Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 06/404/02. The contractual start date was in May 2008. The draft report began editorial review in March 2013 and was accepted for publication in July 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Chris Williams has been a past president of the British Association for Behavioural and Cognitive Psychotherapies (BABCP), a workshop leader and an author of various book and online self-help resources addressing depression. He is Director of Five Areas Ltd, which licenses cognitive behavioural therapy (CBT) self-help and training resources. Wilem Kuyken is co-founder of the Mood Disorders Centre, teaches nationally and internationally on CBT, and has co-authored a cognitive therapy book (Collaborative Case Conceptualization, published by Guilford Press). Anne Garland is clinical lead for the Nottingham Specialised Depression Service, principal investigator to the CLAHRC-NDL (Collaboration for Leadership in Applied Health Research and Care – Nottinghamshire, Derbyshire and Lincolnshire)-funded Depression Study, a past president of the BABCP, a CBT workshop leader, both nationally and internationally, and author of texts on depression.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Wiles et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Plain English summary
Many patients with depression who are prescribed antidepressants by their doctor do not get better after 6 weeks of treatment. Cognitive behavioural therapy (CBT) is a type of ‘talking therapy’ that has been shown to help patients with previously untreated depression but there is little evidence about its effectiveness as a ‘next-step’ treatment for those patients whose depression has not responded to medication. To answer this question we studied 469 patients with depression who had been taking antidepressants for at least 6 weeks and who had not got better. All continued with usual care from their general practitioner, including medication, but half (234) received CBT in addition. We followed up participants for 1 year and found that those who had CBT as well as usual care were approximately three times more likely to have fewer depressive symptoms than those in the usual-care group. The treatment was good value for money over the 12 months. Participants sometimes found therapy to be a challenging and difficult process, but felt that the techniques learned from CBT helped them better manage their depression. This study has provided high-quality evidence that receiving CBT, in addition to continuing on antidepressants as part of usual care, is an effective treatment for patients with depression who have not got better on medication alone.
Chapter 1 Introduction
Depression is ranked among the top five contributors to the global burden of disease, and by 2030 is predicted to be the leading cause of disability in high-income countries. 1 Antidepressants are often the first-line treatment for depression and the number of prescriptions for antidepressants has risen dramatically in recent years in the UK2 and elsewhere. 3,4 Over 46 million prescriptions were issued in England in 2011, at a cost of more than £270M. 5 However, the recent STAR*D (Sequenced Treatment Alternatives to Relieve Depression) study found that only one-third of patients responded fully to pharmacotherapy and that half did not experience at least a 50% reduction in depressive symptoms following 12–14 weeks of antidepressant medication. 6 The reasons for this non-response are complex but include (1) intolerance to medication; (2) treatment resistance (when an adequate dose and duration of treatment has been given) and (3) non-adherence to the treatment regime (both in terms of adherence to medication and failure to attend follow-up). Under-treatment of depression is also a recognised problem. 7 The high prevalence of depression means that effective interventions have the potential to substantially impact on the economic cost of this condition (to the NHS, patients and society). 8
Defining treatment resistance
It is important to separate treatment resistance from a lack of tolerance of medication. For the latter, the clinician’s ‘next step’ would be to switch the patient to a different antidepressant to find one that could be tolerated and determine the outcome for an adequate trial of such medication before seeking alternative treatment options based on the problem of resistance.
Many definitions of treatment resistance have been proposed. These definitions cover a broad spectrum ranging from failure to respond to at least 4 weeks of antidepressant medication given at an adequate dose9 to classification systems based on non-response to multiple courses of treatment. 10,11 Irrespective of the definition used, it is clear that treatment-resistant depression (TRD) has a considerable impact on individuals, health services and society.
In the 2004 depression guidelines,12 the National Institute for Health and Care Excellence (NICE) defined TRD as failure to respond ‘to two or more antidepressants given sequentially at an adequate dose for an adequate time’, but this could include individuals who have failed to respond to two different antidepressants that have the same pharmacological action. More recent guidance advocates that general practitioners (GPs) should reconsider treatment options if there has been little or no response after 4–6 weeks of antidepressant medication. 13 Options for the ‘next-step’ treatment include increasing the dose of medication, switching to a different antidepressant (either within or across pharmacological class) or augmentation with another pharmacological or psychological treatment. 13 However, there is currently little evidence to guide management after the initial 6 weeks of treatment. Hence for the research reported here we propose a more inclusive definition of TRD, directly relevant to UK primary care, given the uncertainty about what course of action to recommend to patients who have not responded to at least 6 weeks of antidepressant medication. These patients will be heterogeneous in terms of prior treatment, but all will have not responded to at least 6 weeks’ medication, thus ensuring trial results are generalisable.
Existing evidence on the management of treatment-resistant depression
The evidence from randomised controlled trials (RCTs) investigating pharmacological and psychological interventions for patients with TRD was summarised up to January 2001. 14 This systematic review included all studies in which patients with unipolar depression had not responded to at least 4 weeks of antidepressant medication at an adequate dose. Despite the broad inclusion criteria, the authors concluded that there was little evidence to guide the management of this patient group. Augmentation of pharmacotherapy was the most common treatment approach for such patients, but the RCTs were very small and of poor methodological quality and hence no precise treatment effects could be obtained. At that time, no RCTs had examined a psychological intervention for TRD. In December 2004, NICE published guidelines for the management of depression suggesting that ‘the combination of antidepressant medication with [face-to-face] cognitive behavioural therapy (CBT) should be considered’ for patients with TRD. 12 However, this report acknowledged ‘significant limitations to the current evidence base’ [grade B evidence (no RCTs)]12 and there are few data on what constitutes ‘usual care’ for this patient group.
A review on the evidence for the effectiveness of psychological interventions for adults with TRD is currently being prepared for publication in The Cochrane Library (www.thecochranelibrary.com). In short, despite 10 years having elapsed since the earlier publication by Stimpson et al. ,14 the evidence base for this area remains weak. Three small studies (n < 50) of CBT have been published. One was our pilot study (n = 25) for the present CoBalT (Cognitive behavioural Therapy as an adjunct to pharmacotherapy for primary care patients with treatment resistant depression) RCT. 15 The second (n = 44) examined the effect of augmentation with CBT among partial responders to antidepressants16 and the third study (n = 36), conducted among inpatients, was published only as a conference abstract. 17 Neither of the last two studies included a group who remained on antidepressants in order to evaluate the effectiveness of CBT as an adjunct to antidepressant medication (comparators: lithium augmentation16 and augmentation with supportive therapy17).
The REVAMP (Research Evaluating the Value of Augmenting Medication with Psychotherapy) trial18 recruited patients with chronic depression (defined as persistent depressive symptoms for > 2 years), who had not responded or only partially responded to 12 weeks of antidepressant medication. However, they found no difference in depression outcomes (symptoms, ‘response’ or ‘remission’) between those who switched to the next step in a medication algorithm and those who received psychotherapy [cognitive behavioural analysis system of psychotherapy (CBASP) or brief supportive psychotherapy (BSP)], and no difference in outcomes between the two different psychotherapies (CBASP vs. BSP). 18
The large US STAR*D study recruited 2876 patients with depression, and invited those who failed to respond to up to 14 weeks’ treatment with citalopram (Celexa®, Forest Laboratories Inc.) to take part in a RCT of various treatment options, including switching or augmenting with either antidepressants or CBT. 19–21 However, the STAR*D RCT included patients who could not tolerate citalopram, as well as those who were treatment resistant. This makes it difficult to apply the findings to clinical practice because, in practice, the clinician would try to find an antidepressant that the patient could tolerate and prescribe this for an appropriate interval at an adequate dose before deciding that the patient was treatment resistant and seeking alternative treatment options. However, more importantly, in STAR*D there was no comparison group of patients who continued on citalopram, so the effect of augmenting antidepressant medication with CBT as a ‘next-step’ treatment option cannot be ascertained from the STAR*D RCT. Given frequent patient preference for ‘talking therapy’,22 the lack of evidence of effectiveness of psychological interventions in this patient group is an important deficit. Furthermore, it is important that such trials are conducted in the UK, as data from other countries may not generalise to UK primary care owing to differences in health services and the clinical characteristics of patients presenting therein. Moreover, the UK needs information on cost-effectiveness that can be applied to the NHS. While it has become more commonplace for an economic evaluation to run alongside a RCT, this has not been the case historically. There are currently few data on the cost-effectiveness of CBT interventions. Those that exist relate to CBT for relapse prevention23 or CBT delivered in ‘real time’ over the internet using an instant messaging service. 24 Other reports on cost-effectiveness of CBT, for example, Durham et al. ,25 are confined to different patient populations (anxiety/psychosis) and varied CBT interventions (including low-intensity interventions comprising, on average, four sessions with a contact time of just 2.6 hours) and cannot be generalised more widely.
Ongoing studies
There are two small studies of CBT for TRD that are registered as ongoing on the clinical trials registers [‘www.controlled-trials.com’ and ‘www.clinicaltrials.gov’, searched 21 February 2013 (search terms: CBT/cognitive therapy and depression)]. The first, a UK-based study, aimed to recruit 24 patients from secondary care to determine the effectiveness of CBT compared with usual care for TRD (ISRCTN53305823). The study commenced in 2006 but was not completed owing to unexpected staffing changes (Stephen Barton, Newcastle University, 14 March 2012, personal communication). In addition, a US pilot study (n = 30) aimed to examine the effectiveness of CBT and desipramine compared with medication alone in outpatients with TRD (NCT00000376: anticipated start/end dates: 03/1996–02/1999). Although the anticipated end date of the trial was given as February 1999, no results have been published to date.
In addition, there are a number of ongoing studies of other psychological interventions for patients with TRD. For example, the Tavistock Adult Depression Study is examining the effectiveness of 60 sessions of weekly psychoanalytic psychotherapy compared with usual care in 90 patients with TRD (www.tavistockandportman.nhs.uk/adultdepressionstudy, ISRCTN40586372). The REFRAMED trial (www.reframed.org.uk, anticipated start/end dates: 1 January 2012 to 1 September 2014; ISRCTN85784627) is evaluating the effectiveness of Dialectical Behaviour Therapy (DBT) for TRD. A Canadian study (NCT01141426), currently in set-up, will evaluate the effectiveness of intensive short-term dynamic psychotherapy (ISTDP) compared with usual care in patients with an inadequate response to at least 6 weeks’ antidepressant medication. Others are recruiting to a RCT to examine the clinical effectiveness and cost-effectiveness of a specialist expert mood disorder team for refractory unipolar depressive disorder (NCT01047124). 26 CBT will be provided as part of the treatment offered by this specialist team to secondary care patients. Finally, the PATH-D study (NCT01021254) will examine the effectiveness of mindfulness-based cognitive therapy (MBCT) compared with a health-enhancement programme for patients with TRD.
Cognitive behavioural therapy for treatment-resistant depression
Many patients with depression express a preference for ‘talking therapies’,22 and some have estimated a need for 250 psychological treatment centres. 8 In England,27,28 and elsewhere,29,30 there have been initiatives to improve access to psychological therapies. CBT is the most widely available structured psychotherapy for depression in specialist mental health services in the NHS, and has been shown to be more effective than other psychotherapies in improving outcome in depression. 31 Nonetheless, although CBT is an effective treatment for previously untreated episodes of depression,31 this evidence is not specific to patients with TRD. Yet, in practice in the NHS, CBT is often reserved for those who have not responded to pharmacotherapy in primary care (i.e. those who are treatment resistant). The roll-out of the Improving Access to Psychological Therapies (IAPT) project27,28,32,33 means that it is important (and timely) to examine the effectiveness of CBT for patients with TRD.
Cognitive behavioural therapy has been proposed as an adjunct to pharmacotherapy in patients with TRD. Although there is currently no evidence for the effectiveness of CBT in this group, there are indications that psychological treatments may be effective. In patients with residual depressive symptoms, who were randomised to receive CBT (16 sessions over 20 weeks), the relapse rate was significantly reduced (after 68 weeks) compared with usual care. 34 This benefit was lost fully only 3–4 years after the end of treatment. 35 There is also an increasing literature on the use of combination therapy for depression, which suggests that there is a small benefit of combination therapy compared with medication alone in reducing depressive symptoms,36,37 although the evidence base for patients with TRD is lacking. Furthermore, others have noted some benefit of combined treatment for those with chronic depression (defined as at least 2 years’ duration). For example, Keller et al. 38 found that the combination of nefazodone (now withdrawn) and psychotherapy was more effective than either component alone in reducing depressive symptoms after 12 weeks.
From clinical practice it is known that not all patients who are offered psychotherapy will complete the course. Although no figures specific to this patient group and intervention are available, it has been estimated that around 47% of individuals do not complete a course of psychotherapy. 39 Quantitative studies have suggested that non-completion is more common among those of lower socioeconomic background, but there are few other consistent predictors. 39 Importantly, as there has been little qualitative research in this area, there is only limited understanding of why patients adhere to psychotherapy or not, and, likewise, for the rationale behind their decisions. Researchers have used qualitative methods to explore people’s experiences of CBT40,41 and their work shows the value of listening to patients, particularly in relation to better understanding patients’ views on both the therapeutic process and outcomes of therapy. To our knowledge, to date no one has focused specifically on the views and experiences of patients with TRD who have received CBT.
Qualitative research methods are increasingly being used within RCTs to investigate patients’ experiences of participation and trial processes (e.g. how interventions are implemented and delivered42,43) and the UK Medical Research Council (MRC) recommends that both qualitative and quantitative methods are used to evaluate complex interventions. 44 Data collected during in-depth interviews or focus groups with trial participants can provide detailed insight into their experiences of the trial and intervention, and the extent to which they view a particular treatment as acceptable and effective. Such data can also illuminate possible reasons for the quantitative findings and give another viewpoint from which to evaluate the treatment being delivered.
Summary of rationale for randomised controlled trial
In summary, given the frequently expressed patient preference for talking therapies, the recent initiatives to widen access to ‘talking therapies’27–30 and the paucity of evidence for interventions for patients with TRD, there is clearly a need for a large-scale pragmatic RCT to examine the effectiveness and cost-effectiveness of CBT as an adjunct to pharmacotherapy, as a ‘next-step’ option for patients who have not responded to antidepressant medication. As indicated earlier, there are reasons to believe that a CBT intervention may be effective and such a trial would make a major contribution to the evidence base for the ‘next-step’ options for the treatment of depression. It is important that any such trial uses an inclusive definition of treatment resistance (based on non-response to at least 6 weeks of antidepressant medication) that is directly relevant to the manner in which depression is typically treated in UK primary care. 13
Given the high prevalence of depression in primary care, an effective intervention has the potential to have a substantial impact on the economic burden associated with this patient group. Currently, the lack of evidence means that clinicians are increasingly faced with a dilemma as to what action to recommend to patients who do not respond to antidepressants.
Research objectives
Amongst patients with TRD (defined as those who have significant depressive symptoms following at least 6 weeks’ treatment with antidepressant medication at an adequate dose) in primary care, to determine (1) the effectiveness of CBT in addition to pharmacotherapy in reducing depressive symptoms and improving quality of life over the following 12 months (compared with usual care that includes pharmacotherapy) and (2) the cost-effectiveness of this intervention. In addition, this study will incorporate a qualitative study to (1) explore patients’ views and experiences of CBT; (2) identify patients’ reasons for completing or not completing therapy and (3) describe ‘usual care’ for this patient group.
Chapter 2 Methods
Study design
The CoBalT study was a multicentre, pragmatic RCT with two treatment groups: a usual-care group and an intervention group. The intervention consisted of 12–18 sessions of CBT in addition to usual care. For both groups, usual care included antidepressant medication as well as continued support and advice from the GP.
An economic evaluation was conducted alongside the RCT in order to evaluate the cost-effectiveness of the intervention (see Chapter 4 ). The nested qualitative study is described in more detail (see Chapter 5 ). The trial protocol has been published. 45
Ethical approval and research governance
Ethical approval for the study was given by West Midlands Multicentre Research Ethics Committee – ref. no. NRES/07/H1208/60. Site-specific approvals were obtained from the relevant Local Research Ethics Committees and primary care trusts (PCTs)/health boards covering the three study sites (Bristol, Exeter and Glasgow). In addition, research governance approval was obtained from Avon & Wiltshire Mental Health Partnership NHS Trust, who were the employers of, and whose premises were used, on occasion, by the Bristol therapists. The trial was registered with the International Standard Randomised Controlled Trial Number Register (ISRCTN38231611). A summary of the changes made to the original protocol is given in Table 1 .
| Description | Submitted | Approved |
|---|---|---|
| Original submission | 19 October 2007 | 26 February 2008 |
Amendment 1 (protocol v3)
|
12 March 2008 | 15 April 2008 |
Amendment 2 (protocol v4)
|
8 August 2008 | 3 September 2008 |
Amendment 3 (protocol v5)
|
30 September 2009 | 14 October 2009 |
Amendment 4 (protocol v6)
|
9 February 2010 | 28 April 2010 |
Amendment 5 (protocol v7)
|
17 July 2010 | 29 July 2010 |
Amendment 6 (protocol v8)
|
27 September 2010 | 10 October 2010 |
Amendment 7 (protocol v9)
|
10 May 2011 | 20 May 2011 |
Participants
The study sought to recruit patients with TRD from 73 general practices across the three centres.
Inclusion criteria
Eligible patients were those aged 18–75 years, who were currently taking antidepressant medication, and had done so for at least 6 weeks at an adequate dose [based on the British National Formulary (BNF: www.bnf.org.uk/bnf) and advice from psychopharmacology experts)] (see Appendix 1 ). In addition, it was necessary for eligible patients to have adhered to their antidepressant medication, score ≥ 14 on the Beck Depression Inventory, second version (BDI-II)46 and meet International Classification of Diseases and Related Health Problems, Tenth Edition (ICD-10), criteria for depression, assessed using the revised Clinical Interview Schedule (CIS-R). 47
It is difficult to measure adherence to medication. Therefore, our definition of treatment resistance was operationalised using the Morisky scale, a four-item self-report measure of adherence,48 which had previously been validated against electronic monitoring medication bottles. A score of ‘0’ (range 0–4) on this scale was indicative of at least 80% adherence. 49 Given the relatively long half-life of antidepressant medication, an additional item (‘Did you miss 2 days’ antidepressant tablets in a row?’ yes/no) was added to this scale to ensure that only individuals who had missed more than one consecutive dose were excluded.
Exclusion criteria
General practitioners were asked to exclude those patients who fulfilled any of the following exclusion criteria at the time of the record search:
-
patients who had bipolar disorder, psychosis or major alcohol or substance abuse problems
-
patients who were not able to complete the study questionnaires
-
patients who were currently receiving CBT or other psychotherapy or secondary care for depression, or who had received CBT in the past 3 years
-
women who were pregnant (women who became pregnant during the trial were able to continue to participate with consent and approval of their GP).
Patients who were currently taking part in another intervention study were excluded, although they were offered the opportunity to participate in CoBalT once their involvement in the other research study had ended. In addition, GPs excluded any patients whom they considered it would be inappropriate to invite.
Recruitment of participants
Eligible participants were identified using a three-stage process ( Figure 1 ).
FIGURE 1.
CoBalT trial design.
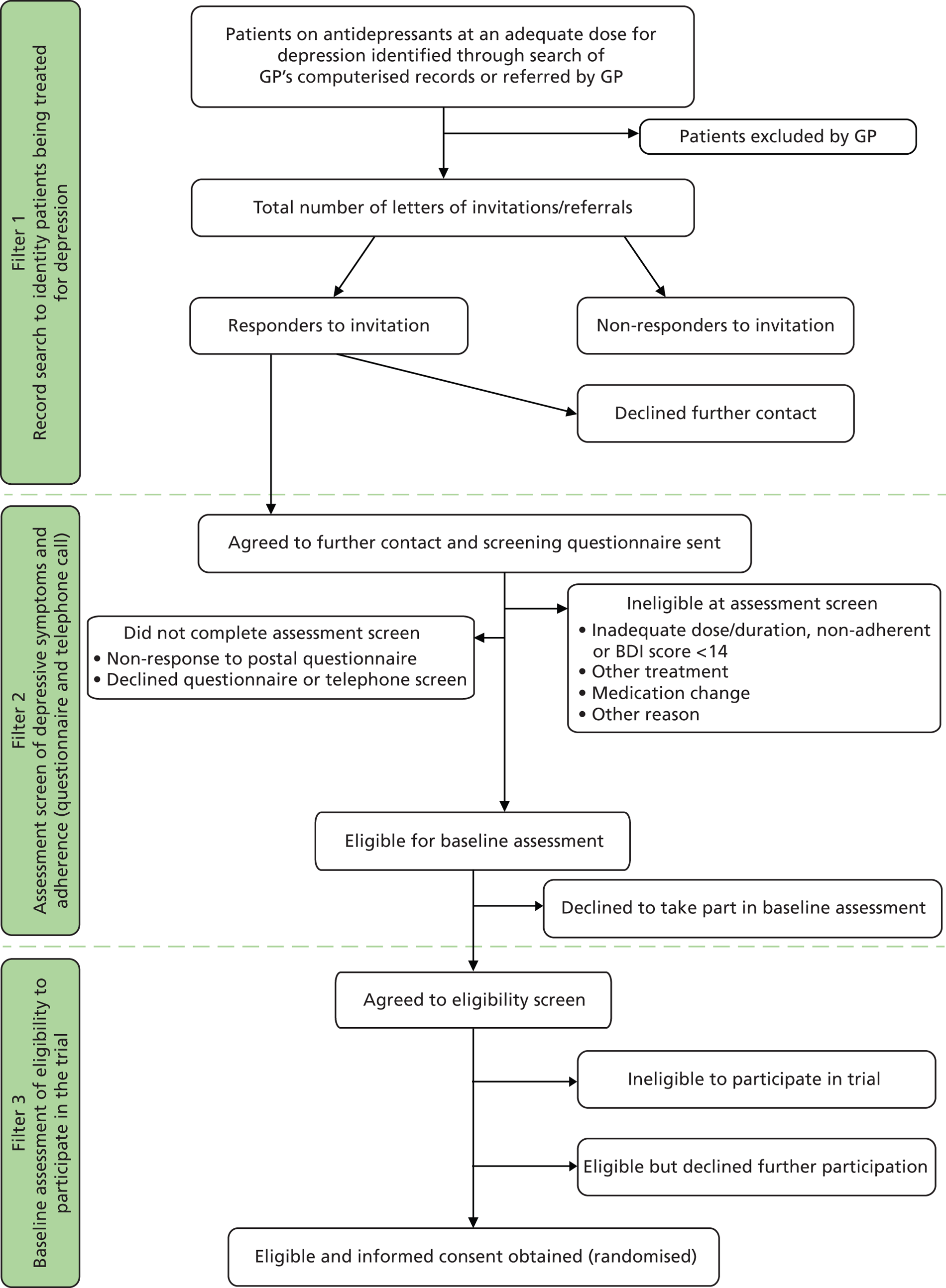
Filter 1: search of general practitioner computerised records to identify patients being treated for depression
The search of GP computerised records identified all patients in the appropriate age range who had received repeated prescriptions for an antidepressant during the previous 4 months, and who were currently being prescribed an antidepressant medication at an adequate dose for depression.
General practitioners then screened this list of patients and excluded those who fulfilled any of the exclusion criteria listed above. A letter of invitation and brief information leaflet about the study was sent by the general practice to the remaining potential participants. This letter sought permission for the research team to contact them and to send a questionnaire asking about their depressive symptoms and adherence to antidepressant medication. Patients replied directly to the study team, indicating whether or not they agreed to be contacted. One reminder was sent to those who did not respond to the initial letter of invitation.
On the reply slip, those who did not wish to participate were asked to indicate their age, gender and reason for non-participation. In addition, non-participants were asked to indicate their willingness to take part in a brief telephone interview to discuss their reasons for non-participation. This would provide more detailed insight into the reasons for non-participation and, in particular, the potential demand for CBT by this patient group.
Anonymised data on age and gender of those patients who were mailed an invitation to participate but who did not respond (or refused to participate when invited during the consultation) were collected to assess the generalisability of the study findings. GPs could also invite patients to take part in the study during a consultation. In such cases, the GP provided the patient with an information leaflet about the study and obtained permission from the patient to pass their contact details to the research team. The research team then mailed a questionnaire to the patient asking them about their depressive symptoms and adherence to antidepressant medication (as earlier).
Filter 2: assessment of depressive symptoms and adherence to antidepressants
All of those who agreed to be contacted by the research team (either in response to the postal invitation or to a direct invitation from their GP during the consultation) were sent a postal questionnaire. This questionnaire collected data on the following:
-
sociodemographic variables (age, gender, marital status, ethnicity, educational qualifications, employment status, home ownership and financial difficulties)
-
severity of depressive symptoms, using the BDI-II46
-
duration of antidepressant treatment, dose of medication and adherence to medication. 48
One reminder was sent to those individuals who did not return a completed postal questionnaire within 2 weeks.
Those who met the definition of TRD (based on severity of depressive symptoms and adherence to antidepressants at an adequate dose for at least 6 weeks) were contacted by a researcher by telephone to ascertain their eligibility with respect to current/past psychological treatment and current secondary care for depression. Those who were not currently receiving (or scheduled to start) CBT or secondary care for their depression, and who had not received CBT in the past 3 years, were invited to attend a face-to-face appointment with a researcher to discuss participating in the trial and to assess their eligibility. The date, time and location of the baseline appointment were confirmed by letter. A detailed patient information leaflet and a leaflet that provided an introduction to CBT were also enclosed, and the patient was asked to read both of these prior to attending the baseline appointment.
Those who were currently receiving non-CBT therapy [e.g. computerised CBT (cCBT) or counselling] were given the option of being contacted again for a rescreen once their current treatment had finished. An ‘end of therapy’ date was taken and the patients were mailed a second postal screen after this point to assess whether they were eligible to participate in the trial.
Those who completed the screening process (postal questionnaire with or without telephone questionnaire), but were not eligible to participate, received a letter informing them of this and thanking them for taking part. The letter explained that their GP had also been informed and would continue to care for them as usual. The GP received a letter that explained that the patient was ineligible as they had not met one or more of the eligibility criteria, but that the GP could refer the patient back to the trial if these factors changed. If the patient had given permission in the postal questionnaire, the GP also received a report that gave more detail about the inclusion criteria that were/were not satisfied, as well as the individual’s score on the BDI-II.
Filter 3: baseline assessment
Baseline assessments to establish eligibility were conducted in the patients’ own home, at their GP surgery or at nearby NHS/university premises. Only those patients who fulfilled ICD-10 criteria (F32) for their current depressive episode (assessed using the CIS-R47), had a BDI-II score of ≥ 14 and who were continuing to take the prescribed antidepressants at an adequate dose were eligible to participate in the trial.
As well as completing the CIS-R and BDI-II in order to assess eligibility, the baseline assessment also collected information on life events, social support, smoking habits and use of alcohol. 50 Sociodemographic details were also recorded (age, gender, ethnicity, marital status), together with information on a number of socioeconomic markers (including employment status, housing situation, financial stress). Questions on post-traumatic stress disorder (PTSD) (PC-PTSD:51 Primary Care Post-traumatic Stress Disorder) and personality (Big Five neuroticism scale52) were also collected at baseline. Additional measures such as the Patient Health Questionnaire-9 items (PHQ-9),53 the Generalised Anxiety Disorder Assessment-7 items (GAD-754), panic (from the Brief Patient Health Questionnaire),55 the Short-Form questionnaire-12 items (SF-12)56 and European Quality of Life-5 Dimensions-3 Levels (EQ-5D-3L)57 were collected at baseline and at subsequent follow-ups, as were measures of dysfunctional attitudes58 and metacognitive awareness59 (see Secondary outcomes, below, for more detail).
Prior to randomisation, participants were asked to indicate whether or not they had any preference for treatment (‘prefer CBT (in addition to usual care)’, ‘prefer usual care’ or ‘don’t mind’) and also asked about whether or not they thought that CBT would help them (based on a list of five response options: ‘CBT would definitely help me’; ‘CBT would probably help me’; ‘I don’t know if CBT would help me’; ‘CBT would probably not help me’; and ‘CBT would definitely not help me’). The latter information was used in an a priori subgroup analysis to examine the influence of patient expectation of effectiveness of the trial intervention on outcome.
If a patient was not eligible to take part in the trial, they were informed of this and thanked for their time. It was explained to them that their GP would also be informed and that a summary giving more detail about the inclusion criteria that were/were not fulfilled, and their depressive symptoms (scores on the BDI-II at postal screen and baseline) could be fed back to their GP, if they had given permission. If the patient was eligible to participate, the individual was asked whether or not they were happy to proceed and, if so, written informed consent was obtained.
Prior to the start of the baseline assessment, patients were asked to provide informed, written consent for the storage and processing of the data collected at the time of the assessment. This covered the data collected from both those who were found to be ineligible to participate in the trial as well as those who were eligible, thus enabling the trial to be reported in line with CONSORT (Consolidated Standards of Reporting Trials) guidelines. 60 Those patients who were identified as eligible to participate in the trial were asked to provide additional informed, written consent for this purpose. The original signed and dated consent forms were held securely as part of the trial site file, with copies for both the participants and their GPs for their records.
Randomisation, concealment of allocation and blinding
Randomisation was at the level of the individual, with eligible and consenting patients randomised at the end of their baseline assessment to one of two treatment groups: ‘Usual care’ or ‘Usual care plus CBT’. At the point of randomisation, all patients were taking antidepressants and had agreed with their GP to continue with such medication as part of their usual care.
To conceal the allocation of treatment from those conducting the research, randomisation of individual participants to one of the two treatment arms was undertaken using an automated telephone randomisation system that was administered remotely and used a computer-generated code. The randomisation service was provided by the Bristol Randomised Trials Collaboration, a UK Clinical Research Collaboration-registered trials unit (www.bris.ac.uk/social-community-medicine/centres/brtc). Once the randomisation procedure had been completed, the outcome and further details about the allocated treatment was immediately communicated by the researcher to the participant. Given the nature of the intervention, it was not possible to blind participants, GPs, researchers or the CBT therapists to the treatment allocation.
Randomisation was stratified by centre (n = 3), with minimisation used to ensure balance in the following variables:
-
baseline BDI-II score (mild 14–19; moderate 20–28; severe ≥ 29)
-
whether the general practice had a counsellor (yes/no)
-
prior treatment with antidepressants (yes/no)
-
duration of their current episode of depression (< 1 year, 1–2 years, ≥ 2 years).
Minimisation with a probability weighting of 0.8 was used in order to reduce predictability. 61
Treatment group allocation
Eligible participants were randomised to either ‘usual care’ or ‘usual care plus CBT’.
Usual care
There were no restrictions on treatment options for patients randomised to be managed as usual by their GP. Patients could be referred for counselling or to secondary care (including for CBT) if it was deemed clinically appropriate by the GP. It was felt unethical to withhold the option of counselling or to restrict access to secondary care if the GP deemed it appropriate. Although the roll-out of IAPT services had improved access to psychological therapies in England, there could still be long waiting times (as is the situation in Scotland), so any such contamination was considered unlikely to substantially influence the primary outcome. Nonetheless, such treatments were recorded as part of the follow-up questionnaires.
Usual care plus cognitive behavioural therapy
Cognitive behavioural therapy manuals
Therapists used the seminal CBT depression treatment manuals. 62,63 Given that the study population had TRD, where appropriate therapists used elaborations on the manual designed to address treatment resistance. 64 The Moore and Garland manual64 emphasises approaches that overcome cognitive and behavioural avoidance, and formed the basis for the treatment manual used in an earlier MRC trial that examined the effectiveness of CBT for patients with residual depression. 34 The therapists were flexible in responding to problems raised by the patient, for example by targeting symptoms of anxiety using appropriate cognitive behavioural models if these were considered important. Emphasis was also given to formulating the psychopathology in terms of conditional beliefs.
Therapy: length and number of sessions
Details of those patients randomised to receive CBT were passed over to the therapy team based in each centre. The allocated therapist contacted the patient to arrange the first appointment at a mutually convenient time and place.
Patients randomised to CBT received a course of 12 sessions, with (up to) a further six sessions if deemed clinically appropriate by the therapist. Sessions typically lasted 50–60 minutes. Therapy usually took place in the patients’ GP surgery, or at nearby NHS premises. In a few exceptional cases the therapy took place at the patient’s home, or by telephone.
Therapists
As this was a pragmatic trial, we aimed to recruit therapists who were representative of CBT therapists working within NHS psychological therapy services, for example ‘high-intensity’ IAPT practitioners who had postgraduate CBT qualifications or equivalent experience, and were accredited (or eligible for accreditation) by the British Association for Behavioural and Cognitive Psychotherapies (BABCP: www.babcp.com).
Across the three sites, 11 therapists working part time [ranging from 0.1 full-time equivalent (FTE) to 0.8 FTE] delivered the therapy (Bristol n = 6; Exeter n = 3; Glasgow n = 2). Ten of the eleven (91%) therapists were female and the mean age of the therapists was 39.2 years [standard deviation (SD) 8.1, range 27–58 years]. The professional background of the therapists varied: four had a mental health nursing background, six were clinical psychologists and one had completed a MSc in Psychological Therapies. On average, the trial therapists had practised as a therapist for 9.7 years (SD 8.1; range 0 (newly qualified)–30). Before the trial, seven of the therapists had received formal CBT training (Masters or Postgraduate Certificate/Diploma) and two of these individuals completed a MSc in CBT during their employment with the study.
Training and supervision of therapists
There were four therapists employed at the start of recruitment, who received 4 days’ training with one of the authors of the manual designed to address treatment resistance (AG). 64 There were five additional training days (including 4 days with AG) over the course of the trial. All therapists attended at least one of these additional training days and received at least 1 day’s training, specific to the trial, from AG.
Therapists received weekly hourly supervision sessions (in groups of two or three) from an experienced therapist based at each centre. This arrangement met the standards for clinical supervision set out by the BABCP (defined as at least 1 hour per month: www.babcp.com/Accreditation/Practitioner/Practitioner_Accreditation.aspx) and the more stringent requirements set out by IAPT (at least 1 hour of individual supervision on a weekly basis for full-time staff: www.iapt.nhs.uk/silo/files/iapt-supervision-guidance-revised-march-2011.pdf). Therapists in two sites were supervised by two of the principal investigators (Bristol, GL; Exeter, WK).
Fidelity to the cognitive behavioural therapy model
Therapy sessions were recorded, with the patient’s consent, using a digital voice recorder. Individual permission was sought to use these recordings for teaching and/or research purposes. A random sample of recordings was selected, and fidelity to the CBT model evaluated by three independent raters from the Oxford Cognitive Therapy Centre using a recognised CBT rating scale. 65 This evaluation was restricted to the recordings of the CBT sessions for the nine therapists who delivered the majority (97%) of the intervention. Assessment (session 1) and review sessions (final session for those who completed therapy) were excluded from the pool of potential sessions for selection, as these sessions are different and would not typically provide a demonstration of competence in CBT.
As three raters were undertaking this evaluation, the first step was to demonstrate inter-rater reliability in Cognitive Therapy Scale-revised (CTS-R) ratings. To ensure an approximately equal distribution between sessions sampled from earlier or later in therapy, one therapist was selected at random and one session selected at random from earlier/later sessions of therapy dependent on the frequency distribution of number of sessions. The remaining eight therapists were then ranked in terms of the number of patients allocated to them (thus including those who completed therapy as per the study protocol; those who withdrew from therapy; and those who were discharged for non-compliance). Four therapist pairs were then defined on the basis of caseload and within each therapist pair, a random number was generated to determine whether an earlier or later therapy session (selected at random) was evaluated. Across the nine therapists, this ensured an approximately equal distribution in the number of earlier and later sessions rated. This process generated one CTS-R rating for each of the nine therapists rated by three independent raters and these data were used to establish inter-rater reliability. If the audio-recording for the selected session was missing (either because the audio-file was missing or the patient withdrew consent for that session to be recorded) or incomplete (the recording failed for technical reasons) then an alternative session for the same patient was randomly selected from within the specified stratum (early/late). If there were no alternative sessions within the same stratum, another patient was selected at random and an early/late session sampled at random, as appropriate.
If there was a handover between therapists during the course of therapy and the selected session was one delivered by the second therapist then the nearest session delivered by the first therapist was selected for evaluation. This corresponded to the therapist who was listed as the allocated therapist and thus prevented sessions from any individual therapist being over-/under-sampled.
It was specified in advance that if at least ‘moderate’ reliability [intraclass correlation coefficient (ICC) > 0.666] between the three raters was observed then each of the three raters would be asked to rate a further 18 sessions. For each of the nine therapists, six patients would be selected at random. A random number would be generated to determine whether an earlier or later therapy session (selected at random) should be evaluated for each of these six patients. This would result in three earlier and three later sessions being sampled for each of the nine therapists (giving a total of 54 sessions for evaluation). The procedure for selecting an alternate recording in the event of a missing/incomplete audio-recording or change in therapists, as outlined earlier, applied.
If there was insufficient inter-rater reliability (ICC ≤ 0.60) then each of the three raters would be asked to rate the same 18 sessions. In this case, one earlier and one later session would be selected at random for each of the nine therapists.
A mean CTS-R rating across all therapists is reported accounting for therapist caseload using sampling weights. For the 12-item CTS-R, a cut-point of ≥ 36 is deemed appropriate, equating to a mean item score of 3.0 ‘competent’. 65
Follow-up
A flow chart outlining CoBalT follow-up procedures is provided in Figure 2 . Follow-up data collection took place at four time points: 3, 6, 9 and 12 months post randomisation. Measurement of the primary outcome took place at the 6-month follow-up, as it was expected that most of those in the intervention group would have attended all (or most) of their CBT sessions by this time. The 12-month follow-up was designed to enable the investigation of any longer-term effects on study outcomes.
FIGURE 2.
CoBalT trial follow-up stages and data collected. DAS-SF2, Dysfunctional Attitudes Scale-Short Form (version 2); MAQ, Metacognitive Awareness Questionnaire; UC, usual care.
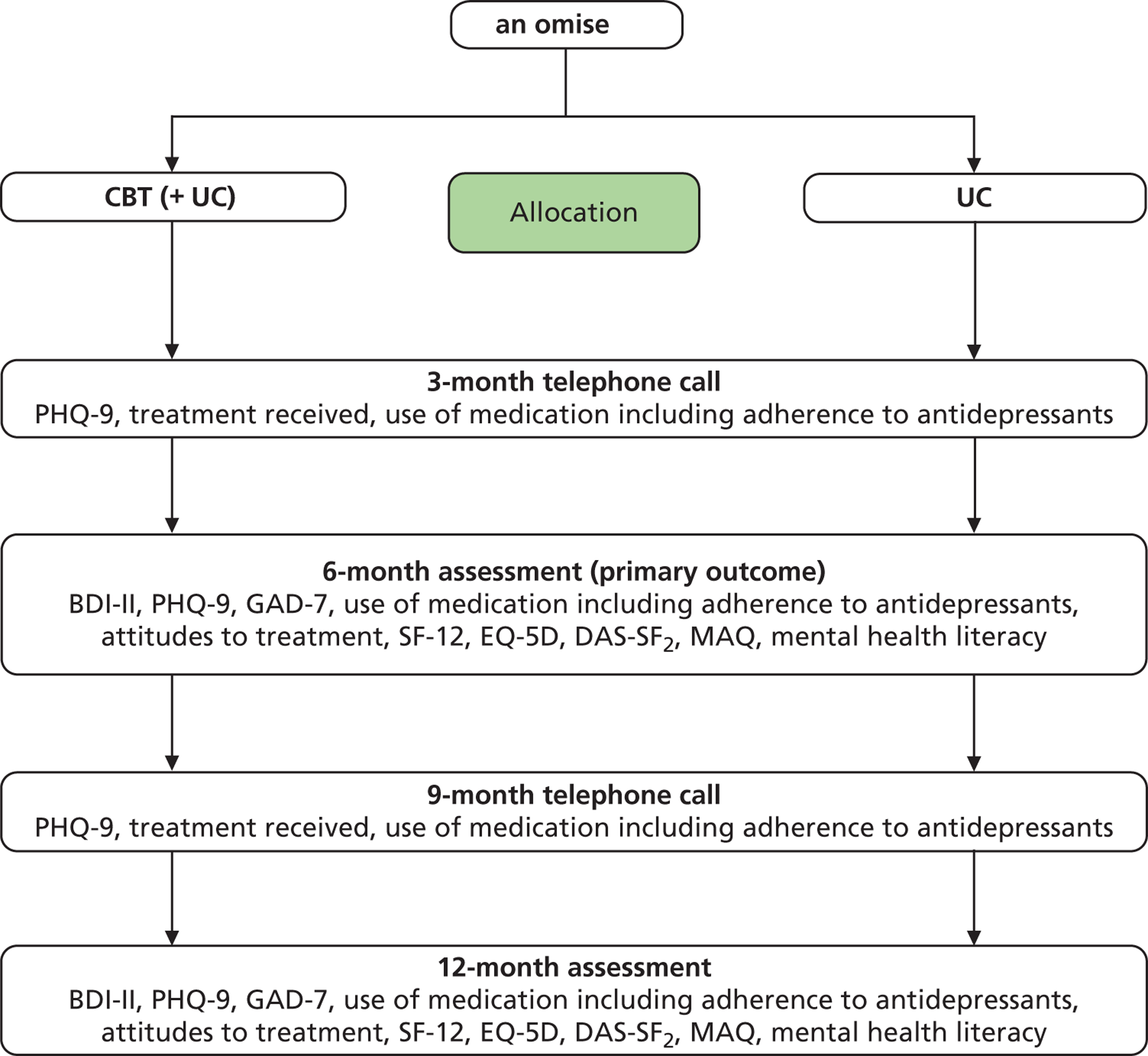
To maximise response rates, wherever possible the researcher arranged to meet the patient at 6- and 12-month follow-up at the participant’s home, in their GP surgery or at university premises. A small number of participants chose to return the questionnaire(s) by post.
The 3- and 9-month follow-ups were conducted by telephone. These follow-ups were designed to maintain contact with participants and enable collection of brief outcome data in terms of depressive symptoms, use of antidepressant medication and receipt of other treatments.
Data collection and management
To standardise processes across the three centres and maximise data quality, researchers were trained to use detailed standard operating procedures for each stage of data collection. A number of cross-checks were routinely performed as a means of ensuring that any data inconsistencies arising from either baseline assessment or follow-up were identified and resolved at the earliest opportunity. Trial data were entered into a Microsoft Access 2003 database (Microsoft Corporation, Redmond, WA, USA) at each centre, before being merged into one central database following the end of data collection. A range of data validation checks were carried out in both Microsoft Access and Stata 11.2 (StataCorp LP, College Station, TX, USA) to minimise erroneous or missing data.
Measures
Primary outcome
The primary outcome was the BDI-II score at 6 months post randomisation – specifically a binary variable representing response defined as a reduction in depressive symptoms of at least 50% compared with baseline. A threshold of 50% improvement in symptoms is a widely used definition of improvement67 and used to compare treatment effects in the systematic review of interventions for TRD. 14
The BDI-II is a 21-item self-report instrument to measure the severity of depressive symptoms occurring over the previous 2 weeks and has been widely used in depression trials. The 21 items are rated on a four-point severity scale (0–3) and are summed to give a total score (range 0–63). A higher score on the BDI-II denotes more severe depression.
Secondary outcomes
The BDI-II was also completed at 12 months to assess the longer-term effect of the intervention. Secondary outcomes included the BDI-II as a continuous score, and a further binary version representing remission of symptoms (defined as a BDI-II score of < 10).
Other outcome measures included at the 6- and 12-month follow-ups assessments are listed below:
-
SF-12 (version 2) A 12-item Short Form Health Survey measuring quality of life. 56 The SF-12 is an abbreviated form of the SF-36 (Short Form questionnaire-36 items), a 36-item instrument for measuring subjective health status. It consists of 12 self-report items, selected from the SF-36. The CoBalT study used a revised version of the SF-12, the SF-12v2, which was introduced in 2002. The algorithms used to score data are dependent on the recall period. The CoBalT study used the acute (1-week recall) survey. Norm-based scores for the physical and mental subscales were calculated. Higher scores indicate better health and functioning.
-
PHQ-9 The Patient Health Questionnaire, a brief nine-item depression scale53 developed for use in a primary care setting. The questionnaire is designed to assess the patient’s mood over the previous 2 weeks and scores for each of the nine items range from 0 (not at all) to 3 (nearly every day). Items are summed to give a total score (range 0–27), with a higher score denoting more severe depression.
-
GAD-7 The Generalised Anxiety Disorder Assessment – a measure of generalised anxiety disorder (GAD). 54 The GAD-7 is a brief self-report questionnaire designed to detect probable cases of GAD and to provide a measure of its severity as recalled over the previous 2 weeks. As with the PHQ-9, scores for each item range from 0 (not at all) to 3 (nearly every day). Items are summed to give a total score (range 0–21), with a higher score denoting more symptoms of anxiety. The PHQ-9 and GAD-7 form part of the core IAPT outcome data set (www.iapt.nhs.uk/silo/files/iapt-outcome-framework-and-data-collection.pdf) and will enable comparison with national IAPT data.
-
Panic (Brief PHQ) The presence of panic disorder was measured using the panic module of the self-report version of the PRIME-MD questionnaire (Brief PHQ). 55 The measure consists of five items. The first item asks individuals to report whether or not they have experienced an anxiety attack within the last 4 weeks; if they have they are asked four further questions about their experience. Each question elicits a response of ‘Yes’ or ‘No’. The total number of panic items endorsed therefore ranges from 0 to 5.
-
EQ-5D-3L A standardised measure of health status developed by the EuroQol Group to provide a simple, generic measure of health for clinical and economic appraisal. 57 The EQ-5D-3L is used in CoBalT as a measure of health outcome for the economic evaluation. Scores were calculated using standard algorithms, with higher scores indicating better health.
Bespoke measures relating to patient’s treatment experience and mental health literacy were also recorded at 6 and 12 months.
Self-reported adherence to antidepressants was collected at each of the four time points. 48 With consent, data on antidepressant medication received (additional prescriptions, changes in dose and/or changes in the antidepressant prescribed) and other medications prescribed during the course of the study were recorded from GP records, together with details of consultations in primary care. Data on antidepressants prescribed during the year prior to entry to the study were also recorded, when consent was given to access medical records. Data on health care utilisation in primary and secondary care, private treatments, and complementary and alternative treatments were collected as part of the 6- and 12-month follow-up questionnaires and were used to inform the economic evaluation.
Process measures of dysfunctional attitudes and metacognitive awareness were also collected at the 6- and 12-month assessments:
-
Dysfunctional Attitudes Scale-Short Form (version 2) (DAS-SF 2 ) The DAS-SF2 is a self-report questionnaire containing nine items that was developed from Weissman’s original Dysfunctional Attitude Scale,68 using item response analysis to provide an efficient and accurate assessment of dysfunctional attitudes among depressed individuals. 58
-
Metacognitive Awareness Questionnaire (MAQ) The MAQ59 assessed whether or not patients with depression view their negative thoughts as reflecting reality. The scale consists of nine self-report items and has the same seven-point response format as the Dysfunctional Attitudes Scale (DAS), with higher MAQ scores reflecting greater metacognitive awareness.
The brief telephone follow-ups at 3 and 9 months comprised the PHQ-9, use of antidepressant medication, including adherence to antidepressants48 and other treatments received.
Table 2 shows the measures and when they were collected.
| Concept | Measure | Time points | |||||
|---|---|---|---|---|---|---|---|
| Screen | Baseline | 3 months | 6 months | 9 months | 12 months | ||
| Sociodemographics | a | ✓ | ✓ | ||||
| Alcohol use | AUDIT-PC | ✓ | |||||
| ICD-10 diagnosis | CIS-R | ✓ | |||||
| Depression severity | BDI-II | ✓ | ✓ | ✓ | ✓ | ||
| Depression severity | PHQ-9 | ✓ | ✓ | ✓ | ✓ | ✓ | |
| Adherence | Four-item Morisky scale | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Post-traumatic stress | PC-PTSD | ✓ | |||||
| Anxiety | GAD-7 | ✓ | ✓ | ✓ | |||
| Panic | Brief PHQ-Panic | ✓ | ✓ | ✓ | |||
| Quality of Life | SF-12 | ✓ | ✓ | ✓ | |||
| Economic evaluation | SF-6D | ✓ | ✓ | ✓ | |||
| Economic evaluation | EQ-5D-3L | ✓ | ✓ | ✓ | |||
| Dysfunctional attitudes | DAS-SF2 | ✓ | ✓ | ✓ | |||
| Metacognitive awareness | MAQ | ✓ | ✓ | ✓ | |||
| Personality | Big Five (neuroticism subscale) | ✓ | |||||
| Mental health awareness | Bespoke questionnaire | ✓ | ✓ | ✓ | |||
| Treatment experience | Bespoke questionnaire | ✓ | ✓ | ||||
Handling missing items
For outcomes on the BDI-II, PHQ-9, GAD-7, DAS-SF2 and MAQ, the trial dealt with any missing data at an individual item level by adopting the following rule. If > 10% of the items were incomplete then the data collected on that measure for that participant were disregarded. However, if < 10% of items on a particular measure were missing, missing item(s) were imputed using the mean of the remaining items (rounded to an integer). Therefore, when an individual had completed 19 or 20 items for the primary outcome measure (BDI-II) then the remaining one or two items were imputed. For all other measures (PHQ-9, GAD-7, DAS-SF2, and MAQ) the 10% rule meant that only a single item would be imputed. Data were complete for the majority of the sample; the number of cases for which values were imputed are reported in Table 3 .
| Instrument | Maximum no. of items imputed | No. of cases in which imputation of individual items was undertaken | ||||
|---|---|---|---|---|---|---|
| Baseline | 3 months | 6 months | 9 months | 12 months | ||
| BDI-II | 2 | 0 | – | 1 | – | 5 |
| PHQ-9 | 1 | 0 | 0 | 1 | 0 | 3 |
| GAD-7 | 1 | 3 | 0 | 2 | 0 | 0 |
| DAS-SF2 | 1 | 0 | – | 0 | – | 2 |
| MAQ | 1 | 0 | – | 1 | – | 2 |
The scoring manuals for the SF-12 or EQ-5D-3L, which require the application of complex scoring algorithms, indicated that if any item was missing, the scale score should not be calculated. In the case of greater item non-response or missing follow-up data, sensitivity analyses were conducted using the method of multiple imputation by chained equation (MICE) to examine the impact of missing data on the main findings (see Sensitivity analyses to examine the impact of missing data, below).
