Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/166/01. The contractual start date was in January 2012. The draft report began editorial review in March 2013 and was accepted for publication in November 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Fortnum et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
Otitis media with effusion
Otitis media with effusion (OME) is the most common cause of impaired hearing in children of > 6 months of age. Treating the condition with insertion of grommets is the most common reason for surgical operations in children worldwide. OME is defined as the occurrence of thick sticky fluid behind the eardrum in the middle ear, without signs or symptoms of an ear infection, which leads to a variable and fluctuating hearing loss. The lack of infection distinguishes the condition from acute otitis media (AOM). 1 This build-up of sticky fluid has led to the adoption of the more commonly used term ‘glue ear’ among both clinicians and parents. In the short term, glue ear causes discomfort for the child and reduced auditory input. Hearing losses can be up to 35–40 dB, which, although mild, can lead in the longer term to delays in speech and language development; these have an even greater impact in children with co-existing learning and communication difficulties, such as children with Down syndrome. This can lead, in turn, to behavioural problems as both children and parents become frustrated. Optimising the management of communication disorders can therefore improve social integration and enhance quality of life for children and their families. Most cases of OME resolve spontaneously. Causes for concern are persistence of > 3 months and bilateral hearing loss of ≥ 25 dB.
(Note: throughout this report we will, when appropriate, use the term glue ear as synonymous with OME.)
Down syndrome
Down syndrome is the most common chromosomal disorder in the UK, with an incidence of 1 in 1000 live births. 2,3 Phenotypically, facial dysmorphism leading to ear and upper airway abnormalities,4,5 including stenotic ear canals6 and Eustachian tube dysfunction,7 combined with poor immune function, results in the development of upper airway obstruction, obstructive sleep apnoea, subglottic stenosis, ear infections and middle ear effusions. 8,9 OME is almost universal in children with Down syndrome, begins at a younger age and persists to older ages than in children without Down syndrome. It has been reported that between 55% and 93% of children with Down syndrome have a conductive hearing loss that is dependent on age, and the majority of these losses are caused by OME. 2,10–13 Developmental delay is associated with Down syndrome, affecting multiple areas that are important in any assessment of hearing function, including non-verbal cognition, language learning and social behaviour. 14 In addition, specific difficulties with speech and intelligibility, which are exacerbated by hearing loss, are associated with Down syndrome over and above delay. 15
Interventions for otitis media with effusion
The commonest intervention to release the middle ear fluid is insertion of tympanostomy (T-tubes) or ventilation tubes more commonly known as grommets. Insertion of grommets is the commonest paediatric surgical procedure worldwide. However, grommet insertion can be difficult or sometimes impossible in children with Down syndrome, as the morphological features of Down syndrome lead to narrow ear canals. Forty per cent of cases can have stenotic external ear canals,16 making examination of the tympanic membrane impossible or difficult.
Amplification devices are alternative interventions to alleviate hearing losses consequent upon glue ear. Conventional behind-the-ear hearing aids (HAs) are often not tolerated by children with Down syndrome and pose hygiene problems if copresent acute or suppurative otitis media leads to eardrum rupture and discharge. Softband attachments for bone vibrators applied to the mastoid bone [bone-anchored hearing aid (BAHA) technology] are offered by some clinicians and may be tolerated better, although a controlled trial is lacking. Watchful waiting (WW) or active observation before determining definite need for intervention is now accepted to be good practice. Some patients have a period of aiding before having grommets, and some children choose aiding after previous grommet surgery.
National Institute for Health and Care Excellence guidelines
Guidelines from the National Institute for Health and Care Excellence (NICE) published in 200817 reviewed the evidence for surgical management of OME in children and recommended guidelines for treatment in children with uncomplicated OME, and also in children with Down syndrome or cleft palate. The report found only limited studies of OME in children with Down syndrome, and reviewed just three studies in detail, concluding that existing studies evaluating effectiveness of interventions are of poor quality.
Two comparative studies of grommet insertion18,19 found poorer hearing thresholds and resolution rates in children with Down syndrome than in control children. In addition, grommets in children with Down syndrome were more likely to fall out and to be associated with complications. A case series using aggressive medical and surgical treatment16 suggested improvement in hearing thresholds in 98% of cases but that this efficacy was short lived owing to early extrusion of grommets. Shott et al. 16 suggest repeated grommet insertion, but this, in turn, may lead to eardrum perforations.
The NICE guidelines17 acknowledged that children with Down syndrome who have glue ear present particular problems of assessment and management because of the earlier age of onset, the prolonged course of the disease, the greater risk of complications and the potential treatment difficulties. The clinical recommendations, based on the evidence available, were for regular multidisciplinary assessments with expertise in assessing and treating children with Down syndrome, HAs to be offered as a first intervention, and consideration of the following before grommet insertion: severity of hearing difficulties, age, practicality of insertion, clinical risks and likelihood of early extrusion.
The NICE report17 recommended research and national audit projects to evaluate the acceptability, effectiveness and consequences of treatment strategies for children with Down syndrome who have glue ear. It is important to assess both the benefit and harm, and the resource costs and savings of all possible interventions. This would imply a randomised controlled trial (RCT) but, among other restrictions, any such trial requires not only the measurement of robust, relevant and measurable outcomes, but also, crucially, that parents and professionals would be willing to randomise the children. 20,21 The NICE report17 acknowledged that RCTs might not necessarily be the most cost-effective use of research resources and if proposed would need to be multicentre. It was recommended that ‘high-quality national audits with statistical control for baseline characteristics’ would provide data on natural history and the outcomes of varying clinical practices to inform best practice.
Measurement of effectiveness of interventions
Any definitive evaluation of intervention options requires identification of outcome measures of importance or concern to both parents and professionals. Testing hearing, and speech and language development requires particular expertise. For a population of children with delayed development, any assessment must be developmentally appropriate rather than age appropriate. 9 In terms of language learning, language comprehension is usually stronger than language production, and phonology and syntax is particularly challenging,22 making the results of speech tests difficult to assess. It is therefore important to consider other outcome measures for success of treatment. Karkanevatos and Lesser23 explored this issue with parents of children with Down syndrome and reported that symptoms such as earache, disturbed sleep, balance problems, general health, hearing difficulties and misunderstandings, behavioural problems and social skills, and speech and language development were considered to be important. In addition, clinical otological outcomes, such as ear infection and discharge, persistent perforation, scarring and cholesteatoma, are important to consider.
Summary
In summary there remains clinical uncertainty of the benefits and costs of different treatment options for children with Down syndrome who have glue ear. This feasibility study was designed to assess the extent of this lack of knowledge and determine if pursuing further information would be practical, beneficial and cost-effective. It assesses the clinical and economic value of, and the potential practical barriers to, undertaking a future RCT or multicentre prospective cohort study of children with Down syndrome who have glue ear. Such a future trial might compare active surgical treatment, i.e. grommet insertion with or without adenoidectomy, with provision of standard air conduction HAs, provision of soft-band bone conduction HAs or WW (active observation). This may not be straightforward and this report documents the findings of research to contribute information from several areas to any decision about the requirement for future research.
Objectives
-
To assess the level and practical effect of the current uncertainty around treatment options for children with Down syndrome and OME.
-
To assess the feasibility of studying the options for management of OME in children with Down syndrome via a RCT or multicentre prospective cohort study.
-
To evaluate the willingness of parents to agree to randomisation for their children.
-
To evaluate the willingness of clinicians to recruit participants to a definitive study.
-
-
To explore relevant and practically measurable outcome domains for use in a definitive study.
-
To assess the feasibility and practical requirements for collecting these outcome measures of the relevant type.
-
To undertake a value of information (VOI) analysis to assess the level and clinical impact of current uncertainty, and the likelihood of further research reducing that uncertainty and minimising the clinical impact of any uncertainty.
Structure of the report
The project comprised four elements to address the objectives.
-
A targeted review of the current literature to assess the level of current uncertainty around treatment options for children with Down syndrome and OME building on the review undertaken by NICE (objective 1) and to explore facilitators and barriers to participation in research (objective 2) (see Chapter 2).
-
An exploration of the views and opinions of parents of children with Down syndrome (objectives 2i, 3 and 4) (see Chapter 3).
-
An exploration of the views and opinions of health-care professionals and teachers concerned with the care of children with Down syndrome (objectives 2ii, 3 and 4) (see Chapter 4).
-
A VOI analysis (objective 5) (see Chapter 5).
Each of the above elements is described in a separate chapter reporting objectives, methods, results and a summary. Finally, Chapter 6 includes the discussion for each element, including strengths and limitations, and recommendations for future research.
Patient and public involvement
To enhance the quality of the methodological approach to this project a multidisciplinary steering group was established at the start of the project. The group was chaired by a speech and language therapist (SLT), who is currently a registered intermediary with expertise in Down syndrome, and included representatives of the relevant professional disciplines, professional organisations, parent support associations, parents and the Down syndrome population (see Appendix 1 for the list of members of the steering group). At the initial meeting in January 2012 the group offered comment on drafts of the proposed questionnaires, information leaflets and consent documentation, and continued to contribute via e-mail as these documents were finalised. At the second meeting in January 2013, and subsequently by e-mail, the group offered comment on the interpretation of the results and presentation of the findings.
Research team
The research was undertaken by a multidisciplinary team of academics and clinicians based in the University of Nottingham and local UK NHS trusts in Nottingham.
Ethical approval
The initial project proposal and subsequent amendments received a favourable opinion from West Midlands – Staffordshire Research Ethics Committee (11/NW/0874).
Chapter 2 Literature review
Objectives
To identify and assess the level and practical effect of current uncertainty around treatment options for children with Down syndrome and OME.
Methods
Given the recent NICE systematic review of the evidence for intervention in OME and the recognition that studies of OME in Down syndrome were very limited and of poor quality, this review was not designed to be a systematic review of all the evidence. Rather, the primary aim of this targeted review was to identify and assess the current state of literature regarding OME in children with Down syndrome to provide an informed background to the study and to contribute to the VOI analyses.
This review summarises the published research into glue ear in children with Down syndrome including the efficacy of interventions, cost-effectiveness and the barriers to recruitment and participation in health research, specifically in terms of studies incorporating RCT methodology.
Search strategy
The search strategy involved an electronic search of the following 15 electronic databases from inception through to June 2012:
-
The Cochrane Library
-
EconLit
-
Tufts Cost-Effectiveness Analysis (CEA) Registry
-
MEDLINE
-
PubMed
-
PsycINFO
-
EMBASE
-
Applied Social Sciences Index and Abstracts (ASSIA)
-
Maternity and Infant Care
-
PsycARTICLES
-
Web of Science
-
Web of Knowledge (WoK)
-
British Psychological Society (BPS)
-
American Psychological Society (APS)
-
Centre for Reviews and Dissemination (CRD).
The search terms used included ‘children’, ‘Down* syndrome’, ‘otitis media with effusion’, ‘OME’, ‘glue ear’, ‘infant’, ‘research’. The terms were used in multiple combinations. Criteria for inclusion were (1) studies that were published in English; (2) studies that were published in peer-reviewed journals or government policy documentation; (3) studies that focused on children with Down syndrome who were experiencing OME; (4) studies that demonstrated the efficacy of treatments for OME (including surgical procedures such as grommets, adenoidectomy, HAs, antibiotics and any other appropriate treatments); and (5) RCTs that involved children with Down syndrome.
Criteria for exclusion were (1) studies that were focused on other forms of otitis media (unless specifically relevant) or other hearing problems (e.g. sensorineural hearing loss); (2) studies that were focused on Down syndrome in the general population, not affected by otitis media (e.g. studies of motor development); (3) studies of otitis media not in the Down syndrome population or other relevant populations (e.g. general population); (4) studies that were published in the grey literature or letters; and (5) studies that were focused on adults and not children.
Inclusion
Publications identified from the electronic search strategy were scrutinised by title and abstract to determine inclusion. Those reporting evidence that met the inclusion criteria were retrieved, and read by one reviewer for final decision on whether or not the data would contribute. The reference lists of included publications were searched to identify any further relevant studies.
Data extraction
A narrative synthesis was adopted. It was expected that included studies would be heterogeneous owing to the broad nature of the review. Relevant articles were reviewed to identify the topic of the article, the results and the methods used.
Results
The literature review confirmed the findings of the NICE report,17 in that there is a paucity of research/literature concerning the treatment options and effectiveness of such treatments for OME in children who have Down syndrome. No systematic reviews specifically concerning the efficacy of OME interventions in children with Down syndrome were identified. For example, Browning et al. 21 in their evaluation of RCTs on the efficacy of grommets (or ventilation tubes) for hearing loss associated with OME acknowledged the exclusion of Down syndrome from clinical trials, referring to participants with Down syndrome to note only how RCTs excluded them as a group.
Prevalence
Prevalence rates of glue ear at various ages have been reported as 59%,11 54.9%,12 77%,13 68%2 and 93%,2 as detailed below.
Schwartz and Schwartz11 investigated 39 non-institutionalised infants and children (mean age 3.1 years) with Down syndrome in the USA during the summer period and found that 59% showed evidence of ‘at least unilateral middle-ear effusion’.
In a survey using parent questionnaires and interviews in Australia to explore health problems and checks in 204 children with Down syndrome, Selikowitz12 reported that a glue ear diagnosis had been made in 112 children (54.9%).
Tomasevic13 reported a total population of 93 children, aged 18 months to 18 years, with Down syndrome, in Oxford, UK. Glue ear was diagnosed in 54 children (77%) and grommets were inserted in 29 children (54% of 54).
Barr et al. ,2 reporting findings from a prospective database set up since 2004 to capture the ear, nose and throat (ENT) health status of every preschool child with Down syndrome (aged 9 months to 6 years) in Greater Glasgow, accessing the community-based surveillance clinic, note that the prevalence of ENT problems is high in children with Down syndrome and that surgical treatment is frequently required. Between September 2004 and September 2008, data were available for 79 (91%) of the children. The prevalence of glue ear was 93% at 1 year old, although not all were symptomatic, dropping to 68% by 5 years old. During the time frame of the study, 37% of children were listed for surgery at some point, either adenotonsillectomy for obstructive symptoms or grommet insertion for OME. Barr et al. 2 recommend an active approach of regular ENT and audiology observation followed by early intervention, aimed at maximising long-term health and educational attainment.
Outcomes
The hearing loss associated with glue ear in children who do not have Down syndrome mediates the emergence of difficulties in other areas. It may affect other developmental abilities in the long term and, for example, may hinder a child’s speech and language development, such that their understanding and production of language (fluency, grammar and syntax) are compromised. 24 Other cognitive and social abilities that may be negatively affected as a secondary consequence of glue ear are attention skills,25 behaviour,26,27 and learning and educational progress, particularly literacy and social interaction,28 with a consequent impact on quality of life. 29
Analyses of several unpublished large databases (OM8-30, Q-16, Eurotitis 2) provide valuable information regarding parental concerns for children with glue ear but without Down syndrome (M Haggard, University of Cambridge, February 2013, personal communication). The Q-16 database indicates that the concerns mentioned most frequently by parents and carers for children without the learning difficulties and medical problems associated with Down syndrome were hearing (20.8%) and school progress (20.1%) and five other categories each accounted for > 5%. However, it is not clear to what extent these findings would be altered for particular populations of children, such as those with Down syndrome.
For children without Down syndrome there may be differences between the views of parents/carers, teachers and ENT surgeons concerning the relative importance of the impact of glue ear on different domains of development. A questionnaire study28 surveyed the perception of the impact of glue ear over four areas involving language and education, hearing, behaviour and balance between three groups involving parents, teachers and ENT consultants. The results indicated that teachers weighed language and education more highly than parents and ENT consultants, but that parents weighed hearing more highly than any other group. ENT professionals were least likely to weigh hearing as important. Differences attributed to the importance of different outcomes by stakeholders may influence the path that treatment takes.
Management of glue ear
There are a number of potential treatments for glue ear noted in the wider literature, but the evidence base is equivocal and only surgery and HAs are recommended by NICE guidelines. 17
Many reviews are available regarding the effectiveness of intervention for glue ear in children who do not have Down syndrome. These include a clinical evidence review of all interventions,30 and systematic reviews on the effectiveness of treatment of OME in the general population of grommet insertion,21,31 adenoidectomy,32 autoinflation,33 antibiotics,34 antihistamines and decongestants,35 oral and topical intranasal steroids36 and zinc supplements. 37 Most refer to participants with Down syndrome only to note that they were excluded from RCTs. Few studies16,18,19 and no reviews report the effectiveness of interventions for children with Down syndrome who have OME.
Surgical intervention
Grommets/ventilation tubes
Grommets [also known as ventilation tubes, pressure equalisation (PE) tubes and T-tubes] are tiny tubes that are inserted into the eardrum. They allow air to pass through the eardrum, between the outer and middle ear, which keeps the air pressure on either side equal. The surgeon makes a tiny hole in the eardrum and inserts the grommet or ventilation tube into the hole. It usually stays in place for 6–12 months and then falls out. This is typical and not considered to affect a child.
There are conflicting reports in the literature of grommet insertion being successful as an intervention,16 and not successful,18 for children with Down syndrome who have glue ear.
Rovers et al. 20 conducted an individual patient data (IPD) meta-analysis of grommets used for glue ear in order to identify subgroups of children who might benefit more than others from having ventilation tubes inserted. Rovers et al. 20 noted the following:
Subgroups that might benefit more from treatment with ventilation tubes include those with speech or language delays, behaviour and learning problems, Down’s syndrome, or children with cleft palate. These could not be studied in this IPD meta-analysis as these subgroups were excluded in individual trials. The experience of many clinicians that these subgroups of children benefit more from treatment with ventilation tubes has not yet been evidenced in RCTs. As the question whether to treat these children with ventilation tubes is very relevant for clinical practice, future trials studying these subgroups are justified.
Reproduced from [Archives of Disease in Childhood. Grommets in otitis media with effusion: an individual patient data meta-analysis. Rovers M, Black N, Browning G, Maw R, Zielhuis G, Haggard M. 90: 480–5, 2005.] with permission from BMJ Publishing Group Ltd. p. 48420
Davies38 reports an early study of hearing and middle ear dysfunction in 100 children with Down syndrome and the findings are contrasted with those of previous studies. Normal hearing was indicated in only 16% of the study sample. Decongestants and antibiotics were established to be of limited use, with surgery frequently carried out. Davis reports a number of problems with surgery including (1) the anaesthetic risks posed by children because of respiratory problems or congenital heart disease; (2) the difficulty of surgery caused by stenosis of the ear canal; (3) the ineffectiveness of the results in the long term; and (4) a heightened risk of post-surgery middle ear infection. Davis reports that 32 children had to have grommets inserted on up to three occasions, with only two children showing continued improvement after 2 years.
Selikowitz18 examined the short-term efficacy of T-tubes or grommets for secretory otitis media (SOM) in 24 children with Down syndrome, aged 6–14 years. Children were tested with audiometry at 6–9 weeks after having T-tubes inserted for bilateral SOM. There was no hearing improvement in 40% of ears compared with only 9% of 21 age-matched control children who also presented with bilateral SOM. The paper concluded that T-tubes for SOM in children with Down syndrome have a pronounced short- and long-term failure rate and recommended that this should be clarified with the parents prior to insertion. It was suggested that management should involve making sure that adequate intervention was provided to allow patients to hear as much as possible. Continuing hearing loss may necessitate the use of HAs.
Iino et al. 19 recommend a conservative approach, suggesting that the use of ventilation tubes should be reserved for cases in which hearing loss, secondary to middle ear secretion, is severe or when there are physical changes to the tympanic membrane (e.g. atelectasis). This was one of the studies reviewed by the NICE report,17 and was a longitudinal observational study following 28 children with Down syndrome, up to at least 7 years of age, and 28 age-matched control children who had T-tubes inserted for > 2 years (n = 50 ears in each group). 19 Children were assessed every month for 6 months post surgery and then thereafter every 2 months. At the time of the last visit, 11 out of 50 ears in the Down syndrome group and 39 out of 50 ears in the control group had normal or retracted eardrums. The authors define this as cured. Complications such as atelectasis, permanent perforation and cholesteatoma were found in 15 of the children with Down syndrome and six of the control children. Improvements in hearing levels to < 25 dB at the last visit were recorded for 10 out of 50 ears in the children with Down syndrome and 40 out of 50 ears in the control children. Iino et al. 19 concluded that the insertion of T-tubes was much less effective in children with Down syndrome than in control children. They made the following recommendation:
For the treatment of OME in children with Down syndrome, we propose that conservative management should be the approach of first choice and that indications for the insertion of tympanostomy tubes should be limited only when hearing loss due to middle ear effusion is in a severe degree and when pathological changes of the eardrum, such as adhesion and deep retraction pocket formation, are going to occur.
Reproduced from [International Journal of Pediatric Otorhindaryngology. 49(2). Lino Y, Imamura Y, Harigai S, Tanaka Y. Efficacy of tympanostomy tube insertion for otitis media with effusion in children with Down Syndrome. 143–9 Copyright Elsevier Science Ireland Ltd (1999)]. with permission from Elsevier. p. 14819
In the more positive study reviewed in the NICE guidelines17 conducted in Cincinnati, USA, Shott et al. 16 advocate, prior to the age of 2 years, an early and aggressive medical and surgical treatment of OME, which can involve grommet insertion. 16 This 5-year longitudinal study followed 48 children with Down syndrome every 6 months. Forty of the children (83%) required insertion of PE tubes because of chronic otitis media, with 55% requiring between two and four sets of PE tubes. After treatment (PE tubes and/or antibiotics) 97.7% of the children had normal to borderline hearing levels (undefined).
In a retrospective review of 29 children with Down syndrome aged 1–10 years referred for common ENT problems to a paediatric otolaryngology clinic in New Mexico, 13 children had PE tubes inserted bilaterally and 7 out of 26 procedures led to a complication. 39
Complications of grommet insertion are more common in children with Down syndrome. Tomasevic,13 in a series of 93 children in Oxford, reported that insertion and retention of grommets was an issue, with a repeat insertion rate of 59%. Otitis externa was noted in 28% and residual tympanic membrane perforation in 10%.
Adenoidectomy
Adenoidectomy is surgical removal of the adenoid, which is a focus of lymphoid tissue located in the space at the back of the nose above the soft palate. Adenoid removal has been shown to increase the benefit from grommet insertion in certain groups of children without Down syndrome. 40 Adenoidectomy, often in combination with tonsillectomy, may be performed in children with Down syndrome to relieve upper airway obstruction. This surgery is not without complications, in particular bleeding, and often specialist postoperative care is required, depending on the size and age of the child and on the severity of their airway obstruction.
Casselbrant et al. ,41 in a study of children without Down syndrome, concluded that adenoidectomy with or without tube insertion, provided no advantage to children aged 2–4 years with chronic OME compared with tube insertion alone, and was not recommended as first-line surgical treatment. In the UK TARGET trial (Trial of Alternative Regimens for Glue Ear Treatment),40 adjuvant adenoidectomy was reported to double the benefit from short-stay grommets in children without Down syndrome, aged 3–6 years, with persistent glue ear.
A retrospective review of the outcomes of adenoidectomy operations compared 27 children with Down syndrome (age 1–15 years) with 53 age- and sex-matched control children in the USA. 42 Long-term follow-up data were collected by telephone interview. Children with Down syndrome had less improvement than control children in middle ear effusion (23.1% vs. 68.0%) and also in symptoms related to nasal obstruction. Children with Down syndrome were 7.7 times more likely than control children to suffer chronic ear drainage.
Device intervention
Behind-the-ear hearing aids
Air conduction aids comprise an ear mould (to hold the aid in place and deliver the amplified sound into the ear canal) and a behind-the-ear digital aid. They can be used for any type of hearing loss. It is necessary to have accurate hearing thresholds measured to ensure the amplification is set appropriately. For some children with Down syndrome the shape of their outer ear can make it difficult to get good-fitting, secure ear moulds, which makes it difficult to keep the HAs in place. Wearing ear moulds can also exacerbate ear infections in some children, which can mean they are unable to wear their HAs until the infection has cleared.
No studies were identified that specifically explored the use of conventional behind-the-ear HAs in children with Down syndrome.
Bone-anchored hearing aid technology, including soft band
Bone-anchored hearing aids are bone conduction aids that utilise a permanent titanium implant to route sounds directly through the skull to the inner ear. They are suitable for patients with any type of hearing loss and who have problems wearing air conduction HAs. For children who are too young for a permanent implant owing to the lack of sufficient thickness of skull bone to hold the device or whose conductive loss is likely to be temporary, the BAHA can be worn on a softband that holds the aid firmly against the child’s head. Skull thickness in very young children and the quality of the bone (it is too soft) means that surgical placement of a permanent fixture is usually delayed until the child is at least 4 years old. Although the softband is easy to wear and put on, some children dislike wearing anything around their heads and do not tolerate the sensation of the band well. Mostly this can be overcome by a slow, structured introduction to wearing the band but, in some cases, the child does not accept it and an alternative has to be found.
Ramakrishnan et al. 43 report a retrospective, anonymised, cross-sectional survey in the UK using two assessment measures: the Glasgow Benefit Inventory and the Listening Situations Questionnaire (parent version) which were completed at least 3 months after the device was fitted. Of the 109 patients (age 6 months to 26 years), 22 were children with syndromes (or ‘syndromic’ children), nine of whom were young people with Down syndrome. Of these nine, six were fitted with a softband BAHA and three had implanted devices. Improvements were seen in both outcome measures. Ramakrishnan et al. 43 conclude that using BAHAs and softband BAHAs leads to appreciable improvements in quality of life for hearing-impaired children and young people, including those with Down syndrome, and suggest that there is major underutilisation of BAHAs in children with ‘skull and congenital abnormalities’ (e.g. Down syndrome).
Kunst et al. 44 report a series from the Netherlands of 22 patients (7–73 years) with moderate ‘mental retardation’, 12 of who had Down syndrome, fitted with implanted BAHAs. They also demonstrated improvements in domains assessed by the Glasgow Children’s Benefit Inventory (GCBI) and in learning and listening assessed by the Listening Inventory for Education.
McDermott et al. 45 report a study of 15 children with Down syndrome (aged 2–15 years) who were implanted with a BAHA over a 15-year period. All were long-term users, and benefits were also demonstrated in scores on the GCBI.
A survey46 of the 81 centres performing BAHA surgery in the UK in 2005 reported that 18 had provided BAHAs to patients with Down syndrome. Forty patients aged < 30 years were included and all received implantable devices. There was a high rate of complications (58%) but the authors concluded that BAHA was an effective option in patients with Down syndrome whose HAs or ventilation tubes had been unsuccessful. Softband BAHAs were not assessed in any of these last three studies. 44–46
Active observation/watchful waiting
A NHS Quality Improvement Scotland review47 suggested that WW, compared with immediate grommet insertion, does not lead to disruption in the development of language, behaviour or social interaction for children with persistent bilateral glue ear diagnosed before 3 years of age and with no other disabling health conditions. However, the Scotland review47 notes that it is unclear whether or not the ‘safe use of WW’ can be applied to other children who have already presented with language and behavioural difficulties at diagnosis. As children with Down syndrome are characterised as having development that is delayed (at the outset), it might be argued that the use of WW as a management strategy for glue ear in this group might indeed be problematic.
Antibiotics
Although glue ear is defined as being non-infective, in a number of cases bacteria are identifiable in the middle ear fluid. In such cases, antibiotics could be hypothesised to be effective. A Cochrane review34 concluded that the benefits were seen only after long-term administration of antibiotics, and that these benefits did not outweigh the risks of resistance. Studies of children with Down syndrome were specifically excluded.
Other treatments for glue ear
The sections above looked at studies that investigate the effectiveness of treatment options commonly used in health-care settings. There is little research concerning the benefits of these management options in children with Down syndrome. The limited empirical studies there are appear to focus mostly on surgical treatments in this group. However, there are other options that have been used to help manage glue ear in children without Down syndrome. These include autoinflation,33,48 decongestants/antihistamines,38 vaccines,49–51 topical intranasal steroids36,52 and zinc supplements. 37
Barriers to research
Of particular interest to this feasibility study are the views and opinions of parents and professionals concerning participation of children with Down syndrome in any future health research, particularly that involving a RCT design.
Most of the literature is concerned with recruitment to RCTs in a general population although one study did consider participation of people with learning disabilities. 53
Barriers identified by professionals
In the particular situation explored in this feasibility study, some professional groups (such as ENT surgeons) would be more often responsible for directly recruiting to a trial involving surgical intervention. However, professionals within other disciplines (such as paediatricians, audiologists and SLTs) may also influence the decision of a parent or carer as to whether or not their child should participate in a research trial. Although an individual’s experience of recruitment may vary, research suggests that there are a number of factors that may be important in deciding to recruit to a trial or encouraging a parent to agree to their child taking part.
A systematic review of 78 studies54,55 reported barriers to professionals recruiting to RCTs as lack of time, lack of training, concern about doctor–patient relationships, concern for patients, loss of professional autonomy, difficulties with the consent procedure, lack of reward and recognition, and the research asking a question that was not sufficiently interesting. Spaar et al. ,56 in a postal survey of 55 physicians in Switzerland, involved in a trial of rehabilitation options for patients with chronic obstructive airways disease, again highlighted time constraints as the most challenging barrier to recruitment, and the only other factor reported was difficulties with actually including eligible patients because the patient did not want to be randomised to a non-attractive treatment option or saw the process as too complex.
Caldwell et al. ,57 exploring the recruitment of children to RCTs with paediatricians in Australia in a qualitative study, conclude that balancing risk and benefit often resulted in clinician non-participation. Many paediatricians saw RCTs as a hindrance rather than a help to the doctor–patient relationship, which they valued highly, because they had to explain the concept of clinical equipoise. Negative elements were again more work, less money and lack of control. Paediatricians recognised that the health status of the child was an important influence for themselves and for parents. If the child has a poor prognosis or the parents are desperate for help, they may be less likely to accept randomisation that involved a placebo or ‘do nothing’ arm. Equally, even if there is genuine clinical equipoise, clinicians are reluctant to lose control of the intervention offered. Poor communication with researchers and lack of detailed information and feedback were seen as barriers to participation.
Barriers identified by parents
Prescott et al. ’s review54 identified uncertainty, additional demands, patient preference and concern about information and consent as barriers to patient participation.
Caldwell et al. ,57 exploring parents’ attitudes to children’s participation in RCTs in Australia, report that the perceived benefits include an offer of hope, the opportunity for better access to new treatments, professionals and information, meeting with parents in a similar situation and helping others. Perceived risks included side effects, being randomised to an ineffective treatment and inconvenience. Agreement to participation would be influenced by factors to do with the parents themselves (knowledge, beliefs and emotional response), with their children (health status, child preference), with the trial (the use of placebos) and with the clinician (recommendation, communication of information). The authors concluded that in order to increase parents’ willingness to agree to participation of their child there is a need to educate parents about trials, improve communication between researchers, clinicians and parents, increase incentives and decrease inconvenience.
The special position of parents as givers of proxy consent for their child is addressed by Shilling and Young. 58 This special position results in a dilemma for many parents between wanting to do the best for their child while also protecting them from any potential adverse events.
Nabulsi et al. 59 report data from a study of parents in Lebanon, defined as a developing country, exploring differences with the developed world literature. Findings were in fact similar, demonstrating that a parent is a parent regardless of the development status of their country. Facilitators in this study were reported to be direct benefit to the child, trust in the doctor or the institution, financial gain and positive previous experience. Barriers were lack of understanding of randomisation and complex consent forms.
A study by Robotham and Hassiotis53 addressed participation by people with learning disabilities and emphasised the importance of including people who were representative of the study population in the design stage of the research.
