Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 06/36/04. The contractual start date was in June 2008. The draft report began editorial review in October 2013 and was accepted for publication in February 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Dawn Skelton and Susie Dinan are directors for Later Life Training, who deliver FaME and OEP training to health and leisure professionals across the UK. The other authors declare that they have no competing interests.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Iliffe et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background: why this study was needed
The health benefits of physical activity (PA) include reductions in the risk of cardiovascular disease, type 2 diabetes, osteoporosis and certain cancers. 1 There is growing evidence of an association between regular PA and a reduced risk of all-cause mortality,2 and of the potential savings for NHS budgets from exercise promotion for older adults. 3 Sedentary behaviour increases the risk of dependence, falls and fractures. Sustained levels of PA in adulthood maintain bone strength and can prevent fragility fractures in later life. Research has shown that a lifetime’s history of regular PA can reduce the risk of hip fracture by up to 50% and much of this benefit is thought to result from a reduction in falls. 4 It is now clear that habitual PA and improved access to exercise opportunities is an important public health approach to the prevention of functional decline that can lead to frailty, falls and fractures. 5
Falls are common in people aged ≥ 65 years and can have serious consequences, including injury, pain, impaired function, loss of confidence in carrying out everyday activities, loss of independence and autonomy, and even death. 6,7 There is evidence that interventions providing some form of exercise may be effective in preventing falls among older people8 and that health-care costs9,10 could be reduced if the number of falls was reduced. 7,11–14
Current PA recommendations propose a target of 150 minutes of moderate to vigorous physical activity (MVPA) per week. 15 However, surveys have consistently shown a high prevalence of physical inactivity in the UK population. 16 A systematic review comparing 17 randomised controlled trials (RCTs) with different interventions designed to encourage sedentary, community-dwelling adults to do more PA17 concluded that interventions were effective in the short- and mid-term, at least in middle age, and that there were no significant increases in adverse events (AEs) in the four studies that reported them. However, it is unclear which individual interventions (e.g. home- or facility-based) are the most effective in increasing PA in the long term or in specific groups (e.g. older people).
Promoting physical activity
The NHS has attempted to address the problem of inactivity in a variety of ways, including exercise referral schemes in primary care (‘exercise on prescription’), which were provided by approximately 90% of primary care trusts (PCTs) in the 2000s and usually involved referring patients to local leisure centres. 18 Although exercise on prescription has been shown to be feasible and effective in vulnerable older people,19 there appear to be significant barriers to the uptake of exercise classes in leisure centres. For many older people, home exercise or group exercise in non-intimidating environments (e.g. community halls) may be more appealing, and result in higher uptake of exercise programmes and longer continuation of exercise. Peer activity mentors have also been shown to be effective in increasing uptake and adherence to exercise. 20-23
There are currently two existing exercise programmes designed for use in community settings with people aged ≥ 65 years. The first is a home-based programme, the Otago Exercise Programme (OEP), and the second is a community-based group exercise programme, the Falls Management Exercise (FaME) programme.
The OEP24–30 and FaME programme31,32 are both designed for use in community settings, specifically for people aged ≥ 65 years, to reduce falls. FaME is based on the components of fitness and principles of programming for all older adults (i.e. warm-up, mobility, stretches, strength and balance, endurance and a cool down), while OEP includes brief warm-up and strength and balance exercises appropriate for the age group. Both programmes involve strength and balance training which is tailored to the individual’s ability and health status.
The OEP is a home-based exercise programme for older people which is effective in reducing falls and fall-related injuries, improving balance, strength and confidence in performing everyday activities without falling, and has been shown to be cost-effective for people aged ≥ 80 years. 24–30 It was designed to be delivered by physiotherapists, and nurses trained and supervised by physiotherapists. A 1-year evaluation of the OEP showed considerable improvements in outdoor activities (walking, shopping, gardening and other outside leisure activities) after 6 months (Professor A J Campbell, University of Otago, 2007, personal communication) with participants continuing to exercise after completing the programme. It also showed significant improvements in executive function after 6 months. 30 While the OEP has been evaluated in four controlled trials of older primary care patients in New Zealand and one RCT in Canada, it has not been tested in a primary care setting in the UK for its feasibility, impact, acceptability and cost-effectiveness.
The FaME programme is a group exercise programme which was developed and tested in a controlled trial in the UK,31 but not in a primary care population. It aims to improve balance33 and was designed to be delivered by qualified postural stability instructors (PSIs). 32 It has been shown to be effective in reducing falls, and injuries resulting from falls. 16,31 Good adherence was demonstrated with the FaME programme and nearly two-thirds of people participating in FaME continued in group exercise programmes for over 1 year after trial completion (Professor D A Skelton, Glasgow Caledonian University, 2007, personal communication). The FaME programme remains to be evaluated for its impact, acceptability and cost-effectiveness within primary care.
This trial aimed to fill the gaps in the current evidence base by evaluating the delivery, impact, acceptability and cost-effectiveness of a community-based exercise programme (FaME) and a home-based exercise programme (OEP) supported by similarly aged (peer) mentors (PMs), compared with usual care for primary care patients. The underlying assumption was that the exercises would produce sufficient subjective well-being and improved mobility to encourage continuation of higher levels of PA after the cessation of the intervention. Each exercise programme was compared with usual care for effectiveness in producing sustained change in PA. The two programmes would be compared for cost-effectiveness if both were effective in promoting sustained change in PA. Our primary hypotheses at the start of the study were (1) both exercise programmes would produce sustained changes in PA compared with usual care and (2) OEP would be more cost-effective than FaME.
Chapter 2 Study design, including interventions
This chapter describes the trial as originally designed and is a summary of the full protocol. 34
Objectives
The primary objective of the ProAct65+ study was to determine the effect of two evidence-based exercise programmes designed for older people compared with usual care (i.e. with no special interventions to promote PA), on the achievement of recommended PA targets 12 months after cessation of intervention.
The secondary objectives were to:
-
determine the health benefits of the two programmes to participants starting at various levels of PA – particularly the effects on physical and psychological status, health status, health-related quality of life and quality-adjusted life-years (QALYs)
-
estimate the costs of the exercise interventions, and possible cost offsets, and to assess the cost-effectiveness of community group exercise, and home-supported exercise compared with usual care
-
determine the acceptability of the programmes, adherence rates, enabling factors and barriers to future implementation
-
compare the time course of responses by participants, in terms of exercising at the recommended levels, at 0, 6, 12, 18 and 24 months after cessation of the intervention, between those undergoing the exercise programmes and those receiving usual care
-
determine participants’ perceptions of the value of exercise and the predictors of continued exercise.
Design
The ProAct65+ study was based on a three-arm, parallel design, cluster-controlled trial comparing a community centre-based group exercise programme (FaME), with a home-based exercise programme and walking plan (OEP) and with usual care, and using minimisation for allocation at the level of general practice in two UK centres (London and Nottingham/Derby). We initially planned 2 years’ follow-up post intervention to determine the impact, acceptability and adherence to the programme, longer-term continuation of exercise and cost-effectiveness. The Consolidated Standards of Reporting Trials (CONSORT) diagram35 summarises the design (see Figure 7).
Participants and inclusion/exclusion criteria
Participants were patients aged ≥ 65 years registered with participating general practices who gave informed consent to participate.
Inclusion criteria for practices
Inclusion criteria for practices were (1) a commitment to participate over the duration of the study and (2) the availability of a suitable community venue in the practice catchment area.
Inclusion criteria for participants
Those aged ≥ 65 years who could walk independently both indoors and outdoors (with or without a walking aid and without help from another person), and who would be physically able to take part in a group exercise class, were eligible to participate in the trial.
Exclusion criteria for participants
Those with any of the following criteria were excluded:
-
three or more self-reported falls in the previous year
-
resting blood pressure (BP) > 180/100 mmHg; tachycardia > 100 beats per minute; those considered by their general practitioner (GP) to have uncontrolled hypertension; significant drop in BP during exercise previously recorded in the participant’s medical records, or found at initial assessment
-
psychiatric conditions which would prevent participation in an exercise class (e.g. psychotic illness)
-
uncontrolled medical problems, which the GP considered would exclude patients from undertaking the exercise programme (e.g. acute systemic illness such as pneumonia, poorly controlled angina, acute rheumatoid arthritis, unstable or acute heart failure)
-
conditions requiring a specialist exercise programme (e.g. uncontrolled epilepsy, significant neurological disease or impairment; unable to maintain seated upright position or unable to move about independently indoors)
-
not living independently (e.g. living in residential or nursing homes)
-
significant cognitive impairment (resulting in the individual being unable to follow simple instructions)
-
already receiving long-term physiotherapy or already in an exercise programme.
Exclusion criteria were checked at the recruitment appointment by the researcher. This assessment included measurement of resting BP and pulse, functional assessments and completion of a health questionnaire. GPs were asked to confirm eligibility for each potentially eligible participant. A further exclusion criterion of those already exercising at, or above, the target level was introduced early in the trial (see Chapter 3 for details).
Recruitment of practices
General practices were recruited through the Primary Care Research Networks (PCRNs) in London and Nottingham/Derby. The PCRNs were asked to identify potential participant practices. Mailed invitations, telephone contact with practice managers and personal contact with local GP opinion leaders were used as necessary. 36–38
Recruitment of participants
Practices produced a single numbered list of patients aged ≥ 65 years. Practice clinical staff were allowed to make and justify their own exclusions in liaison with the research team. The research team provided the practices with a random number list to select the sample of patients to be approached after exclusions had been made. Our intention was that the sampling would vary depending on practice size. In practices with fewer than 450 patients aged ≥ 65 years, all patients aged ≥ 65 years would be invited to participate. In larger practices random sampling would be used to identify 450 patients aged ≥ 65 years who would be invited to participate. Patients were then sent invitation letters about the trial by their usual GP.
Interventions
There were three arms to the trial:
-
home-based exercise programme and walking plan (OEP)
-
community centre-based group exercise programme (FaME)
-
usual care.
Home-based Otago Exercise Programme
This consisted of a 30-minute programme of leg muscle strengthening and balance retraining exercises, progressing in difficulty, to be performed at home at least three times per week, and a walking plan to be undertaken at least two times per week for 24 weeks. Each participant received an instruction booklet and ankle cuff weights (starting at 1 kg) to provide resistance for the strengthening exercises. The OEP intervention is described as ‘moderate’ intensity by the original authors,24 and is designed to be performed unsupervised in the patient’s home and is less intense than the FaME programme.
The programme was introduced to participants by trained research staff, at an appropriate starting level determined at an initial assessment, in either a group setting or at home, depending on circumstances. Mentor support has been shown to be effective in increasing adherence,20–22 so in the initial plan of this intervention trained PMs then contacted and visited the participants at home to start the exercise programme, and followed them up at home with up to three more visits (as the participants required). Participants were asked to record the days they carried out the programme and mentors telephoned them fortnightly to encourage activity and prompt progression of exercises. Mentors recorded and reported any problems encountered with the exercise programme to the research team using an AE form developed for the study (see Appendix 1). The delivery of the OEP was standardised through training of PMs before the trial started, and there was regular contact with the participants and PMs to check that exercise protocols were being followed.
Community-based Falls Management Exercise programme
The FaME programme comprises 1-hour-long group exercise class in a local community centre for a maximum of 15 participants and two 30-minute home exercise sessions (based on the OEP) per week for 24 weeks. These classes were run by PSIs, trained to promote exercise with older people. Participants were advised to walk at least twice per week for up to 30 minutes at a moderate pace. The FaME intervention is a more comprehensive intervention, containing both floor exercises and cardiovascular exercises that the OEP does not contain, and is more intense. The balance section is challenging. The programme included leg muscle strengthening and balance retraining that progressed in difficulty. Progressive trunk and arm muscle strengthening, bone loading, endurance (including walking) and flexibility training, functional floor skills (see below) and adapted tai chi completed this evidence-based programme. Ankle cuff weights, TheraBands™ (elastic resistance training bands) and mats are also used throughout the programme. The group exercises include retraining of the ability to get up from the floor and floor exercises to improve strength, balance and coping strategies to reduce the risk of complications resulting from a long lie. 32 The delivery of the FaME programme was standardised through training of PSIs before the trial started and there were regular quality assurance visits to the FaME classes to check that intervention delivery protocols were being followed.
The PSIs kept a register of attendance and recorded tailoring of the programme and any feedback from participants. They followed up non-attenders by telephone as necessary, recording any positive or negative feedback and notified the research team about reasons for non-attendance or drop out. Participants were given a personalised booklet containing their home exercise instructions.
Initially we planned that FaME groups would have 9 or 10 participants, so there would be four or five classes per week for each of the practices allocated to this arm. The number of PSIs running these classes was determined by their availability, but the aim was to maximise continuity and standardisation of PA training, so the ideal arrangement was to have one PSI leading all groups in one practice. We expected to follow a similar approach to continuity of PMs for participants in the OEP arm.
A starting level for both interventions was determined from baseline assessments and instructor observation in week 1 in FaME, and at the technique instruction class at the beginning of the OEP. Experienced exercise instructors carried out standardised quality assurance visits to FaME classes and reviewed PM paperwork for evidence of tailoring of exercises and of progression in exercise intensity.
General practitioners in participating practices allocated to either the FaME programme or OEP were discouraged from referring participants involved in the trial to other exercise therapy programmes outside of the study.
Usual care
Participants in the usual-care arm were not offered either the OEP or FaME programme, but were free to participate in any other exercise just as they would if they were not participating in the trial.
Cultural and ethnic sensitivity
Cultural and religious requirements were accommodated within the exercise programmes. The recommendations from the Help the Aged Minority Ethnic Elders Falls Prevention Programme (www.helptheaged.org.uk/meefp) were followed. In addition, the research team were advised by the English Disability Sports Federation and the Integrated Fitness Initiative’s ‘Physical Activity Provision for Ethnic Minority Groups’ Project Development Team. In particular, the FaME group class leaders ensured that recommendations for attire respected cultural customs and religious beliefs for a range of ethnic groups.
We made provision for single-sex exercise groups to be scheduled as required, and separate changing facilities and same gender instructors were available wherever possible. Windows in the exercise classrooms were screened as appropriate. Family support was encouraged and classes were provided at different times of the day. The OEP also respected participants’ preferences regarding family support and participation in the home exercise programme.
All research material and exercise manuals had a maximum reading age of 9 years. Inability to read the material was not a formal exclusion criterion as the individual may be able to follow movement and correction accurately in classes and family members were allowed to act as interpreters. Where possible, invitation letters and information sheets were translated into local languages.
Outcome measures
The primary and secondary outcome measures were chosen to reflect the needs of participants (e.g. functional outcomes, falls, confidence, quality of life, participant costs), and of commissioners of exercise services in primary care and policy makers (e.g. PA, falls, NHS costs).
The primary outcome was the proportion of participants reaching the recommended PA target of at least 30 minutes of activity of moderate intensity on at least 5 days each week, measured using the Community Healthy Activities Model Program For Seniors scale (CHAMPS) questionnaires. Although measures were taken at 0, 6, 12, 18 and 24 months after the intervention, our primary analysis was of data collected at 12 months post intervention, as a previous study in New Zealand had suggested that this was the time when the effect of the intervention was maximal. 39
The secondary outcomes included:
-
the direct health benefits, i.e. functional and psychological status, the rate of falls (the major safety outcome measure), the number and nature of falls, and fear of falling
-
self-efficacy for exercise and physical self-perception (self-esteem relative to the physical domain), which includes measurement of perceived importance (the degree to which participants value their physical condition, body image and physical strength) to inform predictors of exercise adherence and continuation, and participants’ judgement of the value or importance of PA
-
health-related quality of life and QALYs40
-
the NHS and private (participant) costs of each exercise programme, and possible cost offsets, identified from a comparison of health and social service utilisation of participants in all groups during the study period.
Ascertainment of outcomes
The following functional assessments were used by researchers at baseline and at the end of the interventions (and at 6 months after allocation in the usual-care arm):
-
Modified Clinical Romberg Static Balance test, eyes open and closed41
-
timed get-up and go (TUG) as a measure of balance and falls risk42
-
functional reach as a measure of balance and falls risk43
-
30-second chair rise as a measure of lower limb strength and power. 44
The following validated tools were used at baseline and as self-completion questionnaires at follow-up:
-
Confidence in balance as measured by the Confidence in Balance (ConfBal) scale. 45 A total score is provided as a measure of confidence.
-
Confidence in carrying out a range of basic activities of daily living without falling as measured by the Falls Efficacy Scale-International (FES-I). 46
-
Readiness to change as measured by the transtheoretical model,47 applying it to exercise behaviour to determine perceived barriers48 and self-efficacy for exercise. 49 Expectations of exercise were measured with the Outcome Expectation for Exercise (OEE) scale-2, a 13-item measure with two subscales: positive and negative OEE. 50
-
Quality of life was measured using the Older People’s Quality of Life Questionnaire (OPQoL). 51–53
-
Social network size and density was measured using the brief Lubben Social Network scale (LSNS)54 and perceived social support was measured by the Multidimensional Scale of Perceived Social Support (MSPSS). 55
-
Subjective habitual PA was assessed using a number of validated questionnaires to ensure all domains of activity and sport are considered, including the Phone-FITT, Physical Activity Scale for the Elderly (PASE) and CHAMPS22,56,57 and the current level of activity questions used in the Household Survey. 58
-
Attitudes and beliefs about falls prevention interventions were measured using the Attitudes to Falls-Related Interventions Scale (AFRIS) questionnaire. 59
-
Falls risk was measured by the Falls Risk Assessment Tool (FRAT). 60
-
Health-related quality of life was measured by the Short Form questionnaire-12 items (SF-12). 61 Quality-adjusted Life-years, which are the main outcome for the economic analysis, were based on European Quality of Life-5 Dimensions (EQ-5D) utility weights obtained by transforming SF-12 scores. 40
In addition, demographic information, co-morbidity, medication, use of general practice and hospital and community social services were recorded at baseline and updated at subsequent assessments. Falls were ascertained by self-completed fall diaries (completed 4-weekly during the intervention period and at longer intervals thereafter – see Chapter 3), with follow-up of non-responders and telephone contact with fallers to ascertain the type of fall and any injury and health-care usage that resulted.
For the purposes of the economic analysis, the resources used in the delivery of the interventions were collected from records kept by PSI instructors (FaME) and the research staff and PMs (OEP). The use of facilities and equipment, and the time spent on travel and instruction, were included and monetary costs were assigned according to market rates.
In addition, the use of health and social care services (GP, community, outpatient, hospital admission) was recorded for participants in all groups by means of the self-completion diaries. Self-reported service utilisation was verified from the primary care medical records of consenting patients after the follow-up period. Costs of services were obtained from local and national sources. 62 Health and social care costs in the exercise groups were compared with each other and with the usual-care (no exercise) group to assess the extent to which the costs of the exercise intervention may be offset by savings elsewhere in the health and social care system.
No other encouragement to continue with PA was given to participants, and all potential reinforcements in the form of diaries and 6-monthly contacts were given to participants in all three arms of the trial. We provided information about local exercise opportunities to all participants at the end of the intervention period (i.e. 24 weeks after randomisation).
Baseline data collection
Baseline assessment included all functional assessments plus administration of all questionnaires described above.
Follow-up data collection
Follow-up assessments occurred at 24 weeks after the commencement of the intervention, at 6, 12, 18 and 24 months after the completion of the intervention for participants in both intervention arms, at 24 weeks after randomisation and at 6, 12, 18 and 24 months after completion of the 24-week assessment in the control arm. The 24-week functional assessment was identical to the baseline assessment plus administration of all questionnaires described above and administration of the Phone-FITT questionnaire by telephone.
Assessments at 6, 12, 18 and 24 months after completion of the intervention or after completion of the 24-week assessment in the usual-care arm consisted of postal administration of the questionnaires described above, plus the Phone-FITT questionnaire administered by telephone.
The primary outcome was the proportion of participants reaching the recommended PA target of at least 150 minutes of MVPA each week, measured using the CHAMPS questionnaire, at 12 months after the intervention.
Sample size
Sample size estimates were based on the numbers of participants needed to detect differences in proportions of participants in intervention and control groups:
-
participating in PA (defined as reaching the national target recommendations of five sessions of ≥ 30 minutes of at least moderate activity per week)
-
self-perceived health as measured by the EQ-5D index, from which mean QALY scores and the incremental cost-effectiveness ratio (ICER) could be calculated.
Under individual randomisation, sample size calculations for a small effect size (0.3)63 equivalent to a mean difference of 0.05 in the EQ-5D index in general community samples would have required 176 participants per study group in an individually randomised trial. 64 Published evidence of participants in a cluster randomised trial of PA promotion shows the proportions of participants achieving the same recommended targets for PA to be 14.6% (intervention subjects) compared with 4.9% (control subjects). 65 A total of 215 participants in each study group would have been required to detect this difference between study groups with 90% power (5% two-sided significance) in an individually randomised trial. Policy at the time when the trial was designed sought a 1% increase in the number of people achieving the PA target of five sessions of ≥ 30 minutes of at least moderate activity per week, year on year. 1
Data from 24 general practices in the British Regional Heart study suggested that an intrapractice correlation coefficient (ICC) not exceeding 0.02 was appropriate for PA outcomes among middle-aged men, but this study aimed to represent the full range of cardiovascular disease prevalence across the UK and the range was assumed to be less in the ProAct65+ study as it was less geographically dispersed. 66 In addition, ICCs collected for a range of variables in primary care settings have typically averaged 0.01. 67
Based on an intraclass correlation coefficient of 0.01, the design effect was estimated as 1.31, because 32 participants per practice were expected to provide data (see below). If 215 participants per arm were to be required (before allowing for attrition) for an individually randomised design (90% power, 5% two-sided significance), then 282 per arm would be required for the clustered design. Allowing for 30% attrition, this equated to 403 participants per arm. The sample size was based on detecting differences between each intervention (exercise programme) and the control arm: we did not expect enough power to detect modest differences in outcome between the two intervention arms.
Assuming an average practice size of 6000 patients, 15% (900) of whom would be aged ≥ 65 years68 and that a random one in two sample of patients would be approached to take part in the study, we calculated that 450 patients aged ≥ 65 years would need to be approached. Assuming a minimum of 10% of these patients agree to participate (approximately 45 per practice), and allowing for an attrition rate of 30%, outcome data would be obtained on 32 participants per practice.
For small practices, we expected that all or most patients in each practice would be invited to join the trial. In larger than average practices, however, where the patient list was very large, we anticipated that a stratified random sample of 450 patients would be drawn. Response rates from each practice were recorded.
Randomisation
Owing to the relatively small number of practices in the trial, minimisation was used to allocate practices to treatment arms to ensure maximum balance. 69 After all participants from a practice had been recruited, the practice was individually allocated to a study arm by the London co-ordinating centre. Practices were given an identification number and treatments were assigned by the senior statistician for the trial using computer-generated random number tables, embedded in a computer program for minimisation. The variables used in the minimisation process were trial centre (London/Nottingham and Derby), practice size (≥ median practice size/< median practice size) and the index of multiple deprivation (IMD) 2007 (IMD2007)70 (≥ median IMD2007/< median IMD2007). Minimisation was undertaken using the MINIM program (www-users.york.ac.uk/~mb55/guide/minim.htm). 71 Practice recruitment and allocation were performed concurrently in the two centres. Median practice size and IMD2007 values for the whole of England were used as cut-points for the minimisation process.
Concealment of allocation
Practices were allocated to intervention or usual care, only after all participants had been recruited. The practices, their patients and the researchers undertaking baseline assessments were all blinded to allocation until this point.
Blinding
It is difficult for participants to be blind in trials of exercise interventions and for researchers to remain blind to the allocation of participants as they recruited them, or undertook baseline or follow-up assessments. The researchers assessing outcomes were not blinded for pragmatic reasons alone; the study was funded to support only enough researchers to carry our recruitment and follow-up simultaneously. However, general practices and their participants, and researchers having contact with practices and participants, did not have foreknowledge of the treatment arm allocation of the practice, which was not disclosed until after all participants within a practice had been recruited.
Withdrawals
Participants could withdraw from the trial either at their own request or be withdrawn at the discretion of the chief investigator after discussion with the chairperson of the trial steering committee (TSC). Participants were made aware (via the information sheet and consent form) that withdrawal from the trial would not affect their future care, and that the data collected to date may still be used in the final analysis. Any requests to withdraw data made by individuals withdrawing from the trial were respected. The research teams at each site advised discontinuation of exercise or withdrawal from the trial if the exercise intervention posed a hazard to the safety of themselves or other participants. Those who withdrew from the trial were not replaced.
Contamination
Usual-care arm participants may have been disappointed and might have sought their own way of increasing PA, but the monthly diaries and the 6-monthly reviews should have captured this information.
Statistical methods
Characteristics of participants were compared with population norms at baseline (see Chapter 3). Linear regression models were used for continuous outcome variables, logistic models for binary outcome variables (in particular the primary end point, namely attainment of recommended exercise level at 12 months after the intervention), and negative binomial models for data on rate of falls. The assumptions for using each model were checked and analyses adjusted accordingly. For a few quantitative outcome measures found to have positively skewed distributions, logarithmic transformations were carried out. For the outcome of minutes of MVPA, as measured by the CHAMPS score, there were a substantial number of zeros in the data at each time point, so the MVPA values were transformed to loge(CHAMPS score + 1). Estimates of effects of each intervention against usual care were then back-transformed to provide an estimate of the multiplicative effect of each intervention on MVPA. However, the primary outcome was defined by dichotomising MVPA, whether or not it exceeded 150 minutes per week (as recommended by guidelines), and binary logistic regression was applied.
All analyses were undertaken adjusted, (a) for variables used for minimisation (centre, deprivation and practice size) and (b) for baseline values of the outcome measure. Multilevel models were applied to take account of clustering at the practice level (applicable to all arms of the study). Our primary analysis focused on participants with complete data at 12 months, but analysis using multiple imputation72 was also carried out on the quantitative form of the primary outcome [loge(CHAMPS score + 1)]. Some participants provided Phone-FITT scores through telephone interview, even though they had not returned a questionnaire to calculate a CHAMPS score. Therefore, imputation of the CHAMPS score at 12 months was first carried out for those who provided a Phone-FITT score at 12 months. Second, all the variables in the analytical model named above were entered into an imputation model for all participants, where all variables had missing data imputed through chained equations. In each case, 50 imputed data sets were created, analysis carried out and the 50 estimates of effects of the interventions were combined using Rubin’s rules. 73 Differential effects of the intervention by age and by sex were assessed for the primary outcome measure by adding terms for the interaction between age (grouped into those aged < 75 years and ≥ 75 years at baseline) and sex and treatment arm to the regression models. This analysis was confined to the quantitative form of the primary outcome [loge(CHAMPS score + 1)] to maximise power.
As the study consists of two intervention arms and one control arm, primary analysis consisted of comparing each intervention group with the control group. No formal adjustment of p-values was made, as the sample size had been specifically designed to test each intervention separately. Stata version 12 (StataCorp LP, College Station, TX, USA) and SPSS version 21 (IBM Corporation, Armonk, NY, USA) were used for analyses, with the Stata mi command for multiple imputation. Multilevel analyses were carried out using the xtmixed and xtmelogit commands in Stata for quantitative and binary outcomes, respectively, and negative binomial regression was carried out in SPSS for the falls outcome.
Economic evaluation
An economic evaluation was conducted alongside the clinical trial. The predefined aims were to:
-
estimate the costs of the exercise interventions, from the NHS and participant perspectives
-
explore the impact of the exercise interventions on participants’ utilisation of health and social services during the 6-month intervention period, and for 12 months post intervention, to assess the extent to which the costs of the interventions are offset by savings elsewhere in the system
-
assess the cost-effectiveness of the interventions, compared with usual care (no exercise intervention), using QALYs as the main measure of effectiveness.
Data collection and analysis related to each aim are described separately, below.
Intervention costs
NHS perspective
The resources involved in the delivery of each intervention (OEP and FaME), and the physical amounts used, were gathered from study records at each site (London and Nottingham/Derby). Resources fell into four categories: set-up and management of the exercise interventions (appointment of PMs for OEP and PSIs for FaME, securing venues for FaME exercise classes, organising staff reimbursements, etc.); hire of facilities for FaME classes; procurement of exercise equipment, such as TheraBands, weights and mats; human resources (cost of time input of PSIs) for FaME and PMs for OEP; and, travel and telephone expenses associated with delivering the interventions. PSIs and PMs recorded all contacts with participants on forms designed for the purpose. Resources associated with the research, such as recruiting participants and gaining informed consent, were not included.
The interventions were delivered in 2010 and 2011, and full economic costs were calculated in British pounds in 2011. Actual expenditures were used for the cost of non-human resources. PSIs were specifically hired for the purposes of the research and paid a fixed fee per one-hour class of £50. In the costing study, the cost of PSIs was based on the unit costs of an equivalent NHS grade, namely a community physiotherapist. Two hours were allowed per class, to include preparation, clear up and travel time. Use of unit costs has the advantage of taking account of salary on-costs, qualifications and management, administrative and capital overheads. 74 The value of volunteer PM time for OEP was established by replacement cost methods using the unit cost to the NHS of community clinical support workers. 73 The cost of training PSIs and PMs in the FaME and OEP interventions (provided by the research team) was included. The total cost of each intervention in each site was established and the cost per participant was calculated.
Private/participant perspective
Participants in all three groups reported out-of-pocket expenses related to exercising. This information was collected in monthly diaries (six) during the 24-week intervention period, and in four subsequent diaries with 3-month recall up to 18 months beyond the end of the intervention. They were asked to report if they have bought anything to help them to exercise (e.g. special clothing such as stretchy trousers) and, if so, what they bought and how much it cost. The diaries were also used for falls reporting and were mailed back to the research team at the end of each reporting period. Diary data were collated at the individual participant level and aggregated to provide total and average (per-participant) costs for each site and study group.
The costs for participants in the FaME group of attending the group exercise venue were estimated from information collected at the 24-week (end of intervention) postal assessment. A short structured form was devised that asked them to report the distance they travelled (in miles, counting both ways); how long they usually spent travelling to and from the exercise class (< 15 minutes, 15–30 minutes, 30–45 minutes, 45–60 minutes, > 1 hour); the mode of travel they usually used (train/tram/bus/taxi and fare both ways, car and payment for parking or congestion charge, walk, other – specifying what method and cost per class). This form also asked what other activity they gave up to attend the exercise class (work, caring, leisure, etc.) to gain an indication of opportunity cost and the societal (productivity) effects. ProAct65+ targets people aged ≥ 65 years and it was expected that many participants would be retired.
Service use
Exercise interventions have the potential to affect utilisation of health and social services in two ways. First, exercise may result in general health benefits and therefore reduce other service utilisation and, thereby, offset the cost of the intervention. Second, although designed to improve stability and reduce falls, there is a possibility that additional engagement in exercise may increase the incidence of falls. Monitoring falls, health and social care utilisation and costs associated with them was thus an important component of the analysis of service use.
Health effects of exercise
Participants (all three groups) were asked to report at baseline their service use in the last month: specifically how many times (0, 1, 2, 3 or 4, ≥ 5) in the last week they had visited their GP; had a home visit from the GP; seen a nurse or other health professional at the GP surgery; had a home visit from a nurse or other health professional; visited the hospital as an outpatient; stayed in hospital (number of nights); bought or received prescribed medicines (number of items); not been able to do paid employment or normal activities as a result of a health problem; had help at home from social care; and had needed friends or relatives to help out at home. This information was used to compare groups at baseline.
Subsequently, service use data (same items as at baseline) were collected from participants in all groups through the diaries (submitted monthly during the 6-month intervention and every 3 months thereafter until 18 months post intervention). However, diary return was patchy, so a small pilot study was conducted to explore the implications of collecting service use data from GP records (enabling all participants to be included). Data were extracted for 27 participants (nine per study arm) for the 12 months prior to recruitment and 18 months post recruitment, covering the same items as in the diary and with separate documenting of service use related to physical injury and falls. The findings from this pilot study showed (1) generally low numbers of contacts, except with GPs and other primary health-care professionals and (2) that only a small proportion of recorded utilisation related to physical injury. It was therefore decided to use GP records as the source of data on service use and to focus on primary care contacts (number of GP, practice nurse, out-of-hours GP and other primary care contacts) at practice or clinic/home/by telephone. Information on the number of falls, and service use associated with those falls [accident and emergency (A&E) attendances, hospital admissions and number of inpatient nights] was also collected. All data gathered from GP records covered the 18 months post recruitment (i.e. 6 months of the intervention and 12 months post intervention). Data were collected manually onto a specially designed proforma and transferred to a SPSS database by a researcher working to a standard operating procedure.
Utilisation of each item of health and social care was recorded at the individual participant level and aggregated to provide total and average (per-participant) utilisation for each site and study group. The costs of health and social care utilisation were obtained by applying published unit cost data75 to physical number of contacts for each service type. Group total and average costs were calculated for the 18-month period post recruitment.
Falls
Falls were recorded in the study by two means. First, participants self-reported falls in diaries (according to the same schedule as for service use): no fall versus fall with no injury, fall with bruise or cut, fall with muscle or ligament injury, fall with broken bone. Reporting of any fall was followed up by the study team for the purpose of AE reporting, but details of service use related to falls was not requested. Similarly, the service use reported in diaries was not specifically related to the falls that were reported and could refer to general health care that had been accessed. Secondly, data on falls [number, A&E attendances as a result of falls, hospital admissions (and number of nights) as a result of falls], was collected as part of the GP record extraction for the 18 months post recruitment. Concordance between the reporting of falls for 53 participants in diaries and from GP records was explored. The findings showed good agreement for people reporting no falls, but poor agreement where falls were reported. Of the 53, 16 had no diary data or incomplete diary data. Of the 37 participants with both diary and GP data over the 12-month period, there was disagreement between the sources regarding the number of falls for 10 records; in three of these, the GP data recorded higher falls than the diaries, and in seven it was the other way round. Of the 27 cases where there was complete agreement between the GP and diary data, 25 were ‘nil’ returns (i.e. no falls reported). On the assumption that falls giving rise to medical treatment are most consistently likely to appear in the GP records, and as diary returns were incomplete, GP data were used in the economic analysis as the primary source of information on service use associated with falls.
The number of falls, and A&E attendances and hospitalisations as a result of falls were collated at the individual participant level and aggregated to provide total and average (per-participant) utilisation for each site and study group. The costs of health and social care utilisation associated with falls were obtained by applying published unit cost data74 to the number of A&E visits and hospital stays. Group total and average costs were calculated for the 18-month period post recruitment.
Economic analysis
Standard techniques of economic appraisal were applied. 76 The main measure of cost-effectiveness was the mean difference in QALY scores at 12 months after the end of the intervention, after adjustment for baseline measures in an analysis of covariance (as described in the statistical analysis section). Quality-adjusted life-year scores were obtained by transforming SF-12 health-related quality-of-life scores into EQ-5D utility weights. Transformation of SF-12 version 1 can be conducted using a published algorithm,40 but as version 2 had been used in the study, an amended algorithm was obtained from the authors (Dr Oliver Rivero-Arias, Oxford University, 2013, personal communication). The prepublished protocol specified that, if statistically significant differences in mean-adjusted QALYs were found between groups at the primary end point, comparisons between the usual-care (no exercise) group and each type of exercise programme would be conducted, ICERs would be calculated, and a probabilistic sensitivity analysis undertaken.
Secondary cost-effectiveness analyses were conducted using the primary PA outcome [proportions in each group reaching the recommended PA target of at least 30 minutes of activity of moderate intensity on at least 5 days each week (150 minutes per week), measured using the CHAMPS and Phone-FITT questionnaires] at 12 months after the end of the intervention.
The planned economic evaluation was based on NHS intervention costs only. Service use costs, and those associated with falls, would be added to the analysis if significant differences in these variables were found between groups.
Data sets
Missing outcome data were assumed to be ‘missing at random’, conditional on prespecified key predictors of ‘missingness’ (in particular baseline values of the response variable, treatment arm and measures of compliance post randomisation). Multiple imputation of outcome variables was carried out using these predictors of missingness. 77
The full analysis set comprised all randomised participants for whom one postbaseline assessment of the primary outcome measure was available. The per-protocol set comprised all randomised participants who are deemed to have no protocol violations. The safety set was all randomised participants who undertake at least one OEP session or FaME class.
