Notes
Article history
This issue of Health Technology Assessment contains a project originally commissioned by the MRC but managed by the Efficacy and Mechanism Evaluation Programme. The EME programme was created as part of the National Institute for Health Research (NIHR) and the Medical Research Council (MRC) coordinated strategy for clinical trials. The EME programme is funded by the MRC and NIHR, with contributions from the CSO in Scotland and NISCHR in Wales and the HSC R&D, Public Health Agency in Northern Ireland. It is managed by the NIHR Evaluation, Trials and Studies Coordinating Centre (NETSCC) based at the University of Southampton. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from the material published in this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Rogers et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background and rationale
Despite advances in medical therapy and percutaneous coronary interventions (PCIs) there is good evidence that coronary artery bypass grafting (CABG) offers superior survival and freedom from repeat intervention in patients with multivessel coronary artery disease (CAD). 1–5 For example, in the published New York State registry of almost 60,000 patients, after risk stratification for cardiac and non-cardiac comorbidity, there was a significant reduction in mortality (absolute difference of 5%) and a sevenfold reduction in the need for repeat interventions at 3 years in patients undergoing CABG rather than PCI using stents. 2 Predictions that drug-eluting stents will significantly reduce the need for CABG are premature because, although these stents reduce the incidence of restenosis compared with bare metal stents, three large meta-analyses have shown that they do not improve survival or reduce the incidence of subsequent myocardial infarction (MI). 6–8 There are two reasons why CABG is likely to remain a superior treatment to PCI over the longer term: (1) CABG protects whole zones of proximal myocardium (as the graft is placed to the midcoronary vessel beyond all proximal disease);9 and (2) PCI frequently results in incomplete revascularisation, which adversely affects survival proportional to the incompleteness of revascularisation. 10 Currently around half a million patients worldwide undergo CABG each year. There is a real possibility that these numbers will increase with a growing elderly population, an increasing epidemic of diabetes and obesity which all predispose to the development of CAD, and an increasing realisation that PCI may merely delay definitive treatment.
Conventional CABG uses cardiopulmonary bypass (CPB) (‘on-pump’) to support the circulation while the heart is temporarily stopped. CPB causes a systemic inflammatory response syndrome, which leads to multiorgan dysfunction, and, although mild and reversible in most, can contribute to mortality and overt morbidity, particularly in higher-risk patients. 11–19 Evidence from randomised controlled trials (RCTs) in low-risk populations shows that ‘off-pump’ CABG (OPCABG) is at least as safe as ‘on-pump’ CABG (ONCABG) in terms of mortality and that it reduces several aspects of morbidity but may lead to a higher need for subsequent reintervention. 11–14
However, the exclusion of high-risk patients from these RCTs is of key importance because there are consistent findings from large observational studies that OPCABG appears to reduce mortality and morbidity in such patients. 15–19 These studies, summarised in Table 1 , have used propensity scoring and/or logistic regression to take account of different baseline characteristics in the OPCABG and ONCABG groups but are still prone to all the limitations of non-randomised studies.
| Reference number | Effect measure | Number of patients | Mortality (%) | OPCABG risk reduction in mortality (%) | p-value | ||
|---|---|---|---|---|---|---|---|
| ONCABG | OPCABG | ONCABG | OPCABG | ||||
| 15 | O/E ratio for death | 106,423 | 11,717 | 1.02 | 0.81 | 20 | 0.001 |
| 16 | O/E ratio for death | 10,631 | 1929 | 1.25 | 0.61 | 49 | 0.001 |
| 17 | Bayes’ risk based mortality | 5163 | 2223 | 2.9 | 1.4 | 52 | 0.001 |
| 18 | Death within 30 days among patients with a EuroSCORE of > 6 | 510 | 510 | 5.9 | 3.1 | 47 | 0.04 |
| 19 | Mortality in 422 very high-risk patients | 211 | 211 | 11 | 4 | 64 | < 0.05 |
Only 15–20% of all CABG in Europe and the USA are performed as OPCABG owing to concerns that it may result both in fewer grafts and in lower graft patency. The Prague-4 RCT of 400 patients in a single centre reported similar 30-day clinical outcomes but a reduction in 1-year saphenous vein graft patency (49% in OPACBG group vs. 59% in ONCABG group) in the OPCABG group. 20 In contrast, in the Surgical Management of Arterial Revascularisation Therapies trial, a single-centre, single-surgeon RCT of 197 patients, Puskas et al. 21 reported 1-year angiographic graft patencies of 94% for OPCABG (mean of 3.2 grafts) and 96% for ONCABG (mean of 3.4 grafts). In the Beating Heart Against Cardioplegic Arrest Studies,22 two single-surgeon RCTs of 401 patients in total, 7-year follow-up has shown graft patency of 86.2% and 85.4%, respectively.
Past research
Research published before commencement of the trial
When the Coronary artery bypass grafting in high-RISk patients randomised to off- or on-Pump surgery (CRISP) trial was conceived, there had been two meta-analyses11,12 and two consensus statements13,14 addressing the issue of OPCABG versus ONCABG. The key summary points of these, and of two other meta-analyses23,24 published before recruitment to CRISP began, are reproduced below. It should be noted that these papers report, in effect, analyses of the same primary data from RCTs. Two earlier meta-analyses,25,26 with fewer patients and listed in several publications, were statistically less rigorous and are not described.
Meta-analysis 1: Cheng et al. 200511
In this meta-analysis of 37 RCTs (3369 patients) of OPCABG versus ONCABG, no significant differences were found for 30-day mortality [odds ratio (OR) 1.02, 95% confidence interval (CI) 0.58 to 1.80], MI (OR 0.77, 95% CI 0.48 to 1.26), stroke (OR 0.68, 95% CI 0.33 to 1.40), renal dysfunction (OR 0.58, 95% CI 0.25 to 1.33), intra-aortic balloon pump (IABP) requirement, wound infection, rethoracotomy or reintervention. However, OPCABG significantly decreased atrial fibrillation (AF), transfusion, inotrope requirements, respiratory infections, ventilation time, intensive care unit stay and hospital stay. Patency and neurocognitive function results were inconclusive. In-hospital and 1-year direct costs were higher for ONCABG. Therefore, this meta-analysis demonstrates that mortality, stroke, MI and renal failure were not statistically significantly reduced in OPCABG; however, selected short- and mid-term clinical and resource outcomes were improved compared with ONCABG.
Meta-analysis 2: Wijeysundera et al. 200512
These authors carried out a meta-analysis of 37 RCTs (3449 patients) and 22 risk-adjusted (logistic regression or propensity score) observational studies (293,617 patients). In RCTs, OPCABG was associated with a reduced incidence of AF and trends towards reduced 30-day mortality (OR 0.91, 95% CI 0.45 to 1.83) and reduced incidence of stroke (OR 0.52, 95% CI 0.25 to 1.05) and MI (OR 0.79, 95% CI 0.50 to 1.25). Observational studies showed OPCAB to be associated with reduced 30-day mortality (OR 0.72, 95% CI 0.66 to 0.78) and a reduced incidence of stroke (OR 0.62, 95% CI 0.55 to 0.69), MI (OR 0.66, 95% CI 0.50 to 0.88) and AF (OR 0.78, 95% CI 0.74 to 0.82). At 1–2 years, OPCABG was associated with trends toward reduced mortality, but also increased repeat revascularisation (RCT: OR 1.75, 95% CI 0.78 to 3.94; observational: OR 1.35, 95% CI 0.76 to 2.39). The conclusions that can be drawn include that the RCTs did not find, aside from AF, the statistically significant reductions in short-term mortality and morbidity demonstrated by observational studies. 12 These discrepancies may be due to differing patient-selection and study methodology. Future studies must focus on improving research methodology, recruiting high-risk patients and collecting long-term data.
Meta-analysis 3: Sedrakyan et al. 200623
This was a meta-analysis of 41 RCTs (3996 patients) of OPCABG versus ONCABG. No statistically significant differences were found for mortality [relative risk (RR) 0.96, 95% CI 0.58 to 1.60], MI (RR 0.80, 95% CI 0.54 to 1.19), renal failure (RR 0.61, 95% CI 0.26 to 1.45), reintervention (RR 1.90, 95% CI 0.92 to 3.90) or recurrence of angina. However, OPCABG significantly decreased AF (RR 0.70, 95% CI 0.57 to 0.84), stroke (RR 0.52 95% CI 0.37 to 0.74) and wound infection.
Meta-analysis 4: Moller et al. 200824
In this meta-analysis of 66 RCTs (5537 patients) of OPCABG versus ONCABG, no significant differences were found for mortality (RR 0.98, 95% CI 0.66 to 1.44), MI (RR 0.95, 95% CI 0.65 to 1.37), repeat revascularisation (RR 1.34, 95% CI 0.83 to 2.18) or stroke (RR 0.62, 95% CI 0.32 to 1.19); however, OPBCABG significantly decreased AF (RR 0.69, 95% CI 0.57 to 0.83). To increase the strength of evidence regarding which method to prefer, large RCTs with longer-term follow-up and blinded outcome assessment, recruiting consecutive high-risk patients, are needed.
American Heart Association scientific statement: Sellke et al. 200513
One of the most hotly debated and polarising issues in cardiac surgery has been whether CABG without the use of CPB or cardioplegia (OPCABG) is superior to that performed with the heart–lung machine and the heart chemically arrested (standard CABG). Various clinical trials are reviewed comparing the two surgical strategies, including several large retrospective analyses, meta-analyses and the randomised trials that address different aspects of standard CABG and OPCABG. 13 Although definitive conclusions about the relative merits of standard CABG and OPCABG are difficult to reach from these varied randomised and non-randomised studies, several generalisations may be possible. Nevertheless, there appear to be trends in most studies. These trends include less blood loss and need for transfusion after OPCABG, less myocardial enzyme release after OPCABG up to 24 hours, less early neurocognitive dysfunction after OPCABG and less renal insufficiency after OPCABG. Fewer grafts tend to be performed with OPCABG than with standard CABG. Length of hospital stay, mortality rate and long-term neurological function and cardiac outcome appear to be similar in the two groups. To answer definitively the remaining questions of whether either strategy is superior, and in which patients, a large-scale prospective randomised trial is required.
Recommendations of the National Heart, Lung, And Blood Institute working group on the future direction in cardiac surgery. Off-pump coronary artery bypass: Baumgartner et al. 200514
Although CPB may reduce the technical difficulty of performing CABG surgery, it also contributes to the risk of specific complications, such as perfusion-related embolisation, hypoperfusion, generalised inflammatory response and anaemia. Consequently, a number of surgeons perform OPCABG, in which CPB is avoided, in an effort to avoid perfusion-related complications. Definitive data establishing the superiority of one technique over the other are lacking. Retrospective reviews of large databases suggest that OPCABG is associated with a decrease in risk-adjusted mortality and morbidity. Smaller prospective, randomised clinical trials comparing OPCABG with pump-based CABG have produced varying results, even when only graft patency is examined. Such conflicting information has led to adoption of OPCABG in a haphazard manner that poorly serves the large patient population with CAD. Currently, fewer than 25% of coronary revascularisations are performed without CPB and this percentage of OPCABG procedures has not increased over the last 3 years. A large, multicentre, randomised clinical trial comparing OPCABG and CABG is needed to resolve uncertainty regarding their relative benefits.
Although these meta-analyses of RCTs showed clinically important effect sizes (similar to those in the observational studies), they were underpowered for statistical significance. The CRISP trial was set up to test the hypothesis that, in high-risk patients, OPCABG reduces mortality and morbidity without causing a higher risk of reintervention, with the aim of recruiting almost 50% more patients than included in the meta-analyses.
Research published after commencement of the trial
There have been eight further meta-analyses and a Cochrane systematic review published since 2009, when recruitment to the CRISP trial began. 27–35 Six of the meta-analyses were restricted to RCTs,27–30,33,35 one considered both RCTs and observational studies32 and the other was a meta-analysis of propensity score analyses. 31 The largest of these meta-analyses, which was similar in size to the Cochrane systematic review (86 RCTs, 9906 patients), examined the association between outcome and risk. 30 Superior results with OPCABG were reported in patients with a lower ejection fraction for mortality and the incidence of AF, but not for the incidence of stroke or MI. No effect modification was seen for age and sex.
The Cochrane review published in 201234 includes 86 RCTs (10,716 patients). It includes results from four large trials (> 300 participants) published since the previous meta-analysis by the same group:24 the Medicine angioplasty or surgery study,36 the Randomised On/Off BYpass (ROOBY) trial,37 the Best Bypass Surgery (BBS) trial38 and the Danish On-pump Off-pump Randomisation Study (DOORS; published in abstract form only). 39 The review does not include the more recently published CABG off- or on-pump revascularisation (CORONARY) trial. 40 All-cause mortality to 30 days (death within 30 days of surgery) favoured OPCABG, but not significantly so (RR 0.63, 95% CI 0.33 to 1.20). However, when including follow up beyond 30 days, a significantly increased risk of death with OPCABG was found (RR 1.24, 95% CI 1.01 to 1.53). There was no difference with respect to MI, either in the first 30 days (RR 1.16, 95% CI 0.83 to 1.64) or overall (RR 1.00, 95% CI 0.80 to 1.26). In contrast, the risk of stroke in the first 30 days was reduced (RR 0.56, 95% CI 0.32 to 0.99) but, again, a difference in overall risk was not found (RR 0.76, 95% CI 0.54 to 1.06). OPCABG conferred a non-significantly increased risk of coronary reintervention (RR 1.25, 95% CI 0.94 to 1.65) and a significantly reduced risk of postoperative AF (RR 0.78, 95% CI 0.63 to 0.96); the incidence of renal insufficiency was similar (RR 0.86, 95% CI 0.62 to 1.20). On average, OPCABG patients had fewer distal anastomoses (−0.28, 95% CI −0.40 to −0.16). The authors acknowledged that mainly patients with low risk of postoperative complications were enrolled and patients with three-vessel coronary disease and impaired left ventricular (LV) function were under-represented. The majority of trials were assessed as having a high risk of bias owing to the open-label design. There was no heterogeneity in all-cause mortality between trials with a low risk of bias. Within this subgroup of trials, both single-surgeon, single-centre and multicentre trials were represented. The review did not consider subgroups of patients because the trials did not report results of subgroups and included only three trials focusing on high-risk patients. 38,41,42 The authors concluded that ONCABG should be the standard treatment but that OPCABG should be considered for patients with contraindications to aortic cannulation and cardiac arrest. They also suggested that large high-quality RCTs recruiting experienced surgeons and focusing on patients with impaired ventricular function and in whom ONCABG is contraindicated are needed.
The Canadian-led CORONARY trial recruited 4752 patients from 79 centres in 19 countries. 40 The trial had a coprimary composite outcome of death, non-fatal stroke, non-fatal MI or new renal failure requiring dialysis at 30 days after randomisation. There was no significant difference in the rate of this primary composite outcome [hazard ratio (HR) 0.95, 95% CI 0.79 to 1.14] or in any of its individual components. OPCABG significantly reduced the rates of blood transfusion (RR 0.80, 95% CI 0.75 to 0.85), reoperation for bleeding (RR 0.61, 95% CI 0.40 to 0.93), acute kidney injury (AKI) (RR 0.87, 95% CI 0.80 to 0.96) and respiratory complications (RR 0.79, 95% CI 0.63 to 0.98) but increased the rate of early repeat revascularisations (HR 4.01, 95% CI 1.34 to 12.0).
Aims and objectives
The CRISP trial was set up to address the limitations highlighted in the meta-analyses, namely to test the hypothesis that OPCABG in high-risk patients reduces mortality and morbidity, without causing a higher risk of reintervention. It complemented the CORONARY trial, which recruited predominantly lower-risk patients. Overall, 5.6% of CORONARY trial participants had impaired LV function (impairment was defined as LV function < 35%) and only 17.7% had a European system for cardiac operative risk evaluation (EuroSCORE) of > 5. 40
This report describes the results of the CRISP trial. The trial closed early, on the grounds of futility, after less than 2% of the target sample size had been reached. The challenges faced and the outcomes for the small cohort of patients recruited are described.
Chapter 2 Methods
Study design
The CRISP trial was a designed as an international, multicentre, open, parallel-group RCT of isolated OPCABG versus ONCABG in high-risk patients with an additive EuroSCORE of ≥ 5. The study received research ethics approval (reference 08/MRE00/58) and was registered (reference ISRCTN29161170).
The preferred method of randomisation when CRISP was set up was expertise based, i.e. patients were randomised to surgery carried out by an experienced off-pump surgeon or by an experienced on-pump surgeon. Evaluating surgical interventions using an expertise-based trial design was first proposed in 1980,43 but was rarely used until more recently. 44 The advantages of an expertise-based design have been discussed in detail by Devereaux et al. ,45 Cook46 and in the orthopaedic setting by Scholtes et al. 47 The rationale for choosing an expertise-based design for the CRISP trial was as follows: individual surgeons, because of their training and experience, are generally more proficient in a particular technique and so are likely to use primarily a single surgical approach. This could compromise the validity of a conventional RCT as the surgical expertise may be skewed toward the technique which is best established, most widely used or easiest to perform; a conventional RCT also has limited applicability since, by design, only surgeons experienced in OPCABG can take part. Surgical procedures that require a ‘learning curve’ are clearly disadvantaged as a minimum number of cases need to be performed and considerable experience is needed before a surgeon feels at ease with both techniques. Unless participating surgeons have expertise in both procedures, there is also a potential for differential crossover in the two arms of the trial (i.e. more crossovers in one direction than the other). OPCABG is less frequently performed than ONCABG, technically more demanding and may have a more prolonged ‘learning curve’. Previous conventional RCTs have been criticised for recruiting ‘inexperienced’ OPCABG surgeons, resulting in poor OPCABG results with an excess of graft occlusion and not the best ONCABG surgeons. 48 Expertise-based randomisation was chosen to avoid these problems. The surgeon eligibility criteria for participation in the CRISP trial are described in Settings.
Changes to trial design after commencement of the trial
After CRISP had been recruiting for 10 months, the Trial Steering Committee (TSC), in reviewing the recruitment challenges CRISP was experiencing at the time (see Chapter 3, Barriers to recruitment for further detail), agreed that the randomisation method should be relaxed and that both expertise-based and within-surgeon randomisation should be permitted, but with expertise-based randomisation remaining the preferred option when staff availability and logistics permitted its use. The CRISP randomisation system was then updated to record prospectively which allocation method, expertise based or within surgeon, was intended to be used for each patient recruited.
Participants
Eligibility criteria
Patients having isolated CABG surgery were eligible if they satisfied the following criteria:
-
additive EuroSCORE of ≥ 549
-
non-emergency surgery
-
operation to be carried out via a median sternotomy
-
written informed patient consent.
Patients with an additive EuroSCORE of five or more are at higher risk of mortality and morbidity. The EuroSCORE is made up of 17 components:
-
Age (one additive EuroSCORE point per 5 years from age 60 years).
-
Sex (one additive EuroSCORE point if female).
-
Chronic obstructive pulmonary disease (one additive EuroSCORE point if on bronchodilators or steroids for lung disease).
-
Extracardiac arteriopathy (two additive EuroSCORE points if claudication, carotid stenosis > 50%, previous or planned surgery of the abdominal aorta, limb artery or carotid).
-
Neurological dysfunction (two additive EuroSCORE points if disease severely affects ambulation or day-to-day function).
-
Previous cardiac surgery (three additive EuroSCORE points if pericardium opened previously).
-
Creatinine (two additive EuroSCORE points if > 200 µmol/l).
-
Active endocarditis (three additive EuroSCORE points if on antibiotics for endocarditis).
-
Critical preoperative state [three additive EuroSCORE points if on inotropes, IABP, acute renal failure (oliguria < 10 ml/hour), aborted sudden death, intermittent positive-pressure ventilation, ventricular tachycardia (VT), ventricular fibrillation (VF)].
-
Unstable angina (two additive EuroSCORE points if on intravenous nitrates until arrival in operating theatre).
-
Left ventricular ejection fraction (one additive EuroSCORE point if between 30% and 50%, three additive EuroSCORE points if < 30%).
-
Recent MI (two additive EuroSCORE points if MI < 90 days before surgery).
-
Pulmonary hypertension (two additive EuroSCORE points if systolic pulmonary artery pressure > 60 mmHg).
-
Emergency surgery required (two additive EuroSCORE points).
-
Not isolated CABG (two additive EuroSCORE points if major cardiac procedure with or without CABG).
-
Surgery on the thoracic aorta (three additive EuroSCORE points if ascending, arch or descending aorta).
-
Post-MI ventricular septal defect (four additive EuroSCORE points).
Note that the last four components are exclusion criteria from the trial and, therefore, patients would not accrue any EuroSCORE points from these components.
Patients having isolated CABG surgery were not eligible if they satisfied any of the following criteria:
-
additive EuroSCORE of < 5
-
emergency operation (immediate revascularisation for haemodynamic instability)
-
concomitant cardiac procedure with CABG
-
operation to be carried out via an incision other than a median sternotomy (e.g. anterolateral left thoracotomy)
-
known contraindication to ONCABG or OPCABG (e.g. calcified aorta, calcified coronaries, small target vessels).
Changes to trial eligibility criteria after commencement of the trial
Following the first CRISP investigators meeting, held in November 2009, participant age of < 70 years was removed as an exclusion criterion. This change was implemented from January 2010.
Settings
Patients were recruited to the CRISP trial from specialist cardiac surgery centres in the UK and Kolkata, India.
The preferred method of randomisation for CRISP was expertise-based randomisation (see Study design). Surgeons at participating centres using this preferred method were eligible to join CRISP if they had a stated preference for either OPCABG or ONCABG and were approved by the TSC as being sufficiently experienced in their preferred technique (i.e. at least 100 operations).
If, after detailed discussion with the research team, it was agreed that expertise-based randomisation was not possible at a centre, stratified within-surgeon randomisation was used. Centres and surgeons that planned to use within-surgeon randomisation required approval from the TSC (prior to the randomisation criteria being relaxed part-way through the trial; see Study design). The surgeons concerned were required to provide evidence that they have expertise in both techniques (at least 100 operations carried out using each method) and that they used both techniques with similar frequency.
Interventions
Trial patients were randomised to
-
CABG without CPB, i.e. OPCABG on the beating heart, via a median sternotomy incision, or
-
CABG with CPB, i.e. ONCABG on a chemically arrested heart, via a median sternotomy incision.
The anaesthetic technique and method of myocardial protection used were in accordance with established local protocols. These aspects were not specified in the trial protocol as there is a consistent 30-day mortality of around 2% for CABG across most UK centres, suggesting that minor differences in anaesthetic technique and methods of myocardial protection do not have a major influence on perioperative mortality. Surgical details were recorded on the case report form (CRF).
The only requirement was that the centre/surgeon followed the randomisation allocation. If it proved necessary to convert from OPCABG to ONCABG during the operation, this was recorded on the CRF.
Outcomes
Primary outcome
The primary outcome was a composite end point of death or serious morbidity (CRISPSw) within 30 days of surgery (i.e. up to and including day 30). The components were (1) all-cause death after Cardiac surgery, (2) new onset Renal failure, (3) MI, (4) Stroke, (5) Prolonged initial ventilation and (6) Sternal wound dehiscence.
New-onset renal failure was defined as a postoperative creatinine value of > 200 µmol/l, a percentage increase from preoperative creatinine of ≥ 40% and the need for renal replacement therapy (RRT). Dialysis/haemofiltration during CPB only did not constitute a requirement for RRT, and any patient who received RRT in the month prior to surgery was not eligible for this end point. The highest creatinine prior to any RRT was measured, along with preoperative and day 2 postoperative creatinine measurements for all patients.
Myocardial infarction was defined by (1) troponin I level of > 0.5 µg/l or troponin T level of > 0.2 µg/l and new pathological Q-waves with documented new wall motion abnormalities except in the septum, (2) creatine kinase MB isozyme (CK-MB) level of ≥ 10 upper limit of normal (non-Q MI), or (3) electrocardiographic (ECG) changes consistent with infarction (new significant Q-waves ≥ 0.04 cm or a reduction in R-waves of > 25%, in at least two contiguous leads). It was originally intended that if blood results did not indicate a MI but ECG suggested a MI had occurred, then the results would be adjudicated by an independent committee masked to the randomised allocation. However, after a blinded review of the data, it was decided that blood results and preoperative and postoperative ECGs for all patients would be adjudicated in this manner and MI defined on consensus of the adjudicators. ECG and blood samples (troponin T or troponin I, when possible; CK-MB was only used only if these tests were not available) were taken for the assessment of cardiac markers on day 5 postoperatively and all tests were redone if there was any indication of a suspected MI at any other time.
Stroke was defined as new acute focal neurological deficit thought to be of vascular origin, with signs or symptoms lasting longer than 24 hours and confirmed by a neurologist. Imaging was encouraged to further delineate between an ischaemic or haemorrhagic event.
Prolonged ventilation was defined as 96 hours or more, excluding any periods of reintubation following the initial extubation.
Sternal wound dehiscence was defined as requiring non-pharmacological intervention (e.g. vacuum-assisted closure dressing or reoperation). Any component events that occurred either prior to surgery or > 30 days after surgery were recorded but not included in the 30-day composite outcome.
Secondary outcomes
Secondary outcomes were:
-
duration of cardiac intensive care unit (CICU) stay during the index hospital admission (excluding any periods when the patient was returned to CICU after initial discharge), calculated as the time from operation end to initial discharge from CICU
-
duration of hospital stay during index hospital admission, calculated as the time from operation to discharge from the cardiac unit
-
quality-of-life (QoL) assessment at recruitment and 4–8 weeks after surgery, measured using Rose Angina Questionnaire (short),50 Canadian Cardiovascular Society (CCS) angina class,51 European QoL-5 Dimensions (EQ-5D)52 and Coronary Revascularisation Outcome Questionnaire (CROQ)53
-
resource utilisation, determined by hospital resources during index admission
-
cost-effectiveness, determined by within-trial cost per CRISPSw event averted, extrapolated cost per life-year gained and per quality-adjusted life-year (QALY) gained.
In addition, UK centres were randomised such that all patients operated at that centre received one of three different EQ-5D questionnaires: (1) the standard EQ-5D three-level questionnaire, (2) an extended five-level version with descriptors for all five levels, (3) an extended five-level version with descriptors for just the three original levels54 (see Appendix 2 ). An intended substudy of CRISP was to compare patient responses using the three scoring systems in patients undergoing coronary surgery.