Cost-effectiveness
The literature search identified very little health economic literature on the subject. Ackerman et al. 60 constructed an economic model to estimate the costs associated with several childhood illnesses in children with Down syndrome. These were respiratory, gastrointestinal and related to the ears (otitis media and sinusitis). The estimated mean cost per episode of otitis media per child with Down syndrome was US$301 (in 1999). The study modelled the impact of a preschool intensive infection control programme. Before the intervention, the estimated cost of illness was US$1235 per child, which was reduced to US$615 per child after the intervention, giving cost savings of around US$620 per child. However, this cost savings estimate is spread over multiple conditions, only one of which is otitis media, therefore the cost savings attributable to a decline in otitis media are potentially less. Hellstrom et al. 61 reported that ventilation tubes were cost-effective; however, no details were given as to the economic analysis performed, and no incremental cost-effectiveness ratio (ICER) was reported, giving no justification of this statement. Berman et al. 29 investigated a hypothetical case of a 13-month-old boy with bilateral middle ear effusion, using effectiveness data generated by a meta-analysis of clinical trials. The analysis was conducted from a private health insurance perspective, using 1992 Medicaid reimbursement rates to estimate costs. They estimated that the most cost-effective strategy was a corticosteroid plus antibiotic at 6 weeks after diagnosis, further antibiotics for non-responders at 9 weeks, and finally, ventilation tubes for non-responders at 12 weeks. It was estimated that this strategy cost US$600.91, increasing to US$1088.54 with a 6-month follow-up. The estimated difference in national expenditures between the most cost-effective strategy and sequential antibiotics was US$643.6M and the study recommended the implementation of the cost-effective strategy. However, from a UK perspective, this study is not particularly useful, as antibiotics are not a currently accepted method for treating OME, and therefore the results have little application to the UK. The only other model with relevance was the economic model developed for the NICE guidance. 17 This model estimated the cost-effectiveness of two surgical interventions – grommets and grommets plus adenoidectomy – against HAs and ‘do nothing’. The model used HAs as the base intervention, with zeroed benefits. The model allowed for repeated surgical interventions, with a child having up to three sets of grommets. However, once receiving a particular treatment, the child would always receive that one treatment – they would not be switched to another. The model estimated that HAs had an expected cost of £752, whereas the ‘do nothing’ cost was £187, the cost of ventilation tubes was £1208, and the cost of ventilation tubes plus adenoidectomy was £1354. HAs were dominated, as they had the same effectiveness as ‘do nothing’; meanwhile, when compared with the ‘do nothing’ intervention, grommets had an ICER of £16,041 per quality-adjusted life-year (QALY). This is below the general NICE threshold value of £20,000 per QALY, but the authors suggested caution when interpreting the results, as the ICERs rose to > £20,000 per QALY in the sensitivity analyses (SAs), in which the HAs were assumed to have some degree of effectiveness, and reported ICERs of approximately £13,500. The economic model developed for the guidance is applicable for only the general population, and the parameters and costs in the model reflect this. Therefore, the results of the economic model must be taken with a degree of caution when applying to the population with Down syndrome.
Summary
-
The evidence concerning effective and efficient treatment of glue ear specifically in children with Down syndrome is sparse.
-
Clinical decisions must currently be based on experience, and evidence in populations who do not have Down syndrome.
-
Other clinical and developmental problems in children with Down syndrome mean that standard interventions (grommets and HAs) are not necessarily the best management options or strategy.
-
There is a need for good-quality evidence to support intervention decisions in children with Down syndrome who have glue ear.
Chapter 3 Exploration of the views of parents
Objectives
To:
-
assess the feasibility of studying the options for management of OME (glue ear) in children with Down syndrome via a RCT or multicentre prospective cohort study
-
evaluate the willingness of parents to agree to randomisation for their children
-
determine relevant and practically measurable outcome domains for use in a definitive study
-
assess the feasibility and practical requirements for collecting these outcome measures.
Methods
Parents of children with Down syndrome aged 1–11 years were identified by paediatricians who were responsible for the health service provision to the children through lists held by the local children’s service.
Our catchment area included all three of the main centres of population in the East Midlands and south Yorkshire (Nottingham, Sheffield and Leicester). Inclusion of smaller services in Derby, Mansfield and Chesterfield provided complete geographic coverage of Nottinghamshire and Derbyshire. Areas were included on the basis of having a named person who had responsibility for children with Down syndrome and who had an accessible database of such children of the appropriate age. We accessed a total population known to community child health and/or hospital-based services in each area. All known families were initially identified but paediatricians did not contact families for whom they felt that circumstances were such that an approach would be distressing for the family, for instance if the child or another close family member was seriously or terminally ill.
We chose to invite participation from a general population rather than identifying families through a special interest association, such as the Down’s Syndrome Association, to enhance the generalisability of the results.
Questionnaires
The questionnaire for parents was developed with input from a number of sources, including databases developed from the TARGET trial (OM8-30, Q-16 and Eurotitis 2),40,62,63 existing OME literature,16,17 parent surveys from other areas of clinical/health research and feedback from the project steering/advisory group. The questions for the survey were also developed and refined via recursive feedback received from members of the multidisciplinary research team. These professionals had expertise in a number of disciplines, including speech and language therapy, paediatrics, epidemiology, psychology, audiology, ear, nose and throat surgery, and health services research. The information on the TARGET trial40 databases was obtained from Professor Mark Haggard, an advisor to the study. These databases are very important in the field and certainly relevant to any study on OME in children. They address issues of the relative importance of concerns expressed by parents. For example, in the Q-16 database (which looks at the impact of OME on children without Down syndrome), the concerns mentioned most frequently for children without the learning difficulties and medical problems associated with Down syndrome, were hearing (20.8%) and school progress (20.1%) and five other categories each accounted for > 5%. However, it is not clear how much this spectrum would be altered for children with Down syndrome.
The questionnaire for parents was piloted with two parents of children who have Down syndrome. These parents, who were members of the project steering group, were asked to give feedback concerning the design and scope of the questionnaire and their experiences of completing it. Feedback was generally positive, but one parent reported that certain sentences were ambiguous (owing to medical terminology or descriptions) and needed to be made clearer. Consequently, certain sentences were rewritten to aid clarity or were deleted. This improved the questionnaire’s accessibility. The questionnaire was also piloted with staff of the research unit who had children without Down syndrome but of an appropriate age.
The questionnaire (see Appendix 2) and a letter and information sheet explaining the project were sent to each family from a paediatrician known to them in May and June 2012 as a complete batch in all centres except Leicester, where the questionnaire distribution was staggered over several weeks. One reminder was sent to each family (unless they had returned the questionnaire and indicated their name and address) in June and July 2012. A poster advertising the existence of the project and offering families the opportunity to request a further questionnaire was sent to the consultant paediatricians in September 2012, together with a number of blank questionnaires. The paediatricians were asked to display the posters in clinical areas that children with Down syndrome were likely to attend. Some of the paediatricians also verbally reminded families about the project during consultations.
Questionnaires were returned directly to the research team and consent was implied and inferred by completion. Questionnaires were written in English and the front page included (in three locally common languages: Urdu, Punjabi and Polish) an offer to provide a translation of the questionnaire if required. Both closed, forced-choice questions and open-ended questions were included.
The questionnaire explored, for each respondent:
-
experience of glue ear and general health of their child
-
effects of glue ear experienced by their child
-
interventions and treatment received and his/her views on its effects
-
views on taking part in research and circumstances that would encourage or discourage participation
-
views on the importance of various outcome domains to them and for their children
-
demographic variables.
The fifth bullet point in the above list concerning the importance of various outcome domains was included to provide more detailed input into the design of any future trial.
The outcome measures we chose to explore covered a broad range of domains, and were relevant to children with Down syndrome, appropriate to the age range in which it would be practicable to conduct a trial, clear to non-professional respondents and describable by a label that is widely understood and of which the importance can be explicitly judged. Measurement of outcomes in a future trial would need to have the capacity to demonstrate reliable differences between treatment arms but more importantly to be seen as important to parents.
We offered a set of outcomes as a short forced-choice list, which was not comprehensive, but met the criteria above on the basis of general knowledge of the main effects of the disease in children without Down syndrome.
With these considerations in mind we decided to elicit judged importance for four domains: hearing, developmental/educational progress in nursery or school, speech/language and social participation. To elicit a little more information on the valuation hierarchy, we offered a first and second priority among the four.
The final question of the questionnaire asked if the family/parent was interested in taking part in an interview and/or focus group. If so, they were asked to provide contact details but, if not, the questionnaire could be returned anonymously.
Responses to closed or forced-choice questions are reported descriptively, and open textual responses have been coded via a thematic analysis and summarised.
Interviews
(See Appendix 3 for discussion areas for interviews.)
Greater depth and more detail about family experiences of treatment and attitudes towards future research were collected using qualitative, semistructured interviews. The rationale for questions asked in the interviews was informed by the ticked responses and written comments in returned questionnaires. To assess any difficulties with the questions asked (such as ethical concerns due to sensitivity of subject areas or problems with the recording equipment) the proposed questions and subject areas to be explored were piloted with a parent of a child with Down syndrome in the Nottinghamshire region in July 2012 at the research unit (NIHR Nottingham Hearing Biomedical Research Unit). This pilot interview lasted about 1 hour. On the basis of the pilot interviewee’s feedback, the interview duration, questions and subject areas were found to be appropriate both in terms of research aims and ethics. The recording equipment was found to be effective in recording a two-way conversation accurately enough to be transcribed without being intrusive in the context of the interview.
A selected sample of parents was identified from among those who indicated a willingness to participate further in the research. The sample included those with different experiences of treatment options, those with younger children and those with older children, those who were positive about future research, and those who were noting concerns about research. None of those expressing a willingness to take part in an interview requested an interpreter and all interviews were conducted in spoken English.
Parents were contacted by telephone by the researcher and an appointment for the interview was arranged. Each interview was undertaken on a single occasion, face to face, and all but one were conducted in the family’s home. Informed consent was confirmed at the beginning of the interview.
Questioning explored further the lines of enquiry in the questionnaire but offered greater scope for families to reflect in more detail upon their experiences. In addition, families were offered the opportunity to introduce and raise relevant topics that were not covered in the survey research. Views on randomisation and about the most appropriate domains for outcomes to be measured in any future trial were explored in detail. Interviews were conducted by a research fellow (LB) and audio-recorded with permission. Recordings were transcribed in full and data was handled using the NVivo computer package version 10 (QSR International, Warrington, UK).
Focus groups
(See Appendix 4 for focus group discussion areas.)
Further qualitative data were collected during focus groups through which findings from both questionnaires and interviews were reviewed and refined. A second purposively selected sample of parents was drawn from those previously involved in the project (both questionnaire and interview stages). All parents previously interviewed who had indicated either ‘yes’ or ‘don’t know’ to an interest in taking part in a focus group (n = 18), and 15 parents who had not previously been interviewed but who had expressed an interest in taking part in a focus group, were invited by post or e-mail to do so and two dates were offered. Non-response was followed up by telephone.
Two focus groups were held on consecutive mornings at the research unit in Nottingham, each lasting 2–3 hours. Each focus group was led and facilitated by two members of the research team (PL and LB), audio-recorded with permission and transcribed in full. All parents taking part had an understanding of spoken English and interpreters were not requested. The descriptive statistics from the questionnaire survey and the analytic framework from the interviews acted as prompts for discussion during these groups. Parents were asked to further explore opinions and perspectives on treatment and on clinical research, including issues around randomisation, with the aim of determining a parental view on the design of any future study.
Parents taking part in interviews or focus groups received full details of the process when initially invited, and again from a member of the research team when the interviews or focus groups took place. All of those taking part in interviews or focus groups signed a consent form before taking part and consented to audio-recording.
Qualitative data analyses
Qualitative data were analysed to explore parents’ experiences of treatment, and to investigate their attitudes towards clinical research, including willingness to participate in clinical trials, strategies for recruiting participants and the acceptability of randomisation. These data may contribute to the generation of a tailored recruitment strategy for any future trial, and the development of bespoke information packs for trial participants that reflect parents’ understanding and concerns. Analysis followed the conventions of framework analysis,64–66 allowing each interview to be considered independently, and enabling the development and refinement of cross-cutting themes.
Framework analysis is a hierarchical, matrix-based method developed for applied or policy-relevant qualitative research. It is a highly structured, transparent and rigorous approach to qualitative data, which is well suited to research for which timescales are limited, and the goals of research are clearly defined at the outset (in this case supporting the development of a future trial). Broadly, framework analysis sees qualitative data mapped on to a thematic framework, from which it can then be interrogated to address information needs and research objectives. Through the process of building, revising and populating a thematic framework, findings can be generated that both directly address research objectives while also being strongly rooted in the responses of research participants.
In the present research, an initial thematic framework was constructed from the literature on clinical treatment and clinical trial recruitment, and the findings of the parent survey. It reflected the research objectives and contained several main themes: the challenges of Down syndrome and OME; diagnosis and treatment pathways; treatments – experiences and opinions; applied health research (AHR); and study design detail. Each main theme was subdivided into a number of topics. For example, theme 1 – The challenges of Down syndrome and OME – included three topics: symptoms, consequences and priorities for improvement. The adequacy of this framework was tested by coding a subset of the interview scripts and themes and subtopics were amended if, for example, they captured no data or an excess of data. New themes and topics were added if participants raised issues that were not otherwise evident in the analytic framework. For example, for theme 1, a topic of ‘Other un-categorised comments’ was added. Once the framework had been finalised, all interview data were mapped on to it – one table for each main theme, with topics as columns and individual cases as rows. The convention in framework analyses is to record summaries of original data rather than extensive chunks of text and this was adhered to in this analysis. Interview transcript page references were recorded to enable linkage to the original data (Table 1).
| Challenges of Down syndrome and OME (theme) | ||||
|---|---|---|---|---|
| 1.1 Symptoms (topic) | 1.2 Consequences (topic) | 1.3 Priorities for improvement (topic) | 1.4 Other uncategorised comments (topic) | |
| Int.1 |
|
etc. | etc. | etc. |
| Int.2 |
|
|||
| Int.3 |
|
|||
| etc. | ||||
Once all data had been ‘charted’ in this way it was assessed to address the research objectives. Broad summaries of themes (complete tables) and topics (individual columns) were generated and key experiences, opinions and attitudes were identified. Each key feature was marked as a subtopic and a final analytic framework constructed (see Appendix 5). Links to original data enable each theme, topic and subtopic to be developed more fully to illustrate a particular phenomenon or perspective.
Outcomes acceptability
One of the objectives of this project was to explore the relevant outcome domains for use in a future trial and whether or not the measurement of these outcomes would be practical and feasible for the relevant population.
The acceptability and value of measures of outcome within relevant areas were explored with parents (through questionnaires, interviews and focus groups) and with professionals (through a Delphi technique) (see Chapter 4) to inform the design of any future trial.
To assess outcomes in terms of communication and outcomes specifically related to glue ear we asked parents who had previously taken part in an interview and/or a focus group to review and assess three instruments designed to measure progress in outcomes. Two of these instruments were the MacArthur–Bates Communicative Development Inventories (CDIs) ‘Words and Sentences’ for older children and ‘Words and Gestures’ for younger children (available at www.brookespublishing.com/resource-centre/screening-and-assessment/cdi/). These instruments assess language and communication skills through parental report but are not designed specifically for children with learning difficulties or developmental delays. However, they assess a child’s understanding across a wide range of modalities and therefore could be useful with children with Down syndrome.
In terms of a tool to measure outcomes specifically related to glue ear, we invited parents to comment on the shorter OMQ-14 instrument (see Appendix 6), developed from the OM8–30 by Professor Haggard. This includes the items that best predicted quality of life in the TARGET study40 and is designed for children aged 3–9 years, but we felt that many of the questions were sufficiently generic that parents of younger children would be able to answer appropriately. An impartial review62 suggested that OM8–30 has the best psychometric properties overall of any instrument available, and it has a strong pathophysiological and developmental rationale and a growing set of applications (construct validity) plus a range of useful facilities. A particular attraction of the OM8–30 is that it has recently been mapped to the universal Utility Scale [via the standard Health Utilities Index Mark 3 (HUI3)] for generic quality of life. 63
The three instruments were sent to a sample of 17 parents who had taken part in either an interview or a focus group. They were asked to look through the instruments and answer three questions for each one:
-
Do you think the questions would apply to a child with Down syndrome?
-
Are there particular things about the questionnaires that would make it difficult to answer for a child with Down syndrome?
-
Are there things about the questionnaires that are particularly good for a child with Down syndrome?
Parent rating of importance
There is potential value, in terms of policy and dissemination, in documenting any large differences in the perceived importance of domains between OME in Down syndrome and OME in children without Down syndrome. To explore comparison between our data and the outcome measure instruments developed from the TARGET trial40 we engaged with Professor Haggard as a special adviser to the project. He provided analysis of the Eurotitis-2 database (∼2000 cases currently with OM8–30, approximately 900 in English and another 1100 in 15 other European languages) and of the open-ended response data from TARGET baseline on aspects of concern to parents within a category scoring system that showed high intercoder agreement. The latter were from > 1100 parents of children with uncomplicated but persistent OME and they establish adequate item coverage and good content validity for the measures of outcome used, as well as the weightings needed to combine facet scores into aggregates. To align the priority data between the two studies, we compared the relative weighting of necessary basic categories of outcome facets from our work with (1) the number of open-ended mentions of concern in uncomplicated glue ear and (2) the empirically optimal (regression coefficient) weights seen in the Dakin et al. mapping63 referred to above (see Outcomes acceptability).
Insofar as our qualitative research may show a similar list of facets to those seen in uncomplicated glue ear, a large body of research on glue ear, including the OM8–30 becomes applicable, perhaps with an altered set of weighting coefficients. If there is a considerable divergence of results, then the implication is that new measures would need to be developed to cover the outcome domains efficiently.
Results
Representativeness
The population covered by this study is broadly based within the East Midlands (Nottingham, Derby, Mansfield, Chesterfield and Leicester) and Sheffield in south Yorkshire. For the purposes of assessing representativeness within the UK and the potential impact on a future study, it is appropriate to use the East Midlands as a basis for comparison. The population of the East Midlands is broadly representative of the diversity present in the wider UK population in terms of socioeconomic classification (Table 2),67 although there are some statistically significant differences in the classifications used here (Table 2).
| Socioeconomic classification | East Midlands (%) | UK (%) | Significance (p-value) |
|---|---|---|---|
| Higher managerial and professional | 9.7 | 10.7 | NS |
| Lower managerial and professional | 21.6 | 22.2 | NS |
| Intermediate occupations | 9.3 | 10.0 | NS |
| Small employers and own account workers | 7.4 | 7.5 | NS |
| Lower supervisory and technical | 10.5 | 9.1 | 0.018 |
| Semiroutine occupations | 13.8 | 12.9 | NS |
| Routine occupations | 11.9 | 9.4 | 0.032 |
| Never worked, unemployed and not elsewhere classified | 15.7 | 18.1 | 0.002 |
In terms of ethnicity, Table 3 indicates a lower proportion of non-white individuals in the East Midlands than in England and Wales. All comparisons in the categories presented in Table 3 are statistically significant at the p < 0.05 level.
| Ethnicity | 2011 | |||
|---|---|---|---|---|
| East Midlands (total 4,533,222) | England and Wales (total 56,075,912) | |||
| n | % | n | % | |
| White: British, Irish, other white | 4,046,356 | 89.26 | 48,209,395 | 84.97 |
| Mixed: white and black African, white and black Caribbean, white and Asian, other mixed | 86,224 | 1.90 | 1,224,400 | 2.18 |
| Asian or Asian British: Indian, Pakistani, Bangladeshi, other Asian | 293,423 | 6.47 | 4,213,531 | 7.51 |
| Black or black British: black Caribbean, black African, other black | 81,484 | 1.79 | 1,864,890 | 3.32 |
| Other ethnic group: Arab | 9746 | 0.21 | 230,600 | 0.41 |
| Other ethnic group: any other | 15,989 | 0.35 | 333,096 | 0.59 |
However, we consider these differences to be practically small and, given our initial selection of a total population sample, we are confident that the project design had the potential to provide information from a sufficiently diverse and representative population to be relevant in any consideration of the value of a future RCT.
For each of the following sections reporting questionnaire data, reference is made to the section of the questionnaire contributing the data (see Appendix 2).
Response rate
Questionnaire
A nominated consultant paediatrician with responsibility for the care of children with Down syndrome in each of the NHS paediatric services in Chesterfield, Derby, Leicester, Mansfield, Nottingham and Sheffield identified parents of children with Down syndrome, aged 1–11 years, whom they considered met the inclusion criteria for the project. The number of families identified in each centre is shown in Table 4.
| Centre | Questionnaires distributed | Responses after 3 weeks | Responses as of November 2012 | |
|---|---|---|---|---|
| n | % | |||
| Chesterfielda | 10 | 0 | 0 | 0 |
| Derby | 63 | 5 | 13 | 20.6 |
| Leicester | 114 | 10 | 42 | 36.8 |
| Mansfield | 21 | 2 | 4 | 19.0 |
| Nottingham | 107 | 12 | 38 | 35.5 |
| Sheffield | 77 | 10 | 25 | 32.5 |
| TOTAL | 392 | 39 | 122 | 31.1 |
The total response was just > 30%, which is lower than the 50% that we anticipated, despite postal reminders, posters in clinics and personal reminders from the paediatricians. The response rate across centres varied from 0% (of 10 questionnaires sent from Chesterfield) to 36.8% (of 114 questionnaires sent from Leicester).
All parents completed the questionnaire in English. One parent requested the questionnaire to be translated into Punjabi but did not then return the translated version.
In Nottingham the responsible paediatrician was a member of the research team (EM) and agreed to gather some basic anonymised information from her clinical records on the families who had not responded. We returned to EM a list of 20 Nottingham responders who had given us their name and address. In addition, we provided details of the child’s age and gender, number of siblings, mother’s age and first three digits of the post code for the remaining 18 responders. From this information, EM was able to identify 35 of the 38 respondents and provide some anonymous general information on the remaining 72 families who were sent a questionnaire (this number of 72 includes the three respondents who could not be identified). Table 5 compares the responders and non-responders in Nottingham by age of the child, and indicates that there is no difference in the age of the children in the responding families compared with the non-responding families (Mann–Whitney U-test; p = 0.088). If aggregated into preschool age and school age there is still no significant difference (Fisher’s exact test; p = 0.139).
| Age of child (years) | Responders | Non-responders | ||
|---|---|---|---|---|
| n | %a | n | %a | |
| < 2 | 3 | 8.8 | 10 | 13.9 |
| 2 | 7 | 20.6 | 4 | 5.6 |
| 3 | 4 | 11.8 | 7 | 9.7 |
| 4 | 4 | 11.8 | 5 | 6.9 |
| 5 | 7 | 20.6 | 9 | 12.5 |
| 6 | 2 | 5.9 | 6 | 8.3 |
| 7 | 1 | 2.9 | 7 | 9.7 |
| 8 | 1 | 2.9 | 10 | 13.9 |
| 9 | 2 | 5.9 | 6 | 8.3 |
| 10 | 3 | 8.8 | 6 | 8.3 |
| 11 | 0 | 0 | 2 | 2.8 |
| Aggregated age group | ||||
| Preschool age ≤ 4 years | 18 | 52.9 | 26 | 36.1 |
| School age ≥ 5 years | 16 | 47.1 | 46 | 63.9 |
| Missing | 4 | 0 | ||
| Total | 38 | 72 | ||
We did not specifically ask for the mother’s age in the questionnaire – rather we asked for ‘your age’ from the person filling in the questionnaire. We can only assume, based on clinical experience, that it is more commonly the mother who answers such questionnaires rather than the father, even if the answers are discussed within the family.
In the group of parents who responded, 4 of the 32 with a known parental age (12.5%) were < 30 years old when the child was born. EM reports that routinely collected data for the last few years indicate that approximately one-third of mothers of children with Down syndrome in Nottingham are < 30 years of age when the child is born. This suggests that the parents responding to this survey in Nottingham were older at the time of the birth.
Interviews
Sixty-four respondents to the questionnaire (53.3%) expressed an interest in taking part in an interview. Twenty-four parents were approached and invited to take part in an interview. Twenty-three parents from 21 families were interviewed. For two interviews both parents were present. Each interview was conducted over 1–2 hours on one occasion in the family’s home. The distribution of parents participating by site is shown in Table 6.
| Site | No. agreeing to interview (% of parents/families completing the questionnaire) | No. of families interviewed (% of parents/families agreeing to be interviewed) |
|---|---|---|
| Derby | 5 (41.7) | 2 (40.0) |
| Leicester | 20 (48.8) | 4 (20.0) |
| Mansfield | 1 (25.0) | 0 |
| Nottingham | 22 (57.9) | 8 (36.4) |
| Sheffield | 16 (64.0) | 7 (43.8) |
| Total | 64 (53.3) | 21 (32.8) |
Parents were selected to receive an invitation to an interview to try to achieve (1) a balance of parents with children of different ages and (2) a mix of parents who had differing levels of knowledge about research, and both positive and negative views on future research. This information was indicated by their response to questions, which asked if parents would agree to their child taking part in research involving a RCT and/or an observational approach (see Appendix 2) (Table 7).
| Age of child (years) | How much do you know about research in general? | Imagine if you were asked to agree to your child taking part in a RCT comparing different treatments for glue ear – would you agree? | Imagine if you were asked to agree to your child taking part in an observational study comparing different treatments for glue ear – would you agree? |
|---|---|---|---|
| 2 | Quite a lot | Don’t know | Yes |
| 2 | Nothing | Yes | Yes |
| 2.5 | A little bit | Yes | Yes |
| 3 | A little bit | Yes | Yes |
| 3 | Nothing | Don’t know | Don’t know |
| 4 | A fair amount | Yes | Yes |
| 4 | A fair amount | Don’t know | Yes |
| 5 | Quite a lot | No | Yes |
| 5 | A fair amount | Don’t know | Yes |
| 5 | A little bit | Yes | Yes |
| 6 | Nothing | Don’t know | Yes |
| 6 | A little bit | No | Yes |
| 6 | Nothing | Yes | Yes |
| 7 | Nothing | Don’t know | Don’t know |
| 7 | A fair amount | Don’t know | Yes |
| 7 | A fair amount | Not answered | Yes |
| 8 | A fair amount | Don’t know | Yes |
| 8 | Quite a lot | No | No |
| 9 | Nothing | Yes | Yes |
| 9.5 | A little bit | No | Yes |
| 10 | A little bit | Yes | Yes |
Focus groups
Eighteen of the parents who had taken part in the interviews and indicated a willingness to take part in a focus group were invited to do so. Seven declined to be involved. Two parents were unavailable for either of the dates offered and two cancelled nearer the time owing to unforeseen circumstances (e.g. child’s ill health). Seven parents were available on the suggested dates and five parents from five families attended.
Of the 15 parents who had not taken part in interviews but had expressed a willingness to take part in a focus group, 10 parents initially indicated that they would be free to attend. Four of these parents cancelled attendance nearer the time owing to unforeseen circumstances (e.g. having to attend a child’s school review). Nine parents from six families attended the focus group.
Six parents (from five families) attended the first parent focus group (a.m. Thursday 4 October 2012) and eight parents (from six families) attended the second (a.m. Friday 5 October 2012).
The distribution of parents by site is shown in Table 8.
| Site | No. agreeing to focus group (% of parents/families completing the questionnaire) | No. of families attending focus group (% of parents/families agreeing to take part in a focus group) |
|---|---|---|
| Derby | 2 (16.7) | 0 |
| Leicester | 13 (31.7) | 4 (30.7) |
| Mansfield | 0 | 0 |
| Nottingham | 18 (47.4) | 5 (27.8) |
| Sheffield | 8 (32.0) | 2 (25.0) |
| Total | 41 (34.2) | 11 (26.8) |
Table 9 presents data on the 11 families taking part in the focus groups in terms of the age of the child and their views on future research.
| Age of child (years) | Already been interviewed | Would take part in RCT | Would take part in observational study | Knowledge of research |
|---|---|---|---|---|
| Focus group 1 | ||||
| 2.5 | Yes | Yes | Yes | A little bit |
| 4 | Yes | Don’t know | Yes | A fair amount |
| 4 | Yes | Yes | Yes | A fair amount |
| 6a | No | Yes | Yes | Nothing |
| 9.5 | Yes | No | Yes | A little bit |
| Focus group 2 | ||||
| 2.5a | No | Yes | Yes | A little bit |
| 4.5 | No | Yes | Yes | Not answered |
| 5 | No | Don’t know | Yes | A little bit |
| 6 | No | Yes | Yes | A little bit |
| 6 | Yes | Don’t know | Yes | Nothing |
| 8a | No | Yes | Yes | A little bit |
Survey of outcome instruments
Seventeen parents were sent (by post) the three instruments which might be considered when assessing outcomes. Seven responded, one by telephone, one by e-mail and five in writing.
Descriptive demographics
(See Appendix 2, section 7.)
Figure 1 illustrates the distribution of ages for the children of families who responded to the questionnaire and provided the age of the child, and Tables 10 and 11 document the age and gender distribution. There is no significant difference between centres in terms of age of the children (Fisher’s exact test: p = 0.090) or gender (Fisher’s exact test: p = 0.801).
FIGURE 1.
Distribution of ages for the children of families who responded to the questionnaire.
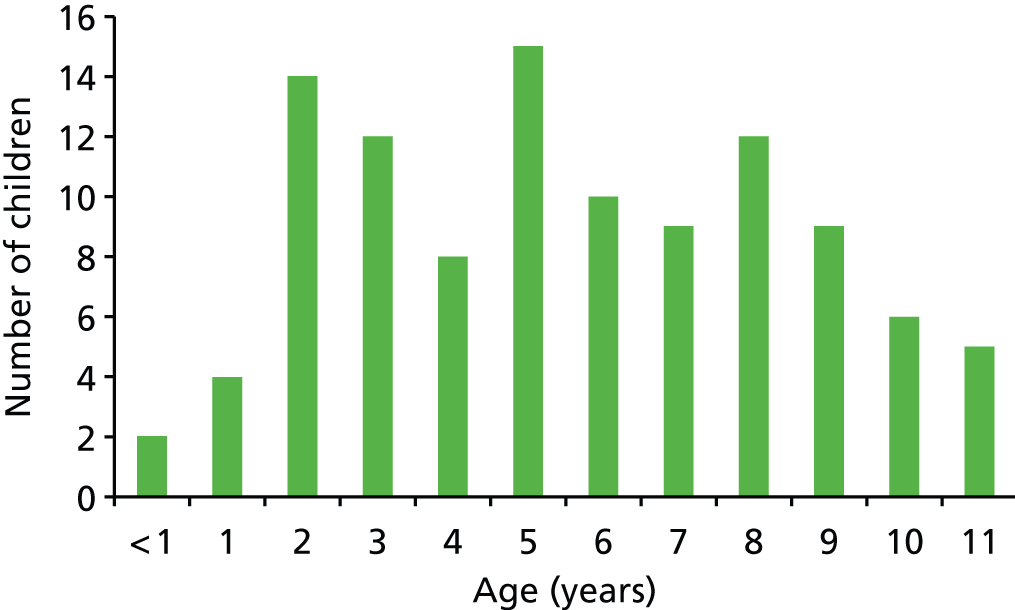
| Site | Preschool ≤ 4 years | School age ≥ 5 years | Total | |||
|---|---|---|---|---|---|---|
| n | % per sitea | n | % per sitea | n | % of totala | |
| Derby | 4 | 36.4 | 7 | 63.6 | 11 | 10.4 |
| Leicester | 13 | 37.1 | 22 | 61.8 | 35 | 33.0 |
| Mansfield | 1 | 33.3 | 2 | 66.6 | 3 | 2.8 |
| Nottingham | 18 | 52.9 | 16 | 47.1 | 34 | 32.1 |
| Sheffield | 4 | 17.4 | 19 | 82.6 | 23 | 21.7 |
| Total | 40 | 38.1 | 66 | 61.9 | 106 | 100 |
| Site | Male | Female | Total | |||
|---|---|---|---|---|---|---|
| n | % per site | n | % per site | n | % of total | |
| Derby | 6 | 54.5 | 5 | 45.5 | 11 | 10.3 |
| Leicester | 20 | 55.5 | 16 | 44.4 | 36 | 33.6 |
| Mansfield | 1 | 33.0 | 2 | 66.0 | 3 | 2.8 |
| Nottingham | 15 | 44.1 | 19 | 55.9 | 34 | 31.8 |
| Sheffield | 13 | 56.5 | 10 | 43.5 | 23 | 21.5 |
| Total | 55 | 52 | 107 | 100 | ||
Interviews, focus groups and outcome evaluation
Demographic data for the parents involved in the interviews, focus groups and outcome evaluation confirmed that the samples were representative of the parents who responded to the questionnaire. They are not reported here or in the following sections, as to do so – with the small numbers of parents involved – would possibly enable identification of individuals.