Justification of sample size
To calculate the required sample size, we assumed that usual care would involve GPs acting in line with NICE guidance,12,13 i.e. increasing the dose of antidepressant, switching to another antidepressant or augmenting the medication. Data from the large US STAR*D study suggested that, among those who had not responded to up to 14 weeks treatment with citalopram, only 30% would ‘respond’ when their antidepressant medication was switched19 or augmented. 20
Original sample size calculation
The expected treatment effect for the CoBalT study was based upon systematic review evidence for the effectiveness of CBT,31 which concluded that there was a threefold increased odds of recovery for CBT compared with treatment as usual (TAU). 31 However, a reduced effect was expected given that this review did not focus specifically on patients with TRD. Therefore, the original sample size calculation for the CoBalT trial was based on detecting an odds ratio (OR) of 2 (or a difference of 16 percentage points between groups). Other trials of residual depression,34 or chronic depression,38 report effect sizes that are not dissimilar.
Thus, the original plan was to recruit 200 patients in each treatment group to yield 90% power to detect a difference between 30% and 46% response (defined as at least a 50% reduction in depressive symptoms) or an OR of 2, at a 2-sided 5% significance level. Allowing for 15% loss to follow-up at 6 months, the sample size was initially calculated to be 472.
Revised sample size calculation
However, a slightly delayed start to recruitment, a slightly lower than anticipated recruitment rate in one centre and difficulties matching recruitment rates to therapist capacity in two centres (to avoid a lengthy delay between randomisation and the start of therapy) necessitated a request for extended funding. This request was submitted in October 2009. A revised power calculation was presented as part of this application ( Table 4 ). The original power calculation was amended to reflect a reduced sample size of 432. This reduced sample size would have 87% power to detect the originally specified difference of 16 percentage points, and would have 90% power to detect a 17 percentage point difference in the binary ‘response’ outcome (between 30% and 47%).
| Sample size calculation | N randomised | n for primary analysis | Power to detect originally specified difference (%)a | Detectable difference with: | |
|---|---|---|---|---|---|
| 80% power | 90% power | ||||
| Original | 472 | 400 | 90 | 14 percentage points (30% vs. 44% = OR 1.84) | 16 percentage points (30% vs. 46% = OR 2.00) |
| Revised | 432 | 367 | 87 | 15 percentage points (30% vs. 45% = OR 1.89) | 17 percentage points (30% vs. 47% = OR 2.07) |
Statistical analysis
The analysis and reporting of this trial was undertaken in accordance with CONSORT guidelines. 60,69,70 All statistical analysis was undertaken in Stata 11.2 (StataCorp LP, College Station, TX, USA), following a pre-defined analysis plan agreed with the Trial Steering Committee (TSC). The primary comparative analyses between the randomised groups were conducted on an intention-to-treat (ITT) basis without imputation of missing data.
Preliminary analyses
Descriptive statistics of the key sociodemographic and clinical variables were used to ascertain any marked imbalances at baseline, and to inform any additional adjustment of the primary and secondary analyses as appropriate.
Primary analysis
The primary analysis used logistic regression to compare the groups as randomised in terms of the primary (binary) BDI-II outcome at 6 months, adjusting for stratification and minimisation variables (the ‘design variables’: centre, baseline BDI-II score, access to a counsellor, prior treatment with antidepressants and duration of the current depressive episode), which included adjustment for the baseline measurement of the outcome (BDI-II score as a continuous variable). The ORs of ‘response’ in the intervention group compared with the usual-care group is presented along with a 95% confidence interval (CI) and the p-value for the comparison.
Secondary analyses
Secondary analyses of the primary outcome included additional adjustment for any prognostic variables demonstrating marked imbalance at baseline (ascertained using descriptive statistics). The BDI-II was also considered as a continuous outcome, with an associated increase in power. Secondary analyses were conducted for other outcomes measured at 6 and 12 months.
Repeated measures analyses were used to incorporate the outcome data from both 6 and 12 months post randomisation (or, in the case of the PHQ-9, from 6, 9 and 12 months) to examine whether or not any treatment effects were sustained or emerged later. This was tested formally by the introduction of an interaction between treatment group and time. In the absence of any time effect, repeated measures analyses generate an average effect size over the duration of follow-up. In all analyses, ORs (or regression coefficients for continuous outcomes), with 95% CIs and p-values, are reported.
Potential clustering by therapist
There is the potential for clustering by therapist within this trial, although clustering effects will operate in only one arm of the trial. Secondary analyses therefore used generalised linear and latent mixed models to obtain a fully specified heteroscedastic model (described in Roberts and Roberts71) to examine the influence of clustering by therapist on the results.
Subgroup analyses
Two subgroup analyses were specified a priori and were conducted by introducing an appropriate interaction term to the regression model for the primary outcome. This permitted investigation of any differential effects of treatment on outcome according to two predefined factors: (1) patient expectation of outcome (defined as three levels: ‘CBT would definitely help me’; ‘CBT would probably help me’; ‘I don’t know if CBT would help me/would probably not help me’) and (2) degree of treatment resistance [six levels based on duration of symptoms (< 1, 1–2, ≥ 2 years) and past treatment with antidepressant medication (yes/no)].
Sensitivity analyses to examine the impact of missing data
The pattern of missing data was investigated by identifying those variables recorded at baseline that were associated with ‘missingness’ of the primary outcome (BDI-II score) at p < 0.20 at either the 6-month follow-up and/or 12-month follow-up. Sensitivity analyses were conducted using the method of MICE72 to impute missing data (Stata ice procedure, version 1.9.5, dated 15 April 2011). The imputation model included all of those variables that were part of the substantive ITT model, together with the variables associated with missingness (as identified above) and all available measures of depressive symptoms (BDI-II and PHQ-9) and anxiety (GAD-7), irrespective of statistical significance. The baseline CIS-R score was also included in the imputation model as another marker of severity. Variables included in the imputation model were declared as continuous, binary, categorical or ordinal variables, as appropriate. The match procedure was used to handle non-normally distributed variables that could not be successfully transformed. In total, 25 data sets were generated and 10 switching procedures were used.
Treatment efficacy
Complier-Average Causal Effect (CACE) estimates73 for those who were viewed as ‘on track’ to receive the full course of CBT treatment at the time of the 6-month follow-up (defined as having completed nine or more sessions) were estimated using instrumental variables regression methods. As the primary outcome ‘response’ was a binary variable, a probit transformation was used, and the primary ITT analysis repeated on this transformed scale for comparison with the CACE estimates.
Complier-Average Causal Effect estimates were also determined for the longer-term outcome at 12-months. For the latter, those who had received ≥ 12 sessions were regarded as having received CBT in line with the treatment protocol.
The CACE methodology compares the outcomes for those who ‘complied’ with the intervention with a similar group of ‘would-be compliers’ from those randomised to usual care, thus avoiding the biases inherent in crude per-protocol analyses. The original definition of a ‘complier’ included those where the therapy goals were achieved in fewer than 12 sessions, as agreed between the patient and therapist. However, in practice, this included individuals who had an ‘agreed end’ of fewer than eight sessions, and thus a stricter definition was adopted, as outlined above.
Safety reporting and disclosure
Although CoBalT was not a Clinical Trial of an Investigational Medicinal Product (CTIMP), it was recognised that both psychopharmacology and psychotherapy have risks and benefits for patients. 74 Therefore, in accordance with good clinical practice the CoBalT team recorded and reported any serious adverse events to comply with the National Research Ethics Service annual safety report requirements of non-CTIMP trials.
In the case of CoBalT, GPs were responsible for the ongoing clinical care of participants. Therefore, researchers and therapists had a duty of care to ensure that the GP was aware of any suicidal ideation expressed by participants. Researchers were not clinically trained and therefore adhered to the study’s safety and disclosure policy and did not engage in any assessment of risk. This policy stated that if at any time the researcher believed that there was a significant suicide risk with a patient who was participating in the study (which had not been communicated to his/her GP), the researcher would ask the patient for their consent to pass this information on to their GP. If the patient refused, the researcher would consult the appropriate clinician (or nominated deputy) at the research site. This person would examine the patient’s data and, if it was considered necessary, would assess the patient. If it was concluded that there was a significant risk, the patient’s GP would be notified without the patient’s consent. The need to break confidentiality in situations where there was significant concern about harm to the individual (or others) was explained in the patient information leaflet.
It was expected that therapists would use their clinical judgement to assess the seriousness of risk and follow normal clinical procedures with respect to communicating disclosure to the participant’s GP.
Trial monitoring
The trial was independently supervised by a TSC, chaired by an academic psychiatrist. Other members included an independent statistician, another senior academic with trials experience, a service user, an observer from the funder [National Institute for Health Research (NIHR) Health Technology Assessment], the chief investigator (NW), principal investigator (GL), Trial Statistician (TP) and the trial co-ordinator. The trial was also supervised by an independent Data Monitoring and Ethics Committee, chaired by an academic GP and also including a statistician and an academic clinical psychologist. Members of these committees are named in Acknowledgements.
Chapter 3 Results: clinical effectiveness
Practice details
One hundred and six general practices across the three centres were approached to participate in the CoBalT study. Eighty-eight practices agreed to collaborate. Record searches were conducted and mailings sent out from 73 general practices. A summary of the practice characteristics for the 73 participating practices are presented in Table 5 . The average number of patients invited to participate in the study per practice was similar in all three centres, although the practices in Glasgow were smaller in size than the other two centres (Bristol and Exeter).
| Characteristic | Bristol | Exeter | Glasgow | Total |
|---|---|---|---|---|
| No. of practices | 26 | 22 | 25 | 73 |
| Practice list size: median (IQR) | 9435 (7400 to 12,026) | 7500 (6677 to 11,000) | 4281 (3200 to 6500) | 7400 (4286 to 10,368) |
| No. of full-time GPs per practice: mean (SD)a | 5.8 (2.3) | 5.1 (3.0) | 2.6 (1.3) | 4.5 (2.6) |
| No. of patients per practice | ||||
| Invited: median (IQR) | 158 (104 to 203) | 128 (96 to 157) | 143 (104 to 172) | 149 (98 to 180) |
| Screened: median (IQR) | 49 (36 to 57) | 38 (31 to 55) | 27 (17 to 37) | 37 (26 to 53) |
| Randomised: mean (SD) | 7.3 (4.4) | 7.3 (3.9) | 4.7 (2.2) | 6.4 (3.8) |
Flow of participants into the trial
As it was not possible to easily identify those patients with TRD from general practice records, a screening process was used to firstly identify those patients being treated for depression (filter 1) and then assess their depressive symptoms and adherence to antidepressant medication (filter 2) in order to identify the target population who was potentially eligible to participate in the CoBalT trial. Potential participants were then invited to a baseline assessment with a researcher to establish eligibility (filter 3). The flow of participants through these three stages is described below. The screening process commenced in November 2009, and the final patient was randomised to the trial on 30 September 2010. All follow-up data were collected between March 2009 and 31 October 2011.
Search of general practitioner computerised records to identify patients being treated for depression
In total, 15,379 patients, aged 18–75 years, who had received repeated prescriptions for antidepressant medication over the previous 4 months and who were currently being prescribed antidepressants at an adequate dose for depression were identified through searches of the practice computerised records. A further 37 individuals were referred to the study directly by their GP. GPs excluded 4750 individuals who were ineligible ( Figure 3 – filter 1).
FIGURE 3.
Case CONSORT flow chart illustrating flow of participants into the CoBalT trial.
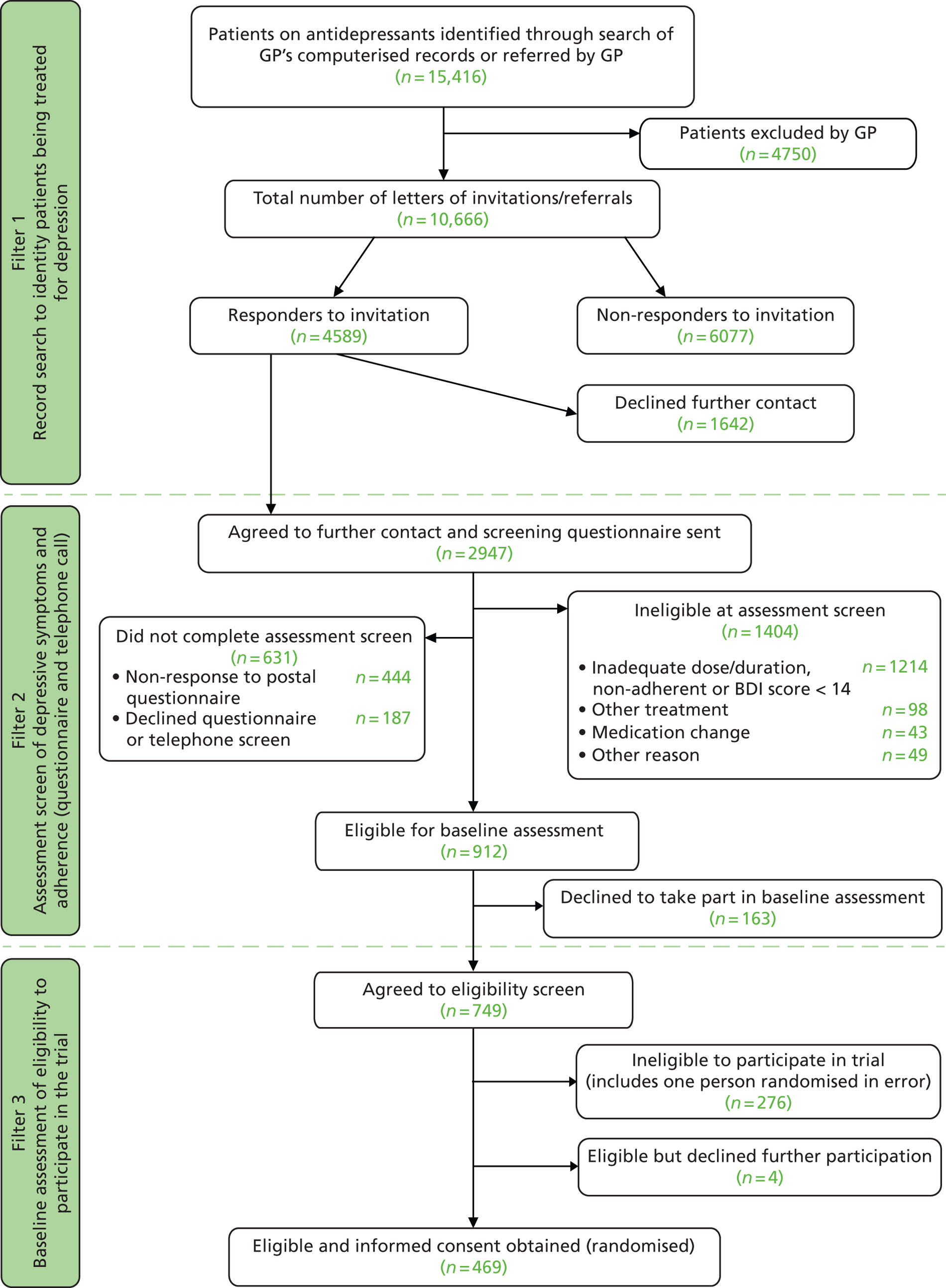
In all, 10,666 patients were mailed a letter of invitation to participate in the study asking for their permission for the research team to contact them or were invited to take part by their GP during the consultation. Of these, 4589 individuals (43.0%) responded to the letter of invitation, with the majority, 2947 (64.2%) agreeing for the research team to contact them (see Figure 3 – filter 1).
When available, information on age and gender was recorded from GP records. Older individuals were more likely to decline to participate [analysis of variance (ANOVA), p < 0.001]. Women were more likely to respond to the invitation to participate and to give permission for the research team to contact them (‘accepted invitation’) (chi-squared test: p = 0.001) ( Table 6 ).
Assessment of depressive symptoms and adherence to antidepressant medication
A screening questionnaire was mailed to 2947 potential participants (see Figure 3 – filter 2). In total, 631 individuals did not complete the assessment screen, either not responding to the postal questionnaire or declining to complete this questionnaire or the additional questions to assess eligibility asked over the telephone (see Figure 3 – filter 2). Older individuals were more likely to complete the assessment screening process (t-test: p = 0.007) ( Table 7 ). There was no evidence of a difference in gender between those who did or did not complete the assessment screen (chi-squared test: p = 0.13).
| N | Age | Female | |||||
|---|---|---|---|---|---|---|---|
| n a | Mean | SD | n a | n | % | ||
| Did not complete screen | 631 | 599 | 46.8 | 13.3 | 615 | 455 | 74.0 |
| Completed assessment screen | 2316 | 2185 | 48.5 | 13.6 | 2244 | 1590 | 70.9 |
The primary aim of filter 2 was to identify those with TRD. A large number of respondents (n = 1214) were not eligible as their depression was below the threshold on the BDI-II (score of < 14) or they were not adhering to their antidepressant medication or did not meet the criteria for an adequate dose/duration of treatment (see Figure 3 – filter 2). In total, 912 participants were eligible to attend a baseline appointment with a researcher to establish an ICD-10 diagnosis of depression but 18% declined (see Figure 3 – filter 2). There were no differences in age or gender between those who did or did not agree to attend a baseline appointment ( Table 8 ) (t-test age: p = 0.48; chi-squared test gender: p = 0.37).
| Agreed to attend a baseline appointment with a researcher? | N | Age | Female | ||||
|---|---|---|---|---|---|---|---|
| n a | Mean | SD | n a | n | % | ||
| No (declined) | 163 | 154 | 50.7 | 13.3 | 155 | 113 | 72.9 |
| Yes (agreed) | 749 | 711 | 49.9 | 12.5 | 749 | 519 | 69.3 |
However, those who agreed to attend a baseline assessment were more highly educated than those who declined ( Table 9 ) (chi-squared test: p = 0.009).
| Agreed to attend a baseline appointment with a researcher? | N | n a | A-levels/Higher Grade or above | Other qualifications | No formal qualifications | |||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| No (declined) | 163 | 160 | 58 | 36.3 | 53 | 33.1 | 49 | 30.6 |
| Yes (agreed) | 749 | 739 | 366 | 49.5 | 199 | 26.9 | 174 | 23.6 |
Baseline assessment of eligibility to participate in the randomised controlled trial
A total of 749 participants agreed to attend a face-to-face appointment with a researcher to discuss participating in the CoBalT trial, establish eligibility and obtain written informed consent (see Figure 3 – filter 3). The baseline assessment took place, on average (median), 34 days [interquartile range (IQR) 21–55] following completion of the screening questionnaire. Two hundred and seventy-six individuals were ineligible to participate (including one person who was randomised in error). The majority (n = 233) were ineligible because they did not meet ICD-10 criteria for depression. In total, 469 individuals were eligible to participate, gave written informed consent and were randomised (see Figure 3 – filter 3).
There were no differences between those who were and were not eligible to participate in the CoBalT trial in terms of age ( Table 10 ) (t-test: p = 0.20), although women were more likely to be eligible (chi-squared test: p = 0.02).
| Eligibility status | N | Age | Female | ||||
|---|---|---|---|---|---|---|---|
| n a | Mean | SD | n a | n | % | ||
| Eligible (including declined to participate) | 473 | 473 | 49.6 | 11.7 | 473 | 342 | 72.3 |
| Ineligible at baseline | 276 | 274 | 50.8 | 13.3 | 274 | 176 | 64.2 |
There were no differences in educational qualifications among those who were or were not eligible to participate in the trial ( Table 11 ) (chi-squared test: p = 0.12).
| Eligibility status | N | n a | A-levels/Higher Grade or above | Other qualifications | No formal qualifications | |||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Eligible (including declined to participate) | 473 | 467 | 218 | 46.7 | 131 | 28.1 | 118 | 25.3 |
| Ineligible at baseline | 276 | 272 | 148 | 54.4 | 68 | 25.0 | 56 | 20.6 |
Summary of recruitment by centre
A summary of the key recruitment statistics by centre is provided in Table 12 . Between 3000 and 4000 invitations were mailed out from each of the three centres. The percentage of patients who agreed for the research team to contact them was around 30% in both Bristol and Exeter. The percentage agreeing to be contacted was lower in Glasgow but, otherwise, the percentage completing the assessment screen, who were eligible to attend a baseline assessment and who were randomised, was similar in all three centres.
| Characteristic | Bristol | Exeter | Glasgow | Total |
|---|---|---|---|---|
| No. of practices | 26 | 22 | 25 | 73 |
| Invitations/GP referrals | ||||
| GP referrals | 13 | 19 | 5 | 37 |
| Total invitations | 4088 | 3066 | 3512 | 10,666 |
| No. returned | 1793 | 1607 | 1189 | 4589 |
| No. accepted | 1185 | 984 | 778 | 2947 |
| Percentage accepted | 29.0 | 32.1 | 22.2 | 27.6 |
| Assessment screen completed | 915 | 805 | 596 | 2316 |
| Percentage completing assessment screen | 77.2 | 81.8 | 76.6 | 78.6 |
| Eligible for baseline assessment | 369 | 313 | 230 | 912 |
| Percentage eligible | 40.3 | 38.9 | 38.6 | 39.4 |
| Baseline assessments | 313 | 251 | 185 | 749 |
| Randomisations | ||||
| No. | 190 | 161 | 118 | 469 |
| Percentage of baseline assessments | 60.7 | 64.1 | 63.8 | 62.6 |
| Average/month | 9.5/month, 20 months | 8.9/month, 18 months | 6.6/month, 18 months | 8.4/month |
Follow-up of participants in the trial
Of the 469 randomised, 234 were allocated to receive CBT in addition to usual care and 235 were allocated to continue with usual care. All participants were taking antidepressant medication at the point of randomisation, with the expectation that this would continue as part of usual care for this patient group. The CONSORT flow diagram for the CoBalT trial is presented in Figure 4 . The number of individuals who withdrew from the study, were lost to follow-up or died is reported, as well as the number who could not be contacted at a particular follow-up but who were contacted later (‘unable to contact’ in Figure 4 ).
FIGURE 4.
Consolidated Standards of Reporting Trials (CONSORT) flow chart.
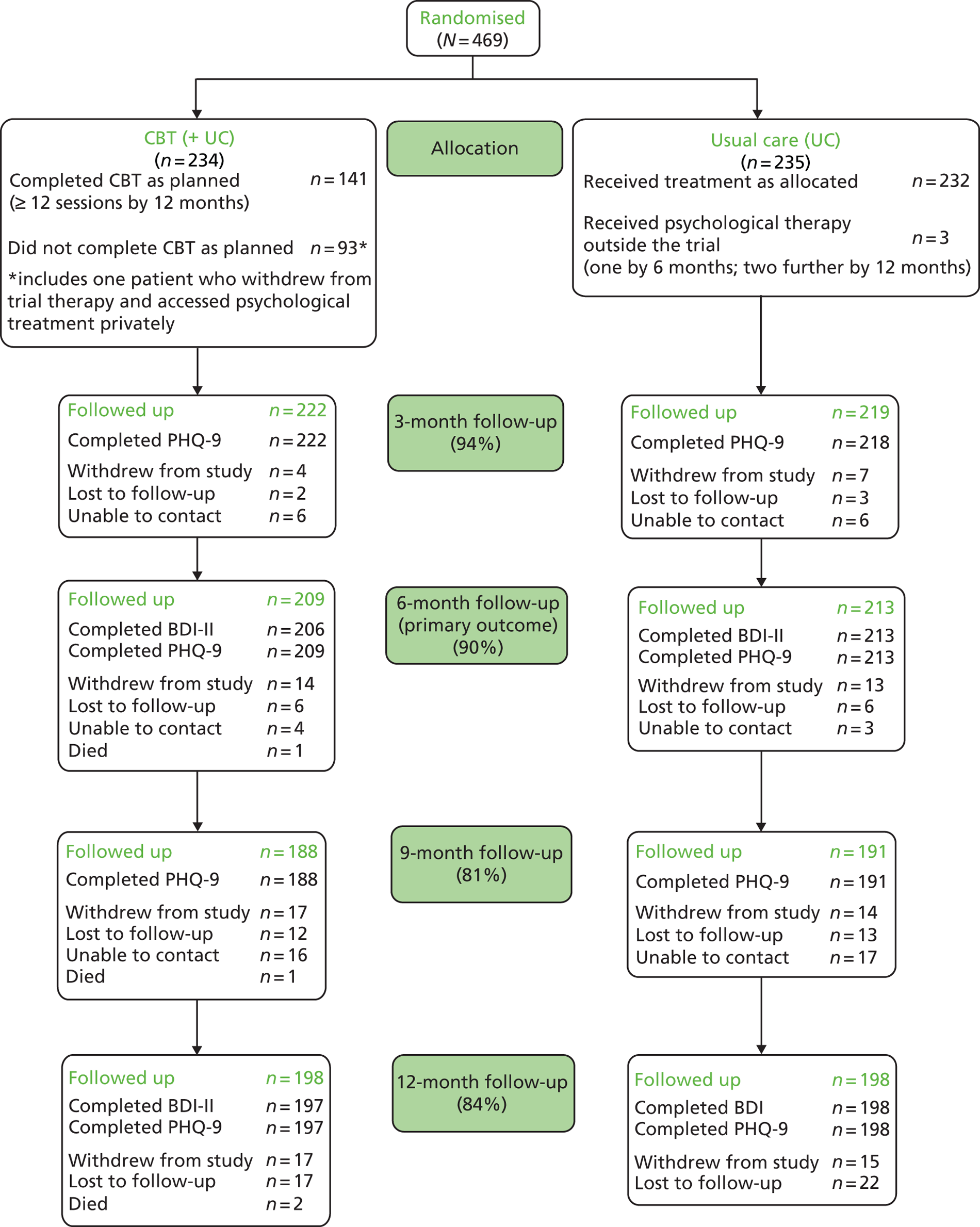
A summary of the follow-up rates by centre is given in Table 13 . Follow-up rates in Glasgow were lower than the other two centres. Part-way through recruitment, the Glasgow research team received additional support from the Scottish Mental Health Research Network (SMHRN), which enabled the research team to devote more resources to maximising the number of trial participants who were successfully contacted at the 12-month follow-up. This was prioritised over attaining a high follow-up rate at the brief 9-month telephone follow-up, hence explaining the lower follow-up rate at this time point. [The other two centres received additional support from the Mental Health Research Network (MHRN) from earlier in the study due to differences in the timing of the establishment of this network in England and Scotland.]
| Follow-up (months) | Bristol (n = 190) | Exeter (n = 161) | Glasgow (n = 118) | Total (n = 469) | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| 3 | 180 | 94.7 | 157 | 97.5 | 104 | 88.1 | 441 | 94.0 |
| 6 | 176 | 92.6 | 156 | 96.9 | 90 | 76.3 | 422 | 90.0 |
| 9 | 166 | 87.4 | 152 | 94.4 | 61 | 51.7 | 379 | 80.8 |
| 12 | 164 | 86.3 | 150 | 93.2 | 82 | 69.5 | 396 | 84.4 |
Follow-up assessments were scheduled to take place at 3, 6, 9 and 12 months following randomisation. These time frames were not always achieved. However, the mean time to each follow-up was close to the target ( Table 14 ) and the vast majority of follow-up assessments were conducted within 2 months of the target (see Table 14 ).
| Follow-up (months) | n | Mean (months) | SD | Within x ± 2 months | |
|---|---|---|---|---|---|
| n | % | ||||
| 3 | 441 | 3.1 | 0.4 | 437 | 99.1 |
| 6 | 422 | 6.4 | 0.7 | 406 | 96.2 |
| 9 | 379 | 9.1 | 0.4 | 376 | 99.2 |
| 12 | 396 | 12.6 | 1.2 | 367 | 92.7 |
Baseline characteristics of randomised participants
Sociodemographic characteristics
Of the 469 participants, 339 (72.3%) were women and the mean age was 49.6 years (SD 11.7). Forty-four per cent (n = 206) were in paid employment (full or part time). Forty-seven per cent (n = 217/463) were educated to A-level/Higher Grade or above, although, conversely, 25% (n = 116) had no formal qualifications. Twenty-seven per cent (n = 128) reported financial difficulty (finding it difficult or very difficult to ‘make ends meet’).
Severity and history of depression, and symptoms of anxiety
The mean BDI-II score at baseline was 31.8 (SD 10.7) denoting, on average, severe symptoms (defined as BDI-II score ≥ 29). This figure was very similar to the mean BDI-II score for the same individuals from the earlier screening questionnaire [mean 31.9 (SD 10.1)]. Fifty-eight per cent of participants met ICD-10 criteria for a moderate depressive episode and 28% (n = 129) met ICD-10 criteria for a severe depressive episode. The duration of the current episode of depression was 2 years or longer for 59% (n = 276) of participants. Seventy per cent (n = 327) of participants had been on their current course of antidepressant medication for > 12 months. Eighty per cent (n = 377) of participants had taken antidepressants prior to their current course.
The majority of participants (n = 415, 88%) had suffered from depression in the past and 52% (n = 245) reported having experienced five or more prior episodes of depression. Forty per cent of participants (n = 188) had previously been referred to a psychiatrist for their depression and 65% (n = 307) reported a family history of depression.
At baseline, the mean PHQ-9 score was 16.6 (SD 5.7) and the mean GAD-7 score was 11.7 (SD 5.1). Eighty-nine per cent of patients (n = 416) had a PHQ-9 score of ≥ 10 at baseline and 33% (n = 155) had a PHQ-9 score of ≥ 20, which is regarded as severe depression. Seventy-six per cent (n = 356) had a score of ≥ 8 on the GAD-7. Ninety-four per cent (n = 439) were a ‘case’ (based on the definitions used in IAPT32,33) on either the PHQ-9 or GAD-7 at baseline.
Secondary diagnoses
Although all participants fulfilled ICD-10 criteria for a depressive episode, most (n = 463, 99%) also had a secondary psychiatric diagnosis according to the CIS-R. Of these secondary diagnoses, generalised anxiety, mixed anxiety and depression and panic disorder were the most common ( Table 15 ).
| Diagnosis | n | % |
|---|---|---|
| Generalised anxiety disorder | 245 | 52.9 |
| Mixed anxiety and depression | 116 | 25.1 |
| Panic disorder | 67 | 14.5 |
| Specific (isolated) phobia | 17 | 3.7 |
| Agoraphobia | 10 | 2.2 |
| Social phobia | 8 | 1.7 |
| Total | 463 |
Antidepressant medication at baseline and in the year prior to randomisation
Sixty-one per cent of participants were taking the selective serotonin reuptake inhibitor (SSRI) citalopram or fluoxetine at doses of 20–80 mg daily at baseline ( Table 16 ). Other commonly prescribed antidepressants were venlafaxine, mirtazapine and paroxetine. A range of other medications were prescribed from all classes of antidepressants [SSRIs, serotonin–norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs) and noradrenaline reuptake inhibitors (NARIs)]. In total, 35 participants (7.5%) were taking two antidepressants at baseline (see Table 16 ).
| Antidepressant medication | Dose (mg) | CBT (n = 234) | Usual care (n = 235) | Total (n = 469) | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Class, name | |||||||
| SSRI, citalopram | 20–80 | 82 | 35.0 | 71 | 30.2 | 153 | 32.6 |
| SSRI, fluoxetine | 20–60 | 63 | 26.9 | 70 | 29.8 | 133 | 28.4 |
| SNRI, venlafaxine | 75–300 | 17 | 7.3 | 18 | 7.7 | 35 | 7.5 |
| OTHER, mirtazapine | 30–60 | 18 | 7.7 | 15 | 6.4 | 33 | 7.0 |
| SSRI, paroxetine | 20–40 | 15 | 6.4 | 12 | 5.1 | 27 | 5.8 |
| SSRI, sertraline | 100–400 | 9 | 3.8 | 6 | 2.6 | 15 | 3.2 |
| TCA, lofepramine | 140–210 | 8 | 3.4 | 5 | 2.1 | 13 | 2.8 |
| TCA, dosulepin | 150–175 | 2 | 0.9 | 6 | 2.6 | 8 | 1.7 |
| SSRI, escitalopram | 10–40 | 3 | 1.3 | 3 | 1.3 | 6 | 1.3 |
| NARI, reboxetine | 8–12 | 2 | 0.9 | 1 | 0.4 | 3 | 0.6 |
| TCA, amitriptyline | 150–180 | 0 | 0 | 3 | 1.3 | 3 | 0.6 |
| SNRI, duloxetine | 60–90 | 0 | 0 | 2 | 0.9 | 2 | 0.4 |
| TCA, trazodone | 150–300 | 0 | 0 | 2 | 0.9 | 2 | 0.4 |
| TCA, clomipramine | 250 | 1 | 0.4 | 0 | 0 | 1 | 0.2 |
| Combination treatment | |||||||
| Citalopram and amitriptyline | 20–50/10–150 | 2 | 0.9 | 5 | 2.1 | 7 | 1.5 |
| Citalopram and mirtazapine | 20–40/15–22.5 | 3 | 1.3 | 2 | 0.9 | 5 | 1.1 |
| Citalopram and trazodone | 40/50 | 1 | 0.4 | 0 | 0 | 1 | 0.2 |
| Fluoxetine and amitriptyline | 20–40/20–120 | 3 | 1.3 | 9 | 3.8 | 12 | 2.6 |
| Mirtazapine and amitriptyline | 30/50 | 2 | 0.9 | 0 | 0 | 2 | 0.4 |
| Paroxetine and bupropion | 30/300 | 1 | 0.4 | 0 | 0 | 1 | 0.2 |
| Fluoxetine and citalopram | 80/20 | 0 | 0 | 1 | 0.4 | 1 | 0.2 |
| Nortriptyline and sertraline | 100/100 | 0 | 0 | 1 | 0.4 | 1 | 0.2 |
| Paroxetine and amitriptyline | 40/20 | 0 | 0 | 1 | 0.4 | 1 | 0.2 |
| Sertraline and mirtazapine | 50–300/15–45 | 2 | 0.9 | 0 | 0 | 2 | 0.4 |
| Venlafaxine and amitriptyline | 150/225 | 0 | 0 | 1 | 0.4 | 1 | 0.2 |
| Venlafaxine and citalopram | 75/10 | 0 | 0 | 1 | 0.4 | 1 | 0.2 |
In total, 453 participants (96.6%) gave their consent for the research team to access their medical records. Data on antidepressant prescriptions in the year prior to randomisation were available for 413 individuals. On average (median), participants received eight prescriptions for antidepressant medication over the previous year (IQR 6–12). In total, 339 individuals (82.1% of 413) were prescribed only one type of antidepressant in the year prior to randomisation.
Self-reported long-standing illness, disability or infirmity
Seventy-five per cent of participants (n = 351) reported that they had a long-standing illness, disability or infirmity, based on a list of six closed responses ( Table 17 ). This cannot be taken to indicate just physical illness, as this was not clear from the question. Therefore, some of who indicated ‘another illness’ may have included depression in their response.
| Chronic illness | n | (%) |
|---|---|---|
| Diabetes | 16 | 4.6 |
| Asthma | 28 | 8.0 |
| Arthritis | 38 | 10.8 |
| Heart disease | 9 | 2.6 |
| High blood pressure | 27 | 7.7 |
| Lung disease | 5 | 1.4 |
| More than one of the above | 79 | 22.5 |
| Another illness (not listed above) | 149 | 42.5 |
Patient preferences and expectations of the effectiveness of cognitive behavioural therapy
Prior to randomisation, participants were asked whether or not they had a preference for treatment. Sixty per cent of participants (n = 281) indicated that they would prefer to receive CBT in addition to usual care, 39% (n = 183) had no preference and a small number (n = 5; 1%) indicated that they would prefer to continue with usual care. Participants were also asked to indicate whether they thought that CBT (in addition to antidepressant medication) would be an effective treatment for them. Almost half of participants were unsure whether or not CBT would help them, although 50% thought that the intervention would ‘probably or definitely’ help them ( Table 18 ).
| Response option | n | (%) |
|---|---|---|
| CBT would definitely help me | 98 | 20.9 |
| CBT would probably help me | 138 | 29.4 |
| I don’t know if CBT would help me | 228 | 48.6 |
| CBT would probably not help me | 5 | 1.1 |
| CBT would definitely not help me | 0 |
Baseline comparability of randomised groups
Table 19 provides a summary of the key descriptive statistics used to assess the baseline comparability of the randomised groups. Data on a large number of variables including sociodemographic factors, severity of depression, history of depression and treatment, and comorbidity were collected at baseline. A number of imbalances were observed. The intervention group comprised more men, a greater proportion in paid employment, more who reported financial difficulty, fewer individuals with caring responsibilities, fewer individuals with long-standing illness/disability and better physical function (on the SF-12). A smaller proportion of those in the intervention group reported five or more prior episodes of depression but a greater proportion had a family history of depression. A smaller proportion of the intervention group reported taking their current course of antidepressant for > 12 months.
| Characteristic | Intervention (n = 234) | Usual care (n = 235) |
|---|---|---|
| Stratification variable: centre n (%) | ||
| Bristol | 95 (40.6) | 95 (40.4) |
| Exeter | 79 (33.8) | 82 (34.9) |
| Glasgow | 60 (25.6) | 58 (24.7) |
| Minimisation variables: n (%) | ||
| Previously prescribed antidepressants | 187 (79.9) | 190 (80.9) |
| BDI-II score: | ||
| 14–19 | 24 (10.3) | 28 (11.9) |
| 20–28 | 78 (33.3) | 75 (31.9) |
| ≥ 29 | 132 (56.4) | 132 (56.2) |
| GP practice has a counsellor | 112 (47.9) | 116 (49.4) |
| Duration of current episode of depression (years) | ||
| < 1 | 58 (24.8) | 52 (22.1) |
| 1–2 | 40 (17.1) | 43 (18.3) |
| > 2 | 136 (58.1) | 140 (59.6) |
| Sociodemographic variables | ||
| Age (years): mean (SD) | 49.2 (11.9) | 50.0 (11.5) |
| Female: n (%) | 161 (68.8) | 178 (75.7) |
| Ethnic group, white: n (%) | 231 (98.7) | 228 (97.0) |
| Marital status: n (%) | ||
| Married/living as married | 120 (51.3) | 128 (54.5) |
| Single | 44 (18.8) | 45 (19.2) |
| Separated/divorced/widowed | 70 (29.9) | 62 (26.4) |
| Employment status: n (%) | ||
| In paid employment (full/part-time) | 109 (46.6) | 97 (41.3) |
| Not in employment | 58 (24.8) | 75 (31.9) |
| Unemployed owing to ill health | 67 (28.6) | 63 (26.8) |
| Highest educational qualification: n (%)a | ||
| A-level, Higher Grade or above | 112 (48.3) | 105 (45.5) |
| GCSE, Standard Grade or other | 63 (27.2) | 67 (29.0) |
| No formal qualifications | 57 (24.6) | 59 (25.5) |
| Financial difficulty: n (%) | ||
| Living comfortably/doing all right | 74 (31.6) | 93 (39.6) |
| Just about getting by | 91 (38.9) | 83 (35.3) |
| Finding it difficult/very difficult to make ends meet | 69 (29.5) | 59 (25.1) |
| Caring responsibilities: n (%) | 29 (12.4) | 35 (14.9) |
| Long-standing illness or disability: n (%) | 170 (72.7) | 181 (77.0) |
| No. of life events in past 6 months: mean (SD) | 1.3 (1.2) | 1.2 (1.1) |
| Social support score: mean (SD) | 11.8 (3.9) | 12.2 (3.7) |
| History of depression | ||
| Suffered from depression in past: n (%) | 206 (88.0) | 209 (88.9) |
| No. of prior episodes of depression: n (%) | ||
| 0–1 | 46 (19.7) | 45 (19.2) |
| 2–4 | 72 (30.8) | 61 (26.0) |
| ≥ 5 | 116 (49.6) | 129 (54.9) |
| Previous referral to a psychiatrist for depression: n (%) | 95 (40.6) | 93 (39.6) |
| Family history of depression: n (%) | 159 (68.0) | 148 (63.0) |
| Length of current course of antidepressants (months): n (%) | ||
| < 6 | 26 (11.1) | 23 (9.8) |
| 6–12 | 51 (21.8) | 42 (17.9) |
| > 12 | 157 (67.1) | 170 (72.3) |
| CIS-R score: mean (SD) | 30.1 (9.1) | 30.0 (8.8) |
| ICD-10 primary diagnosis: n (%) | ||
| Mild | 35 (15.0) | 31 (13.2) |
| Moderate | 135 (57.7) | 139 (59.2) |
| Severe | 64 (27.4) | 65 (27.7) |
| BDI-II score: mean (SD) | 31.8 (10.5) | 31.8 (10.9) |
| Suicidal ideation (CIS-R thoughts/plans): n (%) | 73 (31.1) | 75 (31.9) |
| PHQ-9 score: mean (SD) | 16.6 (5.7) | 16.6 (5.7) |
| GAD-7 score: mean (SD) | 11.7 (5.0) | 11.8 (5.1) |
| Panic score: median (IQR)b | 3 (0 to 5) | 3 (0 to 5) |
| SF-12 mental subscale: mean (SD)c | 28.5 (9.0) | 28.7 (9.3) |
| SF-12 physical subscale: mean (SD)c | 45.3 (13.0) | 41.6 (13.7) |
Losses to follow-up
Follow-up rates were similar in both arms at all time points (3, 6, 9 and 12 months). At 6 months, the time of the measurement of the primary outcome, 90% of participants were followed up. Eighty-four per cent of participants were followed up at 12 months (see Follow-up of participants in the trial and Figure 4 , above).
Missing data
The pattern of missing data was investigated by identifying those variables recorded at baseline that were associated with ‘missingness’ of the primary outcome (BDI-II score) at the 6- and 12-month follow-ups. For brevity, Table 20 presents a summary of the baseline variables associated with missingness at p < 0.20 at either 6-month follow-up (see Table 20a ) or 12-month follow-up (see Table 20b ).
| Baseline variable | Missing (n = 50) | Present (n = 419) | p-value |
|---|---|---|---|
| Age (years): mean (SD) | 47.8 (11.0) | 49.8 (11.8) | 0.27 |
| Female: n (%) | 31 (62.0) | 308 (73.5) | 0.09 |
| Marital status: n (%) | |||
| Married/living as married | 25 (50.0) | 223 (53.2) | 0.20 |
| Single | 14 (28.0) | 75 (17.9) | |
| Separated/divorced/widowed | 11 (22.0) | 121 (28.9) | |
| Highest educational qualification: n (%)a | |||
| A-level, Higher Grade or above | 23 (46.9) | 194 (46.9) | 0.02 |
| GCSE, Standard Grade or other | 7 (14.3) | 123 (29.7) | |
| No formal qualifications | 19 (38.8) | 97 (23.4) | |
| Financial difficulty: n (%) | |||
| Living comfortably/doing all right | 10 (20.0) | 157 (37.5) | 0.03 |
| Just about getting by | 20 (40.0) | 154 (36.8) | |
| Finding it difficult/very difficult to make ends meet | 20 (40.0) | 108 (25.8) | |
| At least one car in household: n (%) | 27 (54.0) | 283 (67.5) | 0.06 |
| Home ownership: n (%) | |||
| Home owner | 20 (40.0) | 226 (53.9) | 0.10 |
| Tenant | 26 (52.0) | 152 (36.3) | |
| Other | 4 (8.0) | 41 (9.8) | |
| Smoking habits: n (%) | |||
| Current smoker | 29 (58) | 131 (31.3) | 0.001 |
| Ex-smoker | 10 (20) | 147 (35.1) | |
| Never smoked | 11 (22) | 141 (33.7) | |
| Social support score: mean (SD) | 11.3 (4.3) | 12.1 (3.7) | 0.16 |
| History of depression: n (%) | |||
| Suffered from depression in the past | 41 (82.0) | 374 (89.3) | 0.13 |
| ICD-10 primary diagnosis: n (%) | |||
| Mild | 5 (10.0) | 61 (14.6) | 0.05 |
| Moderate | 24 (48.0) | 250 (59.7) | |
| Severe | 21 (42.0) | 108 (25.8) | |
| Suicidal ideation (CIS-R thoughts/plans): n (%) | 17 (34.0) | 131 (31.3) | 0.69 |
| Big Five neuroticism scale: mean (SD)b | 4.1 (0.5) | 4.1 (0.6) | 0.48 |
| PC-PTSD score: mean (SD)c | 2.3 (1.6) | 2.0 (1.5) | 0.29 |
| Panic score: median (IQR)d | 3.5 [0, 5] | 3 [0, 5] | 0.51 |
| SF-12 physical subscale: mean (SD)e | 40.7 (13.7) | 43.7 (13.4) | 0.14 |
| Dysfunctional attitudes score: mean (SD) | 33.8 (10.1) | 37.7 (10.9) | 0.08 |
| Characteristic | Missing (n = 74) | Present (n = 395) | p-value |
|---|---|---|---|
| Age (years): mean (SD) | 47.4 (11.8) | 50.0 (11.6) | 0.08 |
| Female: n (%) | 46 (62.2) | 293 (74.2) | 0.03 |
| Marital status: n (%) | |||
| Married/living as married | 35 (47.3) | 213 (53.9) | 0.40 |
| Single | 18 (24.3) | 71 (18.0) | |
| Separated/divorced/widowed | 21 (28.4) | 111 (28.1) | |
| Highest educational qualification: n (%)a | |||
| A-level, Higher Grade or above | 27 (37.0) | 190 (48.7) | 0.003 |
| GCSE, Standard Grade or other | 16 (21.9) | 114 (29.2) | |
| No formal qualifications | 30 (41.1) | 86 (22.1) | |
| Financial difficulty: n (%) | |||
| Living comfortably/doing all right | 12 (16.2) | 155 (39.2) | < 0.001 |
| Just about getting by | 30 (40.5) | 144 (36.5) | |
| Finding it difficult/very difficult to make ends meet | 32 (43.2) | 96 (24.3) | |
| At least one car in household: n (%) | 38 (51.4) | 272 (68.9) | 0.003 |
| Home ownership: n (%) | |||
| Home owner | 28 (37.8) | 218 (55.2) | 0.02 |
| Tenant | 38 (51.4) | 140 (35.4) | |
| Other | 8 (10.8) | 37 (9.4) | |
| Smoking habits: n (%) | |||
| Current smoker | 42 (56.8) | 118 (29.9) | < 0.001 |
| Ex-smoker | 17 (23.0) | 140 (35.4) | |
| Never smoked | 15 (20.3) | 137 (34.7) | |
| Social support score: mean (SD) | 11.6 (3.9) | 12.1 (3.8) | 0.28 |
| History of depression: n (%) | |||
| Suffered from depression in the past | 62 (83.8) | 353 (89.4) | 0.17 |
| ICD-10 primary diagnosis: n (%) | |||
| Mild | 8 (10.8) | 58 (14.7) | 0.16 |
| Moderate | 39 (52.7) | 235 (59.5) | |
| Severe | 27 (36.5) | 102 (25.8) | |
| Suicidal ideation (CIS-R thoughts/plans): n (%) | 30 (40.5) | 118 (29.9) | 0.07 |
| Big Five neuroticism scale: mean (SD)b | 4.2 (0.6) | 4.0 (0.6) | 0.17 |
| PC-PTSD score: mean (SD)c | 2.4 (1.5) | 2.0 (1.5) | 0.05 |
| Panic score: median (IQR)d | 4 (0 to 5) | 1 (0 to 5) | 0.04 |
| SF-12 physical subscale: mean (SD)e | 41.3 (13.5) | 43.8 (13.5) | 0.15 |
| Dysfunctional attitudes score: mean (SD) | 36.0 (10.9) | 36.4 (10.8) | 0.75 |
There was evidence that older participants, women and those from higher socioeconomic backgrounds (as indicated by educational qualifications, financial difficulty, car ownership, smoking status and home ownership) were less likely to have missing outcome data, as were those with greater social support. In contrast, those who were single were more likely to have missing outcome data. There was some evidence that those with more severe depression at baseline (based on ICD-10 diagnosis and suicidal ideation) were more likely to have missing outcome data. Those who scored more highly on a brief measure of symptoms of PTSD were more likely to have missing outcome data, as were those who scored more highly on measures of panic or neuroticism. Those with better physical function at baseline (SF-12 physical subscale score) were less likely to have missing outcome data. Similarly, those with higher scores on the DAS were also less likely to have missing outcome data.
Excluded from this table are the stratification/minimisation variables that would be included by default in the imputation model as they as part of the ITT model. In addition to the variables described above, all available measures of depressive symptoms (BDI-II and PHQ-9) were included in the imputation model. Measures of symptoms of anxiety (GAD-7) that are likely to be closely correlated with depressive symptoms were also included in the imputation irrespective of statistical significance, as was the baseline CIS-R score, another marker of severity.
Comparison of the imputed values with the observed outcomes, in terms of BDI-II scores, at 6 and 12 months suggested that those who were missing outcome data had a worse outcome than those who were followed up ( Table 21 ).
| Data | Follow-up | |||
|---|---|---|---|---|
| 6-month | 12-month | |||
| n | Mean BDI-II score | n | Mean BDI-II score | |
| Observed | 419 | 21.8 | 395 | 19.3 |
| Imputeda | 50 | 25.6 | 74 | 22.6 |
Delivery of, and adherence to, the intervention
The average (median) time elapsed from randomisation to the first session of CBT was 29 days (IQR 17–46) (range 3–126 days). In 10% of participants (n = 23), the time from randomisation to the first CBT session was > 66 days. The longer intervals between randomisation and start of CBT reflected a number of factors. Some participants asked to postpone the start of therapy because of other commitments, and others proved hard to contact following randomisation to arrange a date for their first CBT session. Therapists in Bristol and Exeter reached capacity part-way through recruitment. This also contributed to a lengthier interval between randomisation and the start of CBT for a short period until additional therapists were employed to work on the trial.
On average, the total time in therapy (from randomisation) was 6.3 months (SD 3.0 months). The range was from 0 months (for those who declined to take up CBT following randomisation) to 16.5 months. The total time in therapy reflected the wait from randomisation, the number of sessions attended and the time elapsed through any sessions that were cancelled or not attended.
