Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 05/516/06. The contractual start date was in February 2007. The draft report began editorial review in February 2013 and was accepted for publication in June 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Snowdon et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
If randomised controlled trials (RCTs) are to generate a sound evidence base to support the care of sick children and to improve survival and disability rates in settings where children are most at risk then the critically ill – who are most at risk of death and disability – are the clear target population for inclusion in research. This is especially the case in some of the trials conducted in neonatal and paediatric intensive care (NIC/PIC) settings. In such trials, comparison of mortality rates between intervention arms is a central analytical strategy. This reflects an explicit understanding that, given the severity of the condition of many of the children in the target population, some of those enrolled do not survive their illness or their clinical circumstances. It is the work of the trial to determine the effect that an intervention might have on a range of outcomes, including mortality. Whether mortality rates are lowered, raised or unaffected by a given intervention is considered at a group level, but behind the collective mortality rates are individual families who make the decision to enrol their child in a trial and whose child does not survive.
The last decade has witnessed a highly co-ordinated response in the UK to the shortfall in research evidence for many paediatric medicines and clinical interventions, which was acknowledged around the turn of the century. 1–3 The establishment of the National Institute for Health Research (NIHR) Medicines for Children Research Network (MCRN) to provide an infrastructure for UK research in this field, and the attendant development and expansion of Local Research Networks (LRNs) throughout the UK, is intended to encourage and facilitate paediatric research. Along with the aim of encouraging more high-quality trials comes an increase in the number of families who will be involved in clinical research. It is important that the ways in which those families are cared for in the context of the research to which they give their consent are also of a high standard and, where possible, evidence based. Although there has been some empirical consideration of the perspectives of parents on trials involving their children, much of the work carried out so far has focused on aspects of the decision-making process for trial entry and has predominantly involved parents of children who have survived.
The forms of communication and the means of support that trial teams and their clinician representatives might offer to the bereaved families of research participants can be considered as being as much a part of trial processes and methods as the information leaflets, enrolment discussions and consent forms that were used when their child was entered into a trial. The management of bereavement subsequent to trial enrolment has been almost completely overlooked, however, and has rarely been the focus of empirical study. It is of concern that so little is known about the possible views and experiences of bereaved parents and those involved in their care in the trial context, especially as their particular contribution to clinical research is so important to medical progress. Without research that describes the perspectives of these central players, it is not possible to know whether current trial-related practice is appropriate and whether it meets their needs. It is important to find out exactly how parents, in all their variety, wish to be treated and what subsequent relationships, if any, they wish to have with research. It is also important to consider the views of clinicians who act as intermediaries between trial teams and parents. Other trial team members, such as non-clinical investigators, managers, statisticians and trial advisors, do not necessarily have contact with bereaved parents, but their views and the decisions that they make about the science and the day-to-day conduct of a trial may shape parental experiences overtly or subtly, directly or over time. Understanding how trial teams respond to bereavement and why they do so in particular ways, and how bereaved parents view their experiences and how they would like to be treated, is a complex task given the range of individuals whose views must be considered, and the variety of views and responses that are likely to exist in this very sensitive setting.
In 2006 the NIHR Health Technology Assessment (HTA) programme funded the BRACELET (Bereavement and RAndomised ControlLEd Trials) study in order to generate information and insights into death and bereavement in this context, and to consider methods for research into this area. BRACELET was the first study to specifically focus on the complex and sensitive issues raised when death and bereavement occur in the context of RCTs.
This multidimensional study was conducted in two phases:
-
The quantitative phase (Phase I) sought to ascertain the numbers of babies and children who had been enrolled in NIC/PIC in a given period, the number who had survived and the number who died. By identifying the proportion and distribution of deaths in these trials, this component of the study would provide a valuable overview of the field, as well as giving a sense of the size of the population of parents whose babies and children have died subsequent to their enrolment in a trial. It would also provide a sound base from which to move on to the second qualitative phase of the study. Phase I of the study has been published4 (see Appendix 1 ).
-
The qualitative phase (Phase II) sought to examine how death in a trial setting is handled and experienced by parents, clinicians and trial teams, and to explore how they feel possible needs for communication and support, relating to bereavement, might be addressed.
The BRACELET study was also designed as a methodological study, focusing on two main aspects: methods to support the future running of trials involving children where deaths are expected, and methods for conducting qualitative research in this area, based on the experiences of carrying out Phase II of BRACELET. Given the lack of precedent for research in this area, BRACELET was planned as a feasibility study, determining the extent to which such sensitive research is possible, and assessing the value of the data produced. It aimed to consider the challenges of identifying a research population appropriate to the field of study, as well as how to inform and recruit potential participants, and how to conduct interviews in relation to such a sensitive topic. The plan for Phase II was also to test the value of flexible options for participation. As a scene-setting study working with potentially vulnerable populations, it was important to consider both the challenges of responding to bereavement as an element of clinical trial practice and developing methodological guidance for the conduct of future empirical work on this topic.
Thus, the empirical and methodological dimensions of BRACELET were closely tied, and were considered by the research team to be of equal importance.
The BRACELET study was carried out over the 6-year period 2007–12, a reflection of the multidimensional, multimethod work needed to gain insight into this underexplored field of research. The structure of this report reflects the three main bodies of work involved. After an introduction to the topic (see Chapter 2 ), the methods and the findings from the study are presented in three main sections and a concluding chapter as shown below:
-
Section A Quantitative survey of clinical trials and clinical centres (see Chapter 3 )
-
Section B Qualitative study of bereavement-related practice and experiences in neonatal clinical trials (see Chapters 4–9 )
-
Section C Methodological evaluation of tools and approaches to recruitment and participation in the BRACELET study (see Chapter 10 )
-
Conclusions Implications for practice and research (see Chapter 11 ).
This division of the three main components of the study suggests three discrete areas and emphasises their different methodological approaches. In fact there was much overlap and cross-fertilisation of ideas across the different areas of the study, with the insights gained in one area shaping methodological choices and guiding reflection in other areas. In the final methodological section of this report the benefits accrued from taking a mixed-method approach are considered (see Chapter 10 ).
Although BRACELET is a wide-ranging, multimethod, multidisciplinary study, it is intended to be the first step in gaining some insight into the range of experiences and responses to bereavement that might be encountered. It was not designed to explain fully the phenomenon of bereavement subsequent to RCT enrolment in NIC/PIC trials, but to provide methodological foundations upon which further research would build to refine understanding with examination of different situations, settings, practices and interventions, in paediatrics as well as in other settings in which bereavement can be a salient issue.
Chapter 2 Related research and research plan
Related research
The BRACELET study was developed in order to describe and explicate a specific and underexplored area of clinical trials practice, but much of the ground to be covered is not entirely new research territory. Although there has been little research on death subsequent to trial enrolment, the reactions of clinicians and parents to their experience in neonatal intensive care units (NICUs) and paediatric intensive care units (PICUs), including experiences of death in these setting, have been described by researchers from a wide range of disciplinary perspectives and have been the focus of a number of reviews. Bereavement care packages offered by attendant clinicians in NICUs have been described in a growing literature (see Harvey and colleagues5 for a review). Clinician and parental views of their involvement in RCTs is another burgeoning area of research. Although this broad body of available data provides a helpful empirical contextual base for BRACELET, consideration of the gaps in this literature points to important shortfalls to be addressed. An overview is provided below.
Experiences in neonatal intensive care units and paediatric intensive care units
A number of studies describe responses to NIC and PIC (for a review of qualitative studies of parental experiences of NICU stay, see Obeidat and colleagues;6 for a review of ongoing sequelae for parents after PICU stay, see Nelson and Gold7). They amply demonstrate that the intensive care unit (ICU) is an extraordinarily stressful environment for the adults concerned: for parents of neonates,8–12 when admission takes place shortly after birth, and for parents of older babies and children,13–16 when an admission may be the result of chronic or acute illness, trauma or violence. It can also be a challenging professional environment,17–22 but clinicians build up expertise over time and often develop strategies to deal with and manage difficult situations. 23
Death in the NICU or PICU can bring with it a number of very difficult experiences for parents in addition to the loss of their child, such as end-of-life decision-making,24–30 the experience of withdrawal of care and the impact of lingering death,31,32 loss of a multiple (e.g. a twin or triplet)28 and multiple losses,33 and the need to consider post-mortem examinations34 (for a review of studies of end-of-life decision-making in the NICU see Eden and Callister;32 for decision-making more generally in the PICU, see Madrigal and colleagues35). Studies of parental experiences of death in the ICU suggest that they value clear information given around the time of their child’s death,36 appreciate acts of kindness from ICU staff in their bereavement,37 and often want further contact with ICU clinicians. 38 When death occurs, clinicians can find their own emotional resources being strained. 39–42 They can be confronted with situations that challenge their own ethical positions and stretch their capacity to cope. 19,43–45 Research also shows that despite the difficulties, caring for the needs of parents in these circumstances can be a valued and satisfying aspect of clinical work. 46,47
Participation in neonatal and paediatric intensive care trials
Decisions about whether or not to enrol an infant or a child into a trial involve discussions between parents and recruiting clinicians, and the outcome of their discussion sets in train the experiences of interest here. All parties therefore have relevant insights of interest and value, and a substantial literature exists on the subject of clinical research involving babies and children (see Shilling and colleagues48 for a review). There are, however, a number of biases and gaps in relation to intensive care trials. The views of parents are better represented than those of clinicians, with only a small number of papers describing the research-related experiences and views of intensivists who have responsibility for recruiting to NIC trials,49–51 and there is a dearth of research specifically assessing the views of clinicians involved in PIC trials. Although parental involvement in PIC trials is similarly limited, with few examples identified,52,53 parents’ experiences and views of NIC trials have been relatively well studied: we have identified 21 papers. 49,51,54–72 Many of these papers focus on issues at the ‘front end’ of trials, such as comprehension, decision-making and views of enrolment in more than one trial. They highlight areas of potential difficulty around enrolment for parents with responsibility for proxy decision-making, while also dealing with the stresses and strains of having a sick child, as well as the admission to ICU. They also suggest the importance of parental responsibility as parents seek to make good decisions in relation to the care of their child, and reluctance for others to make that decision for them. There are few data that describe the ongoing experience of being involved in a NIC trial, or later responses after discharge or death when time has passed and parents’ thoughts about a trial may be worked through away from the ICU. Notably data are available to describe parents’ ongoing views and interest in feedback of trial results, which usually occurs some years after recruitment, but only for parents whose child has survived. 56 Whether bereaved parents are similarly interested in the outcome of a trial, or whether they have left this part of their experience in the past and do not wish to have the subject raised, is simply not known.
The gap at the intersection
It is notable that despite the availability of overlapping bodies of literature on parenting and bereavement in the ICU, and on participation in ICU trials, the intersection of the three has been left unattended. In fact the knowledge sets that have built up have done so as if there were no common ground between bereavement and trial participation ( Figure 1 ). Even with the passage of time since BRACELET was funded to address this shortfall, there is still very little research available that describes the experiences of parents whose baby was enrolled in a NIC or PIC trial and subsequently died, or which explores whether the decision to enrol adds, positively or negatively, to the experiences of the families and clinicians involved. There is nothing to give an indication of how bereaved parents would like to be treated in relation to their links with a trial, or what might be appropriate practice and what might not. We found no editorials or opinion papers reflecting on this issue, and no practice-based papers in which clinicians describe how they handle this situation. This gap in research is in sharp contrast with the attention received by other elements of clinical trials practice, such as recruitment strategies and informed consent procedures.
FIGURE 1.
The research gap.
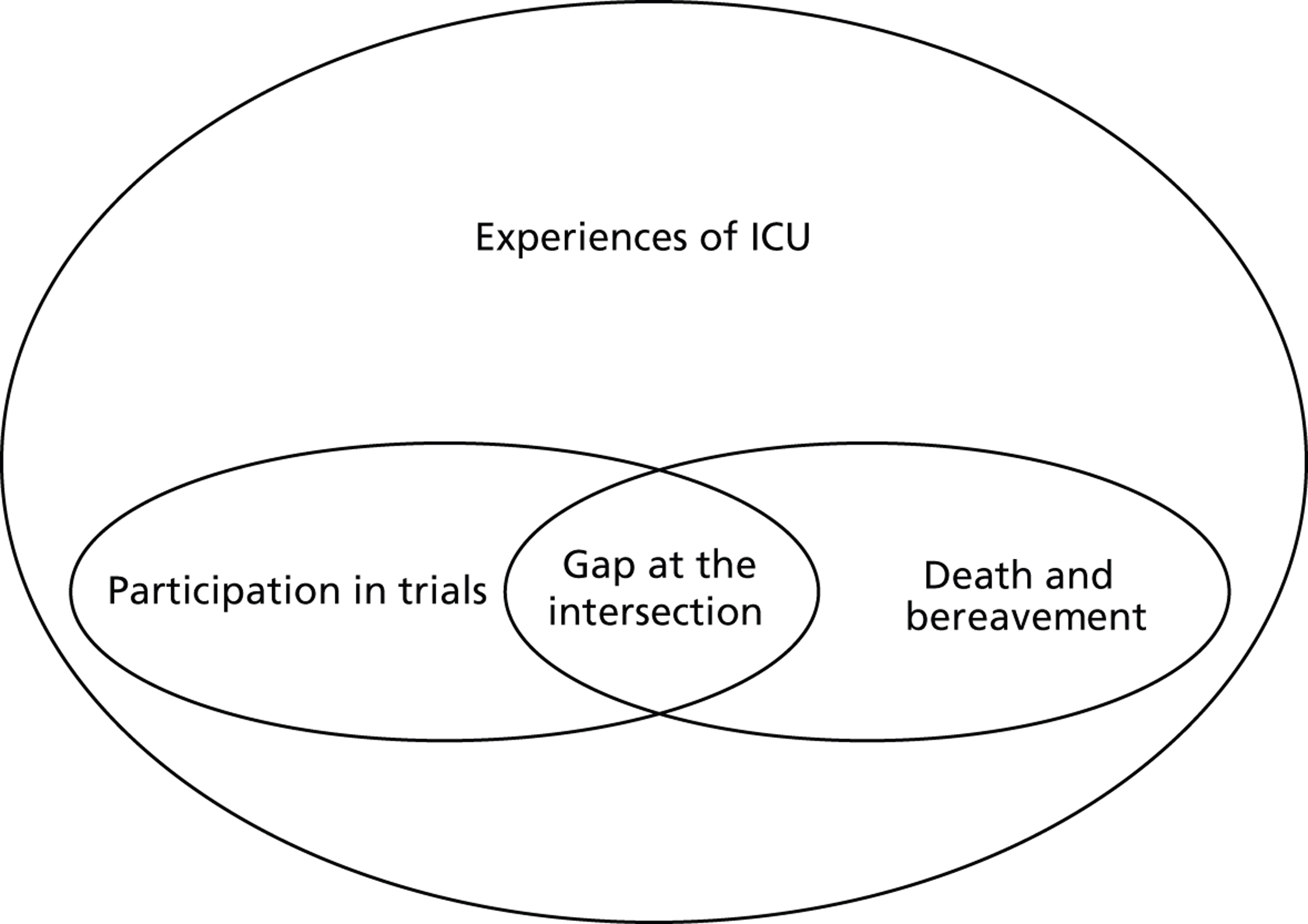
The lack of specific research in this area, as well as the exclusion of bereaved parents from more general research on participation, means that their voice, whether or not it is distinct from those of other parents, does not inform the evidence base for how to carry out a well-conducted RCT. The voice of the parents of survivors stands for all. As an example, Morley and colleagues63 considered parents’ views of participation in multiple trials. The choice to recruit parents to this study in the third week of their baby’s life meant that parents who had been invited to enrol their baby into more than one trial and were subsequently bereaved were unable to give their views. Their views may or may not differ from parents of surviving babies, but the evidence to sanction recruitment to multiple studies is put forward without exploration of this possibility. This is not at all unusual. In some studies it is stated that bereaved parents were actively excluded from research samples, either because this could have been upsetting to them58 or no reason was given. 49,68 This bias in the evidence base seems to have gone almost unnoticed, and passes largely without remark other than an early comment on the absence of bereaved parents in one of our studies: ‘For perfectly understandable ethical reasons . . . bereaved parents were not included in Snowdon’s study . . . however, if approached sensitively, many are helped by discussing related events and their feelings, so that there may in fact be no “ethical no-go-zone” preventing research in this area.’73
Few papers other than our own include bereaved parents in their samples; only three of the 20 studies listed above do so and in each they constitute a small minority of their sample. 61,64,71 We know of only two papers,74,75 a paired set of our own and both from the same study, in which intensivists74 and parents75 have been asked to consider in detail particular issues involved in bereavement and research. Although these papers relate specifically to neonatal trial-related post-mortems, and involve only 11 interviews with 18 bereaved parents (a subsample from a larger study), we were able to gain insights from the views and experiences these parents described. Even in this relatively small subsample there was variety and range, from those who felt that enrolment into a trial was an almost insignificant part of their experience, to others where it complicated and added a difficult element to their baby’s death. The study included one couple who were very pleased to have contributed to research, feeling that it was an important and helpful positive element in a dire situation. Another couple described how horrified they were to learn of allocation to a control group, and how they were left with the persistent feeling that their baby had been deprived of a treatment and so died without every effort being made on her behalf. 76 (This last case is presented in detail in Snowdon and colleagues.)76
Although there are limitations to these data, they are useful in suggesting that separate and dedicated study of bereavement in this context is appropriate. They also suggest that parental responses to ICU trials after the death of a child may, like the experience of bereavement itself, be highly varied, reflecting both parental differences in experiences and reactions, but also changing responses to bereavement over time. Some parents may be keen for further contact with a trial/clinical team after the death of their child, to gain further information, clarification of events and possibly for feedback of the results of the trial to which they contributed; others may shun any such contact.
The views of parents of survivors cannot simply be taken as universal accounts of ICU trial participation, nor should they. The availability of research on the impact of death in the ICU, on experiences at the time and on the long-term effects, suggests that research with bereaved parents on delicate and sensitive topics is possible and is informative. It is the particular combination of bereavement and trial participation that appears to have so far placed this population of parents beyond the scope of research into their views of this aspect of their ICU experience, meaning that recommendations or decisions about practice based on current literature are made on the basis of the views of parents of survivors only. Research on parents of survivors is clearly important as they form the majority of the parents involved, but bereaved parents may differ in terms of circumstances, experiences and views and these differences may inform and shape their reactions to trials. The experience of being invited to take part in a trial is part of a larger narrative of their child’s condition and care. A parent whose child does not survive may have a different set of prior experiences to those whose child recovers. If their child is already severely compromised and at high risk of death, they may be asked to consider trial participation with a different set of expectations and assumptions, and may be more likely to have made their decision in crisis. Reactions to a trial may be forged in the possibility of imminent and then actual bereavement, and any research that considers the views of parents must acknowledge the possible ongoing effects of their child’s death on their subsequent views.
If we are to use research data to inform practice in NIC and PIC trials then a more nuanced account of responses to research is needed. Such an account needs to draw on the views of the different parties involved to gain purchase on the broader responses to death in the context of a trial, and this, in turn, needs to be placed in the context of clinical trials practice in the UK. Addressing the research deficit in relation to bereaved parents, and the views of those who design and implement the trials in which they are involved, marks an important step towards that aim.
Plan of research
The bodies of work described above suggest something of the complexity of considering experiences of trial participation and bereavement. These experiences are part of a larger narrative set of pregnancy, birth, critical illness, care, research and death. They involve the interplay of many individuals, all with different roles, responsibilities and views, yet studies that consider the views and experiences of those involved in trials often look at a group in isolation, such as recruiting clinicians or participants and/or their proxies, and their views are often divorced from the larger context of the trials involved.
There are clearly challenges for any research study that seeks to rigorously explore and fairly represent this situation. The BRACELET study was designed and funded as a substantial first step in addressing the shortfall in knowledge identified here, as a foundation study aiming to tap the multifaceted, multilayered elements of this phenomenon.
The starting point for the study was the likelihood that a range of perspectives on bereavement in trials might exist and that these must be understood within the larger context of trials, their recruiting centres and trials-related practice, as well as with reference to the close detail of the roles played within a trial. It was therefore necessary to look beyond the views of single groups and to consider the topic of bereavement in trials in relation to both the infrastructure of trials-based research and to the role-related infrastructure of individual trials. How to carry out such a study in the light of the sensitivities that surround this topic, in ways that are acceptable to participants and that produce sound data, was also an important consideration.
This yielded a study in three parts using multiple methods to serve its different but overlapping aims. The structure and components of the study are described below.
Phase I: the quantitative component
An essential prerequisite to considering bereavement and trials was to ascertain the magnitude and distribution of mortality post-trial enrolment. This part of BRACELET therefore aimed to determine:
-
trial activity in UK NICUs and PICUs
-
the number and proportion of deaths among babies and children participating in NIC and PIC trials
-
variation in mortality across units, and across trials
-
whether any provision was made for bereavement within trials.*
*Key elements of these data were published during the course of The BRACELET study (Snowdon and colleagues4). The methods of data collection and findings are presented in the following chapters in more detail.
Phase II
Phase II involved both a qualitative and a methodological component, which, together, were intended to open up a new and important field of enquiry, considering throughout the factors that facilitate and impede further research in such a sensitive and difficult area. The aim was not to fully explicate the phenomenon of bereavement subsequent to trial enrolment but to generate new data that would produce important insights and generate further questions for the clinical and trials communities. With this in mind, the specific objectives of Phase II of the study were as follows.
For the qualitative component:
-
to start to address the shortfall that exists on the subject of bereavement subsequent to trial enrolment by initiating enquiry into this new research territory
-
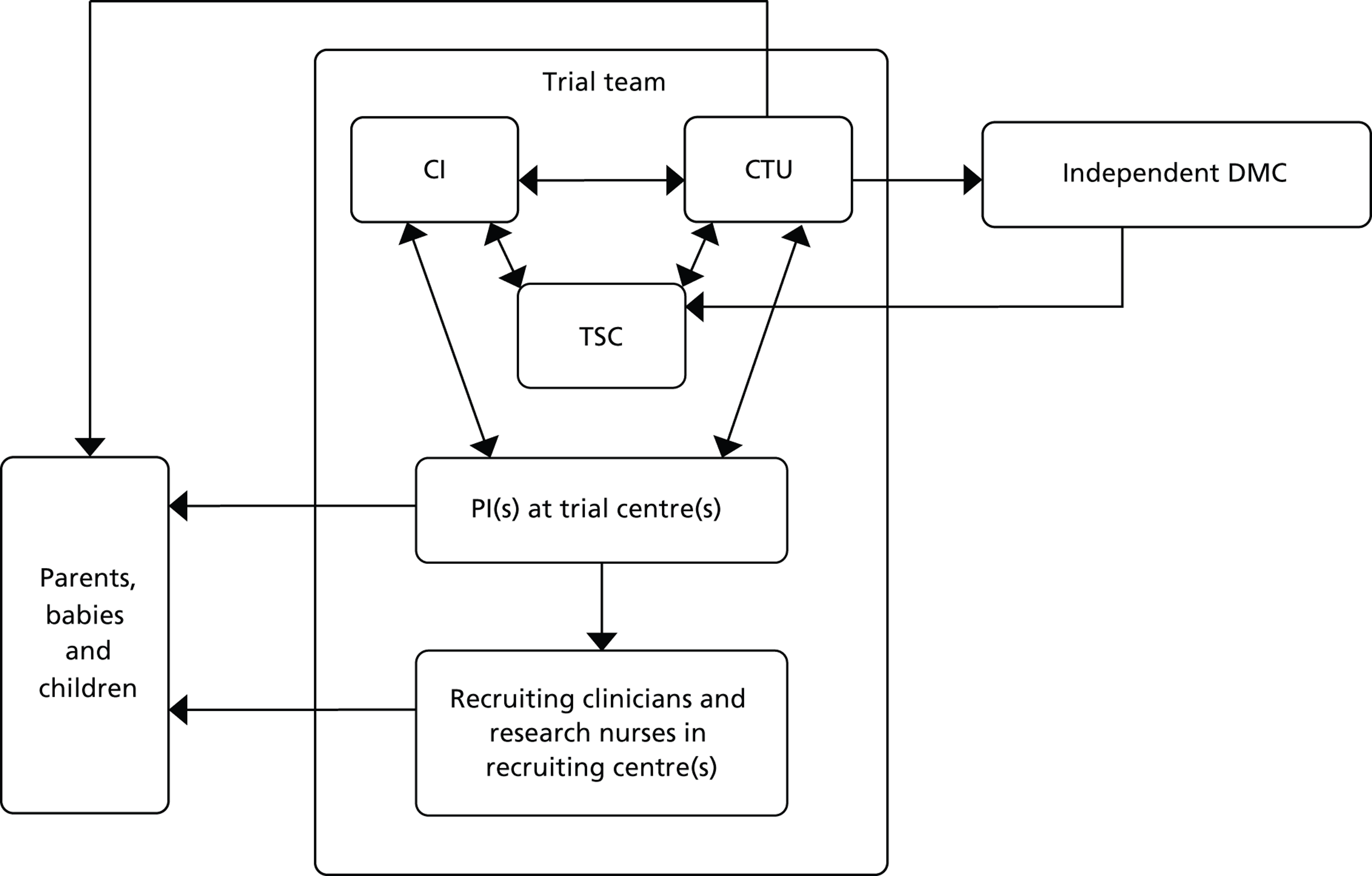
to start to delineate the relevance of trial enrolment to bereavement in this context, by describing and exploring the experiences and views of key people involved in NIC and PIC trials. The methodological starting point for this component of the BRACELET study was therefore to consider the typical structure of a multicentre trial, which involves a central trial team, comprising the chief investigator (CI), Clinical Trials Unit (CTU) and Trial Steering Committee (TSC), and the recruiting centre teams comprising principal investigators (PIs), recruiting clinicians and research nurses. Trials often work in conjunction with an independent Data Monitoring Committee (DMC), which advises but stands apart from the trial team. Although DMCs are not part of the trial team itself, DMC members are likely to have an important perspective of how a trial is run. This broad grouping was defined as one of the major populations of interest to BRACELET and, for convenience, the different roles involved were all considered under the generic term of ‘trial team.’ The other major population to consider was the bereaved parents whose children were enrolled in a trial ( Figure 2 )
-
to consider the similarities and differences in how bereavement is approached in the policies and practices of central trial teams and teams in recruiting centres. This information would allow for best practices to come to light and highlight any actions or omissions which the key people involved consider to be problematic.
FIGURE 2.
Typical structure of a multicentre trial.
For the methodological component:
-
to act as an important foundation for future research, ascertaining the feasibility, and acceptability of research in this area, through reflection and through feedback from study participants
-
to consider and reflect on issues for future researchers encountered during the course of the research process such as, gaining trust and facilitating collaboration (trial teams and clinical centres), approaches to recruitment, the ethical and sensitive conduct of interviews and potential information and support needs post interview.
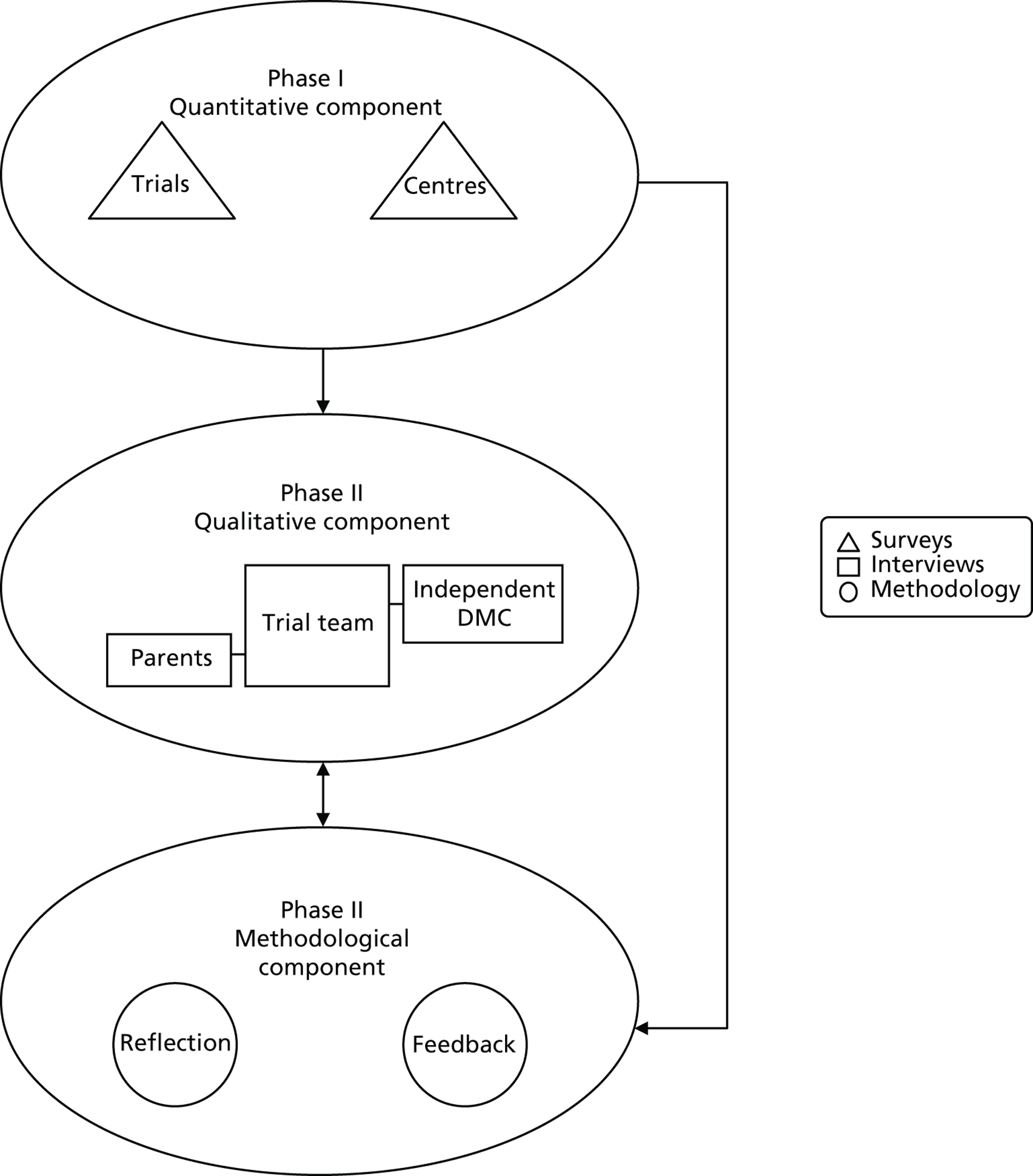
This two-phase, three-part structure uses multiple methods, which are described in Chapter 3 (Phase I – quantitative component), Chapter 4 (Phase II – qualitative component) and Chapter 11 (Phase II – methodological component). The overall structure is shown in Figure 3 .
FIGURE 3.
Structure of the BRACELET study.
Section A
Chapter 3 Quantitative survey of trials and clinical centres
Methods
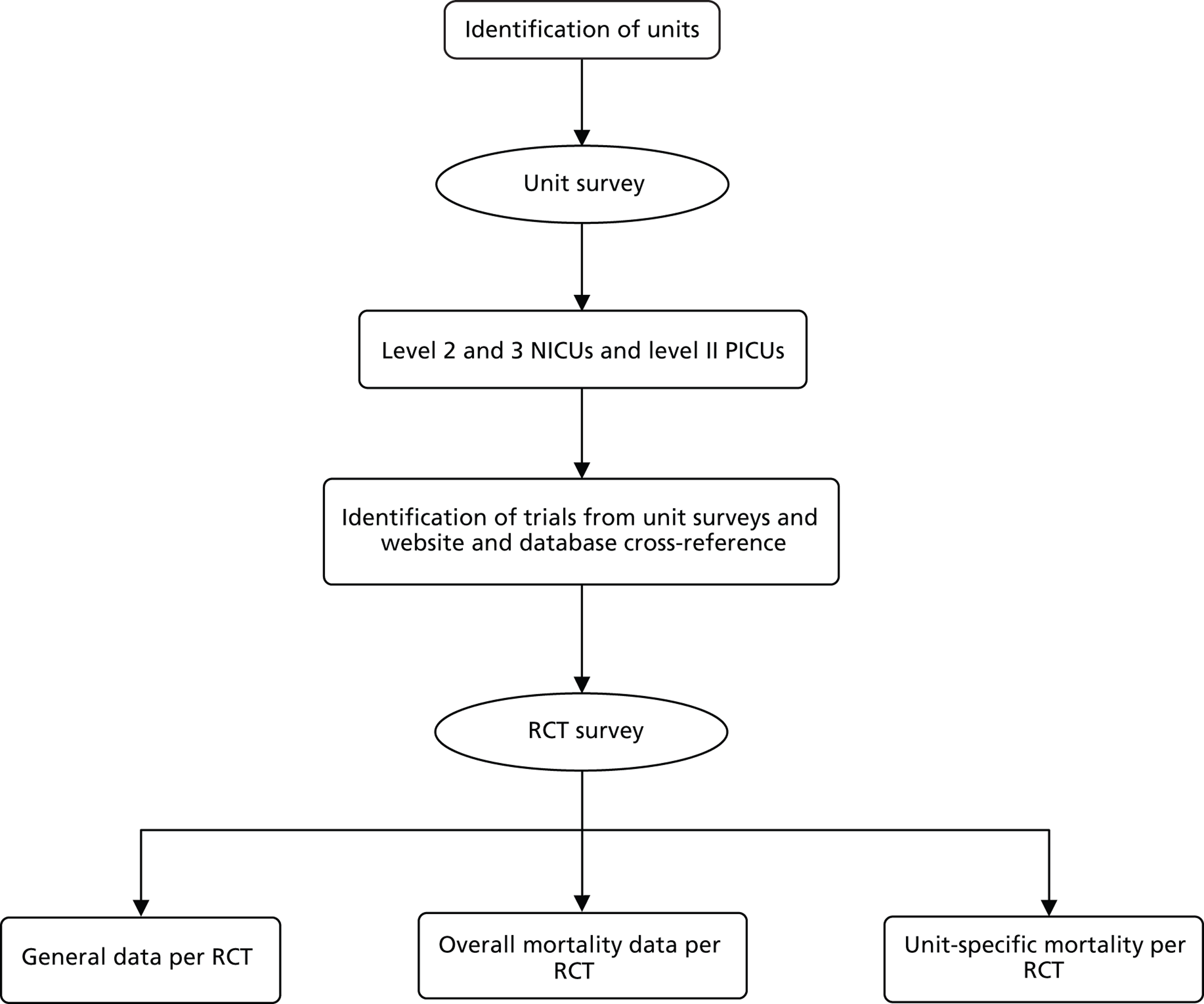
Phase I involved two linked surveys, which aimed to identify all NIC and PIC trials open to recruitment in the UK from 1 January 2002 to 31 December 2006, and to map where and in what sorts of trials deaths occurred, in what numbers and proportions. RCT activity was determined by conducting a survey of all UK NICUs and PICUs to determine their involvement in trials, followed by a survey of the trials identified to determine the number and proportion of deaths of babies and children in their samples. The structure is shown in Figure 4 and explained further below.
FIGURE 4.
Structure of BRACELET study surveys strategies to support the survey response rate.
Unit surveys
In order to carry out the two surveys, appropriate sampling frames were developed.
Neonatal intensive care unit sampling frame
In 2005, researchers at the National Perinatal Epidemiology Unit (NPEU) had reported that there were 218 neonatal units in the UK in 2004–5. 77 To ensure accuracy for 2007, units listed in the Directory of Critical Care78 and on the British Association of Perinatal Medicine (BAPM) website79 were cross-checked with data provided by the NPEU80 and an ongoing study also developing a sampling frame for neonatal units. 81 From this, 220 neonatal units including neonatal surgical units, were identified in the UK (183 in England, 17 in Scotland, 13 in Wales, and seven in Northern Ireland).
Neonatal units are categorised by ‘levels of care’, as described in the BAPM Standards for Hospitals Providing Neonatal Intensive and High Dependency Care (second edition) published in 200182 ( Table 1 ). Given the focus of BRACELET, we aimed to survey only Level 2 and Level 3 NICUs, which would have higher mortality rates than Level 1 units.
| Level of care | Criteria |
|---|---|
| 1 | Provides special care but does not aim to provide any continuing high-dependency care or intensive care. This term includes units with or without resident medical staff |
| 2 | Provides high-dependency care and some short-term intensive care as agreed within the network |
| 3 | Provides the whole range of medical neonatal care but not necessarily all specialist services, such as neonatal surgery |
Designated level of care is not a fixed status, and so for some NICUs this changes over time. Also, for some neonatal units, this information was missing in the source data for the sampling frame. All 220 units were therefore sent a questionnaire developed for the survey (see below for further details), which asked the respondent to indicate his/her unit’s designation at the time of completing the questionnaire, based on the BAPM guidelines and as agreed by the local network. Level 1 units were not asked to complete the rest of the questionnaire but simply to return it with confirmation of their designated level of care. Only representatives of Level 2 and 3 units were asked to go on to complete the rest of the survey questionnaire.
Designation of care as Level 2 or 3 was confirmed for 149 NICUs.
Paediatric intensive care unit sampling frame
As for the NICU survey, the BRACELET focus on deaths meant that the PICU survey concentrated on those units likely to have higher mortality rates. PICUs are classified based on the criteria described in the Paediatric Intensive Care Society (PICS) Standards Document83 ( Table 2 ).
| Level of care | Nurse to patient ratio | Criteria |
|---|---|---|
| 1: high-dependency care | 0.5 : 1 | Close monitoring and observation required but not requiring acute mechanical ventilation |
| 2: intensive care | 1 : 1 | Requires continuous nursing supervision, usually intubated and ventilated; also includes unstable non-intubated or recently extubated child |
| 3: intensive care | 1.5 : 1 | Requires intensive supervision at all times and needs additional complex therapeutic procedures and nursing, e.g. unstable ventilated child on vasoactive drugs and inotropic support with multiple organ failure |
| 4: intensive care | 2 : 1 | Requires most the intensive interventions such as unstable or Level 3 children managed in a cubicle. Includes those on ECMO and children undergoing renal replacement therapy |
The PICU sampling frame for BRACELET therefore comprised PICUs with a paediatric intensivist in post, providing Level 2 intensive care and above. A database of UK PICUs was constructed using data provided by the Paediatric Intensive Care Audit Network (PICANet)84 and by checking the Directory of Critical Care 2006. 78 Thirty-two PICUs that fulfilled the above criteria were identified. It was unnecessary to first check the designation with a PICU representative as these level of care categories were more stable over time than for NICUs. Data were therefore requested from all PICUs with a paediatric intensivist in post, which provided at least Level 2 intensive care.
Unit questionnaire: development and mail-out
Given that neonatal and paediatric intensive care have different criteria to describe the level of care that units provide, two versions of a questionnaire were developed for the survey: one for NICUs and one for PICUs (see Appendices 2 and 3 ). The questionnaires were piloted in two NICUs and two PICUs. In this pilot phase, clinicians were asked to complete the questionnaire and provide feedback, including the clarity of instructions, the length of time that it took to complete, any sections that were difficult to complete, general layout, etc. Only minor revisions were required following the pilot surveys and it was not necessary to ask the pilot units to complete another questionnaire.
Representatives at the 149 Level 2 or 3 NICUs and at the 32 Level 3 PICUs were asked to complete the revised questionnaire in April 2007. In most cases this was the clinical lead or the person presumed to be the clinical lead for the unit. All unit representatives had been given prior notification of the survey by letter briefly describing the BRACELET study and the purpose of the survey (see Appendix 4 ). A survey pack was dispatched to units about 1 week after the pre-notification letter. The pack included the survey questionnaire, a study information leaflet (see Appendix 5 ), a prepaid, pre-addressed return envelope and a covering letter (see Appendix 6 ) explaining the purpose of the survey. Given that a short time cue can be effective in stimulating responses and specification of a deadline may increase the speed of the response,85 recipients were asked to respond within 2 weeks. Approximately 1 week after the deadline date had passed, a reminder letter was sent either via e-mail or mail. Again, recipients were asked to respond within 2 weeks. A second reminder was sent about 2 weeks after the second deadline had passed. The final reminder included a shortened version of the questionnaire containing a list of the multicentre RCTs that had been identified at that point (see Appendix 7 ). Telephone reminders were used where appropriate, and the questionnaire was also made available to clinicians in electronic form and placed on the study website (www.bracelet-study.org.uk) for download to facilitate returns.
Recipients were asked to indicate which RCTs (single- and multicentre) they had enrolled babies or children into during the period 1 January 2002 to 31 December 2006. All RCTs were eligible, regardless of size, intervention and outcome. International multicentre RCTs run from outside of the UK were also included. The questionnaires asked for brief details of all these RCTs.
The clinical lead for each unit was asked to indicate for each RCT listed in the questionnaire whether they would grant permission for the BRACELET study team to request their unit-specific, anonymised mortality data from the trial CI. We also asked about the provision of bereavement care for their unit, including the availability of a bereavement counsellor and other sources of emotional support for bereaved parents.
In addition to the questionnaire reminder process outlined above, a number of strategies were used to ensure a good response rate for the unit survey.
The BRACELET study was adopted by the MCRN and the study team worked very closely with the LRN managers and clinical research nurse practitioners who provided assistance with following-up non-responders. Members of the BRACELET team also spoke with clinicians from non-responding units in person at relevant meetings and conferences, and contact was made with some clinicians by e-mail or by calling their unit directly to encourage return of the questionnaires.
Clinicians could also be directed to the study website (www.bracelet-study.org.uk) for further documentation.
The BRACELET study addresses a sensitive topic and there was some concern among the team that perhaps units might be reluctant to take part in a study exploring issues relating to mortality in the context of their RCTs. During the data collection process the team did receive some feedback in which clinicians expressed discomfort over release of their mortality data. A letter was therefore sent from the then-director of the NPEU (also a BRACELET study investigator – PB) addressing these potential concerns, stressing the importance of BRACELET and providing reassurance about the confidentiality of the data (see Appendix 8 ).
Data collection was concluded in May 2008.
Trials survey
Trials sampling frame
Although new trials are increasingly being registered, especially those involving new medicinal products, there is no single repository of trials through which all trials conducted in the UK over specified time periods and particular specialties can be identified. The unit survey generated a list of trials, and this was supplemented by searches of relevant specialised databases and websites, including the UK Department of Health National Research Register [replaced by the UK Clinical Research Network (CRN) database]; the UK Department of Health Research Findings Register (replaced by the UK CRN database); PubMed; the NPEU website; the PICS website; the BAPM website; and the European Society of Paediatric and Neonatal Intensive Care websites.
For all RCTs identified as being potentially eligible for the trials survey, the trial CI, trial manager or other relevant contact was asked to confirm that the RCT fulfilled the eligibility criteria below.
Inclusion and exclusion criteria
Randomised controlled trials were eligible for inclusion if:
-
allocation was randomised
-
the trial was enrolling babies or children during the period 1 January 2002 to 31 December 2006
-
the intervention was delivered to the baby or child within the NICU or PICU setting (or, if it was initiated outside of the unit, it was delivered by, or under the auspices of, a neonatologist or paediatric intensivist leading to admission to the unit for ongoing care)
-
the intervention was delivered to the parent(s) but was designed to impact the baby or child’s ‘outcome’.
Randomised controlled trials were excluded if the intervention was delivered to unit staff or was delivered at a unit level and did not require informed consent from individual parents of babies or children.
Trials questionnaire
A pilot questionnaire was completed by trial managers for two multicentre RCTs and a CI for a single-centre RCT. Minor revisions were made to the questionnaire following their feedback.
Once eligibility had been confirmed, trial investigators were asked to complete the revised trial questionnaire. A questionnaire was dispatched to the trial investigators in May 2007, which sought information for the 5-year study period in relation to two areas. Part 1 sought general information about the RCT, including start and end dates of recruitment, numbers enrolled, aims/hypotheses, inclusion and exclusion criteria, primary and secondary outcomes, sample size, source(s) of funding, details of participating units and collection of post-mortem data (see Appendix 9 ). Part 2 sought the total numbers of UK survivors and non-survivors before discharge from hospital for the RCT overall, irrespective of randomised allocation (overall mortality data), and the numbers of survivors and non-survivors before discharge from hospital, irrespective of randomised allocation, for every participating unit that had given permission for these data to be released to the BRACELET study team (unit-specific mortality data) (see Appendix 10 ). The questionnaire information was supplemented by data from published papers, relevant websites and personal communication, where appropriate.
Respondents were also asked to provide copies of their protocol, parent information leaflets, information leaflets relating to bereavement, letters sent to bereaved parents or any other relevant documents.
Categorisation of randomised controlled trials
Based on the eligibility criteria and the types of units that took part, RCTs were categorised as being NIC or PIC RCTs. They were further categorised as follows:
-
Single-centre Participants were recruited from one unit only in the UK.
-
Multicentre Participants were recruited from two or more units in the UK only. This included RCTs that were conducted in two or more units within the same NHS Trust.
-
International Participants were recruited from two or more units, of which at least one was in the UK.
Analysis
Descriptive data are presented as proportions and ranges, as appropriate. Analysis used the statistical package Stata 10 (StataCorp, College Station, TX, USA). Variations in the denominators for some of the numbers reported in the Results section reflect different response rates for the unit survey and the trials survey, and incomplete release of mortality data by some units and some trials.
Results
Neonatal intensive care unit and paediatric intensive care unit survey
Overall, of the 220 NICUs surveyed, responses were received from 191 (86.8%), of which 149 were eligible units – 82 providing Level 2 care and 67 providing Level 3 care ( Table 3 ).
| Unit | Total | Responded, n (%) |
|---|---|---|
| NICU: | ||
| Level 1 | 42 | 42 (100.0) |
| Level 2a | 96 | 82 (84.4) |
| Level 3b | 80 | 67 (83.8) |
| Not known | 2 | 0 (0.0) |
| Total | 220 | 191 (86.8) |
| Total NICU: Levels 2 and 3 | 176 | 149 (84.7) |
| PICU (≥ Level 2) | 32 | 28 (87.5) |
| Overall NICU and PICU | 252 | 219 (86.9) |
In most cases (n = 142, 95.3%) a member of the unit staff filled in the questionnaire. A small number of questionnaires were completed either via MCRN contact with unit staff (n = 3), via MCRN contact with the hospital’s Research and Development (R&D) department (n = 3) or via one of the study investigators telephone contact with unit staff (n = 1).
Of the 32 PICUs providing Level 2 intensive care and above, responses were received from 28 (87.5%) (see Table 3 ). Of these, one questionnaire was filled in via MCRN contact with the hospital’s R&D department. The remaining questionnaires were filled in by a member of the unit staff.
Fifty RCTs (36 NIC and 14 PIC trials) were identified as having enrolled babies or children during the 5-year period 1 January 2002 to 31 December 2006.
Randomised controlled trial survey
For the trials survey, a response to Part 1 of the RCT survey, which collected general information about the RCT, was received for 43 (32 NIC and 11 PIC) of the 50 RCTs.
Overall UK mortality data (i.e. data for the whole trial) up to discharge from hospital were released for Part 2 of the RCT survey for 37 trials (28 neonatal, nine paediatric). Unit-specific mortality data up to discharge from hospital were released by 33 trials (24 neonatal, nine paediatric) for those ICUs that had permitted release of their data to BRACELET in the unit survey.
Findings of the surveys
Characteristics of randomised controlled trials
Of the 36 NIC trials, 10 were international, 14 UK multicentre, and 12 single centre. Of the 14 PIC trials, nine were international, and five single centre. Of the 10 international NIC RCTs, six were run from the UK, two from the USA and two from Canada. Of the nine international PIC RCTs, two were run from the UK, two from Canada, two from the US, and one each from Australia, the Netherlands and France – i.e. 32 out of 36 (88.9%) NIC RCTs and 7 out of 14 (50.0%) PIC RCTs were UK led.
Further characteristics of the 50 RCTs by type (NIC or PIC) are reported in Table 4 . The source(s) of funding for the trial was reported by a trial representative in the questionnaire or was obtained from the published papers for 37 (74.0%) RCTs. Most RCTs were funded by public sector or charitable organisations. For nine RCTs, funding was from two or more sources. Investigators for one single-centre RCT and one multicentre RCT (with two participating units) reported that they received no direct funding. In both NIC and PIC RCTs, the interventions that were most frequently evaluated were either drugs and food (including nutritional supplements and feeding regimens) or non-medicines interventions, which included temperature regulation, mechanical ventilation and surgical procedures.
| Characteristics of RCTs | NIC (N = 36), n (%) | PIC (N = 14), n (%) | Total (N = 50), n (%) |
|---|---|---|---|
| Fundinga | |||
| Public sector | 11 (30.6) | 4 (28.6) | 14 (28.0) |
| Charity | 8 (22.2) | 6 (42.9) | 14 (28.0) |
| Commercial – academic led | 5 (13.9) | 1 (7.1) | 6 (12.0) |
| Commercial – company led | 2 (5.6) | 1 (7.1) | 3 (6.0) |
| None | 2 (5.6) | – | 2 (4.0) |
| Not reported | 4 (11.1) | 1 (7.1) | 5 (10.0) |
| Non-responder | 5 (13.9) | 3 (21.4) | 8 (16.0) |
| Types of intervention | |||
| Drugs and foods (including blood products, anaesthesia, oxygen and food supplements) | 19 (52.8) | 7 (50.0) | 26 (52.0) |
| Physical therapies (including cooling, heating, mechanical ventilation, surgery) | 15 (41.7) | 7 (50.0) | 22 (44.0) |
| Monitoring | 1 (2.8) | 0 (0.0) | 1 (2.0) |
| Psychosocial (including parenting support) | 1 (2.8) | 0 (0.0) | 1 (2.0) |
| Mortalityb | |||
| Primary – sole outcome | 2 (5.6) | 2 (14.3) | 4 (8.0) |
| Part of composite – primary outcome | 7 (19.4) | 3 (21.4) | 10 (20.0) |
| Secondary outcome | 10 (27.8) | 4 (28.6) | 14 (28.0) |
| Not reported as an outcome | 13 (36.1) | 5 (35.7) | 18 (36.0) |
| Non-responder | 8 (22.2) | 3 (21.4) | 11 (22.0) |
Information about primary outcomes was obtained from either a BRACELET questionnaire (n = 6), the trial protocol (n = 26) or the published paper (n = 7), and so these data were available for 39 RCTs. Eighteen of the RCTs (46.2%) did not specify mortality as an outcome in either a BRACELET questionnaire (n = 3), or the trial protocol (n = 11), or the published paper (n = 4). Of the remaining 21 RCTs, 14 measured mortality as a primary outcome (four as sole primary and 10 as part of a composite) and 14 had mortality as a secondary outcome (including some with mortality as one component of a composite primary outcome).
Neonatal intensive care unit and paediatric intensive care unit randomised controlled trial activity
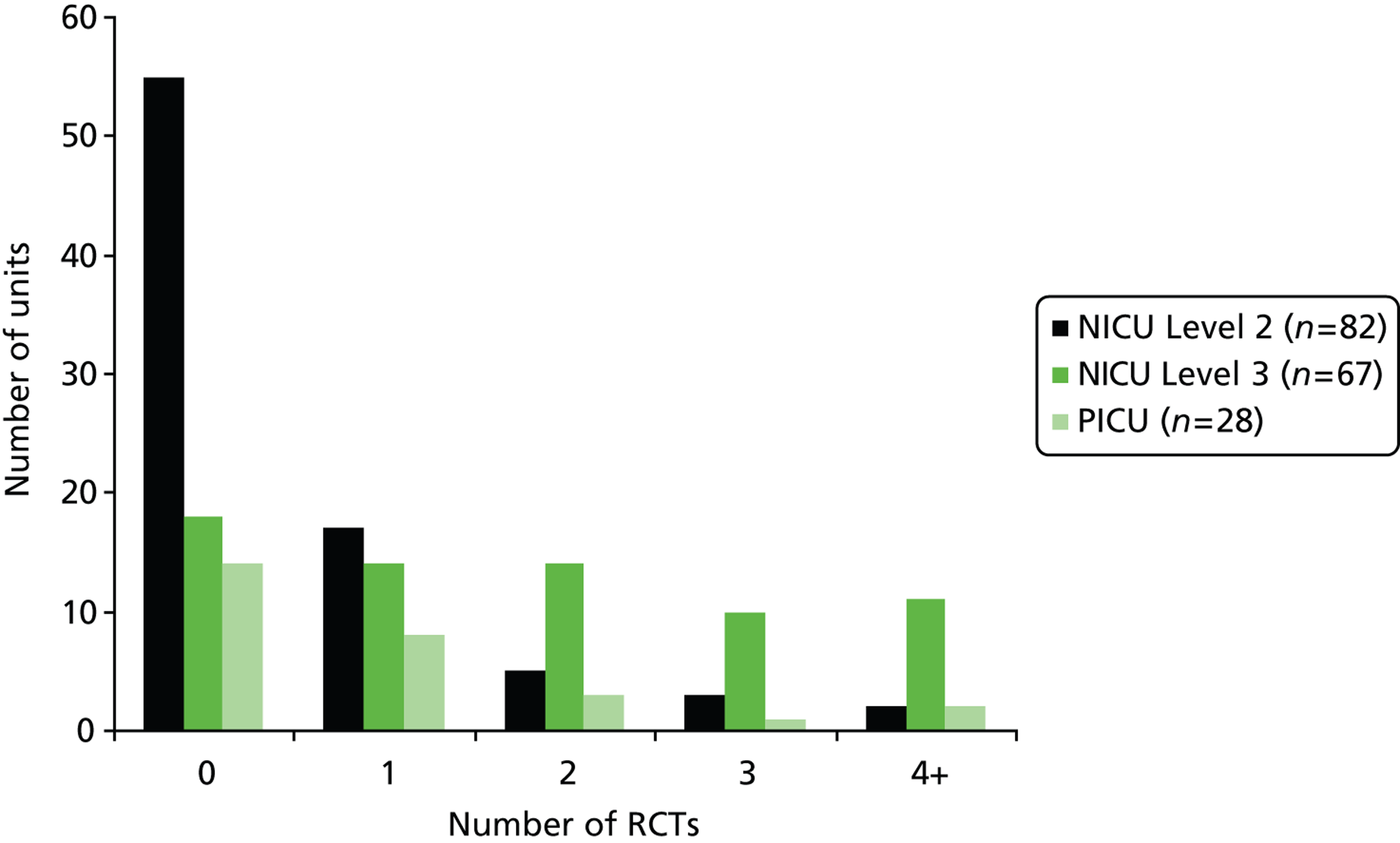
Randomised controlled trial activity was determined by whether a NICU or PICU had enrolled at least one baby or child into a RCT during the period 1 January 2002 to 31 December 2006 (this was confirmed by the trial CI or trial manager in the trials survey). Half of the units enrolled one or more participants in one or more trials during the 5-year study period [76/149 NICUs (51.0%), 14/28 PICUs (50.0%)]. Participation in RCTs was higher in the Level 3 NICUs; 73.1% reported having enrolled babies into one or more trials compared with 32.9% of Level 2 NICUs. Of the 28 PICUs for which questionnaire information was available, 50.0% had enrolled at least one child into one or more RCTs ( Table 5 and Figure 5 ).
| No. RCTs | NICUs | PICUs, N = 28, n (%) | ||
|---|---|---|---|---|
| Level 2, N = 82, n (%) | Level 3, N = 67, n (%) | Total, N = 149, n (%) | ||
| 0 | 55 (67.1) | 18 (26.9) | 73 (49.3) | 14 (50.0) |
| 1 | 17 (20.7) | 14 (20.9) | 31 (20.8) | 8 (28.6) |
| 2 | 5 (6.1) | 14 (20.9) | 19 (12.8) | 3 (10.7) |
| 3 | 3 (3.7) | 10 (14.9) | 13 (8.7) | 1 (3.6) |
| ≥ 4 | 2 (2.4) | 11 (16.4) | 13 (8.7) | 2 (7.1) |
| ≥ 1 RCT | 27 (32.9) | 49 (73.1) | 76 (51.0) | 14 (50.0) |
FIGURE 5.
Participation in RCTs: by type of NICU and by PICU.
Most of the NICUs and PICUs participated in international RCTs (n = 77) or UK multicentre RCTs (n = 39). A small number of Level 3 NICUs (n = 9; 13.4%) and PICUs (n = 5; 17.9%) reported having conducted single-centre RCTs. This was more common for PICUs for which 5 of the 14 responding paediatric units ran single-centre trials (17.9% of units) compared with 9 of the 149 NICUs (6% of units). Notably all neonatal single-centre trials were conducted in Level 3 units ( Table 6 ).
| Type of RCT | NICUs | PICUs, n = 28, n (%) | ||
|---|---|---|---|---|
| Level 2, N = 82, n (%) | Level 3, N = 67, n (%) | All, N = 149, n (%) | ||
| International | 23 (28.0) | 45 (67.2) | 68 (45.6) | 9 (32.1)a |
| Multicentre | 11 (13.4) | 27 (40.3) | 38 (25.5) | 1 (3.6)b |
| Single centre | 0 (0.0) | 9 (13.4) | 9 (6.0) | 5 (17.9) |
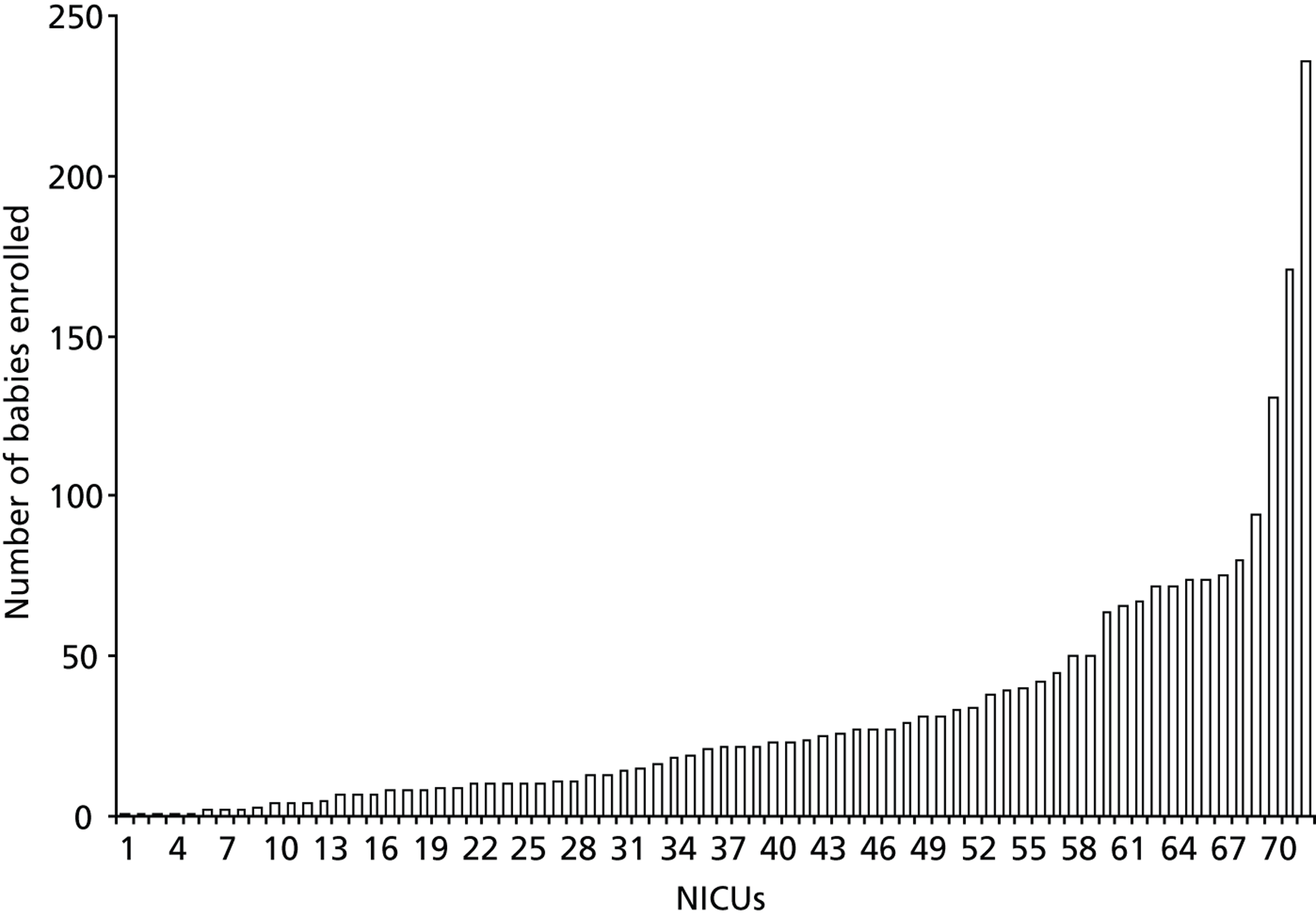
Numbers of babies and children enrolled into randomised controlled trials
Of the 76 NICUs that enrolled to a trial, 72 provided details of the number of babies enrolled. A total of 3117 babies were enrolled by these neonatal units into the 29 neonatal trials for which some enrolment data for the 5-year study period were available ( Table 7 ). The number of babies enrolled per neonatal unit ranged from 1 to 236 [median 22, interquartile range (IQR) 8–40] ( Figure 6 ). Most of those enrolled were being treated in Level 3 NICUs.
FIGURE 6.
Total numbers of babies enrolled across NICUs. Figure excludes 4 out of the 76 NICUs that participated in RCTs but for which numbers of babies enrolled were not obtained.
| Trials | No. enrolled from neonatal units | No. enrolled from paediatric units | Total no. enrolled |
|---|---|---|---|
| Neonatal trials | |||
| No. of trials | 29 | 2 | 29a |
| No. of units | 36 | 2 | 38 |
| No. of babies enrolled | 3117 | 20 | 3137 |
| No. of babies enrolled per recruiting unit; median (IQR) | 1–236; 222 (8 to 40) | 4 and 16b | 1–236; 20 (8 to 39) |
| No. of babies enrolled per trial; median (IQR) | 1–1322; 40 (14 to 104) | 4 and 16b | 5–1326; 40 (15 to 104) |
| Paediatric trials | |||
| No. of trials | 9 | 9 | |
| No. of units | 11 | 11 | |
| No. of children enrolled | 210 | 210 | |
| No. of children enrolled per recruiting unit; median (IQR) | 1–53 | 1–53; 11.5 (2 to 34) | |
| No. of children enrolled per trial; median (IQR) | 2–53 | 2–53; 11 (6 to 39) | |
| All neonatal/paediatric trials | |||
| No. of babies/children enrolled | 3117 | 230 | 3347 |
| No. of trials | 29 | 11 | 38a |
An additional 20 babies were recruited into two multicentre NIC RCTs by two PICUs, bringing the total enrolled in NIC trials to 3137 babies. Of these, 480 (15.3%) were recruited into single-centre trials and 2657 (84.7%) into multicentre trials (UK and international) (see Table 8 ).
Of the 14 PICUs that enrolled into a paediatric trial, 11 provided details of the number enrolled. A total of 210 children were enrolled by these paediatric units into nine PIC trials for which some enrolment data for the 5-year study period were available. The number of children enrolled per paediatric unit into PIC trials ranged from 1 to 53 (median 7, IQR 2–34). Of these, 94 (44.8%) were enrolled into single-centre trials and 116 (55.2%) to multicentre trials (all of which were international) (see Table 8 ).
Mortality* following enrolment into a randomised controlled trial
(*Mortality data refer to deaths before discharge from hospital.)
Overall UK mortality data were available for 28 NIC and nine PIC RCTs. In total, 534/3288 (16.2%) children died before leaving hospital following enrolment in the 37 trials.
The numbers of babies who did and did not survive to leave hospital following enrolment into a NIC RCT are presented in Table 8 by type of RCT. These figures are based on data provided by the trial investigators or reported in the published papers. The 28 NIC trials enrolled 3088 babies, of whom 522 (16.9%) died. Mortality was higher for babies enrolled into international trials (21.3%) than multicentre (11.4%) and single-centre trials (9.8%).
| Type of RCT | Total enrolled, n | Survived, n (%) | Died, n (%) | N/K, n |
|---|---|---|---|---|
| International, n = 7a | 1800 | 1415 (78.6) | 383 (21.3) | 2 |
| Multicentre – UK, n = 10b | 808 | 716 (88.6) | 92 (11.4) | 0 |
| Single centre, n = 11 | 480 | 433 (90.2) | 47 (9.8) | 0 |
| Overall, n = 28c | 3088 | 2564 (83.0) | 522 (16.9) | 2 |
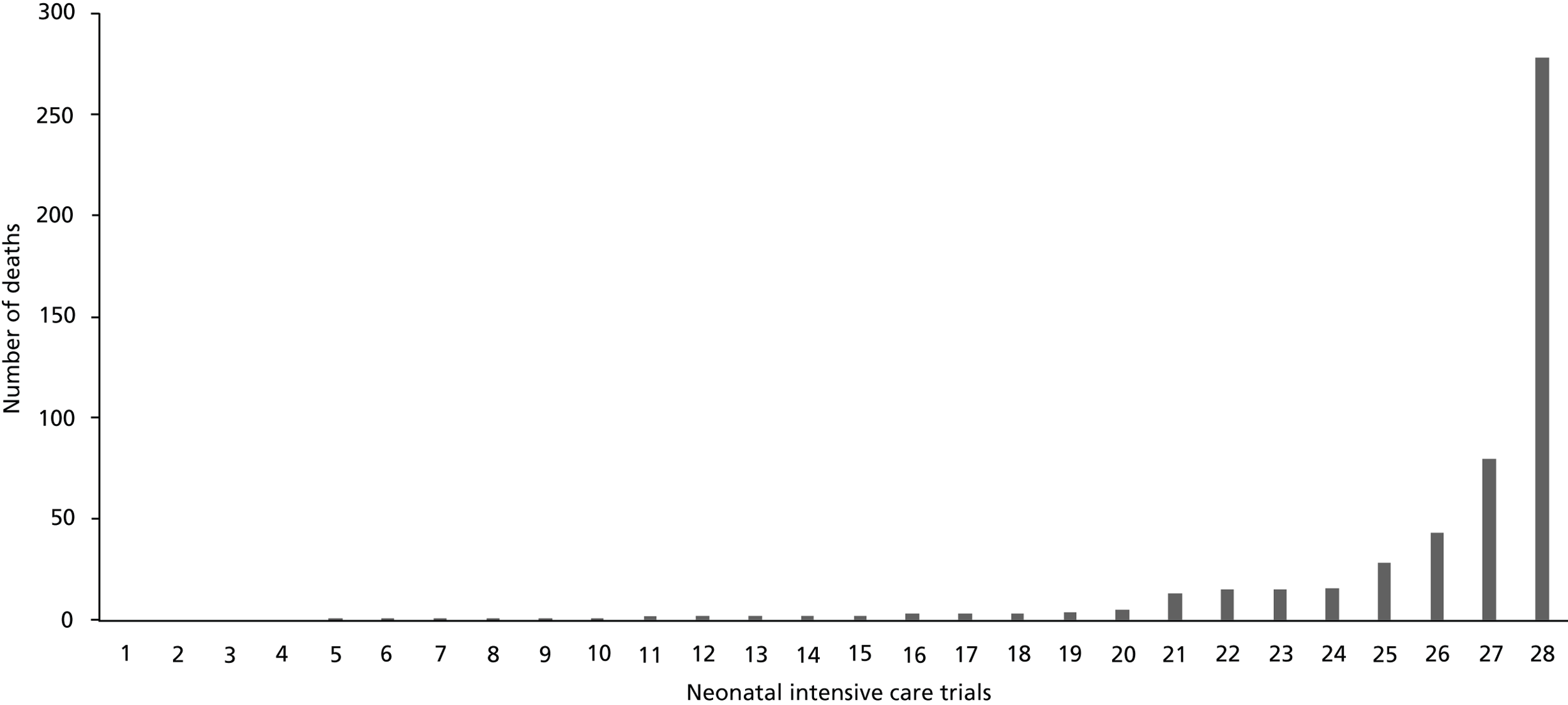
The number of deaths per neonatal trial ranged from 0 to 278 (median 2, IQR, 1–15) ( Figure 7 ). Of the 28 neonatal trials, 24 had at least one death. The highest mortality rate among these trials was 29% (80 deaths). Most reported small numbers of deaths, with only eight trials reporting more than 10 deaths. The majority of deaths [429/522 (82.2%)] occurred in four trials, three of which were multicentre (n = 278 + 80 + 43 deaths) and one single centre (n = 28 deaths). Single-centre trials reported fewer deaths and a lower death rate (47/480, 9.8%) than multicentre trials (475/2608, 18.2%).
FIGURE 7.
Variation in numbers of deaths across neonatal trials. (The high number of deaths in some of these trials is likely to be due to the numbers recruited, the severity of their condition and the types of interventions in these trials.)
In the nine PIC trials for which mortality data were available, 12 (6.0%) out of 200 children died following enrolment into a trial ( Table 9 ). Six of the nine trials had a least one death, with the number of deaths ranging from 0 to 4. Very few deaths occurred in single-centre PIC trials (2/94, 2.1%) compared with those in the single-centre NIC trials (47/480, 9.8%) and the multicentre PIC trials 10/106 (9.4%).
| Type of RCT, n | Total enrolled, n | Survived, n (%) | Died, n (%) |
|---|---|---|---|
| International, 6 | 106 | 96 (90.6) | 10 (9.4) |
| Multicentre UK, 0 | 0 | 0 | 0 |
| Single centre, 3 | 94 | 92 (97.9) | 2 (2.1) |
| All RCTs, 9 | 200 | 188 (94.0) | 12 (6.0) |
Overall, mortality rates were lower for all types of PIC RCTs than for NIC RCTs. However, there appeared to be a similar pattern of higher mortality for children enrolled into international RCTs (10.0%) than for those enrolled into a multicentre (6.2%) or single-centre RCTs (2.1%). There may be many reasons for this, for example the ‘severity’ of the condition being studied in the trial.
Variation in mortality across units (unit-specific mortality)
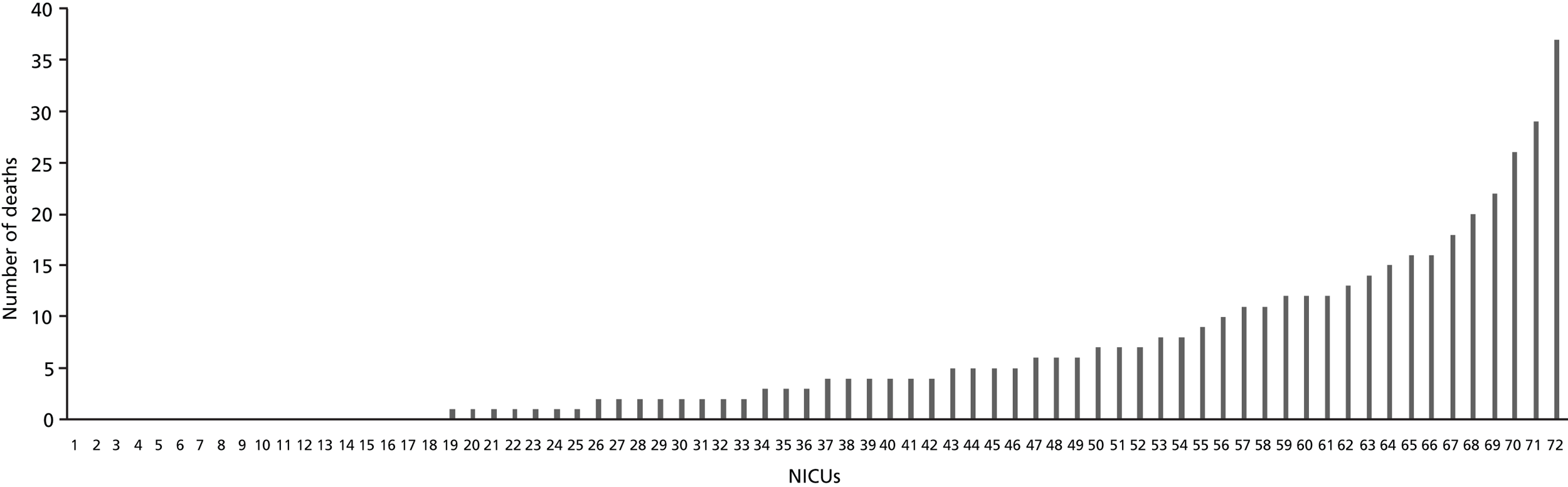
Unit-specific mortality data on 434 deaths were released by 24 neonatal trials for 72 NICUs with the permission of the units in question. The number of deaths per unit ranged from 0 to 37 (median 4, IQR 0–9) ( Figure 8 ). Although 54 units saw at least one death following enrolment in a trial, more than half (42/72, 58.3%) saw fewer than five deaths over this 5-year period ( Table 10 ). Five Level 3 NICUs had larger numbers (n = 37, 29, 26, 22 and 20) and 30.9% of all deaths recorded by the units occurred in these five units. In around half of the units, the proportion of babies who died following trial enrolment was 20% or more ( Table 11 ).
FIGURE 8.
Variation in numbers of deaths across NICUs. (The high number of deaths in some of these NICUs is likely to be due to the numbers recruited, and the severity of the condition of the babies in these units.)
| No. of deaths | NICUs | PICUs |
|---|---|---|
| No. (%) of units (n = 72) | No.a of units (n = 12b) | |
| None | 18 (25) | 6 |
| 1–4 | 24 (33) | 5 |
| 5–9 | 13 (18) | 1 |
| 10–14 | 8 (11) | 0 |
| 15–19 | 4 (6) | 0 |
| ≥ 20 | 5 (7) | 0 |
| Total no. of deaths in these units | 434 | 14 |
| No. (%) of units seeing at least one death | 54 (75) | 6 (43) |
| Proportion of deaths | NICUs | PICUs |
|---|---|---|
| No. (%) of units (n = 72) | No. of unitsa (n = 12b) | |
| 0 | 18 (25) | 6 |
| > 0–0.1 | 10 (14) | 3 |
| > 0.1–0.2 | 14 (19) | 3 |
| > 0.2–0.3 | 16 (22) | |
| > 0.3–0.4 | 7 (10) | |
| > 0.4–0.5 | 5 (7) | |
| ≥ 0.5 | 2 (3) |
Nine PIC trials released unit specific mortality data for 14 PICUs. The number of deaths per unit ranged from 0 to 7 (median 1, IQR 0–2), with six units witnessing at least one death (see Table 10 ). In all of these units, the proportion of children who died following trial enrolment was under 20% (see Table 11 ).
Trials survey: practices after the death of a child
Collection of post-mortem data
For the 36 NIC RCTs, 23 trial investigators reported whether they had collected post-mortem data for the trial. All reported that collection of post-mortem data was not part of the trial protocol. For nearly half (47.8%) this was because these data were not considered necessary. Nine trial investigators reported that although they did not specifically request post-mortem examinations to be conducted on their behalf, they did request to see post-mortem reports if they were available ( Table 12 ).
| Reason for not collecting post-mortem data | N | Requested copies of post-mortem reports if available? | ||
|---|---|---|---|---|
| Yes, n | No, n | Not reported, n | ||
| Not necessary | 11 | 2 | 7 | 2 |
| Desirable but unlikely to be successfully obtained | 5 | 4 | 1 | |
| Other | 1 | 1 | ||
| No reason given | 6 | 3 | 3 | |
| Total | 23 | 9 | 9 | 5 |
For the 14 PIC RCTs, nine trial investigators reported whether they had collected post-mortem data for the trial. All reported that collection of post-mortem data was not part of the trial protocol mainly because it was not necessary. None of the trial investigators had requested to see post-mortem reports if they were available ( Table 13 ).
| Reason for not collecting post-mortem data | N | Requested copies of post-mortem reports if available? | ||
|---|---|---|---|---|
| Yes, n | No, n | Not reported, n | ||
| Not necessary | 6 | 5 | 1 | |
| Desirable but unlikely to be successfully obtained | 1 | 1 | ||
| Other | 1 | 1 | ||
| No reason given | 1 | |||
| Total | 9 | 0 | 7 | 1 |
Contact with bereaved parents from the trial team
Of the 50 RCTs, investigators for just over half (n = 27) provided a copy of the full trial protocol. None of the protocols documented a policy relating to the care of parents bereaved following enrolment of their child into the RCT.
Parent information leaflets were provided for 29 of the 50 trials. These were generally of a standard format that included information about the purpose of the trial, details of the investigators organising the trial, what taking part would involve, the benefits and risks of taking part, what would happen if the parents declined their baby or child taking part, what would happen if something went wrong, the rights of the parents to withdraw their baby or child from the trial at any time, and confidentiality. Some also referred to the publication of the trial results, and whether and when these would be available to parents. [For those trials in which parents were told – when their baby was alive at enrolment – that they would be sent the results of the trial, there could be a clash between this expectation having been set up if, later, bereaved parents were not asked when permission was sought to send the results (e.g. at discharge from hospital or at follow-up)].
Of the 50 RCTs, investigators for two NIC trials (one multicentre and one UK-led international RCT) had produced an information leaflet specifically for bereaved parents. Both leaflets were produced from the same CTU and were of the same format as follows.
The trial investigators started by offering their sincere condolences to the parents and thanked them for allowing their baby to participate in the trial. They expressed their hope that the parents would find some consolation in the fact that their baby’s contribution to the trial would play an important part in future decisions about the best way to care for babies with similar medical conditions. They offered reassurances that all the information collected would be stored safely and confidentially, and would be used in the analysis at the end of the trial. Information about organisations, such as SANDS (the Stillbirth and Neonatal Death Charity: www.uk-sands.org) and BLISS (the National Charity for the Newborn: www.bliss.org.uk) were provided, including telephone helpline numbers. The leaflet ended by offering the parents the opportunity, if they wished, to ask questions about the trial, either relating to their baby or more generally, by contacting either the doctor or nurse who cared for their baby or by contacting a named person at the trial co-ordinating centre.
One clinical trial investigator for a single-centre NIC trial reported having a policy on contact with bereaved parents but took a different approach. Three deaths occurred following enrolment into this trial and the investigator had sent a personal letter to each set of parents to thank them for allowing their child to take part in the trial and to offer contact should they wish to discuss the trial or the continued use for their child’s data in the trial.
Provision of bereavement support in neonatal intensive care units and paediatric intensive care units
Of the 149 NICU respondents, 110 (75%) provided information about bereavement care. Of these, 41 (37%) reported that there was a specific bereavement counsellor for their unit. Of the 69 (63%) NICUs that did not have a specific bereavement counsellor, most reported other sources of support, but two respondents reported that there were no other sources of support for bereaved parents ( Table 14 ).
| NICU | Bereavement counsellora | |
|---|---|---|
| Yes, n (%) | No, n (%) | |
| NICU Level 2, n = 61 | 23 (37.7) | 38 (62.3) |
| Other sources of support: | ||
| Yes | 18 | 38 |
| No | 3 | – |
| Not reported | 2 | – |
| NICU Level 3, n = 49 | 18 (36.7) | 31 (63.3) |
| Other sources of support: | ||
| Yes | 16 | 28 |
| No | 2 | 2 |
| Not reported | – | 1 |
| All NICUs, n = 110 | 41 (37.3) | 69 (62.7) |
| Other sources of support: | ||
| Yes | 34 | 66 |
| No | 5 | 2 |
| Not reported | 2 | 1 |
Of the 28 PICU respondents, 21 (75%) provided information about bereavement care. Of these, 13 (62%) reported that there was a specific bereavement counsellor for their unit. Nearly all respondents reported that other sources of support were available as well. Of the eight (38%) PICUs without a specific bereavement counsellor, three respondents did not report whether other sources of support for bereaved parents were available ( Table 15 ).
| PICU | Bereavement counsellora | |
|---|---|---|
| Yes, n (%) | No, n (%) | |
| PICUs, n = 21 | 13 (61.9) | 8 (38.1) |
| Other sources of support | ||
| Yes | 11 | 5 |
| No | 2 | – |
| Not reported | – | 3 |
The most frequently reported source of support for bereaved parents was the hospital chaplaincy service. Unit-led support was usually from midwives or nurses in the NICU or PICU who were trained counsellors ( Table 16 ).
| Other sources of support | NICUs, N = 100, n (%) | PICUs, N = 21, n (%) |
|---|---|---|
| Chaplaincy services | 89 (89.0) | 12 (57.1) |
| Unit-led support | 23 (23.0) | 9 (42.9) |
| Hospital or department counselling/support services | 18 (18.0) | 8 (38.1) |
| Local support group | 7 (7.0) | 3 (14.3) |
| Social workers | 3 (3.0) | 0 (0.0) |
Discussion
The BRACELET study is currently the only study to investigate RCT activity in UK NICUs and PICUs, to report the numbers of babies and children enrolled into these trials, and to determine the extent and distribution of mortality involved. The study showed that in a 5-year period (2002–6), approximately 50% of the NICUs and PICUs from which a response was received participated in one or more of 50 RCTs, enrolling over 3000 babies and children. However, most of the RCTs were conducted in a much smaller number of units. Less than 20% of NICUs and around 10% of PICUs had taken part in three or more RCTs during the 5-year period. For the majority of international and multicentre RCTs very low numbers of UK units took part. For example, fewer than five UK units took part in six out of nine international RCTs and in 8 out of 11 multicentre RCTs conducted in NIC for which this information was available. Level 3 NICUs were more likely than Level 2 NICUs to have participated in RCTs, including conducting their own single-centre RCTs. This is probably because the types of interventions being evaluated in a RCT, especially those aimed at the sickest babies and children, are more likely to delivered in these Level 3 units. Such units also tend to be located within teaching hospitals where there are more doctors and nurses with academic appointments and better resources for research activity.
In both NIC and PIC trials, the interventions that were most frequently evaluated were drugs but only a very small number of RCTs received funding from commercial organisations. There is widespread concern that most medicines used in the field of paediatrics are unlicensed or off-label. 2,86 It has been suggested that the pharmaceutical industry has been reluctant to conduct clinical trials of medicines because of the small market for the sale of drugs for babies and children, making investment in expensive research less attractive. Other reasons may include concerns about the ethics of conducting research in children, difficulties with blood sampling and recruiting sufficient numbers. 2
Nearly three-quarters of the RCTs identified involved NIC. This is perhaps not surprising given that PIC is a relatively new specialty and that there are only a small number of PICUs in the UK, reflecting the smaller numbers of children needing intensive care. The majority of RCTs in both NICs and PICs were multicentre (whether in the UK only or international). NIC RCTs were more likely to have been UK led than PIC RCTs, with the majority (72.2%) of NIC trials having been conducted within the UK only. There were more multicentre than single-centre NIC RCTs, of which most were collaborations between two or three NICUs. Of the international RCTs conducted within NIC, 60% were led from the UK.
Although PICUs were clearly taking part in collaborative research, the lack of UK-based multicentre RCTs over the period of this survey suggests that collaborative networks within the UK still needed to be established. Since then, three UK-based multicentre PIC trials were funded by HTA: SLEEPS: Safety profiLe, Efficacy and Equivalence in Paediatric intensive care Sedation (www.hta.ac.uk/1613); CATheter infections in CHildren – the CATCH trial (www.hta.ac.uk/1867); and Control of Hyperglycaemia In Paediatric intensive care trial – the CHIP trial (www.hta.ac.uk/project/1533.asp).
For over half of the RCTs for which the information was available, mortality was either a primary and/or secondary outcome measure. Although for other RCTs, mortality was not an end point, these data were still being recorded as trial investigators were able to provide numbers of hospital survivors and non-survivors for the BRACELET study. Over 500 deaths (around 16% of the total enrolled) were reported, predominantly in the neonatal context. The causes of the deaths were usually based on clinical, rather than pathologist reports, as there has been a general decline in neonatal and paediatric autopsy rates over the last 20 years. 87–89 It is not surprising therefore that all of the trial investigators who completed the section of the questionnaire about post-mortem data reported that these data were not specifically collected as part of the trial protocol. Most PIC investigators (66.7%) reported that post-mortem data were unnecessary compared with less than half (47.8%) of NIC investigators. None of the PIC investigators requested to see post-mortem reports, whereas 39.0% of NIC investigators reported that they had.
Whatever the cause of deaths, the variation in the distribution of the deaths identified in the survey suggests that a complex relationship exists between mortality, RCTs and units. For instance, in both NIC and PIC RCTs, higher proportions of babies and children died following enrolment into international RCTs than those enrolled into multicentre or single-centre RCTs. This could be because international RCTs were studying sicker populations, or needed to be international to accrue the larger numbers of units and babies or children to assess mortality differences, but there were also cases where there was such high mortality (e.g. owing to the severity of the condition at trial entry) that the necessary numbers could be enrolled in UK-only trials.
The most obvious division, however, is between the NIC and the PIC contexts. Although the same proportion of NICUs and PICUs took part in at least one trial, the numbers of deaths and the mortality rates that were identified were very different (n = 522 vs. n = 14 and 16.9% vs. 6.4%, respectively), and less than half of the PICUs that enrolled children into one or more RCTs witnessed a death following enrolment. Only one PICU reported more than one death. This is not surprising, as PIC mortality is generally lower in PIC (4.2% in 2009) than in NIC. 90 As death is a ‘rare event’, it is seldom used as a primary outcome measure in PIC RCTs. Rather the focus is other end points such as ‘ventilator-free days at 30 days after randomisation’91 or ‘nosocomial infection rate’. 92 This may mean that for the PIC specialty overall, death following RCT enrolment may not be a particularly salient issue, although for individual trials in the future that involve critically ill populations and those involving interventions that aim to improve survival, it will still be a potentially important consideration.
In contrast, a high proportion of NICUs (71.1%) that enrolled babies into at least one RCT witnessed one or more deaths following enrolment. The number and proportion of deaths in the NIC setting do suggest the salience of the issue both for the specialty and for the NIC research community. The degree of salience might well differ, however, across NICUs given the uneven distribution of deaths. Given that Level 3 NICUs enrolled more babies into RCTs than Level 2 NICUs, it is not surprising that the total number of deaths was also higher in these units. Even so, the proportion of babies who died overall was similar for the two types of units. There was however, a marked difference in the proportion of babies who died following enrolment into a multicentre RCT. Mortality was higher in the Level 3 units than in the Level 2 units but this is probably because more of the Level 3 NICUs took part in a large multicentre RCT that was enrolling much sicker babies.
Some NICUs did not participate in any trials and some that did, did not record any deaths. The majority of the reported deaths occurred in small numbers per unit; over half of the units that recruited to a trial reported five or fewer cases in the study period and so saw one or fewer cases per year. There was, however, a group of particularly research-active NICUs with much greater experience of death in this context. Five units witnessed 20 or more deaths in the study period, the highest number being 37, and one-quarter of the deaths for the neonatal sample as a whole (134/520) occurred in these units. All five were Level 3 units, which had high recruitment rates to RCTs with high mortality rates, and to RCTs with lower mortality rates but large samples. The RCTs to which these five units recruited were a mix of international, multicentre and single-centre RCTs.
The overall mortality figures for the NIC RCTs themselves were also variable, but with a far wider range and a far more skewed distribution than was seen for individual units. Again, some trials reported no or very small numbers of deaths, but the vast majority (82%) of reported deaths were concentrated in only four RCTs and more than half occurred in one high-recruiting RCT. This RCT reported 278 deaths before discharge from hospital in the 5-year period in the UK and a 21% mortality rate. [This trial has since been published,93 with a total of 579 deaths before discharge (16.6%), although these were not all in the UK and spanned more than the 5-year period covered by BRACELET.]
As further RCTs are initiated and accrue more participants, the population of parents bereaved after agreeing to enrol their child in a trial will accumulate; it is already sufficiently sizeable to warrant attention, but whether and how to respond to this population are complex questions.
Provision for bereavement is almost universally made within clinical centres but this body of parents, with potentially diverse experiences and needs, is largely scattered across a number of recruiting clinical centres; most deaths within trials occurred as relatively isolated cases and the majority of centres witnessed small numbers of deaths per year. This is likely to make it difficult for many of the clinical centres to develop, assess and sustain specialised responses to post-trial bereavement themselves. The patterns of mortality revealed by the BRACELET study also suggest, however, that there were pockets of NICUs and NIC trials with substantial numbers of deaths. In general, large units draw upon well-developed bereavement services,5 and research-active centres such as these may be appropriate candidates to develop and assess dedicated trial-related bereavement practices. In trials in which a substantial number of deaths is anticipated, it may also be possible to develop and assess trial-related bereavement practices. The BRACELET study showed that three trials had already developed a response to bereavement, such as preparing a bereavement leaflet for use in clinical centres or sending condolence letters directly to parents (for an example leaflet, see www.npeu.ox.ac.uk/down-loads/nest/NEST-Bereavement-Leaflet.pdf). What other forms that trial-related bereavement practices might take is unclear. This is discussed later in the monograph (see Chapter 9 ).
The BRACELET study has demonstrated that bereavement occurs in relation to RCTs of any size and type and with a range of clinical foci. The four trials that reported the majority of deaths in the 5-year period assessed very different interventions, from routine care practices to potentially life-saving technologies. They involved very different populations and were conducted in single-centre, multicentre and international contexts. This suggests that bereavement in a trial context may be an issue of broad relevance in specialties such as intensive care, and that it could be particularly appropriate for large trials – or trials focusing on high-risk situations – to plan for and assess their approach to bereavement with substantial research populations.
Trials are complex, highly collaborative endeavours between recruiting clinical centres and trial teams – groups that may feel a shared interest in and responsibility for parents bereaved in trials. Their collaboration might be exploited to good effect if experts within these fields take collective responsibility for the potential needs of the population identified here. If those trials and clinical centres with the greatest experience of post-trial bereavement develop effective approaches to care for and support bereaved parents, other smaller trials and centres may draw upon their recommendations and follow their lead. Even in the PIC context in which deaths occurred infrequently, PICUs were more likely than NICUs to have a dedicated bereavement counsellor, and individual trials may still involve severely compromised populations and so find that post-trial bereavement care is a salient issue.
It is, however, important that recommendations in this novel area should be based, from an early stage, on sound empirical evidence that draws upon views of all relevant parties with their potentially different perspectives and insights. Clinical teams often recruit to a number of trials concurrently and see bereaved parents in a variety of circumstances; they may be best placed to consider the broad range of bereavement-related issues that might occur in clinical contexts. Trial teams, by comparison, consider parents in the relatively more uniform circumstances set by the eligibility criteria for their particular trial; they may be best placed to consider bereavement practices that are tailored to fit the population and circumstances of a given trial. Research in this area is sensitive but it is essential that bereaved parents should also be consulted. Studies have demonstrated that bereavement-related research is feasible26,27,36,60,74,75,93 and suggest that bereaved parents might be willing and helpful participants on this challenging and sensitive subject. The task ahead is for those with relevant insight and expertise, to collaborate to find a range of approaches that are sensitive to the variety of parents seen by clinicians and applicable and adaptable to the specific circumstances addressed in individual trials.
Strengths and weaknesses
A major strength of Phase I of the BRACELET study is that a high response rate to the unit survey was achieved, owing, most likely, in part, to the evidence-based strategies used to maximise responses. 85 Efforts were made to ensure that all RCTs were identified by searching relevant research databases and websites in addition to surveying units. We are reasonably confident that most of the larger international and large multicentre RCTs (five centres) were found. However, other smaller RCTs with only two or three participating centres may have been missed. It is very likely that there may have been under-reporting of single-centre RCTs. For example, small trials conducted for university doctorates or master’s degree projects may have been overlooked by those filling in the questionnaires, particularly if the results were never published. The establishment of mandatory trial registration is beginning to facilitate the searching process for future studies, although the variety of registers and the differences in search terms is still a problem. 94
One approach we could have used to validate the unit survey data would have been to contact the relevant R&D departments, given that all research in the UK NHS now requires their approval. However, this was not possible because of the limited resources available. We did find that in a small number of cases, in which information was initially provided by the R&D department, their records were not necessarily accurate or complete. This is likely to vary across NHS hospital trusts.
There are, however, clear limitations to the study. As data were not available for every RCT for every unit, the total numbers of babies and children enrolled into a RCT during the 5-year period is under-reported. For the same reasons, it is possible that the numbers of deaths are under-reported as well. Also, the surveys have a narrow focus on figures for deaths up to discharge from hospital; post-discharge deaths were not included. Other adverse outcomes for parents and families, such as disability and loss of quality of life in surviving babies, are also extremely important but were beyond the remit of the study.
Next steps
The implications of the finding from these surveys are that that the numbers of deaths in the context of a RCT are not insignificant, and further studies may therefore be needed to examine the views of the people most affected by these deaths – bereaved parents, clinicians and triallists. As a first step in this process, the BRACELET study included a second qualitative component that aimed to explore death, dying and bereavement in the context of NIC RCTs from these multiple perspectives. This is described further in the following chapters of Section B of this monograph.
Section B: Qualitative study of bereavement-related practice and experience in neonatal trials
Chapter 4 Research methods and samples
The research sequence: from Phase I to Phase II
The BRACELET study was designed as a sequential study, with components of the research carried out in several steps so that the earlier findings might guide subsequent research questions and processes. This was an effective approach as the insights gained from the quantitative surveys carried out for Phase I shaped both the structure and focus of Phase II in important ways.
The Phase I surveys showed that almost 17% of the babies enrolled into NIC RCTs in the 5-year study period had died, but a much lower proportion, 6%, of children enrolled in PIC RCTs had died. It was clear that far fewer trials were conducted in the PIC context and far fewer deaths had occurred – 12 in the 5-year period compared with 522 in the NIC context. This prompted a major design shift for Phase II; on the grounds of salience and feasibility the study was refocused to consider mortality and bereavement only in the neonatal context.
The Phase I surveys also showed that most of the neonatal deaths occurred in small numbers scattered across many NICUs. There was, however, a core of five NICUs, which dealt with their region’s sickest babies and were high recruiters to trials, which saw ≥ 20 deaths each in the 5-year study period; collectively they saw over one-quarter of all reported deaths in Phase I. Phase I also showed that the vast majority of deaths occurred in only four RCTs.
This clear pattern of high recruitment in specialised centres to a key group of trials set the structure for Phase II. Access was successfully negotiated to each of the four trials and the five NICUs, with a view to interviewing trial team members, recruiting clinicians and bereaved parents for the multiperspective qualitative interview study. From then on these trials were referred to as the core trials, and the five recruiting NICUs as the core centres.
Phase I informed the structure of Phase II ensuring that the sample of trials and interviewees were assembled on the grounds of relevance and expertise, but its methodological impact extended much further into the qualitative study. The patterns of recruitment and mortality, and the distribution of the deaths that were identified in the core trials and core centres offered new research material to the BRACELET team, genuinely informing design and direction. Consideration of the similarities and differences in this particular combination of trials triggered new research questions even before the qualitative work had begun, yielding valuable preliminary insights into the topic. The issue of trial size is one example. The highest number of deaths in the core trials occurred in an international multicentre trial co-ordinated by a major UK CTU. This trial reported 278 deaths in the UK over the 5-year study period and these were distributed across many UK centres. The core centre with the highest number of deaths reported to BRACELET (n = 37) did not, however, accumulate these from this large trial; the vast majority of these deaths were associated with another core trial, their own single-centre trial in which there was 28 deaths. In contrast with the scattered pattern of deaths in the larger trial, the deaths in this single-centre trial occurred within a narrow geographical area under the auspices of a small team that had both clinical and research responsibilities for the trial population.
The Phase I findings highlighted the importance of considering logistical and structural management of trials, their different clinical settings, research foci and populations, and parental circumstances at recruitment. The surveys made it clear that, in many UK centres, death in the context of a clinical trial was a rare event, and so the clinicians in these centres, although they may be experienced in bereavement care, would be unlikely to have built up specific experience and direct insights into the parental situation of bereavement subsequent to trial enrolment. The findings also suggested that in the specialist core centres, at which there were more deaths, clinicians may well have personal experiences of recruitment and subsequent support of bereaved parents, which might be explored in interview, and that the sample of bereaved parents could be recruited for Phase II from these centres.