Family demographics
Parent’s age
Table 12 illustrates the distribution of parental age at the time of completion of the questionnaire and when the child was born (calculated).
Parental educational qualifications
As a measure of socioeconomic status we collected grouped information on the educational qualifications of the person completing the questionnaire. This is detailed for each centre in Table 13 and compared with data from the 2001 UK Census for the population of the East Midlands (www.ons.gov.uk/ons/guide-method/census/census-2001/index.html). (UK Census data for 2011 was not available to the same degree of detail.) Although the categories in the Census data do not match exactly the categories used in the questionnaire, it is possible to note that, whereas the general population is relatively evenly spread across the categories defined, it is apparent that the parents responding to this survey had achieved a higher educational level, with 48% having gained a university degree or higher compared with only 21.5% of the general population (Fisher’s exact test: p = 0.001). If we assume that the parents of children with Down syndrome are representative of the general population in this regard then the parents in this study are not representative of the population of parents of children with Down syndrome. There are two possible alternatives: (1) parents who responded to our survey were more likely to have achieved higher educational qualifications than those who did not respond; (2) parents of children with Down syndrome are more likely to have achieved higher educational qualifications than parents of children without Down syndrome. Alternative 1 should be addressed in a future study by ensuring that participation is equally accessible to all. Alternative 2 may represent specific biases in the population of families of children with Down syndrome. Women who delay having their babies until they are older are at more risk of having a baby with Down syndrome and may well be skewed to higher educational attainment. Alternatively, there may be a bias towards older women continuing a pregnancy after antenatal diagnosis of Down syndrome, i.e. pregnancy may be considered to be more precious as there may be fewer further chances of pregnancy, or there may be a bias towards continuing pregnancy in a more educated population.
| Source | Left school before age 15 years | Usual examinations for age 15–16 years | Usual examinations for age 17–18 years | Further qualification but not university degree | University degree | PG qualification | Missing | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | %a | n | %a | n | %a | n | %a | n | %a | n | %a | |||
| Questionnaire | 1 | 1.0 | 13 | 12.5 | 5 | 4.8 | 35 | 33.6 | 26 | 25.0 | 24 | 23.1 | 18 | 122 |
| No qualification | Level 1 | Level 2 | Level 3 | Level 4/5 | ||||||||||
| East Midlands data from Census (20–54 years)b | 429,333 | 23.0 | 440,018 | 23.5 | 410,578 | 22.0 | 186,519 | 10.0 | 403,275 | 21.5 | 21.5 | 130,649 | 2,000,372 | |
Number of other children in the family
The number of other children in the families responding to the questionnaire varied from zero (n = 19) to more than five (n = 2) with an average number of between one and two. Siblings ranged in age from 3 months to 34 years with a mean of just less than 10 years.
Questions on the general health of child
(See Appendix 2, section 1.)
In the questionnaire we asked specifically about medical problems with the child’s ears and generally about other medical problems. We asked if the child had ever had any medical problems with their ears and 83 out of 122 (68.0%) parents responded ‘sometimes’ or ‘a lot of the time’. The average age of children in these two categories was just less than 6 years (n = 77, 71.8 months) compared with just less than 5 years (n = 26, 58.3 months) for those reporting no medical problems with the child’s ears.
More specifically we asked if a doctor had ever diagnosed the child with glue ear and 69 out of 122 (56.6%) responded ‘yes’. A follow-up question to those 69 respondents asked how often the child had had glue ear since their first birthday. The answers are shown in Table 14.
| How often has your child had glue ear since their first birthday? (N = 69) | ||||||||
|---|---|---|---|---|---|---|---|---|
| All of the time | At least every month | Three to four times a year | Once a year | Less than once a year | Don’t know | Missing | Total | |
| n | 25 | 4 | 17 | 5 | 6 | 11 | 1 | 69 |
| % | 36.2 | 5.8 | 24.6 | 7.2 | 8.7 | 15.9 | 1.4 | 100 |
| Age range (years) | 2–11 | 6–10 | 2–11 | 3–11 | 4–10 | 2–8 | ||
| Age, mean | 5 years, 8 months | 8 years, 0 months | 6 years, 5 months | 7 years, 0 months | 7 years, 3 months | 5 years, 3 months | ||
By design of the sampling, all parents in the interviews and focus groups described that their child had experienced glue ear, although it is notable that on one occasion a parent indicated that glue ear had not explicitly been diagnosed or mentioned by name by any clinician involved in their child’s care. Talk of glue ear mirrored the more general discussions about hearing problems, in that it is typified by fluctuations in symptoms, uncertainty about its impact (hearing loss, development delay and/or glue ear) and uncertainty about treatment. (Some form of uncertainty about symptoms, consequences or treatment of glue ear was manifest in all of the families who were interviewed or participated in a focus group.)
[Question: How has glue ear affected health and behaviour?] Well, you see, this is the thing with Down syndrome because there are so many issues going on, you never quite know which of the one in the balance is affecting the thing . . . but as I said, she has got the visual problems and she has got the mobility problems so then you don’t know which of the elements is playing up, if we could knock out one of them, would there be an improvement.
(Theme 1.1.3 complexity of symptoms and comorbidities and theme 1.2.1 uncertain consequences – cannot distinguish from other features of Down syndrome.)
It is perhaps worth noting that when prompted to talk about their child’s health, and health problems associated with Down syndrome, parents rarely considered hearing issues without this being prompted by the interviewer. In those cases where the parent did independently identify hearing as an issue this was not the first issue to be discussed or perceived to be the most significant issue affecting their child.
Discussion of hearing issues most commonly included reflection upon early years hearing tests as well as concerns about hearing loss, and its broader impact upon communication and development. (Concerns about hearing loss and communication development were universal.) Fluctuations in hearing were described, and the challenge of establishing hearing loss in a child with Down syndrome was also flagged as an important and complicating factor. Parents described concerns that developmental problems might mask (or falsely indicate) hearing problems.
She certainly enjoys listening to things, listening to music and things, and I don’t know. She is always wanting the volume at the top volume whatever it is; but because of A’s character, personality, I do think there is an element that she would just want it really loud any way . . . I think there are times when she is doing something and I am asking her a question and she doesn’t react to me and sometimes there is definitely an element of her ignoring me, she has heard me but she has ignored me.
(Theme 1.2.1 uncertain consequences – cannot distinguish from other features of Down’s syndrome and theme 2.1.1 uncertain diagnosis – complex comorbidities)
A number of parents found difficulties with the hearing tests.
He would get bored with the test halfway through and I think he can hear that sound but he is just not turning around to look at the cat in the box because he is more interested in the toys on the table . . . if they started with the low frequency sounds they would say his low frequency seems to be okay but he is not hearing the high frequency so well. Well, that is because he got bored before we got there, you know, we got bored half way through.
(Theme 2.1.1 uncertain diagnosis – complex comorbidities and theme 2.1.3 failure of hearing test)
Other health problems were noted in questionnaire responses by 89 parents and are categorised as shown in Table 15. The majority noted more than one problem.
| Other health problems | n | %a |
|---|---|---|
| Cardiac | 39 | 43.8 |
| Respiratory | 20 | 22.5 |
| Gastrointestinal | 19 | 21.3 |
| Orthoptic | 15 | 16.9 |
| Endocrinological | 13 | 14.6 |
| Auditory/ENT | 11 | 12.4 |
| Sleep disorder | 8 | 8.9 |
| Disorders of diet/feeding (5), immunity (6), musculoskeletal system (4), tear ducts (4), hair loss (2), neurological system (2) and leukaemia (1) |
Heart conditions were also most commonly presented as important in the interviews and focus groups, even by those whose child had not had heart problems.
[Question: Any kind of significant health issues over the years?] His heart was absolutely fine when he was born. They have checked that and rechecked it since and it seems to be, touch wood, no major anomalies at all. He has in fact had very little illness. I mean, he has had things. His main problems have been bowel problems after starting school . . . He has had a couple of bouts of chest infection but which in fact cleared quite quickly, much more quickly than we feared. We had feared, from talking to other people that you know, chest can be a real difficult one for children with Down’s. He did have glue ear which was picked up quite early on . . .
(Theme 1.4.2 other health priorities)
Questions about the effects of glue ear
(See Appendix 2, section 2.)
Parents who indicated that their child had had glue ear (n = 69) were asked which aspects of the child’s life and functioning had been affected by the condition. The results are shown in Figure 2.
FIGURE 2.
Aspects of a child’s life and functioning that had been affected by glue ear.
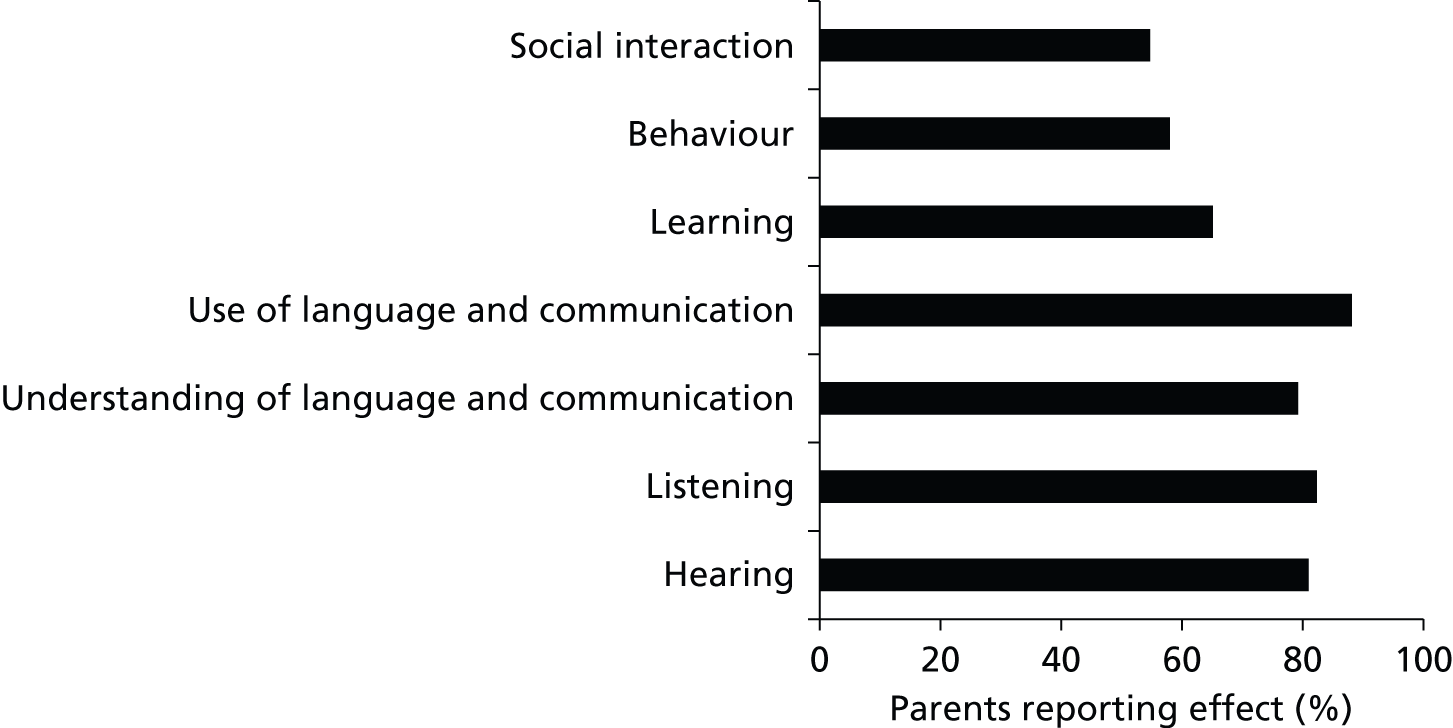
In addition one parent reported effects on sleep and eating and another mentioned safety. Eight (11.6% of 69) parents added comments in the questionnaire responses about the fact that it was difficult to say exactly what, when or how glue ear specifically impacts upon the child, as any hearing loss, however slight, merely adds to the difficulties that a child with Down syndrome already has in progressing in all the areas.
Similar concerns about isolating specific symptoms associated with glue ear were evident in the qualitative interviews and focus group data. Moreover, parents expressed some uncertainty about the extent to which their child’s glue ear and their Down syndrome could or should be considered separately:
It shouldn’t be managed as though it is a child that is presenting with glue ear and they don’t have Down syndrome. I do feel as if it is just, they treat it as glue ear.
And they don’t see the other side. For example, my daughter when she has colds and things like that her hearing is quite badly affected. So it is other things that trigger the hearing off as well and I just feel that this research could kind of separate the glue ear and the types of management it needs for children with Down’s other than just treating it as going through a system . . .
And I think also it might be useful just to look at the broad picture and see how things are in the existing situation. Our children who have Down syndrome have to be treated differently from developing children.
(Theme 1.1.3 complexity of symptoms and comorbidities, and theme 2.2.2 pathway not working)
Explicit questioning in the interviews and focus groups about the consequences of glue ear for children with Down syndrome drew similar responses to the questionnaire data. Concerns about speech, communication and about how glue ear might exacerbate developmental delay were emphasised consistently (by approximately 75%) and repeatedly, perhaps more so than less specific concerns for hearing difficulties (hearing loss, volume to maximum, difficulties in group settings) and other characteristics, such as congestion and infections.
It’s the dominoes effect, isn’t it? Because glue ear itself isn’t all that serious. It’s not life threatening, it’s not you know; and it could come and go as he gets older and whatever. But obviously, it is impacting on him. It definitely is.
(Theme 1.2.2 Multiplier effect – exaggerates other issues)
I think you see in the communication area, so if we assume that because she has got glue ear that is also affecting her communication and her development of her speech, then she has certainly got quite frustrated and angry at times when she can’t get her message across because her speech hasn’t developed at the same rate as another four-year-old’s. So sometimes her behaviour is more like, say a two-year-old’s behaviour with their lack of speech.
(Theme 1.2.2 multiplier effect – exaggerates other issues, theme 1.2.4 social interaction and behavioural, and theme 1.2.5 educational/developmental)
Questions about interventions received for glue ear
(See Appendix 2, section 3.)
Sixty-two parents responding to the questionnaire (89.8% of 69 reporting glue ear) said the child had received help for glue ear. The number of parents answering positively for each intervention is shown in Figure 3 by the green bars. The two most common interventions were the provision of a standard behind-the-ear air conduction HA and insertion of grommets, but a significant number of children had experienced a variety of other interventions.
FIGURE 3.
Number of parents reporting interventions for glue ear received, and reporting improvement in symptoms. Green bars: number of respondents answering positively for each intervention. Black bars: number of respondents answering positively for the intervention leading to an improvement. a, Other (n = 4): treatment for gastric reflux, speech and language therapy, surgery to remove infected tissue from eardrum, ENT operation (not specified); b, alternative therapy (n = 10): osteopathy, craniopathy, herbal fluid drainage, vapour rub and steam baths, acupuncture, restricted diet and cranial osteopathy.
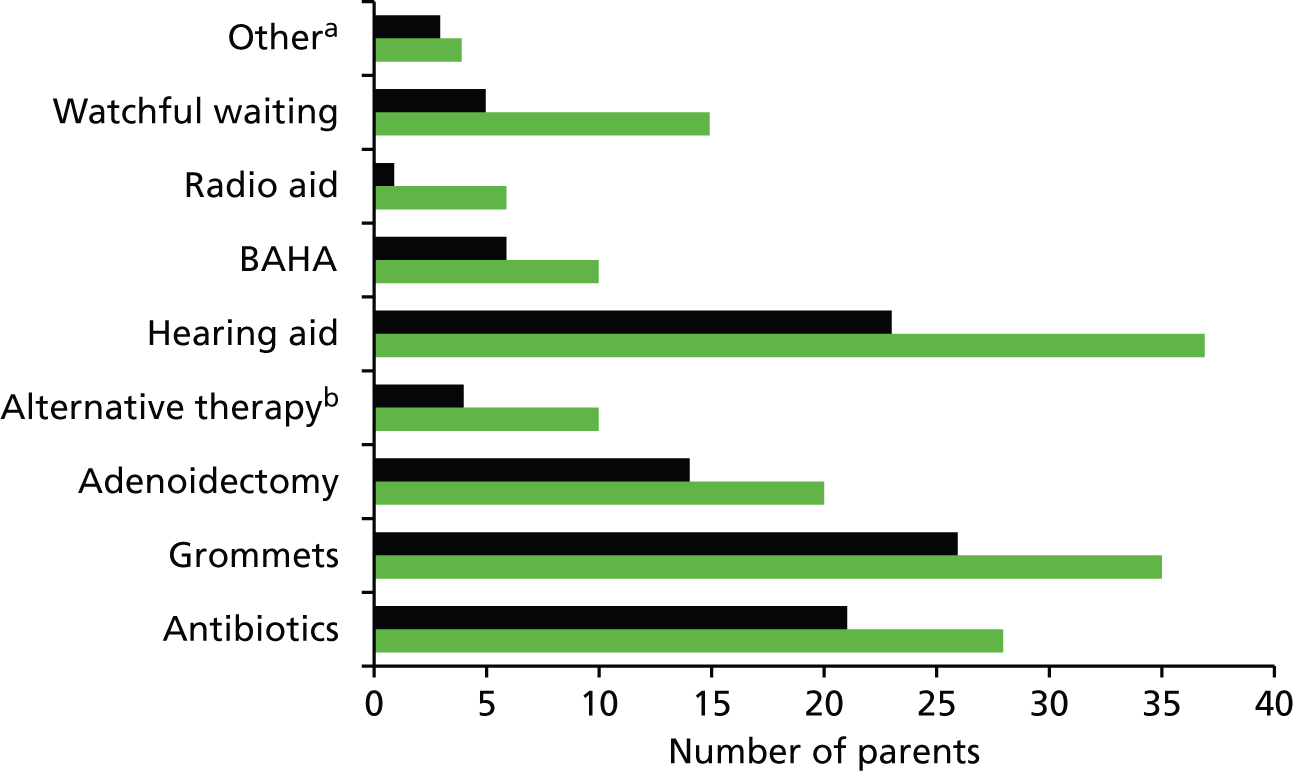
The question also asked if the parent thought that the intervention had led to an improvement. The number who answered yes for each category is shown by the black bars. The proportion reporting an improvement was 60% or more for antibiotics (75.0%), grommets (74.3%), adenoidectomy (70.7%), HAs (62.2%), BAHA (60.0%) and ‘other’ (75.0%) but was 40% or less for alternative therapies (40.0%), WW (33.3%) and radio aids (16.7%).
Additional comments on options for treatment included issues around lack of knowledge, limited choice and tolerance of treatment options particularly HAs and BAHAs and concerns about surgical interventions. These issues were also reflected in the interviews and focus groups. Experience of treatment was highly personalised, and no single treatment option was either favoured universally or totally rejected across the data. On various occasions parents described the benefits of grommets, of HAs (of all types), of adenoidectomy, of antibiotics, and even one case of craniopathy (although this was the only complementary therapy described). Equally, difficulties of grommets falling out and HAs not being accepted by children were presented by parents.
My daughter tried to put them down the toilet, I mean, how many times can you say to a consultant and say, ‘My daughter has put the hearing aid down the toilet?’ It is not going to kind of wash for very long, is it? You know, they are not going to like it.
(Theme 3.2.2 grommets have failed, and theme 1.1.3 complexity of symptoms and comorbidities)
A trial and error approach to treating glue ear and the use of WW left some parents feeling uncertain about clinical decision-making and reinforced the need for clearer and more consistent treatment pathways. Complaints about individual doctors or services were uncommon (although not non-existent), but rather parents presented a lack of confidence in the way that glue ear is managed more generally, and complained about a lack of treatment options (or the availability of treatment options). These more explicit ‘complaints’ were more evident in the focus group data than individual interviews.
My personal experience is when S was born, she is seven now. When she was born they thought she was deaf. It has generally taken seven years to kind of come up with a soft band hearing aid. It is the first time she has been offered it in terms of to help hearing. So it has taken seven years to kind of get her to the point where we are thinking we are actually actively helping her with her hearing and you know, I think realistically for it to have taken seven years, something has got to be worked out for future children or for, you shouldn’t have to struggle with your hearing for seven years just because you have got Down’s, because effectively they didn’t know what to do with her really and I think in the 21st century, it is not really good enough.
(Theme 2.1.2 failure of diagnosis and theme 2.2.2 pathways not working)
For some parents this left them with feelings of heightened responsibility for managing their child’s glue ear, and having to make ‘clinical’ decisions, and to some extent feeling deserted by medical professionals. Although this sort of complaint was less common in the interview data (perhaps one-third of parents alluding to this), it was evident in both focus group discussions:
Yes, I feel as a parent I am kind of left to manage it all by myself even though I do have contact with health care professionals; I don’t have a regular audiologist who my daughter goes to see on a regular basis. It was kind of left at the last appointment ‘it is up to you to decide when E’s hearing is down and you, as a parent have to look out for it because although she has glue ear, there is nothing we can do about it until she is so many years old and we are not prepared to treat it. So you are just going to have to manage it as well as you can.’ … I was so annoyed by, you know, the NHS system and the care that is in place or the protocols that they follow for children with Down syndrome in XX … So I just feel like there is no harmony in the process.
I would just echo that completely. The kind of watchful waiting thing, it is a huge burden on the parents.
(Theme 2.2.2 pathways not working, and theme 2.4.1 heightened parental responsibility for managing care)
It would be easy to conclude from the focus group data, for which parents were perhaps emboldened by the support of their peers, that there was a broad dissatisfaction with treatment pathways. An important part of this was the perception that cost and financial constraints are often key factors in clinical decision-making and in the treatment options available to their children.
I don’t have much confidence in GPs these days . . .
I think some of the logics of the GP leaves [a lot] to be desired.
It is all about saving money so things just get passed.
They only tend to say, ‘We want to look at one thing first, if you go with two things, we can only listen to one.’ . . . The logic, isn’t there? I accept the logic is for saving money.
(Theme 2.2.2 pathways not working) and theme 2.3.3 professionals influenced by non-clinical factors)
They [GPs] just don’t know. I think realistically they just don’t know, do they?
It goes back down to costings and GPs having to do budgets and everything else. It’s ridiculous.
You see, I think the BAHA band is very expensive. I have been told it is very expensive.
(Theme 2.2.2 pathways not working, and theme 2.3.3 professionals influenced by non-clinical factors)
Questions about views on research
(See Appendix 2, sections 4 and 5.)
We asked parents (in the questionnaire) how much they knew about research in general (Figure 4).
FIGURE 4.
Parents’ knowledge of research in general.
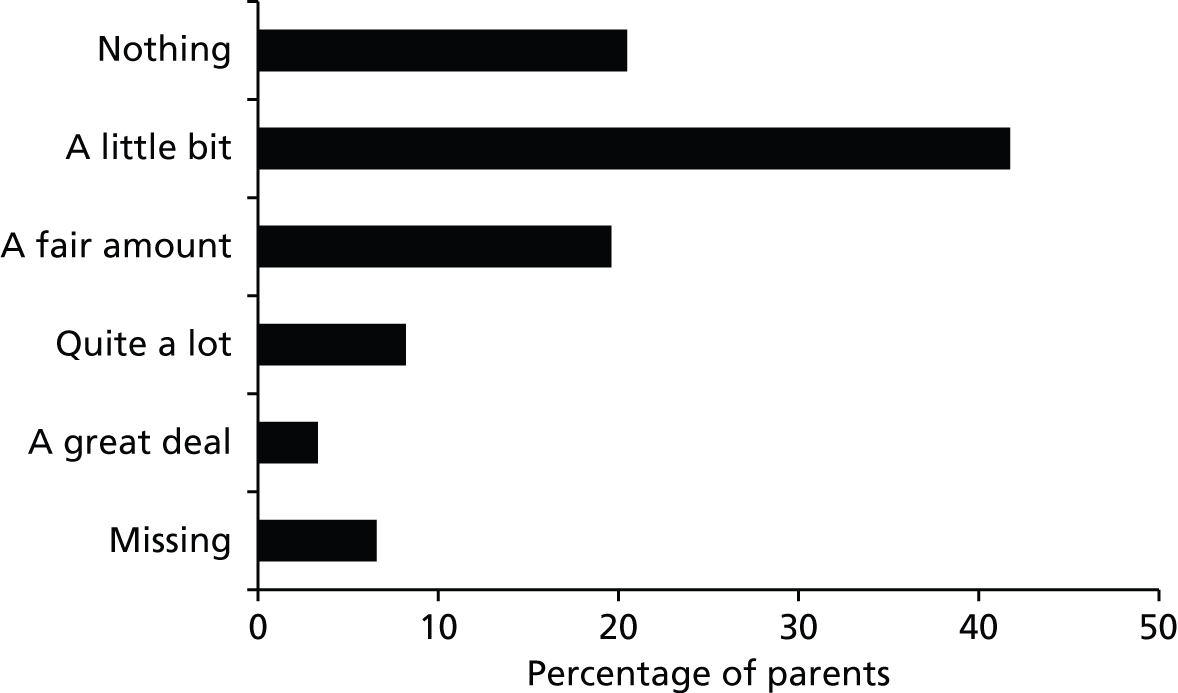
Later in the questionnaire (see section 5) we asked respondents if they would agree to their child taking part in a research study that was either a RCT or an observational study. We provided a summary of what each methodology involved on the second page of the questionnaire (see Appendix 2).
Forty-six respondents of the 115 who completed the question about RCTs (40.0%) said they would agree and 23 (20.0%) said they would not but another 46 (40.0%) answered ‘don’t know’. We recognise that positive response about willingness to agree to participate in future research may reduce when faced with a real choice. However, these findings provide useful information documenting the number of parents who express strong views either way about treatment options that might threaten any randomisation process.
Table 16 correlates parents’ willingness for their child to take part in research with their knowledge of research described in Figure 4. Owing to the small numbers, categories of ‘quite a lot’ and ‘a great deal’ have been combined. There was no significant difference in the extent of knowledge documented by those who expressed a willingness to take part in a RCT (Fisher’s exact test: p = 0.275) or in an observational study (Fisher’s exact test: p = 0.609).
| Taking part in: | Knowledge of research in general | Total | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nothing | A little bit | A fair amount | Quite a lot or a great deal | Missing | |||||||||||||
| n | %a | %b | n | %a | %b | n | %a | %b | n | %a | %b | n | %a | %b | n | % | |
| RCT | |||||||||||||||||
| Yes | 14 | 30.4 | 58.3 | 17 | 37.0 | 34.0 | 8 | 17.4 | 34.7 | 6 | 13.0 | 42.9 | 1 | 2.2 | 25.0 | 46 | 100 |
| No | 3 | 13.0 | 12.5 | 10 | 43.5 | 20.0 | 4 | 17.4 | 17.4 | 5 | 21.7 | 35.7 | 1 | 4.3 | 25.0 | 23 | 100 |
| Don’t know | 7 | 15.2 | 29.2 | 23 | 50.0 | 46.0 | 11 | 23.9 | 47.8 | 3 | 6.5 | 21.4 | 2 | 4.3 | 50.0 | 46 | 100 |
| Total | 24 | 100 | 50 | 100 | 23 | 100 | 14 | 100 | 4 | 100 | 115 | 100 | |||||
| Observational study | |||||||||||||||||
| Yes | 15 | 20.5 | 62.5 | 30 | 41.1 | 60.0 | 15 | 20.5 | 65.2 | 11 | 15.1 | 78.6 | 2 | 2.7 | 40.0 | 73 | 100 |
| No | 4 | 36.4 | 16.7 | 3 | 27.3 | 6.0 | 2 | 18.2 | 8.7 | 1 | 9.1 | 7.1 | 1 | 9.1 | 20.0 | 11 | 100 |
| Don’t know | 5 | 15.6 | 20.8 | 17 | 53.1 | 34.0 | 6 | 18.8 | 26.1 | 2 | 6.2 | 14.3 | 2 | 6.2 | 40.0 | 32 | 100 |
| Total | 24 | 100 | 50 | 100 | 23 | 100 | 14 | 100 | 5 | 100 | 116 | 100 | |||||
Thirty per cent of those who said ‘yes’ to taking part in a RCT say they know nothing about research in general compared with 20.5% of those who said ‘yes’ to an observational study. Looking at this another way, 58.3% of those who know nothing of research in general said ‘yes’ to a RCT, and 62.5% said ‘yes’ to an observational study.
It is unsurprising that awareness and knowledge of clinical research varied considerably across the sample [from those with no knowledge to those exposed to research at work (as a nurse)], and perhaps most concern about health/clinical research was demonstrated by those who displayed more awareness of health research activity and events. For example, the nurse was sceptical of medical research owing to her association of it with pharmaceutical representatives and big business.
In the interviews and focus groups, in general terms, research into medical conditions was viewed positively by all, and this is perhaps the key message to be taken from this data.
I think it can only be a positive thing and not negative, you know, and I don’t think necessarily people are being guinea pigs. I think they are doing it because they are trying to get to find out what works better and what can help other people. So I am all up for that. I think it is a positive thing, definitely.
(Theme 4.1.1 AHR is important a positive thing, and theme 4.1.2 AHR can improve treatment)
It is notable that a number of interviewees automatically associated health research with a clinical trial design (around one-third made this automatic assumption), and this could be problematic given the concerns that were communicated about randomisation. This was most explicitly described in interview 15, but other parents used phrases such as ‘guinea pig’, ‘(clinical) trial’ and ‘placebo’, and alluded to adverse events reported in the press (in early phase clinical/drug trials) when invited to consider what they knew about health research.
My understanding is when new treatments need to be evaluated and assessed there can be different ways of doing that. You can sometimes have blind studies where people are not receiving any sort of treatment whatsoever, sometimes they’re aware of that, sometimes they’re not. That people will be given two or three different sorts of treatment and then a comparison will be made at the end. Sometimes that can require a leap of faith from patients to buy into that process.
(Theme 4.2.3 misunderstanding of purpose, and theme 4.2.4 misunderstanding of process)
The qualitative data demonstrate support for further research into the management of glue ear in children with Down syndrome. No interviewee or parent taking part in a focus group rejected the need for, or potential to undertake, research in this area even though many were uncertain about what this might actually mean. It also highlights that willingness to participate in research is complicated by families negotiating complex circumstances, that circumstances (such as the presence and/or severity of glue ear) vary through time, and that different families have widely differing experiences (more or less severe glue ear, exposure to different treatments, more or less health issues, more or less behavioural issues). It is this juxtaposition of need with potential barriers that is perhaps the most important feature to recognise in the qualitative data.
That there is no common experience of glue ear and Down syndrome, and that there is perhaps no consistent manifestation of glue ear in children with Down syndrome, is a problem for the conception and formulation of any future research study.
I think the key is, although operating on a higher plain you would love to think [that] for the greater good I will put my child into whatever trial, actually when it comes down to it, it is your child; you are making the decision on their behalf, that they can’t input into often and you, when push comes to shove, people may have their own concerns about treatment options for all different reasons. Somebody might be offered grommets and think actually I don’t want to put my child through an operation. Other people might be offered watchful waiting and think, I couldn’t live with myself for not intervening when something might have, you know so everybody is going to have a very personal response to the treatment that they might be offered in a randomised trial and that is what makes it difficult I think, particularly because it is children who are involved.
(Theme 4.5.2 willingness context specific – treatment options, and theme 5.1.5 RCT: willingness context specific – treatment options)
It is perhaps worth noting that no study design (RCT or observational) was automatically or conclusively dismissed by parents. It is true that parents indicated a greater willingness to (hypothetically) include their child in an observational study, and that randomisation was perceived as a significant barrier to accepting RCT design by many parents. However, arguments about the strength of RCT research were accepted by some, and for others the simple assertion that any research is better than no research meant that RCTs would not be dismissed.
