Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/63/01. The contractual start date was in November 2009. The draft report began editorial review in February 2012 and was accepted for publication in March 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors:
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Cooper et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Abnormal uterine bleeding
Abnormal uterine bleeding (AUB) affects women of both reproductive (pre-menopausal women) and post-reproductive (post-menopausal women) age, but the implications of diagnosis and need for treatment of AUB are completely different according to menopausal status.
In pre-menopausal women, AUB manifests itself primarily as excessive cyclical bleeding. This heavy menstrual bleeding (HMB) affects one in five women of reproductive age, with 5% of women aged 30–49 years consulting their general practitioner (GP) each year because of the condition, and accounts for one-third of all gynaecology referrals. 1 The overall prevalence of HMB in England and Wales has been estimated at 1.5 million women. 2 The number and cost of consultations and treatments impose substantial demands on health service resources. 3,4 Moreover, HMB can cause significant distress to women by affecting their performance at work as well as their social activities, and imposes a substantial adverse impact upon health-related quality of life (HRQL). 5–7
Post-menopausal bleeding (PMB) is also a common clinical problem in both general practice and secondary care hospital settings. Women are most likely to present with PMB in the seventh decade of life, with consultation rates in primary care among this age group of 14.3 per 1000 population. 2 In contrast to HMB, PMB is rarely heavy or indeed persistent, but it causes significant alarm and anxiety to women, who recognise vaginal bleeding after their periods have ceased as abnormal and potentially indicative of underlying malignancy. This fear is justified, as between 5% and 10% of women with PMB will have endometrial cancer. 8,9 Prompt referral to secondary care is recommended to exclude the possibility of malignant disease. Thus, it is not surprising that abnormal patterns of uterine bleeding account for up to 50% of all gynaecological consultations in the peri- and post-menopausal years. 10
Heavy menstrual bleeding
Definition of heavy menstrual bleeding
The National Institute for Health and Care Excellence (NICE), in its 2007 guideline on the management of HMB, recommended that the condition be defined as ‘excessive menstrual blood loss which interferes with the woman’s physical, emotional, social and material quality of life, and which can occur alone or in combination with other symptoms’ (p. 30). 11 This clinical definition is the most useful one, as objective measurement, with loss of > 80 ml of blood per cycle considered definitive of HMB,12 is impractical. More applicable semi-objective measurement, using pictorial blood loss assessment of sanitary ware13,14 as a surrogate for objective measurement, has been tried but the correlation between objective and semi-objective quantification has been questioned. 13 In any case, objective quantification of menstrual loss does not correlate in many cases with a woman’s subjective complaint of HMB. 15–17
Causes of heavy menstrual bleeding
Heavy menstrual bleeding has been reported to be caused by a variety of underlying pathologies. 11 However, while many conditions have been linked to HMB, in practice most cases are attributed to fibroids, endometrial pathology or dysfunctional uterine bleeding (DUB), and subsequent treatment is dictated by the presence or absence of these conditions (Table 1).
| Cause | Definition |
|---|---|
| Dysfunctional uterine bleeding | The occurrence of irregular or excessive uterine bleeding in the absence of pregnancy, infection, trauma, new growth or hormone treatment (i.e. the absence of identifiable organic pathology)11 |
| Uterine fibroids | Smooth-muscle tumours of the uterus, generally benign although occasionally (< 1%) malignant. They vary greatly in size, from millimetres to tens of centimetres, and are associated with heavy periods, pressure symptoms and occasionally pain. They are responsive to the female hormones oestrogen and progesterone, generally shrinking to a degree at the menopause11 |
| Endometrial pathology | |
| Polyps | Endometrial polyps are focal outgrowths that can occur anywhere within the uterine cavity. They contain a variable amount of glands, stroma and blood vessels, the relative amounts of which influence their macroscopic appearance. The vast majority are benign, with < 1% malignant18 |
| Hyperplasia | Endometrial hyperplasia is a proliferation of endometrial glands with structural abnormalities and crowding. Atypical hyperplasia designates a proliferation of glands exhibiting cytological atypia in the nuclei and is considered pre-malignant |
| Cancer | Well-differentiated carcinoma is distinguished from atypical hyperplasia by the presence of endometrial stromal invasion.19 These conditions are rare in pre-menopausal women |
Diagnosis of heavy menstrual bleeding
The current NICE guideline advocates full gynaecological examination and taking a full blood count to exclude the possibility of anaemia. 11 This guideline recognises the need for diagnostic tests to evaluate the uterus, namely endometrial biopsy, ultrasound scan and hysteroscopy, in specific cases. These tests are described in Table 2.
| Test | Description | Capability |
|---|---|---|
| Tests primarily for detecting structural abnormalities | ||
| Transvaginal scan | A method of imaging the genital tract in women. The ultrasound machine sends out high-frequency sound waves that bounce off body structures to create a picture on a screen. With the transvaginal technique, the ultrasound transducer (a hand-held probe) is inserted directly into the vagina. It is therefore able to get closer to pelvic structures than the conventional transabdominal technique (with the probe on the skin of the abdomen) | Diagnoses endometrial, focal (polyps, SMFs, other intracavity), myometrial (adenomyosis, fibroids) and adnexal pathology |
| Saline infusion ultrasound | A minimally invasive ultrasound technique used to view the inside of the uterus. Sterile saline is injected into the endometrial cavity through a small cervical catheter, while a transvaginal scan is performed. This allows real-time imaging of the uterus as the saline is injected. The saline fills and distends (expands) the endometrial cavity, providing visualisation of the anatomical structures within | As for transvaginal scan but with enhanced diagnosis of focal pathology |
| Outpatient hysteroscopy | A hysteroscopy is an examination of the inside of the womb (uterus) using a hysteroscope. Hysteroscopy allows direct visualisation of the inside of the womb. The hysteroscope is carefully passed through the vagina and cervix, and into the womb. During the procedure a biopsy may be taken for examination | Diagnoses endometrial and focal (polyps, SMFs, other intracavity) pathology |
| Tests primarily for detecting histological abnormalities | ||
| Endometrial biopsy | A test that involves obtaining a sample of endometrium and subjecting it to histological analysis. The endometrium is obtained blindly using a sampler (a miniature plastic tube passed through the cervix that uses suction to obtain endometrial tissue) | Diagnoses endometrial diseases (pre-malignant condition of endometrial hyperplasia with or without cytological atypia and endometrial carcinoma) |
| Dilatation and curettage | A procedure performed under general anaesthetic in which the lining of the uterus is blindly biopsied by scraping with an instrument (‘curette’). The neck of the womb (cervix) needs to be dilated to allow passage of the curette | As for endometrial biopsy |
Current diagnostic pathways for heavy menstrual bleeding
It is only in the last 25 or so years that evaluation of the uterine cavity, in response to HMB symptoms, has moved on from the so-called ‘D&C’: dilatation of the cervix and curettage of the endometrium lining the uterine cavity (dilatation and curettage). This test is now used only in exceptional circumstances as it requires general anaesthesia and has been superseded by outpatient endometrial biopsy, which obtains endometrial tissue samples for histological analysis in a convenient outpatient setting without the need for anaesthesia. 20,21 Moreover, the development of high-resolution transvaginal scan (TVS) has allowed the female pelvic structure, including the uterus, to be visualised. The ‘inside’ of the uterus (i.e. the uterine cavity) cannot normally be seen without effecting distension using a fluid or gaseous medium to separate the opposing walls of the uterus. This potential limitation of TVS has been overcome by the advent of saline infusion sonography (SIS)22–24 and outpatient hysteroscopy (OPH). The latter test was previously restricted to the operating theatre owing to the relatively large diameter of the endoscopes, which required dilatation of the cervix in order to successfully instrument the uterus. Advances in instrumentation, namely miniaturisation, improved optics and digital imaging, have made direct endoscopic visualisation of the uterus a simple and acceptable outpatient investigation. 25–28 These tests described in Table 2 provide different albeit overlapping information, and diagnostic accuracy varies according to the particular pathology under scrutiny. NICE guidance from 2007 recognised that ‘. . . particular investigative methods were better for identifying certain types of pathology than others’ (p. 41). 11
Thus, the availability of different, easy-to-use, miniature and increasingly portable ‘bed-side’ tests has created uncertainty as to how best to employ them. This is particularly true with HMB, where different aetiologies need to be considered and the preceding clinical history, and more often than not the examination too, is unable to predict causation with accuracy. A rational basis for subsequent testing strategies cannot be reliably formulated and current testing is, therefore, eclectic, depending upon the vagaries of individual clinicians and availability of resources locally.
Literature review of cost-effectiveness studies for the diagnostic work-up of heavy menstrual bleeding
A systematic search was performed of the MEDLINE (from 1950 to February 2012) and EMBASE (from 1980 to February 2012) electronic bibliographic databases using the terms ‘heavy menstrual bleeding’ and ‘cost-effectiveness’ along with their MeSH terms. Three hundred and fifty articles were identified once duplicates had been removed. Three relevant economic evaluations of diagnostic tests used for evaluating HMB were identified. One evaluation took place alongside a randomised controlled trial (RCT)29 and the other two were economic modelling studies. 11,30
Cost-effectiveness was examined in a RCT conducted between 1999 and 2001 in Scotland, comparing three outpatient diagnostic tests (outpatient biopsy, ultrasound and hysteroscopy) for the evaluation of AUB in certain test combinations. 29 Women were split into high-, moderate- and low-risk groups for endometrial cancer. Resource use tended to be higher in the moderate- and low-risk women, because of the need to manage their persistent abnormal bleeding symptoms. Minimal difference in cost-effectiveness was found between investigation options in the high-risk group (post-menopausal), with the option involving hysteroscopy being marginally better than ultrasound (£88 per woman, compared with the other options). The most cost-effective investigation in the moderate-risk group was biopsy alone (saving £128–212 per woman better) and in the low-risk group ultrasound (£74–452 per woman better).
The mixed population of women with AUB, that is to say women of reproductive age with HMB and post-menopausal women with unexpected vaginal bleeding, limits clinical inferences to influence decision-making from this RCT. 29 This is because the aim of investigation of women with PMB is to exclude the possibility of endometrial cancer, whereas in pre-menopausal women it is to optimise management of benign uterine pathologies associated with HMB (i.e. selection of appropriate treatment modalities). The authors of this RCT29 highlight this themselves by stating ‘. . . in future research into the evaluation and management of AUB, postmenopausal women should be studied separately from premenopausal women with menstrual bleeding problems’ (p. 69). Furthermore, the primary end point defining ‘effectiveness’ was based upon the premise that a satisfactory diagnosis must have been reached once no further investigation had been carried out, as identified by retrospective case note review. Clearly, such an indirect assumption of effective diagnosis, while expedient, is unlikely to be a reliable or valid measure of clinical effectiveness and does not take account of patient-centred outcomes (e.g. satisfaction, reduction in bleeding, survival, etc.). Moreover, as diagnostic testing generally precedes the institution of treatments, the use of this outcome measure does not account for all treatment costs when calculating cost-effectiveness. This is important, as most women with AUB have either no identifiable pathology (‘dysfunctional uterine bleeding’) or benign pathologies (e.g. polyps or fibroids), conditions which are often amenable to less invasive, cheaper, and potentially outpatient treatments.
As well as economic data from effectiveness studies, an alternative approach to assessment of cost-effectiveness of diagnostic testing is to employ decision-analytic modelling. Two economic evaluations of diagnostic testing in HMB using decision-analytic modelling have been published. 11,30 The first of these analyses was conducted from the Dutch health-care system perspective and compared the percentage of patients treated successfully and the cost of six strategies for the evaluation of HMB: (0) hormonal treatment, (I) treatment of all patients with balloon ablation, (II) TVS and therapeutic hysteroscopy, (III) TVS, SIS and therapeutic hysteroscopy, (IV) SIS and therapeutic hysteroscopy, and (V) diagnostic hysteroscopy and therapeutic hysteroscopy. Hormonal treatment was considered to be the reference strategy with which the five strategies were compared. The study found that the strategy starting with SIS (IV) and the strategy with diagnostic hysteroscopy (V) revealed the highest number of patients treated successfully for HMB. However, the diagnostic strategy based upon initial evaluation with SIS was the most cost-effective strategy for successful treatment of HMB, especially when the prevalence of intracavity pathology [polyps or submucosal fibroids (SMFs)] was high. Study weaknesses limit to some degree the validity and stability of these findings. These included problems with construction of the decision model (limited pathologies were taken into account, e.g. diagnosis of intramural fibroids and endometrial disease were not considered). The authors used outmoded and restricted medical and surgical treatments; for example, use of long-term systemic progestogen and overlooked ambulatory outpatient-based treatment. 31 Failure rates of testing were unaccounted for, the precision and quality of data sources used for estimating test accuracy were questionable and the definition of therapeutic effectiveness was unclear. The findings of the analysis were sensitive to changes in the key assumptions limiting the robustness of clinical inferences.
The other decision-analytic model was developed to examine the cost-effectiveness of three imaging techniques, TVS, SIS and hysteroscopy, from a NHS perspective. 11 The model showed that TVS was more accurate and less costly than either SIS or hysteroscopy. For a cohort of 1000 women examined for the presence of structural abnormalities, ultrasound generated 810 correct diagnoses at a cost of £107,490 compared with 735 correct diagnoses at a cost of £145,110 using SIS and 696 correct diagnoses at a cost of £209,720 using hysteroscopy. Although the economic analysis was conducted from a NHS perspective, the general applicability of the model is limited due to its simplistic construction. Women were assumed to have one of two health states, no intrauterine pathology or any intrauterine pathology, and the outcome measure chosen was correct diagnosis. This was a pragmatic choice given the scope of the guideline11 such that it was not possible to construct a model designed to take into account the range of pathologies under consideration for HMB, and the associated range of treatment pathways. The impact on cost-effectiveness of women falsely diagnosed was not considered (the model did not follow women beyond an initial diagnosis), and so the model does not reflect the true longer-term costs and outcomes associated with each diagnostic method. Moreover, diagnosis was restricted to one test, which does not reflect contemporary practice where multiple testing is likely, either conducted simultaneously or conditional on previous test results.
The relative dearth of comprehensive diagnostic cost-effectiveness data in women with HMB reflects the complexity of care pathways (i.e. the varied outpatient tests available, the range of uterine pathologies, the relatively recent introduction of minimally invasive, ‘ambulatory’ or ‘outpatient’ treatments, and patient factors including comorbidities and preferences).
Current treatment of heavy menstrual bleeding
Medical therapy
According to the recent NICE guideline on HMB, medical treatments should be considered (i) where structural and histological abnormalities of the uterus have been excluded; (ii) for fibroids < 3 cm in diameter which do not appear to distort the cavity of the uterus or (iii) where future fertility is required. 11 The first-line recommended medical treatment by NICE is the levonorgestrel-releasing intrauterine system (LNG-IUS or Mirena®, Bayer Healthcare Pharmaceuticals, Pittsburgh, PA, USA) which is an effective non-surgical treatment for HMB, is reversible, contraceptive and fertility sparing. In the majority of cases, the device is fitted easily within a few minutes in the outpatient setting. Endometrial proliferation is suppressed as a result of local release of the synthetic progestin LNG and this leads to a reduction in estimated menstrual blood loss of up to 96% by 12 months, with up to 44% of users reporting amenorrhoea,32,33 at a cost which is one-third of that for hysterectomy. 34 However, the LNG-IUS can lead to troublesome breakthrough bleeding and vaginal discharge in some women, causing early discontinuation of the device. The LNG-IUS works effectively in a relatively normal-sized uterus (< 11 cm sound length) without distortion by focal pathology,35 that is to say in DUB or the presence of small uterine fibroids (< 12-week uterine size) which do not encroach into the uterine cavity. 36 Local release of progestin can also reverse endometrial hyperplasia. 37,38 Thus, the LNG-IUS is applicable for most aetiologies of HMB, with the exception of focal pathologies distorting the uterine cavity, large uterine fibroids (> 12 weeks’ size) or the presence of endometrial cancer.
Surgical treatment
Long-term medical treatment with the LNG-IUS is unsuccessful or unacceptable in many cases and surgical alternatives may be required. 39 Traditional surgical treatment of HMB refractory to medical intervention has been with hysterectomy, but now removal of the uterus is generally restricted to women where conservative, uterine-sparing surgical procedures have been unsuccessful [i.e. hysteroscopic surgery including endometrial ablation (EA)], in the presence of large uterine fibroids (> 12-week size) where medical or conservative surgical approaches are likely to fail, or in the presence of endometrial cancer or pre-cancer. EA is a technique whereby a semi-automated device is placed in the uterine cavity and thermal energy is applied to the endometrium and superficial myometrium. Various ablative modalities are available including fluid-filled thermal balloons, free-circulating warmed saline, bipolar radiofrequency ablative systems and cryotherapy. All systems aim to conform to the shape of the uterine cavity to achieve a uniform, global and permanent destruction of the endometrial lining, thereby inducing amenorrhea or substantially reducing menstrual blood loss. 18 EA is recommended as a second-line treatment where fertility is not desired and medical treatment has failed in DUB, but can also be used in the presence of uterine fibroids where there is a relatively normal-sized and -shaped uterine cavity. Hysteroscopic resection of focal intracavity lesions, including polyps40,41 and SMFs,42–46 has been shown to improve HMB symptoms found in association with these pathologies. Hysteroscopic removal is standard practice in the UK47 and in the case of polyps can be achieved in the majority of cases in the outpatient setting. 48–51 The procedures involve the use of electrosurgical cutting electrodes placed down a small operative working channel in the hysteroscope or a formal hysteroscopic resectoscope using a larger loop electrode.
In the presence of significant fibroids associated with an estimated uterine size of > 12 weeks, and where retention of fertility is not required, hysterectomy is usually recommended. Uterine artery embolisation (UAE) is a less invasive, uterine-sparing, radiological intervention. 52–54 This procedure is normally restricted to women with medical or surgical risk factors for open surgery. Myomectomy (removal of fibroids with conservation of the uterus) is sometimes offered but, as it is as invasive as hysterectomy but less effective,55 the technique is generally reserved for women wanting to retain their fertility or to improve fecundity in those women with subfertility associated with a large fibroid uterus.
Defining treatment success in heavy menstrual bleeding
Menstruation is a woman’s monthly bleeding from the reproductive (vaginal) tract, as a consequence of cyclical changes in hormonal activity. It is also called menses, menstrual period or period. 11 The menstrual blood loss consists of blood and glandular tissue and fluid secretions from the inside of the uterus, which pass via the cervix into the vagina and out of the body. Menstruation is normal for women of reproductive age and so defining ‘successful treatment’ can be problematic. The primary aim of treating HMB is not to eradicate bleeding altogether, although some interventions do induce amenorrhoea, but to ameliorate bleeding symptoms to a tolerable level. As we have discussed, objective measurement of reduction in menstrual bleeding is impractical and lacks relevance. Many studies have tried to measure the impact of interventions upon patient’s quality of life and/or satisfaction with treatment outcome.
Health-related quality of life
Generic HRQL measures have been used, but many have not been validated for use in HMB and fail to capture the cyclical nature of the symptom. 56,57 In addition, they lack sensitivity as most women suffering with HMB are otherwise healthy and can continue to function in most generic health domains during menstruation. 6,7 Condition-specific measures have been developed for HMB but either assess only surgical interventions58 (as opposed to medical ones) or have been sparsely utilised to date, limiting a full assessment of their inherent psychometric qualities. 59
Satisfaction
Patient satisfaction is widely used as a primary outcome measure in studies of treatments for HMB and guidelines. 2,11,60,61 Satisfaction is a subjective and relative concept and represents the extent to which a service meets users’ expectations. A variety of questions and scales have been used to elicit satisfaction with treatment in HMB studies. This lack of uniformity precludes meta-analysis of data across studies. 2,40 Furthermore, the validity of current patient satisfaction measures is questionable in light of the lack of published studies examining the development and application of specific satisfaction measures in HMB. However, despite these deficiencies, we selected satisfaction as our primary outcome to assess HMB given the more widespread availability of such data and in keeping with the approach used in a recent, extensive systematic review with individual patient data (IPD) meta-analysis evaluating the relative effectiveness of hysterectomy, endometrial destruction and levonorgestrel intrauterine systems in HMB. 61 Moreover, patient satisfaction is deemed to be the co-primary measure of importance (together with menstrual bleeding) by the Cochrane Menstrual Disorders & Subfertility Group for reviews of interventions for HMB. 62
Post-menopausal bleeding
Definition of post-menopausal bleeding
The cessation of menstruation as a result of ovarian failure occurs at around the age of 52 years on average. 63 The menopause is assumed clinically once 12 months have elapsed without any further menstrual periods. There is a lack of follicular activity within the ovary and consequent absence of production of the main female sex steroid hormone and most potent oestrogen, β-oestradiol. Thus, organs and tissues responsive to oestrogen are affected, giving rise to characteristic menopausal symptoms. One such organ to be affected is the uterus and, specifically, the endometrium. Privation of oestrogen results in a quiescent, non-proliferative endometrium. The absence of ovarian activity and subsequent inactive endometrium is manifest clinically as permanent termination of menstruation and infertility. Post-menopausal bleeding is a term used in gynaecological practice to describe unexpected vaginal bleeding in a woman who has reached the menopause, taken as at least 12 months’ amenorrhoea. As described above, PMB is a common clinical problem in general practice and secondary care, associated with substantial morbidity and heavy use of health-care resources.
Urgent evaluation is recommended for PMB as in around 5% of cases the underlying cause is life-threatening endometrial malignancy. 64 Prompt diagnosis increases the chance of survival as the disease is likely to be detected at an earlier stage where curative treatment is possible. 65 Endometrial hyperplasia is considered potentially pre-malignant, especially where cytological atypia is present. The remaining causes of PMB represent a range of benign pathologies (Table 3).
| Cause | Definition |
|---|---|
| Malignant/premalignant | |
| Endometrial cancer | Well-differentiated carcinoma is distinguished from atypical hyperplasia by the presence of endometrial stromal invasion.19 This abnormal endometrium bleeds easily, which leads to early diagnosis in the majority of women18 |
| Endometrial hyperplasia with atypia | Atypical endometrial hyperplasia designates a proliferation of endometrial glands exhibiting cytological atypia in the nuclei.19 This endometrium is abnormal and bleeds easily |
| Benign | |
| Endometrial hyperplasia | Endometrial hyperplasia is a proliferation of endometrial glands with structural abnormalities and crowding19 |
| SMFs | Smooth-muscle tumours of the uterus that lie underneath the endometrium and indent the uterine cavity. The fragile overlying endometrial vasculature can bleed easily and cause PMB18 |
| Endometrial polyps | Endometrial polyps are focal outgrowths that can occur anywhere within the uterine cavity. They contain a variable number of glands and blood vessels and varying amount of stroma, which influences their macroscopic appearance. The fragile vascular network can bleed and cause PMB. The vast majority are benign with < 1% malignant18 |
| Chronic endometritis | Inflamed fragile endometrium associated with IUCD use, pelvic inflammatory disease and retained products of conception18 |
| Atrophic endometrium | No endometrial tissue remains but the vascular support provided by the underlying stroma is fragile and can become ulcerated with petechial haemorrhages18 |
| HRT | HRT leads to an unstable endometrium when the progestogenic support is inadequate. Poor compliance and absorption can exacerbate symptoms. HRT also increases the prevalence of endometrial polyps18 |
| Coagulation defects | Women with coagulation defects (iatrogenic or inherent) have a propensity to bleed from the endometrial vessels, even after the menopause18 |
Diagnosis and post-menopausal bleeding
Post-menopausal bleeding is a ‘red flag’ symptom, indicating the possibility of an underlying endometrial malignancy. Thus, the main reason for investigating PMB is to detect or exclude endometrial cancer. Current practice for the investigation of women presenting for the first time with PMB is to perform a gynaecological pelvic examination and undertake a TVS to measure the double-layer thickness of the endometrium. 66 As with the investigation of HMB, tests to evaluate the uterus include endometrial biopsy, ultrasound scan and hysteroscopy, and descriptions of these tests are provided in Table 2.
The majority of women with PMB will not have endometrial cancer. However, where it is suspected, either from the symptom alone or after testing with TVS or OPH, endometrial tissue sampling to allow histological confirmation is necessary before proceeding with treatment. This is now most commonly done by the use of endometrial biopsy (EBx), in which miniature plastic suction devices are placed into the uterine cavity in an outpatient clinic setting. This approach has largely superseded the traditional D&C. 67,68 Previous economic analysis69 did not find the routine use of EBx for all women presenting for the first time with PMB to be a cost-effective approach compared with the use of TVS. TVS is thus recommended66,69,70 as an initial test to identify higher-risk women in whom EBx could then be used in a more targeted, efficient way.
It should be noted that, in contrast to the evaluation of the uterus in HMB, SIS is not widely employed. 11 This stems from the fact that the prime purpose of diagnostic work-up of PMB is to evaluate the endometrium for serious, potentially life-threatening disease. SIS is used primarily in women of reproductive age with AUB or fertility problems after the initial TVS shows irregularity of the endometrium suggestive of a focal pathology within the uterine cavity (e.g. polyps or SMFs). However, in the diagnostic work-up of PMB, an irregular or thickened endometrium warrants an EBx and so the additional information about the uterine cavity obtained from proceeding with a SIS is not generally considered as informative as it is in the case of HMB. Furthermore, SIS is more challenging in women after the menopause because of cervical stenosis and vaginal atrophy as a consequence of chronic oestrogen deficiency.
Current diagnostic pathways for post-menopausal bleeding
While the current recommendation is to implement testing, ideally with TVS in all women presenting for the first time with PMB, an additional and often overlooked consideration relates to the role of the preliminary consultation: that is to say, history taking and clinical examination. Prevalence estimates in the UK for women presenting for the first time with PMB are of the order of 5%,64,71 although higher prevalence has been reported elsewhere. 9,72 However, while these population estimates of pre-testing probability of disease are used to inform the need for diagnostic testing, they are crude estimates. There has been recent interest in trying to individualise risk-based before instituting testing, based upon identifying the presence of certain clinical characteristics or ‘risk factors’ from the history and clinical examination. 64,73,74 Relevant individual patient characteristics include age, time since menopause, obesity, hypertension, diabetes mellitus, and reproductive factors. For example, the probability of endometrial cancer in women with PMB rises from 1% in women younger than 50 years to 23.8% in women older than 80 years and the incidence of malignancy is, regardless of age, higher in women with PMB and obesity (18%) or diabetes (21%) compared with women without one of these risk factors. 75–81
The attractiveness of integrating the clinical process with diagnostic testing is that unnecessary testing can be avoided and that the accuracy, effectiveness and, ultimately, cost-effectiveness of current management approaches can be improved upon. In light of this, we developed two multivariable prediction models using IPD to estimate the risk of endometrial cancer in patients with PMB, taking into account their clinical characteristics,73 to be used along with the established testing technologies within this economic analysis.
Literature review of cost-effectiveness of tests for the diagnostic work-up of post-menopausal bleeding
A systematic search was performed of the MEDLINE (from 1950 to February 2012) and EMBASE (from 1980 to February 2012) electronic bibliographic databases, using the terms ‘postmenopausal bleeding’ and ‘cost-effectiveness’ along with their MeSH terms. One hundred and four articles were identified once duplicates had been removed, and four of these were selected as being relevant. 29,69,70,82 One of these was the analysis performed alongside a RCT which has been appraised in the HMB section of this introduction. 29 The other three studies were based upon economic modelling. 69,70,82
The RCT which looked at cost-effectiveness compared three outpatient diagnostic tests (outpatient biopsy, ultrasound and hysteroscopy) for the evaluation of AUB in certain test combinations. 29 The effectiveness outcome measure was ‘need for no further tests’. Women were split into high-, moderate- and low-risk groups for endometrial cancer and the high-risk group comprised entirely post-menopausal women. This group of women was randomised between two investigation strategies: hysteroscopy plus biopsy or ultrasound plus biopsy. The most cost-effective combination was hysteroscopy and biopsy (£632), although ultrasound and biopsy was only £88 more expensive (£720). The study was not sufficiently powered to investigate the sensitivity and specificity of the tests for diagnosing endometrial cancer. 29 In post-menopausal women the purpose of investigation is to exclude endometrial cancer, and so, although this analysis looks at resource cost, its main limitation is that it did not examine the cost of making the correct diagnosis and the effect of this on survival.
The three economic modelling analysis were based upon three different health-care systems, with one study being from the USA,82 one from the Netherlands70 and the final one from the UK. 69
The US study82 used a decision-analytic model to evaluate six diagnostic strategies to diagnose pathology in women with peri- and post-menopausal women, with the aim of determining whether initial diagnosis with EBx or TVS minimises cost. 82 The six strategies started with EBx or TVS and added in additional testing with hysteroscopy, biopsy (if TVS was used initially) or SIS until a diagnosis was reached or bleeding was resolved. HRT was used as a treatment for 3 months if the biopsy was negative or if the TVS showed no treatable pathology with an endometrial thickness of less than 5 mm. Patients left the model if pathology was diagnosed and they had treatment or if their bleeding had resolved after 3 months of HRT. Otherwise they continued to have further sequential diagnostic tests, until a diagnosis was made or the strategy was completed. Total costs for each of the strategies were evaluated for (i) polyps, (ii) SMFs, (iii) atrophic endometrium, (iv) proliferative/secretory endometrium and (v) atypical hyperplasia/endometrial cancer. Short- and long-term patient outcomes were not included within the analyses; hence, it was a cost-minimisation study and not an evaluation of cost-effectiveness. To account for the difference in reported prevalence of endometrial hyperplasia and cancer, the strategies were performed for a range of prevalence values to determine at what level biopsy became cost-minimising. 82 They found that when the prevalence of atypical hyperplasia/endometrial cancer was between 7% and 31%, a strategy that starts with TVS and is followed by biopsy was the cheapest. Below a prevalence of 7%, however, a strategy of TVS followed by SIS, if the endometrium was thickened or bleeding did not resolve, became the cheapest. For a strategy which tests initially with EBx to be cost-efficient, the prevalence of atypical hyperplasia/endometrial cancer would have to be at least 31%. Thus, the findings of this study suggest that initial TVS investigation of patients with AUB was the cheapest method; however, they could not show whether or not it was cost-effective as survival was not used as an outcome measure. The mixed population of peri- and post-menopausal women suggests that the accuracy data used to populate the tree were not specific to post-menopausal women and, thus, the inferences cannot be reliably applied to this population.
The Dutch study70 used economic modelling to evaluate strategies for investigation of PMB. The first strategy was to have a TVS and, if the endometrium was thickened, for the patient to go on to be treated with hysterectomy. The second strategy also looked at TVS but, in this strategy, if the endometrium appeared thickened, the patient went on to have an endometrial biopsy. In the third strategy, if thickened endometrium was diagnosed by TVS, the patient underwent hysteroscopy, and in the fourth strategy patients underwent biopsy only and if the result showed atypical hyperplasia or endometrium the patient underwent hysterectomy. 70 The evaluations with TVS were examined using three cut-off levels to define an abnormal TVS: endometrial thicknesses of 3, 6 and 9 mm. Each of the four strategies was compared with a base-case strategy in which patients underwent no diagnostic testing and the outcome measure used was life expectancy. For TVS alone, using a cut-off of 9 mm was the most cost-effectives strategy at all prevalences in women aged 60. If the prevalence of endometrial cancer in the population was < 15.3%, TVS and biopsy was found to be the most cost-effective option, whereas if the prevalence was ≥ 15.3%, biopsy alone became the most cost-effective strategy. The cost-effectiveness of TVS using a high 9 mm endometrial thickness cut-off reflected the low cancer prevalence (< 15%) and the costs associated with false-positive scans associated with lower endometrial thickness thresholds. However, current guidance66 recommends, and general gynaecological practice utilises, lower endometrial thickness thresholds of 4–5 mm because of better sensitivity83,84 and fears over the implications of false-negative diagnoses.
The UK study69 was a cost-effectiveness analysis based upon an economic model which was constructed to represent clinical evaluation of women with PMB with 12 different investigation strategies, to diagnose endometrial cancer. 69 The strategies looked at EBx, TVS and OPH, used alone and in combination. TVS was assessed when endometrial thickness cut-off levels were set at 4 mm and at 5 mm. One of the 12 strategies represented women having no investigation at first presentation and only having diagnostic testing after a second episode of PMB. This strategy was used as a base case with which to compare the other strategies. The outcome measure used was cost per life-year gained. The economic model was populated using data from systematic reviews with meta-analysis and other published studies and the costs were derived from local and NHS sources. The most cost-effective strategy when compared with no treatment was using TVS with a cut-off of 5 mm for endometrial thickness, with an incremental cost-effectiveness ratio (ICER) of £11,470. When the non-dominated strategies were compared with TVS with a 5-mm cut-off, the ICERs ranged from £37,652 for the initial strategy using TVS 4 mm and £149,219 for the strategy EBx + OPH per additional life-year gained. However, these were reduced if, at the same visit, an EBx was taken following a positive result. If prevalence of endometrial cancer was 10%, EBx and TVS with a cut-off of 4 mm became more competitive options, although the additional cost over TVS with a 5-mm cut-off was still more than £20,000 per life-year gained. This study was based upon high-quality meta-analyses and focused on making a specific diagnosis (endometrial cancer) in a specific population (women with PMB).
In post-menopausal women, the primary goal of the clinician is to exclude endometrial cancer and thus prolong life. Therefore, studies that do not look at survival29,82 cannot reliably comment on the cost-effectiveness of diagnostic strategies except as a superficial assessment of the initial costs of diagnostic work-up. Despite the limitation of these studies, all of the economic analyses identified support the current recommended practice of investigating women with PMB using TVS as the initial test. 66 However, none of the studies integrated the preceding clinical process (history and examination) into the testing strategy. Important patient factors which are key in the development of endometrial cancer include obesity, parity, age, diabetes mellitus, use of hormone replacement therapy (HRT) or tamoxifen, late menopause and hypertension; these factors all increase the risk of endometrial cancer75,77,80 and some of them will also reduce life expectancy. Moreover, while the available tests themselves have not changed much over the last 10 years, advances in technology mean that the feasibility and accuracy of tests may have increased, while the relative costs of specialist equipment may have decreased. Furthermore, outpatient ‘one-stop’ testing settings have become ‘the norm’ and this method of contemporary service delivery will affect costs, satisfaction and quality of life. Therefore, any new assessment of cost-effectiveness should take the aforementioned considerations into account.
Treatment of endometrial cancer
The main aim of rapid diagnosis in cases of PMB is to diagnose endometrial cancer early and thus improve survival. Endometrial cancer can be successfully treated by surgery if it is diagnosed early and is confined to the uterus (stage 1), with 5-year survival rates of up to 90% for stage IA tumours. 85 Once the carcinoma has spread beyond the myometrium, radiotherapy and chemotherapy may be offered. 65 Women tend to present early as PMB is an alarming symptom and the majority of women will have a potentially curable stage of disease (70% present as stage 1).
Defining treatment success in post-menopausal bleeding
The prime reason for the prompt referral and immediate investigation of PMB is to detect or exclude endometrial cancer. Timely treatment can be implemented following a diagnosis of malignant endometrial disease with the aim of eradicating the disease to effect a cure. Where disease has spread beyond the confines of the uterus, treatment is palliative rather than curative. However, the timelier the diagnosis and the earlier the disease stage, the longer the survival post treatment will be. Thus, accurate diagnostic work-up allows prompt treatment, leading to increased survival. Survival is not only an appropriate, relevant measure of effectiveness but is also objectively determined. HRQL is an alternative outcome measure. In contrast to HMB, PMB is rarely heavy and persistent, and so is not as much of a physical burden. However, HRQL may be adversely impacted upon because of fear of cancer or anxiety post diagnosis. As the current evaluation of PMB investigation is focused on the diagnosis of endometrial cancer, rather than diagnoses of benign pathologies, we chose to measure effectiveness in terms of 5-year survival rather than HRQL or patient satisfaction.
Project objectives
Abnormal uterine bleeding is an important problem in women’s health, associated with morbidity and heavy usage of health service resources. Optimal treatments can be implemented only following accurate diagnosis. Therefore, decision models were designed with the purpose of allowing economic evaluation of the diagnostic work-up possibilities in AUB. Specifically, the objectives of this HTA report were:
-
to determine the most cost-effective diagnostic testing strategy for the diagnosis and treatment of HMB
-
to determine the most cost-effective diagnostic testing strategy for the diagnosis and treatment of PMB.
Chapter 2 Methods
Heavy menstrual bleeding model
Construction of decision model for the diagnosis and treatment of heavy menstrual bleeding
A clinically informed cost-effectiveness model was drawn as a decision tree using TreeAge software (TreeAge Software Inc., Williamstown, MA, USA) to reflect current service provision for the diagnostic work-up of women presenting with HMB. The tree was constructed to examine the effectiveness of different diagnostic testing strategies for women referred to secondary care by their GP. The tests evaluated were TVS, SIS, global EBx and OPH. The tree structure was informed by clinical input. As there is no consensus regarding how best to investigate women with HMB, initial investigation utilising all tests either alone or in combination were included in the model. Therefore, the tree consisted of the four tests available deployed in isolation or in various clinically relevant combinations following initial presentation. The need for any additional subsequent tests was conditional upon the preceding test result(s). This resulted in the formation of 11 clinically relevant, alternative testing strategies. In addition, two scenarios were developed where testing was dispensed with and treatment of HMB instituted immediately regardless of diagnosis. The treatments chosen were the most effective medical treatment (the LNG-IUS) and surgical treatment (hysterectomy). This allowed us to compare the various approaches to diagnostic work-up with the option of ‘no investigation’. In view of the fact that NICE guidance11 recommends the use of the LNG-IUS as first-line treatment in HMB, this arm was used as the base-case scenario to compare all other strategies against. An incremental approach was used for reporting the results. Thus, in total there were 13 different scenarios evaluated in the decision model (11 testing and two treatment-alone strategies) which are listed below:
-
LNG-IUS alone
-
hysterectomy alone
-
OPH
-
TVS
-
EBx
-
SIS
-
OPH and EBx
-
TVS and EBx
-
SIS and EBx
-
OPH and SIS
-
OPH and TVS
-
SIS, OPH and EBx
-
TVS, OPH and EBx.
Structure of the model
A series of decision trees evaluating various testing strategies for HMB were developed to represent alternative decision options and their possible consequences. The trees explicitly illustrate the patient pathway from suspected pathology underlying the clinical presentation through to the outcome of testing, distinguishing between correct and incorrect diagnosis. Then, conditional on the accuracy of the diagnostic testing strategy, the outcome of treatment for HMB was analysed at 1 year post initial presentation. Disease prevalence, diagnostic test accuracy, and treatment effectiveness along with associated costs were used to populate the relevant branches of the decision tree. The basic tree structure is illustrated in Figure 1. The 13 trees representing the diagnostic testing options for HMB are detailed in Appendix 2; however, the trees themselves are too large to display completely, so a branch of one tree has been expanded as an example and a table has been included which details the data from the remaining branches of the tree.
FIGURE 1.
Example decision tree for evaluating the cost-effectiveness of diagnostic testing in HMB.
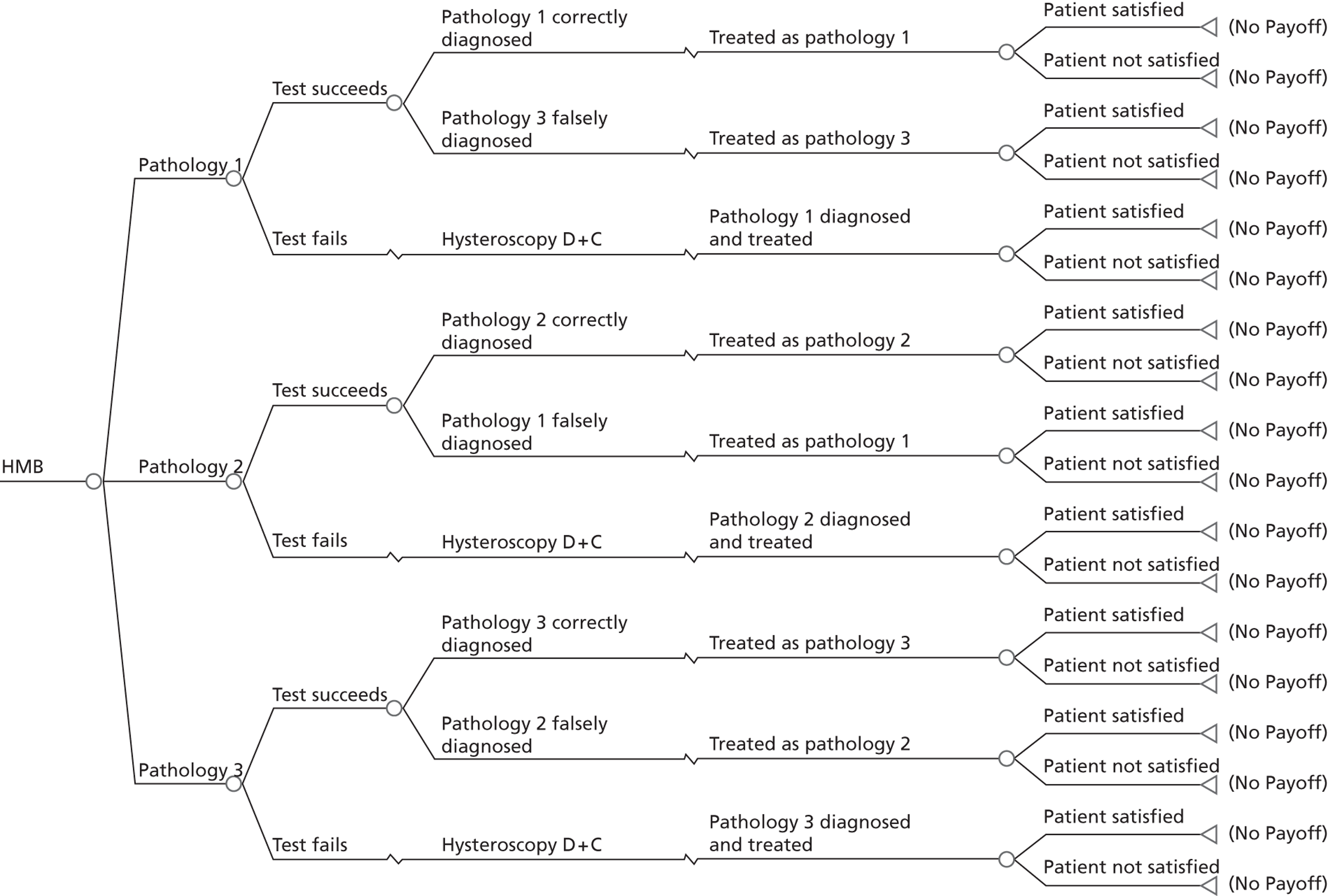
Deterministic results were obtained using point estimates of the parameters to estimate the expected cost, outcome (satisfaction) and incremental cost-effectiveness (additional cost per extra patient satisfied). The stability of the results was then tested through sensitivity analysis.
Clinical assumptions
The following section details the main clinical assumptions required to develop the economic model. An expert panel of senior gynaecologists was convened to ensure that the model structure and clinical inputs reflected contemporary practice. The panel of gynaecologists were practising within the UK or the Netherlands and they were selected based upon their reputation and experience in the field of gynaecology. Owing to financial and time constraints the clinicians were contacted by e-mail, as well as by telephone and face-to-face interviews. Seventeen consultant gynaecologists (three of the authors of this report and 14 external clinicians) responded to various queries made by us; however, inevitably not all clinicians responded to all questions. Initial correspondence concerned the structure of the model and clinical management. The size of the model precluded us from presenting it as a whole to the consultants and so when the clinical management of a scenario appeared contentious we invited opinion from the panel by presenting the individual scenarios to them and asking how they would manage the patients. Once we started to populate the tree with data it became clear that we would not be able to identify all values from the literature. This was particularly true for ‘satisfaction’ values after inappropriate treatments (e.g. women with large fibroid uteri being treated with the LNG-IUS system or EA) because these scenarios arise only when women are unknowingly treated with unsuitable medications or procedure and hence the numbers are small and the data are not collected. In the absence of a consensus view, the opinion of the majority was adopted or, when data were concerned, the median values were used.
Demographics, pathologies and treatments
It is assumed that women presenting with HMB have a mean age of 45 years and have no wish for fertility. Forty-five years was selected for two reasons: firstly, NICE recommends that EBx should not be performed routinely for women with HMB before this age11 as prevalence of endometrial premalignant or malignant disease is low; and, secondly, because HMB is most prevalent in parous women over 40 years, most of whom have completed their families86 and are then eligible for all potential treatment options (the desire for current or future fertility restricts treatment options in HMB, i.e. avoidance of hormonal contraceptive medical treatments or the surgical interventions EA or hysterectomy.
All women were assumed to have been referred from primary care and had not previously been seen for testing or treatment in secondary care with HMB. A single underlying aetiology was assumed to be causative and concurrent pathologies were not considered. This assumption is in keeping with the majority of HMB cases11 and prevented unnecessary model complexity.
Our premise (and presumably that of all gynaecologists who employ diagnostic testing in HMB) is that optimal treatment of HMB is dependent upon correctly diagnosing the underlying cause so that appropriate, tailored treatment is arranged. The model was constructed based upon the true underlying diagnosis. The true diagnosis was assumed to fall within one of the following categories:
-
intrauterine resectable pathology (endometrial polyps, SMFs)
-
fibroid uterus < 12 weeks’ size (intramural and subserosal fibroids only)
-
fibroid uterus > 12 weeks’ size (intramural and subserosal fibroids only)
-
endometrial disease (complex endometrial hyperplasia or endometrial hyperplasia with atypia or cancer)
-
DUB.
When choosing the pathology categories (see Table 1), we decided that endometrial polyps and SMFs should be grouped together as they are treated in the same way (i.e. hysteroscopic resection of focal lesions). The majority of polyps were assumed to be treated in the outpatient setting,49,87,88 while the majority of submucous (intracavity) fibroids were assumed to be treated under general anaesthesia,43,44 70% of which also required pharmaceutical endometrial down-regulation with gonadotropin-releasing hormone (GnRH) analogues for 3 months prior to surgery following outpatient diagnosis. Only 70% were pre-treated with GnRH analogues because not all women would require or tolerate pre-treatment and not all gynaecologists use it. A minority of women would undergo hysteroscopy and D&C under general anaesthesia because the planned outpatient testing was unsuccessful. In these circumstances, where a polyp or SMF was detected at hysteroscopy and D&C, it was assumed that the focal lesion would be treated simultaneously; in the case of a SMF, this meant that GnRH analogue pre-preparation of the endometrium would not have been used.
Submucosal fibroids can occur in isolation or together with other uterine fibroids. A SMF was assumed to be the more significant pathology in the presence of a fibroid uterus < 12-week size, but not when found in conjunction with a fibroid uterus > 12 weeks.
Intramural (confined to the myometrium) and subserosal (extending beyond the myometrium to distort the serosal surface of the uterus) fibroids were grouped according to size. This was because smaller fibroids, which do not substantially distort the shape of the uterine cavity or increase the uterine size beyond the equivalent size of a 12-week gravid uterus (the size at which the uterus becomes palpable abdominally), do not contraindicate the use of LNG-IUS or EA,89–91 treatments which are successful in the majority of women. 61,62,92,93 Thus, small fibroids without cavity encroachment are, in practice, treated in the same way as DUB (i.e. no identified structural uterine pathology). In contrast, large fibroids increasing the uterine size beyond 12 weeks’ size tend to be treated with invasive surgical interventions (abdominal hysterectomy or laparotomic myomectomy), as the LNG-IUS and minimally invasive surgery (EA or hysteroscopic resection of focal lesions) are either contraindicated due to cavity size or ineffective. 89,90 An alternative, less invasive, radiological intervention for large uterine fibroids is UAE, but a RCT of UAE and hysterectomy53 found no statistically significant differences between them in terms of satisfaction and effectiveness. Furthermore, hysterectomy is the gold standard definitive treatment and is more widely available than UAE; therefore, it was chosen as the treatment for fibroid uteri beyond 12 weeks’ size.
The majority of women with HMB have a benign, functional endometrium. However, overproliferation of the endometrium can lead to endometrial hyperplasia, which in the minority of cases (< 5%19), if left untreated, can result in the development of endometrial cancer. Endometrial cancer is rare in pre-menopausal women, but hyperplasia is not infrequently encountered as result of anovulation and a relative excess of unopposed oestrogen stimulating the endometrium. Histological assessment of the endometrium is the only way to reliably diagnose endometrial hyperplasia and cancer, and so EBx is mandatory where these are suspected. Endometrial hyperplasia is treated hormonally with progestogens delivered either systemically or, more often now, locally by fitting a LNG-IUS. Hysterectomy is recommended where the hyperplastic process does not respond to progestogen treatment or in the presence of cytological atypia. This is because the likelihood of developing malignant disease is increased to around 25% in the presence of atypia. 19 Endometrial cancer is generally treated with hysterectomy with or without radiotherapy depending upon the stage and type of cancer. Over 70% of endometrial cancers are diagnosed early, as they present with a visible early warning sign: vaginal bleeding after the menopause. These International Federation of Gynecology and Obstetrics (FIGO) stage 1 cancers are confined to the uterus and can be cured by timely hysterectomy. 65 Given the rarity of malignant endometrial disease in pre-menopausal women and the aforementioned staging statistics, for the purposes of this modelling exercise we assumed that where endometrial cancer was encountered it would be a well-differentiated FIGO stage 1A endometrioid cancer, treated by hysterectomy alone.
Dysfunctional uterine bleeding, although not a distinct pathology, is a diagnosis of exclusion and the recommended first-line medical treatment is the LNG-IUS. 11 This was, therefore, the chosen treatment in the model for DUB.
Setting and decision-making
The clinical setting was assumed to be an efficient, contemporary ‘one-stop’ or ‘see and treat’ service run by a consultant gynaecologist. This setting meant that the expertise and infrastructure were available to perform all stipulated tests at the same visit. It also meant that therapeutic management could be implemented without unnecessary delay. Where outpatient treatment was indicated, such as the fitting of a LNG-IUS or hysteroscopic removal of a uterine polyp, this was done at that visit. Interventions that required a general anaesthetic in a formal theatre setting (e.g. hysterectomy or EAs) were scheduled for a later date, assumed to be within 8 weeks.
The results of all imaging tests would be available in real time to the senior clinician performing the test. However, in the case of EBx, the result would be delayed for around 3 days until the tissue sample had been prepared, analysed and reported by the pathologist. We therefore assumed that for the testing strategy based upon initial investigation with EBx, or where an EBx was performed because endometrial disease was suspected, any treatment or a treatment plan could not be instigated immediately. The expert clinical panel felt that a second appointment, to discuss the diagnosis and institute a treatment, or to formulate a treatment plan, would be required. However, where a testing strategy involved the initial use of EBx in combination with OPH which showed a probable benign cause for HMB [normal appearance, i.e. DUB or a focal lesion (polyp or SMF) seen], expert opinion dictated that treatment would be initiated at that first appointment. If endometrial disease was then unexpectedly diagnosed once the biopsy result became available, an alternative treatment would be instigated at a further appointment if felt to be a more appropriate treatment option.
Imaging tests (OPH, TVS and SIS)94–96 can discriminate to some degree between normal and abnormal endometria, but are unable to accurately differentiate between histological subtypes of abnormal endometria: complex hyperplasia, complex hyperplasia with cytological atypia, or cancer. 95,97 In keeping with clinical practice (opinion of expert panel), where imaging tests diagnose an abnormal endometrium it was felt by the expert panel that no clinician would treat these suspected endometrial conditions without a histological tissue diagnosis. We therefore included a confirmatory histopathological test if abnormal endometria were suspected by imaging; EBx will provide a result in 91% (21) of women but the remaining 9% would need to undergo formal D&C under a general anaesthetic as a day case because of failed procedures, non-diagnostic samples or patient preference. Therefore, the cost for a confirmatory test was a composite value calculated as 91% of the cost of EBx plus 9% of the cost of D&C.
Formal D&C, with its requirement for general anaesthesia, was considered a second-line diagnostic test and was restricted, for consistency, to the minority of women in whom initial diagnostic testing was unsuccessful because of, for example, failure to complete the test. This diagnosis was considered final and the clinical decision was endorsed by the expert panel.
Combination testing strategies and discordant results
If combinations of tests were used, the overall testing strategy was considered successful only if both tests were completed successfully. Failure of one or both tests was considered a failure of the testing strategy. This assumption seemed reasonable on clinical grounds and from a modelling point of view; success of one test in a dual testing strategy would simply replicate the analysis for the corresponding single-test strategy in the model, rendering it redundant. For the two testing strategies evaluating triple tests used together, to avoid unnecessary model complexity, the expert panel was content for the same rule to be applied, that is to say all tests successful for the strategy to be considered successful.
When a testing strategy involved more than one test applied simultaneously, the decision trees for each test were combined (appearing in series within the trees) to provide the additional information associated with combined testing. The final diagnoses were based upon the results from combination tests. Tests in agreement presented no uncertainty, but where test results were modelled as being discordant, decision-making, as regards the assumed diagnosis or need for further testing, was determined by the consensus view of the expert clinical panel.
False diagnoses
Table 4 lists the false-negative diagnoses which the expert panel considered plausible for particular true pathologies according to testing modality. The rationale underpinning the assumptions made by the expert clinical panel is also described.
| True pathology | False diagnoses | Rationale (clinical consensus) |
|---|---|---|
| TVS | ||
| Intrauterine polyp or SMF | Fibroids < 12 weeks | Focal pathology can be easily missed by 2D imaging without cavity distension. Endometrial polyps can appear cystic and thus be mistaken for endometrial disease. A small fibroid encroaching into the endometrial cavity (SMF) could be erroneously considered intramural forming part of a small fibroid uterus |
| Endometrial disease | ||
| DUB | ||
| Fibroid uterus < 12 weeks’ size | Polyp/SMF | Intramural fibroids may be wrongly diagnosed as submucosal. Small fibroids could be missed and a thickened, functional endometrium could appear hyperplastic |
| Endometrial disease | ||
| DUB | ||
| Fibroid uterus > 12 weeks’ size | Polyp/SMF | Large fibroids would be rarely overlooked entirely, but it is possible to underestimate their size or incorrectly classify fibroid location |
| Fibroids < 12 weeks | ||
| Endometrial disease | Polyp/SMF | A thickened hyperplastic or cancerous endometrium could be misdiagnosed as containing a polyp. Small fibroids may be incorrectly identified within the myometrium. The endometrium may appear to be normal |
| Fibroids < 12 weeks | ||
| DUB | ||
| DUB | Polyp/SMF | A normal, thickened endometrium could be considered falsely to be some form of endometrial disease (hyperplasia or cancer) or focal lesion (e.g. folds of normal endometrium mistaken for a polyp). Small fibroids may be incorrectly identified within the myometrium |
| Fibroids < 12 weeks | ||
| Endometrial disease | ||
| SIS | ||
| Intrauterine polyp or SMF | Fibroids < 12 weeks | Focal pathologies could be missed on imaging, but this will occur less compared with TVS because of cavity distension with fluid. A small fibroid encroaching into the endometrial cavity (SMF) could be erroneously considered intramural forming part of a small fibroid uterus. Cystic-looking polyps may be mistaken for endometrial hyperplasia |
| Endometrial disease | ||
| DUB | ||
| Fibroid uterus < 12 weeks’ size | Polyp/SMF | Intramural fibroids may be wrongly diagnosed as submucosal. Small fibroids could be missed and a thickened, functional endometrium appears hyperplastic |
| Endometrial disease | ||
| DUB | ||
| Fibroid uterus > 12 weeks’ size | Polyp/SMF | Large fibroids would be rarely overlooked entirely, but it is possible to underestimate their size or incorrectly classify them as being submucosal |
| Fibroids < 12 weeks | ||
| Endometrial disease | Polyp/SMF | A thickened hyperplastic or cancerous endometrium could appear as a polyp. Small fibroids may be incorrectly identified within the myometrium. The endometrium may appear to be normal |
| Fibroids < 12 weeks | ||
| DUB | ||
| DUB | Polyp/SMF | A normal, thickened endometrium could be considered falsely to be some form of endometrial disease (hyperplasia or cancer) or focal lesion (e.g. folds of normal endometrium mistaken for a polyp). Small fibroids may be incorrectly identified within the myometrium |
| Fibroids <12 weeks | ||
| Endometrial disease | ||
| Outpatient hysteroscopy | ||
| Intrauterine polyp or SMF | Endometrial disease | Focal pathologies could be missed on imaging, but this will occur less compared with TVS because of cavity distension with fluid. OPH cannot visualise the myometrium and so, in contrast to sonography (TVS, SIS), presence of fibroids cannot be falsely diagnosed. Polyps may be mistakenly diagnosed as endometrial disease |
| DUB | ||
| Fibroid uterus < 12 weeks’ size | Polyp/SMF | The myometrium is not visualised by OPH and preceding clinical examination is not sensitive enough to identify small fibroids. Thus, at OPH a normal cavity would be found in the presence of the true pathology (small intramural fibroids). Possible false diagnoses would be when normal, functional thickened endometrium is considered falsely to be some form of endometrial disease (hyperplasia or cancer) or focal lesion (e.g. folds of normal endometrium mistaken for a polyp) |
| Endometrial disease | ||
| Fibroid uterus > 12 weeks’ size | Polyps/SMF | Large fibroids would be missed at OPH because the myometrium is not visualised, although the majority would be detected on preceding clinical examination.a At OPH a normal cavity would be found in the presence of the true pathology (large intramural fibroids). Possible false diagnoses would be when normal, functional thickened endometrium is considered falsely to be some form of endometrial disease (hyperplasia or cancer) or focal lesion (e.g. folds of normal endometrium mistaken for a polyp) |
| Endometrial disease | ||
| Endometrial disease | Polyp/SMF | A thickened hyperplastic or cancerous endometrium could appear normal or as a polyp |
| DUB | ||
| DUB | Polyp/SMF | A normal, thickened endometrium could be considered falsely to be some form of endometrial disease (hyperplasia or cancer) or focal lesion (e.g. folds of normal endometrium mistaken for a polyp, especially in the secretory phase of the menstrual cycle) |
| Endometrial disease | ||
| EBx | ||
| Intrauterine polyp or SMF | Endometrial disease (hyperplasia but not cancer) | Endometrial polyp tissue could be mistaken for normal or complex endometrial hyperplasia but it would be extremely unlikely to erroneously diagnose a polyp as endometrial cancer. The focal lesion may be missed by the biopsy |
| DUB | ||
| Fibroid uterus < 12 weeks’ size | Polyp/SMF | Cystic pieces of endometrium can be mistaken for endometrial polyps. Fibroids can distort the uterine cavity and compact areas of endometrium. If these areas are sampled they can mistaken for complex endometrial hyperplasia |
| Endometrial disease (hyperplasia not cancer) | ||
| Fibroid uterus > 12 weeks’ size | Polyps/SMF | As for small fibroids above |
| Endometrial disease (hyperplasia not cancer) | ||
| Endometrial disease | Polyp/SMF | Polyp or DUB were considered the only plausible false diagnoses |
| DUB | ||
| DUB | Polyp/SMF | An endometrial polyp or SMF were considered the only possible false diagnoses. It was felt to be extremely unlikely to mistakenly diagnose any endometrial disease from a normal sample |
Treatment failure
Following diagnosis, patients were booked for treatment and the most suitable treatment (Table 5) was instituted. Only one treatment was considered for each diagnosis. In view of the cyclical nature of HMB symptoms and the delayed treatment effects associated with the LNG-IUS and EA, most treatment outcomes for HMB can be reliably assessed only after at least 6 months. It was assumed that dissatisfied women would attend their GP and be referred back to secondary care to be reviewed by a gynaecologist, who would undertake a further specific, second-line treatment (see Table 5). The exception to this strategy was dissatisfaction after initial treatment with hysterectomy because no further treatment is possible in the absence of a uterus. They were assumed to attend their GP for a consultation only. Women who remained dissatisfied following a second treatment were assumed to receive ‘rescue treatment’ consisting of a GP visit, a further hospital gynaecology outpatient appointment and a total abdominal hysterectomy (TAH) (unless hysterectomy had been performed already, in which case they were assumed to attend their GP for a consultation only). Patients were assumed to undergo the first two treatments within a 12-month period. All clinical decisions were made following consultation with the expert clinical panel.
| Diagnosis | Treatment 1 | Treatment 2 (performed only if patient ‘not satisfied’ with treatment 1) |
|---|---|---|
| Endometrial polyp | Outpatient polypectomy | LNG-IUS |
| SMF | Transcervical resection of fibroid | LNG-IUS |
| Fibroids < 12 weeks’ size | LNG-IUS | EA |
| Fibroids > 12 weeks’ size | Total abdominal hysterectomy | GP visit |
| Complex hyperplasia | LNG-IUS | TAH |
| Complex hyperplasia with atypia/endometrial cancer | TAH | GP visit |
| DUB | LNG-IUS | EA |
Adaptations of the base-case tree to assess alternative clinical scenarios
Women being managed during multiple clinic visits
The base-case tree was designed to reflect a contemporary, ‘one-stop’ clinic to ensure that the results remain relevant and do not quickly become outdated as services evolve. However, this approach has not yet been widely adopted across the UK and, therefore, the base-case tree was adapted to reflect a patient attending a standard gynaecology outpatient clinic and then being referred on for further investigations, followed by a follow-up appointment to institute treatment. In this analysis all tests were performed at separate appointments except for EBx, which would be taken at the initial consultant appointment. TVS and SIS were assumed to be performed in the ultrasound department at a later date with the patients having a follow-up appointment to review the results. OPH required a further consultant appointment for the hysteroscopic assessment. If polyps were diagnosed, whether by scan or hysteroscopy, patients required a further hysteroscopy appointment for removal. SMFs were removed under general anaesthesia and treatment with the LNG-IUS, hysterectomy and EA was performed as in the base-case tree.
Women refractory to levonorgestrel intrauterine system treatment
Alternative analysis was performed by adapting the model to fit with the scenario that all women referred to secondary care had already received treatment with a LNG-IUS in primary care but their symptoms had not resolved. This was to reflect current NICE guidance which recommends that women receive a LNG-IUS in a primary care setting as first-line treatment for HMB11 and attend secondary care only if their symptoms are refractory or structural abnormality is expected. The prevalence of disease changed within this tree as it was assumed that patients treated appropriately with the LNG-IUS (for DUB, endometrial hyperplasia or fibroids < 12 weeks’ size) would be less likely to be referred to secondary care than women who were being treated inappropriately (for fibroids > 12 weeks’ size, polyps, SMFs or endometrial cancer) because their symptoms would be more likely to have resolved. Satisfaction rates for treatment of each of the pathologies were used to recalculate the disease prevalence. LNG-IUS was no longer a possible treatment within this tree as women had previously failed to respond to it. The exception to this rule was for women who were dissatisfied following removal of a polyp or SMFs as they now had a ‘normal’ uterine cavity, whereas previously there had been a structural abnormality compromising the clinical effectiveness of the LNG-IUS. These women received a LNG-IUS as their second treatment following removal of focal pathology. EA became the first treatment to be offered to women who were thought to have a ‘normal’ uterine cavity and if this failed, hysterectomy was offered as the next treatment option. Given that patients already have a LNG-IUS in situ when they attend secondary care in this scenario the tree was adjusted so that the comparative strategy was ‘no further treatment’ to represent patients coming to clinic but not having any further treatment (i.e. woman attending the clinic but ultimately deciding to continue with the LNG-IUS).
Women wishing to retain their fertility
The base-case analysis was revised to reflect a population who wished to maintain their future fertility. This meant that EA and hysterectomy were no longer possible treatments except in the case of endometrial cancer, where hysterectomy was still selected as the treatment of choice. Myomectomy and UAE were introduced as possible treatments in this tree, as they are far more likely to be offered to women who wish to have children than to women who have completed their families. Myomectomy was assumed to be selected over UAE by 80% of women as it is thought to improve fertility to a greater extent than UAE. 98 Following UAE or myomectomy, patients who were ‘not satisfied’ with their treatment were offered the other treatment. Hysterectomy was not offered as a treatment for any benign cause of HMB and women who remained ‘not satisfied’ after two treatments, or after one if no fertility-preserving treatment could be offered, received a GP visit and a new gynaecology outpatient appointment as ‘rescue treatment’.
Clinical data collection
We intended to use, where possible, IPD to populate the HMB decision tree extracted from a prospective database of over 500 women, which recorded the investigation and management of women who had presented to Birmingham Women’s Hospital (BWH) with HMB between 2004 and 2006. However, it became apparent that useful, comprehensive diagnostic and treatment data were not available. While the majority of women had undergone TVS as part of initial diagnostic work-up, further testing with OPH or EBx was sporadic (SIS was not practised) and usually undertaken in response to an abnormal TVS (i.e. limiting any assessment of accuracy data to ‘positive’ results on TVS). Furthermore, systematically collected outcome data were lacking, which precluded reliable estimation of treatment response at specific time points post therapeutic intervention. An added complexity was that the many women whose data were collected had already had multiple investigations and treatments. The scope of this project did not include time and resources for prospective data collection. However, we did use the primary IPD available to corroborate literature-derived data, especially if published data were imprecise with regard to disease prevalence and treatment choices.
All literature-derived data were obtained following systematic searches (detailed in the relevant report sections). Again, we had hoped to use IPD from published systematic quantitative reviews to facilitate estimation of test accuracy when tests were used in combination. However, published systematic reviews94–96,99 contained a small number of primary studies, of which many evaluated single tests only. Moreover, preliminary checks with study and review authors reinforced the view that pursuing test accuracy IPD (especially given the time and resources of the project) with a view to meta-analysis was futile. This was felt to be the case even assuming perfect compliance with requests for original data from primary study authors because test combination data were sparse and outdated in some instances given the length of time since publication and advances in imaging technologies. Thus, we used test accuracy data from published systematic reviews and meta-analyses where possible, followed by data derived from primary, well-conducted test accuracy studies and, finally, clinical data held at the BWH and expert clinical opinion.
For treatment data we regarded systematic quantitative reviews using IPD as the highest level of data, followed by systematic reviews of study-level data. RCTs were acknowledged as the third step down the hierarchy, followed by large comparative cohort studies and then uncontrolled observational series. Prospectively collected data from studies with large populations were considered superior to small studies and those with retrospectively collected data. When possible, we used data from a purely pre-menopausal population; however, occasionally, data came from studies of AUB incorporating both pre- and post-menopausal women. Where mixed populations of women with AUB were encountered, we aimed to stratify data by menopausal status if possible.
Disease prevalence
For prevalence of disease underlying HMB symptoms, based on expert clinical consensus, we defined a gold standard test for confirmation of diagnosis (Table 6).
| Pathology | Confirmatory test |
|---|---|
| Polyps | OPH |
| SMFs | OPH |
| Uterine fibroids < 12 weeks | Pelvic ultrasound |
| Uterine fibroids > 12 weeks | Pelvic ultrasound |
| Endometrial disease | Histological sampling |
| DUB | Diagnosis of exclusion |
To inform our economic analysis, we conducted a systematic literature review to estimate the prevalence of pathologies as estimated by the most appropriate confirmatory test. The searches for prevalence are reported in Appendix 3. As DUB is a diagnosis of exclusion, we did not use a diagnostic test as one of the search terms. The prevalence of each diagnosis was determined from published studies, using systematic reviews when possible. We developed a quality scoring system to assess studies reporting disease prevalence. We used seven quality criteria (Table 7) and awarded a score from 1 to 3 for each criterion, giving a score out of 21. Data from the highest scoring papers were used to populate the decision model.
| Criterion | Points awarded | ||
|---|---|---|---|
| 3 | 2 | 1 | |
| Data collection | Prospective | Retrospective | Not clear |
| Consecutive patients | Yes | No | Not reported |
| Population size | > 500 | 100–500 | < 100 |
| Menopausal status | Pre-menopausal | Mixed but > 50% pre-menopausal | Post-menopausal |
| Data collection | All have the gold standard test | Selection prior to gold standard test | Inferior test |
| Proportion having the gold standard test | > 90% | < 90% | |
| Pathology clearly defined | Clear definition | Unclear definition | No definition |
Data regarding the prevalence of fibroids (intramural and subserosal) were taken from a database of 500 women with HMB, held at BWH, in the absence of a better quality published data set.
Data regarding the prevalence of DUB were often not specified within studies, in contrast to organic pathologies. As the overall prevalence of disease must add up to 1 (100%) in the economic model, it was decided that the prevalence of DUB (which is a diagnosis of exclusion) would be altered to become the remaining proportion once the prevalence of other pathologies had been estimated. The impact of this manipulation was tested with sensitivity analyses.
Test success and accuracy
To identify diagnostic data regarding the feasibility and accuracy of the tests under evaluation, we kept our database searches sensitive rather than specific by using broad search terms. We used the test name, variations of it and the associated MeSH terms. If searches retrieved a large numbers of studies, we restricted data abstraction to review articles only. In the case of searches for SIS, the search was qualified by the population under scrutiny (i.e. ‘heavy menstrual bleeding’ and its associated terms) because preliminary, broad searches were retrieving a large number of articles which evaluated the test in post-menopausal women and women with infertility. Duplicate articles were removed, the abstracts of all remaining articles were read and the full text of relevant papers was retrieved based upon the following selection criteria:
-
population: HMB
-
intervention: outpatient test to evaluate the uterus (TVS, SIS, EBx or OPH)
-
outcome: feasibility (success rate) or test accuracy for uterine pathology
-
study design: restricted to systematic reviews if available.
Although systematic reviews retrieve a large amount of higher quality aggregated data of feasibility and/or test accuracy, the data reported did not give relevant accuracy data for all of the pathologies associated according to each test. In these circumstances restriction on study design (systematic reviews) was removed and in the absence of any relevant primary study data, additional searches to look for data regarding the accuracy of the test without regard for population characteristics (HMB) were undertaken, as were searches using specific pathologies as the population of interest. The quality of the studies was assessed using the criteria in Table 8. The highest scoring studies were selected for each of the pathologies. If studies were of equal quality, the largest was selected and, if this did not discriminate, blinding and interval between tests were taken into consideration.
| Criterion | Explanation |
|---|---|
| Population size | > 100 women |
| Type of bleeding | > 75% HMB |
| Menopausal status | > 70% pre-menopausal |
| Data collection | Prospective |
| Reference test | Appropriate gold standard test applied (see Table 6) |
| Blinding | Present (diagnostic test and the gold standard test are performed by different, blinded clinicians) |
| Interval between tests | Within the same menstrual cycle |
| Cross-tabulation | Data presented in a 2 × 2 table |
| Total | Eight points maximum (one point awarded for each criterion present) |
Details of searches are given in Appendix 3. An unsuccessful test was defined as failure of the test to provide a diagnosis. This may arise for a number of reasons such as an inability to correctly site the technology [e.g. transvaginal placement of ultrasound probe (TVS, SIS)], instrument the uterine cavity (SIS, EBx or OPH) or inadequate visualisation (imaging modalities – TVS, SIS, OPH). In the case of EBx, failure also included a successfully completed test but subsequent histological analysis of obtained endometrial tissue was reported as ‘insufficient’ (i.e. non-diagnostic).
Accuracy data tended to be reported as sensitivity and specificity along with their respective 95% confidence intervals (CIs). These true-positive and -negative rates were then used to calculate the false-positive and -negative rates (Table 9).
| Accuracy value | Calculation |
|---|---|
| True-positive rate | Sensitivity |
| True-negative rate | Specificity |
| False-positive rate | 1 – specificity |
| False-negative rate | 1 – sensitivity |
Where data were reported as likelihood ratios, sensitivity and specificity values were derived. Sensitivity and specificity, along with their 95% CIs, were calculated from raw data where necessary. In order to simplify our decision model, when possible, underlying pathologies were grouped, that is to say similar aetiologies and/or commonly associated treatments (see assumptions section). One problem with this approach was encountered for the pathology categories ‘intrauterine resectable pathology’ (i.e. endometrial polyps and SMFs) and ‘endometrial disease’ (i.e. endometrial hyperplasia, endometrial hyperplasia + atypia/endometrial cancer). Accuracy data for these categories were often reported separately for each constituent. To overcome this, we combined the figures, weighting them according to the proportion of this pathology category they made up. Taking EBx as an example test:
endometrial hyperplasia = 60% of the endometrial disease category
atypical endometrial hyperplasia/cancer = 40% of the endometrial disease category
the sensitivity value for EBx for hyperplasia is 0.81
the sensitivity value for EBx for atypia/cancer is 0.86
combined sensitivity is (0.6 × 0.81) + (0.4 × 0.86)
sensitivity = 0.83.
A final data manipulation around test accuracy was needed when tests were reported in the literature as having a sensitivity or specificity of 1 for particular pathologies. Perfect accuracy data were rounded down to 0.99 as, although we can say that a test has a high predictive value, no test can be reported as completely accurate. Also, putting false-positive rates (FPRs) of zero in the tree would make the following branches redundant.
Treatment satisfaction data
Systematic searches of the literature were conducted to identify patient satisfaction data at 1 year post treatment. The electronic bibliographic databases EMBASE (1980 to November 2011) and MEDLINE (1950 to November 2011) were searched using search terms of the relevant treatment, combined with menorrhagia or HMB, and satisfaction along with their alternatives. Searches and outputs are detailed in Appendix 3.
For the purposes of our model, effectiveness data in terms of patient satisfaction were needed according to underlying pathology. While treatment outcome data are reported for women with HMB, in some cases the underlying diagnoses were not ascertained or treatments were not utilised (quite correctly) where contraindicated. However, the diagnostic model required data for treatment outcomes not only for treatments applied to appropriate pathologies, but also where false diagnoses are made on testing and so suboptimal or inappropriate treatments would be applied. When we were unable to identify the relevant data from the published literature, we presented our expert clinical panel with the relevant scenarios for their opinions as to likely treatment efficacy. The median values obtained in this way were used as the satisfaction rate and the range of values recorded to be used in sensitivity analysis.
Systematic reviews with meta-analysis were considered the highest quality data, followed by RCTs. We used data for satisfaction at 12 months post treatment in keeping with our model time frame. When these data were not available, we used the data reported closest to 12 months. If the time of reporting was not stated within the study and no other appropriate data had been identified, we used the reported data. For EA, we aimed to use data that exclusively examined second-generation techniques,18 as first-generation methods are seldom used.
Results of clinical data collection
Disease prevalence
Uterine polyps
The searches for prevalence of endometrial polyps identified 845 studies (see Appendix 3). Seven studies were selected and the papers were obtained for further analysis. The majority of studies were rejected because they did not report prevalence or referred to post-menopausal or infertile populations. The highest quality study (score 20/21) reported a uterine polyp prevalence of 18%. 100 The range in prevalence was wide, however, with the next two best studies reporting a prevalence of 3.7%101 and 33.9%. 102
Submucosal fibroids
Searches for the prevalence of fibroids in women with HMB (see Appendix 3) identified 134 papers, three of which were selected and assessed for quality and the prevalence data were extracted. Papers were discarded if they did not report prevalence of SMFs in pre-menopausal women and if the reference test used was not the selected gold standard. The two highest scoring papers reported differing values of 21.9% and 7.4% for the prevalence of SMFs,100,102 with 21.9% coming from the best paper100 (quality score 18 out of 21).
We planned to use the values for the prevalence of polyps and SMFs from the highest quality studies within our decision tree; however, while examining papers that reported the accuracy of hysteroscopy, we identified a systematic review of diagnostic hysteroscopy which reported the prevalence of polyps and SMFs. 99 This systematic review meta-analysed over 3000 procedures. The prevalence of polyps was reported as 21% and that of SMFs as 25%. However, polyps and SMFs are estimated to coexist in approximately one-third of women103 and so we reduced the value of SMFs by one-third to 17% to account for this. Thus, the derived prevalence rate used for the pathology category ‘endometrial polyps/SMF’ was 38% (0.38). In view of the wide variation in our previous estimates, we decided after consultation with our expert panel (and with reference to data from the IPD held at the BWH which concurred with this estimate of prevalence) to use the data from the systematic review. 99
Fibroids
Two studies were identified that looked at the prevalence of intramural or subserosal fibroids out of 134 studies identified from the original search (see Appendix 3). One study only contained 80 participants103 and the second was a study of women being scanned for a variety of symptoms (pain, worry, AUB, suspected fibroids) and not just HMB. 104 The prevalence values reported by the two studies were 57.7% and 23.5%, respectively. Neither study specified whether or not SMFs were excluded and the patient populations were heterogeneous, preventing meta-analysis. Both studies scored 17 for quality but in view of the small size of the first study (80 patients), the poorly defined population in the second study and the large discrepancy in the reported values, we decided to use data from our clinical HMB database of 473 women held at the BWH. When analysing the BWH HMB database, we found that 19% (88 out of 473) women had intramural fibroids < 12 weeks’ size and that 6% (28 out of 473) had fibroids > 12 weeks’ size; thus, these data were used as our prevalence values within the decision tree.
Endometrial disease
The searches for prevalence of endometrial hyperplasia (see Appendix 3) diagnosed by histology samples identified 86 studies, of which five were chosen for review. Two studies were of high quality (both scoring 20 out of 21) and reported similar values for the prevalence of endometrial hyperplasia without atypia (3.0% and 2.4%). One study was a retrospective review of histology samples105 and the second a retrospective audit of hysteroscopy findings. 100 The same two studies were also identified as the best from our searches for studies reporting the prevalence of endometrial cancer (374 studies identified, four assessed further) (see Appendix 3). Both studies were large and comprised only pre-menopausal women. We performed meta-analysis of these two studies to calculate the prevalence data more precisely. We meta-analysed data for endometrial hyperplasia without atypia separately from data for endometrial hyperplasia with atypia and endometrial cancer because the clinical implications of diagnosis and optimal therapeutic interventions differ. Meta-analysis was performed by converting the values from the studies to log odds and standard errors using Microsoft Excel (Microsoft Corporation, Redmond, WA, USA); these data were then copied across to RevMan (The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark) and analysed as the generic inverse variance using random effects analysis. The output from RevMan is the odds, so these were copied back into Excel and converted to values to give our prevalence rates with 95% CIs. The value we calculated for prevalence of endometrial hyperplasia without atypia was 3% and that for endometrial hyperplasia with atypia or endometrial cancer was 2%. Therefore, the value for ‘endometrial disease’ was the sum of the two, 5%.
Dysfunctional uterine bleeding
In all decision trees the sum of all the branches from a common stem must add to 1. It was inevitable that our prevalences would not add up to 1 as our data came from a variety of sources, and so it was decided that dysfunctional bleeding would be the value remaining to make the prevalences add to 1 (given that DUB is a diagnosis assigned after exclusion of other identifiable ‘organic’ pathologies), and so for the base-case tree the prevalence of DUB was set at 32%. The expert panel agreed that this derived figure seemed reasonable from a clinical perspective.
A summary of the derived disease prevalences is given in Table 10.
| Disease | Prevalence | Sensitivity analysis (range) | Source |
|---|---|---|---|
| Polyps/SMFs | 0.38 | 0.2 to 0.5 (EP estimate) | SR100 |
| Fibroids < 12 weeks’ size | 0.19 | 0.15 to 0.22a | BWH database |
| Fibroids > 12 weeks’ size | 0.06 | 0.04 to 0.08a | BWH database |
| Complex endometrial hyperplasia | 0.03 | 0.02 to 0.03a | Meta-analysis of two studies100,105 |
| Atypical hyperplasia/cancer | 0.02 | 0.01 to 0.02a | Meta-analysis of two studies100,105 |
| Endometrial disease (all hyperplasias and cancer) | 0.05 | 0.03 to 0.05a | Meta-analysis of two studies100,105 |
| DUB | 0.32 | Remaining proportion | Remaining proportion used so that total sums to 1 |
To calculate the prevalence of disease within the decision tree of women already treated with a LNG-IUS when referred to secondary care, we used the proportion of women who would not be satisfied with a LNG-IUS (see rates later on in this chapter) and weighted them by dividing each by the total, so that the sum of them all came to 1 and they could be used within the disease prevalence branches of the decision tree (Table 11).
| Disease | Original prevalence (a) | Proportion dissatisfied with LNG-IUS (b) | Proportion coming to gynaecology (a × b) (c) | New prevalence (c/total c) |
|---|---|---|---|---|
| Polyps/SMFs | 0.38 | 0.60 | 0.228 | 0.592 |
| Fibroids < 12 weeks’ size | 0.19 | 0.17 | 0.032 | 0.083 |
| Fibroids > 12 weeks’ size | 0.06 | 0.71 | 0.043 | 0.111 |
| Endometrial disease | 0.05 | 0.56 | 0.028 | 0.073 |
| DUB | 0.32 | 0.17 | 0.054 | 0.141 |
| Total | 0.3853 | 1 |
Test success
Success data for each of the four separate tests came from systematic reviews and meta-analysis (Table 12).
| Test | Success rate | Sensitivity analysis (range) | Source |
|---|---|---|---|
| OPH | 0.97 | SR99 | |
| TVS | 0.99a | SR107 | |
| Endometrial pipelle biopsy | 0.91 | 0.89 to 0.93b | SR21 |
| SIS | 0.95 | 0.94 to 0.96b | SR94 |
Outpatient hysteroscopy
The search strategy for OPH identified three systematic reviews95,96,99 from 1095 studies. Two studies reported test success. 95,99 The OPH value came from one of these systematic reviews99 of diagnostic hysteroscopy. This quantitative systematic review reported success data separately for all women having OPH and also for pre-menopausal women. However, the data for OPH included some post-menopausal women and the data for pre-menopausal women contained some women undergoing hysteroscopy as an inpatient under general anaesthesia. In total 2643 out of 3158 (84%) procedures were done as OPHs and only 306 women were specified as being post-menopausal; therefore, we decided to use the success rate value for OPH (0.97). This value was supported by the second review of predominantly pre-menopausal women (71%) but included both inpatient and outpatient hysteroscopies. The reported success rate in this meta-analysis was similar at 95.8% (95% CI 95.5 to 96.1%). 95
Transvaginal scan
There were no systematic reviews of pre-menopausal women undergoing TVS that reported test success rate. Only one small study of 43 women reported the success rate of TVS, and this was 100%. 106 A previously used systematic review of women with PMB reported a mean success rate of 100% with a standard deviation of 2% when data from 16 studies were meta-analysed. 107 We used a figure of 0.99 because no test is 100% successful and, in the opinion of our expert clinical panel, occasionally women do refuse to have the test, cannot tolerate it, or visualisation on imaging is too poor to make a diagnosis.
Saline infusion sonography
Searches for reviews of SIS identified 257 studies, two of which were systematic reviews96,108 and were selected for data extraction. The first systematic review included meta-analysis of the accuracy of SIS in a population of women with AUB (> 50% pre-menopausal) and reported that the success rate of the test in pre-menopausal women was 94.8% with 95% CIs of 93.5% to 96.1%. 94 This success rate of 95% was supported by the second review examining SIS in pre-menopausal women. 96
Endometrial biopsy
Searches for reviews of EBx identified two systematic reviews: one for the diagnosis of endometrial hyperplasia20 and one for the diagnosis of endometrial cancer. 21 The latter review of EBx for diagnosis of endometrial cancer had a mainly post-menopausal population. We therefore used the study looking at endometrial hyperplasia in which the majority of women were pre-menopausal (50% were known to be pre-menopausal, 25% were known to be post-menopausal and in 25% menopausal status was unknown). This study found that 76 out of 881 (8.6%) tests failed or were insufficient for histological diagnosis,20 and so we used a failure rate of 9% (0.09) and used the raw data to calculate CIs. The expert clinical panel were consulted regarding this estimate and the consensus view was that the derived estimate was reasonable for our study population.