Study design for the qualitative study
The structure of the qualitative study was developed as a result of the work for Phase I and involved core trial team members [i.e. trial investigators, members of the managerial team, TSC members, DMC members (the term ‘trial team members’ is used as a convenient shorthand, although we recognise that DMC members are not strictly part of the trial team as they are independent, as, indeed, are some members of a TSC)], core centre recruiting clinicians, bereaved parents whose baby was offered enrolment into a core trial in a core centre. [We did not wish to exclude from the sample parents who had declined trial participation and so the aims for recruitment included those who were offered enrolment regardless of their decision. In practice, it was unlikely that parents who declined would be able to enter the study, as the core trial records used for contacting parents included only those who had enrolled into the trial. We therefore generally refer to ‘enrolled’ rather than ‘invited to enrol’ unless there might be ambiguity (but see also alternative recruitment methods, Chapter 10 ).]
The core trials
The initial core trials considered in BRACELET were ‘Non-specific intravenous immunoglobulin therapy for suspected or proven neonatal sepsis (INIS)’,95,96 ‘Whole body hypothermia for the treatment of perinatal asphyxial encephalopathy (TOBY)’,97–99 ‘Prophylactic Granulocyte–macrophage colony-stimulating factor (GM-CSF) to reduce sepsis in preterm neonates (PROGRAMS)’100,101 and the ‘Extreme Preterm Nutrition study: Improving post-natal head growth in very preterm infants: a randomised controlled trial of hyperalimentation (ExPN) feeding study’. 102,103 These are described in more detail in the following pages. During the course of BRACELET, in response to recruitment difficulties that will be described below, the decision was made to open the study to recruitment of parents through an additional NIC trial – ‘Benefits of oxygen saturation targeting for very preterm babies (BOOST-II UK)’. 104,105 This trial raised issues that were especially relevant to the research topic, and so was latterly classed as an additional core trial, bringing the total number of trials considered to five. Through BOOST-II UK, an additional two NICUs joined the study so that seven NICUs contributed to BRACELET. These latter two NICUs centres were considered to be ancillary rather than core centres because they were brought into the study at a stage when recruitment of clinicians and Trial team members was complete and only parents could be recruited.
Recruitment to BRACELET was also expanded to include an additional route in to the study for parents responding to specific publicity pathways approved by the Research Ethics Committee (REC), and this allowed bereaved parents whose baby was enrolled in any NIC RCT to take part (see below and Section C of this report for more details). Most of the parents who entered the study in this way were in fact still linked to the core trials, and so the initial design and structure held. A small number of parents from two additional trials, I2S2 [multi-centre randomised controlled trial which is considering iodine supplementation in preterm infants (www.npeu.ox.ac.uk/i2s2)] and SUPFOR [single centre randomised controlled trial in preterm infants receiving expressed breast milk comparing the use of breast milk fortification versus supplementation using a preterm formula (www.controlled-trials.com/ISRCTN92790284)] opted into the study. Although their interviews are included in the analysis and the parents’ accounts are referred to in this report, these two trials are not themselves considered as core trials and these interviews did not count towards the target number of interviews.
Brief details of the five core trials are shown below, with further information and reflection presented in Chapter 5 :
-
INIS International Neonatal Immunotherapy Study. This trial examined whether intravenous immunoglobulin, a blood product containing human antibodies, helps babies of any gestation who have a serious infection.
-
TOBY Whole body hypothermia for the treatment of perinatal asphyxial encephalopathy. This trial assessed whether cooling babies who have suffered perinatal asphyxia (lack of oxygen to the brain before birth) makes a difference to their chances of recovery.
-
PROGRAMS PROphylactic GRAnulocyte–macrophage colony-stimulating factor (GM-CSF) to reduce sepsis in preterm neonates. This trial considered whether granulocyte–macrophage colony-stimulating factor (GM-CSF) helps very premature babies who have a serious infection.
-
BOOST-II UK Benefits of Oxygen Saturation Targeting for very preterm babies. This trial was trying to find out whether higher or lower oxygen saturation levels are better for extremely preterm babies in neonatal intensive care.
-
ExPN Extreme Preterm Nutrition study – Improving postnatal head growth in very preterm infants: a randomised controlled trial of hyperalimentation. This trial looked at the effects of giving extra nutrition to extremely preterm babies in the ICU.
There were a number of contrasting features of the trials, which made them interesting subjects for study. INIS involved babies with a known infection (the majority of whom were preterm), TOBY involved term babies affected by birth asphyxia – both situations in which babies are severely compromised and the trial intervention had the potential to affect survival. PROGRAMS assessed a prophylactic intervention, seeking to prevent infection in a group of preterm growth-retarded babies. ExPN assessed a feeding intervention and was unlikely to affect mortality but was conducted in a population at very high risk of death – extremely preterm neonates of ≤ 27 weeks’ gestation. BOOST-II UK involved a similar extremely preterm population but focused on the delivery of oxygen. Four of the trials were co-ordinated by the NPEU CTU (hereafter referred to as NPEU), and involved experienced administrative and analytical teams. They drew widely on the expertise available in the neonatal community with a TSC and a DMC, as well as the input of recruiting clinicians in the participating clinical centres. The single-centre trial was designed, co-ordinated and analysed by a small expert team of local clinicians. These features of the trials are explored further in Chapter 7 .
Plans for recruitment
The aim was to carry out interviews with members of the core trials and their recruiting clinicians and with bereaved parents of babies who were enrolled in these trials. The structure that this produced is shown in Figure 9 .
Recruitment strategy for clinicians and core trial team members
Rationale
The recruitment strategy for the interviews with core trial team members and clinicians was largely purposive, aiming to recruit according to role. This was to ensure that the sample would represent the different skill sets that are involved in the running, implementation and delivery of a RCT. The intention was to recruit two broad groups of individuals: those with a range of protocol development and organisational roles within a core trial, and those with clinical roles for a core trial in a core centre.
The protocol development and organisational roles were those relating to the management of the scientific, ethical or logistical elements of a trial, such as investigators, managers and administrators, and members of TSCs and DMCs. The aim here was to capture the views of those whose decisions and practice had shaped the design, evaluation and management of the core trials. The clinical roles were those where individuals had direct contact with trial participants and their families, and had responsibility for implementing trial interventions. These were PIs in the core centres who often took a lead in recruitment and took responsibility for the local conduct of the research, the recruiting clinicians and the staff who worked with them on trials at a local level such as dedicated research nurses.
The aim was to produce a sample that fairly represented the multidisciplinary profile of the collective body of trial staff and associated experts that co-ordinated and ran each of the core trials, but was also structured to permit consideration of the cross-cutting of roles that occurs in trials more generally, for instance for membership of a DMC and being a recruiting clinician in another trial, and the particular issues relating to death and bereavement that they might face. This structure is shown in Figure 9 .
FIGURE 9.
Structure of qualitative phase of BRACELET.
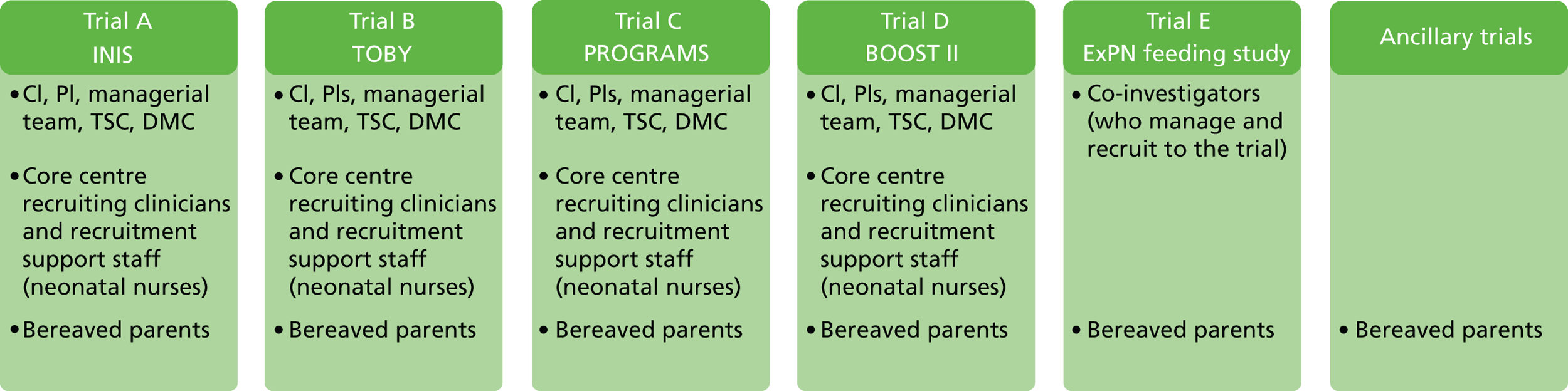
Delineation of responsibilities within a trial in this way was not straightforward. The individuals with the organisational roles of interest could mostly be identified through publically available core trial material in protocols, publications and websites, and some details were provided by the trial co-ordinators. It was clear from the lists of names that were generated that some trial team members had responsibilities to more than one of the core trials, and that some had developmental, organisational and clinical responsibilities within and across the core trials, for instance acting as an investigator for one trial, a TSC member for another, and a recruiting clinician for another ( Figure 10 ).
FIGURE 10.
Representation of roles within and across the core trials.
These multiple and overlapping roles identified in the lists were of particular interest, as they pointed to a degree of expertise and overview among the senior members of the academic, clinical and trials communities, many of whom had played a part in some of the developments in neonatology, which would make the interviews especially informative. Familiarity and an extensive range of trial-related experience was likely to overspill the formal boundaries of the five core trials and this would allow BRACELET to look more closely at issues that were relevant to NIC RCTs generally, as well as those that might be a product of the unique circumstances set up by an individual trial. However, the overlap also meant that trial role as originally conceived was not necessarily the best way to conceptualise the expertise within the sample.
The BRACELET team had expected a degree of overlap in the trial-related roles but the interconnection that was emerging even before any interviews were carried out was even more extensive than anticipated. The original typology was therefore refined to refer to three subgroups of trial-related professionals, i.e.:
-
core trial team members (non-recruiting)
-
core trial team members (recruiting)
-
core centre recruiting clinicians and related support staff (non-trial team members).
The original intention was to recruit 25 trial team members (those with protocol development and organisational roles) and 25 clinicians. This was modified to the aim of recruiting a combined target of 50 clinicians and trial team members, with the breakdown between the groups unspecified.
Recruitment
The multidisciplinary and collaborative nature of trials meant that the sample of clinicians and trial team members was widely distributed across the UK and across many different institutions, hospitals, universities, funding bodies, research organisations, charities and special interest groups. Although the names of most of the core trial team members and their roles were readily available, details of recruiting clinicians were not in the public domain. This meant that different approaches to identifying and recruiting individuals were required in relation to their role in a trial.
Contact with many of the trial team members concerned was facilitated through the core trial office or mediated through trial CIs and PIs. Some individuals were well known to the BRACELET team and they were contacted directly. Recruitment of the core centre recruiting clinicians was more complicated. Recruiting clinicians were eligible to take part in BRACELET if they were a named signatory on the consent form signed by bereaved parents when they had initially enrolled their baby into a core trial at a core centre. Named research nurses who supported these clinicians in their recruitment roles, and who had responsibility for care of the babies in the trial and data collection in core centres, were also identified by the core trial office staff. In three cases, additional nurses who were not named trial team members, but who collectively supported the running of a trial in one of the core centres, were suggested as appropriate invitees by a PI. The aim in linking clinicians to cases when babies had died was not to ask them to comment on those individual cases but to draw into the study a group of clinicians with direct experience of the situation of interest. Once potential interviewees were identified, by whichever route, a letter inviting participation was sent electronically or by post, with a reply slip and an information booklet (see Appendix 11 ). The booklet highlighted a key issue about confidentiality as shown in the extract below ( Box 1 ).
We always keep the details of research participants strictly confidential. Any information that we use would have your name and affiliation removed.
We should point out, however, that it may be possible for others who are familiar with the field to make a guess at the identity of some of those involved in the study, especially those with very specific roles within a trial. If you have a very specialised role in one of the core trials, you should agree to participate only if this is acceptable to you.
Recruitment for bereaved parents
Interviews with bereaved parents in Phase II of the BRACELET study were carried out between March 2010 and July 2012. There were two main periods of recruitment for these parents, and these involved different strategies. The first strategy operated from September 2009 until May 2010 and was replaced by the second, two-part strategy, which started in July 2011 and continued until the target sample size from the core trials was achieved in July 2012. The details underlying the different recruitment strategies will be discussed further in Section C, Chapter 10 , and details of supporting paperwork can be found in the appendices detailed in Chapter 10 , but sufficient information is provided here to permit an understanding of the qualitative study in Section B, Chapters 4 – 7 and 9 .
Initial plans were to recruit a sample of parents from within the four core trials (ExPN, INIS, PROGRAMS and TOBY) in the five core centres identified in Phase I. This involved the following five steps:
-
The core trial co-ordinating team was asked to identify core cases from their own data sets and initiate a screening process. For INIS, TOBY and PROGRAMS this was carried out by trial staff at NPEU. For ExPN this was carried out by staff in the local R&D office where trial records were held.
-
The neonatal consultant in the core centre in which a baby was enrolled into the core trial then approved or vetoed contact with the parents, and informed the core trial co-ordinating team. (The criteria for these decisions are explained further in Chapter 10. )
-
The core trial co-ordinating team then contacted the GP practice manager for the ‘approved’ cases, to confirm registration and identify the appropriate GP for these parents. The practice manager then passed on the study paperwork letter to this GP.
-
The GPs were provided with a prepaid envelope addressed to the core trial co-ordinating centre, indicating whether or not they had any objection to the parents being invited to take part in the study. GP practices were sent a £20 store voucher on return of their reply slip. The GPs also had the option of sending a letter to parents to let them know that they have agreed that the trial team may contact them about the BRACELET study. If GPs did not respond to the request from the core trial co-ordinating centre, reminder letters were sent, backed up by a telephone reminder from the co-ordinating centre, and, if necessary, a third joint letter from the co-ordinating centre and the director of National Institute of Health Research Primary Care Research Network (NIHR PCRN) was sent.
-
If there were no objections from the consultant or GP, the remaining eligible parents were sent a study pack by the core trial co-ordinating team, including an introductory letter outlining the BRACELET study from the PIs of the trials; a BRACELET study information booklet; a reply slip for each parent to indicate whether or not they wished to be interviewed; a questionnaire about the contact processes for the BRACELET study; and a prepaid envelope addressed to Claire Snowdon (CS). No reminders were sent to bereaved parents and if a negative reply slip was received there was no further contact with the parents. Parents who did not wish to be interviewed but who wanted to make comments in a different way also had the alternative option of completing an online questionnaire. If a positive reply slip was received, CS made contact to arrange a time and location for interview.
Strategy 1 was very difficult to implement and there was a high level of attrition. Consequently, recruitment was suspended in 2010, and a new two-part strategy was put in place following REC approval and a funding extension.
Strategy 2 involved a revision to the initial recruitment strategy (see a, below) and the introduction of a new approach to recruitment to allow access to a broader population of bereaved parents (see b, below):
-
a. After a neonatologist had screened the core trial cases, GPs were informed of the plan to contact parents about the BRACELET study and were given 4 weeks in which to state an objection. If no objection was received, a letter was sent to the parents by the senior neonatologist from the neonatal unit that cared for their baby, giving them advance notice that he or she would be telephoning them about a study that they might be interested in. The letter referred to their baby by name so that it was clear to parents that the call would relate to their baby. The consultant then called, not to ask parents for a decision about participation but to ask if they would be interested to see the letter of invitation to participate in BRACELET, and, if they were interested, for permission to pass their details on to the BRACELET study team. CS then sent information to the parents as in step 5 in strategy 1 (above). During this second wave of recruitment a fifth core trial was added to the study – BOOST-II UK. This trial was closed to recruitment at the end of 2010 and parents were sent details of the trial findings. As this sending out included bereaved parents, the addition to the BRACELET study of this trial, also co-ordinated by NPEU, offered the possibility of including an additional group of parents into the study with more recent experiences than those represented in the other core trials. The inclusion of BOOST-II UK required the introduction of an additional two clinical centres into the study raising the number of centres involved in BRACELET to seven.
-
b. Eligibility for the study was widened to include bereaved parents whose baby was enrolled in any UK neonatal trial in neonatal intensive care. An advertisement was produced, which invited bereaved parents who were interested in taking part in BRACELET to make contact with the study team by e-mail, telephone or online through the BRACELET study website. The advertisement was carried by a number of key organisations, such as SANDS, BLISS and Tiny Lives (charity linked to the Royal Victoria Infirmary Neonatal Service, Newcastle upon Tyne: www.tinylives.org.uk/index.asp). This publicity-based approach allowed parents who were interested in the study to opt in directly and without an intermediary. It meant that the advertisement could also be placed on the parental pages of the websites for the core trials, which were run from NPEU, and NPEU was able to include a paper copy along with a mail-out to parents involved in INIS. (Note: the BRACELET study advertisement was not included in the mail-out for all parents; it was added only for those who were bereaved.)
Ethics approval
Research Ethics Committee approval was given for Phase II of the BRACELET study by the North West Research Ethics Committee (NWREC) in April 2009 (ref. no. 08/H1010/113) and in June 2009 by the REC for the London School of Hygiene and Tropical Medicine (ref. no. 5533), which acted as sponsor for the study. A substantial amendment to the study was submitted to the NWREC in September 2010 and this was also approved. Research and Development approval was given for all participating clinical centres.
The sample
Recruitment totals for the BRACELET study
The BRACELET study as a whole involves 90 interviews (58 clinicians and trial team members and 32 parents) with 112 individuals (58 clinicians and trial team members and 54 parents) from the five core trials under consideration (INIS, TOBY, PROGRAMS, BOOST-II UK and ExPN) and from two ancillary trials (I2S2 and SUPFOR).
Clinicians and trial team members
Eighty-seven potential participants were identified. Eight were excluded from the study: in four cases their trial role related only to follow-up of survivors, in one the individual was known to be unwell, and in the remaining three cases the trial team was no longer in contact with the individuals concerned. Of the remaining 79 individuals, 60 were named trial team members and 19 were clinicians identified from recruitment records for core centres. Forty of the 79 were involved in recruitment to one or more of the core trials and 39 were not. Of the 79 eligible professionals, 72 were invited to take part. The remaining seven potential interviewees were not excluded; recruitment was staggered, and the sample was already complete before they were invited. A total of 60 of the 72 invited agreed to take part in the study, giving an 83% acceptance rate. Only three invitees declined to participate: one felt that the study focused on issues beyond his/her expertise, another felt that the issues involved were too personal, and another declined on the grounds of time pressures. No response was received for a further nine. Two of those who opted into the study were not in fact interviewed for logistical reasons. Hence, 58 interviews were carried out for this part of the study ( Table 17 ), an over-recruit of eight interviews.
| Identified | Excluded | Not invited | Invited | Did not respond | Declined | Accepted – not interviewed | Interviewed |
|---|---|---|---|---|---|---|---|
| 87 | 8 | 7 | 72 | 9 | 3 | 2 | 58 |
| Consent rate = 83% (60/72) | |||||||
The original target of 50 interviews was exceeded for a number of reasons:
-
There was a very high acceptance rate (83%).
-
A staggered invitation process was used to avoid long lags between opt-in and interview. Although there was some deliberate clustering of interviews according to trial in order to maintain a focus on the specific issues involved in each, where possible invitations were issued to interviewees who were geographically close to each other to allow for efficiency gains. The high acceptance rate meant that the target of 50 interviews was reached before invitations had been sent out.
-
During the earlier interviews, it became clear that the trial research nurses had very interesting experiences and useful insights to offer. They also appeared to have their own support needs in the context of deaths in trials. It was therefore decided to make a particular effort to represent this group and more invitations to nurses were sent than originally planned. Again there was a high opt-in rate and 8 out of 10 of this group of invitees agreed to participate in the study (although one of these potential interviewees was not in fact interviewed for logistical reasons).
-
Particular efforts were also made to include as many members of core trial DMCs as possible and 12 of these individuals opted in to the study. DMCs bear responsibility for important recommendations for stopping or continuing a RCT based on mortality and morbidity statistics, and are required to balance the scientific and human benefits and costs involved. These were important considerations in the BRACELET study. Few such data are currently in the public domain and the study team decided this element of the study would be a useful contribution to the literature on the conduct of RCTs.
Sample characteristics
Many of the clinicians had both university and hospital appointments. Academics could have both clinical and non-clinical roles. The latter included statisticians and academic triallists. The sample involved many of the senior members of the UK neonatal clinical trials community, with 17 professors (clinical and non-clinical), and 21 non-professorial consultants.
As was anticipated before recruitment started, the interviewees also held a range of more specific roles in relation to the core trials: investigators, trial managers and administrators, TSC members, DMC members, recruiting clinicians and recruitment-related staff. A sizeable number of the 58 interviewees (n = 17) had more than one role in a single core trial (e.g. investigator and recruiter) or had roles in more than one of the trials (e.g. DMC member on one trial, TSC member for another). Some held roles in multiple trials; one interviewee was a CI for four trials, another was a member of the trial management team for four trials. The maximum number of roles recorded across the core trials for one individual was five; a senior clinician acted as CI and recruiting clinician for one trial, TSC member for another trial and DMC member for two further trials. Some of the interviewees have a wider involvement in trials other than the core trials considered here.
The sample included 37 core trial team members who were involved in protocol development and organisational aspects of a core trial, and 21 core centre clinicians. Most of the core trial team members (n = 22) did not have a role in recruitment to the five trials, but some (n = 15) did have a recruiting role (although not necessarily for a baby who went on to die, and not necessarily in a core centre). In the core centres, PIs, clinicians (neonatologists) and nursing staff with an identifiable link to a core trial were eligible to take part. Recruitment of core centre clinicians focused on those who had enrolled a baby into a core trial and subsequently the baby died. Twenty-one individuals are included in the sample from the core centres, 16 of whom were involved in recruiting and five of whom were non-recruiting nursing staff. Specific trial roles and whether or not individuals were involved in recruitment are shown in Table 18.
| Core trial team members and core centre staff | Roles | Non-recruiting | Recruiting | Interviewees per category | ||
|---|---|---|---|---|---|---|
| Core trial team members | Investigators | Philip | Bill | Mia | 14 | |
| Sylvia | Donald | Harvey | ||||
| Annette | Domenic | Naomi | ||||
| Nicholas | Arthur | |||||
| Roger | Alan | |||||
| Marc | Harvey | |||||
| Trial managers | Una | Bridget | 5 | |||
| Belinda | Astrid | |||||
| Corinne | ||||||
| TSC | Gordan | Hugo | Noel | 12 | ||
| Dexter | Celia | Max | ||||
| Frances | Daphne | Duncan | ||||
| Ryan | Matthew | Roger | ||||
| DMC | Austin | Hugo | Simon | 12 | ||
| Dulcie | Polly | Naomi | ||||
| Harriet | Hilary | Roger | ||||
| Dexter | Celia | Duncan | ||||
| Total = 37 | 22 (+ 2 repeats) | 15 (+ 4 repeats) | ||||
| Core centre staff | PIs in core centresa | Seb | 2 | |||
| Dean | ||||||
| Recruiting clinicians | Honor | Mary | 12 | |||
| Irwin | Leonie | |||||
| George | Joe | |||||
| Judith | Olivia | |||||
| Avril | Craig | |||||
| Greg | Eric | |||||
| Named trial nurses | Hayley | Aiden | 4 | |||
| Jenny | Sascha | |||||
| Non-named nurses | Selina | 3 | ||||
| Connie | ||||||
| Grace | ||||||
| Total = 21 | 5 | 16 | ||||
| 27 | 31 | Total = 58 | ||||
Most of the interviewees were interviewed in relation to INIS and TOBY (collectively n = 52), a reflection of the number of individuals involved in these trials at a developmental and organisational level, as well as working as recruiting clinicians in the seven clinical centres involved in BRACELET. The smallest number of interviewees was interviewed in the ExPN feeding study, a single-centre trial that did not have the same substantial staffing structure as the larger trials. Table 19 shows numbers of interviews and the same specific roles as shown in Table 18 , per core trial.
| Trial teams | INIS | TOBY | PROGRAMS | BOOST-II UK | ExPN | Total interviews |
|---|---|---|---|---|---|---|
| Investigators | Philipc | Donalda | Nicholasa,c | Philip c | Miaa | |
| Billa,c | Domenica | Philip c | Bill a,c | Roger a,c | ||
| Alana | Arthura | Naomi c | Harvey c | |||
| Sylvia | Harveya,c | Nicholas a,c | ||||
| Annette | Marca | |||||
| Philip c | ||||||
| Managers and administrative staff | Unac | Bridget | Astrid | Una c | ||
| Belinda | Una c | Una c | ||||
| Corinne | ||||||
| TSC | Rogerc | Noel b | Matthew | Duncan c | ||
| Gordan | Ryan | Max a,b | ||||
| Dexterc | Hugo | |||||
| Frances | Celiac | |||||
| Daphne | ||||||
| DMC | Austin | Simonb | Duncan c | Hilary | ||
| Dulcie | Hugo | Celia c | Roger a,c | |||
| Harriet | Polly | Dexter c | ||||
| Naomia,c | Roger a,c | |||||
| PIs in core centres (other than investigators) | Dean | Maxa,b | Seb a | |||
| Recruiting clinicians in core centres | Honor | Greg | Eric b | Max b,c | ||
| Noelc | Mary | Simon c | ||||
| Irwin | Leonie | |||||
| George | Joe | |||||
| Judith | Ericb | |||||
| Jenny | Olivia | |||||
| Avril | Seba | |||||
| Craig | ||||||
| Dean | ||||||
| Named trial nurses in core centres | Hayley | Aiden | ||||
| Sascha | ||||||
| Non-named nurses in core centres | Selina | |||||
| Connie | ||||||
| Grace | ||||||
| Recruiting clinician not in a core centre | Duncanc | |||||
| Total interviews per trial | 26 | 33 | 11 | 11 | 2 | – |
| Cumulative total interviews | 26 | 27 | 3 | 1 | 1 | 58 |
Tables 18 and 19 show the distribution of clinicians and trial team members through their pseudonyms so that it is possible to see multiple roles for a given individual. Pseudonyms were allocated to all interviewees in BRACELET to preserve confidentiality. Only first names were pseudonymised for clinicians and trial team members (as was the case for parents and their babies – see below). There are advantages and disadvantages of using pseudonyms for first names or family names; the former preserves details of gender, the latter if accompanied by a title preserves something of the background of an interviewee (Mrs X, Dr Y, Professor Z). The decision to pseudonymise first names for all interviewees here was made on the grounds of equity. (The use of pseudonyms rather than numbers allows personhood as well as individual views to be more readily available to the reader. It allows the reader to make their own connections with data taken from an individual account, building up their own sense of the study participants as threads are picked up as data are presented. As the same pseudonyms will be used in all subsequent publications, this effect will extend beyond the limits of this report. In a study, which, in part, aims to give voice to a group of individuals who have previously been unrepresented, this is more than a convention or an organisational device.)
Demographic characteristics of clinicians and trial team members
Clinicians and trial team members were asked to complete a questionnaire (see Appendix 12 ) to provide basic demographic information that was supplemented by information from interviews. The sample comprised 38 men and 20 women, 49 of whom provided details of age ( Figure 11 ).
FIGURE 11.
Age of clinicians and trial team members.
Bereaved parents
A flow chart showing the numbers of potential interviewees identified, excluded, invited and recruited via strategies 1 and 2A are shown in Figure 12 .
FIGURE 12.
Recruitment: strategies 1 and 2a.
The two interviews (with four parents) achieved via strategy 1 were based on the 155 originally identified at NPEU and LWH. Following screening by the trial centres, the site consultant and the GP, 54 were approved to allow a letter to be sent to the parents. Thirty-three of these were not sent while strategy 1 was suspended, 21 letters were sent out and two interviews were achieved (9.5% of those sent a letter but 1.3% of the original 155).
Most of the interviews (n = 18, with 30 parents) were achieved with the assistance of neonatologists in the seven clinical centres who sent a pre-call letter to 71 parents on behalf of the study in strategy 2a. These were based on a combination of the 33 letters not sent under strategy 1, and 38 families for whom there was either agreement or no response from a GP (strategies 1 and 2a). The 18 interviews resulted from the 50 letters and information packs sent out (36.0% of those sent a letter but 11.6% of the original 155).
The remaining interviews (n = 12, with 20 parents) were achieved from parents responding to information about the study that was circulated via charities, research organisations and special interest groups (strategy 2B). It was not possible to produce a similar flow chart for this strategy, as the parents recruited themselves.
This combined recruitment strategy was essential to achieving the target number of interviews for this hard-to-reach, hard-to-recruit population.
The target sample of 30 interviews with bereaved parents from the five core trials was reached, with interviews involving 51 parents in total (29 women and 22 men). An additional two interviews were carried out with two women and one man whose babies were enrolled in trials other than the core trials. A further interview was carried out with a couple whose characteristics did not fit the eligibility criteria for interviews. This last couple identified themselves as bereaved parents on opting in to the study after seeing the BRACELET study publicity but on interview it transpired that their bereavement related to a previous pregnancy and that their child who was enrolled into a NIC RCT had survived. This interview informed the study, in that the parents discussed how their prior bereavement shaped their response to research in their subsequent pregnancy, but as the parents fell outside the eligibility criteria this interview was not included in the analysis.
The final total number of parental interviews was therefore 32, a slight over-recruitment, with 31 women and 23 men represented in the sample. Ten of the interviews were carried out with one parent: nine women and one man; in only two of these interviews were the women no longer in a relationship with their baby’s father. In 22 interviews, couples were interviewed together.
Table 20 shows the distribution of interviews and interviewees by trial and recruitment methods, and includes the pseudonyms used for the study.
| Interviews | INIS | TOBY | PROGRAMS | ExPN | BOOST-II UK | Other | Total interviews | Total parents |
|---|---|---|---|---|---|---|---|---|
| Interviews via core trials | Marion and Doug | Hesther and Stuart | Rhona and Karl | Shirley and Warren | Milly and Adam | 20 | 34 | |
| Stefanie and David | Amanda | Caitlin and Pete | Chloe | |||||
| Julia and Lewis | Liza and Wesley | Danielle | ||||||
| Alice and Ivan | Lesley and Stan | |||||||
| Justine and Francis | Karen and Tony | |||||||
| Amy and Chris | Dawn | |||||||
| Diane | ||||||||
| Beverley | ||||||||
| Interviews via publicity | Sara and Gareth | Laura and Wilf | – | – | Jill and Ethan | Abbyb | 12 | 20 |
| Anita and Sean | Robert | Linda and Danc | ||||||
| Fiona and Keith | Hannah and Ryana | |||||||
| Sophie and Nat | ||||||||
| Dora | ||||||||
| Jana | ||||||||
| Total interviews | 12 | 5 | 1 | 3 | 9 | 2 | 32 | |
| Total parents | 22 | 8 | 2 | 6 | 13 | 3 | 54 |
Parents were asked to complete a pre-interview questionnaire (see Appendix 13 ) about their age, work and educational status, which was supplemented by information from interviews. (Separate versions were available for men and women; they were identical other than the heading. Only the women’s version is appended.)
Forty out of the 54 interviewees were in paid work. More of the women were in part-time work (12 compared with 3 of the men), and more of the men were in full-time work (16 compared with 9 women). Ten of the women were not in outside work (compared with three of the men). For one male interviewee, no information was available on employment status.
Fifty-one parents provided information about their age. There were 29 women with a median age of 39 years (IQR 32–43), and 22 men with a median age of 40 years (IQR 38–43). The age distribution is shown in Figure 13 .
FIGURE 13.
Age of parents.
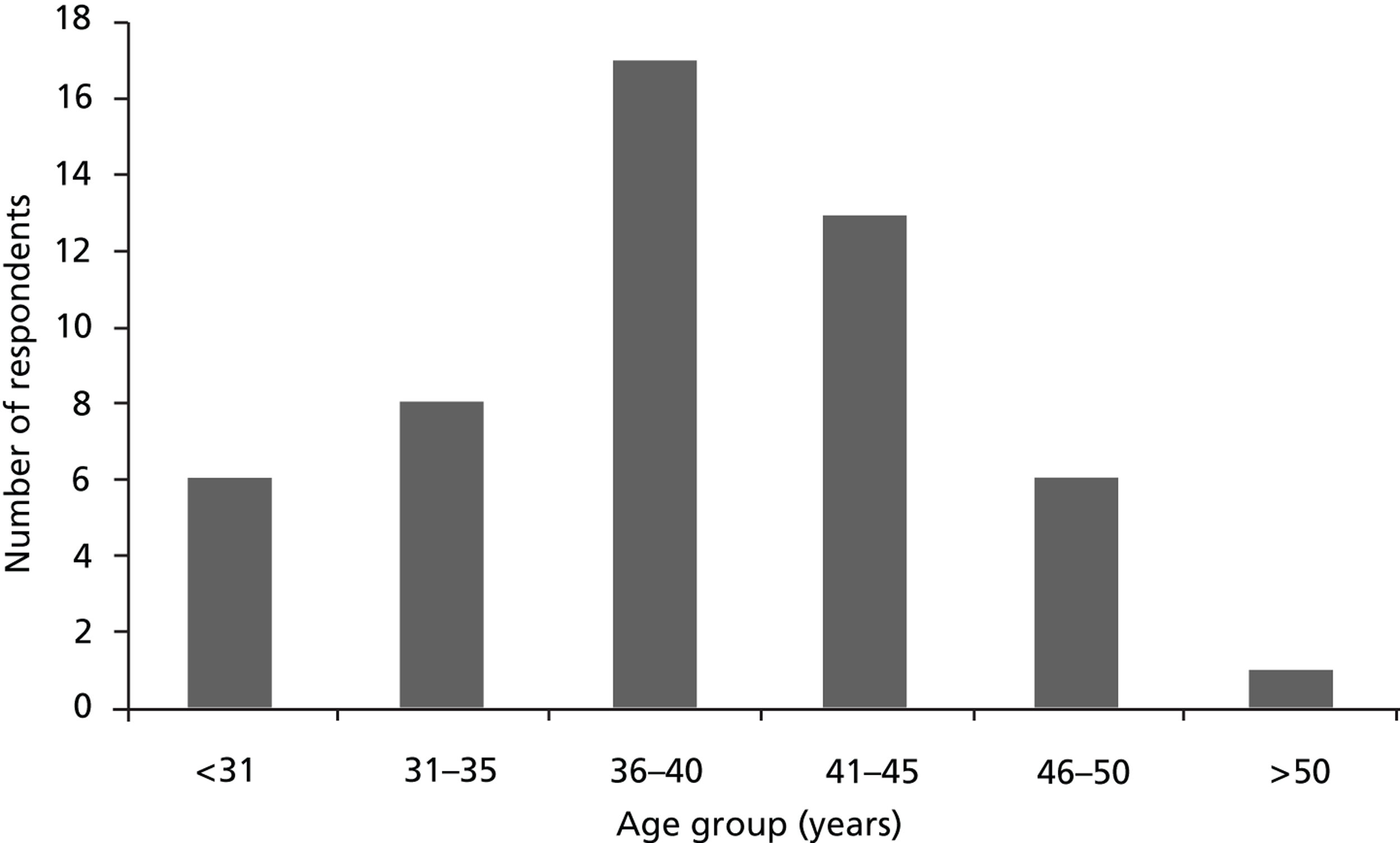
This age range reflects, in part, the length of time between the death of their child and the interview. Although in one case the interview was before the first anniversary of the death, as these parents were recruited via the publicity strategy and their baby did not take part in a core trial, most interviews (n = 16) took place at between 1 and 5 years after the birth ( Table 21 ). A further 15 interviews were carried out considerably longer after their child’s birth (and therefore death), including one 14 years later (from the pilot study before the TOBY trial), where the parents also opted in the publicity strategy. These long intervals from birth to interview explain why many of the women were no longer of reproductive age by the time of the BRACELET interviews. One man was much older than his partner, which explains the high mean age.
| Time from birth to interview (years) | No. of cases |
|---|---|
| 0 | 1 |
| 1 | 1 |
| 2 | 7 |
| 3 | 3 |
| 4 | 0 |
| 5 | 5 |
| 6 | 4 |
| 7 | 3 |
| 8 | 4 |
| 9 | 1 |
| 10 | 2 |
| 14 | 1 |
Fifty participants provided information on educational status, including age at leaving full-time education ( Figure 14 ) and subsequent return to either full- or part-time education. Approximately one-third (n = 18) had left school by the age of 17 years and a very high percentage (n = 29; 58%) either stayed in education until or beyond 21 years (n = 17) or, having left earlier, returned to education (n = 12).
FIGURE 14.
Educational status of parents.
Interviews with clinicians, trial team members and bereaved parents
Sequence of interviews
The BRACELET study aimed to recruit a substantial number of interviews with the expectation that they would all be carried out by one interviewer (CS). The complex mix of trials and roles involved, and the time that it would take to carry out this number of interviews was a methodological challenge, not least because the last interviews would be carried out at some time distant from the first. Although a study has overall research aims, the aims for individual interviews are not necessarily all the same. Certain interviews in a sample can serve particular purposes and the likelihood that they will trigger new insights and lines of enquiry can be anticipated. It was therefore important that the interviews were deliberately sequenced to maximise the likelihood of gaining rather than obscuring and overwhelming insights into the study area. Essentially the sequence of interviews should be exploited to best effect.
The BRACELET study involved interviews with those associated with one of five NIC RCTs. Each trial varied in terms of the interventions under examination, as well as the clinical circumstances of the trial participants and the setting in which the interventions were delivered. It was important to gain a good working understanding of each RCT at an early stage of the data collection period, as well as an overview of the similarities and differences of each. This would allow interviews for a particular trial to be conducted by a researcher who was mindful of how the views and experiences being reported fitted with other accounts of the same trial, and contrasted and compared with those reported for the other trials; in effect, the data could be placed into a larger context at the time of the interviews, maximising the chances of responsive interviewing in the initial and the subsequent interview period.
The intended sequence of interviews for this study was therefore divided into three stages, with primary, secondary and tertiary informants. (‘Primary’, ‘secondary’ and ‘tertiary’ here relate to the time-ordered sequence of the interviews and there is no suggestion that there was any different value placed on interview subgroups.)
The primary informants were interviewed with the aim of gaining a rapid sense of the RCTs, why they were developed and how they were run. The informants for the initial core trials (i.e. excluding BOOST-II UK) were:
-
a senior investigator with responsibility for multiple trials
-
a senior trial manager with responsibility for multiple trials
-
four trial managers/administrators with responsibility for day-to-day running of the trials.
The first interview was with the senior investigator and this served as an agenda-setting exercise, as well as an opportunity to collect the views and experiences of the individual concerned. Layder106 argued in his ‘Adaptive Theory’ approach to qualitative research that the researcher brings his/her own experiences and priorities to the research agenda and that these should be made explicit. In this instance we were aware that the priorities and concerns of the research team in developing the study might produce a subtly different research agenda from those involved in running trials, and that this would shape the research questions that would be asked. The primary informants were invited to consider the questions that the study might explore, and to suggest their own areas of interest and/or concern that might be drawn into the study. In this way the research questions, in part, arise from trials-related issues and clinical practice, and were sensitive to a wider range of priorities over and above those of the research team.
Once these interviews were conducted there was a formal First Stopping Point in the data collection. These interviews were considered in detail and the interview schedules were adapted in the light of the information and insights gained.
The secondary informants were interviewed with the aim of gaining further detailed understanding of the trials, from the perspectives of those involved at a senior level, for example trial co-investigators and some PIs from clinical centres. These interviewees were involved with the trials at different levels. Our previous research indicated that a RCT and its protocol can be perceived and interpreted in very different ways in different clinical centres, and this tier of interviews offered the opportunity to develop understandings of the trials in the light of the views of those involved at a national and a local level. Once these interviews were conducted, a second stopping point was envisaged, at which, again, the interview schedules for the remainder of the interviews would be reconsidered and revised if necessary. In practice, the geographical spread of the interviewees meant that some secondary informants were interviewed later than initially planned, and the boundaries between these and the next set of informants were not clearly defined.
The tertiary informants were all remaining interviewees, and comprised trial team members, clinicians and parents from the core trials and core centres. These interviews with the trial team members did not need to be carried out in any particular order, and were conducted opportunistically throughout the remaining data collection period.
The interviews with clinicians and trial team members and parents were intended to be conducted concurrently to allow insights from the different interviewees to be taken up in later interviews if appropriate. In practice, delays in being able to contact parents (see Chapter 11 ) meant that most of the interviews with clinicians and trial team members had already taken place by the time the parent interviews were under way. In practice, logistics and recruitment delays made these intended re-interviews problematic. One of the primary informants was interviewed a second time and this allowed issues arising in the interviews to be explored in the light of his/her particular expertise and this provided additional and useful data.
Conduct of interviews
Interviews were conducted in each country in the UK, and in one continental European country. They were carried out with clinicians and trial team members between June 2009 and July 2012, with the bulk of interviews (n = 46) taking place in 2010. Interviews with parents were carried out between March 2010 and July 2012. After the first parent interview in 2010, recruitment was suspended in order to revise the recruitment strategy, resuming in July 2011 and continuing until the target sample size from the core trials was achieved in July 2012.
The interviews with clinicians and trial team members mainly took place at their place of work, in the interviewee’s office or in another private room. Six interviewees asked to be interviewed at home: four because they were retired and two to fit in with work patterns. Two interviewees were interviewed at a public location of their choice. Two interviews took place when interviewees were travelling, with one in a booked room at an academic venue and another in the interviewer’s office at the London School of Hygiene and Tropical Medicine. Although there was variety in the circumstances and location of the interviews, discussions were not limited by the settings, possibly because the environment was chosen by the interviewees themselves.
The interviews with parents mainly took place in their own homes. One interviewee asked for the interview to be carried out at his place of work, as he was concerned that his wife who was not participating in the study might be upset.
The interviews covered sensitive ground and the strategies that were developed in relation to this, and interviewees responses to their involvement in an interview and in the study, are considered in detail in Chapter 10 .
Unusually for a qualitative study, different interviews involved different approaches to data collection.
For the clinicians and trial team members it was important that the interviewer engaged with the expertise and experience of the interviews. This was necessary both for the opportunity that the interviews offered for ‘crystalisation points’ 107 – detailed exploration of key areas of interest to BRACELET which would be familiar territory for those individuals – but also to ensure that the interviews were engaging for the interviewer and would therefore generate valuable data. Had the interviewees felt that they had to explain points of clinical trials practice discussion would have been restricted to more simple and less sophisticated territory. Pfadenhauer108 argues that to maximise productivity the interviewer must become a ‘quasi expert’. In this spirit it was important to demonstrate where possible a shared understanding of issues involved with clinical trials practice and the approach taken to the interviews was therefore closer to an academic discussion, with the interviewer at times taking a more active role in drawing out interviewees opinions than would be the case in a narrative interview.
For the parents the approach to interviews followed principles of interpretive phenomenology. 109 This approach emphasises the importance of context, sees personal experiences as socially situated, explores the meaning of experiences for individuals and acknowledges the potentially shaping role of the analyst in interpreting the data. 109,110 Parents told the story of the birth and death of their baby often in great detail, and in data collection and analysis the interviewer sought to fairly represent their individual parental experiences of the phenomenon under scrutiny. Later connections were also made across the data set, to move beyond individual experiences to create a more nuanced account of experiences of bereavement and neonatal trials.
Analysis
All interviews were recorded and transcribed. For each interview a detailed and reflexive summary was created to gain an initial understanding of the interview and the interview processes. These field notes helped to organise initial thoughts and to gain an overview of the substantial data set, and marked the first steps in analysis. BRACELET team members were provided with these summaries as they were written to allow them to engage with both the data and the initial emergent areas of interest for analysis. Extracts from the field notes are used later in this report. The full transcriptions were analysed using the qualitative package ATLAS.ti. 111
Two members of the team, CS and DE, were primarily responsible for analysis. CS is a qualitative researcher specialising in participants’ views of perinatal trials; DE is a senior triallist familiar with qualitative research in this field. The interviews were read by CS and DE and data were initially organised according to the areas of interest identified in the field notes. Analysis was iterative, and became more specific and detailed as analysis progressed. Some themes were clearly evident from an early stage in the analysis and went on to become dominant themes. Others were triggered by an individual comment that led to an idea being traced through and explored in a number of interviews in comparative cycles of analysis. Ambiguities in coding were resolved by joint consideration of the fit between data and codes by CS and DE.
Chapter 5 The research setting
The importance of context
A central feature of qualitative research is the examination of the context in which a phenomenon is situated, experienced and constructed, and contextualisation is one of the standards by which qualitative studies are judged. 112,113 This can involve a relatively quick description of key features of a given setting to orientate the reader, or it can be part of the analysis itself, with description providing the foundation for later conceptualisation of the data. 114 At the most extreme end of the methodological spectrum, some anthropologists immerse themselves as participants in their setting of interest, seeking to understand through direct experience the multidimensional complexity of its workings and effects.
Context is not just the backdrop against which interesting experiences occur; it is a collection of shaping forces that affect experiences in subtle and not-so-subtle ways. It is an environment, an atmosphere perhaps, which is integrated into personal narratives, experienced, interpreted and reinterpreted over time. Attempts to understand a context or setting, and the responses it engenders, can be challenging, as some elements are clearly evident but others are less easily discernable, especially perhaps to those involved. For the BRACELET study this task is complicated by the fact that it effectively considers multiple settings in the form of five NIC RCTs, each viewed from multiple perspectives. Each trial has its own particular set of circumstances and operates in unique ways. For this study then it is appropriate to consider contexts within contexts.
This does, however, raise the methodological question of whether analysis of unique situations can illuminate other situations, a question that has its parallel in the issue of transferability of results for quantitative research. Some qualitative researchers argue that it is not the responsibility of the researcher to address comparability or transferability of qualitative research findings as the researcher sets up only the ‘sending context’; the reader considers the ‘receiving context’, working out from detailed and rich descriptions how much of the research findings can be applied to their context of interest. 113 In the BRACELET study, the involvement of five NIC RCTs helps in this matter, increasing the receiving range, but it also adds to the complexity of the task. Although exploring and examining multidimensional contexts is a complex endeavour, it increases the value of the research as it has allowed the BRACELET study team to address both trial-specific and comparative questions.
In this chapter we consider in detail particular elements of the larger context in which trials are developed and implemented, and within that larger context explore key aspects of the design and conduct of the five trials. Data are drawn from information gathered during the course of conducting the study, from the NIC RCT publications and from some of the interviews with senior clinicians and trial team members. The aim of this chapter is to develop insights into the research settings of the core trials as products of a NIC research community and as shaping forces that drive and mediate personal experiences. This will provide a basis for the presentation of qualitative data, which will describe and explore those experiences in later chapters. We will return to the task of contextualising the data relating to parental experiences around the birth and care of their babies in Chapter 6 , where the NICU into which research is introduced will be described.
One way of contextualising a phenomenon is to use ‘thick description’115 to give a sense of the sample, and to convey the fine detail and texture of the setting. This allows researchers’ own impressions, based on information and details gathered in the field, and interviewees’ own words to be used to promote a sense of their particular insights and understandings. Both strategies give greater depth to the account of the research settings considered in the BRACELET study, the background and foreground into which NIC RCTs are introduced.
Research in neonatal intensive care
The relevance of specialty
Different specialties in medicine are thought to have their own features and characteristics, some of which have been the focus of in-depth study. A number of researchers have set out to understand the environment and ways of working of a specialty, such as Bosk116 on an American genetic clinic; Atkinson117 on the work of UK haematologists; Smith and colleagues118 on the acquisition of expertise amongst UK anaesthetists; and Mesman119 on practice in Dutch NIC. They have also explored the ways in which a subgroup in a specialty might function in the light of a specific purpose or context, for instance surgical teams working in a war zone. 120
How a specialty responds to and manages its research has not been explored for many areas of medicine. When such data exist they can be helpful indicators of cultures within cultures that can grow up around research, offering the opportunity to parallel or draw contrasts with other specialties. One area where there are useful data on this topic is surgery, where the use of RCTs is not particularly common,121–123 and where concern has been expressed about the standard of research that is produced. 124,125 Some of the difficulties identified by studies in this setting relate to particular challenges of working with a research protocol in surgery, for instance blinding is difficult126 and concerns exist that variations in the skills of different surgeons may affect delivery of a trial intervention, undermining faith in RCTs as useful tools in surgical research. 127 A disinclination among surgeons to embrace the concept of uncertainty that underpin trials methods and ethics is also thought to be a barrier to research. 128 These attitudes may be changing as support for RCTs has been shown among younger surgeons who will gradually gain precedence in the field. 129,130
It seems reasonable to suggest that there is a link between the features of a research culture and experiences of research collaboration and participation. Where RCTs are less common, and where practitioners are less familiar with or unsupportive of their science and their conduct, this will shape the ways in which trials are implemented, and how they are implemented, in turn, will affect how they are experienced. A useful starting point for the BRACELET study is therefore to consider how RCTs are developed and run in NIC.
Research in neonatal intensive care
The first data from the BRACELET study that gave some sense of research in this field came from Phase I, in which differences between the NIC and PIC settings were evident. There were more NIC RCTs overall and they involved collaboration with a large network of NICUs across the UK. They were more likely than the PIC RCTs to be initiated within the UK (89% of the NIC RCTs compared with 50% of the PIC RCTs were UK led) and most of international NIC trials, including the TOBY and INIS studies (two of the BRACELET core trials), were initiated and co-ordinated in the UK, with international partners joining the UK-led venture (6/10, were UK led). In the PIC setting the opposite was true; a minority of trials (n = 2/10) were initiated and co-ordinated within the UK, and PICUs more commonly joined international trials run from outside the UK. Some of the larger NIC trials were co-ordinated through an experienced CTU (NPEU) and drew on the support of substantial teams of clinical and non-clinical experts in their TSCs and DMCs. Single-centre RCTs were carried out in both NIC and PIC settings in the same proportions (one-third of trials for each) and these trials could not draw on the same infrastructure and degree of support.
These differences in part reflect the longer history of NIC, for which a larger number of clinical centres have been established and where there has been more time for research to be integrated into practice. PIC is a relatively new area of paediatrics, and paediatric intensivists are members of a relatively new specialism, but as this specialty grows its research profile may well change: the recently completed CHiP trial is a landmark PIC RCT, the first large UK-led collaborative trial to be carried out in multiple UK centres. 131
The data that describe the profile of NIC RCTs identified in Phase I suggest a very active and committed research community, but it should be pointed out that much of the research work was concentrated in a subgroup of NICUs. There were many NICUs that were not involved in research, and even among those considered to be research-active according to the BRACELET study definition, i.e. they recruited at least one baby or child to at least one RCT in the 5-year period, many recruited rarely or sporadically and were not major contributors to research.
One of the primary informants, Philip, a senior clinical trials investigator, was well placed to give a sense of this uneven profile of research. He argued that the recent historical roots of neonatology have produced a research-orientated culture and community that is ‘extremely familiar with trials’ but that this did not necessarily cut across all of NIC:
[It’s not that long ago that neonatologists] ventilated the first baby . . . whereas if you think of [obstetrics] to a large extent practices have not changed for a very long time. We’ve been doing caesarean sections for a very long time, we’ve been doing forceps for a very, very long time. You know, there hasn’t been the same rate of change, whereas for neonatology, from ventilating the first baby and not being able to keep [any baby] under twenty-eight weeks alive to where we are now, has been a very rapid rate of change in an environment where there’s been a greater appreciation of evidence and different methodologies to get that. So neonatologists, I think, see the benefits of this research on their practice and are continuing to see that because there’s, there’s always new things to be done and evaluated and I think that’s just perhaps not the case in some other specialties . . . When you go into a room of neonatologists to talk about trials you often don’t have to start from the basic . . . You’re not constantly justifying why randomisation is important and why the last three trials . . . could produce no useful results . . . A lot of their practices have been determined by a trial, they’re very pro-trial, they’re very familiar with the methodology; they think they’re a good idea . . . You know there are so many neonatal units in this country who will be taking part in three or four trials at any one time and lots of other studies at the same time. I think they really see the neonatal unit as a place where clinical research really should be done to improve the care that they give ‘cos they’ve got real examples of where it has improved the care that they’ve given . . . It’s not universal, and there’ll be some neonatal units who’ve probably never taken part in research . . . some neonatal units, particularly Level 2 neonatal units [which] are staffed by paediatricians with an interest in neonatology as opposed to dedicated neonatologists, and I think that’s important to separate those two out.
The Phase I data suggest this same gradation of involvement in research, and this adds emphasis to the value of focusing closer in on the core trials and their core centres.
The neonatal core trials community
Randomised controlled trials are produced and implemented within communities of varying sizes – some local, some national and some international. The BRACELET study sample includes individuals and teams with experience in each of these dimensions, and so gives a useful window into the workings of the larger NIC RCTs community. The sample is bounded, however, in methodological terms by its involvement in the five core trials and it is an artefact of the BRACELET study method of recruitment to suggest that they are members of an actual ‘core trials community’. Nevertheless, it is helpful to conceptualise the 58 interviewees as forming such a collective, as collectively they have produced and shaped the core trials. In many respects the sample is highly connected as many of the interviewees are longstanding colleagues within and outwith research. At the same time, the sample involves some interviewees with few connections other than their core trial team role. The sampling approach has brought in senior triallists who have helped to drive forward NIC research, but it has also drawn in several interviewees who do not work in NIC medicine at all: they are clinicians in other specialties, statisticians, lay representatives, who are trial team members because of their particular expertise and experience. The interviewees are therefore both connected and separate, they form groups around a core trial but their interest and influence extends to other RCTs and beyond. They reflect the real constituents of trial teams but this mix and range of contributions to individual trials has rarely been tapped.
Useful information about the approach to RCTs in the core trial community started to emerge in preparation for the Phase II interviews, when the distribution of clinicians and trial team members across the trials suggested a highly collaborative field. This was confirmed as the sample was recruited. The extent of the interconnection of roles and responsibilities across the RCTs, as described in Chapter 6 , was effectively a finding in its own right. Some of the core trial team members took on multiple roles in their trials: 15/37 recruiting clinicians also had senior roles in the same trials. These were largely investigators recruiting to their ‘own’ RCT. The collaborative links across trials were also important, demonstrating how they draw on available experience and expertise in the wider research community. In fact the professional demographic of the sample as a whole gives an indication of the high level of clinical and research-related expertise that exists in this community. The sample of 58 individuals includes 17 professors (12 of whom are neonatologists, two are clinical academics in specialties other than neonatology, and three are non-clinical academic statisticians), and 21 non-professorial consultants. Eleven interviews were carried out with members of core trial DMCs. The invitation to serve on a DMC indicates a level of expertise and esteem that members had often accrued through their involvement with major RCTs.
The BRACELET study sample therefore highlights the experienced, multidisciplinary and interconnected nature of the core trial community, and demonstrates the well developed team-based infrastructure that has been established for some of the major trials it has generated.
Academic culture and research in the core centres
The BRACELET study sample includes a number of senior academic clinicians who have witnessed advances and some setbacks in NIC during their own careers. Some had been involved in landmark RCTs and took pride in the developments achieved. At least one senior academic clinician was interviewed in each of the core centres. Each was a professor in NIC medicine and had multiple roles in one or more of the core trials. They all acted as a recruiting clinician, as well as a trial team member and they therefore span every category in the BRACELET study clinician and trial team member sample (core trial team member, recruiting clinician, core centre staff, clinician and academic). They were useful informants as they not only described the research ethos that they felt existed in their centres, but also were able to place this in the larger context of the NIC research community.
Harvey, one of these senior academic clinicians is an emeritus professor at core centre A and had been an investigator for two of the core trials. He gave a sense of how a NIC research culture had grown out of neonatology’s earlier links with obstetrics, and how the new specialty, free of received wisdom, was able to be critical of its own new clinical practices.
Obstetricians way back were very interested in research . . . and how to manage placenta praevia and develop the very conservative management of that, which saved lots of women’s lives. Well, I suppose that was way back in the 1940s. I guess that since neonatal intensive care has been set up we’ve been interested in research – it’s a fairly new thing set up in the 1970s and I guess many of us had a sort of critical mind and didn’t want to adopt techniques and interventions until they’d been properly evaluated. And I think we’ve tried to keep that going now as a routine. I hope that the next generation of neonatologists will keep it going although there are pressures now on doing research.
He felt that the way that research was run in centre A had changed over the years, with a lessening emphasis on academic medicine.
Traditionally we’ve done a lot of research here and we’ve been the co-ordinating centre for many trials. I guess more lately we’ve been collaborators within other’s research. The real difficulty now is to get young training paediatricians to be interested in research, to take 2 years out to do an MD or 3 years to do a PhD. They get poor funds for that, they don’t see any benefit at the end of that for their career. They get promoted probably far more quickly for being able to do a clinical job than to have an academic interest or research interest.
A number of interviewees with a range of roles raised the issue of academic medicine, and research in particular, becoming increasingly difficult, but this did not appear to diminish the sense of its importance. Another emeritus professor, Noel, from core centre B, who sat on a DMC for one of the core trials, described his NICU as having ‘a very strong academic tradition:’
It probably has more papers than virtually any other NICU . . . so it’s very strong academically and clinically . . . The two previous Chairs . . . although general paediatricians, had a research interest in the newborn, both of them. That’s 25 years ago. And I was appointed and my main interest was neonatology, so it continued and I think gathered strength, and all the clinicians, whether they have academic appointments or not, publish here.
He felt that his centre has ‘a better recruitment to clinical trials probably than any other unit in the UK’ and mentioned several RCTs for which they had been the highest UK recruiters. He said that ‘the fact that people are academic is the major stimulus’ but also felt that the collegiate ethos among their consultants was an important factor, as was the fact that they organised recruitment so that it was carried out ‘almost invariably by the principal investigator or the research worker on the trial, rather than the registrar who happens to be on’.
This sense of a local academic culture driving research was also evident in the interview with Roger, a senior triallist and NIC consultant at core centre C. Roger was an investigator for one of the core trials, sat on the TSC for another, and on the DMCs for a further two core trials. He said that he hoped that his centre had ‘a reputation for being scientifically based and research centred’ and that the results of research were fed directly back into practice at core centre C.
I like to think that we have an evidence-based unit. For instance, all the consultants have higher degrees, which is unique in the country I think. All our protocols, as far as they can be, are evidenced based. And there’s a protocol for just about everything . . . in this place. And underneath each protocol, it has the references, so you can go and look up where the evidence came from, and we think this helps the juniors particularly to understand that what they’re doing isn’t just on the whim of a consultant, but it is actually based on some sort of science. Where we have protocols that are not evidence based, because there isn’t any evidence, we state it, we actually say that there is no evidence to support this, but this is what we’re doing. All the consultants agree to use the same protocols, so there’s no change from one consultant to another, and most of the consultants, well they’ve all been involved in research themselves.
Over the course of his career it had become increasingly important to Roger that the evidence base was directly applicable to the babies in his care. He described how he and his colleagues would evaluate a protocol and if necessary ‘made local adjustments’ to fit their practice and population. The way they now carried out research and applied its results was very different from the work that he had carried out himself many years previously, which had little application to practice or population. He described it as ‘pretty well pure science’.
[It] probably had no immediate benefit as far as the patients were concerned. It was almost physiology research we were doing with some of these children. The stuff that we’re involved in now is all with a fairly practical aim in sight . . . I think the sort of work that we’ve been engaged with here for the last 20 years has almost all been with an . . . immediate clinical application if the right answer comes up.
Both Noel and Roger discussed the importance of research collaboration, and both took on multiple roles in the core trials. Dominic, a NIC consultant and professor at core centre D, and investigator for one of the core trials, presented a different model of research:
When I first started [here] all the neonatologists were all academics. Now, that was unsustainable as the complexity of the NHS systems grew, and now it’s divided out into NHS and academic senior staff. But the culture’s still the same . . . There’s a very strong research culture here, and it’s something that everybody buys into.
He explained that much of the research that they carry out is initiated themselves:
We are a large academic unit. We have a lot of funding and therefore a large responsibility . . . and so we’re very self-driven. We – we are initiators rather than participators. We don’t mind participating, but actually one of the problems we have is the . . . opportunity cost of every trial. So every trial we go into means that that baby may be unavailable for another one so we have to be careful of what we enter. So we’re almost entirely self-starting in our research.
While Noel and Roger had described a collegiate approach to research in their centres, with consultants proposing new research to be discussed at a group level, Dominic described a different approach to the process of deciding whether or not to collaborate in an external trial.
We’re probably not representative because by and large I decide. And when I’ve decided I’ll talk to my consultant colleagues about it, and my senior lecturer colleagues about it. It’s a bit autocratic in that sense, but then we are a very single-mindedly driven group . . . Our goal as a research group . . . is to reduce the number of children that suffer brain damage and survive with brain damage. That’s our goal and we’re very focused on that, and we do a lot of things around that field. We’re not against being involved with other people’s trials.
Nicholas, a professor of neonatal medicine who was also an investigator in one of the core trials, described enthusiasm for collaboration in core centre E and the importance of integrating the conduct of research into everyday care.
We recruited highly to the oscillation trial, we recruited quite well to TOBY, we recruited to INIS and other things, and with minimum problems actually . . . One of the things we used to pride ourselves on [was that] we would always have four or five trials running at any one time . . . [P]arents would say to each other, you know, ‘which trial are you in?’ So it was a matter of fact we were doing that. That was part of our culture, part of the way we did things, and I think people were very accepting of it . . . They were just part of the unit culture so . . . it wasn’t a big deal to be on a trial, and that was the whole point about trying to run the unit that way, because you didn’t want it to be a big deal. You wanted it to be something that . . . you could discuss rationally with parents in the knowledge that, you know, these were things you did all the time. [It was] part of the philosophy of what we do.
These thoughts on the local research ethos from senior clinicians and triallists give a flavour of some of the different priorities and conditions that exist across this setting. They give a sense of the importance of academic medicine as part of a tradition and in driving ongoing research and practice; they show how research can shape and be integrated into practice, mapping on to care so that it becomes a normal part of the work of a NICU, and how the evidence that is produced then feeds back into future clinical practice in the form of revisions to the evidence base.
The core trials were a major part of the research field in which these clinicians and triallists were involved. In order to understand the views and experiences of those involved in these trials it is important that they are not treated as a single entity (RCTs), but that the particular features of each trial and the circumstances that were created are described and understood.
Narrative description of core trials
Although RCTs may share some methodological features such as randomisation or blinding, they cannot be treated in generic terms. Each trial has unique features. The conditions that are created around a trial stem from how it is set up, how it functions and how it is perceived and experienced by those involved. These conditions are central to a study such as BRACELET that seeks to understand practice and provision for parents and other participants. A first step towards understanding the shared and the unique features of the core trials is to describe their development, characteristics and something of the circumstances they create for clinicians, parents and the babies involved. The core trials considered in detail in the BRACELET study are INIS, TOBY, PROGRAMS, BOOST-II UK and the ExPN feeding study. They are considered, in turn, below.
International Neonatal Immunotherapy Study: non-specific intravenous immunoglobulin therapy for suspected or proven neonatal sepsis95,96
Infection is a serious problem in NIC where it is a major cause of death and long-term disability. Immunoglobulin, a blood product containing human antibodies, was a promising treatment because newborn babies, those born preterm, have low levels of immunoglobulin and antibodies necessary for fighting infection. A number of RCTs had suggested that it may be helpful in the treatment of babies with a serious infection, but in order to demonstrate an effect on a common condition a large trial was needed. An international collaboration was set up and the resulting trial, INIS, recruited in 113 centres in nine countries. INIS was a placebo-controlled, double-blind randomised trial in which babies with a proven or suspected infection who were receiving antibiotics were allocated either to receive two doses of intravenous immunoglobulin or two doses of placebo. The trial primary outcome measure was a composite of death and disability at 2 years of age.
The majority of the babies involved in INIS were preterm. In each case, concern would be developing about the possibility or actuality of a developing infection. If a baby was showing signs of a serious infection then clinicians and parents would be aware of the threat to life that this represented. According to the trial protocol, enrolment could take place at any time during NICU stay and up to 28 days from expected date of delivery for babies readmitted with suspected or proven infection; this suggests that time would be available for discussion but, in fact, the likelihood of an infection meant that treatment should be initiated as soon as possible after diagnosis, and this introduced a degree of urgency into the enrolment process. The inclusion of ‘suspected infection’ in the eligibility criteria deliberately allowed clinicians a degree of interpretation over recruitment, and this resulted in considerable variation between recruiting centres. Some centres enrolled babies as they initiated antibiotic treatment on suspicion of an infection, but others did not do so unless they had a definite diagnosis. In some centres, this resulted in recruitment of babies at the sicker end of the spectrum permitted within the protocol. The intervention (two doses of immunoglobulin or placebo) was given using the intravenous access that was already in place. As the trial was double blind, neither clinicians nor parents knew whether or not a baby received the intervention.
The INIS trial was the largest trial identified in Phase I of the BRACELET study, and by the time the results were reported, it had recruited 3493 babies. (The figures reported for all of the core trials differ from figures collected for Phase I of the BRACELET study in that they refer to the whole trial and not just the 5-year period of interest, and are not restricted to recruitment in the UK.) Of these, 628 had died by 2 years, a mortality rate of 18.1%. (Mortality rates have been calculated from the number of recruits and the number of deaths reported in the trial papers. These figures do not necessarily appear in the papers themselves.) The trial showed no significant effect of the intervention. The results with 2-year follow-up data were published in 2011 (4–10 years since recruitment). The trial team had maintained contact with parents of surviving babies for follow-up purposes but not with bereaved parents. Results were sent to parents who had indicated at the 2-year follow-up that they wanted to receive them. All bereaved parents were contacted by post and offered the results. Not all parents were still living at the address registered with the trial at the time of their enrolment and it is therefore not known how many parents received this offer. All parents who received the results were given the option of being unblinded to their baby’s trial allocation.
TOBY: whole-body hypothermia for the treatment of perinatal asphyxial encephalopathy97–99
Babies who are born after complicated deliveries can experience a potentially damaging lack of oxygen to the brain before or during birth (perinatal asphyxia, leading to hypoxic–ischaemic encephalopathy). The brain damage they experience occurs not only at the time of oxygen deprivation, but also evolves over some hours after the event (so-called ‘secondary neuronal death’). Historically, there was little to offer these babies in terms of clinical interventions, and outcomes for encephalopathic asphyxiated babies could include a wide range of neurological sequelae (e.g. severe motor and sensory disability, cerebral palsy and neurocognitive developmental delay). In recent years, it was becoming clear that that there was a potential time window after the initiating event in which it may be possible to intervene to prevent further damage from happening. Growing interest in the neuroprotective potential for hypothermia led to RCTs exploring two different methods of cooling: cooling only the head was used in the CoolCap trial,132 whereas TOBY involved whole-body cooling. TOBY assessed whether cooling term babies for 72 hours to a temperature of 33–34 °C after they had suffered perinatal asphyxia, makes a difference to their chances of recovery. Babies in the treatment arm were compared with those in a control group who were given standard NIC without cooling. The primary outcome measure was combined death or disability at 18 months of age, as there was a concern that increased survival might be at the price of increased disability.
Because of the narrow treatment window, it was important to initiate cooling as soon as possible after birth. For the intervention to start early enough, randomisation and initiation of cooling had to take place within 6 hours of birth. All babies involved in the trial were transferred to a Level 3 NICU that was able to provide the specialist ‘cooling’ therapy. For the recruiting clinicians, the pace and setting of recruitment was a challenge. The difficulties that often preceded the development of perinatal asphyxia, and the consequences for babies and families that might ensue, meant that TOBY was conducted against a potentially litigious backdrop, with obstetric teams often being the focus of queries or enquiries about mismanagement. Recruiting NIC clinicians had to enter this difficult arena at a time of extreme crisis for parents and for babies. If babies were not born in a ‘cooling’ NICU, clinicians sometimes had to travel to the referring unit, assess the baby and discuss the trial with the parents, take consent, and randomise the baby within the stipulated 6 hours. If > 6 hours elapsed the baby could not be included in the RCT, even if this process was well under way. Whatever the challenges, it was an innovative and promising area of research and interviewees often presented their involvement very positively, seeing TOBY as a cutting edge, exciting and prestigious trial.
For the parents, the trial offered different challenges. By the time it was introduced they had been through extremely difficult experiences during labour and delivery, and their baby was unexpectedly receiving NIC with an uncertain future. They had to make rapid decisions about cooling and the RCT. Cooling was not otherwise available, and if parents wanted their baby to be cooled, the only route was the 50% chance of allocation to the intervention arm. In open trials, such as TOBY, in which it is not possible to conceal allocation, where babies are critically ill and the trial intervention may offer some hope, allocation to the control arm can be a very disappointing experience for parents. 54 For TOBY, parents also had to consider the issue of transfer and possible separation; if they were not in a cooling centre their baby would be transferred to the nearest available trial NICU and this included those allocated to the control arm, who would be transferred but not cooled. For some families this would involve separation of mother and baby if the mother was too sick post delivery to be moved. It could also involve care at some distance from the parental home.
The TOBY trial recruited 325 babies, and 86 had died by the age of 18 months, a mortality rate of 26.5%. The trial showed that cooling did not reduce the combined outcome of death or severe disability but did lead to improved neurological outcomes in survivors. The results and follow-up data were published in 2009. The trial team had maintained contact with parents through newsletters and change of address cards, and all parents who had indicated that they wanted to know the outcome of the trial, including those who were bereaved, were sent the results.
PROGRAMS: Prophylactic Granulocyte–macrophage colony-stimulating factor to reduce sepsis in preterm neonates100,101
Like the INIS study, the PROGRAMS study focused on the problem of infection for vulnerable preterm babies in the short and long term, but in this RCT the intervention was not aimed at treating an infection: it was intended as prophylaxis for babies at risk of developing an infection. Neonates with neutropenia, a deficit of a particular type of white blood cell (neutrophils), are more likely to develop infections and research had suggested that a protein, GM-CSF, may address this deficit and so reduce the risk of infection. PROGRAMS was described by one of the trial investigators, Nicholas, as involving a ‘subtle’ intervention. The effects of GM-CSF could only be observed on blood tests and it was intended to maintain rather than change a situation (i.e. not developing an infection).
Most of the babies who were eligible for the PROGRAMS study were preterm, born at < 32 weeks’ gestation, and were small for gestational age (SGA). Often growth problems would have been detected antenatally, especially for twins where one baby is often smaller than the other, so parents may well have felt concern over the well-being of their baby for some time before birth. They may have expected for some weeks that their baby would be cared for in a NICU and poor growth may have been the stimulus for an induced delivery. There was no immediate urgency for enrolment into the PROGRAMS study as babies could be recruited up to 72 hours after birth and parents could be offered time to make their decision. The PROGRAMS study involved a daily subcutaneous injection for five days, only in the intervention group. This was potentially an important consideration for parents. For SGA babies with little subcutaneous fat, injections might be a concern and a barrier to participation for some parents. Babies in the control arm had ‘usual care’ (with no placebo injections).
The PROGRAMS study recruited babies in the period 2000–6. This was a longer recruitment period than intended as the trial was stopped for 18 months because of problems with the supply of GM-CSF. In all, 280 babies were recruited and 62 died before discharge – a mortality rate of 22.1%. The trial showed no significant effect of GM-CSF on rates of infection or on 2-year outcomes. Initial results were published in 2009 and 2-year outcomes in 2012. Parents who opted to receive newsletters (whether or not they were bereaved) were informed of the 2-year results of the RCT in 2012.
BOOST-II UK: benefits of oxygen saturation targeting104,105
The BOOST-II UK study also involved very preterm neonates but focused on a different aspect of care. Delivery of supplemental oxygen has long been known to be an essential element of life support for extremely sick babies, but considerable uncertainty has existed over the correct level of oxygen to deliver: too little and babies can develop respiratory or neurological problems, which threaten their survival; too much and they can develop retinopathy of prematurity, which can cause full or partial blindness. The question of where pulse oximetry haemoglobin oxygen saturation (SpO2) thresholds should be set is of particular importance as ventilatory support and supplementary oxygen are central to NIC and the question has concerned neonatologists for many years. 133,134 This uncertainty is reflected in the variation in practice which was identified in preparation for the trial; units reported a wide range of SpO2 targets as their standard practice. The BOOST-II UK study was designed as part of an international collaboration between UK, Australia and New Zealand, the USA and Canada to determine whether, within this wide range of standard practice, higher or lower SpO2 levels are better for extremely preterm babies in NIC. The collaboration included plans for a prospective meta-analysis with ongoing trials.
The BOOST-II UK study involved babies born at < 28 weeks’ gestation, who were recruited within 12 hours of birth. They were allocated to either a higher or a lower target level of SpO2 (i.e. targeted higher levels were 91–95%, and targeted lower levels were 85–89%). To achieve blinding, the trial pulse oximeters – the devices that monitored SpO2 – were modified by the manufacturer so that although they worked as normal when saturations levels were < 85% or > 95%, they displayed and stored a figure that had been ‘offset’ either 3% above or 3% below the true value when saturation levels were within the 85–95% range. Using the trial oximeters centres therefore aimed to maintain all recruited babies within a range of 88–92%.
It was important to start delivery of the intervention quickly and so parents were approached as near to initiation of ventilatory support as was possible. Some women were approached antenatally if they were inpatients and it was clear that their baby would be born at < 28 weeks’ gestation, giving them time to discuss and consider the trial. Parents could also be approached in the hours before preterm delivery. Unlike the INIS and PROGRAMS studies through which delivery of the trial intervention might not be witnessed by parents, in the BOOST-II UK study the trial pulse oximeter was permanently visible at the bedside.
The BOOST-II UK study recruited 973 babies by the time it was stopped. Of these, 895 babies were reported in the early publication, of whom 173 died by 36 weeks postmenstrual age, a mortality rate of 19.3%. During the course of BOOST-II UK another trial, SUPPORT (Surfactant Positive Airway Pressure and Pulse Oximetry Randomized Trial), reported its findings,135 suggesting increased survival with higher levels of SpO2. An interim analysis of the BOOST-II UK data from UK, Australia and New Zealand was therefore carried out. Data were pooled with the data from the SUPPORT Trial and a significant difference in mortality between higher and lower SpO2 targets was identified, whereupon the DMCs recommended the trials should be stopped early. Following emergency meetings of the TSCs, the BOOST-II UK studies stopped recruitment on Christmas Eve 2010, and all hospitalised babies in the trial were transferred to be nursed with non-trial oximeters and their clinicians were informed that the higher oxygen target was associated with greater survival. These initial results were published in 2011 and were communicated to all parents as a study update. (Although the primary outcome is when the children are aged 2 years, we refer to this study update hereafter as results.)
The process of communicating the results of the BOOST-II UK study was very different from those used in the TOBY and INIS studies. Because the BOOST-II UK study was stopped early, findings were made public as soon as a publication was possible. The parents recruited later in the trial will therefore have been contacted within a relatively short time since the birth (and death) of their baby. This ranged from < 1 year to 4 years for the BOOST-II UK study; for the TOBY study it was 3–7 years, and for the INIS study (which took longer to recruit) it was 4–10 years before parents received the results.
Unlike the INIS and PROGRAMS studies, which showed no statistically or clinically significant benefits or harmful effect of the trial interventions, the BOOST-II UK RCTs generated data that allowed the team of investigators to make a recommendation to their NIC colleagues. The publication acknowledged the lack of follow-up data at that point, and while waiting for the outcome of disability-free survival to be determined (expected in 2014), the publication stated that the authors considered it prudent not to target a SpO2 of 85–89% in infants born at < 28 weeks’ gestation.
ExPN: Extremely Preterm Nutrition feeding study – Improving post-natal head growth in very preterm infants: a randomised controlled trial of hyperalimentation102,103
Providing extremely preterm babies with the nutrients that they need is difficult because they may be ventilated, too sick or too immature to suck, and their digestive systems are often unable to digest milk. Fluids and nutrition are given intravenously but growth may still be slow. Clinicians at a single centre were aware that in their local population they saw babies with smaller than expected head circumference at discharge and this was attributed to postnatal growth failure. Funds were sought for a research fellow to conduct a trial assessing the impact of giving additional calories and protein on head growth compared with a standard feeding regimen, with a follow-up to consider whether this might correlate to longer-term outcomes. ExPN was driven, in part, by uncertainty over the cost–benefit ratio of giving additional calories: an increase in glucose can potentially induce a ‘diabetic’-like state, which increases both the chances of hyperglycaemia and infection. Babies receiving the intervention may then require administration of insulin and treatment of infection. Infection, especially the possibility of necrotising enterocolitis (NEC), is a major threat to such small babies, and any possible increase in infection rates would be a major concern. The trial was partially blinded: the trial assessors were unaware of the allocation but parents and clinicians administering nutrition were not blinded. This caused a number of problems for the trial, as some clinicians, sensitised to the issue of postnatal growth failure in this population as a result of the trial, may have compensated babies in the standard feeding group by giving extra calories. However, there was a clear separation between the trial groups as significantly more calories and protein were given to the intervention group.
Babies were eligible for the ExPN study if they were born at < 29 weeks’ gestation. The trial included babies at the lowest gestations to be admitted to NIC, around 24 weeks’ gestation, an extremely high-risk population born at the limit of viability. It included a substantial proportion of twins who are at greater risk of preterm delivery and growth-related complications. In some cases mothers with an identified complication, such as twin–twin transfusion, may have had a planned delivery despite the early gestation. Delivery at this gestation can be an unstoppable crisis or can be preceded by a potentially lengthy period of threatened delivery with some women as inpatients, hoping to extend their pregnancy but expecting to give birth any day. Parents were asked to consider the trial within a week of the delivery and given that time frame there was no urgency over the decision, but some parents would still be in the very difficult immediate aftermath of unexpected preterm birth.
The ExPN study differed from the other core trials in several ways. It was conducted in a single centre with all recruitment taking place locally and carried out by only two clinicians. Often several babies were enrolled into the trial at the same time and parents could discuss this with other parents, and compare information and progress. The ExPN study explored the impact of food rather than a drug, gas or a medical device as in the other core trials, and this may have affected how parents viewed the potential risks and benefits.
Between 2004 and 2007, 142 babies were recruited to the trial, of whom 33 died by 9 months postmenstrual age, a mortality rate of 23.2%. The ExPN study demonstrated no significant effect of the intervention on growth, and there was no increased incidence of NEC or other infection between the trial groups. Initial trial results and follow-up data were published in 2008 with no feedback of results to parents.
Complexity and the core trials
Many different components are present in the narrative descriptions of the five trials. Some are unique features of a single trial, others are shared with several trials. Collectively they are extremely complex, and this reflects a real complexity that exists in NIC research where the different demands and implications of a wide range of components are worked through in practice. Research active centres take part in multiple RCTs concurrently, and experienced triallists develop their own trials while at the same time are in demand to serve as experts on those of their colleagues in the field.
For our purposes it is helpful to draw out these different components of design and management as essential contextual features so that any implications and effects that they might have can be explored. Too often, studies consider reactions to trials in broad terms, without tracking back to these source elements which can shape experiences of collaboration in clinical centres and participation for trial participants and proxies. We therefore suggest the importance of thinking about the features of the core trials in terms of three broad categories: structural, procedural and synergistic components, as defined in Table 22 . How the components of each trial fit into these categories can then be taken into account in subsequent analysis.
| Structural components | The design features of individual RCTs create a structure that is set at the beginning of the trial and commonly does not change. These include the location of the trial (e.g. national/international), support infrastructure, interventions under study, the target population, the recruitment window, trial design, comparison and outcome measures. Table 23 lays out examples of the structural components of the core trials |
|---|---|
| Procedural components | The strategies used by a trial team to organise the running of the trial are also important research components, and this includes how any contact with parents is managed. These strategies depend as much on the infrastructure available, as on views of appropriate trial management. Although the day-to-day organisation of a trial is extensive, and many components could be included here, the procedural components of most relevance to the BRACELET study would include whether or not a trial has policies on contact with parents by means of newsletters and web pages, whether or not a trial team offers feedback of trial results to parents and the timing of doing so, and whether or not unblinding is offered. Table 24 lays out examples of procedural components of the core trials |
| Synergistic components | Some aspects of trials cannot be pinned down in a protocol because they are beyond the control of the trial team or because they are unpredictable. This includes the actual recruitment period (as opposed to the planned recruitment period), unexpected events and the final results of the trial. The ways in which the protocol is implemented in clinical centres, how parents respond and how data accrue are components that grow from synergistic connections between research teams, clinical colleagues and parents. Table 25 lays out examples of synergistic components of the core trials as far as possible, but these elements of trial processes do not lend themselves well to tabulation. Some data cannot be completed as they have not been measured or reported |
| Structural components | INIS | TOBY | PROGRAMS | BOOST-II UK | ExPN |
|---|---|---|---|---|---|
| Location of the trial | International, UK led | International, UK led | Multicentre, UK | UK led but run in parallel with other international trials | Single centre |
| Infrastructure | CTU support | CTU support | CTU support | CTU support | No CTU support |
| Intervention under study | Immunoglobulin for sepsis | Hypothermia for perinatal asphyxia | GM-CSF as prophylaxis for sepsis | Targeted SpO2 levels | Hyperalimentation – extra calories and extra protein |
| Target population | Proven/suspected infection, on antibiotics | Term neonates with perinatal asphyxia | SGA neonates of < 32 weeks | Neonates of < 28 weeks | Neonates of < 29 weeks |
| Recruitment window | As soon as an infection is suspected at any time on NICUa | Within 6 hours of birth | Within 72 hours of birth | Within 12 hours of birth if inborn; within 24 hours of birth if outborn | Within 7 days of birth |
| Trial design | Placebo-controlled double-blind RCT | Unblinded RCT for parents and caregivers, but blinded outcome assessment | Unblinded RCT for parents and caregivers, but blinded outcome assessment | Double-blind RCT | Unblinded RCT for parents and caregivers, but blinded outcome assessment for primary outcome/MRI analysis |
| Comparison | Two doses of intravenous immunoglobulin vs. two doses of placebo | Moderate cooling for 72 hours in a cooling centre vs. best standard care in a cooling centre | Daily intravenous injection of GM-CSF for 5 days vs. best standard care (no placebo injection) | SpO2 targeted at 91–95% vs. 85–89% | Increase in calories in protein vs. no increase in calories or protein |
| Primary outcome measure | Combined death or major disability at 2 years | Combined death or severe disability at 18 months | Sepsis-free survival at 14 days from trial entry | Death or severe neurosensory disability at 2 years’ postmenstrual age | Occipitofrontal head circumference at 36 weeks’ postmenstrual age |
| Procedural components | INIS | TOBY | PROGRAMS | BOOST-II UK | ExPN |
|---|---|---|---|---|---|
| Contact policy | Details updated for parents of survivors only for follow-up | Contact with all parents updated for newsletters and parents of survivors for follow-up | Contact with all parents updated for newsletters and parents of survivors for follow-up | Details updated for parents of survivors only for follow-up | Contact with parents of survivors only for follow-up |
| Additional points of contact | None | Newsletters Condolence letter Message board |
Newsletters | None | None |
| Availability of results | Main results published 2011 | Main results published 2009 | Initial results published 2009; 2-year follow-up) published 2012; 5-year follow-up not yet published | Initial results published 2011; follow-up data not yet published | Initial results and; follow-up data published 2008 |
| Results offered to parents | All contactable parents offered results | All contactable parents sent results unless they had previously opted out | 2-year follow-up results sent to parents who had asked to receive the trial newslettersa | All contactable parents were sent the study update | No feedback of trial results to parents |
| Time from enrolment to sending results | 4–10 years | 3–7 years | 3–9 years | < 1–4 years | N/A |
| Parents offered unblinding | Yes | N/A | N/A | No | No |
| Synergistic components | INIS | TOBY | PROGRAMS | BOOST-II UK | ExPN |
|---|---|---|---|---|---|
| Recruitment period | October 2001 to September 2007 | December 2002 to November 2006 | June 2000 to June 2006 | December 2007 to December 2010 | January 2004 to January 2006 |
| Consent rate | Not recorded | 30/494 declined to participate | Not recorded | Not recorded | 26/176 declined to participate |
| Unexpected events | On advice of DMC that primary outcome more frequent than estimated, target sample size reduced | Planned sample size achieved ahead of schedule, but enrolment continued when results of other trials suggested larger sample would be valuable | Recruitment stopped for 18 months for supply problems | DMC recommended stopping the trial early | Some dilution of separation of policies as caregivers became more sensitised to nutrition issues |
| Trial results | No significant effect on suspected or proven sepsis | Intervention did not reduce combined outcome of death or severe disability but did lead to improved neurological outcomes in survivors | No significant effect of intervention on sepsis, survival or short-term outcomes | Significant difference in mortality up to 36 weeks postmenstrual age (preliminary result) | No significant effect of intervention on occipitofrontal circumference at 36 weeks’ postmenstrual age, but reduction in time to regain birthweight and days on parenteral nutrition |
The features of the core trials described above are important aspects of the context that the BRACELET study was set up to explore, and the typology of components provides a useful framework for reflection on the different elements within a RCT. It is also a basis for further exploration, as the differences between trials highlighted above suggest the value of an even closer focus on the origins, effects and implications of the methodological decisions that were made, in illuminating further the contextual similarities and differences for each of the core trials.
Trial design issues for the core trials
The differences between the RCTs are complex but they are not haphazard. They are a product of a developmental process that produces a trial protocol with a specific set of research methods. The methods defined in a protocol have to be appropriate for use in the clinical arena of NIC, as they are passed into the hands of local clinicians who then dovetail them in their clinical practice. The developmental process is iterative as explained by one of the senior trial investigators:
Although . . . you might start off with the science . . . you have to then meld that with the practicality of it . . . You don’t sit down and just design a trial and then think, ‘Now we’ve got to think about the other aspects of it.’ It goes round and round and round and all of these things get taken into account . . . That’s why you need a group. That’s why you don’t have one person like me sitting there and designing something which is impossible to run.
The core trials all involved populations of babies at high risk of death. The core trial CIs and one lead neonatologist (appointed for one of the trials as the CI was not a neonatologist) were asked how their trial methods were shaped by the likelihood of death in their target populations (pseudonyms are not used in this section of the report to break the link between comments here, in which the interviewees are potentially identifiable, and views expressed elsewhere in this report). This raised discussion of several different methodological features of the trials. Their accounts of why particular features were set as they were, and what implications they had for their trial, demonstrate a number of shaping influences on trial design. These are explored below for the following features:
-
eligibility criteria
-
randomisation
-
management of the control group
-
outcome measures.
Eligibility criteria
One of the most important issues in a RCT is who should be included and excluded from the sample. 136 How the target population is defined will determine the boundaries around the evidence that can be produced and this important consideration was discussed for two of the trials: TOBY and INIS.
In the TOBY study, this issue was central to the trial design and a high threshold of severity for eligibility was set. (Babies were eligible if they had at least a 10-minute Apgar score of ≤ 5, or a continued need for resuscitation, or acidosis within 60 minutes after birth, as well as moderate to severe encephalopathy and either hypotonia, abnormal reflexes, an absent or weak suck, or clinical seizures. In addition, they had to have abnormal background activity for at least 30 minutes or seizures on amplitude integrated electroencephalography.) The CI commented.
Study design is a very important, is critical for the success of a clinical trial, and there are rules . . . you have to follow assiduously if the trial, you know, is going to be . . . useful . . . Selection criteria, if you want, are determined by the type of trial you’re doing. In the TOBY trial this was an early-phase study, even though it was a relatively large study and went over many years. It started out at a time when. . . no previous studies had been completed so it was one of the first clinical trials . . . Although it was large enough that some people would call it a phase-three trial, [it was] in effect an early-stage trial focused on . . . a very high-risk population.
The CI felt that the trial would have been very difficult to run had it included a lower-risk population of babies, but using a more narrowly defined population also raises some difficulties. It has implications for the evidence base generated, and affects the possibility of carrying out future research with a lower-risk population. Now that the TOBY study has shown cooling to be effective in improving neurological outcomes in survivors in a high-risk population, the CI felt that a trial in a low-risk population is unlikely.
That’s a dilemma for commissioners: . . . do they have to stick to the trial protocols, or do they start widening the protocols a bit to include children who didn’t quite meet the criteria but seem to have a similar problem? . . . The problem is that the group that were not included in the trial would have a low risk of adverse events, so you’d need a very, very large trial to demonstrate a small change in that group of babies, and I think because of that many people feel it’s not feasible to do that trial.
The focus on the highest risk babies raises other issues in the running as well as the design of the trial. One issue was how to determine whether a baby at the sicker end of the spectrum should in fact be enrolled. The trial eligibility criteria were very clearly determined at the lower threshold of eligibility, but the upper threshold was less clear. Babies who were moribund were to be excluded on the grounds of futility but how this should be determined was deliberately not defined in the eligibility criteria. The CI explained that whether or not a baby is on a trajectory towards death is difficult to determine:
If the baby’s bradycardic it’s quite clear the baby’s dying, but if the baby’s circulation is fine but is otherwise completely encephalopathic, you know, is that moribund? So I did not feel I could define it so clearly.
This was a real issue for the trial and there were cases where careful judgement was required as to whether or not an extremely sick baby might be included. The CI’s explanation of this conveys something of the fraught clinical circumstances in which the trial was set, and the close interweaving of the circumstances of care into research. He described a delicate balance between the need for good science and the desire to ‘do something’ for an extremely compromised baby:
People wanted to try and formalise this by having specific guidance and direction and I always felt it’s actually very difficult to specify the scenarios, and that anyway neonatal clinicians are having to deal with these grey areas all the time, with uncertainty all the time. And I just wanted the attending clinician to continue to have a say. Whether they felt, ‘actually, no, I don’t think this baby should be in your trial. I think, I think this baby’s going to die, and I think we should just redirect care around that . . .’. If they thought the baby was so ill it was moribund, or likely to be moribund, they did not need to make the referral. In practice, what we found was . . . that these were difficult cases, and clinicians were very keen to try and . . . do something active . . . I’ve been called to cases in other hospitals, where the baby clearly was very poorly and no intervention was going to – could possibly do anything, where the clinician felt the right thing to do would be to have the baby in the trial.
The clinical judgement about who should and should not be referred to the trial shapes the trial population and the inferences that can be drawn from the data that are produced. In the TOBY study it was important that very sick (but not moribund) babies were included so that the trial could explore whether or not cooling was helpful for those babies, but it was important that a wider range of very sick babies should be enrolled in the trial. This was another matter for clinical judgement in the recruiting centres.
[At the] early stage . . . we were in equipoise even for the sick babies. If we didn’t recruit them we didn’t know. We had this problem in TOBY that we were likely to be referred the sickest babies initially, and . . . if you only refer the sickest babies then there was no prospect of seeing any effect of your treatment . . . If you have a trial that went on as long as TOBY did . . .[with] numbers of centres increasing dramatically over the period . . . the type of patient you get referred changes as well. Centres who referred a few patients [at the start] are likely to refer more and more patients [and] centres only just starting on often start off with the bad cases.
One of the CIs interviewed here for another core trial also had links with TOBY and commented on the borderline for eligibility and the decisions that might be made in clinical centres. He felt that they should transcend concerns about which babies should and should not be entered:
In almost all interventions, those that are slightly less than extremely sick if you like, are the ones most likely to benefit, rather than the sickest ones, because the sickest ones are always likely to die, regardless of what you do, because they’ve gone too far . . . I think that when it comes to deciding whether to put a child in a trial, we try to capture everybody who’s eligible rather than say ‘Oh we’ll do this one and not this one.’ It doesn’t always work that way . . . everybody who’s eligible really ought to be going in, people shouldn’t be picking and choosing. We try not to do that here.
This overview from the CI and the view from within a referring centre suggest a dynamic between centres and a trial that can be fixed by local policy or can change over time, and which directly shapes the progress of a trial and the evidence it can produce.
A similar phenomenon was seen in the INIS study, in which, again, interpretation of eligibility criteria was dependent on clinical judgement, which would vary from centre to centre and clinician to clinician.
The eligibility criteria for INIS is so difficult because it’s confirmed or probable sepsis where there are loads of contaminants, so . . . the severity of sickness that babies were recruited at varied a lot between centres . . . [The centres] . . . made their decisions locally about . . . at what threshold they would recruit . . . Some was quite low and some was very high as in, you know, they were expecting these babies to die and this was the sort of last chance.
The CI went on to describe how this sometimes played out in the clinical centres:
There used to be ward rounds where the PI would say, ‘Why wasn’t this baby recruited to INIS?’ ‘Well it wasn’t sick enough.’ ‘What do you mean, it wasn’t sick enough?’ ‘Well . . . the white cell counts were only this and blah, blah’. . . There were these intense debates about whether that baby was or was not sick enough and there’s no right answer to that so I think it was quite difficult.
Trials can be characterised along a continuum from a pragmatic trial, which assesses an intervention within clinical practice, and usually has broad entry criteria reflecting clinical uncertainty, and emphasising an intention-to-treat (ITT) analysis, to an explanatory trial which evaluates an intervention within more tightly controlled conditions, to assess efficacy under ideal circumstances, often emphasising a per-protocol analysis. Many trials have elements of both characteristics. The design differences between the INIS study (a pragmatic RCT that looked at immunoglobulin in the wider context of everyday practice) and the TOBY study (which could be considered to have components of an explanatory trial, as it had tightly controlled eligibility criteria) are important here. Although they both open up discussion of who is and is not eligible and whether or not enrolment in the trial was appropriate, in fact the more open nature of the eligibility criteria for the INIS study mean that a wide variety of babies were included in the trial and this was ‘entirely compatible with the protocol’.
Randomisation
Once eligibility is confirmed and parental permission has been given, the next step is randomisation. Issues around the management of this aspect of RCT methods was discussed for two of the trials: the ExPN and BOOST-II UK studies.
Given the inclusion of extremely preterm babies in their trials, the investigators for both studies could anticipate that a proportion of their sample would come from multiple births. As siblings would be eligible for enrolment at the same time, an important consideration was how to manage their allocation in scientific and in compassionate terms. The trials used opposite randomisation policies. In the ExPN study, twins were randomised together to ensure allocation to the same treatment, and triplets and higher-order multiples were excluded. In the BOOST-II UK study, there was no exclusion of higher-order multiples and each baby was randomised individually, thus introducing the possibility of allocation to different arms of the trial. The CI for the ExPN study explained their rationale:
I took some advice on this, statistical advice, and they said I could [randomise twins together]. What we actually did was they were stratified for gestational age and for singleton, multiple, so we could analyse them apart, as well as together, without losing randomisation. And the twins were both randomised to the same intervention, control or intervention, and the reason I did that [was that] I felt that it was difficult for parents to have one twin treated one way, and one twin treated the other way.
Given these concerns about the impact of allocation for twins on parents, one option would have been to exclude twins from the trial. This would, however, have raised issues of statistical power, and of the applicability and clinical relevance of the trial. It was important that the ExPN study population should be representative of the clinical population so that the evidence would be clinically generalisable. The ExPN study CI commented:
I certainly didn’t want to exclude twins from the study because they make up [a] quarter of our babies, so they’ve got to be included.
In the BOOST-II UK study, babies of multiple births were randomised separately. Whereas in the ExPN study this methodological decision was led by the CI, in the BOOST-II UK study the randomisation policy for the CTU for all of its trials was the determining factor. This meant that the lead neonatologist, was not directly involved in setting this policy, but he said that he was ‘totally comfortable with it’. In the ExPN study, one of the concerns was how parents would react to different outcomes for their babies if they had been allocated to different arms of the trial. This was not seen as problematic by the lead neonatologist for the BOOST-II UK study who felt confident in the explanation that could be given to parents.
I explain to parents that whatever happens with their baby we won’t know what was the explanation for that happening because their baby will be getting a whole package of measures, only one of which will be the trial, so ultimately they’ll know what happened to their baby, they might know what the trial showed, but not all babies in one arm of the trial will have the same result and all babies in the other arm the opposite.
He also argued that an individualised approach in the trial would be commensurate with individualised care which is given outside of the trial context.
We don’t treat twins exactly the same way throughout their course. We tailor their care to meet their needs so I don’t have a problem with them being randomised differently and I haven’t really had parents’ feedback to me that they do either . . . [A] lot of people feel strongly about it and it’s a question you’re always asked when you present to people a trial that you want them to participate in, but I think that . . . the clinicians almost feel more uncomfortable about it than anything I’ve ever heard expressed by the families . . . You have just got to make a decision about your protocol and it’s going to weaken the research to randomise them as pairs.