Nine out of the eleven CoBalT therapists delivered 97% of the intervention sessions. Among these nine therapists, the number of patients assigned to them ranged from 13 (5.6% of the total) to 41 (17.5%), reflecting the range in the FTE basis on which the therapists were employed and their duration of employment ( Table 22 ).
| Centre | Therapist ID | n | % |
|---|---|---|---|
| Bristol | 101 | 26 | 11.1 |
| 102 | 29 | 12.4 | |
| 103 | 20 | 8.6 | |
| 104 | 13 | 5.6 | |
| 105 | 4 | 1.7 | |
| 106 | 3 | 1.3 | |
| Exeter | 201 | 41 | 17.5 |
| 202 | 15 | 6.4 | |
| 203 | 23 | 9.8 | |
| Glasgow | 301 | 39 | 16.7 |
| 302 | 21 | 9.0 |
In total, the therapists delivered 2630 sessions of CBT primarily at the patients’ GP surgery or other NHS premises ( Table 23 ). There were differences in where the sessions took place between the three centres, which reflected differences in how existing psychological services were provided. In Bristol, most patients were seen at their GP surgery. In Exeter, most sessions were held at the centrally located Mood Disorders Centre, as would be the case for patients referred for psychological therapy on the NHS. In Glasgow, patients were mostly seen at the NHS Clinical Research Facility.
| Location | Bristol | Exeter | Glasgow | Total |
|---|---|---|---|---|
| GP surgery | 1079 (95.8) | 311 (31.2) | 80 (15.8) | 1470 (55.9) |
| Mood disorders centre | 0 | 682 (68.4) | 0 | 682 (25.9) |
| Clinical research facility | 0 | 0 | 384 (75.7) | 384 (14.6) |
| Home | 12 (1.7) | 0 | 0 | 12 (0.5) |
| Other | 35 (3.1) | 4 (0.4) | 43 (8.5) | 82 (3.1) |
| Total | 1126 | 997 | 507 | 2630 |
Twenty participants (8.5%) did not attend any sessions of CBT ( Table 24 ). By 6 months, on average (median), those randomised to the intervention had received 11 sessions of CBT (IQR 5–13). One hundred and forty-four participants (61.5%) had received at least nine sessions of CBT by the 6-month follow-up. By 12 months, the median number of sessions received was 12 (IQR 6 to 17) and a total of 141 participants (60.3%) had received at least 12 sessions of CBT (see Table 24 ).
| No. of sessions attended | n | % |
|---|---|---|
| 0 | 20 | 8.5 |
| 1–2 | 16 | 6.8 |
| 3–5 | 22 | 9.4 |
| 6–7 | 9 | 3.8 |
| 8–9 | 11 | 4.7 |
| 10–11 | 15 | 6.4 |
| 12–13 | 35 | 15.0 |
| 14–15 | 26 | 11.1 |
| 16–17 | 23 | 9.8 |
| 18–19a | 57 | 24.4 |
Twenty-seven participants (11.5%) were discharged for non-adherence to the intervention (repeatedly having failed to attend appointments) ( Table 25 ) [median number of sessions attended 4 (IQR 1–10); range 0–16], and 47 (20.1%) withdrew from CBT [median number of sessions attended 2 (IQR 0–4); range 0–11]. Twenty-three participants reached an ‘agreed end’ in less than 12 sessions.
| Therapy outcome | Bristol | Exeter | Glasgow | Total |
|---|---|---|---|---|
| Discharged for non-adherence to the intervention | 11 (11.6) | 5 (6.3) | 11 (18.3) | 27 (11.5) |
| Withdrew from therapy | 19 (20.0) | 14 (17.7) | 14 (23.3) | 47 (20.1) |
| ‘Agreed end’ | 65 (68.4) | 60 (76.0) | 35 (58.3) | 160 (68.4) |
| a‘Agreed end’ excluding those who reached an ‘agreed end’ in < 12 sessions | 60 | 49 | 28 | 137 |
Although patients in Glasgow appeared to be more likely to be discharged for non-adherence to the intervention (see Table 25 ), such differences were no greater than would be expected by chance (chi-squared test: p = 0.18).
Fidelity of the cognitive behavioural therapy intervention
Based on a sample of nine recordings (one for each of the nine main therapists working on the trial), inter-rater reliability for the three raters evaluating fidelity to the CBT model was high (ICC 0.81), indicating very good agreement between the raters. Using a sample of a further 54 sessions selected at random, the mean CTS-R rating (adjusted for caseload) was 38.8 (95% CI 36.7 to 40.8), exceeding the cut-point of 36 that equates to a mean item score of 3.0 or ‘competent’.
Primary outcome
Depressive symptoms considered in terms of ‘response’ at 6 months
The primary outcome at 6 months was ‘response’, which was defined as at least a 50% reduction in depressive symptoms measured using the BDI-II compared with baseline. Forty-six per cent of those randomised to the intervention ‘responded’ compared with 22% of those in usual care ( Table 26 ). The results are presented in Table 26 as an OR of ‘response’ in the intervention group compared with the usual-care group. An OR of > 1 indicates that ‘response’ was more likely in the intervention group at 6 months.
| N | n | (%)a | ORb | 95% CI | p-value | ORc | 95% CI | p-value | |
|---|---|---|---|---|---|---|---|---|---|
| Intervention | 206 | 95 | (46.1) | 3.26 | 2.10 to 5.06 | < 0.001 | 3.48 | 2.17 to 5.57 | < 0.001 |
| Usual care | 213 | 46 | (21.6) | ||||||
| Total N | 419 | 419 | 415 | ||||||
In the primary ITT analysis, those in the intervention group had a threefold increased odds of ‘response’ compared with those in the usual-care group, with the 95% CI excluding the possibility that there was no difference between the groups (column ‘ORb’ in Table 26 ). Additional adjustment for the time (in days) from randomisation to completion of the 6-month follow-up did not change the effect estimate [OR 3.25 (95% CI 2.09 to 5.05) p < 0.001]. Adjustment for the variables that were imbalanced between treatment groups (see Baseline comparability of randomised groups, above) slightly increased the magnitude of the treatment effect (column ‘ORc’ in Table 26 ).
Subgroup analyses
For the two a priori subgroup analyses, there was no evidence that patient expectation of outcome or degree of treatment resistance had any effect on the difference between intervention and usual-care groups (p-values for interaction with treatment group: 0.16 and 0.88, respectively).
In a post hoc subgroup analysis there was no evidence that study centre had any effect on the difference between the intervention and usual-care groups (p-value for interaction between treatment allocation and centre: 0.61).
Clustering effects by therapist
The ICC across therapists was calculated using a random-effects logistic regression model. There was little evidence of clustering of outcomes: ICC before adjustment for baseline BDI-II score 0.00058, attenuated substantially to 0.000009 following adjustment for baseline BDI-II score. Considering the BDI-II score as a continuous outcome, the ICC was also very small (0.051 and 0.0027, respectively, before and after adjustment for baseline BDI-II score).
In a fully heteroscedastic model that accounted for the possibility of clustering by therapist, the OR for the primary outcome of ‘response’ was 3.26 (95% CI 2.10 to 5.06). This is identical to that obtained from the primary ITT analysis (see Table 26 ), demonstrating that there was no evidence that clustering by therapist had any influence on the findings.
Sensitivity analyses to examine the impact of missing data
Table 27 presents the results of the ITT analysis for the 419 ‘complete cases’, with BDI-II outcome data at 6 months and the results from an ITT analysis for which the missing data had been imputed using the method of MICE. The results imputing missing data were consistent with the results of the primary ITT ‘complete-case’ analysis, although the OR had decreased very slightly using the imputed data set.
| ‘Response’ | n | ORa | 95% CI | p-value |
|---|---|---|---|---|
| ITT adjusted for design variables | 419 | 3.26 | 2.10 to 5.06 | < 0.001 |
| MICE estimates | 469 | 3.10 | 2.00 to 4.80 | < 0.001 |
Secondary outcomes
Depressive symptoms on the Beck Depression Inventory (second version) at 6-month follow-up
Beck Depression Inventory (second version) as a continuous score
Table 28 summarises the means and differences in mean BDI-II scores at 6 months. Those in the intervention group had a BDI-II score at 6 months that was, on average, 5.7 points lower (less depressed) than those in the usual-care group, with a 95% CI ranging from a three- to eight-point reduction in BDI-II score see (see Table 28 ). The mean difference between groups equated to an effect size of 0.53 SD [baseline SD for BDI-II (pooled): 10.7]. Additional adjustment for the variables that were imbalanced at baseline did not make any difference to the observed effect.
| n | Mean | SD | Difference in meansa | 95% CI | p-value | Difference in meansb | 95% CI | p-value | |
|---|---|---|---|---|---|---|---|---|---|
| Intervention | 206 | 18.9 | 14.2 | −5.7 | −7.9 to −3.4 | < 0.001 | −5.5 | −7.8 to −3.2 | < 0.001 |
| Usual care | 213 | 24.5 | 13.1 | ||||||
| Total N | 419 | 419 | 415 |
The results imputing missing data were consistent with the results of the ITT ‘complete-case’ analysis [MICE estimates: –5.5 (95% CI –7.8 to –3.3), p < 0.001].
‘Remission’ at 6-month follow-up
Table 29 summarises the percentages and ORs of ‘remission’ (defined as having a BDI-II score of < 10) at 6 months. Those in the intervention group had a twofold increased odds of ‘remission’ at 6 months compared with those in the usual-care group (see Table 29 ). Adjustment for those variables that were imbalanced between treatment groups at baseline had very little effect, if anything resulting in a slight increase in the estimated effect.
| N | n | %a | ORb | 95% CI | p-value | ORc | 95% CI | p-value | |
|---|---|---|---|---|---|---|---|---|---|
| Intervention | 206 | 57 | 27.7 | 2.30 | 1.39 to 3.81 | 0.001 | 2.35 | 1.37 to 4.02 | 0.002 |
| Usual care | 213 | 32 | 15.0 | ||||||
| Total N | 419 | 419 | 415 | ||||||
The results imputing missing data were again very consistent with the results of the ITT ‘complete-case’ analysis [MICE estimates: OR 2.34 (95% CI 1.43 to 3.85), p = 0.001].
Depressive symptoms on the Beck Depression Inventory (second version) over the duration of the study
‘Response’
Table 30 summarises the percentages and ORs of ‘response’ (defined as a reduction in depressive symptoms on the BDI-II of at least 50% relative to baseline) at 6 and 12 months. Based on a repeated measures logistic regression analysis using data from both 6 and 12 months, the intervention group had overall a threefold increased odds of ‘response’ over the 12 months compared with those in the usual-care group, with the 95% CI surrounding this estimate ranging from a two- to fourfold increased odds (see Table 30 ). There was no evidence that the difference between the intervention and usual-care groups varied over time (interaction between treatment and time: p = 0.49).
| Follow-up | ||||||
|---|---|---|---|---|---|---|
| 6 months (n = 419) | 12 months (n = 395) | |||||
| N | n | %a | N | n | %a | |
| Intervention | 206 | 95 | 46.1 | 197 | 109 | 55.3 |
| Usual care | 213 | 46 | 21.6 | 198 | 62 | 31.3 |
| N | ORb | 95% CI | p-value | |||
| 6-month follow-up | 419 | 3.26 | 2.10 to 5.06 | |||
| 12-month follow-up | 395 | 2.79 | 1.84 to 4.24 | |||
| Repeated measures | 814 | 2.89 | 2.03 to 4.10 | < 0.001 | ||
Beck Depression Inventory (version 2) as a continuous score
Table 31 summarises the means and differences in mean BDI-II scores at 6 and 12 months. Based on a repeated measures linear regression analysis using data from both 6 and 12 months, the intervention group had a BDI-II score that was, on average, 5 points lower (less depressed) than those in the usual-care group over the 12 months (see Table 31 ). There was no evidence that the difference between the intervention and usual-care groups varied over time (interaction between treatment and time: p = 0.36).
| 6 months (n = 419) | 12 months (n = 395) | |||||
|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | |
| Intervention | 206 | 18.9 | 14.2 | 197 | 17.0 | 14.0 |
| Usual care | 213 | 24.5 | 13.1 | 198 | 21.7 | 12.9 |
| N | Difference in meansa | 95% CI | p-value | |||
| 6-month follow-up | 419 | −5.7 | −7.9 to −3.4 | |||
| 12-month follow-up | 395 | −5.0 | −7.3 to −2.6 | |||
| Repeated measures | 814 | −5.1 | −7.1 to −3.1 | < 0.001 | ||
‘Remission’
Table 32 summarises the percentages and ORs of ‘remission’ (defined as a BDI-II score of < 10) at 6 and 12 months. Based on a repeated measures logistic regression analysis using data from both 6 and 12 months, overall the intervention group had a threefold increased odds of ‘remission’ over the 12 months compared with those in the usual-care group (see Table 32 ). There was no evidence that the difference between the intervention and usual-care groups varied over time (interaction between treatment and time: p = 0.20).
| 6 months (n = 419) | 12 months (n = 395) | |||||
|---|---|---|---|---|---|---|
| N | n | %a | N | n | (%)a | |
| Intervention | 206 | 57 | 27.7 | 197 | 78 | (39.6) |
| Usual care | 213 | 32 | 15.0 | 198 | 36 | (18.2) |
| N | ORb | 95% CI | p-value | |||
| 6-month follow-up | 419 | 2.30 | 1.39 to 3.81 | |||
| 12-month follow-up | 395 | 3.30 | 2.04 to 5.34 | |||
| Repeated measures | 814 | 2.74 | 1.82 to 4.13 | < 0.001 | ||
Depressive symptoms on the Patient Health Questionnaire-9 items at 6 months and over the duration of the study
A beneficial effect of the intervention in terms of depressive symptoms was confirmed when PHQ-9 scores were compared for the intervention and control groups ( Table 33 ). Those in the intervention group had a PHQ-9 score that was, on average, 3 points lower than those in the usual-care group at 6 months. The difference in means between groups equated to an effect size of 0.53 SD [baseline SD for PHQ-9 (pooled): 5.7]. There was very little effect when additional adjustment for those variables that were imbalanced between groups at baseline was made.
| Group | n | Mean | SD | Difference in meansa | 95% CI | p-value | Difference in meansb | 95% CI | p-value |
|---|---|---|---|---|---|---|---|---|---|
| Intervention | 209 | 9.5 | 6.7 | −3.0 | −4.2 to −1.8 | < 0.001 | −2.8 | −4.0 to −1.7 | < 0.001 |
| Usual care | 213 | 12.5 | 6.6 | ||||||
| Total N | 422 | 422 | 417 | ||||||
The means and differences in mean PHQ-9 scores at 3, 6, 9 and 12 months are summarised in Table 34 . Based on a repeated measures linear regression analysis using data from 6, 9 and 12 months, the intervention group had a PHQ-9 score that was, on average, just under 3 points lower (less depressed) than those in the usual-care group over the 12 months (see Table 34 ). There was weak evidence that the difference between the intervention and usual-care groups varied over time (interaction between treatment and time: p = 0.059), reflecting the smaller difference in means between treatment groups at 12 months. Data from the 3-month follow-up was not included in the repeated measures analysis because the purpose of this follow-up was to maintain contact with trial participants and provide data that could be useful in imputing later missing outcomes. Moreover, it was not expected that there would be any difference between the treatment groups at 3 months because those in the intervention group would only have attended a small number of CBT sessions at this point.
| Follow-up | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3 months (n = 440) | 6 months (n = 422) | 9 months (n = 379) | 12 months (n = 395) | |||||||||
| n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | |
| Intervention | 222 | 12.4 | 6.4 | 209 | 9.5 | (6.7) | 188 | 8.8 | (6.9) | 197 | 9.0 | (7.0) |
| Usual care | 218 | 13.0 | 6.0 | 213 | 12.5 | (6.6) | 191 | 12.0 | (6.2) | 198 | 10.9 | (6.4) |
| N | Difference in meansa | 95% CI | p-value | |||||||||
| 6-month follow-up | 422 | −3.0 | −4.2 to −1.8 | |||||||||
| 9-month follow-up | 379 | −3.3 | −4.5 to −2.0 | |||||||||
| 12-month follow-up | 395 | −2.0 | −3.2 to −0.8) | |||||||||
| Repeated measures | 1196 | −2.8 | −3.7 to −1.8 | < 0.001 | ||||||||
Anxiety on the Generalised Anxiety Disorder Assessment-7 items at 6 months and over the duration of the study
Those in the intervention group had a GAD-7 score that was, on average, 2.5 points lower than those in the usual-care group at 6 months ( Table 35 ). There was, therefore, a benefit in terms of a greater reduction in symptoms of anxiety for those who received the intervention. The difference in means between groups equated to an effect size of 0.49 SD [baseline SD for GAD-7 (pooled): 5.1]. As for the other outcomes, adjustment for those variables that were imbalanced at baseline had very little effect.
| Group | n | Mean | SD | Difference in meansa | 95% CI | p-value | Difference in meansb | 95% CI | p-value |
|---|---|---|---|---|---|---|---|---|---|
| Intervention | 207 | 7.0 | 5.9 | −2.5 | −3.4 to −1.5 | < 0.001 | −2.4 | −3.4 to −1.5 | < 0.001 |
| Usual care | 213 | 9.5 | 5.6 | ||||||
| Total N | 420 | 420 | 416 | ||||||
The means and differences in mean GAD-7 scores at 6 and 12 months are summarised in Table 36 . Based on a repeated measures linear regression analysis using data from 6 and 12 months, the intervention group had a GAD-7 score that was, on average, 2 points lower (less anxious) than those in the usual-care group over the 12 months (see Table 36 ). There was no evidence that the difference between the intervention and usual-care groups varied over time (interaction between treatment and time: p = 0.19).
| Follow-up | ||||||
|---|---|---|---|---|---|---|
| 6 months (n = 420) | 12 months (n = 395) | |||||
| n | Mean | SD | n | Mean | SD | |
| Intervention | 207 | 7.0 | 5.9 | 197 | 6.7 | 6.2 |
| Usual care | 213 | 9.5 | 5.6 | 198 | 8.5 | 5.8 |
| N | Difference in meansa | 95% CI | p-value | |||
| 6-month follow-up | 420 | −2.5 | −3.4 to −1.5 | |||
| 12-month follow-up | 395 | −1.9 | −3.0 to −0.9 | |||
| Repeated measures | 815 | −2.2 | −3.0 to −1.3 | < 0.001 | ||
Panic symptoms at 6 months and over the duration of the study
Those in the intervention group scored, on average, 0.6 points lower on the panic symptom scale than those in the usual-care group at 6 months ( Table 37 ), and this did not change following adjustment for baseline imbalances. In terms of an effect size, the difference between groups was relatively small at 0.26 SD [baseline SD for panic (pooled): 2.3].
| Group | n | Mean | SD | Difference in meansa | 95% CI | p-value | Difference in means | 95% CI | p-value |
|---|---|---|---|---|---|---|---|---|---|
| Intervention | 205 | 1.6 | 2.1 | −0.6 | −1.0 to −0.3 | 0.001 | −0.6 | −1.0 to −0.3 | 0.001 |
| Usual care | 213 | 2.1 | 2.2 | ||||||
| Total N | 418 | 418 | 414 | ||||||
The means and differences in mean panic scores at 6 and 12 months are summarised in Table 38 . Based on a repeated measures linear regression analysis using data from 6 and 12 months, the intervention group had a panic score that was, on average, 0.5 points lower than those in the usual-care group over the 12 months (see Table 38 ). There was no evidence that the difference between the intervention and usual-care groups varied over time (interaction between treatment and time: p = 0.16).
| Follow-up | ||||||
|---|---|---|---|---|---|---|
| 6 months (n = 418) | 12 months (n = 393) | |||||
| N | Mean | SD | N | Mean | SD | |
| Intervention | 205 | 1.6 | 2.1 | 195 | 1.5 | 2.1 |
| Usual care | 213 | 2.1 | 2.2 | 198 | 1.7 | 2.2 |
| N | Difference in meansa | 95% CI | p-value | |||
| 6-month follow-up | 418 | −0.6 | −1.0 to −0.3 | |||
| 12-month follow-up | 393 | −0.3 | −0.7 to 0.05 | |||
| Repeated measures | 811 | −0.5 | −0.8 to −0.2 | 0.001 | ||
Quality of life
Short-Form questionnaire-12 items mental subscale scores at 6 months and over the duration of the study
Table 39 presents the means and difference in mean SF-12 mental subscale scores for both treatment groups at the 6-month follow-up. A positive difference indicates a better outcome among those in the intervention group. On average, those in the intervention group experienced an improvement of 6 points on the SF-12 mental subscale more than the usual-care group. There was very little effect of adjustment for those variables that were imbalanced at baseline. In terms of an effect size, the difference between groups equated to 0.63 SD [baseline SD for SF-12 mental subscale (pooled): 9.2].
| Group | n | Mean | SD | Difference in meansa | 95% CI | p-value | Difference in meansb | 95% CI | p-value |
|---|---|---|---|---|---|---|---|---|---|
| Intervention | 201 | 39.1 | 14.1 | 5.8 | 3.5 to 8.2 | < 0.001 | 6.0 | 3.6 to 8.5 | < 0.001 |
| Usual care | 209 | 33.7 | 12.6 | ||||||
| Total N | 410 | 410 | 410 | ||||||
The means and differences in mean SF-12 mental subscale scores at 6 and 12 months are summarised in Table 40 . Based on a repeated measures linear regression analysis using data from 6 and 12 months, the intervention group had a SF-12 mental subscale score that was, on average, 5 points higher than those in the usual-care group (see Table 40 ). There was no evidence that the difference between the intervention and usual-care groups varied over time (interaction between treatment and time: p = 0.11).
| Follow-up | ||||||
|---|---|---|---|---|---|---|
| 6 months (n = 410) | 12 months (n = 389) | |||||
| n | Mean | SD | N | Mean | SD | |
| Intervention | 201 | 39.1 | 14.1 | 194 | 39.1 | 14.6 |
| Usual care | 209 | 33.7 | 12.6 | 195 | 35.4 | 12.8 |
| N | Difference in meansa | 95% CI | p-value | |||
| 6-month follow-up | 410 | 5.8 | 3.5 to 8.2 | |||
| 12-month follow-up | 389 | 4.1 | 1.6 to 6.7 | |||
| Repeated measures | 799 | 4.8 | 2.7 to 6.9 | < 0.001 | ||
SF-12 physical subscale scores at 6 months and over the duration of the study
Table 41 presents the means and difference in mean SF-12 physical subscale scores for both treatment groups at the 6-month follow-up. Based on a comparison of the absolute mean values at 6 months, it would appear that those in the intervention group had a better outcome in terms of a higher mean SF-12 physical subscale score at 6-month follow-up. There was, however, a substantial imbalance in baseline SF-12 physical subscale scores (mean scores: intervention 45.3; usual care 41.6) such that the adjusted difference in means indicates that those in the intervention group had a slightly worse outcome (negative difference) compared with those in the usual-care group. In any case, the 95% CI surrounding this estimate just included the possibility of no difference between the groups and in terms of an effect size, the difference between groups was small at 0.12 SD [baseline SD for SF-12 physical subscale (pooled): 13.5].
| Group | n | Mean | SD | Difference in meansa | 95% CI | p-value | Difference in meansb | 95% CI | p-value |
|---|---|---|---|---|---|---|---|---|---|
| Intervention | 201 | 44.1 | 14.2 | −1.6 | −3.3 to 0.05 | 0.057 | −1.7 | −3.4 to 0.02 | 0.052 |
| Usual care | 209 | 42.1 | 14.0 | ||||||
| Total N | 410 | 410 | 410 |
The means and differences in mean SF-12 physical subscale scores at 6 and 12 months are summarised in Table 42 . Based on a repeated measures linear regression analysis using data from 6 and 12 months, the intervention group had a SF-12 physical subscale score that was, on average, 0.7 points lower than those in the usual-care group (see Table 42 ). There was weak evidence that the difference between the intervention and usual-care groups varied over time (interaction between treatment and time: p = 0.047). The interaction appeared to be driven by the small difference in SF-12 physical subscale scores between the treatment groups observed at 6 months. There was no evidence of a difference in SF-12 physical subscale scores between the treatment groups at 12 months (see Table 42 ).
| Follow-up | ||||||
|---|---|---|---|---|---|---|
| 6 months (n = 410) | 12 months (n = 389) | |||||
| n | Mean | SD | n | Mean | SD | |
| Intervention | 201 | 44.1 | 14.2 | 194 | 44.6 | 13.2 |
| Usual care | 209 | 42.1 | 14.0 | 195 | 41.1 | 13.5 |
| N | Difference in meansa | 95% CI | p-value | |||
| 6-month follow-up | 410 | −1.6 | −3.3 to 0.05 | |||
| 12-month follow-up | 389 | 0.3 | −1.4 to 2.0 | |||
| Repeated measures | 799 | −0.7 | −2.1 to 0.8 | 0.35 | ||
Treatment efficacy
Complier average causal effect analyses were performed to estimate the efficacy of the intervention in improving depressive symptoms among those in the intervention group compared with those in usual care using instrumental variable regression methods. The results of these analyses are shown in Table 43 . As the primary outcome ‘response’ was a binary variable, a probit transformation has been used and the primary ITT analysis repeated for comparison on this transformed scale. The CACE analysis at 6 months showed that there was a larger effect of the intervention among those who were ‘on track’ to complete the intervention (at least 9 sessions by 6 months; n = 144) compared with the standard ‘ITT’ estimate of the ‘offer’ of treatment.
| Analysis | n | Probit regression coefficienta | 95% CI | p-value |
|---|---|---|---|---|
| ITT | 419 | 0.71 | 0.45 to 0.97 | < 0.001 |
| CACE | 419 | 0.99 | 0.65 to 1.34 | < 0.001 |
Using the secondary outcome of BDI-II score as a continuous variable, the CACE analysis demonstrated that, on average, those who were ‘on track’ to complete the intervention improved by 8 points on the BDI-II, compared with a 6-point difference in a standard ITT analysis ( Table 44 ).
| Analysis | n | Difference in meansa | 95% CI | p-value |
|---|---|---|---|---|
| ITT | 419 | −5.7 | −7.9 to −3.4 | < 0.001 |
| CACE | 419 | −8.2 | −11.4 to −5.0 | < 0.001 |
Extending the CACE analysis for the continuous BDI-II outcome at 12 months and defining ‘compliers’ as those who received at least 12 sessions of CBT (n = 141) confirmed the beneficial effect of adhering to the intervention ( Table 45 ).
| Analysis | n | Difference in meansa | 95% CI | p-value |
|---|---|---|---|---|
| ITT | 395 | −5.0 | −7.3 to −2.6 | < 0.001 |
| CACE | 395 | −7.1 | −10.4 to −3.8 | < 0.001 |
Contamination: receipt of non-CoBalT psychological therapy
Participants were asked about non-CoBalT psychological therapy that they had received over the duration of the study. For the 388 participants who provided data at 6 and 12 months, only 66 participants (17%) reported having had any counselling or other ‘talking therapy’ during the 12 months [usual care: n = 41 (21.0%); intervention: n = 25 (13.0%)]. For the majority (n = 50), this comprised sessions lasting at least 50 minutes (usual care, n = 32; intervention, n = 25).
In response to a series of closed questions, those who indicated that they had had individual face-to-face ‘talking therapy’ in sessions lasting ≥ 50 minutes, and who had completed homework and thought diaries as part of this therapy, were regarded as having received CBT outwith the trial (non-CoBalT CBT). Only a very small minority received such treatment.
By the 6-month follow-up, two participants had attended at least nine sessions of individual face-to-face non-CoBalT CBT with a private therapist. One of these participants had been randomised to receive the intervention but had withdrawn from CBT (not attending any sessions). The other individual had been randomised to usual care.
By the 12-month follow-up, a total of three participants in the usual-care group and two participants in the intervention group had attended at least 12 sessions of non-CoBalT CBT. The second intervention participant had received 12 sessions of CBT in the NHS following completion of the trial intervention (n = 14 sessions). Those in the usual-care group had accessed CBT through a private provider, voluntary organisation or the NHS.
Estimates of treatment efficacy at 6 and 12 months were recalculated for the continuous BDI-II outcome incorporating the contamination through the receipt of non-CoBalT CBT; not unexpectedly, given the small numbers involved, the CACE estimates did not change (data not shown).
Use of antidepressant medication over the study
At the point of randomisation, all participants were taking antidepressant medication and they were asked to report whether or not they were still taking antidepressants (and whether or not there had been any change in their medication) at each follow-up. The vast majority of participants continued to take antidepressant medication over the 12 months of the study ( Table 46 ). At 12 months, a smaller percentage of those randomised to the intervention were still taking antidepressants compared with those randomised to continue with usual care. In a repeated measures analyses, there was some evidence that the difference in antidepressant use varied over time (interaction between treatment and time: p = 0.034) and hence it was not appropriate to generate a summary OR for the difference.
| Follow-up | Intervention | Usual care | Difference | 95% CI | ||||
|---|---|---|---|---|---|---|---|---|
| N | n | % | N | n | % | |||
| 3 months | 222 | 218 | 98.2 | 219 | 211 | 96.4 | 1.9 | −1.2 to 4.9 |
| 6 months | 209 | 194 | 92.8 | 213 | 199 | 93.4 | −0.6 | −5.4 to 4.2 |
| 9 months | 186 | 166 | 89.3 | 191 | 180 | 94.2 | −5.0 | −10.5 to 0.6 |
| 12 months | 198 | 174 | 87.9 | 198 | 183 | 92.4 | −4.5 | −10.4 to 1.3 |
Adherence to antidepressant medication over the previous 6 weeks was assessed as part of the screening questionnaire and the same questions were included as part of the baseline and follow-up questionnaires. Table 47 shows the number and percentage of participants in each group who met criteria for ‘adherence’ as applied at the initial screening stage. The majority of participants continued to adhere to their antidepressants over the 12 months. In a repeated measures analysis there was only very weak evidence that those in the intervention group were more likely to adhere to their medication [summary OR for n = 1516 observations: 1.41 (95% CI 0.92 to 2.16), p = 0.12] and no evidence that the difference between groups varied over time (interaction between treatment and time: p = 0.83).
| Follow-up | Intervention | Usual care | Difference | 95% CI | ||||
|---|---|---|---|---|---|---|---|---|
| N | n | % | N | n | % | |||
| Baseline | 234 | 223 | 95.3 | 235 | 219 | 93.2 | 2.1 | −2.1 to 6.3 |
| 3 months | 217 | 202 | 93.1 | 210 | 187 | 89.1 | 4.0 | −1.4 to 9.4 |
| 6 months | 193 | 178 | 92.2 | 198 | 174 | 87.9 | 4.3 | −1.6 to 10.3 |
| 9 months | 166 | 159 | 95.8 | 179 | 166 | 92.7 | 3.0 | −1.8 to 7.9 |
| 12 months | 173 | 160 | 92.5 | 180 | 168 | 93.3 | −0.8 | −6.2 to 4.5 |
Outcomes that can be compared with the Improving Access to Psychological Therapies data set
In the IAPT project,32,33 outcomes are considered in terms of whether an individual is a ‘case’ on the PHQ-9 (defined as a score of ≥ 10) and/or GAD-7 (defined as a score of ≥ 8).
Of the 416 participants who were a ‘case’ on the PHQ-9 at baseline, 374 were followed up and completed the PHQ-9 at 6 months. In total, 51.3% (n = 96) of those randomised to CBT and 32.1% (n = 60) of those randomised to usual care had a PHQ-9 score of < 10 (‘non-case’) at 6 months.
Of the 356 who were a ‘case’ on the GAD-7 at baseline, 320 were followed up and completed the GAD-7 at 6 months. In total, 59.4% (n = 94) of those randomised to CBT and 33.5% (n = 54) of those randomised to usual care had a GAD-7 score of < 8 (‘non-case’) at 6 months.
In total, 439 patients were a ‘case’ on the PHQ-9 or GAD-7 at baseline, 393 were followed up and completed both the PHQ-9 and GAD-7 at 6 months. Of these, 47.6% (n = 91 of 191) randomised to CBT were a ‘non-case’ on both the PHQ-9 and GAD-7 at 6 months compared with 28.7% (n = 58 of 202) of those randomised to usual care.
Chapter 4 Economic evaluation: methods and results
Introduction
Aim
The aim of the economic evaluation was to assess the cost-effectiveness of individual face-to-face CBT as an adjunct to usual care compared with usual care alone, for primary care patients with TRD. The economic evaluation was carried out alongside the RCT, where patients recruited to the study were randomly allocated to CBT plus usual care or usual care alone.
The interventions
Control: Patients allocated to the control group received TAU. There were no restrictions imposed on GPs, and treatment could include any combination of pharmacotherapy, counselling, CBT and secondary care if considered to be clinically appropriate.
Intervention: Patients allocated to the intervention group were offered between 12 and 18 sessions of face-to-face CBT in addition to TAU. The intervention was delivered by qualified therapists who received at least one day of training specific to the trial and who all used the same CBT manuals for depression; these were informed by the Moore and Garland manual on chronic depression. 64 Therapy took place at the patients’ GP surgeries or at nearby NHS or University premises.
Methods
Form of analysis
The economic evaluation was based on all costs incurred during the 12 months following randomisation. The main perspective was that of the health and social care provider, although personal costs incurred by patients and productivity costs due to time off work were also considered. These are presented separately.
A cost–consequences approach was used to compare cost from all three perspectives (health and social care, patients, and lost productivity) with a range of outcomes.
A cost-effectiveness (utility) analysis was carried out in line with the recommendations of NICE75 to compare health and social care costs with quality-adjusted life-years (QALYs).
Outcomes
The primary outcome for the trial was a reduction of at least 50% in the BDI-II score46 at 6 months compared with baseline. In addition, several outcomes were measured at 12 months, including the BDI-II score as a continuous variable; remission defined as BDI-II score of < 10, the SF-12 mental and physical subscales56 and the EQ-5D-3L. 76 All outcomes were measured at baseline, 6 months and 12 months.
Quality-adjusted life-years over the 12-month period were estimated from responses to the EQ-5D-3L at baseline, 6 months and 12 months. Utility values – representing health-related quality of life on a scale between 0 (death) and 1 (best imaginable health) – were derived using the valuation tariff obtained from a UK general population survey. 77 QALYs were computed from these values using the area under the curve approach and adjusted for any difference between the groups at baseline. 78
Identification of relevant costs
The analysis included the cost of caring for all patients during the 12 months between randomisation and final follow-up. This included the following.
Direct costs to the health service provider (the NHS)
-
The cost of delivering the CBT intervention.
-
Primary and community care: all primary and community care was included, irrespective of the reason for the encounter because the nature of primary care makes it difficult to identify consultations that are for underlying mental ill health. This included:
-
face-to-face consultations with GP, practice nurse, nurse practitioner, specialist nurse, health-care assistant
-
telephone consultations with GP, practice nurse, nurse practitioner
-
home visits by a GP
-
out-of-hours consultations with GP, nurse
-
other primary and community care: district nurse, community health workers, referral to alternative therapists, referral to exercise on prescription, use of NHS Direct
-
prescribed medication
-
-
Hospital care, restricted to care relating to mental health, which was identified according to the specialist involved and/or the reason for the encounter:
-
visits to accident and emergency (A&E)
-
outpatient appointments
-
inpatient stays.
-
Direct costs of Personal Social Services
This included:
-
social worker
-
home help
-
self-help groups run by social services
-
day centre use.
Costs to patients and their carers
-
Travel to trial CBT sessions.
-
Travel to appointments at GP surgery, exercise on prescription.
-
Over-the-counter medication and remedies.
-
Private and alternative therapies and associated travel.
-
Self-help groups and associated travel.
-
Prescription costs.
-
Loss of earnings owing to time off work.
-
Disability payments received.
Productivity costs
-
Time off work.
Measurement of resource use
Trial cognitive behavioural therapy
During the trial, the CBT therapists recorded details of all sessions booked and attended by participants. These records were used to measure – for each patient in the intervention group – how many sessions each attended, how many were booked but were not attended and how many were booked but cancelled in time to allow the therapist to make alternative arrangements.
No extra equipment or other capital was required by the therapists.
Health and social care
Information about all primary care encounters over the 12-month period was extracted from GP records for participants who gave consent. Information extracted included type of professional seen (e.g. GP, practice nurse), where the encounter took place (e.g. face-to-face, over the telephone) and number of encounters. Details of prescribed medication were extracted in the same way; the information recorded included name of drug, method of delivery (e.g. capsules, liquid), strength and amount prescribed.
A questionnaire was administered to participants at 6 months and 12 months to obtain resource-use data (about use of health and social care) that were not available from GP records. The 6-month questionnaire asked about resource use for the period between randomisation and 6 months, and the 12-month questionnaire asked about the time period between 6 months and 12 months.
The questionnaires were designed in two parts. Part 1, which was largely self-completed with a researcher present, contained ‘yes/no’ questions about each item of resource use to find out which categories each participant had used at all. Those who answered ‘yes’ to any category were then asked detailed questions, contained in part 2, by the researcher to gain more information. In the case of NHS hospital use, for example, part 2 of the questionnaire asked participants about the reason for the encounter, the name of the clinic attended or the ward in which they stayed, and information about treatment received. The design of the questionnaire allowed for free text to be entered as comments, to facilitate accurate costing. Detailed information about the use of social services was obtained in the same way, covering use of a social worker, any home help, self-help groups run by social services and use of day centres.
Personal expenditure and time off work
Data on personal out-of-pocket expenditure was obtained using the questionnaires administered at 6 months and 12 months, as described above. Part 1 of the questionnaire asked if each category of resource was used at all and if a participant responded ‘yes’ to any category, part 2 of the questionnaire, administered by a researcher, was used to obtain detailed information about distances travelled to appointments, the estimated cost of over-the-counter medication and remedies, and use and cost of private and alternative therapies and self-help groups. Participants were also asked if they normally pay for prescriptions and whether or not a prescription payment certificate (PPC) was used.
Disability payments
Data on disability payments received was obtained using the questionnaires administered at 6 months and 12 months, as described above. Part 1 of the questionnaire asked whether or not any payments were received, and if the participant responded ‘yes’ then part 2 of the questionnaire, administered by a researcher, was used to obtain detailed information about the type of benefit and the amount received.
Productivity losses owing to time off work
Information about time off work was obtained using the questionnaires administered at 6 months and 12 months, as described above. Part 1 of the questionnaire asked whether or not the participant took any time off work because of their illness and if they responded ‘yes’ then part 2 of the questionnaire, administered by a researcher, was used to obtain detailed information about frequency and length of absences.
Valuation of resource use
The principle of opportunity cost was applied to the valuation of resources; however, in most cases market prices were used as a proxy for opportunity cost. All costs were valued in pound sterling at 2010 prices, adjusted for inflation where necessary. 79
Trial cognitive behavioural therapy
Methods used by Curtis in the Unit Costs for Health and Social Care 79 were used to estimate a unit cost for the trial CBT sessions ( Table 48 ). Most of the therapists in the trial were Band 8a on the NHS Agenda for Change scale,80 and this was used in the base-case analysis. After allowance for National Insurance, superannuation, overheads and non-contact time of 50%, this equated to £73 per hour of face-to-face contact. The first CBT session was 90 minutes in length with subsequent sessions lasting 60 minutes. Appointments that were cancelled were not included in the costing but when a patient did not cancel but failed to attend we included an amount equal to half the usual rate, reflecting the fact that therapists would make some use of the time but would not be fully productive. Supervision was generally carried out in groups of two or three. Therapist time for this was subsumed in the non-contact time but we included an estimate of £2.50 per hour of therapy delivered to cover the cost of the supervisors’ time. This was based on the salary of a consultant psychiatrist/consultant clinical psychologist.
| Costs and unit estimation | Value (£) |
|---|---|
| Basic annual salary (Band 8a) | 41,000 |
| Oncosts: National Insurance and superannuation | 10,065 |
| Capital and overheads | 6422 |
| Total cost per annum | 57,487 |
| Hours worked per year | 1575 |
| Cost per hour | 36.50 |
| Cost per hour face to face allowing for 50% non-contact time | 73 |
| Supervision per hour of therapy | 2.50 |
| Cost of first session (90 minutes): attended | 113.25 |
| Cost of follow-up sessions (60 minutes): attended | 75.50 |
| Cost of first session (90 minutes): did not attend | 54.74 |
| Cost of follow-up sessions (60 minutes): did not attend | 36.50 |
Direct costs to the health service provider (the NHS)
Primary and community health and social services
The majority of unit costs for primary and community health, and social services, were taken from Curtis. 79 These are shown in Table 49 .
| Type of consultation | Cost per consultation (£) |
|---|---|
| Primary and community health care | |
| GP | |
| Surgery | 28.00 |
| Telephone | 17.00 |
| Home visit | 94.00 |
| Practice nurse | |
| Surgery | 10.00 |
| Telephone consultation | 5.00 |
| Nurse practitioner | |
| Surgery | 14.00 |
| Telephone consultation | 7.00 |
| Diabetic nurse | 11.92 |
| District nurse | 16.33 |
| Health visitor | 24.67 |
| Midwife | 24.67 |
| Phlebotomist | 6.92 |
| Podiatrist | 11.00 |
| Occupational therapist | 75.00 |
| Exercise on prescription | 8.00 |
| Alternative therapy | 30.00 |
| PSS | |
| Home help | 21.40 |
| Self-help groups | 15.00 |
| Day centre use | 21.00 |
The cost of out-of-hours care was based on estimates obtained from a local provider (BrisDoc), of walk-in centres81 and NHS Direct82 from published evaluations, adjusted to 2010 values using the pay and prices index. 79 These are shown in Table 50 .
Prescribed medication
The values of prescriptions issued in general practice were based on costs published in the BNF,83 by item name, strength and amount prescribed. These were adjusted to allow for:
Hospital care
Unit costs for hospital use were taken from the NHS National Schedule of Reference Costs for 2010–11. 85 Costs used in the analysis are shown in Table 51 .
| Type of consultation | Cost per consultation/visit (£) |
|---|---|
| A&E | |
| Investigation and minor treatment followed by discharge | 112.33 |
| Observation followed by admission | 150.00 |
| Outpatient appointments and clinic visits | |
| Clinical psychology | 71.00 |
| Community psychiatric nursing | 71.00 |
| Neurology: first attendance | 206 |
| Neurology: follow-up visits | 146 |
| Psychiatry: first attendance | 284 |
| Psychiatry: follow-up visits | 165 |
| Mental Wellbeing Clinic | 58.50 |
| Hospital-based support group | 71.00 |
| Inpatient stays | |
| Observation only, for overdose, panic attack | 291.00 |
| Treated by occupational therapist | 354.00 |
Patients and their carers
Travel costs
Travel costs were reported directly by the participants in their questionnaire responses, with the exception of travel by car, which was reported as mileage. Information about mileage costs was obtained from the Automobile Association schedule of motoring costs86 and a value of 60.5p per mile was used.
Personal expenditure
Information on personal expenditure on over-the-counter medication and remedies, private and alternative therapies, and attendance at self-help groups was obtained from the participants’ questionnaire responses.
Prescription costs
The cost of individual prescriptions and PPCs was obtained from the Department of Health. 87
Loss of earnings due to time off work
Information on loss of earnings owing to time off work was obtained from the participants’ questionnaire responses.
Disability payments received
Information on disability payments received was obtained from the participants’ questionnaire responses.
Productivity costs
The human capital approach was used to value time off work. Median hourly earnings by age and sex were obtained from the Office for National Statistics 2010 Annual Survey of Hours and Earnings. 88 These are shown in Table 52 .
| Age (years) | Men | Women |
|---|---|---|
| 18–21 | 285.9 | 268.3 |
| 22–29 | 421.2 | 401.3 |
| 30–39 | 573.7 | 507.9 |
| 40–49 | 613.7 | 472.2 |
| 50–59 | 582.7 | 440.9 |
| 60+ | 483.0 | 389.0 |
Data analysis
Resource use by patients in the intervention and usual-care groups were compared using frequencies, means and medians of the number of encounters. The level of uncertainty around the point estimate was determined using SDs and IQRs as appropriate.
Resource use was combined with unit costs to obtain mean cost per participant for each category of cost.
Missing data
The MICE procedure was used to address the issue of missing cost and QALY data. 72 The ice command (version 1.9.5 PR/IW 15apr2011) in Stata version 12 was used to generate five data sets using 10 switching procedures, in addition to a range of cost and EQ-5D-3L variables, the model also included randomisation group, age and sex.
Uncertainty resulting from patient variation
Uncertainty in the estimates of cost and QALYs was captured using SDs around the point estimates and CIs around incremental differences.
Uncertainty around the incremental cost-effectiveness ratio (ICER) was captured using the bootstrapping technique. A total of 5000 replicates of the ICER were generated and these were used to estimate (1) CIs around the net benefit statistic and (2) a cost-effectiveness acceptability curve.
Sensitivity analyses
Sensitivity analyses were used to investigate the effect of uncertainty on the results. Four separate one-way analyses were undertaken as follows:
-
Grade of therapist In the trial the therapists were mainly Band 8a, although in practice they are often appointed at a lower grade. Sensitivity analysis was used to estimate the effect on cost per QALY if all therapists were costed at Band 7.
-
Hospital costs Patients in this trial were all recruited from (and largely cared for by) primary care. Secondary care is relatively uncommon but expensive and can affect results disproportionately. The potential effect of this was assessed by removing hospital costs from the analysis.
-
QALY weights The base-case analysis used the EQ-5D-3L to derive QALY weights; sensitivity analysis tested the robustness of these weights by applying the Short Form questionnaire-6 Dimensions (SF-6D) algorithm89 to responses to the SF-12 plus four questions from the SF-36.
-
Missing data The effect of imputing missing data was assessed by comparing the imputed results with those restricted to complete cases, i.e. including only those participants for whom complete cost and QALY data were available at both 6 and 12 months.
Discounting
Costs and outcomes were not discounted, as the study was limited to a period of 12 months.
Results
Data completeness
Nearly all participants (97%) gave permission for the research team to access their GP notes to obtain data about primary care encounters and prescribed medication. Data obtained from the questionnaire were slightly less complete: 83% of participants returned the questionnaire at both 6 and 12 months, although not all completed all sections at both time points. Complete cost and QALY data were available for 368 (78%) participants and complete personal cost data were available for 274 (58%) participants.
Resource use
Trial cognitive behavioural therapy sessions
The number of CBT sessions attended by participants is shown in Table 53 , which includes the number of sessions arranged but not attended. Sessions cancelled ahead were not costed and are not included. Most participants received between 12 and 18 sessions as per protocol, although a small number (8.5%) received no therapy.
| Frequency | No. |
|---|---|
| First session | |
| Attended | 214 |
| Did not attend | |
| 1 | 11 |
| 2–4 | 8 |
| Follow-up sessions | |
| Attended | |
| 1–4 | 30 |
| 5–11 | 62 |
| 12–18a | 114 |
| Did not attend | |
| 1 | 42 |
| 2 | 15 |
| 3 | 8 |
| 4–9 | 4 |
Primary care
Results of primary care resource use are presented in Table 54 using frequency of use for all participants who provided data. Frequencies, means and median number of encounters are presented, along with SDs and IQRs, to indicate the level of uncertainty around the point estimates.
| Frequency of consultations | Intervention | Usual care | ||
|---|---|---|---|---|
| No. | Percentage | No. | Percentage | |
| GP consultations | ||||
| 0 | 25 | 11 | 24 | 11 |
| 1–5 | 74 | 32 | 59 | 26 |
| 6–10 | 64 | 28 | 74 | 33 |
| 11–15 | 42 | 18 | 45 | 20 |
| 16+ | 24 | 10 | 22 | 10 |
| 229 | 100 | 224 | 100 | |
| Mean (SD) | 7.62 (7) | 8.26 (6.2) | ||
| Median (IQR) | 7 (3 to 11) | 7 (4 to 12) | ||
| Nurse consultations | ||||
| 0 | 100 | 44 | 99 | 44 |
| 1–5 | 109 | 48 | 107 | 48 |
| 6–9 | 14 | 6 | 13 | 6 |
| 10+ | 6 | 3 | 5 | 2 |
| 229 | 100 | 224 | 100 | |
| Mean (SD) | 2.03 (4.10) | 1.95 (2.81) | ||
| Median (IQR) | 1 (0 to 3) | 1 (0 to 3) | ||
| Other surgery-based consultations | ||||
| 0 | 146 | 64 | 136 | 61 |
| 1–5 | 76 | 33 | 80 | 36 |
| 6–9 | 4 | 2 | 3 | 1 |
| 10+ | 3 | 1 | 5 | 2 |
| 229 | 100 | 224 | 100 | |
| Mean (SD) | 0.88 (2.16) | 1.23 (2.56) | ||
| Median (IQR) | 0 (0 to 1) | 0 (0 to 2) | ||
| Out of hours, walk-in centres and NHS Direct | ||||
| 0 | 164 | 87 | 163 | 89 |
| 1 | 13 | 7 | 13 | 8 |
| 2+ | 11 | 6 | 6 | 3 |
| 188 | 100 | 182 | 100 | |
| Mean (SD) | 0.23 (0.73) | 0.20 (0.83) | ||
| Median (IQR) | 0 (0) | 0 (0) | ||
| Other primary and community care therapies and interventions: no. using the service | ||||
| Intervention | Intervention | Usual care | ||
| n | No. (%) | n | No. (%) | |
| Exercise on prescription | 191 | 11 (6) | 194 | 10 (5) |
| Alternative therapies | 191 | 21 (11) | 194 | 21 (11) |
| cCBT and CBT | 195 | 14 (7) | 195 | 23 (12) |
Most participants (89%) saw their GP at least once during the year and, on average, they had about eight consultations. There was very little evidence of a marked difference in primary care resource use between participants in the intervention group and those in the usual-care group. The usual-care group appear to be consistently higher users of services but the difference is small and the level of variation is high. One in eight participants in the usual-care group accessed (non-trial) CBT or cCBT at least once during the 12 months compared with one in 14 of those offered trial CBT.
Prescribed medication
Information about the amount of prescribed medication is given in Table 55 .
| Frequency | Intervention | Usual care | ||
|---|---|---|---|---|
| No. | Percentage | No. | Percentage | |
| Medication: no. of antidepressant prescriptions | ||||
| 0 | 24 | 10 | 19 | 8 |
| 1–5 | 40 | 17 | 39 | 17 |
| 6–10 | 84 | 37 | 94 | 42 |
| 11–15a | 56 | 24 | 50 | 22 |
| 16+ | 25 | 11 | 22 | 10 |
| 229 | 100 | 224 | 100 | |
| Mean (SD) | 8.95 (6.84) | 8.71 (5.92) | ||
| Median (IQR) | 7 (5 to 12) | 7 (5 to 12) | ||
| Medication: no. of non-antidepressant prescriptions | ||||
| 0 | 39 | 17 | 25 | 11 |
| 1–10 | 67 | 29 | 63 | 28 |
| 11–20 | 25 | 11 | 36 | 16 |
| 21–30 | 25 | 11 | 17 | 8 |
| 31–40 | 14 | 6 | 16 | 7 |
| 41–50 | 11 | 5 | 14 | 6 |
| 51+ | 48 | 21 | 53 | 24 |
| 229 | 100 | 224 | 100 | |
| Mean (SD) | 28.4 (36.8) | 34.0 (43.3) | ||
| Median (IQR) | 13 (3 to 41) | 19 (4 to 47) | ||
Over 90% of participants had at least one prescription for an antidepressant, and at least 10% had more than 15. There was little evidence of a difference between the two groups.
More participants in the usual-care group had more non-antidepressant medication than those in the intervention group, although variation was high, with the number of prescriptions ranging from 0 to > 150 in both groups.
