pulmonary embolism which may be asymptomatic or may result in signs and symptoms such as chest pain, dyspnoea, tachypnoea, cyanosis, coughing, haemoptysis, dizziness and sweating. 153 Pulmonary emboli composed of marrow material displaced by the cement appear to be more common than emboli formed of the cement itself. Healthy individuals can tolerate small pulmonary emboli without symptoms, but a large cement leak can lead to pulmonary infarct, and multiple emboli may lead to pulmonary compromise and even death due to respiratory compromise. 187 Sharp and elongated spike-like cement fragments may also perforate blood vessels or the heart. 188
-
Complications relating to balloon rupture in BKP. 87
-
Systemic reactions to the bone cement, including hypotension and death. 91 The mechanisms underlying PMMA-induced systemic reactions are not clear: hypotheses include potential toxic, vasodilating or allergic effects of the cement, as well as possible bone marrow microemboli. 189
-
Complications relating to other aspects of the procedure such as patient positioning and anaesthesia: these include fracture of the rib or sternum in severely osteoporotic patients190 and systemic infection. 191
Some of these adverse events are acute (e.g. bleeding at puncture site, local infection, cement leakage and pulmonary embolism); other sequelae are delayed (e.g. adjacent vertebral fracture, cement dislodgement and pyogenic spondylitis). Some are minor, requiring no surgical intervention, whether immediate or delayed. Others are serious, requiring surgical intervention or resulting in death or significant disability. 16
It has been noted that the number of cement leaks which are identified is related to the method used to identify them. For example, in a recent retrospective case series of 181 patients with 277 levels treated with PVP, Martin et al. 192 found that CT detected leakage in 149 patients (82%), while procedural dictation and plain radiography detected leaks in 62 (34%) and 77 patients (50%). The differences in detection rates between each of these methods and CT were statistically significant (both p < 0.01). CT is therefore viewed as the most sensitive method for detecting cement extrusion, as small pulmonary cement deposits which remain undetected on chest radiographs are readily apparent on CT. 193
However, the added sensitivity provided by CT may be of limited benefit. For example, in the open-label VERTOS II RCT,194 perivertebral cement leakage was observed in 80% of treated vertebrae, and all leakages remained clinically silent. 188 Although the long-term implications of such small extrusions remain poorly understood, Venmans et al. 188 have argued that some leakage during PVP is difficult to avoid, and that the real issue concerns clinically relevant leakages.
In addition to adverse events affecting patients, PVP and BKP pose hazards to health-care professionals. These relate to:
-
exposure to bone cement
-
exposure to radiation.
In addition to the adverse events of treatment listed above, Medtronic have put forward evidence drawn from large population-based data sets to suggest that vertebral augmentation also has beneficial effects in terms of reduced mortality and morbidity; the reductions in morbidity are not directly captured by the outcomes included in the current review, while the included studies are not large enough to demonstrate significant differences in mortality. (Academic-in-confidence information has been removed.)
Study quality
Internal validity
The included studies varied in terms of internal validity ( Figure 2 ). The potential sources of bias are discussed in turn below.
FIGURE 2.
Risk of bias summary: review authors’ judgements about each risk of bias item for each included study (+ = low risk; – = high risk; ? = unclear risk).

Risk of selection bias
All the included studies were described as randomised. However, as Blasco et al. 127 stated only that ‘randomisation was done with a previously defined randomised computer list’, there was lack of clarity about the method of both assignment to treatment groups and concealment of allocation. Liu et al. 139 provided no information regarding concealment of allocation.
In multicentre trials of interventional procedures, stratification of randomisation by treatment centre is important to avoid potential imbalances associated with differences in techniques and skills. Two of the five multicentre studies (Buchbinder et al. 102 and INVEST103) specified that randomisation was stratified by treatment centre; in a third (FREE137), although randomisation was stratified, treatment centre was not one of the variables. In VERTOS136 and VERTOS II,17 randomisation is not said to have been stratified. Of the remaining four studies, that by Blasco et al. 127 was definitely a single-centre study, and those by Liu et al. 139 and Rousing et al. 128 were probably also single centre. In the study by Farrokhi et al. ,135 although two hospitals appear to have been involved, the same surgeon seems to have undertaken the procedure in both. 197 Thus, the risk of bias owing to differences in techniques and skills appears to be higher in FREE, VERTOS and VERTOS II than in the remaining studies.
Risk of performance bias
Few studies appear to be at risk of bias associated with co-interventions as they offered the same oral analgesics to both the treatment and control groups. In the study by Blasco et al. ,127 the methods section suggests that rescue therapy by intrathecal infusion (25 µg fentanyl and 1.5 mg bupivacaine) was only offered to patients in the control group in whom drug therapy had proved ineffective (VAS > 7) or intolerable. If there was then no improvement in pain, conservative treatment was deemed to have failed and the patient was considered for PVP. However, it is clear from the results section that this rescue therapy was also made available to patients who had received PVP. 127 The risk of performance bias was unclear in the study by Rousing et al. :128 this did not explicitly define conservative treatment or detail the steps taken to avoid co-interventions, but appeared to offer brace treatment to the control group but not the PVP group.
Some of the studies were at risk of bias because of crossover – that is to say patients who were randomised to one intervention receiving the other intervention. Crossover is generally more common in unblinded than in blinded studies because, in unblinded studies, the patients in the control group are aware that they have not received the experimental intervention. Three of the included studies appear to be free of crossover: it was not allowed in the studies by Buchbinder et al. 102 and Rousing et al. ,128 and was presumably impossible in Liu et al. ’s139 comparison of PVP with BKP. In the remaining six studies, patients randomised to one treatment might choose, after a minimum period of time, to receive the other treatment; in the FREE study, they were then considered to have withdrawn from the study, but were included in the intention to treat analysis at 1 year. 137 In the blinded INVEST study,103 patients were informed at recruitment that they would be allowed to cross over to the other procedure 1 month or more after the intervention if adequate pain relief was not achieved; specific numerical pain thresholds were not used to determine the adequacy of pain relief and therefore eligibility to cross over. 103 Although crossover was permitted, blinding was maintained for the full year. 147 In the unblinded studies, crossover was reported to be in one direction only, from conservative treatment to the intervention; however, in the Blasco et al. ,127 FREE137 and VERTOS II17 studies, some patients allocated to PVP or BKP did not receive the intervention for various reasons, and the investigators did not appear to adjust for non-treatment. The blinded INVEST study103 reported crossover from PVP to the OPLA as well as from the sham procedure to PVP: by 3 months, 8 out of 68 (12%) of patients allocated to PVP had crossed over, compared with 27 out of 63 (43%) allocated to OPLA103 ( Table 6 ).
| Study | Allocated to PVP but did not receive surgery, or crossed over to control | Allocated to BKP but did not receive surgery | Allocated to control intervention but crossed over to receive PVP or BKP | Time point to which data uncontaminated by crossover |
|---|---|---|---|---|
| Blasco 2012127 | 7/64 (11%) (five refused PVP, two improved spontaneously) | N/A | 7/61 (11%) | Data contaminated from baseline by patients in PVP group who refused intervention; not clear when patients crossed over from the control group |
| Buchbinder 2009102 | 0 | N/A | 0 | Data appear uncontaminated to end of follow-up |
| Farrokhi135 | 0 | N/A | 10/42 (26%) | Data uncontaminated to 1 month |
| FREE137 | N/A | 10/149 (7%) | 14/151 (9%) | Data contaminated from baseline by patients in BKP group who refused intervention; some patients crossed over from control group at < 1 month |
| INVEST103 | 8/68 (12%) (crossed over to control intervention) | N/A | 27/63 (43%) | Not clear: crossover not permitted before 1 month but one patient in the VPV group and two in the control group underwent the crossover intervention at < 1 month |
| Liu 2010139 | 0 | 0 | N/A | Data appear uncontaminated to end of follow-up |
| Rousing 2009128 | 0 | N/A | 0 | Data appear uncontaminated to end of follow-up |
| VERTOS136 | 0 | N/A | 14/16 (88%) | Data appear uncontaminated up to 2 weeks |
| VERTOS II17 | 8/101 (8%) (two withdrew consent, three refused PVP, three improved spontaneously) | N/A | 15/101 (15%) | Data contaminated from baseline |
Several studies which were theoretically at risk of bias owing to crossover attempted to avoid such bias by reporting results only up to the point at which crossover was permitted. Thus, the INVEST study,103 which permitted crossover after 1 month, reported comparative results only up to that point; 2 out of 42 patients (5%) appear to have crossed over from OPLA to PVP before 1 month. 103 The VERTOS study,136 which permitted crossover at 2 weeks, was stopped prematurely at that point because 88% of the control group (14/16) requested PVP. 136 In the study by Blasco et al. ,127 7 out of 61 patients allocated to conservative therapy (11%) underwent PVP,127 but the date at which this occurred was not reported. Farrokhi et al. 135 permitted crossover after 1 month; 10 of the 42 control patients (24%) had undergone PVP by 1 year, and a further 10 apparently underwent PVP after 1 year. 135 In the FREE study,137 14 out of 151 patients in the control group (9%) underwent BKP, nine of them (6%) before 1 month. 137 In VERTOS II,17 5 out of 101 patients withdrew from the control group before treatment because they wanted PVP; because they withdrew consent, the vertebroplasty procedure could not be documented and analysed in those patients. A further 10 control patients (10%) requested PVP at various points during the study. 17 Thus, the studies with the highest rates of crossover (INVEST103 and VERTOS136) report comparative results only for the period preceding any substantial crossover, and the 1-month data from the study by Farrokhi et al. 135 are also unaffected. However, there is some potential for bias owing to crossover in the study by Blasco et al. 127 and the FREE137 and VERTOS II17 studies.
Only two studies (Buchbinder102 and INVEST103) sought to avoid bias by blinding patients to their treatment allocation; both used an OPLA to do so. In the INVEST study, the success of blinding was evaluated: at 14 days, 51% of patients in the PVP group and 63% in the control group correctly guessed their allocation, but in both cases their degree of confidence in the accuracy of their guess was only moderate. 103 Buchbinder et al. planned to evaluate the success of blinding at the end of the study148 but do not appear to have reported the results. In the study by Liu et al. , patient blinding was presumably feasible, as the patients received PVP or BKP under general anaesthesia, but, as patient blinding was not mentioned, it seems unlikely that it was done, particularly as it was stated that some outcomes were assessed by blinded assessors. 139 The remaining studies made no attempt to blind patients to their treatment allocation. Consequently, as many of the outcomes were patient-reported and subjective in nature, the risk of bias associated with lack of blinding in these studies is substantial.
Risk of detection bias
Because of the radio-opaque nature of the cement used for PVP and BKP, it is impossible to blind the assessors of radiographic outcomes (vertebral body height, kyphotic angle and incident fracture) to treatment allocation. However, there is no reason why blinded assessors should not have been used to collect data relating to other outcomes, yet only four studies (Buchbinder et al. ,102 Farrokhi et al. ,135 INVEST103 and Liu et al. 139) stated that they used blinded outcome assessors for at least some outcomes. Three studies (Blasco et al. 127, FREE137 and VERTOS II186) stated that the radiopacity of the bone cement made it impossible to blind outcome assessors, and appeared to make no attempt to use blinded assessors for non-radiological outcomes. There appeared to be no blinding of outcome assessors in the Rousing et al. 128 and VERTOS136 studies. As many of the non-radiological outcomes were subjective patient-reported outcomes, which may be modified by contact with non-blinded outcome assessors, such data are at risk of bias in all except the Buchbinder et al. 102 and INVEST103 studies, and possibly also that by Liu et al. 139
In the majority of studies, outcomes appeared to be assessed at comparable times in both groups. However, in the study by Liu et al. , the timing of the assessment of some outcomes was not clear. 139 In VERTOS II, the timing of assessment of both baseline characteristics and subsequent outcomes appears not to be comparable because baseline was said to be the day of randomisation for the control group but the day of PVP for the intervention group; moreover, PVP was said to have been performed a mean of 9.4 (SD 8.1) days after randomisation. 17 This has two major consequences:
-
Although the inclusion criteria stipulate that participants should have had back pain for no more than 6 weeks, many patients in the PVP group would have undergone the intervention more than 6 weeks after pain onset, and thus would have subacute rather acute VCFs, whereas all of the control group would have had acute fractures at baseline. Consequently, Doidge et al. 57 suggest that between-group differences in baseline variables such as the EQ-5D, which Klazen et al. 17 ascribe to chance, may in fact be due to differences in fracture acuity.
-
In the control arm of VERTOS II, the mean pain score fell by 1.9 points (25%) during the first week following randomisation. 17 A comparable reduction might presumably be expected in the PVP group between randomisation and the assessment of baseline characteristics on the day of the intervention. However, as the ‘baseline’ pain score was 7.8 in the PVP group compared with 7.54 in the control group, if such a reduction occurred, it implies both a substantial disparity between groups at randomisation and an implausibly high mean pain score in the PVP group at that point in time.
There is thus considerable lack of clarity in relation to the timing of assessments in VERTOS II, and clarification has been sought, but not received, from the study first author.
Risk of attrition bias
Five studies (Buchbinder et al. ,102 Farrokhi et al. ,135 INVEST,103 Rousing et al. 128 and VERTOS II17) followed up at least 80% of participants. Liu et al. 139 made no reference to attrition: this may be because there was none, but this is not specified. In the Blasco et al. 127 and FREE137 studies, only 76% and 78%, respectively, completed follow-up at 12 months; in the FREE study, there was a marked disparity between treatment groups, with 83% in the BKP group completing follow-up at 12 months compared with 74% in the control group,137 whereas in the study by Blasco et al. follow-up was higher in the control group (79% vs. 73%). 127 In the VERTOS study, only 74% overall completed 2 weeks’ follow-up. The data are poorly presented, making a full comparison of drop-out rates in the two treatment groups impossible; six patients are said to have withdrawn from the control group because they wanted PVP, and two patients from the PVP group because they wanted the control intervention, but details are not given of the treatment allocation of the four patients who refused to complete questionnaires at 2 weeks, nor are their outcomes reported at 1 day. 136
All studies except Liu et al. 139 gave information relating to the reasons for withdrawal. While the absence of such information in Liu et al.’s study may further suggest that there were no withdrawals, this was not explicitly stated.
With the exception of VERTOS,136 all studies specified how many patients were randomised to each group. Moreover, all but Liu et al. 139 stated clearly how many were included in the final analysis, and it is possible that Liu et al. 139 did not provide this information because there were no withdrawals. 139 The majority of studies, including all the studies which reported crossover, reported using intention-to-treat analyses.
Risk of reporting bias
Only three studies (Blasco et al.,127 Farrokhi et al. 135 and VERTOS II17) appeared to be free of selective reporting. The Buchbinder et al.,102 FREE137 and INVEST103 studies reported most but not all of the clinical outcomes specified in the study protocol. It is understood that the longer-term outcomes from the Buchbinder et al. study (presumably including the incidence of new vertebral fractures at 12 and 24 months), although not yet reported, are to be published; however, this does not explain the failure to report the results of the timed ‘up and go’ test and the patients’ perceptions of fatigue and overall health. The FREE study did not report functional outcome and the results of objective functionality tests or data relating to vertebral body height (for details, see Appendix 9 , Table 101 ); the last of those omissions is the most surprising, given that one of the particular merits of BKP is said to be its effect on VBH. The INVEST study did not report adjacent fractures at 12 months or implant-related inflammation. No study protocol could be found for the Liu et al.,139 Rousing et al. 131 or VERTOS136 studies, and therefore the risk of reporting bias in these studies is not clear.
Risk of other bias
Studies may be at risk of bias if their intervention and control groups differ in baseline factors which are strongly related to outcome measures. 196 In four studies (Buchbinder et al. ,102 Farrokhi et al. ,135 FREE137 and INVEST103), the treatment groups appeared to be comparable at baseline. Liu et al. reported so few baseline characteristics that it was difficult to assess comparability. The remaining studies appeared to be potentially at risk of bias because of differences between groups at baseline. In the study by Blasco et al. , mean baseline pain, opioid use and QUALEFFO scores were lower in the control group than in the PVP group. The investigators, who subdivided opioid use into major and minor opiate derivatives, stated that none of the differences were statistically significant;127 however, if the data are aggregated, total opioid use is significantly higher in the PVP group than in the control group (risk ratio 1.08, 95% CI 1.08 to 1.93, p = 0.01). Rousing et al. noted that the mean baseline pain score was significantly lower in the PVP group than in the control group (7.5 vs. 8.8, p = 0.02); no information was presented on baseline analgesic use. Rousing also reported a statistically significant difference in EQ-5D scores, favouring PVP; there were also noticeable between-group differences, favouring PVP, in all domains of the DPQ. Despite these differences, results were not reported as changes from baseline and statistical significance was attributed to the unadjusted data. 128 In VERTOS, the number of treated fractures was significantly higher in the PVP group (p = 0.04), and there were also significantly more wedge fractures, and fewer biconcave fractures, in the PVP group than in the control group (p = 0.02);136 the potential impact of these differences on the success of PVP is not clear. Finally, in VERTOS II, there were said to be significant differences between groups in EQ-5D, QUALEFFO and RDQ scores;17 in each case, the status of the PVP group was worse than that of the control group. However, adjusted results were reported.
External validity (generalisability) and precision
External validity
The external validity of the included trials is summarised in Figure 3 . Most of the included studies specified their eligibility criteria, thus enabling assessment of the nature of their patient populations. However, many reported that a substantial proportion of patients declined to participate, and only Farrokhi et al. 135 specifically stated that the rate of refusal to participate was low (see Table 7 ). It is not always clear whether patients refused to participate before or after they were found to meet the study inclusion criteria. Consequently, the figures included in Table 7 are presented as percentages of the total number of potential patients who were said to have been screened for each study, and not of the (lower) number remaining following subtraction of those who were subsequently excluded because they did not meet the inclusion criteria or for other reasons; the figures may thus be regarded as conservative. As refusal to participate is a patient’s decision rather than one made by the study investigators, such a decision made prior to randomisation may be expected to affect both treatment groups equally and seems unlikely to affect study validity, although it may limit generalisability if the decision to participate is influenced by symptom severity. However, in the INVEST study, a comparison of data relating to eligible patients at the lead site who did and did not enrol found no significant differences in age, proportion of women or RDQ score; data relating to pain were collected differently in the two groups and were therefore not directly comparable. The authors therefore suggested that the results of the INVEST study should be generalisable to all patients who would have been eligible for enrolment in that study. 197
| Study | Potential participants who refused to participate |
|---|---|
| Blasco 2012127 | Not reported |
| Buchbinder 2009102 | 141/468 (30%) |
| Farrokhi135 | 2/105 (2%) |
| FREE137 | 209/1279 (16%) |
| INVEST103 | 300/1813 (17%) |
| Liu 2010139 | Not reported |
| Rousing 2009128 | Not reported |
| VERTOS136 | Not clear how many potential participants were screened; the study states that, ‘of approximately 1 in 4 potential study candidates, a total of 46 patients consented initially to participate in the study’ |
| VERTOS II17 | 277 a/934 (30%) |
Although most of the included studies provided an explicit description of the interventions, Rousing et al. provided only a relatively cursory description of the control treatment. 128 Traditionally, PVP has been performed by radiologists and BKP by surgeons. 198 However, few of the included studies provided adequate details of the clinical background and specific procedure-related training of the clinician who performed PVP or BKP, or of their relevant experience (i.e. the number of procedures they had completed before the study), although such information is important for interpreting study results. Studies involving inexperienced clinicians and centres in the early stages of introducing PVP or BKP may include ‘learning curve’ data, whereas studies involving more experienced clinicians and centres may have more favourable results. The information provided was judged unclear if only the specialism (e.g. radiology or neurosurgery) was reported; it was only judged adequate if details were also given of the specific training in PVP or BKP which the operators had received or the number of such procedures which they had previously performed. Thus, Buchbinder et al. 102 specified that PVP was performed by experienced interventional radiologists who had undertaken formal training in vertebroplasty, had appropriate certification, were actively performing the procedure, and all adhered strictly to a detailed, standardised protocol, while in the INVEST study103 PVP was said to be performed by highly experienced practitioners who had performed a mean of approximately 250 procedures (range 50–800). Blasco et al. ,127 Farrokhi et al. 135 and Rousing et al. 128 provided information relating to the clinicians’ specialism (respectively experienced neurointerventional radiologists, a neurosurgeon, and orthopaedic surgeons specialising in spine surgery) but did not specify their training or level of experience of PVP. The remaining studies (FREE,137 Liu et al. ,139 VERTOS136 and VERTOS II17) provided no relevant information.
All studies used relevant outcome measures, and all but Liu specified that they used valid instruments. All assessed short-term outcomes. However, some studies did not either measure or report long-term outcomes; as noted earlier, 1-year assessments were planned in INVEST103 and VERTOS136 but because of crossover, VERTOS was stopped at 2 weeks,136 while INVEST followed patients up for 1 year but reported outcome data only at 1 month. 103 Buchbinder et al. 102 followed patients up for 2 years, and the 1- and 2-year data are currently being prepared for publication, as is a separate paper on radiological outcomes (Rachelle Buchbinder, Monash University, 2012, personal communication).
Most studies provided an adequate description of adverse events.
Precision
Only three of the included studies (Blasco et al. ,127 FREE137 and INVEST103) appeared to be adequately powered for at least their primary outcome: pain as measured on an 11-point scale (Blasco et al. and INVEST) and change from baseline to 1 month in the SF-36 PCS scale (FREE). However, because of difficulties with recruitment, the power of the INVEST study was reduced from a power of more than 80% to detect a 2.5-point difference between groups on the RDQ score and a 1.0-point difference on an 11-point pain scale to a power of more than 80% to detect a 3.0-point difference on the RDQ score and a 1.5-point difference on the pain scale. 103 Because most studies were underpowered for most outcomes, the absence of a statistically significant difference between treatment groups does not necessarily mean that such a difference would not be found in a larger study.
Almost all studies except that by Blasco et al. 127 published point estimates and measures of variability; Blasco kindly supplied additional data in that format (Jordi Blasco Andaluz, Hospital Clinic de Barcelona, Spain, 2012, personal communication).
FIGURE 3.
External validity and precision summary: review authors’ judgements about each included study (+, good generalisability/precision; –, poor generalisability/precision; ?, unclear generalisability/precision).

Summary of internal and external validity
The quality of the included studies is generally not very high. Much of this is owing to the widespread lack of blinding: the studies at least risk of bias are the double-blinded Buchbinder et al. 102 and INVEST103 studies, which compare PVP with an OPLA. The studies which compare PVP with non-invasive management (Blasco et al. ,127 Farrokhi et al. ,135 Rousing et al. ,131 VERTOS136 and VERTOS II17) vary in quality, that by Farrokhi et al. being at least risk of bias.
The FREE study137 the only study to compare BKP with non-invasive management, is at risk of bias because of the lack of blinding of patients and outcome assessors, failure to follow up at least 80% of participants, the unexpected imbalance in dropouts and selective reporting of outcomes.
The only study to compare PVP with BKP, that by Liu et al. ,139 is poorly reported and potentially at risk of bias from a number of sources. It also appears to be underpowered to identify statistically significant differences in effectiveness between the two interventions.
The external validity of the included studies is limited by the fact that only two (Buchbinder et al. 102 and INVEST103) provided adequate information about the operating clinicians’ training and experience. This makes it difficult to assess to what extent study results may be replicable elsewhere. In addition, the current lack of long-term outcome data in the Buchbinder et al. ,102 INVEST,103 Liu et al. 139 and VERTOS136 studies make it difficult to assess the value of the procedure; however, long-term data from the study by Buchbinder et al. are to be published.
Only three studies (Blasco et al. ,127 FREE137 and INVEST103) appeared to be adequately powered for at least their primary outcomes (pain score in Blasco et al. and INVEST, change in SF-36 PCS score from baseline to 1 month in FREE). Because most studies were underpowered for most outcomes, the absence of a statistically significant treatment effect should not necessarily be taken as evidence that no such difference exists.
Assessment of effectiveness
Clinical effectiveness
Health-related quality of life
Only one study (Buchbinder et al. 102) reported AQoL scores. No difference was found between the PVP and control groups (see Appendix 10 , Table 104 ).
As noted in Discussion of individual outcome measures included in the review, despite its name the DPQ was not designed to evaluate pain per se but the impact of chronic pain on various aspects of a patient’s life: lower scores indicate better quality of life. 152
Only one study, that by Rousing et al. , used the DPQ to evaluate PVP. Rousing et al. claim that, although the other results are not statistically significant, the result for work and leisure at 3 months reaches statistical significance, favouring PVP. 128,131 This is indeed true of the unadjusted score. However, in each domain, baseline scores were noticeably lower in the PVP group than in the control group. Once this is adjusted for by comparing changes from baseline in each group rather than crude scores, it is clear that all the point estimates favour conservative management whereas, previously, all except that for social interest at 12 months had favoured PVP ( Table 8 ). Moreover, the differences are statistically significant for all outcomes except work and leisure at 3 months and anxiety and depression at both 3 and 12 months.
| Domain | Time point | PVP (95% CI) | Control (95% CI) | Mean difference between groups (95% CI) (negative values favour intervention) | p-value |
|---|---|---|---|---|---|
| Daily activities | Baseline | 47.8 (22.5 to 73.1) | 68.5 (47.0 to 90.1) | –20.7 | |
| 3 months | 47.1 (32.9 to 61.4) | 57.4 (40.7 to 74.1) | –10.3 | 0.33 | |
| Change from baseline at 3 months | –0.7 (–7.75 to +6.3) | –11.1 (–17.24 to –4.96) | +10.4 (+0.83 to +19.97) | ||
| 12 months | 53.0 (38.3 to 67.7) | 53.6 (34.8 to 72.5) | –0.6 | 0.95 | |
| Change from baseline at 12 months | +5.2 (–1.89 to +12.92) | –14.9 (–21.33 to –8.47) | +20.1 (+10.25 to +29.95) | ||
| Work and leisure | Baseline | 41.1 (20.7 to 61.5) | 68.7 (47.8 to 89.6) | –27.6 | |
| 3 months | 44.5 (30.4 to 58.7) | 65.2 (50.4 to 80.1) | –20.7 | 0.04 | |
| Change from baseline at 3 months | +3.4 (–2.28 to +9.08) | –3.5 (–9.29 to +2.29) | +6.9 (–1.31 to +15.11) | ||
| 12 months | 46.1 (31.4 to 60.9) | 49.2 (31.5 to 66.9) | –3.1 | 0.78 | |
| Change from baseline at 12 months | +5.0 (–0.75 to +10.75) | –19.5 (–25.78 to –13.22) | +24.5 (+15.93 to +33.07) | ||
| Anxiety and depression | Baseline | 31.5 (12.6 to 50.4) | 43.0 (19.9 to 66.1) | –11.5 | |
| 3 months | 28.7 (15.1 to 42.3) | 40.0 (20.8 to 59.2) | –11.3 | 0.30 | |
| Change from baseline at 3 months | –2.8 (–9.54 to +3.94) | –3.0 (–11.93 to +5.92) | +0.2 (–11.45 to +11.85) | ||
| 12 months | 31.3 (16.5 to 46.2) | 35.3 (20.4 to 50.2) | –4.0 | 0.70 | |
| Change from baseline at 12 months | –0.2 (–7.30 to +6.90) | –4.7 (–12.81 to +3.41) | +4.5 (–6.72 to +15.72) | ||
| Social interest | Baseline | 23.8 (9.9 to 37.7) | 41.0 (23.3 to 58.7) | –17.2 | |
| 3 months | 24.1 (13.2 to 35.0) | 30.7 (15.9 to 45.5) | –6.6 | 0.46 | |
| Change from baseline at 3 months | +0.3 (–3.77 to +4.37) | –10.3 (–15.17 to –5.43) | +10.6 (+4.07 to +17.13) | ||
| 12 months | 32.9 (18.9 to 46.9) | 30.7 (16.5 to 44.8) | +2.2 | 0.82 | |
| Change from baseline at 12 months | +9.1 (+4.60 to +13.60) | –10.3 (–15.28 to –5.32) | +19.4 (+12.49 to +26.31) |
Five studies (Buchbinder et al. ,102 FREE,137 INVEST,103 Rousing et al. 128 and VERTOS II17) collected EQ-5D data. However, two studies did not collect relevant data from all participants, although in both cases non-collection of EQ-5D data does not appear to be related to patient characteristics. Buchbinder et al. only added this outcome to their protocol in June 2005 to allow comparison with the INVEST trial study, and therefore EQ-5D scores were available for only 30 out of 38 participants (79%) in the intervention group and 29 out of 40 (73%) in the control group. 102 Similarly, Rousing et al. collected EQ-5D data only from November 2004, when a PhD study was affiliated to the trial: thus, scores were available for only 15 out of 26 participants (58%) in the intervention group and 17 out of 24 (71%) in the control group. 128 VERTOS II collected EQ-5D data throughout the study and reported baseline values, but the investigators did not report follow-up values, although they used them to estimate quality-adjusted life-years (QALYs). 17
The two blinded RCTs (Buchbinder et al. 102 and INVEST103) found no significant difference between PVP and conservative treatment in terms of short- or medium-term outcomes (see Appendix 10 , Table 105 ). As Doidge et al. note, the CIs include effects which might favour either group, suggesting that the studies were underpowered to detect clinically important differences in this outcome. 57 However, when 1-month data from the Buchbinder and INVEST studies were combined in a meta-analysis of IPD,110 the result was not statistically significant (see Appendix 10 , Table 107 ) and, because the MCID is 0.08,150 the CI for the pooled data only just includes the possibility of a clinically important difference favouring PVP. In the study by Rousing et al. , the changes from baseline at 3 and 12 months favour conservative treatment and suggest that the difference between groups is clinically important.
The FREE study found statistically significant differences in outcomes, favouring BKP over non-surgical management, at 1, 12 and 24 months (see Appendix 10 , Tables 105 and 106 ). However, although at each time point the point estimate is greater than the MCID, the CIs at 3, 6, 12 and 24 months include the possibility of clinically unimportant effects.
Figures 4 , 5 , 6 and 7 graphically represent these change in EQ-5D observed in the Buchbinder et al.,102 FREE,137 INVEST103 and Rousing et al. 131 trials respectively.
Only four studies (Blasco et al.,127 Buchbinder et al.,102 VERTOS136 and VERTOS II17) assessed HRQOL using the QUALEFFO (in which higher scores represent worse HRQoL). In the study by Blasco et al. ,127 although the point estimates suggest that PVP is associated with better short- and medium-term total QUALEFFO scores than conservative treatment at all time points, the CIs indicate that the difference is not statistically significant (see Appendix 10 , Tables 108–110 ). However, while Buchbinder et al. stated that the only statistically significant QUALEFFO result in their study, at 1 week, favoured OPLA,102 the figures they report suggest that it in fact favoured PVP (see Appendix 10 , Table 108 ). As no MCID has been proposed for the QUALEFFO, the clinical significance of this result is not clear. The VERTOS study also found that PVP was associated with a significantly better short-term total QUALEFFO score than conservative treatment. In VERTOS II, after adjusting for baseline differences, there was said to be a significant difference in QUALEFFO scores at 1 year which favoured PVP (p < 0.0001);17 however, this result was not quantified.
Three studies (FREE,137 INVEST103 and Rousing et al. 131) collected data relating to HRQoL at baseline and follow-up using the SF-36. However, only the FREE study reported mean utility scores; (academic-in-confidence information has been removed) (see Appendix 10 , Table 111 ). Academic in confidence data regarding SF-36 data in the FREE study was supplied by Professor Wardlaw (Aberdeen Royal Infirmary, 2012, personal communication).
All three studies reported SF-36 PCS scores. In the FREE study,137 mean SF-36 PCS scores, and improvements from baseline in those scores, were reported in several publications, with some discrepancies in the results reported in the different publications: where there are discrepancies, data from the later publications have been utilised here as they are likely to be more complete. The FREE study found significant differences in medium-term outcomes; these favoured BKP. However, the between-group difference dwindled steadily from 1 month, when the result also suggested clinical importance; at 3 and 6 months, the CIs included the possibility of failing to achieve clinical importance, while after 6 months there was no statistically significant difference between treatment groups. The INVEST103 and Rousing131 studies found no significant differences between treatment groups at any point (see Appendix 10 , Tables 112 and 113 ).
All three studies also reported psychological well-being as assessed by the SF-36 MCS and identified no statistically significant differences between treatment groups, although the CIS include the possibility of potential clinically important treatment effects favouring the intervention at time points up to 6–12 months (see Appendix 10 , Tables 114 and 115 ).
Back-specific functional status/mobility
All of the studies, except that by Liu et al., 139 reported some measure of back-specific functional status or mobility.
Five studies (Buchbinder et al. ,102 FREE,137 INVEST,103 VERTOS136 and VERTOS II17) assessed back-specific functional status using the RDQ. Buchbinder et al. 102 and INVEST137 used the modified, 23-point, version of the RDQ; FREE used the original 24-point version, as apparently did VERTOS136 and VERTOS II. 17 In both versions, higher scores represent worse disability; whichever version is used, the MCID appears to be at least 2 points.
Only Buchbinder et al. ,102 INVEST103 and VERTOS136 reported short-term outcomes (see Appendix 10 , Table 116 ). In terms of the between-group difference in change from baseline, all of the point estimates favour PVP, but the results from the Buchbinder et al. 102 and INVEST103 studies are not statistically significant; unfortunately, the statistical significance of the result from the VERTOS136 study could not be calculated because of the way in which the investigators reported the results.
Buchbinder et al. 137 and INVEST102 found no significant between-group differences in medium-term outcomes. The FREE103 study found that BKP was associated with significantly better outcomes at 1 and 12 months, but not at 24 months; moreover, at 12 months the CIs include the possibility of failing to achieve clinical importance (see Appendix 10 , Table 117 ). VERTOS II17 reported a statistically significant difference in improvement over time which favoured PVP at 1 year (p < 0.0001); however, this was not quantified, and its clinical importance was not indicated.
Meta-analysis of IPD from the Buchbinder et al. 102 and INVEST103 studies indicated no significant difference between treatment groups at 1 month in terms of mean RDQ scores (see Appendix 10 , Table 118 ).
In the INVEST103 study, a post-hoc analysis was performed to identify the proportion of patients who achieved a clinically meaningful improvement in physical disability related to back pain at 1 month: this improvement was not defined, but was presumably measured in terms of a reduction in the RDQ score. There was no significant difference between the proportion of patients in each group who achieved a clinically meaningful improvement (40% of the PVP group vs. 41% of the control group, p = 0.99). 103 Meta-analysis of IPD from the Buchbinder et al. 102 and INVEST103 studies found no significant difference in the proportion showing an improvement of at least three units or of at least 30% in RDQ scores (see Appendix 10 , Table 119 ).
None of the included studies used the original ODI. However, Farrokhi et al. 135 used a questionnaire based on the ODI which replaced the sex life dimension with a question relating to change in the degree of pain. PVP was associated with a statistically significant difference in change from baseline in the modified ODI score at all times from 1 week to 36 months135 (see Appendix 10 , Table 120 ). Moreover, as the MCID for the ODI appears to be 4 points, these differences seem to be clinically meaningful throughout.
Only one study, that by Rousing et al. , reported functional outcomes using the Barthel Index, using the version scored from 0 to 20, with lower scores indicating greater disability. As data were collected only from November 2004, they are available for only a subset of the study population. 128 Rousing et al. state that, at 12 months, the absolute score was significantly better in the PVP group than in the control group. 131 However, once the difference in baseline scores is taken into account, the difference between groups is no longer statistically significant (see Appendix 10 , Table 121 ). It is difficult to know how much importance to attribute to this result as it may reflect a ceiling effect whereby, because the baseline measurement is relatively high, there is little scope for the intervention to improve the outcome beyond the extent to which it would improve under the control treatment.
The INVEST study103 reported mean SOF-ADL scores at baseline and 1 month. There was no statistically significant difference between treatment groups in change from baseline (see Appendix 10 , Table 122 ).
Three studies (Farrokhi et al. ,135 FREE137 and Rousing et al. 131) provided information relating to other indicators of disability. Farrokhi et al. noted that all 40 patients in the PVP group could walk 1 day after PVP, but only 1 out of 42 in the control group (2%) could walk at the equivalent point in time,135 indicating a relative risk of 28.32 (95% CI 5.88 to 136.45, p < 0.0001).
The FREE study137 reported the use of walking aids, back braces, miscellaneous aids and physiotherapy: the data relating to the use of walking aids are presented in Table 9 . BKP was associated with a statistically significant reduction in the risk of needing walking aids at 1 month but not at 12 months. However, the data are not robust because, in the control group, the number of patients requiring walking aids at 12 months is smaller than the number for whom data are missing (44/107).
| Time point | BKP | Control | Relative risk (95% CI) | p-value |
|---|---|---|---|---|
| Baseline | 49/148 (33%) | 55/151 (36%) | 0.91 (0.67 to 1.24) | 0.55 |
| 1 month | 33/136 (24%) | 54/129 (42%) | 0.58 (0.40 to 0.83) | 0.003 |
| 12 months | 30/121 (25%) | 38/107 (36%) | 0.70 (0.47 to 1.04) | 0.08 |
The FREE study137 also provided data relating to the number of patients who reported one or more days of bed rest owing to back pain in the previous 14 days. Again, BKP was associated with a statistically significant reduction in the risk of needing bed rest at 1 month but not at 12 months ( Table 10 ). However, the 12-month data in both groups are not robust because the numbers of patients for whom data are missing outnumber the numbers of those who report the outcome of interest. At 1 month, patients in the BKP group reported on average 2.9 fewer days of restricted activity because of back pain in the previous 14 days than did control subjects (95% CI 1.3 days to 4.6 days, p < 0.001), but at 12 months the difference was no longer statistically significant (1.6 days, 95% CI –0.1 days to 3.3 days, p = 0.0678). 136 The actual numbers of days of restricted activity in each group were not reported.
| Time point | BKP | Control | Relative risk (95% CI) | p-value |
|---|---|---|---|---|
| Baseline | 85/146 (58%) | 92/144 (64%) | 0.91 (0.76 to 1.10) | 0.32 |
| 1 month | 30/133 (23%) | 51/121 (42%) | 0.54 (0.37 to 0.78) | 0.001 |
| 12 months | 5/120 (4%) | 8/106 (8%) | 0.55 (0.19 to 1.64) | 0.28 |
Only one study, that by Rousing et al. ,131 reported three observer-assessed tests of physical function: the Tandem test, the timed ‘up and go’ test, and the repeated chair test. Although the timed ‘up and go’ test was also an outcome measure in the study by Buchbinder et al. ,150 only baseline values were reported. 102 In the study by Rousing et al. , data were available for only a subset of the study population. No statistically significant differences between groups were noted at 3 or 12 months128,131 but, as baseline values were not reported, the clinical meaningfulness of this result in terms of change from baseline is not clear.
Pain/analgesic use
Only one study, the FREE study,137 reported pain using the recommended measure of global pain severity, the bodily pain subscale of the SF-36,169 in which higher scores represent better health. The only result which has been published from this study using this measure is the difference between treatment groups in average improvement over a period of 12 months: this was 9.2 points greater in the BKP group than in the control group (95% CI 3.9 to 14.6, p = 0.0008). 137 Fuller confidential data presented in supplementary document 8 of the manufacturer’s submission35 indicate that (academic-in-confidence information has been removed) (see Appendix 10 , Table 112 ). (Academic-in-confidence information has been removed.)
All nine studies reported pain measured on either a numeric rating scale or a VAS, with higher scores indicating more severe pain. Farrokhi et al. 135 and INVEST103 asked patients to report their average pain over the previous 24 hours, while Buchbinder et al. 102 and FREE137 asked them to do so over the previous week, and the remaining studies did not specify the time period. However, empirical data indicate that broadly comparable results are obtained regardless of whether patients are asked to report average pain over the previous 24 hours or the previous week. 199 Academic in confidence data were provided for the Buchbinder et al. 102 RCT (Margaret Staples, Monash University, 2012, personal communication) for the VAS scores at 12 and 24 months.
As noted in Chapter 2 (see Decision problem), the VAS is less responsive than the numeric rating scale; this is presumably the reason why Doidge et al. have suggested that data collected by the two methods should not be combined in a meta-analysis. 57 The majority of the included studies (Buchbinder et al. ,102 Farrokhi et al. ,135 FREE,137 INVEST,103 Liu et al. ,139 VERTOS136 and VERTOS II17) clearly used a numeric rating scale; although some termed it a VAS, they also referred to it as a 10-point scale. It is not wholly clear whether or not Blasco et al. 127 actually used a VAS, although they claimed to do so. Rousing et al. 131 specified that they used a 10-cm VAS,128 and presumably did so at most time points, but they clearly used a numeric scale in a supplementary telephone interview in which, after all but three had completed 12 months’ follow-up, patients were asked to rate their back pain 1 month after discharge from hospital on a scale of 0–10. 131 As Doidge et al. have pointed out,57 because these data were collected almost 1 year after the event, they are at high risk of recall bias.
Farrokhi et al. ,135 FREE,137 Rousing et al. 131 and VERTOS II17 found statistically significant differences between groups in short- and medium-term changes from baseline in pain following PVP or BKP; FREE and VERTOS II also found statistically significant long-term differences between groups (see Appendix 10 , Tables 124–126 ). However, the double-blinded studies (Buchbinder et al. 102 and INVEST103), and the small VERTOS136 study, found no significant differences between treatment groups, while in the study by Blasco et al. 127 statistical significance in change from baseline was only reported at 2 months, when the result favoured PVP. There appears to have been no significant difference between treatment groups in terms of change from baseline at 12 months, and Blasco et al. attribute the similar prevalence of moderate and/or severe residual pain to the more frequent use of rescue therapy in the control group and the higher number of new clinical fractures associated with PVP in the intervention group. 127 Moreover, the favourable result reported by Rousing et al. 128 at 1 month (see Appendix 10 , Table 125 ) is unreliable because of the high risk of recall bias discussed above. Liu et al. 139 found no significant differences between PVP and BKP, but the study does not appear to have been powered to do so.
Meta-analysis of IPD from the Buchbinder et al. 102 and INVEST103 studies again found no significant difference at 1 month in terms of mean pain scores (see Appendix 10 , Table 127 ).
A comparison of longitudinal trends in pain reduction between the differently treated groups proves instructive. Figures 8 to 13 graphically represent these trends for PVP, BKP, OPLA, and conservative treatment respectively. Graphs for the longitudinal pain changes in individual trials are also included in Appendix 12 . Among the cohorts treated with PVP and BKP, there is a rapid post-procedural reduction in pain which appears to stabilise at approximately 1 month. The OPLA-treated cohorts reveal a somewhat similar pattern: there is a rapid reduction in pain, which seems to stabilise at 1 month. In contrast to PVP and BKP, however, there appears to be a small, temporary worsening of pain between 1 day and 1 month, at which point pain once again reduces and stabilises. A rather different pattern emerges with respect to those treated with OPM. There is no dramatic initial drop in pain; rather, there is a more gradual reduction until approximately 3 months, at which point the pain level stabilises and becomes comparable to that of those treated with PVP.
FIGURE 8.
Longitudinal pain reduction trends in vertebroplasty without AiC data.
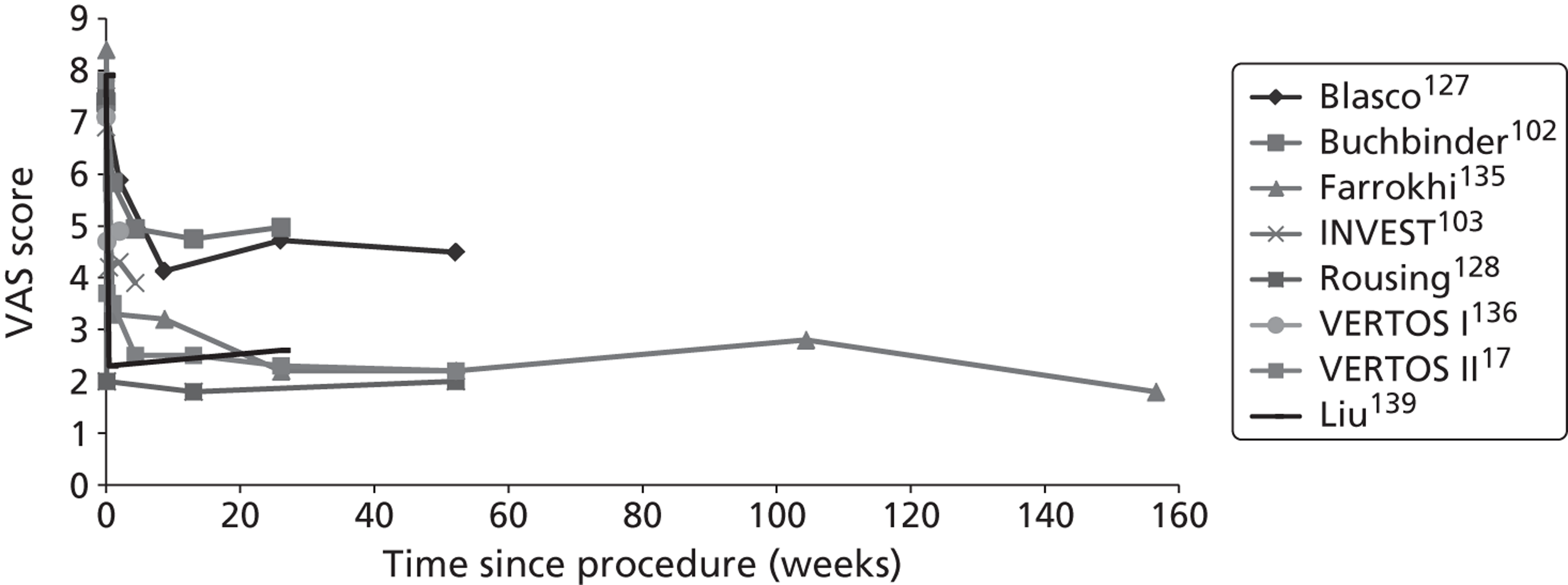
FIGURE 9.
Longitudinal pain reduction trends in vertebroplasty with AiC data. (Academic-in-confidence information has been removed.)
FIGURE 10.
Longitudinal pain reduction trends in BKP.
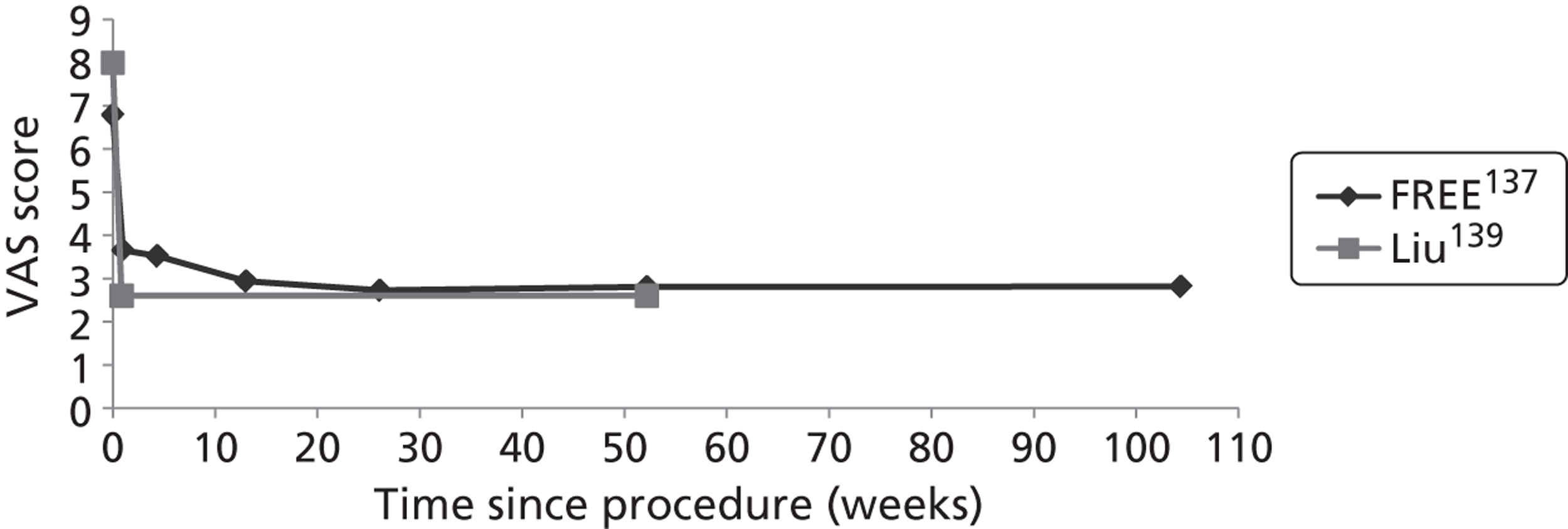
FIGURE 11.
Longitudinal pain reduction trends in OPLA excluding AiC data.
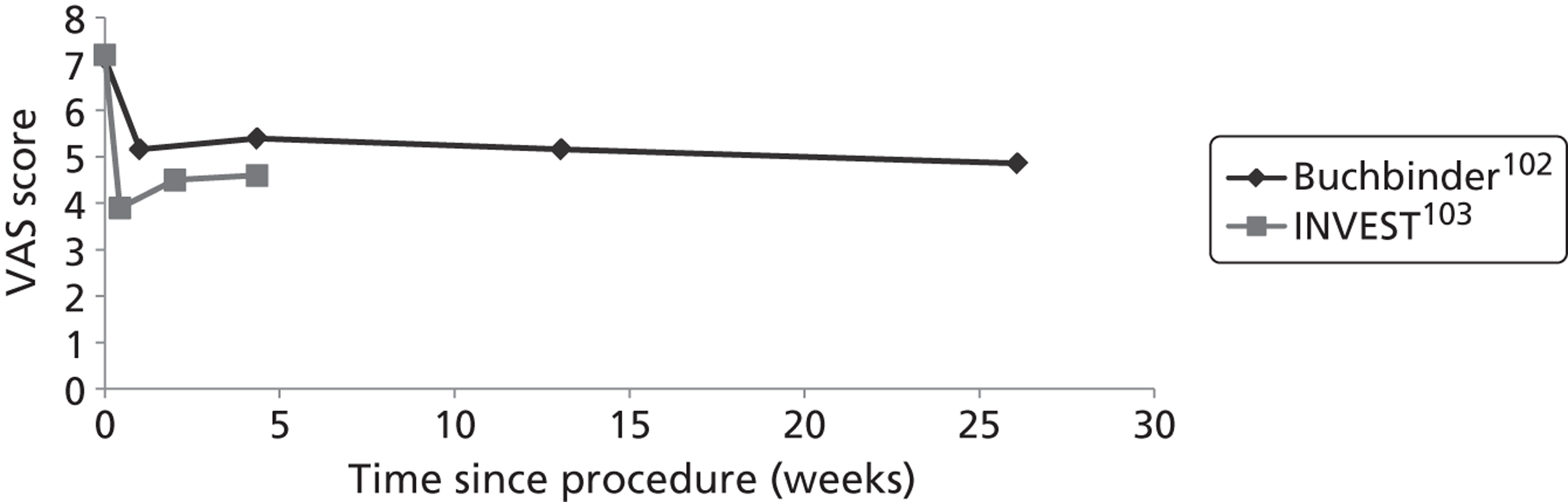
FIGURE 12.
Longitudinal pain reduction trends in OPLA including AiC data. (Academic-in-confidence information has been removed.)
FIGURE 13.
Longitudinal pain reduction trends in OPM.
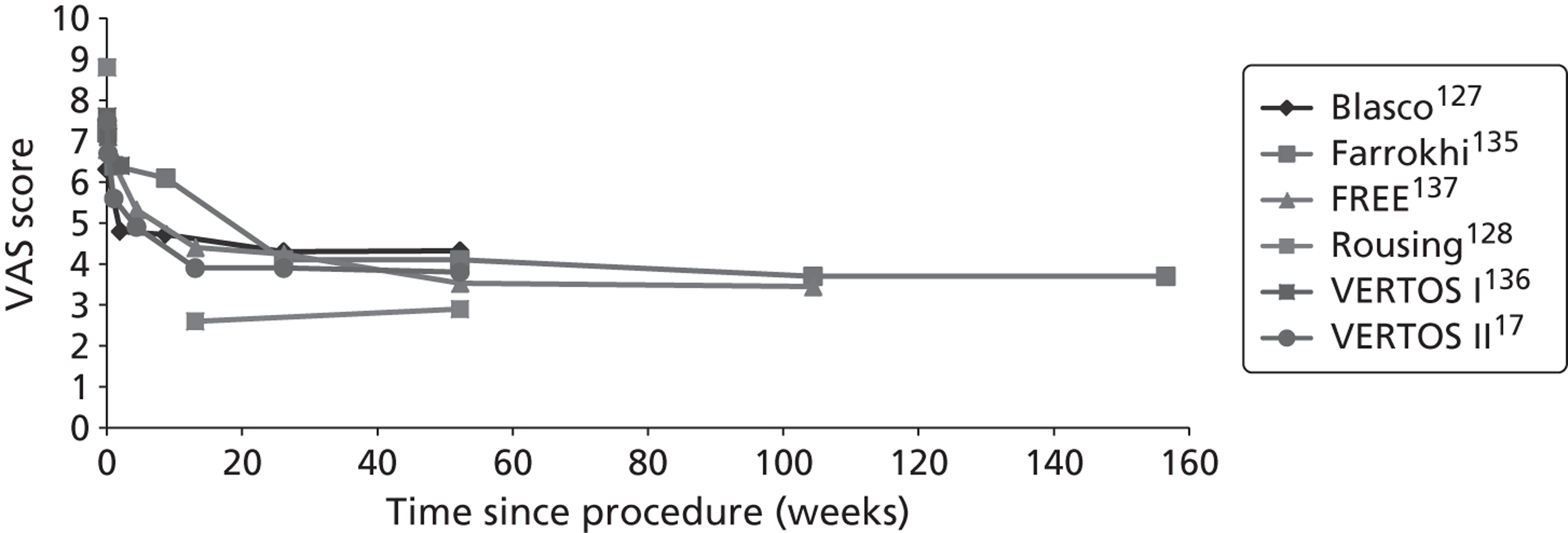
The gradual reduction in pain seen in conservatively treated patients coheres with a regression to the mean as would be expected from the natural history of healing in osteoporotic VCFs. The patterns seen in the PVP and OPLA groups, on the other hand, pose some more interesting interpretive questions. Whitehouse has suggested that the initial ‘dip’ seen in the OPLA cohorts represents a strong initial placebo effect before regression to the mean, while the early and sustained reductions in the PVP cohorts is suggestive of specific mechanisms of efficacy. 95 However, owing to the truncated line from INVEST, interpretations should be made with caution.
(Academic-in-confidence information has been removed.)
If, as indicated in Decision problem, a difference between groups of 2 or more points indicates a clinically meaningful difference, then most of the short- to medium-term results which are statistically significant also appear to be clinically meaningful. However, although FREE137 and VERTOS II17 both reported statistically significant longer-term results, in the FREE study these results did not appear to be, and in VERTOS II the 95% CI included the possibility that they were not, clinically meaningful (see Appendix 10 , Table 126 ).
The INVEST study also stated that 64% of patients randomised to PVP and 48% of those randomised to OPLA reported a clinically meaningful improvement in pain (i.e. a decrease of 30% or more) at 1 month (p = 0.06). 103 It has been suggested that this trend towards favouring PVP might have achieved statistical significance if the trial had recruited more participants, as was originally planned. 200 However, when these data were combined with those from the Buchbinder et al. 102 study in a meta-analysis of IPD, no significant difference at the 5% level was found in the proportion showing a clinically meaningful improvement in pain at 1 month, whether this was defined as a decrease in pain of at least 3 units or of at least 30% ( Table 11 ).
| Outcome | PVP | Control | Relative risk (95% CI) | p-value |
|---|---|---|---|---|
| Improvement in pain of ≥ 3 units | 55/102 (53.9%) | 43/99 (43.4%) | 1.3 (0.8 to 1.9) | NS |
| Improvement in pain of ≥ 30% | 61/102 (59.8%) | 45/99 (45.5%) | 1.3 (1.0 to 1.8) | NS |
In VERTOS II, survival analysis indicated that significant pain relief (apparently defined as a decrease from baseline in pain score of 3 points or more) was achieved earlier, and in more patients, after PVP than with conservative treatment [29.7 days (95% CI 11.45 days to 47.97 days) vs. 115.6 days (95% CI 85.87 days to 145.40 days) (χ2 = 55.6, p < 0.0001)]. 17
Blasco et al. ,127 Buchbinder et al. 102 and VERTOS136 also reported pain outcomes in terms of QUALEFFO pain scores. The reported figures are not directly comparable as they appear to use different scales, although this is poorly reported: Blasco appears to report the domain score (scored from 0 to 5) whereas Buchbinder reports pain scores on a scale of 0 to 100, and it is not clear what potential range of scores is represented by the VERTOS data. Blasco et al. and Buchbinder et al. found no statistically significant difference between the groups; the significance of the VERTOS results unfortunately could not be calculated (see Appendix 10 , Table 128 ).
The INVEST study also reported on the frequency with which participants experienced pain and the impact of pain on their daily lives, both measured on a scale of 0–4. In both groups, pain frequency and pain bothersomeness decreased between baseline and 1 month; however, although the point estimates favoured the intervention, the results were not statistically significant103 (see Appendix 10 , Table 129 ). Moreover, as Doidge et al. note, the CIs did not include a 1-unit effect (the smallest possible threshold of clinical importance) in either direction. 57
Buchbinder et al. 102 collected data on perceived pain: this was classified as ‘better’ if the patient indicated that it was moderately or a great deal better than before the intervention, and ‘worse’ if they reported that it was moderately or a great deal worse. They found no statistically significant between-group differences in the proportion of patients in these categories at any time point102 (see Appendix 10 , Table 130 ).
Six studies (Blasco et al. ,127 Buchbinder et al. ,102 FREE,137 INVEST,103 VERTOS136 and VERTOS II17) reported analgesic use. Blasco et al. 127 divided analgesic use into four categories: no treatment; minor analgesics (paracetamol and/or NSAIDs); minor opiate derivatives; and major opiate derivatives. They found no significant changes between groups in the analgesia used throughout the study (chi-squared test, adjusted p-values > 0.05)127 (see Appendix 10 , Table 131 ). However, rescue therapy by intrathecal infusion of 25 µg of fentanyl and 1.5 mg of bupivacaine was offered to patients in either group with a pain score of ≥ 7 over the 12-month study period, and was required by substantially more patients in the control group than in the PVP group [15/61 (25%) compared with 3/64 (5%), p = 0.0015],127 suggesting greater pain in the control group.
Three studies (Buchbinder et al. ,102 FREE137 and INVEST103) reported the number of patients in each group who took opioids for pain at baseline and at follow-up. For comparability with these studies, Blasco et al. ’s data on minor and major opiate derivatives were pooled to produce a total number of patients taking opioids. Review Manager was then used to calculate the relative risks of taking opioids for each of the four studies; for the INVEST study, the numerator in each group was inferred at 1 month from the denominator and proportion. In the Buchbinder et al. 102 and INVEST103 studies, the number of patients taking opioids for pain decreased over time in both the PVP and the control groups; in the Blasco et al. study127 no significant between-group differences were observed other than at baseline. However, in the FREE study137 BKP was associated with a significantly reduced risk of requiring opioid medication at 1 month and 6 months, but not at 12 or 24 months. The results from the study by Blasco et al. 127 are difficult to interpret. This is partly because a statistically significantly higher proportion of participants in the PVP group required opioid analgesia at baseline, but the picture thereafter is puzzling: in the PVP group, the proportion of participants requiring opioid medication falls noticeably from baseline to 2 weeks and then gradually thereafter, as might be expected, whereas in the control group it rises steeply at 2 weeks and remains elevated for 6 months, then falling substantially at 12 months ( Table 12 ). However, in this study, in both treatment groups the number of patients requiring opioid analgesia at 12 months is smaller than the number for whom data are missing (23/64 randomised to PVP and 19/61 randomised to control), and therefore the data are not robust.
| Study | Time point | PVP | BKP | Control | Relative risk (95% CI) | p-value |
|---|---|---|---|---|---|---|
| Blasco et al. 127 | Baseline | 47/64 (73%) | N/A | 31/60 (52%) | 1.45 (1.08 to 1.93) | 0.01 |
| 2 weeks | 33/56 (59%) | N/A | 36/58 (62%) | 0.95 (0.71 to 1.28) | 0.73 | |
| 2 months | 30/52 (58%) | N/A | 33/56 (60%) | 0.98 (0.71 to 1.35) | 0.90 | |
| 6 months | 26/49 (53%) | N/A | 31/52 (60%) | 0.89 (0.63 to 1.26) | 0.51 | |
| 12 months | 22/41 (54%) | N/A | 17/42 (40%) | 1.33 (0.83 to 2.11) | 0.23 | |
| Buchbinder 2009 et al. 102 | Baseline | 30/38 (79%) | N/A | 34/40 (85%) | 0.93 (0.75 to 1.15) | 0.49 |
| 1 week | 27/38 (71%) | N/A | 27/40 (68%) | 1.05 (0.78 to 1.41) | 0.73 | |
| 1 month | 26/38 (68%) | N/A | 25/40 (63%) | 1.30 (0.5 to 3.32) | 0.58 | |
| 3 months | 19/38 (50%) | N/A | 23/40 (58%) | 0.74 (0.30 to 1.81) | 0.51 | |
| 6 months | 13/38 (34%) | N/A | 16/40 (40%) | 0.78 (0.31 to 1.96) | 0.60 | |
| FREE137,138 | Baseline | N/A | 103/140 (73.6%) | 99/146 (67.8%) | 1.08 (0.93 to 1.26) | 0.28 |
| 1 month | N/A | 53/114 (46%) | 74/115 (64%) | 0.48 (0.28 to 0.82) | 0.007 | |
| 6 months | N/A | 37/124 (29.8%) | 48/112 (42.9%) | 0.70 (0.49 to 0.98) | 0.04 | |
| 12 months | N/A | 33/118 (28.0%) | 34/101 (33.7%) | 0.83 (0.56 to 1.24) | 0.36 | |
| 24 months | N/A | 10/114 (8.8%) | 10/105 (9.5%) | 0.92 (0.40 to 2.12) | 0.85 | |
| INVEST103 | Baseline | 38/68 (56%) | N/A | 40/63 (63%) | 0.88 (0.66 to 1.17) | 0.38 |
| 1 month | 36/67 (54%) | N/A | 26/61 (43%) | 1.26 (0.78 to 1.82) | 0.22 |
In their meta-analysis of IPD from the Buchbinder et al. 102 and INVEST103 studies, Staples et al. 110 found that, after adjusting for baseline opioid use, patients randomised to PVP were more likely to be taking opioids at 1 month than patients randomised to OPLA (relative risk 1.25, 95% CI 1.14 to 1.36, p < 0.001). 110 They consequently suggest that the trend observed in their meta-analysis, towards a higher proportion of patients in the PVP group achieving an improvement of 30% or more in pain scores at 1 month, may have been influenced by the fact that the PVP group was more likely than the OPLA group to be using opioids at that point. 110 This contrasts with the FREE study137 in which opioid use was similar in both groups at baseline, but the BKP group was significantly less likely than the control group to be using opioid analgesia at 1 month and 6 months (see Table 12 ), while the reduction in pain at 1 month was significantly greater in the BKP group than in the control group; the significance of the result at 6 months is not clear (see Appendix 10 , Table 125 ).
Data from VERTOS136 and VERTOS II17 are not comparable with data from the four studies reported above. VERTOS recorded opioid use at baseline, but data at 1 day and 2 weeks were only reported in terms of a mean analgesic use score derived by classifying no medication as 0, paracetamol as 1, NSAIDs as 2, and opiate derivatives as 3. There was said to be no significant between-group difference in the use of pain medications at baseline (p = 0.5). However, at both 1 day and 2 weeks, the mean analgesic use score had reduced in the PVP group and increased in the control group,136 resulting in statistically significant differences which favoured PVP (see Appendix 10, Table 132 ). At the same points in time, there were significantly greater reductions in pain in the PVP group than in the control group (see Appendix 10 , Table 124 ), and thus pain and analgesic use had reduced in parallel.
In VERTOS II, the class of drugs used for pain relief was said to be similar in both groups at baseline (see Appendix 10 , Table 106 ): although 53% of patients in the PVP group used either weak or strong opioid derivatives compared with 46% in the control group, this difference was not statistically significant (p = 0.34). Analgesic use was said to be significantly reduced in the PVP group compared with the control group at 1 day, 1 week and 1 month (p < 0.0001, < 0.001 and 0.033 respectively), but not at later stages of follow-up;17 however, the actual figures were not presented. Pain scores were also lower in the PVP group than in the control group at 1 day, 1 week, and 1, 3, 6 and 12 months, though the between-group difference in change from baseline was said to be statistically significant only at 1 month and 12 months (see Appendix 10 , Tables 125 and 126 ).
Vertebral body height and angular deformity
Four studies (Blasco et al. ,127 Farrokhi et al. ,135 FREE137 and Liu et al. 139) reported changes in VBH and/or angular deformity. However, their results are not necessarily comparable as it is not clear whether or not they used the same methods of measuring vertebral height. Farrokhi et al. 135 calculated the mean VBH by taking the mean of the height of the anterior wall plus the height of the posterior wall, while Blasco et al. 127 and Liu et al. 139 referred to the mean height without specifying how it was measured. Farrokhi et al. specified that they used the sagittal index to measure angular deformity135 whereas the FREE study137 and Liu et al. 139 used the kyphotic angle (see Chapter 2, Decision problem). It is not clear whether or not Liu et al. 139 measured postoperative VBH and angular deformity at 3 days or at 6 months. Because of these potential sources of heterogeneity, it did not seem appropriate to pool data relating to VBH or angular deformity.
Surprisingly, the FREE study did not report changes in VBH even though maintenance of VBH was one of its secondary outcome measures202 and is one of the respects in which BKP might be expected to provide additional benefit compared with PVP. However, although kyphotic angle was measured in both groups, the study protocol stated that VBH was to be measured only in patients undergoing BKP,202 thus making comparison with control subjects impossible.
Blasco et al. 127 found no significant difference between treatment groups in change in VBH from baseline at 12 months. By contrast, Farrokhi et al. 135 found that PVP was associated with significant improvements in mean VBH which were sustained throughout the first year but not thereafter, and with significant improvements in angular deformity which were sustained throughout the 36-month follow-up period (see Appendix 10 , Tables 134 and 135 ). They suggest that the significant differences from pre-treatment values in mean VBH and sagittal index seen at 1 week in the PVP group (p < 0.002 and < 0.011 respectively) but not in the control group (p = 0.22 and < 0.80 respectively) may be related either to the prone position used during PVP or to the high pressure produced within the vertebra by the injected cement, both of which can expand the vertebra and correct kyphotic deformity to some extent. 135
The FREE study only reported improvement from baseline in the kyphotic angle of the index fracture at 24 months, without reporting the absolute figures at either point in time. They reported a statistically significant result in favour of BKP;201 however, the clinical significance of this result is not clear. In the study by Liu et al. ,139 BKP was associated with significantly greater improvements in both postoperative VBH and angular deformity than was PVP (see Appendix 10 , Tables 134 and 135 ).
Progression of treated fractures
Only one study, VERTOS II, reported data relating to the progression of treated fractures during follow-up; in the control group, all vertebrae which showed bone oedema on baseline MRI were considered to be treated vertebrae. At last follow-up (mean 11.4 months, median 12.0 months, range 1–24 months), moderate or severe height loss was seen in 11 vertebrae in 11 out of 91 patients (12%) in the PVP group compared with 39 vertebrae in 35 out of 85 patients (41%) in the control group (p < 0.001)186 (see Appendix 10 , Table 136 ).
Adverse effects
All-cause mortality
Six of the included studies reported all-cause mortality. 17,127,128,135–137 Liu et al. 139 made no reference to any deaths, thus implying that none occurred; however, this was not explicitly stated. None of the individual studies found any statistically significant differences in overall mortality between treatment groups (see Appendix 10 , Table 137 ). However, this is unsurprising, as they were not powered for this outcome. None of the reported deaths appear to be related to treatment: the patient in VERTOS II who died as a result of gastric bleeding had used morphine as his or her only analgesic. 17
Three studies (Blasco et al. ,127 Rousing et al. 131 and VERTOS II17) reported overall mortality at the same time point (12 months). Data from these studies were combined by meta-analysis; inclusion of data from other studies which reported mortality at different time points was not considered appropriate. Statistical significance was still not achieved when the data from these studies were pooled, although the point estimate favours PVP ( Figure 14 ).
FIGURE 14.
Overall mortality at 12 months. M–H, Mantel–Haenszel.
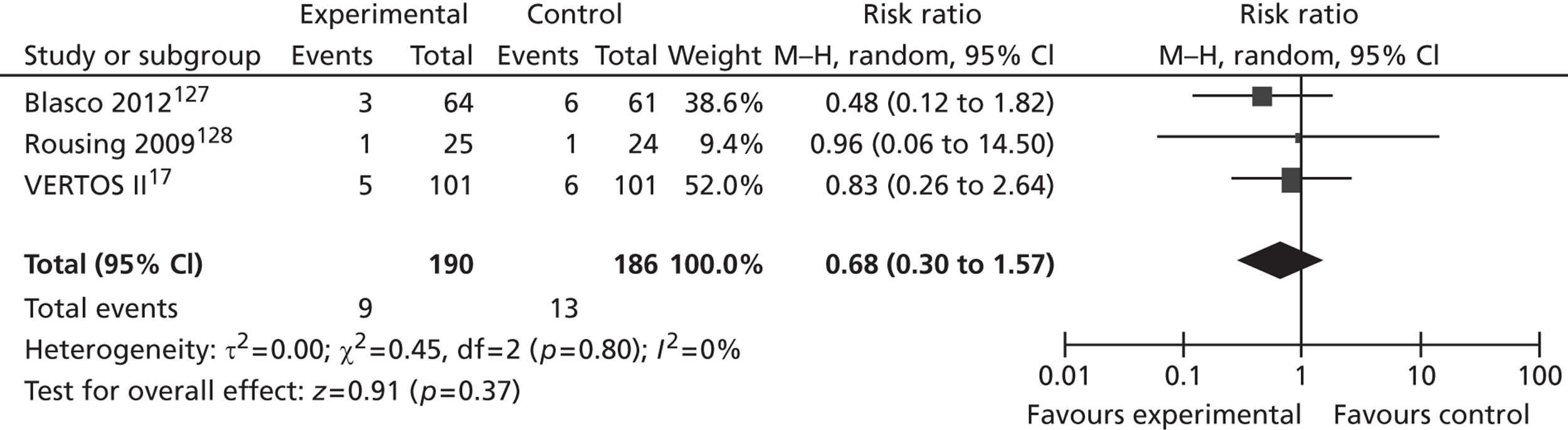
Symptomatic and asymptomatic cement leakage
Seven studies (Blasco et al. ,127 Buchbinder et al. ,102 Farrokhi et al. ,135 FREE,137 Rousing et al. ,128 VERTOS136 and VERTOS II17) reported cement leakages identified using imaging equipment. Four studies (Blasco et al. ,127 Buchbinder et al. ,102 Farrokhi et al. 135 and Rousing et al. 128) appear to have reported only leakages identified by fluoroscopy during the procedure, whereas two (VERTOS136 and VERTOS II17) performed CT immediately after PV to identify possible cement leakage or other local complications; this technique is likely to identify more leaks than fluoroscopy. The FREE study assessed cement extravasation using both intraoperative fluoroscopy and postoperative radiographs,137 thus increasing their likelihood of identifying leaks compared with the use of fluoroscopy alone (see Decision problem). All seven studies stated that they used PMMA cement; none referred specifically to high-viscosity cement and it is therefore assumed that low-viscosity cement was used in all studies.
For PVP, the number of treated vertebrae in which cement leakages were reported ranged from none in the small VERTOS136 study to 72% in VERTOS II;17 the pooled data suggest an incidence of 44% for PVP compared with 27% for BKP. However, this approach may conceal a relationship between the volume of cement injected and the likelihood of leakage, or between the sensitivity of the method of detection used and the detection rate: thus, the highest incidence is seen in VERTOS II,17 which also reports the highest mean volume of cement injected per vertebra, and which specifically scanned patients postoperatively using CT scanning, the most sensitive method of detection, to identify possible leakages ( Table 13 ).
| Study | Mean (SD) volume of cement injected (per vertebra) (ml) | PVP | BKP |
|---|---|---|---|
| Blasco 2012127 | NR | 67/140 (49%, 95% CI 41 to 57%) | N/A |
| Buchbinder 2009102 | 2.8 (1.2) | NRa | N/A |
| Farrokhi 2011135 | 3.5 (range 1–5.5) | 14/100 (14%, 95% CI 7% to 21%) | N/A |
| FREE137 | NR | N/A | 51/188b (27%, 95% CI 21% to 33%) |
| Rousing 2009128 | NR | NRc | N/A |
| VERTOS136 | 3.2 (range 1–5) | 0/29 | N/A |
| VERTOS II17 | 4.1 (1.5, range 1–9) | 97/134 (72%, 95% CI 64% to 80%) | N/A |
| Total | N/A | 178/403 (44%, 95% CI 39% to 49%) | 51/188 (27%, 95% CI 21% to 33%) |
The importance of cement leaks relates to their potential clinical sequelae. These may be immediate or delayed. Blasco et al. found that, although the cement leaks which they reported were not associated with immediate clinical complications, cement leakage into the inferior disk was associated with an increased risk of incident vertebral fracture [OR 7.17 (95% CI 1.69 to 69.30), p = 0.0008]. 127 Farrokhi reported 13 asymptomatic leaks (five into the discal space and eight into the paravertebral space) and one symptomatic leakage into the epidural space. The symptomatic leakage caused severe right lower-extremity pain and weakness but, following immediate decompression through a bilateral laminectomy and evacuation of bone cement, the patient could walk unassisted with no radicular pain after 2 months. 135 Rousing stated that none of the cement leaks caused neurological symptoms. 128 In VERTOS II, most leakages were discal or into segmental veins; none was into the spinal canal. All patients remained asymptomatic, even though fluoroscopy showed cement migration into the venous system towards the lungs in one patient; a follow-up chest CT after 1 year showed no perifocal inflammatory pulmonary changes in this patient. In this study, an asymptomatic cement deposition in a segmental pulmonary artery was also reported, presumably in another patient. 17 Fifty-four PVP patients subsequently underwent CT after a mean follow-up of 22 months (median 21 months, range 6–42 months). Although during the procedure the operators had not reported fluoroscopically visible cement migration towards the lungs in any of these patients, at follow-up 14 out of 54 (26%, 95% CI 16% to 39%) had pulmonary cement embolism visible on CT. The emboli varied in size between 1 mm and 12 mm and were randomly distributed in the periphery of the lungs; six patients had a single cement embolus, while the remaining eight had between 2–35 cement depositions randomly scattered in the peripheral portions of both lungs. All of the affected patients were asymptomatic. 193 In the FREE study, most leaks were endplate or discal leakages, with one foraminal leakage, no leakages to the spinal canal and no cement embolisms. 137
Periprocedural balloon rupture
Neither of the studies of BKP reported periprocedural balloon rupture.
Peri- and postoperative complications (including infection)
Seven studies (Buchbinder et al. ,102 Farrokhi et al. ,135 FREE,137 INVEST,103 Rousing et al. ,128 VERTOS136 and VERTOS II17) provided some information relating to peri- or postoperative complications.
In the INVEST study, one patient had an injury to the thecal sac during PVP which resulted in hospitalisation. In addition, one patient who had received OPLA was hospitalised overnight after the procedure with tachycardia and rigors of unknown cause. 103
In the VERTOS study, in a patient originally randomised to optimum pain medication who requested PVP after 2 weeks, an intrapedicular cement spur broke on manipulation by the bone biopsy needle and caused a small cortical chip fracture at the medial border of the pedicle. The patient recorded an increase in pain score at 1 day but the pain was relieved using analgesics and local anaesthetic infiltration of the involved pedicle; there were no neurological sequelae. 136
In VERTOS II, patients required additional intravenous analgesia in 30% of procedures (31/98); two patients needed atropine because of pain-induced vasovagal reactions. In one case, the procedure had to be stopped because the patient developed an acute asthma exacerbation during vertebroplasty; the procedure was performed successfully 1 week later. 17
Rousing et al. stated that no conversions to open surgery were necessary in their study. 128 While this is not specified in relation to any of the other included studies, it seems likely that, had such conversions been required, they would have been reported.
Three studies (Buchbinder et al. ,102 FREE137 and VERTOS II17) reported postoperative infections which were potentially related to treatment. Farrokhi et al. specified that no infections occurred,135 while Rousing indicated this by stating that there were no adverse events other than cement leaks. 128 In the remaining four studies (Blasco et al. ,127 INVEST,103 Liu et al. 139 and VERTOS136), no postoperative infections were mentioned, again suggesting that none may have occurred. In the study by Buchbinder et al. , prophylactic cefalotin was usually administered intravenously immediately after cement injection. 148 Osteomyelitis developed in a patient who did not receive such prophylaxis because of multiple drug allergies; surgical drainage and antibiotic treatment were required approximately 2 weeks after randomisation and the patient then recovered fully. 102 In the FREE study, a recurrent urinary tract infection (UTI) was exacerbated by catheterisation; this patient also developed spondylitis near the cement in the vertebral body 376 days after surgery and the inflammation had not resolved by 24 months despite antibiotic therapy. 137 Sepsis/septic shock was reported in one patient in the BKP group but also in three patients in the conservative treatment group. 138 In VERTOS II, one patient developed a UTI after vertebroplasty. 17
Wardlaw et al. noted that, in the FREE study, three patients who underwent BKP subsequently had pulmonary embolisms; the earliest of these occurred 46 days postoperatively. 137 The significance of these embolisms is not discussed.
Incidence of new vertebral fractures
Only three studies (Blasco et al. ,127 FREE137 and VERTOS II17) reported the number of patients who suffered new radiographic vertebral fractures during the study period. None of these studies found a statistically significant difference between treatment groups ( Table 14 ). However, in the FREE study,137 loss to follow-up is higher in the control group than in the BKP group [34/149 (23%) vs. 56/151 (37%)]; as the dropout rate outnumbers the event rate in the control group, the fracture incidence data may be biased.
Rousing et al. reported only the number of new radiographic fractures rather than the number of patients who suffered such fractures, and therefore the relative risk of fracture cannot be calculated. They found that, over 12 months, there were more radiographic fractures in the PVP group than in the control group (7 vs. 4, statistical significance not reported). 131
Although the study protocols for the Buchbinder et al. and INVEST studies specified the incidence of new vertebral fractures as an outcome,147,148 the relevant results have not yet been published.
| Study | Length of follow-up | Mean time from estimated fracture onset to intervention (weeks) | No. of patients with incident VCF | Relative risk (95% CI) | p-value | ||
|---|---|---|---|---|---|---|---|
| PVP | BKP | Control | |||||
| Blasco 2012127 | 12 months | 20.0 (13.7)/20.4 (18.6) | 17/64 (26.6%) | N/A | 8/61 (13.1%) | 2.03 (0.94 to 4.35) | 0.07 |
| FREE137,138 | 12 monthsa | 5.6 (4.4)/6.4 (5.2) | N/A | 38/115 (33%) | 24/95 (25%) | 1.31 (0.85 to 2.02) | 0.22 |
| 24 months | N/A | 56/118 (47.5%) | 45/102 (44.1%) | 1.08 (0.81 to 1.44) | 0.62 | ||
| VERTOS II17 | 12 monthsb | 4.2 (2.4)/3.8 (2.3) | 15/91 (16.5%) | N/A | 21/85 (24.7%) | 0.67 (0.37 to 1.21) | 0.18 |
As noted in Discussion of individual outcome measures included in the review, data from populations with differences in length of follow-up are not directly comparable. However, as all three studies17,127,137 reported results at 12 months, we have performed an exploratory meta-analysis combining data from the three studies which reported the number of patients who had suffered new radiographic vertebral fractures by that time. Although the point estimate favours control, statistical significance was not achieved ( Figure 15 ).
FIGURE 15.
Patients with new incident radiographic vertebral fractures at 12 months. M–H, Mantel–Haenszel.
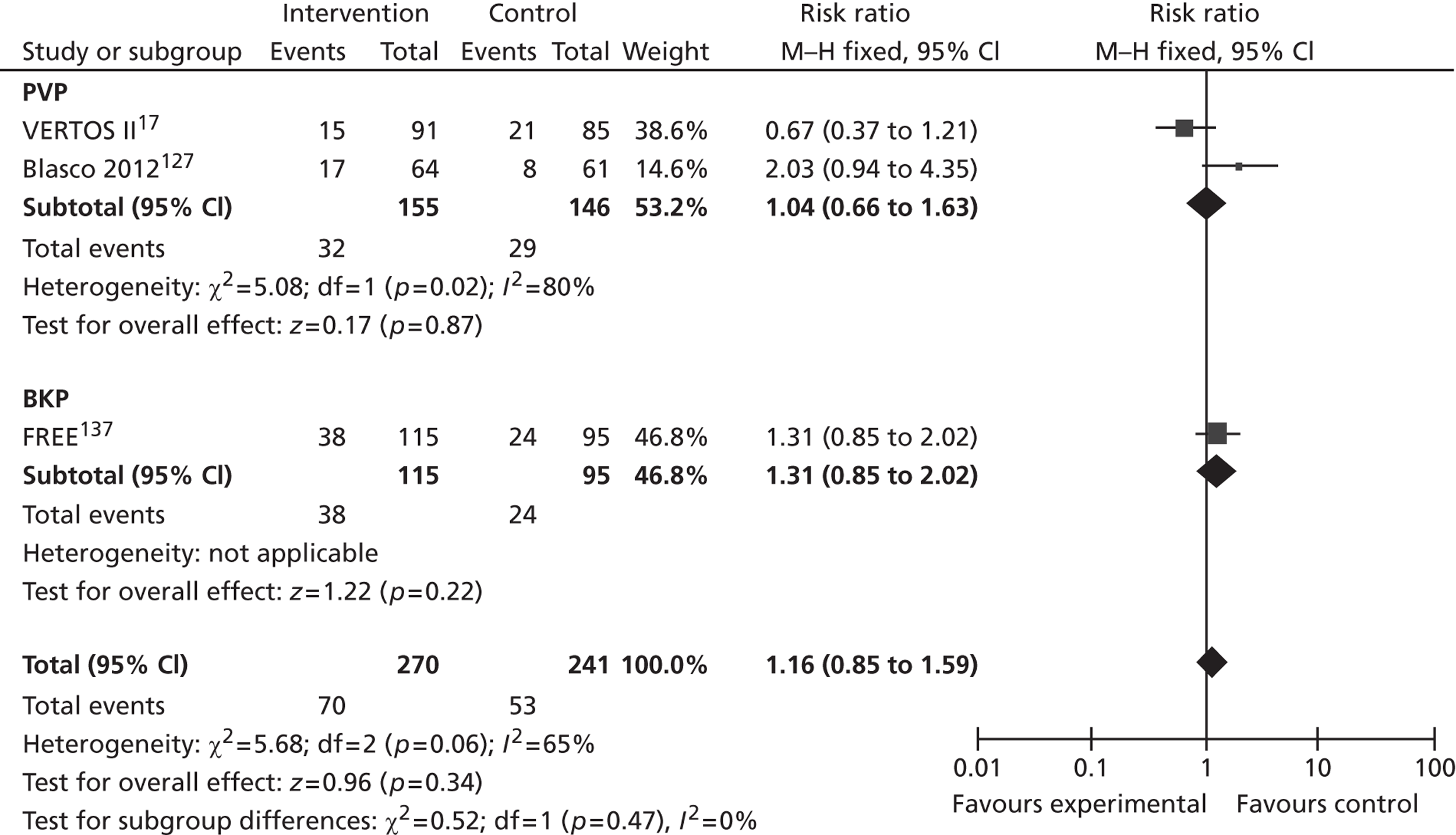
It has been observed that vertebrae adjacent to those treated with PVP or BKP may be particularly susceptible to subsequent fractures (detailed later in Incidence of new vertebral fractures). Thus, fractures in adjacent vertebrae are more likely to be associated with therapy than fractures in more distant vertebrae. Blasco et al. found that 82% of new fractures in the PVP group were adjacent to the index vertebra compared with 27% in the control group (OR 16.00, 95% CI 1.03 to 835.12, p = 0.0101). 127 The FREE study reported that 28 out of 118 patients in the BKP group (23.7%) and 17 out of 102 in the control group (16.7%) suffered a radiographic fracture adjacent to the index fracture;138 however, the difference was not statistically significant (relative risk 1.42, 95% CI 0.83 to 2.45, p = 0.20). Similarly, Klazen et al. reported that, in VERTOS II, the risk of adjacent rather than distant fracture was not significantly different in the intervention and control groups (p = 0.23), nor did such fractures occur significantly sooner in the PVP group than in the conservative therapy group (4.6 ± 5.4 vs. 6.1 ± 5.9 months, p = 0.48). The only risk factor for either the occurrence or the number of new fractures was the number of vertebral fractures at study entry, which is itself an indicator of the severity of osteoporosis. 186
As detailed subsequently, the most meaningful fracture outcome measure is the proportion of patients who experience at least one clinically important fracture in an adjacent vertebra. However, this outcome is not well reported. Only five studies (Buchbinder et al. ,102 Farrokhi et al. ,135 FREE,137 Rousing et al. 131 and VERTOS136) reported the overall incidence of new clinical vertebral fractures, and one of these (VERTOS) did so only for the PVP group. 136 Blasco et al. stated that 71% of the radiographic fractures in the PVP group were clinical compared with 9% in the control group (OR 25.67, 95% CI 3.04 to 216.8, p = 0.029);127 however, the number of patients who suffered clinical vertebral fractures was not reported. None of the other three studies102,128,135 which reported this outcome in both treatment groups identified a statistically significant difference between treatment groups ( Table 15 ).
| Study | Length of follow-up | Time from estimated fracture onset to intervention (weeks) | No. of patients with incident VCF | Relative risk (95% CI) | p-value | |||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | PVP | BKP | Control | ||||
| Buchbinder 2009102 | 6 months | Median: 9.0 (3.8–13.0) | Median: 9.5 (3.0–17.0) | 3/38 (7.9%) | 4/40 (10.0%) | 0.79 (0.19 to 3.30) | 0.75 | |
| Farrokhi 2011135 | 24 months | Median: 27 (4–50) | Median: 30 (6–54) | 1/38 (2.6%) | 6/39 (15.4%) | 0.17 (0.02 to 1.35) | 0.09 | |
| FREE137,138 | 12 months | Mean: 5.6 (4.4) | Mean: 6.4 (5.2) | 21 (14%) | NR | Not calculable | ||
| 24 months | 31/149 (20.8%) | 27/151 (17.9%) | 1.16 (0.73 to 1.85) | 0.52 | ||||
| Rousing 2009128 | 12 months | Mean: 1.2 (0.5–1.9) | Mean: 1.0 (0.3–1.6) | 0/26 | 3/24 (12.5%) | 0.13 (0.01 to 2.44) | 0.17 | |
| VERTOS136 | 2 weeks | Mean: 4.2 (2.4) | Mean: 3.8 (2.3) | 2/18 (11.1%) | NR | Not calculable | ||
Liu reported that adjacent segment fractures occurred at 41 and 50 days after surgery in two patients in the BKP group. 139 As these fractures were reported as adverse events, they were presumably clinical rather than radiographic fractures. No such fractures were reported in the PVP group. However, it is not clear whether or not fractures occurred but were not reported in non-adjacent vertebrae. In the FREE study, at 24 months 11 patients in the BKP group (7.4%) were said to have had clinical fractures which were considered ‘possibly or probably related’ to the intervention. 138
Other adverse events
The included studies varied considerably in their reporting of other adverse events. Six studies (Blasco et al. ,127 INVEST,103 Liu et al. ,139 Rousing et al. ,131 VERTOS136 and VERTOS II17) did not report any other adverse events. Farrokhi et al. stated only that no emboli occurred;135 these were clearly envisaged as different from cement leakages, which were reported separately.
Buchbinder et al. 102 reported a number of adverse events during the first 6 months of follow-up ( Table 16 ). The figures appear to refer to the number of events, not to the number of patients suffering the event.
| Event | PVP | Control | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 week | 1 month | 3 months | 6 months | Total | 1 week | 1 month | 3 months | 6 months | Total | |
| Incident non-vertebral fracture | ||||||||||
| Hip | 0 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Rib | 1 | 0 | 1 | 0 | 2 | 2 | 0 | 0 | 2 | 4 |
| Pelvis | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 |
| Osteomyelitis | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| Tightness in back or ribcage | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 2 | 0 | 2 |
| Pain or burning in thigh or leg | 3 | 0 | 1 | 0 | 4 | 1 | 0 | 1 | 0 | 2 |
| Stomach pain | 1 | 0 | 0 | 1 | 2 | 0 | 0 | 1 | 0 | 1 |
| Increased pain or muscle cramping around puncture site | 1 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 1 | 1 |
| Chest pain | 3 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 0 | 0 |
The FREE study provided extensive data relating to adverse events. 137,138 Data relating to serious adverse events (defined as adverse events which resulted in death, life-threatening injury or permanent impairment, or which required extended hospital stay or intervention to prevent impairment) are summarised in Table 17 . Few of these serious adverse events were considered to be related to BKP. However, a haematoma which occurred at the surgical site within 2 days of the intervention was considered to be procedure related, as was the exacerbation of a recurrent UTI by catheterisation, also within 2 days of surgery. The patient with the UTI also developed spondylitis near the cement in the vertebral body 376 days after surgery and was treated with antibiotics; however, the inflammation had not resolved by 24 months. None of the adverse events which resulted in death (12 in the BKP group and 11 in the control group) were considered to be related to the device or procedure. 138
| BKP | Control | |
|---|---|---|
| Any serious AEs within 24 months | 74 | 73 |
| Anaemia | 3 | 2 |
| Back pain | 5 | 12 |
| Spondylitis | 1a | 0 |
| Cardiovascular and vascular disorders | ||
| Angina pectoris | 2 | 5 |
| Arrhythmia | 2 | 5 |
| MI | 2 | 3 |
| Pulmonary embolism | 5b | 3 |
| Stroke | 4 | 1 |
| Haematoma | 1 | 1 |
| Other | 1c | 1 |
| Infections | ||
| Sepsis/septic shock | 1 | 3 |
| UTI | 2a | 3 |
| Neoplasms/cancer | 7 | 9 |
| Psychiatric disorders – depression | 3 | 1 |
| Respiratory disorders | ||
| Pneumonia | 8 | 6 |
| Dyspnoea | 1 | 4 |
Subgroups
The evidence relating to the subgroups specified in the protocol (see Appendix 1 ) is discussed in turn below.