These issues may differ in a double-blind trial such as BOOST-II UK compared with a unblinded trial such as TOBY. Blinding is discussed further in the next section.
Management of the control group
On randomisation, babies are allocated to the intervention or the control arm of the RCT. Whether or not allocation to an intervention is concealed is an important issue in trial design. 137 The core trials varied in the extent to which allocation remained concealed after randomisation; the INIS study was a placebo-controlled, double-blind trial, the BOOST-II UK study was double blind, the ExPN and PROGRAMS studies were blinded only at the outcome assessment stage, and the TOBY study was an open trial.
In an open trial, in which the intervention brings about a major and obvious change to the management of a baby, important methodological and clinical decisions have to be made about the trial regimen for those allocated to the control arm who will not receive the trial intervention. With no blinding to allocation, management decisions are explicit and can feature prominently in the experiences of those involved. This issue has been faced previously in other NIC RCTs, notably the UK Collaborative extracorporeal membrane oxygenation (ECMO) trial,138 for which all of the babies had severe respiratory failure. Those in the intervention arm were transferred to a specialist centre and those in the control arm continued receiving the standard of care in their original NICUs with no further changes to care. Allocation to standard of care was often experienced as ‘doing nothing’ and could be a difficult experience for parents. 54
The TOBY study also involved very ill term babies and evaluated an intervention that may be desired by clinicians as well as parents. In contrast with the ECMO trial,138 all babies in the TOBY study, regardless of allocation, were cared for in Level 3 NICU cooling centres and this could involve transfer for babies in the control as well as in the intervention arm of the trial.
There were some babies in the TOBY study who were moved for the purposes of the trial (i.e. to assess whether cooling was neuroprotective) but for most of the babies in the control group a transfer in the TOBY study was consistent with their clinical needs. The CI explained that an essential clinical consideration related to the standard of care needed for asphyxiated babies, and said, ‘these were very sick babies and needed to be in intensive care’ and this could not always be offered in the less specialised units where some of the babies were born.
[T]he cooling centres were in effect the most specialised units . . . and most of the babies in the trial were born in those centres . . . [A] number of babies were born outside the treating centres . . . [T]he vast majority of those that were moved came from lower-level centres, and the package of care would not have been easily delivered in their hospital of birth.
The package of care to which the CI refers is the standard of care required for the baby and as a comparator within TOBY. This example highlights the ways in which the clinical characteristics of the population under study, and the methodological response to that, can shape a trial in ways which will then have a direct impact on what happens to babies and their families when a baby is enrolled into a trial. It shows how decisions about research cannot be separated from the duty of care in the clinical context in which they are set.
How the intervention for the control group might best be managed for the PROGRAMS study was an important methodological issue of a different type as it addressed clinicians’ responses to the allocation. The trial intervention involved daily subcutaneous injections for 5 days and the trial team wished to avoid giving five placebo injections to babies in the control group. Placebo injections would have concealed the allocation from the parents but not from the clinicians involved in the baby’s care or from the trial assessors. The CI described the concerns they had:
The result of the treatment was immediately obvious to anyone looking at the baby’s blood counts and, therefore, the whole purpose of the placebo was negated and that raised some very interesting methodological issues. I’m delighted that with the help of brainstorming amongst our collaborators, we came up with a very nifty way of, of dealing with it . . . [We made] sure that the outcome assessment was done blind to the results of the full blood counts . . . so it was done by an independent group of people . . . In other words, it wasn’t the clinician looking after the baby who was responsible for capturing the data that determined whether or not the baby had had an outcome but it was an independent group of people.
Whether or not the trials used a placebo, and how this might affect parents who go on to be bereaved, was a common point of discussion in the BRACELET interviews and this topic will be discussed further in Chapters 6 and 7 .
Outcome measures
An important element in the methods laid down for any trial is the setting of outcome measures. 136 Primary outcomes define what are considered to be the most important potential changes that might be observed in the trial population, and these will vary according to the population and the intervention being used. Death is a common primary outcome but this is not always relevant if a trial involves patients at low risk of death or assesses an intervention that is focused on affecting a particular aspect of care, length of stay or initiation of antibiotics. Trials are often looking for a number of potential changes and more than one primary outcome can be used. Secondary outcomes detect less important but significant changes such as side effects. Sometimes if the population is at high risk of death, mortality is included as a secondary outcome even if it is unlikely to be affected by the trial intervention per se. Death may also be part of a composite primary outcome even if the intervention is not predicted to affect the risk of death: rather it is included so that there can be an ITT analysis.
The five core trials all involved babies at risk of death but they each used different outcome measures, as shown in Table 26 .
| Primary outcome measure for trial: | ||||
|---|---|---|---|---|
| INIS | TOBY | PROGRAMS | BOOST-II UK | ExPN |
| Combined death or major disability at 2 years | Combined death or severe disability at 18 months | Sepsis-free survival at 14 days from trial entry | Death or severe neurosensory disability at 2 years’ postmenstrual age | Occipitofrontal circumference at 36 weeks’ postmenstrual age |
Although all of the core trials involved substantial mortality rates, and the likelihood of death in these critically ill populations could be predicted, not all of the trials measured death as an outcome. The ExPN study did not, but this trial focused on the effects of providing more of something that is considered standard care (i.e. extra nutrition rather than usual nutrition) and it is difficult to make a direct, short-term plausible biological link from more food to death. The primary outcome for the ExPN study was an increase in head circumference measurement at 36 weeks’ postmenstrual age (i.e. there is a potential link between more food and better growth in the short term). Secondary outcomes included a range of clinical events. Some, such as the development of NEC, would be a major threat to the life of a baby, but NEC, and not mortality, was the outcome measured, as there could be a biological connection between overfeeding and NEC. That is not to say that the trial team was not focused on the potential for mortality in their trial: they ensured that deaths in the trial population came under scrutiny. In the ExPN study there was no formal DMC but an expert neonatologist acted as a ‘one man DMC’. He was asked to look over the first 50 cases in the trial, and this raised some issues relating to mortality. On further analysis the effect ‘disappeared’ and the trial continued. Exactly the same issue was faced in the PROGRAMS trial: the DMC examined a possible difference in mortality between the arms, which proved to be an early trend that was not present in the final data set.
Some of the outcomes for the ExPN study were measured at 2 years, meaning that data collection was spread out over a longer time, with follow-up sometimes years after the initial involvement in a trial. This was managed in stages with multiple consent processes, and only the feeding element of the trial was raised with parents initially:
We wanted just to get them on the feeding bit and in fact, that was perhaps the most important bit because we wanted to look at the growth of the child. Subsequently we wanted to do the MRI because that would actually show you which bits of the brain hadn’t grown. Then we wanted to follow up to see whether it was all worth it . . . So there were three consents . . . and most people went through the whole lot. Quite a few opted out of MRI, but allowed the follow-up.
The inclusion of long-term outcomes has implications for data collection and the running of the trial. The outcome measure closest to the time of the intervention in any of the core trials was used in the PROGRAMS study, which looked at sepsis-free survival 14 days after trial entry. The very short time frame was set in this way because this trial was initially looking at whether it was possible to address neutropenia (see explanation above) and whether this in turn might affect infection rates. Both of these changes could be determined very quickly and so the primary outcome for this trial was measured at 14 days.
When combined or composite outcomes are used (such as death or disability, or sepsis-free survival), an outcome is said to have occurred if a participant experiences one or more of the events. 139 Composite outcomes offer the advantage that sample sizes can be lower and trials can therefore complete recruitment more quickly because there is no need to collect sufficient data to look at each outcome separately. But this approach brings with it some difficult issues, which are a particular challenge in this setting. One of the CIs explained how NIC trials differ from those in other specialties such as cancer.
In neonatal intensive care, almost all the deaths are at the beginning and then there’s nothing, there’s almost nothing later on so all your deaths are early and your disability has to wait until 2-years of age to assess . . . It’s a unique situation.
Predetermined outcomes are used to focus in on the key questions for a trial and to mark out the data that are required to answer those questions. They are used by DMCs to determine whether or not to recommend a trial should continue, and as this last quotation suggests, this can be a particularly difficult methodological and ethical issue in NIC RCTs. At interim analyses the DMC members see the trial data by allocation. They can therefore see, while a trial is still recruiting, whether or not there seems to be a difference in mortality between the groups. Sometimes, as in the case of the ExPN and PROGRAMS studies, further analysis will show that that difference is due to chance and will disappear as more data accrue. Sometimes, as in the BOOST-II UK study, an early difference in mortality will be sufficient to make it inappropriate to carry on with the trial, and the trial is stopped. It is difficult, however, if trials are not designed to look at death alone but at a composite outcome of death and longer-term disability combined as the primary outcome. If an intervention does have an effect, the essential question here is more subtle than simply a matter of survival; it is whether increased survival is associated with an increase in disability or lower disability. Mortality then is clearly only part of the question but this places the DMC in a difficult position.
You may be sitting on a difference in mortality waiting to get some supporting evidence that you aren’t getting an increase in severe disability which may offset the difference in mortality and what do we mean by offset, whose, whose judgement is that? . . . If the primary outcome is death or severe disability at 2 years and there’s no difference between the groups but one has a much lower risk of death and the other one has a much higher risk of disability . . . parents will interpret that result differently. Some will say, ‘I don’t care if my baby’s disabled, I want my baby to survive’ and others will say, ‘I don’t want a severely disabled baby, it’s not fair on the child . . . it would seem kinder for them.
This is exacerbated by the need to think beyond the confines of the trial in hand. If a trial is stopped early on the recommendation of a DMC because of a difference in mortality, this affects whether or not future trials may be funded and carried out in another attempt to address the longer-term question. One of the CIs explained that this is a major balancing problem when trials have composite outcomes:
You’re terrified of doing harm, and of being accused of not stopping it in time, but equally you don’t want to throw away the only opportunity you may well have of answering that question and benefiting children in the future.
This decision also shapes the evidence that is produced, which will, in turn, affect how an intervention is used or not used in practice. One of the CIs argued that for this reason it can be very important to push on:
Sometimes it is important to continue to get a very clear answer because, if the aim is to influence clinical practice for the betterment of the patients, there is a very strong argument for having a very clear answer which people can’t argue with. So having a result which is just significant may not persuade people to change their practice, in which case it may be justifiable and I use the term ‘may be’ because these are awful decisions, to go on to accumulate more deaths in order to change practice in order for the myriad of babies out there for years to come who may benefit from that intervention. Those are very, very difficult decisions for DMCs to take.
These very difficult decisions flow directly from the earlier methodological decisions that were made about outcome measures. Reducing the timing of the end point for assessment can help but it is not always possible. Two of the core trials, which had composite outcomes, the INIS and BOOST-II UK studies, measured these at 2 years after birth, as this is the point at which evidence of disability in preterm infants is becoming clear. The point at which death and disability was assessed was earlier for the TOBY study at 18 months following a term delivery. This difference is important, as it is possible – if a trial is set to recruit for a number of years – for some follow-up data to start to accrue during recruitment if the lag between birth and assessment of outcomes is not too great. One of the investigators explained that this difference is set, in part, by the trial population, but his comments also suggest that methodological opinion, which will vary between individuals, is also important. (This individual is not a CI but data from his interview were informative and so they are included here.)
We usually measure prem outcomes at 2 years you see. TOBY was 18 months [and] ECMO was 1 year . . . My view [is] that actually term babies you could do at a year. I still think TOBY should have been done at a year. But even so, your trial’s mainly over by the time you’ve got your outcome, so actually when you’re running the trial you, you really find that it’s very difficult for you to take cognisance of the disability side of the outcome.
Given these difficulties it was not surprising to hear CIs state that trials which do not use composite outcomes that are ascertained at different time points are more simple.
Components and considerations in the design and management of the trials
A range of components have been identified in the five core trials and categorised as structural, procedural and synergistic. The accounts of the lead researchers for these trials describing the development of these components suggests that key features of the trial designs are shaped by a number of considerations.
Four considerations were identified here: scientific issues, clinical issues, the impact on parents, and ethics. This list is not exhaustive, and other considerations, such as finance51 and logistics, are also likely to exert their effects on design and management. It is useful, however, to highlight these four considerations, as the accounts of their relevance reported above show how such factors are incorporated into the trial designs. The data reported above also show how these considerations reflect the interest and concerns of different parties associated with trials: the trial investigators, the CTU, clinical colleagues and parents. This is shown in Figure 15 below. These different components and considerations can also be picked out in Table 27 in the description of one aspect of the trial that flows from an element in the design of the TOBY study.
FIGURE 15.
Parties of influence and considerations in setting design features for the core trials.
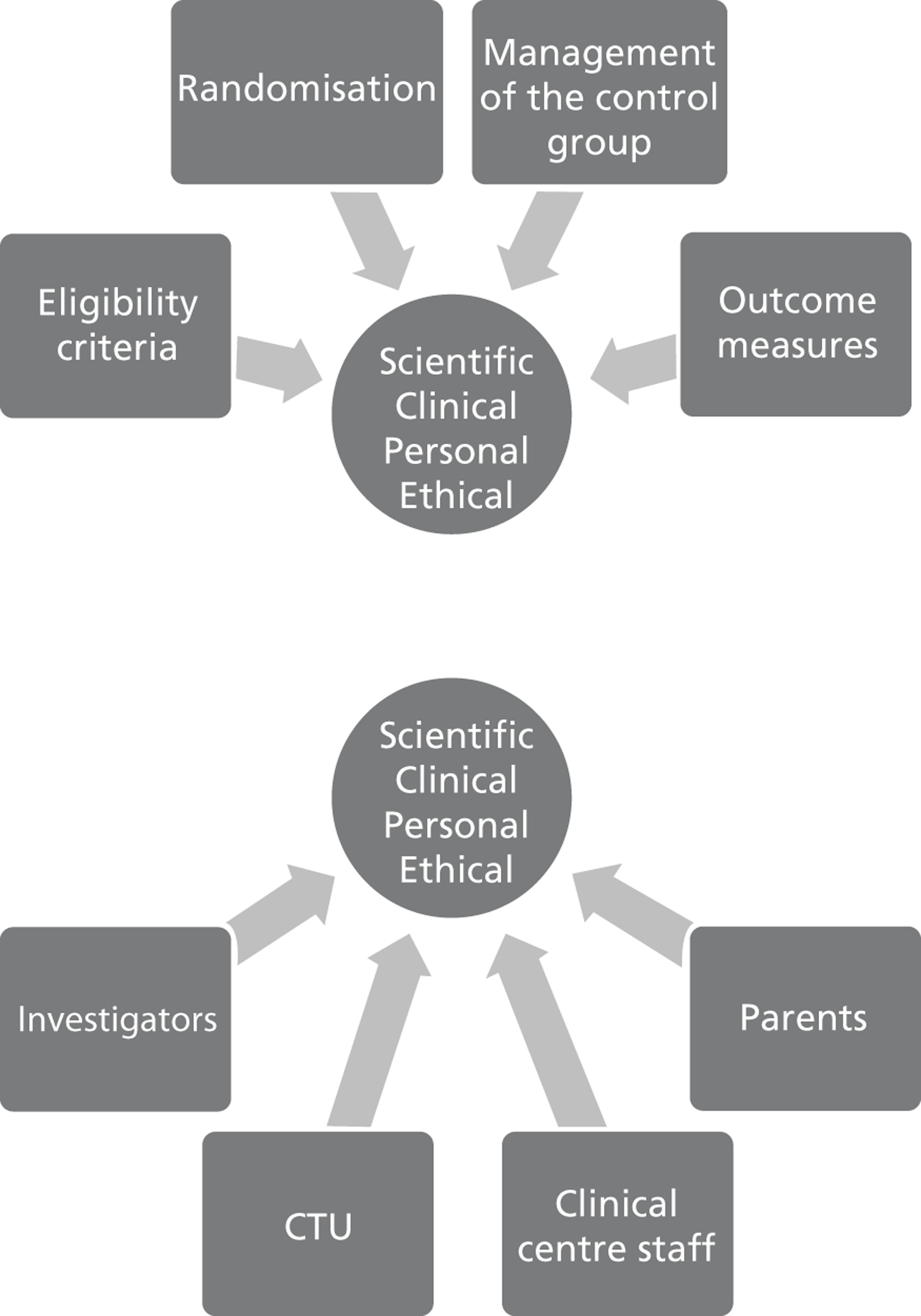
| Features of the trial | Components | Consideration | Parties |
|---|---|---|---|
| Key features of this trial grew out of the hypothesis that the potentially neuroprotective effects of cooling could work only if initiated soon after birth, before irreparable neurological damage occurred. This meant that a tight time frame for recruitment, allocation, transfer and cooling had to be observed. The need for random allocation of treatment and a comparison group are standard elements of trial design . . . | Structural components | Scientific | Investigators |
| . . . but how the care of the comparison group was managed, and the data that would be produced as a result of that management were determined by the views of the team in a number of ways. In this trial all babies, regardless of allocation, were cared for in a Level 3 NICU with cooling facilities. This involved a transfer, which required recruiting clinicians to work to extraordinarily tight time frames in very difficult circumstances | Synergistic components | Scientific + clinical | Clinicians |
| Transfer required parents of all outborn babies to sanction moving their baby to a cooling centre with attendant risks and the possibility of separation from themselves or other family members | Synergistic components | Personal | Parents |
| Arguably this situation could have been avoided at least for some of the babies in the control arm who were receiving adequate care but had to be moved to a trial centre. Essentially those babies were moved for the purpose of the trial. The decision was based on the view that all babies should have the same baseline of care in a centre of excellence to ensure a fair comparison of outcomes | Structural components |
Scientific + clinical + ethical | Investigators |
| All parents of babies enrolled in the TOBY study, whatever the allocation and whatever the outcome, were offered further contact with the trial team via newsletters and when available, access to the trial results | Procedural components | Personal + ethical | CTU management |
Discussion
In order to understand experiences of taking part in a NIC RCT, whether as clinicians and trial team member or as a parent, it is necessary to understand the context in which those experiences are set. In this chapter we have considered different layers in that context, exploring the five core trials as products of a specialty, of trial development teams and their clinical colleagues in collaborating centres. At the most superficial level we considered the specific design features of the trials i.e. their structural components. This makes clear the overt ways in which a trial is set up (e.g. it may involve babies of a given gestation, it may use a placebo, it may involve transfer between hospitals). A focus on structural characteristics allows comparisons of trials with similar or different features. We also took note of the procedural components of a trial, i.e. the decisions that are made about the day-to-day running of research. They are of interest as they set up different patterns of contact between the trials and the parents, and this establishes the extent and terms of a relationship with a trial. These structural and procedural components provide a useful framework for thinking about the range of features which combine to produce a protocol. They are key contextual elements in the setting of interest to the BRACELET study and from these it is possible to start to see how a trial might work in practice, how it might shape experiences and which are the important questions to address, such as:
-
What was it like to offer a trial to parents when the recruitment window was very tight?
-
What was it like when you heard that your baby was not going to receive the trial treatment?
-
Should bereaved parents be sent a copy of the trial results?
Why design features are set as they are is not often considered as part of the context of a RCT, but this is especially informative, as design is part of the ethos of a trial. Each methodological choice reflects a rationale and aspirations for the data to be collected, as well as a judgement about the management and care of the participants who will provide those data. The choices are driven by multiple considerations, by science, by clinical issues, by a sense of the personal impact on parents and by ethics. These are subjective considerations, however, and we have identified a number of parties of influence who may have different views on the considerations involved: investigators, CTUs, clinical centre staff and parents. As we have shown, people differ in what they think is good science, and can differ in their views of the right thing to do. The accounts of the different choice of randomisation method made for multiples by two CIs in the ExPN and BOOST-II UK studies make this point; both felt that their strategy was the most appropriate scientific method and both felt that it would be the most suitable approach for parents.
The team-based approach to protocol development described by a CI is used to ensure that a trial design is an agreed strategy, a product of multiple inputs, but this does not mean that subjectivity is removed. A strategy that works well at the analytical level for experienced triallists and statisticians may still seem to be odd science or inappropriate practice for clinicians who work outside research. The previously mentioned concern of surgeons that variations in surgical skills would undermine trial findings is a case in point. (This required an appreciation of the difference between an explanatory trial and a pragmatic trial. In the latter design, variation is accepted as it reflects clinical practice.) Many triallists have found that their well-considered design does not fit with the priorities of the clinicians, who have responsibility for its implementation. Clinicians sometimes adhere to a protocol, and sometimes modify or ‘tinker’140 with it to fit it with the prevailing sense of equipoise in their centre. 141 Just from this initial work with a small subset of the BRACELET study interviews it is possible to gain a sense of the push-and-pull that can exist between clinical trial teams and clinical centres. The CIs for the TOBY study and for the INIS study both described difficulties with the interpretation of eligibility criteria and for TOBY these changed over time. Only the sickest babies were recruited to the trial in the early days but, as confidence in the clinical centres grew, a wider range of babies were referred. With this shift in context will come different types of parental experiences. These synergistic components of a trial can be captured to some extent but can be subtle and elusive.
Some of these details can be found in the trial narratives that are heard in interviews. Individual trials gain reputations often in relation to their design, their outcome and because of the demands they made of clinicians and parents. One trial might be known for difficulties in recruitment, and another for generating concern or enthusiasm among colleagues. The TOBY study for instance, was often seen as an exciting trial that held promise for clinical practice. It was also the trial that required parents to make decisions at a time of major trauma. The BOOST-II UK study was the trial that has given the NICU community some guidance on oxygen targets for preterm neonates. It was also the trial that was stopped urgently on Christmas Eve because it showed increased survival in one arm.
In this chapter we have shown that there is not a single context to be considered in the BRACELET study but multiple nested and overlapping contexts. The trials that are considered here are produced by multidisciplinary teams who make a range of decisions about how they will be run. The trials’ structural and procedural components reflect the expertise and values of those teams. The implementation of trials in clinical centres introduces another contextual layer as methods are put into practice, but how this is done can change with location and over time, thus introducing an unpredictable degree of synergy into the context. We have also demonstrated that the five core trials are the responsibility of highly interlinked teams. Investigators who develop trial methods also recruit to their trials, and senior triallists who are steering one trial, as a member of its TSC may also be examining data on the DMC of another trial. Clinicians with no role in developing a trial, but who recruit or who support the running of a trial, work at the frontline with parents, and their views can be extremely influential, as they directly shape who is and is not referred to a trial. It is therefore important to see these trials in all of their complexity, their features, the course they take, and the legacy that they have in the field, as essential elements in the context considered in the BRACELET study.
Context then is more than the observable features of the trials. It is dynamic and synergistic, the product of scientific, clinical, interpersonal and ethical considerations, which are, in turn, subjective and mutable. These contextual features are essential to our understanding of the setting of the core trials and inform the analysis of our central research question about the management of bereavement in NIC RCTs.
Chapter 6 Bereaved parents’ initial experiences of neonatal trials
Over the years, a body of empirical evidence has accumulated, which describes the circumstances in which parents are offered and consider enrolment in a neonatal trial for a baby who is, or is likely to be, extremely sick. Collectively these studies address something of the different circumstances in which trial entry might be discussed. They highlight some of the challenges of decision-making for women in labour,142,143 and in the post partum period while their baby is receiving intensive care. 51,64,66 They convey something of the emotions and pressures involved, describing tight timescales for many decisions,51,70 and the hopes that can be pinned to allocation to a particular intervention arm. 54 We know from these studies of the difficulties some parents can experience in following and retaining complex and unfamiliar information about neonatal trials. 49,58,61
Although these data are informative, they are collected almost exclusively from parents whose babies have gone on to survive and so the information available, and perhaps responses to the trials involved, are shaped by a sense of danger averted. They are usually clustered around the point of enrolment, and as a rule do not address issues such as the ongoing experience of involvement in a trial. This is not surprising as the field exploring trial participation more generally is top heavy with research on recruitment and consent processes, but this focus on a single event is at the expense of exploration of other aspects of involvement in trials, which take place over time and are situated in a wider context. If we are to use qualitative data to inform neonatal trial practice then more nuanced accounts of reactions to the introduction of research into the parental experience are needed. Such accounts should consider how parents, including those who go on to be bereaved, view their involvement with neonatal trials in relation to their antecedent and subsequent experiences. The inclusion of bereaved parents is necessary, as those who have gone on to be bereaved may differ from those whose babies survived in a number of ways, and enrolment in a trial may play a different part in their stories from those represented in the extant literature.
To move beyond the constraints of a narrow focus on decision-making, and to consider the larger sequence of events and experiences that surround trial-related decision-making, the following areas were explored with bereaved parents in the BRACELET interviews:
-
Stage 1 Experiences prior to participation.
-
Stage 2 Decision-making about trial participation around admission to NICU.
-
Stage 3 Subsequent experiences of participation in a trial.
-
Stage 4 Response to the trial following bereavement.
This focus on four different temporal stages locates parental involvement in a trial in the larger narratives of the births and deaths of their babies, and their subsequent and ongoing bereavement. This chapter focuses on Stage 1, the circumstances into which the core trials were introduced, and Stage 2, the decision-making process for the five core trials. Chapter 7 covers Stage 3, the period subsequent to decision-making up to the death of the babies involved. Following Chapter 8 , in which the focus is on the clinicians and trial team members, Chapter 9 considers parental reactions to trials in bereavement; here parents review their association with a trial over time and give their views on policies for ongoing contact between trial teams and bereaved parents. In presenting parental accounts of these different periods, several reporting techniques are used. Descriptive data retain links to the narrative accounts that the parents gave, and give a sense of the ways in which decisions about trials could be incorporated into different areas of parental experiences. Thematic data are presented to compare and draw out in greater depth different parental responses to the core trials. Throughout, summaries of individual cases based on interpretive field notes, written as part of the analytic process, are included to pinpoint key observations, and extracts from interviews are included in these summaries to flesh out the observations and to convey a sense of the lived experience behind the data.
Stage 1: Experiences prior to participation in a neonatal trial
The run-up to the time when the subject of trial enrolment was raised with parents was highly varied, and this is reflected in the different points at which the narrative thread was picked up in the interviews. Parents were asked to start their story wherever made sense for them. Some started at the delivery room, some with a problem pregnancy, some with difficulties conceiving, and some with difficulties in previous pregnancies. To capture the variety in their experiences and in the routes that people take into a trial, we will focus here on pregnancy, delivery and transition to neonatal intensive care.
Pregnancy
Many parents of babies admitted to a NICU have not had a straightforward reproductive history. Some have had difficulty conceiving, and, if assisted, their pregnancies can be problematic, as assisted reproduction and different types of underlying infertility are associated with higher rates of obstetric complications and perinatal mortality. 144 Multiple pregnancy, whether assisted or naturally conceived, often brings babies and their parents into the NICU after preterm birth or if growth-related problems necessitate delivery. For some women facing maternal health problems, sustaining a pregnancy can be difficult. Outcomes of previous pregnancies, such as miscarriage or preterm birth, are not only part of the emotional background of subsequent pregnancies, but also they represent significant risk factors for further adverse events. 145
Although it is clear that experiences of pregnancy are also of significance to male partners, this section refers only to the women involved in BRACELET and the management of their pregnancy and delivery. This is partly for convenience in reporting but also to reflect the fact that some of the women were alone at the time of significant conversations and at delivery. In one instance, a woman who is referred to was not interviewed for BRACELET and she is described as ‘Robert’s partner’ as no pseudonym has been allocated. This is to allow Robert’s experiences of the birth of his baby to be included here.
The women interviewed for BRACELET were almost evenly split as to whether or not the index pregnancy (i.e. the pregnancy in which the baby was enrolled into a trial and subsequently died) was their first. Eighteen of the women had had no previous pregnancies (for these parents the death of their baby left them childless, unless they had a multiple pregnancy with a survivor). For six of these women their pregnancy was achieved through assisted conception; two of these pregnancies were with singletons, three were with twins and one with triplets. (In one of the twin pregnancies a baby was not recruited to a trial at all; Abby, declined to enrol him into a trial after his twin brother died. She is not included in the main body of 30 core trial, interviewees, but her account is included here as an ancillary interview because of its relevance to this topic.) Trials of treatments for extremely preterm neonates involve a high proportion of babies from multiple births and almost half of the BRACELET interviews were with parents of multiples (n = 13 with 22 parents: 21 parents of twins and one mother of triplets). Among the 14 women who had had a previous pregnancy, eight had encountered no problems but seven had experienced reproductive loss. Two had experienced miscarriages, one woman had undergone two ectopic pregnancies and the removal of a fallopian tube, two had experienced a previous preterm neonatal death and one had experienced both miscarriage and preterm neonatal death. Two women had a preterm delivery after which their baby survived ( Table 28 ).
| Parity | Parent(s) | Previous loss | Previous experience of NICU | Events of this pregnancy | Events post delivery |
|---|---|---|---|---|---|
| INIS | |||||
| Primiparous | Stefanie and David | Assisted conception | Death of twin | ||
| Julia and Lewis | |||||
| Anita and Sean | Assisted conception | ||||
| Fiona and Keith | |||||
| Alice and Ivan | |||||
| Jana | Assisted conception | ||||
| Amy and Chris | |||||
| Multiparous | Marion and Doug | Scan suggested baby may be affected by Turner’s syndrome – not confirmed | |||
| Sara and Gareth | Preterm baby survived | ||||
| Sophie and Nat | |||||
| Justine and Francis | Miscarriage × 8 | ||||
| Death in delivery room | |||||
| Dora | |||||
| TOBY | |||||
| Primiparous | Laura and Wilf | ||||
| Hesther and Stuart | Assisted conception | ||||
| Robert’s partnera | |||||
| Hannah and Ryan | |||||
| Multiparous | Amanda | Miscarriage × 1 | |||
| PROGRAMS | |||||
| Multiparous | Rhona and Karl | Miscarriage × 2 | Twin–twin transfusion – underwent laser correction of flow | ||
| ExPN | |||||
| Primiparous | Liza and Wesley | ||||
| Multiparous | Shirley and Warren | Ectopic × 2 | |||
| Caitlin and Pete | |||||
| BOOST-II UK | |||||
| Primiparous | Milly and Adam | Assisted conception | Death of twin | ||
| Karen and Tony | |||||
| Chloe | |||||
| Lesley and Stan | |||||
| Multiparous | Jill and Ethan | ||||
| Danielle | Stillbirth | Preterm baby survived | |||
| Dawn | |||||
| Diane | |||||
| Beverley | Stillbirth | ||||
| Other | |||||
| Primiparous | Abby | Assisted conception | Death of twin | ||
| Leigh and Dan | |||||
Women had often experienced difficulties in the index pregnancies. Some pregnancies were known from a very early stage to be at risk, because of a reproductive history or because twins or triplets were identified. For some of the parents a specific risk to the babies was identified antenatally, such as growth restriction, or problems with blood flow between mother and baby or from twin to twin. When problems arose for the women themselves, they commonly spoke of pain, intermittent or persistent bleeding, or leaking amniotic fluid. This situation could continue over a number of weeks and was physically debilitating and emotionally draining. Although there were a number of women who were frustrated by antenatal clinical staff’s reassurances that they could continue as normal when they were sure that something was wrong, some women found themselves in hospital, sometimes for protracted periods. There were other women interviewed for BRACELET who had no expectation of problems until they went into early labour, or until their delivery went seriously awry.
Ten of the women in this study gave birth around at 23/24 weeks’ gestation. Their babies went on to be enrolled in ExPN and BOOST-II UK, which included babies born at these gestations ( Table 29 – the pattern of gestation at delivery and enrolment in the core trials). When they arrived at hospital in preterm labour, or were admitted for observation and bed rest, some had difficult conversations with clinical staff about the implications of their gestational stage for decisions about care. NICUs commonly set boundaries around the care that they offer, as their facilities determine their lower thresholds for care. 146–149 Some NICUs can care for babies of lower gestations, and several women were transferred from their original hospital to a specialised centre when delivery became inevitable. Although the threshold for treatment is lower in those centres, usually around 24 weeks, at such low gestations, decisions about whether intensive care would be initiated is often managed on a case-by-case basis, and depends upon the condition of individual babies at birth. Parents who were around the 23-/24-week cusp not only had to contend with the worry of whether or not their babies would survive and in what condition, but also they were faced with the possibility that intensive care would not be mobilised in their case. At the borderline of viability they had to wait to see whether their baby attempted to breathe to find out whether or not care would be initiated. This sense of ambiguity could be heightened when women were given antenatal steroids to help to mature their babies’ lungs and improve their chances of survival, while also being faced with the possibility of no active care being offered postnatally.
| Gestation at delivery (weeks) | Singleton pregnancies | Weeks’ gestation: range by trial | Multiple pregnancies | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| INIS | TOBY | BOOST-II UK | ExPN | I2S2 | INIS | BOOST | ExPN | PROGRAMS | TOBY TOBY | INIS | PROGRAMS | BOOST-II UK | ExPN | SUPFOR | ||
| 23 | Jill | Leigh | Milly | |||||||||||||
| Lesley | Chloe | |||||||||||||||
| 24 | Karen | Shirley | Caitlin | Abby | ||||||||||||
| Beverley | ||||||||||||||||
| 25 | Julia | Danielle | Stefanie | |||||||||||||
| 26 | Dora | Diane | Liza | Jana | Dawn | |||||||||||
| Amy | ||||||||||||||||
| 29 | Marion | Rhona | ||||||||||||||
| Anita | ||||||||||||||||
| Alice | ||||||||||||||||
| Justine | ||||||||||||||||
| 32 | Fiona | |||||||||||||||
| 34 | Sara | |||||||||||||||
| 36 | Sophie | |||||||||||||||
| 37 | Hester | |||||||||||||||
| Term (weeks not specified) | Amanda | |||||||||||||||
| 40+ | Laura | |||||||||||||||
| Robert | ||||||||||||||||
| Hannah | ||||||||||||||||
| 4 | 5 | 6 | 2 | 1 | 8 | 1 | 3 | 1 | 1 | |||||||
Lesley was one of the women who gave birth at this very low gestation. She was transferred from her local hospital to find that things were not clear cut at the specialist centre:
When I got down to [the hospital], basically they said if he doesn’t show signs that he’s fighting when he’s born, they’re not gonna bother.
What was that like? Did you agree with that decision. Or . . .
No. I didn’t, but in a – in a way I did, and in a way I didn’t, because the way I see it is, if he’s got a little bit of life left in him then that little bit of help they give him could have made a big difference and – but because he showed fight . . . when he was born, then that’s why . . . they made sure they helped him.
Several of the women interviewed for BRACELET described this uncertain state in which they oscillated between almost certain death of the baby and hope, between no care being possible and all possible support being mobilised, a state that continued right up to decisions about delivery. Jill explained that when delivery became inevitable at 23 weeks she was given the alternatives of ‘leaving him to die inside me or doing an emergency section ’. Although 24 weeks is not a fixed point for care or no care, women whose pregnancies were in difficulties around this gestation saw it as a gateway to care and described willing themselves to get through each additional day to reach that target. Chloe was admitted to hospital at 23 weeks. She was upset to be told at this stage that she was having a miscarriage, a term that did not fit with her sense of her pregnancy or of her babies. Her twins were born at 24 weeks. She described the change that took place over that week: ‘When we were admitted, they weren’t willing to help us at all. By the time we had them, they could have the help.’ She explained that she did not expect her sons to be born alive and ‘when they did come out and we heard a bit of noise, then that was a joy, and anything after that was a privilege really ’.
The overwhelming sense from the parents interviewed for BRACELET was that at the time they wanted their babies to be cared for and for everything possible to be done. A minority of the parents who were facing extremely preterm birth came to their own decisions about care, which pre-empted NICU policy on admission. For Dora, her period as an inpatient and her discussions with neonatologists about the implications for her baby if born around 24 weeks, allowed her to crystallise her views on management post delivery. She said:
I can remember the paediatricians coming to see me every single day and telling me that, what was gonna, you know, what the chances of Gerry’s survival were and the level of his disability and all things like that. So by the time it came to his birth, when it actually happened, I’d made the decision that if he wasn’t breathing when he came out, I didn’t want to have him resuscitated.
Beverley’s previous experience of a preterm birth and death at 20 weeks led her to believe that should she go on to deliver at 24 weeks it would not result in a live baby, but nevertheless she and her husband considered the different possibilities that lay ahead:
I really wanted her to go into the special care baby unit, there was no doubt about that. If she was born alive then I’d have wanted that. But we did have the talk about resuscitation as well, if she wasn’t born breathing, and we said we didn’t want her resuscitated if she wasn’t born breathing . . . [W]e just thought that, if she was born that preterm and she wasn’t born strong enough to breathe then we didn’t think that she had any chance of pulling through really.
When women were inpatients with a likelihood of delivery, the subject of enrolling their baby in a trial might have been raised for some trials. Although this would allow parents more time to consider their choice, it is not without some difficulties. For trials that focus on babies of a particular gestation, parents can be approached antenatally but is likely that some would go through the decision-making process but their babies would not be born within the specified recruitment window and would then be ineligible for recruitment. Almost all trial-related discussions for the parents involved in BRACELET had taken place on the NICU.
Beverley, whose views on initiation of care were quoted above, and Danielle, both of whom had a complicated reproductive history, were the only interviewees asked about a trial as inpatients. Danielle’s experiences of antenatal recruitment to BOOST-II UK were strikingly similar to Beverley’s but they described different positions on altruism and enrolment. For Danielle her sense of altruism and the potential benefits for others was sustained throughout her association with the trial, but for Beverley it was more transient and dissipated somewhat once she was able to engage with the reality of her daughter’s initial survival, condition and needs. Their stories are given in Boxes 2 and 3 .
Danielle had experienced three pregnancies, all of which were unplanned, complicated and ended early. Her first baby, a daughter, was stillborn at 24 weeks’ gestation. Her next baby, Alistair, was born at 27 weeks’ gestation and was admitted to NICU. He developed MRSA and was placed in isolation. He then developed meningitis and hydrocephalus. He survived with mild cerebral palsy affecting one foot and has generalised educational delay. He also has a stent in place for the hydrocephalus.
Danielle conceived Todd 6 years later with a new partner. She was asked to consider BOOST-II UK after the decision was made at 25 weeks’ gestation to proceed to an emergency CS when a Doppler scan indicated a problem with placental flow, which threatened both Danielle and Todd. She was approached by a doctor who had cared for Alistair during his NICU stay years previously. She was pleased to see him and said, ‘I trust him with my life. I trusted him with Alistair’s life. I trust him with Todd’s life.’
As Danielle made her decision antenatally, before Todd’s exact condition and care needs were known, and before neonatal intensive care had been initiated, she based her decision in part on her previous experiences. She was familiar with neonatal intensive care and ventilators, and was reassured about Todd’s chances. She thought that he too would survive. She said:
We just thought he’s gonna be like Alistair. He’s gonna have a tough time, but we’ll, you know, we’ll be taking him home. But I just said, ‘Oh, yeah, I don’t mind taking part in the trial.’ They explained that it, you know, they’re gonna get the same sort of thing, but it’s gonna be put in a different way. I mean I was fine with that.
Danielle said that she went though the BOOST-II UK information booklet and although she was not entirely clear what the trial was about, she had no concerns about taking part and said that she was ‘quite happy’ with her decision. She did not feel that it was likely to make much difference to Todd and her decision was largely made with a view to helping other children. She said, ‘I will do anything to help other babies not to go through that situation.’
CS, caesarean section; MRSA, methicillin-resistant Staphylococcus aureus.
Beverley had given birth to two healthy term babies. Her third baby, a son, was born and died at 20 weeks’ gestation. She regretted that she did not see or hold him. Around 23 weeks’ gestation in her next pregnancy with her daughter Ruth, Beverley went to hospital in pain, feeling that she was in labour. She was told that she was not contracting and that she had constipation. She went home but the pain continued. She bled and lost fluid and was admitted, still in pain, but was still thought not to be in labour. By this time she felt that she was ‘going a bit soft’.
She was still in pain when she was approached about BOOST-II UK and was closer to delivery than staff suspected. She is a nurse, and her husband a doctor, and she said that they agreed to the trial because they ‘know how important research is’ and felt that ‘it is the right thing to do’ but she felt some unease. She said:
I was a bit unsure about it. It was more my husband that was saying ‘Oh yeah, we should do it because it could help other babies and whatever, and if this had been done sooner, and we’d have had the results, then it might have helped Ruth.’ And it is all true, but it’s not necessarily the way your mind works at that point.
Beverley’s comments suggested that although she felt some unease, the fact that she was asked about the trial before her baby was born was a crucial element in her decision. Even though she was having what she felt were contractions, and had discussed with her partner the possibility of not initiating care should the baby be born, she described a reaction which was based on positive thinking:
I think because it was before, you’re thinking at the back of your mind she’s not going to be born for another six weeks or whatever, and that it wouldn’t be as bad.
Ruth’s head was delivered unexpectedly after Beverley went to the bathroom in her room on the antenatal ward. She said, ‘I just had to hold the head and get back to the bed with her.’
She was born crying and she was crying for quite a long time, there on the bed. She was just lying on the bed. I couldn’t see her. And there was just like all panic going on because the placenta had shot back in or something. And so it was quite a while before she was actually taken away, because I was saying to them ‘Where’s the paediatricians?’ And they were stood outside wanting to come in, but they didn’t want them to come in because of what other complications was going on.
Given her previous experiences she was aware of the possibility of her baby being stillborn, and that care might not be initiated if her baby was born alive but in a poor condition. The tentative altruism that she that she described antenatally would have had no place for her once her baby was born and was alive, and she had engaged with the actuality of her condition. She felt that had she been asked about the trial postnatally she would probably have declined to take part.
Seeing how fragile . . . she was, because she was only twenty-four weeks, she only weighed one pound, a little tiny thing. And you think if there’s any chance that she might be in the wrong category for anything I just don’t think you would have done it. You would have been more protective of her I suppose then.
Delivery and transition to neonatal intensive care
The births that the women in the study experienced were often fraught. For all of those who were delivered early there was a sense of threat to their baby, whether it was preceded by the protracted period of uncertainty described above, or a fast-moving crisis, such as a placental abruption.
The difficulties that the women encountered can be seen in Table 30 , which details the mode of delivery in the sample as a whole. Fifteen women in total underwent a caesarean section (CS), the majority of which (n = 13) were emergencies. Eight of the women who had a CS also went through labour prior to surgery. With the exception of two induced deliveries, the remainder were spontaneous vaginal deliveries but of these only Sophie experienced a normal, non-emergency delivery. All remaining vaginal deliveries, other than that of Robert’s partner, were difficult, not because they were technically complicated but because they were preterm or extremely preterm.
| Labour | Mode of delivery | INIS | TOBY | PROGRAMS | BOOST-II UK | ExPN | Other | Total | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Y | Spontaneous vaginal | Amy | Robert’s partner | Chloe | Diane | Shirley | Liza | Abby | 15 | |
| Alice | Lesley | Beverley | Leigh | |||||||
| Julia | Dawn | |||||||||
| Justine | ||||||||||
| Sophie | ||||||||||
| Y | Induced vaginal | Dora | Hannah | 2 | ||||||
| Y | Emergency CS – with labour | Fiona | Laura | Rhona | Karen | Caitlin | 8 | |||
| Stefanie | Hesther | |||||||||
| Amanda | ||||||||||
| N | Emergency CS – without labour | Jana | Jill | 5 | ||||||
| Marion | Milly | |||||||||
| Danielle | ||||||||||
| N | Planned CS | Sara | 2 | |||||||
| Anita | ||||||||||
| Total | 12 | 5 | 1 | 9 | 3 | 2 | 32 | |||
The mode of delivery left women in different circumstances as their babies transitioned on to the NICU. Some of the women who delivered at low gestations did so without lasting physical trauma to themselves, mainly because their babies were so small. These women were able to move around and were often able to visit the NICU relatively quickly.
All women who had a CS, some of whom needed additional interventions, such as Laura, who underwent surgical removal of her placenta, and Caitlin, who had reparative bowel surgery after damage caused during delivery, had to deal with the aftermath of their birth and surgery and their initial experiences in the NICU at the same time. Milly was one of the women who underwent an emergency CS with a general anaesthetic. She had bled heavily before delivery and woke to find that major events had taken place while she was unconscious. She said, ‘I was like knocked out for [the CS] and then the next thing I woke up, I’d had the babies, lost one . . . Lee was the biggest one but he died straight away . . . and Cameron, they put him in the incubator.
Where women were debilitated postnatally this could limit their contact with their babies. Although a number were taken to the NICU in a wheelchair or their bed, when babies were moved to another hospital it was not always possible for the women to accompany them. Laura and Robert’s partner, for instance, were unable to be moved with their baby on transfer.
The mode and pace of delivery also impacted upon the men. Some of the men did not arrive at the hospital in time for the birth, as events could be unexpected and fast moving. When women underwent an emergency CS, men could be excluded from the birth, a potentially isolating and anxiety-provoking experience. When Hannah was taken to theatre, her partner Ryan said that he waited on his own for 3 hours and described how awful it was to feel so abandoned and to have so little information at such a worrying time. Stuart also found his time alone while his partner Hester was in theatre to be very difficult. He said, ‘I [was] in a room on my own. Nurse put me there. No one came. In a room on my own [on a unit] with all the proud parents with little ones.’ Being present at a complicated birth can be a distressing experience, however, and Amanda explained that her partner, who was not himself interviewed for BRACELET, had been deeply traumatised by the actions and events he witnessed (see Box 3 for further details of Amanda’s story).
Once babies were delivered and taken to the NICU, there was usually a lag before parents were able to visit. Some of the parents were frustrated at not being allowed to go to the NICU with their baby and the wait for news or to be allowed to visit could seem interminable. Beverley described herself as ‘a nervous wreck’ and kept asking her partner to go and ask the clinical staff whether their daughter was still alive. Parents sometimes decide to wait and visit the baby for the first time together but if the women were incapacitated the men often went ahead to the NICU to see their babies. Some saw themselves as a link between the baby and their partner, reporting back on progress; some saw themselves as the baby’s advocate and this could extend to travelling with them to another hospital, as was the case for Wilf and for Robert. The subject of a trial could be flagged up in this early transitional stage – before the baby is settled in to the NICU that will be responsible for ongoing care – for later discussion when their partners would be able to take part in a conversation. (Robert’s experience was the exception, as he was asked about the trial on his own. Further details are given in Box 6 .)
Most of the parents who were asked to consider taking part in a trial in this very early post partum transitional stage of their experiences were parents of babies born at term who went on to be involved in TOBY (see Table 31 ). In all five of the interviews with parents involved in TOBY, the stories they told were of deeply traumatic experiences, with complicated labours that deprived their previously healthy baby of oxygen. As a narrow window of time for recruitment was used in TOBY, the decision about trial participation took place in the hours immediately after delivery. Discussion of the trial therefore required emotionally strained parents and physically debilitated mothers to engage not only with the information and choice offered to them in relation to cooling and the trial, but also confronted them at an early stage in their experience with the possibility of brain damage and disability for their newborn. Parents still reeling from delivery had to think about both the immediate and the long-term future. In these circumstances Hesther was unable to take in much information about the implications of the trial for her son Joel.
Joel was born after a very quick labour, an ambulance journey in the night and an emergency CS immediately on arrival at the hospital as his heartbeat was found to be very low. Events had moved so quickly that Hesther and Stuart felt that when they agreed to enrol Joel into TOBY they still had not taken on board the seriousness of the situation. Hesther said that it had not occurred to them that cooling might not work for him. The discussion about the trial took place when Hesther was still feeling physically overwhelmed by the drugs she had been given around the time of her delivery: ‘They were telling me that things were at the worst they could be and I just dropped asleep. . . Some things did register, but I think the drugs just took over.’ Both parents said that they did not take much time to think about the trial. As was the case for several other parents, it seemed that the nature of the intervention itself was immaterial in comparison with the possibility that it might benefit their child, a position which they described as ‘anything to help’.
In contrast, Wilf explained how he was hungry for any information that he could get about the condition and plans for the care of his son Archie. He welcomed the discussion of the trial and said:
I would have wanted to know two minutes after he was born. I wanted to know straight away. I don’t want to be mollycoddled or . . . treated with cotton gloves . . . They were letting us know in a very professional manner their complete lack of certainty as to the outcome. But they were telling us stuff, and we wanted to know. It was horrible knowing, but it was much worse not knowing and being kept in the dark for what seemed like a very long time.
Wilf – TOBY
Hannah and Ryan’s interview indicated how experiences of the stress of delivery, initial involvement with a trial, and the postnatal aftermath were overlapping rather than discrete elements in their experience. After a difficult forceps delivery they agreed that their daughter, Eleanor, could be enrolled into the pilot study for TOBY. Hannah was then taken to the NICU in a wheelchair, to see her daughter for the first time since she was born. As Hannah watched her being prepared for transfer to a cooling centre, she felt ill and a post partum haemorrhage became evident at the bedside. They described what happened:
Ryan and the midwife. they pushed me up to the neonatal, and literally I was sort of at the doors, and she was in an incubator . . . I could sort of see her there, and there was a big team of about 15 people, weren’t there, around her? And they said, ‘Oh, they’re nearly ready, and then you can have a quick look before they take her.’ So I said, ‘Oh, okay’. . . and [then] I just said, I said, ‘I don’t think I can wait here any longer.’ ‘It’s only going to be a couple of minutes.’ I said, ‘No!’
I said to the midwife that you looked really grey, because the colour just drained out of you all of a . . . really quick, like all of a sudden.
And I could feel myself sort of going, and I just said, ‘I’m not going to make . . . I can’t wait.’ And, and with that, the midwife looked round, and I was just sitting in a pool of blood. And they said, ‘Oh my God, she’s, she’s haemorrhaging. We need to get her back now.’
Hesther and Stuart were the only parents to describe fast moving events managed with efficiency. In each of the other interviews with parents involved in TOBY there was a sense of crisis, delay and mismanagement: in two cases, of Liza and Wilf, and Hannah and Ryan, there was a formal inquiry into the deaths of their babies, and Amanda received a letter of apology from her hospital. In four of the cases, the baby was their first born. Amanda’s story is given in Box 4 .
Amanda had experienced difficulties in her first labour when the cord was found to be around her baby’s neck. She said that her doctor was ‘awesome ’ but this time she felt that things were different. She had a sense that things were going wrong but that her concerns were not being taken seriously.
This felt different. It felt, uncontrolled . . . [T]his just felt completely – I don’t know, just out there, and my midwife went for her tea and another midwife came in and I said to her, you know, ‘This isn’t going right. I really want a section.’ And she was busy with her magazine and it was like, ‘That’s not how it works,’ and went back to her magazine . . . The doctor wasn’t interested in me really because I was a second-time mum, and there was a first-time mum who was having problems in another room . . . The consultant was called. It was a Sunday night, so there was nobody there. She was called in for the other girl and, but saw that things weren’t going right for me, and got the scanner out and saw that Simone’s heartbeat wasn’t right, tried getting her out and in the end just, it just was pandemonium, it was just absolute pandemonium. I was dragged across the room, the, the drips were crashing to the ground, my necklace was being ripped off my neck and brought into the – the operating theatre and I was struggling, they were trying to get the mask over my mouth and I was struggling and struggling.
Her husband was watching through an open door. Amanda had very long hair which hung over the edge of the bed, creating a vivid image for him. He later told her that it was like watching her ‘being crucified.’ Amanda said that he still has flashbacks to that scene.
The move from the hospital in which problems arose, into the care of a new team not associated with, or implicated in, the initial trauma, is not just a matter of accessing specialist care, but also a feeling that the baby is in different, safer hands. For Amanda the ‘safe hands,’ a doctor who represented both the hospital that would take over the care of Simone, and the TOBY trial, was waiting for her at around 3 a.m. to discuss the move and the trial when she came round from her anaesthetic.
[W]e were brought down to see her and she was all wired up in, in the incubator thing and Dr S was there, so I think that was the first time [my partner] had saw her since she was rushed past him, as far as I know . . . I was wheeled down ‘cos I was very ill afterwards and Dr S was there and basically he told us . . . we had an option of her doing this TOBY trial in the [second hospital]. We didn’t really discuss it between us, but it was, we were both in the same agreement that the [second hospital]. . . has always felt as if it’s the elite hospital and we thought, ‘Well, if she’s any chance, she has a better chance [there],’ you know. So we thought . . . that’s the best we could do for her, you know?
Amanda was asked what she thought about the idea of cooling. She focused less on the details of cooling and the trial and more on the possibility of a solution, a strategy for managing the situation.
I was just happy that she was getting some help, you know. I didn’t care what they did to her, to be honest . . . I was just open to anything. They could have done anything to her, really, I just, if it was going to help, or there was a chance it would help, I really didn’t care, you know.
Amanda was asked whether she had any worries. Her reply suggests that it was the sense of care and expertise that was important. She said, ‘Not about the actual trial, no again, it seems stupid but the [hospital always has such a fabulous name. ’ For her, the prospect of leaving one situation was as important in her decision as the transition into the new setting, and her responses to TOBY were inextricably linked into this sense of escape and hope.
Stage 2: Decision-making about trial participation after admission to neonatal intensive care unit
In order to explore the decisions that parents made to enrol their babies into a trial, it is important to consider the immediate context in which they made their choice, as well as the reasons they gave.
The context of parental decisions
Once a baby is admitted to a NICU parents face their next set of challenges. The neonatal intensive care environment is difficult in itself. 150 Parents are confronted with alien sights and sounds. They have to adapt to new routines, and work out how they will manage their time, especially if they are at a distance from their home, if they have other children or work-related demands. They have to become conversant with their baby’s condition, treatment and the equipment used in their care. The sense of threat to their baby can heighten and recede from hour to hour, and parents often have a strong sense of their suffering. They experience their own life events and witness those of the other parents around them, and they can be asked to make life-changing decisions for their baby and themselves. One of the women (from a non-’core’ trial) described the initial impact of the NICU for her:
The first time you walk on to that neonatal unit, you’ll never forget that as long as you live. It’s the scariest place, because there’s alarms everywhere, and it’s dark and all these little cribs. It’s like another world, like a hospital within the hospital. It’s such a strange place. To think that all of that is going on in that hospital – it’s so weird! But it was just awful walking in there and then being led to her and just seeing this tiny little bundle with all the masks on and wires coming out of her everywhere and – yeah, it’s terrifying.
Leigh – I2S2
Parents respond to admission to NICU in very different ways and this can be seen in their initial approach to being on the unit. For parents of babies born on the borders of viability around 24 weeks’ gestation, who have been through the uncertainties described above and who have to varying extents anticipated their baby’s death, there can be a need to adjust to initial survival and transfer to a NICU. Diane felt that over several weeks while she was still pregnant that it was made clear that her baby ‘wasn’t going to make it,’ and she did not expect him to survive. When he was not stillborn she ‘didn’t know what to think’. Shirley similarly anticipated a stillbirth and vividly described her reaction when she saw her daughter properly for the first time:
I was wheeled into neonatal, must have been about 9 p.m., 10 p.m. that night, and I was still in denial she was alive and it was such a shock. I’m not a dramatic person anyway but I just screamed. I couldn’t believe she was alive and I couldn’t believe what I was seeing. You know, the shock of seeing a baby lying, you know, wires and, you know . . . She was ventilated at this point and all I can remember was seeing this tiny little thing that – I know it sounds awful but I’ll be honest – that to me shouldn’t have been alive. Just didn’t – it just didn’t look right.
Shirley – ExPN
Stefanie explained how she was initially reluctant to confront the reality of her twins’ situation. She was asked ‘numerous times’ by midwifery staff if she would like to visit her sons but said that she ‘didn’t want to go’. When she did see them it was very difficult: ‘I just couldn’t take this in . . . [I was] in the wheelchair in the middle of two incubators and I didn’t know what I was supposed to look at.’ Like Shirley, Stefanie found that this fear subsided once she had spent some time with her babies and she quickly acclimatised to the NICU.
Some parents seemed to be more at ease from the start. For Dawn it was hard to see her babies so ill but she felt that she was able to see beyond their prematurity. She described seeing them for the first time: ‘it didn’t faze [me that] they were smaller, they didn’t scare [me] . . . because they were mine. I didn’t see them as small.’
For most of those who go on to be bereaved, all of their parenting takes place on the NICU. (All but one of the babies in the BRACELET study died without going home; Sophie and Nat’s son was admitted after initial discharge post-delivery.) Although there are many difficult experiences encountered in this setting, it is possible for parents to carve out positive times and memories in this setting. Chloe and her partner had been together for only 7 months when their twins were born at 23 weeks. Chloe saw the time spent together on the NICU as a bonding experience for them as a couple, as well as between them and their babies. They stayed at the hospital and managed their visiting time very carefully:
As strange as it sounds, we did have good times in that hospital, and we’d have a laugh together, and we bonded . . . [W]e wouldn’t sit all day long with them . . . We’d sit up to like 5, 6 o’clock in the morning just kind of chilled out, lights dimmed, no other parents around, and we’d just sit around all night long and then let the other parents come in for day, and then we’d go back of a night. And you know, it just became our life then . . . Maybe family would come visiting us and they’re not allowed in special care, so we’d just spend a bit of time with them, and then the night was ours and sit with them really.
Chloe – BOOST-II UK
Clearly parents come to neonatal intensive care via a range of different clinical pathways. Their reactions to the environment and to their babies are similarly varied and highly personal, bringing together emotions from recent and sometimes more distant events. It is against this background of threat, initial survival and initiation of care that they made their decisions about trial participation.
In almost half of the interviews for BRACELET (n = 12), the parents had agreed to enrol their baby in a trial on the same day that they were born, but others were enrolled much later, days (n = 4) or weeks (n = 6) after birth ( Table 31 ). In two of the core trials, babies became eligible at the time that a specific clinical crisis was looming or had developed: for INIS this was a proven or suspected infection and for TOBY it was diagnosis of ischaemic encephalopathy. In the other three core trials, eligible babies may not have had such a specific diagnosis but were at risk of developing an infection because they were preterm and small (PROGRAMS), were receiving ventilatory support (BOOST-II UK) or were requiring parenteral nutrition (ExPN). Parents of multiples could be asked to make their choice about a trial for one or all of their babies and their circumstances could be particularly complex.
| Timescale for trial entry | INIS | TOBY | PROGRAMS | BOOST-II UK | ExPN | Total |
|---|---|---|---|---|---|---|
| Antenatal | Danielle | 2 | ||||
| Beverley | ||||||
| Same day as born | Fiona and Keith | Laura and Wilf | Jill and Ethan | Caitlin and Pete | 12 | |
| Amanda | Milly and Adam | |||||
| Hesther and Stuart | Karen and Tony | |||||
| Robert | Chloe | |||||
| Hannah and Ryan | Dawn | |||||
| Days | Julia and Lewis | Lesley and Stana | 4 | |||
| Anita and Sean | ||||||
| Amy and Chris | ||||||
| Weeks | Stefanie and David | 6 | ||||
| Sara and Gareth | ||||||
| Sophie and Nat | ||||||
| Alice and Ivan | ||||||
| Justine and Francis | ||||||
| Jana | ||||||
| No timescale could be identified in the interview | Marion and Doug | Rhona and Karl | Diane | Shirley and Warren | 6 | |
| Dora | Liza and Wesley | |||||
| Total | 30 |
In order to highlight and compare contextual elements in these settings, we consider below the situations described by some of the parents involved in two of the trials, INIS and ExPN and the particular situation of parents of multiples.
International Neonatal Immunotherapy Study
The babies who were eligible for INIS were either suspected or known to have developed an infection. These wide inclusion criteria framed the discussions for parents in particular ways. For some parents the possibility of an infection did not feature prominently in their accounts. Dora could not recall any concerns over infection and Fiona and Keith similarly enrolled both of their twins on to INIS within a few hours of delivery without a specific reference to an infection or a trigger for the trial that they could remember. Instead, from Keith’s description it seemed part of developing a more general strategy of care:
About couple of hours after they’d been in there, they asked us if . . . they’d been given the injection for the lungs . . . it helps the lining, to get that developed . . . They’d been given that, which was normal anyway, and then I think they’d sort of said, ‘Look, there’s this trial going on. Would you like to take part?
Keith – INIS
The condition of sick neonates can change very quickly and some of the babies whose parents were interviewed progressed well, sometimes for several days, but then made a very rapid and shocking decline if an infection did take hold. This type of parental experience was often characterised as ‘a rollercoaster’ and the possibility of enrolment in INIS could be raised at the heart of that situation. In four interviews (Julia and Lewis, Sara and Giles, Alice and Ivan, Justine and Francis) parents described what happened when their babies developed NEC, a bowel condition in which sections of the intestine die. NEC causes distressing symptoms and is a serious threat to life, requiring surgical exploration and removal of sections of necrotic bowel. Such surgery for sick preterm infants is, in turn, another serious threat. The situation described by these parents was extremely grave and INIS was discussed against this backdrop of risk and distress. Ivan described their particular rollercoaster:
The twins were delivered at 32 weeks, both good weights; 4lb 6 and 4lb 2. Did really well. Both of them were only in the high-dependency for 2 days . . . and then moved to medium-dependency and then low-dependency within a week. So they were both doing really well, breathing on their own; no problems . . . When Oliver was 10 days old he developed an infection called necrotising enterocolitis . . . [W]ithin 24 hours he became probably from one of the wellest babies on the unit to probably the sickest baby on the unit. He went downhill very quickly . . . they called in one of the consultants during the night, that night, to put a drain in to his abdomen and they took him to [another hospital] the following day just to have a look at what was going on to see if they could perhaps cut the bit of bowel out that was, you know not working and re-joining with maybe a stoma or a you know. However, they told us after that, that . . . they didn’t think that there was anything that was survivable, if you like. Most of it was too damaged for them to do anything, but in rare cases, they can start to improve themselves. So I think it was probably around that point that we were asked about the INIS trial and . . . we were still hoping that, you know there was still some possibility that his bowel would start to recover . . . [but] I think it was about day 13; . . . he was opened up again and they basically took the bowel out and looked at every inch of it and basically told us there was just no way that he was going to survive.
Ivan – INIS
Ivan mentioned INIS right in the middle of this difficult time, as a direct response to the threat of serious infection for their baby. This places the trial into a therapeutic framework, and parents spoke of their hopes that INIS would make a difference, but by this point the gravity of their situation was sometimes clear and the trial could seem like a long shot rather than a magic bullet.
That was certainly the case for Sara and Gareth whose son Aiden, one of twins, also developed NEC after a period of stability. For the first week Aiden had seemed stable on NICU, but he developed NEC and his bowel ruptured at 10 days old and a substantial section of bowel had to be removed. Sara and Gareth were told that he would need a bowel transplant and Gareth commented that ‘he was going to be facing a very difficult life if he had made it’. He added that once they were aware of the severity of the situation it was ‘[a] case of accepting that the worst outcome was the more likely’. The parents made their choice about a trial in the knowledge that their baby was unlikely to survive. Gareth said:
We knew he had a fairly low chance of survival, it sort of became a case of, well this might help him, and it might not . . . if it does help, then great, and if it doesn’t well it’s unlikely to make anything any worse.
Gareth – INIS
Extremely Preterm Nutrition feeding study
The subject of the ExPN feeding study could be raised with parents at any time within their first week on the NICU and decision-making for this trial was not urgent. The babies who were eligible were extremely preterm, all under 28 weeks’ gestation, and they were therefore a very vulnerable population, all born in fraught circumstances. The offer of the trial, however, was disassociated from the crisis that the parents had experienced, partly because of the flexibility over timing and partly because the intervention related to a routine management of care. Three interviews were carried out with parents associated with this trial and in each the trial seemed to be something of a non-issue. The CI for ExPN explained in his interview (cited in Chapter 5 ) that there was a careful balance to be struck between increasing nutrition and increasing the risk of diabetes-related problems, but none of the parents interviewed referred to these concerns. Shirley did not see feeding as an issue, it being secondary to medical management in her view, and similarly Pete said that feeding was not a major concern for Caitlin and himself: ‘The milk side of things . . . yes it’s important but it’s about . . . these infections . . . this is what’s important.’ Liza and Wesley knew that ExPN related to feeding but did not remember much of the details. Liza saw it as a simple and obvious choice: ‘They just told me it was better for her. So I just agreed to it . . . It couldn’t harm her in any way, it was only going to help her.’
It seemed that in these interviews, despite the extra time that was available for decision-making, the issues that underpin and drive the trial were not clear, or at least were not retained and incorporated into the parental narratives, and that the importance of the issue of nutrition in the shorter term for longer-term outcomes is not clear. In all three cases the babies survived for some weeks so it would have been possible for the parents to have been given reminders and ongoing information about their involvement in the trial.
The particular situation of multiple births
Almost half of the parents interviewed for BRACELET were parents of multiples (see Table 29 above). This is not surprising, as trials of treatments for extremely preterm neonates involve a high proportion of babies from multiple births. Prior to delivery parents are often aware that there are no guarantees that any of their babies will survive to be admitted to NICU. In some cases a single baby does not survive this stage and parents enter the NICU already bereaved. This was the case for Abby, Stefanie and David, Milly and Adam, who were bereaved when they were asked to make a decision about a trial for their (then) surviving twin.
When all babies survive to admission to NICU, parents can face an uncertain and often challenging course for more than one baby, and there can be competing demands upon their time. Where there are no surviving babies, multiple deaths can be close together or spaced apart, each situation bringing with it its own complications and responses. When parents are both bereaved and have a surviving baby they can face a different set of complicated emotions.
As parents of twins, Stefanie and David’s interview illuminates several areas of interest to BRACELET. They described in rich detail their time on the NICU and the stressors involved, the ways in which their experiences of multiple birth and parenting twins became intertwined with their decision about INIS. Their account is summarised in Box 5 .
Zander and Callum were conceived after a long period of infertility and a fourth cycle of IVF. Stefanie was an inpatient for much of the last 4 weeks of her pregnancy with recurrent bleeding, When she bled at 23 weeks she was told that if the twins were born at this stage they would not be taken to NICU. Stefanie said this was ‘horrific to hear because obviously, you know, you just assumed that no matter what age they were born at, they would be put in’. She bled again heavily in the shower at 25 weeks and at that point an emergency CS delivery was carried out.
Stefanie felt calm about the plan to deliver, but the delivery itself was a ‘traumatic’ experience, an ‘absolutely full scale emergency’. By the time David reached the hospital their sons had been born. He saw them in the NICU but at first Stefanie could not bring herself to visit or look at photographs. It was soon decided that Zander needed to be transferred to another hospital in the same city for surgery for a problem with his lungs. Although David went with Zander as he was being put into the ambulance for transfer, Stefanie was told not to go. She thought that this was in case she slowed the process down but deeply regretted not being allowed to be involved in this small act of care. She also regretted the fact that they both then stayed at the original hospital with Callum and that no-one was with Zander. She had a strong sense of parental care misaligned, that something had gone wrong that she could not put right.
Zander declined postoperatively and Stefanie was woken at 1 a.m. to go to him and David was called at home. Stefanie said she knew at this point that he would not survive. When they arrived at the NICU caring for Zander he had stabilised but the decision was made to withdraw care. They were given him to hold and expected him to die, but after several hours he was ‘put back into the incubator’. At this point the original hospital asked them to return, as Callum was ill. As they were leaving, a member of staff stopped their car to tell them that Zander had declined. They returned and stayed with Zander until he died then rushed across the city to be with Callum. Stefanie said, ‘It’s amazing how you can get up and run down a corridor when you need to, I can tell you, in a pair of slippers, holding your stomach.’
She described the trauma of this experience very vividly, especially the dreadful experience of leaving Zander so quickly after his death. Looking back on that 24-hour period she said, ‘How can so much happen to a person in such a short period of time?’ David said that ‘it was just unbelievable, it was just surreal’ and Stefanie said that she left the hospital ‘bewildered’.
Callum survived the night but gradually declined over the following week, during which time his brother’s funeral took place. He developed pneumonia, and Stefanie and David were distressed to see him on an oscillator; they hated the noise involved. They were especially upset when at one point they were asked to leave and returned to see ‘his wee head all shaven and wee leads going into it’. Stefanie said it was ‘horrendous’, especially as his eyes had opened for the first time, which David felt was due to ‘the duress’ of the procedure.
They were asked about the INIS trial only hours before Callum died and very much saw it as a last attempt to save him. Stefanie said that she quizzed the consultant who approached them, asking if the trial would be detrimental to Callum. She said that she was satisfied that ‘it definitely wouldn’t’. They both said that they agreed to the trial only because he was so ill, and that had he been in less need they would have declined. Stefanie said that if that been the case she would not have wanted to ‘rock his boat’ and would not have been ‘brave enough’, as the idea of a trial ‘terrified’ her. Both parents ‘squeezed’ their names on to the trial consent paper in an act of joint responsibility, as it was ‘just too big a decision to make by yourself’ (Stefanie).
IVF, in vitro fertilisation.
a The parents referred to the incubator not the ventilator, but it is likely that Zander was re-ventilated.
The decisions
The different settings of crisis and relative stability, and the common background emotional upheaval, all fed into the decisions that parents made about enrolment in a trial. What implications parents saw for their baby should they agree to take part are considered below.
There are a number of potential outcomes of trial participation that might be taken into account by parents. A baby may be allocated to receive an intervention that might have a positive effect for a baby, it might have no effect, or it might have a negative effect. Allocation may be to a control arm using a placebo or best standard care as a comparison. These possible outcomes were variously combined (and sometimes recombined later in an interview) in a number of ways in the parental accounts of their decisions. In some of the interviews parents explained that they also considered the potential for there being a beneficial effect of their participation on other babies. Three main models of the potential effect of a trial could be identified in the data ( Figure 16 ), which were characterised in the analysis as:
-
might help, won’t harm
-
might help, might harm
-
won’t harm, might help others.
FIGURE 16.
Parental models of the potential effect of a trial.
It was possible to identify one of the three models in the views of all but four of the parents. In some instances there were very clear statements that clearly aligned parental views with one of the models, but it was sometimes necessary to consider views expressed throughout the interview to assemble evidence for one model or another. This was difficult in cases where parents said that there might be risks but then stated that there would not or could not be any possibility of a trial having a detrimental effect. All cases were reviewed and the categorisation was agreed between two members of the team (CS and DE). By far the most commonly identified model was ‘might help, won’t harm’, with views expressed by 35 parents (69%) placed in this category. These views were common in all of the five core trials, with no discernible pattern emerging in the data ( Table 32 ). It was notable, however, that where parents’ views were categorised as ‘might help, might harm’ this was not necessarily a model shared by both partners; in seven interviews each member of a couple gave a different model of the trial.
| Parents’ views of potential impact | INIS | TOBY | PROGRAMS | BOOST-II UK | ExPN | Total |
|---|---|---|---|---|---|---|
| Might help, won’t harm | Nat | Amanda | Rhona and Karl | Ethan | Shirley | 35 |
| Alice | Hannah and Ryan | Adam | Liza and Wesley | |||
| Amy and Chris | Hesther and Stuart | Karen and Tony | ||||
| Anita and Sean | Laura and Wilf | Lesley and Stan | ||||
| Jana | ||||||
| Stefanie and David | ||||||
| Justine and Francis | ||||||
| Sara and Gareth | ||||||
| Julia | ||||||
| Fiona | ||||||
| Marion and Doug | ||||||
| Might help, might harm | Sophie | Robert | 5 | |||
| Lewis | ||||||
| Keith | ||||||
| Ivan | ||||||
| Won’t help, might help others | Dora | Milly | Caitlin and Pete | 7 | ||
| Danielle | ||||||
| Jill | ||||||
| Chloe | ||||||
| Might help, might help others | Beverley | 1 | ||||
| No view identified | Dawn | Warren | 3 | |||
| Diane | ||||||
| Total | 51 |
This phrase and slight variants were commonly used in the interviews to characterise the potential impact on a baby of taking part in one of the core trials. Superficially it seems a simple idea, that the baby would either benefit from taking part, or there would be no effect, but in fact it was a multilayered position which drew on information about a trial but was also grounded in hopes and aspirations, circumstances, relationships and responsibilities ( Figure 17 ).
FIGURE 17.
‘Might help, won’t harm’ model of the potential effect of a trial.
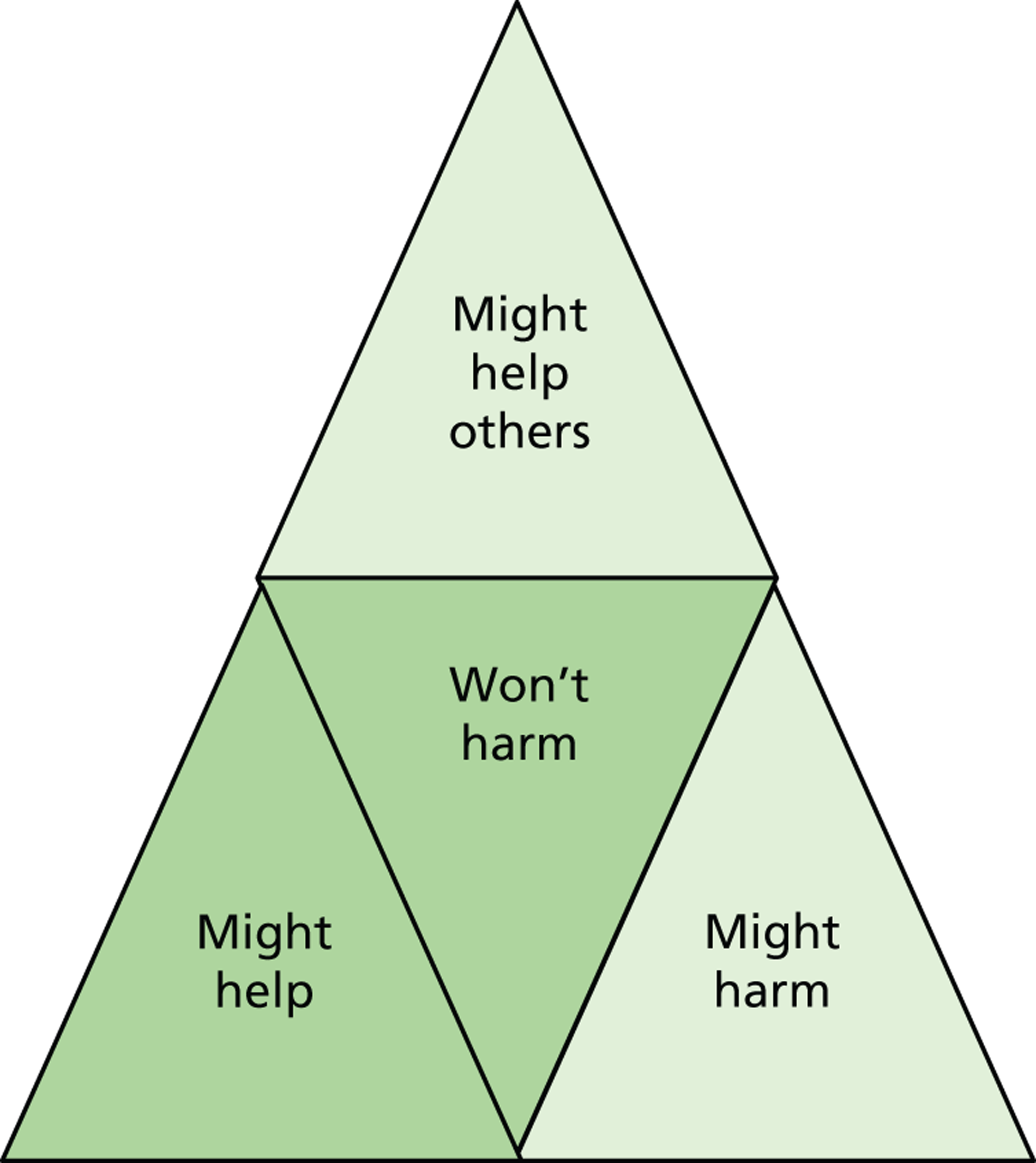
At its most simple level, the view that a trial ‘might help but won’t harm’ was based on what parents felt they were told. There was often a sense in the interviews that they had sanctioned a plan for which their doctors needed their permission, rather than having evaluated and weighed an option themselves. In a model in which all possible outcomes were benign there was little to worry about, and the doctors were quickly given the permission they needed to allow them to ‘get on with it’. The decision to proceed then required little further consideration. Keith, who agreed to enrol his twins in INIS explained how he had focused on the potentially positive effects of the trial: ‘Well if it can help, it can help.’ So the answer’s just ‘Yes. Get on with it!’ Alice similarly presented their decision as approving their doctor’s plan of action. She paraphrased their conversation.
Would you like to try this trial?’ and the doctor sort of saying, ‘There’s probably no harm and there may be some benefit,’ and we just went, ‘Yeah.’ Because I knew there was no – you know – decision-making wasn’t really at the top of our agenda then and it was like, ‘If you think it might help; just do it.’
Alice – INIS
The confidence parents felt in relation to the safety of the trial could in part relate to their view of the intervention. Anita explained how she saw the immunoglobulin used in INIS as ‘a natural thing that your body makes. It was never going to do any harm’. In ExPN the intervention involved extra calories. Although the trial was driven by dual concerns over inadequate levels of nutrition for growth, and the potential impact of increasing calories, Shirley’s model of the trial related only to looking to see whether extra nutrition would help, and this required a relatively straightforward decision. She said:
We were told it would help her, that’s why we consented to it and anything that uses the word nutrition sounds good, doesn’t it? So, you know, we just said ‘yes’ straight away.
Shirley – ExPN
She drew a distinction between feeding research, which she did not see as being of consequence, and drug-related research, which she felt would be risky and more problematic for a baby in such a poor condition.
Now if somebody said to me, ‘We don’t know whether it will be beneficial or not until we’ve obviously administered it and, and monitored it and see what the outcome was’ I think I would have said no . . . because she was so unwell.
Shirley – ExPN
A sense of confidence in the safety of a trial could also be set up by contextual elements. Parents were being offered the trial by a doctor who was involved in the care of their baby, and who was prepared to administer, or initiate administration of, the trial intervention. The offer in itself therefore suggested approval of both the trial and the intervention and for Fiona, who was asked to consider enrolment of twins into INIS, this was sufficient to allay her initial concerns:
The only thing that crossed my mind was the sort of the question of . . . what’s the likelihood of it doing any harm? And I don’t think I even asked; I just sort of stuck through it with the presumption that no one’s going to give a sick baby something that could do them any harm.
Fiona – INIS
In some of these accounts parents were clear that they had not been entirely sure what the trial involved when they made their decisions. To some extent the details were seen as immaterial, even decisions made in relation to TOBY, the trial which offered the least familiar and potentially the most challenging of the trial interventions considered here. Amanda said:
[I was] willing to try anything. I’m not sure if I’d heard about it before or since. I know I’ve heard about it more, maybe more recently. . . . I was just happy that she was getting some help, you know. . ... I think I was just open to anything. They could have done anything to her, really. . . if it was going to help, or there was a chance it would help, I really didn’t care, you know.
Amanda – TOBY
A number of parents made this same point explaining that it was in fact the hope that they were being offered, and to which they had responded, rather than the details of the intervention itself. Ethan said that it did not matter what the treatment was, they would have said ‘yes’:
Frankly at that point he could have said, ‘if you wear blue underpants over the top of your trousers for the next month’ we’d have given it a go.
Ethan – BOOST-II UK
Although these accounts suggest, for whatever reason, that a limited engagement in the details of a trial might set up the might help, won’t harm position, some of the parents who expressed this view, explained how they had pushed their doctors to make sure that they understood the terms of the trial. They proceeded only when they felt that they had the assurances they needed that there was no possibility of harm through a trial. Jana and her partner were asked to consider enrolling all three of their triplets into INIS. She explained how important it was to her to know that they would not be introducing any additional risks into their situation. Once they had that information their decision was then quite simple.
We understood from the information given that . . . there is no risk for the babies whatsoever. It’s something that may or may not help, but nothing that would – that would be in any way harmful. So for us, I think it was like feeling ‘Okay, we can help the trial and you know if it is a sensible intervention, then we can help establish that. If not we don’t risk anything. But maybe, you know, if it is sensible, that we may be helping our children as well . . . And actually we didn’t think about it much. We just went ‘Okay, let’s do it. That’s all right.’ It felt all right for us.
Jana – INIS
Later in the interview Jana drew a distinction between trying to gain a potential advantage – which was acceptable – and the possibility of introducing risk – which wasn’t:
I think it was [because of] the hope that it may help, you know that we said okay, we can go with it, but I don’t think I would be able to add any more risks.
Jana – INIS
Like Jana, Stefanie and David wanted more information about INIS as they worried about upsetting a delicate balance, but they were concerned that the trial was a pivot point, and a decision was required at a time when things could go either way. For some parents there was a sense that at the time of making their decision the balance for their baby had already tipped. Images of ‘grasping’ (Ivan), ‘grabbing’ (Hannah) and ‘clutching’ (Sean) at an option were used repeatedly in the interviews to convey the desperate circumstances in which they found themselves. These parents felt that they could see how things were going for their baby and this positioned them somewhat differently in relation to the might help, won’t harm model of the trial. The approach of this small group of parents can be characterised as one in which there was nothing to lose by taking part in a trial, a phrase used by Francis in the quotation below. For them the dire situation led to a sense that they might as well give a trial a try:
At that point I was already, you know, it’s getting desperate. We need to do anything, basically. So I was more than happy . . . If it works it works. If it doesnae, well, there’s nothing to lose sort of thing . . .You know, there’s lots of things you can take that have side effects, and you’re made aware of them, but they said there wasn’t anything that would cause any bother, so I was confident enough that what they were doing was, was right.
Francis – INIS
Although some of the views described above suggested that parents proceeded when fears for their vulnerable baby were assuaged, in this subtly different approach the dire situation took the potential for harm out of the equation. Hannah explained that things were going so badly for their baby that the trial could not make the situation any worse. In the light of the effects of doing nothing, and in relation to worries about the imminent possibility of death, Hannah had no worries about the trial.
Yeah. I just felt to myself, well, at the end of the day, something’s better than nothing, doing nothing. So it was almost like grabbing at [a] lifeline really.
And did you have any concerns about it?
No.
Was there anything that you found worrying?
No. It didn’t, you know, I thought, it can’t get any worse.
Francis’ sense that there was nothing to lose, and Hannah’s view that things simply could not be made worse by taking part in a trial, were both echoed in Gareth’s comments:
We knew he had a fairly low chance of survival, it sort of became a case of ‘Well this might help him, and it might not, but ultimately if he doesn’t . . . if it does help, then great, and if it doesn’t well it’s unlikely to make anything any worse. And even if it does make anything any worse, are we going to know?
Gareth – INIS
Gareth’s comments about whether or not they would know if the trial had made things worse were not glib. In the case of such a sick, probably moribund child, disentangling the role of a drug in what he saw as an inexorable progress towards death would be impossible. It was, though, a complex and possibly ambiguous comment that triggered an important exchange in the interview, which revealed further layers in the might help, won’t harm position. His partner, Sara, said, in response to his comments, that she had not considered the possibility of the trial making anything the worse;
At worst, it was going to be a placebo that was going to just be like a saline solution going in, that wouldn’t make any difference. If he was getting the drugs then there was a chance they might help, but there was no guarantee of that either.
Sara – INIS
For Sara then the binary might help, won’t harm position reflected the binary set up of the trial: the baby will get the treatment that might help or will get the placebo that won’t harm, rather than being two possible outcomes of an intervention. On hearing this, Gareth then explained that until that point he had not known that the trial had involved a placebo. The possibility of receiving a placebo had ‘never occurred’ to him: ‘In my head . . . we were being asked to participate in a trial of the treatment, and it didn’t occur to me that he wouldn’t get it. ’ Both parents had therefore arrived at the might help, won’t harm position by different routes and a key design element of the trial for one was not part of the equation for the other.
The might help, won’t harm position was commonly expressed in the interviews, but was articulated in different ways and for different reasons. Often it was a straightforward and constant view but it could also be a complicated and fluid interplay of ideas, as shown in the interview with Anita and Sean, in which it was possible to see the notion of risk ebbing and flowing as Anita worked through her thoughts:
We didn’t know how it was going to affect her. We didn’t know whether it was going to be something good, something bad, something indifferent. We weren’t to know. But the way we looked at it was that, you know, it’s got to be good. It’s got to. You know, surely. It’s either going to do nothing or it’s going to help. She can’t – she’s in – she’s not in a good position at the moment because she’s got such a serious infection. So why don’t we just go with it and give it a go, and, and then take it from there and see what happens?
Anita – INIS
She said that it was an easy decision to make because Josephine ‘was just so poorly’ and her partner, Sean, like Ivan quoted above, described them as ‘clutching at straws’.
Might help, might harm
A few parents were clear that a trial might offer benefits for their baby, but that these benefits needed to be considered alongside the possibility of harm. This possibility was carefully explored in the interviews and this brought to light varying degrees of engagement with the nature of trials and how this might impact upon their baby. This went beyond the tacit and generic acceptance of the possibility of side effects that can exist for any treatment that was sometimes articulated by parents who took the might help, won’t harm position. The parents who took the might help, might harm position ( Figure 18 ) demonstrated some knowledge of the potential for risks as well as the potential for benefit as features of the trial that they were asked to consider.
FIGURE 18.
‘Might help, might harm model’ of the potential effect of a trial.
The clearest articulation of the terms of a trial was given by Lewis. His understanding largely came from his own prior knowledge of research. He is a medical scientist, testing patient samples in a hospital laboratory, and he was familiar both with trials and with ‘the science of immunoglobulins’, the category of drug used in INIS which they were considering for their son. Lewis said that when they made their decision to enrol Cory into INIS, he took into account the possibility that the trial might have three outcomes. His presentation however made clear that the technical knowledge of the potential for different outcomes was incorporated into a much more personal and contextualised response to research. In discussing INIS during the interview he said:
[T]here’s two sides to a trial . . . well three sides I suppose. It can be very beneficial, or it can [have] no outcome or a negative outcome . . . But you don’t concentrate on the negatives, you always focus on the positives, . . . and any trials that have been performed, . . . their aim is to be positive.
Lewis – INIS
Although Lewis was himself clear on how trials work, his partner Julia explained that at the time that they had made their decision she had felt that there were just two possibilities, and she clearly articulated a might help, won’t harm position. The risk that concerned her was the possibility of Cory being allocated to the control group and so not receiving the trial intervention, and she sought reassurances from their consultant that this would not happen. It was only later after an explanation from Lewis that she came to understand the nature of the trial, and his explanations to Julia continued into the interview.
The other thing – important thing – that I was aware of in the trial was that the drug can be detrimental to the health of the child. So although you’re thinking of ‘Oh give him the drug; it’s going to help him’.
If it helps him, yeah.
– you give him the drug and it could [make him] deteriorate faster.
I know, when you said that to me afterwards I went, ‘Oh right.’ [Said in a quiet, shocked tone.]
So that hadn’t occurred to you.
That hadn’t occurred to me initially.
Although Lewis accepted the risk from the start, Julia felt that had she appreciated the element of risk in the situation her decision would have been more difficult. She did feel, however, that they would ultimately have made the same decision to enrol.
The difference between Lewis’s and Julia’s appreciation of the situation was not as simple as prior knowledge for Lewis, or not taking on board challenging information for Julia. Julia explained that she had tried to push a reluctant Lewis to read an information booklet about intensive care, which mentioned the possibility of babies dying. She had felt that the information in the booklet had been important to her understanding of their situation and what might lie ahead for them, and that for her this was an important coping strategy. She said, ‘I just want to know everything. You know, that’s the type of person I am. I just want to be really aware, all the options and the possibilities.’ Despite being an information-gatherer, and being prepared to work through very sensitive information, when it came to her approach to the trial, Julia focused only on the potential benefits of INIS saying that in such dreadful circumstances, ‘That wee bit of negativity that there could be a downside of this, doesn’t really take over’. Similarly Lewis, despite his knowledge of trials, focused only on the potential upside of a trial. He could not unlearn what he already knew, but he could set aside for a time the more uncomfortable negatives in the light of the threat of advancing infection (NEC) for his son, and focus on what he felt were ‘the positives’.
Unlike Lewis, Sophie, who also enrolled her son into INIS, did not have prior professional knowledge of trials, but she did describe a clear model of the nature of the trials and of the implications in their situation. She too held a different model from that of her partner, Nat. They had accepted INIS for their son Brendan at a time when things were extremely difficult and Nat said that, for him, the decision was made in ‘desperation’.
I just wanted something, at that point . . . on that day, to change his fortune. I just wanted to see that blood pressure start going back up, and, you know, for him not to be in so much pain.
Nat – INIS
Although Nat saw the trial as something that might help, Sophie indicated that when they agreed to enrol Brendan into INIS she had worried.
Obviously you, you worry that what you’re doing is going to directly impact him in a negative way, because nobody knows. I certainly did think, actually, you know, are we – you know, could this – could this really be a bad thing to be doing right now – that we’re doing actively to opt in – to our child, you know? So certainly I – I did think – [Sophie paused and asked Nat]. Did you not think at any point that it could actually be a . . . ? [Sophie did not finish her question and the conversation moved on.]
Sophie – INIS
Nat indicated later in the interview that he had not, until that evening, considered that there could have been any risks in their situation. (This difference is explored further in Chapter 9 in relation to Sophie and Nat’s reactions to the results of the trial.) Their interview was very informative as they had in fact made two decisions about trials in the same NICU, one in which they accepted INIS for Brendan when he was critically ill, and a different trial for their next son, Jacob, which they declined when he was stable after cardiac surgery. They felt that it was the difference in the condition of the two babies that made them view risk differently on each occasion. Nat explained how he had seen a trial in the non-critical setting for Jacob:
[H]e was doing well at that point, you know, he’d come out of his surgery . . . He was stable. He was doing the things that they wanted him to do. What they were telling us he should be doing, he was doing, and at that point, when they told us about the study, my mind was very much, I just do not want to risk him.
Nat – INIS
Although Nat put forward a might help, won’t harm model, there is an understanding here in a stable situation, that a trial might have some downsides, that it might ‘rock the boat,’ but that in crisis this possibility is obscured or set aside to the point of becoming irrelevant.
This sense of the irrelevance of risk could predominate at the time of decision-making but be eroded over time. Adam described a dawning sense that there had been a possibility of risk in the trial to which he and his partner Milly had consented, as he later pieced together his thoughts. He came to feel that the situation might be more complicated than he had originally thought. Their twin sons were born at 23 weeks as Adam was being rushed back from overseas military service. He arrived at the hospital to find that one baby, Lee, had died in the delivery room and their surviving son Cameron was being cared for in the NICU. He and Milly were asked to consider enrolling Cameron, in BOOST-II UK. Adam said that he readily agreed. He described his experience:
I could only think of the possible benefits. I mean, at the time and looking – this is all looking back and realising – I didn’t think about the fact that it’s a trial, so he could be better or he, he could be worse off. The only thing I had, in my head at the time was I thought, this could help and I sort of blindly agreed to it, not really knowing the full – the full facts behind it and then later on I had a – I caught myself thinking a couple of times, well what if it was detrimental?
Adam – BOOST-II UK
When risks are evident at the time this can complicate an already difficult experience. Robert’s account of his experience of considering his options demonstrates how uncomfortable this can be. (Robert was interviewed alone and so his partner is not given a pseudonym.) His story, which conveys most starkly some of the experiences that surround the introduction of a trial, is told in detail in Box 6 . Like many of the parents interviewed, he was in a dire and traumatic situation. His daughter was enrolled into TOBY in another European country where he was not fluent in the language, and from his description of his experiences, his appreciation of what the trial involved and the nature of a trial situation did not arise from having received a clear explanation from staff. Robert explained how he saw the potential benefits and risks of the trial, and how he weighed these in relation to the context in which he found himself, and in relation too of his daughter’s life and future. He said:
It is a risk, but then in some ways there was no choice. In my opinion we had to take a risk because she was seriously ill so we had to do something.
Robert – TOBY
Robert and his partner were living in Hungary at the time of the birth of their first child. His partner was 2 weeks overdue when she gave birth in a private English-speaking clinic. She had been concerned early in labour that she was losing a lot of blood but was reassured that it was normal. Adele was born during the night not crying. The seriousness of the situation was apparent to Robert.
I knew something was seriously wrong and there were other children who’d been born in the hospital at the same time, you could hear other babies crying, which was incredibly difficult for us, I mean there [was something] totally wrong with our little girl.
After several hours an ambulance was mobilised to transfer her to a hospital 45 minutes away. Robert followed but was only allowed to see Adele briefly once. It was still night time and there were no parents in the NICU. He felt extremely isolated and most of his time was spent alone in a side room ‘with a couple of cracked plastic chairs and some boxes’, an experience he described as ‘almost like being in solitary confinement’.
I remember being – feeling – very isolated not being able to speak to anybody . . . it must have been about three in the morning, two or three in the morning at this stage so it was still very dark. . . I felt tired ‘cos I’d been up all day and all night, the adrenalin and this feeling sick and I didn’t know what to do, couldn’t speak to anybody. . .. I was sitting in this sort of odd little room with boxes everywhere and just told to wait and wait and wait. I didn’t hear anything.
By the time he was asked about TOBY he was at a low ebb. He described his conversation with a doctor about the trial:
The guy who was the, the main doctor there had very broken English and he, he tried his hardest . . . I have to say he looked like an incredibly stressed doctor who had too much to do and I’m sure he tried his damnedest but it was – it was not a nice environment . . . [He] asked me if I’d be willing to allow Adele . . . to go through this process of sort of induced hypothermia I suppose. They were saying that, ‘With brain damaged children, which your child will be brain damaged’ – which was, ‘Oh God!’ which you sort of knew but you didn’t want to hear and hear quite so bluntly put, ‘. . . this could perhaps prevent some of the damage.’ And they gave us some information in English . . . So I was sitting there going, ‘Oh Christ I’ve got to . . . my daughter’s there, I’ve just found out she’s more than likely seriously brain damaged, she may or may not live, they want me to put her into this type of experiment! What is it?’ There’s a sheet of paper, which sort of explained it which I couldn’t – it was in English so that’s fine but you couldn’t make sense of it ‘cos your emotions were so much – and I wanted to speak to my wife, I didn’t want to make that decision without consulting her. My instinct was to say, ‘Yes, anything that might help her.’
Robert called his partner. He very much wanted to accept the offer of the trial and was concerned that she may not agree. He said that she knew ‘there was a risk’ but they also felt that TOBY offered the first ‘glimmer of hope’ that they had had. He described his feelings at the time.
There was a helplessness and this comes along, so almost gives you a feeling there’s something you could do which could be helpful, but it is risk but then in some ways there was no choice. In my opinion we had to take a risk because she was seriously ill so we had to do something . . . . I knew that it was one or the other and I knew it wasn’t a cure and I knew that what it did was to offer a possibility that the damage that was happening might be . . . reduced but I sort of knew in my heart of hearts that either my daughter was badly brain damaged or would die, and you sort of knew it but I didn’t want to accept it and – It was like one of these awful things where you’re sitting. . . there going, ‘Oh God I want her to live but oh God I don’t actually want her to live as a very badly disabled brain damaged child. I want her to have a quality of life’ and it, that was an awful sort of dilemma for me. What was I was actually praying for?
The risks that Robert saw in the trial were outweighed by the hope that cooling might offer to them, and his own need for action. His partner agreed that Adele should be enrolled in the trial. His account draws attention to the need to act in a context of powerlessness. Robert talked about wanting to do something for his daughter as he felt that he had not been able to do anything for her up to the point of being asked about the trial. He saw making this choice about the trial as ‘the positive thing in, in a totally hideous experience’.
I wanted to do something for her . . . as a father . . . I hadn’t done anything for her in her life and this was something I could actually do and that was the positive thing in – in a totally hideous experience . . . I didn’t have any false expectation or false hope . . . my interpretation now was there’s a 50 : 50 your child would be in this trial or not, it’s an experimental trial, we don’t really know, we think it might make some difference for certain children at certain times and it works better if it’s done earlier rather than later in the window . . . And I – I sort of knew that it wouldn’t do . . . yeah, I’m sort of contradicting myself but really I sort of knew that my daughter was probably too far gone to have had any benefit, but I thought that the idea of actually making a positive decision to even throw her that little lifeline, we had to make that choice and do it . . . [N]inety nine times out of a hundred nothing would happen, I knew that, but at least there was one little bit of something we could do.