Adverse events
Expected events were specified in the CRISP protocol (see Appendix 3 ). The protocol states that events listed are expected in the period from surgery and discharge from hospital after the operation. Any event outside this window is considered unexpected. Expected events were captured through purpose-designed CRFs (see Appendix 4 ). Unexpected events were captured in free-text format.
Changes to trial outcomes after commencement of the trial
Some small changes were made after the trial commenced at the recommendation of the Data Monitoring and Safety Committee (DMSC). First, the need to independently adjudicate blood test and ECG results for inconsistencies in the reporting of the MI primary outcome element was added. Second, in order to reduce any possible systematic bias, the definition of the new onset renal failure primary outcome element was changed from the need for RRT alone to the need for RRT and the fulfilment of clinical creatinine criteria. Finally, the collection of patient-reported CCS angina class was added to complement the Rose angina class also being collected.
The original intention of the trial was to follow-up patients for 1 year post surgery, but this was reduced to 4–8 weeks owing to the premature termination of the trial. Amendments were required to secondary outcomes to accommodate this: (1) all QoL outcomes were changed from assessment at recruitment, 4–8 weeks and 1 year post surgery to recruitment and 4–8 weeks alone; (2) resource use was changed from during 1 year to during the index hospital admission and (3) intended secondary outcomes of survival free from death or serious morbidity at 1 year and survival at 3 months were removed.
Sample size
The study sample size was set at 5418 patients (2709 per group). Pooled data collected from Bristol and Oxford cardiac databases were used to inform the sample size calculation. The data suggested an expected incidence of the composite primary outcome of 9.3% for patients with a preoperative EuroSCORE of ≥ 5. As all patients randomised to a given surgeon under expertise-based randomisation will have had their operations using the same technique, they cannot be regarded as independent of each other. Assuming that 80 surgeons would take part in the trial, the resultant intraclass correlation coefficient (ICC) was estimated from data from Bristol and Oxford cardiac databases to be 0.005. Using these assumptions, a sample size of 5418 patients had 90% power to detect a 30% reduction in RR with 5% significance (two tailed).
The DMSC periodically reviewed the safety data. At the start of the trial, two interim analyses of clinical outcomes were proposed: (1) when 50% of participants had been followed up to 30 days and (2) when 50% of participants had completed the trial (i.e. had been followed up for 12 months after surgery, the end of follow-up according to the original trial design). It was proposed that the trial should continue as planned unless there was a statistically significant difference between the two surgical approaches, with p ≤ 0.001. These interim analyses were not undertaken owing to the premature termination of the trial (see Chapter 3, Decision to close the trial early).
Randomisation
Randomised treatment allocations were internet based and generated by Sealed Envelope Ltd, London, UK. 55 Allocations were stratified by centre and cohort-minimisation used to minimise imbalance of key prognostic factors (age, sex, urgency of operation, poor LV function, impaired renal function, previous stroke, redo CABG and significant pulmonary disease) across the OPCABG and ONCABG groups. Patients were randomly assigned in a 1 : 1 ratio.
Using an internet-based randomisation system ensured that allocations were concealed until all data that could uniquely identify the patient, confirm eligibility and establish stratification and cohort minimisation groups were entered. Access to the system was password protected and only available for designated site staff. Randomisation was carried out after the trial co-ordinator or research nurse had obtained written informed patient consent. The timing of expertise-based randomisation was carefully chosen to leave enough time to schedule the surgery, but also to minimise the time between randomisation and surgery and, therefore, reduce the possibility of outcome events or cancellation of surgery occurring in this period. Within-surgeon randomisation was usually carried out as close as possible to surgery. Any patients who were unexpectedly rescheduled retained their study numbers and randomised allocation and every effort was taken to ensure the rescheduled operation was carried out by an appropriate surgeon participating in the trial, according to the randomisation method used and the assigned allocation.
Blinding
It was not possible to blind the surgeons or those involved in the postoperative care of the patients. However, at most centres, postoperative care follows strict protocols that are not ONCABG or OPCABG specific. Patients were not explicitly informed of their allocation and the external signs of surgery were similar for both groups. The careful choice of objective, clinically defined primary outcome components should minimise bias. In addition, the individuals undertaking the adjudication of MI data were masked to the treatment allocation.
Data collection
Data collection was performed both while the patient was under the care of the cardiac unit and again at their standard 4–8 week postoperative outpatients appointment to identify any elements of the primary outcome and/or adverse events (AEs). Data were collected from clinical records by research nurses and/or clinical trial co-ordinators. Purpose-designed CRFs were used to record data at each stage of a patient’s journey through the trial (see Appendix 4 ), with the key data collection points being pre surgery, the period from surgery to discharge and the routine 4–8-week follow-up appointment. Completed CRFs were then entered into the trial database via a password-protected web–based interface.
A bespoke trial database was designed using SQL server (2008). The database was intended to act as both a data storage facility and a trial management resource. For example, the database issued reminders when 4–8 week postoperative assessments were due, managed payment schedules to sites and provided facilities for tracking the progress of serious adverse event (SAE) reporting. Owing to the intended large sample size, a considerable amount of data validation was applied to the database. The validation rules were determined as a result of detailed discussions between clinical trial co-ordinators, research nurses, statisticians and database developers working on the study and were refined following any feedback from sites. Validation broadly included rules such as (1) the correct ordering of any dates and times, e.g. the date and time of CICU, high-dependency unit (HDU) or ward admission must be after the operation end date and time but prior to hospital discharge; (2) agreement of data on postoperative complications between the study CRFs and SAE forms for sponsor reporting, e.g. if there is an AE classified as serious on the CRFs an SAE form should be completed; (3) patient details (e.g. sex, age) and stratification/cohort minimisation data entered on the study CRFs should match that entered on the internet-based randomisation system; and (4) miscellaneous validation of related data recorded on different CRF pages, e.g. if the patient is recorded as being reintubated twice, there should be two reintubation and re-extubation dates and times entered on the relevant CRF. See Appendix 4 , Figure 1 , for an example of a message to the user if validation rules were not met.
Statistical methods
Analyses of the primary and secondary outcomes were carried out on the basis of intention to treat (ITT). The analysis (ITT) population consisted of all randomised patients excluding patients who died prior to surgery, patients who withdrew prior to surgery as it was decided not to perform surgery and patients who withdrew at any time and were unwilling for any data collected to be used. Continuous variables were summarised using the mean and standard deviation (SD) [or median and interquartile range (IQR) if the distribution was skewed] and categorical data were summarised as a number and percentage. All treatment comparisons are presented as effect sizes with 95% CIs, and p-values of < 0.05 from likelihood-ratio tests have been considered statistically significant. However, as the trial was stopped early, it was very underpowered to detect clinically important differences.
It was intended to adjust all formal comparisons of OPCABG versus ONCABG for surgeon and the factors used in the cohort minimisation. However, owing to the reduced sample size and resultant low event rates of some of the cohort minimisation factors, all models were adjusted for age, sex and operative priority as fixed effects and surgeon as a random effect. All underlying model assumptions were checked using standard methods (e.g. residual plots, tests for normality or for proportional hazards). Outlying observations that meant models did not fit the data adequately were excluded from analyses and are indicated in table footnotes.
The primary analysis is the proportion of patients experiencing the composite outcome of death or major morbidity (CRISPSw) up to 30 days and has been analysed using logistic regression with the treatment effect reported as an OR. Component events have been presented separately by occurrence pre or post hospital discharge. The duration of CICU stay and hospital stay were analysed as time to event outcomes, with patients who die prior to CICU/hospital discharge censored at the time of their death. Comparisons were performed using Cox proportional hazards models and treatment comparisons are presented as HRs. The validity of the assumption of proportional hazards was tested and, if violated, a model with a time-dependent covariate (the interaction term between the treatment and the survival time) was used. Random effects terms were fitted via the use of shared frailty terms. 56
For all QoL data, standard rules have been used to derive outcome measures. Rose angina and CCS angina class both result in ordinal outcomes ranging from no angina symptoms to ordered grades of angina symptoms. EQ-5D data are in two sections, the first consisting of five ordinal questions (which, for the patients who used the standard EQ-5D questionnaire, is converted into an EQ-5D single summary index) and the second a visual analogue scale. Finally, data from CROQ questionnaires are used to derive seven continuous scores, including an overall ‘core total’ score.
Rose and CCS angina class data at 4–8 weeks post surgery have been dichotomised into any angina symptoms versus no angina symptoms. Treatment groups have been compared using logistic regression, adjusting for the appropriate preoperative angina class as a categorical outcome, with treatment effects reported as ORs. Formal statistical comparisons of treatment effects have been performed only if > 10 patients in total experience the angina outcome (with at least one event in each treatment group). Responses to the five EQ-5D ordinal questions have been tabulated but no formal analyses undertaken (see Appendix 2 , Table 21 ). EQ-5D single summary index and visual analogue scale data and the CROQ core total score have been analysed using linear mixed effects methodology. Pre and postoperative values were modelled jointly to avoid the necessity to either exclude cases with missing preoperative measures or to impute missing preoperative values. Multivariate normal models were fitted incorporating separate parameter estimates for the mean baseline response and for each treatment at the 4–8 week time point (i.e. saturated model with time fitted as a categorical variable).
Safety data have been reported on the safety population, defined as all randomised patients excluding patients who withdrew prior to surgery, as it was decided not to perform surgery, and patients who withdrew at any time and were unwilling for any data collected to be used. Expected events (i.e. listed in the study protocol as expected prior to hospital discharge following cardiac surgery) and unexpected events (any event not listed in the protocol occurring before discharge and any event occurring after hospital discharge) have been tabulated separately (see Tables 15 – 17 ), with events that meet the criteria (prolonged an ongoing hospitalisation/resulted in hospitalisation, resulted in death, was life-threatening or resulted in persistent or significant disability/incapacity) of a SAE identified. Events have been presented and grouped by the treatment received, rather than the treatment allocated, and no formal comparisons between treatment groups have been made.
No formal corrections have been made for multiple testing, but the number of statistical comparisons has been limited and our interpretation of the results takes into account the magnitude and consistency of effect estimates. No subgroup or sensitivity analyses were performed. A planned subgroup analysis to compare the treatment effect in patients with an additive EuroSCORE of < 8 and ≥ 8 was planned but not performed owing to the early termination of the trial.
Missing data in all tables are indicated by footnotes. There were no missing data for the primary outcome or the time to event outcomes. Missing data for QoL outcomes were infrequent (< 5%) and, therefore, cases with missing postoperative values have been excluded from analyses. For cases with complete postoperative but missing preoperative data, the joint modelling of continuous data avoids the necessity to impute such data under the assumption that data are missing at random, but for categorical data the most common category across both treatment groups has been imputed. Owing to the low levels of missing data, it was judged that more complex missing data approaches (e.g. multiple imputation) would be unlikely to recover any additional information.
All statistical models were fitted in Stata version 12.0 (StataCorp LP, College Station, TX, USA). All other analyses and data management were performed in SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
Health economics
Given the early cessation of the trial (see Chapter 3, Decision to close the trial early), unit cost estimates for valuing resource utilisation data had not yet been collected. This, plus the small sample sizes at trial cessation, precludes the calculation of the costs associated with each method of CABG, as well as estimates of cost-effectiveness. Resource utilisation data reported for each arm of the trial are, therefore, limited to key items consumed during the index hospital admission for surgery, including duration of operation, duration on ventilation, time in CICU, time in HDU and time on a ward.
Following general guidance issued by the National Institute for Health and Care Excellence, continuous data are presented using mean and SDs for each group. Differences between groups are presented using the mean difference (MD) and 95% (bootstrapped) CI for the difference.
Chapter 3 Results
Centres
The CRISP trial planned to recruit patients from 40 centres, 20 in the UK and 20 overseas. In the recruitment period from October 2009 to March 2011, patients were recruited from eight centres in the UK and one centre in India. A total of 39 surgeons participated: 19 ONCABG specialists and 20 OPCABG specialists. The number of surgeons at each centre ranged from two to nine ( Table 2 ). The proportion of consultant surgeons at a centre participating in CRISP ranged from 20% to 100%.
| Centre | Number of surgeons | |
|---|---|---|
| ONCABG surgeons | OPCABG surgeons | |
| Basildon | 1 | 2 |
| Blackpool | 2 | 3 |
| Bristol | 3 | 6 |
| King’s College | 3 | 1 |
| Oxford | 1 | 1 |
| Papworth | 1 | 1 |
| Sheffield | 2 | 1 |
| Wolverhampton | 4 | 2 |
| India | 2 | 3 |
| Total | 19 | 20 |
In addition to the nine participating centres, a further five UK centres (University College London; Sussex Cardiac Centre, Brighton; The Cardiothoracic Centre, Liverpool; Nottingham University Hospital; and South Tees Hospital, Middlesbrough) had the necessary approvals in place to start but had not recruited any trial participants before the study closed to recruitment in March 2011 at the request of the funder (see Decision to close the trial early). Two UK centres, in Edinburgh and Cardiff, and 10 overseas centres were at various stages of the research approvals process when the study closed (see Appendix 1 ).
Screened patients
A total of 787 patients were assessed for potential inclusion in the trial. Six hundred and eighty-one were excluded: 523 were ineligible, 82 were eligible but not approached, 74 were approached but did not consent and two were omitted for other reasons. The numbers of patients screened, found to be ineligible, not approached, did not consent and randomised are given by centre in Table 3 and demonstrate different proportions of ineligible patients between centres (range 0% to 76%). This reflects the fact that some centres did not screen all potential patients.
| Centre | Screened (n) | Excluded from study | ||||||
|---|---|---|---|---|---|---|---|---|
| Ineligible | Not approached | Did not consent | Other reason | Randomised | ||||
| n | %a | n | n | n | n | %a | ||
| Basildon | 13 | 2 | 15 | 1 | 4 | 0 | 6 | 46 |
| Blackpool | 44 | 17 | 39 | 2 | 4 | 0 | 21 | 48 |
| Bristol | 436 | 330 | 76 | 39 | 41 | 0 | 26 | 6 |
| King’s College | 64 | 40 | 63 | 11 | 7 | 1 | 5 | 8 |
| Oxford | 132 | 93 | 70 | 15 | 5 | 0 | 19 | 14 |
| Papworth | 48 | 22 | 46 | 12 | 9 | 0 | 5 | 10 |
| Sheffield | 27 | 17 | 63 | 1 | 1 | 1 | 7 | 26 |
| Wolverhampton | 17 | 2 | 12 | 1 | 3 | 0 | 11 | 65 |
| India | 6 | 0 | 0 | 0 | 0 | 0 | 6 | 100 |
| Total | 787 | 523 | 66 | 82 | 74 | 2 | 106 | 13 |
The majority of ineligible patients [493 out of 523 (94%)] had an additive EuroSCORE of < 5. Other reasons for ineligibility, non-approach and non-consent are given in Figure 1 . Reasons for eligible patients not being approached included (1) cancellations and transfers to another surgeon’s list, (2) a decision not to operate, (3) time constraints and (4) a surgeon’s decision.
FIGURE 1.
Flow of participants. a, The exclusions section is incomplete as not all sites have entered full screening data; b, some patients may be ineligible for more than one reason; c, one further patient (not included on flowchart) withdrew pre surgery but was happy for data collection to continue; therefore, the patient is included in all applicable analyses (for details of all withdrawals see Table 8 ); and d, for further details see Table 9 .
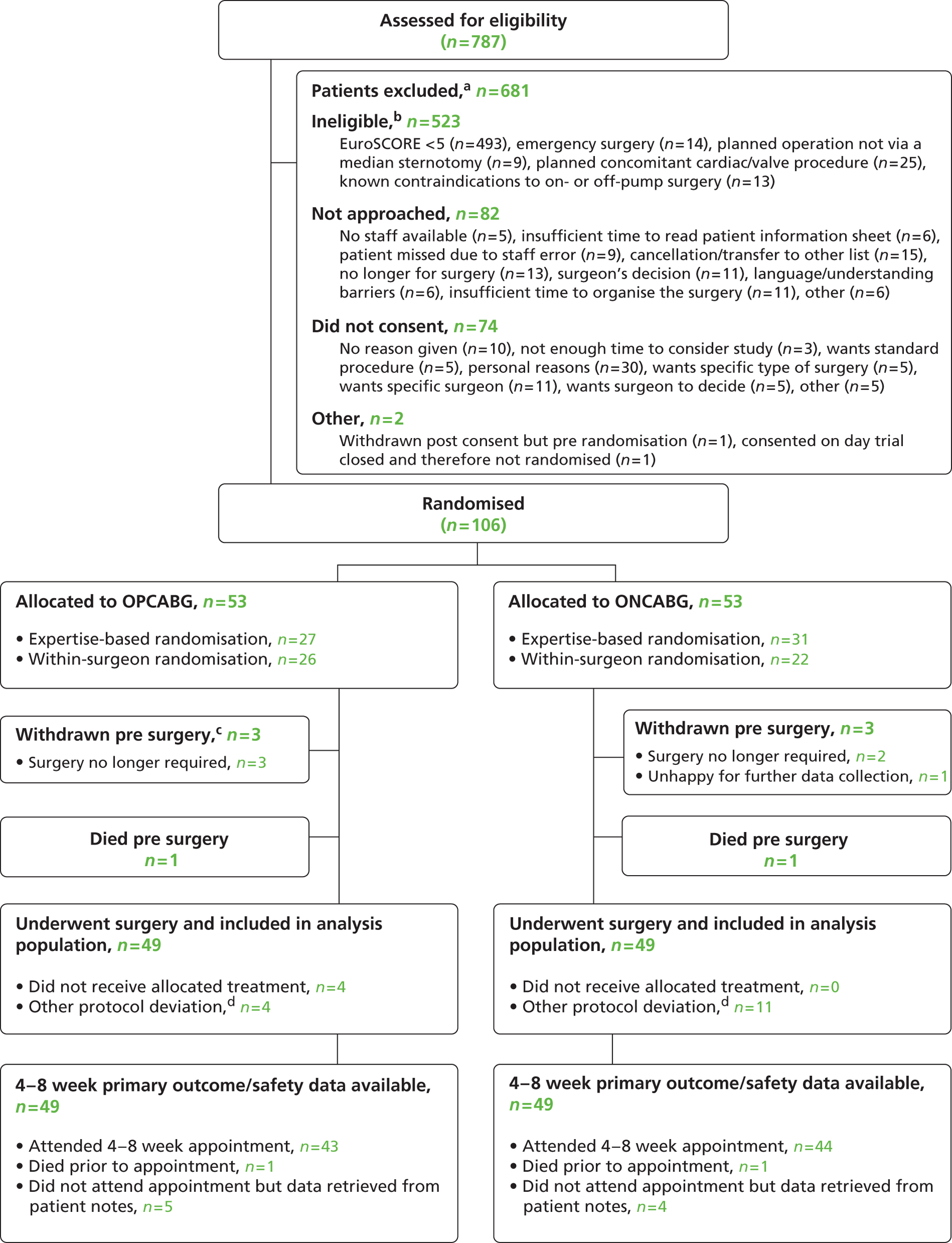
The main reason given for patients declining to take part was personal reasons, followed by the patient having a preference for a specific surgeon.
Even at the Bristol and Oxford centres, where the screening data were most complete, 75 and 39 eligible patients, respectively, were identified each year on average: significantly fewer than the average 300 eligible patients identified retrospectively from an institutional database of all cardiac procedures over the same period in Bristol. The main reasons for the deficit were (1) not all surgeons were participating in CRISP, (2) only willing OPCABG surgeons could participate if logistical problems (e.g. time constraints or surgeon unavailability) required a within-surgeon allocation, (3) other trials were recruiting from the same pool of patients in the same time period (although CRISP was prioritised over other trials in Bristol).
Recruitment
A total of 106 eligible patients were recruited into the study from October 2009 to March 2011. Patient follow-up was completed in June 2011. The study was closed to recruitment in March 2011 at the request of the funder (see Decision to close the trial early).
Recruitment pathway
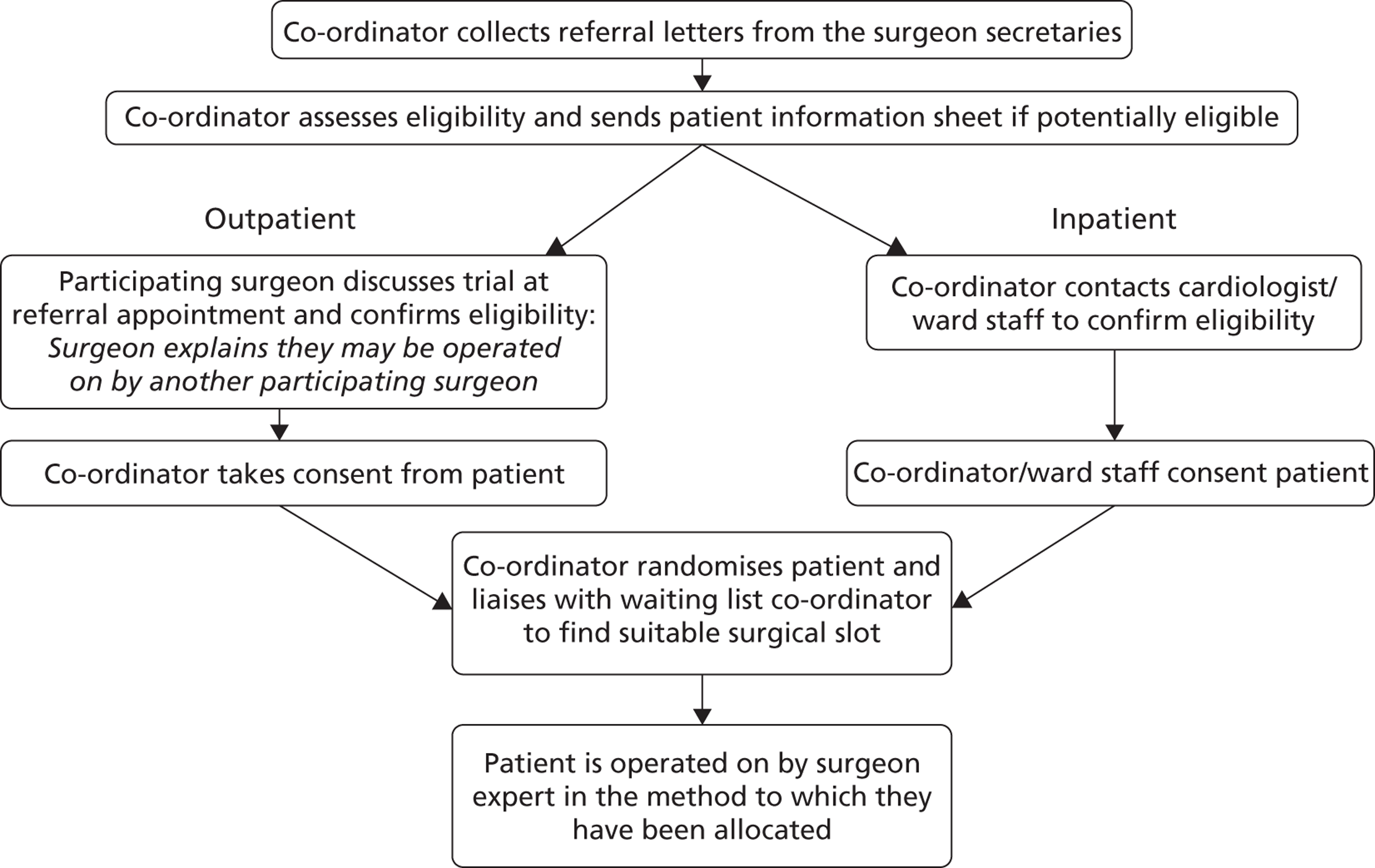
The logistics of identifying eligible patients, recruiting them into the trial and organising the surgery within an expertise-based allocation framework was recognised as the key challenge for participating centres. It was acknowledged that the recruitment pathway could vary between centres in order for them to meet this challenge while continuing to work and operate within national and local protocols. The recruitment pathway envisaged before commencement of the trial, modelled on the recruitment pathway at the Bristol centre, is described in Figure 2 .
FIGURE 2.
Recruitment pathway (Bristol model).
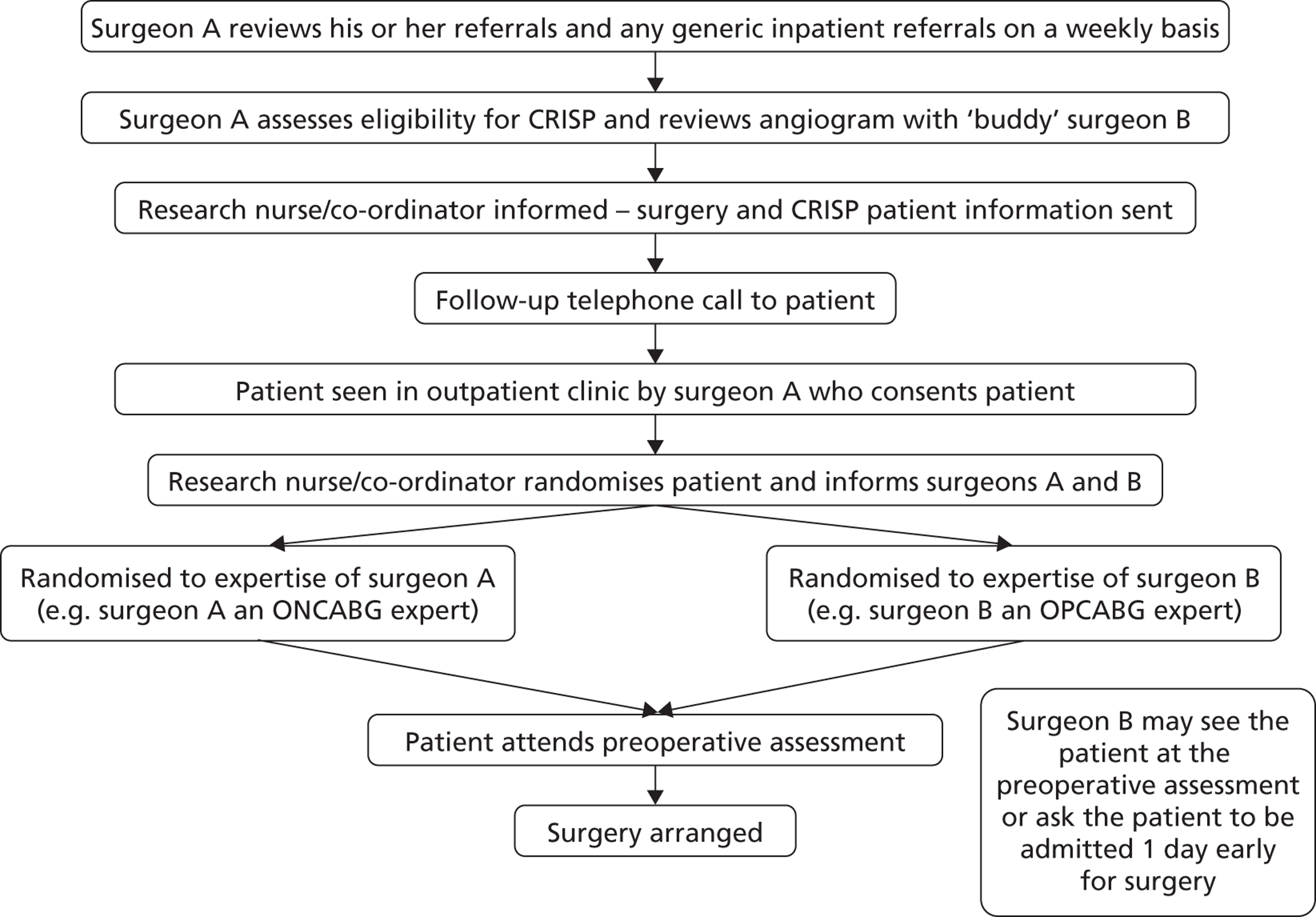
When presenting the study at site initiation visits it became apparent that this exact model would not be applicable at all centres. The model developed at Wolverhampton, where the majority of referrals are to a named surgeon, is shown in Figure 3 .