Time from fracture to intervention
Of the included studies, only INVEST reported data by baseline pain duration. A post-hoc subgroup analysis of the effect of treatment on pain at 1 month by baseline pain duration categories found no significant difference (p comparing all three categories = 0.58, Table 18 ). 103
| Duration of pain at baseline | T1 | T2 | Treatment effect (95% CI) | p-value |
|---|---|---|---|---|
| < 13 weeks | NR | NR | 0.8 (–0.8 to 2.4) | 0.31 |
| 14–26 weeks | NR | NR | 1.3 (–0.8 to 3.4) | 0.23 |
| 27–52 weeks | NR | NR | 0.0 (–1.7 to 1.6) | 0.96 |
As the INVEST study103 was underpowered for this analysis, Staples et al. undertook a meta-analysis of IPD from the INVEST103 and Buchbinder et al. 102 studies to assess the effectiveness of PVP in patients with fracture pain of recent onset (≤ 6 weeks) compared with pain of longer duration. 110 Because the INVEST study103 allowed crossover after 1 month, outcomes were compared only up to that time point. No statistically significant differences in RDQ scores, EQ-5D scores or pain scores were identified between participants whose pain was of recent onset and those whose pain duration exceeded 6 weeks ( Tables 19 to 21 ).
| Duration of pain | PVP | Control | Adjusteda mean between-group difference (95% CI) (negative values favour intervention) | p-value |
|---|---|---|---|---|
| ≤ 6 weeks | –3.8 (5.9) | –4.4 (5.4) | 0.2 (–3.0 to 3.4) | NS |
| > 6 weeks | –4.2 (6.0) | –3.7 (6.3) | –1.0 (–3.0 to 1.0) | NS |
| All patients | –4.1 (5.9) | –3.9 (6.1) | –0.8 (–0.9 to 2.4) | NS |
| Duration of pain | PVP | Control | Adjusteda mean between-group difference (95% CI) (positive values favour intervention) | p-value |
|---|---|---|---|---|
| ≤6 weeks | 0.15 (0.24) | 0.15 (0.30) | 0.03 (–0.06 to 0.13) | NS |
| > 6 weeks | 0.11 (0.18) | 0.09 (0.20) | 0.03 (–0.03 to 0.09) | NS |
| All patients | 0.12 (0.19) | 0.11 (0.23) | 0.03 (–0.02 to 0.08) | NS |
| Duration of pain | PVP | Control | Adjusteda mean between-group difference (95% CI) (negative values favour intervention) | p-value |
|---|---|---|---|---|
| ≤ 6 weeks | –3.1 (3.3) | –2.8 (4.0) | –0.1 (–1.6 to 1.4) | NS |
| > 6 weeks | –2.7 (2.9) | –2.0 (2.7) | –0.8 (–1.8 to 0.1) | NS |
| All patients | –2.8 (3.0) | –2.2 (3.2) | –0.6 (–1.4 to 0.2) | NS |
Presence of fracture-related deformity before treatment
No data were identified relating to subgroups with and without fracture-related deformity before treatment.
Receipt of inpatient care before treatment
None of the studies provided information on the number of patients who were inpatients at the time of randomisation, and no data were identified relating to this subgroup.
Baseline pain severity
In the absence of data relating specifically to patients who had received inpatient care immediately preceding the intervention, it may be relevant to note that Staples et al. ’s analyses of IPD from the Buchbinder et al. 102 and INVEST103 studies include patients grouped by baseline pain severity. While p-values were not reported, no statistically significant differences in RDQ scores, EQ-5D scores or pain scores were identified between participants with severe pain (score ≥ 8 on a 0–10 rating scale) or mild to moderate pain (score < 8) at baseline. 110 In both treatment groups, the decrease in pain was greater in the subgroup which had more severe pain at baseline than in the subgroup with less severe baseline pain ( Tables 22 to 24 ), but this presumably simply reflects a greater potential for improvement.
| Baseline pain score | PVP | Control | Adjusteda mean between-group difference (95% CI) (negative values favour intervention) | p-value |
|---|---|---|---|---|
| < 8 | –4.2 (6.0) | –4.4 (6.4) | –0.2 (–2.5 to 2.1) | NS |
| ≥ 8 | –4.1 (5.9) | –3.3 (5.6) | –1.4 (–3.9 to 1.2) | NS |
| All patients | –4.1 (5.9) | –3.9 (6.1) | –0.8 (–0.9 to 2.4) | NS |
| Baseline pain score | PVP | Control | Adjusteda mean between-group difference (95% CI) (positive values favour intervention) | p-value |
|---|---|---|---|---|
| < 8 | 0.09 (0.17) | 0.07 (0.21) | 0.02 (–0.04 to 0.09) | NS |
| ≥ 8 | 0.16 (0.21) | 0.15 (0.25) | 0.05 (–0.03 to 0.12) | NS |
| All patients | 0.12 (0.19) | 0.11 (0.23) | 0.03 (–0.02 to 0.08) | NS |
| Baseline pain score | PVP | Control | Adjusteda mean between-group difference (95% CI) (negative values favour intervention) | p-value |
|---|---|---|---|---|
| < 8 | –1.9 (2.8) | –1.1 (2.8) | –0.8 (–1.9 to 0.3) | NS |
| ≥ 8 | –3.9 (2.9) | –3.5 (3.2) | –0.3 (–1.5 to 0.8) | NS |
| All patients | –2.8 (3.0) | –2.2 (3.2) | –0.6 (–1.4 to 0.2) | NS |
The evidence relating to pain severity prior to PVP therefore suggests that there is no reason to suppose that outcomes would differ between patients who were inpatients prior to treatment and those who were not. This view is strengthened by the fact that receipt of inpatient care following VCF may be influenced by factors other than clinical factors such as pain severity: patients who are bedridden with severe pain may not be hospitalised if they have adequate support networks in terms of both family/friends and community services. No subgroup data are available for BKP.
Summary of evidence for the clinical effectiveness of percutaneous vertebroplasty and balloon kyphoplasty
The volume of available evidence of clinical effectiveness is greater for PVP than for BKP, and the methodological quality of some of that evidence is also higher than that of any study of BKP. Thus, the studies at least risk of bias are the double-blinded Buchbinder et al. 102 and INVEST103 studies comparing PVP with an OPLA. The studies which compare PVP with conservative management (Blasco et al. ,127 Farrokhi et al. ,135 Rousing et al. ,131 VERTOS136 and VERTOS II17) vary in quality, that by Farrokhi et al. being at least risk of bias.
The FREE study,137 the only study to compare BKP with conservative management, is at risk of bias because of the lack of blinding of patients and outcome assessors, the relatively high loss to follow-up, the unexpected imbalance in dropouts and the selective reporting of outcomes.
The study by Liu et al. ,139 the only study to compare PVP with BKP, is poorly reported and potentially at risk of bias from a number of sources. It is also underpowered to identify statistically significant differences in effectiveness between the two interventions.
In relation to PVP, the studies least at risk of bias (Buchbinder et al. 102 and INVEST103) found no significant differences between treatment groups in terms of change from baseline in HRQoL other than in terms of the total QUALEFFO score at 1 week in the Buchbinder study:102 this favoured PVP. No significant differences were observed in any measure of functional status or pain (whether measured in terms of mean pain scores or numbers of patients reporting clinically meaningful improvements in pain). Although the INVEST study103 reported a trend towards a greater number of patients in the PVP group reporting a clinically meaningful improvement in pain at 1 month, pooled data from the Buchbinder et al. 102 and INVEST103 studies indicate that, after adjusting for baseline opioid use, patients randomised to PVP were more likely than those randomised to the OPLA to be taking opioids at 1 month. Consequently, it is impossible to exclude the possibility that PVP was associated with worse outcomes which were masked by greater opioid use.
What evidence there is from the open-label studies of PVP (Farrokhi et al. ,135 Rousing et al. ,131 VERTOS136 and VERTOS II17) regarding HRQoL is not consistent: VERTOS136 and VERTOS II17 suggest that PVP is associated with better HRQoL as measured by the QUALEFFO, while Blasco et al. 127 found no significant difference between treatment groups. The data reported by Rousing et al. 131 indicate that conservative management is generally associated with better HRQoL, as measured by the EQ-5D and DPQ. By contrast, the evidence from these studies relating to functional status appears to favour PVP; the most convincing evidence comes from Farrokhi et al. ,135 the unblinded study at least risk of bias, which found that, as measured by a modified version of the ODI, PVP was associated with significantly improved functional status at all times from 1 week to 36 months. In the Farrokhi et al. study,135 mobility at 1 day was also dramatically better in the PVP group than in the control group. The Blasco et al. ,127 Farrokhi et al. ,135 Rousing et al. 131 and VERTOS II17 studies found that PVP was associated with significant improvements in pain, although in the study by Blasco et al., 127 statistical significance was seen only at 2 months; moreover, in those studies which report analgesic use (Blasco et al. ,127 and VERTOS II17), these improvements do not appear to be associated with increased analgesic use in the PVP group. Farrokhi et al. ,135 also found that PVP was associated with sustained improvements in VBH and angular deformity; however, Blasco et al. ,127 found no significant difference between groups in VBH.
The unblinded FREE study137 of BKP found that, compared with conservative management, BKP was associated with significantly greater improvements from baseline in HRQoL, although these diminished over time. It was also associated with an improvement in functional status as measured by the RDQ at 1 month and 12 months but not at 24 months, and with a significantly reduced risk of needing walking aids or bed rest/restricted activity at 1 month but not at 12 months. BKP was also associated with significant short- and medium-term reductions in pain and with significant reductions in opioid use up to, but not beyond, 6 months. The effect of BKP on VBH was not reported; a statistically significant improvement in kyphotic angle was reported but its clinical significance is not clear.
In theory, the additional benefits of BKP compared with PVP are:
-
the restoration of vertebral height and spinal alignment
-
a lower incidence of cement leaks because the cement is injected at lower pressure.
In the study by Liu et al. ,139 BKP was said to be associated with greater improvements than PVP in VBH and angular deformity (both p < 0.001). Cement leaks were not reported. While data from the included RCTs and observational studies do indeed suggest that the incidence of cement leaks is lower with BKP than with PVP, because this finding is not derived from a randomised head-to-head comparison it is possible that it may reflect differences in patient selection.
The study by Liu et al. 139 did not attempt to assess HRQoL or functional status. It did not identify a statistically significant difference between PVP and BKP in terms of pain, nor was it powered to do so.
Subgroup analyses conducted by Staples et al. 110 using individual patient data from the Buchbinder et al. 102 and INVEST103 studies found no differential benefit for PVP in relation to either baseline pain duration or pain severity. No subgroup data relating to BKP are available.
Adverse effects and contraindications: observational studies
As RCTs generally perform poorly at detecting long-term or rare adverse events, it was decided that large case series (n ≥ 200) and individual case reports would be examined in order to gain a rough estimate of incidence of more common adverse events from large cohorts, while also scoping the rarer but serious events which are often published as individual case reports. We hoped in this way to be able both to identify the range of potential adverse events associated with PVP and BKP and to quantify the incidence of the more common adverse events. Previous systematic reviews of adverse events have been criticised for focusing on pre-defined adverse events – an approach which may miss unexpected but potentially important information. 121 By our inclusion of case reports relating to adverse events which were not reported in the large case series combined with our decision not to define adverse events of interest a priori, we sought to avoid this pitfall.
Our searches identified no publications of registry data which were specific to patients with osteoporotic VCF. If such studies had been identified, they would have been included either as large case series, if they presented data relating to all patients undergoing PVP or BKP regardless of outcome, or as an agglomeration of individual case reports if they presented data only relating to patients who had suffered adverse events. However, the Medtronic submission34 included two unpublished reports which compared mortality and complication risks for operated and non-operated patients with osteoporotic VCF: their findings are summarised below.
Registry data
(Academic-in-confidence information has been removed.)
Data from these studies are summarised in Appendix 11 ; they will also be discussed below in the relevant contexts. (Academic-in-confidence information has been removed.) Additionally, a formal critique of the data is provided in Appendix 13 , and summarised later.
Large case series
A total of 10 large case series (n > 200) provided data on adverse events following PVP or BKP on fractures of osteoporotic origin only. 203–212 One of these studies211 reported data for fractures of non-osteoporotic origin, but the corresponding author provided separate data for osteoporotic fractures, which were included in the review. The adverse event data for BKP and vertebroplasty are summarised in Tables 25 and 26 , respectively.
| Study | Total patients, N | Mean follow-up duration (range) | Total treated VB | Total (n) treated levels with leakage | Inpatients, n | Location(s) of leakages | Neurological complications | Other |
|---|---|---|---|---|---|---|---|---|
| Blattert et al. 2010203 | 314 | Minimum 2 years | 352 | 32 | NR | Epidural space (n = 6), others NR | NR | Intraoperative balloon perforation (n = 6) |
| Diel et al. 2010204 | 320 | 7 months (20–389 days) | 391 | 70 | 62 | Intervertebral disc (n = 28); paravertebral vessels (n = 13); epidural space (n = 4); other (n = 25) | Radiculopathy due to cement extrusion (n = 3) | Intraoperative balloon rupture (n = 1); fracture of vertebral wall with displacement of balloon catheter (n = 1); interruption of surgery for unspecified reason (n = 1); non-specified complication (n = 1) |
| Majd et al. 2005205 | 222 | 21 months (6–36 months) | 360 | 38 | NR | NR | Radiculopathy caused by cement leakage into foramen (n = 1) | Unspecified medical complications (n = 10): ‘most . . . were related to pre-existing cardiac, pulmonary, or liver disease’ One patient required surgical debridement, irrigation, and closure of wound 3 weeks post procedure One infection at kyphoplasty site 2 months post procedure, leading to abscess formation at L3, and consequent cardiovascular failure and death |
| Study | Total patients, N | Mean follow-up duration (range) | Total treated VB | Total (n) treated levels with leakage | Inpatients, n | Location(s) of leakages | Neurological complications (in patients, n) | Other |
|---|---|---|---|---|---|---|---|---|
| Álvarez et al. 2005206 | 260 | 12 months (3 weeks to 96 months) | 423 | 305 | NR | Spinal canal (n = 3); vertebral disc (n = 43); lumbar venous plexus (n = 44); epidural veins (n = 132) | Transitory radicular pain (n = 12); transitory parapesia (n = 1) | Rib fractures in five patients |
| Diel et al. 2009207 | 203 | 2 months | 1137 | 126 | NR | NR | NR | Temporary hypotension (n = 8); pulmonary embolism (n = 1) |
| Evans et al. 2003208 | 245 | Median 7.2 months (IQR: 3.1–13.6 months) | NR | NR | NR | Transitory radicular pain (n = 2) | Rib fractures in seven patients; post-procedural worsening of pain (n = 3) | |
| Lee and Chen 2004209 | 200 | NR | 200 | 29 | 29 | Disc space or paravertebral space (distribution NR) | NR | NR |
| Masala et al. 2009a210 | 285 | Up to 3 years | 429 | 21 | NR | Disc space or paravertebral veins (distribution NR) | NR | NR |
| aMpotsaris et al. 2011211 | 896 | Up to 12 months | NR | NR | 108 | Paravertebral venous plexus | NR | NR |
| Ryu and Park 2009212 | 215 | 15 months (6–22 months) | 383 | NR | 187 | Epidural space (n = 157); paravertebral space (n = 18); intradiscal space (n = 12) | NR | NR |
Serious adverse events related to BKP and vertebroplasty, though relatively rare, are of sufficient importance to warrant consideration in clinical decision-making. Statistical aggregation of data relating to the more common adverse events was not possible because, as shown in Tables 25 and 26 , the data were heterogeneous in terms of what was reported and how it was reported.
All-cause mortality
No deaths were noted in the large observational studies of PVP. However, one procedure-related death was noted by Majd and colleagues205 in a case series of 222 patients who had 360 vertebral bodies treated by BKP. This patient developed an infected shunt and subsequent abscess formation at the site of kyphoplasty. He underwent a discectomy with anterior plus posterior spinal fusion and instrumentation, but did not recover well and subsequently died from cardiovascular failure. It is noteworthy that this patient had previously received a kidney transplant and was taking antirejection medications and prednisone.
(Academic-in-confidence information has been removed.)
A formal critique of the evidence on mortality provided by Medtronic is provided in Appendix 13 , with a summary presented here ( Table 27 ). Observational data can be subject to confounding factors, although methods to adjust for these exist, such as regression analyses using observed variables as covariates and propensity matching. However, neither method can produce a robust estimate of the variable of interest if there is selection of the intervention provided based on unobserved data. Where this may be likely, instrumental variable methods using a variable correlated with an intervention but which is only correlated with the outcome through its effect on the intervention can be employed. However, the validity of an instrumental variable is subjective and can be open to debate.
Evidence on mortality benefit associated with BKP and PVP was submitted by Medtronic in the form of four studies,213–216 all using observational data: two from a claims database from the USA and two from a health insurance fund in Germany. A variety of methods are used, including Cox regression using covariates, matching methods and instrumental variable estimation. The results involved paired comparisons between different groups rather than simultaneous comparisons of the three treatments which may introduce inaccuracy. It is unclear how generalisable these results are to patients treated in England and Wales.
(Academic-in-confidence information has been removed.)
In summary, it is possible that there is a causal difference in mortality between patients treated using OPM and patients receiving BKP or PVP given the size of the effect. Appropriately taking into account the potential endogeneity of the treatment would tend to reduce the point estimate of the effect size but may or may not eliminate it completely. It is not possible to say with certainty if there is a difference in mortality between patients undergoing BKP and PVP as a result of the treatment based on the data presented in the studies included here. There is also considerable uncertainty, were BKP and PVP assumed to have a mortality benefit, regarding whether or not OPLA would also produce a mortality benefit, but no data are available on this.
| Group | Comparison | Cox regression; adjusted HR (95% CI) | Propensity score matching and Cox regression, HR (95% CI) | IV at 3 years; relative increase in survival | Propensity score matching; difference in survival rates, % (p-value) |
|---|---|---|---|---|---|
| Edidin et al. (2011):213 mortality risk 4 years | |||||
| All | OP vs. OPM | 0.63 (0.62 to 0.64) | |||
| BKP vs. OPM | 0.56 (0.55 to 0.57) | ||||
| PVP vs. OPM | 0.76 (0.75 to 0.77) | ||||
| BKP vs. PVP | 0.77 (0.75 to 0.78) | ||||
| Survival > 1 year | OP vs. OPM | 0.82 (0.81 to 0.84) | |||
| BKP vs. OPM | 0.76 (0.74 to 0.77) | ||||
| PVP vs. OPM | 0.93 (0.91 to 0.95) | ||||
| BKP vs. PVP | 0.82 (0.80 to 0.85) | ||||
| Operated | BKP vs. PVP | 11.82% | |||
| Exponent (2012):214 mortality risk 5 years | |||||
| All | OPM vs. OPa | AiC information has been removed | AiC information has been removed | ||
| OPM vs. BKP | AiC information has been removed | AiC information has been removed | |||
| OPM vs. PVP | AiC information has been removed | AiC information has been removed | |||
| BKP vs. PVP | AiC information has been removed | AiC information has been removed | |||
| OVCF | OPM vs. OPa | AiC information has been removed | AiC information has been removed | ||
| OPM vs. BKP | AiC information has been removed | AiC information has been removed | |||
| OPM vs. PVP | AiC information has been removed | AiC information has been removed | |||
| BKP vs. PVP | AiC information has been removed | AiC information has been removed | |||
| OVCF Survival > 1 year |
OPM vs. OPa | AiC information has been removed | AiC information has been removed | ||
| OPM vs. BKP | AiC information has been removed | AiC information has been removed | |||
| OPM vs. PVP | AiC information has been removed | AiC information has been removed | |||
| BKP vs. PVP | AiC information has been removed | AiC information has been removed | |||
| Lange and Braun (2012a,b):215,216 mortality risk 5 years | |||||
| OVCF | OP vs. OPM | AiC information has been removed | |||
| BKP vs. PVP | AiC information has been removed | AiC information has been removed | AiC information has been removed | ||
| OVCF Survival > 1 year |
OP vs. OPM | AiC information has been removed | |||
| BKP vs. PVP | AiC information has been removed | ||||
Note that HRs have been reported rather than statistics such as median or mean survival. This is owing to relatively large numbers of patients remaining alive at the end of the follow-up period. For example, in the Edidin et al. 213 publication median survival had not been reached at 4 years since VCF diagnosis in any of the arms.
Symptomatic and asymptomatic cement leakage
The most common risk associated with vertebral augmentation procedures is cement leakage outside the target vertebral body. The majority of articles reported the incidence of cement leakage in terms of treated vertebral bodies, while four reported it in terms of treated patients. Only Diel’s study of kyphoplasty204 and Lee and Chen’s study of vertebroplasty209 provided leakage incidence data for both treated vertebrae and treated patients. Taken in isolation, data relating to either the number of vertebrae or the number of patients are potentially misleading and could introduce systematic bias towards under-reporting of incidence.
The location of cement leakages has important implications for safety; intradiscal leakages are unlikely to lead to morbidity, but leakages into the epidural space or venous system have the potential to cause serious complications. 217 Three studies (Majd,205 Diel207 and Evans208) did not report the location of cement leakages. Poor reporting of follow-up duration and completeness was also a problem for interpreting these data. Lee and Chen209 did not report follow-up duration, while in most of the other studies it was unclear what proportion of the cohort was lost to follow-up at what time points, and why.
When reported in terms of treated vertebral bodies, the incidence of leakage ranged from 5%210 to 72%206 for vertebroplasty, and from 9%204 to 18%205 for kyphoplasty. By contrast, when reported in terms of treated patients, leakage incidence was higher, ranging from 12%211 to 87%212 for vertebroplasty. Only one kyphoplasty study204 reported incidence in terms of treated patients: it reported a rate of 19%. It is not clear why such wide variations in incidence were observed, but factors such as practitioner skills and experience, clinical setting, cement viscosity and thoroughness of follow-up may have played a part.
Epidural leaks appeared to be common in vertebroplasty cohorts. Ryu and Park212 reported epidural leaks in 157 of 215 treated patients (73%), and Álvarez et al. 206 reported three leaks into the spinal canal and 132 into the epidural veins in a cohort of 260 patients with 423 treated vertebrae (52% of patients). These complications did not appear to be as common in kyphoplasty cohorts. Blattert et al. 203 and Diel et al. 204 reported six and four leaks into the epidural space in cohorts of 314 and 320 respectively (2% and 1% respectively). Because of the nature of follow-up in the cohorts, the long-term clinical implications of these cement leaks are unknown. Several investigators undertook long-term follow-up: Masala and colleagues210 reported a follow-up duration of up to 3 years, although only 68 patients (24%) had data available at that time point; Majd et al. 205 reported a follow-up duration of up to 36 months, although data were not available on how many patients had data available at given time points; Blattert and Josten203 reported a minimum 2-year follow-up and did not report any missing data; and Álvarez et al. 206 reported follow-up of up to 96 months though, again, it was unclear how many patients were followed up at particular time points.
Other reported adverse events which may be related to cement leakage included pulmonary embolism,207,218 radiculopathy204,205 (which in the study by Diel et al. 204 was specifically due to cement extrusion), temporary radicular pain208 and temporary and permanent motor deficits or parapesia of the legs. 206
Intraoperative balloon rupture
Intraoperative balloon rupture appears to be a relatively rare complication of BKP: in the two studies which report it (Blattert et al. 203 and Diel et al. 204), it occurred in 6 out of 352 procedures (1.7%) in Blattert et al. ’s cohort,203 and in 1 out of 391 (0.3%) in Diel et al. ’s study. 204 Neither of these studies discussed the clinical implications of balloon rupture. However, Saliou et al. 219 discussed some of the potential implications in a smaller case series in which it was more common (n = 51, treated levels: 75, balloon rupture in 5 vertebrae of 5 patients). Although no symptomatic complications due to balloon rupture were observed in that study, the authors point out that this complication could lead to contrast leakage, procedural delay or gas embolism.
Other peri- and postoperative complications (including infection)
In the included case series, peri- and postoperative complications were relatively rare. Majd et al. 205 reported 10 medical and three surgical complications in 222 patients undergoing BKP. Most of the medical complications related to pre-existing cardiac, pulmonary or liver disease. In one case, a patient was treated with local anaesthesia because medical comorbidities made general anaesthetic inadvisable, and developed electrocardiogram abnormalities during the procedure; treatment of a second VCF had to be postponed for 4 days while the patient was assessed by a cardiologist. In addition to one case of infection discussed under All-cause mortality, above, and one cement leak causing radiculopathy, also discussed above, the surgical complications included one patient who needed surgical debridement, irrigation and closure of the wound 3 weeks after BKP. 205
Diel et al. reported one instance where the vertebral wall was fractured, with displacement of the balloon catheter, in a patient undergoing BKP,204 and eight cases of temporary hypotension following cement injection in 202 patients undergoing PVP for osteoporotic fracture (3.9%). 207
Incidence of new vertebral fractures
New vertebral fractures have been identified as an important source of postoperative morbidity among people with osteoporotic VCFs treated with PVP or BKP. The observational studies by Harrop et al. ,220 Kulcsar et al. ,221 Tseng et al. 222 and Uppin et al. 223 specifically set out to study the overall incidence of new vertebral fractures in osteoporotic patients following PVP or BKP. However, as these patients are by definition at increased risk of vertebral fracture, the data are difficult to interpret in the absence of a control group of similar patients who have not undergone PVP or BKP. Similarly, although a number of retrospective reviews of new vertebral fractures in patients treated with PVP or BKP were identified185,220–236 and reported incidence rates ranging between 6.8% over a 25.6-month follow-up period229 to 22.2% during a 1-year follow-up,233 it is difficult to know how to interpret these data. However, it should be noted that, because new fractures were generally identified only when patients returned to clinic with recurring back pain, the reported figures probably represent a conservative estimate of true fracture incidence.
Arguably of greater relevance was the finding from the case series by Harrop et al. ,220 Kulcsar et al. ,221 Tseng et al. 222 and Uppin et al. 223 that new VCFs are significantly more likely to occur in vertebrae adjacent to treated levels than in non-adjacent vertebrae. Although two reports found a similar crude incidence rate of adjacent and non-adjacent fractures,232,234 it should be added that patients would typically have a greater number of non-adjacent vertebral bodies that could fracture, so even these data may represent a greater likelihood of fracture at adjacent levels. 236 In addition, some studies show that, following vertebral augmentation, adjacent fractures are likely to occur sooner than non-adjacent fractures. Donovan et al. 224 reported the case of a 50-year-old woman who developed several new fractures 8 days after a kyphoplasty procedure, and concluded that the ‘temporal relationship between the kyphoplasty procedure . . . with documented fractures of six adjacent vertebrae . . . is highly suggestive of causality’ (p. 712). A larger retrospective analysis of time between vertebroplasty and new adjacent fractures234 found times to diagnosis of new adjacent and nonadjacent fractures of 55 and 127 days, respectively (p < 0.0001). Further evidence was supplied by Mudano and colleagues,237 who compared the rate of new fractures in a cohort of patients treated with PVP or BKP against a cohort of patients with VCFs and no cement augmentation. A significantly higher incidence was observed in the treated cohort at 90 days (adjusted OR 6.8, 95% CI 1.7 to 26.9) and 360 days (adjusted OR 2.9, 95% CI 1.1 to 7.9).
A number of prognostic factors have been associated with higher risk of subsequent vertebral fractures: these include increased age and number of treated vertebrae,228 presence of clefts in the treated VCFs,235,238 and spinal instability measures. 227 There is also a growing body of evidence suggesting biomechanical explanations for the higher rate of adjacent fractures. A number of studies have demonstrated that bone cement can increase the stiffness of the treated vertebra, resulting in an increase in loading on the adjacent vertebrae. 239–242 Cement leakage may also play a part: Han et al. 225 found that, when adjacent VCFs occurred, fractures were more likely to be close to extraneous cement. In contrast to these studies, Farooq et al. 243 demonstrated that vertebroplasty could partially reverse fracture-induced changes including decompression of the adjacent nucleus and higher neural arch load-bearing.
However, in the absence of well-controlled randomised studies, neither time from surgery to new VCF nor a higher incidence of adjacent vertebral fractures (compared with non-adjacent fractures) can be considered as definitive evidence of causation.
Rib fractures
Two large observational studies reported rib fractures related to vertebroplasty: Álvarez et al. 206 reported five fractures in a cohort of 260 patients, while Evans et al. 208 reported seven in a cohort of 245. No rib fractures were reported in the kyphoplasty case series.
Refracture of treated vertebrae
It has been suggested that a treated vertebra may refracture either because too little cement was injected or because the vertebra was extremely fragile and therefore at risk of refracture even when adequate quantities of cement were injected. 187 However, as none of the included case series reported this complication, incidence is likely to be low.
Percutaneous vertebroplasty and BKP may be associated with transitory increase in post-procedural pain. However, among the large case series, only Evans et al. 208 reported this complication: three patients from a cohort of 245 experienced worsening of pain, although no biomechanical causes could be found. While the other case series did not report worsening of pain as an adverse event, it was unclear whether this was because it did not occur or because the authors did not view transitory increases in pain as an adverse event per se.
Need for repeat procedure
A small proportion of patients may require repeat vertebroplasty because of adverse events. Yang et al. 244 presented data relating to 22 patients who required repeat PVP: 20 out of 1523 consecutive patients who underwent VP for osteoporotic fracture in their centre between 2000 and 2006 (1.3%) who had recurrent back pain after a short period of pain relief following first-time vertebroplasty, and a further two patients with neurological deficits following first-time vertebroplasty were referred from other hospitals for revision surgery. The reasons for revision surgery and the nature of the intervention required are presented in Table 28 . Most patients were discharged from hospital within 2 weeks, but those with infections required longer hospitalisation because they received at least a 6-week course of parenteral antibiotics. Four patients required a third surgical procedure.
| Complication | Number of patients | Intervention required |
|---|---|---|
| Residual vacuum cleft or poor cement augmentation | 5 | Repeat vertebroplasty |
| Poor cement augmentation and progressive kyphosis and instability | 2 | Posterior surgery (instrumentation and fusion) |
| Infection (pyogenic spondylitis) | 8 | Anterior and posterior surgery |
| Cement dislodgement | 3 | Anterior or anterior and posterior surgery |
| Cement fragmentation | 2 | Anterior surgery |
| Neurological deficit | 2 | Anterior and posterior surgery |
Case reports
In general, case reports were included only if they reported adverse events which had not been reported in the larger observational studies. However, an exception was made in the case of pulmonary cement embolism, the most commonly reported complication of vertebral augmentation, where all identified case reports were included in order to indicate subsequent therapy, if any.
Pulmonary cement embolism
The search identified 46 case reports245–290 and 47 patients in whom a pulmonary cement embolism caused by venous PMMA leakage was detected. These reports related to 41 vertebroplasty and five kyphoplasty procedures. Four deaths due to pulmonary embolism were reported in vertebroplasty patients; no deaths were identified in kyphoplasty patients. Sixteen embolisms were reported as asymptomatic, while 29 were symptomatic; for the remaining two, no details were provided on symptomatology. Symptomatic manifestations of pulmonary embolism include dyspnoea, tachycardia, chest pain, dizziness and sweating. Asymptomatic pulmonary embolism is more difficult to detect and, furthermore, it is difficult to gain understanding the long-term clinical implications of these silent pulmonary emboli from the available data. The case study data relating to pulmonary embolism are summarised in Table 29 .
| First author | Number of patients | Procedure (PVP/BKP) | Symptomatic/asymptomatic | Therapy |
|---|---|---|---|---|
| Abdul-Jalil245 | 2 | PVP | One symptomatic, one asymptomatic | Low-dose heparin |
| Agko246 | 1 | BKP | Asymptomatic | Surgical embolectomy |
| Baumann247 | 1 | PVP | Asymptomatic | Coumarin 3 months |
| Bernhard248 | 1 | PVP | Asymptomatic | NR |
| Biega249 | 1 | PVP | Asymptomatic | NR |
| Bonardel250 | 1 | PVP | Asymptomatic | Coumarin 6 months |
| Cadeddu251 | 1 | PVP | Asymptomatic | NR |
| Caynak252 | 1 | PVP | Symptomatic | Anticoagulants and pulmonary physiotherapy |
| aChen253 | 1 | PVP | Symptomatic | CPR |
| Dastidar254 | 1 | PVP | Symptomatic | Inferior vena cava filter placement |
| Finch255 | 1 | PVP | Symptomatic | NR |
| Francois256 | 1 | PVP | Symptomatic | Coumarin 6 months |
| Freitag257 | 1 | PVP | Asymptomatic | Coumarin 6 months |
| Grahe258 | 1 | PVP | Symptomatic | Anticoagulation and oxygen therapy |
| Harris259 | 1 | PVP | Symptomatic | NR |
| Jang260 | 2 | PVP | Symptomatic | Anticoagulants and heparin |
| Kim261 | 1 | PVP | Symptomatic | Surgical embolectomy |
| Kovalenko262 | 1 | PVP | NR | NR |
| Lee263 | 1 | PVP | Symptomatic | Surgical embolectomy |
| Leroux264 | 1 | PVP | Symptomatic | NR |
| Liliang265 | 1 | PVP | Symptomatic | None |
| Lim266 | 1 | PVP | Symptomatic | Surgical embolectomy |
| Lim267 | 1 | PVP | Symptomatic | Surgical embolectomy |
| MacTaggert268 | 1 | PVP | Asymptomatic | NR |
| Moll269 | 1 | BKP | Symptomatic | Anticoagulation 3 months |
| aMonticelli270 | 1 | VP | Symptomatic | CPR |
| Moon271 | 1 | VP | NR | Anticoagulation |
| Müller272 | 1 | BKP | Asymptomatic | NR |
| Neuwirth273 | 1 | PVP | Asymptomatic | NR |
| Perrin274 | 1 | PVP | Symptomatic | Low-dose heparin |
| Pleser275 | 1 | PVP | Asymptomatic | Heparin and coumarin 6 months |
| Pott276 | 1 | PVP | Symptomatic | Low-dose heparin |
| Quesada277 | 1 | PVP | Asymptomatic | NR |
| Radcliff278 | 1 | BKP | Symptomatic | Conservative treatment |
| Righini279 | 1 | PVP | Symptomatic | Coumarin 6 months |
| Schneider280 | 1 | PVP | Asymptomatic | NR |
| Schoenes281 | 1 | PVP | Symptomatic | Surgical embolectomy |
| Scroop282 | 1 | PVP | Symptomatic | None |
| Seo283 | 1 | PVP | Asymptomatic | Surgical embolectomy |
| Shalshin284 | 1 | BKP | Asymptomatic | Short-term enoxaparin |
| Son285 | 1 | PVP | Symptomatic | Surgical embolectomy |
| aStricker286 | 1 | PVP | Symptomatic | Definitive airway |
| Torres Machi287 | 1 | PVP | Symptomatic | Anticoagulation |
| Tozzi288 | 1 | PVP | Symptomatic | Coumarin 3 months |
| aYoo289 | 1 | PVP | Symptomatic | Surgical embolectomy |
| Zaccheo290 | 1 | PVP | Symptomatic | Low-dose heparin |
Postoperative infection
A number of case reports291–302 have described postoperative infectious complications. While such infections can occasionally be managed with a medical approach, they often necessitate further surgical intervention. 293,294,296–298,300,302 One team292 reported the death of a patient from septic multiple organ failure after antibiotic treatment and local surgical interventions.
Other adverse events
A number of case reports noted rare but serious cardiovascular complications related to vertebral cement augmentation, including cardiac perforation,281,285,303,304 inferior vena cava syndrome,305 venous air embolism,306 vena cava thrombus,307 acute pericarditis,308 lumbar artery pseudoaneurism309 and stroke. 310
Biafora et al. 311 reported an injury to a segmental branch of the L4 lumbar artery in an 84-year-old patient: this manifested clinically in bleeding from the kyphoplasty site and was successfully treated with torpedo embolisation of a small branch of the right L4 lumbar artery. Heo and Cho312 reported a L2 segmental artery injury, which was also successfully treated with endovascular embolisation. Hard et al. 313 reported a transpedicular needle penetrating the margin of the T5 vertebral body by 15 mm. Injury to the posterior aortic wall was confirmed and an improvised injection of PMMA was used to seal the aortic wall. No related complications were seen during 2 years of follow-up.
Ozturk et al. 314 reported a case of irreversible complete paraplegia due to cement leakage into the spinal canal. Lee et al. 315 presented a case of complete motor and sensory deficits at T11 due to cement leakage, which was treated with surgical decompression. Birkenmaier et al. 316 reported a transitory paraplegia in an 82-year-old patient following a massive epidural haematoma compressing the cauda equine and the conus medullaris. The haematoma was drained, resulting in the loss of 3 litres of blood and requiring transfusion of packed red blood cells and fresh-frozen plasma. However, 48 hours post procedure, full neurological function had been regained. Lopes and Lopes317 also reported paraplegia due to spinal cord and root compression, which was successfully remedied with surgical decompression.
Lim et al. 318 reported two cases of subarachnoid haemorrhage: both patients were treated successfully with medical management. Other rare complications included heterotopic ossification,319 addisonian crisis,320 lumbar disc herniation,321 posterior spinal epidural abscess322 and a fatal fat embolisation with no evidence of cement leakage. 323
Summary and discussion of data relating to adverse events
The evidence drawn from the included RCTs, case series, and case reports suggests that PVP and BKP may be associated with a number of adverse events. Treatment-related deaths appear to be rare, but cement leakage is common, particularly with PVP: pooled data from the RCTs indicate an incidence of 44% of treated vertebrae for PVP and 27% for BKP (see Table 13 ), while the case series indicate a range of 5% to 72% for PVP and 9% to 18% for BKP (see Tables 25 and 26 ). While many cement leaks were not associated with immediate clinical complications, others were associated with serious problems such as pulmonary embolism, radiculopathy, and temporary or permanent motor deficits. A number of procedure-related deaths have been noted. Moreover, there is as yet no good evidence to prove that that leaks which are asymptomatic in the short term do not have long-term implications.
Peri- and postoperative complications other than cement leak appear to be rare, though potentially serious. In particular, infectious complications are potentially fatal and frequently require treatment with further surgical intervention. To reduce the risk of such complications, it has been recommended that PVP or BKP should not proceed until the patient has made a complete recovery from any existing infections, and that, in cases of recent infection, either antibiotics should be prescribed on a long-term basis to avoid deep infection or a cement–antibiotic mixture should be used. 293 Intraoperative balloon perforation during kyphoplasty seems unlikely to lead to any serious complications. Nevertheless, Saliou et al. 219 have suggested a number of methods to minimise the incidence of rupture: (1) purge any trapped air from the balloon to prevent gas embolism, (2) increase balloon inflation pressure very slowly, to allow the balloon to adapt to the solid, sharp, bony environment, and (3) use a curette to break bone bridges and constitutive bone fragments before inflating the balloon.
While it seems likely that PVP and BKP may be associated with increased rates of new vertebral fractures, and in particular adjacent fractures, as yet the quality of the evidence for this is not good.
It is also unclear which of PVP or BKP is the safer of the two approaches to vertebral augmentations, as direct comparisons were unavailable. However, Yang et al. 324 conducted a review which found that rates of specific complications (cement leakage, new compression fractures, pulmonary embolism and radiculopathy) were all significantly higher with vertebroplasty than with kyphoplasty (all p < 0.05). They also found that cement leakage rates were lower in procedures carried out in neurosurgery departments (20.6%) and orthopaedic departments (24.7%) than in radiology departments (52.9%). This could, however, be confounding if the vertebroplasties were carried out by the radiologists. In addition, Medtronic claim that, in BKP, the creation of a cavity within the vertebral body allows for the insertion of a pre-known volume of a more viscous cement at a lower pressure, which reduces the risk of cement leakage and consequent complications compared with PVP. 34
None of the included studies referred to the radiation risks to patients associated with PVP and BKP. These risks, though low, are not trivial. Perisinakis et al. 325 estimated, on the basis of a case series of 11 patients undergoing kyphoplasty with fluoroscopic guidance, a rate of 741 fatal cancers and 5.4 hereditary effects per million treated patients. However, Fitousi et al. 326 found a relatively high level of radiation exposure in a case series of 11 patients undergoing vertebroplasty with fluoroscopy, and estimated a fatal cancer risk of 1 in 580 and a risk of hereditary effects of 1 in 20,000.
Finally, it should be noted that, although PVP and BKP may be associated with the adverse events discussed above, the alternative treatment (conservative management with analgesics, back bracing and bed rest) is linked to a number of potentially serious complications. Bed rest can lead to muscle wasting and deconditioning, and these effects have been associated with DVT, pulmonary emboli, reduced muscle blood flow, red cell volume, capillarisation and oxidative enzymes. 327,328 Narcotic analgesics are associated with a number of undesirable side effects including cognitive impairment and nausea, while NSAIDs are associated with gastrointestinal problems. 55 The registry studies indicate that (academic-in-confidence information has been removed) (for details, see Appendix 11 ). (Academic-in-confidence information has been removed.)
Discussion of clinical effectiveness
Internal validity
The evidence for the clinical effectiveness of PVP is not consistent. The best quality studies, the blinded Buchbinder et al. 102 and INVEST103 studies, show no benefit, whereas some benefit is seen in the lower-quality unblinded studies.
The unblinded FREE137 study suggests some benefit from BKP, although any benefits diminished over time.
Various suggestions have been put forward to explain the inconsistency between the results of the blinded and unblinded studies of PVP. These suggestions relate to:
-
patient selection (fracture acuity and pain severity)
-
operator technique (volume of injected cement and/or technique used for injection)
-
nature of the OPLA
-
outcome measurement
-
use of blinding.
These are discussed in turn below.
Patient selection
The Buchbinder et al. 102 and INVEST103 studies included patients whose fractures were up to 12 months old, as did the studies by Blasco et al. 127 and Farrokhi et al. 135 Clark et al. 108 have argued that most VCFs heal within 8 weeks. It would therefore follow that in these four studies, in which the average time since fracture ranged from 9.5 to around 30 weeks, PVP was carried out on fractures which in most patients had already healed. This would make it unlikely that vertebral augmentation would have any effect on fracture pain by means of fracture fixation. 113 By contrast, VERTOS II recruited patients who had pain of no more than 6 weeks’ duration,17 and it has therefore been suggested that it provides the best evidence relating to the effectiveness of PVP in patients with acute osteoporotic VCF. 113 This assertion is misleading. In addition to the lack of blinding in VERTOS II, which reduces the quality of the study, the delay between recruitment and performance of PVP (9.4 days ± 8.1) meant that many patients would have pain of more than 6 weeks’ duration by the time PVP was performed (see Study characteristics). Furthermore, aggregation of data from the two blinded OPLA-controlled trials showed that outcomes did not differ between those with acute (≤ 6 weeks) and subacute fractures (> 6 weeks). 110 When associations between fracture age and clinical outcomes have been explored in large case series, most of the evidence also suggests no association. 205,206,208 The one exception to this was the study by Ryu et al. 212 who found significant correlations between fracture age and pain, activity, and analgesic use. It was not clear why this discrepancy with the other case series was observed, and the authors seem to have controlled for confounding factors through the use of multiple regression.
Moreover, there is considerable debate about the appropriate timing of PVP and BKP because of evidence that a substantial proportion of VCFs heal without intervention. In VERTOS II, 53% of patients who initially met the inclusion criteria and were willing to participate in the study subsequently became ineligible because their pain score had spontaneously fallen below 5 between screening and randomisation. 17 As noted in Chapter 1 (see Description of health problem), a small study by Klazen et al. found that, by 6 months, 63% of conservatively treated patients with acute radiographically diagnosed VCF reported significant pain relief. 19 They therefore suggest that, to avoid unnecessary interventions, PVP or BKP should be offered only to patients in whom the pain of acute VCF persists for 6 months, but recognise that, during that 6 month wait, a proportion of patients will suffer unnecessary pain and days lost from normal activity. 19 Consequently, studies which include patients with pain of more than 6 weeks’ duration are likely to be more representative of the group of patients who will be considered for vertebral augmentation in clinical practice than those which are limited to patients with pain of less than 6 weeks’ duration.
Some authors have suggested that PVP is only effective for patients with more severe pain which is unresponsive to treatment with analgesics. 107,108,329 However, as emphasised throughout this review, pain is open to a number of confounding influences, which makes its reliability as an eligibility parameter questionable. In addition, the individual patient meta-analysis of the two OPLA-controlled trials of vertebroplasty failed to demonstrate a between-group difference when pain severity (≥ 8 or < 8) was controlled for as a covariate. 110
Gangi and Clark113 have argued that the use of plain radiograph for fracture identification in the INVEST study103 was inadequate. Rather, they suggest that MRI is necessary to identify the presence of marrow oedema and therefore confirm the VCF as the source of pain. Similarly, Whitehouse95 argues that, unless there is an un-united fracture cleft within the vertebra, confirmed by MRI, pain which persists past 10 weeks is likely to be multifactorial as true fracture pain will have been succeeded by mechanical back pain. He also notes that research has suggested that some types of fracture, as seen on initial radiographs, seem to progress, and that cement augmentation is likely to be particularly beneficial in such fractures as it will prevent fracture progression, whereas conservative treatment should be recommended initially in patients whose fracture morphology suggests that it is unlikely to progress, unless they have uncontrollable pain. 95
Operator technique
The technique used in the Buchbinder study to inject cement (i.e. 13-gauge needles and cement hand-injected using 1-cc syringes102,148) has been criticised on the grounds that, to achieve adequate filling of the vertebral body in lumbar fractures, either an 11-gauge needle or a high-pressure injecting system should have been used. 113
It has also been suggested that the volume of cement injected by Buchbinder et al. was too low. 21,113 Aebi has also criticised the INVEST study103 on this basis, suggesting that as the mean amount of cement injected in both studies was inadequate, the investigators in essence compared two placebo operations. 21 However, Kaufmann et al. 330 noted that greater cement volumes may have better outcomes but higher risks of adverse events. Moreover, although Al-Ali et al. 331 found a mean volume of cement injected of 5.1 ± 2.2 ml in 600 osteoporotic fractures treated by vertebroplasty, the range was 1.0 ml to 16.0 ml, and no correlation was observed between the volume of cement and pain improvement. However, they noted that the volume injected was sufficient to fill the intravertebral cleft and, as long as that was done, the volume of cement used was not a determining factor in the degree of pain relief. 331
Debate is ongoing with respect to the impact of the ‘sham’ procedures in the Buchbinder et al. 102 and INVEST103 studies. In the unblinded LABEL study, Brinjikji et al. 332 investigated the efficacy for pain relief of injected lidocaine and bupivacaine at the site of painful osteoporotic compression fractures (n = 19 consecutive patients presenting for consideration of vertebroplasty between April 2009 and January 2010). They compared the changes in the RDQ and average 24-hour pain at days 1 and 3 post injection with those recorded in blinded control patients from the INVEST lead site (n = 16) and found that an unblinded injection of local anaesthetic was ineffective in treating pain from osteoporotic VCFs; significantly greater improvements were seen in the INVEST control patients. This appears to suggest that factors other than local anaesthesia were responsible for that observed improvement. Miller et al. 333 also noted the possibility of high placebo response in any interventional procedure. By contrast, a recent non-randomised case series84 found that facet joint injections resolved pain arising in up to one-third of patients with VCF who were considered suitable for treatment with PVP, while vertebral augmentation appeared to be clinically effective in patients who failed to respond to the facet joint injections. The authors noted that it was not possible to pre-select those patients who would respond to facet joint injection, but suggested that their results supported the hypothesis that PVP was potentially effective in those patients whose pain arose largely from the VCF itself.
A North American Spine Society (NASS) commentary questioned the measurement of pain in both Buchbinder et al. 102 and INVEST,103 arguing that neither study appeared to make any attempt to assess whether or not baseline pain was specific to the fracture. 107 These authors suggested that investigators should percuss or palpitate the spinal levels systematically in order to ascertain the area of maximum focal tenderness. However, only three of the open-label trials (Farrokhi et al. ,135 VERTOS136 and VERTOS II17) reported undertaking such a procedure. Moreover, as the NASS commentary noted, the improvements observed in the vertebroplasty group of the Buchbinder et al. 102 and INVEST103 studies were not dissimilar to those observed in unblinded trials. Perhaps a more important issue is the likelihood of confounding in subjective ratings of pain. 84 However, this is an issue in any trial using visual or numeric pain rating scales and is not specific to Buchbinder and INVEST. As is argued in Chapter 2 (see Decision problem), measures of functional status are more useful than self-reported pain for assessing the impact of vertebral fractures on the patient’s daily life because they are more objective; they have therefore been given priority in the results section.
Also related to the issue of pain measurement, Lotz334 has suggested that, in the INVEST study, the significant difference in crossover rates between treatment and control groups (12% vs. 43%) indicates a degree of patient dissatisfaction with the OPLA procedure which was not fully captured by the pain scales. Indeed, as Doidge et al. point out, those who crossed over at 1 month had worse outcomes for pain and functional status, irrespective of treatment group. This may suggest crossover is a reliable proxy for global effectiveness. 57 However, in an analysis of the crossover data from INVEST, Brinjikji et al. 335 noted that baseline pain duration and treatment site were associated with ability to correctly guess treatment allocation in the control group only. That is, poor responders in the control group were able to guess their allocation, while good responders in the vertebroplasty group were not. Furthermore, Kallmes et al. argue that, as nearly all crossovers occurred after 30 days, they did not affect the primary conclusion that there were no important differences in outcomes between the groups at 1 month. 336
Length of follow-up presents a further set of interpretive challenges. On the one hand, the benefits associated with vertebral augmentation from the open-label trials were all in the short to medium term, with few benefits seen after 6 months. Indeed, this pattern is to be expected in terms of fracture healing and regression to the mean. However, Aebi has suggested that follow-up of 1 year is short to capture the consequences of osteoporotic VCFs with increasing kyphosis which may ultimately lead to death. 21 It is possible that the evidence linking vertebral augmentation to improved survival rates35 may be showing the effect of kyphosis, though this hypothesis is yet to be addressed in clinical trials.
Perhaps the most convincing reasons put forward for discrepancies between findings from the open-label trials of PVP, and those of Buchbinder and INVEST, relate to the use of blinding. Wood et al. 337 and Psaty and Prentice338 have presented empirical evidence that lack of blinding results in an average 25% overestimate of relative treatment benefit. Exaggerations of effectiveness are also likely to be high in any interventional procedure. 333 Factors such as strong patient and physician expectations of effectiveness, and reconfigurations of meaning within the illness experience, are all likely to play a part in determining the apparent strength of an effect. Indeed, such factors have been shown to have an objective neurophysiological impact on pain pathways. 339,340 This is not to say, therefore, that PVP lacks efficacy per se, but rather that the mechanisms that lead to improvement may be unrelated to the injection of bone cement.
In addition to the potential influence of the OPLA response, the apparent disparity between the OPLA trials and some of the open-label trials may be partly explained by response bias. Miller et al. 333 explored this possibility, suggesting that the lack of blinding in the VERTOS II trial may have led to a preponderance among participants in the OPM arm to exaggerate their pain levels owing to dissatisfaction with not having received the procedure. Conversely, the participants in the PVP arm may have exaggerated their improvements either because of expectations that they should be getting better after the intervention or to please the investigators. While it would be difficult to maintain that response bias can account for more objective functional outcomes, it seems probable that the combination of response bias and OPLA effects could explain a substantial degree of the interstudy variability.
External validity
Several factors may affect the external validity of the included studies. The first relates to the potential learning curve relating to the vertebral augmentation procedures. It seems reasonable to assume, and indeed is in some cases stated, that the procedures reported in those studies were performed by experienced personnel, and therefore their results may differ from those obtained by less experienced practitioners. There is little evidence to indicate how many procedures a practitioner needs to perform to achieve a high standard. McDonald et al. compared the outcomes of PVP performed in the Mayo Clinic, MI, USA, by two interventional neuroradiologists with substantial previous vertebroplasty experience and five experienced interventional neuroradiologists who were initially new to the procedure; the ‘experienced’ operators estimated that they had performed at least 150 PVPs prior to the commencement of the study. Patient outcomes appeared to be broadly similar regardless of operator experience, although loss to follow-up limited exploration of long-term outcomes. However, both the volume of cement used and postoperative pain (as assessed by pain at rest and RDQ scores 1 week after PVP) were higher with ‘novice’ than with experienced operators, but decreased as the ‘novice’ operators gained more experience. 341 Thus, although a learning curve can be observed, its effects seem to be relatively limited. However, as the authors caution, the ‘novice’ operators in their study were all highly skilled interventional radiologists with substantial clinical experience prior to the study, and the results are therefore not necessarily generalisable to less skilled personnel. 341
There is potentially an issue about the type of cement used in the studies, as newer generation cements (including those which are highly viscous) have been designed to reduce the risk of cement leakage. 36
-
Syed et al. conducted a RCT of PVP in patients with osteoporotic VCF comparing PMMA with Cortoss,™ a bioactive composite – but this abstract342 does not report that comparison.
-
Blattert et al. 343 RCT compared BKP in patients with osteoporotic VCF (including burst fractures) with PMMA and Norian SRS, a calcium phosphate/carbonate cement. Each cement was associated with leaks in 5 out of 30 vertebrae. PMMA was associated with vascular embolism in two patients; however, with Norian SRS, there were nine cases of cement failure, (i.e. at follow-up at 6 weeks they showed radiographic signs of early cement fracture) all in burst fractures. The investigators therefore did not recommend its use in BKP (there was also persistent haemorrhaging from one vertebral body which partially washed out the cement before it could set – this is less likely to happen with PMMA). (An advantage of calcium phosphate-based cements is that they set at a significantly lower temperature than PMMA, and therefore there is less risk of thermal damage to adjacent structures.)
-
Anselmetti et al. 344 in a single-centre RCT compared PVP with standard low-viscosity PMMA and high-viscosity PMMA designed for injection through a proprietary delivery system (Confidence Type I, Disc-O-Tech, Israel) in patients with VCF of any origin; all procedures were performed by the same experienced operator. CT scans were performed 1 hour after PVP to evaluate cement perfusion, leakages and possible complications; when a venous leak was detected, a CT scan of the lungs was performed to assess the possibility of PMMA embolism. No symptomatic cement leaks occurred in either group; asymptomatic venous leaks were significantly less common in vertebrae treated with high-viscosity PMMA than in those treated with low-viscosity PMMA, but the reduction in the number of leaks into the disk was not statistically significant ( Tables 30 and 31 ).
| High-viscosity PMMA | Low-viscosity PMMA | p-value | |
|---|---|---|---|
| All patients | |||
| Venous leaks | 6/30 (20%) | 24/30 (80%) | NR |
| Leaks into the disk | 6/30 (20%) | 11/30 (36.6%) | NR |
| Osteoporotic patients | |||
| Venous leaks | 4/23 (17.4%) | 19/23 (82.6%) | NR |
| Leaks into the disk | 5/23 (21.7%) | 8/23 (34.8%) | NR |
| High-viscosity PMMA | Low-viscosity PMMA | p-value | |
|---|---|---|---|
| All patients | |||
| Venous leaks | 8/98 (8.2%) | 38/92 (41.3%) | < 0.0001 |
| Leaks into the disk | 6/98 (6.1%) | 12/92 (13.0%) | 0.1374 |
| Osteoporotic patients | |||
| Venous leaks | 6/77 (7.8%) | 30/71 (42.3%) | NR |
| Leaks into the disk | 5/77 (6.5%) | 9/71 (12.7%) | NR |
Summary of key findings
Summary
The included studies measured back-specific functional status using a number of instruments, including the RDQ and SOF-ADL, and indicators of disability such as walking aids. The FREE study137 reported significantly better RDQ outcomes in the BKP group at 1 month and 12 months, although the 95% CIs included the possibility of a lack of MCID at both time points. The VERTOS II study17 reported a significant difference favouring PVP at 12 months. However, the two blinded OPLA-controlled studies102,103 found no statistically significant between-group differences. Farrokhi et al. measured functional status with an instrument based on the ODI, and found a statistically significant difference favouring PVP at all follow-up time points. 135
Five studies (Buchbinder,102 FREE,137 INVEST,103 Rousing131 and VERTOS II17) measured quality of life using the EQ-5D. However, VERTOS II did not report follow-up values, and both Rousing and Buchbinder only began to collect EQ-5D data part way through the trials. Aggregation of IPD from the Buchbinder and INVEST trials110 found no significant difference in EQ-5D in short- or medium-term outcomes. Conversely, the FREE trial found significant differences favouring BKP throughout follow-up. Another commonly used quality of life measure was QUALEFFO, which was reported in four studies (Blasco et al. ,127 Buchbinder et al. ,102 VERTOS136 and VERTOS II17). Broadly speaking, there was a tendency towards favouring PVP. However, the OPLA trial found no significant between group differences on any of the QUALEFFO subscales at any time point.
Four open-label studies (Farrokhi et al. ,135 FREE,137 Rousing et al. 131 and VERTOS II17) found statistically significant differences between groups in short- and medium-term improvement in pain. Conversely, the two double-blinded, OPLA-controlled studies of vertebroplasty, Buchbinder et al. 102 and INVEST103 found no statistically significant between-group differences in pain, and the trend towards a higher rate of clinically meaningful improvement in the INVEST trial may be confounded by greater opioid use in the PVP group. INVEST103 also measured pain frequency and pain bothersomeness on a scale of 0 to 4 and did not find a statistically significant, or clinically meaningful, difference between groups. A similar picture emerged for analgesic use. INVEST and Buchbinder reported reductions in opioid use from baseline in both the PVP and control group, with no statistically significant between-group differences at any time point. However, VERTOS and VERTOS II reported greater short-term reductions in analgesic use among patients treated with PVP, while short- and medium-term differences favouring BKP were reported in the FREE trial.
None of the studies reported statistically significant differences in mortality between treatment groups. However, none of the studies were powered to detect this outcome. A meta-analysis was performed on the three studies which reported all-cause mortality at 12 months. 17,127,131 Although the pooled result slightly favoured PVP, the effect failed to reach statistical significance. Furthermore, there are plausible biomechanical explanations as to why vertebral augmentation may increase life expectancy; these include improvement of lung function due to correction of kyphotic deformity345 and mitigation of impaired physical function through pain relief. 345,346 However, the absence of randomisation in this cohort means that confounding factors cannot be ruled out. A formal critique of mortality data from observational databases (see Appendix 13 ) concludes that:
It is possible that there is a causal difference in mortality between patients treated using OPM and OP patients given the size of the effect. Appropriately taking into account the potential endogeneity of the treatment would tend to reduce the point estimate of the effect size but may or may not eliminate it completely. It is not possible to say with certainty if there is a difference in mortality between patients undergoing BKP and PVP due to the treatment based on the data presented in the studies included here.
There is also considerable uncertainty, were BKP and PVP assumed to have a mortality benefit, in whether or not OPLA would also produce a mortality benefit. However, there were no data on this.
Percutaneous vertebroplasty and BKP appear to be reasonably safe procedures, with a low rate of intra- and postoperative complications. However, when complications do arise from vertebral augmentation, they can be serious. Among the several large case series analysed in this review, one reported a death, though it seemed likely that factors other than the vertebral augmentation played a part. 205 However, several deaths directly related to augmentation procedures have been noted in case reports. Cement extrusion was common, including extrusion into the epidural space. However, most reported extrusions remained asymptomatic. Vertebral augmentation does seem to be associated with a higher risk of new adjacent fractures. A substantial number of case reports of pulmonary embolism, and several case reports of rare but potentially serious complications were identified.
Discussion of potential subgroups
The included studies were too small to permit the identification of subgroups of patients who might benefit from PVP or BKP. While there has been considerable debate on whether vertebral augmentation is more effective in the treatment of acute or chronic fractures,334,336,347,348 analysis of individual patient data from the two double-blinded RCTs of PVP suggested that effectiveness was unrelated to fracture acuity. 110
It has been suggested that PVP and BKP are more successful in patients with a mobile pseudarthrotic cleft pattern of fracture than in those with the more common non-mobile fracture,202 but further research is required to explore this possibility. It has also been suggested that there is a substantial subgroup of patients whose pain is not a direct result of VCF but of overload of facet joints, paraspinal muscles and impingement of spinous processes; such patients respond to facet joint injection while those who do not respond to this treatment have an excellent response to vertebroplasty. 84 However, further evidence from clinical trials would be required to confirm the importance of such subgroups.
Conclusions for clinical effectiveness
PVP and BKP perform significantly better than OPM in reducing pain and disability, and improving HRQoL. There is some evidence that PVP and BKP may lead to reductions in mortality; however, this effect has not yet been confirmed in clinical trials with a randomisation procedure, so the causal mechanisms remain unclear. As yet, there is no convincing evidence that PVP and BKP perform better than blinded administration of local anaesthetic to the affected area in terms of reducing pain and improving HRQoL. However, this may be due, at least in part, to inadequate patient selection methods with some patients (who cannot be identified a priori) receiving considerable benefit. Although the incidence of severe complications arising from vertebral augmentation is low, leakage of cement into the epidural space can pose a serious risk to health, and a small number of procedure-related deaths have been noted in previous case reports.
Chapter 4 Assessment of cost-effectiveness
Systematic review of existing cost-effectiveness evidence
Previously published economic models of percutaneous vertebroplasty and balloon kyphoplasty
From the literature review 243 potential data sources were identified, with full copies of 43 requested. Only one mathematical model assessing the cost-effectiveness of BKP or PVP in the defined population was found. This concurred with the conclusions presented by Medtronic. The identified manuscript was authored by Ström et al. 31 This used a Markov cohort methodology to ascertain the cost-effectiveness of BKP compared with OPM. The model simulated the experiences of hypothetical patients until death or age 100 years, with EQ-5D scores taken directly from the FREE study. 137 It was assumed that the EQ-5D scores would be independent of intervention 3 years post BKP or OPM with a linear decline between 12 months and 36 months. The risks of future vertebral fracture and the risks of mortality after vertebral fracture were incorporated.
The base case assumed a cohort of 70-year-old women and men with a T-score of –2.5 SD and estimated that BKP would be associated with an additional cost of £1494 to obtain 0.169 QALYs at a ratio of £8840 per QALY gained.
The model of Ström et al. 31 was updated to include PVP as an intervention and incorporate the potential beneficial effect of BKP and PVP on mortality and used within the Medtronic submission. 34 As such, it is deemed that the results presented have been superseded.
The models submitted by the manufacturers
The Johnson & Johnson model
Johnson & Johnson submitted a de novo cost-effectiveness model to determine the cost effectiveness of PVP, BKP, OPLA (denoted as ‘invasive control procedure’ and also as ‘sham’) and OPM (termed ‘non-invasive management’). 36 The perspective of the analysis was that of direct NHS and personal and social services costs. In the base case the time horizon was that of 1 year, with discounting of both costs and benefits at 3.5% per annum in sensitivity analyses extending beyond a 1-year time horizon.
The base case assumed a 1-year time horizon assuming that all benefit was lost at 1 year, excluded OPLA as a comparator and included clinical evidence from all relevant studies identified (Buchbinder et al. ,102 Chen et al. ,349 Chen et al. ,350 Farrokhi et al. ,135 Klazen et al. ,17 Kallmes et al. ,103 Liu et al. ,139 Rousing et al. 131 and Wardlaw et al. 137). A ‘target population’ was also denoted where only the results from Chen et al. ,350 Klazen et al. ,17 Liu et al. ,139 Rousing et al. 131 and Wardlaw et al. 136 were included as these were reported as including only fractures within the previous 3 months. The assessment group note, however, that no differential effects of vertebroplasty compared with OPLA were observed when the duration of pain was divided into categories of ≤ 6 weeks and > 6 weeks. 110 Eight alternative scenarios were evaluated, which are detailed fully in the Johnson & Johnson submission36 (pp. 136–7 and cross-references):
-
incorporating data from the OPLA trials
-
incorporating data from the OPLA trials but assuming that these could be pooled with OPM
-
extending the time horizon to beyond 1 year
-
as (3) but using target population results
-
using an alternative bottom-up costing methodology and payment by results tariff
-
as (5) but using target population results
-
using direct EQ-5D values directly
-
as (7) but using target population results.
Within the model, patients are assigned a VAS score at baseline and then at 2 weeks dependent on the intervention received. The treatment-dependent VAS is updated at 1 month, 6 months and 12 months. The values for treatment-dependent VAS were estimated from a network meta-analysis. For information, table 78 of the Johnson & Johnson submission,36 for scenario 1, which includes OPLA trials, is replicated in Table 32 ; other analyses are contained within their submission.
| Treatment | VAS over time, mean (95% CI) | |||
|---|---|---|---|---|
| 2 weeks | 1 month | 6 months | 12 months | |
| Vertebroplasty | 3.360 (2.810 to 3.900) | 2.530 (1.430 to 3.630) | 2.410 (1.880 to 2.940) | 2.170 (1.570 to 2.780) |
| Kyphoplasty | 3.650 (3.10 to 4.190) | 2.990 (1.780 to 4.200) | 2.420 (1.880 to 2.960) | 2.830 (2.220 to 3.440) |
| NIM | 5.920 (5.530 to 6.310) | 4.940 (3.980 to 5.900) | 4.110 (3.820 to 4.410) | 3.810 (3.530 to 4.080) |
| OPLA [Invasive control procedure (‘sham’)] | 3.090 (2.180 to 4.020) | 3.080 (1.720 to 4.440) | 2.410 (0.860 to 3.970) | Not available – assumed equal to the 6-month value |
The assessment group makes two comments regarding the network meta-analysis conducted by the manufacturer. Firstly, there was no attempt to extrapolate or interpolate data from RCTs if they did not report VAS scores at the designated time intervals; this could cause discrepancy within the longitudinal data. Secondly, a further trial, Blasco et al. ,127 was published after the completion of the manufacturer’s systematic review. This trial had similar VAS scores for both PVP and OPM, with both values being relatively high. If the manufacturer had included this study, the VAS scores in all arms would have increased and the relative difference between OPM and both PVP and BKP would have been reduced.
A regression analysis was conducted to translate VAS scores into utility from which QALYs can be calculated. The formula was EQ-5D = 0.9242 – 0.0955 × VAS score. Covariance between the intercept and the slope did not appear to be incorporated.
The submission undertook analyses on potential adverse events, looking specifically at cement leakage and refracture rates. For full details refer to the manufacturer’s submission. 36
It was concluded that cement leakage was highest using low-viscosity cements, and that cement leakage using high-viscosity cements was equivalent to that in BKP. Table 71 of the manufacturer’s submission is reproduced in Table 33 . It is commented that the pooled odds figure relates to the odds that a patient will experience an event if receiving a treatment, rather than being ORs.
| Treatment | Pooled odds | SE |
|---|---|---|
| NIM | 0 | 0 |
| Vertebroplasty LV | 0.814 | 0.776 |
| Vertebroplasty HV | 0.167 | 1.385 |
| Unilateral/unipedicular kyphoplasty | 0.131 | 1.149 |
| Bilateral/bipedicular kyphoplasty | 0.074 | 1.679 |
Regarding fracture rates it was stated that it could not be concluded that there was a significant difference between any of the treatments in terms of refracture rates. Table 72 of the manufacturer’s submission is reproduced in Table 34 . It is commented by the assessment group that these values include all fracture rates and that the conclusions may differ if only adjacent fractures were considered.
| Treatment comparison | Mean HR | Median HR | Lower 95% credible interval | Upper 95% credible interval |
|---|---|---|---|---|
| Vertebroplasty LV vs. NIM | 0.63 | 0.61 | 0.34 | 1.07 |
| Kyphoplasty vs. NIM | 1.24 | 1.20 | 0.71 | 2.03 |
| OPLA [Invasive control procedure (‘sham’)] vs. NIM | 1.21 | 0.80 | 0.15 | 4.70 |
| Vertebroplasty HV vs. NIM | 13.41 | 1.49 | 0.11 | 48.8 |
| Vertebroplasty Cortoss vs. NIM | 0.56 | 0.52 | 0.23 | 1.15 |
| Vertebroplasty CHC vs. NIM | 7.57 | 0.76 | 0.05 | 26.6 |
The assumed acquisition costs
The list prices for PVP using the CONFIDENCE SPINAL CEMENT SYSTEM™ were taken from the Johnson & Johnson submission. 36 The costs vary according to the number of levels that need to be treated and are reported to be £1358 at one level, £1784 at two levels and £1848 for a three-level approach. It is noted that table 83 of the Johnson & Johnson submission appears to contradict the text regarding the cement required in the two-level procedure. 36 The text states that 11 cc would be required, whereas table 83 assumes cc were sufficient. The distribution of operations between one, two and three levels were extracted for Johnson & Johnson by Dr Foster, and are 58.9%, 20.5% and 20.5%, respectively. The costs per level were multiplied by these proportions to arrive at a weighted cost of £1472. If 11 cc of cement were assumed in the two-level operation rather than 7 cc this value would increase to £1546.
The Johnson & Johnson submission36 inflated the price of a BKP operation reported in Ström et al. 31 and assumed a cost of £2842 for BKP. The manufacturer also assumed that the cost of OPLA would equal the cost of PVP; this may be questionable, particularly when it is assumed that high-viscosity cement would be used in the OPLA rather than cheaper low-viscosity cement.
The costs of the preliminary phase, the operating phase and the postoperative phase have previously been reported in Ström et al. 31 These were inflated to 2009–10 prices within the Johnson & Johnson submission,36 and are partially replicated in Table 35 . The table in the Johnson & Johnson appears to misreport the operating room costs, which should be £275, as used in their mathematical model. This has been amended in Table 35 . It was assumed that these costs are applicable to both BKP and PVP.
| Cost per hour (£) | No. of hours | Cost of resource (£) | Percentage of pts | Cost (£) | |
|---|---|---|---|---|---|
| Preliminary phase | |||||
| Surgeon | 106 | 0.25 | – | – | 26 |
| Radiologist | 85 | 0.50 | – | – | 42 |
| Nurse | 16 | 1.00 | – | – | 16 |
| Spine radiograph | – | – | 76 | 100% | 76 |
| MRI | – | – | 275 | 100% | 275 |
| ECG | – | – | 68 | 100% | 68 |
| Blood test(s): | – | – | 21 | 100% | 21 |
| Pain therapy | – | – | 16 | 100% | 16 |
| Sum of preliminary phase | 540 | ||||
| Operating phase | |||||
| Anaesthetist | 106 | 1.00 | – | – | 106 |
| Nurse, anaesthesiology | 12 | 1.00 | – | – | 12 |
| Drugs | – | – | 38 | 100% | 38 |
| Surgeon | 106 | 1.00 | – | – | 106 |
| Radiographer | 32 | 1.00 | – | – | 32 |
| Nurse, surgery | 17 | 2.00 | – | – | 34 |
| Consumables | – | – | 95 | 100% | 95 |
| Operating room | – | – | 275 | 100% | 275 |
| Sum of operating phase | 698 | ||||
| Postoperative phase | |||||
| Nurse | 4.8 | 24.00 | – | – | 114 |
| Surgeon | 106 | 0.50 | – | – | 53 |
| Spine radiograph | – | – | 76 | 100% | 76 |
| Sum of postoperative phase | 243 | ||||
| Total sum | 1479 | ||||
The assessment group comments that there appeared to be a typographical error in the manufacturer’s mathmatical model in which only 10% of patients receiving BKP were assumed to consume operating room resources; it was assumed that this value was intended to be 100%. As such the overall cost-effectiveness results are likely to be favourable to BKP.
Johnson & Johnson also undertook bottom-up costing within the operating phase. This approach used data on procedure times and number of vertebral levels treated, collected using a bespoke iPad (Apple Inc., Cupertino, CA, USA) application designed to measure the total duration of the operating room episode in minutes. These data were collected at five hospitals (details confidential). Data from the iPad app were used in conjunction with data from an audit of vertebroplasty procedures, obtained from two hospitals (identity confidential) currently offering vertebroplasty, to generate estimated costs for the procedure. While the average weighted operating cost per procedure can be made public, the breakdown of constituent parts remain confidential. Data obtained included the cost and volumes of surgical consumables, medication costs (including sedation and antibiotics), theatre costs and staff costs. The data from this analysis are replicated in Tables 36 and 37 .
| Number of levels | Split, % | Average procedure duration, minutes |
|---|---|---|
| 1 | 59 | 31.75 |
| 2 or more | 41 | 46.20 |
| Weighted average duration (minutes) | 37.69 |
| Resource based costs (per hour) | Hourly rate (inc. on costs) | Percentage of pts | Average cost per procedure |
|---|---|---|---|
| Consultant | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Anaesthetist | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Theatre staff (includes three theatre staff) | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Radiographer | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Recovery | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Theatre session (per hour) | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Sedation | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| General anaesthetic | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Surgical consumables | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Average weighted cost per procedure | £527.55 |
The costs associated with hospitalisation stay
In the Johnson & Johnson submission,36 a third party (Dr Foster Intelligence) was employed to extract data based on the ICD code (M80*) and the OPCS 4.5 code (V444 for PVP, V445 for BKP and blank for those treated with OPM).
Johnson & Johnson assumed a cost of £232 per day based on the payment by results national tariff price for an excess bed day associated with vertebroplasty/kyphoplasty and non-invasive management Healthcare Resource Group (HRG) codes (HRGs HC04C, HC05C and HD36C). 351 These values are summarised in Table 38 .
| Johnson & Johnson | |||
|---|---|---|---|
| Intervention | Length of stay in days (standard error) | Cost per day (£) | Total cost (£) |
| PVP | 3.24 (0.49) | 232 | 752 |
| BKP | 4.48 (0.89) | 232 | 1039 |
| OPM | 12.61 (0.27) | 232 | 2926 |
Results presented by Johnson & Johnson
The base-case deterministic results are replicated in Table 39 . It is seen that PVP was shown to dominate (that is to say, producing more QALYs at a lower cost) BKP. The cost-effectiveness plane and cost-effectiveness acceptability frontier are shown in Figure 16 . The incremental cost-effectiveness ratio (ICER) between PVP and OPM was £4392 per QALY gained. Explicit comparison between the PVP and BKP results indicated that the ICER between PVP and BKP was 99.86% likely to be below £20,000 per QALY gained.
| Treatment | Costs | QALYs |
|---|---|---|
| PVP | £3702 | 0.684 |
| BKP | £5113 | 0.656 |
| OPM | £2926 | 0.507 |
FIGURE 16.
The cost-effectiveness plane and frontier associated with the base-case deterministic results in the Johnson & Johnson submission. 36 CE, cost-effectiveness.
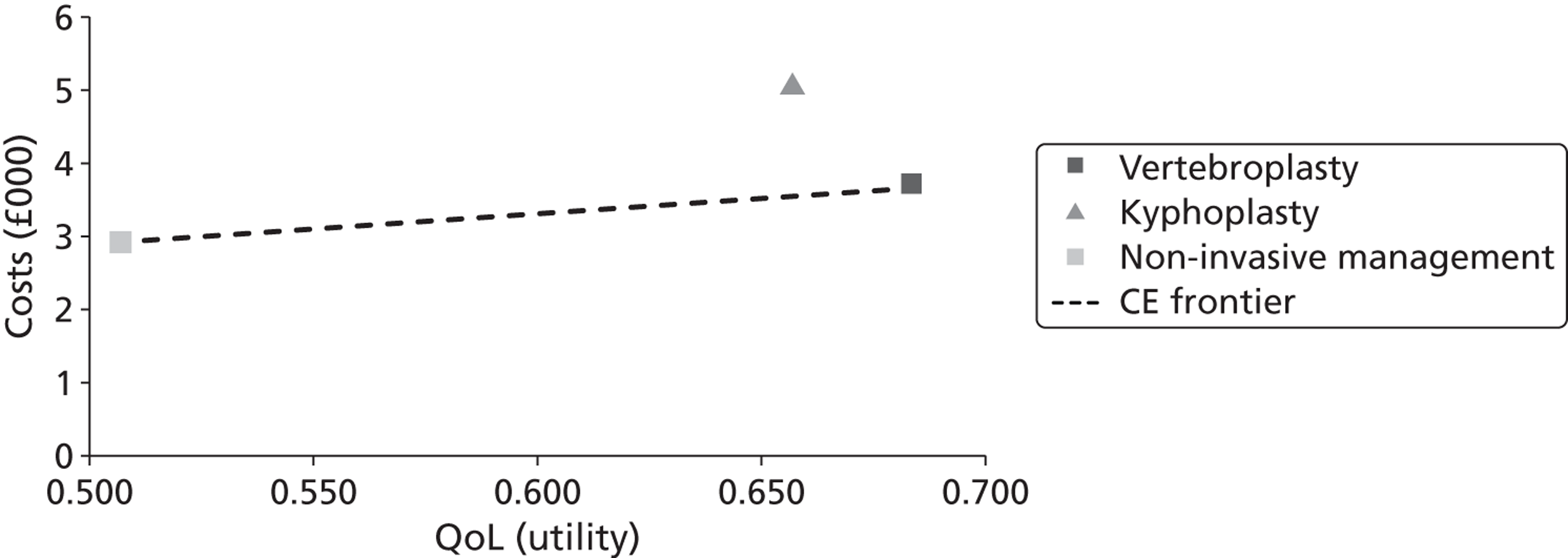
The cost-effectiveness frontiers and ICERs for the target population and the alternative scenarios undertaken by Johnson & Johnson are summarised in Table 40 .
| Scenario | Cost-effectiveness frontier | ICER on cost-effectiveness frontier | |
|---|---|---|---|
| – | Target population | OPM/PVP | £4755 |
| 1 | Incorporating data from the OPLA trials | OPM/PVP | £4392 |
| 2 | Incorporating data from the OPLA trials but assuming that these could be pooled with OPM | OPM/PVP | £4982 |
| 3 | Extending the time horizon to 10 years with decline in benefit across time | OPM/PVP | £1054 |
| 4 | As (3) but using target population results | OPM/PVP | £1168 |
| 5a | Using an alternative bottom up costing methodology and payment by results tariff | PVP | – |
| 5b | Using payment by results tariff | OPM/PVP | £13,595 |
| 6a | As (5a) but using target population results | PVP | – |
| 6b | As (5b) but using target population results | OPM/PVP | £14,718 |
| 7 | Using direct EQ-5D values directly | OPM/PVP | £5516 |
| 8 | As (7) but using target population results | OPM/PVP | £5516 |
Johnson & Johnson undertook univariate sensitivity analysis comparing PVP with OPM and PVP with BKP. 36 The analyses for PVP and OPM are reproduced in Figure 17 . The analyses comparing PVP and BKP are not reproduced as in all analyses undertaken PVP dominated BKP.
FIGURE 17.
A tornado plot of univariate sensitivity comparing PVP with NIM in the base case. NIM, non-invasive management; VP, vertebroplasty.
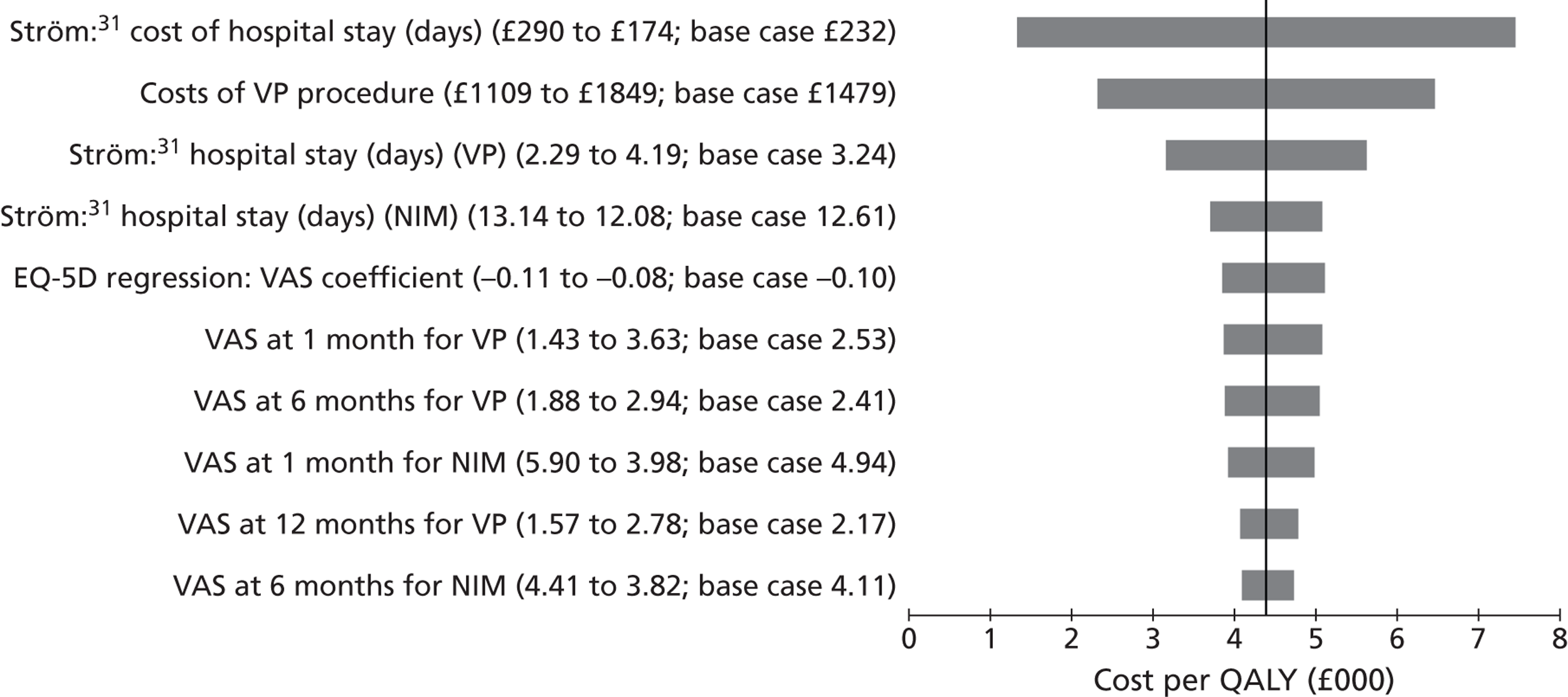
A threshold analysis was undertaken on the parameters contained in Figure 17 in the base case. These values are reproduced in Table 41 . Similar analyses were presented for the target population with broadly similar results.
| Variable | Base case | £20,000 per QALY | £30,000 per QALY |
|---|---|---|---|
| Ström:31 cost of hospital stay (days) | £232 | –£63a | –£251a |
| Costs of VP procedure | £1479 | £4239 | £6008 |
| Ström:31 hospital stay (days) (VP) | 3.24 | 15.14 | 22.76 |
| Ström:31 hospital stay (days) (OPM) | 12.61 | 0.71 | –6.91 |
| EQ-5D regression: VAS coefficient | –0.10 | –0.02 | –0.01 |
| VAS at 1 month for VP | 2.53 | 8.79 | 9.38 |
| VAS at 6 months for VP | 2.41 | 5.56 | 5.86 |
| VAS at 1 month for OPM | 4.94 | –1.32a | –1.91a |
| VAS at 12 months for VP | 2.17 | 7.95 | 8.49 |
| VAS at 6 months for OPM | 4.11 | 0.96 | 0.66 |
The results from the probabilistic analyses were very similar to those for the deterministic base case. PVP was still estimated to dominate BKP, with the ICER between PVP and OPM being £4388 per QALY gained compared with £4392 deterministically. The comparison between the PVP and BKP results indicated that the ICER between PVP and BKP was 98.55% likely to be below £20,000 per QALY gained. As the individual cost and QALY components were very similar in both the probabilistic and deterministic analyses, it was deemed that the model was linear and, for brevity, only the deterministic values have been reported.
The Medtronic model
Medtronic submitted a Markov tunnel model using a patient lifetime approach. 34 The tunnel approach allows the time in a health state to be reflected in model parameters such as transition probabilities, costs and utilities. A time cycle of 6 months was used and a NHS perspective was employed. Measures of both health and costs were discounted at 3.5% per annum. The diagram of the model presented by Medtronic is replicated in Figure 18 . The objective of the model was to determine the cost-effectiveness of PVP, BKP and OPM (termed non-surgical management).
FIGURE 18.
The diagrammatic representation of the Medtronic model. Reproduced with permission from Medtronic.
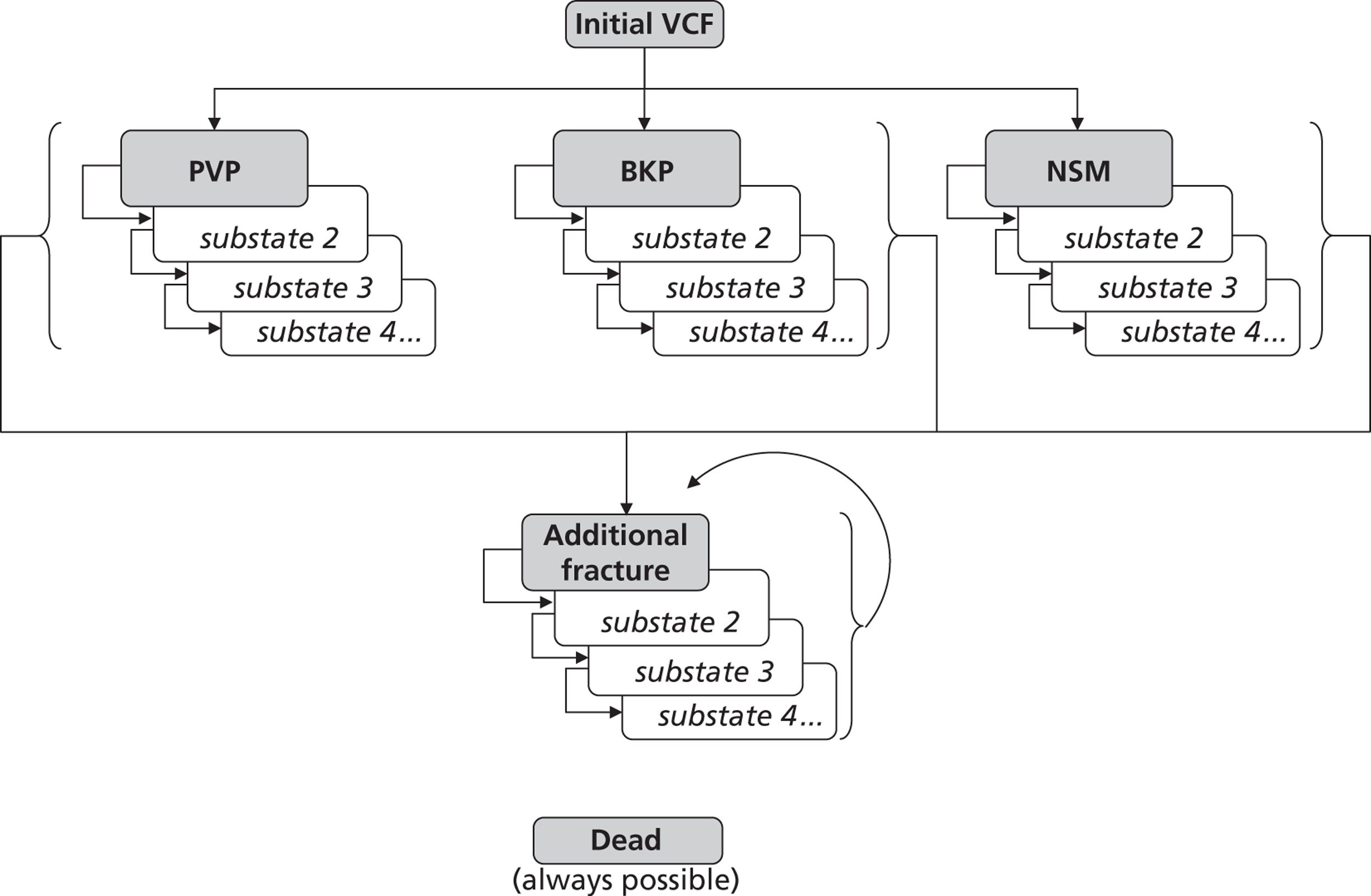
Medtronic assumed that the patient population was those patients hospitalised for vertebral fracture, the stated rationale being that the FREE trial,137 which is the pivotal BKP trial, was conducted in hospitalised patients and that BKP is predominantly an inpatient procedure137 in the UK. In the base case it was assumed that patients were 70-year-olds with a T-score of –3.0 SD, which was reported to be commensurate with the data within the FREE trial and VERTOS II. 17
The patient remained in their initial treatment health state (progressing through the substates) until an additional vertebral fracture occurred or the patient died. For all patients a subsequent vertebral fracture was assumed to be treated using non-surgical management. The transition probabilities for further vertebral fractures were calculated from equations that are a function of the patient’s BMD compared with that of a young woman, age, previous fracture status and the imputed ratio between hip and vertebral fractures at each age, assuming that the Swedish ratio was applicable to the UK. The transition probabilities to death used data from the Human Mortality Database for UK patients352 and the relative risks of mortality reported in Ström et al. 31 for people with a prior vertebral fracture.
The health utilities for BKP and OPM were taken directly from the FREE trial. 137 The utility for PVP was estimated assuming that the difference between PVP and OPM reported in the VERTOS II trial17 could be directly added to the OPM scores in the FREE trial. As the QALY data for VERTOS II were presented only at baseline, 1 month and 12 months, the manufacturer inferred the average utility across the 1-year time horizon. The estimate of the undiscounted QALYs gained in the first year for each treatment is provided in Table 42 .
| Time period | Intervention | ||
|---|---|---|---|
| OPM | BKP | PVP | |
| 0–6 months | 0.219 | 0.276 | 0.273 |
| 6–12 months | 0.255 | 0.311 | 0.309 |
| 13–18 months | 0.260 | 0.307 | 0.305 |
| 18–24 months | 0.265 | 0.307 | 0.305 |
| 24–30 months | 0.264 | 0.292 | 0.291 |
| 30–36 months | 0.264 | 0.278 | 0.277 |
| 36–42 months | 0.263 | 0.263 | 0.263 |
It was assumed that the difference in utility between BKP and OPM would linearly decline across 1 year such that there was no difference 3 years after the intervention. For PVP it was assumed that the utility after the first year (which was not recorded) would progress similarly to that for BKP. It was assumed that the utility of patients would decline after 2 years in accordance with population norm data. The source for these data as apparent from the mathematical model was Ara et al. 353
The model assumes that both BKP and PVP are associated with a mortality benefit compared with OPM. The relative risk for BKP was set at 0.61 and for PVP was set at 0.78. The manufacturer notes that these values have since been updated, but these data became available too close to their submission date to incorporate them within the model.
No adverse events were included in the model bar recurrent fracture, with lack of data being the reported reason for the omission, although the submission does state that associated consequences may be ‘substantial’. The rate and consequences of additional fractures were assumed independent of treatment as neither FREE136 nor VERTOS II17 studies detected a significant difference in the incidence of new fractures among treatments.
The assumed acquisition costs
The list price for a BKP kit (£2600.50) has been taken from the Medtronic submission;34 Medtronic additionally quote a lower price as an average selling price but this value (£1900) is not consistently available to all customers within the NHS. An additional £96 has been added for devices used in the operation.
Medtronic assume a cost of PVP of (commercial-in-confidence information has been removed) reported to be the average selling price of De Puy’s spine PVP plus an additional £53 for devices used in the operation.
The costs associated with the operation
The costs of the preliminary phase, the operating phase and the postoperative phase have previously been reported in Ström et al. 31 Medtronic updated these costs in table 49 of their submission, which are replicated in Table 43 .
| BKP | PVP | NSM | |
|---|---|---|---|
| Procedure costs | |||
| Devices | 96 | 53 | 0 |
| Consumables | 1900 | CiC information has been removed | 0 |
| Other procedure costs | |||
| Preliminary phase | |||
| Interventional radiologist | 0 | 107 | 0 |
| Surgeon | 107 | 0 | 0 |
| Nurse | 16 | 18 | 0 |
| Rx spine | 77 | 77 | 0 |
| MRI | 176 | 176 | 0 |
| ECG | 68 | 68 | 0 |
| Blood test | 21 | 21 | 0 |
| Drugs | 16 | 16 | 0 |
| Operating phase | |||
| Anaesthetist | 107 | 107 | 0 |
| Nurse – anaesthesia | 12 | 13 | 0 |
| Drugs | 38 | 22 | 0 |
| Radiologist | 0 | 107 | 0 |
| Surgeon | 107 | 0 | 0 |
| Nurse – operation | 17 | 17 | 0 |
| Cost of operating room | 160 | 160 | 0 |
| Postoperative phase | |||
| Nurse | 41 | 41 | 0 |
| Drugs | 27 | 63 | 0 |
| Total procedure costs | 2986 | CiC information has been removed | 0 |
The costs associated with hospitalisation stay
In the Medtronic submission34 the length of stay was reported to be taken from Hospital Episode Statistics 2010–11 data. These values are summarised in Table 44 .
| Intervention | Length of stay in days | Cost per day (£) | Total cost (£) |
|---|---|---|---|
| PVP | 6.2 | 457 | 2833 |
| BKP | 5.1 | 457 | 2331 |
| OPM | 9.5 | 457 | 4342 |
In the Medtronic submission34 the assumed cost per day in hospital was taken from NHS reference costs 2009/10/11, and was £457.