Test combinations
When combinations of tests were performed, the success rates of the individual tests were multiplied within the tree to calculate the success rate (see Combination testing strategies and discordant results, above). The success rates of combined tests will always be worse than tests performed individually and this value will reflect this. However, we were not able to take into account whether or not the failure of one test is dependent upon the next; for example, if a hysteroscopy fails because of a stenosed cervical canal, an EBx would be very likely to also fail.
Test accuracy
Outpatient hysteroscopy
The accuracy data for hysteroscopy came from the two systematic reviews (see Appendix 3) used for test success and were identified by our OPH database searches. 95,99 The first review looked at the accuracy of hysteroscopy for diagnosing intrauterine abnormalities in women presenting with AUB (pre- and post-menopausal) using histopathology specimens as the reference standard. This study provided the data for polyps, SMFs and DUB. This study has limitations as it has a mixed population and only 84% of procedures are specified as outpatient hysteroscopies. However, no other large data sets reporting accuracy exist. Data for the sensitivity and specificity of polyps (0.94 and 0.92 respectively) and SMFs (0.87 and 0.95 respectively) are clearly reported in the paper and we combined the values to create data for our combination group of polyps/SMFs. Studies included in this large, systematic quantitative review of hysteroscopy report the accuracy of a test for diagnosing pathology rather than a normal cavity. Thus, we were unable to identify data for the accuracy of OPH in diagnosing DUB and this reflects the fact that DUB is considered a diagnosis of exclusion. We therefore used the data from the review and reversed the values, that is to say we used the proportion not diagnosed as abnormal and assigned these women as DUB. In practice, this results in the sensitivity and specificity being reversed. The sensitivity and specificity reported for diagnosing abnormalities were 0.94 and 0.89, respectively, and so for diagnosing ‘no’ abnormality the values reverse and the sensitivity becomes 0.89 and specificity becomes 0.94.
The data regarding the accuracy of OPH for diagnosis of endometrial disease comes from the second identified review,95 which specifically looks at this question in a mixed population with 29% of the women specified as postmenopausal. This large systematic review meta-analysed data from 65 studies (26,346 women) that compared OPH with endometrial histology results and reported that the sensitivity for diagnosis of endometrial disease (cancer and hyperplasia) was 0.78 and that the specificity was 0.96;95 hence, these are the values which we used in our decision tree.
Transvaginal scan
The search for TVS accuracy data included terms for AUB and yielded 420 studies once duplicates had been removed (see Appendix 3). Thirty-seven studies were selected for further assessment regarding accuracy of TVS. Only one of the selected papers was a systematic review96 but, as the studies included were heterogeneous, no meta-analysis was performed and we were unable to extract useful data from this study. The 37 studies we identified were assessed for quality, and accuracy data were extracted for the different pathologies.
A prospective comparative study was selected as the highest quality paper reporting the accuracy of TVS for diagnosing polyps and SMFs. 109 The study compared the TVS diagnosis with hysteroscopic diagnosis. The tests were performed by different clinicians and each one was blinded to the other results. The tests were performed within 24 hours of each other and the majority of women included in the study were pre-menopausal. The sensitivity and specificity of TVS for diagnosing polyps and SMFs were reported as 0.45 (95% CI 0.32 to 0.58) and 0.78 (95% CI 0.62 to 0.89), and these data were used to populate the decision tree. Data from the same study were used for accuracy of TVS for diagnosing endometrial disease reported as sensitivity 0.57 (95% CI 0.19 to 0.90) and specificity 0.66 (95% CI 0.55 to 0.76).
Only one study was identified which reported the accuracy of TVS for diagnosing intramural or subserosal fibroids. The study aimed to assess the accuracy of TVS for diagnosing adenomyomas and fibroids and its ability to distinguish between the two pathologies by comparing the scan results with hysterectomy specimens. 110 The mean age of women included in the study was 46.7 (range 35.7–51.8) years, and 172 of the 206 women had menorrhagia or dysmenorrhoea. The sensitivity and specificity of TVS for diagnosing fibroids were reported as 95.1% and 82.0%, respectively, and were used as the accuracy values for TVS diagnosis of fibroids.
As with OPH, no studies reported the accuracy of TVS for diagnosing a normal uterus, so this had to be derived indirectly. We selected the largest high-quality study (7 points) that reported the accuracy of TVS for diagnosing abnormality111 and reversed the sensitivity and specificity. This study evaluated 770 women with HMB to establish the accuracy of TVS for diagnosing a composite of all pathologies labelled ‘intrauterine disease’ by comparing the scan results with the results of hysteroscopy. As the reported sensitivity and specificity for abnormality were 0.96 (95% CI 0.934 to 0.972) and 0.86 (95% CI 0.823 to 0.898), they were reversed, and 0.86 (95% CI 0.823 to 0.898) was used as the sensitivity and 0.96 (95% CI 0.934 to 0.972) used as the specificity.
Saline infusion sonography
Searches for the accuracy of SIS identified 157 studies of saline scan for menorrhagia (see Appendix 3). Forty-one papers were selected and assessed for quality and any reported accuracy data were extracted. Two systematic reviews were identified,94,96 one of which was the study with no meta-analysis96 that we could not use. The second was a systematic review and meta-analysis of diagnostic studies that compared SIS with either hysteroscopy or histopathology obtained at hysteroscopy or hysterectomy, and reported the accuracy of the test for diagnosing intrauterine abnormalities. 94 The analysis included 24 studies and more than 50% of the population was pre-menopausal. The accuracy of SIS for diagnosing endometrial polyps and SMFs was reported as a secondary outcome after meta-analysis of 15 homogenous studies. We used the data for polyps and SMFs and combined them with weighting to calculate values for the two pathologies combined. We used these values in the decision tree. Once again, data for a normal cavity were not available, and so we reversed the data for abnormalities, making the sensitivity the specificity and vice versa.
The accuracy of SIS for diagnosing endometrial disease was not reported in the systematic reviews. Of the 39 remaining studies, four reported the accuracy of SIS for diagnosis of endometrial disease (hyperplasia and or cancer). The quality of the studies was assessed according to our quality criteria. Two of the studies scored 6 points,109,112 but the values that they reported for sensitivity were very different, with one reporting a sensitivity for endometrial hyperplasia of 0.94112 and the second reporting sensitivity for endometrial hyperplasia and cancer as 0.29. 109 The study sizes were very similar, as were the proportions of pre-menopausal women; however, one of the studies had a much higher prevalence of endometrial disease (17%) than would be expected in a mainly pre-menopausal population112 and the interval between the SIS and the reference test was up to 14 days, whereas in the second study it was just 24 hours. 109 The first study112 used a cut-off threshold for women of reproductive age of 8 mm for diagnosing abnormality. This threshold to define abnormality was considered low by our expert panel for women of reproductive age and will lead to increased test sensitivity, while decreasing the test specificity. Moreover, simply using an endometrial thickness cut-off level is crude and more applicable to a post-menopausal population in which endometrial thickness is constant rather than changeable according to the menstrual cycle. In contrast, the second study diagnosed abnormalities based upon clinical features seen at SIS rather than defining abnormalities. 109 The culmination of these factors resulted in our decision to use data from the second study109 to populate the decision tree.
Endometrial biopsy
Searches for accuracy data of EBx identified four studies with data regarding accuracy of the test. One of the studies was a RCT which looked at the use of three diagnostic tests, including endometrial biopsy, in groups of women at a specified risk of endometrial cancer. 29 The population included pre- and post-menopausal women and reported only the accuracy of pipelle for diagnosing endometrial cancer. Two of the remaining selected studies were systematic reviews, one of which looked at diagnosis of endometrial hyperplasia20 while the other looked at endometrial cancer. 21 Both studies used histopathology samples as the reference standard. These studies were also limited in that they had pre- and post-menopausal women in their populations. As post-menopausal women have an atrophic endometrium, focal lesion are more likely to be sampled and, thus, the effect of the post-menopausal women within these three studies may increase the sensitivity of the test above what may be expected in a purely pre-menopausal population. As we rated systematic reviews with meta-analysis as higher quality evidence than RCT data, we used the systematic reviews20,21 for accuracy data for endometrial disease. In these papers, likelihood ratios were reported, but in order to populate our model, we used the sensitivities and specificities which were reported in the thesis from which the papers were taken (TJ Clark, Birmingham Women’s NHS Foundation Trust, 2004, personal communication).
The fourth study reported the accuracy of EBx for diagnosis of endometrial polyps. 113 One hundred and seventy-six consecutive patients (77% pre-menopausal) who were scheduled for D&C underwent TVS and endometrial pipelle® biopsy prior to their surgery. The biopsy samples and the curettings were examined by different pathologists who were blinded to the other result. The paper reports these results in a cross-tabulated fashion, enabling calculation of the sensitivity and specificity of EBx for the diagnosis of endometrial polyps114 as 0.997 (95% CI 0.973 to 1) and 0.003 (95% CI 0 to 0.027) respectively. These values were converted to the true- and false-positive values which were used in the decision tree. We used the some cross-tabulation to calculate the accuracy for diagnosis of DUB by looking at the accuracy for benign endometrium (excluding endometrial polyps and hyperplasia). The sensitivity was calculated as 0.953 (95% CI 0.895 to 0.98) and specificity as 0.971 (95% CI 0.902 to 0.992) and the corresponding true- and false-positive values were used accordingly.
No studies reported the use of EBx for diagnosis of SMFs, and so further searches were performed that did not include ‘heavy menstrual bleeding’ or its associated terms in the searches. One study of 330 post-menopausal women was identified that reported the accuracy of EBx for diagnosis of SMFs. The prospective study compared Novak catheter samples with histopathology samples obtained during surgery to establish accuracy of the blind biopsy. The reported sensitivity and specificity of the Novak catheter were 13% and 100% respectively. The specificity was reduced to 99% (no ‘perfect’ values were deemed to be plausible) and the values were converted accordingly for use in the decision tree.
The accuracy estimates, along with ranges for use in sensitivity analyses and data sources, are summarised in Table 13.
| Variable | Baseline sensitivity | Sensitivity analysis (95% CI) | Source |
|---|---|---|---|
| OPH polyps | 0.94 | 0.92 to 0.96 | SR99 |
| OPH submucous fibroids | 0.87 | 0.81 to 0.92 | SR99 |
| OPH polyps/SMF | 0.91 | 0.87 to 0.94 | Composite of polyp and SMF values |
| OPH endometrial disease | 0.78 | 0.76 to 0.80 | SR95 |
| OPH DUB | 0.89 | 0.87 to 0.90 | SR99 values reversed so for no pathology |
| TVS polyps/SMF | 0.45 | 0.32 to 0.58 | Prospective comparative study109 |
| TVS intramural fibroids | 0.95 | Prospective observational110 | |
| TVS endometrial disease | 0.57 | 0.19 to 0.90 | Prospective comparative study109 |
| TVS DUB | 0.86 | 0.82 to 0.90 | Prospective observational study111 values reversed so for no pathology |
| EBx polyps | 0.41 | 0.14 to 0.76 | Observational prospective cohort113 |
| EBx SMF | 0.13 | – | Prospective comparative study115 (NB PMB) |
| EBx polyp/SMF | 0.27 | 0.14 to 0.76 | Composite of polyp and SMF values |
| EBx endometrial hyperplasia | 0.66 | 0.47 to 0.81 | SR20 |
| EBx cancer/atypia | 0.94 | 0.84 to 0.99 | SR21 |
| Pipelle Bx endometrial disease | 0.78 | 0.62 to 0.88 | Composite of hyperplasia and cancer/atypia values |
| EBx DUB | 0.95 | 0.90 to 0.98 | Observational prospective cohort113 2 × 2 created and benign data used to calculate |
| SIS polyps | 0.86 | 0.81 to 0.91 | SR94 |
| SIS SMF | 0.87 | 0.79 to 0.92 | SR94 |
| SIS polyps/SMF | 0.87 | 0.80 to 0.92 | Composite of polyp and SMF values |
| SIS endometrial disease | 0.29 | 0.05 to 0.71 | Prospective comparative study109 |
| SIS DUB | 0.88 | 0.85 to 0.92 | SR94 values reversed so for no pathology |
False-positive rates
Although the FPRs were calculated from the specificity data (1 – specificity = FPR), the derived values could not always be used in their pure form within the decision model. This is because where a false diagnosis is made there are several possible erroneous options. However, the branches from one stem in the tree have to sum to 1. This means that all of the possible false diagnoses must have FPRs which add to 1. To overcome this we decided that if one of the erroneous diagnoses was DUB (i.e. normal), we would use the FPRs for the other pathologies and make the value of the FPR for DUB make up the remainder. When DUB was not a possible false diagnosis, we divided each FPR by the sum of them together to weight the respective values appropriately:
Example 1: let us assume that for TVS the possible false diagnoses were polyp/SMF (FPR = 0.22), endometrial disease (FPR = 0.34) and normal (FPR = 0.04). The only value which changes is the value for normal (i.e. DUB) which becomes 1–(0.34 + 0.22) = 1 – 0.56 = 0.44.
Example 2: let us assume that for TVS the possible false diagnoses are polyp/SMF (FPR = 0.22) or fibroids < 12 weeks’ size (FPR = 0.18). Both values are then divided by the combined FPRs [(0.22 + 0.18) = 0.40] to weight them so that the sum of the two values equals 1 (e.g. 0.22/0.40 = 0.55 and 0.18/0.40 = 0.45).
This rule was used consistently throughout the decision tree. Weighting the values meant that we were unable to use the reported CIs in subsequent sensitivity analyses. We calculated values to use in place of CIs by calculating beta distributions within the tree. Table 14 details the FPRs that were used within the tree as well as detail explaining how they were derived.
| Test | True diagnosis | False diagnosis | Value | Why? |
|---|---|---|---|---|
| TVS | Polyp/SMF | Fibroids < 12 weeks | 0.18 | TVS FPR for intramural fibroids |
| Endometrial disease | 0.34 | TVS FPR for endometrial disease | ||
| Normal | 0.48 | Remaining | ||
| Fibroids < 12 weeks’ size | Polyp/SMF | 0.22 | TVS FPR for polyp/SMF | |
| Endometrial disease | 0.34 | TVS FPR for endometrial disease | ||
| Normal | 0.44 | Remaining | ||
| Fibroids > 12 weeks’ size | Polyp/SMF | 0.55 | Unconditional FPRs weighted to add to 1 | |
| Fibroids < 12 weeks | 0.45 | |||
| Endometrial disease | Polyp/SMF | 0.22 | TVS FPR for polyp/SMF | |
| Fibroids < 12 weeks | 0.18 | TVS FPR for Intramural fibroids | ||
| Normal | 0.60 | Remaining | ||
| DUB | Polyp/SMF | 0.30 | Unconditional FPRs weighted to add to 1 | |
| Fibroids < 12 weeks | 0.24 | |||
| Endometrial disease | 0.46 | |||
| OPH | Polyp/SMF | Endometrial disease | 0.04 | OPH FPR for endometrial disease |
| Normal | 0.96 | Remaining | ||
| Fibroids < 12 weeks’ size | Polyp/SMF | 0.6 | Unconditional FPRs weighted to add to 1 | |
| Endometrial disease | 0.4 | |||
| Fibroids > 12 weeks’ size | Polyp/SMF | 0.6 | Unconditional FPRs weighted to add to 1 | |
| Endometrial disease | 0.4 | |||
| Endometrial disease | Polyp/SMF | 0.06 | OPH FPR for polyp/SMF | |
| Normal | 0.94 | Remaining | ||
| DUB | Polyp/SMF | 0.6 | FPRs weighted | |
| Endometrial disease | 0.4 | |||
| EBx | Polyp/SMF | Normal | 0.95 | Remaining |
| Comp hyp | 0.05 | EBx FPR for complex hyperplasia | ||
| Fibroids < 12 weeks’ size | Polyp/SMF | 0.038 | Unconditional FPRs weighted to add to 1 | |
| Comp hyp | 0.962 | |||
| Fibroids > 12 weeks’ size | Polyp/SMF | 0.038 | Unconditional FPRs weighted to add to 1 | |
| Comp hyp | 0.962 | |||
| Endometrial disease | Polyp/SMF | 0.002 | EBx FPR for polyp/SMF | |
| Normal | 0.998 | Remaining | ||
| DUB | Polyp | 1 | As no alternative disease | |
| SIS | Polyp/SMF | Fibroids < 12 weeks’ size | 0.18 | TVS FPR for intramural fibroids |
| Endometrial disease | 0.02 | SIS FPR for endometrial disease | ||
| Normal | 0.80 | Remaining | ||
| Fibroids < 12 weeks | Polyp/SMF | 0.13 | SIS FPR for polyp/SMF | |
| Endometrial disease | 0.02 | SIS FPR for endometrial disease | ||
| Normal | 0.85 | Remaining | ||
| Fibroids > 12 weeks | Polyp/SMF | 0.42 | Unconditional FPRs weighted to add to 1 | |
| Fibroids < 12 weeks’ size | 0.58 | |||
| Endometrial disease | Polyp/SMF | 0.13 | SIS FPR for polyp/SMF | |
| Fibroids < 12 weeks’ size | 0.18 | TVS FPR for intramural fibroids | ||
| Normal | 0.69 | Remaining | ||
| DUB | Polyp/SMF | 0.39 | Unconditional FPRs weighted to add to 1 | |
| Fibroids < 12 weeks’ size | 0.55 | |||
| Endometrial disease | 0.06 |
Test combinations
As the test combination trees display tests in series, no new values needed to be calculated for data accuracy for the combination trees.
Treatment satisfaction data
Levonorgestrel intrauterine system
The medical database searches identified 2987 studies using the terms ‘levonorgestrel intrauterine device’, ‘heavy menstrual bleeding’ and their associated phrases. Eighty-two studies reported data regarding the effectiveness of the LNG-IUS. They were selected based on whether or not they reported effectiveness data for the LNG-IUS in any form for any pathology. The highest quality data for each pathology were then selected and used within the analysis (see Appendix 3). The LNG-IUS works optimally when used to treat DUB and so we looked for data that reported patient satisfaction when used in women with DUB as their underlying pathology first, so that we could use this as a reference. One of the selected studies was a systematic review with IPD meta-analysis, which looked at the relative effectiveness of hysterectomy, endometrial destruction and LNG-IUS. 61 The review used 12-month follow-up data to report rates of dissatisfaction when comparing the different treatments. The dissatisfaction reported for LNG-IUS overall at 12 months was 17% (22 out of 128). We converted this to a satisfaction rate of 83% and calculated CIs from the data so that the values used for satisfaction with LNG-IUS when used to treat DUB were 0.83 (95% CI 0.76 to 0.89).
No suitable studies were identified that reported the satisfaction level of pre-menopausal women with polyps, fibroids, endometrial hyperplasia or cancer if they were treated with a LNG-IUS. Any studies reporting this outcome either were very small (< 50 patients) or had a mainly post-menopausal population. One study reported predictors of outcome for LNG-IUS and stated that small fibroids were not predictive of outcome36 and so we extrapolated from this and used the same data for fibroids < 12 weeks’ size that we used for DUB from the IPD study. 61 For polyps/SMFs and for fibroids > 12 weeks’ size, no studies were identified that reported patient satisfaction associated with the use of the LNG-IUS, and so the expert panel were asked to estimate how effective the device would be in women with theses pathologies. The values of presumed treatment satisfaction for polyps/SMFs were inconsistent, ranging from 20% to 85% with a median value of 40%. For fibroids > 12 weeks’ size the range was similarly imprecise, varying from 10% to 75% with a median value of 29%. The median values formed the point estimates and the ranges were used in the sensitivity analysis.
One study was identified that reported the regression of endometrial disease with the use of the LNG-IUS;37 however, only 37 of the women were pre-menopausal. The study reported that, at 12 months, 69 out of 80 (86%) with complex hyperplasia had regressed and that six out of nine (66%) with atypical hyperplasia had regressed. This study was reported from data collected at BWH in 2008; however, the database has continued to be updated and we were able to use up-to-date data to produce values for endometrial hyperplasia and cancer. The database records follow-up data for women who have been diagnosed with endometrial hyperplasia with and without atypia and are being treated with systemic or local progestins. Women who were being treated with a LNG-IUS for endometrial disease were identified and their 6- and 12-month follow-up data were examined. If they were still using the LNG-IUS at 12 months it was assumed that they were satisfied with it. If they had undergone hysterectomy or had the LNG-IUS removed they were counted as unsatisfied. One hundred and one pre-menopausal women, 95 with complex hyperplasia and six with complex hyperplasia with atypia, were identified in the database. Thirteen of the women with complex hyperplasia had undergone a hysterectomy before 12 months, and so 82 out of 95 (86%) women were considered satisfied. This value is consistent with the original study. 37 However, this treatment success rate is greater than the aforementioned literature-derived estimates for successful treatment outcomes in women without uterine pathology (DUB). This was considered by the expert panel to be highly unlikely, and so the value for the satisfaction rate of treatment with LNG-IUS in women with endometrial hyperplasia was reduced to 83% to make it the same value as for the DUB population. CIs were calculated for the original data but they were varied around a point estimate of 0.83 instead of 0.86. Of the six women who were treated for complex endometrial hyperplasia with atypia, three had undergone hysterectomy by 12 months, giving a satisfaction rate of 50%.
It was assumed that the patients whose underlying disease was endometrial cancer would not be satisfied with the LNG-IUS treatment, as the inappropriate treatment would mean persistence or worsening of their symptoms. In the database, the ratio of pre-menopausal women with atypical endometrial hyperplasia to pre-menopausal women with endometrial cancer was 0.59 : 0.41, and so each was multiplied by the satisfaction value to produce a composite ‘satisfaction’ value for the group ‘atypia/cancer’:
atypical hyperplasia: satisfaction rate = 0.50, prevalence = 0.59
endometrial cancer: satisfaction rate = 0.00, prevalence = 0.41
overall composite satisfaction rate is (0.50 × 0.59) + (0 × 0.41) = 0.295
value used for treatment satisfaction = 0.3.
Similarly, the value for complex hyperplasia was used proportionally with the value for ‘atypia/cancer’ to produce an overall satisfaction value for ‘endometrial disease’.
complex hyperplasia: satisfaction rate = 0.83, prevalence = 0.60
atypia/cancer: satisfaction rate = 0.30, prevalence = 0.40
overall composite satisfaction rate (0.60 × 0.83) + (0.30 × 0.40) = 0.44
value used for treatment satisfaction = 0.44.
Endometrial ablation
To populate the decision tree, values were needed for satisfaction after EA for DUB and for fibroid uteri. As EA requires women to undergo an EBx and either a TVS, SIS or OPH prior to the procedure, it was assumed that any intrauterine pathology would be picked up by one of these pre-ablation tests and then treated appropriately. Thus, outcome data for polyps/SMF and endometrial disease were not required. As second generation techniques are the most widely used destruction methods, we looked for studies that reported satisfaction after second-generation EA. Searches for EA identified 319 relevant studies, eight of which were systematic reviews, with four containing meta-analysis61,62,93,116 (see Appendix 3). Three of these studies appeared to be updated versions of the same Cochrane review and the fourth study was the IPD meta-analysis used for satisfaction with LNG-IUS. 61 The most recent Cochrane review62 and the IPD meta-analysis61 were evaluated further. The IPD study was found to include 12-month satisfaction data from a larger overall population of women. It also had the benefit of using IPD data, in keeping with our original objective, and so was selected as the preferable study. We used the reported dissatisfaction rates for second-generation EA (110 out of 1034) and converted this to satisfaction rates which we then used to calculate CIs (0.893, 95% CI 0.874 to 0.912). These values were used in the economic analysis for satisfaction after endometrial ablation for DUB.
An electronic bibliographic database search was performed (see Appendix 3) to identify studies that reported outcome after EA in the presence of fibroids. Once duplicates had been removed, 315 studies were identified, of which 17 were selected for further evaluation because they reported the use of EA in the presence of fibroids. None of the studies reported satisfaction after second generation EA in the presence of intramural or subserosal fibroids; however, one study reported that the presence of a small fibroid uterus did not increase the hysterectomy rate. 117 We extrapolated from this and used the same data for small fibroid uteri as we did for satisfaction after second-generation EA for DUB. For large fibroid uteri we asked our expert panel their estimation of satisfaction rates, 1 year post EA in the absence of published data. We used the median value of 0.575 as the point estimate and the range of values as the data for sensitivity analysis (0.075–0.85)
Hysterectomy
As we had used the IPD meta-analysis for treatment satisfaction data following the use of a LNG-IUS and after an EA, we used the same review to obtain values for satisfaction after hysterectomy for DUB. 61 The rates reported were 409 out of 432 patients satisfied which equates to a value of 0.95 and CIs of 0.93 to 0.97. The studies included in this IPD looked at HMB and included women with polyps and fibroids. Only three studies supplied data regarding treatment outcome in the presence of focal uterine pathology. The presence of endometrial polyps and fibroids was found not to be a statistically significant indicator of outcome. Thus, we assumed that satisfaction would be the same whether hysterectomy was performed for DUB, polyps/SMFs or fibroids < 12 weeks’ size and the same values were used.
For large fibroids (> 12 weeks’ uterine size), we performed database searches to identify studies reporting satisfaction after hysterectomy for fibroids. No systematic reviews were identified that examined this outcome directly. However, a study of 397 women that retrospectively followed up women who had had either hysterectomy or UAE reported that 88% of women who had undergone hysterectomy felt that their symptoms were better and that 70% would recommend their treatment to a friend. 118 A disadvantage of this study was that the mean follow-up time was 8.6 years. By scrutinising the reference list of this study, we identified two similar studies. 53,54 The first was a randomised trial of UAE versus hysterectomy, with 51 women in the hysterectomy arm. 53 Women were included if they had fibroids of at least 2 cm in diameter (no upper limit) which caused symptoms and which a clinician thought justified surgical treatment. At 12 months, 93% of women would recommend their treatment to a friend. The second study randomly allocated women with uterine fibroids up to 10 cm in size and menorrhagia to two groups. The women in group 1 were offered UAE as an alternative to hysterectomy for their fibroids and the women in group 2 were not offered the alternative and all had hysterectomy. 54 In total, 17 women underwent hysterectomy and at 6 months 88% reported that they would have the same treatment again, suggesting that they were satisfied with the surgery. 54 After evaluating the data from these studies we decided to use 0.88 as our satisfaction level as this was reported in the large retrospective study118 and supported by the smaller randomised study. 54 For the sensitivity analysis we used the proportion of women who would recommend their treatment to a friend from the two separate studies (0.70 and 0.93). 53,118
No studies were identified that reported satisfaction rates after hysterectomy for endometrial disease in pre-menopausal women. We assumed that women having hysterectomy for atypical hyperplasia or cancer would be 100% satisfied because they would be prevented from developing cancer or treated for their cancer. For sensitivity analysis we used the lowest hysterectomy satisfaction value (i.e. 0.88 for fibroids) and the highest satisfaction value (1.0 for cancer). For complex endometrial hyperplasia we decided to use the same values as for DUB because complex hyperplasia is a benign condition and so can be grouped with other benign causes of HMB without organic pathology (i.e. DUB). Secondly, we found that treatment satisfaction after LNG-IUS was the same for complex hyperplasia as for DUB and so this extrapolation regarding hysterectomy does not seem unreasonable. For endometrial disease overall, we once again used a composite value of the atypia/cancer value and the hyperplasia value. This was calculated as 0.97. For sensitivity analysis we used the lowest and highest satisfaction rates from the two categories (i.e. 0.95 for hyperplasia to 1.0 for cancer).
Polyp/submucosal fibroids removal
Satisfaction after removal of endometrial polyps and SMFs was calculated as a composite of values for the two pathologies. For endometrial polyps, two systematic reviews were selected40,41 from the 216 studies identified by database searches. Neither systematic review included any meta-analysis because of the heterogeneity of the studies. The more recent review41 identified all of the studies used in the older one40 as well as more recently conducted studies. Satisfaction with polyp removal for AUB was reported as 75–100% so we examined the high-quality studies for the most appropriate data. Three studies were prospectively conducted. 27,119,120 The first study was a cohort controlled study comparing the effectiveness of outpatient and day-case endometrial polypectomy, which included 58 women, predominantly post-menopausal. At 6 months, 34 women responded to a follow-up questionnaire that asked them about satisfaction with the treatment. Seventy-eight per cent of women in the outpatient group and 88% women from the inpatient group were satisfied with their treatment, which equates to an 82% satisfaction rate overall. 27 The second trial was a cohort study which looked at 21 women with abnormal menstrual bleeding and evaluated the change in their symptoms following endometrial polypectomy. 119 At 6 months there was a statistically significant reduction in menstrual blood loss (p < 0.001). Thirteen of the women had HMB, which persisted in 10 (77%) of them at 6 months, although they had a statistically significant reduction in pictorial blood loss assessment chart (PBAC) scores (p = 0.001). Overall, 86% of the women felt that they were cured or that their symptoms had been relieved by endometrial polypectomy. 119 The third study randomised 150 pre-menopausal women with AUB to polypectomy or conservative management and found no difference in PBAC scores at 6 months. There was a significant difference between the groups for some of the secondary outcome measures including mean periodic blood loss measured by visual analogue scale (p = 0.02) and occurrence of gynaecological symptoms (intermenstrual bleeding or pain); however, satisfaction was not reported and so could not be used. 120 As the two comparative studies reporting satisfaction were small, we combined the populations and meta-analysed the data using Excel and RevMan to calculate a satisfaction value of 0.86.
For satisfaction with transcervical resection of submucosal fibroids (TCRF) we identified 32 studies from medical database searches. Nine studies reported satisfaction after transcervical myomectomy but seven were rejected because they had fewer than 40 patients121,122 or reported satisfaction data from more than 3 years after treatment. 44,123–126 The two remaining studies42,46 were both prospectively conducted and had populations of over 100 women, with more than 90% being pre-menopausal. The first study examined resection of SMFs using a resectoscope under general anaesthesia (90%) or local anaesthesia with sedation. The average follow-up period was 2.3 years. Satisfaction with treatment was reported as 71.4%. 42 The second study examined removal of fibroids using a bipolar intrauterine operating system and performed 38% of them under local anaesthesia. The average follow-up period was 2.6 years. Satisfaction with treatment was reported as 86%. 46 In our decision tree we used resection for SMF removal, as it is the gold standard. We also have stated that TCRF would be performed as day-case surgery, under general anaesthesia. Therefore, we chose the satisfaction rate from the first study described, as it was the most appropriate for our analyses. 42
The data from the polypectomy meta-analysis and the selected SMF resection study were used to create a value for the combined diagnostic group ‘polyps/SMF’ weighting the data according to the disease prevalence within the group (50% polyps, 50% SMFs). The value calculated was 0.79 and was used as the satisfaction value for removal of polyps and SMFs.
To account for removal of erroneously diagnosed focal pathology (i.e. when no intrauterine pathology was present so normal endometrium is being resected) we looked for satisfaction rates for D&C as the patients will essentially be having normal endometrial tissue removed. Two hundred and seventy-four studies were identified from database searches but none reported patient satisfaction following the procedure. We identified one study which reported relief of HMB after D&C which stated that the menstrual blood loss was reduced for the first month but returned to pre-operative levels after that. 127 This does seem plausible as D&C is primarily a diagnostic procedure and removing the superficial endometrium will only be therapeutic until it grows back. As we had no additional data, we used this value and allocated a satisfaction score of zero for satisfaction following ‘virtual removal’ when no intrauterine lesion was present.
Myomectomy and uterine artery embolisation
The alternative analysis which looked at treating women who wished to preserve their fertility included UAE and myomectomy as treatment options. Searches were performed to look at patient satisfaction after both treatments at 1 year (see Appendix 3). For myomectomy, one systematic review128 was identified and selected from 120 studies and for UAE, one systematic review129 was identified and selected from 169 studies. Both systematic reviews compared UAE with surgical treatments (hysterectomy and myomectomy)128,129 and both found no difference in patient satisfaction or quality of life between UAE, hysterectomy and myomectomy. Therefore, the same value for satisfaction after hysterectomy was used for UAE and myomectomy (0.88).
Costs
Cost values were mainly taken from Healthcare Resource Group (HRG) codes for 2009–10. 130 We used the ‘national average unit cost’ as our cost and the reported upper and lower quartile values for sensitivity analysis. The diagnostic and treatment codes for hysteroscopy include the cost of the consultation as well as any diagnostic or therapeutic procedures but the other diagnostic test codes equate to just the cost of the test. Within our decision tree, patients could undergo multiple diagnostic tests and treatments at one appointment. However, if we then used the relevant HRG code costs for each aspect we would be including the cost of the consultation multiple times. In order to give an accurate reflection of the additional costs of multiple tests including hysteroscopic treatment, we removed the cost of consultation from the diagnostic hysteroscopy costs and also for therapeutic hysteroscopy codes. We subtracted the cost of consultation and diagnostic hysteroscopy so that the value remaining was the additional cost of performing the therapeutic hysteroscopic procedure alone. We could then add up the relevant costs depending on the tests and treatments that the patients had. For example:
cost of new gynaecology consultation = £139
cost of OPH (consultation + diagnostic OPH) = £216
cost of OPH polypectomy = £263 (consultation + diagnostic OPH + polypectomy)
cost of transcervical resection of fibroid = £1344
cost of TVS (test only) = £55
cost of GnRH analogues = £226.
So the cost of the diagnostic hysteroscopy is actually £216 – £139 = £77 and the additional cost of hysteroscopic polypectomy is £263 – £216 = £47.
If a woman (assigned to the TVS diagnostic pathway) comes to clinic and has a diagnosis of ‘polyp/SMF’ made by TVS and then goes on to have removal of the lesion, the costs will equate to all the women having a consultation and a scan, half of them having an OPH polypectomy (ratio of polyps to SMFs, 50 : 50) and half of them returning at a later date for a scheduled transcervical resection of fibroid (with 70% of these women having endometrial pre-treatment with GnRH analogues) (see Chapter 2, Methods, Clinical assumptions)
However, if the HRG code was used for hysteroscopic polypectomy alone the cost would be considered as £263 or if the code for TCRF was used it would be £1344. Our derived estimate for resection of uterine polyps or SMFs of £1007.10 was felt to be reasonable and more realistic by the expert clinical panel. For the cost of LNG-IUS and GnRH analogues we used the costs reported in the British National Formulary (BNF). 131 The cost of UAE could not be identified in the HRG codes so the value used in the decision tree came from the REST study53 as reported and used by NICE in an economic analysis for treatment of fibroids in their HMB guideline. 11 Although this cost was published in 2007, the cost stated for hysterectomy was comparable with the cost stated in the HRG codes 2009–10 (£2566 vs. £2961) which are used in this economic analysis; therefore, no adjustment was made for inflation. For the cost of a GP appointment we referred to data from the Personal Social Services Research Unit (PSSRU). 132 Table 15 details all of the costs, ranges and sources of data.
| Variable | Decision tree name | Cost (£) | Lower quartile | Upper quartile | Explanation |
|---|---|---|---|---|---|
| Consultations | |||||
| GP visit | cGP_visit | 36 | PSSRU (www.pssru.ac.uk/uc/uc2010contents.htm).132 11-minute appointment. Including direct staff | ||
| New gynaecology outpatient appointment | cGynaeNew | 139 | 111 | 161 | 502 HRG consultant service code |
| Follow-up gynaecology outpatient appointment | cGynaeFU | 97 | 73 | 109 | 502 HRG consultant service code |
| Diagnostic tests | |||||
| Diagnostic OPH | cOPH | 77 | 43 | 111 | MA10Z HRG less cost of new gynaecology outpatient appointment |
| TVS | cTVS | 55 | 40 | 66 | HRG code RA23Z |
| Endometrial biopsy | cEBx | 34 | 16 | 34 | HRG code DAP824 |
| SIS | cSIS | 71 | 54 | 83 | HRG code RA24Z |
| Confirmatory test | cConfirmatory_test | 115.72 | 77.56 | 130.66 | (0.91 × cEBx) + (0.09 × cDandC) |
| Treatments | |||||
| LNG-IUS fitting | cLngIUS_fitted | 85.66 | |||
| Endometrial polypectomy | cPolypectomy | 47 | 0 | 120 | MA12Z HRG less the cost of diagnostic hysteroscopy MA10Z |
| Transcervical resection of fibroid with D&C cost | cTCRF | 1344 | 981 | 1541 | HRG code MA09Z |
| Transcervical resection of fibroid without D&C cost | cTCRF_noDandC | 401 | 281 | 433 | HRG code MA09Z less the cost of D&C MA10Z |
| Use of GnRH analogue | cGnRHanalogue | 225.73 | BNF131 | ||
| Hysterectomy for benign disease | cHysterectomy | 2961 | 2346 | 3406 | HRG code MA07D |
| Hysterectomy for malignant disease | cHysterectomy_malignant | 3898 | 3052 | 4464 | HRG code MA06Z |
| Second laparotomy after cancer diagnosis to remove ovaries, lymph nodes, etc. | cReturn_for_BSO | 3898 | 3052 | 4464 | HRG code MA06Z |
| EA | cEndoAblation | 896 | 697 | 1024 | HRG code MA12Z |
| D&C | cDandC | 942 | 700 | 1108 | HRG code MA10Z |
| Rescue treatment | cRescue_Treatment | 3136 | 2493 | 3606 | cGP_visit + cGynaeNew + cHysterectomy |
| Myomectomy | cMyomectomy | 2961 | 2346 | 3406 | HRG code MA07D |
| UAE | cUAE | 1685 | 1465 | 1905 | REST study53 |
Post-menopausal bleeding model
Construction of decision model for the diagnosis and treatment of post-menopausal bleeding
Decision-analytic model
A decision-analytic approach was used to evaluate the outcomes of diagnostic strategies for investigating PMB. Model assumptions and input parameters were based on the earlier reported model based study by Clark et al. 69 However, in this analysis, instead of duration of survival, 5-year survival was used as the primary health outcome.
The diagnostic strategies evaluated included EBx, TVS and OPH used alone and in combination, as well as individualised strategies integrating patient characteristics with TVS and the reference case of withholding immediate investigation at initial presentation and instituting diagnostic work-up only if PMB recurred. The original analysis used D&C as the reference case. This strategy was kept in the updated analysis as an alternative reference case because it is still widely employed in the UK for investigation of PMB, albeit usually following investigative imaging. This resulted in 12 outpatient strategies for the initial clinical investigation of women with PMB for endometrial cancer. These strategies were:
-
no initial evaluation
-
history only
-
TVS 5 mm cut-off
-
selective TVS with history
-
history + TVS
-
EBx
-
OPH
-
TVS + EBx
-
TVS + OPH
-
EBx + OPH
-
TVS + EBx + OPH
-
D&C.
The constructed decision trees are detailed in Appendix 4.
The decision model133,134 was constructed to reflect current service provision. The outpatient tests available for evaluation of the uterus and endometrium have been described in Table 2. The current recommendation for investigation of PMB is to use TVS with a 5-mm endometrial thickness threshold to define for abnormality as the first-line test. 66,69,135–137 Abnormal TVS requires subsequent outpatient EBx to obtain tissue for histological analysis. 66,69,135–137 Despite these recommendations, practice remains variable for a number of reasons including available skills and clinical preference. Perhaps more importantly, technological advances, namely the miniaturisation and portability of imaging technologies, have facilitated a ‘one stop’ approach to diagnosis. This means efficient same-day testing carried out by a senior gynaecologist within a single outpatient clinic setting without the traditional dependence upon referral to other departments with the requirement for multiple appointments and delayed diagnosis. 18,138–141 Therefore, a model was developed that assessed all available tests used either alone or in combination, compared with the baseline reference option of ‘no initial testing’, that is to say awaiting re-presentation because of recurrent or persistent PMB.
The prime reason for investigating women promptly with PMB is to exclude endometrial cancer,142 which is present in between 5% and 10% of cases. Individual patient risk factors for the development of endometrial cancer have been recognised for some time and these include obesity, diabetes and advancing age. 75–81 There has been recent interest in the integration of information easily obtainable from the preceding clinical history and examination into the overall diagnostic work-up. 64,73,74 Therefore, two multivariable prediction models that had previously been developed to estimate the risk of endometrial cancer in patients with PMB, taking into account clinical characteristics,73 were integrated into the diagnostic work-up. These models were developed with data obtained from a prospective cohort study of 614 women presenting with PMB in one university hospital and seven teaching hospitals in the Netherlands. 79 These two models were used in three different diagnostic strategies for PMB to examine whether or not integration of germane clinical information with the currently recommended strategy of first-line testing with TVS66,69 improves cost-effectiveness. These integrated strategies were:73
-
‘History only’ – probability estimates based on characteristics of the women. If the probability of (pre-)malignancy exceeded 4%, EBx was performed. In this strategy TVS is not performed.
-
‘Selective TVS with history’ – recourse to TVS if the probability of endometrial cancer, based on characteristics of the women, exceeded 4%. EBx is then performed if the TVS-derived endometrial thickness exceeded 4 mm.
-
‘History and TVS’ – all women have a TVS and the probability of cancer estimate is generated based on both characteristics of the women and the TVS results. EBx is performed when the combined TVS and history probability of cancer exceeds 4%.
These models have been externally validated using two prospectively collected databases:
-
Breijer database: between January 2009 and April 2011, all women presenting with PMB at the TweeSteden Hospital, Tilburg, the Maxima Medical Center, Veldhoven, and the St. Antonius Hospital, Nieuwegein, in the Netherlands, were included in a prospective study. Women with a history of hysterectomy were excluded. Age, body mass index (BMI), parity, years since menopause, HRT, presence of hypertension, diabetes, use of anticoagulants and endometrial thickness measured by TVS were recorded. If double endometrial thickness was > 4 mm, EBx was performed.
-
Valentin database: between November 2002 and June 2009, all women presenting with PMB at the Skåne University Hospital, Malmö, Sweden, at the PMB clinic, were included in a prospective cohort study. Age, age at menopause, parity, HRT, weight, height, hypertension, diabetes, current use of anticoagulants and endometrial thickness measured by TVS were recorded. If double endometrial thickness was ≥ 4.5 mm, EBx was taken.
Patients from both databases were asked to contact the hospital if they had further episodes of bleeding. The patients from the Breijer database were followed up by collecting data from patient records, and, in the Valentin database, cases were matched to the national cancer register. For the purpose of the study, all patients with a thin endometrium without EBx and without recurrent bleeding were considered negative for endometrial cancer.
The ability of the models to discriminate between women with and without endometrial cancer was assessed for both the ‘patient characteristics’ (history only) and the ‘patient characteristics and TVS’ (the two TVS strategies) models. The two samples available for validation consisted of 413 and 622 non-HRT-using patients, respectively. Table 16 shows the characteristics of patients in the two databases. Age, time since menopause, anticoagulant use and BMI were significantly different between the two validation populations.
| Characteristic | Valentin (n = 413) (SD) | Breijer (n = 622) (SD) | p-value |
|---|---|---|---|
| Age (years) | 67.0 ± 12.1 | 62.2 ± 10.2 | < 0.01a |
| Diabetes mellitus | 64 (15.5) | 85 (13.7) | 0.42b |
| Hypertension | 164 (39.7) | 227 (36.5) | 0.30b |
| Use of anticoagulants | 76 (31.9) | 114 (18.3) | < 0.01b |
| BMI (kg/m2) | 27.9 ± 6.6 | 29.9 ± 7.9 | < 0.01a |
| Time since menopause (years)c | 15 (5–27) | 6 (2–14) | < 0.01d |
| Nulliparity | 49 (12.0) | 69 (13.9) | 0.40b |
| Endometrial thickness (mm)c | 5.0 (3.1–12.1) | 5.7 (2.5–10.0) | 0.05d |
| Endometrial cancer | 54 (13.1) | 75 (12.1) | 0.63b |
Figure 2 shows the receiver operating characteristic (ROC) curves for the two prediction models in both validation data sets. The area under the ROC curve for the patient characteristics only model was 0.68 (95% CI 0.62 to 0.75) and 0.69 (95% CI 0.64 to 0.74) in the Breijer and Valentin populations, respectively, and the area under the ROC curve for the patient characteristics and TVS model was 0.87 (95% CI 0.84 to 0.90) and 0.89 (95% CI 0.85 to 0.91), respectively, in the Breijer and Valentin populations. As a reference, Figure 2c shows the ROC curve of the two models in the development database. The results show that the existing multivariable models maintained their diagnostic accuracy in two independent patient cohorts.
FIGURE 2.
Receiver operator characteristic curves of the patient characteristics only model and the patient characteristics and TVS model for PMB. (a) Validation database I. Breijer; (b) validation database II. Valentin; and (c) development database Van Doorn.
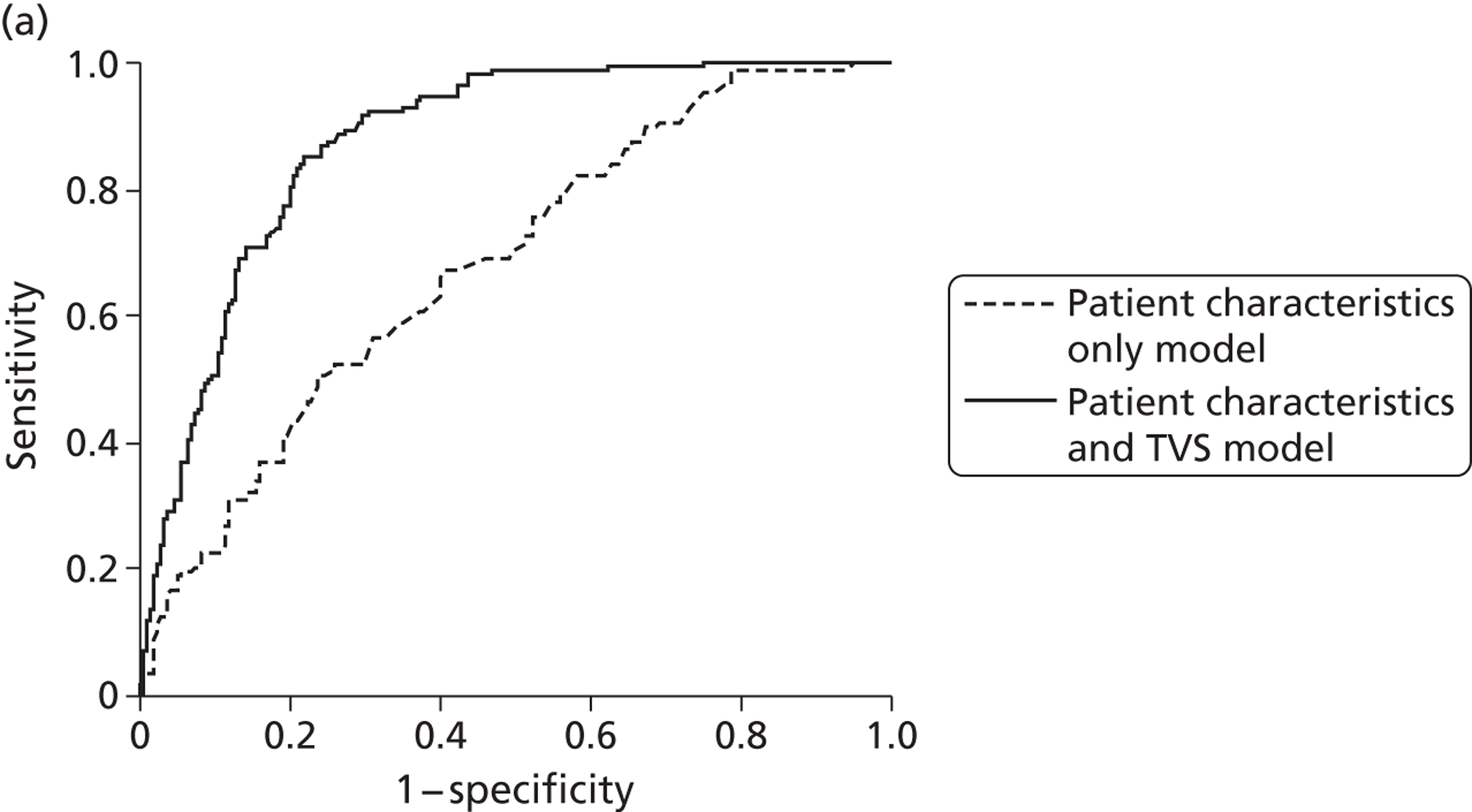
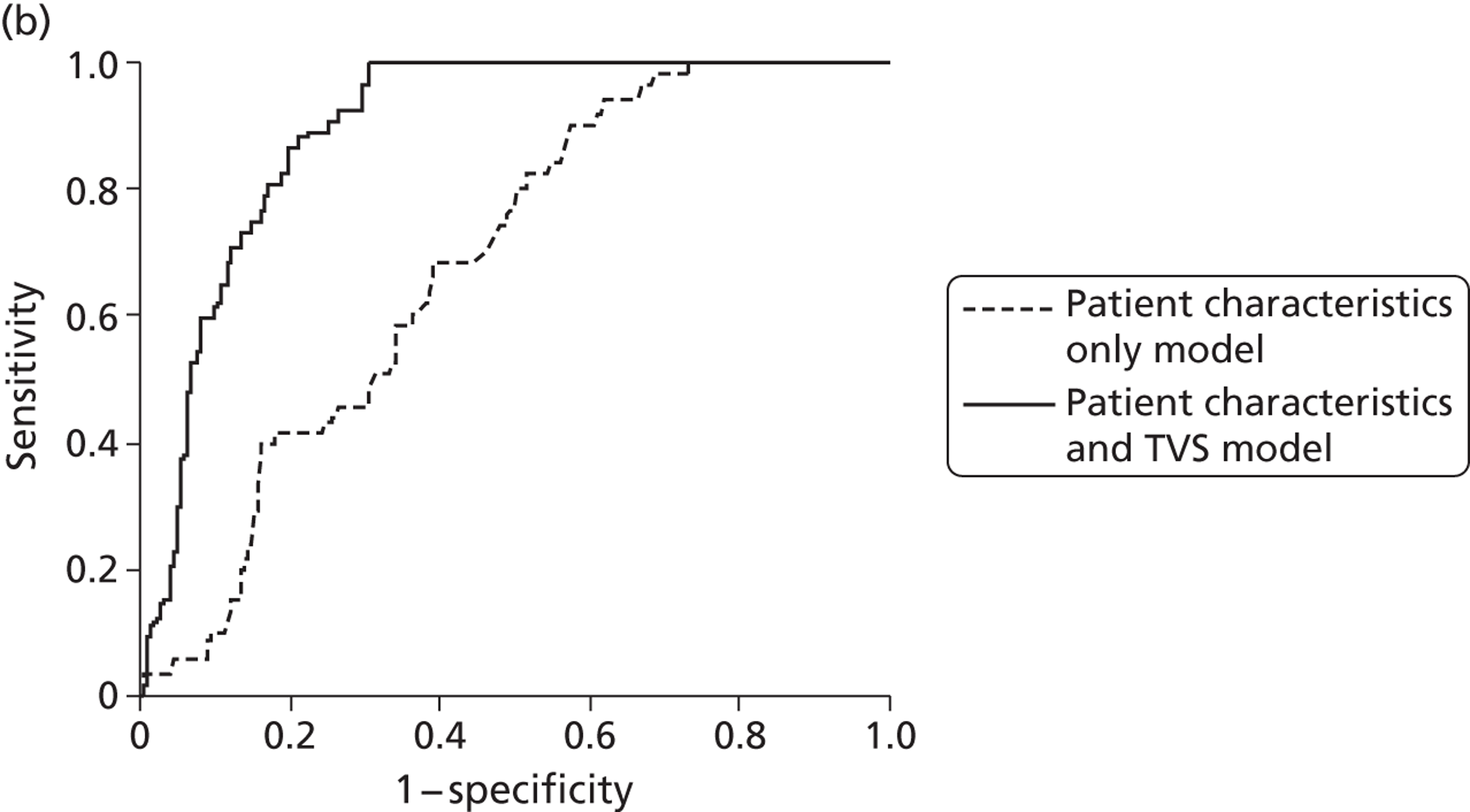
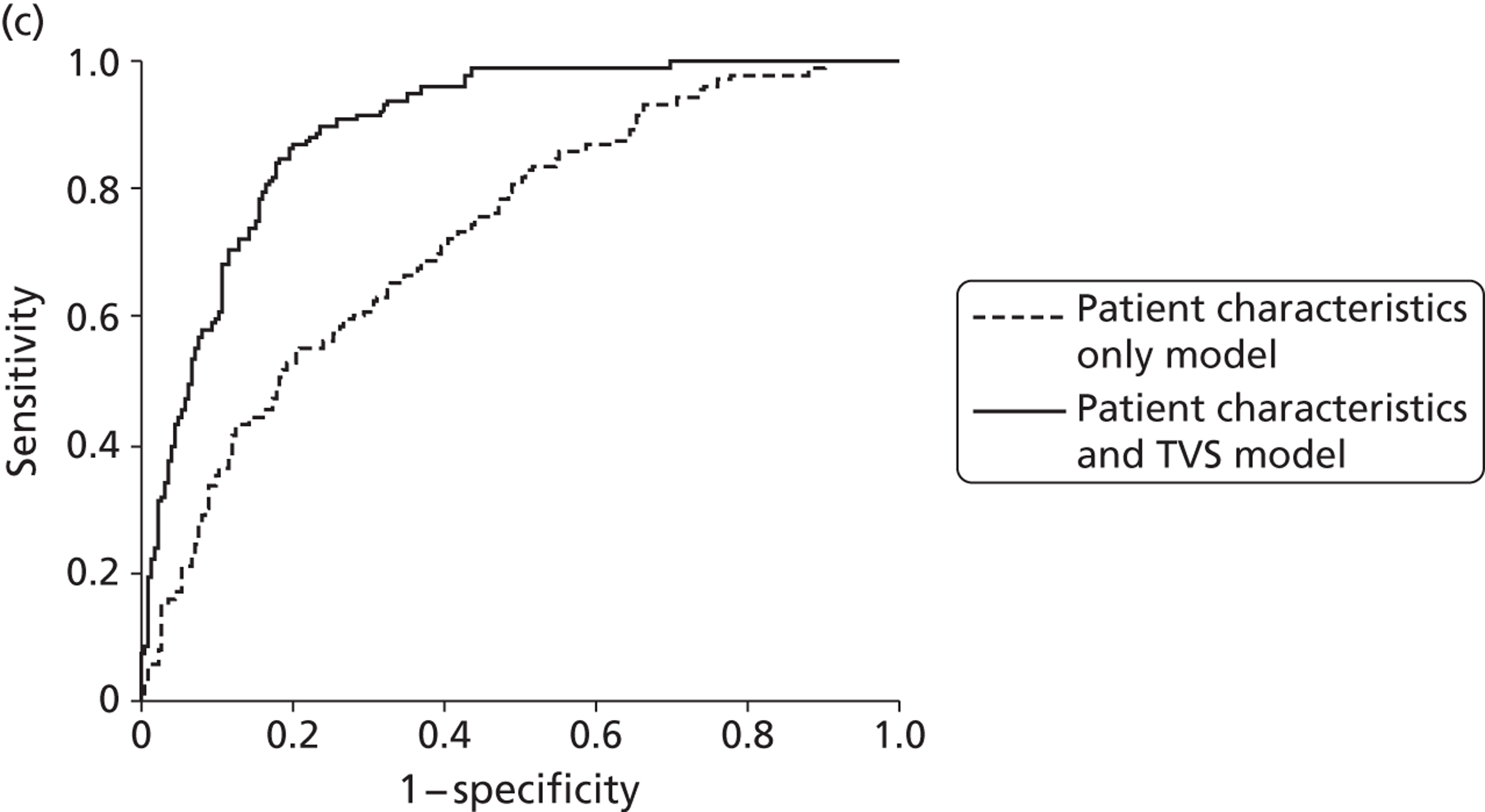
Decision Analysis TreeAge Pro 2009 Suite software (TreeAge Software Inc., Williamstown, MA, USA: www.treeage.com) was used to specify the decision model.
Data sources and modelling assumptions for decision analysis
The modelling assumptions were as previously reported in an earlier analysis. 69 The assumptions of the expert panel consulted at that time were assumed to still be valid and so they were not readdressed by the new panel of gynaecologists who had advised on the HMB model. It was assumed that the hypothetical presentation with PMB represented the first episode. No post-menopausal woman was assumed to be less than 45 years old and no other significant aetiology (e.g. other genital tract malignancy) was considered. The woman was considered to be otherwise healthy with a normal age-adjusted life expectancy. The probability of endometrial cancer in women presenting with PMB is between 5% and 10%. 64,74 A 5% prevalence of malignant disease was used for the base-case analysis.
All assumptions regarding the clinical setting and care pathways were the same as for the HMB analysis.
Diagnostic tests
Data for failure rates and estimates of diagnostic accuracy were obtained from high-quality published systematic quantitative reviews of the diagnostic literature for EBx, USS and OPH21,84,95 (Table 17). Failure rates for initial strategies utilising test combinations were estimated by the consensus panel based on the definition of a failed strategy as any test making up the strategy failing and on available failure rate data from individual tests. 21,84,95,143 Similarly, failure rates were also adjusted for tests performed in a diagnostic strategy conditional on the success of preceding tests. 64,144 As over 95% of women with endometrial cancer present with PMB,64 it was assumed that all women who were erroneously discharged following the initial presentation (i.e. false negatives) remained symptomatic. The interval to re-presentation was thus taken to be short, and all these women were then assumed to undergo reinvestigation with all outpatient tests where perfect test success and accuracy was assumed.
| Variable | Baseline | Sensitivity analysis (95% CI) | Source |
|---|---|---|---|
| Failure rates | |||
| EBx | 0.12 | 0.09 to 0.15 | SR21 |
| TVS | 0.0 | 0.0 to 0.02 | SR84 |
| OPH | 0.05 | 0.04 to 0.07 | SR95 |
| Ultrasound scan + OPH | 0.04 | 0.03 to 0.06 | EP |
| Ultrasound scan + EBx | 0.12 | 0.09 to 0.17 | EP |
| Ultrasound scan + EBx+ OPH | 0.12 | 0.09 to 0.17 | EP |
| EBx after successful OPH | 0.07 | 0.05 to 0.10 | EP |
| EBx after successful ultrasound scan | 0.12 | 0.09 to 0.15 | EP |
| Complication rates | |||
| Outpatient diagnostic procedures (EBx, TVS, OPH) | 0 | 0 | SRs21,84,95,143 |
| TPRs | |||
| EBx | 0.94 | 0.84 to 0.99 | SR21 |
| TVS scan 5 mm | 0.97 | 0.94 to 0.98 | SR84 |
| OPH | 0.86 | 0.8 to 0.89 | SR95 |
| D&C | 0.96 | 0.82 to 1.00 | EP |
| History only | 0.99 | 0.97 to 1.00 | Meta-regression73 |
| History and TVS | 0.99 | 0.96 to 1.00 | Meta-regression73 |
| Conditional TPRs | |||
| EBx if OPH positive | 0.94 | 0.93 to 0.97 | EP |
| EBx if ultrasound positive | 0.94 | 0.94 to 0.95 | EP |
| EBx if history only positive | 0.94 | 0.93 to 0.97 | Meta-regression73 |
| EBx if history and TVS positive | 0.94 | 0.94 to 0.95 | Meta-regression73 |
| OPH if EBx negative | 0.86 | 0.83 to 0.87 | EP |
| OPH if ultrasound positive | 0.86 | 0.86 to 0.87 | EP |
| Ultrasound scan 5 mm if EBx negative | 0.97 | 0.80 to 0.99 | EP |
| Ultrasound scan 5 mm if OPH negative | 0.97 | 0.91 to 0.99 | EP |
| FPRs | |||
| EBx | 0.01 | 0.0 to 0.02 | SR21 |
| Ultrasound scan 5 mm | 0.45 | 0.43 to 0.47 | SR143 |
| OPH | 0.01 | 0.0 to 0.06 | SR95 |
| D&C | 0.01 | 0.0 to 0.03 | EP |
| History only | 0.80 | 0.72 to 0.87 | Meta-regression73 |
| History and TVS | 0.41 | 0.39 to 0.44 | Meta-regression73 |
| Probability of stage II–IV (re-presentation) | 0.35 | 0.3 to 0.6a | EP |
| Prevalence | 0.05 | 0.03 to 0.10 | PL64 |
| Surgical stage at hysterectomy (FIGO) | |||
| Probability of stage I (first presentation) | 0.7 | 0.6 to 0.8a | FIGO85 |
| Probability of stage II–IV (first presentation) | 0.3 | 0.2 to 0.4a | FIGO85 |
| Probability of stage I (re-presentation) | 0.65 | 0.4 to 0.7a | EP |
| 5-year survival rates | |||
| Stage I | 0.89 | FIGO85 | |
| Stage II–IV | 0.54 | FIGO85 | |
No serious complications were assumed to be associated with any of the ambulatory procedures (ultrasound, hysteroscopy and EBx) based on evidence from systematic reviews of the available literature. 21,84,95,143 Mortality rates were assumed to be negligible for all the diagnostic tests. 21,84,95,143
Treatment
For the base-case analysis, it was assumed that all women not discharged underwent initial treatment by TAH and bilateral salpingo-oophorectomy with or without pelvic node sampling (i.e. all were fit for surgery and none had primary radical radiotherapy). All women were therefore assumed to be surgically staged. 85 There is some variation in practice in the treatment of endometrial cancer regarding the relative roles of surgery and radiotherapy/chemotherapy. 85,145 The treatment pathways in this model were based on published recommendations and reports of current practice. 65,146 All epidemiological statistics relating to endometrial cancer were taken from the FIGO results of treatments of gynaecological cancers. 85 For the base-case analysis, the cost of treating a woman correctly diagnosed with endometrial cancer on first presentation was based on the assumption that 70% of such women had localised (FIGO stage I) disease and 30% advanced (FIGO stages II–IV) disease. 85 To account for delayed diagnosis experienced by women with endometrial cancer who were erroneously discharged initially (false negatives) it was estimated that this group of women had a 5% increased probability of advanced-stage endometrial cancer (stage II–IV) in the absence of relevant data (see Table 17). Those with advanced disease (stages II, III or IV) underwent radiotherapy (adjuvant/palliative) and/or chemotherapy. 65 Women with stage IC disease or poorly differentiated (histological grade 3) stage IA or IB disease were assumed to have adjuvant radiotherapy. 65 The proportion of women undergoing additional non-surgical treatment is shown in Figure 3.
FIGURE 3.
Decision-analytic model (common pathway for further treatment of endometrial cancer following initial hysterectomy).
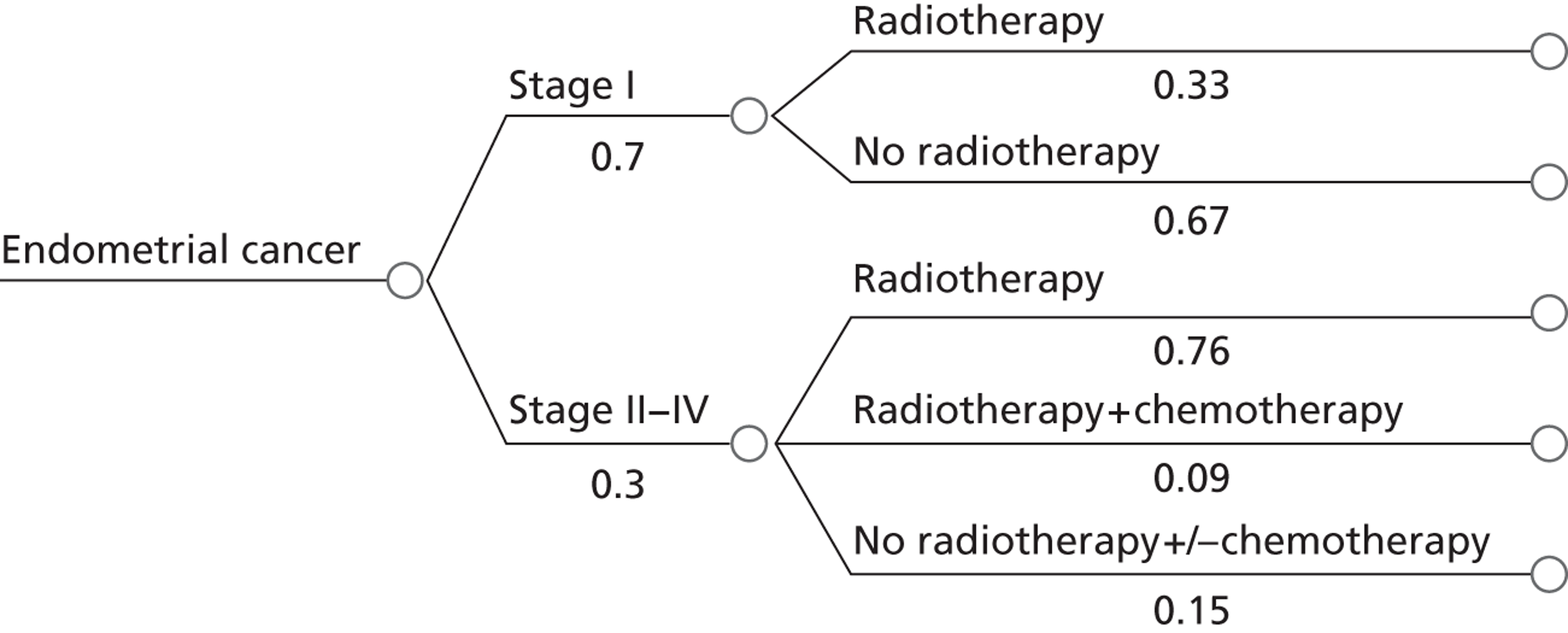
Standardised radiotherapy and chemotherapy regimens were assumed regardless of disease stage; radiotherapy consisted of a 5-week course of external beam radiotherapy giving a total of 25 fractions. Chemotherapy consisted of standard cytotoxic cycles. 65,146 Compliance with treatment was assumed to be 100%. The 5-year survival rates were assumed to be 89% for stage I disease and 54% for advanced (stage II–IV) disease. 85
Cost data
The diagnostic codes and costs used for single and combination testing were as described for investigating HMB (see Table 15). When women re-presented after being wrongly diagnosed as not having endometrial cancer, the cost allocated to their attendance was the sum of a new gynaecology outpatient appointment and investigations with TVS, OPH and EBx. All modelled costs are incurred in the first year. Table 18 details the costs used within the PMB analysis for re-presentation (cRep) and treatments for endometrial cancer.
| Variable | Decision tree name | Cost (£) | Explanation |
|---|---|---|---|
| Consultations | |||
| Re-presentation | cRep | 305 | cGynaeNew + cOPH + cTVS + cEB |
| Treatments | |||
| Hysterectomy for malignant disease | cTAH | 3898 | HRG code MA06Z |
| Radiotherapy | cRadiotherapy | 2182 | HRG code SC23Z multiplied by 25 |
| Chemotherapy | cChemotherapy | 3200 | HRG code SB13Z plus 5 × HRG code SB15Z |
| Treating stage I endometrial cancer | cTreatEC_SI | (0.33 × cRadiotherapy) | |
| Treating stage II–IV endometrial cancer | cTreatEC_SII_IV | (0.76 × cRadiotherapy) +[0.09 × (cRadiotherapy + cChemotherapy)] | |
| Treating endometrial cancer diagnosed immediately | cCancerIMM | pStageI × cTreatEC_SI+[(1 – pStageI) × cTreatEC_SII_IV] | |
| Treating endometrial cancer diagnosed after a delay | cCancerDEL | [(pStageI – pUpstage) × cTreatEC_SI]+[(1 – pStageI + pUpstage) × cTreatEC_SII_IV] | |
Clinical outcomes
The effectiveness of each competing diagnostic strategy was determined by comparing survival using the outcome measure 5-year survival. We differentiated between expected survival of women where malignancy is immediately detected and treated appropriately and women where the first diagnostic procedure fails to detect endometrial cancer, after which survival decreases due to the delayed diagnosis. The extent to which survival decreases is based on an estimated increase in disease stage from local (FIGO stage I) to advanced (FIGO stages II–IV)147 endometrial cancer and associated 5-year mortality rates. 85 The costs in terms of 5-year survival and average cost-effectiveness ratios (cost per additional woman surviving 5 years) were determined for each diagnostic strategy.
Methods for the cost-effectiveness analysis
For each type of analysis, a decision tree was constructed using TreeAge Pro 2009. Model inputs included probabilities and costs. Effectiveness was measured in terms of positive outcomes: patient satisfaction for HMB and 5-year survival for PMB. Therefore, each branch of the tree had an effectiveness outcome of 1 for a positive outcome and 0 if otherwise. Costs were unit costs for the various tests and treatments and were therefore treated as known with certainty, while probabilities depended on data and were treated as uncertain, to be varied in probabilistic sensitivity analysis (PSA). Note that the uncertainty in overall effectiveness for a given strategy is fully accounted for in the uncertainty in the probabilities, so there is no need to vary the outcome parameters.
The model was first run using the point estimates of the branch probabilities. The results, known as ‘base case’ results, are shown in terms of mean costs and effectiveness (overall proportion of positive outcomes) for each strategy modelled. These are tabulated and shown in a cost-effectiveness plane, with the mean cost shown on the vertical axis and the mean effectiveness shown on the horizontal axis. In some cases, a further plot was made of selected strategies to show more clearly the relationship between points that were close together on the first graph.
Any strategy which has greater cost and worse effectiveness than some other strategy is said to be simply dominated. Such a strategy can be excluded from consideration. An ICER can be calculated between any two non-dominated strategies. The ICER is calculated by dividing the difference in cost by the difference in effectiveness. If the ICER is less than the maximum willingness to pay (WTP) for an additional positive outcome, then the more effective strategy can be said to be cost-effective relative to the other strategy.
There is a further reason for excluding strategies, known as extended dominance. This can apply only when there are three or more non-dominated strategies. In this case, two different strategies (incorporating a cheaper but less effective strategy and a more effective, more costly strategy relative to a third strategy) can be mixed together, with a proportion of patients getting one or other of the strategies such that the third strategy now becomes dominated (i.e. is more expensive and less effective) than the blended strategies. Suppose that A, B and C are non-dominated strategies in order of increasing cost. As they are non-dominated, they must also be in order of increasing effectiveness. Now suppose that the ICER of B over A is higher than the ICER of C over A. Then, if B is cost-effective compared with A, so also must C be cost-effective compared with A and B. In such a case, there is no value of WTP per positive outcome at which B will be the preferred strategy, and the strategy B can be excluded by extended dominance. Extended dominance can be seen on a cost-effectiveness plane. The point for strategy B will be above the straight line joining the points for strategies A and C.
Once all dominated strategies have been excluded, whether for simple or extended dominance, the remaining strategies are potentially cost-effective. Which will be preferred depends on the WTP for an additional positive outcome.
To test for the effect of uncertainty in the model inputs, two types of sensitivity analysis were used: probabilistic and deterministic.
In PSA, probability distributions are placed around the point estimate for each model parameter. If there is correlation between the uncertainties, joint or conditional distributions may be used. For the models in this report, beta distributions were used to represent the uncertainty around the branch probabilities. The beta distribution is the standard distribution for a proportion. It has two parameters, a and b, with mean a/(a + b) and variance essentially decreasing as a and b increase.
For individual parameters of the models, the information available was in the form of a point estimate and a 95% CI. In all cases, the beta distribution was selected with mean equal to the point estimate. Usually the distribution also matched the width of the CI, but there were two main exceptions to this:
-
If the point estimate is either 0 or 1, then it is not possible to find a beta distribution with that mean. While it can be argued that the true mean estimate of the probability should be strictly between 0 and 1, taking any actual numerical value would risk overcompensating. Accordingly, it was decided to treat such probabilities as fixed, thereby preserving the mean but slightly underestimating the uncertainty in the model.
-
If either parameter a or b is less than 2, the beta distribution gives an unreasonably high proportion of values very close to the extreme values 0 or 1. To avoid this, in such cases the values of a and b were increased to preserve the mean of the distribution but ensure that both values were at least 2. Again, this slightly underestimates the overall uncertainty.
In some cases, only a point estimate was available. In such cases, it is not appropriate to assume that the value is fixed. Instead, the widest possible uncertainty was modelled subject to the constraint that both parameters a and b should be at least 2, for the reasons given in the previous paragraph.
In the HMB analysis of SIS for detecting fibroids, point estimates of true-positive rates (TPRs) and FPRs were assumed to be the same as for TVS. In these cases, independent samples were taken from beta distributions with the same parameters, a and b.
As the costs in the model are all unit costs of specific procedures, these were treated as fixed, and the only parameters to be varied were the probabilities in the tree, which are proportions of patients expected to follow each branch.
When the models were run for PSA, 1000 replications were made, sampling from distributions for all branch probabilities simultaneously. It is generally accepted that 1000 replications are sufficient to give a clear picture of the uncertainty.
The parameters for this beta distribution used for the PSA of the HMB tree are shown in Table 19 and the parameters for the PMB tree are shown in Table 20.
| Parameter | aa | ba | Low | High |
|---|---|---|---|---|
| Prevalence of disease | ||||
| EndometrialDisease | 5 | 95 | 0.02 | 0.10 |
| FibroidLarge | 6 | 94 | 0.02 | 0.11 |
| FibroidSmall | 19 | 81 | 0.12 | 0.27 |
| PolyporSMF | 38 | 62 | 0.29 | 0.48 |
| Success rates | ||||
| EBx | 728 | 72 | 0.89 | 0.93 |
| OPH | 67.9 | 2.1 | 0.92 | 1.00 |
| SIS | 1520 | 80 | 0.94 | 0.96 |
| TVS | 198 | 2 | 0.97 | 1.00 |
| TPRs | ||||
| ED_EBx | 28.86 | 8.14 | 0.64 | 0.90 |
| ED_OPH | 1248 | 352 | 0.76 | 0.80 |
| ED_SIS | 2.03 | 4.97 | 0.05 | 0.65 |
| ED_TVS | 3.249 | 2.451 | 0.19 | 0.90 |
| Fibroids_SIS | 38 | 2 | 0.87 | 0.99 |
| Fibroids_TVS | 38 | 2 | 0.87 | 0.99 |
| Normal_EBx | 95 | 5 | 0.90 | 0.98 |
| Normal_OPH | 1424 | 176 | 0.87 | 0.90 |
| Normal_SIS | 264 | 36 | 0.84 | 0.91 |
| Normal_TVS | 258 | 42 | 0.82 | 0.90 |
| PolyporSMF_EBx | 2.16 | 5.84 | 0.04 | 0.60 |
| PolypsorSMF_OPH | 227.5 | 22.5 | 0.87 | 0.94 |
| PolypsorSMF_SIS | 104.4 | 15.6 | 0.80 | 0.92 |
| PolypsorSMF_TVS | 22.5 | 27.5 | 0.32 | 0.59 |
| FPRs | ||||
| CompHyp_EBx | 7.5 | 142.5 | 0.02 | 0.09 |
| ED_OPH | 1000 | 24000 | 0.04 | 0.04 |
| ED_SIS | 2 | 98 | 0.00 | 0.05 |
| ED_TVS | 27.2 | 52.8 | 0.24 | 0.45 |
| Fibroids_SIS | 2.16 | 9.84 | 0.03 | 0.43 |
| Fibroids_TVS | 2.16 | 9.84 | 0.03 | 0.43 |
| PolypSMF_EBx | 2 | 998 | 0.00 | 0.01 |
| PolypSMF_OPH | 33 | 517 | 0.04 | 0.08 |
| PolypSMF_SIS | 3.9 | 26.1 | 0.04 | 0.27 |
| PolypSMF_TVS | 7.7 | 27.3 | 0.10 | 0.37 |
| pExDetectsFibroids | 8 | 2 | 0.52 | 0.97 |
| pHyperplasia | 3 | 2 | 0.19 | 0.93 |
| Probability of being satisfied | ||||
| EA_DUB | 924 | 110 | 0.87 | 0.91 |
| EA_Fibroids | 2.76 | 2.04 | 0.17 | 0.92 |
| Hysterectomy_AtypCa | Parameter fixed at value 1 | |||
| Hysterectomy_ED | 164.9 | 5.1 | 0.94 | 0.99 |
| Hysterectomy_Fibroids | 24.64 | 3.36 | 0.74 | 0.97 |
| Hysterectomy_HMB | 380 | 20 | 0.93 | 0.97 |
| Hysterectomy_Hyperpl | 380 | 20 | 0.93 | 0.97 |
| LngIUS_DUB | 107.9 | 22.1 | 0.76 | 0.89 |
| LngIUS_ED | 9.68 | 12.32 | 0.24 | 0.65 |
| LngIUS_Fibroids | 2.03 | 4.97 | 0.05 | 0.65 |
| LngIUS_Hyperplasia | 91.3 | 18.7 | 0.75 | 0.89 |
| LngIUS_PolypSMF | 420 | 580 | 0.39 | 0.45 |
| Removal | 11 | 3 | 0.55 | 0.95 |
| Removal_CavityNormal | Parameter fixed at value 0 | |||
| Parameter | aa | ba | Low | High |
|---|---|---|---|---|
| Success rates | ||||
| EB | 396 | 54 | 0.85 | 0.91 |
| EB after HO | 396 | 54 | 0.85 | 0.91 |
| EB after ‘history + TVS’ positive | 396 | 54 | 0.85 | 0.91 |
| EB after OPH | 372 | 28 | 0.90 | 0.95 |
| EB after TVS | 396 | 54 | 0.85 | 0.91 |
| EB after TVS_OPH | 372 | 28 | 0.90 | 0.95 |
| EB_OPH | 220 | 30 | 0.84 | 0.92 |
| OPH | 760 | 40 | 0.93 | 0.96 |
| EB_TVS | 220 | 30 | 0.84 | 0.92 |
| TVS_EB_OPH | 220 | 30 | 0.84 | 0.92 |
| TVS_OPH | 624 | 26 | 0.94 | 0.97 |
| TPRs | ||||
| D&C | 48 | 2 | 0.89 | 1.00 |
| EB | 32.9 | 2.1 | 0.84 | 0.99 |
| EB after HO pos | 470 | 30 | 0.92 | 0.96 |
| EB after ‘history + TVS’ positive | 3760 | 240 | 0.93 | 0.95 |
| EB if OPH positive | 470 | 30 | 0.92 | 0.96 |
| EB if TVS positive | 3760 | 240 | 0.93 | 0.95 |
| History only | 198 | 2 | 0.97 | 1.00 |
| History + TVS | 198 | 2 | 0.97 | 1.00 |
| OPH | 645 | 105 | 0.83 | 0.88 |
| OPH if EB negative | 1032 | 168 | 0.84 | 0.88 |
| TVS | 261.9 | 8.1 | 0.95 | 0.99 |
| TVS after EB negative | 64.99 | 2.01 | 0.92 | 1.00 |
| TVS if history positive | 234.6 | 3.6 | 0.97 | 1.00 |
| TVS if OPH negative | 64.99 | 2.01 | 0.92 | 1.00 |
| FPRs | ||||
| D&C | 2 | 198 | 0.00 | 0.03 |
| EB | 4 | 396 | 0.00 | 0.02 |
| History only | 80 | 20 | 0.72 | 0.87 |
| History + TVS | 615 | 885 | 0.39 | 0.43 |
| OPH | 2 | 198 | 0.00 | 0.03 |
| TVS | 1080 | 1320 | 0.43 | 0.47 |
| Other parameters | ||||
| Probability of EC stage I | 58.1 | 24.9 | 0.60 | 0.79 |
| Probability of upstaging from I to II–IV owing to delayed diagnosis | 3.75 | 71.25 | 0.01 | 0.11 |
| Mortality by TAH procedure | 2.8 | 197.2 | 0.00 | 0.03 |
For models (such as Markov models) in which there is a non-linear relationship between model inputs and outputs, it is appropriate to give a table of mean results from the PSA, as the Bayesian viewpoint is that the mean results from the PSA are the appropriate basis for decision-making. However, in the case of the models presented here, these results would be statistically equivalent to the base-case results and, therefore, there is no need to produce such a table. Results that have been shown are as follows:
A cost-effectiveness scattergraph: this shows, on a single graph, the uncertainty in the absolute expected cost and effectiveness for each option separately. For each option, the results of the 1000 replications of the model were shown each as a single point. In practice, the printed symbols used merge to form a ‘cloud’ giving the general range of uncertainty in the results. The vertical spread of this cloud reflects the uncertainty in the overall cost and the horizontal spread the uncertainty in overall effectiveness, while the centre of the cloud, where the points are most densely packed, indicates the most likely cost and effectiveness.
A cost-effectiveness acceptability frontier (CEAF): at any given WTP, the preferred option is determined by the mean outcomes, which in the case of the models here are the same as the base-case results described earlier. The CEAF shows the proportion of model replications for which this option remained the preferred strategy, as a function of the WTP for an additional positive outcome.
While the graphs described above are the only convenient ways of showing results comparing all the modelled options, they are applicable only to a decision in which exactly those options are included. For other purposes, it is helpful to look at pairwise comparisons between strategies. The results shown from a pairwise comparison are helpful to any decision problem in which both those strategies are included. Pairwise comparisons were made between successive non-dominated options (in order of increasing cost or effectiveness), and others where a dominated option was close to another option.
For pairwise comparisons, the incremental cost-effectiveness scattergraph was shown. In this type of graph, there is a single point for each of the 1000 replications of the model, showing the difference in cost and effectiveness between the two strategies. If the ‘clouds’ shown in the cost-effectiveness scattergraph for the two strategies overlap, it may be for one of two reasons. First, it may be because there is genuine uncertainty as to which is the more costly and/or more effective strategy. Second, it may be that one strategy is consistently more costly and/or more effective than the other, but this consistent difference is small compared with the uncertainty in the absolute costs and/or effectiveness. The incremental cost-effectiveness scattergraph distinguishes between these two cases and shows the relevant uncertainty for a decision-maker.
The other graph plotted for the pairwise comparisons was the cost-effectiveness acceptability curve (CEAC). This shows the proportion of model replications in which one of the strategies is cost-effective compared with the other, across a range of values of WTP per additional positive outcome.
In deterministic sensitivity analysis, one or more model inputs are varied systematically and the effect on the model outcomes is noted. This was used in the HMB analysis to test the effect of reducing the prevalence of polyps and SMFs and increasing the prevalence of DUB and also to examine the effect when the unit cost of SIS was reduced. For prevalence of the various pathologies, a Dirichlet distribution was used. This is the generalisation of the beta distribution for more than two options. Given that the prevalence data came from different sources, it was necessary to take a compromise between the effective sample sizes indicated by those sources. An effective sample size of 100 was assumed. The distribution of any prevalence parameter on its own then follows a beta distribution.