I know I argued against randomised but I see the element because I think at the beginning we all said, ‘Yes please’ [to research]. So if the choice is randomised or nothing, and leaving us with no research and nothing to change the way we are offered stuff, then I suppose you lean towards, ‘Please do something. Find out something,’ isn’t it? Rather than just leave it down to each individual doctor who is doing it based upon nothing and offering you really no treatment, as such.
(Theme 4.3.2 a need for OME research – care lacks foundation)
The devil of course is in the detail and it is perhaps worth speculating that it is treatment options more so than study design that might most influence a parent to involve their child in a future study. Concerns to avoid certain treatments or to gain access to new treatments were evident in most interviews (> 75%) and a strong focus for discussion in the focus groups.
[Question – would you involve your child in an RCT that had surgery as an option?] Well, because I am totally against him having surgery unnecessarily I would probably say no. But if it was something else I would probably say yes, okay but I really don’t want him to have surgery. [if surgery was involved] I would definitely say no . . . because knowing my luck I would be in the surgery [group].
(Theme 5.1.5 RCT: Willingness context specific – treatment options)
As is evident from the above, parents displayed a greater awareness of clinical trial research than they did of observational studies. Clinical trials were discussed with more confidence and to a much greater degree of detail than observational studies, knowledge of which seemed scant and speculative.
The benefits of a clinical trial design were recognised explicitly in a couple of cases, but in most, the discussion of trial design simply took a harder and more certain tone. Tests and testing, scientific, drugs, new treatments, controlled studies, etc. were all used to describe how a clinical trial was understood. There was some consideration that this more scientific type of research might come with risks attached, in that it frequently involves testing new things where the outcome is uncertain and unknown, and might result in people being given things that they do not require. However, clinical trial research was broadly recognised as the way that research is done to show that new things work.
When you hear the term clinical trial, it is sort of . . . It gives it a sort of gravitas. It gives it a sort of, you know, an importance, a believability factor that goes up. That is what I am trying to say . . . yes, a controlled trial, that sounds good, ‘Oh, yes that must mean that they know what they are doing.’
(Theme 5.1.3 RCT as gold standard)
That clinical trials were not automatically associated with randomisation in many cases perhaps indicates most clearly the lack of a complete understanding about research processes that informed the majority of participants’ thought and comments. Discussion of randomisation (following explanation by the interviewer) left most parents feeling uncomfortable about it, and uncertain as to whether or not they would be happy to recruit their child to a study where they were randomly allocated a treatment. Concerns about being left with no treatment or being exposed to ‘the wrong’ treatment for their child were communicated.
I think my one issue would be if she was – and I know it’s randomised – but if she was in the group where nothing, where she was just being observed, I would be concerned that, perhaps however long the, the trial took place – say six months – over that time that might be a crucial time in her development and she’s not having any treatment for something that she should be having treatment for, therefore it affects her speech delays or whatever, then that would be my concern.
(Theme 5.2.2 randomisation = risk)
I think I said I was nervous about involving him in a randomised control trial because that could lead to him being randomly placed in a group where he gets a treatment option that I don’t agree is right for him at that time.
(Theme 5.2.3 randomisation removes choice/control)
Focus group discussion explored that it might be important to undertake research using randomisation, but in the main the added sense of responsibility that parents feel for their children meant that exposing them to greater perceived ‘risk’ (associated with the uncertainty of randomisation) would be a significant difficulty.
Everyone has expressed their concerns, I mean if that [randomisation] is the way that the research is designed and that is the best way to get results, even though everyone has their concerns, they have got their opinions, then maybe that is the way to push it. Do you know what I mean? If you are wanting to get your results and you have looked at other ways of doing the research and unfortunately randomisation is the only way forward . . . then unfortunately that is probably the best way it is going to be to get your results, if that makes sense.
I think the problem with that is that you are making a judgment for your child. So you might make that judgment for yourself and be willing to put up with that but you know our children as a general go through an awful lot - have to put up with a lot. Life’s not a bed of roses for them at all and so definitely pain and discomfort . . . I think that is quite hard to do as a randomised thing, if you think you are making a judgment with no informed consent from your child . . . [and you can’t say] ‘I know this is the best option for you because the doctors have told me.’
And so therefore you are doing that, rather than, ‘I have put you in a study and actually this may not turn out to have been the best I could have done for you.’ That is quite hard to do as a parent.
(Theme 5.1.3 RCT as gold standard, and theme 2.4.1 parental responsibility for care heightened, and theme 5.2.2 randomisation = risk)
That observational research was perceived to involve active decision-making (on the part of clinician and/or family), based on the child’s clinical need, meant that this type of research was presented as more acceptable by the majority of parents, most indicating that they would be more likely to consent their child’s participation to this type of study than one involving randomisation. However, it should be remembered that these conclusions followed precise descriptions of what an observational study involves and were not entirely without precursor in the interview discussion, i.e. most were not really aware of what observational research involved and conceived research to be a ‘clinical trial’. In setting up any study this lack of knowledge/inaccurate perception would need to be addressed to ensure positive recruitment.
If someone had said to me when we made the decisions ‘Let’s not try grommets, let’s try hearing aids with J, do you mind if we see how that goes if we see how his hearing is over whatever period . . .’ I would have said yes . . . I’d be more likely to do that, than him being randomly selected . . . It goes back to the main thing at the start, whichever treatment he’s having should be a decision that his parents have made, rather than it being made randomly or for them.
(Theme 5.2.3 randomisation removes choice/control) and theme 5.3.3 observational research + active decision making)
Yes, if he was already on the waiting list for an operation for grommets then yes, I would be quite happy to have somebody sort of investigate, take away his notes or come and look at his hearing or whatever else you wanted to follow up, if I had already made that decision that grommets was the right way to go, or he was going to go and get a hearing aid . . . I think people are more likely to say yes under those circumstances because you are not, you, because you already have taken a decision about treatment and it is your decision about treatment and it is not a random decision about treatment. Again, yes, you have taken the decision already and it may involve very little extra appointments. It may just be pro formas in the notes that the clinicians have to fill in as they are going along rather than extra trips back and forwards to the hospital.
(Theme 5.3.3 observational research + active decision making, and theme 5.3.4 observational research is non-intrusive)
In the questionnaires, parents were asked to comment freely on the benefits and problems for their child being involved in health research using observational studies and using RCTs (Table 17). Parents who responded to the questionnaire were aware of the benefits of research for the treatment of children in the future over and above any immediate benefit for their child and many noted no problems, but problems that were noted tended to be for the individual including availability of time and disruption to routine and difficulties with co-operation from the child. There were parents who were concerned about RCT research, as it would mean that they would have no say in the treatment offered to their child.
| Benefits | RCT (n = 73 parents) | Observational (n = 91 parents) | ||
|---|---|---|---|---|
| n | %a | n | %a | |
| For children in the future | 24 | 32.9 | 44 | 48.4 |
| For individual child | 14 | 19.2 | 12 | 13.2 |
| None | 0 | 0 | 9 | 9.9 |
| Providing information/understanding | 8 | 10.9 | 9 | 9.9 |
| Closer follow-up and monitoring | 4 | 5.5 | 9 | 9.9 |
| Less intrusive as continue on same treatment | 0 | 0 | 6 | 6.6 |
| Providing treatment options | 6 | 8.2 | 0 | 0 |
| Saving money in the long term | 7 | 9.6 | 0 | 0 |
| Randomisation means reduced bias | 5 | 6.8 | 0 | 0 |
| Problems | RCT (n = 78 parents) | Observational (n = 84 parents) | ||
| n | %a | n | %a | |
| None | 12 | 15.4 | 31 | 36.9 |
| Co-operation of the child | 17 | 21.8 | 19 | 22.6 |
| Too many appointments | 6 | 7.7 | 8 | 9.5 |
| Time and disruption to routine | 14 | 17.9 | 26 | 30.9 |
| Children are different therefore treatment should be tailored | 9 | 11.5 | N/A | – |
| Would not want particular treatment | 4 (surgery) | 5.1 | N/A | – |
| 8 (no treatment) | 10.2 | |||
| Treatment allocated might not work | 16 | 20.5 | 0 | 0 |
| Wasting valuable (developmental) time with ineffective treatments | 4 | 5.1 | 0 | 0 |
The interviews and focus groups explored this further. Both focus groups started with an explicit question about the need for further research into the management of glue ear in children who have Down syndrome. With one exception the very clear answer to this was that research is required and/or would be valued by families (the dissenting voice was unsure and rather vehemently against research). In some ways the certainty of this response challenges the uncertainty of the interview data (where hearing is not prioritised in the main, uncertainty about research processes, etc.), and it could be that this direct question garnered the response that the participants felt the researcher wanted. However, in their construction focus groups are intended to avoid this form of interviewer bias and further prompting generated a range of thought-out reasons as to why research would be valued. These reasons might be summarised into three broad categories: perceptions of inconsistent care, perceptions of care built upon uncertain foundations (i.e. a lack of confidence in treatment), and feelings of being left alone to manage their child’s glue ear (from monitoring and practical management to clinical decision-making). At the start of these group discussions there was a clear sense that parents felt that glue ear is not currently adequately managed, and that research might lead to improvements.
I mean it is really interesting because this shows as we have all said you need to do research to get a clear pathway that actually works because it seems to be everybody is trying something and there doesn’t seem to be a lot of success.
(Theme 4.3.1 a need for OME research – inconsistent provision, and theme 2.2.2 pathways not working)
Again, it was evident that in some of their responses parents were (independently) alluding to a broader community of families who have a child with Down syndrome, and demonstrating a commitment to improving outcomes for all even if research offers them no direct immediate improvements.
Some kind of consistent approach which you get from research that is what I think. And if it doesn’t benefit us or anyone in this room, I mean, if it does, brilliant but if it – the next people coming through, then because people with Down syndrome have benefited from people 20 years ago with Down’s Syndrome, it has moved on, hasn’t it? So that is why I think it is important.
(Theme 4.4.3 willingness to participate in OME research – to help other families, and theme 1.3.3 a more consistent pathway)
Parents were asked in the questionnaire to choose from a closed list of situations that would encourage or discourage them from taking part in future research using RCT or observational study methodology if their child needed treatment for glue ear. Some of these situations complemented each other, for example encouragement by having the time to take part, discouragement from lack of the time to take part and they are reported in Table 18. Data are reported as a percentage of the number of respondents who answered the questions (n = 112 for the statements about encouragement and n = 107 for the statements about discouragement).
| Something that would encourage research participation | Something that would discourage research participation | ||
|---|---|---|---|
| Time to take part | 62.5% (n = 70) | Lack of time to take part | 64.5% (n = 69) |
| Knowing what will happen | 71.4% (n = 80) | Uncertainty about what will happen | 50.5% (n = 54) |
| Payment of travel expenses | 32.1% (n = 36) | Having to cover the cost of travelling | 25.2% (n = 27) |
| Learning about new treatments | 74.1% (n = 83) | Lack of knowledge about research | 31.8% (n = 34) |
| Child being offered a treatment that would really help | 90.2% (n = 101) | Child may be offered a treatment that does not really help | 55.1% (n = 59) |
| Othera | 9 | Otherb | 12 |
For 60–65% of respondents having the time to take part was important but fewer people were concerned about the cost of taking part.
The opportunity for the child to be offered a treatment that would really help, but to which they would otherwise not have access, would be an encouragement to take part for 90% of parents, and the fact that the child may be offered a treatment that would not really help would discourage only 55.1% of parents from taking part. Treatments that have not worked in the past would discourage participation; new treatments or treatments that have been restricted might encourage participation.
Concerns about surgery and anaesthesia are understandable and more commonly voiced in the qualitative data from the interviews and focus groups.
No, I wouldn’t because he has had it before. I think one of the reasons is because he has had the operation before . . . as you probably know children with Down syndrome have smaller air ways and smaller, all the tubes and things are smaller, so they don’t cope very well with anaesthetic. A friend of mine, her son, he went in for something to do with his tear ducts and he was in there a whole week because she said he had an adverse reaction to the anaesthetic and he was in there a week and they had to give him antibiotics eventually because they said something had happened. He’d got liquid, fluid on his lungs or something all because of this and she said, ‘I only went in to have his tear ducts done,’ and it is just scary the whole thought of surgery for anything is just totally scary unless obviously if it is a heart condition or something that needs to be done then that is fine but it is just totally scares the life out of me.
(Theme 3.1.2 anxiety about surgery, and theme 3.1.3 anxiety about anaesthesia)
Watchful waiting also drew comments and concern. Some saw this is as no different to what happens normally (either as part of clinical treatment or just normal parenting), whereas others perceived it to be removal of treatment and potentially a cause of wasted time.
[Question – would you involve your child in an RCT that had watchful waiting as an option?] . . . if we ended up in the watchful waiting group, I would find that really difficult. In a situation like that, you can’t dictate which group you’re in. I think because I’m so sort of anxious about S’s speech development, because that has such a massive on going effect on her life. If her speech doesn’t develop, in terms of her growing up and going out into the wider world and making herself understood . . . Inability to communicate has such a massive impact upon that and so I would find that really difficult. I think if it was watchful waiting and her speech didn’t develop well . . . That [participation in a study] would be really hard . . . if it was a randomised trial and it was either having grommets or having hearing aids – that would be a different scenario for me.
(Theme 5.1.5 willingness context specific – treatment options, theme 5.2.3 randomisation removes choice/control, and theme 3.4.2 WW = doing nothing)
It is notable that in both interview 3, concerning surgery, and interview 15, concerning WW, the concerns communicated are not general in nature but very much rooted in their own parenting experiences. This brings us back to the point made previously about the importance of personal or familial experiences in guiding attitudes towards treatment and research, and in influencing the likelihood of parents being willing to involve their child in a research study.
Parents had the opportunity via an open question in the questionnaire to add comments on anything else that they thought would make it easier for parents of children with Down syndrome who have glue ear to take part in research. Eighty-two parents took that opportunity and the categorised list is presented in Table 19 in full, as it contributes to the way future research might be conducted.
| Comment | n | %a |
|---|---|---|
| Receiving a detailed explanation of the study and clear information including risks, and the advantages and disadvantages of different options | 33 | 40.2 |
| Appointments carried out locally at home or in school; data collection remotely/online | 29 | 35.4 |
| Appointments at a convenient time, e.g. in school holidays | 19 | 23.2 |
| Confidence in the treatment options offered | 10 | 12.2 |
| No unnecessary treatment or lack of treatment | 9 | 11.0 |
| Limited travel or help with travel expenses | 6 | 7.3 |
| Child care | 5 | 6.1 |
A wide range of reasons were offered in the interviews and focus groups in support of the value of future research into the management of glue ear in children with Down syndrome. Perceived difficulties with current care, and models of care, have already been mentioned but others were evident to a greater or lesser degree. That other aspects of the health of children with Down syndrome (such as heart conditions) are already well researched, whereas hearing has not been previously considered, alongside perceived problems with specific aspects of hearing care (hearing tests specifically), might all be considered good reasons for research in this area. That involvement in a research study might mean improved/more consistent care for those involved was explicitly considered throughout focus group 2. That research might generate improvements in care for future generations was perhaps a more consistent recognition across all data. However, if one argument should be singled out more than any other it was the recognition that hearing problems might contribute to, or exacerbate, other difficulties associated with Down syndrome. All parents recognised that addressing hearing problems removes barriers to development and enhances potential for future development.
. . . if this child has glue ear and the longer you leave it, for a child with Down’s then they miss out on 6 or 7 months of not hearing at crucial listening time, when they [should] pick up speech and develop their speech. That has like a knock-on effect of 2 or 3 years in some children. Whereas a child who doesn’t have Down’s, who has glue ear, will have the ability to [make-up time and] pick up speech. Do you understand what I’m saying? . . . [improved hearing might] see a change in her behaviour, so if I saw even small changes in her behaviour. If I was talking and she looked at me – made eye contact and nodded like she understood – and that was a result of [improved hearing] that would be an improvement.
(Theme 1.2.2 multiplier effect – exaggerates other issues, theme 1.2.4 social interaction and behaviour, and theme 1.2.5 educational/developmental)
Although the potential benefits of research were widely recognised, and parents throughout advocated doing research, the complexity and complications of researching within this community were also widely recognised and reported. A decision about whether or not to include a child in a research study was clearly a very difficult decision, and one that could not be divorced from specific child or familial circumstances. The nature and severity of symptoms, previous exposure to treatment, and the treatments provided within a study might all influence whether or not a family would consent to participate in a research study. The following illustrative discussion about surgery as a treatment option demonstrates a broad agreement among parents about how prior personal experience might influence a willingness to expose a child to a treatment option (either within or outside of a research study).
So from your point of view it is like, ‘I would do anything rather than subject my child to an operation.’ There will be other parents out there who think, ‘Please just give my child the operation.’
Yes.
And that will depend totally on the child’s background.
On their background.
And the severity.
And individualised approach.
Yes. The severity of what the child is suffering.
Yes.
I mean, if the child has really, really got it badly.
Yes.
That is going to be a much easier choice.
Yes.
But if it’s a variable, not too bad, you know, some winters it is worse, it makes making that decision is far harder at that level.
Yes.
Probably it is easy for me because my child is so young. So I have only started to see the effects of it as well and I think that might influence what parents decide.
Okay.
It is because you know, I haven’t been through the mill yet, several times, obviously as some parents have.
. . .
So far, H has not really had too many problems with her ears or whatever, so we are looking from that way. If H had had problems all the time with it, we would probably be looking at it in a different way. So it depends on your background as to which way you are possibly looking at it.
I don’t know, does it depend on what other interventions your child has already had? You know, your sort of response to, you know, if your child has undergone other major surgery, is your attitude towards ear surgery more risk, a pro risk then?
Yes.
Than if you . . .
What you mean, you have already had it horrible, so it would be too awful.
Yes, I think if you have already seen your child undergo a major operation, is it like that is nothing, whereas if you have seen your child undergo no operations at all. I don’t know; I am only throwing that out as an idea.
I think that is a really good question. That could be playing a part.
(Theme 4.5.3 willingness context specific – severity of condition, and theme 4.5.2 willingness context specific – treatment options)
In the questionnaire parents were asked to state their level of agreement with four statements about why people take part in research (see Appendix 2, section 5). These were:
-
to feel that I am helping people with Down syndrome by participating in research
-
to feel like I have been given a choice to participate in research by health-care professionals and that I have been consulted by them
-
to feel like a good parent, that I have some control over the effective care of my child’s glue ear
-
to have access to increased support from health-care professionals.
Figure 5 illustrates the extent to which respondents agreed or disagreed with the statements. It appears that respondents indicated altruism in their strong agreement with the idea that taking part in research might result in future benefit.
FIGURE 5.
Level of agreement with four statements about why people take part in research.
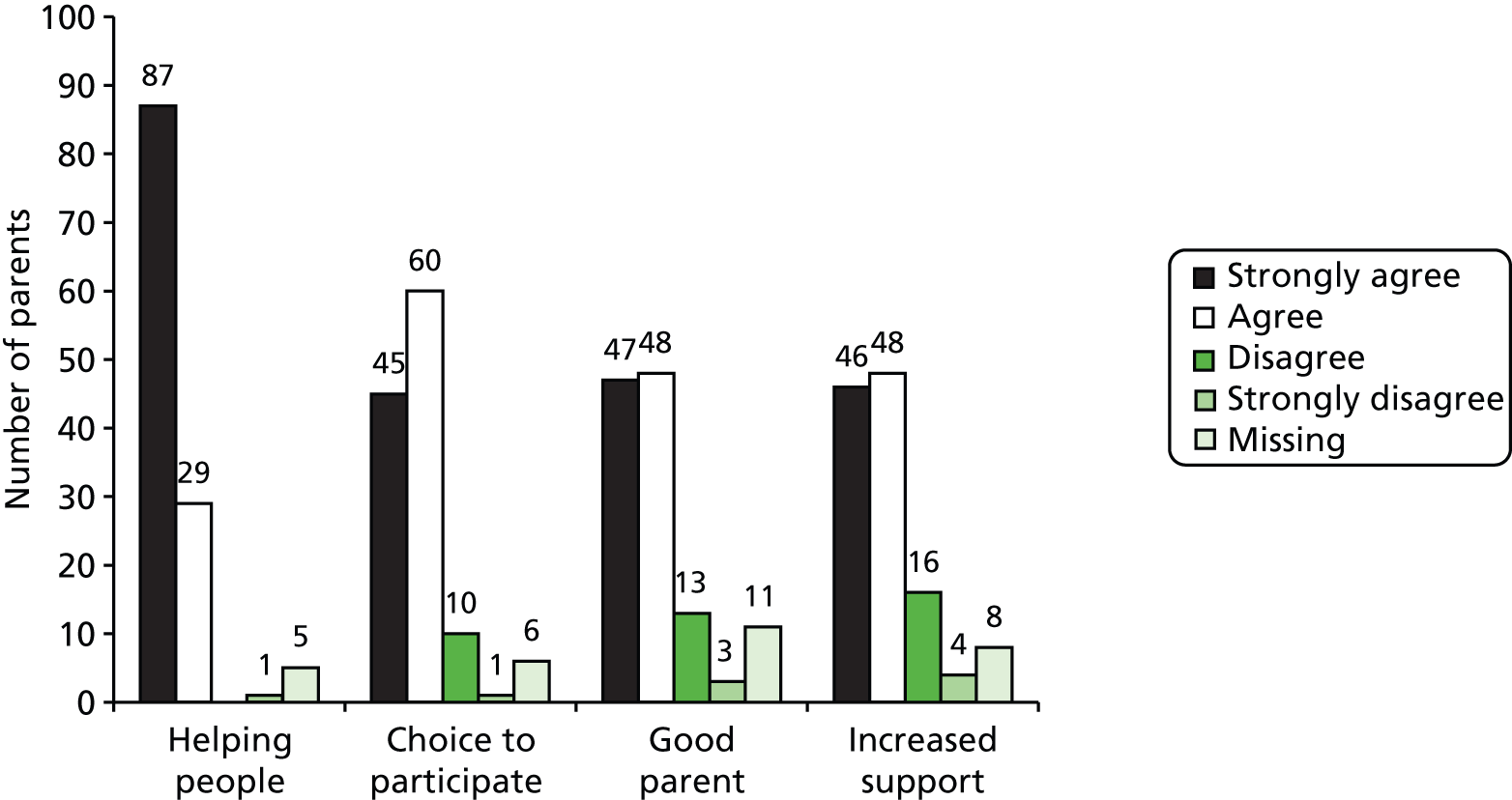
Other reasons given in response to an open question included the desire to increase knowledge and optimise treatment options both for their own child and for others in the future.
Questions about benefits for the child of taking part in research
(See Appendix 2, section 6.)
In order to explore the importance to parents of potential outcomes to be measured in any future study we asked parents responding to the questionnaire to consider, if future research had to look at one or two good outcomes from a health research study comparing different treatment options for glue ear in children with Down syndrome, which of four options would they consider to be most important and which second in importance. The four options were:
-
my child’s speech, language and communication with me and others to improve
-
my child’s hearing to improve
-
my child to make progress at school/nursery
-
my child’s social interaction with others (e.g. with friends and family members) to improve.
Forty-eight respondents (39.3%) failed to answer the question correctly, ticking three or four options (rather than only two). The remaining 74 respondents ranked the outcome as shown in Figure 6.
FIGURE 6.
Importance to parents of potential outcomes to be measured in any future study.
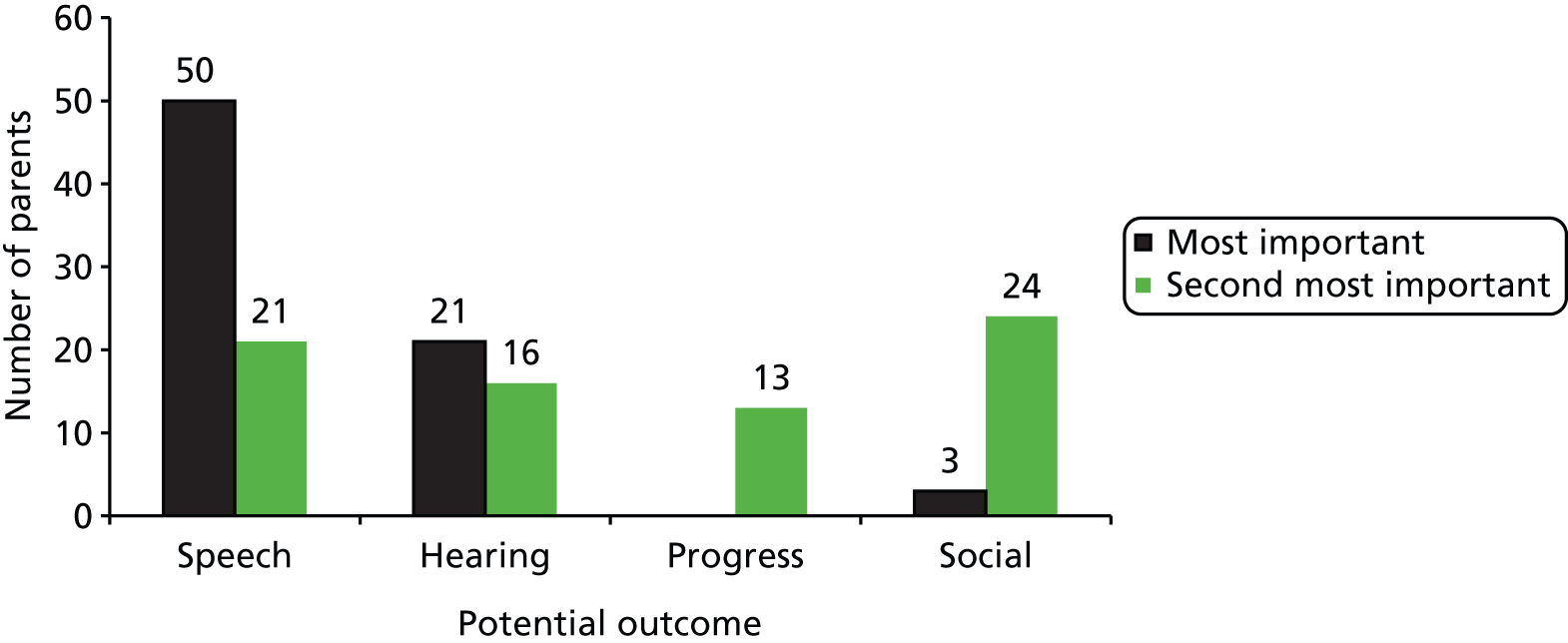
The majority of parents ranked improvements in speech, language and communication to be the most important outcome (67.6%) with no parent choosing progress at school or nursery. The choices for second most important outcome were more evenly spread across the four options. Table 20 shows the distribution of second choices against first choices.
| Most important outcome | Second most important outcome | Total | |||
|---|---|---|---|---|---|
| Speech | Hearing | Progress | Social | ||
| Speech | N/A | 15 | 13 | 22 | 50 |
| Hearing | 19 | N/A | 0 | 2 | 21 |
| Progress | 0 | 0 | N/A | 0 | 0 |
| Social | 2 | 1 | 0 | N/A | 3 |
| Total | 21 | 16 | 13 | 24 | 74 |
It may be that parents would have different views on the importance of outcomes dependent on the age of the child. Figure 7 indicates the percentage of parents with younger and older children who noted each possible outcome as the most important. It is clear that improvements in speech, language and communication are still valued most highly regardless of the age of the child and that progress in school or nursery is never considered the most important outcome.
FIGURE 7.
Percentage of parents reporting the importance of potential outcomes to be measured in any future study for younger and older children.
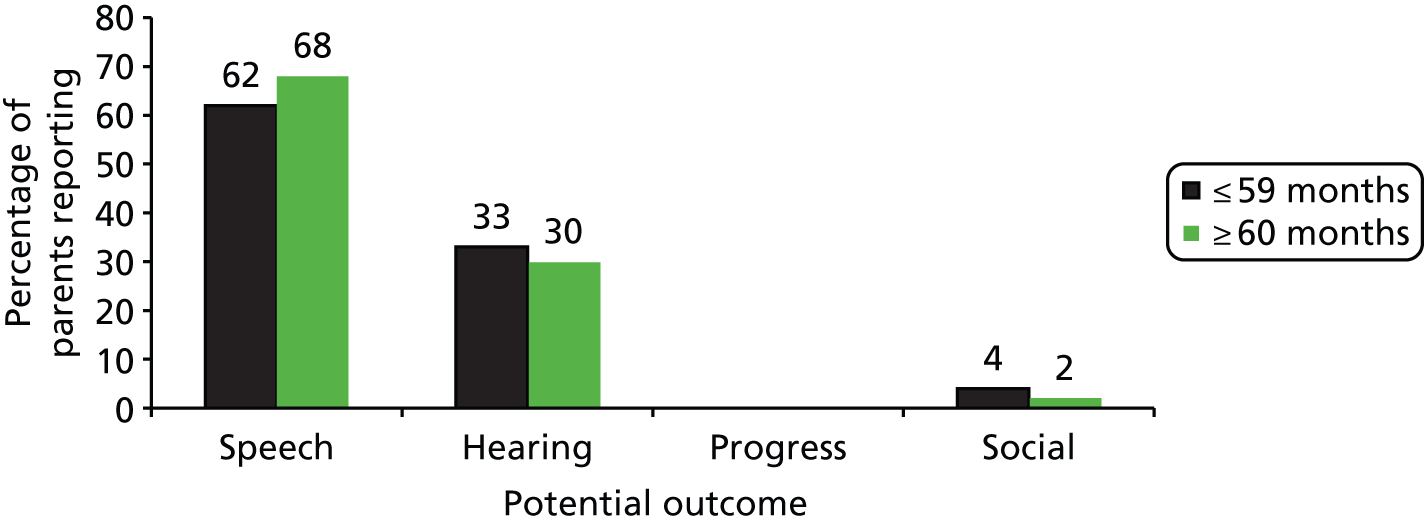
When asked what else is important for you and your child to gain from research into different treatment options, many of those who commented said it was important to be better informed about the condition and the treatment options (n = 34).
In the interviews and focus groups, without exception, improvements in a child’s hearing was deemed to be an appropriate goal for any research in this area. However, hearing was rarely referred to in isolation, and was most commonly associated with improvements in speech, communication and behaviour.
I think the main thing would be his speech and when he is responding to us, to be honest. If he was progressing with his speech or not. . . . linking his words together, because he is still only just starting to do that now. So in theory if his hearing was bad at the moment and then it was relieved by grommets or whatever I would expect it to actually, to start linking at least in the next six months. But if his hearing was bad then he would just stay with single words.
(Theme 1.3.2 speech and communication)
If his hearing was improved then his speech would improve, his communication and therefore, his behaviour … Behaviour is often linked to lack of communication, but it’s also attention seeking and you can’t judge whether or not an operation’s been successful on behaviour, because life’s full of so many different things going on. It’s very complex.
(Theme 1.3.1 hearing, theme 1.3.2 speech and communication, theme 1.2.4 social interaction and behaviour, and theme 1.2.2 multiplier effect – exaggerates other issues)
During the focus group discussion, a more abstract goal of improving patient pathways and enabling more consistent treatment was flagged as a possible outcome of any research but this did not supersede language and communication when participants were asked explicitly to identify what they would like research to achieve.
Speech. So from hearing comes speech.
And language acquisition, you know, and therefore that will spread out into reading and . . .
Behaviour problems frustration, don’t you – when you can’t hear and you can’t . . .
Absolutely.
It is, it is like you have said before, it’s a domino effect, isn’t it? It triggers it all off.