The results presented by Medtronic
Medtronic presented both deterministic and probabilistic results for OPM, PVP and BKP; OPLA was not considered a comparator. Medtronic estimated that all three treatments lay on the cost-effectiveness frontier. In the deterministic analysis the ICER between OPM and PVP was £2053 per QALY gained, while that for BKP compared with PVP was £2510. The deterministic results presented by Medtronic are reproduced in Table 45 .
| Technologies | Total costs (£) | Total LYG | Total QALYs | Incremental costs (£) | Incremental LYG | Incremental QALYs | ICER (£) vs. NSM (QALYs) | ICER (£) incremental (QALYs) |
|---|---|---|---|---|---|---|---|---|
| Deterministic analysis | ||||||||
| OPM | 5394 | 9.851 | 4.976 | |||||
| PVP | 6112 | 10.113 | 5.325 | 718 | 0.26 | 0.35 | 2053 | 2053 |
| BKP | 6403 | 10.319 | 5.441 | 1008 | 0.47 | 0.47 | 2167 | 2510 |
| Probabilistic analysis | ||||||||
| OPM | 5394 | 4.975 | ||||||
| PVP | 6132 | 5.327 | 738 | 0.00 | 0.35 | 2100 | 2100 | |
| BKP | 6385 | 5.443 | 991 | 0.00 | 0.47 | 2118 | 2174 | |
Sensitivity analyses were conducted by Medtronic on the time horizon (table 25 of Medtronic’s submission); the discount rate for costs (table 26); the discount rates for QALYs (table 27); the proportion of health-utility benefit from the pivotal trial (table 28); the health-utility offset time (table 29); post-fracture mortality rates (table 31); the price of PVP compared with BKP (table 33); the unit costs per bed day (table 34); the assumed T-score of the cohort (tables 37 and 38); the age of the cohort (tables 39 and 40); the removal of bisphosphonate treatment (table 41) and the assumption that all patients were male (table 42). 34 In each instance the conclusion that BKP produced most QALYs had an ICER below £15,000 per QALY gained compared with either PVP or OPM remained constant. The assessment group comments that the results in table 28 may lack face validity as the OPM QALY value increased when the benefits of the trial rose from 25% to 50% but remained constant when the benefits were assumed to increase from 50% to 75%.
The fact that BKP had an ICER of £12,353 per QALY compared with PVP when the price of PVP was set to zero (Medtronic’s table 33) highlights that the assumed mortality effect (which was more favourable to BKP than PVP) was a key driver of the cost-effectiveness results as seen in table 30 of Medtronic’s submission (reproduced below in Table 46 ). When it was assumed that there was no mortality benefit associated with either PVP or BKP then the ICER of BKP compared with PVP was £27,340 per QALY gained. It is noted that the ICERs for both PVP and BKP compared with non-surgical management remained low, with the key change being the ICER between BKP and PVP.
| Technology | Total costs (£) | Total QALYS | ICER – PVP vs. NSM | ICER – BKP vs. NSM | ICER – BKP vs. PVP |
|---|---|---|---|---|---|
| 0% mortality benefit | |||||
| Deterministic analysis | |||||
| NSM | 5394 | 4.976 | 3245 | 4325 | 27,340 |
| PVP | 6094 | 5.191 | |||
| BKP | 6371 | 5.201 | |||
| 50% mortality benefit | |||||
| Deterministic analysis | |||||
| NSM | 5394 | 4.976 | 2511 | 2881 | 4562 |
| PVP | 6103 | 5.258 | |||
| BKP | 6387 | 5.320 | |||
| 75% mortality benefit | |||||
| Deterministic analysis | |||||
| NSM | 5394 | 4.976 | 2258 | 2472 | 3233 |
| PVP | 6107 | 5.292 | |||
| BKP | 6395 | 5.380 | |||
The sensitivity analysis conducted by Medtronic on the assumed length of hospital stay following BKP (table 32 in the Medtronic submission) also increased the ICER of BKP compared with PVP to over £20,000 per QALY gained. The lengths of stays for OPM and PVP were maintained at 9.5 days and 6.2 days, respectively, while BKP was increased from the base case of 5.1 days. 34 These results are reproduced in Table 47 .
| Technology | Total costs (£) | Total QALYS | ICER – PVP vs. NSM | ICER – BKP vs. NSM | ICER – BKP vs. PVP |
|---|---|---|---|---|---|
| 7.65 days | |||||
| Deterministic analysis | |||||
| NSM | 5394 | 4.976 | 2053 | 4670 | 12,572 |
| PVP | 6112 | 5.325 | |||
| BKP | 7568 | 5.441 | |||
| 10.2 days | |||||
| Deterministic analysis | |||||
| NSM | 5394 | 4.976 | 2053 | 7174 | 22,634 |
| PVP | 6112 | 5.325 | |||
| BKP | 8733 | 5.441 | |||
A further sensitivity analysis was conducted assuming no benefit beyond those seen in the FREE137 and VERTOS II17 trials (table 36 of the Medtronic submission). This is partially replicated in Table 48 , with the assessment group amending the table to correctly implement extended dominance. It is commented that the trials were not directly comparable as FREE reported EQ-5D values for 2 years, whereas VERTOS II reported values for only 1 year.
| Technologies | Total costs (£) | Total LYG | Total QALYs | Incremental costs (£) | Incremental LYG | Incremental QALYs | ICER (£) vs. NSM (QALYs) | ICER (£) incremental (QALYs) |
|---|---|---|---|---|---|---|---|---|
| Deterministic analysis | ||||||||
| NSM | 5394 | 9.85 | 4.98 | |||||
| PVP | 7602 | 9.85 | 5.08 | 2208 | 0.00 | 0.11 | 20,881 | Extendedly dominated |
| BKP | 8381 | 9.85 | 5.17 | 2987 | 0.00 | 0.19 | 15,655 | 9160 |
The probabilistic results are replicated in Table 49 . When compared with the results in Table 45 it is seen that deterministic and probabilistic results were similar. The assessment group notes that it is unclear why results for total life-years gained were not reported in the probabilistic analyses.
| Technologies | Total costs (£) | Total LYG | Total QALYs | Incremental costs (£) | Incremental LYG | Incremental QALYs | ICER (£) vs. NSM (QALYs) | ICER (£) incremental (QALYs) |
|---|---|---|---|---|---|---|---|---|
| Probabilistic analysis | ||||||||
| OPM | 5394 | 4.975 | ||||||
| PVP | 6132 | 5.327 | 738 | 0.00 | 0.35 | 2100 | 2100 | |
| BKP | 6385 | 5.443 | 991 | 0.00 | 0.47 | 2118 | 2174 | |
A cost-effectiveness acceptability curve (CEAC) was submitted by Medtronic, which is reproduced in Figure 19 .
FIGURE 19.
The CEAC presented in the Medtronic submission. 34 Reproduced with permission from Medtronic.
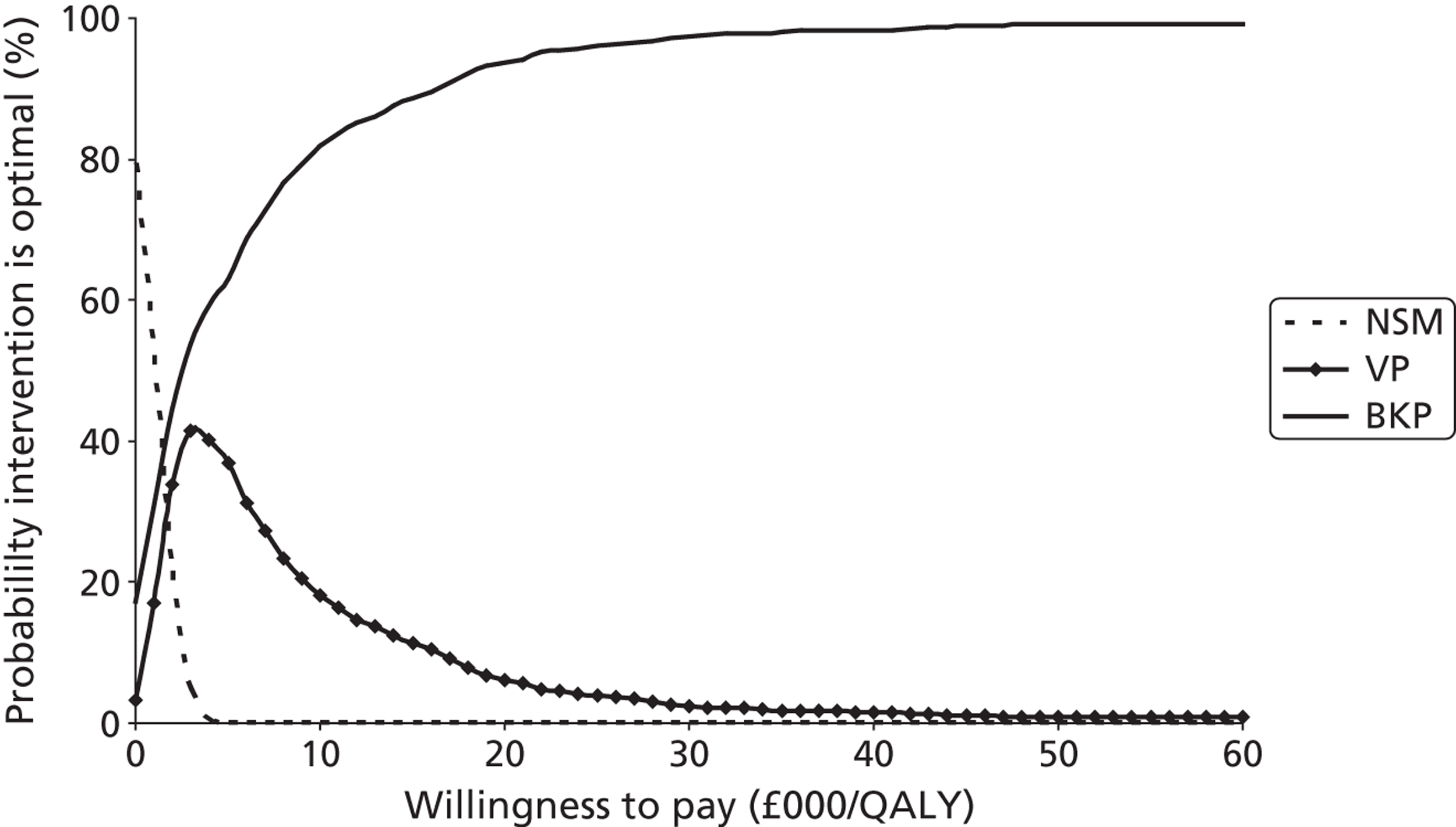
A further comparison of the results between BKP and PVP was provided (and replicated in Figure 20 ). This indicated that in the large majority of cases BKP provided more QALYs than PVP, with an approximately even distribution between whether BKP or PVP was the more expensive procedure.
FIGURE 20.
A scatterplot of the paired BKP and PVP results. Reproduced with permission from Medtronic.
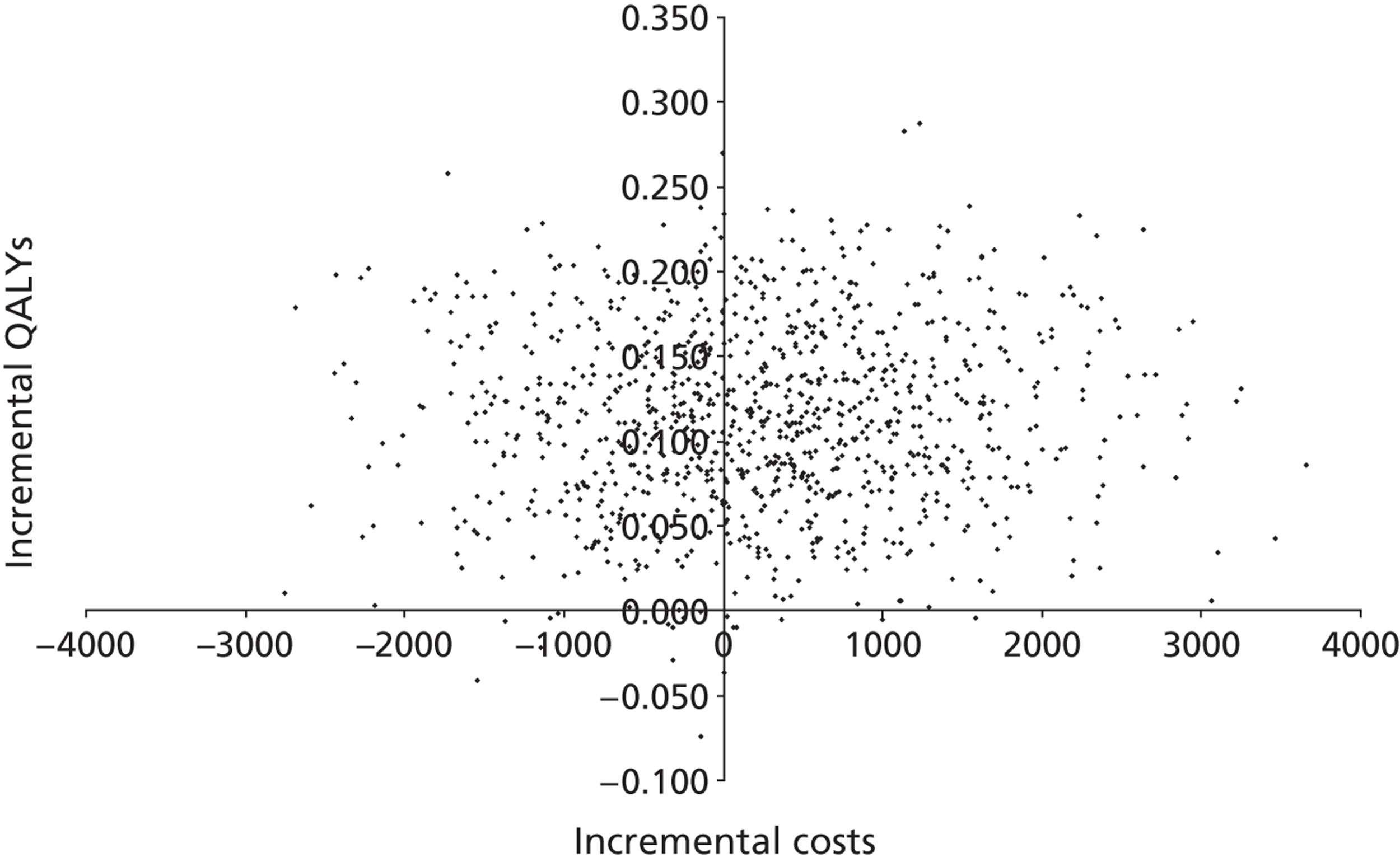
The assessment group model
The decision problem
While in principle the decision problem appears straightforward in comparing PVP (with different cement viscosities), BKP and OPM, in reality, the addition of OPLA as a potential comparator adds significant controversy to the evaluation.
As detailed in the clinical review chapter, and analysed further through the means of a network meta-analysis within this chapter, the result in terms of difference in the level of patient benefit between PVP and OPLA is considerably less than the difference in change in the level of patient benefit between PVP and OPM. This indicates that at least part of the response for PVP (and through the network of evidence, also therefore BKP) compared with OPM in the open-label trials appears to be placebo driven.
The decision to include or exclude OPLA as a comparator can be criticised regardless of the actual conclusion. If OPLA is included then there will be criticism that the use of OPLA treatment within the NHS could, in itself, be deemed unethical and a non-cost-effective use of scarce resources. There may be an additional issue regarding whether or not the components of OPLA, which include providing local anaesthesia and a small incision in the back, meet the criteria for non-invasive management which is the comparator in the in the protocol (see Appendix 1 ) and the scope. 354
If OPLA is not considered an option, then there is a danger of the results from the open-label studies being taken at face value, with the potentially strong placebo effect ignored. In health technology assessments, the highest level of evidence of effect is taken from double-blinded trials which specifically attempt to minimise placebo response. If the trials which attempted to control for the placebo effect were excluded then this would inflate the effectiveness of the interventions and potentially result in a recommendation of interventions that are not a cost-effective use of scarce resources. Additionally, it could be argued that if OPLA provides benefits comparable with those of PVP and BKP, then it may be unethical to perform the active interventions which carry a small, but definite, risk of adverse events.
As detailed in the discussion of clinical effectiveness section, there is insufficient evidence to understand the exact nature of the placebo effect. Further research will be needed to determine whether a good clinical response can be obtained without resorting to BKP, PVP or OPLA, or whether the observed (potentially psychologically driven) benefits compared with OPM can only be achieved through a person being prepared for surgery.
Owing to the uncertainty regarding whether or not OPLA should be included as a comparator (the Johnson & Johnson submission36 included OPLA whereas the Medtronic submission34 did not), where it is indicated that OPLA lies on the cost-effectiveness frontier analyses and where both BKP and PVP have an ICER of > £20,000 per QALY gained, additional results are presented with OPLA excluded as a comparator.
The decision problem is further complicated by the possibility that BKP and PVP (and potentially OPLA) may have a beneficial effect on mortality. The clinical belief for this is that patients who regain mobility quicker remove fluid from the lungs, regain their appetite and are less prone to infection. Observational data indicate that this is the case. The publications indicating the mortality benefit have been formally critiqued (see Appendix 13 ), and it has been concluded that it is not possible to say with certainty if there is a difference in mortality between patients undergoing BKP and PVP as a result of the treatment. As such, separate analyses are presented where different assumptions regarding the mortality effect have been made.
In addition to the direct use of BKP and PVP, an exploratory analysis was considered by the assessment group assuming that all patients are provided with a facet joint injection prior to vertebroplasty as detailed in Wilson et al. 84 Such an intervention is becoming more common in clinical practice, according to our clinical advisor.
Stentoplasty was not considered owing to a dearth of robust evidence.
The conceptual model
The conceptual model was constructed to account for two main factors: firstly, the potential difference in EQ-5D (mapped from VAS or taken directly from the trial) within the short term due to the intervention and secondly, the need to model differential mortality rates which are dependent on the intervention. As there were potentially different mortality rates, it was deemed prudent to also model expensive events related to the osteoporotic VCF to take into consideration the fact that patients who live longer may have other disease-related events. Thus, the risks of subsequent hip and vertebral fractures were also modelled.
The model consisted of five health states: post-osteoporotic VCF, for which BKP, PVP, OPLA or OPM has been undertaken (which is the starting state for all patients); a subsequent additional vertebral fracture; a subsequent hip fracture; both a subsequent vertebral and hip fracture; and death (an absorbing state). For simplicity, only one further vertebral fracture and one hip fracture were permitted. The conceptual model is depicted in Figure 21 . The model employed a time horizon of 50 years, which was assumed to represent patients’ lifetimes, and employed 36 monthly time cycles followed by 47 yearly time cycles. The rationale for the different cycle length was that there may be a utility difference between interventions in the initial period following a procedure which was more easily incorporated into monthly time cycles. A life table methodology was employed to take into consideration that all transitions did not take place at the end of the time cycle. 355 Both costs and benefits were discounted at 3.5% per annum. 356 The model did not include the potential disutility associated with anxiety regarding the prospect of future fractures or the potential reduction in BMD associated with prolonged bed rest.
FIGURE 21.
Diagram of the conceptual model.
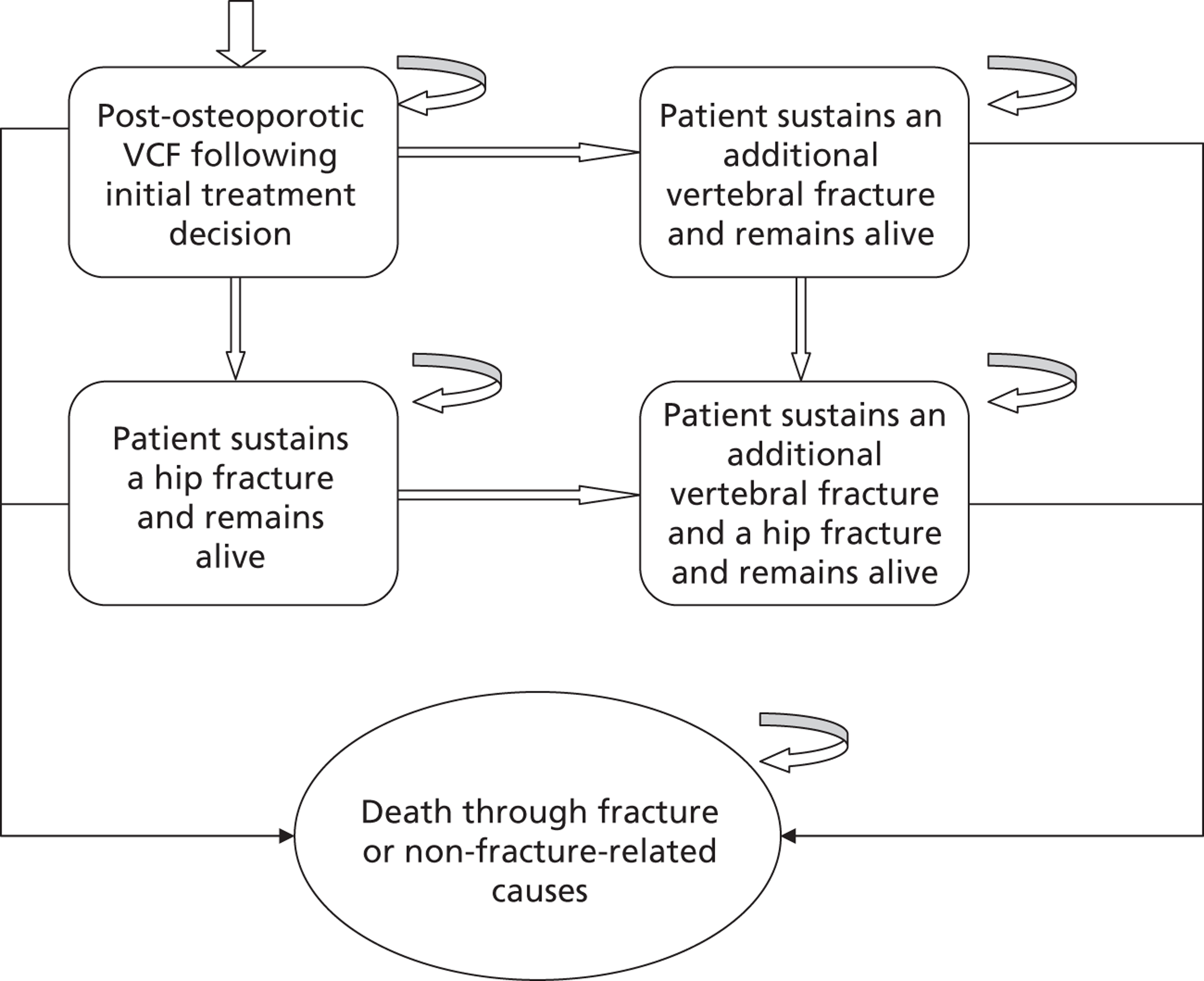
The assumed transition probabilities
Transition probabilities are dependent on patient age, patient sex, T-score, the assumed effect of the procedure on mortality, whether or not bisphosphonates are prescribed and the assumed efficacy of bisphosphonates. A T-score is defined as the number of SDs from the average BMD of healthy young women. For simplicity it was assumed that generic weekly alendronate was the bisphosphonate taken by all patients.
The transition probabilities are detailed in four categories, which represent the health states from which a patient could exit: ‘post-osteoporotic VCF following initial treatment decision’; ‘patient sustains an additional vertebral fracture and remains alive’; ‘patient sustains a hip fracture and remains alive’ and ‘patient sustains an additional vertebral and a hip fracture and remains alive’.
Transition probabilities from ‘post-osteoporotic vertebral compression fracture following initial treatment decision’
To ‘patient sustains an additional vertebral fracture and remains alive’
The transition rates were taken from the values used in Stevenson et al. 357 These values were derived from an exponential fit to population data provided in Singer et al. 37 and adjusted to provide a risk for patients with a T-score of –2.5 SD and no previous fracture (table 20 of Stevenson et al. ). These values were then multiplied by 1.5 to take into account the additional risks following an initial fracture for patients aged 70 years or over. Further detail on this calibration is provided on p. 43 and illustrated in figure 23 of Stevenson et al. 357
Alternative T-scores were considered by using the equations provided by Marshall et al. ,54 which indicate that risk of vertebral fracture increased by 1.8 to the power of the patient’s Z-score. The Z-score is defined as the number of SDs from the average BMD of women of the same age as the patient. Assuming that the SD of BMD remains constant as a population ages, the risk of fracture at a T-score of –3.5 SD was therefore assumed to be 1.8 times greater than that of a patient with a T-score of –2.5 SD.
The annual vertebral fracture risks assumed for patients of a given age, and given T-score on entry to the model, are provided in Table 50 .
| Age group (years) | T-score (SD) | |||
|---|---|---|---|---|
| –2.0 | –2.5 | –3.0 | –3.5 | |
| 65–69 | 0.41% | 0.56% | 0.74% | 1.00% |
| 70–74 | 0.46% | 0.62% | 0.83% | 1.11% |
| 75–79 | 0.55% | 0.74% | 0.99% | 1.32% |
| 80–84 | 0.65% | 0.87% | 1.17% | 1.57% |
| 85–89 | 0.78% | 1.05% | 1.41% | 1.89% |
To take into consideration that a patient’s bone density is likely to deteriorate over time, a decrease of 0.255 SD per 5-year age group was incorporated in accordance with data from Holt and Khaw338 presented in Stevenson et al. 359 Thus, when a patient became 5 years older the risk of a vertebral fracture increased by 1.80.255, which is an increase of 16% compared with a person of the same age, with a T-score equal to that of the patient 5 years previously. For simplicity, it was assumed that women and men with the same T-score would have the same risks of fracture.
If a patient were assumed to be taking a bisphosphonate, the effect on vertebral fractures was assumed to be a relative risk of 0.58 (95% CI 0.50 to 0.67) from data reported in table 27 of Stevenson et al. 357 This effect was assumed to last for 5 years, with a linear wane in effect over a 5-year period, so that the relative risk was 1 after 10 years.
To ‘patient sustains a hip fracture and remains alive’
The transition rates were taken from the values used in Stevenson et al. 357 These values were derived from an exponential fit to population data provided in Singer et al. 37 and adjusted to provide a risk for patients with a T-score of –2.5 SD and no previous fracture (table 20 of Stevenson et al. 357). These values were then multiplied by 1.5 to take into account the additional risks following an initial fracture for patients aged 70 years or over. Further detail on this calibration is provided on p. 43 and illustrated in figure 23 of Stevenson et al. 357
Alternative T-scores were considered by using the equations provided by Marshall et al. ,54 which indicate that risk of vertebral fracture increased by 2.6 to the power of the patient’s Z-score. Assuming that the SD of BMD remains constant as a population ages, the risk of fracture at a T-score of –3.5 SD was therefore assumed to be 2.6 times greater than that of a patient with a T-score of –2.5 SD.
The annual risks of hip fracture assumed for patients of a given age, and given T-Score on entry to the model, are provided in Table 51 .
| Age group (years) | T-score (SD) | |||
|---|---|---|---|---|
| –2.0 | –2.5 | –3.0 | –3.5 | |
| 65–69 | 0.41% | 0.56% | 0.74% | 1.00% |
| 70–74 | 0.46% | 0.62% | 0.83% | 1.11% |
| 75–79 | 0.55% | 0.74% | 0.99% | 1.32% |
| 80–84 | 0.65% | 0.87% | 1.17% | 1.57% |
| 85–89 | 0.78% | 1.05% | 1.41% | 1.89% |
To take into consideration that a patient’s bone density is likely to deteriorate over time, a decrease of 0.255 SD per 5-year age group was incorporated in accordance with data from Holt and Khaw358 presented in Stevenson et al. 359 Thus, when a patient became 5 years older the risk of a vertebral fracture increased by 2.60.255, which is an increase of 28% compared with a person of the same age, with a T-score equal to that of the patient 5 years previously. For simplicity, it was assumed that women and men with the same T-score would have the same risks of fracture.
If a patient were taking a bisphosphonate, the effect on hip fractures was assumed to be a relative risk of 0.72 (95% CI 0.58 to 0.88) from data reported in table 27 of Stevenson et al. 357 This effect was assumed to last for 5 years, with a linear wane in effect over a 5-year period, so that the relative risk was 1 after 10 years.
To ‘death through fracture or non-fracture related causes’
The mortality rate associated with hip fracture was taken from table 21 of Stevenson et al. ,357 who report that an estimated 6% of people aged 70–79 years living in the community die from causes related to a hip fracture in the year of fracture. Corresponding figures for patients aged 80–89 years and 90 years or over were 11% and 16%, respectively. For simplicity, it was assumed that all patients lived in the community prior to the osteoporotic VCF.
The mortality rate associated with vertebral fracture was taken from a UK study360 comparing mortality in those with osteoporosis (and no fracture) with mortality in those with osteoporosis and a previous clinically apparent vertebral fracture. The HR was 4.4 (95% CI 1.85 to 10.6) and was used in the model to inflate the underlying death rate through other causes. The number of years for which a vertebral fracture was assumed to affect mortality rates was user defined with a base-case estimate of 5 years. The effect then linearly dissipated across a user-defined period (5 years in the base case). When a patient was simulated to have a subsequent vertebral fracture, the model had the facility to allow an increase risk of mortality in the year of subsequent fracture in accordance with data from Jalava. 360 Any effects in subsequent years were not incorporated to limit the number of health states required.
It was assumed that the mortality rate following hip fracture could not be lower than either the mortality rate associated with a vertebral fracture or that of general mortality in the underlying age- and sex-matched population. In such circumstances the rate of mortality following hip fracture was increased to equal the higher value.
The underlying death rate through other causes than fracture was taken from the Office of National Statistics’ Interim Life Tables. 361 For simplicity, it was assumed that all patients would die in their 101st year.
There has been published evidence that mortality may be influenced by initial procedure213 and further data have been provided in the submission by Medtronic. 34 This is critiqued in Appendix 13 . Where BKP, PVP or OPLA were assumed to have positive mortality effects compared with non-invasive management (NIM), these were incorporated in the model for a user-defined period (set to 5 years in the base case). It was assumed that mortality benefit does not wane in a linear fashion, but would cease immediately after the user-defined period. The relative risks associated with treatment were assumed to apply to the all-cause mortality rates and to the increase associated with vertebral fractures, but not to the value following hip fracture.
Transition probabilities from ‘patient sustains an additional vertebral fracture and remains alive’
To ‘patient sustains an additional vertebral fracture and a hip fracture and remains alive’
It was assumed that the risk of hip fracture for patients was independent of whether or not the patient was simulated to have a subsequent vertebral fracture. Therefore, the methodology for calculating the risk of hip fracture was identical to that between the ‘post-osteoporotic vertebral compression fracture following initial treatment decision’ and the ‘patient sustains an additional vertebral fracture and remains alive’ states.
To ‘death through fracture or non-fracture related causes’
The methodology for calculating the risk of mortality from fracture and non-fracture causes is identical to that from the ‘post-osteoporotic vertebral compression fracture following initial treatment decision’ state.
Transition probabilities from ‘patient sustains a hip fracture and remains alive’
To ‘patient sustains an additional vertebral fracture and a hip fracture and remains alive’
It was assumed that the risk of vertebral fracture for patients was independent of whether or not the patient was simulated to have sustained a hip fracture. Therefore, the methodology for calculating the risk of hip fracture was identical to that between the ‘post-osteoporotic vertebral compression fracture following initial treatment decision’ and the ‘patient sustains an additional vertebral fracture and remains alive’ states.
To ‘death through fracture or non-fracture related causes’
The methodology for calculating the risk of mortality from fracture and non-fracture causes is identical to that from the ‘post-osteoporotic vertebral compression fracture following initial treatment decision’ state.
Transition probabilities from ‘patient sustains an additional vertebral fracture and a hip fracture and remains alive’
To ‘death through fracture or non-fracture related causes’
The methodology for calculating the risk of mortality from fracture and non-fracture causes is identical to that from the ‘post-osteoporotic vertebral compression fracture following initial treatment decision’ state.
Analyses of the mortality effects associated with the interventions
A stand-alone critique of the data and methodology used to indicate a mortality benefit associated with PVP and BKP is provided in Appendix 13 . This concludes that ‘it is possible that there is a causal difference in mortality between patients treated using OPM and OP patients given the size of the effect. Appropriately taking into account the potential endogeneity of the treatment would tend to reduce the point estimate of the effect size but may or may not eliminate it completely. It is not possible to say with certainty if there is a difference in mortality between patients undergoing BKP and PVP owing to the treatment based on the data presented in the studies included here. There is also considerable uncertainty, were BKP and PVP assumed to have a mortality benefit, in whether or not OPLA would also produce a mortality benefit. However, there were no data on this.
Given that there is considerable uncertainty regarding whether or not there is a mortality effect, it was deemed prudent to explore three scenarios: that BKP had the greatest effect, followed by PVP and then OPM; that BKP and PVP had the same effect which was beneficial compared with OPM; and that BKP, PVP and OPM had the same long-term mortality outcomes. The effectiveness of OPLA was varied in sensitivity analyses. The evidence that was deemed most appropriate was taken from the Cox regression performed on the osteoporotic VCF group who had survived beyond 1 year that was reported in the Exponent214 report contained in the Medtronic submission. 34 These values are contained in Table 52 , and have been inverted compared with the Exponent report to compare each treatment with OPM rather than presenting OPM compared with each treatment.
| Hazard ratio (95% CI) | ||
|---|---|---|
| Scenario | BKP | PVP |
| Differential effects | AiC information has been removed | AiC information has been removed |
| Pooled effects | AiC information has been removed | AiC information has been removed |
| No effect | 1 | 1 |
Data regarding the effect of OPLA on mortality were not available. For initial analyses, it was assumed that the effect was half of that observed for PVP, as this was observed with VAS data (detailed later) which equated to a HR of (academic-in-confidence information has been removed) when a differential effect was assumed, (academic-in-confidence information has been removed) when a pooled effect was assumed and 1 when no effect was assumed. The effect of OPLA on mortality was adjusted in sensitivity analyses to acknowledge the arbitrary value used in the initial analyses.
Analyses of the visual analogue scale scores associated with the interventions
Each trial presented results in terms of VAS scores. These are shown graphically, by intervention, in Figures 8 to 13 . From a visual inspection it appeared plausible that the underlying VAS scores had stabilised at 1 month post intervention for patients treated with PVP, BKP and sham. However, for OPM the time to stability appeared longer than for PVP, BKP and OPLA, and was assumed to be 3 months. It was assumed that the VAS scores would be independent of the type of cement used in the procedure.
It is assumed that the VAS score following an active intervention remains constant until either the patient moves to another health state or the utility mapped from this value (see later) is greater than the underlying population norm value at the patient’s age adjusted for the impact of a vertebral fracture (assuming that a vertebral fracture was associated with an ongoing utility multiplier of 0.909 as detailed in table 24 of Stevenson et al. ). 357 In the latter circumstance, the utility was set equal to the adjusted population value for the given age. The assumed values for the utilities of the population were taken from the mean values reported by Ara et al. 353 The rationale for choosing a constant utility was an analysis of the VAS scores data for the active interventions which is shown in Figures 8 to 13 . The results for each trial are depicted in Appendix 12 .
Additionally, it was assumed that eventually the utility would be the same regardless of treatment. On a combination of clinical advice and data from the Farrokhi et al. 135 and FREE137 trials, which showed a differential persisting beyond 1 year, it was assumed that at 2 years the VAS score difference (and hence mapped utility difference) between different treatments would begin to converge in a linear fashion, such that at 3 years post osteoporotic VCF the VAS scores for all treatments would be equal to the VAS score for the treatment generating the greatest patient benefit.
This methodology is illustrated in Figure 22 using the following assumptions: that there are two treatments (PVP and NIM); a starting VAS score of 7.5; that the stable VAS score for PVP was 3.0 while the stable score for NIM was 4.0 and that no further events have happened at 5 years post osteoporotic VCF.
FIGURE 22.
An illustrative example of methodology regarding VAS scores post intervention.
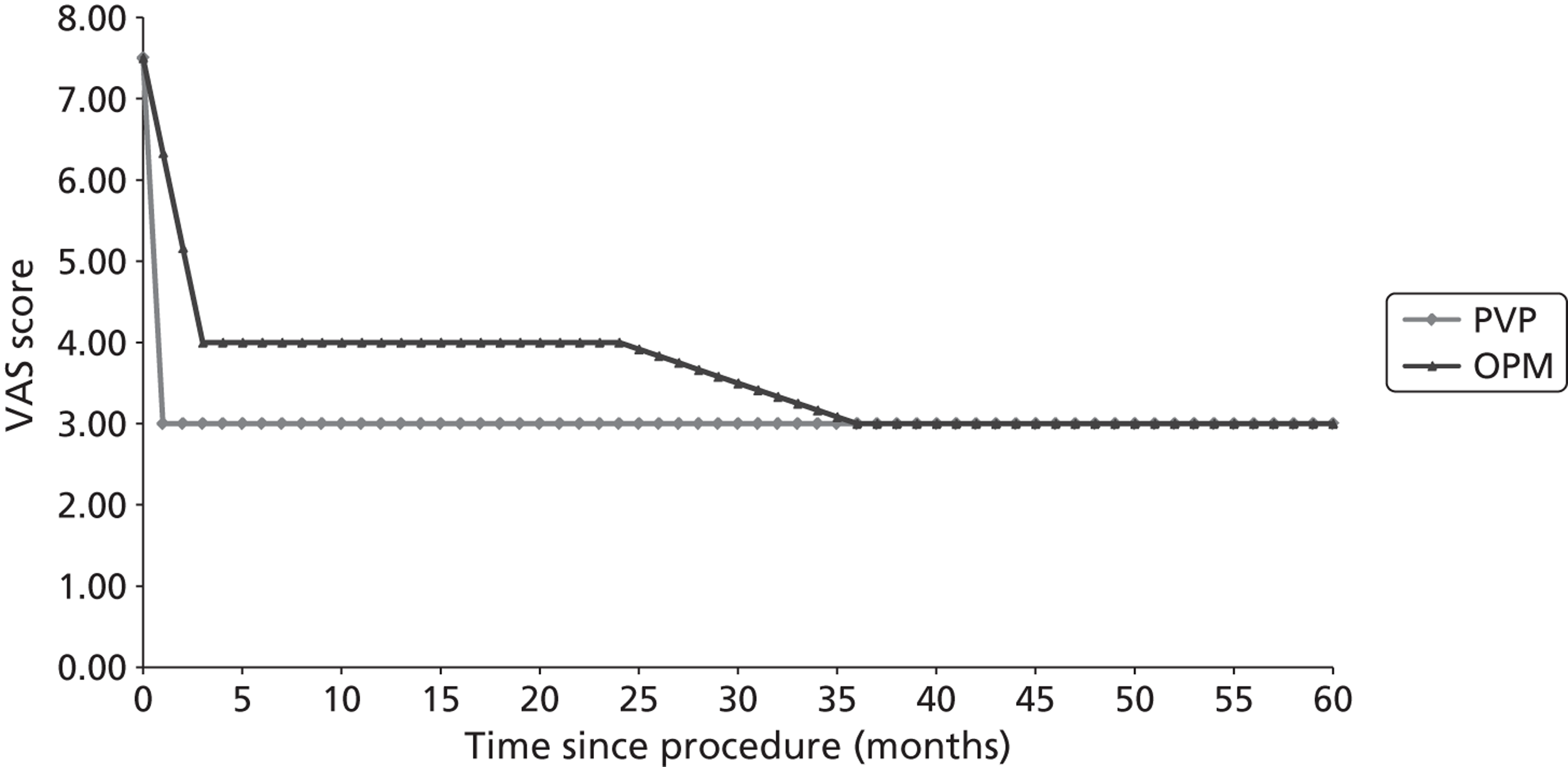
Estimation of the initial visual analogue scale scores of patients within the studies
The initial VAS data from both arms of each trial were analysed using WinBUGS (MRC Biostatistics Unit, Cambridge, UK) to estimate the likely distribution of initial VAS scores for similar patient populations. The CODA output from WinBUGS was used within the model; however, summary statistics are provided for the reader. The mean was a VAS of 7.36, with the 2.5th percentile and the 97.5th percentile values being 6.18 and 8.53 respectively.
Estimation of the stable visual analogue scale scores for the interventions
The estimated stable VAS scores, which were assumed to occur at 1 month post operation for PVP, BKP and OPLA and at 3 months post ‘treatment’ for OPM, were calculated in WinBUGS. A stand-alone report on this process (by Dr Sofia Dias and Professor Tony Ades) is contained in Appendix 14 , with key messages summarised within the main text. Within this process it was assumed that all data were not confounded. The level of crossover is reported in Table 6 . In three trials the crossover rate was more than 25% in an arm. These were VERTOS,136 where data were not used to estimate the stable VAS; INVEST,103 where only data at 1 month were used as crossover was prohibited up to this point; and Farrokhi et al. ,135 in which 26% of patients crossed over from control to PVP. In all other trials the crossover rate was below 25%. The failure to control for crossover is likely to result in a bias against vertebral augmentation; however, there was insufficient evidence to allow robust adjustments.
Investigation of the appropriateness of assuming a stable visual analogue scale score
Analyses were undertaken to ascertain if the assumption that the stable VAS score was independent of initial VAS score was appropriate. Figure 23 provides data on the initial VAS and the simple average of VAS scores within the stable period, defined as 1 month and beyond for BKP, PVP and OPLA, and 3 months and beyond for OPM. In order to allow the graphs to be presented, the academic-in-confidence VAS data from Buchbinder et al. 102 have not been shown. The exclusion of these data did not alter the broad conclusions.
FIGURE 23.
The relationship between initial VAS score and stable VAS score.
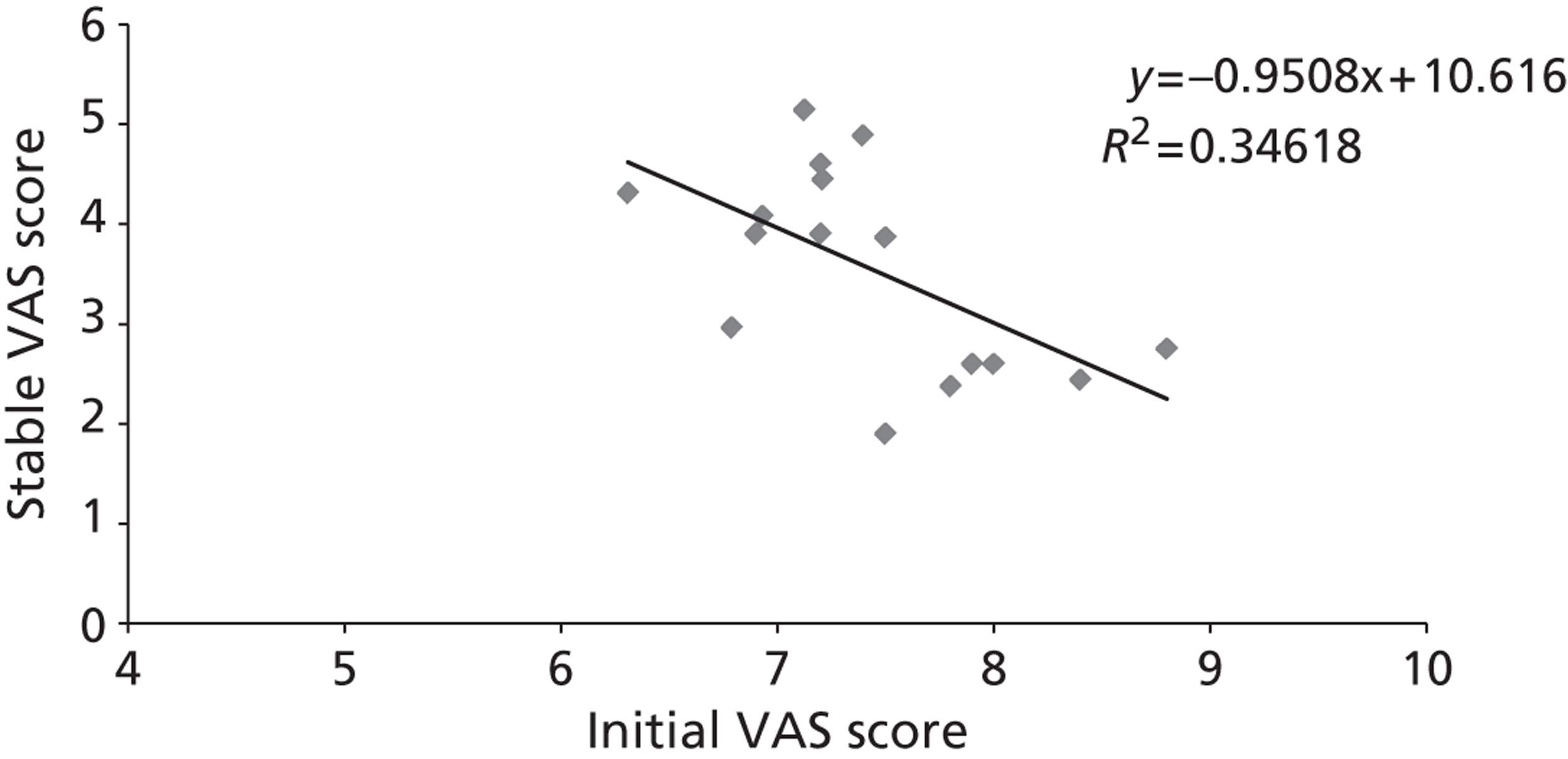
A moderate relationship was shown between initial VAS score and stable VAS (R 2 = 0.346). However, it was seen that the greater the initial VAS score, the lower the stable VAS score: it is unclear whether this is caused by fractures causing more pain being more responsive to treatment; whether there are psychological aspects and that the same pain is rated differently if the preceding pain was worse; or whether the relationship observed is through chance. This conclusion held when analysing the initial VAS score against last VAS score recorded (data not shown)
Further analyses were undertaken to ascertain whether or not the potential relationship between initial VAS score and the stable VAS score could bias the results. An analysis of the difference in the average VAS (the mean of the two arms) at the start of a trial and the difference in stable VAS scores is presented in Figure 24 .
FIGURE 24.
The relationship between the mean initial VAS score and the difference in the stable VAS score.
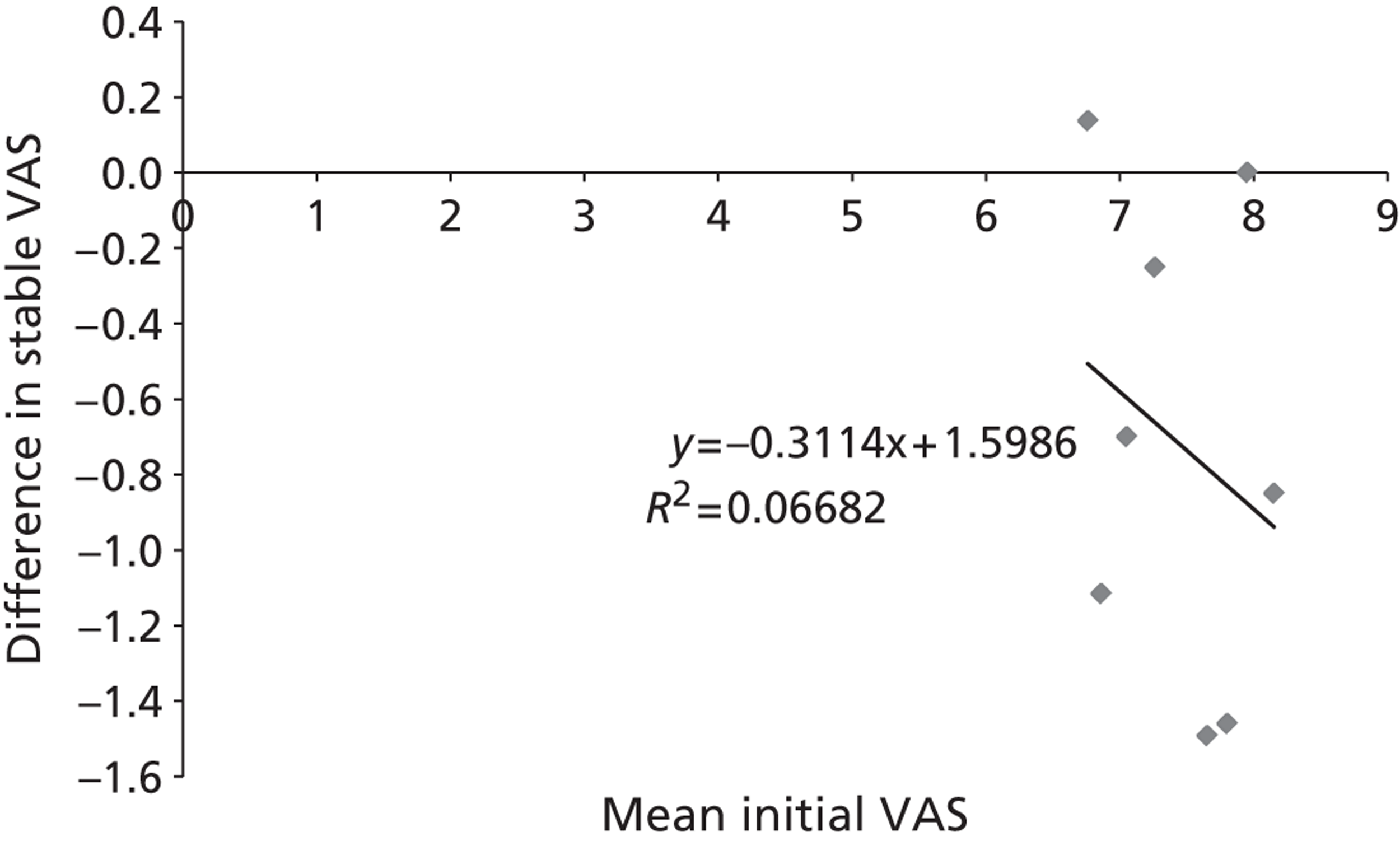
This indicates that there was largely little correlation between the difference in the stable VAS and initial VAS values (R 2 = 0.06). However, these data could be confounded by the different intervention being compared, and so a repeat analysis using only the PVP versus OPM trials was conducted ( Figure 25 ). This showed a better fit (R 2 = 0.53) but it is unclear the effect that a smaller number of data points has had on this (by definition, a regression of only two data points would have a R 2 value of 1).
FIGURE 25.
The relationship between the mean initial VAS score and the difference in the stable VAS score for PVP vs. OPM trials only.
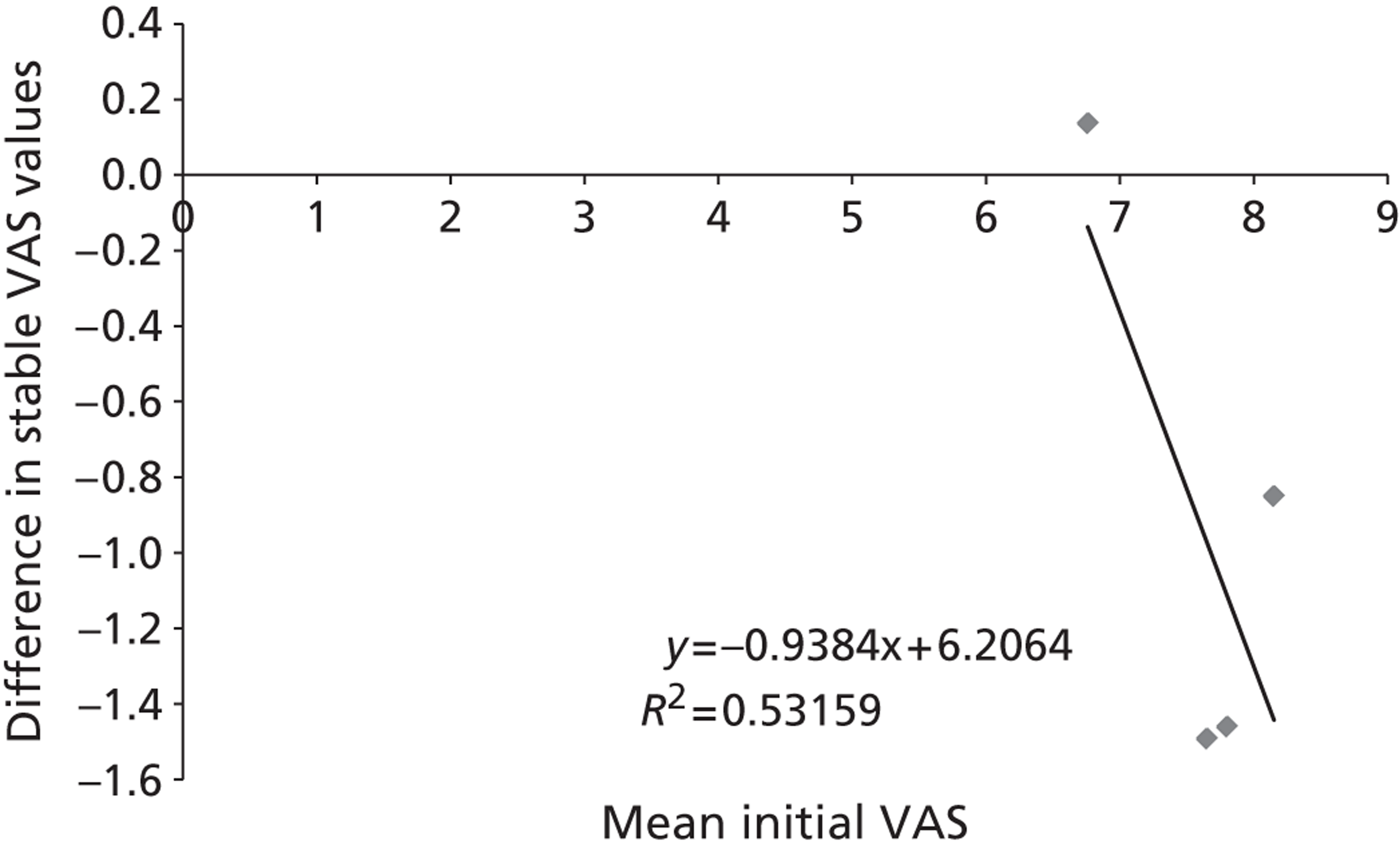
Therefore, an analysis of the difference in the initial VAS values between the arms of the trial and the difference in the stable VAS values was undertaken. This is shown in Figure 26 .
FIGURE 26.
The relationship between the difference in the initial VAS score and the difference in the stable VAS score.
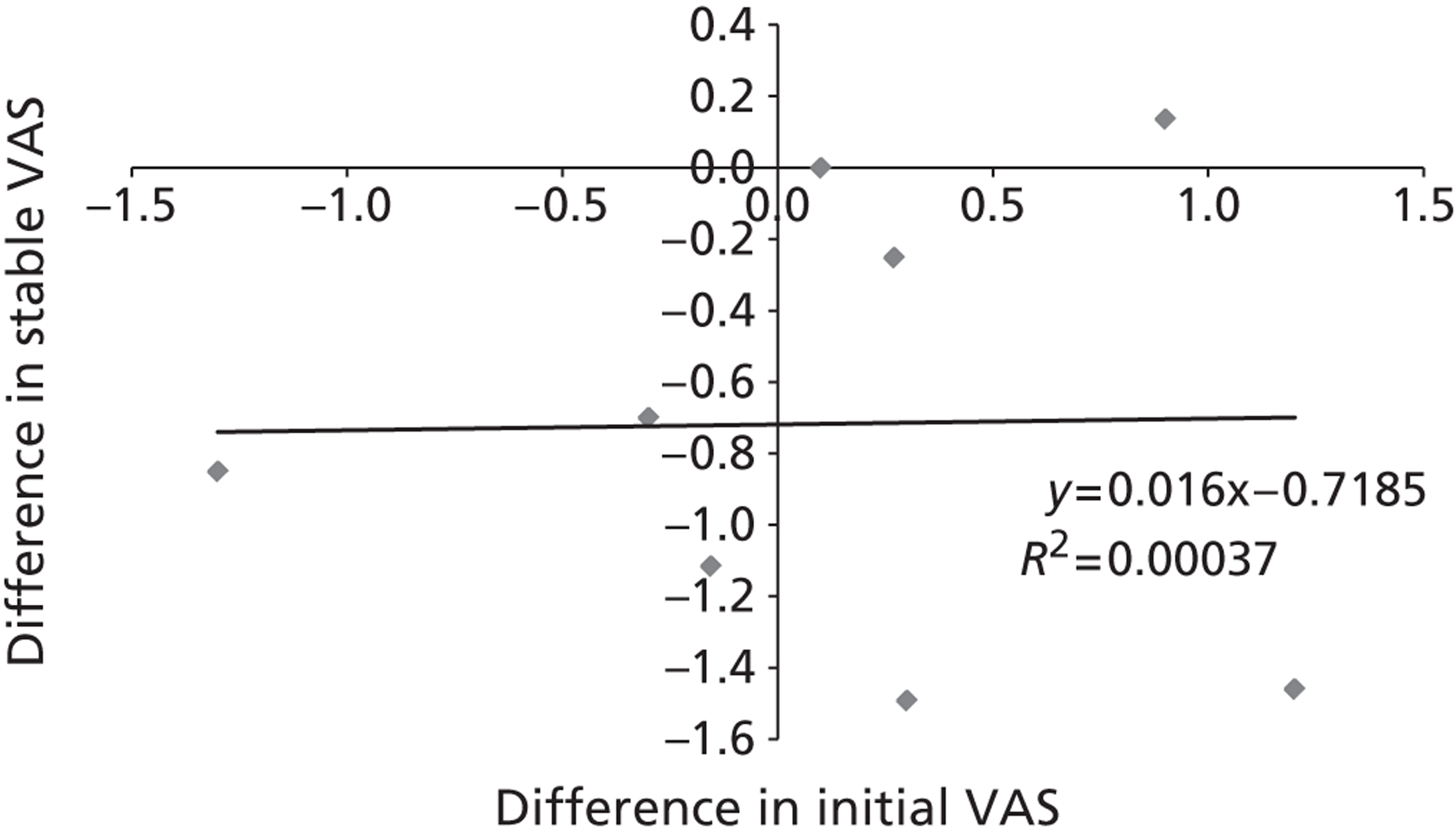
This indicates that there was largely little correlation between the difference in the stable VAS and initial VAS values (R 2 < 0.001). Similar conclusions were drawn when analysing only the PVP compared with OPM trials ( Figure 27 ).
FIGURE 27.
The relationship between the difference in the initial VAS scores and the difference in the stable VAS scores using only the OPM vs. PVP trials.
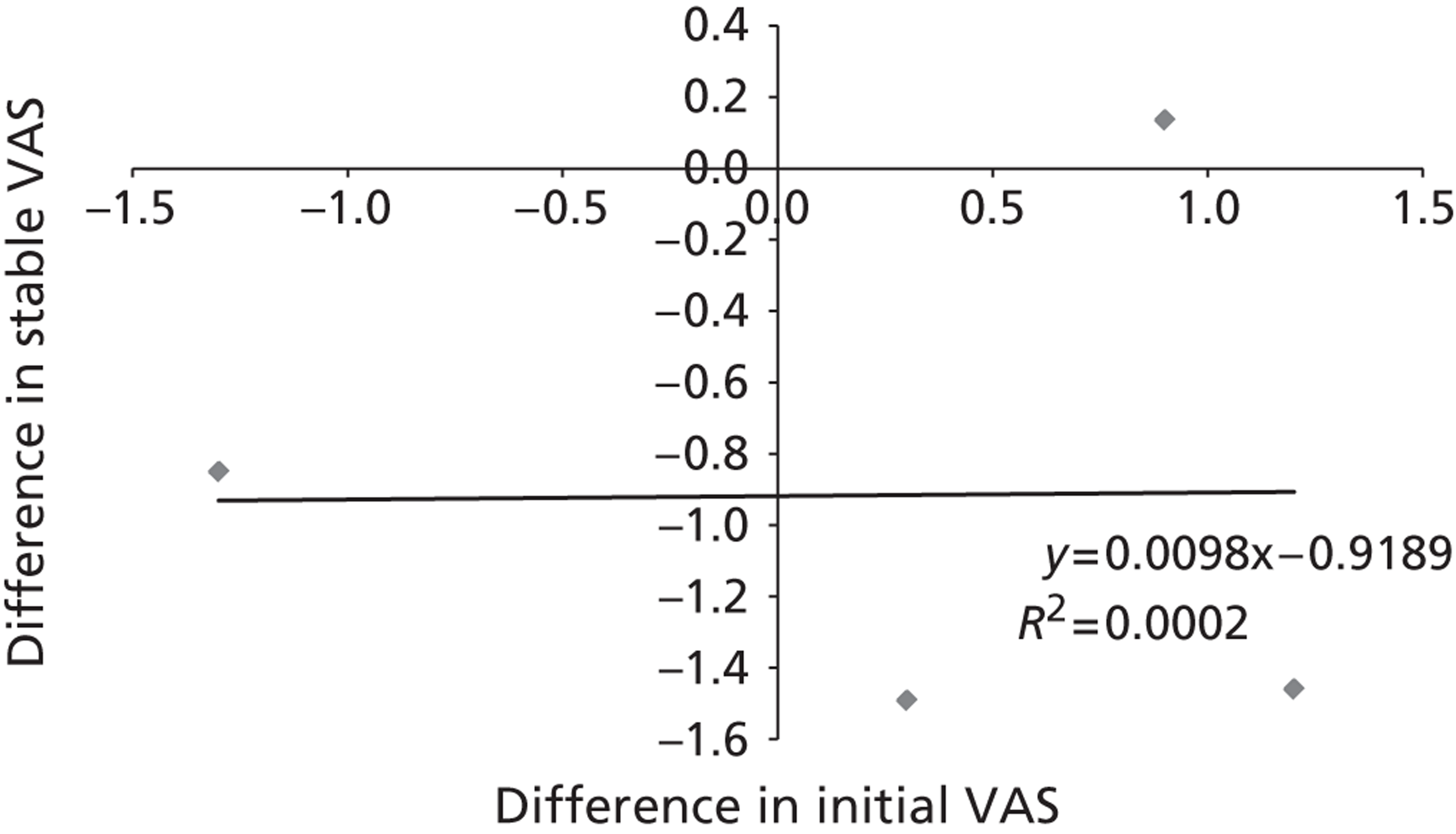
The authors of this report believe that given the analyses undertaken (see Figures 25 – 29 ) there appears to be little bias introduced by assuming a stable VAS score which is not dependent on the initial VAS score.
Results from the network meta-analyses
The network of evidence is depicted in Figure 28 , with the WinBUGS output depicted in the form of a caterpillar plot in Figure 29 . For a full discussion of the methods used, see Appendix 14 . Note that there were considered too few trials (four) to undertake meta-regression on the four treatments.
FIGURE 28.
The network of evidence regarding VAS scores.
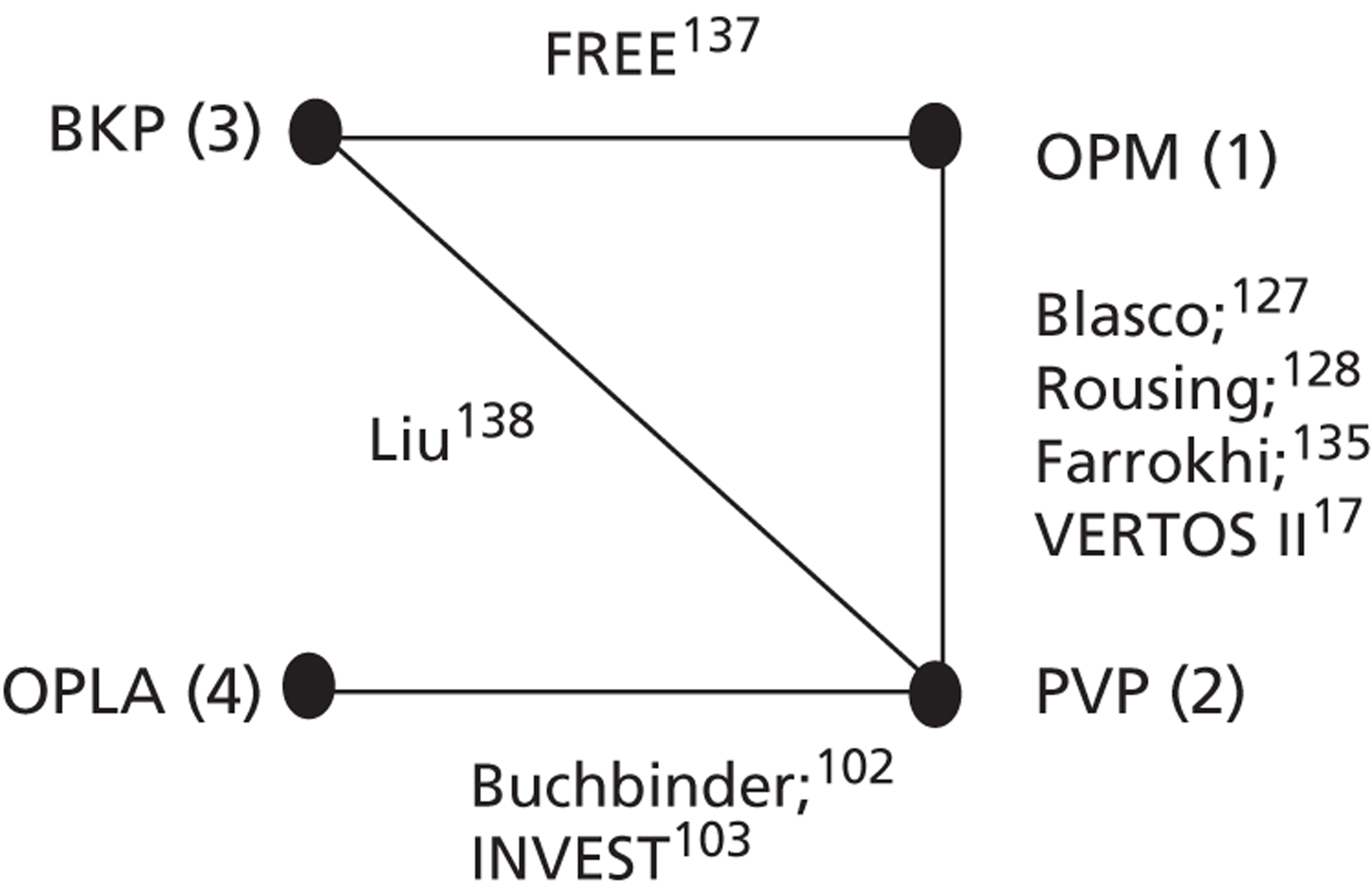
FIGURE 29.
The relative VAS scores.
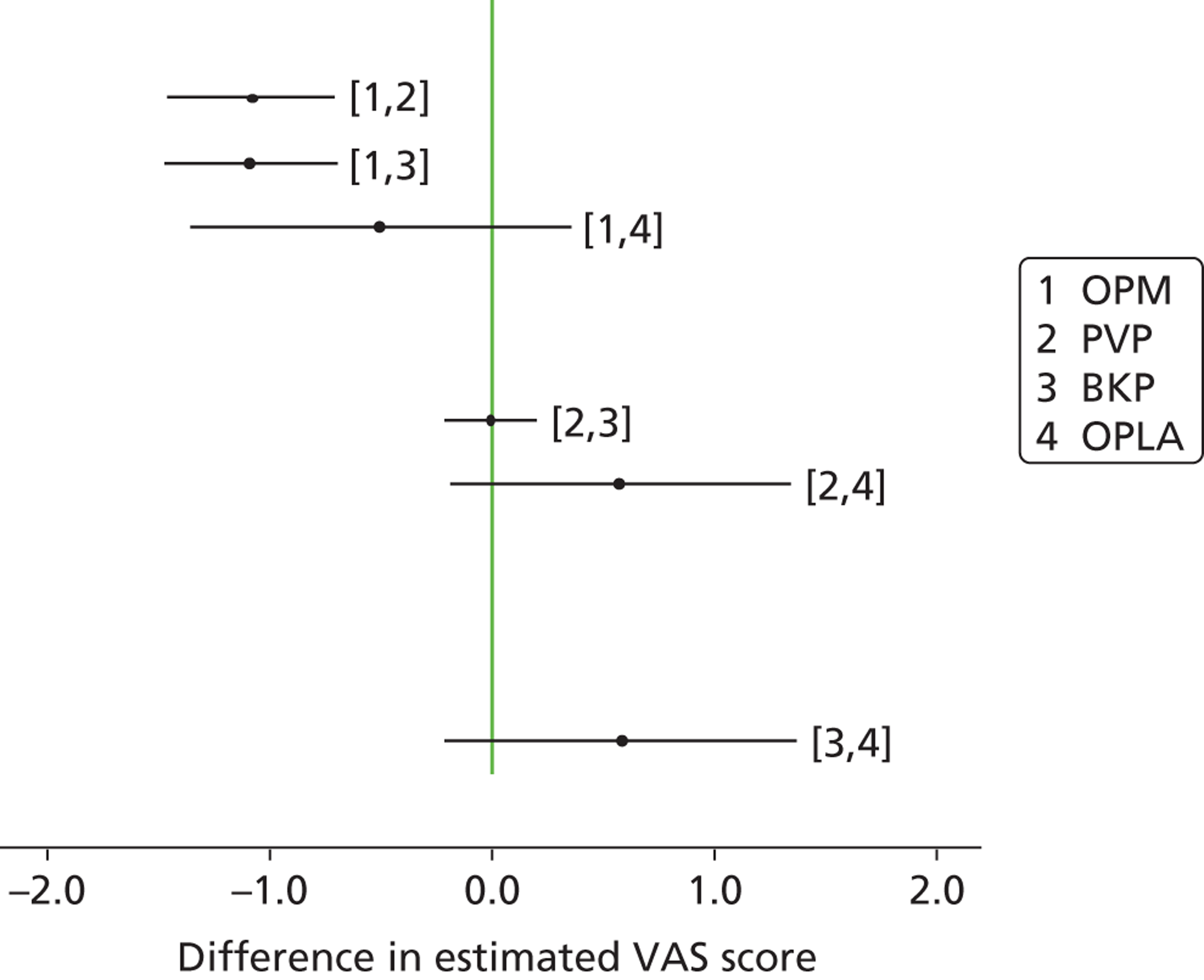
Within Figure 29 , 1 denotes OPM, 2 denotes PVP, 3 denotes BKP and 4 denotes OPLA. Values to the left of the no-effect line indicate that the higher numbered intervention produces a lower VAS score.
Thus, a summary of the results estimated by the network meta-analyses of VAS scores is as follows: BKP and PVP appear to be the best treatments with very little difference between them; OPM is significantly worse than both BKP and PVP; there is a possibility that OPLA may produce equivalent results to BKP and PVP or equivalent results to OPM. However, as with standard meta-analyses, the quality of the evidence should be appraised, and it is stressed that only two of the trials are of the highest standard (Buchbinder et al. 102 and INVEST103). These trials also recorded EQ-5D data which indicate that, with respect to change in EQ-5D from baseline, OPLA was equal or marginally inferior to PVP. The implications of this caveat are explored within the results produced by the assessment group by undertaking multiple analyses, as detailed later.
Mapping analyses
Only a few studies incorporated the EQ-5D, which is the metric recommended in NICE’s reference case. 356 To meet the reference case, a mapping between an alternate metric and the EQ-5D was required. The initial mapping used VAS scores as all of the studies included incorporated a measure of pain using the VAS score. However, as detailed in the clinical section, VAS scores are subjective and may be confounded. Owing to this, an analysis of the relationship between RDQ and EQ-5D was also conducted. As the data were provided only at an aggregated level, the mapping was undertaken using the mean values for VAS and RDQ scores and EQ-5D. Mapping has the advantage of incorporating data from all studies and thus will not discard data, although this will not be as precise as using EQ-5D directly from the trials. Analyses directly using the EQ-5D data reported in the trials have also been conducted and are described later.
Mapping between visual analogue scale and European Quality of Life-5 Dimensions
Data providing both EQ-5D and VAS scores were taken from the FREE study,137 Buchbinder et al. ,102 INVEST,103 VERTOS II17 and Rousing et al. 131 Data were obtained from the authors of the FREE study137 via personal communication (Professor Wardlaw, Aberdeen Royal Infirmary, 2012). The plot of absolute VAS and absolute EQ-5D is shown in Figure 30 and indicates a relatively good fit, with a R 2 of 0.62. The resultant formula was EQ-5D = 0.8053 – 0.0674 × VAS. The variance on the intercept was 0.00216 and the variance on the slope was 0.00008 with a covariance of –0.00038, with these values used in probabilistic sensitivity analyses.
FIGURE 30.
A plot of absolute VAS vs. absolute EQ-5D.
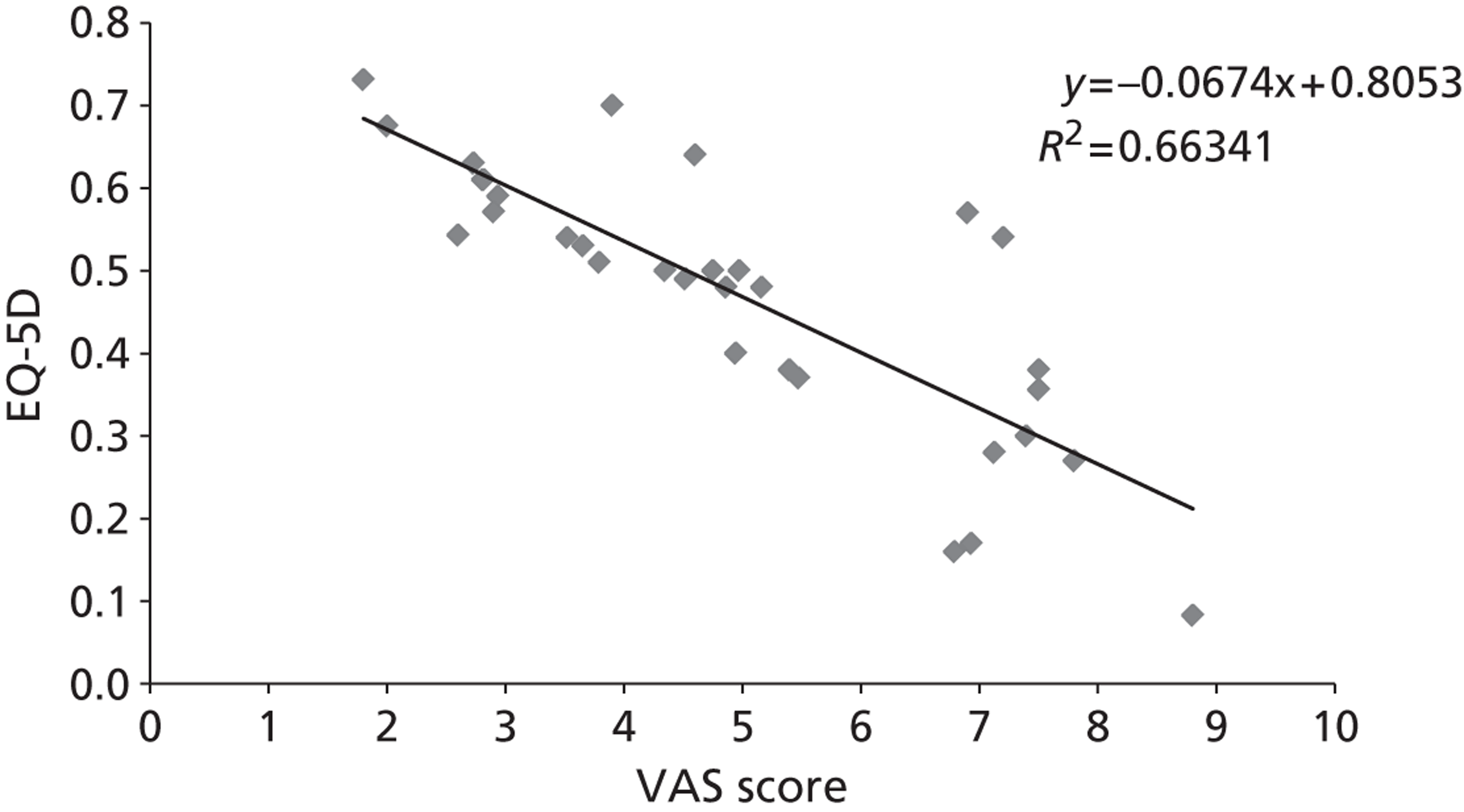
A further analysis was undertaken removing the Rousing et al. 131 and VERTOS II17 studies, as these had used a continuous VAS scale whereas the remainder of studies had used a numeric rating scale. However, this reduced the explanatory power of the fit (R 2 = 0.50) and the full data set was used, assuming that the continuous VAS scale and the numeric rating scale were interchangeable.
It was possible that the four points considerably above the line were potentially outliers as they all came from the same study (INVEST). 103 If these data were not included in the mapping, as shown in Figure 31 the fit improved considerably (with a R 2 of 0.86). The resultant formula was EQ-5D = 0.8392 – 0.0722 × VAS. The variance on the intercept was 0.00095 and the variance on the slope was 0.00003 with a covariance of –0.00017, with these values used in probabilistic sensitivity analyses.
FIGURE 31.
A plot of absolute VAS vs. absolute EQ-5D excluding INVEST data.
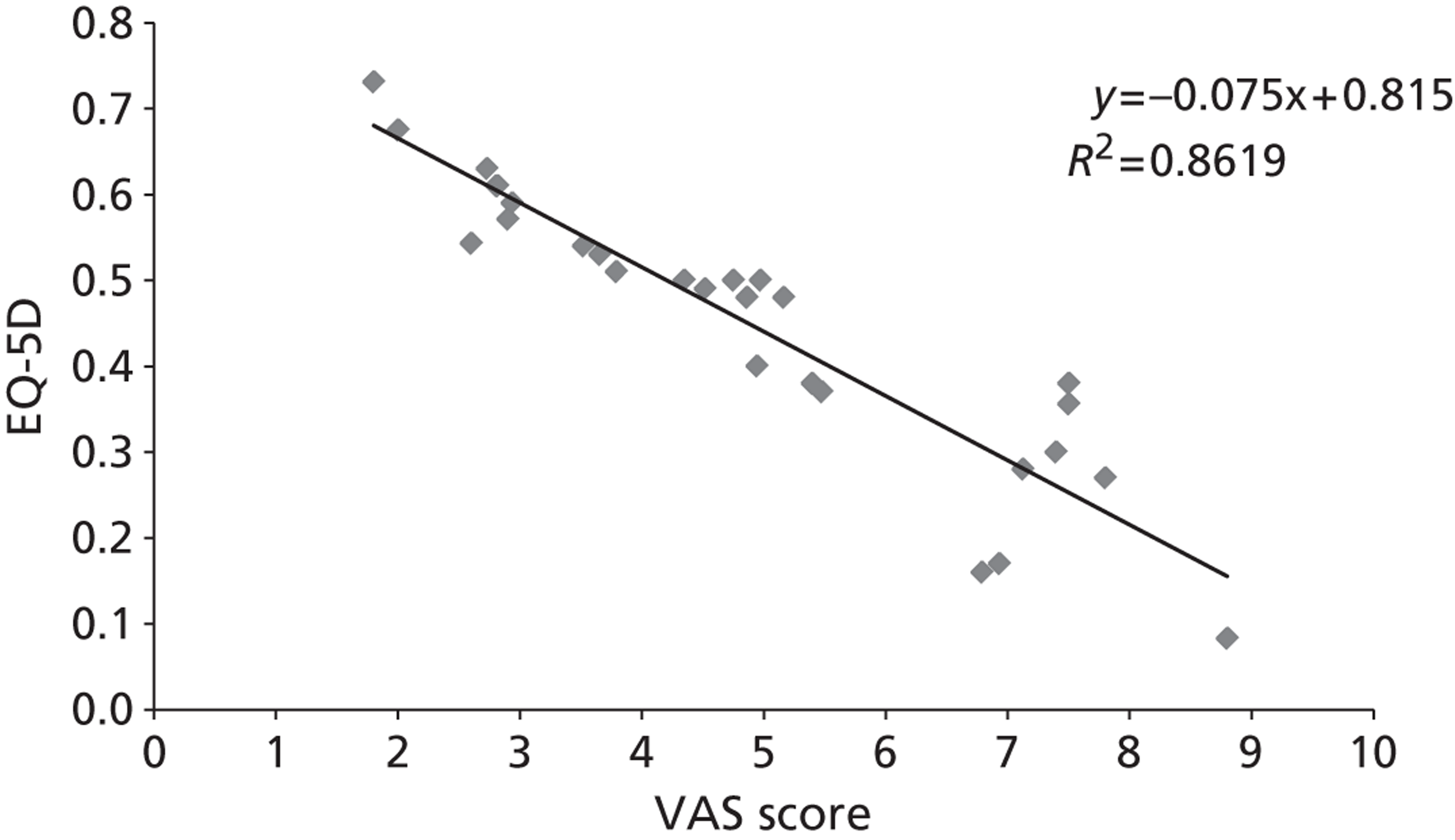
Mapping of Roland–Morris Disability Questionnaire onto European Quality of Life-5 Dimensions
Figures 32 and 33 show the relationship between RDQ and EQ-5D depending on whether or not INVEST data were included. The R 2 values were 0.55 using the full data and 0.84 excluding the INVEST data.
FIGURE 32.
A plot of absolute RDQ vs. absolute EQ-5D.
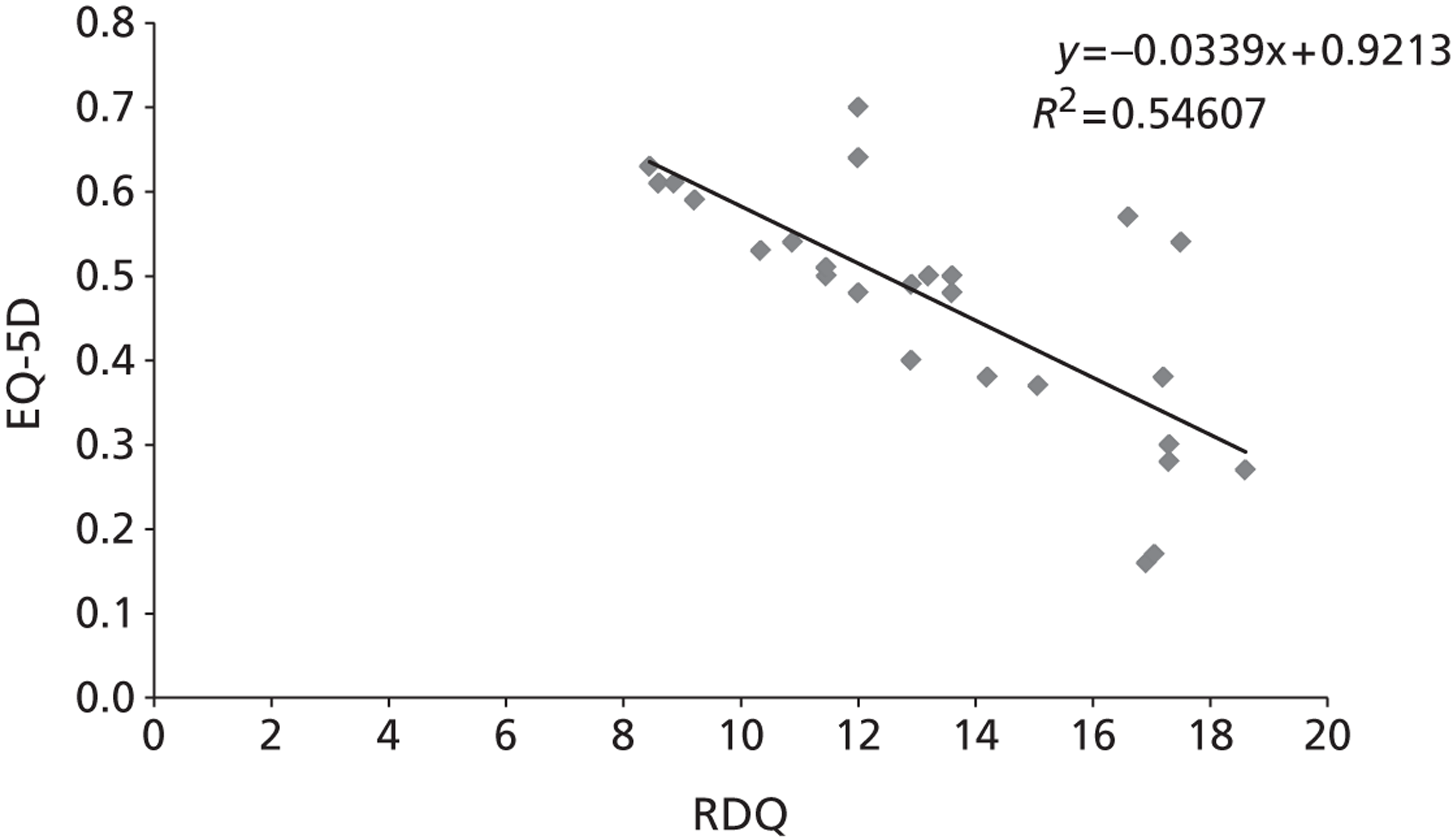
FIGURE 33.
A plot of absolute RDQ vs. absolute EQ-5D excluding INVEST data.
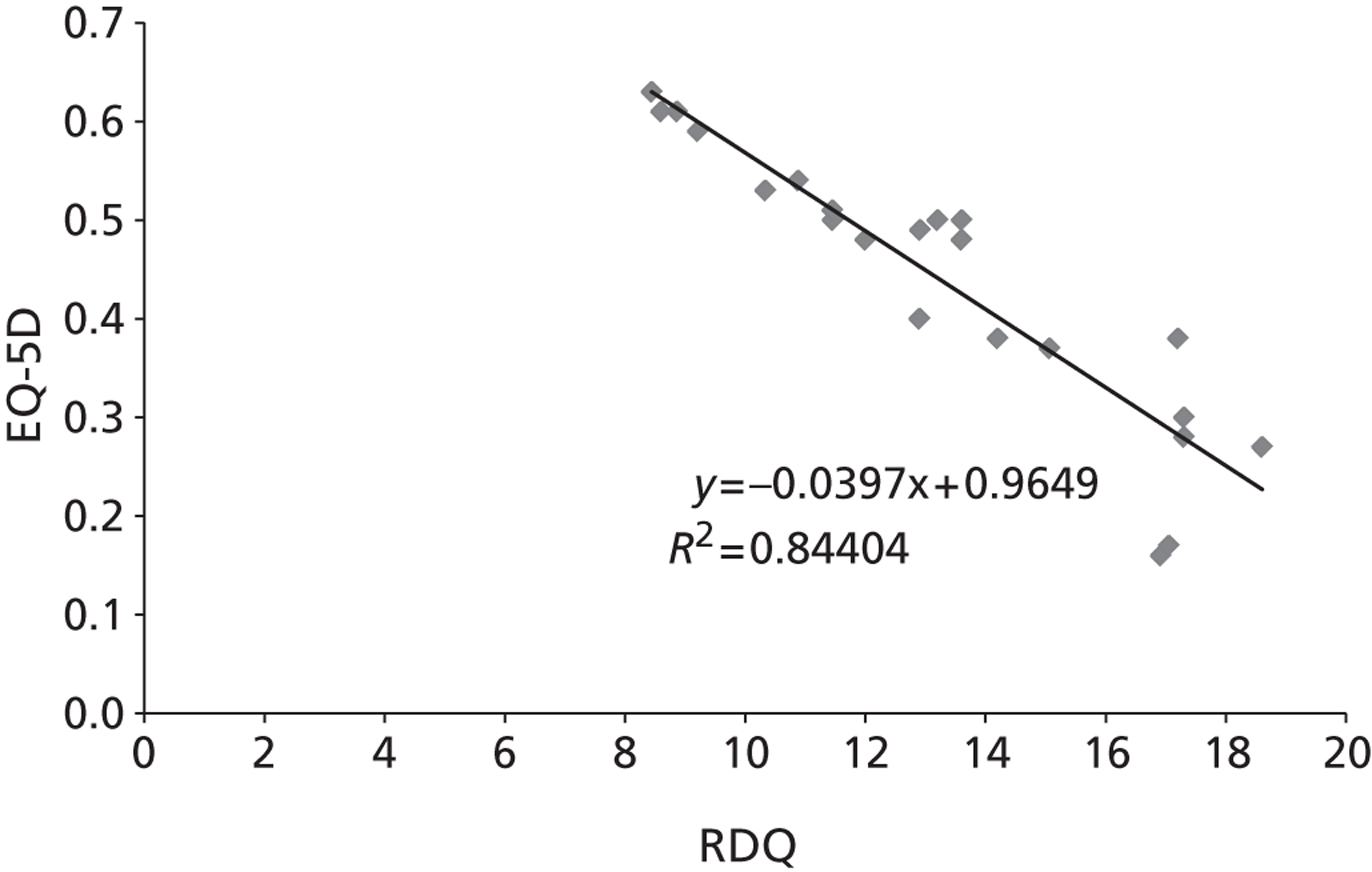
Choice of mapping methodology used
It was seen that the R 2 values were greater in the VAS and EQ-5D regression than in the RDQ and EQ-5D regression. Given that all studies reported VAS scores while not all studies reported RDQ, it would be preferable to assume a mapping from VAS to EQ-5D rather than from RDQ to EQ-5D.
Both statistical relationships (with and without the INVEST103 data) were used in the modelling to test the robustness of the results to the choice of fit. It is commented that the better fit without the INVEST data does not mean that this trial should be excluded; it could be that the mapping between VAS and EQ-5D does in reality contain noise, and it is noted that the INVEST data were removed after evaluating the initial regression. It is further commented that the mapping has been undertaken assuming that all points from all trials have been given the same weight regardless of study quality.
Thus, the VAS score presented in Figure 22 could be converted to an EQ-5D score. For illustrative purposes it is assumed that the deterministic value applies (using all data) and thus EQ-5D = 0.8053 – 0.0674 × VAS, and this is represented in Figure 34 . It is assumed that the predicted EQ-5D scores are lower than those of the age- and sex-mixed population and thus need no adjustment. The area under the curve would then equate to the QALYs accrued post treatment. In this example there would be undiscounted QALY values of 3.00 for PVP and 2.82 for OPM in the initial 5-year period.
FIGURE 34.
An illustrative example of the conversion of VAS scores to EQ-5D.