Chapter 3 Economic evaluation: heavy menstrual bleeding
Deterministic results: base case
Heavy menstrual bleeding causes significant morbidity to sufferers, but is rarely caused by malignant uterine disease. 11 Diagnostic tests have become less invasive and portable such that they can be carried out during the initial gynaecological consultation, but there is no consensus among clinicians as to how best to investigate women with HMB. The lack of evidence as to the effectiveness and cost-effectiveness of diagnostic strategies has not only allowed eclectic practice, but is reflected in the most recent national guidelines for the management of HMB,11 where no routine uterine evaluation is recommended outside of a clinical pelvic examination. The current recommended first-line treatment for HMB in women not desiring immediate fertility is the LNG-IUS, otherwise known as the Mirena® coil (Bayer HealthCare Pharmaceuticals, Pittsburgh, PA, USA). 11 The majority of women with HMB have no uterine pathology (known as DUB) or benign uterine pathologies such as small uterine fibroids or endometrial hyperplasia and all these conditions respond well in general to the LNG-IUS. 36,37
Thus, given the negligible chance of life-threatening disease, the lack of recommendations stipulating the need for routine diagnostic testing, and the known effectiveness and applicability of the LNG-IUS in HMB, we chose our base-case scenario (strategy LNG-IUS) as medical treatment with the LNG-IUS without any preliminary diagnostic testing. The costs and effects of the various clinically relevant diagnostic testing strategies were compared against this base-case strategy. In addition, a recent cost-effectiveness analysis informed by IPD analysis of published trials for treatment of HMB suggested that surgical treatment with hysterectomy was more cost-effective than the LNG-IUS. 2 We therefore also compared hysterectomy without diagnostic work up with the LNG-IUS treatment-alone base-case strategy.
Table 21 reports the deterministic results, referencing all other diagnostic or treatment strategies to the LNG-IUS treatment-alone base-case strategy.
| Strategy | Cost (£) | Effectiveness (satisfaction) |
|---|---|---|
| LNG-IUS alone | 1067 | 0.933327 |
| OPH alone | 1078 | 0.964122 |
| SIS alone | 1083 | 0.962914 |
| TVS alone | 1085 | 0.955106 |
| TVS + OPH | 1139 | 0.964382 |
| OPH + EBx | 1149 | 0.967421 |
| SIS + OPH | 1170 | 0.96445 |
| EBx alone | 1209 | 0.945963 |
| SIS + EBx | 1223 | 0.964271 |
| TVS + OPH + EBx | 1227 | 0.964933 |
| TVS + EBx | 1231 | 0.953851 |
| SIS + OPH + EBx | 1256 | 0.965028 |
| Hysterectomy alone | 3182 | 0.9335 |
Outcomes
Direct treatment without preliminary diagnostic testing was less clinically effective than treatment instigated after diagnostic testing. The least effective approach was the base-case strategy of LNG-IUS treatment alone, followed closely by surgical treatment with hysterectomy, both approaches resulting in satisfaction rates of around 93.3–93.4%. The effectiveness of HMB management was similar across all testing strategies, ranging from 94.6% to 96.7% rates of satisfaction. The most effective strategy was combination testing with OPH and EBx.
Costs
The LNG-IUS treatment-alone base-case strategy was the cheapest, costing £1067 per woman treated for HMB in a secondary care setting, and the strategy of hysterectomy for all women bypassing the need for diagnostic work-up was the most expensive, at £3182 per woman treated, that is to say £2116 more than the approach of LNG-IUS treatment alone. The cheapest of the nine diagnostic testing strategies was the use of OPH alone, costing £1078 for every woman treated, that is to say £11 more than universal LNG-IUS treatment.
Cost-effectiveness and dominance
Only the testing strategies OPH alone and OPH combined with EBx (OPH + EBx) remain non-dominated by alternative empirical treatment or diagnostic testing strategies. It is clear from our analysis that the strategy OPH alone dominates the testing strategies SIS alone and TVS alone. The combination testing strategy TVS + OPH is excluded by extended dominance between OPH alone and OPH + EBx. The remaining seven alternative strategies are dominated by OPH + EBx. Table 22 presents the deterministic analysis restricted to the non-dominated competing strategies.
| Strategy | Total cost (£) | Incremental cost (£) | Effectiveness (satisfaction) | Incremental effectiveness | ICER (£) |
|---|---|---|---|---|---|
| LNG-IUS alone | 1067 | 0.9333 | |||
| OPH alone | 1078 | 11 | 0.9641 | 0.0308 | 359 |
| OPH + EBx | 1149 | 71 | 0.9674 | 0.0033 | 21,500 |
Thus, the cheapest strategy is the base-case scenario of no testing and universal treatment with the LNG- IUS alone of all women presenting to secondary care with HMB. The most effective strategy is the combination of initial testing with OPH + EBx, but this comes at a greater cost, generating an ICER of £21,500, that is to say the strategy requires an investment of £21,500 to gain an extra woman satisfied following treatment for HMB compared with investigation with OPH alone. When compared with blanket LNG-IUS treatment for all, an additional £21,859 is required to gain an additional satisfied patient when investigating with OPH + EBx. The single-test strategy of OPH is slightly less effective than the strategy of OPH with the addition of EBx, but is substantially less costly. The ICER for OPH is approximately £360, that is to say an additional financial outlay of £360 is necessary to acquire an extra woman satisfied following treatment for HMB.
Figure 4 shows the total costs and effectiveness of alternative strategies for the diagnosis and treatment of HMB in secondary care. The line presented graphically joins the non-dominated strategies (OPH alone and OPH + EBx). Any strategy plotted above this line is not considered cost-effective in relation to the non-dominated alternatives.
FIGURE 4.
Cost-effectiveness plane showing the results of deterministic analysis of the strategies for investigation of women with HMB.
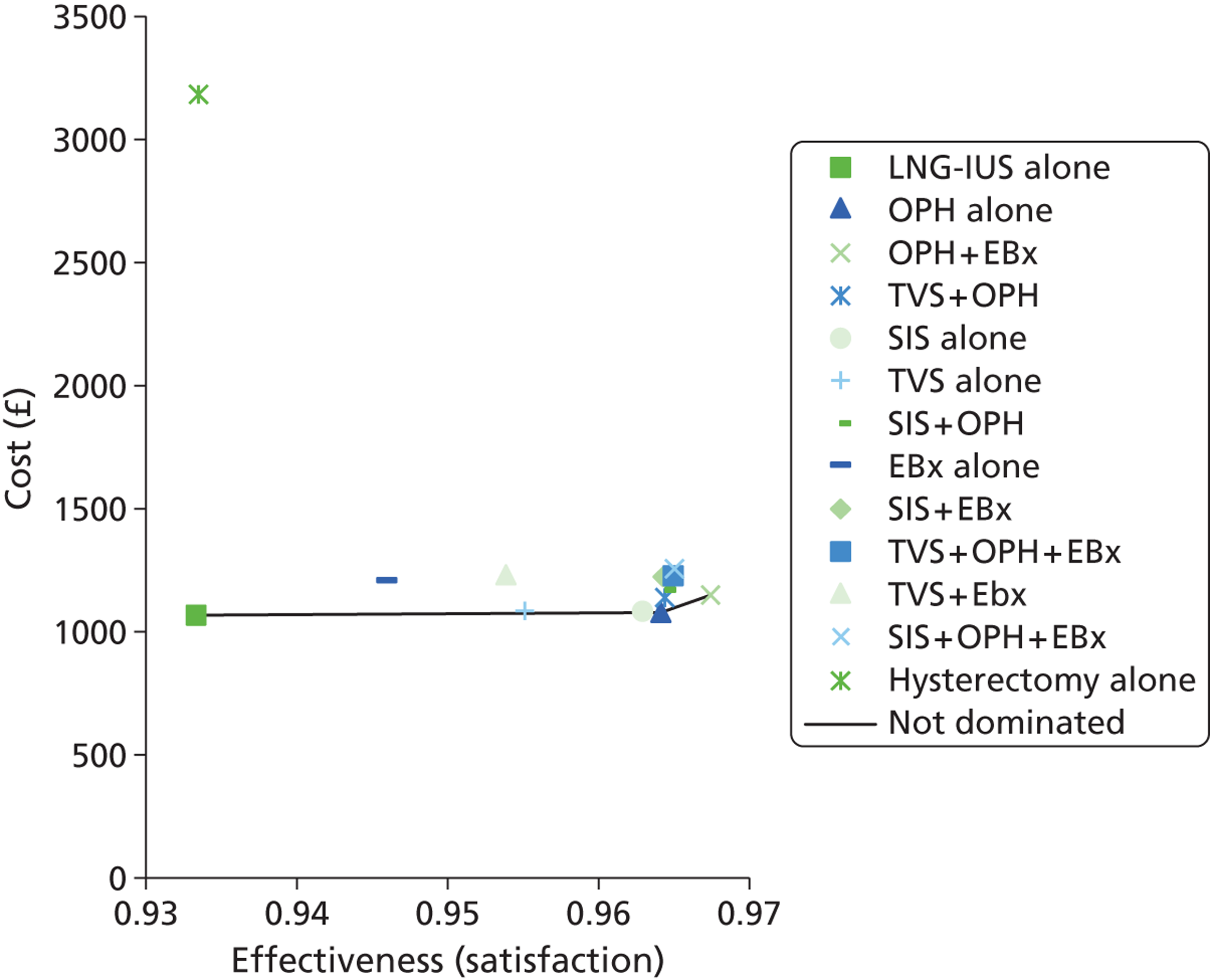
It is clear that the option of direct treatment with hysterectomy is the least cost-effective strategy by some considerable margin. Replicating the figure excluding the hysterectomy reduces the scale of the y-axis, allowing closer examination of the remaining testing strategies. Figure 5 reveals that the options ‘TVS alone’ and ‘SIS alone’ are sufficiently close to the boundary of dominance that it is worth checking for the uncertainty between these dominated alternatives in addition to the non-dominated options ‘OPH’ and ‘OPH + EBx’ through sensitivity analyses.
FIGURE 5.
Cost-effectiveness plane showing the results of deterministic analysis of the strategies for investigation of women with HMB – hysterectomy excluded.
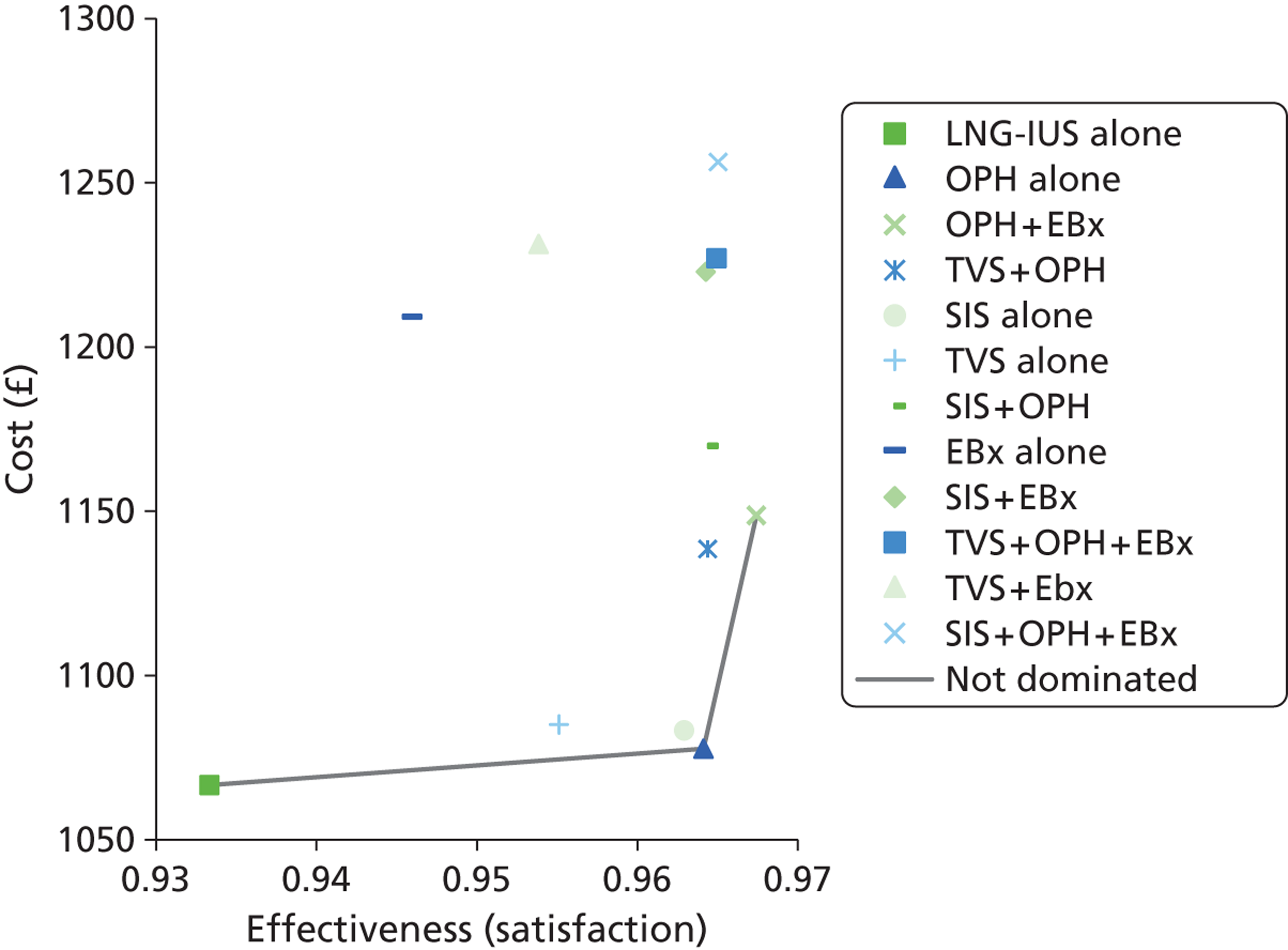
Probabilistic sensitivity analysis results: base case
Figure 6 demonstrates the uncertainty surrounding the absolute expected cost and effectiveness of each of the strategies, with the true value lying somewhere within the ‘cloud’ of plotted points, probably where they are most densely clustered. It shows that hysterectomy is too expensive to be a competitive option but that there is overlap between the remaining strategies.
FIGURE 6.
Scatterplot showing the uncertainty in costs and effectiveness within the model for each of the individual strategies for investigation of women with HMB.
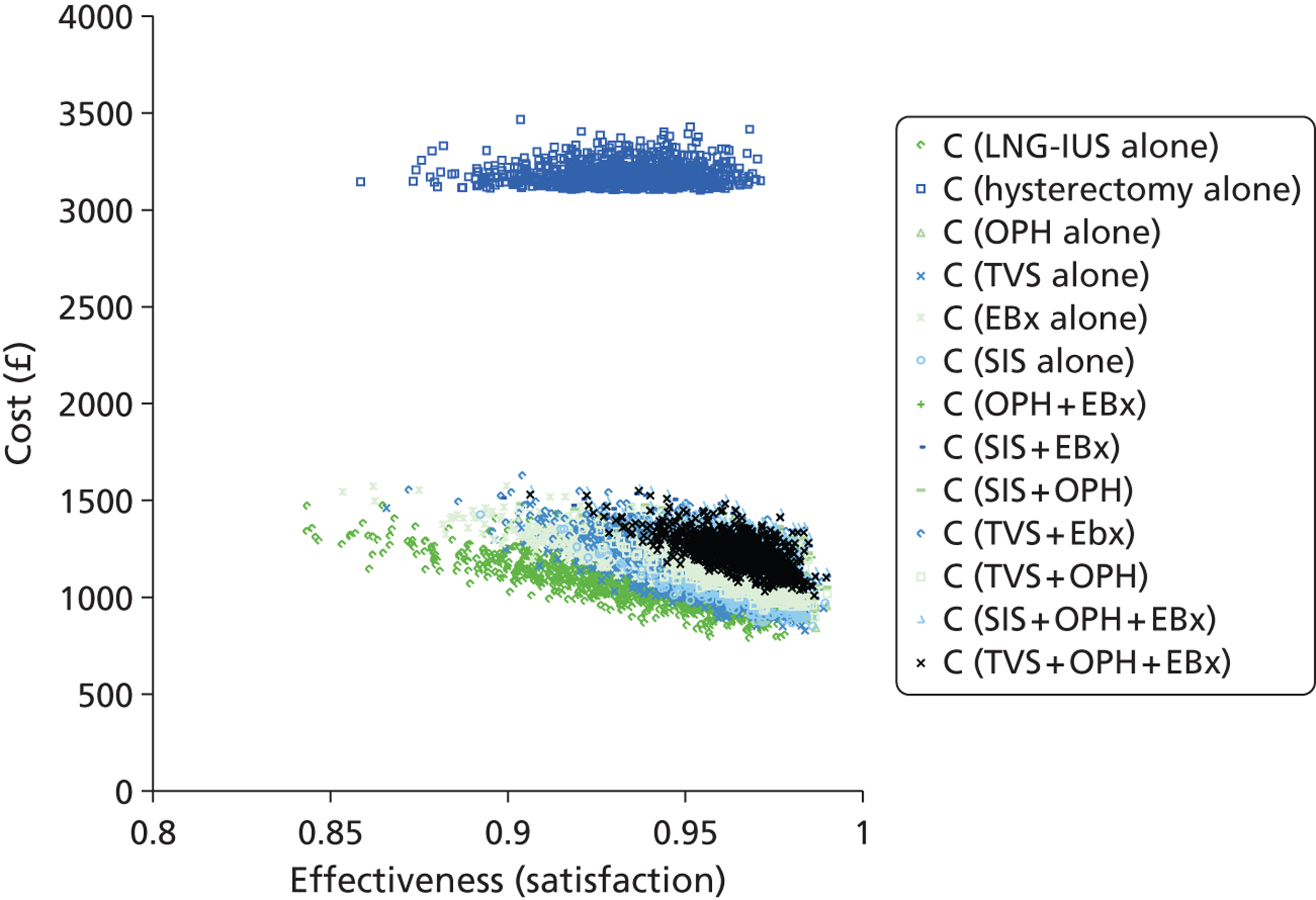
Figure 7 illustrates the overall uncertainty related to the optimal decision across a range of plausible WTP values, where here the WTP is measured in cost per additional case satisfied. In principle, the optimal decision is determined by the mean of the PSA. 148 However, when a model is linear in all parameters and they are sampled independently, the mean of the probabilistic results will be the same as the deterministic result. This is true for the current model, as the only parameters which are not independently sampled are the prevalences, and these do not interact in the calculation of the model results. The CEAF (see Figure 7) is generated as follows. First, for any WTP, the optimal option is determined based on the mean results. Then the proportion of model replications for which that was the optimal option is found and plotted. For example, if we consider a WTP of £10,000 per case satisfied, the preferred option based on the mean results is OPH alone, and this was optimal in around 60% of the model replications. Thus, there is an estimated probability of 40% that there is a better option than OPH at that WTP. By definition, only the options which are not dominated in the mean results can appear on the CEAF. Sometimes the probability shown will be lower than 50%. It will often be the case that the option preferred on mean values is also the preferred option in the highest proportion of model replications, but this is not always so (see the textbook by Briggs et al. 148 for a fuller discussion of this issue). As the WTP crosses the ICER between two non-dominated options, the choice of optimal option changes, and there will usually be a discontinuity in the curve.
FIGURE 7.
Cost-effectiveness acceptability frontier showing the optimal investigative strategy across a range of WTP thresholds for the sensitivity analysis of strategies to investigate women with HMB. (For any WTP per additional case satisfied, the optimal strategy is determined by the mean results.)
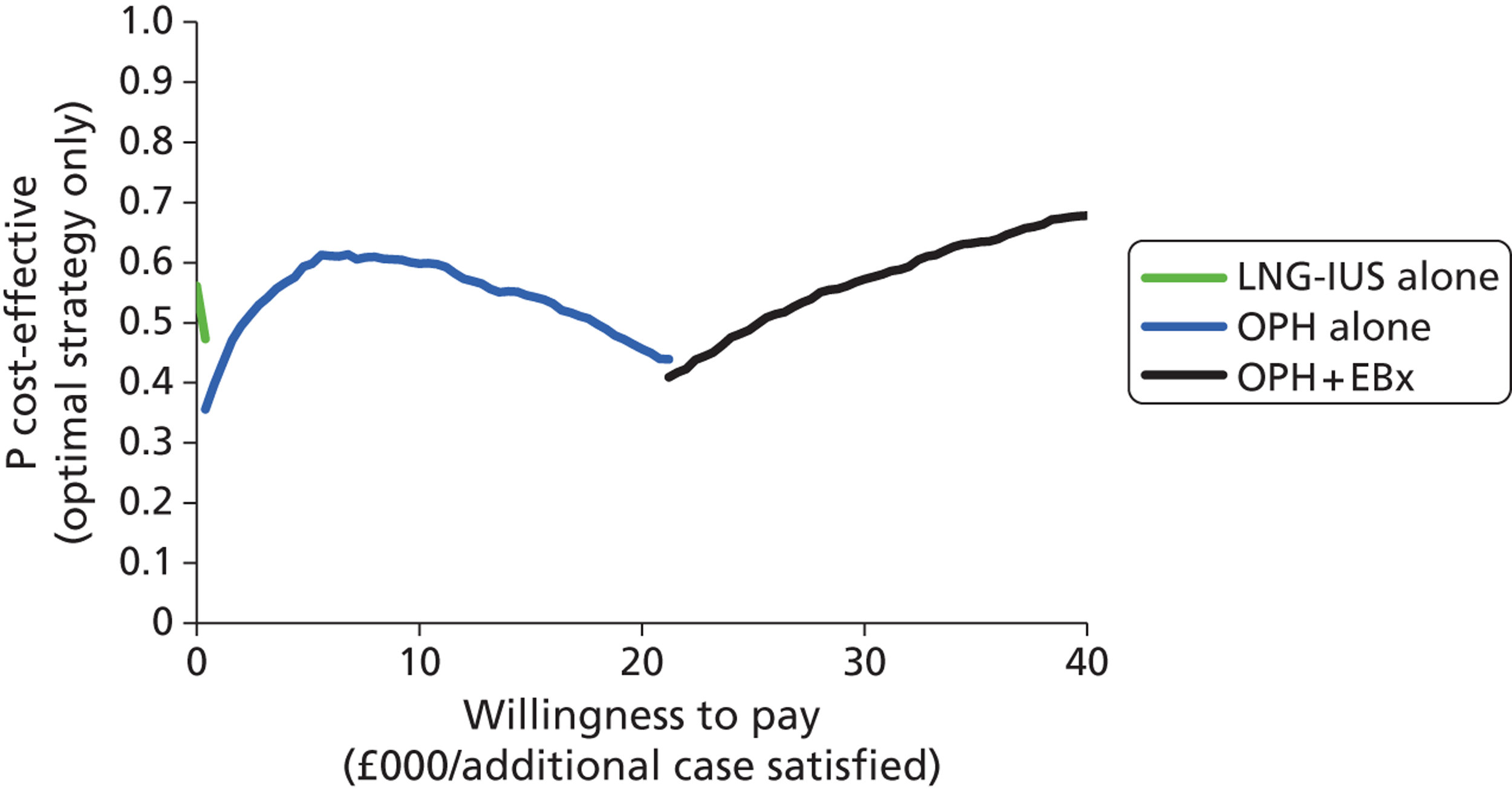
For the current model, the CEAF shows the same range of preferred options already shown in Table 20, but indicates that there is appreciable uncertainty about the preferred option across the whole range of WTP values plotted. To explore the uncertainty more fully, it is helpful to consider a range of appropriate pairwise comparisons between the different options. Comparisons are shown between adjacent non-dominated options. There are also options that are dominated on mean values, but whose mean values are close to the non-dominance lines shown on Figure 5. These options are compared with relevant non-dominated options.
Outpatient hysteroscopy compared with levonorgestrel-releasing intrauterine system
The cost-effectiveness plane (Figure 8a) shows the modelled uncertainty in the difference in costs between OPH alone and LNG-IUS alone. It shows that OPH alone is consistently more effective than LNG-IUS alone, and is likely (but not certain) to be more costly. The CEAC (Figure 8b) shows the proportion of model replications for which OPH alone is preferred to LNG-IUS alone at any given WTP. OPH is the preferred option at any WTP over £360 per additional case satisfied, but there is considerable uncertainty when the WTP is just above this figure. However, by the time the WTP exceeds £8000 per additional case satisfied, it is almost certain that OPH is preferred to LNG-IUS.
FIGURE 8.
Cost-effectiveness plane (a) and CEAC (b): OPH-alone strategy relative to the LNG-IUS-alone strategy.
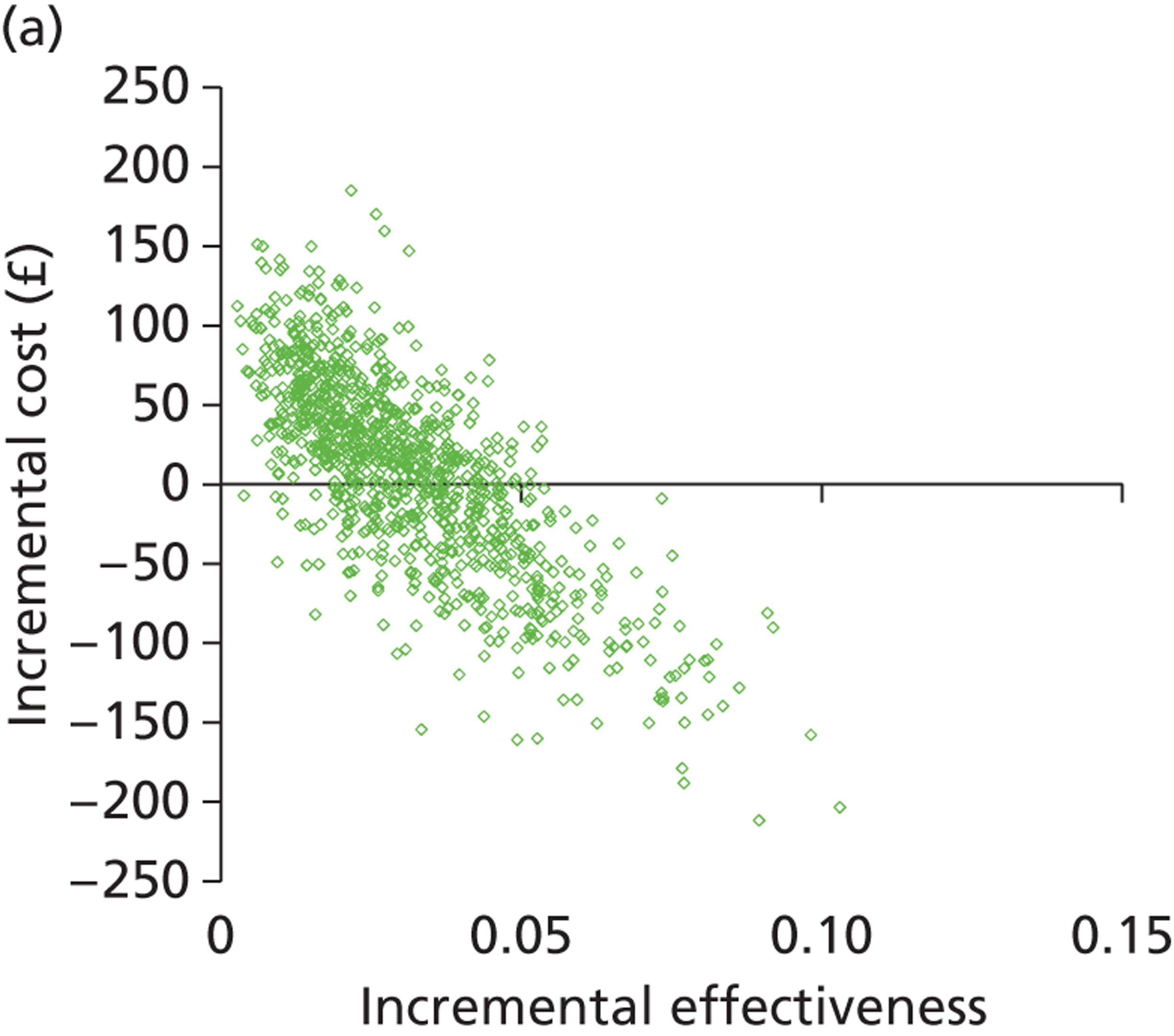
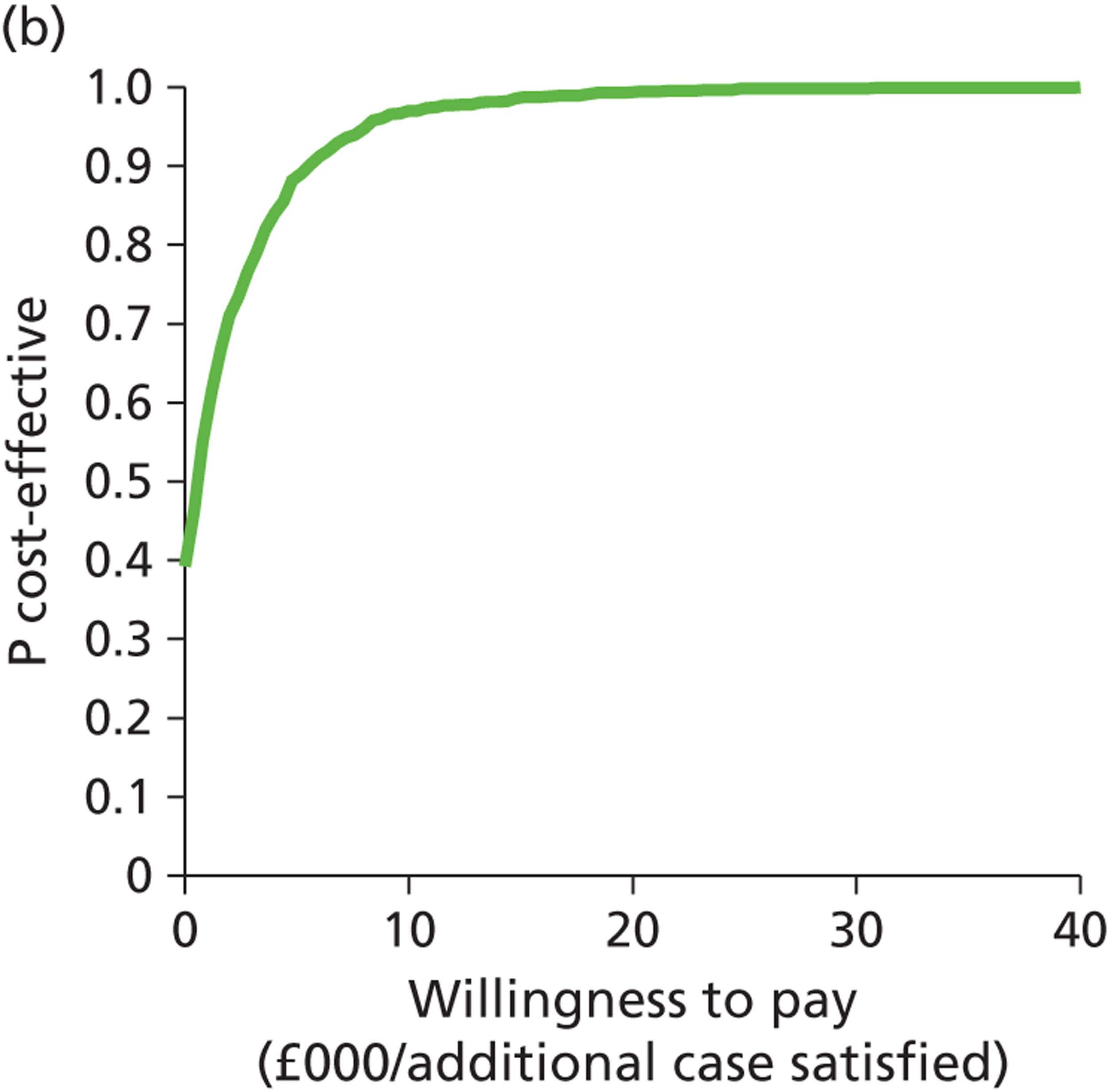
Outpatient hysteroscopy and endometrial biopsy compared with outpatient hysteroscopy alone
The graph (Figure 9a) shows the modelled uncertainty in the difference in costs between OPH + EBx and OPH alone. It shows that adding EBx to OPH increases the cost and is very likely to increase the effectiveness. The CEAC (Figure 9b) shows the proportion of model replications for which OPH + EBx is preferred to OPH alone at any given WTP per additional case satisfied. It is more likely than not that OPH + EBx is cost-effective compared with OPH above a WTP threshold of around £23,000. However, there is considerable uncertainty throughout the range of WTP values shown. About 30% of replications favour OPH alone, even if the WTP is as high as £40,000 per additional case satisfied.
FIGURE 9.
Cost-effectiveness plane (a) and CEAC (b): OPH and EBx strategy relative to the OPH-alone strategy.
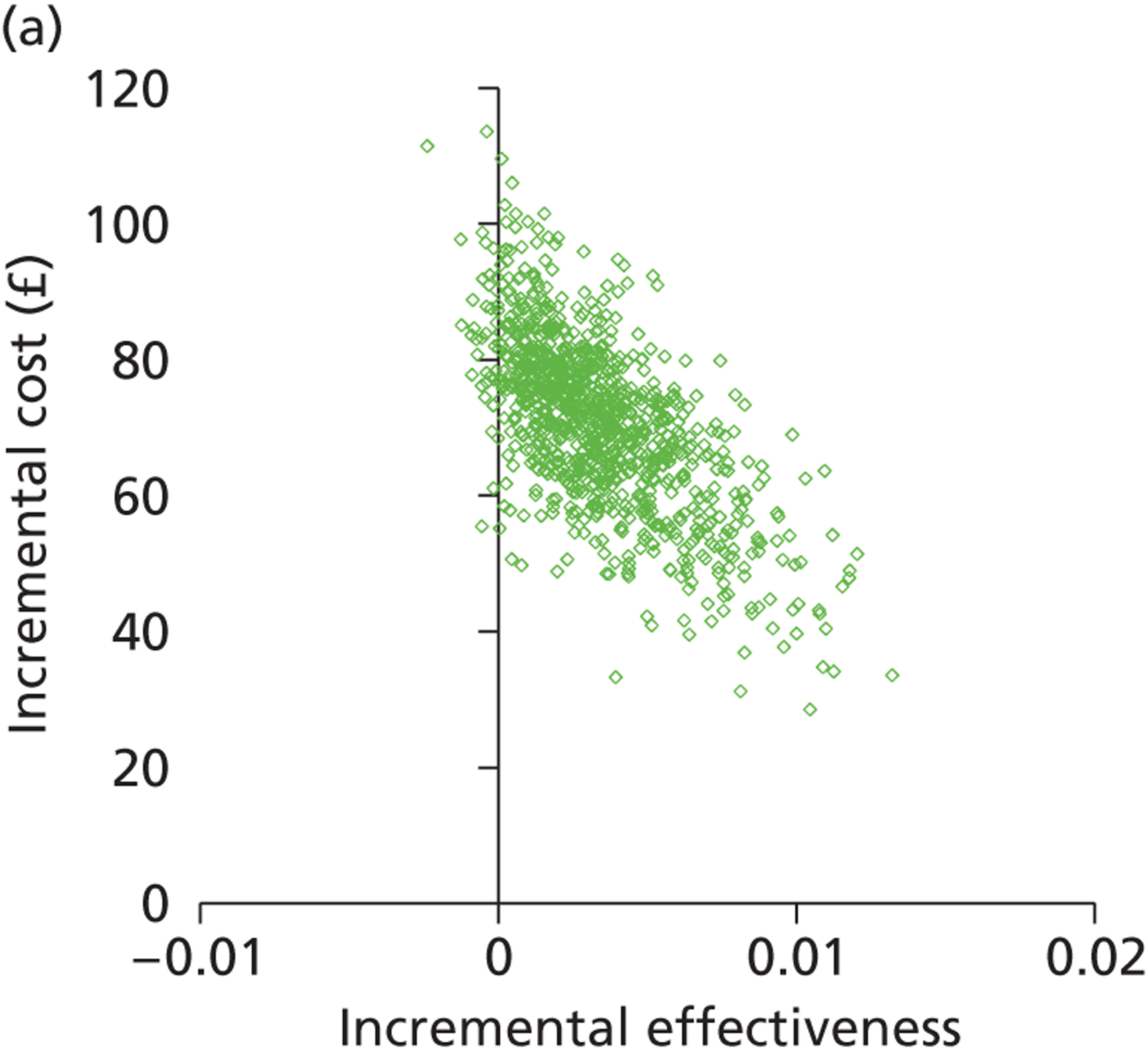
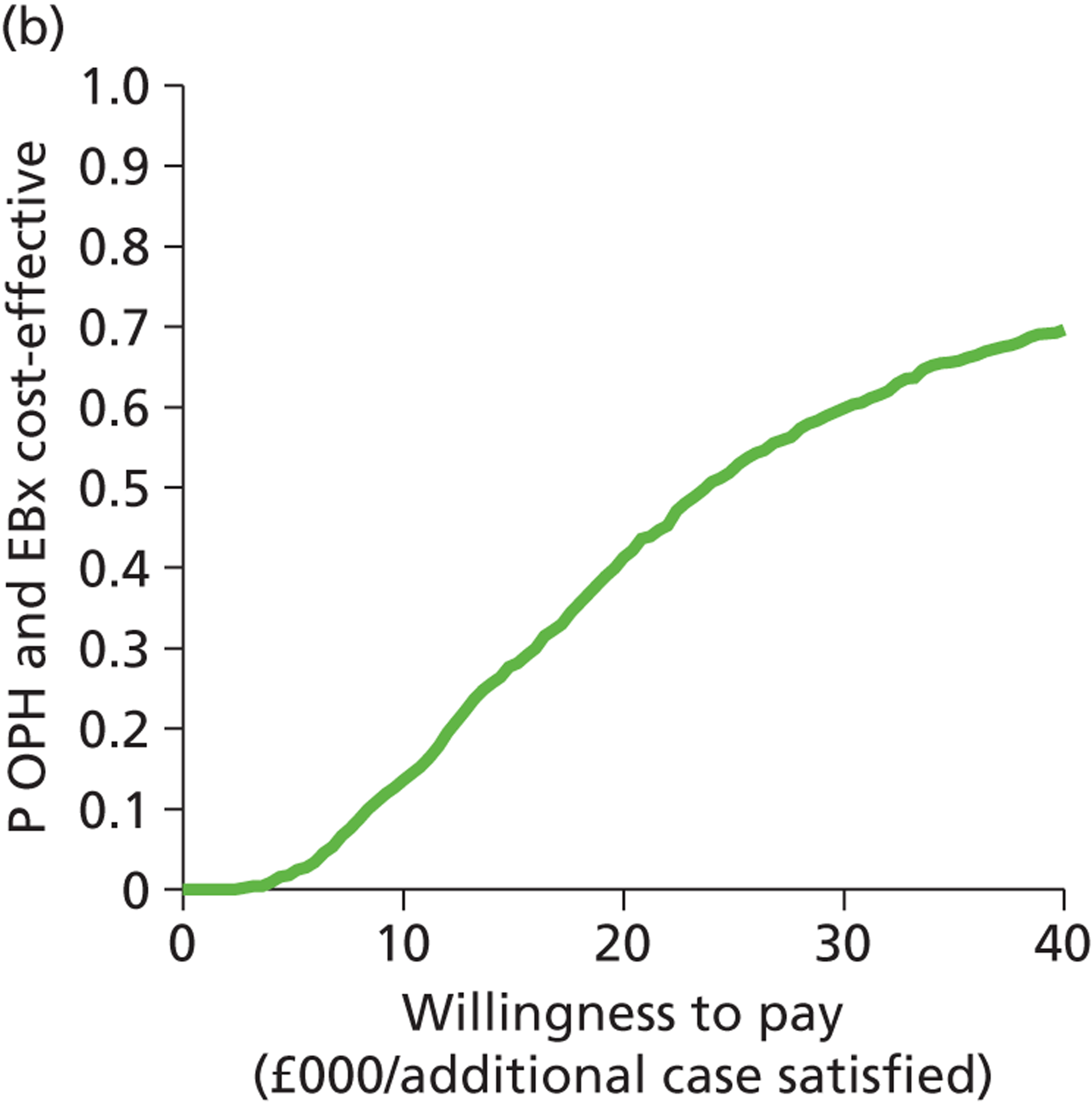
Assessment of potentially cost-effective competing strategies
In addition to the non-dominated testing strategies ‘OPH alone’ and ‘OPH + EBx’, the single testing strategies TVS and SIS were sufficiently close to the boundary of dominance that we felt it prudent to explore the level of uncertainty within the model pertaining to these two dominated alternative options.
Transvaginal scan compared with levonorgestrel-releasing intrauterine system
The graph (Figure 10a) shows the modelled uncertainty in the difference in costs between TVS alone and LNG-IUS alone. It shows that TVS alone is consistently more effective than LNG-IUS alone, and is likely (but not certain) to be more costly. The CEAC (Figure 10b) shows the proportion of model replications for which TVS alone is preferred to LNG-IUS alone at any given WTP per additional case satisfied. It is more likely than not that TVS is cost-effective compared with LNG-IUS above a WTP threshold of around £1000. This is almost certainly the case at WTP thresholds beyond £9000.
FIGURE 10.
Cost-effectiveness plane (a) and CEAC (b): TVS-alone strategy relative to the LNG-IUS-alone strategy.
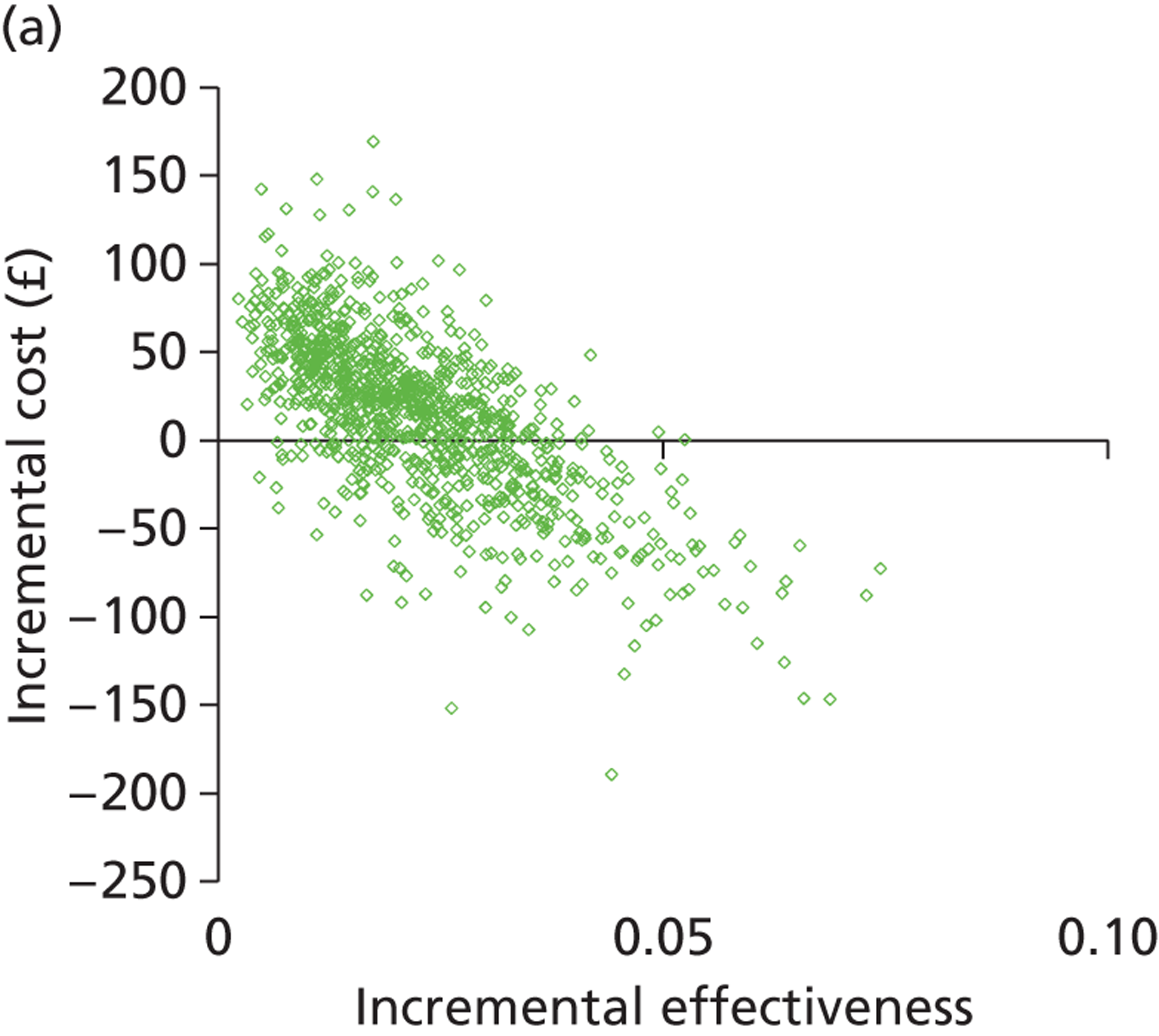
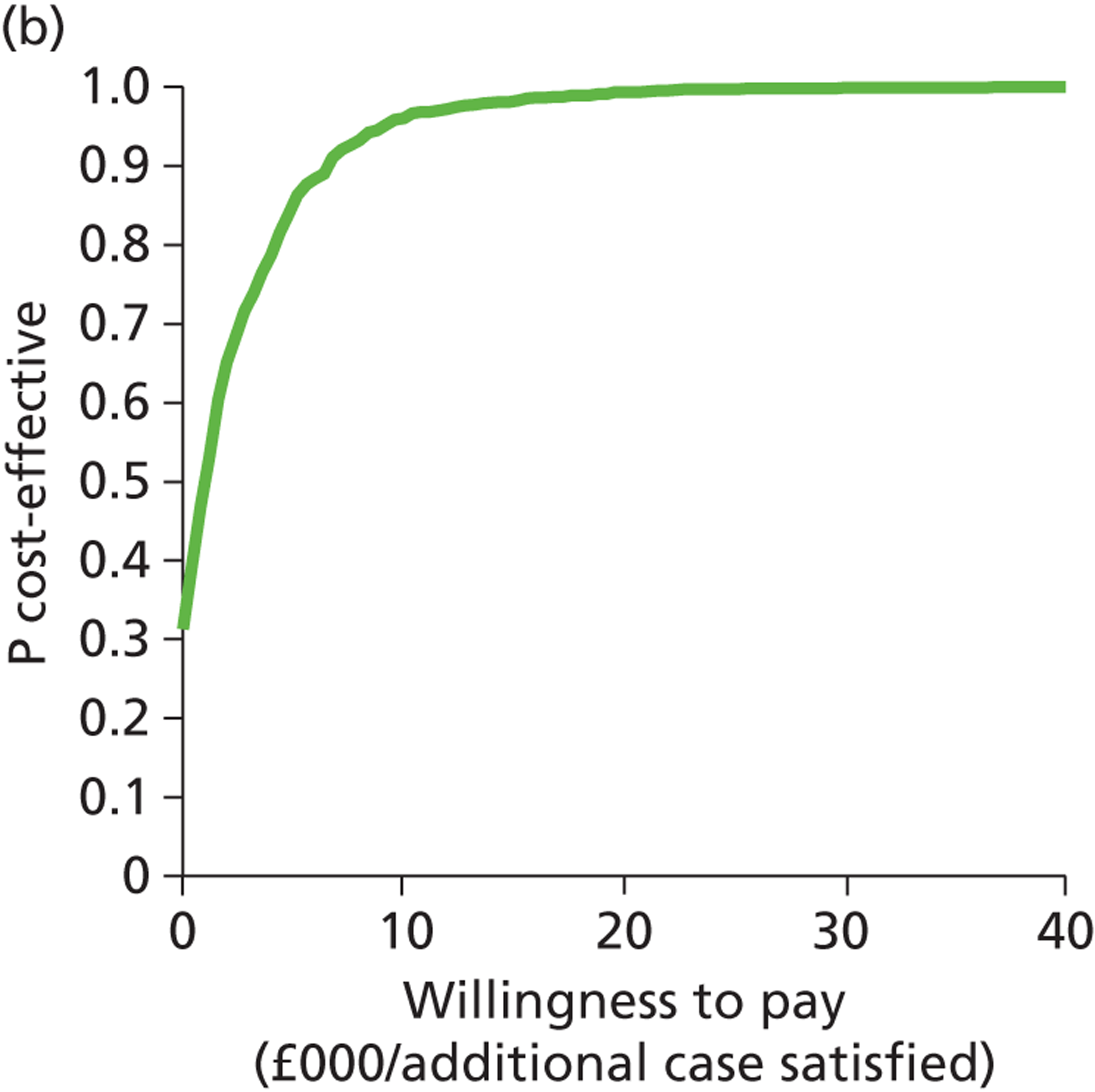
Saline infusion sonography compared with levonorgestrel-releasing intrauterine system
The graph (Figure 11a) shows the modelled uncertainty in the difference in costs between SIS alone and LNG-IUS alone. It shows that SIS alone is consistently more effective than LNG-IUS alone, and is likely (but not certain) to be more costly. The CEAC (Figure 11b) shows the proportion of model replications for which SIS alone is preferred to LNG-IUS alone at any given WTP per additional case satisfied. The likelihood is that SIS is cost-effective compared with LNG-IUS above a WTP threshold of around £800. This is almost certainly the case at WTP thresholds beyond £8000.
FIGURE 11.
Cost-effectiveness plane (a) and CEAC (b): SIS-alone strategy relative to the LNG-IUS-alone strategy.
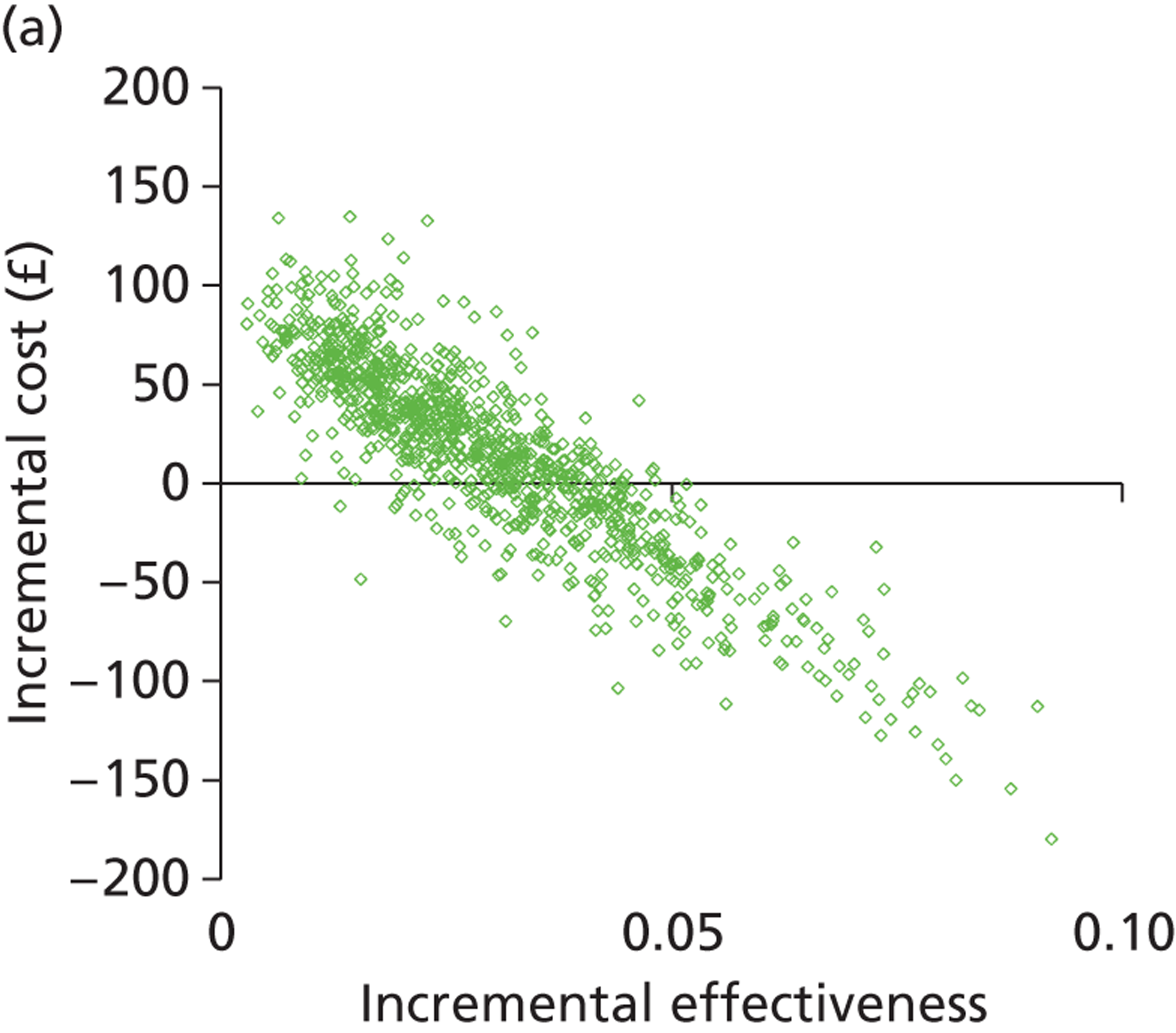
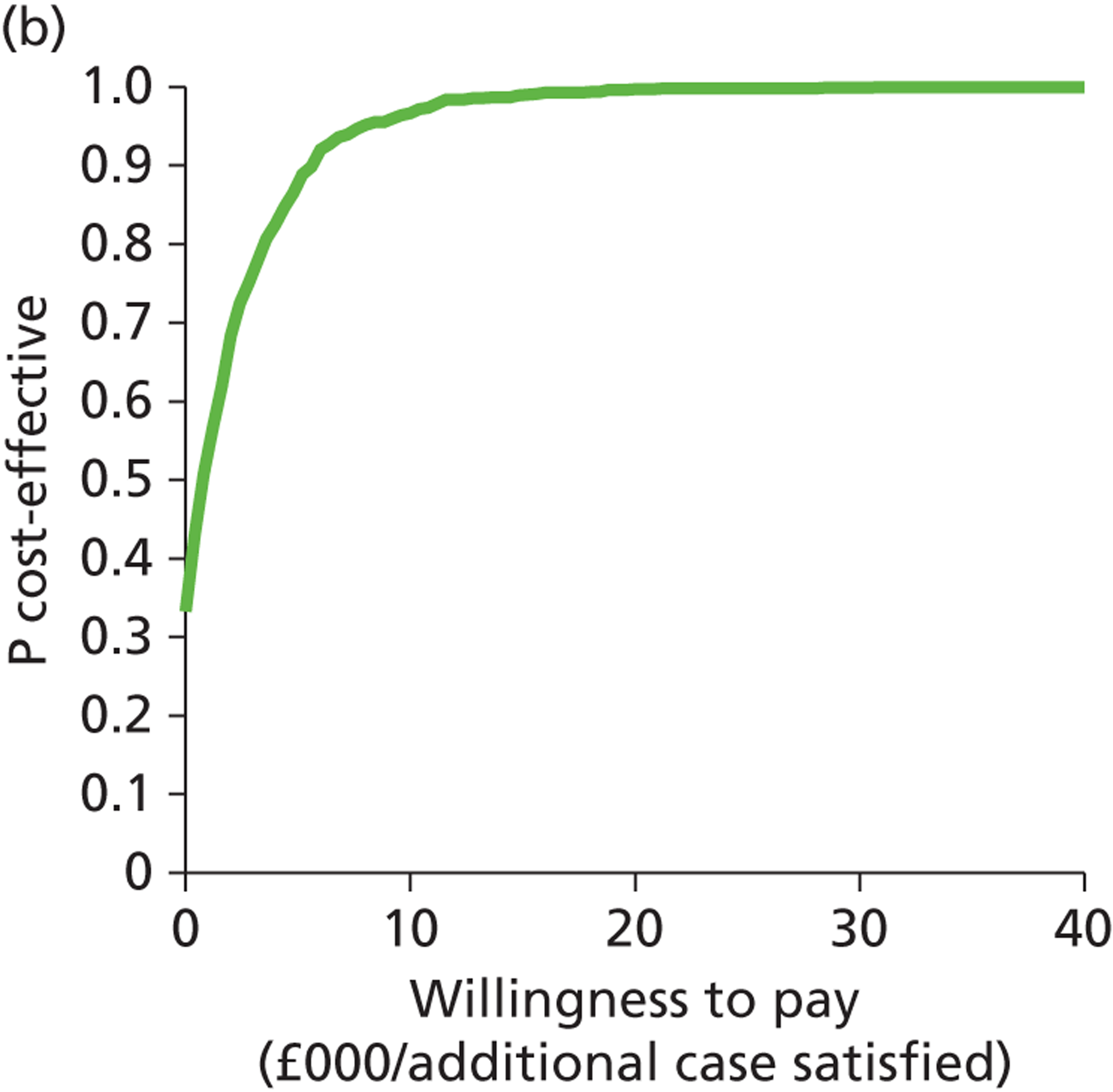
Outpatient hysteroscopy compared with transvaginal scan
The graph (Figure 12a) shows the modelled uncertainty in the difference in costs between OPH alone and TVS alone. It shows that OPH alone is almost certainly more effective than TVS alone but it is unclear whether or not it is more costly. The CEAC (Figure 12b) shows the proportion of model replications for which OPH alone is preferred to TVS alone at any given WTP per additional case satisfied. The likelihood is that OPH is cost-effective compared with TVS above any WTP threshold. This is almost certainly the case at WTP thresholds beyond £9000.
FIGURE 12.
Cost-effectiveness plane (a) and CEAC (b): OPH-alone strategy relative to the TVS-alone strategy.
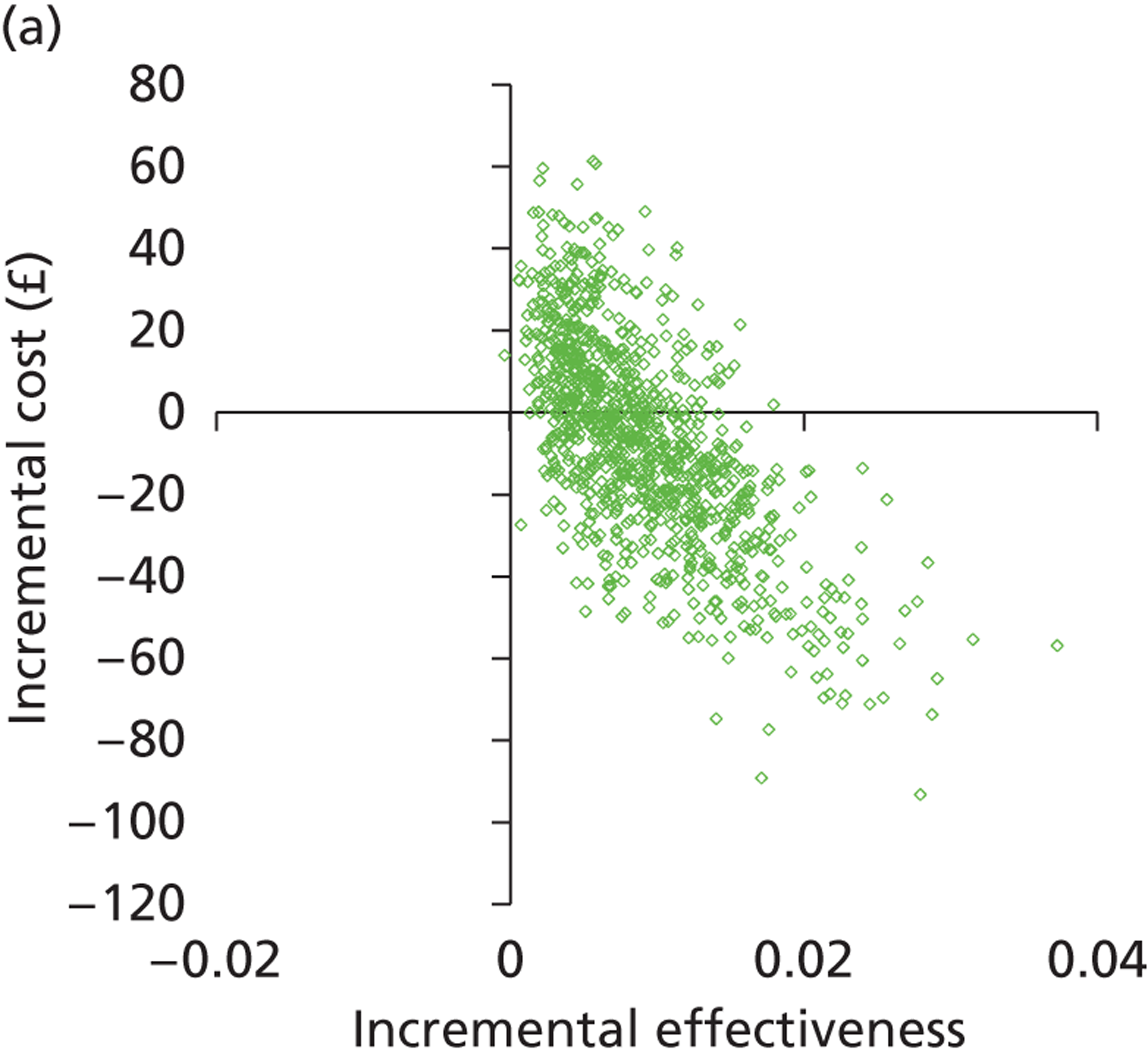
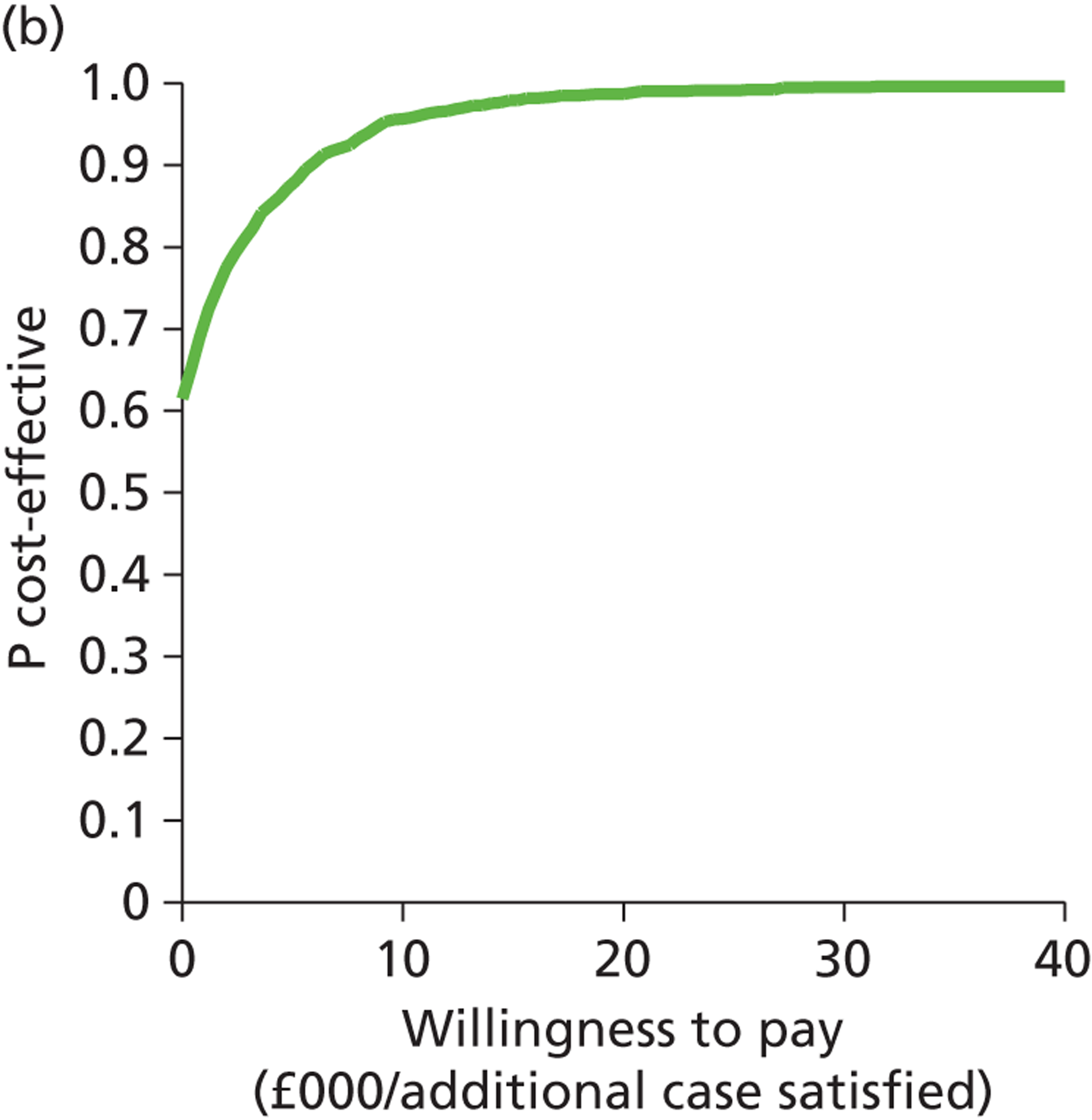
Outpatient hysteroscopy compared with saline infusion sonography
The graph (Figure 13a) shows the modelled uncertainty in the difference in costs between OPH alone and SIS alone. It shows that OPH alone is likely to be more effective than SIS alone, and there is considerable uncertainty as to which is more costly. The CEAC (Figure 13b) shows the proportion of model replications for which OPH alone is preferred to SIS alone at any given WTP per additional case satisfied. The likelihood is that OPH is cost-effective compared with TVS above any WTP threshold, although there is considerable uncertainty throughout. Even at a WTP of £40,000 per additional case satisfied, SIS is preferred in 20% of model replications.
FIGURE 13.
Cost-effectiveness plane (a) and CEAC (b): OPH-alone strategy relative to the SIS-alone strategy.
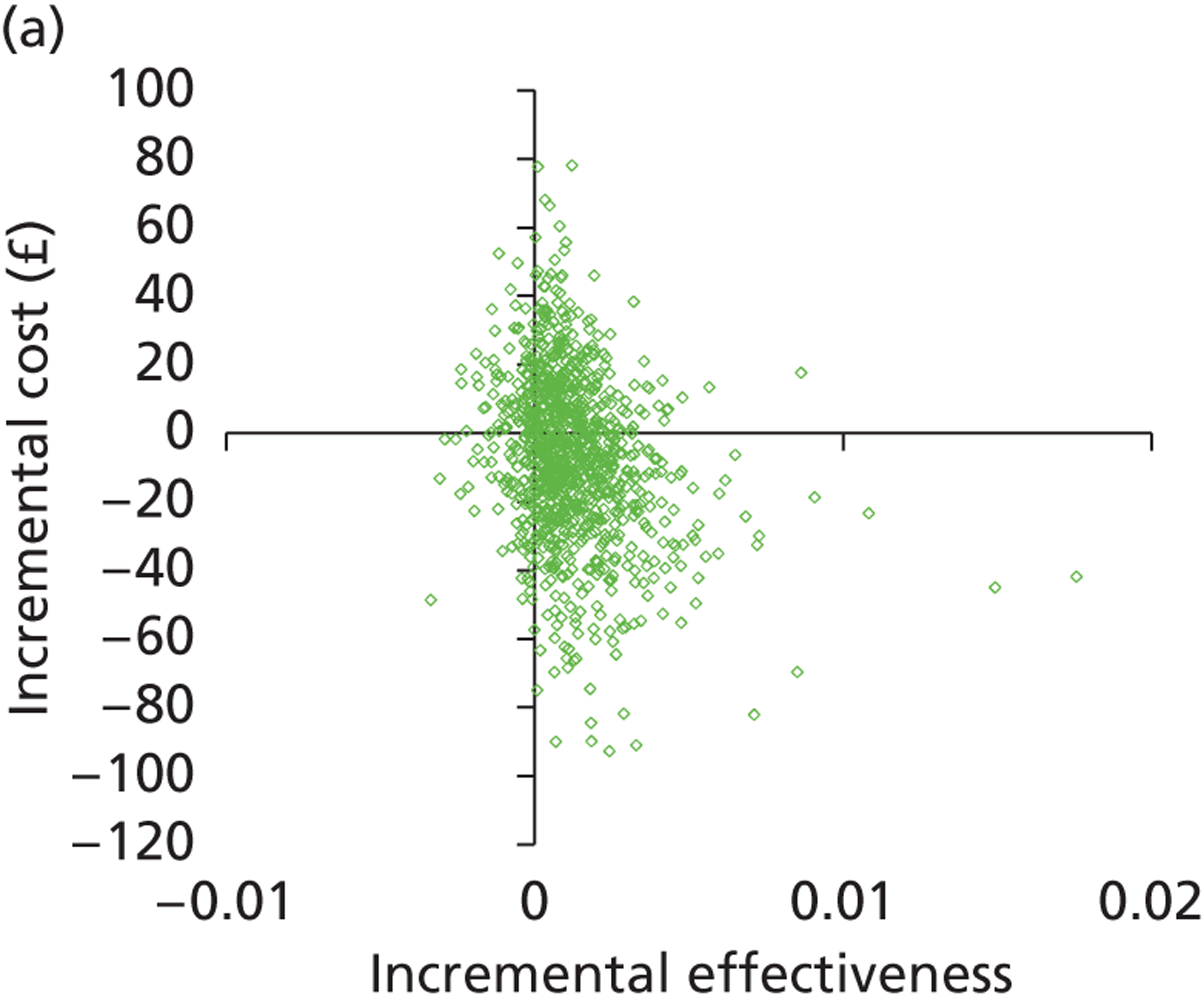
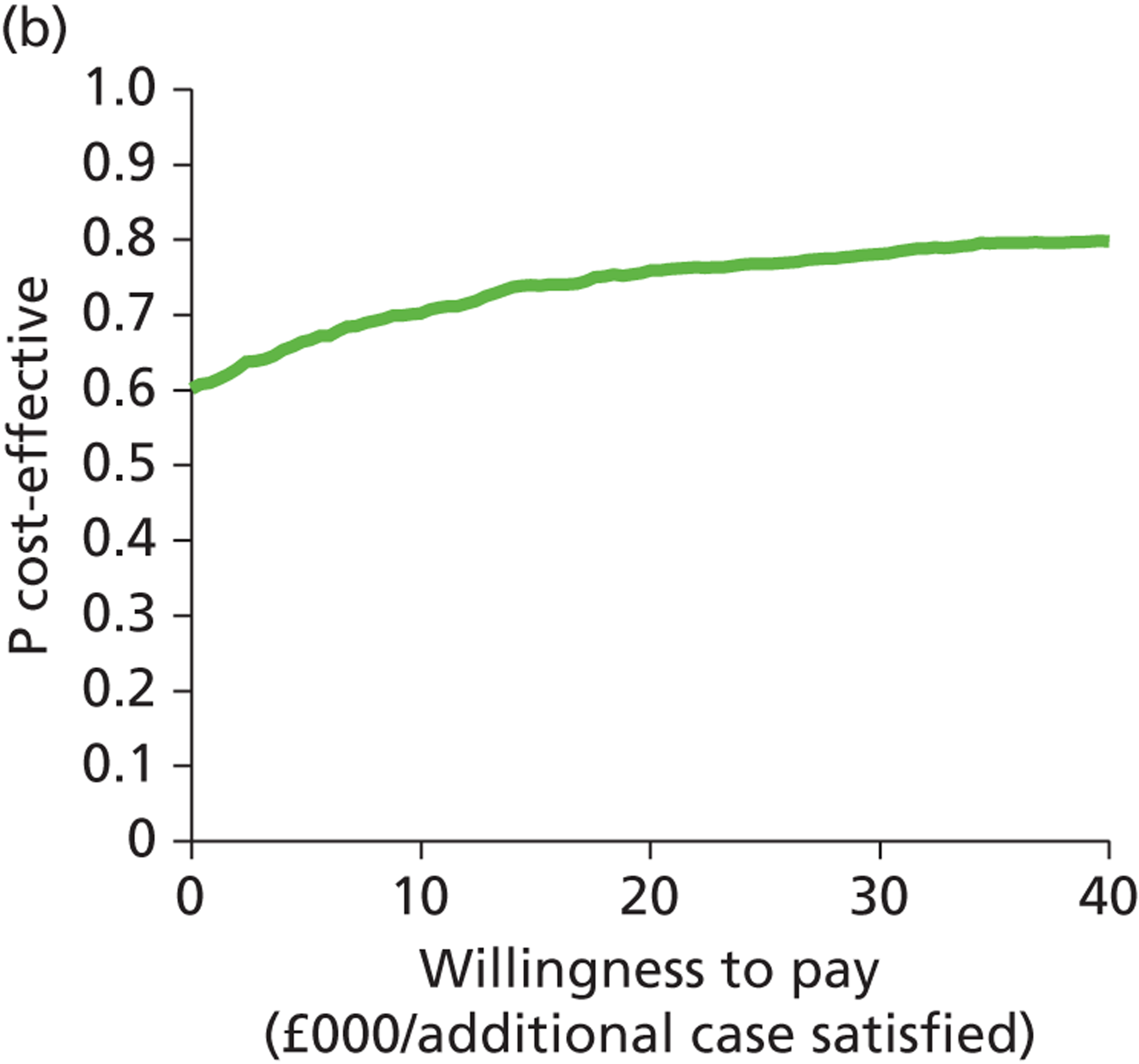
Deterministic sensitivity analysis results
Assessment of the impact of reducing the cost of saline infusion sonography
The probabilistic sensitivity analyses show uncertainty around whether OPH or SIS is the most cost-effective investigative strategy. To assess this uncertainty further, deterministic sensitivity analysis was performed to reduce the cost of SIS and determine at what cost it would become more cost-effective than OPH. Table 23 details the ICER values when the cost of SIS is reduced.
| SIS cost (£) | ICER (£) |
|---|---|
| 65 | 76 |
| 60 | 4006 |
| 55 | 7937 |
| 53 | 9509 |
| 52 | 10,295 |
| 50 | 11,867 |
| 45 | 15,797 |
| 35 | 23,658 |
In this analysis, the unit cost for SIS was reduced from £71 (base-case cost), keeping all other variables fixed. When the cost was reduced to £65, the strategy ‘SIS alone’ was no longer dominated by ‘OPH alone’. However, the modelled ICER was £76 per additional case satisfied, suggesting that OPH alone is still highly cost-effective compared with SIS alone. As the cost of SIS reduces further, the ICER increases (Figure 14). Considering an illustrative WTP of £10,000 per case satisfied, the ICER goes above this figure when the cost of SIS drops to £52. In that case, OPH is no longer cost-effective compared with SIS, and SIS becomes the preferred strategy (of the two) on cost-effectiveness grounds. It should also be noted that at a unit cost for SIS of £53 or lower, the strategy ‘SIS alone’ becomes less costly, as well as remaining more effective, than ‘LNG-IUS alone’.
FIGURE 14.
Incremental cost-effectiveness ratios between OPH and SIS when the cost of SIS is varied.
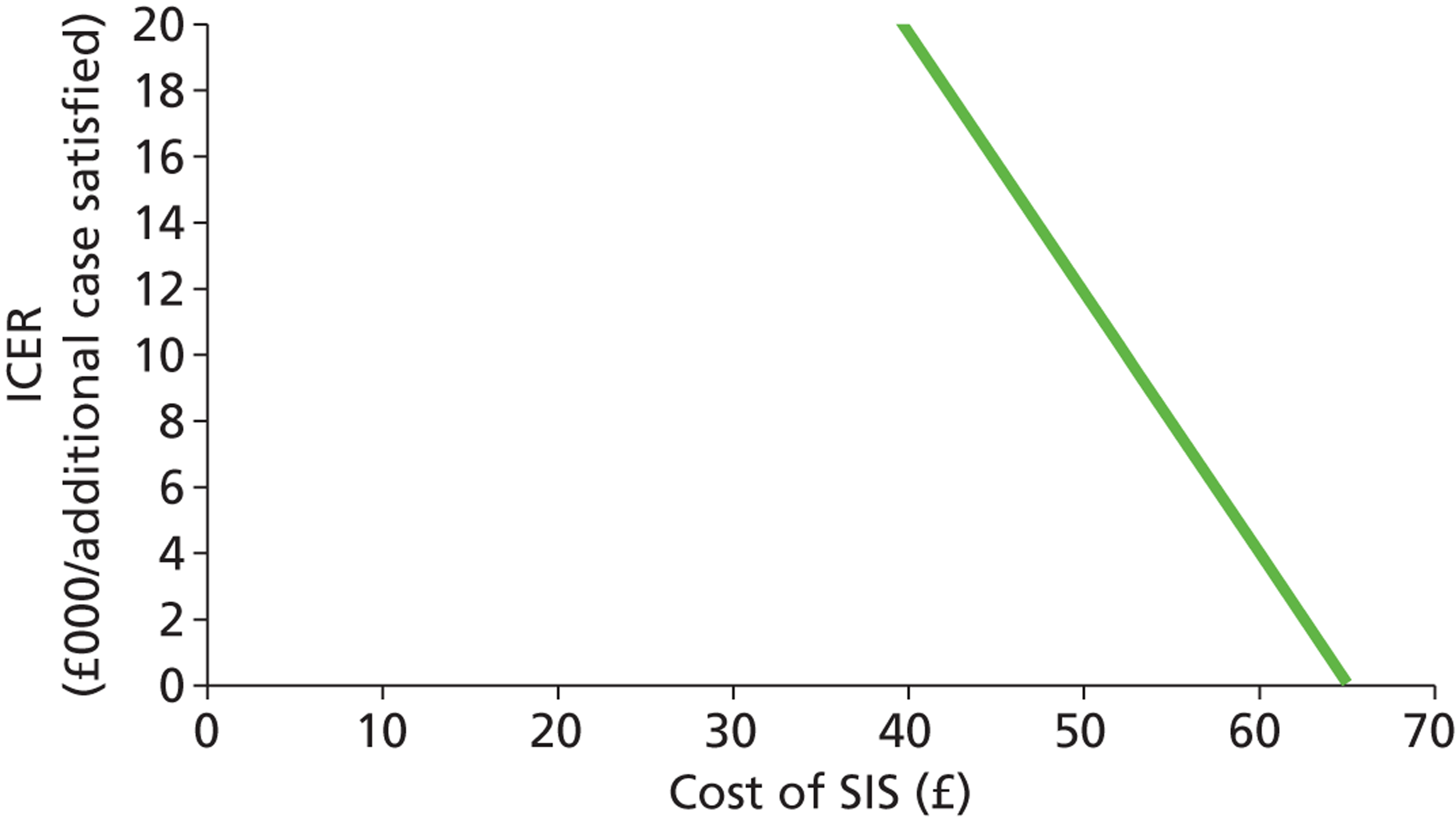
Assessment of the impact of the prevalence of focal uterine pathology
A high prevalence of intracavity focal endometrial lesions will favour OPH over the imaging technologies TVS and SIS because it is more likely to diagnose the lesions and treatment can be initiated with only a small additional cost during the diagnostic procedure (the so-called ‘see and treat’ approach). If the prevalence of endometrial polyps and SMFs is overestimated within the decision tree, OPH will falsely appear the most cost-effective. Similarly, the prevalence of DUB may be an underestimate. To assess the effect of prevalence on the analysis, the prevalence of polyps/SMFs was varied, keeping the prevalence of fibroids and endometrial disease fixed. The prevalence of DUB was changed inversely to compensate for the change in prevalence of polyp or SMF. All other variables in the model were fixed at their point estimates. Figure 15 shows the preferred option at a range of values of WTP per additional case satisfied, varying the prevalence of polyp or SMF. For example, at a prevalence of 30%, the combination of OPH and EBx is preferred if the WTP per additional case satisfied is more than about £27,000, while OPH alone is preferred if this WTP is between £2000 and £27,000. Only at a WTP below £2000 per case satisfied is LNG-IUS alone preferred. For this prevalence, other options are dominated and so not preferred at any WTP value.
FIGURE 15.
Incremental cost-effectiveness ratios between non-dominated options at a variety of prevalence values for polyps/SMFs and DUB.
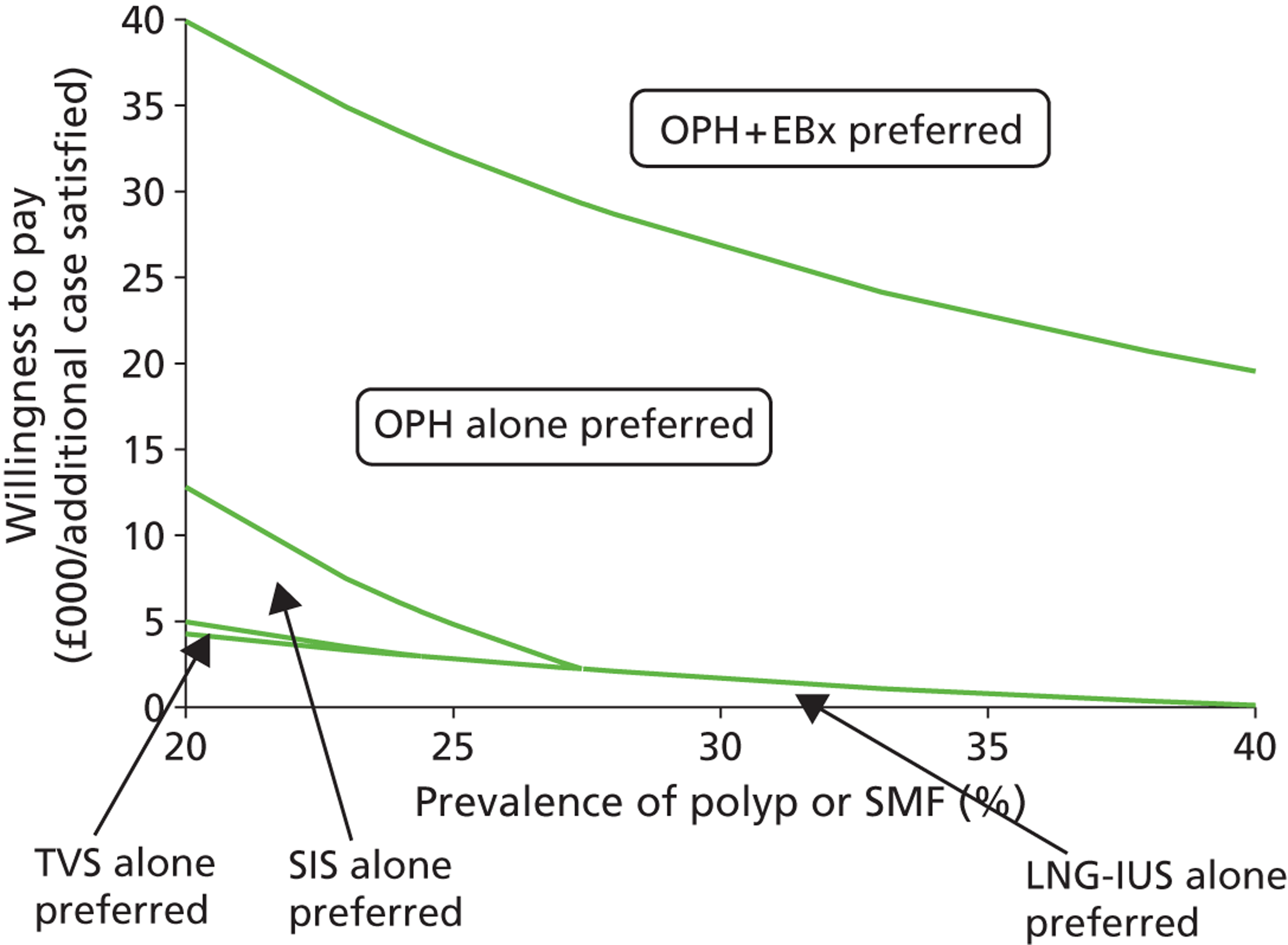
Deterministic results: women being managed during multiple clinic appointments
To reflect ‘traditional’ investigation and treatment of patients, over the course of multiple clinic appointments, the base-case tree was adapted and the results of the deterministic analysis are displayed in Table 24.
| Strategy | Cost (£) | Effectiveness (satisfaction) |
|---|---|---|
| LNG-IUS alone | 1067 | 0.9333 |
| TVS alone | 1204 | 0.9551 |
| EBx alone | 1214 | 0.9460 |
| SIS alone | 1217 | 0.9629 |
| TVS + EBx | 1266 | 0.9540 |
| SIS + EBx | 1274 | 0.9643 |
| OPH alone | 1288 | 0.9641 |
| TVS + OPH | 1315 | 0.9644 |
| OPH + EBx | 1317 | 0.9674 |
| SIS + OPH | 1343 | 0.9644 |
| TVS + OPH + EBx | 1391 | 0.9649 |
| SIS + OPH + EBx | 1418 | 0.9650 |
| Hysterectomy alone | 3182 | 0.9335 |
Outcomes
As with the base-case analysis, the ‘no investigation’ strategies were the least effective strategies for managing women. The most effective strategy for investigating women using a multiple clinic attendance as opposed to a ‘one-stop’ approach was the combination of OPH and EBx.