FIGURE 3.
Recruitment pathway (Wolverhampton model).
Recruitment rate
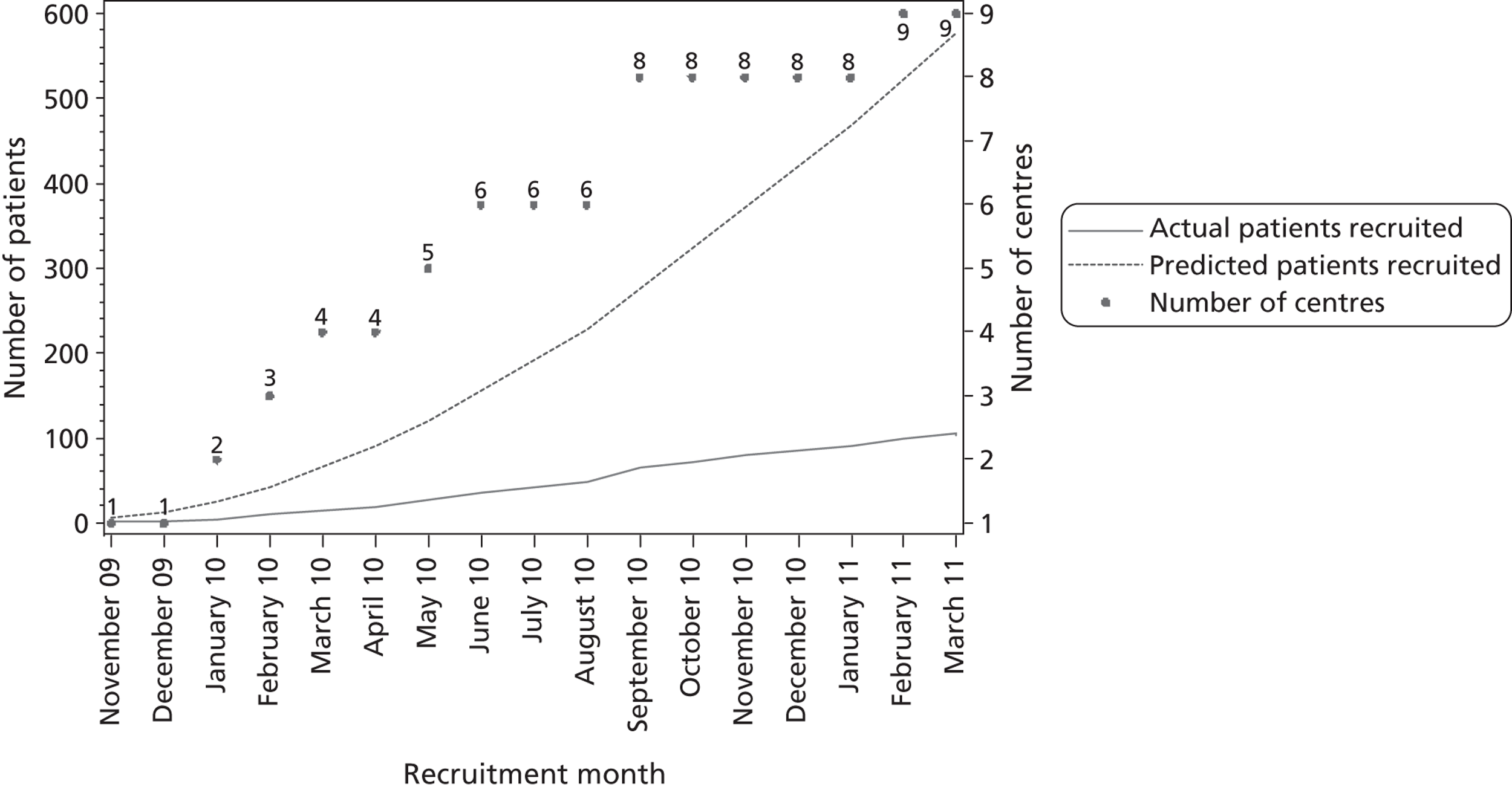
When the CRISP trial was designed, it was estimated that each centre would recruit at least six patients per month. This estimate was based on data from the Bristol and Oxford centres, where between 200 and 300 eligible patients underwent CABG each year. Based on previous trials, it was anticipated that 40% of eligible patients would be recruited,22 which would have resulted in an annual recruitment rate of between 80 and 120 patients per year. This target was not met at any participating centre. Two centres (Blackpool and Bristol) recruited five patients in 1 month and three centres (Blackpool, Wolverhampton and India) each recruited four patients in 1 month. The number of patients recruited by month and centre is shown in Table 4 and cumulative predicted and actual recruitment is shown in Figure 4 .
| Centre | Month of randomisation | Total | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2009 | 2010 | 2011 | ||||||||||||||||
| November | December | January | February | March | April | May | June | July | August | September | October | November | December | January | February | March | ||
| Basildon | 0 | 0 | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 6 |
| Blackpool | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 5 | 2 | 2 | 2 | 0 | 1 | 0 | 3 | 1 | 1 | 21 |
| Bristol | 1 | 1 | 1 | 3 | 0 | 1 | 2 | 1 | 2 | 1 | 3 | 1 | 5 | 0 | 1 | 2 | 1 | 26 |
| King’s College | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 5 |
| Oxford | 0 | 0 | 0 | 0 | 3 | 3 | 3 | 1 | 1 | 1 | 3 | 1 | 2 | 1 | 0 | 0 | 0 | 19 |
| Papworth | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 2 | 1 | 0 | 0 | 0 | 0 | 0 | 5 |
| Sheffield | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 1 | 0 | 0 | 1 | 0 | 1 | 0 | 7 |
| Wolverhampton | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 2 | 0 | 3 | 0 | 2 | 0 | 11 |
| India | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 4 | 6 |
| Total | 1 | 1 | 2 | 7 | 3 | 4 | 10 | 8 | 6 | 6 | 18 | 6 | 8 | 5 | 5 | 9 | 7 | 106 |
FIGURE 4.
Predicted and actual recruitment. The predicted number of patients assumes six patients recruited per centre per month (predictions in study protocol).
Barriers to recruitment
During study visits to centres and through a survey of the UK centres, we sought to gain information on the characteristics and key challenges of the recruitment process at each of the CRISP centres. The information provided by the UK study centres is summarised in Table 5 . The centres not listed did not respond. Five key barriers to recruitment emerged from the information gathered:
-
The number of participating surgeons. Recruitment using an expertise-based randomisation system was severely hampered if only two surgeons in a centre were taking part.
-
Access to potentially eligible patients. In some centres, urgent inpatients were transferred to the specialist cardiac centre several days before surgery, which provided sufficient time to gain the patient’s consent and organise the surgery. In other centres, patients were not transferred until late on the day before surgery and the time window for recruitment was invariably too short.
-
Referral system. Some centres operated a generic referral system for all patients (i.e. patients were placed in a pool) while, in other centres, there was a mixture of generic referrals and referrals to a named surgeon. In some centres, the vast majority were named referrals. Surgeons were reluctant to ‘share’ patients referred to them whom they had met in clinic, as they believed that the patients would want to stay with the surgeon they had met.
-
Targets. The need to meet referral-to-treatment targets and other performance targets imposed locally.
-
Insufficient information in the referral letter to determine eligibility. The EuroSCORE is made up of several components, and frequently the information provided on referral was inadequate to allow the score to be calculated accurately.
| Centre | Patient pool | Recruitment opportunities and key challenges | Participating surgeons |
|---|---|---|---|
| Basildon | Aimed to recruit from urgent patient pool because few (< 20%) of the elective patients would be eligible (EuroSCORE of ≥ 5) | Urgent inpatients referred for surgery are transferred to the cardiac centre at least 3 days prior to surgery. Patients would be recruited, randomised and the surgery arranged in this 3-day window | One ONCABG, two OPCABG |
| Blackpool | Approximately 200 operations in eligible patients per year. Aimed to recruit from urgent patient pool because elective patients are allocated to a surgeon on the basis of their ‘geographic patch’ and the centre was of the opinion that patients want to stay with the allocated surgeon they meet in clinic | Inpatients referred for surgery are transferred to the cardiac centre several days prior to surgery. Patients would be recruited, randomised and the surgery arranged in this window. Soon after recruitment started, the centre stopped screening elective patients for the trial | Two ONCABG, three OPCABG |
| Bristol | Approximately 200 to 300 operations in eligible patients per year. Aimed to recruit eligible patients from both the elective and urgent inpatient pool | Three ONCABG, six OPCABG | |
| King’s College | Aimed to recruit eligible patients primarily from the elective patient pool | Urgent inpatients waiting in a ‘feeder’ hospital are not transferred to the cardiac centre until the night before surgery, which does not give enough time for patients to be given trial information, make a decision and the surgery to be arranged according to an expertise-based allocation | Three ONCABG, one OPCABG |
| Oxford | Patients are referred to named surgeons. The centre was of the opinion that patients want to stay with the allocated surgeon they meet in clinic | With only two surgeons participating, patients can only be recruited and randomised using an expertise-based allocation when both surgeons are available to operate, otherwise national or local protocols could be breached | One ONCABG, one OPCABG |
| Papworth | With only two surgeons participating, patients can be recruited and randomised using an expertise-based allocation only when both surgeons are available to operate. Otherwise national or local protocols could be breached | One ONCABG, one OPCABG | |
| Sheffield | Aimed to recruit from urgent patient pool | Urgent inpatients referred for surgery are transferred to the cardiac centre a couple of days prior to surgery. Patients would be recruited, randomised and the surgery arranged in this window | Two ONCABG, one OPCABG |
| University College, Londona | No specific research nurse or trial co-ordinator support was available – the centre was dependent on secretarial staff to run the trial. The centre was encouraged to contact the CLRN for research support | ||
| Wolverhampton | The majority of patients are referred to a named surgeon | Established a buddy system to facilitate recruitment and the allocation within the expertise-based allocation framework | Four ONCABG, two OPCABG |
The trial team and the participating centres worked hard to try and overcome these challenges. Meetings with referring cardiologists were arranged to increase awareness of the trial and the importance of providing complete referral data. Despite the team providing purpose-designed stickers with tick-boxes that could be added to the referral letters, the quality of the referral data did not improve. Options for seeking consent from urgent inpatients before the transfer to the cardiac centre were explored in the centres with a policy of transferring urgent inpatients the night before surgery. However, this proved unsuccessful; for example, in the Bristol area, the lead research nurse for the comprehensive local research network (CLRN) was not comfortable with asking her team of local research nurses to explain and seek consent for a trial that was taking place in another hospital. The option of a research nurse from the cardiac centre visiting the feeder hospital was also explored, but, in the UK, this requires explicit research and development approval at the feeder hospital, the need to identify a local principal investigator at each feeder hospital and the agreement of the patient’s referring cardiologist. As there was no research funding available and no cardiac surgeon with an interest in the trial employed at the feeder hospitals, this proved impossible to achieve. The study had ethical approval to allow trial information to be faxed to a feeder hospital to allow potential participants time to consider the trial in advance any discussion with a surgeon and this approach was used at the Bristol centre. However, at other centres, e.g. Basildon, faxing patient information to feeder hospitals was not permitted.
In the centres outside the UK, the main barriers that hampered the set-up were (1) obtaining approved translations and back-translations of all essential documents, (2) insurance/indemnity issues (some centres, particularly in North America, required additional insurance/indemnity, which had cost implications) and (3) the per-patient funding available, which several potential investigators felt was insufficient.
Actions taken to increase recruitment
In August 2010, the TSC agreed that the expertise randomisation was a significant barrier to recruitment and that to alleviate the logistical challenges and improve recruitment, a change to within-surgeon randomisation was needed. The TSC agreed that the study could still deliver important data with the revised design and was mindful that the CORONARY trial40 also began with an expertise-based design and changed to a within-surgeon allocation to alleviate recruitment difficulties (Professor David Taggart, University of Oxford, 2010, personal communication).
This TSC decision was communicated to CRISP centres via a study newsletter. Several OPCABG experts expressed their concerns about the decision. A significant number indicated that they would not be willing to operate ONCABG on high-risk patients and so they were effectively withdrawing from the trial. At a further meeting, held in October 2010, the TSC reviewed this feedback and agreed that a balance was needed, whereby recruitment could be improved through within-surgeon randomisation (thereby overcoming some logistical challenges by allowing late referrals to be included and recruitment to continue when the ONCABG expert is unavailable) and some expertise-based randomisation (to maintain the trial’s unique design and allow all participating surgeons to remain in the trial). They therefore agreed to allow both methods of randomisation within a centre and the randomisation database was changed to record prospectively the randomisation method to be used for each recruited patient.
In summer 2010, the study team asked the Research Ethics Committee (REC) to allow an amendment relaxing the time between a potential participant being provided with the patient information sheet and consent being requested. When REC approval was first sought, this time was set at a minimum of 24 hours. The REC agreed to this time restriction being removed to allow urgent cases identified at short notice to be included in the study, provided patients were given sufficient time to consider the information and ask any questions.
Proposals to increase recruitment
The CRISP study team, the DMSC and TSC were all mindful that, even after relaxing the randomisation criteria and removing the 24-hour ‘thinking time’ restriction, the target 5418 of patients recruited was unlikely to be achieved in a realistic time scale. In order to address this, other changes to the trial design were considered.
-
Widening the inclusion criteria. There was no support for this. It was agreed that the trial needed to focus on high-risk patients.
-
Changes to the primary outcome to reduce the study size (two alternative changes to the primary outcome were considered).
-
Replacing the composite end point with a new primary end point: time from surgery to ‘fitness for hospital discharge’. The definition of fitness was made up of six objective components, chosen to avoid the subjective non-clinical factors associated with hospital discharge that can bias open trials. The six components (precise definitions for each of the components were to be agreed) proposed were:
-
normal temperature
-
normal pulse
-
normal rate of respiration
-
normal oxygen saturation
-
bowels opened since surgery
-
ability to walk 70 m or a flight of stairs (or reach pre surgery level of fitness if unable to do this pre surgery).
-
-
Each component would be assessed on a daily basis from the medical notes, with the first day on which all the criteria were met being defined as day the patient was deemed ‘fit for discharge’. Sample size calculations suggested that a 2-day difference in median time to fitness (8 vs. 10 days) could be detected with a sample size of approximately 1000 patients (with 90% power).
-
The TSC felt that use of a fitness for discharge measure could demonstrate a material benefit, in terms of costs, as well as acting as a surrogate for the major clinical end point events included in the composite primary end point. However, the DMSC members were less convinced. The DMSC agreed that the end point should be changed in such a way that would allow a reduction in the sample size but was not in favour of a fitness for discharge measure on the grounds that it was not ‘major’ enough for such a large trial, that the scientific community would not value its clinical significance and that it favoured OPCABG.
-
Extending the composite 30-day outcome to include (1) reoperation for bleeding, (2) low cardiac output, (3) new onset of atrial arrhythmia and (4) replacing new-onset renal failure with the less severe AKI. It was estimated that this revised composite outcome would have had occurred in approximately 30% of patients and that this increased incidence would have reduced sample size from 5418 to 1094 patients (90% power to detect a 30% reduction in RR). This option was presented to the funder (see Decision to close the trial early).
-
-
Seeking REC approval to randomise eligible patients prior to consent – this was suggested by several investigators as a solution to the logistic challenges of expertise-based randomisation that would allow the patient to meet the allocated expert surgeon in clinic prior to surgery. It was not pursued for several reasons, (1) ethical concerns, (2) the potential for bias and the opportunity for the surgeon to influence the patient’s decision to participate or not and (3) potential for imbalance between the groups if the consent rates differed between those allocated to an ONCABG or OPCABG expert.
Decision to close the trial early
After the TSC meeting in August 2010, which was attended by representatives from the funder, the study team were asked to prepare a recovery plan. This plan, which included the following recommendations, was submitted to the funder in September 2010.
-
The original research question remained very important to surgeons, and to the NHS, and was substantially different from the question being addressed by the CORONARY trial.
-
The primary end point should be revised to reduce the study size, as it was anticipated that recruitment would need to be extended to the year 2015 in order to reach the original target study size. A revised protocol, with a change to the primary end point (see Proposals to increase recruitment), would have allowed the two main aspects of the research question: (1) efficacy of off- versus on-pump methods in high-risk patients and (2) the methods compared among both off- and on-pump surgeons, to be answered within a shorter time frame and with significant saving of research costs.
Following further discussions regarding the primary end point with the TSC and DMSC in October and November 2010, respectively, this was followed up with a detailed proposal for the revised primary end point, based on extending the composite end point to include (1) reoperation for bleeding, (2) low cardiac output, (3) new onset of atrial arrhythmia and (4) replacing new-onset renal failure with the less severe AKI (see Proposals to increase recruitment). Using this revised end point, with revised recruitment rates based on the CRISP experience (two to three patients per centre per month) and recruiting from 20 centres, rather than the original target of 40 centres, the trial team estimated that the revised target sample size could be achieved by December 2012, with a financial saving of approximately £500,000 owing to the reduced sample size.
This recovery plan was considered by the NIHR-EME Board in February 2011 and the trial team were informed in March 2011 that the CRISP trial was to close. The Board did not feel that it would have funded the trial with the proposed revised end point and also owing to the overlap with the US funded CORONARY trial. The last CRISP patient was randomised on 11 March 2011.
Recruited patients
Screening data are compared between ineligible, eligible but non-consenting and randomised patients in Table 6 . Ineligible patients were on average younger, less likely to be female and less likely to have preoperative conditions that result in higher additive EuroSCORE, e.g. chronic pulmonary disease, extracardiac arteriopathy, unstable angina or recent MI.
| Eligibility criteria | Ineligible (N = 523) | Eligible but non-consenting (N = 74) | Randomised (N = 106) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Urgent operation | 228 | 44 | 26 | 35 | 50 | 47 |
| EuroSCORE of ≥ 5 | 30 | 6 | 74 | 100 | 106 | 100 |
| EuroSCORE, median (IQR) | 3 (1–3) | – | 6 (5–8) | – | 6 (5–8) | – |
| Age | ||||||
| < 60 years (0 points) | 132 | 25 | 0 | 0 | 2 | 2 |
| 60–64 years (1 point) | 118 | 23 | 0 | 0 | 5 | 5 |
| 65–69 years (2 points) | 109 | 21 | 6 | 8 | 11 | 10 |
| 70–74 years (3 points) | 115 | 22 | 19 | 26 | 18 | 17 |
| 75–79 years (4 points) | 42 | 8 | 31 | 42 | 34 | 32 |
| 80–84 years (5 points) | 4 | 1 | 15 | 20 | 31 | 29 |
| 85–89 years (6 points) | 3 | 1 | 3 | 4 | 5 | 5 |
| 90–94 years (7 points) | 0 | 0 | 0 | 0 | 0 | 0 |
| ≥ 95 years (8 points) | 0 | 0 | 0 | 0 | 0 | 0 |
| Female (1 point) | 70 | 13 | 24 | 32 | 25 | 24 |
| Chronic pulmonary disease (1 point) | 25 | 5 | 10 | 14 | 14 | 13 |
| Extracardiac arteriopathy (2 points) | 16 | 3 | 16 | 22 | 32 | 30 |
| Neurological dysfunction (2 points) | 2 | 0 | 3 | 4 | 3 | 3 |
| Previous cardiac surgery (3 points) | 3 | 1 | 2 | 3 | 3 | 3 |
| Serum creatinine level > 200 µmol/l (2 points) | 10 | 2 | 2 | 3 | 3 | 3 |
| Active endocarditis (3 points) | 0 | 0 | 0 | 0 | 0 | 0 |
| Critical preoperative state (3 points) | 4 | 1 | 2 | 3 | 3 | 3 |
| Unstable angina (2 points) | 6 | 1 | 7 | 9 | 11 | 10 |
| LV functiona | ||||||
| Good (> 50%) (0 points) | 371 | 81 | 47 | 66 | 60 | 57 |
| Moderate (30–50%) (1 point) | 71 | 16 | 17 | 24 | 41 | 39 |
| Poor (< 30%) (3 points) | 15 | 3 | 7 | 10 | 5 | 5 |
| Pulmonary hypertensionb (2 points) | 5 | 1 | 0 | 0 | 5 | 5 |
| Recent MI (2 points) | 55 | 11 | 28 | 38 | 53 | 50 |
Differences in randomisation practices between centres are shown in Table 7 . There was wide variation in the proportion of patients randomised using expertise-based randomisation and the median times from randomisation to surgery, although the numbers of randomised patients per centre are small. Overall, patients were randomised earlier using expertise-based randomisation (median 17.5 days prior to surgery, IQR 7–42 days) than using within-surgeon randomisation (median 3.5 days, IQR 1–16 days).
| Centre | Randomised | Expertise-based randomisation | Time from randomisation to surgerya , b | Operative priority | |||
|---|---|---|---|---|---|---|---|
| n | n | %c | Median | IQR | Electived | Urgentd | |
| Basildon | 6 | 4 | 67 | 2 | 1–14 | 3 (1) | 3 (3) |
| Bristol | 26 | 15 | 58 | 35.5 | 2–43 | 16 (13) | 10 (2) |
| Blackpool | 21 | 17 | 81 | 10 | 8–17 | 1 (0) | 20 (17) |
| King’s College | 5 | 0 | 0 | 1 | 0–3 | 4 (0) | 1 (0) |
| Oxford | 19 | 12 | 63 | 5 | 1–34 | 15 (9) | 4 (3) |
| Papworth | 5 | 2 | 40 | 11 | 0–34 | 4 (2) | 1 (0) |
| Sheffield | 7 | 2 | 29 | 10 | 4–43 | 4 (1) | 3 (1) |
| Wolverhampton | 11 | 0 | 0 | 26 | 11–50 | 9 (0) | 2 (0) |
| India | 6 | 6 | 100 | 1 | 1–1 | 0 (0) | 6 (6) |
| Total | 106 | 58 | 55 | 10 | 2–37 | 56 (26) | 50 (32) |
The numbers of urgent and elective patients recruited varied across centres (see Table 7 ). In Blackpool and the centre in India, the patients were predominantly urgent cases (20 out of 21 in Blackpool and 6 out of 6 in India), while in Oxford and Wolverhampton the majority were elective (15 out of 19 and 9 out of 11, respectively). At the other centres, similar numbers of elective and urgent cases were recruited.
Patient withdrawals
Eight of the 106 randomised patients were excluded from the analysis population: six patients withdrew prior to surgery and two patients died prior to surgery. Therefore, 98 patients underwent surgery and have been included in the principal analysis population, 49 in the OPCABG group and 49 in the ONCABG group (see Figure 1 ).
Five patients were withdrawn because it was decided that surgery was no longer required and one patient withdrew on the day of randomisation with no further details given. A further patient (OPCABG group) also withdrew their consent preoperatively owing to anxiety that they were not randomised to ONCABG; however, they were happy to be followed-up and for their data to be used and so remained in the analysis cohort. Table 8 shows a summary of withdrawals; for full details, see Appendix 2 , Table 20 .
| Withdrawal | Randomised to OPCABG (N = 53) | Randomised to ONCABG (N = 53) | Overall (N = 106) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Any withdrawal | 4 | 8 | 3 | 6 | 7 | 7 |
| Decision taken by | ||||||
| Patient | 1 | – | 1 | – | 2 | – |
| Clinician | 3 | – | 2 | – | 5 | – |
| Reason for withdrawal | ||||||
| Surgery no longer required | 3 | – | 2 | – | 5 | – |
| Type of surgery allocated to | 0 | – | 1 | – | 1 | – |
| Patient did not give reason | 0 | – | 1 | – | 1 | – |
| Other reason | 1 | – | 1 | – | 2 | – |
Protocol deviations
There were 21 protocol deviations in 19 patients ( Table 9 ). Four patients randomised to OPCABG did not receive their allocation and there were no crossovers in the ONCABG group. Reasons for not receiving the allocated treatment were (1) development of ST segment on ECG during manipulation of the heart, (2) unplanned additional procedure required, (3) VF/VT arrest and (4) myocardial ischaemia with ST changes and low blood pressure. Other types of protocol deviations were (1) patient did not meet eligibility criteria (n = 4), (2) the operating surgeon was not on the approved list of trial surgeons (n = 6), (3) expertise-based randomisation was used but the surgeon was not an expert in the allocated surgery type (n = 6), and (4) within-surgeon randomisation was used with an ONCABG surgeon (n = 1). Data on all patients for whom there was a protocol deviation were included in the trial analyses on an intention-to-treat basis.
| Protocol deviation | Randomised to OPCABG (N = 49) | Randomised to ONCABG (N = 49) | Overall (N = 98) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Any protocol deviation | 8 | 16 | 11 | 22 | 19 | 19 |
| Did not receive allocated treatmenta | 4 | 8 | 0 | 0 | 4 | 4 |
| Did not meet eligibility criteriab | 3 | 6 | 1 | 2 | 4 | 4 |
| Surgeon not on list of trial surgeons – expertise-based randomisation | 0 | 0 | 6 | 12 | 6 | 6 |
| Surgeon not on list of trial surgeons – within-surgeon randomisation | 0 | 0 | 0 | 0 | 0 | 0 |
| Expertise-based randomisation used but the surgeon not an expert in allocated surgery type | 2 | 4 | 4 | 8 | 6 | 6 |
| Within-surgeon randomisation used with ONCABG surgeonc | 0 | 0 | 1 | 2 | 1 | 1 |
Patient follow-up
Follow-up data 4–8 weeks after surgery were obtained for all 98 patients in the principal analysis population: 87 patients attended their follow-up visit, two patients died prior to their visit and nine did not attend but data were retrieved from their clinical notes and/or general practitioners.