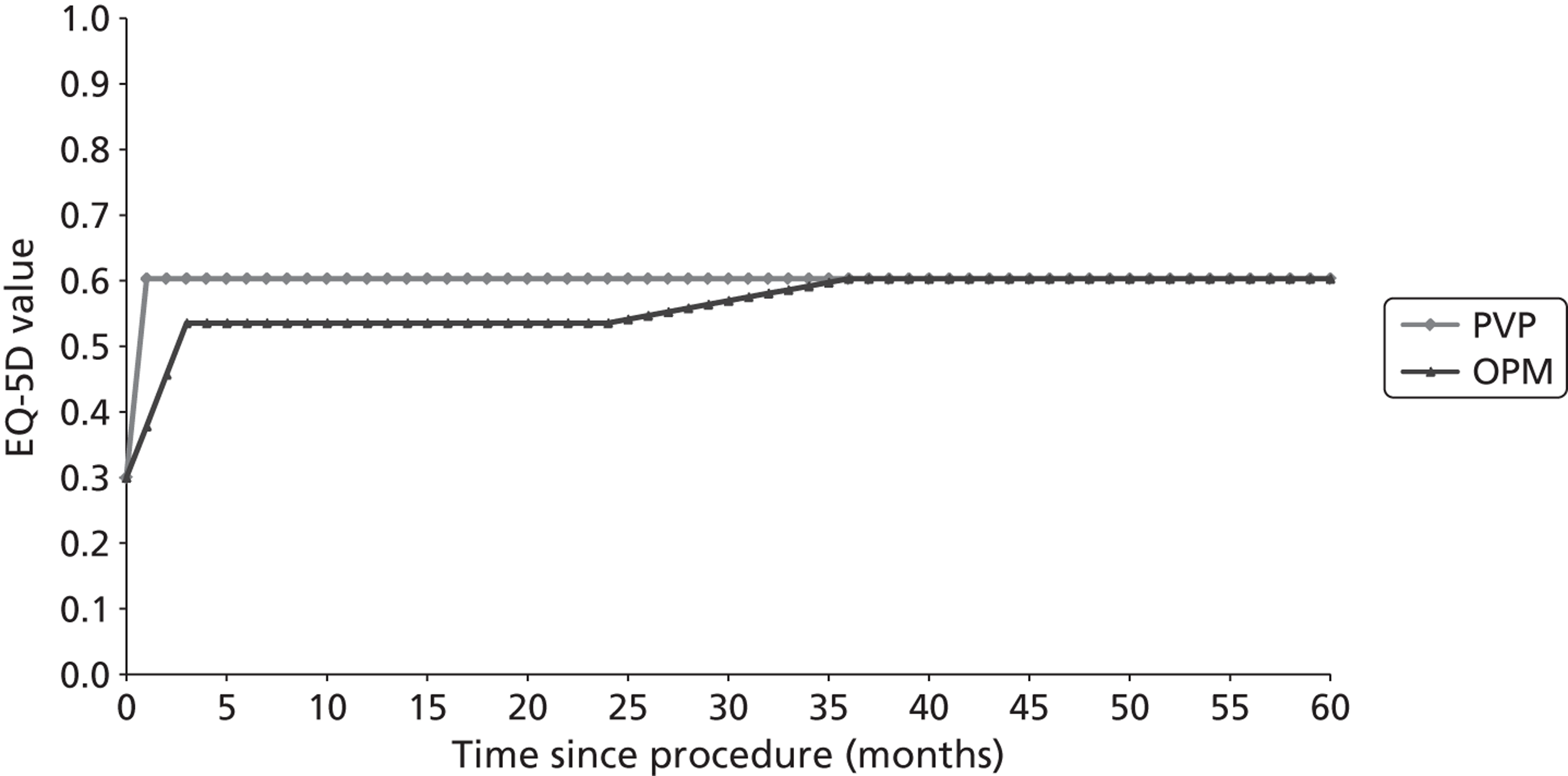
Utility values
The assumed utility within each health state
The utilities within each state are dependent upon a multitude of factors that are detailed for each health state.
Post-osteoporotic vertebral compression fracture following initial treatment decision
The utility of the patient is assumed to be a function of patient sex; patient age; the procedure undertaken (NIM, PVP, BKP or OPLA); the time since the procedure; the time at which patients treated with NIM were assumed to have the same utility as patients treated with an active intervention (PVP, BKP or OPLA); the disutility associated with vertebral fractures that occurred more than 1 year ago and the mapping of VAS scores onto the EQ-5D.
Patient sustains an additional vertebral fracture and remains alive
In addition to the factors that influence the utility of a patient in the ‘post-osteoporotic VCF following initial treatment decision’ state, the disutility associated with a vertebral fracture in the year of occurrence is considered relevant.
In the cycle of the subsequent vertebral fracture, a QALY decrement is automatically applied to account for the associated pain. This QALY decrement is calculated based on the assumed multiplier in the year of the fracture (0.626), the assumed multiplier in subsequent years (0.909)357 and the estimated utility score for the patient if no further events had occurred. The patient utility is assumed to be the lower of two values: the population value matched by age and sex, modified by the prevalent vertebral fracture, and the utility following the initial osteoporotic VCF as depicted in Figure 34 . As an illustration, were a patient to have an estimated utility of 0.5, then the QALY decrement would be assumed to be 0.206, calculated as 0.5/0.909 (the QALY expected in subsequent years following the vertebral fracture) × 0.626 (the QALY expected in the year of the vertebral fracture).
People within this state where the additional vertebral fracture occurred more than 1 year previously would have the population value matched by age and sex multiplied by 0.909 to take the prevalent vertebral fracture into account.
Patient sustains a hip fracture and remains alive
In addition to the factors that influence the utility of a patient in the ‘post-osteoporotic VCF following initial treatment decision’ state, the disutilities associated with a hip fracture in the year of occurrence and in subsequent years are considered relevant.
In the cycle of the hip fracture, a QALY decrement is automatically applied to account for the associated pain. This QALY decrement is calculated based on the assumed utility multiplier in the year of the fracture (0.792) and in subsequent years (0.813)357 and on the underlying utility scores for a patient of the same age and sex. This calculation uses the same methodology as for patients with a subsequent vertebral fracture who remain alive.
People within this state where the hip fracture occurred > 1 year previously would have the population value matched by age and sex multiplied by 0.813.
Patient sustains an additional vertebral fracture and a hip fracture and remains alive
In addition to the factors that influence the utility of a patient in the ‘post-osteoporotic VCF following initial treatment decision’ state, the disutilities associated with a vertebral fracture in the year of occurrence, with a hip fracture in the year of occurrence and in subsequent years, are considered relevant.
This state can be reached from two health states: either by sustaining an additional vertebral fracture following a hip fracture or by sustaining a hip fracture following an additional vertebral fracture. In the first route, a QALY decrement is applied using the methodology described for ‘patient sustains an additional vertebral fracture and remains alive’ with the population utility having been adjusted for a prevalent hip fracture. In the second route, a QALY decrement is applied using the methodology described for ‘patient sustains a hip fracture and remains alive’.
People within this state where the most recent fracture occurred more than 1 year previously would have the underlying population value multiplied by 0.909 (for the prevalent vertebral fracture) and by 0.813 (for the prevalent hip fracture).
Dead
By definition the utility within this health state is zero.
Additional cost and quality-adjusted life-year consequences associated with adverse events
The model includes the facility to allow a QALY decrement to be applied to take serious adverse events into consideration. These are calculated crudely based on the likely incidence of serious adverse events and the severity of each event and are subjected to sensitivity analyses. An initial analysis was conducted assuming that there were no cost or QALY implications of adverse events, with a sensitivity analysis conducted assuming that the QALY losses associated with BKP and PVP were 0.02. This value was estimated assuming that the rate of mortality was 1 in 1000 (with an assumed average loss of 10 discounted QALYs) and the rate of morbidity was 1 in 100 (with an assumed average loss of 1 discounted QALY). When summated this equated to 0.02 discounted QALYs. A threshold analysis is presented to estimate the additional QALY losses at which low-viscosity cement would have an equal net benefit to high-viscosity cement, assuming a threshold of £20,000 per QALY.
Costs
The assumed costs within each health state
This section focuses on the costs associated with each health state. The values within the health states have largely been taken from table 25 of Stevenson et al. 357 and inflated to 2010–11 prices using the Hospital and Community Health Services inflation indices reported by Curtis et al. 362
Post-osteoporotic vertebral compression fracture following initial treatment decision
It was assumed that ongoing costs following the initial vertebral fracture would equate to £229 per year.
Patient sustains an additional vertebral fracture and remains alive
The cost of a vertebral fracture was assumed to be £3081, assuming that all fractures occurred in people aged 70 years or over. This value includes a component for home help. The ongoing costs of £229 per annum associated with vertebral fracture are also continued.
Patient sustains a hip fracture and remains alive
The cost of a hip fracture was assumed to be £7536, assuming that all fractures occurred in people aged 70 years or over. This value includes a component for home help. For simplicity, it was assumed that no patients required nursing home care following a hip fracture, an assumption which is acknowledged to underestimate the costs of a hip fracture, although this is not expected to significantly affect the results. The ongoing costs of £229 per annum associated with vertebral fracture are also continued.
Patient sustains an additional vertebral fracture and a hip fracture and remains alive
This state can be reached from two health states either by sustaining an additional vertebral fracture following a hip fracture or by sustaining a hip fracture following an additional vertebral fracture. In the first route the costs are the same as for ‘patient sustains an additional vertebral fracture and remains alive’; in the second route the costs are the same as for ‘patient sustains a hip fracture and remains alive’. The ongoing costs of £229 per annum associated with vertebral fracture are also continued.
Dead
It was assumed that death carried no further cost.
Costs associated with the initial osteoporotic vertebral compression fracture
The costs associated with the initial osteoporotic VCF have been classified into three categories: the acquisition costs of the interventions, the costs associated with the operation and the costs associated with the length of stay.
The acquisition costs of the interventions
The cost of the Confidence Spinal Cement System™ was taken from the Johnson & Johnson submission,36 although it was assumed that 11 cc of cement was needed for a two-level procedure rather than 7 cc. This resulted in an average cost of £1546 per operation.
In addition to high-viscosity cement, low-viscosity cements are also available to purchase at prices that are lower than that of high-viscosity PMMA cement. The list price for such cements was obtained through NICE, and on clinical advice it was estimated that the costs using lower-viscosity cements, incorporating injection kit, needles, cement and assorted consumables, would be in the region of £660, £720 and £780 for one-, two- and three-level procedures respectively. When weighted for the proportion of operations that are one-, two- and three-level procedures, this would equate to an estimated value of £697. However, our clinical expert estimated that 15% of cases are more complex and would require Cortoss® cement, collation or thicker cement, while younger patients would need bone-absorbable cement. It was assumed that the added cost of these complex cases would add slightly over £100 to the average cost of an operation, resulting in an assumed cost of £800 per low-viscosity cement PVP procedure. Given that the estimate includes a component for using higher-viscosity cement, the price used within the analysis could be equated to a strategy where low-viscosity cement is used within the majority of patients, while higher-viscosity cements are used in a small proportion where the clinician believes that this is appropriate. Sensitivity analyses were undertaken on these average values.
The list price of BKP (£2600.50 per kit) has been inflated to take into consideration that a proportion of patients will require BKP at more than one level. On clinical advice it was assumed that the percentages reported for PVP were also applicable to BKP, an assumption also stated in the Johnson & Johnson submission. 36 On clinical advice it was assumed that each level would need an additional pack of Kyphon® HV-R® Bone Cement priced at £62 per pack, with the remaining instruments being reused. This resulted in the average price per patient increasing to £2639 for BKP. It is noted that this is noticeably less than the £4202 that would be predicted were a new kit required for each level as is implied in the Medtronic BKP brochure. It is commented that our value is significantly higher than that assumed by Medtronic as they did not use the list price but used the average selling price. The NICE Methods guide356 (section 5.5.2 in the guide) is clear that ‘analyses based on price reductions for the NHS will only be considered when the reduced prices are transparent and can be consistently available across the NHS, and if the period for which the specified price is available is guaranteed’ (p. 40). As such, only the list price is used.
The cost of OPLA treatment is contentious. The Medtronic submission34 did not consider OPLA to be a comparator. Johnson & Johnson submission36 did consider OPLA a comparator but assumed that the cost of this treatment was equal to that associated with PVP. Given the nature of the OPLA, the extent of any cost savings compared with vertebroplasty is uncertain. The impact of the potential cost savings has been evaluated within sensitivity analyses. Table 53 summarises the acquisition costs of the interventions assumed by the assessment group.
| Intervention | Assumed cost of intervention (£) |
|---|---|
| PVP – high viscosity | 1546 |
| PVP – low viscosity | 800 |
| BKP | 2639 |
| OPLA | To be explicitly considered in a sensitivity analysis – see text |
| OPM | 0 |
The costs associated with the operation
The costs of the preliminary phase, the operating phase and the postoperative phase have previously been reported in Ström et al. 31 and Johnson & Johnson reported that these prices were inflated. 36 However, there were discrepancies between the two submissions in the values reported (£1479 for Johnson & Johnson and £990 for PVP and £1013 for BKP in the Medtronic submission). Our clinical expert (DW) reviewed the values reported by Johnson & Johnson. While it was deemed there were discrepancies with current UK practice (e.g. in the description of the clinician seeing the patient; in the potential overuse of spinal radiographs; and in the fact that the operation would most likely take place in an interventional suite rather than an operating room), it was concluded that the prices were broadly correct for the preliminary and postoperative phases. Therefore, these were used by the assessment group. However, for the operating phase the expert was of the opinion that the bottom-up costs provided by Johnson & Johnson were a more realistic estimation than those of Ström et al. , and thus these were used, although these were marked as academic-in-confidence by the manufacturer. The costs used by the assessment group are summarised in Table 54 .
| Phase | Estimated cost (£) |
|---|---|
| Preliminary phase | 540 |
| Operating phase | 528 |
| Postoperative phase | 243 |
| Total cost | 1311 |
The costs associated with hospitalisation stay
The length of stay following each intervention
There appears to be considerable uncertainty regarding the lengths of stay associated with each intervention. It is noted that our clinical advisor was surprised by the values presented by both manufacturers (summarised in Table 55 ), commenting that the majority of interventions (PVP or BKP) are undertaken in Oxford as day procedures and that patients would not be admitted to hospital to have these interventions performed. This is further reinforced by the synthesis of data reported within the pivotal trials, which do not indicate a significant length of stay following any of the procedures.
| Intervention | Johnson & Johnson | Medtronic |
|---|---|---|
| PVP | 3.24 (0.49) | 6.2 |
| BKP | 4.48 (0.89) | 5.1 |
| OPM | 12.61 (0.27) | 9.5 |
It is commented that the manufacturer of PVP presented data showing that PVP was associated with shortest length of stay, whereas the manufacturer of BKP presented data showing that BKP was associated with the shortest length of stay.
The assumed hospitalisation costs per day
The manufacturers again present divergent results. Johnson & Johnson assumed a cost of £232 per day based on the payment by results national tariff price for an excess bed day associated with vertebroplasty/kyphoplastry and non-invasive management HRG codes (HRGs HC04C, HC05C and HD36C). 351 Medtronic assumed a cost of £457 per day for hospitalisation, citing NHS reference costs 2009/10/11. These values are summarised in Tables 56 and 57 . The different estimates of hospitalisation costs are depicted in Figure 35 .
FIGURE 35.
The different assumed hospitalisation costs within the manufacturers’ submissions and assessment group base case.
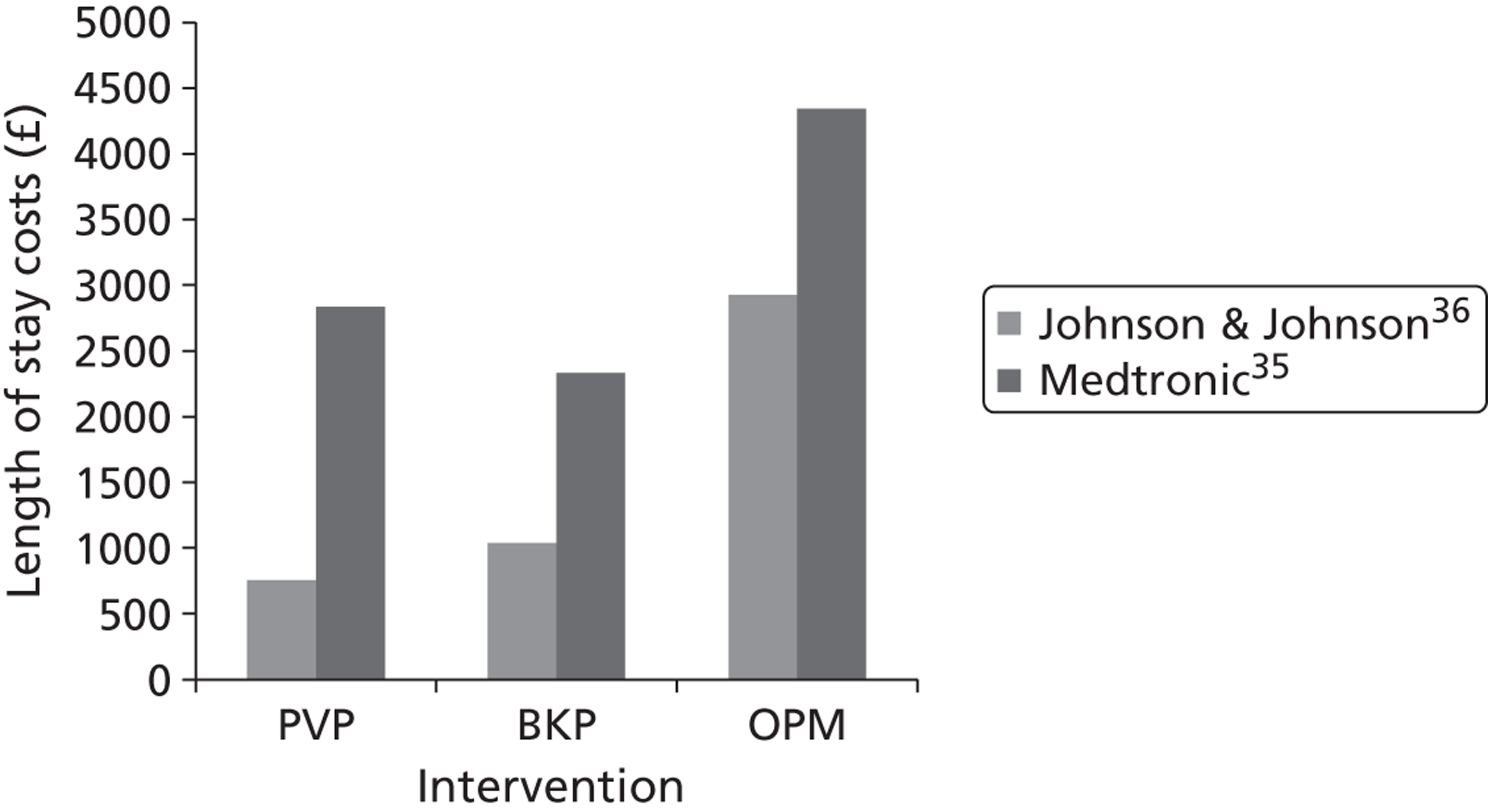
| Intervention | Length of stay, days (standard error) | Cost per day (£) | Total cost per hospital stay (£) |
|---|---|---|---|
| PVP | 6.2 (0.94) | 232 | 1438 |
| BKP | 5.1 (1.01) | 232 | 1183 |
| OPM | 9.5 (0.20) | 232 | 2204 |
Following clarification from Johnson & Johnson that the Dr Foster data used the same data set as the standard HES data, the assessment group decided that the length of stay data presented by Johnson & Johnson were most appropriate as fractures of non-osteoporotic aetiology (e.g. trauma or tumour) were excluded. The assessment group deemed that the cost values presented by Johnson & Johnson (£232 per day) were most appropriate to the decision problem. While the £232 per day value was acknowledged to likely underestimate the total costs in each arm, it was deemed more likely to accurately assess the incremental difference between strategies, which would relate to the latter part of hospital stays. As the incremental differences rather than the total values are used in the cost-effectiveness calculations, this approach was assumed reasonable. Sensitivity analyses were performed using both the Medtronic values, and using the Medtronic data for length of stay and Johnson & Johnson data for cost per day. In addition, an exploratory analysis looking at the impact on the results were the length of stay data assumed equal for all interventions, which was operationally achieved by setting the assumed length of stay to zero for all interventions. The values assumed by the assessment group are shown in Table 57 . It was assumed that the outcomes associated with PVP were also applicable to OPLA. It was assumed that the ratio of standard error to mean associated with the Johnson & Johnson length of stay data was applicable to the Medtronic data.
The assessment group values were consistently lower than those of Medtronic owing to the lower cost per bed-day. Compared with Johnson & Johnson, both PVP and BKP were more expensive owing to the longer assumed hospital stay, although the costs associated with OPM were lower.
Our clinical advisor was extremely surprised by the length of stay information, commenting that over the > 2000 vertebral augmentation procedures he has undertaken the average length of stay would be less than 6 hours. To incorporate this information, which may represent current practice better than the bundled HES data, sensitivity analyses would be performed assuming that there was no cost difference in length of stay, which was achieved operationally by setting the cost per bed-day to £0. Unfortunately, there is a paucity of reported data regarding length of stays within the trial to ensure that the cost and clinical data align. The lack of these data may indicate that the length of stay was briefer than suggested by HES data.
The aggregated costs associated with the osteoporotic vertebral compression fracture
A summary table presenting the values used by the assessment group, Johnson & Johnson, and Medtronic is given in Table 57 .
| Assessment group | Johnson & Johnson | Medtronic | |
|---|---|---|---|
| Acquisition cost of PVP using low-viscosity cement where possible | 800 | – | – |
| Acquisition cost of PVP using high-viscosity cement | 1546 | 1472 | 1193 |
| Acquisition cost of BKP | 2639 | 2842 | 1996 |
| Acquisition cost of OPLA | Evaluated in sensitivity analyses | 1472 | – |
| Operation costs | 1311 | 1479 | 990 (PVP); 1013 (BKP) |
| Hospital stay costs – PVP | Johnson & Johnson data but additionally evaluated in scenario analyses, using the Medtronic value and a combination of the sources, and assuming zero costs | 752 | 2833 |
| Hospital stay costs – BKP | 1039 | 2331 | |
| Hospital stay costs – OPLA | 752 | – | |
| Hospital stay costs – OPM | 2926 | 4342 | |
| Total costs – PVP | Dependent on the scenario analyses being conducted | 3702 | 5804 |
| Total costs – BKP | 5360 | 5527 | |
| Total costs – OPLA | 3702 | – | |
| Total costs – OPM | 2926 | 4828 |
Comparison of the model structures and population
Table 58 provides a summary of the comparison of mathematical models structure developed by the assessment group, Johnson & Johnson, and Medtronic.
| Assessment group | Johnson & Johnson | Medtronic | |
|---|---|---|---|
| Model structure | State transition model | Area under curve simulation | State transition model |
| Base-case time horizon | Lifetime (maximum 101 years) | 1 year | Lifetime (maximum 101 years) |
| OPLA included as a comparator | Yes | Yes (but assumed dominated) | No |
| Consideration of differential mortality effects related to treatment | Yes | No | Yes |
| Network meta-analysis undertaken to estimate VAS scores | Yes, with an assumption of stable VAS independent of initial VAS | Yes, but with no interpolation or extrapolation and the Blasco trial was published after their search | Discussed but not considered appropriate |
| Analyses using direct EQ-5D data | Yes | Yes | Yes |
| Consideration that people with vertebral fracture may have a poorer survival prognosis | Yes (limited to 5 years). UK data used362 and assumed independent of age and time since fracture | No | Yes (limited to 5 years). Age and time since fracture-dependent data derived from Sweden used |
| Consideration of subsequent vertebral fractures | Yes (limited to one additional). UK fracture rates used | No | Yes. Fracture rates calculated using hip fracture data and hip: vertebral fracture ratio seen in Sweden |
| Consideration of increased mortality after subsequent vertebral fractures | Yes (limited to 1 year). Data as above | No | Yes (limited to 5 years). Data as above |
| Consideration of subsequent hip fractures and associated mortality | Yes (limited to one additional) | No | No |
| Consideration of serious AEs related to vertebral augmentation | Yes (in sensitivity analyses) | Assumed none | Assumed none |
Comparison of the results produced by the assessment group model when using (largely) the same data as each manufacturer
In order to assess the level of agreement between the model structures, the assessment group model was populated so that it resembled, as closely as could be achieved relatively easily, each of the deterministic base-case models submitted by the manufacturers. This repopulation did not extend to importing the vertebral fracture rates; the underlying all cause mortality rates; and the underlying population utility assumed by Medtronic.
The results when the assessment group model was populated with Johnson & Johnson data are provided in Table 59 . The results when the assessment group model was populated with Medtronic data are provided in Table 60 .
| Treatment | Cost (£) | QALYs | ΔCost (£) | ΔQALY | ICER (£) | |
|---|---|---|---|---|---|---|
| Johnson & Johnson | OPM | 2926 | 0.507 | |||
| PVP | 3702 | 0.684 | 777 | 0.177 | 4392 | |
| BKP | 5113 | 0.656 | 1410 | –0.027 | Dominated | |
| Assessment group | OPM | 2926 | 0.509 | |||
| PVP | 3702 | 0.683 | 777 | 0.173 | 4480 | |
| BKP | 5113 | 0.658 | 1410 | –0.025 | Dominated |
| Treatment | Cost (£) | QALYs | ΔCost (£) | ΔQALY | ICER (£) | |
|---|---|---|---|---|---|---|
| Medtronic | OPM | 5394 | 4.976 | |||
| PVP | 6112 | 5.325 | 718 | 0.35 | 2053 | |
| BKP | 6403 | 5.441 | 291 | 0.12 | 2510 | |
| Assessment group | OPM | 6995 | 6.047 | |||
| PVP | 7800 | 6.474 | 805 | 0.43 | 2057 | |
| BKP | 8179 | 6.708 | 379 | 0.23 | 2508 |
It is seen that the results are very close, with the discrepancy arising as a result of the number of days assumed in a year (365.25 days in the assessment group model and 365 days in the Johnson & Johnson model) and the way these interact with the monthly EQ-5D scores which fluctuate across time.
It is seen that while the costs and QALYs predicted in the assessment group model are both higher than in the Medtronic model, the incremental values are similar and the ICERs are very similar, indicating that the differences are unlikely to affect the conclusions. The assessment group believes that the discrepancy is caused by the difference in both the fracture rates assumed (the assessment group use vertebral fracture data from Scotland while Medtronic estimate rates based on UK hip fracture data and the Swedish ratio of hip : vertebral fractures), different risks of mortality following subsequent vertebral fracture and the assumed duration of this risk (1 year in the assessment group model; 5 years in the Medtronic model).
The assessment group concluded that, given the results presented in Tables 59 and 60 , the programming of the conceptual models into modelling packages was unlikely to be a key driver of the ICER, in comparison with the assumption made regarding the presence of a mortality benefit (which was the cause for the different conclusions in the Johnson & Johnson and the Medtronic models) on the assumed utilities associated with each intervention and whether or not OPLA should be included as a comparator, and at what cost if so. Accordingly, it was deemed that the assessment group model that had produced ICERs very similar to those of the manufacturers’ models when populated with similar data was of sufficient quality to use in the calculation of all forthcoming results.
Methodology for estimating the scenarios to run
There is a large number of potential structural uncertainties that could be evaluated. In order to restrict the quantity of data presented, the following methodology was used:
-
Two foundation analyses were established, which represented two of many plausible scenarios. The assumptions and data used in the foundation analyses are detailed below with the difference between the analyses being that one assumed a mortality benefit associated with BKP, PVP and OPLA while the other did not. The deterministic results from the foundation analysis were calculated.
-
Univariate sensitivity analyses were conducted, varying structural or parameter values. The effect of the change on the net monetary benefit (NMB) (assuming a willingness to pay of £20,000 per QALY) of the foundation analysis was evaluated. The majority of analyses were undertaken on the model assuming no mortality difference as, where a mortality benefit was assumed, the impact of this assumption was far bigger than that of the variables altered.
-
If the change in the NMB was deemed minimal by the authors then the structural uncertainty or parameter was not considered a key driver and would not require a separate scenario analysis in the full analyses. An exception to this rule was allowed if the authors believed that the variable could be important owing to an interaction with another variable in multivariate analyses.
-
Those parameters which had a large impact in univariate sensitivity analyses or were believed could have an impact in multivariate analyses were used to derive the scenario analyses and sensitivity analyses undertaken by the assessment group.
Net monetary benefit has been used for these exploratory analyses as it is relatively simple to assess which intervention is the most cost-effective (denoted by the intervention with the largest value) and also whether the ICER is < £20,000 per QALY gained (the NMB value is > £0) or > £20,000 per QALY (the NMB value is < £0).
The assumptions used in the foundation analyses
The following are the assumptions and parameters used in the foundation analyses. In the analyses conducted without an assumed mortality benefit, the assumed duration of treatment-related mortality benefit was set to zero. The values in parentheses indicate the values tested in univariate sensitivity analyses:
-
patient age: 70 years (65, 80)
-
sex: female (male)
-
T-score: –3 SD (–2.5, –3.5)
-
length of bisphosphonate use: 5 years (0)
-
fall time associated with bisphosphonates: 5 years (0)
-
the assumed duration of a treatment-related mortality benefit: 5 years (0)
-
the assumed duration of the relative risk of mortality following a vertebral fracture: 5 years (0)
-
the assumed wane time associated with the relative risk of mortality following a vertebral fracture: 5 years (0)
-
include an added risk of mortality in year of subsequent vertebral fracture: true (false)
-
costs associated with hospital stay: Johnson & Johnson (Medtronic; Medtronic length of stay/Johnson & Johnson costs; 0)
-
cost of PVP: low-viscosity cement £800 (high-viscosity cement £1546)
-
discount rate costs: 3.5% (0, 6)
-
discount rate benefits: 3.5% (0, 6)
-
QALY loss associated with PVP and BKP = 0 (0.02)
-
HR on general mortality for BKP and PVP: (academic-in-confidence information has been removed)
-
mortality effect of OPLA: half that of PVP (no effect, equal to PVP)
-
the regression mapping VAS to EQ-5D (using all data, excluding INVEST data)
-
assumed point at which VAS scores converge: 24 months (12 months)
-
cost of OPLA: equal to PVP (20%, 40%, 60% and 80% of PVP).
The following parameters were assumed fixed: costs and utility losses associated with fractures; the acquisition costs of BKP; the distributions of efficacy associated with alendronate; the costs of alendronate; the distribution on the risk of mortality following vertebral fracture; the mortality rate following hip fracture and the general mortality rate.
The analyses conducted
Having undertaken the analyses determining the variables to which the model was sensitive, it was clear that the assumption regarding the mortality benefits of the active interventions was crucial to the cost-effectiveness ratios. As such, it was deemed sensible to present the results split into three categories based on the underlying assumption: a differential effect assumed for BKP and PVP with both better than OPM; a pooled analyses where the effects of BKP and PVP were assumed identical with both better than OPM and where no mortality benefit of BKP or PVP was assumed. The effect of OPLA was varied in sensitivity analyses for the first two categories and assumed equal to OPM in the third.
Furthermore, there was known to be a difference in results based on whether the EQ-5D was taken directly from the RCTs for the four trial reporting such values (INVEST,147 FREE,137 Buchbinder et al. 148 and Rousing et al. 131) or whether the mapping of stable VAS scores from the network meta-analyses to EQ-5D was the preferred method. Analyses of the change in EQ-5D data showed that the Rousing trial was unable to be used meaningfully owing to the imbalance in EQ-5D at the start of the trial between the PVP and control arms. Thus, analyses were conducted on just the Buchbinder, FREE and INVEST studies.
As such, there were deemed six plausible scenarios, depicted in Figure 36 . The results for these were calculated using both deterministic and probabilistic methods. For each of the scenarios, sensitivity analyses were undertaken exploring the effects of changing structural assumptions and parameter values that were deemed important in the exploratory analyses.
FIGURE 36.
Derivation of the assessment group’s six scenarios.
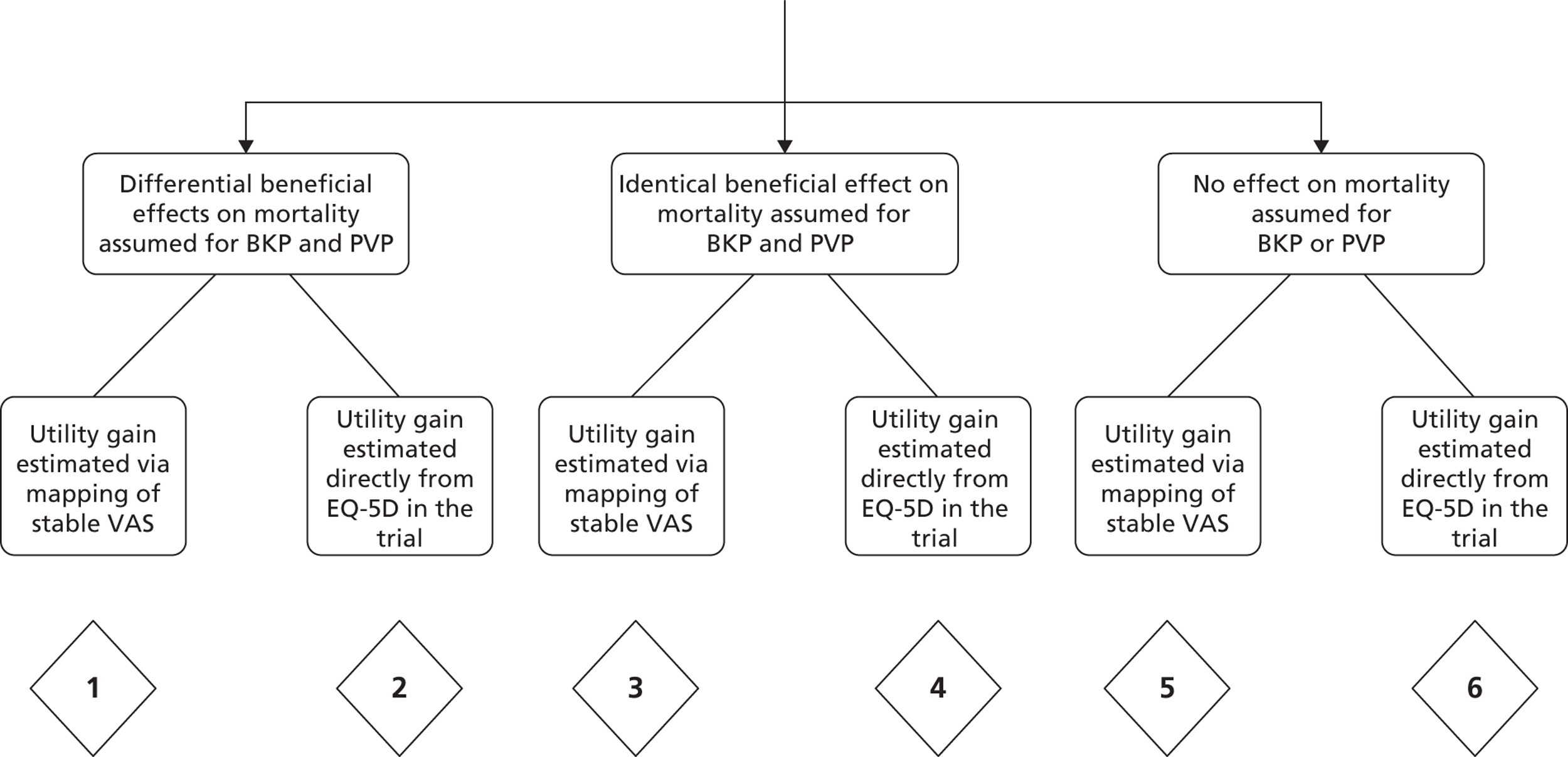
Sensitivity analyses will be undertaken for each of the six scenarios.
Scenarios 2, 4 and 6 will also be subdivided into the results from Buchbinder et al. , the FREE trial, and INVEST.
The assessment group’s results
Foundation analyses
Using the foundation analyses the costs and QALYs associated with each intervention were as depicted in Tables 61 and 62 . An intervention being extendedly dominated indicates that a combination of two other interventions can provide the same health gain at a lower cost. Interventions which are neither dominated nor extendedly dominated form the cost-effectiveness acceptability frontier.
| Procedure | Costs (£) | QALYs | ICER (cost per QALY gained)a (£) | NMBb (£) |
|---|---|---|---|---|
| PVP | 6118 | 4.91 | Dominating | 3512 |
| OPLA | 6118 | 4.83 | Dominated | 1821 |
| OPM | 6181 | 4.74 | Dominated | – |
| BKP | 8244 | 4.91 | Dominated | 1379 |
| Procedure | Costs (£) | QALYs | ICER (cost per QALY gained)a (£) | NMBb (£) |
|---|---|---|---|---|
| OPLA | 6163 | 4.89 | 3030 | |
| OPM | 6181 | 4.74 | Dominated | – |
| PVP | 6210 | 5.04 | 312 | 5958 |
| BKP | 8507 | 5.27 | 9806 | 8346 |
It is stressed that these results do not represent a base case, but one of a number of plausible scenarios.
The results from the univariate analyses have been grouped into seven categories, the first six of which were tested assuming no mortality benefit. These are illustrated in Figures 37 to 43 .
FIGURE 37.
Univariate analyses regarding patients’ characteristics.
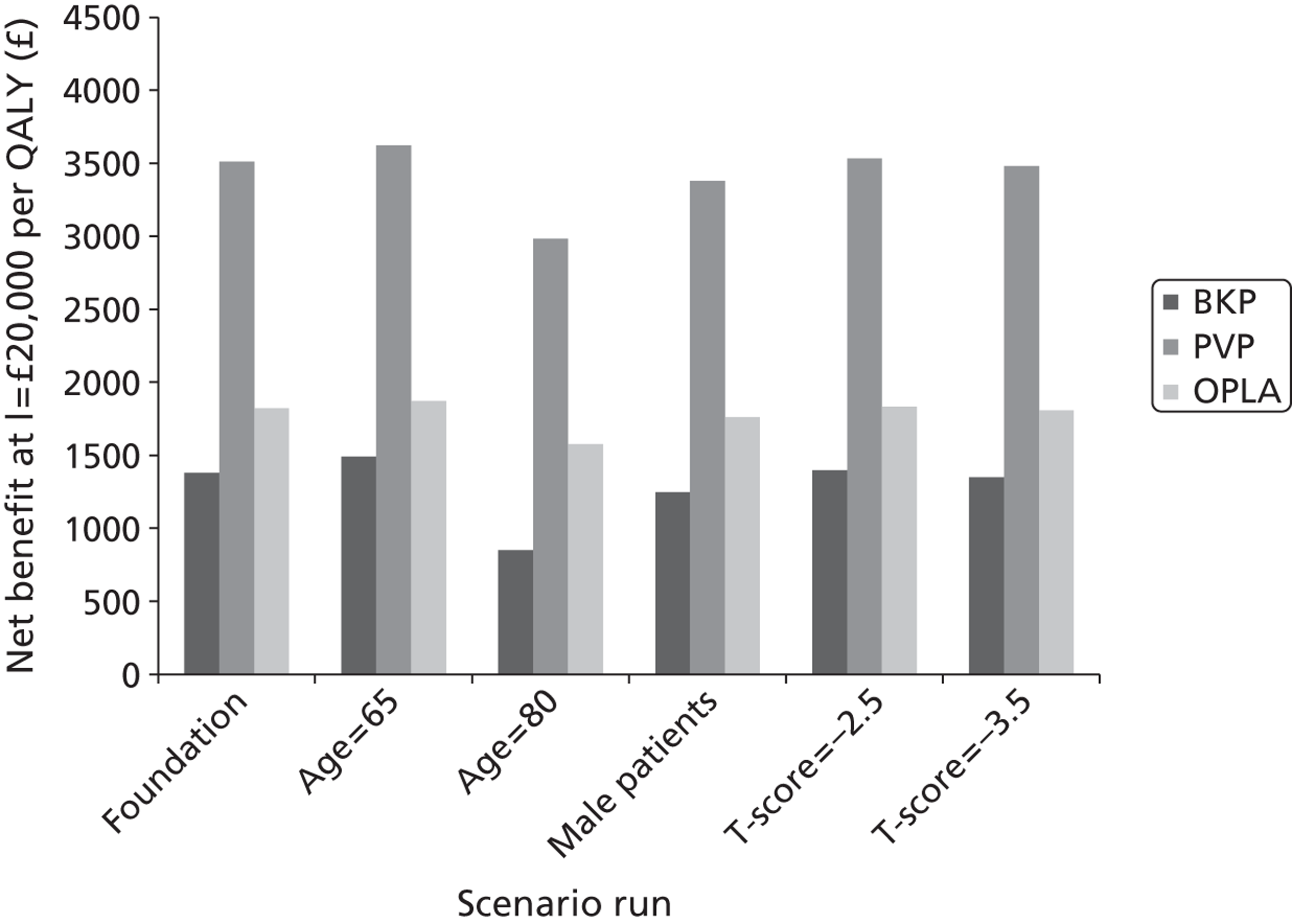
FIGURE 38.
Univariate analyses regarding hospitalisation costs, operation cost and cement costs.
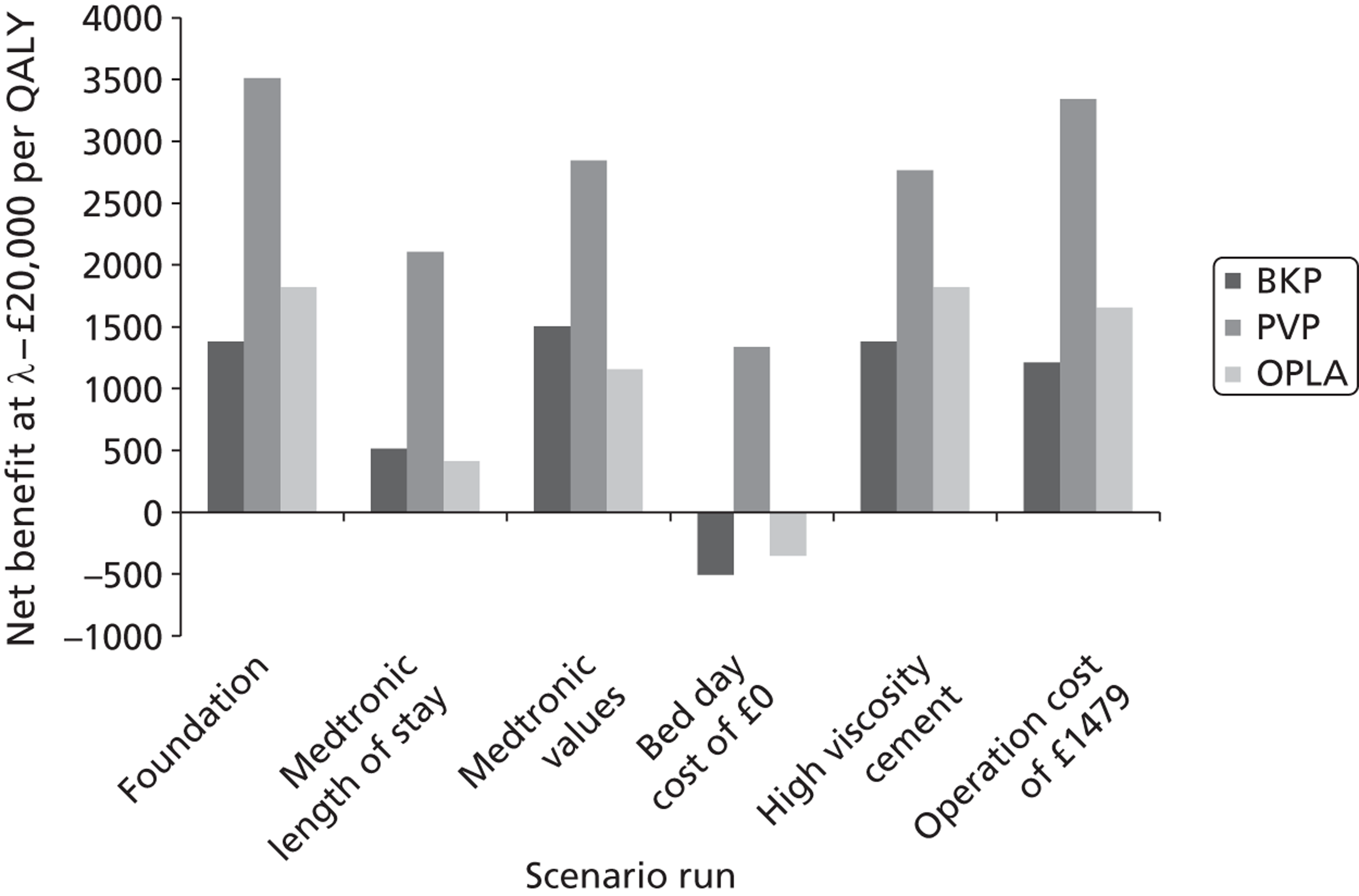
FIGURE 39.
Univariate analyses regarding the costs of equipment required for OPLA and the cost of the procedure when undertaking OPLA. A, Cost of OPLA equipment set to 20% of the cost of PVP equipment; B, cost of OPLA equipment set to 40% of the cost of PVP equipment; C, cost of OPLA equipment set to 60% of the cost of PVP equipment; D, cost of OPLA equipment set to 80% of the cost of PVP equipment; E, cost of OPLA procedure set to 50% of PVP procedure.
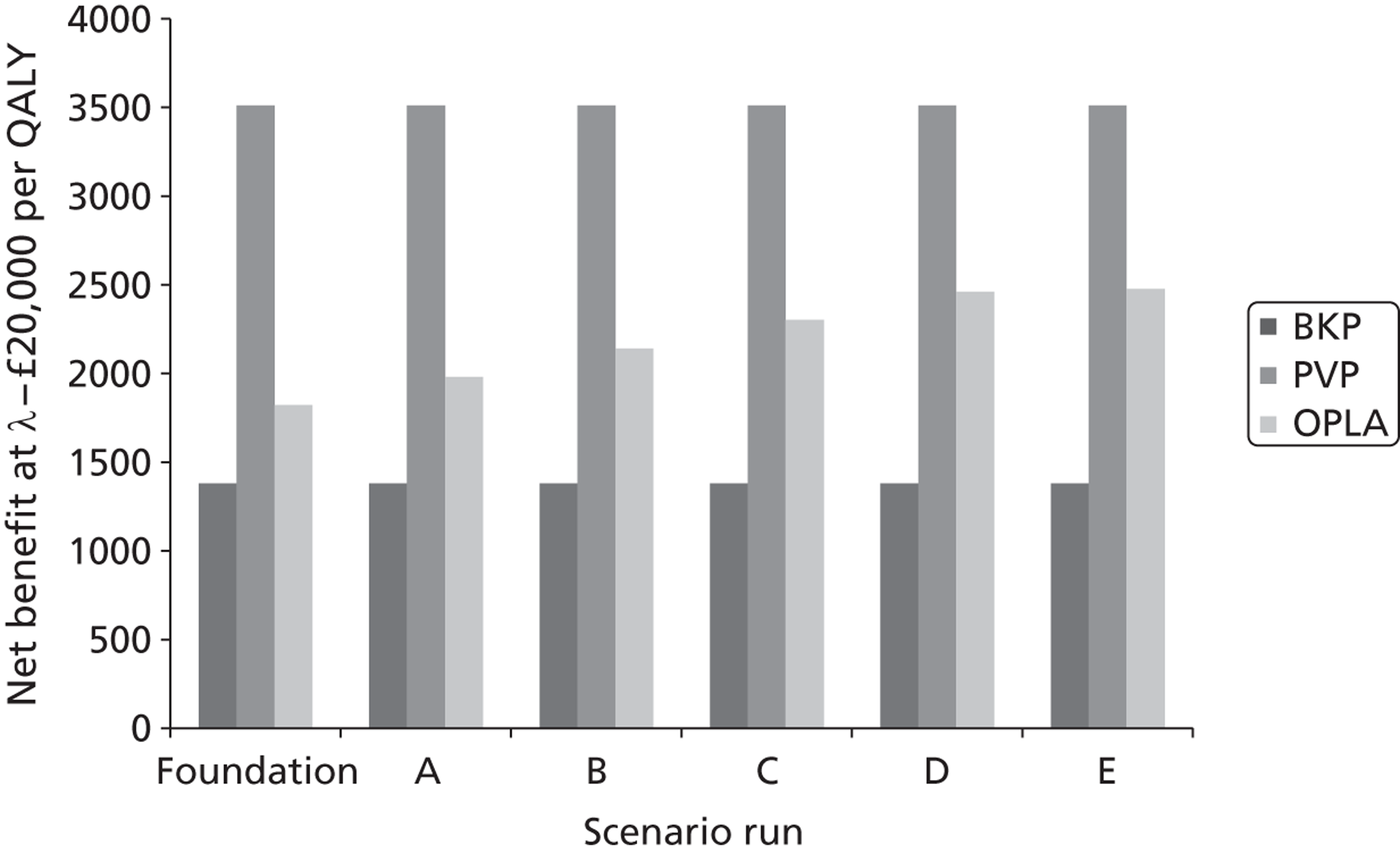
FIGURE 40.
Univariate analyses regarding discount rates, bisphosphonate usage and bisphosphonate wane period. A, Discount rate for future costs set to 0% per annum; B, discount rate for future costs set to 6% per annum; C, discount rate for future benefits set to 0% per annum; D, discount rate for future benefits set to 6% per annum; E, an assumption that no woman was taking bisphosphonates; F, the wane period following bisphosphonate treatment set to 0 years.
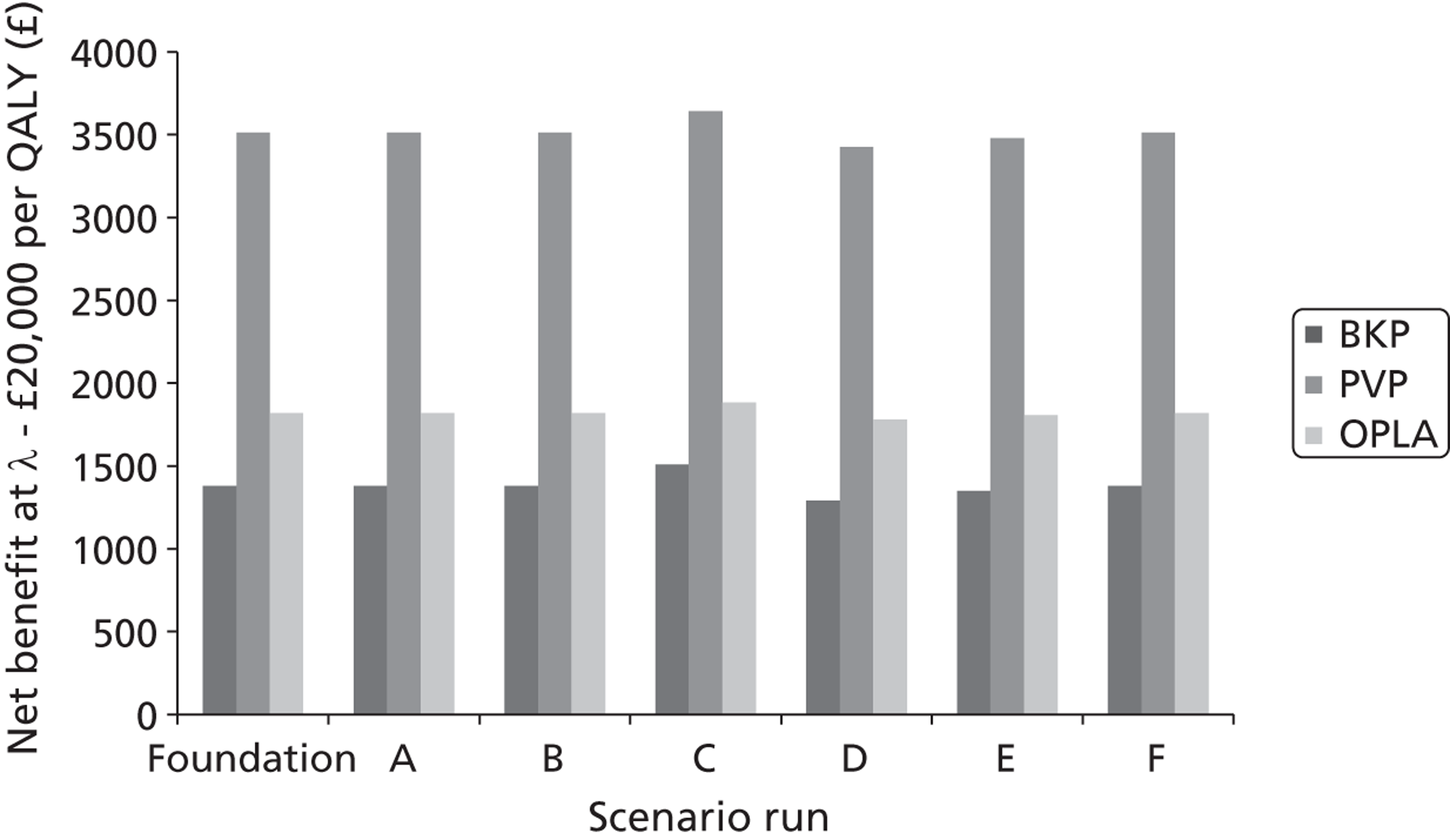
FIGURE 41.
Univariate analyses regarding the assumed time of convergence, the trials used in the VAS to EQ-5D mapping and the inclusion of adverse events associated with treatment.
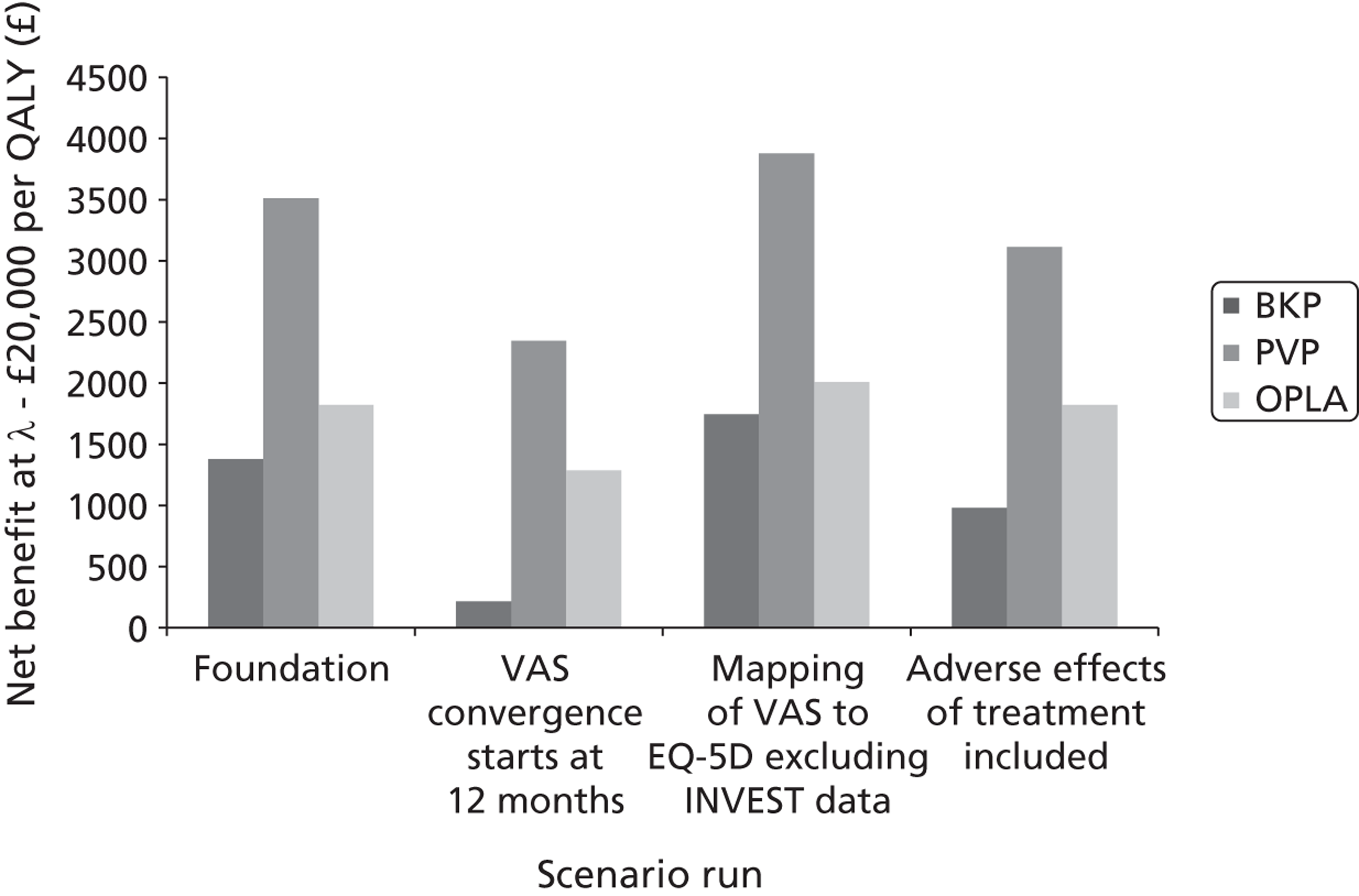
FIGURE 42.
Univariate analyses using change in EQ-5D data directly from trials. A, Using data from the FREE trial;137 B, using data from Buchbinder et al. 102 and assuming convergence between 24 and 36 months; C, using data from Buchbinder et al. 102 and assuming convergence between 12 and 24 months; D, using data from the INVEST trial103 and assuming convergence between 24 and 36 months; E, using data from the INVEST trial103 and assuming convergence between 12 and 24 months.
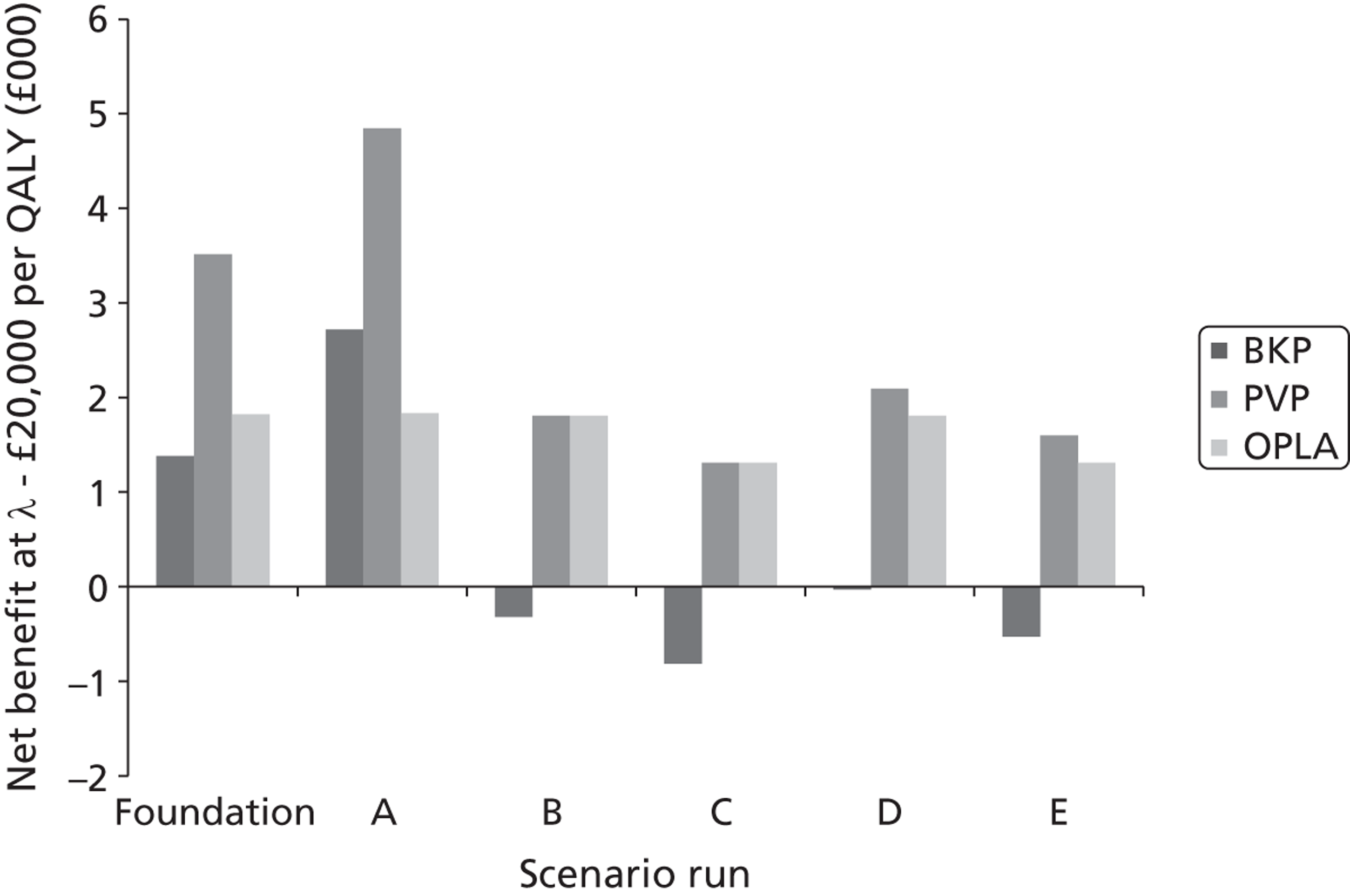
FIGURE 43.
Univariate analyses regarding mortality and fracture rates. A, No mortality benefit for any treatment (the foundation analysis in the earlier figures); B, a pooled mortality benefit for BKP and PVP; C, no mortality benefit for OPLA; D, the mortality benefit for OPLA set equal to PVP; E, no increased mortality risk following the initial vertebral fracture; F, no waning period of the increased mortality risk following the initial fracture; G, no increased risk of mortality in the year of additional vertebral fractures.
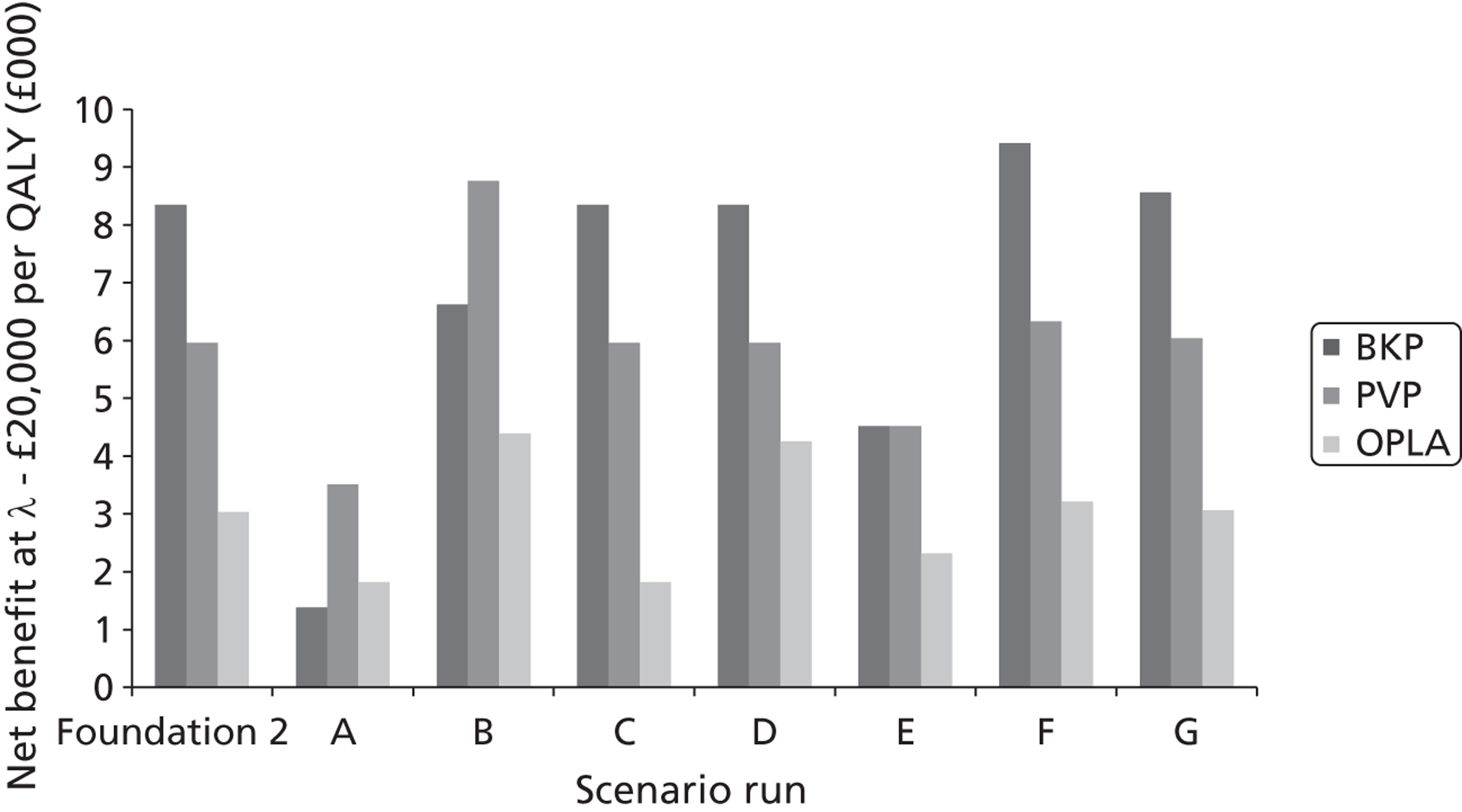
Those related to a patient’s characteristics (age, sex and T-score)
In this univariate analysis, the assumed age of the woman was altered to 65 years and 80 years, the sex of the patient was assumed to be male, and the T-score was altered to –2.5 SD and –3.5 SD. The authors did not deem that any of these parameters made a noticeable difference to the conclusions of the foundation analyses, and these variables remained constant in the full analyses.
Those affecting costs of hospitalisation, operation cost and the price of cement
In this univariate analysis, the costs of hospital stay were altered to the costs proposed by Johnson & Johnson36 and Medtronic,34 the hospitalisation costs were set equal among interventions (by reducing bed-day cost to £0), the cost of high-viscosity cement was used, and the operation cost was set to £1479.
The authors did not deem that any of these parameters made a noticeable difference to the conclusions of the foundation analyses. However, as the length of stay data are contentious, the analysis using a cost per bed-day of £0, which sets the costs equal among interventions, would be retained. The QALY threshold gain at which high-viscosity cement is more cost-effective than low-viscosity cement will additionally be calculated.
Those associated with the costs of equipment required for operative placebo with local anaesthesia and the cost of the procedure when using operative placebo with local anaesthesia
In this univariate analysis, the costs of PVP equipment and the cost of the OPLA procedure were altered.
The analyses presented did not alter whether or not OPLA was adjudged to be the most cost-effective intervention. However, because these analyses were purely univariate, the effects of a change in both the cost of OPLA equipment and the cost of the OPLA procedure were not calculated. It is plausible that multiple changes would affect the conclusions and these parameters were changed in the full analyses.
Those associated with the discount rate and bisphosphonate use
In this sensitivity analyses, the discount rates for both costs and benefits were altered. The assumption that women were being prescribed bisphosphonates was removed, and the assumed residual benefit of 5 years’ linear decline following cessation of bisphosphonates was set to zero.
The assessment group did not believe that any of these sensitivity analyses markedly affected the conclusions and thus these values were left constant in the main analyses.
Those associated with time of convergence, mapping visual analogue scale to European Quality of Life-5 Dimensions, and adverse events
A sensitivity analysis altered the assumption regarding the time at which the VAS score was assumed independent of intervention, and the time point at which convergence started. In the foundation model it was assumed that VAS scores were identical at 36 months and started converging at 24 months, while the sensitivity analysis assumed 24 and 12 months, respectively. The effect of mapping VAS to EQ-5D excluding the INVEST trial103 was analysed, as was assuming a 0.02 QALY loss associated with BKP and PVP.
The change in convergence assumption noticeably reduced the net benefit of all interventions and was maintained in the sensitivity analyses, as was the effect of an assumed (and acknowledged to be arbitrary) 0.02 QALY decrement for PVP and BKP. While the mapping without INVEST favoured BKP, PVP and OPLA, it was assumed that this would not change the conclusions and was omitted.
Those associated with using the European Quality of Life-5 Dimensions directly from the trials where possible rather than mapping from visual analogue scale to European Quality of Life-5 Dimensions
In this sensitivity analysis, the difference between the change in EQ-5D in the FREE,137 Buchbinder et al. 102 and INVEST103 trials was used directly. Data from Rousing et al. 131 were discarded owing to the large baseline difference in EQ-5D. Convergence was tested at both the 24-month and 12-month periods for Buchbinder et al. and INVEST, but only at the 24-month period for FREE as the data collection was of 2 years’ duration.
For all of the analyses (FREE,137 Buchbinder et al. 102 and INVEST103) it was assumed that the EQ-5D value for BKP would equal the value for PVP. This decision was made given the results from the Liu et al. trial139 and supported by the mid point estimate from the network meta-analysis.
When using the FREE137 data, it was assumed that the values for OPM and OPLA remained at those values mapped from VAS, while the EQ-5D values for BKP and PVP were estimated as the OPM values plus the difference in change in EQ-5D between BKP and OPM. A limitation of this analysis is that it was assumed that changes to the values for BKP and PVP did not affect the values for OPLA.
For the Buchbinder et al. 102 and INVEST103 trials, the EQ-5D values for OPM and OPLA remained at the VAS mapped values, while the EQ-5D value for PVP and BKP was estimated to be the OPLA value plus the difference in change in EQ-5D between PVP and OPLA. A potential limitation of these analyses is that it was assumed that the BKP and PVP values were reduced to nearer the OPLA and OPM network meta-analyses values rather than increasing the OPLA value to nearer the BKP and PVP meta-analyses values. The methodology reduces the difference in EQ-5D between BKP and PVP compared with OPM and reduces the apparent cost-effectiveness of BKP and PVP compared with OPM. The ICERs between BKP/PVP and OPM using the alternative method would be the same as produced in scenario 5. It is stressed that the ICERs between BKP/PVP and OPLA are independent of the method chosen.
All three analyses potentially affected the conclusion, with the net benefit for BKP becoming positive when applying the data from FREE;137 when the Buchbinder et al. 102 and INVEST103 trials were used, the net benefit difference between PVP and OPLA was noticeably reduced. Given these results the assessment group decided to explore the impacts of using the three studies within the full analysis.
Those affecting mortality or fracture rates
This scenario assumed that there was a differential rate in mortality with a HR of (academic-in-confidence information has been removed) for BKP, (academic-in-confidence information has been removed) for PVP and (academic-in-confidence information has been removed) for OPLA. Other scenarios assumed no mortality benefit for any treatment; a pooled value of (academic-in-confidence information has been removed) for both BKP and PVP [(academic-in-confidence information has been removed) for OPLA]; HRs of 1 and (academic-in-confidence information has been removed) for OPLA; no increased mortality risk following the initial vertebral fracture; no wane time after 5 years’ increased mortality risk following the initial vertebral fracture and no increased risk of fracture in the year of additional vertebral fractures.
It is seen that the assumed mortality effect is a key driver of the results. Removing this for all interventions resulted in PVP having a greater net benefit than BKP, as did assuming that the mortality effects of PVP and BKP were identical. Assuming that the patients did not have a higher risk of mortality for 5 years owing to the prevalent vertebral fracture also resulted in PVP having a higher net benefit than BKP, because the differential mortality benefit of BKP was now applied to a lower underlying rate of mortality. All of the sensitivity analyses performed, with the exception of F and G, were deemed worthy of additional exploration in the full analyses.
Conclusions from the exploratory univariate analyses
It is clear that whether or not the interventions have a mortality benefit (and the extent of this if a benefit is assumed) has a considerable effect on the relative cost-effectiveness of the strategies. Additionally, the conclusions appeared to be influenced by whether the EQ-5D data were mapped from VAS or taken directly from the trials. These combinations are the six scenarios defined in Figure 36 .
Additional sensitivity analyses conducted on these scenarios were assuming a bed-day cost of £0 to set hospitalisation costs equal; altering the assumed cost of equipment for OPLA and the cost of the procedure; altering the time of convergence and including potential QALY losses associated with adverse events.
Full results for each of the six scenarios including the incorporation of sensitivity analyses
The six scenarios are shown diagrammatically in Figure 36 . The results from each are discussed in turn. Each scenario is subjected to sensitivity analysis exploring the impacts of changes to the following assumptions: assuming a bed-day cost of £0 to set hospitalisation costs equal; altering the assumed cost of equipment for OPLA and the cost of the procedure; altering the time of convergence and including potential QALY losses associated with adverse events. It is noted that combinations of these sensitivity analyses may represent arguably more plausible central estimates of the cost-effectiveness of the interventions than the unadulterated scenarios and should be provided with equal weight. For example, assuming that the costs of OPLA are identical to the costs of PVP is likely to be favourable to PVP when a comparison with OPLA is made. In analyses where both BKP and PVP have a cost per QALY gained value > £20,000, a figure in parenthesis denotes the cost per QALY gained with OPLA removed.
However, for brevity, plots of the cost-effectiveness plane and the CEACs have been provided only for the unadulterated scenario. When running the probabilistic scenarios it was noted that the model was non-linear. This was owing to the assumed distribution for the increased risk of mortality following fracture which was a HR of 4.40 (95% CI 1.85 to 10.60). 360 The mean of this log-normal distribution is 4.86 which increased the risks of dying for all interventions. As such, the sensitivity analyses are presented having undertaken probabilistic sensitivity analyses.
The results for PVP have been estimated, assuming the use of low-viscosity cement. The assessment group’s assumed cost of low-viscosity cement was £800 per operation, while the cost of high-viscosity cement was £1546, resulting in an estimated increase of £746 per operation associated with the use of high-viscosity cement. Exploratory analyses of assuming high-viscosity cement for all patients have been undertaken.
Scenario 1: differential beneficial effects on mortality assumed for balloon kyphoplasty and percutaneous vertebroplasty, utility gain estimated via mapping of stable visual analogue scale
The deterministic results are presented in Table 63 , with the cost-effectiveness plane depicted in Figure 44 .
| Intervention | Costs (£) | QALYs | ICER (£) |
|---|---|---|---|
| OPLA | 6164 | 4.89 | |
| OPM | 6181 | 4.74 | Dominated |
| PVP | 6211 | 5.04 | 312 |
| BKP | 8507 | 5.27 | 9802 |
FIGURE 44.
A plot of the deterministic results produced by assessment group – scenario 1.
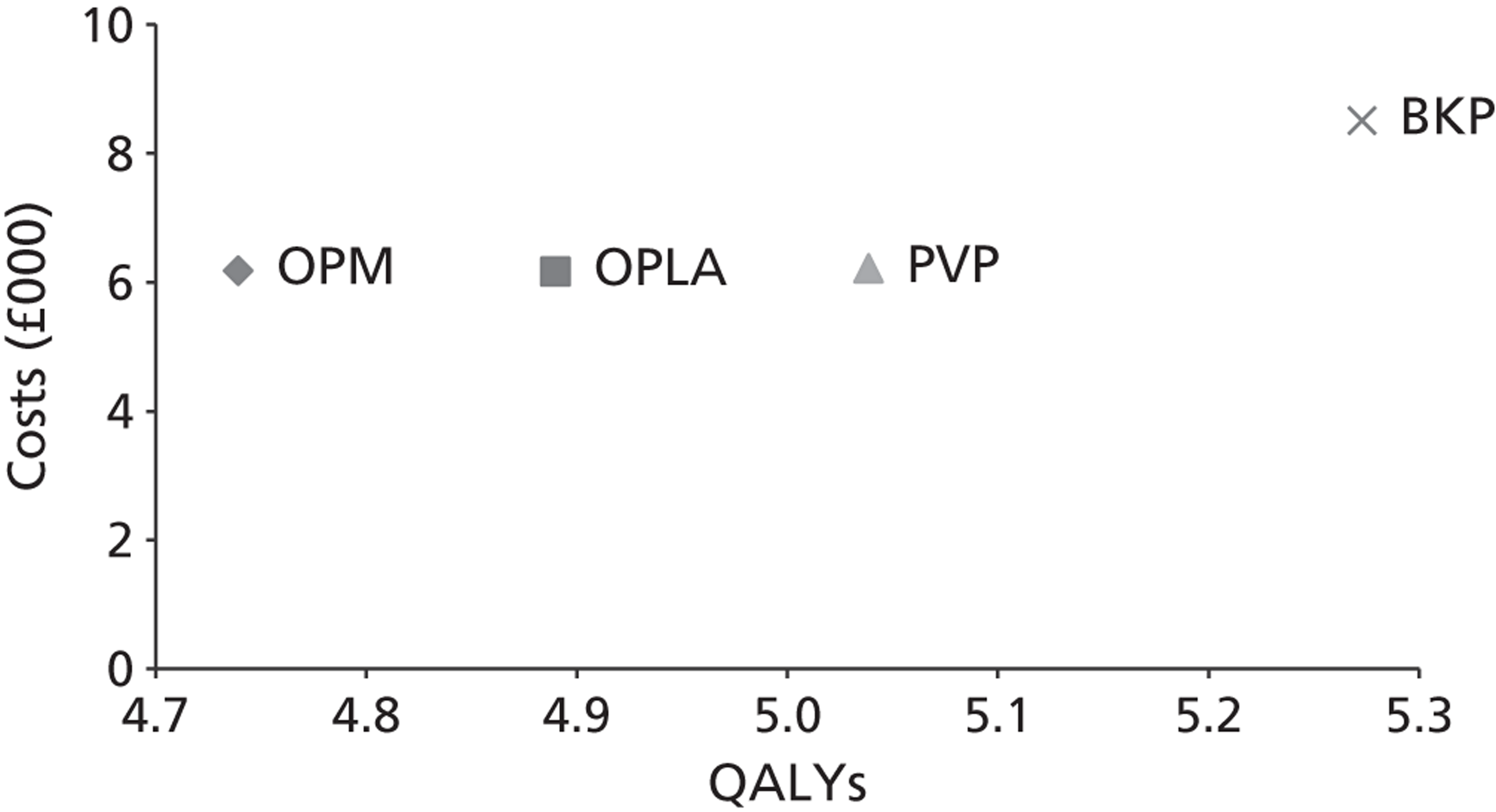
Probabilistic sensitivity analyses were conducted. These results are detailed in Table 64 , with an assessment of the uncertainty of the adoption decision displayed in a CEAC ( Figure 45 ).
| Intervention | Costs (£) | QALYs | ICER (£) |
|---|---|---|---|
| OPLA | 6104 | 4.03 | |
| OPM | 6121 | 3.89 | Dominated |
| PVP | 6149 | 4.16 | 338 |
| BKP | 8440 | 4.35 | 11,992 |
FIGURE 45.
The CEAC produced by the assessment group – scenario 1.
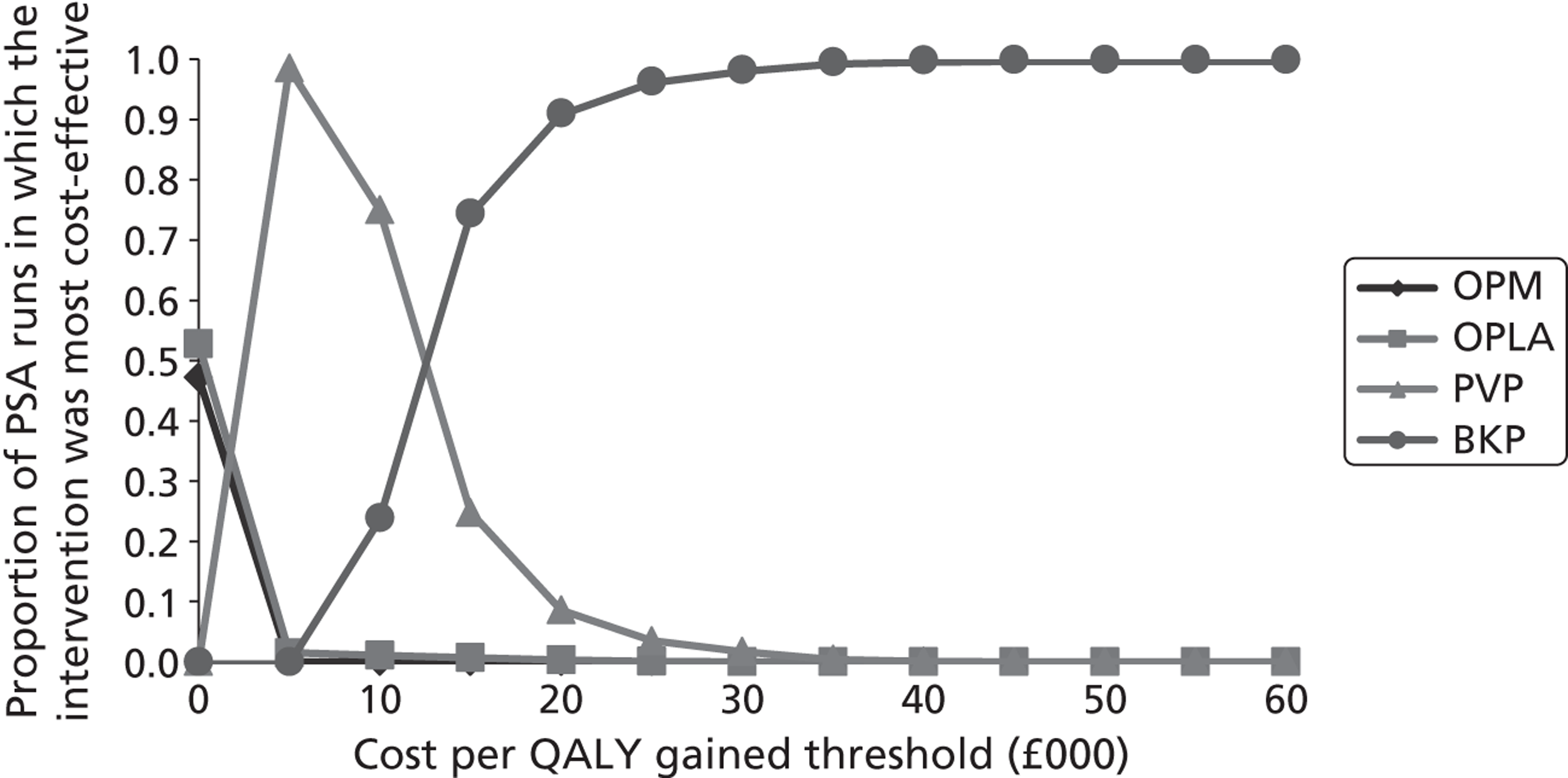
It is seen that the results from the probabilistic sensitivity analyses differed slightly from the deterministic values.
Sensitivity analyses conducted on scenario 1
Table 65 details the sensitivity analyses conducted by the assessment group. Given the non-linearity of the model, the results from the probabilistic sensitivity analyses are presented.
| Intervention | Costs (£) | QALYs | ICER (£) |
|---|---|---|---|
| The probabilistic results produced when hospitalisation costs were set to £0 per day | |||
| OPM | 3196 | 3.89 | |
| OPLA | 5351 | 4.03 | Extendedly dominated |
| PVP | 5396 | 4.16 | 8184 |
| BKP | 7400 | 4.35 | 10,490 |
| The probabilistic results produced when the cost of the OPLA procedure was set to 50% that of PVP and the cost of OPLA equipment was set to 60% that of PVP | |||
| OPLA | 5129 | 4.03 | |
| OPM | 6121 | 3.89 | Dominated |
| PVP | 6149 | 4.16 | 7684 |
| BKP | 8440 | 4.35 | 11,992 |
| The probabilistic results produced when it was assumed that convergence of EQ-5D scores began at 12 months and were equal at 24 months | |||
| OPLA | 6104 | 4.06 | |
| OPM | 6121 | 3.95 | Dominated |
| PVP | 6149 | 4.16 | 436 |
| BKP | 8440 | 4.35 | 11,975 |
| The probabilistic results produced when it was assumed that BKP and PVP were associated with 0.02 QALY loss | |||
| OPLA | 6104 | 4.03 | |
| OPM | 6121 | 3.89 | Dominated |
| PVP | 6149 | 4.14 | 398 |
| BKP | 8440 | 4.33 | 11,992 |
| All of the above sensitivity analyses combined | |||
| OPM | 3196 | 3.95 | |
| OPLA | 4376 | 4.06 | 10,672 |
| PVP | 5396 | 4.14 | Extendedly dominated |
| BKP | 7400 | 4.33 | 11,033 |
Scenario 2: differential beneficial effects on mortality assumed for balloon kyphoplasty and percutaneous vertebroplasty, utility gain estimated via trials reporting European Quality of Life-5 Dimensions
These analyses have been subdivided into three categories based on whether the FREE data,137 the Buchbinder et al. data102 or the INVEST data103 were used.
Analyses using the FREE data
The deterministic results are presented in Table 66 , with the cost-effectiveness plane depicted in Figure 46 .
| Intervention | Costs (£) | QALYs | ICER (£) |
|---|---|---|---|
| OPLA | 6164 | 4.98 | |
| OPM | 6181 | 4.83 | Dominated |
| PVP | 6211 | 5.20 | 214 |
| BKP | 8507 | 5.44 | 9541 |
FIGURE 46.
A plot of the deterministic results produced by the assessment group – scenario 2: FREE data. 137
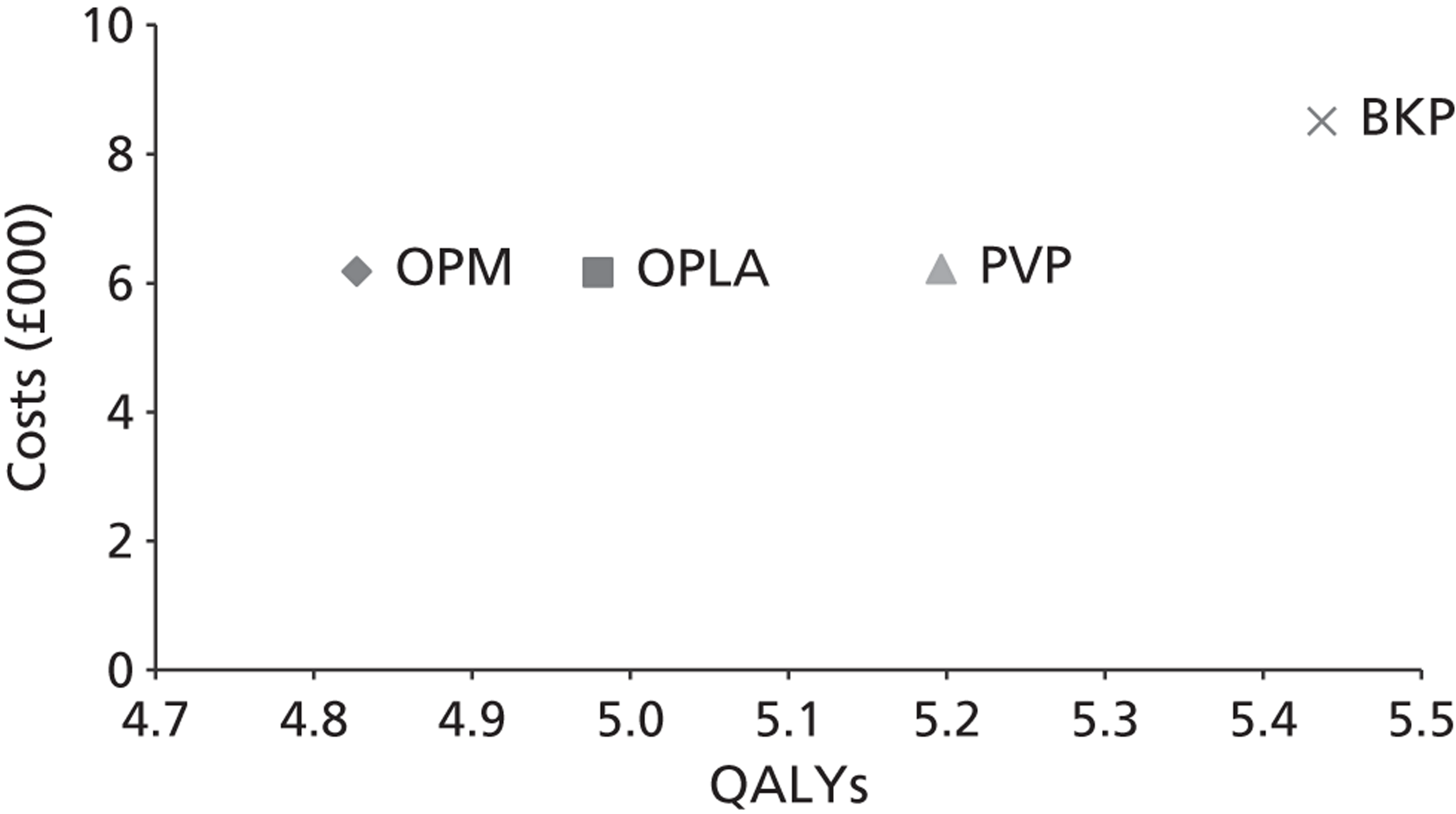
Probabilistic sensitivity analyses were conducted. These results are detailed in Table 67 , with an assessment of the uncertainty of the adoption decision displayed in a CEAC ( Figure 47 ).
| Intervention | Costs (£) | QALYs | ICER (£) |
|---|---|---|---|
| OPLA | 6104 | 4.90 | |
| OPM | 6121 | 4.75 | Dominated |
| PVP | 6149 | 5.05 | 302 |
| BKP | 8440 | 5.35 | 7616 |
It is seen that the results from the probabilistic sensitivity analyses differed slightly from the deterministic values.
FIGURE 47.