Costs
Costs for the ‘no investigation’ strategies (LNG-IUS alone and hysterectomy alone) remained unchanged. The costs of the investigative strategies, however, increased due to the additional appointments required, with the costs for the investigation strategies ranging from £1204 to £1418 in this alternative analysis compared with £1078 to £1256 in the base-case analysis.
Cost-effectiveness and dominance
The strategies ‘EBx alone’, ‘TVS + EBx’, ‘OPH alone’, ‘SIS + OPH’, ‘TVS + OPH + EBx’, ‘SIS + OPH + EBx’, and ‘hysterectomy alone’ are excluded by simple dominance and the strategies ‘TVS alone’, ‘SIS + EBx’ and ‘TVS + OPH’ are excluded by extended dominance. The remaining three strategies are not dominated. In contrast to the base-case analysis the strategy SIS alone is no longer dominated, whereas ‘OPH alone’ is. Table 25 displays the non-dominated strategies from deterministic analysis.
| Strategy | Cost (£) | Incremental cost (£) | Effectiveness | Incremental effectiveness | ICER (£) |
|---|---|---|---|---|---|
| LNG-IUS alone | 1067 | 0.9333 | |||
| SIS alone | 1217 | 150 | 0.9629 | 0.0296 | 5070 |
| OPH + EBx | 1317 | 100 | 0.9674 | 0.0045 | 22,100 |
Using SIS to investigate women in this strategy costs an additional £5070 to make an extra woman satisfied compared with not investigating and giving all women a LNG-IUS. OPH + EBx costs an additional £22,100 to the cost of SIS to gain a further satisfied patient. OPH alone does not appear as a non-dominated option in this analysis. The line on the cost-effectiveness plane in Figure 16 links the non-dominated strategies.
FIGURE 16.
Cost-effectiveness plane showing the results of deterministic analysis for strategies to investigate women presenting with HMB managed over multiple clinic appointments (hysterectomy removed).
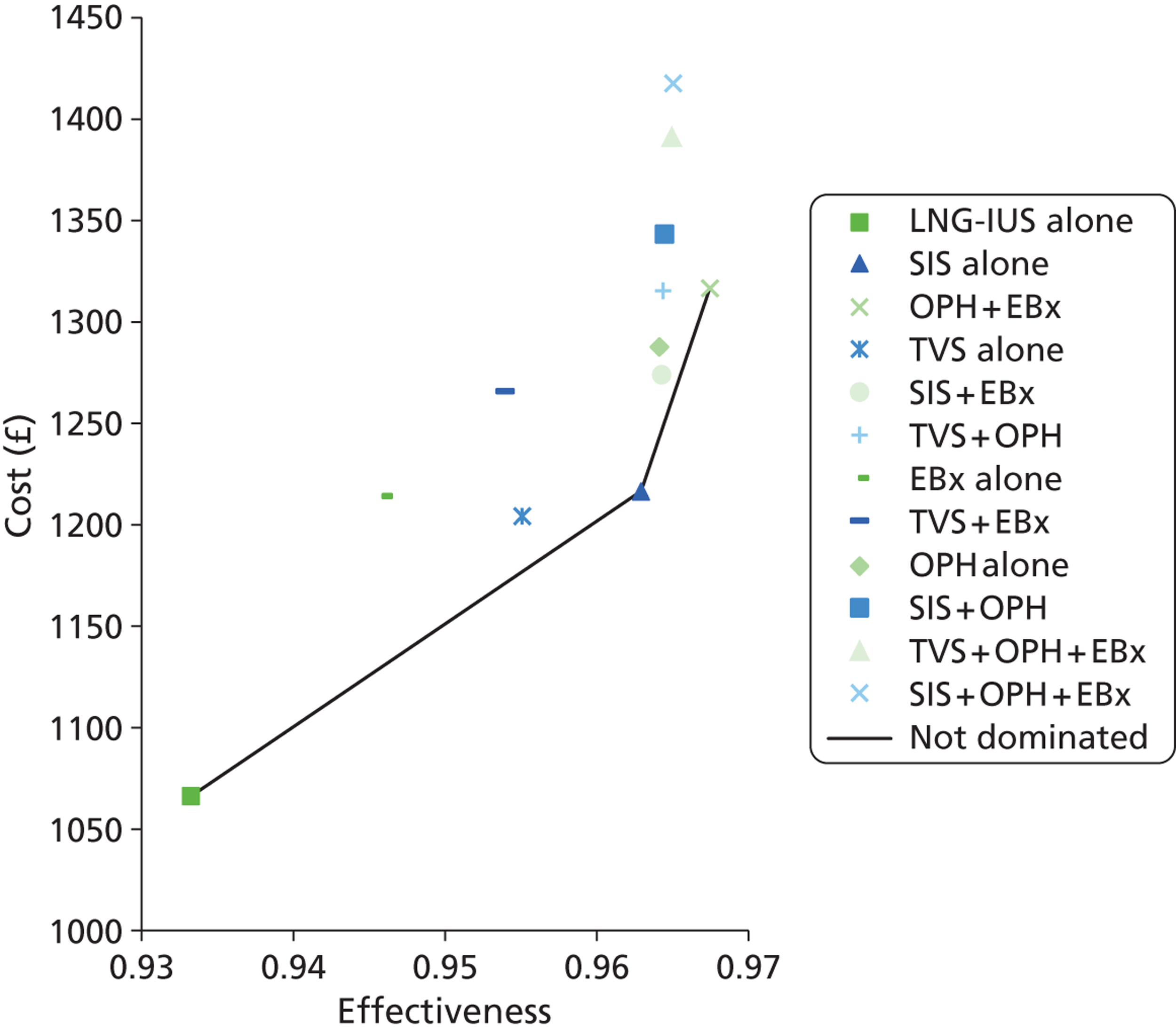
Probabilistic sensitivity analysis results: women being managed during multiple clinic appointments
In the cost-effectiveness scatterplot below (Figure 17) it is clear from the degree of overlap of the diagnostic strategies that there is uncertainty regarding which one might be considered most cost-effective when a range of values is sampled from the distributions of the data values.
FIGURE 17.
Scatterplot showing the uncertainty in costs and effectiveness within the model for each of the individual strategies for investigating women with HMB managed over multiple clinic appointments.
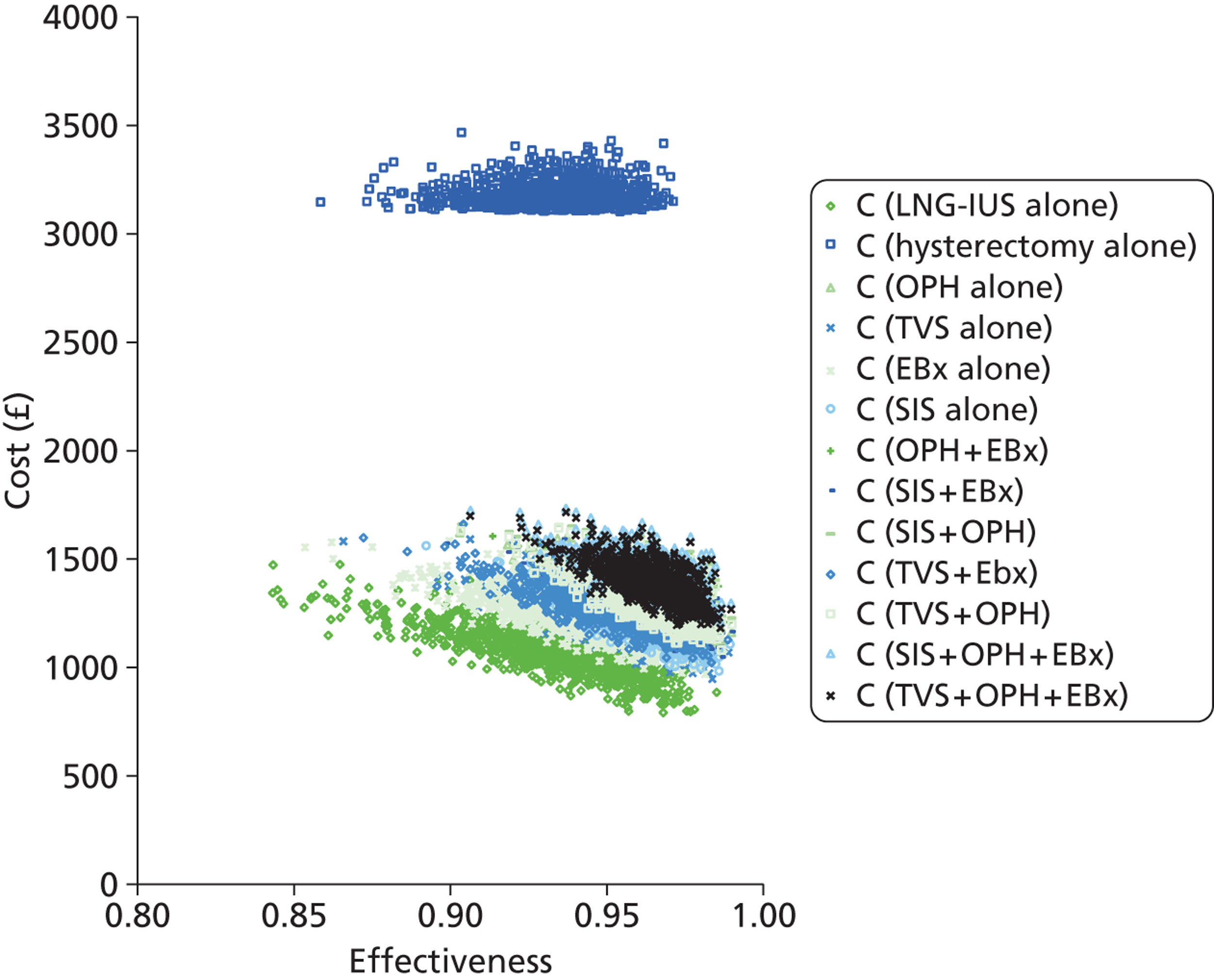
The CEAF (Figure 18) illustrates the overall uncertainty related to the optimal decision across a range of plausible WTP values, where here the WTP is measured in cost per additional case satisfied. It appears that up to a WTP value of approximately £5000, LNG-IUS alone is cost-effective; however, at a WTP between £5000 and £20,000, SIS alone may be preferable, but there is reasonable uncertainty whether or not this really is the optimal strategy, with the probability lying between 40% and 60%. Above £20,000, OPH + EBx becomes the preferred strategy.
FIGURE 18.
Cost-effectiveness acceptability frontier showing the optimal investigative strategies for women with HMB managed over multiple clinic appointments.
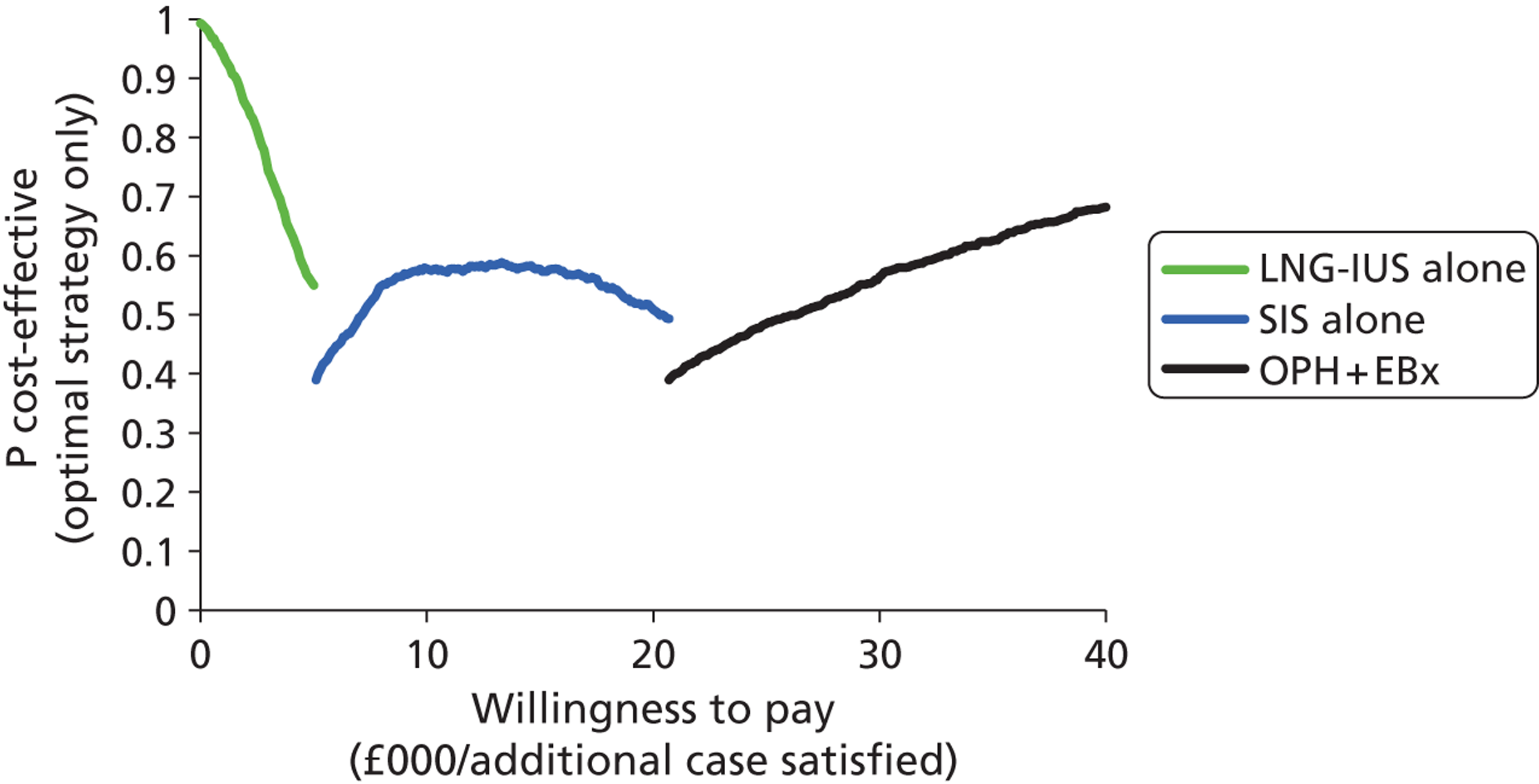
Probabilistic pairwise comparisons were made between the non-dominated strategies to explore the uncertainty between the strategies. TVS was also compared with SIS alone because of its proximity to the line of non-dominance. The results of these analyses are displayed in Appendix 5 and confirm the findings from the deterministic analysis.
Deterministic results: prior treatment with the levonorgestrel-releasing intrauterine system
The base-case economic analysis assumed that the women presenting to the gynaecologist with HMB had not had any treatment in primary care. NICE recommend that women with HMB should be treated with a LNG-IUS in primary care and referred to a gynaecologist only if symptoms persist, structural abnormality is expected or contraindications exist. 11 In practice, only around 25% of women referred from primary care have received prior treatment with the LNG-IUS. 149 However, this report could be criticised for neglecting the fact that, ideally, women coming to clinic will already have had a LNG-IUS, and therefore an alternative analysis was performed to examine this ‘ideal’ scenario and assess whether or not the preferred cost-effective investigative strategies delineated in the base-case analysis are altered. Thus, disease prevalence was adjusted to reflect the fact that women treated appropriately with a LNG-IUS in primary care (i.e. without intracavity pathology, endometrial cancer or large fibroids) were less likely to have persistent symptoms and need referral to a gynaecologist. It was assumed that fertility was not desired in this analysis, as in the base-case analysis. The strategy LNG-IUS alone could no longer be used as the comparison strategy (now being redundant) and was replaced by a strategy of ‘no further intervention’ (i.e. attending clinic with a LNG-IUS in situ, or seeing a gynaecologist but deciding not to have any further intervention).
Outcomes
Direct treatment without preliminary diagnostic testing was less effective than treatment instigated after diagnostic testing. The most effective strategy was combination testing with TVS and EBx; however, the difference between all of the diagnostic strategies was minimal, ranging from 96.28% to 96.39%.
Costs
The results presented in Table 26 show that all costs have increased compared with the base-case analysis, reflecting the increased prevalence of organic uterine pathology requiring more expensive treatments. Adopting no further treatment and persevering with the LNG-IUS treatment alone (reference strategy) was the cheapest option, costing £1355 per woman treated for HMB in a secondary care setting. The strategy of hysterectomy for all women bypassing the need for diagnostic work-up was the most expensive at £3218 per woman treated, that is to say £1863 more than the approach of LNG-IUS treatment alone. The cheapest diagnostic testing strategy was the use of OPH alone, costing £1681 for every woman treated, that is to say £326 more than continuation with LNG-IUS treatment.
| Strategy | Cost (£) | Effectiveness (satisfaction) |
|---|---|---|
| No further intervention | 1355 | 0.9039 |
| OPH alone | 1681 | 0.9633 |
| SIS alone | 1711 | 0.9633 |
| TVS + OPH | 1746 | 0.9633 |
| SIS + OPH | 1775 | 0.9633 |
| TVS alone | 1785 | 0.9633 |
| OPH + EBx | 1796 | 0.9628 |
| TVS + OPH + EBx | 1840 | 0.9628 |
| SIS + EBx | 1846 | 0.9628 |
| SIS + OPH + EBx | 1864 | 0.9629 |
| EBx alone | 1942 | 0.9628 |
| TVS + EBx | 1980 | 0.9639 |
| Hysterectomy alone | 3218 | 0.9378 |
Cost-effectiveness and dominance
The testing strategies OPH alone and TVS combined with EBx (OPH + EBx) remain non-dominated by alternative empirical treatment or diagnostic testing strategies. All of the remaining strategies are dominated by OPH alone except for hysterectomy, which is dominated by TVS and EBx. Table 27 presents the deterministic analysis restricted to the non-dominated competing strategies.
| Strategy | Cost (£) | Incremental cost (£) | Effectiveness | Incremental effectiveness | ICER (£) |
|---|---|---|---|---|---|
| No further intervention | 1355 | 0.9039 | |||
| OPH alone | 1681 | 326 | 0.9633 | 0.0594 | 5480 |
| TVS + EBx | 1980 | 299 | 0.9639 | 0.0006 | 516,000 |
The line on the graph in Figure 19 joins the non-dominated strategies, LNG-IUS only, OPH alone and TVS + EBx. When LNG-IUS and hysterectomy are removed the relationship of the other strategies to the line of non-dominance is clearer (Figure 20).
FIGURE 19.
Cost-effectiveness plane showing the results of deterministic analysis for strategies to investigate women with HMB with a LNG-IUS in situ.
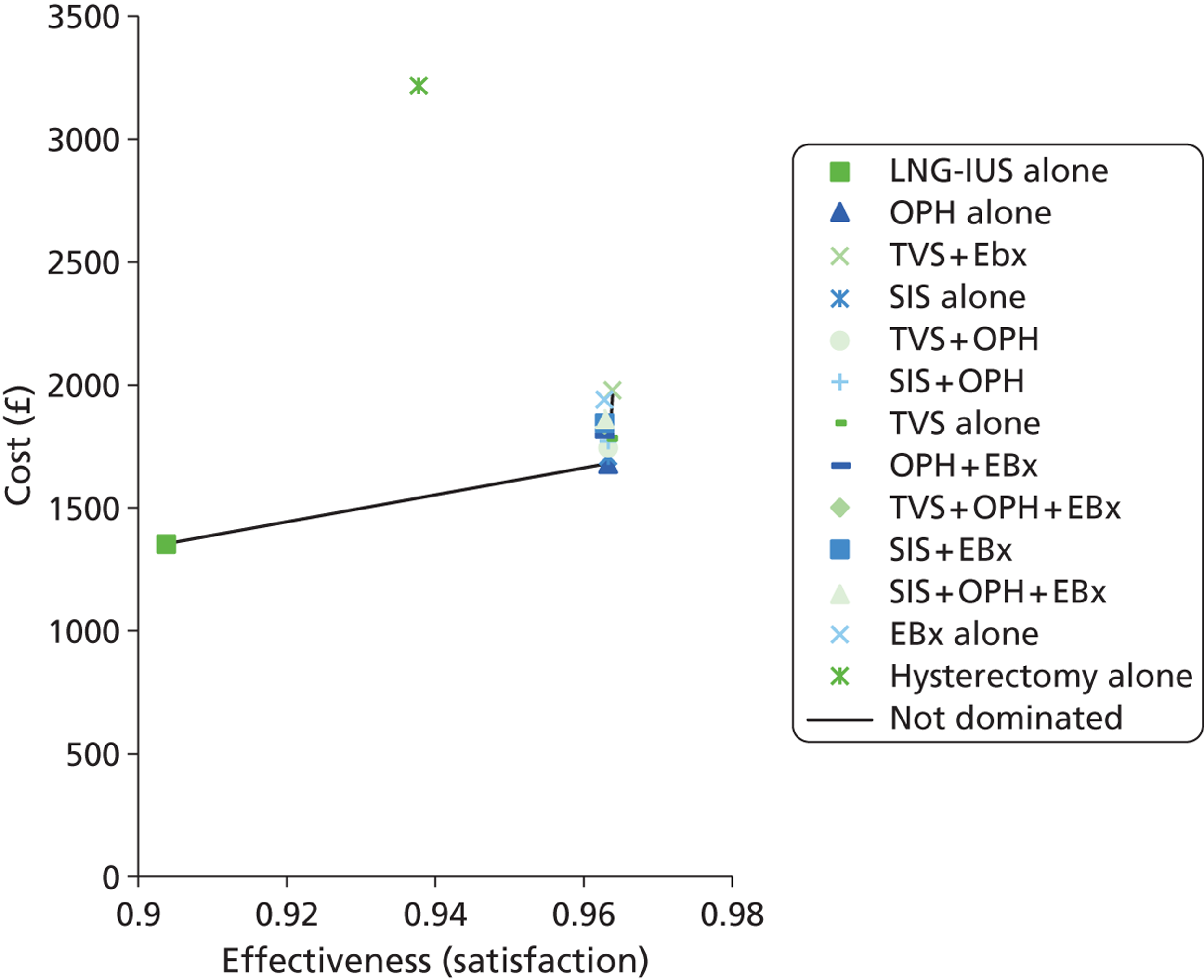
FIGURE 20.
Cost-effectiveness plane showing the results of deterministic analysis for strategies to investigate women with HMB with a LNG-IUS in situ (hysterectomy alone and LNG-IUS alone not shown).
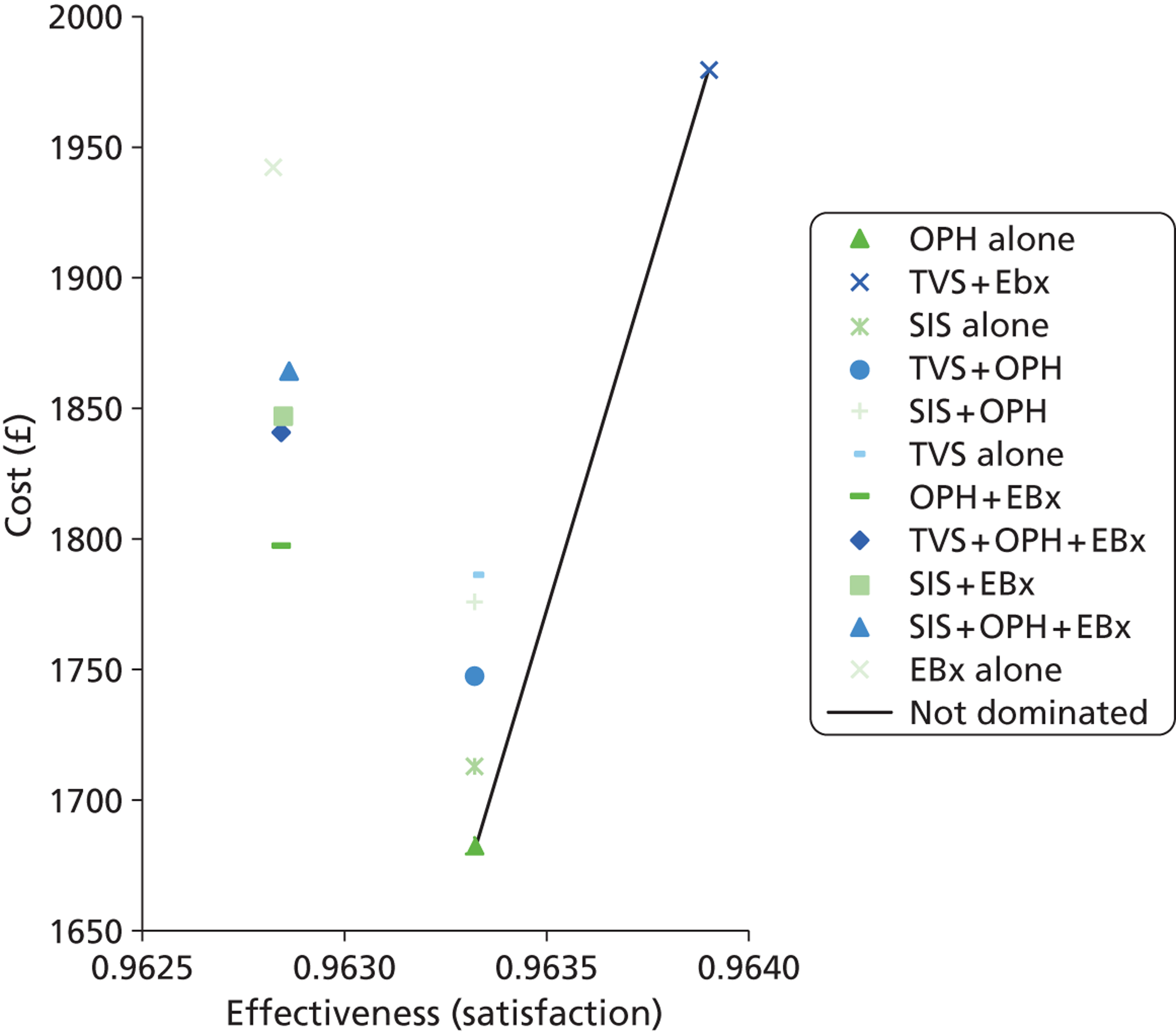
Probabilistic sensitivity analysis results: prior treatment with the levonorgestrel-releasing intrauterine system
In the cost-effectiveness scatterplot below (Figure 21) it is clear from the degree of overlap of the diagnostic strategies that there is uncertainty regarding which one might be considered most cost-effective when a range of values is sampled from the distributions of the data values.
FIGURE 21.
Scatterplot showing the uncertainty in costs and effectiveness within the model for each of the individual strategies for investigating women with HMB with a LNG-IUS in situ.
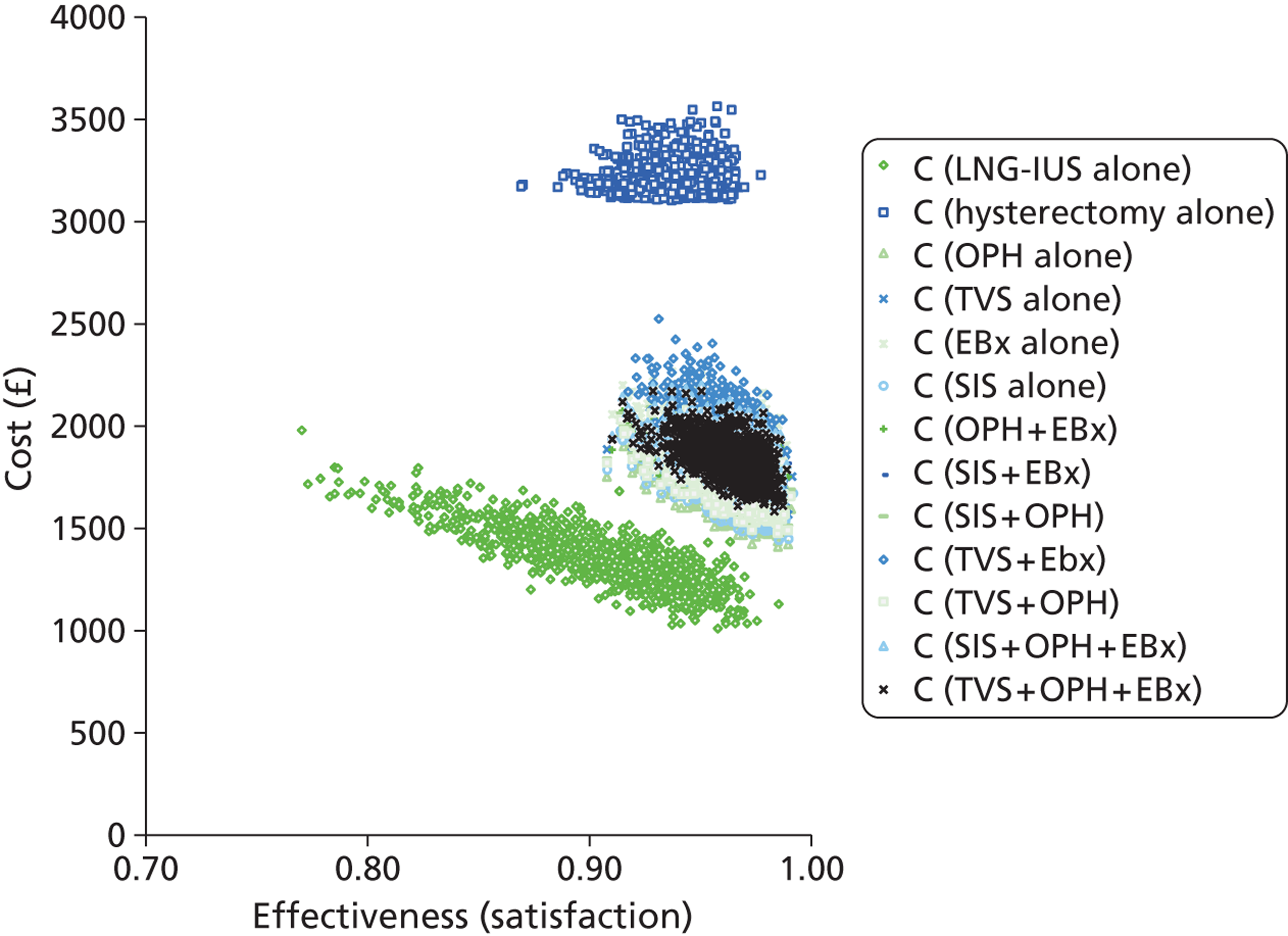
The CEAF (Figure 22) illustrates the overall uncertainty related to the optimal decision across a range of plausible WTP values, where here the WTP is measured in cost per additional case satisfied. The likelihood is that OPH is cost-effective compared with continuing with the LNG-IUS treatment at WTP thresholds of around £20,000. It can be seen that OPH is the preferred option at lower WTP values but that there is considerable uncertainty as the WTP falls below £10,000.
FIGURE 22.
Cost-effectiveness acceptability frontier showing the optimal investigative strategies for women with HMB with a LNG-IUS in situ.
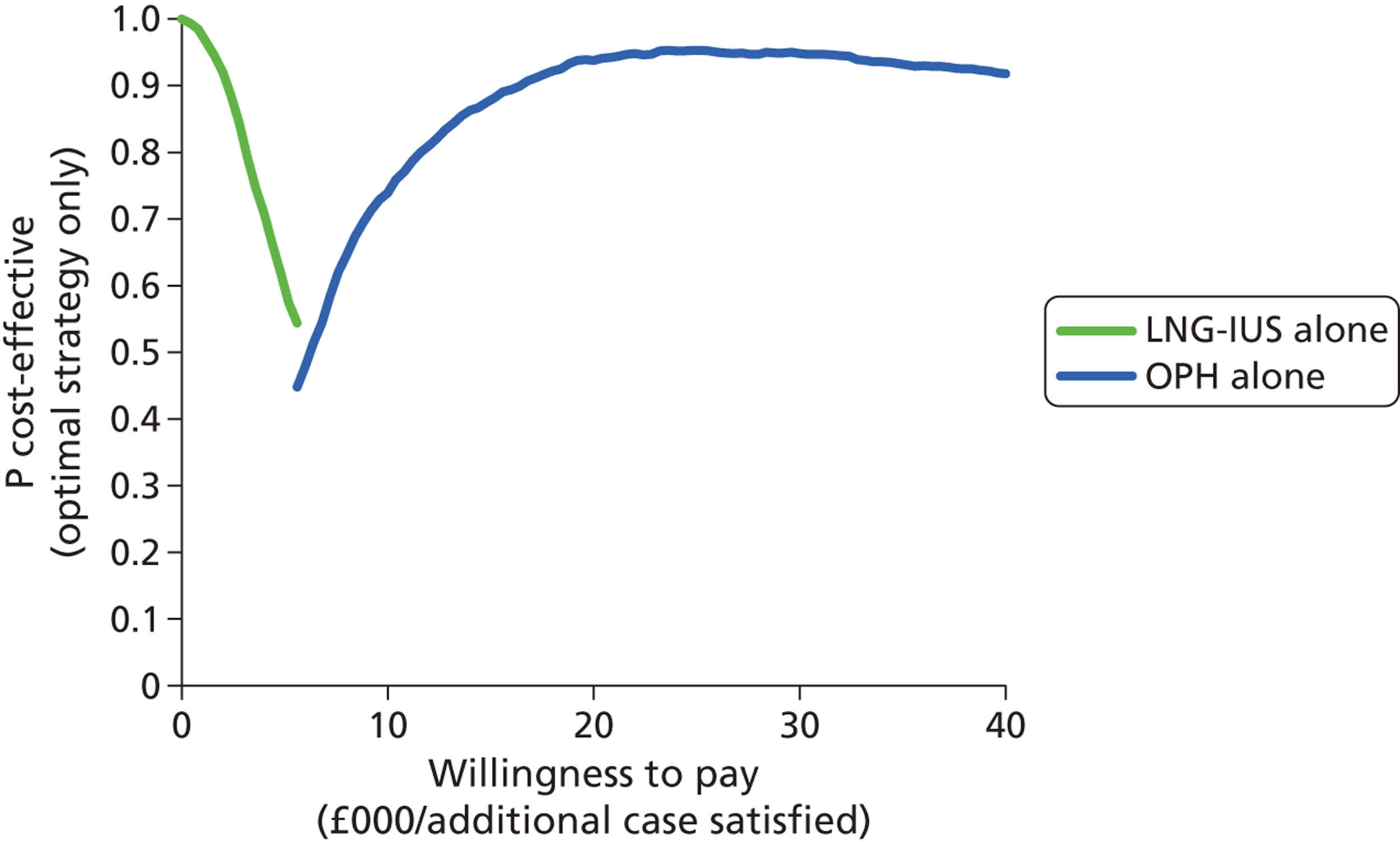
Probabilistic pairwise comparisons were made between the non-dominated strategies to explore the uncertainty between the strategies. SIS was also compared with OPH alone because of its proximity to the line of non-dominance. The results of these analyses are displayed in Appendix 6 and they show that OPH alone was likely to be the most cost-effective strategy for investigating women with HMB who had already received treatment with a LNG-IUS in primary care.
Deterministic analysis: women wishing to preserve their fertility
Table 28 shows the deterministic analysis of the 12 strategies following reconfiguration of the decision trees to reflect women who would not have a hysterectomy or an EA to treat their HMB because they wished to maintain their long-term fertility.
| Strategy | Cost (£) | Effectiveness (satisfaction) |
|---|---|---|
| LNG-IUS alone | 421 | 0.6557 |
| SIS alone | 800 | 0.8467 |
| OPH alone | 844 | 0.8629 |
| SIS + OPH | 944 | 0.8649 |
| TVS alone | 740 | 0.8033 |
| EBx alone | 754 | 0.7419 |
| TVS + EBx | 870 | 0.8020 |
| TVS + OPH | 913 | 0.8389 |
| OPH and EBx | 914 | 0.8618 |
| SIS + EBx | 971 | 0.8473 |
| TVS + OPH + EBx | 971 | 0.8572 |
| SIS + OPH + EBx | 1003 | 0.8584 |
Outcomes
Satisfaction rates are reduced when compared with the original analysis, as optimal surgical interventions precluding future fertility are not available for women with large fibroids or those resistant to the LNG-IUS. This is reflected by the lower satisfaction rate of 65.6% for this analysis compared with 93.33% in the original when women receive the LNG-IUS without any investigation. Satisfaction rates for the investigative strategies range from 74.19% for EBx to 86.49% for SIS + OPH. There is greater variation between the satisfaction rates in this analysis than in the base case, when values varied marginally between 94.6% and 96.7%.
Costs
It should be noted that the costs decreased when compared with the original analysis. Cost is decreased because the more expensive treatments tend to be the surgical options (EA and hysterectomy) which are contraindicated in women desiring preservation of their fertility. The cost of LNG-IUS alone has decreased to £421 from £1066 in the base-case analysis because the women identified to have large fibroids do not undergo a hysterectomy and women who are dissatisfied with the LNG-IUS cannot be offered any further treatment. The cheapest investigative strategy in this analysis is TVS alone, costing £740 per patient. The most expensive strategy is the combination of SIS, OPH and EBx, costing £1003 per patient.
Cost-effectiveness and dominance
The strategy of EBx alone is dominated by TVS alone (i.e. this is a cheaper and more effective option), which in turn is dominated by extended dominance due to a blend of LNG-IUS alone and SIS alone. The remaining strategies are dominated by either OPH alone or SIS and OPH together. Once the dominated strategies were removed, the testing strategies which remained were SIS alone, OPH alone, and SIS and OPH together. This can be more clearly appreciated in Table 29. In contrast to the base-case analysis, a combination strategy of OPH + EBx is not potentially cost-effective. Moreover, SIS alone or in combination with OPH is non-dominated, whereas in women without the need to preserve their fertility (base case), SIS and related strategies were not cost-effective.
| Strategy | Cost (£) | Incremental cost (£) | Effectiveness (satisfaction) | Incremental effectiveness | ICER (£) |
|---|---|---|---|---|---|
| LNG-IUS alone | 421 | 0.6557 | |||
| SIS alone | 800 | 378 | 0.8467 | 0.1910 | 1980 |
| OPH alone | 844 | 44 | 0.8629 | 0.0162 | 2720 |
| SIS + OPH | 955 | 100 | 0.8649 | 0.0020 | 50,300 |
The line on the cost-effectiveness plane (Figure 23) joins the non-dominated strategies, starting with the base case of LNG-IUS which joins to SIS, followed by OPH and then SIS + OPH. TVS lies close to this line and, therefore, when exploring the results, TVS was included to see whether or not analysing the spread of results might suggest that TVS could become cost-effective when values are varied around their point estimates.
FIGURE 23.
Total costs and effectiveness of the alternative strategies for the diagnostic work up of HMB for women wishing to preserve their fertility, excluding the strategy of hysterectomy.
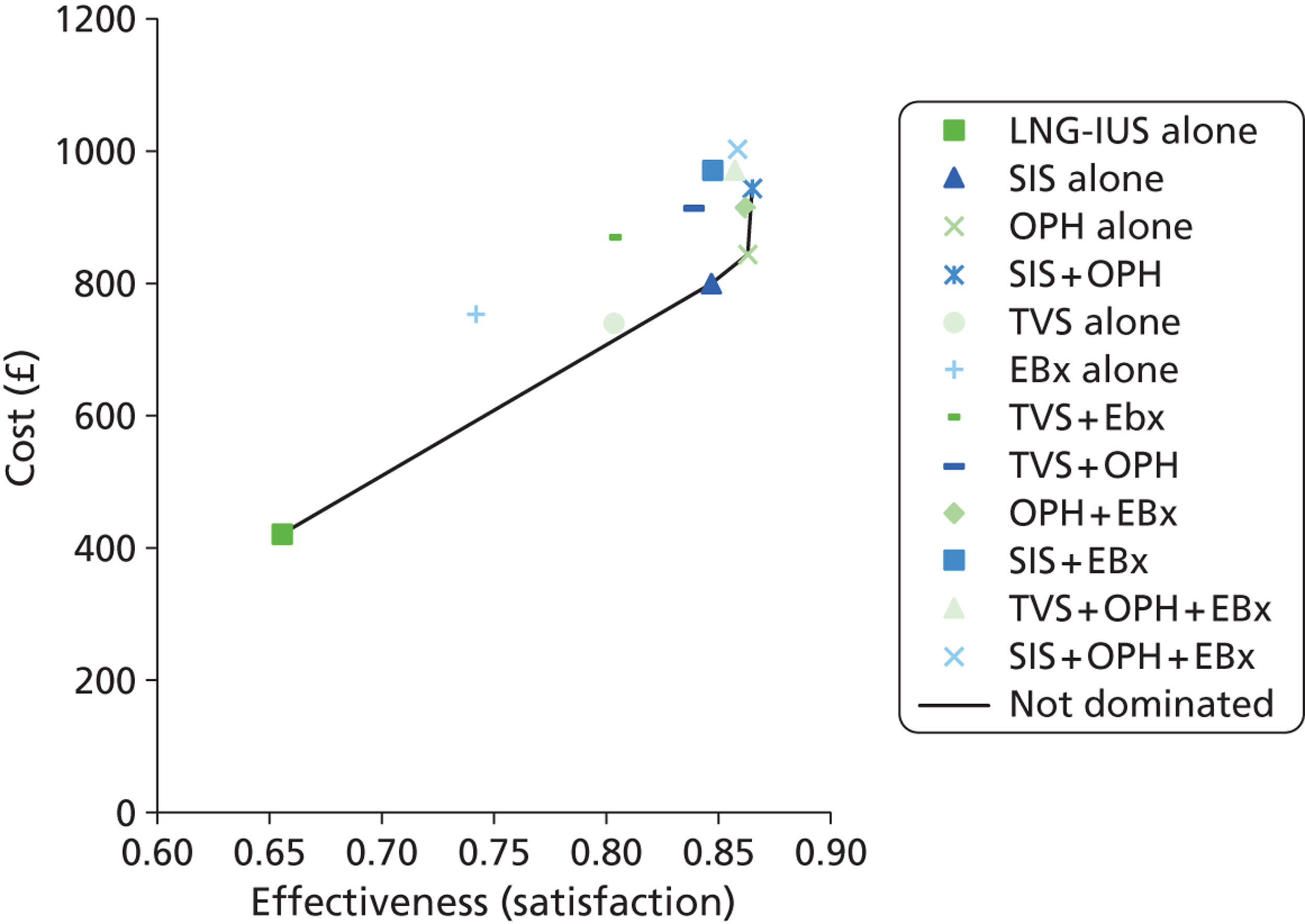
Probabilistic sensitivity analysis results: women wishing to preserve their fertility
Figure 24 shows the uncertainty around the absolute cost and effectiveness values for each of the strategies. Hysterectomy alone has been removed as it is too expensive to be a competing strategy and removing it allows clearer presentation of the other strategies. There is overlap between strategies.
FIGURE 24.
Scatterplot showing the uncertainty in costs and effectiveness within the model for each of the individual strategies for investigating women with HMB who wish to preserve their fertility.
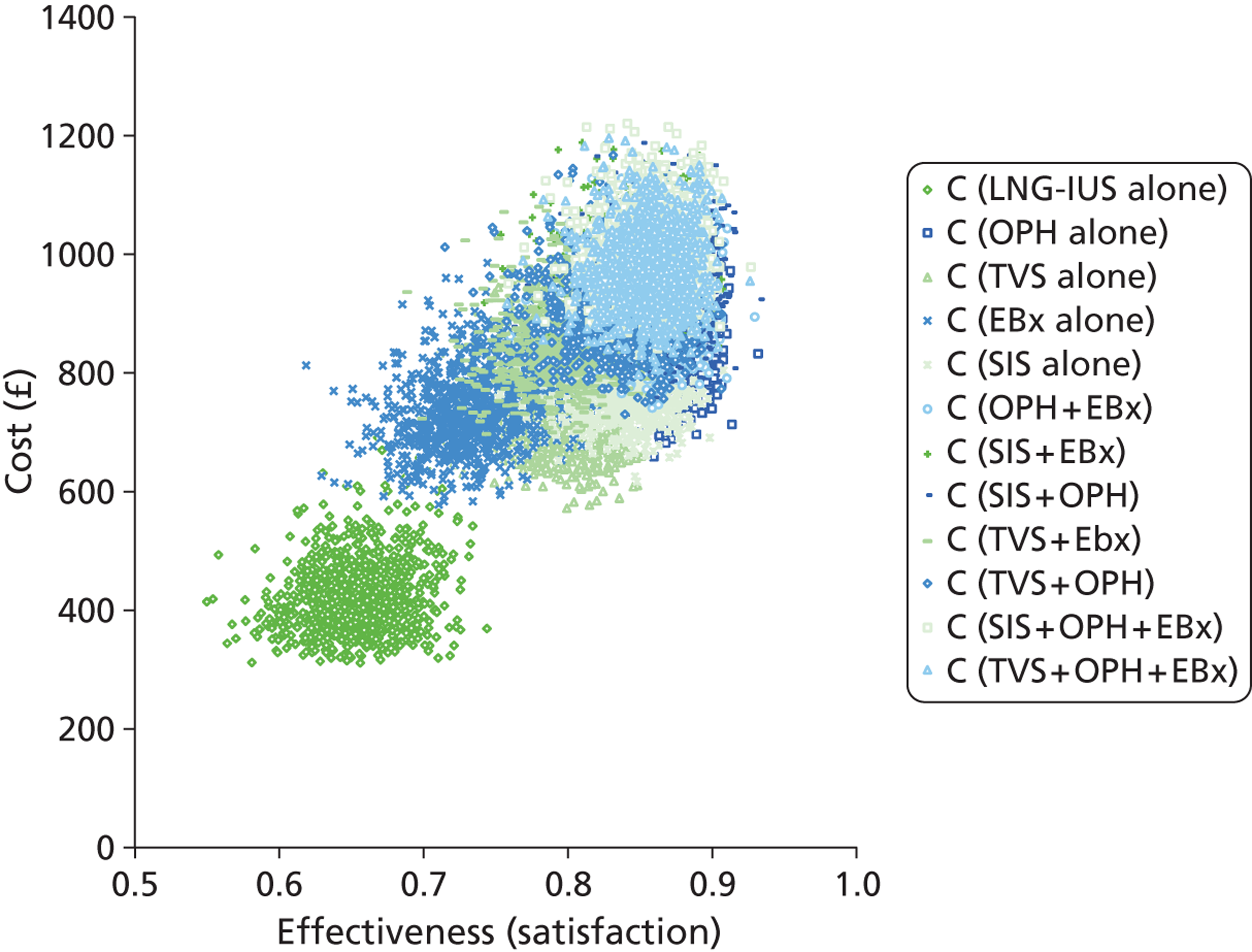
The acceptability frontier in Figure 25 shows how likely the non-dominated strategies are to be the most cost-effective option at a range of WTP thresholds. The strategy SIS + OPH is not plotted on the CEAF because it only becomes cost-effective at a WTP too high to be acceptable to the NHS.
FIGURE 25.
Cost-effectiveness acceptability frontier showing the preferred diagnostic strategy over a range of WTP thresholds for women who wish to preserve their fertility.
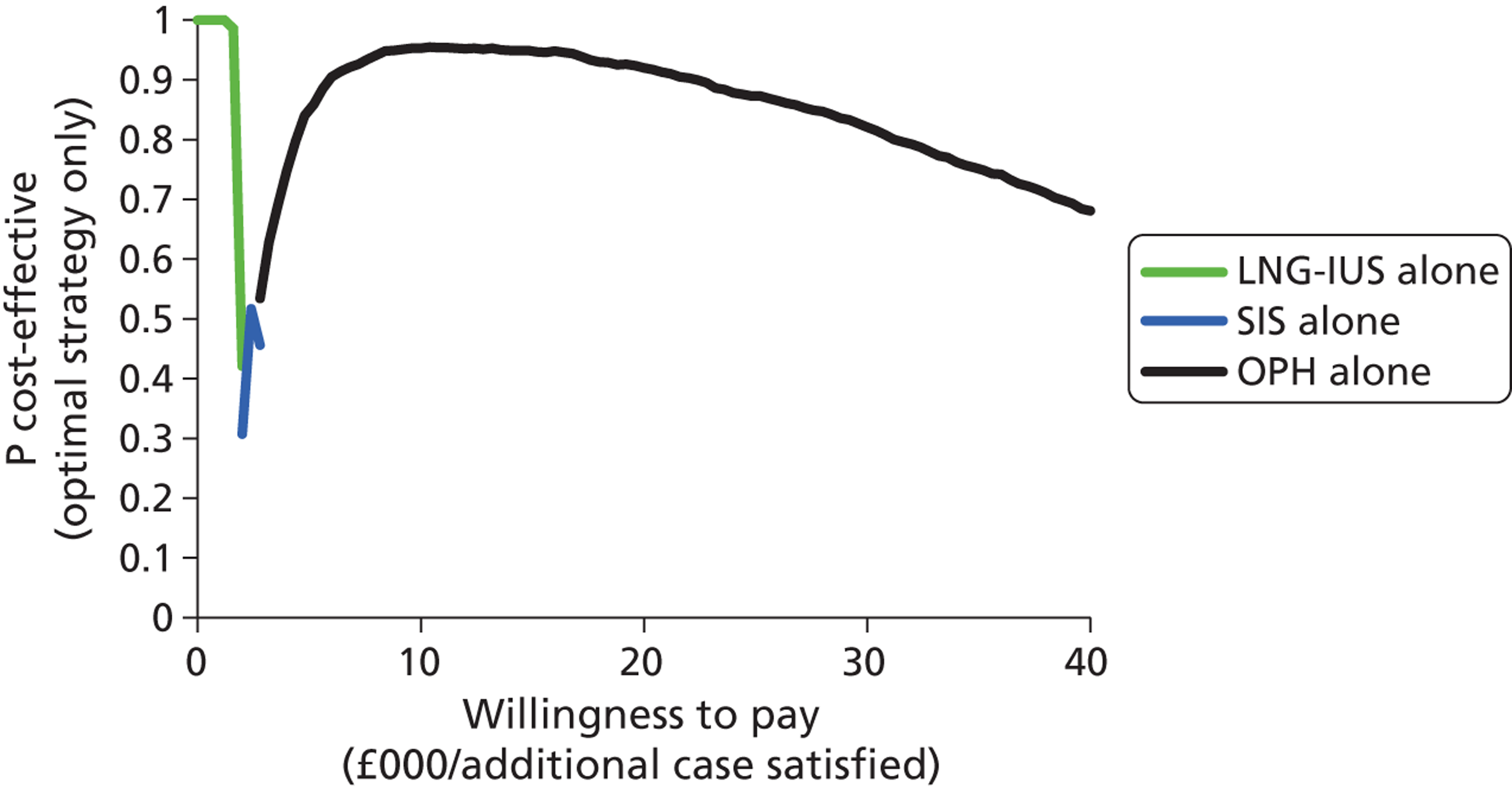
The uncertainty represented in the CEAF is explored by considering pairwise comparisons between the adjacent non-dominated options. TVS is also explored because its mean is close to the non-dominance line. The results of the pairwise comparisons are displayed in Appendix 7 and confirm that OPH is likely to be the most cost-effective strategy for investigating women with HMB who wish to preserve their fertility.
Chapter 4 Economic evaluation: post-menopausal bleeding
Deterministic results of the economic analysis of strategies to investigate post-menopausal bleeding
Table 30 reports the deterministic results, referencing all other diagnostic strategies to the option of no diagnostic work-up.
| Strategy | Cost (£) | Effectiveness (5-year survival) |
|---|---|---|
| No (initial) work-up (reference case) | 439 | 0.987675 |
| Selective TVS with history | 537 | 0.988430 |
| OPH | 550 | 0.988382 |
| History + TVS | 554 | 0.988437 |
| TVS 5 mm | 561 | 0.988415 |
| History only | 567 | 0.988385 |
| EBx | 602 | 0.988367 |
| TVS + OPH | 646 | 0.988471 |
| EBx + OPH | 679 | 0.988404 |
| TVS + EBx | 684 | 0.988403 |
| TVS + EBx + OPH | 727 | 0.988404 |
| D&C (reference case) | 1400 | 0.988382 |
Outcomes
Some form of patient evaluation in PMB, whether this be confined to obtaining germane patient information from clinical history taking alone, employing testing modalities or some combination of both approaches, was more effective than the reference case of no initial testing at all. In contrast to costs, effectiveness in terms of 5-year survival rates was similar regardless of investigative strategy adopted. A decision to undertake no testing on initial presentation, representing the least effective approach with PMB, was associated with a 5-year survival rate of 98.77%. EBx was the least effective investigative strategy with an associated 5-year survival rate of 98.84%, whereas the most effective testing strategy, ‘TVS + OPH’, was associated with a 5-year survival rate of 98.85%. Thus, only small improvements in survival were observed regardless of strategy adopted and this reflects the relatively small incidence of endometrial cancer (5%), a malignancy associated with a predominantly early stage at diagnosis amenable to curative therapy in many.
The acquisition of patient characteristics from the clinical history was more effective if used to identify higher risk women for TVS (‘selective TVS with history’) compared with universal application of these characteristics without TVS (‘history alone’). Corresponding 5-year survival rates were similar.
Costs
The reference option of ‘no initial diagnostic testing’ (diagnosis being delayed until representation with continuing PMB symptoms) was the cheapest strategy, costing £439 per woman investigated for PMB in a secondary care setting. The combination testing strategy of TVS with EBx and OPH was the most expensive at £727 per woman treated, that is to say £288 more than the approach of no initial testing. The cheapest of the 11 investigative strategies was the taking account of patient characteristics by acquiring the patient history and performing a TVS for those deemed to be high risk and then performing EBx when a thickened endometrium was diagnosed. This approach cost £537 (£98 more than no initial testing), whereas the least expensive of the diagnostic testing strategies, OPH alone, cost £550 for every woman investigated, that is to say £13 more than selective TVS with history and £111 more than the reference case – no initial testing. D&C was too expensive at £1400 to be used as a reference case for the analysis.
Cost-effectiveness and dominance
Three of the 11 diagnostic strategies, ‘selective TVS with history’, ‘history + TVS’ and ‘TVS + OPH’, remained non-dominated by alternative investigation approaches for first presentation of PMB. ‘OPH alone’ is dominated by ‘selective TVS with history’. ‘TVS with a cut-off of 5 mm’, ‘history only’ and ‘EBx alone’ are all dominated by ‘history + TVS’, and ‘TVS + EBx + OPH’, ‘TVS + EBx’ and ‘EBx + OPH’ are all dominated by ‘TVS + OPH’. There were no cases of extended dominance.
Table 31 presents the deterministic analysis restricted to the non-dominated competing strategies.
| Strategy | Cost (£) | Incremental cost (£) | Effectiveness | Incremental effectiveness | ICER (£ per additional 5-year survival) |
|---|---|---|---|---|---|
| No (initial) workup (reference case) | 439 | 0.987675 | |||
| Selective TVS with history | 537 | 98 | 0.988430 | 0.000755 | 129,000 |
| History + TVS | 554 | 17 | 0.988437 | 0.000007 | 2,410,000 |
| TVS + OPH | 646 | 92 | 0.988471 | 0.000034 | 2,690,000 |
The cheapest strategy is the base-case scenario of no diagnostic work-up at initial presentation with PMB. The most effective strategy is the combination of testing at the outset with TVS and OPH, but this approach comes at a greater cost, generating an ICER of £2.69M to gain an extra woman with PMB surviving at 5 years. Other non-dominated options have ICERs of over £2M per additional survivor, compared with the next most effective strategy, and are thus most unlikely to be cost-effective.
Figure 26 shows the total costs and effectiveness of alternative strategies for the diagnostic work-up of women presenting for the first time with PMB for endometrial cancer to secondary care. The line presented graphically joins the non-dominated strategies (‘selective TVS with history’, ‘history + TVS’ and ‘TVS + OPH’) to the reference case, no initial investigation. Any strategy plotted above this line is not considered cost-effective in relation to the non-dominated alternatives.
FIGURE 26.
Total costs and effectiveness of each of the 12 alternative strategies to investigate OMB. History only, strategy selecting women for EBx based on the patient characteristics model; history + TVS, strategy selecting women for EBx based on the patient characteristics and TVS model; No work up, no initial diagnostic testing nor consideration of patient characteristics from the history, diagnostic testing being delayed until representation with continuing PMB symptoms was used as the reference case; Selective TVS with history, strategy with selective use of TVS in high-risk women only based on the patient characteristics model.
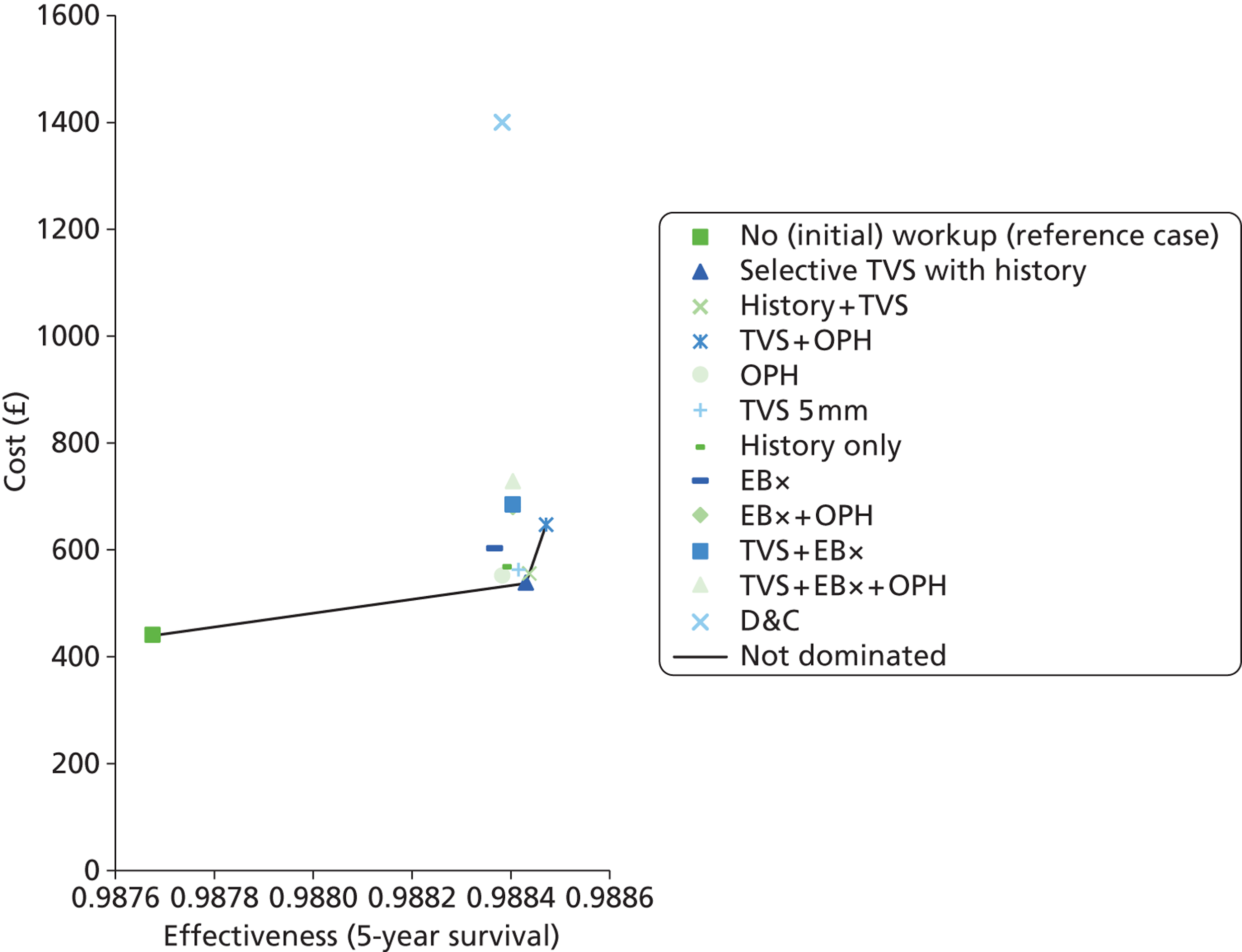
Removing the options ‘no initial work-up’ and ‘D&C’ from Figure 26 allows a clearer view of the relationship between the results for other options, as shown in Figure 27.
FIGURE 27.
Total costs and effectiveness of selected strategies to investigate PMB. History only, strategy selecting women for EBx based on the patient characteristics model; history + TVS, strategy selecting women for EBx based on the patient characteristics and TVS model; No work up, no initial diagnostic testing nor consideration of patient characteristics from the history, diagnostic testing being delayed until representation with continuing PMB symptoms was used as the reference case; Selective TVS with History, strategy with selective use of TVS in high-risk women only based on the patient characteristics model.
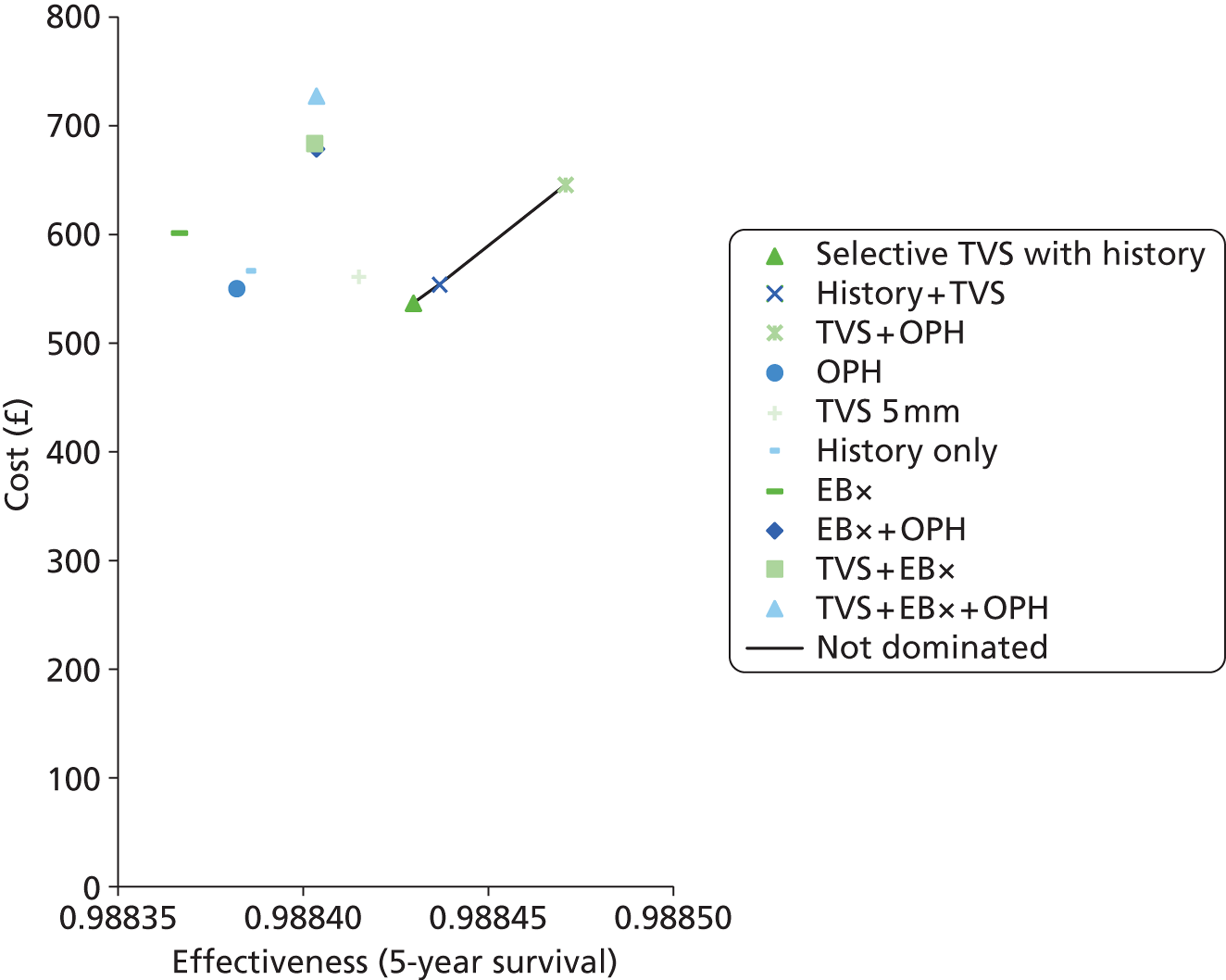
Probabilistic results of the economic analysis of strategies to investigate post-menopausal bleeding
The uncertainty surrounding the costs and effectiveness for diagnostic work-up in PMB according to the individual adopted strategy is illustrated in Figure 28. With the exception of the reference scenario of no initial diagnostic testing, the overlapping scatterplots on the cost-effectiveness plane demonstrate that there exists some uncertainty about the estimates of effectiveness (5-year survival) used in the model. In general, there is less uncertainty pertaining to the costs, although some uncertainty is apparent, especially for the three strategies involving the application of clinical characteristics derived from the patient history. These scatterplots of costs against effectiveness do not indicate, however, whether or not any of these individual options are consistently better than any other. For that, incremental scatterplots are required (see Figures 30–35).
FIGURE 28.
Scatterplot showing the uncertainty between diagnostic strategies to investigate PMB. History only, strategy selecting women for EBx based on the patient characteristics model; history + TVS, strategy selecting women for EBx based on the patient characteristics and TVS model; no work-up, no initial diagnostic testing nor consideration of patient characteristics from the history, diagnostic testing being delayed until representation with continuing PMB symptoms was used as the reference case; selective TVS with history, strategy with selective use of TVS in high-risk women only based on the patient characteristics model.
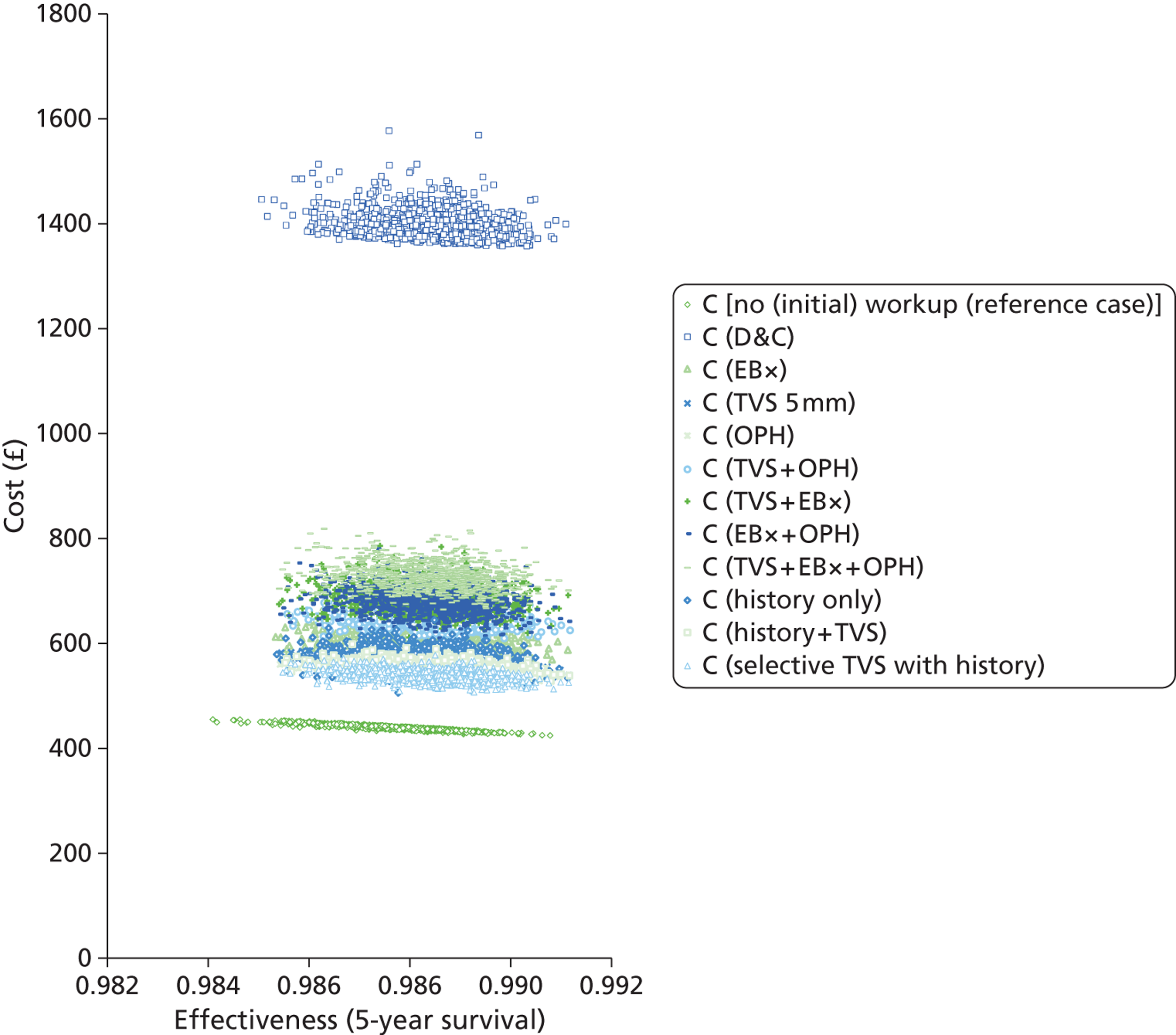
Figure 29 is a cost-effectiveness frontier designed to illustrate graphically which of the non-dominated strategies is optimal, based on the probability that it is the most cost-effective one, across a range of values representing the maximum amount the NHS may be prepared to pay for an additional woman with PMB surviving 5 years. The figure depicts the frontiers for the reference case scenario ‘no diagnostic work-up’ along with the alternative potentially cost-effective strategy ‘selective TVS with history’. ‘TVS with history’ and ‘TVS + OPH’ are not shown on the figure as their ICER values are too expensive to be considered by the NHS. The graph shows the proportion of model replications for which a particular strategy remains optimal. As the WTP threshold approaches zero, the decision is effectively to select the cheapest option (in this case no initial diagnostic work-up). At a WTP level of up to £30,000 there is certainty (probability = 1) that no initial diagnostic testing is the most cost-effective option. However, as the WTP threshold increases, the probability of this being the most cost-effective test decreases so that at a WTP of £150,000 the preferred option, as illustrated here, may be that of selectively performing TVS based upon the history to discriminate between the need for a subsequent EBx to provide histological confirmation of endometrial cancer.
FIGURE 29.
Cost-effectiveness acceptability frontier showing the optimal diagnostic strategy for investigating PMB across a range of WTP thresholds. (For any WTP per additional case satisfied, the optimal strategy is determined by the mean results.) No work-up, initial diagnostic testing or consideration of patient characteristics from the history, diagnostic testing being delayed until representation with continuing PMB symptoms; selective TVS with history, strategy with selective use of TVS in high-risk women only based on the patient characteristics model.
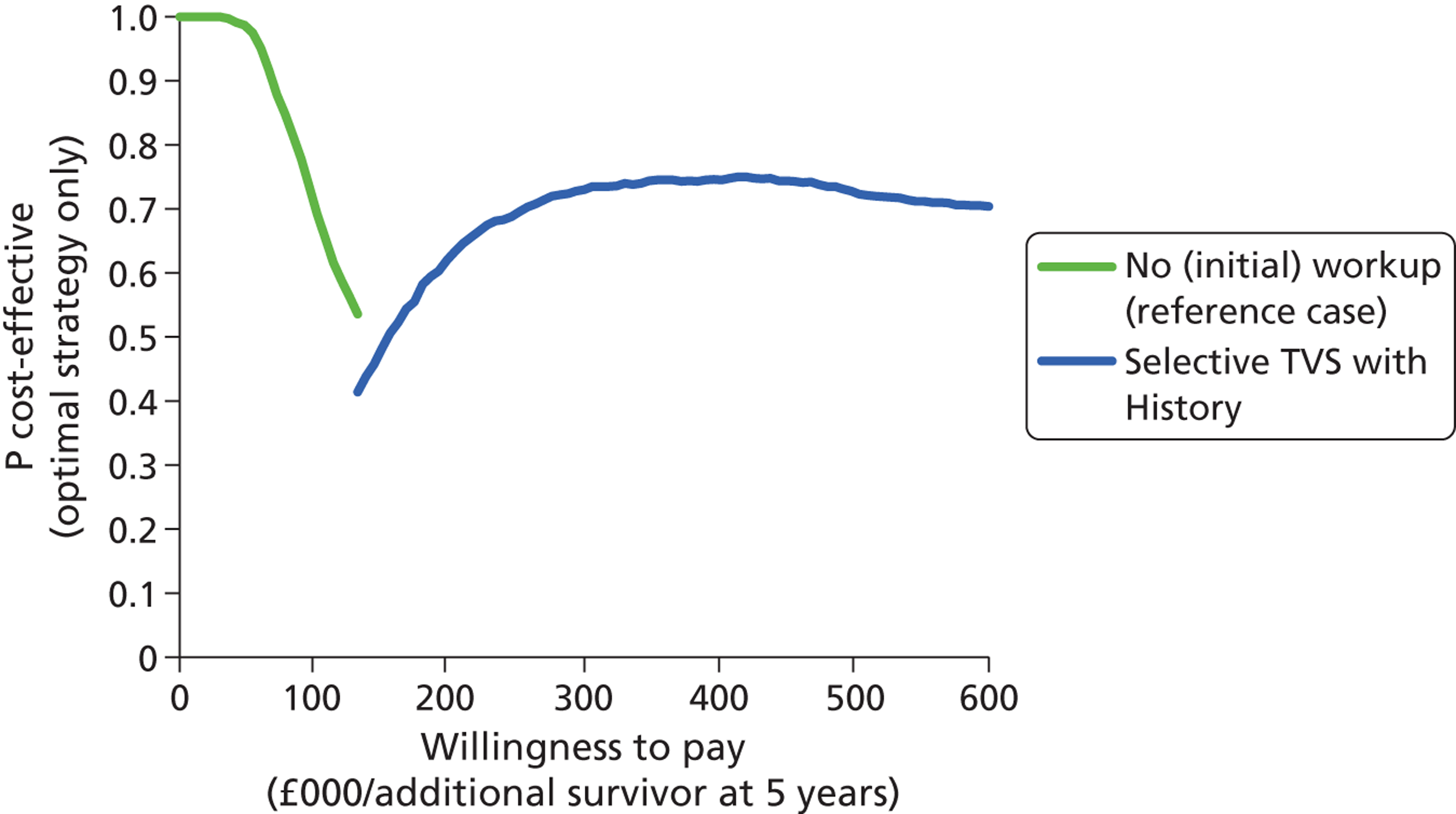
In order to assess whether or not the non-dominated strategies were consistently better than one another, incremental scatterplots were developed along with their corresponding CEACs.
Selective transvaginal scan with history compared with no (initial) work-up for investigating women with post-menopausal bleeding
The graph (Figure 30a) shows the modelled uncertainty in the difference in costs between a strategy of selective TVS with history and no initial diagnostic work-up. It shows that selective TVS with history-taking is consistently more effective and between £80 and £120 more costly than the strategy of no diagnostic testing per extra woman investigated for PMB. The CEAC (Figure 30b) shows the proportion of model replications for which selective TVS with history is preferred to no initial diagnostic work-up at any given WTP per additional case surviving 5 years. Above a WTP threshold of approximately £150,000, it is probable that selective TVS with history is more cost-effective than no-workup; however, this becomes more certain above a WTP of £350,000.
FIGURE 30.
Cost-effectiveness plane (a) and CEAC (b): selective TVS with history strategy relative to the no diagnostic work-up strategy for investigating women with PMB.
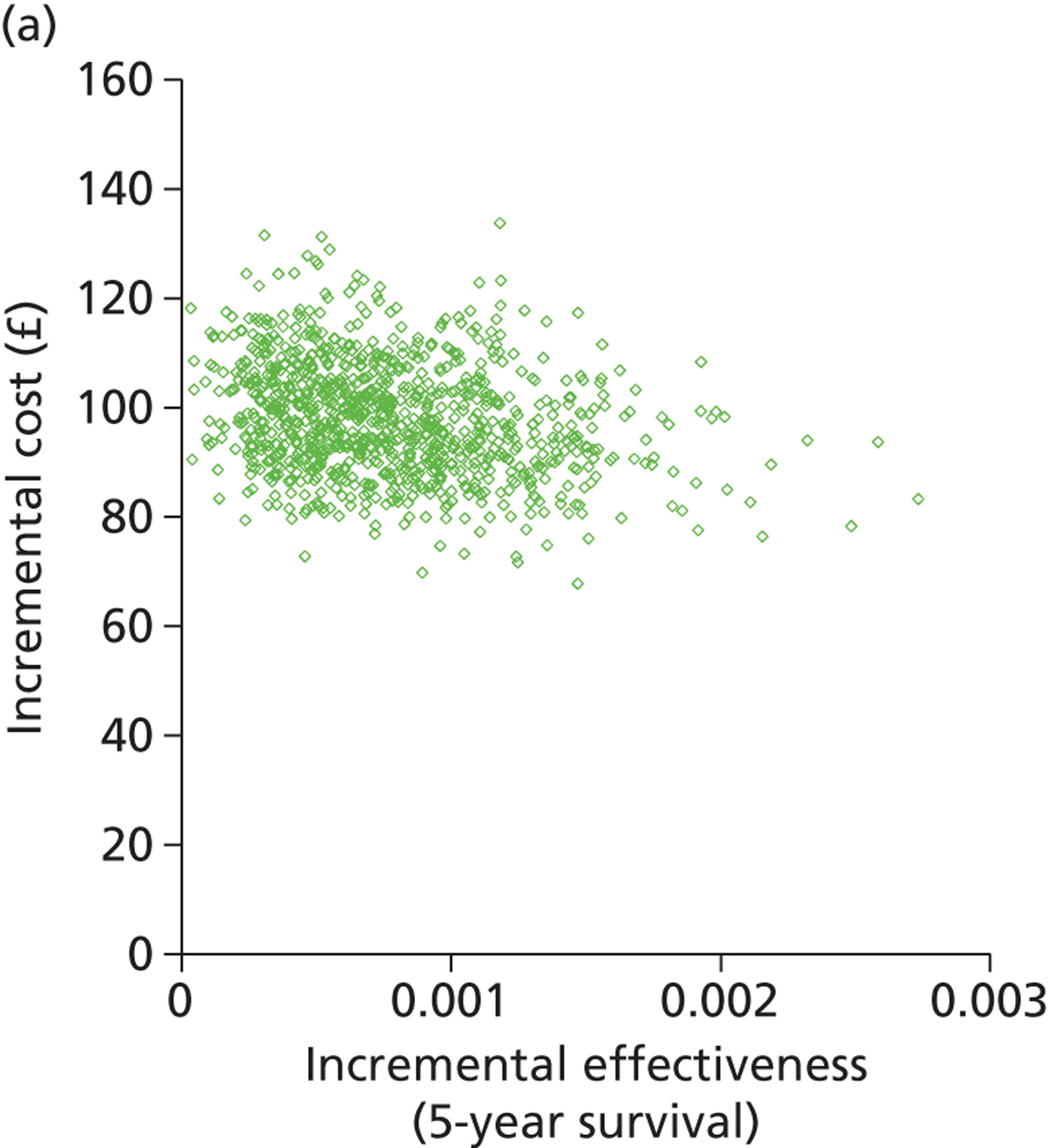
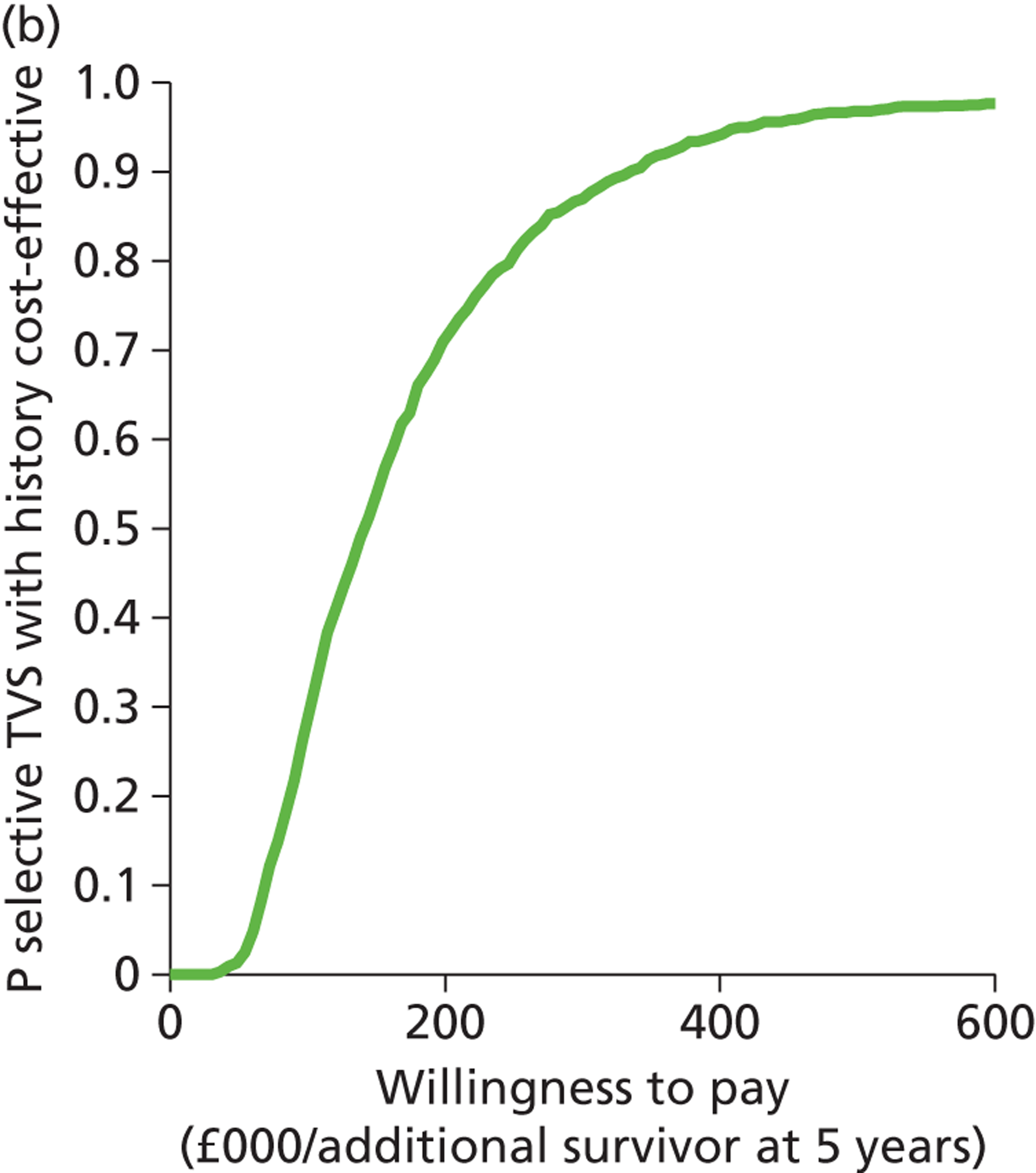
History and transvaginal scan compared with selective transvaginal scan with history for investigating women with post-menopausal bleeding
The graph (Figure 31a) shows the modelled uncertainty in the difference in costs between ‘history and TVS’ and ‘selective TVS with history’. It shows that routine testing with ‘history and TVS’ is likely to be more costly and possibly slightly more effective than ‘selective TVS with history’. The CEAC (Figure 31b) shows that ‘history and TVS’ is cost-effective compared with ‘selective TVS with History’ in a small proportion of model runs, but this proportion does not reach 20% across the range of WTP values plotted.