Numbers analysed
Ninety-eight patients in the principal analysis population were included in all tables of demographic and operative characteristics and analyses of the primary outcome and duration of CICU/hospital stay. Ninety-seven patients were included in QoL analyses: (1) 90 patients with both preoperative and 4–8 weeks postoperative data, (2) six patients with preoperative data only and (3) one patient with postoperative data only. One hundred patients were included in the safety analyses: the 98 patients in the principal analysis population plus the two patients who died prior to surgery.
Baseline data and operative characteristics
Patient demographics and preoperative characteristics are presented in Table 10 . The median EuroSCORE was 6 (IQR 5–8), the median age 77.1 years (IQR 71.9–80.6 years) and 23 patients (23%) were female. Most patients (95%) had good or moderate LV function and low proportions of patients (< 15%) experienced the remaining EuroSCORE components, with the exception of extracardiac arteriopathy (32%) and recent MI (49%). The majority of patients were non-diabetic (76%), were past or current smokers (62%) and had triple-vessel disease (77%). Approximately half (45%) of procedures were classified as urgent. Characteristics were generally similar between the two groups. However, more patients in the OPCABG group than in the ONCABG group had New York Heart Association (NYHA) functional classification of heart failure as Grade I (46% vs. 20%, respectively) and no patients in the OPCABG group had an abnormal heart rhythm or a pacemaker, but five patients in the ONCABG group had an abnormal heart rhythm and four had a pacemaker. Conversely, slightly more patients in the OPCABG group had > 50% disease in the left main stem and hypertension requiring treatment (39% vs. 27% and 84% vs. 76%, respectively). In addition, angiotensin-converting enzyme (ACE) inhibitor/angiotensin receptor blocker (ARB) II and beta blocker use was more common in the OPCABG group (82% vs. 65% and 84% vs. 65%, respectively). Finally, the average heart rate was lower in the OPCABG group [median 63 beats per minute (b.p.m.) (IQR 58–72 b.p.m.) vs. median 70 b.p.m. (IQR 60–85 b.p.m.)].
| Patient characteristic | Randomised to OPCABG (N = 49) | Randomised to ONCABG (N = 49) | Overall (N = 98) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| EuroSCORE | ||||||
| EuroSCORE, median (IQR) | 6 (5–8) | – | 6 (6–8) | – | 6 (5–8) | – |
| EuroSCORE components | ||||||
| Age (years)a | ||||||
| Median (IQR) | 76.1 (73.0–80.6) | – | 77.7 (71.7–80.6) | – | 77.1 (71.9–80.6) | – |
| Mean (SD) | 76.4 (5.8) | – | 75.7 (7.7) | – | 76.1 (6.8) | – |
| Sex,a female (1 point) | 11 | 22% | 12 | 24% | 23 | 23% |
| Chronic pulmonary disease (1 point) | 5 | 10% | 8 | 16% | 13 | 13% |
| Extracardiac arteriopathy (2 points) | 15 | 31% | 16 | 33% | 31 | 32% |
| Neurological dysfunction (2 points) | 1 | 2% | 2 | 4% | 3 | 3% |
| Previous cardiac surgerya (3 points) | 2 | 4% | 1 | 2% | 3 | 3% |
| Serum creatinine > 200 µmol/la (2 points) | 2 | 4% | 1 | 2% | 3 | 3% |
| Active endocarditis (3 points) | 0 | 0% | 0 | 0% | 0 | 0% |
| Critical preoperative state (3 points) | 0 | 0% | 3 | 6% | 3 | 3% |
| Unstable angina (2 points) | 7 | 14% | 3 | 6% | 10 | 10% |
| LV functiona | ||||||
| Good (> 50%) (0 points) | 30 | 61% | 27 | 55% | 57 | 58% |
| Moderate (30–50%) (1 point) | 17 | 35% | 19 | 39% | 36 | 37% |
| Poor (< 30%) (3 points) | 2 | 4% | 3 | 6% | 5 | 5% |
| Pulmonary hypertensiona (2 points) | 1 | 2% | 3 | 6% | 4 | 4% |
| MI within last 90 days (2 points) | 25 | 51% | 23 | 47% | 48 | 49% |
| Other cardiac history | ||||||
| NYHAb | ||||||
| I | 22 | 46% | 10 | 20% | 32 | 33% |
| II | 17 | 35% | 20 | 41% | 37 | 38% |
| III | 8 | 17% | 18 | 37% | 26 | 27% |
| IV | 1 | 2% | 1 | 2% | 2 | 2% |
| Previous MI at any time | 35 | 71% | 34 | 69% | 69 | 70% |
| Time between MI and surgery (months),c median (IQR) | 1 (0–3.5) | – | 1 (0–3) | – | 1 (0–3) | – |
| Congestive cardiac failure | 1 | 2% | 1 | 2% | 2 | 2% |
| Previous PCI | 6 | 12% | 10 | 20% | 16 | 16% |
| Doppler carotid stenosis ≥ 70% | ||||||
| No | 16 | 33% | 18 | 37% | 34 | 35% |
| Yes | 7 | 14% | 5 | 10% | 12 | 12% |
| Not known | 26 | 53% | 26 | 53% | 52 | 53% |
| Heart rhythmd (sinus) | 49 | 100% | 44 | 90% | 93 | 95% |
| Pacemakere | 0 | 0% | 4 | 8% | 4 | 4% |
| Number of vessels with coronary disease | ||||||
| Single | 1 | 2% | 0 | 0% | 1 | 1% |
| Double | 9 | 18% | 12 | 24% | 21 | 21% |
| Triple | 38 | 78% | 37 | 76% | 75 | 77% |
| Quadruple | 1 | 2% | 0 | 0% | 1 | 1% |
| > 50% disease in left main stem | 19 | 39% | 13 | 27% | 32 | 33% |
| Other cardiac history | 8 | 16% | 7 | 14% | 15 | 15% |
| Non-cardiac history | ||||||
| BMI (kg/m2), mean (SD) | 26.8 (4.3) | – | 27.6 (5.2) | – | 27.2 (4.7) | – |
| Smoking status | ||||||
| No | 18 | 37% | 19 | 39% | 37 | 38% |
| Ex-smoker > 1 month | 26 | 53% | 26 | 53% | 52 | 53% |
| Yes | 5 | 10% | 4 | 8% | 9 | 9% |
| Diabetesf | 11 | 22% | 13 | 27% | 24 | 24% |
| Hypertension requiring treatment | 41 | 84% | 37 | 76% | 78 | 80% |
| Haemofiltration/dialysis | 0 | 0% | 0 | 0% | 0 | 0% |
| Previous strokea | 3 | 6% | 5 | 10% | 8 | 8% |
| Previous stroke or TIA | 6 | 12% | 7 | 14% | 13 | 13% |
| Peripheral vascular disease | 5 | 10% | 7 | 14% | 12 | 12% |
| Urgent operative prioritya , g | 20 | 41% | 24 | 49% | 44 | 45% |
| Preoperation tests | ||||||
| Creatinine (µmol/l), median (IQR) | 101 (87–121) | – | 99 (87–116) | – | 101 (87–121) | – |
| Haemoglobin (g/dl), mean (SD) | 13.0 (1.6) | – | 12.6 (1.8) | – | 12.8 (1.7) | – |
| Mean arterial pressure (mmHg), mean (SD) | 92.2 (13.0) | – | 90.4 (13.4) | – | 91.3 (13.2) | – |
| Heart rate (b.p.m.), median (IQR) | 63 (58–72) | – | 70 (60–85) | – | 66 (59–75) | – |
| Drugs on admission | ||||||
| ACE inhibitors or ARB II | 40 | 82% | 32 | 65% | 72 | 73% |
| Beta blockers | 41 | 84% | 32 | 65% | 73 | 74% |
| Calcium antagonists | 15 | 31% | 16 | 33% | 31 | 32% |
| Statins | 45 | 92% | 46 | 94% | 91 | 93% |
| Aspirin and/or clopidogrel | 49 | 100% | 46 | 94% | 95 | 97% |
| Time (days) aspirin/clopidogrel stopped pre operation, median (IQR) | 5 (1–7) | – | 5 (1–6) | – | 5 (1–7) | – |
Operative characteristics are given in Table 11 . Fewer patients had three or four grafts in the OPCABG group (63% vs. 79%, respectively). Use of the partial aortic clamp was more frequent in the ONCABG group than in the OPCABG group (88% vs. 65%, respectively) and use of a cell saver more frequent in the OPCABG group than in the ONCABG group (78% vs. 47%, respectively). More patients in the ONCABG group were paced than in the OPCABG group (27% vs. 14%, respectively) and more received red blood cell transfusions (31% vs. 16%, respectively). The blood product activated factor VII, which may or may not be given postoperatively, was not given to any participants in this cohort. There were no clear differences in terms of the grafts used and there were no deaths during surgery. The duration of the operation measured from the start of the operation (knife to skin) to the end of the procedure (patient leaves theatre) was slightly shorter in the OPCABG group (median 3.2 hours, IQR 2.7–3.9 hours) than in the ONCABG group (median 3.4 hours, IQR 3.0–4.2 hours). The MD was 0.22 of an hour (approximately 13 minutes).
| Operative characteristic | Randomised to OPCABG (N = 49) | Randomised to ONCABG (N = 49) | Overall (N = 98) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Number of grafts | ||||||
| 2 | 18 | 37 | 10 | 20 | 28 | 29 |
| 3 | 24 | 49 | 34 | 69 | 58 | 59 |
| 4 | 7 | 14 | 5 | 10 | 12 | 12 |
| Use of partial aortic clamp | 32 | 65 | 43 | 88 | 75 | 77 |
| Yes, median (IQR) number of times | 1 (1–2) | – | 1 (1–2) | – | 1 (1–2) | – |
| Significant calcification of ascending aortaa (> 50%) | 4 | 8 | 3 | 6 | 7 | 7 |
| Sinus heart rhythm on chest closureb | 47 | 96 | 47 | 96 | 94 | 96 |
| Defibrillation | 4 | 8 | 4 | 8 | 8 | 8 |
| Tranexamic acid | 26 | 53 | 27 | 55 | 53 | 54 |
| Yes, median (IQR) (g) | 2 (2–2) | – | 2 (2–4) | – | 2 (2–2) | – |
| Cell saver set up | 38 | 78 | 23 | 47 | 61 | 62 |
| Yes, median (IQR) (ml) | 170 (0–410) | – | 400 (0–680) | – | 251 (0–500) | – |
| IABP | 3 | 6 | 3 | 6 | 6 | 6 |
| Inotropes (excluding noradrenaline) | 10 | 20 | 7 | 14 | 17 | 17 |
| Noradrenaline | 16 | 33 | 12 | 24 | 28 | 29 |
| Vasodilators | 12 | 24 | 11 | 22 | 23 | 23 |
| Pacing | 7 | 14 | 13 | 27 | 20 | 20 |
| Red blood cells used | 8 | 16 | 15 | 31 | 23 | 23 |
| Yes, median (IQR) units | 1.5 (1–2.5) | – | 2 (1–2) | – | 2 (1–2) | – |
| Plasma used | 2 | 4 | 0 | 0 | 2 | 2 |
| Platelets used | 4 | 8 | 5 | 10 | 9 | 9 |
| ONCABG surgery specific detailsc | Randomised to OPCABG (N = 4) | Randomised to ONCABG (N = 49) | Overall (N = 53) | |||
| n | % | n | % | n | % | |
| Myocardial protection | ||||||
| Warm temperature | 1 | 25 | 14 | 29 | 15 | 28 |
| Blood solution | 4 | 100 | 47 | 96 | 51 | 96 |
| Antegrade infusion moded | 4 | 100 | 44 | 92 | 48 | 92 |
| Continuous timing | 1 | 25 | 4 | 8 | 5 | 9 |
| Cumulative cross-clamp time (minutes), median (IQR) | 41 (19.5–77) | – | 45 (35–57) | – | 44 (35–57) | – |
| Total bypass time (minutes), median (IQR) | 91.5 (60.5–146) | – | 71 (62–92) | – | 71 (62–95) | – |
| Graft details | Grafts of patients randomised to OPCABG (N = 136) | Grafts of patients randomised to OPCABG (N = 142) | Overall (N = 278) | |||
| n | % | n | % | n | % | |
| Carotid endarterectomye | 0 | 0 | 1 | 1 | 1 | 0 |
| Proximal | ||||||
| Aorta | 63 | 46 | 71 | 50 | 134 | 48 |
| LIMA (in situ) | 51 | 38 | 44 | 31 | 95 | 34 |
| RIMA (in situ) | 4 | 3 | 5 | 4 | 9 | 3 |
| Gastroepiploic (in situ) | 0 | 0 | 2 | 1 | 2 | 1 |
| Saphenous vein (piggyback/skip) | 8 | 6 | 13 | 9 | 21 | 8 |
| Radial artery (piggyback/skip) | 7 | 5 | 1 | 1 | 8 | 3 |
| LIMA (piggyback/skip) | 3 | 2 | 4 | 3 | 7 | 3 |
| RIMA (piggyback/skip) | 0 | 0 | 1 | 1 | 1 | 0 |
| Arch/great vessels | 0 | 0 | 1 | 1 | 1 | 0 |
| Conduitf | ||||||
| Saphenous vein | 71 | 52 | 75 | 54 | 146 | 53 |
| Radial artery | 9 | 7 | 7 | 5 | 16 | 6 |
| LIMA | 46 | 34 | 44 | 31 | 90 | 33 |
| RIMA | 10 | 7 | 6 | 4 | 16 | 6 |
| Cryopreserved | 0 | 0 | 8 | 6 | 8 | 3 |
| Distal | ||||||
| Left anterior descending artery | 47 | 35 | 50 | 35 | 97 | 35 |
| Diagonal 1 | 17 | 13 | 11 | 8 | 28 | 10 |
| Diagonal 2 | 1 | 1 | 3 | 2 | 4 | 1 |
| Obtuse marginal 1 | 33 | 24 | 31 | 22 | 64 | 23 |
| Obtuse marginal 2 | 8 | 6 | 6 | 4 | 14 | 5 |
| Posterolateral circumflex | 4 | 3 | 4 | 3 | 8 | 3 |
| Main right coronary artery | 4 | 3 | 5 | 4 | 9 | 3 |
| Posterior descending artery/posterior interventricular | 21 | 15 | 31 | 22 | 52 | 19 |
| Posteroventricle | 1 | 1 | 1 | 1 | 2 | 1 |
Primary outcome
In both the OPCABG and ONCABG groups, 6 out of 49 (12%) patients experienced the primary outcome in the first 30 days ( Table 12 ). The estimated treatment effect, adjusted for age, sex, operative priority and surgeon, was OR = 1.07 (95% CI 0.27 to 4.14; p = 0.93).
| Component of the primary outcome | Randomised to OPCABG (N = 49) | Randomised to ONCABG (N = 49) | OR (95% CI) | p-value | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| At any time | ||||||
| Primary outcome | 6 | 12 | 6 | 12 | 1.07 (0.27 to 4.14) | 0.93 |
| Death | 0 | 0 | 1 | 2 | ||
| New-onset renal failurea | 2 | 4 | 1 | 2 | ||
| MI | 3 | 6 | 3 | 6 | ||
| Stroke | 2 | 4 | 1 | 2 | ||
| Prolonged ventilationb | 1 | 2 | 2 | 4 | ||
| Sternal wound dehiscencec | 0 | 0 | 1 | 2 | ||
| Pre hospital discharge | ||||||
| Primary outcome | 5 | 10 | 6 | 12 | ||
| Death | 0 | 0 | 1 | 2 | ||
| New-onset renal failure | 2 | 4 | 1 | 2 | ||
| MI | 3 | 6 | 3 | 6 | ||
| Stroke | 1 | 2 | 1 | 2 | ||
| Prolonged ventilation | 1 | 2 | 2 | 4 | ||
| Sternal wound dehiscence | 0 | 0 | 1 | 2 | ||
| Post hospital discharge | ||||||
| Primary outcome | 1 | 2 | 0 | 0 | ||
| Death | 0 | 0 | 0 | 0 | ||
| New-onset renal failure | 0 | 0 | 0 | 0 | ||
| MI | 0 | 0 | 0 | 0 | ||
| Stroke | 1 | 2 | 0 | 0 | ||
| Sternal wound dehiscence | 0 | 0 | 0 | 0 | ||
The most commonly occurring component of the primary outcome was MI (occurring in six patients) and the rarest were death and sternal wound dehiscence (experienced by one patient each). All but one of the constituent events occurred prior to discharge from hospital following cardiac surgery.
Secondary outcomes
Quality of life
Quality-of-life data are presented in Table 13 . For both angina classifications there is no evidence of any statistically significant differences between the groups in comparing any angina versus no angina (Rose angina class: OR = 1.89, 95% CI 0.54 to 6.61; p = 0.30; CCS angina class: OR = 0.79, 95% CI 0.23 to 2.65; p = 0.70).
| Quality-of-life measure | Preoperative | 4–8 weeks postoperative | Effect (95% CI) | p-value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Randomised to OPCABG (N = 48) | Randomised to ONCABG (N = 48) | Randomised to OPCABG (N = 46) | Randomised to ONCABG (N = 45) | |||||||
| n | % | n | % | n | % | n | % | |||
| Rose and CCS angina class | ||||||||||
| Rose anginaa | ||||||||||
| No angina | 21 | 44 | 14 | 29 | 35 | 76 | 37 | 82 | OR 1.89 (0.54 to 6.61)b | 0.30 |
| Grade I | 11 | 23 | 18 | 38 | 6 | 13 | 5 | 11 | ||
| Grade II | 16 | 33 | 16 | 33 | 5 | 11 | 3 | 7 | ||
| CCS classc | ||||||||||
| Asymptomatic | 10 | 21 | 9 | 19 | 37 | 84 | 34 | 81 | OR 0.79 (0.23 to 2.65)b | 0.70 |
| Grade I | 11 | 23 | 6 | 13 | 2 | 5 | 3 | 7 | ||
| Grade II | 15 | 31 | 18 | 38 | 4 | 9 | 5 | 12 | ||
| Grade III | 9 | 19 | 10 | 21 | 0 | 0 | 0 | 0 | ||
| Grade IV | 3 | 6 | 5 | 10 | 1 | 2 | 0 | 0 | ||
| EQ-5D categorical responsesd | ||||||||||
| Mobility | ||||||||||
| No problems walking about | 26 | 54 | 21 | 44 | 31 | 67 | 26 | 58 | ||
| Slight problems walking about | 5 | 10 | 2 | 4 | 3 | 7 | 3 | 7 | ||
| Some problems walking about | 16 | 33 | 20 | 42 | 12 | 26 | 14 | 31 | ||
| A lot of problems walking about | 0 | 0 | 4 | 8 | 0 | 0 | 1 | 2 | ||
| Confined to bed | 1 | 2 | 1 | 2 | 0 | 0 | 1 | 2 | ||
| Self-care | ||||||||||
| No problems with self-care | 44 | 92 | 40 | 83 | 43 | 93 | 41 | 91 | ||
| Slight problems washing or dressing | 0 | 0 | 1 | 2 | 2 | 4 | 1 | 2 | ||
| Some problems washing or dressing | 3 | 6 | 6 | 13 | 1 | 2 | 3 | 7 | ||
| A lot of problems washing or dressing | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Unable to wash or dress | 0 | 0 | 1 | 2 | 0 | 0 | 0 | 0 | ||
| Usual activitiese | ||||||||||
| No problems with usual activities | 23 | 49 | 20 | 42 | 26 | 57 | 17 | 38 | ||
| Slight problems with usual activities | 5 | 11 | 3 | 6 | 5 | 11 | 8 | 18 | ||
| Some problems with usual activities | 16 | 34 | 20 | 42 | 14 | 30 | 19 | 42 | ||
| A lot of problems with usual activities | 0 | 0 | 0 | 0 | 1 | 2 | 0 | 0 | ||
| Unable to perform usual activities | 3 | 6 | 5 | 10 | 0 | 0 | 1 | 2 | ||
| Pain/discomforte | ||||||||||
| No pain or discomfort | 20 | 43 | 21 | 44 | 26 | 57 | 23 | 51 | ||
| Slight pain or discomfort | 10 | 21 | 5 | 10 | 7 | 15 | 10 | 22 | ||
| Moderate pain or discomfort | 15 | 32 | 18 | 38 | 13 | 28 | 11 | 24 | ||
| A lot of pain or discomfort | 1 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Extreme pain or discomfort | 1 | 2 | 4 | 8 | 0 | 0 | 1 | 2 | ||
| Anxiety/depressionf | ||||||||||
| Not anxious or depressed | 31 | 65 | 28 | 60 | 38 | 83 | 33 | 77 | ||
| Slightly anxious or depressed | 7 | 15 | 6 | 13 | 3 | 7 | 2 | 5 | ||
| Moderately anxious or depressed | 7 | 15 | 9 | 19 | 5 | 11 | 6 | 14 | ||
| Very anxious or depressed | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 2 | ||
| Extremely anxious or depressed | 3 | 6 | 4 | 9 | 0 | 0 | 1 | 2 | ||
| EQ-5D single summary index and visual analogue scaleg | ||||||||||
| Single summary indexh | ||||||||||
| Median (IQR) | 0.69 (0.69–0.78) | – | 0.73 (0.51–0.84) | – | 0.81 (0.73–1.00) | – | 0.71 (0.69–1.00) | – | ||
| Mean (SD) | 0.666 (0.295) | – | 0.653 (0.278) | – | 0.829 (0.123) | – | 0.748 (0.260) | |||
| Visual analogue scalee | ||||||||||
| Mean (SD) | 68.6 (15.9) | – | 65.9 (17.0) | – | 76.5 (13.6) | – | 70.8 (15.2) | – | MD 4.92 (−0.94 to 10.8)i | 0.11 |
| CROQg | ||||||||||
| Core totalj | ||||||||||
| Median (IQR) | 51.4 (47.4–55.0) | – | 48.8 (44.4–54.4) | – | 52.0 (48.1–53.9) | – | 51.5 (45.4–53.4) | – | MD 1.10 (−0.97 to 3.17)i | 0.30 |
| Symptomsj | ||||||||||
| Median (IQR) | 74.1 (60.4–86.9) | – | 68.5 (54.2–85.7) | – | 94.6 (89.9–100) | – | 92.9 (85.7–98.2) | – | ||
| Physical functioning | ||||||||||
| Median (IQR) | 71.9 (46.9–87.5) | – | 50.0 (25.0–81.3) | – | 75.0 (62.5–93.8) | – | 68.8 (50.0–81.3) | – | ||
| Cognitive functioningj | ||||||||||
| Median (IQR) | 80.0 (60.0–96.7) | – | 86.7 (53.3–96.7) | – | 93.3 (73.3–100) | – | 90.0 (70.0–100) | – | ||
| Psychosocial functioningj | ||||||||||
| Median (IQR) | 66.6 (58.9–86.6) | – | 62.5 (47.3–84.8) | – | 83.9 (75.0–91.1) | – | 85.7 (71.0–91.1) | – | ||
| Satisfactionk | ||||||||||
| Median (IQR) | 86.4 (75.8–100) | – | 83.3 (69.4–91.7) | – | ||||||
| AEs | ||||||||||
| Median (IQR) | 89.8 (79.5–93.2) | – | 84.1 (79.5–90.9) | – | ||||||
The results presented in Table 13 combine the results over the three versions of the EQ-5D questionnaire. EQ-5D data split by questionnaire type (three-level, five-level with five descriptors, five-level with three descriptors) are given in Appendix 2 , Tables 21 and 22 . The single summary index score was generated by applying the social tariff to patients’ responses to the standard three-level version of the EQ-5D (n = 29 patients). A tariff to convert responses on the five-level version of the EQ-5D to a single index value is currently under development.
In general, for each of the five categorical response EQ-5D questions, slightly more patients in the OPCABG group than in the ONCABG group were classified as having no problems/symptoms both preoperatively and postoperatively; however, no formal statistical comparisons were made and the numbers of patients are low. The single summary index scores for the subset of patients completing the standard three-level version of the EQ-5D were similar at baseline, while postoperatively, the difference in the mean score between the groups was on average 0.081 (95% CI −0.076 to 0.237) higher in the ONCABG group. Similarly, on average, patients in the OPCABG group scored slightly higher on the EQ-5D visual analogue scale. However, a formal treatment comparison of postoperative scores adjusting for preoperative scores was not statistically significant (MD = 4.92, 95% CI −0.94 to 10.8; p = 0.11).
The CROQ QoL data also suggest that, on average, patients in the OPCABG score slightly higher both preoperatively and postoperatively, albeit with no statistically significant postoperative treatment differences (core total MD = 1.10, 95% CI −0.97 to 3.17; p = 0.30).
A small number of QoL data were collected at 1-year follow-up (see Appendix 2 , Table 24 ).