The cost-effectiveness acceptability curve produced by the assessment group – scenario 2: FREE data. 137
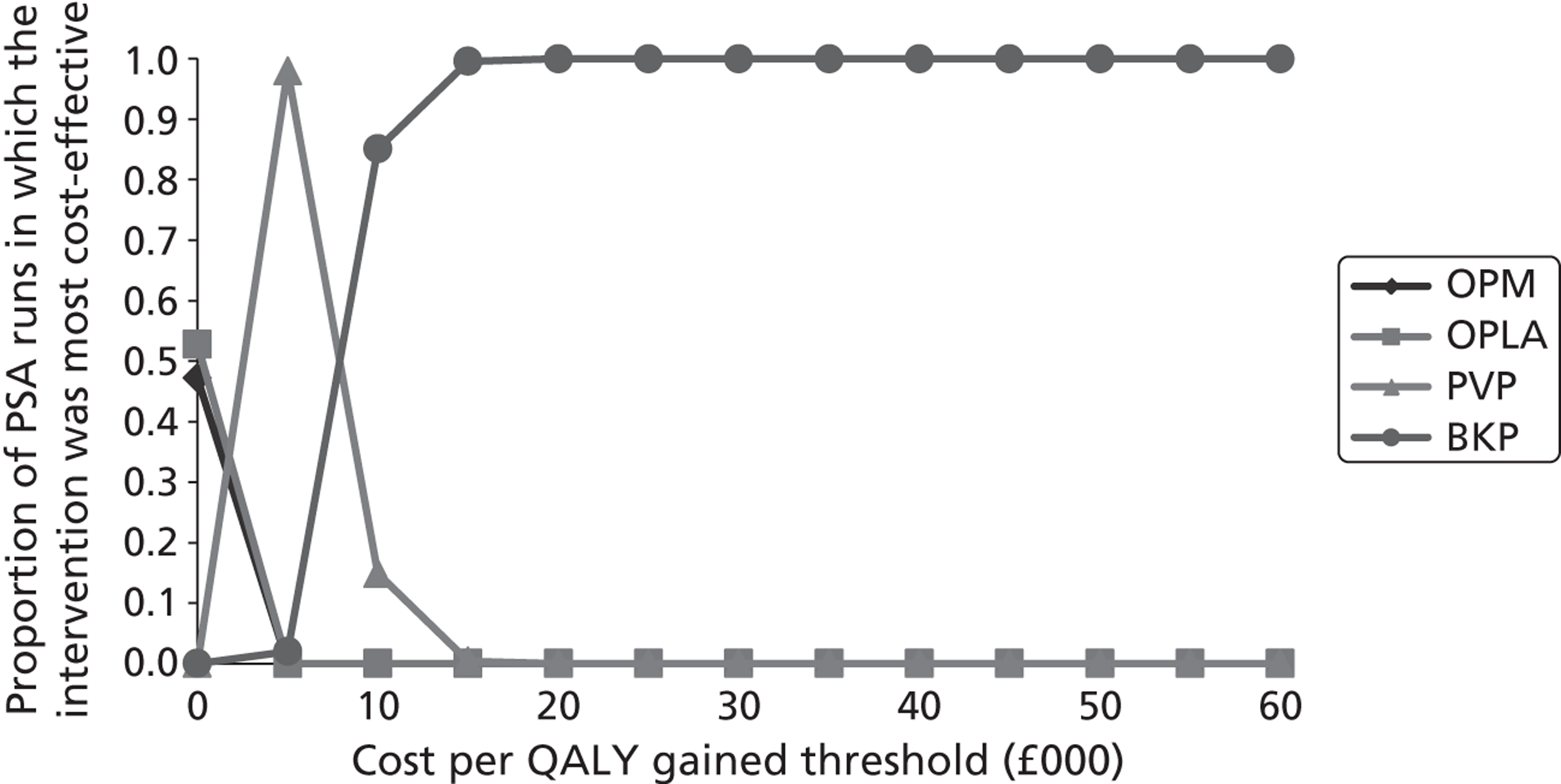
Table 68 details the sensitivity analyses conducted by the assessment group. Given the non-linearity of the model, the results from the probabilistic sensitivity analyses are presented.
| Intervention | Costs (£) | QALYs | ICER (£) |
|---|---|---|---|
| The probabilistic results produced when hospitalisation costs were set to £0 per day | |||
| OPM | 3196 | 4.75 | |
| OPLA | 5351 | 4.90 | Extendedly dominated |
| PVP | 5396 | 5.05 | Extendedly dominated |
| BKP | 7400 | 5.35 | 7012 |
| The probabilistic results produced when the cost of the OPLA procedure was set to 50% that of PVP and the cost of OPLA equipment was set to 60% that of PVP | |||
| OPLA | 5129 | 4.90 | |
| OPM | 6121 | 4.75 | Dominated |
| PVP | 6149 | 5.05 | 6870 |
| BKP | 8440 | 5.35 | 7616 |
| The probabilistic results produced when it was assumed that BKP and PVP were associated with 0.02 QALY loss | |||
| OPLA | 6104 | 4.90 | |
| OPM | 6121 | 4.75 | Dominated |
| PVP | 6149 | 5.03 | 349 |
| BKP | 8440 | 5.33 | 7616 |
| All of the above sensitivity analyses combined | |||
| OPM | 3196 | 4.75 | |
| OPLA | 4376 | 4.90 | Extendedly dominated |
| PVP | 5396 | 5.03 | Extendedly dominated |
| BKP | 7400 | 5.33 | 7254 |
Analyses using the Buchbinder et al. data
The deterministic results are presented in Table 69 with the cost-effectiveness plane depicted in Figure 48 .
| Convergence between 12 and 24 months | Convergence between 24 and 36 months | |||||
|---|---|---|---|---|---|---|
| Intervention | Costs (£) | QALYs | ICER (£) | Costs (£) | QALYs | ICER (£) |
| OPLA | 6164 | 4.88 | 6164 | 4.88 | ||
| OPM | 6181 | 4.75 | Dominated | 6181 | 4.73 | Dominated |
| PVP | 6211 | 4.94 | 731 | 6211 | 4.94 | 731 |
| BKP | 8507 | 5.17 | 9853 | 8507 | 5.17 | 9853 |
FIGURE 48.
A plot of the deterministic results produced by the assessment group – scenario 2: Buchbinder data102 convergence starts at 24 months.
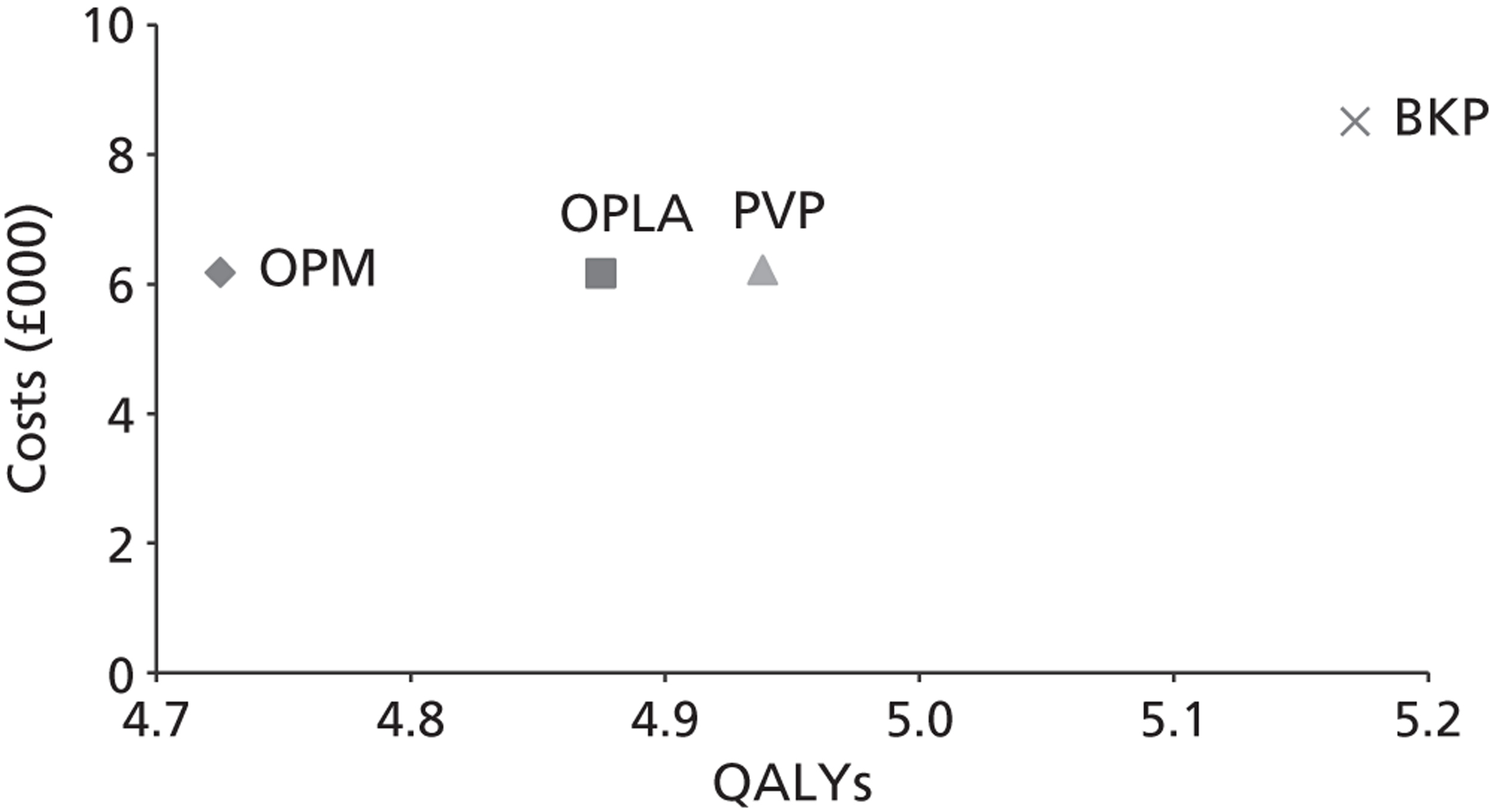
Probabilistic sensitivity analyses were conducted. These results are detailed in Table 70 , with an assessment of the uncertainty of the adoption decision displayed in a CEAC, assuming convergence starts at 24 months ( Figure 49 ).
| Convergence between 12 and 24 months | Convergence between 24 and 36 months | |||||
|---|---|---|---|---|---|---|
| Intervention | Costs (£) | QALYs | ICER (£) | Costs (£) | QALYs | ICER (£) |
| OPLA | 6104 | 4.79 | 6104 | 4.80 | ||
| OPM | 6121 | 4.67 | Dominated | 6121 | 4.65 | Dominated |
| PVP | 6149 | 4.86 | 725 | 6149 | 4.86 | 725 |
| BKP | 8440 | 5.08 | 10,072 | 8440 | 5.08 | 10,073 |
It is seen that the results from the probabilistic sensitivity analyses differed slightly from the deterministic values.
Table 71 details the sensitivity analyses conducted by the assessment group. Given the non-linearity of the model, the results from the probabilistic sensitivity analyses are presented.
| Convergence between 12 and 24 months | Convergence between 24 and 36 months | |||||
|---|---|---|---|---|---|---|
| Intervention | Costs (£) | QALYs | ICER (£) | Costs (£) | QALYs | ICER (£) |
| The probabilistic results produced when hospitalisation costs were set to £0 per day | ||||||
| OPM | 3196 | 4.67 | 3196 | 4.65 | ||
| OPLA | 5351 | 4.79 | Extendedly dominated | 5351 | 4.80 | Extendedly dominated |
| PVP | 5396 | 4.86 | Extendedly dominated | 5396 | 4.86 | Extendedly dominated |
| BKP | 7400 | 5.08 | 10,196 | 7400 | 5.08 | 9625 |
| The probabilistic results produced when the cost of the OPLA procedure was set to 50% that of PVP and the cost of OPLA equipment was set to 60% that of PVP | ||||||
| OPLA | 5129 | 4.79 | 5129 | 4.79 | ||
| OPM | 6121 | 4.67 | Dominated | 6121 | 4.65 | Dominated |
| PVP | 6149 | 4.86 | Extendedly dominated | 6149 | 4.86 | Extendedly dominated |
| BKP | 8440 | 5.08 | 11,445 | 8440 | 5.08 | 11,445 |
| The probabilistic results when it was assumed that BKP and PVP were associated with 0.02 QALY loss | ||||||
| OPLA | 6104 | 4.79 | 6104 | 4.79 | ||
| OPLA | 6121 | 4.67 | Dominated | 6121 | 4.65 | Dominated |
| PVP | 6149 | 4.84 | 1071 | 6149 | 4.84 | 1071 |
| BKP | 8440 | 5.06 | 10,072 | 8440 | 5.06 | 10,072 |
| All of the above sensitivity analyses combined | ||||||
| OPM | 3196 | 4.67 | 3196 | 4.65 | ||
| OPLA | 4376 | 4.79 | 9590 | 4376 | 4.79 | 7998 |
| PVP | 5396 | 4.84 | Extendedly dominated | 5396 | 4.84 | Extendedly dominated |
| BKP | 7400 | 5.06 | 11,230 | 8963 | 5.06 | 11,230 |
Analyses using the INVEST data
The deterministic results are presented in Table 72 , with the cost-effectiveness plane depicted in Figure 50 .
| Convergence between 12 and 24 months | Convergence between 24 and 36 months | |||||
|---|---|---|---|---|---|---|
| Intervention | Costs (£) | QALYs | ICER (£) | Costs (£) | QALYs | ICER (£) |
| OPLA | 6164 | 4.88 | 6164 | 4.88 | ||
| OPM | 6181 | 4.75 | Dominated | 6181 | 4.73 | Dominated |
| PVP | 6211 | 4.95 | 595 | 6211 | 4.95 | 595 |
| BKP | 8507 | 5.19 | 9850 | 8507 | 5.19 | 9850 |
FIGURE 50.
A plot of the deterministic results produced by the assessment group – scenario 2: INVEST data103 convergence starts at 24 months.
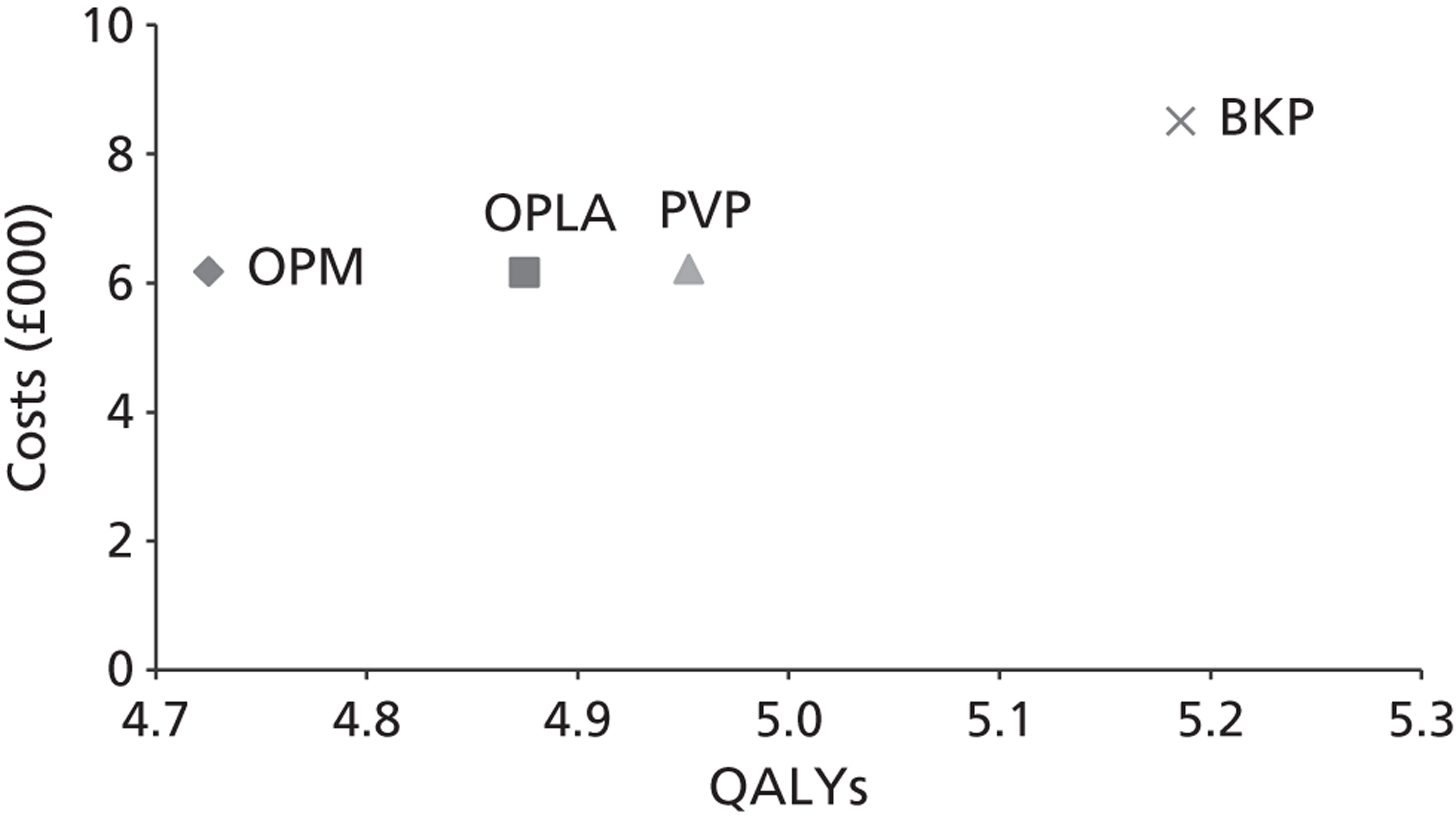
Probabilistic sensitivity analyses were conducted. These results are detailed in Table 73 , with an assessment of the uncertainty of the adoption decision displayed in a CEAC, assuming convergence starts at 24 months ( Figure 51 ).
| Convergence between 12 and 24 months | Convergence between 24 and 36 months | |||||
|---|---|---|---|---|---|---|
| Intervention | Costs (£) | QALYs | ICER (£) | Costs (£) | QALYs | ICER (£) |
| OPLA | 6104 | 4.79 | 6104 | 4.79 | ||
| OPM | 6121 | 4.67 | Dominated | 6121 | 4.67 | Dominated |
| PVP | 6149 | 4.87 | 588 | 6149 | 4.87 | 588 |
| BKP | 8440 | 5.10 | 10,070 | 8440 | 5.10 | 10,070 |
It is seen that the results from the probabilistic sensitivity analyses differed slightly from the deterministic values.
Table 74 detail the sensitivity analyses conducted by the assessment group. Given the non-linearity of the model, the results from the probabilistic sensitivity analyses are presented.
| Convergence between 12 and 24 months | Convergence between 24 and 36 months | |||||
|---|---|---|---|---|---|---|
| Intervention | Costs (£) | QALYs | ICER (£) | Costs (£) | QALYs | ICER (£) |
| The probabilistic results produced when hospitalisation costs were set to £0 per day | ||||||
| OPM | 3196 | 4.67 | 3196 | 4.65 | ||
| OPLA | 5351 | 4.79 | Extendedly dominated | 5351 | 4.79 | Extendedly dominated |
| PVP | 5396 | 4.87 | Extendedly dominated | 5396 | 4.87 | Extendedly dominated |
| BKP | 7400 | 5.10 | 9850 | 7400 | 5.10 | 9316 |
| The probabilistic results produced when the cost of the OPLA procedure was set to 50% that of PVP and the cost of OPLA equipment was set to 60% that of PVP | ||||||
| OPLA | 5129 | 4.79 | 5129 | 4.79 | ||
| OPM | 6121 | 4.67 | Dominated | 6121 | 4.65 | Dominated |
| PVP | 6149 | 4.87 | Extendedly dominated | 6149 | 4.87 | Extendedly dominated |
| BKP | 8440 | 5.10 | 10,900 | 8440 | 5.10 | 10,900 |
| The probabilistic results when it was assumed that BKP and PVP were associated with 0.02 QALY loss | ||||||
| OPLA | 6104 | 4.79 | 6104 | 4.79 | ||
| OPM | 6121 | 4.67 | Dominated | 6121 | 4.65 | Dominated |
| PVP | 6149 | 4.85 | 796 | 6149 | 4.85 | 796 |
| BKP | 8440 | 5.08 | 10,070 | 8440 | 5.08 | 10,070 |
| All of the above sensitivity analyses combined | ||||||
| OPM | 3196 | 4.67 | 3196 | 4.65 | ||
| OPLA | 4376 | 4.79 | 9590 | 4376 | 4.79 | 7998 |
| PVP | 5396 | 4.85 | Extendedly dominated | 5396 | 4.85 | Extendedly dominated |
| BKP | 7400 | 5.08 | 10,657 | 7400 | 5.08 | 10,657 |
Scenario 3: equal beneficial effects on mortality assumed for balloon kyphoplasty and percutaneous vertebroplasty, utility gain estimated via mapping of stable visual analogue scale
The deterministic results are presented in Table 75 , with the cost-effectiveness plane depicted in Figure 52 .
| Intervention | Costs (£) | QALYs | ICER (£) |
|---|---|---|---|
| OPM | 6181 | 4.74 | |
| OPLA | 6216 | 4.96 | 157 |
| PVP | 6316 | 5.18 | 449 |
| BKP | 8442 | 5.18 | Dominated |
FIGURE 52.
A plot of the deterministic results produced by the assessment group – scenario 3.
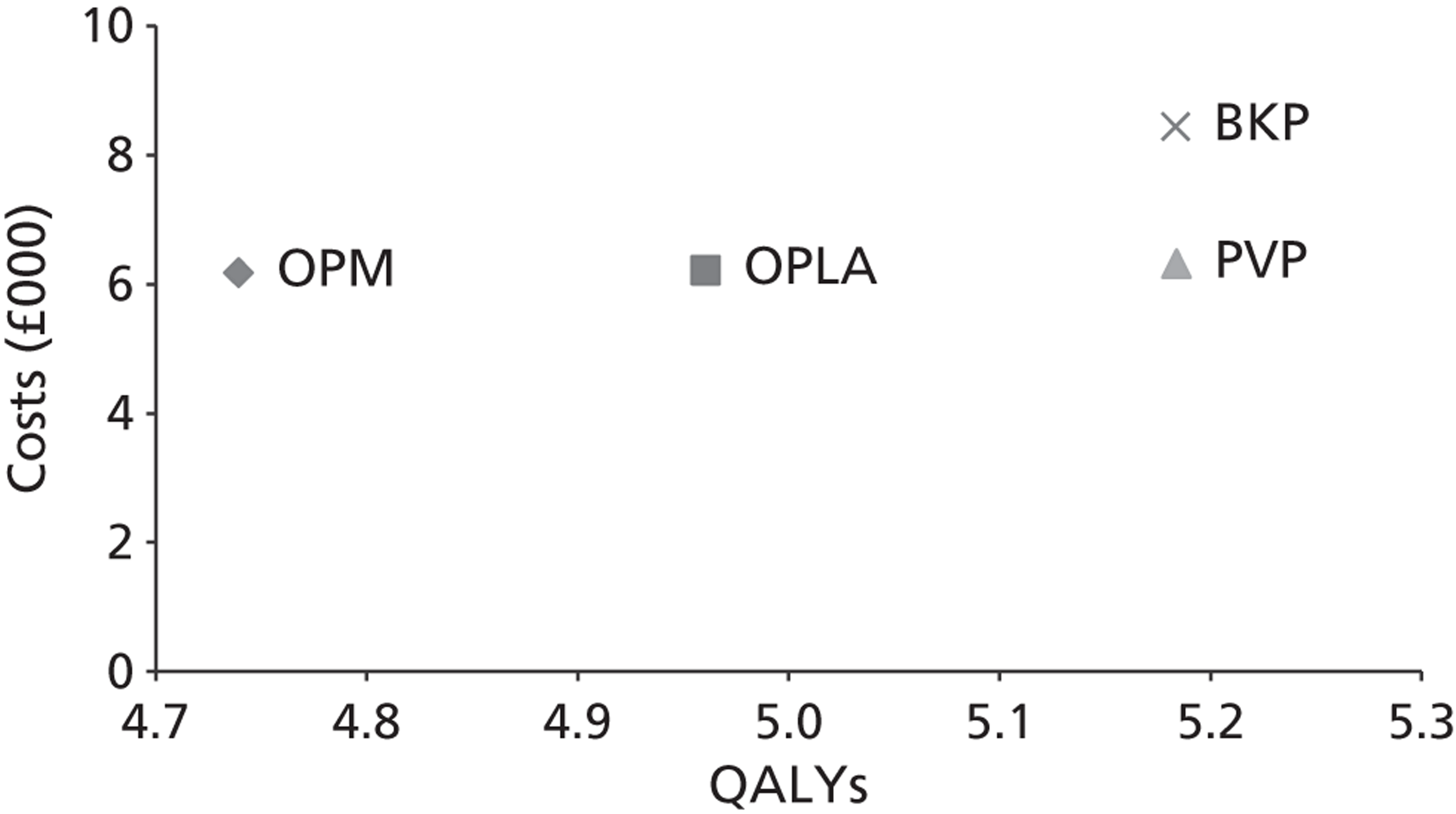
Probabilistic sensitivity analyses were conducted. These results are detailed in Table 76 , with an assessment of the uncertainty of the adoption decision displayed in a CEAC ( Figure 53 ).
| Intervention | Costs (£) | QALYs | ICER (£) |
|---|---|---|---|
| OPM | 6121 | 3.89 | |
| OPLA | 6154 | 4.09 | 169 |
| PVP | 6251 | 4.28 | 501 |
| BKP | 8377 | 4.28 | Dominated |
FIGURE 53.
The CEAC produced by the assessment group – scenario 3.
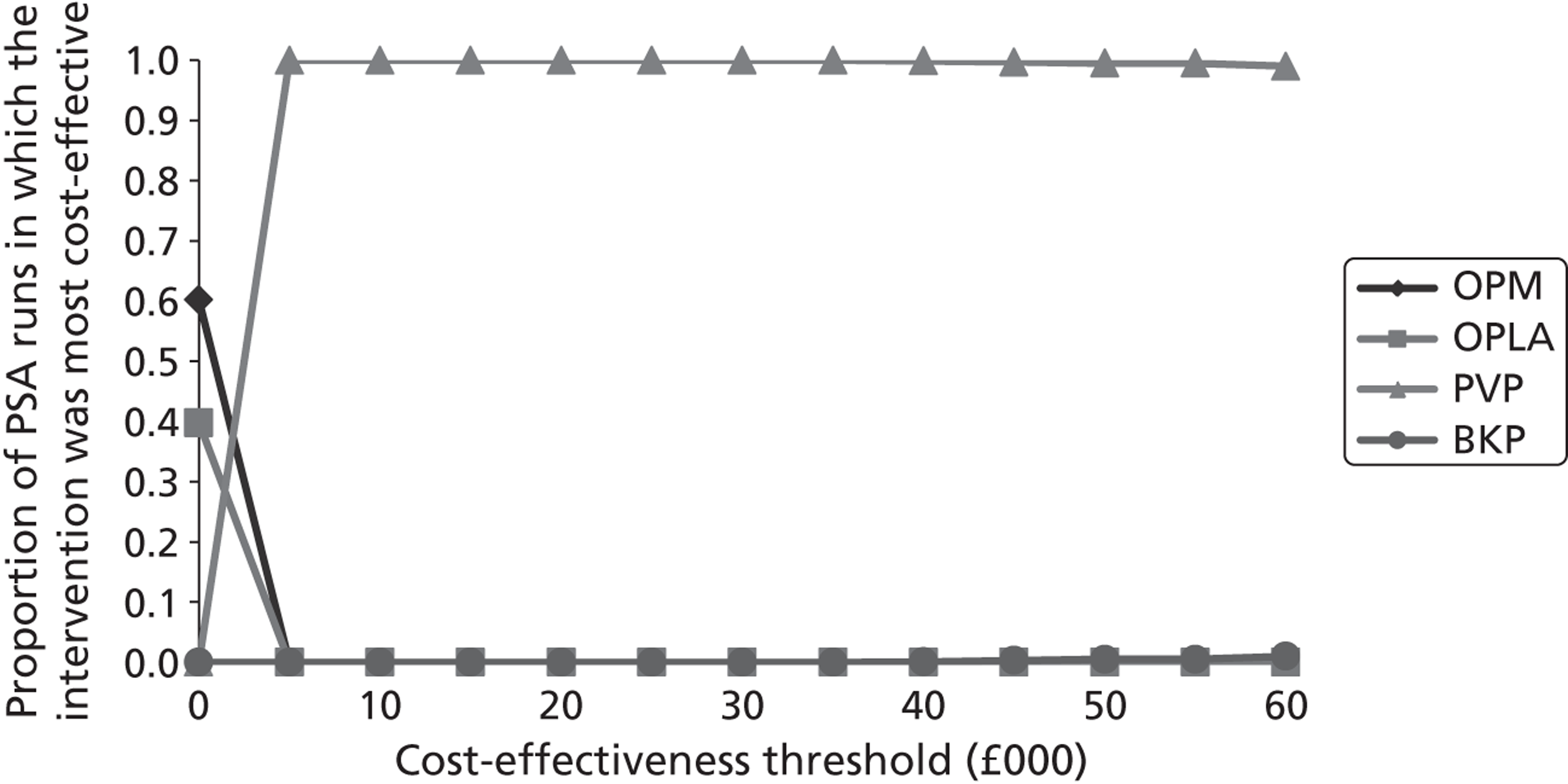
Sensitivity analyses conducted on scenario 3
Table 77 details the sensitivity analyses conducted by the assessment group. Given the non-linearity of the model, the results from the probabilistic sensitivity analyses are presented.
| Intervention | Costs (£) | QALYs | ICER (£) |
|---|---|---|---|
| The probabilistic results produced when hospitalisation costs were set to £0 per day | |||
| OPM | 3196 | 3.89 | |
| OPLA | 5401 | 4.09 | Extendedly dominated |
| PVP | 5498 | 4.28 | 5941 |
| BKP | 7337 | 4.28 | Dominated |
| The probabilistic results produced when the cost of the OPLA procedure was set to 50% that of PVP and the cost of OPLA equipment was set to 60% that of PVP | |||
| OPLA | 5179 | 4.09 | |
| OPM | 6121 | 3.89 | Dominated |
| PVP | 6251 | 4.28 | 5529 |
| BKP | 8377 | 4.28 | Dominated |
| The probabilistic results produced when it was assumed that convergence of EQ-5D scores began at 12 months and were equal at 24 months | |||
| OPM | 6121 | 3.95 | |
| OPLA | 6154 | 4.11 | 195 |
| PVP | 6251 | 4.28 | 594 |
| BKP | 8377 | 4.28 | Dominated |
| The probabilistic results produced when it was assumed that BKP and PVP were associated with 0.02 QALY loss | |||
| OPM | 6121 | 3.89 | |
| OPLA | 6154 | 4.09 | 169 |
| PVP | 6251 | 4.26 | 559 |
| BKP | 8377 | 4.26 | Dominated |
| All of the above sensitivity analyses combined | |||
| OPM | 3196 | 3.95 | |
| OPLA | 4425 | 4.12 | 7308 |
| PVP | 5498 | 4.26 | 7458 |
| BKP | 7337 | 4.26 | Dominated |
| As above plus mortality effect of OPLA set to equal BKP and PVP | |||
| OPM | 3196 | 3.95 | |
| OPLA | 4523 | 4.23 | 4723 |
| PVP | 5498 | 4.26 | 31,304 (7377) |
| BKP | 7337 | 4.26 | Dominated (dominated) |
Scenario 4: equal beneficial effects assumed for balloon kyphoplasty and percutaneous vertebroplasty, utility gain estimated via trials reporting European Quality of Life-5 Dimensions
These analyses have been subdivided into three categories based on whether the FREE data137 the Buchbinder et al. data102 or the INVEST data103 were used.
Analyses using the FREE data
The deterministic results are presented in Table 78 , with the cost-effectiveness plane depicted in Figure 54 .
| Intervention | Costs (£) | QALYs | ICER (£) |
|---|---|---|---|
| OPM | 6181 | 4.83 | |
| OPLA | 6216 | 5.05 | 154 |
| PVP | 6316 | 5.35 | 342 |
| BKP | 8442 | 5.35 | Dominated |
FIGURE 54.
A plot of the deterministic results produced by the assessment group – scenario 4: FREE data. 137
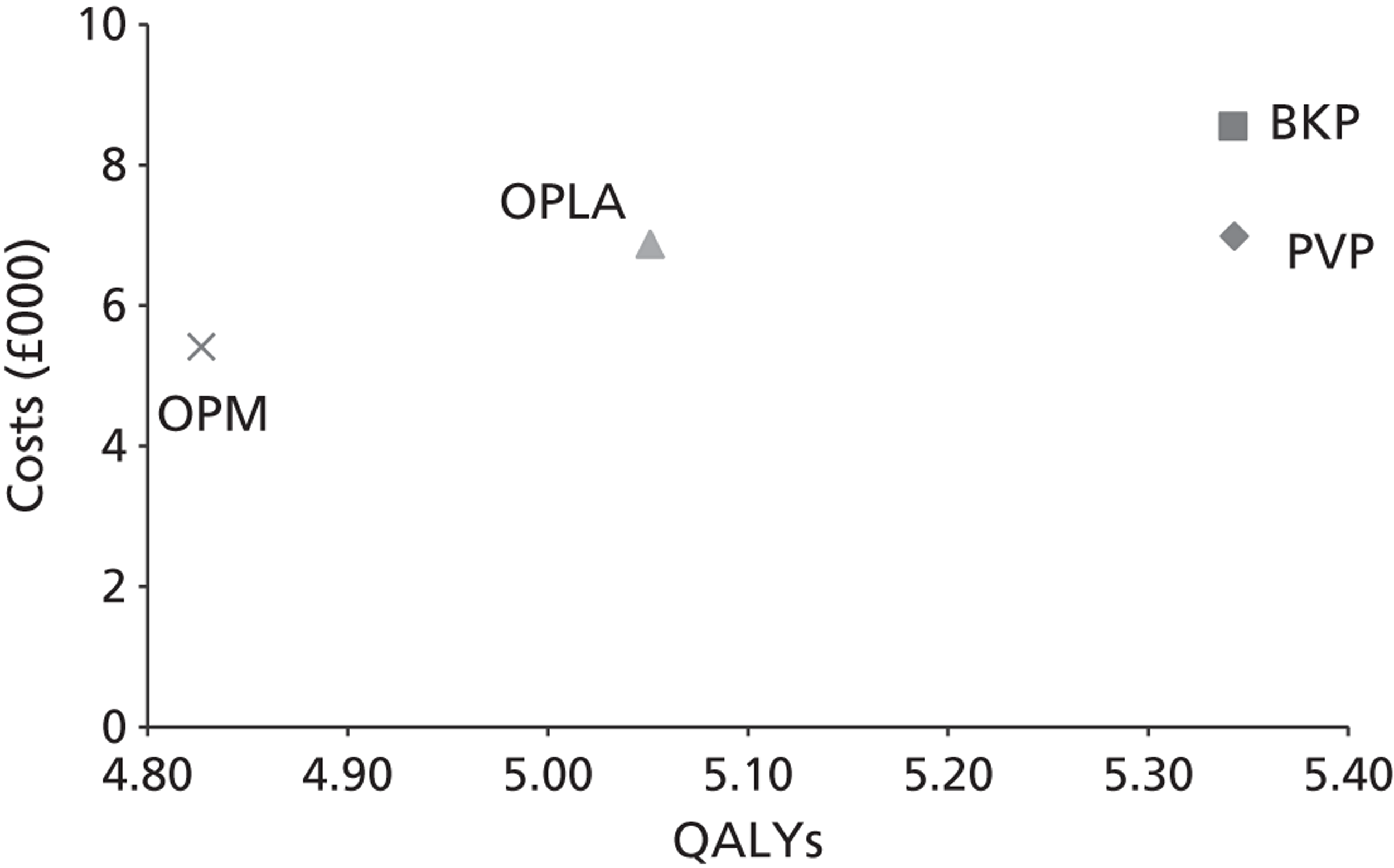
Probabilistic sensitivity analyses were conducted. These results are detailed in Table 79 , with an assessment of the uncertainty of the adoption decision displayed in a CEAC ( Figure 55 ).
| Intervention | Costs (£) | QALYs | ICER (£) |
|---|---|---|---|
| OPM | 6121 | 4.75 | |
| OPLA | 6154 | 4.97 | 149 |
| PVP | 6251 | 5.26 | 336 |
| BKP | 8377 | 5.26 | Dominated |
It is seen that the results from the probabilistic sensitivity analyses differed slightly from the deterministic values.
Table 80 details the sensitivity analyses conducted by the assessment group. Given the non-linearity of the model, the results from the probabilistic sensitivity analyses are presented.
| Intervention | Costs (£) | QALYs | ICER (£) |
|---|---|---|---|
| The probabilistic results produced when hospitalisation costs were set to £0 per day | |||
| OPM | 3196 | 4.75 | |
| OPLA | 5401 | 4.97 | Extendedly dominated |
| PVP | 5498 | 5.26 | 4513 |
| BKP | 7337 | 5.26 | Dominated |
| The probabilistic results produced when the cost of the OPLA procedure was set to 50% that of PVP and the cost of OPLA equipment was set to 60% that of PVP | |||
| OPLA | 5179 | 4.97 | |
| OPM | 6121 | 4.75 | Dominated |
| PVP | 6251 | 5.26 | 3705 |
| BKP | 8377 | 5.26 | Dominated |
| The probabilistic results produced when it was assumed that BKP and PVP were associated with 0.02 QALY loss | |||
| OPM | 6121 | 4.75 | |
| OPLA | 6154 | 4.97 | 149 |
| PVP | 6251 | 5.24 | 361 |
| BKP | 8377 | 5.24 | Dominated |
| All of the above sensitivity analyses combined | |||
| OPM | 3196 | 4.75 | |
| OPLA | 4425 | 4.97 | Extendedly dominated |
| PVP | 5498 | 5.24 | 4697 |
| BKP | 7337 | 5.24 | Dominated |
| All of the above sensitivity analyses combined plus mortality effect of OPLA set to equal BKP and PVP | |||
| OPM | 3196 | 4.75 | |
| OPLA | 4523 | 5.10 | 3705 |
| PVP | 5498 | 5.24 | 7386 |
| BKP | 7337 | 5.24 | Dominated |
Analyses using the Buchbinder data
The deterministic results are presented in Table 81 , with the cost-effectiveness plane depicted in Figure 56 .
| Intervention | Convergence between 12 and 24 months | Convergence between 24 and 36 months | ||||
|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ICER (£) | Costs (£) | QALYs | ICER (£) | |
| OPM | 6181 | 4.75 | 6181 | 4.73 | ||
| OPLA | 6216 | 4.95 | 178 | 6216 | 4.95 | 158 |
| PVP | 6316 | 5.08 | 731 | 6316 | 5.08 | 731 |
| BKP | 8442 | 5.08 | Dominated | 8442 | 5.08 | Dominated |
FIGURE 56.
A plot of the deterministic results produced by the assessment group – scenario 4: Buchbinder data,102 convergence starts at 24 months.
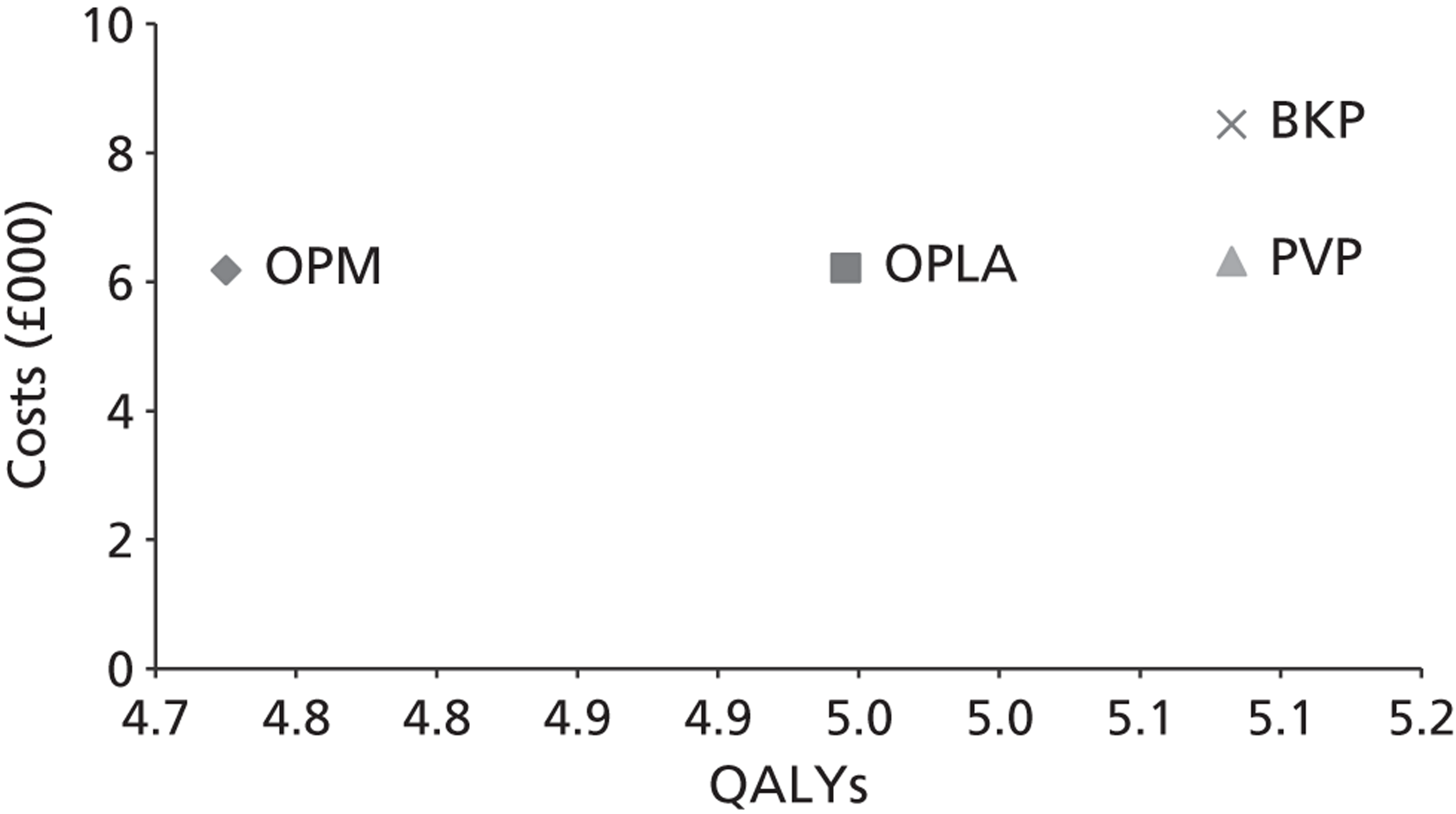
Probabilistic sensitivity analyses were conducted. These results are detailed in Table 82 , with an assessment of the uncertainty of the adoption decision displayed in a CEAC, assuming convergence starts at 24 months ( Figure 57 ).
| Intervention | Convergence between 12 and 24 months | Convergence between 24 and 36 months | ||||
|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ICER (£) | Costs (£) | QALYs | ICER (£) | |
| OPM | 6121 | 4.67 | 6121 | 4.65 | ||
| OPLA | 6154 | 4.86 | 171 | 6154 | 4.86 | 152 |
| PVP | 6251 | 5.00 | 725 | 6251 | 5.00 | 725 |
| BKP | 8377 | 5.00 | Dominated | 8377 | 5.00 | Dominated |
It is seen that the results from the probabilistic sensitivity analyses differed slightly from the deterministic values.
Table 83 details the sensitivity analyses conducted by the assessment group. Given the non-linearity of the model, the results from the probabilistic sensitivity analyses are presented.
| Intervention | Convergence between 12 and 24 months | Convergence between 24 and 36 months | ||||
|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ICER (£) | Costs (£) | QALYs | ICER (£) | |
| The probabilistic results produced when hospitalisation costs were set to £0 per day | ||||||
| OPM | 3196 | 4.67 | 3196 | 4.65 | ||
| OPLA | 5401 | 4.86 | Extendedly dominated | 5401 | 4.86 | Extendedly dominated |
| PVP | 5498 | 5.00 | 7065 | 5498 | 5.00 | 6572 |
| BKP | 7337 | 5.00 | Dominated | 7337 | 5.00 | Dominated |
| The probabilistic results produced when the cost of the OPLA procedure was set to 50% that of PVP and the cost of OPLA equipment was set to 60% that of PVP | ||||||
| OPLA | 5179 | 4.86 | 5179 | 4.86 | ||
| OPM | 6121 | 4.67 | Dominated | 6121 | 4.65 | Dominated |
| PVP | 6251 | 5.00 | 7997 | 6251 | 5.00 | 7997 |
| BKP | 8377 | 5.00 | Dominated | 8377 | 5.00 | Dominated |
| The probabilistic results when it was assumed that BKP and PVP were associated with 0.02 QALY loss | ||||||
| OPM | 6121 | 4.67 | 6121 | 4.65 | ||
| OPLA | 6154 | 4.86 | 171 | 6154 | 4.86 | 152 |
| PVP | 6251 | 4.98 | 852 | 6251 | 4.98 | 852 |
| BKP | 8377 | 4.98 | Dominated | 8377 | 4.98 | Dominated |
| All of the above sensitivity analyses combined | ||||||
| OPM | 3196 | 4.67 | 3196 | 4.65 | ||
| OPLA | 4425 | 4.86 | 6413 | 4425 | 4.86 | 5687 |
| PVP | 5498 | 4.98 | 9399 | 5498 | 4.98 | 9399 |
| BKP | 7337 | 4.98 | Dominated | 7337 | 4.98 | Dominated |
| All of the above sensitivity analyses combined plus mortality effect of OPLA set to equal BKP and PVP | ||||||
| OPM | 3196 | 4.67 | 3196 | 4.65 | ||
| OPLA | 4523 | 5.00 | 4071 | 4523 | 5.00 | 3773 |
| PVP | 5498 | 5.00 | Dominated (7527) | 5498 | 5.00 | Dominated (6943) |
| BKP | 7337 | 5.00 | Dominated (dominated) | 7337 | 5.00 | Dominated (dominated) |
Analyses using the INVEST data
The deterministic results are presented in Table 84 , with the cost-effectiveness plane depicted in Figure 58 .
| Intervention | Convergence between 12 and 24 months | Convergence between 24 and 36 months | ||||
|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ICER (£) | Costs (£) | QALYs | ICER (£) | |
| OPM | 6181 | 4.75 | 6181 | 4.73 | ||
| OPLA | 6216 | 4.95 | 178 | 6216 | 4.95 | 158 |
| PVP | 6316 | 5.10 | 662 | 6316 | 5.10 | 662 |
| BKP | 8442 | 5.10 | Dominated | 8442 | 5.10 | Dominated |
FIGURE 58.
A plot of the deterministic results produced by the assessment group – scenario 4: INVEST data,103 convergence starts at 24 months.
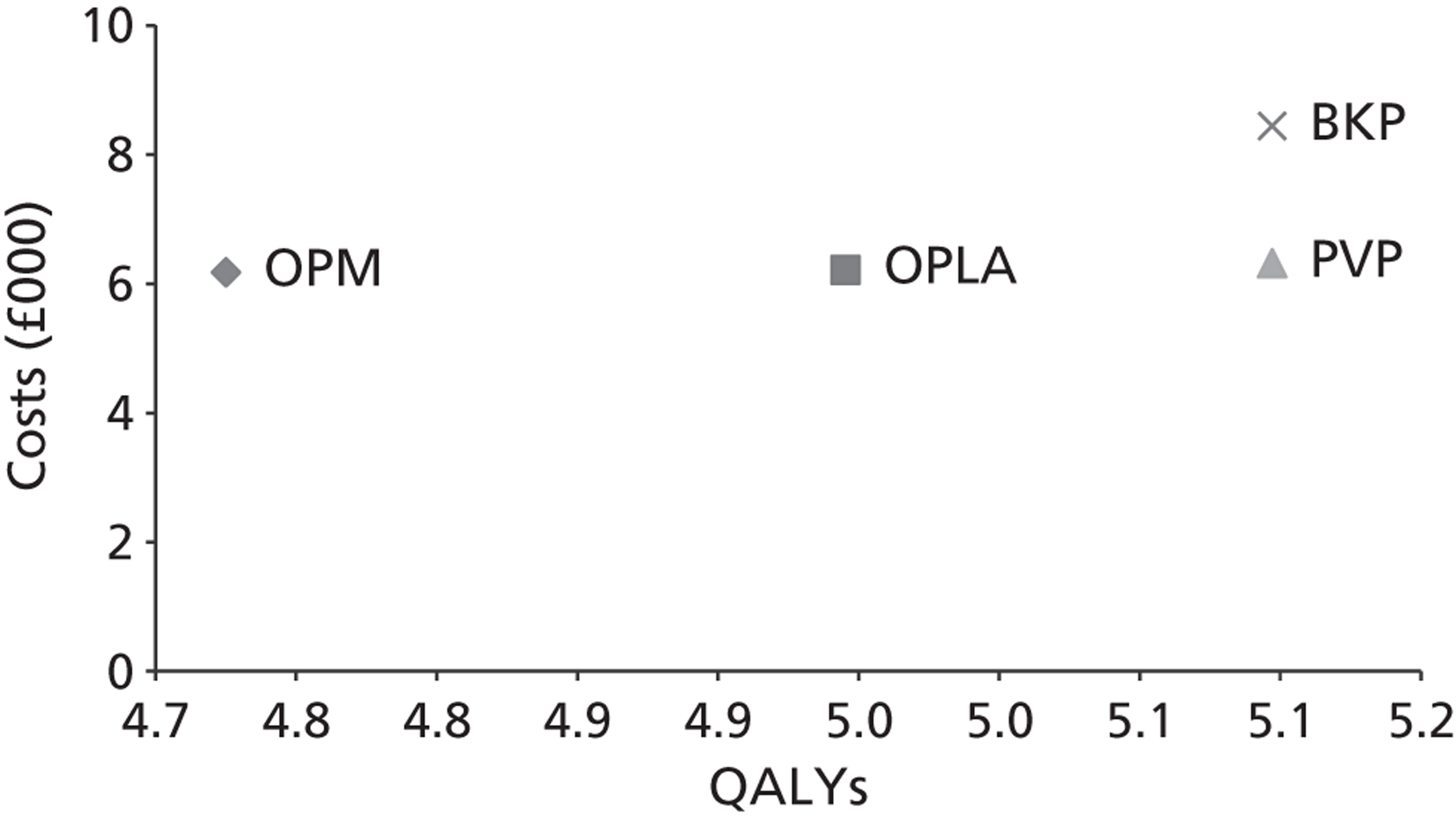
Probabilistic sensitivity analyses were conducted. These results are detailed in Table 85 , with an assessment of the uncertainty of the adoption decision displayed in a CEAC, assuming convergence starts at 24 months ( Figure 59 ).
| Intervention | Convergence between 12 and 24 months | Convergence between 24 and 36 months | ||||
|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ICER (£) | Costs (£) | QALYs | ICER (£) | |
| OPM | 6121 | 4.67 | 6121 | 4.65 | ||
| OPLA | 6154 | 4.86 | 171 | 6154 | 4.86 | 152 |
| PVP | 6251 | 5.01 | 655 | 6251 | 5.01 | 655 |
| BKP | 8377 | 5.01 | Dominated | 8377 | 5.01 | Dominated |
It is seen that the results from the probabilistic sensitivity analyses differed slightly from the deterministic values.
FIGURE 59.
The cost-effectiveness acceptability curve produced by the assessment group – scenario 4: INVEST data. 103
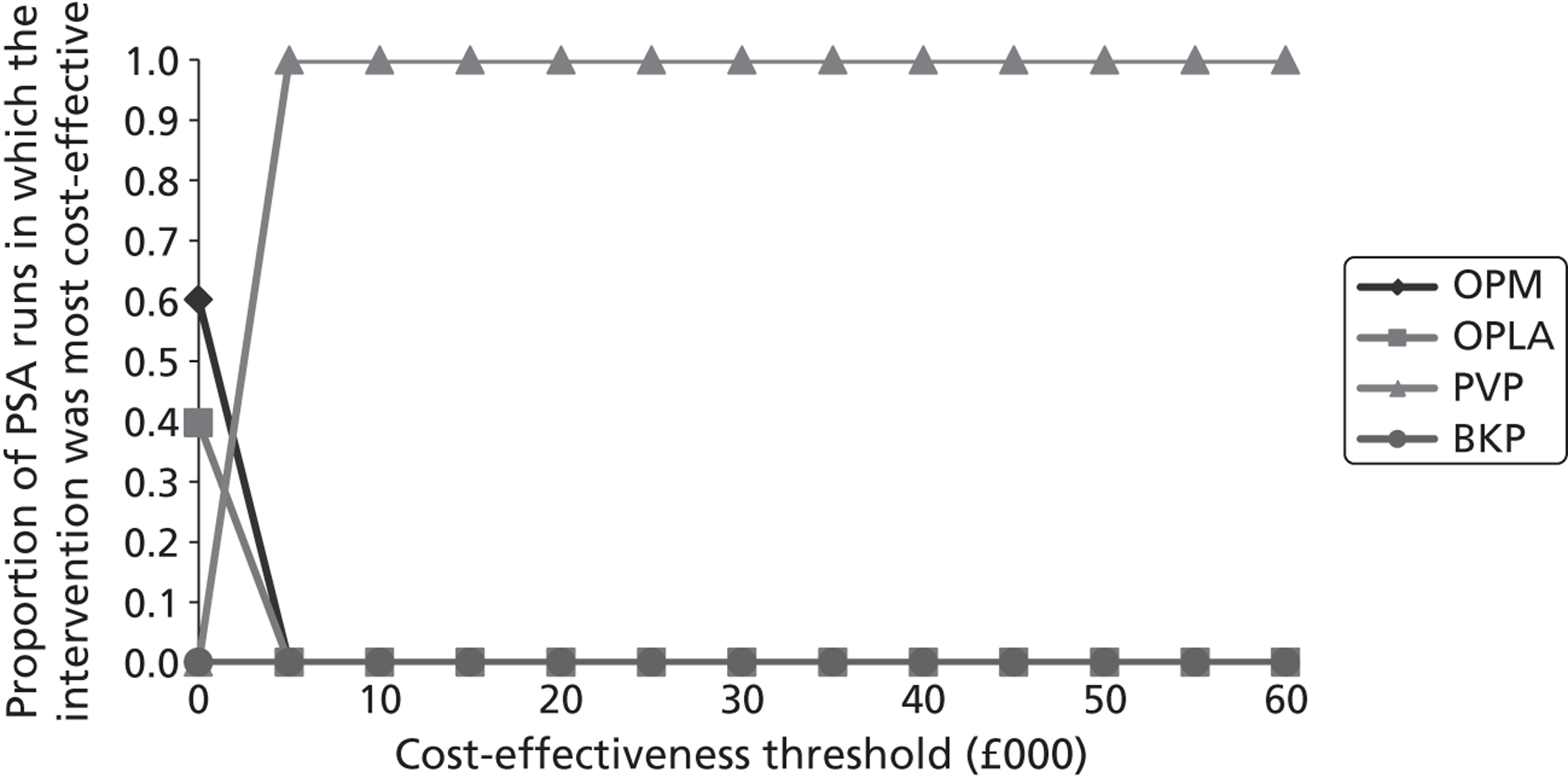
Table 86 details the sensitivity analyses conducted by the assessment group. Given the non-linearity of the model, the results from the probabilistic sensitivity analyses are presented.
| Intervention | Convergence between 12 and 24 months | Convergence between 24 and 36 months | ||||
|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ICER (£) | Costs (£) | QALYs | ICER (£) | |
| The probabilistic results produced when hospitalisation costs were set to £0 per day | ||||||
| OPM | 3196 | 4.67 | 3196 | 4.65 | ||
| OPLA | 5401 | 4.86 | Extendedly dominated | 5401 | 4.86 | Extendedly dominated |
| PVP | 5498 | 5.01 | 6765 | 5498 | 5.01 | 6311 |
| BKP | 7337 | 5.01 | Dominated | 7337 | 5.01 | Dominated |
| The probabilistic results produced when the cost of the OPLA procedure was set to 50% that of PVP and the cost of OPLA equipment was set to 60% that of PVP | ||||||
| OPLA | 5179 | 4.86 | 5179 | 4.86 | ||
| OPM | 6121 | 4.67 | Dominated | 6121 | 4.65 | Dominated |
| PVP | 6251 | 5.01 | 7219 | 6251 | 5.01 | 7219 |
| BKP | 8377 | 5.01 | Dominated | 8377 | 5.01 | Dominated |
| The probabilistic results when it was assumed that BKP and PVP were associated with 0.02 QALY loss | ||||||
| OPM | 6121 | 4.67 | 6121 | 4.65 | ||
| OPLA | 6154 | 4.86 | 171 | 6154 | 4.86 | 152 |
| PVP | 6251 | 4.99 | 756 | 6251 | 4.99 | 756 |
| BKP | 8377 | 4.99 | Dominated | 8377 | 4.99 | Dominated |
| All of the above sensitivity analyses combined | ||||||
| OPM | 3196 | 4.67 | 3196 | 4.65 | ||
| OPLA | 4425 | 4.86 | 6413 | 4425 | 4.86 | 5687 |
| PVP | 5498 | 4.99 | 8342 | 5498 | 4.99 | 8342 |
| BKP | 7337 | 4.99 | Dominated | 7337 | 4.99 | Dominated |
| All of the previous sensitivity analyses combined plus mortality effect of OPLA set to equal BKP and PVP | ||||||
| OPM | 3196 | 4.67 | 3196 | 4.65 | ||
| OPLA | 4523 | 5.00 | 4071 | 4523 | 5.00 | 3773 |
| PVP | 5498 | 5.00 | Dominated (7187) | 5498 | 5.00 | Dominated (6652) |
| BKP | 7337 | 5.00 | Dominated (dominated) | 7337 | 5.00 | Dominated (dominated) |
Scenario 5: differential beneficial effects assumed for balloon kyphoplasty and percutaneous vertebroplasty, utility gain estimated via mapping of stable visual analogue scale
The deterministic results are presented in Table 87 , with the cost-effectiveness plane depicted in Figure 60 .
| Intervention | Costs (£) | QALYs | ICER (£) |
|---|---|---|---|
| OPLA | 6118 | 4.83 | Dominated |
| PVP | 6118 | 4.91 | Dominating |
| OPM | 6181 | 4.74 | Dominated |
| BKP | 8244 | 4.91 | Dominated |
FIGURE 60.
A plot of the deterministic results produced by the assessment group – scenario 5.
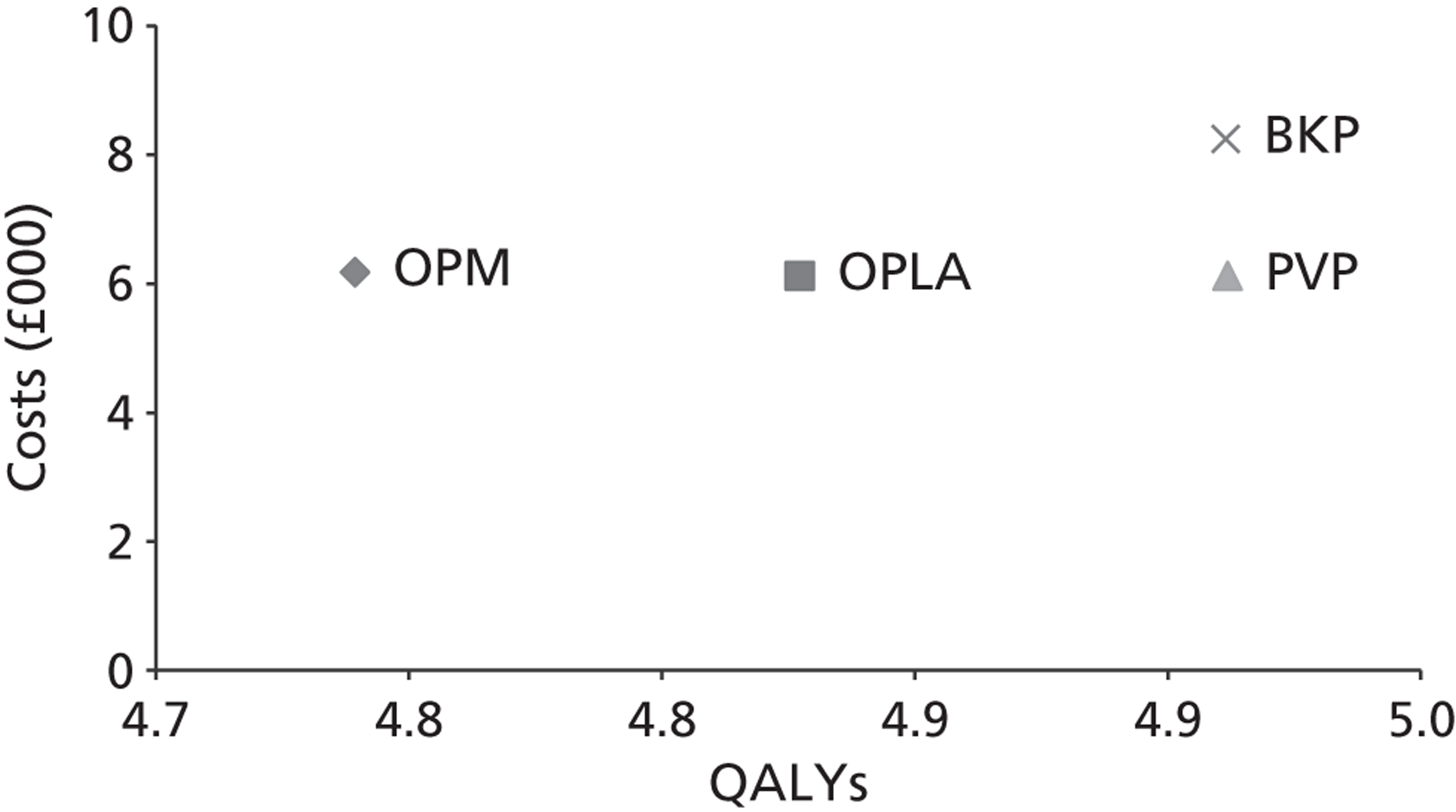
Probabilistic sensitivity analyses were conducted. These results are detailed in Table 88 , with an assessment of the uncertainty of the adoption decision displayed in a CEAC ( Figure 61 ).
| Intervention | Costs (£) | QALYs | ICER (£) |
|---|---|---|---|
| OPLA | 6060 | 3.98 | Dominated |
| PVP | 6060 | 4.06 | Dominating |
| OPM | 6121 | 3.89 | Dominated |
| BKP | 8186 | 4.06 | Dominated |
FIGURE 61.
The CEAC produced by the assessment group – scenario 5.
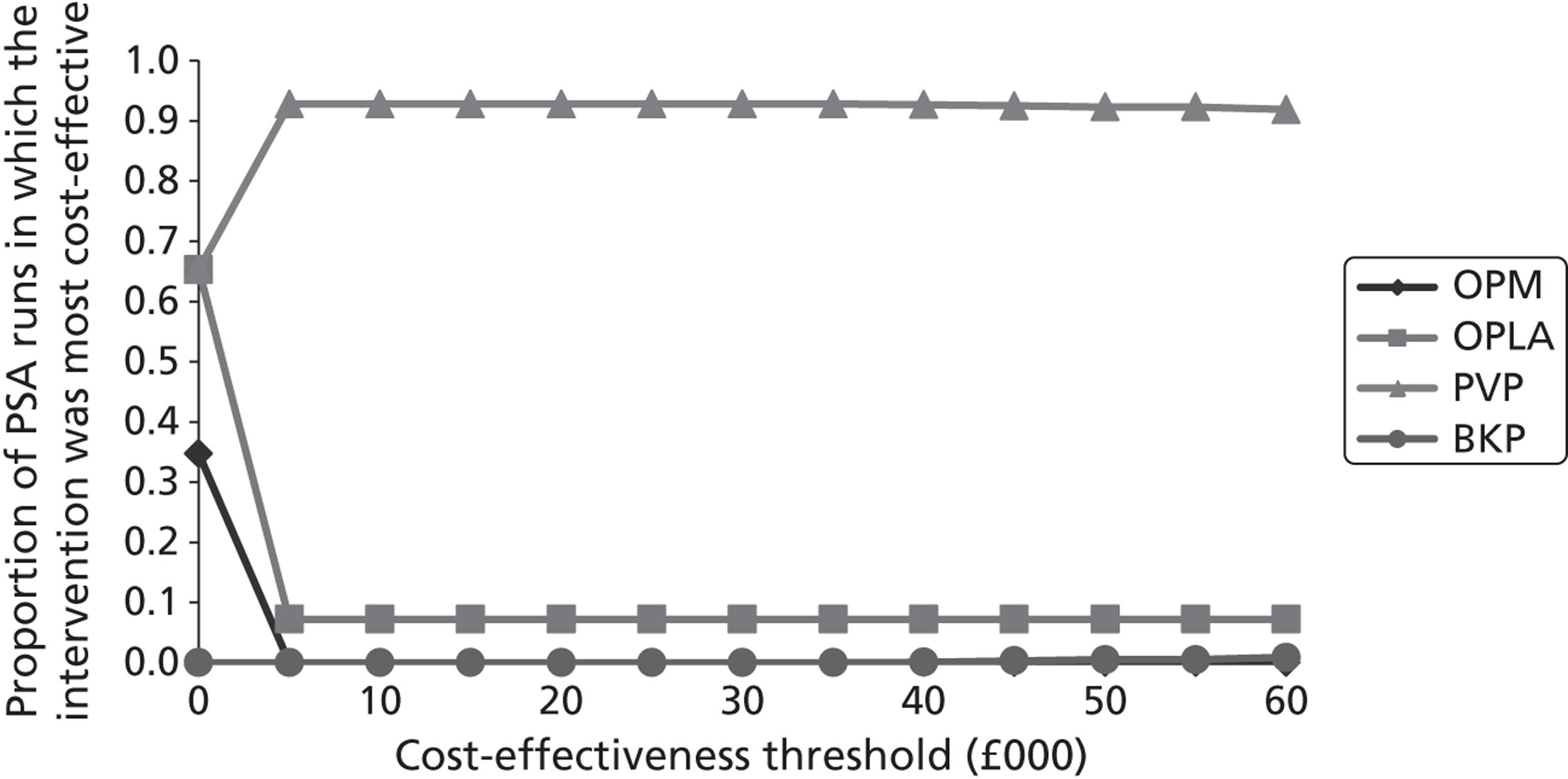
Sensitivity analyses conducted on scenario 5
Table 89 details the sensitivity analyses conducted by the assessment group. Given the non-linearity of the model, the results from the probabilistic sensitivity analyses are presented.
| Intervention | Costs (£) | QALYs | ICER (£) |
|---|---|---|---|
| The probabilistic results produced when hospitalisation costs were set to £0 per day | |||
| OPM | 3196 | 3.89 | |
| OPLA | 5307 | 3.98 | Dominated |
| PVP | 5307 | 4.06 | 12,757 |
| BKP | 7146 | 4.06 | Dominated |
| The probabilistic results produced when the cost of the OPLA procedure was set to 50% that of PVP and the cost of OPLA equipment was set to 60% that of PVP | |||
| OPLA | 5085 | 3.98 | |
| PVP | 6060 | 4.06 | 12,144 |
| OPM | 6121 | 3.89 | Dominated |
| BKP | 8186 | 4.06 | Dominated |
| The probabilistic results produced when it was assumed that convergence of EQ-5D scores began at 12 months and were equal at 24 months | |||
| OPLA | 6060 | 4.01 | Dominated |
| PVP | 6060 | 4.06 | Dominating |
| OPM | 6121 | 3.96 | Dominated |
| BKP | 8186 | 4.06 | Dominated |
| The probabilistic results produced when it was assumed that BKP and PVP were associated with 0.02 QALY loss | |||
| OPLA | 6060 | 3.98 | Dominated |
| PVP | 6060 | 4.04 | Dominating |
| OPM | 6121 | 3.89 | Dominated |
| BKP | 8186 | 4.04 | Dominated |
| All of the above sensitivity analyses combined | |||
| OPM | 3196 | 3.95 | |
| OPLA | 4332 | 4.01 | 19,109 |
| PVP | 5307 | 4.04 | 31,953 (23,469) |
| BKP | 7146 | 4.04 | Dominated (dominated) |
Scenario 6: equal beneficial effects assumed for balloon kyphoplasty and percutaneous vertebroplasty, utility gain estimated via trials reporting European Quality of Life-5 Dimensions
These analyses have been subdivided into three categories based on whether the FREE data,137 the Buchbinder et al. data102 or the INVEST data103 were used.
Analyses using the FREE data
The deterministic results are presented in Table 90 , with the cost-effectiveness plane depicted in Figure 62 .
| Intervention | Costs (£) | QALYs | ICER (£) |
|---|---|---|---|
| OPLA | 6118 | 4.92 | Dominated |
| PVP | 6118 | 5.06 | Dominating |
| OPM | 6181 | 4.83 | Dominated |
| BKP | 8244 | 5.06 | Dominated |
FIGURE 62.
A plot of the deterministic results produced by the assessment group – scenario 6: FREE data. 137
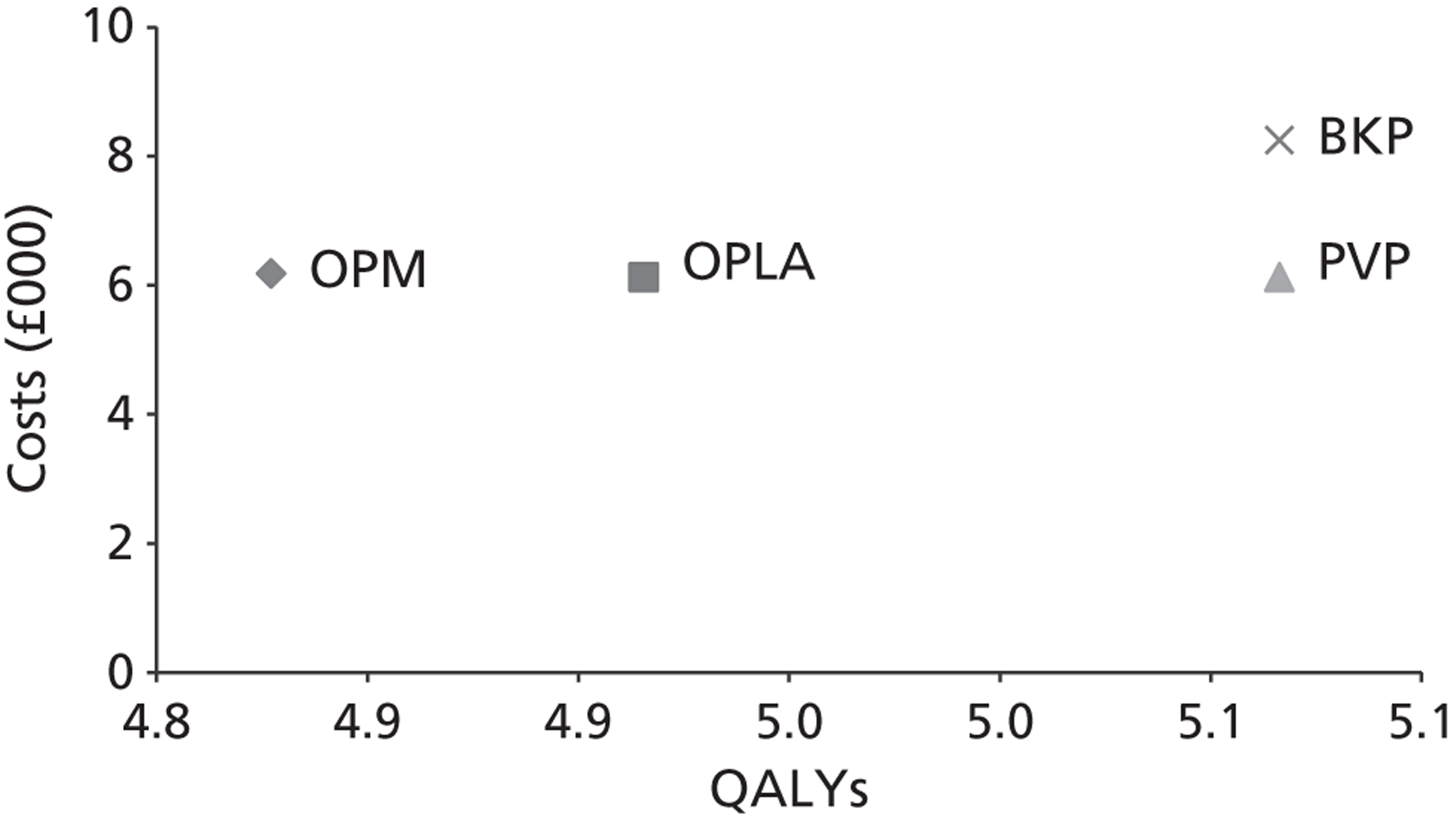
Probabilistic sensitivity analyses were conducted. These results are detailed in Table 91 , with an assessment of the uncertainty of the adoption decision displayed in a CEAC ( Figure 63 ).
| Intervention | Costs (£) | QALYs | ICER (£) |
|---|---|---|---|
| OPLA | 6060 | 4.83 | Dominated |
| PVP | 6060 | 4.98 | Dominating |
| OPM | 6121 | 4.75 | Dominated |
| BKP | 8186 | 4.98 | Dominated |
It is seen that the results from the probabilistic sensitivity analyses differed slightly from the deterministic values.
Table 92 details the sensitivity analyses conducted by the assessment group. Given the non-linearity of the model, the results from the probabilistic sensitivity analyses are presented.
| Intervention | Costs (£) | QALYs | ICER (£) |
|---|---|---|---|
| The probabilistic results produced when hospitalisation costs were set to £0 per day | |||
| OPM | 3196 | 4.75 | |
| OPLA | 5307 | 4.83 | Dominated |
| PVP | 5307 | 4.98 | 8885 |
| BKP | 7146 | 4.98 | Dominated |
| The probabilistic results produced when the cost of the OPLA procedure was set to 50% that of PVP and the cost of OPLA equipment was set to 60% that of PVP | |||
| OPLA | 5085 | 4.83 | |
| PVP | 6060 | 4.98 | £6514 |
| OPM | 6121 | 4.75 | Dominated |
| BKP | 8186 | 4.98 | Dominated |
| The probabilistic results produced when it was assumed that BKP and PVP were associated with 0.02 QALY loss | |||
| OPLA | 6060 | 4.83 | Dominated |
| PVP | 6060 | 4.96 | Dominating |
| OPM | 6121 | 4.75 | Dominated |
| BKP | 8186 | 4.96 | Dominated |
| All of the above sensitivity analyses combined | |||
| OPM | 3196 | 4.75 | |
| OPLA | 4332 | 4.83 | Extendedly dominated |
| PVP | 5307 | 4.96 | 9701 |
| BKP | 7146 | 4.96 | Dominated |
Analyses using the Buchbinder et al. data
The deterministic results are presented in Table 93 , with the cost-effectiveness plane depicted in Figure 64 .
| Intervention | Convergence between 12 and 24 months | Convergence between 24 and 36 months | ||||
|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ICER (£) | Costs (£) | QALYs | ICER (£) | |
| OPLA/PVP | 6118 | 4.81 | Dominating | 6118 | 4.81 | Dominating |
| OPM | 6181 | 4.75 | Dominated | 6181 | 4.73 | Dominated |
| BKP | 8244 | 4.81 | Dominated | 8244 | 4.81 | Dominated |
FIGURE 64.
A plot of the deterministic results produced by the assessment group – scenario 6: Buchbinder data,102 convergence starts at 24 months.
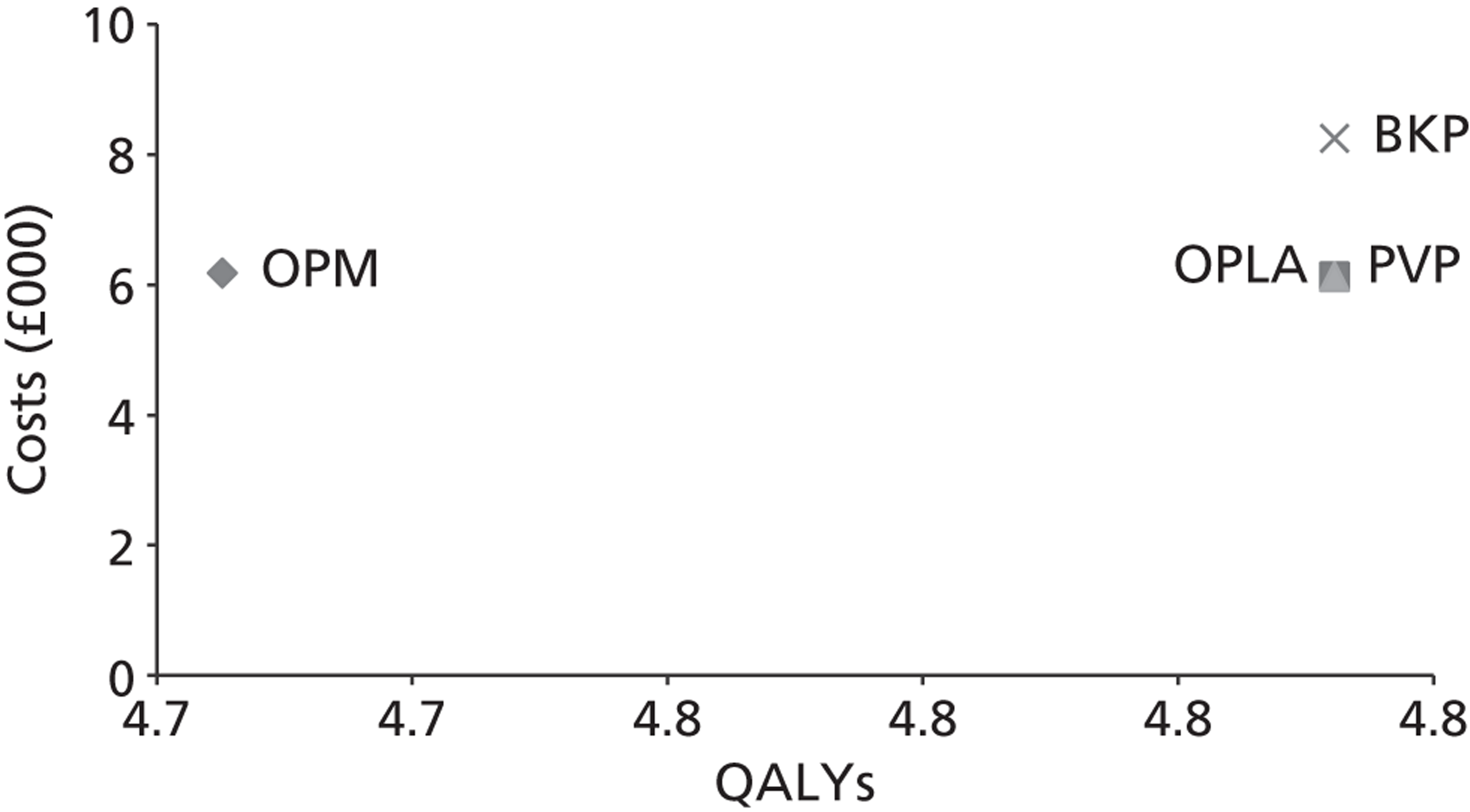
Probabilistic sensitivity analyses were conducted. These results are detailed in Table 94 , with an assessment of the uncertainty of the adoption decision displayed in a CEAC, assuming convergence starts at 24 months ( Figure 65 ).
| Intervention | Convergence between 12 and 24 months | Convergence between 24 and 36 months | ||||
|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ICER (£) | Costs (£) | QALYs | ICER (£) | |
| OPLA/PVP | 6060 | 4.73 | Dominating | 6060 | 4.73 | Dominating |
| OPM | 6121 | 4.67 | Dominated | 6121 | 4.65 | Dominated |
| BKP | 8186 | 4.73 | Dominated | 8186 | 4.73 | Dominated |
It is seen that the results from the probabilistic sensitivity analyses differed slightly from the deterministic values.
Table 95 details the sensitivity analyses conducted by the assessment group. Given the non-linearity of the model, the results from the probabilistic sensitivity analyses are presented.
| Intervention | Convergence between 12 and 24 months | Convergence between 24 and 36 months | ||||
|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ICER (£) | Costs (£) | QALYs | ICER (£) | |
| The probabilistic results produced when hospitalisation costs were set to £0 per day | ||||||
| OPM | 3196 | 4.67 | 3196 | 4.65 | ||
| OPLA/PVP | 5307 | 4.73 | 33,963 | 5307 | 4.73 | 24,336 |
| BKP | 7146 | 4.73 | Dominated | 7146 | 4.73 | Dominated |
| The probabilistic results produced when the cost of the OPLA procedure was set to 50% that of PVP and the cost of OPLA equipment was set to 60% that of PVP | ||||||
| OPLA | 5085 | 4.73 | 5085 | 4.73 | ||
| PVP | 6060 | 4.73 | Dominated (dominating) | 6060 | 4.73 | Dominated (dominating) |
| OPM | 6121 | 4.67 | Dominated | 6121 | 4.65 | Dominated |
| BKP | 8186 | 4.73 | Dominated (dominated) | 8186 | 4.73 | Dominated (dominated) |
| The probabilistic results when it was assumed that BKP and PVP were associated with 0.02 QALY loss | ||||||
| OPLA | 6060 | 4.73 | 6060 | 4.73 | ||
| PVP | 6060 | 4.71 | Dominated (dominating) | 6060 | 4.71 | Dominated (dominating) |
| OPM | 6121 | 4.67 | Dominated | 6121 | 4.65 | Dominated |
| BKP | 8186 | 4.71 | Dominated (dominated) | 8186 | 4.71 | Dominated (dominated) |
| All of the above sensitivity analyses combined | ||||||
| OPM | 3196 | 4.67 | 3196 | 4.65 | ||
| OPLA | 4332 | 4.73 | 18,268 | 4332 | 4.73 | 13,106 |
| PVP | 5307 | 4.71 | Dominated (50,076) | 5307 | 4.71 | Dominated (31,679) |
| BKP | 7146 | 4.71 | Dominated (dominated) | 7146 | 4.71 | Dominated (dominated) |
Analyses using the INVEST data
The deterministic results are presented in Table 96 , with the cost-effectiveness plane depicted in Figure 66 .
| Intervention | Convergence between 12 and 24 months | Convergence between 24 and 36 months | ||||
|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ICER (£) | Costs (£) | QALYs | ICER (£) | |
| OPLA | 6118 | 4.81 | Dominated | 6118 | 4.81 | Dominated |
| PVP | 6118 | 4.83 | Dominating | 6118 | 4.83 | Dominating |
| OPM | 6181 | 4.75 | Dominated | 6181 | 4.73 | Dominated |
| BKP | 8244 | 4.83 | Dominated | 8244 | 4.83 | Dominated |
FIGURE 66.
A plot of the deterministic results produced by the assessment group – scenario 6: INVEST data,103 convergence starts at 24 months.
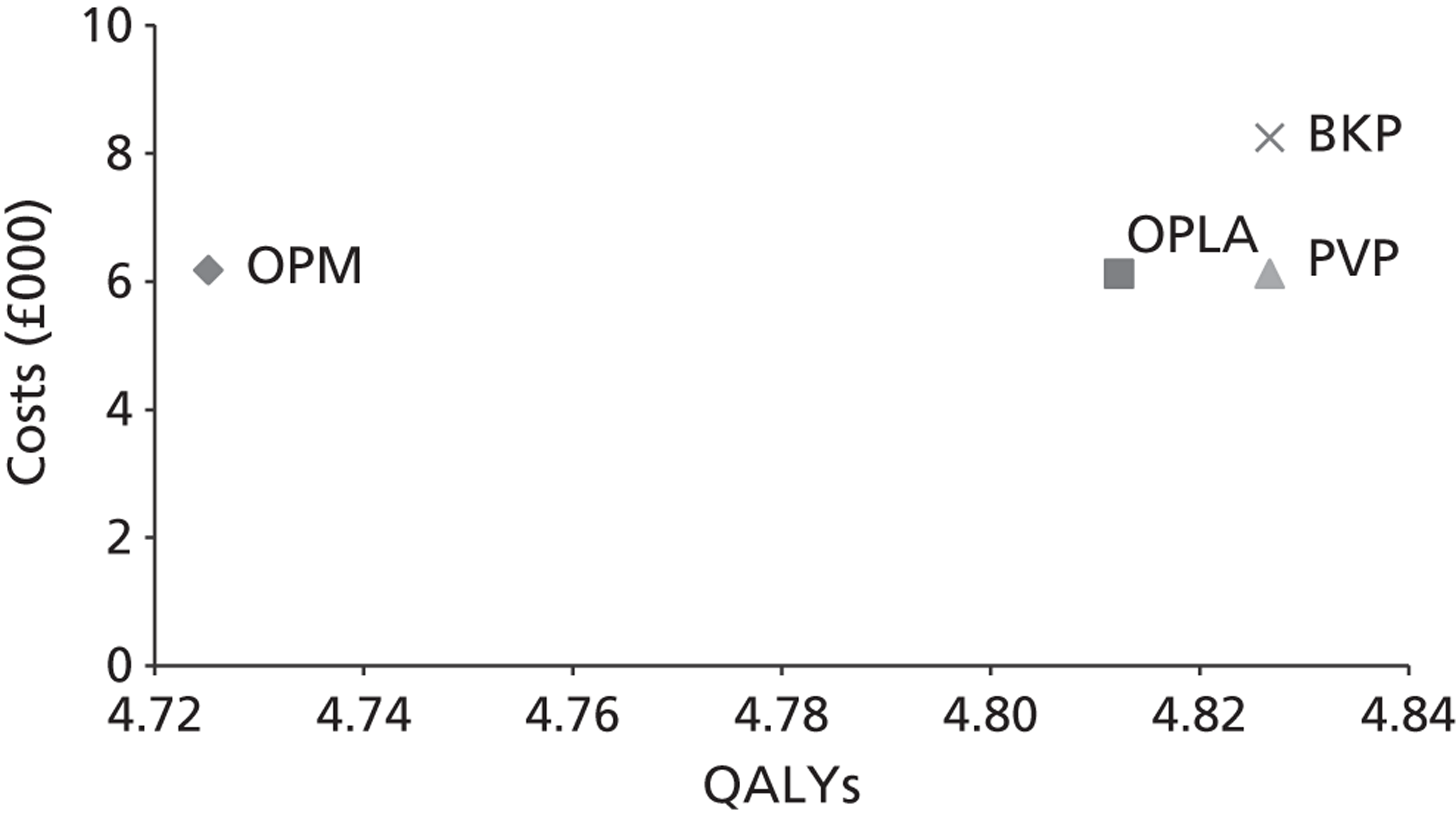
Probabilistic sensitivity analyses were conducted. These results are detailed in Table 97 , with an assessment of the uncertainty of the adoption decision displayed in a CEAC, assuming convergence starts at 24 months ( Figure 67 ).
| Intervention | Convergence between 12 and 24 months | Convergence between 24 and 36 months | ||||
|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ICER (£) | Costs (£) | QALYs | ICER (£) | |
| OPLA | 6060 | 4.73 | Dominated | 6060 | 4.73 | Dominated |
| PVP | 6060 | 4.75 | Dominating | 6060 | 4.75 | Dominating |
| OPM | 6121 | 4.67 | Dominated | 6121 | 4.65 | Dominated |
| BKP | 8186 | 4.75 | Dominated | 8186 | 4.75 | Dominated |
It is seen that the results from the probabilistic sensitivity analyses differed slightly from the deterministic values.
FIGURE 67.
The cost-effectiveness acceptability curve produced by the assessment group – scenario 6: INVEST data. 103
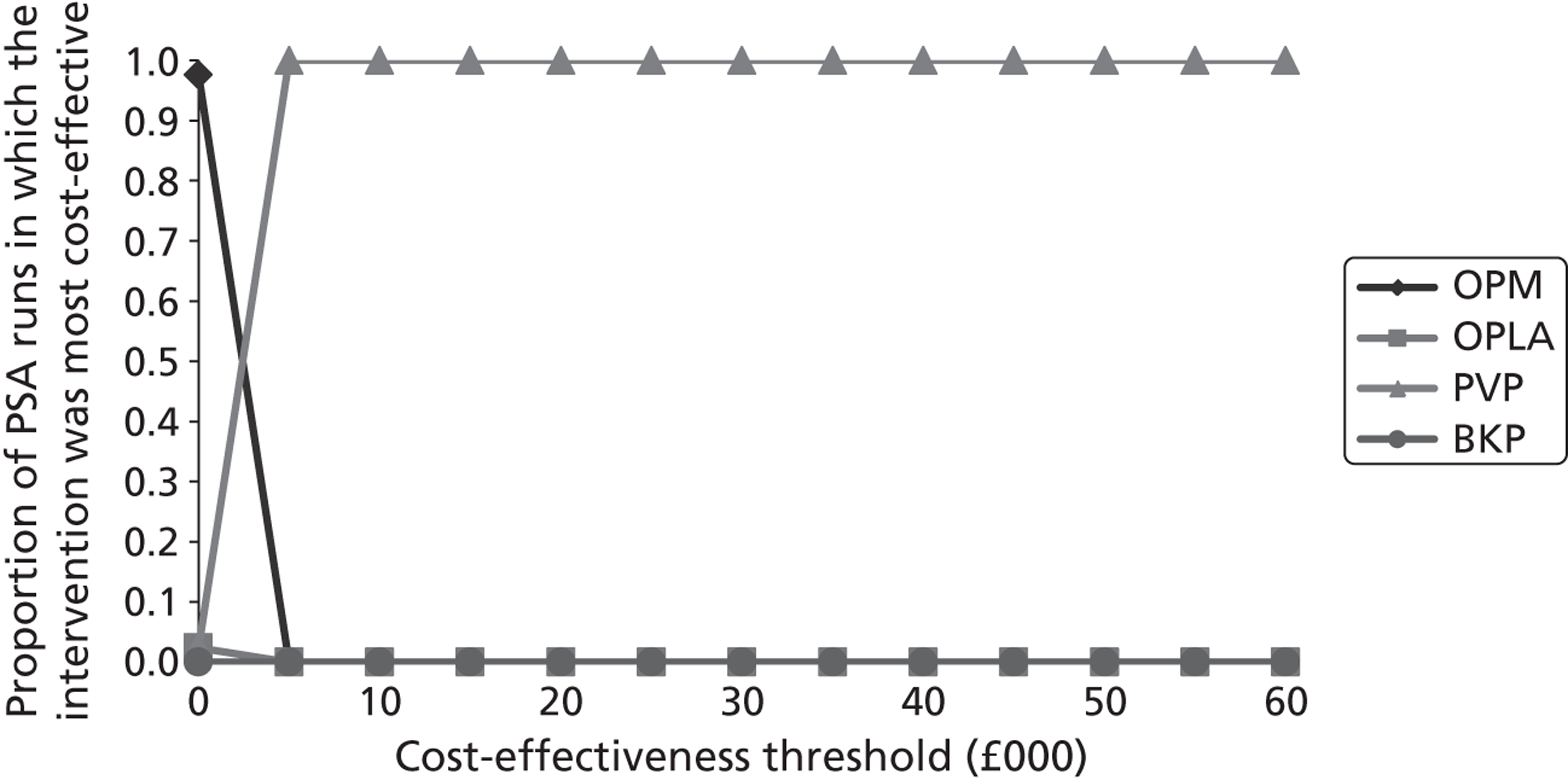
Table 98 details the sensitivity analyses conducted by the assessment group. Given the non-linearity of the model, the results from the probabilistic sensitivity analyses are presented.
| Intervention | Convergence between 12 and 24 months | Convergence between 24 and 36 months | ||||
|---|---|---|---|---|---|---|
| Costs (£) | QALYs | ICER (£) | Costs (£) | QALYs | ICER (£) | |
| The probabilistic results produced when hospitalisation costs were set to £0 per day | ||||||
| OPM | 3196 | 4.67 | 3196 | 4.65 | ||
| OPLA | 5307 | 4.73 | Dominated | 5307 | 4.73 | Dominated |
| PVP | 5307 | 4.75 | 27,577 | 5307 | 4.75 | 20,895 |
| BKP | 7146 | 4.75 | Dominated (dominated) | 7146 | 4.75 | Dominated (dominated) |
| The probabilistic results produced when the cost of the OPLA procedure was set to 50% that of PVP and the cost of OPLA equipment was set to 60% that of PVP | ||||||
| OPLA | 5085 | 4.73 | Dominated | 6060 | 4.73 | Dominated |
| PVP | 6060 | 4.75 | 67,780 (dominating) | 6060 | 4.75 | 67,780 (dominating) |
| OPM | 6121 | 4.67 | Dominated | 6121 | 4.65 | Dominated |
| BKP | 8186 | 4.75 | Dominated | 8186 | 4.75 | Dominated |
| The probabilistic results when it was assumed that BKP and PVP were associated with 0.02 QALY loss | ||||||
| OPLA | £6060 | 4.73 | Dominating | 6060 | 4.73 | Dominated |
| PVP | 6060 | 4.73 | Dominated (dominating) | 6060 | 4.73 | Dominating |
| OPM | 6121 | 4.67 | Dominated | 6121 | 4.65 | Dominated |
| BKP | 8186 | 4.73 | Dominated (dominated) | 8186 | 4.73 | Dominated (dominated) |
| All of the above sensitivity analyses combined | ||||||
| OPM | 3196 | 4.67 | 3196 | 4.65 | ||
| OPLA | 4332 | 4.73 | 18,268 | 4332 | 4.73 | 13,106 |
| PVP | 5307 | 4.73 | Dominated (37,331) | 5307 | 4.73 | Dominated (26,052) |
| BKP | 7146 | 4.73 | Dominated (dominated) | 7146 | 4.73 | Dominated (dominated) |
Interpretation of the results
If differential mortality effects with BKP being more effective than PVP were assumed (scenarios 1 and 2), then BKP always provided the most QALYs and always below a cost of £20,000 per QALY gained. This was maintained even if the cost of BKP was increased, assuming a separate kit was needed for each level (data not shown).