Risks
Participants completed a health questionnaire at recruitment which was sent to their GP to confirm exclusion criteria, prior to commencement of either exercise programme. Previous evaluation of the OEP showed significant reductions in falls and injuries. 13 No adverse effects occurred in previous evaluations of either the OEP or FaME programme. 31
Safe exercise guidelines were followed, pre-exercise assessment was conducted and exercise intensity and difficulty were increased with caution, to minimise the risk of injury. All participants and their GPs were informed of the potential risk of injury from any exercise programme in the information documents provided for participants and practices, so that consent was obtained with full knowledge of such risks.
Adverse events
An AE was defined as any unfavourable and unintended sign, symptom, syndrome or illness that develops or worsens during the period of observation in the trial. This included:
-
exacerbation of a pre-existing illness
-
increase in frequency or intensity of a pre-existing episodic event or condition
-
condition detected or diagnosed after the intervention, even though it may have been present prior to the start of the study
-
continuous persistent disease or symptoms present at baseline that worsen following the start of the study.
A serious adverse event (SAE) was defined as any AE occurring following study-mandated procedures, having received the OEP or FaME programme or usual treatment that results in any of the following outcomes:
-
death
-
a life-threatening AE
-
inpatient hospitalisation or prolonging of existing hospitalisation
-
a disability/incapacity.
Important medical events that did not result in death, were not life-threatening and did not necessitate hospitalisation were considered a SAE when, based on appropriate medical judgement, they jeopardised the participant’s health and required medical or surgical intervention to prevent one of the outcomes listed above. All AEs were assessed for seriousness, expectedness and causality. All AEs were recorded and closely monitored until resolution, stabilisation, or until it had been shown that the study intervention was not the cause.
Participants were asked to contact the trial site immediately in the event of any SAE. The chief investigator was informed immediately and determined seriousness and causality in conjunction with any treating medical practitioners. A SAE that was deemed directly related to, or suspected to be related to, the trial intervention was reported to the TSC and the ethics committee.
Informed consent
Written informed consent was obtained from all participants. The decision regarding participation in the study was entirely voluntary. The researcher emphasised to potential participants that consent regarding study participation could be withdrawn at any time without penalty and without affecting the quality or quantity of future medical care, or loss of benefits to which the participant was otherwise entitled. No trial-specific interventions were undertaken before informed consent had been obtained.
Ethics committee approval
Ethical approval was granted to the trial from Nottingham Research Ethics Committee 2 (application number 08/H0408/72). National Health Service Research & Development approval were granted by NHS Nottinghamshire County and Westminster, Brent, Harrow, Hounslow and Barnet & Enfield PCTs, and other relevant PCTs as practices were recruited to the study.
Management of the trial
A trial management committee made up of all co-applicants and research staff at each site met regularly, face to face or by teleconference, to review the trial’s progress. Patient and public involvement (PPI) in the study was ensured by involvement in the management group of two lay experts from Nottingham University’s PPI forum. A combined TSC and data management committee met twice yearly to review progress of the trial.
Summary
The ProAct65+ trial was a primary care-based exercise intervention for older people with wide inclusion criteria. The pragmatic trial design replicated the approach taken in successful primary care trials in New Zealand39 and differed from the majority of trials which focus on falls reduction in selected groups by having continuation of PA as its primary outcome. The problems that we anticipated were (1) biases in recruitment, with those already exercising at a relatively high level being more likely to volunteer for this trial; (2) limited retention of recruits to the study, which we hoped to minimise by relatively frequent, but brief, contact with participants after the end of the exercise programmes; (3) variation in ‘doses’ of exercise, which we hoped to avoid through our quality assurance processes; and (4) an increase in falls risk, as in previous studies,39 which we countered through training of staff, risk reduction and risk management programmes.
Because the trial documented the levels of activity of participants (which could then be compared with population norms), the number screened, the number who were ineligible and the number who refused, its findings are generalisable, and can contribute to policy on exercise promotion and falls prevention among older people. They are relevant to older people and to policy-makers working in health, social care and leisure arenas, health and social care commissioners and providers, leisure providers and charities and voluntary organisations working with older people.
Chapter 3 Modification of trial processes and procedures
This chapter describes the challenges faced during the ProAct65+ trial and the modifications made to the trial protocol. 34
The protocol was amended in six main ways:
-
The number of practices was increased, the numbers invited from each practice were also increased, and the recruitment period was extended, in order to recruit the target number of participants.
-
Telephone screening of possible participants prior to the initial assessment was introduced to exclude those already exercising at, or above, the target level before they were given an appointment for the baseline assessment.
-
The criteria for the recruitment of PMs for the OEP arm, and the intensity of their role, were changed in an attempt to overcome problems of recruitment and retention.
-
A quality control system was incorporated into the FaME arm to aid standardisation of class activities.
-
The number of diaries participants were asked to complete during the follow-up period was reduced to minimise the burden of diary completion and to optimise data collection about falls, service use and costs.
-
An AE typology was developed and a system for checking it was applied consistently between sites, to ensure governance of risks to participants.
Each of these changes will be summarised here and a detailed description can be found elsewhere. 78
Improving the recruitment of general practices and participants
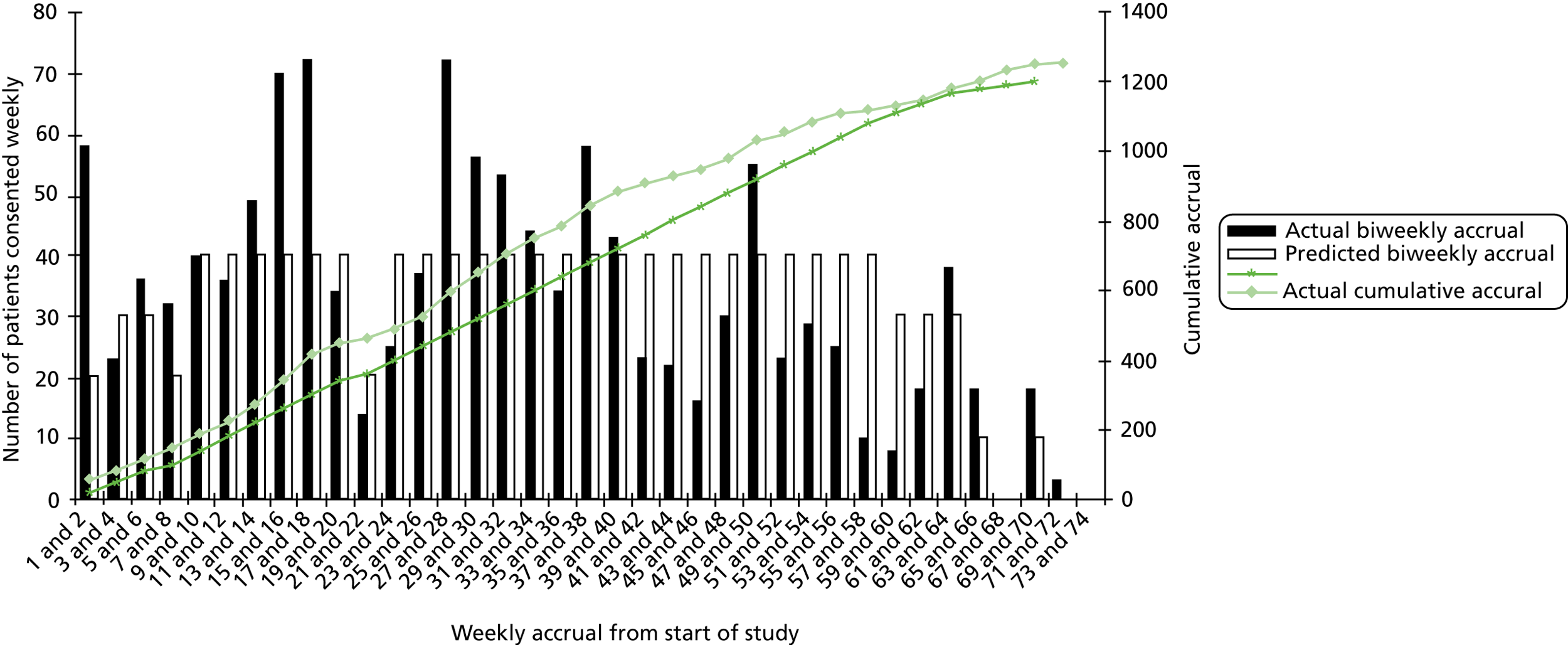
The flow path of participant recruitment to the trial is shown in Figure 1. The trial initially aimed to recruit 30 practices (15 at each site) and 45 patients per practice over a period of 3 weeks, to achieve a sample size of 1200 participants aged ≥ 65 years. The proportion of those who expressed an interest varied between practices, from 8% to 19% in London and from 7% to 21% in Nottingham/Derby, with a mean of 13.4%. In order to achieve the recruitment target, the number of invitations to eligible patients was increased from 450 per practice to 600 to adjust for the lower than anticipated recruitment, and more practices were recruited.
FIGURE 1.
Flow chart of the recruitment and assessment process in the ProAct65+ trial.
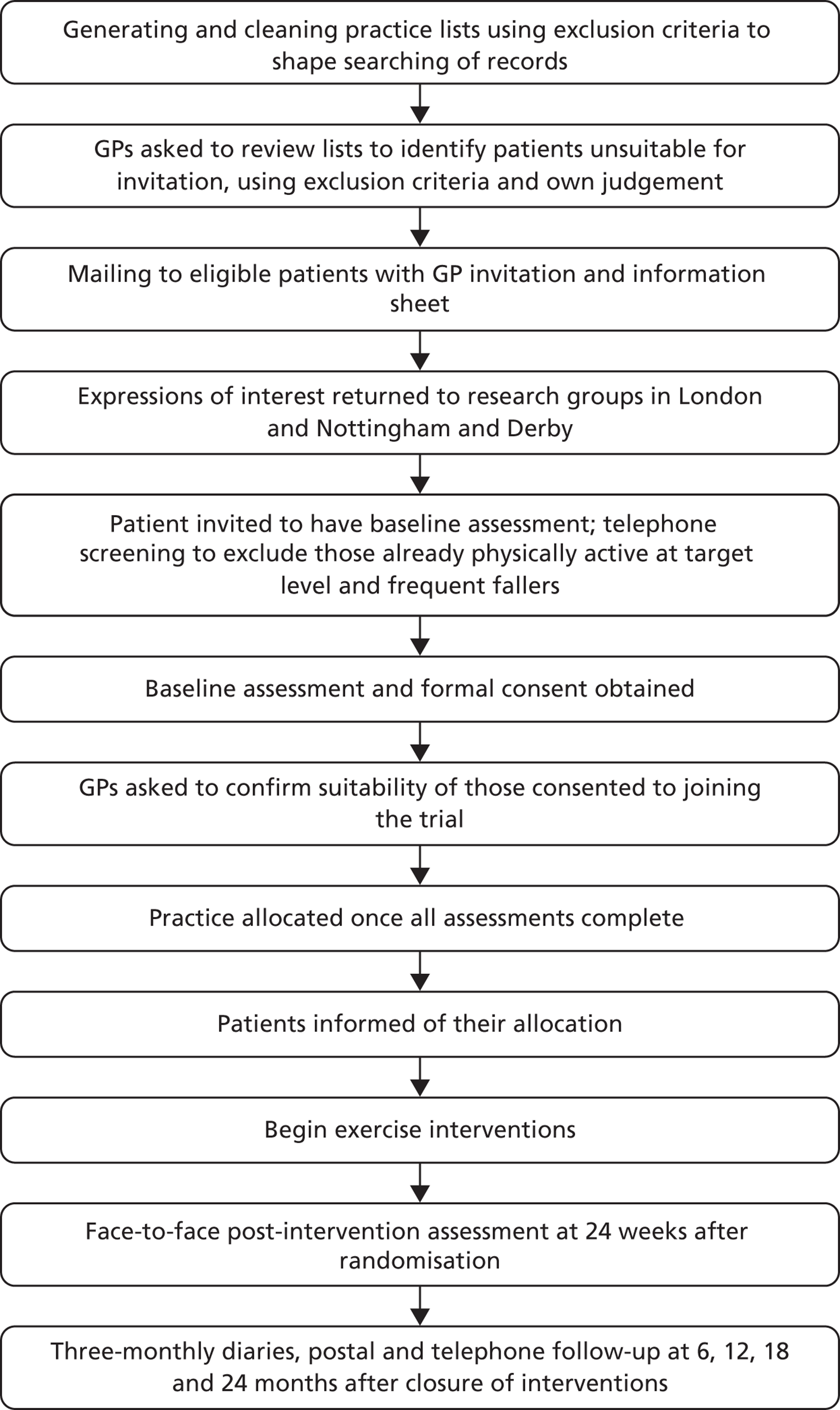
Stratified random sampling was planned, whereby eligible patients would be stratified into age groups 65–74 years and ≥ 75 years. To simplify the tasks for the practices and to encourage their co-operation this stratified sampling approach was abandoned and patients were sampled from one list of patients aged ≥ 65 years.
Room availability in practices for baseline assessments was limited and it took up to 6 weeks in some practices to assess and recruit the target number of participants. The recruitment phase of the trial was 9 months longer than anticipated because of the need to recruit more practices at both sites and to allow more time at each practice to undertake recruitment. This extension of the recruitment period altered the time scale of the trial and potentially limited data collection for the 18- and 24-month follow-up. In total, 43 general practices and 1256 participants were finally recruited: 22 practices and 605 participants in London and 21 practices and 651 participants in Nottingham/Derby.
Adding an eligibility screen
Although there were multiple steps for screening eligible patients (including electronic and manual patient searches by the general practices), in the first practices recruited researchers encountered patients at the baseline assessment and consent stage who were ineligible because they met exclusion criteria, particularly falling fewer than three times in the previous 12 months or already exercising at the target level of five sessions of 30 minutes of moderate exercise per week. To limit the number of assessments of participants who would be found to be ineligible, researchers asked questions over the telephone about falls in the last year and current levels of exercise when arranging the baseline assessment appointment, and excluded those who met these criteria.
Peer mentor recruitment and training
Volunteer PMs were recruited to support the participants during the exercise programme. Recruitment was slow (Figure 2) and time-consuming. Despite intense efforts the number of PM who joined the trial did not reach the target. After 8 months of PMs recruitment, the age criterion for PMs was altered to allow the enrolment of adults aged ≥ 50 years. This led to an additional eight PMs being enrolled in London, but no more in Nottingham/Derby.
FIGURE 2.
Number of PMs trained.
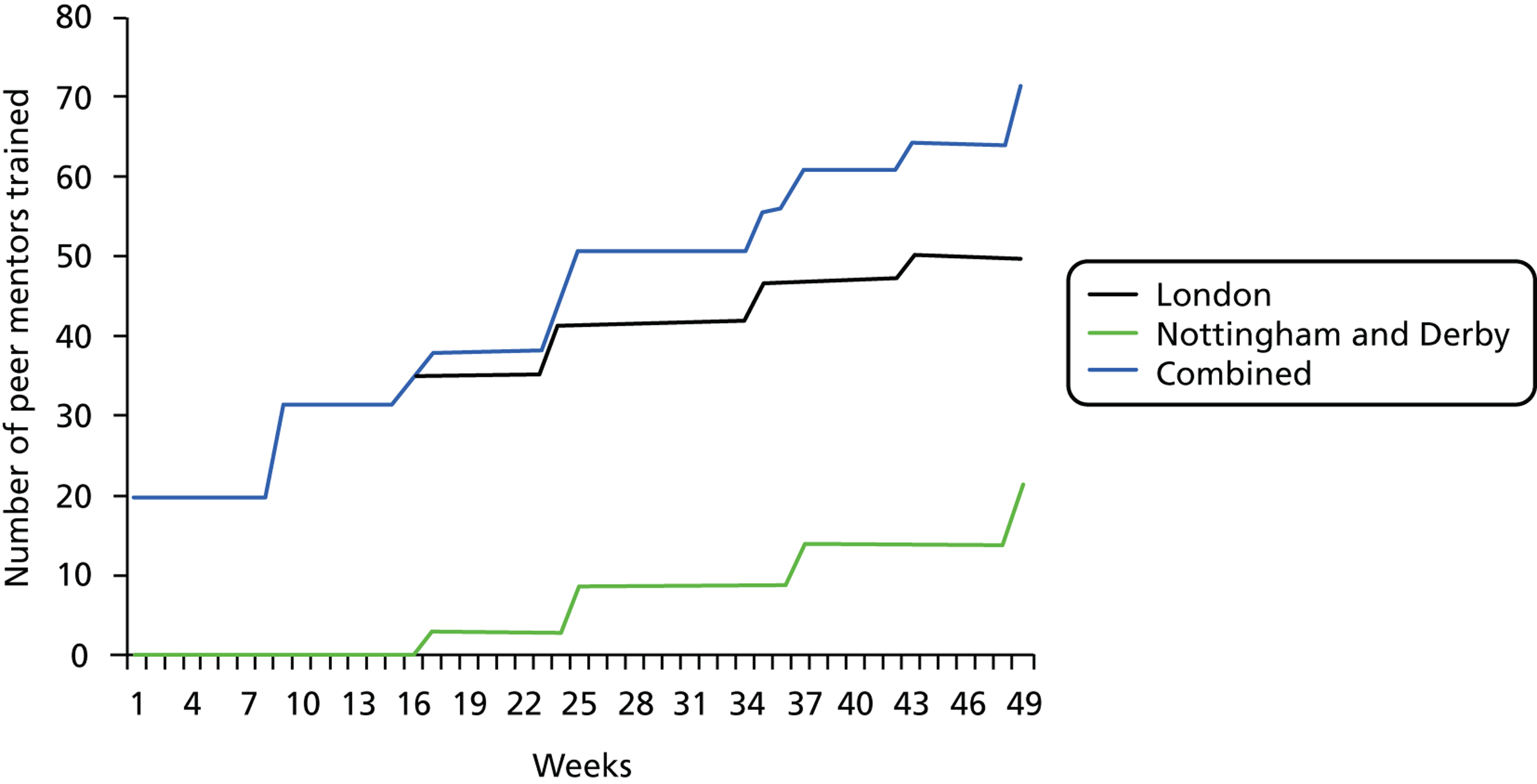
Table 1 shows the length of time spent on recruiting PMs, numbers of individuals who expressed an interest in becoming a PM, numbers of individuals trained, the number who subsequently disengaged from the study, and the final number of PMs who volunteered and were allocated participants. There was a large difference in the number of people who expressed an interest in becoming a PM and those that were trained. Feedback from PMs suggests that disengagement was as a result of, in part, the length of time between training and beginning work. This period was long because of the time needed to obtain research management and governance approvals for the PMs, and because of the staggered recruitment and randomisation of the practices. Disengagement was also as a result of, in part, the distance PMs would need to travel to support participants.
| Time scale and recruitment | London | Nottingham/Derby |
|---|---|---|
| Time spent on recruitment by staff (months) | 12 | 15 |
| Expressed interest (n) | 130 | 79 |
| Trained (n) | 50 | 21 |
| Disengaged (n) | 19 | 14 |
| Volunteered (n) | 31 | 7 |
| Time from trained to deployed (days) | Mean 132 (range 21–255) | Mean 155 (range 75–257) |
Each PM in the trial mentored a mean of three participants (range 1–13) in London, and a mean of three participants (range 1–5) in Nottingham/Derby. Overall, both sites fell short of the target of four to five participants per PM. All participants, regardless of their PM support, received the initial exercise training session and a booklet with tailored exercise instructions. Not all participants received a PM because of the difficulties recruiting them. In London, 123 (53%) participants and in Nottingham/Derby, 21 (12%) participants had a PM.
Despite using the same recruitment methods, recruitment difficulties were greater in Nottingham/Derby, possibly because the trial was competing with existing PM PA programmes for older people in Nottingham/Derby. The TSC advised to keep the intervention true to usual practice in the NHS, i.e. one instruction session plus a manual of exercises. Therefore, where there were insufficient PMs for all participants, they were not supplemented by an alternative person and some participants had no PM support at all.
In another attempt to increase the number of PMs and encourage them to support more participants, the number of their supportive contacts with participants was reduced. Initially PMs were scheduled to visit participants in their home on four occasions and telephone them 12 times during the 24-week intervention. This was reduced to two visits and eight telephone calls. Over both sites, the number of home visits ranged from zero to five (mean 2) and the number of telephone call contacts ranged from 0 to 18 (mean 6). Modification of the number of contacts did not increase PM recruitment or their case load.
Quality control of the Falls Management Exercise programme
The FaME intervention was a weekly group-based exercise session, supplemented with additional home exercises (modified from the OEP) described in a booklet. Postural stability instructors were recruited to lead the classes. The trial aimed to recruit 12 PSIs per site. In London, 16 PSIs were recruited, with a total of seven working on the trial. As there were few qualified PSIs available to recruit in Nottingham/Derby, the trial recruited and trained physiotherapists and exercise professionals who were interested in becoming a PSI and working on the trial. Sixteen individuals embarked on the PSI training course (15 completed the training) and seven of them worked on the trial. Some PSIs were not employed on the trial because of their limited availability. Additionally the complex and lengthy process of completing research governance approvals resulted in losing some available PSIs. The recruitment target was reached with 32 PSIs recruited and trained over both sites. Of these, 14 (44%) delivered the intervention, enabling the intervention to be fully staffed.
In order to quality assure and standardise the FaME intervention, two quality assurance members of the trial oversaw the intervention delivery by attending four exercise sessions over the 24-week intervention period for each PSI in all of the FaME practices. The quality assurers went to the sessions individually, except the first two sessions when they attended together to standardise their method. Overall, 45 FaME classes in London and 38 in Nottingham/Derby were quality assured. Using a standard checklist (Figure 3), the quality assurers observed the PSI leading the exercise class and then gave them feedback and an action plan in order to improve intervention delivery, optimise participants’ ability to undertake progressively demanding exercises and standardise the exercise intervention as much as possible.
FIGURE 3.
Postural stability instructors quality assurance checklist.
Measuring falls, service use and physical activity
During the intervention self-completion diaries were posted to participants every 4 weeks. During the follow-up period, participants were posted self-completion diaries every 3 months, larger self-completion questionnaires every 6 months and telephoned for a short questionnaire every 6 months. See Table 2 for the schedule of questionnaires at different time points and Chapter 2 for full details of questionnaires.
| Outcome and tool | Face-to-face, telephone and postal assessments | Telephone and postal assessments | ||||
|---|---|---|---|---|---|---|
| Baseline | End of intervention (24 weeks) | 6 months | 12 months | 18 months | 24 months | |
| PA | ||||||
| Subjective habitual PA (Phone-FITT, PASE and CHAMPS) | O, F, U | O, F, U | O, F, U | O, F, U | O, F, U | O, F, U |
| Self-completed exercise diaries (4-weekly during intervention and then 3-monthly during follow-up) | O, F, U | O, F, U | O, F, U | O, F, U | O, F, U | O, F, U |
| Direct health benefits | ||||||
| Modified Clinical Romberg Static Balance test | O, F, U | O, F, U | ||||
| TUG | O, F, U | O, F, U | ||||
| Functional reach | O, F, U | O, F, U | ||||
| 30-second chair rise | O, F, U | O, F, U | ||||
| Falls risk (FRAT) | O, F, U | O, F, U | ||||
| Falls (falls dairies 4-weekly during intervention, 3-monthly during follow-up) | O, F, U | O, F, U | O, F, U | O, F, U | O, F, U | O, F, U |
| ConfBal scale | O, F, U | O, F, U | O, F, U | O, F, U | O, F, U | O, F, U |
| FES-I | O, F, U | O, F, U | O, F, U | O, F, U | O, F, U | O, F, U |
| Social network size and density (brief LSNS) and perceived social support (MSPSS) | O, F, U | O, F, U | O, F, U | O, F, U | O, F, U | O, F, U |
| Quality of life | ||||||
| Quality of life (OPQoL and SF-12) | O, F, U | O, F, U | O, F, U | O, F, U | O, F, U | O, F, U |
| Stage of change, self-efficacy for exercise, physical self-perception and value or importance of PA | ||||||
| Self-efficacy for exercise | O, F, U | |||||
| AFRIS | O, F | |||||
| Demographic information, medication | O, F, U | O, F, U | ||||
| Comorbidity | O, F, U | |||||
| Use of primary, secondary care and social care services from falls diaries | O, F, U | O, F, U | O, F, U | O, F, U | O, F, U | O, F, U |
Because non-monetary incentives are known to assist retention in trials,17 small incentives were sent to participants to encourage completion of postal questionnaires. With diary 6 and 12, participants received a ProAct65+ pen and with diary 8 and 10, they received a ProAct65+ cotton shopping bag. Participants were also sent an annual Christmas card and brief newsletters with each diary they received.
Research staff at both sites telephoned participants every 3 months to remind them to return questionnaires. Up to three contacts with participants were made to undertake each telephone interview. Some participants did not return self-completion diaries and/or questionnaires and some were not available for a telephone interview as a result of a variety of reasons, including being on holiday, at work, too busy, or forgetting or losing the questionnaires.
The self-completion diaries requested information on participants’ health and social service use, falls and current exercise levels. 79 Initially, it was planned for participants to receive monthly prospective diaries to complete throughout the full length of the trial. When participants said that they wished to withdraw from the trial because of the quantity and frequency of questionnaires they were offered the opportunity to remain in the trial but complete only the 6-monthly questionnaires and not receive further diaries. By doing this, the trial retained 52 participants in London and 28 in Nottingham/Derby (6% of total trial participants) who would otherwise have withdrawn from the trial. To further limit the number of participants who withdrew from the trial because of the burden of the questionnaires and diaries the frequency of the diaries sent during the 2-year follow-up phase was reduced from monthly to quarterly. The diaries sent during the follow-up phase required the participants to recall their service use and falls from the last 3 months and record a 1-week prospective snap-shot of their exercise activities.
Capturing adverse events
Adverse events were monitored throughout the trial to assess the trial’s safety and manage participant risks. This is especially important as exercise within this age group may be associated with an increased risk of falls. 39,80 The ProAct65+ trial used a risk management pathway for capturing, classifying and dealing with participant AEs (Figure 4), which initially categorised all occurrences as SAEs, AEs, adverse reactions (ARs) or adverse incidents (AIs). All data were logged and any SAEs were reported to the TSC. The original risk management pathway and the definitions of events, reactions and incidents are reported in the trial protocol. 34
FIGURE 4.
The ProAct65+ risk management pathway. CI, chief investigator.
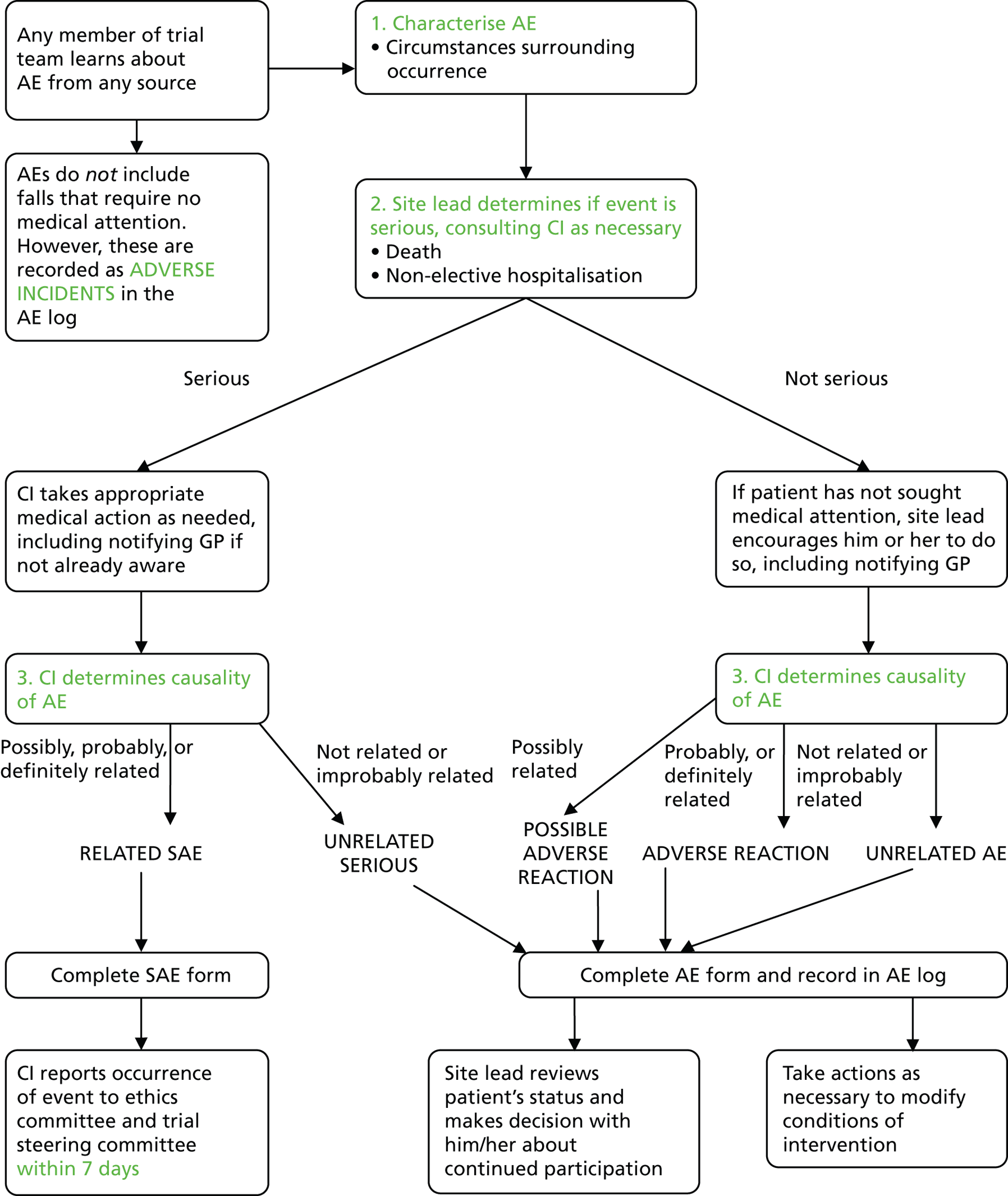
A comparison of all events between trial sites was carried out towards the end of the trial’s intervention phase. There were noticeable differences in the numbers of ARs recorded between sites with London categorising 5%, and Nottingham/Derby categorising 16% of their total events as ARs. A cross-checking system was therefore implemented between sites in an attempt to standardise categorisation. All events from each site, except AIs, were checked by the other site. If the other site’s categorisation was different to the original categorisation, this was deemed a mismatch. Mismatches between sites were identified, and blinded forms then passed to the principal investigators who discussed and agreed a final categorisation. The initial calculation of mismatches was performed towards the end of the intervention phase, when there were 51 mismatches, giving a mismatch rate between sites of 19%.
The decision whether or not an event is ‘possibly related’ to the trial is open to subjective interpretation. Consequently, 45 of the 51 (88%) discrepancies in the categorisation of events recorded at each site were between AEs and ARs. The category ‘possible adverse reaction’ (possible AR) was therefore added. After the introduction of the possible AR category, the mismatch rate (prior to discussion between principal investigators) fell to 2.6%.
After advice from the TSC, the categorisation was further modified to enable unrelated SAEs to be distinguished from non-SAEs. The final categories applied to the trial’s events were, therefore, SAEs, unrelated SAEs, AEs, ARs, possible ARs and AIs (see Figure 4).
Chapter 4 Recruitment of practices, postural stability instructors, peer mentors and participants
Recruitment of general practices
Forty-three practices were recruited to the trial, to ensure that the target study population could be reached (see Chapter 3 for details). The characteristics of practices that joined the trial are shown in Table 3.
| Practice (n = 43) characteristics | Number (%) or median (IQR) |
|---|---|
| Training practices | 24 (55.8) |
| Number of GPs | 4 (3–7) |
| Number of nurses | 2 (1–3) |
| Use of exercise referral scheme | 32 (76.2) [1] |
| IMD2007 scorea | 20.98 (14.50–34.97) |
| List size | 6532 (4046–8509) |
| Number of patients aged ≥ 65 years | 895 (495–1390) |
Postural stability instructors
The target of recruiting 12 PSIs per site was achieved and FaME-arm classes were fully staffed. In London, 16 PSIs were recruited with a total of seven working on the trial, whilst in Nottingham/Derby 15 completed the training, and seven of them worked on the trial. The mean class size was less than planned, at five not nine. The quality assurance reviewers noted that PSIs largely achieved standardisation of the intervention, although they varied most in progression of the exercise programme, and needed reminding about collecting data for the trial.
Peer mentors
Thirty-eight PMs were recruited, trained and deployed in the trial, 31 in London and seven in the Nottingham/Derby practices (details of the recruitment processes can be found in Chapter 3). The planned and actual engagement by PMs with participants is shown in Table 4. Research staff carrying out quality assurance through discussions with PMs concluded that, as a whole, PMs made only a limited attempt to standardise the intervention (i.e. to implement the individualised plan given to the participant at the first encounter) and the participants’ progression was also limited, even though the PMs tailored their advice in other aspects of exercise. They returned trial paperwork (follow-up sheets detailing call and visit information, and time and travel log for the economic analysis) promptly.
| Contacts | Planned | Actual | Duration |
|---|---|---|---|
| Home visits | 2 | Mean 2 (range 0–5) | 25–95 minutes, median 38.5 minutes |
| Telephone calls | 8 | Mean 6 (range 1–18) | 3– 20 minutes, median 5.0 minutes |
Recruitment of participants
Steps that were taken to ensure recruitment to the trial are described in Chapter 3. Figure 5 shows the recruitment of 1256 participants to the study. In total, 20,507 patients were invited to participate (Nottingham/Derby, 10,738; London, 9769). Expressions of interest were received from 2752 (13%) (Nottingham/Derby, 1481; London, 1271) and 1256 (6% of those approached) consented (Nottingham/Derby, 651; London, 605).
FIGURE 5.
Recruitment of participants to the trial.