Hospital care
Results of hospital resource use are presented in Table 56 using frequency of use for all participants who provided data. Frequencies, means and median number of encounters are presented, along with SDs and IQRs, to indicate the level of uncertainty around the point estimates.
| Frequency | Intervention | Usual care | ||
|---|---|---|---|---|
| No. | Percentage | No. | Percentage | |
| A&E visits | ||||
| 0 | 185 | 96 | 184 | 96 |
| 1 | 4 | 2 | 8 | 4 |
| 2 | 2 | 1 | 0 | 0 |
| 3 | 1 | 1 | 0 | 0 |
| 192 | 100 | 192 | 100 | |
| Mean (SD) | 0.06 (0.33) | 0.04 (0.20) | ||
| Median (IQR) | 0 (0) | 0 (0) | ||
| Outpatient attendances | ||||
| 0 | 179 | 93 | 183 | 94 |
| 1 | 8 | 4 | 9 | 5 |
| 2 | 2 | 1 | 2 | 1 |
| 3 | 3 | 2 | 0 | 0 |
| 192 | 100 | 194 | 100 | |
| Mean (SD) | 0.11 (0.46) | 0.07 (0.29) | ||
| Median (IQR) | 0 (0) | 0 (0) | ||
| Inpatient attendances | ||||
| 0 | 189 | 98 | 190 | 99 |
| 1 | 1 | 1 | 1 | 1 |
| 2 | 2 | 1 | 1 | 1 |
| 192 | 100 | 192 | 100 | |
| Mean SD | 0.03 (0.22) | 0.02 (0.16) | ||
| Median (IQR) | 0 (0) | 0 (0) | ||
Few participants accessed hospital care: 15 (4%) visited A&E, 24 (6%) attended at least one outpatient clinic and five (1%) were admitted overnight. The difference in mean number of outpatient appointments between the two groups is due to three patients in the intervention group having three appointments, whereas none in the usual-care group had more than two.
Personal Social Services
Reported use of social services because of mental health was low, with no evidence of a difference between the two groups ( Table 57 ).
| Intervention | Intervention | Usual care | ||
|---|---|---|---|---|
| n | No. (%) | n | No. (%) | |
| PSS: no. using services | ||||
| Social work | 191 | 3 (2) | 195 | 5 (3) |
| Home help | 192 | 3 (2) | 194 | 4 (2) |
| Self-help groups | 192 | 3 (2) | 194 | 4 (2) |
| Day centre use | 192 | 6 (3) | 194 | 3 (2) |
Time off work
The amount of time off work by taken participants because of their condition is shown in Table 58 .
| Frequency | Intervention | Usual care | ||
|---|---|---|---|---|
| No. | Percentage | No. | Percentage | |
| Time off work: no. of days reported | ||||
| 0 | 142 | 78 | 143 | 77 |
| 1–14 | 20 | 11 | 16 | 9 |
| 15–30 | 4 | 2 | 5 | 3 |
| 31–60 | 5 | 3 | 8 | 4 |
| 61–180 | 7 | 4 | 10 | 5 |
| 181+ | 4 | 2 | 3 | 2 |
| 182 | 100 | 185 | 100 | |
| Mean (SD) | 12.0 (44.8) | 12.1 (38.4) | ||
| Median (IQR) | 0 (0) | 0 (0) | ||
Just under one-quarter (22%) of participants reported having some time off work because of their illness, and for these the mean number of days off work was 54. Seven participants (2%) reported that they were unable to work at all during the 12 months. There was no evidence of a difference between the two groups.
Cost analysis
Trial cognitive behavioural therapy
Table 59 details the costing of the intervention CBT. The total cost of 18 sessions was £1400 but allowing for those who had < 18, the mean cost per participant in the intervention group was £910. The overall mean cost of one session was £81.
| Type of appointment | No. | Unit cost (£) | Mean cost (£) per participant (n = 234) |
|---|---|---|---|
| First session: attended | 214 | 113.25 | 103.57 |
| Follow-up sessions: attended | 2416 | 75.50 | 779.52 |
| First session: DNA | 36 | 54.75 | 8.42 |
| Follow-up sessions: DNA | 120 | 36.50 | 18.72 |
| Overall mean cost of therapy | 910.23 |
Direct costs to the health service provider (the NHS) and Personal Social Services
Mean cost per participant, by category and allocation group, is given for all available data; Table 60 gives NHS and PSS costs.
| Cost category | Intervention | Usual care | Difference (95% CI), £ | ||
|---|---|---|---|---|---|
| n | Mean (SD) cost, £ | n | Mean (SD) cost, £ | ||
| GP consultations | 229 | 198.50 (183.85) | 224 | 217.69 (163.87) | −19.20 (−51.38 to 12.99) |
| Nurse consultations | 229 | 19.88 (41.54) | 224 | 19.33 (28.56) | 0.55 (−6.04 to 7.15) |
| Other primary care consultations | 229 | 7.69 (23.29) | 224 | 10.27 (23.00) | −2.59 (−6.86 to 1.69) |
| Out-of-hours care, walk-in centres and NHS Direct | 188 | 14.35 (42.44) | 184 | 9.96 (32.22) | 4.38 (−3.31 to 12.08) |
| Other community-based interventions | 188 | 57.52 (199.43) | 183 | 73.38 (282.60) | −15.86 (−65.69 to 33.97) |
| All primary and community care services | 188 | 319.92 (354.17) | 183 | 344.67 (366.12) | −24.75 (−98.29 to 48.79) |
| Antidepressant medication | 229 | 51.58 (63.42) | 224 | 69.42 (130.95) | −17.84 (−36.78 to 1.09) |
| Other prescribed medication | 229 | 304.62 (602.35) | 224 | 351.05 (557.76) | −46.42 (−153.67 to 60.83) |
| All primary care prescribed medication | 229 | 356.20 (619.49) | 224 | 420.47 (565.99) | −64.27 (−173.90 to 45.37) |
| All primary and community health care | 188 | 700.28 (830.90) | 183 | 755.04 (707.33) | −54.76 (−212.49 to 102.97) |
| A&E visits | 192 | 8.80 (48.66) | 192 | 7.02 (37.78) | 1.78 (−6.96 to 10.52) |
| Outpatient visits | 192 | 47.38 (296.56) | 194 | 21.96 (113.74) | 25.42 (−19.45 to 70.28) |
| Inpatient stays | 192 | 8.23 (69.09) | 192 | 4.55 (46.86) | 3.69 (−8.16 to 15.53) |
| All hospital costs | 192 | 64.41 (355.01) | 192 | 33.76 (137.09) | 30.65 (−23.35 to 84.66) |
| All NHS services | 188 | 766.07 (967.03) | 182 | 786.32 (718.03) | −20.25 (−194.82 to 154.31) |
| Social worker visits | 191 | 10.04 (82.60) | 195 | 9.83 (65.93) | 0.21 (−14.74 to 15.15) |
| Home help | 192 | 6.24 (80.51) | 194 | 1.10 (15.36) | 5.14 (−6.43 to 16.71) |
| Self-help groups | 192 | 0.47 (4.83) | 194 | 1.01 (12.96) | −0.54 (−2.50 to 1.43) |
| Day centre attendance | 192 | 1.75 (24.25) | 194 | 0.32 (4.52) | 1.43 (−2.06 to 4.91) |
| All PSS | 191 | 18.54 (117.27) | 194 | 12.31 (73.01) | 6.23 (−13.32 to 25.77) |
| All NHS and PSS | 188 | 784.91 (1015.20) | 182 | 799.45 (725.10) | −14.54 (−195.41 to 166.32) |
The mean cost, per patient, of caring for those in the usual-care group was slightly higher than for the intervention group, due mainly to more visits to the GP and more non-antidepressant medication. However, the wide CIs around all the categories of cost reflect considerable variation and there is no conclusive evidence of a meaningful difference between the two groups.
Patients and their carers
Mean cost per participant, by category and allocation group, is given for all available data; Table 61 gives personal out-of-pocket expenditure.
| Cost category | Intervention | Usual care | Difference (95% CI), £ | ||
|---|---|---|---|---|---|
| n | Mean (SD) cost, £ | n | Mean (SD) cost, £ | ||
| Travel to intervention | 214 | 12.94 (1.60) | – | – | – |
| All other travel | 190 | 1.74 (2.64) | 195 | 2.02 (3.19) | −0.27 (−0.86 to 0.31) |
| Cost of non-trial CBT | 190 | 4.50 (40.94) | 186 | 28.81 (215.30) | −24.31 (−55.58 to 6.97) |
| Alternative therapy cost | 190 | 19.46 (116.82) | 193 | 32.32 (150.78) | −12.86 (−39.99 to 14.27) |
| Exercise on prescription | 188 | 8.48 (105.28) | 193 | 20.44 (264.43) | −11.95 (−52.70 to 28.79) |
| Self-help groups | 194 | 0.00 (0.00) | 194 | 0.00 (0.07) | 0.00 (−0.01 to 0.00) |
| Voluntary services | 194 | 0.05 (0.65) | 195 | 0.00 (0.00) | 0.05 (−0.04 to 0.14) |
| Day centres | 194 | 0.05 (0.61) | 194 | 0.00 (0.00) | 0.05 (−0.04 to 0.14) |
| Home help services | 192 | 2.03 (28.15) | 192 | 0.00 (0.00) | 2.03 (−1.96 to 6.03) |
| Cost of prescriptions | 221 | 29.41 (43.51) | 220 | 29.40 (43.58) | 0.00 (−8.15 to 8.15) |
| Over-the-counter medication costs | 193 | 3.83 (22.36) | 192 | 1.78 (9.98) | 2.05 (−1.42 to 5.53) |
| Loss of earnings | 161 | 693.68 (4823.87) | 162 | 517.46 (2464.35) | 176.22 (−661.62 to 1014.05) |
| All personal costs (excluding DLA) | 141 | 852.61 (5151.27) | 133 | 516.51 (1952.22) | 336.09 (−601.03 to 1273.22) |
| Amount of DLA received (negative valuea) | 158 | −220.24 (985.74) | 154 | −171.39 (684.54) | −48.85 (−238.36 to 140.65) |
| All personal costs (including DLA) | 111 | 478.98 (5253.48) | 103 | 422.29 (2331.14) | 56.69 (−1053.13 to 1166.51) |
Out-of-pocket personal expenses were higher in the usual-care group than in the intervention group because of greater expenditure on alternative therapies. However, the wide CIs around all the categories of cost reflect considerable variation and there is no conclusive evidence of a meaningful difference between the two groups.
Lost productivity
Table 62 gives the cost to society of lost productivity due to time off work. This is shown by age for intervention and control participants separately. Despite marked differences between the groups, by age, the mean value of time off work (lost productivity) was similar between the two groups and there is no evidence of a meaningful difference.
| Age group (years) | Intervention | Usual care | Difference (95% CI), £ | ||
|---|---|---|---|---|---|
| n | Mean (SD) cost, £ | n | Mean (SD) cost, £ | ||
| 18–21 | 3 | 125 (217) | 1 | 751 (–) | −626 (–) |
| 22–29 | 12 | 2301 (3595) | 9 | 2069 (4762) | 232 (−3577 to 4041) |
| 30–39 | 21 | 334 (927) | 20 | 462 (1013) | −128 (−7414 to 484) |
| 40–49 | 49 | 973 (2519) | 50 | 1673 (4948) | −700 (−2271 to 871) |
| 50–59 | 56 | 1353 (5302) | 54 | 1182 (3031) | 170 (−1470 to 1811) |
| 60+ | 41 | 871 (4138) | 51 | 546 (2663) | 325 (−1091 to 1742) |
| All ages | 182 | 1067 (3887) | 185 | 1102 (3529) | −36 (−797 to 726) |
Cost consequences
The cost–consequences matrix in Table 63 presents disaggregated cost, by perspective and broad category, along with primary and secondary outcomes. These results are for all available data, by category.
| Cost category | Intervention | Usual care | Difference in mean cost (95% CI), £ | ||
|---|---|---|---|---|---|
| n | Mean (SD) cost, £ | n | Mean (SD) cost, £ | ||
| Trial CBT | 234 | 910 (467) | – | – | – |
| Total NHS cost | 188 | 766 (967) | 182 | 786 (718) | –20 (–154 to 194) |
| PSS | 191 | 19 (117) | 194 | 12 (73) | 6 (–13 to 26) |
| Personal expenditure | 165 | 80 (12) | 159 | 127 (35) | –47 (–120 to 25) |
| Out-of-pocket loss of earnings | 161 | 694 (4824) | 162 | 517 (2464) | 176 (–662 to 1014) |
| Lost productivity | 182 | 1067 (3887) | 185 | 1102 (3529) | –36 (–797 to 726) |
| Outcome | Intervention | Usual care | OR or adjusted difference in means (95% CI) | ||
| N | n or mean (% or SD) | N | n or mean (% or SD) | ||
| Response (50% reduction in BDI-II)a | 197 | 109 (55.3) | 198 | 62 (31.3) | 2.89 (2.03 to 4.10) |
| BDI-II score (mean)a | 197 | 17.0 (14.0) | 198 | 21.7 (12.9) | –5.1 (–7.1 to –3.1) |
| Remission (BDI-II < 10)a | 197 | 78 (39.6) | 198 | 36 (18.2) | 2.74 (1.82 to 4.13) |
| SF-12 mental subscale (mean)a | 194 | 39.1 (14.6) | 195 | 35.4 (12.8) | 4.8 (2.7 to 6.9) |
| SF-12 physical subscale (mean)a | 194 | 44.6 (13.2) | 195 | 41.1 (13.5) | –0.7 (–2.1 to 0.8) |
| QALYs | 192 | 0.62 (0.22) | 195 | 0.56 (0.25) | 0.053 (0.019 to 0.087) |
As reported, all three clinical outcomes based on the BDI-II indicated that patients in the intervention group improved more than those receiving usual care alone. The mean score at 12 months was 5.1 points lower for these patients and twice as many in this group recorded a 50% reduction in BDI-II score compared with those receiving usual care. This clinical difference was reflected in the QALY estimates; patients in the intervention group experienced a better health-related quality of life as measured by QALYs (0.615 vs. 0.562) giving a gain of 0.053, a value that equates to 19 extra days of good health.
Excluding the cost of the intervention, the mean cost per patient was similar between the two groups from all three perspectives (NHS and PSS, personal expenditure, lost productivity).
Cost–utility analysis
The cost–utility analysis in Table 64 uses imputed data to allow summary cost totals to be calculated. This analysis is restricted to costs to the NHS and PSS, in line with NICE recommendations. 75 Cost is combined with QALYs to produce an ICER, and the uncertainty around this statistic is indicated by the results of the bootstrapping and the probability that there is a positive NMB.
| Intervention, n = 234: mean (SD) cost, £ | Usual care, n = 235: mean (SD) cost, £ | |
|---|---|---|
| All primary care | 267 (285) | 294 (241) |
| Prescribed medication | 352 (614) | 418 (558) |
| Hospital care | 64 (323) | 38 (128) |
| PSS | 20 (107) | 14 (67) |
| NHS and PSS services | 704 (938) | 763 (697) |
| Cost of CBT | 910 (467) | – |
| Total cost NHS and PSS perspective | 1614 (1100) | 763 (697) |
| QALYs: Mean (SD) | 0.608 (0.22) | 0.551 (0.24) |
| Incremental cost (95% CI) | £850 (683 to 1017) | |
| Incremental benefit: QALY gain (95% CI) | 0.057 (0.015 to 0.099) | |
| ICER: cost per QALY gain | £14,911 | |
| Median NMB and (probability that NMB > 0): | ||
| Willingness to pay (λ) = £20,000 per QALY | £289 (0.74) | |
| Willingness to pay (λ) = £30,000 per QALY | £859 (0.91) |
The cost of the intervention (£910) is slightly offset by the higher cost of health and social care in the usual-care group (£59), giving an incremental cost of £850. Combining this with the estimated QALY gain of 0.057 gives an ICER of £14,911.
If society is willing to pay £20,000 per QALY, as suggested by NICE,75 the NMB per patient per year is £289 (95% CI –£603 to £1182) and the probability that the intervention is cost-effective is 0.74 ( Figure 5 ). These rise to £859 (95% CI –£455 to £2179) and 0.91 at a threshold of £30,000 per QALY.
FIGURE 5.
Cost-effectiveness acceptability curve showing the probability that the intervention is cost-effective at different levels of willingness-to-pay.
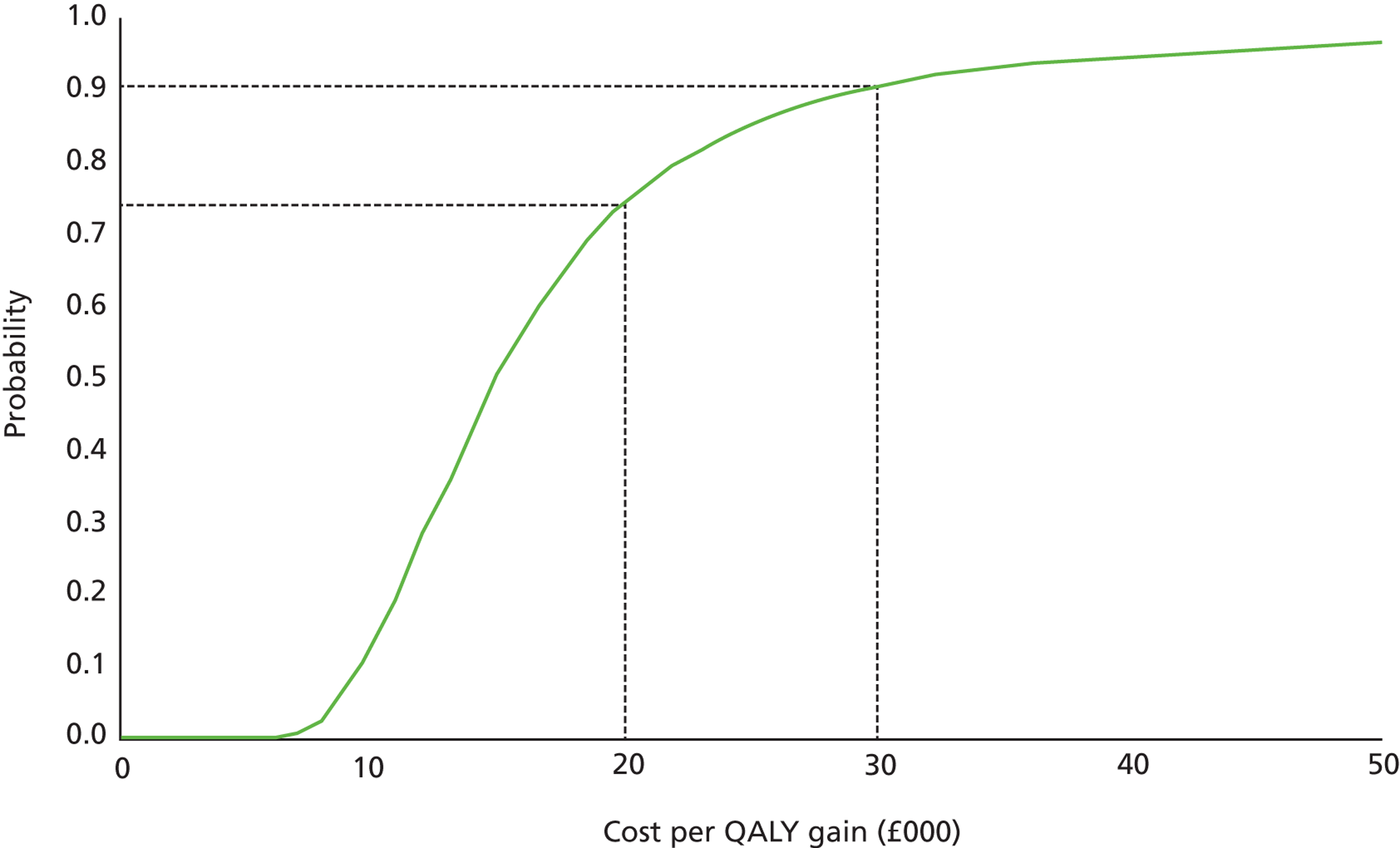
Sensitivity analyses
The results of the four sensitivity analyses are presented in Table 65 .
| Sensitivity analysis | Intervention | Usual care | Difference (95% CI) | |
|---|---|---|---|---|
| Base case | Mean and SD cost (£) | 1614 (1100) | 763 (697) | 850 (683 to 1017) |
| QALYs | 0.608 (0.22) | 0.551 (0.24) | 0.057 (0.015 to 0.099) | |
| ICER (£) | 14,911 | |||
| Median NMB (£) and p(NMB > 0) at λ = £20,000 | 289 (0.74) | |||
| (1) Grade of therapist | Mean and SD cost (£) | 1505 (1071) | 763 (697) | 742 (578 to 905) |
| ICER (£) | 13,006 | |||
| Median NMB (£) and p(NMB > 0) at λ = £20,000 | 391 (0.80) | |||
| (2) Excluding hospital costs | Mean and SD cost (£) | 1550 (1003) | 726 (681) | 824 (669 to 980) |
| ICER (£) | 14,453 | |||
| Median NMB (£) and p(NMB > 0) at λ = £20,000 | 326 (0.76) | |||
| (3) QALYs using the SF-6D | Mean and SD QALYs | 0.613 (0.09) | 0.584 (0.09) | 0.029 (0.014 to 0.043) |
| ICER (£) | 29,626 | |||
| Median NMB (£) and p(NMB > 0) at λ = £20,000 | −274 (0.08) | |||
| (4) Complete cases | Mean and SD cost (£) | 1810 (1119) | 799 (725) | 1011 (817 to 12,304) |
| QALYs | 0.614 (0.24) | 0.559 (0.24) | 0.055 (0.202 to 0.090) | |
| ICER (£) | 18,361 | |||
| Median NMB (£) and p(NMB > 0) at λ = £20,000 | 82 (0.57) | |||
The sensitivity analyses showed that:
-
If therapists were employed at Band 7, the ICER would be lower at £13,006.
-
As hospital costs were similar in the two groups, excluding these made very little difference to the cost per QALY.
-
Using data from the SF-12 to estimate QALYs, gave slightly higher estimates for both groups than using the EQ-5D-3L and the difference between them was narrowed, which resulted in a higher ICER of £29,626.
-
Participants for whom we had complete data on NHS and PSS cost and QALYs at both 6 and 12 months had more therapy sessions on average than those with missing data (12.8 vs. 11.2). Other costs and QALYs were similar but the greater cost of therapy (£1020 vs. £910) produced an estimated ICER of £18,361 for this subset of participants, somewhat higher than the base-case estimate of £14,911.
Chapter 5 Qualitative findings
Introduction
Qualitative methods are increasingly being used within RCTs to explore the views and experiences of trial participants. Data gathered can be an essential part of a trial’s evaluation and can highlight possible reasons for quantitative findings. As stated in earlier (see Chapter 1 , Research objectives), the qualitative study nested within the CoBalT trial had three main aims: to (1) explore patients’ views and experiences of CBT; (2) identify patients’ reasons for completing or not completing therapy; and (3) describe what usual care entails for this patient group. This chapter details the findings from this study.
Methods
Sampling and recruitment to the qualitative study
At the baseline assessment for the trial, patients were informed about the nested qualitative study and invited to consent to the possibility of being contacted by the qualitative researcher with regards to participating in a face-to-face interview.
Patients were contacted about taking part in the qualitative study after they had completed their primary outcome measures for the trial at 6 months post randomisation, in case the experience of being interviewed affected their views of CBT, of usual care, or of the trial in general.
A purposeful sampling strategy was used to ensure interviews were held with individuals in both arms of the trial. Conducting interviews with patients in both trial arms would enable us to fully address the aims of the qualitative study and to illuminate possible reasons for the main trial finding, i.e. why CBT in addition to usual care was or was not found to be as effective as usual care alone. Within this sampling approach we aimed for maximum variation in relation to study centre (Bristol, Exeter, Glasgow), participants’ age, gender, socioeconomic background, and whether or not their BDI-II score indicated at least a 50% reduction in depressive symptoms 6 months post randomisation. When sampling participants from the intervention arm, we also aimed to sample individuals who varied in their levels of treatment adherence, i.e. those who had or had not completed therapy.
Patients were defined as having completed therapy if they had stopped receiving CBT having reached an ‘agreed end’ with their therapist, regardless of how many sessions they had received, or had received the maximum number of sessions permitted within the trial (‘completers’). ‘Non-completers’ were defined as patients who had never started CBT having been randomised to receive this treatment, had requested to end treatment or had been discharged by their therapist having cancelled or not attended three sessions without giving a reason.
We aimed to interview all of those sampled within 2 months of the 6-month follow-up assessment to give people maximal chance of remembering their experiences of treatment. However, this was not always possible for some of the completers, as some of these patients were still receiving CBT 2 months after the 6-month follow-up. Thus, a decision was made that for these individuals, we would interview them after they had completed their 6-month outcome measures and their treatment. Furthermore, because we could not interview patients before they had completed their primary outcome measures, this meant that for some of the non-completers, for example patients who had not started CBT or withdrawn from treatment shortly after being randomised, it was several months between making the decision to withdraw from treatment and being interviewed.
Two researchers conducted the qualitative interviews (SS, then MB). The researcher made contact with the patient to discuss the qualitative component of the trial. This contact was usually by telephone and occasionally by email if the patient had indicated a preference for this form of contact. Having made contact, the researcher asked the individual if he/she would be willing to take part in an interview about their views and experiences of depression and any treatments they had received during or prior to participating in the trial. If the patient agreed to be interviewed, a confirmation letter and information leaflet was then posted to him/her.
Interviews and analysis
A topic guide was used to ensure consistency across the interviews. The same guide was used for completers, non-completers and participants from the usual care arm, with questions tailored to suit certain areas ( Table 66 ). The guide was developed in light of the aims of the qualitative study, the design of the trial and relevant literature.
| Type of interview | Topic area |
|---|---|
| Usual care | Health prior to starting CoBalT Current health and health status Experiences of depression Experiences of any treatment/support for depression before study Views and use of antidepressants Views of CBT Any other care received during study Views on CBT plus antidepressant medication |
| Intervention arm (completer and non-completer) | As above, plus:
|
The interviews were conducted on a face-to-face basis and patients could choose to be interviewed at home or in their own GP surgery. Each interview was audiotaped and fully transcribed. Data collection and analysis proceeded in parallel. Recruitment ended when data saturation had been reached.
The interviews were analysed thematically, as this allowed comparisons to be made within and across the interviews, and for the views expressed in relation to a particular issue to be highlighted, for example patients’ experiences of CBT. This analytical approach entailed individual members of the research team (KT, SS, MB and AOS) reading and re-reading transcripts in order to gain an overall understanding of the participants’ views and experiences, and to identify emerging themes. A coding frame was then developed, based on the themes identified, and the same members of the research team independently coded transcripts and then met to discuss areas of consensus and discrepancy. This led to further codes being developed and to existing codes being defined more clearly. Transcripts were then imported into the software package ATLAS.ti to allow electronic coding and retrieval of data. Once all the transcripts had been coded, data were systematically analysed using a framework approach. 90 Using this method, what participants had said in relation to specific issues were summarised in tables and comparisons then made across and within the interviews to identify thematic patterns and deviant cases, and to determine if, for example, there appeared to be any differences between the views expressed by men and women or individuals of different ages.
Results
Forty interviews were conducted in total between April 2010 and February 2011 ( Table 67 ). Nineteen were held in Bristol, 10 in Exeter and 11 in Glasgow. The first seven interviews were conducted by SS. The remainder were undertaken by MB. The interviews lasted between 24 minutes and 1 hour 43 minutes, the average being around 1 hour. Half of the interviews took place in patients’ homes and half in their local GP surgeries. The average (median) time from end of therapy to the qualitative interview was 96 days, and for those interviewed who did not complete therapy it was 168 days.
| Characteristic | Intervention (completers), n = 17 | Intervention (non-completers), n = 9 | Usual care, n = 14 | Total, n = 40 | |
|---|---|---|---|---|---|
| Centre | Bristol | 7 | 6 | 6 | 19 |
| Exeter | 5 | 1 | 4 | 10 | |
| Glasgow | 5 | 2 | 4 | 11 | |
| Age at baseline (years) | 20–36 | 2 | 2 | 3 | 7 |
| 37–52 | 10 | 3 | 7 | 20 | |
| 53–67 | 5 | 3 | 4 | 12 | |
| Sex | Women | 11 | 5 | 10 | 26 |
| Men | 6 | 4 | 4 | 14 | |
| Highest educational qualification | None | 2 | 0 | 4 | 6 |
| GCSE | 5 | 4 | 2 | 11 | |
| A-level/Higher Grade | 6 | 4 | 5 | 16 | |
| Degree | 4 | 0 | 3 | 7 | |
| Employment | Student | 0 | 1 | 0 | 1 |
| Housewife | 1 | 0 | 3 | 4 | |
| Ill health | 2 | 2 | 3 | 7 | |
| Jobseeker | 2 | 0 | 0 | 2 | |
| Part time | 5 | 2 | 4 | 11 | |
| Full time | 4 | 3 | 2 | 9 | |
| Retired | 3 | 1 | 2 | 6 | |
| Housing | Homeowner | 14 | 6 | 8 | 28 |
| Tenant | 2 | 3 | 5 | 10 | |
| Living with relative | 1 | 0 | 1 | 2 | |
| No. of sessions of CBT attended (median) | 14 | 2 | – | – | |
Bristol patients were slightly over-represented in the non-completers (six of the nine non-completers interviewed were Bristol patients). This group was particularly difficult to recruit and would often cancel appointments. Thus, for pragmatic reasons, non-completers tended to be recruited from the interviewer’s home city.
All of the patients interviewed were white British. More women than men agreed to be interviewed and over half of the participants had an A-level/Scottish Higher or university degree, and owned their own home. Many of the interviewees had other health problems, such as multiple sclerosis, emphysema, ankylosing spondylitis, diabetes or osteoporosis. Some of these conditions disabled the individuals and/or resulted in physical pain.
Within this chapter, findings have been presented within three main sections that reflect the aims of the research: patients’ views and experiences of CBT (including the impact of therapy on their symptoms); reasons for completing and not completing therapy; and what usual care entailed for this patient group.
All quotes reproduced have been tagged according to whether the patient was a completer, non-completer or in the usual care arm, and according to the patient’s gender, age and study site. Quotes from individuals in the intervention arm have also been tagged with information on whether their symptoms had improved by 50% (+) or not (–) between baseline and the 6 month outcome assessment, according to the BDI-II.
Patients’ views and experiences of cognitive behavioural therapy
Few patients talked about having any previous knowledge or experience of CBT prior to their involvement with CoBalT, and in terms of expectations, most patients said they did not expect to be ‘cured’. The majority of patients interviewed who had received CBT during the trial, having been allocated to the trial’s intervention arm, stated that they had found CBT beneficial, particularly in terms of learning techniques to help them manage their symptoms. This included individuals who described how they had struggled with CBT, and both completers and non-completers.
On analysing the data, it was apparent that patients’ views and experiences of the CBT related to the therapy sessions they had received, the homework that they had been asked to complete between sessions and the impact of CBT. When talking about the therapy sessions, patients detailed their experiences ‘in session’ and their relationship with their therapist.
Therapy sessions
Cognitive behavioural therapy in sessions
For many patients, the initial sessions could make them feel uncomfortable because the session’s content and format were outside their previous experience and they were not sure how to respond to the therapy/therapist. However, in most cases patients described working through this preliminary discomfort to gaining from the sessions:
[I felt] panic at the first one because I’d never been in this situation before and it was outside of my comfort zone. [After that] It was great because I actually looked forward to them . . . They just want to talk to you about how you’re feeling about things and how it affects you and all this. And you could open up to [therapist’s name] and it was good.
Completer, male, 65 years, Glasgow +
Some patients described how they felt that CBT did not address the cause of their depression; they talked about feeling that there was not enough exploration of their early lives and the root of their problems or about getting enough insight into why they felt the way they did:
I would maybe have liked to talk, have talked about my earlier life a wee bit more because I feel a lot of the foundations of this all kind of started back then.
Completer, female, 39 years, Glasgow –
The lack of depth could be perceived as being caused by lack of time because of finite sessions or as something fundamental to the model of CBT itself:
So CBT . . . I think it touches the surface without going in too deep . . . I do think it would be nice just to go a little bit further to understand a bit more.
Completer, female, 37 years, Exeter –
This desire for more understanding about their depression than CBT provided was expressed mainly among interviewed patients who were educated to A-level and above and who were in their late thirties and forties.
Other comments made about CBT indicated how an individual’s comorbidity could complicate the therapeutic process. Despite the fact that therapists are trained to work with patients who experience chronic physical pain, it was apparent that such patients could struggle to see how CBT could help them personally:
The only thing that really gets me low is when it’s hurting, the MS is hurting a lot and I found it difficult to often put the model on to dealing with pain, really . . . I did find it difficult just to apply the logic to something which is not an emotional thing, it’s a physical thing. I think that’s probably why I don’t think I was really a 100% right for the study . . . with pain, it doesn’t matter which way you look at it, it’s still the same.
Completer, male, 46 years, Bristol +
For other patients, the learning and insights gained as part of the therapy process could be distressing. CBT was often described as painful owing to discovering aspects of themselves or revisiting difficult periods of their lives, for example realising that one has social anxiety:
I learned things about myself that I never knew and social anxiety came up. I always just thought I was antisocial, a loner, but when I read about it and everything and it exactly fit me and some of it was really upsetting . . .
Completer, female, 43 years, Bristol –
Although patients may have felt challenged by the ‘discovery’ element of therapy – and would consider halting therapy because of it – they would often talk about the insights and benefits to be gained from going through this process:
I’ve found it quite painful at times because you sort of go back to your childhood almost and err yeah it was, it was quite difficult. And first of all when I started I didn’t think I would carry on with it but I persevered and um I think it has done me good actually.
Completer, female, 64 years, Bristol –
Each week, during therapy, patients were asked to complete a short questionnaire (BDI-II) to measure their depressive symptoms. Some patients talked about using the BDI-II score to measure their progress or as a starting point for discussion within the therapy session. BDI-II scores were also talked about as a way of calibrating their experience of depression, for example as evidence that their depression was improving, even if the patient felt the same.
Views and experiences of the therapist
Patients described their therapist’s personal qualities, role and skills, the therapist–patient relationship, and how these impacted on their experience of treatment.
Most patients described their therapist as calm, friendly, trustworthy and caring, all of which encouraged patients to feel comfortable and talk freely during the therapy sessions:
. . . it probably matters a great deal that you get on with your therapist and I suspect that, it’s like most talking therapies, if you don’t get on with your therapist you’re buggered . . . I think [it’s] something about somebody making you feel safe to discuss these things but you know it’s not a friend, it’s a therapist. You are safe to cry and you don’t feel that you can’t come in next week.
Completer, female, 57 years, Exeter +
Most patients considered the role of their therapist was to aid them in coping with and managing their symptoms of depression through giving them ‘tools’ to change their thinking, particularly in separating their thoughts from their emotions:
[The therapist] would draw diagrams and say ‘so OK this happened and then from there what were your thoughts? From there what did you then feel? From there what were you then thinking?’ and then you can see how it turns up a circle and then you can discuss how you stop it from becoming a circle . . . what could you counter that thought with?
Completer, female, 41 years, Exeter +
The therapist skills that were identified and appreciated were largely to do with getting patients to talk and non-judgemental listening. These skills were appreciated by all patients regardless of gender, age and education:
I can’t praise her enough, she was helpful, I suppose she just seemed to see all the things. Like the time when I wanted to storm off sort of thing, I think a month before she would have changed the subject but she almost knew to push and perhaps she could see that I needed it out.
Completer, male, 49 years, Exeter +
[Her role] was for me to sound off to . . . and she done it very nicely. She was very very pleasant and it was just . . . I’ve never thought about this before but it was just that it was pleasant to have someone like that to sit and talk to you know? Because it’s never happened to me before.
Completer, male, 65 years, Glasgow +
The therapists’ ability to steer and guide patients through the structure and agenda of the session was also appreciated, although not necessarily at the time:
The therapist was very good at making sure we stuck to an agenda . . . skilled in leading me in the right direction, skilled at picking out things from what I was saying and bringing about more discussion.
Completer, female, 41 years, Exeter +
If the therapist felt that I needed to go back over something then she wouldn’t lose that, she would make me do it next time, much to my begrudgement.
Completer, female, 46 years, Exeter +
Other skills mentioned were the therapists’ valued knowledge and advice, which enabled patients to gain insight into their thought processes. Patients, particularly women, also talked about how they had gained in self-confidence having worked with their therapist.
Most patients had a ‘good’ relationship with their therapist and could not praise them enough. An important aspect of this relationship was the feeling it was ‘genuine’, that the therapist cared, was trustworthy and collaborative:
After a wee while it was fine. Um, because I genuinely felt that [the therapist] wanted to help.
Non-completer, male, 44 years, Glasgow +
I would always leave there feeling fantastic, like really excited because of the discoveries we had made and it really did feel like ‘we’ . . . you sort of uncover them together.
Completer, female, 41 years, Exeter +
Some female patients also described the relationship they had with their therapist as similar to a teacher–pupil relationship. It was these patients who were most likely to speak about how committed they were to CBT and how fulfilling they had found the process:
[The therapist] was almost like my teacher [laughs] but not in a bad way or anything . . . was there for me to talk to and to tell me – not how stupid I was being, but to help me to get my head around what I was thinking.
Completer, female, 42 years, Glasgow +
[The therapist] was quite formal but I quite liked that because it was almost like the teacher and pupil and we never got pally and so everything was my decision . . . suddenly I had to do everything for myself, not ask somebody else to lean on, so I found that really really helpful.
Completer, female, 59 years, Bristol +
Conversely one non-completer actively refuted the teacher role while going on to describe the therapist role in similar terms to the above women:
[The therapist was] a sounding board, sometimes just giving you ideas really. You know . . . it’s not a teaching one it is literally just kind of wiser or giving a number of options and letting you choose which one is more personally appropriate.
Non-completer, male, 50 years, Bristol –
Although most patients detailed how they had felt comfortable with their therapist, some patients talked about feeling uncomfortable with their therapist and/or under pressure to come up with the ‘right’ answer. The feeling that they were being guided to answer did not, in these cases, feel like a positive aspect of the therapy relationship and is similar to patients’ feelings that the therapy itself was too inflexible for their needs:
I felt a little bit like I was having to run through the maze and . . . take the lefts and rights when I was told to . . . it was easier because I didn’t have to make the decisions so much but you did feel you kept being nudged back on the path . . . the therapist I had basically didn’t really want to know about my MS at all, really. But that was the reason for the depression, and yet . . . I felt like I had to try and disregard that . . . and felt a little bit like I was a square peg trying to be pushed into a round hole [laughs]. I felt a little bit uncomfortable with the fact that . . . [the therapist] was trying to push, put another reason behind why I was having the depression.
Completer, male, 46 years, Bristol +
In addition, a couple of patients commented on the age or perceived experience of their therapist in comparison to their own knowledge and experience, having lived with depression for years. This might also have been a barrier in the therapeutic process:
She was quite young. So I suppose I wouldn’t have thought, well, you know, not saying that she’s not knowledgeable of it or anything but, I was more, in some respects, I was more knowledgeable really because I’ve dealt with it [depression] for so many years . . . I’ve done a lot of research on it . . . and I’ve done a lot of different things. I think if I’d seen, I don’t know, someone who was like, 40 . . . you know, I might have thought, well you know, you may have been doing this for quite a few years now.
Non-completer, female, 28 years, Bristol +
Overall, non-completers talked less about their relationship with their therapist than completers. This could be an artefact of having had less therapy, although three of the non-completers had attended eight or more sessions and their therapist had not featured heavily in their accounts. These patients may have felt less engaged with their therapist – thus dropping out of therapy early – alternatively their individual coping mechanism may have been one of avoidance of difficult situations or ‘facing up’ to problems.
Cognitive behavioural therapy homework
Patients described homework as the most challenging aspect of CBT, in particular the thought and activity diaries. The accounts given indicated that individuals had struggled with homework tasks for both emotional and practical reasons.
Emotional reasons given by patients for why they struggled with homework were often linked to fear of failure, which they, in turn, associated with their school homework experiences and being judged for what they had or had not done. This lack of confidence in patients was also manifested in the concern about ‘not doing it [homework] right’:
Homework never liked it anyway, never liked homework at all but forcing yourself at the start to start writing these down and writing out how you feel. It’s a very difficult thing to write out how you feel it’s difficult to put it into words and you don’t feel you’re doing it right and for me if I don’t feel I’m doing something right then that’s it! So you know it was a double edged sword I couldn’t write it down and then I thought, ‘Och well you can’t write it down because you’re rotten at it anyway’.
Completer, female, 39 years, Glasgow –
Doing homework could also be distressing because it required having to think either about the causes of their depression and/or situations that could not be changed:
Erm [long pause] I think the majority of the time I didn’t particularly want to do it. I didn’t want to dwell on it because well obviously thinking about situations and that you’re dwelling on perhaps certain things you, you can’t deal with . . . that seemed depressing having to think about the depression [yeah] and think of things that perhaps I would normally block out.
Completer, male, 42 years, Bristol –
Homework could be a painful process if it conflicted with individual coping mechanisms of denial. A couple of patients even referred to making things up to complete their thought diaries because they felt they did not have anything to put in it and/or they did not want to have to think about the cause of their depression.
In terms of practical difficulties, some patients felt their depression did not lend itself to separating out thoughts and feelings and then writing them down. One patient detailed how he felt the CBT homework model was too structured for his depression:
I would think ‘oh I’ve got to record something every five minutes, I’ve got to record and make a note of everything’ and it’s just my gosh! What a tall order, because she wants to analyse it, why isn’t it saying enough? I started to get frustrated because I am not being listened to . . . because the homework was set at these, I had times to write something down or whatever and I was looking at it thinking, well 6 hours I don’t feel nothing, 12 hours I didn’t think nothing, oh and then I have 3 hours of all this and I’ve got to try and cram in. And I thought… do they think that your depression is a structured, standard thing that can comply and can conform into their models? Cos it isn’t, not for me it isn’t anyway.
Non-completer, male, 34 years, Bristol +
Some patients spoke about writing at work or at home as a public act and one, therefore, which came with the risk of getting ‘caught’. The riskiness of the endeavour was related to stigma associated with depression, or with the risk of family members reading their private thoughts:
I did struggle first of all you know.. um trying to get my mind into the frame that putting something on paper about how I felt and all that sort of thing you know. I used to say ‘I don’t want anyone to see this and you know I’d hate my family to see what I’ve written down’. I did cope with it in the end but I found it difficult at first.
Completer, female, 64 years, Bristol –
To address the practicalities (and risk) of writing during work time, patients would often report leaving their homework until the end of the day. However, this delay brought its own set of problems in not being able to remember or re-live thoughts and emotions from earlier. Sometimes, simply finding the time at all was the most difficult part of completing homework. Lack of time could threaten completing the course:
The only problem I found was trying to commit the time to doing them [homework sheets]. That was the difficult bit . . . at one stage I did have a discussion with the therapist to say ‘look I don’t feel I’m committing enough time to the homework tasks, you know I want to commit more time but I just cannot find more time.. I might drop out of the study’.
Completer, female, 43 years, Bristol +
Accounts of time/practical pressures could also contain emotional reasons for not doing homework:
I was finding because of the pressures of work and the pressures of everything and the fact that I wasn’t coping, I couldn’t even go sometimes to work and so the homework became an issue for me. And trying to give it the level of time - it fell apart after about five or six weeks. We kind of reviewed it and you know there were times when I said ‘look I haven’t done it’ and there were times where I was trying to do it and so even the fact of not doing it became an issue for me as well.. And part of my problems are my avoidance issues.
Non-completer, male, 50 years, Bristol –
Motivation was also a factor for some in committing to the homework, when a lack of motivation was a large part of their depressive symptoms. This could be interpreted as an emotional, as well as a practical reason for finding homework difficult:
It was just having the motivation really, that’s something that I was really struggling with at that time and have done in my life struggled with motivation, especially in periods of bad depression and the worse the depression is at any time, it sort of can be measurable by my motivation.
Non-completer, male, 34 years, Bristol +
Overall, reports of practical or avoidance issues with the homework tended to be given by male rather than female patients interviewed.
Whilst most patients found the homework difficult, there was a group who associated it (and its associated difficulties) directly with the level of benefit gained through CBT. The only patients who spoke about homework in this positive way were completers and all bar one were women whose BDI-II scores had improved by more than 50%. Direct gains were described from actually ‘seeing’ patterns of thoughts, emotions and behaviours written down:
Just having the actual writing and seeing it. So it’s a visual thing. I mean you can think as much as you like about your routine and how you’re going wrong with it and how it’s damaging you but until you see it on paper, that’s almost a slap in the face . . . which is good for me.
Completer, female, 46 years, Exeter +
I found it was really good and I actually, you know if only I’d been as good as that at school, but I kind of liked it all written down . . . and it all became so much clearer to me.
Completer, female, 36 years, Glasgow +
Patients talked about homework making them engage with the process and work between sessions; the effort invested was equal to the improvement felt:
I did struggle with the homework definitely and I often felt gosh if there hadn’t been any homework you could become very lazy and just keep expecting your therapist to keep bringing something up to talk about or not think at all about anything during the week . . . I guess homework is one of the ways of helping you to continue with things learned in the sessions and at the end of the day of you want to improve how you’re feeling, it’s not going to just happen without any hard work or effort.
Completer, female, 41 years, Exeter +
Patients’ views and experiences of CBT also included accounts of the impact of therapy on their condition.
Impact of cognitive behavioural therapy: fewer bad days
The majority of patients interviewed, who had received CBT during the trial, said they would recommend CBT to others. In addition, even those who did not complete a full course of CBT felt that they had benefited from receiving this treatment:
It’s [CBT] made me more aware of the fact that it’s just feeling an emotion really. It doesn’t really govern your life. And it’s given me the tools to recognise when I’m becoming more depressed . . . and to try to implement things to change that.
Non-completer, male, 44 years, Glasgow +
Random negative thoughts in depressed people tend to be turned up like a volume so that’s something that I’ll always have. And when I started thinking about that and became conscious and aware of it, I was able myself to turn them [negative thoughts] down and think of a positive alternative which is a skill I’m supposed to have been taught.
Non-completer, male, 34 years, Bristol –
When talking more specifically in terms of what they had gained from having CBT, patients talked about learned techniques and tools, a changed understanding of self and behaviours, better communication with their family, and the trigger to healthy lifestyle changes.
Patients most commonly praised the techniques or tools they had learned in CBT that had helped them to manage their depression. Patients especially valued the skill of questioning their negative thoughts to change their behaviour:
It just made me look at the way I look at myself, and other people look at me and it’s just made me feel more positive and question things that I do. ‘Why are you doing that cause if you do X and Y, then Z will happen and you’ll feel miserable’. Whereas if you do something else it could be A, B and C that happen and you feel totally more positive.
Completer, female, 44 years, Bristol +
Patients often talked about gaining a better understanding of themselves and how this had helped them manage their condition. Understanding was usually couched in terms of what had been learned through CBT and how this translated into their thinking and behaving differently:
[The therapist] talked me through why did I feel bad about asking for help and it wasn’t a sign of failure – because I always thought that if I asked for help it was a sign of failure . . . I don’t do those things anymore. I don’t feel bad asking for help and I do get the kids to help and I don’t feel it’s a failure to ask for help now.
Completer, female, 42 years, Glasgow +
Patients also described the gains to be made from learning how to manage their situation. Making small changes to their usual routine could have very beneficial effects, for example putting tasks to one side until one is having a ‘better’ day or changing a routine chore:
It’s dealing with problems, I have learnt now to sort of say, bad letter comes in or something, ‘look I don’t feel right today, let’s deal with it tomorrow’. I have learnt to do that, whereas before I would do it and just get more and more frustrated and lashing out verbally sort of thing. So I have learnt to do that, because before I have always been, what’s the point of putting things off, I have always, whatever has needed doing I have done it straight off.
Completer, male, 49 years, Exeter +
My routine was actually causing me to be depressed as well. I’d actually got myself in this rut, almost like self-harming that is difficult to get out of and you know is not good for you. And then we changed the routine, even if it was something simple like changing your shopping day . . . we started off very small.
Completer, female, 46 years, Exeter +
Some patients felt that CBT had been effective in teaching them better communication techniques and that this had produced a positive effect on family life in particular:
The daughter and I, as the wife says, are two of a kind. You know? We argue like buggery. We used to . . . now . . . I say ‘right, okay’ and walk away, and then come back maybe and say, ‘well, if we do it this way and do it that way, I’ll do it your way’ and that’ll get things done. Before, I would have stood there arguing with her about it. Now, I’ll just say, ‘ah well, fair enough, right’. And walk away.
Completer, male, 65 years, Glasgow+
Cognitive behavioural therapy could also encourage or act as a trigger to wider, healthy lifestyle changes:
I don’t feel cured, I feel that I’ve got some pieces of equipment in my head and my gut that I can use and have to remind myself to use sometimes . . . I’ve done a bit better at saying ‘no’ if I don’t want to do something without feeling that means I’ll never be invited again. I’m better at making myself achieve something in the day however little, even bad days when walking hurts, I know I’ll feel better at the end if I achieve something. l walk the dog more and I’ve lost about a stone and a half. It actually gave me the kick up the bum to do something cos I’d got into a rut and when you get into a rut or a deep hole it’s very hard to get yourself out of it, so it give me the incentive to do some things I’d talked about.
Non-completer, female, 57 years, Exeter –
However, some patients talked about finding it difficult to put what they had learned from the CBT into action, and these tended to be male patients whose symptoms had not generally improved:
I don’t think it’s [CBT] ever been unhelpful but sometimes difficult to actually put into practice. I mean if you’ve got a depressive thought that may block out thoughts of ‘Oh CBT, I must use that’. So you may not automatically think of the thought cycle you should be using to help you.
Completer, male, 42 years, Bristol –
It was also possible for patients to put in the hard work and not feel better or have met the criteria for ‘response’ based on their BDI-II score:
I worked pretty hard, I got the books, I did everything that I could and I did really find a benefit from it yes at the time. I can’t say I enjoyed it because you wouldn’t enjoy it really would you? It did make me feel a lot better and at the time I was really optimistic that I was going to get a lot better and I suppose that was why it was quite bad afterwards to be quite ill again.
Completer, female, 43 years, Bristol –
Both completers and non-completers described having ‘less bad days’ and ‘more good days’ than before their involvement in CoBalT, owing to the therapy. Patients also talked about their symptoms being less intense:
My bad days aren’t half as bad as what they were.
Completer, female, 46 years, Exeter +
Overall, patients gave a very rational and careful account of their current symptoms following CBT. No-one claimed to be cured or that they would not get depressed again. They hoped that they now had the techniques to cope with and better manage their symptoms:
Well I am coping with things better. I can’t say it’s going to be like that next week, but you know I said this money thing was quite a big issue and I am not saying it did not annoy and frustrate me and make me depressed again, because it did. But I got over it or I dealt with it so much better than I would have 4 months ago.
Completer, male, 49 years, Exeter +
I feel at times slightly better, though I have to say there is times when you feel . . . oh, God I wish I’d never got up this morning you know? But it doesnae seem to last as long as it did before.