Contrastingly, if it was assumed that the mortality effects of PVP and BKP were identical, with OPLA providing half the benefit (scenarios 3 and 4), BKP was estimated to be dominated by PVP providing effectively the same QALYs at a higher cost, with PVP having an ICER of below £10,000 per QALY gained compared with OPM and OPLA. In the analyses where OPLA was assumed to have an identical mortality benefit to BKP and PVP, PVP became dominated by OPLA when OPLA cost less than PVP and when QALY losses due to adverse events for PVP and the EQ-5D data from the RCTs were used. However, if OPLA was not deemed to be an appropriate comparator, the ICER of PVP compared with OPM remained below £10,000 per QALY gained.
In the analysis where it was assumed that no intervention provided a mortality benefit compared with OPM (scenarios 5 and 6) then the conclusions altered depending on the assumptions made, in particular the assumed hospitalisation costs. Using the HES data from Dr Foster, these indicated that PVP was typically the dominant procedure; however, assuming equal hospitalisation costs for all interventions increased the cost per QALY of PVP to > £20,000 within the Buchbinder and INVEST scenarios, which represent the data from the blinded RCTs. The most pessimistic sensitivity analyses evaluated indicated that PVP was dominated by OPLA and had an ICER in excess of £25,000 per QALY compared with OPM, should OPLA be considered an inappropriate comparator.
Thus, the intervention that is estimated to be most cost-effective is heavily dependent on the assumptions chosen. Given the uncertainty regarding the mortality effects of the treatments (including for OPLA), the proportions of patients who receive vertebral augmentation as a day-case procedure and the potential limitations of data from unblinded RCTs, a definitive conclusion cannot be provided.
Additional exploratory analyses
The use of high-viscosity cement for all patients
The definition of PVP within the analyses is that of low-viscosity cement use for the majority of patients and with high-viscosity cement being used in the estimated 15% of patients in whom our clinical advisor (DW) believed that this was a clinical necessity. An alternative strategy (and the one used by Johnson & Johnson)36 is to assume that all patients receive Johnson & Johnson’s high-viscosity cement at an additional cost of £746 per patient. Figure 68 shows the exploratory analysis undertaken regarding the cost per QALY gained of using high-viscosity cement when the assumed QALY increases associated with the use of high-viscosity rather than low-viscosity cement are used.
FIGURE 68.
An exploratory analysis of effect of assuming additional QALY gains associated with high-viscosity cement compared with low-viscosity cement.
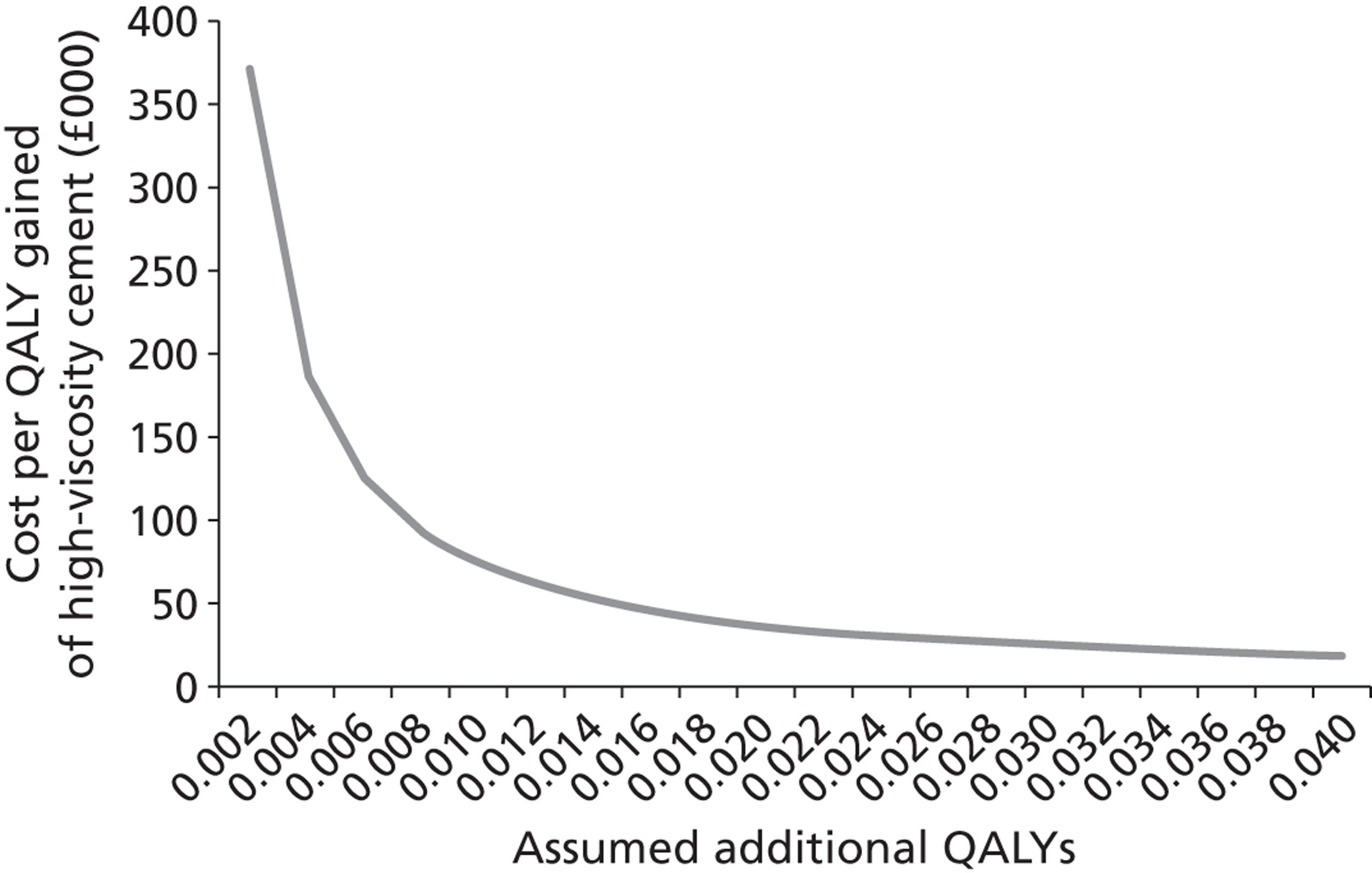
It is calculated that there would need to be an additional 0.037 QALYs for the cost per QALY gained to be equal to £20,000 per QALY gained. It is noted that this value is greater than the value of 0.02 discounted QALYs assumed in the sensitivity analyses that was estimated assuming that 1 in 1000 people died (incurring a loss of 10 discounted QALYs) and that 1 in 100 people experienced morbidity resulting in a loss of 1 discounted QALYs when using low-viscosity cement. As such, it is unlikely that the ICER of high-viscosity cement compared with low viscosity cement would be < £20,000 per QALY gained.
The above analysis assumes that costs would remain constant, whereas there is a possibility that operations would need to be reperformed were there to be a problem with low-viscosity cement. In order to explore this impact, the costs per QALY gained of high-viscosity cement at different levels of reoperation rates were estimated. These data are shown in Figure 69 . In order for the graph to be shown, the operation cost associated with PVP of £1479 was assumed to be correct rather than the academic-in-confidence value used by the assessment group. It was assumed that all reoperations would be undertaken using high-viscosity cements.
FIGURE 69.
An exploratory analysis of effect of assuming additional reoperations associated with high-viscosity cement compared with low-viscosity cement.
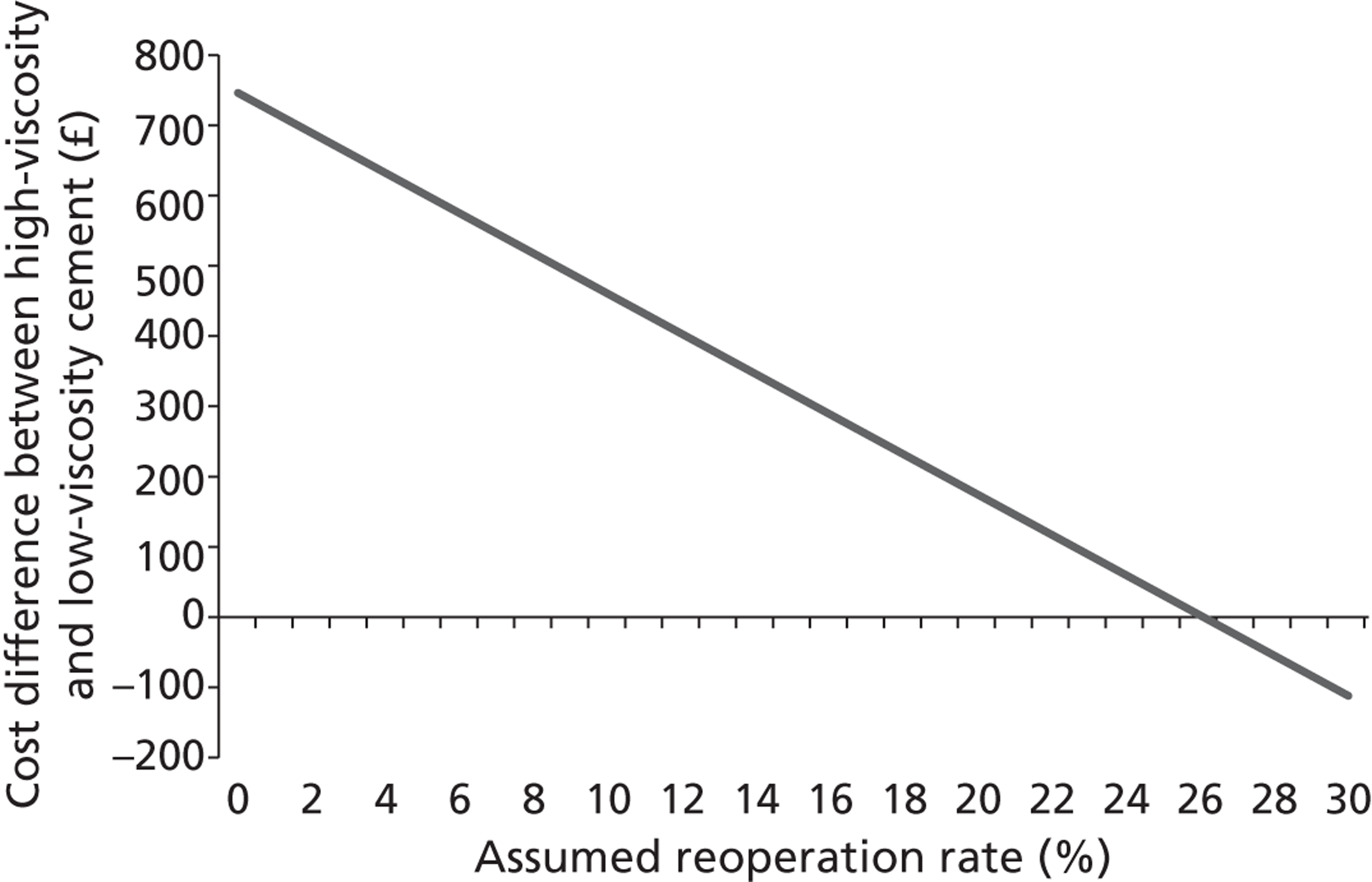
It is calculated that there would need to be a reoperation rate in excess of 25% in order for a strategy of using high-viscosity cement in all patients to be cheaper than using it in a selected 15% of patients, assuming that QALYs remained unaltered. Such values have not been reported, with the only identified estimate being less than 1.5%244 and uncertainty regarding whether or not high-viscosity cement would have prevented the reoperation in each case.
However, it is likely that if reoperations were required then there would also be a QALY effect, and thus the cost per QALY gained of selected combinations has been calculated as shown in Table 99 .
| Assumed QALY increase associated with high-viscosity cement | Assumed reoperation rate | ||||
|---|---|---|---|---|---|
| 0% | 5% | 10% | 15% | 20% | |
| 0.000 | Dominated | Dominated | Dominated | Dominated | Dominated |
| 0.005 | £149,200 | £120,630 | £92,060 | £63,490 | £34,920 |
| 0.010 | £74,600 | £60,315 | £46,030 | £31,745 | £17,460 |
| 0.015 | £49,733 | £40,210 | £30,687 | £21,163 | £11,640 |
| 0.020 | £37,300 | £30,158 | £23,015 | £15,873 | £8730 |
| 0.025 | £29,840 | £24,126 | £18,412 | £12,698 | £6984 |
| 0.030 | £24,867 | £20,105 | £15,343 | £10,582 | £5820 |
Based on the results shown in Table 99 it is unlikely that a strategy of using high-viscosity cement in all patients rather than a subset selected by the clinician would have an ICER of < £20,000 per QALY gained.
Facet joint injections
Consideration was given to explicitly modelling the cost-effectiveness of a pathway involving an initial facet joint injection. Our clinical advisor indicates that a facet joint injection is an outpatient procedure, requiring 15–20 minutes of fluoroscopy room time as well as a radiologist and radiographer for this period, and incurs approximately £60 of drugs and consumables per case. Our clinical advisor (DW) indicated that the cost of a facet joint injection was unlikely to exceed £200 per patient. As such, it is significantly cheaper than PVP or BKP and has been shown both to reduce the numbers of patients progressing to vertebral augmentation and to improve the response rate of those requiring PVP. 84 It is currently unclear whether the increased response is due to removing patients who would have healed naturally without augmentation or whether there is a placebo response to the injection. As anecdotally the use of initial facet joint injections appears to be widespread in the UK, and as facet joint injections were neither interventions nor comparators in the NICE evaluation,363 it was decided to not model facet joint injections.
However, if the use of facet joint injections increases the likelihood of patients responding to vertebral augmentation and facet joint injection-experienced patients were excluded from the RCTs, then the benefit of PVP or BKP may be underestimated. An exploratory analysis of the effect of prior facet joint injection has been undertaken. If it is assumed that one-third of patients would respond to a facet joint injection84 and that these would have exhibited identical VAS/EQ-5D effects regardless of the treatment arm, then the average VAS or EQ-5D difference shown in the entire population would be estimated to be increased by 50% when just considering those who did not respond to the facet joint injection. If it is assumed that the entire QALY difference is due to VAS/EQ-5D scores (rather than adverse events) then the ICER would be reduced by one-third, implying that ICERs of £30,000 may be reduced to £20,000 if all patients had a facet joint injection initially. These analyses are particularly pertinent for vertebral augmentation, which is being considered to be undertaken as a day-case procedure, and hence where large hospitalisation costs are inappropriate.
Within the NICE appraisal process it was commented that it was unclear if facet joint injections were widely undertaken in England and Wales. Additional enquiries were undertaken to gather more information on the prevalence of routine facet joint injections in the UK. Thirty-five vertebroplasty practitioners were emailed to ask if they routinely screen patients by performing facet joint injection prior to considering cement augmentation. Four emails bounced and 10 centres did not respond within the required timescale. Of the 21 centres that responded (locations ranging from Exeter to Dundee), 10 routinely performed facet joint injections and 11 did not. Given that a facet joint injection is relatively commonly used, relatively inexpensive and may have considerable benefit in up to one-third of patients,84 it is likely that this is considered an appropriate first measure.
Additional patient education
The double-blinded trials showed minimal difference between PVP and OPLA, indicating that the benefit seen within the unblended trials could be driven by a response to the OPLA intervention. It is unclear whether or not this response can be generated by less intensive methods than preparing a patient for an operation but performing OPLA rather than vertebral augmentation. An exploratory analysis has been performed to estimate the maximum expenditure that could be provided in educating patients in order that the OPLA responses were assumed to be generated in people receiving OPM, while maintaining a cost-per-QALY-gained ratio of PVP compared with OPM of > £20,000.
The analysis assumed that there was no beneficial mortality effect of either PVP or BKP, as in this instance the ICERs for vertebral augmentation were cost-effective compared with OPLA. The exploratory analysis evaluated three scenarios, namely scenario 5, scenario 6: Buchbinder, and scenario 6: INVEST, as these are the studies that explicitly take the relationship between OPLA and PVP into account. It was assumed that OPM would have identical results to those for OPLA and that, ignoring patient education costs, OPM would cost £2111 less than PVP, comprising a cost of £800 for the PVP equipment and £1311 associated with the operation. As the results from Buchbinder et al. 102 produced identical improvements from PVP and OPLA, then for this study the decision simplified into cost-minimisation (excluding the possibility of adverse events) and OPM would be seen to dominate PVP if the education costs were below £2111 per person and be dominated if the cost was above this value. For scenario 5 and scenario 6: INVEST, Figure 70 indicates the cost per QALY given different assumed costs of patient education. It is seen that the cost of patient education (per person) would need to be > £500 in scenario 5 and > £1800 in scenario 6: INVEST for PVP to have a cost per QALY gained of < £20,000 compared with patient education.
FIGURE 70.
An exploratory analysis of the cost per QALY gained of PVP vs. OPM, assuming that the response of OPLA could be generated in OPM patients through education.
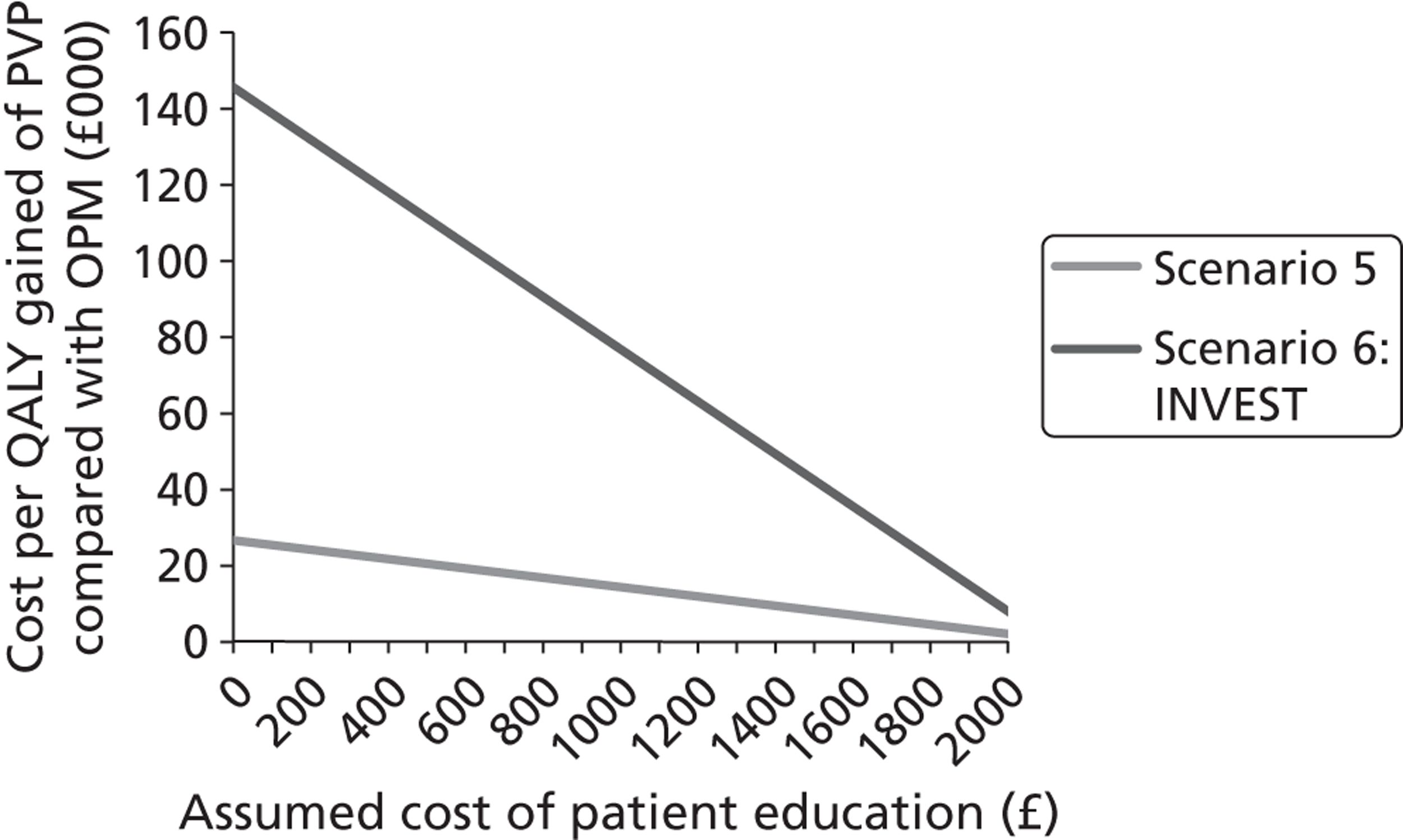
It is commented that this analysis is exploratory. It is not known whether or not the OPLA response could be generated by education nor are the likely costs of patient education known.
Chapter 5 Assessment of factors relevant to the National Health Service and other parties
Place of percutaneous vertebroplasty and balloon kyphoplasty in the treatment pathway
Balloon kyphoplasty and PVP are remedial measures, and the prevention of VCFs should ideally be pursued to avoid unnecessary surgical procedures. There is evidence of suboptimal utilisation of pharmacological treatments, such as bisphosphonates, for at-risk patients. 364,365 Proactive case selection strategies have shown promise for enhancing appropriate prescribing. 365
Care providers will need to consider at what point in the treatment pathway PVP or BKP should be offered. Wilson et al. have suggested that PVP and BKP should not be considered as a first line of treatment. 366 The same authors have found evidence to suggest that facet joint injections may be clinically effective in a substantial minority of patients. 84 Hence, these investigators recommended an initial period of conservative management followed by a facet joint injection, with augmentation offered only after failure of both of these less invasive approaches.
Similarly, previous NICE guidance from 2003367 suggests that PVP should be used only if pain is refractory to non-surgical pain management, while NHS Oxfordshire guidance368 suggests that PVP should be offered only after at least 4 weeks’ OPM, including local anaesthetic/steroid injection to the affected area.
Ethical issues and the placebo response
Findings from the existing literature suggest that vertebral augmentation is substantially better than conservative management, but it is uncertain whether or not it is more beneficial than OPLA. It has been suggested that the lack of evidence of a demonstrable benefit over OPLA represents a powerful placebo response to PVP, owing to factors such as the positive expectations of patients and clinicians and activation of pain-reducing neurobiological pathways. 333
This raises important issues with respect to medical ethics. If the positive effect of PVP is unrelated to the injection of cement, one would have to ask whether or not the benefits outweigh the known risks of the procedure. Miller et al. 333 have argued that it is not necessarily unethical to provide a minimally invasive procedure with the aim of generating a powerful placebo response. They note a growing body of research showing that the placebo effect is associated with real neurophysiological effects that may lead to clinically meaningful improvements. 369–371 With respect to pain, for example, there is evidence that the ‘placebo response’ involves the activation of endogenous opioids and dopamine pathways – that is, it has specific mechanisms for efficacy. 372,373 There has been some evidence provided that the use of an initial facet joint injection may produce clear benefits in around one-third of patients, with a reduced risk profile and low cost, and this should be considered as an initial first response.
The registry data showing improved survival rates following vertebral augmentation214 further complicate the ethical issues related to PVP and BKP. The implications of these findings hinge to a substantial degree on whether or not the improvement was owing to biomechanical factors directly associated with the injection of cement (e.g. correction of kyphotic wedge angle and vertebral body height). On the other hand, there may have been unobserved selection factors for the procedure which were directly related to mortality, and there were a number of methodological issues with the registry findings, as discussed in Appendix 13 .
Chapter 6 Discussion
Statement of principal findings
In unblinded trials, PVP and BKP perform significantly better than OPM in improving HRQoL, functional ability and pain in the short to medium term. However, there is no convincing evidence that vertebral augmentation provides any substantial benefits above OPLA. In addition, OPLA is not associated with the serious adverse events that can result from vertebral augmentation. The two double-blind, OPLA-controlled trials102,103 provide the highest level of clinical effectiveness evidence to date, although these studies were not large and may have had patient selection issues. The ongoing VERTOS IV trial374 will provide important additional evidence on the PVP versus OPLA comparison.
(Academic-in-confidence information has been removed) and pooled 12-month mortality rates from three RCTs17,127,131 are slightly suggestive that vertebral augmentation may have a mortality benefit. However, there were limitations associated with these analyses to the extent that no definitive statement on the presence and size of any mortality benefit could be made. The potential presence of a mortality benefit is a key issue for both the clinical effectiveness and cost-effectiveness of vertebral augmentation. If differential benefits for PVP and BKP (favouring BKP) are assumed, then BKP is more efficacious and is associated with a cost per QALY gained value of below £20,000. If equivalent gains for both PVP and BKP are assumed, then PVP is estimated to dominate BKP and to have an ICER below £10,000 per QALY gained in all bar one scenario where OPLA was assumed to have an identical effect on mortality. If BKP and PVP are assumed to have no mortality benefit, then PVP dominated BKP, although the ICER compared with OPLA and OPM was dependent on the assumptions made. If data from the two blinded studies were used then OPLA was estimated to be the most cost-effective, and if this was not considered an appropriate comparator then the ICER between PVP and OPM was estimated to range from £15,000–£40,000 per QALY. The analyses for PVP were assumed using low-viscosity cement for the majority of patients, with selected patients receiving high-viscosity cements. Exploratory analyses assuming that all patients received high-viscosity cement indicate that the cost-effectiveness of this strategy was likely to be > £20,000 per QALY gained.
Strengths and limitations of the assessment
The robustness of this review was enhanced by a comprehensive search strategy, including a broad search of databases, contact with clinical experts, and manual searches of the bibliographies of retrieved studies. Furthermore, two reviewers independently undertook data extraction, assessment of quality and study inclusion. The assessment of clinical effectiveness included RCTs only, while the assessment of safety included data from RCTs, large case series and individual case reports. The analyses conducted the most robust mapping of VAS to EQ-5D of which we are aware, and undertook a network meta-analysis on the VAS data. Extensive scenario and sensitivity analyses were conducted to explore a wide range of different assumptions. Insufficient evidence, particularly on the impact of BKP, PVP and OPLA on mortality rates, means that no definitive conclusion can be made.
However, a systematic review can only be as good as the studies it includes. With respect to the data set, the most serious methodological problem was the lack of blinding in all studies except INVEST103 and Buchbinder et al. 102 Unblinding in surgical studies has been linked to a 25% overestimation of treatment effect. 337 As Buchbinder and Kallmes have pointed out, the improvement in the treatment groups of the blinded trials was not dissimilar to that seen in the treatment groups of the unblinded trials. 375 The assessment of BKP’s clinical effectiveness was particularly limited, as only one open-label RCT comparing BKP with non-surgical management was available, while the only study to compare BKP with PVP showed a number of potential sources of bias.
A further limitation of these findings was the use of pain as a primary outcome. As others have argued, pain measurement may be confounded by a number of factors, including pain threshold, analgesia and level of activity. 84 Back pain-related disability and quality of life may provide more objective and clinically meaningful measures. 84 However, these outcomes were measured in heterogeneous ways among the trials, precluding statistical aggregation of the data. Measures of vertebral body height and angular deformity may also be more useful clinical outcomes than pain, insofar as improvements could enhance mobility and stave off deterioration of cardiopulmonary function. 345 Four studies (Blasco et al. ,127 Farrokhi et al. ,135 FREE137 and Liu et al. 138) reported these outcomes, but it was not possible to aggregate their findings owing to the heterogeneous approaches that were taken to wedge angle and vertebral body height measurement.
This review was specific to the population of people with painful osteoporotic VCFs; hence, the results are not necessarily generalisable to VCFs of other origins (e.g. multiple myeloma or traumatic). Discussions of generalisability among the studies were usually cursory. For example, several studies did not present data on the ethnic composition of their samples, nor did they comment on the implications of this for generalisability. On the other hand, the age and sex make-up of the study samples was fairly representative of that of the wider osteoporotic population in the UK. A higher proportion of women took part in the trials (typically around 70%) and the mean sample age was usually early to mid 70s. In addition, as all studies with the exceptions of INVEST103 and FREE137 were carried out exclusively outside the UK, the generalisability of the findings to the UK population of people with painful osteoporotic VCFs is unclear.
Perhaps most importantly, we were unable to establish whether or not percutaneous vertebral augmentation leads to changes in rates of mortality. (Academic-in-confidence information has been removed.) However, owing to lack of data on causes of death and other confounding factors, causal mechanisms other than the augmentation procedures cannot be ruled out at this stage. Data on 12-month mortality from three RCTs17,127,131 were pooled in this review. Although the point estimate slightly favoured PVP, it was not possible to rule out no effect. More problematically, as noted by Aebi,21 12 months is unlikely to be long enough to capture longer-term implications of kyphotic deformity and impaired cardiopulmonary function associated with VCFs.
Uncertainties
A key uncertainty is whether or not vertebral augmentation provides a mortality effect over OPM or OPLA. A definitive causal relationship cannot be inferred from the available observational data, and it would be difficult to conduct RCTs with adequate power and follow-up duration to fully explore this. While it seems likely that PVP is no more effective than OPLA in improving functional ability, pain and quality of life, there is yet to be a head-to-head comparison of BKP and OPLA, although evidence from the network meta-analysis indicates that this too would not be expected to be more effective than OPLA in improving functional ability, pain and quality of life. It is also not known if there may be ways to generate the apparently high clinical response without resorting to cement injection or OPLA, although an exploratory analysis has been conducted to indicate how much could be spent on patient education with the cost per QALY of PVP remaining above £20,000 per QALY gained compared with education. This value was seen to be at least £500 per patient and could be considerably more.
The length of stay associated with patients receiving OPM, PVP and BKP is not known with certainty, with both the pivotal trials and clinical advice suggesting that the length of stay is considerably shorter than hospital database values suggest. A prospective study to record such values would be beneficial. Analyses undertaken showed that the results were sensitive to the assumed hospitalisation costs where no mortality benefit was assumed.
Finally, further evidence for the effect of vertebral augmentation on restoration of vertebral body height and sagittal balance is required.
Other relevant factors
Risks to staff
There has been some discussion over the past decade concerning the risk to staff performing vertebroplasty and kyphoplasty procedures of radiation exposure: this risk is low, but is of potential importance. Fitousi et al. 326 estimated that vertebroplasty practitioners could perform 150 vertebroplasty procedures annually without exceeding annual dose constraints, while Harstall et al. 376 estimated an annual risk of 0.0025% for fatal cancer of the thyroid, and a small to medium risk of developing any cancer of 0.025%. However, these risks can be somewhat mitigated by following a number of precautionary measures377 including the use of protective lead gloves378 or other shielding techniques,379 and the use of a combined CT–fluoroscopy approach to imaging as opposed to fluoroscopy only. 380
In addition, some staff have experienced an idiosyncratic reaction or asthma exacerbation in response to PMMA vapour, even though exposure during a typical PVP case or list is below the established occupational exposure limits for staff working with PMMA. 381
Chapter 7 Conclusions
Implications for service provision
Percutaneous vertebroplasty is likely to provide greater clinical benefits than conservative management, and may be one way to mitigate some of the problems associated with the latter approach. However, two blinded RCTs indicate that PVP does not appear to be any more effective than administration of local anaesthesia to the affected area in improving pain, quality of life or back-related functional ability. As yet, there are no well-designed, double-blind, OPLA-controlled trials of BKP. Hence, although this procedure is likely to be beneficial in comparison with conservative treatment, its effectiveness compared with local anaesthetic was estimated through a network meta-analysis, which indicated that PVP and BKP had similar long-term VAS scores. Although some data suggest that PVP and BKP may lead to long-term reductions in mortality, it is not yet clear whether this effect is owing to a specific mechanism of the procedures or to other extraneous factors.
The cost-effectiveness of each intervention is strongly influenced by the assumed mortality benefit. As the evidence for mortality benefits for PVP and BKP is limited, it is unclear whether or not these uptakes of the interventions will increase or decrease; hence, the implications for service provision are unknown.
Suggested research priorities
The effect of vertebral augmentation on mortality is a potentially important area for further exploration, as this was a key driver in the cost-effectiveness model. Data from the USA and Germany suggested that PVP and BKP were associated with reduced mortality rates compared with people with VCFs who did not undergo cement augmentation. 382 However, formal analyses of the data provided to the assessment group highlighted potential methodological limitations meaning that a definitive conclusion could not be formed. Notwithstanding the various methodological and ethical issues which must be carefully addressed in RCTs of surgical procedures,383 it would be desirable to have additional double-blinded RCTs of vertebral augmentation with adequate power and follow-up length to investigate this possible effect further. Alternatively, a prospective observational database containing as many covariates as were feasible could provide beneficial data on the likely mortality impacts.
The effect of local anaesthesia on functional and pain-related improvements in people with VCFs remains a contentious issue. Buchbinder and Kallmes have argued that injecting a short-acting anaesthetic over the pedicle of the fractured vertebral body (as per the INVEST study) would be unlikely to provide sustained benefits. 375 Others have argued that anaesthesia injection may have specific mechanisms of efficacy,112,200 and there is some limited evidence to support this proposition. For example, Riew et al. 114 conducted a RCT of the efficacy of selective nerve root blocks in the treatment of lumbar radiculopathy, and found that local anaesthetic showed an effect long after its expected duration. More recently, Wilson et al. 84 found that administering local anaesthetic with steroid facet joint injection to the most painful level led to substantial improvements in approximately one-third of a cohort of 75 patients with painful VCFs. However, the facet joint block may provide additive benefits to those provided by anaesthesia alone. A study comparing anaesthesia and facet joint injection with anaesthesia only would be useful to explore any possible placebo effects in these approaches.
There are few clinical trials of vertebral augmentation with proper blinding and experimental controls. The total number of patients in the Buchbinder and INVEST studies was low, even when aggregated (n = 209). Further double-blinded, OPLA-controlled RCTs would be helpful in confirming the findings of these trials. In addition, there are currently no double-blinded, OPLA-controlled RCT data on BKP.
The HRQoL data in the reviewed studies were limited and, consequently, missing EQ-5D data were modelled on VAS data from the relevant studies. Although this mapping was based on a reasonably good fit, it would have been preferable to have direct EQ-5D data. It is therefore suggested that EQ-5D be incorporated as a measure in all future evaluations of vertebral augmentation, ideally along with VAS data in order to allow data from earlier studies which reported only VAS data to be mapped more accurately.
There is ambiguity regarding patient selection for PVP and BKP. Critical commentaries on the Buchbinder and INVEST trials have suggested that only patients with < 6 weeks of pain should be treated. 107,334,347 However, a division of patients in the Buchbinder and INVEST trials into ≤ 6 weeks of pain and > 6 weeks of pain indicated very similar adjusted between group differences, indicating that this theory was incorrect. 110 As pain tends to spontaneously improve during the acute phase, others have suggested that, in order to avoid unnecessary surgical interventions, PVP and BKP should be used only in cases of intractable and long-term pain. 336 To this end, more IPD analyses comparing clinical effectiveness in acute and long-term pain from blinded trials would be helpful.
Both spinal deformity and sagittal balance are important measures of VCF severity. If balance becomes unstable then this may be a further pain generator alongside micro-movement of fractures and pain on adjacent joints. Moreover, increasing kyphosis and reduction of vertebral body height can lead to deterioration in cardiopulmonary function and, ultimately, death. However, the clinical effectiveness of PVP and BKP in restoring these morphometric parameters is yet to be studied in high-quality trials.
Acknowledgements
Tony Ades, Professor of Public Health Science, Centre for Academic Primary Care, University of Bristol.
We would like to thank the following individuals for their generous responses to our data requests: Anastasios Mpotsaris, Klinik für Radiologie, Neuroradiologie und interventionelle Therapie, Klinikum Vest, Recklinghausen; Rachelle Buchbinder, Director, Monash University Department of Epidemiology, and Professor in the Monash University Department of Epidemiology & Preventive Medicine; Margaret Staples, biostatistician, Monash University Department of Clinical Epidemiology, Cabrini Hospital; Douglas Wardlaw, Professor, Orthopaedic Department, Woodend Hospital, Aberdeen; and Jordi Blasco Andaluz, Neurointerventional Department, Centre Diagnòstic per la Imatge, Hospital Clinic, Barcelona.
The authors wish to thank Gill Rooney, Programme Administrator, ScHARR, for her help in preparing and formatting the report.
Contributions of authors
Matt Stevenson was the assessment group lead, undertook the cost-effectiveness review and was involved in constructing the cost-effectiveness model and interpreting the results.
Myfanwy Lloyd Jones and Tim Gomersall undertook the clinical effectiveness review.
Andrew Rawdin was involved in constructing and running the cost-effectiveness model; Sofia Dias constructed the network meta-analysis.
Monica Hernández critiqued the data indicating a mortality benefit for percutaneous vertebroplasty and percutaneous balloon kyphoplasty.
David Wilson provided clinical expertise.
Angie Rees performed the literature searches.
About ScHARR
The School of Health and Related Research is one of the four schools that constitute the Faculty of Medicine at the University of Sheffield. ScHARR brings together a wide range of medical and health-related disciplines including public health, general practice, mental health, epidemiology, health economics, management sciences, medical statistics, operational research and information science. It includes the Sheffield unit of the Trent Institute for Health Services Research, which is funded by NHS R&D to facilitate high-quality health services research and capacity development.
The ScHARR Technology Assessment Group (ScHARR-TAG) synthesises research on the clinical effectiveness and cost-effectiveness of health-care interventions for the NIHR Health Technology Assessment Programme on behalf of a range of policy-makers, including NICE. ScHARR-TAG is part of a wider collaboration of a number of units from other regions including Southampton Health Technology Assessment Centre (SHTAC), University of Southampton; Aberdeen Health Technology Assessment Group (Aberdeen HTA Group), University of Aberdeen; Liverpool Reviews & Implementation Group (LRiG), University of Liverpool; Peninsular Technology Assessment Group (PenTAG), University of Exeter; the NHS Centre for Reviews and Dissemination, University of York; Warwick Evidence, The University of Warwick; the BMJ Group and Kleijnen Systematic Reviews.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Glaser DL, Kaplan FS. Osteoporosis. Definition and clinical presentation. Spine 1997;22:12-6. http://dx.doi.org/10.1097/00007632-199712151-00003.
- Kanis JA. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int 1994;4:368-81. http://dx.doi.org/10.1007/BF01622200.
- Consensus development conference: diagnosis, prophylaxis, and treatment of osteoporosis . Am J Med 1993;94:646-50.
- Cummings SR, Melton LJ. Epidemiology and outcomes of osteoporotic fractures. Lancet 2002;359:1761-2. http://dx.doi.org/10.1016/S0140-6736(02)08657-9.
- Lindsay R, Silverman SL, Cooper C, Hanley DA, Barton I, Broy SB, et al. Risk of new vertebral fracture in the year following a fracture. JAMA 2001;285:320-3. http://dx.doi.org/10.1001/jama.285.3.320.
- Holroyd C, Cooper C, Dennison E. Epidemiology of osteoporosis. Best Prac Res Clin Endocrinol Metab 2008;22:671-8. http://dx.doi.org/10.1016/j.beem.2008.06.001.
- Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 1993;8:1137-48.
- Fink HA, Milavetz DL, Palermo L, Nevitt MC, Cauley JA, Genant HK, et al. What proportion of incident radiographic vertebral deformities is clinically diagnosed and vice versa?. J Bone Miner Res 2005;20:1216-22. http://dx.doi.org/10.1359/JBMR.050314.
- Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR. Vertebral fractures and mortality in older women: a prospective study. Arch Intern Med 1999;159:1215-20. http://dx.doi.org/10.1001/archinte.159.11.1215.
- Ross PD. Clinical consequences of vertebral fractures. Am J Med 1997;103:S30-42. http://dx.doi.org/10.1016/S0002-9343(97)90025-5.
- Lyritis GP, Mayasis B, Tsakalakos N, Lambropoulos A, Gazi S, Karachalios TH, et al. The natural history of the osteoporotic vertebral fracture. Clin Rheumatol 1989;8:66-9. http://dx.doi.org/10.1007/BF02207237.
- De Smet AA, Robinson RG, Johnson BE, Lukert BP. Spinal compression fractures in osteoporotic women: patterns and relationship to hyperkyphosis. Radiology 1988;166:497-500.
- Melton LJ. Epidemiology of spinal osteoporosis. Spine 1997;22:2-11. http://dx.doi.org/10.1097/00007632-199712151-00002.
- Kamath S, Venkatanarasimha N, Silver DAT. Percutaneous vertebroplasty. CPD J Radiol Update 2007;6:82-96.
- Silverman SL. The clinical consequences of vertebral compression fracture. Bone 1992;13:27-31. http://dx.doi.org/10.1016/8756-3282(92)90193-Z.
- Gangi A, Sabharwal T, Irani FG, Buy X, Morales JP, Adam A, et al. Quality assurance guidelines for percutaneous vertebroplasty. Cardiovasc Intervent Radiol 2006;29:173-8. http://dx.doi.org/10.1007/s00270-005-0146-5.
- Klazen CAH, Lohle PNM, Jansen FH, Tielbeek AV, Blonk MC, Venmans A, et al. Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures (Vertos II): an open-label randomised trial. Lancet 2010;376:1085-92. http://dx.doi.org/10.1016/S0140-6736(10)60954-3.
- Burton AW, Rhines LD, Mendel E. Vertebroplasty and kyphoplasty: a comprehensive review. Neurosurg Focus 2005;18. http://dx.doi.org/10.3171/foc.2005.18.3.2.
- Klazen CA, Verhaar HJ, Lampmann LE, Juttmann JR, Schoemaker MC, van Everdingen KJ, et al. Clinical course of pain in acute osteoporotic vertebral compression fracture. J Vascular Interventional Radiol 2010;21:1405-9. http://dx.doi.org/10.1016/j.jvir.2010.05.018.
- van Staa TP, Dennison EM, Leufkens HGM, Cooper C. Epidemiology of fractures in England and Wales. Bone 2001;29:517-22. http://dx.doi.org/10.1016/S8756-3282(01)00614-7.
- Aebi M. Vertebroplasty: about sense and nonsense of uncontrolled “controlled randomized prospective trials”. Eur Spine J 2009;18:1247-8. http://dx.doi.org/10.1007/s00586-009-1164-9.
- Schlaich C, Minne HW, Bruckner T, Wagner G, Gebest HJ, Grunze M, et al. Reduced pulmonary function in patients with spinal osteoporotic fractures. Osteoporos Int 1998;8:261-7. http://dx.doi.org/10.1007/s001980050063.
- Culham GE, Jimenez HA, King CE. Thoracic kyphosis, rib mobility, and lung volumes in normal women and women with osteoporosis. Spine 1994;19:1250-5. http://dx.doi.org/10.1097/00007632-199405310-00010.
- Leech JA, Dulberg C, Kellie S, Pattee L, Gay J. Relationship of lung function to severity of osteoporosis in women. Am Rev Respir Dis 1990;141:68-71. http://dx.doi.org/10.1164/ajrccm/141.1.68.
- Melton LJ, Kallmes DF. Epidemiology of vertebral fractures: implications for vertebral augmentation. Acad Radiol 2006;13:538-45. http://dx.doi.org/10.1016/j.acra.2006.01.005.
- Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B. Excess mortality after hospitalisation for vertebral fracture. Osteoporos Int 2004;15:108-12. http://dx.doi.org/10.1007/s00198-003-1516-y.
- Cooper C, Atkinson EJ, Jacobsen SJ, O’Fallon M, Melton LJ. Population-based study of survival after osteoporotic fractures. Am J Epidemiol 1993;137:1001-5.
- Browner WS, Seeley DG, Vogt TM, Cummings SR. Non-trauma mortality in elderly women with low bone mineral density. Lancet 1991;338:355-8. http://dx.doi.org/10.1016/0140-6736(91)90489-C.
- Black DM, Cummings SR, Karpf DB, Cauley JA, Thompson DE, Nevitt MC, et al. Randomised trial of effect alendronate on risk of fracture in women with existing vertebral fractures. Lancet 1996;348:1535-41.
- Cauley JA, Thompson DE, Ensrud KC, Scott JC, Black D. Risk of mortality following clinical fractures. Osteoporos Int 2000;11:556-61. http://dx.doi.org/10.1007/s001980070075.
- Ström O, Leonard C, Marsh D, Cooper C. Cost-effectiveness of balloon kyphoplasty in patients with symptomatic vertebral compression fractures in a UK setting. Osteoporos Int 2010;21:1599-608. http://dx.doi.org/10.1007/s00198-009-1096-6.
- Ström O, Borgström F, Kanis JA, Compston J, Cooper C, McCloskey EV, et al. Osteoporosis: burden, health care provision and opportunities in the EU. Arch Osteoporos 2011;6:59-155. http://dx.doi.org/10.1007/s11657-011-0060-1.
- Percutaneous Stentoplasty (Vertebral Body Stenting) for the Treatment of Osteoporotic Vertebral Fractures. Synthes Submission for the NICE Multiple Technology Appraisal on Osteoporotic Vertebral Fractures. West Chester, PA: Synthes GmbH; 2012.
- Percutaneous Vertebroplasty (PVP) and Balloon Kyphoplasty (BKP) for the Treatment of Osteoporotic Vertebral Compression Fractures (OVCFs). Minneapolis, MN: Medtronic; 2012.
- Wroth C, Wiles A. Key Population and Vital Statistics. Population and Vital Statistics by Area of Usual Residence in the United Kingdom, 2007. London: Office for National Statistics; 2009.
- Vertebroplasty and Kyphoplasty for the Treatment of Osteoporotic Vertebral Fractures. A submission by Johnson & Johnson for the Confidence Spinal Cement System™. New Brunswick, NJ: Johnson & Johnson; 2012.
- Singer BR, McLauchlan GJ, Robinson CM, Christie J. Epidemiology of fractures in 15 000 adults. The influence of age and gender. J Bone Joint Surg Br 1998;80–B:243-8. http://dx.doi.org/10.1302/0301-620X.80B2.7762.
- Gold DT. The clinical impact of vertebral fractures: quality of life in women with osteoporosis. Bone 1996;8:185-9.
- Lyles KW, Gold DT, Shipp KM, Pieper CF, Martinez S, Mulhausen PL. Association of osteoporotic vertebral compression fractures with impaired functional status. Am J Med 1993;94:595-601. http://dx.doi.org/10.1016/0002-9343(93)90210-G.
- Kado DM, Browner WS, Palermo L, Nevitt MC, Genant HK, Cummings SR. Vertebral fractures and mortality in older women. A prospective study. Arch Int Med 1999;159:1215-20. http://dx.doi.org/10.1001/archinte.159.11.1215.
- Ettinger B, Black DM, Palermo L, Nevitt MC, Melnikoff S, Cummings SR. Kyphosis in older women and its relation to back pain, disability and osteopenia: the Study of Osteoporotic Fractures. Osteoporos Int 1994;4:55-60. http://dx.doi.org/10.1007/BF02352262.
- Ensrud KE, Nevitt MC, Yunis C, Cauley JA, Seeley DG, Fox KM, et al. Correlates of impaired function in older women. J Am Geriatr Soc 1994;9:1429-32.
- Cook DJ, Guyatt GH, Adachi JD, Clifton J, Griffith LE, Epstein RS, et al. Quality of life issues in women with vertebral fractures due to osteoporosis. Arthritis Rheum 1993;36:750-6. http://dx.doi.org/10.1002/art.1780360603.
- Greendale GA, Barrett-Connor E, Ingles S, Haile R. Late physical and functional effects of osteoporotic fracture in women: the Rancho Bernardo Study. J Am Geriatr Soc 1995;43:955-61.
- Ryan PJ. A clinical profile of back pain and disability in patients with spinal osteoporosis. Bone 1994;15:27-30. http://dx.doi.org/10.1016/8756-3282(94)90887-7.
- Ettinger B, Block JE, Smith R, Cummings SR, Harris ST, Genant HK. An examination of the association between vertebral deformities, physical disabilities and psychosocial problems. Maturitas 1988;10:283-96. http://dx.doi.org/10.1016/0378-5122(88)90064-3.
- Scane AC, Sutcliffe AM, Francis RM. The sequelae of vertebral crush fractures in men. Osteoporos Int 1994;4:89-92. http://dx.doi.org/10.1007/BF01623230.
- Dolan P, Torgerson D. The cost of treating osteoporotic fractures in the UK female population. Osteoporos Int 1998;8:611-17.
- Puffer S, Torgerson DJ, Sykes D, Brown P, Cooper C. Health care costs of women with symptomatic vertebral fractures. Bone 2004;35:383-6. http://dx.doi.org/10.1016/j.bone.2004.03.035.
- Cooper C, Atkinson EJ, O’Fallon WM, Melton LJ. Incidence of clinically diagnosed vertebral fractures: a population-based study in Rochester, Minnesota, 1985–1989. J Bone Miner Res 1992;7:221-7.
- Ioannidi G, Papaioannou A, Hopman WM, Akhtar-Danes, N, Anastassiades T, Pickard L, et al. Relation between fractures and mortality: results from the Canadian Multicentre Osteoporosis Study. CMAJ 2009;181:265-71.
- Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ 2003;81:646-56.
- Kanis JA. Diagnosis of osteoporosis and assessment of fracture risk. Lancet 2002;359:1929-36. http://dx.doi.org/10.1016/S0140-6736(02)08761-5.
- Marshall DA, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 1996;312:1254-9. http://dx.doi.org/10.1136/bmj.312.7041.1254.
- Prather H, Hunt D, Watson JO, Gilula LA. Conservative care for patients with osteoporotic vertebral compression fractures. Phys Med Rehabil Clin North Am 2007;18:577-91. http://dx.doi.org/10.1016/j.pmr.2007.05.008.
- Maksymowych WP. Managing acute osteoporotic vertebral fractures with calcitonin. Can Fam Physician 1998;44:2160-6.
- Doidge J, Merlin T, Liufu Z, Tamblyn D, Jia LY, Hiller J. Review of Interim Funded Service: Vertebroplasty and New Review of Kyphoplasty. Canberra, ACT: Commonwealth of Australia; 2011.
- Suzuki N, Ogikubo O, Hansson T. The course of the acute vertebral body fragility fracture: its effect on pain, disability and quality of life during 12 months. Eur Spine J 2008;17:1380-90. http://dx.doi.org/10.1007/s00586-008-0753-3.
- Denaro L, Longo UG, Denaro V. Vertebroplasty and kyphoplasty: reasons for concern?. Orthoped Clin North Am 2009;40:465-71. http://dx.doi.org/10.1016/j.ocl.2009.05.004.
- Nairn RJ, Binkhamis S, Sheikh A. Current perspectives on percutaneous vertebroplasty: current evidence/controversies, patient selection and assessment, and technique and complications. Radiol Res Prac 2011. doi:10.1155/2011/175079.
- Hu SS. Internal fixation in the osteoporotic spine. Spine 1997;22:43-8. http://dx.doi.org/10.1097/00007632-199712151-00008.
- Jensen ME. Percutaneous vertebroplasty: a new therapy for the treatment of painful vertebral body compression fractures. Appl Radiol 2000;9:7-12.
- Frey ME. Redo kyphoplasty with vertebroplasty technique: a case report and review of the literature. Pain Physician 2009;12:645-9.
- Novartis . Miacalcin® n.d. www.pharma.us.novartis.com/product/pi/pdf/miacalcin_nasal.pdf (accessed 12 April 2011).
- Stevenson M, Davis S, Kanis JA. The hospitalization costs and outpatient costs of fragility fractures. Womens Health Med 2006;3:149-51.
- Borgstrom F, Jonsson, B, Ström O, Kanis JA. An economic evaluation of strontium ranelate in the treatment of osteoporosis in a Swedish setting: based on the results of the SOTI and TROPOS trials. Osteoporos Int 2006;17:1781-93. http://dx.doi.org/10.1007/s00198-006-0193-z.
- Stevenson M, Lloyd Jones M. The cost effectiveness of an RCT comparing alendronate with Vitamin K1. Med Decis Making 2011;31:43-52.
- Francis RM, Aspray TJ, Hide G, Sutcliffe AM, Wilkinson P. Back pain in osteoporotic vertebral fractures. Osteoporos Int 2008;9:895-903.
- Papaioannou A, Watts NB, Kendler DL, Yuen CK, Adachi JD, Ferk N. Diagnosis and management of vertebral fractures in elderly adults. Am J Med 2002;113:220-8. http://dx.doi.org/10.1016/S0002-9343(02)01190-7.
- Rapado A. General management of vertebral fractures. Bone 1996;18:191-6. http://dx.doi.org/10.1016/8756-3282(95)00501-3.
- Brown FL, Bodison S, Dixon J, Davis W, Nowoslawski J. Comparison of diflunisal and acetaminophen with codeine in the treatment of initial or recurrent acute low back strain. Clin Ther 1986;9:52-8.
- Innes GD, Croskerry P, Worthington J, Beveridge R, Jones D. Ketorolac versus acetaminophen-codeine in the emergency department treatment of acute low back pain. J Emerg Med 1998;16:549-56. http://dx.doi.org/10.1016/S0736-4679(98)00044-4.
- Tramer MR, Moore RA, Reynolds DJ, McQuay HJ. Quantitative estimation of rare adverse events which follow a biological progression: a new model applied to chronic NSAID use. Pain 2000;85:169-82. http://dx.doi.org/10.1016/S0304-3959(99)00267-5.
- Tederko P, Kiwerski J, Barcinska-Wierzejska I. Complex management of osteoporotic vertebral fracture. Ortop Traumatol Rehabil 2002;4:157-63.
- Jellema P, van Tulder MW, van Poppel MN, Nachemson AL, Bouter LM. Lumbar supports for prevention and treatment of low back pain: a systematic review within the framework of the Cochrane Back Review Group. Spine 2001;26:377-86. http://dx.doi.org/10.1097/00007632-200102150-00014.
- Longo UG, Loppini M, Denaro L, Maffulli N, Denaro V. Osteoporotic vertebral fractures: current concepts of conservative care. Br Med Bull 2012;102:171-89. http://dx.doi.org/10.1093/bmb/ldr048.
- Tamayo-Orozco J, Arzac-Palumbo P, Peon-Vidales H, Mota-Bolfeta R, Fuentes F. Vertebral fractures associated with osteoporosis: patient management. Am J Med 1997;103:S44-8. http://dx.doi.org/10.1016/S0002-9343(97)90026-7.
- Furlan AD, Imamura M, Dryden T, Irvin E. Massage for low-back pain. Cochrane Database Syst Rev 2008;4. http://dx.doi.org/10.1002/14651858.CD001929.
- French SD, Cameron M, Walker BF, Reggars JW, Esterman AJ. Superficial heat or cold for low back pain. Cochrane Database Syst Rev 2012;1. http://dx.doi.org/10.1002/14651858.CD004750.
- Brewer L, Williams D, Moore A. Current and future treatment options in osteoporosis. Eur J Clin Pharmacol 2011;67:321-31. http://dx.doi.org/10.1007/s00228-011-0999-2.
- Bolland MJ, Avenell A, Baron JA, Grey A, MacLennan GS, Gamble GD, et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ 2010;341. http://dx.doi.org/10.1136/bmj.c3691.
- Torgerson DJ, Bell-Syer SE. Hormone replacement therapy and prevention of vertebral fractures: a meta-analysis of randomised trials. BMC Musculoskelet Disord 2001;2.
- Tavani A, La VC. The adverse effects of hormone replacement therapy. Drugs Aging 1999;14:347-57. http://dx.doi.org/10.2165/00002512-199914050-00003.
- Wilson DJ, Owen S, Corkill RA. Facet joint injections as a means of reducing the need for vertebroplasty in insufficiency fractures of the spine. Eur Radiol 2011;21:1772-8. http://dx.doi.org/10.1007/s00330-011-2115-5.
- Oxfordshire Priorities Forum (NHS Oxfordshire) . INTERIM Policy Statement 154: Percutaneous Vertebroplasty for Osteoporotic Vertebral Fractures 2010. www.oxfordshirepct.nhs.uk/professional-resources/priority-setting/lavender-statements/documents/LS154_Vertebroplasty.pdf (accessed June 2012).
- Interventional Procedure Guidance 12: Percutaneous Vertebroplasty. London: NICE; 2003.
- Interventional procedure guidance 166: Balloon kyphoplasty for vertebral compression fractures. London: NICE; 2006.
- Ploeg WT, Veldhuizen AG, The B, Sietsma MS. Percutaneous vertebroplasty as a treatment for osteoporotic vertebral compression fractures: a systematic review. Eur Spine J 2006;15:1749-58. http://dx.doi.org/10.1007/s00586-006-0159-z.
- Interventional Procedure Overview of Percutaneous Vertebroplasty (Methyl Methacrylate). London: NICE; 2003.
- Wiles MD, Nowicki RWA, Hancock SM, Boszczyk B. Anaesthesia for vertebroplasty and kyphoplasty. Curr Anaesth Crit Care 2009;20:38-41. http://dx.doi.org/10.1016/j.cacc.2008.07.006.
- Nussbaum DA, Gailloud P, Murphy K. A review of complications associated with vertebroplasty and kyphoplasty as reported to the Food and Drug Administration medical device related web site. J Vasc Interv Radiol 2004;15:1185-92. http://dx.doi.org/10.1097/01.RVI.0000144757.14780.E0.
- Hill JM. Vertebroplasty: a new radiographic technique for treating painful spinal compression fractures. Images 2001;20:6-8.
- Goto K, Hashimoto M, Takadama H, Tamura J, Fujibayashi S, Kawanabe K, et al. Mechanical, setting, and biological properties of bone cements containing micron-sized titania particles. J Mater Sci Mater Med 2008;19:1009-16. http://dx.doi.org/10.1007/s10856-007-3076-8.
- Wren AW, Coughlan A, Placek L, Towler MR. Gallium containing glass polyalkenoate anti-cancerous bone cements: glass characterization and physical properties. J Mater Sci Mater Med 2012;23:1823-33. http://dx.doi.org/10.1007/s10856-012-4624-4.
- Whitehouse RW. (i) Patient selection criteria for vertebroplasty or kyphoplasty in painful osteoporotic fracture. Orthopaed Trauma 2011;25:79-82. http://dx.doi.org/10.1016/j.mporth.2011.01.003.
- Medtronic . Kyphon® Balloon Kyphoplasty Procedure 2012. www.kyphon.com/us/physician.a.spx?contentid=61 (accessed February 2012).
- Sandberg J. Medtronic Launches Bone Cement With Hydroxyapatite in the United States. KYPHON®; ActivOsTM10 Bone Cement Is the Latest Offering from Medtronic for Treatment of Vertebral Compression Fractures 2010. www.orthospinenews.com/medtronic-launches-bone-cement-with-hydroxyapatite-in-the-united-states-kyphon%C2%AE-activos%E2%84%A210-bone-cement-is-the-latest-offering-from-medtronic-for-treatment-of-vertebral-compression-fractur (accessed February 2012).
- Rotter R, Martin H, Fuerderer S, Gabl M, Roeder C, Heini P, et al. Vertebral body stenting: a new method for vertebral augmentation versus kyphoplasty. Eur Spine J 2010;19:916-23. http://dx.doi.org/10.1007/s00586-010-1341-x.
- Destouet JM, Gilula LA, Murphy WA, Monsees B. Lumbar facet joint injection: indication, technique, clinical correlation, and preliminary results. Radiology 1982;145:321-5.
- Peh W. Image-guided facet joint injection. Biomed Imaging Interv J 2011;7.
- Ryan PJ, Evans P, Gibson T, Fogelman I. Osteoporosis and chronic back pain: a study with single-photon emission computed tomography bone scintigraphy. J Bone Miner Res 1992;7:1455-60. http://dx.doi.org/10.1002/jbmr.5650071213.
- Buchbinder R, Osborne RH, Ebeling PR, Wark JD, Mitchell P, Wriedt C, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med 2009;361:557-68. http://dx.doi.org/10.1056/NEJMoa0900429.
- Kallmes DF, Comstock BA, Heagerty PJ, Turner JA, Wilson DJ, Diamond TH, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med 2009;361:569-79. http://dx.doi.org/10.1056/NEJMoa0900563.
- FDA . Class II Special Controls Guidance Document: Polymethylmethacrylate (PMMA) Bone Cement: Guidance for Industry and FDA 2011. www.fda.gov/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm072795.htm (accessed November 2011).
- FDA Center for Devices and Radiological Health . Class II Special Controls Guidance Document: Polymethylmethacrylate (PMMA) Bone Cement; Guidance for Industry and FDA 2002. www.fda.gov/downloads/MedicalDevices/DeviceRegulationandGuidance/GuidanceDocuments/ucm072837.pdf (accessed November 2011).
- Interventional Procedures Overview of Balloon Kyphoplasty for Vertebral Compression Fractures. London: NICE; 2005.
- Bono CM, Heggeness M, Mick C, Resnick D, Watters WC. North American Spine Society. Newly released vertebroplasty randomized controlled trials: a tale of two trials. Spine J 2010;10:238-40. http://dx.doi.org/10.1016/j.spinee.2009.09.007.
- Clark WA, Diamond TH, McNeil HP, Gonski PN, Schlaphoff GP, Rouse JC. Vertebroplasty for painful acute osteoporotic vertebral fractures: recent Medical Journal of Australia editorial is not relevant to the patient group that we treat with vertebroplasty. Med J Aust 2010;192:334-7.
- Rad AE, Kallmes DF. Correlation between preoperative pain duration and percutaneous vertebroplasty outcome. AJNR Am J Neuroradiol 2011;32:1842-5. http://dx.doi.org/10.3174/ajnr.A2617.
- Staples MP, Kallmes DF, Comstock BA, Jarvik JG, Osborne RH, Buchbinder R. Effectiveness of vertebroplasty using individual patient data from two randomised placebo controlled trials: meta-analysis. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d3952.
- Hansen GR, Streltzer J. The psychology of pain. Emerg Med Clin North Am 2005;23:339-48. http://dx.doi.org/10.1016/j.emc.2004.12.005.
- Bolster MB. Consternation and questions about two vertebroplasty trials. Cleve Clin J Med 2010;77:12-6. http://dx.doi.org/10.3949/ccjm.77a.09161.
- Gangi A, Clark WA. Have recent vertebroplasty trials changed the indications for vertebroplasty?. Cardiovasc Interv Radiol 2010;33:677-80. http://dx.doi.org/10.1007/s00270-010-9901-3.
- Riew KD, Yin Y, Gilula L, Bridwell KH, Lenke LG, Lauryssen C, et al. The effect of nerve-root injections on the need for operative treatment of lumbar radicular pain. A prospective, randomized, controlled, double-blind study. J Bone Joint Surg Am 2000;82–A:1589-93.
- Noonan P. Randomized vertebroplasty trials: bad news or sham news?. Am J Neuroradiol 2009;30:1808-9. http://dx.doi.org/10.3174/ajnr.A1875.
- Kaptchuk TJ. Placebo studies and ritual theory: a comparative analysis of Navajo, acupuncture and biomedical healing. Philos Trans R Soc Lond B Biol Sci 2011;366:1849-58. http://dx.doi.org/10.1098/rstb.2010.0385.
- Green SA. Surgeons and shamans: the placebo value of ritual. Clin Orthop Relat Res 2006;450:249-54. http://dx.doi.org/10.1097/01.blo.0000224044.69592.65.
- The Treatment of Symptomatic Osteoporotic Spinal Compression Fractures. Guideline and Evidence Report. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2010.
- Ostelo RWJG, Deyo RA, Stratford P, Waddell G, Croft P, Von Korff M, et al. Interpreting changes scores for pain and functional status in low back pain. Spine 2008;33:90-4. http://dx.doi.org/10.1097/BRS.0b013e31815e3a10.
- Loke YK, Price D, Herxheimer A. Systematic reviews of adverse effects: framework for a structured approach. BMC Med Res Methodol 2007;7. http://dx.doi.org/10.1186/1471-2288-7-32.
- Golder S, Loke Y, McIntosh HM. Room for improvement? A survey of the methods used in systematic reviews of adverse effects. BMC Med Res Methodol 2006;6.
- Brewer T, Colditz GA. Postmarketing surveillance and adverse drug reactions: current perspectives and future needs. JAMA 1999;281:824-9. http://dx.doi.org/10.1001/jama.281.9.824.
- Cranney A, Tugwell P, Wells G, Guyatt G. Systematic reviews of randomized trials in osteoporosis: Introduction and methodology. Endocr Rev 2002;23:497-50.
- Mousavi P, Roth S, Finkelstein J, Cheung G, Whyne C. Volumetric quantification of cement leakage following percutaneous vertebroplasty in metastatic and osteoporotic vertebrae. J Neurosurg 2003;99:56-9. http://dx.doi.org/10.3171/spi.2003.99.1.0056.
- Cotten A, Dewatre F, Cortet B, Assaker R, Leblond D, Duquesnoy B, et al. Percutaneous vertebroplasty for osteolytic metastases and myeloma: effects of the percentage of lesion filling and the leakage of methyl methacrylate at clinical follow-up. Radiology 1996;200:525-30.
- Furlan AD, Pennick V, Bombardier C, van Tulder M. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine 2009;34:1929-41. http://dx.doi.org/10.1097/BRS.0b013e3181b1c99f.
- Blasco JA, Martinez-Ferrer A, Macho Fernández J, San Roman L, Pomés J, Carrasco J, et al. Effect of vertebroplasty on pain relief, quality of life and the incidence of new vertebral fractures. A 12-month randomised follow-up, controlled trial. J Bone Miner Res 2012;27:1159-66. doi:10.1002/jbmr.1564.
- Rousing R, Andersen MO, Jespersen SM, Thomsen K, Lauritsen J. Percutaneous vertebroplasty compared to conservative treatment in patients with painful acute or subacute osteoporotic vertebral fractures. Three-months follow-up in a clinical randomized study. Spine 2009;34:1349-54. http://dx.doi.org/10.1097/BRS.0b013e3181a4e628.
- Blasco J, Garcia A, Manzanera LSR, MacHo JM, Peris P, Jaume P, et al. Randomized trial comparing vertebroplasty and conservative treatment analyzing pain relief and quality of life on the long term basis. Cardiovasc Interven Radiol 2010;33:182-3.
- Martinez-Ferrer A, Blasco J, Carrasco JL, Monegal A, Pomes J, Guaabens N, et al. Effect of vertebroplasty on the quality of life of patients with pain related to osteoporotic vertebral fractures preliminary results of a randomized trial. Bone 2011;48. http://dx.doi.org/10.1016/j.bone.2011.03.356.
- Rousing R, Hansen KL, Andersen MO, Jespersen SM, Thomsen K, Lauritsen JM. Twelve-months follow-up in forty-nine patients with acute/semiacute osteoporotic vertebral fractures treated conservatively or with percutaneous vertebroplasty. A clinical randomized study. Spine 2010;35:478-82. http://dx.doi.org/10.1097/BRS.0b013e3181b71bd1.
- Muijs SP, Van Erkel AR, Dijkstra PDS. Treatment of painful osteoporotic vertebral compression fractures: a brief review of the evidence for percutaneous vertebroplasty. J Bone Joint Surg Br 2011;93–B:1149-53. http://dx.doi.org/10.1302/0301-620X.93B9.26152.
- Do HM, Marcellus ML, Weir RU, Marks MP. Percutaneous Vertebroplasty Versus Medical Therapy for Treatment of Acute Vertebral Body Compression Fractures: A Prospective Randomized Study 2002. http://members.asnr.org/abstracts/2002/02-O-1027-ASNR.pdf (accessed February 2012).
- Kallmes DE, Jensen ME, Marx WF, Sinha, RS, Schweickert PA, Jarvik JG. A Pilot Study for a Sham-Controlled, Randomized, Prospective, Crossover Trial of Percutaneous Vertebroplasty 2002. http://members.asnr.org/abstracts/2002/02-O-632-ASNR.pdf (accessed February 2012).
- Farrokhi MR, Alibai E, Maghami Z. Randomized controlled trial of percutaneous vertebroplasty versus optimal medical management for the relief of pain and disability in acute osteoporotic vertebral compression fractures. J Neurosurg Spine 2011;14:561-9.
- Voormolen MHJ, Mali WPTM, Lohle PNM, Fransen H, Lampmann LEH, van der Graaf Y, et al. Percutaneous vertebroplasty compared with optimal pain medication treatment: short-term clinical outcome of patients with subacute or chronic painful osteoporotic vertebral compression fractures. The VERTOS study. Am J Neuroradiol 2007;28:555-60.
- Wardlaw D, Cummings SR, Van Meirhaeghe J, Bastian L, Tillman JB, Ranstam J, et al. Efficacy and safety of balloon kyphoplasty compared with non-surgical care for vertebral compression fracture (FREE): a randomised controlled trial. Lancet 2009;373:1016-24. http://dx.doi.org/10.1016/S0140-6736(09)60010-6.
- Boonen S, Van MJ, Bastian L, Cummings SR, Ranstam J, Tillman JB, et al. Balloon kyphoplasty for the treatment of acute vertebral compression fractures: 2-year results from a randomized trial. J Bone Miner Res 2011;26:1627-37. http://dx.doi.org/10.1002/jbmr.364.
- Liu JT, Liao WJ, Tan WC, Lee JK, Liu CH, Chen YH, et al. Balloon kyphoplasty versus vertebroplasty for treatment of osteoporotic vertebral compression fracture: a prospective, comparative, and randomized clinical study. Osteoporos Int 2010;21:359-64. http://dx.doi.org/10.1007/s00198-009-0952-8.
- ClinicalTrials.gov . KAVIAR Study – Kyphoplasty And Vertebroplasty in the Augmentation and Restoration of Vertebral Body Compression Fractures 2012. http://clinicaltrials.gov/ct2/show/NCT00323609?term=NCT00323609&rank=1 (accessed February 2012).
- Longo UG, Loppini M, Denaro L, Brandi ML, Maffulli N, Denaro V. The effectiveness and safety of vertebroplasty for osteoporotic vertebral compression fractures. A double blind, prospective, randomized, controlled study. Clin Cases Miner Bone Metabol 2010;7:109-13.
- ClinicalTrials.gov . Percutaneous Vertebroplasty Versus Conservative Treatment of Pain 2008. http://clinicaltrials.gov/ct2/show/NCT00203554?term=00203554&rank=1 (accessed September 2010).
- ClinicalTrials.gov . Cost Effectiveness and Efficacy of Kyphoplasty and Vertebroplasty Trial 2011. http://clinicaltrials.gov/ct2/show/NCT00279877?term=NCT00279877&rank=1 (accessed February 2012).
- ClinicalTrials.gov . Comparison of Balloon Kyphoplasty, Vertebroplasty and Conservative Management in Acute Osteoporotic Vertebral Fractures (OSTEO-6) 2011. http://clinicaltrials.gov/ct2/show/NCT00749060?term=NCT+00749060&rank=1 (accessed September 2012).
- ClinicalTrials.gov . Comparison of Balloon Kyphoplasty and Vertebroplasty in Subacute Osteoporotic Vertebral Fractures (OSTEO+6) 2011. http://clinicaltrials.gov/ct2/show/NCT00749086?term=00749086&rank=1 (accessed September 2011).
- ClinicalTrials.gov . A Trial of Vertebroplasty for Painful Acute Osteoporotic Vertebral Fractures (VERTOS IV) 2011. http://clinicaltrials.gov/ct2/show/NCT01200277?term=01200277&rank=1 (accessed November 2011).
- Gray LA, Jarvik JG, Heagerty PJ, Hollingworth W, Stout L, Comstock BA, et al. INvestigational Vertebroplasty Efficacy and Safety Trial (INVEST): a randomized controlled trial of percutaneous vertebroplasty. BMC Musculoskeletal Disord 2007;8. http://dx.doi.org/10.1186/1471-2474-8-126.
- Buchbinder R, Osborne RH, Ebeling PR, Wark JD, Mitchell P, Wriedt CJ, et al. Efficacy and safety of vertebroplasty for treatment of painful osteoporotic vertebral fractures: a randomised controlled trial [ACTRN012605000079640]. BMC Musculoskeletal Disord 2008;9. http://dx.doi.org/10.1186/1471-2474-9-156.
- Bombardier C. Outcome assessments in the evaluation of treatment of spinal disorders. Summary and general recommendations. Spine 2000;25:3100-3. http://dx.doi.org/10.1097/00007632-200012150-00003.
- Walters SJ, Brazier JE. Comparison of the minimally important difference for two health state utility measures: EQ-5D and SF-6D. Qual Life Res 2005;14:1523-32. http://dx.doi.org/10.1007/s11136-004-7713-0.
- Hawthorne G, Osborne R. Population norms and meaningful differences for the Assessment of Quality of Life (AQoL) measure. Aus NZ J Pub Health 2005;29:136-42. http://dx.doi.org/10.1111/j.1467-842X.2005.tb00063.x.
- Longo UG, Loppini M, Denaro L, Maffulli N, Denaro V. Rating scales for low back pain. Br Med Bull 2010;94:81-144. http://dx.doi.org/10.1093/bmb/ldp052.
- Medical Advisory Secretariat . Percutaneous vertebroplasty for treatment of painful osteoporotic vertebral compression fractures: an evidence-based analysis. Ontario Health Technol Assess Series 2010;10:1-45.
- Ware JE. SF-36 health survey update. Spine 2000;25:3130-9. http://dx.doi.org/10.1097/00007632-200012150-00008.
- Copay AG, Glassman SD, Subach BR, Berven S, Schuler TC, Carreon LY. Minimum clinically important difference in lumbar spine surgery patients: a choice of methods using the Oswestry Disability Index, Medical Outcomes Study questionnaire Short Form 36, and pain scales. Spine J 2008;8:968-74. http://dx.doi.org/10.1016/j.spinee.2007.11.006.
- Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthrit Care Res 2001;45:384-91. http://dx.doi.org/10.1002/1529-0131(200108)45:4<384::AID-ART352>3.0.CO;2-0.
- Wiebe S, Matijevic S, Eliasziw M, Derry PA. Clinically important change in quality of life in epilepsy. J Neurol Neurosurg Psychiatry 2002;73:116-20. http://dx.doi.org/10.1136/jnnp.73.2.116.
- Lips P, Leplège A. Development and validation of a quality of life questionnaire for patients with vertebral fractures: Qualeffo-41. Qual Life Res 2000;9:763-6.
- Oleksik A, Lips P, Dawson A, Minshall ME, Shen W, Cooper C, et al. Health-related quality of life in postmenopausal women with low BMD with or without prevalent vertebral fractures. J Bone Miner Res 2000;15:1384-92. http://dx.doi.org/10.1359/jbmr.2000.15.7.1384.
- van Schoor NM, Knol DL, Glas CA, Ostelo RW, Leplège A, Cooper C, et al. Development of the Qualeffo-31, an osteoporosis-specific quality-of-life questionnaire. Osteoporos Int 2006;17:543-51.
- Bombardier C. Outcome assessments in the evaluation of treatment of spinal disorders. Spine 2000;25:3097-9. http://dx.doi.org/10.1097/00007632-200012150-00002.
- Trout AT, Kallmes DF, Gray LA, Goodnature BA, Everson SL, Comstock BA, et al. Evaluation of vertebroplasty with a validated outcome measure: the Roland–Morris Disability Questionnaire. AJNR Am J Neuroradiol 2005;26:2652-7.
- Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Md State Med J 1965;14:56-61.
- Roland M, Fairbank J. The Roland–Morris Disability Questionnaire and the Oswestry Disability Questionnaire. Spine 2000;25:3115-24. http://dx.doi.org/10.1097/00007632-200012150-00006.
- Lauridsen HH, Hartvigsen J, Manniche C, Korsholm L, Grunnet-Nilsson N. Responsiveness and minimal clinically important difference for pain and disability instruments in low back pain patients. BMC Musculoskeletal Disord 2006;7.
- Patrick DL, Deyo RA, Atlas SJ, Singer DE, Chapin A, Keller RB, et al. Assessing health-related quality of life in patients with sciatica. Spine 1995;20:1899-908. http://dx.doi.org/10.1097/00007632-199509000-00011.
- Kopec JA. Measuring functional outcomes in persons with back pain. A review of back-specific questionnaires. Spine 2000;25:3110-14. http://dx.doi.org/10.1097/00007632-200012150-00005.
- Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 1995;332:556-61. http://dx.doi.org/10.1056/NEJM199503023320902.
- Von Korff M, Jensen MP, Karoly P. Assessing global pain severity by self-report in clinical and health services research. Spine 2000;25:3140-51. http://dx.doi.org/10.1097/00007632-200012150-00009.
- Johnson C. Measuring pain. Visual analog scale versus numeric pain scale: what is the difference?. J Chiropr Med 2005;4:43-4. http://dx.doi.org/10.1016/S0899-3467(07)60112-8.
- DeLoach LJ, Higgins MS, Caplan AB, Stiff JL. The Visual Analog Scale in the immediate postoperative period: intrasubject variabiltiy and correlation with a numeric scale. Anesthes Analges 1998;86:102-6. http://dx.doi.org/10.1097/00000539-199801000-00020.
- Bolton JE, Wilkinson RC. Responsiveness of pain scales: a comparison of three pain intensity measures in chiropractic patients. J Manipulative Physiol Ther 1998;21:1-7.
- Grotle M, Brox JA, Vøllestad NK. Concurrent comparison of responsiveness in pain and functional status measurements used for patients with low back pain. Spine 2004;29:E492-501. http://dx.doi.org/10.1097/01.brs.0000143664.02702.0b.
- Klazen CAH, Verhaar HJJ, Lampmann LEH, Juttmann JR, Blonk MC, Jansen FH, et al. VERTOS II: percutaneous vertebroplasty versus conservative therapy in patients with painful osteoporotic vertebral compression fractures; rationale, objectives and design of a multicenter randomized controlled trial. Trials 2007. http://dx.doi.org/10.1186/1745-6215-8-33.
- McKiernan F, Faciszewski T, Jensen R. Reporting height restoration in vertebral compression fractures. Spine 2003;28:2517-21. http://dx.doi.org/10.1097/01.BRS.0000092424.29886.C9.
- McKiernan FE. Kyphoplasty and vertebroplasty: how good is the evidence?. Curr Rheumatol Rep 2007;9:57-65. http://dx.doi.org/10.1007/s11926-007-0023-0.
- McKiernan F, Jensen R, Faciszewski T. The dynamic mobility of vertebral compression fractures. J Bone Miner Res 2003;18:24-9. http://dx.doi.org/10.1359/jbmr.2003.18.1.24.
- Farcy JP, Weidenbaum M, Glassman SD. Sagittal index in management of thoracolumbar burst fractures. Spine 1990;15:958-65. http://dx.doi.org/10.1097/00007632-199009000-00022.
- Jiang G, Eastell R, Barrington NA, Ferrar L. Comparison of methods for the visual identification of prevalent vertebral fracture in osteoporosis. Osteoporos Int 2004;15:887-96. http://dx.doi.org/10.1007/s00198-004-1626-1.
- Glassman SD, Bridwell K, Dimar JR, Horton W, Berven S, Schwab F. The impact of positive sagittal balance in adult spinal deformity. Spine 2005;30:2024-9. http://dx.doi.org/10.1097/01.brs.0000179086.30449.96.
- National Osteoporosis Foundation Working Group on Vertebral Fractures . Assessing vertebral fractures. J Bone Miner Res 1995;10:518-23.
- Genant HK, Jergas M. Assessment of prevalent and incident fractures in osteoporosis research. Osteoporos Int 2003;14:S43-55.
- Grigoryan M, Guermazi A, Roemer FW, Delmas PD, Genant HK. Recognizing and reporting osteoporotic vertebral fractures. Eur Spine J 2003;12:S104-12. http://dx.doi.org/10.1007/s00586-003-0613-0.
- Ferrar L, Jiang G, Adams J, Eastell R. Identification of vertebral fractures: an update. Osteoporos Int 2005;16:717-28. http://dx.doi.org/10.1007/s00198-005-1880-x.
- Fribourg D, Tang C, Sra P, Delamarter R, Bae H. Incidence of subsequent vertebral fracture after kyphoplasty. Spine 2004;29:2270-6. http://dx.doi.org/10.1097/01.brs.0000142469.41565.2a.
- Klazen CAH, Venmans A, de Vries J, van Rooij WJ, Jansen FH, Blonk MC, et al. Percutaneous vertebroplasty is not a risk factor for new osteoporotic compression fractures: results from VERTOS II. AJNR Am J Neuroradiol 2010;31:1447-50. http://dx.doi.org/10.3174/ajnr.A2148.
- Mathis JM. Percutaneous vertebroplasty: complication avoidance and technique optimization. Am J Neuroradiology 2003;24:1697-706.
- Venmans A, Klazen CA, van Rooij WJ, de Vries J, Mali WP, Lohle PN. Postprocedural CT for perivertebral cement leakage in percutaneous vertebroplasty is not necessary – results from VERTOS II. Neuroradiology 2011;53:19-22. http://dx.doi.org/10.1007/s00234-010-0705-6.
- Jensen ME, Evans AJ. Cardiovascular collapse and death during vertebroplasty – response. Radiology 2003;228:902-3.
- Jensen ME, Evans AJ, Mathis JM, Kallmes DF, Cloft HJ, Dion JE. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral body compression fractures: technical aspects. AJNR Am J Neuroradiol 1997;18:1897-904.
- Belinson SE. Percutaneous vertebroplasty or kyphoplasty for vertebral fractures caused by osteoporosis. Tec Assessment Program 2011;25:1-56.
- Martin DJ, Rad AE, Kallmes DF. Prevalence of extravertebral cement leakage after vertebroplasty: procedural documentation versus CT detection. Acta Radiol 2012;53:569-72. http://dx.doi.org/10.1258/ar.2012.120222.
- Venmans A, Klazen CAH, Lohle PNM, van Rooij WJ, Verhaar HJJ, de Vries J, et al. Percutaneous vertebroplasty and pulmonary cement embolism: results from VERTOS II. AJNR Am J Neuroradiol 2010;31:1451-3. http://dx.doi.org/10.3174/ajnr.A2127.
- Klazen C, Lohle P, Jansen F, Schoemaker M, Elgersma O, Van EK, et al. 1-year results of the VERTOS II trial: vertebroplasty versus conservative therapy. CardioVasc Interv Radiol 2009;32.
- Iranian Registry of Clinical Trials . The Comparison of Results of PV (Percutaneous Vertebroplasty) and Conservative Treatment in Patients With Osteoporotic VCF (vertebral Compression Fracture) 2010. www.irct.ir/searchresult.php?keyword=IRCT138804252193N1&id=2193&number=1&field=a&prt=1&total=1&m=1 (accessed February 2012).
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 2011. www.cochrane-handbook.org/ (accessed April 2012).
- Kallmes DF, Comstock BA, Gray LA, Heagerty PJ, Hollingworth W, Turner JA, et al. Baseline pain and disability in the Investigational Vertebroplasty Efficacy and Safety Trial. AJNR Am J Neuroradiol 2009;30:1203-5. http://dx.doi.org/10.3174/ajnr.A1519.
- Mathis JM, Ortiz O, Zoarski GH. Vertebroplasty versus kyphoplasty: a comparison and contrast. AJNR Am J Neuroradiol 2004;25:840-5.
- Khosla A, Turner JA, Jarvik JG, Gray LA, Kallmes DF. Impact of pain question modifiers on spine augmentation outcome. Radiology 2010;257:477-82. http://dx.doi.org/10.1148/radiol.10100237.
- Orr RD. Vertebroplasty, cognitive dissonance, and evidence-based medicine: what do we do when the ‘evidence’ says we are wrong?. Cleve Clin J Med 2010;77:8-11. http://dx.doi.org/10.3949/ccjm.77a.09146.
- Van Meirhaeghe JK, Boonen S, Bastian L, Cummings S, Ranstam J, Tillman J, et al. A randomized trial of balloon kyphoplasty and nonsurgical care for patients with acute vertebral compression fractures: two year results. Osteoporos Int 2010;21:S667-8.
- ClinicalTrials.gov . FREE Study – Fracture Reduction Evaluation 2010. http://clinicaltrials.gov/ct2/show/NCT00211211?term=kyphoplasty&rank=17 (accessed November 2011).
- Blattert TR, Josten C. Treatment of osteoporotic vertebral body fractures by means of percutaneous Balloon kyphoplasty: long term results of a prospective, clinical trial. Osteoporos Int 2010;21.
- Diel P, Reuss W, Aghayev E, Moulin P, Roder C. SWISSspine Registry Group. SWISSspine-a nationwide health technology assessment registry for balloon kyphoplasty: methodology and first results. Spine J 2010;10:961-71. http://dx.doi.org/10.1016/j.spinee.2009.08.452.
- Majd ME, Farley S, Holt RT. Preliminary outcomes and efficacy of the first 360 consecutive kyphoplasties for the treatment of painful osteoporotic vertebral compression fractures. Spine J 2005;5:244-55. http://dx.doi.org/10.1016/j.spinee.2004.09.013.
- Alvarez L, Perez-Higueras A, Granizo JJ, de M I, Quinones D, Rossi RE. Predictors of outcomes of percutaneous vertebroplasty for osteoporotic vertebral fractures. Spine 2005;30:87-92.
- Diel P, Merky D, Roder C, Popp A, Perler M, Heini PF. Safety and efficacy of vertebroplasty: Early results of a prospective one-year case series of osteoporosis patients in an academic high-volume center. Indian J Orthopaed 2009;43:228-33.
- Evans AJ, Jensen ME, Kip KE, DeNardo AJ, Lawler GJ, Negin GA, et al. Vertebral compression fractures: pain reduction and improvement in functional mobility after percutaneous polymethylmethacrylate vertebroplasty retrospective report of 245 cases. Radiology 2003;226:366-72. http://dx.doi.org/10.1148/radiol.2262010906.
- Lee ST, Chen JF. Closed reduction vertebroplasty for the treatment of osteoporotic vertebral compression fractures. Technical note. J Neurosurg 2004;100:392-6. http://dx.doi.org/10.3171/spi.2004.100.4.0392.
- Masala S, Mammucari M, Angelopoulos G, Fiori R, Massari F, Faria S, et al. Percutaneous vertebroplasty in the management of vertebral osteoporotic fractures. Short-term, mid-term and long-term follow-up of 285 patients. Skeletal Radiol 2009;38:863-9. http://dx.doi.org/10.1007/s00256-009-0712-z.
- Mpotsaris A, Abdolvahabi R, Hoffleith B, Nickel J, Harati A, Loehr C, et al. Percutaneous vertebroplasty in vertebral compression fractures of benign or malignant origin: a prospective study of 1188 patients with follow-up of 12 months. Deutsches Arzteblatt International 2011;108:331-8.
- Ryu KS, Park CK. The prognostic factors influencing on the therapeutic effect of percutaneous vertebroplasty in treating osteoporotic vertebral compression fractures. J Korean Neurosurg Soc 2009;45:16-23. http://dx.doi.org/10.3340/jkns.2009.45.1.16.
- Edidin AA, Ong KL, Lau E, Kurtz SM. Mortality risk for operated and nonoperated vertebral fracture patients in the medicare population. J Bone Miner Res 2011;26:1617-26. http://dx.doi.org/10.1002/jbmr.353.
- Mortality and Complication Risks for Operated and Non-Operated Vertebral Fracture Patients in the Medicare Population. Washington DC: Exponent; 2012.
- Lange A, Braun S. Survival Analysis Using German Claims Data of Patients with Osteoporotic Vertebral Compression Fractures (OVCF) 2012.
- Lange A, Braun S. Survival Analysis Using German Claims Data of Patients with Osteoporotic Vertebral Compression Fractures (OVCF). Propensity Score Matching Results (BKP vs. VP). Hannover: Herescon Gmbh; 2012.
- Padovani B, Kasriel O, Brunner P, Peretti-Viton P. Pulmonary embolism caused by acrylic cement: a rare complication of percutaneous vertebroplasty. AJNR Am J Neuroradiol 1999;20:375-7.
- Masala S, Mastrangeli R, Petrella MC, Massari F, Ursone A, Simonetti G. Percutaneous vertebroplasty in 1,253 levels: results and long-term effectiveness in a single centre. Eur Radiol 2009;19:165-71. http://dx.doi.org/10.1007/s00330-008-1133-4.
- Saliou G, Rutgers DR, Kocheida EM, Langman G, Meurin A, Deramond H, et al. Balloon-related complications and technical failures in kyphoplasty for vertebral fractures. AJNR Am J Neuroradiol 2010;31:175-9. http://dx.doi.org/10.3174/ajnr.A1783.
- Harrop JS, Prpa B, Reinhardt MK, Lieberman I. Primary and secondary osteoporosis’ incidence of subsequent vertebral compression fractures after kyphoplasty. Spine 2004;29:2120-5. http://dx.doi.org/10.1097/01.brs.0000141176.63158.8e.
- Kulcsar Z, Marosfoi M, Berentei Z, Szikora I. Frequency and risk factors of adjacent fractures after vertebroplasty. Neuroradiology 2009;51.
- Tseng YY, Yang TC, Tu PH, Lo YL, Yang ST. Repeated and multiple new vertebral compression fractures after percutaneous transpedicular vertebroplasty. Spine 2009;34:1917-22. http://dx.doi.org/10.1097/BRS.0b013e3181ac8f07.
- Uppin AA, Hirsch JA, Centenera LV, Pfiefer BA, Pazianos AG, Choi IS. Occurrence of new vertebral body fracture after percutaneous vertebroplasty in patients with osteoporosis. Radiology 2003;226:119-24. http://dx.doi.org/10.1148/radiol.2261011911.
- Donovan MA, Khandji AG, Siris E. Multiple adjacent vertebral fractures after kyphoplasty in a patient with steroid-induced osteoporosis. J Bone Miner Res 2004;19:712-13. http://dx.doi.org/10.1359/jbmr.040207.
- Han IH, Chin DK, Kuh SU, Kim KS, Jin BH, Yoon YS, et al. Magnetic resonance imaging findings of subsequent fractures after vertebroplasty. Neurosurgery n.d.;64:740-4. http://dx.doi.org/10.1227/01.NEU.0000339120.41053.F1.
- Hierholzer J, Fuchs H, Westphalen K, Baumann C, Slotosch C, Schulz R. Incidence of symptomatic vertebral fractures in patients after percutaneous vertebroplasty. Cardiovasc Interv Radiol 2008;31:1178-83. http://dx.doi.org/10.1007/s00270-008-9376-7.
- Kim HS, Jeon KH, Choi WJ, Kim KT, Ju CI, Kim SW, et al. Spinal instability predicting score (SIPS) for following fractures after vertebroplasty in patients with osteoporotic vertebral compression fractures. Spine J 2010;10. http://dx.doi.org/10.1016/j.spinee.2010.07.280.
- Lee WS, Sung KH, Jeong HT, Sung YS, Hyun YI, Choi JY, et al. Risk factors of developing new symptomatic vertebral compression fractures after percutaneous vertebroplasty in osteoporotic patients. Eur Spine J 2006;15:1777-83. http://dx.doi.org/10.1007/s00586-006-0151-7.
- Lo YP, Chen WJ, Chen LH, Lai PL. New vertebral fracture after vertebroplasty. J Trauma-Inj Infect Crit Care 2008;65:1439-45. http://dx.doi.org/10.1097/TA.0b013e318169cd0b.
- Nene A. Vertebroplasty-happily ever after?. Osteoporos Int 2010;21.
- Nunley PD, Jawahar A. A comparison of clinical outcomes and adjacent level fractures in patients receiving vertebroplasty for osteoporotic compression fractures using Cortoss or Poly Methyl Methacrylate: prospective, randomized trial. Neurosurgery 2009;65. http://dx.doi.org/10.1227/01.neu.0000358697.94292.6a.
- Syed MI, Patel NA, Jan S, Harron MS, Morar K, Shaikh A. New symptomatic vertebral compression fractures within a year following vertebroplasty in osteoporotic women. Am J Neuroradiol 2005;26:1601-4.
- Tatsumi RL, Ching AC, Byrd GD, Hiratzka JR, Threlkeld JE, Hart RA. Predictors and prevalence of patients undergoing additional kyphoplasty procedures after an initial kyphoplasty procedure. Spine J 2010;10:979-86. http://dx.doi.org/10.1016/j.spinee.2010.08.027.
- Trout AT, Kallmes DF, Kaufmann TJ. New fractures after vertebroplasty: adjacent fractures occur significantly sooner. AJNR Am J Neuroradiol 2006;27:217-23.