Baseline characteristics of the study population
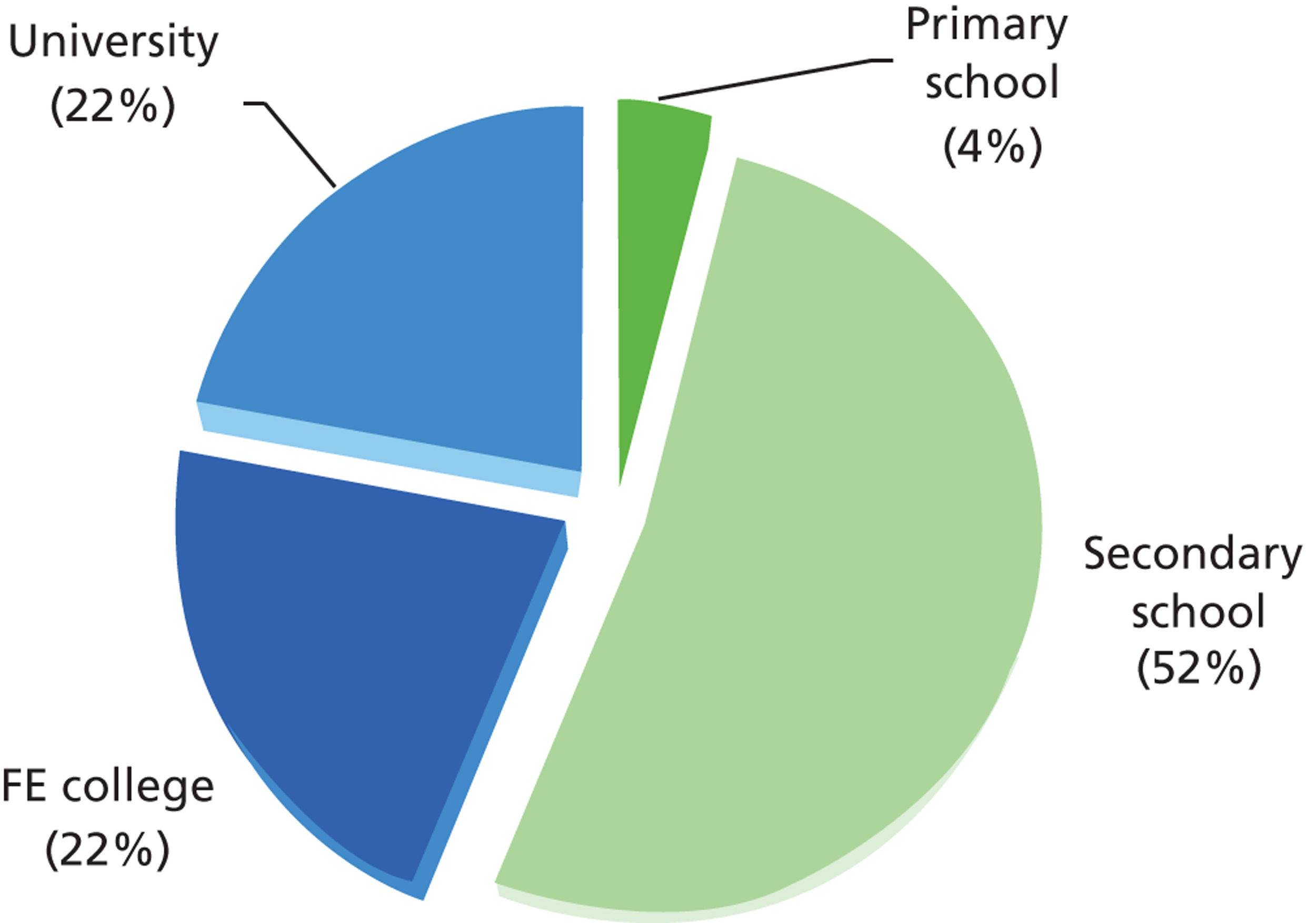
The average age of participants was 73 years (range 65–94 years), with 84% of participants aged less than 80 years, and 62% of participants were female. Thirty-four languages were spoken (33 in London and 12 in Nottingham/Derby) and 14% of participants were non-white, with greater ethnic diversity among the London participants. A total of 44% of participants had completed some form of further education, as shown in Figure 6. On average, each participant had 1.7 comorbidities [range 0–7, standard deviation (SD) 1.4 comorbidities] and was taking 3.7 medications on repeat prescription (range 0–18, SD 3.7 medications).
FIGURE 6.
Educational attainment among trial participants. FE, further education.
Baseline characteristics of participants in the trial are compared with normative data in Table 5. Trial participants performed below normative levels on most scales, except for Phone-FITT, PASE, ConfBal and OPQoL, but similarly to normative values on the AFRIS questionnaire. The normative values for Phone-FITT apply to an older (mean age 81 years) male population, so the higher level of household PA and the lower level of recreational activity in the ProAct65+ population may reflect its lower median age and the predominance of female participants. The normative values for ConfBal were calculated from the published data, which were derived from a population attending day centres, so the better performance of trial participants is not surprising.
| Outcome measure | ProAct65+ mean (SD) | Normative mean (SD) | Normative reference |
|---|---|---|---|
| TUG | 11.08 seconds (5.94 seconds) | 9.4 seconds (95% CI 8.9 to 9.9 seconds) | Bohannon 200681 |
| 30-second chair rise | 10.40 stands (3.26 stands) | Rikli 199944 | |
| Women 10.03 stands (3.02 stands) | Women 12.7 stands (4.0 stands) | ||
| Men 11.06 stands (3.54 stands) | Men 14.2 stands (4.6 stands) | ||
| Functional reach | 25.46 cm (8.03 cm) | Duncan 199043 | |
| Men 26.34 cm (8.38 cm) | Men 33.43 cm (1.55 cm) | ||
| Women 24.93 cm (7.77 cm) | Women 26.59 cm (3.53 cm) | ||
| Romberg test (scored out of 28) | 20.19 (6.98) | None published as a score | |
| FRAT (scored out of 5, ≥ 3 high risk of future fall) | Mean score not useful. Proportion at high risk = 6% | Does not state what % of recruited population scored ≥ 3 | Nandy 200460 |
| SF-12 | Physical summary score 36.90 (6.59) | Physical summary score 47.42 ± 0.40 | Ware 199661 |
| Mental summary score 48.76 (6.29) | Mental summary score 53.82 ± 0.30 | ||
| Phone-FITT | 39 (14) | Gill 200856 (population is male, mean age 81 years) | |
| Household PA score 26.28 (9.86) | Household PA score 19.2 (9.0) | ||
| Recreational PA score 12.30 (8.42) | Recreational PA score 14.9 (11.3) | ||
| CHAMPS | Calorific expenditure per week in at least moderate intensity physical activities 915 (1306) calories | Calorific expenditure per week in at least moderate intensity physical activities 1486 (1472) calories | Stewart 200120 |
| Frequency per week in at least moderate intensity physical activities 2 (4) | Frequency per week in at least moderate intensity physical activities 5.7 (4.5) | ||
| Calorific expenditure per week in all listed physical activities 2238 (2136) calories | Calorific expenditure per week in all listed physical activities 2420 (1831) calories | ||
| Frequency per week in all listed physical activities 10 (11) | Frequency per week in all listed physical activities 13.1 (8.0) | ||
| PASE (total score) | 117 (61) | 102.9 (64.1) | Washburn 199357 |
| AFRIS (range for each item 1–7, high score is good) | Group exercise median Attitude, 5.5 Subjective norm, 6.0 Perceived behavioural control, 5.0 Identity, 5.0 Intention, 7.0 |
Median Attitude, 5.5 Subjective norm, 5.5 Perceived behavioural control, 6.0 Identity, 6.0 Intention, 6.0 |
Yardley 200746 |
| Home exercise median Attitude, 5.0 Subjective norm, 6.0 Perceived behavioural control, 6.0 Identity, 5.0 Intention, 6.0 |
|||
| ConfBal (scored between 10 and 30, low score is good) | 12.55 (3.887) | 17.59 (not published – SG calculated from their data) | Simpson 200945 (population attending day centres) |
| FES-I (range for each item = 1–4, 1 = not at all concerned, 4 = very concerned, items are matched by question although item numbers differ) | Item 1 1.18 (0.54) | Item 2 1.50 (0.81) | Yardley 200559 |
| Item 2 1.37 (0.72) | Item 4 2.09 (1.09) | ||
| Item 3 1.14 (0.49) | Item 6 1.49 (0.79) | ||
| Item 4 1.44 (0.76) | Item 7 2.06 (1.08) | ||
| Item 5 1.41 (0.75) | Item 9 2.14 (1.11) | ||
| Item 6 1.34 (0.69) | Item 15 2.46 (1.16) | ||
| Item 7 1.18 (0.54) | Item 16 1.85 (1.06) | ||
| MSPSS (average score) | 5.50 (1.37) | 6.40 (0.75) | Stanley 199882 |
| OPQoL (total score) | 129.87 (13.27) | 114.538 (10.718) | Bowling 200983 |
Retention of participants
Of the 1256 randomised study participants, 426 (33.9%) did not reach 12 months’ follow-up after the end of the intervention period. Of these, 69 were excluded by their GP, 12 died, three withdrew at an unknown time point, one withdrew before providing any baseline data and one withdrew but asked for their data to be destroyed. Overall, 340 participants were defined as lost to attrition. Almost half of these dropouts withdrew within the first 3 months of the intervention (49.7%). A total of 830 participants were retained in the trial at 12 months’ follow-up. Figure 7 summarises the pattern of attrition over time, for all arms and sites.
FIGURE 7.
The CONSORT diagram.
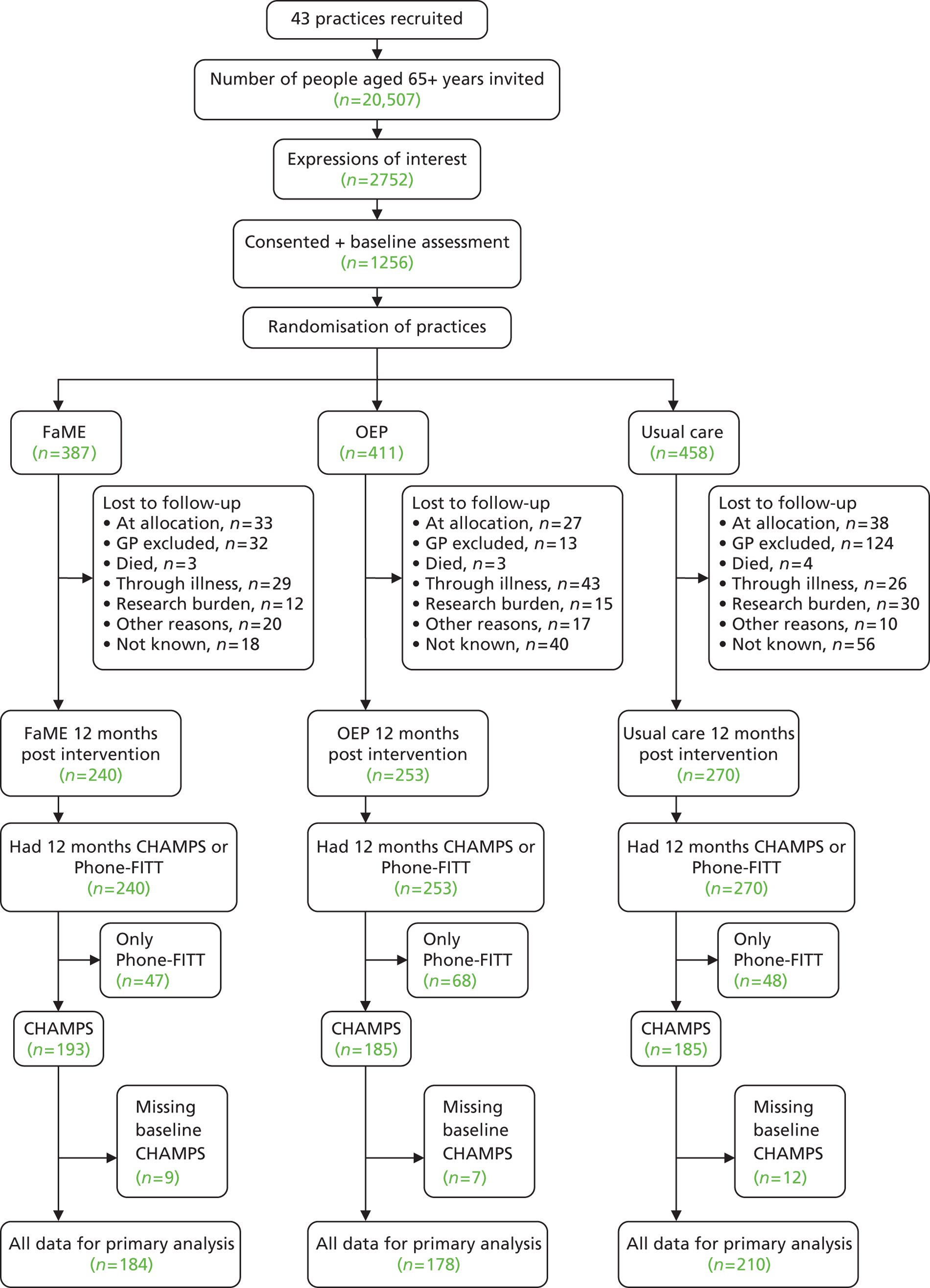
Illness events were common in this study population and 30% of those who dropped out cited illness (their own or others’) as their reason for discontinuing with the study. Disappointment at allocation and research burden (principally related to the number of questionnaires and diaries to complete) were responsible for at least 18% and 11% of dropouts, respectively.
Tables 6 and 7 describe participants’ characteristics by withdrawal status. Associations of baseline measures with later withdrawal were investigated using logistic regression, with robust standard errors calculated to allow for clustering of participants within practices.
| Characteristics | Retained | Withdrew | OR (95% CI) | p-value |
|---|---|---|---|---|
| Number of participants (n = 1170) | 830 (70.9) | 340 (29.1) | ||
| Sex (% female) | 518 (62.4) | 219 (64.4) | 1.09 (0.90 to 1.32) | 0.380 |
| Age (years) median (IQR) | 71 (68–76) | 74 (69–79) | 1.05 (1.03 to 1.08) | < 0.001 |
| Group allocation | ||||
| FaME | 256 (30.8) | 95 (27.9) | 1.00 | 0.750 |
| OEP | 278 (33.5) | 114 (33.5) | 1.11 (0.77 to 1.58) | |
| Usual care | 296 (35.7) | 131 (38.5) | 1.19 (0.74 to 1.92) | |
| BMI (kg/m2) mean (SD) | 26.7 (4.9) [20] | 27.2 (5.1) [26] | 1.02 (0.99 to 1.05) | 0.140 |
| Number of comorbidities | [1] | |||
| 0 comorbidities | 157 (18.9) | 46 (13.5) | 1.00 | 0.007 |
| 1 or 2 comorbidities | 404 (48.7) | 157 (46.2) | 1.33 (0.90 to1.96) | |
| ≥ 3 comorbidities | 268 (32.3) | 137 (40.3) | 1.74 (1.22 to 2.49) | |
| Number of current medications median (IQR) | 3 (1–6) [5] | 4 (2–6) [3] | 1.06 (1.02 to 1.10) | 0.004 |
| English main language | 738 (89.9) [9] | 290 (86.3) [4] | 0.71 (0.50 to 1.01) | 0.060 |
| White self-reported ethnicity | 707 (87.2) [19] | 291 (86.6) [4] | 0.95 (0.67 to 1.34) | 0.780 |
| Living alone | 285 (34.6) [5] | 122 (35.9) | 1.06 (0.78 to 1.44) | 0.710 |
| Children < 18 years living at home | 8 (1.0) [2] | 6 (1.8) | 1.84 (0.68 to 4.98) | 0.230 |
| Living with dependent adults | 40 (5.0) [35] | 27 (8.2) [9] | 1.68 (0.95 to 2.95) | 0.073 |
| Education | [13] | [3] | ||
| Primary/secondary school | 436 (53.4) | 206 (61.1) | 1.00 | 0.030 |
| College/university | 381 (46.6) | 131 (38.9) | 0.73 (0.55 to 0.97) | |
| Employed full- or part-time | 69 (8.4) [8] | 33 (9.7) [1] | 1.18 (0.76 to 1.82) | 0.470 |
| NS-SEC job gradeb | [35] | [15] | ||
| 1–2: managerial and professional occupations | 354 (44.5) | 125 (38.5) | 1.00 | 0.047 |
| 3–4: intermediate occupations | 229 (28.8) | 84 (25.9) | 1.04 (0.76 to 1.41) | |
| 5–7: routine and manual occupations | 205 (25.8) | 109 (33.5) | 1.51 (1.08 to 2.11) | |
| 8–9: never worked and long-term unemployed | 7 (0.9) | 7 (2.2) | 2.83 (1.01 to 7.95) | |
| Annual household income ≥ £20,000 | 291 (40.1) [104] | 110 (40.0) [65] | 1.00 (0.75 to 1.32) | 0.980 |
| Smoking status | [2] | |||
| Non smokers | 444 (53.6) | 148 (43.5) | 1.00 | 0.011 |
| Ex-smokers | 341 (41.2) | 172 (50.6) | 1.51 (1.15 to 1.99) | |
| Current smokers | 43 (5.2) | 20 (5.9) | 1.40 (0.70 to 2.76) | |
| Characteristics | Retained | Withdrew | OR (95% CI) | Unadjusted p-value |
|---|---|---|---|---|
| Number of participants (n=1170) | 830 (70.9) | 340 (29.1) | ||
| Use public transport easily | 788 (95.6) [6] | 312 (92.3) [2] | 0.55 (0.30 to 1.01) | 0.052 |
| Use a walking aid | 112 (13.5) [2] | 51 (15.0) [1] | 1.13 (0.78 to 1.64) | 0.520 |
| FRATb | ||||
| History of any fall in the previous year | 134 (16.2) [1] | 67 (19.8) [1] | 1.28 (0.94 to 1.75) | 0.120 |
| On ≥ 4 medications per day | 361 (43.6) [1] | 174 (51.3) [1] | 1.37 (1.07 to1.75) | 0.014 |
| Diagnosis of stroke or Parkinson’s disease | 14 (1.7) [1] | 12 (3.5) [1] | 2.14 (0.91 to 5.03) | 0.082 |
| Any self-reported problems with their balance | 197 (23.9) [5] | 83 (24.8) [5] | 1.05 (0.80 to 1.38) | 0.730 |
| Unable to rise from a chair of knee height | 25 (3.0) [3] | 19 (5.6) [3] | 1.92 (1.01 to 3.63) | 0.046 |
| ConfBal score median (IQR)c | 10 (10.0–13.0) [81] | 12 [10–15) [82] | 1.08 (1.05 to 1.12) | <0.001 |
| High concern about falling (measured by short FES-I)d | 123 (16.3) [74] | 64 (24.2) [75] | 1.64 (1.15 to 2.34) | 0.007 |
| CHAMPS (minutes/week MVPA)e | [64] | [70] | ||
| 0 minutes | 163 (21.3) | 99 (36.7) | 1.00 | <0.001 |
| 1–149 minutes | 274 (35.8) | 82 (30.4) | 0.49 (0.35 to 0.70) | |
| ≥ 150 minutes | 329 (43.0) | 89 (33.0) | 0.45 (0.32 to 0.62) | |
| SF-12 PCS mean (SD)f | 37.6 (6.2) [4] | 36.3 (6.9) [2] | 0.97 (0.95 to 0.99) | <0.001 |
| SF-12 MCS mean (SD)g | 48.7 (6.1) [3] | 48.4 (6.7) [2] | 0.99 (0.97 to1.02) | 0.540 |
| Socially isolated (based on LSNS-6)h | 149 (20.0) [85] | 72 (27.4) [77] | 1.51 (1.06 to 2.15) | 0.023 |
| Perceived social support (MSPSS) median (IQR)i | 70 (58.0–79.0) [130] | 70 (55.0–78.0) [95] | 0.99 (0.99 to1.00) | 0.250 |
| OPQoL mean (SD)j | 131.0 (13.0) [169] | 126.9 (13.8) [126] | 0.98 (0.96 to 0.99) | <0.001 |
| OEE-positive subscale median (IQR)k | 3.9 (3.6–4.2) [107] | 3.8 (3.4–4.2) [93] | 0.78 (0.62 to 0.98) | 0.035 |
Those participants who dropped out were significantly more likely to be older, have three or more comorbidities, have more medications, have a lower level of education, have worked or currently work in a routine or manual occupation, be an ex-smoker, be unable to rise from a chair of knee height, be less confident about their balance, have a high concern about falling, be inactive, perceive their physical health as poor, be at risk of social isolation, have a lower subjective quality of life, have lower outcome expectations for exercise, take longer than 13.5 seconds to complete the TUG test, have a reduced functional reach, score lower on the Romberg test and perform fewer sit to stands in 30 seconds.
Chapter 5 The primary outcome and safety
The primary outcome (see Chapter 2) was the proportion of participants achieving or exceeding the PA target of 150 minutes of MVPA per week. In this analysis, we dichotomised CHAMPS scores, according to whether or not MVPA exceeded 150 minutes per week. Table 8 and Figure 8 demonstrate that the proportion achieving or exceeding the MVPA target increased in those allocated to the FaME intervention from 40% at baseline to 49% at 12 months post intervention. By contrast, the proportions remained at 38% in the usual-care arm and increased only marginally, from 41% to 43%, in those allocated to the OEP arm. The benefit observed in the FaME arm at 12 months remained at 18 months.
| Percentage reaching 150 minutes MVPA, by group at each follow-up | Randomisation group | ||
|---|---|---|---|
| Usual care | FaME | OEP | |
| Baseline | |||
| n | 400 | 342 | 362 |
| Number ≥ 150 minutes | 150 | 136 | 150 |
| Per cent ≥ 150 minutes | 37.50% | 39.77% | 41.44% |
| Post intervention | |||
| n | 264 | 224 | 224 |
| Number ≥ 150 minutes | 109 | 121 | 96 |
| Per cent ≥ 150 minutes | 41.29% | 54.02% | 42.86% |
| 6 months post intervention | |||
| n | 242 | 195 | 194 |
| Number ≥ 150 minutes | 107 | 79 | 85 |
| Per cent ≥ 150 minutes | 44.21% | 40.51% | 43.81% |
| 12 months post intervention | |||
| n | 222 | 193 | 185 |
| Number ≥ 150 minutes | 84 | 95 | 79 |
| Per cent ≥ 150 minutes | 37.84% | 49.22% | 42.70% |
| 18 months post intervention | |||
| n | 221 | 181 | 179 |
| Number ≥ 150 minutes | 81 | 89 | 78 |
| Per cent ≥ 150 minutes | 36.65% | 49.17% | 43.58% |
FIGURE 8.
Proportion achieving or exceeding MVPA target, by arm over time.
Table 9 shows that the difference in attaining the exercise target 12 months post intervention, after adjustment for dichotomised baseline activity, practice, practice deprivation, list size and site, was statistically significant when comparing FaME patients with those allocated to usual care [odds ratio (OR) 1.78, 95% confidence interval (CI) 1.11 to 2.87; p = 0.018]. There was no significant difference when comparing OEP with usual-care patients (OR 1.17, 95% CI 0.72 to 1.92; p = 0.52).
| Outcome | CHAMPS minutes of moderate or greater intensity activity (per week) (< 150 minutes vs. ≥ 150 minutes) | ||
|---|---|---|---|
| Usual care | FaME | OEP | |
| Number in model | 210 | 184 | 178 |
| Estimate (OR) | N/A | 1.782 | 1.173 |
| 95% CI | N/A | 1.106 to 2.872 | 0.718 to 1.918 |
| p-value | N/A | 0.018 | 0.524 |
If 38% of patients in the usual-care arm would be meeting the target of 150 minutes of MVPA per week at 12 months post intervention, an OR of 1.78 associated with FaME 12 months post intervention would mean that 52% of participants would be meeting the guideline, an absolute increase of 14%.
To increase statistical power, we carried out analysis using the quantitative form of the primary outcome, namely [loge(CHAMPS score + 1)] at 12 months after the end of the intervention period. The positively skewed nature of the distribution necessitated the use of logarithmic transformation. Figure 9 presents box and whisker plots of minutes of MVPA by treatment arm and time, illustrating the distributions and highlighting the occurrence of zero values in all treatment arms at 12 months post intervention; indeed, there were 20% of participants reporting no activity at this time point.
FIGURE 9.
Box and whisker plot of minutes of MVPA 12 months post intervention by group, according to CHAMPS questionnaire.
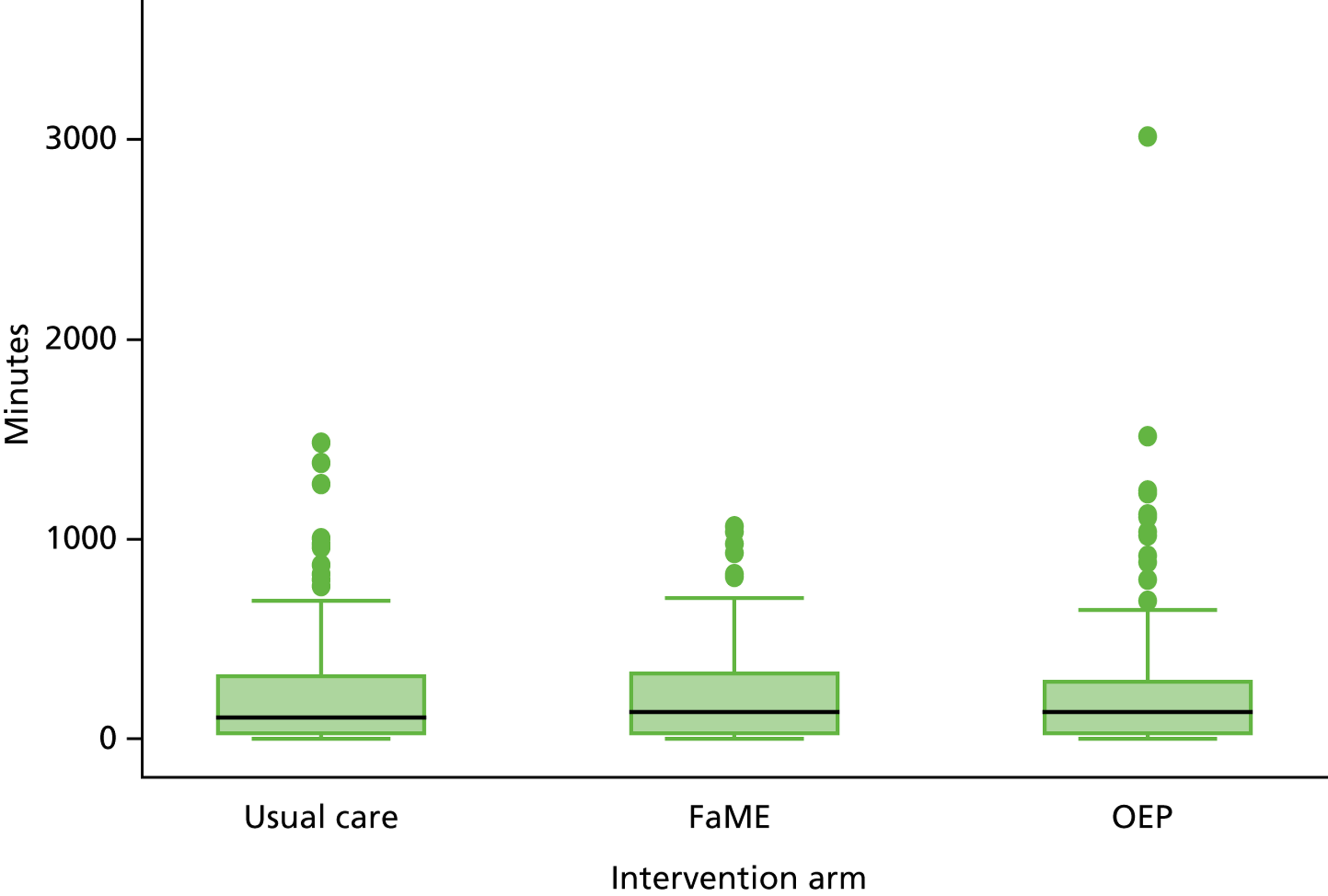
Figure 10 shows the geometric mean number of minutes of MVPA per week, as measured by CHAMPS, by time and group. This was obtained by calculating means of log-transformed CHAMPS scores, then back transforming. Figure 10 shows this graphically, plotted on a logarithmic scale. Physical activity increased in the FaME arm compared with usual care at 12 months after intervention ceased. The increase, although slightly attenuated, still appears 18 months after intervention. Mean MVPA was higher in the OEP arm at 12 months than for usual care, but a difference had already existed at baseline, prior to randomisation.
FIGURE 10.
Geometric means of number of minutes of MVPA by group and time, according to CHAMPS questionnaire.
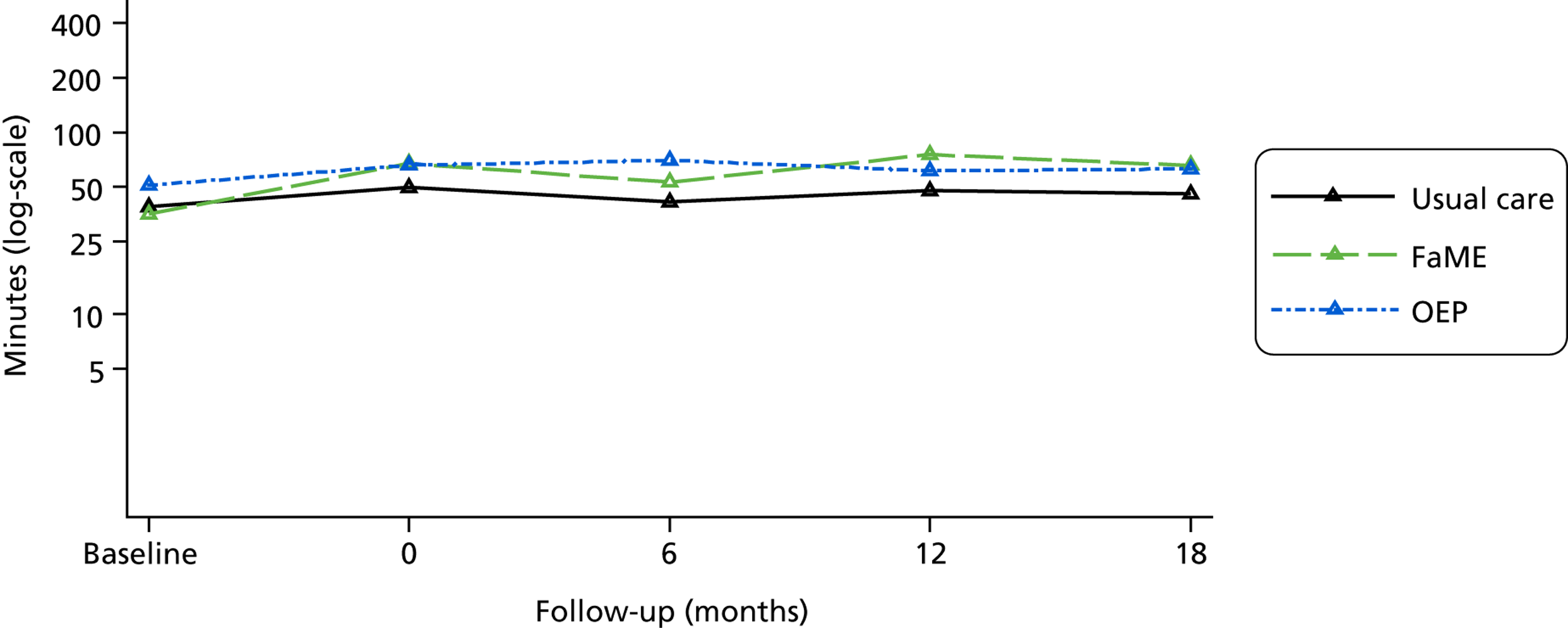
Means of the loge(CHAMPS score + 1)-transformed data were calculated and then back-transformed by taking the exponential of the mean (for each group/time combination) and subtracting 1.
Formal analysis was carried out on the loge(CHAMPS score + 1) values 12 months post intervention (see Chapter 2); baseline level of loge(CHAMPS score + 1), practice size, site and deprivation level were covariates. A multilevel model was fitted, allowing for the clustering by general practice in the study design.
Differences between each intervention arm and the usual-care arm are shown in Table 10, with 95% CIs. The first column represents a complete case analysis (the primary analysis in our plan) and shows a significant increase (p < 0.001) in the mean log-transformed minutes of MVPA in the FaME arm of 0.689 (95% CI 0.312 to 1.065) compared with the control arm. This may be interpreted as a multiplicative effect on minutes of MVPA by a factor of 1.99 (95% CI 1.37 to 2.90). The effect of OEP was positive (0.245, 95% CI –0.150 to 0.639) but non-significant (p = 0.22), representing a multiplicative effect on minutes of MVPA of 1.28 (95% CI 0.86 to 1.89).
| Model | Complete case analysis | Imputed 12-month post-intervention CHAMPS, based on participants with 12-month Phone-FITT | Imputed 12-month post-intervention CHAMPS, based on all participants | |
|---|---|---|---|---|
| Multilevel modelling results (group effects for FaME and OEP vs. usual care)a | ||||
| Number in model | 572 | 707 | 1254 | |
| FaME | Estimate [difference in mean loge(CHAMPS score + 1)] | 0.689 | 0.671 | 0.645 |
| 95% CI | 0.312 to 1.065 | 0.307 to 1.035 | 0.292 to 0.998 | |
| p-value | < 0.001 | < 0.001 | < 0.001 | |
| Multiplicative effect on MVPA | 1.99 | 1.96 | 1.91 | |
| 95% CI | 1.37 to 2.90 | 1.36 to 2.82 | 1.34 to 2.71 | |
| OEP | Estimate | 0.245 | 0.229 | 0.207 |
| 95% CI | –0.150 to 0.639 | –0.161 to 0.618 | –0.152 to 0.566 | |
| p-value | 0.22 | 0.25 | 0.26 | |
| Multiplicative effect on MVPA | 1.28 | 1.26 | 1.23 | |
| 95% CI | 0.86 to 1.89 | 0.85 to 1.86 | 0.86 to 1.76 | |
Other columns of Table 10 show results after applying multiple imputation. The second column shows results using only participants who had responded to the telephone-administered Phone-FITT questionnaire and imputing the CHAMPS measure of MVPA using the Phone-FITT response. The multiplicative effect for FaME on time in MVPA was barely altered at 1.96 (95% CI 1.36 to 2.82; p < 0.001), whereas for OEP it was 1.26 (95% CI 0.85 to 1.86; p = 0.25). The last column shows a full imputation model using all variables included in the substantive model. The benefit of FaME was still highly significant and of comparable magnitude, now having a multiplicative effect on time in MVPA by 1.91 (95% CI 1.34 to 2.71; p < 0.001), whereas for OEP it was 1.23 (95% CI 0.86 to 1.76; p = 0.26).
Our primary analysis estimated a multiplicative effect on MVPA of 1.99. The median time in the usual-care group at 12 months was 105 minutes, so this multiplicative effect would have added a further 104 minutes (almost 15 minutes per day). The more conservative estimate after applying a fully imputed model would suggest adding an extra 95 minutes per week (13–14 minutes per day).
Finally, Table 11 and Figure 11 show the percentage of participants who did no MVPA per week, as measured by CHAMPS, by arm and over time. This was carried out as a post hoc analysis as it was observed that many participants reported zero activity. The percentage of inactive participants changed little in the usual-care arm, declined slightly in the OEP arm, but fell markedly in the FaME arm.
| Measurement point | Randomisation group | ||
|---|---|---|---|
| Usual care | FaME | OEP | |
| Baseline | |||
| n | 400 | 342 | 362 |
| Number = 0 minutes | 109 | 100 | 80 |
| Per cent = 0 minutes | 27.25% | 29.24% | 22.10% |
| Post intervention | |||
| n | 264 | 224 | 224 |
| Number = 0 minutes | 64 | 46 | 40 |
| Per cent = 0 minutes | 24.24% | 20.54% | 17.86% |
| 6 months post intervention | |||
| n | 242 | 195 | 194 |
| Number = 0 minutes | 66 | 44 | 29 |
| Per cent = 0 minutes | 27.27% | 22.56% | 14.95% |
| 12 months post intervention | |||
| n | 222 | 193 | 185 |
| Number = 0 minutes | 54 | 30 | 36 |
| Per cent = 0 minutes | 24.32% | 15.54% | 19.46% |
FIGURE 11.
Proportion of participants recording 0 minutes of MVPA per week, by arm over time.