Completer, male, 65 years, Glasgow +
For those that felt they still had more bad days than good – most of whom had symptoms which had not improved by 50% on the BDI-II – the barriers to alleviation of their symptoms tended to be related to a comorbidity and/or to a feeling of hopelessness:
Sometimes it like you don’t notice it [the depression]. You think you’re alright, going on quite happily . . . But things do run through your head sometimes you know, with this health thing, and I think to myself ‘what’s the point in being here? Can’t do anything. Can’t hold a pen for more than 5 minutes because of my hand . . . I drop things and then I start thinking stupid things, although I wouldn’t do it. But I do start thinking about it. Couldn’t do it to my son to be honest. Tried it before and I don’t want him to think his mother killed herself you know.
Non-completer, female, 57 years, Bristol –
Sometimes I get these waves of hopelessness. You know like I feel I have just got no future sometimes. That is what I feel like all the time, I feel like there is no point to me basically. I feel like it all the time really but when I am really depressed it is a lot worse.
Completer, female, 43 years, Bristol –
There was little cause for optimism among this group of patients when thinking about their future health:
I don’t see an end to it. I don’t see anything that is actually going to turn a light on and I will be different.
Non-completer, male, 50 years, Bristol –
Those patients who had completed CBT and felt positive about the experience were, unsurprisingly, positive about the future and their condition. Patients would often marry the hope of continued lessening of symptoms with being able to reduce or stop their antidepressant medication:
Well, I’m hoping that I can keep up using CBT myself to the extent that I can at least reduce the antidepressants – I mean at the moment my goal would be how to reduce or stop them for the summer and winter with minimum pills and to keep using CBT.
Completer, female, 57 years, Exeter +
I’m hoping that I’ll be able to cut down on antidepressants . . . whether it’ll be in 6 months I don’t know but (laughs) the plan ahead is that. And just to keep going the way I am and keep questioning everything that I think and do and say and not be too hard on myself. It’s as simple as that (laughs).
Completer, female, 42 years, Glasgow +
Many of the elements of the impact of CBT described by patients are summarised in the following quote from a Glasgow patient who, despite her BDI-II score not improving by at least 50%, had committed to CBT and felt it had changed her life:
Well I’m not saying that there’s not going to be times where I maybe fall off the wagon and end up depressed or low again, but it won’t be for the want of trying to keep myself on an even keel. Now that I know there are ways to avoid that. So I’m hoping it’s just going to go from strength to strength and given that it is the end of November and I’m usually in the pits of depression at this time of the year I am really feeling quite good about everything. I know it’s not just medication I know it’s because I’m feeling better about myself as a person as well.
Completer, female, 39 years, Glasgow –
Reasons for completing and not completing therapy
Patients tended to give more than one reason for completing or not completing their course of CBT. These reasons were often interwoven with their accounts of their views and experiences of CBT. Overall, patients talked about whether or not they were able to fit CBT into their existing life in terms of practicalities and other commitments. For completers this was a relatively easy process. For non-completers, CBT could prove difficult owing to work factors and associated stigma, caring responsibilities, emotional responses to the process or the complications of their own ill health. These findings are discussed further below.
Reasons for completing therapy
For the majority of patients who completed their CBT course, the practicalities involved with attending sessions were largely unproblematic. The workplace was described as being ‘good’ and ‘flexible’ and/or the location and timing of the appointment was acceptable:
My work’s really good. Any time I’ve ever had . . . they just gave me the day off or somebody would swap shifts with me or whatever.
Completer, female, 36 years, Glasgow +
It was just at my local surgery, which I still attend, so I felt it was home from home almost . . .
Completer, female, 59 years, Bristol +
For others, not working owing to retirement, ill health or job-seeking meant that attending therapy was quite straightforward:
I didn’t have a lot going on my life at the time except parents . . . so that was OK.
Completer, female, 57 years, Exeter +
When work proved less flexible for one patient, leading her to almost dropping out of therapy, it was the therapist who was accommodating:
I went through a tricky phase where I couldn’t make the appointments at a certain time because of work and things but the therapist was really good and changed, changed the times and we managed to get through it cause at one stage I was really close to just dropping out of the study because everything was just getting too much just to get there but we worked through that and got out the other side.
Completer, female, 43 years, Bristol+
The location of sessions could be regarded as tricky by some patients owing to their condition (e.g. agoraphobia alongside depression) or when therapy sessions took place at a location other than the patient’s own GP surgery. For most of these patients it was a surmountable obstacle, either by being able to change location or making the journey part of their exercise regime:
[the] hospital was where I was asked to go originally and at that time, the way I felt, navigating two or three buses – I just couldn’t have done it. It just wasn’t feasible. Whereas the routine of coming down here [GP surgery] it’s fine for me.
Completer, female, 39 years, Glasgow –
For the completers, ending therapy was broadly described as a decision taken between themselves and their therapist at a point where they defined themselves as being ready to end therapy, feeling better or feeling that there was no more to be gained from continuing therapy. Patients went on to describe the ending as being suggested by the therapist and agreed by themselves.
There could be some ambivalence expressed about the cessation of therapy, even among those who had the maximum number of sessions allowed in the trial. Some patients described their anxiety about stopping CBT:
I was quite looking forward to it really [ending] and it is mixed feelings. You are apprehensive because there is someone there for you every week, someone who listens to you and goes through things with you and I knew I was going to really miss that. But then it is quite nice not to have to do it you know.
Completer, female, 43 years, Bristol –
Anxieties about ending therapy could be alleviated by feeling there were processes in place to support the patient after sessions had finished:
I did panic at [the end of therapy] because in a way the therapist is like that crutch when you are offered it. But we discussed that because she said. ‘you know this will come to an end and we need to look at how you are going to carry on and cope and have little things in place.’
Completer, female, 59 years, Bristol +
For some patients their change in BDI-II score recorded in therapy sessions was considered as part of the decision to end CBT as it was tangible evidence of their progress:
Well I suppose the scores you know I was up in the 20s a few times and then I was like 5 or 6 so I think it was the scores I probably outtalked myself I suppose . . . we could both see progress there and it was a good cutting off point.
Completer, male, 49 years, Exeter +
For one man, the stress involved in attending sessions outweighed any benefits:
I started toward the end thinking, I don’t really want to do any more of these sessions. I’m starting to get stressed about going to them [laughs]. The last three sessions I didn’t really feel I was gaining a lot more because I’d taken time off work and I didn’t feel I was getting enough benefit against what I was having to [put in].
Completer, male, 46 years, Bristol+
Reasons for not completing therapy
Explicit reasons given by participants for not completing therapy grouped around the themes of work issues and stigma, practical reasons (such as other commitments), feeling CBT was not for them, and ill health.
It was apparent that some of the non-completers had found it hard to commit to CBT because they felt they needed to prioritise other commitments in their lives, such as work or caring for others, over completing therapy. If they worked, the possible censure of work colleagues could also influence patients’ ability to commit to CBT. For those in employment, the perceived cost–benefit of attending sessions compared with attending work could lead to early dropout from CBT. For some, the threat of work colleagues finding out about their depression, linked with the imagined moral judgement about taking time off work, became an insurmountable obstacle to finishing the course of therapy:
You know I was skint, I hadn’t had work for a whole year I think and I’d started working. And it just became more and more difficult, you know, I could do a few Fridays, a few Tuesdays but then after that it was just like ‘Oh bloody hell he’s off again on Tuesday ‘. . . It conveys the wrong message. People think ‘Oh where’s he going? Is he still signing on?’ So I tried to schedule them [appointments] so as I could keep seeing her for as long as I could and then it just got to a point where it was like, ‘No I don’t’ think I can make it any more’.
Non-completer, male, 44 years, Glasgow +
As indicated in the above quote, financial priorities were also taken into account when weighing up the costs and benefits of continuing therapy.
The fear of being potentially judged by work colleagues was a theme more common in the accounts given by male than female patients. Similarly, a female patient did not want her work colleagues to know about her appointments as the workplace was very ‘male’ and she feared would consequently be less understanding of mental, as opposed to physical, health problems:
[The therapist] that I was seeing, had to change to a Wednesday which would have meant having to leave work early and I really didn’t want to do that . . . especially where I work as well it’s very male orientated . . . I don’t think it would have gone well as I didn’t really want to go into my business with them anyway about that. And also I don’t think they would have been so understanding about something like that you know . . . and I didn’t really want anyone saying ‘oh why’s [interviewee’s name] leaving early?’ And you know, the office thing.
Non-completer, female, 47 years, Exeter –
One patient who was randomised to CBT felt unable to start therapy due to work commitments, and it was apparent that several participants who were parents or carers had not started or completed therapy because they had prioritised their caring duties over their own health needs:
I was to go ahead with it [therapy] but then because my husband’s health had deteriorated . . . I just felt that to focus on both things and trying to take part in that when a lot of my time was spent going back – some weeks we were at the hospital every single day you know for six and seven weeks at a time . . . I just prioritised and at that point in time his health was more important to me.
Non-completer, female, 53 years, Glasgow –
One patient who attended only three sessions, being reminded of distressing events was a reason to stop CBT. For another patient, her emotional response to the homework diaries, with its intimations of school (‘I rebelled’) was a large part of her reasoning for not continuing with CBT:
I had to fill in this . . . these papers about how I felt every day and I didn’t want to do it. I think that was what put me off as well, filling in these things every day of how I felt . . . I looked at it and I thought, ‘How can you do that?’ And yet I’m not stupid, but I just felt like I was . . . I kind of rebelled. I didn’t want to do it.
Non-completer, female, 57 years, Bristol –
Ill-health could also be a barrier to completion of CBT, for example a woman spoke about her mental health as getting worse during the time of the study and of taking an overdose:
I was very disappointed actually that I had to come out of the CBT bit of this study and that was due to my ill health and things that I did feel that the therapist I was actually working with, I think she [the therapist] probably would have ended up breaking the cycle [of my condition], I think just because of the questions that she’d actually asked. So therefore you know I did feel disappointed that I had to come out . . . But unfortunately I think I only made about three sessions, because once again my health took a real dip and I got myself really down and I ended up taking extra tablets [overdose].
Non-completer, female, 57 years, Bristol –
For another individual, physical comorbidity was a factor:
In my head like it’s [the depression is] ongoing for me because a lot of it is tied in with the health. And that ain’t getting any better and I don’t know how to get it better. Like it’s the pain is there all the time.
Non-completer, female, 57 years, Bristol –
The above quote was part of a patient’s account of why she dropped out of therapy after one session and included the disabling nature of her conditions (osteoporosis, emphysema, diverticulitis) and associated feelings of unease outside the comfort of her own home.
Attendance could be an issue due to the symptoms of depression, for example a patient struggled with attendance due to his lack of motivation/inability to speak when he was having a bad day.
Usual care for this patient group
The accounts of patients in both arms of the trial, detailing their experiences of usual care, indicated that this mainly entailed them taking antidepressants. These accounts also highlighted that the extent to which patients received support from their GP varied greatly between patients. In terms of other sources of support, it was apparent that some patients had received invaluable support from family and friends, and had developed their own personal strategies to help them cope.
Patients’ views and experiences of taking antidepressants
Most patients talked about how they had been on antidepressants for years. Patients described being on a ‘maintenance’ dose, which kept them ‘ticking over’, and detailed how the dose they were on had changed over time, in response to their needs:
I’ve been on them [antidepressants] for years. I know when I’m sort of having a bad episode and I need to increase the dose. So I can go to the GP and say ‘Look you know something’s not quite right here . . . I think the dose needs to be increased’ and then when I’m feeling better again, it can be decreased.
Completer, female, 44 years, Bristol +
It was apparent that whilst some patients had been on the same type of antidepressant for some time, others had been on several different types over the years. Reasons given for changing the type of antidepressant were experiencing side effects, and restarting on a different type of antidepressant having been off medication for a while and/or now being under the supervision of a different GP. Comments made by one individual, however, suggested that no explicit reason may be given by the GP for changing medication type. His comments also suggested that, although some patients described going back on the same type of antidepressant, this had not been his experience:
I’ve been through a large number of them [antidepressants] now, I think.
Why is that, they keep changing?
Changing yeah, sometimes you go with a problem and they say, ‘Oh just change it, just stop, change it.’ They say, ‘We’ll try you on this one’ and they don’t say, ‘Well you’re better on that one, so we’ll put you back on that one until’, you never go back again. I don’t know whether that’s part of the protocols or whatever but you never go back.
Usual care, male, 60 years, Glasgow –
A few patients described how when they first started taking antidepressants, they had experienced side effects, such as headaches and feeling dizzy. In addition, one individual recounted how initially she had felt suicidal. Other side effects described, which stayed beyond the initial period of starting them, were feeling more tired, feeling numb and less aware of what is going on around them, weight gain and experiencing a lack of libido.
Only a few patients stated antidepressants had improved their mood. Most patients described the effect of medication as keeping their mood stable or at a certain level:
I had erm lots of different antidepressants . . . Although I’ve not been happy, I’ve not been depressed, I’ve just been even sort of like flat, which has been easier to contend with than having depression.
Non-completer, female, 57 years, Bristol –
I think they [antidepressants] help me, they sort of give me a sort of baseline to work from . . . as if I’m not going to drop too far down . . . it gives me a sort of baseline to work from, em even a buffer. I don’t know quite how to explain it, but it makes me feel safe.
Usual care, female, 53 years, Bristol +
A few patients described still feeling low or experiencing low periods despite taking antidepressants. In addition, several patients commented that they were unsure whether or not being on antidepressants was having any effect on their mood, and/or described not knowing if they were feeling better because they were better or because their medication was working. These patients argued that the only way they could establish what impact antidepressants were having, would be to stop taking them; something which some of them had attempted to do and which had left them feeling worse:
The thing is you only know with these things that they’re doing some sort of good when you try to give them up and then suddenly you feel twice as bad.
Usual care, male, 60 years, Exeter –
Within the accounts given by those who had received CBT during the trial, there was the suggestion that antidepressants could help an individual to feel less tearful and therefore more able to work on the source of their depression:
If used correctly it [antidepressants] can be a really good platform as a starting point for people . . . I think generally speaking that they are a necessary thing sometimes. You know just to get you to a point where you can think clearly to resolve some issues you know and stop the crying.
Completer, female, 41 years, Exeter +
Most patients in both arms of the trial described how they wanted to come off medication. A range of reasons were given for this view: experiencing side effects, feeling that antidepressants only addressed the symptoms and not the cause of their depression, concerns about becoming dependent on medication, wanting to be ‘normal’, viewing antidepressants as ‘unnatural’ and having concerns about what they were doing to their bodies, wondering if the antidepressants themselves were causing the individual to be more depressed and feeling that they should be able to cope with life without the support of medication. These patients remained on medication because they had attempted to come off them before and then experienced nightmares or a significant drop in mood; they feared a relapse in mood; their GP had not suggested they came off them; they became agitated when they forgot to take them; or because they felt they should wait until life was less stressful before doing so, for example when they retire. It was also apparent that, being on medication for years could mean taking antidepressants became a habit:
Before you know it I think you are on them 5 years and they are not really doing you any good you know? But you are so used to taking them; it just becomes a habit I think to take them.
Completer, female, 43 years, Bristol –
Only a few patients appeared to be comfortable with taking medication. Interestingly, all were female. Their accounts implied that this was a pragmatic view rather than one based on viewing antidepressants in a positive light:
If it’s a choice between taking two tablets a day and feeling okay or not taking two tablets a day and feeling absolutely crap, then I know which I would rather do.
Completer, female, 44 years, Bristol +
I don’t have a problem [with taking antidepressants] if it’s making me feel better I don’t have a problem.
Usual care, female, 41 years, Bristol –
Never particularly bothered me being on them because it’s always made the difference between staying in bed and being up.
Completer, female, 57 years, Exeter +
Patients in both arms of the trial talked about feeling ready to come off mediation because they felt their mood or situation had improved enough to enable them to do this. There was no clear evidence that this feeling was more common among those who had received CBT during the trial. However, a few patients who had been randomised to receive CBT did talk about feeling differently about being on antidepressants because they now felt they had other ways of coping.
Contact with the general practitioner
Only a few patients described regularly seeing their GP and their medication being actively monitored. Most patients detailed how they simply got their antidepressants via repeat prescriptions and if they did see their GP, this was because of other health issues. This lack of direct contact, and the limited amount of time patients had with their GPs, may have meant some patients were on antidepressants longer than they needed to be:
I think maybe I have been on them slightly longer than I could have . . . because the doctor just sees you for two minutes, says ‘how are you doing?’ and that is the way it goes.
Completer, female, 59 years, Bristol +
Patients may also have been on medication longer than they needed to be because they did not feel GPs were well placed to help them, not only in terms of lacking in time but also in terms of knowledge about depression:
People keep telling me I should actually go to see the doctor again [and talk about coming off antidepressants], but I haven’t got a lot of faith in GPs because I know they’re GPs, they’re general practitioners, they don’t know about these things. I mean a lot try more than others but they haven’t got much time, you know they’re, it doesn’t do a lot of good seeing your GP really.
Usual care, male, 60 years, Exeter –
There was no evidence to suggest that patients who had been allocated to usual care saw their GP more frequently than patients who had been randomised to CBT plus usual care, or sought additional GP appointments or alternative sources of support in order to ‘compensate’ for not being allocated to the intervention arm. Financial constraints may have been one reason for this latter point, as usual-care patients talked about wanting counselling but not being able to afford it. Also, there was little evidence to suggest that GPs had offered extra support or referred patients for counselling, having established that the patient was in the usual-care arm; only one patient in the usual-care arm mentioned that his GP had referred him for counselling.
When talking about their GPs, some patients described them in a negative light. GPs were described as individuals who were focused on making money and reaching targets, and who had little understanding of depression. There was also evidence to suggest that some GPs had not treated their patients with much sympathy or respect:
I went to see him erm back in November . . . because he’d halved my antidepressants before that, and I said I want them doubled up because I feel quite low. He said, ‘You feel low because you’re fat.’ . . . I walked out that doctor’s surgery and I wanted to dig a hole and bury myself and disappear.
Usual care, female, 38 years, Exeter –
Others however, described their GP in a very positive way. Attributes mentioned included listening and having time for their patients, providing continuity of care and believing the patient when he/she said he/she felt down:
I’m lucky here because I’ve got a doctor I can go to who understands me, who believes me when I say I’m down, who trusts me when I say this is worse than I felt a couple of months ago.
Usual care, female, 53 years, Bristol +
Various comments made throughout the interviews indicated that it was very important to this patient group that they were not viewed as ‘time-wasters’, as individuals who exaggerated their symptoms or were ‘crazy’, and it was apparent that the possibility of being viewed as such could deter a patient from accessing services:
In the last 6 months I haven’t had to visit him (GP) very much. I’ve felt like a proper crazy person in the last sort of 2 years because I was constantly at the doctors and it’s horrible. And I’ll tell you something every time I have to go and see a GP or anything like that now, all I can think about in the back of my head is all they’re thinking of me is that she’s this crazy anxiety person and I hate that. And it’s, it’s just horrific.
Usual care, female, 26 years, Exeter +
Another theme that was evident within the accounts relating to GPs was a lack of treatment options for this patient group. Patients often talked about their GP offering them only antidepressants. In addition, comments made by one individual, who had received CBT within the trial, indicated that within the NHS, there were only so many treatments for which a patient may be eligible and, that, having received them all, no further help could be given:
I had had the CBT, I had had the therapy, you know, I had had the antidepressants and that was all like the NHS could do for me and the doctor even said that. You know like he said that there is nothing else, he wouldn’t sort of refer me to a psychiatrist or anything and he just said that there is nothing else that the NHS can do for me and I think I felt like I was like 44 and I was sort of like stuck on the scrapheap and he kept saying ‘You know you are still relatively young, you know our aim is to get you off medication into you know live your life again’ and all I could think of was well how do I do that? Because when you are feeling down, really down, it is hard to do that on your own and I think he just thought you know I have had what you know I am entitled to and that is it, and that really did send me in a downward spiral because I just thought that is it for me from now on.
Completer, female, 43 years, Bristol –
Other sources of support
Many patients described how they had received invaluable support from family and friends. This support could take the form of simply listening to the individual, distracting them from their negative thoughts, providing encouragement and advice. Friends who had knowledge of depression, either because they had experienced it personally or knew someone who had, were described as being particularly helpful in terms of giving the individual advice and understanding their situation:
[Friend’s name] actually sat me down and explained to me what it was like [to have depression], what it was about, actually having anxiety and depression and stuff, so every time I’d be like, ‘This is happening, this is how I’m feeling, is that normal?’ She actually just sat there and went, ‘It’s normal mate don’t worry.’
Usual care, female, 26 years, Exeter +
My friend [name] I’ve known know for 11 years and she understands as well, her mother suffers from depression. So she knows where I’m coming from when I’m having the low times and I’ve been friends with a neighbour for quite a few years whose husband has depression. And again, somebody else who knows what’s happening, understands where you’re coming from and these are people that I can sit down and talk about anything to . . . Things that you wouldn’t bring into normal conversation, silly things [hmm mm] irrational thoughts, stuff like that, I can throw them at them and they can say, ‘Och now you know that’s not true’ and I start the next thing.
Completer, female, 39 years, Glasgow –
Patients mentioned treatments they had tried outside the NHS. Some of these were sought in order to help with their depression or anxiety levels (e.g. counselling, hypnotherapy), whereas others had been used to address other illnesses the individual experienced, some of which were viewed as contributing to or underpinning their depression, for example self-management course for chronic fatigue. Patients also described how they had developed personal strategies to help them cope with their depression. These included avoiding situations that they found stressful, exercising, meditating, doing t’ai chi or self-hypnosis, wearing a hat to block out noise and listening to relaxation tapes. Patients’ descriptions of when and how they used these strategies suggested they were experienced as helpful because they reduced the individual’s levels of anxiety or helped them to feel removed from reality or the immediate situation:
If I find I’m getting anxious at work . . . I will sit down and hopefully I don’t go to sleep but, erm, perhaps on occasions, I will nevertheless sit down there and, I mean I don’t necessarily have to have my eyes closed to meditate so again it will, you know, I’ll use meditation from that sort of point of view.. I can meditate when I’m walking along . . . it doesn’t mean that I am separate from other things that are going around . . . to me obviously it’s wonderful, but a wonderful little, big I suppose in some sense, er, scenario that I can, when I’m really upset, I can sort of put myself into in my mind . . . I can put myself there and wash my troubles away for want of a term, you know.
Usual care, male, 61 years, Exeter –
In addition, some patients referred to counselling they had had in the past, including CBT, and it was apparent that lessons learnt during that time were still being employed:
I looked at things very much as if they were black and white [mm], you know, erm, I mean, and after that accident . . . CBT taught me that there was an awful lot of greys in the middle of it . . . if you’re prepared to work at it, it gradually makes you aware that life isn’t against you all the time.
Usual care, male, 61 years, Exeter –
This lady used to say to me, ‘If you are feeling bad one day and somebody says to you how are you feeling’, she said ‘Don’t say to them I feel fine’ because you don’t, she said tell them how you feel. So now I do actually, if I am feeling bad I do say to people, obviously only people I can trust who understand me. I don’t say to somebody, somebody in the supermarket queue [laughter]. But yeah I usually say, I admit to how I’m feeling, I listen to myself, listen to my body, listen to my mind, whatever . . . And I think because I do that I’m more honest with myself I suppose, I’m not trying to keep things suppressed.
Usual care, female, 53 years, Bristol +
A couple of patients talked about using the internet to find out about treatments, or social networking sites, such as Facebook, to ‘talk’ to other people in similar situations. One patient also described how she had read several books on how to cope with depression, such as, ‘Mind Over Mood’, which she had found helpful.
Although the accounts given by some of the patients suggested that they had proactively sought out and accessed treatments and resources that could help them with their depression, it was clear that many individuals had not attempted to do this. This may have been due to a lack of motivation to seek help. Some patients talked about a symptom of their depression being a lack of energy or motivation, and comments by some individuals suggested that they had come to accept their depression and to feel there was nothing which could change their situation. A lack of financial resources could be another reason, as patients talked about not being able to access counselling or alternative therapies because they could not afford them. Not being located within a social network may also have been a factor, as it was clear that not everyone had someone they felt they could turn to when they needed help:
I haven’t always got the support when I want it . . . You know, there’s no one at the end of the phone if I feel really low and I feel like I want to smack one of the kids . . . there’s no one I can ring up and say, ‘Help’ or ‘Can I chat for five minutes?’
Usual care, female, 39 years, Exeter –
Discussion
Summary of findings
Overall, patients who had CBT were appreciative of the methods used and felt that they had been given the techniques to help them better manage their symptoms. Patients could describe components of CBT that they struggled with, or were a barrier to them completing the therapy, and still feel they had benefited from the sessions. In particular, in learning to question negative thought patterns and behaviours and, therefore, better managing their condition. Patients’ accounts of usual care indicated that this consisted mainly of taking antidepressants.
Views and experiences of cognitive behavioural therapy
Most patients had little or no knowledge of CBT prior to taking part in the trial. Most patients settled down to enjoy the process of the sessions, often after some initial discomfort due to the unfamiliar situation. For some patients, however, there were barriers to the therapy process in sessions. Patients could feel that the CBT model did not necessarily address the cause of their depression and provide the depth of understanding that they required to make sense of their condition.
Cognitive behavioural therapists are trained to deal with physical and/or psychological comorbidity by bringing the focus of treatment onto the here and now and how their condition is linked to current depressive symptoms. However, for a few individuals their comorbidities could complicate the therapeutic process; in a few instances chronic pain caused by physical condition(s) was perceived not to ‘fit’ the CBT model.
Most patients thought the role of the therapist was to aid them in learning to cope with and manage their symptoms through giving them tools to change their thinking, for example, separating their thoughts and emotions. Therapist skills identified and appreciated by most were to do with getting patients to talk and non-judgemental listening. Indeed, overall, patients in the study reported their relationship with their therapist as genuine, trustworthy and collaborative. For a few individuals the therapeutic relationship was an uncomfortable one. They related this to feeling under pressure to come up with the ‘right’ answer.
The qualitative study highlighted the fact that patients often struggled with the homework element of CBT. The hourly/daily thought and activity diaries were emotionally charged exercises for some patients, who associated them with negative school homework experiences and feared being judged at a time when they were feeling vulnerable or lacking in self-confidence or motivation. Having to work through feelings and events between therapy sessions could also be distressing if the individual’s coping mechanism was one of avoidance. Avoiding homework could, in some cases, be a reason to stop therapy.
Some patients felt completing thought and activity diaries could be risky, as family or work colleagues might see them writing private thoughts. As a consequence, some patients would describe leaving completing homework until the end of the day and then experience difficulties in trying to remember thoughts and emotions. Although it is important for GPs and patients to be aware of the commitment to (and engagement with) the homework element of CBT as part of the decision-making process about CBT referral, it should also be noted that for some completers in our study, homework and the attendant difficulties were associated directly with the level of benefit experienced from CBT. For instance, in ‘seeing’ patterns of thoughts and behaviour written down that previously had not been apparent to them.
Impact of cognitive behavioural therapy
Many patients expressed opinions on why they thought CBT was effective. They described a changed understanding of self and behaviour and how this translated into thinking and behaving differently; learning how to manage their depression through small goals and changes; better communication with others and its positive effect on family life; and how CBT had triggered changes to a healthier lifestyle.
Barriers to the efficacy of CBT were to do with feelings of failure when patients could not put what they had learned into action or the continuing (and deteriorating) nature of their comorbidities. These findings may inform the continued dialogue between therapist and patient during therapy.
Interestingly, there was no obvious association among the interviewed patients between not liking CBT and not benefiting from it, based on their BDI-II score and their interview accounts. In fact, a substantial number of those who said they found the process challenging and difficult had improved in terms of their BDI-II scores.
Reasons for completing and not completing therapy
Patients tended to describe more than one reason for completing or not completing CBT. For most completers, the practicalities of fitting CBT into their existing life were largely unproblematic; if people were not working, or were working but their workplace and therapist were flexible and supportive during therapy, attendance was straightforward. For non-completers, however, commitment and attendance could prove difficult because of needing to prioritise caring for a family member, work or financial commitments over their own health. In addition, if employed, patients feared censure from work colleagues for missing work and their depression being ‘found out’ by others. The perceived judgement of work colleagues was a particular worry among men.
Patients’ experiences of usual care
Most patients described antidepressants as stabilising rather than improving their mood. Many patients wanted to come off medication. Reasons for continuing were linked to fears of withdrawal symptoms or relapse, waiting for the right moment to stop taking antidepressants or for their GP’s suggestion that they do so.
Despite being on medication, it was evident that most patients interviewed were not in regular contact with their GP, as they usually received their medication via repeat prescription. Contact may also have been limited because some patients had not found their GP supportive or did not feel GPs were in a position to help them. Furthermore, it was apparent that patients may be reluctant to see their GP in case they were viewed as wasting his/her time, and some described critical comments from GPs that reinforced these fears. The limited contact some patients had with their GP may have meant they were on medication longer than necessary.
Patients talked about receiving invaluable support from family and friends, and some patients described strategies they had developed to help them cope with their depression. The internet and books were used as sources of information. There was no evidence to suggest that patients randomised to usual care had received or sought additional treatment or support to compensate for not receiving CBT during the trial.
Strengths and limitations
We used a purposeful sampling approach to maximise the diversity of patients across the three study centres and continued data collection until data saturation was reached (using ATLAS.ti). Having managed to recruit patients from both arms of the trial, and interview both completers and non-completers, we were able to address the aims of the qualitative study. However, the extent to which our findings are applicable to other patients with depression may be limited because we sampled patients who had agreed to take part in a trial of CBT and thus may have held particular views or expectations of treatment. Also, the patients we interviewed were primarily white British and individuals of other ethnic backgrounds may hold different views towards CBT. In addition, most of the patients we interviewed who had not completed therapy were based in Bristol. However, the qualitative sample was reflective of the CoBalT sample in general and interviewing both completers and non-completers ensured we gathered data from a range of perspectives. In addition, the reasons patients gave for not completing therapy were similar across the three centres. Furthermore, patients in the CoBalT study were individuals with TRD, and it is likely that primary care patients who are referred for CBT are those who have refused or not responded to medication for their depressive symptoms.
The interviews were conducted by researchers who were not involved in recruitment/follow-up of the trial participants, which may have helped elicit a high degree of openness from the participants. This proposition is supported by the fact that patients gave negative as well as positive views of CBT. The fact that we could not interview patients before they had completed their primary outcome measures for the trial or had completed treatment, meant the accounts given were open to recall bias and post hoc reconstruction of events. Patients, however, appeared able to remember their experiences of treatment during the trial, and what benefits and difficulties had arisen during this time period. A number of researchers worked together on the analysis of the data, and a software package was used to aid data management. This ensured a rigorous, systematic analysis of the material gathered.
Comparison with existing literature
Cognitive behavioural therapy requires a significant commitment from patients in terms of attending a number of therapy sessions, engaging with a therapist and completing homework between sessions. 62,63 However, engagement with CBT may be problematic and there are no studies directly exploring what patients find difficult or dislike about CBT. Greater adherence to therapy should equal greater effectiveness. 41 Thus, there is a need to identify barriers to adherence. Patients often say they would prefer talking therapies22,91–93 but for GPs to refer, it is necessary to explain what therapy involves. An exploration of these issues has the potential to guide GPs in their discussion with patients at the point of possible referral for CBT, as well as therapist’s initial and ongoing relationship with the patient.
Qualitative studies exploring patients’ perspectives of CBT have tended to focus on patient expectations and experiences of written or cCBT40,94–96 or aspects of group CBT97,98 for a range of conditions. For example eating disorders and alcohol dependency, not just depression. The few qualitative studies that have explored patients’ experiences of face-to-face CBT have reported on patients with psychosis,41,96 described the techniques by which patients used CBT after leaving therapy97 or compared patient experiences of CBT and psychodynamic therapy. 98
Our study found that patients could struggle with the focus of CBT if they felt their past or current situation – such as physical symptoms or what they felt was their primary condition – was not being explored sufficiently to address the cause of their depression. Patients sometimes expressed dissatisfaction with aspects of the relationship with their therapist. These findings have something in common with research reporting that dissatisfied CBT patients (in comparison with other psychotherapy patients) considered the therapist to be applying a rigid and predetermined therapy design, and felt steered by the therapists’ ideas. 98 However, our study highlights the particular difficulties of addressing the comorbidities in patients; although CBT therapists are trained to take into account comorbidity and how it affects the patient currently, patients do not necessarily engage with the CBT as relevant to their physical pain(s).
From a therapeutic standpoint, patients struggle with having to think about distressing aspects of their lives and completing homework tasks can be viewed as a result of cognitive or emotional avoidance of issues that can prolong depressive mood. Patients with depression for many years learn to avoid stressful situations or painful thoughts and discussions. 64 The process of avoidance can have major implications for the conduct of CBT, which focuses on ‘emotionally charged’ thoughts and feelings. These difficult issues can be dealt with through the collaborative nature of the therapist–patient relationship, and this includes obtaining feedback from patients regarding their understanding of the therapist’s communications and exploring any counterproductive reactions to the therapist’s manner, technique or suggestions. 62 However, one of the themes that emerged from our data was that this level of collaboration is not always perceived as present by patients and this can have important implications for patient engagement, particularly when the patient perceives the therapist is not as experienced as they would like.
The literature around CBT homework tends to focus on evidence that engagement with homework is an important mediator of outcome. 99 Although it is suggested that problems with homework seem to be the norm rather than the exception,100 there is little research into patients’ perspectives of why this may be. To the best of our knowledge, only one other study has explored patients’ views of CBT homework and this was among patients with psychosis,96 whose views may not generalise to those with depression. Factors identified in this earlier study that affected completion of homework were a lack of motivation, difficulty in filling in worksheets, putting off assignments, and not understanding the rationale and perceived benefits. Although some of our findings regarding motivation and avoidance overlapped with this previous study, our nested qualitative study provided insight into the specific difficulties for depressed patients, for example associations with school homework, fear of judgement and failure; issues that can then be addressed by the GP or therapist in dialogue with the patient.
It is of note that although patients may have disliked aspects of the CBT, most felt they had gained some insight into how to manage their depression; in particular how to challenge their negative thought patterns. This is in contrast with previous research, which found that it was the ‘high compliance’ patients with psychosis who felt that CBT had given them effective skills to realise their goals. 96
Patients’ accounts of how antidepressants affected their mood suggested medication stabilised their emotions, giving them a platform from which they could then function and engage with others. Similar findings have been reported by Knudsen et al. ,101 who found that women viewed antidepressants as enabling them to lead ordinary lives. We also found patients were unsure of whether or not being on antidepressants affected their mood. Such uncertainty has been reported by others. 102
Most of our patients described wanting to come off antidepressants. Other studies have also reported that patients view this as preferable103,104 and, like us, identified a moral dimension to this view, for example patients feeling that they would only be ‘normal’ once off medication. 104 It has been reported that patients may stay on antidepressants because they fear relapse and withdrawal symptoms. 102,104 Such fears were also described by our patients, some of whom had been tempted to stop their medication but then experienced withdrawal symptoms. There is increasing evidence to suggest that withdrawal symptoms are more likely to occur if a patient discontinues his/her medication abruptly, and has been on medication long term. 104
Most patients received their medication through repeat prescription. This limited the amount of direct contact they had with their GP and may have discouraged regular review of their medication. Johnson et al. 105 recently commented on the fact that currently there are no formal processes in primary care to support routine review of patients on long-term antidepressants and results of a prospective observational cohort study they conducted, suggested that reviewing patients can lead to appropriate reductions in prescribing. As the patients we interviewed had been eligible to enter the CoBalT trial, it is likely that they still needed to be on medication for their depression. However, the lack of contact some patients had with their GP was of concern. Some patients described still feeling very low despite being on medication, and it was evident that not all patients had access to support from family and friends, and that some patients were not proactive in seeking help.
Patients may be reluctant to approach their GP about their depression and may feel people are not sympathetic to their situation. 106 We found evidence to suggest that GPs are not always supportive towards patients with depression. Patients described how they had received invaluable support from friends and relatives, and it was apparent that patients viewed individuals who had had experienced depression as being particularly well placed to understand their situation. Patients with depression have described the ‘ideal’ person to confide in, as being someone who can draw on their own personal experience of depression and who has recovered. 106
Implications
Our qualitative findings contribute to the body of work on patient views on the acceptability of, and adherence to, CBT. The practical implications of the findings can be applied on two levels: in the initial discussion between the GP and patient in primary care regarding referral for CBT and in the first therapy session, and ongoing therapist–patient dialogue.
These accounts of patients’ experiences of therapy have the potential to help GPs in their discussions with patients at the time of referral. A conversation with the GP that includes acknowledgement of the difficulties and challenges of CBT may better prepare patients to make an informed choice about referral for therapy. In particular, homework and its potentially negative associations, issues around feeling judged on written work, the risky nature of writing things down, recall problems and avoidance strategies. Being aware of these perceived or actual barriers to completion of CBT by some patients again may aid the informed decision-making process.
It is also possible that therapists may be able to address some of these issues by using alternative media. For example, there are now thought and activity diary ‘apps’ available for smartphones and tablets, which can be completed in a discrete manner in the workplace or home.
By addressing possible difficulties between therapist and patient in the first instance, and emphasising the collaborative nature of the relationship, adherence may well be maintained or improved over the course of therapy. Our findings also underline the importance of eliciting negative thoughts and feelings that may induce the patient to leave treatment, these feelings being more likely to surface and be addressed if there is good rapport between therapist and patient and what is known as ‘engagement with therapy’. However, it is clear that not everyone will like CBT. Our data also suggest that physical comorbidities can also be an important part of the experience of depressed patients, and a perception of a lack of flexibility on the part of therapists concerning these comorbidities may be a barrier to engagement with therapy. This may be of particular relevance to therapists working with patients from primary care.
Overall, patients in our study, despite struggling with some aspects of CBT, reported that they had found CBT beneficial. The majority of patients reported gaining insight and learning skills enabled them to deal more effectively with their depression.
If we can increase both patient and clinicians’ knowledge about what the patient is signing up for when being referred to CBT, and continue the dialogue with therapists about possible barriers to engagement, completion rates of therapy may be improved and the efficiency of the use of these resources can be maximised.
In terms of improving patients’ experiences of usual care, GPs should aim to regularly review their patients’ medication to assess whether or not the patient needs to continue with antidepressants, and if so, to determine whether or not they are on the correct dose. GPs should also use these consultations to identify what other forms of support the patient is able to access and to consider whether there are resources within the community or on the internet, such as patient support groups or websites, from which the patient may benefit. GPs need to reassure patients they will not be judgemental and should support patients who are ready to stop medication, as this transition can be difficult.
Chapter 6 Discussion and conclusions
Summary of findings
Cognitive behavioural therapy, when given as an adjunct to usual care, which included antidepressant medication, was effective in reducing depressive symptoms in primary care patients with TRD. In order to be eligible to take part in the trial, it was necessary for participants to have not responded to at least 6 weeks of treatment with an antidepressant at an adequate dose for depression; however, many of the CoBalT participants presented with severe chronic depression with physical and/or psychological comorbidity. At baseline, 29% fulfilled ICD-10 criteria for a severe depressive episode, and 70% had been on their antidepressant medication for > 12 months. Although a greater proportion of the intervention group ‘responded’ at 6 months compared with those in the usual-care group, a beneficial effect of the intervention was also observed with respect to the more stringent criteria of ‘remission’ (BDI-II score of < 10) at this time. The difference in mean BDI-II score between groups equated to an effect size (0.5 SD), which exceeded the target effect size of ≈0.3 SD, which has been suggested as corresponding to a clinically important difference. 12 Estimates of treatment efficacy based on a CACE model found an even larger positive effect (0.8 SD) on depressive symptoms at 6 months for those who were regarded as ‘on track’ to receive the full course of CBT at this time. The beneficial effect of the intervention was sustained from 6 to 12 months, both in terms of the primary outcome of ‘response’ and the secondary ‘remission’ outcome. Furthermore, a reduction in symptoms of anxiety and panic was also found for those in the intervention group compared with those in the usual-care group over the 12 months. The intervention was also effective in improving quality of life (SF-12 mental subscale score) over the 12 months.
Cognitive behavioural therapy in addition to usual care was also shown to be cost-effective using the methods and criteria recommended by NICE. The ICER, from the perspective of the health-care provider, was £14,911, considerably less than the accepted threshold of £20,000–30,000. The probability of the intervention being cost-effective was estimated to be 0.74 at the £20,000 threshold, and 0.91 at the higher level of £30,000. This finding was shown to be robust under a range of scenarios tested in the sensitivity analysis.
Out-of-pocket personal expenditure was dominated by loss of earnings, and the value of lost productivity was substantial, but neither of these differed between the two groups.
Cognitive behavioural therapy requires a significant commitment from patients in terms of regular attendance at therapy sessions, engaging with the therapist and completing ‘homework’ between sessions. Patients described how they had found CBT to be a challenging and difficult process at times, and had struggled to complete homework tasks for emotional and practical reasons. Understanding the aspects of CBT that patients find difficult will aid therapists in engaging the patients with treatment. Moreover, the findings from the qualitative interviews will enable GPs and patients with depression to discuss the possible challenges and benefits of committing to a course of CBT. This will enable patients to make more informed decisions about whether or not to be referred for CBT. This may reduce failure to complete therapy, which, in turn, may result in efficiencies in the provision of this limited resource.
Patients recruited into the CoBalT trial had depression that had not responded to antidepressant medication alone, but most had moderate or severe depression, with symptoms that had been ongoing for several years. The fact that this intervention both reduced depressive symptoms and was cost-effective has important implications for the management of this patient group whose depression is difficult to treat and who would otherwise incur considerable costs to both the NHS and society.
Strengths and limitations
The CONSORT statement60 and the extensions for pragmatic trials70 and trials of non-pharmacological interventions69 were followed in terms of both the conduct and reporting of the CoBalT trial. Allocation was concealed through the use of a remote telephone randomisation system. The primary analyses were conducted according to the principle of ITT following an analysis plan that was agreed in advance with the TSC.
Follow-up rates at 6 and 12 months were high (90% and 84%, respectively) and exceeded the target follow-up rate at 6 months (85%). Follow-up rates in Glasgow were lower but this may have reflected greater deprivation and higher mobility among this population. Importantly, there was no evidence for a difference in completion rates of therapy between centres, and, in a post hoc subgroup analysis there was no evidence that study centre had any effect on the difference between the intervention and usual-care groups.
Given the high follow-up rates overall, the potential impact of missing data on the findings was minimised. Sensitivity analyses that imputed missing outcome data were consistent with the results from the primary ITT analysis, and hence there was no evidence that the missing data had biased findings. Moreover, although a number of imbalances were evident when the baseline comparability of the two groups was examined, additional adjustment for such imbalances did not substantially affect the findings.
It was not possible to blind the treatment allocation from participants, researchers or from those delivering the intervention. Outcomes were therefore collected by means of a self-report questionnaire in order to eliminate the possibility of observer bias.
Our primary outcome was depressive symptoms on the BDI-II. This instrument was also used by the therapists within CBT sessions; hence, for those in the intervention group, the responses on this specific measure may have been influenced by the process of therapy. Nevertheless, results were consistent for the other mental health outcomes (that were not used in therapy), including for the PHQ-9, which is part of the core outcome data set within UK psychological services. 27,28
The identification of those with TRD from primary care was challenging. There is no single accepted definition of treatment resistance. Therefore, we used an inclusive definition of TRD, that was directly relevant to UK primary care, given the uncertainty about what course of action to recommend to patients who have not responded to (at least) 6 weeks of antidepressant medication. 13 However, as highlighted earlier, many of those recruited to CoBalT had severe and chronic depression. Seventy per cent had been taking their current antidepressant medication for > 12 months, and only 10% of those recruited had taken their current medication for < 6 months. Hence, the sample recruited was more chronic than originally envisaged. Nonetheless, patients recruited to CoBalT were a heterogeneous group in terms of prior treatment and duration of their current depressive episode, so this inclusive definition ensured that trial results were as generalisable as possible.
Measurement of adherence to medication is also challenging, especially as tablet counts are difficult to interpret. Although, electronic monitoring bottles are considered as the ‘gold standard’ for assessing adherence, using such bottles to document adherence to medication prior to trial entry was impractical and prohibitive on the basis of cost. We therefore relied upon a self-report measure of adherence48 that had been validated against electronic monitoring bottles. 49 Although some of those recruited may not have adhered to their medication (i.e. were false positives), the vast majority had been on their medication for > 12 months and the long half-life of many antidepressant medications would minimise the effect of any non-adherence.
There was no ‘attention control’ group as this was regarded of limited value in the context of a pragmatic trial. Therefore it is not possible to exclude the possibility that this could explain the findings, but there is little evidence that counselling is effective over the long term. 107
The trial benefited from an earlier feasibility study,15 as part of which different methods of collecting the data for the economic evaluation were examined. Nonetheless, not unexpectedly, there were some missing data for the economic evaluation. However, the CBT intervention comprised the largest component of cost, and complete data were available on this. The vast majority (90%) of other health and social care costs were based on data extracted from primary care medical records (consultations in primary care and prescribed medication) and such data were 97% complete. QALY data were 83% complete. Hence, our base-case scenario is a cost–utility analysis, with the small amount of missing data imputed. Sensitivity analyses, using complete cases, demonstrate the robustness of our findings. Under all scenarios, the cost per QALY was below the upper threshold of £30,000 used by NICE, hence confirming that this is a cost-effective intervention.
There was more missing data in relation to personal costs and the cost of lost productivity. Therefore, we presented these costs in a cost–consequences format, using all available data by category to ensure transparency.
Cognitive behavioural therapy takes an educational approach in order to help patients incorporate therapeutic strategies into their everyday activities, thereby enabling them to better manage their mood. If they are successful in this then it seems reasonable to suggest that they may have better health in the future and better work attendance. The results of this study are limited to outcomes at 12 months, so we cannot comment on the longer-term costs and benefits of this type of therapy. However, there may be evidence from this study and others that could enable modelling of future costs and benefit.
Other methodological issues
The sample size calculation was revised following a slightly delayed start to recruitment, recruitment difficulties in one centre and problems matching recruitment rates to therapist capacity in two centres. The original target of 472 was reduced to 432, with only a small reduction (87% vs. 90%) in power to detect the pre-specified target difference of 16 percentage points. In the event, though, there was a surge in recruitment in the final months, and the final number of 469 randomised participants was only three short of the original target.
In the context of a pragmatic trial of a non-pharmacological intervention, a key methodological consideration is the background and experience of the health-professionals delivering the intervention. 69,70 In the CoBalT trial, the aim was to recruit therapists who were representative of those working within NHS psychological services (such as IAPT). Although it is difficult to recruit a truly representative sample, the therapists working on CoBalT came from a range of professional backgrounds, and although the majority had practised as a therapist for a number of years, two of the trial therapists were newly qualified (≤ 18 months experience), reflecting the variation seen in clinical services. In line with standards for ‘high-intensity’ IAPT practitioners (www.iapt.nhs.uk/silo/files/national-curriculum-for-high-intensity-cognitive-behavioural-therapy-courses.pdf), the majority of the CoBalT therapists had completed postgraduate CBT training. An independent evaluation of the therapy delivered in CoBalT found good fidelity to the CBT model, with the mean CTS-R score for the nine therapists who delivered the majority of the intervention indicating that the therapy was delivered at a standard that met conventional levels of ‘competence’. 65
Therapists were supervised by an experienced therapist at each centre. Supervision arrangements for therapists met the standards for clinical supervision set out by the BABCP (www.babcp.com/files/Accreditation/CBP/Reaccreditation/CBP-Reaccreditation-CriteriaGuidelines-V2–1009.pdf) and the more stringent requirements set out by IAPT (www.iapt.nhs.uk/silo/files/iapt-supervision-guidance-revised-march-2011.pdf).
All of the CoBalT therapists were employed on a part-time basis and hence allocation of patients to therapists was based on matching patient availability with therapist availability. This non-random assignment of patients to therapists meant that it was not possible to examine the effect of therapist characteristics on outcome in an unbiased manner. The potential for clustering of outcomes by therapist was nonetheless explored using appropriate statistical techniques72 and there was no evidence of any such clustering effects.
Eighty-five per cent of CBT sessions took place in the patient’s GP surgery or in other local NHS premises. Differences in the provision of NHS psychological services between the three centres were reflected in variations between sites in terms of where the majority of the CoBalT CBT sessions took place. In Bristol, 96% of patients were seen at their GP surgery, reflecting the model used by the local IAPT services (www.bristol.nhs.uk/your-health/mental-health-and-wellbeing/help-in-bristol.aspx). In Exeter, a partnership between the NHS and University (www.exeter.ac.uk/mooddisorders/) meant that NHS patients in Exeter and the surrounding area were expected to travel to the centrally located Mood Disorders Centre for therapy, a pattern that was replicated in the trial, whereas patients registered at more remote practices were seen by the therapists in their GP surgery. In Glasgow, patients were primarily seen at the Glasgow Clinical Research Facility (www.glasgowcrf.org.uk/), which is located on the site of one of the main Glasgow hospitals. If patients had been referred to psychological services on the NHS in Glasgow, they would have been expected to travel to their local primary care mental health team (PCMHT) for therapy. The distance travelled by CoBalT participants to the Clinical Research Facility would have, on average, mirrored the distance travelled to their local PCMHT. Again, such variation reflects the pragmatic design of the trial.
Finally, there were concerns that the roll-out of the IAPT services in England could have led to a substantial number of those randomised to usual care receiving CBT on the NHS (outwith the trial). The potential for such contamination was of concern because it would reduce any difference in the treatment effect between the two groups. In the event, only a very small minority of those who participated in CoBalT (three participants in the usual-care group and two participants in the intervention group) received a course of ‘non-CoBalT’ CBT. This contamination had little impact on the findings.
Comparison with existing studies
Prior to CoBalT there was no existing RCT evidence on the effectiveness of CBT as an adjunct to usual care that included pharmacotherapy as a ‘next-step’ treatment option for primary care patients whose depression had not responded to antidepressant medication. However, the effects observed are similar to those found in an earlier RCT of combined psychological and pharmacological treatment for chronic depression. 38
Using a similar definition of response, Keller et al. 38 found that 48% of those randomised to the antidepressant nefazodone (Serzone, Bristol-Myers Squibb – now withdrawn) ‘responded’ after 12 weeks treatment, compared with 73% of those randomised to receive both medication and psychological treatment (16 sessions of CBASP). The difference between these two groups was similar to the difference in response rates between treatment groups found in CoBalT (24%). The response rate among those randomised to medication (48%) in the Keller trial38 was higher than in CoBalT (22%), and may be due to differences in the patient populations. Those recruited by Keller et al. 38 were chronically depressed (mean duration of current episode of depression 7.8 years), but patients with TRD (defined as an absence of response to three previous trials of at least two different classes of antidepressants) were excluded.