Robert seemed to have access to very little information and once he had consented there was no further information about allocation or discussion of the trial. and he was not told whether Adele was cooled or not. He said that he came to a position where, because he was hoping that she would be cooled, and that perhaps it was better that he did not know.
[You] invest some hope no matter how tenuous that hope is. [If] I’d have spoken to my wife and would have got her emotional engagement, and they’d turned round and said, ‘Well actually we’re going to put her in control group, that would just destroy me.
What parents felt they were risking was not always clear as the terms ‘help,’ ‘harm’ or ‘hurt’ could be used without further qualification. Sometimes parents referred to risk in terms of side effects, and this had particular implications. It made them more manageable than the risks considered by Lewis, Sophie and Robert who were concerned that they might worsen the condition of their baby. Parents who presented risk in these terms could view those risks as irrelevant as their baby did not survive to experience any side effects. Keith, who agreed with his partner Fiona, to enrol their twin boys into INIS, presented the ‘repercussions’ of taking part in a trial as something that he was aware of but did not see as considerations at the time.
At that point in time it was just do whatever you can to help the children. You sign the forms. The fact that there was a risk, i.e. you don’t know what the side effects were going to be, you just . . . your kids have got a 50 : 50 chance of surviving, you’re better to try something than nothing at that point in time, and not even worry about all the repercussions. So logically it didn’t matter. It was just do something.
Keith – INIS*
*Keith was categorised as holding the might help, might harm position because of his statement about the potential for consequences, but the spirit of his comments in which risk is something that did not matter is similar to views expressed might help, won’t harm position. Keith’s comment links harm only to a longer-term future that did not transpire, rather than to outcomes relevant to the shorter term. Keith argued that the possibility of side effects had become irrelevant for their baby who died; his concern at the time of interview was for their surviving twin, Kelvin, who was also enrolled into INIS.
Our worst-case scenario in all of this is if we’d had a letter saying ‘We’ve just found out at the age of eight Kelvin’s going to get leukaemia or a brain disorder, or something, because of the drug.’ That’s a consequence. And I don’t know how you deal with that because . . . so the death bit I’m actually less concerned about. It’s side effects and serious side effects that I’d be concerned about. So the fact he gets eczema – fine, you know. He’s alive, and he gets a skin rash. Not a bad thing. Is it a consequence? Yes, but not in a way that I deem . . . yeah, useful to know about.
Keith – INIS
If the possibility of side effects is conceived of at the level of eczema, then, in comparison with death, it is negligible. If it is a side effect which is thought to be of consequence only if a baby survives then this will foster a ‘cross that bridge when we come to it’ approach. Either way the possibility of harm is acknowledged but then screened out.
Alice and Ivan saw INIS as offering a valuable opportunity that outweighed any risk. Alice said that a doctor had explained to them ‘This is a trial. We think it might be beneficial but we’re not sure. You know there might be side effects but we’re not sure.’ She said that the possibility of side effects had not worried her, that ‘you can blot that out of your mind if you’re in a desperate situation’ and Ivan said, ‘I don’t even think we thought about it to be honest. ’ For them side effects were relative, and would be a by-product of success. Ivan said:
[L]et’s just say, for the sake of argument, the trial had been successful and it had turned things around for Oliver and he’d had some side effects from it; I think the mere fact that he was here . . .
Ivan – INIS
Alice finished his sentence by saying:
Side effects aren’t as bad as being dead.
Alice – INIS
The most poignant ways in which parents discussed harm occurred in only a small number of interviews, where parents discussed their worries that their baby might survive as a result of taking part in a trial, but to live a life that they would not wish for them. Robert touched on this when he described himself praying after he was told that his daughter may not survive or may survive with brain damage, but not feeling clear about which outcome he was praying for (see Box 6 , above). A mother who decided that she could not take part in an interview submitted a written account of her experience to BRACELET, in which she carefully described how she saw the possibility of harm in relation to TOBY. Her text is reproduced in Box 7 .
At the time our baby daughter Melanie was born and we were told she had lost oxygen due to the cord being so tight around her neck in delivery, we were asked to take part in the TOBY study. During this traumatic time, we really were unsure what to do but felt that maybe research could prevent other babies dying because of this.
In hindsight, I am glad that Melanie was selected to not be treated as part of the TOBY study. Maybe things would have been different had she lived, but I think at that traumatic time parents really are not fully aware of how their choices could affect their child’s survival or not. Melanie died 2.5 days after birth. If she had survived as a result of having received temperature-control treatment, and having had so much brain damage, her life and our lives would have been so much different. I’m not sure how I feel about this. I just think that maybe it’s not appropriate to ask parents who are going through such a traumatic situation to take part in research because really they are not in a position to make a conscious decision. We are lucky in that we don’t have any questions regarding Melanie’s death other than the cord got caught around her neck during delivery. I know how important research is but I also know, from personal experience, it is very very hard to take medical information on board when you are experience a tragedy like we did. God forbid, but if it ever happened again and I was in a similar situation, I don’t think I’d agree to take part.
[W]hether or not the results of the trial prove or don’t prove that reducing the temperature can or cannot reduce the damage to brain cells following oxygen starvation would really have no impact on us as Melanie had died. The only results parents in this situation want to know is if their child is going to live or die. Simple as that. Statistics mean nothing in this situation. I remember being told something along the lines of 20% survival rate in babies who have been starved of oxygen during delivery – but this figure meant nothing at all when Melanie died. She fell into the 80% category who don’t survive. Again, this is my personal opinion based on what happened to us.
I found it quite difficult thinking back to this research study. I know we made a decision at the time but now – almost 5 years later – I find it too difficult thinking about the whole medical side around Melanie’s death.
Won’t harm, might help others
In each of the conceptual groupings of parental thoughts about help and harm, there were parents who talked about the value of contributing to research and the hope that the trial might benefit other children. This is explored in more detail in Chapter 9, where we consider parents’ presentation of the meaning that trial participation holds for them. Here our focus is not on the potential for a trial to benefit others, but how this won’t harm, might help other position related to parental perceptions of the potential impact of a trial for their baby ( Figure 19 ).
FIGURE 19.
Won’t harm, might help others model of the potential effect of a trial.
Two positions were identified:
-
trial is benign and unlikely to have an effect on their baby, but others might benefit
-
baby is unlikely to survive and trial is unlikely to change this, but others might benefit.
Dora’s son Gerry was born at 26 weeks. Dora is a nurse but this did not help her to cope with the NICU. She said, that it was an environment in which she felt ‘totally lost’. When she described the time in which she had decided to enrol Gerry in INIS, she said that she had done so without knowing much about the trial. She could remember very little but said that this was partly because she had taken so little on board during the week when her son was alive. She did not see INIS as something that had been likely to help him but was interested in the fact that it might help other babies in the future. (Dora’s thoughts on this are considered further in Chapter 9 .) She said, ‘There wasn’t a lot of thought went into it . . . , it was just something I automatically wanted to do. There was something within me that just wanted to do it. ’ Some parents pin their hopes on a trial but Dora is one of the parents who saw it as incidental, as something that was unlikely to change anything.
I can remember them asking me about it and me just readily agreeing really. I – I don’t remember even thinking about it, it was just, like, well, it’s not going to make any difference to the outcome but if it helps somebody else.
Dora – INIS
Dora was not able to say exactly why she felt that the trial would not make a difference, but described how her ideas about the trial came from a sense of trust.
I probably didn’t ask a lot of questions. I would’ve imagined – I would’ve just signed . . . I suppose I would have trusted them. I know that sound – that sounds bad, doesn’t it? But it’s like I trusted that – you know – I knew it would be a fair study, it would have been looked into before it started, it wouldn’t just be a random thing that anyone would do.
Dora – INIS
Danielle is one of only two interviewees in BRACELET who was asked about a trial antenatally (see Box 1 above for details). She was asked to consider BOOST-II UK for her baby after the decision was made to proceed to an emergency CS.
You know, once they’d said, ‘Right, this is gonna happen,’ I think it was then they said, ‘Oh, well, would you mind taking part in a trial?’ And, and they sort of explained it was – and I can’t remember off the top of my head. It was something to do with breathing. Instead of doing it one way they were going to do it another way, but he would still get the same level of care and everything else. I said, ‘no, that’s fine.’
Danielle – BOOST-II
It had not been a big decision and was largely made with a view to helping other children. Danielle did not seem to feel that it was likely to make much difference to her baby. She had no concerns and was not entirely clear what the trial was about, and saw it as something intended to benefit babies in the future rather than something that might affect her baby. She said, ‘I will do anything to help other babies not to go through that situation.’
It was just a case of helping other babies in the future. It wasn’t a case of, you know, ‘well, Todd’ll be better off on this,’ or, ‘well, he won’t be better off on this.’ It was just a case . . . I didn’t even think about it in that way or anyway. I just went, ‘oh, yeah, I don’t mind.’
Danielle – BOOST-II UK
Caitlin and Pete agreed that their twin sons could be enrolled in ExPN. Caitlin remembered that it was ‘something that would help’ and something that was ‘to do with bringing them on’ but Pete’s account was different. For him there was no sense that anything would change as a result of a decision to take part. Pete said, ‘It was just a data collate rather than anything that they were going to do to benefit like our two.’
It was very early on; literally within the first day of, to them being in the unit. To be honest it was presented quite, and don’t take this the wrong way, quite quickly and – ‘Right this is what we’re doing. If you’re in agreement, great; if you’re not, don’t worry about it. And for us . . . I don’t think it was a case of ‘Let’s discuss this Caitlin. What do you think you’d want?’ It was a case of, ‘Is there any danger to the child as a result of what you’re doing here?’ ‘No.’ ‘There’s no other impact other than you collating data or whatever?’ ‘Yes.’ ‘In which case; happy to help’ and that was really it, you know. Once I’d ironed those two issues out, that was it, great, sign the dotted and away we go.
Pete – ExPN
In this interview the ExPN study was presented as a very simple thing that they had agreed to, a very small part of their experience. Caitlin and Pete had given it very little thought, not because it seemed the obvious thing to do or because they were jumping at a chance to benefit their baby, as in lots of the other interviews – it just didn’t seem to be a big issue at the time or since. The issues of undernourishment, diabetes and infections, and the balancing of those risks in relation to shorter- and longer-term outcomes that drove this trial were not present in the parental narratives. An important factor that also seemed to shape Caitlin and Pete’s views of the trial is that neither parent actually foresaw a long-term future for their babies. Pete said that he was told in the delivery room that the outlook was very poor and he never expected their babies to survive. Caitlin commented on this in relation to the trial:
I don’t ever think that I thought it would benefit the babies. I just thought it would benefit the research along with the wider issues . . . . maybe that’s telling me that in my subconscious, there was no future.
Caitlin – ExPN
As the parents felt that ExPN was unlikely to be of benefit to their babies, and would not change the elements of care that they felt were most important for their babies (fighting infections), the trial did not map on to their needs. Their decision to take part was intended primarily to help others, and they did not pin any hopes on the trial.
Milly’s view was similar to that of Caitlin and Pete, but her partner Adam viewed chances of survival differently for their son. One of their twins had died on delivery, and their other baby was enrolled into BOOST-II UK after admission to the NICU. Milly felt that at 23 weeks his chances of survival were slim, but Adam was optimistic.
I knew. The first time I seen like Cameron in that little incubator, I knew then that he wasn’t gonna survive. I could see it, ‘cos he was just too – so I’m quite strong-willed so I sort of talked myself into that. I already knew he wasn’t gonna last ‘cos I could see he was just too tiny and frail.
And I was – I was the other way. I was like, he’s gonna be alright.
He’s, he’s full of positivity.
This difference in their positions also shaped their two responses to the trial. Adam, whose views were described earlier, was very hopeful that the trial would benefit Cameron but Milly focused elsewhere. She said that she is ‘up for doing things’ and referred to her willingness to consider neonatal organ donation. (As neonatal organ donation is not currently practiced in the UK, Milly’s comment is hypothetical and it does not suggest that they had been asked to consider donation.)
I’m quite up for things like that, anything where it could help like, if they’d needed like his you know, eye, you know kidneys, anything – I would happily. I’m one of those people who would say, ‘Yeah take.’
Milly – BOOST-II UK
She said in relation to the trial ‘I’m happy to try anything.’ She was pushed as to whether she made her decision for Cameron’s benefit and said that it was ‘for others’. The following exchange highlights Milly and Adam’s contrasting positions.
I already knew in my heart – I could see that he wasn’t [going to] survive, so I was thinking if they could help somebody else, ‘cos at the end of the day we wanted to have more kids so you never know, in the future that, that could help us. So that’s how I see things.
Well, whereas I, I could only think of the possible benefits.
Might help, might help others
Beverley’s account of her decision-making for BOOST-II UK did not fit neatly into any of three main categories. She articulated all of the components as some points in her interview, but was clear that some of the views expressed related to her later feelings, rather than to her views antenatally, which was when she made her decisions about whether or not to enrol her baby into the trial. It seemed that her husband, a doctor, was particularly keen that they should enrol Ruth in the trial. She recalled that her husband said:
We should do it because it could help other babies and whatever, and if this had been done sooner, and we’d have had the results, then it might have helped Ruth.
Beverley – BOOST-II UK
She agreed with this, saying:
And it is all true.
Beverley – BOOST-II UK
On recalling this discussion Beverley said nothing about harm or risk, neither dismissing them nor worrying about them, but, at another point in the interview, her comments suggested a degree of ambivalence. On the one hand she said, ‘No, I didn’t really have any concerns’ but when she described the last couple of days of her baby’s life, she said:
I did have concerns . . . I was thinking then, we shouldn’t have done this study . . . I think if they’d have asked us afterwards, I probably would have said no.
Beverley – BOOST-II UK
Beverley’s fluctuating account of her views demonstrate some of the difficulties for parents in balancing potential benefits and harms, and how these views may shift over time, and some of the complexities for researchers in attempting to capture and represent these views.
Discussion
The data presented here cover the first two phases of involvement in a trial for the parents who took part in BRACELET. They detail some of the harrowing events that can precede and influence trial-related decision-making: difficult pregnancies and traumatic births, maternal complications, transition to NICU, separation of mothers and babies, separation of multiples, infections, surgery, and, in some instances, the death and funeral of a sibling. If BRACELET had worked with a more narrow focus on decision-making just in the NICU, the background and significance of birth trauma would have been lost. Although these experiences precede the discussion in which the possibility of a trial is introduced, sometimes by days or even weeks, they are important features in both parental narratives and in the larger data set. They detail the route that parents and babies took, and the mindset they had developed, by the time they reached that point.
A broad and inclusive focus has, in addition, yielded insights into the significance of the different relationships that are established during this initial period. The interviews included accounts of parents’ experiences of not knowing until the last minute whether their baby will breathe, and if he or she does, whether this would demonstrate enough ‘fight’ for the neonatal team, who were waiting on standby, to initiate their fight for the baby in response. When their baby does breathe the threat of waiting for death is removed and replaced with the activity that surrounds admission to neonatal intensive care. The interviews also suggested the significance of the move away from the setting of a physically and emotionally traumatic birth, where some have felt that care was compromised, into the care of a new clinical team. If a transfer was involved, this effect was heightened as they associated the new team with increased expertise and more sophisticated facilities. Although the need to initiate intensive care, or to transfer a baby to a higher level of care, are both indications of the severity of the situation, in each of these situations admission to a NICU, or transfer to a new NICU with more specialised facilities, represented hope and the possibility of rescue. It also seemed to be a more positive environment. Although complaints about antenatal and obstetric care were common, and a sense of powerlessness was often present in accounts of pregnancy and birth, none of the parents complained of the neonatal care given; instead their accounts referred to respect, support and inclusion. Parents and the new clinical team were in accord, and new and important relationships were forged with those striving to save their baby.
The offer of a trial was most commonly made by a member of this new team. Often the doctor who raised the subject of a trial was someone would go on to play a major part in a baby’s life. By the time of the interviews the parents recounted the offer of a trial in the context of a relationship which had in some cases developed to the point of parents using the doctor’s first name, the doctor being involved in decision-making around the time of death, and of parents being aware of the impact of the death on the doctor. In some instances they had visited bereaved parents at home, or had attended their baby’s funeral. The offer of a trial was imbued with this atmosphere of fight and rescue and a retrospective sense of care given. This was heightened if a trial had been offered as a baby took a downwards turn or if only one of multiples was eligible, as the trial was often then presented by parents as a response to individual need. Even when babies were relatively stable, and where a trial was offered as a matter of routine, or was offered to twins or triplets at the same time, the sense of need and the corresponding value of anything that might help was persuasive when parents knew their babies to be fragile and vulnerable. In these situations, it was the broader possibilities offered rather than the close detail that was important; a trial raised the possibility of hope when things were becoming hopeless and the possibility of action when options were running out. The imagery of desperation was commonly used to convey the emotions that could surround trial entry; the knowledge that all of the parents involved went on to be bereaved brings additional resonance to the image of clutching at straws.
The prevalence of the might help, won’t harm model that arose in this setting and was identified for the majority of parents was not necessarily a surprise. It predominated in the parental accounts, and cut across the trials as well as the different backdrops of enrolment in routine and in crisis situations. The same filtering out of risk was identified in our earlier work with parents of survivors,51 and by Ward,66 who has argued that in her study parents ‘discounted the risks of research or did not believe them to be applicable to their own situation’. This cannot be as simple an issue of difficulties in understanding information. Parents of babies receiving intensive care have to take on board on a daily basis, information about physiology, equipment and treatment, and can become experts in their child’s condition; it would be surprising if they could not also process information about the possibility of risk. To determine the origins of this model though, whether a positive filtering out of risk, or an effect of information given or not given, or a more complex interplay of factors including the framing effects discussed above, would require a different study with an observational component. What is of interest here is the significance of this risk-free model in terms of parental responses to the trial in which their baby was enrolled.
For some parents the risk-free model still endured at the time of the interview. They were clear that they had agreed to a trial without any substantive engagement with the information, describing fast and superficial discussions in which they quickly sanctioned a trial, and this position had not been revisited or re-evaluated. Some parents, however, did gradually wonder whether they had given the trial sufficient consideration and Adam, who described how he had readily agreed to a trial but with little sense of to what he had consented, was troubled by his lack of knowledge by the time of the interview. In situations when parents felt that the doctors were offering something that the baby needed, parents often saw their own role as one of sanctioning a decision rather than taking a substantive part in the decision-making process. Here some of the parents presented their role as facilitators as the doctor needed their permission to get on with enrolling their baby in a trial; formally seeking their permission was largely a nod to their parental responsibility.
It is important to note that parental responses to risk were presented in the interviews from the position of bereavement, from the vantage point of knowing that their baby died regardless of their enrolment in a trial. In a might help won’t harm model the fact that taking part in a trial did not save their baby’s life (whether or not the intervention involved has that potential) can be incorporated into a sense that it was worth trying and that nothing was lost by doing so. With a model of risk based on implications which would only be relevant should a child survive, risk for bereaved parents could be removed from consideration. This will be considered further in later chapters.
The sense of risk, if present, was not necessarily clear and stable. It was layered in with the potential for benefit, and in some accounts the possibility of risk rose to the surface and dropped away at different times in the interview. Even some of the parents who were clearest about the implications in the offer of a trial, who were often able to draw on pre-existing knowledge of research and research methods gained outside of the discussions in the NICU, could set aside risk as immaterial or for later consideration. To some extent these parents thought about the trial that they were offered in a different way from those adopting the might help, won’t harm model; they were aware that the trial may involve some risks for their baby and accepted this as part of the situation in which they found themselves, but the extent to which potential disadvantages were worrisome varied. Although these parents identified the possibility of risk this was most often in the broad sense of making things worse rather than with reference to specific risks for the trial under consideration. Their focus on advantages, although cognisant of potential disadvantages, was in fact not particularly dissimilar from other parents in the study.
The won’t help, might help others model, identified for a much smaller group of parents, was a new element in the data. It was linked for some to the sense that whatever was done for their baby would not change the outcome; those parents felt before they made their decision that that their baby was not going to survive. These parents were not the only interviewees to discuss their hope that by agreeing that their baby could take part in a trial there might be some benefit to other babies. There was a strong wish expressed by many parents that through their child’s participation in a trial, other babies and parents might be ‘helped’. The views of the parents in the won’t help, might help others mode were particular in seeing their baby as not being a potential beneficiary of the trial.
Understanding more of the experiences that surround and shape the decisions that parents make gives an important window into their involvement in a trial. Uncovering their views of the trial, and why they made the decision they made, aid understanding of the terms on which they entered the trial. Although this has already been deemed important in the trials community, the standards of consent given being an ethical barometer by which wider standards in research are judged, perceptions of the terms of the agreement that parents entered into also has an additional significance for ongoing relationships with trials and trial teams. Views of the terms of a trial, and the highly individualised responses to the ‘deal on the table’, are important key points when parents might revisit the choices they made. This chapter has taken us as far as most studies go in exploring reactions to trials but a larger relationship can be captured only by considering experiences beyond decision-making, of participation, and, for these parents, of further reflection in bereavement. The following chapter therefore considers parents’ experiences of participation in a trial, after the decision to enrol their baby and up to their baby’s death.
Chapter 7 Bereaved parents’ subsequent experiences of neonatal trials
In Chapter 6 , we considered the two earlier stages in parents’ experiences of their involvement in a neonatal trial, the circumstances in which babies became eligible for the trial and the context that this created for parents, and the decision-making process around enrolment into a trial. In this chapter we consider experiences of participation beyond this point, and up to their initial experiences of bereavement.
Stage 3: Experiences of participation in a trial
A substantial literature exists which involves trial participants as informants, but few papers consider their experience of trial participation itself. 151,152 The literature that goes beyond decision-making tends to skip forward to examine later events, such as reactions to the end of a trial152–155 or to feedback of results. 155,156 The decision to join a trial marks the start of a relationship with research, and whether that relationship is a major or a minor part of the larger experiences of illness and care, and in this instance parenting and bereavement, and what significance that relationship has for those involved, is useful information. An understanding of trial participation will offer those involved in trial design and those involved in the care of trial participants and their families, insights into the ways in which research can shape experiences and even perhaps life events. It is an important precursor to understanding the overall significance and meaning of trial participation generally, and specifically in this instance, in bereavement.
It was very clear from the BRACELET interviews with parents that very different experiences can flow from the decision to take part, depending on the focus and set up of the trial. The extent to which a trial was visible and an influential part of events during the NICU stay varied, with TOBY bringing about the greatest material change to circumstances and to care. For the other trials the extent to which a trial was prominent in parental accounts varied according to how events unfolded and were managed by clinical staff, and how parents themselves responded to their ongoing involvement in research. In this chapter we will therefore consider the experiences associated with the prominent and obvious effects of being involved in a trial as a major feature in the foreground of parental experiences, and those that did not appear to bring about change, in which a trial seemed to be a background or minor feature in events. We refer to this as trials in the foreground, trials in the background, and trials disappeared ( Figure 20 ).
FIGURE 20.
Prominence of trials during NICU stay.
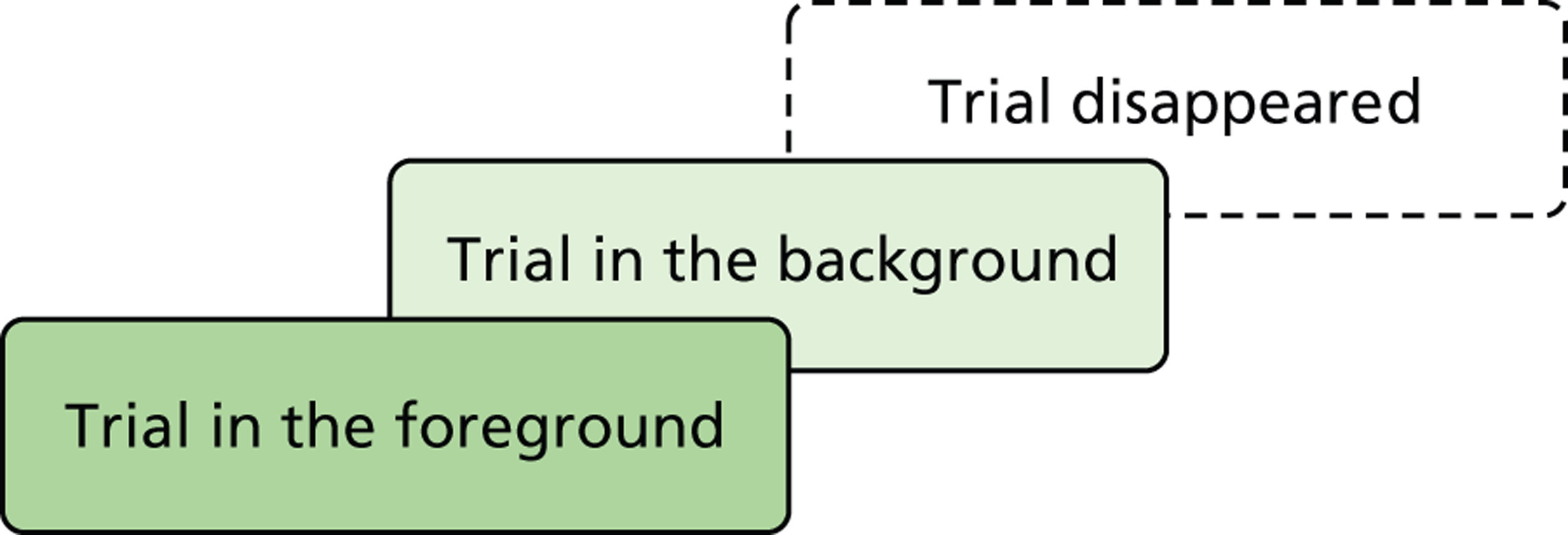
Trials in the foreground
For two sets of parents in INIS the trial appeared in the foreground of their experience because of its proximity to the death of their baby, and these experiences are considered below, but the trial which brought about the most obvious change to the circumstances of the parents and babies was TOBY. This trial required treatment in a Level 3 NICU which was designated as a cooling centre and babies born elsewhere were transferred to such a centre by a specialist neonatal transfer team. The trial intervention involved 72 hours of hypothermia, an intervention that parents are unlikely to have encountered before and, because a baby receiving hypothermic care is cool, the active intervention involves an obvious and observable physical change.
Five interviews were carried out with eight parents involved in TOBY, and these interviews demonstrate the variety of ways in which a trial can affect what happens to a family, and how they view their experiences. This variation can stem from:
-
the pace of recruitment for the trial and the decision-making process required (considered in the previous chapter)
-
whether or not transfer of care is necessary, particularly from one hospital to another
-
allocation to the intervention or control arm of the trial
-
reactions to the trial in relation to the prognosis for the baby
-
enrolment in close proximity to death.
This categorisation can also be applied to non-TOBY trials, although transfer between hospitals for the purpose of a trial is an unusual (but not unique) feature of TOBY.
Transfer
In four of the TOBY interviews, babies were transferred to a cooling centre. (In Robert’s case his daughter was transferred because she required a higher level of care than could be given in the centre in which she was born, and she was transferred before the subject of the trial was raised. The question of trial participation, which was raised on arrival, was therefore entirely divorced from the issue of transfer. His experience of the trial is considered later in this chapter.) For two of the couples interviewed, Hannah and Ryan, and Laura and Wilf, it was clear that the transfer for the trial was very closely linked to their wider experiences of care and research. Hannah and Ryan’s interview showed how parents viewed and experienced transfer in these tight time frames, and this part of their story is presented below (see Box 8 ).
The recruitment window for TOBY was narrow and was rigorously observed. Recruiting clinicians interviewed for BRACELET described how they had to work within a very tight time frame to go through consent processes, stabilise and randomise the baby all within 6 hours of birth, always mindful of the possibility of being ‘timed out’, in which case a baby could not proceed to enrolment into the trial.
All of the parents interviewed in relation to this trial were aware of the need to act quickly. Amanda found a recruiting clinician waiting for her when she first went to visit Simone in the NICU (see Box 4 ) and Robert described calling his wife who was being cared for in a different hospital to try and make a joint decision straight away (see Box 6 ).
Laura and Wilf were aware of the situation and quickly made their decision to permit trial enrolment and transfer. Although the prospect of a move to another hospital was challenging, and Laura and Wilf did not see it as being without risks, they felt that they had to balance this against the possible chance of benefit for their son. Archie was their first baby, born almost 2 weeks post-term after an induction, a failed ventouse attempt and then a forceps delivery. He was born not breathing, and without a paediatrician present. (Their case was the focus of a formal enquiry.) For Laura, one of the most important issues was that the trial offered them some hope in the context of an unexpected disaster:
I wasn’t prepared for bad news in any way when he was born and so . . . being told that there is a glimmer of hope, perhaps, kind of opens you up to the idea that there might be something that might benefit him in some way.
Laura – TOBY
Wilf felt that the policy of transferring all babies would be an important advantage of taking part in TOBY, and said, ‘the fact it also offered us another hospital when we had very little regard for the hospital we were in, was an added incentive to say yes.’ He explained that when they were offered the trial it was important to weigh the risks of the transfer itself against potential benefit at a new centre:
The chances were that he might not make it to [the second hospital], but if he wasn’t going to make it to [the second hospital], probably he wouldn’t have made it staying in [the first hospital] either.
Wilf – TOBY
Once they decided to take part in TOBY things moved very quickly. Wilf said that they were ‘on a clock as soon as they mentioned it. They then said they had to get moving. ’ Both parents felt that despite the timescales the move was managed well. Wilf felt that it was ‘relatively rapid but it didn’t seem inappropriately rapid’ and Laura was appreciative of the way her leave-taking of Archie was managed. Archie was briefly brought to her bedside before transfer and Laura said that ‘they were very respectful in terms of bringing his cot to me and giving me time to say goodbye’. She was emotional during the interview when she recalled this time.
[T]he last thing you want to do is see your baby leave you, especially if you’ve been separated for hours. Then the thought of someone taking them away again is a difficult decision to make. But you think what might be best for your child in the long term . . . They were very reassuring . . . [T]hey came and he said goodbye, or I said goodbye to him, with the knowledge that he might not survive the journey.
Laura – TOBY
She was devastated at not being able to go with him and was well aware that in permitting him to be transferred she risked possibly missing his last hours and not being with him when he died. Laura said that once Wilf left to follow the ambulance to the cooling centre she was ‘left pretty much on my own for hours and hours’ with limited communication.
They put me in a room on my own, but they just, they kept me in the bed that I’d had surgery in, and hadn’t cleaned me and . . . and they just kind of left me.
Laura – TOBY
This experience of women who are too sick to be moved, or who cannot be moved to a centre which does not have obstetrics facilities, is not exclusive to TOBY, but the decision to join the trial did have some impact on this situation. As she waited on her own, Laura did not know which arm of the trial Archie was allocated to, and was left with this uncertainty unresolved. She did not find out that he was not being cooled until she was also transferred 6 hours later.
Robert’s partner was in a similar position. We do not have her own account in BRACELET as only Robert was interviewed (see Box 6 ), but we know from him that she remained in the original hospital when her daughter was transferred and that Robert called her so that they could make a shared decision about whether or not to enrol Adele into TOBY. Once they had made their decision, she had no further information about the allocation as Robert had limited means to call her. He decided to leave the NICU to go and see his wife in the original hospital but shortly after he arrived they received a call to say that Adele had deteriorated. A member of staff was driving them between hospitals when they received another call to say that Adele had died. She died at 23 hours without Robert’s wife seeing her again.
Random allocation
The time of random allocation to either the intervention or the control arm of a trial marks a potentially influential point in the course of a baby’s care (if the allocation is ‘open i.e. the parents know which group the baby is allocated to. For trials where this is not known, for example INIS, the allocation may be less influential’). In the UK ECMO trial (see Chapter 5 ), allocation to the control arm was often a source of major disappointment and sometimes loss of hope for parents. 54 In a trial such as TOBY, which similarly offers access to a potentially helpful therapy, allocation could play an important part in the parental experience.
Although five interviews were carried out with parents involved in TOBY, only one of the interviews was with parents whose baby was allocated to be cooled. Hesther and Stuart’s son, Joel, was allocated to the cooling group after a rapid delivery by emergency CS. Hesther struggled to take on board information at the time and said that she did not develop a preference for him being cooled and was not aware that it might not happen. She said, ‘I just remember him talking about this cooling, and he’d got to go and make a phone call to see if he could do it.’ Stuart said that he knew that it was possible that Joel might not be cooled but that he accepted this as something they could do little about. He said, ‘That’s the decision they make, isn’t it? There’s nothing we could have done . . . it’s been taken out of my hands.’ The allocation process was very quick with the doctor taking ‘no longer than five minutes’ and ‘they took him away and put him on TOBY’.
Joel was in fact cooled for only a short time and Hesther could not remember details of this period. Stuart described what it was like to see his baby being cooled. He explained that Joel’s temperature continued to drop.
Very strange, seeing that we were hot, and then putting your hand into somebody with cold, but knowing that is helping him. It’s a strange thing. Knowing that, you know, this cold that’s bringing the temperature down is helping him. It’s very strange. And, yeah, I can’t explain. It’s a funny thing to experience, you know, seeing your child in this – and then all of a sudden cooling down to what temperature they’re trying to get. I don’t know what they’re trying to get down to. But it was really cold. And then once he went really cold, they put blanket on trying get his temperature back up.
Stuart – TOBY
Cooling was discontinued, Joel was re-warmed and care was withdrawn shortly afterwards.
In Wilf’s interview he explained that he felt that the offer of the trial was persuasive almost irrespective of allocation: ‘Whether he was cooled or not cooled, being on the trial would almost guarantee him the best possible care in a hospital that was more geared up for these sorts of things’. Archie was allocated to the control arm of the trial and Wilf described his reaction as: ‘Disappointment, yes. Not massive disappointment. It was, well it was a 50 : 50 chance and he didn’t get it, but he’s still getting the best care.’ He still felt that the trial brought with it many benefits, especially in relation to the policy of moving control babies to cooling centres so that all babies receive the same standard of care. He described sitting by Archie’s incubator through the night, talking to a registrar and to the neonatal nurses, feeling involved and cared for himself. Laura arrived the next morning and both parents felt that they were treated very well and provided for with great sensitivity in the cooling centre. They spoke very positively about the staff, the facilities, the care and the management of their son’s decline and death and felt that their involvement in TOBY had positively shaped such difficult experiences. They articulated very clearly that they had benefited in a number of ways from the trial. The main benefit was the transfer away from a place which was not associated with the disastrous delivery and their sense of neglect, to a place that they felt was better for their baby. They saw care at the first hospital as the origin of their problems and the trial, whatever the allocation, as part of an attempted solution.
Amanda’s experience of the allocation process was very different. The story of Amanda’s birth is given in Box 4 . She explained how she had focused only on the positive sides of cooling when the possibility was put to her, saying:
There was no thoughts about, you know, the pros and the cons of it. It was just all pro. It was [Hospital X], and she was possibly going to be cooled, and it was going to help . . . In my mind, it was all pro.
Amanda – TOBY
Amanda said that she was aware that there was a possibility that Simone would not be cooled, but that she was ‘so hopeful that she would be cooled, that it nearly wasn’t an option.’ Amanda broke down in the interview when she explained that Simone was in fact allocated not to be cooled and how hard that was to cope with, at the time and still, 8 years later.
Because she was in . . . the part of the trial where she wasn’t cooled, I was hugely disappointed, you know, but.. you know, we’d . . . offered our baby for the trial, and yet she wasn’t cooled (begins to cry).
Amanda – TOBY
Amanda felt that she did not clearly understand the process by which this decision was made and asked for information about the trial and the allocation mechanism in the interview. Simone died two days later, something that Amanda was not prepared for. She said:
I didn’t think she would die. It wasn’t, wasn’t something that happens, you know? In 2004, babies don’t die because of lack of oxygen?
Amanda – TOBY
Prognosis
As parents described how events unfolded, another layer in their experiences became apparent. Having described their initial enthusiasm for cooling as a source of hope, they went on to explain how they came to see the trial and the intervention as the extent of the damage their baby had undergone became clear.
Hannah and Ryan, whose daughter was not cooled, described a similar sense of comfort with regard to the trial, which also seemed to be linked to information they received during Eleanor’s care about the extent of her injuries. Hannah said:
When they did the CAT scan, they said, ‘oh, you know, she is severely brain damaged, and we didn’t realise that at the time’. . . And they said, ‘even if she’d had the technique done, we don’t think the outcome would have been what we initially thought it could have been.’
Hannah – TOBY
Based on this information Hannah and Ryan agreed to withdraw care, and revised their view of the possible role of the cooling in her care, and for the future that they had anticipated for her ( Box 8 provides further details).
An investigation into Laura and Wilf’s case was carried out after Archie died. A specialist report was written, which detailed his birth and the implications of his injuries (see Chapter 9 for further discussion of parental reactions to the trial later in their bereavement). With this new knowledge came a reassessment of the role that cooling may have played for him. Wilf said that although it had not been clear at the time, they now know that: ‘He was well beyond anybody’s help by the time he was born’. Laura added:
He was deprived of oxygen for such a long period of time that the cooling treatment, if it had happened, probably wouldn’t have made any difference whatsoever to the final outcome. Definitely! So the fact that he wasn’t cooled doesn’t really come into it.
Laura – TOBY
Amanda came to the conclusion herself that cooling would not have helped. Although this is a similar approach to that of Laura and Wilf, and Hannah and Ryan, it is subtly different. They said that they were comfortable with the trial and the fact that their baby was not cooled, as they felt that cooling would not have made a difference. In Amanda’s case, however, there was a feeling that this is what Amanda needed to think. She said, ‘I felt she was so bad that cooling wouldn’t help, and that’s how I get by. ’ She held her hand up in the interview in a ‘halt’ gesture as she was making this statement and added, ‘Don’t correct me if I am wrong because this is how I cope. ’
Hannah and Ryan were involved in the pilot study for TOBY in 1998 and were asked to consider cooling for their daughter Eleanor at a time when very few babies had been cooled. In Chapter 6 we described Hannah’s initial experiences of a forceps delivery followed by a post partum haemorrhage some hours later. Eleanor had been born in a poor condition and took a very long time to cry. Hannah described watching the minutes tick by, waiting to hear her. From the time taken to eventually resuscitate her, Hannah and Ryan were in no doubt that they were fighting to save a baby who would have some disabilities but they also felt that there was hope. Hannah said:
[She was a] chubby baby, healthy, you know, so the only thing wrong with her was a lack of oxygen in the brain, so if they could correct that or help that bit, you know, there’s nothing else that [was wrong] . . . at that time I just kept thinking, ‘Well the only thing that’s wrong with her is just the brain, you know, so if something can be done with just that, everything else is fine.’
The pilot study for the trial offered access to a technique that Hannah hoped would mitigate the effects of Eleanor’s birth. (Note that as the study was prior to the main TOBY trial, many of the documents were different and there was no randomisation process.) Hannah felt that with cooling, disabilities might be mild.
She’d been quite some time without oxygen anyway. They said, ‘oh, you know, you could be looking at, you know, a child with severe cerebral palsy, or just minor learning difficulties. At this stage, we don’t know at what degree or how much brain damage has, has been occurred.’ So I’m thinking . . . if you can eliminate the worst, and hopefully end up with a child with just minor learning difficulties then that’s fine. You know, so that’s why, in my mind, I was thinking, well, yeah, let’s just do it . . . [E]ven if she just got mild brain damage, if they do this then she won’t hardly have anything. You know, anybody wouldn’t even notice, you know. She might just be a little bit slow at reading or . . . You know, I’m thinking it might be something so mild that if she has this it’s going to be hardly detected.
The trial also offered transfer away from an extremely difficult environment that the parents associated with mismanagement and harm. Hannah said, ‘the damage was done there’ and Ryan said that they were ‘elated’ at the prospect of a move. An important element in their thinking was that Eleanor would be transferred to a hospital with a good reputation, one which they knew from television programmes and they were sure that everything would be well managed. Hannah said, ‘They are like leaders in the field and . . . I just thought, ‘Well, you know, she couldn’t be in anybody better’s hands in the whole country, in the whole world really. ’ This tied research to reputation and gave them confidence.
The transfer process was protracted, as there were delays in the ambulance reaching their hospital. The ambulance team got lost, and the possibility of a police escort for the return journey was raised but did not materialise. Eleanor was transferred first and Hannah followed after receiving a blood transfusion. Eleanor was in a poor condition on arrival and later Hannah and Ryan met with her consultant.
A consultant called us in, wasn’t it, in the afternoon? And he sort of said, ‘oh, we’ve assessed her and, to be honest, she didn’t really get here . . . quick enough’. . . And he said, ‘due to the amount of brain damage that she’s had, it’s too late to start that technique now.’ So that was a really big sort of disappointment, wasn’t it? Because, you know, we’ve got her here, and then it was just a bit too late.
Hannah went on to explain how she saw the decision not to proceed to cooling when they had had high hopes for the benefits it might have had for Eleanor. If it had just been a matter of speed Hannah said, ‘I would have even hired a private helicopter, you know. Yeah, I think you would cut your arm off, limbs off, anything you could do to physically make that happen. ’ She was not entirely convinced, however, that they really were timed out. She wondered whether it was kinder than saying to them that it was not appropriate to cool Eleanor given the information that was emerging about their daughter’s condition and the implications of this for her future.
I think they know that . . . we’re going there on a hope that something’s going to be done, and it’s very sad for it not to be done, not to be carried out . . . [I]n the back of your mind, you think, oh, I wonder if it was done, if that would have made any – you know, you get those questions, don’t you? But I think at the end of the day . . . she was a full-term baby with minor brain [damage but] . . . she’d been without oxygen at birth for 10 minutes, 15 minutes! . . . I think it wasn’t until they’d got her to the hospital, and then suddenly realised, well, actually, you know, she has, she’s fitted. She’s not showing good signs. She’s not, you know, acting like a baby with just minor brain damage. I think they had a few indications and possibly they maybe even decided at that point, it’s not even worth going down the road . . . I think they may have just said, ‘oh, just . . . we’ll say to the parents, ‘she just got here a little bit too late to do the treatment.’ But we could do the treatment [but] it’s not going to make any difference’. . . They never really sort of told us that, because I think that might have been too cruel at the time.
Although Ryan was also initially enthusiastic about the possibility of cooling, he expressed ambivalence in the interview about the decision that they had made in the light of the information that they were subsequently given about the likelihood of disability.
I think the hardest decision is what you actually want to get out of the treatment, isn’t it? And what is going to be achieved by the treatment . . . If it’s going to make someone who’s at death’s door 40%, 50% better, is that going to be good enough for a, a good life? And would you want that? I don’t know . . . . Because the doctors said there was no hope: that she won’t talk, she won’t know you, she won’t, won’t do anything. She’ll live, but she’ll never walk, never talk . . . at what point do you change your mind?’
He speculated on what it would have been like if cooling had gone ahead and Eleanor had survived:
Say we’d got there earlier and they done the technique . . . would I want that for my daughter, just to be able to sit in a bed for the rest of her life, just to be able to talk? To make me feel happy. Oh God.
Did you – did you do any of that weighing up when you were deciding to transfer her to [the second hospital]?
No . . . None of them come into your mind. Not one.
Hannah and Ryan achieved a sense of peace with regard to the trial and this seemed in part to be linked to information they received about the extent of Eleanor’s injuries, which caused them to revise their view of the possible role of the cooling in her care, and the future that they had anticipated for her. Hannah said that the focus then switched to managing Eleanor’s care until she died. Although Eleanor was not cooled, Hannah and Ryan felt that they had all benefited from taking part in the research and were pleased to have done so. Partly they sincerely hoped that other babies would benefit but also they felt that they had gained by being transferred away from the original hospital and into a place that was calm and where they felt that they were looked after very well.
Trials in close proximity to death
Although those involved in TOBY largely experienced major changes as a result of enrolment in the trial, for two sets of parents their involvement in INIS was very short and involved little material change, but the trial was kept at the fore as their babies died so close to enrolment. Stefanie and David’s son Callum, and Fiona and Keith’s son Timothy, both one of twins, were both enrolled in INIS only a few hours before their deaths, and the trial became a prominent feature in their experiences of their final hours.
Stefanie had been aware of INIS before they agreed to enrol Callum, but was not sure what it was. There was another baby in the NICU who was enrolled in the trial:
I was always aware of this wee sticker. You know, we’d never, obviously had no reason to know what it was about. I think it said, ‘I am an INIS baby’. . . [It] always stuck in my mind, you know, always when I passed – ‘Wonder what that means?’ you know, and little did I know, unfortunately I would know what it meant.
Stefanie – INIS
Stefanie said that once Callum was enrolled in INIS he quickly received the first dose of medication (immunoglobulin or placebo) for the trial:
I remember . . . them doing it. We were there you know, thinking ‘Right, hopefully this is okay to have done this.’ You know. Very frightening thing when you’re not – everything that you were learning in that place on a day-to-day basis was all brand new to you.
Stefanie – INIS
David and Stefanie were already bereaved when they were asked to make a decision about a trial for their (then) surviving twin. David found it frustrating to think that a placebo might have been used when their son was so sick. Both parents felt that the offer of the trial at such a late stage had raised false hopes for them and Stefanie questioned whether they should have been asked at all.
It was so hazy that night, it was within the last few hours I think wasn’t it, of his life? . . . You know, but he didn’t live for very long after, it was only a matter of hours wasn’t it, after that decision was made, I think. You know certainly was late in the day anyway . . . [I]t seemed like as if it was a last resort thing.
Definitely late in the day.
You know, telling somebody literally ‘Your son really isn’t going to make it here, you know but this isn’t going to harm him in any way, and it’s a trial and it’s a good thing.’ And you understood where they were coming from that way, but . . . definitely you know, late in the day . . . We’d only [just] buried you know a wee child, why would they think [to] give [us] something else to think of? . . . I don’t know what the reason was.
Stefanie felt that they should either have been asked about the trial at an earlier stage or not at all. It had affected their last few hours with their son and had had an ongoing effect for her as she was left with questions about the impact and consequences of the trial.
Sounds strange, but I’ve always waited for my letter in the post or whatever, to tell me, you know, that the trial had finished and this was the outcome of the trial.*
Stefanie – INIS
(*This will be discussed further in Chapter 9 .)
Fiona and Keith’s twin boys Timothy and Kelvin were born at 32 weeks’ gestation after almost 6 weeks of hospitalisation for Fiona. At 27 weeks she lost amniotic fluid, and while she was in hospital she continued to lose fluid and to bleed. The twins were born by emergency CS at 08.30 in the morning after Fiona went into spontaneous labour. Initially both babies were stable and the subject of INIS was raised for both babies in this early period.
Keith argued that it is important to trust doctors and presented the trial very positively, arguing that because of ‘the Hippocratic do no harm thing they’re not going to suggest anything that’s a bad idea.’ Fiona said that in other circumstances she would have ‘interrogated them for about half an hour’ but when asked if she had asked any questions she said, ‘No. I didn’t. I don’t think we did ask any questions’. Keith felt that the trial ‘created no false hope at the time. You didn’t sort of think ‘‘Well here’s the miracle cure. The kids are going to be fine’’.’
Fiona added:
There is no promise that it was believed that the drug was going to help. If someone even suggested that they believed it would, then you’d be quite upset that you might get a placebo. But on the basis that the trial was sort of, so, I suppose experimental in that stage, you know, you accepted it.
Fiona – INIS
By the afternoon, concerns were being raised about Timothy and the decision was made to move him to another hospital 40 miles away. By early evening a transfer team arrived to move Timothy, but the decision was made that he was not fit for transfer. He deteriorated further and care was withdrawn that evening, 12 hours after birth. Keith said:
I watched them remove all the equipment, they dressed him in his little baby suit and you know I got to hold him and then Fiona did later.
Keith – INIS
As Fiona and Keith were going through the process of withdrawal of care for Timothy, his brother Kelvin deteriorated. The transfer team that had arrived for Timothy stabilised Kelvin, and he was then transferred in lieu of Timothy within 3 hours. The parents described the pace of these events.
You were in this bizarre situation that one minute you’ve lost a child and the next minute your other child is in a transportable incubator.
Yeah, disappearing.
You got to say goodbye and that was it, he was gone.
It’s a strange explosion of motherhood.
Keith summarised their situation:
So you got Fiona who obviously again with the Caesarean was sort of stuck in hospital, couldn’t go anywhere, you got our child being taken to [the second hospital] 40 [miles] away, and we obviously had Timothy in a room and passed away.
Keith – INIS
Like Stefanie and David, Fiona and Keith left a deceased twin to go to a surviving twin. Keith reflected on this:
This is always your challenge . . . and whenever you think back to it is ‘Did we ever do enough for Timothy?’ Not in a ‘Could we have done more to help him live?’ but kind of got rejected or neglected or those sort of words, and it’s like well should we have spent longer with him, or should we have done this or that?
Keith – INIS
When they reflected on their involvement in INIS at this time, like Stefanie and David they felt that it had not been raised at the best time for them. Fiona commented on how she had not been able to consider her choice with the care she would usually give to such decisions. ‘We were in such a shaky, it was such a surreal situation . . . you’re not in a frame of mind, however calm, rational you think you are, to actually make a rational decision about it.’
Although they did not feel that it had raised hope, it did not feel appropriate to them that they had had to consider the trial at this point. Keith was not even sure that it was a decision that he could be meaningfully involved in and characterised his view as:
If you think it’s going to help. You’re the expert – do it! In fact, don’t even ask me the question, because I can’t – who am I to make a decision? Other than to give you the comfort of me saying ‘Yes.’ Fiona, if you’d asked her the question 2 days earlier probably would have gone ‘Don’t like the idea but okay, maybe.’ Now would have gone ‘Yes, yes, yes.’
Keith – INIS
Like Stefanie and David, they felt that it would have been better for them if the possibility of the trial had been mentioned to them antenatally. With Fiona’s long period of hospitalisation Keith felt that this would have made them ‘prime candidates’ for such a discussion. The trial would not have been introduced in ‘crisis mode, it’s not last minute, and still the decision’s last minute, but the knowledge has already happened . . . it’s always going to be a last minute decision, but the knowledge can be shared, introduced earlier, in a way that’s, you know. And be very clear, it is a trial, you know, it isn’t a cure.’ Fiona expanded on Keith’s view:
The notion that clinical trials go on in hospitals shouldn’t be news to anybody . . . Someone like myself who’d been in for six and a half weeks – they wouldn’t let me leave the ward on my own [and] I was forbidden to put foot outside the hospital building because they said I was very high risk . . . having, you know, actually having someone come round breaks up the boredom.
Fiona – INIS
For them their experience of the trial was not related to the delivery of the trial intervention itself but was an integral part of a time of dramatic shifts: it could not be separated from the final hours of their son’s life, which included both the anticipation of something that might help and shortly afterwards withdrawal of care.
Trials in the background
In comparison with TOBY, the four other core trials, INIS, PROGRAMS, BOOST-II UK and ExPN, generally involved many fewer obvious interventions, and mostly these did not materially change parental experiences. In two of the trials, the interventions were not very different from the care the baby was already receiving; INIS involved delivering the intervention or placebo via the intravenous access that was already in place; ExPN involved a supplement added to milk already being given. In both trials, the interventions were given for short periods, two doses over 2 days in INIS and supplementation for 5–7 days in ExPN. PROGRAMS involved one injection each day over 5 days, and only for those in the intervention arm. The intervention in BOOST-II UK was potentially more obvious for parents as the pulse oximeter attached to the baby, which maintained that oxygen within the allocated range was visible for the duration of the period of ventilatory support, or until the baby reached a postmenstrual age of 36 weeks. When parents were aware of trial-related activity, however, it was often a background rather than a foreground feature in their experiences.
In making their decisions about enrolment into a trial, the parents often expressed their hopes that the trial might help. The concept of help was often unspecific, but in some instances the aim was linked to the need to tackle a particular problem such as an escalating infection in INIS. The urgency of the situation was clear to parents who, as described in the previous chapter, often gave rapid decisions to facilitate initiation of treatment in a trial. In such circumstances it might be expected that parents would be very aware of any trial-related activity, but only a small number described any sense of the trial being implemented after their decision to proceed. When it featured in their accounts post consent it was not a particularly prominent feature in relation to their wider experiences, and when the topic of a trial came to the fore, parents said that this was when they themselves had reflected during that time on the decision that they had taken.
For babies enrolled in BOOST-II UK there were visual reminders of the trial in the pulse oximeters attached to their baby, and a card kept in each incubator to identify BOOST-II UK babies to the ophthalmology team. Chloe (whose views are presented under Trials disappeared below) had kept the trial cot card in her son’s memory box. She said, ‘they’d wrote BOOST on it’. Danielle had given her consent to take part in BOOST-II UK antenatally, but by the time her son Todd was born she had forgotten all about the trial. She asked a member of staff about the equipment, asking ‘How come that’s so and so?’ Although all babies receiving supplemental oxygen would be nursed on oximeters, in many centres these would have incorporated into the monitoring system rather than the stand-alone monitors used in the trial. When Danielle was told what the pulse oximeters were she said, ‘Oh, that’s what the trial is!’
The pulse oximeters were essential equipment in the trial and were central elements in data collection. In the interview with Lesley and Stan there was some sense of how the parents saw the day-to-day management of the trial being carried out around their son Lloyd. Notably, the process they described did not seem to change anything for Lloyd.
It was just a box.
On top of his incubator.
Was it on top?
Yeah.
I know it was around his incubator.
It was on top.
And did you know what it did? Did they give you an explanation for what it was doing?
I can’t remember.
‘Cos it was something to do with oxygen.
Yeah. I know they just came in every day and they did something on it, signed the form and then like went.
They described a process of monitoring and data collection that seemed to be incidental to the care Lloyd received and in which they had no involvement themselves. Similarly Ethan, whose son James was also enrolled in BOOST-II UK, said that for him the trial was ‘just an aside to what happened. It just happened to be going on at the same time’.
In two of the interviews with parents involved in the ExPN feeding study, the trial was presented as a very simple thing that they had agreed to, a very small part of their experience. Pete, like Lesley and Stan for BOOST-II UK felt that for ExPN ‘they were just collecting information; that’s the way it came across to me’. As the interview progressed, Pete and Caitlin discussed the fact that something was being added to Caitlin’s milk and tried to work out between them the mechanism for that happening and how it would be given to their babies.
But how did you give the baby the milk? I can’t remember that. I thought it was on a drip, you know, drip form so unless it had gone in through the drip.
Was it dripping into the . . . the syringe?
That’s what I mean, that, but I don’t know.
Liza, whose daughter took part in the same trial, remembered more of the details but these related to her own role of expressing and storing her milk for the trial rather than what would happen for her baby.
You know when you express your milk, they put them into these like tubs and they go into these drawers . . . . and basically they have the names on for each baby and that, like the top ones were the ones who were taking part in the trial and the bottom ones who weren’t. So they like separated them. Yeah, I think if you ask them they would have, you know, gone into more detail, but I just wanted what was best really.
Liza – ExPN
Some of the parents described having fleeting thoughts of the trial in this period and some of the concerns that this raised for them. Adam, who was flown back from military service when his partner Milly went into preterm labour, had made a decision about BOOST-II UK in the fervent hope that it would help his one surviving twin. He described how he gradually developed concerns:
The only thing I had in my head at the time was I thought this could help, and I sort of blindly agreed to it not really knowing the full – the full facts behind it. And then later on I had a – I caught myself thinking a couple of times, well what if it was detrimental?
Adam – BOOST-II UK
Similarly, when Beverley’s daughter Ruth went into respiratory failure this raised questions for her about the possible implications of having taken part in BOOST-II UK I was saying,’ Is it because of which oxygen group she’s in?’ and they said that wouldn’t have made any difference. Julia, whose son was enrolled in INIS, said that she did worry at first, commenting ‘I did ponder over it for a day or two afterwards. I come back to it every now and then – was that the right thing to do?’ They were not aware of the trial intervention taking place, and INIS faded from their day-to-day experience. They did say, however, that they would have been interested in talking to someone about the trial at some point but that they would not have initiated this themselves. They would have been very pleased for a doctor to have raised the subject with them. Julia articulated very clearly how the trial can be both a minor part of their experience, but is also an important aspect of what was happening to them. She said:
Even though it’s a small part of everything we were experiencing at the time, we still took time over whether to be part of it or not. And it still mattered to us that he was part of that trial.
Julia – INIS
Trials disappeared
The most common experiences described by parents accounting for the period after deciding to take part in a trial and up to their baby’s death, was that the trial disappeared from their experiences. This was the case even for parents who, like Nat and Robert, had invested in a trial with a significant degree of hope as a means of helping their babies. The mechanisms by which the trials disappeared from view were several:
-
the trial interventions were not readily apparent
-
the trial was subsumed into other aspects of care
-
the trial was seen as irrelevant to the death of the baby.
Trial not readily apparent
In trials such as TOBY, the intervention itself is readily apparent to parents and there is no possibility of carrying out a blinded or placebo-controlled trial. In such a trial, the allocation is therefore quite explicit and it is likely that clinicians would engage in further conversation with parents to inform them of the outcome of the randomisation process, and, if allocated to an unconcealed intervention, to explain what would happen next. Over the 72 hours of cooling for those in the intervention group, further explanations may be given to parents, and questions may arise. The trial in fact included in its protocol a specific strategy of continuous consent by which the initial information given at enrolment should be revisited and further explanations offered. 64 In a placebo-controlled trial such as INIS, or a trial such as ExPN, in which only the assessor was blinded (see Chapter 5 ), there is not necessarily a follow-on discussion with parents about allocation post randomisation, as there is no news to pass on about which treatment a baby would be having. In INIS the trial intervention, whether active or placebo, would be administered via the intravenous systems already in place and the parental interviews made it clear that often the parents were unaware of it being delivered. This could be because of the difficult circumstances that prevailed around the time of decision-making. For Alice, her sense of the trial was lost in the context of everything that was happening in that initial stressful time:
Almost as soon as he went on it, I’d forgotten he was on it . . . As soon as we’d have made that decision it was out of my mind. It was like, you’re doing the best – whether that included some trial or not; I wasn’t aware really.
Alice – INIS
For Amy and Chris, it had been difficult to engage with the details of the INIS trial as they made their decision to enrol their son Alby, and they found that subsequently the details slipped from their memory very quickly as the exchange below shows:
I didn’t really know what it was for . . . I actually don’t even remember or even know what the actual drug was trialling out.
Did you know that at the time and you’ve forgotten it since, or do you think you didn’t know it at the time?
D’you know, I don’t know, I don’t, I don’t know. I’m sure I was told but I don’t even think . . . .
It sounds strange right now because –
Yeah, I don’t even think . . . I took it in what it was about.
No.
It appeared that, as important as the decision to join the trial had seemed to be, and however much hope was pinned to the trial, if parents received insufficient details to understand the workings of the trial without other prompts, then it was often not at the front of their mind. Few parents recalled any subsequent explanations from staff to inform or remind them about the trial. None of them described being told that a trial intervention had been delivered, or that the course of intervention was complete, and none described being invited to ask questions about how the trial was being managed at a later date.
Jana, who agreed to enrol all three of her triplet sons into INIS, felt that she and her partner had made a quick decision about the trial, and realising that she did not have a full appreciation of the trial, she would have welcomed the opportunity to speak to someone again about this. She was curious but also felt that the doctors around her ‘had so much going on’ that she didn’t feel that she could raise the subject. She did say, however, that ‘if there was a person from the study that would you know, offer this possibility [then] maybe we have, we would have taken it.’ This sense of ongoing curiosity but with no further outlet was also present in the interview with Fiona and Keith.
One of the surprises in the BRACELET interviews related to Robert, whose daughter was enrolled in TOBY in one of the international collaborating centres. He was asked about the trial after she was moved to a specialist centre. He was asked what happened after he gave his decision and his account was more like that of parents in a placebo-controlled trial than one in which there was an overt intervention.
What then happened in relation to the research once you’d said, ‘Yes we’ll take part?’
Absolutely no idea. I don’t even know if it happened. I have no idea whether she was chilled, whether she was too far gone in terms of her deterioration . . . We were given a telephone number if we wanted to speak to somebody about it which was I think through, through the UK but because the UK was an hour behind where Europe was and this was in the morning I don’t think it was open so I couldn’t speak to anybody because the timing was wrong and I have no idea.
As his daughter died without either parent being present, the trial intervention was not apparent to them. In a trial such as TOBY not knowing the allocation, or having any feedback about whether a baby was cooled or not, is probably a relatively rare event. Robert and his wife were left without this information at the time and without any subsequent clarification. Although this left Robert with some questions, a number of non-TOBY parents, including Marion and Doug, Karen and Tony, Sara and Gareth, Caitlin and Pete, and Diane, said that they had no further discussions with staff about the trial after they had given their consent.
For Tony and Liza, the first prompt that they had had to think about the trial again was the letter inviting them to take part in BRACELET. Liza was initially puzzled and ‘I was a bit shocked’ at receiving the invitation but described her reaction on reading it. She said, ‘now I remember, yeah, I do remember it! It’s all coming back!’ For Nat the first reminder came with a letter relating to the results of INIS. He said, ‘until we got a letter – sort of saying about the outcome of the trial. I hadn’t really thought about it from when he passed away to, to that point.’
Trial subsumed
A trial could seem important at first but in the context of long-term care could be left in the past. In four of the interviews, parents had made a decision about trial participation early in their baby’s protracted stay in NIC. In contrast, with parents such as Hesther and Stuart (TOBY), or Stefanie and David (INIS), whose trial-related experiences were part of fast-moving events in a short and intense period, these accounts of involvement in a trial are considered over time.
Shirley, whose initial trauma and reaction to seeing her daughter for the first time, unexpectedly alive, was described in Chapter 6 . Beth was born at 24 weeks and was a patient in the NICU for 5 months. The ExPN feeding study was raised at the beginning of this period and Shirley and Warren agreed to enrol her into the trial. Although ExPN was set up to address the issue of poor nutrition for extremely preterm babies, with the specific hope of improving longer-term outcomes, Shirley saw it as relating to day-to-day nutrition. She said that there were days when it was simply not possible to feed Beth and that getting medication into her on a daily basis was more important. She had accepted that feeding difficulties were a normal part of caring for extremely preterm babies and was not particularly looking for a solution to this when the trial was discussed. Her model of the trial was that it was looking to see whether extra nutrition would help, but that it was an extra, non-essential aspect of her care. With no particular hopes pinned on the trial, and a general feeling that it might help, her sense of being involved in the trial was hazy. She said, ‘I can’t remember how long she was on the study for and at what point it stopped. ’ Shirley had a keen sense of her daughter’s suffering and explained that she came to question the care that she was receiving. She did not see ExPN as an intervention that had the potential to save her daughter as it related to a feeding regimen, but for her wider care she felt that ‘maybe a lot of the treatment she was given she shouldn’t have been.’
I remember writing in my diary about the roller coaster. One day I’d be fine about it but the next day I’d think, you know ‘This isn’t right . . .’ just didn’t feel right. But then we’d have episodes where’d she’s slightly improve, you know, and I’d think ‘Oh this is just like winning the lottery, it’s fantastic,’ you know, and we’d all be on a high, and then within hours we’d be, you know, back down again. So I think after about four, maybe five months we were getting to the point where we couldn’t see, really see any light at the end of the tunnel, you know, and it was at that point I started really questioning what was happening.
Shirley – ExPN
For Anita and Sean, INIS similarly became a small part of their experiences, as their daughter was in the NICU for 4 months. Anita said that it had been an easy decision to take up the offer of the trial because Josephine had ‘such a serious infection’ and ‘was just so poorly’. Sean described them as ‘clutching at straws’. Once they agreed that Josephine, one of twin girls, could take part they did not discuss the trial with anyone again. Josephine recovered from the infection but Anita said that ‘she had so many other problems and so many other things later on . . . they overshadowed INIS.’
She had hydrocephalus and . . . she’d had to have a shunt put in in the January . . . They were both born..[in] November . . . I think it was . . . [in] January that she had a shunt done, wasn’t it? So she was still only, like, a tiny baby. You know, she wasn’t, she wasn’t even five pound at that stage . . . She’d only just been off the ventilator, just over two weeks . . . Because she used to go round and round in circles, with herself on the ventilator, off the ventilator, back on it again, and then she’d stay on it, cling to it for dear life, and she’d end up on steroids to wean her off it again. So she had so much going on, and because, because she’d had the, the bleed on her brain, which had led to the hydrocephalus, she was so severely brain-damaged anyway. Every, all that, overshadowed how she’d been when she’d first been born, didn’t it?
Anita – INIS
And you said that in this time afterwards, the INIS trial was sort of not really something that . . . popped up again.
Not at all.
Did anybody ever talk to you about it again?
I don’t actually think they did.
No.
Over the course of Josephine’s NICU stay, her condition changed so much and so often that the infection that triggered her involvement in INIS was no longer a significant concern. It had been dealt with and was in the past, and, by the time she died, INIS was entirely separated from the time and circumstances of her death. In the interview, however, Anita speculated on whether her involvement in INIS had got her through the initial infection:
We’ll never know whether it helped her or not. That’s something we’re never, ever gonna know. But . . . one way or another, you know, she got through that short period of her life when she was really seriously ill with the infection. And, you know, whether it helped . . . You know, if it did, good; if it didn’t, well, she got through it anyway.
Anita – INIS
Although Shirley questioned the appropriateness of having fought to save her daughter in the early days, this issue, and the potential for a trial to affect an initial course for a baby, did not arise in the interview with Anita and Sean.
For the other parents of babies receiving long-term care, there was a sense that the trial was just one detail in a long period during which other priorities were at the fore. Diane was asked to consider BOOST-II UK when her son Kirk was born at 24 weeks. She found it difficult to engage with the information she was given about the trial.
They just asked us if I would like to take part in it, and give us some information. I think I just agreed with it because – I don’t know – I wasn’t really paying much attention half the time.
Diane – BOOST-II UK
Kirk survived for 5 months and Diane focused in her interview on the time she spent with him. She had other children at home but feeling that they were well cared for by her family, she made Kirk her priority, spending as much time with him as she could, day and night.
I just seemed to cope with it. I was always at the hospital. I didn’t spend no time at home. I was just constantly at the hospital, but I don’t think my head was anywhere else apart from focusing on Kirk.
Diane – BOOST-II UK
By the time he died he was no longer receiving the trial intervention. The trial seemed to have been a peripheral detail in Diane’s experience, one which she said she did not think about again.
Rhona and Karl similarly presented the trial as something that they barely remembered. They had experienced a rollercoaster time, with multiple miscarriages, diagnosis of twins in a third pregnancy and then a risky antenatal intervention to manage twin–twin transfusion. It seemed significant that they completed the whole story up until their daughter Ava’s death at 10 weeks without referring to PROGRAMS. Rhona said that before the interview she was ‘trying to think’ about the trial. She said, ‘I honestly can’t remember that at all.’ During the interview she was able to recall details, including ‘her having a little tag at the end of the incubator’. For Karl, the difficulty in recall related to the position they were in at the time that they made their decision and he found it hard to pick out the trial from everything else that went on. He said, ‘It kinda blended into one, you know, one and the same thing really, because it, it had been such a sort of dramatic time.’ With the trial indistinct at the start of their experience, it was subsumed into other events by the time Ava died at 10 weeks old.
Box 9 describes Dawn’s account of decision-making for a trial that was rapidly subsumed into other experiences.
Dawn conceived twins naturally and at 16 weeks’ gestation she started to lose amniotic fluid from around one of the babies. Fluid loss continued and there was a high risk of miscarriage. She was offered selective feticide but rejected this option, saying ‘If I’m gonna miscarry, I’m gonna miscarry both . . . I’ll just see what happens, you know. Let nature take its course.’ A debilitating period followed in which it was hard for her to leave the house as she regularly bled and lost fluid. She was hospitalised for some of this time and her pregnancy ended at 26 weeks with a spontaneous labour.
Dawn described the impact of having her babies taken away to the NICU:
Your baby [is] taken away from you and then obviously it’s not your baby. It’s the nurses who are doing the cares, and there’s nothing you can do. So they weren’t my – they weren’t my babies, and it was harder to bond with Gemma it was . . . Because I did struggle. I did what I needed to do for her, but I did struggle. So what I mean is, them getting taken away, that – that spoilt – you know, I couldn’t get the . . .
Dawn struggled to complete her sentence but it was clear that in the period in the NICU it was very difficult for her to connect with her babies and their needs.
During her pregnancy she and her (now ex-) partner had experienced some relationship problems and she felt that she had little support. Decisions were left to her, and she found this burdensome. She said, ‘It might have looked to the hospital that I had support there, as in a partner and my mam. But . . . I felt so alone.’ When she was approached to consider enrolling her twins into BOOST-II UK she said that her partner ‘took a back step’ in the discussion and decision-making and she ‘wasn’t in the head space to really sign the form’.
I just didn’t really want to talk . . . I just signed the . . . At the end of the day, I wanted you, I wanted them hospitals to give the care Gemma and Mitchell wanted, whether it was the oxygen or was the medication given. Anything to make them to get home to us. So I would have signed anything at the time.
Dawn was very clear in her statement that the information about the trial was not of interest to her:
I didn’t even read the form. I flicked through that, and I’ve read that . . . I wasn’t thinking, to be honest with you. I just signed a bit of paper, say, ‘Oh, yes, I can do the trial,’ without . . . I know he did sit us down, and he did explain it to me as . . . Well, I didn’t know the difference. As long as they were getting oxygen, I don’t care which machine it was coming from.
Dawn was reluctant to engage with information about a trial and although management of oxygen featured in her story, and she described her surviving baby’s move to CPAP and her use of oxygen at home, when asked if she thought about the trial again she said, ‘No, I never really. Some things go out of your mind.’
When Mitchell was 3 days old Dawn took the decision to withdraw care. She said that she made the decision on her own.
Trial seemed irrelevant to the death of the baby
For most of the parents involved in BRACELET, their time in the NICU ended with the death of their baby. (Jana, Dawn, and Fiona and Keith still had surviving babies who continued to receive intensive care.) This part of their experiences was covered in varying details in the interviews with some parents, such as Jana, describing the process of death in careful detail, and others choosing not to revisit that experience. Parents took the lead in this area of the interview. As details of withdrawal of care were, to some extent, at the periphery of our topic of trials and bereavement, if they did not describe this experience they were not pushed to do so.
In describing the time of the baby’s death, the trial was not spontaneously referred to by the parents, suggesting that they did not couple the two events. This is not surprising as the trial had already faded from the experience of a lot of parents. Although some expressed some concerns that arose for them at a later state in their bereavement (see Chapter 9 ), this was mostly in the light of later information. A number of parents explicitly stated at other times in the interview that they saw no connections between their baby’s death and the trial. Some took the position that the trial intervention did not have the potential to save their baby. Marion and Doug said on a couple of occasions that nothing could have saved their daughter, Dee, that they felt that she was beyond help – Marion said, ‘there was nothing no doctors, consultant or anybody could have done. Not even prayers . . . ’ and Doug added ‘It doesn’t matter how far advanced they would have been medically, there was nothing they could actually do. ’ The sense of a baby being beyond help emerged as a clear theme in the data. Laura and Wilf, and Hannah and Ryan, came to this position because of post-mortem information and the testimony of experts, but others, such as Gareth, drew their own conclusions about the severity and impact of their baby’s condition:
Perhaps if it had been suddener or it had been a shocking, a shocking death, we might have been searching around for more potential causes. But the fact is, he got NEC because he was small and he died because he had NEC, and that’s, you know, it’s very, there’s a very linear relationship there between those things. And INIS to me sits aside from that and is not on the critical path of his lifespan essentially.
Gareth – INIS
Here the focus was on the impossibility of saving a moribund child, but there was also the possibility that parents might feel that a trial was implicated in their baby’s death. Danielle was keen to make clear that this was not her position and said before her interview started that she did not blame BOOST-II UK for her son Ted’s death. In bereavement, parents are often said to self-blame, and guilt over seemingly irrelevant actions or decisions can be debilitating in grief. Some of the parents did discuss feelings of guilt, especially in relation to early labour, and Jana spoke of her distress and self-blame when her son developed an unspecified infection after her breast milk was introduced for the first time. She said, ‘I thought oh maybe there was, I don’t know, a drop of my sweat getting into the milk or whatever, with some bacteria, and causing this but we don’t, we don’t know. ’ Jana described the time of Anton’s death very carefully and explained how she went back to see him afterwards:
I did it twice and again it was very important. It was, yes. I felt you know that as a parent you feel you know, your job is to protect your child, that’s your role. If you cannot do it, it’s – I felt like a failure. I felt like I wasn’t able – I felt so sorry, and I felt such a need to tell him that I’m so sorry.
Jana – INIS
We did not find that when parents described their baby’s death that they attached blame to the trial or linked it to their decision to participate. In Jana’s case, as for a number of other parents who took part in INIS, their decision to enrol their baby into a trial was taken in an attempt to fight the infection that eventually killed their child. In this situation the trial was constructed not as something to be concerned about or to connect with feelings of guilt, but was seen as part of an attempt to mobilise resources and to find a solution. Amy said, ‘It wasn’t that that what killed him . . . it didn’t make him any worse. It was just one of these things that’s gonna happen.’
One final area to consider was whether parents felt that had their baby received an active treatment, or one particular treatment, their baby might have been saved. There was some evidence to support this position, and this will be considered in Chapter 9 , but more common was the sense that no treatment, whether within or without a trial, could have changed the inexorable decline that the parents witnessed. Jill, who we saw in Chapter 6 , considered even at trial entry that the trial was unlikely to help her baby, said in relation to BOOST-II UK that ‘Nobody knows whether it’s better to have less oxygen or more oxygen. So I guess – I don’t think it would have affected James either way, for the worse or the better.’ Anita explained how she saw the use of placebo when she was making her decision about INIS, and since.
We knew that to get the right evidence, and to get the right information . . . that they’d have to use a placebo anyway. It was like, well, you know, whether she gets one or whether she gets the other, it doesn’t matter. You know, it’s just giving her a chance no matter what . . . And if she’d have had the placebo, it wouldn’t have bothered us. Even now, it wouldn’t because I don’t think it would have made any difference to her, at the end of the day.’
Anita – INIS
The feeling that the trial was immaterial to the outcome might be later tested for parents if a trial shows a clear benefit or a clear disadvantage, but at the time of their bereavement it seemed that for these parents the beyond help model held sway.
The trial recedes
For the majority of the parents, the trial was not something that stayed in the foreground of their experiences once they had made their decision to enrol their baby. In over half of the 30 interviews, the parental account was classified as the trial having disappeared (n = 17) and for some parents (n = 7) it was classified as something that they were aware of in the background but was not an element of their baby’s care that they particularly focused on again. In only a small group of parents (n = 6) did the trial play a major part of their experiences in the NICU, shaping what happened to their baby, and occupying their thoughts at the time ( Table 33 categorises the prominence of each trial per interview).
| Position | INIS | TOBY | PROGRAMS | BOOST-II UK | ExPN | Total interviews |
|---|---|---|---|---|---|---|
| Trial in the foreground | Stefanie and David | Laura and Wilf | 6 | |||
| Fiona and Keith | Hesther and Stuart | |||||
| Amanda | ||||||
| Hannah and Ryan | ||||||
| Trial in the background | Jill and Ethan | Caitlin and Pete | 7 | |||
| Milly and Adam | Liza and Wesley | |||||
| Danielle | ||||||
| Lesley and Stan | ||||||
| Beverley | ||||||
| Trial disappeared | Marion and Doug | Robert | Rhona and Karl | Chloe | Shirley and Warren | 17 |
| Sophie and Nat | Karen and Tony | |||||
| Alice and Ivan | Dawn | |||||
| Amy and Chris | Diane | |||||
| Anita and Sean | ||||||
| Jana | ||||||
| Justine and Francis | ||||||
| Dora | ||||||
| Sara and Gareth | ||||||
| Julia and Lewis | ||||||
| Total interviews | 30 |
The BRACELET interviews gave a sense of the range of experiences that existed while parents were in the NICU with their baby, from those for whom their involvement in a trial shaped what was happening to them on a daily basis, to those who entirely forgot that their baby was taking part in a trial. An important theme emerged in the analysis of the data, which cut across these different experiences and helps to account for this variety; the sense that a trial recedes, by whatever mechanism, from the foreground to the background, or from the background to having disappeared, gives some purchase on the sense that came through in the interviews of a fading out of links with a trial. This allows us to conceptualise parental experiences as ones where a trial does or does not recede and to consider for each, why this happens, and whether the place that a trial occupies in their experience suggests that any further input might be appropriate for future parents, for instance to clarify trial participation post consent or to offer additional support.
What we see in looking at the recession of a trial is first a period of interest and engagement around decision-making for a trial, at varying levels, as explored in Chapter 6 , from parents who made a quick choice to allow the doctor to do their job, to those who pinned their hopes to a trial and to the allocation or their baby. In looking closely at the parental experiences it seems that where the trial recedes, it does so at different points, for different reasons and to different degrees. We see, by the various mechanisms described above, that for some a trial recedes gradually and sometimes it is almost instantly forgotten ( Figure 21 ).
FIGURE 21.
Interest in a trial and subsequent recession.

We can see that parental views on the significance of a trial were varied and were the product of their different circumstances and individual responses. One of the interviews showed how responses to the trial were not necessarily fixed and could be mediated by changing circumstances. In Chloe’s account there was a sense of the trial rising and sinking repeatedly over time ( Box 10 ).
Chloe’s experience of deciding about BOOST-II UK for her twin sons was described in Chapter 6 .
Her account of her involvement with BOOST-II UK was categorised as fitting into the ‘trial disappeared’ category but her experience was in fact more complicated than this suggests. She had dealt with the discussion of the trial around the time of her sons’ birth very quickly and then set it aside. She explained that she had given the decision very little thought as she had n0t expected their participation in the trial would make much difference to their outcome. She said:
[A]s soon as he’d explained it and we’d agreed, and we’d signed off on it, I forgot about it then. I didn’t need to know any more.
At this point the trial disappeared but it re-emerged for her as an issue on the death of one of her sons, Lucas. When Lucas died, the trial moved back into the foreground for her, and she requested that his brother Drake should be withdrawn from the trial. She said that she did not think that there was any great risk but just felt that she had to do it to be sure.
[It was] out of fear more than anything else . . . We didn’t for a second blame the trial. We know that was nothing to do with it, but there is always something in the back of your mind ‘Well if he hadn’t been in that trial, would he have took that turn? Who knows.’ And just out of fear, we pulled his brother then out of the trial. But that’s about as much thought as we gave it really.
Chloe was asked what it was in particular that had made them feel anxious about the trial. She explained:
[It was] the only thing we had control over. We couldn’t stop them doing anything else, but we – it was up to us if he was in that trial. So I think just in a blind panic we were like ‘we want him off the trial, just the sooner the better’. And we never felt like judged or anything for that. They couldn’t have been nicer about it.
Chloe added that it was a chance that they simply felt they could not take. She said:
[E]ven at the time of pulling him out, I think part of me thought ‘Well what difference does it make? They’ve told us it doesn’t affect his health and it could, the results could help them out. Are we being selfish?’ But just that little millionth of a chance that it could have was enough to override the potential to help other people’s babies who weren’t even born yet.
The decision to withdraw was made at a difficult time, a time when they were also ‘thinking funeral arrangements’. Once they had made their decision, the trial then disappeared for her for a second time. She said:
Once we done it we kind of just forgot it really, we didn’t think about it again. Didn’t really speak about it again.
When Drake died some time later, the issue of his previous trial participation did not seem to be relevant. Chloe explained:
[H]ow Drake died, the way he was, he wasn’t urinating and he was bloating and it was affecting his organs. I think because it was so obviously nothing to do with his oxygen, it just didn’t even figure . . . Whereas Lucas it was to do with his lung function when he took a turn. So I think it was more of an issue with him really.’
What Chloe wanted was for the trial intervention to stop. She did not have a detailed conversation with anyone about the terms of the withdrawal, but said that she would have been prepared to have discussed the decision. From the information available to the BRACELET study, it seems likely that Lucas’s details still formed part of the trial records, but that Drake’s details, and so his contribution were removed. Chloe said that this was not what she intended to happen:
It’s funny thing really, because if they’d asked me at the time I’d have been more than happy for them to keep any information they got up to that point, I would be thrilled for them if it could have helped them. I just didn’t want him carrying on any more. I have no problem with the results they collated thus far, but it never – it never got broached with me at all.
Like Tony and Liza who, having forgotten about the trial were somewhat taken unawares by the invitation to take part in BRACELET invitation, when Chloe’s letter arrived she initially struggled to make the connection with the trial. She noticed the stamp on the envelope from the hospital where her sons had died.
I thought ‘What else could it be if not about my boys?’ But I couldn’t think of any reason why there would be anything. I totally forgot about the trial.
Chloe’s account shows the trial disappearing and reappearing over time. In Chapter 9 we will consider the re-emergence of the trial for Chloe through her BRACELET interview.
Key points for the recession of a trial for parents interviewed here were after the decision making process had taken place; as other clinical events took over, and then on the death of their baby ( Figure 22 ).
FIGURE 22.
Trial recedes.
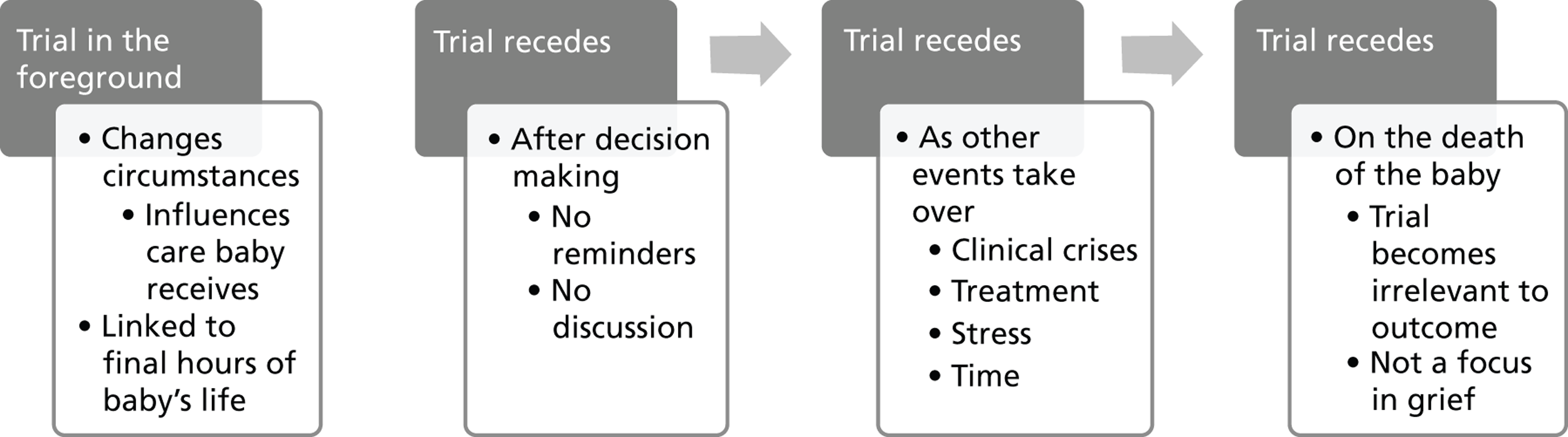
Discussion
Whether parents experience a trial as a major part of their experiences in the NICU, or whether it recedes, seems to relate to the nature of the trial involved, how long the baby survived, and local implementation and management of the trial.
It was very clear that the trial that most obviously stayed in the foreground of parental experiences was TOBY. The trial spoke directly to their most pressing concerns; the threat to survival and the possibility of disability. The rapid recruitment window and action around their baby, created a sense of urgency to which the parents interviewed responded, making their decisions quickly and giving their permission for transfer. The hope that the parents invested in the trial, and their preparedness to take the risks of transfer and the difficulties of separation, are likely to have contributed to the continued salience of the trial. The changes brought about by the trial were experienced not exclusively as research but as part of the care of their child, and at a level that was greatly appreciated. In the case of Laura and Wilf, and Hannah and Ryan, their access to good-quality care, and subsequently to sensitive palliative care, was something that they felt had flowed directly from their involvement in the trial. The interviews did not, however, suggest a straightforward or uniform picture of the means by which TOBY stayed in the foreground of parental experiences. In only two interviews (Laura and Wilf, Hannah and Ryan) was this so clearly related to the transfer of their babies. For Amanda, whose daughter was also transferred, the prominence of the trial in her account related to the possibility of accessing an intervention that she felt would help her daughter and then having this possibility removed. That she went on to die without Amanda gaining a firm sense of resolution to this issue meant that it was still a concern to her at the time of the interview, 8 years after her daughter’s death. For Hesther and Stuart, the trial was a feature of their son’s final hours (see below). For Robert, however, the trial disappeared out of his experience almost as soon as he had made his decision.
The trials could also stay at the fore not because of the nature of the intervention or because it required change, but because it was so closely tied in to major life events. This was the case for Hesther and Stuart, for whom TOBY was an important feature without their baby having been moved, and for Stefanie and David, and Fiona and Keith for INIS. In all three cases their babies died within hours of their decision to join a trial and this decision for them was part of the fight to save their babies. In the latter two interviews the parents questioned having been asked to consider the trial at such a late stage.
The other trials considered here did not in the main bring about change of the same magnitude. They each involved an intervention that had to be administered to the babies involved, but in ways which, unlike TOBY, were not readily observable and apparent to parents. It seemed that the details of the trials were not always particularly clear for parents after their decision-making process. If parents find information difficult, or are reluctant to engage with the details of a trial, it is likely that they proceed with a limited understanding of what the trial is and how it will be carried out. Unless they seek out further explanations themselves, or are offered a further opportunity to discuss the trial, it is unlikely to figure further in their experiences. From the parental accounts it would seem that once they had agreed to enrol their baby in INIS, PROGRAMS BOOST-II UK or ExPN there was little, if no, involvement of them further. There were no reports of doctors or nurses explaining to parents that they were administering an intervention, or that all doses had been completed. Evidence of the trial in the form of equipment or stickers was sometimes noted, and trial-related activity was sometimes seen, but from the parents’ perspective these trials largely seem to have been conducted in a manner quite separate from their recollections of their child’s care. (To provide data on how trial-related activity is managed, an observational or ethnographic study would be required.) The data reported here can inform us only about the impressions parents are left with over time. Parents often did not know when a trial started, when it had finished, or even, in the case of Robert, whether the trial intervention had taken place at all.
One potentially important factor is whether a trial is tackling the issue that was of greatest concern to the parents at that time. Many parents constructed the trial in rather generic terms, as shown in Chapter 6 , as something that might help, and although unspecific, it could still be compelling at the time. Without details of the trial at the forefront, it may be easy for awareness of the trial to slip away, and the same may be true in situations in which a trial addressed an incidental concern rather than parents’ main priority, for instance feeding rather than other challenges, such as infections for Pete and for Shirley in ExPN. When a baby dies, and where the trial did not deliver the hoped-for help, with the exception of Amanda’s situation, this does not seem to have caused major difficulties in this sample. Although some parents such as Adam and Beverley did describe intrusive thoughts as to whether it has been the right things to do, the trial largely receded, sometimes to a point of irrelevance. As Francis explained:
It sort of becomes irrelevant, if you like, when he dies. You’re like, well, doesn’t really matter whether he got it or not. Later on you may be (. . .) thinking, wonder if he did? I remember sitting thinking that a few times, ‘I wonder if he did get it?’
Francis – INIS
This is useful as it suggests initial reactions in the period considered here and hints at the possibility of changing reactions to trials over time.
It seems likely that some trials disappear from people’s lives, some trials linger, and some trials stay present and relevant while their baby is receiving intensive care. This might relate to trial type, for instance ExPN seemed, for these parents at least, to disappear; TOBY seemed more likely to stay present. Trials in which an intervention is ongoing such as BOOST-II UK might linger at the time. The local conditions set by those who implement a trial are likely to be influential: how they present information and the extent to which parents are included in any ongoing trial-related activity. Trial type and trial conditions however cannot be divorced from individual parental responses. How parents respond may also relate to much more individual differences; for Amanda, TOBY was still present at the time of the interview and she still struggled with the fact that her baby was allocated to control. For Laura and Wilf, whose baby was also allocated to the control group in TOBY, the trial was present as a continuing source of satisfaction. For Pete and Caitlin, ExPN was such a minor part of their experiences that it had largely disappeared, and Lewis had completely forgotten about INIS, until contacted for BRACELET.
By the time the parents interviewed for BRACELET had left the NICU, for most the trial had receded – it was forgotten, overtaken or subsumed in their grief. The trial-related experiences described here, whether in the fore or subsumed, are the ones that parents take forward with them into bereavement. Chapter 8 considers whether and how clinicians and trial teams feel parents’ bereavement might be responded to in the context of a trial. Chapter 9 revisits parental views and experiences to consider how they go on to view trials in their bereavement and in the longer term.
Chapter 8 Bereavement and neonatal trials
Views of clinicians and trial team members
When babies are recruited into a neonatal trial, they have dual status as NIC patients and as clinical trial participants. When a baby dies, bereaved parents may be offered a range of support services by their local clinical team. These can include in-house support systems offered by the neonatal consultants and nurses who have cared for the family, and/or hospital-led provision either by bereavement counsellors or chaplaincy services. Although the precise form and origin of support available to parents is likely to vary, common elements would include initial support around the time of death, bereavement follow-up, and for some parents, referral on to specialist bereavement care. These various services are offered in response to the baby’s status as a patient; whether any response to bereavement might flow from the fact that a baby was also a trial participant, has not previously been considered in the research literature. The BRACELET study was in part designed to consider whether any response for bereaved parents might be triggered by a baby’s status as a trial participant, what form any response might take, and how this might be approached by clinical and trial teams. To this end, the interviews with clinicians associated with the core trial recruiting centres (n = 30), and with trial team members responsible for the co-ordination and management of the core trials (n = 7) were used to explore their views of parental needs in relation to their baby’s enrolment in a trial and to determine the extent of any provision made for bereavement in this context.
Views and practice among core trial recruiting centre clinicians
Thirty interviews were carried out with clinicians who were based at one of the original five core trial recruiting centres. (Interviews with clinicians and trial team members were already complete by the time the two additional recruiting centres for BOOST-II UK joined the study. The aim in extending the study to include the additional centres was to recruit bereaved parents for interview.) The interviewees were 23 neonatal consultants who had a role as an investigator, local PI, and/or recruiting clinician for one or more of the core trials, and seven nurses with a research-related role in a core trial ( Table 34 ).
| core centres | Centre A | Centre B | Centre C | Centre D | Centre E | Total |
|---|---|---|---|---|---|---|
| Trials recruited to: | TOBY, INIS | INIS, BOOST-II UK | TOBY, ExPN | TOBY | TOBY, BOOST-II UK, PROGRAMS | |
| Investigators based in core centres | Harvey | Bill | Mia | Donald | Nicholas | 7 |
| Roger | Dominic | |||||
| PIs and recruiting clinicians in core centres | Dean | Honor | Max | Mary | Seb | 16 |
| Avril | Noel | Leonie | ||||
| Greg | Irwin | Joe | ||||
| Craig | George | Eric | ||||
| Judith | Olivia | |||||
| Nurses in core centres | Hayley | Jenny | Aiden | 7 | ||
| Selina | Sascha | |||||
| Connie | ||||||
| Grace | ||||||
| 9 | 7 | 5 | 7 | 2 | 30 interviews |
Seven of the interviews were carried out with senior consultants at professorial level (Harvey, Roger, Donald, Dominic, Nicholas, Noel and Max). These interviewees have extensive experience of neonatal care, and of supporting parents around the time of the death of their babies and in the aftermath. Eight interviews were with well-established consultants (Bill, Dean, Irwin, George, Judith, Mary, Eric and Seb) and eight were with consultants who were relatively newly appointed (Mia, Avril, Greg, Craig, Honor, Leonie, Joe and Olivia); interviewees in this latter group were consultants at the time of their interview but had been registrars at the time of recruiting to the core trials in question and so had more limited experience of providing bereavement follow-up support.
At the time of drawing up the interview schedule for the BRACELET study, there was no information in the public domain which described whether trial participation was considered in any way in the course of bereavement support for parents, and, if it was, how it was managed. The interview schedule was therefore deliberately open, with the aim of describing practice and exploring the views that underpinned and drove decisions about practice in the core centres. To open up discussion, interviewees were asked if they would raise the subject of a baby having participated in a trial with parents at a bereavement follow-up.
Clinicians who would not raise the topic of trial participation
As the interviews accumulated it became clear that interviewees would rarely raise the subject of trial participation with parents who were bereaved. A number of interviewees very quickly pointed out, sometimes before the interview had started, that they would not change their approach to supporting parents in any way because a baby had been enrolled in a trial. Noel was one of the consultants who explained that he felt that they offered good bereavement support and that there was no need for modifications. He said, ‘Our bereavement [support] wouldn’t be any different at all, whether a baby was in a trial or not’ (Noel – Recruiting Clinician, INIS). His colleague, Irwin, described their bereavement care programme as being ‘really quite extensive’ and he too felt that there was no need for additional input in relation to a trial.
Parents who are struggling, we’ve got a bereavement counsellor. Parents . . . at the time of the baby’s death, they’re cuddling their baby, they’re given arm bands, photographs . . . and then they’re given a little bereavement box. They can take their baby home. They go home, and . . . the nurses send them a card. They come back to the bereavement clinic. They’ll sometimes sit for an hour and a half, two hours talking with the nurse that knew them best and the consultant who’s responsible . . . and the consultant obstetrician very often, talking things through. We always have a member of staff who goes to the funeral. So there are lots of supportive things, and that would apply equally to babies who’ve been in studies or not been in studies.
Irwin – recruiting clinician, INIS
He went on to explain his view that trial participation was not pertinent at this time:
I don’t think it goes round my mind at all in the bereavement clinic ‘This was a baby who was in a study’. That’s probably because a lot of the babies are in studies, and so they’re not exceptional.
Irwin – recruiting clinician, INIS
This was a position that was encountered in several interviews as well as a view given by two senior neonatologists for declining to take part in BRACELET. (In both instances the clinicians who declined saw the topic as being of limited relevance to practice and did not feel that it was appropriate for study. Like Irwin, the heart of their concern appeared to be the sense that bereaved parents whose baby was in a trial were no different from other parents who are bereaved and that they are well cared for through standard bereavement services provided within neonatal care.) Harvey, an investigator for two of the core trials, made a similar comment:
I’ve never thought of bereavement as being different within a trial as without a trial. I’ve thought of it just being similar.
Harvey – investigator, TOBY and BOOST-II UK
A number of the consultants indicated that trial participation was a topic that they had not previously considered introducing themselves, for instance Honor said, ‘It’s certainly not something that I particularly think about’ (Honor – recruiting clinician, TOBY), Judith commented ‘I’ve never brought that up in a bereavement session, ever’ (Judith – Recruiting Clinician, INIS), and George said, ‘I don’t consistently think about mentioning the fact their baby’s been in a trial’ (George – recruiting clinician, INIS). Greg, who explained that he was not entirely convinced of the topic covered by BRACELET, commented:
I can’t really see the situation where I would be talking to a family afterwards about a child’s death and they were enrolled in a trial . . . I can’t really see that situation arising.
Greg – recruiting clinician, INIS
It was also an aspect of care that was not seen as particularly memorable or prominent for the clinicians. Avril, a recruiting clinician for INIS said, ‘I can’t remember when any of the babies who were recruited to INIS died, I’m not sure if we ever found out.’ Her colleagues, Greg and Connor, became aware that a baby that they had recruited to trial had gone on to die only because this was how their eligibility for participation in BRACELET was determined. Judith, who recruited to the same trial in a different centre, argued that trial participation was not a particularly notable feature in the information that they held about a baby, which would be used to prepare for bereavement follow-up:
It’s interesting, it doesn’t actually feature very highly even on the discharge summary. We don’t have specific boxes to check to see if this baby’s enrolled in such and such a study or not. The registrars will sometimes remember to include that in the text, but . . . it’s often not something that I’ve picked up when I’ve gone through the notes on that baby and I also don’t necessarily – I wouldn’t necessarily feel it was appropriate to talk about their involvement in the trial.
