Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/32/01. The contractual start date was in June 2012. The draft report began editorial review in August 2012 and was accepted for publication in February 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Whiting et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Description of the health problem
Cystic fibrosis (CF) is the most common, life-threatening, autosomal recessive disorder in Caucasian populations; it has an estimated carrier rate of 1 in 25 and incidence of 1 in 2500 live births. 1 It affects around 9000 people in the UK with a prevalence of 1.37 in 10,000. 2 CF was first recognised as a distinct disease in 1938. 3 It is characterised by abnormal transport of chloride and sodium, leading to thick viscous secretions in the lungs, pancreas, liver, intestine and reproductive tract, and to an increased salt content in sweat gland secretions. 4 Most of the morbidity and mortality is from pulmonary disease, which is characterised by bronchial and bronchiolar obstruction with thick tenacious secretions that are difficult to clear, colonisation by pathogenic bacteria and repeated infections. 1 There is chronic inflammation and progressive lung destruction can lead to bronchiectasis, altered pulmonary function and respiratory failure. CF can also lead to CF-related diabetes (CFRD), male infertility and liver involvement. In addition to repeated chest infections, symptoms of CF can include a troublesome cough, prolonged diarrhoea and poor weight gain. 1 The treatment burden associated with this condition is significant. Patients undertake a minimum of twice-daily chest physiotherapy frequently augmented by nebulised therapies to aid sputum clearance, take prophylactic antibiotics both orally and nebulised twice daily, take fat-soluble vitamins, take pancreatic enzyme supplements owing to pancreatic insufficiency with every fat-containing meal, and live as normal a life as possible. All of these therapies are time-consuming and are non-curative. The recurrent chest infections from which this group suffer are severe and prolonged, resulting in long courses of antibiotics, often intravenous (i.v.), and ultimately irreversible lung damage. The benefits of this regime are seen only if the treatments are adhered to which, given their burden, is not always the case. Most patients with CF eventually succumb to lung disease; however, the life expectancy of patients with CF is currently around 30 years, a considerable increase from around 6 months when the disease was first identified,4 and is expected to increase to at least 50 years for children born in 2000. 2
Cystic fibrosis is caused by mutations in the CF transmembrane conductance regulator (CFTR) gene, which was discovered in 1989. 5 It sits on chromosome 7, is some 250 kb in length, and encodes a protein of 1480 amino acids. This protein is a chloride channel present at the surface of epithelial cells in multiple organs and is responsible for aiding in the regulation of salt and water absorption and secretion. Over 1000 disease-causing alleles within this gene have been identified, although only 23 have been demonstrated to cause sufficient loss of CFTR function to confer CF disease. 6 The most common mutation is the ∆F508 mutation which is present on around 67% of CF chromosomes worldwide. 7 The G551D (glycine to aspartate change in nucleotide 1784 in exon 11) mutation, which affects approximately 5.7% of patients with CF in the UK,8 is of interest as a new treatment has been developed targeted specifically at patients with this mutation. CFTR protein channels with the G551D mutation have a greatly reduced fraction of time that the channel spends in the open state, or ‘open probability’, and, therefore, have limited chloride transport ability.
Diagnosis of cystic fibrosis and genetic testing
Cystic fibrosis can be diagnosed through the sweat test, newborn screening or genetic testing. 6 The sweat test tests for elevated levels of chloride in sweat with a diagnosis of CF being made at levels above 60 mmol/l, and a possible diagnosis of CF at levels above 30 mmol/l. Newborn-screening tests have been introduced in many countries, and have been routine throughout the UK since October 2007. 9 These involve a small sample of blood being taken (‘heel prick test’) which is tested for high levels of immunoreactive trypsinogen (IRT). If an abnormal IRT value is identified, most newborn-screening programmes perform a combination of deoxyribonucleic acid (DNA) testing to identify known CFTR mutations and repeat IRT testing. 10 IRT testing alone has a sensitivity of 82–100%, double IRT testing increases sensitivity to 89–100%, and IRT and DNA testing has a sensitivity of 94–100%; specificity is > 99% for all testing strategies. 11 In the UK screening programme, the initial DNA test involves testing for four mutations (ΔF508, G551D, G542X and 621+1G>T); if only one CF mutation is detected, then further DNA analysis based on 29 or 31 mutations is recommended. A range of commercial kits are available for diagnostic testing. The diagnosis is then confirmed using the sweat test. 10
Treatment of cystic fibrosis
There is no cure for CF and current treatments generally target the complications rather than the cause of the disease. 4 Treatments can be broadly classified as nutritional repletion (e.g. pancreatic enzyme supplementation and nutritional supplementation), relief of airway obstruction (e.g. physiotherapy, drugs to improve sputum clearance, bronchodilators), treatment of airway infection (e.g. antibiotics), suppression of inflammation (e.g. steroids, high-dose ibuprofen) and lung transplantation. 4
Ivacaftor
Ivacaftor (Kalydeco®, Vertex Pharmaceuticals) is the first in a new class of drugs known as CFTR potentiators that represent a new therapeutic approach to the treatment of patients with CF by targeting the underlying protein defect of CF. The drug facilitates increased chloride transport by potentiating the channel-open probability (or gating) of the G551D-CFTR protein. 12
Ivacaftor is a designated orphan medicinal product. 13 It has been approved by the US Food and Drug Administration (FDA)14 and European Commission15 for the treatment of CF in patients aged ≥ 6 years who have the G551D mutation in the CFTR gene. No active comparator agents that target the underlying CFTR protein defect in CF disease exist. 16
Chapter 2 Objective
This review aims to appraise the clinical effectiveness and cost-effectiveness of ivacaftor (150 mg twice-daily tablet for oral administration) for the treatment of CF in patients aged ≥ 6 years who have at least one G551D mutation in the CFTR gene. We will aim to determine the category of patients most likely to benefit from ivacaftor by assessing whether or not the effects vary according to disease severity and age.
Chapter 3 Systematic review methods
Methods for assessing clinical effectiveness
We conducted a systematic review of the evidence on the clinical effectiveness of ivacaftor (150-mg tablet for oral administration twice daily) for the treatment of CF in patients aged ≥ 6 years who have at least one G551D mutation in the CFTR gene. The review followed the general principles recommended in the PRISMA statement and Centre for Reviews and Dissemination (CRD) report 4. 17,18
Identification of studies
Systematic searches were undertaken to locate randomised controlled trials (RCTs) assessing ivacaftor. Searches were not limited by date, language or publication status (unpublished or published). The following databases were searched from inception in May 2012 with searches updated in July 2012:
-
MEDLINE (OvidSP) 1946–April 2012 week 4
-
MEDLINE In-Process & Other Non-Indexed Citations (OvidSP) up to 2 May 2012
-
MEDLINE Daily Update (OvidSP) up to 2 May 2012
-
EMBASE (OvidSP) 1974–2012 week 17
-
Latin American and Caribbean Health Sciences Literature (LILACS) (Biblioteca Regional de Medicina) up to 4 May 2012
-
Cochrane Database of Systematic Reviews (CDSR) (Wiley Online Library) up to Issue 4:2012
-
Cochrane Central Register of Controlled Trials (CENTRAL) (Wiley Online Library) up to Issue 4:2012
-
*Database of Abstracts of Reviews of Effects (DARE) (Wiley Online Library) up to Issue 2:2012 (CRD) up to 3 May 2012
-
*NHS Economic Evaluation Database (NHS EED) (Wiley Online Library) up to Issue 2:2012 (CRD) up to 3 May 2012
-
*Health Technology Assessment (HTA) Database (Wiley Online Library) up to Issue 2:2012 (CRD) up to 3 May 2012.
*For completeness DARE, NHS EED and HTA databases were searched through both the Wiley Online Library and the CRD host sites.
The EMBASE strategies were independently peer reviewed by a second information specialist, using the Peer Review of Electronic Search Strategies Evidence-Based Checklist (PRESS-EBC). 19 Supplementary searches were undertaken to identify unpublished and ongoing studies on the following resources:
-
metaRegister of Controlled Trials (internet): www.controlled-trials.com
-
National Institutes of Health Clinicaltrials.gov (internet): www.clinicaltrials.gov
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (internet): www.who.int/ictrp/en/.
The following conference proceedings were searched, where possible, from 2007 until the most recent conference (up to July 2012):
-
European Cystic Fibrosis Society (ECFS) conference: www.ecfs.eu/conferences/main
-
North American Cystic Fibrosis Conference (NACFC): www.nacfconference.org/
-
International Congress on Pediatric Pulmonology (CIPP): www.cipp-meeting.org/index.htm.
The bibliographies of retrieved articles and relevant systematic reviews were screened for additional studies. Identified references were downloaded into EndNote bibliographic management software (Thomson Reuters, CA, USA) for de-duplication and then into Microsoft Access (Microsoft Corporation, Redmond, WA, USA) for further assessment and handling. Details of the search strategies can be found in Appendix 1.
Inclusion and exclusion criteria
Studies that fulfilled the following criteria were eligible for inclusion.
Population
Children (≥ 6 years) and adults with CF who had the G551D mutation on at least one CFTR allele. Patients with all severities of disease were eligible.
Intervention
Ivacaftor tablets.
Comparator
Any reported comparator.
Outcomes
The primary outcome was lung function [e.g. forced expiratory volume in 1 second (FEV1)]. Other eligible outcomes included mortality, weight, body mass index (BMI), sweat chloride, respiratory symptoms, reduction in pulmonary exacerbations, exercise tolerance, adverse effects of treatment, health-related quality of life (HRQoL) and utilisation of hospital resources.
Study design
For the review of clinical effectiveness, only RCTs were included. Criteria were relaxed for consideration of adverse events and longer-term outcomes (> 12 months), for which open-label studies were eligible. Studies that reported only short-term outcomes (< 3 months) were excluded.
The results of the searches were screened for relevance independently by two reviewers. The full text of studies identified as potentially relevant was obtained and assessed for inclusion by one reviewer and checked by a second. Disagreements were resolved through discussion or referral to a third reviewer where necessary.
Data extraction strategy
Data were extracted using a standardised data extraction form by one reviewer and checked by another. Disagreements were resolved through discussion or referral to a third reviewer where necessary. Data were extracted on the primary outcome, lung function, and the following additional outcomes, where reported: mortality, weight, BMI, sweat chloride, respiratory symptoms, reduction in pulmonary exacerbations, exercise tolerance, adverse effects of treatment, HRQoL and utilisation of hospital resources. Data were extracted for 24-week follow-up (intermediate) and after the longest duration of follow-up reported. If data were available for different patient subgroups (e.g. age, disease severity, region) then data were extracted separately for each subgroup. If composite end points were reported, data were extracted on the definition of the end point, results, and, if sufficient data were available, the events that contributed to the end point. There were some discrepancies in data reported in different sources. In such situations data were extracted from a single source based on the following hierarchy: supplementary results report > journal article > conference abstract > manufacturer’s dossier > press release. Details on discrepancies in figures reported in the different reports are summarised in Appendix 5.
Critical appraisal strategy
Trials were assessed for methodological quality using the Cochrane risk of bias tool. 20 This includes items covering selection bias (random sequence generation and allocation concealment), performance bias (participant blinding), detection bias (blinding of outcome assessors), attrition bias (incomplete outcome data) and reporting bias (selective reporting of results). Each domain was assigned a rating of high, low, or unclear. Each trial was assigned an overall rating of the risk of bias. If at least one of the domains was rated as ‘high’ the trial was considered at high risk of bias and if all domains were judged as ‘low’ the trial was considered at low risk of bias; otherwise, the trial was considered at ‘unclear’ risk of bias. The risk of bias assessment was incorporated into the data extraction form and was conducted as part of the data extraction process.
Methods of data synthesis
We did not have sufficient data to conduct a formal meta-analysis. Data were tabulated and discussed in a narrative review. Where possible, results were grouped by age, lung function, disease severity and prior treatment (including consideration of intolerance to treatments). Dichotomous data were summarised as relative risks (RRs) or hazard ratios together with 95% confidence intervals (CIs). If continuous data were normally distributed then mean differences (MDs) and 95% CIs were calculated; otherwise, we reported the results of non-parametric statistical analyses conducted by the study authors. Where sufficient data were available, results were displayed graphically using forest plots. Publication bias was not formally assessed because of the very small number of trials included.
Methods for reviewing cost-effectiveness evidence
A comprehensive search was undertaken to identify literature that might inform a cost-effectiveness study of ivacaftor. The search focused on original papers that reported on cost, cost-effectiveness or cost–utility analyses, studying either the diagnostic phase (genetic testing for CF mutations), the therapeutic phase (management of patients with confirmed CF), or a combination. The search was not restricted to studies on ivacaftor; evaluations of any treatment for CF were eligible. We identified cost studies, utility studies and full economic evaluations, that is to say those that explicitly compared different decision options. The intention was not to perform a systematic review, but to use the studies identified to support the critical review of the economic model provided by the manufacturer and where necessary the estimation of modified model input parameters that would address the objectives of this project.
Cost-effectiveness
Focused searches were undertaken to identify literature on cost-effectiveness and CF. Searches were limited to the last 10 years. The following resources were searched:
-
MEDLINE (OvidSP)
-
MEDLINE In-Process & Other Non-Indexed Citations (OvidSP)
-
EMBASE (OvidSP)
-
NHS EED (CRD).
Health-related quality of life
Focused searches were undertaken to identify literature on HRQoL and CF. Searches were not limited by date and the following resources were searched:
-
MEDLINE (OvidSP)
-
MEDLINE In-Process Citations & Other Non-Indexed Citations (OvidSP)
-
EMBASE (OvidSP)
-
Cost-effectiveness analysis (CEA) Registry (internet).
Guidelines and guidance
The following resources were searched for guidelines and guidance related to CF:
-
National Institute for Health and Care Excellence (NICE) Guidance (internet): http://guidance.nice.org.uk
-
TRIP database (limited to guidelines) (internet): www.tripdatabase.com
-
Guidelines International Network (GIN) (internet)
-
National Guidelines Clearinghouse (internet): www.guidelines.gov
-
Cystic Fibrosis Trust: www.cftrust.org.uk.
The results of the searches were independently screened by two reviewers; disagreements were resolved through consensus. Studies were rated as include, background or exclude based on the following criteria:
Include: Studies that had potential to inform parameters within the de novo analysis of cost-effectiveness.
Background: Studies that had the potential to inform methodological issues associated with parameters within the model, albeit that parameter estimates may be absent.
Exclude: All other studies.
Full-text copies of studies rated as ‘include’ or ‘background’ based on the above criteria were obtained. Two reviewers independently assessed each full-text study for inclusion according to the following criteria:
-
Likely to inform care processes in a UK setting. In some instances, a non-UK study could be included if it provided unique information (not available in UK studies) that was nonetheless informative for UK care profiling/outcome measurement.
-
Focused on CF population. For reviews that focused on screening programmes for CF, these were included only if they contained useable information on the lifetime costs and/or effects of having CF.
-
Reported long-term effects (> 6 months).
-
Reported data that were < 10 years old (i.e. 2002 or later). Studies that included data from 2002 or earlier were included only if they provided unique information that was nonetheless informative for current UK care profiling/outcome measurement.
-
Reported on at least one of the following: FEV1, percentage predicted FEV1, exacerbations, European Quality of Life-5 Dimensions (EQ-5D), Short Form questionnaire-36 items (SF-36), mortality, quality-adjusted life-years (QALYs).
Studies that fulfilled these criteria were further classified based on the following:
-
cost-effectiveness study (Y/N)
-
health-care costs reported (Y/N)
-
social care costs reported (Y/N)
-
disease-specific utility (FEV1, percentage predicted FEV1 and exacerbations) reported (Y/N)
-
generic utility (e.g. EQ-5D, SF-36, QALY) reported (Y/N)
-
incidence/prevalence reported (Y/N)
-
mortality or similar reported (Y/N)
-
model and/or probabilities reported (Y/N).
In addition the following elements were recorded within the resulting database:
-
year of study data (text)
-
setting (text)
-
follow-up period (text)
-
relevant CF study population (text).
This report contains references to confidential information. This information has been removed from the report and the results, discussions and conclusions of the report do not include the confidential information. These sections are clearly marked in the report.
Chapter 4 Systematic review results
Results of clinical effectiveness review
Quantity and quality of research available
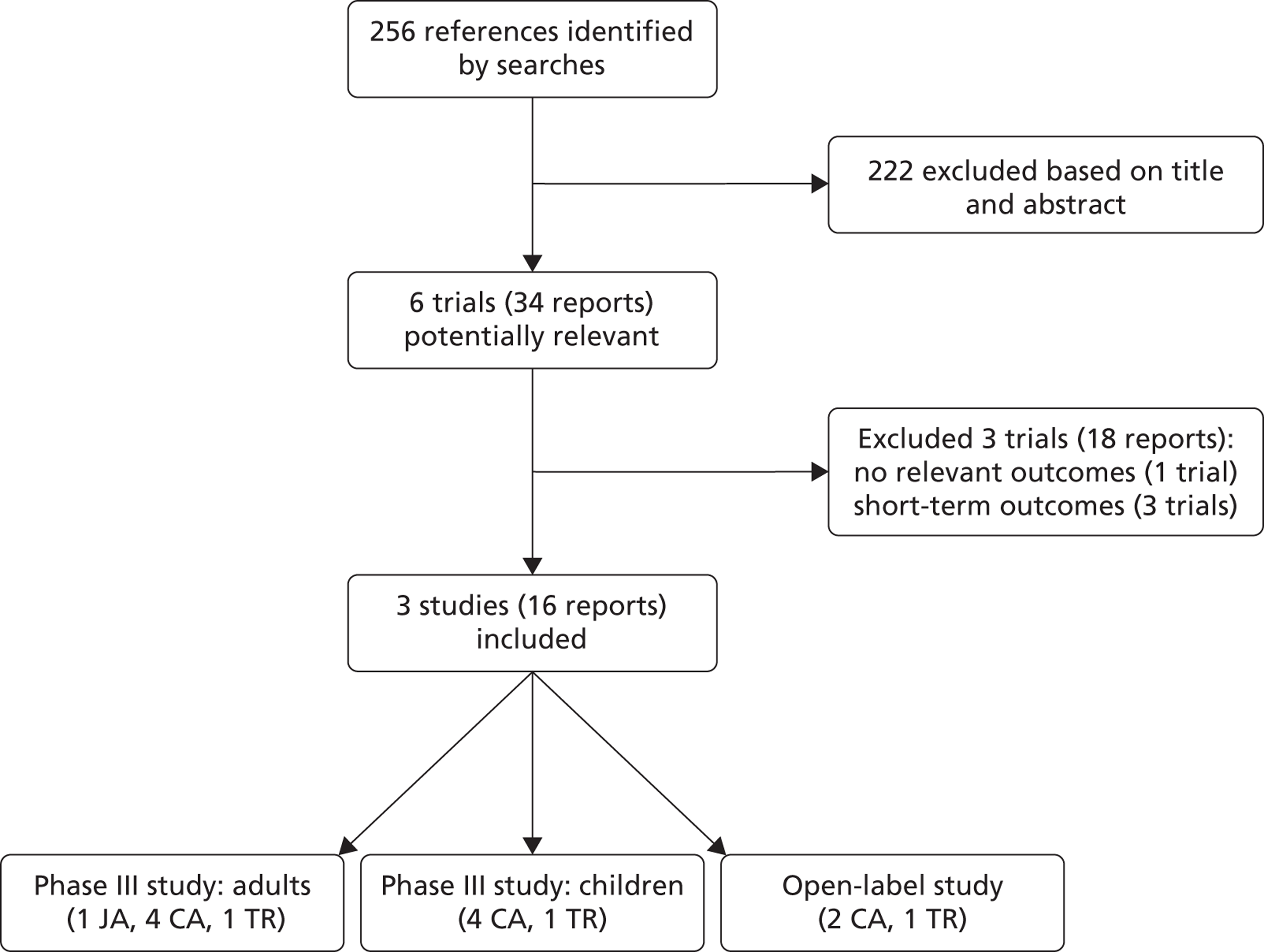
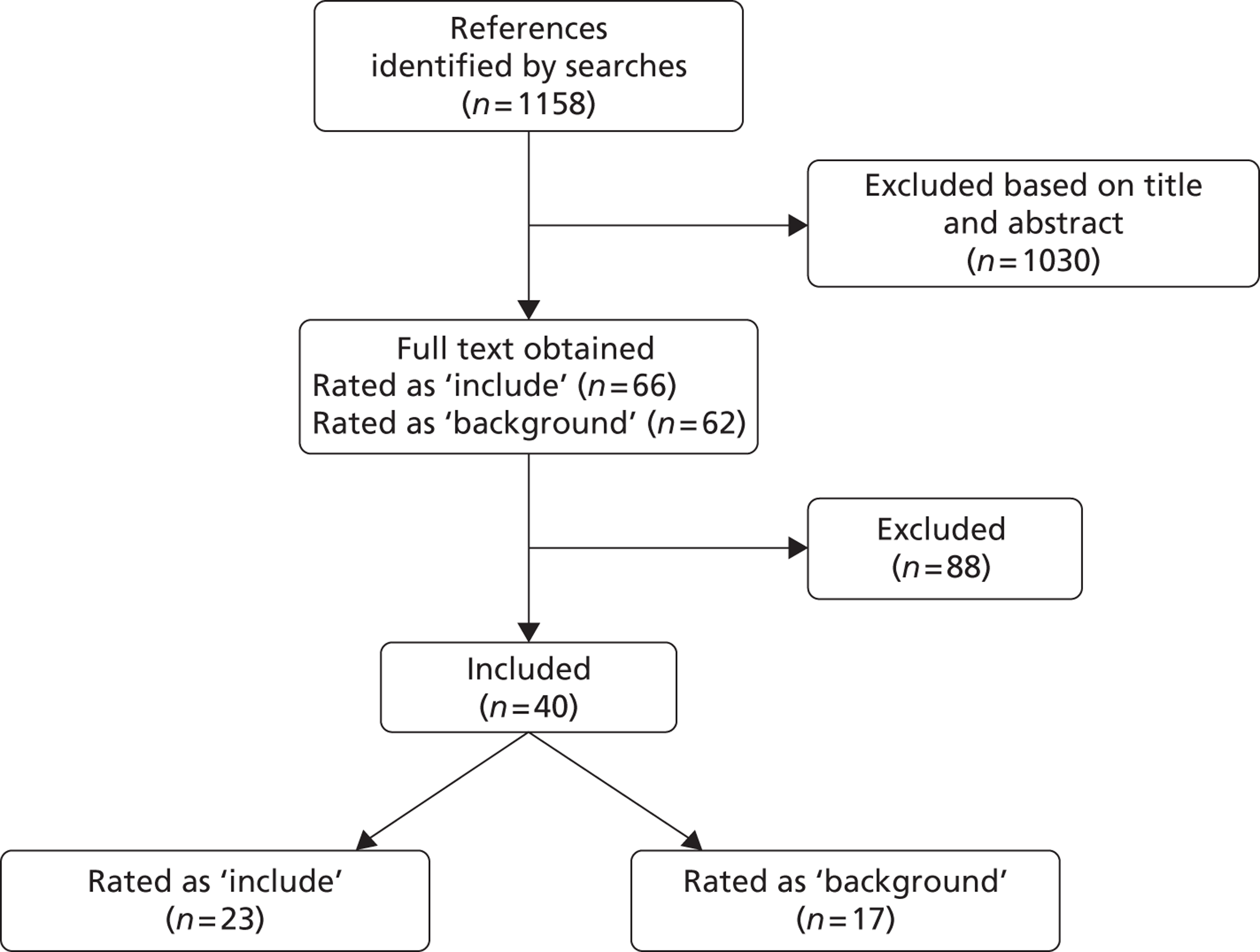
The searches identified 256 references, of which 29 reports were considered potentially relevant and full-text copies were obtained. Three studies (16 reports) fulfilled the inclusion criteria: two Phase III RCTs and one open-label study (Figure 1). Three Phase II RCTs were excluded; all reported short-term outcomes only and one trial did not report any of the outcomes specified in the inclusion criteria. Details of these studies are summarised in Appendix 4.
FIGURE 1.
Flow of studies through the review. CA, conference abstract; JA, journal article; TR, trial registry entry.
Summary of included studies
The first RCT was conducted in adults (‘adults’ study’) and was published as a full-text report,21 with the study protocol and further results available as supplementary information from the journal website. Details were also reported in four conference proceedings22–25 and one trial registry entry. 26 The second RCT was conducted in children (‘children’s study’) and full results have not yet been reported. Details were available only from four conference abstracts,23,24,27,28 a press release29 and a trial registry entry. 30 An open-label extension study of the two included RCTs was also included. Details were available only from two conference abstracts,31,32 a press release29 and a trial registry entry. 33 Additional details on all three studies were submitted by Vertex Pharmaceuticals, the manufacturer of ivacaftor,16 and from FDA reports prepared to support the licensing recommendation. 34–36 Confidential information provided by the manufacturer has been removed throughout this report.
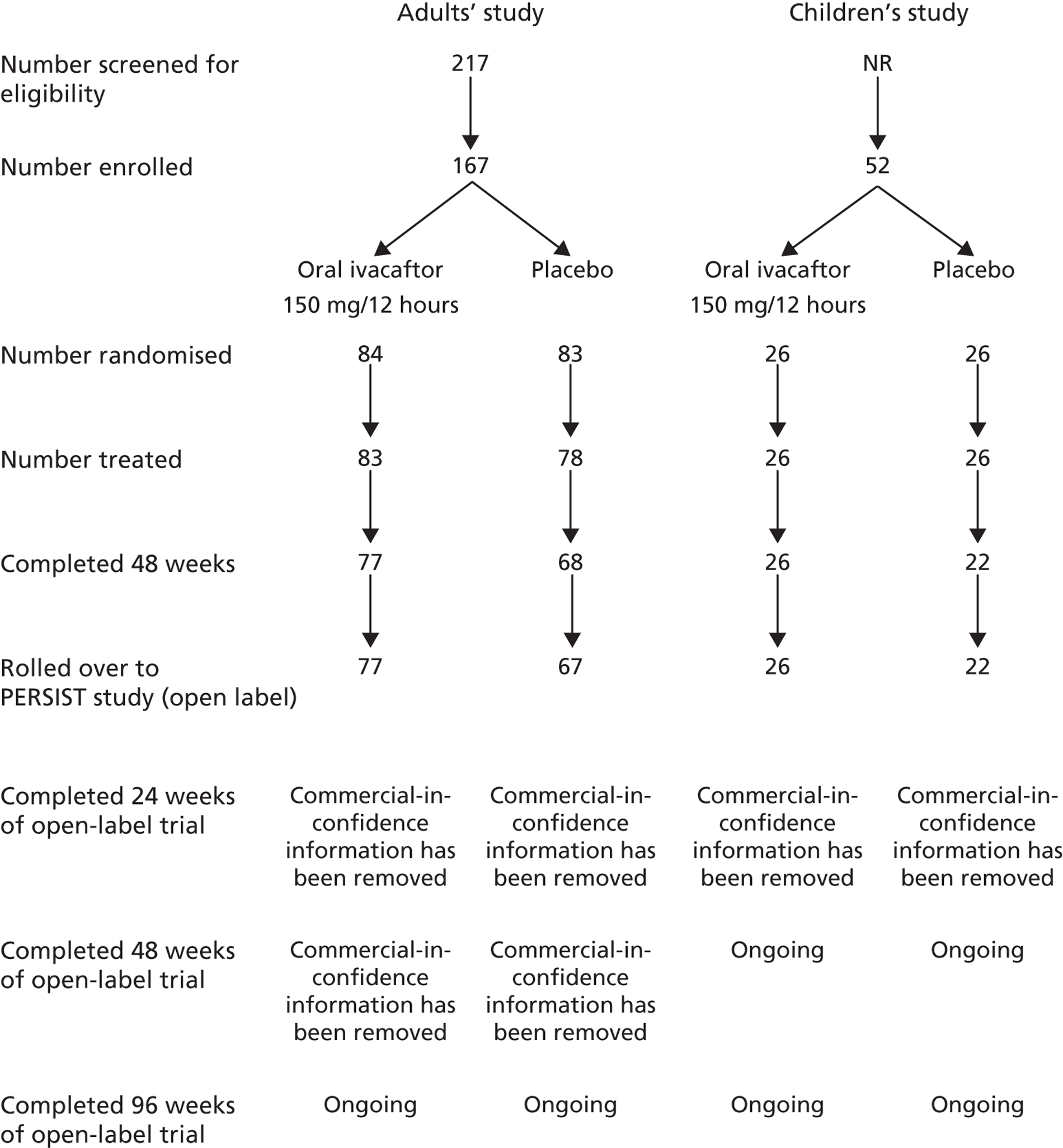
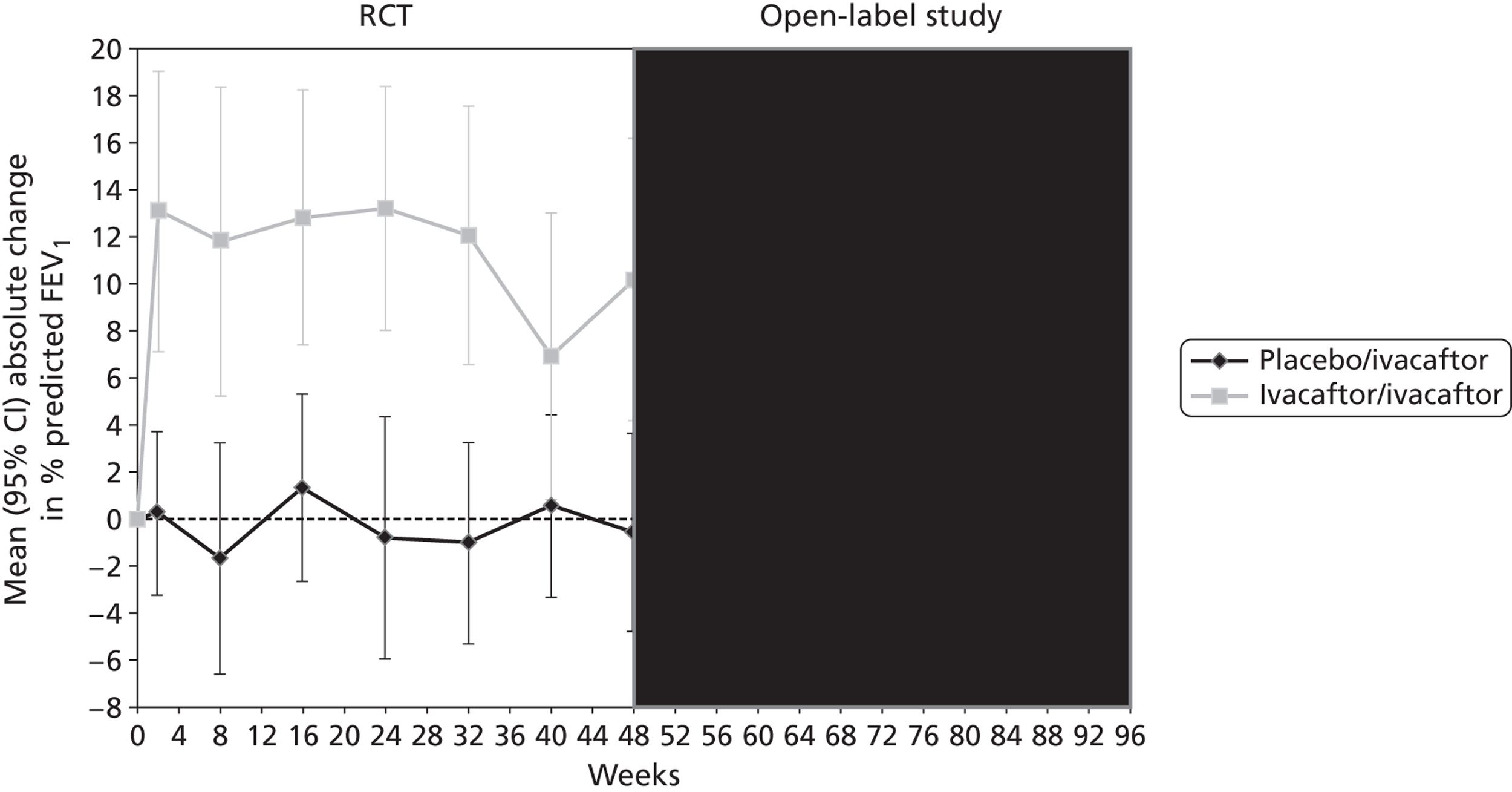
Baseline details of the two included RCTs are summarised in Table 1. The flow of patients through each study is summarised in Figure 2. Both studies were funded by Vertex Pharmaceuticals and were conducted in centres across the USA, Australia and Europe. Inclusion and exclusion criteria were similar with the exception of age: the study in adults enrolled adults and children aged ≥ 12 years; the study in children was restricted to children aged 6–11 years. All patients enrolled in the adults’ and children’s studies were eligible for inclusion in the open-label study. One patient who was in the placebo arm of the adults’ study did not enter the open-label study. Oral ivacaftor tablets were administered at a dose of 150 mg every 12 hours; the two RCTs also included an arm in which patients received matching placebo. The two RCTs were 48 weeks in duration. The open-label study was a further 96 weeks in duration with results currently available for 48 weeks’ follow-up (96 weeks’ ivacaftor treatment) in adults and 24 weeks’ follow-up (72 weeks’ ivacaftor treatment) in children.
| Features | Adults’ study | Children’s study |
|---|---|---|
| Country | North America, Europe, Australia | North America, Europe, Australia |
| Funding source | Vertex Pharmaceuticals (VX08-770-102) | Vertex Pharmaceuticals |
| Inclusion criteria | Aged ≥ 12 years; diagnosis of CF with at least one G551D mutation; FEV1 40–90% predicted | Children aged 6–11 years; G551D mutation; FEV1 40–105% predicted |
| Exclusion criteria | Ongoing illness; pulmonary exacerbation, changes in therapy for pulmonary disease, use of inhaled hypertonic saline treatment within 4 weeks of treatment, abnormal liver function, abnormal renal function, low haemoglobin; history of prolonged QT/QTc interval; history of solid organ or haematological transplantation; colonisation with organisms associated with more rapid decline in pulmonary status; concomitant use of inhibitors or inducers of CYP3A4 | Acute respiratory tract infection, pulmonary exacerbation, changes in therapy for pulmonary disease, use of inhaled hypertonic saline treatment within 4 weeks of treatment, abnormal liver function, abnormal renal function, low haemoglobin |
| Age (years), mean (SD) | 26 (9.5) | 9 (1.9) |
| Proportion male, % | 48 | 48 |
| Proportion white, % | 98 | 87 |
| Weight (kg), mean (SD) | 61 (14.1) | 31 (8.6) |
| Height (cm), mean (SD) | 167 (10.2) | 134 (13.3) |
| Percentage predicted FEV1, mean (SD) | 64 (16.4) | 84 (18.1) |
| Sweat chloride (mmol/l), mean (SD) | 100 (10.3) | 105 (11.9) |
| Positive for Pseudomonas aeruginosa, % | 76 | NR |
| Comorbidities | NR | NR |
| Co-interventions | Pre-study medication except hypertonic saline | NR |
FIGURE 2.