(Theme 1.3.1 hearing, theme 1.3.2 speech and communication, theme 1.2.4 social interaction and behaviour and theme 1.2.2 multiplier effect – exaggerates other issues)
Seventy-four families of children with Down syndrome had complete and valid data noting two and only two outcomes as important. To compare these data with data from the open-ended question responses collected in the TARGET study,40 352 families who produced responses spontaneously mentioning at least two of the four domains included in the current study were selected. (Over 1100 families had supplied some data but many had mentioned only two domains and many of the domains mentioned are not in the present scheme; generally, there was similar ranking of domains within multiple-mention data, as with mentions of two or one.) The alignment between the study questions is not perfect. The current study asked respondents to rank a fixed set of outcomes, whereas the TARGET respondents were asked an open, non-prompted question. Not all of the families may have been aware of the considerations in glue ear. The analyses are shown in Table 21. For speech, hearing and educational progress there are significant differences between the groups (p < 0.001), whereas for social development there is a marginal association only. The data show a strong preponderance for the importance of speech/language for families of children with Down syndrome relative to families of children without Down syndrome, and a strong preponderance of hearing, and also of progress in education, as important for families of children without Down syndrome relative to families of children with Down syndrome.
| Outcome measure | Parents of children with Down syndrome (n = 74) | Parents of children in the TARGET trial (n = 351) | Significancea (p-value) | ||
|---|---|---|---|---|---|
| Important | Not important | Important | Not important | ||
| Speech | 71 (96%) | 3 (4%) | 96 (27%) | 255 (73%) | < 0.001 |
| Hearing | 37 (50%) | 37 (50%) | 252 (72%) | 99 (28%) | < 0.001 |
| (Educational) Progress | 13 (17.5%) | 61 (82.5%) | 273 (78%) | 78 (22%) | < 0.001 |
| (Social) Development | 27 (36.5%) | 47 (63.5%) | 177 (50%) | 174 (50%) | 0.052 |
Looking at the preponderance of concerns on a dichotomous basis, the ordering for families of children with Down syndrome is (high importance first) speech/language, hearing, social development and then education, whereas for TARGET study40 families it is education, hearing, social development and then speech/language.
The ordering in this breakdown of the results is highly credible, based on the pathological contrast between uncomplicated glue ear and the constellation of problems in Down syndrome of which one may be glue ear. Speech, language and communication are of concern for parents of children with Down syndrome. The extent to which any middle ear disease exacerbates these will be important. The fact that the pattern is not too surprising does not undermine the usefulness of having direct data on it.
Outcomes evaluation
Seven parents responded to the invitation to comment on the MacArthur–Bates CDI and the OMQ-14 instruments. All had completed the original questionnaire and had taken part in either an interview or a focus group, and were aware of the aims of the research.
They were asked to assess the instruments against three questions. The first asked if they felt that the questions in the instruments would apply to a child with Down syndrome. A summary of the results is shown in Table 22.
| Do you think the questions would apply to a child with Down syndrome? | OMQ-14 | MacArthur–Bates CDI ‘Words and Gestures’ (younger children) | MacArthur–Bates CDI ‘Words and Sentences’ (older children) |
|---|---|---|---|
| Yes | 2 | 6 | 4 |
| No | 1 | 0 | 1 |
| In part | 4 | 1 | 2 |
The second and third questions asked if there were things about the instruments that would make it difficult to answer or if there were things about the instruments that were particularly good for a child with Down syndrome.
The OMQ-14 (see Appendix 6), which is designed for children aged 3–9 years who are experiencing ear problems, was generally not thought to be wholly appropriate for children with Down syndrome. Of the 14 questions, nine were cited by at least one parent to be difficult to answer. Reasons stated were that the children often exhibit these behaviours regardless of ear problems because they may have difficulties with concentration, understanding, speech development and responding to requests, and generally require more attention. The early questions (1–4) were thought to be easier to answer, and the fact that the questions addressed impact and not just speech was valued.
Both of the MacArthur–Bates instruments were thought to be very long. The one for younger children was generally thought to be more appropriate for children with Down syndrome (of all ages) because it did not concentrate on speech and contained a section about actions and gestures rather than words and grammar. For both instruments, four of the seven parents noted that they could complete the questions more positively if they included signing as well. It was noted that it can be difficult to assess understanding and that some questions dealt with concepts such as time, which are affected less by hearing and more by learning difficulties. Some of the parents noted the value of the CDI instrument as a developmental log.
Summary
Glue ear and its consequences
-
Sixty-eight per cent of our sample reported that their child had difficulties with hearing and 56% reported a diagnosis of glue ear, although it may be that these figures represent an under-reporting. Interview data suggests that glue ear is not always diagnosed by name nor explicitly referred to as such by professionals in the care of children with Down syndrome.
-
Difficulties of diagnosis (with specific queries about hearing tests), fluctuation in symptoms, uncertainty about treatment (‘what works?’, ‘what is available?’) and uncertainty about the impact of glue ear each contribute to a recognition that this is a difficult condition to manage.
-
Parents associate glue ear with hearing difficulties and problems with communication. Although hearing is perceived to be the primary symptom of glue ear, parents see its greatest impact in listening, understanding language and using language.
-
Parents found it difficult to isolate the symptoms of glue ear from other aspects of Down syndrome. That hearing difficulties might exacerbate developmental delay was considered an important reason for more effective management of glue ear in this population.
Glue ear and its treatment
-
Standard HAs and grommets were the most commonly reported interventions received by our sample, with a variety of other interventions (including antibiotics and WW) also described.
-
No intervention was reported as generating improvements in all cases, although antibiotics, grommets and adenoidectomy each led to improvements reported by > 70% of parents. No single treatment option was universally favoured or universally rejected by the parents in this study.
-
Complaints about specific interventions were rare, although parents did demonstrate some uncertainties about the clinical management of glue ear in general:
-
Parents perceived inconsistent care with different interventions advocated by different clinicians, and different interventions available in different parts of the country.
-
Parents perceived unclear clinical pathways based upon uncertain foundations and limited knowledge of glue ear in this population.
-
WW was perceived to absolve clinicians of their responsibilities and to place additional pressure upon families to make ‘clinical’ decisions about their child’s care and treatment.
-
The value of research
-
Applied health research was perceived positively and the need for further research into the management of glue ear in children with Down syndrome was supported.
-
The perceived benefits of future research might include addressing difficulties with current clinical pathways and bringing about improved clinical and developmental outcomes for children with Down syndrome.
-
That the outcome of any research might benefit future generations, rather than current patients, was not considered a barrier.
-
Parents were aware that although they might advocate further research in this topic they can also identify a number of barriers that would prevent them from participating in any such study.
The form of research
-
No study design (i.e. RCT or observational study) was automatically dismissed by parents.
-
Parents indicated that to be meaningful research should seek improvements in a child’s speech, language and communication, more so than a focus upon hearing in isolation.
-
Parents demonstrated a greater awareness of clinical trial type research than of observational research, although understanding of research processes varied widely and often included inaccurate assumptions. Common misunderstandings were that all research takes the form of a clinical trial (i.e. comparing treatments often involving a placebo) and that randomisation is not necessary in a clinical trial.
-
Randomisation was perceived by parents as a significant barrier that might prevent them from consenting their child to a research study. Randomisation might mean that their child gets no treatment or the wrong treatment; that treatment is allocated by chance was viewed as an important difficulty.
-
That observational research would involve treatment actively allocated by a clinician was perceived to make this type of research more acceptable. For this reason, parents indicated that they would be more likely to get involved in a study of this kind.
-
Parents expressed concerns about surgery being included in a study indicating that the risks associated with surgery and anaesthetic would discourage them from getting involved. In a similar vein, some parents indicated that the inclusion of WW would discourage their involvement, for fear of not receiving treatment and disadvantaging their child.
-
It is most likely to be personal circumstances – more so than study design or research outcomes – that will influence a parent’s decision about involving their child in research. Whether symptoms are well managed, whether treatment options have already been tried, experiences of previous treatments, etc. will all influence a parent’s willingness, although it may be that this willingness changes over time as symptoms fluctuate, and health and behavioural difficulties increase or decrease.
Chapter 4 Exploration of the views and opinions of health-care professionals and teachers
Objectives
To:
-
assess the feasibility of studying the options for management of otitis media in children with Down syndrome via a RCT or multicentre prospective cohort study
-
evaluate the willingness of clinicians to recruit participants to a definitive study
-
determine relevant and practically measurable outcome domains for use in a definitive study
-
assess the feasibility and practical requirements for collecting these outcome measures of the relevant type.
Methods
Online questionnaire survey
Professionals with clinical/professional responsibility for children with Down syndrome and/or otitis media were approached to complete an online questionnaire. ENT surgeons, paediatricians, audiologists, SLTs, and teachers were contacted in July and August 2012 via regional and national professional organisations and special interest groups. These involved ENT UK, British Association of Community Child Health (BACCH), Down Syndrome Medical Interest Group, Trent Regional Paediatric Audiologists Group, British Association of Teachers of the Deaf (BATOD), Royal College of Speech and Language Therapists (RCSLT), British Academy of Audiology (BAA), British Society of Audiology (BSA), Down Syndrome Special Interest Group for Speech & Language Therapists, Regional Interest Group for Speech & Language (Northampton & Nottingham) and East Midlands Speech & Language Therapy Managers (Leicestershire, Derbyshire & Nottinghamshire). Details of the project and a link to an online questionnaire were distributed via e-mail lists, electronic and paper newsletters, online fora and social media sites.
The questionnaire was developed by the research team including representatives of the main professional groups involved in the care of children with Down syndrome (ENT surgeon, paediatrician, audiologist, SLT). Assessment of the practicalities of online administration was piloted with members of staff of the research unit.
The questionnaire explored, for each participant:
-
their caseload of children with Down syndrome and the proportion who experience glue ear
-
approaches to clinical management
-
opinions on frequency and significance of the consequences of glue ear for this population
-
the importance of different outcome measures
-
opinions of interventions and their role in future research
-
their views on health research and the facilitators and barriers to participation and recruitment in research involving RCTs.
A text version of the questionnaire is included as Appendix 7.
Respondents were made aware that a further stage in the research would involve using the Delphi technique to try to reach a consensus view and were invited to express an interest in taking part. Follow-up questionnaires were not possible, although ENT UK agreed to distribute the questionnaire to their mailing list on a second occasion.
None of the organisations were able to tell us how many people the questionnaire would potentially reach.
Delphi technique
The Delphi technique is a consultative process that seeks to establish consensus among an expert panel by using an iterative approach to scoring, revising and rescoring a series of structured statements until a designated level of agreement has been reached (or a designated number of scoring rounds have been completed). 68 It is an approach that is widely used in health research and in the development of clinical and/or diagnostic criteria. 69,70
Although lacking precise rules for implementation (e.g. in number of scoring rounds, number of participants, accepted score for consensus, etc.) the approach is underpinned by four core principles: (1) a selected expert panel; (2) numerous iterations and controlled feedback; (3) statistical feedback of whole group responses; and (4) anonymity of responses. 61,71,72 The review undertaken here consulted a range of clinical experts. It was pragmatically restricted to three rounds of scoring to ensure maximum retention of participants. Summary statistics of all responses were reported to panel members between scoring rounds. The review was delivered electronically using the online ‘SurveyMonkey’ tool (Survey Monkey Inc., Palo Alto, CA.) allowing participants anonymity.
The questionnaire described above constituted the starting point for this review and statements for round 1 were informed by a preliminary analysis of data generated during the survey. At an interim point of survey data collection research group members (LB, HF and PL) considered the emerging findings. Independently, they identified important, interesting, expected and unexpected features of the data. Particular attention was paid to those questions for which there was an evident uniformity of professional response and those for which there was evident disagreement across respondents.
Statements were generated collaboratively by these research group members, with the purpose of both informing the broad sweep of the study (i.e. to demonstrate a need for more research in this field) and clarifying specific details relating to the nature and form of any future research study. Some statements were generated to reflect the emerging findings, others to clarify or challenge emerging findings, and a final (small) set of statements were informed by preliminary analysis of the qualitative data generated in interviews and focus groups with parents.
For example:
-
The statement ‘I would support the need for further research in this field’ sought to build upon analysis showing that more than 90% of respondents (at that point in data collection) indicated that research ‘is essential for developing practice’.
-
Statements such as ‘I would be willing to recruit families to a RCT study in this field’ and ‘Because there is uncertainty about which treatment option offers the best outcomes a RCT in this field [and random allocation of treatment] is justified’ sought to challenge the finding that only 35% of respondents (at that point in data collection) indicated that they would recruit families to a RCT design study.
-
The statement ‘Current guidelines are adequate and do not require improvement’ sought to explore further the finding that only 21% of respondents (at that point in data collection) indicated that they knew ‘a great deal’ about NICE guidelines.
-
The statement ‘A study of glue ear in children who have Down syndrome should focus upon clarifying organisational matters and refining clinical pathways rather than clinical/non-clinical outcomes for the child’ directly reflected comments made by parents during a focus group.
To reflect the heterogeneous nature of the expert panel an 80% threshold of agreement or disagreement was considered to be an indicator of group consensus.
The Delphi review
A multidisciplinary group of respondents selected from those responding to the preceding survey questionnaire and indicating a willingness to take part in a Delphi review were invited to take part in the process. This group reflected the range of clinical and professional expertise involved in the care of children with Down syndrome among the original respondents to the online survey. Those who agreed were sent, via e-mail, a link to an electronic survey comprising a number of statements developed from the responses to the preceding questionnaire, and they were asked to provide an indication of the level of their agreement or disagreement with each statement on a five-point scale (strongly agree, agree, neutral, disagree, strongly disagree). All responses were anonymous. After each round the responses and opinions were summarised and returned to respondents. In rounds 1 and 2, statements reaching a consensus level of ≥ 80% were removed. In subsequent rounds, respondents had the opportunity to see statements that had reached consensus in previous rounds or to skip to the statements for that round. Statements which had not reached consensus were repeated either in the same format or amended for clarity. The development of the Delphi review is included in Appendix 8.
In round 1, 43 statements were scored. These were organised under three subheadings:
-
Is further research worthwhile? (14 statements).
-
The nature and scope for further research (18 statements).
-
Barriers to undertaking future research (11 statements).
In round 2, 25 statements were scored. From round 1, 16 statements were excluded because consensus had been reached (13 agreement and three disagreement). One statement was removed because it asked about preferred research study design and therefore did not require rescoring. Four statements were amended to aid clarity. Five statements were condensed to form two new statements. Two entirely new statements were added.
Statements were organised under four subheadings:
-
Is further research worthwhile? Reasons for further research (six statements).
-
The nature and scope for further research (six statements).
-
Outcome measures (six statements).
-
Recruitment to future studies (seven statements).
In round 3, 11 statements were scored. From round 2, nine statements were excluded because consensus had been reached. Five statements were removed because they did not require rescoring. Three statements were amended to aid clarity.
Statements were organised under four subheadings:
-
Is further research worthwhile? Reasons for further research (two statements).
-
The nature and scope for further research (three statements).
-
Outcome measures (three statements).
-
Recruitment to future studies (three statements).
For round 3, all statements were scored on a four-point Likert scale (strongly agree, agree, disagree, strongly disagree). The neutral response option was removed to encourage more positive scoring. In addition, participants were invited to comment on the list of statements for which consensus had been established.
Results
See Appendix 7 for a text version of the online questionnaire as sent to professionals. Reference is made to the relevant section of the questionnaire when reporting the results.
See Appendix 8 for detail of the development of the statements in the Delphi review, together with more details of the responses and consensus.
Response rate
Questionnaire survey
Questionnaires were submitted by 102 participants. Three respondents answered that they had no children with Down syndrome aged between 1 and 11 years on their caseload and therefore did not complete the rest of the questionnaire.
The professional discipline of the remaining 99 respondents is shown in Table 23. (Note: given that the number of respondents is 99, results are, in most cases, presented as the number of respondents rather than as a percentage, as number and percentage are assumed to almost equate.)
| Professional discipline | n (equivalent to %) |
|---|---|
| Paediatrician | 24 |
| Audiologist | 21 |
| SLT | 18 |
| ENT surgeon | 18 |
| ToD | 6 |
| Audiological physician | 4 |
| Other (ToD for deaf–blind) | 1 |
| Missing | 7 |
| Total | 99 |
Ninety-six respondents said they did have children with Down syndrome aged between 1 and 11 years on their caseload, two were unsure and one did not complete the question. These three respondents went on to answer further questions, the answers to which indicated that they did indeed care for children with Down syndrome and hence these three respondents have been included in the final total of 99.
Delphi review
Forty-two experts who indicated a willingness to take part in the Delphi review were invited to participate. The distribution by discipline of those invited and those responding is shown in Table 24. Whereas paediatricians and audiologists dominated the questionnaire responses (24% and 21%), ENT surgeons were the largest group in the Delphi review (34.5%). The distributions by discipline for both the questionnaire and Delphi review responses should be borne in mind when considering the results presented here.
| Professional discipline | Invited | Responded | % of total Delphi group | |
|---|---|---|---|---|
| n | % of invitees | |||
| Paediatrician | 11 | 6 | 54.5 | 22.2 |
| Audiologist | 7 | 6 | 85.7 | 22.2 |
| SLT | 7 | 3 | 42.9 | 11.1 |
| ENT surgeon | 12 | 9 | 75.0 | 33.3 |
| ToD | 2 | 1 | 50.0 | 3.7 |
| Audiological physician | 3 | 3 | 66.7 | 7.4 |
| Total | 42 | 28 | 64.3 | 100.0 |
Of the initial 28 respondents, all completed at least some of the statements in round 1. This was reduced to 25 in round 2, and 24 in round 3.
Overview
The closing bank of statements in the Delphi review included 42 statements. [From the original 43 statements, five statements were revised to form two new statements and two entirely new statements were created (43 – 5 + 4 = 42).] Of these, consensus was established in 31 statements, five remain unresolved and six were one-off questions that did not seek consensus. In those statements for which consensus was established, 21 concluded in agreement and 10 concluded in disagreement.
Experience of Down syndrome
The number of children on the caseload of questionnaire respondents ranged from 2 to 200, with an average of 27 (excluding those who answered ‘hundreds’, ‘not sure’, and ‘lots’ and six respondents who did not answer the question).
Respondents were asked if they considered themselves to have a specific professional interest in the care of children with Down syndrome. Forty-six per cent claimed a great deal of interest, 52% a little or a fair amount of interest and one respondent claimed no interest. This indicates that the majority of respondents, not surprisingly, had sufficient interest to complete the questionnaire, and it means that the responses reported here make a valid and valuable contribution to the issues being addressed.
Glue ear in children with Down syndrome
The proportion of children with Down syndrome who had experienced glue ear was reported as ≤ 30% for six respondents and 31–60% for eight respondents. The majority (n = 85) estimated that > 60% of children had experienced glue ear with nearly one-third of all respondents (n = 31) estimating that all children with Down syndrome in their care had experienced the condition.
Delphi data demonstrated consensus about the significance of the condition for this population, and the challenge that it poses in clinical management:
Effective treatment and management of glue ear in children who have Down syndrome is particularly important because of the high prevalence of glue ear in this population. (100% agreement in round 1.)
Effective treatment and management of glue ear in children who have Down syndrome is particularly important because difficulties with hearing can contribute to other developmental and/or behavioural problems. (100% agreement in round 1.)
The treatment and management of glue ear in a child who has Down syndrome poses distinctive challenges compared with other children. (92% agreement in round 1.)
It is difficult to be entirely confident of effective treatment and management of glue ear in a child who has Down syndrome. (80% agreement in round 2.)
For the fourth statement above no individual indicated a ‘strong agreement’ with the premise that it is ‘difficult to be entirely confident’ and it should be noted that no consensus was reached on more specific statements about reasons why a clinician might not be ‘entirely confident’ (including ‘fluctuation in symptoms’, ‘individualised response to treatment’ and ‘variety of symptoms’).
Approaches to clinical management
In section 2 of the questionnaire (see Appendix 7, section 2, questions 1–3) we asked respondents how confident they felt in explaining the benefits and risks of three possible interventions to parents of a child who has glue ear. Responses are shown in Table 25. Confidence is lower overall for surgical interventions.
| How confident are you that you can explain to a parent and their child with Down syndrome, who has glue ear, the benefits and risks of . . . | Very confident | Fairly confident | A little confident | Not at all confident |
|---|---|---|---|---|
| Surgical intervention | 24 | 32 | 27 | 16 |
| Non-surgical intervention | 39 | 34 | 18 | 8 |
| Active observation (WW) | 35 | 38 | 22 | 4 |
Confidence in this area varied by professional discipline. Taking the four disciplines represented by the most respondents (audiology, ENT, paediatrics, and speech and language therapy), ENT surgeons expressed most confidence with the surgical option (88.9% very or fairly confident) and SLTs the least (44.4%, not at all confident). For non-surgical options, 100% of audiologists said they were very or fairly confident, as did all but two (11.1%) of the ENT surgeons. Paediatricians were consistent across all of the three interventions, with 62.5% claiming to be very or fairly confident for each one. SLTs indicated most confidence with the WW option (33.3% very or fairly confident) but over all three of the interventions the majority indicated little or no confidence.
We asked respondents for views on national guidelines and the impact that new guidelines would have on their practice (see Appendix 7, section 2, questions 4 and 5). Table 26 illustrates the responses with regard to understanding by discipline.
| How much do you understand about the NICE guidelines concerning the care pathway for children with Down syndrome who have glue ear? | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Professional discipline | Nothing | A little bit | A fair amount | A great deal | Missing | Total | ||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Paediatrics | 2 | 8.3 | 7 | 29.2 | 10 | 41.7 | 5 | 20.8 | 0 | 0 | 24 | 100 |
| Audiology | 3 | 14.3 | 6 | 28.6 | 8 | 38.1 | 4 | 19.0 | 0 | 0 | 21 | 100 |
| ENT | 0 | 0 | 0 | 0 | 7 | 38.9 | 10 | 55.5 | 1 | 5.5 | 18 | 100 |
| SLT | 7 | 38.9 | 8 | 44.4 | 2 | 11.1 | 1 | 5.5 | 0 | 0 | 18 | 100 |
| Othera | 8 | 44.4 | 3 | 16.7 | 3 | 16.7 | 4 | 22.2 | 0 | 0 | 18 | 100 |
| Total | 20 | 20.2 | 24 | 24.2 | 30 | 30.3 | 24 | 24.2 | 1 | 1 | 99 | 100 |
The ENT surgeons appear to understand more (10/18, 55.5%, a great deal) and SLTs less (7/18, 38.9%, nothing), but only 24 of the total of 99 respondents knew a great deal about the guidelines. There was a broad disagreement in the Delphi review to the proposition ‘Current guidelines are adequate and do not require improvement’, although consensus was not reached in two scoring rounds (a neutral response was offered by 41% in round 1, and by 32% in round 2). A more positive formulation in an additional statement in round 2, however, did reach a consensus score.
There is a need for new and improved clinical guidelines for the treatment and management of glue ear in children who have Down syndrome. (87% agreement in round 2.)
That both RCT and observational research might provide evidence to improve current guidelines was agreed, and although agreement about observational research was universal (100%), more ‘strongly agreed’ responses were evident for RCT research (44% compared with 22%).
Evidence from a well-founded observational study could improve current guidelines for clinical practice in the treatment and management of glue ear in children who have Down syndrome. (100% agreement in round 1.)
Evidence from a well-founded RCT study could improve current guidelines for clinical practice in the treatment and management of glue ear in children who have Down syndrome. (96% agreement in round 1.)
The next questionnaire question (see Appendix 7, section 2, question 5) asked about the impact any new guidelines might have on the current practice of respondents. Only eight respondents said they would immediately change their practice in line with the guidance [two teachers of the deaf (ToDs), one SLT, one audiologist, one ENT and three missing] and the majority (88/99) said that they would consider the guidelines on the basis of the evidence presented. One respondent said that he/she would continue with current practice regardless of the guidelines, and one pointed out that he/she was unable to change treatment owing to caseload demands (both SLTs).
Consequences of glue ear in children with Down syndrome
Section 3 of the questionnaire explored views on the consequences of glue ear in terms of the frequency, significance for the child/family and degree of challenge for the professional. Eight potential consequences were listed with the option to add any other areas. Respondents were asked to indicate the top three in each area in order of importance. Figures 8–10 present the results for the consequences that were indicated to be the most important.
FIGURE 8.
Consequences of glue ear for a child with Down syndrome who has glue ear, considered to be the most frequent. a, Iatrogenic complications of repeated grommet surgery.
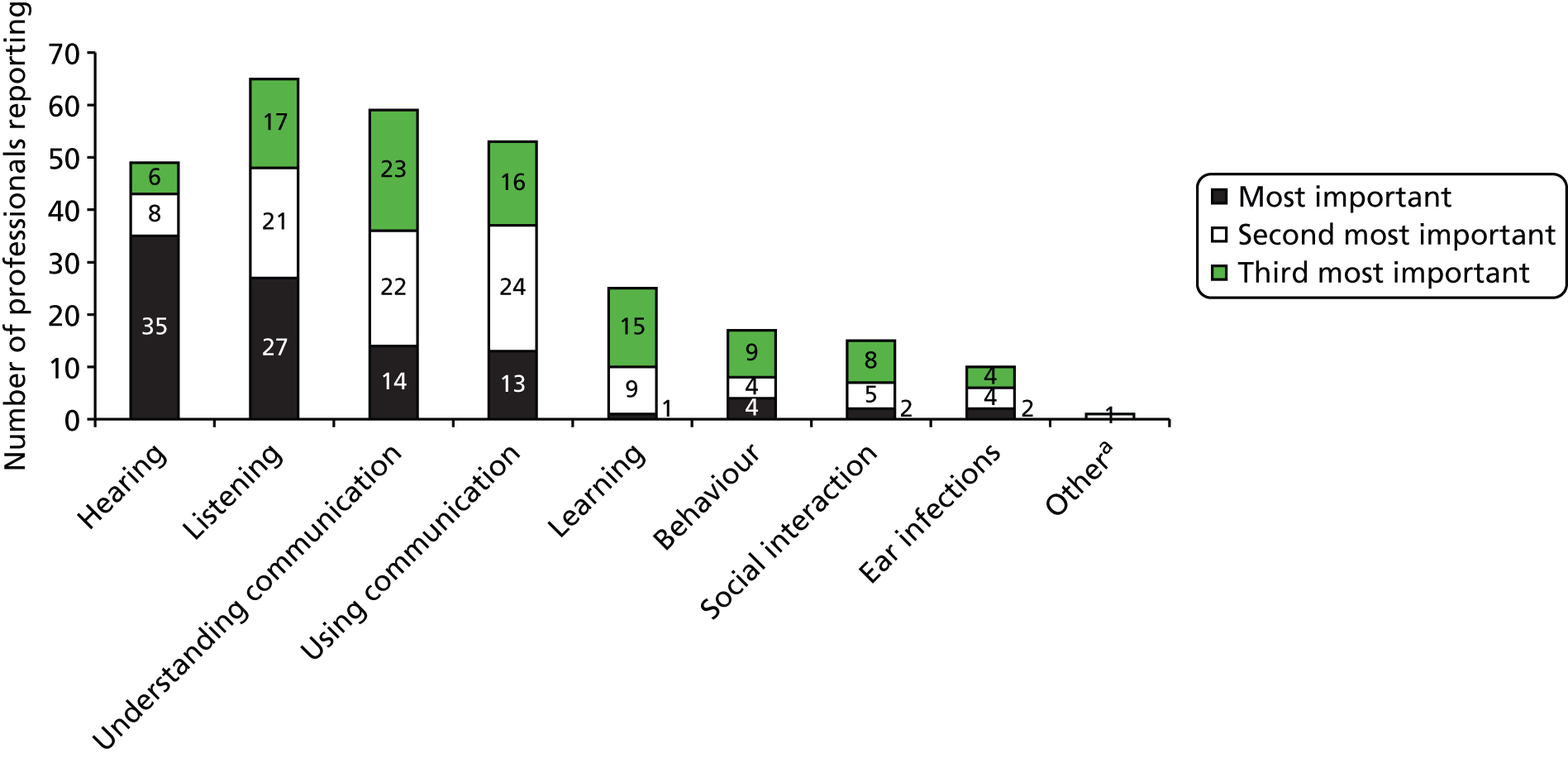
FIGURE 9.
Consequences of glue ear for a child with Down syndrome who has glue ear, considered to be the most significant for the child/family a, Difficulties in understanding the impact of hearing vs. other developmental issues and coexisting conditions.
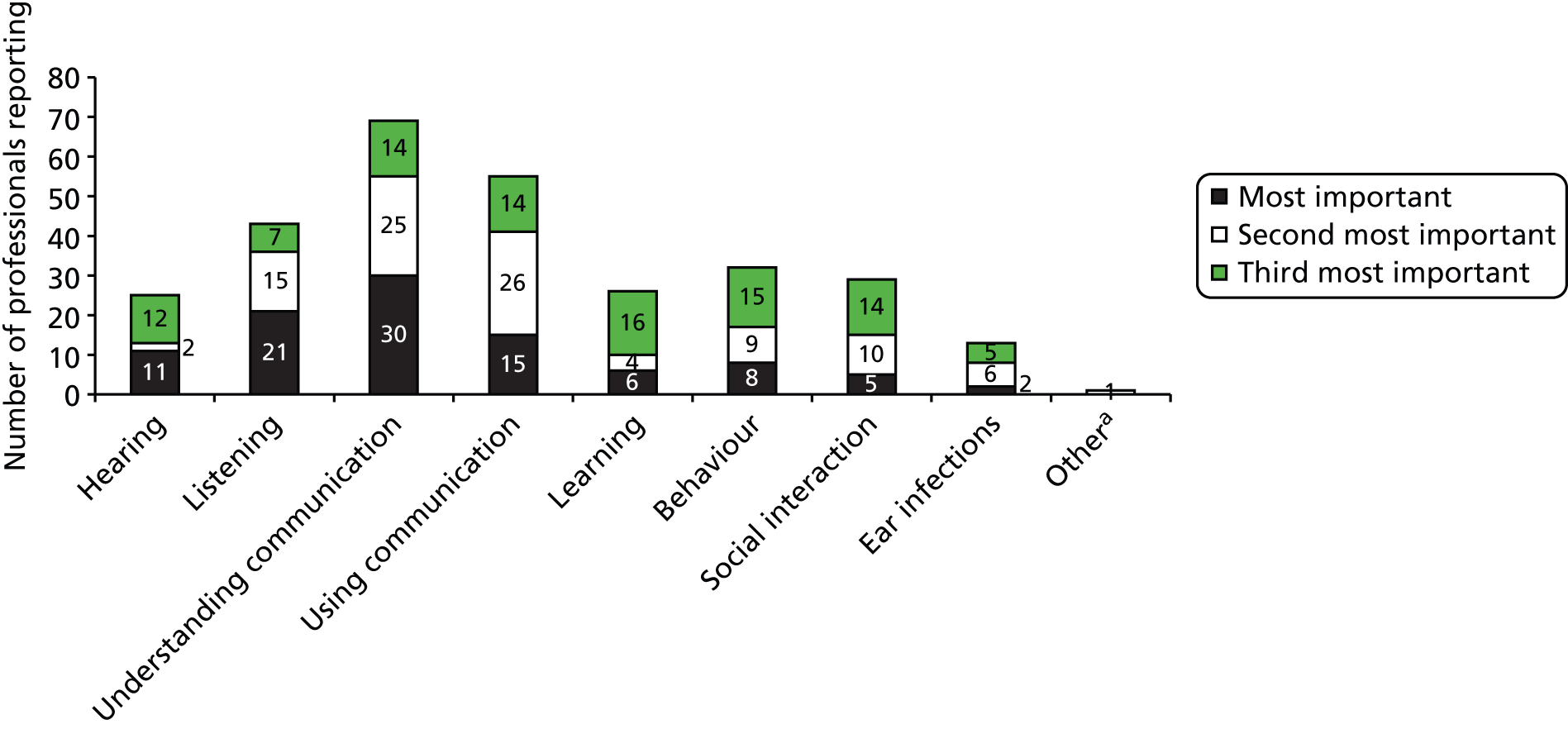
FIGURE 10.
Consequences of glue ear for a child with Down syndrome who has glue ear, considered to be the most challenging in terms of management. a, Irreversible tympanic membrane and middle ear problems; assessment/management/validation of management; narrow ear canals; parents choosing a non-surgical management but the child not wanting to wear HAs.