Resource use
Resource-use data are summarised in Table 14 . On average, patients randomised to ONCABG spent 0.22 of an hour (approximately 13 minutes) longer in surgery than patients randomised to OPCABG. Time on ventilation after surgery, measured from the time the operation ended to the time the patient was extubated, was longer for patients in the ONCABG group (median 7.1 vs. 5.7 hours). On average, patients randomised to ONCABG also spent longer in the CICU (median 27.7 vs. 26.0 hours), although this difference was not statistically significant ( Figure 5 ). Of those admitted to HDU, the stay was, on average, 37.3 hours (1.6 days) longer in the ONCABG group. In total, six patients were not admitted to a ward; one (in the ONCABG group) had died postoperatively but prior to hospital discharge. On average, of the patients admitted to a ward, length of stay was again longer in the ONCABG group. After surgery, patients randomised to ONCABG spent longer in hospital than patients randomised to OPCABG (median 8 vs. 7 days, Figure 6 ).
| Resource | Randomised to OPCABG (N = 49) | Randomised to ONCABG (N = 49) | Effect (95% CI)a | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Intraoperative | |||||
| Duration of surgery (hours)b | |||||
| Median (IQR) | 3.2 (2.7–3.9) | – | 3.4 (3.0–4.2) | – | |
| Mean (SD) | 3.39 (1.18) | – | 3.61 (0.86) | – | MD −0.22 (−0.601 to 0.209) |
| Postoperative | |||||
| Red blood cells used | 25 | 51 | 25 | 51 | |
| If yes, median (IQR) units | 1.0 (1.0–2.0) | – | 2.0 (2.0–4.0) | – | |
| Plasma used | 7 | 14 | 3 | 6 | |
| Platelets used | 9 | 18 | 5 | 10 | |
| Any haemostatic agents used | 16 | 33 | 17 | 35 | |
| Tranexamic acid | 10 | 20 | 11 | 22 | |
| Activated factor VII | 0 | 0 | 0 | 0 | |
| Other haemostatic agent | 6 | 12 | 8 | 16 | |
| Duration of ventilation (hours)c , d | |||||
| Median (IQR) | 5.7 (4.9–11.3) | – | 7.1 (4.9–14.3) | – | |
| Mean (SD) | 12.0 (23.2) | – | 17.5 (36.4) | – | MD −5.48 (−18.13 to 6.36) |
| CICU stayc , e | |||||
| Median hours (IQR) | 26.0 (21.3–65.1) | – | 27.7 (20.7–66.5) | – | HR 1.15 (0.69 to 1.91) |
| Mean hours (SD) | 45.9 (49.4) | – | 55.1 (58.8) | – | MD −9.20 (−30.2 to 11.7) |
| Mean days (SD) | 1.91 (2.06) | – | 2.29 (2.45) | – | MD −0.38 (−1.26 to 0.48) |
| Admitted to HDU | 29 | 59 | 27 | 55 | |
| HDU stayc , f | |||||
| Median hours (IQR) | 41.0 (25.8–72.0) | – | 48.8 (29.0–100) | – | |
| Mean hours (SD) | 57.95 (52.03) | – | 95.23 (145.07) | – | MD −37.28 (−99.2 to 8.86) |
| Mean days (SD) | 2.41 (2.17) | – | 3.97 (6.04) | – | MD −1.55 (−4.36 to 0.38) |
| Admitted to ward | 48 | 98 | 44 | 90 | |
| Ward stayf | |||||
| Median hours (IQR) | 98.0 (70.9–139) | – | 94.8 (72.5–143) | – | |
| Mean hours (SD) | 110.0 (56.37) | – | 136.7 (126.9) | – | MD −26.7 (−68.5 to 15.1) |
| Mean days (SD) | 4.58(2.35) | – | 5.69 (5.29) | – | MD −1.11 (−2.97 to 0.47) |
| Hospital stayg | |||||
| Median days (IQR) | 7 (6–9) | – | 8 (6–10) | – | HR 1.26 (0.81 to 1.95) |
| Mean days (SD) | 8.49 (4.98) | – | 10.12 (7.39) | – | MD −1.63 (−4.03 to 0.83) |
| Reoperationh | 0 | 0 | 4 | 8 | MD −0.082 (−0.159 to −0.005) |
| Other unplanned procedurei | 3 | 8 | 0 | 0 | MD 0.079 (−0.007 to 0.165) |
| Medications at discharge | |||||
| ACE inhibitors/ARB II | 26 | 53 | 22 | 45 | |
| Beta blockers | 38 | 78 | 35 | 71 | |
| Calcium antagonists | 5 | 10 | 0 | 0 | |
| Statins | 45 | 92 | 47 | 96 | |
| Aspirin/clopidogrel | 47 | 96 | 47 | 96 | |
| Medications at 4–8 weeks | |||||
| ACE inhibitors/ARB II j | 24 | 50 | 26 | 55 | |
| Beta blockers j | 35 | 73 | 34 | 72 | |
| Calcium antagonists j | 7 | 15 | 2 | 4 | |
| Statins j | 42 | 88 | 43 | 91 | |
| Aspirin/clopidogrel j | 46 | 96 | 42 | 89 | |
FIGURE 5.
Duration of CICU stay.
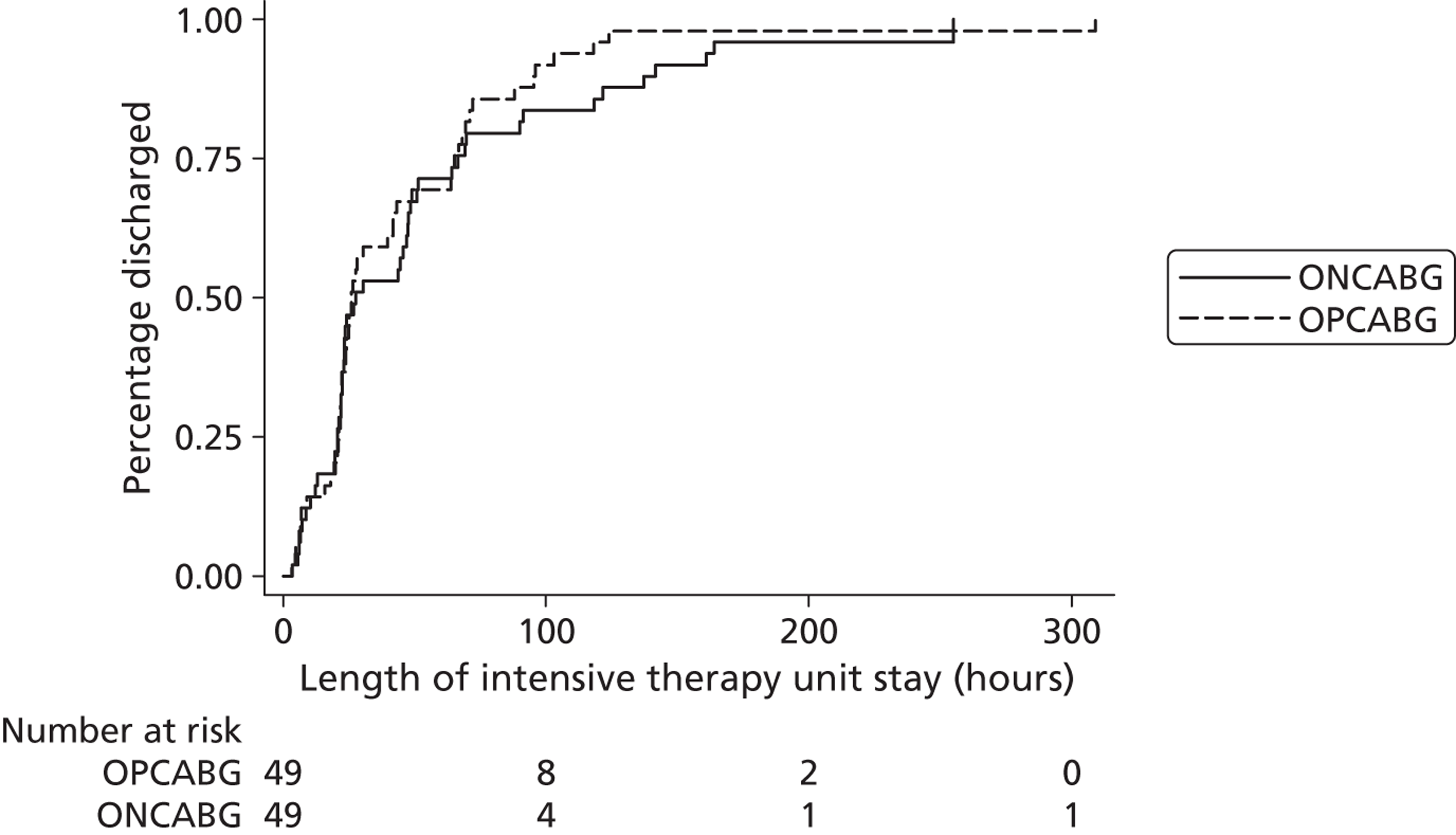
FIGURE 6.
Duration of postoperative hospital stay.
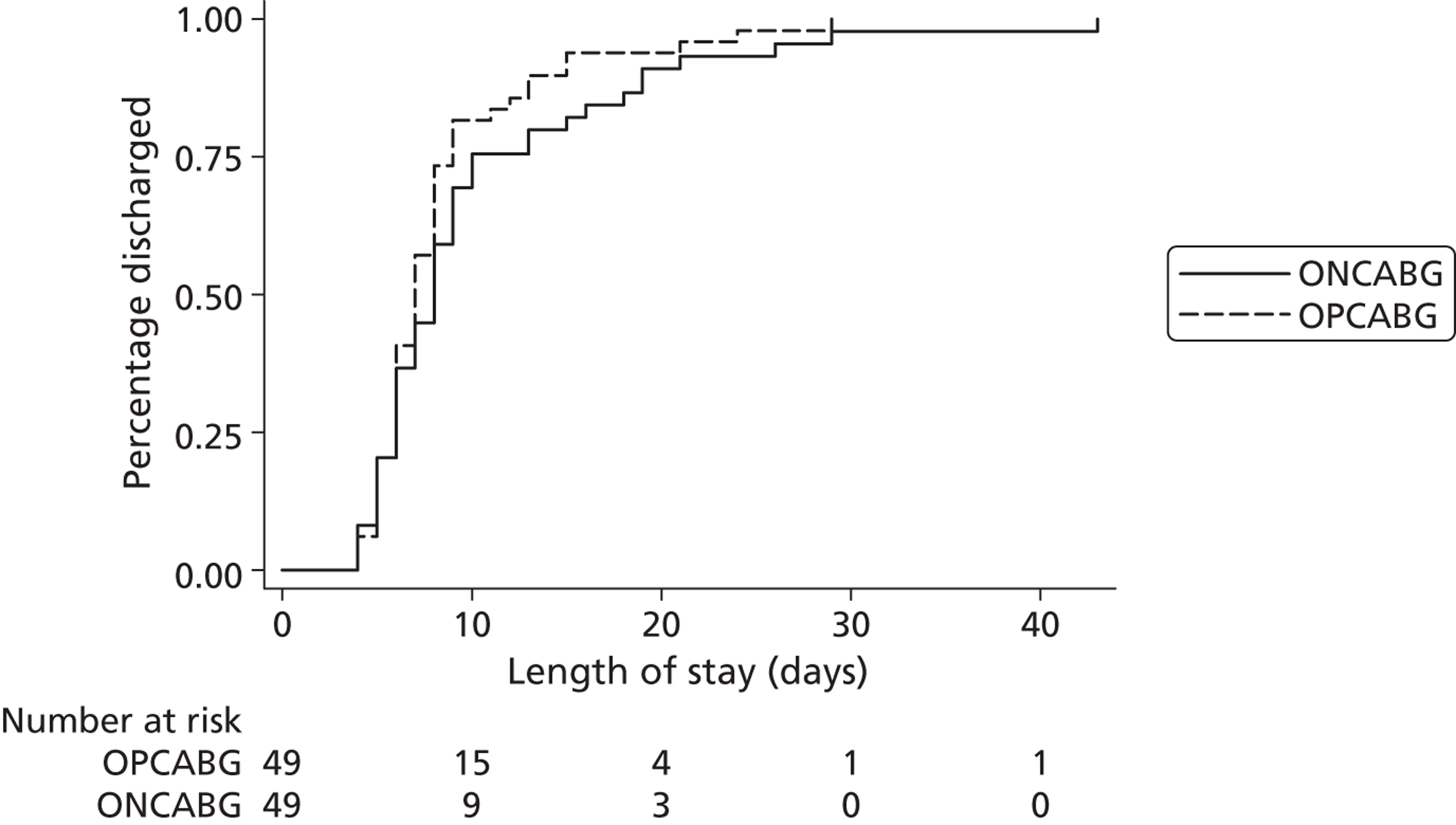
Adverse events and postoperative complications
Expected adverse events
There were 74 expected AEs (i.e. listed in the study protocol as expected prior to discharge after cardiac surgery) ( Table 15 ). Slightly fewer events occurred in patients who received OPCABG: 32 events in 27 out of 46 (59%) patients, compared with 42 events in 27 out of 54 (50%) patients who received ONCABG. Eight of these events were deemed to meet the criteria of a SAE: one event in a patient who received OPCABG and seven events occurring in six patients who received ONCABG. The most common expected AE was AF and the most common expected SAEs were respiratory infection and AF. There were eight instances of wound infections in patients who received ONCABG and three in patients who received OPCABG. There were no cases of coronary angiography, PCI or repeat CABG, or the need for a LV assist device (LVAD). No patient experienced acute respiratory distress syndrome, deep-vein thrombosis, pulmonary embolism, heparin-induced thrombocytopenia or a transient ischaemic attack (TIA).
| AE | Received OPCABG (n = 46) | Received ONCABG (n = 54) | Overall (n = 100) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AE | % | SAEa | % | AE | % | SAEa | % | AE | % | SAEa | % | |
| Total number of events | 32 | 1 | 42 | 7 | 74 | 8 | ||||||
| Patients with one or more events | 27 | 59 | 1 | 2 | 27 | 50 | 6 | 11 | 54 | 54 | 7 | 7 |
| Reoperated | 0 | 0 | 0 | 0 | 4 | 7 | 1 | 2 | 4 | 4 | 1 | 1 |
| Use of IABP | 2 | 4 | 0 | 0 | 3 | 6 | 1 | 2 | 5 | 5 | 1 | 1 |
| Respiratory infection | 8 | 17 | 0 | 0 | 9 | 17 | 3 | 6 | 17 | 17 | 3 | 3 |
| Tracheostomy | 1 | 2 | 0 | 0 | 1 | 2 | 0 | 0 | 2 | 2 | 0 | 0 |
| AF | 16 | 35 | 1 | 2 | 16 | 30 | 1 | 2 | 32 | 32 | 2 | 2 |
| Superficial wound infection: chest | 1 | 2 | 0 | 0 | 4 | 7 | 0 | 0 | 5 | 5 | 0 | 0 |
| Superficial wound infection: leg | 2 | 4 | 0 | 0 | 4 | 7 | 1 | 2 | 6 | 6 | 1 | 1 |
| Superficial wound infection: arm | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gastrointestinal complication | 2 | 4 | 0 | 0 | 1 | 2 | 0 | 0 | 3 | 3 | 0 | 0 |
Unexpected adverse events
Unexpected AEs (i.e. not listed in the study protocol or occurred after discharge from hospital) are given in Table 16 . Again, slightly fewer events occurred in patients who received OPCABG: 12 events occurred in 11 patients (24%) versus 20 events in 16 patients (30%) who received ONCABG. The most common events were post-discharge wound infections. A summary of unexpected SAEs is given in Table 17 . There were 37 unexpected SAEs, with slightly fewer in the patients who received OPCABG (12 vs. 25). Nine patients (20%) who received OPCABG and 15 patients (28%) who received ONCABG experienced one or more unexpected SAEs. Most unexpected SAEs occurred post discharge, and the most common reason for classifying as an event as serious was prolonging an ongoing hospitalisation/causing hospitalisation. Five post-discharge events were classified as possibly related to the method of surgery (two events in the OPCABG group, both breathing difficulties/shortness of breath, and three in the ONCABG group: stroke, sternal wound reopening and death following hospital admission) and one event was classified as probably related to the method of surgery (in the ONCABG group: shortness of breath and palpitations). Owing to the reduction in the follow-up period of the trial, seven of the events reported in Table 17 (two in the OPCABG group and five in the ONCABG group) took place after the patient’s 4- to 8-week follow-up appointment.
| AE | Received OPCABG (N = 46) | Received ONCABG (N = 54) | Overall (N = 100) | |||
|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |
| Total number of events | 12 | 20 | 32 | |||
| Patients with one or more events | 11 | 24 | 16 | 30 | 27 | 27 |
| Events pre-hospital discharge | ||||||
| Cardioverted for atrial flutter | 0 | 1 | 1 | |||
| Coffee ground vomit | 0 | 1 | 1 | |||
| CPAP for bibasal collapse | 0 | 1 | 1 | |||
| Diarrhoea | 0 | 1 | 1 | |||
| Dual chamber ICD implant – planned prior to intervention | 0 | 1 | 1 | |||
| Left pleural effusion: ICD inserted and 500 ml drained | 0 | 1 | 1 | |||
| Pleural effusion: right side | 0 | 1 | 1 | |||
| Renal impairment (acute) (creatinine raised 273 max.) | 1 | 0 | 1 | |||
| Required blood transfusions for haemophilia | 0 | 1 | 1 | |||
| Urinary retention: failed trial without catheter. Commenced on tamsulosin – successful trial without catheter pre discharge | 1 | 0 | 1 | |||
| UTI | 1 | 1 | 2 | |||
| VT | 1 | 0 | 1 | |||
| Wheezing | 0 | 1 | 1 | |||
| Events post hospital discharge | ||||||
| Attended accident and emergency with shortness of breath, underwent chest radiography and was diagnosed with fluid on the lung. Possible reoccurrence of pleural effusion. Prescribed diuretics and sent home that day | 0 | 1 | 1 | |||
| Respiratory infection | 1 | 3 | 4 | |||
| Superficial wound infection: chest | 1 | 0 | 1 | |||
| Superficial wound infection: leg | 6 | 5 | 11 | |||
| Radiograph taken for a suspected chest infection | 0 | 1 | 1 | |||
| AE | Received OPCABG (N = 46) | Received ONCABG (N = 54) | Overall (N = 100) | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Total number of events | 12 | 25 | 37 | ||||
| Patients with one or more events | 9 | 20 | 15 | 28 | 24 | 24 | |
| Description of events | |||||||
| Pre operative | |||||||
| Death | 1 | 1 | 2 | ||||
| Other events | Emergency admission prior to surgery | 0 | 1 | 1 | |||
| MI | 0 | 1 | 1 | ||||
| Post operative but pre discharge | |||||||
| Death | 0 | 1 | 1 | ||||
| Cardiac events | Reintubation and mechanical ventilation | 1 | 0 | 1 | |||
| Ventilator-associated pneumonia: heart failure | 0 | 1 | 1 | ||||
| Acute coronary syndrome and pulmonary oedema | 0 | 1 | 1 | ||||
| Other events | Critical illness neuropathy | 0 | 1 | 1 | |||
| Diarrhoea/vomiting | 0 | 1 | 1 | ||||
| Post discharge | |||||||
| Death | 2 | 1 | 3 | ||||
| Cardiac events | AF | 1 | 0 | 1 | |||
| Heart failure secondary to AF | 0 | 1 | 1 | ||||
| Shortness of breath/difficulty in breathing | 3 | 2 | 5 | ||||
| Pulmonary events | Fluid on lungs | 0 | 1 | 1 | |||
| Pulmonary embolism | 0 | 1 | 1 | ||||
| Pulmonary oedema | 1 | 0 | 1 | ||||
| Infectious events | Chest infection | 0 | 1 | 1 | |||
| Wound infection | 0 | 1 | 1 | ||||
| Cellulitis | 0 | 2 | 2 | ||||
| Hospital-acquired pneumonia | 0 | 1 | 1 | ||||
| Clostridium difficile infection | 0 | 1 | 1 | ||||
| Other events | Stroke | 2 | 1 | 3 | |||
| Anaemia and hypotension | 1 | 0 | 1 | ||||
| Fall due to hypotension | 0 | 1 | 1 | ||||
| Diarrhoea/vomiting | 0 | 2 | 2 | ||||
| Sternal wound reopening: failure to heal | 0 | 1 | 1 | ||||
| Timing of events | Pre surgery | 1 | 3 | 4 | |||
| Post surgery but pre discharge | 1 | 5 | 6 | ||||
| Post discharge | 10 | 17 | 27 | ||||
| Maximum intensity | Mild | 0 | 2 | 2 | |||
| Moderate | 5 | 8 | 13 | ||||
| Severe | 7 | 15 | 22 | ||||
| Reason event classified as SAE | Resulted in death | 3 | 3 | 6 | |||
| Is/was life-threatening | 2 | 5 | 7 | ||||
| Resulted in persistence of significant disability/incapacity | 4 | 7 | 11 | ||||
| Prolonged ongoing hospitalisation/caused hospitalisation | 9 | 20 | 29 | ||||
| Other | 0 | 2 | 2 | ||||
| Relatedness to the method of surgery | Not related | 6 | 10 | 16 | |||
| Unlikely to be related | 4 | 11 | 15 | ||||
| Possibly related | 2 | 3 | 5 | ||||
| Probably related | 0 | 1 | 1 | ||||
Thirty-three out of 46 patients (72%) who received OPCABG, 39 out of 54 patients (72%) who received ONCABG and 72 out of the total 100 patients (72%) experienced either the primary outcome or any AE. Similarly, 13 out of 46 patients (28%) who received OPCABG, 19 out of 54 patients (35%) who received ONCABG and 32 out of the total 100 patients experienced either the primary outcome or any SAE.
Chapter 4 Discussion
Main findings: study conduct
The main findings of the CRISP trial are that expertise-based randomisation is challenging to implement and make work in a tertiary surgical setting. For a range of logistical reasons, the trial failed to recruit in time and to target, and the proposal to extend the primary outcome to include (1) reoperation for bleeding, (2) low cardiac output, (3) new onset of atrial arrhythmia and (4) AKI, and thereby reduce the study size, was not accepted by the funder. The trial was closed prematurely on the grounds of futility and also because of the perceived overlap between CRISP and the Canadian-led CORONARY trial.
Some of the challenges faced in CRISP were due to the context and nature of the service provision in the UK. Cardiac surgery is a tertiary service. As a consequence, patients are referred from a large geographical area and a significant proportion of referrals are urgent inpatients waiting in neighbouring ‘feeder’ hospitals for a suitable surgical slot to become available. The information provided at referral was often limited, making the assessment of eligibility for the trial by a research nurse or co-ordinator difficult. CRISP was marketed as a trial in high-risk patients. It was therefore important that only patients likely to be eligible were contacted, to avoid undue stress to patients at lower risk of complications.
Optimising the recruitment pathway was difficult, and the challenges varied according to how the local service was organised. Elective patients were usually seen at least once before surgery in an outpatient referral and/or preoperative assessment clinic. These contacts provided opportunities for the local research team to engage with potential participants, discuss the trial and seek consent, but often patients were unwilling to take part because they either wished to stay with the surgeon they met at the first appointment or wanted the surgeon to decide which type of surgery was best for them. Frequently, the need for surgery was not discussed until this first appointment so contacting a patient in advance of this was not considered appropriate. Urgent patients presented a different challenge. In the centres with a policy of transferring patients to the cardiac centre 2 or 3 days before surgery, the recruitment window was adequate and expertise-based randomisation was achievable, provided experts in both ONCABG and OPCABG were available to carry out the surgery. In centres where the policy was to transfer the patient as close to surgery as possible, recruitment and expertise-based randomisation was severely hampered. The CLRN was not long established when CRISP was set up and support from CLRN research nurses working at ‘feeder’ hospitals to facilitate recruitment was not forthcoming. This may not be the case now. Research governance issues were also a limiting factor, the concept of the research passport was not working well at that time and the need to identify local principal investigators at hospitals where the study was not taking place and in a speciality that was not theirs proved impossible.
Some of these issues were relevant to the context and setting in which CRISP was based only, but others were not. The availability of an expert surgeon to carry out the operation within a time scale that does not breach local and national targets for treatment applies to any surgical trial using expertise-based randomisation. The allocation of patients to surgeons through a system of named referrals, or via a generic pool, and the willingness or otherwise of surgeons to work together and ‘share’ their patients is a challenge and potential barrier to recruitment into any trial using expertise-based randomisation. The majority of surgeons continue to work autonomously, but this is gradually changing with the appointment of a clinical director or a chief of service; however, this is by no means widespread, particularly in the UK. When a patient is referred directly to an individual surgeon, that surgeon becomes responsible for that patient. Surgeons are often reluctant to transfer the patient to another surgeon, especially after meeting the patient and the ‘doctor–patient bond’ has formed. In addition, there continues to be a strongly held belief that the length of a surgeon’s waiting list reflects his or her surgical ability. Similarly, understanding the recruitment pathway and optimising when and how to introduce the trial to patients to ensure that surgeon preferences do not influence patient decisions is relevant to all surgical trials.
Many of these barriers to recruitment have been encountered previously. Ross et al.,58 in 1999, identified time constraints, lack of staff and training, worry about the impact on the doctor–patient relationship, concern for patients, loss of professional autonomy, difficulty with the consent procedure and lack of rewards and recognition as the key clinical-based barriers, while, for patients, the main barriers were the additional demands of the trial, patient preferences, worry caused by uncertainty and concerns about information and consent. A survey from 2011 of centres recruiting to three trials in head and neck surgery, all of which were significantly delayed and behind target, identified patient and surgeon preferences, insufficient time in the NHS clinic, lack of research nurse support, insufficient funding for excess treatment costs and delays in the approval process as the key barriers. 59 Complex recruitment pathways involving staff across different specialties/centres have also hindered recruitment in other trials. 60
In addition, for a trial such as CRISP to recruit successfully in the UK health-care setting, there has to be an agreement when the research is funded that a centre as a whole will participate in the study. The surgical autonomy needs to be broken down and the structure of the NHS, with consultants responsible for their own patients, is a stumbling block that is not limited to expertise-based recruitment. Surgeons need to work together and there need to be improved links between those responsible for service delivery and for the research. In the UK, the NHS is under huge pressure to deliver services and treatment to target, while at the same time reducing costs. Expertise-based recruitment, with a limited number of surgical experts, will almost inevitably lead to longer waiting times for some patients. For it to be implemented successfully in a surgical trial, the service providers and the health-care commissioners need to be committed to the research and be prepared to allow some flexibility in the targets in order for the research to succeed. Similarly, research needs to be considered an integral part of the service provision of a hospital; strategies for reducing hospital-based costs often impact on research. For example, patients are increasingly spending less time in hospital before their surgery and so the opportunities for recruitment are restricted. This was a particular problem for high-risk urgent in-hospital transfers (ideal candidates for the CRISP trial) as these patients will not have attended the cardiac centre previously and so there were no opportunities for earlier recruitment. Similarly, there needs to be a greater flexibility in the implementation of the research governance framework in NHS hospitals and within the CLRN. The need for local principal investigators at ‘feeder’ hospitals and the unwillingness of CLRN nurses at these hospitals to facilitate recruitment caused particular frustration.
Main findings: study results
The CRISP trial did not find statistically significant differences between the OPCABG and ONCABG groups owing to the limited power (< 2% of the target number of patients was recruited). However, the question that the trial set out to address remains important. The Cochrane review, published in 2012,34 acknowledged that mainly patients with low risk of postoperative complications were enrolled in the 86 trials reviewed and patients with three-vessel coronary disease and impaired LV function were under-represented.