Flow of patients through the RCTs.
Risk of bias
The full results of the risk of bias assessment, including the support for judgement, are reported in Appendix 3. The rating of each bias criterion for each of the two RCTs is summarised in Table 2. The open-label study was not assessed for risk of bias, as this was a continuum of the two RCTs with all patients receiving ivacaftor and so issues relating to randomisation and blinding no longer applied. The study in adults was clearly reported and the availability of the study protocol meant that each of the risk of bias criteria could be assessed in detail. This study was rated as low risk of bias for all criteria. Fewer details were available for the study in children, as this has not yet been published in full. Randomisation, allocation concealment and incomplete outcome data were rated as unclear as there was insufficient information to make a judgement on these. The study was reported to have been double-blinded and so both blinding criteria were judged as low risk of bias. The study was also judged to be at low risk of bias for selective outcome reporting, as it appears that results for all relevant outcomes were reported.
| Criteria | Adults’ study | Children’s study |
|---|---|---|
| Randomisation | Low | Unclear |
| Allocation concealment | Low | Unclear |
| Blinding: participant | Low | Low |
| Blinding: outcome assessor | Low | Low |
| Incomplete outcome data | Low | Unclear |
| Selective reporting | Low | Low |
Lung function
Both RCTs reported significantly greater changes from baseline in all measures of lung function in patients receiving ivacaftor compared with those receiving placebo at 24 and 48 weeks (Table 3 and Figure 3). The primary outcome was the absolute change from baseline in the percentage predicted FEV1, expressed as a percentage of the predicted values for patients with similar height, age and sex. The MD between ivacaftor and placebo in ‘relative change from baseline in percentage predicted FEV1’ and the MD in ‘actual FEV1’ at 24 and 48 weeks were also assessed. The study in adults indicated that differences in lung function between the ivacaftor and placebo groups were due to improvements in lung function in the ivacaftor group while lung function in those in the placebo group stayed approximately the same or showed very slight decreases (Figure 4). (Commercial-in-confidence information has been removed.) The mean change from baseline in the adults’ and children’s trials and in the open-label extension at the various measurement points for each treatment group are shown in Figures 4 and 5. The improvement in lung function occurred very soon after treatment initiation with a MD between ivacaftor and placebo in change from baseline in percentage predicted FEV1 of 9.17% after 2 weeks of treatment in the adults’ study. Results from the children’s study supported this initial improvement with a MD of 12.85% after 2 weeks of treatment (commercial-in-confidence information has been removed).
| Outcomes | Studies | Mean change ivacaftor (SD) | Mean change placebo (SD) | MD in change (95% CI) | p-valuea |
|---|---|---|---|---|---|
| 24 weeks’ follow-up | |||||
| Percentage predicted FEV1: absolute change, percentage points | Adults | 10.4 | –0.2 | 10.6 (8.6 to 12.6) | < 0.0001 |
| Children | 12.6 | 0.0 | 12.5 (6.6 to 18.3) | < 0.0001 | |
| Percentage predicted FEV1: relative change, % | Adults | 17.6 | 0.7 | 16.9 (13.6 to 20.2) | < 0.0001 |
| Children | 21.7 | 4.3 | 17.4 (NR) | < 0.0001 | |
| FEV1, litres | Adults | 0.4 | 0.0 | 0.4 (0.3 to 0.4) | < 0.0001 |
| 48 weeks’ follow-up | |||||
| Percentage predicted FEV1: absolute change, percentage points | Adults | 10.1 | –0.4 | 10.5 (8.5 to 12.5) | < 0.0001 |
| Children | NR | NR | 10.0 (4.5 to 15.5) | 0.0006 | |
| Percentage predicted FEV1: relative change, % | Adults | 17.5 | 0.8 | 16.8 (13.5 to 20.1) | < 0.0001 |
| Children | NR | NR | 15.1 (NR) | NR | |
| FEV1, litres | Adults | 0.4 | 0.0 | 0.4 (0.3 to 0.4) | < 0.0001 |
FIGURE 3.
Mean difference in change from baseline in percentage predicted FEV1 (95% CI) in patients receiving ivacaftor compared with placebo in the adults’ study.
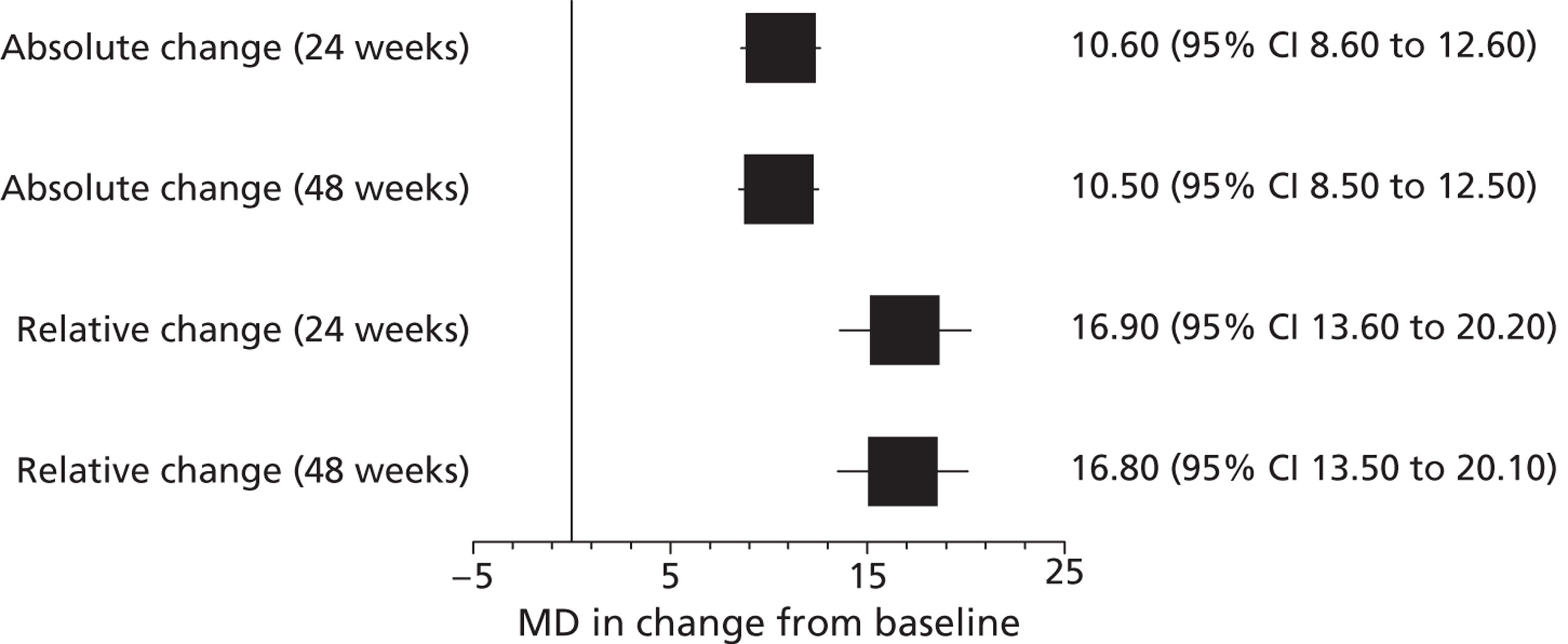
FIGURE 4.
Mean absolute change from baseline in percentage predicted FEV1 in adults in the RCT and open-label study. (Commercial-in-confidence information has been removed.)
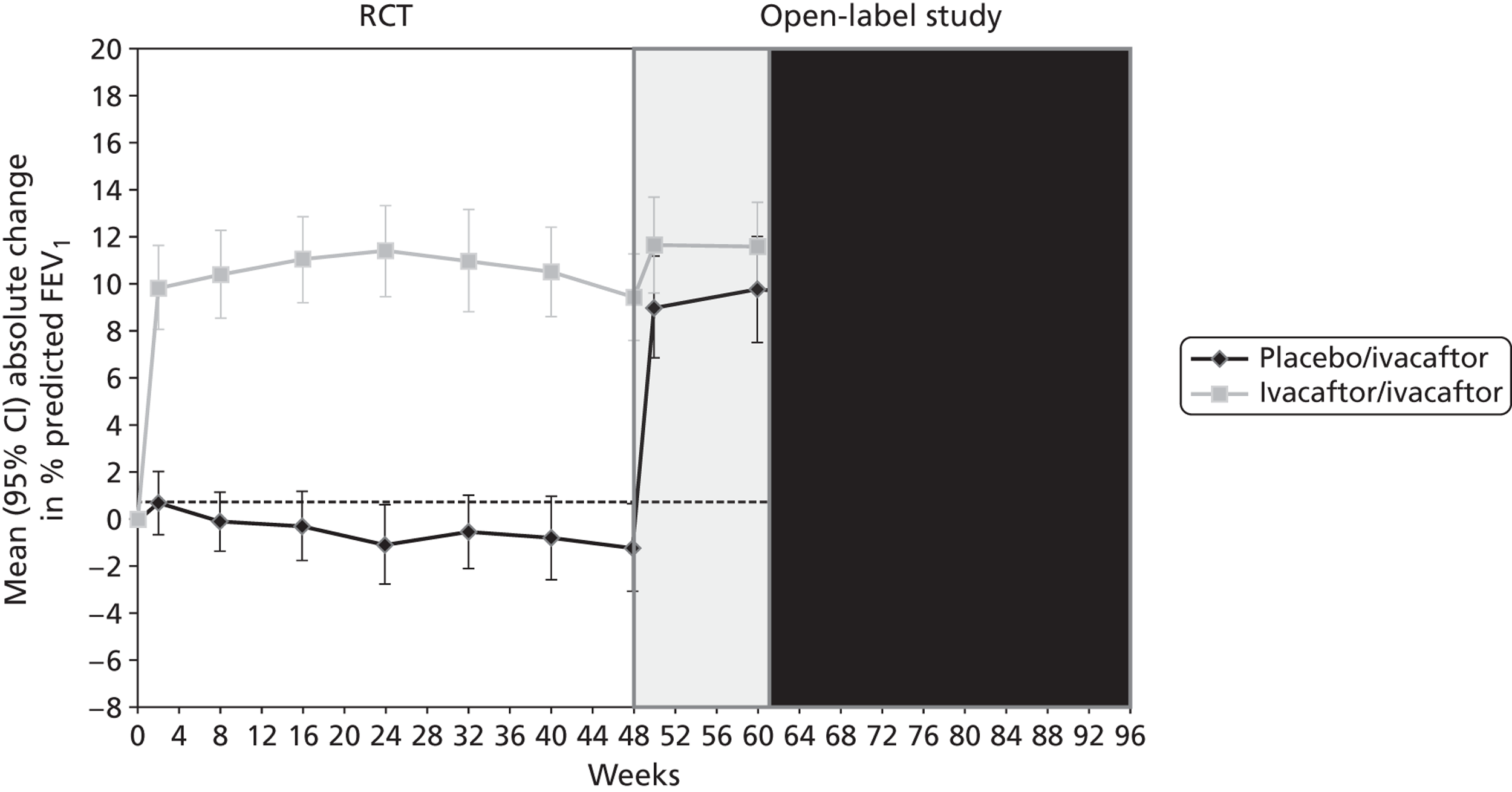
FIGURE 5.
Mean (95% CI) absolute change from baseline in percentage predicted FEV1 in children in the RCT and open-label study. (Commercial-in-confidence information has been removed.)
The adults’ study also reported results stratified according to age, sex, study region and baseline lung function. Ivacaftor treatment resulted in significant improvements in absolute change in FEV1 for all subgroups investigated (Table 4). CIs around estimates stratified according to subgroup were not reported and so it was not possible to formally investigate differences between subgroups. (Commercial-in-confidence information has been removed.) The children’s study did not report numerical results separately according to subgroups but results were presented graphically. This suggested that ivacaftor was associated with significantly greater improvements in absolute change in FEV1 compared with placebo for the following subgroups: Europe, ≤ 90% predicted FEV1 and girls. No significant differences were found for Australia, North America, > 90% predicted FEV1 or for boys, although all point estimates favoured ivacaftor. The small number of children in each subgroup means that the study may have lacked power to detect significant differences in these subgroups.
| Subgroups | Categories | MD in change from baseline (95% CI) | p-value |
|---|---|---|---|
| Age | < 18 years | 11.4 | 0.005 |
| ≥ 18 years | 9.9 | < 0.001 | |
| Sex | Male | 11 | < 0.001 |
| Female | 11.6 | < 0.001 | |
| Region | Australia | 11.9 | 0.008 |
| Europe | 9.9 | < 0.001 | |
| North America | 9 | < 0.001 | |
| Lung function | Percentage predicted FEV (≥ 70%) | 10.3 | < 0.001 |
| Percentage predicted FEV (< 70%) | 10.6 | < 0.001 | |
| Genotypea | G551D/ΔF508 | 10.3 (7.2 to 13.3)b | NR |
| G551D/other | 12.1 (6.8 to 17.4) | NR |
Pulmonary exacerbations
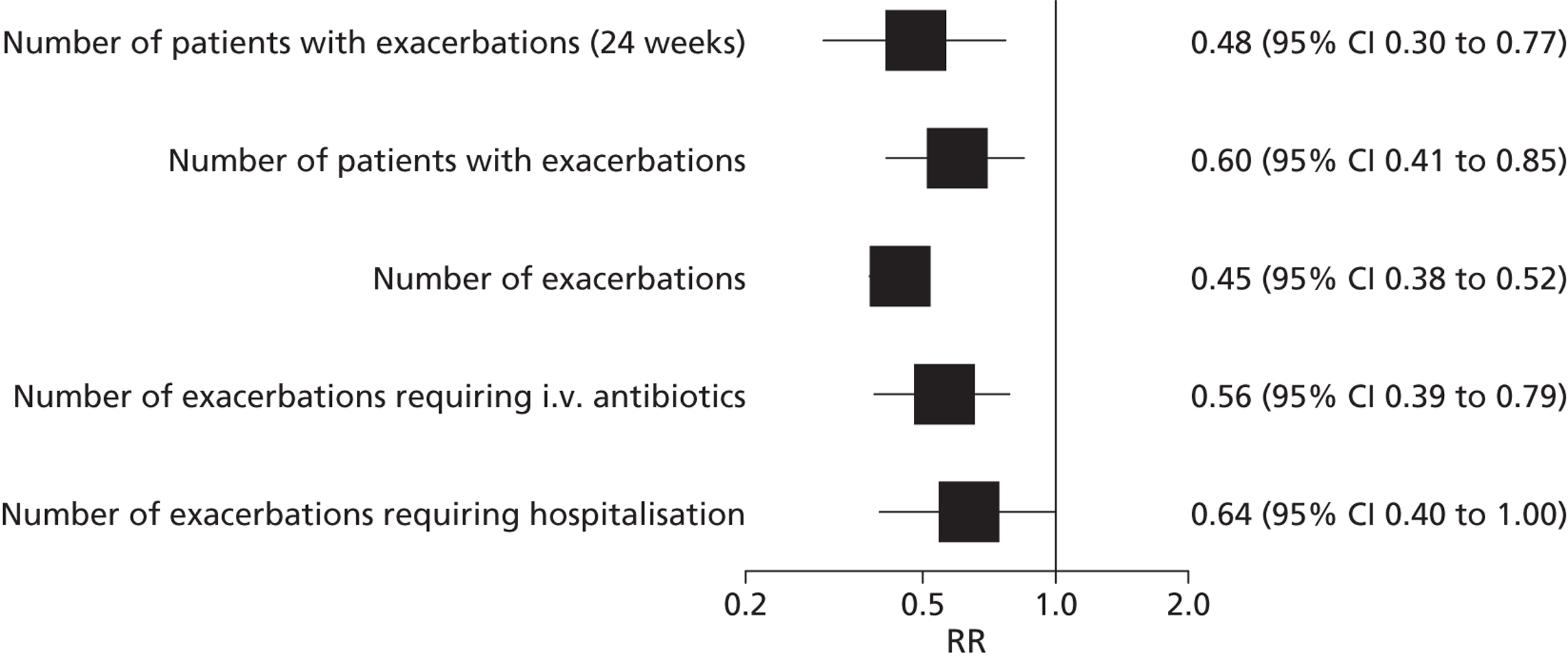
Pulmonary exacerbations were defined in the adults’ study using modified Fuchs criteria38 of new or a change in antibiotic therapy [intravenous (i.v.), inhaled or oral] for any four or more of the following symptoms: new or increased haemoptysis; increased cough; increased dyspnoea; malaise, fatigue or lethargy; temperature above 38 ºC; anorexia or weight loss; sinus pain or tenderness; change in sinus discharge; change in physical examination of the chest; decrease in pulmonary function by 10%; radiographic change indicative of pulmonary infection. The number and severity of pulmonary exacerbations (patients with pulmonary exacerbation and total exacerbations) at both 24 and 48 weeks were significantly reduced in the ivacaftor group compared with placebo group in the adults’ study (Table 5 and Figure 6). The mean number of days with pulmonary exacerbations, mean number of days with exacerbations requiring i.v. antibiotics and number of days hospitalised with exacerbations were also significantly lower among the ivacaftor treatment group (Table 6). (Commercial-in-confidence information has been removed.) The authors of the study in children reported that exacerbations were uncommon in both groups.
| Outcomes | Adults | Children | ||||||
|---|---|---|---|---|---|---|---|---|
| Ivacaftor | Placebo | Ivacaftor | Placebo | |||||
| Events | n | Events | n | Events | n | Events | n | |
| 24 weeks’ follow-up | ||||||||
| Number of patients with exacerbations | 18 | 83 | 35 | 78 | NR | NR | NR | NR |
| 48 weeks’ follow-up | ||||||||
| Number of patients with exacerbations | 28 | 83 | 44 | 78 | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Number of exacerbations | 47 | 83 | 99 | 78 | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Number of exacerbations requiring i.v. antibiotics | 28 | 83 | 47 | 78 | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Number of exacerbations requiring hospitalisation | 21 | 83 | 31 | 78 | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| CiC information has been removed | ||||||||
| CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
FIGURE 6.
Relative risk (95% CI) of exacerbations in adults receiving ivacaftor compared with placebo (data are at 48 weeks’ follow-up unless otherwise stated).
| Outcomes | Ivacaftor, mean (SD) | Control, mean (SD) | p-valuea |
|---|---|---|---|
| Days with exacerbations | 13.5 (27.3) | 36.7 (49.5) | 0.0007 |
| Days with i.v. antibiotics for exacerbations | 6.68 (19.43) | 11.03 (20.36) | 0.0183 |
| Days hospitalised for exacerbations | 3.92 (13.62) | 4.15 (8.71) | 0.0275 |
Other outcomes
Other outcomes reported in the studies included change from baseline in ivacaftor and placebo groups for quality of life (QoL) [measured using the respiratory domain of the Cystic Fibrosis Questionnaire Revised (CFQ-R)], sweat chloride, weight, BMI and BMI-for-age z-score. Each of these outcomes was reported in both the adults’ and children’s studies at 24 and 48 weeks’ follow-up and quality of life and weight were also reported in the open-label study. There were significantly greater improvements in the ivacaftor group than in the placebo group for all outcomes at all time points with the exception of quality of life in children, which failed to reach statistical significance at either 24 or 48 weeks’ follow-up (Table 7, Figure 7). Patients who had received ivacaftor in the RCT continued to gain weight in the open-label study. (Commercial-in-confidence information has been removed.) The mean absolute change from baseline in CFQ-R respiratory domain scores in adults and children are summarised in Figures 8 and 9 respectively.
| Outcomes | Studies | Change from baseline | p-value | ||
|---|---|---|---|---|---|
| Ivacaftor | Placebo | MD (95% CI) | |||
| 24 weeks’ follow-up | |||||
| Quality of life: CFQ-R respiratory domaina | Adults | NR | NR | 8.1 (4.7 to 11.4) | < 0.001 |
| Children | 6.31 | 0.25 | 6.1 (–1.4 to 13.5) | 0.1092 | |
| Sweat chloride, mmol/lb | Adults | –48.7 | –0.8 | –47.9 (–51.3 to –44.5) | < 0.001 |
| Children | –55.53 | –1.21 | –54.3 (–61.8 to –46.8) | < 0.0001 | |
| Weight, kgc | Adults | 3.0 | 0.2 | 2.8 (1.8 to 3.7) | < 0.0001 |
| Children | 3.7 | 1.8 | 1.9 (0.9 to 2.9) | 0.0004 | |
| 48 weeks’ follow-up | |||||
| Quality of life: CFQ-R respiratory domaina | Adults | 5.9 | –2.7 | 8.6 (5.3 to 11.9) | < 0.001 |
| Children | 6.1 | 1 | 5.1 (–1.6 to 11.8) | 0.1354 | |
| Physical functioning domain | Adults | NR | NR | 4.4 | 0.005 |
| Social functioning domain | Adults | NR | NR | 4.3 | 0.0026 |
| Eating disturbances domain | Adults | NR | NR | 3.3 | 0.0021 |
| Treatment burden domain | Adults | NR | NR | 3.3 | 0.0419 |
| Sweat chloride, mmol/lb | Adults | –48.7 | –0.6 | –48.1 (–51.5 to –44.7) | < 0.0001 |
| Children | 56 | 3 | –53.5 (–60.9 to –46.0) | < 0.0001 | |
| Weight, kgc | Adults | 3.1 | 0.4 | 2.7 (1.3 to 4.1) | < 0.0001 |
| Children | 5.9 | 3.1 | 2.8 (1.3 to 4.2) | 0.0002 | |
| BMI, kg/m2 | Adults | NR | NR | 0.9 | < 0.0001 |
| Children | NR | NR | 1.1 | 0.0003 | |
| BMI-for-age z-score | Adults | NR | NR | 0.33 | 0.0490 |
| Children | NR | NR | 0.45 | < 0.0001 | |
FIGURE 7.
Mean difference (95% CI) in change from baseline in (a) sweat chloride, (b) weight and (c) quality of life (measured using the CFQ-R) in patients receiving ivacaftor compared with placebo.
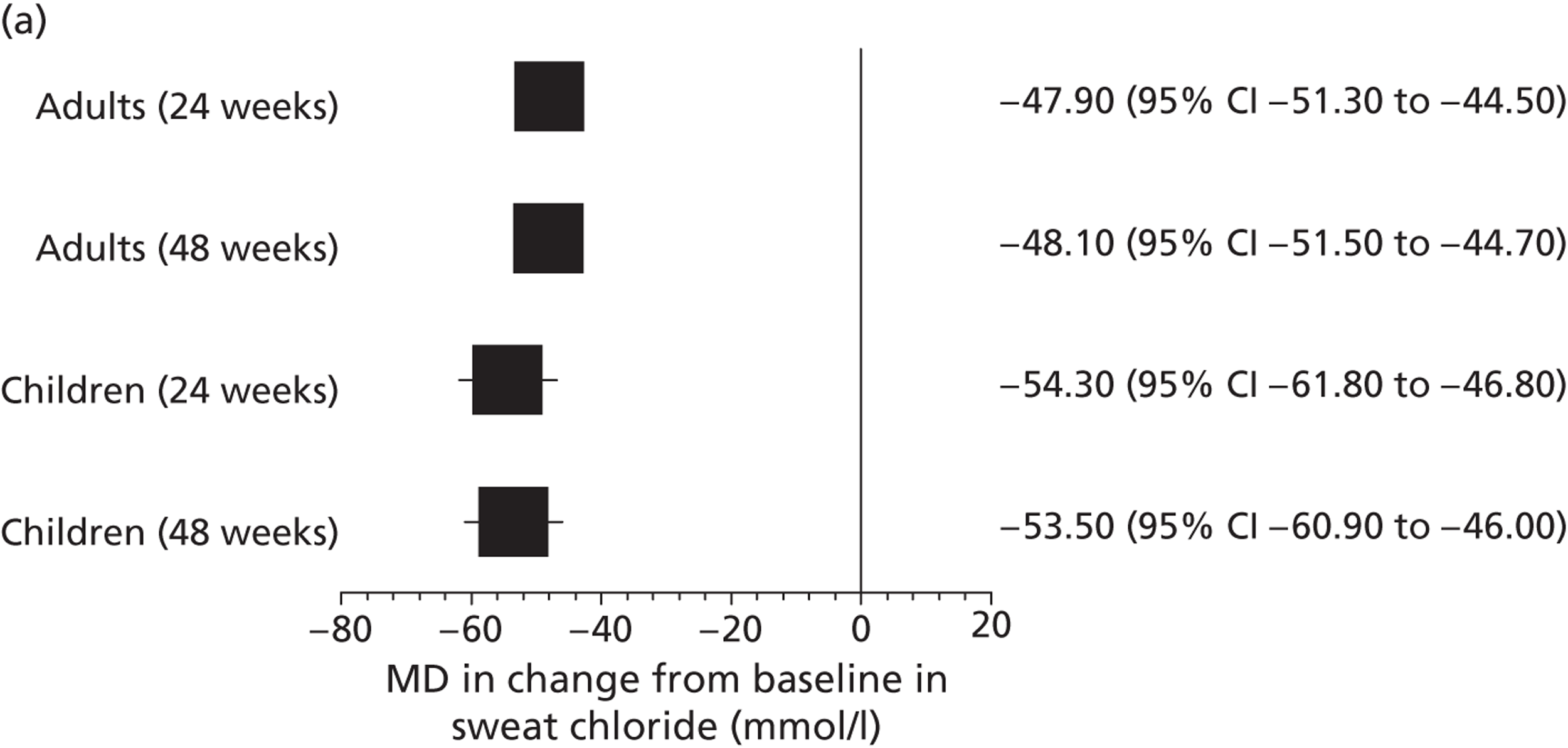
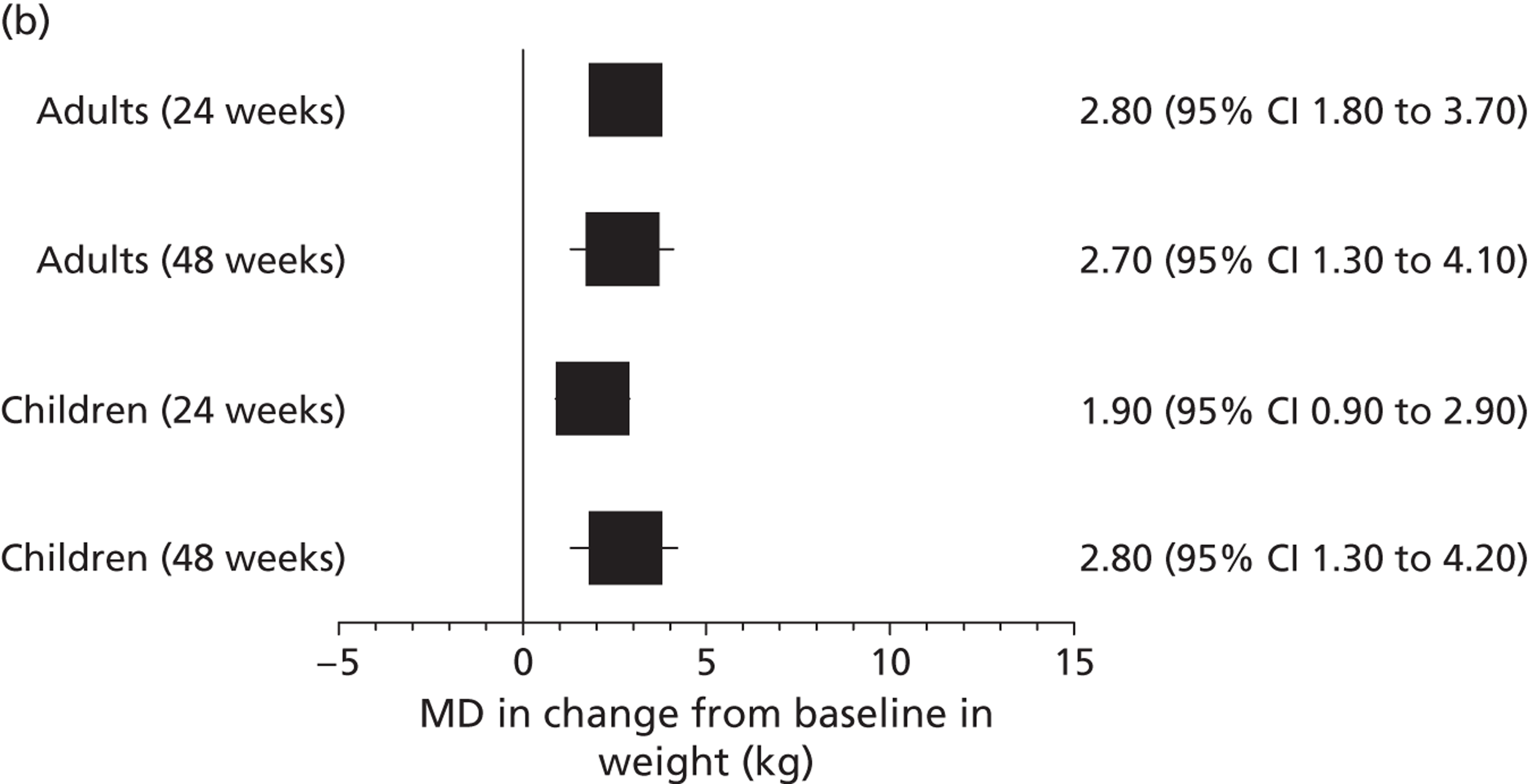
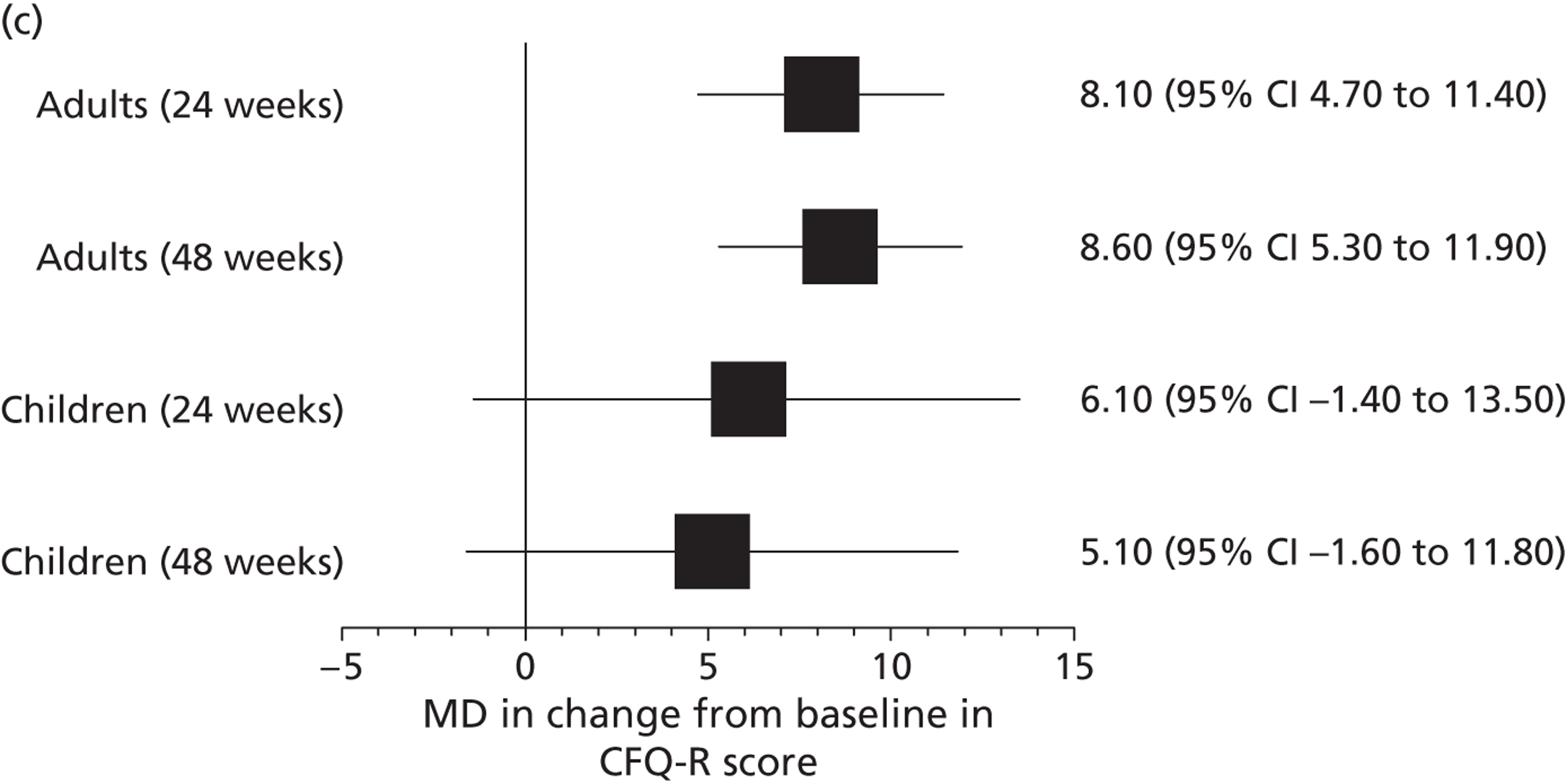
FIGURE 8.