- Trout AT, Kallmes DF, Lane JI, Layton KF, Marx WF. Subsequent vertebral fractures after vertebroplasty: association with intraosseous clefts. AJNR Am J Neuroradiol 2006;27:1586-91.
- Trout AT, Kallmes DF. Does vertebroplasty cause incident vertebral fractures? A review of available data. AJNR Am J Neuroradiol 2006;27:1397-403.
- Mudano AS, Bian J, Cope JU, Curtis JR, Gross TP, Allison JJ, et al. Vertebroplasty and kyphoplasty are associated with an increased risk of secondary vertebral compression fractures: a population-based cohort study. Osteoporos Int 2009;20:819-26. http://dx.doi.org/10.1007/s00198-008-0745-5.
- Lin CC, Yen PS, Wen SH. Fluid sign in the treated bodies after percutaneous vertebroplasty. Neuroradiology 2008;50:955-61. http://dx.doi.org/10.1007/s00234-008-0430-6.
- Baroud G, Bohner M. Biomechanical impact of vertebroplasty. Postoperative biomechanics of vertebroplasty. Joint Bone Spine 2006;73:144-50. http://dx.doi.org/10.1016/j.jbspin.2005.02.004.
- Berlemann U, Ferguson SJ, Nolte LP, Heini PF. Adjacent vertebral failure after vertebroplasty. A biomechanical investigation. J Bone Joint Surg Br 2002;84:748-52. http://dx.doi.org/10.1302/0301-620X.84B5.11841.
- Baroud G, Nemes J, Heini P, Steffen T. Load shift of the intervertebral disc after a vertebroplasty: a finite-element study. Eur Spine J 2003;12:421-6. http://dx.doi.org/10.1007/s00586-002-0512-9.
- Baroud G, Heini P, Nemes J, Bohner M, Ferguson S, Steffen T. Biomechanical explanation of adjacent fractures following vertebroplasty. Radiology 2003;229:606-7. http://dx.doi.org/10.1148/radiol.2292030378.
- Farooq N, Park JC, Pollintine P, Annesley-Williams DJ, Dolan P. Can vertebroplasty restore normal load-bearing to fractured vertebrae?. Spine 2005;30:1723-30. http://dx.doi.org/10.1097/01.brs.0000171906.01906.07.
- Yang SC, Chen WJ, Yu SW, Tu YK, Kao YH, Chung KC. Revision strategies for complications and failure of vertebroplasties. Eur Spine J 2008;17:982-8. http://dx.doi.org/10.1007/s00586-008-0680-3.
- Abdul-Jalil Y, Bartels J, Alberti O, Becker R. Delayed presentation of pulmonary polymethylmethacrylate emboli after percutaneous vertebroplasty. Spine 2007;32:E589-93. http://dx.doi.org/10.1097/BRS.0b013e31814b84ba.
- Agko M, Nazzal M, Jamil T, Castillo-Sang M, Clark P, Kasper G. Prevention of cardiopulmonary embolization of polymethylmethacrylate cement fragment after kyphoplasty with insertion of inferior vena cava filter. J Vasc Surg 2010;51:210-13. http://dx.doi.org/10.1016/j.jvs.2009.07.110.
- Baumann A, Tauss J, Baumann G, Tomka M, Hessinger M, Tiesenhausen K. Cement embolization into the vena cava and pulmonal arteries after vertebroplasty: interdisciplinary management. Eur J Vasc Endovasc Surg 2006;31:558-61. http://dx.doi.org/10.1016/j.ejvs.2005.11.008.
- Bernhard J, Heini PF, Villiger PM. Asymptomatic diffuse pulmonary embolism caused by acrylic cement: an unusual complication of percutaneous vertebroplasty. Ann Rheum Dis 2003;62:85-6. http://dx.doi.org/10.1136/ard.62.1.85.
- Biega TJ, Lettieri CJ, Levy LM, Venbrux AC. Linear pulmonary opacities in an asymptomatic patient. Respiration 2006;73:705-7. http://dx.doi.org/10.1159/000092955.
- Bonardel G, Pouit B, Gontier E, Dutertre G, Mantzarides M, Goasguen O, et al. Pulmonary cement embolism after percutaneous vertebroplasty: a rare and nonthrombotic cause of pulmonary embolism. Clin Nucl Med 2007;32:603-6. http://dx.doi.org/10.1097/RLU.0b013e3180a1ad5a.
- Cadeddu C, Nocco S, Secci E, Deidda M, Pirisi R, Mercuro G. Echocardiographic accidental finding of asymptomatic cardiac and pulmonary embolism caused by cement leakage after percutaneous vertebroplasty. Eur J Echocardiogr 2009;10:590-2. http://dx.doi.org/10.1093/ejechocard/jep030.
- Caynak B, Onan B, Sagbas E, Duran C, Akpinar B. Cardiac tamponade and pulmonary embolism as a complication of percutaneous vertebroplasty. Ann Thorac Surg 2009;87:299-301. http://dx.doi.org/10.1016/j.athoracsur.2008.05.074.
- Chen HL, Wong CS, Ho ST, Chang FL, Hsu CH, Wu CT. A lethal pulmonary embolism during percutaneous vertebroplasty. Anesth Analg 2002;95:1060-2. http://dx.doi.org/10.1097/00000539-200210000-00049.
- Dastidar J. History of PE/DVT + vertebroplasty + dyspnea =?. J Hosp Med 2011;6.
- Finch L, Cheng SG, Steinberg KP, Stern EJ. Polymethylmethacrylate pulmonary emboli. Clin Pulm Med 2002;9:133-4. http://dx.doi.org/10.1097/00045413-200203000-00013.
- Francois K, Taeymans Y, Poffyn B, Van NG. Successful management of a large pulmonary cement embolus after percutaneous vertebroplasty: a case report. Spine 2003;28:E424-5. http://dx.doi.org/10.1097/01.BRS.0000092345.00563.E0.
- Freitag M, Gottschalk A, Schuster M, Wenk W, Wiesner L, Standl TG. Pulmonary embolism caused by polymethylmethacrylate during percutaneous vertebroplasty in orthopaedic surgery. Acta Anaesthesiol Scand 2006;50:248-51. http://dx.doi.org/10.1111/j.1399-6576.2005.00821.x.
- Grahe JS, Casey L, White G. Pulmonary cement embolism following percutaneous vertebroplasty. Chest 2004;126.
- Harris B, Briggs G, Dennis C. Cement pulmonary embolism as a consequence of vertebroplasty. Int Med J 2007;37:196-7. http://dx.doi.org/10.1111/j.1445-5994.2006.01282.x.
- Jang JS, Lee SH, Jung SK. Pulmonary embolism of polymethylmethacrylate after percutaneous vertebroplasty: a report of three cases. Spine 2002;27:E416-18. http://dx.doi.org/10.1097/00007632-200210010-00021.
- Kim YJ, Lee JW, Park KW, Yeom JS, Jeong HS, Park JM, et al. Pulmonary cement embolism after percutaneous vertebroplasty in osteoporotic vertebral compression fractures: incidence, characteristics, and risk factors. Radiology 2009;251:250-9.
- Kovalenko B, Rao Q, Hughes T. Pulmonary embolism caused by acrylic cement from prior vertebroplasty. CardioVasc Interv Radiol 2009;32.
- Sang EL, Chang SA, Kim MS, Kim SY, Han JK, Jang HJ, et al. Acrylic cement foreign body and thrombus in right atrium causing pulmonary embolism after percutaneous vertebroplasty. Korean Circ J 2006;36:713-15.
- Leroux G, Costedoat-Chalumeau N, Chiras J, de Gennes C, Piette JC. A vertebroplasty with dyspnea. Revue E Med Interne 2007;28:492-4.
- Liliang PC, Lu K, Liang CL, Tsai YD, Hsieh CH, Chen HJ. Dyspnoea and chest pain associated with pulmonary polymethylmethacrylate embolism after percutaneous vertebroplasty. Injury 2007;38:245-8. http://dx.doi.org/10.1016/j.injury.2006.08.031.
- Lim KJ, Yoon SZ, Jeon YS, Bahk JH, Kim CS, Lee JH, et al. An intraatrial thrombus and pulmonary thromboembolism as a late complication of percutaneous vertebroplasty. Anesth Analg 2007;104:924-6. http://dx.doi.org/10.1213/01.ane.0000256974.84535.7a.
- Lim SH, Kim H, Kim HK, Baek MJ. Multiple cardiac perforations and pulmonary embolism caused by cement leakage after percutaneous vertebroplasty. Eur J Cardio-Thorac Surg 2008;33:510-12. http://dx.doi.org/10.1016/j.ejcts.2007.12.012.
- MacTaggart JN, Pipinos II, Johanning JM, Lynch TG. Acrylic cement pulmonary embolus masquerading as an embolized central venous catheter fragment. J Vasc Surg 2006;43:180-3. http://dx.doi.org/10.1016/j.jvs.2005.09.002.
- Moll S, Kuzma C. Images in vascular medicine: cement pulmonary embolism. Vasc Med 2010;15:339-40. http://dx.doi.org/10.1177/1358863X10365180.
- Monticelli F, Meyer HJ, Tutsch-Bauer E. Fatal pulmonary cement embolism following percutaneous vertebroplasty (PVP). Forensic Sci Int 2005;149:35-8. http://dx.doi.org/10.1016/j.forsciint.2004.06.010.
- Moon S, Lee S, Kong G, Kim J, Lee E. Large pulmonary embolus after percutaneous vertebroplasty in osteoporotic compression fracture – a case report. Bone 2009;44. http://dx.doi.org/10.1016/j.bone.2009.01.192.
- Muller M, Biedermann M, Strecker W. A complication during kyphoplasty. Cement penetration through the azygos vein into the superior vena cava. Orthopade 2006;35:1183-6.
- Neuwirth J, Weber JC, Kohler B. Pulmonary cement embolism after vertebroplasty in multiple osteoporotic vertebral fractures. Dtsch Med Wochenschr 2006;131:2275-6.
- Perrin C, Jullien V, Padovani B, Blaive B. Percutaneous vertebroplasty complicated by pulmonary embolus of acrylic cement. Rev Mal Respir 1999;16:215-17.
- Pleser M, Roth R, Worsdorfer O, Manke C. Pulmonary embolism caused by PMMA in percutaneous vertebroplasty. Case report and review of the literature. Unfallchirurg 2004;10:807-11.
- Pott L, Wippermann B, Hussein S, Gunther T, Brusch U, Fremerey R. PMMA pulmonary embolism and post interventional associated fractures after percutaneous vertebroplasty. Orthopade 2005;34:698-702.
- Quesada N, Mutlu GM. Images in cardiovascular medicine. Pulmonary embolization of acrylic cement during vertebroplasty. Circulation 2006;113:e295-6.
- Radcliff KE, Reitman CA, Delasotta LA, Hong J, DiIorio T, Zaslavsky J, et al. Pulmonary cement embolization after kyphoplasty: a case report and review of the literature. Spine J 2010;10:e1-5. http://dx.doi.org/10.1016/j.spinee.2010.07.394.
- Righini M, Sekoranja L, Le GG, Favre I, Bounameaux H, Janssens JP. Pulmonary cement embolism after vertebroplasty. Thromb Haemost 2006;95:388-9.
- Schneider L, Plit M. Pulmonary embolization of acrylic cement during percutaneous vertebroplasty. Intern Med J 2007;37:423-5. http://dx.doi.org/10.1111/j.1445-5994.2007.01377.x.
- Schoenes B, Bremerich DH, Risteski PS, Thalhammer A, Meininger D. Cardiac perforation after vertebroplasty. Anaesthesist 2008;57:147-50.
- Scroop R, Eskridge J, Britz GW. Paradoxical cerebral arterial embolization of cement during intraoperative vertebroplasty: case report. AJNR Am J Neuroradiol 2002;23:868-70.
- Seo JS, Kim YJ, Choi BW, Kim TH, Choe KO. MDCT of pulmonary embolism after percutaneous vertebroplasty. AJR Am J Roentgenol 2005;184:1364-5. http://dx.doi.org/10.2214/ajr.184.4.01841364.
- Shalshin A, Brar N, Chawla S, Islam T. Pulmonary cement embolism: rare complication of Kyphoplasty. Chest 2004;138. http://dx.doi.org/10.1378/chest.10723.
- Son KH, Chung JH, Sun K, Son HS. Cardiac perforation and tricuspid regurgitation as a complication of percutaneous vertebroplasty. Eur J Cardio-Thorac Surg 2008;33:508-9. http://dx.doi.org/10.1016/j.ejcts.2007.11.027.
- Stricker K, Orler R, Yen K, Takala J, Luginbuhl M. Severe hypercapnia due to pulmonary embolism of polymethylmethacrylate during vertebroplasty. Anesth Analg 1000;98:1184-6. http://dx.doi.org/10.1213/01.ANE.0000104585.83801.C5.
- Torres Machi ML, Suarez RV, Medina RC, Gil BF, Ojeda BN, Rodriguez-Perez A. Pulmonary embolism caused by cement following vertebroplasty. Rev Esp Anestesiol Reanim 2003;50:489-91.
- Tozzi P, Abdelmoumene Y, Corno AF, Gersbach PA, Hoogewoud HM, von Segesser LK. Management of pulmonary embolism during acrylic vertebroplasty. Ann Thorac Surg 2002;74:1706-8. http://dx.doi.org/10.1016/S0003-4975(02)03962-0.
- Yoo KY, Jeong SW, Yoon W, Lee J. Acute respiratory distress syndrome associated with pulmonary cement embolism following percutaneous vertebroplasty with polymethylmethacrylate. Spine 2004;29:E294-7. http://dx.doi.org/10.1097/01.BRS.0000131211.87594.B0.
- Zaccheo MV, Rowane JE, Costello EM. Acute respiratory failure associated with polymethyl methacrylate pulmonary emboli after percutaneous vertebroplasty. Am J Emerg Med 2008;26:636-7. http://dx.doi.org/10.1016/j.ajem.2007.10.013.
- Gaye M, Fuentes S, Pech-Gourg G, Benhima Y, Dufour H. Spondylitis following vertebroplasty. Case report and review of the literature. Neurochirurgie 2008;54:551-5.
- Ivo R, Sobottke R, Seifert H, Ortmann M, Eysel P. Tuberculous spondylitis and paravertebral abscess formation after kyphoplasty: a case report. Spine 2010;35:E559-63. http://dx.doi.org/10.1097/BRS.0b013e3181ce1aab.
- Lee CB, Kim HS, Kim YJ. Pyogenic spondylitis after vertebroplasty – a report of two cases. Asian Spine J 2007;1:106-9. http://dx.doi.org/10.4184/asj.2007.1.2.106.
- Mummaneni PV, Walker DH, Mizuno J, Rodts GE. Infected vertebroplasty requiring 360 degrees spinal reconstruction: long-term follow-up review. Report of two cases. J Neurosurg Spine 2006;5:86-9.
- Schmid KE, Boszczyk BM, Bierschneider M, Zarfl A, Robert B, Jaksche H. Spondylitis following vertebroplasty: a case report. Eur Spine J 2005;14:895-9. http://dx.doi.org/10.1007/s00586-005-0905-7.
- Schofer MD, Lakemeier S, Peterlein CD, Heyse TJ, Quante M. Primary pyogenic spondylitis following kyphoplasty: a case report. J Med Case Rep 2011;5. http://dx.doi.org/10.1186/1752-1947-5-101.
- Shin JH, Ha KY, Kim KW, Lee JS, Joo MW. Surgical treatment for delayed pyogenic spondylitis after percutaneous vertebroplasty and kyphoplasty. Report of 4 cases. J Neurosurge Spine 2008;9:265-72. http://dx.doi.org/10.3171/SPI/2008/9/9/265.
- Syed MI, Avutu B, Shaikh A, Sparks H, Mohammed MI, Morar K. Vertebral osteomyelitis following vertebroplasty: is acne a potential contraindication and are prophylactic antibiotics mandatory prior to vertebroplasty?. Pain Physician 2009;12:E285-90.
- Vats HS, McKiernan FE. Infected vertebroplasty: case report and review of literature. Spine 2006;31:E859-62. http://dx.doi.org/10.1097/01.brs.0000240665.56414.88.
- Walker DH, Mummaneni P, Rodts GE. Infected vertebroplasty. Report of two cases and review of the literature. Neurosurg Focus 2004;17. http://dx.doi.org/10.3171/foc.2004.17.6.6.
- Wendling D, Runge M, Toussirot E, Bertolini E, Prati C. Vertebral osteitis adjacent to kyphoplasty. Joint Bone Spine 2010;77:67-9. http://dx.doi.org/10.1016/j.jbspin.2009.11.004.
- Yu SW, Chen WJ, Lin WC, Chen YJ, Tu YK. Serious pyogenic spondylitis following vertebroplasty–a case report. Spine 2004;29:E209-11. http://dx.doi.org/10.1097/00007632-200405150-00023.
- Farahvar A, Dubensky D, Bakos R. Perforation of the right cardiac ventricular wall by polymethylmethacrylate after lumbar kyphoplasty. J Neurosurg Spine 2009;11:487-91. http://dx.doi.org/10.3171/2009.5.SPINE08517.
- Kim SY, Seo JB, Do KH, Lee JS, Song KS, Lim TH. Cardiac perforation caused by acrylic cement: a rare complication of percutaneous vertebroplasty. AJR Am J Roentgenol 2005;185:1245-7. http://dx.doi.org/10.2214/AJR.04.1443.
- Kao FC, Tu YK, Lai PL, Yu SW, Yen CY, Chou MC. Inferior vena cava syndrome following percutaneous vertebroplasty with polymethylmethacrylate. Spine 2008;33:E329-33. http://dx.doi.org/10.1097/BRS.0b013e31816f6a10.
- White JB, Thielen KR, Kallmes DF. Putative risk of substantial venous air embolism during vertebroplasty: a technical observation. Spine 2009;34:1526-8. http://dx.doi.org/10.1097/BRS.0b013e3181a90b44.
- Dash A, Brinster DR. Open heart surgery for removal of polymethylmethacrylate after percutaneous vertebroplasty. Ann Thorac Surg 2011;91:276-8. http://dx.doi.org/10.1016/j.athoracsur.2010.06.106.
- Park JH, Choo SJ, Park SW. Images in cardiovascular medicine. Acute pericarditis caused by acrylic bone cement after percutaneous vertebroplasty. Circulation 2005;111.
- Puri AS, Colen RR, Reddy AS, Groff MW, DiNobile D, Killoran T, et al. Lumbar artery pseudoaneurysm after percutaneous vertebroplasty: a unique vascular complication. J Neurosurg Spine 2011;14:296-9. http://dx.doi.org/10.3171/2010.10.SPINE1082.
- Marden FA, Putman CM. Cement-embolic stroke associated with vertebroplasty. AJNR Am J Neuroradiol 2008;29:1986-8. http://dx.doi.org/10.3174/ajnr.A1159.
- Biafora SJ, Mardjetko SM, Butler JP, McCarthy PL, Gleason TF. Arterial injury following percutaneous vertebral augmentation: a case report. Spine 2006;31:E84-7. http://dx.doi.org/10.1097/01.brs.0000197596.88416.02.
- Heo DH, Cho YJ. Segmental artery injury following percutaneous vertebroplasty using extrapedicular approach. J Korean Neurosurg Soc 2011;49:131-3. http://dx.doi.org/10.3340/jkns.2011.49.2.131.
- Hard JM, Gonda RL, Kadakia SR. A novel approach to treatment of unexpected vertebroplasty complication. Cardiovasc Interv Radiol 2008;31:1249-51. http://dx.doi.org/10.1007/s00270-008-9353-1.
- Ozturk C, Tas I, Hepguler S, Dusunceli Y. Paraplegia as a complication of vertebroplasty: a case report. Osteoporos Int 2007;18.
- Lee BJ, Lee SR, Yoo TY. Paraplegia as a complication of percutaneous vertebroplasty with polymethylmethacrylate: a case report. Spine 2002;27:E419-22. http://dx.doi.org/10.1097/00007632-200210010-00022.
- Birkenmaier C, Seitz S, Wegener B, Glaser C, Ruge MI, von LA, et al. Acute paraplegia after vertebroplasty caused by epidural hemorrhage. A case report. J Bone Joint Surg Am 2007;89:1827-31. http://dx.doi.org/10.2106/JBJS.F.01612.
- Lopes NM, Lopes VK. Paraplegia complicating percutaneous vertebroplasty for osteoporotic vertebral fracture: case report. Arq Neuro Psiquiatr 2004;62:879-81. http://dx.doi.org/10.1590/S0004-282X2004000500027.
- Lim JB, Park JS, Kim E. Nonaneurysmal subarachnoid hemorrhage: rare complication of vertebroplasty. J Korean Neurosurg Soc 2009;45:386-9. http://dx.doi.org/10.3340/jkns.2009.45.6.386.
- Heo DH, Cho SM, Cho YJ, Cho JH, Sheen SH. Heterotopic ossifications after vertebroplasty using calcium phosphate in osteoporotic vertebral compression fractures: report of 2 cases. World Neurosurg 1000;73:207-9. http://dx.doi.org/10.1016/j.surneu.2009.07.038.
- Nemeth AJ, Lie-Nemeth TJ, Marota JJ, Pryor JC, Rabinov JD, Hirsch JA. Vertebral augmentation complicated by perioperative addisonian crisis. Pain Physician 2006;9:257-60.
- Sonmez E, Yilmaz C, Caner H. Development of lumbar disc herniation following percutaneous vertebroplasty. Spine 2010;35:E93-5. http://dx.doi.org/10.1097/BRS.0b013e3181b52e8e.
- Soyuncu Y, Ozdemir H, Soyuncu S, Bigat Z, Gur S. Posterior spinal epidural abscess: an unusual complication of vertebroplasty. Joint Bone Spine 2006;73:753-5.
- Syed MI, Jan S, Patel NA, Shaikh A, Marsh RA, Stewart RV. Fatal fat embolism after vertebroplasty: identification of the high-risk patient. AJNR Am J Neuroradiol 2006;27:343-5.
- Yang CT, Hou SM, Hou CH, Lin FL, Lin CC, Yang RS. Balloon kyphoplasty and vertebroplasty in the management of vertebral compression fracture: Does complication rate differ in countries or specialties of operators?. Osteoporos Int 2010;21:S767-8.
- Perisinakis K, Damilakis J, Theocharopoulos N, Papadokostakis G, Hadjipavlou A, Gourtsoyiannis N. Patient exposure and associated radiation risks from fluoroscopically guided vertebroplasty or kyphoplasty. Radiology 2004;232:701-7. http://dx.doi.org/10.1148/radiol.2323031412.
- Fitousi NT, Efstathopoulos EP, Delis HB, Kottou S, Kelekis AD, Panayiotakis GS. Patient and staff dosimetry in vertebroplasty. Spine 2006;31:E884-9. http://dx.doi.org/10.1097/01.brs.0000244586.02151.18.
- Slipman CW, Lipetz JS, Jackson HB, Vresilovic EJ. Deep venous thrombosis and pulmonary embolism as a complication of bed rest for low back pain. Arch Phys Med Rehabil 2000;81:127-9. http://dx.doi.org/10.1053/apmr.2000.0810127.
- Convertino VA, Bloomfield SA, Greenleaf JE. An overview of the issues: physiological effects of bed rest and restricted physical activity. Med Sci Sports Exerc 1997;29:187-90. http://dx.doi.org/10.1097/00005768-199702000-00004.
- Munk PL, Liu DM, Murphy KP, Baerlocher MO. Effectiveness of vertebroplasty: a recent controversy. Can Assoc Radiolog J 2009;60:170-1. http://dx.doi.org/10.1016/j.carj.2009.08.001.
- Kaufmann TJ, Trout AT, Kallmes DF. The effects of cement volume on clinical outcomes of percutaneous vertebroplasty. AJNR Am J Neuroradiol 2006;27:1933-7.
- Al-Ali F, Barrow T, Luke K. Vertebroplasty: what is important and what is not. AJNR Am J Neuroradiol 2009;30:1835-9. http://dx.doi.org/10.3174/ajnr.A1732.
- Brinjikji W, Comstock BA, Gray L, Kallmes DF. Local Anesthesia with Bupivacaine and Lidocaine for vertebral fracture trial (LABEL): a report of outcomes and comparison with the Investigational Vertebroplasty Efficacy and Safety Trial (INVEST). AJNR Am J Neuroradiol 2010;31:1631-4. http://dx.doi.org/10.3174/ajnr.A2145.
- Miller FG, Kallmes DF, Buchbinder R. Vertebroplasty and the placebo response. Radiology 2011;259:621-5. http://dx.doi.org/10.1148/radiol.11102412.
- Lotz JC. Trials of veretebroplasty for vertebral fractures. N Engl J Med 2009;361.
- Brinjikji W, Comstock BA, Heagerty PJ, Jarvik JG, Kallmes DF. Investigational Vertebroplasty Efficacy and Safety Trial: detailed analysis of blinding efficacy. Radiology 2010;257:219-25. http://dx.doi.org/10.1148/radiol.10100094.
- Kallmes DF, Heagerty PJ, Jarvik JG. Trials of vertebroplasty for vertebral fractures. N Engl J Med 2009;361:2099-100.
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta-epidemiological study. BMJ 2008;336:601-5. http://dx.doi.org/10.1136/bmj.39465.451748.AD.
- Psaty BM, Prentice RL. Minimizing bias in randomized trials: the importance of blinding. JAMA 2010;304:793-4. http://dx.doi.org/10.1001/jama.2010.1161.
- Brody HB, Brody D. Placebo and health–II. Three perspectives on the placebo response: expectancy, conditioning, and meaning. Adv Mind Body Med 2000;16:216-32.
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron 2007;55:325-36. http://dx.doi.org/10.1016/j.neuron.2007.06.028.
- McDonald RJ, Gray LA, Cloft HJ, Thielen KR, Kallmes DF. The effect of operator variability and experience in vertebroplasty outcomes. Radiology 2009;253:478-85. http://dx.doi.org/10.1148/radiol.2532081370.
- Syed M, Shaikh A, Akhter T, Morar K, Cortoss S. Does age of fracture affect the outcome of vertebroplasty? Results from a prospective multicenter FDA-IDE study. J Vasc Interv Radiol 2011;22.
- Blattert TR, Jestaedt L, Weckbach A. Suitability of a calcium phosphate cement in osteoporotic vertebral body fracture augmentation: a controlled, randomized, clinical trial of balloon kyphoplasty comparing calcium phosphate versus polymethylmethacrylate. Spine 2009;34:108-14. http://dx.doi.org/10.1097/BRS.0b013e31818f8bc1.
- Anselmetti GC, Zoarski G, Manca A, Masala S, Eminefendic H, Russo F, et al. Percutaneous vertebroplasty and bone cement leakage: clinical experience with a new high-viscosity bone cement and delivery system for vertebral augmentation in benign and malignant compression fractures. Cardiovasc Interv Radiol 2008;31:937-47. http://dx.doi.org/10.1007/s00270-008-9324-6.
- Yang HL, Zhao L, Liu J, Sanford CG, Chen L, Tang T, et al. Changes of pulmonary function for patients with osteoporotic vertebral compression fractures after kyphoplasty. J Spinal Disord Tech 2007;20:221-5. http://dx.doi.org/10.1097/01.bsd.0000211273.74238.0e.
- Alvarez L, Alcaraz M, Perez-Higueras A, Granizo JJ, de M I, Rossi RE, et al. Percutaneous vertebroplasty: functional improvement in patients with osteoporotic compression fractures. Spine 2006;31:1113-8.
- Clark W, Lyon S, Burnes J. Trials of vertebroplasty for vertebral fractures. N Engl J Med 2009;361:2097-8.
- Buchbinder R, Osborne R, Staples M. Trials of vertebroplasty for vertical fractures. N Engl J Med 2009;361.
- Chen C, Chen L, Gu Y, Xu Y, Liu Y, Bai X, et al. Kyphoplasty for chronic painful osteoporotic vertebral compression fractures via unipedicular versus bipedicular approachment: a comparative study in early stage. Injury 2010;41:356-9. http://dx.doi.org/10.1016/j.injury.2009.09.021.
- Chen L, Yang H, Tang T. Unilateral versus bilateral balloon kyphoplasty for multilevel osteoporotic vertebral compression fractures: a prospective study. Spine 2011;36:534-40. http://dx.doi.org/10.1097/BRS.0b013e3181f99d70.
- Department of Health . Confirmation of Payment by Results (PbR) Arrangements for 2011–12 2012. www.dh.gov.uk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_124356 (accessed 12 May 2012).
- Max Planck Institute for Demographic Research (Germany) . Human Mortality Database 2012. www.mortality.org (accessed 12 May 2012).
- Ara R, Brazier JE. Populating an economic model with health state utility values: moving toward better practice. Value Health 2010;135:509-18. http://dx.doi.org/10.1111/j.1524-4733.2010.00700.x.
- Percutaneous Vertebroplasty and Percutaneous Balloon Kyphoplasty for the Treatment of Osteoporotic Vertebral Fracture. London: NICE; 2011.
- Barendregt JJ. The half-cycle correction: banish rather than explain it. Med Decis Making 2009;29:500-2. http://dx.doi.org/10.1177/0272989X09340585.
- Guide to the Methods of Technology Appraisal. London: NICE; 2008.
- Stevenson M, Lloyd-Jones M, Papaioannou D, Stevenson M, Lloyd-Jones M, Papaioannou D. Vitamin K to prevent fractures in older women: systematic review and economic evaluation. Health Technol Assess 2009;13.
- Holt G, Khaw KT, Reid DM, Compston JE, Bhalla A, Woolf AD, et al. Prevalence of osteoporotic bone mineral density at the hip in Britain differs substantially from the US over 50 years of age: implications for clinical densitometry. Br J Radiol 2002;75:736-42.
- Stevenson M, Jones ML, De NE, Brewer N, Davis S, Oakley J, et al. A systematic review and economic evaluation of alendronate, etidronate, risedronate, raloxifene and teriparatide for the prevention and treatment of postmenopausal osteoporosis. Health Technol Assess 2005;9.
- Jalava T, Sarna S, Pylkkänen L, Mawer B, Kanis JA, Selby P, et al. Association between vertebral fracture and increased mortality in osteoporotic patients. J Bone Miner Res 2003;18:1254-60. http://dx.doi.org/10.1359/jbmr.2003.18.7.1254.
- Interim Life Tables, 1980–82 to 2008–10. London: Office of National Statistics; n.d.
- Curtis L. Unit Costs of Health and Social Care 2011. Canterbury: PSSRU: University of Kent; 2011.
- Percutaneous Vertebroplasty and Percutaneous Balloon Kyphoplasty for the Treatment of Osteoporotic Vertebral Fractures. London: NICE; 2011.
- Torgerson DJ, Dolan P. Prescribing by general practitioners after an osteoporotic fracture. Ann Rheum Dis 1998;57:378-9. http://dx.doi.org/10.1136/ard.57.6.378.
- Morrison LS, Tobias JH. Effect of a case-finding strategy for osteoporosis on bisphosphonate prescribing in primary care. Osteoporos Int 2005;16:71-7. http://dx.doi.org/10.1007/s00198-004-1644-z.
- Wilson DJ. Vertebroplasty for vertebral fracture. On the basis of current evidence, cannot be recommended as first line treatment. BMJ 2011;343:104-5.
- Percutaneous Vertebroplasty. London: NICE; 2003.
- NHS Oxfordshire n.d. www.oxfordshirepct.nhs.uk/professional-resources/priority-setting/lavender-statements/documents/LS154_Vertebroplasty.pdf (accessed 3 April 2012).
- Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. Lancet 2010;375:686-95. http://dx.doi.org/10.1016/S0140-6736(09)61706-2.
- Benedetti F, Amanzio M. The placebo response: how words and rituals change the patient’s brain. Patient Educ Couns 2011;84:413-19. http://dx.doi.org/10.1016/j.pec.2011.04.034.
- Benedetti F, Mayberg HS, Wager TD, Stohler CS, Zubieta JK. Neurobiological mechanisms of the placebo effect. J Neurosci 2005;25:10390-402. http://dx.doi.org/10.1523/JNEUROSCI.3458-05.2005.
- Amanzio M, Benedetti F. Neuropharmacological dissection of placebo analgesia: expectation-activated opioid systems versus conditioning-activated specific subsystems. J Neurosci 1999;19:484-94.
- Levine JD, Gordon NC, Fields HL. The mechanism of placebo analgesia. Lancet 1978;2:654-7.
- Firanescu C, Lohle PNM, de Vries J, Klazen CA, Juttmann JR, Clark W, et al. A randomised sham controlled trial of vertebroplasty for painful acute osteoporotic vertebral fractures (VERTOS IV). Trials 2011;12. http://dx.doi.org/10.1186/1745-6215-12-93.
- Buchbinder R, Kallmes DF. Vertebroplasty: when randomized placebo-controlled trial results clash with common belief. Spine J 2010;10:241-3. http://dx.doi.org/10.1016/j.spinee.2010.01.001.
- Harstall R, Heini PF, Mini RL, Orler R. Radiation exposure to the surgeon during fluoroscopically assisted percutaneous vertebroplasty: a prospective study. Spine 2005;30:1893-8. http://dx.doi.org/10.1097/01.brs.0000174121.48306.16.
- Seibert JA. Vertebroplasty and kyphoplasty: Do fluoroscopy operators know about radiation dose, and should they want to know?. Radiology 2004;232:633-4. http://dx.doi.org/10.1148/radiol.2323040968.
- Synowitz M, Kiwit J. Surgeon’s radiation exposure during percutaneous vertebroplasty. J Neurosurg Spine 2006;4:106-9. http://dx.doi.org/10.3171/spi.2006.4.2.106.
- Kruger R, Faciszewski T. Radiation dose reduction to medical staff during vertebroplasty: a review of techniques and methods to mitigate occupational dose. Spine 2003;28:1608-13. http://dx.doi.org/10.1097/01.BRS.0000076832.18944.00.
- Tappero C, Barbero S, Costantino S, Bergui M, Ropolo R, Bradac G, et al. Patient and operator exposure during percutaneous vertebroplasty. Radiologia Medica 2009;114:595-607. http://dx.doi.org/10.1007/s11547-009-0385-7.
- Cloft HJ, Easton DN, Jensen ME, Kallmes DF, Dion JE. Exposure of medical personnel to methylmethacrylate vapor during percutaneous vertebroplasty. AJNR Am J Neuroradiol 1999;20:352-3.
- Edidin A, Ong KL, Lau E, Kurtz S. Mortality risk for operated and non-operated vertebral fracture patients in the U.S. medicare population. J Vasc Interv Radiol 2011;22. http://dx.doi.org/10.1016/j.jvir.2011.01.010.
- Lilford R, Braunholtz D, Harris J, Gill T. Trials in surgery. Br J Surg 2004;91:6-16. http://dx.doi.org/10.1002/bjs.4418.
- Appel NB, Gilula LA. Percutaneous vertebroplasty in patients with spinal canal compromise. AJR Am J Roentgenol 2004;182:947-51. http://dx.doi.org/10.2214/ajr.182.4.1820947.
- Baerlocher MO, Munk PL, Liu DM, Tomlinson G, Badii M, Kee ST, et al. Clinical utility of vertebroplasty: need for better evidence. Radiology 2010;255:669-74. http://dx.doi.org/10.1148/radiol.10092107.
- Becker S, Garoscio M, Meissner J, Tuschel A, Ogon M. Is there an indication for prophylactic balloon kyphoplasty? A pilot study. Clin Orthopaed Relat Res 2007;458:83-9. http://dx.doi.org/10.1097/BLO.0b013e318034032c.
- Bian J, Mudano A, Allison J, Briggs D, Cope J, Curtis J, et al. Vertebroplasty/kyphoplasty increases the risk of secondary vertebral compression fractures. J Bone Miner Res 2006;21.
- Boonen S, Cummings S, Van Meirhaeghe JK, Bastian L, Tillman J, Ranstam J, et al. A randomized trial of balloon kyphoplasty and non surgical care for patients with acute vertebral compression fractures: two year results. Osteoporos Int 2010;21:S21-2.
- Buchbinder R, Osborne RH, Wark JD, Mitchell P, Wreidt C, Graves S, et al. Efficacy and safety of vertebroplasty for treatment of painful osteoporotic vertebral fractures: A randomised double-blind placebo-controlled trial. Intern Med J 2009;39.
- Buchbinder R, Osborne RH, Kallmes D. Vertebroplasty appears no better than placebo for painful osteoporotic spinal fractures, and has potential to cause harm. Med J Aus 2009;191:476-7.
- Cummings SR, Wardlaw D, Van MJ, Bastian L, Tillman JB, Ranstam J, et al. A randomized trial of balloon kyphoplasty and nonsurgical care for acute vertebral compression fracture. Bone 2009;44:S51-2. http://dx.doi.org/10.1016/j.bone.2009.01.136.
- Figueiredo N, Barra F, Moraes L, Rotta R, Casulari LA. Percutaneous vertebroplasty: a comparison between the procedure using the traditional and the new side-opening cannula for osteoporotic vertebral fracture. Arq Neuro Psiquiatr 2009;67:377-81. http://dx.doi.org/10.1590/S0004-282X2009000300001.
- Gray LA, Kallmes DF. A pilot study of the use of pain questionnaires in vertebroplasty research. AJNR Am J Neuroradiol 2009;30:1364-5. http://dx.doi.org/10.3174/ajnr.A1510.
- Holden L, Cheung G, Chow E, Finkelstein J, Danjoux C. Prospective evaluation of functional status and quality of life in patients undergoing percutaneous vertebroplasty. Int J Cancer 2002.
- Mao KY, Liu BW, Wang Y, Tao S, Wang JF, Liu ZS, et al. Effect of carbonated hydroxyapatite cement for filing vertebral body on the vertebral heights and pain in patients with osteoporotic vertebral compression fractures. J Clin Rehabil Tissue Eng Res 2007;11:188-90.
- Ramaswamy D, Teitelbaum GP, Horwitz DA, Ehresmann GR. Comparison of efficacy of percutaneous vertebroplasty with polymethylmethacrylate in providing pain relief in patients with acute vs chronic osteoporotic vertebral compression fractures. Arthrit Rheum 2000;43.
- Smith FW, Boonen S, Van MJ, Bastian L, Wardlaw D. A randomized trial of balloon kyphoplasty and nonsurgical care for acute vertebral compression fracture: 2-year results. Cardiovasc Interv Radiol 2009;32:313-14.
- ClinicalTrials.gov . Quality of Life After Vertebroplasty Versus Conservative Treatment in Patients With Painful Osteoporotic Fracture 2012. http://clinicaltrials.gov/ct2/show/NCT00994032 (accessed Jan. 2012).
- ClinicalTrials.gov . FREE Study – Fracture Reduction Evaluation 2012. http://clinicaltrials.gov/ct2/show/study/NCT00211211?term=00211211&rank=1 (accessed February 2012).
- Wardlaw D, Boonen S, Bastian L, Van Meirhaeghe J, St Jan AZ. An international multicenter randomized comparison of balloon kyphoplasty and nonsurgical care in patients with acute vertebral body compression fractures. J Bone Miner Res 2007;22.
- Cameron AC, Trivedi PK. Microeconometrics: Methods and Applications. New York, NY: Cambridge University Press; 2005.
- Rosebaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika 1983;70:41-50.
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 2: A Generalised Linear Modelling Framework for Pair-Wise and Network Meta-Analysis 2011. www.nicedsu.org.uk (accessed 9 May 2012).
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 4: Inconsistency in Networks of Evidence Based on Randomised Controlled Trials 2011. www.nicedsu.org.uk (accessed 9 May 2012).
Appendix 1 Percutaneous vertebroplasty and percutaneous balloon kyphoplasty for the treatment of osteoporotic vertebral fractures: a systematic review and cost-effectiveness analysis
FINAL PROTOCOL
21st October 2011
1. Title of the project:
Percutaneous vertebroplasty and percutaneous balloon kyphoplasty for the treatment of osteoporotic vertebral fractures: a systematic review and cost-effectiveness analysis
2. Name of TAR team and project ‘lead’
School of Health and Related Research (ScHARR) Technology Assessment Group, The University of Sheffield.
Lead: Matt Stevenson, Reader in Health Technology Assessment,
ScHARR, University of Sheffield, Regent Court, 30 Regent Street,
Sheffield S1 4DA
Tel: 0114 2220691
Fax: 0114 272 4095
Email: m.d.stevenson@sheffield.ac.uk
Address for correspondence
All correspondence should be sent to the Project Lead (Matt Stevenson, M.D.Stevenson@sheffield.ac.uk), the Systematic Reviewer (Myfanwy Lloyd Jones, m.lloydjones@sheffield.ac.uk), the Managing Director of ScHARR-TAG (Eva Kaltenthaler, e.kaltenthaler@sheffield.ac.uk) and the Project Administrator (Gill Rooney, g.rooney@sheffield.ac.uk).
3. Plain English Summary
Many people suffer from osteoporosis, a condition in which the mineral content of their bones decreases, making the bones weaker and more brittle. Primary osteoporosis is generally associated with aging, and is particularly common in postmenopausal women. Other people may develop osteoporosis secondary to certain medical conditions (e.g. hyperthyroidism, and malignant disease) or prolonged steroid therapy. 1
Osteoporosis itself has no symptoms. However, bones weakened by osteoporosis can break easily, with little or no identifiable trauma. The most common osteoporosis-related fractures are vertebral compression fractures. These develop as the weakened vertebrae are compressed and distorted. Most vertebral compression fractures do not come to clinical attention, and do not appear to be associated with a significant increase in back pain. 2 However, some cause substantial pain and functional impairment; these are often termed “symptomatic” fractures. Most people who suffer symptomatic fractures can be treated successfully with conservative therapy,3 but others have persistent pain and limited mobility,4 and some may require hospitalisation, long-term care, or both. 5
Percutaneous vertebroplasty is a procedure in which acrylic bone cement is injected into a fractured vertebra under radiological guidance with the aim of relieving pain and/or stabilising the fracture. 3,6 The procedure may be done under general anaesthetic, but is more often performed using conscious sedation and local anaesthesia. 6 Clinical complications following percutaneous vertebroplasty appear to be rare,3 but can be serious, potentially including compression of the spinal cord. 6
Percutaneous balloon kyphoplasty is a variant of vertebroplasty in which a balloon-like device is inserted into the fractured vertebra and then slowly inflated until the vertebral body is restored to its normal height or the balloon reaches its highest achievable volume. The balloon is then deflated and removed, and the ensuing cavity is filled with bone cement. Like percutaneous vertebroplasty, balloon kyphoplasty is performed under local or general anaesthesia. 7
In 2003, NICE issued Interventional Procedure Guidance 12, which stated that percutaneous vertebroplasty may be considered for the provision of pain relief in patients with severe painful osteoporosis with loss of height and/or compression fractures of the vertebral body only if their pain is refractory to more conservative treatment. 8 Interventional Procedure Guidance 166, issued in 2006, stated that balloon kyphoplasty may be considered in patients with vertebral compression fractures whose condition is refractory to medical therapy and in whom there is continued vertebral collapse and severe pain. 9
The aim of this review is to systematically evaluate and appraise the long-term efficacy, safety, and cost-effectiveness of percutaneous vertebroplasty and percutaneous balloon kyphoplasty in people with symptomatic osteoporotic vertebral compression fractures.
4. Decision problem
4.1 Purpose of the decision to be made
The assessment will address the question “What is the long-term efficacy, safety, and cost-effectiveness of percutaneous vertebroplasty and percutaneous balloon kyphoplasty (with or without vertebral body stenting) as a treatment for osteoporotic vertebral compression fractures?”
4.2 Clear definition of the intervention
Percutaneous vertebroplasty is a procedure in which acrylic bone cement is injected into a fractured vertebra under radiological guidance in order to relieve pain and/or stabilise the fracture. 3,6 The procedure may be done under general anaesthetic, but is more commonly performed using conscious sedation and local anaesthesia. 6
Percutaneous balloon kyphoplasty is a variant of vertebroplasty in which a balloon-like device is inserted into the vertebral body and then slowly inflated until the vertebral body is restored to its normal height or the balloon reaches its highest achievable volume. The balloon is then deflated and removed, and the ensuing cavity is filled with bone cement. The procedure may be performed under either local or general anaesthetic. 7 Stents may be used to prevent the vertebral body from collapsing after the balloon is deflated, ensuring that the maximum vertebral height is retained. 10
4.3 Place of interventions in the treatment pathway
Percutaneous vertebroplasty and percutaneous balloon kyphoplasty are usually offered as a last resort to people with symptomatic vertebral compression fractures in whom alternative treatments have not been successful. 6,7
4.4 Relevant comparators
The relevant comparators are the interventions themselves, and non-invasive management. There is no gold standard for non-invasive management: the American Academy of Orthopaedic Surgeons considers the strength of the evidence for the various non-invasive treatment options (such as physiotherapy, analgesia, and the use of anti-osteoporotic agents such as a bisphosphonate or strontium ranelate) to be generally weak to inconclusive, although they provide a recommendation of moderate strength for the short-term use of calcitonin. 11
In addition to the comparators specified above, comparison with a sham procedure or no treatment is relevant in terms of safety outcomes, and also because percutaneous vertebroplasty or percutaneous balloon kyphoplasty have a potential role in people who cannot tolerate the relevant active comparator interventions, and for whom the only relevant alternative is therefore no treatment.
4.5 Population and relevant subgroups
The relevant population is people of any age and either gender with painful osteoporotic vertebral compression fractures. If the evidence permits, consideration will be given to subgroups defined by:
-
time from fracture to the intervention
-
presence of fracture-related deformity before treatment
-
receipt of inpatient care before treatment.
4.6 Key factors to be addressed
The review aims to:
-
evaluate the clinical effectiveness of percutaneous vertebroplasty and percutaneous balloon kyphoplasty in reducing pain and disability in people with osteoporotic vertebral compression fractures
-
evaluate the adverse effect profile of percutaneous vertebroplasty and percutaneous balloon kyphoplasty
-
estimate the cost effectiveness of percutaneous vertebroplasty and percutaneous balloon kyphoplasty in reducing pain and disability associated with osteoporotic vertebral compression fractures
-
identify key areas for primary research
-
estimate the possible overall cost of introducing percutaneous vertebroplasty and percutaneous balloon kyphoplasty for people with osteoporotic vertebral compression fractures in England and Wales.
4.7 Areas of agreement at the scoping workshop that are outside the scope of the appraisal and therefore do not require any detailed assessment
It was agreed at the scoping workshop that people with malignancy-related vertebral fractures, and those with neuropathy in the absence of osteoporotic compression fractures, should not be included the scope of this appraisal.
5. Report methods for synthesis of evidence of clinical effectiveness
A systematic review of the evidence for clinical effectiveness will be undertaken following the general principles outlined in ‘Systematic Reviews: CRD’s guidance for undertaking reviews in health care’12 and the principles recommended in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (http://www.prisma-statement.org/). 13
5.1 Search strategy
The search strategy will comprise the following main elements:
-
Searching of electronic databases
-
Contact with experts in the field
-
Scrutiny of bibliographies of retrieved papers.
5.1.1 Electronic searches
A comprehensive search will be undertaken to systematically identify clinical and cost-effectiveness literature pertaining to percutaneous vertebroplasty and percutaneous balloon kyphoplasty as treatments for osteoporotic compression fractures in men and women of all ages. Search strategies will be used to identify relevant studies (as specified in the inclusion criteria) and systematic reviews/meta-analyses (for the identification of additional trials). Searches will not be restricted by language or publication date, nor will they be restricted by publication type or study design, as studies which do not meet the review inclusion criteria may be important in identifying further relevant papers and current research. The proposed MEDLINE search strategy is provided in Appendix A . A comprehensive database of relevant published and unpublished articles will be constructed using Reference Manager © software.
5.1.2 Databases
The following electronic databases will be searched from inception: MEDLINE (Ovid); MEDLINE in Process; CINAHL; EMBASE; EconLit; the Cochrane Library including the Cochrane Database of Systematic Reviews, Cochrane Controlled Trials Register (CENTRAL), DARE, NHS EED and HTA databases; Science Citation Index (SCI).
Current research registers (e.g. the NIHR CRN Portfolio, Current Controlled Trials, Clinical Trials.gov) will also be searched and relevant professional and research organisations contacted. Citation searches of key included studies will be undertaken using the SCI citation search facility.
5.2 Inclusion/exclusion criteria
5.2.1 Inclusion criteria
The inclusion criteria are as reported in sections 5.2.1.1–5.2.1.5 below. The review of clinical effectiveness will include any intervention studies which report at least one of the primary outcomes. This criterion will be relaxed for consideration of adverse events, when studies which do not report any of the primary outcomes may be included.
5.2.1.1 Population
The population will comprise people of any age and either gender with osteoporotic vertebral compression fractures. Studies which also include participants with non-osteoporotic vertebral fractures of other aetiologies (e.g. fractures associated with trauma, myeloma, or metastatic cancer) will be included if data relating to participants with osteoporotic fractures can be extracted separately.
5.2.1.2 Interventions
Percutaneous vertebroplasty or percutaneous balloon kyphoplasty.
5.2.1.3 Comparators
The comparators will be the interventions themselves, non-invasive management, a sham procedure, or no treatment.
5.2.1.4 Outcomes
5.2.1.5.1 Primary outcomes
-
Pain/analgesic use
-
Back-specific functional status/mobility
-
Vertebral body height and angular deformity
-
Progression of treated fracture
-
Incidence of new vertebral fractures
-
Health-related quality of life
5.2.1.4.2 Secondary outcomes
-
All-cause mortality
-
Symptomatic and asymptomatic leakage of cement (egg into adjacent intervertebral discs)
-
Periprocedural balloon rupture
-
Post-operative complications (including infection)
-
Other adverse events
-
Resource utilisation
-
Cost utility.
5.2.1.5 Study design
For the review of clinical effectiveness, the best available level of evidence will be utilised, with priority given to randomised controlled studies, if available. However, this criterion will be relaxed for the consideration of adverse events, for which observational studies may be included.
5.2.2 Exclusion criteria
Reviews of primary studies will not be included in the analysis, but will be retained for discussion and identification of additional trials. Studies which are considered methodologically unsound in terms of either study design or the method used to assess outcomes will be excluded from the results. The following publication types will also be excluded from the analysis:
-
Animal models
-
Preclinical and biological studies
-
Narrative reviews, editorials, opinions
-
Reports published as meeting abstracts only, where insufficient methodological details are reported to allow critical appraisal of study quality.
5.3 Data extraction strategy
Retrieved studies will be selected for inclusion through a two-stage process according to the inclusion/exclusion criteria specified in section 5.2. Studies will be assessed for relevance first by title/abstract, and then finally by full text, excluding at each step studies which do not satisfy those criteria; abstract-only studies will be included. One reviewer will examine titles and abstracts for inclusion, and a second reviewer will check ten per cent of citations, with a kappa coefficient calculated to measure inter-rater reliability. Discrepancies will be resolved by discussion, with involvement of a third reviewer when necessary.
Full manuscripts of selected citations will be retrieved and assessed by one reviewer against the inclusion/exclusion criteria. Data will be extracted by one reviewer using a standardised data extraction form (see Appendix B ) and a second reviewer will check ten per cent of data extraction forms. Discrepancies will be resolved by discussion, with involvement of a third reviewer when necessary. Where multiple publications of the same study are identified, data will be extracted and reported as a single study. Handling data obtained from the manufacturer’s submission is detailed in Section 7.
5.4 Quality assessment strategy
The methodological quality of all studies which meet the inclusion criteria will be assessed according to criteria based on those proposed by Ploeg et al. for the assessment of studies of percutaneous vertebroplasty3 (see Appendix C ).
5.5 Methods of analysis/synthesis
Data will be tabulated and discussed in a narrative review. If appropriate (i.e. if a number of studies which report data relating to a given outcome are comparable in terms of key features such as their design, populations, and interventions), meta-analysis will be employed to estimate a summary measure of effect on relevant outcomes based on intention to treat analyses.
Meta-analysis will be carried out using fixed and random effects models, using the Cochrane Collaboration ReviewManager© software (version 5.1). 14 Heterogeneity will be explored through consideration of the study populations, methods, and interventions, by visualisation of the results, and, in statistical terms, by the χ2 test for homogeneity and the I2 statistic.
If the evidence permits, a network meta-analysis will be undertaken to determine efficacy. This will be populated with all identified trials involving an intervention or a comparator deemed relevant to the decision problem. Where a full network incorporating all interventions and comparators of interest cannot be constructed, indirect comparisons will be undertaken where applicable.
6. Report methods for synthesising evidence of cost-effectiveness
6.1 Identifying and systematically reviewing published cost-effectiveness studies
The sources detailed in section 5 will be used to identify studies of the cost effectiveness of percutaneous vertebroplasty or percutaneous balloon kyphoplasty. Stand-alone cost analyses based in the UK NHS will also be sought. Relevant studies identified and included in the manufacturer’s submission will also be included. The quality of economic literature will be assessed using a combination of key components of the British Medical Journal checklist for economic evaluations15 together with the Eddy checklist on mathematical modelling. 16
6.2 Systematic literature search for other data related to cost-effectiveness
A search of the broader literature on outcomes following percutaneous vertebroplasty or percutaneous balloon kyphoplasty, or in patients eligible but where neither intervention was performed will be undertaken to identify the evidence base on HRQoL (i.e. health state values). The literature search will identify relevant values for appropriate health states. Primary data collection will not be undertaken.
6.3 Assessment group economic model
A new economic evaluation is likely to be carried out from the perspective of the UK NHS. The model structure will be determined in consultation with clinical experts. The TAR team has extensive experience and publication track-record using state transition modelling, discrete event simulation, individual patient modelling, meta-modelling, and the use of decision trees in economic evaluation and also of evaluating pharmaceuticals for the prevention of fractures.
The time horizon of our analysis will be a patient’s lifetime in order to reflect the chronic nature of the disease. The perspective will be that of the National Health Services and Personal Social Services. Both cost and benefits will be discounted at 3.5% per annum.
Cost and utility data from published sources associated with osteoporotic fracture will be incorporated into the above model in order to allow the economic, as well as clinical, implications of treatment to be assessed. Ideally, evidence on the impact of these therapies on HRQoL will be available directly from the trials included within the review. In the absence of such evidence, the mathematical model may use indirect evidence on quality of life from alternative sources. Quality of life data will be reviewed and used to generate the quality adjustment weights required for the model.
The key model outputs will be the discounted incremental costs and discounted incremental quality adjusted life years gained for percutaneous vertebroplasty and the comparators in a full incremental analysis. Univariate sensitivity analysis will be undertaken to identify the key parameters that determine the cost-effectiveness of the intervention with the objective of identifying how secure the results of the economic analyses are, given the available evidence. Probabilistic sensitivity analyses will be undertaken to determine how robust the results of the economic analysis are, given the current level of evidence, and to provide a more informative estimation of cost-effectiveness.
7. Handling the company submission(s)
All data submitted by the manufacturers/sponsors will be considered if received by the TAR team no later than 15th February 2012. Data arriving after this date may not be considered. If the data meet the inclusion criteria for the review, they will be extracted and quality assessed in accordance with the procedures outlined in this protocol. Any economic evaluations included in the company submission, provided it complies with NICE’s advice on presentation, will be assessed for clinical validity, reasonableness of assumptions, and appropriateness of the data used in the economic model. If the TAR team judge that the existing economic evidence is not robust, then further work will be undertaken, either by adapting what already exists or by developing de-novo modelling.
Any ‘commercial in confidence’ data taken from a company submission will be underlined and highlighted in turquoise in the assessment report (followed by an indication of the relevant company name, e.g. in brackets). Any academic in confidence data will be underlined and highlighted in yellow.
8. Competing interests of authors
None.
9. Appendices
Appendix A
Draft MEDLINE clinical effectiveness search strategy (Ovid)
-
Vertebroplasty/
-
Kyphoplasty/
-
vertebroplasty.ti,ab.
-
kyphoplasty.ti,ab.
-
bone void fill*.ti,ab.
-
injectable bone cement*.ti,ab.
-
osteoplastic procedure*.ti,ab.
-
vertebral* augmentation*.ti,ab.
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8
MEDLINE Economics Strategy (SIGN Strategy)
-
Economics/
-
"costs and cost analysis"/
-
Cost allocation/
-
Cost-benefit analysis/
-
Cost control/
-
Cost savings/
-
Cost of illness/
-
Cost sharing/
-
"deductibles and coinsurance"/
-
Medical savings accounts/
-
Health care costs/
-
Direct service costs/
-
Drug costs/
-
Employer health costs/
-
Hospital costs/
-
Health expenditures/
-
Capital expenditures/
-
Value of life/
-
Exp economics, hospital/
-
Exp economics, medical/
-
Economics, nursing/
-
Economics, pharmaceutical/
-
Exp "fees and charges"/
-
Exp budgets/
-
(low adj cost).mp.
-
(high adj cost).mp.
-
(health?care adj cost$).mp.
-
(fiscal or funding or financial or finance).tw.
-
(cost adj estimate$).mp.
-
(cost adj variable).mp.
-
(unit adj cost$).mp.
-
(economic$ or pharmacoeconomic$ or price$ or pricing).tw.
-
Or/1-32
Appendix B
Draft data extraction form
| Study & Design | Data Extraction | |
|---|---|---|
| Trial | Review Details | |
| Author, year | ||
| Study design | Objective | |
| Study design (egg RCT, before-and-after study) | ||
| Publication type (i.e. full report or abstract) | ||
| Country of corresponding author | ||
| Language of publication | ||
| Sources of funding | ||
| Interventions | ||
| Focus of interventions (comparisons) | ||
| Description | ||
| T1: Intervention group | ||
| T2: Control group | ||
| Intervention site (country) | ||
| Length of follow-up | ||
| Study Characteristics | ||
| Method of randomisation | ||
| Description | ||
| Generation of allocation sequences | ||
| Allocation concealment? | ||
| Blinding level | ||
| Numbers included in the study | ||
| Numbers randomised | T1: T2: |
|
| Population Characteristics | ||
| Target population (describe) | ||
| Inclusion/exclusion criteria (n) | ||
| Recruitment procedures used (participation rates if available) | ||
| Characteristics of participants at baseline | ||
| Age (mean yr.) | ||
| Gender | ||
| Ethnicity | ||
| Primary or secondary osteoporosis | ||
| Number of vertebral fractures (mean) | ||
| Other relevant information | ||
| Were intervention and control groups comparable? |
| Outcomes | |
| Definition of primary outcomes | |
| Definition of secondary outcomes | |
| Definition of tertiary outcomes | |
| Definition of other outcomes | |
| Analysis | |
| Statistical techniques used | |
| Intention to treat analysis | |
| Does technique adjust for confounding? | |
| Power calculation (priori sample calculation) | |
| Attrition rates (overall rates) i.e. Loss to follow-up | |
| Was attrition adequately dealt with? | |
| Number (%) followed-up from each condition | |
| Results | |
| Adverse events | |
| Other information | |
| Summary | |
| Authors’ overall conclusions | |
| Reviewers’ comments |
Appendix C
Draft quality assessment scale (adapted from Ploeg et al. 20063)
| Criterion | Yes | No | Unclear | Not applicable |
|---|---|---|---|---|
| Is a control group present? If yes: | ||||
| Was a method of randomisation performed? | ||||
| Was the treatment allocation concealed? | ||||
| Were co-interventions avoided or comparable? | ||||
| Was the outcome assessor blinded to the intervention? | ||||
| Were the outcome measures relevant? | ||||
| Was the withdrawal/drop-out rate described and acceptable? | ||||
| Was the timing of the outcome assessment comparable in both groups? | ||||
| Did the analysis include an intention-to-treat analysis? | ||||
| Were the eligibility criteria specified? | ||||
| Were the groups similar at baseline regarding the most important prognostic indicators? | ||||
| Were the index and control interventions explicitly described? | ||||
| Were adverse effects described? | ||||
| Was a short-term follow-up measurement performed? | ||||
| Was a long-term follow-up measurement performed? | ||||
| Was the sample size for each group described? | ||||
| Were point estimates presented for the primary outcome measures? | ||||
| Were measures of variability presented for the primary outcome measures? | ||||
| Was a valid questionnaire, e.g. concerning pain and quality of life, used? |
Appendix D
Critical appraisal checklist for economic evaluations using key components of the British Medical Journal checklist for economic evaluation15 together with the Eddy checklist on mathematical models employed in technology assessments.16
| Title | ||
| Authors | ||
| Year | ||
| Modelling assessments should include: | Yes/No | |
|---|---|---|
| 1 | A statement of the problem; | |
| 2 | A discussion of the need for modelling vs. alternative methodologies | |
| 3 | A description of the relevant factors and outcomes; | |
| 4 | A description of the model including reasons for this type of model and a specification of the scope including; time frame, perspective, comparators and setting. Note: n=number of health states within sub-model | |
| 5 | A description of data sources (including subjective estimates), with a description of the strengths and weaknesses of each source, with reference to a specific classification or hierarchy of evidence; | |
| 6 | A list of assumptions pertaining to: the structure of the model (e.g. factors included, relationships, and distributions) and the data; | |
| 7 | A list of parameter values that will be used for a base case analysis, and a list of the ranges in those values that represent appropriate confidence limits and that will be used in a sensitivity analysis; | |
| 8 | The results derived from applying the model for the base case; | |
| 9 | The results of the sensitivity analyses; unidimensional; best/worst case; multidimensional (Monte Carlo/parametric); threshold. | |
| 10 | A discussion of how the modelling assumptions might affect the results, indicating both the direction of the bias and the approximate magnitude of the effect; | |
| 11 | A description of the validation undertaken including; concurrence of experts; internal consistency; external consistency; predictive validity. | |
| 12 | A description of the settings to which the results of the analysis can be applied and a list of factors that could limit the applicability of the results; | |
| 13 | A description of research in progress that could yield new data that could alter the results of the analysis | |
Additional information that is needed by NCCHTA and NICE.
Please send this as a WORD document when you submit your protocol to Htatar@soton.ac.uk.
Details of TAR team
Matt Stevenson
Reader in Health Technology Assessment and Technical Director of the ScHARR Technology Assessment Group
ScHARR, University of Sheffield, Regent Court, 30 Regent Street, Sheffield S1 4DA
Tel: 0114 222 0691
Fax: 0114 272 4095
E-mail: M.D.Stevenson@sheffield.ac.uk
Myfanwy Lloyd Jones, Senior Research Fellow,
ScHARR, University of Sheffield, Regent Court, 30 Regent Street, Sheffield S1 4DA
Tel: 0114 222 0698
Fax: 0114 272 4095
Email: m.lloydjones@sheffield.ac.uk
Angie Rees, Information Specialist
ScHARR, University of Sheffield, Regent Court, 30 Regent Street, Sheffield S1 4DA
Tel: 0114 222 0725
Fax: 0114 272 4095
Email: a.rees@sheffield.ac.uk
Gill Rooney
Project Administrator
ScHARR, University of Sheffield, Regent Court, 30 Regent Street, Sheffield S1 4DA
Tel: 0114 222 0800
Fax: 0114 272 4095
E-mail: g.rooney@sheffield.ac.uk
Intended Clinical Advisors
Eugene McCloskey
Professor of Adult Bone Diseases
Academic Unit of Bone Metabolism
Metabolic Bone Centre
Northern General Hospital
Herries Road
Sheffield S5 7AU
Email: e.v.mccloskey@sheffield.ac.uk
Dr Adrian Highland
Consultant Radiologist
Northern General Hospital
Herries Road
Sheffield
S5 7AU
Email: Adrian.Highland@sth.nhs.uk
Address for correspondence
All correspondence should be sent to the project lead, Matt Stevenson (M.D.Stevenson@sheffield.ac.uk), the systematic reviewer Myfanwy Lloyd Jones (m.lloydjones@sheffield.ac.uk), the managing director of ScHARR-TAG (Eva Kaltenthaler, e.kaltenthaler@sheffield.ac.uk), and the project administrator (Gill Rooney, g.rooney@sheffield.ac.uk).
Timetable/milestones
| Milestone | |
|---|---|
| Draft protocol | 5th October 2011 |
| Final protocol | 26th October 2011 |
| Progress report | 24th February 2012 |
| Draft assessment report | 30th April 2012 |
| Assessment report | 25th May 2012 |
REFERENCES
- Melton LJ. Epidemiology of spinal osteoporosis. Spine 1997;22:2-11.
- O’Neill TW, Cockerill W, Matthis C, Raspe HH, Lunt M, Cooper C, et al. Back pain, disability, and radiographic vertebral fracture in European women: a prospective study. Osteoporos Int 2004;15:760-5.
- Ploeg WT, Veldhuizen AG, The B, Sietsma MS. Percutaneous vertebroplasty as a treatment for osteoporotic vertebral compression fractures: a systematic review. Eur Spine J 2006;15:1749-58.
- Hall SE, Criddle RA, Comito TL, Prince RL. A case–control study of quality of life and functional impairment in women with long-standing vertebral osteoporotic fracture. Osteoporos Int 1999;9:508-15.
- Buchbinder R, Osborne RH, Ebeling PR, Wark JD, Mitchell P, Wriedt C, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med 2009;361:557-68.
- National Institute for Clinical Excellence Interventional Procedures Programme . Interventional Procedure Overview of Percutaneous Vertebroplasty (methyl Methacrylate) 2003.
- National Institute for Health and Clinical Excellence . Interventional Procedures Overview of Balloon Kyphoplasty for Vertebral Compression Fractures. 2005.
- National Institute for Clinical Excellence . Interventional Procedure Guidance 12: Percutaneous Vertebroplasty 2003.
- National Institute for Health and Clinical Excellence . Interventional Procedure Guidance 166: Balloon Kyphoplasty for Vertebral Compression Fractures 2006.
- Rotter R, Martin H, Fuerderer S, Gabl M, Roeder C, Heini P, et al. Vertebral body stenting: a new method for vertebral augmentation versus kyphoplasty. Eur Spine J 2010;19:916-23.
- American Academy of Orthopaedic Surgeons . The Treatment of Symptomatic Osteoporotic Spinal Compression Fractures 2011.
- Centre for Reviews and Dissemination . systematic reviews. CRD’s Guidance for Undertaking Reviews in Health Care 2009. URL: http://www.york.ac.uk/inst/crd/SysRev/!SSL!/WebHelp/SysRev3.htm (accessed Oct. 2011).
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Ann Intern Med 2009;151.
- Copenhagen: The Nordic Cochrane Centre; 2011.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. BMJ 1996;313:275-83.
- Eddy DM. Assessing medical technologies. Washington, DC; 1985.
Appendix 2 Literature search strategies
MEDLINE clinical effectiveness search strategy (Ovid)
-
Vertebroplasty/
-
Kyphoplasty/
-
vertebroplasty.ti,ab.
-
kyphoplasty.ti,ab.
-
bone void fill*.ti,ab.
-
injectable bone cement*.ti,ab.
-
osteoplastic procedure*.ti,ab.
-
vertebral* augmentation*.ti,ab.
-
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8
MEDLINE economics strategy (SIGN Strategy)
| 1 | Economics/ |
| 2 | “costs and cost analysis”/ |
| 3 | Cost allocation/ |
| 4 | Cost-benefit analysis/ |
| 5 | Cost control/ |
| 6 | Cost savings/ |
| 7 | Cost of illness/ |
| 8 | Cost sharing/ |
| 9 | “deductibles and coinsurance”/ |
| 10 | Medical savings accounts/ |
| 11 | Health care costs/ |
| 12 | Direct service costs/ |
| 13 | Drug costs/ |
| 14 | Employer health costs/ |
| 15 | Hospital costs/ |
| 16 | Health expenditures/ |
| 17 | Capital expenditures/ |
| 18 | Value of life/ |
| 19 | Exp economics, hospital/ |
| 20 | Exp economics, medical/ |
| 21 | Economics, nursing/ |
| 22 | Economics, pharmaceutical/ |
| 23 | Exp “fees and charges”/ |
| 24 | Exp budgets/ |
| 25 | (low adj cost).mp. |
| 26 | (high adj cost).mp. |
| 27 | (health?care adj cost$).mp. |
| 28 | (fiscal or funding or financial or finance).tw. |
| 29 | (cost adj estimate$).mp. |
| 30 | (cost adj variable).mp. |
| 31 | (unit adj cost$).mp. |
| 32 | (economic$ or pharmacoeconomic$ or price$ or pricing).tw. |
| 33 | Or/1-32 |
Appendix 3 Data extraction form
Randomised controlled trials data extraction form
Based on NHS CRD Report No. 4. (NHS Centre for Reviews and Dissemination. Report 4: Undertaking systematic reviews of research on effectiveness; CRD’s guidance for those carrying out or commissioning reviews. York: University of York; 2001.)
| Study and design | Data extraction |
|---|---|
| Trial | Review details |
| Author, year | |
| Study design | Objective |
| Publication type (i.e. full report or abstract) | |
| Country of corresponding author | |
| Language of publication | |
| Sources of funding | |
| Interventions | |
| Focus of interventions (comparisons) | |
| Description | |
| T1: Intervention group | |
| T2: Control group | |
| Intervention site (health care setting, country) | |
| Procedure performed by | |
| Length of follow up | |
| Study characteristics | |
| Method of randomisation | |
| Description | |
| Generation of allocation sequences | |
| Allocation concealment? | |
| Blinding level | |
| Numbers included in the study | |
| Numbers randomised | |
| Population characteristics | |
| Target population (describe) | |
| Inclusion/exclusion criteria (n) | |
| Recruitment procedures used (participation rates if available) | |
| Characteristics of participants at baseline | |
| Age (mean years) | |
| Female | |
| Median duration of back pain in weeks (IQR) | |
| Duration of symptoms < 6 weeks | |
| BMI | |
| Median duration of corticosteroid use in years (IQR) | |
| Pain score (scale of 0–10) | |
| Overall | |
| At rest | |
| In bed at night | |
| QUALEFFO total score | |
| AQoL score | |
| RDQ score | |
| EQ-5D score | |
| Timed ‘up and go’ test (seconds) | |
| Medication for osteoporosis | |
| Any | |
| Calcium supplements | |
| Vitamin D | |
| Bisphosphonates | |
| One or more previous vertebral fractures | |
| Opioids for pain | |
| BMD T-score ≤ 2.5 | |
| Lumbar | |
| Femoral neck | |
| Severity of fracture (no./total no. of fractures) assessed by Genant’s semiquantitative system | |
| Mild | |
| Moderate | |
| Severe | |
| No of vertebral bodies treated | |
| 1 | |
| 2 | |
| Other information | |
| Were intervention and control groups comparable? | |
| Outcomes | |
| Definition of primary outcomes | |
| Definition of secondary outcomes | |
| Definition of tertiary outcomes | |
| Definition of other outcomes | |
| Analysis | |
| Statistical techniques used | |
| Intention to treat analysis | |
| Does technique adjust for confounding? | |
| Power calculation (priori sample calculation) | |
| Attrition rates (overall rates), i.e. loss to follow-up | |
| Was attrition adequately dealt with? | |
| Number (%) followed up from each condition | |
| Results | |
| Adverse events | |
| Other information | |
| Summary | |
| Authors’ overall conclusions | |
| Reviewers’ comments |
Appendix 4 Original quality assessment checklist (adapted from Ploeg et al. 200688)
| Criterion | Yes | No | Unclear | Not applicable |
|---|---|---|---|---|
| Is a control group present? If yes: | ||||
| Was a method of randomisation performed? | ||||
| Was the treatment allocation concealed? | ||||
| Were co-interventions avoided or comparable? | ||||
| Was the outcome assessor blinded to the intervention? | ||||
| Were the outcome measures relevant? | ||||
| Was the withdrawal/drop-out rate described and acceptable? | ||||
| Was the timing of the outcome assessment comparable in both groups? | ||||
| Did the analysis include an intention-to-treat analysis? | ||||
| Were the eligibility criteria specified? | ||||
| Were the groups similar at baseline regarding the most important prognostic indicators? | ||||
| Were the index and control interventions explicitly described? | ||||
| Were adverse effects described? | ||||
| Was a short-term follow-up measurement performed? | ||||
| Was a long-term follow-up measurement performed? | ||||
| Was the sample size for each group described? | ||||
| Were point estimates presented for the primary outcome measures? | ||||
| Were measures of variability presented for the primary outcome measures? | ||||
| Was a valid questionnaire, e.g. concerning pain and quality of life, used? |
Appendix 5 Revised quality assessment checklist
| Criterion | Yes | No | Unclear | Not applicable | |
|---|---|---|---|---|---|
| Internal validity | |||||
| Selection bias | Was the method used to assign participants to treatment groups really random? (see Note A) | ||||
| What method of assignment was used? | |||||
| Was the allocation of treatment concealed? (see Note B) | |||||
| What method was used to conceal treatment allocation? | |||||
| Performance bias | Were cointerventions avoided or comparable? | ||||
| Were the participants who received the intervention blinded to the treatment allocation? | |||||
| Was the success of the blinding procedure assessed? | |||||
| Detection bias | Were the outcome assessors blinded to the treatment allocations? | ||||
| Was the success of the blinding procedure assessed? | |||||
| Was the timing of the outcome assessment comparable in both groups? | |||||
| Attrition bias | Were at least 80% of the participants originally randomised to treatment followed up in the final analysis? | ||||
| Were the reasons for withdrawal stated? | |||||
| Was the number of participants randomised to each group stated? | |||||
| Did the report state the number of participants in each group who were included in the final analysis? | |||||
| Were there any unexpected imbalances in dropouts between groups? | |||||
| If there were unexpected imbalances, were they explained or adjusted for? | |||||
| Was an intention-to-treat analysis included? | |||||
| If an intention-to-treat analysis was included, was it appropriate, and were appropriate methods used to account for missing data? | |||||
| Reporting bias | Is there any evidence to suggest that the authors measured more outcomes than they reported? | ||||
| Other bias | Were the groups similar at baseline in terms of the most important prognostic indicators? | ||||
| External validity (generalisability) | |||||
| Were the eligibility criteria for study entry specified? | |||||
| Were the index and control interventions explicitly described? | |||||
| Were the skills, training, and experience of the operator described? | |||||
| Were the outcome measures relevant? | |||||
| Was a valid instrument used to measure each outcome? | |||||
| Was a short-term follow-up measurement performed? (< 3 months after randomisation) | |||||
| Was a long-term follow-up measurement performed? (≥ 12 months after randomisation) | |||||
| Were adverse effects adequately described? | |||||
| Precision | |||||
| Was the study powered to detect differences in outcome? | |||||
| Were point estimates presented for the primary outcome measures? | |||||
| Were measures of variability presented for the primary outcome measures? | |||||
Appendix 6 Details of studies which were potentially relevant to the review of clinical effectiveness, copies of which could not be obtained within the study timescale
Proceedings of the 39th Annual Meeting of the Spanish Society of Neuroradiology SENR – 6th Congress of the Portuguese Society of Neuroradiology, SPNR. Neuroradiology Conference.
Baier M, Meeder P, Grafe I, Nöldge G, Kasperk C. Pain reduction and verbal redressement by kyphoplasty. Osteologie 2007;16. 3:173–5.
Bobra S, Maus TP, Thielen KR, Wald JT, Everson S. Early outcomes in osteoporotic patients with painful vertebral body fractures treated with percutaneous vertebroplasty. Radiology 2001;221:136.
Carlier RY, Gordji H, Mompoint DM, Vernhet N, Feydy A, Vallée C. Percutaneous vertebroplasty and local kyphosis correction. Radiology 2002;225.2:514.
Hoffmeister E. Balloon kyphoplasty: continuing evidence of efficacy in treating vertebral collapse and fracture. Bone Joint 2007;13.6:61–5.
Kasperk C. Lkypho-vertebroplasty and non-pharamcologic treatment. Annal Rheum Dis 2006;65:14.
Kim AK, Jensen ME, Dion JE, Kallmes DF. Modified transpedicular approach for percutaneous vertebroplasty: Holo-vertebral body filling using a single injection. Radiology 2000;217:510.
Kobayashi T, Takanaka T, Matsui O. Percutaneous vertebroplasty-guided by CT fluoroscopy. Radiology 2000;217:527.
Kraus J, Achatz W, Gorzer HG. Pelvic and crural phlebothrombosis as complication of percutaneous vertebroplasty. Rofo-Fortschritte Auf dem Gebiet der Rontgenstrahlen und der Bildgebenden Verfahren 2003;175.4:565–6.
Mallampati GK, Kanamalla US, Gupta K, Jain N, Weinik MM, Kochan JP. Functional outcome and pain modification following vertebroplasty. Radiology 2002;225:614.
Oka M, Westesson PA. Vertebroplasty can improve pain and mobility. Radiology 2002;225:513.
Ruefenacht DA, Martin J, Jean B, Muster M, Murphy K, Piotin M. Vertebroplasty: clinical results and follow-up. Radiology 1999;213P:416.
Sehgal M, Gilula LA, Brown DB. Vertebroplasty in patients with symptoms for greater than one year in duration. Radiology 2002;225:513–14.
Theodorou DJ, Wong W, Duncan T, et al. Percutaneous balloon kyphoplasty: a novel technique for reducing pain and spinal deformity associated with osteoporotic vertebral compression fractures. Radiology 2000;217:S511.
Wang XF, Yang YY, Yu ZH, Li CQ, Wu YS. [Percutaneous vertebroplasty and conservative therapy for osteoporotic vertebral compression fractures: a clinical comparative study.] J Interv Radiol 2008;17.9:663–7.
Westesson PA, Numaguchi Y. Vertebroplasty: physical examination, plain film, and bone scan can be misleading in preoperative evaluation. Radiology 2001;221:618.
Westesson PA, Numaguchi Y. Vertebroplasty: subsequent compression fracture is a common reason for recurrent pain. Radiology 2001;221:617.
Appendix 7 Details of included studies relating to trials which met the inclusion criteria for the review of clinical effectiveness
Asterisks indicate the major publications for the study.
Blasco 2012
Blasco J, Garcia A, Manzanera LSR, MacHo JM, Peris P, Jaume P, et al. Randomized trial comparing vertebroplasty and conservative treatment analyzing pain relief and quality of life on the long term basis. Cardiovasc Interv Radiol 2010;33(Suppl. 2):182–3.
*Blasco JA, Martinez-Ferrer A, Macho Fernández J, San Roman Manzanera L, Pomés Talló J, Carrasco Jordan JLl, et al. Effect of vertebroplasty on pain relief, quality of life and the incidence of new vertebral fractures. A 12-month randomised follow-up, controlled trial. J Bone Miner Res 2012;27:1159–66.
Martinez-Ferrer A, Blasco J, Carrasco JL, Monegal A, Pomes J, Guaabens N, et al. Effect of vertebroplasty on the quality of life of patients with pain related to osteoporotic vertebral fractures preliminary results of a randomized trial. Bone 2011;48(Suppl. 2):S161.
Buchbinder 2009
Buchbinder R, Osborne RH, Ebeling PR, Wark JD, Mitchell P, Wriedt CJ, et al. Efficacy and safety of vertebroplasty for treatment of painful osteoporotic vertebral fractures: a randomised controlled trial [ACTRN012605000079640]. BMC Musculoskel Disord 2008;9:156.
*Buchbinder R, Osborne RH, Ebeling PR, Wark JD, Mitchell P, Wriedt C, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med 2009;361:557–68.
Staples MP, Kallmes DF, Comstock BA, Jarvik JG, Osborne RH, Buchbinder R. Effectiveness of vertebroplasty using individual patient data from two randomised placebo controlled trials: meta-analysis. BMJ 2011;343:d3952.
Farrokhi 2011
*Farrokhi MR, Alibai E, Maghami Z. Randomized controlled trial of percutaneous vertebroplasty versus optimal medical management for the relief of pain and disability in acute osteoporotic vertebral compression fractures. J Neurosurg Spine 2011 May;14:561–9.
FREE
Bastian L, van MJ, Boonen S, Ramstam J, Cummings S, Wardlaw D. 1-year results of a randomised, controlled, international, multi-centre study to compare balloon kyphoplasty and non-surgical care of acute compression fractures of vertebral bodies. Med Klin 2008;103:16.
Boonen S, Wardlaw D, Bastian L, Lips P, Van Meirhaghe J, Cummings S. Balloon kyphoplasty and non-surgical management in patients with acute vertebral body compression fractures: A randomized comparative trial. Calcif Tissue Int 2007;80(Suppl. 1):S33.
Boonen S, Cummings S, Wardlaw D, Eastell R. Impact of balloon kyphoplasty on quality of life and risk of recurrent vertebral fractures: A randomized trial in patients with acute vertebral compression fractures. Calcif Tissue Int 2008;82(Suppl. 1):S40–1.
Boonen S, Van MJ, Bastian L, Cummings SR, Ranstam J, Tillman JB, et al. Balloon kyphoplasty for the treatment of acute vertebral compression fractures: 2-year results from a randomized trial. J Bone Miner Res 2011;26:1627–37.
Van Meirhaeghe JK, Boonen S, Bastian L, Cummings S, Ranstam J, Tillman J, et al. A randomized trial of balloon kyphoplasty and nonsurgical care for patients with acute vertebral compression fractures: two year results. Osteoporos Int 2010;21(Suppl. 5):S667–8.
Wardlaw D, Boonen S, Bastian L, Van Meirhaeghe J, St Jan AZ. An international multicenter randomized comparison of balloon kyphoplasty and nonsurgical care in patients with acute vertebral body compression fractures. J Bone Miner Res 2007;22:1119.
*Wardlaw D, Cummings SR, Van Meirhaeghe J, Bastian L, Tillman JB, Ranstam J, et al. Efficacy and safety of balloon kyphoplasty compared with non-surgical care for vertebral compression fracture (FREE): a randomised controlled trial. Lancet 2009;373:1016–24.
INVEST
Brinjikji W, Comstock BA, Heagerty PJ, Jarvik JG, Kallmes DF. Investigational Vertebroplasty Efficacy and Safety Trial: detailed analysis of blinding efficacy. Radiology 2010;257:219–25.
Gray LA, Jarvik JG, Heagerty PJ, Hollingworth W, Stout L, Comstock BA, et al. INvestigational Vertebroplasty Efficacy and Safety Trial (INVEST): a randomized controlled trial of percutaneous vertebroplasty. BMC Musculoskel Disord 2007;8:126.
*Kallmes DF, Comstock BA, Heagerty PJ, Turner JA, Wilson DJ, Diamond TH, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med 2009;361:569–79.
Kallmes DF, Comstock BA, Gray LA, Heagerty PJ, Hollingworth W, Turner JA, et al. Baseline pain and disability in the Investigational Vertebroplasty Efficacy and Safety Trial. AJNR Am J Neuroradiol 2009;30:1203–5.
Staples MP, Kallmes DF, Comstock BA, Jarvik JG, Osborne RH, Buchbinder R. Effectiveness of vertebroplasty using individual patient data from two randomised placebo controlled trials: meta-analysis. BMJ 2011;343:d3952.
Liu 2010
*Liu JT, Liao WJ, Tan WC, Lee JK, Liu CH, Chen YH, et al. Balloon kyphoplasty versus vertebroplasty for treatment of osteoporotic vertebral compression fracture: a prospective, comparative, and randomized clinical study. Osteoporos Int 2010;21:359–64.
Rousing 2009
*Rousing R, Andersen MO, Jespersen SM, Thomsen K, Lauritsen J. Percutaneous vertebroplasty compared to conservative treatment in patients with painful acute or subacute osteoporotic vertebral fractures. Three-months follow-up in a clinical randomized study. Spine 2009;34:1349–54.
Rousing R, Hansen KL, Andersen MO, Jespersen SM, Thomsen K, Lauritsen JM. Twelve-months follow-up in forty-nine patients with acute/semiacute osteoporotic vertebral fractures treated conservatively or with percutaneous vertebroplasty. A clinical randomized study. Spine 2010;35:478–82.
VERTOS
*Voormolen MHJ, Mali WPTM, Lohle PNM, Fransen H, Lampmann LEH, van der Graaf Y, et al. Percutaneous vertebroplasty compared with optimal pain medication treatment: short-term clinical outcome of patients with subacute or chronic painful osteoporotic vertebral compression fractures. The VERTOS study. AJNR Am J Neuroradiol 2007;28:555–60.
VERTOS II
Klazen CAH, Verhaar HJJ, Lampmann LEH, Juttmann JR, Blonk MC, Jansen FH, et al. VERTOS II: percutaneous vertebroplasty versus conservative therapy in patients with painful osteoporotic vertebral compression fractures; rationale, objectives and design of a multicenter randomized controlled trial. Trials 2007;8.
Klazen C, Lohle P, Jansen F, Schoemaker M, Elgersma O, Van EK, et al. 1-year results of the VERTOS II trial: Vertebroplasty versus conservative therapy. Cardiovasc Interv Radiol 2009;32(Suppl. 2):313.
*Klazen CAH, Lohle PNM, Jansen FH, Tielbeek AV, Blonk MC, Venmans A, et al. Vertebroplasty versus conservative treatment in acute osteoporotic vertebral compression fractures (Vertos II): an open-label randomised trial. Lancet 2010;376:1085–92.
Klazen CAH, Venmans A, de Vries J, van Rooij WJ, Jansen FH, Blonk MC, et al. Percutaneous vertebroplasty is not a risk factor for new osteoporotic compression fractures: results from VERTOS II. AJNR Am J Neuroradiol 2010;31:1447–50.
Venmans A, Klazen CAH, Lohle PNM, van Rooij WJ, Verhaar HJJ, de Vries J, et al. Percutaneous vertebroplasty and pulmonary cement embolism: results from VERTOS II. AJNR Am J Neuroradiol 2010;31:1451–3.
Venmans A, Klazen CA, van Rooij WJ, de Vries J, Mali WP, Lohle PN. Postprocedural CT for perivertebral cement leakage in percutaneous vertebroplasty is not necessary – results from VERTOS II. Neuroradiology 2011;53:19–22.