The two largest trials to compare ONCABG and OPCABG, the ROOBY37 and the CORONARY40 trials, have been published since the CRISP trial began. The ROOBY trial, which contributed 2203 patients to the Cochrane review, has been severely criticised. The operative experience of the surgeons in the OPCABG group was substantially less than that of the ONCABG surgeons (median of 50 patients per surgeon), which was reflected in a high conversion rate from OPCABG to ONCABG (12%), a significant proportion of patients receiving fewer grafts than planned (18% OPCABG vs. 11% ONCABG),37 significantly lower patency rates (arterial conduits: 85.8% vs. 91.4% and saphenous vein grafts: 72.7% vs. 80.4%) at 1 year and fewer patients with effective revascularisation (50.1% vs. 63.9%) with OPCABG compared with ONCABG. 61 The trial also recruited predominantly low-risk patients.
The CORONARY trial, the largest trial to date, recruited a higher proportion of higher-risk patients than the ROOBY trial, although < 20% of participants had a EuroSCORE of > 5. 40 This compares with 74% of patients recruited to CRISP. The participating surgeons were also more experienced than those recruited to the ROOBY trial: all surgeons were required to have > 2 years’ experience and have completed > 100 procedures involving their preferred technique. Trainees were not allowed to be the primary surgeon for any procedure. This experience threshold was consistent with that used in CRISP.
The Cochrane meta-analysis has been updated to include the results from the CORONARY and CRISP trials. The results, for all-cause mortality, MI, stroke and renal failure are summarised in Table 18 . The RR of death and MI reduced from 1.24 (95% CI 1.01 to 1.53) to 1.18 (95% CI 0.98 to 1.40) and from 1.00 (95% CI 0.79 to 1.26) to 0.96 (95% CI 0.82 to 1.12) respectively, while the RR of a stroke and a renal complication increased from 0.76 (95% CI 0.54 to 1.06) to 0.80 (95% CI 0.61 to 1.06) and from 0.86 (95% CI 0.62 to 1.20) to 0.92 (95% CI 0.70 to 1.21), respectively.
| Outcome | Randomised to OPCABG | Randomised to ONCABG | RR (95% CI) | p-value | ||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Deatha | 249/7604 | 3.3 | 220/7570 | 2.9 | 1.18 (0.98 to 1.40) | 0.077 |
| MIb | 301/6710 | 4.5 | 311/6687 | 4.7 | 0.96 (0.82 to 1.12) | 0.60 |
| Strokec | 86/6951 | 1.2 | 112/6943 | 1.6 | 0.80 (0.61 to 1.06) | 0.13 |
| Renal complicationd | 90/4835 | 1.9 | 97/4821 | 2.0 | 0.92 (0.70 to 1.21) | 0.55 |
The Cochrane review identified three trials in high-risk patients: the BBS trial, which recruited 341 patients with a EuroSCORE of ≥ 5 and triple-vessel disease;38,62 a trial by Carrier and colleagues, which recruited 65 patients with at least three of the following criteria: age > 65 years, high blood pressure, diabetes, creatinine > 133 mol/l, LV ejection fraction < 45%, chronic pulmonary disease, unstable angina, congestive heart failure, repeat CABG, anaemia and carotid atherosclerosis;41 and a study in 128 patients with a ST-segment elevation MI. 42 The data from these trials, plus CRISP, have been combined in meta-analyses, the results of which are shown in Figures 7 – 10 . Part (a) of each figure is restricted to early outcomes (30 days or hospital discharge) and part (b) includes outcomes across the full follow-up period of each study. The BBS trial and the trial in patients with a ST-segment elevation MI reported cardiac-related mortality outcomes to 3 years, while CRISP and the trial by Carrier et al. 41 reported outcomes to 30 days only. It was not possible to include the CORONARY trial results in these meta-analyses and the data were not reported for the individual components of the trial’s composite outcome for the subgroup of high-risk patients. In contrast to the Cochrane review, these analyses suggest a lower risk of death with OPCABG in the early postoperative period (RR 0.46, 95% CI 0.20 to 1.04; p = 0.06) and a comparable risk overall (RR 0.90, 95% CI 0.32 to 2.58; p = 0.85). The risk of an MI was also reduced in the early postoperative period (RR 0.59, 95% CI 0.33 to 1.06; p = 0.077). No differences in the risk of a stroke or of renal complications were found.
FIGURE 7.
Meta-analysis of trials in high-risk patients. a, Death up to day 30/hospital discharge; and b, death up to 3 years.
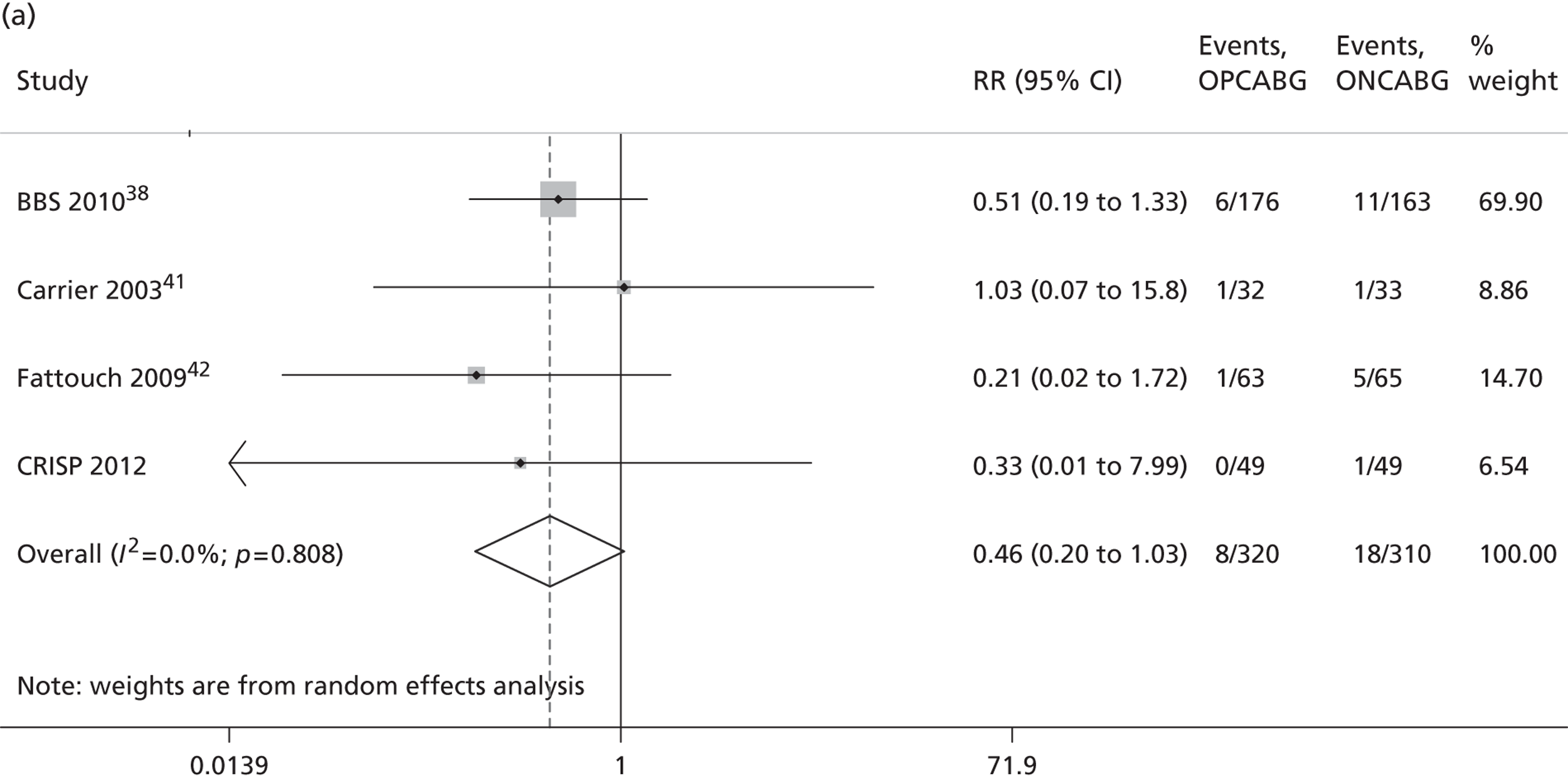
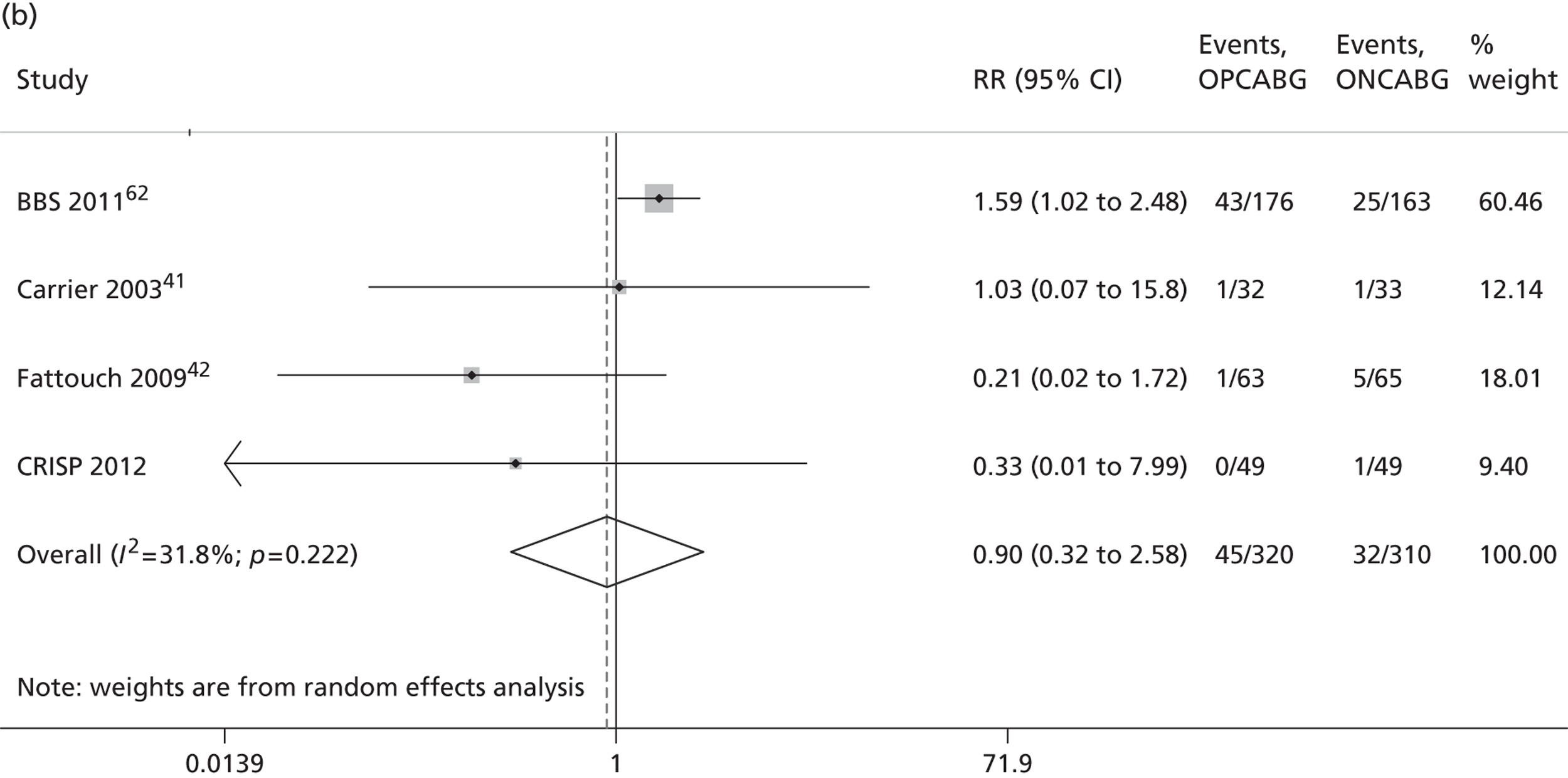
FIGURE 8.
Meta-analysis of trials in high-risk patients. a, MI up to day 30/hospital discharge; and b, MI up to 3 years.
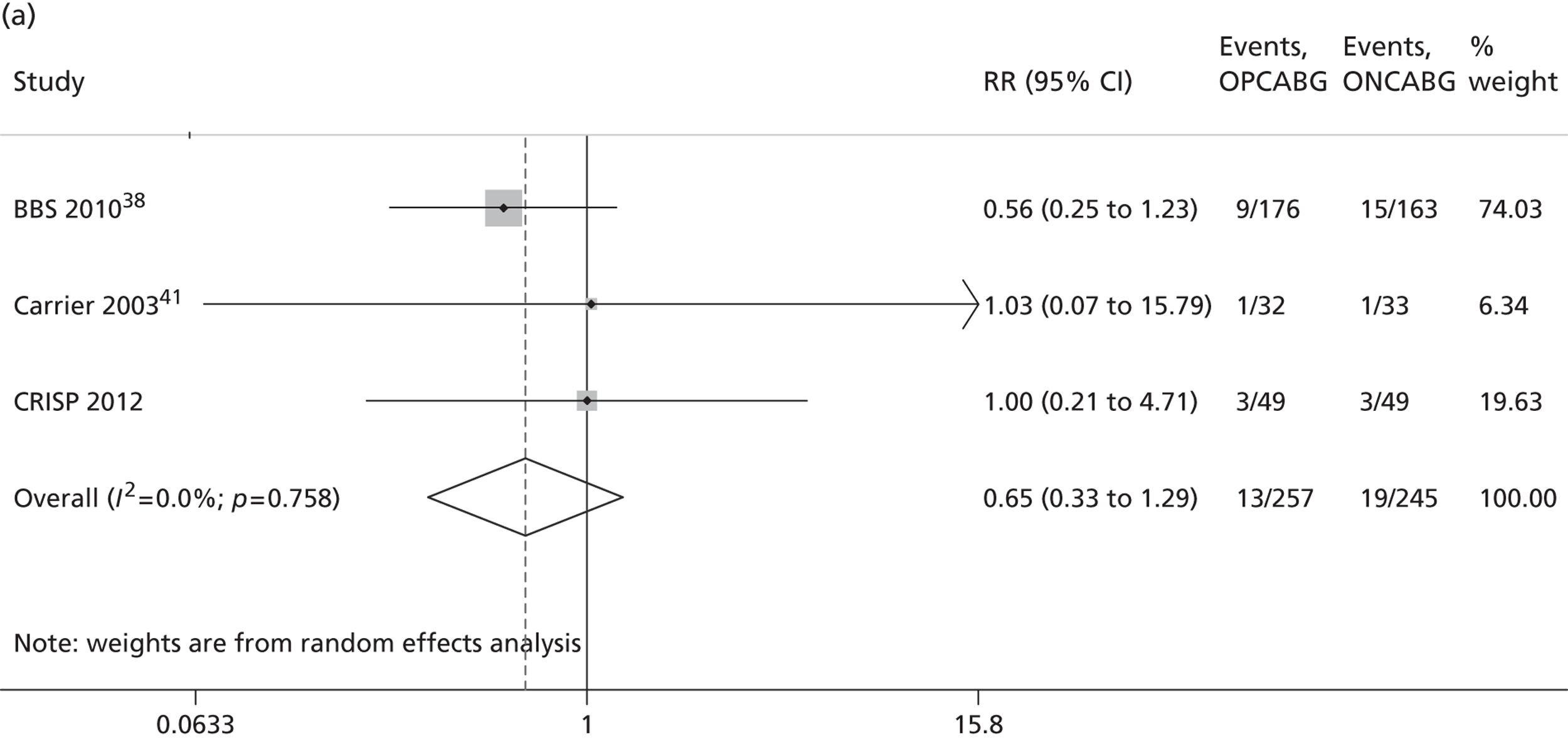
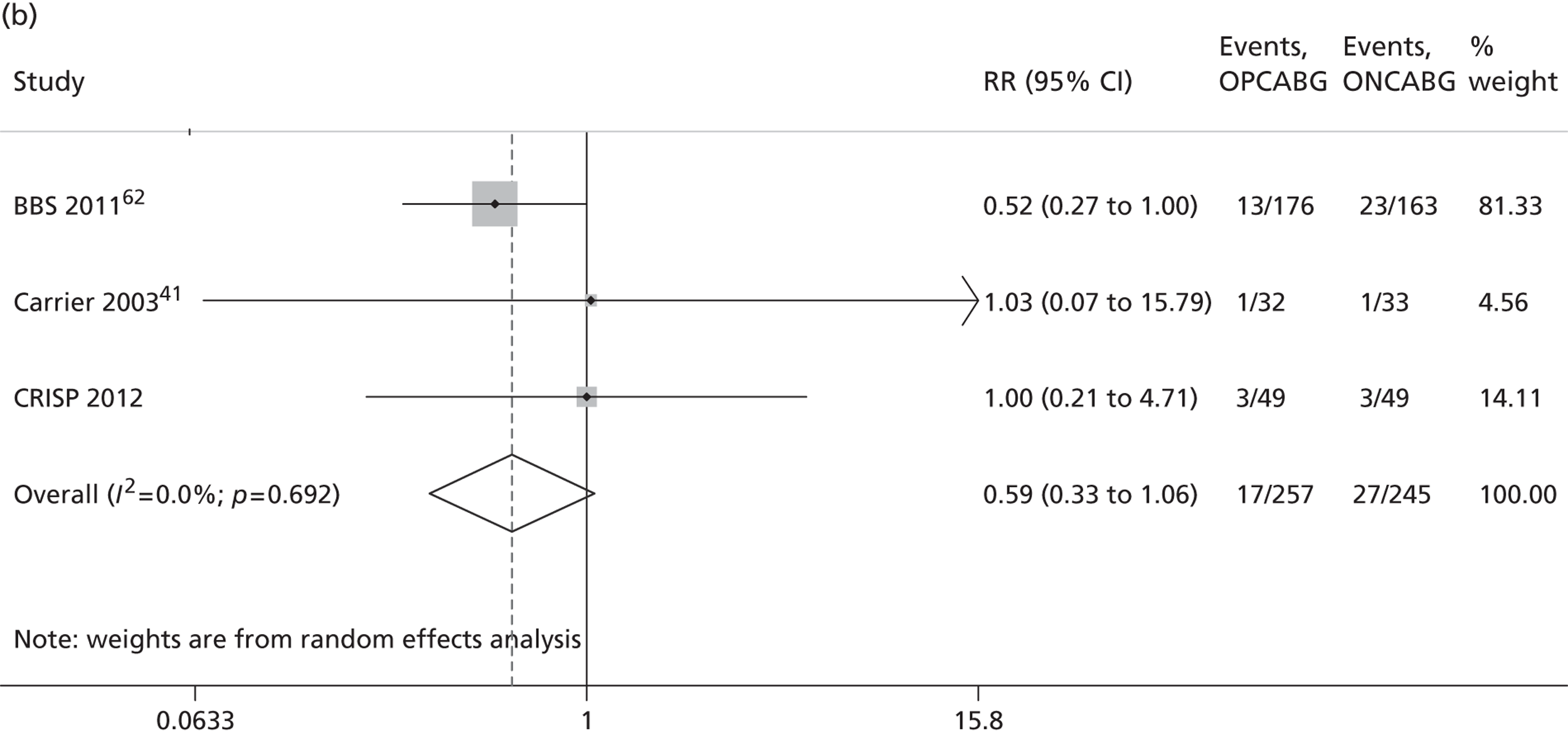
FIGURE 9.
Meta-analysis of trials in high-risk patients. a, Stroke up to day 30/hospital discharge; and b, stroke up to 3 years.
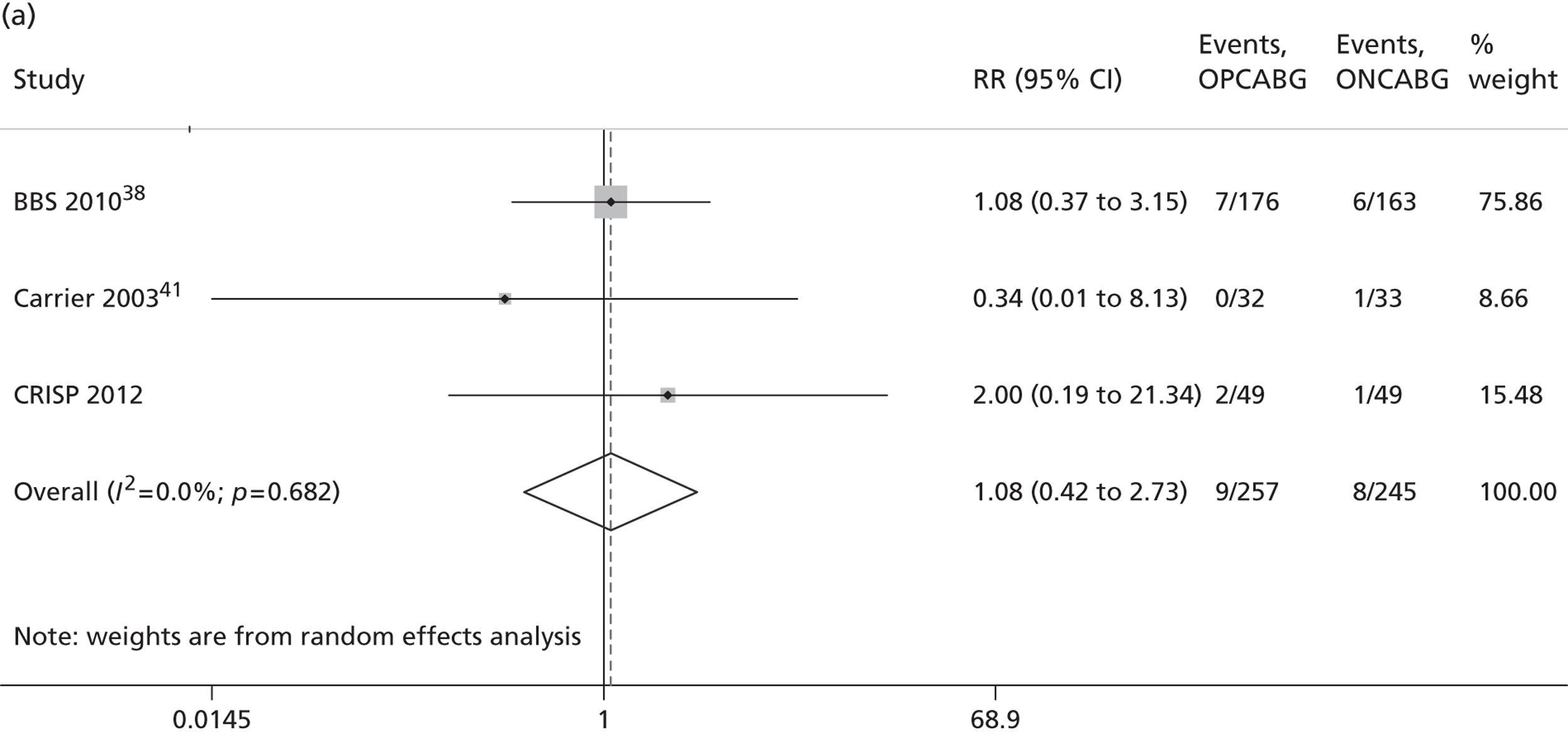
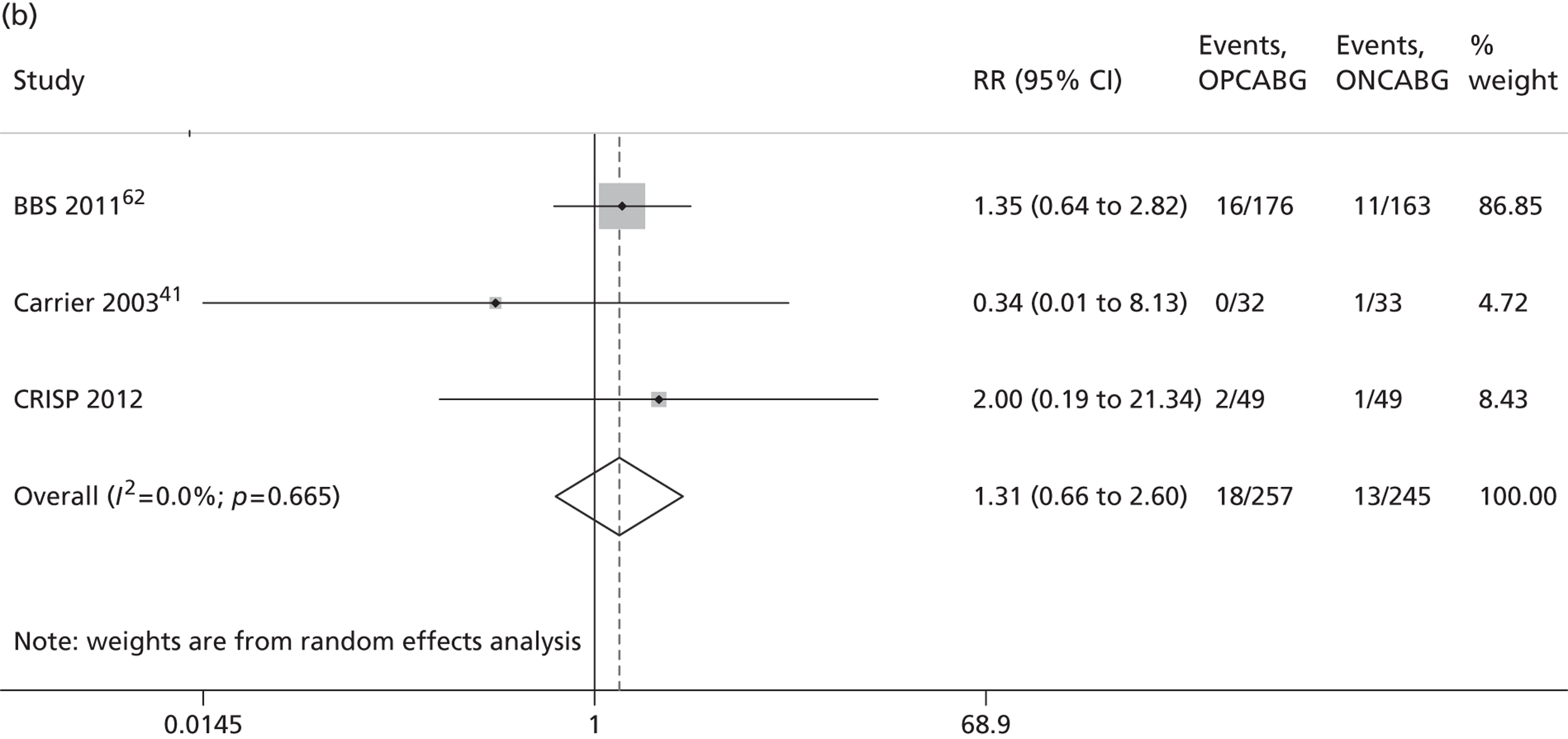
FIGURE 10.