Mean (95% CI) absolute change from baseline in CFQ-R respiratory domain score in adults in the RCT and open-label study. (Commercial-in-confidence information has been removed.)
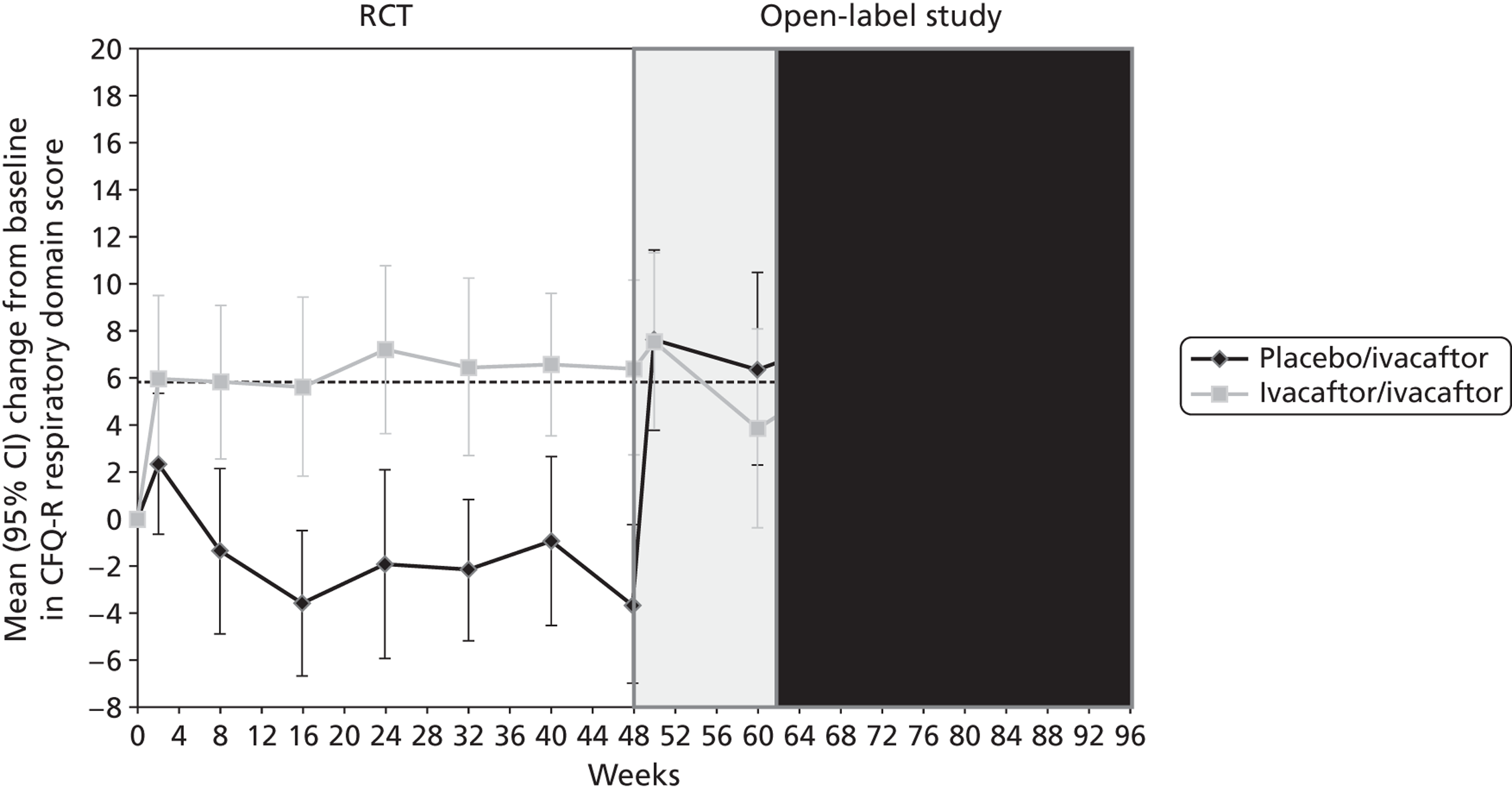
FIGURE 9.
Mean (95% CI) absolute change from baseline in CFQ-R respiratory domain score in children in the RCT and open-label study. (Commercial-in-confidence information has been removed.)
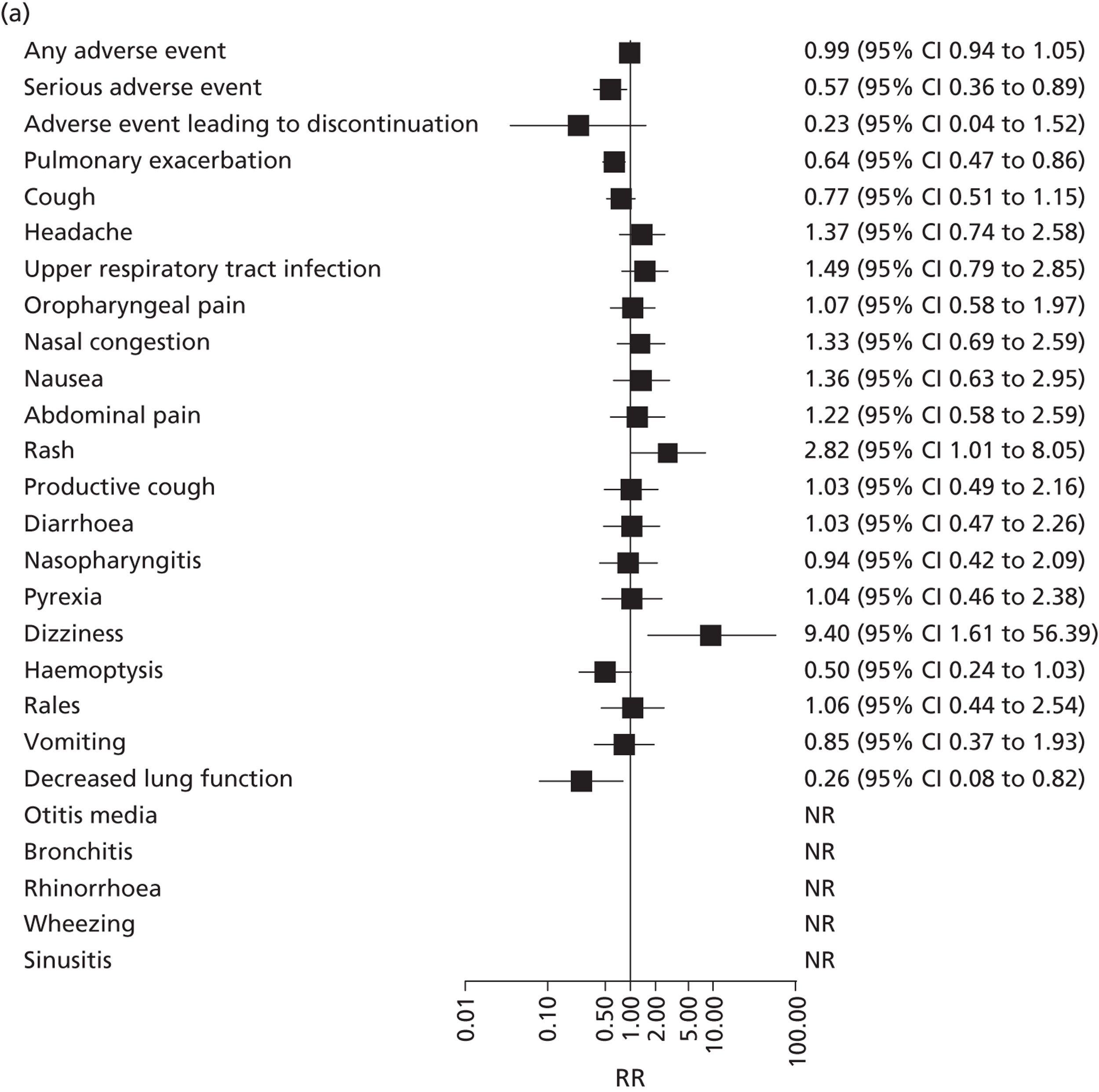
Adverse events and withdrawals
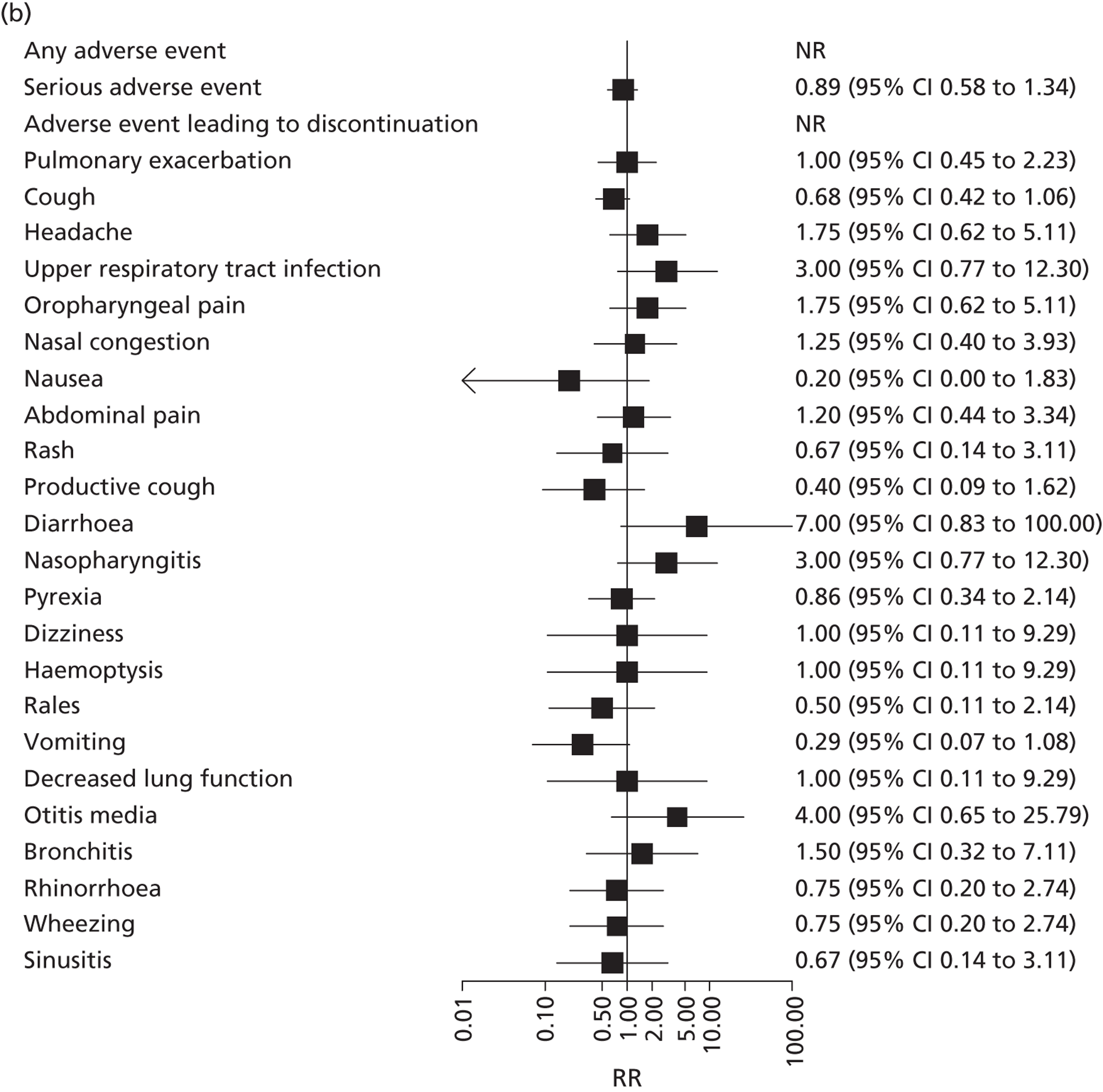
Adverse events were mainly minor and comparable across treatment groups and studies (Table 8). The most commonly reported adverse events were pulmonary exacerbation, cough, headache, upper respiratory tract infection and oropharyngeal pain. Figure 10 shows the RR for each adverse event in intervention compared with control arms for the adults’ and children’s studies. In the adults’ study, there was a greater risk of a serious adverse event (RR 0.67, 95% CI 0.36 to 0.89), pulmonary exacerbation (RR 0.64, 95% CI 0.47 to 0.85), and decreased lung function test (RR 0.25, 95% CI 0.05 to 0.82) in the placebo group and a small increased risk of rash (RR 2.52, 95% CI 1.01 to 8.05) and dizziness (RR 9.40, 95% CI 1.51 to 56.39) associated with ivacaftor. However, these differences were not found in the children’s study, which reported RRs very close to 1 for each of these events. The children’s study did not find any significant differences between treatment groups.
| Adverse events | Adults | Children | Open label | |||
|---|---|---|---|---|---|---|
| Ivacaftor events (n = 83) | Control events (n = 78) | Ivacaftor events (n = 26) | Control events (n = 26) | Placebo/ivacaftor (n = 67) | Ivacaftor/ivacaftor (n = 77) | |
| Any adverse event | 82 | 78 | NR | NR | 47 | 63 |
| Serious adverse event | 20 | 33 | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Adverse event leading to study-drug discontinuation | 1 | 4 | 0 | 0 | NR | NR |
| Pulmonary exacerbation | 34 | 50 | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Cough | 27 | 33 | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Headache | 19 | 13 | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Upper respiratory tract infection | 19 | 12 | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Oropharyngeal pain | 17 | 15 | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Nasal congestion | 17 | 12 | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Nausea | 13 | 9 | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Abdominal pain | 13 | 10 | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Rash | 12 | 4 | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Productive cough | 12 | 11 | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Diarrhoea | 11 | 10 | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Nasopharyngitis | 10 | 10 | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Pyrexia | 10 | 9 | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Dizziness | 10 | 1 | NR | NR | NR | NR |
| Haemoptysis | 9 | 17 | NR | NR | NR | NR |
| Rales | 9 | 8 | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Vomiting | 9 | 10 | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| Pulmonary function decreased | 3 | 11 | NR | NR | 0 | 1 |
| CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed | NR | NR |
| CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed | NR | NR |
| CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed | NR | NR |
| CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed | CiC information has been removed |
| CiC information has been removed | NR | NR | NR | NR | CiC information has been removed | CiC information has been removed |
| CiC information has been removed | NR | NR | NR | NR | CiC information has been removed | CiC information has been removed |
| CiC information has been removed | NR | NR | NR | NR | CiC information has been removed | CiC information has been removed |
FIGURE 10.
Relative risk (95% CI) of adverse events in ivacaftor compared with placebo for the (a) adults’ and (b) children’s studies. NR, not reported.
Both RCTs reported more overall withdrawals and withdrawals due to adverse events in the placebo group than in the ivacaftor group (Table 9). Two patients in the open-label trial discontinued treatment before the 12-week visit, one due to pregnancy and one due to the adverse event of suicidal depression.
| Reasons for withdrawal | Adults | Children | ||
|---|---|---|---|---|
| Ivacaftor | Placebo | Ivacaftor | Placebo | |
| Withdrawals on or before day 1 | ||||
| Illness | 0 | 3 | 0 | 0 |
| Daily panic attacks | 0 | 1 | 0 | 0 |
| FEV1 too low | 0 | 1 | 0 | 0 |
| Wrong genotype | 0 | 1 | 0 | 0 |
| Randomised by error | 0 | 1 | 0 | 0 |
| Required prohibited medication | 1 | 0 | 0 | 0 |
| Withdrawals after day 1 | ||||
| Non-compliance | 2 | 0 | 0 | 0 |
| Required prohibited medication | 1 | 2 | 0 | 0 |
| Pregnancy | 1 | 0 | 0 | 0 |
| Withdrawal of consent | 1 | 1 | 0 | 0 |
| Physician decision | 0 | 1 | 0 | 0 |
| Unclear | 0 | 1 | 0 | 3 |
| Adverse event | 1 | 3 | 0 | 1 |
| Withdrawals during open-label study | ||||
| Adverse event | 0 | 1 | 0 | 1 |
| Pregnancy | 0 | 1 | 0 | 0 |
| Withdrew consent | 2 | 1 | 0 | 0 |
| Non-compliance | 1 | 0 | 0 | 0 |
| Total | 10 | 18 | 0 | 5 |
Results of cost-effectiveness review
The health economics searches identified 1158 titles and abstracts. Of these, 66 were rated as include based on initial screening criteria and 62 were rated as background. After full-text review, 23 studies were rated as include, 17 as background and the remaining 88 were excluded (Figure 11). Details of the studies rated as include and background are summarised in Appendix 6.
FIGURE 11.
Flow of studies through the health economic review.
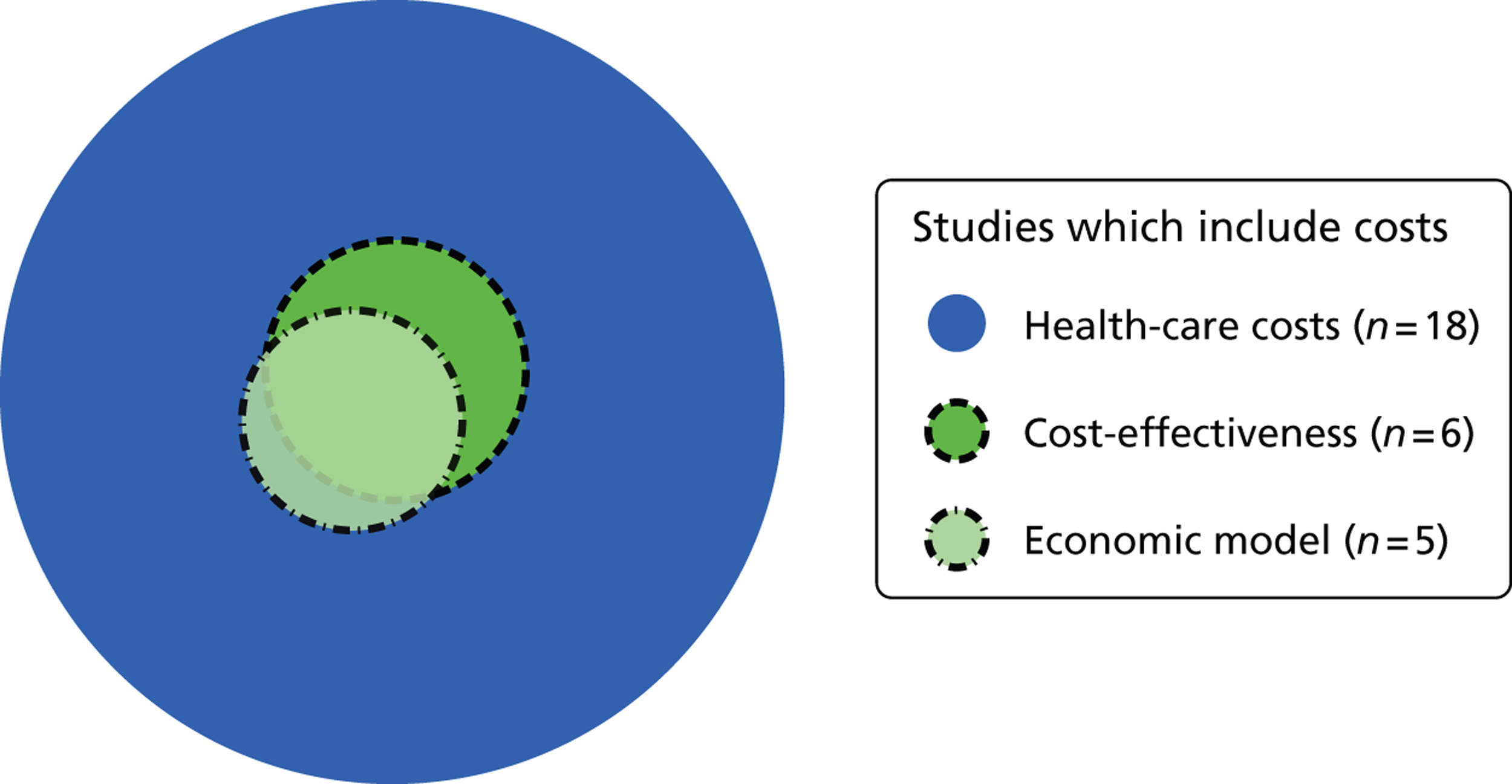
Included studies focused on health-care costs, cost-effectiveness and utility to inform the economic model. None of the included studies contained information that would inform social care costs. Included studies were used to validate and contextualise assumptions in the model.
Eighteen ‘included’ studies that focused on cost elements contained information on health-care costs (Figure 12). Six studies provided data on cost-effectiveness, five of which included data based on economic models. 38–42 These studies provided useful methodological information as well as data that were used to validate the manufacturer’s cost-effectiveness model. However, these studies did not model the cost-effectiveness of ivacaftor. Background studies did not contribute directly to the economic model; they were used only to inform methodological issues.
FIGURE 12.
Venn diagram of included studies that included costs.
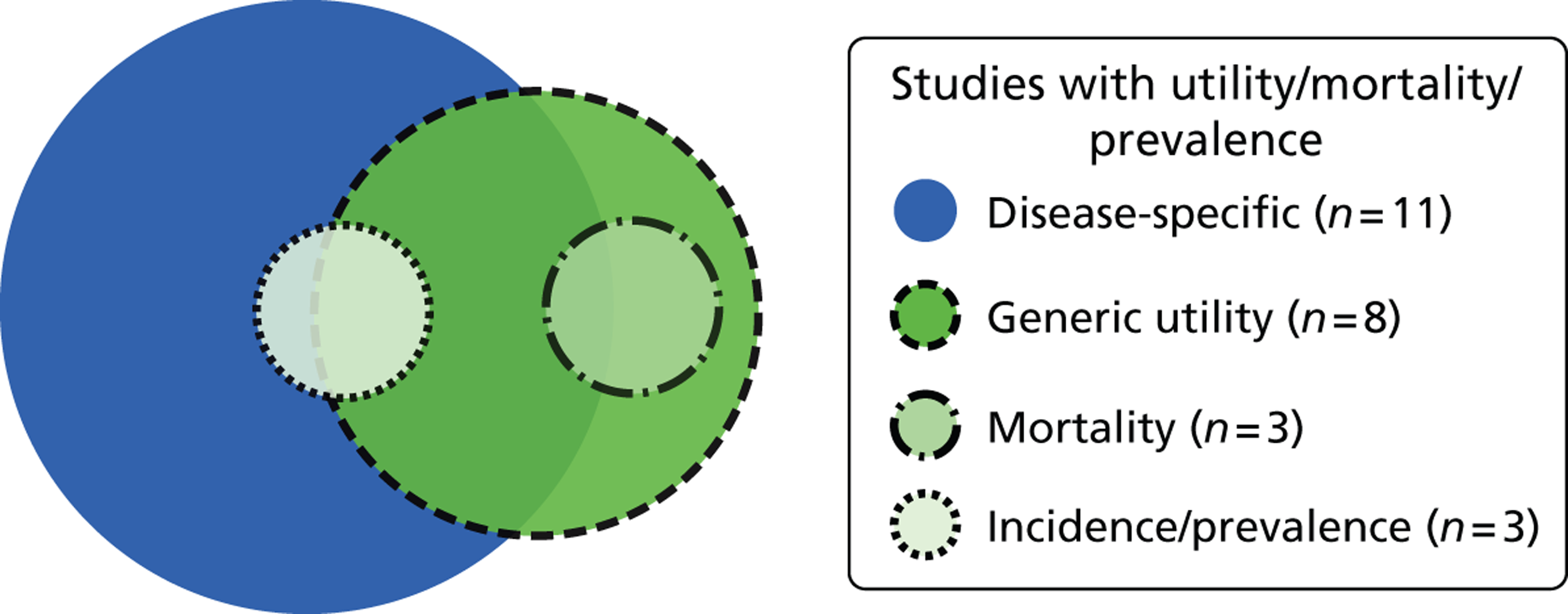
Fourteen studies provided data on utility, mortality or prevalence. Eleven studies focused on disease-specific utility measures, and six studies included valid generic tools (Figure 13). Three studies provided mortality data; two of these used a generic utility measure and the other used a disease-specific utility measure. Three examined incidence and prevalence; two of these also used a generic utility measure and the other used a disease-specific utility measure.
FIGURE 13.
Venn diagram of included studies that included mortality/utility.
Parameter estimates
Three of the 23 included studies contributed to the model. 8,21,43 Additionally, a more contemporary, as yet unpublished, version of the UK CF Registry database (2011) was used to inform cost parameters. 44 The adults’ study included in the review of clinical effectiveness was also included in the economic review. 21 UK-based generic utility values of CF patients by percentage predicted FEV1 category were obtained from Gee et al. 45 and expressed in terms of SF-36. The prevalence rates of diabetes mellitus, Staphylococcus aureus infection and Burkholderia cepacia infection were derived from the UK CF Registry,8 which was also a data source for the proportion of genotyped patients and the proportion who were eligible for, and in receipt of, a lung transplant. Following correspondence with the Cystic Fibrosis Trust, audit data from the 2011 registry were obtained. These were analysed to provide information on costing, particularly in relation to tariff bands, expensive drugs and implantation of venous access devices.
No evidence was derived from the other included studies and the studies included as background. These studies were not useful for the model as, on detailed review, they did not:
-
provide useful evidence for use in a UK setting
-
transparently report costs or effects; or
-
provide utility values that were linked to percentage predicted FEV1 bands.
Chapter 5 Methods for assessing cost-effectiveness
Methodology and structure
The manufacturer of ivacaftor, Vertex Pharmaceuticals, submitted a health economic model for the assessment of the lifetime cost-effectiveness of ivacaftor for the treatment of CF in patients aged ≥ 6 years who have at least one G551D mutation in the CFTR gene. 16 This model was a deterministic patient-level simulation model. We used the manufacturer’s model as the basis for our model, making modifications where necessary. Input values into the model were modified if values used by the manufacturer were not UK-specific or not recent, or better estimates could be found. We took estimates of the treatment effect of ivacaftor from the results of the clinical effectiveness review. All costs and effects were discounted by 3.5% according to the NICE methods guide. 46 The model incorporated a lifetime time horizon to estimate outcomes in terms of QALYs and costs from the perspective of the NHS. There were various uncertainties in the model regarding the input data. The impact of these uncertainties was explored through probabilistic sensitivity analysis (PSA). Below we describe the content and structure of the manufacturer’s model (Figure 14). We then present a summary of the model inputs and any modifications that we made to these for our model.
FIGURE 14.
Overview of model. ICERs, incremental cost-effectiveness ratios.
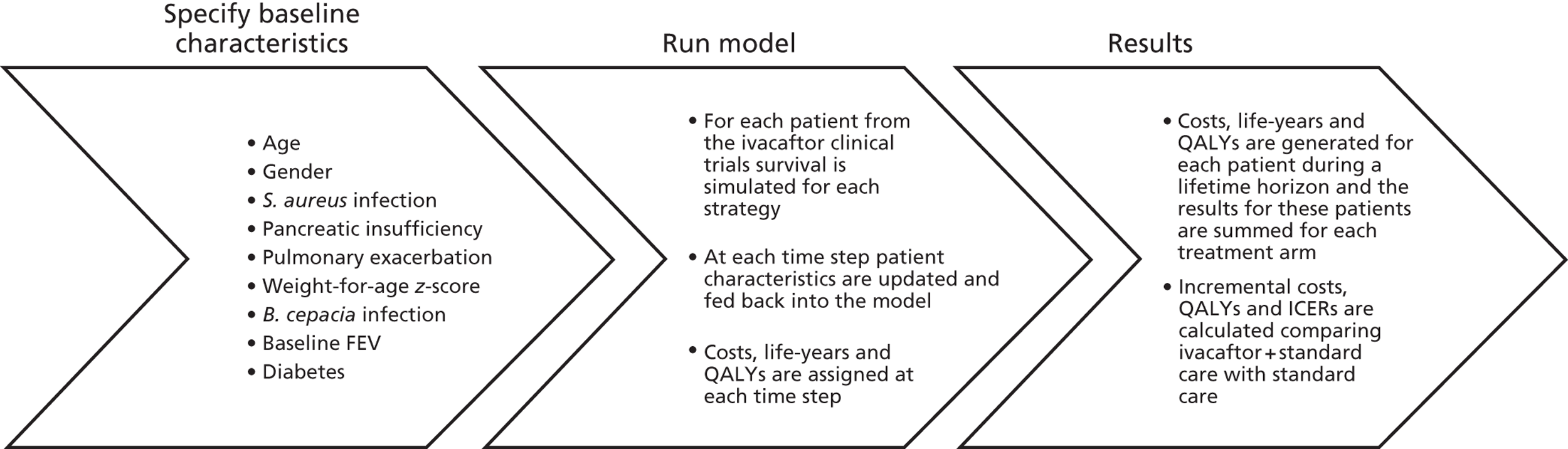
Population
The starting patient population for this individual patient simulation is the population in the two RCTs included in the clinical effectiveness review (adults’ and children’s studies). 21,27 The analysis is therefore based on adults and children aged ≥ 6 years at the time of the start of the clinical trials in 2010. The cost-effectiveness study is conducted from a NHS perspective and so the reference population is the total CF population in England. There is therefore a potential concern regarding the generalisability of the model. To assess this, we compared baseline characteristics of patients included in the RCTs with details of patients included in the UK CF Registry, an anonymised database of all those with CF in the UK, maintained by the Cystic Fibrosis Trust (Table 10). 8 The median age of patients included in the UK CF Registry was lower than that of patients included in the trials, which is explained by the fact that patients aged < 6 years were excluded from the trials. The proportion male to female and median percentage predicted FEV1 were comparable. Table 10 provides a summary of key characteristics of patients in the ivacaftor trials compared with UK CF Registry data. We did not make any modifications to the population in the model.
| Characteristic | Ivacaftor Phase III trials | UK CF Registry |
|---|---|---|
| Median age (years) | 20 | 17 |
| Sex (% female) | 52% | 47% |
| Median baseline percentage predicted FEV1 | 71% | 74% |
| Mean weight-for-age z-score | –0.41 | NA |
| Pancreatic insufficiency | 93% | NA |
| P. aeruginosa infection (age > 12 years) | 76% | 50.4% |
Strategies
To estimate the lifetime impact of ivacaftor in terms of costs and effects (QALYs) on CF patients, standard care (standard care strategy) was compared with ivacaftor plus standard care (ivacaftor + standard care strategy). Standard care consisted of CF-related medication [pancreatic enzymes, dornase alfa (DNase) (Pulmozyme®, Roche), inhaled corticosteroids, bronchodilators, prednisone and antibiotics], devices (oxygen vests, nebulizers and other airway clearance and respiratory devices) and respiratory therapy. 16 We did not make any modifications to the strategies in the model.