Judith – recruiting clinician, INIS
Given the widespread statement that the topic of trial participation was seldom discussed with bereaved parents, and that it was an unlikely area of interest for parents, the interview discussions that ensued largely focused on exploration of why it was seen as unnecessary or inappropriate. During analysis, the main reasons that were identified related to a view that trial participation is:
-
not the issue that parents want to discuss
-
not a topic for which information is available
-
no longer relevant.
Trial participation is not the issue that parents want to discuss
For some of the interviewees there were issues that they felt were much higher on the parental agenda than the trial and they could not foresee situations in which the subject of a trial would be raised by parents. For Greg, the trial would fall outside the parental areas of interest:
Most parents of bereaved children that I have dealt with focus very much on, you know, the events immediately preceding death and once they have their questions answered about that they often don’t want to think too much about, you know, what happened a few days before or weeks before or a month before.
Greg – recruiting clinician, INIS
Roger acknowledged that some parents might be interested in asking about their involvement in a trial but saw this as unusual. He would not therefore discuss a trial unless parents indicated that it was something that they wished to talk about. He said, ‘I’ve only ever dealt with it when it’s been raised by the parents.’
Roger – investigator and recruiting clinician, ExPN
Mary and Max also felt that parents focus on the cause of a baby’s problems and not on the impact of the care that they received:
My recollection is that parents [of babies in TOBY] were much more focused on the reasons why their babies had had encephalopathy and died . . . and almost all the conversation was related to that . . . whether or not their baby could have been saved was always much more focused on, [as well as] what had happened antenatally.
Mary – recruiting clinician, TOBY
I think it’s not inappropriate if people are interested in knowing about it, but mostly what people want to know is why the baby got into that situation in the first place . . . I’m going to be seeing someone later on today for bereavement counselling . . . and it’ll be about what’s led to the baby being born very prematurely or becoming sick or being born at term with severe hypoxic ischemia, as happened with the TOBY babies. The treatment isn’t really a point for discussion unless there’s been a complication, and actually [cooling is] a very safe treatment as it turns out. There are very few complications.
Max – PI, TOBY
One of the consultants, Noel, described his own aims for bereavement counselling sessions, highlighting the importance in his view of both information and emotional support. He saw discussion of trial participation as superfluous to these aims.
My mission at the counselling is to try and inform them why their baby died, and indeed to inform them of the next emotional issues that they’re going to go through. You know, the fact that . . . some people are gonna avoid them, some people are going to talk to them who have never talked to them, and they will have a complete change of friends as a consequence of the baby dying . . . [If] they raised the trial I would certainly fully talk about it to the extent that they wanted, but no, my mission at bereavement counselling is to try and get them fully understanding why their baby died, and whether it’s going to happen again if they get pregnant again, and plan for the future.
Noel – recruiting clinician, INIS
Bill explained that despite his and his colleagues’ preparedness to discuss anything that parents wished to explore, he did not find that this generally included a baby’s involvement in a trial.
[I]t would be very seldom that research came up in those visits in discussion with families and maybe that’s not right – but we – we try and make the visits as open as possible in as much as encouraging the families to talk and enquire about anything they want to.
Bill – investigator and recruiting clinician, INIS
For Dominic, the issue of trial participation was just one feature in a much larger picture, one which was subsumed into other events and experiences. He was asked how significant trial participation might be for parents when a baby has died.
Sometimes it does [take on more significance], although quite rarely actually, surprisingly rarely I think . . . We shouldn’t think of this as in vacuo, because these parents are going through that experience as a totality, and the research actually is quite a small aspect of what they, what they’re going through. And their interaction with the nurses and doctors looking after them, their family, what else has happened, what happened beforehand, their – their previous visions of things, prior beliefs, is all going to have a huge effect on how they react to things. And trying to pull out the research consent moment, or whatever you want to call it, as a single thing, is going to be very biased by all this other stuff as well.
Domenic – investigator and recruiting clinician, TOBY
In a small number of interviews the consultants started to explore whether parents might indeed have questions that are not articulated in the follow-up meetings. George had suggested earlier in his interview that it is difficult for clinicians to understand what parents are really feeling when their baby is sick, and that parents might not be explicit about concerns that they may have at that time. In considering the fact that he rarely encountered parents who raised the subject of trial participation at the bereavement follow-up, he speculated on the possibility that a similar phenomenon might be at work at that point.
A large proportion of our premature babies have been through one trial or another. It’s unusual for a parent to ask, and it’s just been formulating in my mind the question about whether we should – whether going on from my sort of comment that parents don’t always own up to their – to what’s worrying them, I wonder whether it’s a bigger part of what’s going on in their minds than we realise – I’ve started to wonder that.
George – recruiting clinician, INIS
Irwin also suggested that it is possible that some parents have needs that have not been apparent to him:
I haven’t been really aware of anxieties stemming from research studies. That doesn’t mean that they’re not there, just I’m not aware of them . . . I think that many of the parents that we look after have their needs dealt with. There may be as I say an unsatisfied minority who harbour things, uncertainties that I don’t know of.
Irwin – recruiting clinician, INIS
The possibility that parents might have questions that they wish to explore was mentioned occasionally in interviews but, as in Irwin’s comment above, this was seen as a minority position, a need raised for parents who were unusual in having concerns or particular interests. Avril suggested that waiting for the parents to indicate their concerns or interests by raising the topic themselves may not necessarily be appropriate.
[S]ome parents they’re just not ready to talk about it and they’re not prepared to talk about it. They haven’t come to terms with that side of things and they’re just not, not at the point in their – in their journey where they can actually bring that up . . . [We are] assuming, perhaps wrongly, that if there are any queries about the trial or the treatment their baby received or anything to do with the trial that they will ask and they will bring it up.
Avril – recruiting clinician, INIS
Several consultants speculated that the topic of trial participation might be appropriate if parents were concerned that their baby might have survived if allocated to a different arm of the trial, but this was not seen as an issue that often arose. Donald speculated that:
[W]hen things go wrong when parents have been in a trial, they must surely wonder whether they did a good thing, the right thing to be in the trial . . . would feel almost cheated perhaps that their, their child was not helped by being in the trial . . . I think this is a very complex area, and probably you can’t tease out the feeling about the death of a baby being in a trial, as opposed to all the complex feelings of being in a trial itself.
Donald – investigator and recruiting clinician, TOBY
He was asked whether he felt that clinicians are making any response to this. He replied:
There probably is very little or no discussion about . . . being in a trial, how that affects their feelings . . . [I]n our unit we spend so much time trying to support families to deal with the loss of the baby, that the – the – the fact that they’re aware of the trial or are in a trial becomes much less important.
Donald – investigator and recruiting clinician, TOBY
Trial participation is not a topic for which information is available
In looking at the wider comments made by the consultants about the implications of trial participation for the babies concerned, it became clear that an important element for some was the inability to be clear and specific about the role that a trial intervention might have for an individual baby. A trial intervention is one treatment among many, and is administered to babies with challenging illnesses and often multiple complications of prematurity. Max argued that if a death ‘seemed to be related to the therapy, it would be appropriate to talk about that if one knew’ (emphasis added in italic text). Judith explained that unless a baby was involved in a ‘major interventional study’ such as the UK ECMO Trial138 or TOBY where parents might wish to discuss more general issues such as transfer, she ‘wouldn’t necessarily feel it was appropriate to talk about their involvement in the trial’. This concern for her hinged on the uncertain status of the information available.
Well, it would be different if we had results of the trial that . . . maybe related to the outcome of their child . . . [T]he purpose of my bereavement sessions is really to talk about their life and their pathology and what happened during their life, the baby’s life and the post-mortem examination, if the parents are coping, rather than to talk explicitly about every area of the management of the baby.
Judith – recruiting clinician, INIS
Judith’s comments highlight a very practical issue relating to the timing of the bereavement follow-up. For most parents this meeting takes place a short time, weeks or a few months after the death of their baby, and no further information about a trial is available than was available at the time of enrolment. A major role in the bereavement follow-up is clarification of events, but at that point parents can be offered no further information about the possible impact of the intervention delivered in a trial than they were given at recruitment. Nicholas raised the possibility that if there were particular concerns about the potential impact of an intervention, unblinding could be initiated to afford parents some further information, but this was not something that he would usually do:
[O]ne thing you could offer the parent is to say, ‘well, we could ask for the blinding to be lifted and find out what you got’. . . [but] that’s not something I normally would offer parents until the end of the study.
Nicholas – investigator and recruiting clinician, TOBY, PROGRAMS and BOOST-II UK
Avril raised the possibility that uncertainty might exist for parents not in relation to the treatment that the baby received, but the treatment that they did not receive in a trial where the intervention was not masked.
I think being part of the trial and having had the baby cooled [in TOBY] is probably helpful for some parents because they know that absolutely everything was done and that unfortunately there was nothing else that could be done for their babies. I suppose the – the hard part is for the parents whose babies were recruited but weren’t cooled to know whether if their baby had been cooled would things have been different and you can’t answer that, you could never answer that for them, and I suppose those parents may well need more support down the line.
Avril – recruiting clinician, INIS
Honor empathised with parents faced with the uncertainty of treatment in the context of a trial, and although she stated ‘the point of clinical trials is the fact that we really don’t know; we’ve just got to try these things out’, she added:
. . . but certainly if I’d been a parent in that situation, that would be the thing that I would want to know. Did my baby actually get immunoglobulin? And if it didn’t, if it had done, would it have made any difference?
Honor – recruiting clinician, INIS
For most parents, no further details about the effects of an intervention can be given as the uncertainty that drove their inclusion in a trial would still be unresolved at the time of their bereavement follow-up. None of the clinicians interviewed suggested that discussion of that ongoing uncertainty or revisiting the rationale for a trial or trial methods would be an appropriate aspect of bereavement care.
Trial participation is no longer relevant
A small group of the more senior clinicians made comments that helped to explain why trial participation was seen as largely irrelevant in the context of bereavement. They presented it as an experience which belonged to the past; essentially the baby’s involvement in a trial was over and there would be no ongoing connections with the trial. Dominic explained that once a baby had died, in their particular case ‘no one cares about the trial any more’. He argued that for the clinicians concerned with the care of that family, priorities had changed:
The trial’s all dead and gone. The CTU cares about it because they’ve got a number . . . but the doctors don’t care because it’s – it’s gone, you know, and they’re now worried about looking after the family.
Dominic – investigator and recruiting clinician, TOBY
Harvey commented that for the baby and the family ‘the study’s over’. He felt that this reduced the salience of the trial; unlike parents of survivors who would have an ongoing contribution to the research through follow-up, the role of a deceased trial participant is over.
[U]nfortunately, when the baby dies . . . that’s the outcome, that’s the end point . . . Unless there was something that happened within the trial that led to the death of the infant then I think that you would be relying on the parents bringing up anything in relation to the trial at that bereavement interview.
Harvey – investigator TOBY and BOOST-II UK
In these accounts, the dual status that the baby had taken on as both patient and trial participant reverted on death entirely to the original single status as patient. Noel explained that by the time parents came for bereavement follow-up he considered them and their baby only in clinical terms. He said, ‘To me they would be completely patients. Nothing to do with participants.’
Dominic reflected on this reversion to patient-only status. He argued that for a bereaved family his own role similarly shifted, that in providing bereavement care he would revert to ‘medical mode’. He felt that the confidence that trial teams have in the expertise and support systems available in their recruiting centres means that it is not necessary to think about any special support that might be owed to the families of trial participants. To return to the original point made in this chapter, this is because parents would be well served by the support available in the recruiting centres, which came from experience not as researchers, but as clinicians in medical mode used to dealing with death and bereavement.
We all do clinical medicine, and probably the thing that senior consultants do more than anything else now is talk to parents about bad, bad things, because the juniors tend to do the day-to-day stuff and we end up doing most of the talking. So our professional lives [are] very heavily involved with talking about death and disability and awful things like that. And I kind of think that when we write a protocol it’s assumed. So let me give you an example. [In] the TOBY trial . . . death was an expected outcome in about a third of the babies. And we knew that would happen, we knew that would be the case, but the trial itself didn’t really do much else about that because we knew that the units involved in the TOBY study, ours and other people’s, would have a fairly well worked up process for that, which would not be a research issue, it would be a medical issue.
Dominic – investigator and recruiting clinician, TOBY
Noel’s delineation of deceased babies as patients not research participants, and Dominic’s statement of a reversion to ‘medical mode’, essentially a shift from clinician/researcher to clinician only, is illuminating. It suggests that even in clinical centres that pride themselves on their research profile, and when trials are considered to be a prominent sign of excellence, on death there is a parallel redefinition of roles for patients and for clinicians that removes involvement in research from the situation ( Figure 23 ). This redefinition was seen as an appropriate part of offering and delivering high-quality bereavement care.
FIGURE 23.
Redefinition of roles for patients and clinicians on enrolment and on death.
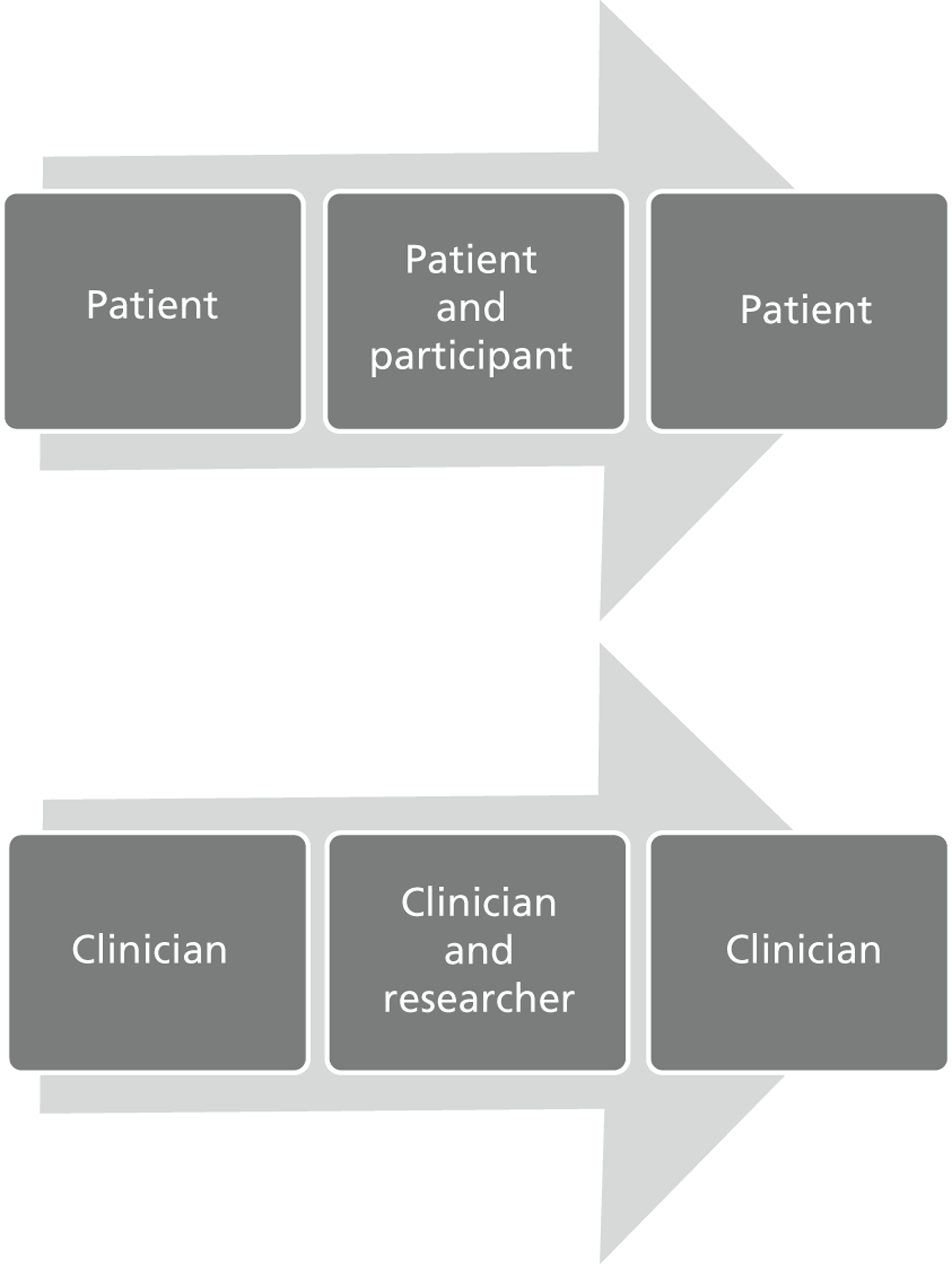
Clinicians who would raise the topic of trial participation
A small group of clinicians indicated in their interview that they did feel that it would be appropriate for the topic of trial participation to be raised with parents at bereavement follow-up. These included Joe and Craig who were relatively recently appointed as consultants. Joe argued that he would do so in the spirit of openness and to acknowledge the trial as part of the baby’s story.
I would definitely mention it, yeah. I think I wouldn’t consider – I wouldn’t set out to conceal any information about their participation . . . [I]t would have been an important aspect of the child’s life when he or she was alive and . . . it would be brought up.
Joe – recruiting clinician, TOBY
Craig argued that raising the topic of trial participation would be ‘appropriate’.
I would say . . . ‘Have you any issues about what happened to your baby while on the unit and do you understand what happened to your baby and why your baby died?’ and I think just part of that would be . . . ‘Have you any issues about the fact that your baby was in the trial?’ I mean you may find the parents will bring it up before you but, no, I think it is appropriate.
Craig – recruiting clinician, INIS
He also felt that it would provide him as a clinician and researcher with important feedback, which would help him in his practice to understand the impact of a trial on parents. He argued that parents might say ‘I wish I’d never been asked’ and commented ‘So how are you gonna find out if you don’t ask?’
Seb, a more established consultant and local PI for TOBY and PROGRAMS, felt that it would be important to raise the subject so that any possible concerns would not be left unattended. He saw the bereavement follow-up as an opportunity for parents to voice concerns, and his role to facilitate that and to provide any explanations that might be helpful.
I’d raise it, mention it, just in case there are any lingering doubts or worries that might [be] in the parents’ minds that the intervention or lack of intervention might have made any difference to their baby’s outcome. So I think I’d – it’s one of the things I would probably talk about . . . I think parents in that situation often have things that they’re worried about that they don’t always verbalise and so to sort of, to mention it just in case, it’s something that’s a nagging doubt in the back of their minds I think – personally I think that’s always useful.
Seb – PI and recruiting clinician, TOBY, PROGRAMS
The clearest statement of the perceived importance of discussing trial participation with bereaved parents was given by Nicholas, who was involved in three of the core trials (PROGRAMS, TOBY and BOOST-II UK). He described his practice and the rationale that underpinned his approach. His views were so strikingly different from most of his fellow interviewees that the extract from his interview is presented in detail in Box 11 so that his own words can be used to describe his sense of the need for this discussion.
I often, you know, see families for bereavement counselling where, you know, they’ve been in a trial, and the first thing is you must always mention the trial.
That’s interesting, because that’s one of my usual questions and I ask people what they do, and people vary hugely as to whether they think it’s appropriate or not. Some people feel it’s inappropriate.
No, no, no, no! You must! The inappropriate thing is hiding things, so if you don’t discuss things openly with them – and so, you know, if someone’s been in TOBY and got – got no cooling, you know, it must be in the parent’s mind, you know, ‘had you cooled my baby, would the baby have survived?’ You . . . really had to, you really had to bring that into the conversation, and, you know, babies who died in TOBY usually had gone too far before we started, and so it’s unlikely that a huge number of them would not have died anyway . . . One can talk very openly about . . . not so much it’s the intervention that’s led to this, because [you might have] elected . . . to stop because the brain injury is so severe. It’s much more, you know, concentrating on the original insult rather than the intervention, but they need to have that conversation with you, because otherwise they’re going to go away and think, ‘well, if I had the cooling he would have been here,’ you know.
And they will think that for many, many, many . . .
They will think that for a long time, yeah. But it’s quite interesting. I mean you do talk to . . . parents for quite a long time about their bereavement, and then you say to them, ‘Well, do you want to come back again?’ and very few of them take you up on that. Even though I always leave the door open . . . they rationalise it themselves, and whether they bury it or whether they deal with it in some other ways I don’t know.
If you felt parents had quite a few questions about a trial, or they had some issues that were unresolved, some concerns or a difficult experience with the trial part of their – their neonatal care experience, is that something that you would prefer to handle yourself, or would it be useful, for you to have somewhere to be able to refer those parents on? I’m trying to think of ways that trials in the future might bring things into their protocols.
Sure, sure. Well, I mean personally I would always try and address it myself, but then I’m someone who’s done a lot of trials and involved in a lot of trials and understand trials. But I think . . . it might be a useful thing to have someone around . . . some resource available who might independently perhaps discuss the trial with the parents.
Views of research nurses
Although the nurses interviewed for BRACELET did not themselves take responsibility for bereavement follow-up meetings with parents, a task that was exclusively carried out by neonatal consultants (at least among the BRACELET study interviewees), nurses were often involved with parents on a NICU around the time of their bereavement and so had a strong sense of parental experiences and needs.
Their involvement in a trial ranged from largely administrative or occasional roles (Jenny and Hayley for INIS) to specialist nursing care for babies in a trial (Aiden and Sasha for TOBY). Sasha described herself as ‘a constant presence’ for the 72 hours of cooling for babies in TOBY and this helped to forge relationships with parents. Both she and Aiden explained how they had been directly involved in withdrawal of care for babies who had been involved in the trial, both at the specific request of the parents involved.
The nurses described this work to support parents around this time as potentially satisfying, but it could also be onerous. Hayley explained that she found it difficult to complete the INIS paperwork to report a death for a baby in the trial: ‘It used to always be awful. I remember like filling in the forms when somebody died, I just found that very, very hard.’ Aiden described coming in to the NICU on his day off to meet a father of a baby in TOBY who asked to speak to him after his baby had died. Sasha linked her decision to relinquish her role as a research nurse on the close of the trial to the impact of bereavement care for the parents of babies in TOBY. She said:
[I]t took a lot of my efforts and energy, time, input. You built up a very, very close relationship with that family and with that baby, and then to lose them was very, very draining.
Sasha – research nurse, TOBY.
When the nurses were asked about the bereavement follow-up care that would be provided for the parents, they largely had to speculate on whether or not a trial would figure in the discussion between consultants and parents. Aiden said, ‘I hope it does,’ Sasha said, ‘as far as I’m aware, yeah, they did [discuss the trial]’ and Connie said that a trial ‘obviously should be talked about.’ Selena felt that ‘it would be addressed at some stage [in] the bereavement counselling session’. She added:
I think it just shows that you’re open and transparent and that you’re not worried in any way that the trial did influence the outcome and I think definitely our guys upstairs would always bring it up and mention it and then if the parents do have any concerns and things they can be addressed too.
Selena – neonatal nurse, TOBY
The most commonly discussed reason for expecting or hoping that the trial would be discussed was to provide parents with some reassurance that their baby had not been harmed by their participation. Grace and Jenny both felt that if the topic were raised by the consultant this might allow parents to articulate fears that might otherwise go unattended.
Well I think it would be very appropriate to actually ask the parents . . . if they felt the trial had been of any benefit or if they were – if they had any concerns about the baby being in the trial . . . [I]f a parent is – or parents are thinking in their mind, ‘Well if my baby hadn’t had cooling he mightn’t have died.’. . . If they’re having that in their mind but they don’t want to vocalise it and the consultant will say to them, ‘You know, how do you feel about your baby having been in TOBY?’ they might then say, ‘Well maybe he wouldn’t have – this wouldn’t have happened’. . . that way it might actually give them answers . . . [and the chance] to ask the question.
Grace – neonatal nurse, TOBY
Jenny felt that discussion of the trial may have a therapeutic effect for parents and that the consultant could facilitate this as well as offering reassurance.
I think they would want reassurance that the baby hadn’t died as a result of anything to do with the trial. I would think, though, they then would take some comfort in the fact that their baby had been involved in something that may then go on to help some other baby, because that seems to be, that seems to be quite comforting to a lot of parents, to know that if they go on and they do something else for someone else, or it wasn’t a complete waste. If something good comes out of [it] . . . then I think that could help them . . . I don’t think it would be a bad thing to mention it, and if you were professional and used to doing these interviews, you could gauge very quickly from the reaction of the parents if it was a talking point or not . . . If you mentioned INIS and they just ignored you, you’d just pass on or say, ‘have you any questions about that?’ And if they say ‘no’, fine. But I think it would be quite nice to offer it as an opportunity if there was any unanswered questions.
Jenny – research nurse, INIS
Connie also felt that it would be important for clinicians to acknowledge the role that a baby had played in a trial, and that a full discussion would allay fears, address any missing information, and in some way be a form of reciprocation for the contribution to the research that the parents and their baby had made.
[I]n the bereavement follow-up that obviously should be talked about . . . that their babies were just too sick to survive, and be able to answer people’s questions, because they often will have questions afterwards . . . I think they need to be told again and again, you know, or not told, discuss with them you know what – why these things happen, in case they’ve forgotten, you know from previous discussions . . . I do think that it would be important to mention it, because after all they have . . . been able to use the information they’ve obtained through the study and also parts given, so I think it should be mentioned to them . . . maybe saying thank you again for taking part in the study, and it has benefited other babies and will benefit people in the future.
Connie – neonatal nurse, TOBY
Connie clearly felt that it was important that this message of thanks should come from the clinical centre staff as well as from the trial team.
[The clinicians have] met the people, [they have] have looked after them. . . and they could say it face-to-face to the people. They might get more out of it than you do in a letter type of thing, I would have thought.
Connie – neonatal nurse, TOBY
Both Aiden and Sasha worked in a centre where the research nurses were directly involved in the recruitment process for TOBY, supporting the clinicians in their discussions with parents and with retrieval of babies from outlying centres. They commented on the potential role of the research nurse for parents who go on to be bereaved. In a trial such as TOBY, for which the research nurses are trained to deliver a specific intervention, the nurses gain a deeper insight into the trial and the treatment, as well as developing relationships with families involved, from recruitment through to discharge or death.
You’ve met them at the initial outset. You’ve probably been there while we’ve turned everything off. So you’re now taking them through the bereavement process and to sort of like tie it all up, all the loose ends, yeah, I think it would [work], for some people, they might have turned round, said, ‘Yeah, that would be great’.
Aiden – research nurse, TOBY
Sasha felt that they could be well placed to discuss a trial with parents who had questions but for whom the bereavement follow-up was not necessarily the best forum to cover question and answers about a trial.
If parents did want to contact me and come back and see me and have questions about the trial then I would have been more than happy to have gone through that with them, if, you know, if it would help them, if it would help answer questions, queries, problems, issues, anything like that they had that was separate to the bereavement meeting, that they felt, ‘oh, we couldn’t bring that up because that was our bereavement meeting with the consultants’.
Sasha – research nurse, TOBY
Views and practice among co-ordinating trial team members
Seven interviews were carried out with individuals with responsibility for co-ordinating and managing the day-to-day running of a core trial. Five of the interviewees worked in a CTU as trial managers and administrators (Una, Bridget, Corinne, Belinda and Astrid) and two were clinicians who both co-ordinated a single centre trial (ExPN) and acted as recruiting clinicians (Mia and Roger) ( Table 35 ). The interviewees were involved in decisions about whether there should be contact between the trial team and bereaved parents and were responsible for implementing any policy that was put in place.
| Trial teams, based at: | INIS | TOBY | PROGRAMS | BOOST-II UK | ExPN centre C | Total |
|---|---|---|---|---|---|---|
| CTU | ||||||
| Managers and administrative staff | Una | (Una)a | (Una) | (Una) | Mia | |
| Belinda | Bridget | Astrid | Roger | |||
| Corinne | ||||||
| Totalb | 3 | 2 (−1) | 2 (−1) | 1 (−1) | 2 | 7 |
Clinical centre-run trial: ExPN
ExPN was the only core trial to be run from a clinical centre by the clinicians directly involved in the care and management of the babies in the trial. Mia’s experience of working with these families was similar to that described by the TOBY research nurses, Aiden and Sasha, who developed links with parents through the day-to-day care required for the trial. Her direct connection with the parents meant that for ExPN there was the possibility that any trial-related follow-up would be conducted in the context of a clinical, social and research-related relationship.
I hadn’t quite expected it, but I found that I developed a relationship with a lot of these families. So from the time of recruitment I’d see them almost on a daily basis because these parents would be sitting at the cotside of their babies, and – and we’d talk nearly every day. And then even after they’d been discharged from hospital, they’d still ring me, even for things that are completely not related to the trial. So it could generally be about the child’s health or a decision they had to make about whether its immunisation or something else, they’d just ring me and we sort of developed that sort of relationship.’
Mia – investigator and recruiting clinician, ExPN
Mia was asked whether a relationship also existed with the parents of the babies in the trial who died.
No, actually it then stopped abruptly. I must say, most of the infants who died, died very early, so perhaps I wouldn’t have built up such a relationship with the parents. But having said that, I did see them every day, and, you know, you’d have a conversation almost every day. There were about, at least half a dozen of the families where the infants [survived] for more than a month, and a couple of them had actually been discharged from the hospital and then died . . . It was harder, because often the – the deaths were quite – I can’t say it’s unexpected because we knew that these infants weren’t doing that well anyway, but it occurred at a time that was quite sudden, and then you’re sort of almost at a loss for words really, knowing how to talk to the parents and how to comfort them. And then certainly once the, the necessary forms and things have been signed and dealt with on the neonatal unit, I’d just never see the parents again.
Mia – investigator and recruiting clinician, ExPN
Mia did not institute any further contact with the parents of babies who died after being recruited to ExPN. In the same way as was described by Dominic for TOBY earlier in this chapter, Mia said that care of the bereaved parents was ‘left to the, to the normal procedure of a neonatal unit.’ She did not at that time have responsibility for bereavement follow-up meetings and received no feedback from colleagues as to whether ExPN had figured in their discussions with parents. She said that she would have liked to have known whether parents raised the topic of the trial and what sorts of questions they might have had.
During the interview Mia explained that she had not thought about making any additional response to bereavement. Although she had considered sending the ExPN results to parents of surviving babies she had not thought about the possibility of including bereaved parents. On reflection she felt that this in part related to what she thought the parents would want, but it also related to her own discomfort.
[M]y feeling was once this whole episode is over, whether parents just want to forget about it and just move on, and perhaps they don’t want to have anything more to do with the neonatal unit if they can help it. So I suppose I was thinking along those lines, so I wasn’t planning to have any more contact with the – those [whose babies] didn’t survive . . . I suppose when I was running the trial, I almost want to sort of forget about those who didn’t make it . . . . Apart from the fact that it’s a figure on – on the data that I collect, I didn’t have anything more to do with them . . . and also, because I knew that it’d be quite awkward for me to talk to the parents afterwards. I didn’t know what to say to them so – so they sort of just dropped off my radar if you like.
Mia – investigator and recruiting clinician, ExPN
She added that she had learned a lot from carrying out the trial and from her interactions with parents and her views had changed. ‘I think that’s changed for me. I think that they deserve to be treated the same as the other[s]’. She also felt that taking part in BRACELET had caused her to reflect on how ExPN was run and without prompting she explained that she felt that she would design another such trial differently.
If I were to run this trial again, I would like to have a closer liaison with the bereavement officers, so that they know that infants who have been involved in a trial have died, and they are, they are supporting parents with that sort of information available to them . . . But also, perhaps a way of them feeding back to the investigators, or me, about whether any issues have cropped up for parents . . . so I could address those issues, and also to offer the parents the opportunity to meet up with investigators a few weeks after the infant’s death, again to talk about it. So, you know, not only are you getting that information from bereavement officers, but also to have direct contact with parents should they want to.
Mia – investigator and recruiting clinician, ExPN
Clinical Trials Unit trials: INIS, TOBY, PROGRAMS, BOOST-II UK
The interviewees from the CTU that co-ordinated INIS, TOBY, PROGRAMS and BOOST-II UK held a number of managerial and administrative roles for these trials. They were asked to describe any provision that the trials made for bereaved parents and were invited to reflect on these approaches and those of their colleagues. An important consideration for the interviewees related to the fact that the four trials were run under the auspices of the same CTU, but they differed in particular ways, and these could impact upon their capacity and inclination to respond to bereavement. They explained that for them their differences did not so much relate to the clinical focus of the trials, but issues of team structure, logistics and timing of the trials.
Each trial was developed in conjunction with different clinical teams. Astrid, Bridget and Una were original trial team members and had been instrumental in developing management systems for the trials, whereas Belinda and Corinne joined their teams later. Corinne, the trial administrator for INIS explained that, given multiple staffing changes for the trial, the current staff were required to work with systems that they had not themselves initiated or established. The trials involved quite different numbers of clinical centres and the sample sizes ranged from TOBY, which recruited 277 babies to INIS, which recruited 1454 babies in the UK. The trial team’s capacity to respond to parents’ requests or perceived needs was partly an issue of numbers, but it also became clear that the timing at which the trials were initially established determined key features in their set up and to some extent this too affected processes for contact with parents.
There was a sense in the interviews of a methodological and administrative progression across the trials over time. The oldest trials were PROGRAMS which started to recruit in 2000, and INIS which opened in 2001. PROGRAMS was described by Corinne as being ‘more paper based’ compared with the most recent trial, BOOST-II UK for which ‘things are a lot more online, [and] computerised’. Corinne also explained that elements of the management of INIS came about in response to a period of regulatory change. She said:
INIS has changed so much since it started. The ethics were completely different then than it is now. We didn’t have regulations in place until half way through the trial, and you had to sort of pull it into line, whereas a trial starting now would have all that set in place.
Corinne – trial administrator, INIS
(In this context, regulations refers to the legislation for the conduct of clinical trials of investigational medical products brought about by the EU Clinical Trials Directive.)
The interviewees were asked about their knowledge or expectations of any response to bereavement that might take place in the trial recruiting centres, about their own systems for managing contact with bereaved parents, and about any response to bereavement that might flow from the trial team.
Expectations of response to bereavement in the recruiting centres
The interviewees were asked about the provision for bereavement that might be made for parents of trial participants in the clinical centres. None was able to categorically state what happened in their recruiting centres but some expressed an expectation that trial participation would be discussed with parents at a bereavement follow-up meeting with their neonatal consultant. Astrid said, ‘I know they do that’ and Corinne said, ‘I would anticipate they would have procedures in place such as that. ’ Bridget, co-ordinator for TOBY, said:
Most places have this sort of post, 6-week post-bereavement counselling visit. So, you know, you’d like to think, you know, it might come up in that, but it’s not something I have any feedback about.
Bridget – trial co-ordinator, TOBY
Belinda, trial director for INIS like the clinicians cited above, felt that bereavement would be dealt with in the clinical centres and that it was a clinical issue, and not an issue for trials teams.
I think from a – a triallist point of view the whole bereavement aspect of participants in a clinical trial is taken care of locally, clinically and not as a research issue . . . In the majority of big pragmatic trials it is just totally left to the local nurse, doctor . . . or whatever the process is locally to take care of . . .They have to fall into the jurisdiction of the clinical team caring for that child because that’s where the contract is if you like, the contract between doctor and parent, and that’s the closest contact . . . and I think in a bereavement situation they’re the ones that actually can judge the situation best.
Belinda – trial director, INIS
She argued that it was important that the parents were seen as linked to the clinical centres and to the clinical staff, and were distanced from the trial once their baby had died.
[W]e would no longer have anything to do with bereaved parents because it’s outside our knowledge and it’s outside the trial and so it would be totally left . . . Personally I think it’s right [to do it this way because] I think it’s the people on the ground that know the parents, have a rapport with parents that need to be supported, and not some . . . sort of nebulous piece of paper from [us] that says . . . ’The INIS Trial sends condolences.’ I mean, I think that would be lip service.
Belinda – trial director, INIS
This sense of distance was, in Belinda’s view, important for another reason, a comment that echoes Mia’s concerns in relation to the management of ExPN quoted earlier in this chapter:
[F]rom the triallist point of view, linking the two together* that causes some sort of anxiety that . . . if a child has been in a trial and has died then it maybe that the parents then start to think, ‘Well is it the trial that’s caused my child to die?’
Belinda – trial director, INIS
*i.e. death and trial participation.
Clinical Trials Unit systems for managing contact with bereaved parents
Within the CTU, one of the ways by which all of the trial teams took bereavement into account was to take great care not to contact bereaved parents as if their baby was still alive. Corinne and Belinda described how they ensure that they have up-to-date information and flagging systems so that parents are not sent a follow-up appointment for their child or a birthday card. As INIS involved thousands of babies, this requires tight organisational systems as well as trial staff looking out for problems and inconsistencies.
Our discharge form would tell us if the child had died before discharge at the hospital, so we would have the information then to let us know . . . [W]e’d rely on the nurses letting us know immediately because . . . that was a fail-safe really . . . Until we knew [a baby] had been discharged home okay, we didn’t make any contact. So I mean that was always sort of an issue that everybody was always aware of in the trial, that we didn’t want to send anything that we shouldn’t be sending home to cause any sort of upset and we were always very careful about that.
Corinne – trial administrator, INIS
Corinne explained that once the INIS team was alerted to a death ‘no further contact would be made, just as simple as that’. An exception would be parents of twins, one of which had died but the sibling had survived. In this case, Corinne said that it was important that they:
Make sure that we have that information on our database so we’re aware if the parents should call or when we contact, just so we know that that is the situation and we need to handle with extra sensitivity.
Corinne – trial administrator, INIS
Astrid, the trial co-ordinator for PROGRAMS felt that, for her, the smaller sample size helped her to keep track of bereaved parents. She felt that making sure that they were not contacted ‘with the routine stuff’ was very important, ‘a huge thing’. She explained:
PROGRAMS didn’t have a huge amount of funding to set up advanced computer systems that would ensure that didn’t happen . . . I think the systems I put in place myself manually have worked. I always found it very worrying writing to parents, sending newsletters and sending the original letter, you know, I used to go over the pile of letters and backwards and forwards looking at the database and almost sleepless nights thinking the last thing I want to do is send a routine letter to the parent of a bereaved child.’
Astrid – trial co-ordinator, PROGRAMS
Trial team responses to bereavement
All interviewees wanted to draw a clear distinction between support, which they felt was the territory of the clinical centres, and an area of specialist expertise, and any response that they might make that would be of a totally different nature. Una, who had oversight for the trial managers for all of the CTU-led core trials considered here, explained how she distinguished between the formal support systems that were available via clinical centres and the informal ‘sympathetic ear’ that trial staff might offer on an ad hoc basis.
I don’t see our role, as triallists, is to do anything, I mean, I think we should provide information about where bereaved families can go to for support, but I don’t see that it’s our role to support them. We’ve got no training, we’ve [got no] expertise in this. Having said that, if a bereaved parent rang up, we would find the right person to ring them back. The trial co-ordinators, are not in a position to – to offer any kind of counselling. Obviously, they could [offer] a sympathetic ear, and I’ve heard them on the phone. When parents ring up, they get a hearing, and, very often, that’s what parents want, is just to talk to someone.
Una – trial manager, INIS, TOBY, PROGRAMS and BOOST-II UK
Astrid expressed a similar view:
For PROGRAMS, we had no contact with the family and most of those babies died within the first month or two whilst in hospital, whilst in the care of their consultant and so I think for us to try and get involved to any degree after that wouldn’t be right. It’s like ‘Well who is this person? I know we signed up for some – but who is this person?’ You know, I think a letter is – that’s enough. A letter with a form giving them the option to contact us, you know, if they want to talk about anything is enough.
Astrid – trial co-ordinator, PROGRAMS
There was an anxiety in the interviews about intervening and making things worse. Belinda was concerned about the impact of intervening in any way, arguing that they could ‘come from the outside and be completely off the wall’.
This distinction between support and a sympathetic ear was important for the CTU interviewees. Although it was not surprising to find staff with managerial and administrative roles expressing a disinclination to be drawn into forms of contact that might involve direct support, reactions to the extent and forms of routine trial communications (such as standard letters, website information, etc.) that were initiated by the different trial teams suggest that decisions about a response to bereavement on any level were ethically and emotionally complicated and logistically difficult. The trial teams for the four CTU-led core trials had different policies in place which determined how they managed contact with bereaved parents. The contact policies included:
-
no contact with parents during the course of the trial following their bereavement
-
early acknowledgement of bereavement
-
newsletters
-
response to late bereavement
-
web-based message board
-
feedback of results.
The contact policies are considered below and Table 36 shows the different bereavement-related strategies used by the core trials.
| Trial teams | INIS | TOBY | PROGRAMS | BOOST-II UK |
|---|---|---|---|---|
| No contact during the course of the trial | ✓ | ✓ | ||
| Early acknowledgement of bereavement | ✓ | |||
| Newsletters sent to bereaved parentsa | ✓ | ✓ | ||
| Response to later bereavement | ✓ | |||
| Web-based message board available to bereaved parents | ✓ | |||
| Feedback of results to bereaved parents | ✓ | ✓ | ✓ | ✓ |
ExPN, INIS and BOOST-II UK had no further contact with parents during the course of the trial following their bereavement (see below for further discussion of this), but the teams for PROGRAMS and TOBY did have a policy of contact in place. They had to take into consideration issues of timing, logistics and content. The various elements that were developed with the assistance of SANDS or lay members of trial advisory groups were approved by a REC as part of the trial processes. (For an exception to this, see Bridget’s comments, below, on feedback of trial results to parents.)
Early acknowledgment of bereavement from the trial team
The earliest point of contact with bereaved parents was initiated in TOBY where a leaflet for use in the recruiting centres was produced (see Appendix 14 ). The leaflet expressed condolences and thanked parents for their decision to allow their baby to take part in the trial. It also included contact details for the trial team. The intention was to provide a sensitive reference point for the trial which was flexible and could be used by local clinical teams in ways that fitted with their local bereavement practice. The leaflets were sent to the TOBY recruiting centres. Decisions about if and how the leaflet might be used, whether added to a bereavement pack or given to parents in person by a doctor or nurse, were entirely passed over to the clinical teams. Bridget received no feedback as to how widely the leaflet had been used and to what effect. [None of the clinicians interviewed in relation to TOBY indicated that he/she gave (or did not give) the leaflets to parents.] She said:
[W]e left at the discretion of the clinicians [how to use it] and I don’t honestly know how widely they used it . . . I’ve got no way of knowing. We didn’t document [it] you know. I never said, ‘Did you give it to them?’ Even if [the parents] got it, who knows if they read it.
Bridget – trial co-ordinator, TOBY
Letters and newsletters
None of the trials made direct contact with the parents initially following their bereavement. Belinda saw a difference between trial-related contact to pass on condolences that might be expressed on behalf of the INIS trial staff based in the CTU, which she saw as tokenistic and potentially problematic, and condolences that might be managed on behalf of the trial in a clinical centre by a local INIS research nurse.
I’m in two minds . . . whether you would send them a . . . a condolence card because I do think from us here . . . from if you like the, the management of the trial, it would be almost lip service . . . I would think if I was a bereaved parent I would rather not have that but I think locally, maybe from our local nurses or something, they could have been [sent out].
Belinda – trial director, INIS
For TOBY, a personalised letter was prepared and sent to parents at home but this was not triggered by the initial information that a baby had died. As the letter included the offer of receiving the next and subsequent trial newsletters, it was timed to precede despatch of newsletters to all interested parents, rather than to fit with a specific number of weeks or months post bereavement. This meant that some parents would receive the letter and reply slip closer to the time of their bereavement than others. The aim of the letter was to express sympathy and to thank parents for their decision to allow their baby to take part in the trial and to determine whether there was interest in further contact with the trial team (see Appendix 15 ). Parents were asked to indicate on a reply slip whether they wanted to be sent newsletters, and later the results of the trial. The reply slip was included with each newsletter so that parents could opt out at any point should they no longer wish to receive them. Bridget said that ‘nobody ever came back to me after [the first reply]’ to change their decision. A change of address card was also included for parents who wanted to keep their records up to date for future contact. Bridget said there were some parents who did not want to receive newsletters or results but that that she was ‘surprised how many’ parents did want to have further contact. Bridget commented that shortly before the interview she had received a change of address card from a family who were some years on from their bereavement. She was asked if parents had given her any indication of their views on the reply slips and she said that they had not.
For PROGRAMS, Astrid explained that ‘Probably 50% of bereaved parents have said “Yes please, do send the newsletter” and probably only two per cent of those, of that fifty per cent have since said “Please don’t send me any more”.’
These systems were developed as a result of reactions to the very first use of newsletters for parents at the CTU. The PROGRAMS trial team had sent newsletters to all parents, including those who were bereaved. This was a deliberate strategy to ensure that bereaved parents were not excluded from the trial on the basis of their bereavement alone. Una explained that this had resulted in two difficult telephone calls for staff in the trial office:
We had two phone calls from quite angry [people] . . . from then on if we know about a bereavement, we write to the parents and say, you know, ‘We’d like to give information to people who took part, would you like this information?’. . . ‘Would you like the newsletters or would you like a summary [of the results]?’ And then they can say what they want, and then that’s what’s sent to them. And, if we don’t hear from them, we take that as a ‘No’.
Una – trial manager, INIS, TOBY, PROGRAMS and BOOST-II UK
The newsletters themselves were brief, and generally kept parents up to date with the progress of the trial. Astrid said that for PROGRAMS they were very aware that the newsletter would be read by bereaved parents, and that as a result ‘a lot of thought goes into the newsletter [and] . . . I think what we say is fairly sensitive.’ She was asked whether they thought that there were any grounds for having slightly different versions of the newsletters for parents who are bereaved and parents who have surviving babies. Astrid was concerned that there would be little to say in a newsletter specifically targeted to bereaved parents.
What could you tell parents who are bereaved? There’s nothing to tell them really, is there? That, well I suppose the only thing you would leave out would be the follow-up, but PROGRAMS now, that’s basically all we’re doing. We’re following up children so I think that there would be really probably no point in sending out a newsletter just for bereaved parents. I don’t think there’s enough to tell them.
Astrid – trial co-ordinator, PROGRAMS
Astrid was asked what she thought parents might think about the newsletters but she said that she had received ‘no feedback at all, no comment at all’.
Response to later bereavement
Occasionally, babies enrolled in a neonatal trial die at a much later stage and systems are in place to alert trial teams to late deaths. This is required for data collection purposes but it was also important in relation to managing contact with parents. As with the early in-hospital deaths, the trial team would wish to avoid sending follow-up appointment letters to parents of babies who had died. Bridget was the only interviewee to say that she also used this information to make personal contact with bereaved parents.
If I don’t happen to hear through the grapevine beforehand then we get the [formal] notifications. And then at that point, you know, even now, I still would . . . just send a blank greeting card or flowers or something . . . not usually a bereavement card as such, but a blank card, and, and just, you know, write in it myself by hand.
Bridget – trial co-ordinator, TOBY
Web-based message board
The TOBY trial was the only trial to put in place a route for parents (whether or not their baby had survived) to make contact with each other. A web-based message board was hosted by BLISS and was open to any parents of babies who were enrolled in TOBY. Bridget explained how the message board came about:
It had crossed my mind that, you know, it’d be nice because obviously they’re not going to meet other TOBY families by chance. It would be very unusual for a unit to have two together and, you know, and they were so spread around the country . . . but of course it was something, you know, I couldn’t possibly do, either from a time point of view or confidentiality. It just wasn’t the right thing.
Bridget – trial co-ordinator, TOBY
Parents were informed about the message board via one of the newsletters and the information was also included in the compliment slip that accompanied follow-up appointment letters for surviving babies. Parents signed up to the message board without the mediation of the CTU. Having an external and independent host circumvented data protection issues and allowed parents to offer mutual support, information and friendship to others who had undergone similar experiences. Bridget felt that it was ‘very successful for the group that fed into it’. It was however noteworthy that none of those who contributed to the message board was a bereaved parent. It was not clear whether this forum did not suit the bereaved parents, or whether they did not know of the existence of the message board. The mechanism for informing parents was largely geared towards the parents of survivors, and Bridget said, ‘I can’t remember if I put those slips in with any of the [letters to] bereaved parents. ’
Feedback of results
All four of the CTU-run core trials fed back results of the trials to parents. How they chose to manage the feedback process for bereaved parents varied from trial to trial. The timing of feedback was an important consideration. Trials may take a long time to recruit, analyse and to report their results and for most of the parents involved, years would have passed between their baby’s enrolment in a trial and the results becoming available, especially if their baby was recruited in the early stages of a trial. The different time between recruitment and feedback of trial results to parents for the four CTU-led core trials are shown in Table 37 below.
| Recruitment and feedback | INIS | TOBY | PROGRAMS | BOOST-II UK |
|---|---|---|---|---|
| Recruitment period | October 2001 to September 2007 | December 2002 to November 2006 | June 2000 to June 2006 | December 2007 to December 2010 |
| Feedback of results | Autumn 2011 | October 2009 | Summer 2009 | May 2011, preliminary results |
| Time between start of recruitment and feedback of results | 4–10 years | 3–7 years | 3–9 years | < 1–4 years |
At the time of the interviews with CTU staff, TOBY was the only trial to have reported and to have finalised a strategy to send out results to parents. The INIS and PROGRAMS teams were still working out the fine details of the feedback strategies.
For PROGRAMS, there were concerns as to whether or not the communication about the results would be received by parents. This trial had the longest time of all of the trials between the start recruitment and feedback of results. The first babies were recruited in 2000 and by the time of the interviews, 9 years later, results had not yet been released to parents. Astrid was concerned that for the bereaved parents, in particular those who had not opted to receive newsletters, there would have been little or no contact with the trial team. She said:
Their address details may be completely out of date and, well, [i] they are out of date or whether they’ve moved I don’t know. So no, I don’t think we can send it out to everybody but I think we could send it to everybody apart from bereaved parents.
Astrid – trial co-ordinator, PROGRAMS
The results were communicated in a final trial newsletter and it was eventually decided that where parents had not opted to receive newsletters, or had opted in and subsequently opted to discontinue, the results newsletter could not be sent. Astrid appreciated that, over time, parents may have changed their minds and be interested in receiving the results, but they had made a clear decision at the time and she felt that there was no way back: ‘You’ve got to leave those and that’s it.’
The PROGRAMS results showed no significant effect of the trial intervention. Astrid felt that this meant that the results would not particularly raise any issues for the bereaved parents.
Had it been the other way round, had they shown that there was a huge benefit for those receiving the drug it may have had implications to those bereaved parents perhaps if the child didn’t get the drug, so I think that’s something that would have to be thought about for trials where it does show [a difference], like the TOBY trial for example, but for PROGRAMS that’s not an issue.
Astrid – trial co-ordinator, PROGRAMS
For INIS, the feedback process was complicated by the fact that there was no specific point of contact with bereaved parents over the course of the trial at which parental interest in receiving the results could be determined. As the trial had not used newsletters, the contact point at which the trial team had offered parents the results was at the first follow-up of survivors. Belinda explained:
The mechanism is on the [follow-up] questionnaire at two [years old], so . . . the bereaved parents if [their baby] died before two, won’t have had the option to opt out or opt in of getting the results.
Belinda – trial director, INIS
At the time of the interview in 2009 it was expected that, as a result of this policy, which was inherited rather than initiated by the current trial team, bereaved parents would not be sent the INIS results. By the time the results were actually available in 2011, a decision was made to write to all bereaved parents to ask whether they would like to have the results. This was a significant gesture as it involved a not-inconsiderable workload for a trial for which, by that point, funding had ended and no dedicated staff were in post. The time from recruitment to feedback was between 4 and 10 years and as there had been no update of addresses for bereaved parents, only addresses given at the time of recruitment could be used. INIS showed no significant effect of the treatment. Parents who chose to receive the results, including those who were bereaved, were sent the results and also offered the option of unblinding should they want to have this information.
The TOBY results were due to be communicated to parents in 2009 and preparations for this were under way at the time of the CTU interviews. As this occurred after the trial was complete, the REC that had approved TOBY, and had considered all of the previous communication strategies for the trial, said this no longer came under their remit.
[W]hen you get to the point of doing your results newsletter for the parents, the grant has finished. You’re no longer under the umbrella of the ethics if you like . . . so there’s really no-one to send it to . . . So, you know, I found that a bit odd. But anyway, but of course we’ve passed this newsletter round (to the rest of the trial team).
Bridget – trial co-ordinator, TOBY
As described previously, the parents could indicate at an early stage that they did or did not wish to have further communication from the trial team. For PROGRAMS parents opting out of contact via the newsletters had the effect of ruling oneself out of receiving the results newsletter. For TOBY the decision about receiving newsletters was set up as independent of the decision about results; parents could opt out of the newsletters but say that they still wanted the results newsletter when available. This meant that for parents who had opted out of the newsletters, but who wanted to be sent the results, the results newsletter would be the first contact in a long time.
For one or two . . . of the bereaved parents, the results newsletter might be the first newsletter they’re getting . . . I think I will have [the covering] letter saying, ‘you have told us in the past that you want to receive this.’
Bridget – trial co-ordinator, TOBY
For these parents the time from that decision to receiving the results of the trial would range from three to seven years. While Bridget saw this as potentially quite difficult, she also expressed some concern about the parents who had wanted no further contact:
Now, obviously if the people have said no to everything then I won’t send it, and I just hope they don’t.
Bridget – trial co-ordinator, TOBY
Bridget did not complete this thought but went on to explain that she was aware that it was possible that parents who had not opted to receive the results may hear about TOBY through other routes. She said, ‘Maybe if there’s any press coverage, you know, they’ll pick it up from there and they can, might choose to come back to us at that point, I don’t know.’ This comment from Bridget highlights the last in a long line of examples of gestures that were made towards bereavement by the trial teams, without feedback or reassurance being given to let them know that they have done the right thing. This sense of working blind underpinned much of their practice in this area.
Some time after the interviews with CTU staff, the preliminary results for BOOST-II UK became available. The trial was stopped early at the end of 2010, after a significant difference in mortality was identified between the trial arms. A publication of the preliminary trial results was quickly arranged and a linked communication was prepared for parents. For this trial, recruitment, and for some parents bereavement, was a more recent event and the trial results were sent to parents in 2011, who were, in some instances, < 1 year on from their baby’s death. This frames the communication very differently from the situation where parents are almost a decade away from these experiences when they receive the results. It was not possible to discuss this turn of events with the CTU staff but parental experiences of receiving results of BOOST-II UK will be described in Chapter 9 .
Overview from the Clinical Trials Unit staff
The bereavement-related practices described here are part of both a broad CTU policy of treating bereaved parents with respect, and more specific individual trial team policies that were implemented according to the strategies developed by each team. Corinne explained the importance of this within the CTU.
[T]rial co-ordinators are particularly careful . . . They’re very aware of what they’re dealing with . . . . We do handle any cases with dignity I think really, even when we’re just looking through a folder of a baby that died three or four years ago . . . looking at some data – we’re all aware this is still a child you know, who died and the death certificate is there, and they had parents that grieved for them, you know and so I don’t think it’s anything that anybody takes lightly or [is] blasé about it.
Corinne – trial co-ordinator, INIS
Belinda argued that the notion of individual respect should inform the management policies of individual trials.
I think the principle is that trials need to care that . . . parents are bereaved and they need to apply the best, if you like, management policy for that trial . . . [T]hen it has to be applied directly to how the trial is going to work and be managed day by day.
Belinda – trial director, INIS
At the CTU the management policies evolved as experience grew and were shared across the different trials, and refined in each trial in relation to its set up and working conditions. Una, with a management role for each of the CTU-led core trials, was well placed to reflect on the development of bereavement strategies for the different trials over time. She pointed out the interplay of multiple factors: the constraints placed around the trial teams by research governance frameworks, the sense that they as a CTU should make some response to bereavement, and a degree of uncertainty about doing so.
I think we’ve been hampered, to a very large extent, by ethics committees not allowing . . . us to interact with bereavement . . . but then, I’m not sure that we’ve actively pursued [it] . . . I’m not sure that that’s our role, either?
Una – trial manager, INIS, TOBY, PROGRAMS and BOOST-II UK
This tentative approach to bereavement, a sense of the need to do something and hoping but not knowing that the policies that were in place would be well received, was widely expressed in the CTU interviews. Corinne felt that for her, taking part in an interview for BRACELET had ‘brought up a whole load of issues’ about how they respond to bereavement. She and Belinda were implementing policies which had been put in place by predecessors and were not necessarily working in a way that most suited their preferences. The complexity of how best to respond was however, becoming evident for her as the BRACELET discussion unfolded. Corinne said:
I came in thinking ‘I think some sort of contact with parents when the child died is a good idea.’ That was my thought, but I don’t know when, how or in what format, you know, how to do it, ‘cos it’s just not as simple as that . . . [Y]ou need to know who’s going to be taking charge of it, and how it’s going to be dealt with logistically, who’s, you know, it’s the collaboration of the centres as well . . . I don’t know whether there should have been . . . [a letter] sent to those parents [whose baby] died, saying ‘We know you entered into the INIS study and we’re sorry to hear that your child died. Please tick here if you want to continue receiving information . . . I think it’s definitely possibly problematic, but then I also think not contacting them is probably desperately problematic, but you just don’t know about it because you haven’t contacted them. So I think there probably are people that we didn’t contact that possibly are unhappy that we didn’t contact them, but . . . I obviously don’t know because we never contacted them.
Corinne – trial administrator, INIS
Discussion
The clinicians in the trial recruiting centres, and the trial co-ordination and management staff, are the two main parties who input to the day-to-day running of trials. In seeking their views on trial-related bereavement practice, we found that although they are engaged in a collaborative research endeavour, and are used to working together with shared set trial processes, approaches to bereavement in any of the core trials were not shared and integrated.
In the clinical centres, among the trial investigators and recruiting clinicians, this was largely due to a sense that bereavement and trials were entirely separate issues; that bereavement-related needs, which the consultants felt should be managed by health-care professionals, were already well served by their standard local procedures, and trial participation, which the majority did not perceive as needing consideration after a death, would be provided for on a case-by-case basis if parents indicated that it was necessary. In the accounts of most of the consultants, by the time parents came back for bereavement follow-up meetings, they viewed a baby’s joint status as trial participant and patient as having reverted to their initial single status as patient, and the consultants dealt with their case in the framework of a doctor–patient/doctor–parent relationship. The nurses who were interviewed did not perceive the same separation of trial participation and bereavement as their colleagues, and largely assumed that the doctors providing bereavement support would discuss trial participation with parents as a matter of routine. For the trial co-ordination and management staff there was a similar expectation that issues relating to trials for bereaved parents would probably be dealt with by clinicians under the auspices of their bereavement care policies.
The consultants largely based their views on their direct observation of parental responses to the bereavement follow-up visits. Although bereavement itself is very varied, the consultants described a somewhat regular response to trial participation at this time, they felt that it was an issue that was not a priority for parents. In the main they felt that the trial disappeared from parental accounts and concerns. They also drew on their understanding of the theories that underpin trial methodology to judge that trial participation was no longer a relevant issue; they drew on ideas of uncertainty (equipoise) that drive both the need for the trial, and legitimate the enrolment of an individual into a trial, to argue that there was generally little information parents could be given about the actual impact of a trial intervention for their baby. With trial participation seen as being of little concern for parents at the bereavement follow-up meeting, and information about the effects of an intervention being uncertain, trial participation did not feature in the neonatologists’ accounts as warranting a particular response.
The confidence of the neonatologists in the systems that they had in place to serve the needs of bereaved parents, was consolidated by their clinical experience with bereavement and their direct encounters with parents. It is in sharp contrast with the more tentative and uncertain approach to bereavement that was discussed with the trial co-ordinators and managers. An important and recurrent theme that ran through this group of interviews was identified; it appeared that in all aspects of their responses to bereavement, the trial staff were working blind. They were at times unsure as to what the most appropriate response, if any, should be. Una explained that their lack of direct contact with parents meant that from the CTU perspective ‘it’s quite hard to know what they must be going through’. When provision was made, the CTU staff had almost no feedback as to how their gestures of condolence or information that they provided were received. They were aware that increasing the time between recruitment and any of the forms of contact used also increased the possibility that the trial teams had an out-of-date address for the bereaved parents and it was often difficult even to know whether their paperwork, leaflets, letters, newsletters and results, had ever reached the parents who had requested them.
An area of particular interest in the interviews with the clinicians, trial co-ordinators and managers related to the idea of support for parents. Although the neonatologists were equipped to give support they did not feel it was necessary to link this to the trial; the trial staff felt that it was probably necessary to offer support but did not feel equipped to give it. These concerns appeared to arise from an assumption that a response to bereavement would be focused on emotional support as distinct from information giving, or acknowledging participation. There were a number of potentially distorting effects of the focus on support as a response to bereavement in trials.
First, it assumes that trial participation is problematic and parents need emotional support to cope with it. That did not fit with clinicians’ views of parental situation; they do not witness trial-related distress that might require their support. The CTU staff had no contact with parents and so could not judge the impact of their experiences or their emotional needs in this regard but were unsettled by the possibility that they may be drawn into areas that were beyond their expertise.
Second, a focus on support distracts from the potential to consider other possible responses to bereavement in a trial, such as addressing any information needs that might exist or acknowledging and explaining the value of the contribution to research made by parents and babies.
Lastly, focusing on what is not done (offering support) obscures what actually is done in response to bereavement in trials. The consultants were clear that any parents who had issues that they wanted to discuss in relation to the trial would be encouraged to do so, and a minority said that they would, or do, raise the subject of trial participation with parents, even if it is just to make sure that there are no unarticulated questions or concerns. An important issue that was not fully acknowledged in the discussions about the need for reassurance that a baby has not been negatively affected by a trial intervention can be addressed only if it is genuinely possible to offer such reassurance. In many trials, neither parents nor clinicians will be aware of which trial arm the baby was in. In addition, the overall results are unlikely to be available at the time of the bereavement visit – and maybe not for a long time afterwards. Even in trials such as BOOST-II UK where one group was shown to have higher levels of mortality than another, it is difficult to move from the overall result to the effect on a specific baby to offer such reassurances.
Some of the clinicians were open to the possibility that parents might have issues or questions of which they have been unaware, or that the bereavement follow-up meeting might not be the forum in which those questions might come to the fore, and a number stated during or after their interviews that they would be thinking through a lot of the issues that had been discussed. The co-ordinating and managerial trial staff were making a number of responses to bereavement in the form of condolences, information and acknowledgement of the value of the contribution, but these gestures were sometimes tentative and are constrained in important ways. As already stated, the lack of feedback they receive means that they cannot know the value (or the cost) of their actions for the parents, and this generated uncertainty. In addition, the limits placed around contact with parents by research governance and data protection systems brings an extra complicating layer into the situation. Unlike clinicians who encounter parents directly and are free to make their own judgements about need and how to respond, who can consider trial participation in relation to a rich picture of the parental story, trial teams have to respond at a group level. They must plan any response ahead and submit their ideas and paperwork to a REC to seek approval for both mechanisms of contact and content. The limits around the information that can be stored mean that the ability to engage, inform and acknowledge, and to gain feedback on the appropriateness of doing so are limited.
These data suggest that at present there is no agreed or integrated response to bereavement, and responses by trial team members and local clinicians to bereavement following participation in a trial are varied. There is no consensus that a response specific to both the trial and the bereavement needs to be in place. The different parties involved have different skill sets, and different opportunities and conduits for a response. Even if some specific response is thought to be necessary, there is no agreement about what it might be, when it might be provided or who might be responsible for it. While uncertainty remains as to the impact on parents of bereavement in the context of a trial, this variable situation is unlikely to change.
These considerations give important direction for further reflection on the parental experiences of bereavement and trial participation. The consultants’ view of the parents having priorities other than their baby’s inclusion in a trial in the weeks or months following the death of their baby fits with the accounts of the parents interviewed for BRACELET in relation to the time around the death of their baby. For most parents, the trial had ‘disappeared’, either at the time of crisis in the NICU or around the time of their bereavement. It is however important to consider how they viewed their involvement in a trial in their ongoing life as bereaved parents. In the following chapter, we explore parents’ views about contact with the clinicians involved in the trials following their bereavement, and their experiences and/or views of trial-led policies of contact and feedback.
Chapter 9 Parents’ responses to bereavement in neonatal trials in the longer term
In Chapter 8 we presented the neonatal consultants’ views of parental needs and preferences for bereavement follow-up. For the majority, trial participation was seen as an issue that was superseded by, and so separate from, bereavement. It was viewed as an unnecessary and possibly inappropriate area for discussion in the follow-up meetings. From the interviews with parents previously presented in Chapter 7 we showed how, for the majority, despite their initial hopes that the trial might help their baby, the trial disappeared or receded over their time in the NICU or on bereavement. This would seem to confirm the consultants’ sense that the follow-up meeting is not in the main a forum for the discussion of trials. The trial co-ordinating and administrative staff for the core trials had a range of policies in place in relation to bereavement in their trial populations, ranging from no further contact for ExPN to a multipart strategy for TOBY, which responded to bereavement from NICU to the point of sending out the results of the trial. For the trial staff, their lack of feedback from bereaved parents meant that it was very difficult for them to know how these gestures and responses to bereavement were received.
Here we consider parental perspectives on practice in the clinical centres and on the various trial-led policies that were in place as described in Chapter 8 . The data cover parents’ experiences of attending bereavement follow-up, as well as determining their views on whether or not it would have been interesting or of value to have discussed their baby’s involvement in a trial at that time. The interviews covered their thoughts on contact with the trial team, through leaflets, newsletters and feedback of the results, as well as contact with other parents through web-based message boards. In many cases, therefore, these views give a much longer term perspective than previous chapters.
Bereavement follow-up via clinical centres
The bereavement follow-up (BFU) meeting is made available to parents to offer emotional support and guidance as well as to review their case and to consider the information available about their baby’s condition, progress and death. Where a post-mortem examination has been carried out the follow-up is usually timed to coincide with the results so that these can be presented and explained to parents. For most, this meeting is the last contact the parents will have with the NICU and the NICU staff, and so their last opportunity to discuss issues of importance to them. The consultants explained that while some parents are keen to come to meet with them, and to see the nurses who cared for their baby, not all elect to attend a BFU meeting. It can be challenging to re-enter the NICU and to revisit such difficult experiences especially in the short time frame that is often used. Occasionally, they find that parents who chose not to attend do still have outstanding questions and request a meeting years later. In the course of recruiting parents for BRACELET, one of the consultants contacted a family to ask if they would be interested in receiving an invitation to take part in an interview. The parents did not wish to participate in BRACELET but the contact with their consultant triggered a number of questions for them and, as a result, a bereavement follow-up visit, several years after the death of their baby, was arranged.
Just over half of the parents interviewed said that they did attend a BFU meeting (n = 17 interviews), and a smaller group did not (n = 6 interviews). In some of the interviews (n = 7) it was not clear whether or not parents had attended, either because they were unsure themselves or because there was some ambiguity in the dialogue of their interview ( Table 38 ). The views of parents who did attend a BFU meeting are considered in two groups; the majority, who did not discuss the trial with their consultants, and the small number who did.
| Discussion of the trial | Attended BFU | Did not attend BFU | Not clear |
|---|---|---|---|
| Did not discuss trial | Laura and Wilf | Marion and Doug | Shirley and Warren |
| Stefanie and David | Robert | Fiona and Keith | |
| Julia and Lewis | Jill and Ethan | Dora | |
| Amanda | Liza and Wesley | Milly and Adam | |
| Sara and Gareth | Chloe | Danielle | |
| Anita and Sean | Dawn | Lesley and Stan | |
| Sophie and Nat | Beverley | ||
| Justine and Francis | |||
| Jana | |||
| Karen and Tony | |||
| Amy and Chris | |||
| Discussed trial | Rhona and Karl | ||
| Hesther and Stuart | |||
| Not clear | Caitlin and Pete | ||
| Alice and Ivan | |||
| Hannah and Ryan | |||
| Diane | |||
| Total | 17 | 6 | 7 |
Parents who did not discuss the trial at their bereavement follow-up meeting
Some of the parents described reactions to the BFU meeting that fitted with the consultants’ observation that trial participation is not a priority for discussion. Their accounts suggest a range of reasons for this. Three positions were identified. Parents did not:
-
want to discuss trial participation
-
have the capacity to discuss trial participation
-
think about discussing trial participation.
Parents did not want to discuss trial participation
Like a number of other parents in the study, Sara and Gareth said that they did not discuss the trial with anyone again. The INIS trial was not something that they particularly thought about in the initial weeks of their bereavement, given what they were experiencing at the time. They felt that they would not have wanted to have discussed the trial at the BFU; they felt no particular need and Gareth would have been ‘surprised if there had been anything to say on it to be honest’. Sara said:
Because he was only in the study for such a short period of time and he obviously didn’t make it . . . I mean how – how much of a chance would it have had to work? So maybe that’s, in my mind it was just closed off.
Sara – INIS
Stefanie and Anita both similarly felt that there was no particular need to discuss the trial at their BFU meeting. They were aware that there would have been very little additional information to give them at that point.
I was clear enough in knowing that it was a trial that had just started, would take a number of years, and that we would find out the outcome.
Stefanie – INIS
The INIS study never, ever came up [and] . . . I think at the time it was fine for what I needed. I think if she’d have brought it up, it wouldn’t have bothered me. I . . . I don’t think I’d have had any more questions about it. Because the research hadn’t been completed . . . there wasn’t really anything she could have [said], or I could have questioned about it.
Anita – INIS
Justine and Francis had no further conversation about INIS in the follow-up meeting, and this seemed appropriate to them. They had had a long and difficult reproductive history with repeated miscarriages, and, with the death of their son Edward, two preterm deaths. This focused their counselling session very firmly on reproductive issues rather than care, and the trial clearly fell outside of these concerns. Justine explained:
It wasn’t so much about what’s actually happened to Edward or Esther. It was more about, you know, if you want to still have a family, what can we do? Is there something we can do?. . . What can we do to stop you having a baby at twenty-four, twenty-nine weeks? We need to get to beyond that.
Justine – INIS
Parents did not have the capacity to discuss trial participation
Amanda’s daughter Simone was allocated to the control arm of TOBY and so was not cooled (see Box 5 ). She had almost no memories of the follow-up visit, which she felt was a response to the stress of that period. Everything from that time was ‘fuzzy’. She commented, ‘I know we were there, but I may as well not have been there’. She said her consultant referred her on to a psychiatrist for support. She felt that the BFU was too soon and that ‘it would be better maybe 6 months, 8 months down the line, when time has passed and heads have cleared a little’. Amanda still had a number of questions about the trial which she wanted to explore during the BRACELET interview. She asked how the decision about whether or not a baby would be cooled was made in TOBY. Even after 8 years had passed, she was clearly interested in understanding the trial and in exploring what had happened to them. She was asked whether it would have been helpful to have had the option of talking about the trial at a later date she said, ‘Absolutely, yes! But we never did.’ For her it seemed that the best option would have been a later appointment in which she could have taken a more active role, and where she could have explored events more fully. She felt that she would then have been able to take part in a discussion and to ask questions, including questions about TOBY, which were important to her. Amanda said that she would rather talk to her neonatal consultant about this than anyone from the trial. He was the doctor who had cared for her daughter and had been involved in their case from the start. (In Chapter 7 , Amanda describes how this same doctor was waiting for her in the NICU when she first visited Simone, to talk to her about transfer and TOBY.) She very much saw this discussion as grounded in an existing relationship, commenting ‘he’d know Simone, know me, know [my partner], but it would need to be a good few months down the line.’ A later timing would however have delayed the opportunity for referral on to specialist care, and that is an important balance that those providing bereavement support services have to make.
Julia, whose son was enrolled in INIS also found the BFU to be a difficult experience. She spoke powerfully of how overwhelming the meeting can be, especially having to go back to the neonatal unit, and of how hard it was to order thoughts and emotions.
[I]n the bereavement follow-up appointment, again you’re very emotional. You’re going back into that – for us back to that vast – back – every time I go past that hospital, that’s all I can think of – back into that hospital, back up to that area, you know, and the last time you were there it was very different. And you’re not thinking [about] the clinical trial, you’re just thinking to get through this, to hear what they have to say, you know. Obviously you’re emotional, your mind’s all over the place . . . I suppose [it is]only when you come out and you’re on your way home, or a week later or you’re thinking about maybe the conversation that you had in the follow-up appointment, you’re thinking ‘I didn’t ask about that. Maybe should have asked about that. He would have mentioned it if it was that important.’
Julia – INIS
Julia did not go to the meeting with a list of questions. She said, ‘all that we wanted to know was what exactly did happen.’ Parents can have limited capacity to take control of the discussion and Julia argued that they can rely on the clinicians to structure things for them and for her this included the clinicians taking the initiative in raising the issue of the trial. It was precisely because it was not high on her agenda at the time, as observed by the consultants, that she felt that someone else should bring the subject to the fore. If the subject of the trial was raised for them, parents could then decide whether or not it was important to them and whether they would like to discuss it.
All that we wanted to know was what exactly did happen . . . and you relived all that again. So the idea of a clinical trial and how that was going wasn’t a big focus in your head. So that’s why I think if the doctor or the clinician . . . [said] ‘You may remember, you know, your son was involved in a clinical trial. Would you like to discuss that? Or are you happy enough to leave that? Or if you want to come back at another time. Or do you want to find out later about how that has gone?’ I think that could be helpful.
Julia – INIS
Like Amanda, Julia felt that she would want to talk to her consultant about a trial, rather than to anyone else, because of his involvement in their case:
[W]e worked with him, he asked our permission for it initially, we had our follow-up appointment with him. So if you’d any questions, he would be the obvious one that you’d want to speak to.
Julia – INIS
Parents did not think about discussing trial participation
Jana, whose three sons were also enrolled in INIS, felt that it would have been appropriate for the topic of INIS to be raised at a BFU, but she and her partner had not thought about raising the topic themselves. For Jana, the potential value of a discussion was unrelated to the significance of the trial at the time, as she had not felt a particular need to discuss it, but, in retrospect, she thought it would be good practice, as shown in the extract from her interview below.
I think when we talked with our consultant, he didn’t raise it and neither did we. Except that moment [of deciding to take part] it was not an issue.
No? Would it have been a good idea for it to have been raised or was it fine the way it was?
I think it would be good.
For Sophie, a discussion about the trial not only would have been valued as a route to information and further discussion about INIS but also would have been received as an empathetic and supportive gesture.
For somebody to say to me, you know, ‘have you got any questions about that?’ You know, I would have received that well . . . [T]o have that opportunity to talk to somebody would, would have been good, and it would have showed, in my view, more empathy for knowing what my baby had been through and the fact that I might have questions.
Sophie – INIS
Julia and Sophie’s comments about not raising the subject of the trial themselves highlight two issues: (1) the role and responsibility of clinicians in responding to the situational aspects of the BFU interview, and how they interpret cues; and (2) the position of vulnerable parents who may or may not know, or be able to, or want to, express their needs. Neither of these parents said that they gave any indication that they wished to discuss the trial, and Amanda was clearly not in a position to do so at the time. They would, however, have welcomed the possibility of further opportunities for discussion, should the need arise, i.e. ‘the door remaining open for them’.
Karen felt that she would have been similarly open to discussion of the trial, but she pointed to differences between her and her husband’s positions, and this highlights the difficulties that clinicians may have in providing for different needs in a single meeting. Karen and Tony’s daughter was enrolled into BOOST-II UK. As described in Chapter 7 , Karen was keen for this to happen but Tony had concerns and took some time to come around to the idea of Tabitha being enrolled into the trial. Tony had to leave the BRACELET interview before the end for work-related reasons, and so only Karen was asked how it would have been for them if the topic of the trial had been raised. She felt that the difficulties and concerns that Tony had experienced initially would have possibly resurfaced for him in the follow-up meeting had they discussed the trial. She said:
Because I like [the consultant] so much, if he had brought it up I wouldn’t have, you know . . . I mean, he was there to talk about Tabitha so I wouldn’t have been worried. But I can see the point as well of parents getting stressed [about it] because Tony would have. Tony would have been one of them parents that would have questioned was it what they done in the trial that may have caused, you know, any of what happened to have happened, ‘cos he’s just – he is a worrier.
Karen – BOOST-II UK
Parents who did discuss the trial at their bereavement follow-up meeting
Only two couples stated that they did discuss their baby’s enrolment in a trial with their consultant. In the first instance the topic was raised by the consultant, in the second the parents went to the meeting with specific questions about the trial that they wanted to address and work through.
Rhona and Karl felt that it was appropriate for their consultant to have mentioned their daughter Ava’s enrolment in PROGRAMS in their BFU meeting. Karl said that it was discussed because it was ‘part of our journey we’d been on’. When asked for their view on the subject of the trial being raised, the response of both parents suggested that they interpreted this as an open and supportive gesture on the part of their consultant. Rhona said, ‘He was completely open with us and when . . . we’ve encountered a problem . . . it always felt like we were a team’ and Karl said, ‘You felt you could speak, and I think probably he felt the same.’ The trial was covered only briefly but that fitted well with its position within their larger experience. Karl said that he felt that there would have been no difficulty for their consultant in raising the subject:
Not with us, not with the trial we had and the way it kinda . . . seemed to fit in to everything that was going [on . . . I]t certainly didn’t seem to be first and foremost, the focus of . . . everything . . . It was very much kinda going on in tandem.
Karl – PROGRAMS
A trial played a much more prominent role in Hesther and Stuart’s time in a NICU and figured more prominently in their account of their bereavement. The events that surrounded the birth of their son, Joel, were fast moving and unexpected. He was born, enrolled into TOBY, cooled and rewarmed all in a short time, and he died only 14 hours after he was born. The parents felt that in such a fast-moving context there were many questions that they had not got around to asking; Hesther said that they still ‘sit here at night . . . asking questions’. Stuart said, ‘we still don’t know if [we] . . . did this right, did this wrong. ’ At the BFU meeting Hesther raised the topic of TOBY herself. She wanted to know whether their consultant had believed that cooling might work for Joel when he offered them the trial. They had a good relationship with the consultant, who was directly involved in the process of withdrawing care. She therefore felt able to ask him what might, for some, be challenging questions. Hesther said that she wanted to know if he was ‘clutching at straws just as much as we were’. She said:
I can remember asking when we went for the post-mortem results, [asking] ’did you truly believe that TOBY would work when you did it . . .? ‘ And he said . . . he did, he did believe it would work, else . . . he wouldn’t have suggested it’. . . [H]e did believe it’d work, it was just that Joel went colder than he should have done.
Hesther – TOBY
This question was raised for them in response to the short time that Joel spent being cooled before he was rewarmed and care was withdrawn. Hesther said, ‘it was because of how quick he was on and then straight back off it. I think that was why. ’ They wanted to know whether what they had been through was worth it for the chance that might have been given to Joel, that there had been a chance of cooling working for them. Their wish to examine and understand the decision-making process through the bereavement follow-up highlights both the potential for that meeting to address any questions that parents might have about the trial, but also suggests that achieving a level of understanding about a trial that meets any existing parental needs may be an important step in bereavement. Hesther wanted to understand an element in their experience that related to the trial and for her the open discussion in the BFU meeting was the right forum; she wanted to know more about their doctor’s motivation but also to fit this with their own decision-making and sense of responsibility for their baby as an important part of her working through her bereavement.
Bereavement contact via Clinical Trials Unit
The bereavement contact strategies developed for the core trials were conducted in two time periods. The initial period of contact ran alongside trial recruitment and follow-up and included the use of:
-
a leaflet prepared by the trial co-ordinating centre for the clinical centre to hand out (TOBY)
-
a letter sent by the trial co-ordinating centre (offering newsletters) (TOBY, PROGRAMS)
-
newsletters sent by the trial co-ordinating centre (TOBY, PROGRAMS)
-
web-based message board hosted by the trial co-ordinating centre (TOBY).
The later period of contact followed analysis of initial or follow-up trial data and involved.
-
feedback of results sent by the trial co-ordinating centre (TOBY, PROGRAMS, INIS and BOOST-II UK).
The bereaved parents involved in BRACELET had varying levels of experience of these strategies. For those involved in ExPN for instance, there had been no communication with the trial after their bereavement; for those involved in INIS and BOOST-II UK communication was linked only to feedback of the results, whereas the parents involved in TOBY and PROGRAMS could have received communications in a number of forms. Parents may not, however, have received information for which they were eligible if the TOBY leaflet was not used in their clinical centre, or if newsletters and results did not reach them as they were no longer living at the address logged in the trial records. The interviews therefore explored parental experiences of CTU communication strategies where possible, but often these were considered in hypothetical terms.
The model of the TOBY bereavement communication strategy was used in the interviews to structure discussion. A file was created which included examples of the communication paperwork which parents could look through and which could be used for reference. This included copies of the TOBY leaflet (see Appendix 14 ), personalised letter offering newsletters (see Appendix 15 ), and the newsletters (see for example Appendix 16 ). Copies of the PROGRAMS newsletters (for example, see Appendix 17 ), and those used for the INIS trial in Australia and New Zealand (for example, see Appendices 18 and 19 ), were also included for comparison, to access parental views of style and content as well as the principle of newsletters.
Initial contact strategy
Condolence leaflet
The TOBY leaflet was prepared by the trial administrator and supplied to the recruiting centres ( Box 12 and Appendix 15 provide full details). (A similar letter was sent to PROGRAMS parents.) It was also available for download on the TOBY trial website. Clinicians could pass on the leaflet to parents in person, or it could be included with other bereavement information routinely supplied to parents on the death of a baby. Clinicians could choose not to use the leaflet if they preferred.
None of the parents had direct experience of receiving a condolence leaflet from a trial, including those whose babies were enrolled in TOBY. They were quite split in their opinions, with some expressing concern or dislike of the idea or content of the leaflet, whereas it was well received by others. For some parents the leaflet would neither have engaged nor upset them.
The leaflet was the only one of the TOBY communications that was not presented in a simple font. It included a photograph of white flowers on the cover, and a sketched picture of blue flowers inside. Two of the parents did not like the font and layout of the leaflet. Robert, whose daughter was enrolled into TOBY, said, ‘I hate that . . . it feels like it’s a – it’s a gravestone and that just feels – I’m sure it’s very authentic but it [doesn’t] feel authentic.’ He later added:
[T]hey’ve probably worked on and thought about it but for me it – it sort of – it feels like overly emotional and I think it is a huge emotional time that you’re going through so don’t add layers of even more on . . . I’m not saying it should be brutal; factual, but don’t . . . try to make it feel as if it’s sympathetic.
Robert – TOBY
Beverley also felt that the production style of the leaflet was not right for her, and explained her association of flowers with her bereavement.
When we come back from the hospital and the house was just full of flowers. And I thought ‘Oh I just don’t like it, it’s like a funeral parlour or something . . . So I would rather if something was to come relating to Ruth that it was a picture of a baby rather than flowers, but that’s just personal to me I suppose.
Beverley – BOOST-II UK
For Sara, it was the generic nature of the leaflet that she did not particularly like. She said:
I don’t think a blanket leaflet is particularly helpful to be honest, if it just says thank you and there’s no personal details or anything particularly about that child, I don’t think that . . . wouldn’t offend me, but it wouldn’t . . . do anything for me particularly.
Sara – INIS
In contrast to Sara, who was somewhat nonplussed, Nat reacted quite strongly to the leaflet. For him, the issue was related not to style or to content but to the timing of the introduction of a reference to the trial in a medium that he felt did not fit the enormity of their experiences:
[A]t the point when I’ve just lost my son the trial would be the last thing on my mind. It’s dealing with the grief of the loss of a child, you know, the whole – the emotion, you know, supporting Sophie and supporting . . . our eldest, Otto, and . . . I wouldn’t have been remotely interested in reading a leaflet, to call a number or . . . I don’t know. I might, actually, that might have been one of those, as you go through the, the emotion and the anger sometimes, that might have been an anger moment for me . . . You’ve lost a child and, you know, and it’s in a leaflet!
Nat – INIS
He was not negative about the content or the general idea of contact, more about the specific timing and the use of a leaflet. He felt that a personalised letter, some months later, saying ‘“We’re awfully sorry” would be appropriate.’ This was more acceptable because of the distance from initial events and orientation to bereavement that would have gone on in the meantime.
[A]fter that period . . . you’ve obviously received lots of sympathy cards and, you know, messages of – of support . . . [Y]ou’re receptive to things coming to you in the post, and having messages of, ‘we’re sorry to hear about your loss’.
Nat – INIS
Francis also viewed the leaflet in the context of a recent bereavement. He said, ‘when your baby dies, nothing else really matters’ and that he would not have read the leaflet. His partner Justine said that a leaflet in relation to INIS was ‘probably not something that would make a difference to me personally’.
Anita’s views bridged our categorisation of parental reactions to the leaflet as she felt that it would have been difficult to have had the leaflet in the initial stages of her bereavement but she would have valued the gesture in the longer term. In their case if a leaflet had been included in the hospital bereavement pack it would not have reached her at the time, or since. She had put the complete pack of information that she received when Josephine died, unopened in her memory box and has been unable to look at it since. Occasionally, her daughter Carrie, Josephine’s surviving twin, goes through the box, but Anita still finds it difficult to engage with the contents. She has, however, kept them as significant, albeit potentially disturbing, items, and they are put away for a stronger time.
I remember getting . . . Josephine’s bereavement pack when we were leaving the hospital, and it’s still sat in the box . . . I know it’s there. I’ve taken it out of the box, but only because Carrie has this thing every now and again: ‘let’s get the boxes out, and let’s look at all my baby things, and need to look at Josephine’s.’ So we’ve taken it out of the box. I can’t look at anything that’s in it. I can’t do it. I just – I’ve never, I’ve never been able to. I can’t explain it. I can’t – I don’t know why. Everything’s in the box. She’s got lots and lots of things in the box. But the bereavement envelope from the hospital, I can’t look at. I just can’t. So that [leaflet] would be lost. But the letter a few weeks down the line, to me, would be perfect.
Anita – INIS
Fiona made a very similar comment:
We got a little pack from the hospital, and I’ve still got it. But I very rarely look at it. So I mean I know everybody’s response is going to be different, but the leaflet in there could quite possibly just sit and be ignored, because you can’t bring yourself to go and look at all that stuff.
Fiona – INIS
Interest in the leaflet
The leaflet included the name of the trial and contact details for the trial office. Milly, whose son was enrolled in BOOST-II UK in fraught circumstances shortly after the death of his twin, felt that it would have been useful to have had a leaflet as an early communication and a reference point. In particular, she felt that a record of the name of the trial was useful. Without this simple piece of information it is difficult for parents to access further information about a trial, and Milly said, ‘I didn’t know what ours was called. ’ This role as a reference point was important for Alice too:
I think it would’ve been good to have had it, definitely and then . . . when you’re kinda going through the packs at, you know, later dates, at least you’ve got someone. I mean, I’m not sure we would’ve contacted anybody but it would’ve been quite nice to have had some information.
Alice – INIS
Some of the parents who expressed interest or approval for the leaflet focused on the acknowledgment of their decision to take part. Shirley thought that having such a leaflet for ExPN would have been ‘brilliant’ and said, ‘A study is really to help other people research isn’t it . . . so it would be nice to be acknowledged for doing that, whether she died or not. ’ Diane said, ‘I think it is nice in a way, because it is actually thanking you for taking part in the study. ’
Marion focused on the supportive nature of the leaflet and described the gesture as ‘amazing’ and ‘a help for the parents’. When asked whether she would have liked to have had such a leaflet for INIS and she said, ‘That would have been brilliant. ’ Jana also felt that there was a supportive element in the leaflet and that she would have found it helpful. After one of her triplets died, his surviving siblings were transferred to a town nearer to their home but where they knew no-one. It seemed that any form of support at a time of isolation would have been appreciated.
The leaflets were also seen by some parents as a memento, although not necessarily one with which they were particularly comfortable. When we discussed the communication strategy, Stefanie said that she thought the bereavement leaflet and condolence letter would ‘Certainly be a good idea’ adding ‘at the time I just wanted every scrap of paper or information or letter, anything I could get hold of.’ Amanda’s interview touched on the same idea as Stefanie, as she would have kept any communication that related to her daughter. She said that it was ‘absolutely’ the right thing to use the leaflet at that early stage, but was also clear that had she received it she would probably not been able to have read it. Like Anita she would have stored it for a stronger time. Amanda had received one communication from the trial, most probably the letter offering the newsletters, but could not remember what it was. She said:
I did get a letter from TOBY, but I’m not sure how long after that was . . . Do you know what, I probably didn’t read it, and I think, I probably saw it’s TOBY on the letter, on the envelope . . . [and] I probably filed it away with Simone’s stuff . . . I mean I maybe opened and glanced at it, but it would be too painful to read it . . . I’m glad I got it.
Amanda – TOBY.
Amanda felt that she would have treated the leaflet in the same way.
Other parents viewed the leaflet differently. Caitlin, whose twin sons were enrolled in ExPN, said that ‘you read everything that’s given to you in a [bereavement] package’. Her partner Peter felt that a leaflet ‘would be a good thing’. In a similar vein, when Dora saw the newsletter she said, ‘That would be something I would want to keep.’
Julia would also have kept a bereavement leaflet had it been used for INIS. As soon as she saw it she said, ‘Oh that’s good!’. She felt that the acknowledgement and thanks for the role a baby had played in research was important even though it was a non-personalised form of communication: She said, ‘[I]t’s still nice and you feel that somebody is acknowledging the fact that you did take part in this, and acknowledging the fact that . . . [your child] has made a difference. ’
Julia reflected on how the leaflet given at this stage could later help parents in their exploration of events and grief. She felt that the reference to the decision to join the trial acknowledged a significant event in parental experiences and that acknowledgement might be an important aid to the emotional work that parents have to do to come to terms with events.
At some point in time I think you go back and visit these things, you know. Because you go through a whole process, as you’re going through the whole processes of grief, and anger is a normal part of that process – why did it have to happen to us and is there a God up there and all these kind of questions that go through your head. Personally I would like something like that, but that’s me personally. And if my child had say, had of died and I found out that they didn’t get whatever drug it was, and it could have helped them, again I might think why, but if you’ve gone through the whole process before that of knowing that they may get it or they may not get it, and you understand that bit, this is a nice acknowledgement that you were willing to take part and maybe you’ve helped another child. And I suppose later on, when maybe you’ve accepted things and you’re living with things and you’ve managed to move on, you can look back at this and say ‘Well maybe it was the right decision to be part of it.’ Personally I think that’s nice. I like something like that.
Julia – INIS
At the end of the interview Julia singled out the leaflet for comments, saying that it was ‘a very good idea’.
The interviews suggested that parents accorded very different status to the idea of the leaflet, from something of little interest, to something inflammatory, from an item that would be potentially too difficult to contemplate, to something that parents would value and keep. Interestingly, some of the parents who found the leaflet to be difficult would still keep it as a memento.
Personalised letter
In the TOBY communication strategy, a covering personalised letter was sent offering parents the trial newsletters ( Box 13 ). It gave condolences and thanked parents for taking part in the trial. A reply slip, which was included for parents to return, indicated that they would like to receive the newsletters. The fact that the letter was personalised, referring to the baby by name, was important. One of the parents, Jana, was very interested to look at the TOBY letter and said, ‘I would be grateful for it, yes and the personal form is really nice. I think it’s really important to make it personal.’
The letter prompted a lot of discussion. Much of the discussion, led by the parents, centred on the idea of acknowledgment and what that meant to them in the context of their bereavement. A number of different aspects of acknowledgement emerged in the data – acknowledgement of:
-
the contribution to research
-
the parents’ loss
-
twins.
Acknowledgement of the contribution to research
The letter offering the newsletters did not specifically thank parents for their decision to enrol their baby into the trial, but it could still be seen as an acknowledgement of links with research. This was the case for Caitlin and Pete, who saw the connection back to the research as a very positive and helpful gesture, even though they had presented the trial in which their sons were enrolled (ExPN) as a minor part of their experience. Caitlin said that the letter ‘would have been great’. She said:
[I]n some ways it just gives you a bit of a – you did something good, you know, a bit of a – not a lifeline but you know a . . . positive from out of a negative situation, you know . . . . [I]n my eyes I think that would have done brilliantly.
Caitlin – ExPN
As Caitlin continued she commented on an aspect of the communication which proved to be important to a number of parents; as well as giving parents a sense of satisfaction at their involvement in the trial, the arrival of the letter meant that they were not forgotten.
I think that’s very welcome. Massively welcome. I think it’s, it’s a bit of a – just a – you’ve not been forgotten . . . . [W]e did it ‘cos we knew if it didn’t help ours it would help other people . . . I think that for a lot of parents . . . would really be, ‘Wow, yeah, we did right there.’ You know, a bit of a positive, a lift if anything.
Caitlin – ExPN
Acknowledgement of loss
The sense of satisfaction at not being forgotten expressed by Caitlin was echoed in the accounts of other parents. who placed the letter in the context of what had been happening in their lives in those early days of their bereavement. Anita described this well. She and her partner, Sean, had a surviving twin, a particularly complex grieving situation. 157,158 At a time when her bereavement could seem to be invisible to the outside world, the letter would be an important acknowledgement of the loss of her daughter.
I remember seeing one of the buses going past across the top of the road and I just wanted to stop the bus and get on it and say, ‘Don’t you know that my daughter’s died? How dare you carry on with your lives?’ But you couldn’t because it’s not happening to them. It’s only happening to you, and it’s only within your . . . It’s within your family . . . but even within your wider family, they’re all getting on with their lives, and they’ve got their own things to be sorting out in their lives. But it’s your family unit that it’s happening to . . . so in some ways, to get something shortly afterwards to say, ‘Well, actually we do remember, and thanks, you know, thanks for the contribution. And, you know, if you want to know what’s going on . . .’ I think in that, in some ways that can provide some comfort.
Anita – INIS
For some of the parents the letter served an important purpose in acknowledging the life of their baby. For this reason in particular it was common for parents to say that they would have kept such a letter, with other significant items. Sara (INIS), who did not particularly like the idea of a leaflet, did like the idea of the letter. She felt that it could have a role in the future in helping her other children, including Jacob, Aiden’s surviving twin, to understand what had happened. She said, ‘I think the letter with the baby’s name on when they say thank you would be nice, just for like a memory box. Not so much for us, but for – just so siblings know. ’
This rang true, as Anita had described how her daughter likes to takes out her sister’s things from the memory box, and, after the interview with Shirley and Warren, their daughter showed the interviewer her sister’s clothes from the box that they had kept.
Julia felt that a letter from the trial team could be ‘tough and challenging’ if it came in the early days, but she would have kept such a letter with her son’s things to explore herself at a later date.
[I]nitially . . . you might feel ‘Oh can’t face this,’ even the letter, personalised letter, but it goes into the wee memory box, and when you’re ready to look at it, you can and you think ‘Yes, we really did make a difference.’
Julia – INIS
Like Caitlin and Anita, she too felt that contact with a trial team meant that ‘your child hasn’t been forgotten. ’ In a similar vein, Justine talked about the letter of a way of keeping their baby connected to the world. Justine (INIS) felt that this could be an important reminder for parents: ‘It would it keeps you kind of thinking, right, okay, well, just because Edward’s died, or any other baby’s died, that they’ve still got his details . . . because he’s taken part in that study. ’
Dora said that what she liked most was the use of her son’s name on the letter (further details of Dora’s response to the TOBY communication package as a whole are presented later in this chapter in Box 15 ). The use of names was also important to Stefanie, who said, ‘I was just obviously totally devastated about the boys but so proud that I’d had them, and wanted to make sure that their names stayed in the [neonatal] unit. ’ She and David and their extended family raised funds for the NICU and plaques with their sons’ names were attached to the equipment they had bought. In considering the TOBY letter she particularly liked the fact that it would refer to her son. She said, ‘It would still give me that wee connection [if it] had Callum’s name. ’ She too said that she would have kept such a letter ‘in the bereavement box’.
None of the parents talked about the letters as administrative items that would be filed or kept with paperwork. The letters in these accounts would very clearly have the status of a memento of a person and a time.