FIGURE 31.
Cost-effectiveness plane (a) and CEAC (b): history and TVS strategy relative to the selective TVS with history strategy for investigating women with PMB.
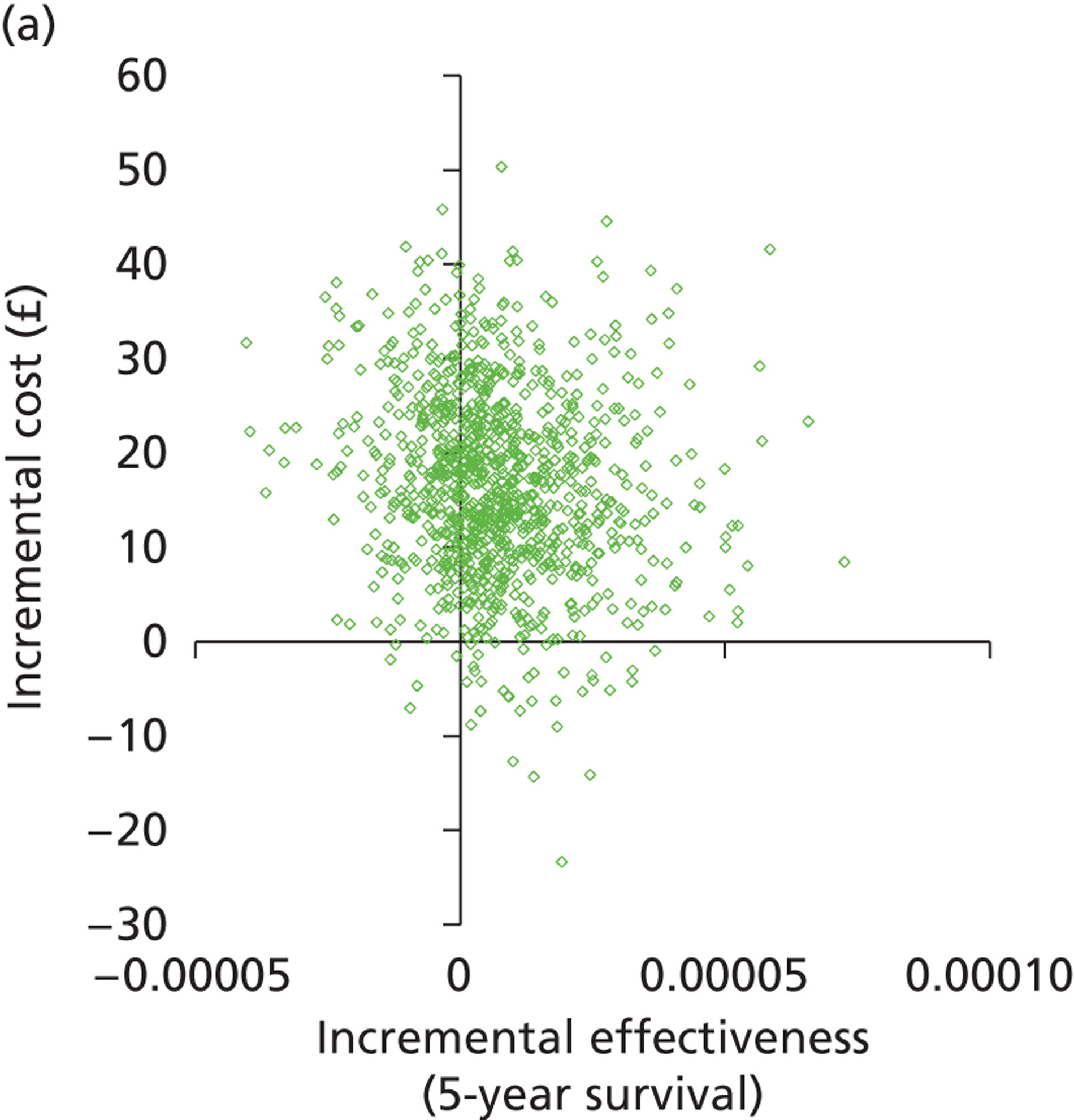
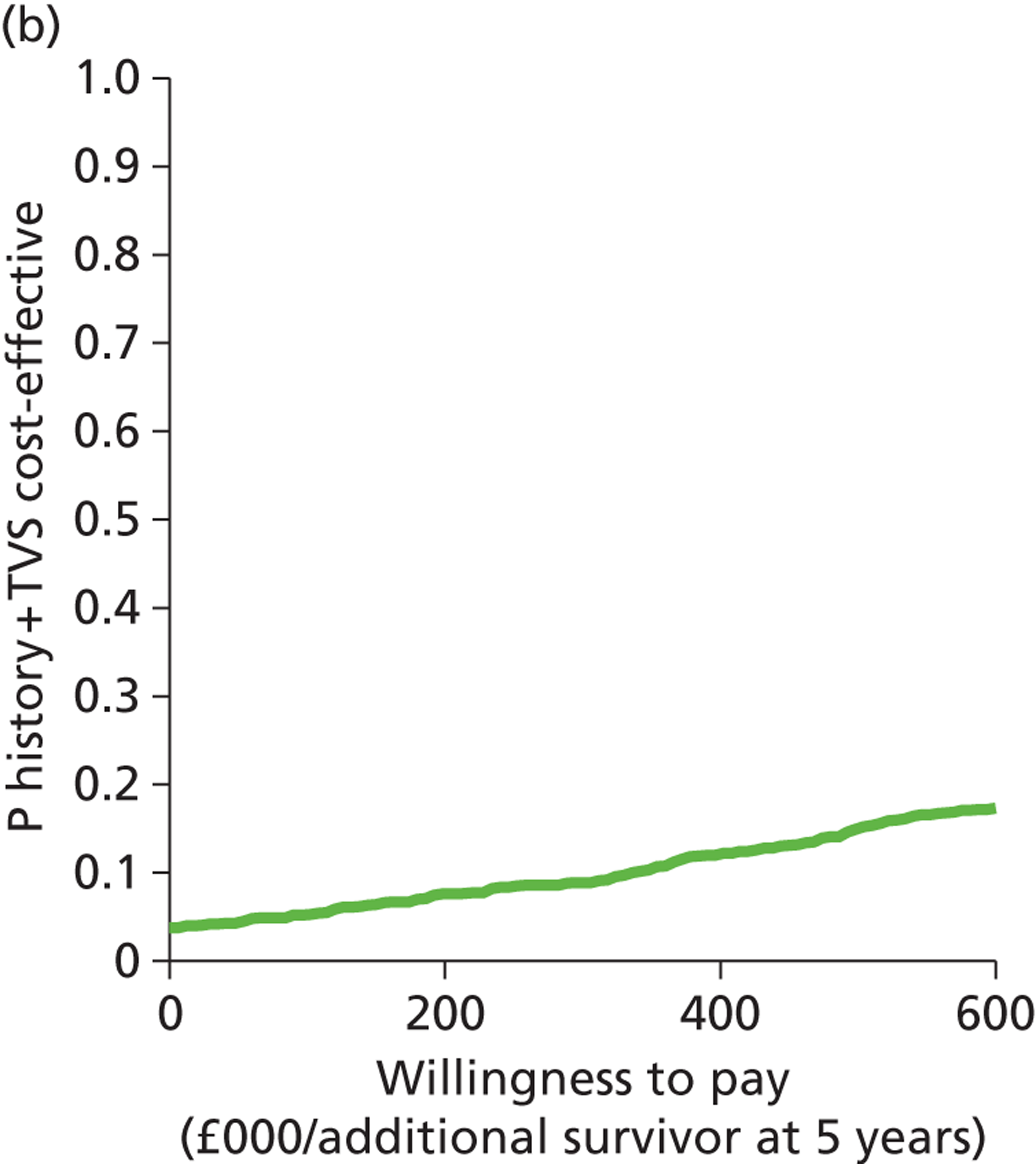
Transvaginal scan and outpatient hysteroscopy compared with history and transvaginal scan for investigating women with post-menopausal bleeding
The graph (Figure 32a) shows the modelled uncertainty in the difference in costs between ‘TVS + OPH’ and ‘history and TVS’. It shows that routine testing with ‘TVS + OPH’ is more expensive and probably more effective than ‘history and TVS’. Figure 32b shows that it is extremely unlikely that TVS + OPH will be cost-effective compared with ‘history and TVS’ across the range of WTP values plotted.
FIGURE 32.
Cost-effectiveness plane (a) and CEAC (b): TVS + OPH strategy relative to the history and TVS strategy for investigating women with PMB.
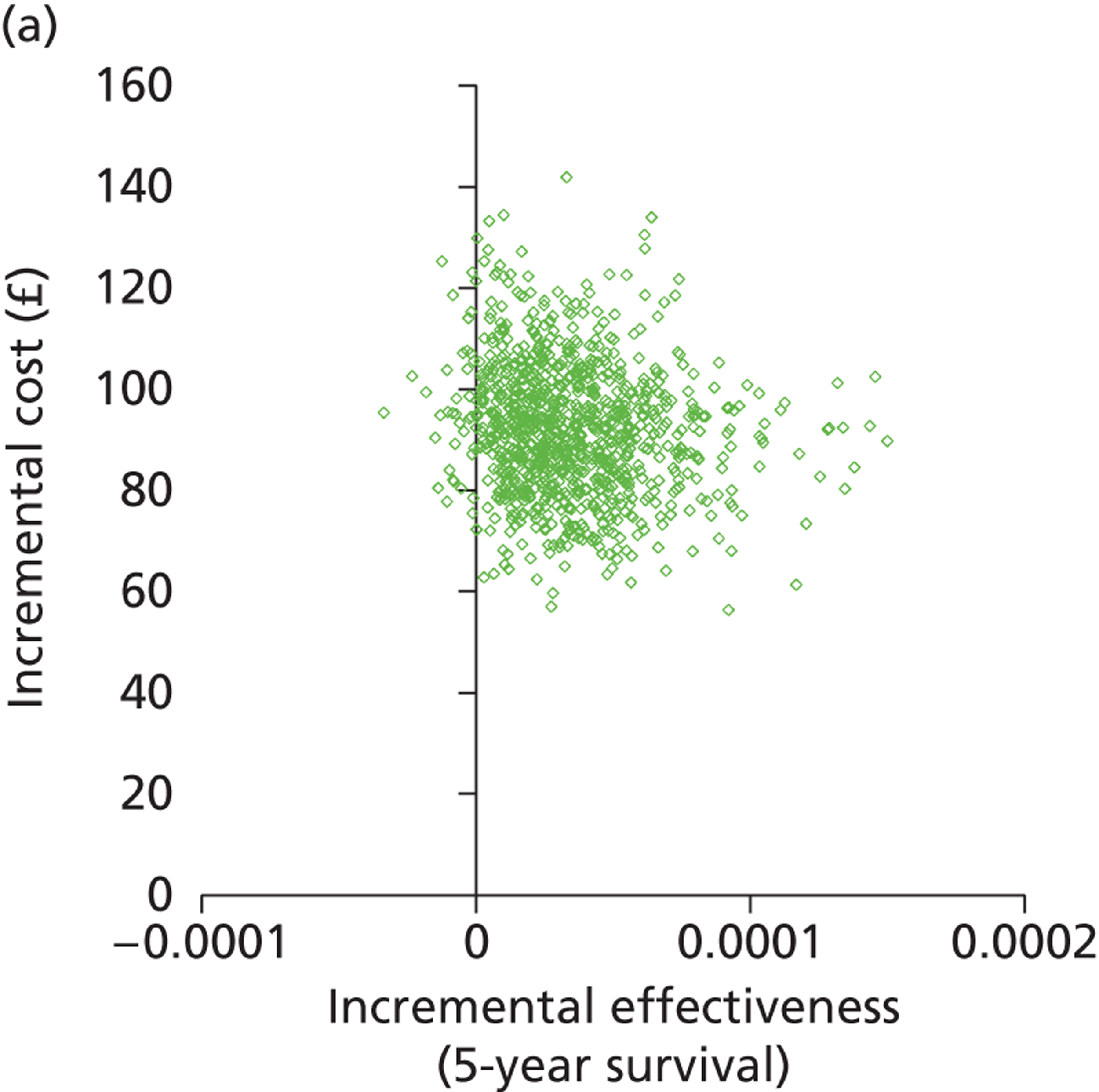
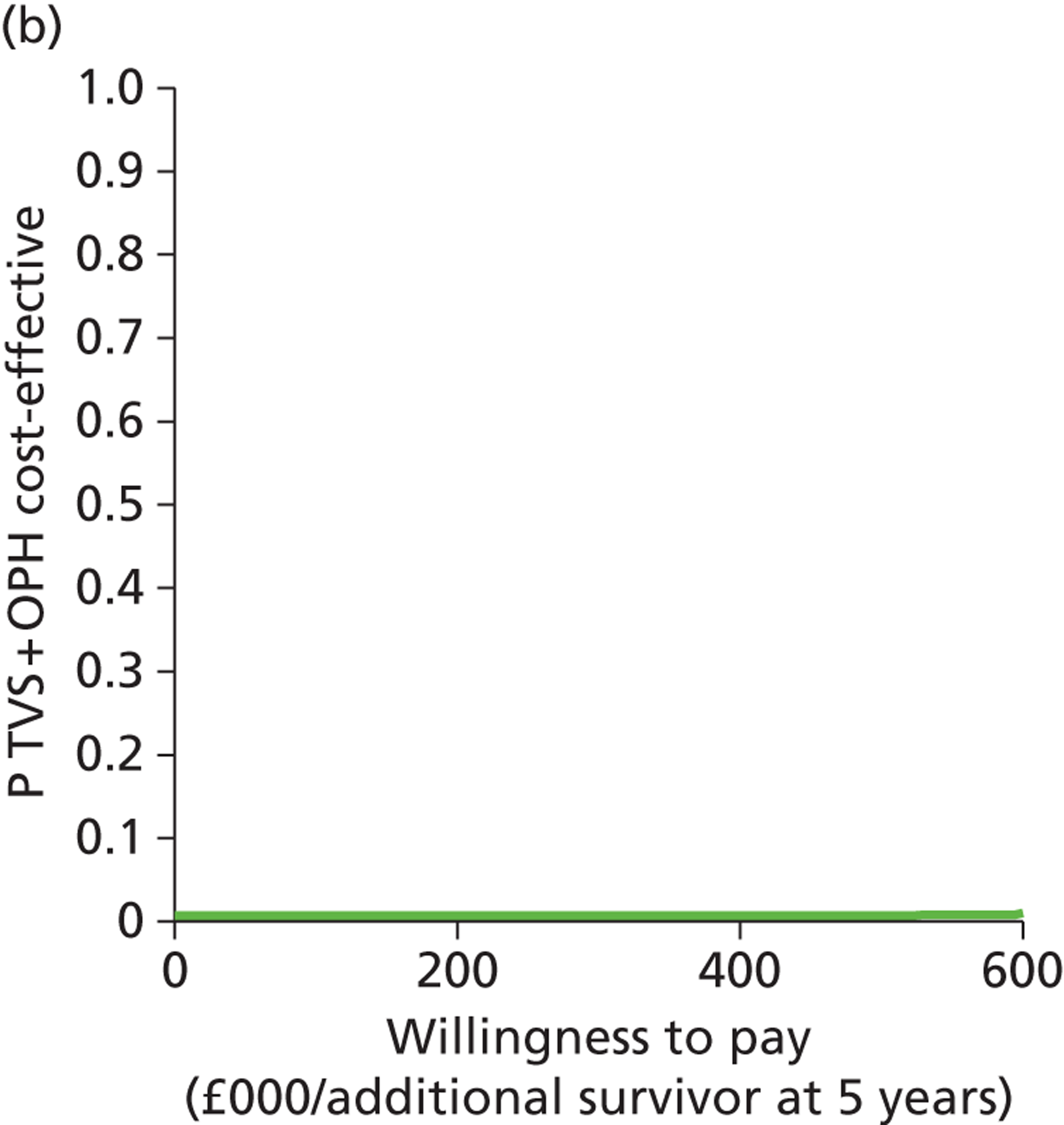
Transvaginal scan with a 5-mm cut-off compared with selective transvaginal scan and history for investigating women with post-menopausal bleeding
‘Selective TVS with history’ was compared with ‘TVS alone’ with a 5-mm cut-off for diagnosis of a thickened endometrium because TVS is the current primary investigation for PMB. The graph (Figure 33a) shows the modelled uncertainty in the difference in costs between a strategy of using ‘TVS with a cut-off of 5 mm’ for diagnosis of a thickened endometrium and ‘selective TVS with history’. It shows that ‘TVS with a 5-mm cut-off’ is more costly than ‘selective TVS with history’ and is probably less effective. The option ‘TVS 5 mm’ is dominated by ‘selective TVS with history’ in the deterministic results. Figure 33b shows that there is a negligible probability that ‘TVS 5 mm’ will be cost-effective across the range of WTP values plotted.
FIGURE 33.
Cost-effectiveness plane (a) and CEAC (b): TVS with a 5-mm cut-off strategy relative to the selective TVS with history strategy for investigating women with PMB.
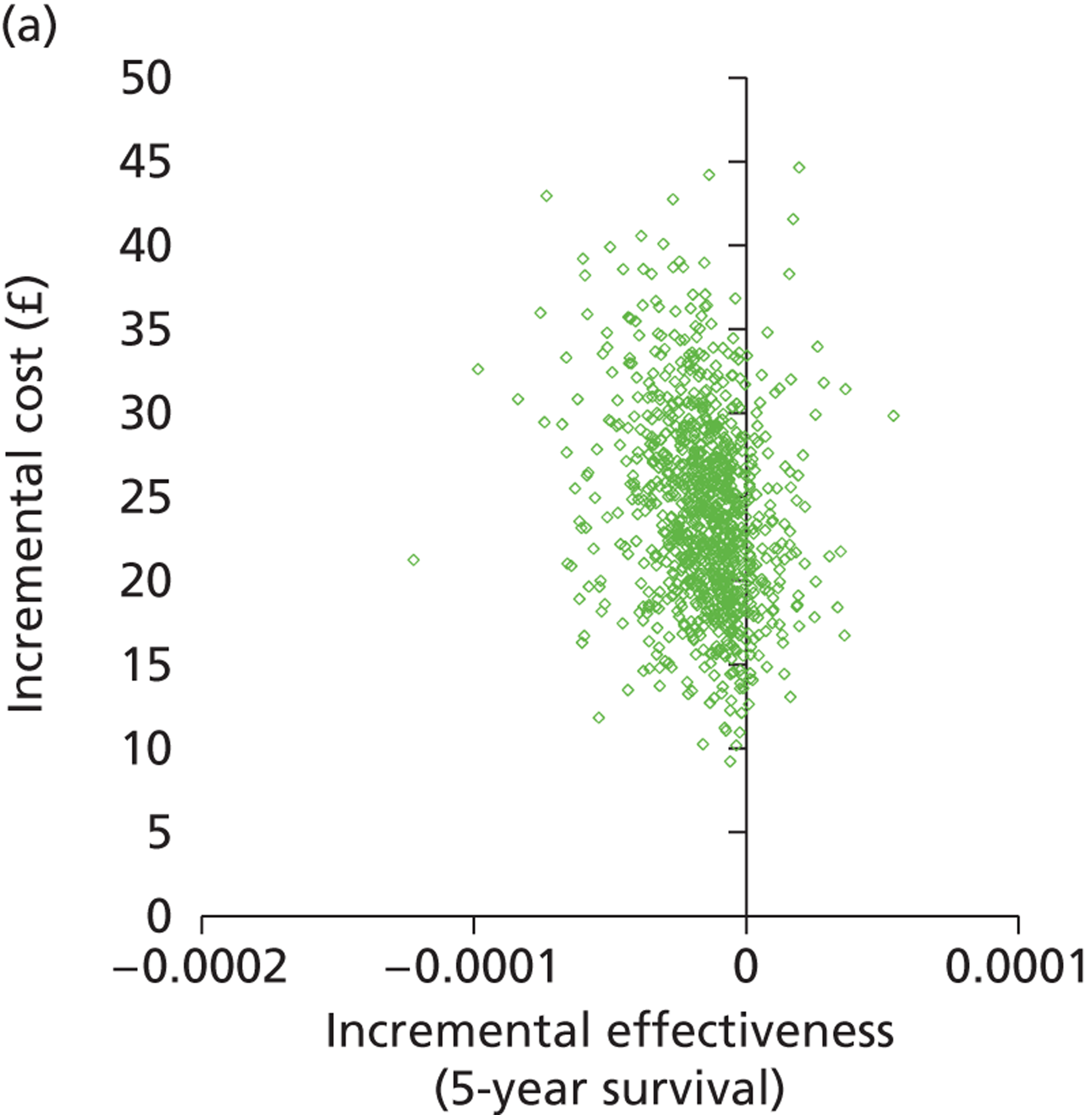
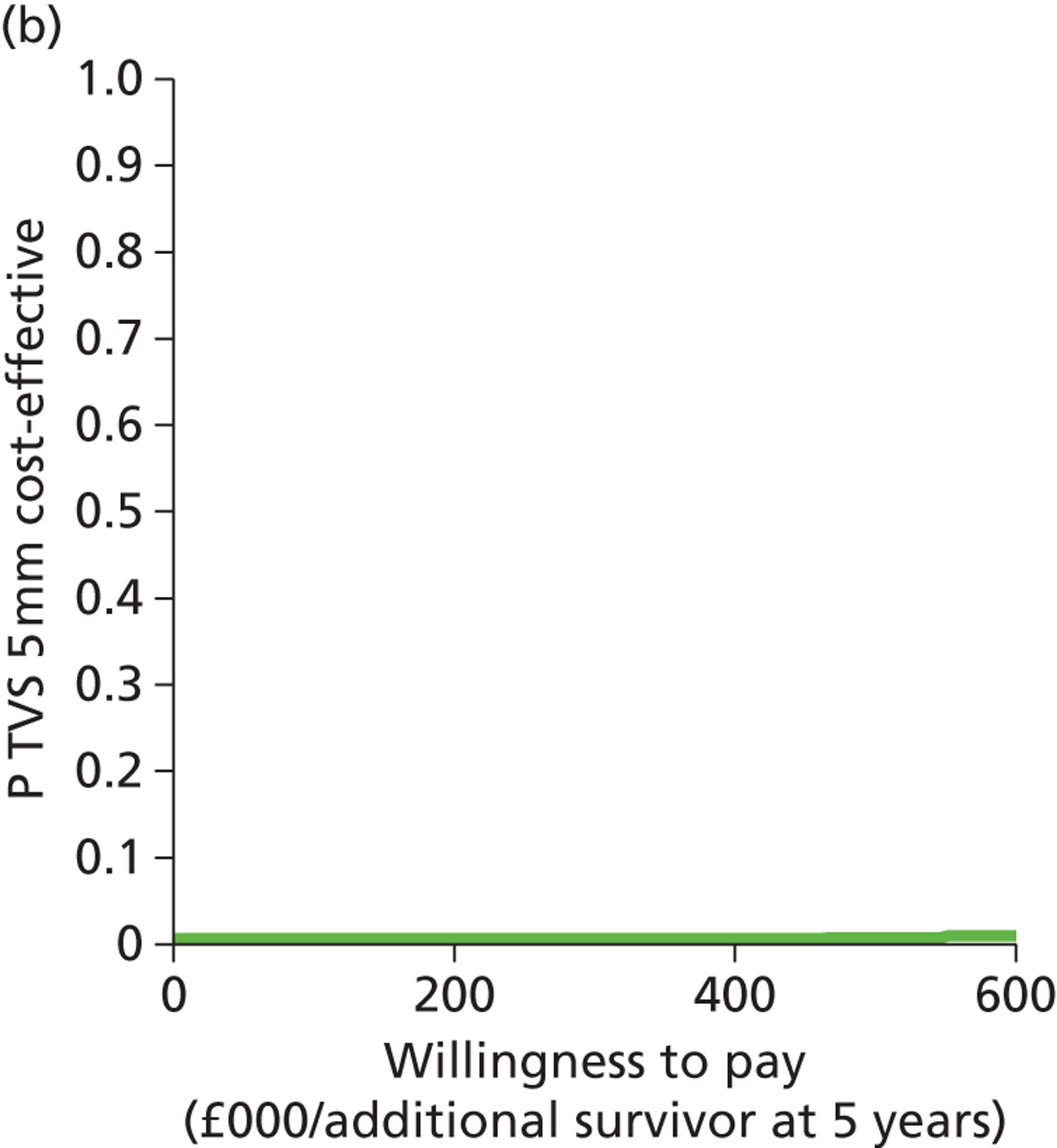
History only compared with selective transvaginal scan with history for investigating women with post-menopausal bleeding
The graph (Figure 34a) shows the modelled uncertainty in the difference in costs between ‘history only’ and ‘selective TVS with history’. It shows that routine testing with ‘history only’ is likely to be more costly than ‘selective TVS with history’ but is unlikely to be more effective. The option ‘history only’ is dominated by ‘selective TVS with history’ in the deterministic results. Figure 34b shows that there is a very low probability that ‘history only’ will be cost-effective across the range of WTP values plotted.
FIGURE 34.
Cost-effectiveness plane (a) and CEAC (b): history only strategy relative to the selective TVS with history strategy for investigating women with PMB.
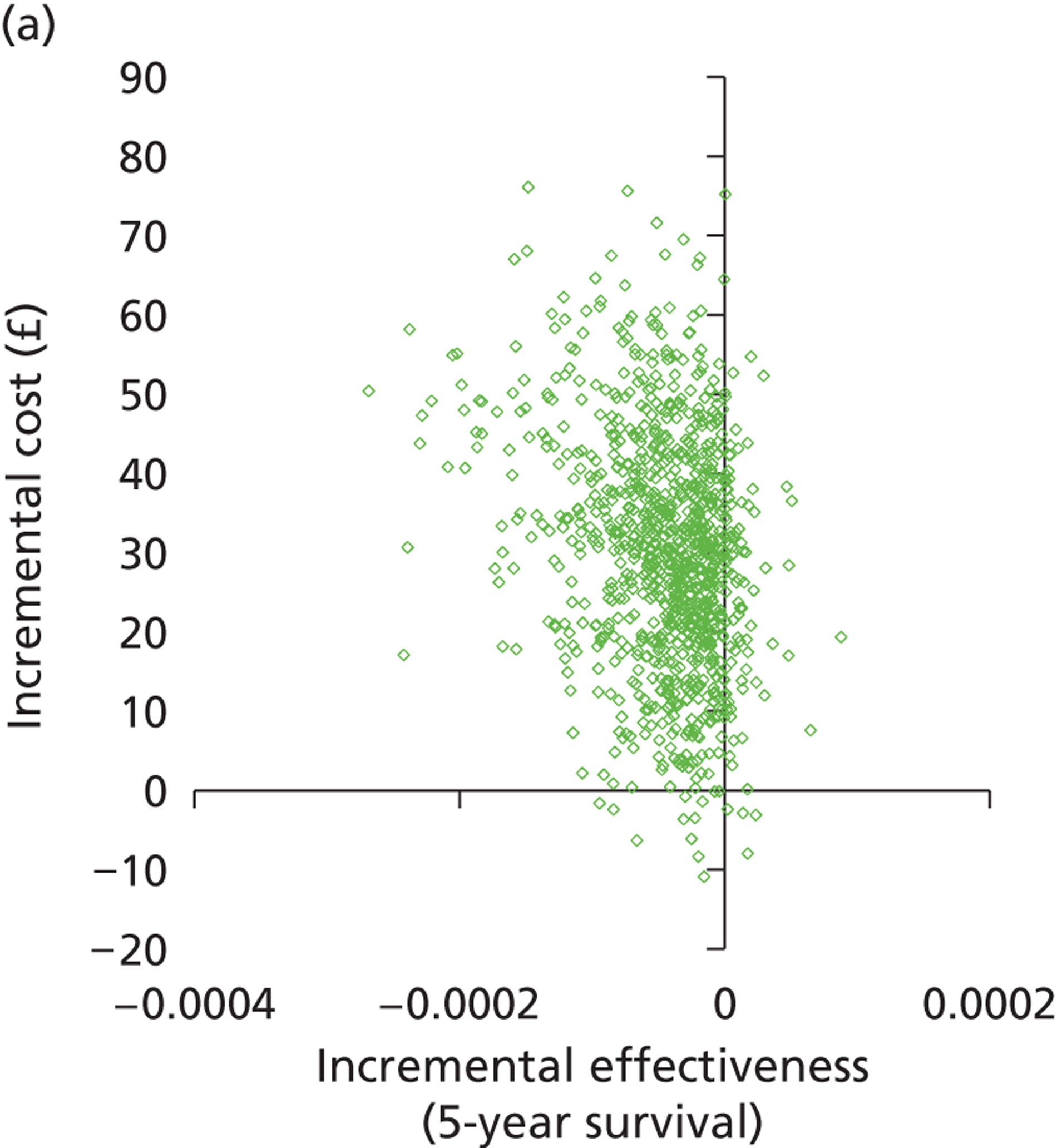
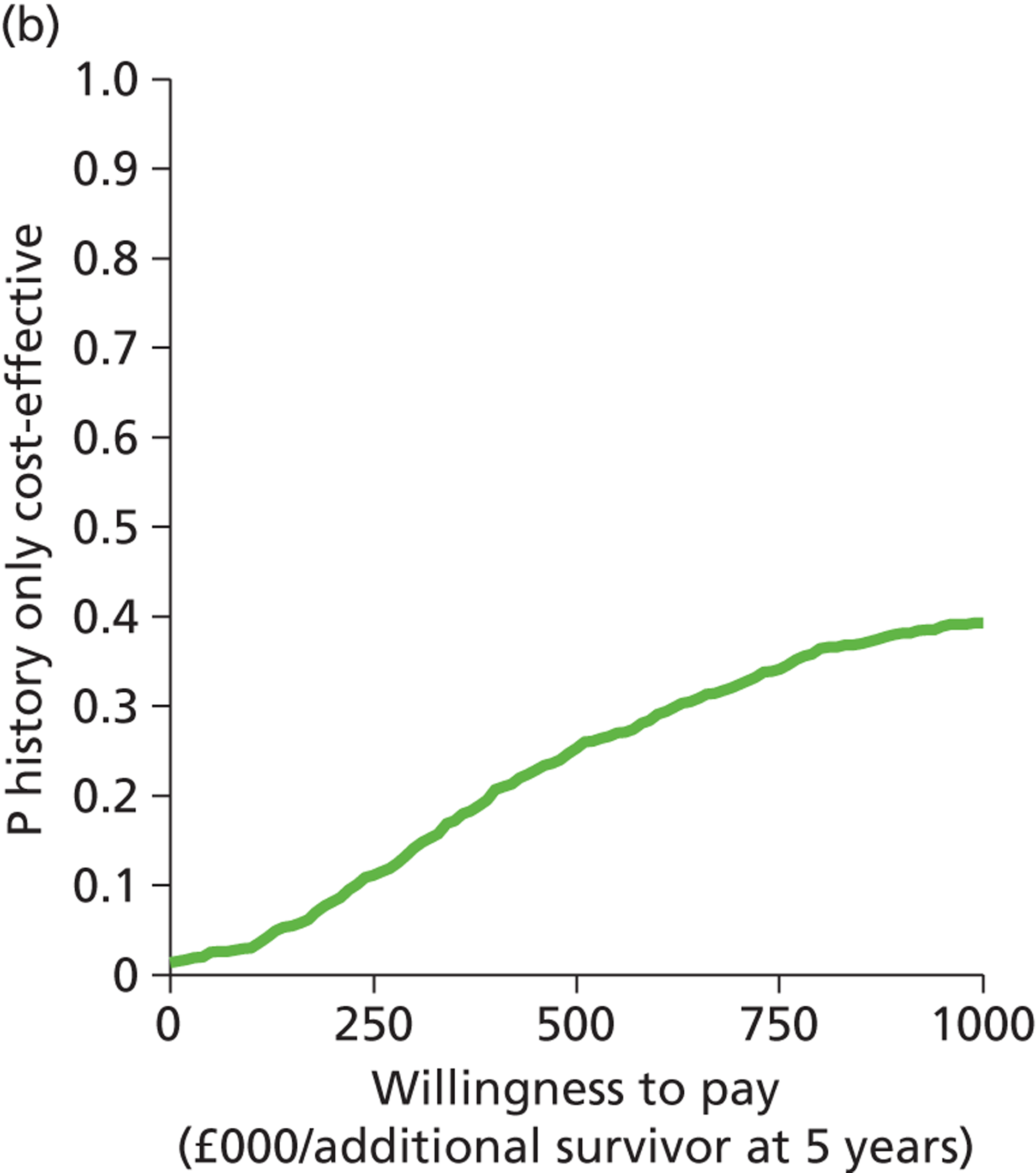
History only compared with history and transvaginal scan for investigating women with post-menopausal bleeding
The graph (Figure 35a) shows the modelled uncertainty in the difference in costs between ‘history only’ and ‘history and TVS’. It shows that routine testing with ‘history only’ is probably more costly than ‘history with TVS’ and unlikely to be more effective. The option ‘history only’ is dominated by ‘history and TVS’ in the deterministic results. In just under 20% of model replications, ‘history only’ was the less costly option, but for most of these it was also less effective than ‘history and TVS’. As the WTP increases, the cost saving indicated by these points is less worth making, and the probability that ‘history only’ is cost-effective reduces (Figure 35b).
FIGURE 35.
Cost-effectiveness plane (a) and CEAC (b): history only strategy relative to the history with TVS strategy for investigating women with PMB.
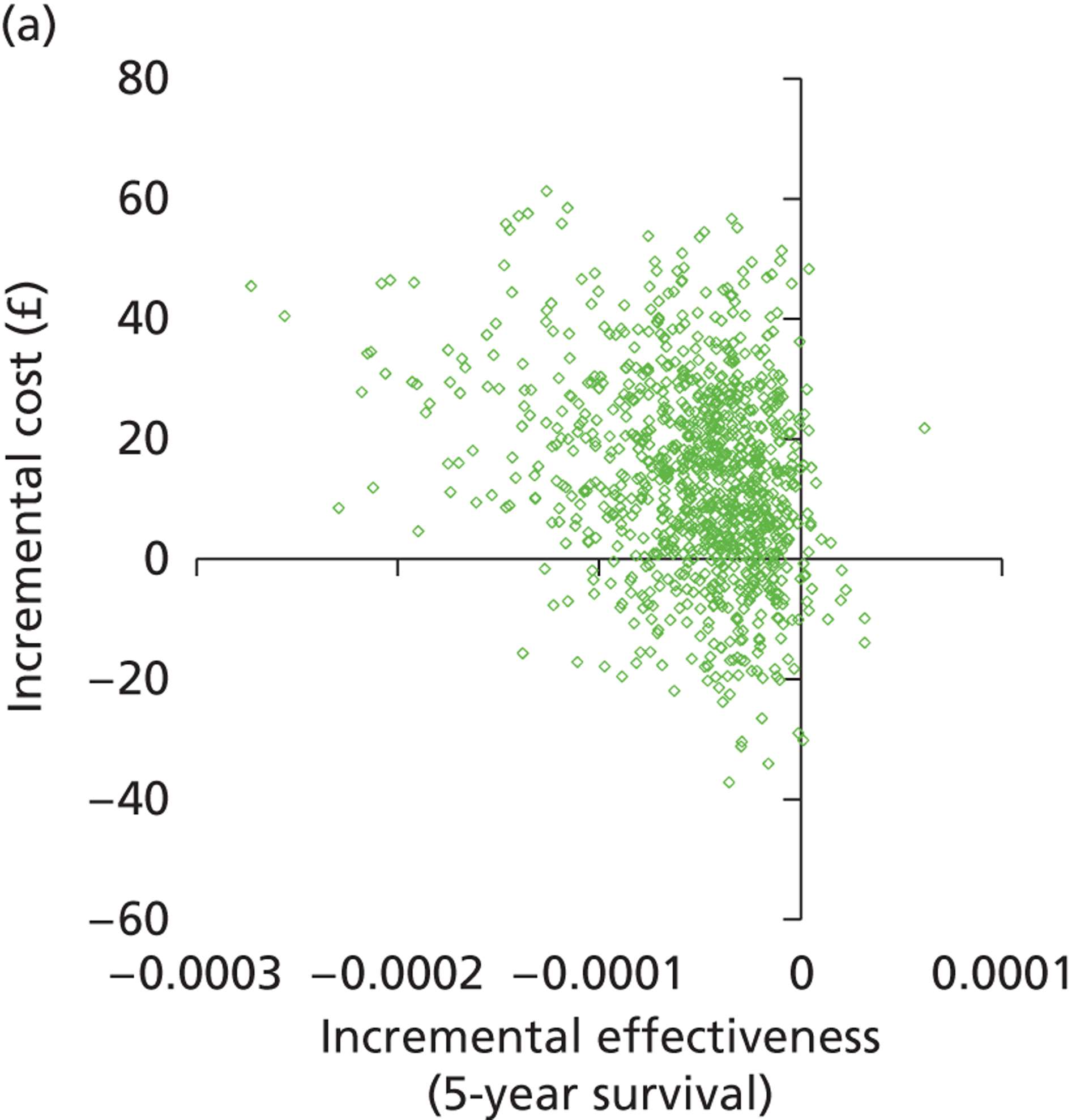
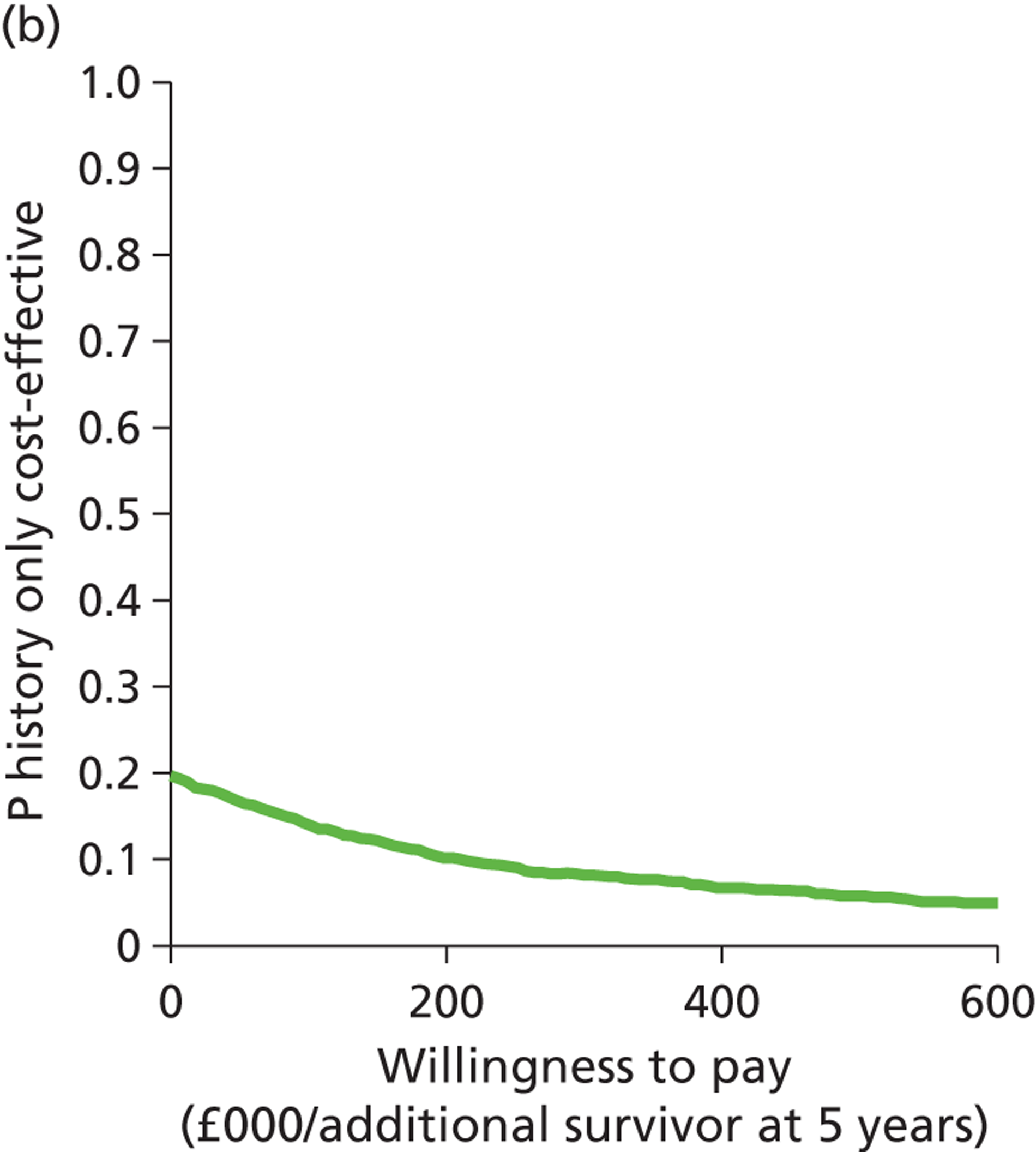
Chapter 5 Discussion
Statement of overall aim and methods
Abnormal uterine bleeding is a common complaint, affecting women of all ages. In women of reproductive age, HMB is the predominant pattern of AUB and, while it is usually of a benign origin, the symptom causes significant morbidity with restrictions on activities of daily living. In older women, PMB is an alarming symptom, causing much anxiety, and potentially mortality if an underlying endometrial cancer is diagnosed. Although the aetiology of AUB varies significantly according to menopausal status, investigation in both HMB and PMB involves a more detailed assessment of the uterus employing convenient, minimally invasive outpatient tests: ultrasound, endoscopy or tissue sampling technologies. However, despite the evolution and availability of outpatient testing, optimal diagnostic testing strategies are not clearly defined and this is reflected in eclectic practice among gynaecologists. Moreover, the investigation and subsequent treatment of both HMB and PMB is associated with the heavy utilisation of scarce health-care resources. It is, therefore, self-evident that defining economically efficient diagnostic pathways for the common presentations of HMB and PMB is very important from both a clinical and an economic perspective.
Our aim was to determine the most cost-effective testing strategies for the diagnosis of AUB after referral from primary care into a contemporary, ambulatory, secondary care, NHS, UK hospital setting. To do this we constructed two clinically informed cost-effectiveness models: one for women of reproductive age with HMB and another for women with PMB. The models were designed to reflect current service provision for the diagnostic work-up of women presenting with either complaint. In view of the lack of consensus regarding how best to investigate women with HMB, initial investigation utilising all tests either alone or in combination were included in the model. A similar methodology was adopted for developing the PMB model, but because existing evidence-based guidelines recommend the use of TVS as a first-line test, additional approaches, examining the use of TVS, were produced. These alternative strategies incorporated patient characteristics obtained from the preceding clinical history.
The tree structures were informed by clinical input and data derived from the literature following systematic searches or from existing clinical data sets. When possible, IPD, data from systematic quantitative reviews and high-quality randomised or observational studies were used to populate the decision trees. Up-to-date costs were obtained using HRG data published by the Department of Health. The effectiveness and costs of the testing strategies were modelled; deterministic results were obtained using point estimates of the parameters to estimate the expected cost (£), outcome (HMB – satisfaction; PMB – 5-year survival) and incremental cost-effectiveness (additional cost per extra outcome achieved). To allow for uncertainty, the stability of the results was then tested through sensitivity analysis. These data were then used to assess the implications for clinical practice and to inform future research.
Summary of main findings
Heavy menstrual bleeding
Our objective was to evaluate the cost-effectiveness of diagnostic testing in HMB, that is to say the effect of diagnostic work-up strategies on improving treatment outcomes. We chose universal treatment with the LNG-IUS without any preliminary investigation as our reference strategy to compare testing options against. This was because (i) not investigating or treating is an unacceptable alternative; (ii) the LNG-IUS is recommended by NICE as first-line treatment of HMB; and (iii) the LNG-IUS is a less invasive and more applicable initial treatment option compared with hysterectomy. We did, however, incorporate the option of bypassing investigation altogether and undertaking hysterectomy for all women with HMB because a recent report in Health Technology Assessment found hysterectomy to be a more cost-effective treatment than LNG-IUS in DUB. 2 We found that treating all women with a LNG-IUS without preliminary testing resulted in high levels of satisfaction (93%) which were increased by approximately 4% if some form of currently available diagnostic testing was undertaken to guide treatment.
Base case
Our analysis identified two potentially cost-effective investigation strategies: initial testing with OPH alone or a combination strategy incorporating OPH with EBx (OPH + EBx). Although a testing strategy of OPH + EBx was marginally more effective, the ICER was approximately £21,000 to gain one more satisfied patient compared with OPH, while OPH has an ICER of under £400 per additional case satisfied compared with LNG-IUS alone. Thus, for relatively little additional investment by the NHS, the adoption of OPH in place of LNS-IUS alone will improve outcomes for women presenting with HMB. This improvement can be increased further if combination testing with OPH + EBx is introduced. This additional cost is contentious, but it can be tested by comparison with the £20,000–30,000 per quality-adjusted life-year (QALY) NICE threshold at which interventions are considered cost-effective to implement within the NHS. Owing to a paucity of HRQL data, we were unable to perform a cost–utility analysis with QALYs directly estimated in the model, but we can make an estimate of the QALY gain per additional case satisfied. It has been estimated that a woman’s quality of life is reduced by 0.5 for the 1 week per month of heavy menses. 150 This means that HMB is associated with a reduction of 0.125 QALY in any year, as the reduction of 0.5 applies for one-quarter of the time overall. Let us consider a 45-year-old woman with 7 symptomatic years until menopause. Discounting future years at 3.5%, the annual QALY loss must be multiplied by [1 + (1/1.035) + (1/1.035)2 + (1/1.035)3 + . . . + (1/1.035)6] or approximately 6.3 to give a total QALY loss of approximately 0.8 QALYs. This means that an ICER of £21,000 per case satisfied is approximately equivalent to an ICER of £26,500 per QALY, which falls within the £20,000–30,000 per QALY threshold range used by NICE. Thus, by this measure, a strategy of OPH + EBx is of borderline cost-effectiveness compared with initial investigation with OPH in isolation.
The certainty of these results was assessed by PSA. OPH remained stable in our sensitivity analyses, remaining more cost-effective than the LNG-IUS reference strategy even at relatively low WTP thresholds. OPH + EBx also remained stable; however, to be at least 70% certain that it was a more cost-effective alternative than OPH alone, the WTP threshold would need to be increased to around £40,000 per patient satisfied (∼£50,000 per QALY). EBx is most useful for diagnosing endometrial disease but the prevalence of endometrial hyperplasia and carcinoma is low in a pre-menopausal population, estimated at around 5%. In populations of women where higher rates of endometrial disease are observed (e.g. epidemics of obesity), the benefit of EBx will have more influence on overall cost-effectiveness. In general, however, substantially higher estimates of endometrial disease in an HMB population are unlikely to be credible.
Ultrasound is a convenient, minimally invasive, portable test that allows assessment of both the uterus and the ovaries. It is universally available and can be incorporated easily into standard gynaecological examination and has therefore been widely, albeit variably, adopted. In view of the popularity of pelvic scanning and the fact that two scanning strategies came close to the boundary of dominance when the cost-effectiveness analysis was performed, further exploratory analyses were undertaken. Sensitivity analysis was performed to examine TVS and SIS to see whether or not there was uncertainty regarding them when compared with the most cost-effective strategy of OPH alone. However, when compared with OPH, TVS was found to be almost certainly less effective and there was also a chance of it being more costly too, therefore ruling it out as a primary diagnostic test. When SIS was compared with OPH, there was significant doubt regarding which was the more effective approach. By increasing the WTP threshold from £0 to £40,000, the likelihood that OPH was more cost-effective than SIS increased further from 60% to 80%. Thus, at viable WTP levels, OPH should be preferred over SIS as a first-line diagnostic test in HMB for those clinicians establishing services in the UK. However, clinicians who currently use SIS for first-line investigation of HMB and are able to easily integrate SIS into their practice at low cost may have less to gain by changing to OPH. Furthermore, patients may receive a certain amount of reassurance from knowing that their ovaries have been examined as well as the uterine cavity, a benefit of scanning that is neglected by OPH.
In sensitivity analysis, the cost of SIS needed to be reduced from £71 to £52 to make it more cost-effective than OPH. This reduction in costs may not, however, be realistic as SIS would have to cost less than the £55 which is the cost of a standard 2D TVS. This is unlikely to be feasible, given that SIS requires purchase of an instillation catheter and takes longer to perform, costs which have been estimated to be an extra 35% on top of the scan in a Dutch study. 151
Prevalence of focal uterine pathology
From the aforementioned, it can be seen that a higher prevalence of intracavity focal pathology favours OPH. Therefore, we conducted further sensitivity analyses where the prevalence of polyps/SMFs was reduced sequentially to determine at what prevalence an alternative option may be favoured. This analysis suggested that even if the estimate of polyps/SMFs was 10% less than had been stated in the base case (i.e. 28% instead of 38%), OPH would still remain the preferred option. The prevalence of polyps and SMFs reported in the better-quality prevalence studies we used in our model reported values for both pathologies of around 20%,99,100 which approximates the 40% prevalence quoted by NICE in their HMB guideline (30% SMFs and 10% endometrial polyps). 11 Our model estimated a more conservative prevalence of focal pathology to account for the fact that these pathologies coexist in one-third of women. 103 If, however, 40% is a true reflection of disease, then OPH is for certain the most cost-effective option. Even in different HMB populations, it seems unlikely that the combined prevalence of endometrial polyps and SMFs would be lower than 28%. We can, therefore, be confident that OPH is the most cost-effective diagnostic strategy in HMB, even in populations with focal uterine pathology prevalence rates at the lower end of the plausible range.
Women managed over multiple clinic appointments
Traditionally, women referred to a gynaecologist would be reviewed in a general outpatient clinic before any investigation or treatment was instigated. The patient would have their history taken and be examined and then the clinician would plan appropriate tests and send the patient away to have these done at a later date. Weeks or even months later, the patient would be seen again in clinic with the results of those investigations and then the clinician would initiate treatment. This approach wastes time and resources, not only for the health service provider but also for the patient. Furthermore, the patient is likely to experience anxiety as a result of uncertainty while awaiting the results of investigations and a clear plan of management. In contrast to traditional multistep investigative models, the so-called ‘one-stop’ approach to managing common gynaecological conditions minimises unnecessary delay and inconvenience for women by avoiding the need for multiple visits to separate hospital departments. The approach is based upon the principle of being able to provide all indicated diagnostic tests, and in many cases simultaneous minor treatments, in a single, outpatient visit. The development of ‘one-stop’ services over the last decade has been facilitated by technological advances such as enhanced digital imaging, data capture, miniaturisation and portability of equipment allowing diagnostic equipment (i.e. OPH, TVS, SIS and EBx) to be housed in a single clinic setting. ‘One-stop’ services are becoming increasingly established, driven by the recognition by health service providers of the feasibility and potential efficiency of such care models. In addition to the UK NHS, health services provision throughout the developed world is rapidly moving to one-stop ‘shops’ providing seamless investigation in hospitals, specific ‘ambulatory centres’ and community settings. Thus, to reflect contemporary practice and ensure the relevance of our work as well as widen the generalisability of our findings, we adopted a ‘one-stop’ setting on which to base our economic models.
However, while ‘one-stop’ services are being increasingly developed, the traditional multistop model of care remains embedded in much of the NHS. In recognition of this we adapted our model in sensitivity analysis to mirror the multistop approach to investigation and treatment of women with HMB. This analysis suggested that SIS alone, at an added cost of £5070 per additional patient satisfied, or OPH + EBx, at an added cost of £22,100, were the most cost-effective strategies. OPH alone was not considered a cost-effective option in this analysis; however, we applied costs to OPH that made it less competitive than the other tests by dictating that concomitant treatment of polyps and fibroids could not be performed at the time of diagnostic hysteroscopy. This approach is practised at some centres across the UK to try to avoid delays in clinic running time; therefore, we used this approach to ensure that we applied the ‘worst-case scenario’ costs to this strategy. Also, in this analysis OPH become relatively expensive because it required an additional consultant appointment, whereas TVS and SIS were performed in the scan department by a sonographer and EBx was performed at the initial consultant gynaecological outpatient appointment. Interestingly, in the pairwise comparison between OPH and SIS, OPH was probably more effective than SIS but it was more expensive under the assumptions of the multistop model of care to a degree that OPH was no longer cost-effective compared with SIS. In both the ‘one-stop’ base-case model of care and the multistop reanalysis, the testing strategy of OPH combined with EBx was a non-dominated strategy. If the same QALY rules are applied to this strategy as to the base-case results (see the discussion under Base case, above) the cost per QALY for OPH + EBx was £27,625, which falls within the NICE threshold of £20,000 to £30,000. Thus, an initial testing approach for women presenting with HMB using OPH with EBx is potentially cost-effective regardless of the model of care. However, those able to provide a contemporary ‘one-stop’ service would need to weigh up the additional costs associated with OPH + EBx over simply OPH alone to gain a further health benefit. Similarly, those units utilising a traditional multistop set-up would need to consider the much-reduced costs of initial testing with SIS against the reduction in health benefit compared with a combination of OPH and EBx.
It seems likely that the drive to provide outpatient testing and, where possible, simple concomitant treatments will continue in light of the convenience for patients and their doctors as well as the ongoing improvement in health technologies. Therefore, because the base-case analysis, based upon the premise of a ‘one-stop’ service, indicated that OPH was the optimal strategy [regardless of wish for fertility or prior treatment with LNG-IUS (as discussed in Fertility preservation and Universal levonorgestrel intrauterine system treatment prior to referral)], it may be consistent to employ combination testing from the outset with OPH and EBx in hospitals that do not yet have ‘one-stop’ facilities in place. This would allow the services in these centres to evolve until they were able to offer ‘one-stop’ care based upon initial testing with OPH in women presenting with HMB. The alternative for these hospitals would be to adopt SIS as the first-line test for investigating HMB, training doctors, nurses and sonographers where necessary in the technique. However, future reinvestment in equipment and training to establish ‘one-stop’ services based upon initial testing with OPH may become necessary if such service models become embedded.
Fertility preservation
Heavy menstrual bleeding is most common in parous women in their fifth decade of life. 86 Thus, the majority of women presenting to secondary care have completed child bearing. However, many women with HMB do want to retain their fertility potential and to take account of this, and to test the generalisability of our base-case findings, we produced an additional model for women wishing to preserve their fertility which precluded certain surgical treatment options. The findings of this additional analysis were consistent with the base-case scenario, with OPH remaining the most cost-effective first-line diagnostic test of choice. SIS was the closest, viable competing testing option compared with OPH. However, by adopting OPH rather than SIS, the additional £2720 needed to achieve an extra woman satisfied is likely to be considered affordable and worthwhile by health services. Moreover, at WTP thresholds of around £5000, there is a > 90% certainty of OPH being the most cost-effective option. Indeed, SIS only appeared to be cost-effective over a very narrow range of WTP values and even then there was considerable observed uncertainty (p > 0.5).
Universal levonorgestrel intrauterine system treatment prior to referral
A further analysis using a modified decision model was based upon the situation envisaged (although not observed in current clinical practice) by NICE in their 2007 report into the management of HMB,11 where all women referred from primary care with HMB had received prior treatment with a LNG-IUS. Even in this scenario, OPH continued to be the favoured, cost-effective option with an ICER of £5480 for each additional woman with HMB satisfied and increasing certainty of cost-effectiveness, with increasing, but viable, WTP levels. Although the combination of TVS and EBx was a more effective approach, its ICER of over £500,000 prohibits its use as a sensible diagnostic strategy for adoption by the NHS.
‘See and treat’
Outpatient hysteroscopy is consistently the preferred front-line testing strategy for women with HMB, irrespective of their desire for future fertility and regardless of pre-referral treatment with the LNG-IUS, considered the most effective medical intervention available for HMB. 11 One consideration driving the economic benefits of OPH over other tests is that the modality allows treatment for most pathology to be initiated at the same time as the diagnosis, thereby reducing the number of patient attendances and the costs. This contemporary ‘see and treat’ ambulatory approach in gynaecological practice is increasingly being adopted. There are also additional benefits of embracing this philosophy which are not accounted for in our analysis, for example improved safety, lowered infection risks, convenience, rapid discharge and recovery. 18 In our model, only 30% of women required a further appointment in order to return for treatment at a later stage in the OPH testing strategy. This was because all women presenting with polyps or pathology suitable for treatment with a LNG-IUS could be treated at their first appointment if diagnosed correctly. For scanning modalities, concomitant treatment was still possible, for the same proportion of women; however, the cost is elevated slightly because of the additional cost of hysteroscopy and polypectomy for the 19% of women with endometrial polyps. Furthermore, the accuracy of TVS without contrast for the diagnosis of focal pathology is reduced compared with OPH. This consideration, combined with the additional costs of an OPH needed to effect the polypectomy for both TVS and SIS scanning approaches, contributes to their reduced cost-effectiveness compared with OPH. This was even more apparent for strategic testing approaches utilising EBx from the outset where no simultaneous treatment was possible because of the necessity to await the EBx result before making a diagnosis and instituting therapy. ‘Real-time’ bed-side testing can be seen to confer an advantage in the efficiency of delivering care. Thus, it is the ability to concomitantly treat as well as diagnose intrauterine pathology with a high degree of accuracy that leads to OPH being the most cost-effective testing strategy in HMB.
Post-menopausal bleeding
A previous cost-effectiveness analysis had identified initial testing with TVS to measure endometrial thickness at a 5-mm threshold as the preferred option. 69 Within this strategy, histological testing using EBx would be restricted to those women with an abnormal TVS endometrial thickness measurement. In our updated analysis, we took account of a contemporary ‘one-stop’ clinical setting and adopted a novel approach using IPD to integrate important clinical characteristics from the history into the relevant testing options to examine whether or not this affected cost-effectiveness. Our analysis identified three potentially cost-effective testing approaches all utilising TVS: (i) ‘selective TVS with history’ where TVS was restricted to women with a > 4% chance of endometrial cancer based upon risk factors identified from the patient history; (ii) ‘history + TVS’, where historical risk factors are taken into account along with the result of a TVS; and (iii) a combination of TVS and OPH. Selective TVS based upon historical risk prediction for the diagnostic work-up of women presenting with PMB generated an ICER compared with our reference strategy of ‘no initial work-up’ of £129,000 per extra woman surviving 5 years. The ICER of combining history and TVS was £2.4M and the combination of OPH and TVS required an investment of £2.7M to acquire one additional woman with PMB living 5 years.
From ONS life tables,153 the life expectancy of a 63-year-old woman (the mean age of women diagnosed with endometrial cancer85) is approximately 22 years. This reduces to approximately 15 years when discounting at 3.5% is applied. Assuming that each extra survivor at 5 years gains a full 15 discounted QALYs, the maximum WTP at the upper NICE threshold of £30,000 per QALY would be £450,000 per additional survivor. Across the NICE threshold range of £20,000 to £30,000 per QALY gained, the option ‘selective TVS with history’ would appear to be cost-effective if each additional 5-year survivor gains 4 to 6 QALYs. This seems plausible and suggests that ‘selective TVS with history’ is the preferred option for investigating PMB at a cost acceptable to the NHS. The two other non-dominated options have ICERs over £2M per additional survivor compared with the next most effective strategy, and are thus most unlikely to be cost-effective. We drew acceptability curves and frontiers as far as a WTP of £600,000 per additional survivor to assess the stability of our findings. The certainty of selective TVS based upon information obtained from the patient history being the most cost-effective of the non-dominated strategies at a WTP investment of £129,000 was approximately 40%, but this rose to over 70% at WTP levels between circa £260,000 and £600,000.
We examined further the certainty of our findings by producing acceptability curves comparing the non-dominated strategies. Although the probabilistic sensitivity analyses showed that ‘history + TVS’ was cost-effective compared with ‘selective TVS with history’ in a small proportion of model runs, this proportion never exceeded 20% across a range of WTP values from £0 to £600,000. Similarly, the likelihood of a strategy combing TVS and OPH being cost-effective compared with ‘history + TVS’ was extremely unlikely, with WTP values up to £600,000. Interestingly, however, qualitative research has suggested that, given the choice, women want to undergo OPH in addition to strategies based upon TVS even despite the marginal additional diagnostic value. 152 Thus, economic analyses from a wider perspective, capturing patient preferences, may make a combination strategy of TVS + OPH more viable. The current recommendation is to use TVS as the first-line diagnostic test for investigating PMB. 66,69 So, despite the fact that TVS alone was dominated in our deterministic analysis by selective TVS based upon preceding patient history, we produced an acceptability curve to explore whether or not TVS alone was likely to become cost-effective over the plausible range of WTP values. Acceptability curves demonstrated that the probability of TVS alone being more cost-effective than a selective approach to TVS was negligible.
Strengths and limitations
The economic evaluations were based upon the construction of comprehensive, contemporary and clinically informed decision trees. All available testing strategies were modelled and a modern, ‘one-stop’ clinical environment for testing was assumed. Test accuracy and performance data were obtained from high-quality systematic quantitative reviews of the literature. Furthermore, in the PMB model, IPD integrated patient characteristics into the testing strategies to reflect the real clinical situation, although these data were available only for strategies utilising TVS. Other clinical parameter inputs, including treatment effectiveness and disease prevalence, were obtained following systematic searches and selection based upon a rigorous evaluation of data quality. Producing a comprehensive series of reviews was not the aim of the economic modelling study. Thus, although data were identified using a systematic approach, ‘systematic reviews’ per se were not conducted for each clinical question because this was not the focus of our project and the quantity of data and resources required were beyond the funding constraints. Full adherence to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) was not, therefore, practical or possible. We had hoped to use IPD derived from published systematic reviews of test accuracy to provide estimates of accuracy of tests used in combination. However, we adapted our approach when it became clear, following literature searches and scrutiny of the primary studies included in the available aggregated reviews, that reporting accuracy data in HMB or PMB for more than one test was rare. Tests in agreement presented no uncertainty, but where test results were modelled as being discordant, decision-making, as regards the assumed diagnosis or need for further testing, was determined by the consensus view of an expert clinical panel. This panel included senior gynaecologists from the UK and the Netherlands and pathologists active in the field. The accord of this representative panel was key to informing the model inputs in other areas of practice where the evidence base was lacking; notably, the effectiveness of treatments for HMB in the presence of undetected pathologies (HMB model) and the effect of delayed diagnosis of endometrial cancer on disease staging and subsequent survival (PMB model).
The economic evaluation took the UK NHS as its perspective. This meant that only costs incurred by the NHS were included, and benefits were measured in terms of survival (PMB model) and patient satisfaction (HMB model). The prime aim of testing in chronic benign conditions such as HMB is to improve patient symptomatic outcomes. Thus, our approach of evaluating effectiveness in terms of patient satisfaction within the HMB analysis is relevant. We chose patient satisfaction (i) in order to ensure consistency with previous research trials undertaken in the field2 and (ii) because patient satisfaction is deemed to be the co-primary measure of importance (together with menstrual bleeding) by the Cochrane Menstrual Disorders & Subfertility Group for reviews of interventions for HMB. 62 Inevitably, this perspective will have excluded other potentially important costs and benefits. For example, the scarcity of HRQL data in AUB precluded a cost–utility analysis and comparison, by health-care decision-makers, with competing health-care interventions. While we took account of the feasibility of testing, we did not consider the morbidity (anxiety, discomfort, complications) and psychological implications for women and their families of undergoing investigation. Similarly, we did not incorporate patient preferences for testing into the models or indeed the added value of individual tests outside of the focus of uterine assessment, for example simultaneous assessment of the ovaries. Indeed, qualitative research has shown that informed women presenting with PMB are desirous of the added reassurance, despite the discomfort, inconvenience and minimal extra diagnostic benefit, of more extensive testing with OPH in addition to evidence-based strategies based upon TVS with recourse to EBx. 152 This suggests that this added reassurance is valued more by patients than clinicians and those tasked with allocating health resources.
We believe our findings to be generalisable to modern practice in the UK because we adopted a contemporary, ‘one-stop’ clinical setting, where all tests are available during a single visit whether they are utilised in combination from the outset or sequentially, conditional on an earlier test finding. Moreover, it was assumed that minor therapeutic interventions were implemented during this visit: an up-to-date ‘see and treat’ approach using all available health technologies. Costs were derived from up-to-date HRG data;154 the costs of individual tests were derived and added to the cost of a standard new consultation. This method allowed us to compare the different strategies fairly by breaking down the different aspects of each appointment. However, while this approach allowed us to apply costs in a contemporary ‘one-stop’ clinical setting, the costs may not accurately reflect the true cost to the NHS. It is unlikely that the HRG codes precisely take account of opportunity costs from the clinician’s perspective, that is to say the additional time and disruption associated with combination testing either from the outset or conditional on preceding tests. For example, the ‘one-stop’ diagnostic testing philosophy combined with the ability to provide concomitant treatment (‘see and treat’) is certainly convenient and efficient but the additional time required (which is somewhat unpredictable) necessitates the allocation of fewer appointment slots within such clinics. In sensitivity analysis we modified our base-case model to reflect multistop diagnostic and treatment pathways to help inform health providers currently still adopting traditional service models.
While it is certainly the case that a ‘one-stop’ approach is efficient, obviating the need to for further appointments, the UK NHS perspective of our economic analysis may have underestimated the total cost savings of ‘one-stop’ service delivery had we adopted a wider social perspective. Minimising interference to women’s home and working lives by avoiding unnecessary follow-up appointments with consequent travel and waiting times is likely to be associated with substantial economic benefits. If we had been able to quantify the absence from paid work and household activities in monetary units, these costs may not have been insignificant. Thus, it is probable that a ‘one-stop’ setting for investigation of AUB from a societal viewpoint would have been even more cost-effective compared with traditional multistop models of service delivery.
A variety of different benign pathologies underlie HMB, and each pathology has its optimal first-line treatment. However, some pathologies, for example DUB and endometrial hyperplasia, share the same ‘optimal’ first-line treatment (i.e. the LNG-IUS). Moreover, erroneously diagnosed pathologies, given ‘incorrect’ treatment, will generally still respond to several treatments, albeit to a suboptimal degree. Thus, the fact that HMB is usually of benign origin and often responds to some degree regardless of pathology to the NICE recommended first-line HMB treatment LNG-IUS has raised uncertainty over the cost-effectiveness of any testing in HMB. It was for this reason we used ‘no investigation’ with LNG-IUS treatment for all as our reference strategy against which all other testing options could be compared. However, the universal prescription of the LNG-IUS in secondary care to treat HMB is unlikely to be acceptable to either women who require an explanation for their symptoms (and may not want a LNG-IUS, although our model did not account for patient preferences) or clinicians who are aware that more effective, tailored treatments are available according to the underlying diagnosis. In primary care the situation is different, with NICE recommending LNG-IUS as a first-line treatment without stipulating the need for diagnostic testing. 11 In view of this, we modelled a scenario in which all women with HMB referred to secondary care were refractory to treatment with the LNG-IUS. The inference from this alternative analysis, that investigation should be based upon first-line testing with OPH, was unchanged. Thus, given the data available, our model is comprehensive, pragmatic and relevant to current clinical practice.
Extensive probabilistic sensitivity analyses were conducted to allow for uncertainty, manipulating parameter inputs for clinical assumptions (disease prevalence, test performance, accuracy, and consequences of false diagnoses and effectiveness of treatments) and costs. Acceptability frontiers were drawn to aid assessment and interpretation. The most cost-effective testing strategies identified for both HMB and PMB remained stable following sensitivity analyses, increasing the confidence of our conclusions regarding recommendations regarding the optimal approaches for testing in AUB for the NHS. To further evaluate the stability and also transferability of our findings in HMB, we produced alternative models to embrace scenarios where referral to secondary care is restricted to women who remain refractory to effective medical treatment11 and where preservation of fertility is required.
Simplifications
We aimed to develop economic models that accurately and explicitly mirrored clinical practice. Some simplification was necessary, driven by a desire to keep the extensive and comprehensive decision trees workable. One problem our analysis had to address was that of how to account for failed testing and discordance of tests within combination strategies. The accuracy of individual outpatient tests varies according to which pathology is under scrutiny. However, this does not mean that combination testing will be more cost-effective. Our model comprehensively evaluated tests used in isolation (with additional testing conditional on the test outcome) and in combination from the outset (again with any further testing being conditional on the combination testing outcome). However, where combination testing was incomplete due to tests within the combination failing, clinicians would then face a dilemma as to what to do next and this was apparent in our inability to obtain expert consensus. We debated at length how best to deal with failed tests within test combinations. In the end we decided to simplify our model with the aim of maintaining consistency and minimising potential bias. Thus, the approach we used was that any test or tests that failed within a combination testing strategy were assumed to have not allowed a diagnosis to be arrived at (i.e. the testing strategy has failed) and recourse to D&C was the result. The limitation of this approach is its clinical validity and relevance. Undoubtedly, many clinicians would consider examination under anaesthesia with D&C the ‘gold standard’ test, whereas others may pragmatically arrive at a diagnosis based upon the information provided by the successful tests within the combination strategy. However, there are a huge number of potential test combinations and failure possibilities to consider. Moreover, it was hard to arrive at any consensus from the expert panel as to what subsequent testing or management decisions would then arise. Thus, we believe that simplification of our model, such that a combination testing strategy was considered incomplete, necessitating recourse to D&C, was reasonable in order to maintain consistency and minimise bias. Moreover, the failure rates of tests are generally low and so the impact of our model simplification should be minimal.
Subtle differences between testing strategies may have been overlooked; for example, in the HMB model we assumed that women could only have one underlying pathology, whereas in practice, pathologies may, in a minority of women, coexist. The most common pathologies underlying HMB were accounted for in our model: DUB, polyps/SMFs, small and large fibroids and significant endometrial disease. Adenomyosis, a condition of the myometrium (uterine muscle) where ectopic endometrial tissue is found, can be associated with HMB but we excluded the diagnosis from our model. This omission can be justified because pain rather than HMB tends to be the presenting symptom of adenomyosis. Moreover, treatment is not affected by the suspicion of adenomyosis and indeed it only generally comes to light after histopathological examination of the uterus post hysterectomy. Thus, it is unlikely that the exclusion of adenomyosis as a potential aetiology of HMB would have had any effect on the outcome of the economic evaluation.
We modelled all relevant, widely employed testing modalities in AUB. Endometrial tissue sampling was assumed to be by outpatient EBx and inpatient D&C in failed cases. EBx can, however, be performed under direct vision by passing miniature forceps down the working channel of an outpatient hysteroscope. We chose not to include directed hysteroscopic biopsy as it is not widely used and is less likely to provide an adequate tissue sample for histological assessment compared with simpler, cheaper and universal outpatient EBx. Furthermore, OPH and SIS are accurate tests for the diagnosis of focal pathology without recourse to histological confirmation. In addition, focal endometrial disease (cancer and hyperplasia) necessitating a directed biopsy is rare, with most endometrial conditions being global hormonally induced phenomena allowing ‘blind’ sampling technologies to obtain representative, diagnostic samples to provide diagnosis with a high level of accuracy. 20,21 Thus, directed biopsy is unlikely to confer an advantage over blind EBx.
Testing with magnetic resonance imaging (MRI) was not examined because NICE recommends that it should not be used for diagnosis in women with HMB on the basis that there are no studies examining its use. 11 One study compared MRI with TVS for the identification of adenomyosis and found no significant difference between them. 155 In addition, MRI is not suitable for real-time outpatient (‘office’) testing and is expensive and rarely used in the diagnosis of HMB unless there are concerns over the nature of uterine fibroids (an infrequent situation) or the suitability for UAE is being assessed.
Our evaluation of PMB was restricted to detecting or excluding endometrial cancer and the impact of testing strategies on survival. The rationale for this approach was based upon the following considerations. In contrast to HMB, the management of PMB is aimed at identifying and treating a potentially life-threatening condition. HMB is invariably of benign origin and the aim of management is to alleviate symptoms to a woman’s satisfaction and enhance life quality by minimising restrictions on daily activities and desires. The majority of PMB is also non-malignant, but endometrial cancer and pre-cancer is present in 10–20%9 of cases and is eminently treatable if diagnosed early. Thus, survival was considered the most appropriate clinical outcome. An evaluation of optimal testing approaches for all possible causes of PMB, benign and malignant, would require a relevant clinical outcome applicable uniformly and, in the absence of HRQL data for the treatment of specific conditions underlying PMB, a unifying, all-encompassing analysis was not feasible. PMB, although an alarming symptom, is not often heavy, persistent or recurrent when associated with benign causes. Thus, the over-riding rationale for prompt diagnostic work-up is aimed at excluding serious endometrial disease rather than relieving symptoms.
Assumptions
As with all economic modelling exercises, assumptions had to be made where contentious clinical decision-making or a paucity of clinical data were encountered and these assumptions were ultimately endorsed by the expert clinical panel after extensive consultation. One such area pertained to disease prevalence, estimates of which came from a variety of sources, and this meant that, to make them sum to 1, we needed to use the prevalence of one ‘disease’, DUB, as a flexible value. The assigned value of 0.32 (32%) may not accurately reflect the true prevalence; however, published data and the expert panel considered this a reasonable estimate. As with the precision of all assumptions, they were subject to sensitivity analyses. In this particular case, the inferences of the base-case analysis were not affected with varied, plausible estimates of DUB prevalence. A similar problem was encountered for some accuracy data such that the reciprocal FPR estimates, which had originated from diverse data sources, had to be manipulated to total 1 and these were tested in sensitivity analysis.
In the HMB model, we selected the unifying outcome as satisfaction with treatment at 1 year because this is one of the most common outcomes measured in RCTs of interventions for HMB61 and is clinically relevant. Moreover, the availability of other patient-reported outcome measures, for example HRQL data (and especially their application to the specific scenarios that arose from the construction of our comprehensive decision trees), is low. Collecting such data was not possible within the time and resource constraints of this project. Approximating data from published studies was decided to be an inaccurate method for producing QALYs and so ‘cost per patient satisfied’ was used alone. In addition, the cyclical and intermittent nature of HMB makes it somewhat problematic to calculate QALYs, particularly as women are affected by the condition for only approximately 25% of the year and symptoms will naturally resolve in time once the menopause is reached. While we endeavoured through systematic searching and quality appraisal to identify the optimal data, with a minority of interventions for particular underlying diagnoses, satisfaction data were not explicitly available and, where this problem was encountered, we assumed that the outcomes reported (e.g. ‘reduced bleeding’, ‘would recommend the treatment’ or ‘cured of cancer’) were indirect measures of satisfaction following consultation with the expert panel.
Occasionally, satisfaction data were not reported at 1 year, in which case we chose the data points closest to 1. This time point, while reasonable in terms of evaluating medium-term response, may favour conservative, ‘uterine-sparing’ procedures (the LNG-IUS, EA and myomectomy) because their effectiveness reduces over time. 2 In contrast, more invasive but definitive hysterectomy is not associated with recurrence of HMB symptoms. Thus, longer-term outcome assessment may have made the option of ‘hysterectomy without diagnostic testing’ a more viable option. However, the objective and emphasis of our analysis was to estimate the cost-effectiveness of diagnostic testing. Thus, as long as the treatment options were appropriate and consistently applied according to the diagnosed pathology, we could be confident that the most cost-effective testing strategies were delineated. Our base-case analysis assumed included women to be 45 years old as this is the modal age for presentation to secondary care with HMB. Thus, they could be expected to have an average of a further 7 years of menstruation before the menopause.
We have highlighted the ability of OPH to facilitate the removal of uterine polyps in addition to simply providing a diagnosis. However, this presumed cost-efficient benefit may be blunted if uterine polyps are not causative of HMB but are simply an incidental finding. NICE were unable to find any data to link uterine polyps and HMB,11 although clinicians do assume some link. 47,156 Two systematic reviews of generally low-quality, observational studies do, however, support the notion of polyp treatment being associated with a 75–100% improvement in HMB complaints. 40,41 A RCT of polyp treatment (www.opt.bham.ac.uk), due to be published in 2014, should be able to clarify this point as data have been collected from 507 women with AUB being treated with polypectomy. 51 Furthermore, even if polyps do not cause HMB, they may negatively affect treatment (e.g. impairing the effectiveness of the LNG-IUS) and ultimately cause dissatisfaction. 157,158 This may also be a psychological effect given that most women and their clinicians are unwilling to accept conservative management of detected uterine polyps. 159 In addition to hysteroscopic surgical interventions such as polypectomy and myomectomy, other uterine-sparing therapeutic interventions, such as fitting of LNG-IUS or EA, may not be successfully completed. For the purposes of our model, however, we assumed that all treatments were successfully performed given that the objective of our economic analysis was to examine the cost-effectiveness of diagnostic testing based upon their test performance and accuracy, rather than an assessment of treatment efficacy.
Despite the need for assumptions arising in response to the ‘holes’ in our evidence base, the analytic modelling methodology allowed us to produce an extensive, comprehensive, contemporary and clinically relevant evaluation. All-embracing RCTs, assessing the wide range of diagnostic strategies defined in our models, would be a huge, impractical and ultimately futile undertaking.
Comparisons with existing guidance
How do the findings from this economic evaluation compare with existing guidance? Current NICE guidance published in 2007 for the management of HMB11 recommends that TVS should be the diagnostic modality of choice when testing is considered and that OPH should be employed if TVS is inconclusive. This advice was predicated upon a cost-effectiveness analysis (discussed in more detail in Chapter 1, Literature review of cost-effectiveness studies for the diagnostic work-up of heavy menstrual bleeding) limited to the assessment of three single testing options: TVS, SIS and OPH. The outcome ‘cost per correct diagnosis’ did not take into account the range of pathologies under consideration for HMB, and the optimal treatments of those pathologies once diagnosed. A fuller evaluation is necessary in order to consider the consequences of erroneous diagnosis given that the raison d’etre of formulating a diagnosis is to inform and optimise clinical management. Women may serendipitously receive appropriate treatment following a false diagnosis but, more often than not, misdiagnosis results in misallocation of resources and consequent morbidity attributable to unnecessary procedures. The comprehensive analysis performed in this report reflects the reality of diagnostic evaluation in day-to-day clinical practice in the UK.
Previous economic analyses69,70,82 support the current recommended guidance for the investigation of PMB, namely that TVS measurement of endometrial thickness at a 5-mm threshold, with recourse to biopsy with an abnormal result, should be the initial testing strategy of choice for investigating women with PMB. 66 However, our updated cost-effectiveness analysis incorporated a contemporary ‘one-stop’ clinical setting for testing and integrated patient characteristics, acquired from the pre-testing clinical process (history and examination). Our analysis is consistent with the policy of basing initial testing of women presenting with PMB on TVS, but supports the selective deployment of TVS based upon clinical risk information.
Chapter 6 Conclusions
Summary of findings
Heavy menstrual bleeding
Our analysis identified two potentially cost-effective testing strategies for the investigation of women with HMB. These were initial testing with OPH alone or in combination with EBx. To adopt a strategy of OPH, £360 needs to be invested to gain one more woman satisfied at 1 year compared with a strategy of empirical treatment with a LNG-IUS. Although a testing strategy of OPH + EBx is marginally more effective, the ICER is approximately £21,000 to gain one more satisfied patient compared with OPH. We estimate that this equates to around £26,500 per QALY, which falls within the £20,000–30,000 per QALY threshold. These findings were stable during sensitivity analyses, varying model inputs including disease prevalence, test feasibility and accuracy, with OPH remaining more cost-effective than the LNG-IUS reference strategy even at relatively low WTP thresholds. In women wishing to preserve their fertility, therapy with EA and hysterectomy are contraindicated. SIS was cost-effective in this situation, with an ICER of approximately £2000, but for an additional financial outlay of £2720, testing instead with OPH produces a further satisfied patient, which is likely to be considered affordable and worthwhile by the NHS. Sensitivity analysis also showed SIS to be a cost-effective testing option along with the combination testing strategy of OPH + EBx within the context of traditional multistop pathways, although such service models are likely to diminish over time with the ongoing improvement and increasing availability of portable diagnostic health technologies. OPH remains the most cost-effective testing option if a scenario is envisaged where only women with HMB refractory to the LNG-IUS, recommended first-line treatment in general practice,11 are referred to secondary care for investigation. In this situation, we estimate that £5480 of extra funding is necessary for each additional woman satisfied. No other testing strategies fell within plausible WTP ranges.
Therefore, our data are consistent in supporting OPH as the diagnostic testing strategy of choice for women referred to secondary care with HMB, irrespective of their desire for future fertility and regardless of pre-referral treatment with the LNG-IUS. A combination strategy of OPH and EBx may be cost-effective at the upper NICE WTP threshold, in women without a desire to retain their fertility who have not undergone pre-referral treatment with the LNG-IUS or in women investigated through a traditional multivisit pathway.
Post-menopausal bleeding
Our economic analysis identified three potentially cost-effective testing approaches for the investigation of PMB, all utilising TVS. Selective TVS conditional upon patient characteristics generated an ICER compared with our reference strategy of no initial testing of £129,000 per extra woman surviving 5 years. Across the NICE threshold range of £20,000 to £30,000 per QALY gained, this option of selective TVS combined with risk factors acquired from the clinical history would appear to be cost-effective if each additional 5-year survivor gains 4 to 6 QALYs. This seems plausible and suggests that this is the preferred option for investigating PMB at a cost acceptable to the NHS. Although combination strategies of TVS with integration of patient characteristics or alternatively TVS with OPH were also non-dominated, these potentially cost-effective approaches both required an investment of over £2M for each additional woman with PMB living 5 years, which is beyond viable funding thresholds. Probabilistic sensitivity analyses showed that ‘history + TVS’ was cost-effective compared with ‘selective TVS with history’ in a small proportion of model runs, but this proportion never exceeded 20% across a range of WTP values from £0 to £600,000, supporting the case for selective TVS after considering the risk associated with adverse patient characteristics.
Implications for the NHS and patients
Standardisation of diagnostic practices for the investigation of AUB in secondary care, based upon the findings of this report, should provide reassurance that patients throughout the UK NHS are receiving an equitable, high standard of care and that health-care resources are being utilised in a rational, cost-effective way.
Heavy menstrual bleeding
Initial investigation of women presenting to secondary care with HMB who do not require preservation of their fertility appears to be a choice between OPH alone or, at WTP thresholds above £20,000 per additional woman satisfied, OPH combined with EBx.