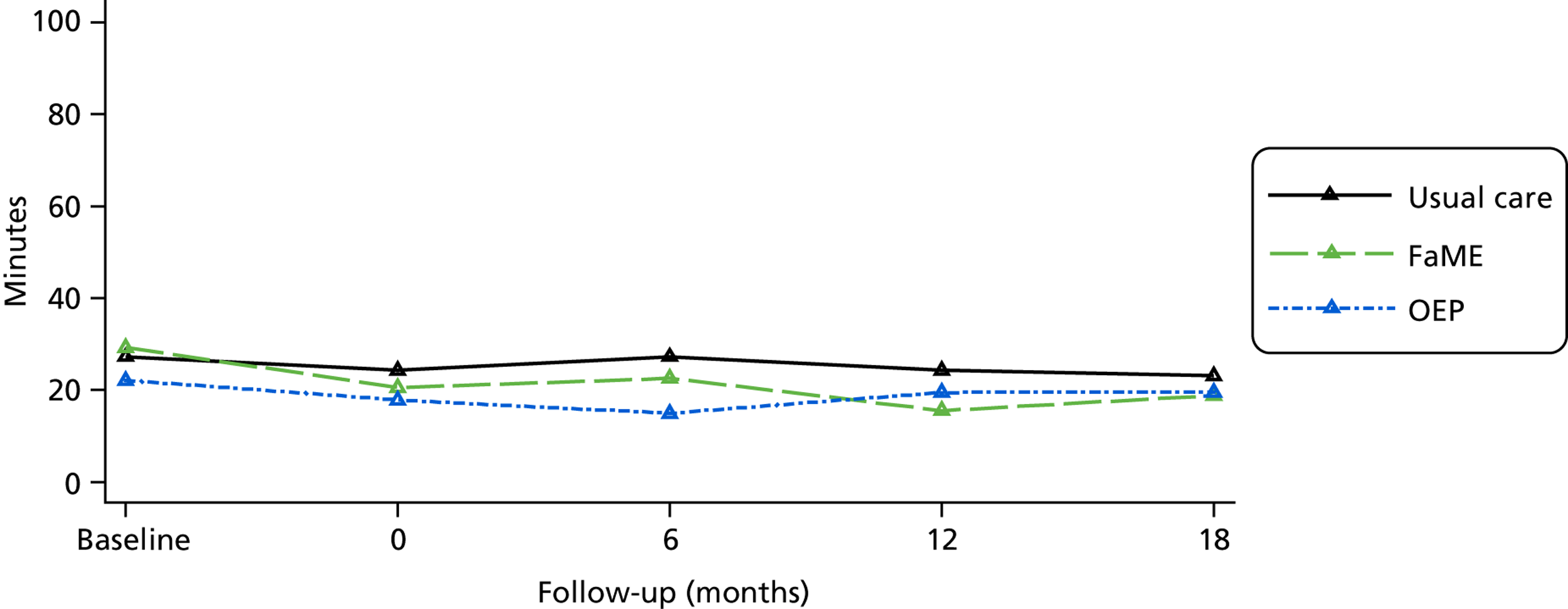
To address the very slight difference in estimates of effectiveness of FaME compared with usual care from the complete case analysis and imputation models, comparisons of baseline levels of CHAMPS and Phone-FITT were made between participants included in the three models (see Appendix 2, Tables 38 and 39). Baseline levels of activity, as recorded by CHAMPS and Phone-FITT, were lower in those who did not provide CHAMPS questionnaire data 12 months post intervention. The differences, however, were comparable between participants allocated to the three arms of the trial. Hence, estimates of intervention effects compared with usual care were almost unaffected.
Possible effect modifications of each intervention according to gender and to age group were tested through fitting interactions; however, there was no evidence for effect modification in either case.
Sensitivity analyses
Analysis of the log(CHAMPS score + 1) outcome was repeated (1) after excluding an outlying value for a participant in the OEP arm (see Figure 10), (2) after excluding an individual whose reported number of falls was extremely high post entry and (3) after excluding 18 participants who were considered ineligible by GPs after randomisation had taken place. In all three analyses, the results concerning estimates of intervention effects were essentially unaltered.
The ICCs for the primary outcome (CHAMPS minutes per week of moderate or greater intensity activity at the 12-month post-intervention follow-up) were for the untransformed outcome 0.000 (95% CI 0.000 to 0.032) and the logarithmic transformation 0.009 (95% CI 0.000 to 0.044).
Adherence analysis
Different definitions were applied to participants in the FaME and OEP arms. In each case, analysis comparing adherent and non-adherent participants was carried out. Comparisons of loge(CHAMPS score + 1) were made, with multilevel modelling as described above.
Falls Management Exercise programme
Expected activity consisted of two 30-minute sessions of home exercise, together with 60 minutes through class attendance, per week for 24 weeks, totalling 2880 minutes. Defining adherence as 75% or more of expected total exercise,31 meant that participants needed to carry out at least 2160 minutes of exercise during the 24-week intervention to be classed as adherent. Two analyses were carried out: firstly assuming that those not returning relevant diaries had not undertaken any exercise during that 4-week period and, secondly, omitting any participant who had not returned all six diaries.
In the first analysis, 387 participants were eligible, of whom 60 (17%) were classed as adherent. In the second analysis, 188 participants were eligible, of whom 58 (31%) were classed as adherent. However, only a subset of eligible participants actually provided CHAMPS data 12 months post intervention, and provision of outcome data was far less common among non-adherers (especially in the first analysis). Among the subsets, there was no evidence of difference in primary outcome between adherers and non-adherers for either analysis (p = 0.67 and p = 0.95, respectively). Appendix 2, Table 40, shows the results.
Otago Exercise Programme
Expected activity consisted of three 30-minute sessions of home exercise per week for 24 weeks, totalling 2160 minutes. Defining adherence as 75% or more of expected total exercise meant that participants needed to carry out at least 1620 minutes of exercise during the 24-week intervention to be classed as adherent.
Three analyses were carried out: the first two were as specified for the analysis of FaME participants above and the third compared participants who were assigned a PM with the remainder. Numbers of participants eligible for the three analyses were 410 (among whom 25% were classed as adherent), 200 (46% classed as adherent) and 366 (39% classed as adherent). As with the FaME analysis, only subsets of these numbers provided CHAMPS data at 12 months, and provision of data was less common among non-adherers, especially in the first analysis. No evidence of difference in outcome was apparent between adherers and non-adherers in any analysis (p = 0.54, p = 0.42 and p = 0.34, respectively, for the three analyses). Appendix 2, Table 41, shows the results.
Safety: adverse events in the trial
There was only one documented SAE that the chief investigator thought could have been because of the trial, and this was reported to the TSC’s chairperson. On further enquiry, this potential SAE was judged by the TSC chairperson to be unrelated to the trial.
Table 12 summarises the categorisation process for harms arising in the participant population that could potentially be attributed to the trial (see Chapter 3 for the categorisation algorithm).
| First screen | Serious | Not serious |
|---|---|---|
| Subcategorisation | Related SAE (report to TSC) | Related AE Possibly, probably or definitely related to the trial |
| Unrelated SAE | Unrelated AE Not related or improbably related to the trial |
|
| AR Possibly, probably or definitely related to the trial |
||
| Possible AR Possibly related to the trial |
||
| Incident Non-injurious falls |
||
Table 13 shows the numbers of events documented during the trial and Table 14 shows AEs, reactions and incidents by arm, during the intervention period and in the 12 months post intervention, per person-month.
| Allocation | Total | Unrelated SAEs (% within category) | AEs (% within category) | ARs (% within category) | Possible ARs (% within category) | Incident (% within category) |
|---|---|---|---|---|---|---|
| FaME | 770 | 8 (28%) | 329 (30%) | 14 (33%) | 52 (31%) | 338 (38%) |
| OEP | 758 | 12 (41%) | 378 (35%) | 26 (62%) | 59 (36%) | 256 (29%) |
| Usual care | 800 | 9 (31%) | 386 (35%) | 2 (5%) | 55 (33%) | 289 (33%) |
| Total | 2328 | 29 | 1093 | 42 | 166 | 883 |
| Intervention arm | No. | Length of observation/months | Person-months | Incidents | AEs | Possible ARs | ARs | Unrelated SAEs | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | Per person-month | No. | Per person-month | No. | Per person-month | No. | Per person-month | No. | Per person-month | ||||
| During intervention | |||||||||||||
| FaME | 322 | 6 | 1932 | 141 | 0.073 | 74 | 0.038 | 11 | 0.006 | 12 | 0.006 | 5 | 0.0026 |
| OEP | 371 | 6 | 2226 | 74 | 0.033 | 91 | 0.041 | 23 | 0.010 | 16 | 0.007 | 5 | 0.0022 |
| Usual care | 396 | 6 | 2376 | 88 | 0.021 | 94 | 0.040 | 16 | 0.007 | 1 | 0.000 | 4 | 0.0017 |
| After intervention | |||||||||||||
| FaME | 255 | 18 | 4590 | 120 | 0.026 | 181 | 0.039 | 35 | 0.008 | 1 | 0.0002 | 8 | 0.0017 |
| OEP | 290 | 18 | 5220 | 162 | 0.031 | 211 | 0.040 | 28 | 0.005 | 2 | 0.0004 | 15 | 0.0029 |
| Usual care | 303 | 18 | 5454 | 163 | 0.030 | 208 | 0.038 | 27 | 0.005 | 1 | 0.0002 | 16 | 0.0029 |
Table 15 shows the clinical conditions categorised as AEs.
| Medical problem | Number in FaME | Number in OEP | Number in usual care |
|---|---|---|---|
| Cancer | 15 | 28 | 18 |
| Cardiovascular | 23 | 51 | 49 |
| Dermatological | 9 | 12 | 16 |
| Endocrine | 9 | 13 | 9 |
| Gastrointestinal | 5 | 16 | 18 |
| Gynaecological | 2 | 1 | 2 |
| Hospitalisation (cause unknown) | 1 | 0 | 1 |
| Musculoskeletal | 28 | 36 | 27 |
| Neurological | 8 | 6 | 4 |
| Ophthalmological | 18 | 11 | 15 |
| Orthopaedic/rheumatological | 59 | 78 | 71 |
| Renal | 3 | 3 | 6 |
| Respiratory | 12 | 14 | 12 |
| Urology | 11 | 4 | 7 |
The number of falls recorded during the trial in diaries and followed up by telephone contact are shown in Table 17 in Chapter 6. The different types of ARs are shown in Appendix 3.
Chapter 6 Secondary outcomes
Other physical activity measures
The CHAMPS questionnaire allowed calculation of weekly calories expended (Table 16). No significant differences were observed whether the measure was analysed as its original value or after logarithmic transformation. The full-time profiles are shown in Appendix 4 (Table 42 and Figure 12). Other questionnaire batteries (PASE and Phone-FITT) measuring reported activity were also analysed. The PASE scores showed a small, but statistically significant, benefit for FaME compared with usual care (difference in means 11.2, 95% CI 0.2 to 20.2; p = 0.046), but no statistically significant benefit for OEP (difference in means 7.5, 95% CI –3.8 to 18.8; p = 0.20). No significant differences were observed according to Phone-FITT (reported through telephone interviews) [Table 17 and Appendix 4 (Table 42 and Figures 13 and 14)].
| Outcome | CHAMPS total calorific expenditure (per week) | Log-CHAMPS total calorific expenditure (per week) | ||||
|---|---|---|---|---|---|---|
| Usual care | FaME | OEP | Usual care | FaME | OEP | |
| Baseline | Baseline | |||||
| Mean (SD) | 2222.3 (2180.9) | 2129.1 (2009.5) | 2314.0 (2009.8) | 6.982 (1.931) | 6.914 (2.013) | 7.227 (1.516) |
| Median (min., max.) | 1713.6 (0.0, 22967.8) | 1690.6 (0.0, 14793.2) | 1782.2 (0.0, 13272.7) | 7.447 (0.000, 10.042) | 7.433 (0.000, 9.602) | 7.486 (0.000, 9.494) |
| n | 391 | 339 | 354 | 391 | 339 | 354 |
| 12 months post intervention | 12 months post intervention | |||||
| Mean (SD) | 2573.6 (2158.8) | 2660.9 (2248.0) | 2787.5 (2771.6) | 7.314 (1.576) | 7.382 (1.568) | 7.530 (1.141) |
| Median (min., max.) | 1829.4 (0.0, 9876.2) | 2079.8 (0.0, 15,272.9) | 2004.1 (0.0, 24,288.5) | 7.512 (0.000, 9.198) | 7.640 (0.000, 9.634) | 7.603 (0.000, 10.098) |
| n | 221 | 192 | 184 | 221 | 192 | 184 |
| Number in model | 569 | 569 | ||||
| Estimate | N/A | 217.7 | 42.1 | N/A | 0.084 | 0.216 |
| 95% CI | N/A | –184.1 to 619.6 | –380.3 to 464.5 | N/A | –0.195 to 0.364 | –0.078 to 0.511 |
| p-value | N/A | 0.288 | 0.845 | N/A | 0.555 | 0.150 |
| Reported falls | FaME | OEP | Usual care |
|---|---|---|---|
| No. of falls during intervention period | 96 | 104 | 116 |
| Person-years at risk during intervention period | 117.9 | 129.8 | 133.9 |
| Falls per person-year during intervention period | 0.81 | 0.80 | 0.87 |
| IRR (95% CI) during intervention period (compared with usual care)a | 0.91 (0.54 to 1.522; p = 0.72) | 0.93 (0.64 to 1.37; p = 0.72) | Ref |
| No. of falls in the 12 months post intervention | 107 | 100 | 158 |
| Person-years at risk in the 12 months post intervention | 187.3 | 184.0 | 221.3 |
| Falls per person-year in the 12 months post intervention | 0.57 | 0.54 | 0.71 |
| IRR (95% CI) in the 12 months post intervention (compared with usual care)a | 0.74 (0.55 to 0.99; p = 0.042) | 0.76 (0.53 to 1.09; p = 0.14) | Ref |
Falls and falls risk
The number of falls was analysed (1) during the intervention period and (2) in the 12 months following the intervention (see Table 18). One very frequent faller (> 100 falls reported during the intervention period) was excluded from analysis, since his rate of falling should have excluded him from the trial, even though his reported fall rate prior to baseline was within inclusion criteria. There was no statistically significant difference in the number of falls among the FaME, OEP and the control arms during the intervention period [adjusted incidence rate ratio (IRR) 0.91 (95% CI 0.54 to 1.52)] for FaME compared with usual care, and IRR 0.93 (95% CI 0.64 to 1.37) for OEP compared with usual care. In the 12 months post intervention there was a statistically significant reduction in falls in the FaME arm compared with the usual-care arm (IRR 0.74, 95% CI 0.55 to 0.99; p = 0.009) and a non-significant reduction in the OEP arm (IRR 0.76, 95% CI 0.53 to 1.09; p = 0.14).
During the intervention period, participants in the FaME arm reported 39 falls with no injury, 31 falls with a bruise or cut, 13 falls with muscle or ligament damage and one fall resulting in a broken bone. In the OEP arm, participants reported 59 falls with no injury, 45 falls with a bruise or cut, 19 falls with muscle or ligament damage, and two falls resulting in a broken bone. In the usual-care arm, participants reported 34 falls with no injury, 59 falls with a bruise or cut, 23 falls with muscle or ligament damag, and six falls resulting in a broken bone.
The FES-I index, which measures participants’ fear of falling, showed no significant differences according to intervention arm (Table 18 and Appendix 4).
| Outcome | FES-I | ||
|---|---|---|---|
| Usual care | FaME | OEP | |
| Baseline | |||
| Mean (SD) | 9.36 (4.08) | 8.99 (3.56) | 8.89 (3.49) |
| Median (min., max.) | 8 (7, 28) | 7 (7, 28) | 7 (7, 28) |
| n | 396 | 333 | 359 |
| 12 months post intervention | |||
| Mean (SD) | 8.94 (3.66) | 9.20 (4.56) | 9.09 (4.19) |
| Median (min., max.) | 7 (7, 28) | 7 (7, 28) | 7 (7, 28) |
| n | 220 | 188 | 185 |
| Number in model | 561 | ||
| Estimate | N/A | 0.102 | 0.045 |
| 95% CI | N/A | –0.653 to 0.856 | –0.740 to 0.831 |
| p-value | N/A | 0.792 | 0.910 |
Quality-of-life measures
No significant differences were apparent at 12 months for either component of the SF-12 (mental or physical), the EQ-5D scores or the OPQoL (Tables 19 and 20). Details of profiles over time are shown in Appendix 4 (Table 44 and Figure 16).
| Outcome | SF-12 PCS | SF-12 MCS | ||||
|---|---|---|---|---|---|---|
| Usual care | FaME | OEP | Usual care | FaME | OEP | |
| Baseline | Baseline | |||||
| Mean (SD) | 38.74 (5.50) | 38.74 (5.64) | 38.78 (5.64) | 49.88 (6.09) | 49.60 (6.02) | 50.15 (5.86) |
| Median (min., max) | 39.23 (15.98, 55.12) | 38.86 (20.02, 55.17) | 38.86 (16.90, 55.20) | 50.59 (9.54, 70.19) | 50.36 (27.10, 67.49) | 50.46 (28.21, 66.46) |
| n | 454 | 386 | 407 | 454 | 387 | 407 |
| 12 months post intervention | 12 months post intervention | |||||
| Mean (SD) | 39.11 (5.00) | 38.85 (4.92) | 39.30 (4.73) | 49.16 (5.60) | 48.74 (5.81) | 49.05 (5.11) |
| Median (min., max.) | 38.86 (20.71, 53.56) | 38.87 (25.52, 55.66) | 39.30 (21.05, 51.90) | 49.86 (31.52, 66.56) | 48.98 (29.59, 63.57) | 49.05 (29.90, 64.18) |
| n | 217 | 186 | 183 | 217 | 186 | 183 |
| Number in model | 583 | 584 | ||||
| Estimate | N/A | –0.211 | 0.278 | N/A | –0.430 | –0.172 |
| 95% CI | N/A | –1.125 to 0.703 | –0.672 to 1.229 | N/A | –1.506 to 0.646 | –1.291 to 0.947 |
| p-value | N/A | 0.651 | 0.566 | N/A | 0.434 | 0.763 |
| Outcome | OPQoL total score | EQ-5D score | ||||
|---|---|---|---|---|---|---|
| Descriptive statistics | ||||||
| Usual care | FaME | OEP | Usual care | FaME | OEP | |
| Baseline | Baseline | |||||
| Mean (SD) | 130.75 (13.53) | 129.36 (13.54) | 129.36 (12.69) | 0.675 (0.082) | 0.672 (0.087) | 0.675 (0.088) |
| Median (min., max.) | 129.00 (93.00, 163.00) | 129.00 (96.00, 163.00) | 128.00 (97.00, 162.00) | 0.688 (0.260, 0.855) | 0.688 (0.388, 0.905) | 0.688 (0.253, 0.922) |
| n | 342 | 273 | 312 | 450 | 380 | 399 |
| 12 months post intervention | Post intervention | |||||
| Mean (SD) | 134.80 (14.82) | 132.31 (15.98) | 133.72 (14.95) | 0.675 (0.072) | 0.667 (0.072) | 0.675 (0.074) |
| Median (min., max.) | 135.00 (91.00, 165.00) | 132.00 (93.00, 163.00) | 134.00 (95.00, 164.00) | 0.683 (0.358, 0.885) | 0.675 (0.381, 0.846) | 0.688 (0.285, 0.841) |
| n | 185 | 169 | 156 | 212 | 179 | 176 |
| Multilevel modelling results (group effects vs. usual care) | ||||||
| Number in model | 444 | 558 | ||||
| Estimate | N/A | –0.794 | 0.374 | N/A | –0.009 | 0.000 |
| 95% CI | N/A | –2.848 to 1.260 | –1.772 to 2.520 | N/A | –0.022 to 0.005 | –0.014 to 0.015 |
| p-value | N/A | 0.449 | 0.733 | N/A | 0.229 | 0.958 |
Balance confidence and social networks
Table 21 shows differences between arms on balance confidence and social network support. Significant improvements in balance confidence were seen in both intervention arms at 12 months post intervention. The mean difference for FaME compared with usual care was –0.529 (95% CI –0.998 to –0.061; p = 0.027) while the mean difference for OEP compared with usual care was –0.545 (95% CI –1.033 to –0.057; p = 0.029). No significant difference in either social network scale (MSPSS or LSNS) was observed when comparing FaME and OEP to the usual-care arm. Further information concerning changes over time is shown in Appendix 4 (Table 45 and Figure 17).
| Outcome | ConfBal | MSPSS | LSNS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Descriptive statistics | |||||||||
| Usual care | FaME | OEP | Usual care | FaME | OEP | Usual care | FaME | OEP | |
| Baseline | Baseline | Baseline | |||||||
| Mean (SD) | 12.55 (3.93) | 12.63 (3.98) | 12.48 (3.76) | 65.81(17.96) | 65.93 (15.57) | 66.60 (15.49) | 15.93 (5.70) | 16.47 (5.76) | 15.44 (5.48) |
| Median (min., max.) | 11 (10, 29) | 10 (10, 30) | 11 (10, 30) | 71 (12, 84) | 69 (12, 84) | 70.5 (12.0, 84.0) | 16 (0, 30) | 17 (3, 30) | 15 (1, 30) |
| n | 389 | 330 | 353 | 375 | 305 | 330 | 392 | 330 | 351 |
| 12 months post intervention | 12 months post intervention | 12 months post intervention | |||||||
| Mean (SD) | 12.38 (4.05) | 12.13 (3.65) | 12.23 (3.71) | 67.23 (16.54) | 63.27 (17.69) | 63.46 (18.14) | 16.41 (5.79) | 15.68 (5.82) | 15.43 (5.35) |
| Median (min., max.) | 10 (10, 30) | 10 (10, 28) | 10 (10, 28) | 71 (12, 84) | 67 (12, 84) | 68 (12, 84) | 17 (4, 30) | 16 (0, 30) | 16 (1, 30) |
| n | 218 | 183 | 179 | 209 | 183 | 171 | 210 | 181 | 180 |
| Multilevel modelling results (group effects vs. usual care) | |||||||||
| Number in model | 546 | 500 | 533 | ||||||
| Estimate | N/A | –0.529 | –0.545 | N/A | −2.480 | −2.373 | N/A | −0.651 | 0.176 |
| 95% CI | N/A | −0.998 to −0.061 | −1.033 to −0.057 | N/A | −5.637 to 0.677 | −5.700 to 0.953 | N/A | −1.411 to 0.110 | −0.624 to 0.976 |
| p-value | N/A | 0.027 | 0.029 | N/A | 0.124 | 0.162 | N/A | 0.093 | 0.666 |
Other secondary outcomes (measures taken only at baseline and immediately post intervention)
Table 22 shows no evidence for effect of either intervention on the FRAT. However, clear benefits were seen on the OEE scale (Table 22). Those in the FaME arm whose expectations of exercise were positive at baseline had significantly increased expectations of exercise at follow-up compared with those in the usual-care arm. Those who were negative about exercise at baseline improved their expectations at follow-up, in both FaME and OEP arms, compared with usual care. Changes from baseline to post intervention are shown in Appendix 4 (Table 46). Results are shown for three functional measures in Table 23. No significant effects of intervention were observed. Table 24 shows outcomes for PASE, Phone-FITT and the mental and physical components of the SF-12 scale. Table 25 shows results for quality of life (OPQoL) and falls risk assessment (FRAT score). Table 26 shows results for the FRAT binary score (0 or ≥ 1). Table 27 shows the results from multilevel modelling of post intervention and 6- and 12-month post-intervention scores on EQ-5D.
| Outcome | FRAT score | OEE positive | OEE negative | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Usual care | FaME | OEP | Usual care | FaME | OEP | Usual care | FaME | OEP | |
| Baseline | Baseline | Baseline | |||||||
| Mean (SD) | 1.029 (0.955) | 0.890 (0.900) | 0.980 (0.899) | 3.84 (0.58) | 3.85 (0.62) | 3.85 (0.60) | 3.85 (0.81) | 3.96 (0.75) | 3.90 (0.85) |
| Median (min., max.) | 1 (0, 4) | 1 (0, 4) | 1 (0, 4) | 3.89 (2.00, 5.00) | 3.89 (1.67, 5.00) | 3.78 (1.67, 5.00) | 4 (1.25, 5.00) | 4 (1.75, 5.00) | 4 (1, 5) |
| n | 453 | 383 | 402 | 372 | 309 | 349 | 367 | 320 | 339 |
| 12 months post intervention | Post intervention | Post intervention | |||||||
| Mean (SD) | 0.987 (0.905) | 0.929 (0.944) | 0.996 (0.951) | 3.85 (0.64) | 4.02 (0.55) | 3.93 (0.65) | 3.96 (0.87) | 4.19 (0.75) | 4.20 (0.71) |
| Median (min., max.) | 1 (0, 4) | 1 (0, 4) | 1 (0, 4) | 3.78 (2.11, 5.00) | 4.00 (2.33, 5.00) | 3.89 (1.00, 5.00) | 4 (1, 5) | 4.25 (1.00, 5.00) | 4 (1, 5) |
| n | 299 | 253 | 263 | 252 | 206 | 211 | 248 | 204 | 203 |
| Number in model | 808 | 614 | 595 | ||||||
| Estimate | N/A | −0.004 | 0.030 | N/A | 0.130 | 0.083 | N/A | 0.200 | 0.252 |
| 95% CI | N/A | −0.160 to 0.152 | −0.127 to 0.189 | N/A | 0.043 to 0.216 | −0.006 to 0.171 | N/A | 0.077 to 0.323 | 0.125 to 0.379 |
| p-value | N/A | 0.960 | 0.708 | N/A | 0.003 | 0.066 | N/A | 0.001 | <0.001 |
| Outcome | Sits to stands (total) | Functional reach | Log-TUG (seconds) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Descriptive statistics | |||||||||
| Usual care | FaME | OEP | Usual care | FaME | OEP | Usual care | FaME | OEP | |
| Baseline | Baseline | Baseline | |||||||
| Mean (SD) | 10.49 (3.31) | 10.48 (3.64) | 10.27 (2.81) | 24.68 (7.43) | 25.60 (6.98) | 25.57 (7.43) | 2.35 (0.32) | 2.33 (0.34) | 2.33 (0.34) |
| Median (min., max.) | 10 (0, 26) | 10 (1, 28) | 10 (3, 20) | 25 (4, 49) | 26 (8, 45) | 26 (4, 55) | 2.29 (1.74, 3.70) | 2.26 (1.41, 4.02) | 2.29 (1.71, 4.58) |
| n | 449 | 377 | 400 | 438 | 371 | 402 | 438 | 337 | 376 |
| Post intervention | Post intervention | Post intervention | |||||||
| Mean (SD) | 11.86 (3.57) | 11.62 (3.77) | 11.40 (3.35) | 27.13 (6.82) | 26.99 (7.28) | 26.84 (7.64) | 2.28 (0.27) | 2.25 (0.30) | 2.27 (0.27) |
| Median (min., max.) | 12 (2, 25) | 11 (3, 29) | 11 (0, 22) | 28 (10, 45) | 27 (7, 46) | 27 (7, 44) | 2.23 (1.79, 4.05) | 2.20 (1.48, 3.60) | 2.23 (1.69, 3.81) |
| n | 285 | 252 | 245 | 293 | 249 | 232 | 273 | 203 | 203 |
| Multilevel modelling results (group effects vs. usual care) | |||||||||
| Number in model | 772 | 749 | 651 | ||||||
| Estimate | N/A | −0.644 | −1.055 | N/A | −0.644 | −1.055 | N/A | −0.008 | −0.011 |
| 95% CI | N/A | −2.583 to 1.295 | −3.031 to 0.921 | N/A | −2.583 to 1.295 | −3.031 to 0.921 | N/A | −0.064 to 0.048 | −0.066 to 0.044 |
| p-value | N/A | 0.515 | 0.295 | N/A | 0.515 | 0.295 | N/A | 0.775 | 0.700 |
| Outcome | PASE total score | Phone-FITT total score | SF-12 PCS | SF-12 MCS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Descriptive statistics | ||||||||||||
| Usual care | FaME | OEP | Usual care | FaME | OEP | Usual care | FaME | OEP | Usual care | FaME | OEP | |
| Baseline | Baseline | Baseline | Baseline | |||||||||
| Mean (SD) | 119.2 (60.4) | 109.1 (52.2) | 119.9 (50.6) | 36.80 (13.65) | 37.68 (13.67) | 41.18 (13.11) | 38.74 (5.50) | 38.74 (5.64) | 38.78 (5.64) | 49.88 (6.09) | 49.60 (6.02) | 50.15 (5.86) |
| Median (min., max.) | 111.1 (0.0, 379.5) | 107.1 (0.0, 356.6) | 116.8 (8.57, 280.60) | 36.0 (0.00, 85.83) | 37.46 (0.0, 102.0) | 40.67 (7.00, 80.83) | 39.23 (15.98, 55.12) | 38.86 (20.02, 55.17) | 38.86 (16.90, 55.20) | 50.59 (9.54, 70.19) | 50.36 (27.10, 67.49) | 50.46 (28.21, 66.46) |
| n | 400 | 342 | 362 | 377 | 316 | 354 | 454 | 386 | 407 | 454 | 387 | 407 |
| 12 months post intervention | 12 months post intervention | 12 months post intervention | 12 months post intervention | |||||||||
| Mean (SD) | 122.5 (51.8) | 124.2 (53.3) | 126.8 (61.3) | 47.71 (17.41) | 49.52 (15.95) | 49.38 (16.50) | 39.11 (5.00) | 38.85 (4.92) | 39.30 (4.73) | 49.16 (5.60) | 48.74 (5.81) | 49.05 (5.11) |
| Median (min., max.) | 118.1 (0.0, 277.7) | 116.0 (0.0, 269.7) | 114.6 (0.0, 356.6) | 47.75 (0.0, 162.33) | 47.63 (8.0, 112.5) | 50.33 (0.00, 97.75) | 38.86 (20.71, 53.56) | 38.87 (25.52, 55.66) | 39.30 (21.05, 51.9) | 49.86 (31.52, 66.56) | 48.98 (29.59, 63.57) | 49.05 (29.90, 64.18) |
| n | 222 | 193 | 185 | 225 | 208 | 237 | 217 | 186 | 183 | 217 | 186 | 183 |
| Multilevel modelling results (group effects vs. usual care) | ||||||||||||
| Number in model | 572 | 628 | 583 | 584 | ||||||||
| Estimate | N/A | 11.19 | 7.48 | N/A | 2.303 | 1.340 | N/A | −0.211 | 0.278 | N/A | −0.430 | −0.172 |
| 95% CI | N/A | 0.194 to 22.191 | −3.826 to 18.794 | N/A | −0.531 to 5.137 | −1.494 to 4.174 | N/A | −1.125 to 0.703 | −0.672 to 1.229 | N/A | −1.506 to 0.646 | −1.291 to 0.947 |
| p-value | N/A | 0.046 | 0.195 | N/A | 0.111 | 0.354 | N/A | 0.651 | 0.566 | N/A | 0.434 | 0.763 |
| Outcome | OPQoL total score | FRAT score | ||||
|---|---|---|---|---|---|---|
| Descriptive statistics | ||||||
| Usual care | FaME | OEP | Usual care | FaME | OEP | |
| Baseline | Baseline | |||||
| Mean (SD) | 130.75 (13.53) | 129.36 (13.54) | 129.36 (12.69) | 1.029 (0.955) | 0.890 (0.900) | 0.980 (0.899) |
| Median (min., max.) | 129.00 (93.00,163.00) | 129.00 (96.00, 163.00) | 128.00 (97.00, 162.00) | 1 (0, 4) | 1 (0, 4) | 1 (0, 4) |
| n | 342 | 273 | 312 | 453 | 383 | 402 |
| 12 months post intervention | Post intervention | |||||
| Mean (SD) | 134.80 (14.82) | 132.31 (15.98) | 133.72 (14.95) | 0.987 (0.905) | 0.929 (0.944) | 0.996 (0.951) |
| Median (min., max.) | 135.00 (91.00, 165.00) | 132.00 (93.00, 163.00) | 134.00 (95.00, 164.00) | 1 (0, 4) | 1 (0, 4) | 1 (0, 4) |
| n | 185 | 169 | 156 | 299 | 253 | 263 |
| Multilevel modelling results (group effects vs. usual care) | ||||||
| Number in model | 444 | 808 | ||||
| Estimate | N/A | −0.794 | 0.374 | N/A | −0.004 | 0.030 |
| 95% CI | N/A | −2.848 to 1.260 | −1.772 to 2.520 | N/A | −0.160 to 0.152 | −0.127 to 0.189 |
| p-value | N/A | 0.449 | 0.733 | N/A | 0.960 | 0.708 |
| Outcome | FRAT binary (score ≥ 1) | ||
|---|---|---|---|
| Usual care | FaME | OEP | |
| Baseline | |||
| Percentage with score ≥ 1 | 65.8% | 60.6% | 65.6% |
| n | 453 | 383 | 402 |
| Post intervention | |||
| Percentage with score ≥ 1 | 65.5% | 62.1% | 64.6% |
| n | 299 | 253 | 263 |
| Number in model | 808 | ||
| Estimate (OR) | N/A | 0.857 | 1.000 |
| 95% CI | N/A | 0.504 to 1.460 | 0.584 to 1.716 |
| p-value | N/A | 0.571 | 0.998 |
| Descriptive Statistics | Usual care | FaME | OEP | Usual care | FaME | OEP | Usual care | FaME | OEP | Usual care | FaME | OEP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post intervention | 6 months post intervention | 12 months post intervention | |||||||||
| Mean (SD) | 0.675 (0.082) | 0.672 (0.087) | 0.675 (0.088) | 0.700 (0.074) | 0.691 (0.083) | 0.705 (0.071) | 0.661 (0.080) | 0.668 (0.085) | 0.674 (0.070) | 0.675 (0.072) | 0.667 (0.072) | 0.675 (0.074) |
| Median (min., max.) | 0.688 (0.260, 0.855) | 0.688 (0.388, 0.905) | 0.688 (0.253, 0.922) | 0.704 (0.400, 0.870) | 0.702 (0.261, 0.870) | 0.706 (0.311, 0.942) | 0.671 (0.271, 0.809) | 0.685 (0.351, 0.845) | 0.685 (0.411, 0.804) | 0.683 (0.358, 0.885) | 0.675 (0.381, 0.846) | 0.688 (0.285, 0.841) |
| n | 450 | 380 | 399 | 296 | 255 | 258 | 225 | 178 | 184 | 212 | 179 | 176 |
| Multilevel modelling results (group effects vs. usual care) | ||||||||||||
| Number in model | N/A | 793 | 576 | 558 | ||||||||
| Estimate | N/A | N/A | N/A | N/A | −0.007 | 0.008 | N/A | 0.008 | 0.018 | N/A | −0.009 | 0.000 |
| 95% CI | N/A | N/A | N/A | N/A | −0.019 to 0.006 | −0.005 to 0.020 | N/A | −0.007 to 0.023 | 0.003 to 0.033 | N/A | −0.022 to 0.005 | −0.014 to 0.015 |
| p-value | N/A | N/A | N/A | N/A | 0.301 | 0.238 | N/A | 0.301 | 0.021 | N/A | 0.229 | 0.958 |
Chapter 7 Economic analysis
Intervention costs – NHS perspective
Otago Exercise Programme
The OEP intervention involved a 30-minute programme of leg muscle strengthening and balance retraining performed at home at least three times per week and a walking plan undertaken at least twice per week, for 24 weeks. Participants attended an induction event, in groups, at a local community centre, which included individual assessment and setting of an exercise regimen. Ankle weights were distributed and an instruction booklet provided.
A PM was assigned to make contact with each participant. PMs received basic training in mentoring skills and were asked to make a home visit to help their mentee start their exercise programme, and up to three more home visits during the course of the 6-month intervention. In addition, the intervention protocol recommended that PMs maintain contact with and provide encouragement and support to their mentee through telephone calls every 2 weeks. PMs kept logs of their contacts with each of their mentees (date, time, duration and method of contact).
Otago Exercise Programme resources
Problems were encountered in recruiting PMs (see Chapter 2), especially in Nottingham, and some participants could not be allocated a PM. Thirty-six PMs were used in London, supporting 122 of 230 participants (53.0%) in OEP practices (mean 3.3, median 2.0 participants per PM). In Nottingham, only seven PMs were hired, supporting 21 of 168 (12.5%) OEP participants (mean 3.0, median 3.0 participants per PM).