Appendix 8 Table of excluded studies with rationale
This is not intended to be an exhaustive list of every study examining the intervention. It includes studies identified by the electronic searches which initially appeared to be relevant to the systematic review of clinical effectiveness but on closer inspection were not deemed to be relevant and/or valid. In addition, it includes RCTs cited in the submission by Johnson & Johnson36 which did not meet the review group’s inclusion criteria. The submissions by Medtronic34 and Synthes33 did not include any such RCTs.
| Study name | Reason for exclusion |
|---|---|
| Anselmetti 2008344 | Cited by Johnson & Johnson. Comparison not relevant (high-viscosity vs. low-viscosity cement) |
| Appel 2001384 | Includes patients with cancer; results for patients with osteoporosis not reported separately |
| Baerlocher 2010385 | Cited by Johnson & Johnson. Discussion paper |
| Becker 2007386 | Cited by Johnson & Johnson. Intervention not relevant (prophylactic BKP) |
| Bian 2006387 | Not clear that this was limited to patients with osteoporotic fracture |
| Boonen 2010388 | Does not include data not found in the included publications relating to the FREE study |
| Buchbinder 2009389 | Does not include data not found in the main publication of the study by Buchbinder et al. |
| Buchbinder 2011390 | Discussion paper |
| Buchbinder 2010378 | Discussion paper |
| Chen C. 2010349 | Cited by Johnson & Johnson. Comparison not relevant (unipedicular vs. bipedicular BKP) |
| Chen L. 2011350 | Cited by Johnson & Johnson. Comparison not relevant (unipedicular vs. bipedicular BKP) |
| Cummings 2009391 | Does not include data not found in the included publications relating to the FREE study |
| Figueiredo 2009392 | Cited by Johnson & Johnson. Comparison not relevant (traditional vs. side-opening cannula for PVP) |
| Firanescu 2011374 | Does not include any results |
| Gray 2009393 | Cited by Johnson & Johnson. Not a randomised study |
| Holden 2002394 | Includes patients with cancer; results for patients with osteoporosis not reported separately |
| Mao 2007395 | Cited by Johnson & Johnson. Comparison not relevant (carbonated hydroxyapatite cement vs. PMMA) |
| Ramaswamy 2000396 | Not randomised: patients divided into groups on the basis of duration of pain |
| Smith 2009397 | Does not include data not found in the included publications relating to the FREE study |
Appendix 9 Data abstraction tables
| Study | Pre-procedural imaging method used to determine fracture characteristics | Background and experience of operator performing vertebroplasty or kyphoplasty | Anaesthetic | Approach for vertebroplasty or kyphoplasty | Type of cement used | Mean number of vertebrae treated in index procedure | Mean (SD) volume of cement injected (per vertebra) |
|---|---|---|---|---|---|---|---|
| Blasco 2012127 | MRI for all patients; if MRI inconclusive, bone scan | Experienced neurointerventional radiologists | Not specified | Mostly bilateral transpedicular | PMMA manufactured by Exolent Spine, Elmdown, London, UK | 2.46 | NR |
| Buchbinder 2009102 | MRI (if MRI not feasible, e.g. because of contraindications, CT scan and bone scan148) | Experienced interventional radiologists with formal training in vertebroplasty and appropriate certification, who were actively performing the procedure | Neurolept sedation/analgesia using midazolam and fentanyl148 | Unipedicular. Satisfactory infiltration of the vertebral body was confirmed radiographically. Injection was stopped when substantial resistance was met or when the cement reached the posterior quarter of the vertebral body, or if cement leaked into extraosseous structures or veins. A bipedicular approach was used only if there was inadequate instillation of cement with the unipedicular approach | PMMA: manufacturer and viscosity not specified | 1.18 | 2.8 (1.2) ml |
| Farrokhi135 | Radiography and MRI for all patients | Neurosurgeon | Conscious sedation (i.v. fentanyl and midazolam) in 10/40 patients; GA in 30/40 patients | Unilateral parapedicular approach in 35 patients (87.5%); bipedicular approach in five patients (12.5%). Cement was injected using fluoroscopic monitoring with a C-arm unit in both planes. A bilateral approach was used only if there fluoroscopy indicated inadequate instillation of cement with the unilateral approach | PMMA: manufacturer and viscosity not specified | 2.5 | 3.5 ml (median 3.1 ml, range 1–5.5 ml) (this figure applies only to patients with a single treated fracture; for patients with several treated fractures, a total figure is given) |
| FREE137 | MRI for all patients | Not specified | Most procedures were done under GA, but 6/149 patients had conscious or deep sedation with local anaesthesia | Bilateral, transpedicular, or extrapedicular | PMMA manufactured by Medtronic Spine LLC. Viscosity not specified | 1.3 | NR |
| INVEST103 | Plain-film radiograph or MRI;147 requirement, for fractures of uncertain age as indicated by pain onset, of marrow oedema on MRI or increased vertebral-body uptake on bone scan | Highly experienced practitioners (discipline not specified, but said by Orr202 to be interventional radiologists) who had performed a mean of approximately 250 procedures (range 50–800) | Conscious or deep sedation according to the treating physician’s usual practice335 | Typically unipedicular.197 PMMA infused under constant lateral fluoroscopy into the vertebral body, and infusion was stopped when the cement reached to the posterior aspect of the vertebral body or entered an extraosseous space such as the intervertebral disk or an epidural or paravertebral vein | Barium-opacified PMMA; manufacturer and viscosity not specified | 1.4 | NR |
| Liu 2010139 | Not specified | Not specified | Intravenous GA (Propofol) plus 2% lidocaine injected locally | PVP was bipedicular. For both PVP and BKP, PMMA was injected under X-ray visualisation using a mobile C-arm X-ray | Barium-opacified PMMA (Zimmer) mixed with an antibiotic (gentamicin); viscosity not specified | NR | PVP 4.91 ± 0.65 ml; BKP 5.56 ± 0.62 ml |
| Rousing 2009128 | Plain radiography for all patients; MRI (stir weighted) or bone scan (spect) for those with > 1 fracture (fractures accepted as new if they showed oedema on MRI or increased bone turnover on bone scan) | Orthopaedic surgeons specialising in spine surgery | Most patients were mildly conscious sedated; all were prepared for GA in case of complications | Unipedicular or bipedicular. PMMA injected under continuous biplane fluoroscopy, and injection terminated in case of extravertebral cement leakage | PMMA: manufacturer and viscosity not specified | Not clear. Probably 1.2 | NR |
| VERTOS136 | Radiography and MRI for all patients | Not specified | Local anaesthesia | Bipedicular. PMMA injected under continuous fluoroscopy. CT scan with multiplanar reconstruction of the treated levels, performed immediately after vertebroplasty to assess the cement deposition and to identify possible extra cement leakage or other local complications that might not have been noted under fluoroscopy | PMMA (Osteopal V, Biomet Merck). Viscosity not specified | 1.6 | 3.2 ml (median 3.0, range 1.0–5.0 ml) |
| VERTOS II17 | Radiography and MRI for all patients | Experienced radiologists174 | Local anaesthesia | Bipedicular using a single or biplane angiography system under fluoroscopic guidance. Cement was injected under continuous fluoroscopic monitoring to identify local cement leakage or migration into the venous system towards the lungs. (Immediately after the procedure, a CT scan of the treated vertebral bodies was done with 2 mm slices to identify cement leakage outside the vertebral body or other possible local complications193) | PMMA (Osteo-Firm, COOK Medical). Viscosity not specified | 1.3 | 4.1 ± 1.5 ml (range 1–9 ml) |
| Study | Pain/analgesic use | Back-specific functional status/mobility | Disability | Vertebral body height/angular deformity | Progression of treated fracture | Incidence of new fractures | HRQoL | All-cause mortality | Complications | Other adverse events |
|---|---|---|---|---|---|---|---|---|---|---|
| Blasco 2012127 | Yes (pain on VAS scale, analgesic use) | No | No | No | No | Yes | Yes (QUALEFFO-41) | Yes | Yes (cement leaks) | No |
| Buchbinder 2009102 | Yes (pain on 0–10 scale, opioid use) | Yes (modified RDQ) | No | No | No | Yes | Yes (QUALEFFO, AQoL, EQ-5D) | No | Yes (cement leaks) | Yes |
| Farrokhi135 | Yes (pain on 0–10 scale) | Yes (Oswestry LBP scale) | Yes (whether or not ambulatory on day 1) | Yes (vertebral body height, kyphotic wedge angle) | Yes | Yes | Yes | Yes | Yes (cement leaks) | Yes |
| FREE137 | Yes (pain on 0–10 scale, analgesic use) | Yes (RDQ) | Yes (days of restricted activity, use of walking aids, back braces, etc.) | Yes (kyphotic angle) | Yes | Yes | Yes (SF-36 PCS, EQ-5D) | Yes | Yes (cement leaks) | Yes |
| INVEST103 | Yes (pain on 0–10 scale, pain frequency index, pain bothersomeness scale, opioid use) | Yes (modified RDQ) | Yes (SOF-ADL) | No | No | No | Yes (SF-36 PCS, EQ-5D) | No | Yes | Yes |
| Liu 2010139 | Yes (pain on VAS scale) | No | No | Yes (vertebral body height, kyphotic wedge angle) | No | Yes (adjacent fractures) | No | No | No | No |
| Rousing 2009128 | Yes (pain on VAS scale) | No | Yes (tandem test, timed ‘up and go’ test, repeated chair test) | No | No | Yes | Yes (SF-36 PCS and MCS, DPQ, EQ-5D, Barthel Index, MMSE) | Yes | Yes (cement leaks) | Yes |
| VERTOS136 | Yes (pain on 0–10 scale, analgesic use) | Yes (RDQ) | No | No | No | Yes | Yes (QUALEFFO) | Not specifically. Implicitly none | Yes | Yes |
| VERTOS II17 | Yes (pain on VAS scale, analgesic use) | Yes (RDQ) | No | Yes | Yes (height loss during follow-up of treated fractures186) | Yes | Yes (EQ-5D, QUALEFFO) | Yes | Yes (cement leaks) | Yes |
| Study | 12–24 hours | 3 days | 1 week | 2 weeks | 1 month | 2 months | 3 months | 6 months | 12 months | 24 months | 36 months |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Blasco 2012127 | ✓ | ✓ | ✓ | ✓ | |||||||
| Buchbinder 2009102 | ✓ | ✓ | ✓ | ✓ | |||||||
| Farrokhi135 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | |||||
| FREE137 | ✓ | ✓a | ✓a | ✓ | ✓ | ||||||
| INVEST103 | ✓ | ✓ | ✓ | ||||||||
| Liu 2010139 | ✓ | ✓ | |||||||||
| Rousing 2009128 | ✓ | ✓b | ✓ | ✓ | |||||||
| VERTOS136 | ✓ | ✓ | |||||||||
| VERTOS II17 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Study | Study protocol available | Clinical outcomes specified in study protocol | Outcomes reported |
|---|---|---|---|
| Blasco 2012127 | Yes398 | Quality of life (QUALEFFO-41 at baseline, 2 weeks, and 2, 6 and 12 months) Pain (VAS at baseline, 2 weeks, and 2, 6 and 12 months) |
Quality of life (QUALEFFO-41 at baseline, 2 weeks, and 2, 6 and 12 months) Pain at baseline, 2 weeks, and 2, 6 and 12 months Analgesic use at baseline, 2 weeks, and 2, 6 and 12 months Symptomatic vertebral fractures |
| Buchbinder 2009102 | Yes148 | Pain (overall, at rest, and in bed at night (11-point scale at 1 week, 1, 3, 6, 12, and 24 months) Quality of life (AQoL, QUALEFFO, EQ-5D at 1 week, 1, 3, 6, 12 and 24 months) Back pain-related disability (modified RDQ at 1 week, 1, 3, 6, 12 and 24 months) Timed ‘up and go’ test (at baseline, 12 and 24 months) Patients’ perception of recovery with respect to pain, fatigue, and overall health (7-point ordinal scales at 1 week, 1, 3, 6, 12 and 24 months) Incidence of new vertebral fractures (radiographs at 12 and 24 months) |
Average pain during 24-hour period; pain at rest, and pain in bed at night (11-point VAS at 1 week, 1, 3 and 6 months) Quality of life (AQoL, QUALEFFO, EQ-5D at 1 week, 1, 3, and 6 months) Back pain-related disability (modified RDQ score at 1 week, 1, 1, 3 and 6 months) Patients’ perception of pain at 1 week, 1, 3, and 6 months Opioid use at 1 week, 1, 3 and 6 months Adverse events at 1 week, 1, 3 and 6 months |
| Farrokhi135 | Yes195 | Fast treatment of VCF (measured by radiography at 1 week and 2, 6, 12, 24 and 36 months) | Average pain during 24-hour period (Huskisson’s 10-point scale at 1 week and 2, 6, 12, 24 and 36 months) Functional quality of life (non-validated Persian translation of Oswestry LBP disability scale at 1 week and 2, 6, 12, 24 and 36 months) Vertebral body height (measured radiographically at 2, 6, 12, 24 and 36 months) Sagittal index (measured radiographically at 2, 6, 12, 24 and 36 months) Mobility on day 1 after start of intervention Cement leakage Adverse events |
| FREE137 | Yes399 | Quality of life (SF-36 PCS at 1 month, EQ-5D and SF-36 at 1, 3, 6, 12 and 24 months) Function (RDQ and objective functionality tests – reaching, ‘get up and go’ – at 1, 3, 6, 12 and 24 months) Pain (11-point scale at 5–10 days – post enrolment for the control group and post kyphoplasty for the kyphoplasty group) Changes in spinal deformity (measured radiographically at baseline, 3, 12 and 24 months) Maintenance of vertebral body height in kyphoplasty-treated subjects only (lateral spine radiographs at baseline and at 3, 12 and 24 month visits) Patient satisfaction (1, 3, 6, 12 and 24 months) Outcome (nursing home, back to status prior to fracture, at 1, 3, 6, 12, 24 months), and hospital days, disabilities, etc. at 1, 3, 6, 12 and 24 months Rate of incident fractures (frequency, timing and location, at 3, 12 and 24 months) Procedural safety (perioperative clinical events) |
Quality of life (SF-36 PCS and EQ-5D at baseline, 1, 3, 6, 12 and 24 months) Function (RDQ score at baseline, 1, 3, 6, 12 and 24 months) Non-pharmacological therapies at baseline, 1 and 12 months Pain (11-point scale and analgesic use at baseline, 1, 12 and 24 months) Changes in spinal deformity (postoperatively and at 24 months) Patient satisfaction at 24 months Days of restricted activity at 1, 12, and 24 months Incident fractures at 12 and 24 months Procedural safety and other adverse events at 12 and 24 months |
| INVEST103 | Yes147 | Back pain-related disability (modified RDQ) Pain (11-point scale, modified Deyo–Patrick Pain Frequency and Bothersomeness Scale) Analgesic use Quality of life (SF-36, EQ-5D) Functional status (SOF-ADL, OPAQ body image domain) Adjacent fractures (radiograph at 12 months) Implant-related inflammation (patients receiving vertebroplasty) |
Back pain-related disability (modified RDQ) Average pain during 24-hour period (11-point scale, modified Deyo–Patrick Pain Frequency and Bothersomeness Scale) Opioid use Quality of life (SF-36 PCS and MCS, EQ-5D) Functional status (SOF-ADL) |
| Liu 2010139 | No | – | Pain on a 10-point scale 3 days and 6 months Postoperative vertebral body height Postoperative kyphotic wedge angle Adjacent fractures |
| Rousing 2009128 | No | – | Pain on a 10 cm VAS at 12–24 hours, 3 and 12 months Quality of life (SF-36 PCS and MCS at 3 and 12 months; also EQ-5D, Barthel Index, and MMSE in subgroup only) Effect of pain on daily life (Dallas Pain Questionnaire) Function (objective functionality tests – tandem test, timed ‘up and go’ test, repeated chair test – at 3 and 12 months, in subgroup only) Incident fractures at 3 and 12 months Intraoperative cement leakage |
| VERTOS136 | No | – | Back pain recorded on an 11-point scale 1 day and 2 weeks after vertebroplasty or initiation of optimal pain medication Analgesic use score 1 day and 2 weeks after vertebroplasty or initiation of optimal pain medication Quality of life (QUALEFFO completed 2 weeks after vertebroplasty or initiation of optimal pain medication) Back pain-related disability (RDQ completed 2 weeks after vertebroplasty or initiation of optimal pain medication) |
| VERTOS II17 | Yes | Pain (11-point scale at baseline, 1 day, and 1, 3, 6 and 12 months; analgesic use over first month) Quality of life (QUALEFFO and EQ-5D at baseline, 1 day, and 1, 3, 6 and 12 months) Back pain-related disability (RDQ) Secondary fractures (radiograph at 1, 3 and 12 months) |
Pain (11-point scale at 1 day, 1 week, and 1, 3, 6 and 12 months; analgesic use at 1 day, 1 week and 1 month) Quality of life (QUALEFFO and EQ-5D) Back pain-related disability (RDQ) Secondary fractures (radiograph at 1, 3 and 12 months) Vertebral body height loss ‘during follow-up’186 |
Appendix 10 Clinical efficacy data
| Time point | PVP | Control | Difference between groups (95% CI) (positive values favour intervention) | p-value |
|---|---|---|---|---|
| Baseline | 0.33 (0.25) | 0.27 (0.26) | ||
| 1 week | NR | NR | ||
| Change from baseline at 1 week | 0.0 (0.2) | 0.0 (0.2) | 0.0 (–0.1 to 0.1) | NR |
| 1 month | NR | NR | ||
| Change from baseline at 1 month | 0.0 (0.2) | 0.1 (0.3) | 0.0 (–0.1 to 0.1) | NR |
| 3 months | NR | NR | ||
| Change from baseline at 1 month | 0.0 (0.2) | 0.1 (0.3) | 0.0 (–0.1 to 0.1) | NR |
| 6 months | NR | NR | ||
| Change from baseline at 1 month | 0.0 (0.3) | 0.1 (0.3) | 0.0 (–0.1 to 0.2) | NR |
| Study | Time point | PVP | BKP | Control | Between-group mean difference (95% CI) (positive values favour intervention) | p-value |
|---|---|---|---|---|---|---|
| Buchbinder 2009102 | Baseline | 0.30 ± 0.32 | 0.28 ± 0.33 | NR | ||
| 1 week | NR | NR | ||||
| Change from baseline at 1 week | 0.1 (0.3) | 0.1 (0.3) | 0.0 (–0.1 to 0.2) | NR | ||
| 1 month | NR | NR | ||||
| Change from baseline at 1 month | 0.1 (0.3) | 0.1 (0.3) | 0.0 (–0.1 to 0.1) | NR | ||
| 3 months | NR | NR | ||||
| Change from baseline at 3 months | 0.2 (0.3) | 0.2 (0.4) | 0.0 (–0.1 to 0.2) | NR | ||
| 6 months | NR | NR | ||||
| Change from baseline at 6 months | 0.2 (0.4) | 0.2 (0.4) | 0.0 (–0.1 to 0.2) | NR | ||
| FREE35,137 | Baseline | 0.17 (0.37) | 0.19 (0.36) | |||
| 1 month | 0.59 (0.32) | 0.40 (0.33) | ||||
| Change from baseline at 1 month | 0.42 | 0.21 | 0.18 (0.08 to 0.28)a | 0.0003 | ||
| 3 months | 0.62 (0.29) | 0.53 (0.33) | ||||
| Change from baseline at 3 months | 0.45 (95% CI 0.37 to 0.53) | 0.34 (95% CI 0.28 to 0.42) | 0.11 (0.00 to 0.22) | |||
| 6 months | 0.63 (0.31) | 0.53 (0.32) | ||||
| Change from baseline at 6 months | 0.46 (95% CI 0.38 to 0.54) | 0.34 (95% CI 0.26 to 0.42) | 0.12 (0.01 to 0.23) | |||
| INVEST103 | Baseline | 0.57 (0.18) | 0.54 (0.23) | |||
| 1 month | 0.70 (0.18) | 0.64 (0.20) | ||||
| Change from baseline at 1 month | 0.13 | 0.10 | 0.05 (–0.01 to 0.11) | 0.13 | ||
| Rousing 2009128 | Baseline | n = 17: 0.356 (95% CI 0.196 to 0.516) | n = 16: 0.083 (95% CI –0.151 to 0.317) | 0.05 | ||
| 3 months | n = 15: 0.731 (95% CI 0.653 to 0.809) | n = 17: 0.543 (95% CI 0.387 to 0.699) | 0.04 | |||
| Change from baseline at 3 months | +0.375 (95% CI 0.33 to 0.42) | +0.460 (95% CI 0.42 to 0.50) | –0.085 (–0.15 to –0.02) |
| Study | Time point | PVP | BKP | Control | Difference between groups (95% CI) (positive values favour intervention) | p-value |
|---|---|---|---|---|---|---|
| FREE35,137,138 | Baseline | 0.17 (0.37) | 0.19 (0.36) | |||
| 12 months | 0.64 (0.29) | 0.54 (0.33)) | ||||
| Change from baseline at 12 months | 0.47 | 0.35 | 0.12 (0.01 to 0.22)a | 0.0252 | ||
| 24 months | 0.63 (0.29) | 0.56 (0.32) | ||||
| Change from baseline at 24 months (mean over 24 months) | 0.46 | 0.37 | 0.12 (0.06 to 0.18)a | 0.0002 | ||
| Rousing 2009128,131 | Baseline | n = 17: 0.356 (95% CI 0.196 to 0.516) | n = 16: 0.083 (95% CI –0.151 to 0.317) | 0.05 | ||
| 12 months | n = 14: 0.675 (0.576 to 0.775) | n = 18: 0.571 (0.448 to 0.694) | 0.19 | |||
| Change from baseline at 12 months | +0.319 (0.27 to 0.37) | +0.488 (0.45 to 0.52) | –0.169 (–0.23 to –0.11) |
| Study | PVP | Control | Adjusted mean between-group difference (95% CI) (positive values favour intervention) | p-value |
|---|---|---|---|---|
| Buchbinder 2009102 | 0.1 (0.3) | 0.1 (0.3) | 0.0 (–0.1 to 0.1) | NR |
| INVEST103 | 0.13 | 0.10 | 0.05 (–0.01 to 0.11) | 0.13 |
| Pooled data110 | 0.12 (0.19) | 0.11 (0.23) | 0.03 (–0.02 to 0.08) | NR |
| Study | Time point | PVP | Control | Mean difference between groups (95% CI) (negative values favour intervention) | p-value |
|---|---|---|---|---|---|
| Blasco 2012 (Blasco, personal communication) | Baseline | 65.19 (SE 2.23) | 59.17 (SE 2.17) | ||
| 2 weeks | 61.16 (SE 2.42) | 58.03 (SE 2.29) | |||
| Change from baseline at 2 weeks | –4.03 (–10.45 to +2.42) | –1.14 (–7.32 to +5.04) | –2.89 (–11.74 to +5.96) | ||
| Buchbinder 2009102 | Baseline | 56.9 (13.4) | 59.6 (17.1) | ||
| 1 week | NR | NR | |||
| Change from baseline at 1 week | –0.5 (7.4) | 3.6 (9.2) | –4.0 (–7.8 to –0.2)a | ||
| VERTOS136 | Baseline | 60 (range 37–86) | 67 (range 38–86) | 0.1 | |
| 2 weeks | 53 (range 28–79) | 67 (range 40–88) | –14 (–24.7 to –3.4) | NR | |
| Change from baseline at 2 weeks | –6.8 | –0.7 | –6.1 (–10.7 to –1.6) | NR |
| Study | Time point | PVP | Control | Mean difference between groups (95% CI) (negative values favour intervention) | p-value |
|---|---|---|---|---|---|
| Blasco 2012 (Blasco, personal communication) | Baseline | 65.19 (SE 2.23) | 59.17 (SE 2.17) | ||
| 2 months | 57.80 (SE 2.39) | 55.65 (SE 2.28) | |||
| Change from baseline at 2 months | –7.39 (–13.80 to –0.98) | –3.52 (–9.69 to +2.65) | –3.87 (–12.62 to +4.88) | ||
| 6 months | 54.13 (SE 2.30) | 51.93 (SE 2.25) | |||
| Change from baseline at 6 months | –11.06 (–17.34 to –4.78) | –7.24 (–13.67 to –1.11) | –3.82 (–12.42 to +4.78) | ||
| Buchbinder 2009102 | Baseline | 56.9 (13.4) | 59.6 (17.1) | ||
| 1 month | NR | NR | |||
| Change from baseline at 1 month | 2.8 (9.3) | 2.4 (12.3) | 0.9 (–4.2 to 6.0)a | ||
| 3 months | NR | NR | |||
| Change from baseline at 3 months | 6.0 (9.6) | 6.1 (13.7) | 0.7 (–4.4 to 5.7)a | ||
| 6 months | NR | NR | |||
| Change from baseline at 6 months | 6.4 (13.4) | 6.1 (13.4) | 0.6 (–5.1 to 6.2)a |
| Study | Time point | PVP | Control | Mean difference between groups (95% CI) (negative values favour intervention) | p-value |
|---|---|---|---|---|---|
| Blasco 2012 (Blasco, personal communication) | Baseline | 65.19 (SE 2.23) | 59.17 (SE 2.17) | ||
| 12 months | 54.38 (SE 2.38) | 52.01 (SE 2.32) | |||
| Change from baseline at 21 months | –10.81 (–17.20 to –4.42) | –7.16 (–13.89 to –0.93) | –3.65 (–12.28 to +4.98) | ||
| VERTOS II17 | Baseline | 58.7 (13.5) | 54.7 (14.4) | > 0.05 | |
| 1 year | NR | NR | NR | ||
| Change from baseline at 1 year | NR | NR | NR | < 0.0001a |
| Time point | BKP | Control | Between-group mean difference (95% CI) (positive values favour intervention) | p-value |
|---|---|---|---|---|
| Baseline | AiC information has been removed | AiC information has been removed | ||
| 1 month | AiC information has been removed | AiC information has been removed | ||
| Change from baseline at 1 month | AiC information has been removed | AiC information has been removed | AiC information has been removed | |
| 3 months | AiC information has been removed | AiC information has been removed | ||
| Change from baseline at 3 months | AiC information has been removed | AiC information has been removed | AiC information has been removed | |
| 6 months | AiC information has been removed | AiC information has been removed | ||
| Change from baseline at 6 months | AiC information has been removed | AiC information has been removed | AiC information has been removed | |
| 12 months | AiC information has been removed | AiC information has been removed | ||
| Change from baseline at 12 months | AiC information has been removed | AiC information has been removed | AiC information has been removed | |
| 24 months | AiC information has been removed | AiC information has been removed | ||
| Change from baseline at 24 months | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Study | Time point | PVP | BKP | Control | Between-group mean difference (95% CI) (positive values favour intervention) | p-value |
|---|---|---|---|---|---|---|
| FREE35,137,138 | Baseline | N/A | AiC information has been removed | AiC information has been removed | ||
| 1 month | N/A | AiC information has been removed | AiC information has been removed | |||
| Change from baseline at 1 month | N/A | AiC information has been removed | AiC information has been removed | 5.2 (2.9 to 7.4)a | < 0.0001 | |
| 3 months | N/A | AiC information has been removed | AiC information has been removed | |||
| Change from baseline at 3 months | N/A | AiC information has been removed | AiC information has been removed | 4.0 (1.6 to 6.3)a | 0.0008 | |
| 6 months | N/A | AiC information has been removed | AiC information has been removed | |||
| Change from baseline at 6 months | N/A | AiC information has been removed | AiC information has been removed | 3.39 (1.13 to 5.64)a | 0.003 | |
| INVEST103 | Baseline | 25.3 (7.8) | N/A | 25.3 (7.3) | ||
| 1 month | 29.7 (9.6) | N/A | 28.7 (8.0) | |||
| Change from baseline at 1 month | +4.4 | N/A | +3.4 | 1.0 (–1.7 to 3.7)b | 0.45 | |
| Rousing 2009128 | Baseline | 36.7 (95% CI 30.0 to 43.4) | N/A | 33.4 (95% CI 26.2 to 40.7) | ||
| 3 months | 34.0 (95% CI 30.1 to 37.9) | N/A | 29.3 (95% CI 24.5 to 34.1) | 0.12 | ||
| Change from baseline at 3 months | –2.7 (95% CI –4.52 to –0.88) | N/A | –4.1 (95% CI –6.16 to –2.04) | +1.4 (–1.38 to +4.18) |
| Study | Time point | PVP | BKP | Control | Between-group mean difference (95% CI) (positive values favour intervention) | p-value |
|---|---|---|---|---|---|---|
| FREE34,138 | Baseline | AiC information has been removed | AiC information has been removed | |||
| 12 months | AiC information has been removed | AiC information has been removed | ||||
| Change from baseline at 12 months | AiC information has been removed | AiC information has been removed | 1.70 (–0.59 to 3.98)a | 0.15 | ||
| 24 months | AiC information has been removed | AiC information has been removed | ||||
| Change from baseline at 24 months | AiC information has been removed | AiC information has been removed | 1.68 (–0.63 to 3.99)a | 0.26 | ||
| Rousing 2009128,131 | Baseline | 36.7 (95% CI 30.0 to 43.4) | 33.4 (95% CI 26.2 to 40.7) | |||
| 12 months | 32.1 (95% CI 27.8 to 36.3) | 30.5 (95% CI 25.2 to 35.7) | 0.63 | |||
| Change from baseline at 12 months | –4.6 (95% CI –6.48 to –2.72) | –2.9 (95% CI –5.00 to –0.80) | –1.7 (–4.57 to +1.17) |
| Study | Time point | PVP | BKP | Control | Between-group mean difference (95% CI) (positive values favour intervention) | p-value |
|---|---|---|---|---|---|---|
| FREE34 | Baseline | AiC information has been removed | AiC information has been removed | |||
| 1 month | AiC information has been removed | AiC information has been removed | ||||
| Change from baseline at 1 month | AiC information has been removed | AiC information has been removed | AiC information has been removed | |||
| 3 months | AiC information has been removed | AiC information has been removed | ||||
| Change from baseline at 3 months | AiC information has been removed | AiC information has been removed | AiC information has been removed | |||
| 6 months | AiC information has been removed | AiC information has been removed | ||||
| Change from baseline at 6 months | AiC information has been removed | AiC information has been removed | AiC information has been removed | |||
| INVEST103 | Baseline | 44.8 (11.8) | 41.5 (14.1) | |||
| 1 month | 46.9 (12.0) | 45.6 (14.8) | ||||
| Change from baseline at 1 month | +2.1 | +4.1 | 1.0 (–3.7 to 4.6)a | 0.83 | ||
| Rousing 2009128 | Baseline | 49.7 (95% CI 43.6 to 55.8) | 49.6 (95% CI 41.9 to 57.3) | |||
| 3 months | 48.9 (95% CI 43.8 to 54.0) | 46.2 (95% CI 39.2 to 53.2) | 0.51 | |||
| Change from baseline at 3 months | –0.8 (95% CI –2.62 to +1.02) | –3.4 (95% CI –5.84 to –0.96) | +2.6 (–0.51 to +5.71) |
| Study | Time point | PVP | BKP | Control | Between-group mean difference (95% CI) (positive values favour intervention) | p-value |
|---|---|---|---|---|---|---|
| FREE34 | Baseline | AiC information has been removed | AiC information has been removed | |||
| 12 months | AiC information has been removed | AiC information has been removed | ||||
| Change from baseline at 12 months | AiC information has been removed | AiC information has been removed | AiC information has been removed | |||
| 24 months | AiC information has been removed | AiC information has been removed | ||||
| Change from baseline at 24 months | AiC information has been removed | AiC information has been removed | AiC information has been removed | |||
| Rousing 2009128,131 | Baseline | 49.7 (95% CI 43.6 to 55.8) | 49.6 (95% CI 41.9 to 57.3) | |||
| 12 months | 48.7 (95% CI 42.7 to 54.6) | 49.0 (95% CI 43.9 to 54.1) | 0.93 | |||
| Change from baseline at 12 months | –1.0 (95% CI –2.99 to +0.99) | –0.6 (95% CI –2.70 to +1.57) | –0.4 (–3.40 to +2.60) |
| Study | Time point | PVP | Control | Mean difference, intervention vs. control (95% CI) (negative values favour intervention) | p-value |
|---|---|---|---|---|---|
| Buchbinder 2009102 | Baseline | 17.3 (2.8) | 17.3 (2.9) | ||
| 1 week | NR | NR | |||
| Change from baseline at 1 week | –1.8 (5.0) | –4.0 (6.8) | –2.1 (–5.2 to 0.9)a | ||
| INVEST103 | Baseline | 16.6 (3.8) | 17.5 (4.1) | ||
| 3 days | 13.0 (5.2) | 12.5 (5.5) | |||
| Change from baseline at 3 days | –3.6 | –5.0 | –0.9 (–2.7 to 0.8)b | 0.30 | |
| 2 weeks | 12.4 (5.8) | 12.3 (5.9) | |||
| Change from baseline at 2 weeks | –4.2 | –5.2 | –0.6 (–2.4 to 1.2)b | 0.35 | |
| VERTOS136 | Baseline | 15.7 (range 8–22) | 17.8 (range 9–24) | 0.2 | |
| 2 weeks | 13 (range 3–22) | 18 (range 9–23) | –5 (–8.4 to –1.2) | ||
| Change from baseline at 2 weeks | –2.7 | +0.2 | –2.9 c |
| Study | Time point | PVP | BKP | Control | Mean difference, intervention vs. control (95% CI) (negative values favour control) | p-value |
|---|---|---|---|---|---|---|
| Buchbinder 2009102 | Baseline | 17.3 (2.8) | 17.3 (2.9) | |||
| 1 month | NR | NR | ||||
| Change from baseline at 1 month | –4.4 (6.6) | –3.1 (6.8) | –1.7 (–5.2 to +1.8)a | |||
| 3 months | NR | NR | ||||
| Change from baseline at 3 months | –3.7 (5.4) | –5.3 (7.2) | –1.5 (–1.7 to +4.8)a | |||
| 6 months | NR | NR | ||||
| Change from baseline at 6 months | –4.1 (5.8) | –3.7 (5.8) | 0.0 (–2.9 to +3.0)a | |||
| FREE34,137,138 | Baseline | 16.79 (4.95) | 17.75 (3.96) | |||
| 1 month | 11.36 (6.14) | 16.32 (4.46) | ||||
| Change from baseline at 1 month | –5.43 | –1.43 | –4.0 (–5.5 to –2.6)b | < 0.0001 | ||
| 3 months | 10.03 (5.55) | 13.40 (6.26) | ||||
| Change from baseline at 3 months | –6.76 | –4.35 | –2.41 | |||
| 6 months | 9.37 (5.82) | 11.92 (6.17) | ||||
| Change from baseline at 6 months | –7.42 | –5.83 | –1.59 | |||
| 12 months | 9.61 (6.24) | 12.07 (6.12) | ||||
| Change from baseline at 12 months | –7.18 | –5.68 | –2.6 (–4.1 to –1.0)b | 0.0012 | ||
| 24 months | 9.79 (5.77) | 10.89 (6.30) | ||||
| Change from baseline at 24 months | –7.00 | –6.86 | –1.43 | 0.51 | ||
| INVEST103 | Baseline | 16.6 (3.8) | 17.5 (4.1) | |||
| 1 month | 12.0 (6.3) | 13.0 (6.4) | ||||
| Change from baseline at 1 month | –4.6 | –4.5 | 0.7 (–1.3 to 2.8)c | 0.49 | ||
| VERTOS II17 | Baseline | 18.6 (3.6) | 17.2 (4.2) | < 0.05 | ||
| 12 months | NR | NR | NR | |||
| Change from baseline at 12 months | NR | NR | NR | < 0.0001 |
| Study | PVP | Control | Adjusted mean between-group difference (95% CI) (negative values favour intervention) | p-value |
|---|---|---|---|---|
| Buchbinder 2009102 | –4.4 (6.6) | –3.1 (6.8) | –1.7 (–5.2 to +1.8) | NR |
| INVEST103 | –4.6 | –4.5 | –0.1 | NR |
| Pooled data110 | –4.1 (5.9) | –3.9 (6.1) | –0.8 (–0.9 to 2.4) | NR |
| Outcome | PVP | Control | Relative risk (95% CI) | p-value |
|---|---|---|---|---|
| Improvement in RDQ score of ≥ 3 units | 49/94 (52.1%) | 46/89 (51.7%) | 1.0 (0.7 to 1.5) | NS |
| Improvement in RDQ score of ≥ 30% | 41/102 (40.28%) | 41/100 (41.0%) | 1.0 (0.7 to 1.4) | NS |
| Study | Time point | PVP | Control | Mean difference, intervention vs. control (95% CI) (negative values favour PVP) | p-value |
|---|---|---|---|---|---|
| Farrokhi 2011135 | Baseline | 52.2 (2.4) | 50.4 (2.8) | ||
| 1 week | 30.1 (3.0) | 44.0 (2.5) | –14.0 (–15.0 to –12.82) | < 0.001 | |
| Change from baseline at 1 week | –22.1 (–23.29 to –20.91) | –6.4 (–7.54 to –5.27) | –15.7 (–17.35 to –14.05) | ||
| 2 months | 15.0 (2.2) | 30.0 (3.1) | –15.0 (–16.76 to –13.24) | < 0.019 | |
| Change from baseline at 2 months | –37.2 (–38.21 to –36.19) | –20.4 (–21.66 to –19.14) | –16.8 (–18.43 to –15.17) | ||
| 6 months | 10.0 (2.0) | 21.0 (2.5) | –11.0 (–12.17 to –7.83) | < 0.011 | |
| Change from baseline at 6 months | –42.2 (–43.17 to –41.23) | –29.4 (–30.54 to –28.27) | –12.8 (–14.03 to –11.30) | ||
| 12 months | 8.0 (3.2) | 20.0 (1.7) | –12.0 (–13.5 to –11.5) | < 0.021 | |
| Change from baseline at 12 months | –44.2 (–45.46 to –42.94) | –30.4 (–31.40 to –29.40) | –13.8 (–15.41 to –12.19) | ||
| 24 months | 8.0 (2.2) | 20.0 (2.0) | –12.0 (–13.32 to –10.68) | < 0.041 | |
| Change from baseline at 24 months | –44.2 (–45.22 to –43.18) | –30.4 (–31.45 to –29.35) | –13.8 (–15.26 to –12.34) | ||
| 36 months | 8.0 (1.7) | 22.0 (1.2) | –14.0 (–14.91 to –13.09) | < 0.01 | |
| Change from baseline at 36 months | –44.2 (–45.12 to –43.28) | –28.4 (–29.33 to –27.47) | –15.8 (–17.11 to –14.49) |
| Study | Time point | PVP | Control | Mean difference between groups (positive scores favour PVP) (95% CI) | p-value |
|---|---|---|---|---|---|
| Rousing 2009128,131 | Baseline | 17.7 (15.6 to 19.8) | 17.0 (14.2 to 19.8) | ||
| 3 months | 19.6 (19.0 to 20.3) | 18.1 (16.8 to 19.4) | 0.07 | ||
| Change from baseline at 3 months | 1.9 (1.26 to 2.54) | 1.1 (0.31 to 1.89) | +0.8 (–0.23 to +1.83) | ||
| 12 months | 19.8 (19.5 to 20.0) | 18.5 (17.6 to 19.3) | 0.02 | ||
| Change from baseline at 12 months | 2.1 (1.49 to 2.71) | 1.5 (0.75 to 2.25) | +0.6 (–0.38 to +1.58) |
| Study | Time point | PVP | Control | Mean difference, intervention vs. control (95% CI) (negative values favour intervention) | p-value |
|---|---|---|---|---|---|
| INVEST103 | Baseline | 10.0 (3.6) | 10.3 (2.8) | ||
| 1 month | 7.7 (3.7) | 8.2 (3.6) | |||
| Change from baseline at 1 month | –2.3 | –2.1 | 0.4 (–0.8 to 1.6)a | 0.51 |
| Time point | BKP | Control | Mean difference, intervention vs. control (95% CI) (positive values favour BKP) |
|---|---|---|---|
| Baseline | 16.91 (13.95) | 15.23 (14.44) | |
| 1 month | 44.34 (24.29) | 29.49 (17.80) | |
| Change from baseline at 1 month | +27.43 (+22.80 to +32.06) | +14.26 (+10.41 to +18.11) | +13.17 (+7.26 to +19.08) |
| 3 months | 54.48 (27.63) | 41.55 (22.57) | |
| Change from baseline at 3 months | +37.57 (+32.38 to +42.76) | +26.32 (+21.63 to +31.01) | +11.25 (+4.52 to +17.98) |
| 6 months | 55.03 (26.78) | 45.85 (24.91) | |
| Change from baseline at 6 months | +38.12 (+33.02 to +43.22) | +30.59 (+25.49 to +35.69) | +7.53 (+0.65 to +14.41) |
| 12 months | 55.03 (26.78) | 49.01 (23.78) | |
| Change from baseline at 12 months | +38.12 (+32.90 to +43.34) | +33.78 (+28.76 to +38.77) | +4.34 (–2.52 to +11.20) |
| 24 months | 57.36 (26.61) | 48.66 (22.96) | |
| Change from baseline at 24 months | +40.45 (+35.19 to +45.71) | +33.43 (+28.59 to +38.27) | +7.02 (+0.20 to +13.85) |
| Study | Time point | PVP | BKP | Control | Mean difference, intervention vs. control (95% CI) (negative values favour intervention) | p-value |
|---|---|---|---|---|---|---|
| Blasco 2012 (Blasco personal communication) | Baseline | 7.21 (SE 0.33) | 6.31 (SE 0.35) | |||
| 2 weeks | 5.87 (SE 0.44) | 4.79 (SE 0.41) | ||||
| Change from baseline at 2 weeks | –1.34 (95% CI –2.13 to –0.55) | –1.52 (95% CI –2.28 to –0.76) | +0.18 (–0.95 to +1.31) | |||
| Buchbinder 2009102 | Baseline | 7.4 (2.1) | 7.1 (2.3) | |||
| 1 week | NR | NR | ||||
| Change from baseline at 1 week | –1.5 (2.5) | –2.1 (2.8) | +0.7 (–0.4 to 1.8)a | |||
| Farrokhi 2011135 | Baseline | 8.4 (1.6) | 7.2 (1.7) | |||
| 1 week | 3.3 (1.5) | 6.4 (2.1) | –3.1 (–3.72 to –2.28) | < 0.001 | ||
| Change from baseline at 1 week | –5.1 (95% CI –5.59 to –4.61) | –0.8 (95% CI –1.39 to –0.21) | –4.3 (–5.11 to –3.49) | |||
| FREE34,137 | Baseline | 6.85 (1.57) | 6.80 (1.53) | |||
| 1 week | NR | NR | ||||
| Change from baseline at 1 week | NR | NR | –2.2 (–1.6 to –2.8)b | < 0.0001 | ||
| INVEST103 | Baseline | 6.9 (2.0) | 7.2 (1.8) | |||
| 3 days | 4.2 (2.8) | 3.9 (2.9) | ||||
| Change from baseline at 3 days | –2.7 | –3.3 | +0.4 (–0.5 to 1.5)c | 0.37 | ||
| 2 weeks | 4.3 (2.9) | 4.5 (2.8) | ||||
| Change from baseline at 1 week | –2.6 | –2.7 | +0.1 (–0.8 to 1.1)c | 0.77 | ||
| Liu 2010139 | Baseline | 7.9 (0.7) | 8.0 (0.8) | |||
| 3 days | 2.3 (0.5) | 2.6 (0.6) | NS | |||
| Change from baseline at 3 days | –5.6 (95% CI –5.78 to –5.42) | –5.4 (95% CI –5.60 to –5.20) | PVP vs. BKP: –0.2 (–0.43 to +0.03) | |||
| Rousing 2009128 | Baseline | 7.5 (95% CI 6.6 to 8.4) | 8.8 (95% CI 8.2 to 9.3) | 0.02 | ||
| 12–24 hours | 2.0 (95% CI 0.9 to 3.2) | NR | ||||
| Change from baseline at 12–24 hours | –5.5 | NR | NR | |||
| VERTOS136 | Baseline | 7.1 (95% CI 5 to 9) | 7.6 (95% CI 5 to 10) | 0.3 | ||
| 12–24 h | 4.7 (95% CI 1 to 8) | 7.1 (95% CI 5 to 10) | –2.4 (–3.7 to –1.0) | |||
| Change from baseline at 12–24 hours | –2.4 | –0.5 | –1.9 d | |||
| 2 weeks | 4.9 (95% CI 0 to 10) | 6.4 (95% CI 3 to 9) | –1.5 (–3.2 to –0.2) | |||
| Change from baseline at 2 weeks | –2.1 | –1.1 | –1.0 (–0.5 to –2.5) | |||
| VERTOS II17 | Baseline | 7.8 (1.5) | 7.5 (1.6) | |||
| 12–24 hours | 3.7 (2.4) | 6.7 (2.1) | < 0.0001 | |||
| Change from baseline at 12–24 h | –4.1 (95% CI –4.52 to –3.70) | –0.8 (95% CI –1.19 to –0.41) | –3.3 (–3.94 to –2.66) | |||
| 1 week | 3.5 (2.5) | 5.6 (2.5) | < 0.0001 | |||
| Change from baseline at 1 week | –4.3 (95% CI –4.74 to –3.86) | –1.9 (95% CI –2.35 to –1.45) | –2.4 (–3.11 to –1.70) |
| Study | Time point | PVP | BKP | Control | Mean difference, intervention vs. control (95% CI) (negative values favour intervention) | p-value |
|---|---|---|---|---|---|---|
| Blasco 2012 (Blasco127, Blasco personal communication) | Baseline | 7.21 (SE 0.33) | 6.31 (SE 0.35) | |||
| 2 months | 4.13 (SE 0.41) | 4.72 (SE 0.41) | ||||
| Change from baseline at 2 months | –3.07 (SE 0.45) | –1.59 (SE 0.42) | –1.48 (–2.94 to –0.02) | 0.0172 | ||
| 6 months | 4.72 (SE 0.36) | 4.30 (SE 0.38) | ||||
| Change from baseline at 6 months | –2.49 (95% CI –3.45 to –1.53) | –2.01 (95% CI –3.02 to –1.00) | –0.48 (–1.84 to +0.88) | |||
| Buchbinder 2009102 | Baseline | 7.4 (2.1) | 7.1 (2.3) | |||
| 1 month | NR | NR | ||||
| Change from baseline at 1 month | –2.3 (2.6) | –1.7 (3.3) | –0.5 (–1.7 to +0.8)a | |||
| 3 months | ||||||
| Change from baseline at 3 months | –2.6 (2.9) | –1.9 (3.3) | –0.6 (–1.8 to +0.7)a | |||
| 6 months | NR | NR | ||||
| Change from baseline at 6 months | –2.4 (3.3) | –2.1 (3.3) | –0.1 (–1.4 to +1.2) | |||
| Farrokhi 2011135 | Baseline | 8.4 (1.6) | 7.2 (1.7) | |||
| 2 months | 3.2 (2.2) | 6.1 (2.1) | –2.9 (–4.90 to –0.82) | < 0.011 | ||
| Change from baseline at 2 months | –5.2 (95% CI –6.04 to –4.36) | –1.1 (95% CI –1.92 to –0.28) | –4.1 (–5.28 to –2.92) | |||
| 6 months | 2.2 (2.1) | 4.1 (1.5) | –1.9 (–3.25 to –0.55) | < 0.021 | ||
| Change from baseline at 6 months | –6.2 (95% CI –7.02 to –5.38) | –3.1 (95% CI –3.79 to –2.41) | –3.1 (–4.17 to –2.03) | |||
| FREE34,400 | Baseline | 6.85 (1.57) | 6.80 (1.53) | |||
| 1 month | 3.42 (2.47) | 5.33 (2.25) | ||||
| Change from baseline at 1 month | –3.43 | –1.47 | –1.9 (–2.5 to –1.3)b | < 0.0001 | ||
| 3 months | 2.88 (2.57) | 4.40 (2.59) | ||||
| Change from baseline at 3 months | –3.97 | –2.40 | –1.57 c | |||
| 6 months | 2.67 (2.38) | 4.24 (2.45) | ||||
| Change from baseline at 6 months | –4.17 | –2.56 | –1.61 c | |||
| INVEST103 | Baseline | 6.9 (2.0) | 7.2 (1.8) | |||
| 1 month | 3.9 (2.9) | 4.6 (3.0) | ||||
| Change from baseline at 1 month | –3.0 | –2.6 | –0.7 (–1.7 to 0.3)d | 0.19 | ||
| Liu 2010139 | Baseline | 7.9 (0.7) | 8.0 (0.8) | |||
| ∼ 6 months | 2.6 (0.6) | 2.6 (0.6) | NS | |||
| Change from baseline at ∼6 months | –5.3 (95% CI –5.56 to –5.04) | –5.4 (95% CI –5.68 to – 5.12) | PVP vs. BKP: +0.1 (–0.28 to + 0.48) | |||
| Rousing 2009128,131 | Baseline | 7.5 (95% CI 6.6 to 8.4) | 8.8 (95% CI 8.2 to 9.3) | 0.02 | ||
| 1 monthe | 3.4 (95% CI 2.2 to 4.8) | 6.4 (95% CI 5.0 to 7.9) | 0.00 | |||
| Change from baseline at 1 month | –4.1 (95% CI –4.46 to –3.74) | –2.4 (95% CI –2.78 to –2.02) | –1.7 (–2.21 to –1.19) | |||
| 3 months | 1.8 (95% CI 0.8 to 2.8) | 2.6 (95% CI 1.2 to 4.0) | 0.33 | |||
| Change from baseline at 3 months | –5.7 (95% CI –5.99 to –5.41) | –6.2 (95% CI –6.52 to –5.88) | –0.5 (–0.05 to –0.95) | |||
| VERTOS II17 | Baseline | 7.8 (1.5) | 7.5 (1.6) | |||
| 1 month | 2.5 (2.5) | 4.9 (2.6) | ||||
| Change from baseline at 1 month | –5.2 (95% CI –4.72 to –5.88) | –2.7 (95% CI –1.98 to –3.22) | –2.6 (–3.37 to –1.74) | < 0.0001 | ||
| 3 months | 2.5 (2.7) | 3.9 (2.8) | 0.025 | |||
| Change from baseline at 3 months | –5.3 (95% CI –5.93 to –4.67) | –3.6 (95% CI –4.28 to –2.92) | –1.7 (–2.62 to –0.78) | |||
| 6 months | 2.3 (2.7) | 3.9 (2.9) | 0.014 | |||
| Change from baseline at 6 months | –5.5 (95% CI –6.14 to –4.86) | –3.6 (95% CI –4.32 to –2.88) | –1.9 (–2.84 to –0.97) |
| Study | Time point | PVP | BKP | Control | Mean difference, intervention vs. control (95% CI) (negative values favour intervention) | p-value |
|---|---|---|---|---|---|---|
| Blasco 2012 (Blasco, personal communication) | Baseline | 7.21 (SE 0.33) | 6.31 (SE 0.35) | |||
| 12 months | 4.49 (SE 0.39) | 4.32 (SE 0.37) | ||||
| Change from baseline at 12 months | –2.72 (95% CI –3.72 to –1.72) | –1.99 (95% CI –2.99 to –0.99) | –0.73 (–2.10 to +0.64) | |||
| Farrokhi 2011135 | Baseline | 8.4 (1.6) | 7.2 (1.7) | |||
| 12 months | 2.2 (2.1) | 4.1 (1.8) | –1.9 (–2.90 to 0.90) | < 0.11 | ||
| Change from baseline at 12 months | –6.2 (95% CI –7.03 to –5.37) | –3.1 (95% CI –3.86 to –2.34) | –3.1 (–4.23 to –1.97) | |||
| 24 months | 2.2 (2.1) | 3.7 (2.0) | –0.5 (–1.39 to 0.50) | < 0.37 | ||
| Change from baseline at 24 months | –6.2 (95% CI –7.03 to –5.37) | –3.5 (95% CI –4.31 to –2.69) | –2.7 (–3.86 to –1.54) | |||
| 36 months | 2.8 (2.0) | 3.7 (2.5) | –1.5 (–9.85 to 6.85) | < 0.81 | ||
| Change from baseline at 36 months | –5.6 (95% CI –6.41 to –4.79) | –3.5 (95% CI –4.44 to –2.56) | –2.1 (–3.36 to –0.87) | |||
| FREE34,137,138 | Baseline | 6.85 (1.57) | 6.80 (1.53) | |||
| 12 months | 2.70 (2.53) | 3.53 (2.41) | ||||
| Change from baseline at 12 months | NR | NR | –0.9 (–1.5 to –0.3)a | 0.0034 | ||
| 24 months | 2.61 (2.55) | 3.45 (2.49) | ||||
| Change from baseline at 24 months | NR | NR | –0.80 (–1.39 to –0.20)a | 0.009 | ||
| Rousing 2009128,131 | Baseline | 7.5 (95% CI 6.6 to 8.4) | 8.8 (95% CI 8.2 to 9.3) | 0.02 | ||
| 12 months | 2.0 (95% CI 1.1 to 3.0) | 2.9 (95% CI 1.6 to 4.1) | 0.29 | |||
| Change from baseline at 12 months | –5.5 (95% CI –5.79 to –5.21) | –5.9 (95% CI –6.20 to –5.60) | +0.4 (–0.03 to +0.83) | |||
| VERTOS II17 | Baseline | 7.8 (1.5) | 7.5 (1.6) | |||
| 12 months | 2.2 (2.7) | 3.8 (2.8) | 0.014 | |||
| Change from baseline at 12 months | –5.7 (95% CI –4.98 to –6.22) | –3.7 (95% CI –3.05 to –4.35) | –2.0 (–2.80 to –1.13) | < 0.0001 |
| Study | PVP | Control | Adjusted mean between-group difference (95% CI) (negative values favour intervention) | p-value |
|---|---|---|---|---|
| Buchbinder 2009102 | –2.3 (2.6) | –1.7 (3.3) | –0.5 (–1.7 to +0.8) | NR |
| INVEST103 | –3.0 | –2.6 | –0.7 (–1.7 to 0.3) | 0.19 |
| Pooled data110 | –2.8 (3.0) | –2.2 (3.2) | –0.6 (–1.4 to 0.2) | NR |
| Study | Time point | PVP | Control | Mean difference, intervention vs. control (95% CI) (negative values favour intervention) | p-value |
|---|---|---|---|---|---|
| Blasco 2012 (Blasco, personal communication) | Baseline | 4.03 (SE 0.12) | 3.68 (SE 0.12) | ||
| 2 weeks | 3.44 (SE 01.4) | 3.40 (SE 0.13) | |||
| Change from baseline at 2 weeks | –0.59 (–0.95 to –0.23) | –0.28 (–0.63 to +0.07) | –0.31 (–0.81 to +0.19) | ||
| 2 months | 3.20 (SE 0.14) | 3.18 (SE 0.13) | |||
| Change from baseline at 2 months | –0.83 (–1.19 to –0.47) | –0.50 (–0.85 to –0.15) | –0.33 (–0.82 to +0.16) | ||
| 6 months | 3.22 (SE 0.13) | 3.12 (SE 0.13) | |||
| Change from baseline at 6 months | –0.81 (–1.16 to –0.46) | –0.56 (–0.91 to –0.21) | –0.25 (–0.73 to +0.23) | ||
| 12 months | 3.05 (SE 0.13) | 2.90 (SE 0.13) | |||
| Change from baseline at 12 months | –0.98 (–1.33 to –0.63) | –0.78 (–1.13 to –0.43) | –0.20 (–0.67 to +0.27) | ||
| Buchbinder 2009102 | Baseline | 72.2 (17.3) | 72.1 (16.5) | ||
| 1 week | |||||
| Change from baseline at 1 week | 7.8 (20.5) | 16.1 (23.1) | –8.5 (–18.2 to 1.1) | ||
| 1 month | NR | NR | |||
| Change from baseline at 1 month | 14.8 (21.2) | 19.3 (27.7) | –4.0 (–15.1 to 7.1) | ||
| 3 months | NR | NR | |||
| Change from baseline at 3 months | 18.1 (21.1) | 21.1 (30.6) | –2.7 (–14.5 to 9.1) | ||
| 6 months | NR | NR | |||
| Change from baseline at 6 months | 20.4 (25.0) | 20.7 (25.0) | –0.5 (–11.2 to 10.2) | ||
| VERTOS139 | Baseline | 19 | 21 | –2 (–3.6 to 0.4) | |
| 2 weeks | 14 | 20 | –6 (–8.5 to –2.5) | ||
| Change from baseline at 2 weeks | –5 | –1 | –4 a |
| Outcome measure | Time point | PVP | Control | Adjusted treatment effect (95% CI) | p-value |
|---|---|---|---|---|---|
| Pain Frequency Index | Baseline | 3.0 ± 0.8 | 3.1 ± 0.8 | ||
| 1 month | 2.1 ± 1.2 | 2.3 ± 1.1 | 0.2 (–0.2 to 0.6) | 0.33 | |
| Pain Bothersomeness Index | Baseline | 2.9 ± 0.7 | 3.1 ± 0.8 | ||
| 1 month | 1.9 ± 1.1 | 2.1 ± 1.1 | 0.2 (–0.2 to 0.6) | 0.33 |
| Perceived pain | PVP | Control | Relative risk for ‘better’ compared with ‘no change’ or ‘worse’ (95% CI) |
|---|---|---|---|
| 1 week | n = 37 | n = 37 | |
| Better | 6 (16%) | 13 (35%) | 0.5 (0.2 to 1.1) |
| No change | 26 (70%) | 23 (62%) | |
| Worse | 5 (14%) | 1 (3%) | |
| 1 month | n = 35 | n = 38 | |
| Better | 12 (34%) | 9 (24%) | 1.5 (0.7 to 3.0) |
| No change | 21 (60%) | 20 (53%) | |
| Worse | 2 (6%) | 9 (24%) | |
| 3 months | n = 36 | n = 37 | |
| Better | 14 (39%) | 12 (32%) | 1.2 (0.6 to 2.2) |
| No change | 19 (53%) | 18 (49%) | |
| Worse | 3 (8%) | 7 (19%) | |
| 6 months | n = 35 | n = 36 | |
| Better | 16 (46%) | 15 (42%) | 1.1 (0.6 to 1.9) |
| No change | 12 (34%) | 16 (44%) | |
| Worse | 7 (20%) | 5 (14%) |
| Time point | No treatment | Minor analgesics | Minor opiate derivatives | Major opiate derivatives | ||||
|---|---|---|---|---|---|---|---|---|
| PVP | Control | PVP | Control | PVP | Control | PVP | Control | |
| Baseline | 9 (14%) | 12 (20%) | 8 (12%) | 17 (28%) | 19 (30%) | 17 (28%) | 28 (44%) | 14 (23%) |
| 2 weeks | 13 (23%) | 12 (21%) | 10 (18%) | 10 (17%) | 13 (23%) | 19 (33%) | 20 (36%) | 17 (29%) |
| 2 months | 15 (29%) | 16 (29%) | 7 (13%) | 7 (13%) | 14 (27%) | 16 (29%) | 16 (31%) | 17 (30%) |
| 6 months | 17 (35%) | 13 (25%) | 6 (12%) | 8 (15%) | 8 (16%) | 14 (27%) | 18 (37%) | 17 (33%) |
| 12 months | 14 (34%) | 17 (40%) | 5 (12%) | 8 (19%) | 7 (17%) | 10 (24%) | 15 (37%) | 7 (17%) |
| PVP (n = 18) | Control (n = 16) | Mean difference, intervention vs. control (95% CI) (negative values favour intervention) | |
|---|---|---|---|
| Pain medication at baseline | |||
| None | 2 (11%) | 1 (6%) | |
| Paracetamol | 4 (22%) | 7 (44%) | |
| NSAIDs | 6 (33%) | 3 (19%) | |
| Opiate derivative | 6 (33%) | 5 (31%) | |
| Mean analgesic use score (range) | |||
| Baseline | 1.9 (0–3) | 1.7 (0–3) | |
| 1 day | 1.1 (0–3) | 2.5 (1–3) | |
| Change from baseline at 1 day | –0.8 | +0.8 | –1.6 (–2.3 to –0.8) |
| 2 weeks | 1.2 (0–3) | 2.6 (2–3) | |
| Change from baseline at 2 weeks | –0.7 | +0.9 | –1.5 (–2.3 to –0.8) |
| Analgesic | PVP (n = 95) | Control (n = 92) |
|---|---|---|
| None | 5 (5%) | 7 (8%) |
| Non-opiate drugs | 40 (42%) | 43 (47%) |
| Weak opiate derivatives | 31 (33%) | 22 (24%) |
| Strong opiate derivatives | 19 (20%) | 20 (22%) |
| Study | Time point | PVP | BKP | Control | Mean difference, intervention vs. control (95% CI) (positive values favour intervention) | p-value |
|---|---|---|---|---|---|---|
| Blasco 2012127 | Baseline | NR | NR | |||
| 12 months | NR | NR | ||||
| Change from baseline at 12 months | –0.27 (0.15) | –0.13 (0.17) | –0.14 a | NS | ||
| Farrokhi 2011135 | Baseline | 2.8 (1.5) | 2.5 (1.3) | |||
| 1 week | 3.2 (1.1) | 2.0 (1.0) | 1.2 (1.73 to 0.67) | < 0.011 | ||
| Change from baseline at 1 week | +0.4 (–0.18 to +0.98) | –0.5 (–1.00 to –0.004) | +0.9 (+0.14 to +1.66) | |||
| 6 months | 3.2 (1.1) | 1.9 (1.4) | 1.3 (2.05 to 0.55) | < 0.027 | ||
| Change from baseline at 6 months | +0.4 (–0.18 to +0.98) | –0.6 (–1.30 to +0.10) | +1.0 (+0.09 to +1.91) | |||
| 12 months | 3.2 (1.5) | 2.0 (1.2) | 1.2 (2.03 to 0.37) | < 0.001 | ||
| Change from baseline at 12 months | +0.4 (–0.27 to +1.07) | –0.5 (–1.04 to +0.04) | +0.9 (+0.04 to +1.76) | |||
| 24 months | 3.0 (1.5) | 2.1 (1.2) | 0.9 (1.75 to 0.05) | < 0.04 | ||
| Change from baseline at 24 months | +0.2 (–0.47 to +0.87) | –0.4 (–0.94 to +0.14) | +0.6 (–0.26 to +1.46) | |||
| 36 months | 3.0 (1.2) | 2.0 (1.0) | 2.0 (1.50 to 0.44) | < 0.01 | ||
| Change from baseline at 36 months | +0.2 (–0.41 to +0.81) | –0.5 (–1.003 to +0.003) | +0.7 (–0.09 to +1.49) | |||
| Liu 2010139 | Baseline | 1.01 (0.22) | 1.13 (0.34) | |||
| ‘Postoperative’ | 1.32 (0.26) | 2.04 (0.41) | < 0.001 | |||
| Change from baseline | +0.31 (+0.22 to +0.40) | +0.91 (+0.76 to +1.06) | Favours BKP: –0.60 (–0.78 to –0.43) |
| Study | Time point | PVP | BKP | Control | Mean difference, intervention vs. control (95% CI) (negative values favour intervention) | p-value |
|---|---|---|---|---|---|---|
| Farrokhi 2011135 | Baseline | 20.0 (5.5) | 21.0 (4.2) | |||
| 1 week | 10.0 (2.5) | 22.0 (2.2) | –12.0 (–12.96 to –11.04) | < 0.027 | ||
| Change from baseline at 1 week | –10.0 (–11.87 to –8.13) | +1.0 (–0.43 to +2.43) | –11.0 (–13.63 to –8.63) | |||
| 6 months | 10.1 (2.6) | 23.0 (2.1) | –13.0 (–13.73 to –11.37) | < 0.031 | ||
| Change from baseline at 6 months | –9.9 (–11.79 to –8.01) | +2.0 (+0.58 to +3.42) | –11.9 (–14.27 to –9.54) | |||
| 12 months | 10.0 (1.0) | 23.0 (2.0) | –13.0 (–13.47 to –12.53) | < 0.001 | ||
| Change from baseline at 12 months | –10.0 (–11.73 to –8.27) | +2.0 (+0.58 to +3.42) | –12.0 (–14.24 to –9.76) | |||
| 24 months | 9.0 (1.0) | 23.0 (2.3) | –14.0 (–14.53 to –13.57) | < 0.001 | ||
| Change from baseline at 24 months | –11.0 (–12.73 to –9.27) | +2.0 (+0.54 to +3.46) | –13.0 (–15.26 to –10.74) | |||
| 36 months | 8.9 (1.0) | 23.0 (2.0) | –14.0 (–14.96 to –13.04) | < 0.011 | ||
| Change from baseline at 36 months | –11.1 (–12.84 to –9.37) | +2.0 (+0.58 to +3.42) | –13.1 (–15.34 to –10.86) | |||
| FREE201 | Baseline | NR | NR | |||
| Postoperatively | NR | NR | ||||
| Change from baseline to ‘postoperatively’ | –3.3 (–2.4 to –4.2) | NR | ||||
| 24 months | NR | NR | ||||
| Change from baseline to 24 months | –3.1 | –0.8 | –2.3 a | 0.03 | ||
| Liu 2010139 | Baseline | 15.5 (4.2) | 17.0 (7.3) | |||
| ‘Postoperative’ | 12.2 (3.6) | 9.0 (5.7) | < 0.001 | |||
| Change from baseline | –3.3 (–5.63 to –0.97) | –8.0 (–9.87 to –6.23) | BKP vs. PVP: –4.7 (7.69 to –1.71) | < 0.001 |
| Height loss during follow-up | PVP (n = 136 vertebrae) | Control (n = 120 vertebrae) | p-value |
|---|---|---|---|
| None (0–3 mm) | 118 | 74 | < 0.001 |
| Moderate (4–7 mm) | 7 | 28 | |
| Severe (≥ 8 mm) | 4 | 11 |
| Study | PVP | BKP | Control | Relative risk (95% CI) | p-value |
|---|---|---|---|---|---|
| Length of follow-up 2 weeks | |||||
| VERTOS136 | 0/18 (0%) | N/A | 0/16 (0%) | Not calculable | |
| Length of follow-up 3 months | |||||
| INVEST103 | 0/68 (0%) | N/A | 0/63 (0%) | Not calculable | |
| Length of follow-up 6 months | |||||
| Buchbinder 2009102 | 2/38 (5.3%) (chest infection, oesophageal cancer) | N/A | 1/40 (2.5%) (acute MI) | 2.11 (0.20 to 22.28) | 0.54 |
| Length of follow-up 12 months | |||||
| Blasco 2012127 | 3/64 (4.7%) (cause not reported) | N/A | 6/61 (9.8%) (cause not reported) | 0.48 (0.12 to 1.82) | 0.28 |
| Rousing 2009128 | 1/25 (4.0%) (cause not reported) | N/A | 1/24 (4.2%) (cause not reported) | 0.96 (0.06 to 14.50) | 0.98 |
| VERTOS II17 | 5/101 (5.0%) (cardiac failure 4, old age 1) | N/A | 6/101 (6.0%) (old age 2, gastric bleeding 1, respiratory insufficiency 1, sepsis 1, cardiac failure 1) | 0.83 (0.26 to 2.64) | 0.76 |
| Length of follow-up 24 months | |||||
| FREE138 | N/A | 12/149 (8.1%) (cardiovascular 5, pneumonia 1, cancer 3, other 3) | 11/151 (7.3%) (cardiovascular 5, pneumonia 2, cancer 2, other 2) | 1.11 (0.50 to 2.43) | 0.80 |
| Length of follow-up 36 months | |||||
| Farrokhi 2011135 | 2/40 (5.0%) (MI) | N/A | 1/42 (2.4%) (cervical cancer) | 2.10 (0.20 to 22.26) | 0.54 |
Appendix 11 Registry data
US registry data
(Academic-in-confidence information has been removed.)
| Treatment | 12 months (95% CI) | 24 months (95% CI) | 48 months (95% CI) |
|---|---|---|---|
| Non-operated | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| PVP | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| BKP | AiC information has been removed | AiC information has been removed | AiC information has been removed |
(Academic-in-confidence information has been removed.)
| 12 months (95% CI) | 24 months (95% CI) | 48 months (95% CI) | |
|---|---|---|---|
| Pneumonia | |||
| Non-operated | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| PVP | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| BKP | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Subsequent hospitalisation | |||
| Non-operated | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| PVP | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| BKP | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| MI/cardiac complications | |||
| Non-operated | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| PVP | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| BKP | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Pulmonary/respiratory complications | |||
| Non-operated | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| PVP | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| BKP | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Pulmonary embolism | |||
| Non-operated | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| PVP | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| BKP | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| DVT | |||
| Non-operated | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| PVP | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| BKP | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| UTI | |||
| Non-operated | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| PVP | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| BKP | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Infection | |||
| Non-operated | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| PVP | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| BKP | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| Subsequent VCF with repair | |||
| Non-operated | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| PVP | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| BKP | AiC information has been removed | AiC information has been removed | AiC information has been removed |
(Academic-in-confidence information has been removed.)
| BKP | VP | ||
|---|---|---|---|
| Pneumonia | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | ||
| Subsequent VCF with repair | AiC information has been removed | AiC information has been removed | |
| Subsequent hospitalisation | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | ||
| MI/cardiac complications | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | ||
| Pulmonary/respiratory complications | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | ||
| Pulmonary embolism | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | ||
| DVT | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | ||
| UTI | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | ||
| Infection | AiC information has been removed | AiC information has been removed | AiC information has been removed |
| AiC information has been removed | AiC information has been removed | ||
German registry data
(Academic-in-confidence information has been removed.)
Appendix 12 Longitudinal pain trends
Visual analogue scale data
FIGURE 73.
(Academic-in-confidence information has been removed.)
Appendix 13 Review of observational studies: estimating mortality differences between treatment for vertebral compression fractures
Background: estimating treatment effects using observational data
The standard problem in treatment evaluation requires the estimation of a causal relationship between the treatment and the outcome. When using observational data, no individual is observed in both the treated and non-treated state and therefore the counterfactual is not observed. Instead, a comparison group is generated usually from the same data source as the treated group and used as a control group to estimate the treatment effect. An intuitive and non-technical description of the methods used to evaluate treatment effects when using observational data is found below. A more technical account of the different methods and assumptions used can be found in Cameron and Trivedi. 401
Different estimators place restrictions on the counterfactuals that can be identified and, hence, on the treatment effects that can be consistently estimated. There are several treatment effects that might be of interest depending on the evaluation, such as the average treatment effect (ATE), the average treatment effect for the treated (ATET) and the average treatment effect for the untreated (ATEU). ATE calculates the expected effect of the treatment if individuals in the population under consideration were randomly allocated to treatment. Depending on the specific evaluation, ATE may or may not be the appropriate treatment effect as it is relevant in cases where the treatment is applicable to the entire population represented by the sample data. ATET is relevant when the interest lies on the effect of the treatment for those who are treated and ATEU is relevant when interest lies on the effect of the treatment for those who have not taken the treatment.
Unlike randomised controlled trials, observational data are generated in an uncontrolled environment which makes it more difficult to identify the causal relationship of interest. Random assignment to treatment implies that no person is assigned to the treatment on the grounds that the expected benefit is large. In observational data the non-random treatment assignment complicates the estimation of the treatment effect because of selection bias. Selection bias is the difference in the base state between the treated and the control groups and arises when the treatment variable is correlated with the error in the outcome equation. This correlation could be owing to incorrectly omitting observable variables that partly determine both the treatment and the outcome (selection on observables) or to the presence of unobserved factors that partly determine both the treatment and the outcome (selection on unobservables).
The problem of selection on observables can be dealt with by using regression and matching methods. When using regression, the estimated outcome equation needs to include all variables that could be correlated with the error term so there are no omitted observable variables that partly determine the treatment and the outcome. A well-specified regression equation identifies the ATE parameter. This method is easy to implement but it usually needs a large set of controls. Furthermore, the functional form employed tends to be quite restrictive and usually assumes that the functions for the treated and non-treated groups conditional on covariates are the same apart from an additional intercept component.
Matching estimators can be used instead of regression to deal with selection on observables. Matching avoids the need of the strong functional form assumptions embedded in a regression model by identifying data from a set of potential comparison individuals (not necessarily from the same population as the treated individuals) with observable characteristics that match those of the treated units up to some specified level of proximity. Matching can be exact or inexact. Exact matching is only possible when there is a small number of discrete covariates and the sample includes a large number of observations for each set of possible covariate values. Inexact matching methods generally use a scalar as the basis for matching individuals. This scalar is obtained as a function of the covariates. The propensity score402 is a popular inexact matching method and it is defined as the conditional probability of receiving treatment given the covariates. To implement matching based on propensity scores, decisions need to be made about whether to match with or without replacement, the number of individuals to use in the control group and what matching method to use. Matching without replacement implies that each observation in the control group is matched to, at most, one treated observation. Matching with replacement allows multiple matches. If the comparison group is small, the matches may not be very close in terms of the propensity score, especially if there is no replacement which will increase the bias of the estimator. When choosing the number of individuals to use in the control group, one needs to take into account the trade-off between the bias and the variance of the estimator. The bias is reduced if one uses only the closest match and the variance of the estimator decreases by using more matched control subjects. However, when using more than one control the bias increases if the extra matches are worse than the first. A compromise solution is to use only matches within a specified radius (calliper matching). In addition, matching is sensitive to the matching method used and the size of the comparison group as well as the amount of overlap between the treated and comparison group. Matching methods are able to identify the ATET parameter.
Matching is more complex than regression and choices about matching methods need to be made and justified on a case-by-case basis. Matching assumes that the treatment does not indirectly affect untreated individuals (selection into treatment on observables only) and, thus, requires a good understanding of the process of selection into treatment in each data set. Matching works best when there exists a rich set of covariates to model this selection: that is, the propensity score. It also assumes that the treated and non-treated groups have comparable observable characteristics (support condition) and needs a large number of potential control subjects.
Neither regression nor matching methods can identify the parameter of interest if there is selection on unobservables, also referred to as endogeneity of the treatment variable. Endogeneity occurs when a variable which is correlated with the treatment is omitted from the regression. The omission of the variable might be an oversight, or owing to the variable not being included within the data set, or because the variable cannot be measured. The omitted variable is therefore included in the error term and, thus, there is an association between the error term and treatment leading to inconsistent estimates of the parameter of interest. In other words, the regression estimate of the parameter measures only the association between the treatment and the outcome rather than the size and direction of the effect. It is often the case that the treatment has a positive effect on the outcome and the unobservable variable is positively correlated with the treatment. This implies that the magnitude of the association obtained using standard regression methods is larger than the true causal effect; that is, the treatment appears more effective than it really is once endogeneity is fully taken into account. In cases like this, instrumental variable (IV) methods are needed to identify a parameter related to the ATE, the local ATE (LATE). The strategy is to choose a variable (the IV) that is correlated with the treatment but only correlated with the outcome through its effect on the treatment. Then the variation on this variable can be used to identify the true causal effect due to the treatment only. The estimated LATE is local, because it measures only the effect of the treatment on those who are induced to take the treatment by the change in the IV. The LATE depends on the particular instrument chosen and also on the values of the IV that are used in the estimation. The essential conditions for IV methods to be able to identify the parameter of interest are that the IV is uncorrelated with the error term (also termed exogenous) and that it is correlated with the treatment. If the IV is not strongly correlated with the treatment variable, then the instrument is a weak instrument and the model is said to be weakly identified. It is extremely important that an instrument is exogenous if the instrument is weak. Even mild endogeneity of the instrument can lead to IV parameter estimates that are more inconsistent than those obtained by methods which do not take into account selection on unobservables. Weak instruments in general can also lead to a loss of precision, especially on the coefficients of the endogenous variables.
There is no test possible of the hypothesis that the instrument is uncorrelated with the error term. Therefore, exogeneity of the instrument is a subjective decision and has to be justified on theoretical grounds. This often leads to disagreement on instrument validity.
Instrumental variable methods are relatively easy to implement but they do involve restrictive functional form and identifying assumptions. In practice, however, good exogenous IVs are difficult to find.
Review of included studies
Summary
There were four studies submitted which analysed data relating to mortality differences between non-operated vertebral fracture patients receiving only OPM and OP patients. Further, the group of OP patients is split into patients who undergo PVP and those who undergo BKP. All four studies use observational data: two of them use the Medicare national inpatient and outpatient claims database in the USA, and the other two use data from the AOK Niedersachsen health insurance fund in Germany. A variety of methods are used, including Cox regression, matching methods and IV estimation. The results involved paired comparisons between different groups rather than simultaneous comparisons of the three treatments. Only three of the papers report results for the group of patients with osteoporotic vertebral compression fractures (OVCF): one using the Medicare database and two using the German health insurance fund data. Patients who undergo BKP are found to have lower mortality rates than patients who undergo PVP and both groups of operated patients are found to have lower mortality rates than patients who receive OPM. The extent of the difference as well as the level of significance depends on the data set and methods used, although the difference between the groups of OP patients and OPM patients is always significant.
Detailed review
Edidin et al. 213 use the Medicare inpatient and outpatient claims between 1 January 2005 and 31 December 2008 in the USA. They analyse the sample of individuals who are 65 or older who did not have a history of VCFs, defined as a VCF diagnosis in the preceding year. After a few other minor exclusions, the final sample size is 858,978 of VCF patients, which amounts to 85.3% of the total sample of patients with vertebral fractures. A total of 182,946 (21.3%) patients were operated on and, of these, 119,253 (13.9%) had a BKP and 63,693 (7.4%) had a PVP. Thus, the number receiving BKP is almost twice the number receiving PVP in this data set.
Methods: the methods employed include Cox regression (including subgroup analysis for different comorbidities, and patients who survived at least 1 year following their VCF) and IV methods (two-step procedure) with a follow-up limited to 3 years owing to data requirements for the IV) for differences in mortality. The paper uses a large number of covariates: age; race/ethnicity; patient health status; (for general health: Charlson comorbidity index groups; for condition-specific: 12 comorbidities that have been identified previously as possible causes of death associated with VCFs: arterial disease; chronic obstructive pulmonary disease; cancer; diabetes; hip fracture; hypertensive disease; ischemic heart disease; other heart disease; pneumonia; pulmonary heart disease; stroke; wrist fracture); type of diagnosed fracture (pathologic, traumatic); site of service (outpatient, inpatient); physician specialty (orthopaedic surgeon, neurosurgeon, interventional radiologist, others); socioeconomic status (per-capita income for county of residence and Medicare buy-in status); year of diagnosis; and census region (Northeast, Midwest, South and West). The paper also considers four different IVs but only one is finally used: physician preference. Hospital preference, census region and physician specialty were also given consideration to be used as IV but judged as inappropriate.
Results: using Cox regression, a significant difference in survival at 4 years is found between OP and OPM patients as well as between BKP and OPM, PVP and OPM, and BKP and PVP patients ( Table 141 ). Groups with specific comorbidities were analysed separately and the treatment effects were found to be similar. In addition, the subsample of patients who survived 1 year after the operation was analysed and although the differences in survival were reduced, they did not completely disappear. Using IV methods and using only the sample of operated patients, they find a relative increase in survival for BKP compared with PVP of 11.82% (at 3 years).
Comments: the analysis uses a sample with both traumatic and osteoporotic fractures and thus assumes that the treatment effect, as well as the rest of the estimated parameters, is the same for both groups apart from a differential intercept term. In the discussion, it is stated that ‘it remains problematic to attribute a causal relationship between operative treatment and improved patient survival based solely on the results of this study’ (p. 1623). This is a fair comment and the sensitivity of the results to the assumptions underlying the methods employed should be explored to establish causality and rule out a simple association.
There are some counterintuitive significant associations in the Cox regressions. Arterial disease (significant in the analysis of OPM vs. OP patients but insignificant in the analysis of BKP vs. PVP), diabetes, hypertension, ischemic heart disease, stroke and wrist fracture were associated with lower mortality risk which might signal missing variables in the regression. Factors such as obesity and smoking were not included owing to lack of data; however, as long as they are not correlated with the choice of treatment it should not significantly affect the estimated treatment effect. However, if the missing factors are correlated with the treatment variable, the IV estimator would be a better estimator of the causal effect as long as the IV is exogenous. Unfortunately, the IV used might not be exogenous. Although it is stated that the instruments were ‘tested’ in terms of their correlation with treatment and survival, there is no such test, and that is the reason why it is very important to be explicit about the correlation between the IVs considered and the treatment and outcome as this is a subjective decision. It would have been useful to see more information in order to be able to judge its appropriateness. Some of the instruments that were considered (census region and physician specialty) were included in the regressions, which would immediately invalidate them as IVs. A condition for the instrument used, physician preference, to be a good IV is that it should be correlated with the treatment but only correlated with survival through its effect on the selection of treatment. If physician preference of treatment happens because the physician is more likely to get a higher success rate and fewer complications in the operation, this variable would not be considered a valid IV. This may well be the case in practice in the UK (David Wilson, Consultant Musculoskeletal Interventional Radiologist, Oxford University Hospitals, 2012, personal communication).
Exponent214 also uses the Medicare inpatient and outpatient claims data in the USA but adds an extra year, using data between 1 January 2005 and 31 December 2009. (Academic-in-confidence information has been removed.)
(Academic-in-confidence information has been removed.)
(Academic-in-confidence information has been removed.)
(Academic-in-confidence information has been removed.)
(Academic-in-confidence information has been removed.)
(Academic-in-confidence information has been removed.)
(Academic-in-confidence information has been removed.)
(Academic-in-confidence information has been removed.)
(Academic-in-confidence information has been removed.)
(Academic-in-confidence information has been removed.)
(Academic-in-confidence information has been removed.)
(Academic-in-confidence information has been removed.)
(Academic-in-confidence information has been removed.)
Summing up, it is possible that there is a causal difference in mortality between patients treated using OPM and OP patients given the size of the effect. Appropriately taking into account the potential endogeneity of the treatment would tend to reduce the point estimate of the effect size but may or may not eliminate it completely. It is not possible to say with certainty if there is a difference in mortality between patients undergoing BKP and PVP owing to the treatment based on the data presented in the studies included here.
| Edidin et al. (2011):213 mortality risk 4 years | Group | Comparison | Cox regression adjusted HR (95% CI) |
IV at 3 years relative increase in survival |
|
|---|---|---|---|---|---|
| All | OP vs. OPM | 0.63 (0.62 to 0.64) | |||
| BKP vs. OPM | 0.56 (0.55 to 0.57) | ||||
| PVP vs. OPM | 0.76 (0.75 to 0.77) | ||||
| BKP vs. PVP | 0.77 (0.75 to 0.78) | ||||
| Survival > 1 | OP vs. OPM | 0.82 (0.81 to 0.84) | |||
| BKP vs. OPM | 0.76 (0.74 to 0.77) | ||||
| PVP vs. OPM | 0.93 (0.91 to 0.95) | ||||
| BKP vs. PVP | 0.82 (0.80 to 0.85) | ||||
| Operated | BKP vs. PVP | 11.82% | |||
| Exponent (2012):214 mortality risk 5 years | Group | Comparison | Cox regression adjusted HR (95% CI) |
Propensity score matching and Cox regression HR (95% CI) | |
| All | OPM vs. OPa | AiC information has been removed | AiC information has been removed | ||
| OPM vs. BKP | AiC information has been removed | AiC information has been removed | |||
| OPM vs. PVP | AiC information has been removed | AiC information has been removed | |||
| BKP vs. PVP | AiC information has been removed | AiC information has been removed | |||
| OVCF | OPM vs. OPa | AiC information has been removed | AiC information has been removed | ||
| OPM vs. BKP | AiC information has been removed | AiC information has been removed | |||
| OPM vs. PVP | AiC information has been removed | AiC information has been removed | |||
| BKP vs. PVP | AiC information has been removed | AiC information has been removed | |||
| OVCF Survival > 1 |
OPM vs. OPa | AiC information has been removed | AiC information has been removed | ||
| OPM vs. BKP | AiC information has been removed | AiC information has been removed | |||
| OPM vs. PVP | AiC information has been removed | AiC information has been removed | |||
| BKP vs. PVP | AiC information has been removed | AiC information has been removed | |||
| Lange and Braun (2012):215,216 mortality risk 5 years | Group | Comparison | Cox regression adjusted HR (95% CI) |
Propensity score matching difference in survival rates % (p-value) |
Propensity score matching and Cox regression HR (95% CI) |
| OVCF | OP vs. OPM | AiC information has been removed | |||
| BKP vs. PVP | AiC information has been removed | AiC information has been removed | AiC information has been removed | ||
| OVCF Survival > 1 |
OP vs. OPM | AiC information has been removed | |||
| BKP vs. PVP | AiC information has been removed |
Appendix 14 Mixed-treatment comparison of mean difference in visual analogue scale during stable period
Summary
Data consist of the mean VAS at various time points throughout a period of stable pain (after the treatment effect is assumed to have operated), from 4.35 to 156.54 weeks.
A simple analysis of the mean difference in VAS scores over this period was carried out, where the data inputs are the averages of the means reported at all time points and their variances. However, the averaging needs to account for the correlation in the observations at different time points. This correlation was assumed to be 0.87 and constant over time. If other sources of information on the within-study correlation at different time points become available, the calculations can easily be redone.
We describe the method used to impute the within-trial correlations at different time points within the same trial, and for calculating the average and variance of correlated outcomes. The resulting averages and variances are used as data inputs into a standard MTC model in WinBUGS. Results from fixed effects (FE) and random effects (RE) models are described and the FE model is recommended.
There is potential for inconsistency in one loop in this network, but no evidence of inconsistency was found.
Data
Data on mean VAS score are available from eight trials, comparing four treatments.
Treatments were coded 1 to 4 ( Table 142 ), the data available are described in table and the network diagram is presented in Figure 81 . OPM was chosen as the overall baseline, or reference treatment.
Methods
Calculating the mean and variance of all means in stable period
For each arm of each study in Table 143 , let y j be the mean VAS score at time point j and s j the standard error of the observations at time point j. For a given study, reporting at J time points (J ≥ 1), we have a vector of observations Y, such that,
where m is a vector of unknown means and V is the variance–covariance matrix, assumed known. Letting Z represent a linear combination of the elements of Y, such that,
where
we have,
For each arm of each study, V has in its diagonal the variances of the mean at each time point, S j 2 , and the off-diagonal elements in row i, column j, will hold ρs j s i , where ρρ = 0.87 and independent of the time lag between observations i and j.
Repeating this method for all arms of each study, we get Z = y i , k * the average of the mean VAS score in arm k of study i, (i = 1,. . .,16, k = 1,2) with variances calculated using equation (4).
The transformed data, on which the MTC will be carried out, are given in Table 144 .
Relative effects model
The data in Table 144 were used to conduct a MTC, using the model and corresponding WinBUGS code in Dias et al. (2011, section 3.4). 403
Briefly, the transformed means are assumed to be normally distributed, so that the likelihood can be written as:
The parameter of interest is the mean, θ ik , of this continuous measure which is unconstrained on the real line. The model can be written as:
with
where d t i 1 , t i k represents the mean effect of the treatment in arm k in trial i, t ik , compared with the treatment in arm 1 of trial i, t i 1 and τ 2 represents the between-trial variability in treatment effects (heterogeneity). Under the exchangeability (consistency) assumption we can write:
For a FE model we replace equation (6) with:
Non-informative N(0,1002) priors are given to the μs and ds. In a RE model a Uniform(0,10) prior was used for τ.
Results
Model fit statistics for the FE and RE models are given in Table 145 . Although the RE model has a slightly better fit, this is at the expense of more parameters, and the DIC does not favour any of the models. We will, therefore, prefer the FE model, owing to its simplicity and easier interpretation. However, a re-examination of the Blasco study (study 1) is recommended as it is showing a relatively poor fit – this appears to be because it is the only study comparing OPM with vertebroplasty and (marginally) favouring OPM, while all other trials favour vertebroplasty.
A plot of the effects (mean differences) of all treatments relative to each other is given in Figure 82 . Differences > 0 favour the lowest numbered treatment.
Vertebroplasty and kyphoplasty (treatments 2 and 3) appear to be the best treatments, although the differences are small (about 1 point) and may not be clinically significant.
Consistency
There is only one evidence loop in this network (see Figure 81 ) formed by treatments 1, 2 and 3. Consistency was checked by comparing the treatment effects obtained from separate pairwise meta-analysis for each pair of treatments using the Bucher approach as recommended in Dias et al. 404 No evidence of inconsistency was found (Bayesian p-value > 0.8).
| Treatment code | Treatment name | Treatment code | Treatment name |
|---|---|---|---|
| 1 | OPM | 3 | Kyphoplasty |
| 2 | Vertebroplasty | 4 | OPLA |
| ID | Weeks | T1 | T2 | T1 | T2 | Trial | ||
|---|---|---|---|---|---|---|---|---|
| Mean | SE | Mean | SE | |||||
| 1 | 8.70 | 1 | 2 | N/A | N/A | 4.13 | 0.41 | Blasco127 |
| 1 | 26.09 | 1 | 2 | 4.30 | 0.38 | 4.72 | 0.36 | Blasco127 |
| 1 | 52.18 | 1 | 2 | 4.32 | 0.37 | 4.49 | 0.39 | Blasco127 |
| 2 | 4.35 | 2 | 4 | 4.94 | 0.38 | 5.40 | 0.50 | Buchbinder102 |
| 2 | 13.04 | 2 | 4 | 4.75 | 0.41 | 5.16 | 0.50 | Buchbinder102 |
| 2 | 26.09 | 2 | 4 | 4.97 | 0.47 | 4.86 | 0.44 | Buchbinder102 |
| 2 | 52.18 | 2 | 4 | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | Buchbinder102 |
| 2 | 104.36 | 2 | 4 | AiC information has been removed | AiC information has been removed | AiC information has been removed | AiC information has been removed | Buchbinder102 |
| 3 | 8.70 | 1 | 2 | N/A | N/A | 3.20 | 0.33 | Farrokhi135 |
| 3 | 26.09 | 1 | 2 | 4.10 | 0.26 | 2.20 | 0.30 | Farrokhi135 |
| 3 | 52.18 | 1 | 2 | 4.10 | 0.32 | 2.20 | 0.29 | Farrokhi135 |
| 3 | 104.36 | 1 | 2 | 3.70 | 0.36 | 2.80 | 0.28 | Farrokhi135 |
| 3 | 156.54 | 1 | 2 | 3.70 | 0.55 | 1.80 | 0.22 | Farrokhi135 |
| 4 | 4.35 | 1 | 3 | N/A | N/A | 3.52 | 0.20 | FREE137 |
| 4 | 13.04 | 1 | 3 | 4.52 | 0.21 | 2.93 | 0.20 | FREE137 |
| 4 | 26.09 | 1 | 3 | 4.35 | 0.21 | 2.73 | 0.20 | FREE137 |
| 4 | 52.18 | 1 | 3 | 3.79 | 0.22 | 2.81 | 0.20 | FREE137 |
| 4 | 104.36 | 1 | 3 | 3.65 | 0.21 | 2.82 | 0.21 | FREE137 |
| 5 | 4.35 | 2 | 4 | 3.90 | 0.35 | 4.60 | 0.38 | INVEST147 |
| 6 | 13.04 | 1 | 2 | 2.60 | 0.71 | 1.80 | 0.51 | Rousing128 |
| 6 | 52.18 | 1 | 2 | 2.90 | 0.64 | 2.00 | 0.48 | Rousing128 |
| 7 | 4.35 | 1 | 2 | N/A | N/A | 2.50 | 0.26 | VERTOS II17 |
| 7 | 13.04 | 1 | 2 | 3.90 | 0.30 | 2.50 | 0.28 | VERTOS II17 |
| 7 | 26.09 | 1 | 2 | 3.90 | 0.32 | 2.30 | 0.29 | VERTOS II17 |
| 7 | 52.18 | 1 | 2 | 3.80 | 0.32 | 2.20 | 0.29 | VERTOS II17 |
| 8 | 26.09 | 2 | 3 | 2.60 | 0.08 | 2.60 | 0.08 | Liu139 |
| Treatments | Number of arms | Data | RefID | ||||
|---|---|---|---|---|---|---|---|
| Arm 1 | Arm 2 | Arm 1 | Arm 2 | ||||
| t1 | t2 | na[] | y | Var1 | y2 | Var2 | |
| 1 | 2 | 2 | 4.310 | 0.131 | 4.447 | 0.137 | Blasco |
| 2 | 4 | 2 | 4.702 | 0.161 | 5.114 | 0.190 | Buchbinder |
| 1 | 2 | 2 | 3.900 | 0.126 | 2.440 | 0.072 | Farrokhi |
| 1 | 3 | 2 | 4.078 | 0.041 | 2.962 | 0.037 | FREE |
| 2 | 4 | 2 | 3.900 | 0.123 | 4.600 | 0.144 | INVEST |
| 1 | 2 | 2 | 2.750 | 0.426 | 1.900 | 0.229 | Rousing |
| 1 | 2 | 2 | 3.867 | 0.090 | 2.375 | 0.071 | VERTOS II |
| 2 | 3 | 2 | 2.600 | 0.006 | 2.600 | 0.006 | Liu |
| resdeva | pD | DIC | Heterogeneity (τ) | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Median | CrI | ||||
| RE | 16.3 | 13.8 | 30.1 | 0.52 | 0.46 | 0.42 | (0.02 to1.64) |
| FE | 18.5 | 11.0 | 29.5 | ||||
FIGURE 81.
Treatment network for mean VAS score in stable period.

FIGURE 82.
Plot of mean differences of all treatments relative to each other. Values to the right of the vertical (green) line favour the lowest numbered treatment. Treatment codes are given in Table 142 .

Glossary
- Bone mineral density
- A measure of the strength of the bones, ascertained by calcium content.
- Kyphosis
- Abnormal curvature of the spine.
- Minimal clinically important difference
- The smallest change in an outcome measure which reflects a change in symptom which can be considered important to patients.
- Osteopenia
- A condition in which bone density is lower than the average in the healthy young population. Diagnosis requires a T-score between –1.0 and –2.5.
- Osteoporosis
- A severe loss of bone mineral density and deterioration of bone microarchitecture. Diagnosis requires a T-score below –2.5.
- Parapesia
- Motor weakness, especially of the legs.
- Radiculopathy
- Pressure on, or other damage to, a nerve root.
- T-score
- The number of standard deviations of an individual’s bone density above or below the bone mineral density of a healthy 30-year-old matched for sex and ethnicity.
- Vertebral augmentation
- The addition of cement into a vertebra affected by a compression fracture in an attempt to stabilise it, and in some cases also to reduce the compression. Vertebral augmentation is a generic term which embraces both percutaneous vertebroplasty and percutaneous balloon kyphoplasty.
- Z-score
- The number of standard deviations that a woman is from the average bone mineral density of women of the same age.
List of abbreviations
- ATE
- average treatment effect
- ATET
- average treatment effect for the treated
- ATEU
- average treatment effect for the untreated
- AQoL
- Assessment of Quality of Life (scale)
- BKP
- balloon kyphoplasty
- BMD
- bone mineral density
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CIRSE
- Cardiovascular and Interventional Radiology Society of Europe
- CT
- computed tomography
- DIC
- deviance information criterion
- DPQ
- Dallas Pain Questionnaire
- DVT
- deep-vein thrombosis
- DXA
- dual X-ray absorptiometry
- EQ-5D
- European Quality of Life-5 Dimensions
- FDA
- Food and Drug Administration
- FE
- fixed effects
- HES
- Hospital Episode Statistics
- HR
- hazard ratio
- HRG
- Healthcare Resource Group
- HRQoL
- health-related quality of life
- HRT
- hormone replacement therapy
- ICER
- incremental cost-effectiveness ratio
- IPD
- individual patient data
- IPG
- Interventional Procedure Guideline
- LATE
- local average treatment effect
- MCID
- minimal clinically important difference
- MCS
- mental component score
- MMSE
- Mini Mental State Examination
- MRI
- magnetic resonance imaging
- NASS
- North American Spine Society
- NCT
- National Clinical Trials
- NICE
- National Institute for Health and Care Excellence
- NIM
- non-invasive management
- NMB
- net monetary benefit
- NSAID
- non-steroidal anti-inflammatory drug
- ODI
- Oswestry Disability Index
- OPLA
- operative placebo with local anaesthesia
- OPM
- optimal pain management
- OR
- odds ratio
- PCS
- physical component score
- PMMA
- polymethylmethacrylate
- PVP
- percutaneous vertebroplasty
- QALY
- quality-adjusted life-year
- QUALEFFO
- Quality of Life Questionnaire of the European Foundation for Osteoporosis
- RCT
- randomised controlled trial
- RDQ
- Roland–Morris Disability Questionnaire
- RE
- random effects
- ScHARR
- School of Health and Related Research
- SD
- standard deviation
- SF-36
- Short Form questionnaire-36 items
- SOF
- Study of Osteoporotic Fractures
- SOF-ADL
- Study of Osteoporotic Fractures-Activities of Daily Living (questionnaire)
- UTI
- urinary tract infection
- VAS
- visual analogue scale
- VBB
- vertebral body balloon
- VBH
- vertebral body height
- VCF
- vertebral compression fracture
Note
This monograph is based on the Technology Assessment Report produced for NICE. The full report contained a considerable number of data that were deemed academic-in-confidence or commercial-in-confidence. The full report was used by the Appraisal Committee at NICE in their deliberations. The full report with each piece of academic-in-confidence and commercial-in-confidence data removed and replaced by the statement ‘academic-in-confidence/commercial-in-confidence information (or data) removed’ is available on the NICE website: www.nice.org.uk.
The present monograph presents as full a version of the report as is possible while retaining readability, but some sections, sentences, tables and figures have been removed. Readers should bear in mind that the discussion, conclusions and implications for practice and research are based on all the data considered in the original full NICE report.