Outpatient hysteroscopy appears to be the most cost-effective first-line diagnostic test used for the investigation of women presenting to secondary care with HMB wishing to preserve their fertility or refractory to previous medical treatment with the LNG-IUS.
A strategy of initial testing with OPH appears to be cost-effective within the context of a contemporary, ‘one-stop’ clinical service, where histological sampling, insertion of LNG-IUS and uterine polypectomy can be performed during the same visit. However, this does not appear to be the case within a more traditional multistop diagnostic and treatment service. A combination of OPH and EBx is potentially cost-effective within both ‘one-stop’ and multistop settings at WTP thresholds between £20,000 and £30,000 per QALY.
Post-menopausal bleeding
The use of transvaginal ultrasonic measurement of endometrial thickness as the first-line test in the diagnostic evaluation of PMB appears to be appropriate. However, the model-based results from this analysis, supporting a more selective use of TVS based upon risk factors for endometrial cancer, should be empirically confirmed with RCTs before current medical practice is changed.
Suggested research priorities
Data from primary test-accuracy studies and secondary systematic reviews with aggregated, or in some instances IPD, meta-analyses are available. However, there is a dearth of data pertaining to the accuracy of combination and sequential testing strategies. Future research should be aimed at generating estimates of diagnostic test accuracy of test combinations from individual patients so that the added value of tests used in combination from the outset or in sequence, conditional on preceding test results, can be more rigorously estimated. These data can then inform future economic evaluations.
The completion of this extensive and comprehensive economic modelling evaluation incorporating all contemporary testing alternatives for HMB and PMB has delineated potential cost-effectives options. Thus, it is now feasible to compare competing cost-effective testing strategies for HMB or PMB within focused RCTs.
Prospective collection of potentially relevant patient characteristics obtained from clinical history and examination should be undertaken so that these features can be integrated into the clinical process. This would allow predictive models to be built and the formulation of rational, tailored testing strategies based upon individual risk assessment.
Primary accuracy studies will be required to examine the use of newer diagnostic technologies, such as 3D ultrasonography and power Doppler imaging of tissues.
Validated, condition-specific patient-reported outcome measures are available for HMB, but these have not as yet been widely applied as outcomes in clinical research. Future clinical trials should employ such measures to better capture the impact of HMB symptoms, and its investigation and management, on HRQL.
The model-based results from the PMB analysis suggesting that women should only be scanned if their history suggests a high risk for endometrial cancer should be empirically confirmed with RCTs.
The management of PMB is aimed at identifying and treating a potentially life-threatening condition. However, while the detection of endometrial cancer is of over-riding importance, PMB in the majority of women has a benign underlying cause. An evaluation of optimal testing approaches for all possible causes of PMB, benign and malignant, would require a relevant clinical outcome, applicable uniformly. To facilitate this more comprehensive assessment requires further studies to capture HRQL data for the treatment of specific conditions underlying PMB.
Diagnostic assessment should incorporate patient preferences and take into account associated morbidities, including psychological implications. To understand these preferences, studies should be designed where participants acquire a detailed knowledge of the accuracy and added value of particular testing approaches as well as become familiar with the experience and risks of adverse effects. An acquaintance with patients’ views will better inform the development of testing strategies, facilitate more individualised testing and maybe enhance compliance. This qualitative knowledge of the whole patient experience will be especially important where choice between potential cost-effective alternatives is contentious.
Our economic models applied to women presenting with AUB for the first time to secondary care and who were evaluated in a ‘one-stop’ setting. Further evaluations of the role of diagnostic testing may want to consider re-presentation because of recurrent HMB or PMB.
Acknowledgements
We thank the following people:
Mr Tyrone Carpenter, Consultant Obstetrician and Gynaecologist, Poole Hospital NHS Foundation Trust, Poole, UK.
Dr Mary Connor, Consultant Obstetrician and Gynaecologist, Royal Hallamshire Hospital, Sheffield, UK.
Professor Jon Deeks, Professor of Biostatistics, University of Birmingham, Birmingham, UK.
Dr Ioannis Gallos, Obstetrics and Gynaceology Trainee, Birmingham Women’s Hospital, Birmingham, UK.
Dr Raji Ganesan, Consultant Pathologist, Birmingham Women’s Hospital, Birmingham, UK.
Professor Janesh Gupta, Professor of Obstetrics and Gynaecology, University of Birmingham, Birmingham, UK.
Professor Sian Jones, Consultant Obstetrician and Gynaecologist, Bradford Teaching Hospitals Foundation Trust, Bradford, UK.
Mr Matthew Parsons, Consultant Obstetrician and Gynaecologist, Birmingham Women’s Hospital, Birmingham, UK.
Ms Mamta Patak, Consultant Obstetrician and Gynaecologist, Worcestershire Royal Hospital, Worcester, UK.
Mr Kevin Phillips, Consultant Obstetrician and Gynaecologist, Castle Hill Hospital, Hull, UK.
Dr Hanny Pijnenborg, Consultant Obstetrician and Gynaecologist, TweeSteden Ziekenhuis, Tilburg, the Netherlands.
Mr Nick Raine-Fenning, Consultant Gynaecologist and Clinical Associate Professor in Reproductive Medicine, University of Nottingham, Nottingham, UK.
Mr Robert Richardson, Consultant Obstetrician and Gynaecologist, Chelsea and Westminster Hospital, London, UK.
Dr Anthony Roberts, Queens Hospital, Burton-upon-Trent, UK.
Ms Manjeet Shehmar, Consultant Obstetrician and Gynaecologist, Birmingham Women’s Hospital, Birmingham, UK.
Dr Andreas Thurkow, Consultant Obstetrician and Gynaecologist, Saint Lucas Andreas Ziekenhuis, Amsterdam, the Netherlands.
Dr Sebastiaan Veersema, Consultant Obstetrician and Gynaecologist, St Antonius Hospital, Utrecht, NL.
Dr Lucet van der Voet, Consultant Gynaecologist, Deventer Ziekenhuis, the Netherlands.
Contributions of authors
Dr Natalie AM Cooper (Clinical Research Fellow, Gynaecology): design of economic models, clinical data collection; economic data collection; contributed to analysis, interpretation of results and drafting of manuscript.
Dr Pelham M Barton (Reader in Mathematical Modelling): design of economic models; led economic analysis; interpretation of results; and revision of manuscript.
Dr Maria C Breijer (Clinical Research Fellow, Gynaecology): design of PMB economic model; revision of HMB economic model; IPD validation for PMB economic model; and revision of manuscript.
Miss Orla Caffrey (Research Fellow in Health Economics): construction of HMB economic models; and economic data collection.
Dr Brent C Opmeer (Assistant Professor Clinical Epidemiology/Health Economics): design of PMB economic model; IPD validation for PMB economic model; and revision of manuscript.
Dr Anne Timmermans (Consultant Obstetrician and Gynaecologist): design of PMB economic model; and IPD validation for PMB economic model.
Professor Ben WJ Mol (Professor of Obstetrics, Gynaecology and Epidemiology): conceived study; advised on economic models and analysis; and supervised the IPD meta-analysis.
Professor Khalid S Khan (Professor of Women’s Health and Clinical Epidemiology): conceived study; and advised on economic models and analysis.
Mr T Justin Clark (Consultant Obstetrician and Gynaecologist, Honorary Reader in Gynaecology): conceived study; design of economic models; supervised clinical data collection; contributed to analysis, interpretation of results and drafting of manuscript.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Coulter A, Noone A, Goldacre M. General practitioners’ referrals to specialist outpatient clinics. I. Why general practitioners refer patients to specialist outpatient clinics. BMJ 1989;299:304-6.
- Bhattacharya S, Middleton LJ, Tsourapas A, Lee AJ, Champaneria R, Daniels JP, et al. Hysterectomy, endometrial ablation and mirena for heavy menstrual bleeding: a systematic review of clinical effectiveness and cost-effectiveness analysis. Health Technol Assess 2011;15.
- Coulter A, McPherson K, Vessey M. Do British women undergo too many or too few hysterectomies?. Soc Sci Med 1988;27:987-94. http://dx.doi.org/10.1016/0277-9536(88)90289-4.
- Coulter A, Kelland J, Long A, Melvill A, O’Meara S. The management of menorrhagia. Eff Health Care 1995;9:1-14.
- Coulter A, Peto V, Jenkinson C. Quality of life and patient satisfaction following treatment for menorrhagia. Fam Pract 1994;11:394-401. http://dx.doi.org/10.1093/fampra/11.4.394.
- Clark TJ, Khan KS, Foon R, Pattison H, Bryan S, Gupta JK. Quality of life instruments in studies of menorrhagia: a systematic review. Eur J Obstet Gynecol Reprod Biol 2002;104:96-104. http://dx.doi.org/10.1016/S0301-2115(02)00076-3.
- Jones GL, Kennedy SH, Jenkinson C. Health-related quality of life measurement in women with common benign gynecologic conditions: A systematic review. Am J Obstet Gynecol 2002;187:501-11. http://dx.doi.org/10.1067/mob.2002.124940.
- Gull B, Karlsson B, Milsom I, Granberg S. Can ultrasound replace dilation and curettage? A longitudinal evaluation of postmenopausal bleeding and transvaginal sonographic measurement of the endometrium as predictors of endometrial cancer. Am J Obstet Gynecol 2003;188:401-8. http://dx.doi.org/10.1067/mob.2003.154.
- Gredmark T, Kvint S, Havel G, Mattsson LA. Histopathological findings in women with postmenopausal bleeding. Br J Obstet Gynaecol 1995;102:133-6. http://dx.doi.org/10.1111/j.1471-0528.1995.tb09066.x.
- Spencer CP, Whitehead MI. Endometrial assessment re-visited. Br J Obstet Gynaecol 1999;106:623-32. http://dx.doi.org/10.1111/j.1471-0528.1999.tb08358.x.
- CG44 Heavy Menstrual Bleeding: NICE Guideline 2007. London: NICE; 2007.
- The Initial Management of Menorrhagia. Evidence-Based Guidelines No. 1. London: Royal College of Obstetricians and Gynaecologists; 1998.
- Reid PC, Coker A, Coltart R. Assessment of menstrual blood loss using a pictorial chart: a validation study. Br J Obstet Gynaecol 2000;107:320-2. http://dx.doi.org/10.1111/j.1471-0528.2000.tb13225.x.
- Wyatt KM, Dimmock PW, Walker TJ, O’Brien PM. Determination of total menstrual blood loss. Fertil Steril 2001;76:125-31. http://dx.doi.org/10.1016/S0015-0282(01)01847-7.
- Chimbira TH, Anderson AB, Naish C, Cope E, Turnbull AC. Reduction of menstrual blood loss by danazol in unexplained menorrhagia: lack of effect of placebo. Br J Obstet Gynaecol 1980;87:1152-8. http://dx.doi.org/10.1111/j.1471-0528.1980.tb04489.x.
- Fraser IS, McCarron G, Markham R. A preliminary study of factors influencing perception of menstrual blood loss volume. Am J Obstet Gynecol 1984;149:788-93. http://dx.doi.org/10.1016/0002-9378(84)90123-6.
- Hallberg L, Nilsson L. Determination of menstrual blood loss. Scand J Clin Lab Invest 1964;16:244-8. http://dx.doi.org/10.3109/00365516409060511.
- Clark TJ, Gupta JK. Handbook of Outpatient Hysteroscopy. A Complete Guide to Diagnosis and Therapy. London: Hodder Education; 2005.
- Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia. A long-term study of ‘untreated’ hyperplasia in 170 patients. Cancer 1985;56:403-12. http://dx.doi.org/10.1002/1097-0142(19850715)56:2%3C403::AID-CNCR2820560233%3E3.0.CO;2-X.
- Clark TJ, Mann CH, Shah N, Khan KS, Song F, Gupta JK. Accuracy of outpatient endometrial biopsy in the diagnosis of endometrial hyperplasia. Acta Obstet Gynecol Scand 2001;80:784-93. http://dx.doi.org/10.1034/j.1600-0412.2001.080009784.x.
- Clark TJ, Mann CH, Shah N, Khan KS, Song F, Gupta JK. Accuracy of outpatient endometrial biopsy in the diagnosis of endometrial cancer: a systematic quantitative review. BJOG 2002;109:313-21. http://dx.doi.org/10.1016/S1470-0328(02)01088-1.
- Goldstein SR. Saline infusion sonohysterography. Clin Obstet Gynecol 1996;39:248-58. http://dx.doi.org/10.1097/00003081-199603000-00023.
- Wolman I, Jaffa AJ, Kupferminz M, Geva E, Gordon D, Lessing JB, et al. Transvaginal sonohysterography: a method for the evaluation of the endometrial cavity – 4 years’ experience. Ultrasound International 1999;5:79-86.
- Ossola MW, Bertulessi C, Iasi L, Bianchini B, Hanozet F, Grassini E, et al. Comparison of saline infusion sonography to transvaginal echography and hysteroscopy in the diagnostic evaluation of abnormal uterine bleeding. Italian J Gynaecol Obstet 1999;11:145-50.
- Bakour SH, Jones SE, O’Donovan P. Ambulatory hysteroscopy: evidence-based guide to diagnosis and therapy. Best Pract Res Clin Obstet Gynaecol 2006;20:953-75. http://dx.doi.org/10.1016/j.bpobgyn.2006.06.004.
- Gulumser C, Narvekar N, Pathak M, Palmer E, Parker S, Saridogan E. See-and-treat outpatient hysteroscopy: an analysis of 1109 examinations. Reprod Biomed Online 2010;20:423-9. http://dx.doi.org/10.1016/j.rbmo.2009.11.024.
- Clark TJ, Godwin J, Khan KS, Gupta JK. Ambulatory endoscopic treatment of symptomatic benign endometrial polyps. A feasibility study. Gynaecol Endosc 2002;11:91-7. http://dx.doi.org/10.1046/j.1365-2508.2002.00485.x.
- Bettocchi S, Nappi L, Ceci O, Selvaggi L. What does ‘diagnostic hysteroscopy’ mean today? The role of the new techniques. Curr Opin Obstet Gynecol 2003;15:303-8. http://dx.doi.org/10.1097/01.gco.0000084241.09900.c8.
- Critchley HOD, Warner P, Lee AJ, Brechin S, Guise J, Graham B. Evaluation of abnormal uterine bleeding: comparison of three outpatient procedures within cohorts defined by age and menopausal status. Health Technol Assess 2004;8.
- De Vries LD, Dijkhuizen FPHL, Mol BWJ, Brolmann HAM, Moret E, Heintz APM. Comparison of transvaginal sonography, saline infusion sonography, and hysteroscopy in premenopausal women with abnormal uterine bleeding. J Clin Ultrasound 2000;28:217-23. http://dx.doi.org/10.1002/(SICI)1097-0096(200006)28:5%3C217::AID-JCU2%3E3.3.CO;2-2.
- Clark TJ. Outpatient hysteroscopy and ultrasonography in the management of endometrial disease. Curr Opin Obstet Gynecol 2004;16:305-11. http://dx.doi.org/10.1097/01.gco.0000136491.26463.c2.
- Milsom I, Andersson K, Andersch B, Rybo G. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol 1991;164:879-83. http://dx.doi.org/10.1016/S0002-9378(11)90533-X.
- Lahteenmaki P, Haukkamaa M, Puolakka J, Riikonen U, Sainio S, Suvisaari J, et al. Open randomised study of use of levonorgestrel releasing intrauterine system as alternative to hysterectomy. BMJ 1998;316:1122-6. http://dx.doi.org/10.1136/bmj.316.7138.1122.
- Hurskainen R, Teperi J, Rissanen P, Aalto AM, Grenman S, Kivela A, et al. Clinical outcomes and costs with the levonorgestrel-releasing intrauterine system or hysterectomy for treatment of menorrhagia: randomized trial 5-year follow-up. JAMA 2004;291:1456-63. http://dx.doi.org/10.1001/jama.291.12.1456.
- Hurskainen R, Teperi J, Rissanen P, Aalto AM, Grenman S, Kivela A, et al. Quality of life and cost-effectiveness of levonorgestrel-releasing intrauterine system versus hysterectomy for treatment of menorrhagia: a randomised trial. Lancet 2001;357:273-7. http://dx.doi.org/10.1016/S0140-6736(00)03615-1.
- Hurskainen R, Teperi J, Aalto AM, Grenman S, Kivela A, Kujansuu E, et al. Levonorgestrel-releasing intrauterine system or hysterectomy in the treatment of essential menorrhagia: predictors of outcome. Acta Obstet Gynecol Scand 2004;83:401-3. http://dx.doi.org/10.1111/j.0001-6349.2004.00440.x.
- Varma R, Soneja H, Bhatia K, Ganesan R, Rollason T, Clark TJ, et al. The effectiveness of a levonorgestrel-releasing intrauterine system (LNG-IUS) in the treatment of endometrial hyperplasia – a long-term follow-up study. Eur J Obstet Gynecol Reprod Biol 2008;139:169-75. http://dx.doi.org/10.1016/j.ejogrb.2008.02.022.
- Gallos ID, Krishan P, Shehmar M, Ganesan R, Gupta JK. LNG-IUS versus oral progestogen treatment for endometrial hyperplasia: a long-term comparative cohort study. Hum Reprod 2013;28:2966-71.
- Cooper KG, Jack SA, Parkin DE, Grant AM. Five-year follow up of women randomised to medical management or transcervical resection of the endometrium for heavy menstrual loss: clinical and quality of life outcomes. BJOG 2001;108:1222-8. http://dx.doi.org/10.1016/S0306-5456(01)00275-3.
- Nathani F, Clark TJ. Uterine polypectomy in the management of abnormal uterine bleeding: a systematic review. J Minim Invasive Gynecol 2006;13:260-8. http://dx.doi.org/10.1016/j.jmig.2006.03.015.
- Lieng M, Istre O, Qvigstad E. Treatment of endometrial polyps: a systematic review. Acta Obstet Gynecol Scand 2010;89:992-1002. http://dx.doi.org/10.3109/00016349.2010.493196.
- Hart R, Molnar V, Magos A. Long-term follow-up of hysteroscopic myomectomy assessed by survival analysis. BJOG 1999;106:700-5. http://dx.doi.org/10.1111/j.1471-0528.1999.tb08370.x.
- Emanuel MH, Wamsteker K, Hart AA, Metz G, Lammes FB. Long-term results of hysteroscopic myomectomy for abnormal uterine bleeding. Obstet Gynecol 1999;93:743-8. http://dx.doi.org/10.1016/S0029-7844(98)00558-4.
- Vercellini P, Zaina B, Yaylayan L, Pisacreta A, De O, Crosignani PG. Hysteroscopic myomectomy: long-term effects on menstrual pattern and fertility. Obstet Gynecol 1999;94:341-7. http://dx.doi.org/10.1016/S0029-7844(99)00346-4.
- Clark TJ, Mahajan D, Sunder P, Gupta JK. Hysteroscopic treatment of symptomatic submucous fibroids using a bipolar intrauterine system: a feasibility study. Eur J Obstet Gynecol Reprod Biol 2002;100:237-42. http://dx.doi.org/10.1016/S0301-2115(01)00485-7.
- Varma R, Soneja H, Clark TJ, Gupta JK. Hysteroscopic myomectomy for menorrhagia using Versascope bipolar system: efficacy and prognostic factors at a minimum of one year follow up. Eur J Obstet Gynecol Reprod Biol 2009;142:154-9. http://dx.doi.org/10.1016/j.ejogrb.2008.10.006.
- Clark TJ, Khan KS, Gupta JK. Current practice for the treatment of benign intrauterine polyps: a national questionnaire survey of consultant gynaecologists in UK. Eur J Obstet Gynecol Reprod Biol 2002;103:65-7. http://dx.doi.org/10.1016/S0301-2115(02)00011-8.
- Litta P, Cosmi E, Saccardi C, Esposito C, Rui R, Ambrosini G. Outpatient operative polypectomy using a 5 mm-hysteroscope without anaesthesia and/or analgesia: advantages and limits. Eur J Obstet Gynecol Reprod Biol 2008;139:210-14.
- Capobianco G, Vargiu N, Dessole F, Milia L, Dessole S. Office hysteroscopy for uterine intracavitary pathologies: see and treat approach. Gynecol Surg 2009;6.
- Marsh FA, Rogerson LJ, Duffy SRG. A randomised controlled trial comparing outpatient versus daycase endometrial polypectomy. BJOG 2006;113:896-901. http://dx.doi.org/10.1111/j.1471-0528.2006.00967.x.
- Clark TJ, Middleton LJ, Cooper NAM, Diwakar L, Denny E, Smith P, et al. A randomised controlled trial of outpatient versus inpatient polyp treatment for abnormal uterine bleeding. Health Technol Assess 2014.
- Pelage JP, Le O, Soyer P, Kardache M, Dahan H, Abitbol M, et al. Fibroid-related menorrhagia: treatment with superselective embolization of the uterine arteries and midterm follow-up. Radiology 2000;215:428-31.
- The REST. Investigators. Uterine artery embolization versus surgery for symptomatic uterine fibroids. N Eng J Med 2007;356:360-70.
- Pinto I, Chimeno P, Romo L, Haya J, De la Cal M, Bajo J. Uterine fibroids: uterine artery embolization versus abdominal hysterectomy for treatment. A prospective randomized and controlled trial. Radiology 2003;226:425-31.
- Lefebvre G, Vilos G, Allaire C, Jeffrey J, Arneja J, Birch C, et al. The management of uterine leiomyomas. J Obstet Gynaecol Can: JOGC 2003;25:396-418.
- Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473-83. http://dx.doi.org/10.1097/00005650-199206000-00002.
- Jenkinson C, Peto V, Coulter A. Making sense of ambiguity: evaluation in internal reliability and face validity of the SF-36 questionnaire in women presenting with menorrhagia. Quality Health Care 1996;5:9-12. http://dx.doi.org/10.1136/qshc.5.1.9.
- Lamping DL, Rowe P, Clarke A, Black N, Lessof L. Development and validation of the Menorrhagia Outcomes Questionnaire. Br J Obstet Gynaecol 1998;105:766-79. http://dx.doi.org/10.1111/j.1471-0528.1998.tb10209.x.
- Shaw RW, Brickley MR, Evans L, Edwards MJ. Perceptions of women on the impact of menorrhagia on their health using multi-attribute utility assessment. Br J Obstet Gynaecol 1998;105:1155-9. http://dx.doi.org/10.1111/j.1471-0528.1998.tb09968.x.
- Crow R, Gage H, Hampson S, Hart J, Kimber A, Storey L, et al. The measurement of satisfaction with healthcare: implications for practice from a systematic review of the literature. Health Technol Assess 2002;6.
- Middleton LJ, Champaneria R, Daniels JP, Bhattacharya S, Cooper KG, Hilken NH, et al. Hysterectomy, endometrial destruction, and levonorgestrel releasing intrauterine system (Mirena) for heavy menstrual bleeding: systematic review and meta-analysis of data from individual patients. BMJ 2010;341. http://dx.doi.org/10.1136/bmj.c3929.
- Lethaby A, Hickey M, Garry R, Penninx J. Endometrial resection/ablation techniques for heavy menstrual bleeding. Cochrane Database Syst Rev 2009;4. http://dx.doi.org/10.1002/14651858.CD001501.pub3.
- Kato I, Toniolo P, Akhmedkhanov A, Koenig KL, Shore R, Zeleniuch-Jacquotte A. Prospective study of factors influencing the onset of natural menopause. J Clin Epidemiol 1998;51:1271-6. http://dx.doi.org/10.1016/S0895-4356(98)00119-X.
- Bachmann LM, ter Riet G, Clark TJ, Gupta JK, Khan KS. Probability analysis for diagnosis of endometrial hyperplasia and cancer in postmenopausal bleeding: an approach for a rational diagnostic workup. Acta Obstet Gynecol Scand 2003;82:564-9. http://dx.doi.org/10.1034/j.1600-0412.2003.00176.x.
- FIGO Committee on Gynecologic Oncology . Staging Classifications and Clinical Practice Guidelines for Gynaecological Cancers 2000. www.igcs.org/professionalEducation/treatmentResources/figoStaging.html (accessed 21 January 2012).
- Investigation of Post-Menopasual Bleeding. Edinburgh: Royal College of Physicians; 2002.
- Ong S, Duffy T, Lenehan P, Murphy J. Endometrial pipelle biopsy compared to conventional dilatation and curettage. Irish J Med Sci 1997;166:47-9. http://dx.doi.org/10.1007/BF02939779.
- Epstein E, Skoog L, Valentin L. Comparison of Endorette and dilatation and curettage for sampling of the endometrium in women with postmenopausal bleeding. Acta Obstet Gynecol Scand 2001;80:959-64. http://dx.doi.org/10.1034/j.1600-0412.2001.801015.x.
- Clark TJ, Barton PM, Coomarasamy A, Gupta JK, Khan KS. Investigating postmenopausal bleeding for endometrial cancer: cost-effectiveness of initial diagnostic strategies. BJOG 2006;113:502-10. http://dx.doi.org/10.1111/j.1471-0528.2006.00914.x.
- Dijkhuizen FP, Mol BW, Brolmann HA, Heintz AP. Cost-effectiveness of the use of transvaginal sonography in the evaluation of postmenopausal bleeding. Maturitas 2003;45:275-82. http://dx.doi.org/10.1016/S0378-5122(03)00152-X.
- Burbos N, Musonda P, Giarenis I, Shiner AM, Giamougiannis P, Morris E, et al. Age-related differential diagnosis of vaginal bleeding in postmenopausal women: a series of 3047 symptomatic postmenopausal women. Menopause Int 2010;16:5-8. http://dx.doi.org/10.1258/mi.2010.010005.
- Ferrazzi E, Torri V, Trio D, Zannoni E, Filiberto S, Dordoni D. Sonographic endometrial thickness: a useful test to predict atrophy in patients with postmenopausal bleeding. An Italian multicenter study. Ultrasound Obstet Gynecol 1996;7:315-21. http://dx.doi.org/10.1046/j.1469-0705.1996.07050315.x.
- Opmeer BC, Van HC, Heintz APM, Burger CW, Bossuyt PMM, Mol BWJ. Improving the existing diagnostic strategy by accounting for characteristics of the women in the diagnostic work up for postmenopausal bleeding. BJOG 2007;114:51-8. http://dx.doi.org/10.1111/j.1471-0528.2006.01168.x.
- Musonda P, Burbos N, Duncan TJ, Crocker SG, Morris EP, Nieto JJ. Comparing the performance of two clinical models in estimating the risk of endometrial cancer in symptomatic postmenopausal women. Eur J Obstet Gynecol Reprod Biol 2011;159:433-8. http://dx.doi.org/10.1016/j.ejogrb.2011.09.005.
- Anderson KE, Anderson E, Mink PJ, Hong CP, Kushi LH, Sellers TA, et al. Diabetes and endometrial cancer in the Iowa women’s health study. Cancer Epidemiol Biomarkers Prev 2001;10:611-16.
- Gull B, Karlsson B, Milsom I, Granberg S. Factors associated with endometrial thickness and uterine size in a random sample of postmenopausal women. Am J Obstet Gynecol 2001;185:386-91. http://dx.doi.org/10.1067/mob.2001.115869.
- McPherson CP, Sellers TA, Potter JD, Bostick RM, Folsom AR. Reproductive factors and risk of endometrial cancer. The Iowa Women’s Health Study. Am J Epidemiol 1996;143:1195-202. http://dx.doi.org/10.1093/oxfordjournals.aje.a008707.
- Tabor A, Watt HC, Wald NJ. Endometrial thickness as a test for endometrial cancer in women with postmenopausal vaginal bleeding. Obstet Gynecol 2002;99:663-70. http://dx.doi.org/10.1016/S0029-7844(01)01771-9.
- van Doorn HC, Opmeer BC, Jitze DM, Kruitwagen RF, Dijkhuizen FP, Mol BW. The relation between age, time since menopause, and endometrial cancer in women with postmenopausal bleeding. Int J Gynecol Cancer 2007;17:1118-23. http://dx.doi.org/10.1111/j.1525-1438.2007.00925.x.
- Weiderpass E, Persson I, Adami HO, Magnusson C, Lindgren A, Baron JA. Body size in different periods of life, diabetes mellitus, hypertension, and risk of postmenopausal endometrial cancer (Sweden). Cancer Causes Control 2000;11:185-92.
- Xu WH, Xiang YB, Ruan ZX, Zheng W, Cheng JR, Dai Q, et al. Menstrual and reproductive factors and endometrial cancer risk: results from a population-based case–control study in urban Shanghai. Int J Cancer 2004;108:613-19.
- Medverd JR, Dubinsky TJ. Cost analysis model: US versus endometrial biopsy in evaluation of peri- and postmenopausal abnormal vaginal bleeding. Radiology 2002;222:619-27.
- Bakour SH, Dwarakanath LS, Khan KS, Newton JR, Gupta JK. The diagnostic accuracy of ultrasound scan in predicting endometrial hyperplasia and cancer in postmenopausal bleeding. Acta Obstet Gynecol Scand 1999;78:447-51. http://dx.doi.org/10.1034/j.1600-0412.1999.780519.x.
- Smith-Bindman R, Kerlikowske K, Feldstein VA, Subak L, Scheidler J, Segal M, et al. Endovaginal ultrasound to exclude endometrial cancer and other endometrial abnormalities. JAMA 1998;280:1510-17. http://dx.doi.org/10.1001/jama.280.17.1510.
- Creasman W, Odicino F, Maisonneuve P, Quinn M, Beller U, Benedet J, et al. Carcinoma of the corpus uteri. Int J Gynecol Obstet 2006;95:S105-43. http://dx.doi.org/10.1016/S0020-7292(06)60031-3.
- Higham JM, Shaw RW. Clinical associations with objective menstrual blood volume. Eur J Obstet Gynecol Reprod Biol 1999;82:73-6. http://dx.doi.org/10.1016/S0301-2115(98)00224-3.
- Fuentes JA. Ginecol Obstet Mex 2007;75:341-6.
- Gonzalez A, Fernandez J, Rodrguez A. Operative office hysteroscopy. Gynecol Surg 2010;7.
- Bayer Healthcare . Mirena® Prescribing Information 2013. http://labeling.bayerhealthcare.com/html/products/pi/Mirena_Pl/pdf (accessed 10 March 2014).
- Hologic Inc . NovaSure® Instructions for Use 2011. www.novasure.com/pdf/info/Instructions.pdf (accessed 10 March 2014).
- Ethicon Women’s Health and Urology . Instructions for Use Gynecare® Thermachoice® III Uterine Balloon Therapy System 2010. www.ethicon360emea.com/sites/default/files/products/C03.23_Thermachoice.pdf (accessed 4 December 2011).
- Kaunitz AM, Bissonnette F, Monteiro I, Lukkari-Lax E, Muysers C, Jensen JT. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol 2010;116:625-32. http://dx.doi.org/10.1097/AOG.0b013e3181ec622b.
- Lethaby A, Shepperd S, Cooke I, Farquhar C. Endometrial resection and ablation versus hysterectomy for heavy menstrual bleeding. Cochrane Database Syst Rev 2000;2.
- de Kroon CD, de Bock GH, Dieben SW, Jansen FW. Saline contrast hysterosonography in abnormal uterine bleeding: a systematic review and meta-analysis. BJOG 2003;110:938-47. http://dx.doi.org/10.1111/j.1471-0528.2003.02472.x.
- Clark TJ, Voit D, Gupta JK, Hyde C, Song F, Khan KS. Accuracy of hysteroscopy in the diagnosis of endometrial cancer and hyperplasia: a systematic quantitative review. JAMA 2002;288:1610-21. http://dx.doi.org/10.1001/jama.288.13.1610.
- Farquhar C, Ekeroma A, Furness S, Arroll B. A systematic review of transvaginal ultrasonography, sonohysterography and hysteroscopy for the investigation of abnormal uterine bleeding in premenopausal women. Acta Obstet Gynecol Scand 2003;82:493-504. http://dx.doi.org/10.1034/j.1600-0412.2003.00191.x.
- Giorda G, Crivellari D, Veronesi A, Perin T, Campagnutta E, Carbone A, et al. Comparison of ultrasonography, hysteroscopy, and biopsy in the diagnosis of endometrial lesions in postmenopausal tamoxifen-treated patients. Acta Obstet Gynecol Scand 2002;81:975-80. http://dx.doi.org/10.1034/j.1600-0412.2002.811013.x.
- Mara M, Maskova J, Fucikova Z, Kuzel D, Belsan T, Sosna O. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol 2008;31:73-85. http://dx.doi.org/10.1007/s00270-007-9195-2.
- van Dongen H, de Kroon CD, Jacobi CE, Trimbos JB, Jansen FW. Diagnostic hysteroscopy in abnormal uterine bleeding: a systematic review and meta-analysis. BJOG 2007;114:664-75. http://dx.doi.org/10.1111/j.1471-0528.2007.01326.x.
- Emanuel MH, Verdel MJC, Stas H, Wamsteker K, Lammes FB. An audit of true prevalence of intra-uterine pathology: the hysteroscopical findings controlled for patient selection in 1202 patients with abnormal uterine bleeding. Gynaecol Endosc 1995;4:237-41.
- Dreisler E, Stampe SS, Ibsen PH, Lose G. Prevalence of endometrial polyps and abnormal uterine bleeding in a Danish population aged 20–74 years. Ultrasound Obstet Gynecol 2009;33:102-8. http://dx.doi.org/10.1002/uog.6259.
- Lasmar RB, Dias R, Barrozo PR, Oliveira MA, Coutinho ES, da Rosa DB. Prevalence of hysteroscopic findings and histologic diagnoses in patients with abnormal uterine bleeding. Fertil Steril 2008;89:1803-7. http://dx.doi.org/10.1016/j.fertnstert.2007.05.045.
- Clevenger-Hoeft M, Syrop CH, Stovall DW, Van Voorhis BJ. Sonohysterography in premenopausal women with and without abnormal bleeding. Obstet Gynecol 1999;94:516-20. http://dx.doi.org/10.1016/S0029-7844(99)00345-2.
- Lurie S, Piper I, Woliovitch I, Glezerman M. Age-related prevalence of sonographicaly confirmed uterine myomas. J Obstet Gynaecol 2005;25:42-3. http://dx.doi.org/10.1080/01443610400024583.
- Iram S, Musonda P, Ewies AAA. Premenopausal bleeding: when should the endometrium be investigated? A retrospective non-comparative study of 3006 women. Eur J Obstet Gynecol Reprod Biol 2010;148:86-9. http://dx.doi.org/10.1016/j.ejogrb.2009.09.023.
- Basu A, Lewis P. Transvaginal scan, saline infusion sonography and hysteroscopy in abnormal uterine bleeding. Int J Gynecol Obstet 2009;107:S120-1. http://dx.doi.org/10.1016/S0020-7292(09)60470-7.
- Smith-Bindman R. Diagnostic imaging in the differential diagnosis of vaginal bleeding and breast mass. Adv Stud Med 2004;4:476-82.
- de Kroon CD, de Bock GH, Dieben SWM, Jansen FW. Saline contrast hysterosonography in abnormal uterine bleeding: a systematic review and meta-analysis. BJOG 2003;110:938-47.
- Grimbizis GF, Tsolakidis D, Mikos T, Anagnostou E, Asimakopoulos E, Stamatopoulos P, et al. A prospective comparison of transvaginal ultrasound, saline infusion sonohysterography, and diagnostic hysteroscopy in the evaluation of endometrial pathology. Fertil Steril 2010;94:2720-5. http://dx.doi.org/10.1016/j.fertnstert.2010.03.047.
- Botsis D, Kassanos D, Antoniou G, Pyrgiotis E, Karakitsos P, Kalogirou D. Adenomyoma and leiomyoma: differential diagnosis with transvaginal sonography. J Clin Ultrasound 1997;26:21-5. http://dx.doi.org/10.1002/(SICI)1097-0096(199801)26:1%3C21::AID-JCU5%3E3.3.CO;2-V.
- Vercellini P, Cortesi I, Oldani S, Moschetta M, De O, Crosignani PG. The role of transvaginal ultrasonography and outpatient diagnostic hysteroscopy in the evaluation of patients with menorrhagia. Human Reprod 1997;12:1768-71. http://dx.doi.org/10.1093/humrep/12.8.1768.
- Aslam M, Ijaz L, Tariq S, Shafqat K, Meher UN, Ashraf R, et al. Comparison of transvaginal sonography and saline contrast sonohysterography in women with abnormal uterine bleeding: correlation with hysteroscopy and histopathology. Int J Health Sci 2007;1:17-24.
- Goldschmit R, Katz Z, Blickstein I, Caspi B, Dgani R. The accuracy of endometrial pipelle sampling with and without sonographic measurement of endometrial thickness. Obstet Gynecol 1993;82:727-30.
- Evidence Based Medicine T . Stats Calculator 2011. http://ktclearinghouse.ca/cebm/practise/ca/calculators/statscalc (accessed 8 November 2011).
- Angioni S, Loddo A, Milano F, Piras B, Minerba L, Melis GB. Detection of benign intracavitary lesions in postmenopausal women with abnormal uterine bleeding: a prospective comparative study on outpatient hysteroscopy and blind biopsy. J Minim Invasive Gynecol 2008;15:87-91. http://dx.doi.org/10.1016/j.jmig.2007.10.014.
- Lethaby AE, Cooke I, Rees M. Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev 2005;4.
- Longinotti MK, Jacobson GF, Hung YY, Learman LA. Probability of hysterectomy after endometrial ablation. Obstet Gynecol 2008;112:1214-20. http://dx.doi.org/10.1097/AOG.0b013e31818c1766.
- Hirst A, Dutton S, Wu O, Briggs A, Edwards C, Waldenmaier L, et al. A multi-centre retrospective cohort study comparing the efficacy, safety and cost-effectiveness of hysterectomy and uterine artery embolisation for the treatment of symptomatic uterine fibroids. The HOPEFUL study. Health Technol Assess 2008;12.
- van Dongen H, Janssen CA, Smeets MJ, Emanuel MH, Jansen FW. The clinical relevance of hysteroscopic polypectomy in premenopausal women with abnormal uterine bleeding. BJOG 2009;116:1387-90. http://dx.doi.org/10.1111/j.1471-0528.2009.02145.x.
- Lieng M, Istre O, Sandvik L, Engh V, Qvigstad E. Clinical effectiveness of transcervical polyp resection in women with endometrial polyps: randomized controlled trial. J Minim Invasive Gynecol 2010;17:351-7. http://dx.doi.org/10.1016/j.jmig.2010.01.019.
- Camanni M, Bonino L, Delpiano EM, Ferrero B, Migliaretti G, Deltetto F. Hysteroscopic management of large symptomatic submucous uterine myomas. J Minim Invasive Gynecol 2010;17:59-65. http://dx.doi.org/10.1016/j.jmig.2009.10.013.
- Clark TJ, Mahajan D, Sunder P, Gupta JK. Hysteroscopic treatment of symptomatic submucous fibroids using a bipolar intrauterine system: a feasibility study. Eur J Obstet Gynecol Reprod Biol 2002;100:237-42. http://dx.doi.org/10.1016/S0301-2115(01)00485-7.
- Bourdel N, Bonnefoy C, Jardon K, Da D, Tognazza E, Rabischong B, et al. Hysteroscopic myomectomy: recurrence and satisfaction survey at short- and long-term. J Gynecol Obstet Biol Reprod 2011;40:116-22.
- Cravello L, Farnarier J, Roger V, D’Ercole C, Blanc B. Hysteroscopic myomectomy. Functional results with an average follow-up of 6 years. J Gynecol Obstet Biol Reprod 1998;27:593-7.
- Munoz JL, Jimenez JS, Hernandez C, Vaquero G, Perez C, Noguero R, et al. Hysteroscopic myomectomy: our experience and review. JSLS 2003;7:39-48.
- Polena V, Mergui JL, Perrot N, Poncelet C, Barranger E, Uzan S. Long-term results of hysteroscopic myomectomy in 235 patients. Eur J Obstet Gynecol Reprod Biol 2007;130:232-7. http://dx.doi.org/10.1016/j.ejogrb.2006.01.014.
- Haynes PJ, Hodgson H, Anderson AB, Turnbull AC. Measurement of menstrual blood loss in patients complaining of menorrhagia. Br J Obstet Gynaecol 1977;84:763-8. http://dx.doi.org/10.1111/j.1471-0528.1977.tb12490.x.
- van der Kooij SM, Bipat S, Hehenkamp WJ, Ankum WM, Reekers JA. Uterine artery embolization versus surgery in the treatment of symptomatic fibroids: a systematic review and metaanalysis. Am J Obstet Gynecol 2011;205. http://dx.doi.org/10.1016/j.ajog.2011.03.016.
- Gupta JK, Sinha AS, Lumsden MA, Hickey M. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev 2006;1.
- NHS Reference Costs 2009–2010. London: Department of Health; 2011.
- British national formulary. London: BMA and RPS; 2009.
- Unit Costs of Health and Social Care 2010. Canterbury: PSSRU, University of Kent; 2010.
- Sculpher M, Fenwick E, Claxton K. Assessing quality in decision analytic cost-effectiveness models. A suggested framework and example of application. Pharmacoeconomics 2000;17:461-77. http://dx.doi.org/10.2165/00019053-200017050-00005.
- Elwyn G, Edwards A, Eccles M, Rovner D. Decision analysis in patient care. Lancet 2001;358:571-4. http://dx.doi.org/10.1016/S0140-6736(01)05709-9.
- Epstein E. Management of postmenopausal bleeding in Sweden: a need for increased use of hydrosonography and hysteroscopy. Acta Obstet Gynecol Scand 2004;83:89-95. http://dx.doi.org/10.1111/j.1600-0412.2004.00357.x.
- Goldstein RB, Bree RL, Benson CB, Benacerraf BR, Bloss JD, Carlos R, et al. Evaluation of the woman with postmenopausal bleeding: Society of Radiologists in Ultrasound-Sponsored Consensus Conference statement. J Ultrasound Med 2001;20:1025-36.
- NVOG (Dutch Society of Obstetrics and Gynaecology) . NVOG-RichtlijnAbnormaal Vaginaal Bloedverlies in De Menopauze 2003. www.artsennet.nl/Richtlijnen/Richtlijn/43092/Abnormaal-vaginaal-bloedverlies-in-de-postmenopauze.htm (accessed 2 February 2012).
- Lotfallah H, Farag K, Hassan I, Watson R. One-stop hysteroscopy clinic for postmenopausal bleeding. J Reprod Med 2005;50:101-7.
- Luk KL, Tang LCH. Modern strategy in the diagnosis of postmenopausal bleeding. Hong Kong Pract 1998;20:421-9.
- MacNab W, Majumudar T, Abdel-Rahman H. Incidence and management of endometrial polyps in women with post-menopausal PV bleeding (PMB) in the office setting. Gynecol Surg 2010;7.
- Panda JK. One-stop clinic for postmenopausal bleeding. J Reprod Med 2002;47:761-6.
- Gupta JK, Wilson S, Desai P, Hau C. How should we investigate women with postmenopausal bleeding?. Acta Obstet Gynecol Scand 1996;75:475-9. http://dx.doi.org/10.3109/00016349609033357.
- Gupta JK, Chien PFW, Voit D, Clark TJ, Khan KS. Ultrasonographic endometrial thickness for diagnosing endometrial pathology in women with postmenopausal bleeding: a meta-analysis. Acta Obstet Gynecol Scand 2002;81:799-816. http://dx.doi.org/10.1034/j.1600-0412.2001.810902.x.
- Clark TJ, Bakour SH, Gupta JK, Khan KS. Evaluation of outpatient hysteroscopy and ultrasonography in the diagnosis of endometrial disease. Obstet Gynecol 2002;99:1001-7. http://dx.doi.org/10.1016/S0029-7844(02)01976-2.
- Rogerson L, Downes E. How do UK gynaecologists manage endometrial carcinoma? A national survey. Eur J Gynaecol Oncol 1998;19:331-2.
- National Cancer Institute . Endometrial Cancer Treatment PDQ 2012. www.cancer.gov/cancertopics/pdq/treatment/endometrial/Patient/page1 (accessed 2 February 2012).
- Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet 2009;105:103-4. http://dx.doi.org/10.1016/j.ijgo.2009.02.012.
- Briggs A, Claxton K, Sculpher M. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Clark TJ, Samuel N, Malick S, Middleton LJ, Daniels J, Gupta JK. Bipolar radiofrequency compared with thermal balloon endometrial ablation in the office: a randomized controlled trial. Obstet Gynecol 2011;117:109-18.
- Sculpher M. A cost-utility analysis of abdominal hysterectomy versus transcervical endometrial resection for the surgical treatment of menorrhagia. Int J Technol Assess Health Care 1998;14:302-19. http://dx.doi.org/10.1017/S0266462300012277.
- Dijkhuizen FP, Mol BW, Bongers MY, lmann HA, Heintz AP. Cost-effectiveness of transvaginal sonography and saline infused sonography in the evaluation of menorrhagia. Int J Gynaecol Obstet 2003;83:45-52. http://dx.doi.org/10.1016/S0020-7292(03)00080-8.
- Timmermans A, Opmeer BC, Veersema S, Mol BW. Patients’ preferences in the evaluation of postmenopausal bleeding. BJOG 2007;114:1146-9. http://dx.doi.org/10.1111/j.1471-0528.2007.01424.x.
- Office for National Statistics . Statistical Bulletin: Interim Life Tables, England and Wales, 2010–2012 n.d. www.ons.gov.uk/ons/rel/lifetables/interim-life-tables/2010-2012/stbilt2012.html (accessed 10 March 2014).
- NHS reference costs 2009–2010. London: Department of Health; 2011.
- Reinhold C, McCarthy S, Bret PM, Mehio A, Atri M, Zakarian R, et al. Diffuse adenomyosis: comparison of endovaginal US and MR imaging with histopathologic correlation. Radiology 1996;199:151-8.
- Timmermans A, van H, Mol BW, Veersema S, Jansen FW. Hysteroscopy and removal of endometrial polyps: a Dutch survey. Eur J Obstet Gynecol Reprod Biol 2008;138:76-9. http://dx.doi.org/10.1016/j.ejogrb.2007.05.016.
- Brechin ST, Cameron AM, Paterson AR, Williams HOD, Critchley. Intrauterine polyps – a cause of unscheduled bleeding in women using the levonorgestrel intrauterine system. Hum Reprod 2000;15:650-2. http://dx.doi.org/10.1093/humrep/15.3.650.
- Mercorio F, De R, Di A, Cerrota G, Bifulco G, Vanacore F, et al. The effect of a levonorgestrel-releasing intrauterine device in the treatment of myoma-related menorrhagia. Contraception 2003;67:277-80. http://dx.doi.org/10.1016/S0010-7824(02)00522-X.
- Timmermans A, Veersema S, Van TC, Van LF, V, Opmeer BC, Bongers MY, et al. Should endometrial polyps be removed in patients with postmenopausal bleeding? – an assessment of study designs and report of a failed randomised controlled trial (ISRCTN73825127). BJOG 2009;116:1391-5. http://dx.doi.org/10.1111/j.1471-0528.2009.02234.x.
Appendix 1 Membership and individual areas of expertise of the project
Dr Natalie A M Cooper
-
Clinical expertise in gynaecology and the management of abnormal uterine bleeding.
-
Epidemiology and systematic reviews.
-
Health technology assessment.
Dr Pelham M Barton
-
Economic modelling and analysis.
Dr Maria C Breijer
-
Clinical expertise in gynaecology and the management of abnormal uterine bleeding.
-
Epidemiology and systematic reviews.
-
Health technology assessment.
Miss Orla Caffrey
-
Economic modelling and analysis.
Dr Brent C Opmeer
-
Economic modelling and analysis.
-
Assessment of diagnostic test accuracy.
-
Epidemiology and systematic reviews.
-
Health technology assessment.
-
Decision-analytic modelling and economic evaluation.
Dr Ann Timmermans
-
Clinical expertise in gynaecology and the management of abnormal uterine bleeding.
-
Epidemiology and systematic reviews.
-
Health technology assessment.
Professor Ben Willem Mol
-
Clinical expertise in gynaecology and the management of abnormal uterine bleeding.
-
Assessment of diagnostic test accuracy.
-
Epidemiology and systematic reviews.
-
Health technology assessment.
-
Decision-analytic modelling and economic evaluation.
-
Economic modelling and analysis.
Professor Khalid S Khan
-
Clinical expertise in gynaecology and the management of abnormal uterine bleeding.
-
Assessment of diagnostic test accuracy.
-
Epidemiology and systematic reviews.
-
Health technology assessment.
-
Decision-analytic modelling and economic evaluation.
Mr T Justin Clark
-
Clinical expertise in gynaecology and the management of abnormal uterine bleeding.
-
Epidemiology and systematic reviews.
-
Health technology assessment.
Appendix 2 Decision trees for the economic analysis of heavy menstrual bleeding
The HMB decision trees are too large to be displayed in their entirety; therefore, an expanded branch from the OPH tree is shown below. Table 32 shows the diagnosis made and the treatments given for all branches within the decision tree.
Expanded branch from the outpatient hysteroscopy strategy for investigation of heavy menstrual bleeding
Diagnosis and treatments from the decision tree for investigation of women with heavy menstrual bleeding
| Strategy | True pathology | Diagnosis from test 1 | Diagnosis from test 2 | Diagnosis from test 3 | Diagnosis from test 4 | First treatment | Second treatment |
|---|---|---|---|---|---|---|---|
| LNG-IUS only | Polyp/SMF | LNG-IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy | ||||
| Fibroids < 12 weeks’ size | LNG-IUS | EA | |||||
| Fibroids > 12 weeks’ size | LNG-IUS Hysterectomy if bimanual examination suggests > 12 weeks’ size |
EA GP appointment |
|||||
| ED | LNG-IUS | Hysterectomy | |||||
| DUB | LNG-IUS | EA | |||||
| Hysterectomy only | Polyp/SMF | Hysterectomy | GP appointment | ||||
| Fibroids < 12 weeks’ size | Hysterectomy | GP appointment | |||||
| Fibroids > 12 weeks’ size | Hysterectomy | GP appointment | |||||
| ED | Hysterectomy | GP appointment | |||||
| DUB | Hysterectomy | GP appointment | |||||
| OPH alone | Polyp/SMF | Polyp/SMF | Resection | LNG-IUS | |||
| ED | Polyp/SMF | LNG-IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy | ||||
| DUB | LNG-IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy | |||||
| Fibroids < 12 weeks’ size | Normal cavity | LNG-IUS | EA | ||||
| Polyp/SMF | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| ED | Normal endometrium | LNG-IUS | EA | ||||
| Fibroids > 12 weeks’ size | Normal cavity | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | |||
| < 12 weeks’ size by bimanual examination | LNG-IUS | ||||||
| Polyp/SMF | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| ED | > 12 weeks’ size by bimanual examination | Normal endometrium | Hysterectomy | GP appointment | |||
| < 12 weeks’ size by bimanual examination | Normal endometrium | LNG-IUS | EA | ||||
| ED | ED | Hyperplasia | LNG-IUS | Hysterectomy | |||
| Cancer | Hysterectomy | GP appointment | |||||
| Polyp/SMF | Resection: histology shows hyperplasia/cancer so get LNG-IUS/hysterectomy | Hysterectomy/GP visit | |||||
| DUB | LNG-IUS | Hysterectomy as EBx prior to EA shows ED | |||||