Acknowledgment of twins
Milly’s and Adam’s twin sons both died, Lee on delivery and Cameron after admission to NICU. They raised an important consideration for communication with parents of twins, where one baby was in the trial and the other was not. Lee had died earlier than Cameron, and Adam said that they ‘were getting a lot of correspondence, sorry that your baby’s, – your baby – child has died . . . [and] we were getting quite upset at the fact that no-one’s acknowledging Lee’. In such a situation they felt that it would be important for a trial team that was writing to acknowledge a bereavement to acknowledge both children. Milly said, ‘It would just be a courtesy . . . [I]t would’ve been nice to say, “Sorry for the loss of your, like both your children”. ’
These comments led to a discussion of how to address this problem, given that a trial team would have no details about a baby who did not participate in their trial. Milly and Adam both felt that it would be acceptable if a letter could at least acknowledge that the mother had had twins and that the baby who was enrolled into the trial had died. Adam appreciated the difficulties and data protection issues involved, and said that had a letter as a minimum made reference to twins without using a name for the non-trial participant: ‘[I’d] recognise that they’d be doing sort of as much as they could.’ In a strategy aimed at acknowledging loss, Milly and Adam felt that it was most important not to acknowledge only a part of that loss, precisely because this happened so commonly in their lives. They added:
It’s still happening now.
. . . as if he was forgotten . . .
It could however also be potentially difficult to receive a letter and this was explored in several interviews. Stefanie (INIS), who had explained how much she values anything that refers to her son by name, also acknowledged that this also causes her pain, saying ‘even though I’m saying I like to see something with his name, it still hits you like a ton of bricks as well’. This balancing of benefit and harm in sending out a letter to bereaved parents is an important consideration and as stated earlier, communication strategies should be viewed in relation to the context of experiences of bereavement into which they would be introduced. In the initial period of their bereavement, parents talked about being ‘in a really bad way’ (Liza – ExPN), ‘in a lot of shock’ (Milly – BOOST-II UK) and ‘in such grips of devastation’ (Hesther – TOBY). For these parents the thought of receiving a letter around that time was challenging.
Liza, like Amanda, said that she had periods in the early months of her bereavement for which she has no memories. She said that she would have found any communication about Kirsty to be distressing in the early years. Liza felt that had a letter from the trial team arrived in that time, she ‘would have been upset, getting a letter like that.’ Her partner, Wesley, said that if he had opened such a letter first he ‘probably would have just thrown it in the bin.’ This was not a response to the information about the trial per se but a response to any communication that related to their daughter. The interview explored the difficulty for a trial team, given that some people might value a letter and others would find it to be upsetting. Liza did not argue that the letters should not be sent. She said, ‘It’s really difficult. You can’t really ask them ‘Do you want [the letter]?. . . I suppose you have to send a letter. If you didn’t want to get involved, then you don’t.’
A similar discussion took place in the interview with Milly and Adam. Milly explained how difficult it was after their twins, Lee and Cameron, died when mail arrived at the house.
Whenever we got anything through the post, when it was with regards to the twins, I just went into shock, I mean as soon as anything came and I seen it, that was it, I was a mess . . . I wasn’t ready for the reminders . . . [A]nything that reminded me that actually I’ve just lost these two babies, I just didn’t want. You know we got like condolence letters, I mean even when we got like sympathy cards, anything that came through the door, it was just a hard reminder . . . and you don’t know what it’s gonna be when it’s letter . . . it could be a bill, it could be anything and then you get that! And sometimes you could be having a good day and you get that, you open it and then it’s just (gasps).
Milly – BOOST-II UK
Adam tried to shield Milly, and possibly himself from painful references. In the first few months he threw out any mail that came to the house relating to baby clubs and parenting, a period he called ‘The Purge’. He found that his interest in the trial re-emerged as he moved through the initial stages of his grief. He realised that in the confusion and stress of fast-moving and emotional events, he had not taken on board sufficient information about the trial, and also that mail from the trial may have been thrown out in ‘The Purge’. Like quite a number of parents, Milly and Adam felt that it would have been helpful to have the name of the trial on the outside of the envelope, Milly to prepare her emotionally for any reference to Cameron, and Adam to signal that it was an important communication that should not be thrown away. Milly said:
[Y]ou’ve got a letter that’s sealed but sort of refers to your baby dying, well you can then put that aside and open it when you’re ready and prepare yourself.
Milly – BOOST-II UK
Hesther and Stuart had received the personalised letter from TOBY. They did not throw it away when it arrived but Hesther did discuss how difficult it was to receive it when she was ‘still quite raw’. As described in Chapter 8 , one of the purposes of the letter was to offer parents the trial newsletters. It was therefore sent out just before the newsletters were due to be despatched. They were not particularly frequent and so it could be quite some months post bereavement when some of the parents received the letter. In Hesther and Stuart’s case, however, it arrived within a month of the death of their son, Joel. Hesther said that she wanted to have the newsletters but that when the letter arrived it ‘probably wasn’t the right time’. In thinking about the best approach, Stuart said, ‘If you leave it a few months you might, they might appreciate it better. ’ Hesther felt that it would have helped if she could have asked for a delay. She said that she would have liked the option of saying ‘yeah, I want it, but give me a bit of time’.
Amy and Chris argued that it was not only the fact that grief recedes that might allow parents to engage with communication from the trial, but also that interest can be rekindled.
Time is a great healer because you do start thinking differently as time goes by. Like after it, that would have meant nothing to me, absolutely nothing . . . The longer it goes the more actually I’ve wanted to know more, but right after it I wasn’t interested in anything. Anything!
Chris – INIS
Amy felt the same: ‘A bit more down the line I would be, you know . . . interested to find [out]. ’
Newsletters
The use of newsletters prompted comments on both a disinclination to receive newsletters, as well as interest.
Disinclination to receive the newsletter
Some of the parents would not have wanted to receive trial newsletters. In a few cases this was because they did not feel that they were particularly relevant to their situation or would not offer them anything particularly interesting.
Shirley, whose daughter Beth took part in ExPN had very little curiosity about the trial and the results. She said that this may be because her baby died and ‘it’s of no relevance to me any more . . . how other children are doing’. This was a surprise as she had earlier talked of her hope that the trial would help to advance knowledge and would benefit others.
[F]or the pre-term nutrition study I would have liked to have known . . . had the baby, Beth, survived I definitely would have like to have known, you know any ongoing – what, how everyone’s, other babies that have taken part in it are doing, yeah and read other stories . . . I have me off days and I feel sad about what happened . . . [I]t wouldn’t offend me or sort of affect me if I was sent [newsletters] . . . but I think once the baby’s passed away I don’t see the relevance of being kept up to date of how the research is going.
Shirley – ExPN
Neither Jill nor Ethan were interested in the newsletters. Jill looked at them in the interview and said, ‘I’m not sure that that would have been very appropriate for me’ and Ethan added, ‘It looks like one of those daft quarterly things you get when corporations are trying to get touchy-feely. ’ (It is not clear from the interview transcript whether Ethan was referring to the TOBY newsletters, which were quite plain, or had seen PROGRAMS or the Australian and New Zealand INIS newsletters, which included colour, photographs and images of babies.)
Mostly the concerns that parents expressed related to the potential emotional difficulties they associated with contact with the trial or the content of the newsletters. Chris, for instance, said that he would not have requested the newsletters as he would not have wanted ‘to open old wounds up or go on about the trial. ’ His partner Amy was more focused on the content. She looked through the examples of trial newsletter and focused on a PROGRAMS newsletter that carried details of the 2-year follow-up. She said:
You wouldn’t want that ‘cos you haven’t got a two-year old . . . so to get a leaflet that says ‘Congratulations – your child’s now two and he’s got his two-year review’. No he hasn’t! So I wouldn’t – no, no way! I wouldn’t want that! ‘Cos [when you] sign up . . . you don’t know [what you are] signing up to . . . you don’t know the content. You’re signing up to a newsletter to be involved with the results and to keep an ongoing thing . . . [Y]ou don’t know you’re suddenly gonna get a thing that says ‘Oh congratulations – your child’s now two’ or gone through it’s two-year review, medical review . . . No I wouldn’t like that.
Amy – INIS
Amanda and her partner had opted not to receive the TOBY newsletters but to receive the results. She was worried that the newsletters would include stories of survivors and did not want to see ‘people flaunting their babies’,’ a similar concern to that raised by Amy in relation to the PROGRAMS newsletters. Success stories of babies having survived after cooling have become increasingly common in the wider media, whereas stories of babies who do not survive an attempted ‘miracle cure’ are not especially newsworthy. (See for example, ‘Three days in a chiller saved my baby from brain damage’ by Jo Kessel, 29 May 2007, www.dailymail.co.uk/health/article-458258/Three-days-chiller-saved-baby-brain-damage.html#ixzz2JMpNNfjV; ‘Charlie's Story-Hope for Cooling Blanket Babies’, http://charliecoolingblanket.blogspot.co.uk; ‘ “Our little miracle” Ella the Ice Baby, Who Died in the Womb and was Stillborn, Amazes Doctors by Coming Back to Life After 25 MINUTES’, www.dailymail.co.uk/health/article-1362132/Amazing-recovery-ice-baby-came-life-25-MINUTES-avoided-brain-damage-placed-state-hypothermia.html#ixzz2JMoU1dzT). Amanda felt this difference. The TOBY newsletters did not carry reports of success stories (the Australian and New Zealand INIS newsletters did occasionally contain stories of babies with successful outcomes. See Appendix 18 for an example) and during the interview Amanda sat and looked through them with interest. She said that if she had known that they were so plain, she would in fact have liked to have had them.
BOOST-II UK did not offer parents newsletters but, in discussing the issue, Adam drew on his experience of making sure that he rid their home of any communication relating to their babies. He saw this as his initial reaction to grief and said that the urge to distance himself from information had completely reversed. By the time of the interview he wanted to know more about the trial and was frustrated to think that he might have thrown out some information relating to BOOST-II UK. He could see, hypothetically, how by this reaction he would have excluded himself, and, by extension, Milly, from newsletters and information that he would have valued at a later point.
Decisions are made . . . in grief and then like, like ourselves, later only when you feel a bit better about it, you want to know more and things you’ve done at the time you, you’ve done hastily . . . so you’ve burnt your bridges really. So I feel . . . definitely do that with your leaflet and your information and letting people know, but later on perhaps, get in touch, six months, twelve months, nine months; something like that and ask again, if they would like more information or to be left alone?
Adam – BOOST-II UK
This image of inadvertently burning bridges was apt as trial policies meant that anyone who opted out of communication did not have a ready way back into the system, especially if they did not know the name of the trial. This issue was relevant for Rhona and Karl. They had initially requested the newsletters for PROGRAMS and continued to receive them for several years. Initially, Rhona was keen. She said, ‘[T]o start with, yes, we did want them. We wanted to still kind of be in touch, in a way, and to kind of, hope that our participation in it had – . . . [they] had found something. ’ Gradually, her view changed. She explained how she felt at the time: ‘I was just finding every time the newsletter came I was in floods of tears again and I just thought, no, this – this is time to kind of put that one to bed. ’ This was not because the material in the newsletters was particularly difficult but because for Rhona it ‘triggered memories’. Karl said that he was ‘not so bothered’ by them but they decided that they did not want to receive any more. They used the reply slip that came with each newsletter to opt out of contact. Although they did not want the newsletters, they both said in their interview that they wanted the results and still expected to receive them. It was not clear to them that in opting out of further contact they had also effectively opted out of the results which had been sent out to parents in the year prior to their interview.
Interest in the newsletters
When parents were interested in the newsletters, they focused on them as:
-
a source of information
-
a connection with the research
-
a connection back to their baby.
These were not discrete categories but closely interwoven strands in the parental data; essentially parents often wanted information about the trial, both as a connection to the ongoing research endeavour and as a connection back to their baby.
This interconnected set of interests was clear in the interview with Laura and Wilf. Their son Archie was enrolled into TOBY and allocated to the control arm. This did not lessen their interest in cooling or in the trial. They both felt that the TOBY newsletters offered both information and a way of connecting to the research endeavour of which their son was part. Laura appreciated the updates and talked about Archie’s contribution as ongoing. She said:
I loved the fact that, even though he wasn’t on the – wasn’t cooled, and he didn’t survive, that perhaps in some way he was contributing to some good, that his death was not in vain, even though he didn’t survive.
Laura – TOBY
Wilf said that trial teams ‘should do it, they should give you information’. He later added:
I would have wanted to know what was happening. If my son only lived 2 days, and on those 2 days he was on a trial, I’d want to know what was going on with that trial, even though he didn’t survive, and that trial didn’t help him. That was one of the most important things in Archie’s very short life, so I’d want to know.
Wilf – TOBY
Justine and Francis had disliked the idea of a bereavement leaflet but were interested in the idea of newsletters. Francis said that they would ‘keep you in the loop’ and Justine saw them as an indication of an ongoing role in INIS for their son. She said, ‘It keeps you kind of thinking, right, okay . . . Edward’s died . . . [but they have] still got his details . . . because he’s taken part in that study.’
This idea of the newsletters as a link to the baby was also present in Anita’s description of what it might have been like to have received a newsletter for INIS.
If I’d have got a newsletter, I would have probably sat down and looked at it, to be honest, because . . . it’s a connection with – with my daughter. It’s a connection that I can get some comfort from, to know . . . what’s happening . . . Because it’s something that she’s been part of, and it’s been part of her life. But, you know it’s interesting to see what’s gone on, and that, actually, what she did and what she went through can be having a good effect. Or it might not be a good effect, but it’s had an effect, and it’s, you know, something she’s contributed to.
Anita – INIS
The TOBY newsletters specifically included an acknowledgement that not all of the babies who were in the trial survived, and that some of the parents reading the newsletter would be bereaved. Rhona said in response to this:
I don’t think there was ever that kind of pointing out that some of the babies that had taken part hadn’t survived . . . [I]t almost kind of recognises that, you know, some of us didn’t get what we’d hoped out of the trial.
Rhona – PROGRAMS
Hesther felt that it was important that the parents of surviving babies were aware of this:
I think that’s good, because – I don’t know how harsh this is going to sound. If this went out to parents whose children had survived with it, I think it – I think it’s good for them to know that they were one the of the lucky ones.
Hesther – TOBY
When we looked through the newsletters in the file, Hesther saw the Australian and New Zealand INIS newsletters, with recipes and ideas for books to read to your children. The newsletter, like the TOBY newsletters, mentioned that not all of the babies in the trial survived but she still felt that it was not sufficiently sensitive. She made the point that with the death of Joel they had no children, and said, ‘Why on earth would I need that!’ They had received the TOBY newsletters. Hesther said that despite the fact that for her it was always ‘a shock to see it land on the door’, she ‘read them all’. She and Stuart had kept three copies of the TOBY newsletter in their memory box, which they brought out during the interview.
Web-based message board
For parents of babies enrolled in the TOBY trial, the web-based message board was hosted and maintained by the neonatal charity BLISS. The parents did not, as a group, enthuse greatly about the idea of a message board to make contact with other parents from their trial. In part, their views related to whether or not they were inclined to use social networks and this was wholly unrelated to the trial or their bereavement. Several commented that it was not something that they could see themselves doing. Beverley said that she would not have used it and Robert could not see how contacting other people via a message board would help. He said:
Let’s be brutally honest . . . what benefit would come to me as a bereaved parent writing e-mail messages to other people I’ve never met before who are also bereaved who are going through their own emotional traumas? I could imagine that if you have gone through [it] and something has been a success then you can sort of reinforce it and say ‘Look, got this but this is where we are now’, so there is almost a positive reinforcing going on, but there’s nothing positive about the loss.
Robert – TOBY
In contrast, Lesley, who was used to using Facebook as a way to contact others, and to deal with her bereavement, was more positive, and to her the idea did not seem strange, and another mother, Dawn, commented ‘That is a good one to have!’ Lesley said:
[W]e’re on Facebook and I’ve got a lot of friends on there now that have been through a similar situation, and to hear their story [and] their outcome now, like they’ve gone on to have other children, and it’s just – it’s, you know there’s like a light at the end of the tunnel. And I think it’s good thing hearing other people’s stories.
Lesley – BOOST-II UK
Although making contact with other parents could be appealing, several parents felt that the trial was insufficient to connect them with others. Neither Sophie nor Nat were interested at all in the idea of a message board, seeing INIS as ‘quite a tenuous link’ (Sophie) between strangers, and Jana felt that she would not have sought contact with other parents from INIS as the trial was not a particularly ‘influential’ part of their experience. Irrespective of her involvement in BOOST-II UK Diane did not warm to the idea of talking to people generally. She had declined the offer of seeing a bereavement counsellor and said, ‘I’m not a person to express how I feel. I’d rather keep things to myself. ’ A message board was, however, seen by Chloe and by Wilf as a way of accessing a group of parents with similar experiences, of which the trial was only one element. Chloe felt that she would have used a message board had one been available for BOOST-II UK.
I think that’s a good idea like to have a support network of other people who’ve been through something similar [so] I think I would have, but only because they’ve had children as premature as mine. It wouldn’t necessarily be because of the trial it would benefit me, it would just be getting put in touch with other mums.
Chloe – BOOST-II UK
Wilf had used a bereavement-based message board before. He said that he ‘would follow some of the threads and look at some of the stories, but I never left a message there’. Although he felt that technologically they are ‘things of the past now’, he could see the value of using more up-to-date methods as a means of accessing people with whom they might have common ground, ‘parents who have similar stories to us’. He said:
Those are the ones you’re most interested in hearing what their experience is, and where your experience might be most helpful to them. So that actually the parents who you might meet . . . or hear about their stories on [the] TOBY message board, would be the ones with the most similar story to us . . . an asphyxiated baby, a baby who died and a baby who did survive for a period of time. So in reality, you would have the closest connection with those . . . I mean this is not about the trial. This is more about managing bereavement.
Wilf – TOBY
Karl had used a twin–twin transfusion message board years previously. He had received a reply to his post and said that ‘that was quite nice’. Another father, Keith, had not used a message board himself, but felt that there was potentially a role for something of this sort for parents.
[H]aving the ability to go on a website every year, like every Kelvin’s anniversary and answer some questions and sharing ideas and little chat room with all the other parents involved – Fiona would probably get nicely involved in something like that because of the curiosity. So you can be anonymous but you kind of get results of what happened with, you know, what’s happening with other children in that sort of age group.
Keith – INIS
Fiona replied, ‘Probably. ’ Keith did, however, point out that his view was shaped by the fact that they had a surviving twin who was also enrolled in INIS. They would have had a particular interest in the stories of the surviving babies, which was not necessarily shared by other parents, although Wilf said that he and Laura might have visited the TOBY message board, had they been aware of it, because they ‘might have wanted to have seen those good news stories’.
Other parents were not particularly surprised to hear that no bereaved parents had used the site. This was largely seen as relating to the fact that it would perhaps be difficult to write about bereavement if the majority of users have surviving babies and are talking about their progress. Rhona explained just how difficult contact with parents of surviving babies could be in everyday life.
[I]t’s still one of the really difficult things to talk to people about because they don’t understand and, you know, to actually talk to another parent about losing a child . . . I still find it quite difficult now and, in that, you’ve got friends and yet at what point do you bring it up, because they’re your friends you’d – you’d like to think that they kind of know – know about you, but you know . . . if you do mention it, yeah, the conversation goes dead . . . and then you get that look of pity come in their eyes, and, and it’s . . . I wish I hadn’t have said that. Yeah, so it is, it’s still very difficult.
Rhona – PROGRAMS
Rhona felt, like several others, that because of this difficulty, bereaved parents would read messages but would not necessarily post themselves.
[I]t’s pretty awful what’s happened, but you don’t want people to feel sorry for you the whole time, and you, it, and you don’t really want it to cause distress to people that have had a normal [baby].
Rhona – PROGRAMS
This led to discussion of whether there might be value in having a separate area for bereaved parents. [This model is used on the Twins and Multiple Births Association (TAMBA) website (www.tamba.org.uk), where there is a specific password-protected area for bereaved parents.] Elsewhere Rhona had argued that she did not want to be excluded or treated differently because her baby had died but in this matter she felt that a dedicated space would be valuable, a contrast with ‘all the little clubs going on but you’re not really allowed to join in on any of them’. Karl felt that this would be a recognition of different circumstances and different needs. He said, ‘The practicalities for both . . . groups of parents, they’re quite different, I think, aren’t they, so, I think, it would be fine to have the, the two separate.’ He concluded, ‘I think message boards is a good, good idea.’
Anita said that she would have read messages on a general site but would probably not have commented. She felt that had there been a board for the bereaved parents from the trial she said that she thought that she probably would have been more actively involved, but, like Sophie, Nat and Jan, was not sure that the trial-related link, rather than bereavement for parents more generally, was of particular value. In contrast, Stefanie felt that she would not have been able to read messages on a site about surviving babies. She felt that bereaved parents ‘wouldn’t want to hear about the other babies’ and for this reason liked the idea of a separate but linked area for bereaved parents.
Definitely something separate . . . because the last thing you want to do is hear about how well everybody else is. It’s an awful feeling to have, and you’re aware of that, but, you know, you don’t need that, you know. No, definitely you don’t need something like that.
Stefanie – INIS
The bereavement contacts describes above may or may not have included any reference to the trial results. These are discussed in the following section.
Later contact strategy: feedback of trial results
The discussion of feedback of trial results took place towards the end of the interviews, after the parents had gone over the story of the birth and death of their baby, and had described their reactions to bereavement and to the trial, initially and over time. Over a succession of interviews it became clear that discussion of this element of parents’ experiences provided an opportunity to reflect back and to re-explore their involvement in a trial. Building on ideas that had started to come to the fore in relation to the initial TOBY communication strategy, and covering some of the same area, discussion of the results of a trial not only provided information about concerns and preferences, it also generated a rich new vein of data about the significance of participation in the context of bereavement.
Parents often discussed feedback of results in relation to where they were when they agreed to take part in the trial, and where they were in their bereavement trajectory at the time of the interview. Commonly parents said that had the results been available earlier in their bereavement it would have been difficult to have considered them, but with the time that had elapsed they were ready and keen to engage with the information. The discussions were often very open and in some instances parental responses revealed a depth and complexity to the personal meaning they attached to trial participation once that whole story, from recruitment to results, was complete.
In 12 interviews it was possible to explore parents’ reactions to having received information about the outcome of the trial, but this discussion was hypothetical for the remaining parents who had not received trial results ( Table 39 ). For four couples this was because the studies in which their babies participated, ExPN and the TOBY pilot, did not send results to parents. Another three couples were involved in a trial that sent results to bereaved parents, but their interviews took place earlier in the BRACELET sequence, and preceded the point at which results had been dispatched. In the remaining 11 interviews, trials results had been dispatched to parents but for different reasons had not been received. In one instance, the mother, Beverley, said that she remembered receiving a letter with a reply slip to opt in to receiving the results but did not think that she had returned it; in another, the parents, Rhona and Karl, had opted out of receiving newsletters and so did not receive the newsletter that reported the trial results. In nine interviews parents had lost contact with the trial team, as they had moved house since their baby was born (indicated by ‘[M]’ in Table 39 ). (These parents were recruited to BRACELET either through publicity, or via the clinical centres that held updated addresses. The new addresses had not been available to the trial teams for despatch of results.)
| Receipt of results | INIS | TOBY | PROGRAMS | BOOST-II UK | ExPN | Total interviews |
|---|---|---|---|---|---|---|
| Results not sent to parents | Stefanie and David | Hannah and Ryan | Shirley and Warren | 7 | ||
| Marion and Doug | Caitlin and Pete | |||||
| Julia and Lewis | Liza and Wesley | |||||
| Results sent, parents had not received results | Alice and Ivan [M] | Robert [M] | Rhona and Karl | Danielle [M] | 11 | |
| Justine and Francis [M] | Dawn [M] | |||||
| Sara and Gareth [M] | Diane [M] | |||||
| Karen and Tony [M] | ||||||
| Chloe [M] | ||||||
| Beverley | ||||||
| Parents had received results | Anita and Sean | Laura and Wilf | Jill and Ethan | 12 | ||
| Fiona and Keith | Hesther and Stuart | Milly and Adam | ||||
| Sophie and Nat | Amanda | Lesley and Stan | ||||
| Amy and Chris | ||||||
| Jana | ||||||
| Dora | ||||||
| Total interviews | 30 |
It was striking that in almost every interview parents said that they would want to have the results. Wesley was the only parent to say that he was not interested at all. Although his partner Liza said that she would have liked to have known the outcome of ExPN Wesley said, ‘I wouldn’t have wanted to keep lingering on about it and on about it. ’ The rest of the parents varied in the strength of their desire for the results, from mild interest to a strong wish for the information. For Robert, Gareth and Sara, and for Pete, their level of interest developed during the course of the interview (see, in particular, Robert’s reaction as detailed in Box 14 ).
This almost unanimous view that the parents should have access to the trial results was an important finding. On a practical level it is useful, as trials do not always include bereaved parents in feedback processes, and this provides the first empirical evidence of interest in this group. As a lead for analysis it was intriguing, as much of the data collected in the earlier parts of the interviews have suggested that, with the exception of trials such as TOBY, which can shape experiences in a very obvious way, trials could often play a relatively minor part in parental experiences once they had been through the decision-making process, and for many parents the trial receded or disappeared. This begged the question then of why details about a trial that did not figure prominently in a parental account of their NICU stay, or which had been almost forgotten, should still be considered to be important information quite some years post enrolment and post bereavement.
On analysis, four closely linked elements were identified in the desire for trial results expressed by the parents. They echoed and developed the ideas which had emerged in relation to the initial contact strategies for TOBY. The parents were interested in the trial results as:
-
information
-
acknowledgement
-
connection
-
commemoration.
These four elements had a number of different dimensions, and Figure 24 demonstrates the complex nature of the parental wish to know the outcome of a trial.
FIGURE 24.
Four elements to the importance of trial results.
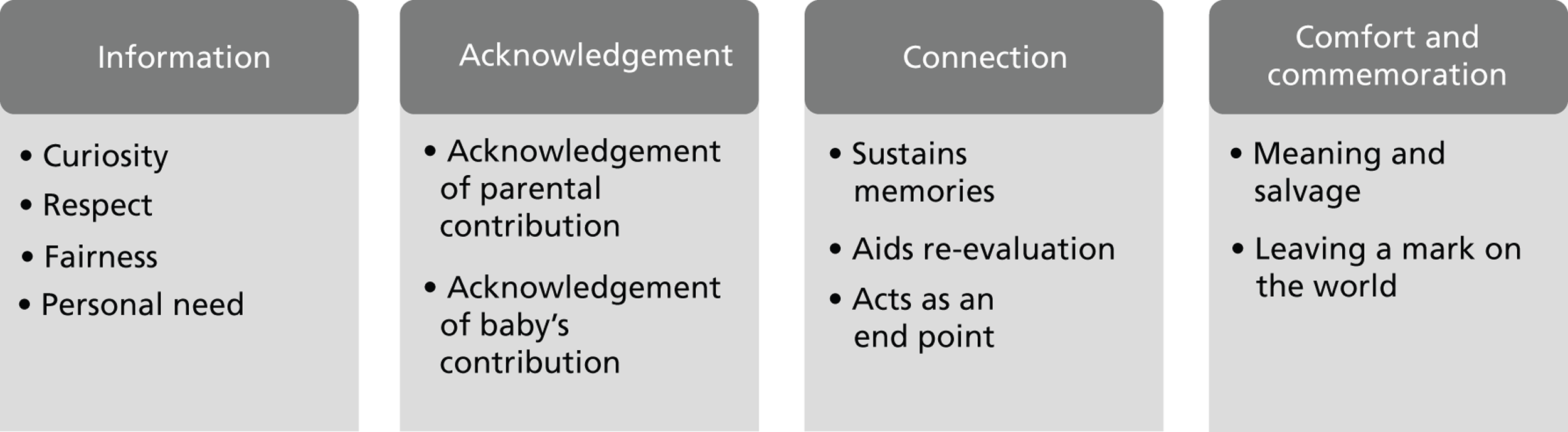
Information
The wish for information about the outcome of the trial was not just a matter of access to factual details. This element itself contained a number of different dimensions:
-
curiosity
-
respect
-
fairness
-
personal need.
Parents wanted to know the outcome of the trial, saying that they were ‘curious’ (Chloe – BOOST-II UK). Karen pointed to the importance of this information:
[Y]ou know, my child passed away, and I would like to see the results, ‘cos it’s like you want to see if, you know, it’s made a difference, and even better if, you know, the findings are positive. ‘Cos you think well wow, it was really worthwhile! It is going to make a difference and maybe other babies born then, you know, may stand a better chance because they are being given more oxygen . . . . [E]ven if it’s not that – even if it’s they’ve realised it doesn’t make a difference, then there’s no argument . . . up and down the country, . . .’Well we’ve done a study. It doesn’t make a difference.
Karen – BOOST-II UK
Where parents felt themselves to be part of the research endeavour, passing on details of the outcome of the trial was a matter of respect and fairness. Laura, whose baby was in the control arm for TOBY and so was not cooled, still felt a great interest and personal investment in cooling and said, ‘We now had some sort of ownership of the trial and wanted the trial to be successful. ’ Stefanie, whose son was enrolled in INIS wanted the information as it was ‘something that we were connected with’. Gareth’s son Aiden took part in INIS a trial that showed no significant effect of the intervention. He felt that this did not alter parents’ right to the information:
Even if there was no definitive result, that is in itself a result . . . and I think, you know, that it should be – it should be published and made available and . . . the parents of participants should be told that the results are available. If they don’t read them, they don’t read them, that’s it, but they should definitely be told that those results are available.
Gareth – INIS
Dora felt that it was important that results are sent to bereaved parents as well as to parents of babies who survive. She found receiving the INIS results to be an emotional experience exactly because the trial did not show the intervention to be useful. Although it was not what she had hoped for, she felt strongly about her right to the information and to be treated fairly:
I would have felt cheated if they’d excluded me from the results, ‘cos, you know, I’d agreed to take part, you know, and I think it’s just decency they should let you know the result.
Several parents said that they had been anticipating the results for some time. Hesther said that she was interested in the newsletters, but it was the final results of TOBY that she was ‘waiting for’, Justine and Francis had clearly expected that they would hear about INIS one day. Francis said that although a long time had passed he did think that the results would arrive. ‘I remember thinking that . . . I’ve never heard about that trial. So it was always still there, still remembered. ’ Justine said, ‘It sort of pops in your head sometimes, and then you think, it’ll come sooner or later, you know.’ Stefanie said:
I’ve always waited for my letter in the post or whatever, to tell me, you know, that the trial had finished and this was the outcome of the trial . . . And maybe I’m wrong in my memory in thinking that the trial may be finished May time, that every May I’m waiting. You know, was 3 years, was it 4 years you know. Waiting for this, some sort of acknowledgement in the post, you know, to say that the end of the trial, and you know this is the outcome.
Stefanie – INIS
The information from the trial results could meet an important personal need for parents where they wished to understand the possible impact for their own child, positive or negative, and to know whether they themselves had made a good decision to participate. Justine and Francis both said of the INIS trial results ‘I would always want to know. ’ They felt that knowing that the intervention made no difference to the babies made their position easier. Justine speculated on how it would have been if the treatment had been beneficial and their son had not received it.
[Y]ou think to yourself, well, if he did get it, would he be here or would he not be? I think they kind of things – go through your head when it comes back as well.
Justine – INIS
Francis was comfortable with the decision that they had made.
At the time, it’s a 50 : 50 thing. He’s either getting or he’s not getting it. So I was – I knew at the time that, you know, it’s a hit or miss, either yes or no.
Francis – INIS
Hesther wanted to know that the ‘idea had worked’ and said that she had not considered that the trial might not have shown cooling to be successful. It was important for her that it would have ‘worked for somebody’, even although it had not worked for their son.
I wanted to know how many it had worked for and how many it hadn’t. You know, just because it hadn’t worked for us, I wanted, I still wanted to know it had been a success. That big decision was made, and within 10 minutes or half an hour . . . I needed to know that it had worked for somebody . . . I needed to know that there was a point to it.
Hesther – TOBY
Hesther was asked how she would have felt if the trial had shown that cooling had not been a success. She replied, ‘Don’t know. It was something that I never considered. I just saw it as something that maybe they wouldn’t have tried if they thought it wasn’t going to be some sort of success. ’
Accessing the information about the trials was not without risk as it contained the possibility that parents might learn that the trial was detrimental to some of the babies involved. Lesley wanted more specific information and felt that parents ‘should get the option’ of knowing which arm their baby was in. Given that she had been clear that she wanted the BOOST-II UK results for reassurance, she was asked how she might feel if she learned that their son, Lloyd, was allocated to the group receiving the lower level of oxygen. She said that she would want the information ‘because it closes a question that we’ve got’. There is a sense in the dialogue shown below that the value of the much-hoped-for information that Lloyd was in the higher oxygen group outstrips the possible negatives of learning that he was not, as they have cognitive strategies in place for coping with that.
It’ll never get easier [but] that one little thing, knowing, say like he was on the high oxygen, knowing that will make it a little bit easier for us, knowing that . . .
It was helping.
Yeah. And it wasn’t – that’s not the reason he passed away, if that makes sense. And he passed away because of all the other problems, which we knew about, but couldn’t have been solved, because he was too early. But if we knew that he was on the high and it was helping him, it’s that little, just that little bit that makes it easier . . . Like I say, it’s never ever going to get like completely gone, do you know what I mean, but a little bit could make it slightly easier.
What if you found out that he was on the low one? Is that . . . ?
I don’t think it would make an awful difference because of the amount of other problems he had.
He had the other problems.
. . . Yeah, yeah. Because he had so many problems, that little thing wouldn’t have made any difference, whether he was on it or not.
Sophie described how she had felt some apprehension about having the results of INIS but still felt a compulsion to know the outcome of the trial:
I remember thinking, when they sent me that letter . . . a real kind of sense of, oh my God. Do I really want to know this if it’s possibly going to be negative? But yes, I’ve got to know it. There’s no way of not knowing it. I want to have the results, for sure.
Sophie – INIS
Nat had not considered the possibility of the information being difficult at all and opened the letter straight away with Sophie watching him. She said:
I remember being really quite nervous . . . I remember thinking, my heart’s in my mouth almost . . . I thought, God, are they gonna say that, you know, actually, you know, it, it was a bad thing to have done, that, you know, could have actually caused even more deterioration in Brendan. So I certainly saw the possible potential downside to doing it.
Sophie – INIS
The possible emotional risks that the results could contain did not necessarily go away. The discussion of the results in Amanda’s interview involved a delicate moment. She described her interpretation of the TOBY results and said that from her reading of them she had concluded that her daughter Simone would have been in the category where cooling would not have helped. As soon as she said this she quickly held her hand up in a ‘halt’ gesture saying ‘Don’t correct me if I am wrong because this is how I cope. ’ There was a strong sense that Amanda needed to feel that the trial would not have saved her baby, and could not risk any threat to this important coping mechanism.
Acknowledgement
There were two aspects to the acknowledgment that parents felt would be signified by feedback of the trial results. Acknowledgement of:
-
parental contribution
-
baby’s contribution.
An acknowledgement of the parental contribution to the trial would recognise what they went through at the time of making their decision, and would repay the trust that they had placed in the clinicians and researchers involved.
Julia felt that not being sent the trial results (she had not received them at the time of the interview) could suggest that parents and their opinion, and their child’s involvement in the trial, were not valued:
If you don’t hear about it again – well did it even matter? Did our child matter? Did our opinion matter? Did anything we contributed matter? . . . Where, if you’re actually given the option of finding out what it resulted in, well then it feels like your opinion matters and what you said matters and your child being involved matters.
Julia* – INIS
*Julia made a comment that was quoted earlier in relation to the TOBY bereavement leaflet but contains ideas that are highly relevant to feedback of the results. Julia reflected on how the leaflet given at this initial stage could later help parents in their exploration of earlier events and their grief. She said, ‘This is a nice acknowledgement that you were willing to take part and maybe you've helped another child. And I suppose later on, when maybe you've accepted things and you're living with things and you've managed to move on, you can look back at this and say “Well maybe it was the right decision to be part of it”. ’
Parents could value the results as an acknowledgment of their own baby’s contribution the trial. It was important that the baby was valued as an individual, and not just for the data contributed to the trial. Sean, who said early in the discussion that he was not particularly keen to have the INIS results, warmed to the idea over the course of the interview and eventually said that the gesture of sending results to parents communicates something important about how his daughter was seen in the context of the trial.
It just proves that she wasn’t just a number . . . She was treated as a person and you remember us and tell us what’s gone on, or given us the opportunity to know.
Sean – INIS
Stefanie, who had not received the INIS results, but had anticipated receiving them for some time, imagined what it would be like.
I’ve always waited for that, and said to David you know, when you get that letter, you know, that has Callum’s name wrote on it from the professor or whatever, you know, that [would give] me great joy to see something with his name on it.
Stefanie – INIS
Connection
Another dimension to the acknowledgment of a baby’s involvement in a trial, related to a recognition of their existence. The trial results could be closely related to the idea of a connection back to the time of the birth and death of a baby. This connection back stimulated three important responses for parents.
-
sustains memories
-
aids re-evaluation
-
acts as an end point.
Several parents valued this idea of the baby’s personhood being acknowledged, and referred to the fact that they were ‘not forgotten’. This filled a gap, helping parents to sustain memories, providing what Stefanie (INIS) referred to as ‘another wee link to him’. Parents explained that this was important because as time passes people refer less and less to the babies who died. There is no contact with the neonatal unit, which, especially in the cases of babies who received long-term care, may have included important relationships and had been an important source of support. There can be few people with whom parents can discuss events, their baby and their bereavement. Fiona and Keith explained that they can only talk to each other, and Karen felt that discussions of their loss were very uncomfortable for her family. (See also Dora’s account in Box 11 , in which she explains that, 10 years on, ‘everyone’s forgotten about him’.) Babies are represented in the memory boxes that parents keep, the photographs that they very commonly had on display, and in the ways that they had found to recognise family connections; Marion and Doug said that they mention Dee every day. Dawn explains how she connects her son, Mitchell, to his surviving twin, Gemma: ‘Gemma’s got lovely piercing blue eyes . . . I know twins . . . boy, girl, are no more identical than brother and sister, so I often sit and wonder would he have had the piercing blue eyes?’ Sometimes Dawn sees her son, healthy, running with his sister in a recurring dream.
If I have a dream . . . I see two healthy children like Gemma running around. The only thing I imagine Mitchell to have one default . . . his thumb . . . came down like that (DAWN MIMED THE POSITION OF MITCHELL’S THUMB) and it stuck there. But to me that’s not – that was part of his feature[s]. What I remember is him, and I’ll always remember. [I see them] playing around, and I’m dreaming of him, and I’ve seen him, because I know his thumb’s like that.
Dawn – BOOST-II UK
The trial results become available, often many years post bereavement, and offer both physical mementoes (memory making) and evidence that the baby was not forgotten. Dora was one of a number of parents to put paperwork from the trial into her son’s memory box. She added the results letter to the consent form from INIS that she had put in there 10 years previously. The results offered her a time to think back and, even if this was not easy, she valued that link.
The trial results can also act as a form of emotional housekeeping, an opportunity to re-engage and re-evaluate events and to tie up another of the ‘loose ends’ that stem from the death of a baby. Julia explained the value of having an end point to the story of the trial very clearly.
[B]ereavement is such a big thing, the trial is just a small part of all that, but it’s still part of the whole experience, and it’s all these loose ends that you want to tie up and have meaning for at the end of it all, and you work your way through that whole process. Initially it mightn’t be even that important to you, but later on down the line, you’re thinking ‘I wonder how that did pan out. And I wonder . . .’ you know.
Julia – INIS
It was striking how much uncertainty some of the parents were living with: uncertainty over the cause of events, over the implications of various actions and decisions, and whether things might have been different. A trial can add to that uncertainty and tying off one of these ‘loose ends’ could possibly be helpful. For instance, a diagnosis had never been made for Marion and Doug’s daughter Dee. A concern had been raised during pregnancy that she might have Turner syndrome but this was later discounted, and her post-mortem was inconclusive. Not knowing, at the time and since, was a major theme in the interview with Marion and Doug, at which point they did not know the outcome of INIS. Both parents wanted to have this information and Doug said:
[T]hat’s gone through my head quite a few times, you know, I wonder if Dee has helped other babies? I’d like somebody to say ‘Oh yes, Dee’s death hasn’t been in vain . . . We have made progress . . . and it has helped’. But I don’t know if it has or not. Nobody’s told me or sent me anything.
Doug – INIS
For Francis, the letter about the trial results would serve as ‘closure’. Stefanie very much wanted to have the INIS results, in part for this same sense of closure to that part of their experience, but she had not received them. She said, ‘It’s just always something that over the years that has been in my mind, just wondering what the outcome was. ’ She later added, ‘There’s always something out there unfinished. ’
Comfort and commemoration
Parents also saw the results as being commemorative, a tribute to their child. Two closely intertwined dimensions emerged as important in commemoration that the information about a trial offered:
-
meaning and salvage
-
leaving a mark on the world.
These ideas hinged on a view of a trial as a worthwhile endeavour. The results could provide parents with some personal meaning and a sense of salvage in a dire situation, a feeling that their baby still has a place and a role in the world through the contribution that they made. Jill made a comment about her son James, which encapsulated both of these ideas:
It’s probably the only kind of, I don’t know, maybe positive thing that came out of him being alive, because . . . you know, he had such a short life.
Jill – BOOST-II UK
This comment was made in the light of Jill’s clear understanding that ‘more oxygen was better than less oxygen’. Jill expressed satisfaction at having helped to answer a question. She said that ‘the trial had done what it set out to do’ and she was pleased to have been part of that. Without explicitly drawing out any implication of the possibility that James was in the group with less oxygen, she added:
[I]t’s not bad news, the results of the study . . . He was part of it, so I don’t feel like it’s bad news. I feel like, you know, it’s relevant to him.
Jill – BOOST-II UK
Sara and Gareth, having initially seemed not so interested in the results, both said after discussing them for a short time, that they would have liked to have feedback from INIS. (Sara and Gareth had moved house and so had not received the results.) Sara made a comment that, although brief, contained links to the various top-level themes of information, connection and commemoration, saying ‘It’s something that Aiden was part of really. So it’s just sort of knowing about something he contributed to. ’ This triggered the following response from Gareth:
He got so little time to achieve anything, that if he was able to . . . contribute to achieving a result it would be a very important factor of his life, essentially. One of the things that I find very difficult with the shortness of his life is that he got no opportunity to do anything with his life, and when you compare that with lots of other people it’s very distressing . . . If he was able to contribute in some way to that study then I think I would like to know, and like to know what happened with the study. But it’s not something that I think about every day.’
Gareth – INIS
Laura and Wilf, whose son Archie was enrolled in TOBY, felt a great sense of satisfaction that their baby had left a mark on the world, even although he was in the control arm and his life was so short. Receiving the results was an important affirmation of this. Laura mentioned the importance of knowing that cooling worked for someone, saying:
You want to know that . . . someone somewhere benefited, a baby survived because someone chose to try something different in the treatment.
Laura – TOBY
Parents often said that they would/did value a sense that something had changed for other people because of their baby. Lesley said that she was ‘glad’ about the BOOST-II UK results, even although they did not know whether or not their son was allocated to the arm that was associated with higher rate of survival.
I’m glad of that because now, right . . . yeah, fair enough we don’t know what Lloyd was on, but we know that it’s helped other people. And like yeah, it could save lives.
Lesley – BOOST-II UK
Karen felt that the ability to help other people was helpful in coming to terms with what had happened to them:
[Y]ou don’t want it to feel like the whole experience was just useless and nothing came of it you know. It’s – you don’t want to feel like it was just a horrific experience and that was it, you go on with your lives.
Karen – BOOST-II UK
Hannah and Ryan were still very interested in NIC and cared about the well-being of sick babies. They both had a strong sense of allegiance to the patient group to which their daughter Eleanor had belonged, and to the parents involved. They are active members of SANDS, running the local group. Hannah saw involvement in research as a way of having an impact for the future.
[Y]our baby, although maybe didn’t live and maybe died, may have been quite a contributing factor on the ones that came along further down the line to keep them alive or we learned by these things that by doing that it didn’t help so we’ve changed it . . .
Hannah – TOBY
After the interview, Hannah spoke again of her hope that by taking part in the pilot study they had somehow helped in a research process that would ultimately benefit other families and babies. She said that one day her own children might have babies in neonatal intensive care and they might be beneficiaries of the contribution that Eleanor had made.
Parents described the value of their baby’s contribution and the mark that this has left in the world. It was clearly something that for some brought pride and consolation. The interview with Robert showed just how meaningful this information was, and is presented in detail in Box 14 .
Robert said in an e-mail before the interview that although he wanted to help he was feeling ‘some trepidation’ and just as we started to record he tapped his heart to indicate nervousness or that he was perhaps feeling moved. The story that he told was one of extremely difficult and devastating experiences, which were detailed earlier in Box 6 .
Once we had gone over the story surrounding his daughter Adele’s birth and very quick death, we turned to the TOBY communication strategy. Robert pointed out what he felt were difficulties with all of the components of the strategy. He disliked the initial bereavement leaflet and said:
[I] would have no, absolutely no interest whatsoever in, in reading that . . . [A]ll hopes have just been dragged away from you and you’re given one of these things, then that would just be inappropriate I think.
He felt more positive about the personalised letter but pointed out how difficult it would be for parents if a baby’s name was misspelled. He felt that that would undo any benefit of sending it. Robert described the web-based message board as ‘just text on a computer’ and added that it would be ‘too impersonal for strangers to be sharing your emotion’. He characterised a message board as ‘all needy people relying on other needy people for emotional support when we’re all at our lowest ebb’. He was not interested in the information that he might access through the newsletters, or any other information about cooling. He said:
[I]f there’s some on the websites or the BBC News and it said something about that and it said that ‘Actually there’s been a great breakthrough . . .’ I’d be quite pleased to know if that was the case, but I wouldn’t seek the information out. In fact I probably haven’t really thought about it for God knows how long, but I would like to know . . . [if] it has come to some benefit. But I don’t want to know the ins and outs of it and do I want to know about other people’s success stories? To be honest, I’m very ambivalent.’
Having been involved in the trial outside of the UK, the UK trial co-ordinators would have had no contact details for Robert and his wife, and so they had not been offered access to the trial results. When the subject of feedback of results to parents was raised, Robert started to say that he would not be interested but very suddenly revised his position. This was quite an emotional reaction. The longer extract from the interview below shows the development of his response.
‘I wouldn’t necessarily want to . . . ‘There you go! This is the results!’ It probably wouldn’t be that important to me, but actually you now have got me thinking I’d actually like to know. Has it made a big difference to children?’
‘Yeah, yeah.’
‘It has? So that sort of made me feel actually – just as you saying that has actually made me . . .’ [AT THIS POINT ROBERT BECAME SLIGHTLY TEARFUL AND DID NOT FINISH HIS SENTENCE]
‘So it’s become a standard treatment now and if you like I can ask the trial team if – if – well I could probably get a copy of the sort of standard letter would explain it.’
‘Yeah, yeah.’
‘But it’s – it’s become, – not only has it become a standard treatment, there’s further research being carried on and with cooling to see whether they can add anything on to . . . refine it further. So at the moment there’s a trial going on. Now that they know cooling helps, the next trial, all of the babies are cooled but half of the babies have a gas added, xenon, and that’s – the theory is that they may have an extra additional protective effect, so it’s not just entered into normal treatment, it’s sort of spawned another piece of research.’
‘Yeah. No I – I – now you’ve told me I actually feel pleased I know.’
‘I wouldn’t have told you if you hadn’t wanted to know. I wouldn’t have forced it on you.’
‘Yeah, no, no, no, no and, and even if I would have asked you and you had said there had been no effect at all . . . I wouldn’t have been saddened. I would have been fine, but I’m actually pleased to know. I don’t actually think my daughter’s situation had very much impact [on the trial] if I’m honest, however, maybe there was just a little bit of extra information if she was involved, I don’t know . . . [I]t might have done something so it’s alright. That’s a little bit of a positive.’
In a very open and reflexive statement Robert summarised and emphasised the new position that he had taken. He said:
Maybe before when I was saying, ‘Oh I’m not that actually [interested]’ maybe that was just my initial reaction. The more I think about it then yeah, if there has been some benefit from what my daughter went through, and also the reason why I’m here now talking to you, it’s about being able to help others, actually I do want to know.
Robert clearly did not expect to respond in this way. His initial reservation about the value of results, and his very sudden opening of himself to the personal meaning of the information, was a significant moment for both interviewer and interviewee. It suggests that parents might appreciate, and even perhaps benefit from, access to information about the outcome of a trial in ways that they do not anticipate when asked to decide whether or not they want to have the results.
After the interview Robert sent an e-mail that ended ‘I wish you every success in your work which I believe is so very important’.
Parents often aware that receiving results might be emotionally difficult
Although the parents wanted to have the results, for all of the reasons presented above, they were aware that introducing the results into their lives is not without potential complications. Parents acknowledged themselves that receiving the results would be difficult in the early stages of bereavement. In the BOOST-II UK trial which stopped early, this is exactly when the results would be communicated for some. Reactions to the results may well be shaped by initial understanding of the trial, essentially whether or not parents appreciated that there were any potential risks involved, by what they knew of the cause of their baby’s death, and how they interpret the results that they are given. They can also be affected by the initial reasons for taking part in the trial. This can be seen in Dora’s reactions to the TOBY communication strategy, and her experience of receiving the INIS results, as described in Box 15 .
Not all parents had considered the possibility that the results could be challenging, or could give them information that they might have in fact preferred not to have. The image of Sophie’s concern as Nat opened the INIS results letter with ease was striking. The parents were aware, however, that opting to receive the results would require re-engagement with a difficult time. This was not seen by most as a reason for not requesting or not sending the results. Parents knew how their own bereavement worked and how to manage information. The parents interviewed here wanted to have the results as part of their story and their experience of being a bereaved parent.
When Dora enrolled her son, Gerry, into INIS she had few expectations the trial would make a difference to him but very much hoped that it would help other babies in the future. (See Chapter 6 for further details.) INIS seemed to be a minor part of events at the time and quickly disappeared from her experience. It was, however, discussed in the interview as a powerful connection back to the brief time she had with her son.
Dora acknowledged that if the TOBY communication strategy had been used for INIS it would have been difficult when the first letter arrived, but this did not mean that she would not have wanted to receive it. Like a number of parents, she felt that although she might not have been able to go through the information initially but that it could be put away for a stronger time. She said, ‘I would have just put it away with his stuff and it’d be there for when I wanted to deal with it. ’ She therefore felt that trial paperwork, even if difficult, should still be made available to parents. She would not have been interested in talking to her consultant about INIS had she gone to a bereavement follow-up, and would not have used a message board. She was, however drawn to the initial letter and to the later results letter. A key feature for her was that they would have included her son’s name.
It just sounds really sad but I know why I want it, because it would have had his name in it, and to me anything that made him exist, was something I wanted to treasure . . . [E]veryone’s forgotten about him apart from me, of course, and the children.
As Dora said the words ‘it would have had his name in it,’ she held out a hand and stroked her palm with a finger, miming stroking Gerry’s name on the paper. Gerry is not mentioned by her friends or family, even on the anniversary of his birth or death. She is no longer in a relationship with Gerry’s father so cannot share memories of the events with someone who shared them at the time. She said, ‘I find that hard that he’s been pushed away. ’ For Dora, the results were an important form of recognition of her son, and serve to sustain a memory. She had placed the results letter in his memory box along with the INIS information leaflet and the copy of her consent form from 10 years earlier. She commented on this, saying ‘I don’t have a lot of stuff. ’ With a short life a memory box can be slight and is seldom added to over the years. Adding the results was therefore a significant act.
When she looked back over the course of her bereavement, Dora felt that the various communications from a trial could have been helpful, but felt that her reactions would probably have been different at different stages. She used the word ‘comfort’, a word used in a number of interviews to describe aspects of the TOBY communication strategy but was clear that this benefit might be slowly accrued.
I would [get] great comfort 10 years down the line, now, but whether I would have in the beginning? . . . I’d like as much paperwork as possible now. I’d like everything, you know yeah, so it would – I think it would have helped me, and I think – I think it would help a lot of people. They might not realise at the time, but coming, sort of, through the other end and realising, you know, how important little bits of paper are with his name on, you know.’
When the letter arrived offering the results, Dora said that receiving it was a shock and it did make her cry. This did not relate to the content but to the connection back to the loss of her son. She did not though think that the letter should not have been sent, she was pleased to have been given this option of making a decision about whether she wanted to have the results rather than receiving them out of the blue. Parents often said that they simply sent off for the results without giving it much thought, but Dora took some time to think through whether or not she wanted to have the results. She was aware that it might not be straightforward. For her the issue was partly about taking her back to a difficult time, and partly the possibility of dangerous information, but she described a compulsion to know the findings. She hoped that the results would not suggest that she had made the wrong decision in accepting the trial. She said, ‘If it hadn’t worked, would I blame myself?’
Given her initial ease about taking part in INIS and her sense at the time that the trial was unlikely to change anything for Gerry, Dora was surprised at how disappointed she was to learn that INIS did not show immunoglobulin to be effective. She explained that if the trial had shown it to be useful, this would have given some significance to her son’s life. She said, ‘I wanted a bit of purpose for him. ’ Part of her disappointment lay in the fact that her aim in taking part in the trial had not been met, a feeling that she summarised as ‘I felt I hadn’t been able to help’. Dora said that she had not picked up the statement in the letter that INIS was still useful because it was important to know not to use immunoglobulin: ‘I didn’t even pick that part of it up! I just looked for the “did it make a difference?” and remember feeling really disappointed. ’
Interestingly the opportunity to take part in BRACELET helped to mitigate the disappointment. She felt that by telling Gerry’s story he would still be doing some good (not her, him).
Even though I was disappointed, when I read the little bit at the bottom that said to me about the research you were doing, I remember thinking to myself ‘Well, he can help that way, maybe.’ Yeah, so that was, sort of, the saving grace for that, really.
Discussion
The narrative accounts given by the parents involved in the BRACELET study described their involvement in a NIC trial, from recruitment (see Chapter 6 ), up to their baby’s death (see Chapter 7 ), to a time usually some years later when trials findings were published (this chapter). This vantage point has offered insights into what it means to parents to be involved in a trial when their baby is receiving NIC, and what forms that association might subsequently take as their bereavement evolves. Bereavement is a complex and changeable phenomenon, and understanding how involvement in a trial might fit within such a variable and highly personal experience is a challenge.
For some of the parents, the trial was a seemingly minor feature in their account of their time in the NICU and decision-making was quick. At the opposite end of the spectrum there were those for whom a trial was tightly woven into their narrative, it being a major decision, a catalyst for change (e.g. transfer to a different hospital), or an event that took place in close proximity to the death of their baby. It seemed that this major/minor spectrum soon dissipated as even a trial upon which parents pinned their hopes for help for their baby, could recede into the fast-moving events, or the slow grind of long-term stay, both common features of care in the neonatal intensive care setting. Here a trial could become a small part of much larger events, and could disappear altogether, especially in the early maelstrom of bereavement.
The longer-term approach taken in the BRACELET study means that we were able to see that this is not necessarily the end of the story. Had the study been carried out with relatively newly bereaved parents, it would probably have concluded that the trial was not a priority for parents, and would not necessarily have been of observable interest at the BFU. If data were collected from more recently bereaved parents and the clinicians who care for them, the study might have suggested that ongoing communication about the trial may not be of particular interest, and may not be effective or well received.
A strong finding of the BRACELET study was that for the majority of parents the significance of a trial continued to change over time and that this was not a straightforward or linear effect. Whatever the parental experiences of their initial involvement in a trial, it became clear that the degree of salience that they later accorded to the trial was often unrelated to the original response. Trials appeared to ebb and flow in and out of parents’ bereavement stories. We were able to see that some parents, such as Stefanie, sustained their interest in a trial themselves, thinking back to their initial involvement and anticipating the results to come. To a large extent, however, the ebb and flow that we identified was influenced by clinician and trial-led policies, which determined whether or not the topic of a trial was brought to the fore for parents. Key points at which interest and engagement may be stimulated were the BFU meeting, and, as initial grief subsided, on receipt of newsletters, and later on receipt of the trial results. (If there were different follow-up periods, there may have been more than one set of results.) The BRACELET interview also proved to be an important point of engagement in which parental interest in discussing their reactions to the trial emerged. By looking at the views of parents some years after their initial involvement in a trial, at a time when results were available for some of the trials (i.e. after completion, analysis and publication) and could be given to parents, we were able to see interest rekindled at these various contact points.
Parents had mixed views on the topic of the trial being raised in the short term, as this would occur at an earlier point in bereavement. Responses to the idea of discussing the trial with their consultant at the BFU suggested that it would often be seen as an appropriate and supportive gesture, and a means of encouraging parents to discuss this aspect of their experiences if they felt that they needed to do so, but that this would not necessarily be an issue high on the parental agenda. None felt that it would have been inappropriate for a consultant to have raised the topic, as it could always be dismissed by parents if not of interest, but some felt that it could have been helpful in initiating a discussion that parents would not themselves have led. This contrasted with the consultants’ view that if trial participation is a relevant issue then parents will raise it themselves; not doing so is taken to be an indication of the personal irrelevance of a trial. The idea of a bereavement leaflet that might be used around this time was warmly received by some parents but soundly rejected by others. At a time when any reference to the baby was fraught with emotions, some desired it as an acknowledgment of the reality of a baby’s existence, and an early acknowledgment of their contribution to a trial, but even quite some time after the event, a small number of parents appeared aggravated or even briefly angry in the interview when they thought about how they may have felt about the leaflet at the time of their bereavement.
Once interest is rekindled there might be a more prominent place for the trial in later bereavement, which it does not have in the shorter term. In the course of an interview, which for most parents took place years after the event, the focus on the trial as a connection back to that time seemed to be highly engaging and valued. Parents for whom the trial was well in the past were able to look back on events and place them into the context of their lived experience of bereavement, and to explain how they would fit new communications, such as letters and newsletters relating to the trial, into their own personal bereavement management strategies. For some, this would involve a careful and cautious approach to contact, and paperwork could be put away for processing at a stronger time. For other parents, communications from the trial could be readily and immediately embraced. It could offer new insights into a story that perhaps had not developed in some time. That parents responding in both of these ways might store communications with precious items, which could be used to record, recall or explain events, suggests something of the importance of anything that links to their time with their baby. The ideas that clearly emerged from discussions about the different forms of communication that a trial might use suggested that both the materiality and the ideas of inclusion and acknowledgement with which they were suffused would be valued.
We found that the bereaved parents in this study did not wish to be excluded from feedback of results. It was striking that even when a trial was a small part of their experience, when they had thought about it very little since, and even if they had little recollection of what the trial was about, there could be a strong interest in receiving the results. This was because parental interest in the outcome of a trial was far more complex than simply accessing information. The process of contacting, acknowledging and informing parents has a symbolic meaning that was valued for the respect and openness that parents perceive in the gesture, and for the sense of contribution that it can foster. It was clear that the actual results had great significance and personal resonance for the parents. They were shown to be important as an acknowledgement, as a connection back to their baby and the time around their birth and death, as a way to tie off loose ends and, potentially, for some parents, as a form of consolation or comfort. Even when parents have not received any formal communication, simply the knowledge of the results could prove to be personally meaningful. Robert was moved when he heard the TOBY results in the interview and, to his surprise, became tearful. He said that he was pleased about the research and pleased that he knew.
The notion of trials offering consolation seemed to be a phenomenon that related more to the longer than the shorter term, to a time when parents felt that something could be salvaged from their difficult experiences. The idea of trials as salvage was important to some of the parents because it spoke to a major difficulty that many faced in relation to the death of their baby, the sense of frustration and sadness articulated so clearly by Gareth, for a life not lived. He was quoted earlier in this chapter as saying, ‘He got so little time to achieve anything, that if he was able to . . . contribute to achieving a result it would be a very important factor of his life. ’ Linked to this is the sense of parenting that could not take place. Robert made a comment, quoted in Chapter 6 (see Box 6 ), which placed the decision that he made about TOBY into the sense of powerlessness he felt at the time: ‘I hadn’t done anything for her in her life and this was something I could actually do and that was the, the positive thing in, in a totally hideous experience. ’ Parents had a keen sense of their baby’s suffering, especially when they had undergone surgery or painful interventions, when their bodies were visibly damaged as a result of injury to the skin, or by the distorting effects of extreme oedema and bruising. Parents carry these difficult experiences with them, and these feelings of frustration, pity and loss, along with other complex emotions, underpin the direct links that were often made between their baby and the outcome of the research in which they participated. These feelings can be seen in Laura and Wilf’s satisfaction at the effectiveness of cooling shown by TOBY, and in Dora’s sadness at feeling that the negative results of INIS did not give ‘a bit more meaning’ to her son’s life, a sense of meaning for which she had been hoping. They can also be seen in Amanda’s concern that things might possibly have been different had her daughter been cooled.
Communication of trial results appeared then to create a number of connections that were important to parents.
-
They connect back to their baby. Effectively the links with the trial which are recognised in newsletters and finally confirmed in the results connect parents back to their baby, and promote re-engagement and re-evaluation of the decisions that were made.
-
They connect out to the wider world. Parents hope that the benefits not accrued by their baby, and so by extension by themselves, might be accrued by another baby, and another family, and this is encapsulated by the commonly used imagery of someone somewhere. They also connect to the trial teams who indicate to parents that they have remembered, that their baby is not forgotten by others outside the family.
-
They link the baby from the past and connect forward to the future through the advancement of care and those who will go on to benefit. Parents carry their knowledge of the results, and their views of the impact that they may have had for their baby, and the role their baby has played, into their own future. If results have to be stowed for a stronger time, they also connect to a future stronger self ( Figure 25 ).
FIGURE 25.
Connections for parents: out, back and forward.
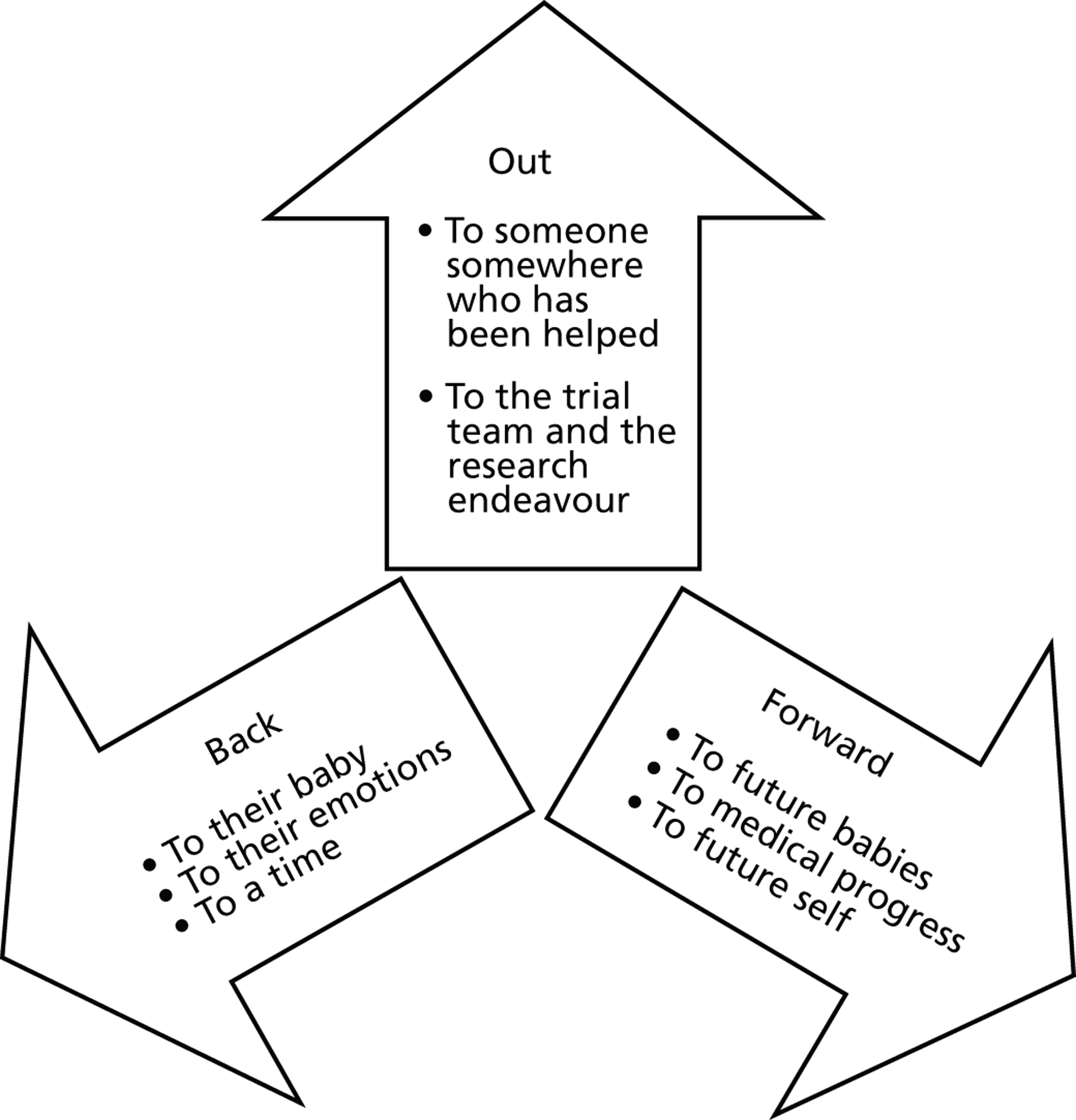
These connections establish a role for the baby in the world. Parents felt this signified that they and their babies were not forgotten. When a life is short and does not exist outside the neonatal unit, the connections embedded into the trial results appeared to have a particular significance and value.
Although these connections appeared to be valued by most of the parents in this sample, this was not a universal response and a degree of caution in our interpretation is required. For a number of the parents unsolicited reference to a baby was acknowledged as difficult but desirable, but for some there was the potential to breach coping strategies. Wesley said, ‘You never forget but you just keep it to yourself basically, don’t you? You just hold it in. ’ Those whose grieving takes the form of minimising rather than embracing thoughts of their baby will not be well served by any approach, which re-opens and connects back. One man called the NICU that cared for his baby after receiving a letter about the BRACELET study to say, and forcefully so, not to contact them again, and when one of the consultants called a family to raise the topic of BRACELET, another father put the telephone down as soon as he introduced himself. Our data tell us only about the views of those who are prepared to re-engage to the extent that they will take part in an interview or fill in a questionnaire about their experiences. (In Chapter 10 we will consider the insights gained from the small number of questionnaires submitted to BRACELET.) It also tells us how parents view communication and feedback after they have spent some time re-engaging with the story of the birth and death of their baby, and their views may, in part, be a product of this process of reflection.
This issue goes beyond the principles of contact that are presented and explored here, given that some trial results may be more problematic for some parents than others. In the BOOST-II UK trial, for instance, it was shown that babies in one arm of the trial had a poorer outcome. This was not picked up and explored as a particular problem for parents who took part in the BRACELET study, either because the parents had not received the results or because they already felt that their baby would not have survived regardless of the effect of the trial. Not all parents in a trial with this type of outcome will have coping strategies in place and for these parents the results may not result in the positive connections outlined above. Even when a trial shows a treatment to be effective, for parents not allocated to that arm there may be issues not tapped here. Amanda who found her experience with TOBY, in particular allocation to the non-cooling arm of the trial, to be a difficult memory, copes with what she knows of the results by keeping them fixed in a form that states that the trial was unlikely to have changed the outcome for her baby. In this form, she can manage the spectre of a potentially effective treatment being withheld.
Where information is made available some of the parents explained that there would be a compulsion to know, even if it might prove to be difficult and unsettling. Lisa and Jill both felt that it would be difficult not to pursue information that they knew was available. If, for instance, a letter arrived from a trial, a sticker with a trial name on the outside could act as a warning that it would include a reference to the trial (it will work in this way if parents remember the trial acronym, or if there is a reminder on the outside of the envelope to remind them. Liza did not remember the name of EXPN at the time of the interview); however, it would also communicate to parents that the content related to their baby, and it is that this would be compelling. Lisa said that although she did not want any reference to her daughter when her grief was raw: ‘If I saw that, I’d have to open it anyway’. Wesley’s comment about throwing away such a letter, a response also described by Adam, suggests that partners might play an important gatekeeping role and might control communication channels, especially if they think they might breach coping strategies, their partners or their own. (The methodological aspects of this finding are discussed further in Chapter 10 .)
The issues that parents raised and discussed, and the value that they saw in the communication strategy, brought to the fore ideas of relationships with research and the meaning of trial participation. When both their contribution and their baby’s contribution is acknowledged, this seems to be welcome and a source of pleasure and satisfaction. When results are fed back to parents, even if the process and content are difficult, it seems that parents would still rather be offered and make their choice than not be offered at all. Although there was a recognition in some interviews of the potential for results to be difficult, and to introduce unsettling information into their lives, this did not mean that they felt that it would be better if the information was concealed. Parents prized openness and honesty, and complained – often outside the interviews – that they were not treated as they would like to be, and did not wish this to extend to a trial that was so closely connected to their experience of bereavement. Stuart said that people should treat them ‘like human beings’ and from her doorstep, as the interviewer was leaving, Julia called out, with reference to sending trial results to bereaved parents, ‘Tell them that it is important!’
Conclusions
When parents agree to enrol their baby into a trial, they enter a clinical and a social contract with researchers. The terms of the contract include well-recognised obligations on the part of the researchers, which are enshrined in international codes of conduct, national research governance systems and data protection legislation. When a baby dies, the terms of that contract become unclear, and what could or should be done for bereaved parents can be dealt with by each trial independently. The relationship can be sustained by judicious and optional forms of contact, or it can be indeterminate, left with no explicit and formal ending. The relationships that we have seen are somewhat unidirectional for both of the parties involved. The trial teams send out information without any real sense of its impact; the parents receive the information without any ongoing dialogue. This is in contrast with the parents of survivors for whom a more reciprocal relationship with the research can be forged, in the form of clinical advice (see Chapter 8 for details of contact between ExPN parents and the ExPN clinician), birthday cards from CTUs and parental involvement in trial follow-up appointments. When opportunities for communication are more limited, as they are for bereaved parents, it is important that they are offered in ways that recognise and support their involvement in research if that is what they wish.
This study suggests that bereaved parents can be interested in a more meaningful relationship with research than is sometimes assumed, even if the research seems like a minor part of their initial experience. Although some of the interviews with clinicians and trial team members have suggested that a trial becomes irrelevant once a baby has died, some of our interviews with parents suggest that although trials do often recede into the background, this is not necessarily where they remain. The feelings that clinicians who are involved with parents in the earliest period in their bereavement are able to observe may not reflect ongoing lack of interest in research but a limited capacity or inclination to engage with research at that time. After time, interest in a trial can re-emerge, either through the concerns and priorities of the parents or rekindled by trial-led communication strategies. This could result in difficult experiences for parents but, for those in this study, painful memories were part of their lived experience of being bereaved and were not necessarily grounds for their exclusion from information or contact.
An important and unexpected finding of the BRACELET study was that the significance of trial communications and feedback of the results, and the depth of the responses that these appeared to trigger for parents, did not necessarily relate to the initial investment that they made in the trial, to the length of time that they were involved, or to their understanding of the aims and conditions of the trial. We also found that parents can be intellectually interested in the outcome of the research, but trial communications could also be suffused with different layers of meaning for parents, which extend their value beyond the information that they contain. If stimulated to review their involvement in a trial over time, as parents were via the BRACELET study interviews, their accounts show their interest and engagement, and the potential value that a more prominent relationship with the research might have held for them, not necessarily because of the focus of the trial, but because it was research in which their baby was involved.
The BRACELET study has highlighted that an interest in a relationship with research is not always catered for. It was not necessarily a glaring omission in practice, as a response to trial participation was not something that people were necessarily requesting; it was concealed in the emotional complexities of bereavement, and emerged over time. Some people had wanted to know more for some time but they were not aware of any clear channels through which they could make their feelings known. Stefanie was still waiting for the results at the time of the interview, and Adam opted in to be interviewed as he very much wanted a route to understand more of the trial that they had agreed to in haste. Commonly, even people who had not previously considered information or feedback as a need warmed to the idea of information and contact, and by the end of the interviews were arguing that they would have liked and would have benefited from such an approach. This suggests that there is much work to do with individual trials to examine what may or may not be gained by a trial population (both bereaved and not bereaved), from ready access to information and a broad approach to communication.
Section C
Chapter 10 The methodological work of the BRACELET study
The BRACELET study was initiated to consider the topic of bereavement and trial participation, an area that is largely unexplored in the trials-related literature. As this was a novel research area, the three-part study design was intended to provide a broad foundation for future research, providing insights into the topic and considering the methods required to do so. Although methodological enquiry runs throughout the study, underpinning the overall aim of adding to knowledge to aid the conduct of trials that anticipate mortality in their research populations, BRACELET also included a specific methodological component that would consider the complexities and challenges of carrying out research in this area. As most of the studies carried out to date have not included bereaved parents in their samples there is little evidence to indicate whether or not their inclusion is feasible and informative. It was therefore necessary to consider the importance of gaining trust and facilitating collaboration with trial teams and clinical centres, as well as the practicalities of how a sample of bereaved parents might be identified and recruited through those agencies and other routes. It was also important to assess through reflection and feedback, the value and impact of the methods used in the qualitative study, and the extent to which research on bereavement and trial participation is acceptable to bereaved parents. It is important to understand how their involvement in research on their difficult experiences might be sensitively managed if they are to be included in future studies to guide the practice of clinical trials.
It is likely that a number of factors contribute to the lack of research in this area. The methodological component focused on three broad issues for which there was already some evidence of difficulty in relation to the topic of bereavement and trial participation. These are:
-
sensitivity of the topic
-
the challenge of recruitment
-
concerns over the experience of participation in an interview.
These are not discrete factors. The sensitivity of the topic relates closely to, and so is likely to affect, recruitment and the experience of study participation, the other areas of methodological interest here. (The term ‘study participation’ is used to denote participation in research relating to views and experiences, and to distinguish this activity from ‘trial participation’.) These issues are relevant to clinicians and trial team members but our main focus here is on bereaved parents. Discussing with parents the decision to enter a baby into research and the subsequent death of that baby is undoubtedly a difficult topic, and it is likely that concerns about this underlie many of the difficulties for research in this area. Concerns clearly exist, as some RECs have declined approval for researchers to include bereaved parents in their samples,61 and researchers have found access to bereaved parents who might participate in their studies blocked by clinicians who have not permitted parents in their care to be invited to take part. 64 The challenges of recruitment are evident in the size of the samples in the small number of trial-related studies that have been open to the inclusion of bereaved parents alongside those whose babies survived. In such studies, bereaved parents were a minority in the research samples. In the study by Allmark and Mason64 only three of 30 interviews were with bereaved parents; Cartwright and colleagues71 included two interviews with bereaved parents in a sample of 16 interviews and Stenson and colleagues61 secured 10 out of 154 questionnaires from bereaved parents. Our earlier research includes the largest numbers of bereaved parents in a trial-related study so far at 18 out of 78 interviewees51,60,76 but this is still a minority of the sample. That bereaved parents are in such a minority in each of the above studies may suggest that particular obstacles may exist to recruitment of this population. Although this may reflect parental choice on recruitment, with bereaved parents choosing not to participate, it may also relate to researchers’ own concerns over the experience of participation, which lead to bereaved parents not being invited to participate. In most studies of parental views and experiences, bereaved parents have not been included, and in one study report the researchers state explicitly that bereaved parents were excluded because of concerns that participation might be upsetting. 58 However, each of these factors do also raise different types of obstacles to research, and these challenges to research practice therefore require careful consideration in their own right.
Throughout the BRACELET study the central issues of sensitivity, recruitment difficulties and the experience of participation were the focus of methodological scrutiny. Dedicated recruitment strategies were developed and multiple options for participation were made available to bereaved parents. Data were collected on parental responses to the research aims and their experiences of participation. These were used throughout the interview period to monitor and reflect upon our own research practices. The methodological work relating to the three areas of interest are described and explored below, and are followed by an overview and reflection on the methodological aspects of the BRACELET study from the position of having completed the study.
Sensitivity of the topic
The lack of research in relation to bereavement and neonatal trials, and the absence of opinion papers and editorials on the subject, meant that at the beginning of the study the topic was something of an unknown entity. So much has been written on trial methods and conduct, and mortality as an outcome measure is such a familiar, everyday concept for triallists, that the relative silence on death in the context of a trial was difficult to interpret. This created a degree of uncertainty about how the topic would be received by potential collaborators and participants. It was possible that the particular intersection of trials and bereavement was underexplored because clinicians and trial teams viewed the subject as a non-issue, irrelevant to research practice, and a minor feature in the parental experience of bereavement. Alternatively, it was equally possible that the topic was a big issue but something of a taboo or the ‘elephant in the room’, an aspect of clinical and research practice and area of personal experience which is difficult to discuss and to contemplate. Either of these positions could account for the topic being under-represented in the trials-related literature and both would shape the conduct and ultimately the findings of BRACELET.
These two possible extremes, and a range of positions in between, hint at some of the complexity confronted at the start of the research process. Whether or not bereavement and trials would prove to be an important topic, it had to be considered sensitive because the data that would offer insights and promote understanding would be contained within, and accessed through, the stories of the birth and death of babies and the subsequent course of parental bereavement. Any research study that focuses in full, or in part, on parental bereavement involves engagement in highly sensitive material and deeply personal experiences and emotions. A number of studies have examined parental experiences of bereavement in the neonatal period and these suggest that sensitivity shapes the way that research is carried out but does not necessarily constitute an insurmountable obstacle to research. 26,28,159–161 The sensitivities that might be associated with the topic considered in BRACELET, however, extend beyond, and possibly heighten, those linked with bereavement per se. Interviews for this study would involve exploration of additional dimensions of parental experiences, such as decision-making about research participation and exposure of a baby to research risks, and for some parents could include discussion of the potential of a trial to change outcomes for a child. Parental experiences of bereavement might be shaped by their involvement in a trial, as might subsequent coping strategies. A trial might affect experiences in relatively small ways if, for example, a baby was included in a trial assessing a minor intervention with outcomes such as shorter length of stay. It could also potentially shape parental experiences in life-changing and emotionally traumatic ways if, for instance, parents felt that doctors had denied a potentially useful treatment to a dying baby allocated to the control arm of a trial, or worried that a treatment that they had consented to had harmed their baby in some way. Whether the focus on bereavement and neonatal trials would make research in this area too difficult to carry out and too difficult for parents to contemplate was an important aspect of the methodological work to be done. It was therefore recognised from the earliest stages of the research that there was the potential for the qualitative component of BRACELET to fail to recruit but that this outcome in itself would be an important methodological finding.
This additional layer of sensitivity was considered at all stages of the research process, and is reflected in decisions that were made about recruitment and data collection. Much of the practical work done in relation to the sensitivity of the topic is therefore described and discussed in the sections below, which focus on recruitment and participation.
The challenge of recruitment
Recruitment to BRACELET was a multifaceted challenge, as the focus of study was not a single hard-to-reach population but rather the interconnection of trials, trial teams, centres, clinicians and bereaved parents. Achieving an appropriate sample for a study of this nature was the most difficult aspect of the research faced by the study team, and this challenge was ongoing as the study developed and necessarily changed over its course. The developmental work to produce the initial design and approach for the study involved wide negotiation to gain access to the areas of study needed. Recruitment strategies for the study were modelled and then remodelled in response to research governance concerns, and remodelled again in response to recruitment difficulties. The element of fluidity that was incorporated into the study was not only essential to its success in producing the sample required, but also it allowed for methodological consideration and reflection on the obstacles and facilitators to recruitment, and their impact on the sample and evidence available for analysis. Crucially, we were aware that we would encounter bereaved parents with very difficult stories to tell and it was essential that the recruitment methods used were safe and acceptable to those we encountered. In this section we describe and explore the following challenging areas of recruitment considered in the methodological component of BRACELET.
-
defining the study area
-
identification of parents
-
checks and approvals
-
recruitment in the clinical centres
-
recruitment by publicity
-
responding to ineligible contacts.
Defining the study area
As BRACELET was the first study of its kind, it was essential that the sample was collected in a way that would be broadly informative and could act as a foundation for other future studies. It was important to consider how best to access data that would offer insights into different types of trials, how they are designed and implemented by trial teams and in clinical centres, and how they are experienced by the parents of the babies enrolled. This meant that the choice of trials and the centres through which trial team members, clinicians and bereaved parents would be recruited was not simply a route to a sample but a key element in the study as a whole. The means of defining the study area for the qualitative study pulled together all three components of BRACELET, with the quantitative Phase I acting as a foundation for qualitative Phase II, as well as informing decisions about how to determine the sample which draw on and feed into the methodological considerations at the heart of the study.
The BRACELET team felt that the choice of trials and centres should not be made on the basis of convenience, but should be made in such a way as to offer the best opportunity to represent practice in the UK. This was achieved by creating a matrix, or ‘map’ from data collected in the first part of the study, which indicated how commonly, and in which clinical centres, and in which trials, deaths had occurred during the 5-year period considered in Phase I. This took some of the selection process away from the research team, as the matrix indicated which centres and trials would be the most appropriate and salient for study. It showed where, in logistical terms, there would be the greatest chance of successful recruitment of the study sample.
The matrix was based on a subset of the 149 NICUs that responded in Phase I. Of these, 76 reported taking part in one or more trials, and 54 of the 76 reported one or more neonatal deaths subsequent to enrolment in a trial. These 54 centres were ranked according to the numbers of deaths accumulated across all trials in the 5-year period. The number of deaths in these centres ranged from 1 to 37 per centre in this period, indicating that the centres varied in their experience of bereavement in the context of a trial.
The first six centres in the ranked matrix were the primary candidates for collaboration as core centres, i.e. those from which clinical and parental interviewees could be recruited. All of these centres were invited to collaborate with BRACELET. Five of the six centres agreed and these are referred to here as centres 1–5. (These centres are referred to in Section B as centres A–E but the original ranking of 1–5 is preserved here as it makes a methodological point. A–E does not map directly on to the 1–5 ranking.) The centre from which the lead clinicians declined to collaborate did so owing to their concerns about the focus and methods to be used in the study. In particular they felt that approaching parents some time after their recruitment, when they had not given prior permission for this to happen, was not ethical.
Collectively, the five collaborating centres recruited to six multicentre trials and to four of their own single-centre trials. Three of the multicentre trials and three of the single-centre trials were excluded from Phase II, as the numbers of deaths per centre were too small to be considered in the qualitative study. The remaining trials, three multicentre trials – INIS, TOBY and PROGRAMS and one single-centre trial, the ExPN feeding study – were the candidates for core trials. The CIs for these trials were invited to collaborate and all agreed. In one trial at one of the core centres, 12 babies were excluded at the request of the trial CI for logistical reasons.
Table 40 shows the distribution of deaths highlighted in the matrix across these five core centres and four core trials (a further trial, BOOST-II UK, and two more clinical centres were added later in the study but are not considered in this description of the initial recruitment processes). As Phase II investigations were not limited to the same 5-year study period as Phase I, the table shows 146 deaths rather than the 117 reported for the restricted study period. After 35 exclusions 111 deaths remained across the initial core centres and core trials. These 111 are referred to as core deaths and represented the target population for the study.
| NICUs | Total deaths by centre | Deaths in excluded trials | Deaths in multicentre core trials | Deaths in single-centre core trials: ExPN | Potential deaths per centre | ||
|---|---|---|---|---|---|---|---|
| INIS | TOBY | PROGRAMS | |||||
| Centre 1 | 40 | 6b | 6 | 28 | 34 | ||
| Centre 2 | 33 | – | 21 | 12c | – | 33 | |
| Centre 3 | 29 | 7b | 22 | – | 22 | ||
| Fourth-ranked Centre declined to participate | 23 | – | 16 | 7 | – | 23 | |
| Centre 4 | 22 | 1b | 14 | 7 | – | 21 | |
| Centre 5 | 21 | 8b | 1 | 12 | – | 13 | |
| Total deaths overall | 168 | 22 | 52 | 35 | 31 | 28 | 146 |
We were aware from the experience of our previous study that the process of gaining access to bereaved parents was time-consuming and complicated, and that some clinicians would feel that there would be some families for whom an approach to offer participation in an interview would be inappropriate. Around one-third of parents who were eligible to take part in that study were lost via this route. Also from our previous experience we anticipated a response rate from bereaved parents of ≤ 50%. It was therefore judged that for the BRACELET study a denominator of the 111 core deaths as a starting point was sufficient to mark out the population from which the target number of 30 interviews with bereaved parents might be achieved. This denominator was based on the restricted 5-year period defined for Phase I and increased when all deaths per core trial in the core centres were included.
Identification of parents
The BRACELET team developed an identification and recruitment strategy in collaboration with the co-ordinating teams for the original four core trials (INIS, TOBY, PROGRAMS and ExPN). This plan underwent a series of modifications over the course of the study, as described below. The original recruitment plan was very much shaped by the requirement for data protection and research governance, as these practices affected who might access information about the parents involved, and what steps needed to taken before parents might be contacted.
The first issue to be tackled was identification of the babies, parents and parental contact details. A population of bereaved parents of babies who have been enrolled in a neonatal trial should not necessarily be difficult to identify. If there had been plans for the trial teams to have direct contact with parents (e.g. to arrange follow-up or to send newsletters or to feed back trial results), the trial teams would hold records and so have details of parental names and addresses as well as those of GPs if checks or permission to make contact with parents are required. (For many multicentre trials, data protection requirements can mean only the recruiting centre has details by name. The co-ordinating centre may have only a trial number, and may not know the name of child or parent.) For a study that is recruiting bereaved parents within a relatively short time after trial enrolment, these records are likely to be up to date. BRACELET was interested, however, in responses to trials over time and in feedback of trial results, and this meant that parents some years into bereavement were the most appropriate target sample. Trial teams often have little or no long-term contact with bereaved parents, and although their records were accurate at the time of recruitment, they were useful for our recruitment only if parents were still living at the same address. Where parents had moved they were lost to contact.
The second issue related to constraints around who was permitted to access the contact details for the bereaved parents, which were held in the trial databases. Data protection legislation meant that the BRACELET team had no rights to access these data, and parental details could not be shared with the BRACELET team without parental permission. This meant that the BRACELET team could not take part in the preliminary checks or in writing to parents, and so recruitment depended entirely upon trial staff for each of the four trials prepared to identify the parents from their records and to take responsibility for the initial checks that were to be made. This was a stumbling block that would not have been faced had the research been carried out within a CTU or by a researcher within the trial team with legitimate access to these data. Nor would it have been as big an issue had BRACELET considered only one trial. For a comparative study with an interviewer who stood outside the trials and institutions in question, it was a major complication.
Checks and approvals
Given the focus on bereavement, we agreed that it would be appropriate to make preliminary checks to ensure as far as possible that an invitation to participate in BRACELET was not sent to parents inappropriately or at an inopportune time, such as around the time of another neonatal loss, or in the event of major parental depression. The protocol stated:
Parents will be ineligible and so excluded if:
-
they have not yet passed the first anniversary of their baby’s death by the time of identification for the study
-
another baby has subsequently died and they have not passed the first anniversary of their subsequent baby’s death by the time of identification for the study
-
another baby is known to be currently receiving or has recently received NIC
-
the clinician judges from his/her knowledge of the family that contact would be inadvisable, for example death of one of the parents, parental depression
-
they would require a translator to take part in an interview (note that it is hoped that future research will consider ways of including such families but this is beyond the scope of the BRACELET study)
-
other circumstances make contact inappropriate, for example in litigation against the hospital.
Given these criteria, a series of three checks were agreed:
-
Central co-ordinating trial teams were asked to exclude any families with whom they felt contact would be inappropriate or not feasible. This could include families known to have left the country, or when the trial team was aware of other reasons why an approach would be inadvisable. A number of families from TOBY had been approached to take part in an earlier study of consent processes in this trial;162 the TOBY CTU staff, in consultation with the trial PI, felt that they should exclude these families.
-
The neonatal consultant who acted as the local trial PI in the centre where a baby was enrolled would be asked to review the remaining babies and to exclude any that they felt would be inappropriate. Although their knowledge of the current family circumstances was not up to date, they would for example be aware of any cases of ongoing litigation between the family and hospital.
-
The family GP was to be contacted and asked to advise the team if there were more recent factors, such as ongoing depression or a subsequent neonatal death, that would make contact inadvisable. We aimed to inform GPs of our intention to write to parents, giving a 4-week window in which to object. We refer to this as the ‘GP opt-out’ model.
The REC application for approval for BRACELET was submitted in November 2008 and was not approved until May 2009. The REC required modifications to the recruitment processes and approved the study only with the undertaking to secure a definite response from a GP before writing to parents to invite them to participate. We refer to this as the ‘GP opt-in’ model. This requirement led to further modifications to the study protocol; the inability of the BRACELET team to work with identifying information meant that the trial teams took on the responsibility for contacting the GPs, a process which, with the GP opt-in model, had become a much greater undertaking. The trial team now had to secure definite responses and to remind non-responding GPs.
The modified design proved to be highly problematic for a number of reasons as described below.
-
GPs could not be identified If no GP was listed in the trial records or if parents were no longer registered with the original practice, no approval from the GP could be secured and so contact with parents could not be made. When parents had changed GPs, practices sometimes offered to update records but most often this was not the case.
-
GPs often did not respond The process of sending repeated reminders was labour intensive and did not always result in permission to contact.
-
Some GPs declined to give permission for contact In some instances this was very useful, for instance contact was avoided with a family in which there had been a recent death of one of the parents, and some GPs indicated that there were families with parental depression, which, in the GPs’ view, made contact inadvisable. In other families GPs declined because they felt that parents had been through a difficult experience and so should not be contacted; it is difficult to judge whether parents themselves would have shared the same view. One GP declined not because of known problems, but because the parents had not visited the surgery in 3 years and so their situation was not known. Some GPs declined on receiving a reminder. It is possible that they had concerns but it is also possible that for busy practitioners it was easier to tick a box to decline than to check notes in order to make a decision.
In this three-part screening process, 14 babies were excluded by the central trial team, and four were excluded by the consultant neonatologist. The GP screen, by contrast, resulted in a big loss to the study, with 83 families removed from the pool of parents who might have been contacted. The attrition via this route is shown in Box 16 .
No response from GP 41
Excluded:
-
GP excluded parents 21
-
GP said could not decide as had not seen parents 1
-
GP checked with parents who did not wish to proceed 1
Could not be contacted:
-
Parents had moved GP 18
-
CTU never had GP details 1
Total loss at this stage of the study 83 families
The target sample for BRACELET was a population for which we did not anticipate a particularly high acceptance rate, and this loss of parents to contact was a major challenge to the study. The 41 families lost because of a lack of a response from the GPs was particularly frustrating.
When permissions to proceed with the original recruitment strategy (strategy 1) were secured, the first group of parents was invited to participate, and it became clear that the difficulty with the imposed ‘GP opt-in’ system was compounded by an especially low response rate from parents. When GP permission was secured, a recruitment pack was sent to parents with a letter of invitation, an information booklet, a reply slip and a questionnaire about the contact process used; 21 recruitment packs were sent to parents and these resulted in one interview request (PROGRAMS), one request for an alternative to an interview (TOBY) and one where parents declined to participate. (This is dealt with below: see Participation options.) This constituted a consent rate for interview of 4.8% for the method of sending a letter alone. An additional request to be interviewed was received from a mother (Shirley), who was informed about the study directly by her GP practice, taking the number of interviews achieved to two (9.5%). (Shirley’s route into the study is considered further in the Discussion, below.)
We had not experienced this level of non-response in any of our previous studies, either for parents of surviving babies or bereaved parents. Recruitment processes required a long and laborious process of identification, the three-step check, and the time allocated for three reminders for non-responding GPs, all of which had to be managed by our collaborators and not the BRACELET team, and only one interview was secured. Because of this combined impact of the lengthy and unwieldy recruitment processes, the impact of the stipulation to secure permission from GPs, and the poor response rate for the parents who were contacted, in April 2010, further attempts to recruit parents to the study were suspended.
The BRACELET team reviewed the recruitment strategy and came to the following conclusions:
-
The recruitment design was inefficient and labour intensive for the trial teams, in particular the requirement for a definite response from GPs.
-
The use of a recruitment pack sent as ‘cold-mailing’ by post did not elicit a positive response from bereaved parents
-
Parents may be more likely to respond to a personal touch in recruitment.
-
Multiple recruitment methods may be necessary to recruit the sample.
-
Access to a significant number of parents had been blocked as they were either lost to contact, excluded by GPs, or had already been invited to participate and had not replied; the pool of remaining parents was small and it would be necessary to recruit from outside the four core trials.
This led to a complete revision of the recruitment strategy, legitimated by the methodological nature of the study. The failure of one approach had been demonstrated and it was important to explore new approaches. The revisions were major and funds were sought to resource further attempts to recruit parents. An application for a substantial amendment to modify the recruitment strategy was made to the REC, to request that the requirement to secure permission from the GP should be lifted. Both applications were successful and in October 2010 REC approval was given and recruitment to the study resumed (strategy 2 and 2a).
The study was revised as follows:
Strategy 2a
-
The REC gave permission for the team to revert to the originally planned design in which GPs were given 4 weeks in which to object to contact with parents. Non-response was taken to signify no objection to contact. The families from the initial wave of recruitment for which GPs had not replied were ‘recycled’ into this second wave on the grounds that the GPs had not lodged an objection, thus reintroducing 41 previously lost families back into the study. In addition, two families in which the parents had moved GP, and five for which GP contact details were obtained late, were added to the 41, allowing 48 families to be contacted under what became strategy 2a.
-
A point of contact was introduced between parents and a clinician from the NICU that cared for their baby. It was agreed in all five collaborating clinical centres that where GPs had not registered an objection, a neonatologist would approach a family to ask if they would view a letter about BRACELET, and to seek their permission to pass on their contact details to the BRACELET team. Interested parents were sent a recruitment pack as described earlier.
Strategy 2b
-
An additional approach to recruitment was developed in which the study would be publicised via charities and special interest groups allowing parents to opt in to the study without an intermediary. A preset text for use as the publicity material was approved by the REC (see Appendix 20 ). A number of organisations were asked to help with this part of the study by placing this text and a link to the BRACELET website on their own websites, or by included the publicity material on their websites, in their newsletters or any mailings to their membership. Trial teams were able to include the study advertisement in their own mailings to parents and to post a link to the BRACELET website on their own trial websites. Parents responding to the publicity material could opt in to BRACELET via the study website or by making direct contact with the BRACELET team. Those preferring not to be interviewed could visit the website and complete a questionnaire or leave their comments (see below for further details).
Also:
-
At a late stage in the recruitment it became clear that one more trial was needed to reach the target of 30 interviews. Under the revised REC approval, which allowed recruitment from any neonatal trial, we sought and received permission from the TSC and trial sponsor to recruit from the BOOST-II UK neonatal intensive care trial. The trial and two of its recruiting centres were introduced to the study in 2011. (The introduction of the trial raised the total number of parents from the five trials and seven centres to the 155 shown earlier in this report in Figure 12 .) BOOST-II UK assessed two different oxygenation strategies (higher/lower) and closed to recruitment at the end of 2010 when a difference in mortality between the two arms was identified. The preliminary results of this trial were communicated to parents in the summer of 2011 (see also Chapter 4 ).
Recruitment in the clinical centres
In each centre at least one consultant neonatologist agreed to act as the first person for contact for parents, and to introduce the study. Ten consultants took on this role, which came after the GP check. When the GP check was complete, parents could still be lost to contact if no telephone details could be traced for the consultant to use, or if they could not be reached by telephone to give their permission for release of their details to BRACELET. These difficulties caused the loss of 22 families; of the initial 81 families that were available to the study under strategy 2a, only 59 proceeded to consultant contact.
In the first instance, a letter was sent to parents to explain that the consultant planned to telephone the following week to tell them about a research study (see Appendix 21 ). The aim of the letter was to forewarn the parents of contact and to offer a means to prevent the call taking place if they wished. The letter gave the telephone number that was held in the records for them, and gave parents a contact telephone number and e-mail address to use if they preferred the consultant not to call. In four instances, parents did make contact to say that they would prefer not to be called, but there were also two where parents called to facilitate contact, one to give an updated telephone number and the other to state that they would be on holiday but that the consultant could call at a later date.