Meta-analysis of trials in high-risk patients. a, Renal complications up to day 30/hospital discharge; and b, renal complications up to 3 years.
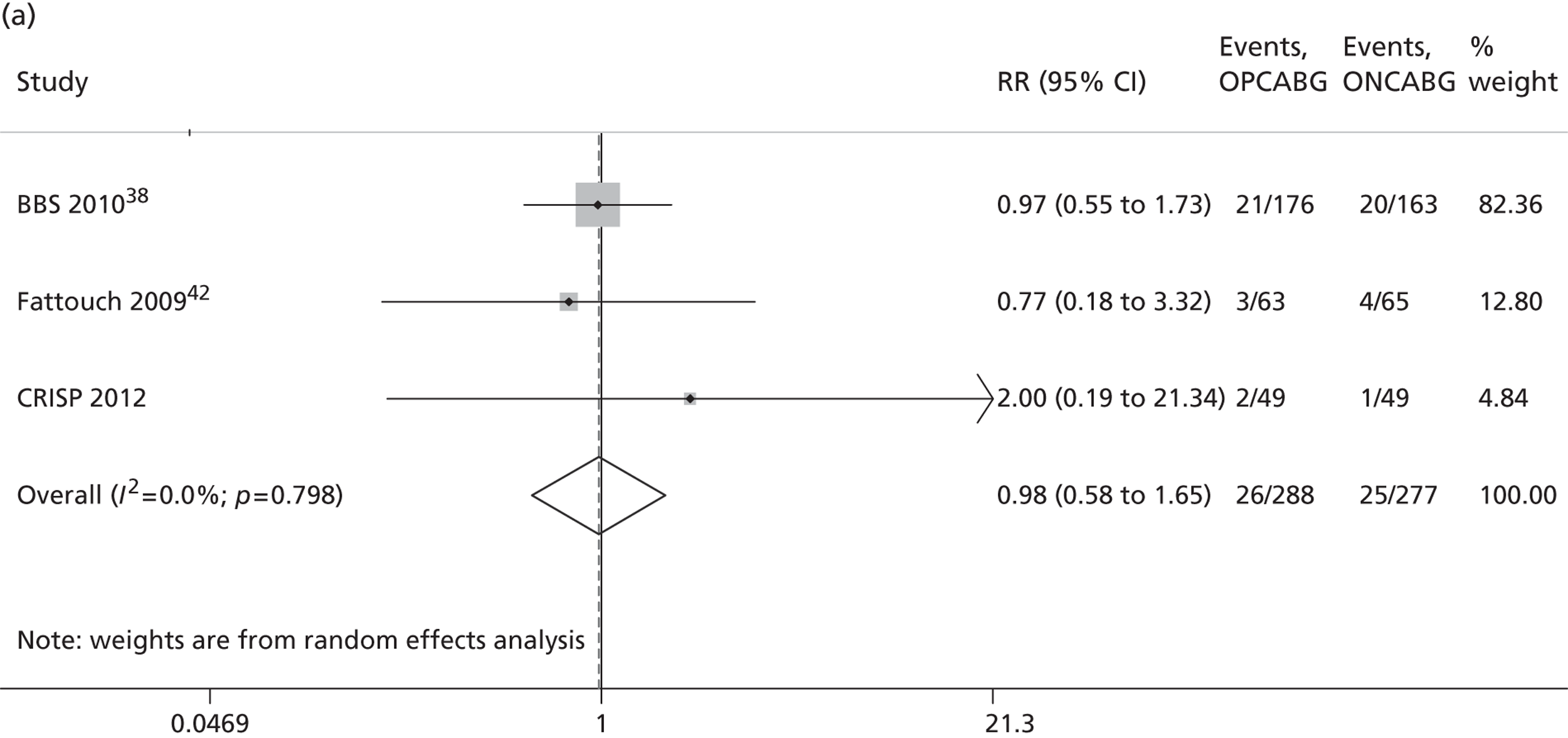
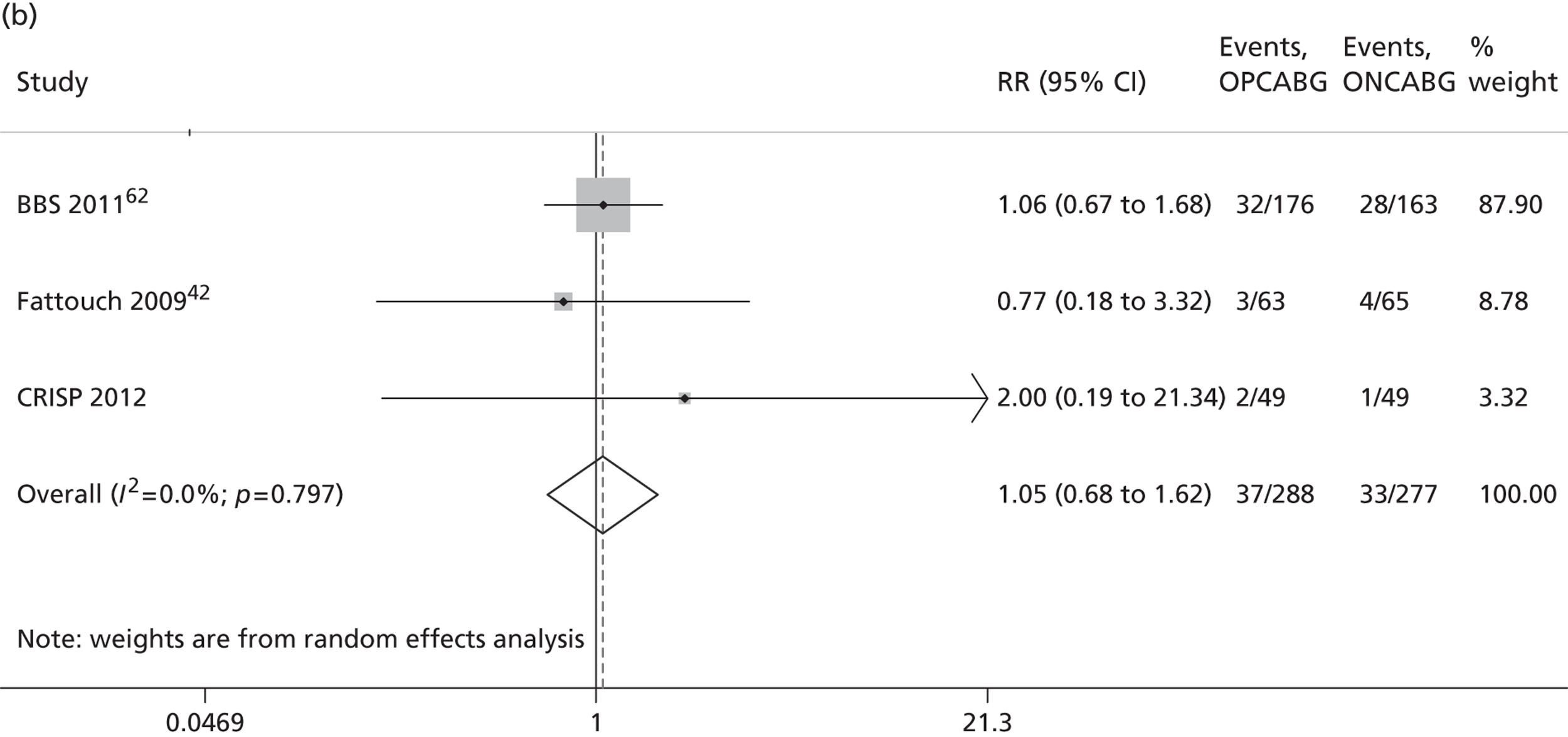
The BBS and CORONARY trials both reported the results of a composite primary outcome at 30 days in high-risk patients and the composites varied across studies. The BBS trial38 used death, MI, cardiac arrest, low cardiac output, stroke and coronary reintervention, while the CORONARY trial40 used death, MI, stroke and new renal failure requiring dialysis. These compare with the CRISP composite of death, new renal failure, MI, stroke, prolonged ventilation and sternal wound dehiscence. The composites from these studies were combined in a meta-analysis and the results are summarised in Table 19 . As anticipated, the pooled estimate reflects the estimate from the large CORONARY trial, but with a narrower CI.
| Study | n | Treatment effect (95% CI)a | p-value |
|---|---|---|---|
| BBS | 341 | RR 0.83 (0.52 to 1.34) | |
| CORONARY | 828 | HR 0.85 (0.58 to 1.25) | |
| CRISP | 98 | OR 1.07 (0.27 to 4.14) | |
| Overallb | 1257 | RR 0.85 (0.64 to 1.14) | 0.28 |
One possible reason for the lack of compelling evidence of a difference between OPCABG and ONCABG in the recent trials is that over time techniques in ONCABG have improved. Different methods of cardioplegia and body temperature cooling have been introduced to reduce myocardial injury and systemic inflammatory response during surgery and a miniaturised CPB circuit has been developed that is associated with a non-significantly reduced risk of adverse outcomes. 63 There may also have been ill-defined temporal improvements in care across both techniques.
Strengths and limitations
Despite the failure of CRISP to recruit to target, the options to improve recruitment were thoroughly tested. There was a strongly held view that the expertise-based randomisation was the key barrier to successful recruitment, but, when we attempted to change to a within-surgeon allocation, many of the OPCABG experts were no longer willing to participate. A survey of orthopaedic surgeons similarly found a strong preference for expertise-based randomisation. 64 We believe that expertise-based randomisation is the only way to evaluate established surgical procedures where there are strongly held preferences but collective equipoise. Furthermore, it avoids the problem of differential expertise bias,65 can protect against crossover as a result of unfamiliarity or less experience with one surgical method and allows for greater surgeon participation. In addition, an expertise-based design provides the participant with the assurance that the surgery will be carried out by a surgeon who has both the appropriate expertise and is comfortable carrying out the procedure. Expertise-based randomisation has been used successfully in other areas, for example in studies comparing coronary angioplasty and CABG66–69 and in orthopaedic surgery. 69 However, we have to recognise that it may not be feasible in a tertiary referral setting, when the referral information to determine patient eligibility is often inadequate, surgeon availability is limited and there is an imbalance in the numbers of surgical experts at a centre.
The trial was methodologically strong; the risk of bias was minimised through concealed allocation and objective definitions for the primary end points. There was a blinded review of the blood results and preoperative and postoperative ECGs of all patients and a postoperative MI defined on consensus of the adjudicators. The database used to collect the data was robust and included extensive within-CRF and cross-CRF validation. The screening data were incomplete for most centres, as indicated by the wide variation in the proportion of screened patients recruited, and this is a weakness that was recognised by the TSC.
Despite the poor recruitment, the CRISP patients reflect the population the trial was designed to study. Using data from the Bristol cardiac surgery database, we compared the characteristics of the CRISP patients with 3364 eligible isolated CABG patients with a EuroSCORE of ≥ 5 who had undergone an operation between April 1997 and August 2012 in Bristol. The cohorts were of similar age (median 77 vs. 74 years) and sex mix (23% vs. 28% female) and comorbidities occurred with similar frequency (diabetes 24% vs. 26%, previous MI 70% vs. 68%, previous stroke 8% vs. 6%, median EuroSCORE was 6 in both cohorts). In addition, similar proportions had triple-vessel disease (77% vs. 73%) and > 50% disease in left main stem (33% vs. 30%). However, there was a lower proportion of patients with poor ejection fraction (5% vs. 11%) and the proportion of patients requiring surgery urgently was lower in the CRISP study (45% vs. 66%), which is reflective of the recruitment difficulties.
The final study size is a clear weakness: the study has low power to detect significant differences between the groups and the value of the trial data is their contribution to meta-analyses. However, the approach to the analysis of the data was strong. An analysis plan was prepared in advance of any comparative analyses of the study data and the number of statistical tests carried out was restricted. Formal statistical comparisons of treatment effects were only carried out if > 10 patients in total experienced the outcome (see Appendix 5 ), to minimise the probability of a type 1 error.
We chose to use an additive EuroSCORE of ≥ 5 as a marker of ‘high risk’. All scoring systems have their limitations and this score is strongly influenced by age (one point for every 5 years from 60 years onwards) and less by a participant’s comorbidity. As a consequence, CRISP recruited more elderly patients than the CORONARY trial (median age 77 vs. 68 years, respectively) and many fewer diabetic patients (24% vs. 47%, respectively), although, in both trials, only 5% of patients had poor LV function. The question of which treatment option is most effective, ONCABG or OPCABG, remains an important question in the large group of patients with poor LV function that cannot be answered by either trial.
Lessons for the future
If we were setting up the CRISP trial now there are many things that we would do differently. First, we would design the trial in two phases, with a feasibility phase followed by a main trial phase. This design is being used in other surgical areas and is an attractive option for funders of difficult-to-do trials.
Second, we would include a qualitative research element, which would involve researchers interviewing the research teams at the study centres in order to gain a full understanding of the recruitment pathway, barriers to recruitment (including a willingness or otherwise to work together and share patients) and the extent of the equipoise. Through feedback and training, the study team (including the surgeons) would be taught how to present the trial in an unbiased way to minimise the number who decline to take part. The strength of the bond formed between surgeon and patient at that first referral would also be explored through interviews with patients who did and did not agree to take part. This approach has been used very successfully in the POTECT trial of surgery versus radiotherapy versus medical management in men with localised prostate cancer. 70 A total of 1500 men were recruited to a trial that many strongly believed would never succeed. Failure to meet the recruitment target is a common problem71 and qualitative methods have been recommended as the most effective for identifying and overcoming barriers to clinician recruitment activity and increasing recruitment. 72
Third, we would focus recruitment equally towards UK and overseas centres from the start. Many of the barriers to recruitment experienced in the UK may not be such a problem overseas. Although the centre in India was actively participating for only a short period before CRISP closed, it recruited six patients in 3 weeks, which was more than was achieved in any UK centre. The CORONARY trial, which successfully recruited 4752 patients at 79 centres, recruited only a small number of patients from the UK (227 patients, < 5%). The biggest contributors were India and China (1307 and 781 patients, respectively).
Future research
The answer to the question of whether OPCABG offers an additional benefit over ONCABG in a high-risk population is unclear. The trial evidence in high-risk patients suggests the outcomes are similar, although the collective evidence across all trials suggests the risk of death is higher with OPCABG (RR 1.18; p = 0.077). Possible reasons for this are fewer grafts, a greater need for subsequent revascularisation and worse patency. Despite recruiting more than 15,000 patients into trials of OPCABG versus ONCABG, the views of members of the surgical community are polarised. A qualitative evaluation of the reasons behind the views held by the advocates of the two techniques, and in particular what evidence would need to be presented in order to change individual practice, is an area for future research.
One possible explanation for the polarisation is the belief that ‘it’s in the surgeon’s hands’. If the surgeons are true ‘experts’ then one might anticipate no difference in outcomes between the two methods. Surgeons that use both techniques, albeit one perhaps slightly more frequently than the other, are likely to be less committed to OPCABG than surgeons who use OPCABG exclusively, and this may be reflected in the results. One way to test this hypothesis would be an individual patient data meta-analysis of the trial data, classifying patients according to the characteristics/experience of the surgeon.
Chapter 5 Conclusion
We firmly believe there is still a role for expertise-based randomisation to evaluate established treatments in which there are strong practitioner preferences and both treatments are used. The CRISP trial was not successful for a range of logistical reasons. Nonetheless, the experience gained will be of value for the design and conduct of future trials, so that some of the pitfalls experienced in CRISP can be avoided.
Acknowledgements
The CRISP investigators thank the following, without whose help the study would not have happened:
-
the CRISP trial participants
-
the CRISP TSC and DMSC members (see Appendix 6 for details)
-
staff at the Bristol Clinical Trials and Evaluation Unit who helped with the set-up and running of the trial: Lucy Dreyer, Jon Evans, Neil Smith, Verity Waine
-
research nurse teams at the participating centres
-
Professor Raimondo Ascione, Chiara Bucciarelli-Ducci and Elisa Mcalindon for the masked independent evaluation of the blood results and ECGs of CRISP participants
-
Carol Wallis, trial administrator, Oxford, for providing a vital link between the Bristol Clinical Trials and Evaluation Unit and the Sponsor’s office.
Contribution of authors
-
Dr Chris A Rogers designed the study with Professors DP Taggart, DG Altman, GD Angelini, A Gray and BC Reeves. She led the team at the Bristol Clinical Trials and Evaluation Unit, which led and managed the trial, and oversaw the analyses and their interpretation. She drafted the report with Miss K Pike.
-
Miss Katie Pike advised on the design of the CRFs and the trial database. She wrote the statistical analysis plan and carried out the statistical analyses under the guidance of Dr Rogers. She drafted the report with Dr Rogers.
-
Dr Helen Campbell contributed to the design of the resources use components of the CRFs and analysed some resource use data. She designed the five-level versions of the EQ-5D questionnaires and analysed the EQ-5D data.
-
Professor Barnaby C Reeves designed the study with Professors DP Taggart, DG Altman, GD Angelini, A Gray and Dr Rogers. He helped to draft the discussion of the trial.
-
Professor Gianni D Angelini designed the study with Professors DP Taggart, DG Altman, A Gray, BC Reeves and Dr Rogers. He actively promoted the trial amongst his clinical colleagues, particularly those based in European centres. He reviewed a draft of the report.
-
Professor Alastair Gray designed the study with Professors DP Taggart, DG Altman, GD Angelini, and BC Reeves and Dr Rogers and led the health economic analyses. He reviewed a draft of the report.
-
Professor Doug G Altman designed the study with Professors DP Taggart, GD Angelini, A Gray, BC Reeves and Dr Rogers. He advised on the use of expertise-based randomisation.
-
Dr Helen Miller was a trial manager at Bristol Clinical Trials and Evaluation Unit. She liaised with sites about the protocol and queries, carried out visits to sites and facilitated the closure of the trial.
-
Miss Sian Wells was a trial manager at Bristol Clinical Trials and Evaluation Unit. She designed the trial CRFs and database, liaised with sites about the protocol and queries, and carried out visits to sites.
-
Professor David P Taggart designed the study with Professors DG Altman, GD Angelini, A Gray, BC Reeves and Dr Rogers. He was chief investigator and the principal applicant in the effort to secure funding. He actively promoted the trial amongst his clinical colleagues. He reviewed a draft of the report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, the MRC, NETSCC, the HTA programme, the EME programme or the Department of Health.
References
- Taggart DP. Surgery is the best intervention for severe coronary artery disease. BMJ 2005;330:785-6. http://dx.doi.org/10.1136/bmj.330.7494.785.
- Brener SJ, Lytle BW, Casserly IP, Schneider JP, Topol EJ, Lauer MS. Propensity analysis of long-term survival after surgical or percutaneous revascularization in patients with multivessel coronary artery disease and high-risk features. Circulation 2004;109:2290-5. http://dx.doi.org/10.1161/01.CIR.0000126826.58526.14.
- Hannan EL, Racz MJ, Walford G, Jones RH, Ryan TJ, Bennett E, et al. Long-term outcomes of coronary-artery bypass grafting versus stent implantation. N Engl J Med 2005;352:2174-83. http://dx.doi.org/10.1056/NEJMoa040316.
- Malenka DJ, Leavitt BJ, Hearne MJ, Robb JF, Baribeau YR, Ryan TJ, et al. Comparing long-term survival of patients with multivessel coronary disease after CABG or PCI: analysis of BARI-like patients in northern New England. Circulation 2005;112:I371-6.
- Smith PK, Califf RM, Tuttle RH, Shaw LK, Lee KL, Delong ER, et al. Selection of surgical or percutaneous coronary intervention provides differential longevity benefit. Ann Thorac Surg 2006;82:1420-8. http://dx.doi.org/10.1016/j.athoracsur.2006.04.044.
- Babapulle MN, Joseph L, Belisle P, Brophy JM, Eisenberg MJ. A hierarchical Bayesian meta-analysis of randomised clinical trials of drug-eluting stents. Lancet 2004;364:583-91. http://dx.doi.org/10.1016/S0140-6736(04)16850-5.
- Hill R, Bagust A, Bakhai A, Dickson R, Dundar Y, Haycox A, et al. Coronary artery stents: a rapid systematic review and economic evaluation. Health Technol Assess 2004;8.
- Nordmann AJ, Briel M, Bucher HC. Mortality in randomized controlled trials comparing drug-eluting vs. bare metal stents in coronary artery disease: a meta-analysis. Eur Heart J 2006;27:2784-814. http://dx.doi.org/10.1093/eurheartj/ehl282.
- Gersh BJ, Frye RL. Methods of coronary revascularization – things may not be as they seem. N Engl J Med 2005;352:2235-7. http://dx.doi.org/10.1056/NEJMe058053.
- Hannan EL, Racz M, Holmes DR, King SB, Walford G, Ambrose JA, et al. Impact of completeness of percutaneous coronary intervention revascularization on long-term outcomes in the stent era. Circulation 2006;113:2406-12. http://dx.doi.org/10.1161/CIRCULATIONAHA.106.612267.
- Cheng DC, Bainbridge D, Martin JE, Novick RJ. Does off-pump coronary artery bypass reduce mortality, morbidity, and resource utilization when compared with conventional coronary artery bypass? A meta-analysis of randomized trials. Anesthesiology 2005;102:188-203. http://dx.doi.org/10.1097/00000542-200501000-00028.
- Wijeysundera DN, Beattie WS, Djaiani G, Rao V, Borger MA, Karkouti K, et al. Off-pump coronary artery surgery for reducing mortality and morbidity: meta-analysis of randomized and observational studies. J Am Coll Cardiol 2005;46:872-82.
- Sellke FW, DiMaio JM, Caplan LR, Ferguson TB, Gardner TJ, Hiratzka LF, et al. Comparing on-pump and off-pump coronary artery bypass grafting: numerous studies but few conclusions: a scientific statement from the American Heart Association council on cardiovascular surgery and anesthesia in collaboration with the interdisciplinary working group on quality of care and outcomes research. Circulation 2005;111:2858-64.
- Baumgartner WA, Burrows S, del Nido PJ, Gardner TJ, Goldberg S, Gorman RC, et al. Recommendations of the National Heart, Lung, and Blood Institute Working Group on future direction in cardiac surgery. Circulation 2005;111:3007-13. http://dx.doi.org/10.1161/CIRCULATIONAHA.104.530154.
- Cleveland JC, Shroyer AL, Chen AY, Peterson E, Grover FL. Off-pump coronary artery bypass grafting decreases risk-adjusted mortality and morbidity. Ann Thorac Surg 2001;72:1282-8. http://dx.doi.org/10.1016/S0003-4975(01)03006-5.
- Mack M, Bachand D, Acuff T, Edgerton J, Prince S, Dewey T, et al. Improved outcomes in coronary artery bypass grafting with beating-heart techniques. J Thorac Cardiovas Surg 2002;124:598-607. http://dx.doi.org/10.1067/mtc.2002.124884.
- Al-Ruzzeh S, Ambler G, Asimakopoulos G, Omar RZ, Hasan R, Fabri B, et al. Off-pump coronary artery bypass (OPCAB) surgery reduces risk-stratified morbidity and mortality: a United Kingdom multi-center comparative analysis of early clinical outcome. Circulation 2003;108:II1-8. http://dx.doi.org/10.1161/01.cir.0000087440.59920.a1.
- Calafiore AM, Di Mauro M, Canosa C, Di Giammarco G, Iaco AL, Contini M. Early and late outcome of myocardial revascularization with and without cardiopulmonary bypass in high risk patients (EuroSCORE > or = 6). Eur J Cardiothorac Surg 2003;23:360-7. http://dx.doi.org/10.1016/s1010-7940(02)00800-x.
- Sharony R, Bizekis CS, Kanchuger M, Galloway AC, Saunders PC, Applebaum R, et al. Off-pump coronary artery bypass grafting reduces mortality and stroke in patients with atheromatous aortas: a case control study. Circulation 2003;108:II15-20. http://dx.doi.org/10.1161/01.cir.0000087448.65888.21.
- Straka Z, Widimsky P, Jirasek K, Stros P, Votava J, Vanek T, et al. Off-pump versus on-pump coronary surgery: final results from a prospective randomized study PRAGUE-4. Ann Thorac Surg 2004;77:789-93. http://dx.doi.org/10.1016/j.athoracsur.2003.08.039.
- Puskas JD, Williams WH, Mahoney EM, Huber PR, Block PC, Duke PG, et al. Off-pump vs conventional coronary artery bypass grafting: early and 1-year graft patency, cost, and quality-of-life outcomes: a randomized trial. JAMA 2004;291:1841-9. http://dx.doi.org/10.1001/jama.291.15.1841.
- Angelini GD, Taylor FC, Reeves BC, Ascione R. Early and midterm outcome after off-pump and on-pump surgery in Beating Heart Against Cardioplegic Arrest Studies (BHACAS 1 and 2): a pooled analysis of two randomised controlled trials. Lancet 2002;359:1194-9. http://dx.doi.org/10.1016/S0140-6736(02)08216-8.
- Sedrakyan A, Wu AW, Parashar A, Bass EB, Treasure T. Off-pump surgery is associated with reduced occurrence of stroke and other morbidity as compared with traditional coronary artery bypass grafting: a meta-analysis of systematically reviewed trials. Stroke 2006;37:2759-69. http://dx.doi.org/10.1161/01.STR.0000245081.52877.f2.
- Moller CH, Penninga L, Wetterslev J, Steinbruchel DA, Gluud C. Clinical outcomes in randomized trials of off- vs. on-pump coronary artery bypass surgery: systematic review with meta-analyses and trial sequential analyses. Eur Heart J 2008;29:2601-16. http://dx.doi.org/10.1093/eurheartj/ehn335.
- Parolari A, Alamanni F, Cannata A, Naliato M, Bonati L, Rubini P, et al. Off-pump versus on-pump coronary artery bypass: meta-analysis of currently available randomized trials. Ann Thorac Surg 2003;76:37-40. http://dx.doi.org/10.1016/S0003-4975(03)00183-8.
- Reston JT, Tregear SJ, Turkelson CM. Meta-analysis of short-term and mid-term outcomes following off-pump coronary artery bypass grafting. Ann Thorac Surg 2003;76:1510-15. http://dx.doi.org/10.1016/S0003-4975(03)01195-0.