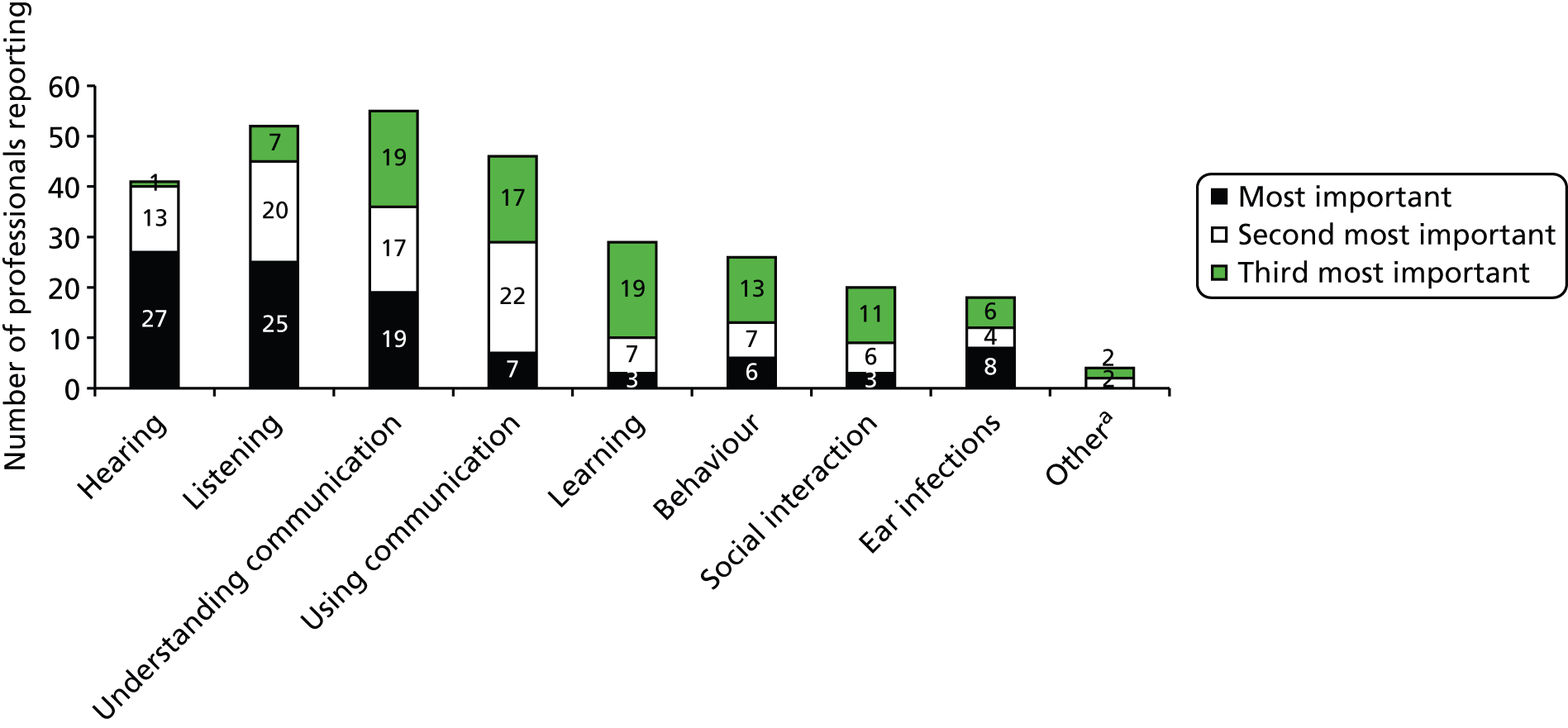
Although hearing was noted by most respondents to be the most important in terms of frequency of the consequence, in total across three choices, there were more mentions for listening, and understanding and using language and communication, i.e. although hearing is the clinical manifestation of glue ear as a condition, the effects (consequences) for the child’s communication are seen to be more frequent.
Understanding communication and listening are noted most often as the most important but in terms of total mentions, using language and communication ranks second in the list.
Hearing and listening were most often listed as most important challenging consequence, but listening and understanding communication received the most total mentions.
These concepts were explored further in the Delphi review. Agreement about the value of research to improve ‘hearing’, ‘communication’, ‘social interaction’ and ‘progress at school’ were all achieved in the Delphi review, although it should be noted that agreement for ‘progress at school’ and ‘social interaction’ was achieved in only the final round of scoring when the opportunity to provide a neutral response was removed.
A study of glue ear in children who have Down syndrome should seek improvements in a child’s hearing to be meaningful. (100% agreement in round 1.)
A study of glue ear in children who have Down syndrome should seek improvements in a child’s speech, language and communication to be meaningful. (81% agreement in round 1.)
A study of glue ear in children who have Down syndrome should seek improvements in the child’s social interaction with others to be meaningful. (82% agreement in round 3.)
A study of glue ear in children who have Down syndrome should seek improvements in the child’s progress at school/nursery to be meaningful. (91% agreement in round 3.)
The importance of research offering clinical (and/or other) benefits to the child was reinforced in the rejection of the proposal that research might address ‘organisational matters/clinical pathway’ independently of impact upon the child.
A study of glue ear in children who have Down syndrome should focus upon clarifying organisational matters and refining clinical pathways rather than clinical/non-clinical outcomes for the child. (88% disagreement in round 2.)
In order to inform the design of any future health research study comparing different treatment options for glue ear in children with Down syndrome, respondents to the questionnaire were asked to rank the importance of improvements in just two outcomes from a closed list of four, with the opportunity to add anything that they felt was more important (see Appendix 7, section 3, question 4) (Figure 11).
FIGURE 11.
Importance (for professionals) of outcomes from a health research study comparing different treatment options for glue ear in children with Down syndrome. a, Impact of conservative management on irreversible middle ear changes.
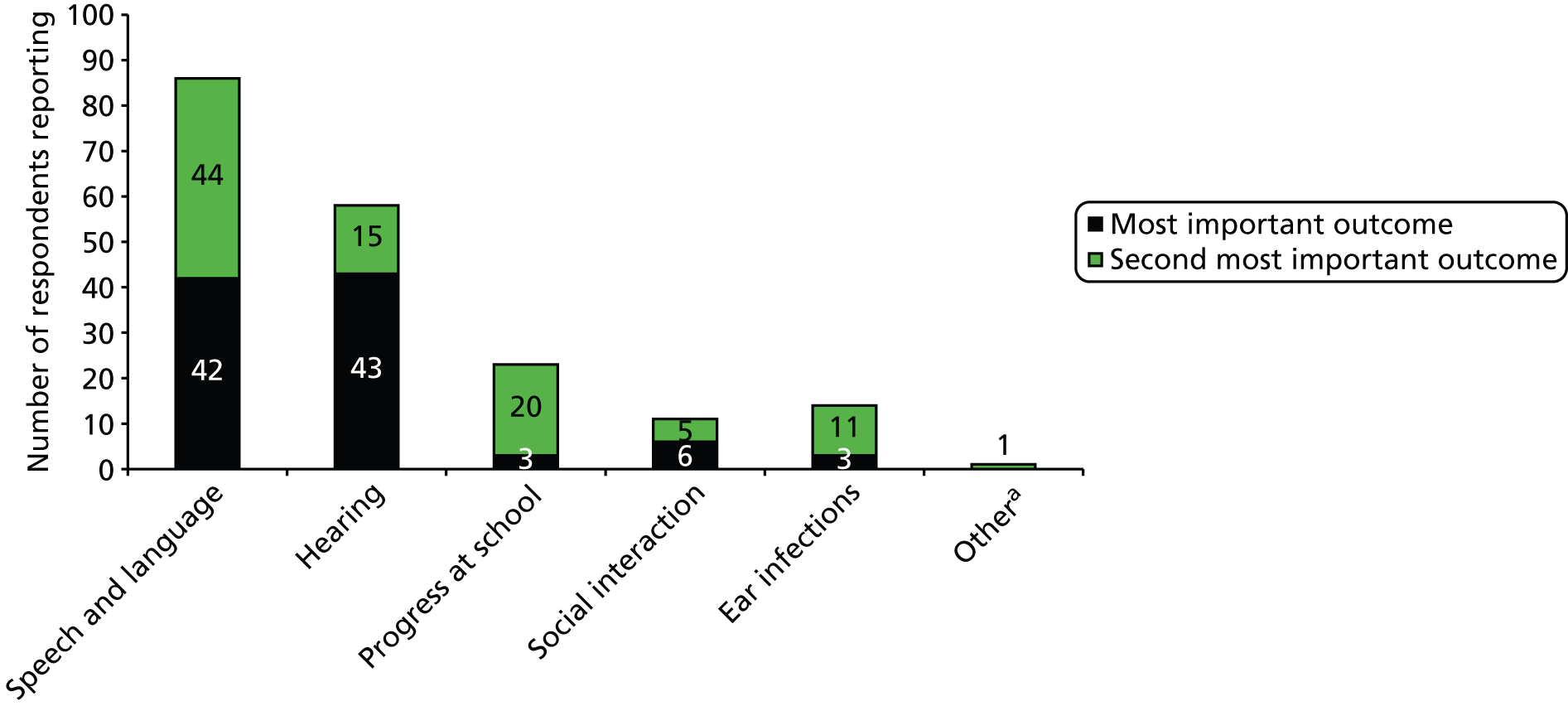
Respondents were also given the opportunity to add free-text comments on the assessment of outcomes. Several commented that many areas were important for these children and also that many outcomes are long term and less easy to measure. It was noted that changes in hearing levels are relatively easy to measure but what is really important is language and communication, which is more difficult to measure. The need for standardised measures and also for subjective measures from parents in addition to medical outcomes was also noted.
One-off explicit questions about primary and secondary outcomes for a research study were posed in the Delphi review. In round 2, where the option of a secondary outcome was included, 84% indicated that ‘improvements in a child’s hearing’ should be the primary outcome measure of any study, and 16% indicated that it should be ‘Improvements in a child’s speech, language and communication’. As a secondary outcome measure, 71% opted for ‘Improvements in a child’s speech, language and communication’, 13% ‘Improvements in a child’s social interaction with others’, 8% ‘Clarifying organisational matters and refining clinical pathways’, 4% ‘Improvements in a child’s hearing’ and 4% ‘Improvements in a child’s progress at school/nursery’.
Interventions
Children with Down syndrome who have glue ear present many clinical challenges in the choice of intervention. All professionals who work with these children provide intervention in some form whether that be fitting a hearing device, prescribing medication, recommending surgery or providing speech and language therapy.
We were interested to know what influences decisions on intervention for the respondents, accepting that each child presents different challenges. We asked respondents to the questionnaire to tell us which factors, from a closed list of eight, influenced their decisions on intervention (see Appendix 7, section 4, questions 1 and 2). Ten respondents answered that a combination of all of the eight suggested factors influenced their decision. A further 13 ticked between one and seven of the eight options, but also ticked the combination option. Excluding these 23 respondents and six who did not answer the question, 70 respondents selected between one and seven factors (total 170) and their choices are shown by the black bars in Figure 12. The green bars indicate the responses when asked to choose only one factor that most influenced the intervention decision.
FIGURE 12.
Factors influencing decisions on intervention for children with Down syndrome who have glue ear (black bars) and those that have most influence (green bars).
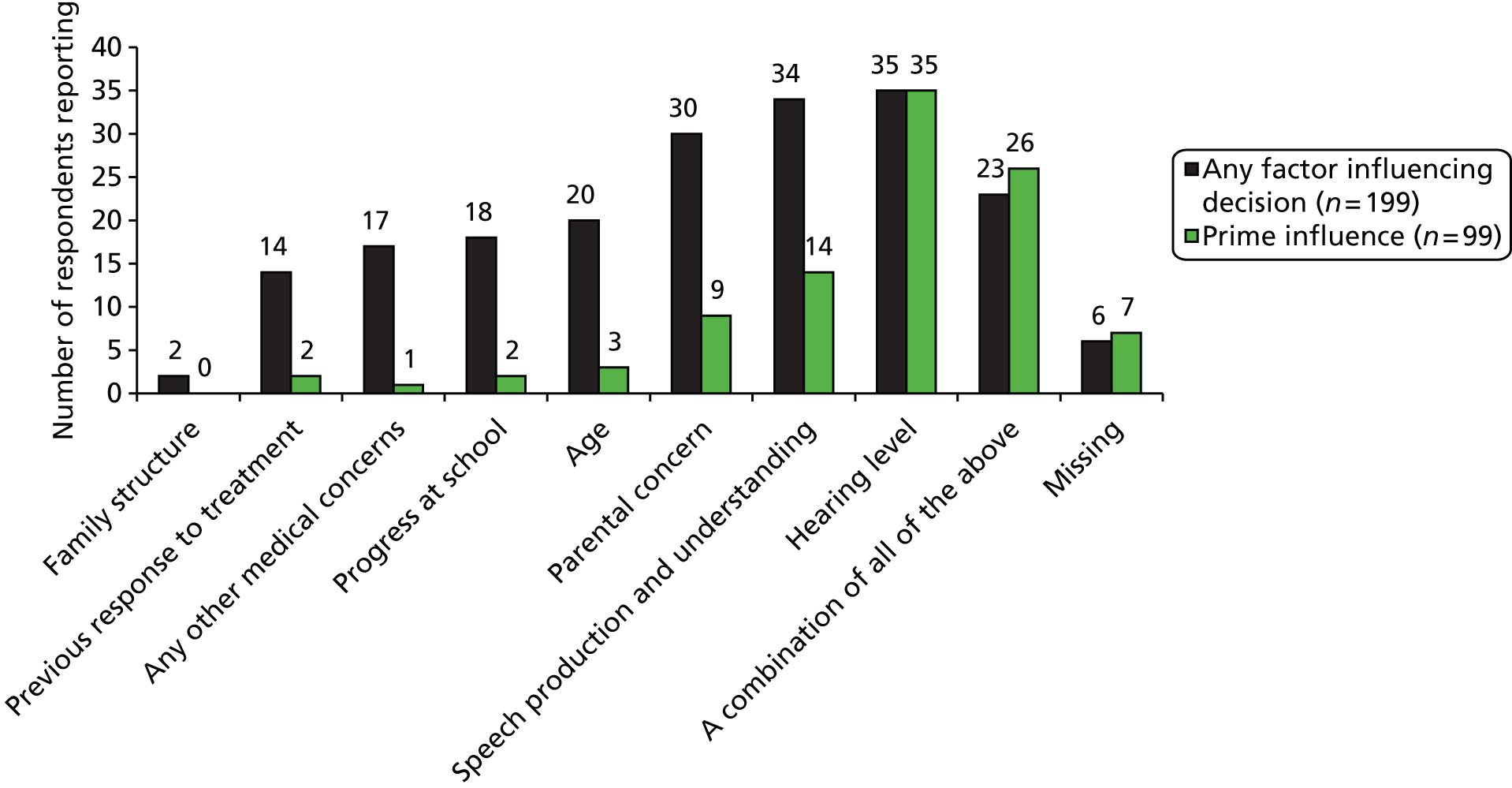
Hearing level is the most important factor here, although a significant number of respondents reported being influenced by several factors and were unable to distinguish individual factors.
From a list of 10 interventions (plus the opportunity to add interventions not included in the list) for glue ear in children with Down syndrome, respondents were asked to indicate the top three effective interventions in order of effectiveness (see Appendix 7, section 4, question 3). The question asked respondents to consider effectiveness in terms of improvements in glue ear and/or hearing loss and/or communication difficulties. Figure 13 illustrates the responses ordered from left to right in terms of the number of ‘most effective’ responses.
FIGURE 13.
Effectiveness of interventions in improving glue ear and/or hearing loss and/or communication difficulties. a, Strategies from family and school to support hearing; T-tubes (long-stay grommet); meatoplasty.
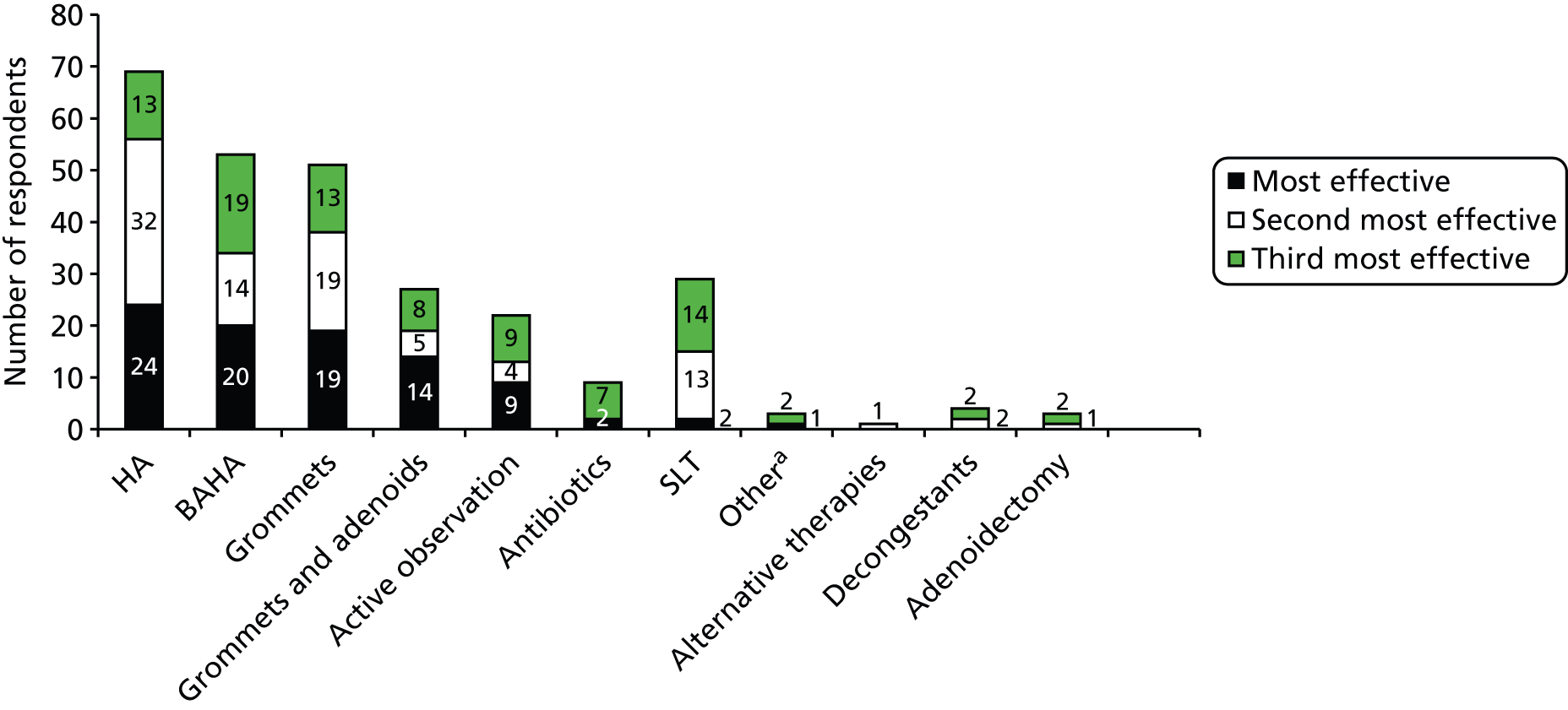
Conventional HAs are considered to be the most effective option and receive the highest number of total mentions followed by BAHAs (permanent or softband) and insertion of grommets. Speech and language therapy is considered to be important but mainly as the second or third option. Active observation does appear to have a role to play, but there is little reported support for antibiotics, alternative therapies, decongestants or adenoidectomy alone.
In open questions, respondents were given the opportunity to specifically reject an intervention for consideration for children with Down syndrome and glue ear (see Appendix 7, section 4, question 4). Twenty-three respondents provided an opinion. There were very strong comments against alternatives therapies (n = 11) and decongestants (n = 5), based mainly on the lack of evidence. Some concerns were expressed about the use of antibiotics (n = 5) and surgery for particular children (n = 2).
A linked question then asked if there were any interventions that the respondent considered should not be used in a research trial comparing interventions (see Appendix 7, section 4, question 5). Fourteen respondents gave an opinion. Again alternative therapies were mentioned (n = 5), together with decongestants (n = 3), antibiotics (n = 2), active observation (n = 2) and any intervention for which there was no evidence (n = 1).
For a similar question in the Delphi review, 22 respondents indicated 27 responses. Fourteen responses indicated that grommets and adenoidectomy should be excluded from any future trial, seven responses indicated that WW should be excluded, four responses suggested exclusion of conventional HAs, one response suggested excluding grommets and one response suggested excluding softband BAHA. Elsewhere in the Delphi review all major treatment options were proposed as possible treatments to be included in a future research study. Consensus about the potential to utilise ‘grommets’, ‘softband BAHA’ and ‘conventional HAs’ was reached in the first round of scoring. Agreement on ‘WW’ was achieved in a second round of scoring, and agreement on ‘grommets and adenoidectomy’ was achieved only when the neutral response category was removed in round 3.
A study of glue ear in children who have Down syndrome should include grommets as a treatment option. (88% agreement in round 1.)
A study of glue ear in children who have Down syndrome should include softband BAHA as a treatment option. (92% agreement in round 1.)
A study of glue ear in children who have Down syndrome should include conventional hearing aid as a treatment option. (88% agreement in round 1.)
A study of glue ear in children who have Down syndrome should include WW as a treatment option. (84% agreement in round 2.)
A study of glue ear in children who have Down syndrome should include grommets and adenoidectomy as a treatment option. (91% agreement in round 3.)
Developing from this, a more helpful investigation of this topic asked Delphi respondents to prioritise two treatment options for inclusion in a research study. Forty-two per cent indicated that softband BAHA should be included; 34%, grommets; 10%, conventional HAs; 8%, grommets and adenoidectomy; and 2% WW. Two additional comments referred to body-worn or hardband bone conduction HAs.
A further open question in the questionnaire exploring any other comments concerning the management of children with Down syndrome who have glue ear (see Appendix 7, section 4, question 6) elicited comments from 24 respondents summarised as shown below.
-
All children are different (mentioned many times).
-
Educational support is important.
-
Family support is important.
-
The importance of glue ear should not be played down simply because the children have suffered much worse and it is not life-threatening.
-
Some children do not tolerate HAs.
-
Management changes as the child gets older.
-
Problems with fluctuating hearing levels.
-
Introduce interventions early to promote tolerance.
-
Grommets should be the last resort.
Views and experience of health research
We explored views on research in general and in particular two methodologies: RCTs and observational health studies (see Appendix 7, section 5, questions 1 and 2). Explanation of each of these methodologies was provided via a link from the questionnaire and are included as Appendix 9.
Six respondents stopped answering questions at this point and the remaining data apply to only 93 respondents.
When asked ‘In general what is your view of health research?’ all were positive, answering ‘It is essential for developing practice’ (n = 84) or ‘It can sometimes be useful’ (n = 9). No-one said that research findings would not change their practice or that health research was a waste of time.
Positive attitudes about the value of health research were reflected in responses to Delphi statements about research into the management of glue ear in children who have Down syndrome. Universal consensus was achieved in round 1 for statements proposing the value of research in this topic.
I believe that irrespective of immediate outcomes (i.e. whether results are positive or negative) further research in the treatment and management of glue ear in children who have Down syndrome will contribute to our understanding of how best to manage this condition, i.e. benefits are not immediate but will improve the development of treatment in the future. (100% agreement in round 1.)
I would support the need for further research in this field. (100% agreement in round 1.)
For the specific methodologies referred to above, respondents to the questionnaire were asked to indicate how confident they were about explaining a particular methodology to a parent of a child with Down syndrome (see Appendix 7, section 5, question 2) (Table 27).
| Very confident | Fairly confident | Not very confident | Not at all confident | |
|---|---|---|---|---|
| How confident are you that you can explain an observational health study to a parent of a child with Down syndrome? | 33 (35.5%) | 54 (58.1%) | 4 (4.3%) | 2 (2.1%) |
| How confident are you that you can explain a RCT to a parent of a child with Down syndrome? | 44 (47.3%) | 42 (45.2%) | 6 (6.4%) | 1 (1.1%) |
Most respondents feel at least fairly confident about explaining research to parents, and there is little difference between the two methodologies, although it should be noted that more respondents felt very confident about explaining a RCT than explaining an observational study. Similar findings are evident in the Delphi data, for which statements proposing difficulties with describing study methodology were rejected, although it is notable that in round 1 a number of respondents (16%) agreed that they would have difficulty explaining a RCT study.
I would find it difficult to describe to parents the rationale and processes associated with an observational study. (88% disagreement in round 1.)
I would find it difficult to describe to parents the rationale and processes associated with an RCT. (84% disagreement in round 2.)
I would find it difficult to describe the potential benefits of involvement in a research study to families who might be recruited to a study. (91% disagreement in round 2.)
Open questions in the questionnaire asked about the benefits or difficulties of taking part in health research for the children with Down syndrome who have glue ear and the benefits for the individual professional, his/her practice and the wider community (see Appendix 7, section 5, questions 3–5). Knowledge of these opinions would play a part in encouraging professionals to take part in future research and might be considered in any approach to maximise involvement. The areas noted by four or more respondents as benefits are listed in Table 28.
| Benefits | Benefit for children (n) | Benefit for professional, practice or wider community (n) |
|---|---|---|
| Provide evidence based practice/guidelines | 22 | 20 |
| Better-informed professionals | 8 | 5 |
| Better-informed parents | 9 | 1 |
| Better management of child | 14 | 11 |
| Close monitoring of child | 4 | |
| Eliminate poor treatments/less surgery | 4 | |
| Determine most (cost) effective treatment | 7 | 8 |
| Improve understanding of condition and consequences | 4 | 6 |
| Active involvement, more confidence in the results | 4 | |
| Continuing professional development and research skills; prestige for institution | 6 | |
| Raise awareness | 4 |
Delphi statements that proposed additional benefits for the child associated with participating in a research study did not reach consensus:
Participation in research will bring ADDITIONAL clinical benefits (e.g. less ear infection, improved hearing, speech and language) to a child with Down syndrome and glue ear. (58% agree, 42% disagree in round 3.)
Participation in research will bring ADDITIONAL non-clinical benefits (e.g. improved social interaction, progress at school) to a child with Down syndrome and glue ear. (58% agree, 42% disagree in round 3.)
Consensus was reached, however, about the professional benefits associated with involvement in a research study:
Further research in this field provides an opportunity for personal professional development which I would find attractive. (84% agreement in round 2.)
Respondents to the questionnaire also commented on the difficulties of participating in research for children with Down syndrome who have glue ear (Table 29). Some comments specifically related to difficulties for the children but others were more general difficulties.
| Difficulties | n |
|---|---|
| Parents agreeing to randomisation | 12 |
| Time (family and professional) | 8 |
| Outcomes and assessment | 8 |
| All children with Down syndrome are different – heterogeneity | 8 |
| Not attending/attrition | 7 |
| Ethical issues | 6 |
| Children with Down syndrome have other problems | 4 |
| Definite views on effective treatments | 4 |
| Extra clinic visits | 3 |
| Consent | 3 |
| Safety (e.g. anaesthetic) | 2 |
| Interdisciplinary collaboration | 2 |
| Seasonality of condition | 2 |
| Additional burden of care | 1 |
| Adoption of recommendations | 1 |
| Achieving big enough study | 1 |
| Not knowing parental view | 1 |
| Lack of resources | 1 |
| Have to use proxy measures | 1 |
| Need to include educators | 1 |
Despite these difficulties, statements that explored barriers to performing research into the management of glue ear in children with Down syndrome were rejected in the Delphi review, each statement passing the threshold for consensus:
The clinical and developmental difficulties associated with children who have Down syndrome make it inappropriate to undertake research with this population. (96% disagreement in round 1.)
The clinical and developmental difficulties associated with children who have Down syndrome make it impossible to objectively capture data on hearing or other outcomes. (96% disagreement in round 1.)
The clinical and developmental difficulties associated with children who have Down syndrome make it impossible to capture the impact of glue ear independently of other health or behavioural traits. (100% disagreement in round 3.)
The complexity of symptoms and/or treatment of glue ear in children who have Down syndrome limit the potential value of further research in this field, i.e. each case is different so treatment will remain individualised irrespective of research findings. (100% disagreement in round 3.)
Forty-one respondents to the questionnaire had participated in a RCT (see Appendix 7, section 5, question 6); 14 as a participant and 27 as a researcher. Of those 41, six responded that they did not know if they would do it again and one said they would not. The concerns of those who were unsure were: depends on treatment options; reservations about involvement of the Down syndrome population; depends on the trial, trust approval and time; RCTs are unethical, particularly in surgery; and time consuming, would depend on design.
More specifically, respondents were asked if they would agree to recruiting parents and their children with Down syndrome to two different types of health research study (Table 30): the first described as a study in which the children receive the intervention for glue ear that the respondent recommended (equivalent to an observational study) and the second described as a study in which the children would be randomly assigned to receive different treatments for glue ear (equivalent to a RCT) (see Appendix 7, section 5, questions 7 and 8).
| Observational study | RCT | TOTAL | ||
|---|---|---|---|---|
| Yes | No | Don’t know | ||
| Yes | 33 | 5 | 14 | 52 (71.2%) |
| No | 4 | 2 | 2 | 8 (11.05) |
| Don’t know | 2 | 2 | 9 | 13 (17.8%) |
| TOTAL | 39 (53.4%) | 9 (12.3%) | 25 (34.2%) | 73a (100%) |
More respondents would agree to recruit to an observational study (71.2%) than to a RCT (53.4%). The highlighted cells are of interest, as these are people who would be uncertain about recruiting to an observational study but who would recruit to a RCT.
Looking at this by discipline, 60% of ENT surgeons and 65% of paediatricians would recruit to a RCT, and 80% (ENT) and 65% (paediatricians) to an observational study, whereas only 27.8% of audiologists and 44.4% of SLTs would recruit to a RCT, and 61.1% (audiologists) and 55.5% (SLTs) would recruit to an observational study.
Delphi data also suggest that there might be a greater willingness to recruit families to an observational study than to a RCT study. A standalone question in round 1 highlighted that 52% favoured an observational study design for any future research, 40% RCT design, and 8% felt that study design was unimportant. Explicit statements about recruiting families to different types of study demonstrated more concern about RCT design than observational design, although a consensus of agreement was achieved for both types of study.
I would be willing to recruit families to an observational study in this field. (93% agreement in round 1.)
I would be willing to recruit families to a RCT study in this field. (84% agreement in round 2.)
In round 1, 42% of responses were neutral or in disagreement about recruiting to a RCT study; in round 2, 16% remained neutral. It should also be reported that consensus was not achieved in further statements that explored the methodological underpinning of any future study.
Because there is uncertainty about which treatment option offers the best outcomes a RCT in this field (and the random allocation of treatment) is justified. (78% agree, 22% disagree in round 3.)
The necessity for individually tailored treatment for glue ear in children who have Down syndrome means that an observational study (with treatment recommended by their clinician) is most appropriate. (70% agree, 30% disagree in round 3.)
Facilitators and barriers to randomised controlled trial recruitment
As reviewed in Chapter 2, research suggests that there may be many factors that encourage clinicians to recruit patients into health research studies. Equally there may be many factors that discourage recruitment but there are few studies that specifically explore the recruitment to trials of children who have Down syndrome.