Otago Exercise Programme costs
Cost items associated with use of PM were calculated as a cost per participant with a PM. Other costs, such as hall hire for the induction sessions that were attended by all OEP participants, were calculated with total OEP participants as the denominator (see Table 28 for OEP costs). The costs per OEP participant with a PM were £88.16 in London and £117.08 in Nottingham. The per-participant costs of ankle weights were higher in London as greater access to PMs meant that more participants graduated to receive additional (highest-weight) cuffs. PM training costs were higher in Nottingham because of the smaller number of PMs attending sessions.
| OEP intervention costs | ||||
|---|---|---|---|---|
| Category | London (N = 230); 122 (53.0%) with PM | Nottingham (N = 168); 21 (12.5%) with PM | ||
| Total | Per participant with PM (n = 122) | Total | Per participant with PM (n=21) | |
| PM traininga | £2771.70 (36 PMs, £76.99 per PM) | £22.72 | £973.60 (7 PMs, £139.09 per PM) | £46.36 |
| PM expenses reimbursementb | £608.40 | £4.99 | £243.76 | £11.61 |
| Imputed value of PM time, for visits and telephone calls to participantsc | 84 visits, 4178 minutes (mean 49.7 minutes, SD 25.6 minutes) = £2017.97 (£24.02/visit) 75 telephone calls, 652 minutes (mean 8.7 minutes, SD 4.7 minutes) = £260.80 (£3.48/telephone call) |
0.69 visits per participant £16.54 0.62 telephone calls per participant £2.14 Both: £18.68 |
21 visits, 893 minutes (mean 42.5 minutes, SD 15.0 minutes) = £431.32 (£20.54/visit) 17 telephone calls, 138 minutes (mean 8.1 minutes, SD 2.6 minutes) = £55.20 (£3.25/telephone call) |
1 visit per participant, £20.54 0.81 telephone calls per participant, £2.63 Both: £23.17 |
| Per all participants (n = 230) | Per all participants (n = 168) | |||
| Induction – hall hired | £1484.00 (31 groups, £47.87 per group; range £28–90) | £6.45 (mean group size 7.6) | £861.44 (18 groups, £47.86 per group; range £18–90) | £5.13 (mean group size 9.3) |
| Induction – trainer timee | £3491.00 | £15.18 | £2550.00 | £15.18 |
| Induction – refreshmentsf | £92.00 | £0.40 | £67.20 | £0.40 |
| Ankle cuffsg | £3918.29 | £17.04 | £2104.56 | £12.53 |
| Instruction bookleth | £621.00 | £2.70 | £453.60 | £2.70 |
| Total | £15,265.16 | £7740.68 | ||
| Per participant, no PM | £41.77 | £35.94 | ||
| Per participant, with PM | £88.16 | £117.08 | ||
Falls Management Exercise programme resources
The FaME intervention comprised a 1-hour group exercise class with PSIs and two 30-minute home exercise sessions per week, for 24 weeks, plus walking twice per week for 30 minutes. Nine or ten participants were allocated to each group, with multiple classes per week for each GP practice. Seventeen groups were run in London by seven PSIs for 162 participants (mean 9.5 per group). There were five PSIs in Nottingham running 20 groups for 194 participants (9.7 per group). PSIs monitored attendance on a weekly basis. The aim was to provide continuity of PSI for each group, although occasionally PSIs would cover for each other for sickness or holidays.
The delivery of the intervention was standardised through training of PSIs and quality assurance visits. Participants are given elastic resistance bands and an instruction booklet for the home exercise component. Exercise mats were purchased for use in the group sessions.
Falls Management Exercise programme costs
The per-participant costs averaged £268.74 in London and £218.43 in Nottingham; PSI reimbursement was the largest cost component, followed by hire of the venues. The higher cost of FaME venues in London reflects differences in rents between the capital and Nottingham, although there was considerable variation within both sites. Participants were recruited by general practice (cluster randomised) and the main criterion for selecting halls was proximity. Table 29 shows FaME costs.
| FaME intervention costs | ||||
|---|---|---|---|---|
| Category | London (n = 162) | Nottingham (n = 194) | ||
| Total | Per participant (cost of item as % of total) | Total | Per participant (cost of item as % of total) | |
| PSI reimbursementa | £27,744.00 (17 groups) | £171.26 (63.7%) | £32,640.00 (20 groups) | £168.25 (77.0%) |
| PSI trainingb | £1550.00 7 PSIs (£214.28 per PSI) | £9.63 (3.6%) | £1405.00 5 PSIs (£281.00 per PSI) | £7.24 (3.3%) |
| Hall hirec | £12,982.00 (£763.65 per group; range £540–938) | £80.63 (30.0%) | £6929.50 (£346.28 per group; range £221–417) | £35.72 (16.3%) |
| Refreshmentsd | £196.42 | £1.22 (0.5%) | £237.08 | £1.22 (0.6%) |
| Matse | £563.50 | £3.50 (1.3%) | £679.00 | £3.50 (1.6%) |
| TheraBandsf | £322.00 | £2.00 (0.7%) | £388.00 | £2.00 (0.9%) |
| Instruction bookletg | £80.50 | £0.50 (0.2%) | £97.00 | £0.50 (0.2%) |
| Total | £43,438.42 | £268.74 | £42,375.58 | £218.43 |
Comparing OEP and FaME
The FaME programme is more expensive than the OEP delivered with PMs (£269 vs. £88 per participant in London; £218 vs. £117 in Nottingham) as a result of more direct participant contact from PSIs and hire of halls for the exercise classes. The difference between groups in per-participant costs would have been smaller if the OEP had been delivered to protocol (the full quota of home visits and telephone calls) and this might have had an impact on effectiveness. The costs of equipment were higher in the OEP (ankle cuffs) than in the FaME programme (resistance bands and mats); the home exercise booklet was also more extensive and costly than that provided to FaME programme participants, but these items represented small proportions of the total costs.
Discussion
The reasons for the low PM contact with mentees in the OEP arm are not fully known. The lower PM input, or lack of PM input for a significant proportion of participants, may have impacted effectiveness to an unknown extent.
Although PMs were volunteers, a cost was applied to PM time, inputted using a replacement cost method (based on a clinical support worker). Use of the opportunity cost method would have required further information from PMs about the activities that they were not doing in order to carry out PM responsibilities. If PMs were retired, the opportunity cost of their time may have been less than the replacement cost used and the per-participant cost in the OEP arm would have been lower. Moreover, if volunteer PMs had obtained positive utility from their contribution, their cost should have been reduced accordingly. 75 Problems with recruiting PMs might have been mitigated if they had been remunerated and this may have also resulted in more active support of mentees.
Per-participant costs in delivery of the FaME programme would be inversely affected by group size, but group size might also affect effectiveness to an unknown extent. In the trial, the mean group size was similar in both sites at allocation (9 or 10 participants, as planned per protocol). However, poor attendance at some groups [mean group attendance rates were 50.5% (range 35–68%) in London and 56.9% (range 28–68%) in Nottingham] indicates that actual costs per attendee were higher and that a larger group size at the outset may be possible so that there is still a core group of active participants after drop-out. Attendance rates of participants assigned to the FaME programme ranged from 0% to 100% in both sites.
Intervention costs – private/participant perspective
Out-of-pocket expenditures
Data on exercise-related out-of-pocket expenditure (clothes, equipment, gym membership, etc.) were analysed for the 592 participants (of 603 recruited) in London and the 594 (of 651) in Nottingham. The remaining 11 and 57 volunteers, respectively, were excluded by their GPs on health grounds (considered too unfit to take part in the interventions). Information on private expenditures was captured through diary returns (six during the intervention period and four in the subsequent 12-month follow-up period). Over 60% of diaries were returned during the intervention, although response rates varied between groups and were lowest in London. Diary returns dropped in London in the follow-up period to below 50% and in the OEP group in Nottingham.
Relatively small numbers of participants reported out-of-pocket expenditures and the average per-participant spend both during the 6-month intervention and in the 12-month follow-up period was < £10, but variable across groups and sites in a non-systematic way (Table 30 shows out-of-pocket expenditures).
| Site | Group | Number recruited | Number after GP exclusion | Number of diaries returned/total possible | % of possible diaries returned | Number of participants reporting an expenditure | Number of items purchased | Total expenditure | Spend per participant after GP exclusions | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | S | C | EE & G | O | |||||||||
| During the 6-month intervention period: diaries 1–6 | |||||||||||||
| London | OEP | 168 | 230 | 841/1340 | 60.9 | 32 | 49 | 20 | 20 | 1 | 8 | £1398.40 | £6.08 |
| FaME | 232 | 162 | 601/972 | 61.8 | 19 | 28 | 8 | 7 | 2 | 11 | £799.47 | £4.93 | |
| Usual care | 203 | 200 | 649/1200 | 54.1 | 26 | 42 | 10 | 5 | 19 | 7 | £1704.96 | £8.52 | |
| Total | 603 | 592 | 2091/3512 | 59.5 | 77 | 119 | 38 | 32 | 22 | 26 | £3902.83 | £6.59 | |
| Nottingham | OEP | 179 | 168 | 728/1008 | 72.2 | 48 | 64 | 17 | 29 | 1 | 17 | £1352.78 | £8.05 |
| FaME | 219 | 194 | 838/1164 | 72.0 | 18 | 23 | 8 | 8 | 0 | 7 | £496.58 | £2.56 | |
| Usual care | 253 | 232 | 958/1392 | 68.8 | 34 | 56 | 9 | 18 | 8 | 21 | £1704.65 | £7.34 | |
| Total | 651 | 594 | 2524/3554 | 71.0 | 100 | 152 | 42 | 66 | 2 | 42 | £3553.01 | £5.98 | |
| During the 12-month post intervention follow-up period: diaries 7–10 | |||||||||||||
| London | OEP | 168 | 230 | 399/920 | 43.4 | 21 | 21 | 10 | 10 | 1 | 0 | £632.72 | £2.75 |
| FaME | 232 | 162 | 299/648 | 46.1 | 26 | 26 | 5 | 9 | 6 | 6 | £1512.98 | £9.34 | |
| Usual care | 203 | 200 | 330/800 | 41.2 | 32 | 31 | 6 | 14 | 7 | 4 | £1121.35 | £5.61 | |
| Total | 603 | 592 | 1028/2364 | 43.5 | 79 | 78 | 21 | 33 | 14 | 10 | £3267.05 | £5.52 | |
| Nottingham | OEP | 179 | 168 | 345/672 | 51.3 | 16 | 16 | 2 | 6 | 1 | 7 | £769.49 | £5.48 |
| FaME | 219 | 194 | 565/776 | 72.8 | 31 | 31 | 9 | 14 | 2 | 6 | £719.30 | £3.70 | |
| Usual care | 253 | 232 | 672/928 | 72.4 | 38 | 38 | 12 | 12 | 7 | 7 | £2453.63 | £10.58 | |
| Total | 651 | 594 | 1582/2376 | 66.6 | 85 | 85 | 23 | 32 | 10 | 20 | £3942.42 | £6.64 | |
Additional expenditure was incurred by participants in the FaME group for travel to exercise classes. Participants were asked to report their usual method of getting to the class at the 6-month (end-of-intervention) assessment point. Responses were received by just over half of participants and showed that the average round-trip distance travelled for classes was 1.5 miles in London and 3.1 miles in Nottingham, reflecting the relative population densities of the areas. Accordingly, higher proportions of participants reported walking to classes, and lower proportions using cars, in London than in Nottingham (FaME travel costs are shown in Table 31).
| Site | Number of participants | Number (%) providing data | Average round-trip distance to class (miles) | Usual method of travel to class Number (%) of participants using |
|||
|---|---|---|---|---|---|---|---|
| Train, tram, bus, taxi | Car | Walka | Other, cycle | ||||
| London | 168 | 85 (50.6) | 1.5 | 17 (20.0) | 24 (28.2) | 42 (49.4) | 4 (4.7) |
| Nottingham | 219 | 126 (57.5) | 3.1 | 11 (8.7) | 93 (73.8) | 32 (25.4) | 0 |
Almost all participants who used public transport reported that they had a free bus pass, so participants incurring out-of-pocket costs associated with travel to classes were mostly those using private cars (28% in London and 74% in Nottingham). The maximum average cost incurred in driving to classes in London (assuming 45 p per mile, the NHS reimbursement rate for staff) was £16.20 for the 24 classes and in Nottingham was £33.48 because of the longer round-trip distance to the venue. Only one individual reported parking charges (of £4.00 per class).
Productivity effects
Participants in the FaME programme were also asked to report at the post-intervention assessment what activity they had given up to attend the exercise classes. This question was answered by < 30% of people assigned to the FaME group. The largest proportion of responders stated that they had given up recreational activities, followed by home making. Only two people stated they had given up paid employment, both in Nottingham. A total of 14 (13%) reported giving up voluntary or caring responsibilities (FaME programme opportunity costs are shown in Table 32).
| Site | Number of participants | Number (%) providing data | What gave up doing to attend class (opportunity cost), number (%) of participants | |||||
|---|---|---|---|---|---|---|---|---|
| Employment | Voluntary work | Home making | Caring | Recreation | Other | |||
| London | 168 | 44 (26.2) | 0 | 6 (13.6) | 14 (31.8) | 2 (4.5) | 20 (45.5) | 2 (4.5) |
| Nottingham | 219 | 63 (28.8) | 2 (3.2) | 2 (3.2) | 23 (36.5) | 4 (6.3) | 26 (41.3) | 6 (9.5) |
Service use
Data on primary care service use covering the 6 months of the intervention and 12 months post-intervention follow-up period were collected from all 21 GP practices (594 participants – 194 FaME, 168 OEP, 232 usual care) in Nottingham, and 19 out of 22 practices (500 participants – 161 FaME, 186 OEP, 153 usual care) in London (access was denied by the other three). Group comparisons of primary care contacts and associated costs were conducted to explore possible impacts of exercise on general health and were offset against the costs of the interventions. Both parametric and non-parametric methods were used to compare group utilisation and costs. Inspection of the histograms showed that total contact and total cost distributions approached normality with few outliers, so the results of the parametric approach are reported. The findings from the non-parametric approach revealed no differences in the findings.
Taking London and Nottingham together (Table 33), there was a tendency for the mean number of primary care contacts to be higher in the OEP group, compared with usual care (p = 0.100), largely as a result of utilisation in Nottingham, but no differences between the other groups. In London, the mean of total contacts was significantly higher in the FaME group than in the usual-care group (p = 0.037) (Table 34). Table 35 shows primary care service use per participant, during the 6-month intervention and the 12-month follow-up in Nottingham.
| Service | Variable | Mean | t-test for equality of means, p-value; 95% CIs of difference between means | ||||
|---|---|---|---|---|---|---|---|
| FaME (N = 350) | OEP (N = 346) | Usual care (N = 381) | OEP vs. usual care | FaME vs. usual care | FaME vs. OEP | ||
| GP | Number of contacts at practice | 7.98 | 7.68 | 7.70 | 0.959; –9.440 to –0.895 | 0.565; –0.673 to 1.232 | 0.531; –0.648 to 1.256 |
| Number of home visits | 0.29 | 0.18 | 0.13 | 0.406; –0.069 to 0.171 | 0.015; 0.030 to 0.279 | 0.205; –0.057 to 0.264 | |
| Number of telephone calls | 1.45 | 0.81 | 0.82 | 0.956; –0.351 to 0.332 | 0.005; 0.192 to 1.079 | 0.002; 0.244 to 1.046 | |
| Total GP contacts | 9.72 | 8.67 | 8.65 | 0.976; –1.071 to 1.105 | 0.087; –0.157 to 2.295 | 0.086; –0.150 to 2.255 | |
| Practice nurse | Number of contacts at practice | 3.49 | 3.36 | 3.42 | 0.839; –0.624 to 0.507 | 0.815; –0.551 to 0.699 | 0.678; –0.495 to 0.761 |
| Number of home visits | 0.01 | 0.04 | 0.00 | 0.141; –0.014 to 0.095 | 0.238; –0.006 to 0.023 | 0.261; –0.088 to 0.024 | |
| Number of telephone calls | 0.18 (n = 349) | 0.25 | 0.10 (n = 380) | 0.008; 0.035 to 0.254 | 0.081; –0.010 to 0.171 | 0.314; –0.192 to 0.062 | |
| Total practice nurse contacts | 3.64 (n = 349) | 3.36 | 3.53 (n = 380) | 0.676; –0.459 to 0.708 | 0.728; –0.523 to 0.749 | 0.973; –0.661 to 0.639 | |
| Out of hours | Number of treatment centre visits | 0.07 | 0.08 | 0.04 | 0.148; –0.014 to 0.092 | 0.261; –0.018 to 0.066 | 0.612; –0.074 to 0.044 |
| Number of home visits | 0.01 | 0.04 (n = 345) | 0.02 | 0.134; –0.007 to 0.051 | 0.648; –0.023 to 0.014 | 0.078; –0.055 to 0.003 | |
| Number of telephone calls | 0.07 | 0.09 | 0.08 | 0.936; –0.063 to 0.069 | 0.551; –0.078 to 0.042 | 0.454; –0.076 to 0.034 | |
| Total out-of-hours contacts | 0.15 | 0.21 (n = 345) | 0.14 | 0.208; –0.036 to 0.165 | 0.976; –0.086 to 0.089 | 0.216; –0.163 to 0.037 | |
| Other senior-level practitionersb | Number of contacts at practice | 0.07 | 0.32 | 0.17 | 0.102; –0.029 to 0.318 | 0.056; –0.206 to 0.002 | 0.003; –0.409 to –0.084 |
| Number of home visits | 0.05 | 0.44 | 0.00 | 0.019; 0.072 to 0.807 | 0.144; –0.017 to 0.114 | 0.040; –0.764 to –0.017 | |
| Number of telephone calls | 0.02 | 0.05 | 0.04 | 0.727; –0.049 to 0.068 | 0.226; –0.064 to 0.015 | 0.247; –0.094 to 0.024 | |
| Other middle-level practitionersb | Number of contacts at practice | 0.16 | 0.12 | 0.03 | 0.022; 0.012 to 0.156 | 0.002; 0.047 to 0.204 | 0.406; –0.57 to 0.140 |
| Number of home visits | 0.05 | 0.01 | 0.03 | 0.072; –0.048 to 0.002 | 0.446; –0.031 to 0.071 | 0.083; –0.006 to 0.091 | |
| Number of telephone calls | 0.00 | 0.00 | 0.00 | 0.318; –0.003 to 0.009 | N/A | 0.318; –0.009 to 0.003 | |
| Other lower-level practitionersb | Number of contacts at practice | 1.30 | 1.70 | 1.19 | 0.017; 0.093 to 0.923 | 0.569; –0.258 to 0.470 | 0.080; –0.853 to 0.048 |
| Number of home visits | 0.00 | 0.08 | 0.01 | 0.067; –0.005 to 0.157 | 0.696; –0.014 to 0.010 | 0.058; –0.159 to 0.003 | |
| Number of telephone calls | 0.01 | 0.03 | 0.02 | 0.471; –0.018 to 0.038 | 0.648; –0.023 to 0.014 | 0.329; –0.044 to 0.015 | |
| Total OPC (all three levels) | 1.66 | 2.75 | 1.50 | 0.001; 0.525 to 1.980 | 0.442; –0.259 to 0.593 | 0.005; –1.840 to –0.331 | |
| Grand total | Number of contacts | 15.06 (n = 349) | 15.27 (n = 345) | 13.85 (n = 380) | 0.100; –0.270 to 3.103 | 0.146; –0.494 to 2.914 | 0.829; –2.082 to 0.669 |
| Service | Variable | Mean | t-test for equality of means, p-value; 95% CIs of difference between means | ||||
|---|---|---|---|---|---|---|---|
| FaME (N = 158) | OEP (N = 180) | Usual care (N = 151) | OEP vs. usual care | FaME vs. usual care | FaME vs. OEP | ||
| GP | Number of contacts at practice | 8.41 | 8.74 | 8.85 | 0.526; –1.944 to 1.169 | 0.560; –1.963 to 1.065 | 0.933; –1.500 to 1.376 |
| Number of home visits | 0.07 | 0.06 | 0.11 | 0.320; –0.134 to 0.044 | 0.413; –0.124 to 0.051 | 0.818; –0.064 to 0.081 | |
| Number of telephone calls | 2.23 | 0.83 | 0.75 | 0.700; –0.322 to 0.479 | 0.000; 0.771 to 2.187 | 0.000; 0.667 to 2.135 | |
| Total GP contacts | 10.71 | 9.36 | 9.72 | 0.681; –2.047 to 1.339 | 0.301; –0.895 to 2.882 | 0.142; –0.455 to 3.150 | |
| Practice Nurse | Number of contacts at practice | 4.51 | 3.65 | 3.16 | 0.194; –0.251 to 1.233 | 0.007; 0.378 to 2.317 | 0.091; –0.139 to 1.851 |
| Number of home visits | 0.01 | 0.01 | 0.00 | 0.158; –0.004 to 0.027 | 0.319; –0.006 to 0.019 | 0.641; –0.025 to 0.015 | |
| Number of telephone calls | 0.18 (n = 157) | 0.37 | 0.13 (n = 150) | 0.019; 0.039 to 0.027 | 0.522; – 0.120 to 0.236 | 0.129; –0.417 to 0.053 | |
| Total practice nurse contacts | 4.60 (n = 157) | 4.03 | 3.29 (n = 150) | 0.066; 0.048 to 1.517 | 0.010; 0.313 to 2.298 | 0.277; –0.460 to 1.602 | |
| Out of hours | Number of treatment centre visits | 0.03 | 0.02 | 0.05 | 0.174; –0.073 to 0.013 | 0.589; –0.068 to 0.039 | 0.464; –0.025 to 0.055 |
| Number of home visits | 0.01 | 0.02 | 0.01 | 0.831; –0.028 to 0.035 | 0.964; –0.026 to 0.025 | 0.798; –0.035 to 0.027 | |
| Number of telephone calls | 0.05 | 0.05 | 0.04 | 0.676; –0.038 to 0.059 | 0.703; –0.045 to 0.067 | 0.983; –0.057 to 0.058 | |
| Total out-of-hours contacts | 0.09 | 0.08 | 0.10 | 0.708; –0.100 to 0.068 | 0.926; –0.098 to 0.089 | 0.806; –0.081 to 0.105 | |
| Other senior-level practitionersb | Number of contacts at practice | 0.12 | 0.07 | 0.02 | 0.345; –0.051 to 0.144 | 0.098; –0.019 to 0.219 | 0.456; –0.088 to 0.195 |
| Number of home visits | 0.01 | 0.00 | 0.00 | N/A | 0.319; –0.006 to 0.019 | 0.319; –0.006 to 0.019 | |
| Number of telephone calls | 0.00 | 0.00 | 0.00 | N/A | N/A | N/A | |
| Other middle-level practitionersb | Number of contacts at practice | 0.06 | 0.04 | 0.02 | N/A | N/A | N/A |
| Number of home visits | 0.04 | 0.00 | 0.01 | 0.611; –0.054 to 0.092 | 0.467; –0.063 to 0.137 | 0.736; –0.0877 to 0.124 | |
| Number of telephone calls | 0.00 | 0.00 | 0.00 | 0.319; –0.039 to 0.013 | 0.237; –0.021 to 0.083 | 0.052; 0.000 to 0.089 | |
| Other lower-level practitionersb | Number of contacts at practice | 0.59 | 0.77 | 0.17 | 0.00; 0.315 to 0.887 | 0.000; 0.211 to 0.647 | 0.317; –0.509 to 0.165 |
| Number of home visits | 0.01 | 0.00 | 0.01 | 0.319; –0.039 to 0.013 | 0.633; –0.035 to 0.022 | 0.319; –0.006 to 0.019 | |
| Number of telephone calls | 0.00 | 0.01 | 0.03 | 0.143; –0.049 to 0.007 | 0.045; –0.052 to –0.001 | 0.350; –0.017 to 0.006 | |
| Total OPC (all three levels) | 0.83 | 0.88 | 0.26 | 0.000; 0.311 to 0.928 | 0.000; 0.280 to 0.861 | 0.809; –0.444 to 0.347 | |
| Grand total | Number of contacts | 15.98 (n = 157) | 14.35 | 13.43 (n = 150) | 0.400; –1.225 to 3.058 | 0.037; 0.158 to 4.938 | 0.172; –0.714 to 3.975 |
| Service | Variable | Mean | t-test for equality of means, p-value; 95% CIs of difference between means | ||||
|---|---|---|---|---|---|---|---|
| FaME (N = 192) | OEP (N = 166) | Usual care (N = 230) | OEP vs. usual care | FaME vs. usual care | FaME vs. OEP | ||
| GP | Number of contacts at practice | 7.64 | 6.83 | 6.95 | 0.23; –1.198 to 0.953 | 0.26; –0.531 to 1.906 | 0.195; –0.417 to 2.037 |
| Number of home visits | 0.46 | 0.31 | 0.15 | 0.173; –0.073 to 0.404 | 0.004; 0.103 to 0.529 | 0.328; –0.152 to 0.452 | |
| Number of telephone calls | 0.81 | 0.78 | 0.86 | 0.777; –0.618 to 0.462 | 0.861; –.590 to 0.493 | 0.883; –0.363 to 0.422 | |
| Total GP contacts | 8.91 | 7.92 | 7.96 | 0.962; –1.458 to 1.389 | 0.244; –0.655 to 2.565 | 0.227; –0.619 to 2.599 | |
| Practice nurse | Number of contacts at practice | 2.66 | 3.05 | 3.59 | 0.201; –1.377 to 0.291 | 0.021; –1.716 to –0.143 | 0.322; –1.153 to 0.380 |
| Number of home visits | 0.02 | 0.08 | 0.00 | 0.195; –0.038 to 0.186 | 0.33; –0.012 to 0.034 | 0.280; –0.177 to 0.051 | |
| Number of telephone calls | 0.18 | 0.12 | 0.09 | 0.365; –0.039 to 0.106 | 0.030; 0.009 to 0.181 | 0.224; –0.038 to 0.162 | |
| Total practice nurse contacts | 2.86 | 3.25 | 3.68 | 0.312; –1.282 to 0.411 | 0.047; –1.636 to –0.010 | 0.338; –1.183 to 0.407 | |
| Out of hours | Number of treatment centre visits | 0.10 | 0.16 | 0.04 | 0.028; 0.12 to 0.214 | 0.082; –0.007 to 0.118 | 0.309; –0.169 to 0.054 |
| Number of home visits | 0.01 | 0.06 (n = 165) | 0.02 | 0.090; –0.007 to 0.093 | 0.603; –0.033 to 0.019 | 0.055; –0.101 to 0.001 | |
| Number of telephone calls | 0.08 | 0.13 | 0.11 | 0.819; –0.102 to 0.129 | 0.475; –0.131 to 0.061 | 0.320; –0.144 to 0.047 | |
| Total out-of-hours contacts | 0.19 | 0.35 (n = 165) | 0.17 | 0.058; –0.006 to 0.349 | 0.844; –0.122 to 0.150 | 0.080; –0.335 to 0.019 | |
| Other senior-level practitionersb | Number of contacts at practice | 0.03 | 0.59 | 0.27 | 0.058; –0.011 to 0.644 | 0.002; –0.392 to –0.093 | 0.000; –0.860 to –0.258 |
| Number of home visits | 0.09 | 0.92 | 0.00 | 0.019; 0.153 to 1.681 | 0.166; –0.035 to 0.204 | 0.035; –1.606 to –0.060 | |
| Number of telephone calls | 0.04 | 0.11 | 0.07 | 0.152; –0.015 to 0.094 | 0.288; –0.107 to 0.032 | 0.201; –0.198 to 0.042 | |
| Other middle-level practitionersb | Number of contacts at practice | 0.24 | 0.20 | 0.04 | 0.014; 0.032 to 0.287 | 0.001; 0.084 to 0.317 | 0.621; –0.121 to 0.203 |
| Number of home visits | 0.06 | 0.02 | 0.04 | 0.207; –0.065 to 0.014 | 0.736; –0.067 to 0.094 | 0.373; –0.047 to 0.126 | |
| Number of telephone calls | 0.00 | 0.01 | 0.00 | 0.319; –0.006 to 0.018 | N/A | 0.319; –0.018 to 0.006 | |
| Other lower-level practitionersb | Number of contacts at practice | 1.88 | 2.71 | 1.87 | 0.021; 0.128 to 1.563 | 0.974; –0.573 to.593 | 0.036; –1.617 to –0.055 |
| Number of home visits | 0.00 | 0.17 | 0.00 | 0.049; 0.001 to 0.336 | N/A | 0.049; –0.336 to –0.001 | |
| Number of telephone calls | 0.02 | 0.05 | 0.01 | 0.152; –0.015 to 0.094 | 0.366; –0.014 to 0.038 | 0.336; –0.083 to 0.028 | |
| Total OPC (all three levels) | 2.35 | 4.78 | 2.31 | 0.000; 1.094 to 3.843 | 0.906; –0.632 to 0.713 | 0.001; –3.852 to –1.004 | |
| Grand total | Number of contacts | 14.31 | 16.27 (n = 165) | 14.12 | 0.112; –0.503 to 4.793 | 0.877; –2.166 to 2.537 | 0.187; –4.889 to 0.970 |
Regarding costs of primary care service utilisation, taking London and Nottingham together, there was a tendency (p = 0.104) for the mean costs in the FaME group to be higher than that in the usual-care group, but no significant difference between the other groups. In Nottingham, the mean cost of services used in OEP tended to be higher than that of usual care (p = 0.095) (Table 36).
| Service | Mean | t-test for equality of means, p-value; 95% CIs of difference between means | ||||
|---|---|---|---|---|---|---|
| London and Nottingham combined | FaME (N = 350) | OEP (N = 346) | Usual care (N = 381) | OEP vs. usual care | FaME vs. usual care | FaME vs. OEP |
| N = 1077 with complete data (eight missing from OEP, five missing from FaME and four missing from usual care) | ||||||
| Total GP (at practice, home, telephone) | 353.95 | 316.29 | 311.22 | 0.803; –34.79 to 44.92 | 0.064; –2.50 to 87.96 | 0.112; –8.81 to 84.13 |
| Total practice nurse (at practice, home, telephone) | 34.79 | 35.17 | 33.94 | 0.674; –4.52 to 6.99 | 0.783; –5.26 to 6.97 | 0.907; –6.73 to 5.97 |
| Total out of hours (at treatment centre, home, telephone) | 7.95 | 14.25 | 8.04 | 0.077; –0.67 to 13.09 | 0.972; –5.33 to 5.14 | 0.090; –13.59 to 0.980 |
| Total other primary care (at practice, home, telephone) | 15.53 | 38.52 | 13.53 | 0.004; 7.87 to 42.11 | 0.428; –2.95 to 6.94 | 0.010; –40.33 to –5.65 |
| Grand total | 412.22 | 404.23 | 366.73 | 0.188; –17.70 to 89.96 | 0.104; –8.57 to 91.14 | 0.866; –54.73 to 65.07 |
| London only | FaME (N = 158) | OEP (N = 180) | Usual care (N = 151) | OEP vs. usual care | FaME vs. usual care | FaME vs. OEP |
| N = 489 with complete data (six missing from OEP, three missing from FaME and two from missing usual care) | ||||||
| Total GP (at practice, home, telephone) | 360.16 | 330.53 | 348.19 | 0.561; –77.42 to 42.10 | 0.711; –51.50 to 75.44 | 0.337; –31.00 to 90.26 |
| Total practice nurse (at practice, home, telephone) | 44.02 (n = 157) | 37.62 | 31.47 (n = 150) | 0.102; –1.23 to 13.54 | 0.009; 3.10 to 22.01 | 0.197; –3.34 to 16.14 |
| Total out of hours (at treatment centre, home, telephone) | 5.68 | 5.58 | 6.22 | 0.859; –7.74 to 6.45 | 0.869; –6.99 to 5.90 | 0.978; –7.10 to 7.31 |
| Total other primary care (at practice, home, telephone) | 9.03 | 6.97 | 2.38 | 0.003; 1.55 to 7.63 | 0.001; 2.21 to 10.70 | 0.381; –2.57 to 6.70 |
| Grand total | 418.89 (n = 157) | 380.70 | 388.26 (n = 150) | 0.761; –74.12 to 54.25 | 0.546; –46.98 to 88.66 | 0.357; –34.83 to 96.38 |
| Nottingham only | FaME (N = 192) | OEP (N = 166) | Usual care (N = 230) | OEP vs. usual care | FaME vs. usual care | FaME vs. OEP |
| N = 586 with complete data (two missing from OEP, two missing from FaME and two missing usual care) | ||||||
| Total GP (at practice, home, telephone) | 348.84 | 300.84 | 286.95 | 0.614; –40.26 to 68.06 | 0.052; –0.41 to 124.19 | 0.181; –22.48 to 118.47 |
| Total practice nurse (at practice, home, telephone) | 27.25 | 32.51 (n = 165) | 35.55 | 0.484; –11.54 to 5.47 | 0.039; –16.18 to –0.42 | 0.205; –13.41 to 2.88 |
| Total out of hours (at treatment centre, home, telephone) | 9.81 | 23.71 | 9.23 | 0.018; 2.49 to 26.46 | 0.884; –7.21 to 8.36 | 0.033; –26.63 to –1.16 |
| Total other primary care (at practice, home, telephone) | 20.88 | 72.73 | 20.86 | 0.004; 17.12 to 86.63 | 0.996; –7.805 to 7.844 | 0.004: –86.93 to –16.78 |
| Grand total | 406.78 | 429.79 (n = 165) | 352.59 | 0.095; –13.37 to 165.95 | 0.128; –15.73 to 124.20 | 0.622; –121.35 to 77.15 |
Falls
Data on falls and A&E and hospital service utilisation associated with falls were collected from GP records at the same time as primary care contact information. The numbers of falls documented are therefore different from those reported in Chapter 6, which were reported in diaries and at telephone follow-up. No differences were found in number of GP-recorded falls, or the A&E and hospital costs associated with falls, between any groups at either site (Table 37).
| Variable | Mean | t-test for equality of means, p-value; 95% CIs of difference between means | ||||
|---|---|---|---|---|---|---|
| London and Nottingham combined | FaME (N = 355) | OEP (N = 354) | Usual care (N = 385) | OEP vs. usual care | FaME vs. usual care | FaME vs. OEP |
| Number of falls | 0.12 (n = 350) | 0.17 (n = 346) | 0.14 (n = 379) | 0.548; –0.063 to 0.119 | 0.645; –0.089 to 0.055 | 0.136; –0.132 to 0.043 |
| Number of A&E visits for falls | 0.06 (n = 350) | 0.07 (n = 347) | 0.06 (n = 379) | 0.423; –0.028 to 0.067 | 0.823; –0.036 to 0.045 | 0.534; –0.062 to 0.032 |
| Number of hospital admissions for falls | 0.01 (n = 350) | 0.03 (n = 347) | 0.02 (n = 380) | 0.351; –0.014 to 0.040 | 0.613; –0.021 to 0.013 | 0.215; –0.045 to 0.010 |
| Number of inpatient nights for falls | 0.01 (n = 351) | 0.16 (n=348) | 0.05 (n = 380) | 0.413; –0.155 to 0.377 | 0.325; –0.115 to 0.038 | 0.271; –0.416 to 0.117 |
| Total cost of falls (A&E and nights) | 12.63 (n = 350) | 39.20 (n = 347) | 19.27 (n = 379) | 0.305; –18.24 to 58.08 | 0.461; –24.33 to 11.05 | 0.146; –62.42 to 9.3 |
| London only | FaME (N = 161) | OEP (N = 186) | Usual care (N = 153) | OEP vs. usual care | FaME vs. usual care | FaME vs. OEP |
| Number of falls | 0.17 (n = 158) | 0.17 (n = 180) | 0.19 (n = 151) | 0.755; –0.137 to 0.100 | 0.819; –0.139 to 0.110 | 0.942; –0.110 to 0.118 |
| Number of A&E visits for falls | 0.09 (n = 158) | 0.08 (n = 180) | 0.06 (n = 151) | 0.539; –0.052 to 0.100 | 0.393; –0.038 to 0.096 | 0.892; –0.071 to 0.082 |
| Number of hospital admissions for falls | 0.02 (n = 158) | 0.02 (n = 180) | 0.01 (n = 151) | 0.600; –0.025 to 0.043 | 0.690; –0.023 to 0.034 | 0.855; –0.038 to 0.032 |
| Number of inpatient nights for falls | 0.02 (n = 159) | 0.28 (n = 181) | 0.10 (n = 151) | 0.552; –0.407 to 0.761 | 0.378; –0.260 to 0.099 | 0.354; –0.803 to 0.288 |
| Total cost of falls (A&E and nights) | 19.82 (n = 158) | 50.78 (n = 180) | 25.41 (n = 151) | 0.502; –48.89 to 99.63 | 0.761; –41.77 to 30.58 | 0.369; –98.64 to 36.7 |
| Nottingham only | FaME (N = 194) | OEP (N = 168) | Usual care (N = 232) | OEP vs. usual care | FaME vs. usual care | FaME vs. OEP |
| Number of falls | 0.08 (n = 192) | 0.17 (n = 166) | 0.11 (n = 228) | 0.396; –0.077 to 0.196 | 0.547; –0.112 to 0.059 | 0.231; –0.225 to 0.055 |
| Number of A&E visits for falls | 0.04 (n = 192) | 0.07 (n = 167) | 0.05 (n = 228) | 0.675; –0.049 to 0.075 | 0.525; –0.066 to 0.034 | 0.327; –0.088 to 0.030 |
| Number of hospital admissions for falls | 0.01 (n = 192) | 0.04 (n = 167) | 0.02 (n = 229) | 0.393; –0.024 to 0.061 | 0.249; –0.303 to 0.009 | 0.181; –0.076 to 0.014 |
| Number of inpatient nights for falls | 0.01 (n = 192) | 0.04 (n = 167) | 0.02 (n = 229) | 0.393; –0.024 to 0.061 | 0.227; –0.032 to 0.008 | 0.181; –0.076 to 0.014 |
| Total cost of falls (A&E and nights) | 6.72 (n = 192) | 26.71 (n = 167) | 15.21 (n = 228) | 0.433; –17.32 to 40.31 | 0.259; –23.24 to 6.27 | 0.188; –49.79 to 9.82 |
Cost-effectiveness analysis
The primary outcome for the economic evaluation was to be QALYs derived from transformation of SF-12, as described in the methods. The main analysis failed to find a significant difference between groups in this outcome, with or without imputation, and after adjusting for baseline values, cluster and other confounders. As a result, the economic analysis focused on cost-effectiveness using the primary clinical outcome, i.e. the proportion of people reaching or exceeding 150 minutes of MVPA per week at 12 months after the end of the intervention.