| DUB | DUB | LNG-IUS | EA | ||||
| Polyp/SMF | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| ED | Normal endometrium | LNG-IUS | EA | ||||
| TVS alone and SIS alone | Polyp/SMF | Polyp/SMF | Resection | LNG-IUS | |||
| Fibroids < 12 weeks’ size | LNG-IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy | |||||
| ED | Polyp/SMF | Resection | LNG-IUS | ||||
| DUB | LNG-IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy | |||||
| Fibroids < 12 weeks’ size | Fibroids < 12 weeks’ size | LNG-IUS | EA | ||||
| Polyp/SMF | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| ED | Normal endometrium | LNG-IUS | EA | ||||
| DUB | LNG-IUS | EA | |||||
| Fibroids > 12 weeks’ size | Fibroids > 12 weeks’ size | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | |||
| < 12 weeks’ size by bimanual examination | LNG-IUS | EA | |||||
| Polyp/SMF | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| Fibroids < 12 weeks’ size | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | LNG-IUS | EA | |||||
| ED | ED | Hyperplasia | LNG-IUS | Hysterectomy | |||
| Cancer | Hysterectomy | GP appointment | |||||
| Polyp/SMF | Resection: histology shows hyperplasia/cancer so get LNG-IUS/hysterectomy | Hysterectomy/GP visit | |||||
| Fibroids < 12 weeks’ size | LNG-IUS | Hysterectomy as EBx prior to EA shows ED | |||||
| DUB | LNG-IUS | Hysterectomy as EBx prior to EA shows ED | |||||
| DUB | DUB | LNG-IUS | EA | ||||
| Polyp/SMF | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| Fibroids < 12 weeks’ size | LNG-IUS | EA | |||||
| ED | Normal endometrium | LNG-IUS | EA | ||||
| EBx alone | Polyp/SMF | Polyp/SMF | Resection | LNG-IUS | |||
| DUB | LNG-IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy | |||||
| Complex hyperplasia | LNG-IUS | Hysterectomy | |||||
| Fibroids < 12 weeks’ size | Normal cavity | LNG-IUS | EA | ||||
| Polyp/SMF | Resection but no lesion seen so LNG-IUS inserted | EA | |||||
| Complex hyperplasia | LNG-IUS | Hysterectomy | |||||
| Fibroids > 12 weeks’ size | Normal cavity | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | |||
| < 12 weeks’ size by bimanual examination | LNG-IUS | EA | |||||
| Polyp/SMF | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | Resection but no lesion seen so LNG-IUS inserted | EA | |||||
| Complex hyperplasia | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | LNG-IUS | Hysterectomy | |||||
| ED | Complex hyperplasia | LNG-IUS | Hysterectomy | ||||
| Atypia/cancer | Hysterectomy | GP appointment | |||||
| Polyp/SMF | Resection: histology shows hyperplasia/cancer so get LNG-IUS/hysterectomy | Hysterectomy/GP visit | |||||
| DUB | LNG-IUS | Hysterectomy as BX prior to EA shows ED | |||||
| DUB | Normal cavity | LNG-IUS | EA | ||||
| Polyp/SMF | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| OPH + EBx | Polyp/SMF | Polyp/SMF | Polyp/SMF | Resection | LNG-IUS | ||
| DUB | Resection | LNG-IUS | |||||
| Complex hyperplasia | Resection followed by LNG-IUS | Hysterectomy | |||||
| ED | Polyp/SMF | LNG-IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy | ||||
| DUB | LNG-IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy | |||||
| Complex hyperplasia | LNG-IUS | Hysterectomy | |||||
| Normal cavity | Polyp/SMF | LNG-IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy | ||||
| DUB | LNG-IUS | EA | |||||
| Complex hyperplasia | LNG-IUS | Hysterectomy | |||||
| Fibroids < 12 weeks’ size | DUB | DUB Polyp/SMF |
LNG-IUS LNG-IUS |
EA EA |
|||
| Complex hyperplasia | LNG-IUS | Hysterectomy | |||||
| Polyp/SMF | DUB | Resection but as normal endometrium LNG-IUS inserted | EA | ||||
| Polyp/SMF | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| Complex hyperplasia | Resection followed by LNG-IUS | Hysterectomy | |||||
| ED | DUB | LNG-IUS | EA | ||||
| Polyp/SMF | LNG-IUS | EA | |||||
| Complex hyperplasia | LNG-IUS | Hysterectomy | |||||
| Fibroids > 12 weeks’ size | DUB | DUB | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||
| < 12 weeks’ size by bimanual examination | LNG-IUS | EA | |||||
| Polyp/SMF | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | LNG-IUS | EA | |||||
| Complex hyperplasia | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | LNG-IUS | Hysterectomy | |||||
| Polyp/SMF | DUB | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | |||
| < 12 weeks’ size by bimanual examination | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| Polyp/SMF | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| Complex hyperplasia | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | Resection followed by LNG-IUS | Hysterectomy | |||||
| ED | DUB | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | |||
| < 12 weeks’ size by bimanual examination | LNG-IUS | EA | |||||
| Polyp/SMF | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | LNG-IUS | EA | |||||
| Complex hyperplasia | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | LNG-IUS | Hysterectomy | |||||
| ED | ED | Complex hyperplasia | LNG-IUS | Hysterectomy | |||
| Cancer/atypia | Hysterectomy | GP appointment | |||||
| Polyp/SMF | LNG-IUS | Hysterectomy as pre-ablation EBx shows ED | |||||
| DUB | LNG-IUS | Hysterectomy as pre-ablation EBx shows ED | |||||
| Polyp/SMF | Complex hyperplasia | Resection followed by LNG-IUS | Hysterectomy | ||||
| Cancer/atypia | Hysterectomy | GP appointment | |||||
| Polyp/SMF | Resection but histology shows complex hyperplasia/cancer so LNG-IUS/hysterectomy | Hysterectomy/GP appointment | |||||
| DUB | Resection but histology shows complex hyperplasia/cancer so LNG-IUS/hysterectomy | Hysterectomy/GP appointment | |||||
| DUB | Complex hyperplasia | LNG-IUS | Hysterectomy | ||||
| Cancer/atypia | Hysterectomy | GP appointment | |||||
| Polyp/SMF | LNG-IUS | Hysterectomy as pre-ablation EBx shows ED | |||||
| DUB | LNG-IUS | Hysterectomy as pre-ablation EBx shows ED | |||||
| DUB | DUB | DUB | LNG-IUS | EA | |||
| Polyp/SMF | LNG-IUS | EA | |||||
| Polyp/SMF | DUB | Resection followed by LNG-IUS | EA | ||||
| Polyp/SMF | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| ED | DUB | LNG-IUS | EA | ||||
| Polyp/SMF | LNG-IUS | EA | |||||
| SIS + EBx and TVS + EBx | Polyp/SMF | Polyp/SMF | Polyp/SMF | Resection | LNG-IUS | ||
| DUB | Resection | LNG-IUS | |||||
| Complex hyperplasia | Resection followed by LNG-IUS | Hysterectomy | |||||
| Fibroids < 12 weeks’ size | Polyp/SMF DUB |
Resection LNG-IUS |
LNG-IUS Resection: lesion diagnosed at pre-ablation hysteroscopy |
||||
| Complex hyperplasia | LNG-IUS | Hysterectomy | |||||
| ED | Polyp/SMF | Resection | LNG-IUS | ||||
| DUB | LNG-IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy | |||||
| Complex hyperplasia | LNG-IUS | Hysterectomy | |||||
| DUB | Polyp/SMF | LNG IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy LNG-IUS | ||||
| DUB | LNG-IUS | Resection: lesion diagnosed at pre-ablation | |||||
| Complex hyperplasia | LNG-IUS | Hysterectomy | |||||
| Fibroids < 12 weeks’ size | Fibroids < 12 weeks’ size | DUB | LNG-IUS | EA | |||
| Polyp/SMF | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| Complex hyperplasia | LNG-IUS | Hysterectomy | |||||
| Polyp/SMF | DUB | Resection but as normal endometrium LNG-IUS inserted | EA | ||||
| Polyp/SMF | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| Complex hyperplasia | Resection but as normal endometrium LNG-IUS inserted | Hysterectomy | |||||
| ED | DUB | LNG-IUS | EA | ||||
| Polyp/SMF | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| Complex hyperplasia | LNG-IUS | Hysterectomy | |||||
| DUB | DUB | LNG-IUS | EA | ||||
| Polyp/SMF | LNG-IUS | EA | |||||
| Complex hyperplasia | LNG-IUS | Hysterectomy | |||||
| Fibroids > 12 weeks’ size | Fibroids > 12 weeks’ size | DUB | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||
| < 12 weeks’ size by bimanual examination | LNG-IUS | EA | |||||
| Polyp/SMF | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| Complex hyperplasia | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| <12 weeks’ size by bimanual examination | LNG-IUS | Hysterectomy | |||||
| Polyp/SMF | DUB | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | |||
| < 12 weeks’ size by bimanual examination | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| Polyp/SMF | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| Complex hyperplasia | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | Resection followed by LNG-IUS | Hysterectomy | |||||
| Fibroids < 12 weeks’ size | DUB | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | |||
| < 12 weeks’ size by bimanual examination | LNG-IUS | EA | |||||
| Polyp/SMF | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| Complex hyperplasia | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | LNG-IUS | Hysterectomy | |||||
| ED | ED | Complex hyperplasia | LNG-IUS | Hysterectomy | |||
| Cancer/atypia | Hysterectomy | GP appointment | |||||
| Polyp/SMF | Resection but histology shows complex hyperplasia/cancer so LNG-IUS/hysterectomy | Hysterectomy/GP appointment | |||||
| DUB | LNG-IUS | Hysterectomy as pre-ablation testing shows ED | |||||
| Polyp/SMF | Complex hyperplasia | Resection followed by LNG-IUS | Hysterectomy | ||||
| Cancer/atypia | Resection followed by hysterectomy | GP appointment | |||||
| Polyp/SMF | Resection but histology shows complex hyperplasia/cancer so LNG-IUS/hysterectomy | Hysterectomy/GP appointment | |||||
| DUB | Resection but histology shows complex hyperplasia/cancer so LNG-IUS/hysterectomy | Hysterectomy/GP appointment | |||||
| Fibroids < 12 weeks’ size | Complex hyperplasia | LNG-IUS | Hysterectomy | ||||
| Cancer/atypia | Hysterectomy | GP appointment | |||||
| Polyp/SMF | Resection but histology shows complex hyperplasia/cancer so LNG-IUS/hysterectomy | Hysterectomy/GP appointment | |||||
| DUB | LNG-IUS | Hysterectomy as pre-ablation testing shows ED | |||||
| DUB | Complex hyperplasia | LNG-IUS | Hysterectomy | ||||
| Cancer/atypia | Hysterectomy | GP appointment | |||||
| Polyp/SMF | LNG-IUS | Hysterectomy as pre-ablation testing shows ED | |||||
| DUB | LNG-IUS | Hysterectomy as pre-ablation testing shows ED | |||||
| DUB | DUB | DUB | LNG-IUS | EA | |||
| Polyp/SMF | LNG-IUS | EA | |||||
| Polyps/SMF | DUB | Resection but as normal endometrium LNG-IUS inserted | EA | ||||
| Polyp/SMF | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| Fibroids < 12 weeks’ size | DUB Polyp/SMF |
LNG-IUS Resection but as normal endometrium LNG-IUS inserted |
EA EA |
||||
| ED | DUB | LNG-IUS | EA | ||||
| Polyp/SMF | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| SIS + OPH and TVS + OPH | Polyp/SMF | Polyp/SMF | Polyp/SMF | Resection | LNG-IUS | ||
| ED | Polyp/SMF | LNG-IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy | ||||
| DUB | LNG-IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy | |||||
| Fibroid < 12 weeks’ size | Polyp/SMF | Resection | LNG-IUS | ||||
| ED | Polyp/SMF | LNG-IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy | ||||
| DUB | LNG-IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy | |||||
| ED | Polyp/SMF | Resection | LNG-IUS | ||||
| ED | Polyp/SMF | LNG-IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy | ||||
| DUB | LNG-IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy | |||||
| DUB | Polyp/SMF | Resection | LNH-IUS | ||||
| ED | Polyp/SMF | LNG-IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy | ||||
| DUB | LNG-IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy | |||||
| Fibroids < 12 weeks’ size | Fibroids < 12 weeks’ size | DUB Polyp/SMF |
LNG-IUS Resection but as normal endometrium LNG-IUS inserted |
EA EA |
|||
| ED | Normal endometrium | LNG-IUS | EA | ||||
| Polyp/SMF | DUB | LNG-IUS | EA | ||||
| Polyp/SMF | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| ED | Normal endometrium | LNG-IUS | EA | ||||
| ED | DUB | Normal endometrium | LNG-IUS | EA | |||
| Polyp/SMF | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| ED | Normal endometrium | LNG-IUS | EA | ||||
| DUB | DUB | LNG-IUS | EA | ||||
| Polyp/SMF | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| ED | Normal endometrium | LNG-IUS | EA | ||||
| Fibroids > 12 weeks’ size | Fibroids > 12 weeks’ size | DUB | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||
| < 12 weeks’ size by bimanual examination | LNG-IUS | EA | |||||
| Polyp/SMF | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| ED | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | Normal endometrium | LNG-IUS | EA | ||||
| Polyp/SMF | DUB | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | |||
| < 12 weeks’ size by bimanual examination | LNG-IUS | EA | |||||
| Polyp/SMF | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| ED | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | Normal endometrium | LNG-IUS | EA | ||||
| Fibroid < 12 weeks’ size | DUB | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | |||
| < 12 weeks’ size by bimanual examination | LNG-IUS | EA | |||||
| Polyp/SMF | >12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| ED | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | Normal endometrium | LNG-IUS | EA | ||||
| ED | ED | ED | Complex hyperplasia | LNG-IUS | Hysterectomy | ||
| Cancer/atypia | Hysterectomy | GP appointment | |||||
| Polyp/SMF | Resection but histology shows complex hyperplasia/cancer so LNG-IUS/hysterectomy | Hysterectomy/GP appointment | |||||
| DUB | Complex hyperplasia | LNG-IUS | Hysterectomy | ||||
| Cancer/atypia | Hysterectomy | GP appointment | |||||
| Polyp/SMF | ED | Complex hyperplasia | LNG-IUS | Hysterectomy | |||
| Cancer/atypia | Hysterectomy | GP appointment | |||||
| Polyp/SMF | Resection but histology shows complex hyperplasia/cancer so LNG-IUS/hysterectomy | Hysterectomy/GP appointment | |||||
| DUB | LNG-IUS | Hysterectomy as pre-ablation testing shows ED | |||||
| Fibroid < 12 weeks’ size | ED | Complex hyperplasia | LNG-IUS | Hysterectomy | |||
| Cancer/atypia | Hysterectomy | GP appointment | |||||
| Polyp/SMF | Resection but histology shows complex hyperplasia/cancer so LNG-IUS/hysterectomy | Hysterectomy/GP appointment | |||||
| DUB | LNG-IUS | Hysterectomy as pre-ablation testing shows ED | |||||
| DUB | ED | Complex hyperplasia | LNG-IUS | Hysterectomy | |||
| Cancer/atypia | Hysterectomy | GP appointment | |||||
| Polyp/SMF | Resection but histology shows complex hyperplasia/cancer so LNG-IUS/hysterectomy | Hysterectomy/GP appointment | |||||
| DUB | LNG-IUS | Hysterectomy as pre-ablation testing shows ED | |||||
| DUB | DUB | DUB | LNG-IUS | EA | |||
| Polyp/SMF | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| ED | Normal endometrium | LNG-IUS | EA | ||||
| Polyp/SMF | DUB | LNG-IUS | EA | ||||
| Polyp/SMF | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| ED | Normal endometrium | LNG-IUS | EA | ||||
| Fibroids < 12 weeks’ size | DUB | LNG-IUS | |||||
| Polyp/SMF | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| ED | Normal endometrium | LNG-IUS | EA | ||||
| ED | DUB | Normal endometrium | LNG-IUS | EA | |||
| Polyp/SMF | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| ED | Normal endometrium | LNG-IUS | EA | ||||
| SIS + OPH + EBx and TVS + OPH + EBx | Polyp/SMF | Polyp/SMF | Polyp/SMF | Polyp/SMF DUB |
Resection Resection |
LNG-IUS LNG-IUS |
|
| Complex hyperplasia | Resection followed by LNG-IUS | Hysterectomy | |||||
| ED | Polyp/SMF | LNG-IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy | ||||
| DUB | LNG-IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy | |||||
| Complex hyperplasia | LNG-IUS | Hysterectomy | |||||
| DUB | Polyp/SMF | LNG-IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy | ||||
| DUB | LNG-IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy | |||||
| Complex hyperplasia | LNG-IUS | Hysterectomy | |||||
| Fibroids < 12 weeks’ size | Polyp/SMF | Polyp/SMF DUB |
Resection Resection |
LNG-IUS LNG-IUS |
|||
| Complex hyperplasia | Resection followed by LNG-IUS | Hysterectomy | |||||
| ED | Polyp/SMF | LNG-IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy | ||||
| DUB | LNG-IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy | |||||
| Complex hyperplasia | LNG-IUS | Hysterectomy | |||||
| DUB | Polyp/SMF | LNG-IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy | ||||
| DUB | LNG-IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy | |||||
| Complex hyperplasia | LNG-IUS | Hysterectomy | |||||
| ED | Polyp/SMF | Polyp/SMF | Resection | LNG-IUS | |||
| DUB | Resection | LNG-IUS | |||||
| Complex hyperplasia | Resection followed by LNG-IUS | Hysterectomy | |||||
| ED | Polyp/SMF | LNG-IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy | ||||
| DUB | LNG-IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy | |||||
| Complex hyperplasia | LNG-IUS | Hysterectomy | |||||
| DUB | Polyp/SMF | LNG-IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy | ||||
| DUB | LNG-IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy | |||||
| Complex hyperplasia | LNG-IUS | Hysterectomy | |||||
| DUB | Polyp/SMF | Polyp/SMF | Resection | LNG-IUS | |||
| DUB | Resection | LNG-IUS | |||||
| Complex hyperplasia | Resection followed by LNG-IUS | Hysterectomy | |||||
| ED | Polyp/SMF | LNG-IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy | ||||
| DUB | LNG-IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy | |||||
| Complex hyperplasia | LNG-IUS | Hysterectomy | |||||
| DUB | Polyp/SMF | LNG-IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy | ||||
| DUB | LNG-IUS | Resection: lesion diagnosed at pre-ablation hysteroscopy | |||||
| Complex hyperplasia | LNG-IUS | Hysterectomy | |||||
| Fibroids < 12 weeks’ size | Fibroids < 12 weeks’ size | DUB | DUB Polyp/SMF |
LNG-IUS LNG-IUS |
EA EA |
||
| Complex hyperplasia | LNG-IUS | Hysterectomy | |||||
| Polyp/SMF | DUB | Resection but as normal endometrium LNG-IUS inserted | EA | ||||
| Polyp/SMF | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| Complex hyperplasia | Resection followed by LNG-IUS | Hysterectomy | |||||
| ED | DUB | LNG-IUS | EA | ||||
| Polyp/SMF | LNG-IUS | EA | |||||
| Complex hyperplasia | LNG-IUS | Hysterectomy | |||||
| Polyp/SMF | DUB | DUB | LNG-IUS | EA | |||
| Polyp/SMF | LNG-IUS | EA | |||||
| Complex hyperplasia | LNG-IUS | Hysterectomy | |||||
| Polyp/SMF | DUB | Resection but as normal endometrium LNG-IUS inserted | EA | ||||
| Polyp/SMF | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| Complex hyperplasia | Resection followed by LNG-IUS | Hysterectomy | |||||
| ED | DUB | LNG-IUS | EA | ||||
| Polyp/SMF | LNG-IUS | EA | |||||
| Complex hyperplasia | LNG-IUS | Hysterectomy | |||||
| ED | DUB | DUB | LNG-IUS | EA | |||
| Polyp/SMF | LNG-IUS | EA | |||||
| Complex hyperplasia | LNG-IUS | Hysterectomy | |||||
| Polyp/SMF | DUB | Resection but as normal endometrium LNG-IUS inserted | EA | ||||
| Polyp/SMF | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| Complex hyperplasia | Resection followed by LNG-IUS | Hysterectomy | |||||
| ED | DUB | LNG-IUS | EA | ||||
| Polyp/SMF | LNG-IUS | EA | |||||
| Complex hyperplasia | LNG-IUS | Hysterectomy | |||||
| DUB | DUB | DUB | LNG-IUS | EA | |||
| Polyp/SMF | LNG-IUS | EA | |||||
| Complex hyperplasia | LNG-IUS | Hysterectomy | |||||
| Polyp/SMF | DUB | Resection but as normal endometrium LNG-IUS inserted | EA | ||||
| Polyp/SMF | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| Complex hyperplasia | Resection followed by LNG-IUS | Hysterectomy | |||||
| ED | DUB | LNG-IUS | EA | ||||
| Polyp/SMF | LNG-IUS | EA | |||||
| Complex hyperplasia | LNG-IUS | Hysterectomy | |||||
| Fibroids > 12 weeks’ size | Fibroids > 12 weeks’ size | DUB | DUB | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | |
| < 12 weeks’ size by bimanual examination | LNG-IUS | EA | |||||
| Polyp/SMF | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | LNG-IUS | EA | |||||
| Complex hyperplasia | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | LNG-IUS | Hysterectomy | |||||
| Polyp/SMF | DUB | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | |||
| < 12 weeks’ size by bimanual examination | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| Polyp/SMF | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| Complex hyperplasia | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | Resection followed by LNG-IUS | Hysterectomy | |||||
| ED | DUB | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | |||
| < 12 weeks’ size by bimanual examination | LNG-IUS | EA | |||||
| Polyp/SMF | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | LNG-IUS | EA | |||||
| Complex hyperplasia | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | LNG-IUS | Hysterectomy | |||||
| Polyp/SMF | DUB | DUB | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||
| < 12 weeks’ size by bimanual examination | LNG-IUS | EA | |||||
| Polyp/SMF | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | LNG-IUS | EA | |||||
| Complex hyperplasia | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | LNG-IUS | Hysterectomy | |||||
| Polyp/SMF | DUB | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | |||
| < 12 weeks’ size by bimanual examination | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| Polyp/SMF | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| Complex hyperplasia | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | Resection followed by LNG-IUS | Hysterectomy | |||||
| ED | DUB | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | |||
| < 12 weeks’ size by bimanual examination | LNG-IUS | EA | |||||
| Polyp/SMF | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | LNG-IUS | EA | |||||
| Complex hyperplasia | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | LNG-IUS | Hysterectomy | |||||
| Fibroids < 12 weeks’ size | DUB | DUB | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||
| < 12 weeks’ size by bimanual examination | LNG-IUS | EA | |||||
| Polyp/SMF | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | LNG-IUS | EA | |||||
| Complex hyperplasia | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | LNG-IUS | Hysterectomy | |||||
| Polyp/SMF | DUB | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | |||
| < 12 weeks’ size by bimanual examination | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| Polyp/SMF | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| Complex hyperplasia | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | Resection followed by LNG-IUS | Hysterectomy | |||||
| ED | DUB | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | |||
| < 12 weeks’ size by bimanual examination | LNG-IUS | EA | |||||
| Polyp/SMF | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | LNG-IUS | EA | |||||
| Complex hyperplasia | > 12 weeks’ size by bimanual examination | Hysterectomy | GP appointment | ||||
| < 12 weeks’ size by bimanual examination | LNG-IUS | Hysterectomy | |||||
| ED | ED | ED | Complex hyperplasia | LNG-IUS | Hysterectomy | ||
| Cancer/atypia | Hysterectomy | GP appointment | |||||
| Polyp/SMF | LNG-IUS | Hysterectomy as pre-ablation testing shows ED | |||||
| DUB | LNG-IUS | Hysterectomy as pre-ablation testing shows ED | |||||
| Polyp/SMF | Complex hyperplasia | Removal followed by LNG-IUS | Hysterectomy | ||||
| Cancer/atypia | Removal followed by hysterectomy | GP appointment | |||||
| Polyp/SMF | Resection but histology shows complex hyperplasia/cancer so LNG-IUS/hysterectomy | Hysterectomy/GP appointment | |||||
| DUB | Resection but histology shows complex hyperplasia/cancer so LNG-IUS/hysterectomy | Hysterectomy/GP appointment | |||||
| DUB | Complex hyperplasia | LNG-IUS | Hysterectomy | ||||
| Cancer/atypia | Hysterectomy | GP appointment | |||||
| Polyp/SMF | LNG-IUS | Hysterectomy as pre-ablation testing shows ED | |||||
| DUB | LNG-IUS | Hysterectomy as pre-ablation testing shows ED | |||||
| Polyp/SMF | ED | Complex hyperplasia | LNG-IUS | Hysterectomy | |||
| Cancer/atypia | Hysterectomy | GP appointment | |||||
| Polyp/SMF | LNG-IUS | Hysterectomy as pre-ablation testing shows ED | |||||
| DUB | LNG-IUS | Hysterectomy as pre-ablation testing shows ED | |||||
| Polyp/SMF | Complex hyperplasia | LNG-IUS | Hysterectomy | ||||
| Cancer/atypia | Hysterectomy | GP appointment | |||||
| Polyp/SMF | Resection but histology shows complex hyperplasia/cancer so LNG-IUS/hysterectomy | Hysterectomy/GP appointment | |||||
| DUB | Resection but histology shows complex hyperplasia/cancer so LNG-IUS/hysterectomy | Hysterectomy/GP appointment | |||||
| DUB | Complex hyperplasia | LNG-IUS | Hysterectomy | ||||
| Cancer/atypia | Hysterectomy | GP appointment | |||||
| Polyp/SMF | LNG-IUS | Hysterectomy as pre-ablation testing shows ED | |||||
| DUB | LNG-IUS | Hysterectomy as pre-ablation testing shows ED | |||||
| Fibroids < 12 weeks’ size | ED | Complex hyperplasia | LNG-IUS | Hysterectomy | |||
| Cancer/atypia | Hysterectomy | GP appointment | |||||
| Polyp/SMF | LNG-IUS | Hysterectomy as pre-ablation testing shows ED | |||||
| DUB | LNG-IUS | Hysterectomy as pre-ablation testing shows ED | |||||
| Polyp/SMF | Complex hyperplasia | Removal followed by LNG-IUS | Hysterectomy | ||||
| Cancer/atypia | Removal followed by hysterectomy | GP appointment | |||||
| Polyp/SMF | Resection but histology shows complex hyperplasia/cancer so LNG-IUS/hysterectomy | Hysterectomy/GP appointment | |||||
| DUB | Resection but histology shows complex hyperplasia/cancer so LNG-IUS/hysterectomy | Hysterectomy/GP appointment | |||||
| DUB | Complex hyperplasia | LNG-IUS | Hysterectomy | ||||
| Cancer/atypia | Hysterectomy | GP appointment | |||||
| Polyp/SMF | LNG-IUS | Hysterectomy as pre-ablation testing shows ED | |||||
| DUB | LNG-IUS | Hysterectomy as pre-ablation testing shows ED | |||||
| DUB | ED | Complex hyperplasia | LNG-IUS | Hysterectomy | |||
| Cancer/atypia | Hysterectomy | GP appointment | |||||
| Polyp/SMF | LNG-IUS | Hysterectomy as pre-ablation testing shows ED | |||||
| DUB | LNG-IUS | Hysterectomy as pre-ablation testing shows ED | |||||
| Polyp/SMF | Complex hyperplasia | LNG-IUS | Hysterectomy | ||||
| Cancer/atypia | Hysterectomy | GP appointment | |||||
| Polyp/SMF | Resection but histology shows complex hyperplasia/cancer so LNG-IUS/hysterectomy | Hysterectomy/GP appointment | |||||
| DUB | Resection but histology shows complex hyperplasia/cancer so LNG-IUS/hysterectomy | Hysterectomy/GP appointment | |||||
| DUB | Complex hyperplasia | LNG-IUS | Hysterectomy | ||||
| Cancer/atypia | Hysterectomy | GP appointment | |||||
| Polyp/SMF | LNG-IUS | Hysterectomy as pre-ablation testing shows ED | |||||
| DUB | LNG-IUS | Hysterectomy as pre-ablation testing shows ED | |||||
| DUB | DUB | DUB | DUB | LNG-IUS | EA | ||
| Polyp/SMF | LNG-IUS | EA | |||||
| Polyp/SMF | DUB | Resection but as normal endometrium LNG-IUS inserted | EA | ||||
| Polyp/SMF | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| ED | DUB | LNG-IUS | EA | ||||
| Polyp/SMF | LNG-IUS | EA | |||||
| Polyp/SMF | DUB | DUB | LNG-IUS | EA | |||
| Polyp/SMF | LNG-IUS | EA | |||||
| Polyp/SMF | DUB | Resection but as normal endometrium LNG-IUS inserted | EA | ||||
| Polyp/SMF | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| ED | DUB | LNG-IUS | EA | ||||
| Polyp/SMF | LNG-IUS | EA | |||||
| Fibroids < 12 weeks’ size | DUB | DUB Polyp/SMF |
LNG-IUS LNG-IUS |
EA EA |
|||
| Polyp/SMF | DUB | Resection but as normal endometrium LNG-IUS inserted | EA | ||||
| Polyp/SMF | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| ED | DUB | LNG-IUS | EA | ||||
| Polyp/SMF | LNG-IUS | EA | |||||
| ED | DUB | DUB | LNG-IUS | EA | |||
| Polyp/SMF | LNG-IUS | EA | |||||
| Polyp/SMF | DUB | Resection but as normal endometrium LNG-IUS inserted | EA | ||||
| Polyp/SMF | Resection but as normal endometrium LNG-IUS inserted | EA | |||||
| ED | DUB | LNG-IUS | EA | ||||
| Polyp/SMF | LNG-IUS | EA |
Appendix 3 Search strategies for collection of data to populate the decision trees for the economic analysis of heavy menstrual bleeding
MEDLINE search strategy for prevalence of endometrial polyps
-
endometrial.ti,ab
-
endometr*.ti,ab
-
uterine.ti,ab
-
uter*.ti,ab
-
exp UTERINE DISEASES/
-
uterus.ti,ab
-
exp UTERUS/
-
1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7
-
polyp.ti,ab
-
polyp*.ti,ab
-
exp POLYPS/
-
9 OR 10 OR 11
-
8 AND 12
-
hysteroscopy.ti,ab
-
exp HYSTEROSCOPY/
-
hysteroscop*.ti,ab
-
14 OR 15 OR 16
-
sensitiv*.ti,ab
-
exp "SENSITIVITY AND SPECIFICITY"/
-
diagnos*.ti,ab
-
DIAGNOSIS/
-
diagnostic*.ti,ab
-
DIAGNOSIS, DIFFERENTIAL/
-
*DIAGNOSIS/
-
18 OR 19 OR 20 OR 21 OR 22 OR 23 OR 24
-
13 AND 17 AND 25
EMBASE search strategy for prevalence of endometrial polyps
-
endometrial.ti,ab
-
endometr*.ti,ab
-
exp ENDOMETRIAL DISEASE/
-
uter*.ti,ab
-
uterine.ti,ab
-
exp UTERUS/
-
uterus.ti,ab
-
1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7
-
polyp.ti,ab
-
polyp*.ti,ab
-
exp POLYP/ OR exp ENDOMETRIUM POLYP/
-
9 OR 10 OR 11
-
8 AND 12
-
hysteroscopy.ti,ab
-
exp HYSTEROSCOPY/
-
hysteroscop*.ti,ab
-
19 OR 20 OR 21
-
18 AND 22
-
sensitiv*.ti,ab
-
exp “SENSITIVITY AND SPECIFICITY”/
-
diagnos*.ti,ab
-
DIAGNOSIS/
-
DIFFERENTIAL DIAGNOSIS/
-
DIAGNOSTIC TEST/
-
24 OR 25 OR 26 OR 27 OR 28 OR 29
-
18 AND 30
-
22 AND 31
MEDLINE search strategy for prevalence of fibroids
-
prevalence.ti,ab
-
exp PREVALENCE/
-
1 OR 2
-
exp LEIOMYOMA/
-
fibroid.ti,ab
-
leiomyoma.ti,ab
-
exp MYOMA/
-
fibromyoma.ti,ab
-
leiofibromyoma.ti,ab
-
fibroleiomyoma.ti,ab
-
fibroma.ti,ab
-
exp FIBROMA/
-
myoma*.ti,ab
-
4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13
-
sonogr*.ti,ab
-
hysterosonogr*.ti,ab
-
ultrasound.ti,ab
-
exp ULTRASONOGRAPHY/
-
((transvaginal scan)).ti,ab
-
hysterosco*.ti,ab
-
exp HYSTEROSCOPY/
-
sonohyster*.ti,ab
-
15 OR 16 OR 17 OR 18 OR 19 OR 20 OR 21 OR 22
-
3 AND 14 AND 23
EMBASE search strategy for prevalence of fibroids
-
prevalence.ti,ab
-
exp PREVALENCE/
-
fibroid*.ti,ab
-
exp LEIOMYOMA/ OR exp UTERUS MYOMA/
-
leiomyoma.ti,ab
-
myoma*.ti,ab
-
exp MYOMA/
-
fibromyoma.ti,ab
-
leiofibromyoma.ti,ab
-
fibroleiomyoma.ti,ab
-
fibroma.ti,ab
-
exp FIBROMA/
-
1 OR 2
-
3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12
-
sonogra*.ti,ab
-
hysterosonogra*.ti,ab
-
sonohyster*.ti,ab
-
ultrasound.ti,ab
-
exp ULTRASOUND/
-
((transvaginal scan)).ti,ab
-
hysterosco*.ti,ab
-
exp HYSTEROSCOPY/
-
15 OR 16 OR 17 OR 18 OR 19 OR 20 OR 21 OR 22
-
13 AND 14 AND 23
MEDLINE search strategy for the prevalence of endometrial hyperplasia
-
exp HEMORRHAGE/
-
bleeding.ti,ab
-
exp BLOOD/
-
blood.ti,ab
-
exp MENORRHAGIA/
-
menorrhagia.ti,ab
-
menstr*.ti,ab
-
exp MENSTRUAL CYCLE/
-
1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8
-
prevalence.ti,ab
-
exp PREVALENCE/
-
10 OR 11
-
uterine.ti,ab
-
uterus.ti,ab
-
exp UTERUS/
-
uter*.ti,ab
-
endometrial.ti,ab
-
exp ENDOMETRIAL HYPERPLASIA/
-
endometr*.ti,ab
-
hyperplas*.ti,ab
-
hyperplasia.ti,ab
-
exp HYPERPLASIA/
-
24 OR 25 OR 26 OR 27 OR 28 OR 30
-
29 OR 31 OR 32 OR 33
-
9 AND 12 AND 34 AND 35
EMBASE search strategy for the prevalence of endometrial hyperplasia
-
hemorrhage.ti,ab
-
exp BLEEDING/
-
bleed*.ti,ab
-
blood*.ti,ab
-
exp BLOOD/
-
bleeding.ti,ab
-
exp MENORRHAGIA/
-
menorrhagia.ti,ab
-
menstr*.ti,ab
-
exp MENSTRUAL CYCLE/
-
1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10
-
prevalence.ti,ab
-
exp PREVALENCE/
-
12 OR 13
-
uterus.ti,ab
-
exp UTERUS/
-
uter*.ti,ab
-
uterine.ti,ab
-
endometrial.ti,ab
-
exp ENDOMETRIAL DISEASE/
-
endometr*.ti,ab
-
hyperplas*.ti,ab
-
exp HYPERPLASIA/ OR exp ENDOMETRIUM HYPERPLASIA/
-
15 OR 16 OR 17 OR 18 OR 19 OR 21
-
20 OR 22 OR 23
-
24 AND 25
-
11 AND 15 AND 27
MEDLINE search strategy for the prevalence of endometrial cancer
-
exp HEMORRHAGE/
-
bleeding.ti,ab
-
exp BLOOD/
-
blood.ti,ab
-
exp MENORRHAGIA/
-
menorrhagia.ti,ab
-
menstr*.ti,ab
-
exp MENSTRUAL CYCLE/
-
6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13
-
prevalence.ti,ab
-
exp PREVALENCE/
-
15 OR 16
-
uterine.ti,ab
-
uterus.ti,ab
-
exp UTERUS/
-
uter*.ti,ab
-
endometrial.ti,ab
-
endometr*.ti,ab
-
cancer.ti,ab
-
exp NEOPLASMS/
-
malignan*.ti,ab
-
((Endometrial cancer)).ti,ab
-
exp ENDOMETRIAL NEOPLASMS/
-
13 OR 14 OR 15 OR 16 OR 17 OR 19
-
20 OR 21 OR 22 OR 23 OR 24
-
9 AND 12 AND 25 AND 26
EMBASE search strategy for the prevalence of endometrial cancer
-
hemorrhage.ti,ab
-
exp BLEEDING/
-
bleed*.ti,ab
-
blood*.ti,ab
-
exp BLOOD/
-
bleeding.ti,ab
-
exp MENORRHAGIA/
-
menorrhagia.ti,ab
-
menstr*.ti,ab
-
exp MENSTRUAL CYCLE/
-
1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10
-
prevalence.ti,ab
-
exp PREVALENCE/
-
12 OR 1
-
uterus.ti,ab
-
exp UTERUS/
-
uter*.ti,ab
-
uterine.ti,ab
-
cancer.ti,ab
-
exp NEOPLASM/
-
malignan*.ti,ab
-
(endometrial AND cancer).ti,ab
-
endometrial.ti,ab
-
exp ENDOMETRIAL DISEASE/
-
endometr*.ti,ab
-
15 OR 16 OR 17 OR 18 OR 23 OR 24 OR 25
-
19 OR 20 OR 21
-
26 AND 27
-
22 OR 28
-
11 AND 14 AND 29
MEDLINE search strategy for reviews of outpatient hysteroscopy
-
exp HYSTEROSCOPY/
-
hysteroscopy.ti,ab
-
hysteroscop*.ti,ab
-
1 OR 2 OR 3
-
4 [Limit to: Review Articles]
EMBASE search strategy for reviews of outpatient hysteroscopy
-
exp HYSTEROSCOPY/
-
hysteroscopy.ti,ab
-
hysteroscop*.ti,ab
-
1 OR 2 OR 3
-
4 [Limit to: (Publication Types Review)]
MEDLINE search strategy for studies of transvaginal scan and heavy menstrual bleeding
-
(transvaginal AND ultrasound).ti,ab
-
exp ULTRASONOGRAPHY/
-
sonogra*.ti,ab
-
transvaginal.ti,ab
-
vaginal.ti,ab
-
2 OR 3
-
4 OR 5
-
6 AND 7
-
1 OR 8
-
“abnormal uterine bleeding”.ti,ab
-
exp METRORRHAGIA/ OR exp MENORRHAGIA/
-
menorrhagia.ti,ab
-
10 OR 11 OR 12
-
9 AND 13
EMBASE search strategy for studies of transvaginal scan and heavy menstrual bleeding
-
(transvaginal AND ultrasound).ti,ab
-
exp ULTRASOUND/
-
sonogra*.ti,ab
-
transvaginal.ti,ab
-
vaginal.ti,ab
-
4 OR 5
-
2 OR 3
-
6 AND 7
-
1 OR 8
-
exp MENORRHAGIA/ OR exp UTERUS BLEEDING/
-
“abnormal uterine bleeding”.ti,ab
-
menorrhagia.ti,ab
-
10 OR 11 OR 12
-
9 AND 13
MEDLINE search strategy for studies of saline infusion sonography and heavy menstrual bleeding
-
exp MENORRHAGIA/
-
menorrhagia.ti,ab
-
hypermenorrhea.ti,ab
-
(heavy ADJ menstrual ADJ bleeding).ti,ab
-
(heavy ADJ menstrua*).ti,ab
-
“abnormal uterine bleeding”.ti,ab
-
1 OR 2 OR 3 OR 4 OR 5 OR 6
-
exp SODIUM CHLORIDE/ AND exp ULTRASONOGRAPHY/
-
(saline AND infusion AND sonography).ti,ab
-
(sono AND hysterosonography).ti,ab
-
(saline AND hysterosonography).ti,ab
-
(saline AND hysterography).ti,ab
-
sonohysterography.ti,ab
-
8 OR 9 OR 10 OR 11 OR 12 OR 13
-
7 AND 14
EMBASE search strategy for studies of saline infusion sonography and heavy menstrual bleeding
-
exp MENORRHAGIA/
-
menorrhagia.ti,ab
-
hypermenorrhea.ti,ab
-
(heavy ADJ menstrual ADJ bleeding).ti,ab
-
(heavy ADJ menstrua*).ti,ab
-
“abnormal uterine bleeding”.ti,ab
-
1 OR 2 OR 3 OR 4 OR 5 OR 6
-
exp SODIUM CHLORIDE/ AND exp ULTRASONOGRAPHY/
-
(saline AND infusion AND sonography).ti,ab
-
(sono AND hysterosonography).ti,ab
-
(saline AND hysterosonography).ti,ab
-
(saline AND hysterography).ti,ab
-
sonohysterography.ti,ab
-
8 OR 9 OR 10 OR 11 OR 12 OR 13
-
7 AND 14
MEDLINE search strategy for reviews of endometrial biopsy
-
(endometrial AND biopsy).ti,ab
-
exp ENDOMETRIUM/
-
endometr*.ti,ab
-
exp BIOPSY/
-
biopsy.ti,ab
-
sampling.ti,ab
-
2 OR 3
-
4 OR 5 OR 6
-
7 AND 8
-
1 OR 9
-
10 [Limit to: Review Articles]
EMBASE search strategy for reviews of endometrial biopsy
-
(endometrial AND biopsy).ti,ab
-
exp ENDOMETRIUM BIOPSY/
-
exp ENDOMETRIUM/
-
endometr*.ti,ab
-
biopsy.ti,ab
-
exp BIOPSY/
-
sampling.ti,ab
-
exp SAMPLING/
-
5 OR 6 OR 7 OR 8
-
3 OR 4
-
9 AND 10
-
1 OR 2 OR 11
-
12 [Limit to: (Publication Types Review)]
MEDLINE search strategy for studies of levonorgestrel intrauterine system for heavy menstrual bleeding
-
menorrhag*.ti,ab
-
exp MENORRHAGIA/
-
“heavy menstrual blee*”.ti,ab
-
menometrorrhagia.ti,ab
-
METRORRHAGIA/
-
hypermenorrh*.ti,ab
-
1 OR 2 OR 3 OR 4 OR 5 OR 6
-
mirena.ti,ab
-
exp LEVONORGESTREL/
-
“intrauterine device”.ti,ab
-
“intrauterine system”.ti,ab
-
INTRAUTERINE DEVICES, MEDICATED/ OR INTRAUTERINE DEVICES/
-
IUS.ti,ab
-
IUD.ti,ab
-
LNG-IUS.ti,ab
-
levonorgestrel-releasing.ti,ab
-
8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15 OR 16
-
7 AND 17
EMBASE search strategy for studies of levonorgestrel intrauterine system for heavy menstrual bleeding
-
menorrhag*.ti,ab
-
exp MENORRHAGIA/
-
“heavy menstrual bleed*”.ti,ab
-
exp MENSTRUATION DISORDER/
-
menometorrhagia.ti,ab
-
hypermenorrh*.ti,ab
-
1 OR 2 OR 3 OR 4 OR 5 OR 6
-
mirena.ti,ab
-
exp LEVONORGESTREL/
-
“intrauterine system”.ti,ab
-
IUS.ti,ab
-
LNG-IUS.ti,ab
-
IUD.ti,ab
-
“intrauterine device”.ti,ab
-
levonorgestrel-releasing.ti,ab
-
8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15
-
7 AND 16
MEDLINE search strategy for patient satisfaction after endometrial ablation
-
“endometrial ablation”.ti,ab
-
exp ENDOMETRIAL ABLATION TECHNIQUES/
-
satisfaction.ti,ab
-
exp PATIENT SATISFACTION/
-
1 OR 2
-
3 OR 4
-
5 AND 6
EMBASE search strategy for patient satisfaction after endometrial ablation
-
“endometrial ablation”.ti,ab
-
exp ENDOMETRIUM ABLATION/
-
satisfaction.ti,ab
-
exp PATIENT SATISFACTION/ OR exp SATISFACTION/
-
3 OR 4
-
1 OR 2
-
5 AND 6
MEDLINE search for endometrial ablation and fibroids
-
fibroid*.ti,ab
-
UTERUS MYOMA/ OR LEIOMYOMA/
-
myoma.ti,ab
-
leiomyoma.ti,ab
-
1 OR 2 OR 3 OR 4
-
(endometrial AND ablation).ti,ab
-
exp ENDOMETRIAL ABLATION TECHNIQUES/
-
6 OR 7
-
5 AND 8
EMBASE search for endometrial ablation and fibroids
-
((endometrial ablation)).ti,ab
-
exp ENDOMETRIUM ABLATION/
-
1 OR 2
-
fibroid*.ti,ab
-
UTERUS MYOMA/ OR LEIOMYOMA/
-
myoma.ti,ab
-
leiomyoma.ti,ab
-
4 OR 5 OR 6 OR 7
-
3 AND 8
MEDLINE search strategy for satisfaction with hysterectomy as a treatment for fibroids
-
exp HYSTERECTOMY/
-
exp LEIOMYOMA/
-
exp PERSONAL SATISFACTION/
-
satisfaction.ti,ab
-
3 OR 4
-
1 AND 2 AND 5
EMBASE search strategy for satisfaction with hysterectomy as a treatment for fibroids
-
exp HYSTERECTOMY/
-
exp LEIOMYOMA/
-
satisfaction.ti,ab
-
exp SATISFACTION/
-
3 OR 4
-
1 AND 2 AND 5
MEDLINE search strategy for satisfaction after endometrial polypectomy
-
polypectomy.ti,ab
-
(endometrial AND polyp).ti,ab
-
exp POLYPS/ AND exp ENDOMETRIUM/
-
removal.ti,ab
-
1 OR 4
-
2 OR 3
-
5 AND 6
EMBASE search strategy for satisfaction after endometrial polypectomy
-
polypectomy.ti,ab
-
exp POLYPECTOMY/
-
(endometrial AND polyp).ti,ab
-
exp ENDOMETRIUM POLYP/
-
removal.ti,ab
-
1 OR 2 OR 5
-
3 OR 4
-
6 AND 7
MEDLINE search strategy for satisfaction after transcervical resection of a fibroid
-
(transcervical AND resection AND fibroid).ti,ab
-
exp LEIOMYOMA/
-
(hysteroscopic AND removal).ti,ab
-
myomectomy.ti,ab
-
exp GYNECOLOGIC SURGICAL PROCEDURES/
-
fibroid.ti,ab
-
(submucosal AND fibroid).ti,ab
-
submuc*.ti,ab
-
6 AND 8
-
2 AND 8
-
1 OR 3 OR 4 OR 5
-
7 OR 9 OR 10
-
11 AND 12
-
satisf*.ti,ab
-
satisfaction.ti,ab
-
14 OR 15
-
13 AND 16
EMBASE search strategy for satisfaction after transcervical resection of a fibroid
-
(transcervical AND resection AND fibroid).ti,ab
-
fibroid.ti,ab
-
exp UTERUS MYOMA/ OR exp LEIOMYOMA/
-
(hysteroscopic AND removal).ti,ab
-
myomectomy.ti,ab
-
exp MYOMECTOMY/
-
submuc*.ti,ab
-
(submucosal AND fibroid).ti,ab
-
2 OR 3
-
7 AND 9
-
8 OR 10
-
1 OR 4 OR 5 OR 6
-
11 AND 12
-
satisf*.ti,ab
-
satisfaction.ti,ab
-
exp SATISFACTION/ OR exp PATIENT SATISFACTION/
-
14 OR 15 OR 16
-
13 AND 17
MEDLINE search strategy for satisfaction after dilatation and curettage
-
D+C.ti,ab
-
exp “DILATATION AND CURETTAGE”/
-
curettage.ti,ab
-
CURETTAGE/
-
((heavy menstrual bleeding)).ti,ab
-
exp MENORRHAGIA/
-
5 OR 6
-
1 OR 2 OR 3 OR 4
-
7 AND 8
EMBASE search strategy for satisfaction after dilatation and curettage
-
D+C.ti,ab
-
exp “DILATATION AND CURETTAGE”/
-
curettage.ti,ab
-
CURETTAGE/
-
((heavy menstrual bleeding)).ti,ab
-
exp MENORRHAGIA/
-
5 OR 6
-
1 OR 2 OR 3 OR 4
-
7 AND 8
MEDLINE search strategy for satisfaction after uterine artery embolisation
-
“uterine artery embolis*”.ti,ab
-
“uterine artery emboliz*”.ti,ab
-
UAE.ti,ab
-
exp UTERINE ARTERY EMBOLIZATION/
-
1 OR 2 OR 3 OR 4
-
satisfaction.ti,ab
-
satisf*.ti,ab
-
6 OR 7
-
5 AND 8
EMBASE search strategy for satisfaction after uterine artery embolisation
-
“uterine artery embolis*”.ti,ab
-
“uterine artery emboliz*”.ti,ab
-
UAE.ti,ab
-
exp UTERINE ARTERY EMBOLIZATION/
-
1 OR 2 OR 3 OR 4
-
satisfaction.ti,ab
-
exp SATISFACTION/ OR exp PATIENT SATISFACTION/
-
satisf*.ti,ab
-
6 OR 7 OR 8
-
5 AND 9
MEDLINE search strategy for satisfaction after myomectomy
-
myomectomy.ti,ab
-
satisf*.ti,ab
-
2 AND 3
EMBASE search strategy for satisfaction after myomectomy
-
myomectomy.ti,ab
-
satisf*.ti,ab
-
2 AND 3
Appendix 4 Decision trees for the economic analysis of post-menopausal bleeding
Decision tree for the strategy ‘no initial work-up’

Decision tree for the strategy ‘dilatation and curettage’
Decision tree for the strategy ‘endometrial biopsy’
Decision tree for the strategy ‘transvaginal scan with a 5-mm cut-off’
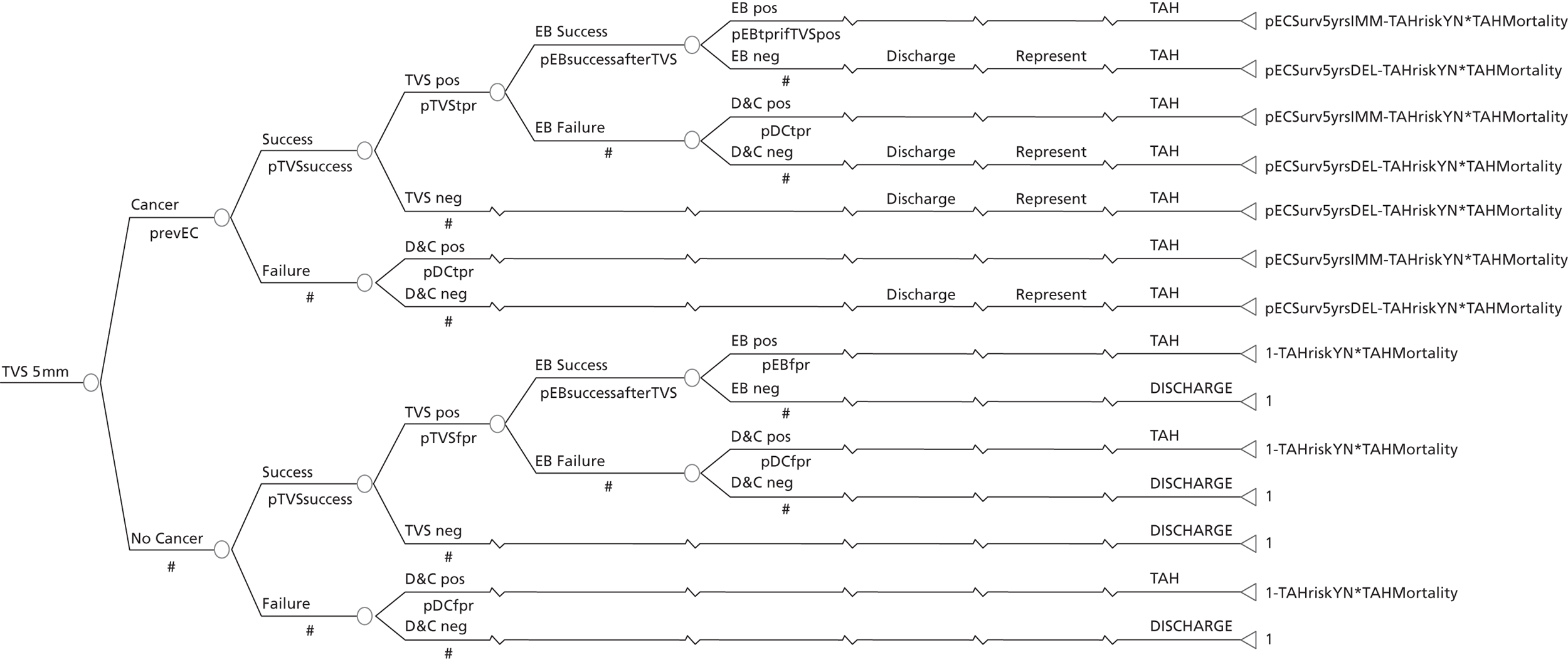
Decision tree for the strategy ‘outpatient hysteroscopy’
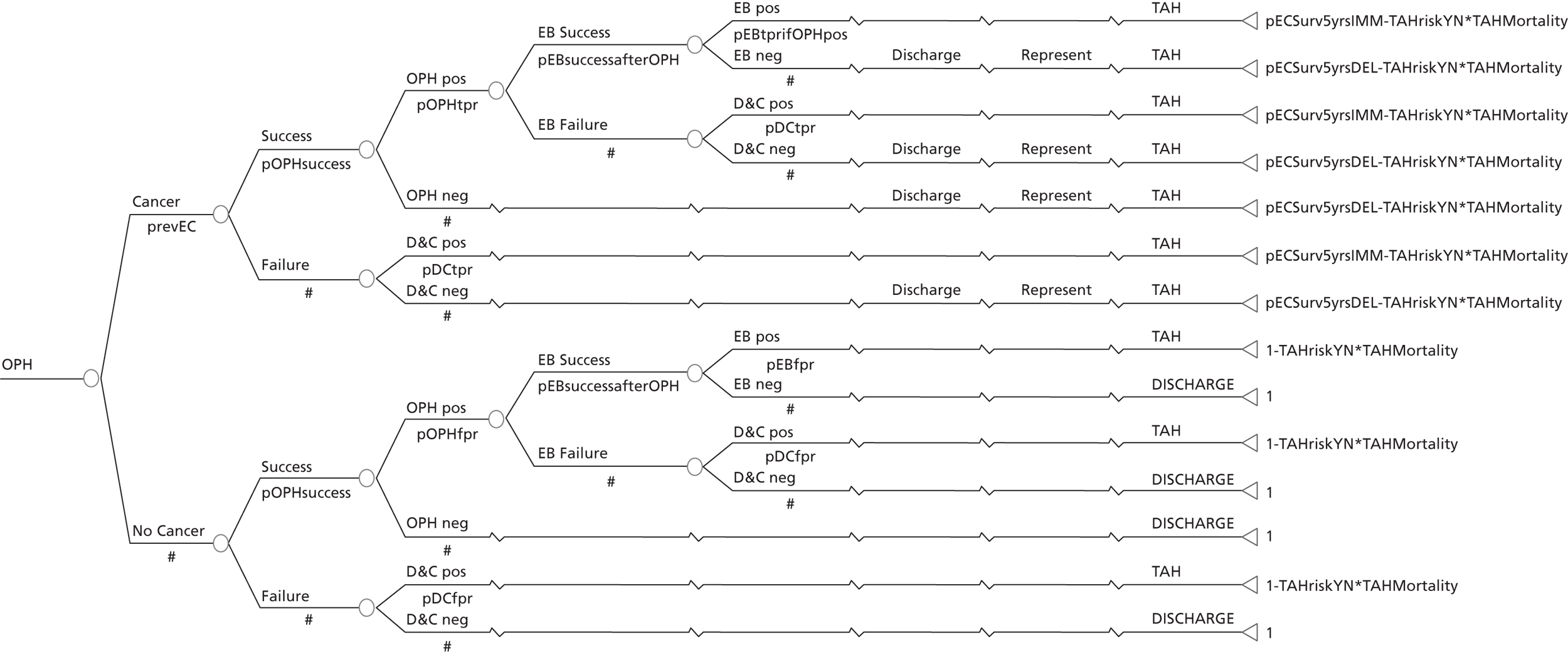
Decision tree for the strategy ‘transvaginal scan and outpatient hysteroscopy’
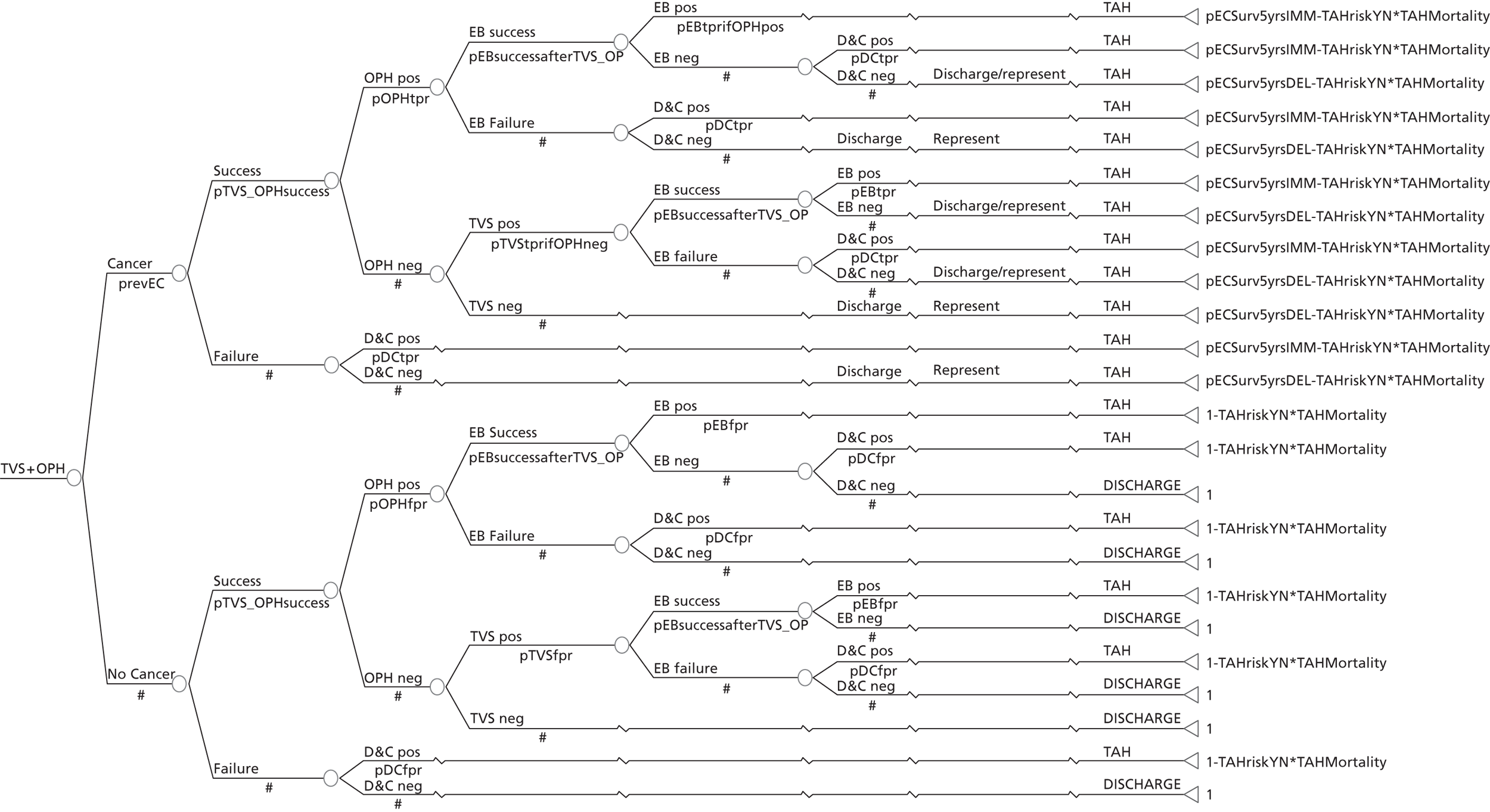
Decision tree for the strategy ‘transvaginal scan and endometrial biopsy’
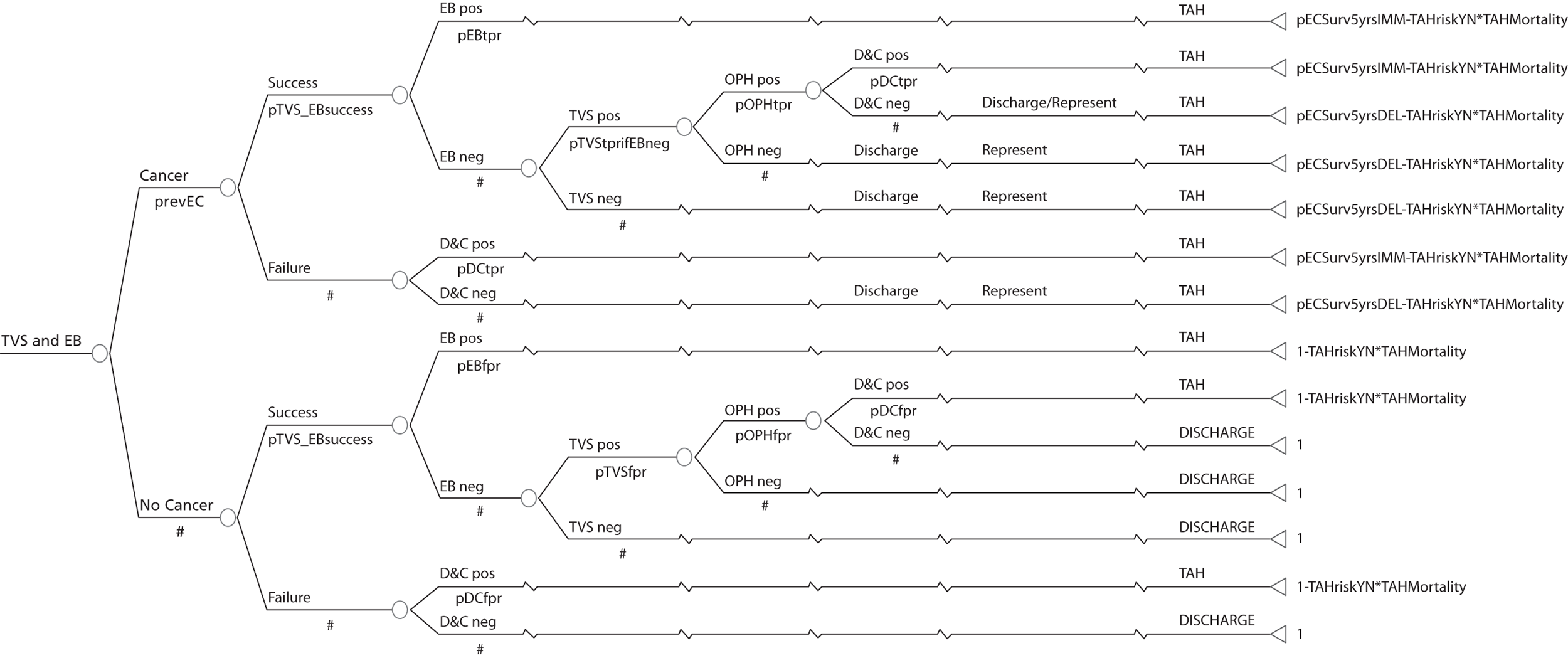
Decision tree for the strategy ‘endometrial biopsy and outpatient hysteroscopy’
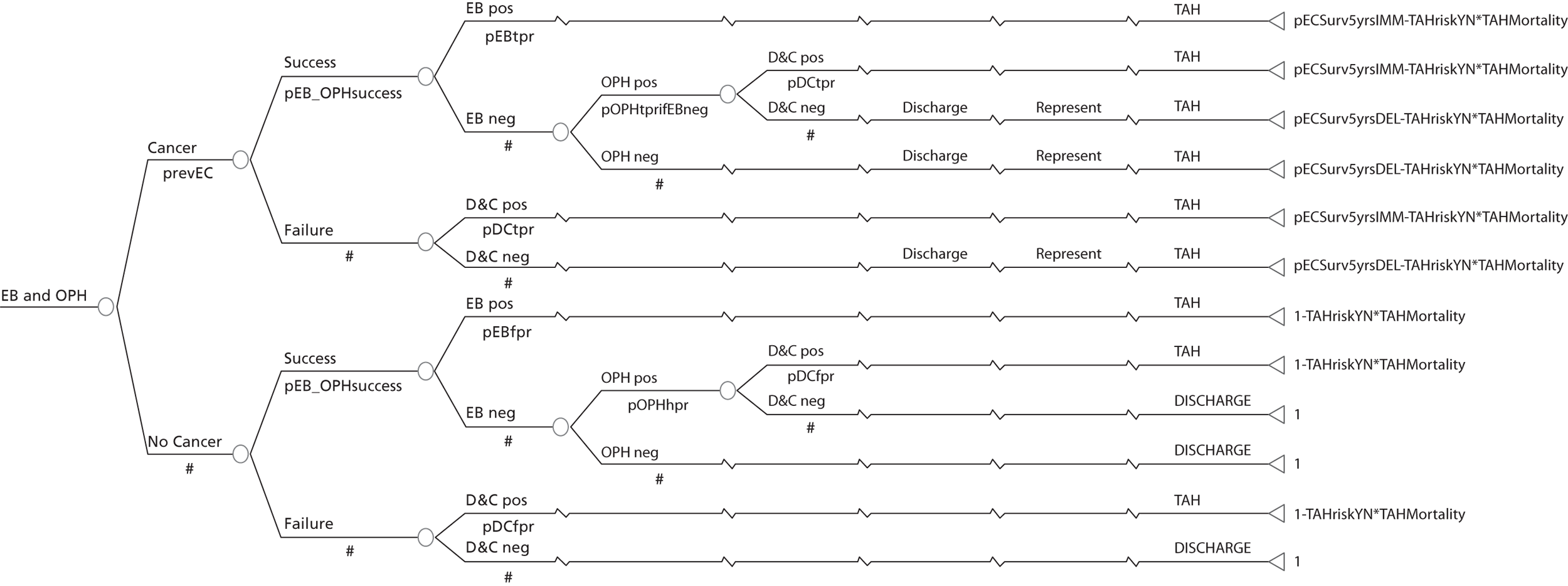
Decision tree for the strategy ‘transvaginal scan, endometrial biopsy and outpatient hysteroscopy’
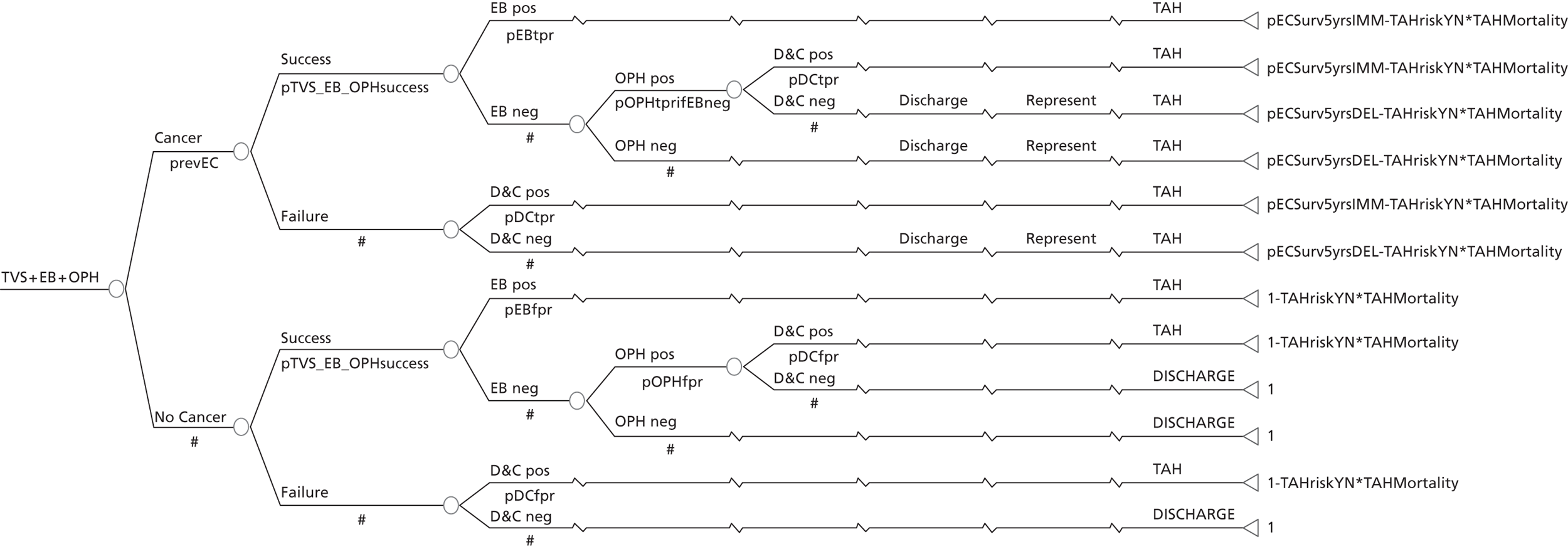
Decision tree for the strategy ‘history only’
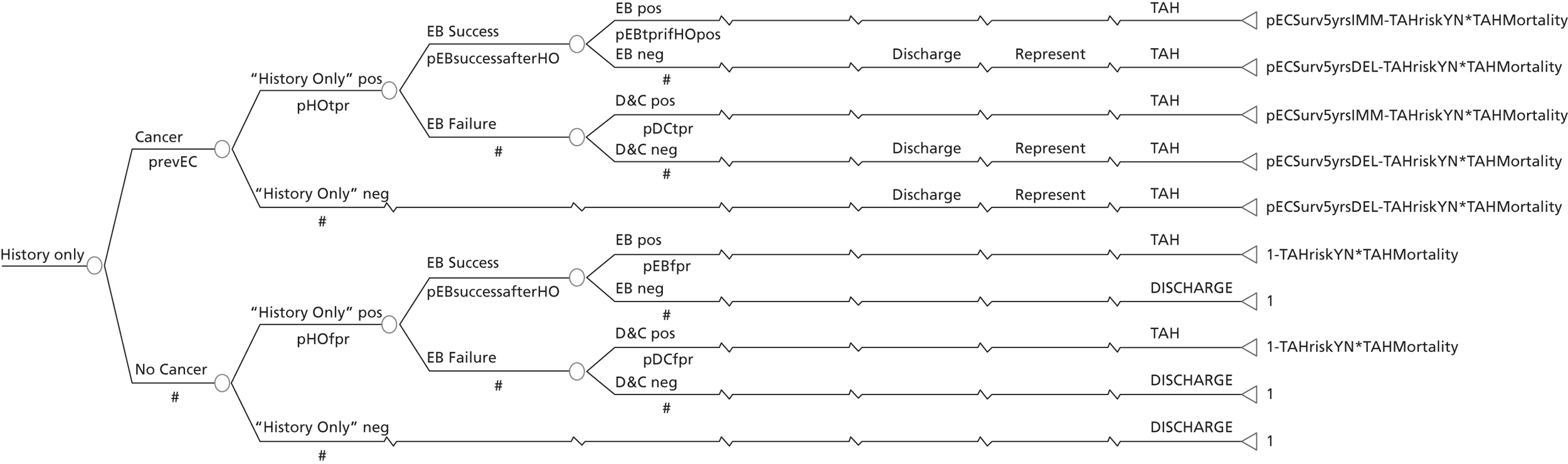
Decision tree for the strategy ‘history and transvaginal scan’
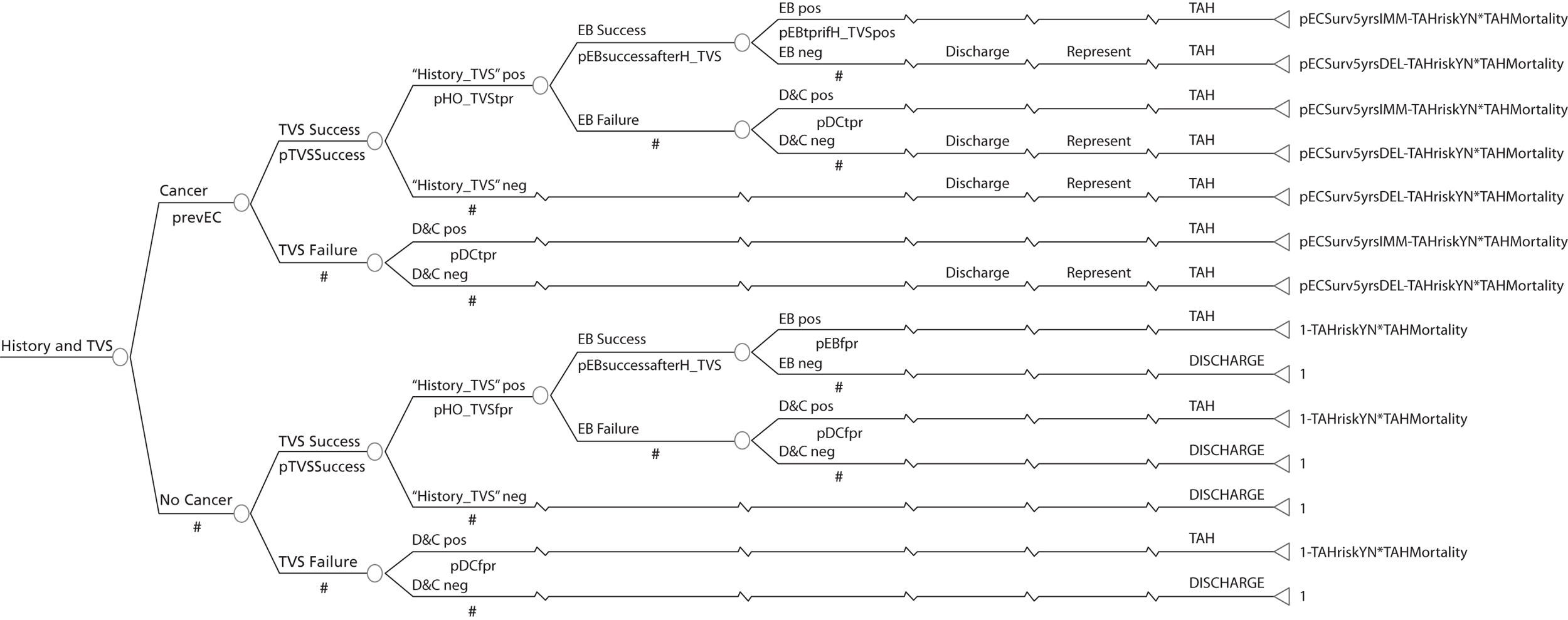
Decision tree for the strategy ‘selective transvaginal scan with history’
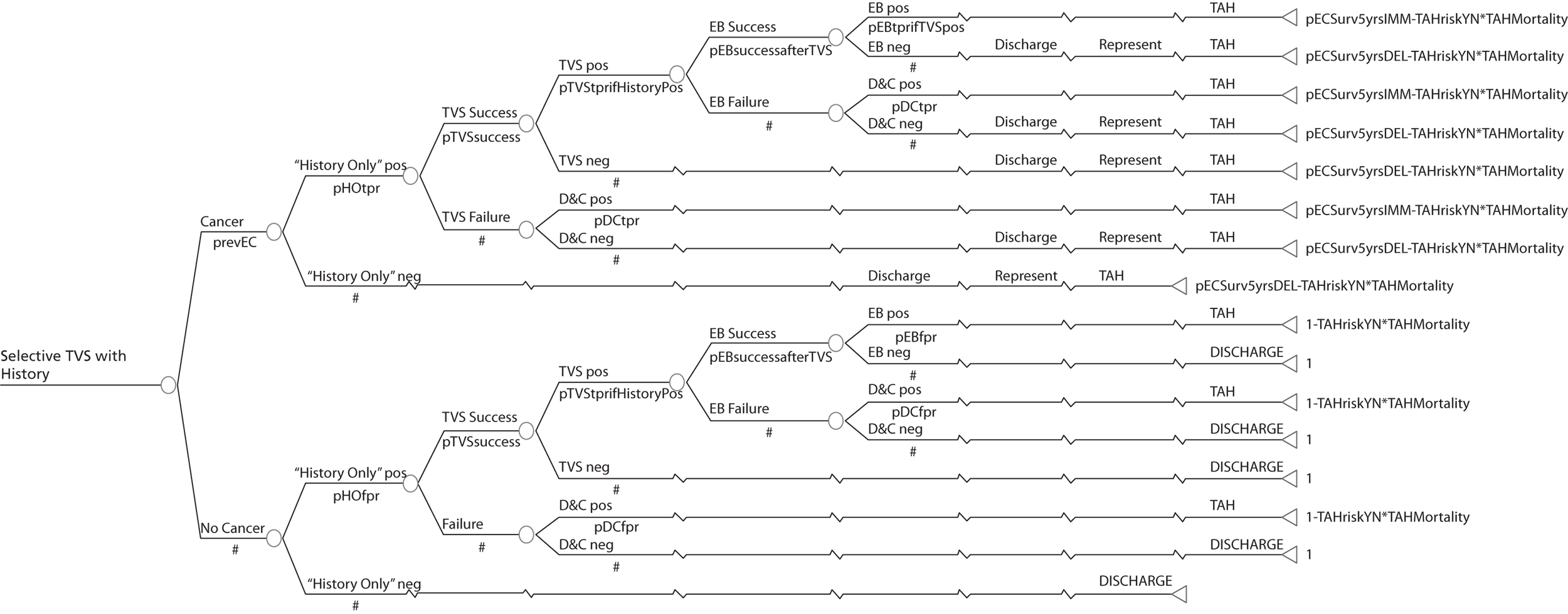
Appendix 5
Transvaginal scan compared with levonorgestrel intrauterine system: women with heavy menstrual bleeding managed over multiple clinic appointments
The scatterplot below (Figure 36a) shows that TVS is more effective than LNG-IUS alone and is very likely to be more costly per patient satisfied. The CEAC (Figure 36b) shows that above a WTP of £8000, TVS is probably a more cost-effective option than LNG-IUS alone per woman satisfied but it is only at WTP above £20,000 that this is almost certain (p > 0.9).
FIGURE 36.
Cost-effectiveness plane (a) and CEAC (b): TVS-alone strategy relative to the LNG-IUS-alone strategy for women with HMB managed over multiple clinic appointments.
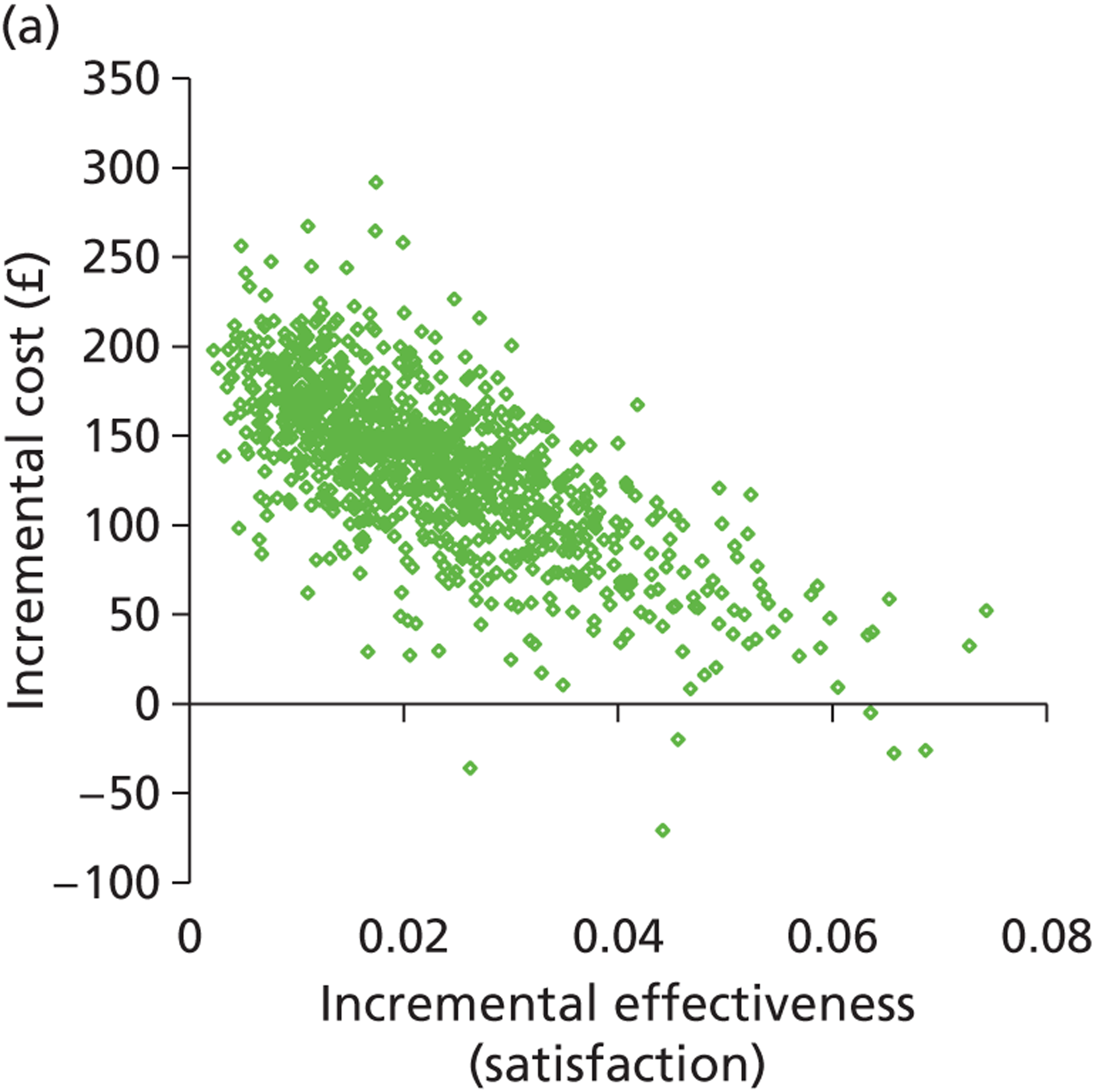
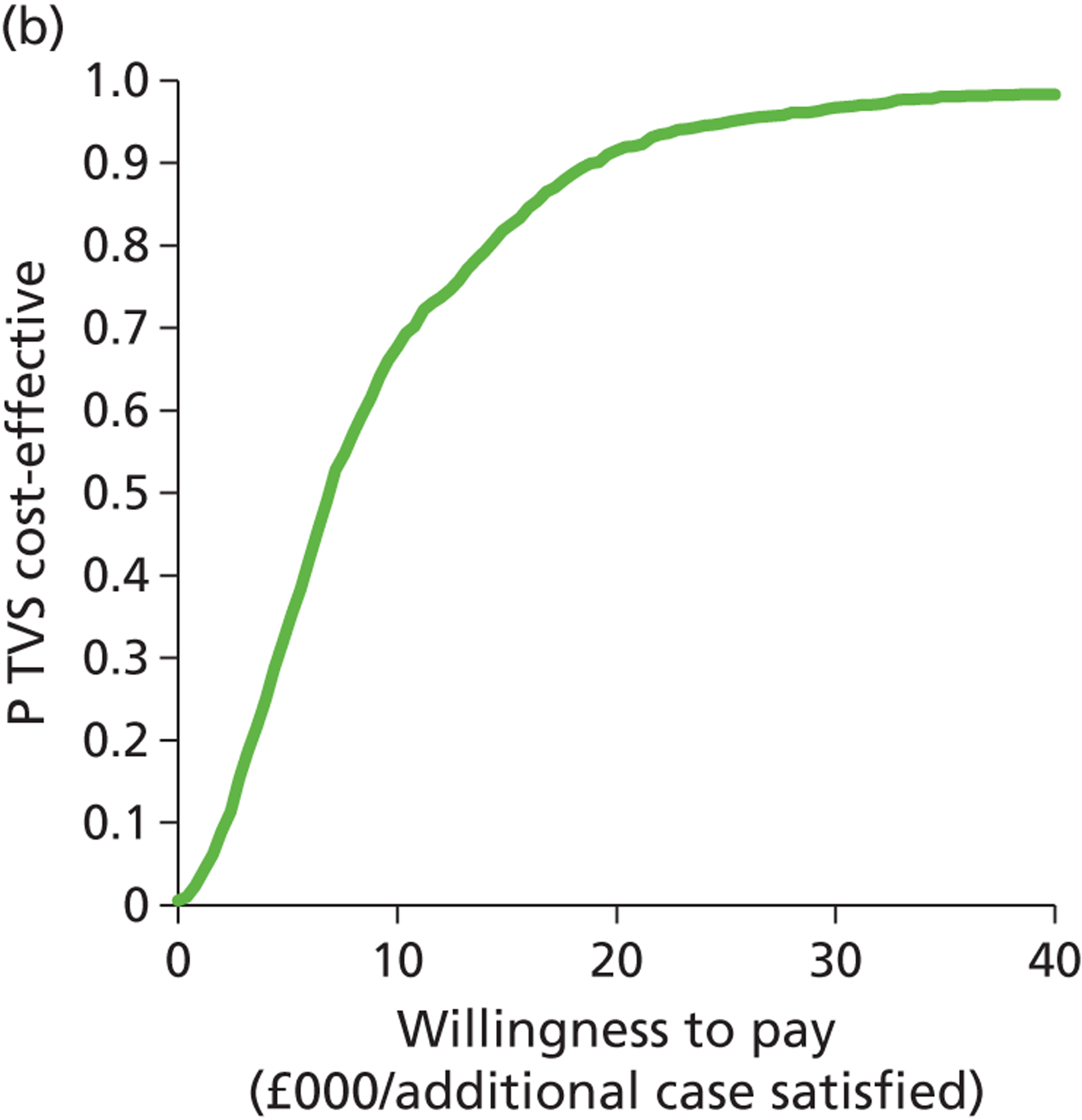
Saline infusion sonography compared with levonorgestrel intrauterine system: women with heavy menstrual bleeding managed over multiple clinic appointments
The scatterplot depicted in Figure 37a shows that SIS is more effective than LNG-IUS alone but it is also probably more expensive. The CEAC (Figure 37b) shows that at a WTP of around £10,000 it is probable that SIS is the most cost-effective option and this becomes almost certain at a WTP of £20,000.
FIGURE 37.
Cost-effectiveness plane (a) and CEAC (b): SIS-alone strategy relative to the LNG-IUS-alone strategy for women with HMB managed over multiple clinic appointments.
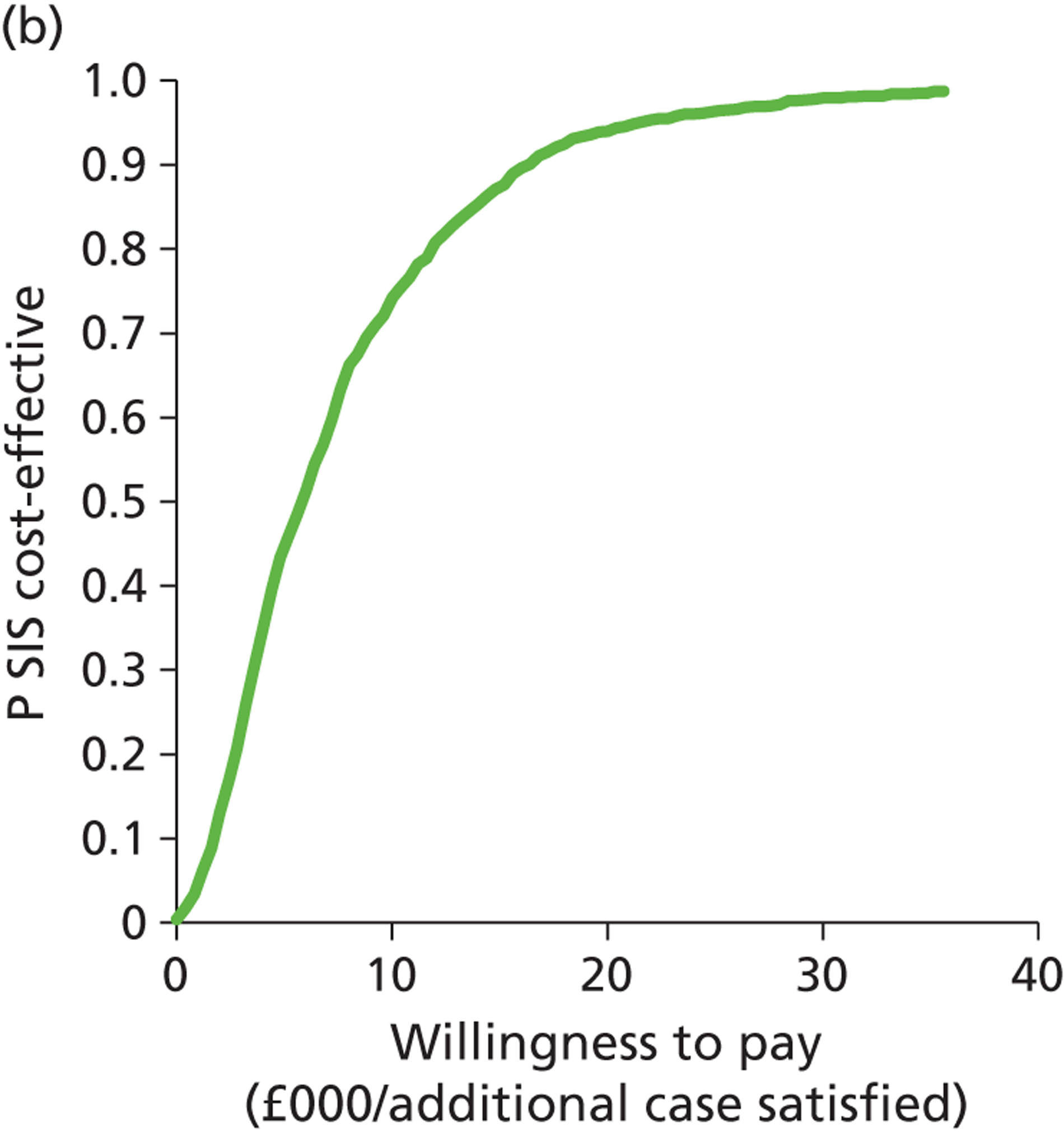
Outpatient hysteroscopy compared with saline infusion sonography: women with heavy menstrual bleeding managed over multiple clinic appointments
Figure 38a suggests that there is uncertainty between SIS and OPH but that OPH may be more effective than SIS. It also suggests that OPH is more expensive than SIS. The CEAC (Figure 38b) shows that even at a WTP of £40,000, OPH is unlikely to be the most cost-effective strategy (p < 0.3).
FIGURE 38.
Cost-effectiveness plane (a) and CEAC (b): OPH-alone strategy relative to the SIS-alone strategy for women with HMB managed over multiple clinic appointments.
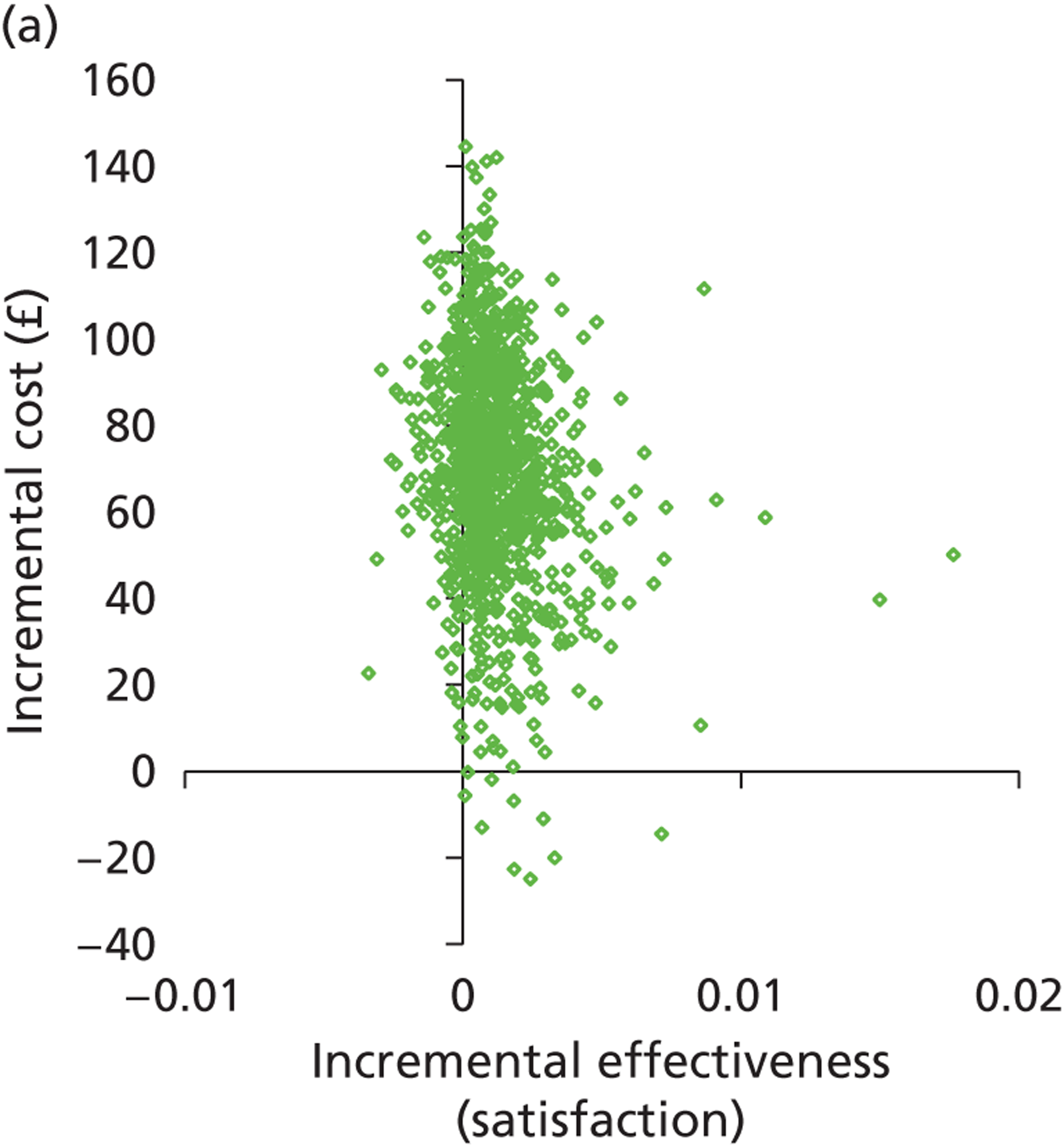
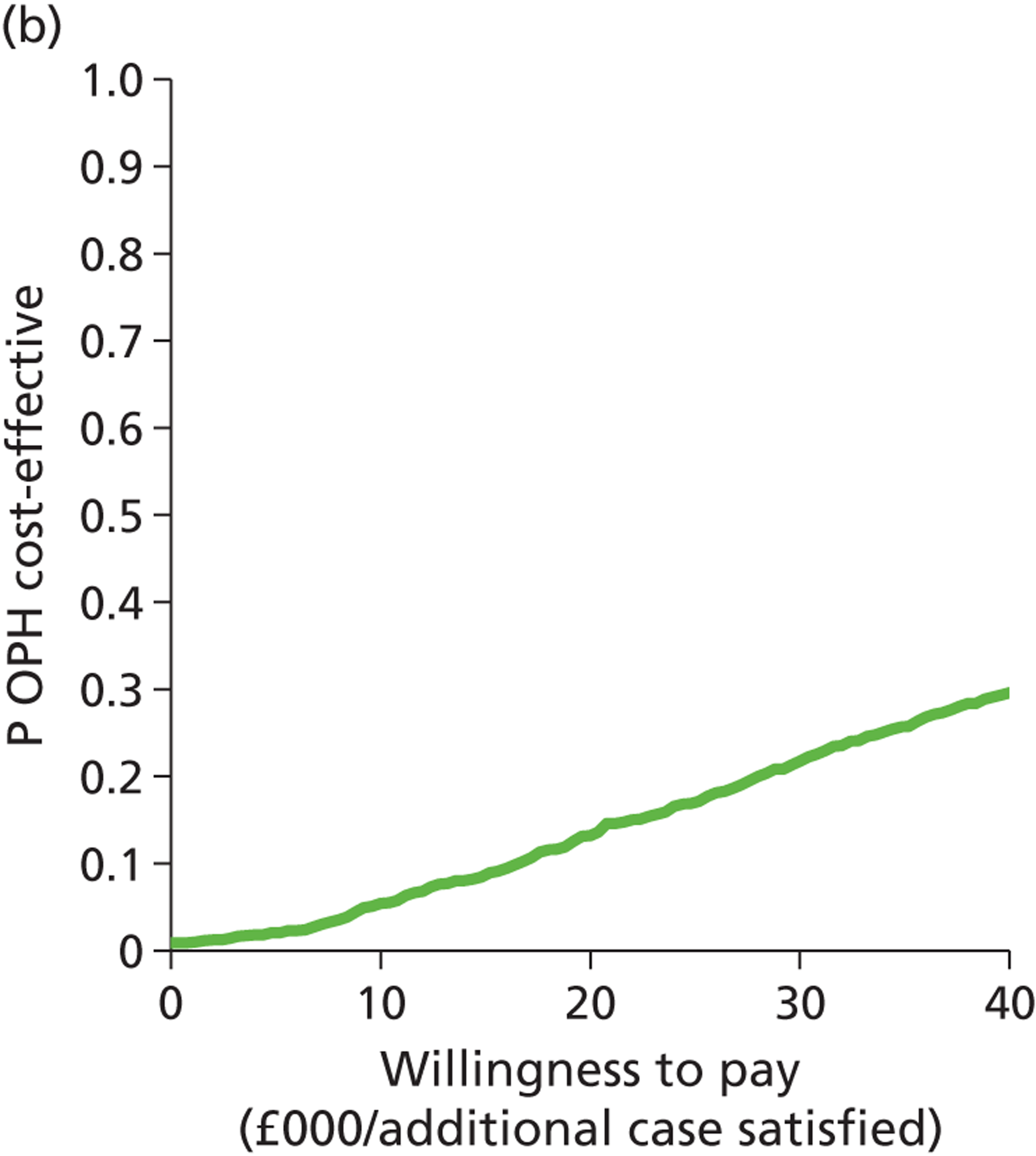
Outpatient hysteroscopy and endometrial biopsy compared with outpatient hysteroscopy: women with heavy menstrual bleeding managed over multiple clinic appointments
Figure 39 shows that OPH + EBx is probably more effective and more expensive than OPH alone but that it is only becomes likely to be the most cost-effective of the two strategies at WTP values of above £20,000, and that even at WTP of £40,000 the probability of it being the most cost-effective strategy is only just above 0.7.
FIGURE 39.
Cost-effectiveness plane (a) and CEAC (b): OPH + EBx strategy relative to the OPH-alone strategy for women with HMB managed over multiple clinic appointments.
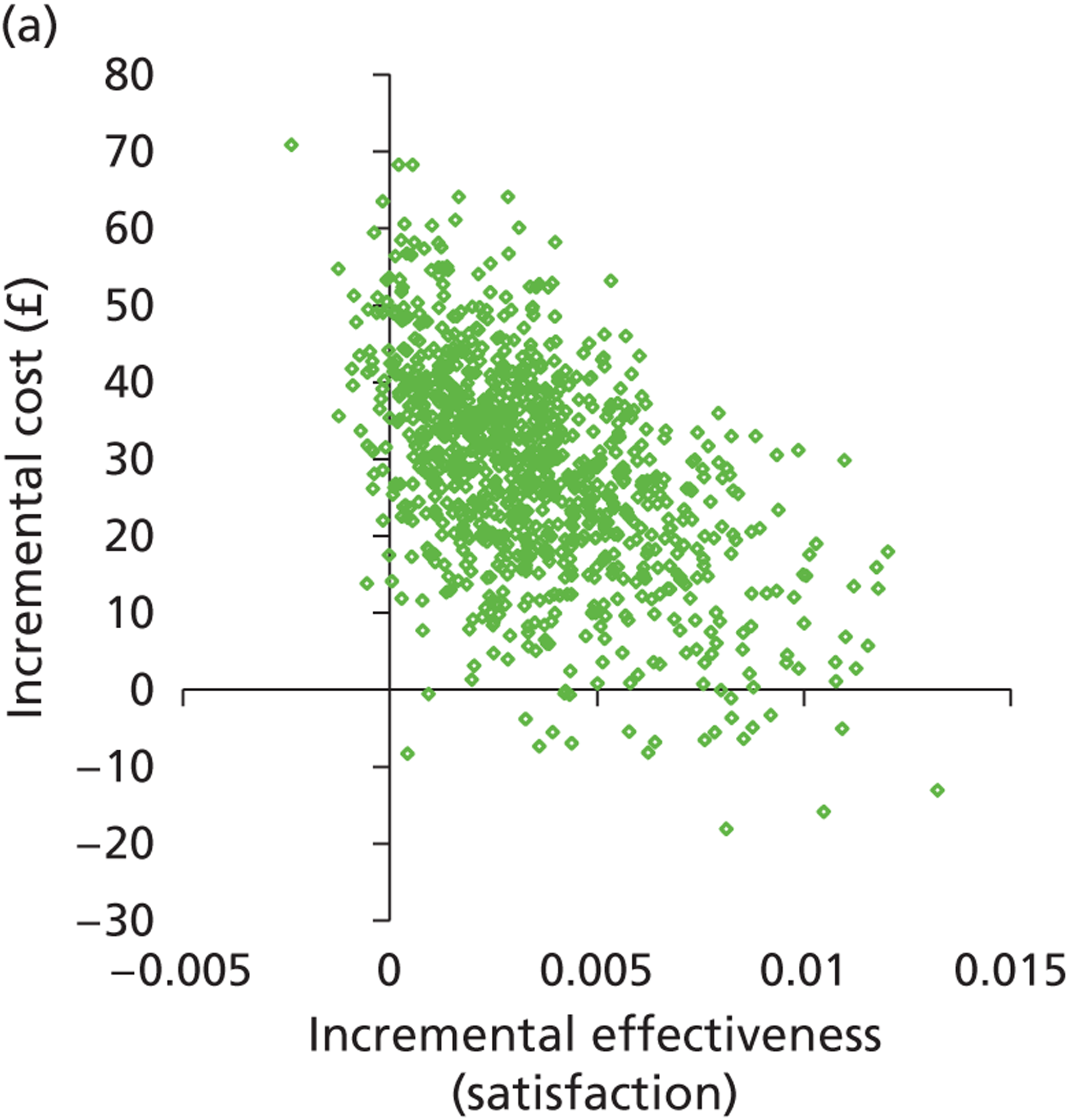
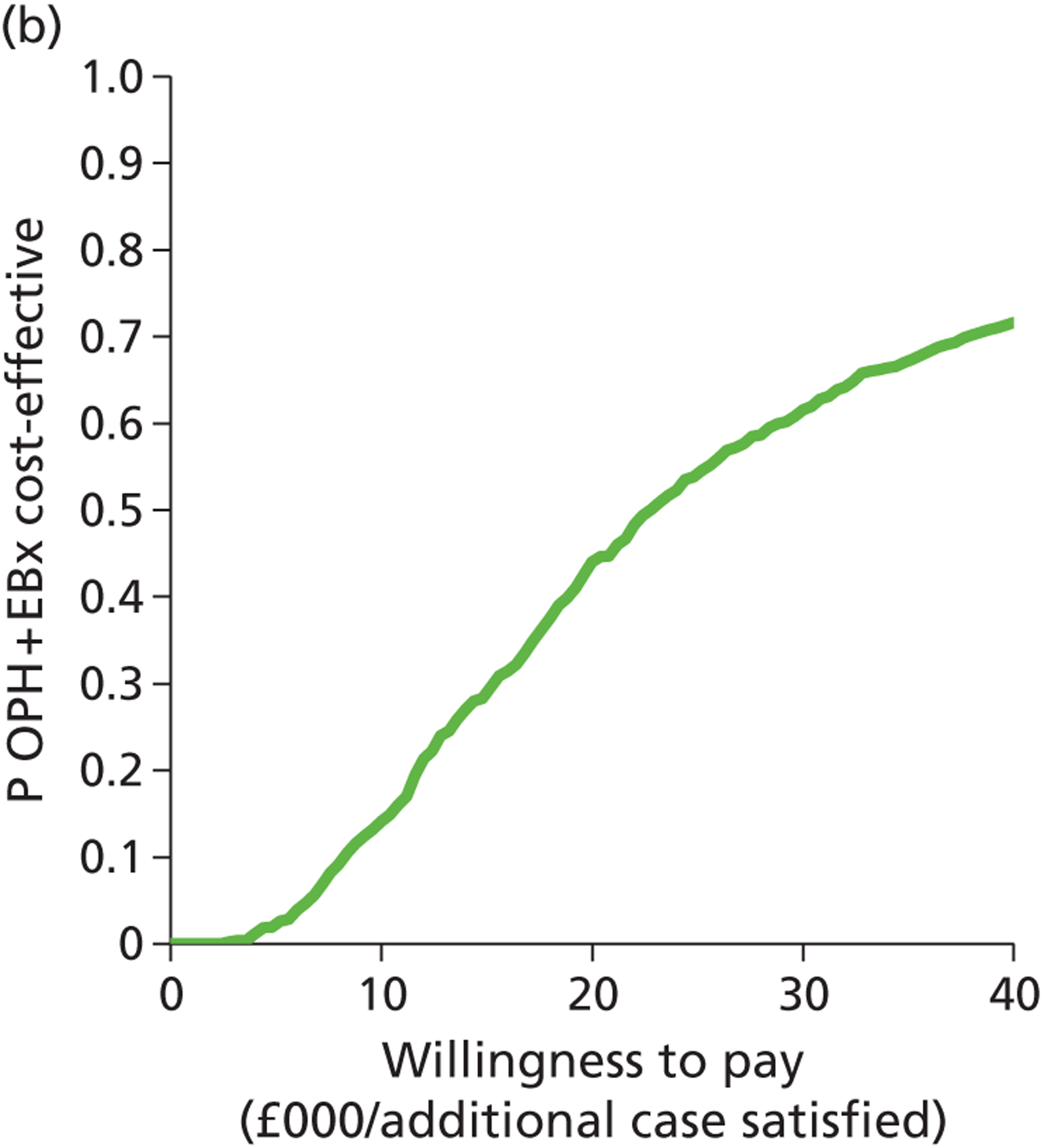
Saline infusion sonography compared with transvaginal scan: women with heavy menstrual bleeding managed over multiple clinic appointments
The scatterplot below (Figure 40a) shows that SIS is more effective than TVS, although it is probably a more expensive strategy. The CEAC (Figure 40b) shows that SIS is likely to be the most cost-effective strategy at a WTP of approximately £3000, but this becomes almost certain (p > 0.9) at just over £10,000.
FIGURE 40.
Cost-effectiveness plane (a) and CEAC (b): SIS-alone strategy relative to the TVS-alone strategy for women with HMB managed over multiple clinic appointments.
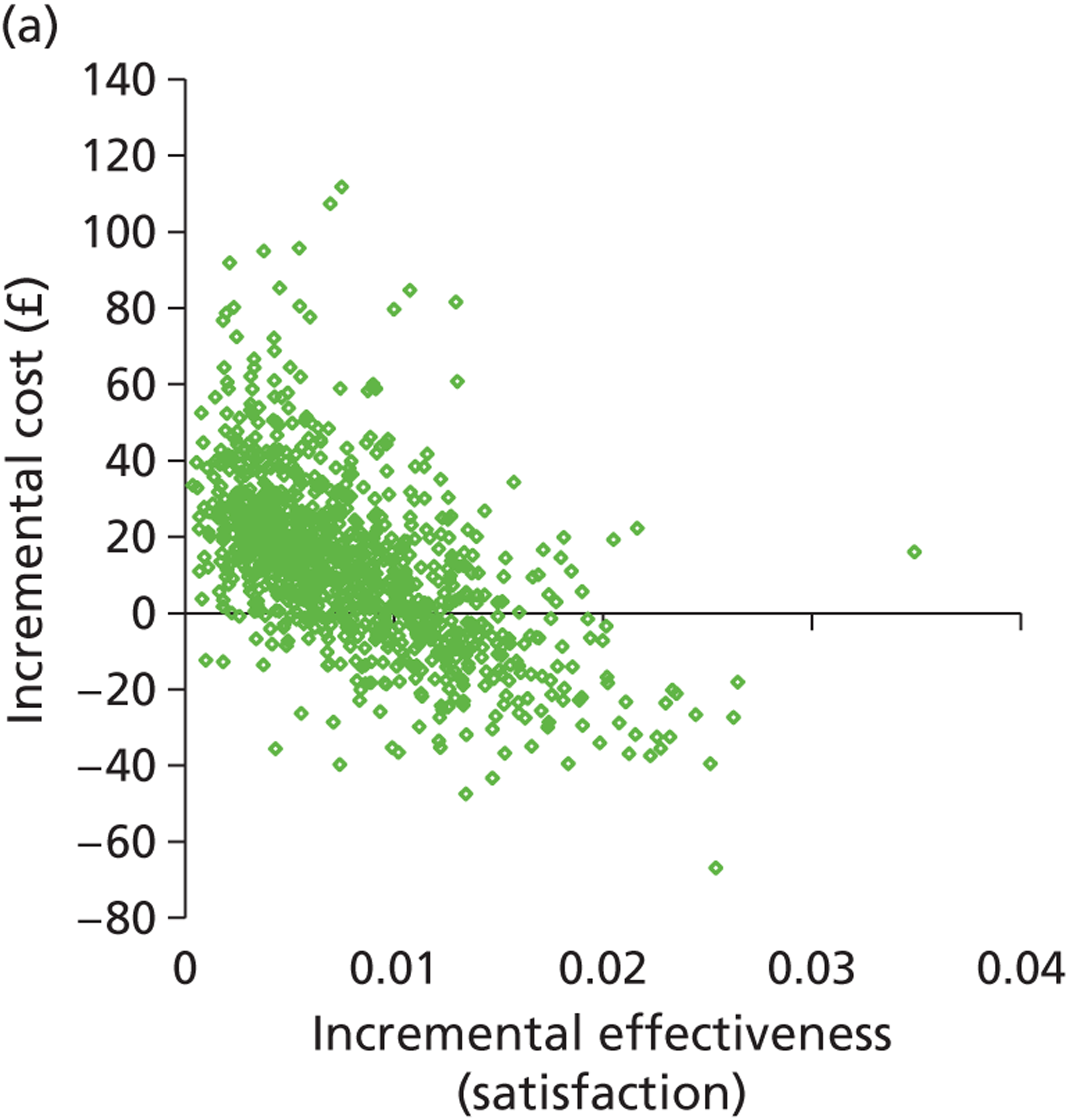
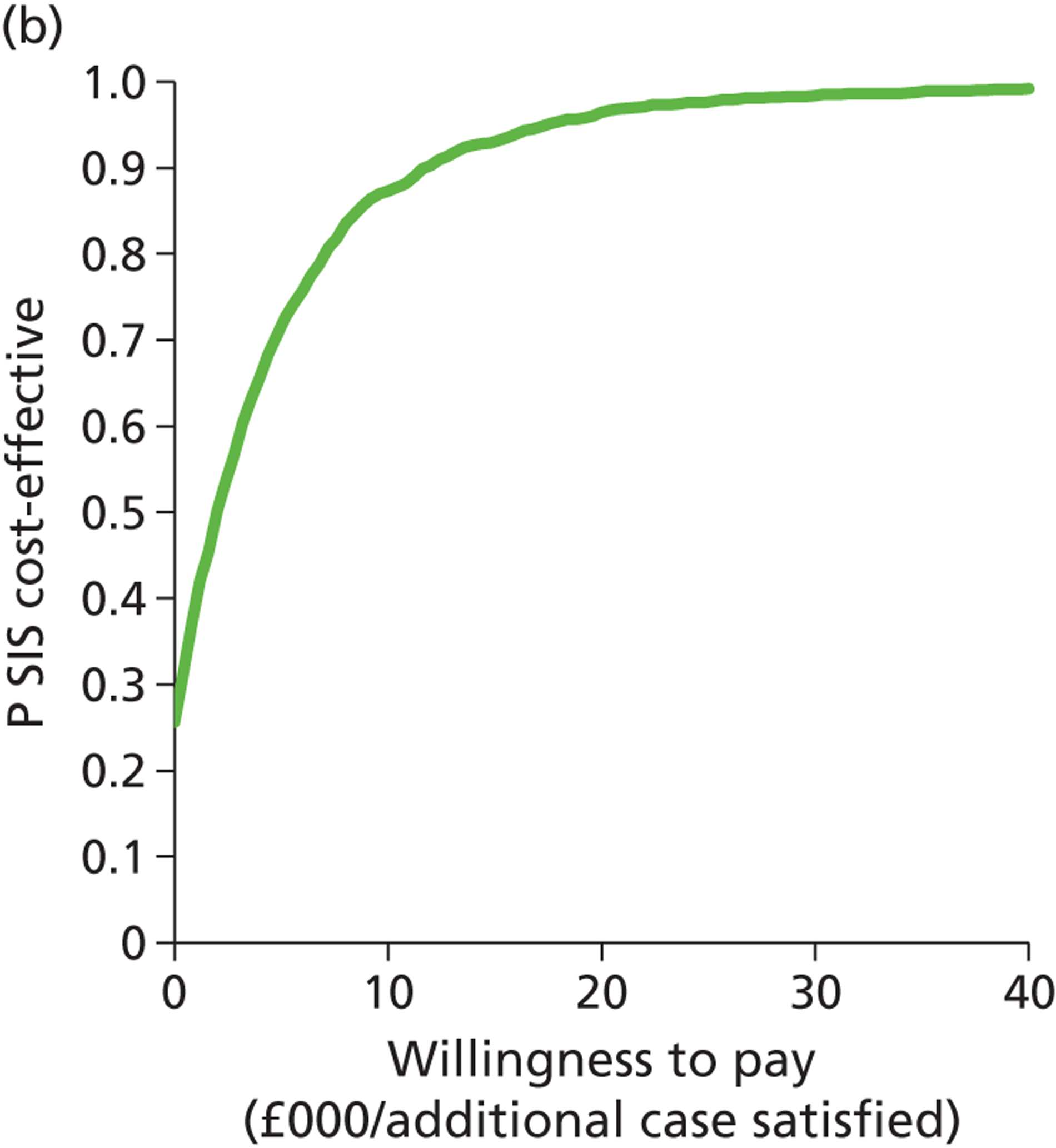
Saline infusion sonography and endometrial biopsy compared with saline infusion sonography: women with heavy menstrual bleeding managed over multiple clinic appointments
Figure 41a shows that SIS + EBx is more effective and more expensive than SIS alone. Figure 41b shows that SIS + EBx is unlikely to be cost-effective at WTP values acceptable to health service providers.
FIGURE 41.
Cost-effectiveness plane (a) and CEAC (b): SIS + EBx strategy relative to the SIS-alone strategy for women with HMB managed over multiple clinic appointments.