Disease progression model
The model simulates the disease progression of CF patients included in the trials beyond the trial duration as an independent decline in percentage predicted FEV1 (no change in any other characteristics, e.g. exacerbation rate). The probability of death is a function of the percentage predicted FEV1, number of pulmonary exacerbations per year, infections with S. aureus (yes or no), infection with B. cepacia (yes or no), diabetes (yes or no), weight-for-age z-score and pancreatic sufficiency status (yes or no) (Table 11).
| Covariates | Liou et al.47 | Manufacturer input values | Sources | (Modified) input values | Sources | |||
|---|---|---|---|---|---|---|---|---|
| β-coefficient (SE) | Reference values | 6–11 years | 12+ years | 6–11 years | 12+ years | |||
| Sex (female = 1, male = 0) | 0.15 (0.074) | 47.0% | Patient dependent | Trial data (Vertex 2012) | Patient dependent | Trial data (Vertex 2012) | ||
| Percentage predicted FEV1 | –0.042 (0.0025) | 67.7% | Patient dependent | Trial data (Vertex 2012) | Patient dependent | Trial data (Vertex 2012) | ||
| Weight-for-age z-scores | –0.28 (0.041) | –0.85 | Patient dependent | Trial data (Vertex 2012) | Patient dependent | Trial data (Vertex 2012) | ||
| Pancreatic sufficiency | –0.14 (0.23) | 5.3% | Patient dependent | Trial data (Vertex 2012) | Patient dependent | Trial data (Vertex 2012) | ||
| Diabetes mellitus | 0.44 (0.098) | 6.1% | 28% | 28% | UK CF Registry 2010 | 28% | 28% | UK CF Registry 2010 |
| S. aureus infection | –0.25 (0.09) | 30.6% | 30% | 30% | UK CF Registry 2010 | 30% | 30% | UK CF Registry 2010 |
| B. cepacia infection | 1.41 (0.19) | 3.2% | 2% | 2% | UK CF Registry 2010 | 2% | 2% | UK CF Registry 2010 |
| Number of acute exacerbations (0–5) | 0.35 (0.024) | 1.10 | 0.00 | 1.40 | Trial data (Vertex 2012) | 0.51 | 0.83 | Goss et al.48 |
| Number of acute exacerbations (0–5) × B. cepacia infection | –0.28 (0.06) | |||||||
Improvement in the percentage predicted FEV1, exacerbations and weight-for-age z-score associated with ivacaftor21 is translated into better survival of the patients. Each individual from the two RCTs with certain baseline characteristics runs through each treatment arm of the model. Every 3 months, patients’ characteristics are updated, based on efficacy outcomes and natural disease progression, and fed back into the model to estimate the survival of the patient. The estimated 3-monthly survival probability is then multiplied by the survival probability at the beginning of the 3-month period leading to cumulative survival probabilities. In addition, the HRQoL and health-care costs for the patients during the 3-month period are calculated. HRQoL values defined in terms of utility values were assumed to be dependent on the level of percentage predicted FEV1 with a decrease in percentage predicted FEV1 resulting in a decrease in utility. Costs were assumed to be dependent on percentage predicted FEV1 and on age with a decrease in percentage predicted FEV1 age resulting in an increase in costs. Adding up all the costs and effects generated in each time step leads to total costs and effects for each individual for both strategies. Average costs and QALYs per strategy are then used to estimate the incremental cost-effectiveness ratio (ICER).
Modified disease progression
The only change made to the structure of the model was the addition of lung transplantations. Lung transplantations were added to the model as ivacaftor has the potential to improve the percentage predicted FEV1, which could lead to fewer lung transplantations and therefore savings in CF-related health-care costs. We have assumed that individuals with a percentage predicted FEV1 ≤ 30% are eligible for lung transplantation, although only 17% of them receive a transplantation. 8,37 Reasons for this include not meeting the requirements for the waiting list and the unavailability of matching donors once on the waiting list. After transplantation patients were assumed to have lower mortality rates and improved QoL. In transplanted patients costs for ivacaftor and CF-related health-care use were assumed to be zero and all inclusive follow-up costs after transplantation were applied. The inclusion of lung transplantations is important because preventing the need for lung transplantation could increase the incremental effectiveness and reduce costs of the ivacaftor + standard care strategy compared with the standard care strategy.
Model parameters
The inputs into the model are summarised below together with changes that we made to improve the model and make it more applicable to the UK setting.
Survival function
The probability of dying was estimated by means of a hazard function adapted from Liou et al. 47 depending on age, sex, percentage predicted FEV1, number of pulmonary exacerbations, infections with S. aureus, infection with B.cepacia, diabetes, weight-for-age z-score and pancreatic sufficiency status (see Table 11). This study found no evidence of an association between other clinical parameters (e.g. height and infection with P. aeruginosa) and survival; these parameters were therefore not included in the survival function. Table 11 presents the original survival function based on Liou et al. ,47 the input estimates used by the manufacturer and our updated input estimates. The hazard function was estimated by subtracting the value of each individual patient characteristic from the reference values listed in Table 11.
The proportion female in the Liou et al. 47 study was 47% and was used as a reference value for the survival function. This reference value is compared with the sex status of patients included in the ivacaftor trials. Baseline values of percentage predicted FEV1 are based on individual baseline estimates of the patients and compared with a reference baseline percentage predicted FEV1 value of 67.7%. 47 The weight-for-age z-score was assumed to be constant over a lifetime period from the baseline score. Individual weight-for-age z-score estimates were used for the simulation of the disease progression and compared with a reference value of –0.85 based on Liou et al. 47 The number of exacerbations, based on trial data,1 was age dependent; patients aged ≥ 12 years treated with standard care were assumed to experience 1.4 exacerbations annually, while patients between 6 and 11 years were assumed to experience zero events annually. These estimations of the exacerbations are kept constant during the entire model duration and were compared with a reference value of 1.1 exacerbations per year. 47 The prevalence of diabetes mellitus, S. aureus infection and B. cepacia infection were not available from the trial data, and therefore age-specific percentages of patients with these conditions were derived from the UK CF Registry Annual Data Report8 and compared with the presented reference values. 47 Pancreatic insufficiency has a negative impact on the survival of patients and was therefore included in the survival function. Individual pancreatic insufficiency status is compared with the reference value. 47
Modified survival function
Generally, we used the same input values as the manufacturer. However, we adjusted the annual exacerbation rates as the manufacturer included an annual exacerbation rate of 1.4 for patients aged ≥ 12 years based on 78 patients receiving standard care during the 48 weeks of the adults’ trial. Patients aged < 12 years of age were assumed to experience no exacerbations in the original model. In our modified model the annual exacerbation rate was assumed to be dependent on percentage predicted FEV1 and age. Based on two figures presented in a paper by Goss et al. 48 we estimated the association between the mean annual pulmonary exacerbation rate and the mean percentage predicted FEV1 separately for patients with CF < and ≥ 18 years of age. The final estimated equations were:
-
mean annual exacerbation rate in patients < 18 years of age = 8.5938 × exp[–0.035 × percentage predicted FEV1]
-
mean annual exacerbation rate in patients ≥ 18 years of age = 3.7885 × exp[–0.026 × percentage predicted FEV1].
The patient-level data on baseline percentage predicted FEV1 from the trials were incorporated into the estimated equations to calculate the exacerbation rate for each patient. Based on these estimates the mean annual baseline exacerbation rate was estimated to be 0.51 for patients < 12 years of age and 0.83 for patients ≥ 12 years of age. These rates were applied in the model for the standard care group.
Survival after transplantation
A major modification to the model was the inclusion of lung transplantation. Patients undergoing lung transplantation have a different mortality rate from other CF patients. We derived the probability of dying after a lung transplantation from 2009–10 UK data (Table 12). 49 Note that we always used the most recent estimate of survival probability available. From this, we derived a probability per cycle of dying in the first year after transplantation of 0.057. For all following years (2–10), we derived one probability of death between years 2 and 10. This probability of 0.57 (1 – 0.895 × 0.824 × 0.585) translates into a probability per cycle of 0.023.
| Years of transplant | Periods | Survival probabilities |
|---|---|---|
| 2005–8 | 1-year survival | 0.790 |
| 2002–4 | Survival second year given survival first year | 0.895 |
| 2002–4 | Survival fifth year given survival second year | 0.824 |
| 1996–8 | Survival tenth year given survival fifth year | 0.585 |
Survival general population
In Treatment effect we will introduce four scenarios in which the use of ivacaftor in a subgroup of patients (children < 12 years with good lung function) leads to survival as in the general UK population. We used age- and sex-specific life tables for these survival probabilities. 50
Annual decline in percentage predicted forced expiratory volume in 1 second
The age-dependent annual decline in percentage predicted FEV1 in the standard care arm was based on expert consultation. An annual decline in percentage predicted FEV1 of 2% was used for patients aged between 10 and 20 years. For patients < 10 years or > 20 years the manufacturer assumed an annual decline of 1%.
Modified annual decline in percentage predicted forced expiratory volume in 1 second
In our modified model the annual decline in percentage predicted FEV1 in the standard care group was based on the Epidemiologic Study of Cystic Fibrosis, a large prospective, multicentre, observational study designed to characterise the natural history of pulmonary disease and growth in a large population of patients with CF in the USA and Canada with predicted FEV1 (Table 13). 51,52
| Age groups (years) | Numbers of patients | Decline in percentage predicted FEV1 |
|---|---|---|
| 6–8 | 1811 | 1.12% |
| 9–12 | 1696 | 2.39% |
| 13–17 | 1359 | 2.34% |
| 18–24 | 2793 | 1.92% |
| 25+ | 1368 | 1.45% |
Treatment effect
Based on the results of the clinical trials, treatment with ivacaftor was assumed to lead to an (almost) immediate improvement in the percentage predicted FEV1, the decline in percentage predicted FEV1 and the weight-for-age z-score, and a reduction in the annual number of exacerbations.
An initial age-dependent absolute improvement from baseline percentage predicted FEV1 was applied to model the impact of ivacaftor on percentage predicted FEV1 and possible reductions in the number of lung transplantations. This improvement (difference between ivacaftor group and placebo), based on the Phase III RCTs, was 10.5% in patients ≥ 12 years at treatment initiation21 and 10% for patients between 6 and 11 years. 27
For the long-term assessment of clinical effectiveness, extrapolation beyond observed data was required. The manufacturer assumed as base-case scenario that owing to the treatment no decline in percentage predicted FEV1 would occur thereafter. Alternative efficacy scenarios for the rate of FEV1 decline in the ivacaftor–standard care treatment arm were also investigated by the manufacturer (Table 14).
| Manufacturer | Current model | ||
|---|---|---|---|
| Scenarios | Descriptions | Scenarios | Descriptions |
| Base-case manufacturer | Patients treated with ivacaftor experience no annual decline in percentage predicted FEV1 over a lifetime horizon | ‘Optimistic’ scenario | Same |
| Scenario 1 | Ivacaftor patients experience an annual decline in percentage predicted FEV1 of 50% the standard care rate, beginning instantly on treatment initiation and continuing over a lifetime time horizon | Not modelled | |
| Scenario 2 | Ivacaftor patients experience no decline in FEV1 for 90 weeks following treatment initiation. After 90 weeks, ivacaftor patients decline at 66% of the annual standard care rate (based on findings from an evaluation of DNase)53 | ‘Intermediate’ scenario | Same but decline starts after 96 weeks |
| Scenario 3 | Ivacaftor patients experience no decline in FEV1 for 90 weeks following treatment initiation. After 90 weeks, ivacaftor patients decline at the same annual rate as standard care patients | ‘Conservative’ scenario | Same but decline starts after 96 weeks |
| Subgroup analysis | Not conducted | Additional ‘optimistic’ scenario: < 12 years, FEV1 > 70% or 90% | No decline in ivacaftor-treated patients, survival and utility as general population |
The trials21,27 showed an improvement in weight-for-age z-score. This improvement was age dependent and was estimated to be 0.33 for patients aged ≥ 12 years at treatment initiation. Children aged between 6 and 11 years at treatment initiation have an initial increase of 0.39. The initial increase remains over a lifetime period.
The annual reduction in the total number of exacerbations due to ivacaftor was estimated in the clinical trials to be 0.8 (RR 0.45). 21 This absolute reduction is used only for patients ≥ 12 years since the model assumed that patients younger than 12 do not experience exacerbations. The absolute decline of 0.8 exacerbations was kept constant over the whole model duration. In the manufacturer’s submission, ivacaftor was assumed not to influence the prevalence of diabetes or infections and therefore the same prevalence was used for the ivacaftor–standard care strategy.
Modified treatment effects
We made a number of changes regarding treatment effects. Instead of assuming a steady decline in percentage predicted FEV1 in the standard care group, we assumed a decline of 0.4% in the first 48 weeks for patients ≥ 12 years, based on the RCT data. 21 After this period, the earlier reported annual decline in percentage predicted FEV1 becomes relevant. For the ivacaftor group, we modelled an immediate increase in percentage predicted FEV1 of 10.1%, which resulted in a difference between the two groups of 10.5%, as observed in the RCT. For patients aged < 12 years, we assumed no decline in the first 48 weeks for the standard care group and an immediate 10% increase for the ivacaftor group.
Additionally, instead of deriving an absolute reduction in exacerbations, we used the rate ratio of 0.45 to estimate the total number of exacerbations in the ivacaftor treatment group using the updated exacerbation rates described in Survival function. For patients aged < 12 years, no reduction in exacerbations was assumed, as exacerbations were rare in both treatment groups.
Finally, in the scenarios investigated, the duration of no decline in percentage predicted FEV1 in the ivacaftor group was changed from 90 weeks to 96 weeks, as the results of the open-label study provided evidence for treatment effects up to this time point.
For our analyses, we have opted to consider the manufacturer base case, scenario 2 and scenario 3 as relevant and thus we present all results for these three scenarios (see Table 14). We considered scenario 1 less relevant, as it does not reflect results found in the clinical studies, and so this scenario was not modelled.
As ivacaftor corrects the underlying protein defect of CF, the assumption could be made that treatment with ivacaftor prevents any further deterioration in individuals who begin taking ivacaftor before any permanent impairment occurs, although evidence for this assumption (as with all assumptions on long-term effectiveness) is lacking. This would imply that ivacaftor might be effective in young children (e.g. < 12 years) who have little or no lung damage at the start of treatment and that no further progression would occur while these patients remain on ivacaftor treatment. This means that the percentage predicted FEV1 would be close to normal (≥ 90%) for the remaining lifetime, which would be equivalent to the life expectancy in the general population. Furthermore, no or very little standard care for CF treatment would be required.
Based on this assumption, we calculated the cost-effectiveness and budget impact for an additional ‘optimistic scenario’ in a subgroup of patients. In this scenario, the individual patient simulation was programmed to select only patients from the total 206 patients included in the manufacturer’s model who were < 12 years and had little or no lung damage. Because the latter criterion is rather arbitrary, two different cut-off values were used to define normal lung function: a percentage predicted FEV1 > 70% or > 90%. Applying the limit of 70% resulted in the inclusion of 36 patients (17.5%) and applying the limit of 90% resulted in only 20 (9.7%) patients. Treatment with ivacaftor in these patient groups was assumed to result in no progression of the disease, which was modelled in three ways: (1) mortality was set to that of the general population, (2) utilities were set to those of the general population and (3) no lung transplant was required. Furthermore, two different assumptions were made for costs of standard care, which were assumed to be either zero or equal to the Band 1 tariff (see Costs of standard care) plus the costs of DNase.
This resulted in four separate analyses:
-
analysis 1: baseline percentage predicted FEV1 > 70%, standard care costs zero
-
analysis 2: baseline percentage predicted FEV1 > 70%, standard care costs Band 1 tariff plus DNase costs
-
analysis 3: baseline percentage predicted FEV1 > 90%, standard care costs zero
-
analysis 4: baseline percentage predicted FEV1 > 90%, standard care costs Band 1 tariff plus DNase costs.
Utilities
The manufacturer used utility values that were measured during the clinical trials. These utility values were obtained using baseline and end-of-trial (i.e. 48-week) EQ-5D scores obtained from patients in the trials. This generic measure of HRQoL was then adjusted by North American/European normative values to determine the utility scores. These utilities were linked to disease severity expressed in percentage predicted FEV1. Table 15 presents the utility values by percentage predicted FEV1 category used in the manufacturer’s model.
| Percentage predicted FEV1 categories | Quality-of-life values |
|---|---|
| Normal (percentage predicted FEV1 ≥ 90%) | 0.97 |
| Mild (percentage predicted FEV1 70–89%) | 0.95 |
| Moderate (percentage predicted FEV1 40–69%) | 0.93 |
| Severe (percentage predicted FEV1 < 40%) | 0.91 |
Modified utilities
The utilities presented by the manufacturer appear unrealistically high as utilities for the general population are reported to range from 0.94 for people aged < 25 years of age to 0.91 for people aged 35–44 years. 54 In addition, utilities in chronic obstructive pulmonary disease are in the range of 0.79 (percentage predicted FEV1 50–80%) to 0.65 (percentage predicted FEV1 < 30%). 55 For our modified model we based the utility estimates on SF-36 health-related utilities derived from Gee et al. 43 (Table 16). These UK estimates of the utility specified by three severity groups seemed to have more face validity.
| Percentage predicted FEV1 categories | Number of patients | Utility (SD) |
|---|---|---|
| Mild (percentage predicted FEV1 > 70%) | 60 | 0.803 (20.1) |
| Moderate (percentage predicted FEV1 40–69%) | 97 | 0.749 (20.5) |
| Severe (percentage predicted FEV1 < 40%) | 66 | 0.688 (20.2) |
We took the utility post transplantation from a study by Anyanwu et al. 56 This study reported quality-of-life data from a cross-sectional study of 255 patients who had had single or bilateral lung or heart–lung transplants. QoL was measured using the EQ-5D. As most CF patients undergo a bilateral transplantation, we used utilities for these types of transplant. In the first 6 months post-transplant QoL is slightly lower than in the following months. We used the weighted average (weighted by months, the group > 36 months was assumed to contribute 24 months) of these utilities (Table 17) as input for the model.
| Times post transplant, months | Utility | SD | n | SE |
|---|---|---|---|---|
| 0–6 | 0.75 | 0.17 | 14 | 0.045 |
| 7–18 | 0.83 | 0.17 | 16 | 0.043 |
| 19–36 | 0.81 | 0.19 | 21 | 0.041 |
| > 36 | 0.82 | 0.19 | 28 | 0.036 |
| Weighted average | 0.81 | 0.040 |
Utilities for the general population used in the additional scenario for the subgroup of patients aged < 12 years with good lung function were obtained from Sullivan et al. (Table 18). 57
| Age groups (years) | EQ-5D |
|---|---|
| 0–9 | 1.00 |
| 10–19 | 0.91 |
| 20–29 | 0.91 |
| 30–39 | 0.88 |
| 40–49 | 0.84 |
| 50–59 | 0.80 |
| 60–69 | 0.77 |
| 70–79 | 0.72 |
| ≥ 80 | 0.66 |
Costs of standard care
The annual costs of CF patients in the manufacturer’s model consist of two components: drug costs of standard care and the cost of CF care. The age-specific annual drug costs of standard care treatment were calculated based on a retrospective claims study of US health-care costs and utilisation among patients with CF. 58 Three sources59–61 were used to obtain relationships between costs, age and disease severity category. Three disease severity categories were defined: mild (percentage predicted FEV1 ≥ 70%), moderate (percentage predicted FEV1 of 40–69%) and severe (percentage predicted FEV1 < 40%). The relative proportions in each age group are multiplied by the costs per disease severity category. US dollars were converted to UK pounds.
Modified costs of standard care
The original cost data used in the model were not UK specific and were therefore updated for the current analyses. The UK uses a yearly banding system. For the period 2012–13 there are seven bands of increasing complexity in treatment used to assign costs to patients with CF (Table 19). The bandings cover most treatment costs directly related to CF for a patient during a financial year. 62 Patient care, outpatient attendances, home care support, home visits by the multidisciplinary team, general support for patients and carers, i.v. antibiotics (delivered in secondary care) and annual review investigations are included in the bandings. Usage of ‘high cost’ inhaled/nebulised drugs [colistimethate sodium, tobramycin, DNase and aztreonam lysine (Cayston®, Gilead)], surgeries, insertion of gastrostomy devices (percutaneous endoscopic gastrostomy) and totally implantable venous access devices (TIVADs) and primary care-prescribed medication are not included in the tariff bands. The tariffs are defined by complications, therapies, hospitalisation and supplemental feeding. Annual costs per patient in a tariff band are provided in Table 19.
| Banding definitions | Bands | |||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 1A | 2 | 2A | 3 | 4 | 5 | ||
| Therapies | Maximum number of total days of i.v. antibiotics | 0 | 14 | 28 | 56 | 84 | 112 | ≥113 |
| Nebulised antibiotics (P. aeruginosa infection) | Yes | |||||||
| Long-term (> 3 months) nebulised antibiotics or DNase | Yes | |||||||
| Long-term (> 3 months) nebulised antibiotics and DNase | Yes | |||||||
| Hospitalisations | Maximum number of total days in hospital | 0 | 7 | 14 | 57 | 112 | ≥113 | |
| Supplemental feeding | Nasogastric feeds | Yes | ||||||
| Gastrostomy | Yes | |||||||
| Complications | CF-related diabetes or ABPA without other complications | Yes | ||||||
| CF-related diabetes and ABPA | Yes and (FEV1 ≥ 60%) | Yes and (FEV1 < 60%) | ||||||
| Massive haemoptysis or pneumothorax | Yes and (FEV1 ≥ 60%) | Yes and (FEV1 < 60%) | ||||||
| CF related Diabetes and Gastrostomy | Yes and (FEV1 ≥ 60%) | Yes and (FEV1 < 60%) | ||||||
| Non-tuberculous mycobacterium treated or difficult-to-treat infections (e.g. MRSA or B. cepacia) requiring other nebulised antibiotics, e.g. meropenem, Cayston®, vancomycin | Yes | |||||||
| Annual costs per patient | £5210 | £7707 | £7707 | £12,457 | £19,067 | £34,388 | £41,458 | |
| Per cent of patients (≥ 6 years, England) | 15% | 1% | 18% | 32% | 24% | 8% | 3% | |
The CF Registry assigns patients to bands according to the matrix in Table 19 and the accompanying instructions62 using data from the UK Cystic Fibrosis Registry. 63 We contacted the CF Registry and obtained the following individual-level data for 2011: date of birth, percentage predicted FEV1, nebulised tobramycin inhaled solution (TOBI) solution (y/n), colistimethate sodium (y/n), DNase (y/n), gastric feeding tube (y/n), and the payment by results tariff band. 63 Data were available on 7329 patients, of whom 6209 were aged ≥ 6 years. Of these patients, 5786 had a percentage predicted FEV1 recorded; missing values were mostly in the younger patients. In order to examine overall spend, we assigned cost estimates to each patient in the registry. We attached annual costs per patient according to each band (see Table 19). Drug usage dosage and pricing assumptions were derived from British National Formulary,64 and then cross-checked with dosage recommendations provided by the CF Trust Antibiotic Working Group. 59 The variable of gastric feeding tube included all prevalent patients rather than incident cases. Thus, we did not attach costs to these. However, as only 7% of the patients aged ≥ 6 years have a gastric feeding tube, the number of incident cases per year may be assumed to be limited. Note that no data were available on implantation of TIVAD or use of aztreonam lysine. The costs that we attached to each occurrence in the database are listed in Table 20. We then added all costs per patient and performed a regression analysis to explore the relationship between costs, percentage predicted FEV1 and age. We considered two models, one with and one without an interaction between age and percentage predicted FEV1. The adjusted R2 of both models were approximately equal (27%), while the tolerance in the model with interaction suggested that there might be a collinearity issue. We have therefore opted to use the model without interaction. The parameter estimates are found in Table 21.
| Drugs | Annual costs64 |
|---|---|
| Nebulised TOBI solution | £7123 |
| Colistin | £3358 |
| DNase | £6041 |
| Variables | β | SE |
|---|---|---|
| Constant | 41083.87 | 588 |
| Age | –100.78 | 12 |
| Percentage predicted FEV1 | –254.34a | 6 |
In the additional scenario for the subgroup of patients < 12 years of age with no or little lung damage, costs for standard care were assumed to be either zero or as high as the Band 1 tariff (£5210 per annum) (Department of Health, 2012) plus the costs for DNase (£6041), that is to say £11,251 per annum for their remaining lifetime. 62 Costs of DNase are added to those of Band 1 because this drug is not covered by the bands but we believe that it is likely that these patients would also receive this drug.
Cost of ivacaftor
The drug costs of standard care and the CF costs are the same in the ivacaftor–standard care strategy as in the standard care strategy. The annual cost of ivacaftor was set at £182,000 (Vertex 2012) (Table 22). The number of years until the patent of ivacaftor expires was assumed to be 14 years; the manufacturer assumed that after this a generic drug would be launched at a lower price of £20,000 (Vertex 2012). 16 The manufacturer used an adherence rate for ivacaftor of 91% based on the Phase III trials,21,27 but they indicated that real-world adherence rates are typically lower than those observed in clinical studies. The observed efficacy of ivacaftor is based on the observed adherence rate of 91%; a reduction of adherence would be likely to impact on efficacy.
| Parameter | Value | Source |
|---|---|---|
| Annual drug costs, brand | £182,000 | Vertex 2012 |
| Annual drug costs, generic | £20,000 | Vertex 2012 |
| Adherence rate | 91% | Vertex 2012 |
Costs of lung transplantation
For the lung transplantation part of the model, two cost estimates are relevant: the cost of the transplantation itself and the costs of follow-up (Table 23). For the cost of the transplantation we used the 2010 reference costs. 65 We combined the costs for elective in-hospital stay with the costs of excess elective hospital days, which resulted in an estimate of £42,018 per transplantation.
| Parameter | Costs per year |
|---|---|
| Procedure | £42,018 |
| Follow-up year 1 | £21,634 |
| Follow-up year 2 | £13,063 |
| Follow-up year 3 | £13,733 |
| Follow-up year 4–10 | £8249 |
| Follow-up subsequent years | £4590 |
The costs of follow-up were based on a study by Anyanwu et al. 66 In this study, costs are reported for up to 15 years after transplantation (between years 10 and 15 based on extrapolation). We used the costs as reported for bilateral transplantation as these are most common (in 2010, 26 out of 29 transplants) in CF patients. As these costs were reported in 1999 UK pounds and were discounted at 6%, we first reversed the discounting and then adjusted the 1999 UK pounds to 2011 UK pounds using a price index of 1.48. 67,68 It is important to realise that the costs per year reported by Anyanwu et al. 66 are per transplanted patient, whereas our model required input per patient still alive. Thus, the costs reported by Anyanwu et al. 66 were adjusted according to the survival rates in the same study. Note that once patients have had a lung transplant, only the above-described follow-up costs apply and thus costs for treatment of CF were assumed to be zero.
Model assumptions
The main model assumptions made in the cost-effectiveness analysis are summarised below:
-
The starting patient population for the individual patient simulation is the population included in the two RCTs included in the clinical effectiveness review. We assumed that this population is representative of the total CF population in England.
-
The efficacy of ivacaftor will translate into better survival based on a survival function presented by Liou et al. 37
-
We assumed that the population under investigation was comparable with the population from Liou et al. 37
-
The age-specific annual decline in percentage predicted FEV1 was based on the epidemiologic study of CF in a large population of patients with CF in the USA and Canada. We assumed that these declines were also appropriate for the UK population.
-
Patients treated with standard care experience a decline in FEV1 as reported in the RCTs for the first 96 weeks; thereafter an age-dependent annual decline based on a large epidemiologic study was used.
-
The weight-for-age z-score was assumed to be constant over a lifetime period from the baseline score.
-
The annual exacerbation rate was assumed to be dependent on percentage predicted FEV1 and age.
-
For patients < 12 years old, no reduction in exacerbations was assumed, as exacerbations were rare in both treatment groups.
-
The QoL of patients after a lung transplant was based on the patients who have undergone a bilateral transplant as the majority of transplants are bilateral.
-
Average dosages are used to estimate the costs of the high-cost drugs.
-
The annual cost of ivacaftor was assumed to be £182,000.
-
The number of years until the ivacaftor patent expires was assumed to be 14 years.
-
The annual cost of a generic drug was assumed to be £20,000.
-
The adherence rate to ivacaftor was assumed to be 91% based on the Phase III trials.
Probabilistic sensitivity analyses
There are various uncertainties in the model with regards to the input data. The impact of these uncertainties was explored through PSA. Values used are summarised in Table 24.
| PSA input parameters | Parameters | Mean | SE | Distribution |
|---|---|---|---|---|
| Coefficients survival curve | b_gender | 0.150 | 0.0740 | Normal |
| b_fev1% | –0.042 | 0.0025 | Normal | |
| b_ # exacerbations | 0.350 | 0.0240 | Normal | |
| b_diabetes | 0.440 | 0.0980 | Normal | |
| b_Sa Infection | –0.250 | 0.0900 | Normal | |
| b_Bc Infection | 1.410 | 0.1900 | Normal | |
| b_panc sufficiency | –0.140 | 0.2300 | Normal | |
| b_wt | –0.280 | 0.0410 | Normal | |
| b_Bc inf * exacer | –0.280 | 0.0600 | Normal | |
| Utility (FEV1 ≥ 70%) | 0.80 | 0.0259 | Beta | |
| Utility (40%≤ FEV1 < 70%) | 0.75 | 0.0208 | Beta | |
| Utility (< 40%) | 0.69 | 0.0249 | Beta | |
| Utility (post Tx) | 0.81 | 0.0400 | Beta | |
| Absolute number of exacerbations per year standard care ≥ 12 years | 0.83 | 0.0308 | Normal | |
| Absolute number of exacerbations per year standard care < 12 years | 0.51 | 0.0500 | Normal | |
| Reduction (RR) in exacerbations for ivacaftor patients | 0.45 | 0.0357 | Normal | |
| Initial increase in FEV1 percentage predicted for ivacaftor patients, ≥ 12 years | 0.10 | 0.0121 | Normal | |
| Initial increase in FEV1 percentage predicted for ivacaftor patients, < 12 years | 0.10 | 0.0630 | Normal | |
| Initial increase in weight-for-age z-score for ivacaftor patients ≥ 12 years | 0.33 | 0.0200 | Normal | |
| Initial increase in weight-for-age z-score for ivacaftor patients, < 12 years | 0.39 | 0.0800 | Normal | |
| Coefficients standard care cost equation | const | £41,084 | £588 | Normal |
| b_age | –£100 | £12 | Normal | |
| b_FEV1 | –£254 | £6 | Normal | |
| Probability lung Tx per cycle | 0.046 | 0.0084 | Beta | |
| Probability of death per cycle after Tx first year | 0.057 | 0.0057 | Beta | |
| Probability of death per cycle after Tx subsequent years | 0.023 | 0.0023 | Beta | |
| Cost Tx | £42,018 | £4202 | Normal | |
| Cost FU Tx first year | £21,634 | £2163 | Normal | |
| Cost FU Tx second year | £13,063 | £1306 | Normal | |
| Cost FU Tx third year | £13,733 | £1373 | Normal | |
| Cost FU Tx years 4–10 | £8249 | £825 | Normal | |
| Cost FU Tx subsequent years | £4590 | £459 | Normal |
Budget impact
When deciding whether or not to fund drugs in England, those which go through the NICE appraisal process are usually compared with an ICER threshold (NICE guidance). However, different decision rules might apply to orphan drugs (for diseases with a prevalence lower than 5 in 10,000)69 and the macro (total population) impact of introducing a new expensive treatment might be an important consideration. The manufacturers included a budget impact analysis but we could not use this as it was based on non-UK values and assumptions. We therefore conducted our own budget impact analysis for England to estimate the budget impact of total lifetime costs and first-year costs. Budget impact is equal to the mean cost per individual × population size. Total lifetime costs per individual were derived from the results of the main cost-effectiveness analysis and first-year costs per individual were estimated by running the cost-effectiveness model for the first year. Different values were obtained for the three different scenarios (conservative, optimistic, intermediate) for lifetime costs. For first-year costs a single value was obtained as the three scenarios were identical for the first year. Discounted costs were used for all analyses.