- Seabra VF, Alobaidi S, Balk EM, Poon AH, Jaber BL. Off-pump coronary artery bypass surgery and acute kidney injury: a meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol 2010;5:1734-44. http://dx.doi.org/10.2215/CJN.02800310.
- Feng ZZ, Shi J, Zhao XW, Xu ZF. Meta-analysis of on-pump and off-pump coronary arterial revascularization. Ann Thorac Surg 2009;87:757-65. http://dx.doi.org/10.1016/j.athoracsur.2008.11.042.
- Takagi H, Matsui M, Umemoto T. Off-pump coronary artery bypass may increase late mortality: a meta-analysis of randomized trials. Ann Thorac Surg 2010;89:1881-8. http://dx.doi.org/10.1016/j.athoracsur.2010.03.010.
- Kuss O, Borgermann J. Do higher-risk patients benefit from off-pump coronary artery bypass grafting? Evidence from an ecologic analysis of randomized trials. J Thorac Cardiovasc Surg 2011;142:e117-22. http://dx.doi.org/10.1016/j.jtcvs.2011.04.032.
- Kuss O, von Salviati B, Borgermann J. Off-pump versus on-pump coronary artery bypass grafting: a systematic review and meta-analysis of propensity score analyses. J Thorac Cardiovasc Surg 2010;140:829-35. http://dx.doi.org/10.1016/j.jtcvs.2009.12.022.
- Nigwekar SU, Kandula P, Hix JK, Thakar CV. Off-pump coronary artery bypass surgery and acute kidney injury: a meta-analysis of randomized and observational studies. Am J Kidney Dis 2009;54:413-23. http://dx.doi.org/10.1053/j.ajkd.2009.01.267.
- Takagi H, Matsui M, Umemoto T. Lower graft patency after off-pump than on-pump coronary artery bypass grafting: an updated meta-analysis of randomized trials. J Thorac Cardiovasc Surg 2010;140:e45-7. http://dx.doi.org/10.1016/j.jtcvs.2009.11.045.
- Moller CH, Penninga L, Wetterslev J, Steinbruchel DA, Gluud C. Off-pump versus on-pump coronary artery bypass grafting for ischaemic heart disease. Cochrane Database Syst Rev 2012;3.
- Afilalo J, Rasti M, Ohayon SM, Shimony A, Eisenberg MJ. Off-pump vs. on-pump coronary artery bypass surgery: an updated meta-analysis and meta-regression of randomized trials. Eur Heart J 2012;33:1257-67. http://dx.doi.org/10.1093/eurheartj/ehr307.
- Hueb W, Lopes NH, Gersh BJ, Castro CC, Paulitsch FS, Oliveira SA, et al. A randomized comparative study of patients undergoing myocardial revascularization with or without cardiopulmonary bypass surgery: the MASS III Trial. Trials 2008;9. http://dx.doi.org/10.1186/1745-6215-9-52.
- Shroyer AL, Grover FL, Hattler B, Collins JF, McDonald GO, Kozora E, et al. On-pump versus off-pump coronary-artery bypass surgery. N Engl J Med 2009;361:1827-37. http://dx.doi.org/10.1056/NEJMoa0902905.
- Moller CH, Perko MJ, Lund JT, Andersen LW, Kelbaek H, Madsen JK, et al. No major differences in 30-day outcomes in high-risk patients randomized to off-pump versus on-pump coronary bypass surgery: the Best Bypass Surgery trial. Circulation 2010;121:498-504. http://dx.doi.org/10.1161/CIRCULATIONAHA.109.880443.
- Houlind K, Kjeldsen BJ, Madsen SN, Rasmussen BS, Holme SJ, Schmidt TA, et al. The impact of avoiding cardiopulmonary by-pass during coronary artery bypass surgery in elderly patients: the Danish On-pump Off-pump Randomisation Study (DOORS). Trials 2009;10. http://dx.doi.org/10.1186/1745-6215-10-47.
- Lamy A, Devereaux PJ, Prabhakaran D, Taggart DP, Hu S, Paolasso E, et al. Off-pump or on-pump coronary-artery bypass grafting at 30 days. N Engl J Med 2012;366:1489-97. http://dx.doi.org/10.1056/NEJMoa1200388.
- Carrier M, Perrault LP, Jeanmart H, Martineau R, Cartier R, Page P. Randomized trial comparing off-pump to on-pump coronary artery bypass grafting in high-risk patients. Heart Surg Forum 2003;6:E89-92.
- Fattouch K, Guccione F, Dioguardi P, Sampognaro R, Corrado E, Caruso M, et al. Off-pump versus on-pump myocardial revascularization in patients with ST-segment elevation myocardial infarction: a randomized trial. J Thorac Cardiovasc Surg 2009;137:650-6. http://dx.doi.org/10.1016/j.jtcvs.2008.11.033.
- van der Linden W. Pitfalls in randomized surgical trials. Surgery 1980;87:258-62.
- Johnston BC, da Costa BR, Devereaux PJ, Akl EA, Busse JW, Expertise-Based RCTWG. The use of expertise-based randomized controlled trials to assess spinal manipulation and acupuncture for low back pain: a systematic review. Spine 2008;33:914-18. http://dx.doi.org/10.1097/BRS.0b013e31816b4be4.
- Devereaux PJ, Bhandari M, Clarke M, Montori VM, Cook DJ, Yusuf S, et al. Need for expertise based randomised controlled trials. BMJ 2005;330. http://dx.doi.org/10.1136/bmj.330.7482.88.
- Cook JA, Boutron I, Ravaud P, Moher D. Randomized Clinical Trials of Nonpharmacological Treatments. Boca Raton, FL: CRC Press; 2012.
- Scholtes VA, Nijman TH, van Beers L, Devereaux PJ, Poolman RW. Emerging designs in orthopaedics: expertise-based randomized controlled trials. J Bone Joint Surg Am 2012;94:24-8. http://dx.doi.org/10.2106/JBJS.K.01626.
- Khan NE, De Souza A, Mister R, Flather M, Clague J, Davies S, et al. A randomized comparison of off-pump and on-pump multivessel coronary-artery bypass surgery. N Engl J Med 2004;350:21-8. http://dx.doi.org/10.1056/NEJMoa031282.
- Nashef SA, Roques F, Michel P, Gauducheau E, Lemeshow S, Salamon R. European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothoracic Surg 1999;16:9-13. http://dx.doi.org/10.1016/S1010-7940(99)00134-7.
- Rose GA. The diagnosis of ischaemic heart pain and intermittent claudication in field surveys. Bull World Health Organ 1962;27:645-58.
- Canadian Cardiovascular Society . Canadian Cardiovascular Society Angina Grading Scale n.d. www.ccs.ca/images/Guidelines/Guidelines_POS_Library/Ang_Gui_1976.pdf (accessed 19 June 2014).
- EQ-5D . EQ-5D Standardised Instrument n.d. www.euroqol.org (accessed 19 June 2014).
- Schroter S, Lamping DL. Coronary revascularisation outcome questionnaire (CROQ): development and validation of a new, patient based measure of outcome in coronary bypass surgery and angioplasty. Heart 2004;90:1460-6. http://dx.doi.org/10.1136/hrt.2003.021899.
- Janssen MF, Birnie E, Bonsel GJ. Quantification of the level descriptors for the standard EQ-5D three-level system and a five-level version according to two methods. Qual Life Res 2008;17:463-73. http://dx.doi.org/10.1007/s11136-008-9318-5.
- Sealed Envelope Ltd . Randomisation and Online Databases for Clinical Trials n.d. www.sealedenvelope.com (accessed 14 March 2012).
- Gutierrez RG. Parametric frailty and shared frailty survival models. Stata J 2002;2:22-44.
- Bridgewater B, Keogh B, Kinsman R, Walton P. Sixth National Adult Cardiac Surgical Database Report 2008. www.scts.org/_userfiles/resources/SixthNACSDreport2008withcovers.pdf (accessed 27 November 2013).
- Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R. Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol 1999;52:1143-56.
- Shaw R. Barriers to Recruitment in Head &Amp; Neck Surgical Trials 2011. www.methodologyhubs.mrc.ac.uk/pdf/Shaw%20-%20Head%20and%20Neck.pdf (accessed 21 November 2012).
- Paramasivan S, Huddart R, Hall E, Lewis R, Birtle A, Donovan JL. Key issues in recruitment to randomised controlled trials with very different interventions: a qualitative investigation of recruitment to the SPARE trial (CRUK/07/011). Trials 2011;12. http://dx.doi.org/10.1186/1745-6215-12-78.
- Hattler B, Messenger JC, Shroyer AL, Collins JF, Haugen SJ, Garcia JA, et al. Off-pump coronary artery bypass surgery is associated with worse arterial and saphenous vein graft patency and less effective revascularization: results from the veterans affairs Randomized On/Off BYpass (ROOBY) trial. Circulation 2012;125:2827-35. http://dx.doi.org/10.1161/CIRCULATIONAHA.111.069260.
- Moller CH, Perko MJ, Lund JT, Andersen LW, Kelbaek H, Madsen JK, et al. Three-year follow-up in a subset of high-risk patients randomly assigned to off-pump versus on-pump coronary artery bypass surgery: the Best Bypass Surgery trial. Heart 2011;97:907-13. http://dx.doi.org/10.1136/hrt.2010.211680.
- Biancari F, Rimpilainen R. Meta-analysis of randomised trials comparing the effectiveness of miniaturised versus conventional cardiopulmonary bypass in adult cardiac surgery. Heart 2009;95:964-9. http://dx.doi.org/10.1136/hrt.2008.158709.
- Bednarska E, Bryant D, Devereaux PJ, Expertise-Based Working Group. Orthopaedic surgeons prefer to participate in expertise-based randomized trials. Clin Orthop Relat Res 2008;466:1734-44. http://dx.doi.org/10.1007/s11999-008-0273-9.
- Cook JA, Ramsay CR, Fayers P. Statistical evaluation of learning curve effects in surgical trials. Clin Trials 2004;1:421-7. http://dx.doi.org/10.1191/1740774504cn042oa.
- Coronary angioplasty versus coronary artery bypass surgery: the Randomized Intervention Treatment of Angina (RITA) trial . Lancet 1993;341:573-80.
- CABRI Trial Participants . First-year results of CABRI (Coronary Angioplasty versus Bypass Revascularisation Investigation). CABRI trial participants. Lancet 1995;346:1179-84.
- (BARI) Investigators . Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. The Bypass Angioplasty Revascularization Investigation (BARI) Investigators. N Engl J Med 1996;335:217-25.
- Finkemeier CG, Schmidt AH, Kyle RF, Templeman DC, Varecka TF. A prospective, randomized study of intramedullary nails inserted with and without reaming for the treatment of open and closed fractures of the tibial shaft. J Orthop Trauma 2000;14:187-93. http://dx.doi.org/10.1097/00005131-200003000-00007.
- Donovan J, Mills N, Smith M, Brindle L, Jacoby A, Peters T, et al. Quality improvement report: Improving design and conduct of randomised trials by embedding them in qualitative research: ProtecT (prostate testing for cancer and treatment) study. Commentary: presenting unbiased information to patients can be difficult. BMJ 2002;325:766-70. http://dx.doi.org/10.1136/bmj.325.7367.766.
- Raftery J, Bryant J, Powell J, Kerr C, Hawker S. Payment to healthcare professionals for patient recruitment to trials: systematic review and qualitative study. Health Technol Assess 2008;12.
- Fletcher B, Gheorghe A, Moore D, Wilson S, Damery S. Improving the recruitment activity of clinicians in randomised controlled trials: a systematic review. BMJ Open 2012;2. http://dx.doi.org/10.1136/bmjopen-2011-000496.
Appendix 1 CRISP study centres and principal investigators
UK Centres
| Centre | Hospital trust | Principal investigator | Status at study closure |
|---|---|---|---|
| Basildon | Basildon and Thurrock University Hospitals NHS Foundation Trust | Andrew Ritchie | Recruited |
| Blackpool | Blackpool Teaching Hospitals NHS Foundation Trust | Augustus Tang | Recruited |
| Bristol | University Hospitals Bristol NHS Foundation Trust | Professor Gianni Angelini | Recruited |
| King’s College | King’s College Hospital NHS Foundation Trust | Jatin Desai | Recruited |
| Oxford | Oxford University Hospitals NHS Trust | Professor David Taggart | Recruited |
| Papworth | Papworth Hospital NHS Foundation Trust | Max Codiposti | Recruited |
| Sheffield | Sheffield Teaching Hospitals NHS Foundation Trust | Norman Briffa | Recruited |
| Wolverhampton | Royal Wolverhampton Hospitals NHS Trust | Patrick Yiu | Recruited |
| Liverpool | Liverpool Heart and Chest Hospital Hospitals NHS Foundation Trust | Brian Fabri | Withdrew |
| Brighton | Brighton and Sussex University Hospitals NHS Trust | Uday Trivedi | Participating but no recruitment |
| University College | University College London Hospitals NHS Foundation Trust | Shyam Kolvekar | Participating but no recruitment |
| Nottingham | Nottingham University Hospital NHS Trust | David Richens | Participating but no recruitment |
| Middlesbrough | South Tees Hospitals NHS Foundation Trust | Enoch Akowuah | Approvals in place, but not started |
| Cardiff | University Hospital of Wales, Cardiff and Vale University Health Board | Dheeraj Mehta | Seeking approvals |
| Edinburgh | Royal Infirmary of Edinburgh, NHS Lothian | Vipin Zamvar | Seeking approvals |
Non-UK Centres
| Country | Centre | Principal investigator | Status at study closure |
|---|---|---|---|
| India | RTIICS, Kalkota | Kunal Sarkar | Recruited |
| Brazil | Pernambuco | Fernando Moraes | Seeking approvals |
| Brazil | Federal University of Sao Paulo | Walter Gomes | Seeking approvals |
| Brazil | Florianopolis, Santa Catarina | Sergio Almeida | Seeking approvals |
| Canada | McGill University Health Centre, Montreal | Patrick Ergina | Seeking approvals |
| Germany | Universität Leipzig | Friedrich Mohr | Seeking approvals |
| Germany | Herz- und Gefäßzentrum, Bad Bevensen | Gerhard Wimmer-Greinecker | Seeking approvals |
| Germany | Herz- und Diabeteszentrum NRW, Bad Oeynhausen | Jochen Borgermann | Seeking approvals |
| Italy | Pasquinucci, Massa Carrara | Mattia Glauber | Seeking approvals |
| Italy | University of Insubria, Varese | Andrea Sala | Seeking approvals |
| Italy | Sacco Hospital, Milan | Carlo Antona | Seeking approvals |
Appendix 2 Additional data tables
Withdrawals
| Allocation | Time of withdrawal | Time from randomisation to withdrawal (days) | Consent withdrawn by | Reason for withdrawal | Received surgery |
|---|---|---|---|---|---|
| ONCABG | Pre surgery | 0 | Patient | No reason given | No |
| ONCABG | Pre surgery | 21 | Clinician | Surgery no longer required, patient and surgeon agreed medical treatment other than surgery | No |
| ONCABG | Pre surgery | 31 | Clinician | Not willing for data to be used | No |
| OPCABG | Pre surgery | 1 | Clinician | Decided to treat medically | No |
| OPCABG | Pre surgery | 2 | Clinician | Patient no longer being considered for surgery as not symptomatic | No |
| OPCABG | Pre surgery | 4 | Patient | Patient withdrew without knowing allocation, wanted to be operated on by the surgeon met in clinic | Yesa |
| OPCABG | Pre surgery | 50 | Clinician | Not willing for data to be used | No |
The European Quality of Life-5 Dimensions responses
| Domain | Levels | Three-level EQ-5D | Five-level with five descriptors EQ-5D | Five-level with three descriptors EQ-5D | |||
|---|---|---|---|---|---|---|---|
| Randomised to OPCABG (n = 14) | Randomised to ONCABG (n = 16) | Randomised to OPCABG (n = 18) | Randomised to ONCABG (n = 13) | Randomised to OPCABG (n = 16) | Randomised to ONCABG (n = 19) | ||
| Mobility | No problems walking about | 7 | 8 | 10 | 3 | 9 | 10 |
| Slight problems walking about | – | – | 5 | 1 | 0 | 1 | |
| Some problems walking about | 7 | 8 | 2 | 5 | 7 | 7 | |
| A lot of problems walking about | – | – | 0 | 4 | 0 | 0 | |
| Confined to bed | 0 | 0 | 1 | 0 | 0 | 1 | |
| Self-care | No problems with self-care | 12 | 13 | 17 | 11 | 15 | 16 |
| Slight problems washing or dressing | – | – | 0 | 0 | 0 | 1 | |
| Some problems washing or dressing | 2 | 3 | 0 | 2 | 1 | 1 | |
| A lot of problems washing or dressing | – | – | 1 | 0 | 0 | 0 | |
| Unable to wash or dress | 0 | 0 | 0 | 0 | 0 | 1 | |
| Usual activitiesa | No problems with usual activities | 5 | 7 | 10 | 6 | 8 | 7 |
| Slight problems with usual activities | – | – | 4 | 2 | 1 | 1 | |
| Some problems with usual activities | 8 | 7 | 3 | 5 | 5 | 8 | |
| A lot of problems with usual activities | – | – | 0 | 0 | 0 | 0 | |
| Unable to perform usual activities | 0 | 2 | 1 | 0 | 2 | 3 | |
| Pain/discomfortb | No pain or discomfort | 5 | 7 | 5 | 4 | 10 | 10 |
| Slight pain or discomfort | – | – | 9 | 4 | 1 | 1 | |
| Moderate pain or discomfort | 8 | 7 | 4 | 4 | 3 | 7 | |
| A lot of pain or discomfort | – | – | 0 | 0 | 1 | 0 | |
| Extreme pain or discomfort | 1 | 2 | 0 | 1 | 0 | 1 | |
| Anxiety/depressionc | Not anxious or depressed | 9 | 10 | 11 | 5 | 11 | 13 |
| Slightly anxious or depressed | – | – | 6 | 6 | 1 | 0 | |
| Moderately anxious or depressed | 3 | 6 | 1 | 1 | 3 | 2 | |
| Very anxious or depressed | – | – | 0 | 0 | 0 | 0 | |
| Extremely anxious or depressed | 2 | 0 | 0 | 1 | 1 | 3 | |
| Domain | Levels | Three-level EQ-5D | Five-level (5 descriptors) EQ-5D | Five-level (3 descriptors) EQ-5D | |||
|---|---|---|---|---|---|---|---|
| Randomised to OPCABG (n = 14) | Randomised to ONCABG (n = 15) | Randomised to OPCABG (n = 17) | Randomised to ONCABG (n = 12) | Randomised to OPCABG (n = 15) | Randomised to ONCABG (n = 18) | ||
| Mobility | No problems walking about | 8 | 6 | 12 | 7 | 11 | 13 |
| Slight problems walking about | – | – | 3 | 2 | 0 | 1 | |
| Some problems walking about | 6 | 9 | 2 | 1 | 4 | 4 | |
| A lot of problems walking about | – | – | 0 | 1 | 0 | 0 | |
| Confined to bed | 0 | 0 | 0 | 1 | 0 | 0 | |
| Self-care | No problems with self-care | 14 | 13 | 15 | 11 | 14 | 17 |
| Slight problems washing or dressing | – | – | 2 | 0 | 0 | 1 | |
| Some problems washing or dressing | 0 | 2 | 0 | 1 | 1 | 0 | |
| A lot of problems washing or dressing | – | – | 0 | 0 | 0 | 0 | |
| Unable to wash or dress | 0 | 0 | 0 | 0 | 0 | 0 | |
| Usual activities | No problems with usual activities | 9 | 6 | 13 | 4 | 4 | 7 |
| Slight problems with usual activities | – | – | 2 | 5 | 3 | 3 | |
| Some problems with usual activities | 5 | 8 | 1 | 3 | 8 | 8 | |
| A lot of problems with usual activities | – | – | 1 | 0 | 0 | 0 | |
| Unable to perform usual activities | 0 | 1 | 0 | 0 | 0 | 0 | |
| Pain/discomfort | No pain or discomfort | 7 | 9 | 12 | 4 | 7 | 10 |
| Slight pain or discomfort | – | – | 4 | 8 | 3 | 2 | |
| Moderate pain or discomfort | 7 | 5 | 1 | 0 | 5 | 6 | |
| A lot of pain or discomfort | – | – | 0 | 0 | 0 | 0 | |
| Extreme pain or discomfort | 0 | 1 | 0 | 0 | 0 | 0 | |
| Anxiety/depressiona | Not anxious or depressed | 12 | 12 | 13 | 9 | 13 | 12 |
| Slightly anxious or depressed | – | – | 3 | 1 | 0 | 1 | |
| Moderately anxious or depressed | 2 | 3 | 1 | 1 | 2 | 2 | |
| Very anxious or depressed | – | – | 0 | 0 | 0 | 1 | |
| Extremely anxious or depressed | 0 | 0 | 0 | 1 | 0 | 0 | |
| Questionnaire | Preoperative | 4–8 weeks postoperative | MD (95% CI) | ||
|---|---|---|---|---|---|
| Randomised to OPCABG | Randomised to ONCABG | Randomised to OPCABG | Randomised to ONCABG | ||
| Three-level EQ-5D | |||||
| n | 13 | 16 | 14 | 15 | |
| Mean (SD) | 68 (15) | 62 (17) | 76 (16) | 66 (16) | 10 (–2 to 22) |
| Five-level (five descriptor) EQ-5D | |||||
| n | 18 | 13 | 17 | 12 | |
| Mean (SD) | 69 (17) | 71 (15) | 77 (16) | 74 (12) | 3 (–7 to 14) |
| Five-level (three descriptor) EQ-5D | |||||
| n | 16 | 19 | 15 | 18 | |
| Mean (SD) | 69 (16) | 66 (18) | 76 (10) | 73 (16) | 3 (–6 to 13) |
| Quality-of-life measure | Randomised to OPCABG (n = 5) | Randomised to ONCABG (n = 2) |
|---|---|---|
| Rose and CCS angina class | ||
| Rose angina | ||
| No angina | 4 | 0 |
| Grade I | 1 | 1 |
| Grade II | 0 | 1 |
| CCS class | ||
| Asymptomatic | 5 | 1 |
| Grade I | 0 | 0 |
| Grade II | 0 | 1 |
| Grade III | 0 | 0 |
| Grade IV | 0 | 0 |
| EQ-5D categorical responses | ||
| Mobility | ||
| No problems walking about | 3 | 1 |
| Slight problems walking about | 0 | 0 |
| Some problems walking about | 1 | 1 |
| A lot of problems walking about | 1 | 0 |
| Confined to bed | 0 | 0 |
| Self-care | ||
| No problems with self-care | 4 | 2 |
| Slight problems washing or dressing | 0 | 0 |
| Some problems washing or dressing | 1 | 0 |
| A lot of problems washing or dressing | 0 | 0 |
| Unable to wash or dress | 0 | 0 |
| Usual activities | ||
| No problems with usual activities | 3 | 1 |
| Slight problems with usual activities | 0 | 0 |
| Some problems with usual activities | 2 | 1 |
| A lot of problems with usual activities | 0 | 0 |
| Unable to perform usual activities | 0 | 0 |
| Pain/discomfort | ||
| No pain or discomfort | 2 | 1 |
| Slight pain or discomfort | 0 | 0 |
| Moderate pain or discomfort | 2 | 1 |
| A lot of pain or discomfort | 0 | 0 |
| Extreme pain or discomfort | 1 | 0 |
| Anxiety/depression | ||
| Not anxious or depressed | 4 | 2 |
| Slightly anxious or depressed | 0 | 0 |
| Moderately anxious or depressed | 1 | 0 |
| Very anxious or depressed | 0 | 0 |
| Extremely anxious or depressed | 0 | 0 |
Appendix 3 CRISP protocol
Appendix 4 CRISP case report forms and database validation
Example of database validation
CRISP case report forms
Appendix 5 CRISP statistical analysis plan
Appendix 6 Trial Steering Committee and Data Monitoring and Safety Committee members
Trial Steering Committee
Professor William Wijns (chairperson)
Dr Jonathan Cook
Professor John Dark
Mr Neville Jones (patient representative)
Dr Belinda Lees
Mr Patrick Magee
Professor John Pepper
Data Monitoring and Safety Committee
Professor Tom Treasure (chairperson)
Dr Tim Clayton
Professor Desmond Julian
Professor Paul Sergeant
List of abbreviations
- ACE
- angiotensin-converting enzyme
- AE
- adverse event
- AF
- atrial fibrillation
- AKI
- acute kidney injury
- ARB
- angiotensin receptor blocker
- BBS
- Best Bypass Surgery
- CABG
- coronary artery bypass grafting
- CAD
- coronary artery disease
- CCS
- Canadian Cardiovascular Society
- CI
- confidence interval
- CICU
- cardiac intensive care unit
- CK-MB
- creatine kinase MB isozyme
- CLRN
- comprehensive local research network
- CORONARY
- CABG off- or on-pump revascularisation trial
- CPB
- cardiopulmonary bypass
- CRF
- case report form
- CRISP
- Coronary artery bypass grafting in high-RISk patients randomised to off- or on-Pump surgery
- CRISPSw
- (1) all-cause death after Cardiac surgery, (2) new onset Renal failure, (3) MI, (4) Stroke, (5) Prolonged initial ventilation and (6) Sternal wound dehiscence
- CROQ
- Coronary Revascularisation Outcome Questionnaire
- DMSC
- Data Monitoring and Safety Committee
- ECG
- electrocardiograph
- EQ-5D
- European Quality of Life-5 Dimensions
- EuroSCORE
- European system for cardiac operative risk evaluation score
- HDU
- high-dependency unit
- HR
- hazard ratio
- IABP
- intra-aortic balloon pump
- ICC
- intraclass correlation coefficient
- IQR
- interquartile range
- LV
- left ventricle/ventricular
- LVAD
- left ventricular assist device
- MD
- mean difference
- MI
- myocardial infarction
- NYHA
- New York Heart Association
- ONCABG
- on-pump coronary artery bypass graft
- OPCABG
- off-pump coronary artery bypass graft
- OR
- odds ratio
- PCI
- percutaneous coronary intervention
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- ROOBY
- Randomised On/Off Bypass trial
- RR
- relative risk
- RRT
- renal replacement therapy
- SAE
- serious adverse event
- SD
- standard deviation
- TIA
- transient ischaemic attack
- TSC
- Trial Steering Committee
- VF
- ventricular fibrillation
- VT
- ventricular tachycardia