The REVAMP trial18 recruited those with chronic depression who were non-responders (or partial responders) to 12 weeks of antidepressant medication (allocated based on a medication algorithm). Of those randomised to switch to the next step in the medication algorithm, 14.7% met criteria for ‘response’ after 12 weeks’ treatment, with a similar figure for response (15.3%) among those allocated to receive medication and psychotherapy (either CBASP or BSP; mean number of sessions: CBASP 12.5, BSP 13.1). The lack of a difference in outcomes between the groups was viewed as surprising18 given the earlier findings of Keller et al. 38 However, although both studies recruited patients with chronic depression, those recruited to the REVAMP trial were, in addition, a treatment-resistant population. Importantly, those randomised to CBASP in the REVAMP trial attended an average of 12.5 sessions, compared with 16 sessions in the earlier trial by Keller et al. ,38 which may also contribute to the explaining the differing trial findings.
There was a higher remission rate among those receiving pharmacotherapy in REVAMP (38.5% at 12 weeks)18 compared with CoBalT (15.0% for the usual-care group at 6 months) but this may be attributed to differences in the patients recruited. In REVAMP, only one-third of participants had previously had an adequate trial of pharmacotherapy in contrast with the CoBalT population, of whom most (80%) had previously been prescribed antidepressants and 70% had been on their current medication for > 12 months.
In CoBalT, the treatment protocol defined the intervention as ‘a course of 12 sessions [of CBT], with (up to) a further 6 sessions if deemed clinically appropriate by the therapist’. 45 Anecdotally, the therapists reported that the CoBalT population included a high proportion of very complex cases with significant comorbidity. This observation was reflected in the fact that, of those who completed therapy as per protocol, the median number of sessions attended was 16. This figure was similar to that in the Keller trial,38 which also reported a beneficial effect for combined pharmacological and psychological treatment but in those with chronic depression rather than non-responders to antidepressant medication.
Only 26% of STAR*D participants agreed to be randomised to CBT as a second-step treatment option21 but those in the CBT augmentation group (n = 65) were similar to CoBalT with most (86%) having a history of depression, with an average of seven prior episodes. 21 The percentage who fulfilled criteria for response based on self-reported depressive symptoms for those in the CBT augmentation group of STAR*D21 was slightly lower than the figure for CoBalT (35% vs. 46.1%), although remission rates were similar. However, as highlighted earlier (see Chapter 1, Existing evidence on the management of treatment resistant depression), STAR*D answered a different question to CoBalT. STAR*D examined alternative treatment approaches to the management of TRD, rather than examining the effectiveness of augmenting antidepressant medication with CBT as a ‘next-step’ treatment option.
Finally, others have shown that those with personality disorder are less likely to benefit from CBT than individuals without such comorbidity,108 and that those with more severe depression are likely to gain greater benefit from CBT than those with mild depression. 109 However, there was no evidence that severity of treatment resistance differentially affected the effectiveness of the CoBalT intervention. Similarly, in the other a priori subgroup analysis, there was no evidence that patient expectation of outcome influenced the magnitude of the treatment effect.
Comparison with data from Improving Access to Psychological Therapies services
Given the investment in the expansion of psychological services in England through the IAPT project,27,28 a pertinent question relates to the generalisability of the findings from CoBalT. As outlined earlier, therapists were recruited to be representative of those in NHS psychological services and the majority of CBT sessions were delivered in GP surgeries or other NHS premises in line with the ‘standard’ provision of psychological services in each of the three centres.
So how does the profile of patients recruited by CoBalT relate to those being seen in IAPT services? Reports from the two demonstration (pilot) sites32 and the review of the first year of the IAPT roll-out33 enable comparison. Of the two pilot sites, data from the Newham site that provided more ‘high-intensity’ CBT are the most relevant. Most patients seen in Newham had a primary diagnosis of depression (46%) or anxiety (43%) and, as in CoBalT, they had chronic symptoms, with 61% reporting that the duration of their current problem had persisted for over 2 years. 32 In Newham, 76% of patients scored ≥ 10 on the PHQ-9 (with 28% being classified as having ‘severe’ depression defined as a PHQ-9 score of ≥ 2032), similar to the figures for CoBalT. Eighty-six per cent of patients seen in Newham were regarded as being a ‘case’ on the PHQ-9 or GAD-7 (scoring ≥ 10 or ≥ 8 respectively), again a figure similar to the CoBalT population (94%).
Data from the first year of the roll-out of IAPT across 32 sites33 confirmed that the majority of those referred to IAPT services (76%) present with a diagnosis of depression and/or anxiety, with the majority (84%) being a ‘case’ on either the PHQ-9 or GAD-7 (PHQ ‘case’, 72.5%; GAD-7 ‘case’, 77.4%). In terms of outcome, 36.8–42.4% of IAPT patients ‘recovered’ (defined as not a ‘case’ on PHQ-9 or GAD-7) after treatment, which is slightly lower than the figure for participants in the intervention group of CoBalT (48%). However, in neither report32,33 are any data given on the proportion of patients who have not responded to antidepressant medication.
Improving Access to Psychological Therapies services are focused on delivering ‘NICE-compliant’ treatment,110 which equates to 16–20 sessions of CBT for depression according to NICE guidelines. 13 However, there are currently few robust data on the average number of sessions delivered in IAPT services. 33 Data from the first year report suggest that < 2% of patients (with depression or generalised anxiety disorder) received 16–20 sessions of CBT, although it is thought that there was a problem with the recording of all treatment sessions. 33 However, knowledge of the number of sessions received by patients treated in IAPT services, together with more details about the patient profile (in terms of their prior history of treatment), are important in terms of generalising the findings of CoBalT and projecting the likely benefits (both in terms of clinical and economic outcomes) that may be obtained.
Implications for health care and suggestions for further research
In 2006, Layard8 suggested that investing in training more therapists deployed in psychological treatment centres across the UK would lead to significant savings in terms of incapacity benefits and NHS costs to the UK government. The subsequent investment and expansion in psychological services in England27,28 was founded on this premise of cost-effectiveness. The results of the CoBalT trial are useful in informing the ‘next step’ for those who do not respond to antidepressant medication. CBT, given as an adjunct to usual care that includes antidepressant medication, is an effective treatment in reducing depressive symptoms and improving quality of life over 12 months when compared with usual care alone. Importantly, the intervention was cost-effective when judged against the criteria used by NICE.
Given the chronic relapsing nature of depression, it is important to examine the long-term outcome of this intervention. It has been argued that CBT has the potential to produce a more sustainable improvement than pharmacotherapy alone. This is because CBT adopts an educational approach that teaches patients skills to help manage their mood and to gain an understanding of persistent unhelpful aspects of their behaviour. In principle, this approach should lead to an improvement in longer-term outcomes that should outlast the duration of the treatment. This is evidenced by the positive outcomes at 12 months in CoBalT and the beneficial effects observed by others in terms of relapse prevention. 111 However, many RCTs of a CBT intervention only report outcomes after 12–16 weeks,38,112 although some have included a longer-term follow-up (at 10–16 months34,113–116). To date, there is little evidence of the effectiveness of CBT over the long term (> 3 years); that which does exist relates to the role of CBT in relapse prevention. 35,117
Hence, the question remains whether the beneficial effects of the intervention observed at 12 months are maintained over subsequent years or if such effects ‘wear off’. Given the educational approach that is key to CBT, it is plausible that a smaller, albeit clinically relevant, difference might still exist 4 years later, although this remains unproven. Furthermore, although clinical effectiveness may decline with time, the intervention could still be cost-effective over the long term. There is an opportunity for future research to develop a decision-analytic model to evaluate CBT for this patient group, taking a long-term societal perspective and weighing up costs invested at the outset against future benefits in term of health, well-being and productivity.
Of those studies examining the long-term outcome of CBT in terms of relapse prevention,35,117 none has examined the cost-effectiveness of such a strategy. Previous reports on cost-effectiveness of CBT (e.g. Durham et al. 25) are confined to different patient populations and varied CBT interventions (including low-intensity interventions with a contact time of < 3 hours) meaning that these findings cannot be generalised to the population of patients with TRD studied in CoBalT. If the intervention was found to be cost-effective over the long term, this would have significant implications for recommendations as to how depression should be managed.
Future investment in psychological services in the UK and elsewhere should take account of the needs of this population for a ‘live’ therapist, who is able to tailor the treatment approach to the individual (the idea of meta-competence in the CBT competencies framework: www.ucl.ac.uk/clinical-psychology/CORE/competence_frameworks.htm). Although cCBT is often promoted as a way of improving access to psychological therapies, such an approach is inflexible to the needs of many depressed patients. As highlighted by the CoBalT population, many of those whose depression does not respond to antidepressant medication have chronic depression, with physical and/or psychological comorbidity. A skilled therapist will be able to deal with such comorbidity and formulate more long-lasting beliefs that, according to cognitive theory, underpin the longer-term risk of depression. Other modes of delivering CBT over the internet109 or by telephone118 could retain such a flexible approach, but may also afford an opportunity for greater efficiency in the provision of such treatment.
Finally, it is important to remember that although nearly half of those in the intervention group met criteria for response, 54% did not. Therefore, it is a priority that the evidence base for the effectiveness of a range of ‘next-step’ treatments for those who do not respond to medication alone is expanded. Alternative ‘next-step’ treatments may include increasing the dose of the antidepressant medication, switching to another antidepressant or augmentation with another pharmacological treatment (either two antidepressants or augmentation with a non-antidepressant medication, such as lithium). 13,119 Combining pharmacological agents broadens the pharmacological actions involved. 120 Although many different strategies have been evaluated, to date there is little robust evidence regarding the effectiveness of many of these strategies,13 with reviews frequently incorporating evidence from uncontrolled studies and/or non-randomised studies as well as RCTs. 121–123 Furthermore, there is little evidence for the effectiveness of other psychological therapies as an adjunct to pharmacotherapy. Although CBT is, at present, the psychological treatment that is most frequently available on the NHS, it is evident that from CoBalT that other psychological treatment approaches may be preferred by some patients. Indeed, evidence from ongoing RCTs investigating the effectiveness of DBT (REFRAMED trial: ISRCTN85784627), ISTDP (NCT01141426) and MBCT (NCT01021254) will be welcome. Only by obtaining robust evidence on the effectiveness and cost-effectiveness of a range of ‘next-step’ psychological and pharmacological interventions will it be possible to reduce the significant burden to patients, NHS and society, which is associated with non-response to the most common first-line treatment for depression in primary care.
Chapter 7 Secondary analyses of the CoBalT study
In addition to the main analyses reporting the clinical effectiveness and cost-effectiveness of the intervention and the results of the nested qualitative study, three additional secondary analyses using data from the CoBalT study were conducted, the aims of which were to:
-
estimate the prevalence of TRD in UK primary care
-
examine whether or not dysfunctional attitudes and metacognitive awareness are mediators of the effect of CBT on depression outcomes
-
examine potential moderators of response to CBT.
These additional analyses will be reported in Chapters 8–10 .
Chapter 8 The prevalence of treatment-resistant depression in primary care
Introduction
Depression is a disabling condition and the third most common reason for consulting a GP in the UK. 124 As outlined earlier (see Chapter 1 ), antidepressants are the first-line treatment for moderate and severe depression in primary care, and there has been a steady rise in antidepressant prescribing in recent years, in the UK and elsewhere. 2–4 However, not all patients respond adequately to antidepressants and there is concern about the impact on both patients and society for those whose symptoms do not respond to such treatment. Much of the cost and disability associated with depression is accounted for by treatment resistance. 125,126 Yet, there are few estimates of the prevalence of TRD.
The large US STAR*D study found that more than half of all patients recruited through primary care and psychiatric clinics did not achieve remission after first-line antidepressant treatment, and one-third did not experience remission after four courses of acute treatment. 127 A multicentre European study (Group for the Study of Resistant Depression) found that 50.7% of depressed patients recruited from specialist referral centres were considered treatment resistant after two consecutive courses of treatment with antidepressants. 128 Although there is no single accepted definition of what constitutes ‘treatment resistance’,129 these data suggest that non-response to medication following antidepressant treatment is a substantial problem. However, it is unclear whether or not these data would generalise to UK primary care.
Accurate estimates of non-response to antidepressant treatment are important to determine whether or not there is unmet need, particularly given the high prevalence of depression among patients presenting to primary care. The CoBalT study provides an opportunity to estimate the prevalence of TRD among those prescribed antidepressants for at least 6 weeks in UK primary care.
Methods
This was a secondary analysis of data collected during the initial screening stage of the CoBalT study (filter 1 and filter 2), which is described in full earlier (see Chapter 2 , Filter 1: Search of general practitioner records to identify patients being treated for depression). Brief details are outlined below.
Identification of participants
A search of computerised records was conducted at each of the 73 collaborating GP practices, to identify patients aged 18–75 years who were currently receiving antidepressants and who had received repeated prescriptions for antidepressants [at an adequate dose for depression (see Appendix 1 ) during the previous 4 months]. GPs excluded individuals with bipolar disorder, psychosis or major alcohol or substance use problems, as well as those who were unable to complete the study questionnaires or for whom the study was regarded as inappropriate. Patients who were currently receiving CBT or other psychotherapy (or who had undertaken CBT in the last 3 years) were also excluded. The remaining patients were mailed an invitation letter and brief information sheet about the study and asked to respond, indicating whether or not they were willing to be contacted by the research team. Anonymised data on age and gender of those patients who were mailed an invitation to participate but who did not respond were collected in order to assess the generalisability of the study findings.
Questionnaire
Patients who agreed to participate were mailed a short screening questionnaire. This questionnaire included a self-report measure of depressive symptoms – the BDI-II46 – and asked for details of their current antidepressant medication, including the duration of their current treatment and their adherence to antidepressants. The latter was assessed using a modified version of the Morisky scale48,49 (see Chapter 2 , Inclusion criteria). The questionnaire also collected data on sociodemographic variables (age, gender, marital status, educational qualifications, employment status, housing situation and financial situation).
Defining treatment resistance
Given the lack of consensus in the definition of TRD, we proposed an inclusive definition, directly relevant to UK primary care. 13 Treatment resistance was defined as those patients who scored ≥ 14 on the BDI-II and who had been taking antidepressant medication at an adequate dose for at least 6 weeks.
Data set
As well as recruiting participants via a search of electronic records, GPs were able to refer patients directly to the research team. However, for the purpose of the present analysis, such individuals (n = 37) were excluded. In addition, for those individuals who were rescreened to ascertain eligibility for the trial, data from only their first postal questionnaire were used. Thus, the estimates of prevalence are based on questionnaire data obtained from one search of patient records from all participating practices.
Statistical analysis
All analyses were conducted in Stata 11.2. The prevalence of TRD was estimated with 95% CI, adjusting for clustering by GP practice. The impact of non-response (to the initial study invitation and screening questionnaire) on estimates of prevalence was assessed using probability weights (inverse of the non-response rate for each GP practice), such that data from practices with higher response rates were given more weight. Weighted estimates of prevalence were calculated using the survey commands in Stata (svy commands). Technical limitations meant that it was not possible to adjust the latter estimates for clustering by GP practice; however, preliminary analyses showed that there was little evidence of clustering by GP practice.
Descriptive data on the type of antidepressant medication taken by those fulfilling our definition of TRD are reported, including the number on combined (defined as two different antidepressant medications at an adequate dose) or augmented antidepressant treatment (with a non-antidepressant medication). Sociodemographic characteristics were compared for the TRD group, with those who were not adhering to medication and with those who had minimal depressive symptoms. Comparisons of age and gender were also made between those who did and did not participate in the study.
Results
Response to study invitation and questionnaire completion
A total of 10,629 patients who were mailed an invitation letter, of whom 4552 (43%) responded ( Figure 6 ). Of these, 64% agreed to being sent a questionnaire by the research team, and subsequently, most (n = 2439, 84%) returned a completed questionnaire (see Figure 6 ).
FIGURE 6.
Flow chart of the recruitment process and number of participants with data for estimating the prevalence of TRD.
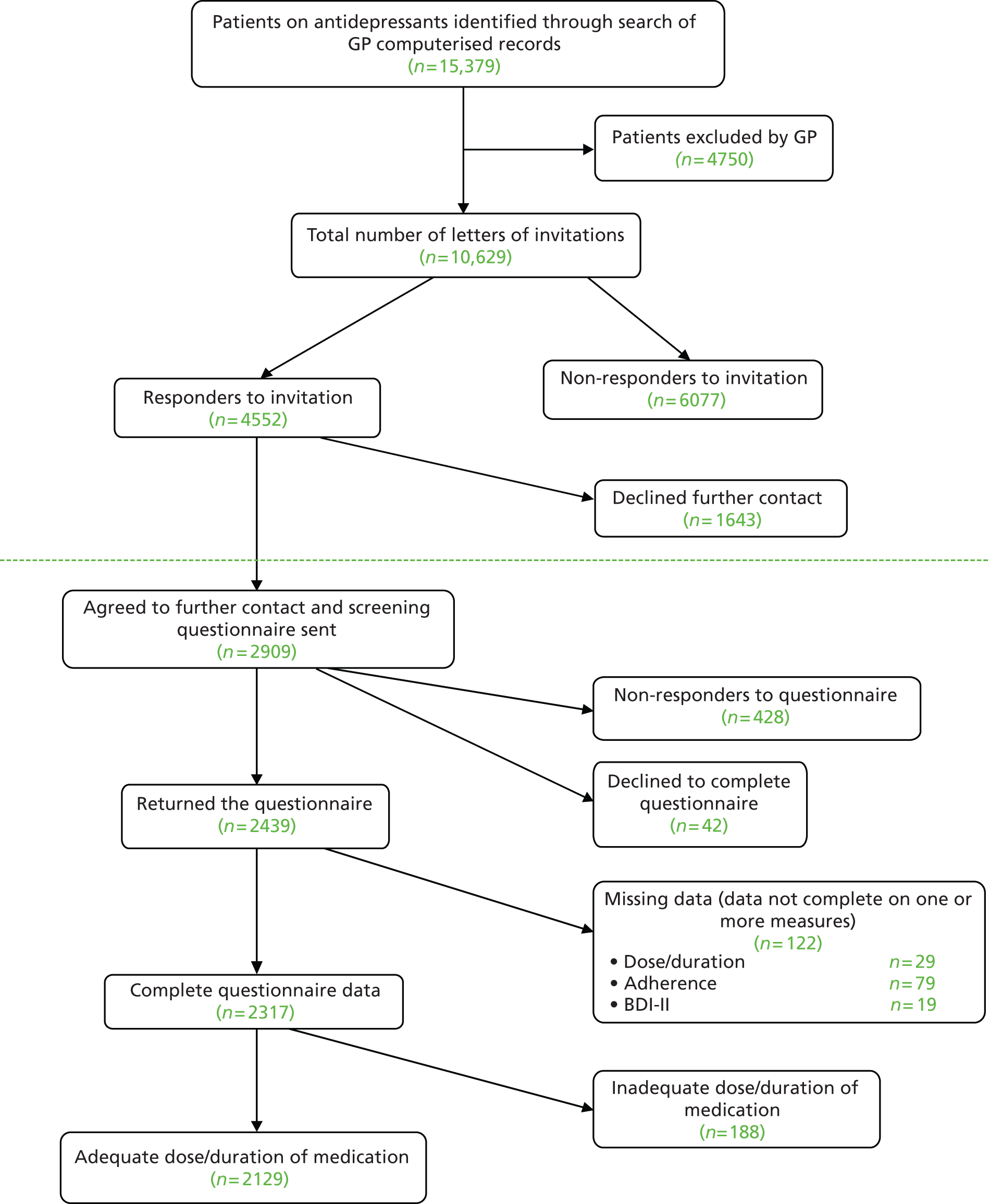
Of those who returned a questionnaire, we had complete data on dose and duration of antidepressant treatment and depressive symptoms from 2317 participants (95%). Of these, 8.8% were not taking an adequate dose of medication or had been taking their medication for < 6 weeks and were excluded from further analyses. This gave a sample of 2129 patients (see Figure 6 ) for whom to estimate the prevalence of TRD.
Data on age and gender were available for most of those who were mailed an invitation and were compared for participants and non-participants (those who did not respond to the invitation, n = 6077; and those who responded but who declined to participate, n = 1643). There were no differences in age between those who returned a completed questionnaire (participants) and those who did not ( Table 68 ). However, women were more likely to participate than men (see Table 68 ).
Prevalence of treatment-resistant depression
Among the 2129 patients who had been prescribed an adequate dose of antidepressant medication for at least 6 weeks, 1635 (77%, 95% CI 75% to 79%) had a BDI-II score of ≥ 14. Overall, 55% met our definition of TRD ( Table 69 ). Twenty-two per cent had a BDI-II score of ≥ 14 but had not adhered to medication, and 23% had minimal symptoms of depression (BDI-II score of < 14) (see Table 69 ). Of those with minimal symptoms, the majority [n = 401; 81% (95% CI 78% to 84%)] had adhered to their medication.
| Description of group | n | % | 95% CIa |
|---|---|---|---|
| BDI ≥ 14 and adhered to medication (TRD) | 1177 | 55.3 | 52.8 to 57.8 |
| BDI ≥ 14 but had not adhered to medication | 458 | 21.5 | 19.4 to 23.6 |
| BDI < 14 (minimal symptoms) | 494 | 23.2 | 20.9 to 25.5 |
Given the non-response to the study invitation and subsequently to the postal questionnaire, we examined the impact on estimates of prevalence. The response rate (for the study invitation and screening questionnaire) varied between 8% and 50% for the 73 practices. Response rate was negatively correlated with prevalence of TRD ( Figure 7 ) and positively correlated with prevalence of minimal depressive symptoms (BDI-II score of < 14) ( Table 70 ) for the 73 practices. However, estimates of prevalence weighted for non-response differed little from the figures reported above [weighted prevalence of TRD: 54.7% (95% CI 52.2% to 57.2%); non-adherers: 21.6% (95% CI 19.5% to 23.7%); minimal symptoms: 23.7% (95% CI 21.4% to 26.0%)].
FIGURE 7.
Prevalence of TRD against response to the postal screening questionnaire for each of the 73 GP practices [weighted according to number of responses (the size of the dot in the graph represents the number of responses per practice: the larger the dot, the greater the number of responses)].
| Practice prevalence of: | Response rate | |
|---|---|---|
| Invitation | Questionnaire | |
| TRD | –0.29 | –0.43 |
| Not adhering to medication | –0.04 | 0.19 |
| Having minimal symptoms | 0.38 | 0.31 |
Antidepressant medication
Among those with TRD, SSRIs were the most common type of antidepressants taken (79%) ( Table 71 ). The two most common medications were citalopram and fluoxetine. Together they accounted for 67% of all antidepressants prescribed, in line with prescribing figures for England for 2011. 5 Most patients were taking one antidepressant (monotherapy), with < 2% of those with TRD receiving combined treatment. Augmented antidepressant treatment was very rare (see Table 71 ). A further 57 patients were taking a second antidepressant medication but at a dose below our definition of ‘adequate’.
| Antidepressant | Dose (mg) | Class | n | % | Percentage of ADs issued in England in 2011a |
|---|---|---|---|---|---|
| Citalopram | 20–80 | SSRI | 448 | 38.0 | 28.9 |
| Fluoxetine | 20–80 | SSRI | 334 | 28.3 | 11.8 |
| Venlafaxine | 75–450 | SNRI | 93 | 7.9 | 5.9 |
| Mirtazapine | 30–60 | Other | 82 | 6.9 | 8.3 |
| Paroxetine | 20–60 | SSRI | 73 | 6.2 | 3.3 |
| Sertraline | 100–400 | SSRI | 39 | 3.3 | 7.8 |
| Escitalopram | 10–40 | SSRI | 25 | 2.1 | 2.6 |
| Lofepramine | 140–350 | TCA | 24 | 2.0 | 0.7 |
| Dosulepin | 150–225 | TCA | 9 | 0.8 | 3.3 |
| Trazodone | 150–300 | TCA related | 8 | 0.7 | 2.1 |
| Duloxetine | 60–90 | SNRI | 8 | 0.7 | 1.7 |
| Amitriptyline | 150–200 | TCA | 6 | 0.5 | 20.5 |
| Reboxetine | 8–12 | NARI | 3 | 0.3 | 0.09 |
| Clomipramine | 250 | TCA | 1 | 0.1 | 0.7 |
| Imipramine | 150 | TCA | 1 | 0.1 | 0.4 |
| Moclobemide | 600 | MAOI | 1 | 0.1 | 0.04 |
| Trimipramine | 150 | TCA | 1 | 0.1 | 0.2 |
| Combined AD treatmentb | 19 | 1.6 | |||
| Augmented AD treatmentc | 2 | 0.2 | |||
Characteristics of patients with treatment-resistant depression
Sociodemographic and clinical variables were compared for those with TRD, for those who had significant depressive symptoms but who had not adhered to their medication (not adherent) and for those with minimal symptoms ( Table 72 ). Differences were evident for a number of variables but tended to reflect differences between those with minimal symptoms compared with the other two groups. For example, those with minimal symptoms were more likely to be married, working (full/part time) and were less likely to be living in rented accommodation or experiencing financial difficulty than the other two groups (see Table 72 ). Almost 70% of patients had been taking their current antidepressant for > 12 months. This was a consistent finding among the three groups of patients described: those with TRD (67.2%); those non-adherent to their antidepressants (67.2%); and those with minimal symptoms (71.9%) (see Table 72 ).
| Characteristic | All, n = 2129 | TRD: BDI-II score ≥ 14 and adhering to medication, n = 1177 | Not adherent: BDI-II score ≥ 14 and not adhering to medication), n = 458 | Minimal symptoms: BDI-II score < 14), n = 494 | p-valuea | |
|---|---|---|---|---|---|---|
| Age (years): mean (SD) | 48.5 (13.4) | 49.6 (12.7) | 43.0 (12.8) | 50.9 (14.1) | < 0.001 | |
| Gender: female: n (%) | 1474 (71.3) | 815 (70.3) | 311 (70.8) | 348 (74.0) | 0.31 | |
| Married/living as married: n (%) | 1113 (52.9) | 622 (53.3) | 193 (42.9) | 298 (60.9) | < 0.001 | |
| BDI-II score: mean (SD)b | 24.6 (13.5) | 29.1 (10.4) | 32.0 (10.6) | 7.0 (4.1) | < 0.001 | |
| Duration of current AD use: n (%)b | 6 weeks to 6 months | 328 (15.4) | 187 (15.9) | 71 (15.5) | 70 (14.1) | 0.40 |
| 6 months to 1 year | 347 (16.3) | 199 (16.9) | 79 (17.3) | 69 (14.0) | ||
| > 1 year | 1454 (68.3) | 791 (67.2) | 308 (67.2) | 355 (71.9) | ||
| Socioeconomic indicators | ||||||
| Educational qualifications: n (%) | A-level or higher | 1047 (49.9) | 559 (48.1) | 197 (44.0) | 291 (59.8) | < 0.001 |
| GCSE/other | 570 (27.2) | 317 (27.3) | 147 (32.8) | 106 (21.8) | ||
| No qualifications | 479 (22.9) | 285 (24.6) | 104 (23.2) | 90 (18.5) | ||
| Working (full or part time): n (%) | 970 (46.1) | 487 (41.8) | 191 (42.4) | 292 (59.8) | < 0.001 | |
| Housing tenure (rented/other): n (%) | 927 (44.0) | 533 (45.6) | 261 (58.0) | 133 (27.1) | < 0.001 | |
| Financial situation – just getting by/difficult: n (%) | 1150 (54.6) | 715 (61.2) | 320 (71.3) | 115 (23.5) | < 0.001 | |
Discussion
Summary
More than three-quarters of primary care patients taking antidepressants for at least 6 weeks had significant residual depressive symptoms. Fifty-five per cent of patients who had taken an adequate dose of antidepressant medication for at least 6 weeks were classified as having TRD, clearly showing that inadequate response to antidepressant medication is an important problem in UK primary care.
Most of these patients (70%) reported having taken their current medication for > 12 months. This finding highlights the chronic nature of depression among many of those treated in primary care, and gives rise to concern about the systematic reassessment and treatment of those on long-term antidepressant therapy.
The frequency of the different types of antidepressant taken by those with TRD, in the main, reflected national prescribing data for England. 5 Amitriptyline was frequently prescribed at low doses that were not regarded as an effective therapeutic dose for depression, which accounted for the lower ranking of this medication among those with TRD in our sample. Venlafaxine was slightly more frequently used compared with national prescribing data,5 suggesting that GPs may have initially prescribed an SSRI in line with NICE recommendations for the treatment of depression, and switched to venlafaxine as an alternative because of a lack of response.
Strengths and limitations
We used data from the recruitment phase of the CoBalT RCT based in UK primary care. The large number of practices covering urban, rural and semi-rural settings across the three centres (Bristol, Exeter and Glasgow) has enabled us to estimate a figure for the prevalence of TRD in UK primary care with a high degree of precision.
Unlike other studies such as STAR*D,127 we distinguished between non-response and non-adherence to medication in defining our treatment-resistant group. Non-adherence to medication is known to be common in depressed patients130 but is difficult to measure. Other studies have relied upon clinician report to gather this information,128 but we know that patients find it difficult to be honest with health professionals about whether they are taking their medication as prescribed. 131 We relied upon a self-report measure of medication use,48 which has been validated against electronic monitoring bottles. 49
There are a number of limitations to our study. Only 43% of those invited to participate responded to the letter from the GP, with 54% subsequently returning a completed postal questionnaire. Our estimates of prevalence may be biased by this non-response. However, there was no difference in estimates of prevalence that were or were not weighted for non-response by practice, suggesting that our estimates were robust.
Our data are cross-sectional and we acknowledge that individuals may have experienced a change in their depressive symptoms between the initial prescription and the time that the questionnaire was completed. A longitudinal study of patients newly starting an antidepressant could help make finer distinctions in terms of defining treatment responders, partial responders and non-responders. Furthermore, although the BDI-II measures severity of depressive symptoms, it is not a diagnostic instrument. Nonetheless, those with TRD had a mean BDI-II score of 29.1, which is indicative of severe depression.
As highlighted earlier, there are many definitions of treatment resistance. The definition of TRD used in the CoBalT study is pragmatic and directly relevant to UK primary care, given the uncertainty about what treatments to recommend to those who do not respond after 4–6 weeks of antidepressant medication. 13
Comparisons with other studies
Reports suggest that the management of TRD is an area that has been poorly investigated, with few robust data to guide treatment. 14,132,133 Although non-response to antidepressants is frequently cited as a key issue in the management of patients with depression, few studies have quantified the magnitude of this problem and, prior to this study, no evidence has existed for UK primary care. Estimates of the prevalence of TRD range from 30%127 to 50%. 128 Our estimate of 55% is at the upper end of this range. It is difficult to compare the estimates of prevalence between studies directly because of differing definitions of treatment resistance, including whether or not diagnostic criteria have been applied to identify those with depression. 128 Nonetheless, our data clearly demonstrate that TRD represents a significant burden for patients and primary care clinicians in the UK.
Clinical implications and directions for future research
Based on our data, the scale of inadequate response to antidepressants in UK primary care is worrying, particularly in the context of the continued increase in prescribing. Little is known about the treatment received by patients with depression after an antidepressant has been prescribed. It is not clear what constitutes usual care. Although the Quality Outcomes Framework has incentivised primary care clinicians to record the severity of depressive symptoms at the start of treatment (DEP6) and again within 12 weeks (DEP7), no incentives are in place with respect to longer-term management. 134 A large number of patients may receive long-term antidepressants without being adequately assessed for treatment response. Our data suggest that the NICE guidelines13 for sequencing treatments after initial inadequate response are not widely followed, as there is very little evidence in our sample of combining or augmenting antidepressant treatments.
Given the lack of motivation that is common among depressed patients, it has been suggested that a more proactive clinician-led approach to the management of this patient population could be of benefit. 135 We would urge repeated monitoring of symptoms, together with recording of medication adherence, at regular reviews. Such an approach may help identify those patients whose symptoms have not responded to medication at an earlier stage when it might be possible to intervene to prevent chronicity and to improve patient outcome.
Chapter 9 Mediated effect of cognitive behavioural therapy on depression outcomes
Introduction
The theory underpinning CBT posits that dysfunctional attitudes (also referred to as ‘rules for living’ or conditional beliefs) are a risk factor for both the onset and maintenance of depression. 62,63 When a person experiences an event (e.g. their partner threatens to leave) that is congruent with a dysfunctional attitude (e.g. ‘If I am not in a relationship, I am unlovable’) this activates the belief and triggers the onset of, and then exacerbates, depression. CBT focuses on behavioural activation and identifying/challenging negative automatic thoughts to provide symptom relief. As therapy progresses, the emphasis switches to identifying and modifying the dysfunctional attitudes (or ‘rules for living’) that underpin the longer-term risk of depression as a way of ensuring longer-term resilience. 62,64 However, although there has been much work on dysfunctional attitudes in depression, there is little evidence that changes in dysfunctional attitudes are specific to CBT. 136 Others have suggested that CBT and other psychotherapies involve a shift in how the individual relates to negative thoughts, such that they relate these thoughts in a different way (a ‘decentring’ approach). 137,138 Rather than viewing such thoughts as a reflection of reality or a representation of themselves, they learn to see these thoughts as mental events (otherwise referred to as increased metacognitive awareness). These arguments suggest that changes in dysfunctional attitudes or metacognitive awareness mediate some or all of the therapeutic benefit of CBT.
Increasingly in the context of pragmatic trials, we are interested in establishing the extent to which proposed mediators, such as dysfunctional attitudes, underpin the effects of treatments, such as CBT, on health outcomes, such as depressive symptoms. Based on such knowledge, it may then be possible to refine interventions to improve efficacy and ultimately patient outcomes. 139 To date, although a number of RCTs have examined how CBT works, there is little evidence that cognitive factors, such as dysfunctional attitudes, act as mediators. 140,141 Moreover, a number of studies have shown that changes in cognitive factors are not specific to CBT interventions [e.g. also being seen in patients receiving pharmacotherapy,141,142 with some attributing such non-specific effects as being a consequence (rather than cause) of treatment143].
Much of the previous work in this area used the Baron and Kenny144 approach to mediation, the limitations of which are now well recognised,145 and others have proposed modified criteria or guidelines for identifying mediators139,146,147 that can be applied using structural equation modelling. 139 Nonetheless, there are difficulties in establishing that a factor is a mediator on the causal pathway between intervention and outcome, even when data from a RCT are available. The key difficulty is that both the proposed mediator and outcome act after the point of random allocation, and so the observed association between the two can be subject to ‘hidden confounding’. The commonly used structural equation modelling approach to evaluating potential mediators does not address this possible confounding. More recent developments in the field are based on the ‘causal inference approach’. These include the potential outcomes framework148,149 and the use of instrumental variable methods150 but the latter is the subject of ongoing development.
In this chapter we analyse data collected in the CoBalT trial, using the structural equation and potential outcomes approaches, to investigate whether or not dysfunctional attitudes and metacognitive awareness mediate the effect of CBT on depression outcomes. Therapists in the CoBalT trial used the seminal CBT for depression manuals62,63 and elaborations designed to address treatment resistance. 64 The latter manual emphasises the cognitive elements of treatment, including focusing on the dysfunctional attitudes. Hence, such mediators may be more relevant in this trial than in prior studies of CBT for less chronic depression.
This chapter will first describe the background to causal inference with reference to the original criteria put forward by Baron and Kenny,144 before proceeding to describe the potential outcomes model that will also be used in these analyses.
Causality and mediation analysis
Causal inference is a key objective in most scientific research where RCTs are considered the gold standard for estimating the effectiveness of interventions. 151 Randomisation is crucial for ensuring comparable groups (intervention and comparator) to obtain causal estimates by eliminating selection bias and minimising confounding. Although pragmatic trials evaluate the effectiveness of an intervention under ‘real-life’ conditions, in practice we are often interested in efficacy as a measure of the benefit under ‘ideal conditions’ as well as how the intervention works.
Examining a cause–effect process may provide better information towards understanding the mechanisms connecting an intervention with the outcome, as in the case of the CoBalT trial. The mechanisms, measured as intervening variables on a causal pathway linking intervention and outcome, are commonly referred to as mediators. With regards to the CBT intervention delivered in the CoBalT trial, the specific variables of interest are measures of dysfunctional attitudes and metacognitive awareness. Mediation analysis aims to establish the role of such potential mechanisms that connect interventions and outcomes. In essence, although causality reveals if a relationship exists, mediation analysis goes further to explain the nature (the ‘how’) of the causal relationship.
In the absence of mediation, a simple model ( Figure 8a ) relates intervention Z (CBT) to outcome Y (depression). However, a simple mediation model (see Figure 8b ) may be schematically presented as a mediator M (e.g. dysfunctional attitudes) in the causal path between intervention Z (CBT) and outcome Y (depression).
FIGURE 8.
The total effect of Z on Y (a); simple mediation model (b).
The seminal approach originally proposed by Baron and Kenny144 used the simple mediation model schema above to develop a basic approach to testing for empirical evidence of mediation using a set of related (structural) equations to fit three regression models:
where αi represent the intercepts, εi the model fit errors (normally distributed), and a, b, c, and c′ the regression coefficients that provide the magnitudes of the associations between the three key variables. The Baron and Kenny framework144 proposed three necessary and sufficient conditions for evidence of mediation – a significant association between:
-
the intervention and the mediator, i.e. a statistically significant regression coefficient a in equation (1)
-
the intervention and the outcome, i.e. a statistically significant regression coefficient c in equation (2)
-
the mediator and the outcome, and also a significant association between the intervention and the outcome, i.e. statistically significant regression coefficients b and c′ in equation (3).
In regression analysis terminology, a statistically significant value of c in equation (2) implies a direct association between intervention and outcome, whereas a statistically significant c′ in equation (3) implies a direct association between intervention and outcome that is independent of mediation (association not mediated by M). On the other hand, b captures the strength and/or direction of association between mediator and outcome while adjusting for intervention.
Total, direct and indirect effects
As illustrated above, mediation is an intermediate step in the causal pathway between intervention and outcome. The relationships between the three variables of interest (intervention, mediator and outcome) may be better understood by decomposing the cause–effect relationship into total, direct and indirect effects. To help visualise the decomposition, we reproduce the basic mediation schema ( Figure 9 ).
FIGURE 9.
Simple mediation model.
We observe that a statistically non-significant regression coefficient for a or b implies no mediation. For the CoBalT trial such a scenario would imply no association between CBT and dysfunctional attitudes or no association between dysfunctional attitudes and depression. As the CoBalT trial showed CBT to be an effective treatment, it is plausible to consider that the partial mediation-provided conditions (1)–(3) above hold. We can then rule out the possibility of a perfect mediation, which can be inferred only if, in addition to conditions (1)–(3), there was no direct association between intervention and outcome.
In the presence of no mediation, c provides the magnitude of total effect of intervention on outcome. On the other hand c′ provides the direct effect of intervention on outcome after adjusting for the potential mediator. The product ab captures the indirect effect of intervention on outcome through the mediator. Hence, if all three variables are observed then c = c′ + ab so that the indirect effect ab (mediation) is obtainable as the difference between the total (c) and direct effect (c′) of intervention on outcome: ab = c – c′.
However, the simple product rule cannot be extended to situations when either the mediator and/or outcome are not continuous. But this may be addressed by standardising the estimates to help obtain the proportion of effects mediated, i.e. rescaling using the SD of the underlying latent variable for the binary variable. 152 However, the resulting standardised indirect/mediation effect computed as a product of two estimates may not be back transformed to make meaningful inference on individual estimates.
The seminal work by Baron and Kenny144 provided the foundation for empirical analysis of mediation. Analysis using the Baron and Kenny method144 provides the magnitude of association between the variables but fails to account for the order in which the variables occur, meaning that it is not possible to make causal inferences. Temporality, which is an integral component of causal inference is also a defining concept in evaluating mediation, i.e. the mediator precedes the outcome as highlighted in the well-known conceptual framework of mediation analysis. 139 Recent advances in this field are based on instrumental variables methods150 and the potential outcomes framework. 148 The latter method can be extended to binary outcomes and has been implemented in a widely used statistical software package but has not, to date, been used to examined mediators within the context of a pragmatic trial of CBT for depression. It is this method that we adopt for the mediation analysis of the CoBalT data.
Potential outcomes framework
The potential outcomes framework is most easily explained using an example. If we consider the two-arm CoBalT trial, then the potential outcome model presupposes that individuals under study have two theoretical (i.e. potential) outcomes, one of which would be observed if they were randomised to the CBT intervention (Y1) and the other one that would be observed if they were to be randomised to usual care (Y0). A causal estimate Y may then be defined as the comparisons of the potential outcomes that would have been observed under the two different exposures of units to treatments: Y = Y1 – Y0.
In our RCT set-up for CoBalT, in which Z denotes a randomisation indicator, such that Z = 1 indicates assignment to CBT and Z = 0 assignment to usual care, each subject has two potential outcomes, Y1 and Y0 (for outcome under CBT and usual care respectively), but at most, only one of the potential outcomes can be realised and observed for any unit. The potential outcomes (for both Y1 and Y0) are generated from the observed outcomes by comparison with the most similar values of other baseline characteristics. A causal estimate is then obtained by comparing the potential outcomes that would have been observed under different exposure of units to treatment. 153 Most statistical software algorithms implementing potential outcomes framework use matching to generate such variables. However, other methods like propensity scores are also applicable.
Unlike in standard statistical practice that ordinarily compares two independent experimental units, causal effects are obtained by comparing two different potential outcomes for the same experimental unit under different treatment levels. But the fact that we can only observe one outcome and not both presents what is often referred to as the ‘fundamental problem of causal inference’, which posits that we can never know the individual-level causal effects. 154 This predicament makes it impossible to make causal inference without making assumptions, for example, by taking expectations over the whole population to provide an average (aggregate) causal effect. However, most of these causal assumptions are not testable using observed data and hence the validity of any causal inference is premised on the plausibility of its considered assumptions.
Estimating mediation effects
A fundamental difference between analysis using observed outcomes (such as in Baron and Kenny;144 Kraemer et al. 139) and potential outcomes in identifying mediators is that although the former focuses on the observed mediator status, the latter approach focuses on the potential mediator status, which permits causal interpretation. As mediator status and outcome are both realised post randomisation, the observed association between them can be distorted by ‘hidden confounders’, such as contextual variables, which promote high levels of mediator and outcome in the same individuals. Although they are generated post randomisation, potential variables are not susceptible to selection bias because they are not affected by the factors determining treatment actually received. As a result, adjustment using potential mediators provides valid causal interpretation.
In the potential outcomes framework, the average causal mediated effects (ACMEs) can be defined as the mean difference in effect between two counterfactual states of a mediator, assuming no change in the intervention. Similarly, the average direct effect (ADE) is the mean difference between two counterfactual states of intervention, assuming no change in the mediator. In comparison with the Baron and Kenny method,144 ADE may be viewed as an estimate of c’ [see equation (3)]. The ACME enables us to understand the underlying causal mechanisms between an intervention and outcome via the mediator. However, because the counterfactual states are not simultaneously observable, additional assumptions must be made to allow ACME and ADE to be estimated without bias.
A defining assumption for identifying ACME and ADE is the sequential ignorability assumption. 149 First, the treatment assignment is assumed to be unrelated to potential confounders, which is plausible in RCTs owing to random allocation of treatment. Next, the observed mediator is assumed to be unrelated to potential confounders once the actual treatment status and pre-treatment confounders are taken into account. A violation of the latter assumption leads to correlation between the two resulting error terms (residuals) from regression models fitting mediator and outcome as outcomes, i.e. no correlation implies the sequential ignorability assumption is valid. 155 So given the crucial role of the (untestable) sequential ignorability assumption, it is necessary to conduct a sensitivity analysis to examine the robustness of the results for different degrees of violation of this key assumption.
Aims
The aim of this secondary analysis was to examine whether dysfunctional attitudes and metacognitive awareness (measured at 6 months) are mediators of the effect of CBT on depression outcomes at 12 months in the CoBalT trial using a ‘causal inference’ approach.
Methods
We used data from the CoBalT trial (described in Chapter 2 ) for these analyses.
Outcomes at 12 months
The BDI-II46 score at 12 months post randomisation was used as the outcome for these analyses. This outcome was analysed both as a continuous variable and as two binary variables denoting ‘response’ (primary outcome for the trial), which was defined as at least a 50% reduction in depressive symptoms compared with baseline, and ‘remission’ (BDI-II score of < 10).
Mediators at 6 months
As outlined in the main description of the trial methods (see Chapter 2 , Secondary outcomes), participants in both groups were asked to complete the DAS and MAQ as part of the 6-month follow-up assessment. We used a short version of the original 40-item DAS,68 the DAS-SF2, which was developed using item response theory as an efficient method of capturing such data for more widespread use by researchers and clinicians. 58 The DAS-SF2 has been shown to be a valid and reliable measure of dysfunctional attitudes. 58 The measure of metacognitive awareness was developed to examine whether or not CBT involves a shift in how the individual relates to their thoughts and has been shown to be associated a greater likelihood of relapse in a RCT of CBT for residual depression. 59 For both instruments, a total score is generated (range 9–63), with higher scores indicating more dysfunctional attitudes or greater metacognitive awareness. Scores on the DAS and MAQ at 6 months (as continuous variables) were explored as potential mediators of the treatment effect.
Statistical analysis
Baron and Kenny method
To evaluate mediation for continuous BDI scores outcome, initially we fitted the standard Baron and Kenny set of equations144 (see Causality and mediation analysis, above). For the binary outcome version of the Baron and Kenny method,144 the Stata command binary_mediation was used to compute the proportion of mediated (indirect) effects. The program provides standardised coefficients for both the linear and the logistic models used. Although the binary_mediation command permits adjustment for multiple mediators, it does not compute standard errors or CIs directly. Therefore, bootstrapping was performed to determine the significance of mediation effects and compute the proportion of the total variance mediated.
Potential outcomes framework
The method of ‘potential outcomes’148 as implemented in the Stata statistical software156 will be used to estimate the ACME and ADE. Under the potential outcomes framework, we used the Stata command medeff, with the medsens command used to conduct sensitivity analyses to explore violation of the key assumption of sequential ignorability that permits valid causal inference. 148,149 We specified ordinary least-squares regression for the continuous BDI-II outcome as a check on the results obtained from the Baron and Kenny method. 144 We specified logistic regression for the binary outcomes. Of note, unlike the binary_mediation command used to implement the Baron and Kenny method144 for the binary outcomes, the medeff command does not have the option to adjust for multiple mediators. ADE and ACME for binary outcomes are produced as standardised estimates, which may not be meaningfully back-transformed to estimates of ORs or similar statistics. Therefore, our analysis will report the proportion of total effects mediated and their corresponding 95% CIs.
All analyses adjusted for the stratification and minimisation (design) variables (baseline BDI-II score, centre, GP practice access to counselling, prior use of antidepressants and duration of depression) as well as the baseline measure of the mediator (DAS or MAQ). The latter variable was included in the model to ensure that the model examined the effect of change in the potential mediator from baseline to 6 months on later depression outcome (measured at 12 months).
Results
A total of 469 patients were randomised into the CoBalT trial, with an equal number of patients in each of the two treatment arms. Tables 73–75 provide descriptive statistics summarising depression outcomes for each treatment arm and mediator (dysfunctional attitudes scale and metacognitive awareness questionnaire) scores at baseline, 6 and 12 months. On average, those in the usual-care group had higher BDI-II scores than those who received the intervention over the duration of follow-up.
| Time point | Intervention | Usual care | ||
|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |
| Baseline | 234 | 31.8 (10.5) | 235 | 31.8 (10.9) |
| 6 months | 206 | 18.9 (14.2) | 213 | 24.5 (13.1) |
| 12 months | 197 | 17.0 (14.0) | 198 | 21.7 (12.9) |
| Time point | Intervention | Usual care | ||
|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |
| Baseline | 234 | 35.8 (11.0) | 235 | 36.9 (10.6) |
| 6 months | 204 | 31.7 (11.4) | 213 | 34.1 (11.6) |
| 12 months | 196 | 29.8 (12.4) | 198 | 33.2 (10.9) |
| Time point | Intervention | Usual care | ||
|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |
| Baseline | 233 | 37.5 (7.7) | 235 | 37.4 (7.6) |
| 6 months | 204 | 42.8 (8.7) | 213 | 40.0 (8.2) |
| 12 months | 196 | 44.2 (9.1) | 198 | 40.7 (7.8) |
Descriptive statistics (see Tables 74 and 75 ) also show that both DAS and MAQ scores were similar at baseline and reduced with time, more so for the intervention arm. Overall, the mean MAQ scores were higher with corresponding smaller SDs compared with DAS scores.
There was moderate to strong correlation between the measures of dysfunctional attitudes, metacognitive awareness and BDI-II scores across the study ( Table 76 ).
| Measure | Baseline | 6 months | 12 months |
|---|---|---|---|
| DAS: MAQ | –0.33 | –0.40 | –0.46 |
| DAS: BDI-II | 0.34 | 0.50 | 0.52 |
| MAQ: BDI-II | –0.23 | –0.41 | –0.50 |
The Baron and Kenny approach to mediation
Beck Depression Inventory (second version) scores as a continuous outcome
We start by considering a continuous BDI-II score outcome to explore mediation using the standard Baron and Kenny144 set of equations. As expected, a linear regression of the effect of the intervention on depression outcome at 12 months [see Causality and mediation analysis, condition (ii), above] demonstrated the effectiveness of the intervention. Those randomised to receive the intervention had, on average, a BDI-II score that was 5 points lower at 12 months than those randomised to continue with usual care (c = –4.96, 95% CI –7.33 to –2.59, p = 0.001).
The dysfunctional attitudes scale score at 6 months was also significantly related to the intervention [see Causality and mediation analysis, equation (1) ], such that those who were allocated to CBT scored, on average, 2 points lower on the DAS than those who continued with usual care (a = –1.96, 95% CI –3.82 to –0.10, p = 0.039). Although the effect of the intervention on BDI-II outcome at 12 months reduced slightly after adjusting for dysfunctional attitudes measured at 6 months, the effect of the intervention remained significant (c′ = –4.06, 95% CI –6.34 to –1.78, p = 0.001), indicating that there may be other mediators of the intervention effect besides dysfunctional attitudes. Similarly, the association between DAS and BDI-II scores at 12 months after adjusting for CBT was significant (b = 0.39, 95% CI 0.27 to 0.51, p = 0.001). As expected for continuous outcomes the results show that c = c′ + ab and we can conclude that dysfunctional attitudes measured at 6 months mediated about 16% (ab/c) of the effect on the intervention on depression outcome at 12 months ( Table 77 ).
| Mediator | Total effects (c) | Direct effect (c′) | Intervention-mediator (a) | Mediator-outcome (b) | Proportion mediated, % (ab/c) |
|---|---|---|---|---|---|
| DAS | –4.96 (–7.33 to –2.59) | –4.06 (–6.34 to –1.78) | –1.96 (–3.82 to –0.10) | 0.39 (0.27 to 0.51) | 16 |
| MAQ | –5.13 (–7.48 to –2.78) | –3.80 (–6.14 to –1.47) | 2.98 (1.50 to 4.47) | –0.43 (–0.58, –0.28) | 25 |
For comparison, results for metacognitive awareness as a potential mediator of the effect of the intervention on depression are also given in Table 77 below. As would be predicted, a reversal in the direction of effect was observed, for example the effect of intervention on MAQ at 6 months (a) is now such that those who received the intervention scored, on average, 3 points higher on the MAQ than those who continued with usual care (a = 2.98, 95% CI 1.50 to 4.47, p < 0.001), reflecting a positive outcome of increased metacognitive awareness (as opposed to a reduction in dysfunctional attitudes seen previously). After adjusting for the intervention, the effect of MAQ reduced BDI-II outcome by, on average, 0.4 points (b = –0.43, 95% CI –0.58 to –0.28, p < 0.001). Overall, a higher proportion of intervention effect was mediated by MAQ (25%) (see Table 77 ).