The aim of the call was to introduce brief information about the study but not to ask parents to make a decision about participation. The consultant explained that at that point no details had been released to the BRACELET team. If the parents were prepared to view information about the study, their permission to release contact details was sought and, crucially, this allowed the BRACELET team to take over the process of inviting parents to participate. This relieved the trial teams of the task of sending out invitations to parents and afforded the BRACELET team some control over the invitation process. An invitation, information booklet and reply slip were sent to parents, along with a questionnaire about the contact process used. Parents were asked to return the reply slip directly to the lead researcher, CS. This method had the advantage of revealing to the BRACELET team the identities of only those who chose to opt in to the study, and concealing from the clinicians involved in the contact process whether or not the individuals concerned chose to participate in the study. No reminders were issued. This process is shown in Figure 26 .
FIGURE 26.
Recruitment via consultant neonatologists in collaborating centres (strategy 2a).
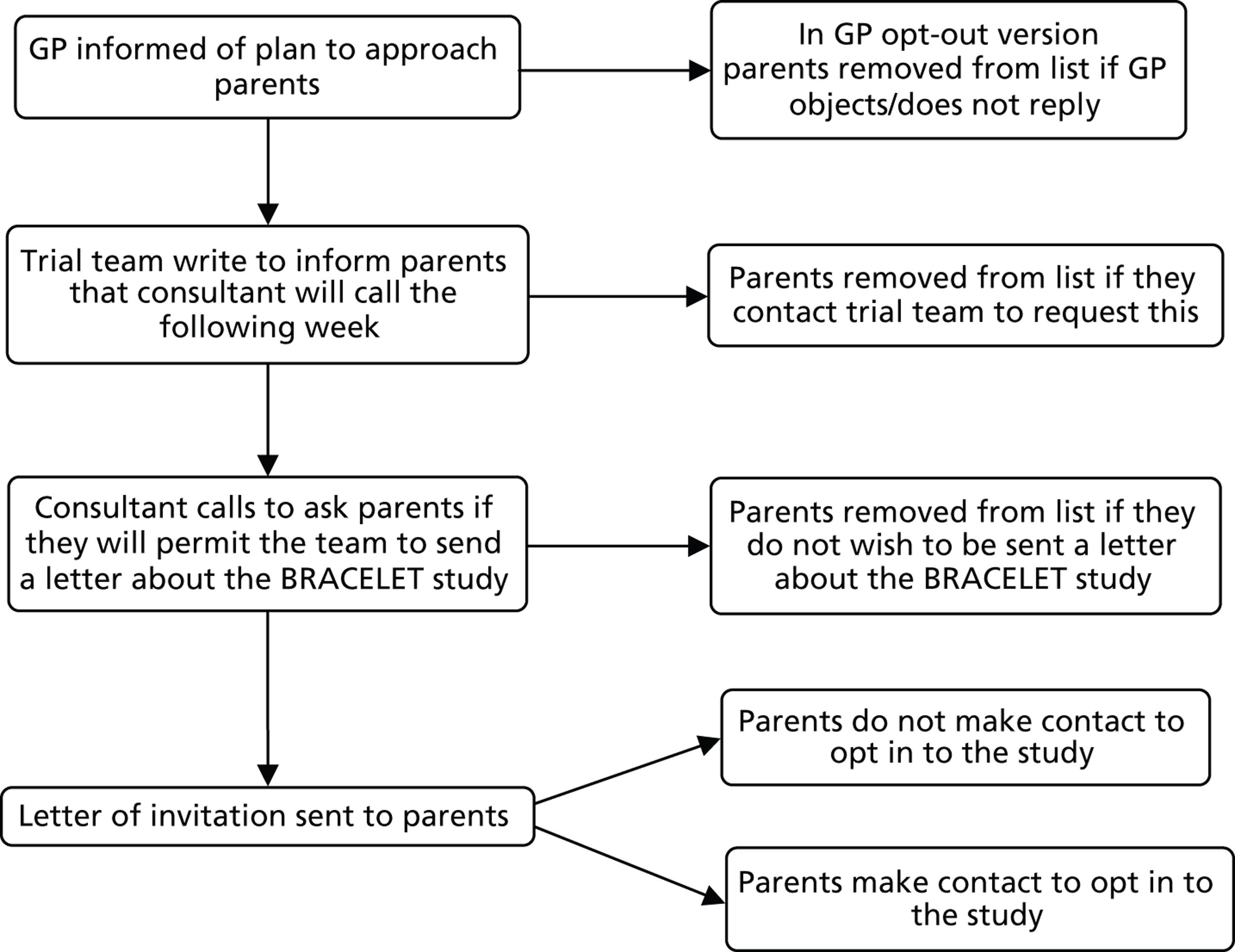
In the 59 households contacted, 50 agreed to view a letter about the study. This resulted in 18 interviews with 30 parents, a 36% consent rate for those who received an invitation ( Table 41 ). Although this is not a substantial consent rate, it is to be compared with the rate of < 10% achieved by letter alone. The observation that fewer than half of those approached elected to participate would suggest that the parents did not experience the contact with the consultant to be coercive.
| Clinical centre | Consultant neonatologists per centre | Possible families | Excluded | Pre-call letter sent | Could not proceed to contact | Families to call | Could not be contacted | Contacted | Declined | Agreed to view details (invite sent by CS) | Did not reply | Declined | Opted in | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Interview | Parents | |||||||||||||
| A | 1 | 12 | 2 | 10 | 0 | 10 | 1 | 9 | 1 | 8 | 4 | 0 | 4 | 7 |
| B | 2 | 11 | 3 | 8 | 1 | 7 | 1 | 6 | 1 | 5 | 2 | 0 | 3 | 6 |
| C | 3 | 31 | 0 | 31 | 0 | 31 | 3 | 28 | 0 | 28 | 22 | 1 | 5 | 8 |
| D | 1 | 3 | 0 | 3 | 1 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| E | 1 | 6 | 3 | 3 | 0 | 3 | 1 | 2 | 1 | 1 | 0 | 0 | 1 | 2 |
| F | 1 | 13 | 1 | 12 | 0 | 12 | 1 | 11 | 6 | 5 | 3 | 0 | 2 | 3 |
| G | 1 | 5 | 1 | 4 | 1 | 3 | 0 | 3 | 0 | 3 | 0 | 0 | 3 | 4 |
| Total | 10 | 81 | 10 | 71 | 3 | 68 | 9 | 59 | 9 | 50 | 31 | 1 | 18 | 30 |
Recruitment by publicity (strategy 2b)
A short article for publicity was written and approved by the REC for use in BRACELET (see Appendix 20 ). The introduction of recruitment via publicity opened up the study to a wider population of parents who could decide, without any intermediaries, whether or not they would like to take part in BRACELET. Although this would potentially bring in parents who would be beyond the initial reach of the study – such as those who took part in a trial more recently than the original target population, or those who had moved house since the death of their baby – we were aware that publicity would have to be on a large scale in order to come to the attention of eligible parents.
Bereaved parents whose babies took part in a trial may (or may not) identify with a wide range of special interest groups, none of which specifically relates to our research topic. They may connect with organisations that pertain to their experience in NICU, such as Bliss, which operates at a national level, or with support and fundraising groups linked to their local hospitals. They may focus on groups that relate to the condition that affected their baby, for instance CDH UK for diaphragmatic hernia. Parents of multiples may identify with organisations, such as TAMBA and the Multiple Birth Foundation. Even with specialist bereavement-related groups, such as SANDS, when the majority of parents have experienced a stillbirth or neonatal death, most will not necessarily have enrolled their baby in a neonatal trial. As we expected the parents of interest to BRACELET to be a minority in the membership of the organisations that were approached, the likely yield from this method of recruitment was unpredictable.
To maximise the chances of bringing the publicity material to eligible parents, a wide range of organisations and groups was approached and asked if they would consider making the BRACELET publicity material available to their membership. The publicity material could be used as reproduced text on a website with a hyperlink to the BRACELET website, it could be included in newsletters and mailings, and/or circulated by e-mail as an attachment. Some organisations agreed to circulate the material very readily and the text was quickly posted on websites. Others agreed to include the information in a future newsletter. Some local groups agreed to mention the subject at their next meeting. Only one organisation declined as the topic did not seem to them to be appropriate for their membership. The CTU that ran four of the core trials placed the information on its website and the INIS team was able to include paper copies of the publicity material in with their mail-out, offering parents the results of the trial. (The A5 publicity flyer was included only with letters to bereaved parents.) Given the variety of responses, it is not possible to know how widely the material was circulated.
Parents who saw the publicity material and wished to take part could opt in to BRACELET via the study website (see below) or could contact the BRACELET team by e-mail or telephone. (A dedicated telephone was bought for the study. E-mails from the study website were streamed to this telephone. CS kept this with her during the recruitment period and this allowed for a quick response to a call, text or e-mail.) Those preferring not to be interviewed could visit the website and complete a questionnaire or leave their comments.
This part of the study yielded 10 interviews with 17 parents and 7 online questionnaires. Although the number of additional interviews seems to be small for the effort involved, this approach achieved one-third of the target sample for a population that was extremely difficult to recruit. This approach was therefore vital to the success of the study. Table 42 details of the parents who were recruited in this way and whose accounts contributed so vividly to the qualitative study.
| Interviews and parents | INIS | TOBY | BOOST-II UK | Total interviews | Total parents | Other |
|---|---|---|---|---|---|---|
| Interviews via publicity | Sara and Gareth | Laura and Wilf | Jill and Ethan | 10 | 20 | Abbyb
Linda and Danc |
| Anita and Sean | Robert | |||||
| Fiona and Keith | Hannah and Ryana | |||||
| Sophie and Nat | ||||||
| Dora | ||||||
| Jana | ||||||
| Total interviews | 6 | 3 | 1 | 10 | 2 | |
| Total parents | 10 | 5 | 2 | 17 | 3 |
In reviewing the responses, two issues came to light. Three of the couples who opted in via publicity – Laura and Wilf, and Hannah and Ryan, who saw the publicity material via a charity, and Sophie and Nat who received the publicity material with the INIS results – had gone through inquiries into the death of their babies. In two of these families the babies were cared for in centres that were taking part in BRACELET and so would have been eligible to have been invited to take part. As the BRACELET team could not reveal the names of the parents who chose to take part in an interview to the trial teams who carried out the screening processes and issued the invitations to parents, it was not possible to check the point at which they were excluded from the recruitment process. They could have been excluded by the trial team, the consultant neonatologist for their centre or by their GP’s exclusion or non-response. The three couples are examples of parents who were highly engaged with BRACELET and were pleased to have given their views but were initially lost to the study through the screening processes. Another couple, Sara and Gareth, also linked to one of the BRACELET centres, had moved house and GP and so were lost to contact. They opted in to the study the same day as receiving an e-mail about BRACELET sent from their local hospital charity, which sent out a message to all members about the study. They are an example of interested parents lost to the study as records for bereaved parents are not currently maintained.
The role of the BRACELET study website
Parents could opt in to the study via the BRACELET study website (which can be viewed at www.bracelet-study.org.uk). This site was developed as a major methodological platform for the study, a response to the challenges inherent in recruiting and making provision for a sample of individuals that is hard to reach, hard to recruit, and may have particular information needs and areas of vulnerability. It was available to those who have received an invitation to participate, as well as to those responding to publicity. The website therefore served a number of purposes linked to recruitment:
-
It served as an information repository for potential participants and those involved in supporting recruitment to BRACELET (trial co-ordinating staff, clinical centre staff, GPs and GP practice managers) giving access to a wide range of information about the study. Relevant study documentation and details of publications could be accessed via the site. The information included for parents went beyond that required by RECs and addressed the sort of questions that parents often ask before an interview: What is an interview like? Who will interview me? Can my children be there during the interview? This section of the website also included three short accounts from participants in previous studies, describing what it was like for them to take part in a research interview (see www.bracelet-study.org.uk//index.php?page = taking-part-in-an-interview).
-
It was a communication route, offering potential participants an alternative way to contact the study team and to opt in to the study.
-
It was a data collection/data salvage tool, offering those less inclined to participate in a personal interview alternative ways to contribute to the study, by questionnaire or free-form writing.
-
The website also offered a means for parents to feedback on the contact processes for BRACELET if they wished to do so.
The image below ( Figure 27 ) shows the parents’ landing page for the website. Similar pages were created for clinicians and trial team members, and for GPs.
FIGURE 27.
Parents’ landing page for the BRACELET website.

The site has proved to be useful not only as an opt-in route but also because we were able to direct two parents to the website as an alternative to being interviewed. One mother contacted us to say that she wished to take part in the study but asked if this could be done by e-mail, as she felt that an interview would be too emotional. Another mother offered to take part in an interview but her baby had not in fact taken part in a trial. Many years previously she was asked informally if her baby’s doctor could try out nitric oxide (then an unevaluated treatment that had been little used) as a last attempt to save her daughter. Her detailed account described what it was like to take this leap of faith, and showed how interested she was, almost 20 years after the death of her baby to know whether anything ever came of nitric oxide. These were precisely the sorts of situations that the website was set up to address. For the first mother, it enabled her inclusion in the study. Without this option she would have been unlikely to have participated; for the second mother it allowed her to describe the experiences and emotions that BRACELET had stimulated and brought to the surface, and to widen the BRACELET team’s appreciation of the topic. Both women were able to make a valuable contribution to the study and the significance of their accounts are considered in Boxes 17 and 18 .
Options for participation
The BRACELET study focused on a population which was particularly hard to reach and this, combined with the potential challenges of being interviewed, made the options for participation important in terms of both the amount and type of data produced. With the development of the BRACELET study website it was possible to offer parents multiple options for participation. These were:
-
in depth interview
-
online questionnaire
-
free-form comments box.
The main form of data collection for BRACELET was the in-depth interview, a method that is ideally suited to collecting information on a complex and sensitive topic. Interviews suit the researcher who requires rich and detailed data, and may well be satisfying for research participants, but they also make demands of participants in terms of time and emotional energy. BRACELET predominantly involves interviews with couples and this introduces additional elements for methodological scrutiny and reflection.
The online questionnaire in contrast was deliberately brief. We were aware that the subject matter and the prospect of taking part in an interview may be a disincentive to participation for parents who have valuable contributions to make. Where accounts of a phenomenon in the literature are particularly rare, and where recruitment may be difficult, every testimony that might be added to the evidence is valuable. Given the potential difficulties of access that the study faced, the online questionnaire offered a means by which reticent individuals with valuable perspectives might be encouraged to add to the research.
The questionnaire was accessed via the BRACELET study website ( Figure 28 and Appendix 22 ). It asked broadly about experiences of trial participation and specifically about some of the practical issues of interest to the BRACELET study, such as whether or not trials should send out condolence letters and newsletters to bereaved parents and whether or not they should offer feedback of trial results. Parents could fill in the questionnaire online, or print out the questionnaire from the study website and return it by post.
FIGURE 28.
The questionnaire was accessed via the BRACELET study website.

We have used the BRACELET study website as a data collection/data salvage tool, offering those less inclined to participate in a personal interview alternative ways to contribute to the study: by questionnaire or free-form writing. This option has been used but only by a small number of parents: seven in total. This small number should be placed, however, in the context of the target population being hard to reach, should be seen in relation to the size of the interview sample for which the target was 30 interviews, and viewed in relation to the yield. The data have in fact proved to be extremely useful in methodological terms, as they:
-
point to the concerns that some parents have in relation to taking part in an interview
-
confirm some of the findings from the interviews, where issues can be seen to arise for parents without the direction and mediation of an interviewer
-
introduce dimensions not commonly seen in the interviews
-
give more parents a means of communicating their views; where they have concerns that are particularly challenging, it may be these issues that make an interview difficult to contemplate.
Table 43 below shows the numbers of parental questionnaires received via the BRACELET study website. These data would otherwise have been lost to the study, hence the term ‘data salvage’.
| Questionnaires | INIS | TOBY | PROGRAMS | ExPN | BOOST–II UK | Other | Total |
|---|---|---|---|---|---|---|---|
| Questionnaires via core trials | – | 1 | – | – | – | 1 | |
| Questionnaires via publicity | 2 | – | – | – | 2 | 2 | 6 |
| Total | 2 | 1 | 2 | 2 | 7 |
In all studies there are questions as to whether the research participants are similar to or different from the larger population from which they are drawn. The issue of generalisability is not as much of an issue for qualitative research as for quantitative studies, as the aim is conceptual rather than statistical generalisability. For some topics, however, it is important that researchers aim to represent as fairly as possible the population of interest. In studies where a very wide range of views may readily be tapped, those who do not participate may not differ so much from those who do; non-participants may make this choice because they are busy, or forget, as well as being less interested in the topic. For a topic such as bereavement, however, it may be that the factors that make participation difficult are also those that need to be understood in a full exploration of a phenomenon.
In order to consider the concerns that some parents have in relation to taking part in an interview, those who completed an online questionnaire were asked as a final question about their preference for an online contribution. This read: ‘Finally, we need to decide whether or not to offer people the option of filling in a questionnaire like this in our future studies. It would help us to know why you preferred to take part in the BRACELET study in this way rather than being interviewed.’ In Table 44 , three views from questionnaire respondents 1, 3 and 4 are contrasted with views given by parents who took part in an interview in a post-interview questionnaire (see Appendix 27 ).
| Completed a questionnaire | Took part in an interview |
|---|---|
|
Respondent 1 (INIS) I don’t like phone calls as our eldest (the surviving twin of the child who died) has only just started school full time. He has two to one care there and we have another boy who takes up a lot of time and energy. I can sit at the computer with a cup of tea in between trying to catch up – we get lots of extra appointments/things to do due to the disability. |
Karen (BOOST-II UK) Spent a lot of time thinking about Tabitha, which was nice. She was a big part of our lives even if it was only for a short period of time. |
|
Respondent 2 (BOOST-II UK) I chose to take part in the questionnaire as I think I would have broken down crying in an interview as our loss is still raw; however, if an interview was the only option then I would have taken part in it. |
Marion (INIS)
The interview was brilliant. It brought out our feelings and it was important to the research. Would do it again and if I can help any way please don’t hesitate in any way. It’s nice to discuss my daughter in great length. |
|
Respondent 4 (TOBY) I would have found a 1 : 1 person-to-person interview too painful, even after 5 years since our loss. This online [questionnaire] is easier emotionally. I felt that it was important to get my views across but also know that it would be too upsetting being interviewed. |
Dora (INIS)
Afterwards I felt sad, though having said that I also felt grateful that someone had listened to me and let me talk about my precious son as I think people tend to avoid difficult conversations like that. I have had a little cry since it all finished but overall I feel good. Happy my opinions do matter and most importantly to me happy to be allowed to talk about my precious little boy. |
Even just from the juxtaposition of these six viewpoints, it is clear that the features of an interview that make it so difficult for one person are those which make it attractive to another. For the three parents who chose to complete questionnaires ( Box 17 ), talking would have been a difficult option; for respondent 1, time was hard to find in the context of her life as a carer, whereas for Karen it was the time taken to explore their experiences that she valued; for respondents 2 and 4, recalling their experiences would have been too emotional and demanding, whereas Marion and Dora saw it as an opportunity to connect with the experiences and to talk in detail about their babies. The questionnaires and the interviews seem to tap very different coping styles for these six parents, and the important issue in methodological terms is whether the choice of participation method might also be associated with different views. It is important to consider that possibility, but it is beyond the scope of the current study, and the numbers available, to adequately address that substantial methodological question.
The views of these same questionnaire respondents were also useful in the reactions that they describe for three of the core trials: INIS, BOOST-II UK and TOBY. Respondent 3 is also included in Box 17 , above, as his/her comments relate to BOOST-II UK. Their comments provided the opportunity to consider whether themes that were proving to be important to parents who took part in an interview, were also present for parents who chose not to. Clearly a larger number of parents would be needed to make any detailed comparisons and to draw any substantive conclusions, but in methodological terms, a comparison of the data from the questionnaires and the interviews is very interesting.
Both of our twins were involved (in the trial). One died and the other lived but has quadriplegic cerebral palsy following birth trauma . . . The trial may have helped but was not going to be harmful. [This mother indicated that she wants to receive the results of the trial and to have allocation details for her babies] . . . but then I am just curious. My husband does not want to know for the twin who died.
Respondent 1 (INIS)
I was quite happy to be involved in the trial as I felt we were helping try to raise survival rates in extremely premature babies . . . Unfortunately our son only lived for 37 hours after birth so we weren’t involved in the trial for long but I felt that everything was handled brilliantly and we were given a lot of information about the trial . . . I don’t think there is much they can really do for parents who are bereaved as our son didn’t die because of the study, he died because he was extremely premature.
Respondent 2 (BOOST-II UK)
At first I wanted to help others in our situation, but as time went on we started to wonder if it was doing any harm . . . Will always wonder if it was a factor in our baby’s death.
Respondent 3 (BOOST-II UK)
At the time our baby daughter Mary was born and we were told she had lost oxygen due to the cord being so tight around her neck in delivery, we were asked to take part in the TOBY study. During this traumatic time, we really were unsure what to do but felt that maybe research could prevent other babies dying because of this. In hindsight, I am glad that Mary was selected to not be treated as part of the (trial). Maybe things would have been different had she lived, but I think at that traumatic time parents really are not fully aware of how their choices could effects their child’s survival or not. Mary died 2.5 days after birth. If she had survived as a result of having received temperature-control treatment and having had so much brain damage her life and our lives would have been so much different. I’m not sure how I feel about this. I just think that maybe it’s not appropriate to ask parents who are going through such a traumatic situation to take part in research because really they are not in a position to make a conscious decision. We are lucky in that we don’t have any questions regarding Mary’s death other than the cord got caught around her neck during delivery. I know how important research is but I also know, from personal experience, it is very very hard to take medical information on board when you are experience a tragedy like we did. God forbid, but if it ever happened again and I was in a similar situation, I don’t think I’d agree to take part.
Respondent 4 (TOBY)
The comments in Box 17 contain some of the same issues as the interviews and some of the themes that were identified in the main analysis can be seen here; that the trial might help, won’t harm, that the baby was too sick to save, and in the connection out to parents in the same situation with the hope that someone somewhere might benefit from their decision to participate. We also see something of the changing response to a trial over time for respondent 3, with doubts arising, a theme that was coded in the analysis but referred to only a minor strand in the data. (Doubts arising were noted for Beverly and for Stan. If they were mentioned for other parents they were usually counterbalanced by a statement, such as the baby was too small or too sick to save.) In raising this issue, respondent 4 entered territory that was explored with clinicians and trial team members but was rarely present in the parental interviews. Respondent 4 raises the possibility that had her daughter received cooling as part of the trial, this may have been the factor that tipped the balance towards survival, and with the disabilities that she was likely to have faced, this mother was unsure whether the trial would then have served her well. It is exactly the balance of death and disability that a trial such as TOBY examines through the use of composite outcome measures. This is a highly sensitive issue which was approached gradually in interview with parents who were already giving some indication that this might be their view. Hannah and Ryan discussed this possibility, as did Robert, but parents often made it very clear that they would have wanted their baby to have survived and were hoping the that trial might help this to come about. In her online questionnaire, this mother drew out, without the mediation of an interviewer, her own complex and emotionally conflicting strands of thought on this situation.
This view that a trial may have ‘inappropriately’ (from the parent’s perspective) saved a baby, and the view expressed by respondent 3 that the trial might have contributed to the baby’s death, are two views that we had expected to have encountered more frequently in the interviews. That we did not may perhaps be an indication that for some parents, recollection of a trial, and indeed the wider setting of decision-making in life-and-death situations in the NICU, takes them into difficult emotional and moral territory. If such parents find re-exploration of these aspects of their experience to be too difficult and too unsettling to face in an interview, it is vital that the methods that will allow expression of their views are made available.
The number of questionnaires is too small to draw major conclusions but they do suggest the value of this multioptioned approach for a study for which potential participants might have some concerns about participation. This approach will still not tap the views of those who find the idea of contributing to research too difficult to contemplate, but it offers a means to represent a middle group that might otherwise be beyond the scope of a single-method, interview-based study.
Responding to ineligible contacts
A final important observation must be added here about the use of publicity as a recruitment tool. The publicity route also attracted some parents who did not fit the formal entry criteria of BRACELET. There were issues about managing this situation so that those who could not be included in the study still felt that their offer of contributing was valued. All who called were given time to discuss their experiences if they wished and thanked for calling. Some of these were very clearly beyond the scope of the study: parents whose babies had died but had not been offered participation in a trial; some whose babies took part in a trial but had not died, and one respondent was the grandmother of a child who had been cared for in a NICU and survived, who called in relation to her own bereavement many years previously. Others were ineligible but their input helped the team to see that the topic overspilled into a much broader area than we had defined for study. In one instance ineligibility did not come to light until an interview was under way and it became clear that the parents were bereaved, but in relation to an earlier pregnancy. The parents had enrolled their subsequent baby into a trial and that baby had survived. On seeing the publicity material about BRACELET, they saw the topic of bereavement and trials as relevant to their situation and felt that they would be able to contribute on the research. The interview continued and covered the useful area of discussing a trial with parents of a critically ill baby when the parents have already experienced bereavement. In another family, Abby’s account of the death of a twin days before being asked to consider a trial for her second twin, seemed to be closely tied and relevant to our topic and interests; as a bereaved parent asked to consider a trial at the time of her bereavement, she was on the edge of eligibility and was interviewed. Like Stefanie and David, who took part in an interview in relation to a core trial, Abby’s bereavement directly informed and shaped the decision about a feeding trial made for her surviving baby. For this reason her interview was presented as an extra interview, which informed our understanding of the breadth of the topic.
One contribution was particularly useful and interesting. A mother who had not formally participated in any research presented an account of an informal experiment which had stayed with her for many years. At the time of submission her account highlighted the issue of the blurred boundaries of the topic, but later the presence of many of the themes explored in analysis of the interview could be readily observed in her questionnaire response. Without the influence or direction of an interviewer this mother spontaneously covered core concepts of importance to the study. Her input is presented in Box 18 , below.
The mother who completed this questionnaire initially requested an interview. On making telephone contact it became clear that she was very interested to take part in the study but that technically she did not fit the eligibility criteria. She explained that she was approached when her daughter was very sick and asked if she would consider allowing the doctors to try using nitric oxide. At that point no trial was under way but this mother definitely felt that she had agreed to something new and novel, so new that there was no worked out system of delivering the gas. She explained that someone came and built a contraption at the end of her baby’s cot to rig up the equipment.
Her ‘Request an interview’ form submitted via the BRACELET website included the following:
I was never told it was a trial, just that it was a treatment that may give my daughter a chance and if I would agree to it then it might help babies in the future. It was that new they had to set up the flow machines and so on on some chip board at the end of the cot. Did I understand it at the time? Not at all I just knew she might have a chance at living. They were testing to see if they could improve her saturation levels by giving her nitric oxide at this time the sats were 20 to maybe 40 – little did I know then how bad levels like that were. The nitric oxide was given in parts per million and over seen by Prof. . . . I wasn’t given any paperwork so I can’t tell you much more.
Although she was not interviewed, she did make an important contribution by going on to complete an online questionnaire in which she described her views and experiences. Her description of her views at the time and since then shows the same ebb and flow of interest in the research situation, the need to understand the value of the contribution, and the role of that information in making sense of events, as was identified in the parental interviews. Her account, which relates to the longest time scale represented in the study, suggests that the experiences that we have identified are present even in relation to being involved with new clinical treatments, and that these views are enduring. Her questionnaire is repeated almost in full below, with only the name of her hospital (Q1) removed.
Taking part in a neonatal clinical trial – online questionnaire for parents2. Which year was your baby born?
1994
3. Which trial was your baby enrolled in? If you can’t remember the name it would help if you could give an idea of what sort of treatment the trial involved so that we can work it out.
All I know it was an experiment that had only been tried ‘successfully with adult heart patients’ they were using nitric oxide to soften the pulmonary artery so to increase the oxygen saturation levels and stop her turning a blue colour and because she was that poorly it might give her a better chance at survival.
4. How did you feel about being involved with the trial?
I just wanted my daughter to be OK and would do anything that the doctor said would give her a better chance. If I knew then what I know now, I would have asked then doctors to let her go. But your in that situation where you would give your own life to save theirs. I remember sitting there with the make shift equipment at the end of the cot, learning about how nitric oxide was a toxin and they have to increase it slowly in parts per million, then they would watch the increasing saturation levels and smile because it looked like it was working and you get new hope, you really believe that your baby is going to beat the odds. 18 years later and I still remember the sounds the smells and the emotions. I also remember that I was alone, my husband and I were both serving in the forces and he had to go back to Ireland so I was making these decisions on my own and very quickly trying to learn as much as I could from the professionals about what was going on . . . the reality is that after becoming a midwife and working on the NNU for a while, it should have been done a lot differently, I mean I should have been approached differently. I wonder was it ethical for them to approach me as they did, not really and I still, even now wonder what if. It was during my training as a midwife I realised that I wouldn’t have agreed to any of it and I would have let nature take its course rather than cause both her, me and my family all the heartache and pain over a 5-month period. But then I balance the guilt of agreeing with the experiment with knowing that they learnt a lot and it has made a difference to the life of other children and that can’t be a bad thing can it?
5. Is there anything else that you would like to tell us about your experiences?
I think vulnerability and guilt can play a massive part in a parent’s decision to participate or not. But I recall the overwhelming emotions and hope that this experiment meant for us and maybe hope was all I needed right then.
6. We would like to know whether parents would have wanted to have had any more contact with the trial after their baby died. This information will be a great help to people who run neonatal trials.
-
a. Would you have wanted to have had a condolence letter from the trial team which also thanked you for agreeing that your baby could take part in the trial?
Not sure. I did get a letter of condolence but the participation wasn’t recognised and I think at the time wasn’t important. Would I have wondered what the treatment did?
-
b. Would you have wanted to be sent trial newsletters telling you how the research was progressing?
Yes. Oh God Yes. I waiting 14 years till I was able to ask a paediatrician about the treatment.
-
c. Would you have wanted to have had the results at the end of the trial?
Yes. You want to know if there was a purpose and had you made the right decision. But then I wonder if the results had been negative, how would I feel then.
-
d. Is there anything else that you think people who run trials should think about doing for parents who are bereaved?
Parents to talk to about their experiences and emotions but then you wouldn’t be able to maintain anonymity.
7. Is there anything else that you would like to add?
Its about time this study was done.
The experience of participation in an interview
Many research topics can be considered to be sensitive but the precise nature and implications of that sensitivity varies from study to study. In the BRACELET study, bereaved parents were asked to talk about neonatal trials and the death of their baby. We were aware from the inception of the study that some concerns existed about the impact on parents of asking them to reflect on these experiences. Years previously, when planning an earlier study, we had discussed with potential collaborators the possibility of including bereaved parents in our research and had been advised by a neonatal consultant that it would be too difficult, that they should be ‘left alone’. In some of the collaborating centres, clinicians agreed that we could approach parents of surviving babies but not those who were bereaved. This view was not expressed only by clinicians; in the same study, a senior bereavement researcher suggested that interviews with bereaved parents would be ill advised, and inappropriate for an interviewer without counselling skills to support parents during and around the interview. This sense of unease about the impact of participation has been echoed in other informal conversations with clinicians and fellow researchers over the years and quickly emerged for BRACELET. When CS met with the REC to seek permission to recruit and interview bereaved parents, one of the concerns expressed by the committee both at that time, and subsequently in writing, was that CS would encounter bereaved parents but was not a qualified bereavement counsellor. A sense of concern was clearly articulated in one of the interviews with a recruiting clinician for INIS. He said:
I must say I’m very much in two minds about your research because on the one hand I’m sort of like, you can’t speak to parents about this, you know, it’s really tough, but then I can’t really answer some of these questions without some more information [about parents].
Greg – INIS
These concerns were taken very seriously by the BRACELET team since the interviews with parents would involve asking potentially vulnerable people to discuss what may be challenging dimensions of their difficult experiences. Two of the team (CS and DE) had already conducted qualitative research interviews with bereaved parents. Like others, we saw the interviews as potentially demanding, and the research topic as sensitive. We did not, however, view either as inappropriate.
The methodological component of BRACELET offered the opportunity to formalise and reflect upon our recruitment and interview strategies. It allowed us to consider our own practice as well as to explore how parents viewed and experienced their involvement in the study. Given concerns over the inclusion of bereaved parents in our research, we were also interested to understand how parents viewed their participation and how they positioned themselves in relation to BRACELET. Did they for instance see themselves as informants? As beneficiaries? Did they feel that they needed the protections that would be built in to the study processes? As part of the methodological work for BRACELET, the study paperwork included questions about reasons for accepting or declining participation (see Appendix 23 , Reply slip), views about contact processes (see Appendix 24 for strategy 1; Appendix 25 for strategy 2a; and Appendix 26 for strategy 2b – these questionnaires were also available online) and reactions to taking part in an interview (see Appendix 27, post-interview questionnaire). (The appendix shows the post-interview questionnaire for mothers; an equivalent questionnaire was available for fathers.)
Drawing first upon our own reflections on practice, and subsequently on parental responses to interviews, this section considers:
-
The interview environment and managing the interview.
-
What does it mean to parents to take part in an interview?
A key feature of the study was the development of a management strategy for the interviews with parents and for data collection, and a focus on the experience of participating in the interview for parents.
The interview environment and management of the interview
In preparation for the interviews, a code of conduct was drawn up, which laid out five key areas for management of the interviews. These were:
-
All interviewees will be treated with courtesy and respect.
-
The interviewees should be given control of the physical environment.
-
The interviewees should be given control over the progress of the interview.
-
The distinction between counselling and research should be clear.
-
The interviewees should have access to information and support after the interview.
These key areas were expanded and the full text of the code of conduct is supplied as Appendix 28 . The expanded text structures and informs the point-by-point discussion of the management of interviews below.
1. All interviewees will be treated with courtesy and respect
The code of conduct for this item stated:
-
The interviewees will be given the time and encouragement to speak freely without censure.
-
They will be allowed the opportunity to make their views clear. If, however, this involves discussion of a named patient, a colleague or a clinician, all names will be removed from a transcript.
This area of the code of conduct is not considered in detail here, as it is a basic principle that should be present in all research involving humans, whether interviews or clinical studies, and its meaning and aspiration are clear.
2. The interviewees should be given control of the physical environment
The code of conduct for this item stated that:
-
Where and when the interview should be conducted.
-
Where and in what arrangement interviewees and interviewer should be seated.
-
Who else should be present, for example children, an invited family member.
-
Whether or not to make any adaptations for the interview, for example switching off a television or a radio. CS will only affect the environment if invited to do so (for instance by choosing where to sit), and will request that volume be reduced only if a recording will be compromised.
The environment in which an interview takes place is created by both interviewer and interviewee. The interviewer has a responsibility to create rapport and to ensure that the interview is conducted in a supportive and non-judgemental manner. There is, however, only so much control that the interviewer has over the environment, especially as interviews commonly take place in the parental home. It was in fact considered an important principle for the BRACELET interviews not to disrupt or change the environment but to allow the interviewees to determine the atmosphere that worked for them. The setting that is created is a reflection of the complex mix of interviewees’ response to participating in the research, to the research topic and to pre-existing patterns of behaviour and relationships that exist within the household. Reflecting on a comfortable or a tense atmosphere, on situations where parents can talk freely or where they are inhibited, brings researchers closer to the data that are produced. To overtly change the situation, for instance by asking for a television to be switched off or for all parties to sit around a table, may disrupt the setting that most suits the interviewees and which facilitates their engagement with the interview.
The field notes made after the interviews were an important tool for capturing the essence of the interviews, and of interviewer reflections and concerns. The field notes convey something of the atmosphere that parents created for this time of re-engagement with their experiences. Parents showed their commitment to the study not only in their preparedness to talk, but by the time they put aside for the interview. Several took time off from work or arranged the interview around their work patterns. Karen and Tony, and Justine and Frances had arranged for someone to look after their children while the interview took place. Jill and Ethan, and Anita and Sean requested evening interviews so that they were freer to talk. This arrangement may have been because of a disinclination to discuss difficult events with children present, but it was often suggested that parents would be better able to focus on the questions.
Some parents were interviewed with their children present: Dawn, and Hannah and Ryan played with their small children as the discussions took place. In a number of interviews older children were present for part of the time and their parents often drew them into the encounter in some way or other. Shirley and Warren’s daughter talked the interviewer through her sister’s memory box, Marion and Doug’s daughter showed an album of photographs, and Julia’s daughter sat with her parents for part of the interview and asked questions about her brother. Older children were introduced in a number of interviews and if they were not present their photographs were often pointed out or shared. The presence of children, either physically or through photographs, seemed to be important in establishing a sense of family life. In only one of the interviews, the extra interview that was carried out with Linda and Dan, did the parents have no children and the contrast was apparent, as the interview was much more tightly focused on the couple and their own views.
In some of the interviews there was a strong sense of focus and calm, as if parents had prepared themselves to engage with their past experiences. Dora asked for the interview to take place in the peace and quiet of her mother’s house, and Jana said as soon as the interviewer arrived how pleased she was to be taking part in BRACELET. This sense of preparedness to engage and readiness to talk was not only helpful to the interviewer, but also it communicated something of the value of the experience for the parents. The three extracts from field notes shown in Box 19 below convey a sense of the highly focused and attentive atmosphere in three of the interviews.
This interview was very emotional but in a calm and ordered sort of way. One of the main lamps in the room was switched off to allow me to plug in the recorder and so we were sitting in quite low lighting. Stefanie was very friendly but clearly slightly nervous and was a little formal when she first started to speak. She sat upright on the sofa looking ahead of her, occasionally rubbing one hand with the other thumb as she spoke. David sat back in an armchair quietly listening. Stefanie soon got into the stride of the story and told it in a lot of detail. I felt that the detail was really important to her and she was clearly remembering it all very vividly. Although it looked emotional and she was describing heartbreaking experiences, she did not cry. I felt that it was very important to her to go over her story.
Sophie and NatThe interview with Sophie and Nat was very calm and focused. I felt that they were pleased to have me there, to tell their story, and to help me with my questions. Whatever I asked them, they took very seriously and considered carefully. We sat in their large living room with me on one large sofa and Sophie and Nat on the other. Sophie sat holding her cup of tea at one end with her feet curled up looking relaxed and at home. Nat sat a bit more formally at the other end. There was a strong sense of unity in the interview and they said that they had supported each other throughout their son’s death and their bereavement.
JanaSome people talk about events in quite a rehearsed way, as they are used to telling their story if they have been involved in SANDS, or have been involved in an inquest. Some tell it in an emotional way. Jana spoke in a beautifully calm way, as if she was trying to be as faithful as she could to the details that she remembered, and as if she was exploring again how events unfolded and how she felt. She vividly described the need to feel the moment of her baby’s death – he died lying on her chest – and of taking note of the changes in his body, so that she could understand and accept the reality of his death. This discussion of the importance of going through pain in order to understand what has happened became important later when we discussed feedback of the results.
Although these parents embraced the idea of re-engagement with the time of the birth and death of their children, volunteering to take part in a research interview about deeply emotional, seldom-shared aspects of personal or professional life is also, to a certain extent, an emotional risk. The interviewer is a stranger and the questions that might be asked are unknown. The interviewee might be asked to think about previously unconsidered aspect of their experiences. If interviewed with a partner, new information and previously unarticulated views may come to light. Although this may well be a positive and welcome aspect of the process, this possibility that it brings with it certain challenges for the interviewees. In a small number of the field notes, the descriptions of the atmosphere of the interviews convey something of the tensions that seemed to be present. In the interview described below ( Box 20 ) these tensions affected the flow of the interview, and although the interview was very useful, they also affected the data that were collected. (Parents are not identified here by their usual pseudonym, as these comments are more personal than would often be made in a report and the distance offered by this approach seems to be an appropriate gesture.)
This was a very fast interview. I was out of the house within an hour. I was not sure at the time why there was less of a rapport than usual as [mother] had been friendly in setting up the interview and she had said that [father] wanted to be there. On looking back at the transcript I think it was perhaps something to do with [father’s] reserve. There was not a strong feeling of discomfort but [mother] did seem a bit self-conscious, and [father] a bit removed. My questions were answered quite quickly with little expansion. The television was on and their children were with us, which might have contributed, but perhaps there was less to say if the trial was a small part of what happened to them. It was probably telling that I mistakenly left without giving them the post interview paperwork so perhaps I felt a bit uncomfortable too.
The parents ranged from those who seemed at ease with the topic, to those who gave a sense of bracing themselves for difficult questions. In these latter interviews, the atmosphere could be less relaxed but the parents were still prepared to talk because they wished to help with the research. In Amanda’s interview for instance, the house was very still and Amanda seemed to be steeled to revisit extremely difficult events. Wherever they sat on the spectrum, the questions were not always easy and some parents set themselves a goal of not crying or getting upset. Some who did not expect to be moved found themselves becoming tearful to their surprise. None of the parents, however, wished to stop his/her interview. It was often recorded in the field notes how careful and attentive the parents were in listening and how thoughtful they were in their answers.
3. Managing the interview: the interviewees should be given control over the progress of the interview
With regard to influencing the pace and content of the interview, the BRACELET code of conduct described the ways in which the interviewees could be given control over this aspect of the interview.
-
They will be asked about time constraints and the interview will be timed to fit.
-
It will be clear that they can choose to pause or end the interview at any time.
-
There will be no pressure to discuss anything that they do not wish to talk about. If it becomes clear in a joint interview that one parent wishes to discuss something that the other finds difficult, CS will check whether he or she is comfortable with continuing. If not, a decision will need to be made on a case-by-case basis between the interviewees and CS over the best way to proceed.
-
The discussion could end.
-
The discussion could continue without the parent who is uncomfortable – they could leave to make tea or take time out.
-
The discussion of this particular aspect of their experience could proceed only with the parent who wishes to continue the discussion but at another time. *
-
It is an absolute premise of data collection in this study that data, however valuable or interesting, should not be prioritised over the needs or preferences of any individual.
-
-
As far as possible the interview will continue as long as the interviewees wish to talk. If it is clear that they wish to continue to talk after data collection is complete and the recorder has been put away, CS will make herself available as far as is practicable and reasonable. Where it is known before the interview that CS will have to leave by a particular time, this will be made clear before the interview starts.
Some of the issues about timing and duration of interviews referred to above are simple matters of logistics and courtesy. Others, however, involve a very careful management of the interview process so that the interviewee can progress at the pace that is appropriate for them, while at the same time the interviewer maintains control of the key areas of interest which need to be covered in the interview. There is always a push and pull in interviews as the researcher seeks answers to core questions, as interviewees cover ground that is important to them and they feel is essential to their story, and as new lines of inquiry emerge and are followed in discussion between the parties. In the BRACELET interviews it was important that this process was carried out in such a way that the parents felt that they had explored their experiences to their own satisfaction, but were not pushed into areas which made them uncomfortable or distressed or where they felt that they have disclosed too much.
It is important that the interviews are conducted in such a way as to record and describe the parental experiences and not to dominate with researcher-led priorities or raise new concerns for parents to consider. Some parents for instance will talk in detail about their baby’s death, whereas others will barely refer to it. Taking an approach which follows the parents’ lead is clearly important when discussing bereavement, and this can involve accepting a degree of compromise in the data, such as limiting the range of questions, skipping particularly difficult parts of the story or ending an interview if appropriate. How much parents talked, what they talked about and how detailed their answers were, provided a useful lead in terms of interview management and this can be seen in the extract from field notes below ( Box 21 ).
This was not a particularly comfortable interview. When I arrived [mother] was curled up on the sofa with a blanket over her legs. She did not get up but did shake my hand. [Father] made me a cup of tea and then sat in the chair opposite. He looked very serious and [mother] was sniffing a little and her eyes looked wet. I was not sure whether she had a cold or was already feeling anxious about the interview. By the end of the interview I still had not worked this out. She did seem to be rather fragile and I was very wary of probing too much. Even answers to simple questions sometimes conveyed a sense that she might not wish to go into more detail . . . This resulted in parts of the interview being somewhat superficial, with many of the areas relating to their personal experiences or feelings being completely skated over and me talking too much. We concentrated for much of the time on the bereavement package that was used by TOBY, the value of newsletters and feedback of the results. I think though that this was fine . . . , and I recorded the outline of what happened to them, their comfort with the trial and their satisfaction with how they were approached. I have more than enough detail to understand their views about the results process and this was very useful.
As indicated earlier, the interviewer and the interviewees co-manage the encounter in unpredictable ways and this in turn creates a co-constructed account of experiences and views. For the researcher, whatever the dynamics of its production, that account stands as fixed, as research data for analysis. For parents it is a re-evaluation, a re-investigation and a re-telling of their story, of familiar events, which, post interview, are suffused with a layer of interpretation introduced by the priorities and interests of the researcher. It is important to be mindful of this difference, as the interviewee is left with a modified version of their experiences, which will, from thereon, include elements of the interview discussion. It is for this reason that data, however valuable or interesting, should not be pursued over the needs or preferences of any interviewee.
It was also considered to be important that interviews were reciprocal. Parents had generously given their time and hospitality and had shared deeply personal memories to meet the purposes of the interviewer, and often parents introduced another period of reflection and engagement at the end of the interview, which they drove themselves. Once the recorded discussion was over, parents would often explore the contents of memory boxes that contained items from when their baby was alive, and would share photographs of their baby’s time in intensive care. Some of the parents also shared items that conveyed to the researcher the reality of their baby’s death, most often with last photographs of their baby after care was withdrawn. Hesther and Stuart showed their photographs of themselves holding their son with family members watching on. Marion and Doug showed the interviewer photographs from her daughter’s funeral and of her grave, and three families showed the caskets in which they kept their babies’ ashes. Some of the parents showed how they remembered their baby, with photographs on display, with special areas in the house, and with tattoos of their baby’s name. This suggested a desire to bring into the interview a strong and tangible sense of the babies who had died as being once living individuals but who had since been lost. This usually took place without the research recorder running and when the data were collected, and seemed to be a special and reflective time for the parents in which they were able to convey something of the physical and material life of their child. Turkle163 points to the importance of objects ‘to think with’ and the objects, as well as the memories described in the interviews, were sources of reflection for both parents and for the interviewer. Wherever possible, interviews were always booked with a generous time slot in case parents wished to extend the discussion in this way.
4. The distinction between counselling and research should be clear
The code of conduct for this item stated that:
-
Unlike a counselling relationship, the interview should proceed with the principle of not seeking to change how the individuals perceive and respond to their experience. The aim of the research is to understand how individuals view and respond to bereavement subsequent to an enrolment in a clinical trial, not to modify their views. Some change may well be inevitable, as any conversation has the potential to effect a response, but this is not the aim of the interview.
-
It is most important that a distinction is made between a supportive environment in an interview where interviewees feel able to speak freely and without pressure or censure, and an intention to provide support. Many interviewees in qualitative studies have indicated that the opportunity to revisit and reconsider their experiences can be positive and cathartic, but this must be considered as collateral benefit rather than the purpose of the interview. It is important that the interview is seen as a point where the interviewer is the beneficiary, and the interviewees are providing assistance for which the research team is grateful. The written material given before a decision is made about the interview will emphasise the value of their contributions to further our understanding of views and experiences of bereavement and clinical trials.
-
CS will not offer to visit or call for the purpose of support but will provide details of sources of support to all interviewees (see below for further details).
The concerns expressed by the REC that CS did not have bereavement counselling skills suggested that for interviews involving bereaved parents, the distinction between the aims of care and research, and the nature of the research contract, were not sufficiently clear. That counselling skills, which are therapeutic in intent, might be considered a prerequisite to interviewing bereaved parents suggests a care-based model of the interview, which presupposes both a particular type of need and a particular type of service. It positions the interviewee as the recipient and beneficiary, rather than the interviewer, and the interviewer as the expert. In a more clearly research-based model, the informant would be viewed as having expertise and insights and, as the giver of privileged information, it is the interviewee who helps the interviewer to gain in experience and understanding.
Having spent time researching the often blurred boundaries between care and research in clinical trials (therapeutic and injurious misconception65), and mindful of the tension between the views of research interviews on sensitive topics as therapeutic at one extreme and potentially harmful at the other, the BRACELET team was clear that we should adopt a position that clearly demarcated our research aims from those of care; here, as in clinical research, both benefit and harm were potential outcomes to be aware of and considered, and for which we needed to prepare. To avoid any potential confusion over the nature of the interviews, it was written into the code of conduct that the distinction between counselling and research should be explicit.
Here we should make it clear that the emphasis on not offering a counselling environment is not to say that the interviews should not be carried out in a supportive environment. There is a growing body of evidence that suggests that bereaved parents can find their involvement in research interviews and the opportunity to discuss emotional experiences with an interested and engaged listener, to be a positive, supportive and sometimes a therapeutic experience. 66,164–167 We hoped that parents would find their involvement in research to be a positive experience, and would feel that their input was valued and valuable, but felt that it was important that they did not enter the research under the impression that it was therapeutic in intent. Where parents felt that they gained from their participation this was viewed as collateral benefit. Given the possibility that some parents might find an interview to be a difficult experience, it would be inappropriate to suggest in information or in dialogue that they may gain from their participation.
To make this distinction between research roles and caring roles clear, parents who took part in an interview with CS were given separate access to the services of a bereavement counsellor who understood neonatal trials and who could provide an initial period of support (see below for further discussion of this).
5. The interviewees should have access to information and support after the interview
This aspect of the code of conduct for this aspect of the management of interviews addressed the issues of need for information arising during the interview and of support needs that might emerge in the aftermath. It stated:
-
Interviewees may wish for no, or varying levels of, support and/or information after their interview. The information leaflet for parents includes details of sources of support and information. After the interview, all interviewees will be given a card that reiterates these sources of support, which they may access according to their self-determined level of need and preferences. Further details are given below (see Ethical considerations).
-
If interviewees initiate a request for more information about a particular trial, CS will make a judgement about their request. Simple questions will be answered at the time but more complex and detailed enquiries will need to be directed to a representative of the core trial in question. CS will offer where possible to facilitate access to that information to preserve confidentiality, but interviewees who wish to access information directly (and so reveal their participation in the study) may do so.
-
If bereaved parents initiate a request for more information about the care of their baby, they will be directed to the clinical team in question. CS will offer to facilitate contact and it will be made clear that in such circumstances it will be difficult to avoid revealing to the clinicians that an individual has been interviewed for the BRACELET study.
-
Interviewees will be informed that all interviewees will be contacted towards the end of the study and asked whether or not they would like to have a summary of the findings. If they state that they do not wish to be contacted again this will be noted and no approach will be made.
-
Interviewees will be given details of the BRACELET study website in their respective information sheets. The study protocol and links to previous publications and later those arising from this research will be posted on the website.
Information about the BRACELET study was made available to all parents in paper form and/or electronic form before an interview was arranged. Paper copies of information were given to parents again in the consent process at the start of the interview. At the end of the interview, details of sources of further information were given to parents, as well as the means to access specialist support arranged for the study participants should the interviews stimulate a need and a wish to talk further (see Appendix 29). The counsellor could discuss how to manage any further support needs that might arise if parents so wished. The aim was not only to provide access to support to parents who feel that they needed this option, but also to clearly mark out the limits of the role of the interviewer; the role of the interviewer was to listen and to gather information in an engaged and supportive environment, but not herself to provide bereavement support.
The issue of access to information did arise during the course of a number of interviews. In the interview with Hannah and Ryan, Ryan brought out his iPad to check details of trials that were being discussed (see below for a description of this), and both Pete and Robert specifically asked about the results of the trials in which their baby had participated. In both instances the interviewer gave basic information but offered to facilitate access to further details after the interview. Adam wanted to understand more about the trial, as he was aware that there were gaps in his knowledge. The BRACELET team sent him a copy of the trial information leaflet after the interview. This included details of the BOOST-II UK website.
An issue that required careful consideration was the situation where parents in INIS and BOOST-II UK had moved house and so had not received the results of the trials in the mail-out from the CTU. In discussion, it was clear that the parents had not received the TOBY bereavement package and had not therefore benefited from the principles of communicating results to bereaved parents. When the parents wished to have the results, the interviewer agreed to pass on their new address, with their permission, to the CTU so that a copy of the information that was previously sent out could be passed on the parents in BRACELET. This was negotiated with the CTU and put in place only when it was clear that the CTU would be able to respond to the requests for further information.
Parents’ views of the recruitment and interview processes
Parents were asked to complete a questionnaire about the process of contacting parents to invite them to participate in the BRACELET study. This contact processes questionnaire was included with all of the invitations sent out to parents so that anyone who found the contact to be difficult could make their views known, even if they did not want to take part. The same questionnaire was available online (see Appendices 28–29 ). The reply slip (see Appendix 23 ) also had a question about contacts processes.
Parents who declined
Two questionnaires were received from parents who did not participate in an interview. In the reply slip from one of these (approached under strategy 2a), the parent had said:
I do not feel an in-depth interview is healthy for me at this time. I generally do not spend time thinking about my baby now, and this has stirred long-bedded emotions. Perhaps at a later date through your website. I wish you well in your research.
She went on to write:
You have been very sensitive. When a baby dies, mostly it is as if he/she wasn’t really here. Seeing their name and DOB written down brings back the reality of their being. That brings its own issue, but re-affirms in my case HER being. Even after a period of time, emotions are stirred. Nonetheless, I welcome the approach.
As the questionnaires are anonymous, we do not know whether this parent did go on to return online comments about her experiences. She nevertheless enclosed a fully completed contact processes questionnaire ( Box 22 ).
1. We decided to wait until the first anniversary of their baby’s death had passed before contacting parents. Was this the right thing to do?
Not sure.
I believe for me it would have been easier to speak about the issue then but I’m not usually like other people in issues like this.
2. A consultant in the neonatal unit at the hospital where babies were cared for was asked to check through a list of names of families and remove any where they felt it might be particularly difficult for the parents if we contacted them. Was this the right thing to do?
No.
I did not especially like my consultant and felt he did not really know me or the family situation. We were just another case.
3. For each set of parents left on the list, their GP was sent a letter to let them know that we planned to contact the parents. If the GP felt that there was any reason why the parents should not be contacted their names were removed from the list. Was this the right thing to do?
Yes.
GP may well be in a better potion to know the individual concerned.
4. For each set of parents who had not been removed from the list, a letter was sent to their home to say that someone from the neonatal ICU would be calling about the BRACELET study. Was this the right thing to do? Please choose an option.
Yes.
A bit of a shock to bring it all back but as an academic, I understand the need for research.
5. A week later someone from the neonatal ICU called to talk to parents about the BRACELET study and ask if they would like to have a letter from the research team about taking part. Was this the right thing to do?
No.
For us the guy couldn’t get through. I am glad. I would not have liked his call. To be contacted by mail was much better.
6. For each set of parents who agreed, a letter, information sheet and reply slip were sent directly to their home. Is there anything you would like to say about these?
They are self-explanatory.
7. In other studies we would often send a reminder letter to people if we had not had a reply from them after a few weeks. This is usually an important way of bringing some extra people into a study. For this study we decided not to send any reminder letters to bereaved parents. Was this the right thing to do?
Not sure.
The other parent who declined (approached under strategy 1), explained:
I don’t wish to take part in this study as I have two other children. My relationship with partner has just broken up and I still feel so guilty over [daughter’s name]. I am sorry about this, but it is all too much for me to cope with. I have been through traumatic 7 years. I am really sorry. I feel like I have let you and [daughter’s name] down but I am not strong enough to carry on with this study. But I wish you all my best wishes and good luck with your study.
This parent also filled in a contact processes questionnaire, agreeing with all the steps in strategy 1 (waiting until after the first anniversary, initial screening by the consultant and the GP, and the ‘no reminder’ policy), but was unsure about the letter and information sheet being sent directly to their home. He or she did not comment further.
Two further parents declined on a reply slip but did not complete a contact processes questionnaire. For one parent ‘the loss was still very raw and painful’, and the other parent wrote ‘at this moment in time, we are unable to participate.’ However, this family respondent explained that there was in error in the letter from the local consultant: ‘it would have been less upsetting if the letter [from the local consultant] would have stated her [daughter’s] name correctly’.
Parents who requested an interview*
(*This includes both those requesting an interview and those who wished to know more before deciding.)
Nine parents returned a written reply slip requesting an interview. Fifteen contacts questionnaires were received. Some parents also made comments during their interview or in the post-interview questionnaire.
On the reply slip, parents were asked:
Is there anything you would like to say about how we got in touch with you about the BRACELET study?
We have put this question here to try to make sure that everyone has a chance to tell us what they think, even if it is just in a few words. If you would be prepared to tell us more there is also a questionnaire about this on our website.
Four out of the nine parents who returned their reply slip replied to this general question about contacts. One saying ‘thoughtful’, and the other three commenting specifically on strategy 2a:
Hesther and Stuart appreciated the personal contact from their neonatologist, and wrote ‘It was very nice to hear the voice of my son’s doctor’.
Chloe was ‘impressed with the measures taken to protect my feelings, first a letter from a neonatologist, then a phone call before even speaking to a researcher. I feel this was extremely thoughtful, as I know how busy they must be’.
Caitlin and Peter also felt the contact process had worked for them:
The method of contacting seemed quite appropriate. First a letter and then a phone call. It doesn’t matter what method is used, there is always a risk of opening up psychological issues associated with premature death of babies. For us – we have dealt with those issues and feel somewhat at peace with the situation, so we are just happy to help with any sort of research that aids the understanding of the causes and best methods of dealing with premature baby death and the associated issues.
There were three versions of contact processes questionnaires, reflecting the three strategies, although some of the questions were common.
Timing of the interviews
One of the factors considered in BRACELET was the significance of the time that had elapsed since parents considered enrolment into a neonatal trial, the death of their baby and the interview. As the purpose of the study was not to assess recall but reactions to trial participation over time, there was no need to recruit parents close to events. It was in fact considered an advantage to carry out interviews at some distance from events so that parents would be able to consider their evolving responses to a trial and to review them in the longer term. Interviewing parents some years after their initial involvement with a trial also increased the chances that trials had reported their findings and so the topic of feedback of results and the outcome of a trial could be explored with the parents in interview.
On the questionnaires about strategies 1 and 2a, the first question was:
We decided to wait until the first anniversary of their baby’s death had passed before contacting parents. Was this the right thing to do?
All 10 parents who answered this question in the contact processes questionnaire agreed it was right to wait. Five gave further comments, four recognising the particular strains around the anniversary. Chloe wrote ‘I certainly appreciated this thoughtfulness’ and Rhona and Karl endorsed this: ‘The first anniversary was the most difficult time.’ Other parents queried the first anniversary timing with Hesther and Stuart suggesting ‘maybe wait 2 years,’ and Stefanie and David wondering whether it would be better to ‘wait until a few months after anniversary due to the build up to anniversary’. Marion and Doug commented, ‘It may have been difficult for parents the first year, but after that time it may be good to talk. I do not think it would do any harm to ask parents.’
In fact this timescale, although fixed in the initial REC approval, became irrelevant once the core trials were identified as all parents were beyond this time point at the time of invitation. No upper time limit was imposed.
When the study recruitment strategy was revised and recruitment via publicity was incorporated into the design, no lower limit was put in place for participation. As parents who opted into this study via this route were not being invited by an unsolicited letter, but were initiating contact with the study themselves in response to publicity, it was deemed that they themselves would be the best judge of whether or not they were ready and willing to discuss their experiences.
The inclusion of publicity, and the fact that recruitment from the core trials was carried out over a much longer period than originally anticipated (March 2010 to July 2012 rather than September 2008 to December 2009, meant that the timescale for parents was much wider than expected, at 1–14 years. (Phase I was extended and delayed the start of Phase II. Recruitment was suspended in 2010 until a funding extension and revised REC approval were in place in 2011. New recruitment processes which involved neonatal consultants calling parents at home were slower. Recruitment continued until 2012.) During the course of the interviews it became apparent that this range was a source of strength, as the study was able to access different types of experiences and views and to consider central issue in the short and in the longer term. The following field note extracts give a sense of the value of looking at an interview with recently bereaved parents as well as with parents some years on from their bereavement. The interview with Linda and Dan was very similar in tone to those of many others in the study and it was clear that they were willing and able research informants who gained satisfaction from their involvement in the original trial and in BRACELET ( Box 23 ).
Linda and Dan are the only parents in the study to have taken part in the same year as their baby’s death. As Suzy was their first baby they do not (yet) have a family. All other parents I have interviewed so far have either already had children, or have gone on to have children after their bereavement and so there was still evidence of children and family life in the home. Their views of taking part in a trial, their aspirations for their baby and their subsequent bereavement are filtered through this larger experience and knowledge of the final outcome. There was a sense in Linda and Dan’s home, and in their account, of a hiatus, of waiting to move on, and that they do not yet know how their story will work out. They did, however, say that they were very interested in taking part in BRACELET and were comfortable telling their story; other parents have said that they were in a position to take part and go over events only given the time that has elapsed but Linda and Dan’s ease with participation shows that there are parents who wish to take part in research even within a year of their bereavement.
In contrast, the interview with Hannah and Ryan ( Box 24 ) took place over 14 years after the death of their first baby, the longest gap in the study. They had gone on to have two children. And their family was complete. Although it was a long time since they were involved in neonatal research they were still interested and readily engaged with the topic. They had no easy way to link their experiences with the pilot study for the TOBY trial to the programme of highly successful clinical research that built on this initial study, but it became clear in the interview that, even with the time that had elapsed, they would have been interested and gratified to have known how the research story had unfolded over the years.
It was clear as we talked that Hannah and Ryan were still very interested in neonatal intensive care and cared about the well-being of sick babies . . . While we were talking at the dining room table, Ryan had his iPad nearby. I showed them the newsletters from TOBY and described the research that has gone on since they took part in the pilot. Ryan asked, ‘So who is TOBY?’ We went on to the web via his iPad and I showed him the NPEU page with the various trials listed. He bookmarked the TOBY page but as we continued to talk he leafed through the different pages for different trials, commenting on things that he found. He planned to have a closer look later. When we were not recording Hannah spoke of her hope that by taking part in the pilot study they had somehow helped in a research process that would ultimately benefit other families and babies. She said that one day her children might have babies in neonatal intensive care and they might be beneficiaries of the contribution that Eleanor had made . . . Hannah and Rory would have been very interested to have known about TOBY, the TOBY Children study and TOBY Xe but there was no further contact with parents in the pilot study.
Screening process involving the neonatologist
Parents were asked for their views of the screening process which involved the neonatologist. On the questionnaires about strategies 1 and 2a, the relevant question was:
A consultant in the neonatal unit at the hospital where babies were cared for was asked to check through the list of names and remove any where they felt it would not be right to contact those particular parents. Was this the right thing to do?
Six parents approved of this but five indicated that they were unsure. Stefanie and David’s response included the view that ‘everybody should have the chance to take part, it should be up to the parent if they feel they want to help’. The same sentiment was expressed by Chloe who said, ‘It’s up to the parents to decide and have that choice in the first place.’
Screening process involving the general practitioner
Parents were asked for their views of the screening process, which involved the neonatologist. On the questionnaires about strategies 1 and 2a, the relevant question was:
For each set of parents left on the list, their GP was sent a letter to let them know that we planned to contact the parents. If the GP felt that there was any reason why the parents should not be contacted their names were removed from the list. Was this the right thing to do?
The responses to a similar question about GP screening were also mixed, with six questionnaires showing agreement, two unsure and two where parents indicated that they did not agree with the involvement of the GP. Marion and Doug gave their reason for agreeing with the process: ‘Perhaps family circumstances have changed. Death of a parent, divorce, remarriage etc. So I think it was the right thing to do.’ Stefanie and David commented, ‘GP should know you more than a neonatal consultant i.e. ongoing issues/mental health,’ whereas Chloe agreed that the ‘GPs are on a more regular familiar term with the parents I imagine;’ she felt ‘It’s still the parents’ choice.’
Rhona and Karl were unsure about the involvement of GPs: they said, ‘GPs don’t really know families these days.’ Hesther and Stuart were more forthright: ‘I don’t think it’s up to the doctors to decide, no matter how bad parents could be feeling, it’s their decision and may help them.’
Direct contact made by the neonatal consultant
Under the revised contact strategy, after these initial CTU, neonatologist and GP screening steps, a letter was sent to the parents by a consultant neonatologist from the neonatal unit that cared for their baby, giving them advance notice that he/she would be telephoning them about a study that they might be interested in. There were two questions about this on the contacts questionnaire for strategy 2a.
For each set of parents who had not been removed from the list, a letter was sent to their home to say that someone from the neonatal ICU would be calling about the BRACELET study. Was this the right thing to do?
A week later someone from the neonatal ICU called to talk to parents about the BRACELET study and ask if they would like to have a letter from the research team about taking part. Was this the right thing to do?
One of the parents was unsure that this was the right approach but did not comment further. The other eight thought it was the right approach. Chloe wrote, ‘I found it helpful to have a heads up and very thoughtful.’ Beverley felt the same, commenting:
The letter was a good warning that there would be a phone call – I think it would have otherwise come as a shock . . . The letter followed by the phone call was good. I wasn’t sure if I wanted to do the study and probably wouldn’t have agreed by reply slip.
Their perception that there was always a risk was borne out by Lewis and Julia’s experience:
The only reason we have been slow to reply is due to timing as it was our son’s birthday on 23rd August and his anniversary on 1st September, so timing may not have been great; however, we do appreciate the sensitive nature in the way we were approached to take part in the study.
To work well, the system that was developed for contact wholly depended on a set process being followed, but in three instances the consultant called the parents before the letter arrived. Although Alice had not found this to be a problem she did recommend that researchers should ‘make sure there is sufficient gap between letter being sent and then contact made’. In the other two cases, the parents approved this step. Stefanie and David’s questionnaire said, ‘I don’t think we received this letter but yes it is a good idea’, and Julia and Lewis’s questionnaire included the comment ‘Although I feel this is the correct approach, we did not receive a letter prior to the phone call from the consultant’.
Lesley mentioned that the initial letter that they had received about BRACELET study had been difficult and a shock. She said that her first reaction was to not read it properly and to put it aside. Karen said that Tony had felt like he had been ‘thumped in the stomach’ when he realised that the letter with a hospital logo related to Tabitha, not the hospital appointment for their other daughter that they were expecting. He was initially unsure about taking part: ‘I thought it may be painful to bring back the emotions.’ Chloe explained in the interview that she ‘crumbled’ when she read the initial letter. Each of these parents said that there had to be a first point of contact and that the initial shock was unavoidable; they still felt that it was right that they were contacted and were pleased to be taking part. Chloe had pinned the letter to her fridge door and was proud of it. When the interviewer arrived she said, ‘Everyone on Facebook knows you are coming today.’
Similar comments were made by other parents around the time of their interviews.
Three parents thought that the study should not be so concerned about the approach to parents. Fiona said, ‘They can always say no’. Wilf made a similar point: ‘I think the ethics oversight should be less worried about approaching parents; we can always say no if asked.’ From a discussion around the time of the interview, Dan, who opted into the study via the publicity route, and whose account was included as an additional interview, was aware of the processes that were used for parents who were contacted by a clinical centre. He put his view more strongly:
I feel the ethics committees are wrong to limit contact with parents. It makes me feel like we are being hidden away. It should be our choice if we are contacted and I would have had no objection to being contacted by GP or via the hospital.
His partner Linda also stated her views robustly:
I would like to make it clear that I have no objection to being contacted through hospital staff or my GP. Whilst I appreciate how important data protection is I also think it’s important to have the opportunity to participate in studies such as this, and think that I have the right to decide for myself who has access to my contact details. Obviously child bereavement is a sensitive subject and must be handled considerately but we can be contacted and can deal with being contacted and talking about the subject. It should be dealt with openly to try and make it less of a taboo subject. As long as it’s made easy to say no and people don’t feel pressured to participate then I think most people would welcome the chance to participate and try to make improvements even if it’s just in a very small way.
The invitation to participate
Once parents agreed to release their contact details to the study and to view details of the research, they were sent a letter and information booklet about the study. These could be sent by post or by e-mail according to their preference. Parents were asked for their views about these:
For each set of parents who agreed, a letter, information sheet and reply slip were sent directly to their home. Is there anything you would like to say about these?
Stefanie and David were appreciative: ‘Received phone call that evening – then an e-mail form and in the post within days – very quick and information well thought out, clear, easy to read and understand’. Dora liked the information: ‘Very simple and informative literature. Wording very good – enough empathy/sympathy but not patronising’.
Reminders
Under the terms of the ethics committee approval, no reminders were sent to parents who did not reply. Seven parents agreed with this strategy. Parents were asked:
In other studies we would often send a reminder letter to people if we had not had a reply from them after a few weeks. This is usually an important way of bringing some extra people into a study. For this study we decided not to send any reminder letters to bereaved parents. Was this the right thing to do?
Rhona and Karl wrote, ‘They probably ignored it for a reason’, and Stefanie and David’s questionnaire included the comment, ‘I don’t think you’d forget to reply to something like this – but some people would probably not want this pain again!’ Four parents were unsure, including Amanda for whom there was a delay in replying to her invitation. Her comments suggest that a reminder might have been useful:
I can understand your reasons for not sending a reminder but, in my case, family life is busy and things get put off even though I am interested in taking part, e.g. Am I too late now?
At the end of her questionnaire, Chloe added, ‘I thought it was all approached very sensitively, which I was appreciative of.’
Contact process (publicity route)
Seven parents who had responded to the publicity about BRACELET gave comments in their questionnaire on this route into the study. Four saw information via a support group, and for the other three, information came from the trial team with the results of the trial in which their baby had been enrolled.
The questionnaire asked whether they agreed with this approach, which did not involve screening by a hospital doctor or a family GP. Caroline and Finn were unsure: ‘It’s OK for me because I’m further down the years but if it’s a newer bereavement then I think go through doctors’. (Caroline and Finn were interviewed but their data could not be included in the analysis as they did not fit the study eligibility criteria. Their baby who was enrolled in a trial survived. They did, however, submit a questionnaire about the use of publicity, and so their comment is included here.) All of the other respondents were happy with this strategy.
Jana said that ‘It would have been OK for us to be contacted by the hospital doctor but there was no need to involve them. Taking part in the study meant that we were engaging with the study team, not with the doctor’. She went further, saying, ‘It would feel artificial – we never [talked] with the GP about our loss.’
Dora also reflected on the GPs role:
GPs do not always have a proper understanding of studies. May affect the way GPs approach subject. GPs often reluctant to be involved in any other work.
Sophie and Nat felt that the distribution of publicity material by the trial team alongside the trial results worked well as a single step:
The promotional flyer asking us to help in the study was sent out along with the results of the INIS study. As such it was directly associated with it and not pushy. It allowed me time to consider whether we’d like to take part or not . . . GP had no involvement in the INIS trial that our son took part in. So would not have been appropriate for them to be involved.’
Set-up of the interviews
When parents agreed to take part in an interview, they were able to choose whether to take part on their own or with a partner. In 22 interviews both parents were present, and in 10 only one parent took part. In two of these 10 cases, the parents were no longer in a relationship. In the post-interview questionnaire parents were then asked about their preference.
Did you want to have the interview on your own or with your partner?
On my own; together; I didn’t mind; do not have a partner
In 38 responses, three parents indicated that they preferred to be interviewed alone, in 22 that they preferred to be together, and in 13 they did not mind.
Where parents chose to be interviewed together, this often related to the shared nature of their experience. This was reflected in Pete’s statement: ‘I felt that we both went through the original emotional ordeal together and it was right for us to both contribute at the interview.’ Julia felt that there were emotional benefits of both parents being present: ‘We both gave our consent for the study and as it can open an “old wound” your partner is good support in that situation.’ David pointed to the practical gains: ‘Between the two of us we could remember what happened.’
In this and our previous studies, female interviewees have always outnumbered male, and all lone interviewees have been women. In BRACELET, Robert chose to be interviewed alone. He also asked that the interview should take place at his office, at the end of the working day. He explained that once we had carried out his interview he would raise the subject with his wife and then would let the BRACELET study team know whether she would also like to take part in an interview. His wife did not wish to participate. Robert made a contribution to BRACELET and without the option of a lone interview away from the family home, his participation may have been lost.
Parental views and experiences of taking part in an interview
The final methodological consideration for BRACELET was to explore the parents’ own views of participating in the study and of taking part in an interview. A number of approaches were used. The reply slip (see Appendix 23 ) that parents could use to opt in to the study included a question about reasons for choosing to take part in the study. Nine parents returned a reply slip before their interviews, all of whom agreed to take part. Interviewees were asked to complete a post-interview postal questionnaire asking them to reflect on their experiences in the BRACELET study, and to suggest any ways in which the research processes might be improved (see Appendix 27 ). Thirty-nine responses were received (nine from mothers, two from fathers, and 14 from both mothers and fathers in a couple. Comments were often made by parents before or after the interview, and when these discussions were informative they were described in the interview field notes. When parents discussed their involvement in the study, or their feelings about being interviewed during the course of the interview, their verbatim comments were available in the interview transcript.
Four dimensions were identified in this wide range of data, and, together, these different aspects of participation built up of the value and meaning of the study and the interview for the parents involved. These dimensions were conceptualised as:
-
helping
-
healing
-
honouring
-
commemorating.
Helping
Just as the image of helping was important in relation to the trials – a trial might help a baby, parents might help a trial, or through trial participation they might help other families – the same image was used when parents described their reasons for deciding to participate in BRACELET. The parental statements about helping showed the same interweaving of different ways of helping.
Wilf specifically focused on participation as a means: ‘To be helpful with future trials’. Alice and Ivan’s reply slip indicated a sense of connection between helping BRACELET to improve the way clinical research is run which will, in turn, improve care:
I feel it is important to support research in the medical profession particularly. With more research and studies this can only help other parents and sick babies in the future. Anything we can do to perhaps help avoid what we went through with our son . . . happening to other families in my view is positive. Without research there cannot be progress and without progress babies will continue to die from perhaps preventable illness.
Caitlin and Pete made the same connection:
After losing Martin and Damon, we both feel that it’s vitally important to assist with any aspect of research into premature babies and associated issues (including how any research is undertaken).
Stefanie and David placed their research contribution in the context of the altruism of parents who had gone before them. They felt that they were direct beneficiaries of the research contributions that those parents made and were ready to take up their role in research as part of a larger cycle. Their comment referred to an aim to help ‘medical staff or parents’.
If I can say something that helps anyone else, either medical staff or parents. Also it was something our son went through and our short time with him was due to medical tests etc. being carried out on other babies in the past – and so the medical processes continues with new information.
Some of the comments made no reference to medical research but linked participation to an aim of helping other parents involved in research in similar circumstances. Julia and Lewis wrote on their reply slip that they would like to take part in BRACELET because it would ‘allow us to share our experiences of involvement in the clinical trial and hopefully contribute to making the process easier for parents in the future’, and Sara and Gareth stated, ‘Looking at it from bereaved parents viewpoint is a positive step.’
The sense of wishing to help was very clearly present in many of the interviews. Sometimes it was specifically mentioned by parents. Robert, for instance, commented, ‘The reason why we’re sitting here is because I want to be able to share my experience and help other people.’ Sometimes it was evident in the care that parents took over answering the questions put to them. At one point in the interview with Hesther and Stuart, Stuart encouraged the interviewer who was struggling to formulate a question. Thinking that the interviewer may be being cautious he said, ‘Go on, just say it!’ Before the interview with Caitlin and Pete, Pete had asked to be sent an outline of some of the questions that would be put to them so that they could think about them beforehand. Jana explained that she read through her diary from the time that her sons were in the NICU to prepare for her interview. These parents clearly wished to contribute to the research in a meaningful way. Parents also commonly offered additional help. Marion and Doug said that they would not mind if BRACELET needed anything from their daughters’ notes and very commonly the interviewer was invited to contact parents again if there was any other way that they could help.
Healing
Both before and after the interviews parents talked about the benefits of talking about their baby and their experiences. Caitlin said before their interview started that she was very pleased to be taking part in BRACELET because she feels that she ‘heals through talking’. It was Caitlin’s connection of healing and talking that provided the name for this dimension of participation. At a later stage Dora explicitly linked participation to healing, which she referred to as ‘recovery’, when she described the emotions that she had gone through at different parts of the interview. She wrote about three different feelings in her post-interview questionnaire:
Angry: When I talked about people forgetting my son’s birthday.
Helpful: Felt positive that I may help others.
Pleased: Pleased that I had agreed to talk about my son Gerry, for my own recovery really.
Although other parents did not use the term, there was a strong sense that they saw the opportunity to talk as positive and beneficial. Some anticipated this in advance of the meeting. Marion and Doug’s reply slip included the comment:
We would welcome an interview. It’s good to talk and explain the way we felt at the time of our daughter’s death. As we had twins we were blessed by God that he allowed us to take one of the twins home. Other parents come home without any baby.
Others reflected on it afterwards, expressing pleasure at the process. Hesther said in her post-interview questionnaire that what she liked about the interview was ‘How easy it was to talk. We were not judged on anything we said and we could talk about everything about Joel, not just TOBY’. The interviews could provide parents with a rare opportunity to revisit and to think through events that are seldom aired, and to extend their thoughts on what had happened. Jana, who told the story of her triplet sons extremely carefully, wrote in her post-interview questionnaire that the interview offered a greatly valued ‘opportunity to talk about my experiences, the opportunity to reflect, the opportunity to think about and answer interesting questions’.
Keith said that they rarely had the opportunity to talk about their experiences because ‘everybody’s very sensitive about it’. He said that friends ‘hardly ever talk to us about it’ and parents ‘never talk to us about it’. Keith explained that ‘if you open a conversation, it soon closes down’ and Fiona added that ‘they look scared and run away’. This left them only with each other and this was not without some difficulties. Keith said, ‘So the worst thing we have is we talk about it among ourselves, and of course I upset Fiona.’ Immediately, Fiona added, ‘But I don’t not want to talk!’ The interviews offered the opportunity not only to talk to someone who was interested in their experiences and would listen for as long as they wished to talk, but also offered couples a framework in which to explore their joint experiences and to hear each other’s views.
The interviews provided an opportunity and for some acted as a stimulus to talk with their partner further after the interview. The post-interview questionnaire asked whether the interviewees talked about things afterwards with their partners. Three answered ‘no’, or ‘not really’ and 26 parents said that they did talk. Fiona and Keith set off for a walk together as the interviewer left the house, and in their post-interview questionnaire mentioned that they discussed the trial: ‘It had been good to talk about it but left us with a lot of questions about the study and our own understanding of what it had been about.’
Comments in both Stefanie and David’s post-interview questionnaires focused on each other. During the interview Stefanie had taken a greater lead in describing events, whereas David listened very carefully. Stefanie’s questionnaire said:
David said it was the first time he heard me talk for so long about the boys and heard a few things he didn’t know. But it was all fine. We just had no real reason to talk like that to each other before as we had both been there at the time together. It was good to hear David talk as he finds it hard [to] talk about that time in our lives.
David said that they discussed the interview afterwards and he mentioned ‘the closure that my wife felt’. Hesther and Stuart felt that their interview, and the opportunity that it gave to talk, was something that they valued. Hesther said in her post-interview questionnaire that they ’both said we hadn’t spoke in length about Joel in such a long time. It was almost like a catch up’. In setting aside the protected time for interview parents could hear a familiar story anew and could learn new things, and Hesther went on to comment on her experience of hearing Stuart describe how difficult it was for him while she was in surgery around delivery. She said, ‘My husband has never said how he felt in the room alone while waiting for me in theatre until today.’ Sara discovered that Gareth ‘. . . didn’t realise there was a placebo in the study. That he feels frustrated that our son never had a chance to do anything with his life’ and Sara mentioned that the interview had taken them into new territory. She wrote, ‘Things we had not discussed between us cropped up and I discovered things I hadn’t known before’.
For parents who could discuss their bereavement with their partners, the interview seemed to have been attractive because of the opportunity to talk about their baby. Both Amanda and Danielle explained that their partner does not like to talk about their experiences, which is why they did not take part in the interview. Amanda said in the post-interview questionnaire that she liked the fact that the interview ‘didn’t leave me feeling I was wrong or silly about what I’d said’. Dora, who was no longer married to her son’s father, could not share her recollection of events with anyone who also remembered that time, and the interview offered an important chance to reflect (see below for further comments on Dora’s response to the interview).
Honouring and commemorating
These two dimensions of participation could be very closely linked and difficult to disentangle in some accounts. Where their different features were separable, subtle differences emerged.
The interviews were grounded in the story of a baby’s birth, life and death, and parents varied in the level of detail and the attention they paid to different aspects of that story. Some were selective, focusing more on the time around their involvement in the trial but skirting around or only alluding to the time of their baby’s death. For other parents, it was important to tell the story in detail, bringing to the interview not only a description of events, but also a strong sense of what it meant both at the time, and how they have come to see events over time. The sense of honouring a baby through the details in the account given in a BRACELET interview came from Chloe, who was very enthusiastic about taking part in the study. Her view of a narrative as a way of ‘honouring’ her sons, captures the importance of telling their baby’s story in close and faithful detail, which could be readily heard in the accounts of a number of other parents in the study.
The narrative accounts that parents gave in an interview were not just the results of parents answering questions. They grew out of a re-engagement with that time in their life and for some became a testimony to their child. Jana described a very significant and personal moment in her interview and it seemed to the interviewer that it was important that this was articulated and acknowledged. There was a sense of tribute in her account as she described with great care the time around Anton’s death, how he looked and felt, and how she reacted to him and to withdrawal of care. She explained how she went back to see him afterwards. She said:
I did it twice and again it was very important. It was, yes. I felt you know that as a parent you feel you know – your job is to protect your child, that’s your role. If you cannot do it, it’s – I felt like a failure. I felt like I wasn’t able – I felt so sorry, and I felt such a need to tell him that I’m so sorry.
In some of the interviewees it seemed that in recalling their experiences, in describing the baby as an individual with their own experiences, emotions, reactions and agency, the parents created a sense of their personhood and were bearing witness to the short lives.
Shirley said that Beth was ‘bombarded with blood samples’ and there was never a long period of time where she was left alone. ’ Lesley said that her son opened his eyes the day before care was withdrawn. She said, ‘He just kept looking at us . . . I think he knew that it was gonna happen, cos of the way he was looking at us. It was . . . like a look that said, “Just let me go”.’ Hesther said that her son Joel took cooling ‘to another level and just carried on going cold’. Anita and Sean’s daughter ‘set all her alarms off’ whenever a particular doctor left her cot side. Eventually the doctor sat down with his notes and worked beside Josephine until Anita and Sean arrived to visit her.
Talking about their children was important and Stefanie said, ‘I like to talk about my boys and let people know how special they were,’ and Karen said, ‘It was lovely to get the chance to talk about Tabitha.’ Karen and Tony had encountered several people, including family members, who could not see why they wanted to take part, seeing an interview as ‘dragging it all up’. They were both positive about the study, and although he was not initially sure about taking part as he ‘thought it may be painful to bring back the emotions’, Tony was sorry to have to leave for work part of the way through the interview. For Dora the opportunity to talk was important, even if it was a painful process.
Afterwards I felt sad; though having said that I also felt grateful that someone had listened to me and let me talk about my precious son as I think people tend to avoid difficult conversations like that. I have had a little cry since it all finished but overall I feel good.
Elsewhere we have discussed the introduction of memory boxes and photographs into the interview and these connections to the reality of the baby, of attempts to help the interviewer to appreciate the person, also seem to be part of the process of honouring a baby through the interview.
Commemorating
The idea of the interview serving to commemorate a baby extended the idea of honouring by recalling the details and emotions in order to do something of significance and importance in their baby’s name. The first time this idea appeared in the field notes was after one of the earliest interviews in the study, Interview 4, in relation to comments made by Stefanie.
Towards the end of the interview Stefanie said that telling me all about the trial and her son was ‘one last thing I had to do for [him]’. The care that she took in telling their story was evidence of the importance she placed on this. It suggested that interviews can be an act of commemoration, a way in which parents, who are the only people who know the story, can tell it in full, revisiting and re-evaluating as they go, bearing witness to the life of their child.
Participation as an act of commemoration was evident in both the interviews and the comments that parents made informally and in their questionnaires. A particular focus was related to sustaining the memory of a child. Chloe said, ‘I welcomed the opportunity to share my experience in memory of my sons’ and Stefanie commented during her interview that when she received the invitation to take part in BRACELET she saw it as ‘one last thing I could do on behalf of my son’.
Some of the parental comments suggested that they wished to record and acknowledge their baby’s legacy; Hesther felt that after giving her account of his life to the study that ‘Joel is still important in some way’ and Rhona felt that if she took part ’my daughter’s life would mean something’. These views are linked to the sense of meaning making we described in Chapter 9 , whereby parents felt that on receiving trial results that their baby would be connected out to the world in which he/she had made a mark.
Commemoration through recounting a narrative gives a sense of making something permanent out of an impermanent situation. This may have been heightened by the fact that each baby’s story was recorded and stored, that parents were assured that the stories were appreciated and would be used. Parents often responded very positively to the fact that the interviewer had travelled a long way to hear those stories. BRACELET was a UK-wide study and interviews took place in each UK country. The interviewer therefore often flew or made long journeys to see parents, and stayed away from home in order to accommodate evening interviews. The parents seemed to appreciate this as it communicated the importance of their accounts to the research and often made particular efforts to help with travel, offering lifts to an airport, rail station or back to a hotel, helping with driving directions, and sometimes offering meals. Fiona said in her interview that her son had now contributed to two pieces of research: INIS and BRACELET. Notably, she saw the contribution to research as his, not her own – as a consequence and a remembrance of his life rather than her actions. Dora explained that her participation in BRACELET was directly connected to her sense of huge disappointment on receiving the results of INIS (see Chapter 9, Box 15, for further details). She had very much wanted to feel that Gerry’s contribution to the trial would have had the effect of benefiting someone else. She was upset at the lost opportunity because she had ‘wanted a bit of purpose for him’. The opportunity to take part in BRACELET mitigated this sense of a lost opportunity as she felt that in telling Gerry’s story she would be ‘doing some good’ and taking part ‘was the one thing that made it all right’.
Even though I was disappointed, when I read the little bit at the bottom that said to me about the research you were doing, I remember thinking to myself, ‘Well, he can help that way, maybe’. Yeah, so that was, sort of, the saving grace for that, really.
Interview – Dora
The linked ideas of honouring and commemorating babies are contained in the larger empathetic impulse to ‘help’ through research participation, through the trial or through the associated interview study. Chloe, whose twin boys both died, made a comment on her reply slip which covered three of the four dimensions of participation that we have identified in only two sentences. She said:
I would be honoured to help with such worthy research to potentially help others going through what I did, and for the memory of my sons. I’m looking forward to honouring our boys through this study.
Her coverage of helping, honouring and commemorating is indicative of the close relationship between these powerful dimensions of what can be a demanding and draining experience.
Was there a price to pay for taking part in an interview?
Although these positive aspects of participation were clear, it was also important to consider whether taking part in an interview came at a cost to the parents involved. In the post-interview questionnaire parents were asked ‘How did you feel afterwards?’ and ‘Was there anything you disliked about the interview?’. For the first questions some parents gave single-word answers such as Pleased, Happy, Calm, Thoughtful, Fine. Hesther said, ‘None different really as what’s happened I can’t change.’
There was clear evidence that parents could find the interviews to be difficult, but Laura was the only parent to say that there was something that she disliked about the interview. She wrote that it ‘opened up some raw and difficult feelings’ but stated that she was ‘happy to have helped’. Even although some parents found aspects of the interview difficult, none indicated in the questionnaire in response to specific questions that he/she had found the interview to be too long or that they had wanted to stop the interview early.
In almost every questionnaire where parents described difficult emotions or feelings such as tiredness or sadness, they coupled this with expressions of pleasure or satisfaction at having taken part. The only exception was Rhona, who said that she felt ‘like I had relived the early days’. Where parents expressed any difficult feelings, their comments are detailed in full in Box 25 .
Glad it was over but we don’t know that we could give much more than our story about the boys – we found it hard to answer questions at the end – which were the main part of the interview.
It had been good to talk about it but left us with a lot of questions about the study and our own understanding of what it had been about.
Happy to be involved. Brings back memories but not in a bad way.
Really tired and a bit flat but glad I’d done it.
Afterwards I felt sad; though having said that I also felt grateful that someone had listened to me and let me talk about my precious son as I think people tend to avoid difficult conversations like that. I have had a little cry since it all finished but overall I feel good. Happy my opinions do matter and most importantly to me happy to be allowed to talk about my precious little boy.
I reflected on that period of my life all for good reasons though.
Drained, relieved, happy to have helped in some way as this is the first time the medical profession has engaged with bereaved parents.
OK, but sad as I always am after talking about my dead child.
Tired – I had to sleep for half an hour. Also pleased that I did the interview.
Like I had relived the early days.
I had to go to work before the interview finished, which was a little strange. Because after talking about everything I felt drained and unfocused towards work and just wanted to relax with my family.
It was emotional to talk about our story, but I felt glad afterwards. It’s nice to talk to someone who is genuinely interested and wants to hear about it.
It is hard to re-live it all in so much detail, although I was happy to participate.
To check whether parents had any concerns, they were asked whether the interviews should have been different in any way. Almost all of the parents made no suggestions for change. Laura wondered whether before the interview started parents should receive some reassurances that they should not feel worried about how they would be seen if they became upset. She said, ‘Perhaps when you are there forewarn them that they may be upset but that’s OK.’ Tony referred to the fact that discussion started very quickly before their interview had formally started. Consent forms had to be signed for permission to start recoding and this stop-and-start affected the conversational flow, which he found uncomfortable. He suggested:
Maybe just have a dictaphone running all the time, as when asked to stop until we were recording made forget things and feel like we were being put on the spot.’
At the end of the interview, the interviewer gave the parents a copy of the post-interview questionnaire (see Appendix 27 ) and the BRACELET study support and information card (see Appendix 29 ), which gave details of a number of sources of support. This included details of how to make contact with a bereavement counsellor with knowledge of both neonatal trials and bereavement. This gesture seemed to be appreciated by parents, even if it seemed to be very clear from our conversation that they did not feel that they would need to draw on further support. Jana said that she appreciated very much the fact that the study team had provided access to counsellor specifically for the study participants. She wrote:
The info about source of support – highly appreciated. Good to know about the BRACELET study counsellor – even if I think I will be all right, it still gives me a feeling of being cared for and respected and this in turn makes me want to help.
Hannah made a similar comment to the interviewer directly after her interview. She felt that this gesture showed that the well-being of the people in the study was being taken seriously.
The extract from the field notes written after the interview with Amanda suggest that the back-up of a trusted colleague was also of value to the interviewer where any concerns arose about parental well-being ( Box 26 ).
We continued talking for a while after the interview and I left when I felt that she was more settled, although I suspect that she may well have cried after I left. I gave her [the BRACELET bereavement counsellor’s] details and was very pleased to be able to do so. She said she had turned to SANDS for support but that it had not been a particularly positive experience so it would be good to think that she had the option of some support if she wanted it via BRACELET.
It was notable that in a study in which a total of 56 parents were interviewed (51 core trial interviewees, three additional interviewees and two parents interviewed but whose data were not presented in the analysis), none chose to access the services of the counsellor. This might suggest that it was not a useful or an appropriate service to offer but from parental reactions at the time this did not appear to be the case. It is beyond the ability of the study to state with any certainty why no contact was made, but from the experience of conducting the interviews it would seem most likely that they did not trigger a particular need for additional support.
The very clear message from the parents who took part in an interview was that this served an important purpose for themselves, hopefully for others, and that any difficult emotions that they experienced were ones that they were prepared to go through in order to give their views. Dora wrote after her interview of her feeling about having taken part in BRACELET:
Personally I feel including parents of bereaved children in studies is essential so as we can improve things. For me talking about Gerry is so good for me. I have clung on to the fact that letting Gerry be involved in the study has made his life have a purpose. I would like to take this opportunity to thank you for letting me be involved in this study for bereaved parents. We do matter and our opinion means as much as any other parent whose children are lucky enough to survive!
Discussion
The qualitative component of the BRACELET study has considered the topic of bereavement and participation in a neonatal trial from multiple perspectives using multiple methods. It has been a complex study in terms of the conceptual and analytical work required to gain insights into barely documented experiences and unexplored areas of clinical and research practice. The processes involved were logistically complex in terms of the need to access a number of different research arena and populations, using different approaches to recruitment and to data collection. This complexity was appropriate; it was required to bring us closer to the phenomenon in question, but it was also a means to explore the different methodological considerations that might be raised in future studies, and the qualitative component undoubtedly provided rich material for methodological study. The novelty of the subject matter, the logistical considerations and the sensitivity of both the setting and focus were, in themselves, sources of data and stimulated methodological reflection. In exploring subjects such as the value of the topic, the feasibility of recruitment and the appropriateness of the research tools for their purpose, BRACELET has been able to contribute important information to help to address the question of whether researchers in the future should consider inclusion of bereaved parents in their samples, and if they should, how they might do so. This is perhaps the crux of the study.
The BRACELET study has demonstrated the value and feasibility of research with bereaved parents involved in neonatal clinical trials, but with some important caveats. These relate to some of the barriers and pitfalls identified in the course of developing and implementing recruitment processes, important considerations in any research, but also to the consequences that these factors have for the sample that is produced. This, in turn, shapes the nature of the evidence on which our conclusions are based and in this sensitive setting we must be mindful of its boundaries and limitations. The study has also generated a number of strategies for interaction with the target population and the management of data collection processes. These have been developed in direct response to the sensitivity of the topic and the setting.
Although recruitment was difficult, these difficulties, in greater part, related to research governance and data protection, and the restriction they place upon the information that might be held in trial databases. The identification and screening system that was initially used for the study so that it would fit with these restrictions was cumbersome and labour intensive, and with the low response from GPs it resulted in very few parents who could be contacted. In the revised recruitment strategy the barrier of GP non-response was lifted, but the workload that fell to the CTU staff who supported the study was still excessive and out of proportion numerically to the yield of interviews. A major obstacle and complicating factor for the recruitment processes was the fact that parents, at the time of enrolling their baby into a trial, are not asked for their permission to be contacted at a later date for further study. Without this permission in place, a very cautious multistep process had to be developed, which was liable to attrition at multiple points. This is a major obstacle for any research that is not concurrently funded and approved alongside the trial under study. BRACELET was funded years after recruitment to the trials took place, and the lack of prior permission was sufficient an objection for one of the target clinical centres to decline to collaborate. If trials had approval to ask parents prospectively for their permission to contact them at a later date, or to inform them of how to register their willingness to be contacted for further research, for instance in a database held by the trial co-ordinators, the way to recruitment for future research would be smoothed. Flagging the opportunities for participation in trial newsletters and on pages for parents on a trial website might be another means of achieving the same end. Any such approach would require collaboration between researchers and RECs to develop procedures that would be acceptable to parents and would fit comfortably within the requirements of research governance systems. This would facilitate novel and responsive research, not only allowing trial teams to collaborate with other researchers, but also to respond to issues that arise in the course of a trial, or to take the opportunity to study an unanticipated event, such as early closure, or feedback of particularly challenging research findings.
Once the screening processes for BRACELET were under way, the effect of the imposition by the REC of the requirement for GP permission emerged as a major challenge to recruitment. Although there was a small number of cases in which the BRACELET team was grateful for the advice not to contact a family, there was also a great cost to the study and a threat to its chances of producing data that might benefit parents in the future, as BRACELET came very close to closure of the parental part of the qualitative study. This issue is known to have affected other studies. Knowles and colleagues168 have described the effects of a similar decision for their clinical study in which they wished to follow up children with congenital heart defect; 24% of their population could not be contacted, primarily owing to the inability to establish contact with the family GPs.
The REC that approved BRACELET took the decision to agree to reverse the terms of their original approval and to permit the proposed system whereby GPs had 4 weeks in which to lodge an objection to an approach to parents. This decision was crucial to the success of BRACELET; without it the study could not have proceeded to recruit the sample of parents, and findings based on clinicians and trial teams only would have provided a very different window on trial participation and bereavement. This then raises interesting issues about how best to protect potentially vulnerable individuals who might be invited to take part in research. It was questionable as to whether requirement for a definite approval from GPs did in fact offer the parents any more protection than the opt-in system, as we would expect that GPs who had any worries about a family would lodge that concern with either system. Some of the parents who were interviewed questioned whether GP involvement would offer the protection it was meant to provide, and it is possible that some of the parents who chose to join the study by the publicity route were initially excluded from contact via their clinical centre as a result of the screening process. A number of the parents who were interviewed felt that their GP would not have been in a position to make a valid judgement about their situation, especially if they did not know their GP, or if they had never discussed their bereavement with them.