The population size (number of patients eligible for treatment with ivacaftor) in England was estimated based on the data in Table 25. These show that the total number of people in England eligible for treatment with ivacaftor is 271.
| Patient groups | Prevalence, % | Number of people | Denominator | Source |
|---|---|---|---|---|
| Number of patients with CF in England | NA | 7329 | NA | CF Trust |
| Number of CF patients (≥ 6) | NA | 6209 | NA | CF Trust |
| Number of CF patients (≥ 6) with the G551D mutation | 4.4%a | 271 | 6209 | Estimate |
| Percentage not genotyped | 4.7a | 289 | 6209 | Estimate |
Results from CF Registry data suggested that 4.7% of patients had unknown gene mutation. With the introduction of ivacaftor, these patients would need to be tested in order to determine if they were eligible for this treatment. We therefore incorporated the costs required to screen the 289 patients (aged ≥ 6) for whom the gene mutation is unknown, which was not considered in the cost-effectiveness analysis. The additional costs of a CF mutation test in these patients were added to the ivacaftor + standard care treatment arm. The cost of the CF mutation test was estimated to be £160. 70
We also conducted a sensitivity analysis for the additional optimistic subgroup analysis. We estimated the number of patients aged 6 to 11 years with FEV1 > 70% and > 90% who would be eligible for ivacaftor treatment based on the data in Table 26. This suggested that 48 children with a FEV1 > 70% and 25 children with a FEV1 > 90% would be eligible for treatment. A further 52 children with a FEV1 > 70% and 27 with a FEV1 > 90% would not have been genotyped.
| Patient groups | Prevalence (%) | Number of people | Denominator | Source |
|---|---|---|---|---|
| Number of CF patients aged 6–12 years | NA | 1283 | NA | CF Trust |
| Number of CF patients aged 6–12 years with percentage predicted FEV1 > 70% | NA | 1096 | NA | CF Trust |
| Number of CF patients aged 6–12 years with percentage predicted FEV1 > 90% | NA | 581 | NA | CF Trust |
| Number of CF patients aged 6–12 years with percentage predicted FEV1 > 70% and G551D mutation | 4.4%a | 48 | 1096 | Estimate |
| Number of CF patients aged 6–12 years with percentage predicted FEV1 > 90% and G551D mutation | 4.4%a | 25 | 581 | Estimate |
| Percentage with FEV1 > 70% not genotyped | 4.7a | 52 | 1096 | Estimate |
| Percentage with FEV1 > 90% not genotyped | 4.7a | 27 | 581 | Estimate |
Chapter 6 Results of cost-effectiveness analyses
Costs, effects and cost-effectiveness
Conservative scenario
In the conservative scenario, the percentage predicted FEV1 of ivacaftor-treated patients stays stable for 96 weeks, after which it declines by the same rate as in the standard care population. Without discounting, treatment with ivacaftor leads to a lifetime additional cost of £2.1M (standard care £0.4M, ivacaftor + standard care £2.5M) while gaining 2.7 life-years (standard care 16.25, ivacaftor + standard care 18.94) or 2.18 QALYs (standard care 12.29, ivacaftor + standard care 14.47). After discounting costs and effects, the additional costs of ivacaftor amount to £1.6M, with a gain in life-years of 1.52 and a gain in QALYs of 1.27, leading to an ICER of £1.27M. The ratio of costs to effects is least favourable for the younger age groups, becoming more favourable as the age at start of treatment increases (Table 27). The PSA suggests that the ICER is likely to be between £980,000 and £1.85M per QALY gained (see Table 30).
| Age groups (years) | Total costs, £ | Total LYs | Total QALYs | Incrementals | ICER | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard care | Standard care + ivacaftor | Standard care | Standard care + ivacaftor | Standard care | Standard care + ivacaftor | Cost, £ | LYs | QALYs | Cost/LY, £ | Cost/QALY, £ | |
| 6–9 | 377,369 | 2,254,478 | 16.64 | 17.90 | 12.78 | 13.91 | 1,877,109 | 1.25 | 1.13 | 1,497,903 | 1,659,110 |
| 10–14 | 336,038 | 2,135,961 | 13.90 | 15.34 | 10.56 | 11.80 | 1,799,924 | 1.44 | 1.25 | 1,249,949 | 1,444,225 |
| 15–19 | 301,868 | 2,031,015 | 12.68 | 14.09 | 9.62 | 10.83 | 1,729,147 | 1.41 | 1.21 | 1,230,573 | 1,433,410 |
| 20–24 | 261,493 | 1,902,274 | 11.33 | 12.74 | 8.64 | 9.77 | 1,640,781 | 1.40 | 1.14 | 1,168,744 | 1,441,148 |
| 25–29 | 214,716 | 1,711,345 | 8.76 | 10.45 | 6.55 | 7.92 | 1,496,630 | 1.69 | 1.37 | 886,942 | 1,090,397 |
| 30–34 | 184,657 | 1,599,947 | 7.84 | 9.62 | 5.87 | 7.31 | 1,415,291 | 1.78 | 1.44 | 795,865 | 983,073 |
| 35–39 | 148,188 | 1,411,962 | 6.30 | 8.16 | 4.70 | 6.17 | 1,263,774 | 1.85 | 1.48 | 681,394 | 854,858 |
| 40–44 | 130,071 | 1,316,147 | 5.63 | 7.51 | 4.18 | 5.68 | 1,186,075 | 1.88 | 1.50 | 630,479 | 789,149 |
| 45+ | 96,733 | 1,071,256 | 3.98 | 5.83 | 2.93 | 4.35 | 974,519 | 1.85 | 1.41 | 526,010 | 689,186 |
| Overall | 267,393 | 1,882,254 | 11.34 | 12.86 | 8.60 | 9.87 | 1,614,861 | 1.52 | 1.27 | 1,062,219 | 1,273,805 |
Optimistic scenario
In the optimistic scenario, the percentage predicted FEV1 of ivacaftor-treated patients stays stable over lifetime, while in standard care patients the percentage predicted FEV1 declines over time. Without discounting, treatment with ivacaftor leads to a lifetime additional cost of £2.5M (standard care £0.4M, ivacaftor + standard care £2.9M) while gaining 19.8 life-years (standard care 16.25, ivacaftor + standard care 36.00) or 16.3 QALYs (standard care 12.29, ivacaftor + standard care 28.66). After discounting costs and effects, the additional costs of ivacaftor amount to £1.8M, with a gain in life-years of 6.20 and a gain in QALYs of 5.26, leading to an ICER of £334,775. The ratio of costs to effects is most favourable for the younger age groups and increases slightly as the age at the start of treatment increases (Table 28). The PSA suggests that, given the parameter uncertainty, the ICER is likely to be between £284,000 and £401,000 per QALY gained (see Table 30).
| Age groups (years) | Total costs, £ | Total LYs | Total QALYs | Incrementals | ICER | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard care | Standard care + ivacaftor | Standard care | Standard care + ivacaftor | Standard care | Standard care + ivacaftor | Cost, £ | LYs | QALYs | Cost/LY, £ | Cost/QALY, £ | |
| 6–9 | 377,369 | 2,330,496 | 16.64 | 23.92 | 12.78 | 19.18 | 1,953,127 | 7.28 | 6.40 | 268,296 | 305,181 |
| 10–14 | 336,038 | 2,260,721 | 13.90 | 21.01 | 10.56 | 16.71 | 1,924,684 | 7.10 | 6.15 | 271,041 | 312,824 |
| 15–19 | 301,868 | 2,178,788 | 12.68 | 19.45 | 9.62 | 15.45 | 1,876,920 | 6.77 | 5.83 | 277,442 | 321,971 |
| 20–24 | 261,493 | 2,064,591 | 11.33 | 17.61 | 8.64 | 13.89 | 1,803,099 | 6.28 | 5.25 | 287,253 | 343,167 |
| 25–29 | 214,716 | 1,895,717 | 8.76 | 14.31 | 6.55 | 11.17 | 1,681,002 | 5.55 | 4.62 | 302,847 | 364,130 |
| 30–34 | 184,657 | 1,784,942 | 7.84 | 13.20 | 5.87 | 10.32 | 1,600,285 | 5.36 | 4.45 | 298,361 | 360,008 |
| 35–39 | 148,188 | 1,593,925 | 6.30 | 10.98 | 4.70 | 8.49 | 1,445,737 | 4.68 | 3.79 | 308,897 | 381,088 |
| 40–44 | 130,071 | 1,492,938 | 5.63 | 10.04 | 4.18 | 7.76 | 1,362,867 | 4.41 | 3.59 | 308,889 | 380,158 |
| 45+ | 96,733 | 1,236,253 | 3.98 | 7.39 | 2.93 | 5.59 | 1,139,521 | 3.41 | 2.66 | 333,780 | 429,077 |
| Overall | 267,393 | 2,029,969 | 11.34 | 17.53 | 8.60 | 13.86 | 1,762,567 | 6.20 | 5.26 | 284,501 | 334,775 |
Intermediate scenario
The intermediate scenario lies between the conservative and optimistic scenarios, with the percentage predicted FEV1 of ivacaftor-treated patients declining after 96 weeks at a rate of 66% of that of standard care patients. Without discounting, treatment with ivacaftor leads to a lifetime additional cost of £2.2M (standard care £0.4M, ivacaftor + standard care £2.6M) while gaining 5.29 life-years (standard care 16.25, ivacaftor + standard care 21.54) or 4.27 QALYs (standard care 12.29, ivacaftor + standard care 16.56). After discounting costs and effects, the additional costs of ivacaftor amount to £1.7M, with a gain in life-years of 2.58 and a gain in QALYs of 2.16, leading to an ICER of £771,297. As in the conservative scenario, the ratio of costs to effects is least favourable for the younger age groups and becoming more favourable as the age at the start of treatment increases (Table 29). The PSA suggests that the ICER is likely to be between £607,699 and £1.05M per QALY gained (Table 30).
| Age groups (years) | Total costs, £ | Total LYs | Total QALYs | Incrementals | ICER | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard care | Standard care + ivacaftor | Standard care | Standard care + ivacaftor | Standard care | Standard care + ivacaftor | Cost, £ | LYs | QALYs | Cost/LY, £ | Cost/QALY, £ | |
| 6–9 | 377,369 | 2,283,223 | 16.64 | 19.33 | 12.78 | 15.15 | 1,905,854 | 2.69 | 2.37 | 708,537 | 803,059 |
| 10–14 | 336,038 | 2,176,018 | 13.90 | 16.62 | 10.56 | 12.89 | 1,839,980 | 2.72 | 2.33 | 677,537 | 788,304 |
| 15–19 | 301,868 | 2,080,394 | 12.68 | 15.29 | 9.62 | 11.85 | 1,778,526 | 2.61 | 2.22 | 682,368 | 799,394 |
| 20–24 | 261,493 | 1,956,552 | 11.33 | 13.83 | 8.64 | 10.69 | 1,695,059 | 2.50 | 2.05 | 677,498 | 825,322 |
| 25–29 | 214,716 | 1,769,591 | 8.76 | 11.28 | 6.55 | 8.60 | 1,554,875 | 2.52 | 2.05 | 617,920 | 758,240 |
| 30–34 | 184,657 | 1,659,432 | 7.84 | 10.41 | 5.87 | 7.96 | 1,474,776 | 2.57 | 2.09 | 574,244 | 705,951 |
| 35–39 | 148,188 | 1,469,508 | 6.30 | 8.78 | 4.70 | 6.69 | 1,321,320 | 2.48 | 1.99 | 532,964 | 663,811 |
| 40–44 | 130,071 | 1,372,635 | 5.63 | 8.07 | 4.18 | 6.14 | 1,242,564 | 2.45 | 1.97 | 507,393 | 631,814 |
| 45+ | 96,733 | 1,123,009 | 3.98 | 6.18 | 2.93 | 4.63 | 1,026,276 | 2.21 | 1.69 | 464,920 | 606,125 |
| Overall | 267,393 | 1,930,690 | 11.34 | 13.91 | 8.60 | 10.76 | 1,663,297 | 2.58 | 2.16 | 645,780 | 771,297 |
| Measures | Conservative scenario | Optimistic scenario | Intermediate scenario | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Incremental cost, £ | Incremental QALY | ICER, £ | Incremental cost, £ | Incremental QALY | ICER, £ | Incremental cost, £ | Incremental QALY | ICER, £ | |
| Mean | 1,613,491 | 1.25 | 1,313,333 | 1,762,287 | 5.24 | 338,293 | 1,655,640 | 2,05 | 814,401 |
| SD | 12,800 | 0.17 | 178,467 | 15,517 | 0.39 | 24,349 | 15,324 | 0.20 | 73,733 |
| Min. | 1,590,459 | 0.87 | 980,700 | 1,708,888 | 4.36 | 284,473 | 1,614,532 | 1.55 | 607,699 |
| Median | 1,613,534 | 1.27 | 1,278,072 | 1,763,910 | 5.21 | 338,723 | 1,654,904 | 2.05 | 811,036 |
| Max. | 1,643,041 | 1.68 | 1,845,752 | 1,802,561 | 6.171 | 400,559 | 1,693,060 | 2.76 | 1,047,179 |
Subgroup analysis: ‘optimistic scenario’ in young children with good lung function
Tables 31 to 34 show that assuming no further disease progression for the proportion of the trial population who were aged < 12 years and had specified baseline lung function would reduce the ICER substantially (range £154,257 to £200,268) in comparison with the optimistic scenario for the whole population, which had an ICER of £334,775 (see Optimistic scenario). This is for three main reasons: reduced mortality (and thus increased life expectancy), increased utility (and thus increased QALYs) and reduced cost of standard care (to zero in scenarios 1 and 3).
| Strategies | Total costs, £ (disc.) | Total costs, £ (undisc.) | Total LYs (disc.) | Total LYs (undisc.) | Total QALYs (disc.) | Total QALYs (undisc.) | Incremental costs, £ (disc.) | Incremental QALYs (disc.) | Cost per QALY, £ (disc.) |
|---|---|---|---|---|---|---|---|---|---|
| Standard care | 360,185 | 606,412 | 16.89 | 27.06 | 13.06 | 20.73 | NA | NA | Reference |
| Ivacaftor + standard care | 2,354,701 | 4,113,770 | 26.18 | 72.18 | 24.08 | 72.87 | 1,994,516 | 11.02 | 180,987 |
| Strategies | Total costs, £ (disc.) | Total costs, £ (undisc.) | Total LYs (disc.) | Total LYs (undisc.) | Total QALYs (disc.) | Total QALYs (undisc.) | Incremental costs, £ (disc.) | Incremental QALYs (disc.) | Cost per QALY, £ (disc.) |
|---|---|---|---|---|---|---|---|---|---|
| Standard care | 360,185 | 606,412 | 16.89 | 27.06 | 13.06 | 20.73 | NA | NA | Reference |
| Ivacaftor | 2,060,130 | 3,301,687 | 26.18 | 72.18 | 24.08 | 72.87 | 1,699,945 | 11.02 | 154,257 |
| Strategies | Total costs (disc.), £ | Total costs (undisc.), £ | Total LYs (disc.) | Total LYs (undisc.) | Total QALYs (disc.) | Total QALYs (undisc.) | Incremental costs (disc.), £ | Incremental QALYs (disc.) | Cost per QALY (disc.), £ |
|---|---|---|---|---|---|---|---|---|---|
| Standard care | 352,444 | 621,317 | 18.01 | 29.86 | 14.07 | 23.07 | NA | NA | Reference |
| Ivacaftor + standard care | 2,351,687 | 4,088,066 | 26.09 | 71.31 | 24.05 | 72.20 | 1,999,243 | 9.98 | 200,268 |
| Strategies | Total costs (disc.), £ | Total costs (undisc.), £ | Total LYs (disc.) | Total LYs (undisc.) | Total QALYs (disc.) | Total QALYs (undisc.) | Incremental costs (disc.), £ | Incremental QALYs (disc.) | Cost per QALY (disc.), £ |
|---|---|---|---|---|---|---|---|---|---|
| Standard care | 352,444 | 621,317 | 18.01 | 29.86 | 14.07 | 23.07 | NA | NA | Reference |
| Ivacaftor | 2,058,197 | 3,285,701 | 26.09 | 71.31 | 24.05 | 72.20 | 1,705,752 | 9.98 | 170,869 |
Budget impact
When we translate the per-patient results from the previous section to population costs, we find that the total lifetime cost of treating all ivacaftor-eligible patients in England (n = 271; see Chapter 5, Budget impact) is £510M for the conservative scenario, £550M for the optimistic scenario and £523M for the intermediate scenario, plus one-time genetic testing costs of 289 × £160 = £46,240. The total lifetime costs of treating patients with standard care would amount to £72M. The incremental costs (additional cost of ivacaftor compared with standard care alone) are £438M for the conservative scenario, £479M for the optimistic scenario and £451M for the intermediate scenario. The total additional costs in the first year (assuming all eligible patients receive ivacaftor) would amount to £43M, while the first-year costs for standard care are £5.6M. Note that in the first year, all scenarios produce the same result.
The estimate for the total lifetime cost of treating all patients aged 6–12 years who have baseline percentage predicted FEV1 > 70% in England (n = 48; see Chapter 5, Budget impact) with ivacaftor is £99M for the scenario with no standard care cost (scenario 2) and £113M for the scenario with Band 1 and DNase costs for standard care (scenario 1). One-time genetic testing costs of £8320 (52 × £160) should be added to each scenario. The incremental costs (additional cost of ivacaftor compared with standard care alone) is £81M for the zero-cost standard care scenario and £96M for the scenario with Band 1 and DNase costs for standard care. In the scenario where standard care is zero for ivacaftor patients, the total additional costs in the first year would amount to £7M whereas assuming that the standard care for ivacaftor patients includes Band 1 and DNase leads to total additional costs in the first year of £7.5M. The first-year costs for standard care patients (i.e. without ivacaftor) are £0.8M.
The estimate for the total lifetime cost of treating all patients aged 6–12 years who have baseline percentage predicted FEV1 > 90% in England (n = 25; see Chapter 5, Budget impact) with ivacaftor is £51.5M for the scenario with no standard care cost and £58.8M for the scenario with Band 1 and DNase costs for standard care. One-time genetic testing costs of £4320 (27 × £160) should be added to each scenario. The incremental costs (additional cost of ivacaftor compared with standard care alone) is £42.6M for the zero-cost standard care scenario and £50.0M for the scenario with Band 1 and DNase costs for standard care. In the scenario where standard care is zero for ivacaftor patients, the total additional costs in the first year would amount to £3.7M, whereas assuming that the standard care for ivacaftor patients includes Band 1 and DNase leads to total additional costs in the first year of £4M. The first-year costs for standard care patients (i.e. without ivacaftor) are £0.4M.
Chapter 7 Discussion
Statement of principal findings
Clinical effectiveness
The clinical effectiveness review found that ivacaftor is an effective treatment for adults and children with the G551D mutation based on two RCTs, one in adults and one in children, and an open-label extension trial of the two included RCTs. The studies were generally well conducted and were rated as low or unclear on all risk of bias domains; limited details available on the study in children resulted in some domains being rated as unclear. All outcomes assessed in the review showed greater improvements in the ivacaftor group than in the placebo group. All differences were statistically significant (p < 0.02) with the exception of QoL in children where differences between ivacaftor and placebo favoured ivacaftor but failed to reach statistical significance at either 24- or 48-week follow-up. Ivacaftor was associated with an absolute increase of around 10% in the percentage predicted FEV1 compared with baseline values while levels in patients treated with placebo stayed around the same. Results from the open-label study showed that improvements in lung function, quality of life and weight were maintained after a further 48 weeks of treatment with ivacaftor (96 weeks’ total treatment). Subgroup analysis showed similar improvements in lung function for all subgroups investigated. This suggests that the effects of ivacaftor do not differ significantly according to age, sex, geographical region or baseline lung function. Ivacaftor does not appear to be associated with an increased risk of adverse events or withdrawals.
Cost-effectiveness
The economic evaluation of ivacaftor showed that the ICER varies between £334,000 and £1.27M per QALY gained, depending on the assumptions made for the long-term effectiveness of ivacaftor. We explored three scenarios: conservative, optimistic and an intermediate scenario. The variation between ICERs was mostly due to large differences in QALYs gained between the scenarios, which varied between 1.27 and 5.26. We found that the impact of the remaining parameter uncertainty is small compared with the uncertainty caused by the long-term extrapolation. An additional optimistic scenario for the subgroup of patients < 12 years of age with no or little lung damage resulted in an ICER of between £154,000 and £200,000 per QALY gained.
We also explored the budget impact for England of introducing ivacaftor to all eligible CF patients. We found that the total additional lifetime costs (discounted) for this cohort would amount to £438M to £479M, whereas the lifetime costs for standard care only would amount to £72M. The total additional costs in the first year would amount to £43M (including the costs for genetic testing) while the costs for standard care would amount to only £5.6M.
When the population treated with ivacaftor is limited to patients < 12 years of age with no or little lung damage, we found that the total additional lifetime costs (discounted) amount to £51M to £113M, whereas the lifetime costs for standard care only would be between £9M and £17M. The total additional costs in the first year would amount to £3.7M to £7.5M (including the costs for genetic testing) while the costs for standard care only would amount to £0.4M to £0.8M.
Strengths and limitations
Clinical effectiveness
Extensive literature searches were conducted in an attempt to maximise retrieval of relevant studies. These included electronic searches of a variety of bibliographic databases, screening references of included publications, as well as screening of clinical trials registers and conference abstracts to identify unpublished studies. Despite this, we were able to identify only two RCTs and one open-label study which met the inclusion criteria for our review. This finding is to be expected because ivacaftor is a very new drug and so has currently been evaluated only by the manufacturer.
Clear inclusion criteria were specified in the protocol for this review. The eligibility of studies for inclusion is therefore transparent. In addition, we have provided specific reasons for excluding all of the studies which were assessed as full-text papers. We restricted inclusion into the review to studies that reported a minimum of 3 months’ follow-up. We felt that follow-up shorter than this was insufficient to establish a sustained treatment effect. This resulted in the exclusion of three small Phase II trials, all of which reported positive effects of ivacaftor. The review process followed recommended methods to minimise the potential for error and/or bias. 18 Search results were independently screened for relevance by two reviewers, and full-text inclusion assessment, data extraction and quality assessment were done by one reviewer and checked by a second. Any disagreements were resolved by consensus. We conducted a formal quality assessment to evaluate the risk of bias in the included studies. This was limited by the lack of published details on the children’s trial, which resulted in this trial being rated as unclear on some domains.
The small number of studies conducted in different patient groups (adults and children) meant that it was not possible to conduct a formal meta-analysis. Instead results were presented grouped on outcome to provide an overview of the evidence available for each outcome. The small number of included studies also meant that it was not possible to formally investigate the potential for publication bias. However, the risk of publication bias is likely to be very low. All trials of ivacaftor identified by the searches (both included and excluded) were conducted by the manufacturers and were registered on trial registries, which were searched as part of our systematic searches. It would not have been possible for anyone other than the manufacturer to conduct a trial of ivacaftor prior to it being licensed. Licensing was only granted in the USA on 31 January 2012,14 and so there would not have been time for new studies to be conducted since these data and for results to have been published that would have fulfilled our inclusion criteria. Details were available from multiple sources for the two included RCTs. The primary report of the adults’ study included a supplementary file containing detailed results information. However, some information was lacking from this report, in particular information on the variability of effect estimates (CIs and standard deviations). Other sources were therefore used to locate this information, in particular documents available via the FDA website34–36 and supplementary data provided by Vertex. The children’s study and the open-label study have not yet been reported as full-text journal articles. Information relating to these studies was therefore obtained from conference abstracts,27 the FDA website,34–36 a Vertex press release,29 and the confidential dossier supplied by the manufacturer. 16 A problem with obtaining results from multiple sources was that there were slight discrepancies in figures reported in different reports. We therefore developed a hierarchy to select a single estimate to contribute to the results of the review. We also included details of differences in results in Appendix 5. These differences were all small and unlikely to have impacted on the conclusions of the review.
The methods of analysis in the included RCTs appeared statistically robust. This was supported by the FDA analysis of these studies, which included various sensitivity analyses using different statistical methods. These analyses all showed similar results for the primary outcome of percentage predicted FEV1 through to week 24. 36
Cost-effectiveness
We performed a critical appraisal of the model submitted by the manufacturer, and were able to improve many model inputs, such as the quality-of-life estimates, the costs of standard care and the natural decline in percentage predicted FEV1. We were also able to include lung transplantation (with its associated survival, quality-of-life and follow-up costs) to the model.
The patient population in the model was based on the patients included in the two RCTs. Comparison of baseline characteristics of these patients with characteristics of all CF patients in the UK obtained from the UK CF registry showed that the patient populations were comparable with respect to the proportion of male to female and the median FEV1. However, the percentage of patients with a chronic P. aeruginosa infection was higher in our patient population (76% in the adult population) compared with 51% in the total UK CF population aged > 12 years. This could indicate that despite the comparable FEV1 the patient population in the model included more patients whose lungs were already severely and irreversibly damaged by CF, which may have an influence on the generalisability of the results.
The survival function used in the model requires an estimate of the annual number of exacerbations per patient. The annual exacerbation rates for the standard care group used in the manufacturer model, 1.4 for patients ≥ 12 years and 0 for patients < 12 years, were based on the small numbers of patients in the two trials (n = 78 and n = 28, respectively) and an observation period of 48 weeks. We adapted these rates because they were based on small sample sizes and obtained from a trial population, which is generally a selective population due to the inclusion and exclusion criteria used. The current clinical trials, for example, included only patients with clinically stable CF without any respiratory infection or exacerbation in the 4 weeks before the start of the study. Furthermore, studies reporting on exacerbation frequencies should at least have a follow-up of 1 year to account for seasonal variation. Therefore, the annual exacerbation rates in the adapted model were based on a large, continuous, observational registry, the US CF Patient Registry, which most likely resulted in exacerbation rates that were more representative for the total CF population than the trial estimates.
In the model, we have included lung transplantation, assuming that patients with a percentage predicted FEV1 < 30% are eligible for a lung transplant. However, in reality, eligibility is increasingly based on additional factors as, in the past years, the survival of patients with a percentage predicted FEV1 < 30% has markedly increased. 71 Additionally, the costs of follow-up of transplanted patients were based on a study by Anyanwu et al. 66 However, it is unclear if these costs also include the potential CF-related costs that will still occur for CF complications in other organs. Thus, it is possible that the post-transplantation costs should be higher. However, both the percentage of patients eligible for lung transplant and the post-transplantation costs have a very minimal impact on the ICER.
Three out of four dimensions on which ivacaftor showed an effect (percentage predicted FEV1, weight and exacerbations) were taken into account in the model. However, the decrease in the number of exacerbations due to ivacaftor was included in the model only in so far as it affected the survival of the patients. A reduction in exacerbations results in decreased mortality and so reduced exacerbations contributes to life-year gains. It is reasonable to assume that a reduction in exacerbations also has a direct effect on lung function, QoL and costs. Studies by Saunders et al. 72,73 show that there is a strong association between frequency of exacerbations and a decline in lung function. In addition, a study by Britto et al. 74 found that pulmonary exacerbations in the past 6 months were negatively associated with both physical and psychosocial measures of quality of life. Furthermore, treatment of pulmonary exacerbations is associated with high health-care costs, especially for inpatient hospital care and medication. 75 A reduction in exacerbations would therefore lead to an increase in quality of life and a reduction of health-care costs. However, due to a lack of valid data on the association between CF exacerbations and QoL (in terms of utility values) and the costs of a CF exacerbation, the independent impact of exacerbations on QoL and costs could not be included in the model. The CF bandings cover most costs for patients with CF but it was not possible to disentangle maintenance costs from treatment costs for exacerbations. Ideally, we would have added an event probability to the model, indicating per cycle what the probability of an exacerbation is, possibly dependent on age and percentage predicted FEV1. However, such data were neither found in the literature nor available in the clinical study reports of ivacaftor. One of the challenges in modelling exacerbations explicitly is that a treatment-related reduction in exacerbations is often a result of both an indirect impact through lung function and a direct impact. In order to avoid double counting, we would have needed patient-level data on all lung function measurements and all dates when exacerbations occurred. Also, information about the severity of the exacerbation would be required. Additionally, valid data would need to be found on the costs of a CF exacerbation. In the data source used as input for the cost of CF care by severity no distinction was made between costs for maintenance treatment and costs for exacerbations. If the treatment effects on exacerbations could have been taken into account then the gain in QALYs in the ivacaftor group might have been higher and the savings in CF-related health-care costs might have been higher, resulting in a lower ICER.
In the model, quality-of-life values and costs were assumed to be dependent on disease severity defined in terms of percentage predicted FEV1. However, a study by Gee et al. 76 showed that this clinical measure explains only part of the variation in QoL and they suggested that other clinical and social factors might also be important, such as social support and coping strategies. Our analysis of the cost data from the CF registry showed the same, that only 26% of variance was explained by age and percentage predicted FEV1. As always in modelling diseases, further refinements of the health states considered would provide a better reflection of the heterogeneity among patients, but as a result it would likely become more difficult to find the data required to inform transitions between health states.
The costs of standard care included in the model are not complete. The CF tariff bands do not include surgery for certain device implantations nor do they include costs of medications prescribed by the general practitioner. Thus, the true costs of standard care in CF will be higher than the costs used in the model, which would decrease the ICER. However, the impact of these additional costs for standard care would probably have a minimal impact on the ICER given that the annual cost of standard care was only a very small fraction of total annual cost given the price of ivacaftor of £182,000.
One of the main challenges in estimating lifetime cost and QALYs is extrapolating from short-term trial data, in this case the time horizon for the randomised component being only 48 weeks, although up to 96 weeks with some open-label data. On this basis we constructed a set of scenarios based on various assumptions regarding the degree of maintenance of the treatment effect. We make no claim as to which scenario is more likely. However, given expert opinion that ivacaftor might permanently correct the underling biochemical disorder, it does seem likely that young children with little or no lung damage will do much better. Therefore, we conducted a subgroup analysis on children up to age 12 with little or no permanent lung damage, which assumed they would have length and quality of life as if they had been cured of the CF.
The budget impact analysis was performed only for the cohort of CF patients as observed in the year 2011. The impact of new patients becoming eligible for the treatment with ivacaftor after that year was not taken into account. Lifetime costs for ivacaftor for this group of patients would have been lower, because these patients will be treated for fewer years before the drug comes off patent.
Uncertainties
Clinical effectiveness
The main uncertainty relates to the long-term clinical effectiveness of ivacaftor. The available RCTs were only 48 weeks in duration. The open-label trial provided information on an additional 48 weeks of ivacaftor treatment in adults and 24 weeks in children, meaning that data are currently available for 96 weeks of treatment with ivacaftor in adults and 72 weeks of treatment in children. The open-label trial is intended to run for 96 weeks; when full data are available from this study, information will be available on the effectiveness of a total of 144 weeks’ (just over 2.5 years’) treatment with ivacaftor in adults and children.