Beck Depression Inventory (second version) scores as binary outcomes of response and remission
Results from bootstrapping showed that both direct and indirect effects were statistically significant. Table 78 shows the proportion of total mediated effects for the two binary outcomes of ‘response’ and ‘remission’ for the two postulated mediators (DAS and MAQ).
| Binary outcome | DAS: proportion of total mediated (%) | MAQ: proportion of total mediated (%) | DAS + MAQ: proportion of total mediated (%) | |||
|---|---|---|---|---|---|---|
| Effects | Variance | Effects | Variance | Effects | Variance | |
| Response | 20 | 12 | 23 | 12 | 31 | 19 |
| Remission | 21 | 12 | 19 | 11 | 28 | 15 |
In general, the proportion of total effect of the intervention mediated was slightly higher for the response outcome compared with the remission outcome, although such effects were only marginal. Adjusting for both mediators resulted in greater proportion of mediated effects than for individual mediators. However, the higher proportion of mediated effect in the model including both mediators was estimated with less precision (higher proportion of variance).
Estimating average causal mediation effects and average direct effects under the potential outcomes framework
Continuous outcome and continuous mediators
Mediation analysis of linear models (continuous outcomes) for both mediator and outcome under the potential outcomes framework produced a mean average direct effect (ADE) for DAS measured at 6 months of –4.05 and ACME of –1.23 ( Table 79 ). The estimate of the ACME indicated that the effect of CBT intervention that was mediated by DAS equated, on average, to a reduction of 1 point in the BDI-II score. This compares with a total treatment effect of, on average, a reduction of 5 points in the BDI-II score (total effect). Hence, overall, the proportion of the total effect of the intervention mediated by dysfunctional attitudes measured at 6 months was 24% (see Table 79 ).
| Outcome at 12 months | Mediator measured at 6 months | ADE (95% CI) | ACME (95% CI) | Total effect (95% CI) | Proportion of effect mediated, % (95% CI) |
|---|---|---|---|---|---|
| BDI-II score (continuous) | DAS | –4.05 (–6.33 to –1.84) | –1.23 (–2.18 to –0.48) | –5.29 (–7.69 to –3.02) | 24 (16 to 41) |
| MAQ | –3.80 (–6.12 to –1.54) | –1.50 (–2.39 to –0.76) | –5.30 (–7.58 to –2.88) | 28 (20 to 52) |
We observe that, on average, the results for total effect and ADE are similar to the total effect c and the direct effect of CBT on BDI-II scores c′, respectively, as obtained from the standard Baron and Kenny method144 above. As expected for linear models, under the sequential ignorability assumption, the ACME estimate, on average, approximates the product of effects ab (i.e. ACME = –1.23, ≈ –3.18 × 0.39).
A sensitivity analysis of the results suggested that the sequential ignorability assumption would be violated (ACME≠0) in the presence of a positive correlation of 0.31 between the two error terms for regression models fitting dysfunctional attitudes and BDI-II scores as outcomes. This may be interpreted to mean that the presence of hidden confounders, which are moderately correlated to both BDI-II scores and DAS, would confound the causal pathway by introducing dependence in the two error terms.
Table 79 also gives the corresponding results for the MAQ as the postulated mediator. This shows a slightly larger ACME of –1.50 compared with an ACME of –1.23 for DAS as the mediator. A slightly higher proportion of the effect of the intervention was mediated by MAQ at 28% compared with DAS (24%), although the CIs for the proportion of mediated effect for MAQ were relatively wider (see Table 79 ). A sensitivity analysis suggested that only a modest degree of ‘hidden’ confounding (a negative correlation of –0.27 between the error terms for regression models with MAQ and BDI-II scores as outcomes) would result in the sequential ignorability assumption being violated.
Binary depression outcomes (response and remission) and continuous mediators
A mediation analysis considering a binary ‘response’ outcome (at least a 50% reduction in BDI-II score relative to baseline) and continuous dysfunctional attitudes (mediator) produced an average proportion of total effects mediated of 19% ( Table 80 ). For a continuous metacognitive awareness mediator, the average proportion of total effects mediated was slightly higher, 23%, although this estimate was accompanied by a wider 95% CI (see Table 80 ).
| Binary outcome | DAS: proportion of total mediated effects, % (95% CI) | MAQ: proportion of total mediated effects, % (95% CI) |
|---|---|---|
| Response | 19 (14 to 30) | 23 (17 to 38) |
| Remission | 20 (15 to 32) | 19 (14 to 32) |
In general, for a binary ‘response’ outcome, the proportion of total mediated effects were slightly higher for MAQ compared with DAS albeit with a corresponding wider 95% CI. On the other hand, the proportion of total mediated effects were, on average, similar for the remission outcome regardless of the mediator considered (MAQ or DAS). Sensitivity analyses for the binary outcomes were not conducted as the results may not be meaningfully interpreted, as the resulting correlation values are based on the standardised coefficients from both linear and logistic models.
Discussion
Our results suggest that approximately 20–25% of the treatment effect observed at 12 months was mediated through changes in dysfunctional attitudes or metacognitive awareness at 6 months. This equated, on average, to a one-point reduction in the difference in mean BDI-II scores between treatment groups. However, these findings need to be interpreted with caution as sensitivity analyses suggested that any hidden confounders with a fairly weak correlation (rho 0.3) with both the mediator (scores on the DAS or MAQ) and outcome (depressive symptoms on the BDI-II) would be an alternative explanation of our observed results.
We used the potential outcomes framework, which is part of the ‘causal inference approach’, to examine mediated treatment effects. To the best of our knowledge, this is the first time that this methodology has been used to examine mediators within the context of a pragmatic trial of CBT for depression. This methodology explicitly permits testing of the assumptions required for making valid causal inferences.
Strengths and limitations
We used data from a large multicentre RCT based in UK primary care to examine potential mediators of the observed treatment effect. When examining mediated treatment effects, one key issue that has been highlighted139,146,157 is ensuring the temporal sequencing of the intervention, mediator and outcome. The Baron and Kenny approach144 does not incorporate information about the temporal sequence of variables and hence relies on the study design to establish this sequence in order to permit causal inference. For the analyses conducted using the potential outcomes framework, a priori, we specified our outcome as depressive symptoms at 12 months and our mediators as scores on the DAS and MAQ at 6 months post randomisation. We also adjusted for the baseline score of the mediator (DAS or MAQ) in these models in order to ensure that we focused on change in the postulated mediator that preceded the depression outcome measured at 12 months. However, it proved difficult to be certain about the temporal sequence of changes in the proposed mediators and the outcome measure.
It is widely acknowledged that measures of cognitive variables, such as dysfunctional attitudes are moderately correlated with depressive symptoms,158 and data from the CoBalT trial supported this (rho 0.34 to 0.52). In the context of a CBT intervention, which takes place over a period of several months, the therapist would hope to observe a shift in the postulated mediator (e.g. dysfunctional attitudes) during the course of therapy and simultaneously to observe a decrease in depressive symptoms. Therefore, although it is possible to use data collected at specified time points in order, at face value, to satisfy the conditions of temporality, it is possible that the changes in mediator and outcome will be occurring more closely in time, and even co-varying depressive symptoms will not truly address this issue. In the case of the CoBalT trial, we also need to acknowledge the fact that the treatment effect was already observed at the 6-month time point, making it more difficult to ensure temporal separation.
A strength of the potential outcomes framework is that it uses counterfactuals in order to identify causal effects. Hence the model compares the observed outcome with that which would have been observed had the individual been randomly allocated to the other treatment group (= potential outcome). Such ‘potential outcomes’ are not susceptible to selection bias because they are not affected by factors determining actual treatment received, unlike approaches that rely solely on observed outcomes. 139,144
Previous approaches to testing for mediated effects (e.g. Baron and Kenny;144 Kraemer et al. 139) implicitly assume that there is no ‘hidden’ (unmeasured) confounding between the observed mediator and outcome but do not address this issue. 159 Although the same issue of ‘hidden confounding’ applies when estimating the ACME under the potential outcomes framework, the latter approach makes explicit this assumption and permits sensitivity analyses to test the plausibility of this assumption. In our study, such sensitivity analyses (for the mediated effects of DAS and MAQ) suggested that a relatively modest correlation (rho 0.3) between error terms for the models with mediator and BDI-II score as the outcome would mean that this assumption was violated and hence that ‘hidden’ confounding could explain the mediated effect. Such a formal test of this assumption is key in enabling researchers to draw robust conclusions regarding potential mediated effects.
Other methodological issues
Implementation of the Baron and Kenny approach144 for binary outcomes (binary_mediation) permits adjustment for multiple mediators, and we found that 20–25% of the treatment effect was mediated by either dysfunctional attitudes or metacognitive awareness. When the effect of both mediators was modelled simultaneously, a slightly higher percentage (approximately 30%) of the treatment effect was explained. Although, on technical grounds, it is currently not possible within the potential outcomes framework to replicate this finding, this multivariable mediator model provides only weak evidence that both dysfunctional attitudes and metacognitive awareness independently contribute to explaining how CBT works.
The modest increase in percentage of mediated effect for the model including both factors suggests that there may be substantial overlap (or measurement error) in the constructs measured by the two instruments. Scores on the scales measuring dysfunctional attitudes and metacognitive awareness were moderately correlated (rho –0.33 to –0.46), as has been noted previously. 59 This raises the question of how well we are measuring the cognitive factors that are thought to result in the improvements in outcome seen following a course of CBT. Although others have suggested that individual styles of responding to questionnaires can also lead to correlation between questionnaire measures of quite different phenomena. 160
We used a short version of the 40-item DAS,68 the DAS-SF2, which was developed using item response theory as a valid and reliable approach to efficiently measuring dysfunctional attitudes. 58 The measure of metacognitive awareness (MAQ) was developed to examine whether or not CBT involves a shift in how the individual relates to their thoughts and was associated with greater likelihood of relapse in a previous RCT of CBT for residual depression. 59 Nonetheless, there is likely to be substantial error in our measurement of the mediator but this issue is difficult to resolve given the nature of the mediators of interest.
Causal inference approaches to mediation analysis make explicit the assumption of no ‘hidden’ confounding that has, so far, been implicit but not addressed by many key papers in this field. 139,144,146,147 However, to date, there has been little discussion about what such confounding factors might be. A greater understanding of these confounders, in terms of both identifying and measuring such factors, would help us to draw more robust conclusions when interpreting the results of sensitivity analyses, particularly when the mediated effects are modest. Methods have been developed to allow for hidden confounding (using instrumental variables);150 however, these cannot easily be applied using standard statistical software.
Conclusions
Increasingly, in the context of pragmatic trials, we are interested in whether or not a treatment is effective and how it works. Recent methodological developments have permitted us to begin to unravel the ‘how’ by using approaches based on causal inference. Using such methods, we found that changes in cognitive variables that we studied (dysfunctional attitudes and metacognitive awareness) accounted for about 20–25% of the observed treatment effect of the CBT intervention. However, sensitivity analyses showed that only a relatively modest degree of ‘hidden’ (unmeasured) confounding between the mediator and outcome would explain the mediated effect. Furthermore, when the proposed mediators are correlated, as are dysfunctional attitudes and depressive symptoms, it is very difficult to establish the temporal order of change in the different variables, leaving conclusions susceptible to ‘reverse causality’. This reinforces the view140,141 that there is little robust evidence for the role of cognitive factors as the mechanism through which CBT works.
Chapter 10 Moderators of treatment response to cognitive behavioural therapy
Introduction
Clinical problem
Depression is a major contributor to the global burden of disease and is projected to be the leading cause of disability in high-income countries by 2030. 161 There is good evidence that CBT is an effective treatment in previously untreated episodes of depression. 162,163 However, there is considerable variation in patient response to CBT, with significant proportions responding either not at all or only partially. It is therefore clinically useful to identify which patients will respond well to which treatments. Reliable evidence informing this issue remains elusive, however, and clinicians often decide which patients to refer to which treatment, based on implicit beliefs about patient suitability. 164 In line with the current drive towards stratified medicine that aims to target interventions at subgroups of patients who are likely to respond,165,166 research is needed to identify reliable moderators or effect modifiers of treatment response.
It is important to distinguish between predictors and moderators. Predictors are prognostic factors associated with disease outcomes irrespective of treatment, whereas moderators or effect modifiers are associated with different treatment responses. 139 In other words, a moderator will lead to a smaller or larger difference between active and comparator groups. Understanding of potential moderators is clinically useful as this would enable clinicians to base treatment choices on the individual’s likelihood of benefiting from a given treatment. A variable is established as a moderator by testing for interactions between that variable and two or more treatment options, ideally within the context of a controlled trial. 167 Studies designed specifically to test for interactions are large, expensive, and therefore rare. Using existing data from good-quality and well-controlled clinical trials is an efficient and cost-effective alternative. 168 Although such secondary analyses suffer from low statistical power, they are prone to false-positive findings owing to multiple testing. Caution is therefore required when interpreting findings from a single study. Consistent findings across studies are required before we can consider moderators as clinically informative and, ideally, the field should aim for meta-analyses of randomised studies using individual patient data to achieve sufficient statistical power. 169
To date, studies reporting moderators of response to CBT in controlled trials have used small sample sizes, randomising fewer than 63 patients per CBT arm,170–174 or have compared CBT to antidepressant treatment,172,173 or have focused on adolescent or elderly populations. 175–177 With such small sample sizes these studies were almost certainly underpowered,167 and although understanding which of two treatment options is likely to produce the best outcomes is important, antidepressants and CBT are often prescribed together in practice. In most health services, antidepressants are widely available and access to psychotherapy is limited. CBT is often reserved for those patients who have not responded to antidepressant medication. We have previously reported effect modifiers for online CBT as an adjunct to usual care, which included the option of antidepressants where prescribed, and just over half the sample were taking antidepressants at baseline. 168 However, to date, no research has examined moderators of response to CBT as a ‘next-step’ treatment for primary care patients who have not responded to antidepressants. Identifying reliable moderator variables in this population will inform treatment options for depressed patients who do not respond to antidepressants. To date, the existing literature in this area is based on studies of CBT in populations without TRD.
Demographic factors
A few studies have examined whether or not demographic variables such as age, gender, education and marital status are moderators of response to CBT in adults using appropriate tests for interaction in controlled studies. 168,172,174 In a stepwise regression, Fournier and colleagues172 found that being married, unemployed or having more antecedent life events were associated with better response to CBT than antidepressants. However, patients often receive CBT as an adjunct treatment in addition to antidepressant medication. In a previous study168 with a sample twice the size of that reported by Fournier and colleagues,172 we found that being separated, widowed or divorced or having fewer recent stressful life events were associated with better responses to CBT compared with a waiting list control. In our study, both arms received care as usual, including antidepressants if prescribed by the GP,168 which may account for the discrepant findings. Furthermore, in contrast with Fournier et al. 172 we divided our ‘unmarried’ participants, into ‘single’ or ‘separated/widowed/divorced’. 168 Educational attainment and age were not found to modify response to CBT. 168,172
Illness characteristics
To date, pre-treatment severity of depression is the most reliable moderator of response to CBT, with the more severely depressed benefiting most. 168,177–179 The evidence suggests that mild depression seems to recover well irrespective of treatment, whereas severe depression gains most from CBT. 168 However, meta-analytic findings that rely on aggregate data179,178 and issues of scaling confuse these severity findings, which may be an artefact of assessing outcomes using continuous measures. For example, a 5-point reduction in scores for someone whose baseline score was 50 is a proportionally smaller improvement than 5 points for someone with a baseline score of 15.
There is no evidence that history, chronicity and type of depression are moderators of CBT response. 168,172 The literature on comorbidity is also mixed. Patients in the STAR*D study with anxious depression who were partial or non-responders to citalopram responded less well to either CBT or an alternative antidepressant as a second-line treatment but there was no evidence for effect modification. 171 Asarnow et al. 180 identified comorbidity, including anxiety, as a moderator, with increased comorbidity associated with increased response to combined CBT and antidepressants relative to antidepressants alone. However, anxiety did not modify response to CBT in a study of depressed adolescents. 175
Personality traits, cognitions and psychological mindedness
Assessing individuals’ suitability for therapy is an important part of clinical practice, which often focuses on interpersonal skills, personality and psychological mindedness. 164 The lack of clear evidence of effect modification from appropriately controlled studies, however, illustrates that this practice is not evidence based. Indeed, few studies have investigated these variables as moderators. To date, there is no evidence that the personality trait neuroticism is a moderator. 172,181 Patients with lower dysfunctional attitudes have been found to do better in treatment arms (CBT and antidepressants) relative to pill placebo,174 whereas other studies found no evidence for effect modification. 172,176 Clinicians believe it is important to individualise treatment in line with particular patient presentations,182 and it seems likely that cognitive-based therapies will be most effective in those with high levels of psychological awareness. However, no studies have directly investigated metacognitive awareness as a moderator in an appropriately controlled trial.
Aims
The aim of the present analysis was to examine potential moderators of response to CBT given as an adjunct to usual care that included pharmacotherapy as a ‘next-step’ treatment for patients whose depression had not responded to treatment with antidepressants using data from the CoBalT trial. By examining moderators in this group, we aim to inform decisions of whether or not to refer such patients for CBT. We examined the modifying effects of demographic, life events, illness, comorbidity, personality traits and cognitive variables.
Methods
Participants
This was a secondary analysis of data collected as part of the CoBalT trial, which has been described in detail earlier (see Chapter 2 ). Brief details are outlined below.
Individuals were eligible for the trial if they were aged between 18 and 75 years, were currently taking antidepressant medication and had been doing so at an adequate dose for at least 6 weeks, scored ≥ 14 on the BDI-II46 and met the ICD-10 criteria for depression (assessed using the CIS-R47,183). Participants were randomised to one of two groups: (1) usual care or (2) CBT in addition to usual care. Treatment allocation was stratified by recruitment centre and minimised by baseline BDI-II score, whether participant’s general practice had a counsellor (yes/no), prior treatment with antidepressants (yes/no) and duration of their current episode of depression (< 1 year; 1–2 years; ≥ 2 years) in order to achieve balance in these important (design) variables across the treatment arms. Participants were followed up at intervals of 3 months for 1 year, with the BDI-II being completed at baseline, 6 and 12 months.
Outcome
The outcome variable used in this secondary analysis was BDI-II score treated as a continuous variable at 6 and 12 months’ follow-up analysed as a repeated measure. We treated BDI-II score as a continuous variable in this exploratory study to retain maximum power and ensure comparability of findings with previous studies of moderation. 168,172 This is in contrast with the main trial, where the primary outcome was a binary variable representing a reduction in BDI-II score of at least 50% compared with baseline (see Chapter 2 , Primary outcome).
Moderators
All data on potential moderators were collected as part of the baseline assessment, prior to randomisation. The potential moderators were grouped into three general classes: (1) demographic and life factors; (2) illness characteristics; and (3) personality, cognition and psychological mindedness.
Demographic and life factors
Age was categorised into the following groups: (1) < 30 years; (2) 30–39 years; (3) 40–49 years; and (4) > 49 years. Level of education was defined as highest educational qualification and categorised as (1) ‘A-level/Higher Grade or above’; (2) ‘Other qualifications – GCSE or equivalent’; and (3) ‘No formal qualifications’. A-levels are UK national qualifications that are generally taken at age 18 years, and qualifications at this level are usually required for entry to university or higher education. GCSEs are also national qualifications generally taken at age 16 years. Marital status was categorised as (1) ‘Single’; (2) ‘Married/living as married’; and (3) ‘Separated/Widowed/Divorced’. Eight questions selected from the Social and Readjustment Rating Scale,184 dealing with bereavement, separation or divorce, serious illness or injury, victim of crime, problems with the police resulting in a court appearance, debt, disputes with friends, relatives and/or neighbours and redundancy within the 6 months prior to randomisation were used to measure adverse life events. The number of life events were summed and categorised as: (1) 0 events; (2) 1–2 events; and (3) ≥ 3 events.
Illness characteristics
Two measures of pre-treatment depression severity were measured: (1) baseline BDI-II score, dichotomised as (i) severe (BDI-II score of > 28) and (ii) less severe (BDI-II score of < 29); and (2) baseline CIS-R depression severity as a continuous variable, generated by summing the depression, depressive ideas, fatigue, concentration and sleep sections of the CIS-R to produce a score ranging from 0 to 21. History of depression was assessed in terms of the number of previous episodes of depression reported and the duration of the current episode. Number of prior episodes of depression was categorised as (1) 0–1 episodes; (2) 2–4 episodes; and (3) ≥ 5 episodes. The duration of the current episode of depression was categorised as (1) < 1 year; (2) 1–2 years; and (3) > 2 years. Anxiety was measured as the score of the CIS-R anxiety section, range 0–4. PTSD was scored as an additive count of symptoms on the PC-PTSD,51 with a possible range of 0–4. Physical comorbidity was investigated based on self-report of participants’ other illnesses: (1) no chronic illness; (2) diabetes; (3) asthma; (4) arthritis; (5) heart disease; (6) high blood pressure; (7) lung disease; (8) more than one of the above; and (9) none of the above but other.
Personality, cognition and psychological mindedness
Dysfunctional attitudes and metacognitive awareness were measured as continuous variables by summing participants’ responses to the DAS-SF2 58 and MAQ59 respectively. Neuroticism was measured using the neuroticism subscale of the ‘Big Five’ Inventory (BFI)52 and examined as a continuous variable as the mean score of the eight test items.
Statistical analysis
Treatment effect was defined as the (adjusted) difference in mean BDI-II outcome score (as a continuous variable) between the usual care and intervention arms. Separate repeated measures linear regression models were carried out for each potential moderator. The model included an interaction term between the moderator and treatment allocation, and adjusted for the design variables (including baseline BDI-II score) and time. Further models, containing a three-way interaction (moderator by treatment allocation by time) were carried out to investigate whether or not effect modification varied across time. Repeated measures regression models were also stratified by each level of the potential moderators to illustrate any interaction effects.
Results
Baseline characteristics
As reported earlier (see Table 19 ), the randomised groups were similar in terms of the stratification and minimisation variables (baseline BDI-II score, whether participant’s general practice had a counsellor, prior treatment with antidepressants, and duration of their current episode of depression), age, gender and demographic factors. The two groups were also similar in terms of the other potential treatment moderators investigated ( Table 81 ) that were not reported earlier.
| Characteristic | Intervention (n = 234) | Usual care (n = 235) |
|---|---|---|
| Demographic and life factors | ||
| Age (categories, years): n (%) | ||
| < 30 | 20 (8.6) | 11 (4.7) |
| 30–39 | 29 (12.4) | 32 (13.6) |
| 40–49 | 64 (27.4) | 69 (29.4) |
| > 49 | 121 (51.7) | 123 (52.3) |
| Life events in the past 6 months: n (%) | ||
| 0 | 62 (26.5) | 71 (30.2) |
| 1–2 | 138 (59.0) | 135 (57.5) |
| ≥ 3 | 34 (14.5) | 29 (12.3) |
| Illness characteristics | ||
| BDI severity group: n (%) | ||
| Less severe | 102 (43.6) | 103 (43.8) |
| Severe | 132 (56.4) | 132 (56.2) |
| CIS-R depression severity score: mean (SD) | 14.8 (3.1) | 14.9 (2.9) |
| Anxiety score: mean (SD) | 2.5 (1.5) | 2.4 (1.5) |
| PTSD score: mean (SD) | 2.0 (1.5) | 2.1 (1.5) |
| Illnesses: n (%) | ||
| No chronic illness | 64 (27.4) | 54 (23.0) |
| Diabetes | 10 (4.3) | 6 (2.6) |
| Asthma | 11 (4.7) | 17 (7.2) |
| Arthritis | 19 (8.1) | 19 (8.1) |
| Heart disease | 5 (2.1) | 4 (1.7) |
| High blood pressure | 11 (4.7) | 16 (6.8) |
| Lung disease | 1 (0.4) | 4 (1.7) |
| More than one of the above | 35 (15) | 44 (18.7) |
| None of the above but other | 78 (33.3) | 71 (30.2) |
| Personality, cognition and psychological mindedness | ||
| Dysfunctional attitudes score: mean (SD) | 35.8 (11.0) | 36.9 (10.6) |
| Metacognitive awareness score: mean (SD) | 37.5 (7.7) | 37.4 (7.6) |
| Neuroticism score: mean (SD) | 4.0 (0.7) | 4.1 (0.6) |
Adherence to the intervention
The level of adherence to the intervention (defined as the mean number of CBT sessions attended) were generally very similar across the levels of the potential moderators investigated ( Table 82 ).
| Moderator | No. of CBT sessions attended: mean (SD) |
|---|---|
| Demographic and life factors | |
| Age (years) | |
| < 30 | 11.0 (6.4) |
| 30–39 | 10.7 (6.6) |
| 40–49 | 11.0 (6.3) |
| > 49 | 11.6 (6.2) |
| Highest level of educationa | |
| A-level/Higher Grade or above | 12.5 (5.9) |
| Other qualifications – GCSE or equivalent | 11.5 (5.9) |
| No formal qualifications | 8.6 (6.5) |
| Marital status | |
| Single | 9.9 (6.6) |
| Married/living as married | 11.6 (6.4) |
| Separated/divorced/widowed | 11.5 (5.8) |
| Life events in the past 6 months | |
| 0 | 11.0 (6.2) |
| 1–2 | 11.5 (6.2) |
| ≥ 3 | 10.7 (6.8) |
| Illness characteristics | |
| Baseline BDI severity | |
| Less severe | 10.8 (6.0) |
| Severe | 11.6 (6.4) |
| Baseline CIS-R depression severityb | |
| Low | 11.1 (6.0) |
| High | 11.3 (6.5) |
| No. of prior episodes of depression | |
| 0–1 | 10.5 (6.4) |
| 2–4 | 10.7 (6.4) |
| ≥ 5 | 11.9 (6.1) |
| Duration of current episode of depression: | |
| < 1 year | 10.2 (6.6) |
| 1–2 years | 12.0 (6.1) |
| > 2 years | 11.5 (6.1) |
| Anxiety scorec | |
| Low | 11.3 (5.9) |
| High | 11.2 (6.4) |
| PTSD scoreb | |
| Low | 10.7 (6.5) |
| High | 11.6 (6.0) |
| Illnesses | |
| No chronic illness | 10.8 (6.3) |
| Diabetes | 7.8 (7.2) |
| Asthma | 11.5 (5.6) |
| Arthritis | 10.7 (7.6) |
| Heart disease | 16.0 (2.9) |
| High blood pressure | 12.4 (4.1) |
| Lung disease | 4.0 (NA) |
| More than one of the above | 10.7 (6.4) |
| None of the above but other | 12.0 (6.0) |
| Personality, cognition and psychological mindedness | |
| Dysfunctional attitudes scoreb | |
| Low | 10.4 (6.5) |
| High | 12.1 (5.9) |
| Metacognitive awareness scoreb | |
| Low | 11.7 (6.2) |
| High | 10.8 (6.3) |
| Neuroticism scoreb | |
| Low | 10.4 (6.5) |
| High | 12.0 (5.9) |
Effect modification by potential moderators
The results obtained from the repeated measures regression models suggested that age was the only variable for which there was any evidence of an interaction between a potential moderator and the intervention, implying that age may modify the effectiveness of CBT. The interaction coefficients became more negative the higher the age category, suggesting that the higher the age category the greater the benefit of treatment (p-value for interaction effect = 0.012; Table 83 ). When age was used as a continuous variable the conclusion was the same, evidence for greater treatment derived benefit the older the subject (interaction coefficient = –0.20, 95% CI = –0.37 to –0.02, p = 0.027).
The regression analyses were also carried out separately at each level of the potential moderator variables in order to illustrate the findings. The adjusted differences in mean BDI-II scores between the levels of the investigated variables were similar, had overlapping CIs and did not show any clear trends except for age, in which older individuals had a larger treatment response (see Tables 84 and 85 ). The three-way treatment × moderator × time interactions suggested that there was no evidence that the relationships between any of the investigated potential moderators and the intervention varied over time ( Table 83 ).
| Moderatora | n b | b c | 95% CI | p-valued | Time interactione | 95% CI | p-valued |
|---|---|---|---|---|---|---|---|
| Demographic and life factors | |||||||
| Age (years) | 0.012 | 0.77 | |||||
| < 30 | 32 | 0 | 0 | ||||
| 30–39 | 50 | –1.5 | –11.5 to 8.5 | 0.9 | –9.3 to 11.2 | ||
| 40–49 | 112 | –5.6 | –14.6 to 3.5 | 0.9 | –8.3 to 10.2 | ||
| > 49 | 209 | –7.2 | –15.9 to 1.5 | –1.4 | –10.2 to 7.4 | ||
| Highest level of education | 0.43 | 0.24 | |||||
| A-level/Higher Grade or above | 198 | 0 | 0 | ||||
| Other qualifications: GCSE or equivalent | 118 | 0.9 | –4.0 to 5.9 | 2.6 | –2.3 to 7.5 | ||
| No formal qualifications | 85 | –2.8 | –8.1 to 2.5 | 4.4 | –1.0 to 9.7 | ||
| Marital status | 0.39 | 0.074 | |||||
| Single | 67 | 0 | 0 | ||||
| Married/living as married | 213 | –4.0 | –9.6 to 1.7 | –3.2 | –8.9 to 2.5 | ||
| Separated/divorced/widowed | 123 | –2.7 | –8.9 to 3.6 | 2.3 | –4.0 to 8.6 | ||
| Life events in the past 6 months | 0.93 | 0.79 | |||||
| 0 | 108 | 0 | 0 | ||||
| 1–2 | 236 | –0.9 | –5.7 to 3.8 | 0.4 | –4.4 to 5.1 | ||
| ≥ 3 | 59 | –0.5 | –7.2 to 6.3 | 2.3 | –4.6 to 9.2 | ||
| Illness characteristics | |||||||
| Baseline BDI severity | 0.56 | 0.58 | |||||
| Less severe | 174 | 0 | 0 | ||||
| Severe | 229 | 1.2 | –2.9 to 5.4 | –1.2 | –5.4 to 3.0 | ||
| Baseline CIS-R depression severity | 403 | 0.0 | –0.7 to 0.7 | 0.91 | 0.48 | –0.2 to 1.2 | 0.18 |
| No. of prior episodes of depression | 0.93 | 0.088 | |||||
| 0–1 | 73 | 0 | 0 | ||||
| 2–4 | 126 | 0.4 | –5.7 to 6.6 | –0.6 | –6.8 to 5.6 | ||
| ≥ 5 | 204 | 1.0 | –4.6 to 6.6 | 4.3 | –1.3 to 9.9 | ||
| Duration of current episode of depression: | 0.72 | 0.12 | |||||
| < 1 year | 101 | 0 | 0 | ||||
| 1–2 years | 68 | 2.7 | –3.8 to 9.2 | –0.6 | –7.1 to 5.9 | ||
| > 2 years | 234 | 1.0 | –4.0 to 6.0 | 4.1 | –0.9 to 9.2 | ||
| Anxiety score | 403 | –0.2 | –1.6 to 1.2 | 0.82 | –0.2 | –1.6 to 1.2 | 0.76 |
| PTSD score | 393 | –0.6 | –2.0 to 0.7 | 0.36 | –0.6 | –2.0 to 0.8 | 0.40 |
| Illnesses | 0.20 | 0.67 | |||||
| No chronic illness | 168 | 0 | 0 | ||||
| Diabetes | 17 | 1.3 | –10.3 to 12.9 | –1.8 | –14.1 to 10.4 | ||
| Asthma | 19 | –7.1 | –16.8 to 2.6 | 4.1 | –5.9 to 14.2 | ||
| Arthritis | 29 | –1.2 | –9.7 to 7.4 | –2.4 | –11.2 to 6.3 | ||
| Heart disease | 10 | –1.8 | –16.6 to 13.0 | 5.3 | –10.3 to 21.0 | ||
| High blood pressure | 19 | –12.0 | –21.7 to –2.4 | –1.3 | –11.3 to 8.6 | ||
| Lung disease | 2 | 0.4 | –23.4 to 24.2 | 3.2 | –20.7 to 27.0 | ||
| More than one of the above | 61 | –7.8 | –14.3 to –1.3 | 2.6 | –4.1 to 9.3 | ||
| None of the above but other | 139 | –3.3 | –8.8 to 2.2 | 4.8 | –0.8 to 10.5 | ||
| Personality, cognition and psychological mindedness | |||||||
| Dysfunctional attitudes score | 403 | 0.1 | –0.1 to 0.3 | 0.46 | 0.1 | –0.1 to 0.3 | 0.51 |
| Metacognitive awareness score | 401 | 0.2 | –0.1 to 0.4 | 0.23 | 0.0 | –0.3 to 0.3 | 0.98 |
| Neuroticism score | 401 | –1.4 | –4.6 to 1.8 | 0.39 | 1.1 | –2.2 to 4.3 | 0.53 |
| Moderator | Baseline | 6 months | 12 months | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Usual care | Intervention | Usual care | Intervention | Usual care | |||||||
| n | Mean | n | Mean | n | Mean | n | Mean | n | Mean | n | Mean | |
| Demographic and life factors | ||||||||||||
| Age (years): | ||||||||||||
| < 30 | 20 | 31.7 | 11 | 35.4 | 17 | 19.2 | 11 | 20.2 | 15 | 15.4 | 10 | 15.3 |
| 30–39 | 29 | 33.7 | 32 | 32.6 | 26 | 20.8 | 28 | 22.2 | 24 | 17.8 | 24 | 16.0 |
| 40–49 | 64 | 33.1 | 69 | 33.5 | 57 | 20.7 | 61 | 27.1 | 55 | 18.1 | 56 | 22.5 |
| > 49 | 121 | 30.6 | 123 | 30.4 | 106 | 17.5 | 113 | 24.1 | 103 | 16.5 | 108 | 23.1 |
| Highest level of education: | ||||||||||||
| A-level/Higher Grade or above | 112 | 31.0 | 105 | 31.0 | 101 | 18.8 | 93 | 23.0 | 97 | 15.8 | 93 | 20.7 |
| Other qualifications: GCSE or equivalent | 63 | 32.2 | 67 | 31.2 | 60 | 19.7 | 63 | 23.6 | 58 | 17.5 | 56 | 19.8 |
| No formal qualifications | 57 | 33.1 | 59 | 34.5 | 44 | 18.6 | 53 | 28.7 | 41 | 19.6 | 45 | 25.3 |
| Marital status: | ||||||||||||
| Single | 44 | 33.5 | 45 | 31.6 | 35 | 22.9 | 40 | 24.5 | 32 | 19.5 | 39 | 19.4 |
| Married/living as married | 120 | 32.4 | 128 | 31.3 | 109 | 18.7 | 114 | 23.5 | 104 | 16.2 | 109 | 22.4 |
| Separated/divorced/widowed | 70 | 29.5 | 62 | 33.2 | 62 | 17.1 | 59 | 26.5 | 61 | 17.2 | 50 | 21.8 |
| Life events in the past 6 months: | ||||||||||||
| 0 | 62 | 29.4 | 71 | 31.4 | 55 | 16.3 | 64 | 21.7 | 53 | 15.8 | 61 | 20.7 |
| 1–2 | 138 | 32.4 | 135 | 31.4 | 120 | 19.7 | 121 | 24.9 | 116 | 17.6 | 113 | 22.1 |
| ≥ 3 | 34 | 33.6 | 29 | 35.0 | 31 | 20.7 | 28 | 29.1 | 28 | 17.1 | 24 | 22.3 |
| Illness characteristics | ||||||||||||
| Baseline BDI severity: | ||||||||||||
| Less severe | 102 | 22.5 | 103 | 22.1 | 88 | 12.4 | 97 | 18.8 | 86 | 11.9 | 93 | 16.5 |
| Severe | 132 | 38.9 | 132 | 39.4 | 118 | 23.8 | 116 | 29.3 | 111 | 21.0 | 105 | 26.2 |
| Baseline CIS-R depression severity:a | ||||||||||||
| Low | 102 | 25.3 | 101 | 25.3 | 90 | 14.6 | 96 | 19.4 | 87 | 12.3 | 89 | 17.6 |
| High | 132 | 36.8 | 134 | 36.7 | 116 | 22.3 | 117 | 28.7 | 110 | 20.8 | 109 | 25.0 |
| No. of prior episodes of depression: | ||||||||||||
| 0–1 | 46 | 32.5 | 45 | 30.0 | 38 | 20.2 | 40 | 24.5 | 35 | 17.1 | 39 | 22.6 |
| 2–4 | 72 | 29.7 | 61 | 27.8 | 64 | 18.8 | 59 | 22.4 | 62 | 14.8 | 53 | 19.9 |
| ≥ 5 | 116 | 32.7 | 129 | 34.4 | 104 | 18.5 | 114 | 25.6 | 100 | 18.4 | 106 | 22.2 |
| Duration of current episode of depression (years): | ||||||||||||
| < 1 | 58 | 29.2 | 51 | 28.4 | 51 | 16.8 | 48 | 21.9 | 50 | 12.8 | 45 | 18.7 |
| 1–2 | 40 | 30.5 | 43 | 31.1 | 35 | 20.5 | 39 | 23.5 | 33 | 16.4 | 38 | 21.6 |
| > 2 | 136 | 33.2 | 140 | 33.3 | 120 | 19.4 | 126 | 25.8 | 114 | 19.1 | 115 | 22.8 |
| Anxiety score:b | ||||||||||||
| Low | 67 | 26.1 | 65 | 26.8 | 60 | 16.0 | 60 | 20.8 | 61 | 15.2 | 57 | 20.2 |
| High | 167 | 34.0 | 170 | 33.8 | 146 | 20.1 | 153 | 26.0 | 136 | 17.9 | 141 | 22.3 |
| PTSD score:a | ||||||||||||
| Low | 93 | 28.4 | 87 | 28.2 | 81 | 16 | 81 | 21.2 | 80 | 16.5 | 77 | 17.9 |
| High | 141 | 34.0 | 148 | 34.0 | 125 | 20.8 | 132 | 26.5 | 117 | 17.4 | 121 | 24.0 |
| Illnesses: | ||||||||||||
| No chronic illness | 64 | 28.5 | 54 | 31.8 | 55 | 18.2 | 48 | 20.9 | 52 | 14.8 | 45 | 18.9 |
| Diabetes | 10 | 30.9 | 6 | 35.8 | 10 | 27.7 | 6 | 29.8 | 7 | 20.4 | 6 | 28.3 |
| Asthma | 11 | 35.3 | 17 | 32.6 | 9 | 13.2 | 13 | 21.0 | 10 | 17.3 | 13 | 22.3 |
| Arthritis | 19 | 27.1 | 19 | 28.9 | 15 | 20.2 | 17 | 22.2 | 14 | 18.7 | 16 | 23.5 |
| Heart disease | 5 | 40.8 | 4 | 40.5 | 5 | 29.4 | 4 | 35.5 | 5 | 25.6 | 3 | 25.7 |
| High blood pressure | 11 | 36.2 | 16 | 29.7 | 9 | 11.8 | 15 | 25.7 | 10 | 14.0 | 13 | 27.3 |
| Lung disease | 1 | 16.0 | 4 | 30.8 | 1 | 6.0 | 4 | 21.3 | 1 | 9.0 | 4 | 21.8 |
| More than one of the above | 35 | 36.7 | 44 | 32.8 | 31 | 20.9 | 41 | 28.8 | 30 | 20.2 | 38 | 25.8 |
| None of the above but other | 78 | 32.0 | 71 | 31.5 | 71 | 18.3 | 65 | 24.5 | 68 | 16.5 | 60 | 18.4 |
| Personality, cognition and psychological mindedness | ||||||||||||
| Dysfunctional attitudes score:a | ||||||||||||
| Low | 115 | 29.2 | 111 | 28.4 | 97 | 18.0 | 99 | 22.7 | 92 | 15.9 | 94 | 21.4 |
| High | 119 | 34.2 | 124 | 34.9 | 109 | 19.8 | 114 | 26.1 | 105 | 18.0 | 104 | 21.9 |
| Metacognitive awareness score:a | ||||||||||||
| Low | 104 | 33.0 | 105 | 34.2 | 96 | 18.2 | 92 | 26.2 | 87 | 15.7 | 84 | 24.0 |
| High | 130 | 30.7 | 130 | 29.9 | 110 | 19.6 | 121 | 23.2 | 110 | 18.0 | 114 | 19.9 |
| Neuroticism score:a | ||||||||||||
| Low | 115 | 28.6 | 102 | 27.8 | 99 | 17.8 | 93 | 21.0 | 95 | 15.7 | 89 | 19.9 |
| High | 119 | 34.8 | 133 | 34.9 | 107 | 20.0 | 120 | 27.6 | 102 | 18.3 | 109 | 23.1 |
| Moderator | b a | 95% CI |
|---|---|---|
| Demographic and life factors | ||
| Age (years) | ||
| < 30 | –2.4 | –8.1 to 3.3 |
| 30–39 | –0.5 | –7.0 to 6.0 |
| 40–49 | –5.0 | –8.9 to –1.1 |
| > 49 | –6.6 | –9.4 to –3.9 |
| Highest level of education | ||
| A-level/Higher Grade or above | –4.6 | –7.5 to –1.7 |
| Other qualifications: GCSE or equivalent | –3.3 | –7.7 to 1.1 |
| No formal qualifications | –7.5 | –12.0 to –3.0 |
| Marital status | ||
| Single | –2.5 | –7.3 to 2.4 |
| Married/living as married | –6.1 | –8.8 to –3.3 |
| Separated/divorced/widowed | –5.0 | –9.2 to –0.7 |
| Life events in the past 6 months | ||
| 0 | –5.0 | –9.0 to –1.0 |
| 1–2 | –5.7 | –8.4 to –3.1 |
| ≥ 3 | –6.9 | –12.2 to –1.5 |
| Illness characteristics | ||
| Baseline BDI severity | ||
| Less severe | –5.8 | –8.1 to –3.5 |
| Severe | –4.6 | –7.8 to –1.3 |
| Baseline CIS-R depression severityb | ||
| Low | –5.4 | –8.0 to –2.8 |
| High | –5.1 | –8.2 to –2.0 |
| No. of prior episodes of depression | ||
| 0–1 | –5.2 | –10.2 to –0.2 |
| 2–4 | –5.5 | –9.1 to –1.9 |
| ≥ 5 | –4.7 | –7.7 to –1.7 |
| Duration of current episode of depression (years) | ||
| < 1 | –6.1 | –10.2 to –2.0 |
| 1–2 | –3.5 | –8.4 to 1.4 |
| > 2 | –5.2 | –7.9 to –2.5 |
| Anxiety scorec | ||
| Low | –4.6 | –7.9 to –1.3 |
| High | –5.3 | –7.9 to –2.8 |
| PTSD scoreb | ||
| Low | –3.5 | –6.6 to –0.5 |
| High | –5.9 | –8.7 to –3.2 |
| Illnesses | ||
| No chronic illness | –2.4 | –6.8 to 2.0 |
| Diabetes | 1.5 | –8.9 to 11.9 |
| Asthma | –8.4 | –18.5 to 1.7 |
| Arthritis | –1.3 | –8.7 to 6.1 |
| Heart disease | –15.4 | –34.5 to 3.7 |
| High blood pressure | –10.1 | –22.8 to 2.5 |
| Lung disease | 0 | Omitted |
| More than one of the above | –9.6 | –15.0 to –4.2 |
| None of the above but other | –4.9 | –8.2 to –1.6 |
| Personality, cognition and psychological mindedness | ||
| Dysfunctional attitudes scoreb | ||
| Low | –5.6 | –8.4 to –2.8 |
| High | –4.7 | –7.7 to –1.7 |
| Metacognitive awareness scoreb | ||
| Low | –8.2 | –11.5 to –4.8 |
| High | –3.0 | –5.7 to –0.3 |
| Neuroticism scoreb | ||
| Low | –3.3 | –6.2 to –0.4 |
| High | –6.4 | –9.4 to –3.3 |
Discussion
Summary of main findings
This is the first study testing for moderators of response to CBT as a ‘next-step’ treatment for primary care patients who have not responded to antidepressants. Of the 14 variables assessed, age was the only variable with some statistical evidence for effect modification, with older patients benefiting the most from CBT. We found no evidence of effect modification by any other demographic, life, illness, personality trait or cognitive variable. Insufficient power prevents conclusive interpretation of such null findings. However, our findings suggest that it would be premature to adopt a stratified approach to prescribing CBT as a ‘next-step’ treatment for individuals who have not responded to antidepressants.
Strengths and limitations
The limitations associated with post hoc subgroup analyses should be borne in mind when interpreting our findings. 167 Although the sample size (n = 469) is one of the largest RCTs of CBT to date, it is small for testing interactions, creating uncertainty about the reliability of the estimates. In addition, multiple testing increases the likelihood of chance findings. However, we tested 14 different variables and found evidence for only one moderator. Given the move towards stratified medicine,165 it is important to discern for which patients CBT is likely to work and for what reasons. Pragmatically, CBT is often reserved for the patients who have not responded to antidepressants. Hence, identifying moderators of CBT response in this population is clearly clinically important, and ours is the first large-scale controlled study to examine effect modifiers of CBT offered as a ‘next-step’ treatment for non-responders to pharmacotherapy.
Demographic and life factors
Age was the only variable with evidence for effect modification, with older patients benefiting the most from CBT. There is no precedent for age as a moderator in previous studies of CBT in populations without TRD,168,172 so we treat our result with caution, and it may be a type I error. In contrast with RCTs of previously untreated episodes of depression, the mean age of patients in CoBalT was higher, with over half of the sample being ≥ 50 years when they entered the study. We would not expect this in itself to influence the findings in terms of the pattern of coefficients, especially given the good balance between the trial arms with respect to age. Yet it may have increased our power to detect this particular interaction compared with other studies with a younger age distribution. Alternatively, it may reflect something specific to the treatment-resistant population. CBT was most effective for patients over 40 years, and least effective in patients aged 30–39 years. It is unclear why CBT was not beneficial in this younger subgroup, but it is worth noting that given the small numbers (n = 61) the CIs around the estimate are wide, providing no evidence for either treatment benefit or harm. Further research is required to assess whether this finding is replicated.
In contrast with previous research,168,172,174 we did not identify marital status or stressful life events as moderators. The point estimates for marital status were consistent with single individuals gaining least from CBT, but there was no statistical evidence for effect modification (p = 0.34). The estimates for life events showed no evidence or even a suggestion of support for previous findings in non-TRD samples. 168,172
Illness characteristics and comorbidity
In contrast with previous studies,168,177–179 pre-treatment severity of depression did not moderate response to CBT. This may reflect the nature of our treatment-resistant sample; in CoBalT patients were selected for their non-response to antidepressants. In our previous study, mild depression seemed self-limiting, improving equally well irrespective of receiving CBT or waiting list control. By contrast, CBT was particularly effective for severe depression, which did not improve in the waiting list arm. By definition, the depression in patients recruited to CoBalT was not self-limiting as we selected patients through the resistance of their symptoms to pharmacotherapy. This may explain the absence of effect modification by severity in this group. It is of note, however, that baseline severity in CoBalT was similar to other RCTs of depression in the UK. 109,185,186 The CoBalT sample was nevertheless more ill in terms of chronicity, number of previous episodes, comorbidities and non-response to medication. This suggests that to capture the extent of illness that we see clinically then we need to account for both severity and chronicity, especially in those whose symptoms are resistant to antidepressants. However, an a priori subgroup analysis conducted in the main trial found no evidence that the degree of treatment-resistance modified response to CBT (see Chapter 3, Subgroup analyses).
Personality, cognitions and psychological mindedness
The literature on assessing individuals’ suitability for CBT is based more on clinical opinion164 than empirical evidence, and often focuses on interpersonal skills (which we were unable to investigate), personality and psychological mindedness. Consistent with previous research assessing personality traits in untreated episodes of depression,172,181 we found no evidence that neuroticism was a moderator of response to CBT in treatment-resistant individuals. Furthermore, dysfunctional attitudes and metacognitive awareness were not associated with differential response. The practice of selecting patients for CBT based on assessments of personality and psychological dysfunction and awareness remains empirically unsupported. Indeed, such practice might be harmful, preventing individuals, who might actually benefit, from receiving CBT.
Clinical implications
Cognitive behavioural therapy as an adjunct to usual care is an effective ‘next-step’ treatment for patients whose depression has not responded to treatment with antidepressants. This is an important finding, as, in practice, limited availability means that referral for CBT often only follows non-response to first-line treatment with antidepressants. However, to further improve patients outcomes by tailoring treatment in line with stratified medicine165 it is helpful to understand if there are any factors associated with differential treatment response. We found that response to CBT differed with age, with older age groups benefiting more. Given the small numbers of patients and wide CIs in this subgroup, we caution against using age to inform treatment decisions until further research replicates this effect. We found no evidence to suggest that non-response varied systematically with other patient characteristics. Until research replicates the age finding, and in the absence of other clear and reliable moderators, consideration should be given to offering CBT to all individuals where antidepressant medication has failed. Not all patients will respond, but, as we have only preliminary evidence as to whom these might be, consideration should be given to offering CBT to all patients in this severely ill group.
Conclusions
Cognitive behavioural therapy as an adjunct to usual care is an effective ‘next-step’ treatment for patients whose depression has not responded to treatment with antidepressants. To move from a stepped care towards a stratified approach requires evidence of reliable and informative moderators of CBT response. To date, the evidence does not support a stratified approach to prescribing CBT in depressed patients who have not responded to antidepressants, and we suggest, therefore, that consideration should be given to offering CBT to all patients in this group. Future studies to investigate moderators of clinical importance will require much larger sample sizes and this may need individual patient data meta-analyses.
Acknowledgements
We are grateful to all the patients, practitioners and GP surgery staff who took part in this research. We would like to thank the members of our TSC [Professor David Kingdon (chairperson), Professor Irwin Nazareth, Dr Sally Kerry and Mr Paul Lanham] and Data Monitoring Committee [Professor Frank Sullivan (chairperson), Dr Rafael Perera and Dr Tony Roth] for their valuable advice and support during the project. We acknowledge the additional support that has been provided by the MHRN, SMHRN, Primary Care Research Network (PCRN) and Scottish Primary Care Research Network (SPCRN).