This begs the question of what parents are being protected from, and how best to offer that protection. The focus in this regard was the recruitment process rather than the interview, and so it seems that parents were being protected from receiving a letter of invitation and a reference to the death of their baby. From the interviews with parents we can see that receiving such communications that refer to the baby, whether they were a trial newsletter or a letter of invitation to take part in BRACELET, could certainly be a challenging and difficult experience for some parents, and this therefore needs to be taken seriously. For the parents who decided to go on to take part in an interview, this initial difficulty was one that passed and turned into a preparedness to engage with research. For those who did not take part we cannot offer the same reassurance. We inferred, from the father who put the telephone down on a consultant who called him, and the angry father who contacted a NICU to say that there should be no call, that it could also be a challenge for parents who were not part of BRACELET, but we have no information about how those reactions unfolded. Although it is right to be cautious about the possible impact on parents with whom we have no further contact, we can perhaps be reassured by the fact that we received no complaints from parents, even although the means to do so was made readily available with the inclusion of the questionnaire about the BRACELET contact process being included in the invitation pack sent to all parents as well as on the study website.
The request to introduce contact between neonatal consultants and parents as a new step in the revised recruitment process did not raise concerns for the REC. All of the consultants in the clinical centres who were asked to take on this role of contacting parents agreed to do so, and we recognise that for them, and for the staff who supported them in doing so, there was a substantial workload involved. While it was labour-intensive for the relatively small number of cases yielded, it was an approach that seemed to suit parents, and resulted in a substantial increase in recruitment over the previous method of contact by letter only. Out of 81 eligible families, 59 were actually called. In 50 instances parents agreed to see an invitation and 18 took part in an interview. No reminders were issued. This was a 36% consent rate for those who received an invitation. It is low but not necessarily surprising. In research settings where potential participants are plentiful and readily accessible to researchers, such an approach would be unnecessary, but where they are difficult to recruit, and where there are potential and unpredictable risks in recruitment processes, a more personal and supportive approach is likely to be good for the potential participants and good for the research.
Our view that this method of recruitment offers a sensitive approach to parents was echoed in an unprompted comment made by one of the consultants who contacted parents on our behalf. He referred to the strategy in an e-mail and wrote:
I actually found it nice to speak to parents and explain/justify why we were writing to them. I don’t like the formality of a single approach letter when you have cared for parents, often got to know them on first name terms, and frequently put your arms around them when the baby dies – to send a formal letter with no personal touch feels ‘cold’ and misrepresents what it means to us. I don’t expect RECs to understand our area of work, and I don’t expect all clinicians will feel as strongly about it, but for our other studies I found it hard to not personalise the letter in any way.
Consultant Neonatologist – centre F
This comment recognises the personal nature of the relationship that research such as BRACELET can build upon. It is a counterpart to the parental comment quoted earlier from a mother who was pleased to have the opportunity to speak to her son’s consultant again after a number of years had passed. It is also, however, in contrast with the small number of parents mentioned above where parents most definitely did not wish to speak to the consultant. Like many aspects of research in this highly sensitive area, every decision that is made about contact with bereaved parents involves balancing potential benefits and hazards, and this is done in an unpredictable context. It is important to proceed with caution and care.
Once parents were contacted and sent a letter of invitation, no reminders were issued. At times this was a frustrating policy to have put in place as parents had in some instances spoken to the study interviewer already on the telephone to give their contact details and had expressed interest in the study. It may have been that some parents did not realise that, having said yes to the consultant passing their name to the BRACELET study, and having given their address to the study researcher, that they still had to return a reply slip to opt in to the study. Without this response, under the terms of the REC approval the parents could not be contacted again. The decision not to use reminders was initiated by the BRACELET study team, but in retrospect a single reminder with a reply slip may have encouraged more parents into the study, raising the response rate and shortening the recruitment period. Whether or not a REC would approve the use of reminders for use with bereaved parents is not yet clear.
As recruitment proceeded, the sample of parents was gradually enrolled into the study. The attrition rate via the clinical centres was extremely high and the sample could not have been achieved without the strategy of recruitment through publicity. This proved to be more than a helpful back-up plan; it coupled well with recruitment via the clinical centres and helped to create a sample that tapped a wider range of experiences than might have been tapped by one method alone. This approach brought in key testimonies to the study: Laura and Wilf, and Sophie and Nat, who had gone through formal inquiries into the deaths of their babies; Robert, who was able to describe his experiences of communication about a trial outside of the UK where he and his daughter’s doctor had very little common language; and questionnaire respondent 7 and Hannah and Ryan, who could tell us what it was like to be involved at the very start of research processes, and showed how this experience could result in an enduring connection to that research. These individuals were screened out, or would not have appeared in the UK core trial database, and so would not appear as eligible for participation. Some of their experiences might be shared with other participants but for these respondents it seemed that it was these key experiences that made them inaccessible to the study in the first place. These experiences are however as much a part of the phenomenon we were trying to understand as those more readily available for recruitment.
Once the sample was recruited, response rates for the two recruitment strategies that were used in the study could be compared. We were able to demonstrate that parents responded to personal contact in a way that they did not to a letter sent cold. While response rates were still low even with the more successful approach, it was possible to meet the target number of interviews and generate a sample of parents whose accounts were informative and capable of extending the existing knowledge base. It was also possible to observe an important bias which is not just a product of the shaping effects on a sample of who does and does not choose to take part. We were obliged to recruit through trial records which dated back to the time of enrolment to a trial. Although these records would be updated for parents of survivors who are involved in clinical follow-up studies, for the bereaved parents they may record only the address and GP details from that original entry point into the trial (unless parents send change of address or change of GP details to the trial co-ordinating centre). Bereavement after perinatal loss is a known factor in relationship breakdown,169 as is the experience of the birth of a very-low-birthweight infant170 and this is likely to result in changes of address within the long timescales that we were considering. That in this recruitment strategy we were only able to access parents who were still at their original address or were registered with the same GP meant that we were approaching a population with a significant degree of stability post bereavement. It was striking that so many of the parents wished to be interviewed as a couple, and that so many men chose to participate. Although inclusion of men is a very positive element of the research, we should acknowledge the inability to represent other men and women who were beyond the reach of our methods.
Although there were limitations in the sample, it was important to have pushed on to recruit the parents, as a central aim was to gain insights into the worth of the data that might be available in future studies. We now have the data to say that bereaved parents can make a worthwhile and important contribution to research on parental views of trials. They have some similar and many different experiences from parents of surviving children, as well as similar and dissimilar things to say, and so their views should be represented. We were also able to look at parental responses to the approaches to data collection that were developed for the study.
We were able to demonstrate the value of interviewing parents some time after their bereavement. This vantage point provided a unique overview of a long-term association with a trial. It would be a fruitful area of enquiry in another study that included both bereaved parents and those whose babies survived and who might be involved in clinical follow-up studies. This longer-term view also provided some emotional distance from bereavement that parents often said was useful. Not only were they at a different point in their personal trajectory of grief than they were in the earlier days, they were also able to look at their involvement in a trial in the context of the personal story that had subsequently unfolded. It is possible, however, that research that seeks to involve those more recently bereaved is still feasible and acceptable. Linda and Dan, whose interview was included as additional data, opted into BRACELET within 1 year of their daughter’s death, which, under the terms of our original approval (a timescale that we had set ourselves), would not have been invited to participate. (They took part in the I2S2 trial and not one of the core trials.) They both argued eloquently that the voices of bereaved parents should be heard. Recently, Ward66 demonstrated the acceptability to parents of discussing their experiences of taking part in neonatal research closer to events, and Breeze and colleagues160 were able to successfully recruit bereaved parents to a questionnaire-based study, considering their views of their very recent decision-making process for post-mortem after perinatal loss. It may be that earlier studies are possible, and this would offer insights into experiences of trial participation that were beyond the scope of BRACELET, but it is likely that a particular profile of parents would take part. This raises interesting methodological question of whether study findings would be shaped by the timing of interviews, not only because of the period that has elapsed since bereavement affects views, but also because of this effect of timing of recruitment on the sample constituency. This highlights the importance of a careful exploration of time since bereavement on views and awareness of the potential for data being shaped by this aspect of the parental experience.
There were other aspects of our approach to the management of data collection that were specifically developed in the course of the study, which proved to be interesting in methodological terms. Three key aspects were not necessarily widely used but still seemed to represent a potentially useful step forward in research practice.
The availability of a wide range of information on a study website, might help potential participants to decide whether or not they wish to participate. It was a useful resource to refer to during the course of the study and a number of interviewees mentioned reading about the research. The site will continue to develop as links to publications are posted. Parents who took part in BRACELET will be alerted when this happens and will also be offered access to paper copies of findings and a summary of the results. This website is an important facility given our own findings about the value of making research findings available to research participants.
The availability of online questionnaires as an alternative to interview, with direct links from websites that displayed the study publicity to the BRACELET site, was also useful. The questionnaires provided a route in to the study for parents who might otherwise not have participated, and gave voice to views that could differ from those of interviewees who would not otherwise have been represented. This approach, which we referred to as data salvage, may prove to be important for other studies with finite, hard-to-reach and potentially vulnerable populations for which every contribution counts. It may also be well used in studies in sensitive settings, in which the target population is larger and more readily accessible than the scattered population searched for in BRACELET, and where interviews may be too challenging for some individuals. Data salvage was an extremely useful approach in BRACELET. With further research it may be shown to be an effective and informative strategy that meets some needs and preferences of some potential participants in other studies.
The services of the bereavement counsellor that were made available to parents were not used, but this does not mean that it was not an effective or appropriate strategy to have used. Although no parents contacted the counsellor, the provision of this resource was appreciated by parents when it was mentioned at the end of the interviews, and was reassuring for the interviewer. In this study the parents were generally well supported and were often comfortable and interested to talk about their experiences. In a study in which the sample represents a wider range of experiences and needs, or which focuses on more recent events, such a service may be particularly important to offer. The key methodological step forward was to connect with a bereavement counsellor who was conversant with neonatal trials. If, for instance, parents’ emotions were stirred in an interview and they were left with thoughts and feelings that centred on the allocation made for their baby, access to a counsellor who understands and can discuss that aspect of their experience is what lifts this strategy above a more simple provision of details of national helplines.
Once parents had participated in an interview for BRACELET they were able to comment on the processes that were used and their own involvement in the study. This was a key element in the methodological component of the BRACELET, given its focus on some of the most difficult aspects of personal and interpersonal experience. It explored aspects of participants’ lives that might be considered to be ‘private, stressful or sacred’. 160 Although this typology was coined with reference to the difficulties of carrying out research in sensitive situations, the notion of tapping the personal and sacred seems to be an appropriate image when parents communicated their sense of helping, healing, honouring and commemoration through their involvement in BRACELET. Although a clinical trial and a qualitative interview-based study sit at opposite ends of methodological and epistemological spectra, responses to participation in the two forms of research were remarkably similar. Both were tied to their baby’s life and both allowed, to varying extents and in complex ways, some sense of satisfaction and meaning making in the larger context of loss.
Taking part in a research interview about deeply emotional, seldom-shared aspects of family life can be challenging, but it can also give parents an opportunity to revisit and explore their experiences with a view to improving the way in which parents are provided for in the future. Researchers are not new to the challenges of working in relation to sensitive topics, and qualitative researchers who often value empathy and reflexivity as key research skills, have reflected upon the issues raised when participants’ lives and views are opened up to their scrutiny. 171–173 There is a careful balancing act in collecting these types of data, to make sure that they are not collected at the expense of the interviewee. It is important, however, to note that it is not just the researchers who manage the impact of research; the interviewees live with their bereavement and have developed their own strategies for grief management. Some display their photographs of their baby after death in their home; some keep them in an album for when they wish to engage with that time in their lives; some would not want to keep such things at all. In the same vein, parents develop strategies to manage information and their links with research. These strategies can result in a letter inviting participation in an interview being put on the fridge door or being put in the bin. They are the same strategies that we saw being used to manage feedback of trial results. It is important that bereaved parents are not only seen as vulnerable, but as individuals with agency who will make their choices about participation according to what suits them. The sensitivity of the topic is not in question. Jill wrote in her post-interview questionnaire that ‘it will always be a difficult thing to recall’, and the discussions in BRACELET undoubtedly brought difficult emotions to the surface, but this was not necessarily something that parents wished to avoid or needed protection from.
The methodological component of BRACELET has highlighted the ineffectiveness of an unsupported postal approach to recruitment, the value of a more personal approach through the involvement of a clinician, and the potential value of publicity through trusted organisations, as an adjunct to recruitment for this hard-to-reach population. It has demonstrated both the impact of recruitment methods on the sample that is produced, and the need for the development and permission for approaches that will encourage higher consent rates in this challenging setting, and will facilitate the inclusion of parents with a wide range of experiences. The bias that currently exists in the field of assessing participants’ views of trials serves to limit our understanding of the impact of trial protocols on personal experiences. For many years researchers in this area have chosen not to include those who are bereaved in studies of participants’ views of trials, and so our understanding of the impact of trials, and any recommendations for change, are based on skewed and incomplete samples. We do not know enough of about what happens to parents in this situation or how to respond to any needs that they might have. This sort of skew in the evidence base would not be tolerated in many other areas of research. It is therefore important that we continue to consider how best to carry out this sort of sensitive research so that research in the broader field, which explores the views of trial teams and trial participants, might flourish. If we are to respond to bereavement in a fair and sensitive way, it is most important that we aim to listen to those who are bereaved and to do this it is necessary to continue to work out methods by which we can do so.
Chapter 11 Overview and conclusions
In the BRACELET study we have approached the previously unexplored topic of bereavement and participation in a trial from different angles and multiple perspectives. In looking at trial practice, clinical practice and the views of the key parties involved, we have been ‘circling reality 174 to gain a closer, more nuanced understanding of a complex and mutable phenomenon. Shenton113 argues that this circling approach produces ‘a better, more stable view of “reality”, based on a wide spectrum of observations from a wide base of points in time space. ’ The topic stemmed from a small number of interviews with bereaved parents who had taken part in two different neonatal trials and who showed a wide range of responses to their involvement in research, from great satisfaction to devastation that a potentially useful treatment had not been used. 51,141 Diversity within a small number of interviews suggested not only the value of the topic, but also the challenges that might exist in any attempts to provide for a heterogeneous group whose involvement in trials derives from a range of circumstances. Little was known about the views and preferences of this population of parents. How triallists and clinicians might respond to bereaved parents in the context of a NIC trial was equally unexplored. On examination, the same deficit existed for PIC trials. The story that was gradually pieced together is summarised below.
The whole story
To gain purchase on this topic we tracked back to the trials themselves in order to understand how commonly babies and children die after enrolment in a NIC or PIC trial. In Phase I of the study we found that almost 17% of the babies enrolled into NIC trials went on to die, which translates into over 500 babies in the 5-year period that we considered. Many of the deaths that were identified were scattered across the UK and it was clear that individual clinicians involved would not encounter this situation particularly commonly in their practice. There was, however, a group of clinical centres of excellence that cared for the sickest babies at greatest risk, wherein levels of recruitment to trials and therefore numbers of deaths in trials, were higher. In these (mostly academic) centres, deaths subsequent to enrolment were not an everyday occurrence, but were sufficiently frequent to suggest that the topic warranted further exploration, and that these centres would be the most appropriate for study. The profile of recruitment and mortality was very different for PIC trials. Here, far fewer trials had been carried out in the 5-year period, far fewer deaths had occurred, and they were a smaller percentage of the total number of children recruited to trials in this setting, only 6%. The questions considered in BRACELET were relevant to paediatric intensive care trials, but with small numbers of trials and bereaved families; it was clear that the research questions that we were considering would be best approached when developments in the field would afford better opportunities for study. BRACELET was therefore reorientated to focus on the neonatal setting.
Phase I data provided a starting point, a link to neonatal research populations. From this point we picked up the thread175 of trial participation and subsequent bereavement, and followed it through the core centres, their core trials, and through the views and experiences of the parties involved.
Interviews with senior triallists for the core trials and senior core centre clinicians showed that trials are valued both as a means to contribute to clinical knowledge and as an indicator of clinical and academic excellence. Trial designs took careful account of the clinical circumstances and needs of the babies involved, and the triallists and clinicians were mindful of the demands that could be placed upon parents by the combination of trial design, recruitment methods and clinical circumstances.
Parents’ accounts described a range of experiences around recruitment. The offer of trial participation came for most parents in the context of care on the NICU. The subject could be raised in relation to relatively routine aspects of intensive care, such as mechanical ventilation, feeding, or in response to an escalating crisis. In all of these situations there was a strong sense that parents and clinicians were acting together. Although some parents pinned their hopes on a trial, for others it could be a minor part of their experiences; whatever its degree of prominence, however, parents very often articulated a view that it might help but would not harm their baby. Commonly, the trial receded for parents as they had no further conversations about the research and did not witness trial-related activity. Other events took over and once a baby had died it was largely subsumed in bereavement.
The interviews with the core centre clinicians demonstrated the care that they take over bereavement support and the pride that they take in the systems that have been developed. It was felt that these provided well for bereaved parents who could make clear any interest or need that they might have in relation to their involvement in a trial. No formal provision for previous trial participation was made in the core centres, and each clinician made their own choice about whether or not to raise the topic of trial participation with parents. The majority chose not to do so, viewing trial participation as over, and saw their links with parents at that time as grounded in a wholly clinical rather than a research relationship. They felt that other issues such as cause of death, implications for future pregnancy, or emotional support are at the fore at the bereavement follow-up meeting and in their experience parents rarely raise the issue of trial participation at this point.
In contrast, the links between parents and those who co-ordinate trials are wholly research based. We found that trial teams varied in their response to bereavement, from no further contact in one trial to a multipart bereavement contact strategy in another, reflecting differences between the trials and the time periods in which they operated. In a CTU that supported most of the core trials, a number of pioneering strategies were in place to recognise the involvement of bereaved parents in a trial, and to provide information about the research, including the use of newsletters and communication of trial results. However, these efforts were complicated and constrained by data protection and research governance, and there was little spontaneous feedback from the parents who were sent communications. It was therefore very difficult for the CTU to know whether or not these were as well received, effective or valued as they hoped. The constraints that do not permit trial teams to seek out updated contact details for bereaved parents, largely because there is no requirement for further follow-up, also meant that CTU staff could not know whether their communications would reach parents, an issue that would only become more problematic as time passed and parents were more likely to change address.
The parents’ responses to the idea, and sometimes to the experience of receiving information and feedback of results from the trials, suggested that even if a trial initially seemed like a minor part of their experience, or was an element which had receded into the background on bereavement, recognition of the contribution made and the information given could be suffused with a different type of meaning than the paperwork might outwardly suggest. Trial results in particular had the potential to serve as connections back to the time of the baby’s care, out to other families who might benefit from their participation, and forward to contribute to the advancement of knowledge. These connections were meaningful for the parents. Trial paperwork was often kept as an update to, and a record of, the story of a baby’s life, with some parents placing letters and newsletters in a memory box. The interviews also suggested that communications from a trial can be difficult, bringing painful memories to the surface and, depending on the results, could be challenging information for parent to process.
Some of the clinicians and trial team members who were interviewed suggested that a trial would become irrelevant once a baby had died. This view was in part borne out in the majority of parents’ accounts of the early stages in their bereavement when a trial was not a particularly major focus and when other factors would indeed take precedence in bereavement follow-up meetings. However, there was scope, according to the parents, for neonatologists raising the topic of trial participation in case this facilitated a helpful discussion. The view of the reduced relevance of trial participation in bereavement might stem from the particular positioning of clinicians at only the start of bereavement. Bereavement trajectories are lifelong and changing, and we found that responses to trial participation were similarly changeable – ebbing and flowing over time. It seemed that an interest in a trial, especially in the outcome of the trial, can fit into the longer-term experience of bereavement at a point that the clinicians do not usually encounter parents. This fits well with the timing of trial outcome and findings that are usually made available some years after recruitment has stopped. In trials such as BOOST-II UK for which early stopping meant that preliminary results were delivered in closer proximity to bereavement, there may be particular challenges that still need to be explored and understood. In discussing the results, parents often spontaneously placed them in the context of their lives as bereaved parents. They discussed how pain and sadness are everyday experiences, and that they have gradually found ways to live around these emotions. Although there were many statements of appreciation of sensitivity, there were often vociferous arguments for treating them as normal. Their reactions to the idea or the actuality of receiving trial results, suggest that whatever information they contain, however difficult, for some parents they would be embraced and incorporated into the balancing act of bereavement.
Our awareness of the potential skew in our sample towards parents who have a degree of stability in their home circumstances and who feel sufficiently comfortable to take part in an interview, suggest that a wider range of views should be explored before we can be sure that the comfort with research and the interest in receiving trial results are positions shared with other bereaved parents. The methodological work carried out for BRACELET helps in this regard in pointing to some of the facilitators and pitfalls for future research. It suggests that bereaved parents can be highly engaged in qualitative studies of their experiences, and are prepared to engage with the topic of bereavement and participation in trials. For the parents in this study, their involvement in BRACELET seemed to be a satisfying experience and the accounts that the parents gave were often seen as a way of honouring and commemorating their baby.
Key lessons learned from BRACELET study: interdisciplinary team views
The story that has been collated and constructed from the BRACELET data offers a number of key lessons for different audiences. Those audiences are represented in the constitution of the interdisciplinary team that was assembled for this study. BRACELET is the product of a team of six academic and clinical researchers from four different universities, each member having particular expertise, which has allowed the study to cover the wide span of methodological and intellectual ground needed to address the research question. The team was assembled, in part, for specific quantitative (DE, SH) or qualitative (CS, DE) skills, and for their investment in the field under study, either as triallists designing and running clinical trials (DE, PB, MWP, RT) or as clinicians with responsibilities to parents and children who enter those trials (MWP, RT). The triallists’ and clinicians’ insights into their own specific fields were important in the design, conduct and analysis of the study, but their influence extended beyond a core set of methodological skills. These team members were part of the world under study; they knew how that world worked, explained what information they felt was needed and were able to guide the process of fitting the study methods to the research questions. They acted as sounding boards to explore and explain emergent findings and they valued the information that the study would yield for their own practice. From the experience gained in conducting earlier qualitative studies in the field, two of the team members (CS, DE) brought different methodological dimensions to the study, as well as a different set of insights and interests. Despite our common investment in the research topic, none of the team members would have designed and delivered the study in the form it finally achieved on their own.
Although the design and implementation of the study has been collaborative, dissemination of the findings needs to be targeted at different audiences – for instance triallists with design considerations, clinicians involved in recruitment, or social scientists carrying out research on participants’ views of trials. The team members served as research advocates during the study, facilitating the progress of the research in their field, and, on completion, they act as ambassadors ensuring that the research findings are disseminated within their particular area of influence. In order to reflect the diverse skills in the team, and the different audiences that might benefit from the insights gained from BRACELET, the authors have drawn out what they feel to be the key lessons from the study that might be learned by their particular field. They focus on the insights gained and how these might be implemented in practice, and in some instances the challenges of doing so. They reflect the priorities of each author and in their ambassadorial capacity they are the messages that they feel are best shared with colleagues and incorporated into their own practices. Each set of key lessons is written in the author’s own words. These are presented in Boxes 27–31 .
The BRACELET study acts an important reminder of the need to consider the potential effects of being in a trial for all participants, from the very early stages of designing and conducting a trial, and to build in systems to support their possible needs, so that these can be incorporated into the submissions to RECs. Not all of the participants in a trial will do well, although it is not easy to predict at the outset who is likely to die. For those who do not survive, the BRACELET study shows that their relatives may have varying needs at different stages in their bereavement. Of course, not all of these will be related to the trial.
Acknowledging bereavementEven though the generalisability of the views expressed by these parents interviewed for BRACELET is difficult to judge, because we have no idea of how representative they are, there are still practical issues for CTUs to consider. In particular, the early condolences communications from CTUs may not be helpful. Despite some parents feeling that this was useful, especially the reference to the value of the contribution that a baby had made to the trial, several reacted negatively to this communication. In contrast, later communication expressing condolences appeared to be better received.
In the weeks and months after the death of their child, parents are often still in contact with staff at the NICU, and a bereavement follow-up visit at the NICU is commonly offered. Even though for many bereaved parents the trial is unlikely to be uppermost in their minds straight away, they still need to be given the opportunity to raise questions about the trial at that encounter if they wish, and to have the door left open to make further contact later. A CTU may be able to support the local clinicians by providing appropriate information about the trial and to facilitate contact with the CTU, if this is wanted later.
NewslettersAs the BRACELET study has shown, some parents feel they would have wanted to have had the opportunity to stay in touch with the trial in the same way as parents of children who survived, and this could be via newsletters. Newsletters for parents are not always used in trials. Where they are, one of their main purposes is to maintain contact to facilitate longer-term follow-up, which means that the content and tone may not be suitable for bereaved parents. This raises questions about whether there should be separate newsletters for bereaved parents, and how these should be offered.
Parents in BRACELET appreciated the use of trial-identifiable envelopes for newsletters and other communications, such as personalised letters expressing condolences and letters asking if parents want to see the results of the trial when available. This is a practice that CTUs might use, although the best timing for these communications needs to be evaluated.
Contacting bereaved parentsThe process of contacting bereaved parents was made more difficult because there was no mention made during recruitment and in the early stages of the trial, that contact with parents may be made in the future for other research, such as BRACELET. Whether it is possible or desirable to include this information in the initial Parents Information Leaflet, to facilitate more direct contact with parents through the CTU, is not clear, and whether this would be acceptable to RECs is also uncertain.
Sending resultsShould results be sent to all parents? Most of the parents interviewed in BRACELET were keen to know the results of the trial and were largely supportive of the way results of the trials were fed back, even when this was many years after their participation. For the parents we interviewed, receiving results was important. However, when bereaved parents are offered the option of receiving results, not all will take up this option initially. From the accounts we have heard, it is clear that this view may change over time. But once parents have indicated that they do not want to receive results, contact with the trial is likely to cease. How trials cope with this evolving process of bereavement where the desire for information may change, is not clear.
It was clear that moving house, particularly for trials that took many years to complete, could mean that contact details of bereaved parents had been lost. This may have prevented them from receiving newsletters as well as results. Maintaining contact details for bereaved families is challenging. For large trials in which many babies may die, this is a substantial burden of work, but even for smaller trials this will involve tracing the contact details of the mother (rather than for follow-up, as for surviving children). Whether this will be acceptable to the bodies that house and control access to these data, when the request for this information from trial teams may not be seen to be for legitimate clinical reasons, is uncertain.
Use of alternative approaches to maintaining contactOne issue that was surprising is that few parents used CTU websites to get information about the trials. Most of the core trials were conducted by one CTU, which includes full details of the trials, including newsletters (where used) and the trial results, on the website. The website address is also included on most correspondence with parents, from the initial Parent Information Leaflet right through to results letters (and the BRACELET study showed that many parents keep these communications in a memory box). In the future, it is possible that websites may be more widely used as a means of providing information for bereaved parents. Similarly, trials recruiting now are more likely to collect a broader range of contact details, including e-mail addresses and mobile telephone numbers. The routes for communication with all parents, including bereaved parents, will change, and the impact this will have on how parents receive and feel about this information needs to be considered.
The significance of trial paperworkWithin CTUs, we need to be aware, when writing trial communications, that the BRACELET study has shown that much of the trial-related paperwork (letters, consent forms, information leaflets, newsletters etc.) might be kept among the different valued items that parents had preserved in memory of their child’s short life. This might argue for producing items which are durable, for instance printed on a high quality paper, and which articulate clearly the importance of the contribution that babies and families make to a trial.
Acknowledgement of multiple births and lossesBRACELET has highlighted a number of different possible configurations of the involvement of multiples in neonatal trials. If parents enrolled all of their babies in a trial, and one died, follow up studies would relate to the baby with an ongoing role in the trial. It is important that any ongoing communication such as feedback of results should acknowledge the contribution of all babies in that family. If parents enrolled only one of their babies in a trial, but both babies die, a letter which expresses condolences may refer to only the enrolled baby. These situations raises yet more challenging issue for CTUs as only the names of babies enrolled in a trial may be retained. Any strategy aimed at acknowledging loss needs, as far as possible, to acknowledge that loss in its entirety, but how to address this issue is one of the questions thrown up, rather than answered by BRACELET.
Evaluation of CTU practicesThe lack of an evidence base about how CTUs and triallists respond to bereaved parents has begun to be addressed by BRACELET, but there are still big gaps in our knowledge. Triallists need to consider ways to evaluate their existing practices and to evaluate any changes to these which may result from new knowledge generated by BRACELET. How to do this is still uncertain. Involving bereaved parents in the design of communication and interventions is an obvious way, but getting a broad range of views to design these approaches may also be very important, as the views of bereaved parents about later contact may vary more than non-bereaved parents. The parents we have interviewed were willing to be interviewed. Although we only have a small number of instances where parents declined to be contacted again, we have many instances where no response was received from bereaved parents. Their views need to be heard if we are to optimise the way we communicate with bereaved parents.
The findings from the BRACELET study raise similar issues for CTUs in many different areas. In adult critical care, patients frequently do not have the capacity to provide informed consent to take part in research. In these situations, relatives are usually asked to make decisions about the patient taking part in a trial. The time frame for decision-making is often very short, because of the urgency of initiating treatment in critically ill patients, and occurs in the context of a very stressful environment at a time when relatives may be receiving a lot of complex information. Many of the issues that have been raised by the BRACELET study also apply in adult critical care settings and warrant further investigation. These include providing opportunities for relatives to discuss the trial and ask questions (relatives may be in contact with the critical care unit in the weeks following the death of the patient, including bereavement follow-up), being offered the option to be informed of the trial results or at the very least, being provided with information about accessing trial results.
Triallists dealing with mortality in other fields may find that our findings are also relevant to their practice. In trials in adult oncology or cardiac care, relatives may support a family member through decisions about trial participation, or take them to clinic appointments. In trauma or dementia trials, for which relatives may have given proxy consent for trial enrolment, there may be even more similarities. Bereaved family members in these different areas of adult care and research may have similar interests and needs to those that we have identified for bereaved parents of babies in neonatal intensive care.
Trial participation can be important to parents after the death of a baby, and may even increase in importance as the immediate grief fades. One might have imagined that the reverse would be true, so this finding is important for all neonatal clinicians to take on board. In particular it has implications for bereavement follow-up: at present it is rare for a consultant to see parents more than once but the BRACELET results provide an argument for offering a further formal appointment several months later at which to pick up the trial issues, should the parents want this.
Acknowledgement of trial participation at discussions following bereavement may be valued by some parents. This raises the question as to whether it should become a routine of the bereavement appointment to discuss trial(s) explicitly. It is clear that parents vary widely in their desire to address trial participation, but in view of their diverse attitudes to receiving trial newsletters and information about trial outcomes, ascertaining their views would provide a perspective that could be fed back to the trial co-ordinators.
Parents may not only remain interested in study findings after the death of their baby, but resolution of one dimension of their grief may depend on knowing trial results. However, the trial co-ordinator may in some cases be contacting them perhaps several years after the bereavement has taken place. This may not occur to parents who are in the midst of grief, and may be a useful point for discussion at a bereavement follow-up, and at any subsequent appointment as suggested above. It might also be helpful for participating neonatologists to clarify at the outset of the trial whether they or the triallists will have responsibility for sharing long-term results, once these are available.
Those working in trial co-ordinating centres clearly have legitimate concerns about contacting bereaved parents once the death of a participating baby becomes known to them. Parents seem to favour personalised communications over leaflets, for very good reasons as articulated in their interviews. However much a leaflet might look appropriate to a trial design committee, or to an Ethics committee, not all parents will like it and some may find it causes additional upset, which makes me think that the idea of leaflets of condolence should be abandoned in favour of personal letters. It might be that co-ordinating centres could work collaboratively with the consultant who is in touch with the parents, which would both lessen the anxieties of the co-ordinators and would allow the consultant to influence the nature of the contact, for example if twins had both died but only one was in the trial.
Because BRACELET addressed trials that mostly pre-dated the child death review procedures introduced in England in 2008, the interface between bereaved parents and conduct of these procedures was not germane to the interviews. However, the issue of bereavement care and parental follow-up is explicitly addressed in the child death review process, and is of interest and concern to the CDOP. 176 It will therefore be important to ensure that BRACELET results are communicated to the CDOP community.
CDOP, Child Death Overview Panels.
In the UK, the organisation and undertaking of RCTs in the ICU is much further developed in the neonatal than paediatric area of practice. It is clear, however, that there are lessons to be learned from the five core studies in Phase II of BRACELET, which should now be translated to research practice in the PICU setting. If it was not obvious before the BRACELET study, it is clearly evident from the interviews with bereaved parents that the child’s family should be considered as essential partners in the activity of clinical research, even after the child’s death. For me, there are therefore a series of implications that will need to be addressed in our clinical practice.
-
In many of the RCTs reviewed in Phase I of BRACELET, death was used as a measure of outcome of the intervention being studied. As death is, by definition, not infrequent in this population, its consequence should influence trial design.
-
The circumstances of the RCT are often interwoven in a parent’s memory of their child’s story, and these ‘parent partners’ often want a relationship, if not some ownership, of the research in which their child participated. Hence, such bereaved parents will need and deserve an ongoing connection with their CTU.
-
The time course of bereavement identified in Phase II of BRACELET means that some bereaved parents may require support and interaction, even years after trial completion.
-
Bereavement in the context of a RCT should be planned as part of trial design in critical care studies. Anticipating this possible need, in the very least, respects the valued contribution that parent partners make in health-care research.
As a practitioner in paediatric critical care, my practice in providing parental support through bereavement is assessed according to nationally accepted professional standards (see PICS Standards for the Care of Critically Ill Children, 4th edn, June 2010: www.ukpics.org.uk/documents/PICS_standards.pdf). However, this standards document does not make provision for parents involved in a RCT and the BRACELET study has identified a particular need beyond our usual support through bereavement. We therefore require a co-ordinated approach to bereavement in the context of clinical research. The following actions should follow:
-
It is imperative that researchers and funding agencies engage in developing a national consensus for parental support through bereavement in the context of a RCT.
-
As individual clinical centres (whether NICU or PICU) will have limited experience of such death, an integrated strategy will be required between units, professional organisations, CTUs and funding agencies.
As a clinical researcher in paediatric critical care studies, the methodology section in Phase II of BRACELET has highlighted that our research community now needs to consider how to apply some of the lessons learned to recent and ongoing studies. For example, there are three major PICU studies with over 1000 patients per study where bereavement in the context of the study will need to be addressed: the recently completed131 Control of Hyperglycaemia in Paediatrics RCT (supported by the UK NIHR-HTA programme), mortality ≈5%; the ongoing Catheter Infection in Children RCT (also supported by the NIHR-HTA programme), mortality ≈5%; the new comparative effectiveness research study on Multiple Medical Therapies for Severe Traumatic Brain Injury (funded by the USA National Institutes of Health for three PICUS from England), mortality ≈20%.
We are now ideally positioned with the lessons learned from BRACELET to examine the arrangements for bereaved parents in these studies, improve our practice and define a national standard of care.
Relationships between researchers and RECs – Some of the difficulties highlighted in BRACELET, such as the requirement for written approval from GPs, stem from the restrictions imposed by the REC. Some, such as not using reminders, were self-imposed. Such restrictions can have major implications for the success of a study, and may preclude rather than facilitate access to those whose accounts are best able to inform future practice. The insights available are not only important for triallists and clinicians, but also offer opportunities for reflection and development for RECs themselves. The introduction of formal GP approval was highly problematic, as it seemed that a standard approach was being applied to a non-standard situation, for example parents were unlikely to be registered with the same GP given the years that had passed. Recruitment had to fail before sufficient evidence accumulated to request a substantial amendment a year later. The RECs reversal of its decision suggested that a more individualised and responsive approach was taken and is an example of how RECs and researchers can work together to find solutions to challenging research situations. Another useful example is the negotiations that took place for a study by Breeze and colleagues160 whereby researchers were permitted to contact a restricted number of bereaved parents for a study of their views around the time of post-mortem. The study went on to yield important information about parents’ views at this time. This sort of dialogue is important, as it will facilitate rather than block research in sensitive settings.
Identification and contact processes – It is important that better ways are found of identifying and contacting potential participants. This is a major challenge as trial teams who maintain trial records have to work within the terms of data protection legislation, as well as the terms of REC approval for their trials. Without improved recruitment processes it is likely that future studies will be subject to some of the same biases that have affected BRACELET and it is most important that the means of representing bereaved parents in all their variety is found.
Researchers who are making contact with parents should be aware that one partner can control communication channels for a household and might act to protect the other from any potential breach of coping strategies. In the process of contacting parents for BRACELET some men have declined and it was not clear whether this was in consultation with their partner; one man called a NICU to say not to contact them again, and another put down the telephone when a consultant introduced himself. Others have acted as intermediaries. One woman asked the consultant to call her husband’s mobile to talk to him instead, and one of the men in the study chose to be interviewed alone away from the family home. Only after he had done this did he ask his wife whether she wanted to take part too; she did not. Two parents described a period in the earlier stages of their bereavement in which they would throw away letters so that their partner did not see them. This raises important issues for researchers trying to contact parents to invite them to give their views. It is possible that some people need the protection that their partners afford them and that others may not gain access to information that they may wish to have. This plurality makes a strong case for creating flexible research methods, in terms of the means of informing potential participants about a study, for instance by publicity as well as direct contact, and for contributing data, alone, with a partner, by questionnaire, by interview, or by other methods not considered here such as private diaries or online blogs. This flexibility would allow parents to participate on terms that suit them. Researchers in sensitive settings have to tread a fine line between facilitating voluntary contributions and overstepping personal and emotional boundaries and the provision of multiple methods for recruitment and participation would seem to be an essential part of a careful and supportive research strategy.
Reflexive interview practice and the importance of planning It is most important that researchers are reflexive about their interview practice in sensitive settings. We developed a Code of Practice that fitted the particular needs of our setting and used this as a guide for interviews with parents throughout the study. Different studies will require different guides. A period of reflection on the challenges that interviews on a particular topic in a particular setting might raise is valuable, and documenting these before the interviews commence is helpful in formalising strategies and preparing the interviewer.
Dedicated bereavement support Researchers working in particularly sensitive areas, such as that explored in BRACELET, may consider offering bereavement support by a counsellor who is skilled in the topic under consideration in the study. Other researchers working with bereaved carers have already offered bereavement support for study participants and have found it to be a positive approach (Ewing and Grande177). We found that parents were appreciative of this option. We felt that the additional dimension of provision of a counsellor with an understanding of our research topic was particularly important. This increased the potential value of this facility was an important safeguard and served as an acknowledgement of responsibility for the well-being of study participants.
Funding of qualitative studies If we are to understand more about the issues and challenges that are raised in the conduct of clinical trials, it is important to consider changes to the current system of stand-alone qualitative studies that are funded and approved separately from the trials they are exploring. The availability of funds to consider practice in individual trials will not only allow for practices to be explored as they are rolled out, but it would also allow qualitative studies to be used in responsive mode, to understand the effects of unanticipated happenings, such as early closure of a trial, and to afford opportunities to learn through study from any management decisions that are made.
Strengths and limitations of the research
The BRACELET study broke new ground in terms of its focus and the methodological work to be done. Some of the difficulties that were faced were anticipated, and it was stated in the initial application for funds that recruitment of bereaved parents was uncertain and may not prove to be possible. It did eventually prove to be possible, and the target numbers of interviews with clinicians and trial team members, as well as with parents were met, but only with the help and cooperation of a large number of individuals and organisations. This was a major success, as recruiting parents provided data that were the keystone in the larger data set, which allowed the whole story to be assembled and to make sense. These data were not easy to achieve, however, and their means of collection and its implications for our findings mean that we must place very clear caveats around the findings for the study as a whole.
As a novel study there are clear strengths and weaknesses in the research in each component.
Phase I: The quantitative component
The quantitative work carried out for Phase I was the first to investigate RCT activity in UK NICUs and PICUs; report the numbers of babies and children enrolled into these trials; and determine the extent and distribution of mortality involved. This component of BRACELET achieved a high response rate to the unit survey, and we are confident that most of the larger international and large multicentre RCTs were identified. It may be the case, however, that the total numbers of babies and children enrolled into a RCT during the 5-year period are under-reported, as data were not available for every RCT for every unit. For the same reasons, it is possible that the number of deaths is also under-reported.
Phase II: The qualitative component
As a foundation study it was important to consider a range of trials, rather than carry out a close analysis of the specific conditions of a single trial. This introduced both strengths and weaknesses into the study. In order to work with a spread of trials, it was only possible to recruit small numbers of parents per trial and clearly there are experiences within a trial that cannot be tapped with this approach. For TOBY, for instance, we were not able to represent parents whose babies were cooled for the full 72 hours, but we were able to account for parental experiences of difficult term deliveries, rapid decision-making and transfer, which are key features of this trial. The ability to contrast these experiences to those involved in ExPN, for which babies were extremely preterm and the intervention was so minor as to go largely unnoticed by the parents involved, meant that we were able to demonstrate the range of experiences involved across trials. This range became a particular strength when we were able to demonstrate the commonality of views such as the trial ‘might help, won’t harm’ and reactions to the results of the trial, whether the trial and the intervention were major or minor parts of the parental experiences. It is important that trial teams and clinicians understand both the diversity and the commonality that exists in this population if they are to make provision for their care and their interests within their practice. If particular dimensions of these experiences require further explication then studies of single trials, or comparative studies of two trials, with larger numbers of interviewees, would be appropriate.
Our exclusive focus on bereaved parents was necessary to achieve our aim of gaining insights into parental experiences of bereavement in the context of trial participation. Had BRACELET been designed as a comparative study, the number of bereaved parents who might have been included would have been halved and this would have limited its potential to offer insights into their particular experiences. Without an internal comparison of bereaved and non-bereaved parents it is clearly inappropriate, however, to attribute some of the views, such as valuing acknowledgment of a contribution to a trial or interest in the results, exclusively to bereaved parents, as these views may well be held by parents of survivors too. It is for this reason that we strongly recommend that future research invites both parents of surviving babies and bereaved parents of babies enrolled in a trial to participate and to contribute their views.
We have been very clear throughout this report that there is bias in our sample. This is not the socioeducational bias that often affects studies but a skew that has two main origins: (1) the restrictions we encountered in accessing parents meant that those who were invited to participate in BRACELET were more likely to be living in the same home, and so still be in the original couple, and (2) the parents had to be prepared to engage with a difficult topic, to invite an interviewer into their home and to discuss experiences of an intensely personal nature. Some had to steel themselves for this experience, and others welcomed the opportunity with enthusiasm. It is likely that there is an inter-relationship between these two factors of stability and preparedness to talk for some parents, although it is conceivable too that parents who have little access to support and few opportunities for reminiscing and reflection would also find participation in a study such as BRACELET to be attractive.
The methodological component
There are limits, even in a substantial programme of work such as BRACELET, to the ground that can be covered, and the study has thrown up more methodological questions than it could answer. We have been able to highlight difficulties with recruitment of a hard-to-reach population, and offered some ways forward, but the strategies that we developed were cumbersome and labour intensive, and further work needs to be carried out in order to find the means to recruit more efficiently and effectively.
The inclusion of multiple options for participation has in part addressed the issue of disinclination to be interviewed referred to above and in a study in which every testimony is important this strategy offered an important opportunity for data salvage. We have been able to demonstrate the value of data that might be achieved with this option in such a sensitive setting. The variety of options also provided an important reassurance, as they suggested that the same themes were present in data collected by interview and from the questionnaires from parents who did not wish to be interviewed. But the numbers were small and the data therefore largely contributed to the methodological component of the study rather than to the main data set.
Recommendations for research
Like clinical studies, a qualitative study usually represents only one step forward, with clear boundaries around the nature of the evidence produced. BRACELET was designed to be a springboard for further research rather than providing answers in its own right, and it does indeed provide the stimulus for the next wave of research. The study has highlighted a number of opportunities for engagement with parents, as well as difficulties that might affect future research, both of which might be addressed in methodological studies.
Further research is needed on bereavement and participation in trials
More research is needed into the experiences of bereavement subsequent to trial enrolment, with studies of any bereavement strategies that trials might introduce being the focus of research and reflection. This should include:
-
Exploration of contact processes such as newsletters, whether common to all participants or developed specifically for bereaved parents, online resources and any other strategies that might be developed. If such research runs alongside a trial and explores views of different forms of communications as they are used, it is important to be mindful of the difficulties that might be encountered in recruitment closer to the time of bereavement. In such a study the strategies used in BRACELET of multiple options for participation may be particularly important and we would recommend that close attention be paid to the sensitivities of the setting.
-
Exploration of whether parents and triallists in PICU trials face the same issues identified in BRACELET. For example, death comes about in different ways in this setting – through accident, illness and planned surgical interventions, and cancer chemotherapies. How might these different conditions shape responses to bereavement in RCTs and what provision might be needed?
-
Many of the issues that have been raised by the BRACELET study also apply in trials in adults (e.g. critical care, cardiac care, trauma, oncology or dementia) and warrant further investigation.
Comparative research should become the new standard approach to research in this field
To achieve a more complete appreciation of views and experiences of trial participation researchers should seek to represent views of samples of all parents, bereaved and not, so their perspectives on research can be understood and compared.
-
It is most important that careful studies of feedback of trial results are carried out, in ongoing trials as this strategy is being used. Examples are needed of how individual trials manage the process of feedback, with accompanying explorations of how results are received and understood by parents of survivors and bereaved parents. Trial findings are highly specific; as we have shown, some show no effect, some show increased survival, and some are stopped early because of specific concerns, such as increased mortality. Careful study of multiple trials is therefore important if we are to gain an understanding of this complex element of trial conduct. This will involve multiple studies of single trials if the richness of data and the depth of understanding that can be offered by qualitative methods are exploited to the full. If this is achieved a body of research will accumulate which will allow comparison of practice across trials and our understanding of feedback processes will gain in sophistication. Trial teams will be better able to serve the variety of needs and interests highlighted here.
-
For parents of multiples (twins or higher order births), BRACELET has highlighted a number of different possible configurations of the involvement in neonatal trials, all of which are complicated for parents and potentially for trial teams and clinicians. In such situations one or more babies may be recruited into a trial, and one or more babies may die. There are important questions to be answered about parental experiences of parenting multiples in trials, which are highly relevant to any trials that include substantial numbers of preterm babies. Multiples and preterm babies are at high risk of death and of survival with disabilities. With the potential for allocation to different arms of a trial (if that is the chosen allocation strategy for a trial), and different outcomes, parental experiences are potentially complicated and need to be understood through careful study if trial teams are to fairly respond to this population.
Methodological and developmental research
-
Developmental research would allow for exploration of the means of including a wider range of parents in future research. Such a study could explore the potential for communicating to parents the opportunities for research participation via publicity and the creation of specialist websites. It might also consider some of the more challenging issues that touch upon data protection and research governance with triallists and REC members, and take further research on alternative methods for recruitment from trial populations.
-
Methodological research is needed to ensure that we have the tools to explore with parents the topics for which they have expertise to offer but which might be challenging as the information is complex or the focus is sensitive. It is important to promote sufficient understanding of the issues involved in clinical trials in order to seek parental views of those issues. A successful example is our own research that carefully explored parents’ views of Zelen’s approach56 to prerandomisation consent. Such research is difficult, and determining the means to carry out this type of exploration of parents’ views is necessary if we are to truly engage with parents as partners in research and to seek their particular expertise to inform trial practice.
Conclusions
The findings from the BRACELET study very clearly indicate that bereavement subsequent to trial participation is a complex and multilayered topic, which elicits a variety of responses from practitioners and parents. It is a topic that was largely unexplored and so reactions and responses were mainly unidentified. Provision for bereavement in this context is therefore, not surprisingly, in its infancy, and, although we have identified examples of good practice, there has been little public dialogue about policy and practice.
We have shown that bereaved parents can be willing participants in research about their experiences. They were keen to make their voices heard and not to be marginalised. The availability of the data reported here means that excluding bereaved parents from research into parental experiences of trial participation can no longer be justified. Exclusion does not treat parents equitably and introduces a skew into research which would not be tolerated in other settings. The questions that need to be asked are undoubtedly difficult but this does not justify not asking them. Trial-related research that takes up the ethical challenge of sensitively including bereaved parents should now be considered essential and parents should be provided with the opportunity and forum to give their views if they wish to do so. RECs and researchers should gain confidence about permitting and conducting this sort of research, but caution is undoubtedly necessary and care does need to be taken at all stages of the research.
The BRACELET study throws up questions not just about bereavement in this context but questions about research relationships and some of the responsibilities that might fall upon trial teams and their clinical collaborators. We have found that parents enter into a contract with research that is initially very specific, with information-giving and the formal signing of the consent form, but later, on bereavement, the nature and terms of that contract can become less clearly delineated. This is perhaps not surprising, as the experience of bereavement plays out away from the view of those with responsibilities for trials. We have a responsibility as triallists, clinicians, and quantitative and qualitative methodologists to improve our understanding of practice and its impact upon those involved, and to develop the means to meet the challenge of addressing complex research questions. Methodological research is crucial in this regard, as without it these complex aspects of trial participation cannot be addressed.
Acknowledgements
We greatly appreciate the help of all survey respondents for Phase I, and all of the bereaved parents, the clinicians and the trial team members who gave their time to be interviewed and contribute their different perspectives for Phase II. The parents were particularly generous in their willingness to share their stories and their engagement with the focus of the study. For reasons of confidentiality we are unable to acknowledge their input by name but we would like to emphasise the central role that they have played in this research.
We would also like to thank:
-
The BRACELET study Advisory Group (Gillian Colville, Richard Cooke, Bobbie Farsides, Merryl Harvey, Andy Leslie, Fiona Lockett, Ursula Bowler) for their comments and support. Fiona Lockett has been a particular source of advice and generously took on the role of providing post-interview telephone support should it be necessary.
-
For Phase I, Roger Parslow, PICANet (for his advice with constructing the PICU sampling frame); the MCRN, in particular Vanessa Poustie and William van’t Hoff; the MCRN LRN Managers for help with surveys; Sara Lewis and Maggie Redshaw, NPEU, and the late Dominique Acolet for assistance with constructing the NICU sampling frame; Ursula Bowler, Brenda Strohm, Mary Logan and Maggie Redshaw, NPEU – for helpful comments on drafts of the NICU/PICU and Trial Team questionnaires; Kevin Morris, Reinout Mildner and Nick Embleton for assisting with the pilot survey and giving helpful comments on the survey questionnaire; and Elizabeth Allen, London School of Hygiene and Tropical Medicine (LSHTM), for statistical support.
-
For Phase II, a number of people have helped in a variety of ways, for example facilitating access, giving permission for access to trials, providing documentation, etc.: Denis Azzopardi, Helen Budge, Wendy Cheadle, Richard Cooke, David Edwards, Nick Embleton, Barbara Farrell, David Field, Angela Garrett, Ann Kennedy, Jenny Kurinczuk, Ian Laing, Gill Mitchell, Neena Modi, Tarn Nozedar, Bernd Reichart, Lynne Roberts, Clare Shakeshaft, Anne Smith, Ben Stenson, Brenda Strohm, Nim Subhdar, David Sweet, Maw Tan, William Tarnow-Mordi, Stephen Wardle, Mike Weindling and Suzanne Williams.
-
Staff at the R&D Office at the Liverpool Women’s Hospital facilitated and supported the study through research governance processes, as well as facilitating recruitment to the study: namely Mark Turner, Gillian Vernon, Louise Harding and Andy Burke.
-
Several individuals in key organisations have facilitated the study in important ways: Sabah Attar, MCRN Portfolio Manager, UK MCRN; MCRN Consumer Involvement Steering Group, INVOLVE, Farrah Pradhan from BLISS, and Erica Stewart from SANDS, for facilitating the development of strategy 2B in Phase II; and the charities and interested organisation that placed information about the BRACELET study on their web pages and in their newsletters – these include BLISS, Child Bereavement Trust, SANDS, SANDS Northern Ireland and multiple SANDS local groups, Simpsons Special Care Babies, TAMBA, Tiny Lives, and Tommy’s, the baby charity.
-
For part of the study CS was accommodated at the Centre for Family Research, Cambridge, and benefited from the support and engagement of colleagues, especially Abby Scott, Helen Statham and Susan Golumbok.
-
Sarah Moncrieff and Barry Cooper (Motivated Design) for BRACELET study logo and website, respectively.
-
Lesley Dodd, Stephen Lemon, Simon Bevan from the National Institute of Health Research Health Technology Assessment programme (NIHR-HTA).
-
For invaluable assistance with preparing this monograph: Joanna Sturgess and Eleanor Morris (LSHTM).
Contributions of authors
Claire Snowdon conceived the study and led on its design, analysis and interpretation of the data, data collection for Phase II, and drafting and revising the report.
Diana Elbourne was involved in the conception and design of the study, and the analysis and interpretation of the data.
Peter Brocklehurst, Robert Tasker and Martin Ward Platt were involved in the conception and design of the study, and interpretation of the data.
Sheila Harvey managed Phase I of the study, and led on the data collection and the analyses.
All authors were involved in drafting and revising the report and approved the version submitted for publication.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
Publications
Snowdon C, Harvey SE, Brocklehurst P, Tasker RC, Ward Platt MP, Allen E, Elbourne, D. BRACELET study: surveys of mortality in UK neonatal and paediatric intensive care trials. Trials 2010;11:65.
Elbourne DR, Snowdon C, Harvey S, Ward Platt M, Tasker R, Brocklehurst P. Neonatal trials in the UK: additional information from the BRACELET study [published online ahead of print 16 February 2011]. Arch Dis Child. URL: http://adc.bmj.com/content/96/6/500/reply#content-block (accessed 24 July 2013).
References
- Turner S, Longworth A, Nunn AJ, Choonara I. Unlicensed and off label drug use in paediatric wards: prospective study. BMJ 1998;316:343-5. http://dx.doi.org/10.1136/bmj.316.7128.343.
- Conroy S, McIntyre J, Choonara I. Unlicensed and off label drug use in neonates. Arch Dis Child 1999;80:F142-5. http://dx.doi.org/10.1136/fn.80.2.F142.
- Caldwell PH, Murphy SB, Butow PN, Craig JC. Clinical trials in children. Lancet 2004;364:803-11. http://dx.doi.org/10.1016/S0140-6736(04)16942-0.
- Snowdon C, Harvey SE, Brocklehurst P, Tasker RC, Platt MPW, Allen E, et al. The BRACELET Study: surveys of mortality in UK neonatal and paediatric intensive care trials. Trials 2010;11. http://dx.doi.org/10.1186/1745-6215-11-65.
- Harvey S, Snowdon C, Elbourne D. Effectiveness of bereavement interventions in neonatal intensive care: a review of the evidence. Semin Fetal Neonatal Med 2008;13:341-56. http://dx.doi.org/10.1016/j.siny.2008.03.011.
- Obeidat HM, Bond EA, Callister LC. The parental experience of having an infant in the newborn intensive care unit. J Perinatal Educ 2009;13:23-9. http://dx.doi.org/10.1624/105812409X461199.
- Nelson LP, Gold JI. Posttraumatic stress disorder in children and their parents following admission to the pediatric intensive care unit: a review. Pediatr Crit Care Med 2012;13:338-47. http://dx.doi.org/10.1097/PCC.0b013e3182196a8f.
- Miles MS, Funk SG, Kasper MA. The neonatal intensive care unit environment: sources of stress for parents. AACN Clin Crit Care Nurs 1991;2:346-54.
- Peebles-Kleiger MJ. Pediatric and neonatal intensive care hospitalization as traumatic stressor: implications for intervention. Bull Menninger Clin 2000;64:257-80.
- Dudek-Shriber L. Parent stress in the neonatal intensive care unit and the influence of parent and infant characteristics. Am J Occup Ther 2004;58:509-20. http://dx.doi.org/10.5014/ajot.58.5.509.
- Carter JD, Mulder RT, Bartram AF, Darlow BA. Infants in a neonatal intensive care unit: parental response. Arch Dis Child 2005;90:F109-13. http://dx.doi.org/10.1136/adc.2003.031641.
- Heermann JA, Wilson ME, Wilhelm PA. Mothers in the NICU: outsider to partner. Pediatr Nurs 2005;31:176-81.
- Miles MS, Carter MC, Riddle I, Hennessey J, Eberly TW. The pediatric intensive care unit environment as a source of stress for parents. Mater Child Nurs J 1989;18:199-206.
- Huckabay LM, Tilem-Kessler D. Patterns of parental stress in PICU emergency admission. Dimens Crit Care Nurs 1999;18:36-42. http://dx.doi.org/10.1097/00003465-199903000-00009.
- Board R, Ryan-Wenger N. State of the science on parental stress and family functioning in pediatric intensive care units. Am J Crit Care 2000;9:106-22.
- Colville G, Pierce C. Patterns of post-traumatic stress symptoms in families after paediatric intensive care. Intensive Care Med 2012;38:1523-31. http://dx.doi.org/10.1007/s00134-012-2612-2.
- Jennings P. Should paediatric units have bereavement support posts?. Arch Dis Child 2002;87:40-2. http://dx.doi.org/10.1136/adc.87.1.40.
- Walsh WF, McCullough KL, White RD. Room for improvement: nurses’ perceptions of providing care in a single room newborn intensive care setting. Adv Neonatal Care 2006;6:261-70. http://dx.doi.org/10.1016/j.adnc.2006.06.002.
- van Zuuren FJ, van Manen E. Moral dilemmas in neonatology as experienced by health care practitioners: a qualitative approach. Med Health Care Philos 2006;9:339-47. http://dx.doi.org/10.1007/s11019-005-5641-6.
- McClendon H, Buckner EB. Distressing situations in the intensive care unit: a descriptive study of nurses’ responses. Dimens Criti Care Nurs 2007;26:199-206. http://dx.doi.org/10.1097/01.DCC.0000286824.11861.74.
- Braithwaite M. Nurse burnout and stress in the NICU. Adv Neonatal Care 2008;8:343-7. http://dx.doi.org/10.1097/01.ANC.0000342767.17606.d1.
- Fujimaru C, Okamura H, Kawasaki M, Kakuma T, Yoshii C, Matsuishi T. Self-perceived work-related stress and its relation to salivary IgA, cortisol and 3-methoxy-4-hydroxyphenyl glycol levels among neonatal intensive care nurses. J Int Soc Invest Stress 2012;28:171-4. http://dx.doi.org/10.1002/smi.1414.
- Baverstock A, Finlay F. What can we learn from the experiences of consultants around the time of a child’s death?. Child Care Health Develop 2008;34:732-9. http://dx.doi.org/10.1111/j.1365-2214.2008.00875.x.
- Jellinek MS, Catlin EA, Todres ID, Cassem EH. Facing tragic decisions with parents in the neonatal intensive care unit: clinical perspectives. Pediatrics 1992;89:119-22.
- McHaffie HE, Laing IA, Lloyd DJ. Follow up care of bereaved parents after treatment withdrawal from newborns. Arch Dis Child 2001;84:F125-8. http://dx.doi.org/10.1136/fn.84.2.F125.
- McHaffie HE, Lyon AJ, Hume R. Deciding on treatment limitation for neonates: the parents’ perspective. Eur J Pediatr 2001;160:339-44. http://dx.doi.org/10.1007/PL00008444.
- Støre Brinchmann B, Førde R, Nortvedt P. What Matters to the Parents? A qualitative study of parents’ experiences with life-and-death decisions concerning their premature infants. Nurs Ethics 2002;9:388-404.
- Pector EA. Views of bereaved multiple-birth parents on life support decisions, the dying process, and discussions surrounding death. J Perinatol 2004;24:4-10. http://dx.doi.org/10.1038/sj.jp.7211001.
- Zawistowski CA, DeVita MA. A descriptive study of children dying in the pediatric intensive care unit after withdrawal of life-sustaining treatment. Pediatr Crit Care Med 2004;5:216-23. http://dx.doi.org/10.1097/01.PCC.0000123547.28099.44.
- Sharman M, Meert KL, Sarnaik AP. What influences parents’ decisions to limit or withdraw life support?. Pediatr Crit Care Med 2005;6:513-18. http://dx.doi.org/10.1097/01.PCC.0000170616.28175.D9.
- McHaffie HE, Lyon AJ, Fowlie PW. Lingering death after treatment withdrawal in the neonatal intensive care unit. Arch Dis Child 2001;85:F8-12. http://dx.doi.org/10.1136/fn.85.1.F8.
- Eden LM, Callister LC. parent involvement in end-of-life care and decision making in the newborn intensive care unit: an integrative review. J Perinatal Edu 2010;19:29-3. http://dx.doi.org/10.1624/105812410X481546.
- Moro TT, Kavanaugh K, Savage TA, Reyes MR, Kimura RE, Bhat R. Parent decision making for life support for extremely premature infants: from the prenatal through end-of-life period. J Perinatal Neonatal Nurs 2011;25:52-60.
- McHaffie HE, Fowlie PW, Hume R, Laing IA, Lloyd DJ, Lyon AJ. Consent to autopsy for neonates. Arch Dis Child 2001;85:F4-7. http://dx.doi.org/10.1136/fn.85.1.F4.
- Madrigal VN, Carroll KW, Hexem KR, Faerber JA, Morrison WE, Feudtner C. Parental decision-making preferences in the pediatric intensive care unit. Crit Care Med 2012;40:2876-82. http://dx.doi.org/10.1097/CCM.0b013e31825b9151.
- Brosig CL, Pierucci RL, Kupst MJ, Leuthner SR. Infant end-of-life care: the parents’ perspective. J Perinatol 2007;27:510-16. http://dx.doi.org/10.1038/sj.jp.7211755.
- Macdonald ME, Liben S, Carnevale FA, Rennick JE, Wolf SL, Meloche D, et al. Parental perspectives on hospital staff members’ acts of kindness and commemoration after a child’s death. Pediatrics 2005;116:884-90. http://dx.doi.org/10.1542/peds.2004-1980.
- Meert KL, Eggly S, Pollack M, Anand KJ, Zimmerman J, Carcillo J, et al. Parents’ perspectives regarding a physician-parent conference after their child’s death in the pediatric intensive care unit. J Pediatr 2007;151:50-5. http://dx.doi.org/10.1016/j.jpeds.2007.01.050.
- Jacobson SF. Stresses and coping strategies of neonatal intensive care unit nurses. Res Nurs Health 1983;6:33-40. http://dx.doi.org/10.1002/nur.4770060107.
- Oates RK, Oates P. Stress and mental health in neonatal intensive care units. Arch Dis Child 1995;72:F107-10. http://dx.doi.org/10.1136/fn.72.2.F107.
- Fischer JE, Calame A, Dettling AC, Zeier H, Fanconi S. Experience and endocrine stress responses in neonatal and pediatric critical care nurses and physicians. Crit Care Med 2000;28:3281-8. http://dx.doi.org/10.1097/00003246-200009000-00027.
- Bratt MM, Broome M, Kelber S, Lostocco L. Influence of stress and nursing leadership on job satisfaction of pediatric intensive care unit nurses. Am J Crit Care 2000;9:307-17.
- Jellinek MS, Todres ID, Catlin EA, Cassem EH, Salzman A. Pediatric intensive care training: confronting the dark side. Crit Care Med 1993;21:775-9. http://dx.doi.org/10.1097/00003246-199305000-00023.
- Solomon MZ, Sellers DE, Heller KS, Dokken DL, Levetown M, Rushton C, et al. New and lingering controversies in pediatric end-of-life care. Pediatrics 2005;116:872-83. http://dx.doi.org/10.1542/peds.2004-0905.
- Okah FA, Wolff DM, Boos VD, Haney BM, Oshodi AA. Perceptions of a strategy to prevent and relieve care provider distress in the neonatal intensive care unit. Am J Perinatol 2012;29:687-92. http://dx.doi.org/10.1055/s-0032-1314889.
- Lee KJ, Dupree CY. Staff experiences with end-of-life care in the pediatric intensive care unit. J Palliat Med 2008;11:986-90. http://dx.doi.org/10.1089/jpm.2007.0283.
- McDonald K, Rubarth LB, Miers LJ. Job satisfaction of neonatal intensive care nurses. Adv Neonatal Care 2012;12:E1-8. http://dx.doi.org/10.1097/ANC.0b013e3182624eb1.
- Shilling V, Young B. How do parents experience being asked to enter a child in a randomised controlled trial?. BMC Med Ethics 2009;10. http://dx.doi.org/10.1186/1472-6939-10-1.
- Mason SA, Allmark PJ. Obtaining informed consent to neonatal randomised controlled trials: interviews with parents and clinicians in the Euricon study. Lancet 2000;356:2045-51. http://dx.doi.org/10.1016/S0140-6736(00)03401-2.
- Garcia J, Elbourne D, Snowdon C. Equipoise: a case study of the views of clinicians involved in two neonatal trials. Clinical Trials 2004;1:170-8. http://dx.doi.org/10.1191/1740774504cn020xx.
- Snowdon C, Elbourne D, Garcia J. ‘It was a snap decision’: parental and professional perspectives on the speed of decisions about participation in perinatal randomised controlled trials. Soc Sci Med 2006;62:2279-90. http://dx.doi.org/10.1016/j.socscimed.2005.10.008.
- Hulst JM, Peters JW, van den Bos A, Joosten KFM, van Goudoever JB. Illness severity and parental permission for clinical research in a pediatric ICU population. Intensive Care Med 2005;31:880-4. http://dx.doi.org/10.1007/s00134-005-2647-8.
- Raymond TT, Carroll TG, Sales G, Morris MC. Effectiveness of the informed consent process for a pediatric resuscitation trial. Pediatrics 2010;125:e866-75. http://dx.doi.org/10.1542/peds.2009-2427.
- Snowdon C, Garcia J, Elbourne D. Making sense of randomization; responses of parents of critically ill babies to random allocation of treatment in a clinical trial. Soc Sci Med 1997;45:1337-55. http://dx.doi.org/10.1016/S0277-9536(97)00063-4.
- Zupancic JA, Gillie P, Streiner DL, Watts JL, Schmidt B. Determinants of parental authorization for involvement of newborn infants in clinical trials. Pediatrics 1997;99. http://dx.doi.org/10.1542/peds.99.1.e6.
- Snowdon C, Garcia J, Elbourne D. Reactions of participants to the results of a randomised controlled trial: exploratory study. BMJ 1998;317:21-6. http://dx.doi.org/10.1136/bmj.317.7150.21.
- Snowdon C, Elbourne D, Garcia J. Zelen randomization: attitudes of parents participating in a neonatal clinical trial. Control Clin Trials 1999;20:149-71.
- Burgess E, Singhal N, Amin H, McMillan DD, Devrome H. Consent for clinical research in the neonatal intensive care unit: a retrospective survey and a prospective study. Arch Dis Child 2003;88:F280-5. http://dx.doi.org/10.1136/fn.88.4.F280.
- Ballard HO, Shook LA, Desai NS, Anand KJ. Neonatal research and the validity of informed consent obtained in the perinatal period. J Perinatol 2004;24:409-15. http://dx.doi.org/10.1038/sj.jp.7211142.
- Snowdon CE, Elbourne D, Garcia J. Perinatal pathology in the context of a clinical trial: attitudes of bereaved parents. Arch Disease Child 2004;89:F208-11. http://dx.doi.org/10.1136/adc.2003.041392.
- Stenson BJ, Becher JC, McIntosh N. Neonatal research: the parental perspective. Arch Dis Child 2004;89:F321-3. http://dx.doi.org/10.1136/adc.2002.021931.
- Hoehn KS, Wernovsky G, Rychik J, Gaynor JW, Spray TL, Feudtner C, et al. What factors are important to parents making decisions about neonatal research?. Arch Dis Child 2005;90:F267-9. http://dx.doi.org/10.1136/adc.2004.065078.
- Morley C, Lau R, Davis P, Morse C. What do parents think about enrolling their premature babies in several research studies?. Arch Dis Child 2005;3:F225-2. http://dx.doi.org/10.1136/adc.2004.061986.
- Allmark P, Mason S. Improving the quality of consent to randomised controlled trials by using continuous consent and clinician training in the consent process. J Med Ethics 2006;32:439-43. http://dx.doi.org/10.1136/jme.2005.013722.
- Snowdon CE, Elbourne D. Declining enrolment in a clinical trial and injurious misconceptions: is there a flipside to the therapeutic misconception?. Clin Ethics 2007;2:193-200. http://dx.doi.org/10.1258/147775007783560193.
- Ward FR. Parents’ views of involvement in concurrent research with their neonates. J Empirical Res Human Res Ethics 2010;5:47-55. http://dx.doi.org/10.1525/jer.2010.5.2.47.
- Shilling V, Williamson PR, Hickey H, Sowden E, Beresford MW, Smyth RL, et al. Communication about children’s clinical trials as observed and experienced: qualitative study of parents and practitioners. PLOS ONE 2011;6. http://dx.doi.org/10.1371/journal.pone.0021604.
- Shilling V, Williamson PR, Hickey H, Sowden E, Smyth RL, Young B. Processes in recruitment to randomised controlled trials of medicines for children (RECRUIT): a qualitative study. Health Technol Assess 2011;15:1-116. http://dx.doi.org/10.3310/hta15150.
- Allmark P, Mason S, Gill AB, Megone C. Obtaining consent for neonatal research. Arch Dis Child 2003;88:F166-7. http://dx.doi.org/10.1136/fn.88.3.F166.
- Hoehn KS, Nathan A, White LE, Ittenbach RF, Reynolds WW, Gaynor JW, et al. Parental perception of time and decision-making in neonatal research. J Perinatol 2009;29:508-11. http://dx.doi.org/10.1038/jp.2009.5.
- Cartwright K, Mahoney L, Ayers S, Rabe H. Parents’ perceptions of their infants’ participation in randomized controlled trials. J Obstetric Gynecologic Neonatal Nurs 2011;40:555-65. http://dx.doi.org/10.1111/j.1552-6909.2011.01276.x.
- Jollye S. An exploratory study to determine how parents decide whether to enrol their infants into neonatal clinical trials. J Neonatal Nurs 2009;15:18-24. http://dx.doi.org/10.1016/j.jnn.2008.07.012.
- Braunholtz DA. A note on Zelen randomization: Attitudes of parents participating in a neonatal clinical trial. Controlled Clin Trials 1999;20:569-71.
- Snowdon CE, Elbourne DR, Garcia J. Perinatal pathology in the context of a clinical trial: attitudes of neonatologists and pathologists. Arch Dis Child 2004;89:F204-7. http://dx.doi.org/10.1136/adc.2002.012732.
- Snowdon CE, Elbourne DR, Garcia J. Perinatal pathology in the context of a clinical trial: a review of the literature. Arch Dis Child 2004;89:F200-3. http://dx.doi.org/10.1136/adc.2002.012740.
- Snowdon CE, Elbourne D, Garcia J, Lavender TE, Alfirevic Z. Demystifying Qualitative Research. London: Quay Books; 2004.
- Redshaw M, Hamilton K. A Survey of Current Neonatal Unit Organisation and Policy. Oxford: National Perinatal Epidemiology Unit, University of Oxford; 2005.
- Directory of Critical Care. Loughborough, UK; 2006.
- British Association of Perinatal Medicine (BAPM) 2012. www.bapm.org/ (accessed 18 August 2012).
- National Perinatal Epidemiology Unit n.d. www.npeu.ox.ac.uk/ (accessed 18 August 2008).
- Acolet D, Jelphs K, Davidson D, Peck E, Clemens F, Houston R, et al. The BLISS cluster randomised controlled trial of the effect of ‘active dissemination of information’ on standards of care for premature babies in England (BEADI) study protocol [ISRCTN89683698]. Implement Sci 2007;2. http://dx.doi.org/10.1186/1748-5908-2-33.
- Standards for Hospitals Providing Neonatal Intensive Care. 2nd edn. London: BAPM; 2001.
- Paediatric Intensive Care Society . Standards of Care 2001. http://careers.bmj.com/careers/advice/view-article.html?id = 2645 (accessed 18 August 2008).
- Paediatric Intensive Care Audit Network (PICANet) 2012. www.picanet.org.uk/ (accessed 18 August 2008).
- McColl E, Jacoby A, Thomas L, McColl E, Jacoby A, Thomas L, et al. Design and use of questionnaires: a review of best practice applicable to surveys of health service staff and patients. Health Technol Assess 2001;5.
- Sammons HM, Gray C, Hudson H, Cherrill J, Choonara I. Safety in paediatric clinical trials: a 7-year review. Acta Paediatr 2008;97:474-7. http://dx.doi.org/10.1111/j.1651-2227.2008.00676.x.
- Kumar P, Angst DB, Taxy J, Mangurten HH. Neonatal autopsies: a 10-year experience. Archives of Pediatr Adolesc Med 2000;154:38-42.
- Brodlie M, Laing IA, Keeling JW, McKenzie KJ. Ten years of neonatal autopsies in tertiary referral centre: retrospective study. BMJ 2002;324:761-3. http://dx.doi.org/10.1136/bmj.324.7340.761.
- Hickey L, Murphy A, Devaney D, Gillan J, Clarke T. The value of neonatal autopsy. Neonatology 2012;101:68-73. http://dx.doi.org/10.1159/000329094.
- Paediatric Intensive Care Audit Network . UK Paediatric Intensive Care Audit Network National Report 2007–2009 2010.
- Vlasselaers D, Milants I, Desmet L, Wouters PJ, Vanhorebeek I, van den Heuvel I, et al. Intensive insulin therapy for patients in paediatric intensive care: a prospective, randomised controlled study. Lancet 2009;373:547-56. http://dx.doi.org/10.1016/S0140-6736(09)60044-1.
- Agus MSD, Steil GM, Wypij D, Costello JM, Laussen PC, Langer M, et al. Tight glycemic control versus standard care after pediatric cardiac surgery. N Engl J Med 2012;367:1208-19. http://dx.doi.org/10.1056/NEJMoa1206044.
- Kavanaugh K. Parents’ experience surrounding the death of a newborn whose birth is at the margin of viability. J Obstetric Gynecologic Neonatal Nurs 1997;26:43-51. http://dx.doi.org/10.1111/j.1552-6909.1997.tb01506.x.
- Raftery JP, Fairbank E, Douet L, Dent L, Price A, Milne R, et al. Registration of noncommercial randomised clinical trials: the feasibility of using trial registries to monitor the number of trials. Trials 2012;13. http://dx.doi.org/10.1186/1745-6215-13-140.
- The INIS Study Collaborative Group . The INIS Study. International Neonatal Immunotherapy Study: non-specific intravenous immunoglobulin therapy for suspected or proven neonatal sepsis: an international, placebo controlled, multicentre randomised trial. BMC Pregnancy Childbirth 2008;8.
- The INIS Study Collaborative Group . Treatment of neonatal sepsis with intravenous immune globulin. N Engl J Med 2011;365:1201-11.
- Azzopardi D, Robertson NJ, Cowan FM, Rutherford MA, Rampling M, Edwards AD. Pilot Study of Treatment With Whole Body Hypothermia for Neonatal Encephalopathy. Pediatrics 2000;106:684-94. http://dx.doi.org/10.1542/peds.106.4.684.
- Azzopardi D, Brocklehurst P, Edwards D, Halliday H, Levene M, Thoresen M, et al. The TOBY Study. Whole body hypothermia for the treatment of perinatal asphyxial encephalopathy: a randomised controlled trial. BMC Pediatr 2008;8. http://dx.doi.org/10.1186/1471-2431-8-17.
- Azzopardi DV, Strohm B, Edwards AD, Dyet L, Halliday HL, Juszczak E, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med 2009;361:1349-58. http://dx.doi.org/10.1056/NEJMoa0900854.
- Carr R, Brocklehurst P, Dore CJ, Modi N. Granulocyte-macrophage colony stimulating factor administered as prophylaxis for reduction of sepsis in extremely preterm, small for gestational age neonates (the PROGRAMS trial): a single-blind, multicentre, randomised controlled trial. Lancet 2009;373:226-33. http://dx.doi.org/10.1016/S0140-6736(09)60071-4.
- Marlow N, Morris T, Brocklehurst P, Carr R, Cowan FM, Patel N, et al. A randomised trial of granulocyte–macrophage colony-stimulating factor for neonatal sepsis: outcomes at 2 years. Arch Dis Child Fetal Neonatal Ed 2013;98:F46-53. http://dx.doi.org/10.1136/fetalneonatal-2011-301470.
- Tan M, Abernethy L, Cooke R. Improving head growth in preterm infants: a randomised controlled trial II: MRI and developmental outcomes in the first year. Arch Dis Child 2008;93:F342-6. http://dx.doi.org/10.1136/adc.2007.124255.
- Tan MJ, Cooke RW. Improving head growth in very preterm infants: a randomised controlled trial I: neonatal outcomes. Arch Dis Child 2008;93:F337-41. http://dx.doi.org/10.1136/adc.2007.124230.
- Askie LM, Brocklehurst P, Darlow BA, Finer N, Schmidt B, Tarnow-Mordi W. NeOProM: Neonatal Oxygenation Prospective Meta-analysis Collaboration study protocol. BMC Pediatr 2011;11.
- Stenson B, Brocklehurst P, Tarnow-Mordi W. UK BOOST II UK trial, Australian BOOST II UK Trial, New Zealand BOOST II UK trial . Increased 36-week survival with high oxygen saturation target in extremely preterm infants. N Engl J Med 2011;364:1680-2. http://dx.doi.org/10.1056/NEJMc1101319.
- Layder D. Sociological Practice: Linking Theory and Social Research. London: Sage; 1998.
- Bogner DA, Littig DB, Menz DW. Interviewing Experts (Research Methods Series). UK: Palgrave Macmillan; 2009.
- Pfadenhauer M, Bogner DA, Littig DB, Menz DW. Interviewing Experts (Research Methods Series). UK: Palgrave Macmillan; 2009.
- Lopez KA, Willis DG. Descriptive versus interpretive phenomenology: their contributions to nursing knowledge. Qual Health Res 2004;14:726-35. http://dx.doi.org/10.1177/1049732304263638.
- Koch T. Interpretive approaches in nursing research: the influence of Husserl and Heidegger. J Adv Nurs 1995;21:827-36. http://dx.doi.org/10.1046/j.1365-2648.1995.21050827.x.
- Friese S. Qualitative Data Analysis with ATLAS.ti. London: SAGE; 2012.
- Mays N, Pope C. Assessing quality in qualitative research. BMJ 2000;320:50-2. http://dx.doi.org/10.1136/bmj.320.7226.50.
- Shenton A. Strategies for ensuring trustworthiness in qualitative research projects. Edu Inform 2004;22:63-75.
- Corbin ASJ. Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. London: SAGE; 2008.
- Ponterotto J. Brief note on the origins, evolution, and meaning of the qualitative research concept ‘Thick Description’. Qual Rep 2006;11:538-49.
- Bosk C. All God’s Mistakes: Genetic Counseling in a Pediatric Hospital. Chicago, IL: University of Chicago Press; 1992.
- Atkinson P. Medical Talk and Medical Work. London: SAGE; 1995.
- Smith A, Goodwin D, Mort M, Pope C. Expertise in practice: an ethnographic study exploring acquisition and use of knowledge in anaesthesia. Br J Anaesthesia 2003;91:319-28. http://dx.doi.org/10.1093/bja/aeg180.
- Mesman J. Uncertainty in Medical Innovation: Experienced Pioneers in Neonatal Care. New York: Palgrave Macmillan; 2008.
- de Rond M. ‘Soldier, surgeon, photographer, spy: organizational ethnography beyond the comfort zone’. Strat Organ 2012;10:256-62.
- Pollock A. The rise and fall of the randomised controlled trial in surgery. Theoret Surg 1989;4:163-70.
- Ko CY, Sack J, Chang JT, Fink A. Reporting randomized, controlled trials: where quality of reporting may be improved. Dis Colon Rectum 2002;45:443-7. http://dx.doi.org/10.1007/s10350-004-6217-x.
- Wente MN, Seiler CM, Uhl W, Buchler MW. Perspectives of evidence-based surgery. Digestive Surg 2003;20:263-9. http://dx.doi.org/10.1159/000071183.
- Anyanwu AC, Treasure T. Surgical research revisited: clinical trials in the cardiothoracic surgical literature. Eur J Cardio-Thoracic Surg 2004;25:299-303. http://dx.doi.org/10.1016/j.ejcts.2003.12.004.
- Agha R, Cooper D, Muir G. The reporting quality of randomised controlled trials in surgery: a systematic review. Int J Surg (London, England) 2007;5:413-22. http://dx.doi.org/10.1016/j.ijsu.2007.06.002.
- Solomon MJ, Laxamana A, Devore L, McLeod RS. Randomized controlled trials in surgery. Surgery 1994;115:707-12.
- Cook JA. The challenges faced in the design, conduct and analysis of surgical randomised controlled trials. Trials 2009;10.
- McCulloch P, Taylor I, Sasako M, Lovett B, Griffin D. Randomised trials in surgery: problems and possible solutions. BMJ 2002;324:1448-51. http://dx.doi.org/10.1136/bmj.324.7351.1448.
- Millat B, Fingerhut A, Flamant Y, Hay JM, Fagniez PL, Farah A, et al. Survey of the impact of randomised clinical trials on surgical practice in France. French Associations for Research in Surgery (AURC and ACAPEM). Association Universitaire de Recherche en Chirurgie. Association des Chirurgiens de l’Assistance Publique pour l’Evaluation Medicale. Eur J Surg 1999;165:87-94.
- Martin RC, Polk HC, Jaques DP. Does additional surgical training increase participation in randomized controlled trials?. Am J Surg 2003;185:239-43. http://dx.doi.org/10.1016/S0002-9610(02)01369-7.
- Macrae D, Pappachan J, Grieve R, Parslow R, Nadel S, Schindler M, et al. Control of hyperglycaemia in paediatric intensive care (CHiP): study protocol. BMC Pediatr 2010;10. http://dx.doi.org/10.1186/1471-2431-10-5.
- Gluckman PD, Wyatt JS, Azzopardi D, Ballard R, Edwards AD, Ferriero DM, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 2005;365:663-70. http://dx.doi.org/10.1016/S0140-6736(05)17946-X.
- Silverman WA. Retrolental fibroplasia: a modern parable. New York: Grune & Stratton; 1980.
- Silverman WA. Personal Reflections on Lessons Learned from Randomized Trials Involving Newborn Infants, 1951 to 1967 2003. www.jameslindlibrary.org/essays/cautionary/silverman.html (accessed 19 January 2014).
- SUPPORT Study Group of the Eunice Kennedy Shriver NICHD Neonatal Research Network . Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med 2010;362:1959-69.
- Pocock S. Clinical Trials: A Practical Approach. Chichester: Wiley; 2006.
- Wright S, Duncombe P, Altman D. Assessment of blinding to treatment allocation in studies of a cannabis-based medicine (Sativex(R)) in people with multiple sclerosis: a new approach. Trials 2012;13.
- Field DJ, Davis C, Elbourne D, Grant A, Johnson A, Macrae D. UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation. Int J Gynecol Obstetrics 1996;55:314-15.
- Cordoba G, Schwartz L, Woloshin S, Bae H, Gøtzsche PC. Definition, reporting, and interpretation of composite outcomes in clinical trials: systematic review. BMJ 2010;341. http://dx.doi.org/10.1136/bmj.c3920.
- Timmermans S, Berg M. The Gold Standard: The Challenge of Evidence-Based Medicine and Standardization in Health Care. Philadelphia: Temple University Press; 2003.
- Snowdon C. Collaboration, Participation and Non-Participation: Decisions about Involvement in Randomised Controlled Trials for Clinicians and Parents in Two Neonatal Trials 2005.
- Ferguson PR. Testing a drug during labour: the experiences of women who participated in a clinical trial. J Reprod Infant Psychol 2000;18:117-31. http://dx.doi.org/10.1080/02646830050008369.
- Kenyon S, Dixon-Woods M, Jackson CJ, Windridge K, Pitchforth E. Participating in a trial in a critical situation: a qualitative study in pregnancy. Qual Saf Health Care 2006;15:98-101. http://dx.doi.org/10.1136/qshc.2005.015636.
- Kuivasaari-Pirinen P, Raatikainen K, Hippelainen M, Heinonen S. Adverse outcomes of IVF/ICSI pregnancies Vary depending on aetiology of infertility. ISRN Obstetrics Gynecol 2012. http://dx.doi.org/10.5402/2012/451915.
- van Oppenraaij RH, Jauniaux E, Christiansen OB, Horcajadas JA, Farquharson RG, Exalto N. Predicting adverse obstetric outcome after early pregnancy events and complications: a review. Human Reproduct Update 2009;15:409-21. http://dx.doi.org/10.1093/humupd/dmp009.
- Wood N, Gibson A, Marlow N, Costeloe K, Hennessy E, Wilkinson A. The EPICure Study: perinatal antecedents and correlates of disability in extremely preterm children. Arch Dis Child 2005;90:F134-40.
- Moore T, Hennessy EM, Myles J, Draper ES, Costeloe KL, Marlow N, et al. Neurological and developmental outcome in extremely preterm children born in England in 1995 and 2006: the EPICure studies. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e7961.
- Costeloe KL, Hennessy EM, Haider S, Stacey F, Marlow N, Draper ES. Short term outcomes after extreme preterm birth in England: comparison of two birth cohorts in 1995 and 2006 (the EPICure studies). BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e7976.
- Smith L, Draper ES, Manktelow BN, Pritchard C, Field DJ. Comparing regional infant death rates: the influence of preterm births < 24 weeks of gestation. Arch Dis Child 2013;98:F103-7. http://dx.doi.org/10.1136/fetalneonatal-2011-301359.
- Gavey J. Parental perceptions of neonatal care. J Neonatal Nurs 2007;13:199-206. http://dx.doi.org/10.1016/j.jnn.2007.06.001.
- Glogowska M, Roulstone S, Enderby P, Peters T, Campbell R. Who’s afraid of the randomised controlled trial? Parents’ views of an SLT research study. Int J Lang Commun Disord 2001;36:499-504.
- Lawton J, Fox A, Fox C, Kinmonth AL. Participating in the United Kingdom Prospective Diabetes Study (UKPDS): a qualitative study of patients’ experiences. Br J Gen Pract 2003;53:394-8.
- Henzlova MJ, Blackburn GH, Bradley EJ, Rogers WJ. Patient perception of a long-term clinical trial: experience using a close-out questionnaire in the Studies of Left Ventricular Dysfunction (SOLVD) Trial. SOLVD Close-out Working Group. Control Clin Trials 1994;15:284-93.
- Sofaer N, Thiessen C, Goold SD, Ballou J, Getz KA, Koski G, et al. Subjects’ views of obligations to ensure post-trial access to drugs, care and information: qualitative results from the Experiences of Participants in Clinical Trials (EPIC) study. J Med Ethics 2009;35:183-8. http://dx.doi.org/10.1136/jme.2008.024711.
- Dixon-Woods M, Jackson C, Windridge KC, Kenyon S. Receiving a summary of the results of a trial: qualitative study of participants’ views. BMJ 2006;332:206-10. http://dx.doi.org/10.1136/bmj.38675.677963.3A.
- Mancini J, Genre D, Dalenc F, Ferrero J-M, Kerbrat P, Martin A-L, et al. Participants’ uptake of clinical trial results: a randomised experiment. Br J Cancer 2010;102:1081-4. http://dx.doi.org/10.1038/sj.bjc.6605592.
- Woodward J. The lone twin: understanding twin bereavement and loss. Twin Res Human Genet 2002;5:249-51. http://dx.doi.org/10.1375/twin.5.3.249.
- Cooper C. Twins & Multiple Births: The Essential Parenting Handbook from Pregnancy to Adulthood. London: Random House; 2004.
- Einaudi MA, Le Coz P, Malzac P, Michel F, D’Ercole C, Gire C. Parental experience following perinatal death: exploring the issues to make progress. Eur J Obstetrics Gynecol Reproduct Biol 2010;151:143-8. http://dx.doi.org/10.1016/j.ejogrb.2010.04.003.
- Breeze ACG, Statham H, Hackett GA, Jessop FA, Lees CC. Attitudes to perinatal postmortem: parental views about research participation. J Med Ethics 2011;37:364-7. http://dx.doi.org/10.1136/jme.2010.038505.
- Caeymaex L, Jousselme C, Vasilescu C, Danan C, Falissard B, Bourrat MM, et al. Perceived role in end-of-life decision making in the NICU affects long-term parental grief response. Arch Dis Child 2013;98:F26-31. http://dx.doi.org/10.1136/archdischild-2011-301548.
- Allmark PJ, Boote J, Chambers E, Clarke, A, McDonnell A, Thompson A, et al. Ethical issues in the use of in-depth interviews: literature review and discussion. Res Ethics Rev 2009;5:48-54. http://dx.doi.org/10.1177/174701610900500203.
- Turkle S. Evocative Objects. Things We Think With. Cambridge, MA: MIT Press; 2011.
- Kavanaugh K, Ayres L. ‘Not as bad as it could have been’: assessing and mitigating harm during research interviews on sensitive topics. Res Nurs Health 1998;21:91-7. http://dx.doi.org/10.1002/(SICI)1098-240X(199802)21:1%3C91::AID-NUR10%3E3.3.CO;2-V.
- Dyregrov K. Bereaved parents’ experience of research participation. Soc Sci Med 2004;58:391-400. http://dx.doi.org/10.1016/S0277-9536(03)00205-3.
- Hynson JL, Aroni R, Bauld C, Sawyer SM. Research with bereaved parents: a question of how not why. Palliat Med 2006;20:805-11. http://dx.doi.org/10.1177/0269216306072349.
- Michelson KN, Koogler TK, Skipton K, Sullivan C, Frader J. Parents’ reactions to participating in interviews about end-of-life decision making. J Palliat Med 2006;9:1329-38. http://dx.doi.org/10.1089/jpm.2006.9.1329.
- Knowles RL, Bull C, Wren C, Dezateux C. Ethics, governance and consent in the UK: implications for research into the longer-term outcomes of congenital heart defects. Arch Dis Child 2011;96:14-20. http://dx.doi.org/10.1136/adc.2008.152975.
- Gold KJ, Sen A, Hayward RA. Marriage and cohabitation outcomes after pregnancy loss. Pediatrics 2010;125:e1202-7. http://dx.doi.org/10.1542/peds.2009-3081.
- Swaminathan S, Alexander GR, Boulet S. Delivering a very low birth weight infant and the subsequent risk of divorce or separation. Matern Child Health J 2006;10:473-9. http://dx.doi.org/10.1007/s10995-006-0146-3.
- Estroff SE, Toombs SK, Barnard D, Carson RA. Chronic Illness: From Experience to Policy. Bloomington, IN: Indiana University Press; 1995.
- Duncombe J, Jessop J. ‘Doing rapport’ and the ethics of ‘faking friendship’. Ethics in Qualitative Research. London: SAGE; 2002.
- Forbat L, Henderson J. ‘Stuck in the middle with you’: the ethics and process of qualitative research with two people in an intimate relationship. Qual Health Res 2003;13:1453-62. http://dx.doi.org/10.1177/1049732303255836.
- Dervin B. An overview of sense-making: concepts, methods, and results to date n.d. http://communication.sbs.ohio-state.edu/sense-making/art/artdervin83.html (accessed 20 March 2014).
- Cronin A, Fielding VDAJ, Moran-Ellis J, Thomas H, Alasuutari P, Brannen J, et al. The SAGE Handbook of Social Research Methods. London: SAGE; 2008.
- Working Together to Safeguard Children: A Guide to Inter-agency Working to Safeguard and Promote the Welfare of Children. London: The Stationery Office; 2010.
- Ewing G, Grande G. Development of a Carer Support Needs Assessment Tool (CSNAT) for end-of-life care practice at home: a qualitative study. Palliat Med 2013;27:244-56. http://dx.doi.org/10.1177/0269216312440607.
Appendix 1 Phase I: the BRACELET study – surveys of mortality in UK neonatal and paediatric intensive care trials (Trials; 2010)
Appendix 2 Phase I: neonatal intensive care unit questionnaire
Appendix 3 Phase I: paediatric intensive care unit questionnaire
Appendix 4 Phase I: pre-notification letter to intensive care unit representatives
Appendix 5 Phase I: BRACELET study summary to intensive care unit representatives
Appendix 6 Phase I: cover letter to intensive care unit representatives
Appendix 7 Phase I: neonatal intensive care unit and paediatric intensive care unit short questionnaire
Appendix 8 Phase I: letter from the National Perinatal Epidemiology Unit director to intensive care unit representatives
Appendix 9 Phase I: trial questionnaire to trial representatives
Appendix 10 Phase I: unit mortality data form to trial representatives
Appendix 11 Phase II: information booklet for clinicians and trial team members and core centre clinicians
Appendix 12 Phase II: demographic questionnaire – clinicians and triallists
Appendix 13 Phase II: demographic questionnaire – bereaved parents (version for women)
Appendix 14 Leaflet for bereaved parents in the TOBY trial
Appendix 15 Letter to bereaved parents about the TOBY trial newsletters
Appendix 16 The TOBY parents’ newsletter issue 4
Appendix 17 The PROGRAMS parents’ newsletter issue 3
Appendix 18 The INIS ANZ parents’ newsletter, winter 2008–9
Appendix 19 The INIS ANZ parents newsletter issue 4
Appendix 20 Phase II: BRACELET amendment – publicity for parents, version 1 170910
Appendix 21 Phase II: BRACELET amendment – preparatory letter to parents, version 1 170910
Appendix 22 Phase II: online BRACELET questionnaire for bereaved parents
Appendix 23 Phase II: reply slip for bereaved parents
Appendix 24 Phase II: questionnaire to bereaved parents about contact processes, strategy 1
Appendix 25 Phase II: questionnaire to bereaved parents about contact processes, strategy 2a
Appendix 26 Phase II: questionnaire to bereaved parents about contact processes, strategy 2b
Appendix 27 Phase II: post-interview questionnaire to bereaved parents (version for women)
Appendix 28 Phase II: code of practice for conduct of the interviews
Appendix 29 Phase II: support and information card for parents
List of abbreviations
- BAPM
- British Association of Perinatal Medicine
- BFU
- bereavement follow-up
- Bliss
- the National Charity for the Newborn
- BOOST-II UK
- Benefits of Oxygen Saturation Targeting
- BRACELET study
- Bereavement and RAndomised ControlLEd Trials study
- CI
- chief investigator
- CRN
- Clinical Research Network
- CS
- caesarean section
- CTU
- Clinical Trials Unit
- DMC
- Data Monitoring Committee
- ECMO
- extracorporeal membrane oxygenation
- ExPN
- Extreme Preterm Nutrition study: Improving post-natal head growth in very preterm infants: a randomised controlled trial of hyperalimentation
- GM-CSF
- granulocyte–macrophage colony-stimulating factor
- GP
- general practitioner
- HTA
- Health Technology Assessment
- ICU
- intensive care unit
- INIS
- International Neonatal Immunotherapy Study
- IQR
- interquartile range
- ITT
- intention to treat
- LRN
- Local Research Network
- LWH
- Liverpool Women’s Hospital
- MCRN
- Medicines for Children Research Network
- NEC
- necrotising enterocolitis
- NIC
- neonatal intensive care
- NICU
- neonatal intensive care unit
- NIHR
- National Institute for Health Research
- NPEU
- National Perinatal Epidemiology Unit
- NWREC
- North West Research Ethics Committee
- PI
- principal investigator
- PIC
- paediatric intensive care
- PICANet
- Paediatric Intensive Care Audit Network
- PICS
- Paediatric Intensive Care Society
- PICU
- paediatric intensive care unit
- PROGRAMS
- PROphylactic GRAnulocyte-Macrophage colony-Stimulating factor (GM-CSF) to reduce sepsis in preterm neonates
- R&D
- Research and Development
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SANDS
- the Stillbirth and Neonatal Death Charity
- SGA
- small for gestational age
- SpO2
- oxygen saturation
- SUPPORT
- Positive Airway Pressure and Pulse Oximetry Randomized Trial
- TAMBA
- Twins and Multiple Births Association
- TOBY
- whole-body hypothermia for the treatment of perinatal asphyxial encephalopathy (trial)
- TSC
- Trial Steering Committee