Ivacaftor has been evaluated only in adults and children ≥ 6 years. Its potential effect in children younger than this is unclear. It may be that treating patients at a very young age with ivacaftor is more effective as it may prevent some of the complications of CF from developing. Ivacaftor works by correcting the underlying deficit in chloride ion transport. This is supported by the results of the systematic review which show improvements in sweat chloride levels, with levels in ivacaftor-treated patients returning to within normal ranges (i.e. below the threshold required for a diagnosis of CF) within 2 weeks of treatment with ivacaftor. It would therefore appear reasonable to hypothesise that treating very young patients in whom no lung damage or infection has yet developed may prevent symptoms of CF from ever developing. However, until trials with long-term follow-up are done in this age group of children the potential harms and benefits remain uncertain. The trials to date focus on the impact of ivacaftor on lung function. If ivacaftor works by correcting the CFTR defect and is given before damage has occurred to other organs such as the pancreas, then it may also prevent damage to these organs, thereby preventing the occurrence of complications associated with CF, such as diabetes. Further evidence on the effects of ivacaftor on other organs is required to address this issue.
The trials evaluated in this review were restricted to patients with the G551D mutation. These represent only around 5.7% of the UK population with CF. A study of 140 patients homozygous for the ∆F508 mutation did not find a significant difference in percentage predicted FEV1 between patients treated with ivacaftor and those receiving placebo after 16 weeks of treatment. Adverse events were similar between the groups and there were some small benefits of ivacaftor on other outcomes. 77,78 A further study is ongoing which is investigating ivacaftor in combination with VX-809, an investigational CFTR corrector, in patients with CF and homozygous for the ∆F508 mutation. 79 This trial is still ongoing but early results suggest potentially beneficial effect of the drug combination. If this combination is proved to be effective it would considerably expand the potential usage of ivacaftor as the ∆F508 is the most common CF-causing mutation in the UK population. 7
Cost-effectiveness
From a cost-effectiveness perspective the long-term effectiveness is also the main uncertainty. The various scenarios explored for this long-term effectiveness show a wide range of ICERs. Only when longer-term data on ivacaftor become available will it be clear which of these ICERs is most relevant.
Additional uncertainty is caused by the long-term costs of ivacaftor. In the model, it is assumed that, after 14 years, a generic version of ivacaftor will be available at £20,000 (as opposed to £182,000 for the branded version). This information was obtained from the manufacturer and therefore assumed to be best possible estimates. It is clear that the costs of ivacaftor are the main driver of the results, and thus the lifelong cost estimates should be interpreted with care. If the patent expiry for ivacaftor were to occur sooner or if the generic costs are lower, the lifetime costs for treatment with ivacaftor would be lower, resulting in a lower cost-effectiveness ratio compared with standard care.
Adoption of a societal perspective might have been interesting; however, this was beyond the scope of the review, which was to estimate the cost-effectiveness of ivacaftor from the perspective of the NHS. However, had such an analysis been undertaken it is likely that savings in costs for informal care and productivity loss in those treated with ivacaftor would have reduced the ICER.
Also, given that ivacaftor is an orphan drug, there is no clear benchmark to indicate whether or not ivacaftor should be considered cost-effective. Several other orphan drugs are in current use despite ICERs being considerably higher than the threshold of £20,000 to £30,000 applied in most NICE appraisals;69 some examples of these are shown in Table 35.
| Products | Conditions | Number of people with condition in UK | Preliminary estimated ICER (£ per QALY) |
|---|---|---|---|
| Agalsidase beta (Fabrazyme®, Genzyme) | Fabry’s disease | 200 | 203,009 |
| Imiglucerase (Cerezyme®, Genzyme) | Gaucher’s disease (types I and III) | 270 | 391,244 |
| Laronidase (Aldurazyme®, Genzyme) | Mucopolysaccharidosis (type I) | 130 | 334,880 |
| Miglustat (Zavesca®, Actelion) | Gaucher’s disease (type I) | 270 | 116,800 |
| Nonacog alfa (BeneFIX®, Wyeth) | Haemophilia B | 350 | 172,500 |
| Iloprost (Ventavis®, Bayer Schering) | Primary pulmonary hypertension | 100 | 23,324 |
Chapter 8 Conclusions
Implications for service provision
The available evidence suggests that ivacaftor is a clinically effective treatment for patients with CF and the G551D mutation. The high cost of ivacaftor may prove an obstacle in the uptake of this treatment; the economic evaluation showed that the ICER for ivacaftor + standard care compared with standard care only varies between £334,000 and £1.27M per QALY gained.
The estimate of the increased cost in the first year of prescribing ivacaftor to about 271 eligible individuals was found to be approximately £43M compared with the total annual cost of caring for all 7329 individuals with CF in England of approximately £150.2M.
On 19 December 2012, the four Specialised Commissioning Groups in England (North of England, South of England, Midlands and East, and London) announced that ivacaftor will be funded by the NHS in England for all patients aged ≥ 6 years with CF and the G551D gene mutation. 80
Suggested research priorities
The main area where further research is required, to inform both clinical effectiveness and cost-effectiveness questions, is the long-term effectiveness of ivacaftor. The main area of uncertainty in the economic model related to how the long-term effects (beyond 96 weeks) of ivacaftor were included in the model. The ongoing open-label trial will go some way to addressing this question but will provide data only on effects up to around 2.5 years of treatment. The effectiveness of ivacaftor in children aged < 6 years is another potentially important question although this may be difficult to address through clinical trials due to the difficulties in conducting such trials in young children. The current evidence only supports the use of ivacaftor in patients with at least one G551D mutation. Such patients represent only around 5% of patients with CF. The potential benefit of ivacaftor in patients with other mutations is therefore also an important area for further research. Clinical trials in patients with certain mutations are ongoing.
From an economic perspective further research to inform how exacerbations can be fully accounted for in an economic model would improve the economic model. Although we have attempted to include these in our model a more sophisticated method of analysis is required which could appropriately model the additional benefits that ivacaftor may have on exacerbations above the effects which it has on percentage predicted FEV1. Further information on the costs of standard care and primary care costs of cystic fibrosis would also be helpful for the model. Although such data would be likely to be dominated by the costs of ivacaftor, they would help to increase the validity of the model.
Acknowledgements
The authors acknowledge the clinical advice and expert opinion provided by Dr Diana Bilton MD FRCP, Consultant Physician/Honorary Senior Lecturer, Department of Respiratory Medicine, Royal Brompton Hospital, London; Dr Andy Clayton Consultant Adult Cystic Fibrosis/Respiratory Medicine, Nottingham University Hospitals NHS Trust; Dr Patrick Oades, Consultant Paediatrician, Royal Devon & Exeter Foundation NHS Trust; Dr Jeremy Hull, Consultant in Paediatric Respiratory Medicine, Children’s Hospital, Oxford; Mr David Turner, Patient Parent Expert Representative on the CF CRG. The authors would also like to thank Vertex Pharmaceuticals Inc., for making the Kalydeco® model available; Ms Elaine Gunn, Cystic Fibrosis Registry Manager, Cystic Fibrosis Trust, for prompt supply of requested data; Mrs Gill Worthy, Statistician, Kleijnen Systematic Reviews Ltd, for assistance in analysing registry data; and Mrs Jeanette Kleijnen, Office Manager, Kleijnen Systematic Reviews Ltd, for formatting of the report.
The views expressed in this report are those of the authors and not necessarily those of the NIHR HTA programme. Any errors are the responsibility of the authors.
Contributions of authors
Penny Whiting and Marie Westwood planned and performed the systematic review and interpretation of evidence.
Maiwenn Al, Laura Burgers and Martine Hoogendoorn planned and performed the cost-effectiveness analyses and interpreted results.
Nigel Armstrong and Steve Ryder contributed to planning and interpretation of cost-effectiveness analysis and acquisition of cost data for modelling. Laura Burgers and Steve Ryder planned and performed the systematic review and interpretation of health economic evidence.
Alex Allen devised and performed the literature searches and provided information support to the project.
Jos Kleijnen and Hans Severens provided senior advice and support to the systematic review and cost-effectiveness analyses, respectively.
All parties were involved in drafting and/or commenting on the report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Standards for the Clinical Care of Children and Adults with Cystic Fibrosis in the UK. London: Cystic Fibrosis Trust; 2011.
- Dodge JA, Lewis PA, Stanton M, Wilsher J. Cystic fibrosis mortality and survival in the UK: 1947–2003. Eur Respir J 2007;29:522-6. http://dx.doi.org/10.1183/09031936.00099506.
- Andersen DH. Cystic fibrosis of the pancreas and its relation to celiac disease. Am J Dis Child 1938;56:344-99. http://dx.doi.org/10.1001/archpedi.1938.01980140114013.
- Davis PB. Cystic fibrosis since 1938. Am J Respir Crit Care Med 2006;173:475-82. http://dx.doi.org/10.1164/rccm.200505-840OE.
- Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, et al. Identification of the cystic fibrosis gene: genetic analysis. Science 1989;245:1073-80. http://dx.doi.org/10.1126/science.2570460.
- Farrell PM, Rosenstein BJ, White TB, Accurso FJ, Castellani C, Cutting GR, et al. Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr 2008;153:S4-1. http://dx.doi.org/10.1016/j.jpeds.2008.05.005.
- The Cystic Fibrosis Genotype-Phenotype Consortium . Correlation between genotype and phenotype in patients with cystic fibrosis. N Engl J Med 1993;329:1308-13.
- Annual Data Report 2010. Bromley, Kent: Cystic Fibrosis Trust; 2011.
- The UK NSC Policy on Cystic Fibrosis Screening in Newborns. London: UK Screening Portal; 2012.
- A Laboratory Guide to Newborn Screening in the UK for Cystic Fibrosis. London: UK Newborn Screening Programme Centre; 2009.
- Serra-Prat M. Neonatal Screening for Cystic Fibrosis. BR01/2000. Barcelona: Catalan Agency for Health Technology Assessment (CAHTA); 2000.
- Davis PB, Yasothan U, Kirkpatrick P. Ivacaftor. Nat Rev Drug Discov 2012;11:349-50. http://dx.doi.org/10.1038/nrd3723.
- Orphan Designation. London: European Medicines Agency; 2012.
- FDA Approves Kalydeco to Treat Rare Form of Cystic Fibrosis. Silver Spring, MD: FDA; 2012.
- Vertex Pharmaceuticals Inc . Vertex Receives European Approval for Kalydeco™ (Ivacaftor), the First Medicine to Treat the Underlying Cause of Cystic Fibrosis in People With a Specific Genetic Mutation (G551D) 2012. http://investors.vrtx.com/releasedetail.cfm?ReleaseID=696069 (accessed 15 August 2012).
- Ivacaftor for the Management of Cystic Fibrosis. Formulary Submission Dossier. Cambridge, MA: Vertex Pharmaceuticals Inc.; 2012.
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264-9. http://dx.doi.org/10.7326/0003-4819-151-4-200908180-00135.
- Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York: University of York; 2009.
- McGowan J, Sampson M, Lefebvre C. An evidence based checklist for the peer review of electronic search strategies (PRESS EBC). Evidence Based Libr Inform Prac 2010;5:1-6.
- Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343. http://dx.doi.org/10.1136/bmj.d5928.
- Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Drevinek P, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 2011;365:1663-72.
- Ramsey B, Dong Q, Yen K, Elborn J. Efficacy and safety of VX-770 in subjects with cystic fibrosis and the G551DCFTR mutation. Paper presented at the 25th North American Cystic Fibrosis Conference, Anaheim, CA, 2–5 November 2011. Pediatr Pulmonol 2011;46:286-7.
- Borowitz D, Ramsey B, Dong Q, Yen K, Elborn JS. Measures of nutritional status in two Phase 3 trials of ivacaftor in subjects with cystic fibrosis who have the G551D-CFTR mutation [WS6.3]. Paper presented at 35th European Cystic Fibrosis Conference, Dublin, Ireland, 6–9 June 2012. J Cyst Fibros 2012;11.
- Quittner AL, Ramsey B, Dong Q, Yen K, Elborn JS. Patient-reported outcomes in Phase 3 trials of ivacaftor in subjects with CF who have the G551D-CFTR mutation. Paper presented at 35th European Cystic Fibrosis Conference, Dublin, Ireland, 6–9 June 2012. J Cyst Fibros 2012;11.
- Griese M, Ramsey B, Rodriguez S, Yen K, Elborn JS. Pulmonary exacerbations in a Phase 3 trial of ivacaftor in subjects with cystic fibrosis who have the G551D-CFTR mutation. Paper presented at 35th European Cystic Fibrosis Conference, Dublin, Ireland, 6–9 June 2012. J Cyst Fibros 2012;11.
- Study of VX-770 in Cystic Fibrosis Subjects Age 12 and Older with the G551D Mutation. Bethesda, MD: National Library of Medicine (US); 2011.
- Aherns R, Rodriguez S, Yen K, Davies JC. VX-770 in subjects 6 to 11 years with cystic fibrosis and the G551D-CFTR mutation. Paper presented at the 25th North American Cystic Fibrosis Conference, Anaheim, CA, 3–5 November 2011. Pediatr Pulmonol 2011;46.
- Davies JC, Li H, Yen K, Ahrens R. Ivacaftor in subjects 6 to 11 years of age with cystic fibrosis and the G551D-CFTR mutation [WS6.5]. Paper presented at 35th European Cystic Fibrosis Conference, Dublin, Ireland, 6–9 June 2012. J Cyst Fibros 2012;11.
- Vertex Pharmaceuticals Inc . Phase 3 Study of KALYDECO (Ivacaftor) in Children Ages 6 to 11 With a Specific Type of Cystic Fibrosis Showed Significant Improvements in Lung Function and Other Measures of Disease Sustained Through 48 Weeks. 2011.
- Study of Ivacaftor in Cystic Fibrosis Subjects Aged 6 to 11 Years with the G551D Mutation (ENVISION). Bethesda, MD: National Library of Medicine (US); 2012.
- McKone EF, Borowitz D, Drevinek P, Griese M, Konstan MW, Wainwright CE, et al. Long-term safety and efficacy of investigational CFTR potentiator, VX-770, in subjects with CF [abstract 204]. Paper presented at the 25th North American Cystic Fibrosis Conference, 2–5 November 2011, Anaheim, CA. Pediatr Pulmonol 2011;46.
- McKone E, Li H, Yen K, Davies JC. Long-term safety and efficacy of ivacaftor in subjects with cystic fibrosis who have the G551D-CFTR mutation [WS6.4]. Paper presented at 35th European Cystic Fibrosis Conference, Dublin, Ireland, 6–9 June 2012. J Cyst Fibros 2012;11.
- Study of VX-770 in Cystic Fibrosis Subjects. Bethesda, MD: National Library of Medicine (US); 2012.
- Summary Review of Regulatory Action: Ivacaftor [Application number: 203188Orig1s000]. Silver Spring, MD: FDA; 2011.
- Medical Review: Ivacaftor [Application number: 203188Orig1s000]. Silver Spring, MD: FDA; 2011.
- Statistical Review and Evaluation: Ivacaftor [Application number: 203188Orig1s000]. Silver Spring, MD: FDA; 2011.
- Balfour-Lynn I. Clinical Guidelines: Care of Children with Cystic Fibrosis 2011. London: Royal Brompton & Harefield NHS Foundation Trust; 2011.
- Simpson N, Anderson R, Sassi F, Pitman A, Lewis P, Tu K, et al. The cost-effectiveness of neonatal screening for cystic fibrosis: an analysis of alternative scenarios using a decision model. Cost Eff Resour Alloc 2005;3:1-11.
- Thornton J, Elliott RA, Tully MP, Dodd M, Webb AK. Clinical and economic choices in the treatment of respiratory infections in cystic fibrosis: comparing hospital and home care. J Cyst Fibros 2005;4:239-47. http://dx.doi.org/10.1016/j.jcf.2005.08.003.
- Baumann U, Stocklossa C, Greiner W, von der Schulenburg J-MG, von der Hardt H. Cost of care and clinical condition in paediatric cystic fibrosis patients. J Cyst Fibros 2003;2:84-90. http://dx.doi.org/10.1016/S1569-1993(03)00024-9.
- Colistimethate Sodium and Tobramycin Dry Powders for Inhalation for Treating Pseudomonas Lung Infection in Cystic Fibrosis. NICE technology appraisal guidance TA276. London: NICE; 2013.
- Riemsma R, Al MJ, Armstrong N, Misso K, Allen A, Manning N, et al. Mannitol Dry Powder for Inhalation for the Treatment of Cystic Fibrosis: a Single Technology Appraisal. York: Kleijnen Systematic Reviews Ltd; 2011.
- Gee L, Abbott J, Conway SP, Etherington C, Webb AK. Validation of the SF-36 for the assessment of quality of life in adolescents and adults with cystic fibrosis. J Cyst Fibros 2002;1:137-45. http://dx.doi.org/10.1016/S1569-1993(02)00079-6.
- UK Cystic Fibrosis Registry . The UK CF Registry homepage n.d. www.cysticfibrosis.org.uk/who-we-are/driving-up-standards/uk-cf-registry.aspx (accessed 12 September 2013).
- Gee L, Abbott J, Conway SP, Etherington C, Webb AK. Development of a disease specific health related quality of life measure for adults and adolescents with cystic fibrosis. Thorax 2000;55:946-54. http://dx.doi.org/10.1136/thorax.55.11.946.
- Guide to the Methods of Technology Appraisal. London: NICE; 2008.
- Liou TG, Adler FR, Fitzsimmons SC, Cahill BC, Hibbs JR, Marshall BC. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol 2001;153:345-52. http://dx.doi.org/10.1093/aje/153.4.345.
- Goss CH, Burns JL. Exacerbations in cystic fibrosis. 1: epidemiology and pathogenesis. Thorax 2007;62:360-7. http://dx.doi.org/10.1136/thx.2006.060889.
- Transplant Activity in the UK 2009/10. London: NHS Blood and Transplant; 2010.
- England and Wales Interim Life Tables. London: Office for National Statistics; 2011.
- Konstan MW, Accurso FJ, Boyle MP, Clancy JP, Ordonez CL, Zha J, et al. Relationship between pulmonary outcomes, biomarkers of CF disease, and serum drug levels in subjects with the G551D-CFTR mutation treated with VX-770, an investigational oral potentiator of CFTR. Paper presented at American Thoracic Society International Conference, New Orleans, LA, 14–19 May 2010. Am J Respir Crit Care Med 2010;181.
- Konstan MW, Wagener JS, Vandevanter DR, Pasta DJ, Yegin A, Rasouliyan L, et al. Risk factors for rate of decline in FEV(1) in adults with cystic fibrosis. J Cyst Fibros 2012;11:405-11.
- Konstan MW, Ratjen F. Effect of dornase alfa on inflammation and lung function: potential role in the early treatment of cystic fibrosis. J Cyst Fibros 2012;11:78-83. http://dx.doi.org/10.1016/j.jcf.2011.10.003.
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. CHE Discussion Paper 172. York: Centre for Health Economics, University of York; 1999.
- Rutten-van Molken MPMH, Oostenbrink JB, Tashkin DP, Burkhart D, Monz BU. Does quality of life of COPD patients as measured by the generic EuroQol five-dimension questionnaire differentiate between COPD severity stages?. Chest 2006;130:1117-28. http://dx.doi.org/10.1378/chest.130.4.1117.
- Anyanwu AC, McGuire A, Rogers CA, Murday AJ. Assessment of quality of life in lung transplantation using a simple generic tool. Thorax 2001;56:218-22. http://dx.doi.org/10.1136/thorax.56.3.218.
- Sullivan PW, Slejko JF, Sculpher MJ, Ghushchyan V. Catalogue of EQ-5D scores for the United Kingdom. Med Decis Making 2011;31. http://dx.doi.org/10.1177/0272989X11401031.
- Vertex Pharmaceuticals Inc . Examination of Costs and Utilization Associated With Progression of Cystic Fibrosis. 2010.
- Antibiotic Treatment for Cystic Fibrosis. London: Cystic Fibrosis Trust; 2009.
- Corey M. Power considerations for studies of lung function in cystic fibrosis. Proc Am Thorac Soc 2007;4:334-7. http://dx.doi.org/10.1513/pats.200611-176HT.
- Lieu TA, Ray GT, Farmer G, Shay GF. The cost of medical care for patients with cystic fibrosis in a health maintenance organization. Pediatrics 1999;103. http://dx.doi.org/10.1542/peds.103.6.e72.
- Cystic Fibrosis Guidance. Leeds: Department of Health; 2012.
- Apply for Data from the CF Registry. Bromley: Cystic Fibrosis Trust; 2012.
- British national formulary. London: BMA and RPS; 2012.
- NHS Reference Costs 2010–11. London: Department of Health; 2012.
- Anyanwu AC, McGuire A, Rogers CA, Murday AJ. An economic evaluation of lung transplantation. J Thorac Cardiovasc Surg 2002;123:411-18. http://dx.doi.org/10.1067/mtc.2002.120342.
- Curtis L. Unit Costs of Health and Social Care 2009. Canterbury: PSSRU, University of Kent; 2009.
- Curtis L. Unit Costs of Health and Social Care 2011. Canterbury: PSSRU, University of Kent; 2011.
- Appraising Orphan Drugs [draft v3]. London: NICE; 2006.
- Cystic Fibrosis Genetic Screening [Cost per Test]. Leeds: Thisismy: Health Screening & Ultrasound Centre; n.d.
- George PM, Banya W, Pareek N, Bilton D, Cullinan P, Hodson ME, et al. Improved survival at low lung function in cystic fibrosis: cohort study from 1990 to 2007. BMJ 2011;342. http://dx.doi.org/10.1136/bmj.d1008.
- Sanders DB, Bittner RCL, Rosenfeld M, Hoffman LR, Redding GJ, Goss CH. Failure to recover to baseline pulmonary function after cystic fibrosis pulmonary exacerbation. Am J Respir Crit Care Med 2010;182:627-32. http://dx.doi.org/10.1164/rccm.200909-1421OC.
- Sanders DB, Bittner RCL, Rosenfeld M, Redding GJ, Goss CH. Pulmonary exacerbations are associated with subsequent FEV1 decline in both adults and children with cystic fibrosis. Pediatr Pulmonol 2011;46:393-400. http://dx.doi.org/10.1002/ppul.21374.
- Britto MT, Kotagal UR, Hornung RW, Atherton HD, Tsevat J, Wilmott RW. Impact of recent pulmonary exacerbations on quality of life in patients with cystic fibrosis. Chest 2002;121:64-72. http://dx.doi.org/10.1378/chest.121.1.64.
- Bell SC, Robinson PJ. Exacerbations in cystic fibrosis: 2. prevention. Thorax 2007;62:723-32. http://dx.doi.org/10.1136/thx.2006.060897.
- Gee L, Abbott J, Hart A, Conway SP, Etherington C, Webb AK. Associations between clinical variables and quality of life in adults with cystic fibrosis. J Cyst Fibros 2005;4:59-66. http://dx.doi.org/10.1016/j.jcf.2004.12.005.
- Flume PA, Borowitz DS, Liou TG, Li H, Yen K, Ordonez CL, et al. VX-770 in subjects with cystic fibrosis who are homozygous for the F508del-CFTR mutation. Paper presented at 34th European Cystic Fibrosis Conference, Hamburg, Germany, 8–11 June 2011. J Cyst Fibros 2011;10.
- Flume PA, Borowitz D, Liou T, Li H, Yen K, Ordonez C, et al. VX-770 in subjects with CF and homozygous for the F508del-CFTR mutation. Paper presented at 25th Annual North American Cystic Fibrosis Conference, Anaheim, CA, 3–5 November 2011. Pediatr Pulmonol 2011;46:284-5.
- Boyle MP, Bell S, Konstan MW, McColley SA, Wisseh S, Spencer-Green G. VX-809, an investigational CFTR corrector, in combination with VX-770, an investigational CFTR potentiator, in subjects with CF and homozygous for the F508del-CFTR mutation. Paper presented at 25th Annual North American Cystic Fibrosis Conference, Anaheim, CA, 3–4 November 2011. Pediatr Pulmonol 2011;46.
- Statement from North of England SCG on Behalf of the Four Specialised Commissioning Groups in England: Ivacaftor (Brand Name Kalydeco) for Cystic Fibrosis. Barnsley: NHS Barnsley; 2012.
- Wertz DA, Chang C-L, Stephenson JJ, Zhang J, Kuhn RJ. Economic impact of tobramycin in patients with cystic fibrosis in a managed care population. J Med Econ 2011;14:759-68. http://dx.doi.org/10.3111/13696998.2011.621004.
- Briesacher BA, Quittner AL, Fouayzi H, Zhang J, Swensen A. Nationwide trends in the medical care costs of privately insured patients with cystic fibrosis (CF), 2001–2007. Pediatr Pulmonol 2011;46:770-6. http://dx.doi.org/10.1002/ppul.21441.
- O’Sullivan AK, Sullivan J, Higuchi K, Montgomery AB. Health care utilization & costs for cystic fibrosis patients with pulmonary infections. Manag Care 2011;20:37-44.
- Woodward TC, Brown R, Sacco P, Zhang J. Budget impact model of tobramycin inhalation solution for treatment of Pseudomonas aeruginosa in cystic fibrosis patients. J Med Econ 2010;13:492-9.
- Braccini G, Festini F, Boni V, Neri AS, Galici V, Campana S, et al. The costs of treatment of early and chronic Pseudomonas aeruginosa infection in cystic fibrosis patients. J Chemother 2009;21:188-92.
- Schreyogg J, Hollmeyer H, Bluemel M, Staab D, Busse R. Hospitalisation costs of cystic fibrosis. Pharmacoeconomics 2006;24:999-1009.
- van den Akker-van Marle ME, Dankert HM, Verkerk PH, Dankert-Roelse JE. Cost-effectiveness of 4 neonatal screening strategies for cystic fibrosis. Pediatrics 2006;118:896-905. http://dx.doi.org/10.1542/peds.2005-2782.
- Iles R, Legh-Smith J, Drummond M, Prevost A, Vowler S. Economic evaluation of Tobramycin nebuliser solution in cystic fibrosis. J Cyst Fibros 2003;2:120-8. http://dx.doi.org/10.1016/S1569-1993(03)00064-X.
- Sansgiry SS, Joish VN, Boklage S, Goyal RK, Chopra P, Sethi S. Economic burden of Pseudomonas aeruginosa infection in patients with cystic fibrosis. J Med Econ 2012;15:219-24.
- Dewitt EM, Grussemeyer CA, Friedman JY, Dinan MA, Lin L, Schulman KA, et al. Resource use, costs, and utility estimates for patients with cystic fibrosis with mild impairment in lung function: analysis of data collected alongside a 48-week multicenter clinical trial. Value Health 2012;15:277-83. http://dx.doi.org/10.1016/j.jval.2011.11.027.
- Abbott J, Hart A, Morton AM, Dey P, Conway SP, Webb AK. Can health-related quality of life predict survival in adults with cystic fibrosis?. Am J Respir Crit Care Med 2009;179:54-8. http://dx.doi.org/10.1164/rccm.200802-220OC.
- Becker CC, Vieira MC, Harrow B, Liou TG, Jansen JP. Disease progression in cystic fibrosis-synthesis of survival evidence. Paper presented at 16th Annual International Meeting of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR), Baltimore, MD, 21–25 May 2011. Value Health 2011;14.
- Loadman M, Holman J, Jackson K, Weinkauf J, Roland N, Kapasi A, et al. Trends in the functional outcomes and quality of life of cystic fibrosis patients following lung transplant. Paper presented at 30th Annual Meeting and Scientific Sessions of the International Society for Heart and Lung Transplantation, Chicago, IL, 21–24 April 2010. J Heart Lung Transplant 2010;29:125-6.
- Eidt-Koch D, Mittendorf T, Greiner W. Cross-sectional validity of the EQ-5D-Y as a generic health outcome instrument in children and adolescents with cystic fibrosis in Germany. BMC Pediatr 2009;9. http://dx.doi.org/10.1186/1471-2431-9-55.
- Elphick HE, Mallory G. Oxygen therapy for cystic fibrosis. Cochrane Database Syst Rev 2009;1.
- Weiner JR, Toy EL, Sacco P, Duh MS. Costs, quality of life and treatment compliance associated with antibiotic therapies in patients with cystic fibrosis: a review of the literature. Expert Opin Pharmacother 2008;9:751-66. http://dx.doi.org/10.1517/14656566.9.5.751.
- Guerriere DN, Tranmer JE, Ungar WJ, Manoharan V, Coyte PC. Valuing care recipient and family caregiver time: a comparison of methods. Int J Technol Assess Health Care 2008;24:52-9. http://dx.doi.org/10.1017/S0266462307080075.
- Radhakrishnan M, van Gool K, Hall J, Delatycki M, Massie J. Economic evaluation of cystic fibrosis screening: a review of the literature. Health Policy 2008;85:133-47. http://dx.doi.org/10.1016/j.healthpol.2007.07.007.
- Elphick HE, Tan A. Single versus combination intravenous antibiotic therapy for people with cystic fibrosis. Cochrane Database Syst Rev 2005;2.
- Yi MS, Tsevat J, Wilmott RW, Kotagal UR, Britto MT. The impact of treatment of pulmonary exacerbations on the health-related quality of life of patients with cystic fibrosis: does hospitalization make a difference?. J Pediatr 2004;144:711-18. http://dx.doi.org/10.1016/j.jpeds.2004.02.032.
- Wark P, McDonald VM. Nebulised hypertonic saline for cystic fibrosis. Cochrane Database Syst Rev 2009;2.
- Mayer-Hamblett N, Ramsey BW, Kronmal RA. Advancing outcome measures for the new era of drug development in cystic fibrosis. Proc Am Thorac Soc 2007;4:370-7. http://dx.doi.org/10.1513/pats.200703-040BR.
- Ashish A, Nazareth D, Tsoulkani A, Priona G, Harris M, Ledson M, et al. Social deprivation and clinical outcomes in adult CF patients. Paper presented at British Thoracic Society Winter Meeting, London, UK, 1–3 December 2010. Thorax 2010;65.
- Smalarz A, Harrow B, Pashos CL, Schoeman O. Cost of pseudomonas aeruginosa in cystic fibrosis patients in the United Kingdom. Paper presented at ISPOR 14th Annual International Meeting, Orlando, FL, 16–20 May 2009. Value Health 2009;12.
- Abbott J, Hart A, Havermans T, Matossian A, Goldbeck L, Barreto C, et al. Measuring health-related quality of life in clinical trials in cystic fibrosis. J Cyst Fibros 2011;10:82-5. http://dx.doi.org/10.1016/S1569-1993(11)60013-1.
- Elborn JS. Key advances along the CF pipeline. Paper presented at 25th Annual North American Cystic Fibrosis Conference, Anaheim, CA, 3–5 November 2011. Pediatr Pulmonol 2011;46:179-80.
- Hofer M, Fiechter Lienert B, Kurowski T, Boehler A. Correlation of physical performance and quality of life in adult patients with cystic fibrosis. Paper presented at Joint Annual Meeting of the Swiss Respiratory Society, Swiss Society of Oto-Rhino-Laryngology, Head and Neck Surgery, Swiss Paediatric Respiratory Society, Swiss Society for Thoracic Surgery, Interlaken, Switzerland, 4–6 May 2011. Respiration 2011;81.
- Bradley J, Blume S, Bal MM, Honeybourne D, Elborn JS. Health related quality of life (HRQoL) and health utility in patients with cystic fibrosis (CF) and chronic Pseudomonas aeruginosa (PA) infection in the UK. Paper presented at 34th European Cystic Fibrosis Conference, Hamburg, Germany, 8–11 June 2011. J Cyst Fibros 2011;10.
- Ratjen F, Grasemann H. New therapies in cystic fibrosis. Curr Pharm Des 2012;18:614-27. http://dx.doi.org/10.2174/138161212799315984.
Appendix 1 Literature search strategies
Clinical effectiveness
EMBASE (OvidSP) 1974–2012 week 17
Searched 3 May 2012.
-
Ivacaftor/ (72)
-
(Ivacaftor or Kalydeco or VX-770 or VX770 or 873054-44-5 or ivacaftorum).af. (138)
-
or/1-2 (138)
MEDLINE (OvidSP) 1946–April 2012 week 4
Searched 3 May 2012.
-
(Ivacaftor or Kalydeco or VX-770 or VX770 or 873054-44-5 or ivacaftorum).af. (10)
MEDLINE In-Process & Other Non-Indexed Citations (OvidSP) up to 2 May 2012
MEDLINE Daily Update (OvidSP) up to 2 May 2012
Searched 3 May 2012.