Finally, we would also like to thank the following colleagues who have contributed to the CoBalT study, through recruitment and retention of patients, provision of administrative support, or delivery of therapy: Joy Farrimond, Mary Yarwood, Katrina Crook, Caroline Baker, Nathan Filer, Alex Burrage, Samantha Green, Meyrem Musa, Emma Riggs, Ros Fortune and Kate Chapman in Bristol; Rachel Winder, Caroline Jenkinson, Alice Garood, Holly Sugg, Miriam Cassell, Rob Kidney and Clare Bootle in Exeter; and Monica Cairns, Seonaid Cleare, Janice Reid, June Anderson, Jacqueline McTaggart, Katy Park and Eileen Riddoch in Glasgow. We would also like to acknowledge the contribution of Catriona Kent (CK) who was the main clinical supervisor for the Glasgow therapists. Finally, we would like to acknowledge the contribution of Professor Debbie Sharp, who was a co-applicant on the original grant application.
Contributions of authors
Nicola Wiles, John Campbell, Sandra Hollinghurst, Bill Jerrom, David Kessler, Willem Kuyken, Jill Morrison, Katrina Turner, Chris Williams, Tim Peters and Glyn Lewis were responsible for the original proposal, securing funding for the trial and drafting the original protocol.
Nicola Wiles, as chief investigator, had overall responsibility for the management of the study and the Bristol site, and, as co-investigators, John Campbell and Willem Kuyken had responsibility for the Exeter site, and Jill Morrison and Chris Williams for the Glasgow site.
All authors (with the exception of Nicholas Turner, Katherine Button, Lang’o Odondi and Chris Metcalfe) contributed to refinement of the trial protocol.
Anne Garland, Willem Kuyken and Glyn Lewis provided training and supervision for the trial therapists.
Laura Thomas, Anna Abel and Nicola Ridgway were responsible for data collection.
Nicola Wiles, Tim Peters and Glyn Lewis wrote the statistical analysis plan.
Laura Thomas and Nicholas Turner carried out data cleaning and analyses (under the supervision of Nicola Wiles).
Nicola Wiles conducted the main analyses of clinical effectiveness, with input from Tim Peters and Glyn Lewis.
Maria Barnes and Sofie Sherlock conducted the qualitative interviews and Maria Barnes analysed the qualitative data under the supervision of Amanda Owen-Smith and Katrina Turner.
Fran Carroll contributed to the collection of data for the economic evaluation and carried out data cleaning and analyses under the supervision of Sandra Hollinghurst.
Laura Thomas drafted the methods chapter and the chapter reporting the prevalence of TRD.
Maria Barnes and Katrina Turner drafted the qualitative chapter.
Sandra Hollinghurst drafted the chapter reporting the results of the economic evaluation.
Nicola Wiles drafted the introduction, main results and discussion chapters.
Lang’o Odondi conducted the analyses (under the supervision of Nicola Wiles and Chris Metcalfe) and drafted the chapter on mediators of CBT.
Nicholas Turner conducted the analyses and Katherine Button drafted, the chapter on moderators of the treatment effect.
All co-investigators and researchers have contributed to critically revising the final report.
Job titles and area(s) of specialty for all authors are listed below (in author order).
| Initials | Job title | Specialty |
|---|---|---|
| NW | Senior Lecturer in Epidemiology | RCT design, management and analysis |
| LT | Research Associate | RCT management |
| AA | Research Fellow | RCT management |
| MB | Research Associate | Qualitative methods |
| FC | Research Assistant | Health economics |
| NR | Research Assistant | RCT management |
| SS | Research Associate | Qualitative methods |
| NT | Research Assistant/NIHR pre-doctoral Research Methods Fellow | Statistics |
| KB | Research Associate | Psychology |
| LO | Research Associate | Statistics |
| CM | Reader in Medical Statistics | Statistics |
| AOS | Research Fellow | Qualitative methods |
| JC | Professor of General Practice and Primary Care | Primary care |
| AG | Nurse Consultant in Psychological Therapies | CBT and treatment of depression |
| SH | Senior Lecturer in Health Economics | Health economics |
| BJ | Director of Psychology | Psychological therapies; CBT |
| DK | Consultant Senior Lecturer | Primary care |
| WK | Professor of Clinical Psychology | RCT design, management and analysis; CBT |
| JM | Professor of General Practice | General practice |
| KT | Senior Lecturer in Primary Care | Qualitative methods |
| CW | Professor of Psychosocial Psychiatry | Psychiatry; CBT; RCT design, management and analysis |
| TP | Professor of Primary Care Health Services Research | RCT design, management and analysis; statistics |
| GL | Professor of Psychiatric Epidemiology | RCT design, management and analysis; psychiatry; CBT |
Ethical approval
Ethical approval was given by the West Midlands Research Ethics Committee (NRES/07/H1208/60) and research governance approval was obtained from the local PCTs/health boards.
Sponsorship and adoptions
The University of Bristol acted as sponsor for the study. The study was adopted by the MHRN, the SMHRN, the PCRN and the SPCRN.
Funding
The Department of Health and the Chief Scientists Office (through central subventions) and local PCTs and NHS Greater Glasgow and Clyde Health Board provided funds to cover the excess treatment associated with the trial. Service support costs were funded by the local PCTs in England. The SPCRN (West node) arranged payment of service support costs for the Glasgow practices.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
Publications
1. Barnes M, Wiles NJ, Morrison J, Kessler D, Williams C, Kuyken W, et al. Exploring patients’ reasons for declining contact in a cognitive–behavioural therapy RCT. Br J Gen Pract 2012;62:e371–7.
2. Thomas LJ, Abel A, Ridgway N, Peters TJ, Kessler D, Hollinghurst S, et al. Cognitive–behavioural therapy as an adjunct to pharmacotherapy for treatment resistant depression in primary care: the CoBalT randomised controlled trial protocol. Contemp Clin Trials 2012;33:312–19.
3. Wiles N, Thomas L, Abel A, Ridgway N, Turner N, Campbell J, et al. Cognitive–behavioural therapy as an adjunct to pharmacotherapy for primary care based patients with treatment resistant depression: results of the CoBalT randomised controlled trial. Lancet 2013;381:375–84.
4. Barnes M, Sherlock S, Thomas L, Kessler D, Kuyken W, Owen-Smith A, et al. No pain, no gain: patients’ experiences of cognitive–behavioural therapy. Br J Clin Psychol 2013;52:347–64. DOI: 10.1111/bjc
5. Barnes M, Sherlock S, Thomas L, Kessler D, Kuyken W, Owen-Smith A, et al. No pain, no gain: depressed clients’ experiences of cognitive behavioural therapy. Br J Clin Psychol 2013;52:347–64.
6. Hollinghurst S, Caroll FE, Abel A, Campbell J, Garland A, Jerrom B, et al. Cost-effectiveness of cognitive behavioural therapy as an adjunct to pharmacotherapy for treatment-resistant depression in primary care: economic evaluation of the CoBalt Trial. Br J Psych 2014;204:69–76.
References
- Mathers CD, Loncar D. Updated Projections of Global Mortality and Burden of Disease, 2002–2030: Data Sources, Methods and Results. Geneva: World Health Organization; 2005.
- Middleton N, Gunnell D, Whitley E, Dorling D, Frankel S. Secular trends in antidepressant prescribing in the UK, 1975–1998. J Public Health Med 2001;23:262-7.
- McManus P, Mant A, Mitchell PB, Montgomery WS, Marley J, Auland ME. Recent trends in the use of antidepressant drugs in Australia. Med J Aus 2000;173:458-61.
- Pincus HA, Tanielian TL, Marcus SC, Olfon M, Zarin DA, Thompson J, et al. Prescribing trends in psychotropic medications. JAMA 1998;279:526-31. http://dx.doi.org/10.1001/jama.279.7.526.
- Prescription Cost Analysis England 2011. London: Health and Social Care Information Centre; 2012.
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 2006;163:28-40. http://dx.doi.org/10.1176/appi.ajp.163.1.28.
- Keller MB, Berndt ER. Depression treatment: a lifelong commitment?. Psychopharmacol Bull 2002;36:133-41.
- Layard R. The case for psychological treatment centres. BMJ 2006;322:1030-2. http://dx.doi.org/10.1136/bmj.332.7548.1030.
- World Psychiatric Association . Symposium on therapy resistant depression. Pharmacopsychiatry 1974;7:69-74.
- Thase ME, Rush AJ. When at first you don’t succeed: sequential strategies for antidepressant nonresponders. J Clin Psychiatry 1997;58:23-9.
- Fekadu A, Wooderson S, Donaldson C, Markopoulou K, Masterson B, Poon L, et al. A multidimensional tool to quantify treatment resistance in depression: the Maudsley staging method. J Clin Psychiatry 2009;70:177-84. http://dx.doi.org/10.4088/JCP.08m04309.
- Clinical Guideline 23. Depression: Management of Depression in Primary and Secondary Care. London: NICE; 2004.
- Depression: The Treatment and Management of Depression in Adults (Update). National Clinical Practice Guideline 90. London: DH; 2009.
- Stimpson N, Agrawal N, Lewis G. Randomised controlled trials investigating pharmacological and psychological interventions for treatment-refractory depression. Systematic review. Br J Psychiatry 2002;181:284-94. http://dx.doi.org/10.1192/bjp.181.4.284.
- Wiles NJ, Hollinghurst S, Mason V, Musa M, Burt V, Hyde J, et al. A randomised controlled trial of cognitive–behavioural therapy as an adjunct to pharmacotherapy in primary care based patients with treatment resistant depression: a pilot study. Behav Cogn Psychother 2008;36:21-33.
- Kennedy SH, Segal ZV, Cohen NL, Levitan RD, Gemar M, Bagby RM. Lithium carbonate versus cognitive therapy as sequential combination treatment strategies in partial responders to antidepressant medication: an exploratory trial. J Clin Psychiatry 2003;64:439-44. http://dx.doi.org/10.4088/JCP.v64n0414.
- Strauss WH. Combined cognitive-behavioral and pharmacotherapy in refractory depression 2002.
- Kocsis JH, Gelenberg AJ, Rothbaum BO, Klein DN, Trivedi MH, Manber R, et al. Cognitive behavioral analysis system of psychotherapy and brief supportive psychotherapy for augmentation of antidepressant nonresponse in chronic depression: the REVAMP Trial. Arch Gen Psychiatry 2009;66:1178-88. http://dx.doi.org/10.1001/archgenpsychiatry.2009.144.
- Rush AJ, Trivedi MH, Wisniewski SR, Stewart JW, Nierenberg AA, Thase ME, et al. Bupropion-SR, sertraline or venlafaxine-XR after failure of SSRIs for depression. N Engl J Med 2006;354:1231-42. http://dx.doi.org/10.1056/NEJMoa052963.
- Trivedi MH, Fava M, Wisniewski SR, Thase ME, Quitkin FM, Warden D, et al. Medication augmentation after the failure of SSRIs for depression. N Engl J Med 2006;354:1243-52. http://dx.doi.org/10.1056/NEJMoa052964.
- Thase ME, Friedman ES, Biggs MM, Wisniewski SR, Trivedi MH, Luther JF, et al. Cognitive therapy versus medication in augmentation and switch strategies as second-step treatments: a STAR*D report. Am J Psychiatry 2007;164:739-52. http://dx.doi.org/10.1176/appi.ajp.164.5.739.
- Dwight-Johnson M, Sherbourne CD, Liao D, Wells KB. Treatment preferences among depressed primary care patients. J Gen Intern Med 2000;15:527-34. http://dx.doi.org/10.1046/j.1525-1497.2000.08035.x.
- Scott J, Palmer S, Paykel ES, Teasdale JD, Hayhurst H. Use of cognitive therapy for relapse prevention in chronic depression: cost-effectiveness study. Br J Psychiatry 2003;182:221-7. http://dx.doi.org/10.1192/bjp.182.3.221.
- Hollinghurst S, Peters TJ, Kaur S, Wiles N, Lewis G. Cost-effectiveness of therapist-delivered online cognitive-behavioural therapy for depression: randomised controlled trial. Br J Psychiatry 2010;197:297-304. http://dx.doi.org/10.1192/bjp.bp.109.073080.
- Durham RC, Chambers JA, Power KG, Sharp DM, Macdonald RR, Major KA, et al. Long-term outcome of cognitive behaviour therapy clinical trials in central Scotland. Health Technol Assess 2005;9.
- Morriss R, Marttunnen S, Garland A, Nixon N, McDonald R, Sweeney T, et al. Randomised controlled trial of the clinical and cost effectiveness of a specialist team for managing refractory unipolar depressive illness. BMC Psychiatry 2010;10. www.biomedcentral.com/1471–244X/10/100 (accessed 1 February 2013).
- No Health without Mental Health. A Cross-government Mental Health Outcomes Strategy for People of All Ages. London: DH; 2011.
- Talking Therapies: A Four-year Plan of Action. London: DH; 2011.
- Pirkis J, Livingston J, Herrman H, Schweitzer I, Gill L, Morley B, et al. Improving collaboration between private psychiatrists, the public mental health sector and general practitioners: evaluation of the Partnership Project. Aust N Z J Psychiatry 2004;38:125-34. http://dx.doi.org/10.1111/j.1440-1614.2004.01314.x.
- Payne KA, Myhr G. Increasing access to cognitive–behavioural therapy (CBT) for the treatment of mental illness in Canada: a research framework and call for action. Health Policy 2010;5:e174-85.
- Churchill R, Hunot V, Corney R, Knapp M, McGuire H, Tylee A, et al. A systematic review of controlled trials of the effectiveness and cost-effectiveness of brief psychological treatments for depression. Health Technol Assess 2001;5.
- Clark DM, Layard R, Smithies R, Richards DA, Suckling R, Wright B. Improving access to psychological therapy: initial evaluation of two UK demonstration sites. Behav Res Ther 2009;47:910-20. http://dx.doi.org/10.1016/j.brat.2009.07.010.
- Glover G, Webb M, Evison F. Improving Access to Psychological Therapies. A Review of the Progress Made by Sites in the First Rollout Year. Durham: North East Public Health Observatory; 2010.
- Paykel ES, Scott J, Teasdale JD, Johnson AL, Garland A, Moore R, et al. Prevention of relapse in residual depression by cognitive therapy: a controlled trial. Arch Gen Psychiatry 1999;56:829-35. http://dx.doi.org/10.1001/archpsyc.56.9.829.
- Paykel ES, Scott J, Cornwall PL, Abbott R, Crane C, Pope M, et al. Duration of relapse prevention after cognitive therapy in residual depression: follow-up of controlled trial. Psychol Med 2005;35:59-68. http://dx.doi.org/10.1017/S003329170400282X.
- Pampallona S, Bollini P, Tibaldi G, Kupelnick B, Munizza C. Combined pharmacotherapy and psychological treatment for depression. Arch Gen Psychiatry 2004;61:714-19. http://dx.doi.org/10.1001/archpsyc.61.7.714.
- Friedman MA, Detweiler-Bedell JB, Leventhal HE, Horne R, Keitner GI, Miller IW. Combined psychotherapy and pharmacotherapy for the treatment of major depressive disorder. Clin Psychol Sci Prac 2004;11:47-68. http://dx.doi.org/10.1093/clipsy.bph052.
- Keller MB, McCullough JP, Klein DN, Arnow B, Dunner DL, Gelenberg AJ, et al. A comparison of nefazodone, the cognitive behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression. N Engl J Med 2000;342:1462-70. http://dx.doi.org/10.1056/NEJM200005183422001.
- Wiezbicki M, Pekarik G. A meta-analysis of psychotherapy dropout. Prof Psychol Res Prac 1993;24:190-5.
- Laberg S, Tornkvist A, Andersson G. Experiences of patients in cognitive–behavioural group therapy: a qualitative study of eating disorders. Scand J Behav Ther 2001;30:161-78.
- Messari S, Hallam R. CBT for psychosis: a qualitative analysis of clients’ experiences. Br J Clin Psychol 2003;42:171-88.
- Donovan J, Mills N, Smith M, Brindle L, Jacoby A, Peters T, et al. Quality improvement report: Improving design and conduct of randomised trials by embedding them in qualitative research: ProtecT (prostate testing for cancer and treatment) study. BMJ 2002;325:766-70. http://dx.doi.org/10.1136/bmj.325.7367.766.
- Oakley A, Strange V, Bonell C, Allen E, Stephenson J. RIPPLE Study Team . Process evaluation in randomised controlled trials of complex interventions. BMJ 2006;332:413-16. http://dx.doi.org/10.1136/bmj.332.7538.413.
- Clinical Trials for Tomorrow: An MRC Review of Randomised Controlled Trials. London: MRC; 2003.
- Thomas LJ, Abel A, Ridgway N, Peters T, Kessler D, Hollinghurst S, et al. Cognitive–behavioural therapy as an adjunct to pharmacotherapy for treatment resistant depression in primary care: the CoBalT randomised controlled trial protocol. Control Clin Trials 2012;33:312-19.
- Beck A, Steer RA, Brown GK. Beck Depression Inventory. San Antonio, TX: The Psychological Corporation; 1996.
- Lewis G, Pelosi AJ, Araya R, Dunn G. Measuring psychiatric disorder in the community: a standardized assessment for use by lay interviewers. Psychol Med 1992;22:465-86. http://dx.doi.org/10.1017/S0033291700030415.
- Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care 1986;24:67-74. http://dx.doi.org/10.1097/00005650-198601000-00007.
- George CF, Peveler RC, Heiliger S, Thompson C. Compliance with tricyclic antidepressants: the value of four different methods of assessment. Br J Clin Pharmaco 2000;50:166-71. http://dx.doi.org/10.1046/j.1365-2125.2000.00244.x.
- Piccinelli M, Tessari E, Bortolomasi M, Piasere O, Semenzin M, Garzotto N, et al. Efficacy of the alcohol use disorders identification test as a screening tool for hazardous alcohol intake and related disorders in primary care: a validity study. BMJ 1997;314:420-4. http://dx.doi.org/10.1136/bmj.314.7078.420.
- Prins A, Ouimette P, Kimerling R, Cameron RP, Hugelshofer DS, Shaw-Hegwer J, et al. The primary care PTSD screen (PC-PTSD): development and operating characteristics. Prim Care Psychiatry 2003;9:9-14.
- John OP, Donahue EM, Kentle RL. The ‘Big Five’ Inventory. Berkeley, CA: University of California, Berkeley, Institute of Personality and Social Research; 1991.
- Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13. http://dx.doi.org/10.1046/j.1525-1497.2001.016009606.x.
- Spitzer RL, Kroenke K, Williams JBW, Lowe B. A brief measure for assessing generalized anxiety disorder. The GAD-7. Arch Intern Med 2006;166:1092-7. http://dx.doi.org/10.1001/archinte.166.10.1092.
- Spitzer RL, Kroenke K, Williams JBW. Patient Health Questionnaire Primary Care Study Group . Validation and utility of a self-report version of PRIME-MD. The PHQ primary care study. JAMA 1999;282:1737-44. http://dx.doi.org/10.1001/jama.282.18.1737.
- Ware J-EJ, Kosinski M, Keller SD. A 12-item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. http://dx.doi.org/10.1097/00005650-199603000-00003.
- The EuroQol Group . EuroQol: a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208.
- Beevers CG, Strong DR, Meyer B, Pilkonis PA. Efficiently assessing negative cognition in depression: an item response theory analysis of the dysfunctional attitude scale. Psychol Assess 2007;19:199-20. http://dx.doi.org/10.1037/1040-3590.19.2.199.
- Teasdale JD, Scott J, Moore RG, Hayhurst H, Pope M, Paykel ES. How does cognitive therapy prevent relapse in residual depression? Evidence from a controlled trial. J Consult Clin Psychol 2001;69:347-57. http://dx.doi.org/10.1037//0022-006X.69.3.347.
- Altman DG, Schulz KF, Moher D, Egger M, Davidoff F, Elbourne D, et al. The revised CONSORT statement for reporting randomized trials: explanation and elaboration. Ann Intern Med 2001;134:663-94. http://dx.doi.org/10.7326/0003-4819-134-8-200104170-00012.
- Brown S, Thorpe H, Hawkins K, Brown J. Minimization – reducing predictability for multi-centre trials whilst retaining balance within centre. Stat Med 2005;24:3715-27. http://dx.doi.org/10.1002/sim.2391.
- Beck A, Rush AJ, Shaw B, Emery G. Cognitive Therapy of Depression. New York, NY: Wiley; 1979.
- Beck J. Cognitive Therapy: Basics and Beyond. New York, NY: Guilford Press; 1995.
- Moore RG, Garland A. Cognitive Therapy for Chronic and Persistent Depression. Chichester: John Wiley & Sons; 2003.
- Blackburn I-M, James IA, Milne DL, Baker C, Standart S, Garland A, et al. The revised cognitive therapy scale (CTS-R): psychometric properties. Behav Cogn Psychother 2001;29:431-46. http://dx.doi.org/10.1017/S1352465801004040.
- Shrout PE. Measurement reliability and agreement in psychiatry. Stat Methods Med Res 1998;7:301-17. http://dx.doi.org/10.1191/096228098672090967.
- Rush AJ, Kraemer HC, Sackeim HA, Fava M, Trivedi MH, Frank E, et al. Report by the ACNP Task Force on response and remission in major depressive disorder. Neuropsychopharmacology 2006;31:1841-53. http://dx.doi.org/10.1038/sj.npp.1301131.
- Weissman A. Dysfunctional Attitudes Scale: A Validation Study. Philadelphia, PA: University of Pennsylvania; 1979.
- Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P. CONSORT group . Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med 2008;148:295-309. http://dx.doi.org/10.7326/0003-4819-148-4-200802190-00008.
- Zwarenstein M, Treweek S, Gagnier JJ, Altman DG, Tunis S, Haynes B, et al. Improving the reporting of pragmatic trials: an extension of the CONSORT statement. BMJ 2008;337. http://dx.doi.org/10.1136/bmj.a2390.
- Roberts C, Roberts SA. Design and analysis of clinical trials with clustering effects due to treatment. Clin Trials 2005;2:152-62. http://dx.doi.org/10.1191/1740774505cn076oa.
- van Buuren S, Boshuizen HC, Knook DL. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med 1999;18:681-94. http://dx.doi.org/10.1002/(SICI)1097-0258(19990330)18:6%3C681::AID-SIM71%3E3.3.CO;2-I.
- Dunn G, Maracy M, Tomenson B. Estimating treatment effects from randomized clinical trials with noncompliance and loss to follow-up: the role of instrumental variable methods. Stat Methods Med Res 2005;14:369-95. http://dx.doi.org/10.1191/0962280205sm403oa.
- Nutt DJ, Sharpe M. Uncritical positive regard? Issues in the efficacy and safety of psychotherapy. J Psychopharmacol 2008;22:3-6. http://dx.doi.org/10.1177/0269881107086283.
- National Institute for Health and Clinical Excellence . Guide to the Methods of Technology Appraisal 2008. www.nice.org.uk/media/B52/A7/TAMethodsGuideUpdatedJune2008.pdf. (accessed February 2013).
- Brooks R. EuroQol Group . Euroqol: the current state of play. Health Policy 1996;37:53-72. http://dx.doi.org/10.1016/0168-8510(96)00822-6.
- Dolan P, Gudex C, Kind P, Williams A. A Social Tariff for EuroQol: Results from a UK General Population Survey. York: University of York; 1995.
- Manca A, Hawkins N, Sculpher M. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. http://dx.doi.org/10.1002/hec.944.
- Curtis L. Unit Costs of Health and Social Care 2010. Canterbury: PSSRU, University of Kent; 2011.
- NHS Agenda for Change Pay Scales – 2010/2011. Royal College of Nursing; 2011.
- Salisbury C, Chalder M, Manku-Scott T, Nicholas R, Deave T, Noble S. The National Evaluation of NHS Walk-in Centres. Bristol: University of Bristol; 2002.
- Munro J, Nicholl J, O’Cathain A, Knowles E, Morgan A. Evaluation of NHS Direct First Wave Sites: Final Report of the Phase 1 Research. Sheffield: University of Sheffield; 2001.
- British National Formulary. London: BMA and RPS; 2010.
- National Health Service . NHS Drug Tariff 2012. www.nhsbsa.nhs.uk/PrescriptionServices/924.aspx (accessed January 2012).
- 2010–11 Reference Costs. London: DH; 2011.
- The Automobile Association . The AA Schedule of Motoring Costs 2005. www.theaa.com/motoring_advice/running_costs/index.html (accessed January 2012).
- National Health Service . Help With Health Costs 2012. www.nhs.uk/NHSEngland/Healthcosts/Pages/Prescriptioncosts.aspx (accessed January 2012).
- Annual Survey of Hours and Earnings. London: ONS; 2011.
- University of Sheffield . SF-6D. n.d. www.shef.ac.uk/scharr/sections/heds/mvh/sf-6d (accessed September 2012).
- Ritchie J, Spencer L, Bryman A, Burgess RG. Analysing Qualitative Data. London: Routledge; 1994.
- Prins MA, Verhaak PF, van der Meer K, Penninx BW, Bensing JM. Primary care patients with anxiety and depression: need for care from the patient’s perspective. J Affect Disord 2009;119:163-71. http://dx.doi.org/10.1016/j.jad.2009.03.019.
- Dwight-Johnson M, Meredith LS, Hickey SC, Wells KB. Influence of patient preference and primary care proclivity for watchful waiting on receipt of depression treatment. Gen Hosp Psychiatry 2006;28:379-86.
- Brody DS, Khaliq AA, Thompson TL. Patients’s perspectives on the management of emotional distress in primary care settings. J Gen Intern Med 1997;12:403-6. http://dx.doi.org/10.1046/j.1525-1497.1997.00070.x.
- Hind D, O’Caithin A, Cooper CL, Parry GD, Isaac CL, Rose A, et al. The acceptability of computerised cognitive–behavioural therapy for the treatment of depression in people with chronic physical disease: a qualitative study of people with multiple sclerosis. Psychol Health 2010;25:699-712.
- Cramer H, Salisbury C, Conrad J, Eldred J, Araya R. Group cognitive–behavioural therapy for women with depression: pilot and feasibility study for a randomised controlled trial using mixed methods. BMC Psychiatry 2011;11. http://dx.doi.org/10.1186/1471-244X-11-82.
- Dunn H, Morrison A, Bentall R. Patients’ experiences of homework tasks in cognitive–behavioural therapy for psychosis: a qualitative analysis. Clin Psychol Psychother 2002;9:361-9. http://dx.doi.org/10.1002/cpp.344.
- Glasman D, Finlay W, Brock D. Becoming a self-therapist: using cognitive–behavioural therapy for recurrent depression and/or dysthymia after completing therapy. Psychol Psychother Theory Res Prac 2004;77:335-51.
- Nilsson T, Svensson M, Sandel R, Clinton D. Patients’ experiences of change in cognitive–behavioural therapy and psychodynamic therapy: a qualitative comparative study. Psychother Res 2007;17:553-66. http://dx.doi.org/10.1080/10503300601139988.
- Flynn H. Setting the stage for the integration of motivational interviewing with cognitive–behavioural therapy in the treatment of depression. Cogn Behav Prac 2011;18:46-54. http://dx.doi.org/10.1016/j.cbpra.2009.09.006.
- Helbig S, Fehm L. Problems with homework in CBT: rare exception or rather frequent?. Behav Cogn Psychother 2004;32:291-30. http://dx.doi.org/10.1017/S1352465804001365.
- Knudsen P, Hansen EH, Eskildsen K. Leading ordinary lives: a qualitative study of younger women’s perceived functions of antidepressants. Pharm World Sci 2003;25:162-7.
- Leydon GM, Rodgers L, Kendrick T. A qualitative study of patient views on discontinuing long-term selective serotonin reuptake inhibitors. Fam Pract 2007;24:570-5. http://dx.doi.org/10.1093/fampra/cmm069.
- Knudsen P, Hansen EH, Traulsen JM, Eskildsen K. Changes in self-concept while using SSRI antidepressants. Qual Health Res 2002;12:932-43. http://dx.doi.org/10.1177/104973202129120368.
- Verfeek-Heida PM, Mathot EF. Better safe than sorry: why patients prefer to stop using selective serotonin reuptake inhibitor (SSRI) antidepressants but are afraid to do so: results of a qualitative study. Chron Illness 2006;2:133-42. http://dx.doi.org/10.1179/174592006X111003.
- Johnson CF, Macdonald HJ, Atkinson P, Buchanan AL, Downes N, Dougall N. Reviewing long-term antidepressants can reduce drug burden: a prospective observational cohort study. Br J Gen Pract 2012;62:e773-9. http://dx.doi.org/10.3399/bjgp12X658304.
- Kadam UT, Croft P, McLeod J, Hutchinson M. A qualitative study of patients’ views on anxiety and depression. Br J Gen Pract 2001;51:375-80.
- Bower PJ, Rowland N. Effectiveness and cost effectiveness of counselling in primary care. Cochrane Database Syst Rev 2006;3. http://dx.doi.org/10.1002/14651858.CD001025.pub2.
- Fournier JC, DeRubeis RJ, Shelton RC, Gallop R, Amsterdam JD, Hollon SD. Antidepressant medications v. cognitive therapy in people with depression with or without personality disorder. Br J Psychiatry 2008;192:124-9. http://dx.doi.org/10.1192/bjp.bp.107.037234.
- Kessler D, Lewis G, Kaur S, Wiles N, King M, Weich S, et al. Therapist-delivered internet psychotherapy for depression in primary care: a randomised controlled trial. Lancet 2009;374:628-34. http://dx.doi.org/10.1016/S0140-6736(09)61257-5.
- Improving Access to Psychological Therapies. Implementation Plan: National Guidelines for Regional Delivery. London: DH; 2008.
- Hollon SD, DeRubeis RJ, Shelton RC, Amsterdam JD, Salomon RM, O’Reardon JP, et al. Prevention of relapse following cognitive therapy vs medications in moderate to severe depression. Arch Gen Psychiatry 2005;62:417-22. http://dx.doi.org/10.1001/archpsyc.62.4.417.
- DeRubeis RJ, Hollon SD, Amsterdam JD, Shelton RC, Young PR, Salomon RM, et al. Cognitive therapy vs medications in the treatment of moderate to severe depression. Arch Gen Psychiatry 2005;62:409-16. http://dx.doi.org/10.1001/archpsyc.62.4.409.
- Miller IW, Norman WH, Keitner GI. Cognitive–behavioural treatment of depressed inpatients: six- and twelve-month follow-up. Am J Psychiatry 1989;146:1274-9.
- Shapiro DA, Barkham M, Rees A, Hardy GE, Reynolds S, Startup M. Effects of treatment duration and severity of depression on the effectiveness of cognitive–behavioral and psychodynamic–interpersonal psychotherapy. J Consult Clin Psychol 1994;62:522-34. http://dx.doi.org/10.1037//0022-006X.62.3.522.
- Kovacs M, Rush AJ, Beck AT, Hollon SD. Depressed outpatients treated with cognitive therapy or pharmacotherapy. Arch Gen Psychiatry 1981;38:33-9. http://dx.doi.org/10.1001/archpsyc.1981.01780260035003.
- Simons AD, Murphy GE, Levine JL, Wetzel RD. Cognitive therapy and pharmacotherapy for depression. Arch Gen Psychiatry 1986;43:43-8. http://dx.doi.org/10.1001/archpsyc.1986.01800010045006.
- Fava GA, Rafanelli C, Grandi S, Canestrari R, Morphy MA. Six-year outcome for cognitive behavioral treatment of residual symptoms in major depression. Am J Psychiatry 1998;155:1443-5.
- Mohr DC, Ho J, Duffecy J, Reifler D, Sokol L, Burns MN, et al. Effect of telephone-administered vs face-to-face cognitive behavioral therapy on adherence to therapy and depression outcomes among primary care patients. A randomized trial. JAMA 2012;307:2278-85. http://dx.doi.org/10.1001/jama.2012.5588.
- Practice Guideline for the Treatment of Patients with Major Depressive Disorder. Virginia: APA; 2010.
- Anderson IM, Ferrier IN, Baldwin RC, Cowen PJ, Howard L, Lewis G, et al. Evidence-Based Guidelines for Treating Depressive Disorders with Antidepressants: a Revision of the 2000 British Association for Psychopharmacology Guidelines. J Psychopharmacol 2008;22:343-96. http://dx.doi.org/10.1177/0269881107088441.
- Carvalho AF, Cavalcante JL, Castelo MS, Lima MCO. Augmentation strategies for treatment-resistant depression: a literature review. J Clin Pharm Ther 2007;32:415-28. http://dx.doi.org/10.1111/j.1365-2710.2007.00846.x.
- Shelton RC, Papakostas GI. Augmentation of antidepressants with atypical antipsychotics for treatment-resistant major depressive disorder. Acta Psychiatr Scand 2008;117:253-9. http://dx.doi.org/10.1111/j.1600-0447.2007.01130.x.
- Cooper C, Katona C, Lyketsos K, Blazer D, Brodaty H, Rabins P, et al. A systematic review of treatments for refractory depression in older people. Am J Psychiatry 2011;168:681-8. http://dx.doi.org/10.1176/appi.ajp.2011.10081165.
- Singleton N, Bumpstead R, O’Brien M, Lee A, Meltzer H. Psychiatric Morbidity Among Adults Living in Private Households, 2000. London: The Stationery Office; 2001.
- Crown WH, Finkelstein S, Berndt ER, Ling D, Poret AW, Rush AJ, et al. The impact of treatment-resistant depression on health care utilization and costs. J Clin Psychiatry 2002;63:963-71. http://dx.doi.org/10.4088/JCP.v63n1102.
- Ivanova JI, Birnbaum HG, Kidolezi Y, Subramanian G, Khan SA, Stensland MD. Direct and indirect costs of employees with treatment-resistant and non-treatment resistant major depressive disorder. Curr Med Res Opin 2010;26:2475-84. http://dx.doi.org/10.1185/03007995.2010.517716.
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006;163:1905-17. http://dx.doi.org/10.1176/appi.ajp.163.11.1905.
- Souery DA, Oswald P, Massat I, Bailer U, Bollen J, Demyttenaere K, et al. Clinical factors associated with treatment resistance in major depressive disorder: results from a European Multicenter study. J Clin Psychiatry 2007;68:1062-70. http://dx.doi.org/10.4088/JCP.v68n0713.
- Berlim MT, Turecki G. What is the meaning of treatment resistant/refractory major depression (TRD)? A systematic review of current randomized trials. Eur Neuropsychopharmacol 2007;17:696-707. http://dx.doi.org/10.1016/j.euroneuro.2007.03.009.
- DiMatteo M, Lepper HS, Croghan TW. Depression is a risk factor for noncompliance with medical treatment: Meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med 2000;160:2101-7. http://dx.doi.org/10.1001/archinte.160.14.2101.
- Carter S, Taylor D, Levenson R. A Question of Choice: Compliance in Medicine Taking: a Preliminary Review. London: Medicines Partnership; 2005.
- Connolly KR, Thase ME. If at first you don’t succeed: a review of the evidence for antidepressant augmentation, combination and switching strategies. Drugs 2011;71:43-64.
- McPherson S, Cairns P, Carlyle J, Shapiro DA, Richardson P, Taylor D. The effectiveness of psychological treatments for treatment-resistant depression: a systematic review. Acta Psychiatr Scand 2005;111:331-40. http://dx.doi.org/10.1111/j.1600-0447.2004.00498.x.
- Quality and Outcomes Framework for 2012/13. Guidance for PCOs and Practices. London: NHS Employers; 2012.
- Trivedi MH. Tools and strategies for ongoing assessment of depression: a measurement-based approach to remission. J Clin Psychiatry 2009;70:26-31. http://dx.doi.org/10.4088/JCP.8133su1c.04.
- Teasdale JD. Assessing cognitive mediation of relapse prevention in recurrent mood disorders. Clin Psychol Psychother 1997;4:145-56. http://dx.doi.org/10.1002/(SICI)1099-0879(199709)4:3%3C145::AID-CPP130%3E3.0.CO;2-Z.
- Teasdale JD, Segal ZV, Williams JMG. How does cognitive therapy prevent depressive relapse and why should attentional control (mindfulness) training help?. Behav Res Ther 1995;33:25-39. http://dx.doi.org/10.1016/0005-7967(94)E0011-7.
- Moore RG. It’s the thought that counts: the role of intentions and meta-awareness in cognitive therapy. J Cogn Psychother 1996;10:255-69.
- Kraemer HC, Wilson T, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry 2002;59:877-83. http://dx.doi.org/10.1001/archpsyc.59.10.877.
- Garratt G, Ingram RE, Rand KL, Sawalani G. Cognitive processes in cognitive therapy: evaluation of the mechanisms of change in the treatment of depression. Clin Psychol 2007;14:224-39. http://dx.doi.org/10.1111/j.1468-2850.2007.00081.x.
- Longmore RJ, Worrell M. Do we need to challenge thoughts in cognitive behavior therapy?. Clin Psychol Rev 2007;27:173-87. http://dx.doi.org/10.1016/j.cpr.2006.08.001.
- Harmer CJ, Goodwin GM, Cowen PJ. Why do antidepressants take so long to work? A cognitive neuropsychological model of antidepressant drug action. Br J Psychiatry 2009;195:102-8. http://dx.doi.org/10.1192/bjp.bp.108.051193.
- Hollon SD, DeRubeis RJ, Leahy RL. Contemporary Cognitive Therapy: Theory, Research and Practice. New York, NY: Guilford Press; 2004.
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic and statistical considerations. J Pers Soc Psychol 1986;51:1173-82. http://dx.doi.org/10.1037//0022-3514.51.6.1173.
- Wu AD, Zumbo BD. Understanding and using mediators and moderators. Soc Indicat Res 2008;87:367-92. http://dx.doi.org/10.1007/s11205-007-9143-1.
- Kazdin AE. Mediators and mechanisms of change in psychotherapy research. Annu Rev Clin Psychol 2007;3:1-27. http://dx.doi.org/10.1146/annurev.clinpsy.3.022806.091432.
- Stice E, Rohde P, Seeley JR, Gau JM. Testing mediators of intervention effects in randomized controlled trials: an evaluation of three depression prevention programs. J Consult Clin Psychol 2010;78:273-80. http://dx.doi.org/10.1037/a0018396.
- Imai K, Keele L, Tingley D. A general approach to causal mediation analysis. Psychol Methods 2010;15:309-34. http://dx.doi.org/10.1037/a0020761.
- Imai K, Keele L, Yamamoto T. Identification, inference and sensitivity analysis for causal mediation effects. Stat Sci 2010;25:51-7. http://dx.doi.org/10.1214/10-STS321.
- Emsley R, Dunn G, White IR. Mediation and moderation of treatment effects in randomised controlled trials of complex interventions. Stat Methods Med Res 2009. http://dx.doi.org/10.1177/0962280209105014.
- Bullock JG, Green DP, Ha SE. Yes, but what’s the mechanism? (Don’t expect an easy answer). J Pers Soc Psychol 2010;98:550-8.
- MacKinnon DP, Dwyer JH. Estimating mediated effects in prevention studies. Eval Rev 1993;17:144-58. http://dx.doi.org/10.1177/0193841X9301700202.
- Little RJA, Rubin DB. Causal effects in clinical and epidemiological studies via potential outcomes: concepts and analytical approaches. Annu Rev Public Health 2000;21:121-45. http://dx.doi.org/10.1146/annurev.publhealth.21.1.121.
- Holland PW. Statistics and causal inference. J Am Stat Assoc 1986;81:945-70. http://dx.doi.org/10.2307/2289064.
- Imai K, Keele L, Tingley D, Yamamoto T. Unpacking the black box of causality: learning about causal mechanisms from experimental and observational studies. Am Political Sci Rev 2011;105:765-89. http://dx.doi.org/10.1017/S0003055411000414.
- Hicks R, Tingley D. Causal mediation analysis. Stata J 2011;11:1-15.
- MacKinnon DP, Fairchild AJ, Fritz MS. Mediation analysis. Annu Rev Psychol 2007;58:593-614. http://dx.doi.org/10.1146/annurev.psych.58.110405.085542.
- Whisman MA. Mediators and moderators of change in cognitive therapy of depression. Psychol Bull 1993;114:248-65. http://dx.doi.org/10.1037//0033-2909.114.2.248.
- Dunn G, Bentall RP. Modelling treatment-effect heterogeneity in randomized controlled trials of complex interventions (psychological treatments). Stat Med 2007;26:4719-45. http://dx.doi.org/10.1002/sim.2891.
- Watson D, Clark LA. Negative affectivity: the disposition to experience aversive emotional states. Psychol Bull 1984;96:465-190. http://dx.doi.org/10.1037//0033-2909.96.3.465.
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLOS Med 2006;3:2011-31. http://dx.doi.org/10.1371/journal.pmed.0030442.
- Butler AC, Chapman JE, Forman EM, Beck AT. The empirical status of cognitive-behavioral therapy: a review of meta-analyses. Clin Psychol Rev 2006;26:17-31. http://dx.doi.org/10.1016/j.cpr.2005.07.003.
- Cuijpers P, van Straten A, Andersson G, van Oppen P. Psychotherapy for depression in adults: a meta-analysis of comparative outcome studies. J Consult Clin Psychol 2008;76:909-22. http://dx.doi.org/10.1037/a0013075.
- Safran JD, Segal ZV, Safran JD, Segal ZV. Interpersonal Process in Cognitive Therapy. New York, NY: Basic Books; 1990.
- Hingorani AD, Windt DA, Riley RD, Abrams K, Moons KG, Steyerberg EW, et al. Prognosis research strategy (PROGRESS) 4: Stratified medicine research. BMJ 2013;346. http://dx.doi.org/10.1136/bmj.e5793.
- Trusheim MR, Berndt ER, Douglas FL. Stratified medicine: strategic and economic implications of combining drugs and clinical biomarkers. Nat Rev Drug Discov 2007;6:287-93. http://dx.doi.org/10.1038/nrd2251.
- Brookes ST, Whitely E, Egger M, Davey Smith G, Mulheran P, Peters TJ. Subgroup analyses in randomized trials: risks of subgroup-specific analyses; power and sample size for the interaction test. J Clin Epidemiol 2004;57:229-36.
- Button KS, Wiles NJ, Lewis G, Peters TJ, Kessler D. Factors associated with differential response to online cognitive–behavioural therapy. Soc Psychiatry Psychiatric Epidemiol 2012;47:827-33.
- Fournier JC, DeRubeis RJ, Hollon SD, Dimidjian S, Amsterdam JD, Shelton RC, et al. Antidepressant drug effects and depression severity: a patient-level meta-analysis. JAMA 2010;303:47-53. http://dx.doi.org/10.1001/jama.2009.1943.
- Elkin I, Gibbons RD, Shea MT, Sotsky SM, Watkins JT, Pilkonis PA, et al. Initial severity and differential treatment outcome in the National Institute of Mental Health Treatment of Depression Collaborative Research Program. J Consult Clin Psychol 1995;63:841-7. http://dx.doi.org/10.1037//0022-006X.63.5.841.
- Farabaugh A, Alpert J, Wisniewski SR, Otto MW, Fava M, Baer L, et al. Cognitive therapy for anxious depression in STAR*D: what have we learned?. J Affect Disord 2012;142:213-18. http://dx.doi.org/10.1016/j.jad.2012.04.029.
- Fournier JC, DeRubeis RJ, Shelton RC, Hollon SD, Amsterdam JD, Gallop R. Prediction of response to medication and cognitive therapy in the treatment of moderate to severe depression. J Consult Clin Psychol 2009;77:775-87. http://dx.doi.org/10.1037/a0015401.
- Leykin Y, Amsterdam JD, DeRubeis RJ, Gallop R, Shelton RC, Hollon SD. Progressive resistance to a selective serotonin reuptake inhibitor but not to cognitive therapy in the treatment of major depression. J Consult Clin Psychol 2007;75:267-76. http://dx.doi.org/10.1037/0022-006X.75.2.267.
- Sotsky SM, Glass DR, Shea MT, Pilkonis PA, Collins JF, Elkin I, et al. Patient predictors of response to psychotherapy and pharmacotherapy: findings in the NIMH Treatment of Depression Collaborative Research Program. Am J Psychiatry 1991;148:997-1008.
- Curry J, Rohde P, Simons A, Silva S, Vitiello B, Kratochvil C, et al. Predictors and moderators of acute outcome in the Treatment for Adolescents with Depression Study (TADS). J Am Acad Child Adolesc Psychiatry 2006;45:1427-39. http://dx.doi.org/10.1097/01.chi.0000240838.78984.e2.
- Jacobs RH, Silva SG, Reinecke MA, Curry JF, Ginsburg GS, Kratochvil CJ, et al. Dysfunctional attitudes scale perfectionism: a predictor and partial mediator of acute treatment outcome among clinically depressed adolescents. J Clin Child Adolesc Psychol 2009;38:803-13. http://dx.doi.org/10.1080/15374410903259031.
- Thompson LW, Coon DW, Gallagher-Thompson D, Sommer BR, Koin D. Comparison of desipramine and cognitive/behavioral therapy in the treatment of elderly outpatients with mild-to-moderate depression. Am J Geriatr Psychiatry 2001;9:225-40. http://dx.doi.org/10.1176/appi.ajgp.9.3.225.
- Driessen E, Cuijpers P, Hollon SD, Dekker JJ. Does pretreatment severity moderate the efficacy of psychological treatment of adult outpatient depression? A meta-analysis. J Consult Clin Psychol 2010;78:668-80. http://dx.doi.org/10.1037/a0020570.
- Kiosses DN, Leon AC, Arean PA. Psychosocial interventions for late-life major depression: evidence-based treatments, predictors of treatment outcomes, and moderators of treatment effects. Psychiatr Clin North Am 2011;34:377-401. http://dx.doi.org/10.1016/j.psc.2011.03.001.
- Asarnow JR, Emslie G, Clarke G, Wagner KD, Spirito A, Vitiello B, et al. Treatment of selective serotonin reuptake inhibitor-resistant depression in adolescents: predictors and moderators of treatment response. J Am Acad Child Psychiatry 2009;48:330-9. http://dx.doi.org/10.1097/CHI.0b013e3181977476.
- Spek V, Nyklicek I, Cuijpers P, Pop V. Predictors of outcome of group and internet-based cognitive behavior therapy. J Affect Disord 2008;105:137-45. http://dx.doi.org/10.1016/j.jad.2007.05.001.
- Kuyken W, Padesky CA, Dudley R. Collaborative Case Conceptualization: Working Effectively with Clients in Cognitive–Behavioural Therapy. New York, NY: Guilford Press; 2009.
- Lewis G. Assessing psychiatric disorder with a human interviewer or a computer. J Epidemiol Community Health 1994;48:207-10. http://dx.doi.org/10.1136/jech.48.2.207.
- Holmes TH, Rahe RH. The social readjustment rating scale. J Psychosom Res 1967;11:213-18. http://dx.doi.org/10.1016/0022-3999(67)90010-4.
- Chalder M, Wiles NJ, Campbell J, Hollinghurst SP, Haase AM, Taylor AH, et al. Facilitated physical activity as a treatment for depressed adults: randomised controlled trial. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.e2758.
- Lewis G, Mulligan J, Wiles N, Cowen PJ, Craddock N, Ikeda M, et al. Polymorphism of the 5HT transporter and response to antidepressants: a randomised controlled trial. Br J Psychiatry 2011;198:464-71.
Appendix 1 A list of commonly used antidepressants with adequate doses for CoBalT
Appendix 2 Patient and public involvement
Service user input was sought during the original application for funding for this study. We asked user representatives to provide feedback on trial documentation and questionnaires during the preparatory phase of the study. A user representative was a member of our independent TSC. The newsletter to participants giving feedback on the study findings was developed in collaboration with the service user member of our TSC.
List of abbreviations
- A&E
- accident and emergency
- ACME
- average causal mediated effect
- ADE
- average direct effect
- BABCP
- British Association for Behavioural and Cognitive Psychotherapies
- BDI-II
- Beck Depression Inventory (second version)
- BFI
- Big Five Inventory
- BNF
- British National Formulary
- BSP
- brief supportive psychotherapy
- CACE
- Complier-Average Causal Effect
- CBASP
- cognitive behavioural analysis system of psychotherapy
- CBT
- cognitive behavioural therapy
- cCBT
- computerised cognitive behavioural therapy
- CI
- confidence interval
- CIS-R
- Clinical Interview Schedule – Revised version
- CoBalT
- Cognitive behavioural Therapy as an adjunct to pharmacotherapy for primary care patients with treatment resistant depression: a randomised controlled trial
- CONSORT
- Consolidated Standards of Reporting Trials
- CTIMP
- Clinical Trial of an Investigational Medicinal Product
- CTS-R
- Cognitive Therapy Scale-Revised
- DAS
- Dysfunctional Attitudes Scale
- DAS-SF2
- Dysfunctional Attitudes Scale-Short Form (version 2)
- DBT
- Dialectical Behaviour Therapy
- EQ-5D-3L
- European Quality of Life-5 Dimensions-3 Levels
- FTE
- full-time equivalent
- GAD-7
- Generalised Anxiety Disorder Assessment-7 items
- GP
- general practitioner
- IAPT
- Improving Access to Psychological Therapies
- ICC
- intraclass correlation coefficient
- ICD-10
- International Classification of Diseases and Related Health Problems, Tenth Edition
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- ISTDP
- intensive short-term dynamic psychotherapy
- ITT
- intention to treat
- MAQ
- Metacognitive Awareness Questionnaire
- MBCT
- mindfulness-based cognitive therapy
- MHRN
- Mental Health Research Network
- MICE
- multiple imputation by chained equation
- MRC
- Medical Research Council
- NARI
- noradrenaline reuptake inhibitor
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NMB
- net monetary benefit
- OR
- odds ratio
- PCMHT
- Primary Care Mental Health Team
- PC-PTSD
- Primary Care PTSD
- PCRN
- Primary Care Research Network
- PCT
- primary care trust
- PHQ-9
- Patient Health Questionnaire-9 items
- PPC
- prescription payment certificate
- PSS
- Personal Social Services
- PTSD
- post-traumatic stress disorder
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- REVAMP
- Research Evaluating the Value of Augmenting Medication with Psychotherapy
- SD
- standard deviation
- SF-6D
- Short Form questionnaire-6 Dimensions
- SF-12
- Short-Form questionnaire-12 items
- SF-36
- Short Form questionnaire-36 items
- SMHRN
- Scottish Mental Health Research Network
- SNRI
- serotonin–norepinephrine reuptake inhibitor
- SPCRN
- Scottish Primary Care Research Network
- SSRI
- selective serotonin reuptake inhibitor
- STAR*D
- Sequenced Treatment Alternatives to Relieve Depression
- TAU
- treatment as usual
- TCA
- tricyclic antidepressant
- TRD
- treatment-resistant depression
- TSC
- Trial Steering Committee