Some professional groups, such as ENT surgeons, are more often responsible for directly recruiting to a trial involving surgical intervention. However, people within other disciplines, such as paediatricians, audiologists, SLTs, etc., may also influence a parent or carer in the decision of whether or not their child should participate in a research trial. We explored the factors that would be important to the respondents in deciding to recruit to a trial or encouraging a parent to agree to their child taking part (Table 31).
| Statement | Mean value | Modal value | Important = 61–100:a equivocal = 40–60:a not important = 0–39a |
|---|---|---|---|
| All out-of-pocket expenses for the family would be reimbursed | 76.8 | 100 | Important |
| The child and his/her family would have the time to take part | 76.2 | 100 | |
| There would be minimal inconvenience to the child and his/her family | 73.5 | 100 | |
| There is genuine uncertainty within the clinical community about which treatment option is better | 71.3 | 100 | |
| Having confidence in my ability to explain the study to parents and to take their consent | 69.8 | 100 | |
| I would be contributing to determining the most effective treatment | 69.3 | 100 | |
| My involvement in the study would cause little or no disruption to my clinical commitments | 65.8 | 85 | |
| I would be helping individuals with Down syndrome by encouraging them to participate in research | 60.8 | 100 | Equivocal |
| The research might take priority over the needs of the patient | 60.1 | 100 | |
| There may be risk of harm or side effects from the treatment options | 55.9 | 0 | |
| I personally am uncertain about which treatment option is better | 52.8 | 0 | |
| I would increase my awareness and experience of research | 51.9 | 0 | |
| Additional non-routine procedures would be involved for the child | 46.4 | 0 | |
| Feeling I have sufficient experience of research | 44.4 | 0 | |
| The research might have a detrimental effect on my relationship with the family | 41.0 | 0 | |
| Not having confidence in my ability to explain the study to parents and to take their consent | 40.3 | 0 | |
| Taking part would improve my CV | 27.0 | 0 | Not important |
| I would lose my professional autonomy to decide which is the best treatment | 25.6 | 0 | |
| Not having research experience | 22.5 | 0 | |
| I would receive some financial reward for taking part | 11.3 | 0 |
Respondents to the questionnaire were asked to imagine a scenario where they were asked to collaborate with researchers conducting a RCT of interventions for glue ear in children with Down syndrome. They were asked to recruit children to the study and had to explain to parents that their child will be randomly assigned to receive one of three intervention options: insertion of grommets; provision of a softband HA; or active observation (WW). Each child would be followed up for 6 months.
For each of the following statements they were asked to indicate the importance of each statement on a scale of 0 (not important at all) to 100 (very important) in informing their decision to try to recruit a parent and their child with Down syndrome to take part in a RCT or to advise them to do so (see Appendix 7, section 6, questions 1 and 2).
In terms of the design of future research, it should be noted that the extent of any inconvenience to the family ranks highest in importance, followed by demonstration of genuine clinical equipoise and professional factors of sufficient confidence and little disruption to clinical commitments. Some of these concerns about professional practice were posed in the Delphi review, in which they were rejected – although it should be noted that concerns about professional autonomy were dismissed only after the neutral response category was removed, and that no consensus was achieved with regard to concerns about the research study overtaking the needs of the child/family.
I would be concerned that the processes of a research study would adversely affect my relationship with the child and their family. (92% agreement in round 2.)
I would be concerned about losing professional autonomy in a research study where treatment is randomly allocated. (88% agreement in round 3.)
I would be concerned about the needs of the research study overtaking the needs of the child and/or family in a research study. (25% agree, 75% disagree in round 3.)
Finally, in the questionnaire respondents were asked what else that was important for them to gain from research into treatment options for glue ear in children with Down syndrome. Twenty-eight respondents added a comment, 11 mentioned the need for standardisation and guidelines in treatment, five mentioned the need for increased awareness and confidence and individual comments referred to the complexities of the condition and the impact of glue ear and treatment, and the need for new ideas, novel treatments and long-term solutions.
Summary
The overwhelming message from professionals was that Down syndrome is a complex condition in a challenging population, and there was a broad recognition that each child is different and poses different challenges.
Glue ear and its consequences
-
Clinicians confirmed that glue ear is an important condition for this population owing to its prevalence and the potential implications that it has for other behavioural/developmental difficulties.
-
Difficulties hearing, listening and communicating, both understanding and using communication, were identified as the most frequent problems associated with glue ear in children with Down syndrome. Hearing was commonly identified as the most important problem, but communication difficulties were frequently identified by clinicians. Difficulties with listening and communication are considered to be more challenging than hearing alone in terms of condition management.
-
Clinicians recognise that it is difficulties with communication (listening, understanding and using communication) that pose families most difficulties.
Glue ear and its treatment
-
Clinicians confirmed that treatment for glue ear in this population is challenging, and that it is difficult to be entirely confident of effective treatment.
-
Confidence in explaining the risks and benefits of different interventions for glue ear varied by profession. Surgeons were confident in explaining surgical intervention, others were less certain and overall surgical intervention was the treatment option for which respondents were least confident in explaining risks and benefits.
-
Hearing level, speech production and parental concern were identified as the strongest influences of clinical decision-making, with hearing level singled out as the most frequent single factor that might inform treatment for glue ear. Many clinicians indicated that no single factor can influence decision-making, and it would more likely be influenced by a combination of clinical, behavioural and familial factors.
-
HAs were presented as the most effective treatment, followed by BAHA and grommets.
National Institute for Health and Care Excellence guidelines
-
Only one-quarter of those surveyed claimed a strong knowledge of NICE guidelines, and the need for new and improved clinical guidelines for the treatment and management of glue ear in this population was established in the Delphi review. That either RCT or observational research might inform future guidelines was also established.
-
Most respondents indicated that they would assess any new guidelines in light of the evidence presented before changing their clinical practice.
The value of research
-
All respondents recognised the value of AHR, indicating in the main that it can importantly inform clinical practice.
-
There was strong support for the need for further research in this area and recognition that research might generate evidence to inform/change guidelines and practice, which may result in improved management of the child.
-
The complexity of the condition was not considered a sufficient barrier to prevent research in this area, similarly the challenges of working with this population were not perceived to be a barrier to any future research.
The form of research
-
It was established that any future research should consider improvements in hearing and, perhaps more importantly, communication (speech and language) to be meaningful. It is perhaps easier to justify and activate improved hearing as the primary outcome of any study, with improved communication as a secondary outcome.
-
If comparing only two treatment options then these should be BAHA and grommets.
-
Clinicians expressed no difficulty in explaining either a RCT or observational study design to parents.
-
Randomisation was identified as a potential barrier to any family committing to participate in a research study, and more clinicians indicated a willingness to recruit families to an observational study than to a RCT. More clinicians indicated a preference for an observational study design. Although it should be noted that statements which explicitly sought to exclude one study design or the other failed to reach consensus (agree or disagree) in the Delphi review.
Facilitators and barriers to randomised controlled trial participation
-
Practical factors for the families, such as having all out-of-pocket expenses reimbursed, time to take part and minimal inconvenience would all strongly encourage professionals to recruit a parent and their child with Down syndrome to take part in a RCT or advise them to do so.
-
Ethical and clinical factors, such as clinical equipoise, having confidence in explaining the study and taking consent, helping to determine best treatment efficacy and minimal disruption to clinical commitments would also strongly encourage recruitment of patients to a RCT.
-
Personal factors, including improvements to curriculum vitae, loss of autonomy in treatment decision-making, lack of research experience and receiving a financial reward for research participation were viewed as having low importance in terms of the decision to try to recruit a parent and their child with Down syndrome to take part in a RCT or to advise them to do so.
Chapter 5 Economic analyses and value of information
Objectives
The purpose of this chapter is twofold: to (1) develop economic models to represent clinical pathways of care and a RCT for children with Down syndrome with hearing loss induced by chronic OME and (2) undertake VOI analyses to demonstrate the potential economic benefit from undertaking further research. It is important to demonstrate the VOI in this area, as any research conducted on hearing loss and OME in children with Down syndrome has to be targeted at the areas where the most economic benefit can be found.
Methods
We undertook deterministic cost–utility analyses in two settings: a clinical care pathways model and a hypothetical simple RCT model. In both cases an averaged cohort approach has been taken. Prior parameter information sourced from practice in either setting is sparse.
The economic models were constructed as decision trees using TreeAgePro® 2012 (TreeAge Software, Inc., Williamstown, MA, USA), with costs and QALYs calculated in Microsoft Excel® 2010 (Microsoft Corporation, Redmond, WA, USA). The perspective of the analyses is the UK NHS and Personal Social Services (PSS) as recommended by NICE. This was chosen as the models were constructed to represent the decisions facing patients and clinicians, and therefore only costs and consequences that are directly attributable to NHS and PSS were deemed relevant. Costs were assumed to inflate by 3.5% per annum (pa). Future incurred costs and QALY gains and losses were discounted to time 0 present values, using a 3.5% pa discount rate. Both models assume that upon entry the child with Down syndrome is aged 3 years and is suffering hearing loss due to chronic OME (i.e. OME that has been persistent for at least 3 months).
The key aggregate parameters are an ICER (£/QALY) and an incremental net benefit (INB; £). A further parameter, the population expected value of perfect information (pop-EVPI; £), was computed as the product of the expected value of perfect information (EVPI; £) and the total population multiplier. The latter estimates the number of Down syndrome children with OME as 541.3, obtained as the number of live births in 2011 (723,000)73 adjusted by the child mortality rate (0.005 per year for children ≤ aged 5 years)74 scaled by the incidence rate of Down syndrome (0.001),2,3 as well as the estimated prevalence rate of OME in children with Down syndrome aged 3 years (0.762): 723,000 × (1–0.005)3 × 0.001 × 0.76 = 541.3. Note that unless stated otherwise INB, EVPI and pop-EVPI are measured at a £20,000/QALY threshold.
Hearing loss
The primary health outcome in both models is hearing loss measured in decibels [decibel hearing level (dB HL)], as a pure tone average (PTA). This is a weaker health outcome than clearance of OME, as the hearing loss induced by OME can be counteracted using symptom management amplification devices, such as HAs, without the need to clear the OME. However, we do incorporate clearance of OME into both models as an exit condition, the assumption being that recovery from OME implies its permanent extinguishment. In the models, any diagnosis of recovery from OME is determined at the end of a cycle.
Hearing loss is mapped to QALY using Dixon’s unpublished formula (Simon Dixon, University of Sheffield, December 2012, personal communication). Dixon gathered PTA data (n = 105) to inform the regression of utility (measured using the HUI3 instrument) against it and age. The study data spanned a range of low to moderate hearing loss due to OME (i.e. most recorded PTA data were ≤ 40 dB) covering the same range of hearing loss found in children with Down syndrome with chronic OME. 75 We applied Dixon’s estimated utility decrement for increasing PTA hearing loss; namely, 0.00874 QALY/dB [95% confidence interval (CI) 0.005 to 0.012]. Improved PTA hearing is treated symmetrically, thus each 1-dB decrease in PTA hearing loss attracts a 0.00874 QALY gain. Dahle and McCollister75 report a PTA loss averaging 26.5 dB across n = 10 ears of children with Down syndrome with OME. At baseline, we assign QALY gains and losses to Dahle’s average relative to the 7.5-dB midpoint of the no hearing loss range (0–15 dB). For example, under these assumptions extinguishment of OME in a child with Down syndrome determines a PTA hearing improvement averaging 19 dB, gaining 0.166 QALY.
Cost determination
Costs have been determined according to the procedures given in NICE Clinical Guidelines 60. 17 The price year is 2011–12 and, where available, NHS reference costs 2011–12 are applied (www.gov.uk/government/publications/nhs-reference-costs-financial-year-2011-to-2012). Typically input are median recorded values; however, some were replaced by the cost at the upper quartile. This was to represent the increased costs associated with treating children with Down syndrome, as it can add complications to the normal procedure as advised by the clinical members of the research team. Tables 32 and 33 list treatment and medical personnel costs used in the modelling.
| Variable | Unit cost (£) | Source | Notes |
|---|---|---|---|
| Insertion of ventilation tubes | 957 | NRC: day cases | CZ08S (lower quartile £630, upper quartile £1166) |
| Insertion of ventilation tubes plus adjuvant adenoidectomy | 1055 | NRC: day cases | CZ02S (lower quartile £685, upper quartile £1303) |
| HA | 85 | NRC: audiological services | DHA1 (the patient is assumed to have bilateral OME so two aids are fitted; lower quartile £65, upper quartile £100) |
| HA fitting assessments | 71 | NRC: fitting of HAs and counselling | AS1A (lower quartile £40, median £59, upper quartile £71) |
| HA fitting | 77 | NRC: fitting of HAs and counselling | AS1FA (lower quartile £46, median £65, upper quartile £77) |
| HA follow-up | 53 | NRC: fitting of HAs and counselling | AS1FU (lower quartile £31, median £46, upper quartile £53) |
| Softband BAHA | 2613 | NRC: day cases | CZ28Z (covering the supply of the processor as well as the programming tuning of it by the audiologist and fixing to the headband; lower quartile £1292, upper quartile £3858) |
| Visit | Unit cost (£) | Source | Notes |
|---|---|---|---|
| Audiology | 53 | NRC: fitting of HAs and counselling | AS1FU (counselling; lower quartile £31, upper quartile £53) |
| GP | 36 | PSSRU, Unit Costs of Health and Social Care 201176 | A complication cost of otorrhoea and granulations; one visit assumed per case |
| ENT | 161 | PSSRU, Unit Costs of Health and Social Care 201176 | Consultant-led services (outpatient follow-up attendances); this is used to cost surgical follow-up |
The costs of surgical complications arising from ventilation tube insertion (grommets) include further surgical procedures to repair damage, as well as use of critical care (Tables 34 and 35).
| Variable | Unit cost (£) | Source | Notes |
|---|---|---|---|
| Medication | 10 | Research team | A complication cost of otorrhoea |
| Surgical arrest of bleeding from internal nose | 1154 | NRC: day cases | CZ13U (a complication cost of bleeding) |
| Tympanoplasty | 1849 | NRC: day cases | CZ10U (a complication cost of perforation of eardrum) |
| Palatoplasty | 1304 | NRC: day cases | CZ03U (a complication cost of palatal insufficiency) |
| Removal of ventilation tubes | 957 | NRC: day cases | CZ08S (lower quartile £630, upper quartile £1166) |
| Critical care | Unit cost (£) | Source | Notes |
|---|---|---|---|
| High dependency per day | 920 | NRC: NHS Trusts Critical Care Services | XB07Z (severe bleeding as a complication of surgery, spend 2 days on a paediatric high-dependency unit) |
| Intensive care per day | 1826 | NRC: NHS Trusts Critical Care Services | XB05Z (patients who die as a complication of surgery spend 1 day on a paediatric intensive care unit) |
Value of information
In Table 36 are listed a range of scenarios that represent varying degrees of perfect information with respect to potential health outcomes related to particular treatment strategies. These are labelled according to the economic model, with clinical care pathways perfect information (CPPI 1–5) examined in the clinical care pathways model and trial perfect information (TPI 1) in the trial model. Each scenario is represented by changing the associated probability values in the baseline economic models.
| CPPI 1 | Perfect surgery: everyone recovers and no complications |
| CPPI 2 | Perfect surgical recovery |
| CPPI 3 | No complications during surgery |
| CPPI 4 | HAs are always tolerated |
| CPPI 5 | BAHA is always tolerated |
| TPI 1 | Perfect intervention surgery: everyone recovers and no complications |
Modelling clinical care pathways
The first model we consider examines VOI in the context of clinical care pathways. In the absence of clearly defined guidelines on how to progress care of the child with Down syndrome suffering with OME, standard practice would appear to proceed along a variety of pathways of treatments. Accordingly, the economic model we constructed permits switching from one type of treatment to another from one cycle to the next, for example treatment in one cycle could be for the child to receive HAs followed in the next cycle by surgery. Each cycle represents one year of life for the child.
Description of treatment strategies
Watchful waiting
Watchful waiting or active observation represents a ‘do nothing’ strategy. It does not address either the OME-induced hearing loss of the child with Down syndrome or the OME itself, although this is rectified should spontaneous recovery from OME occur. Although children will be assumed to have faced a period of observation of at least 3 months before they enter the model, observation is still considered a potential option at any cycle.
Hearing aids
The child is given a pair of behind-the-ear digital HAs to overcome their OME-induced hearing loss. However, HA do not clear OME – rather they manage the hearing loss associated with OME. As with WW (and BAHA next) recovery from OME, when treatment is HA, can only be spontaneous. The continuance of HA treatment depends on whether or not they are tolerated by the child.
Softband bone-anchored hearing aid
A BAHA is an amplification device that can be fitted externally to a headband (the ‘softband’) and worn on the head. It is a symptom management device, so, once again, recovery from OME is only spontaneous. The continuance of BAHA treatment depends on whether or not it is tolerated by the child.
Surgery
The insertion of ventilation tubes (grommets), sometimes combined with an adenoidectomy, has been demonstrated as a cost-effective strategy for clearing OME in the general population. 17 Surgery removes the ‘glue’ and, as a consequence, immediately restores the normal level of hearing in both ears without the need of assistance of hearing devices.
Economic model
The following diagrams (Figures 14–17) outline the structure of the clinical care pathways model. The model was adapted to represent each perfect information strategy.
FIGURE 14.
Clinical care pathways model: truncated version of the decision tree.
FIGURE 15.
Clinical care pathways model: subtree 1, with OME persisting after first-cycle surgery.
FIGURE 16.
Clinical care pathways model: subtree 2, with OME persisting after first-cycle HAs not being tolerated.
FIGURE 17.
Clinical care pathways model: subtree 3, with OME persisting after first-cycle WW.
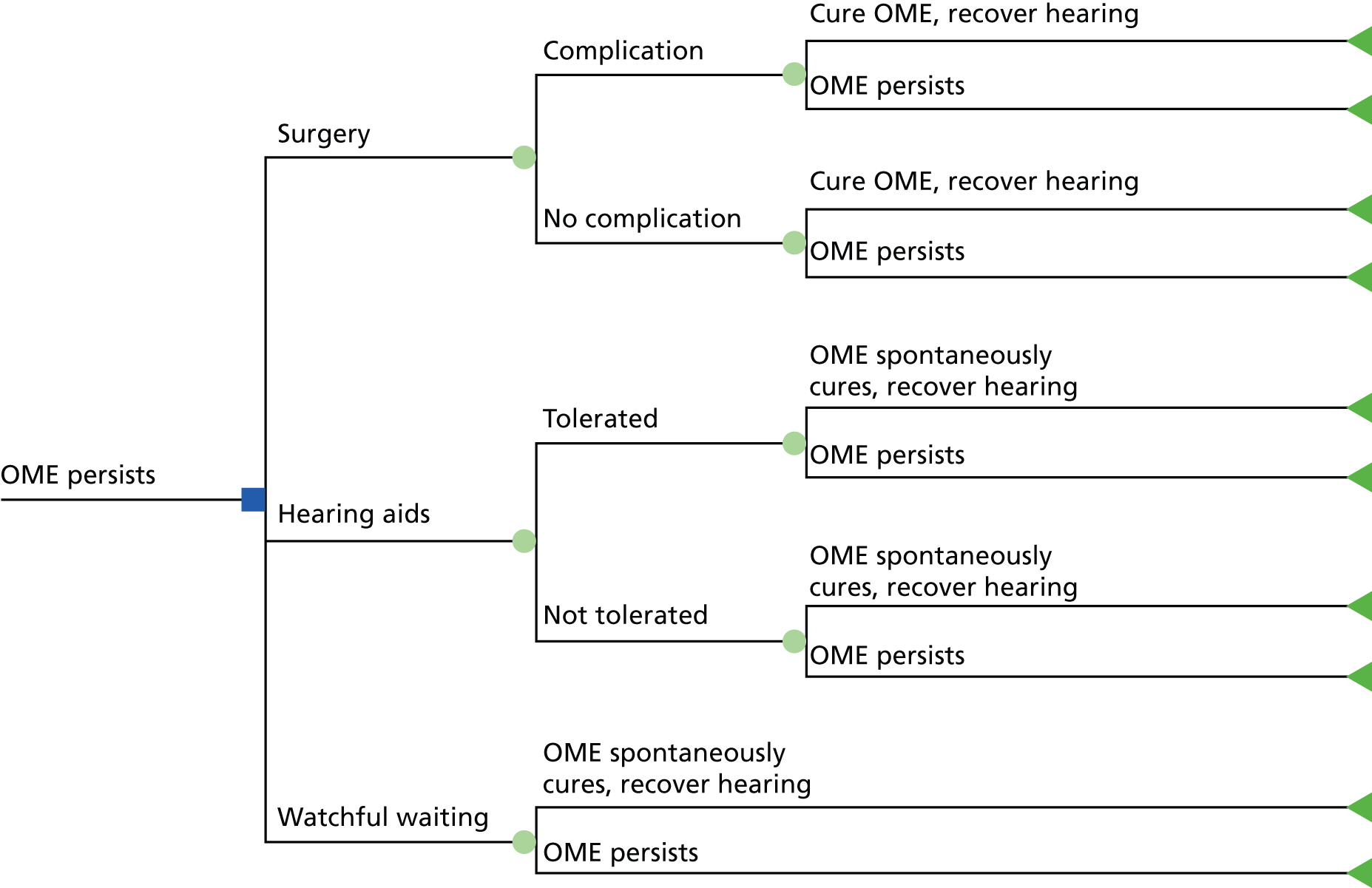
Modelling assumptions and parameters of the clinical care pathways model
We assume the child with Down syndrome entering the model at time 0 is aged 3 years, and, has been suffering OME and experienced hearing loss for at least the previous 3 months. We notionally regard the model’s cycle length to represent 1 year of time, and, with the model running for only two cycles, children are aged 5 years at the end of the model.
The maximum QALY increment possible of 0.166 occurs at time 0 under all care pathways apart from WW; this is because the child’s hearing loss is negated under either surgery or use of hearing devices. In the event of surgical complications (see below) or HA not tolerated, a QALY decrement applies; in the case of HA, this is a full deduction of 0.166 as care reverts to WW. The same assumption applies if BAHA is initiated in cycle 2. Assessment of whether or not OME has cleared (hence restoration of a normal level of hearing) is determined at the end of each cycle, and, if occurring, acts as a terminating state, for it is assumed never to return. OME recovery probabilities can either be spontaneous (p = 0.05), applicable for WW, HA and BAHA, or assumed higher for surgery, which actively targets OME (p = 0.1 for surgery 1 and p = 0.2 for surgery 2).
The costs assigned to WW undertaken from time 0 are four audiologist visits and one ENT visit. Should WW occur after a previous round of treatment (i.e. surgery or HA), costs are assumed to be three audiologist visits. The cost components of WW can be found in Table 33.
The cost of HA involves the units themselves and various appointment costs for assessment, fitting and follow-up. Should HA be tolerated (p = 0.8) then we assume that the child will remain using those aids in subsequent cycles until OME spontaneously clears when unassisted hearing returns to the normal level. Post follow-up, we assume three audiologist visits for the remainder of the cycle, and four visits in the subsequent cycle. We also impute costs for aid replacement due to breakage (two aids per cycle). Should the child not tolerate HA then for the remainder of that cycle they revert to WW and we assign costs of two audiologist visits and one ENT visit. Also, treatment options available at the start of the next cycle would exclude HA. The costs associated with HA are given in Table 32.
Use of a BAHA is a second-line symptom management treatment option, available only if the child does not tolerate HA and therefore appears in only the second cycle of the model. Should the BAHA be tolerated (p = 0.85) then we assume the child would remain using BAHA until OME spontaneously clears. We assume the cost of the BAHA includes a 3-year maintenance contract between supplier and NHS incorporating any repairs as required and one free replacement should the unit become lost or irreparably broken. As the NHS pays the maintenance contract in full on item delivery, the full cost is assigned at initiation. Should the child not tolerate BAHA then for the remainder of that cycle they revert to WW and we assign costs of three audiologist visits and one ENT visit. The costs associated with BAHA are given in Table 32.
For modelling simplicity we assume that first-line surgery (surgery 1) represents a weighted mix of both the grommet insertion procedure (weight 0.7) and the grommet insertion plus adenoidectomy procedures (weight 0.3). Second-line surgery (surgery 2) comprises a mix of grommet extraction (if the grommet is blocked) followed by grommet insertion (weight 0.2) and grommet insertion alone if the previous ones had naturally extruded (weight 0.8). The costs of the procedures are given in Table 32.
Associated with both surgeries are complications that can arise during the procedures, including surgical arrest from bleeding in the internal nose, tympanoplasty (the piercing of the ear drum), otorrhoea (infection of the ear) and granulation. Probabilities are given in Tables 37 and 38.
| Complication | Probability | Source |
|---|---|---|
| Otorrhoea | 0.26 | Kay et al. (2001)77 |
| Granulations | 0.05 | Kay et al. (2001)77 |
| Eardrum perforation | 0.022 | Kay et al. (2001)77 |
| Severe bleeding | 0.01 | NICE CG60 |
| Surgical mortality | 0.000005 | www.netdoctor.co.uk/health_advice/facts/anesthetic.htm |
| Complication | Probability | Source |
|---|---|---|
| Otorrhoea | 0.26 | Kay et al. (2001)77 |
| Granulations | 0.05 | Kay et al. (2001)77 |
| Eardrum perforation | 0.022 | Kay et al. (2001)77 |
| Severe bleeding | 0.01 | NICE CG60 |
| Palatal insufficiency | 0.0006 | www.emedicine.com/ent/topic316.htm |
| Surgical mortality | 0.00005 | Randall and Hoffer (1998)78 |
If there are postoperative complications (p = 0.24) a QALY decrement is incurred equal to 6/52 × 0.166 QALY. This represents the negation of the hearing gain due to surgery fully apportioned to the 6-week postoperative period. The cost of incurring surgical complications is a weighted average of all complications costs (see Tables 34 and 35), where the weights are given in Tables 37 and 38.
A further complications-induced QALY deduction is due to surgical mortality. Death generates a reduction, discounted to time 0, equal to 20.5 QALY; this assumes an average life expectancy of the child with Down syndrome of 60 years,79 scaled by the Down syndrome QALY multiplier of 0.8180 (Table 39).
| Item | Cost (£) | QALY deduction |
|---|---|---|
| Cost when no surgical complications | 161 | 0 |
| Cost of surgery 1 complications | 225 | 0.0195 |
| Cost of surgery 2 complications | 216 | 0.0193 |
Finally, as data were exceedingly limited, a number of parameters were taken from expert opinion as listed in Table 40.
| Parameter | Probability | Source |
|---|---|---|
| Spontaneous recovery from OME | 0.05 | Research team |
| Recovery from OME after surgery 1 | 0.10 | Research team |
| Recovery from OME after surgery 2 | 0.20 | Research team |
| Complication during either surgery | 0.24 | Research team |
| Toleration of HAs | 0.80 | Research team |
| Toleration of softband BAHA | 0.85 | Research team |
| Extraction and reinsertion of grommets | 0.05 | Research team |
Note that recovery from OME is a terminating state in the model, such that if it occurs then OME is assumed permanently extinguished and PTA hearing level returns permanently to the normal level. All treatments for OME-induced hearing loss are ceased.
Modelling a randomised control trial
Economic model
The second economic model examines VOI in the context of an artificial, simple, hypothetical RCT. The trial model takes a narrower focus than the clinical care pathways model but this has distinct advantages in terms of simplification, as treatment pathways can be imposed as conditions of the trial and interventions be characterised only by scenarios involving changes to costs and probabilistic assignments in terms of the model’s parameters. The trial model is shown in the form of a decision tree in Figures 18 and 19.
FIGURE 18.
First cycle of the trial model.
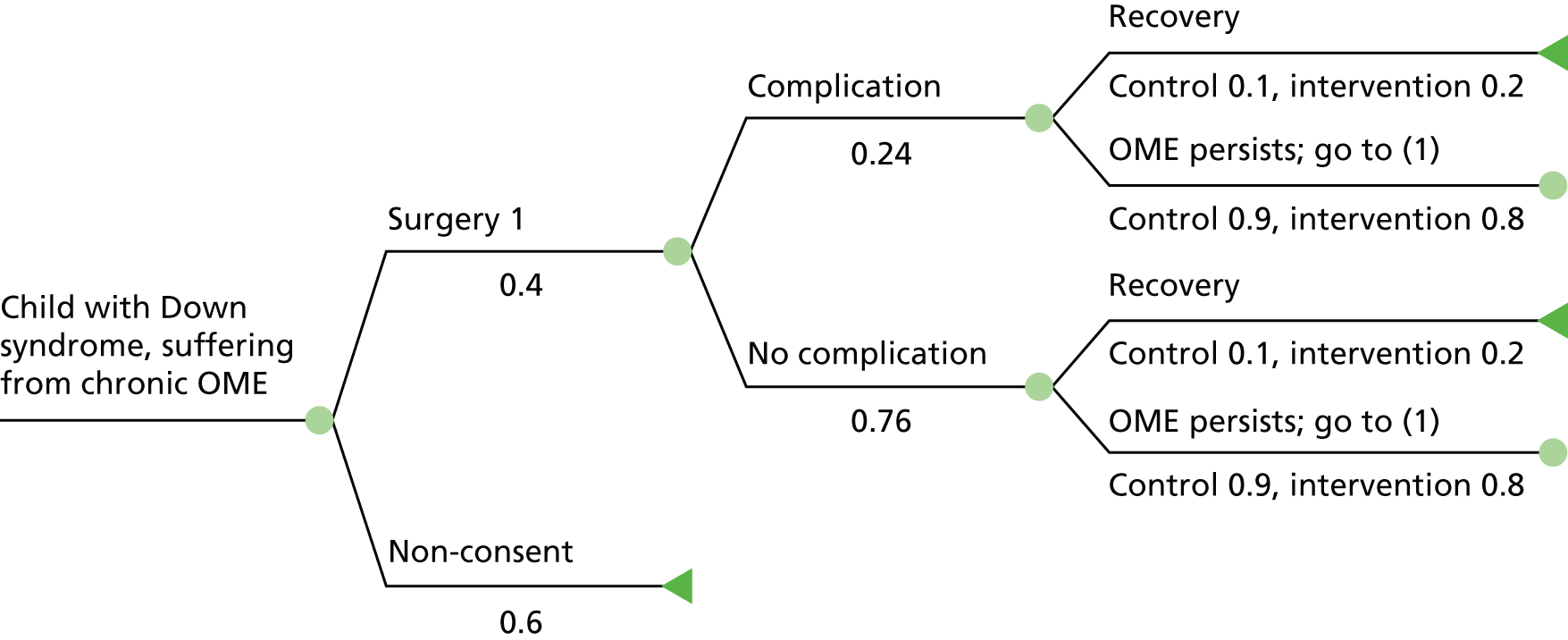
FIGURE 19.
Second cycle of the trial model.
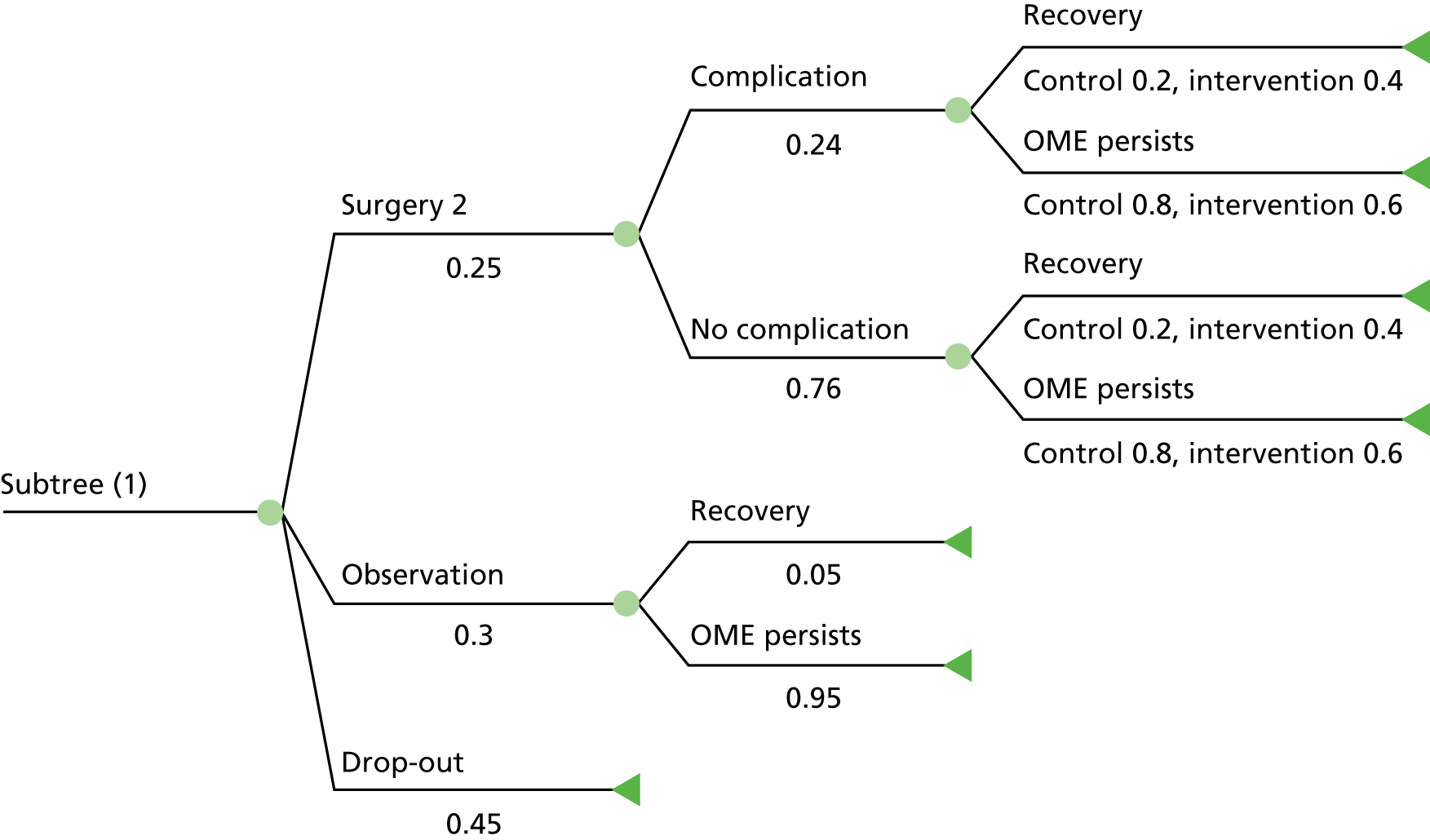
Modelling assumptions and parameters of the trial model
We assume that the child with Down syndrome entering the model at time 0 is aged 3 years and, for at least the previous 3 months, has been suffering OME and experienced hearing loss. We notionally regard the model’s cycle length to represent one year of time and, with the trial running for two cycles, children are aged 5 years at the end of the trial.