-
(Ivacaftor or Kalydeco or VX-770 or VX770 or 873054-44-5 or ivacaftorum).af. (3)
Cochrane Database of Systematic Reviews (CDSR) (Wiley Online Library) up to 2012 issue 4
Cochrane Central Register of Controlled Trials (CENTRAL) (Wiley Online Library) up to 2012 issue 4
Database of Abstracts of Reviews of Effects (DARE) (Wiley Online Library) up to 2012 issue 2
Health Technology Assessment (HTA) Database (Wiley Online Library) up to 2012 issue 2
NHS Economic Evaluation Database (NHS EED) (Wiley Online Library) up to 2012 issue 2
Searched 3 May 2012.
#1 (Ivacaftor OR Kalydeco OR VX-770 OR VX770 OR VX 770 OR 873054-44-5 OR ivacaftorum)
CDSR retrieved no records.
CENTRAL retrieved five records.
DARE retrieved no records.
HTA retrieved no records.
NHS EED retrieved 0 records.
Database of Abstracts of Reviews of Effects (DARE) (CRD) up to 3 May 2012
Health Technology Assessment (HTA) Database (CRD) up to 3 May 2012
NHS Economic Evaluation Database (NHS EED) (CRD) up to 3 May 2012
Searched 3 May 2012.
#1 (Ivacaftor OR Kalydeco OR VX-770 OR VX770 OR VX 770 OR 873054-44-5 OR ivacaftorum)
DARE retrieved no records.
HTA retrieved no records.
NHS EED retrieved no records.
Latin American and Caribbean Health Sciences Literature (LILACS) (VHL)
URL: http://lilacs.bvsalud.org/en
Searched 4 May 2012.
Ivacaftor OR Kalydeco OR VX-770 OR VX770 OR 873054-44-5 OR ivacaftorum (0)
ClinicalTrials.gov
URL: http://clinicaltrials.gov/ct2/search/advanced
Searched 4 May 2012.
Ivacaftor OR Kalydeco OR VX-770 OR VX770 OR VX 770 OR 873054-44-5 OR ivacaftorum (18)
mRCT – metaRegister of Controlled Trials (internet)
URL: www.controlled-trials.com/mrct/search.html
Searched 4 May 2012.
Ivacaftor OR Kalydeco OR VX-770 OR VX770 OR VX 770 OR 873054-44-5 OR ivacaftorum (12)
World Health Organization International Clinical Trials Registry Platform (ICTRP) (internet)
URL: www.who.int/ictrp/en
Searched 4 May 2012.
Ivacaftor OR Kalydeco OR VX-770 OR VX770 OR VX 770 OR 873054-44-5 OR ivacaftorum (18)
European Cystic Fibrosis Society (ECFS) Conferences – searched titles (no abstracts available)
URL: www.ecfs.eu/meetings/ecfs
| Year | Ivacaftor | Kalydeco | VX-770 | G551D | Total abstracts found after deduplication |
|---|---|---|---|---|---|
| 2011 | 0 | 0 | 2 | 1 | 3 |
| 2010 | 0 | 0 | 2 | 1 | 3 |
| 2009 | 0 | 0 | 2 | 2 | 4 |
| 2008 | 0 | 0 | 0 | 1 | 1 |
| 2007 | 0 | 0 | 0 | 0 | 0 |
| Total | 11 |
NACFC: The Annual North American Cystic Fibrosis Conferences
| Year | Ivacaftor | Kalydeco | VX-770 | G551D | Total abstracts found after deduplication |
|---|---|---|---|---|---|
| 2011a | 1 | 0 | 14 | 12 | 17 |
| 2010b | 0 | 0 | 8 | 8 | 10 |
| 2009b | 0 | 0 | 7 | 8 | 13 |
| 2008b | 0 | 0 | 3 | 1 | 3 |
| 2007b | 0 | 0 | 1 | 10 | 10 |
| Total | 53 |
CIPP: International Congress on Pediatric Pulmonology
URL: www.cipp-meeting.org/index.htm
Searched titles (no abstracts available).
| Year | Ivacaftor | Kalydeco | VX-770 | G551D | Total abstracts found after deduplication |
|---|---|---|---|---|---|
| 2011 | 0 | 0 | 0 | 0 | 0 |
| 2010 | 0 | 0 | 1 | 1 | 1 |
| 2009 | No conference | ||||
| 2008 | Unable to search so browsed abstracts | 0 | |||
| 2007 | Abstracts unavailable through website | ||||
| Total | 1 | ||||
Update searches
EMBASE (OvidSP) 2012 week 10–2012 week 26
Searched 6 July 2012.
-
Ivacaftor/ (89)
-
(Ivacaftor or Kalydeco or VX-770 or VX770 or 873054-44-5 or ivacaftorum).af. (156)
-
or/1-2 (156)
-
(20121$ or 20122$).em. (43,3317)
-
3 and 4 (29)
MEDLINE (OvidSP) 2012–June 2012 week 4
Searched 6 July 2012.
-
(Ivacaftor or Kalydeco or VX-770 or VX770 or 873054-44-5 or ivacaftorum).af. (12)
-
2012$.ed. (414,936)
-
1 and 2 (4)
MEDLINE In-Process & Other Non-Indexed Citations (OvidSP) 2012–5 June 2012
MEDLINE Daily Update (OvidSP) 2012–5 June 2012
Searched 6 July 2012.
-
(Ivacaftor or Kalydeco or VX-770 or VX770 or 873054-44-5 or ivacaftorum).af. (3)
-
2012$.ed. (29,802)
-
1 and 2 (1)
Cochrane Database of Systematic Reviews (CDSR) (Wiley Online Library) 2012–Issue 6:2012
Cochrane Central Register of Controlled Trials (CENTRAL) (Wiley Online Library) 2012–Issue 6:2012
Database of Abstracts of Reviews of Effects (DARE) (Wiley Online Library) 2012–Issue 2:2012
Health Technology Assessment (HTA) Database (Wiley Online Library) 2012–Issue 2:2012
NHS Economic Evaluation Database (NHS EED) (Wiley Online Library) 2012–Issue 2:2012
Searched 6 July 2012.
#1 (Ivacaftor OR Kalydeco OR VX-770 OR VX770 OR VX 770 OR 873054-44-5 OR ivacaftorum), in 2012 0
CDSR retrieved no records.
CENTRAL retrieved no records.
DARE retrieved no records.
HTA retrieved no records.
NHS EED retrieved no records.
Database of Abstracts of Reviews of Effects (DARE) (CRD) up to 7 June 2012
Health Technology Assessment (HTA) Database (CRD) up to 7 June 2012
NHS Economic Evaluation Database (NHS EED) (CRD) up to 7 June 2012
Searched 6 July 2012.
#1 (Ivacaftor OR Kalydeco OR VX-770 OR VX770 OR VX 770 OR 873054-44-5 OR ivacaftorum) 0
DARE retrieved no records.
HTA retrieved no records.
NHS EED retrieved no records.
Latin American and Caribbean Health Sciences Literature (LILACS) (VHL)
URL: http://lilacs.bvsalud.org/en
Searched 6 July 2012.
(Ivacaftor OR Kalydeco OR VX-770 OR VX770 OR 873054-44-5 OR ivacaftorum) (0)
ClinicalTrials.gov 1 May 2012–6 July 2012
URL: http://clinicaltrials.gov/ct2/search/advanced
Searched 6 July 2012.
Ivacaftor OR Kalydeco OR VX-770 OR VX770 OR VX 770 OR 873054-44-5 OR ivacaftorum | received from 01/05/2012 to 06/07/2012 (5)
mRCT – metaRegister of Controlled Trials (internet)
URL: www.controlled-trials.com/mrct/search.html
Searched 6 July 2012.
Four out of five registers, i.e. not ClinicalTrials.gov.
Ivacaftor OR Kalydeco OR VX-770 OR VX770 OR VX 770 OR 873054-44-5 OR ivacaftorum (1)
Downloaded into Word file: Trials_Update1.
World Health Organization International Clinical Trials Registry Platform (ICTRP) (internet) 1 May 2012–6 July 2012
URL: www.who.int/ictrp/en
Searched 6 July 2012.
Intervention: Ivacaftor OR Kalydeco OR VX-770 OR VX770 OR VX 770 OR 873054-44-5 OR ivacaftorum (3)
Title: Ivacaftor OR Kalydeco OR VX-770 OR VX770 OR VX 770 OR 873054-44-5 OR ivacaftorum (two duplicates)
Downloaded into Word file: Trials_Update1.
European Cystic Fibrosis Society (ECFS) Conferences
URL: www.ecfs.eu/meetings/ecfs
Searched 19 July 2012.
Searched titles – no abstracts available.
| Year | Ivacaftor | Kalydeco | VX-770 | G551D | Total abstracts found after deduplication |
|---|---|---|---|---|---|
| 2012 | 9 | 0 | 0 | 6 | 9 |
Downloaded into Word file: ECFS_Update1.
NACFC: The Annual North American Cystic Fibrosis Conferences
URL: https://www.nacfconference.org
Searched 19 July 2012.
| Year | Ivacaftor | Kalydeco | VX-770 | G551D | Total abstracts found after deduplication |
|---|---|---|---|---|---|
| 2012 | Conference not occurred yet | ||||
CIPP International Congress on Pediatric Pulmonology
URL: www.cipp-meeting.org/index.htm
Searched Paediatric Respiratory Reviews supplement 1 as e-mailed by CIPP.
Searched 25 July 2012.
Searched titles – no abstracts available.
| Year | Ivacaftor | Kalydeco | VX-770 | G551D | Total abstracts found after deduplication |
|---|---|---|---|---|---|
| 2012 | 1 | 1 | 0 | 0 | 1 |
Cost-effectiveness
MEDLINE (OvidSP) 2002–May 2012 week 1
Searched 11 May 2012.
-
cystic fibrosis/ (26,164)
-
((Cystic adj2 fibrosis) or mucoviscidosis).ti,ab,ot,hw. (34,176)
-
CF.ti,ot. (1276)
-
(pancreas adj3 fibrocystic adj3 disease$).ti,ab,ot,hw. (16)
-
or/1-4 (34,859)
-
economics/ (26,269)
-
exp "costs and cost analysis"/ (164,214)
-
economics, dental/ (1840)
-
exp "economics, hospital"/ (17,877)
-
economics, medical/ (8463)
-
economics, nursing/ (3861)
-
economics, pharmaceutical/ (2325)
-
(economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (359,328)
-
(expenditure$ not energy).ti,ab. (15,019)
-
(value adj1 money).ti,ab. (18)
-
budget$.ti,ab. (15,258)
-
or/6-16 (475,664)
-
((energy or oxygen) adj cost).ti,ab. (2417)
-
(metabolic adj cost).ti,ab. (636)
-
((energy or oxygen) adj expenditure).ti,ab. (13,970)
-
or/18-20 (16,385)
-
17 not 21 (471,962)
-
letter.pt. (745,733)
-
editorial.pt. (297,634)
-
historical article.pt. (282,385)
-
or/23-25 (1,312,332)
-
22 not 26 (446,294)
-
5 and 27 (667)
-
animals/ not (animals/ and humans/) (3,620,832)
-
28 not 29 (657)
-
remove duplicates from 30 (639)
-
limit 31 to yr="2002 -Current" (307)
Economics filter
Centre for Reviews and Dissemination. NHS EED Economics Filter: Medline (Ovid) monthly search [internet].
York: Centre for Reviews and Dissemination; 2010 (cited 28 September 2010).
URL: www.york.ac.uk/inst/crd/intertasc/nhs_eed_strategies.html
MEDLINE In-Process & Other Non-Indexed Citations (OvidSP) 2002–10 May 2012
MEDLINE Daily Update (OvidSP) 2002–10 May 2012
Searched 11 May 2012.
-
cystic fibrosis/ (13)
-
((Cystic adj2 fibrosis) or mucoviscidosis).ti,ab,ot,hw. (860)
-
CF.ti,ot. (153)
-
(pancreas adj3 fibrocystic adj3 disease$).ti,ab,ot,hw. (2)
-
or/1-4 (999)
-
economics/ (3)
-
exp "costs and cost analysis"/ (127)
-
economics, dental/ (0)
-
exp "economics, hospital"/ (16)
-
economics, medical/ (0)
-
economics, nursing/ (0)
-
economics, pharmaceutical/ (2)
-
(economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (28,438)
-
(expenditure$ not energy).ti,ab. (752)
-
(value adj1 money).ti,ab. (2)
-
budget$.ti,ab. (1477)
-
or/6-16 (29,977)
-
((energy or oxygen) adj cost).ti,ab. (158)
-
(metabolic adj cost).ti,ab. (44)
-
((energy or oxygen) adj expenditure).ti,ab. (649)
-
or/18-20 (833)
-
17 not 21 (29,739)
-
letter.pt. (18,035)
-
editorial.pt. (11,136)
-
historical article.pt. (158)
-
or/23-25 (29,322)
-
22 not 26 (29,403)
-
5 and 27 (27)
-
animals/ not (animals/ and humans/) (2139)
-
28 not 29 (27)
-
remove duplicates from 30 (27)
-
limit 31 to yr="2002 -Current" (22)
Economics filter
Centre for Reviews and Dissemination. NHS EED Economics Filter: Medline (Ovid) monthly search [internet].
York: Centre for Reviews and Dissemination; 2010 [cited 28 September 2010].
URL: www.york.ac.uk/inst/crd/intertasc/nhs_eed_strategies.html
EMBASE (OvidSP) 2002–2012 week 18
Searched 11 May 2012.
-
cystic fibrosis/ (41,722)
-
((Cystic adj2 fibrosis) or mucoviscidosis).mp. (48,828)
-
CF.ti,ot. (2503)
-
(pancreas adj3 fibrocystic adj3 disease$).mp. (16)
-
or/1-4 (49,844)
-
health-economics/ (31,568)
-
exp economic-evaluation/ (183,852)
-
exp health-care-cost/ (176,126)
-
exp pharmacoeconomics/ (152,648)
-
or/6-9 (423,153)
-
(econom$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (504,978)
-
(expenditure$ not energy).ti,ab. (20,285)
-
(value adj2 money).ti,ab. (1089)
-
budget$.ti,ab. (20,942)
-
or/11-14 (526,198)
-
10 or 15 (774,146)
-
letter.pt. (784,623)
-
editorial.pt. (406,289)
-
note.pt. (515,518)
-
or/17-19 (1,706,430)
-
16 not 20 (696,305)
-
(metabolic adj cost).ti,ab. (744)
-
((energy or oxygen) adj cost).ti,ab. (2877)
-
((energy or oxygen) adj expenditure).ti,ab. (17,302)
-
or/22-24 (20,179)
-
21 not 25 (691,788)
-
exp animal/ (1,778,262)
-
exp animal-experiment/ (1,613,173)
-
nonhuman/ (3,832,807)
-
(rat or rats or mouse or mice or hamster or hamsters or animal or animals or dog or dogs or cat or cats or bovine or sheep).ti,ab,sh. (4,657,237)
-
or/27-30 (6,588,417)
-
exp human/ (13,530,525)
-
exp human-experiment/ (300,208)
-
32 or 33 (13,531,960)
-
31 not (31 and 34) (52,04,957)
-
26 not 35 (643,011)
-
5 and 36 (1330)
-
limit 37 to embase (1088)
-
remove duplicates from 38 (1086)
-
limit 39 to yr="2002 -Current" (745)
Economics filter
Centre for Reviews and Dissemination. NHS EED Economics Filter: EMBASE (Ovid) (weekly search) [internet].
York: Centre for Reviews and Dissemination; 2010 [cited 28 September 2010].
NHS Economic Evaluation Database Centre for Reviews and Dissemination 2002–3 May 2012
Searched 3 May 2012.
-
MeSH DESCRIPTOR Cystic Fibrosis (86)
-
(Cystic near2 fibrosis) (271)
-
(mucoviscidosis) (0)
-
(pancreas near3 fibrocystic near3 disease*) (0)
-
#1 OR #2 OR #3 OR #4 (271)
-
(#5) IN NHSEED (52)
-
(#6) FROM 2002 TO 2012 (22)
Health Economic Evaluations Database (HEED) (Wiley Online Library) 2002–21 May 2012
Searched 21 May 2012.
Using compound search.
-
All Data: ’CYSTIC FIBROSIS’ OR ’mucoviscidosis’ OR ’CF’ AND
-
Journal Date: >= 2002
Sixty-five references were retrieved.
Health-related quality of life
MEDLINE (OvidSP) 1946–May 2012 week 1
Searched 11 May 2012.
-
cystic fibrosis/ (26,164)
-
((Cystic adj2 fibrosis) or mucoviscidosis).ti,ab,ot,hw. (34,176)
-
CF.ti,ot. (1276)
-
(pancreas adj3 fibrocystic adj3 disease$).ti,ab,ot,hw. (16)
-
or/1-4 (34,859)
-
(sf36 or sf 36 or short form 36 or shortform 36).ti,ab. (12,308)
-
(sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).ti,ab. (1)
-
(sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).ti,ab. (890)
-
(euroqol or euro qol or eq5d or eq 5d).ti,ab. (2653)
-
(hql or hrql or hqol or h qol or hrqol or hr qol).ti,ab. (7311)
-
(hye or hyes).ti,ab. (52)
-
health$ year$ equivalent$.ti,ab. (36)
-
(hui or hui1 or hui2 or hui3 or hui4 or hui-4 or hui-1 or hui-2 or hui-3).ti,ab. (692)
-
(quality of well being or quality of wellbeing or qwb).ti,ab. (315)
-
(Disability adjusted life year$ or Disability-adjusted life year$ or health adjusted life year$ or health-adjusted life year$ or years of healthy life or healthy years equivalent or years of potential life lost or years of health life lost or quality adjusted life year$).ti,ab. (5478)
-
(QALY$ or HRQOL or HRQL or DALY$ or HALY$ or YHL or HYES or YPLL or YHLL).ti,ab. (11,747)
-
(health$ adj3 utilit$).ti,ab. (1526)
-
(Time trade-off or time tradeoff or TTO or Standard gamble).ti,ab. (1266)
-
((Cystic Fibrosis adj2 Questionnaire$) or CFQ).ti,ab. (146)
-
or/6-19 (28,430)
-
5 and 20 (127)
-
animals/ not (animals/ and humans/) (3,620,832)
-
21 not 22 (127)
-
remove duplicates from 23 (125)
MEDLINE In-Process & Other Non-Indexed Citations (OvidSP) up to 10 May 2012
MEDLINE Daily Update (OvidSP) up to 10 May 2012
Searched 11 May 2012.
-
cystic fibrosis/ (13)
-
((Cystic adj2 fibrosis) or mucoviscidosis).ti,ab,ot,hw. (860)
-
CF.ti,ot. (153)
-
(pancreas adj3 fibrocystic adj3 disease$).ti,ab,ot,hw. (2)
-
or/1-4 (999)
-
(sf36 or sf 36 or short form 36 or shortform 36).ti,ab. (719)
-
(sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).ti,ab. (0)
-
(sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).ti,ab. (309)
-
(euroqol or euro qol or eq5d or eq 5d).ti,ab. (209)
-
(hql or hrql or hqol or h qol or hrqol or hr qol).ti,ab. (507)
-
(hye or hyes).ti,ab. (1)
-
health$ year$ equivalent$.ti,ab. (1)
-
(hui or hui1 or hui2 or hui3 or hui4 or hui-4 or hui-1 or hui-2 or hui-3).ti,ab. (56)
-
(quality of well being or quality of wellbeing or qwb).ti,ab. (10)
-
(Disability adjusted life year$ or Disability-adjusted life year$ or health adjusted life year$ or health-adjusted life year$ or years of healthy life or healthy years equivalent or years of potential life lost or years of health life lost or quality adjusted life year$).ti,ab. (431)
-
(QALY$ or HRQOL or HRQL or DALY$ or HALY$ or YHL or HYES or YPLL or YHLL).ti,ab. (841)
-
(health$ adj3 utilit$).ti,ab. (118)
-
(Time trade-off or time tradeoff or TTO or Standard gamble).ti,ab. (75)
-
((Cystic Fibrosis adj2 Questionnaire$) or CFQ).ti,ab. (7)
-
or/6-19 (2158)
-
5 and 20 (7)
-
animals/ not (animals/ and humans/) (2139)
-
21 not 22 (7)
-
remove duplicates from 23 (7)
EMBASE (OvidSP) 1974–2012 week 18
Searched 11 May 2012.
-
cystic fibrosis/ (41,722)
-
((Cystic adj2 fibrosis) or mucoviscidosis).mp. (48,828)
-
(pancreas adj3 fibrocystic adj3 disease$).mp. (16)
-
CF.ti,ot. (2503)
-
or/1-4 (49,846)
-
(sf36 or sf 36 or short form 36 or shortform 36).ti,ab. (17,443)
-
(sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).ti,ab. (1)
-
(sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).ti,ab. (1381)
-
(euroqol or euro qol or eq5d or eq 5d).ti,ab. (4305)
-
(hql or hrql or hqol or h qol or hrqol or hr qol).ti,ab. (10,640)
-
(hye or hyes).ti,ab. (62)
-
health$ year$ equivalent$.ti,ab. (41)
-
(hui or hui1 or hui2 or hui3 or hui4 or hui-4 or hui-1 or hui-2 or hui-3).ti,ab. (935)
-
(quality of well being or quality of wellbeing or qwb).ti,ab. (365)
-
(Disability adjusted life year$ or Disability-adjusted life year$ or health adjusted life year$ or health-adjusted life year$ or years of healthy life or healthy years equivalent or years of potential life lost or years of health life lost or quality adjusted life year$).ti,ab. (7550)
-
(QALY$ or HRQOL or HRQL or DALY$ or HALY$ or YHL or HYES or YPLL or YHLL).ti,ab. (17,567)
-
(health$ adj3 utilit$).ti,ab. (2194)
-
(Time trade-off or time tradeoff or TTO or Standard gamble).ti,ab. (1639)
-
((Cystic Fibrosis adj2 Questionnaire$) or CFQ).ti,ab. (272)
-
or/6-19 (41,148)
-
5 and 20 (251)
-
animal/ or animal experiment/ (3,374,417)
-
(rat or rats or mouse or mice or murine or rodent or rodents or hamster or hamsters or pig or pigs or porcine or rabbit or rabbits or animal or animals or dogs or dog or cats or cow or bovine or sheep or ovine or monkey or monkeys).mp. (5,438,846)
-
or/22-23 (5,438,846)
-
exp human/ or human experiment/ (13,531,960)
-
24 not (24 and 25) (4,385,166)
-
21 not 26 (251)
-
limit 27 to embase (235)
-
remove duplicates from 28 (235)
Cost-effectiveness Analysis Registry (internet)
URL: https://research.tufts-nemc.org/cear4
Searched 14 May 2012.
Searched for ‘Articles’ using the term ‘cystic’ (6)
Searched for ‘Ratios’ using the term ‘cystic’ (14)
Searched for ‘Weights’ using the term ‘cystic’ (23)
A total of 43 references were retrieved.
Guidance
National Institute for Health and Care Excellence Guidance (internet)
URL: http://guidance.nice.org.uk
Searched 11 May 2012.
Searched for ‘cystic fibrosis’.
Limited to information type: guidance.
Six references retrieved.
TRIP database (internet)
URL: www.tripdatabase.com
Searched 11 May 2012.
Searched for ‘cystic fibrosis’ in title.
Limited to guidelines.
Sixteen references retrieved.
Guidelines International Network (internet)
Searched 11 May 2012
Searched for ‘cystic fibrosis’.
Seven references retrieved.
National Guidelines Clearinghouse (internet)
URL: www.guidelines.gov
Searched 11 May 2012.
Searched for ‘cystic fibrosis’ in title.
Three references retrieved.
US Food and Drug Administration
URL: www.fda.gov
Searched 11 May 2012.
Searched for ‘cystic fibrosis’ in title.
Limited to guidance.
Two references retrieved.
Cystic Fibrosis Trust
URL: www.cftrust.org.uk
Searched 11 May 2012.
Browsed website publications.
Seventeen references retrieved.
Appendix 2 Risk-of-bias assessment results
| Criteria | Adults | Children | ||
|---|---|---|---|---|
| Support for judgement | Rating | Support for judgement | Rating | |
| Randomisation | Randomisation in 1 : 1 ratio. Randomisation stratified according to age (≥ 18 years) and pulmonary function (= 70% of predicted FEV1). Randomisation code will be produced by Vertex; exact details not reported (from protocol) | Low | No details | Unclear |
| Allocation concealment | Final randomisation list will be provided to the Interactive Voice Response System or Interactive Web Response System. Copy of the final randomisation list will be archived at Vertex in sealed tamper evidence envelopes (protocol) | Low | No details | Unclear |
| Blinding: participant | Study described as ‘double blind’ and ‘the subjects, all site personnel including the investigator, the study monitor, and the Vertex study team will be blinded’, details of a number of site personnel who will not be blinded or situations in which they will be informed of treatment allocation was provided. Appears that VX-770 and placebo will look the same – ‘similar in size and appearance and will be supplied as blue film-coated tablets’ (protocol) | Low | Described as double blind; no further details | Low |
| Blinding: outcome assessor | All outcomes assessors will be blinded to treatment allocation although for some outcome they will be aware of the results for the outcome (protocol) | Low | Described as double blind; no further details | Low |
| Incomplete outcome data | Five withdrawals prior to treatment in placebo group and one in ivacaftor group. Ten withdrawals after treatment started in placebo group and six in ivacaftor group. Missing data were not imputed. For some outcomes last observation carried forward will be use (protocol). Denominator was total number of patients treated not just completers | Low | Four withdrawals in placebo, none in ivacaftor. No details on analysis | Unclear |
| Selective reporting | All primary and secondary outcomes reported; some tertiary outcomes not reported in paper but appears to be more due to space than problem of selective outcome reported | Low | Results appear to have been reported for all outcomes including the primary outcome although we did not have access to protocol | Low |
Appendix 3 Data extraction tables
Randomised controlled trials
| Study details | Selection criteria | Participants (mean, range/SD) | Sample size and interventions | Withdrawals |
|---|---|---|---|---|
| Trial registry number: NCT0090953221,22,26 Country: North America, Australia, Europe Funding: Vertex Pharmaceuticals (VX08–770–102) Study design: parallel group Study duration: 48 weeks |
Inclusion criteria: diagnosis of CF with at
least one G551D mutation. FEV1 predicted
40–90%. Genotype confirmed using a 32-mutation panel
(Ambry Genetics) Exclusion criteria: other illnesses that confounded the study results; ongoing illness; pulmonary exacerbation or changes in therapy (including antibiotics) for pulmonary disease within 4 weeks before first dose of study drug; abnormal liver tests; abnormal renal function tests; history of prolonged QT/QTc interval; history of solid organ or haematological transplantation; colonisation with organisms associated with more rapid decline in pulmonary status; concomitant use of inhibitors or inducers of CYP3A4; use of inhaled hypertonic saline treatment – this was required to be stopped for at least 4 weeks prior to day 1 of treatment |
Mean age: 26 (12–53)
years Proportion male: 48% Ethnicity: proportion non-Hispanic or non-white: 2% Weight: 62 (30–110) kg BMI: 22 (15–39) kg/m2 Height: 167 (142–190) cm Percentage predicted FEV1: 64 (32–98)% Sweat chloride: 100 (58–128) mmol/l Comorbidities: not stated |
Total number eligible: 217 Total number randomised: 167 Placebo Number randomised: 83 Number treated: 78 Co-interventions: pre-study medication except hypertonic saline: (commercial-in-confidence information has been removed) Intervention: ivacaftor Dose: 150 mg Dose frequency: every 12 hours Number randomised: 84 Number treated: 83 Co-interventions: pre-study medication except hypertonic saline: (commercial-in-confidence information has been removed) |
Placebo: 10 withdrawals (four adverse events,
one physician’s decision, two required prohibited
medication, one withdrew consent, two other); 83 assigned to
placebo; 78 received placebo; 68 completed 48 weeks of
treatment Ivacaftor: Six withdrawals in ivacaftor group (one adverse event, two non-complicans, one pregnancy, one prohibited medication, one withdrew consent); 83 received ivacaftor; 77 completed 48 weeks of treatment All but one of the patients (placebo group) entered the open-label extension study |
| Trial registry number: NCT0090972729,30 Country: North America, Australia, Europe Funding: Vertex Pharmaceuticals Study design: parallel group Study duration: 48 weeks |
Inclusion criteria: children aged 6 to 11
years; G551D-CFR mutation; FEV1
40–105% predicted Exclusion criteria: acute respiratory tract infection, pulmonary exacerbation, changes in therapy for pulmonary disease, use of inhaled hypertonic saline treatment within 4 weeks of treatment, abnormal liver function, abnormal renal function, low haemoglobin |
Mean age: 9 (6–11)
years Male: 48% Ethnicity: NR Weight: 31 (9) kg BMI: 17 (2) kg/m2 Height: 134 (13) cm Percentage predicted FEV1: 84 (18)% Sweat chloride: 105 (12) mmol/l Comorbidities: NR |
Total number eligible: NR Total number randomised: 52 Placebo Number randomised: 26 Number treated: 26 Co-interventions: not stated Intervention: ivacaftor Dose: 150 mg Dose frequency: every 12 hours Number randomised: 26 Number treated: 26 Co-interventions: not stated |
Four withdrawals in placebo group, one due to adverse event. No withdrawals in ivacaftor group |
Open-label trial
| Study details | Selection criteria | Participants (mean, range/SD) | Sample size and interventions | Withdrawals |
|---|---|---|---|---|
| Trial registry number: NCT0111701231 Country: North America, Australia, Europe Funding: Vertex Pharmaceuticals Study design: open label Study duration: 96 weeks; data available only for patients who completed 12 weeks’ follow-up |
Inclusion criteria: all patients enrolled in
STRIVE (adults) and ENVISION (children)
studies (Commercial-in-confidence information has been removed) (Commercial-in-confidence information has been removed) |
(Commercial-in-confidence information has been
removed) Ethnicity: NR Weight: (commercial-in-confidence information has been removed) BMI: NR Height: NR Percentage predicted FEV1: 67.4 (29.1–110.4; SD = 19.1)% Sweat chloride: NR Comorbidities: NR |
Intervention: Ivacaftor 150 mg, every 12 hours Number treated: 144 |
One patient who had previously received placebo discontinued
ivacaftor (Commercial-in-confidence information has been removed) 67 originally in placebo arm, 77 in ivacaftor arm |
Appendix 4 Table of excluded studies
Three Phase II trials reported in 18 publications did not reach the review inclusion criteria:
| Trial registry number | Phase of trial | Number of participants | Reason for exclusion |
|---|---|---|---|
| NCT004578211–14 | Phase II; two separate studies | Trial 1: 20; Trial 2: 19 | Short-term outcomes only (2–4 weeks depending on trial) |
| NCT0126235215 | Phase II; ongoing | Target 20; 10 recruited at time of conference abstract | Short-term outcomes only (4 weeks’ duration) |
| NCT0116153716–18 | Phase II; ongoing | Unclear; four recruited at time of conference abstract | Short-term outcomes only (8 weeks’ duration followed by open-label study). No clinically relevant outcomes as specified in the review protocol |
References
- Accurso FJ, Rowe SM, Clancy JP, Boyle MP, Dunitz JM, Durie PR, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med 2010;363:1991-2003.
- Rowe SM, Van Goor F, Clancy JP, Durie PR, Konstan MW, Dunitz J, et al. Corresponding effects of VX-770 on NPD in vivo & human bronchial epithelial (HBE) cells in vitro. Paper presented at 24th Annual North American Cystic Fibrosis Conference, Baltimore, MD, 21–23 October 2010. Pediatr Pulmonol 2010;45.
- Rowe SM, Clancy JP, Boyle M, Van Goor F, Ordonez C, Dong Q, et al. Parallel effects of VX-770 on transepithelial potential difference in vitro and in vivo. Paper presented at 33rd European Cystic Fibrosis Conference, Valencia, Spain, 16–19 June 2010. J Cyst Fibros 2010;10.
- Rowe SM, Accurso FJ, Clancy JP, Boyle MP, Dunitz JM, Durie PR, et al. Improvement in ion transport biomarkers and spirometry with the investigational CFTR potentiator VX-770 in subjects with cystic fibrosis and the G551D-CFTR mutation. Paper presented at 9th International Congress on Pediatric Pulmonology, Vienna, Austria, 19–21 June 2010. Paediatr Respirat Rev 2010;11.
- Donaldson S, Accurso F, Rowe S, Clancy J, Boyle M, Dunitz J, et al. Improved CFTR and lung function with VX-770, a novel investigational potentiator of CFTR, in subjects with the G551D-CFTR mutation n.d.