A significant effect in favour of FaME, compared with usual care, was identified by the main statistical analysis (see Chapter 5). Against 38% of patients receiving usual care who at least met the exercise target at 12 months post intervention, an OR of 1.78 was associated with FaME, meaning that 52% of participants assigned to that group would be meeting the target, an absolute increase of 14%. This benefit was achieved through NHS expenditure on delivering the FaME interventions in London and Nottingham of £268.75 and £218.43 per participant, respectively (mean £243.59).
A cohort of 100 people assigned to the FaME intervention would therefore incur a total cost to the NHS of £26,875 in London and £21,843 in Nottingham (average £24,359), compared with no cost for usual care. As the FaME programme, compared with usual care, results in 14% more people achieving or exceeding the 150-minute per week moderate or vigorous exercise target at the 12-month post-intervention end point, the cost per extra person exercising can be calculated as the total cost for 100 people divided by 14, i.e. £1919.64 in London and £1560.21 in Nottingham (mean £1739.93).
Discussion
These findings need to be interpreted with caution. The per-participant costs for FaME are based on those recorded in the trial and would be affected by class size, with smaller groups increasing the average costs. Class size (and instructor) might also affect compliance and outcomes, but the impact of these factors is not known. The difference in costs between sites is largely a reflection of the higher costs of facilities hired for group classes in London, but considerable variability was observed within both sites.
The calculations reflect the NHS perspective and do not take account of the expenditures incurred by individuals in travelling to the exercise class venues or other out-of pocket expenses associated with exercise. No allowances are made for offsets against the costs of the interventions because no differences were found between groups in the costs of primary care contacts (used as an indicator or general health effects of exercise) or injurious falls in the 18 months post recruitment. The lack of difference between groups in use of primary care utilisation is consistent with the finding of no difference in health-related quality of life between groups.
Data from participants returning diaries indicated that those in the FaME group reported fewer falls than those in the usual-care group in the 12 months post intervention, but this was not reflected in the GP data (which covered all participants in GP practices that permitted access for data gathering, i.e. all practices except for three in London). Owing to lack of a statistically significant difference in QALYs between groups, a probabilistic sensitivity analysis was not undertaken, so no interpretation of the findings against a cost/QALY gained benchmark is possible. The study recruited volunteers, some of whom were already achieving the 150 minutes of MVPA at baseline. Further analysis is needed to explore the differential impact of the interventions on sustaining exercise among those already at target, and encouraging people not exercising to start, since this may impact on the cost-effectiveness ratios.
Chapter 8 Discussion
What this study shows
Exercise classes using the FaME programme significantly increased PA in older people. The proportions reporting at least 150 minutes of MVPA per week rose from 40% at baseline to 49% at 12 months post intervention in the FaME arm, from 41% to 43% in the OEP arm and from 37.5% to 38.0% in the usual-care arm. The odds of reporting at least 150 minutes of MVPA were 78% higher in the FaME arm than in the usual-care arm, equating to an absolute increase of 14% in the number of participants reaching or exceeding the PA target. In terms of minutes of MVPA, the FaME arm reported an additional 13–15 minutes of MVPA per day (91–105 minutes per week) compared with the usual-care arm, depending on the imputational model used. There was no statistically significant increase in MVPA in the OEP arm compared with the usual-care arm.
In the 12 months post intervention there was a statistically significant reduction in the rate of falls in the FaME arm compared with usual care (IRR 0.74, 95% CI 0.55 to 0.99; p = 0.042). Although the falls rate was lower in the OEP arm than in the usual-care arm, there was no statistically significant difference between these two arms. The PASE scores showed a small, but statistically significant, benefit for FaME compared with usual care (difference in means 11.2, 95% CI 0.2 to 20.2; p = 0.046), but no statistically significant benefit for OEP (difference in means 7.5, 95% CI –3.8 to 18.8; p = 0.20). Significant improvements were seen in balance confidence for both intervention arms at 12 months post intervention. The mean difference for FaME compared with usual care was –0.529 (95% CI –0.998 to –0.061; p = 0.027), while the mean difference for OEP compared with usual care was –0.545 (95% CI –1.033 to –0.057; p = 0.029). Participants in the FaME and OEP arms were significantly less likely to dismiss exercise as not beneficial, and in the FaME arm were more likely to be positive about exercise, 12 months after the end of the interventions.
There were no statistically significant differences between intervention arms and the usual-care arm in self-efficacy, mental and physical well-being, quality of life, social networks, falls risk or functional abilities. The lack of change in quality of life is perhaps not surprising, given the high baseline level of OPQoL scores and the limited likelihood that an extra 15 minutes of PA in relatively active people would change perceptions of quality of life. The interventions were not associated with increased risk of AEs or ARs, during or, after the intervention period.
FaME is more expensive than OEP delivered with PMs (£269 vs. £88 per participant in London; £218 vs. £117 per participant in Nottingham), because of more direct participant contact from PSIs and hire of halls for the exercise classes. The cost per additional person meeting the target of 150 minutes MVPA per week at 12 months post intervention in FaME, compared with usual care, is £1920 in London and £1560 in Nottingham.
There are a number of methodological lessons from this trial. We have demonstrated that it is possible to recruit older people who would benefit from increasing their PA (as shown by their performance on a range of functional and psychological measures) to exercise promotion trials in general practice. As we outlined in Chapter 3, organisational factors in practices (such as room availability and space to carry out functional assessments) mean that planned recruitment rates may overestimate the speed of recruitment. Given that participation in an exercise trial attracts some who are already physically active at or above the recommended target level, telephone prescreening is useful to minimise the conduct of baseline assessments on individuals who are subsequently found to be ineligible. Quality assurance of interventions is necessary to optimise the fidelity of their application. The quality assessment process developed for the FaME intervention proved workable and may be of use in other similar studies. Recruitment of PMs was difficult despite relaxing the eligibility rules and the OEP arm was disadvantaged by this. Finally, frequent request of participants to complete exercise diaries challenged retention, and we lessened this research burden by reducing the frequency of requests. We will return to these methodological issues later in this chapter.
As we described in Chapter 4, those recruited to the trial were more active than their peers (with a median weekly MVPA level at baseline of 105 minutes), but on other characteristics functioned below population norms. Although not inactive, in other respects the participant population was an ideal one for testing PA interventions, as noted above. Retention of trial participants in the study remained problematic, despite the efforts made to increase it described in Chapter 3. However, this may in part be an unavoidable consequence of targeting an older age group, as illness events are common in the older population; 30% of those who dropped out cited illness as their reason for discontinuing with the study. Disappointment at allocation and research burden (the number of questionnaires and diaries to complete – see above and Chapter 3) were responsible for at least 18% and 11% of dropouts, respectively. As Chapter 4 shows, those who dropped out were older and more disabled than those who remained in the study. Those lost to follow-up were the subgroup which would probably have benefited most from increasing PA and may have been those least likely to increase their MVPA as a result of the intervention. This suggests that post-intervention levels of MVPA may overestimate activity levels that could be achieved in the general population of older people with delivery of FaME and OEP programmes. However, as losses to follow-up were similar across treatment arms, it is unlikely that this will have biased our estimates of the difference in MVPA between treatment arms.
Comparison with other studies
Our systematic review of the effectiveness of PA interventions for adults aged ≥ 50 years delivered through general practice84 identified six studies published between 1998 and 2011, with a total of 1522 participants. Four interventions were delivered by GPs or nurses and exercise specialists. 85–88 Three used only exercise specialists86,89 or an exercise counsellor. 90 Two used specific PA measures, such as the PASE and the Auckland Heart Exercise Questionnaire. 89,90 Four studies had 12 months’ follow-up. 86–89 Only two of these studies reported a statistically significant increase in PA levels. Kolt et al. 90 report that moderate-leisure PA increased by 86.8 minutes per week in the intervention participants compared with controls (p = 0.007). More intervention participants than controls reached 2.5 hours per week of moderate/vigorous leisure physical activity at 12 months (42% vs. 23%; OR 2.9, 95% CI 1.33 to 6.32; p = 0.007). Halbert et al. 86 reported that PA increased in both groups (p < 0.05), but more participants in the intervention group than in the control group increased their intention to do PA (p < 0.001). The increase was greater in the intervention than in the control group for all measures, except time spent walking. Two studies showed no significant increase in activity. 85,88
The ProAct65+ trial almost doubles the number of participants in such studies and shows the impact of interventions using standard scales for assessing PA for up to 2 years post randomisation. In contrast to the methodological variability of the other six studies, the ProAct65+ trial reported the method for generating the randomisation sequence, concealment of allocation, blind assessment of outcomes, an intention-to-treat analysis controlled for confounding variables and differences between treatment groups at baseline. In the six other studies, all interventions left participants to motivate and organise their own PA and the quantity of PA undertaken was not monitored, making it difficult to know whether or not the dose of the intervention affected the results. The effectiveness of the FaME arm in increasing self-reported PA may reflect the direction and encouragement provided by PSIs to participants.
Strengths and limitations of the study
To the best of our knowledge this study is the largest general practice-based trial of exercise interventions for older people in the UK, to date, and the first to deploy PMs to augment an exercise programme. Both exercise interventions were evidence based, but also pragmatic (i.e. feasible to use in general practice), and tailored to individual participants’ capabilities.
The ProAct65+ trial largely fulfils the RE-AIM criteria for evaluation of the public health impact of health promotion studies, using five dimensions:91
-
Reach (the proportion of the target population reached and the characteristics of participants compared with the target population). The ProAct65+ trial recruited a large number of people aged ≥ 65 years, whose performance on most of the measures used fell below population norms, despite their relatively high level of PA at baseline. The trial attracted participants who would be likely to benefit from increasing their PA level.
-
Efficacy (how the intervention benefited the participants). Participants in the FaME arm reported increased physical activity (on two measures), had a lower risk of falls and improved balance confidence, as well as becoming more positive about the beneficial effects of exercise. These findings are consistent with the conclusions of the Cochrane review of falls prevention. 5
-
Adoption (engagement of the settings participating in the study). Recruitment through general practice was feasible and 70% of participants remained in the study for 1 year after the intervention period.
-
Implementation (the extent to which the intervention was delivered as intended; including the adherence to the intervention, and the involvement of staff in the setting). Adherence to the intervention was easier to maintain in the FaME arm than in the OEP arm. As reported in Chapter 5, we were unable to show any difference in outcome in either intervention arm attributable to adherence.
-
Maintenance (long-term maintenance of behaviour change, defined as ≥ 2 years). We have reported findings at 12 months post intervention, our predetermined analysis point, but have collected data for up to 24 months post intervention. Significant increases in self-reported MVPA were found in the FaME arm of this study at 12 months post intervention (18 months after allocation); as Chapter 5 shows, this increase persisted, although slightly attenuated, at 18 months post intervention (2 years post allocation). Further studies are needed to measure attenuation of effects and to test the impact of reinforcement of the intervention.
Because of the difficulties of recruiting sufficient PMs we were unable to ensure a consistent dose of peer mentoring, which means that we have not measured the true impact of the OEP intervention.
The trial was reliant on self-report of PA, which is criticised for overestimating actual levels of activity. 92 However, this is less of a limitation than some suggest, for several reasons.
First, associations between self-reported PA and health outcomes93 are the basis of guidelines on 150 minutes of MVPA94 and, therefore, self-report of PA is also an appropriate measure of change in behaviour. 95 Using objective measures to assess compliance with guidelines that are based on evidence from self-reported activity could give an inaccurate picture of the proportion of the population that is insufficiently active. 94
Second, self-reported engagement in activities predicts both self-reported and measured functional ability 3–5 years later96 and all-cause mortality in middle-aged men 21 years later. 97 Self-reported PA scales can have acceptable validity98 and a single question can reflect a physiological measure like VO2max. 99
Third, it appears that social desirability may influence self-reporting of PA, but this bias may also be determined by the type of questions asked100 and the characteristics of respondents. For example, misperception of activity level in one study was associated with older age, female sex, poorer walking performance, lower social support and lower self-efficacy,101 while another found that the difference between self-report and objectively measured MVPA was greatest among older men with lower educational level, at higher activity and intensity levels. 101 In the ProAct65+ trial the treatment arms were well balanced, and factors known to be associated with reporting PA were similar across treatment arms, suggesting social desirability to report PA might be expected to be similar across treatment arms. In addition, although those who were less active at baseline were more likely to withdraw, attrition did not vary significantly between treatment arms, again, suggesting this should not have resulted in differential reporting of PA between treatment arms.
Finally, although self-reported PA may overestimate objectively measured PA, this finding has not been consistent across studies. Some of the discrepancies between self-reported and objectively measured PA are because subjective and objective measures of PA are actually measuring different aspects of activity that are independently associated with biomarkers. 102 For example, PA monitors cannot accurately assess upper-body activities or account for movements that require extra effort, such as walking uphill or carrying loads. 103 Overall, it is not yet possible to draw definitive conclusions about the validity of self-reporting of PA compared with objective measurement,104 and this is an important research topic needing further investigation.
Lessons learned
The challenges faced during the ProAct65+ trial and solutions to these challenges are summarised in Chapter 3. Other research which has faced similar challenges is discussed here, and implications for future research and public health practice are suggested.
Although the trial exceeded its recruitment target, the recruitment process was more difficult and slower than anticipated. The time needed to recruit participants was underestimated and an extension in recruitment time was needed. Other trials recruiting from general practice have found similar slow and difficult recruitment, with lower than anticipated numbers recruited and required time extensions. 105–107 In ProAct65+, the recruitment phase was extended, more general practices were recruited and more patients at each practice were invited to participate to achieve the target numbers. We learned that it is advisable to keep recruitment as straight forward as possible and to minimise the work demanded of general practices. 108
Expressions of interest were received from patients already exercising at the target level of 150 minutes of moderate activity per week, and from frequent fallers. Others have reported that exercise trials can attract the more active part of the population. 109 Telephone prescreening was introduced to exclude such patients before they reached the baseline assessment appointment, but further studies are needed of ways to recruit the less active population.
The use of volunteers to act as PMs proved complicated. As others have found with interventions using volunteers, recruitment can be slow and the numbers deployed may be low. 110 Our PMs had a case load lower than we planned and, because we also had fewer PMs than intended, some participants received little or no PM support. Other studies have also encountered these problems. 111–113 The lower age limit for PMs was reduced, as was the frequency of their contacts with participants, but with only limited benefit to PM recruitment. It will be important for future interventions testing peer mentoring to allow enough time and resources (human and financial) when planning recruitment and training programmes. In order to minimise the time from training a PM to deployment, and to retain interested volunteers, attention needs to be focused on speeding up the process of gaining Criminal Records Bureau checks and Research Management and Governance approvals. Strategies to optimise PM motivation and involvement need further investigation.
In addition, the number of supportive contacts between PMs and participants varied and often differed from the number of contacts advised by the research team, which may reflect the needs of the individual participants. Future projects implementing PM support should be aware of participants’ needs for more or less support, which may lead to varied numbers of contacts with PMs. Overall, our experience of recruiting and retaining PMs within a trial raises questions about the feasibility of doing this in routine provision of exercise programmes in the community. Community-based exercise programmes proposing to use PMs should explore the feasibility of this prior to embarking on the programme.
Failure to ensure the fidelity of interventions is an important source of variation affecting the credibility and utility of research. 114 Quality assurance observation visits to classes were carried out by expert instructors, with verbal and written feedback on performance. Exercise instructors may not always achieve a balance between tailoring exercise and providing a standardised programme, and observations of intervention delivery are recommended. 115
Participants can be burdened by frequent data collection, which can impact on response rates to self-completion questionnaires and falls diaries. Response biases may occur, and we found that those with lower educational attainment and those whose first language is not English were less likely to complete falls diaries. 78 Compromises in the frequency of data collection were made, and the frequency of the self-completion questionnaires and diaries was reduced. However, maintaining between-assessment contacts is important to reduce attrition. 116 Personal contact with the research team improves response rates,117 as do reminders, incentives and printed educational materials. 118,119 Home visits to collect follow-up data are useful and can reduce attrition bias in longitudinal studies. 120 Alternatively, higher response rates to postal questionnaires have been found when they are sent by the general practices rather than by the research team; this may also be a method to aid retention of participants during a trial. 121
The classification of safety events between sites was variable, so a method of cross-checking and standardisation was developed. Both site principal investigators reviewed and discussed discrepancies in categorisation and a new possible AR category was introduced to reduce variability. This method of cross-checking and the classifications of safety events used in ProAct65+ could be applied to future exercise or indeed any multisite trials.
The ProAct65+ trial was a large pragmatic RCT, which, despite difficulties, reached its recruitment target, making it the largest exercise trial in UK general practice to date. The research team’s flexibility in being able to adapt to unexpected problems may have led to the successful implementation of the trial. 106 The lessons learnt during the ProAct65+ trial have been valuable and have potential implications for similar trials in general practice.
Conclusions
Our first hypothesis, that both exercise interventions would increase self-reported PA, has been refuted in this study, as has the second, that the OEP intervention would be more cost-effective. The FaME intervention increased self-reported PA, adding almost 15 minutes per day of MVPA. This effect persisted for 12 months after cessation of classes. The cost of getting one person to achieve or exceed the target level of PA was between £1560 (Nottingham) and £1920 (London). The OEP arm participants did not show any statistically significant increase in self-reported PA 12 months post intervention. This may be as a result of the limited support from PMs experienced by many participants in the OEP arm, and needs further investigation.
Acknowledgements
Daniel Jackson and Caragh Flannery contributed to the economic analysis, and Laura Perry explored factors associated with completion of falls diaries. Mirilee Pearl and Kalpa Kharicha acted as trial managers when other staff were absent, and Philip Prah worked on the early statistical analysis planning. Tessa Hill, Sarah Scott, Tanimola Martins, Tracey McCauley, Marie Ashmore and Caroline Mulvaney all contributed to the implementation of this study. Peter Cass was a lay expert advisor to the trial management group. We thank the clinical and administrative staff of the 43 practices which took part in the trial, for their support throughout, and the PSIs and PMs, who contributed so much.
Contributions of authors
Steve Iliffe conceived and designed the study, submitted it for funding, was chief investigator for the study and drafted this report.
Denise Kendrick conceived and designed the study, submitted it for funding, was the principal investigator for the study in Nottingham and Derby and helped draft this report.
Richard Morris was senior statistician for the trial, led the analyses and helped draft this report.
Tahir Masud conceived and designed the study, submitted it for funding, supported implementation of the study, and contributed to this report.
Heather Gage supported implementation of the study, led the economic analysis and contributed to this report.
Dawn Skelton conceived and designed the study, guided the development of the exercise interventions, and contributed to this report.
Susie Dinan guided the development of the exercise intervention and contributed to this report.
Ann Bowling supported implementation of the study, led on quality-of-life measurement and contributed to this report.
Mark Griffin was trial statistician, supported implementation of the study and contributed to this report.
Deborah Haworth was trial manager, drove implementation of the trial at both sites and contributed to this report.
Glen Swanwick was PPI representative on the management board, supported implementation of the study and contributed to this report.
Hannah Carpenter co-ordinated the Nottingham research team, supported implementation of the study and contributed to this report.
Arun Kumar supported implementation of the study, led the analysis of fear of falling and contributed to this report.
Zoe Stevens was the trial administrator, supported implementation of the study and contributed to this report.
Sheena Gawler was research associate in the London research team, supported implementation of the study and contributed to this report.
Cate Barlow was research associate in the London research team, supported implementation of the study and contributed to this report.
Juliette Cook was research associate in the Nottingham research team, supported implementation of the study and contributed to this report.
Carolyn Belcher was research associate in the Nottingham research team, supported implementation of the study and contributed to this report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
Publications
Perry L, Kendrick D, Morris R, Dinan S, Masud T, Skelton D, et al. Completion and return of fall diaries varies with participants' level of education, first language, and baseline fall risk. J Gerontol A Biol Sci Med Sci 2012;67:210–14. doi: 10.1093/gerona/glr175
Iliffe S, Kendrick D, Morris R, Skelton D, Gage H, Dinan S, et al. Multicentre cluster randomised trial comparing a community group exercise programme with home based exercise with usual care for people aged 65 and over in primary care: protocol of the ProAct 65+ trial. Trials 2010;11:6. doi: 10.1186/1745-6215-11-6
Stevens Z, Barlow C, Kendrick D, Masud T, Skelton D, Dinan-Young S, et al. Effectiveness of general practice-based exercise promotion for older adults: a systematic review. Prim Health Care Res Dev 2013:15;190–201. doi: 10.1017/S1463423613000017
Kumar A, Carpenter H, Morris R, Iliffe S, Kendrick D. Which factors are associated with fear of falling in community dwelling older people? Age Ageing 2014;43:76–84. doi: 10.1093/ageing/aft154
Stevens Z, Carpenter H, Gawler S, Belcher C, Haworth D, Kendrick D, et al. Lessons learnt during a complex, multi-centre cluster randomised controlled trial: the ProAct65+ trial. Trials 2013:14;192. doi: 10.1186/1745-6215-14-192
References
- Choosing Activity: A Physical Activity Action Plan. London: DH; 2005.
- Blair SN, Kampert JB, Kohl HW, Barlow CE, Macera CA, Paffenbarger RS, et al. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA 1996;276:205-10. http://dx.doi.org/10.1001/jama.1996.03540030039029.
- Nicholl JP, Coleman P, Brazier JE. Health and healthcare costs and benefits of exercise. Pharmacoeconomics 1994;5:109-22. http://dx.doi.org/10.2165/00019053-199405020-00005.
- Gregg EW, Pereira MA, Caspersen CJ. Physical activity, falls, and fractures among older adults: a review of the epidemiologic evidence. J Am Geriatr Soc 2000;48:883-93.
- McClure R, Turner C, Peel N, Spinks A, Eakin E, Hughes K. Population-based interventions for the prevention of fall-related injuries in older people. Cochrane Database Syst Rev 2005. http://dx.doi.org/10.1002/14651858.CD004441.pub2.
- Skelton DA, Todd C. What are the Main Risk Factors for Falls Amongst Older People and what are the Most Effective Interventions to Prevent These Falls? How should Interventions to Prevent Falls be Implemented?. Denmark: World Health Organization Health Evidence Network; 2004.
- Close JC, Lord SL, Menz HB, Sherrington C. What is the role of falls?. Best Pract Res Clin Rheumatol 2005;19:913-35. http://dx.doi.org/10.1016/j.berh.2005.06.002.
- Gillespie LD, Robertson MC, Gillespie WJ, Lamb SE, Gates S, Cumming RG, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev 2009 n.d. http://dx.doi.org/10.1002/14651858.CD007146.pub2.
- Lawrence TM, White CT, Wenn R, Moran CG. The current hospital costs of treating hip fractures. Injury 2005;36:88-91. http://dx.doi.org/10.1016/j.injury.2004.06.015.
- Newton JL, Kyle P, Liversidge P, Robinson G, Wilton K, Reeve P. The cost of falls in the community to the North East Ambulance Service. Emerg Med J 2006;23:479-81. http://dx.doi.org/10.1136/emj.2005.028803.
- Tinetti ME, Speechley M. Prevention of falls among elderly. N Engl J Med 1989;320:1055-9. http://dx.doi.org/10.1056/NEJM198904203201606.
- Cryer C, Patel S. Falls, Fragility and Fractures: National Service Framework for Older People: The Case for and Strategies to Implement a Joint Health Improvement and Modernisation Plan for Falls and Osteoporosis. Tonbridge: University of Kent, Centre for Health Services Studies; 2001.
- Robertson MC, Devlin N, Scuffham P, Gardener MM, Buchner DM, Campbell AJ. Economic evaluation of a community based exercise programme to prevent falls. J Epidemiol Community Health 2001;55:600-6. http://dx.doi.org/10.1136/jech.55.8.600.
- Falls: The Assessment and Prevention of Falls in Older People. London: NICE; 2004.
- Be Active, be Healthy: A Plan for Getting the Nation Moving. London: DH; 2009.
- Skelton DA, Young A, Walker A, Hoinville E. Physical Activity in Later Life: Further Analysis of the Allied Dunbar National Fitness Survey and the Health Education Authority National Survey of Activity and Health. London: Health Education Authority; 1999.
- Hillsdon M, Foster C, Thorogood M. Interventions for promoting physical activity. Cochrane Database Syst Rev 2005.
- At Least Five a Week: Evidence on the Impact of Physical Activity and its Relationship to Health. A Report from the Chief Medical Officer. London: DH; 2004.
- Dinan S, Lenihan P, Tenn T, Iliffe S. Is the promotion of physical activity in vulnerable older people feasible and effective in general practice?. Br J Gen Pract 2006;56:791-3.
- Stewart AL, Mills KM, Sepsis PG, King AC, McLellan BY, Roitz K, et al. Evaluation of CHAMPS, a physical activity promotion program for older adults. Ann Behav Med 1997;19:353-61. http://dx.doi.org/10.1007/BF02895154.
- Stewart AL, Verboncoeur CJ, McLellan BY, Gillis DE, Rush S, Mills KM, et al. Physical outcomes of CHAMPS II: a physical activity promotion program for older adults. J Geronto A Bio Sci Med Sci 2001;56:M465-70.
- Stewart AL, Gillis D, Grossman M, Castrillo M, Pruitt L, McLellan B, et al. Diffusing a research-based physical activity promotion program for seniors into diverse communities: CHAMPS III. Prev Chronic Dis 2006;3.
- Laventure RME, Dinan SM, Skelton DA. Someone Like Me: Increasing Participation in Physical Activity Among Seniors With Senior Peer Health Motivators. J Aging Phys Act 2008;16:S76-7.
- Campbell AJ, Robertson MC, Gardener MM, Norton RN, Tilyard MW, Buchner DM. Randomised control trial of a general practice programme of home based exercise to prevent falls in elderly women. BMJ 1997;315:1065-9. http://dx.doi.org/10.1136/bmj.315.7115.1065.
- Campbell AJ, Robertson MC, Gardener MM, Norton RN, Buchner DM. Psychotropic medication withdrawal and a home based exercise programme to prevent falls: a randomised controlled trial. J Am Geriatr Soc 1999;47:850-3.
- Gardener MM, Buchner DM, Robertson MC, Campbell AJ. Practical implementation of an exercise-based falls prevention programme. Age Ageing 2001;30:77-83. http://dx.doi.org/10.1093/ageing/30.1.77.
- Gardener MM, Phty M, Robertson MC, McGee R, Campbell AK. Application of a falls prevention program for older people to primary health care practice. Prev Med 2002;34:546-53. http://dx.doi.org/10.1006/pmed.2002.1017.
- Robertson MC, Devlin N, Gardener MM, Campbell AJ. Effectiveness and economic evaluation of a nurse delivered home exercise programme to prevent falls. 1: Randomised controlled trial. BMJ 2001;322:697-701. http://dx.doi.org/10.1136/bmj.322.7288.697.
- Robertson MC, Campbell AJ, Gardner MM, Devlin N. Preventing injuries in older people by preventing falls: a meta-analysis of individual-level data. J Am Geriatr Soc 2002;50:905-11. http://dx.doi.org/10.1046/j.1532-5415.2002.50218.x.
- Liu-Ambrose T, Donaldson MG, Ahamed Y, Graf P, Cook WL, Close J, et al. Otago home-based strength and balance retraining improves executive functioning in older fallers: a randomized controlled trial. J Am Geriatr Soc 2008;56:1821-30. http://dx.doi.org/10.1111/j.1532-5415.2008.01931.x.
- Skelton DA, Dinan SM, Campbell MG, Rutherford OM. Tailored group exercise (Falls Management Exercise – FaME) reduces falls in community-dwelling older frequent fallers (an RCT). Age Ageing 2005;34:636-9. http://dx.doi.org/10.1093/ageing/afi174.
- Skelton DA, Dinan SM. Exercise for falls management: rationale for an exercise programme aimed at reducing postural instability. Physiother Theory Pract 1999;15:105-20. http://dx.doi.org/10.1080/095939899307801.
- Skelton DA, Kennedy J, Rutherford OM. Explosive power and asymmetry in leg muscle function in frequent fallers and non-fallers aged over 65. Age Ageing 2002;31:119-25. http://dx.doi.org/10.1093/ageing/31.2.119.
- Iliffe S, Kendrick D, Morris R, Skelton D, Gage H, Dinan S, et al. Multi-centre cluster randomised trial comparing a community group exercise programme with home based exercise with usual care for people aged 65 and over in primary care: protocol of the ProAct 65+ trial. Trials 2010;11. http://dx.doi.org/10.1186/1745-6215-11-6.
- CONSORT . CONSORT Flow Diagram n.d. www.consort-statement.org/consort-statement/flow-diagram (accessed 23 October 2013).
- Curran HV, Collins R, Fletcher S, Kee SC, Woods B, Iliffe S. Older adults and withdrawal from benzodiazepine hypnotics in general practice: effects on cognitive function, sleep, mood and quality of life. Psychol Med 2003;33:1223-37. http://dx.doi.org/10.1017/S0033291703008213.
- Downs M, Turner S, Bryans M, Wilcock J, Keady J, Levin E, et al. Effectiveness of educational interventions in improving detection and management of dementia in primary care: cluster randomised controlled study. BMJ 2006;332:692-6. http://dx.doi.org/10.1136/bmj.332.7543.692.
- Wilcock J, Bryans M, Turner S, O’Carroll R, Keady J, Levin E, et al. Methodological problems in dementia research in primary care: a case study of a randomized controlled trial. Prim Health Care Res Dev 2007;8:12-21. http://dx.doi.org/10.1017/S1463423607000035.
- Lawton BA, Rose SB, Elley CR, Dowell AC, Fenton A, Moyes SA. Exercise on prescription for women aged 40–74 recruited through primary care: two year randomised controlled trial. BMJ 2008;337. http://dx.doi.org/10.1136/bmj.a2509.
- Gray A, Rivero-Arias O, Clarke PM. Estimating the association between SF-12 responses and EQ-5D utility values by response mapping. Med Decis Making 2006;26:18-29. http://dx.doi.org/10.1177/0272989X05284108.
- Freeman MA, Dean MR, Hanham IW. The etiology and prevention of functional instability of the foot. J Bone Joint Surg Br 1965;47–B:678-85.
- Podsiadlo DA, Richardson S. The timed ‘Up and Go’: a test of basic functional mobility for frail elder persons. J Am Geriatr Soc 1991;39:142-8.
- Duncan PW, Weiner DK, Chandler J, Studenski S. Functional reach: a new clinical measure of balance. J Gerontol 1990;45:M192-7. http://dx.doi.org/10.1093/geronj/45.6.M192.
- Rikli RE, Jones CJ. Functional fitness normative scores for community-residing older adults aged 60–94. J Aging Phys Act 1999;7:162-81.
- Simpson JM, Worsfold C, Hawke J. Balance confidence in elderly people. The CONFbal Scale (abstract 123). Age Ageing 1998;27. http://dx.doi.org/10.1093/ageing/27.suppl_2.57-b.
- Yardley L, Beyer N, Hauer K, Kemper G, Piot-Ziegler C, Todd C. Development and initial validation of the Falls Efficacy Scale-International (FES-I). Age Ageing 2005;34:614-19. http://dx.doi.org/10.1093/ageing/afi196.
- Prochaska JO, DiClemente CC. Stages and processes of self-change of smoking: toward an integrative model of change. J Consult Clin Psychol 1983;51:390-5. http://dx.doi.org/10.1037/0022-006X.51.3.390.
- Clarke P, Eves F. Applying the transtheoretical model to the study of exercise on prescription. J Health Psychol 1997;2:195-207. http://dx.doi.org/10.1177/135910539700200216.
- Marcus BH, Selby VC, Niaura RS, Rossi JS. Self-efficacy and the stages of exercise behaviour change. Res Q Exerc Sport 1992;63:60-6. http://dx.doi.org/10.1080/02701367.1992.10607557.
- Resnik B. Reliability and validity of the Outcome Expectations for Exercise Scale-2. J Aging Phys Act 2005;13:382-94.
- Bowling A, Banister D, Sutton S, Evans O, Windsor J. A multidimensional model of the quality of life in older age. Aging Ment Health 2002;6:355-71. http://dx.doi.org/10.1080/1360786021000006983.
- Bowling A, Gabriel Z, Dykes J, Dowding LM, Evans O, Fleissig A, et al. Let’s ask them: a national survey of definitions of quality of life and its enhancement among people aged 65 and over. Int J Aging Hum Dev 2003;56:269-306. http://dx.doi.org/10.2190/BF8G-5J8L-YTRF-6404.
- Bowling A, Gabriel Z. An integrational model of quality of life in older age. A comparison of analytic and lay models of quality of life. Soc Indic Res 2004;69:1-36. http://dx.doi.org/10.1023/B:SOCI.0000032656.01524.07.
- Lubben J, Blozik E, Gillmann G, Iliffe S, von Renteln Kruse W, Beck J, et al. Performance of an abbreviated version of the Lubben Social Network Scale among three European community-dwelling older adult populations. Gerontologist 2006;46:503-13. http://dx.doi.org/10.1093/geront/46.4.503.
- Zimet GD, Dahlem NW, Zimet SG, Farley GK. The Multidimensional Scale of Perceived Social Support: a confirmation study. J Pers Assess 1988;52:30-4. http://dx.doi.org/10.1207/s15327752jpa5201_2.
- Gill DP, Jones GR, Zou GY, Speechley M. The Phone-FITT: a brief physical activity interview for older adults. J Aging Phys Act 2008;16:292-315.
- Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol 1993;46:153-62. http://dx.doi.org/10.1016/0895-4356(93)90053-4.
- Ali R, Binmore R, Dunstan S, Greer J, Matthews D, Murray L, et al. General Household Survey, Overview Report 2007.
- Yardley L, Donovan-Hall M, Francis K, Todd C. Attitudes and beliefs that predict older people’s intention to undertake strength and balance training. J Gerontol B Psychol Sci Soc Sci 2007;62:119-25. http://dx.doi.org/10.1093/geronb/62.2.P119.
- Nandy S, Parsons S, Cryer C, Underwood M, Rashbrook E, Carter Y, et al. Development and preliminary examination of the predictive validity of the Falls Risk Assessment Tool (FRAT) for use in primary care. J Public Health 2004;26:138-43. http://dx.doi.org/10.1093/pubmed/fdh132.