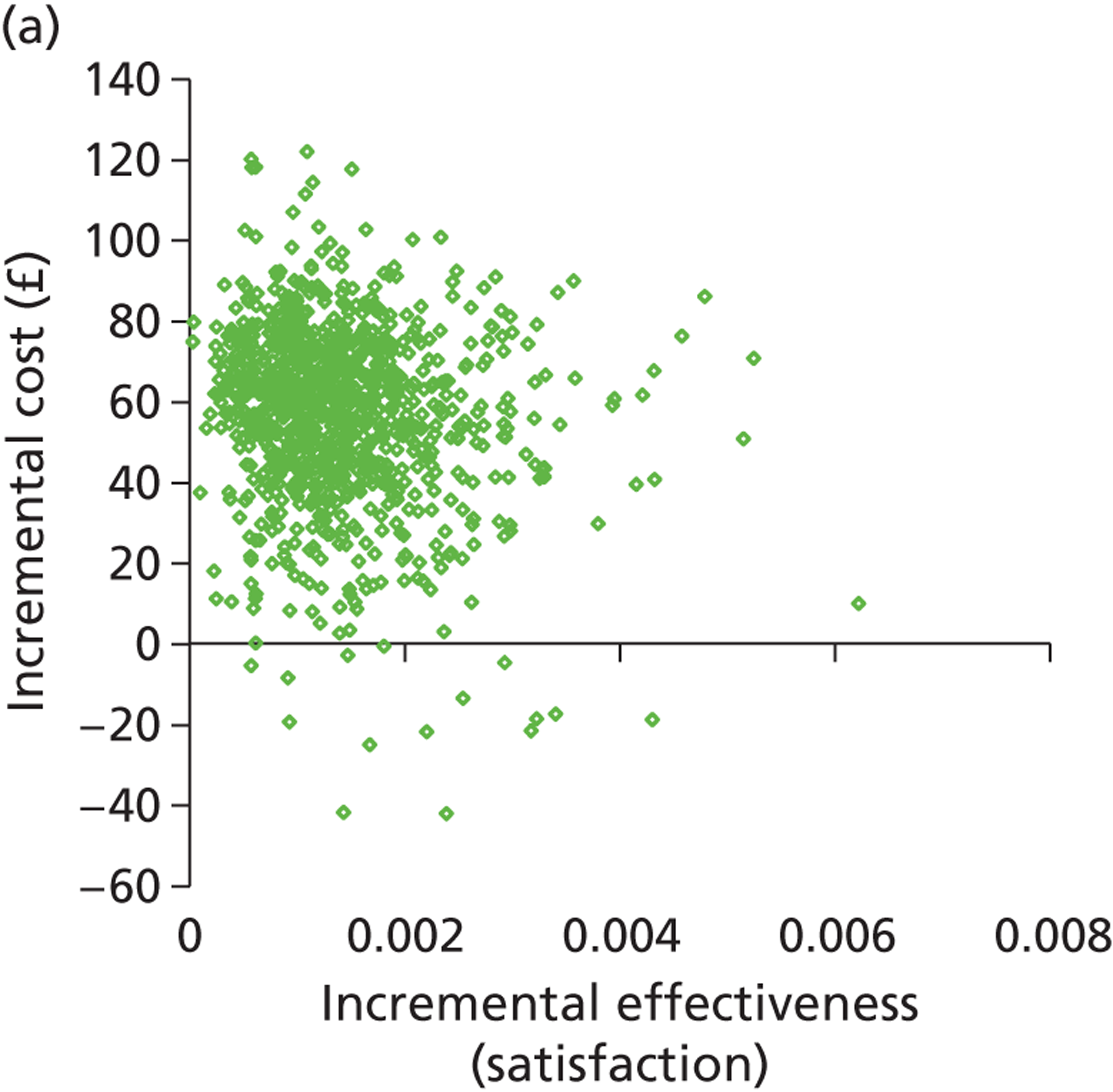
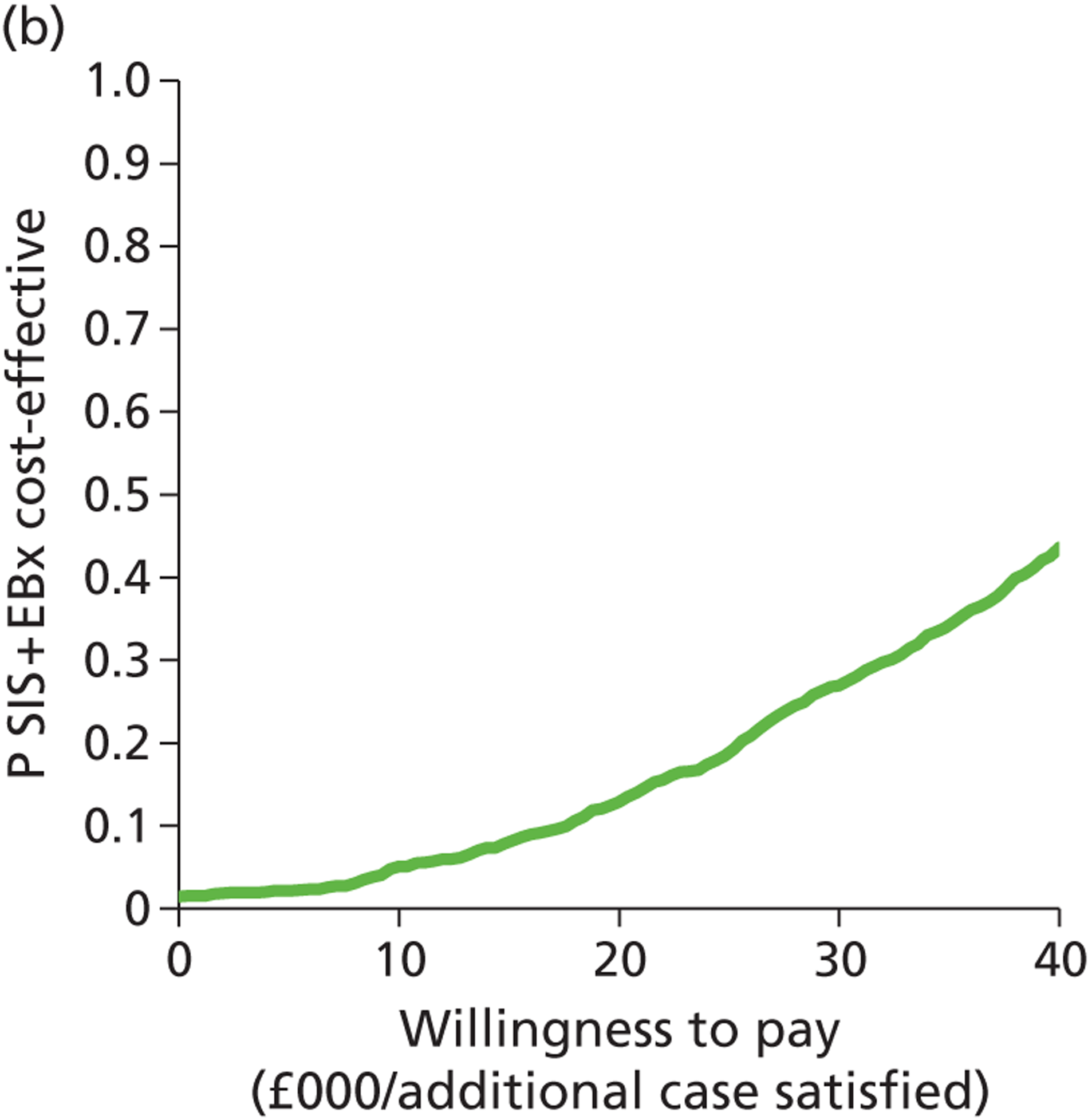
Outpatient hysteroscopy and endometrial biopsy compared with saline infusion sonography and endometrial biopsy: women with heavy menstrual bleeding managed over multiple clinic appointments
The cost-effectiveness plane in Figure 42a shows that OPH + EBx is more effective and more expensive than SIS + EBx. The CEAC (Figure 42b) suggests that above £15,000 OPH + EBx is likely to be the most cost-effective test and that as the WTP increases so does the certainty.
FIGURE 42.
Cost-effectiveness plane (a) and CEAC (b): OPH + EBx strategy relative to the SIS + EBx strategy for women with HMB managed over multiple clinic appointments.
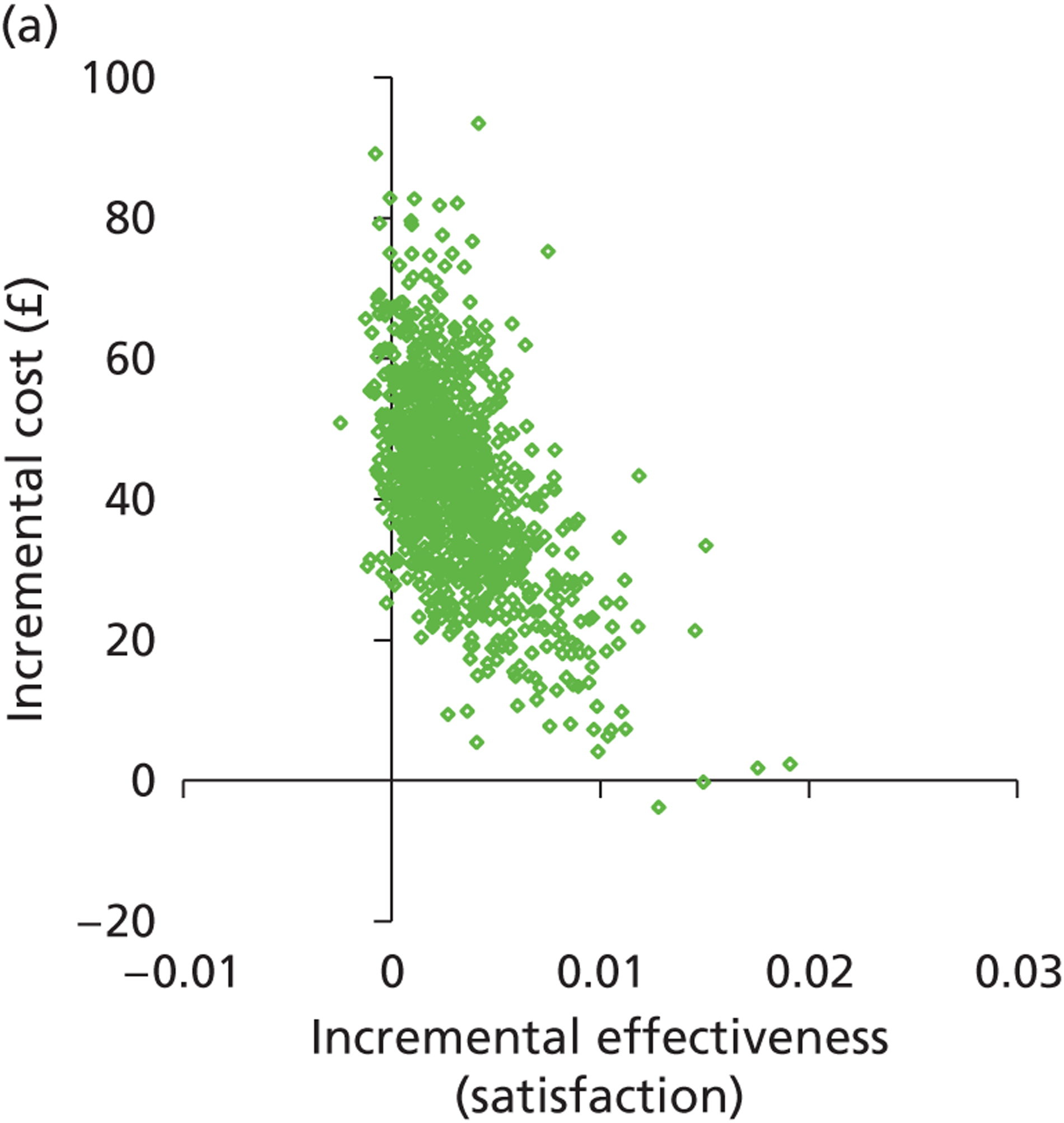
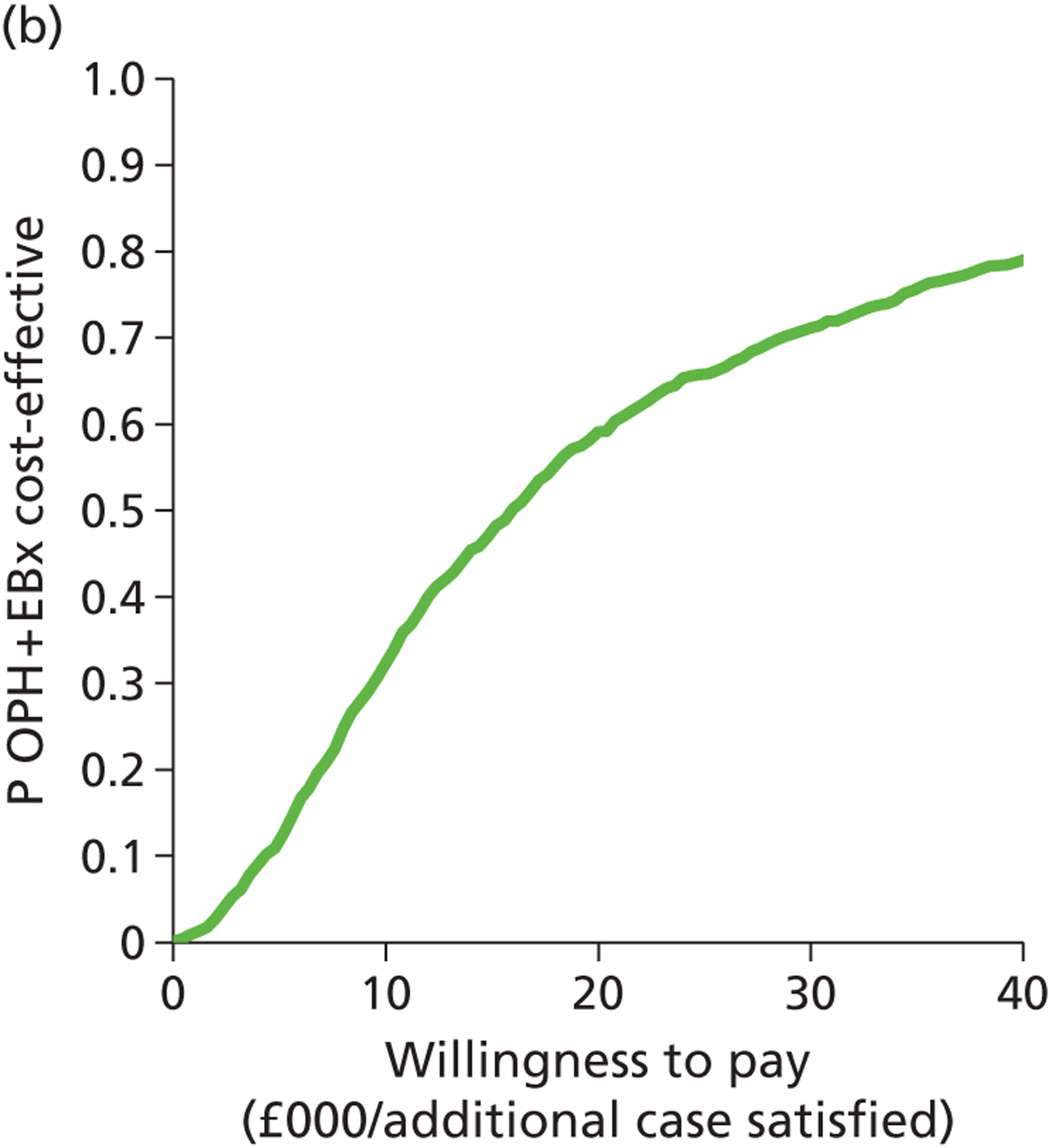
Outpatient hysteroscopy and endometrial biopsy compared with saline infusion sonography: women with heavy menstrual bleeding managed over multiple clinic appointments
The scatterplot below (Figure 43a) shows that OPH + EBx is probably more effective and more expensive than SIS. The CEAC (Figure 43b) shows that above a WTP threshold of £25,000 OPH + EBx is likely to be the most cost-effective strategy, although the probability of this is until only 0.7 at a WTP of £40.000.
FIGURE 43.
Cost-effectiveness plane (a) and CEAC (b): OPH + EBx strategy relative to the SIS-alone strategy for women with HMB managed over multiple clinic appointments.
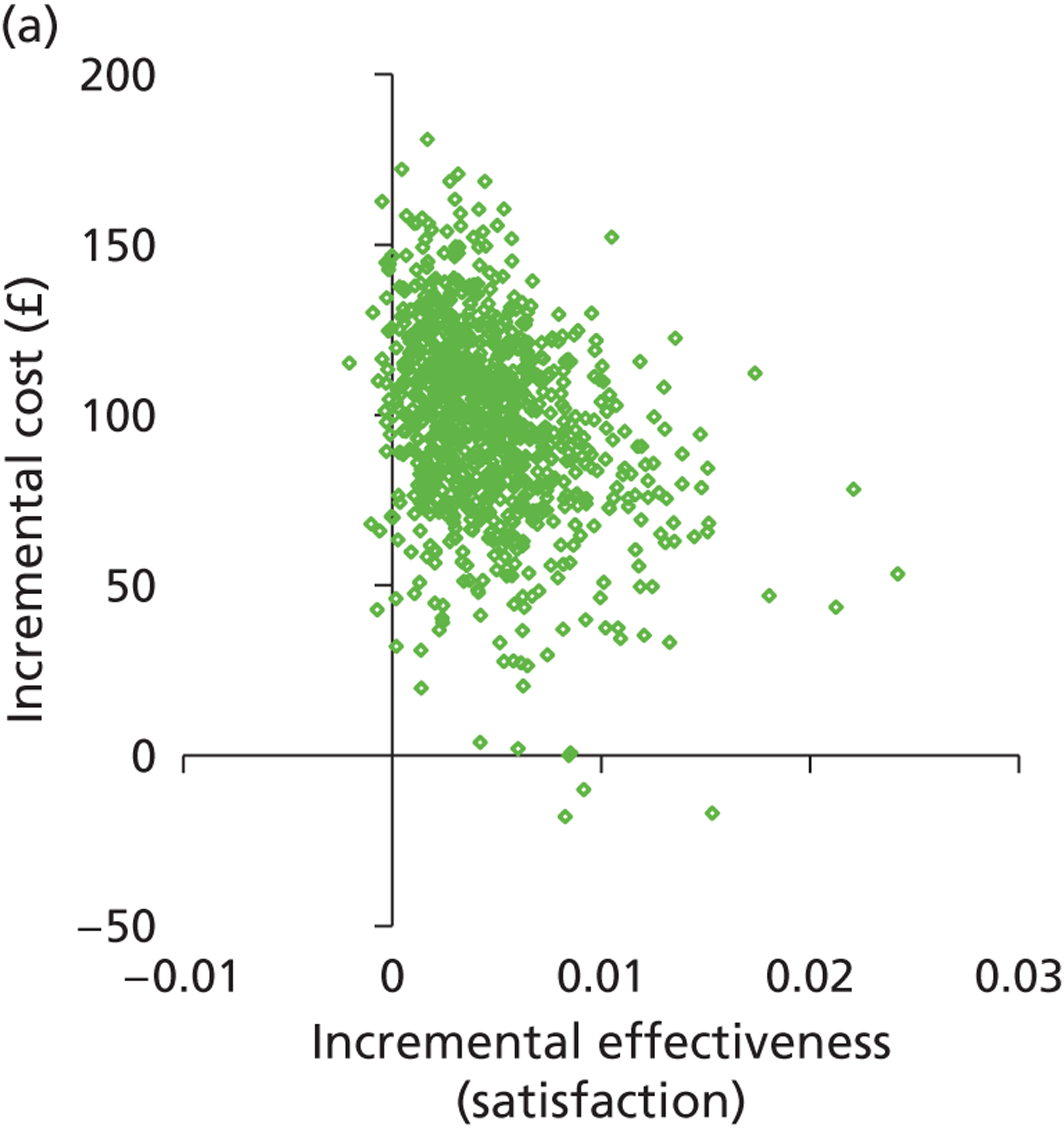
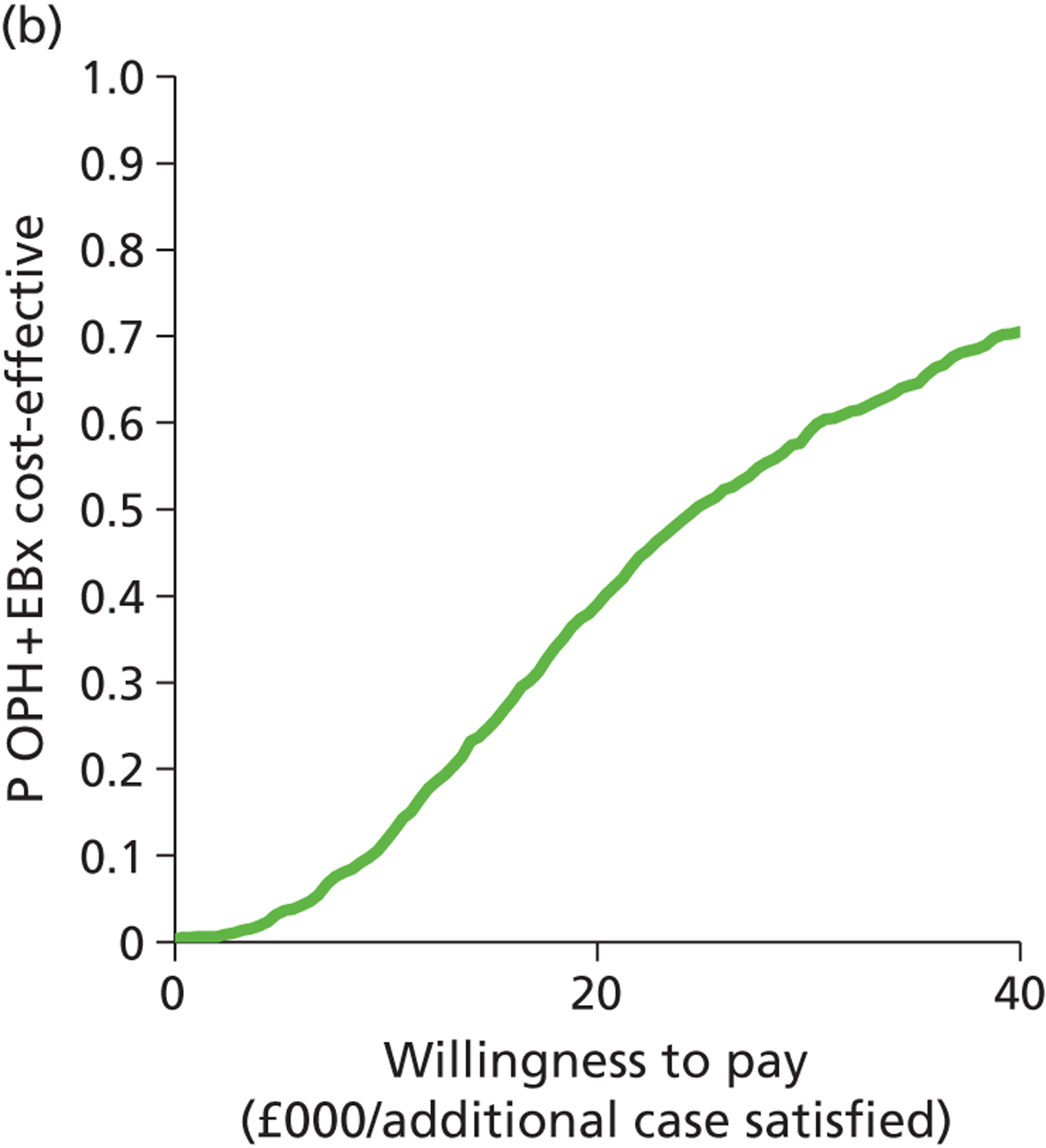
Appendix 6
Outpatient hysteroscopy compared with levonorgestrel intrauterine system: prior treatment with the levonorgestrel intrauterine system
The scatterplot below (Figure 44a) shows that OPH is almost certain to be more effective than LNG-IUS alone but it will be more costly per patient satisfied. The CEAC (Figure 44b) shows that above a WTP of £6000, OPH is probably a more cost-effective option than LNG-IUS alone per woman satisfied. At a WTP above £15,000 this is almost certain (p > 0.9).
FIGURE 44.
Cost-effectiveness plane (a) and CEAC (b): OPH-alone strategy relative to the LNG-IUS-alone strategy for women with HMB with a LNG-IUS in situ.
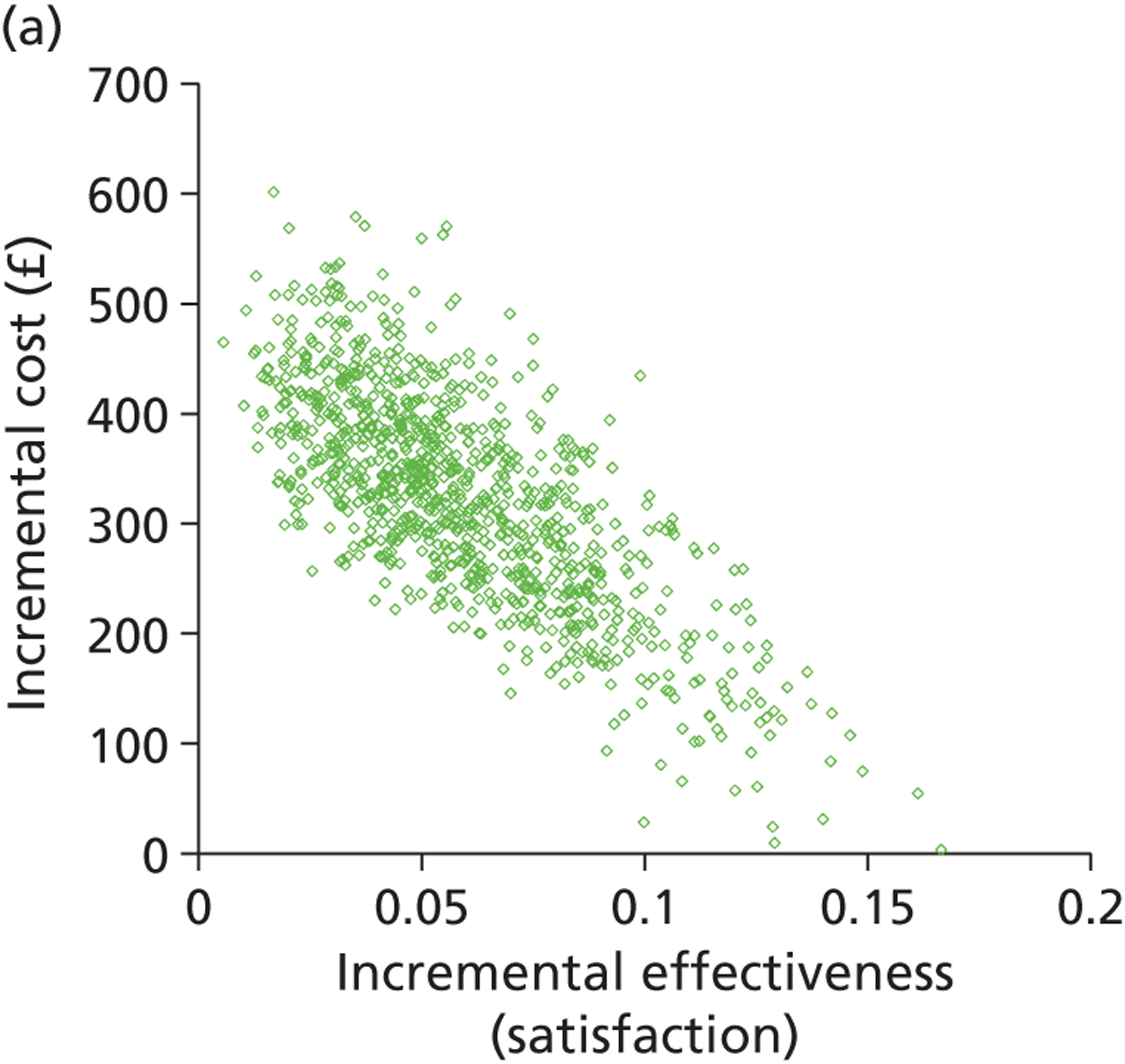
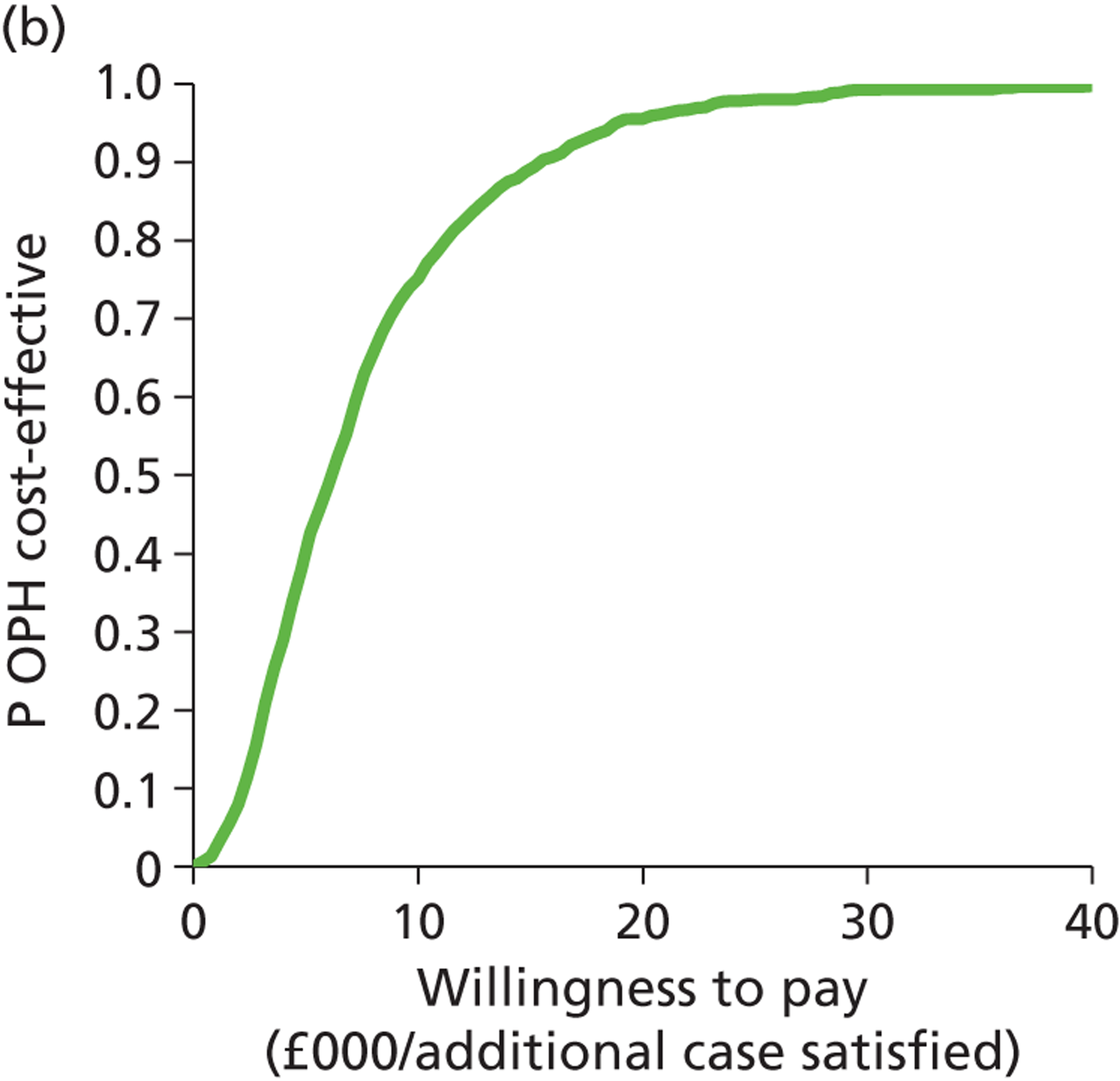
Transvaginal scan and endometrial biopsy compared with outpatient hysteroscopy: prior treatment with the levonorgestrel intrauterine system
The cost-effectiveness plane, Figure 45a, shows that there is considerable overlap between TVS + EBx and OPH alone. It certainly is not clear which is the most effective strategy but TVS + EBx is the more costly of the two. For all WTP values between £0 and £40,000 per extra woman satisfied, OPH alone is almost definitely the most cost-effective option when compared with TVS and EBx (p > 0.98), as displayed in the CEAC below (Figure 45b).
FIGURE 45.
Cost-effectiveness plane (a) and CEAC (b): TVS + EBx strategy relative to the OPH-alone strategy for women with HMB with a LNG-IUS in situ.
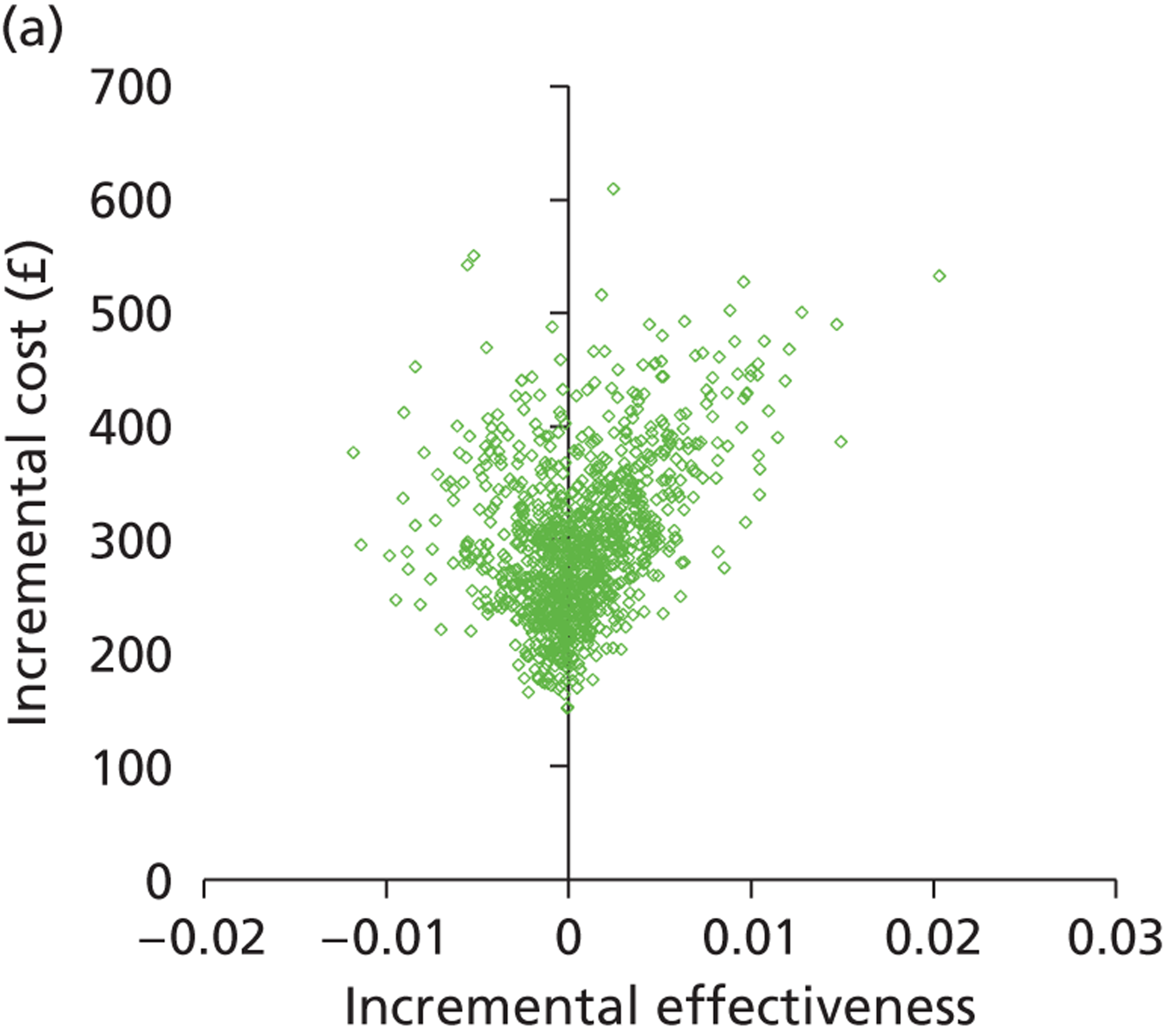
Saline infusion sonography compared with outpatient hysteroscopy: prior treatment with the levonorgestrel intrauterine system
Saline infusion sonography falls close to the line of non-dominance in the deterministic analysis; therefore, a comparison was made between SIS and the most cost-effective, non-dominated strategy: OPH alone. The graph, Figure 46a, shows that although there is likely to be little difference in effectiveness between the two strategies, SIS is almost certain to be the more costly strategy. The CEAC (Figure 46b) comparing SIS and OPH shows that SIS is extremely unlikely to be the most cost-effective option even at a WTP of £40,000 per additional woman satisfied.
FIGURE 46.
Cost-effectiveness plane (a) and CEAC (b): SIS-alone strategy relative to the OPH-alone strategy for women with HMB with a LNG-IUS in situ.
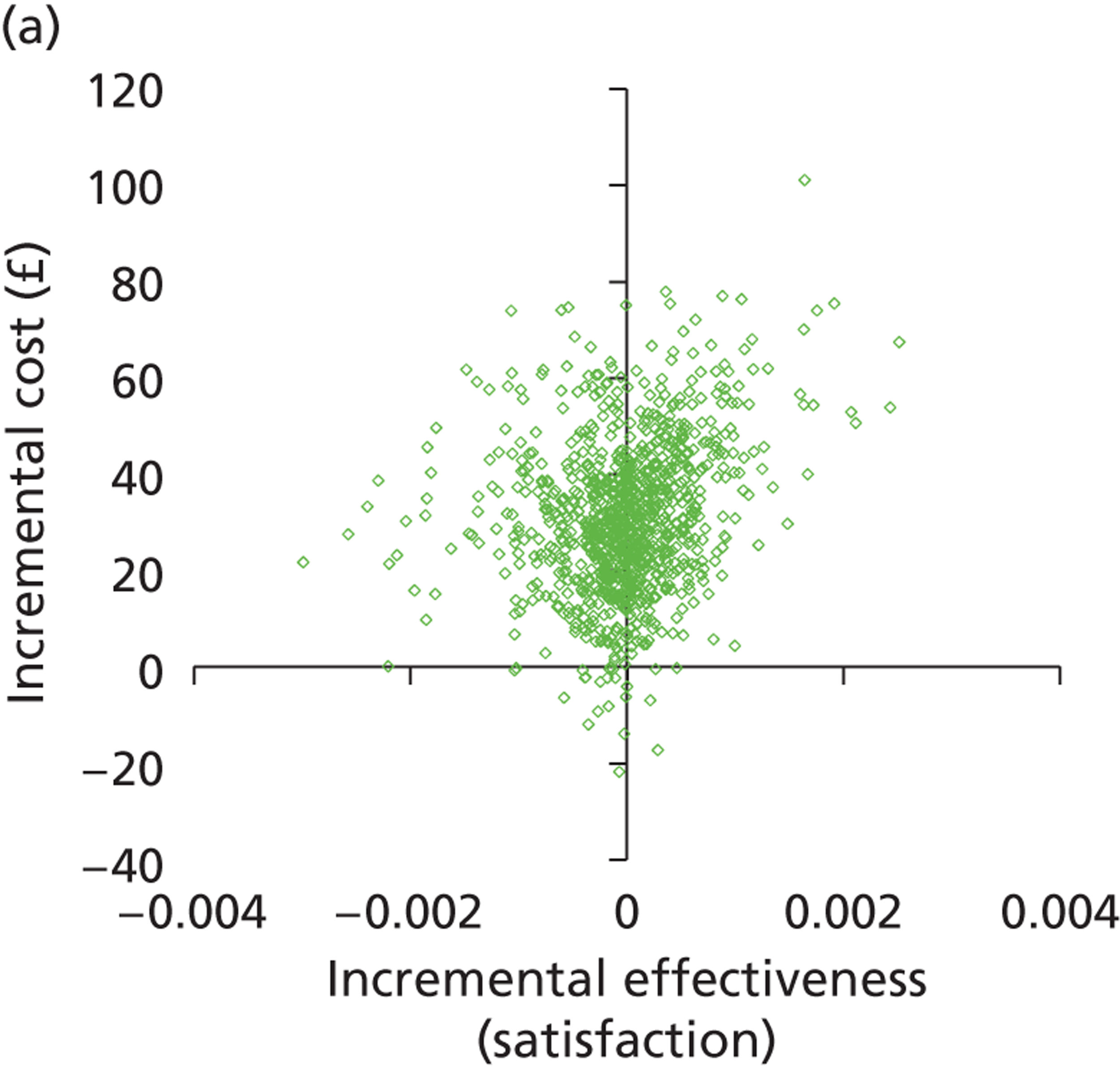
Appendix 7
Saline infusion sonography alone compared with levonorgestrel intrauterine system alone: women wishing to preserve their fertility
Figure 47a shows that SIS is definitely more effective and more costly than LNG-IUS alone. The CEAC (Figure 47b) shows that above a WTP of £2700, SIS is definitely (p = 1) a more cost-effective option than using LNG-IUS alone.
FIGURE 47.
Cost-effectiveness plane (a) and CEAC (b): SIS-alone strategy relative to the LNG-IUS-alone strategy for women wishing to preserve their fertility.
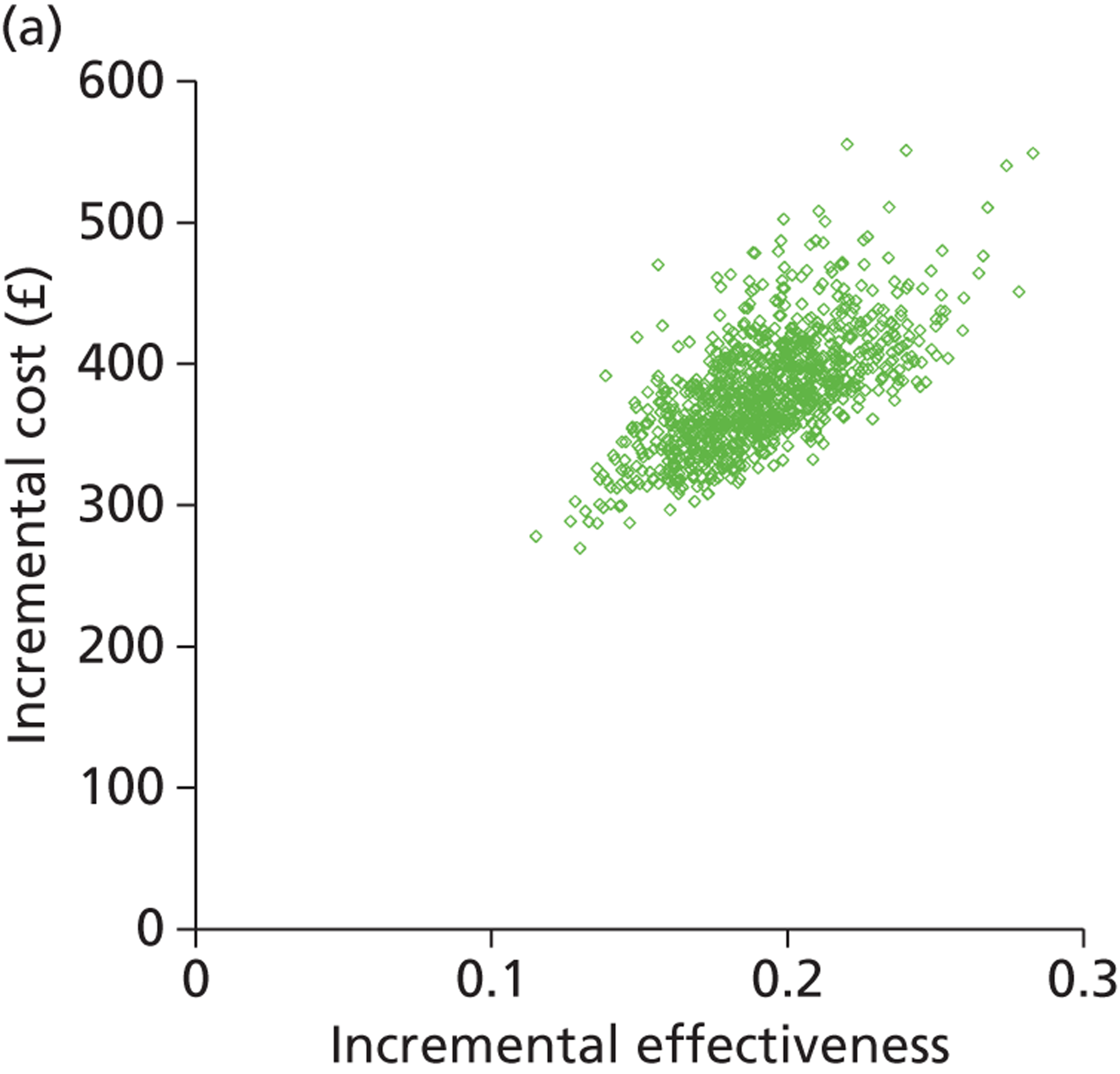
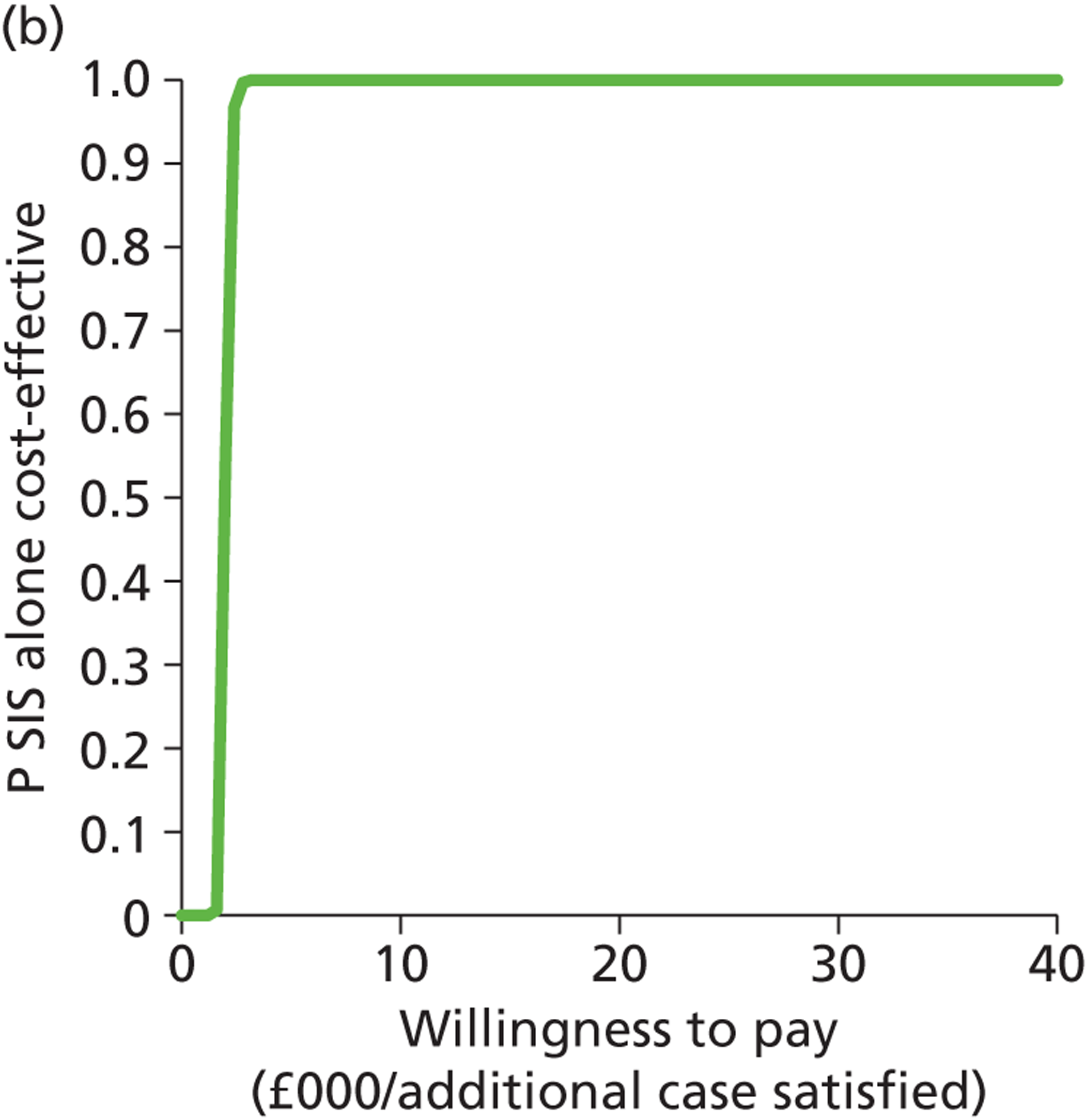
Outpatient hysteroscopy compared with saline infusion sonography: women wishing to preserve their fertility
Figure 48a shows that OPH is likely to be more effective and more expensive than SIS for investigating women with HMB who want to preserve their fertility. The CEAC (Figure 48b) shows that above a WTP of approximately £2500 to make one extra woman satisfied, OPH becomes likely to be the most cost-effective strategy (p > 0.5) and that at a WTP just above £5000, it has a > 90% chance of being the most cost-effective strategy when compared with SIS.
FIGURE 48.
Cost-effectiveness plane (a) and CEAC (b): OPH-alone strategy relative to the SIS-alone strategy for women wishing to preserve their fertility.
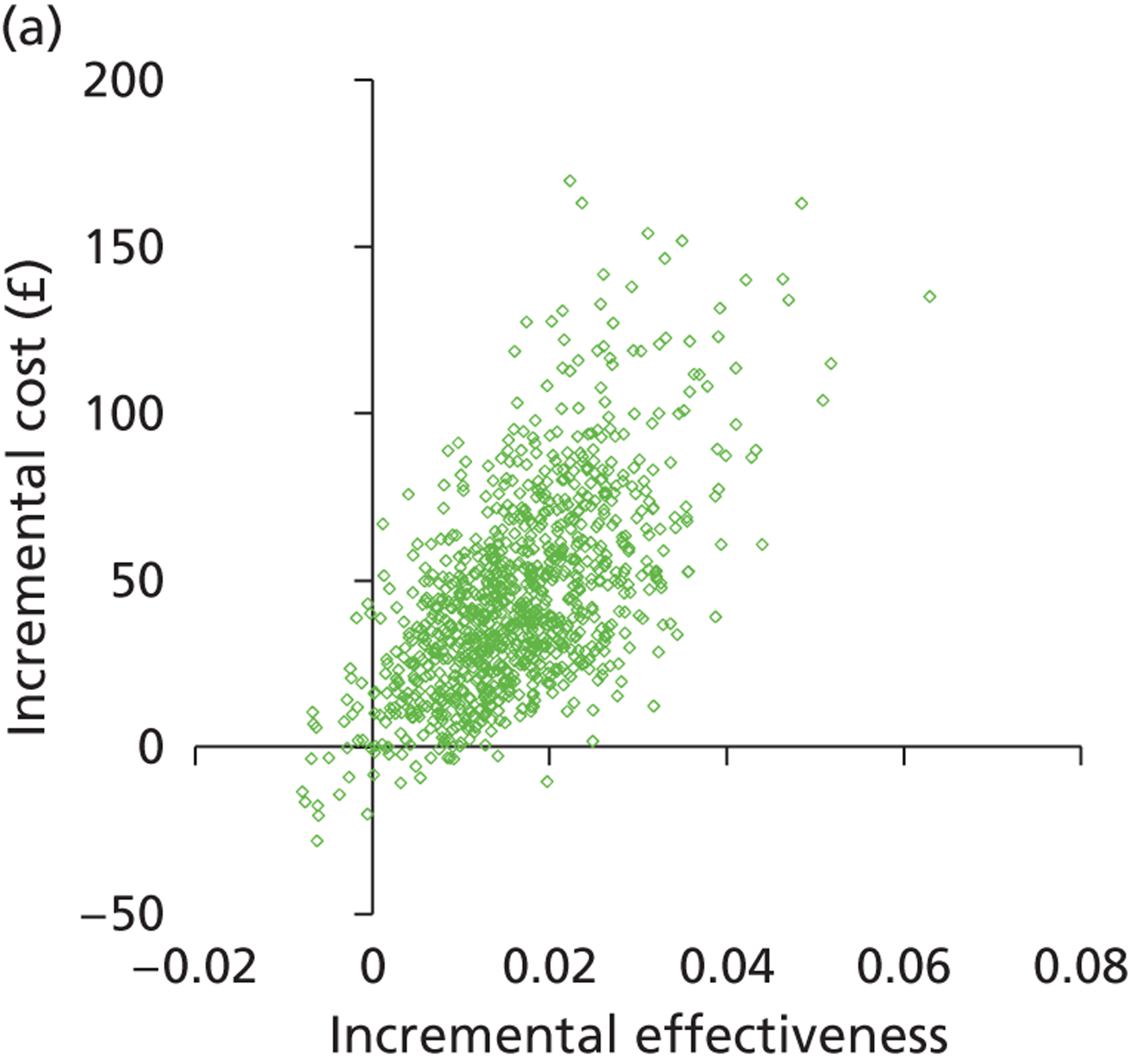
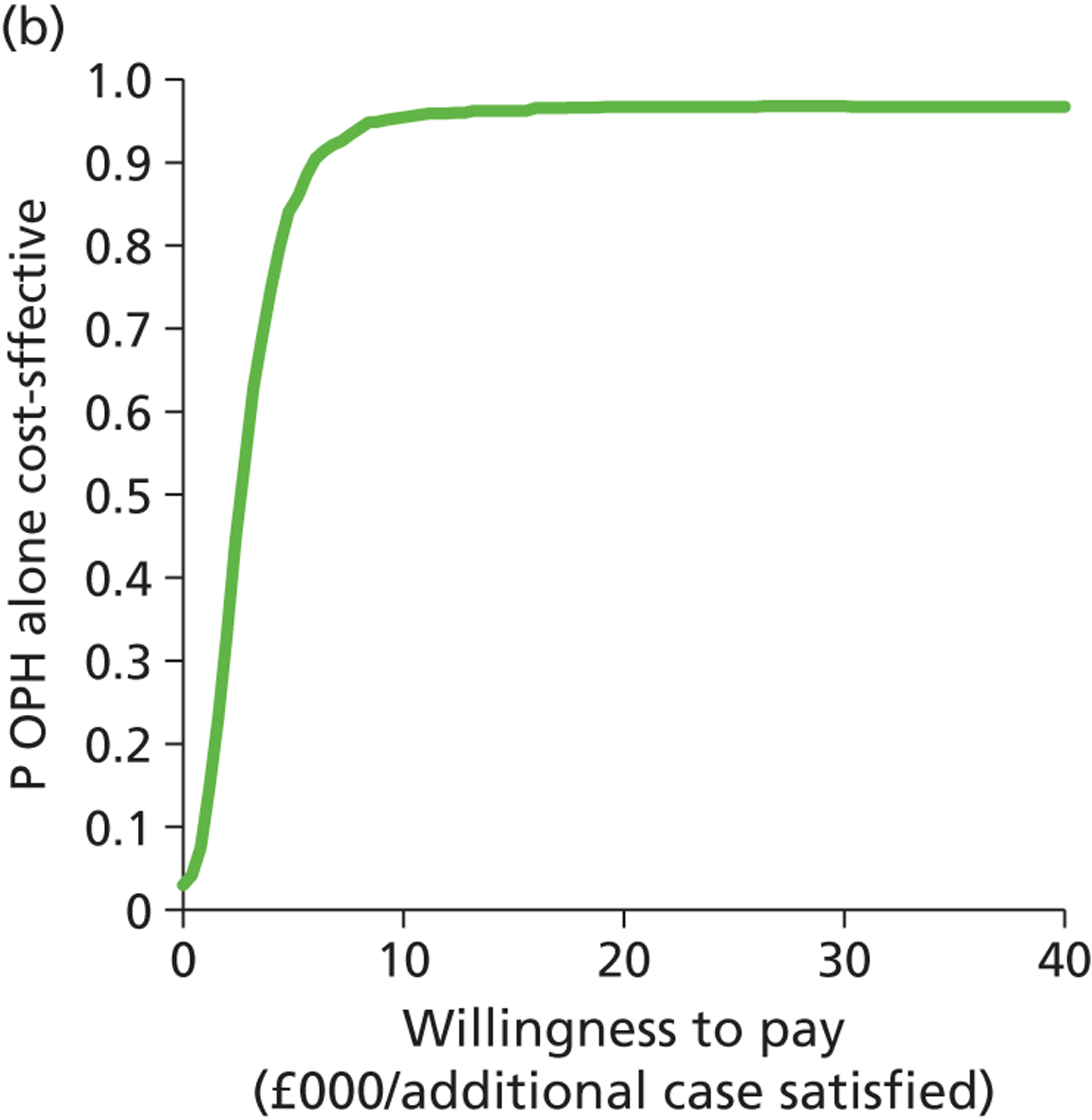
Saline infusion sonography and outpatient hysteroscopy compared with outpatient hysteroscopy: women wishing to preserve their fertility
Figure 49a shows the cost-effectiveness plane comparing OPH + SIS with OPH alone. It shows that SIS + OPH is more costly than OPH alone and that this extra cost is almost certainly between £100 and £120. The two-test combination is also probably, but not definitely, the more effective strategy. The CEAC (Figure 49b) shows the proportion of model replications for which SIS + OPH is preferred to OPH alone at any given WTP per additional case satisfied. It shows that even at a WTP of £40,000, there is considerable uncertainty that SIS + OPH is a more cost-effective strategy than OPH alone (p = 0.2).
FIGURE 49.
Cost-effectiveness plane (a) and CEAC (b): SIS + OPH strategy relative to the OPH-alone strategy for women wishing to preserve their fertility.
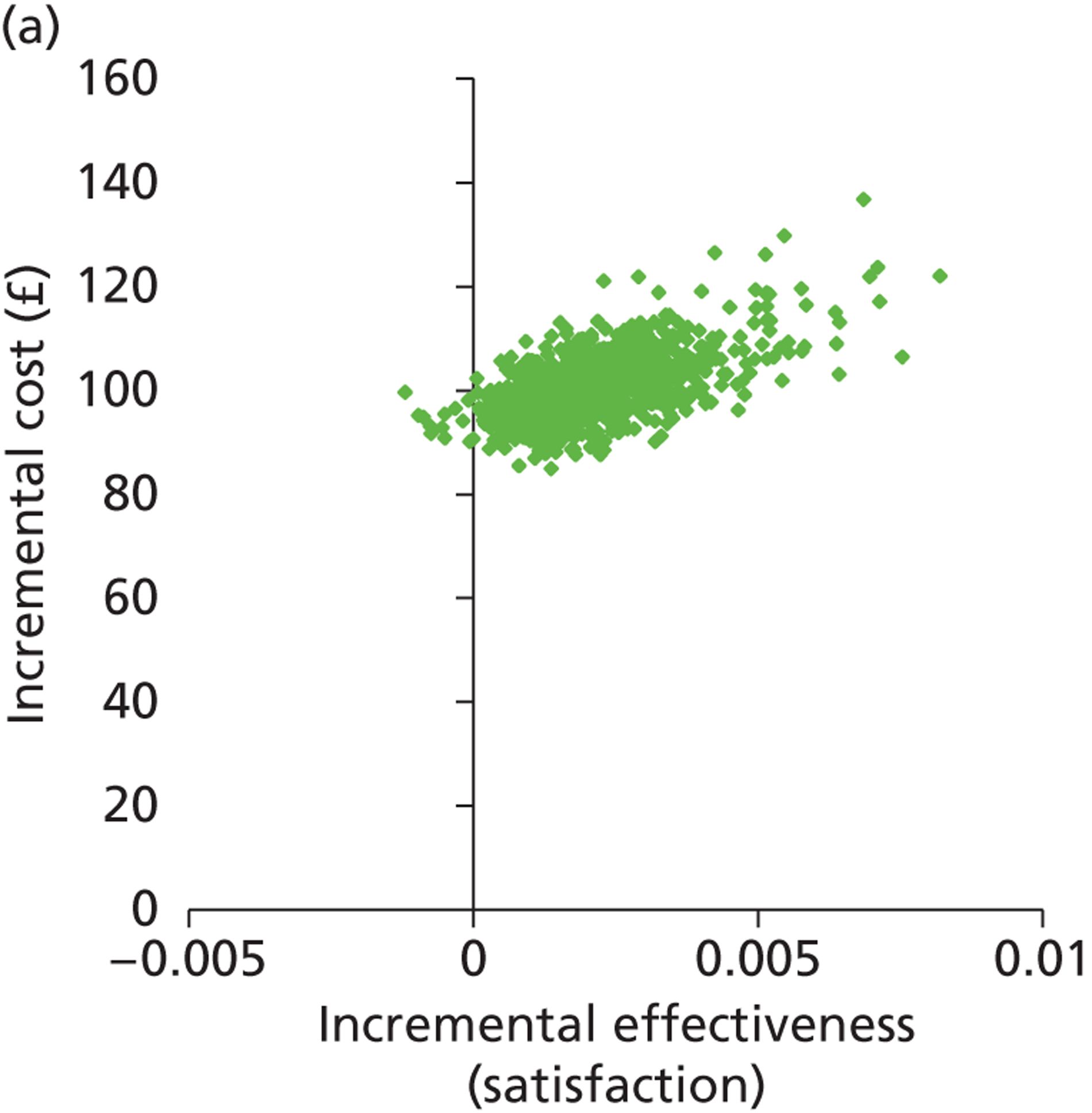
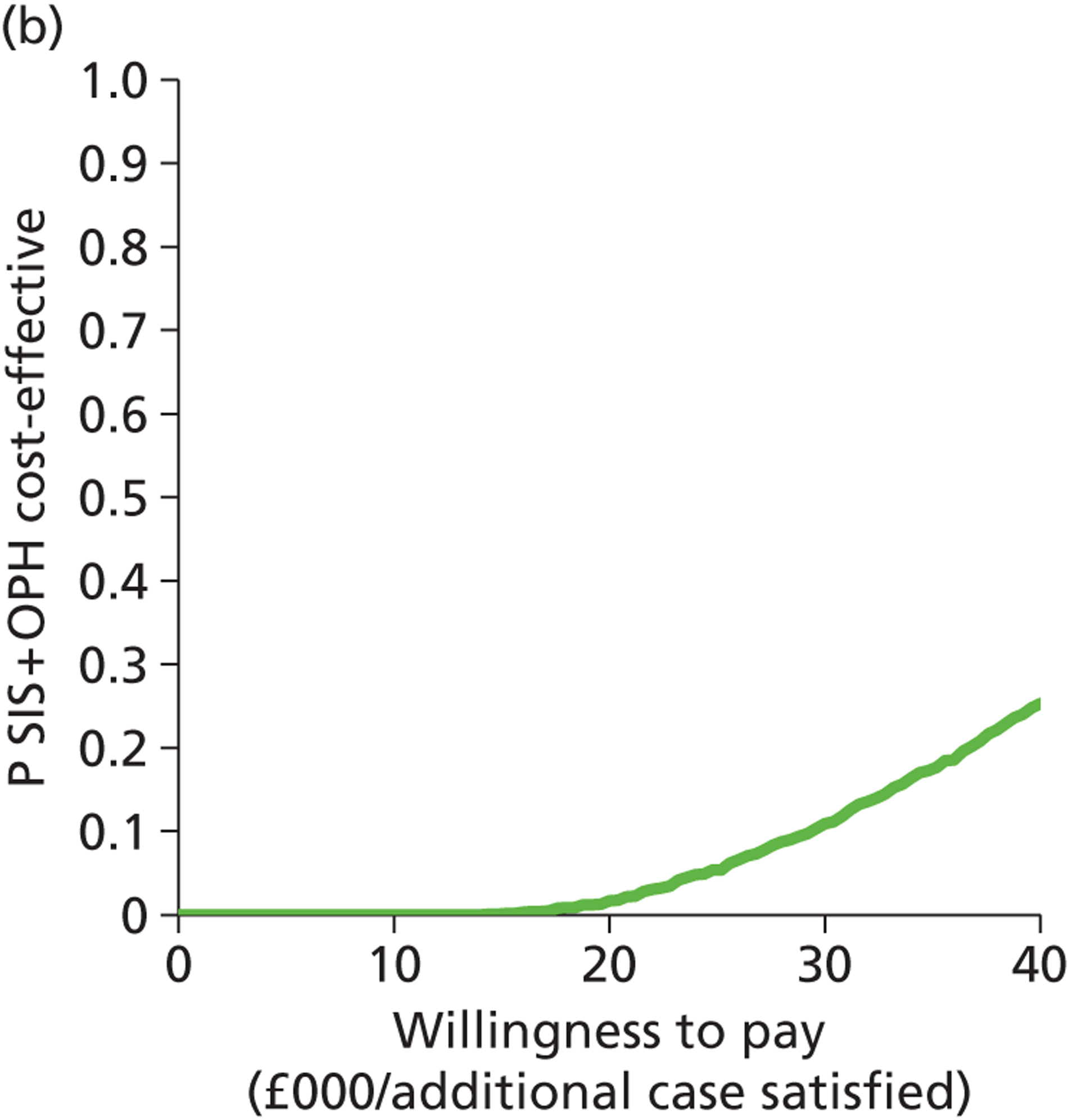
Transvaginal scan compared with levonorgestrel intrauterine system: women wishing to preserve their fertility
The graph (Figure 50a) shows the modelled uncertainty in the difference in costs between TVS alone and LNG-IUS. It shows that TVS is more effective and more costly than giving all women an LNG-IUS without investigation. The CEAC (Figure 50b) shows the proportion of model replications for which TVS is preferred to LNG-IUS alone at any given WTP per additional case satisfied. It shows that above a WTP of £3000, TVS is definitely the most cost-effective option (p = 1).
FIGURE 50.
Cost-effectiveness plane (a) and CEAC (b): TVS-alone strategy relative to the LNG-IUS-alone strategy for women wishing to preserve their fertility.
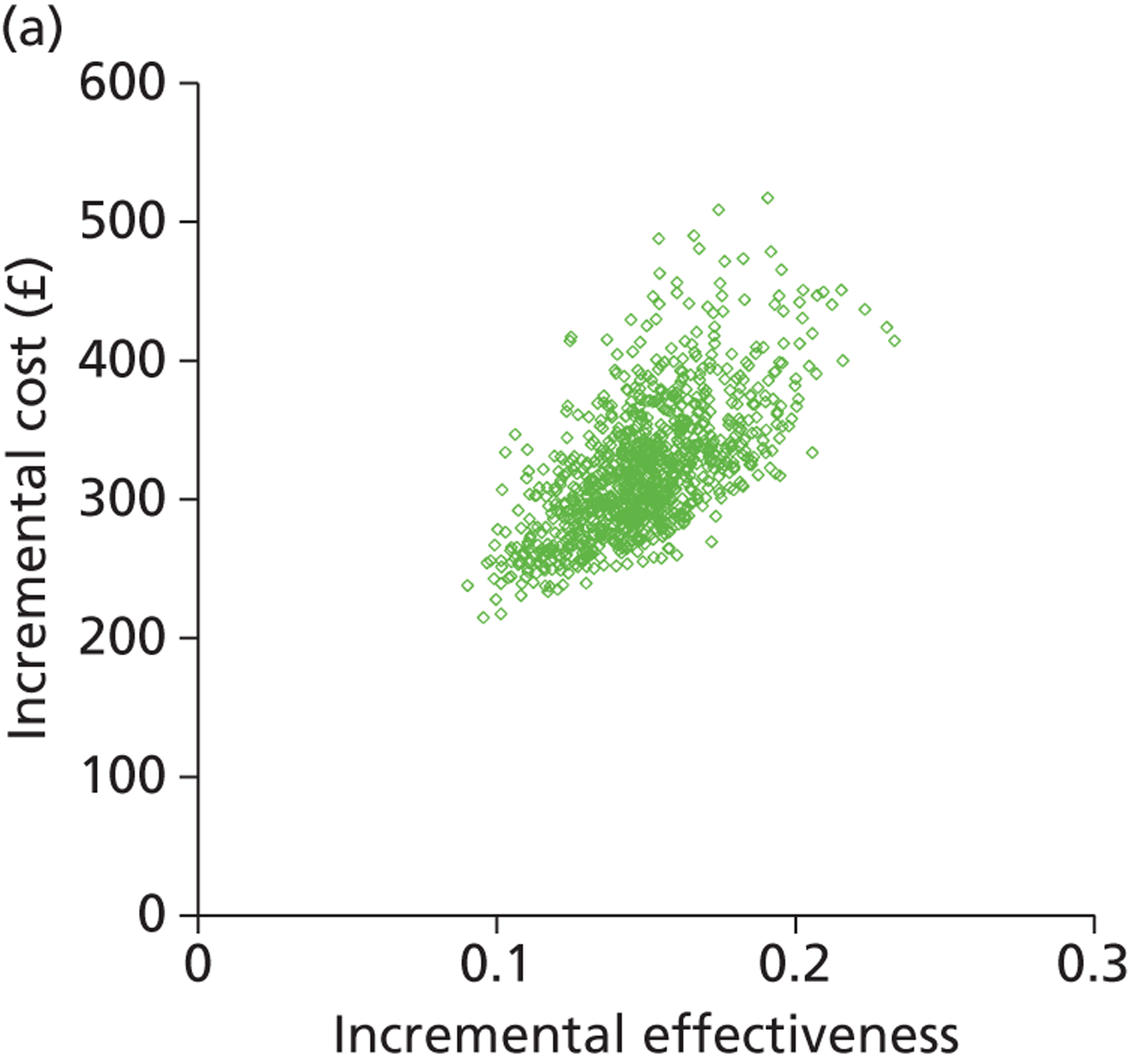
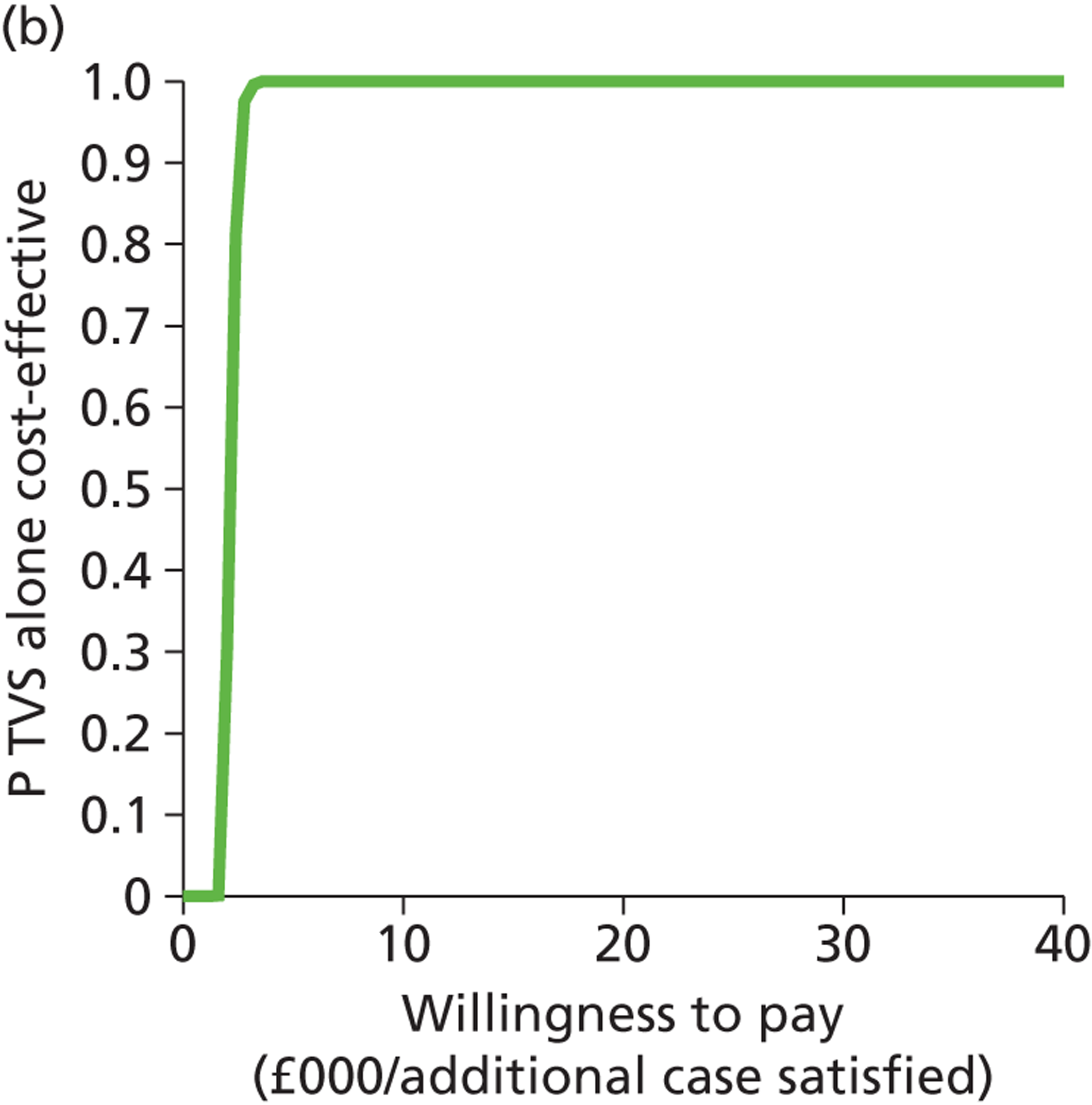
Saline infusion sonography compared with transvaginal scan: women wishing to preserve their fertility
The graph (Figure 51a) shows the modelled uncertainty in the difference in costs between SIS alone and TVS alone. It shows that SIS is probably more effective and more costly than TVS alone. The CEAC (Figure 51b) shows the proportion of model replications for which SIS is preferred to TVS alone at any given WTP per additional case satisfied. It shows that above a WTP of approximately £4000, SIS alone is definitely the most cost-effective option when compared with TVS.
FIGURE 51.
Cost-effectiveness plane (a) and CEAC (b): SIS-alone strategy relative to the TVS-alone strategy for women wishing to preserve their fertility.
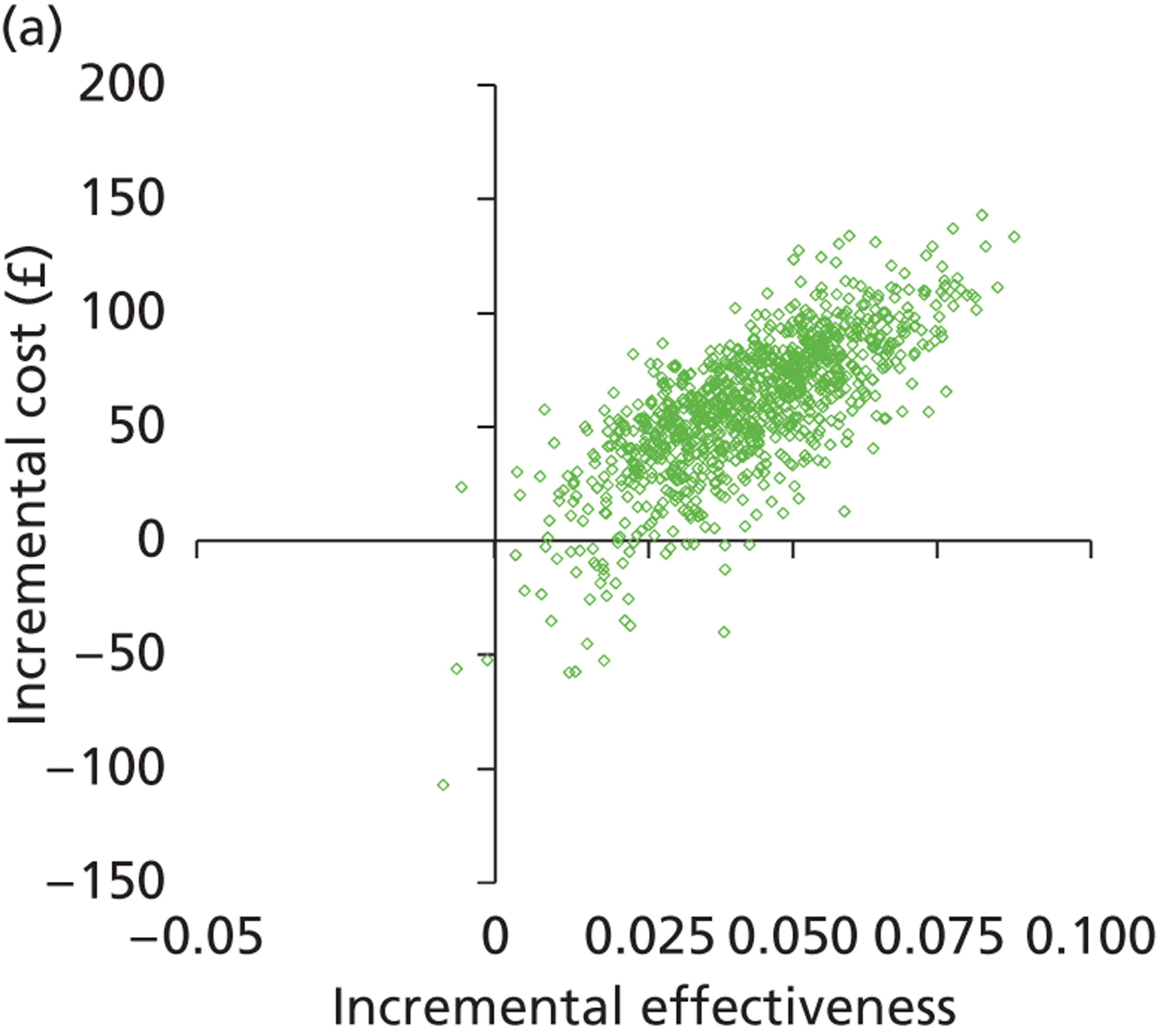
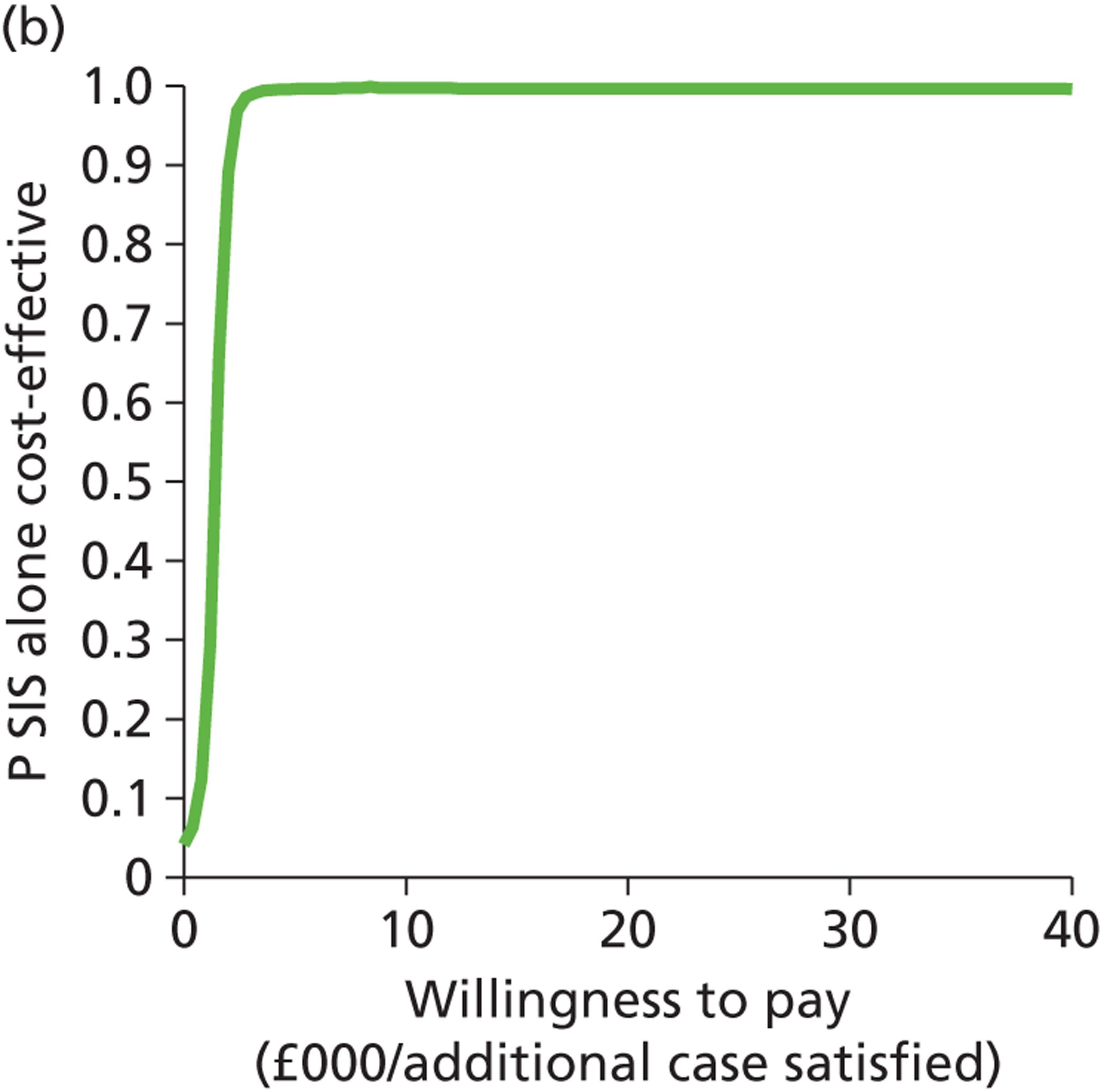
List of abbreviations
- AUB
- abnormal uterine bleeding
- BMI
- body mass index
- BNF
- British National Formulary
- BWH
- Birmingham Women’s Hospital
- CEAC
- cost-effectiveness acceptability curve
- CEAF
- cost-effectiveness acceptability frontier
- CI
- confidence interval
- D&C
- dilatation and curettage
- DUB
- dysfunctional uterine bleeding
- EA
- endometrial ablation
- EBx
- endometrial biopsy
- FIGO
- International Federation of Gynecology and Obstetrics
- FPR
- false-positive rate
- GnRH
- gonadotropin-releasing hormone
- GP
- general practitioner
- HMB
- heavy menstrual bleeding
- HRG
- Healthcare Resource Group
- HRQL
- health-related quality of life
- HRT
- hormone replacement therapy
- ICER
- incremental cost-effectiveness ratio
- IPD
- individual patient data
- LNG-IUS
- levonorgestrel-releasing intrauterine system
- MRI
- magnetic resonance imaging
- NICE
- National Institute for Health and Care Excellence
- OPH
- outpatient hysteroscopy
- PBAC
- pictorial blood loss assessment chart
- PMB
- post-menopausal bleeding
- PSA
- probabilistic sensitivity analysis
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- ROC
- receiver operating characteristic
- SIS
- saline infusion sonography
- SMF
- submucosal fibroid
- TAH
- total abdominal hysterectomy
- TCRF
- transcervical resection of submucous fibroids
- TPR
- true-positive rate
- TVS
- transvaginal scan
- UAE
- uterine artery embolisation
- WTP
- willingness to pay