- Konstan MW, Accurso FJ, Boyle MP, Clancy JP, Ordonez CL, Zha J, et al. Relationship between pulmonary outcomes, biomarkers of CF disease, and serum drug levels in subjects with the G551D-CFTR mutation treated with VX-770, an investigational oral potentiator of CFTR. Paper presented at American Thoracic Society International Conference, New Orleans, LA, 14–19 May 2010. Am J Respir Crit Care Med 2010;181.
- Boyle M, Clancy JP, Rowe SM, Durie P, Dunitz J, Konstan MW, et al. Effect of VX-770, a CFTR potentiator, on spirometry and qol assessment in subjects with CF and the G551D-CFTR mutation n.d.
- Accurso FJ, Rowe SM, Durie PR, Konstan MW, Dunitz J, Hornick DB, et al. Final results of a 14- and 28-day study of VX-770 in subjects with CF. Paper presented at the 32nd European Cystic Fibrosis Society (ECFS) conference, Brest, France, 10–13 June 2009. J Cyst Fibros 2009;8.
- Accurso FJ, Rowe SM, Durie PR, Konstan MW, Dunitz J, Hornick DB, et al. Interim results of phase 2a study of VX-770 to evaluate safety, pharmacokinetics, and biomarkers of CFTR activity in cystic fibrosis subjects with G551D 2008.
- Accurso F, Rowe SM, Durie PR, Konstan MW, Dunitz J, Hornick D, et al. Improvement in sweat chloride concentration by the CFTR potentiator VX-770 in subjects with cystic fibrosis and the G551D-CFTR mutation n.d.
- Clancy JP, Rowe SM, Durie P, Freedman S, Dong Q, Ordonez C, et al. NPD evaluation of ion transport in G551D CF patients treated with a CFTR potentiator n.d.
- Rowe SM, Accurso FJ, Clancy JP, Boyle MP, Dunitz JM, Durie PR, et al. Improvement in ion transport biomarkers and spirometry with the investigational CFTR potentiator VX-770 in subjects with cystic fibrosis and the G551D-CFTR mutation n.d.
- Safety study of ivacaftor in subjects with cystic fibrosis. Bethesda, MD: National Library of Medicine (US); 2010.
- Vertex Pharmaceuticals Inc . A Phase 2a, Randomized, Double-Blind, Placebo-Controlled Study of VX-770 to Evaluate Safety, Pharmacokinetics, and Biomarkers of CFTR Activity in Cystic Fibrosis (CF) Subjects With Genotype G551D n.d. URL: www.clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2007-002657-23 (accessed 14 May 2012).
- Study of the effect of VX-770 on lung clearance index in subjects with cystic fibrosis and the G551D mutation. Bethesda, MD: National Library of Medicine (US); 2012.
- Bethesda, MD: National Library of Medicine (US); 2011.
- Altes T, Johnson MA, Miller GW, Mugler JP, Flors L, Mata J, et al. Hyperpolarized helium-3 magnetic resonance imaging of CFTR potentiator therapy in subjects with cystic fibrosis and the G551D mutation. Paper presented at 25th Annual North American Cystic Fibrosis Conference, Anaheim, CA, 3–5 November 2011. Pediatr Pulmonol 2011;46.
- Altes T, Johnson MA, Miller GW, Mugler JP, Flors L, Mata J, et al. Hyperpolarized helium-3 magnetic resonance imaging of CFTR potentiator therapy in subjects with cystic fibrosis and the G551D mutation n.d.
Appendix 5 Discrepancies in reporting of results
Adults’ trial
Results data which were reported differently in different reports of the same trial:
| Outcomes | Measures | Time periods | Paper21 | Supplementary data, %21 | Dossier16 |
|---|---|---|---|---|---|
| Percentage predicted FEV1: relative change | Mean difference in change from baseline | 24 weeks | 17.1 | 16.9 (13.6, 20.2) | 17.1 and 17.2 |
| Percentage predicted FEV1: relative change | Mean difference in change from baseline | 48 weeks | 16.8 (13.5, 20.1) | 17.0 |
Relative risks reported in publications that were different from those that we calculated using the reported raw data:
| Outcomes | Durations of follow-up | RR (95% CI) | |
|---|---|---|---|
| Paper21 | Calculated from raw data | ||
| Number of patients with exacerbation | 24 weeks | 0.38 (0.22 to 0.64) | 0.48 (0.30 to 0.77) |
| Number of patients with exacerbation | 48 weeks | 0.43 (0.27 to 0.68) | 0.60 (0.41 to 0.85) |
Children’s trial
Results data which were reported differently in different reports of the same trial:
Open-label trial
Results data which were reported differently in different reports of the same trial:
| Outcomes | Measures | Ivacaftor | Placebo | ||||
|---|---|---|---|---|---|---|---|
| CA | Dossier | Press release | CA | Dossier | Press release | ||
| Percentage predicted FEV1: absolute change, percentage points | Mean change from baseline (SD) | 11.1 (9.7) | Same | 11.6 | 10.8 (9.5) | 9.7 (9.7) | 10.9 (NR) |
| Percentage predicted FEV1: relative change, % | Mean change from baseline (SD) | 18.7 (17.0) | Same | 19.4 | 19.9 (20.1) | 17.0 (19.3) | 19.9 (NR) |
Appendix 6 Overview of included and background studies for health economics review
Included studies
| Author | Year | Cost-effectiveness | Health-care cost | Social care cost | Disease-specific utility | Generic utility | Incidence prevalence | Mortality | Model probability | Year of data | Setting | Time horizon | n with CF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Wertz et al.81 | 2011 | – | ✓ | – | – | – | – | – | – | 2004–9 | USA | 2004–9 | 832 |
| Briesacher et al.82 | 2011 | – | ✓ | – | – | – | – | – | – | 2001–7 | USA | 2001–7 | 3273 |
| O’Sullivan et al.83 | 2011 | – | ✓ | – | – | – | – | – | – | 2006 | USA | 12 months | 1064 |
| Woodward et al.84 | 2010 | – | ✓ | – | – | – | – | – | – | 2008 | USA | 4 years | 262 |
| Braccini et al.85 | 2009 | – | ✓ | – | – | – | – | – | – | 2002–6 | Italy | 4 years | 61 |
| Schreyogg et al.86 | 2006 | – | ✓ | – | – | – | – | – | – | 2004 | Germany | 6 months | 131 |
| van den Akker-van Marle et al.87 | 2006 | – | ✓ | – | – | – | – | – | – | 2004 | Holland | NA | NA |
| Thornton et al.39 | 2005 | ✓ | ✓ | – | ✓ | – | – | – | ✓ | 2003 | UK | 12 months | 196 |
| Iles et al.88 | 2003 | ✓ | ✓ | – | ✓ | – | – | – | – | 2002 | UK | 2 years | 71 |
| Baumann et al.40 | 2003 | – | ✓ | – | ✓ | – | – | – | ✓ | 2002 | Germany | 12 months | 138 |
| Sansgiry et al.89 | 2012 | – | ✓ | – | – | – | – | – | – | 2005–8 | USA | 12 months | 358 |
| Dewitt et al.90 | 2012 | – | ✓ | – | ✓ | ✓ | – | – | – | 2008 | USA | 48 weeks | 352 |
| Abbott et al.91 | 2009 | – | – | – | – | ✓ | – | ✓ | – | 1996–2006 | UK | 10 years | 223 |
| Ramsey et al.21 | 2011 | – | – | – | ✓ | ✓ | – | – | – | 2009–11 | USA | 48 weeks | 161 |
| Gee et al.43 | 2002 | – | – | – | ✓ | ✓ | – | – | – | 2002 | UK | 6 months | 223 |
| Becker et al.92 | 2011 | ✓ | ✓ | – | – | – | – | – | – | 2004–8 | US | 12 months | NA |
| Aherns et al.27 | 2011 | – | – | – | ✓ | – | – | – | – | 2010 | UK | 48 weeks | 52 aged 6–11 years |
| Loadman et al.93 | 2010 | – | – | – | – | ✓ | – | ✓ | – | 1986–2008 | Canada | 1–5 years | 72 |
| Simpson et al.38 | 2005 | ✓ | ✓ | – | ✓ | ✓ | – | ✓ | ✓ | 1998 | UK | Lifetime | NA |
| ScHARR41 | 2012 | ✓ | ✓ | – | ✓ | ✓ | ✓ | – | ✓ | NA | UK | Lifetime | NA |
| UK CF Registry report 20108 | 2011 | – | ✓ | – | ✓ | – | ✓ | – | – | 2010 | UK | 1 year | 9385 |
| CFT antibiotic treatment59 | 2009 | – | ✓ | – | – | – | – | – | – | 2009 | UK | NA | NA |
| KSR42 | 2011 | ✓ | ✓ | – | ✓ | ✓ | ✓ | – | ✓ | 2008–9 | UK | Lifetime | 341 |
Background studies
| Author | Year | Cost-effectiveness | Health-care cost | Social care cost | Disease-specific utility | Generic utility | Incidence prevalence | Mortality | Model probability |
|---|---|---|---|---|---|---|---|---|---|
| Eidt-Koch et al.94 | 2009 | – | – | – | – | ✓ | – | – | – |
| Elphick et al.95 | 2009 | – | – | – | ✓ | ✓ | – | – | – |
| Weiner et al.96 | 2008 | – | ✓ | – | ✓ | ✓ | – | – | – |
| Guerriere et al.97 | 2008 | – | – | ✓ | – | – | – | – | – |
| Radhakrishnan et al.98 | 2008 | ✓ | ✓ | ✓ | – | – | – | – | – |
| Elphick et al.99 | 2005 | – | – | – | ✓ | – | – | – | – |
| Yi et al.100 | 2004 | – | – | – | ✓ | ✓ | – | – | – |
| Wark et al.101 | 2009 | – | – | – | ✓ | – | – | – | – |
| Mayer-Hamblett et al.102 | 2007 | – | – | – | ✓ | ✓ | – | – | – |
| Ashish et al.103 | 2010 | – | ✓ | – | – | – | – | – | – |
| Smalarz et al.104 | 2009 | – | ✓ | – | – | – | – | – | – |
| Abbott et al.105 | 2011 | – | – | – | – | ✓ | – | – | – |
| Flume et al.78 | 2011 | – | – | – | ✓ | – | – | – | – |
| Elborn et al.106 | 2011 | – | – | – | ✓ | – | – | – | – |
| Hofer et al.107 | 2011 | – | – | – | ✓ | ✓ | – | – | – |
| Bradley et al.108 | 2011 | – | – | – | ✓ | ✓ | – | – | – |
| Cystic Fibrosis Trust Standards1 | 2010 | – | ✓ | – | – | – | – | – | – |
Appendix 7 Review protocol
Ivacaftor for cystic fibrosis
Protocol
Plain English Summary
Cystic Fibrosis (CF) is one of the most common, inherited diseases in white populations. Around 1 in every 2500 babies born in the UK has CF and there are over 9000 people in the UK with CF. CF is caused by a single faulty gene which controls the movement of salt and water in and out of cells. This results in thick sticky mucous clogging up the internal organs (e.g. lungs, pancreas, liver, intestine and reproductive tract) making it difficult to breathe and digest food. Other symptoms can include a troublesome cough, prolonged diarrhoea and poor weight gain. Most of the illness caused by CF is from diseases of the lungs and repeated infections. There is no cure for CF and most treatments (e.g. physiotherapy, antibiotics for infections, drugs to suppress inflammation) target the symptoms rather than the cause of disease. Median survival of the current UK cohort with CF is estimated as 41 years. Most patients die from lung disease. Life expectancy is increasing and is expected to increase to at least 50 years for children born in 2000.
A large number of different mutations have been identified in the gene that causes CF. New treatments are being developed which target specific mutations. Ivacaftor (brand name Kalydeco, Vertex Pharmaceuticals) is the first of these drugs and targets patients with the “G551D” mutation. Around 4.4% of patients with CF in the UK will have at least one G551D mutation. Ivacaftor represents a new approach to treating patients with CF as it targets the underlying cause of CF. It aims to increase salt movement through the cell by targeting a specific protein. Ivacaftor is classed as an “orphan drug” which means that has been developed specifically to treat a rare disease. It has been approved by the American Food and Drug Administration for the treatment of patients with CF who are at least 6 years old and have the G551D mutation. There are currently no similar drugs which target the underlying protein defect in CF on the market.
This review aims to evaluate the effectiveness of ivacaftor tablets for the treatment of cystic fibrosis (CF) in patients age 6 years and older who have at least one G551D mutation. The review will consider both clinical effectiveness (improvement in patients’ symptoms and adverse events) and cost effectiveness (cost of treatment).
2. Decision problem
2.1 Objectives
This review aims to appraise the clinical and cost effectiveness of ivacaftor 150 mg tablet for oral administration for the treatment of cystic fibrosis (CF) in patients age 6 years and older who have at least one G551D mutation in the CFTR gene. We will aim to determine the category of patients most likely to benefit from Ivacaftor by assessing whether the effects vary according to disease severity and age.
2.2 Background
Cystic Fibrosis is the most common, life-threatening, autosomal recessive disorder in Caucasian populations; it has an estimated carrier rate of 1 in 25 and incidence of 1 in 2500 live births. 1 It affects around 9000 people in the UK with a prevalence of 1.37/10,000. 2 CF was first recognised as a distinct disease in 1938. 3 It is characterised by abnormal transport of chloride and sodium, leading to thick viscous secretions in the lungs, pancreas, liver, intestine, and reproductive tract and to an increased salt content in sweat gland secretions. 4 Most of the morbidity and mortality is from pulmonary disease, which is characterised by bronchial and bronchiolar obstruction with thick tenacious secretions that are difficult to clear, colonisation by pathogenic bacteria and repeated infections. 1 There is chronic inflammation and progressive lung destruction can lead to bronchiectasis, altered pulmonary function, and respiratory failure. CF can also lead to CF related diabetes (CFRD), male infertility and liver involvement. In addition to repeated chest infections, symptoms of CF can include a troublesome cough, prolonged diarrhoea and poor weight gain. 1 Most patients with CF eventually succumb to lung disease and survival of patients with CF is currently around 41 years, a considerably increase from around 6 months when the disease was first identified,4 and is expected to increase to at least 50 years for children born in 2000. 2
CF is caused by mutations in the CF transmembrane conductance regulator (CFTR) gene which was discovered in 1989. 5 It sits on chromosome 7, is some 250 kB in length, and encodes a protein of 1480 amino acids. This protein is a chloride channel present at the surface of epithelial cells in multiple organs and is responsible for aiding in the regulation of salt and water absorption and secretion. Over 1000 disease-causing alleles within this gene have been identified although only 23 have been demonstrated to cause sufficient loss of CFTR function to confer CF disease. 6 The most common mutation is the F508 mutation which is present on around 67% of CF chromosomes worldwide. 7 The G551D (glycine to aspartate change in nucleotide 1784 in exon 11), which affects approximately 4.4% of patients with CF in the UK,8 is of interest as a new treatment has been developed targeted specifically at patients with this mutation. CFTR protein channels with the G551D mutation have a greatly reduced fraction of time that the channel spends in the open state, or “open probability,” and, therefore, have limited chloride transport ability.
Diagnosis of CF and genetic testing
The gold standard for the diagnosis of CF is the sweat test. 6 This tests for elevated levels of chloride in sweat with a diagnosis of CF being made at levels above 60 mmol/L, and a possible diagnosis of CF at level above 30 mmol/L. New born screening tests have been introduced in many countries, and have been routine throughout the UK since October 2007. 109 These involve a small sample of blood being taken (“heel prick test”) which is tested for high levels of immunoreactive trypsinogen (IRT). If an abnormal IRT value is identified, most new born screening programmes perform a combination of DNA testing to identify known CFTR mutations and repeat IRT testing. 10 IRT testing alone has a sensitivity of 82–100%, double IRT testing increases sensitivity to 89–100% and IRT and DNA testing has a sensitivity of 94–100%; specificity is > 99% for all testing strategies. 11 In the UK screening programme, the initial DNA test involves testing for four mutations (F508, G551D, G542X and 621 + 1G > T), if only one CF mutation is detected then further DNA analysis based on 29 or 31 mutations is recommended. A range of commercial kits are available for diagnostic testing. The diagnosis is then confirmed using the sweat test. 10
Treatment of CF
There is no cure for CF and current treatments target the complications rather than cause of the disease. 4 Treatments can be broadly classified as nutritional repletion (e.g. pancreatic enzyme supplementation and nutritional supplementation), relief of airway obstruction (e.g. physiotherapy, drugs to improve sputum clearance, bronchodilators), treatment of airway infection (e.g. antibiotics), suppression of inflammation (e.g. steroids, high-dose ibuprofen) and lung transplantation. 4
Ivacaftor
Ivacaftor (brand name Kalydeco, Vertex Pharmaceuticals) is the first in a new class of drugs known as CFTR potentiators which represents a new therapeutic approach to the treatment of patients with CF by targeting the underlying protein defect of CF. The drug facilitates increased chloride transport by potentiating the channel-open probability (or gating) of the G551D-CFTR protein. 12
Ivacaftor is a designated orphan medicinal product. 13 It has been approved by the FDA for the treatment of CF in patients aged 6 years or older who have a G551D mutation in the CFTR gene14 and is the subject of a European Union marketing authorisation application. No active comparator agents that target the underlying CFTR protein defect in CF disease exist. 16
3. Report methods for synthesis of evidence of clinical effectiveness
We will conduct a systematic review of the evidence on the clinical effectiveness of ivacaftor 150 mg tablet for oral administration for the treatment of cystic fibrosis (CF) in patients age 6 years and older who have at least one G551D mutation in the CFTR gene. The review will follow the general principles recommended in the PRISMA statement and CRD report 4. 17,18
3.1 Search strategy
Literature searches will be undertaken in several stages to identify relevant information, such as eligible studies, evidence-based health technology assessments (HTAs), systematic reviews, economic evaluations, guidelines and health-related quality of life data. The EMBASE strategies will be independently peer reviewed by a second Information Specialist, using the PRESS-EBC checklist. 19
Clinical effectiveness
Searches will be undertaken to locate randomised controlled trials using ivacaftor. They will not be limited by date, language or publication status (unpublished or published). The following databases will be searched:
-
MEDLINE (OvidSP)
-
MEDLINE In-Process & Other Non-Indexed Citations (OvidSP)
-
EMBASE (OvidSP)
-
Latin American and Caribbean Health Sciences Literature (LILACS) (VHL)
-
Cochrane Database of Systematic Reviews (CDSR) (Wiley Online Library)
-
Cochrane Central Register of Controlled Trials (CENTRAL) (Wiley Online Library)
-
Database of Abstracts of Reviews of Effects (DARE) (Wiley Online Library & CRD)
-
NHS Economic Evaluation Database (NHS EED) (Wiley Online Library & CRD)
-
Health Technology Assessment Database (HTA) (Wiley Online Library & CRD)
Supplementary searches will be undertaken on the following resources to identify unpublished and on-going studies:
-
metaRegister of Controlled Trials (internet) (http://www.controlled-trials.com)
-
NIH Clinicaltrials.gov (internet) (http://www.clinicaltrials.gov)
-
WHO International Clinical Trials Registry Platform (ICTRP) (internet) (http://www.who.int/ictrp/en/)
Scanning abstracts and programmes of relevant conferences will enable identification of relevant studies and projects. The following conference proceedings will be searched from 2007–2012:
-
European Cystic Fibrosis Society (ECFS) conference (http://www.ecfs.eu/conferences/main)
-
North American Cystic Fibrosis Conference (NACFC) (https://www.nacfconference.org/)
-
International Congress on Pediatric Pulmonology (CIPP) (http://www.cipp-meeting.org/index.htm)
The bibliographies of retrieved articles and relevant systematic reviews will be checked for additional studies. Identified references will be downloaded into Endnote bibliographic management software for further assessment and handling.
3.2 Inclusion criteria
Studies that fulfil the following criteria will be eligible for inclusion:
Population: Children (6 years and older) and adults with cystic fibrosis who have the G551D mutation on at least one CFTR allele. Patients with all severities of disease will be eligible.
Intervention: Ivacaftor tablets.
Comparator: Any reported comparator.
Outcomes: The primary outcome will be lung function (e.g. per cent predicted forced expiratory volume in one second (FEV1)). Other eligible outcomes include mortality, weight, BMI, sweat chloride, respiratory symptoms, reduction in pulmonary exacerbations, exercise tolerance, adverse effects of treatment, health-related quality of life and utilisation of hospital resources. Studies that only report short-term outcomes (< 3 months only) will be excluded.
Study design: For the review of clinical effectiveness, only RCTs will be included. Criteria will be relaxed for consideration of adverse events, for which open label studies will be eligible.
The results of the searches will be screened for relevance independently by two reviewers. Full text of studies identified as potentially relevant will be obtained and assessed for inclusion by one reviewer and checked by a second. Disagreements will be resolved through discussion or referral to a third reviewer where necessary.
3.3 Data extraction strategy
Data will be extracted by one reviewer using a standardised data extraction form and checked by another. Disagreements will be resolved through discussion or referral to a third reviewer where necessary. Data will be extracted on the primary outcome, lung function (e.g. percept predicted forced expiratory volume in one second (FEV1)), and the following additional outcomes: mortality, weight, BMI, sweat chloride, respiratory symptoms, reduction in pulmonary exacerbations, exercise tolerance, adverse effects of treatment, health-related quality of life and utilisation of hospital resources. Data will be extracted after 24 weeks (intermediate) treatment and after the longest duration of follow-up reported. If data are available for different patient subgroups (e.g. age, disease severity, region) then data will be extracted separately for each subgroup. If composite end points are reported, data will be extracted on the definition of the end point, results, and, if sufficient data are available, the events that contributed to the end point.
3.4 Quality assessment strategy
Trials will be assessed for methodological quality using the Cochrane Risk of Bias tool. 20 This includes items covering selection bias (random sequence generation and allocation concealment), performance bias (participant blinding), detection bias (blinding of outcome assessors) attrition bias (incomplete outcome data), and reporting bias (selective reported). There is also an addition field for other sources of bias. We believe that all important concerns about bias are include in the other domains in the tool and so no further domains will be added. Each domain is assigned a rating of high, low, or unclear. Each trial will be assigned an overall rating of the risk of bias. If at least one of the domains is rated as “high” the trial will be considered at high risk of bias, if all domains are judged as “low” the trial will considered at low risk of bias, otherwise the trial will be considered at “unclear” risk of bias. The risk of bias assessment will be incorporated into the data extraction form and will be conducted as part of the data extraction.
3.5 Methods of analysis/synthesis
We do not anticipate having sufficient data to conduct a formal meta-analysis. Data will be tabulated and discussed in a narrative review. Details of the components of best supportive care, where reported in the included studies, will be clearly described. If sufficient data are available results will be grouped by age, lung function, disease severity, and prior treatment (including consideration of intolerance to treatments). Dichotomous data will be summarised as relative risks or hazard ratios together with 95% confidence intervals (CIs). Continuous outcomes will be summarised as mean differences between treatment groups together with 95% CIs; where appropriate mean differences between groups in mean change from baseline will be calculated. If sufficient data are available, results will be displayed graphically using forest plots. Publication bias will not be formally assessed as we only expect to include a very small number of trials. Standard methods to detect publication bias will therefore not be possible.
4. Report methods for synthesising evidence of cost effectiveness
4.1 Identifying and reviewing published cost-effectiveness studies
Focussed searches will be undertaken to identify literature on cost-effectiveness and cystic fibrosis. Searches will be limited to the last ten years. The following resources will be searched:
-
Medline (OvidSP)
-
Medline In-Process Citations (OvidSP)
-
Embase (OvidSP)
-
NHS Economic Evaluation Database (NHS EED) (CRD)
-
Health Economic Evaluation Database (HEED)
Health-related Quality of Life
Focussed searches will be undertaken to identify literature on HRQoL and cystic fibrosis. Searches will not be limited by date and the following resources will be searched:
-
Medline (OvidSP)
-
Medline In-Process Citations & Other Non-Indexed Citations (OvidSP)
-
Embase (OvidSP)
-
CEA Registry (internet)
Guidelines and guidance
The following resources will be searched for guidelines and guidance related to cystic fibrosis:
-
NICE Guidance (internet) (http://guidance.nice.org.uk/)
-
TRIP database (limited to guidelines) (internet) (http://www.tripdatabase.com/)
-
Guidelines International Network (GIN) (internet)
-
National Guidelines Clearinghouse (internet) (http://www.guidelines.gov)
-
Cystic Fibrosis Trust (http://www.cftrust.org.uk/)
Searches will focus on original papers that report on cost, cost-effectiveness or cost-utility analyses, either studying the diagnostic phase (genetic testing for CF mutations), therapeutic phase (management of patients with confirmed CF), or a combination. Note that this search does not only include studies on ivacaftor, but evaluations of any treatment for CF. For our assessment cost studies, utility studies and full economic evaluations, i.e. those that explicitly compare different decision options will be selected. Clinical trials as well as modelling studies and cohort studies will be relevant within the frame of our project. The intention is not to perform a systematic review, but to use the studies identified to support the development of an economic model and estimation of model input parameters that will aim to answer the research questions of this project.
The results and the methodological quality of the studies selected will be summarised. Data extraction will focus on interventions compared, indicated population, main results in terms of costs and consequences of the alternatives compared, and the incremental cost-effectiveness, but also on methods of modelling used (if applicable), for example relating to extrapolation of study results, analytical methods and robustness of the study findings.
4.2 Evaluation of cost-effectiveness
If an economic evaluation is provided by the manufacturer it will be assessed for clinical validity, reasonableness of assumptions and appropriateness of the data used in the economic model. If the team judge that the existing economic evidence is not robust, then further work will be undertaken, either by adapting what already exists or developing a de novo model. Such de novo economic evaluation will be undertaken from a NHS and social care perspective. The model will draw together evidence from literature and study reports concerning treatment efficacy, withdrawal, treatment related adverse events, relevant diagnostic interventions, chronic care costs, and HRQoL. The model structure will be developed such that the effects of treatment on lung function, exacerbations, quality of life and treatment costs can be incorporated. The level of detail will depend on available evidence. Specifically, the impact of treatment on resource use in pulmonary exacerbations in both the primary and secondary care settings will be taken into account if data allows. If evidence allows, subgroups by age, lung function, disease severity, and prior treatment (including consideration of intolerance to treatments) may be considered. Additionally, the impact of treatment on resource use in pulmonary exacerbations in both the primary and secondary care settings will be taken into account if data allows.
Costs will be identified through literature searches. As genetic testing is essential to the use of ivacaftor it will be part of the assessment. If possible with the data available, the assessment of ivacaftor will consider the impact of treatment on progression through treatment bands over time, and take in to account any service implications (e.g. changes in type/duration/frequency of hospital activity). In line with current recommendations, costs and health outcomes will be discounted at 3.5%. Key health economic outcomes are likely to include the cost per life year gained, and the cost per quality adjusted life year (QALY) gained. The cost-effectiveness of interventions will be compared incrementally against each other.
Sensitivity analysis will be undertaken to examine the key determinants of cost-effectiveness. Probabilistic sensitivity analysis (PSA) will be undertaken to generate information on the likelihood that each treatment produces the greatest amount of net benefit. The results of this PSA will be presented as cost-effectiveness acceptability curves (CEACs).
5. Timetable/milestones
| Milestone | Deadline |
|---|---|
| Protocol Submitted | 22 May |
| Searches | 10 May |
| Reference Screening | 10 May |
| Inclusion assessment | 14 May |
| Data extraction and quality assessment | 24 May |
| SR results section draft | 24 May |
| Health economic results to KSR | 29 June |
| Health economics section complete | 6 July |
| Report to commissioner | 10 July |
| Comments from Commissioner | 31 July |
| Final report | 17 August |
6. Team members’ contributions
Penny Whiting will be the main reviewer on this project and will maintain day-to-day running of the review. Marie Westwood will act as second reviewer. Both reviewers have contributed to the study protocol and will carry out the study selection, data extraction, analysis and production of the final report. Maiwenn Al will be health economic lead for this project, and thus be responsible for the cost-effectiveness study.
Appendix: Draft search strategy
EMBASE (OvidSP): 1974-2012/wk17
Searched 3.5.12
-
Ivacaftor/ (72)
-
(Ivacaftor or Kalydeco or VX-770 or VX770 or 873054-44-5 or ivacaftorum).af. (138)
-
or/1-2 (138)
Appendix 8 PRISMA checklist
| Section/topic | # | Checklist item | Reported on page # |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both | Title page and p. v |
| Abstract | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number | pp. v–vi |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known | Chapter 1, pp. 1–2 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS) | Chapter 2, p. 3 |
| Methods | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g. Web address), and, if available, provide registration information including registration number | p. xix and Appendix 7, PROSPERO, CRD42012002516 www.crd.york.ac.uk/prospero/ |
| Eligibility criteria | 6 | Specify study characteristics (e.g. PICOS, length of follow-up) and report characteristics (e.g. years considered, language, publication status) used as criteria for eligibility, giving rationale | Chapter 3, Inclusion and exclusion criteria, p. 6 |
| Information sources | 7 | Describe all information sources (e.g. databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched | Chapter 3, Identification of studies, pp. 5–6 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated | Appendix 1, pp. 69–81 |
| Study selection | 9 | State the process for selecting studies (i.e. screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis) | Chapter 3, Inclusion and exclusion criteria, p. 6 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g. piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators | Chapter 3, Data extraction strategy, p. 6 |
| Data items | 11 | List and define all variables for which data were sought (e.g. PICOS, funding sources) and any assumptions and simplifications made | Chapter 3, Data extraction strategy, p. 6 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis | Chapter 3, Critical appraisal strategy, pp. 6–7 |
| Summary measures | 13 | State the principal summary measures (e.g. risk ratio, difference in means) | Chapter 3, Methods of data synthesis, p. 7 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g. I2) for each meta-analysis | Chapter 3, Methods of data synthesis, p. 7 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g. publication bias, selective reporting within studies) | Not applicable |
| Additional analyses | 16 | Describe methods of additional analyses (e.g. sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified | Not applicable |
| Results | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram | Chapter 4, Quantity and quality of research available and Figure 1, p. 9 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g. study size, PICOS, follow-up period) and provide the citations | Chapter 4, Summary of included studies, pp. 10–11; Table 1, p. 10; and Appendix 3, pp. 85–8 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12) | Chapter 4, Risk of bias, pp. 11–12; Table 2, p. 12; and Appendix 2, p. 83 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group, (b) effect estimates and confidence intervals, ideally with a forest plot | Tables 3 to 9 and Figures 3 to 10, pp. 12–24 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency | Not applicable |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15) | Not applicable |
| Additional analysis | 23 | Give results of additional analyses, if done [e.g. sensitivity or subgroup analyses, meta-regression (see Item 16)] | Not applicable |
| Discussion | |||
| Summary of evidence | 24 | Summarize the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g. healthcare providers, users, and policy makers) | Chapter 7, Statement of principal findings, p. 51 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g. risk of bias), and at review-level (e.g. incomplete retrieval of identified research, reporting bias) | Chapter 7, Clinical effectiveness, pp. 51–2 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research | Chapter 8, p. 57 |
| Funding | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g. supply of data); role of funders for the systematic review | p. vi and p. xix |
List of abbreviations
- BMI
- body mass index
- CF
- cystic fibrosis
- CFQ-R
- Cystic Fibrosis Questionnaire Revised
- CFRD
- cystic fibrosis-related diabetes
- CFTR
- cystic fibrosis transmembrane conductance regulator
- CI
- confidence interval
- CRD
- Centre for Reviews and Dissemination
- DARE
- Database of Abstracts of Reviews of Effects
- DNA
- deoxyribonucleic acid
- DNase
- dornase alfa
- EQ-5D
- European Quality of Life-5 Dimensions
- FDA
- US Food and Drug Administration
- FEV1
- forced expiratory volume in 1 second
- G551D
- glycine to aspartate change in nucleotide 1784 in exon 11
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- IRT
- immunoreactive trypsinogen
- i.v.
- intravenous
- MD
- mean difference
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- PSA
- probabilistic sensitivity analysis
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- RR
- relative risk
- SF-36
- Short Form questionnaire-36 items
- TIVAD
- totally implantable venous access device
- TOBI
- tobramycin inhaled solution
- WHO
- World Health Organization
Note
This monograph is based on the Technology Assessment Report produced for the North of England Specialised Commissioning Group: Yorkshire and the Humber Office. The full report contained a number of data that were deemed commercial-in-confidence. The present monograph presents as full a version of the report as possible while retaining readability, but some sections, sentences, tables and figures have been removed.