- Ware JE, Kosinsiki M, Keller SD. A 12-item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care 1996;34:220-33. http://dx.doi.org/10.1097/00005650-199603000-00003.
- Curtis L, Netten A. Unit Costs of Health and Social Care. Personal and Social Services Research Unit: University of Kent and LSE; 2008.
- Lipsey MW, Wilson DB. The efficacy of psychological, educational, and behavioural treatment. Confirmation from meta-analysis. Am Psychol 1993;48:1181-209. http://dx.doi.org/10.1037/0003-066X.48.12.1181.
- Roset M, Badia X, Mayo NE. Sample size calculations in studies using the EuroQol 5D. Qual Life Res 1999;8:539-49. http://dx.doi.org/10.1023/A:1008973731515.
- Elley CR, Kerse N, Arroll B, Robinson E. Effectiveness of counselling patients on physical activity in general practice: a cluster randomised controlled trial. BMJ 2003;326:793-8. http://dx.doi.org/10.1136/bmj.326.7393.793.
- Morris RW, Whincup PH, Lampe FC, Walker M, Wannamethee SG, Shaper AG. Geographic variation in incidence of coronary heart disease in Britain: the contribution of established risk factors. Heart 2001;86:277-83. http://dx.doi.org/10.1136/heart.86.3.277.
- Adams G, Gulliford MC, Ukoumunne OC, Eldridge S, Chinn S, Campbell MJ. Patterns of intra-cluster correlation from primary care research to inform study design and analysis. J Clin Epidemiol 2004;57:785-94. http://dx.doi.org/10.1016/j.jclinepi.2003.12.013.
- Office for National Statistics . National Statistics Online n.d. www.statistics.gov.uk/cci/nugget.asp?ID=949 (accessed May 2014).
- Pocock SJ. Clinical Trials: A Practical Approach. Chichester: John Wiley & Sons; 1983.
- Noble M, Wright G, Dibben C, Smith GAN, McLennan D, Anttila C, et al. The English Indices of Deprivation 2004.
- Evans SM, Royston P, Day S. Minim: Allocation by Minimisation in Clinical Trials n.d. www-users.york.ac.uk/~mb55/guide/minim.htm (accessed July 2009).
- Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338. http://dx.doi.org/10.1136/bmj.b2393.
- Carpenter J, Kenward M. Multiple Imputation and its Application. Chichester: John Wiley & Sons; 2013.
- Curtis L. Unit Costs of Health and Social Care 2011 n.d. www.pssru.ac.uk/project-pages/unit-costs/2011 (accessed May 2014).
- van den Berg B, Ferrer-I-Carbonell A. Monetary valuation of informal care: the well-being valuation method. Health Econ 2007;16:1227-44. http://dx.doi.org/10.1002/hec.1224.
- Drummond MF, Sculpher MJ, Torrence GW, O’Brien BJ, Stoddart G. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Carpenter JR, Kenward MG. Birmingham: National Health Service Co-ordinating Centre for Research Methodology; 2007.
- Stevens Z, Carpenter H, Gawler S, Belcher C, Haworth D, Kendrick D, et al. Lessons learnt during a complex, multi–centre cluster randomised controlled trial: the ProAct65+ trial. Trials 2013;14. http://dx.doi.org/10.1186/1745-6215-14-192.
- Perry L, Kendrick D, Morris R, Dinan S, Masud T, Skelton D, et al. Completion and return of fall diaries varies with participants’ level of education, first language, and baseline fall risk. J Gerontol A Biol Sci Med Sci 2012;67:210-14. http://dx.doi.org/10.1093/gerona/glr175.
- Ebrahim S, Thompson PW, Baskaran V, Evans K. Randomized placebo-controlled trial of brisk walking in the prevention of postmenopausal osteoporosis. Age Ageing 1997;26:253-60. http://dx.doi.org/10.1093/ageing/26.4.253.
- Bohannon RW. Reference values for the timed up and go test: a descriptive meta-analysis. J Geriatr Phys Ther 2006;29. http://dx.doi.org/10.1519/00139143-200608000-00004.
- Stanley MA, Beck JG, Zebb BJ. Psychometric properties of the MSPSS in older adults. Aging Mental Health 1998;2:186-93. http://dx.doi.org/10.1080/13607869856669.
- Bowling A. The Psychometric Properties of the Older People’s Quality of Life Questionnaire, Compared with the CASP-19 and the WHOQOL-OLD. Curr Gerontol Geriatr Res 2009. http://dx.doi.org/10.1155/2009/298950.
- Stevens Z, Barlow C, Kendrick D, Masud T, Skelton DA, Dinan-Young S, et al. Effectiveness of general practice-based exercise promotion for older adults: a systematic review. Prim Health Care Res Dev 2013;15:190-201. http://dx.doi.org/10.1017/S1463423613000017.
- Goldstein MG, Pinto BM, Marcus BH, Lynn H, Jette AM, Rakowski W, et al. Physician-based physical activity counseling for middle-aged and older adults: a randomized trial. Ann Behav Med 1999;21:40-7. http://dx.doi.org/10.1007/BF02895032.
- Halbert JA, Silagy CA, Finucane PM, Withers RT, Hamdorf PA. Physical activity and cardiovascular risk factors: effect of advice from an exercise specialist in Australian general practice. Med J Aust 2000;173:84-7.
- Petrella RJ, Koval JJ, Cunningham DA, Paterson DH. Can primary care doctors prescribe exercise to improve fitness? The Step Test Exercise Prescription (STEP) project. Am J Prev Med 2003;24:316-22. http://dx.doi.org/10.1016/S0749-3797(03)00022-9.
- Kerse N, Elley CR, Robinson E, Arroll B. Is physical activity counseling effective for older people? A cluster randomized, controlled trial in primary care. J Am Geriatr Soc 2005;53:1951-6. http://dx.doi.org/10.1111/j.1532-5415.2005.00466.x.
- Harrison RA, Roberts C, Elton PJ. Does primary care referral to an exercise programme increase physical activity one year later? A randomized controlled trial. J Public Health (Oxf) 2005;27:25-32. http://dx.doi.org/10.1093/pubmed/fdh197.
- Kolt GS, Schofield GM, Kerse N, Garrett N, Oliver M. Effect of telephone counseling on physical activity for low-active older people in primary care: a randomized, controlled trial. J Am Geriatr Soc 2007;55:986-92. http://dx.doi.org/10.1111/j.1532-5415.2007.01203.x.
- Glasgow RE, Vogt TM, Boles SM. Evaluating the public health impact of health promotion interventions: the RE-AIM framework. Am J Public Health 1999;89:1322-7. http://dx.doi.org/10.2105/AJPH.89.9.1322.
- Dyrstad SM, Hansen BH, Holme IM, Anderssen SA. Comparison of self-reported versus accelerometer-measured physical activity. Med Sci Sports Exerc 2013 n.d.;46:99-106. http://dx.doi.org/10.1249/MSS.0b013e3182a0595f.
- Gulsvik AK, Thelle DS, Samuelsen SO, Myrstad M, Mowe M, Wyller T. Ageing, physical activity and mortality – a 42 year follow-up study. Int J Epidemiol 2012;41:521-30. http://dx.doi.org/10.1093/ije/dyr205.
- Celis-Morales CA, Perez-Bravo F, Ibañez L, Salas C, Bailey MES, Gill JMR. Objective vs. self-reported physical activity and sedentary time: effects of measurement method on relationships with risk biomarkers. PLoS One 2012;7. http://dx.doi.org/10.1371/journal.pone.0036345.
- Shephard RJ, Vuillemin A. Limits to the measurement of habitual physical activity by questionnaires. Br J Sports Med 2003;37:197-206. http://dx.doi.org/10.1136/bjsm.37.3.197.
- Young DR, Masaki KH, Curb JD. Associations of physical activity with performance-based and self-reported physical function in older men: the Honolulu Heart Program. J Am Geriatr Soc 1995;43:845-54.
- Eaton CB, Medalie JH, Flocke SA, Zyzanski SJ, Yaari S, Goldbourt U. Self-reported physical activity predicts long-term coronary heart disease and all-cause mortalities: Twenty-one-year follow-up of the Israeli Ischaemic Heart Disease study. Arch Fam Med 1995;4:323-9. http://dx.doi.org/10.1001/archfami.4.4.323.
- Kurtze N, Rangul V, Hustvedt BE, Flanders WD. Reliability and validity of self-reported physical activity in the Nord-Trøndelag Health Study: HUNT 1. Scand J Public Health 2008;36:52-61. http://dx.doi.org/10.1177/1403494807085373.
- Aadahl M, Kjaer M, Kristensen JH, Mollerup B, Jørgensen T. Self-reported physical activity compared with maximal oxygen uptake in adults. Eur J Prev Cardiol 2007;14:422-8. http://dx.doi.org/10.1097/HJR.0b013e3280128d00.
- Adams SA, Matthews CE, Ebbeling CB, Moore CG, Cunningham JE, Fulton J, et al. The effect of social desirability and social approval on self-reports of physical activity. Am J Epidemiol 2005;161:389-98. http://dx.doi.org/10.1093/aje/kwi054.
- Visser M, Brychta RJ, Chen KY, Koster A. Self-reported adherence to the physical activity recommendation and determinants of misperception in older adults. J Aging Phys Act 2013;22:226-34. http://dx.doi.org/10.1123/JAPA.2012-0219.
- Atienza AA, Moser RP, Perna F, Dodd K, Ballard-Barbash R, Troiano PP, et al. Self-reported and objectively measured activity related to biomarkers using NHANES. Med Sci Sports Exerc 2011;43:815-21. http://dx.doi.org/10.1249/MSS.0b013e3181fdfc32.
- Sun F, Norman IJ, While A. Physical activity in older people: a systematic review. BMC Public Health n.d.;13. http://dx.doi.org/10.1186/1471-2458-13-449.
- Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act 2008;5. http://dx.doi.org/10.1186/1479-5868-5-56.
- Davey R, Edwards SM, Cochrane T. Recruitment strategies for a clinical trial of community-based water therapy for osteoarthritis. Br J Gen Pract 2003;53:315-17.
- Campbell MK, Snowdon C, Francis D, Elbourne D, McDonald AM, Knight R, et al. Recruitment to randomised trials: strategies for trial enrollment and participation study. The STEPS study. Health Technol Assess 2007;11:ix-105.
- Skoro-Kondza L, See Tai S, Gadelrab R, Drincevic D, Greenhalgh T. Community based yoga classes for type 2 diabetes: an exploratory randomised controlled trial. BMC Health Serv Res 2009;9. http://dx.doi.org/10.1186/1472-6963-9-33.
- Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R. Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol 1999;52:1143-56. http://dx.doi.org/10.1016/S0895-4356(99)00141-9.
- Hillsdon M, Foster C, Thorogood M. Interventions for promoting physical activity. Cochrane Database Syst Rev 2005;1.
- National Evaluation of Partnerships for Older People Projects. London: PSSRU; 2009.
- Hooker SP, Seavey W, Weidmer CE, Harvey DJ, Stewart AL, Gillis DE, et al. The California active aging community grant program: translating science into practice to promote physical activity in older adults. Ann Behav Med 2005;29:155-65. http://dx.doi.org/10.1207/s15324796abm2903_1.
- Murphy CA, Cupples ME, Percy A, Halliday HL, Stewart MC. Peer-mentoring for first-time mothers from areas of socio-economic disadvantage: a qualitative study within a randomised controlled trial. BMC Health Serv Res 2008;8. http://dx.doi.org/10.1186/1472-6963-8-46.
- Dale J, Caramlau I, Sturt J, Friede T, Walker R. Telephone peer-delivered intervention for diabetes motivation and support: the telecare exploratory RCT. Patient Educ Couns 2009;75:91-8. http://dx.doi.org/10.1016/j.pec.2008.09.014.
- Carroll C, Patterson S, Wood S, Booth A, Rick J, Balain S. A conceptual framework for implementation fidelity. Implement Sci 2007;2. http://dx.doi.org/10.1186/1748-5908-2-40.
- Resnick B, Inguito P, Orwig D, Yahiro JY, Hawkes W, Werner M, et al. Treatment fidelity in behavior change research: a case example. Nurs Res 2005;54:139-43. http://dx.doi.org/10.1097/00006199-200503000-00010.
- Davis LL, Broome ME, Cox RP. Maximizing retention in community-based clinical trials. J Nurs Scholarsh 2002;34:47-53. http://dx.doi.org/10.1111/j.1547-5069.2002.00047.x.
- Patel M, Doku V, Tennakoon L. Challenges in recruitment of research participants. Adv Psychiatr Treat 2013;19:229-38.
- Motzer SA, Moseley JR, Lewis FM. Recruitment and retention of families in clinical trials with longitudinal designs. West J Nurs Res 1997;19:314-33. http://dx.doi.org/10.1177/019394599701900304.
- Foy R, Parry J, Duggan A, Delaney B, Wilson S, Lewin-Van Den Broek NT, et al. How evidence based are recruitment strategies to randomized controlled trials in primary care? Experience from seven studies. Fam Pract 2003;20:83-92. http://dx.doi.org/10.1093/fampra/20.1.83.
- Peterson JC, Pirraglia PA, Wells MT, Charlson ME. Attrition in longitudinal randomized controlled trials: home visits make a difference. BMC Med Res Methodol 2012;12. http://dx.doi.org/10.1186/1471-2288-12-178.
- Williamson MK, Pirkis J, Pfaff JJ, Tyson O, Sim M, Kerse N, et al. Recruiting and retaining GPs and patients in intervention studies: the DEPS-GP project as a case study. BMC Med Res Methodol 2007;7. http://dx.doi.org/10.1186/1471-2288-7-42.
Appendix 1 ProAct65+ adverse event report form (from Chapter 2)
Appendix 2 Primary outcome – modelling physical activity (from Chapter 5)
| Baseline CHAMPS | Baseline Phone-FITT | |||||
|---|---|---|---|---|---|---|
| Usual care | FaME | OEP | Usual care | FaME | OEP | |
| Those with a Phone-FITT and CHAMPS recorded at 12 months post intervention | ||||||
| Mean | 216.43 | 182.06 | 220.24 | 38.99 | 39.60 | 42.87 |
| SD | 241.25 | 222.54 | 252.27 | 11.31 | 13.19 | 12.99 |
| Number | 168 | 153 | 164 | 167 | 150 | 160 |
| Those with a Phone-FITT but no CHAMPS recorded at 12 months post intervention | ||||||
| Mean | 174.42 | 186.75 | 170.16 | 38.54 | 34.80 | 41.52 |
| SD | 342.85 | 252.53 | 198.24 | 18.01 | 9.93 | 14.24 |
| Number | 43 | 40 | 61 | 42 | 43 | 66 |
| Those with neither a Phone-FITT nor a CHAMPS recorded at 12 months post intervention | ||||||
| Mean | 130.61 | 152.67 | 149.76 | 33.67 | 35.62 | 39.01 |
| SD | 197.02 | 255.98 | 210.87 | 13.46 | 15.23 | 12.72 |
| Number | 147 | 118 | 123 | 129 | 94 | 116 |
| Log-baseline CHAMPS | |||
|---|---|---|---|
| Usual care | FaME | OEP | |
| Those with a Phone-FITT and CHAMPS recorded at 12 months post intervention | |||
| Mean | 4.254 | 3.915 | 4.309 |
| SD | 2.166 | 2.310 | 2.089 |
| Number | 168 | 153 | 164 |
| Those with a Phone-FITT but no CHAMPS recorded at 12 months post intervention | |||
| Mean | 3.302 | 3.490 | 4.099 |
| SD | 2.496 | 2.595 | 2.030 |
| Number | 43 | 40 | 61 |
| Those with neither a Phone-FITT nor a CHAMPS recorded at 12 months post intervention | |||
| Mean | 2.910 | 3.241 | 3.317 |
| SD | 2.554 | 2.508 | 2.514 |
| Number | 147 | 118 | 123 |
| FaME | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Adherence | CHAMPS minutes of moderate or greater intensity activity (per week) 12 months post intervention | Log-CHAMPS minutes of moderate or greater intensity activity (per week) 12 months post intervention | Multilevel modelling log-CHAMPS (effect of adherent vs. not) | ||||||
| ≥ 75% of total expected activity (1 × 60 minutes’ class exercise plus 2 × 30 minutes’ home exercise per week for 24 weeks = total 2880 minutes). Adherent if ≥ 2160 minutes’ total exercise. (Assumes no diary data = 0 minutes) | |||||||||
| Number (%) | Mean | SD | n | Mean | SD | n | Estimate | 0.109 | |
| No | 321 (82.95) | 199.85 | 207.68 | 136 | 4.219 | 2.109 | 136 | 95% CI | –0.394 to 0.613 |
| Yes | 66 (17.05) | 266.58 | 279.95 | 57 | 4.605 | 2.004 | 57 | p-value | 0.670 |
| Number | 184 | ||||||||
| ≥ 75% of total expected activity (1 × 60 minutes’ class exercise plus 2 × 30 minutes’ home exercise per week for 24 weeks = total 2880 minutes). Adherent if ≥ 2160 minutes’ total exercise. (Only if all six diaries completed) | |||||||||
| Number (%) | Mean | SD | n | Mean | SD | n | Estimate | 0.017 | |
| No | 130 (69.15) | 217.80 | 205.29 | 100 | 4.527 | 1.896 | 100 | 95% CI | –0.492 to 0.525 |
| Yes | 58 (30.85) | 270.87 | 289.62 | 52 | 4.620 | 1.978 | 52 | p-value | 0.949 |
| Number | 145 | ||||||||
| OEP | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Adherence | CHAMPS minutes of moderate or greater intensity activity (per week) 12 months post intervention | Log-CHAMPS minutes of moderate or greater intensity activity (per week) 12 months post intervention | Multilevel modelling log-CHAMPS (effect of adherent vs. not) | ||||||
| ≥ 75% of total expected activity (3 × 30 minutes’ home exercise per week for 24 weeks = total 2160 minutes). Adherent if ≥ 1620 minutes’ total exercise. (Assumes no diary data = 0 minutes) | |||||||||
| Number (%) | Mean | SD | n | Mean | SD | n | Estimate | –0.192 | |
| No | 307 (74.88) | 231.29 | 371.24 | 105 | 4.010 | 2.366 | 105 | 95% CI | –0.801 to 0.417 |
| Yes | 103 (25.12) | 223.69 | 296.75 | 80 | 4.294 | 2.053 | 80 | p-value | 0.537 |
| Number | 178 | ||||||||
| ≥ 75% of total expected activity (3 × 30 minutes’ home exercise per week for 24 weeks = total 2160 minutes). Adherent if ≥ 1620 minutes’ total exercise. (Only if all six diaries completed) | |||||||||
| Number (%) | Mean | SD | n | Mean | SD | n | Estimate | –0.271 | |
| No | 108 (54.00) | 235.25 | 390.78 | 79 | 4.117 | 2.279 | 79 | 95% CI | –0.935 to 0.393 |
| Yes | 92 (46.00) | 217.30 | 296.22 | 74 | 4.219 | 2.096 | 74 | p-value | 0.423 |
| Number | 148 | ||||||||
| PM allocated. (N.B. no information about PM allocation for n = 44) | |||||||||
| Number (%) | Mean | SD | n | Mean | SD | n | Estimate | 0.364 | |
| No | 222 (60.66) | 246.77 | 392.06 | 113 | 4.175 | 2.228 | 113 | 95% CI | –0.388 to 1.116 |
| Yes | 144 (39.34) | 201.43 | 239.11 | 70 | 4.059 | 2.288 | 70 | p-value | 0.343 |
| Number | 176 | ||||||||
Appendix 3 Adverse reactions (from Chapter 5)
Description of all ARs (i.e. events related to the trial) occurring during the ProAct65+ trial.
‘High knee standing’ exercises made patient’s lower-back ache.
Aching pain in calf.
Cold pain in stomach and bad taste in mouth, started after first FaME class. Patient said she would see her GP.
Patient called to say the FaME exercises ‘upset his metabolism’.
Patient feels soreness in muscles and joints.
Patient fell in FaME class, no injuries, potentially as a result of adjusting to new glasses.
Potentially pulled back muscle after doing sit down weights at gym.
Pulled calf muscle while doing our home exercise session ex 3. Sore for 2 days but did not affect normal activities.
Was out power walking and pulled a muscle; did not seek medical attention; rested and massaged leg.
Fell when in FaME class, taken to A&E, let go and in evening said was OK.
Pulled muscle.
Plantar fasciitis started about a year ago and has been getting worse, (first had it 20 years ago), got worse when started doing more walking to improve health. Recently visited podiatrist/foot surgeon (private) for a cortisone injection into left heel.
Pulled muscle.
Knee pains so didn’t attended classes.
History of intermittent knee pains past 2 years assumed osteoarthritis – no medical diagnosis. November 2011, aches in knees. Stopped leg weights exercise. Started again January 2012 and again experienced pain in knees. Still does other exercise tai chi and keep-fit class.
Began to have more back pain from approximately March 2011. Doing our home exercise made it worse. Saw podiatrist then GP who diagnosed Sciatica. Given painkillers. Pain has been on and off for several years.
Burst blood vessel in leg after using Otago weights.
Hurt left wrist/fingertips after using Otago weights to strengthen arms. Ointment prescribed, wrists/fingertips recovered.
Pain in small of back after Otago training.
Plantar fasciitis – started after Otago exercises.
Potentially pulled ligament in right knee.
Patient reported in diary that they had slight hip problem; during follow-up telephone call patient stated that ‘walking backwards during home exercises’ had aggravated hip; OK now stopped exercises.
Patient withdrew from study exercises because of pains in right hip exacerbated by exercise; has sciatic pain – intermittent and ongoing. Also commented in OEP evaluation forms of general health decline and hip and sciatica problems.
Suspected pulled muscle in right foot after Otago training.
Swelling of varicose vein on right leg after using leg weights. Also commented on OEP evaluation forms.
While walking a lot on holiday patient hurt back, patient puts it down to a disc problem in the past and will rest it, will not see the doctor as it is a reoccurrence and just needs rest.
Decided to cycle not walk for exercise. Went up hill and got a hernia. About to go in for operation.
Keyhole surgery on knee.
Left knee/groin pain after doing ankle exercises with weights.
Pain in leg from exercises.
Pulled knee ligaments when doing exercises with weight. Right knee, recently replaced. Saw GP about it. No long-term damage. Is continuing without weights.
Pulled muscle in back.
Pulled muscle in leg after exercising.
Bad knees for years. Advised to see GP.
Hip pain diagnosed with arthritis, patient thinks caused by weights exercise.
Left knee pain after exercises with 2-kg weights on.
Pain behind knee – better now after stopping exercising with weights.
Pain when walking, thinks as a result of OEP training and her existing osteoarthritis.
Sciatic worsened after OEP training.
Sciatica worsened.
Pain in shoulder while using ‘shake weight’.
Worsened pain in knees and lower back. X-ray came back fine; patient said her knees and back were starting to feel better.
Appendix 4 Secondary outcomes (from Chapter 6)
| Measure | Follow-up | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post intervention | 6 months post intervention | 12 months post intervention | 18 months post intervention | |||||||||||
| Randomisation group | Randomisation group | Randomisation group | Randomisation group | Randomisation group | |||||||||||
| Usual care | FaME | OEP | Usual care | FaME | OEP | Usual care | FaME | OEP | Usual care | FaME | OEP | Usual care | FaME | OEP | |
| CHAMPS total calories per week | |||||||||||||||
| Mean | 2222.27 | 2129.06 | 2314.04 | 2662.37 | 2704.59 | 2450.67 | 2444.28 | 2459.29 | 2534.42 | 2573.63 | 2660.90 | 2787.53 | 2374.87 | 2639.04 | 2739.06 |
| Median | 1713.60 | 1690.56 | 1782.20 | 1897.68 | 2191.54 | 2029.00 | 1950.12 | 1919.41 | 1890.24 | 1829.36 | 2079.80 | 2004.08 | 1811.25 | 1979.79 | 2077.62 |
| SD | 2180.93 | 2009.50 | 2009.83 | 2620.97 | 2212.64 | 1891.44 | 2149.83 | 2180.54 | 2154.03 | 2158.80 | 2247.96 | 2771.58 | 2016.37 | 2460.90 | 2382.97 |
| n | 391 | 339 | 354 | 261 | 224 | 220 | 240 | 195 | 192 | 221 | 192 | 184 | 219 | 180 | 178 |
| PASE total score | |||||||||||||||
| Mean | 119.19 | 109.11 | 119.85 | 130.12 | 128.08 | 124.82 | 124.70 | 120.39 | 125.36 | 122.52 | 124.18 | 126.75 | 119.92 | 118.46 | 125.81 |
| SD | 60.42 | 52.21 | 50.60 | 53.12 | 51.15 | 51.42 | 56.03 | 59.88 | 54.13 | 51.81 | 53.34 | 61.29 | 52.06 | 52.17 | 60.18 |
| n | 400 | 342 | 362 | 264 | 224 | 224 | 242 | 195 | 194 | 222 | 193 | 185 | 221 | 181 | 179 |
| Phone-FITT total score | |||||||||||||||
| Mean | 36.80 | 37.68 | 41.18 | 46.56 | 48.32 | 48.39 | 47.87 | 47.93 | 49.01 | 47.71 | 49.52 | 49.38 | 47.87 | 48.59 | 47.95 |
| SD | 13.65 | 13.67 | 13.11 | 16.52 | 14.53 | 14.92 | 15.93 | 15.18 | 16.36 | 17.41 | 15.95 | 16.50 | 17.58 | 14.99 | 18.18 |
| n | 377 | 316 | 354 | 255 | 214 | 259 | 260 | 218 | 245 | 225 | 208 | 237 | 238 | 202 | 221 |
FIGURE 12.
Line graph to show means of total calorie expenditure by time and intervention arm.
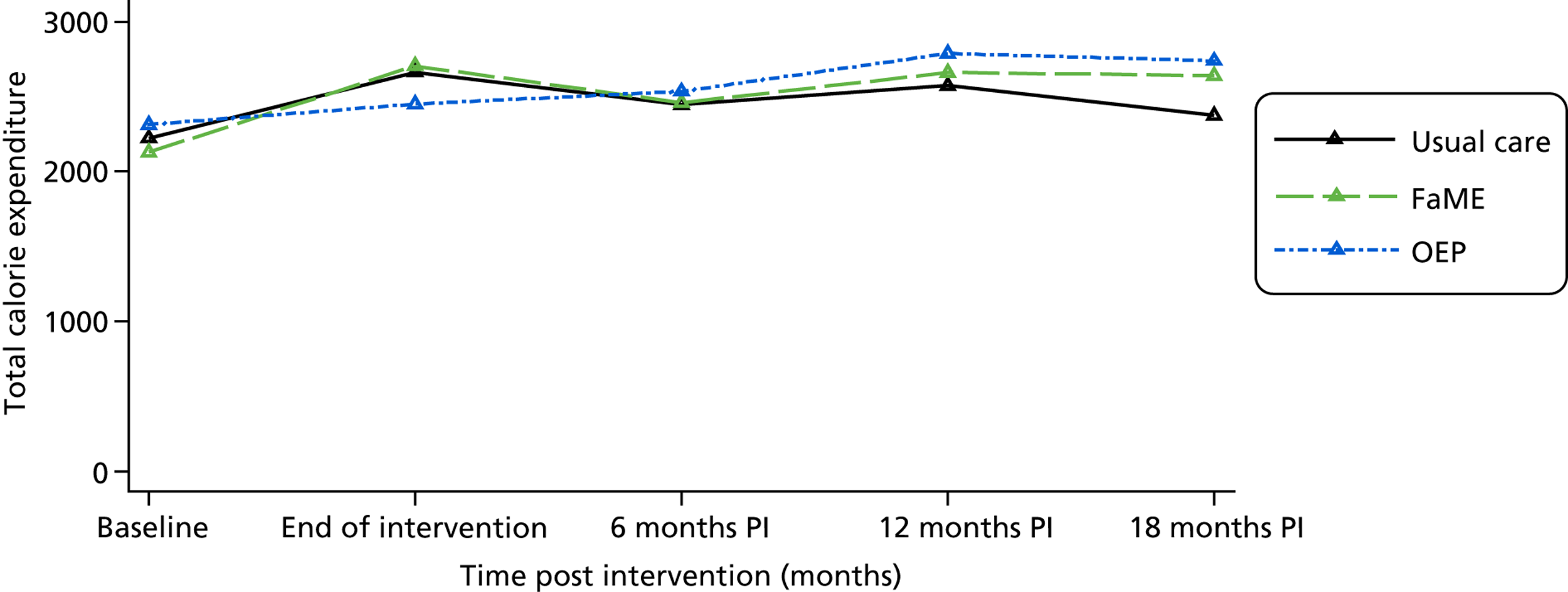
FIGURE 13.
Line graph to show means of PASE score by time and intervention arm.
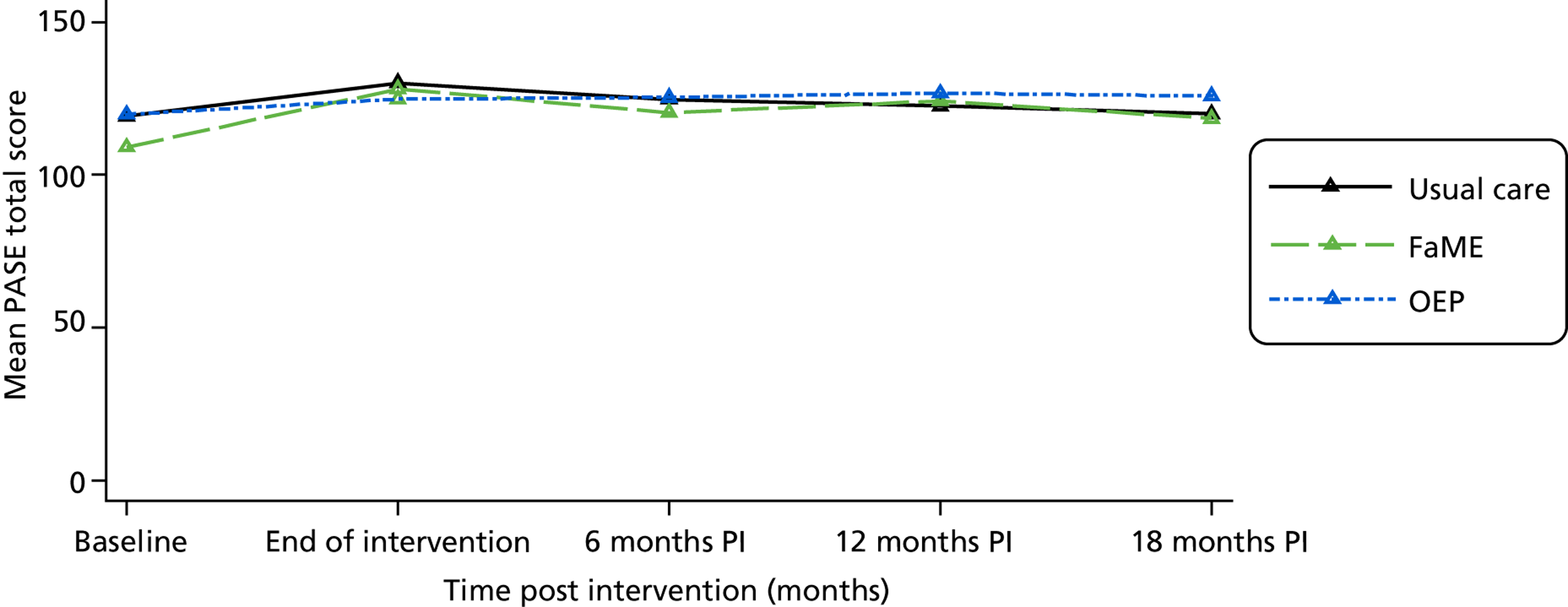
FIGURE 14.
Line graph to show means of Phone-FITT score by time and intervention arm.
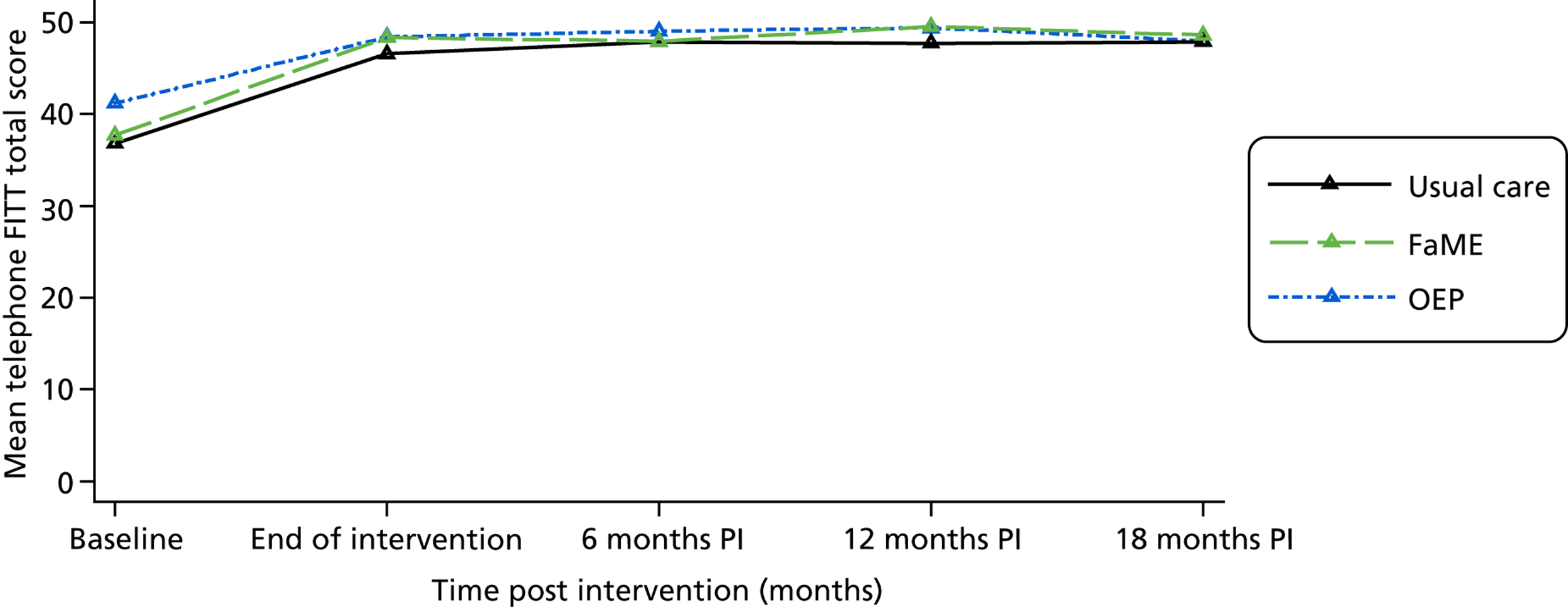
| Measure | Follow-up | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 0 months | 6 months | 12 months | 18 months | |||||||||||
| Randomisation group | Randomisation group | Randomisation group | Randomisation group | Randomisation group | |||||||||||
| Usual care | FaME | OEP | Usual care | FaME | OEP | Usual care | FaME | OEP | Usual care | FaME | OEP | Usual care | FaME | OEP | |
| FES-I total | |||||||||||||||
| Mean | 9.36 | 8.99 | 8.89 | 8.71 | 8.59 | 8.77 | 9.06 | 8.85 | 8.83 | 8.94 | 9.20 | 9.09 | 9.01 | 8.76 | 8.97 |
| Median | 8.00 | 7.00 | 7.00 | 7.00 | 7.00 | 7.00 | 7.00 | 7.00 | 7.00 | 7.00 | 7.00 | 7.00 | 7.00 | 7.00 | 7.00 |
| SD | 4.08 | 3.56 | 3.49 | 3.47 | 3.39 | 3.98 | 3.91 | 3.87 | 4.11 | 3.66 | 4.56 | 4.19 | 3.67 | 3.48 | 3.75 |
| n | 396 | 333 | 359 | 258 | 218 | 221 | 238 | 192 | 190 | 220 | 188 | 185 | 217 | 177 | 178 |
| Number ≥ 11 | 82 | 66 | 61 | 43 | 29 | 29 | 38 | 31 | 26 | 37 | 31 | 33 | 43 | 31 | 34 |
| Per cent ≥ 11 | 20.71 | 19.82 | 16.99 | 16.67 | 13.30 | 13.12 | 15.97 | 16.15 | 13.68 | 16.82 | 16.49 | 17.84 | 19.82 | 17.51 | 19.10 |
FIGURE 15.
Line graph to show means of FES-I score by time and intervention arm.
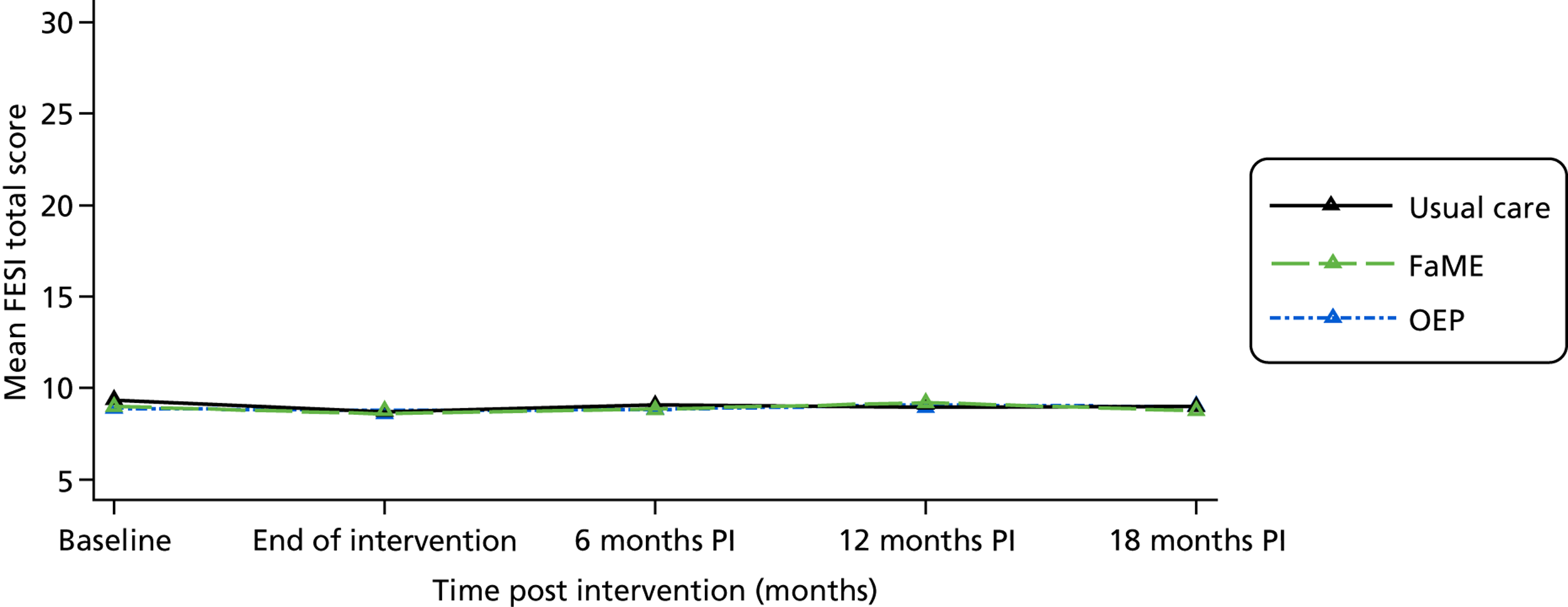
| Measure | Follow-up | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 0 months | 6 months | 12 months | 18 months | |||||||||||
| Randomisation group | Randomisation group | Randomisation group | Randomisation group | Randomisation group | |||||||||||
| Usual care | FaME | OEP | Usual care | FaME | OEP | Usual care | FaME | OEP | Usual care | FaME | OEP | Usual care | FaME | OEP | |
| SF-12 physical health component score | |||||||||||||||
| Mean | 38.73 | 38.74 | 38.78 | 40.37 | 39.64 | 40.52 | 38.68 | 39.30 | 38.83 | 39.11 | 38.85 | 39.30 | N/A | N/A | N/A |
| SD | 5.50 | 5.65 | 5.64 | 5.02 | 4.75 | 4.64 | 4.88 | 5.01 | 4.79 | 5.00 | 4.92 | 4.73 | N/A | N/A | N/A |
| n | 454 | 386 | 407 | 298 | 255 | 261 | 234 | 184 | 187 | 217 | 186 | 183 | 0 | 0 | 0 |
| SF-12 mental health component score | |||||||||||||||
| Mean | 49.88 | 49.60 | 50.15 | 49.50 | 49.91 | 49.66 | 49.20 | 48.34 | 49.62 | 49.16 | 48.74 | 49.05 | N/A | N/A | N/A |
| SD | 6.09 | 6.02 | 5.86 | 5.19 | 5.86 | 4.87 | 5.64 | 6.47 | 5.24 | 5.60 | 5.81 | 5.11 | N/A | N/A | N/A |
| n | 454 | 387 | 407 | 298 | 255 | 261 | 234 | 184 | 187 | 217 | 186 | 183 | 0 | 0 | 0 |
| OPQoL total score | |||||||||||||||
| Mean | 130.75 | 129.36 | 129.36 | 131.36 | 131.48 | 131.89 | 134.21 | 132.68 | 133.45 | 134.80 | 132.31 | 133.72 | 133.75 | 133.60 | 134.53 |
| SD | 13.53 | 13.54 | 12.69 | 16.09 | 14.56 | 13.39 | 14.51 | 15.40 | 13.65 | 14.82 | 15.98 | 14.95 | 14.99 | 14.74 | 14.07 |
| n | 342 | 273 | 312 | 237 | 190 | 199 | 206 | 158 | 156 | 185 | 169 | 156 | 183 | 152 | 154 |
| EQ-5D | |||||||||||||||
| Mean | 0.67 | 0.67 | 0.68 | 0.70 | 0.69 | 0.70 | 0.66 | 0.67 | 0.67 | 0.68 | 0.67 | 0.68 | N/A | N/A | N/A |
| SD | 0.08 | 0.09 | 0.09 | 0.07 | 0.08 | 0.07 | 0.08 | 0.08 | 0.07 | 0.07 | 0.07 | 0.07 | N/A | N/A | N/A |
| n | 450 | 380 | 399 | 296 | 255 | 258 | 225 | 178 | 184 | 212 | 179 | 176 | 0 | 0 | 0 |
FIGURE 16.
Line graph to show means of quality-of-life measures by time and intervention arm. (a) Mean SF-12 physical component score by time and group; (b) mean SF-12 mental component score by time and group; (c) mean OPQoL total score by time and group; and, (d) mean EQ-5D score by time and group.
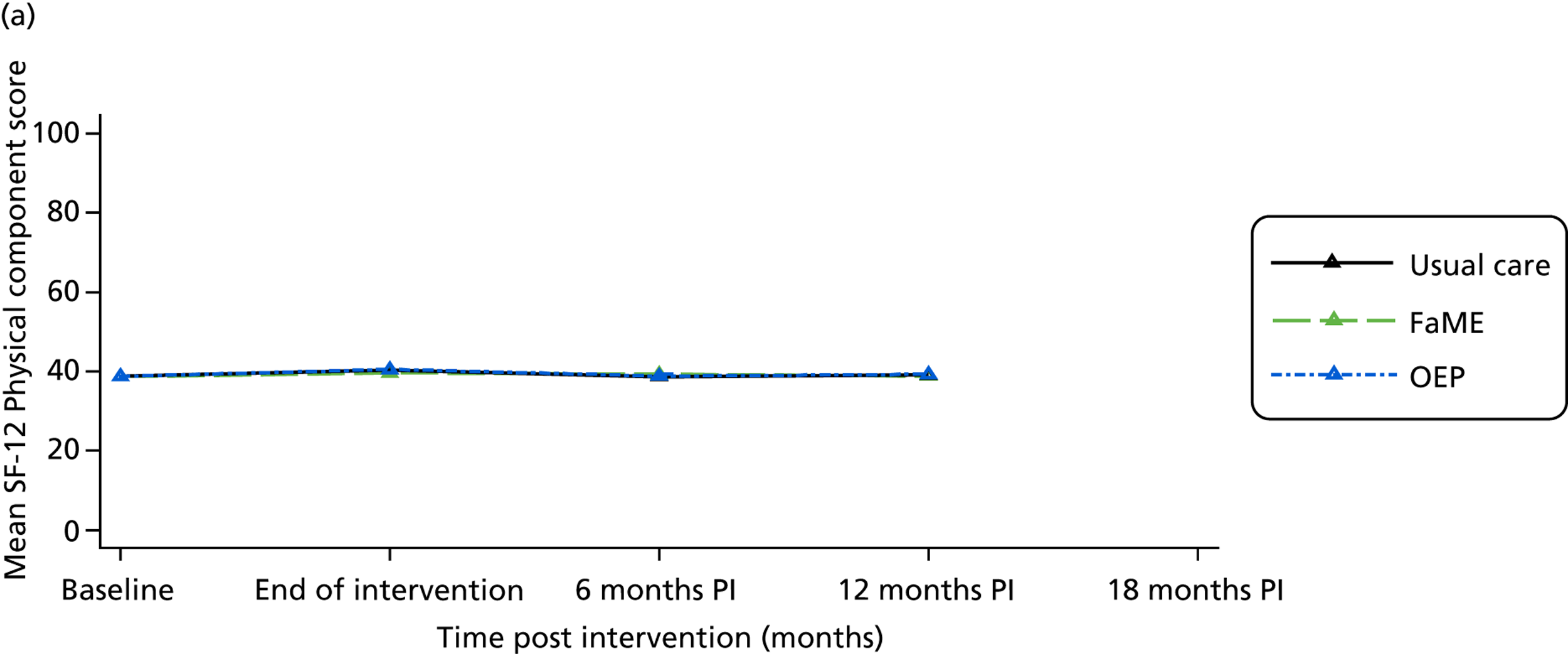
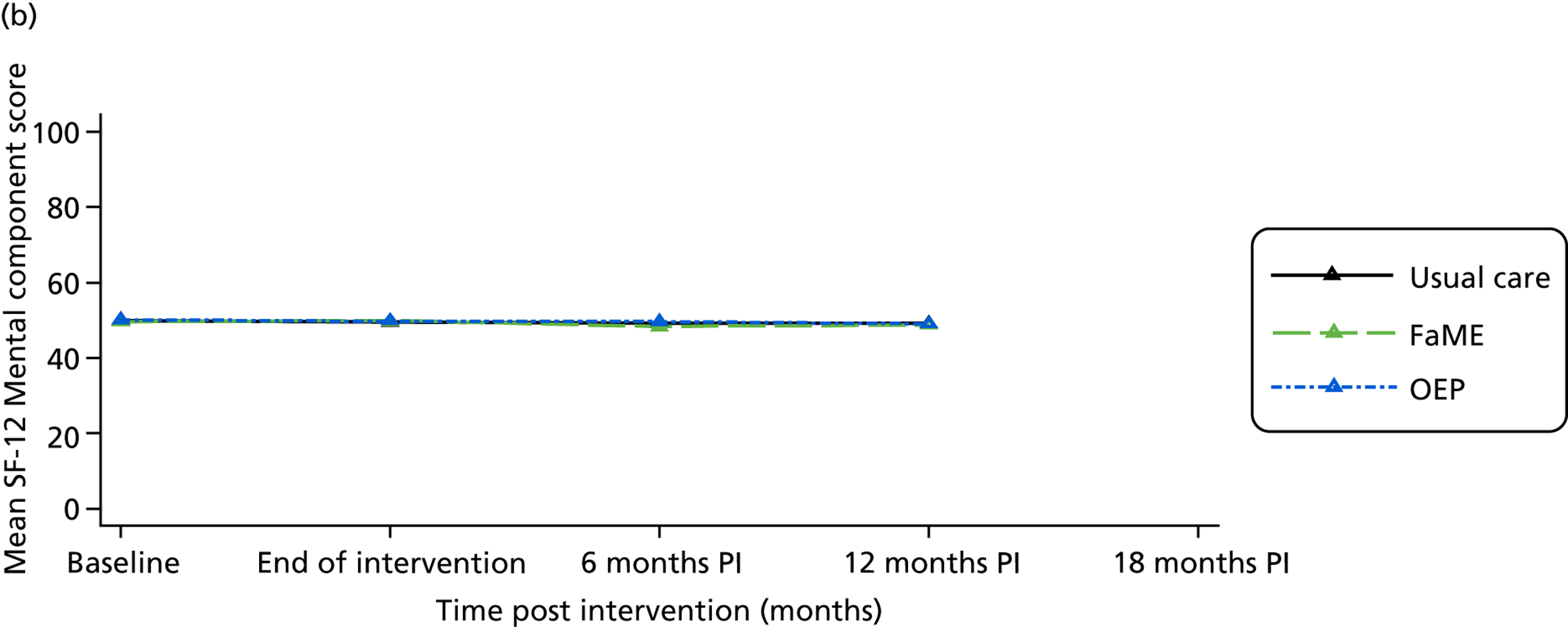
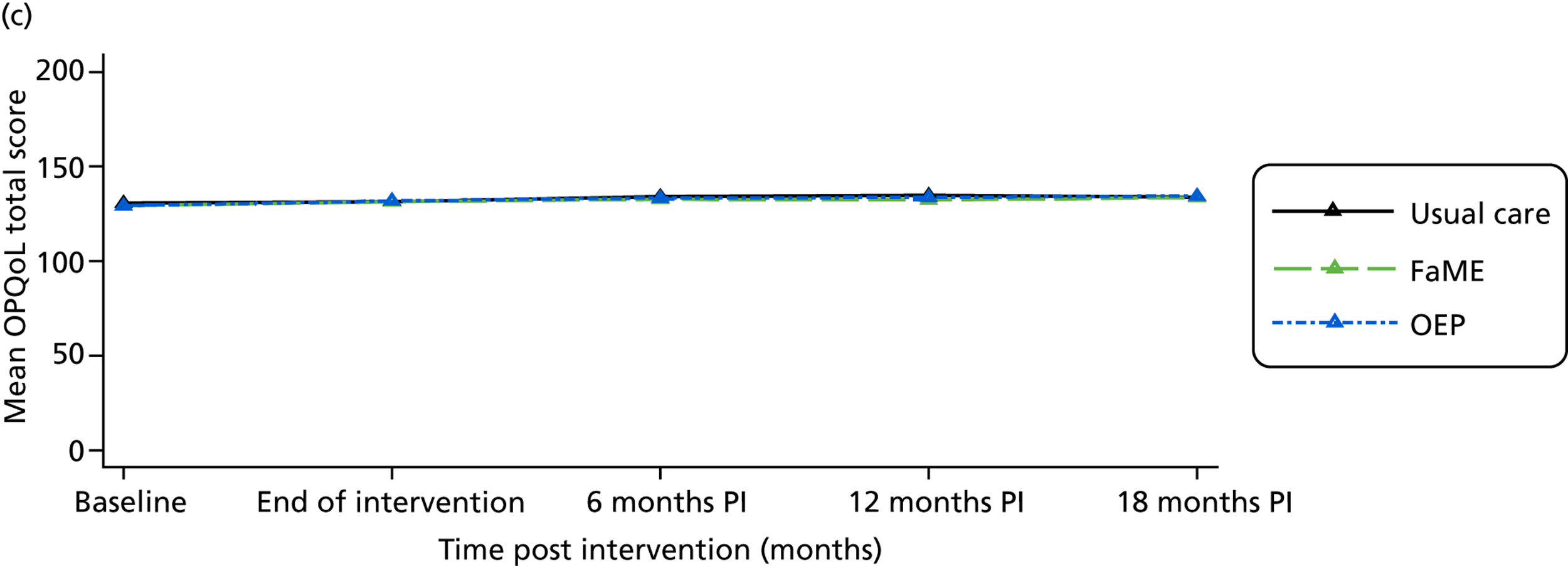
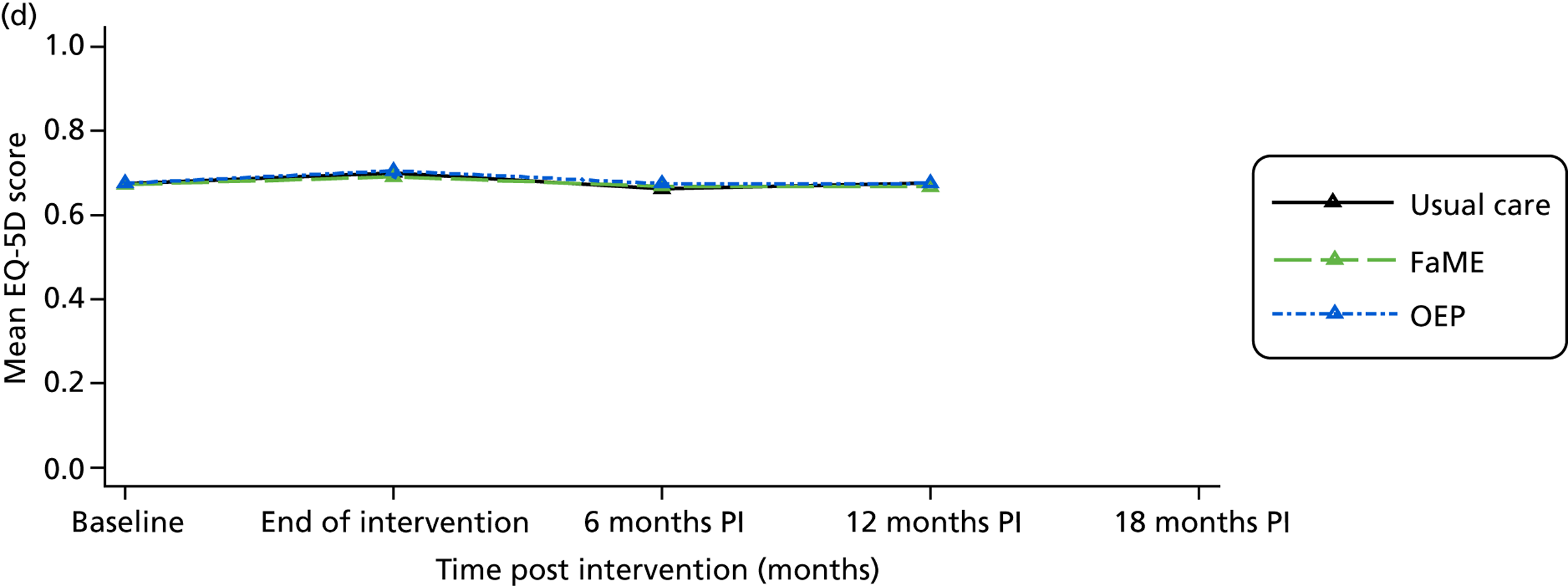
| Measure | Follow-up | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 0 months | 6 months | 12 months | 18 months | |||||||||||
| Randomisation group | Randomisation group | Randomisation group | Randomisation group | Randomisation group | |||||||||||
| Usual care | FaME | OEP | Usual care | FaME | OEP | Usual care | FaME | OEP | Usual care | FaME | OEP | Usual care | FaME | OEP | |
| ConfBal total score | |||||||||||||||
| Mean | 12.55 | 12.63 | 12.48 | 12.08 | 12.17 | 11.81 | 12.25 | 12.09 | 12.07 | 12.38 | 12.13 | 12.23 | 12.47 | 12.11 | 12.28 |
| Median | 11.00 | 10.00 | 11.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 11.00 | 10.00 | 11.00 |
| SD | 3.93 | 3.98 | 3.76 | 3.33 | 3.86 | 3.37 | 3.77 | 3.79 | 3.33 | 4.05 | 3.65 | 3.71 | 3.87 | 3.75 | 3.44 |
| n | 389 | 330 | 353 | 262 | 215 | 217 | 233 | 183 | 186 | 218 | 183 | 179 | 217 | 175 | 174 |
| Number = 10 | 188 | 169 | 172 | 144 | 127 | 122 | 125 | 103 | 95 | 114 | 97 | 93 | 104 | 97 | 85 |
| Per cent = 10 | 48.33 | 51.21 | 48.73 | 54.96 | 59.07 | 56.22 | 53.65 | 56.28 | 51.08 | 52.29 | 53.01 | 51.96 | 47.93 | 55.43 | 48.85 |
| MSPSS total | |||||||||||||||
| Mean | 65.81 | 65.93 | 66.60 | 67.95 | 65.78 | 65.43 | 65.78 | 63.79 | 65.64 | 67.23 | 63.27 | 63.46 | 66.15 | 65.57 | 65.55 |
| Median | 71.00 | 69.00 | 70.50 | 72.00 | 69.00 | 69.00 | 71.00 | 67.00 | 69.00 | 71.00 | 67.00 | 68.00 | 72.00 | 69.00 | 69.00 |
| SD | 17.96 | 15.57 | 15.49 | 15.68 | 15.05 | 16.97 | 18.21 | 17.37 | 16.74 | 16.54 | 17.69 | 18.14 | 18.04 | 15.68 | 17.58 |
| n | 375 | 305 | 330 | 243 | 202 | 210 | 224 | 175 | 181 | 209 | 183 | 171 | 211 | 173 | 166 |
| Number = 84 | 73 | 44 | 43 | 49 | 24 | 30 | 39 | 17 | 28 | 43 | 26 | 18 | 38 | 21 | 32 |
| Per cent = 84 | 19.47 | 14.43 | 13.03 | 20.16 | 11.88 | 14.29 | 17.41 | 9.71 | 15.47 | 20.57 | 14.21 | 10.53 | 18.01 | 12.14 | 19.28 |
| LSNS total | |||||||||||||||
| Mean | 15.93 | 16.47 | 15.44 | 16.23 | 15.91 | 15.84 | 16.98 | 15.91 | 16.04 | 16.41 | 15.68 | 15.43 | 16.76 | 16.18 | 15.61 |
| SD | 5.70 | 5.76 | 5.48 | 5.58 | 5.69 | 5.21 | 5.53 | 5.78 | 5.11 | 5.79 | 5.82 | 5.35 | 5.18 | 5.53 | 5.12 |
| n | 392 | 330 | 351 | 257 | 213 | 218 | 230 | 180 | 188 | 210 | 181 | 180 | 214 | 174 | 174 |
| Number ≤ 11 | 84 | 67 | 88 | 55 | 46 | 46 | 36 | 40 | 39 | 43 | 44 | 39 | 37 | 35 | 37 |
| Per cent ≤ 11 | 21.43 | 20.30 | 25.07 | 21.40 | 21.60 | 21.10 | 15.65 | 22.22 | 20.74 | 20.48 | 24.31 | 21.67 | 17.29 | 20.11 | 21.26 |
FIGURE 17.
Line graphs of other measures. (a) Mean ConfBal total score by time and group; (b) mean MSPSS total score by time and group; and, (c) mean LSNS total score by time and group.
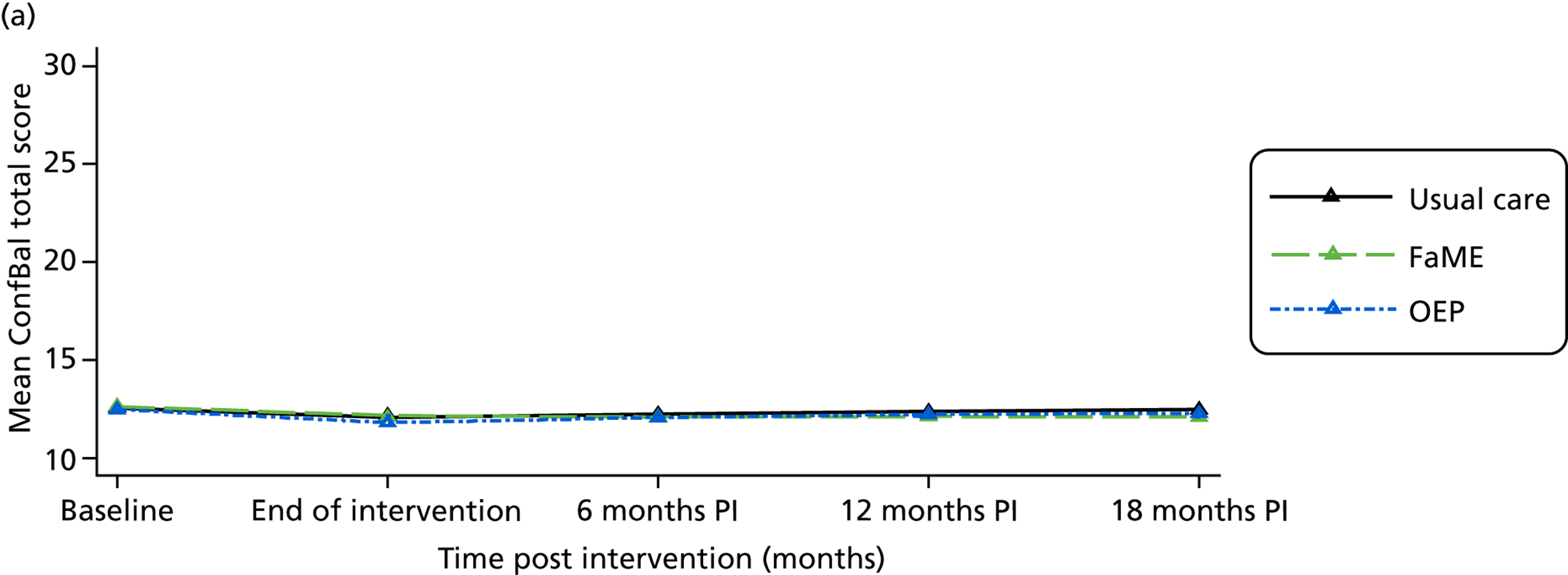
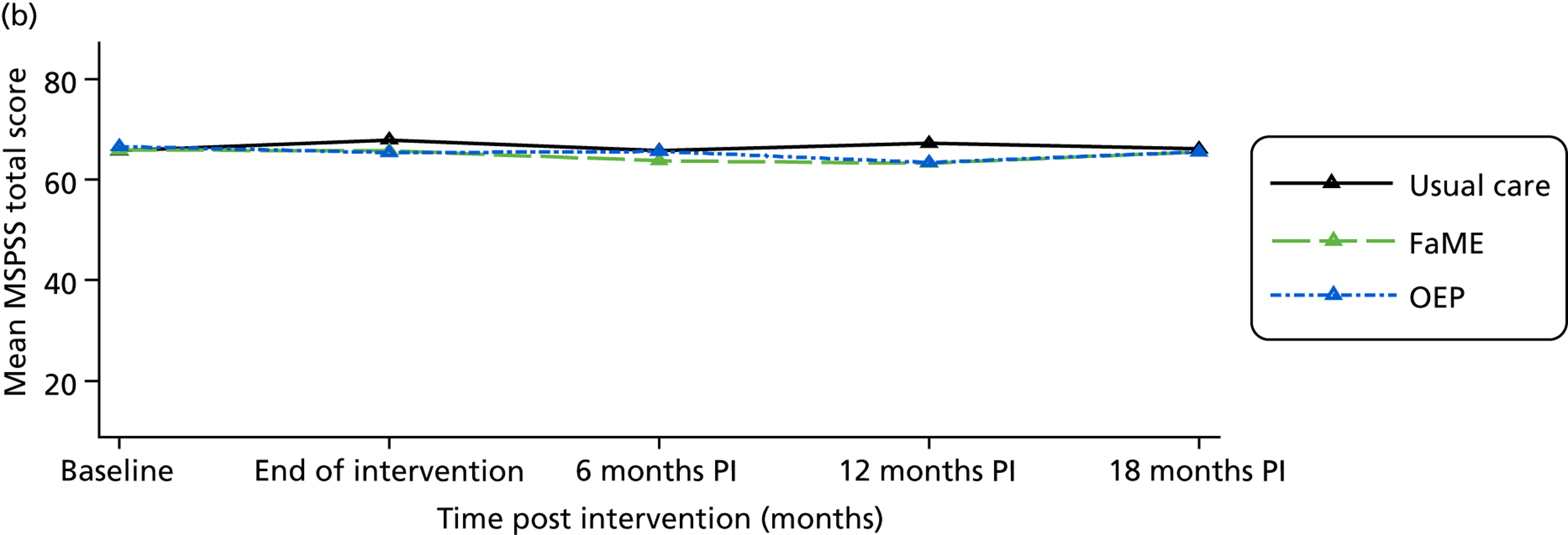
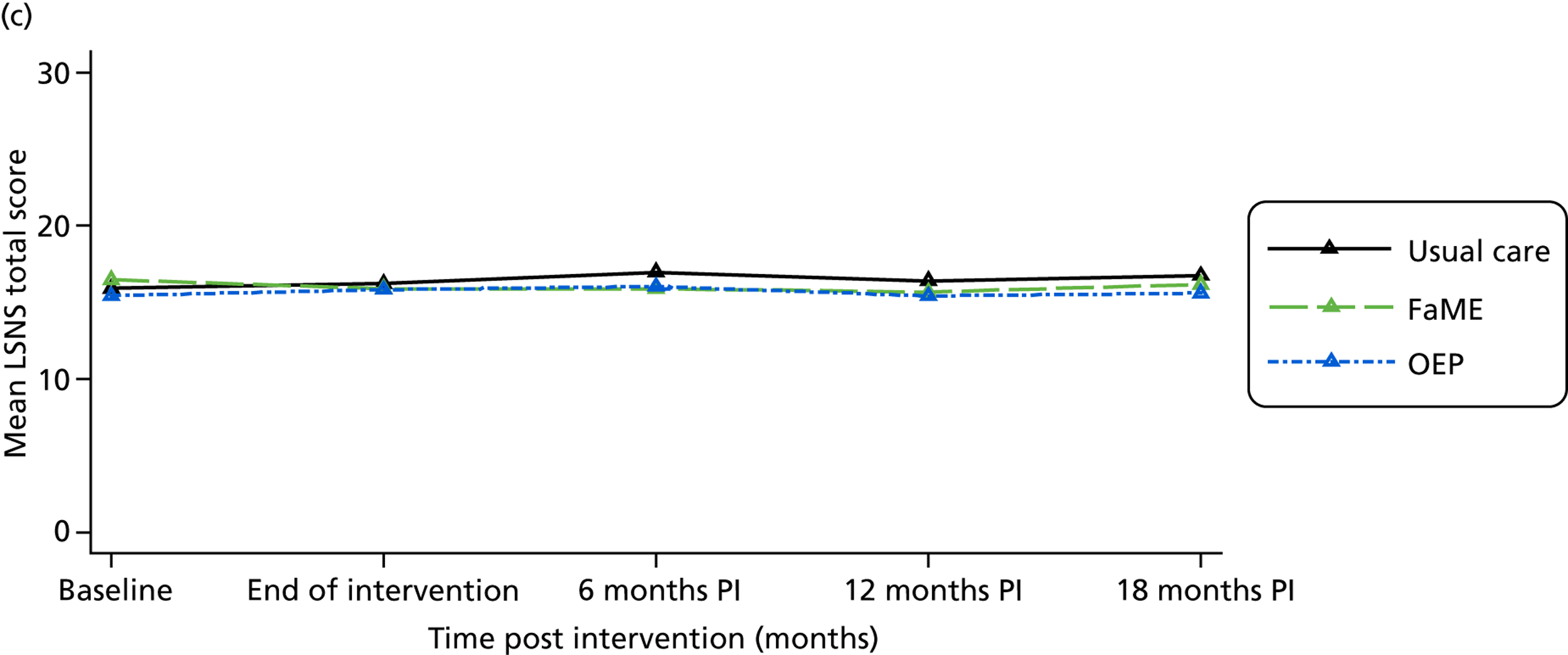
| Measure | Baseline | 0 months | ||||
|---|---|---|---|---|---|---|
| Randomisation group | Randomisation group | |||||
| Usual care | FaME | OEP | Usual care | FaME | OEP | |
| FRAT total score | ||||||
| Mean | 1.03 | 0.89 | 0.98 | 0.99 | 0.93 | 1.00 |
| SD | 0.96 | 0.90 | 0.90 | 0.90 | 0.94 | 0.95 |
| n | 453 | 383 | 402 | 299 | 253 | 263 |
| Number ≥ 1 | 298 | 232 | 264 | 196 | 157 | 170 |
| Per cent ≥ 1 | 65.78 | 60.57 | 65.67 | 65.55 | 62.06 | 64.64 |
| OEE positive | ||||||
| Mean | 3.84 | 3.85 | 3.85 | 3.85 | 4.02 | 3.93 |
| SD | 0.58 | 0.62 | 0.60 | 0.64 | 0.55 | 0.65 |
| n | 372 | 309 | 349 | 252 | 206 | 211 |
| OEE negative | ||||||
| Mean | 3.85 | 3.96 | 3.90 | 3.96 | 4.19 | 4.20 |
| SD | 0.81 | 0.75 | 0.85 | 0.87 | 0.75 | 0.71 |
| n | 367 | 320 | 339 | 248 | 204 | 203 |
| Functional reach (cm) | ||||||
| Mean | 24.68 | 25.60 | 25.57 | 27.13 | 26.99 | 26.84 |
| SD | 7.43 | 6.98 | 7.43 | 6.82 | 7.28 | 7.64 |
| n | 438 | 371 | 402 | 293 | 249 | 232 |
| Sits to stands (total) | ||||||
| Mean | 10.49 | 10.48 | 10.26 | 11.86 | 11.62 | 11.40 |
| SD | 3.31 | 3.64 | 2.81 | 3.57 | 3.77 | 3.35 |
| n | 449 | 377 | 400 | 285 | 252 | 245 |
| TUG (seconds) | ||||||
| Mean | 11.11 | 10.95 | 11.18 | 10.24 | 9.94 | 10.09 |
| Median | 9.88 | 9.63 | 9.84 | 9.30 | 9.00 | 9.30 |
| SD | 4.61 | 4.94 | 7.84 | 4.02 | 3.75 | 3.97 |
| n | 438 | 337 | 376 | 273 | 203 | 203 |
| Log-TUG (seconds) | ||||||
| Mean | 2.35 | 2.33 | 2.33 | 2.28 | 2.25 | 2.27 |
| SD | 0.32 | 0.34 | 0.34 | 0.27 | 0.30 | 0.27 |
| n | 438 | 337 | 376 | 273 | 203 | 203 |
List of abbreviations
- A&E
- accident and emergency
- AE
- adverse event
- AI
- adverse incident
- AFRIS
- Attitudes to Falls-Related Interventions Scale
- AOR
- adjusted odds ratio
- AR
- adverse reaction
- BP
- blood pressure
- CHAMPS
- Community Healthy Activities Model Program for Seniors
- CI
- confidence interval
- ConfBal
- Confidence in Balance scale
- CONSORT
- Consolidated Standards of Reporting Trials
- EQ-5D
- European Quality of Life-5 Dimensions
- FaME
- Falls Management Exercise programme
- FES-I
- Falls Efficacy Scale-International
- FRAT
- Falls Risk Assessment Tool
- GP
- general practitioner
- ICC
- intrapractice correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- IMD
- Index of Multiple Deprivation
- IMD2007
- Index of Multiple Deprivation 2007
- IRR
- incidence rate ratio
- LSNS
- Lubben Social Network Scale
- MSPSS
- Multidimensional Scale of Perceived Social Support
- MVPA
- moderate to vigorous physical activity
- OEE
- Outcome Expectation for Exercise
- OEP
- Otago Exercise Programme
- OPQoL
- Older People’s Quality of Life Questionnaire
- OR
- odds ratio
- PA
- physical activity
- PASE
- Physical Activity Scale for the Elderly
- PCRN
- Primary Care Research Network
- PCT
- primary care trust
- PM
- peer mentor
- PPI
- patient and public involvement
- PSI
- postural stability instructor
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SD
- standard deviation
- SF-12
- Short Form questionnaire-12 items
- TSC
- Trial Steering Committee
- TUG
- timed get-up and go test