Active surgical treatments targeting OME that involve the insertion of ventilation tubes (grommets) are compared. We contrast between intervention and control in that the former is a procedure that, despite adding to costs, improves OME recovery rates. Control outcomes, costs and probabilistic assignments are the same as were applied in the previous model for surgery 1 and surgery 2. The primary health outcome is hearing loss.
Results from the parents’ questionnaire (see Chapter 3) show that there is considerable reluctance on the part of the majority of parents to consent their child with Down syndrome to trial randomisation. For that reason alone the economic model includes a consent decision over whether or not to participate in the trial. The non-consent rate is assigned 60%, in line with the evidence from the parents’ questionnaire. The major implication of non-consent in economic terms occurs in the VOI analysis, as the population multiplier must be modified, now becoming 541.3 × 0.4 = 216.5. We allow for fatigue resulting in dropout from the trial owing to unsuccessful outcomes after completion of the first cycle. The dropout rate at the beginning of the model’s second cycle is hypothesised to be 45%. If dropout occurs, there is, from the perspective of the trial, no cost or benefit derived, and the child exits from the model. Trial participants all receive surgery 1 in cycle 1 (randomisation 1 : 1 between intervention and control). Beginning at the second cycle, options are a second round of surgery (surgery 2; p = 0.25), observation (p = 0.3) and dropout from the trial (p = 0.45).
Recovery from OME in the model is a terminating state and represents permanent extinguishment of OME. The key assumption that distinguishes intervention from control is in the doubling of the probabilities of recovery:
The third avenue for recovery from OME occurs at the end of cycle 2 when under observation; in this case, recovery is assumed to be spontaneous (p = 0.05) under both arms. The intervention is assumed to attract an additional per-procedure cost to the NHS, which is over and above the costs of control, and we vary these in the range £50–600. We further assume independence of outcomes under both arms of the trial, meaning, for example, that if a child recovered under the intervention then that would have no bearing on whether or not recovery would have occurred under control.
The benefit derived by the child with Down syndrome from surgery is, in either arm of the trial, an immediate gain of 0.166 QALY. This is due to the removal of ‘glue’ and restoration to normal levels of hearing in both ears. Should recovery from OME be diagnosed at the end of either cycle then the recovered child exits the trial, as OME is assumed not to recur. As the benefit has already been accrued, there is therefore no further QALY gain upon exit due to recovery. Similarly, there is no gain if assigned to observation in the second cycle or if early dropout is chosen by parents before entering the second treatment cycle of the trial. On the other hand, a QALY decrement is incurred if over the course of a cycle OME is diagnosed to persist. Our assumption in the trial model is the same as was applied in the clinical care pathways model, namely, the child returns to their pre-surgery level of hearing loss, and we apply a 0.166 decrement at the end of the cycle.
Postoperative complications under either intervention or control (p = 0.24) attract the same costs and QALY decrement as were imposed in the clinical care pathways model.
Results
Results for clinical care pathways model
Baseline
We undertook deterministic analyses of the clinical pathways model. Treatment options across the three main types – surgery, HAs, WW – can be taken at time 0 and again the same options are faced at the beginning of the second cycle. With switching permissible from one type of treatment to another, this implies that there are 23 + 1 = 9 pathways in the model’s two cycles (the added one due to HA followed by BAHA). Surgery 1 is imposed initially, noting that, irrespective of whether surgical complications arise or not, the more cost-effective pathway switches to HA in the second cycle. On the other hand, the most cost-effective pathway relative to the £20,000/QALY threshold is initiated with WW, followed by HA in cycle 2, which, if tolerated, continues to be used but, if not, revert back to further WW. Results by initial treatment are shown in Table 41, which shows that WW has least costs on average and initiates the most cost-effective care pathway.
| Initial treatment (cycle 1) | Second treatment (cycle 2) | Cost (£) | QALY | ICER (£)/QALY (vs. WW + HA) | INB (£)a |
|---|---|---|---|---|---|
| WW | HAs | 1303 | 0.131 | ||
| HAs | WW | 1457 | 0.136 | 34,399 | –65 |
| Surgery | HAs | 2338 | 0.134 | 422,114 | –986 |
Value of perfect information
In these analyses, uncertainty is removed by imposing perfect information in a variety of scenarios listed as CPPI 1–5 in Table 36. These scenarios give an upper bound against which the value of further research can be assessed. In each case, perfect information is contrasted against the most cost-effective pathway at baseline (initiated with WW) at the cost-effectiveness threshold of £20,000/QALY.
The highest population EVPI is under CPPI 1 – perfect surgery without complications – closely followed by CPPI2, in which everyone recovers from surgery and CPPI 4 HAs are always tolerated (Table 42). Interestingly, when focus is on surgical complications alone (CPPI 3) there is no gain whatsoever to be made from further research. Thus, there is capacity to gain benefit by directing funds into research about surgical interventions but not if the focus of that further research is on the complications that may arise from surgery.
| Information | Cost-effective treatment path | Cost (£) | QALY | INB (£)a | Pop-EVPIb (£) | |
|---|---|---|---|---|---|---|
| First cycle | Second cycle | |||||
| CPPI 1 | Surgery | N/A | 1342 | 0.166 | 653 | 353,000 |
| CPPI 2 | Surgery | N/A | 1358 | 0.161 | 544 | 294,000 |
| CPPI 3 | WW | HA | 1303 | 0.131 | 0 | 0 |
| CPPI 4 | WW | HA | 1335 | 0.160 | 548 | 297,000 |
| CPPI 5 | HA | BAHA | 1954 | 0.165 | 19 | 10,000 |
Results for randomised control trial model
Baseline
We undertook deterministic analyses of the trial model. First, given our assumptions, at baseline the QALY increment of intervention over control is on average 0.0214 (i.e. 0.0456 – £0.0242). This is a relatively small gain compared with the 0.166 gain possible in the event of recovery from OME without surgical complications, but recovery rates are modest, even when taking into account the assumption of doubling the intervention recovery rate. Moreover, the eightfold magnitude of the difference suggests that a VOI analysis will identify positive worth associated with gaining additional information from research reinforcing that same positive finding in the clinical care pathway analysis.
Turning to costs, suppose per-procedure intervention treatment adds on top of control an additional cost of £300 due to the NHS, this being the mid-point of the assigned cost range. The per-patient costing differential due to the trial is, on average, £297 (i.e. £2183 – £1886). This yields an ICER of £13,861/QALY and a positive INB of £132 when calculated at the £20,000/QALY threshold. Figure 20 depicts the ICER as the continuous line varying against the added per-procedure cost of the intervention over the range of £50–600.
FIGURE 20.
Incremental cost-effectiveness ratio (continuous): SA 1 (long dashes), SA 3 (short dashes).
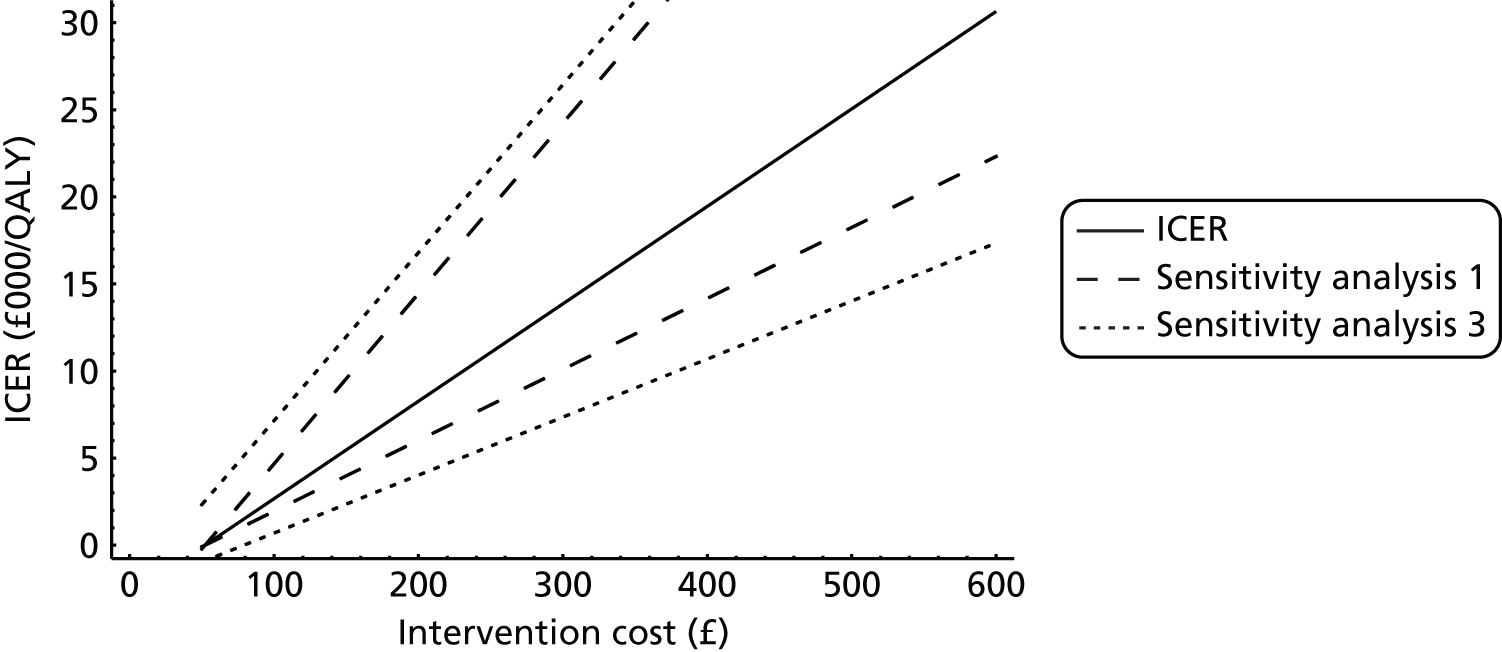
Sensitivity analyses
SA 1: QALY mapping In this deterministic SA the dB–QALY mapping coefficient is varied. Dixon’s estimate 0.00874 used to map hearing loss to QALY is enclosed within a 95% CI (0.005 to 0.012). Raising the mapping value from the point estimate will accentuate the QALY differential between the trial arms resulting in a decrease in the ICER, as the cost differential does not change. Decreases from the point estimate serve to work in the opposite direction. For example, at an added per-procedure intervention cost of £300 the ICER at the lower bound (0.005) is £24,231/QALY, and at the upper bound (0.012) is £10,096/QALY; INB values at the £20,000/QALY threshold are, respectively, –£52 and £292. The long-dashed lines appearing in Figure 20 plot the ICER evaluated at either extreme of the CI.
SA 2: Costs In this deterministic SA, surgery costs are varied. At baseline, surgery costs associated with grommets insertion and combination adenoidectomy plus grommets are applied at median values of NHS reference cost. These we now replace with their respective lower/upper quartile values (see Table 32), and these are applied to both arms. With less/more surgery costs, the ICER increases/decreases, as the relative cost of the intervention compared with control is greater/lesser, as the QALY differential does not vary. For example, at an added per-procedure intervention cost of £400, the ICER, when evaluated at the lower quartile of reference costs, is £19,912/QALY, whereas at the upper quartile it is £19,163/QALY; both of these ICER values enclose closely the baseline ICER reported above. This same pattern of robustness persists as the cost of the intervention is increased.
SA 3: Recovery rates In this deterministic SA, the OME recovery rates under the intervention arm are decreased/increased by 0.05. A lower rate will tend to lessen the apparent difference between intervention and control and therefore the ICER will increase. On the other hand, a higher rate will magnify the difference, making the intervention appear more cost-effective. The short-dashed lines in Figure 20 plot the ICER evaluated at 0.05 on either side of the baseline recovery rates.
Value of perfect information
Information about the target population – children with Down syndrome with OME – is scarce. Even within the confines of our hypothetically constructed trial – albeit one for which we have tried whenever possible to populate our parameter set with values taken from practice – the added value of research to gather further information is expected to be positive. In these analyses, uncertainty is removed by imposing perfect information with respect to recovery from OME and absence of surgical complications. The resulting EVPI can then be used to provide an upper bound against which the value of further research into model parameters can be assessed.
In our hypothetical trial setting we focus on a perfect information scenario pertaining to whether the child with Down syndrome will recover or not from OME simultaneous with the absence of any issues arising from surgical complications (see TPI 1 in Table 36). At baseline, the intervention is preferred over control if its per-procedure additive cost is approximately ≤ £400 when the cost-effective threshold is £20,000/QALY. However, even then decision uncertainty exists, as can be seen in Figure 20, where SAs’ bounds, concerning the QALY mapping coefficient (SA1) and the interventions recovery rate (SA3), are depicted. By focusing on the treatment effect through perfect information about the interventions recovery rates we can thus provide an upper bound on the value of research against which the cost of a RCT can be compared.
The TPI 1 scenario implies a time 0 reassignment of children into the respective trial arms – the mathematically equivalent effect of which is to alter the randomisation rate. For TPI 1, the proportion of known outcomes is Pr(cycle 1 recovery) × [1 – Pr(cycle 1 complications)] = 0.2 × (1 – 0.24) = 0.152, where this proportion of trial participants would be assigned to intervention, as all will receive the full QALY benefit of 0.166 associated with recovery from OME. The remaining proportion of 0.848 is assigned to control, as under TPI 1 there is no information about outcomes under the control arm. Table 43 shows the population EVPI against per-procedure intervention cost at a cost-effectiveness threshold of £20,000/QALY.
| Information | Pop-EVPIa (£) | |
|---|---|---|
| Cost £200 | Cost £400 | |
| TPI 1 Pr(recovery under intervention) = 1 and Pr(complications) = 0 | £588,000 | £648,000 |
We may examine the sensitivity of pop-EVPI to the most sensitive scenario SA1 varying the QALY mapping coefficient. For the per-procedure intervention cost set to £400, pop-EVPI evaluated at the lower/upper QALY mapping coefficient value is, respectively, £465,000 and £808,000.
Summary
Modelling demonstrates the limitations of the information in this topic, and there is potential scope for more research around interventions for OME-induced hearing loss in children with Down syndrome. The main conclusions from both models, given the limitations of their assumptions, are:
-
In clinical management the most cost-effective strategy for a child with Down syndrome experiencing OME-induced hearing loss is WW, followed by symptom management using HAs in those that tolerate them.
-
If further research using RCTs into new OME recovery-improved surgical interventions are to be conducted then, to mitigate uncertainty at conventional ICER threshold levels, economic benefit can be derived, provided that costs do not exceed approximately £650,000.
Chapter 6 Discussion
Background to the need for this study
There is a lack of good-quality clinical evidence to inform clinically effective and cost-effective interventions for glue ear in children with Down syndrome. Further research is needed and the best-quality evidence would come from a RCT, but would parents and professionals be willing to take part in any such trial? Is the extent of the lack of information sufficient to warrant investment of health research money? This feasibility study was designed to address these questions and to determine if pursuing further information through research would be practical, beneficial and cost-effective.
What makes Down syndrome different?
It is hardly surprising, but perhaps worthy of comment, that bringing up a child with Down syndrome is, without exception, presented as a complex and challenging experience. 81,82 The perceived ‘vulnerability’ and ‘dependence’ of the child heightens the parenting role and increases the sense of responsibility felt by the parent for the care and development of their child. A range of other, varied, emotions and feelings are also present in relation to specific personal experiences that are associated with bringing up a child who has Down syndrome. These include anger, resentment, denial, stigma, ‘fight’, ‘being part of a wider community’ and ‘responsibility to others’. The challenge of ‘working things out’, i.e. practical everyday things, was evident throughout the data, alongside some of these more emotional/social features. For example:
You go through something with your child, don’t you and that is the way it always works with a child like J. You learn, pick up hints, tips, whatever has gone on and then you hope to pass that onto somebody else. I mean, [being] ‘mum’, it is so much more with a child like J, it is all about finding out what works, what’s the best thing, doing the best thing for them and if you can then pass that onto future children so they don’t have to, you know, find a way through themselves, absolutely. That is a good thing.
Strengths and weaknesses
The study was informed by a multidisciplinary research team supported by an engaged advisory group contributing the parent, patient and lay perspective, as well as independent clinical advice. As such, we feel that the questions asked in all elements of the research were appropriate and covered relevant issues to inform the feasibility and, if appropriate, design of future research.
This study achieved a lower than expected response rate from both parents and professionals, but arguably provides representative sample sizes for the qualitative elements of the study. It is likely that neither group is truly representative of the population that they were selected to represent. As in all similar research, it is likely that respondents were those who were more interested in the topic expressing more coherently thought-through opinions. The issue here is whether that means future research in a wider population would be more or less feasible?
Responses to the Delphi review were received from 28 professionals, with 24 completing all three rounds. Although a small number, this group encompasses the range of professionals involved in the care of children with Down syndrome and glue ear and the responses in terms of consensus or lack of it, and the pathways to achieving it were similar to previously reported Delphi reviews. A Health Technology Assessment publication on consensus methods68 indicates that as few as 12 participants may be required to ensure reliability and that as numbers increase response rates may diminish, possibly making the process counterproductive, in that bigger numbers might broaden ownership of the consensus and may lead to diminishing response rates that undermine the value of initially including more people.
From the 24 respondents who completed all three rounds of the review it has been possible to draw conclusions in consistent themes. The range of responses and the fact that consensus was not achieved immediately, or in some instances, at all, indicates that differences of attitude do exist between the various professions. Any negative attitudes would need to be controlled for in a future study.
A key element of representativeness for parents was their knowledge and experience of glue ear. From clinical experience we expected the majority of children to have had glue ear and expected the parents responding to be those whose children had experienced the condition but only just > 50% said that a doctor had diagnosed glue ear. This may be because we used the term ‘doctor’ or it may be because professionals do not actually say ‘glue ear’. More parents reported that their child had medical problems with their ears than those who said the child had been diagnosed with glue ear. It is likely that the parents responding are generally representative of those who might be asked to consider participation in a RCT.
Design of future research
For any future study to be successful it must achieve a high level of recruitment, both of parents to agree to their children taking part and of professionals to agree to recruit participants. The key issue in design is to encourage and not discourage participation.
Choice of interventions
There is currently clinical equipoise concerning the ‘best’ treatment for glue ear for children with Down syndrome. Clinicians identified conventional HAs, softband BAHA and grommets to be the most effective treatment options in this population, and these interventions, along with WW, and grommets and adenoidectomy, are possible interventions that might productively be investigated in a future research study. In restricting the scope of a future study to two treatment options, clinicians here prioritised softband BAHA and grommets. BAHAs are not routinely offered in all clinical services in the UK but a research study, appropriately funded could facilitate their provision. However, if found to be the intervention of choice, standard clinical provision would have to be implemented. A study including surgery, i.e. grommet insertion, may not be attractive to all parents, although some would welcome the chance for their child to receive this intervention. The inclusion of WW for this population of children would probably not be appropriate owing to the inferred element of ‘wasting time’. Data generated here also confirm that research should not incorporate alternative therapies or decongestants as treatment options.
There may be an argument for smaller in-depth studies in particular subgroups, for example a study of the role of antibiotics in children with Down syndrome who suffer recurrent infections, as a number of parents in this study reported improvements after antibiotics.
Choice of design
Randomisation was seen as a barrier by both parents and professionals, and an observational study of outcomes from clinically determined interventions might be more successful in recruiting participants.
Barriers and facilitators: parents’ perspective
Any proposed research study needs to respond to the broad similarities of parenting a child with Down syndrome (multiple symptoms, social–developmental issues, practical difficulties, etc.) and also needs to accommodate and negotiate the variety of highly personal perspectives and experiences that families have demonstrated. Individual willingness to participate in any future research is most likely to be influenced by familial experiences of medical professionals, exposure to life-threatening/life-saving medical procedures, and the nature and severity of their child’s symptoms and/or behavioural issues, more so than any general perspective on glue ear, Down syndrome or medical research.
Willingness to participate in a research study will not only vary between families, but also would vary over time. Times when symptoms are not well controlled, when treatment is not working, when the study treatment options are new, might all be times when a family might be more likely to participate in a study, whereas some degree of stability and control in the condition, and treatment options to which the child has already been exposed, might discourage participation. Research would certainly be unattractive at those times when the condition is being managed well. At this time research might be perceived as risking previously gained improvement.
There might exist a similarly complex and possibly contradictory relationship between a child’s age and a family’s willingness to take part in research. Research involving younger children might be perceived to have the greatest impact (most years of benefit, unlikely to have a stable treatment regime to disturb) but is also likely to be perceived as most risky and potentially traumatic to both child and parents, particularly for parents of very young children who are adjusting to parenting a disabled child. Times of transition are known to be times when parents become more concerned about communication, i.e. starting school and transfer from infant to junior to secondary education, particularly if separate schools. Concern often peaks when plans are being considered for the secondary phase of education. The timing of an invitation to participate in research perhaps requires further investigation.
That parents in this study described children with lesser or greater behavioural issues, who are more or less confident in new situations, who are more or less comfortable with treatments and more or less happy with strangers, once again reinforcing the range of circumstances that typify this population. Although clinic visits in a future research study might work for one family, home visits would be essential for another; frequent data collection might be acceptable to one but impossible for another; the involvement of unknown researchers might be unproblematic to some but a source of stress for others, etc. Some general principles might be postulated: recognise and limit disruption to family routines; generate child-friendly environments for appointments; be flexible about appointment times, accommodating school and work; recognise that families often have other children to care for; and be geographically accessible, ensuring that involvement does not require lots of travel (children’s centres were suggested as a possible venue). These are simple principles of good practice in undertaking research but are particularly relevant for this population.
It is hard to find concrete recommendations with respect to recruitment strategies and who is best placed and most likely to succeed in recruiting families to a study. Families would need to feel confident in the person who approached them to become involved; for some this meant someone already involved in the care of their child, whereas for others this meant a member of the team doing the research. Support and recommendation from other families were mentioned on a number of occasions. Overall, it might be summarised that the person who approaches families needs to be ‘credible’, he/she needs to appear knowledgeable about the challenges that families face, and also what the research will involve.
Detail about research processes was indicated to be important. Without detail about what is being asked of them, and what the outcomes might be, families cannot make a fully informed decision. A clear explanation of the chosen research design is essential as evidenced by a lack of understanding of randomisation expressed by some parents. A high level of recruitment could be compromised by parents withdrawing when their child is allocated to an intervention that they definitely do not want.
Barriers and facilitators: professionals’ perspective
Data generated here confirm that clinicians consider glue ear to be an important and challenging condition that can have significant impacts upon the health, behaviour and development of children with Down syndrome. That glue ear can affect hearing and communication – with direct consequences for social and emotional development, behavioural development and educational success – in a population that is already challenged by developmental delay reinforces the importance of effective management in this population. That Down syndrome is typified by a variety of symptoms, by symptoms that come and go, and by different manifestations in different children emphasises the complexity of the syndrome in both manifestation and management. It is consequently not surprising that more than one-quarter of those surveyed here could not isolate a single/prime symptom that might inform their clinical decision-making, and that more than two-thirds (70 out of 99) indicated that knowledge of multiple and varied factors are required to inform the management of glue ear in a child with Down syndrome.
This is a condition that clinicians find difficult to be entirely confident about treating, and a condition for which new evidence-based guidelines would be welcomed and would probably have a positive impact upon clinical management. Clinicians would support future research into the management of glue ear in children with Down syndrome, and the Delphi review demonstrated that significant numbers of clinicians would be willing to recruit to an appropriate study. That the variety of symptoms associated with glue ear, the complexity of management and the clinical and developmental difficulties associated with Down syndrome might act as barriers to the success of any future research study were rejected by the clinical population consulted here.
A consensus that research should seek improvements in a child’s hearing and in a child’s speech, language and communication was easily reached, and this reflects a professional assessment that these symptoms are frequent, difficult for the child and their family, and challenging to manage. A more detailed assessment of these data shows that hearing difficulties are identified as the most frequent problem associated with glue ear and also the most important challenge to address in Down syndrome (ranked first by most clinicians here). However, when all responses are considered (i.e. not just ranking first), listening, understanding communication and using communication all overtake hearing in this professional assessment of the most common and challenging symptoms of glue ear in children with Down syndrome. From a professional point of view, although future research might appropriately take improvements in hearing as its primary outcome, it should also definitely incorporate improvements in communication as a secondary objective. This assessment also reflects the opinion that improvements in hearing might be objectively measured more easily than improvements in communication.
There was some recognition that involvement in research might offer benefits for professional and career development but, in the main, professionals viewed an enhanced evidence base and better management of glue ear in Down syndrome as its key benefits. It should be noted, however, that this positive assessment did not extend to feeling confident that participation in a study would bring immediate clinical or non-clinical improvements for those children included. Most professionals surveyed here indicated that both observational and RCT research design might bring positive results, and most felt confident in describing both the benefits and processes associated with research. It is important to note that more professionals indicated that they would be happier to recruit families to an observational study rather than to a RCT, and that more than one-third of those who said that they would be happy recruiting to an observational study were not sure that they would do so for a RCT.
Differences in the views expressed by professionals might be attributed to a difference between those responsible for provision of interventions for glue ear, i.e. ENT surgeons and audiologists, and those who work with the outcomes of a hearing loss, i.e. SLTs and paediatricians, who support parents and children in daily life. Perhaps these data indicate a desirability for closer collaboration.
Throughout our data it is clear that professionals view this as an important and challenging topic; every child is different and every case requires different clinical management. Cases are complicated, typified by a complex interplay of those symptoms associated with glue ear and those symptoms associated with Down syndrome. These are symptoms that are difficult to distinguish and difficult to isolate, and it is this complex characteristic that makes future study both worthwhile and challenging. Yet this is a challenge that professionals here consider important to tackle; a challenge that all support, in principle at least, and that many, importantly, indicate that they would practically support should any study be undertaken.
Outcomes in a future trial
We explored the relevant areas of outcome rated as important by parents and professionals in order to contribute to the design of any future study through closed questions assessing importance, open discussion in interviews and focus groups, and in comparison with a data set derived from the responses of parents of children with glue ear but not Down syndrome. The outcome domains identified as important included speech, language and communication development, social development, hearing, school progress and quality of life of the children and parents, including issues around behaviour, sleep and general well-being. Children with Down syndrome exhibit wide variation in these measures and, in order to accurately reflect that variation, it would be necessary to include a number of measures in any future study.
We attempted to address this complexity of appropriate domains of outcome needed in future trials and whether or not measurement of these outcomes would be practical and feasible. It is apparent that the complexity due to variation in presentation of children with Down syndrome (particularly in the area of communication) and the overlapping impact of various domains raises challenges in being clear about causality and attribution of any identified improvement in any given domain.
Although speech and language were considered to be most important by parents and professionals as outcomes from any intervention, formal assessment with validated standard tools might be challenging as some existing tools (e.g. those assessed here) are not seen as appropriate for children with Down syndrome. Pre- and post-descriptive data would be most useful and, in relation to speech, an objective intelligibility rating.
In addressing any recommendation of research outcome measures, we have to see treatment in its larger context as attempting to help Down syndrome families, rather than alleviating middle ear disease as such, even though it is the direct manifestation of middle ear disease that can be helped by the treatments available. Previous research in children with glue ear who do not have Down syndrome found some evidence for improvements in hearing, leading to improvements in speech/language (M Haggard, personal communication), but the clinical outcomes in language in children with Down syndrome make it totally unclear whether improvements should be larger (via a synergistic multiplication) or smaller (because of a ceiling that cannot be shifted by therapy at this level.) Not only the parental prioritisation, but also the need for high reliability and ecological validity demands inclusion of a multifaceted assessment of language in any trial with performance as well as rating measures. A single main outcome measure cannot be justified under any circumstances. Outcomes should be multiple, based on the identified domains of concern and others relevant to the treatability of the glue ear, but should not be exclusively oriented to assessment of hearing.
It will be important to incorporate outcome measures that not only address elements of importance to parents and children, but also consider the practicalities of measurement, including time. Impact on communication is central and parental report via questionnaires may contribute a time-efficient measure. At the heart of any measure of effectiveness should be the question ‘Does helping the condition and its consequence, i.e. glue ear and hearing, help the child not only in communication, but also in social and educational progress and in the impact on behaviour and the family?’.
Any measure would first need to be sensitive enough, within subjects, to detect a difference due to an intervention over what would be a relatively short period of time and, second, measure outcomes that are affected mostly by hearing problems rather than by other consequences of Down syndrome. Although standardised assessments are not usually used clinically with children who have Down syndrome, they are used in research, often necessarily with caveats about how performance was affected by other factors. For example, it is difficult to score a child’s expressive responses when they have highly unintelligible speech. Another important issue in this context is the range of cognitive ability in the children and the effect of that on obtaining traditional outcome measures of hearing thresholds, speech discrimination and speech production. Outcomes of any future research study assessing treatment options for glue ear in children with Down syndrome might include speech, language and communication development; social development; hearing; school progress and quality of life of the child and parents, including issues around behaviour, sleep and general well-being. Children with Down syndrome will exhibit wide variation in these measures and, in order to accurately reflect that variation, it would be necessary to include a number of measures in any future trial. Key issues in any assessment of communication are the widespread reliance on signing systems for children with Down syndrome in the preschool years, and the variation in attention control and interaction.
Economic modelling
The intention of the economic modelling was to demonstrate the EVPI for interventions to treat children with Down syndrome with chronic OME. The models we examined had several limitations.
First, they iterate for only two cycles, notionally 2 years of life for the child, up to the age of 5 years. This may be too long a period in the context of a RCT, and too short a period in terms of clinical management. If trials are for shorter periods then the time lapse between repeated rounds of surgery is shortened with possible implications on adverse consequences; the need to extract and/or reinsert grommets in particular may raise. On the other hand, in extending to additional years of clinical management, rates of recovery from surgery and rates of spontaneous recovery improve with the age of the child and this adjustment would need to be incorporated. Our assumption on fixed recovery rates is a conservative estimate of the benefits of interventions for chronic OME.
Second, the model includes softband BAHA, which has a 3-year maintenance contract, while including only the first year of possible benefit from the BAHA. This would suggest that the model underestimates the benefits from the BAHA device. However, it is worth noting that the clinical care pathways model chooses BAHA only to be part of the cost-effective pathway when its costs are considerably lowered and it is always tolerated.
Third, the majority of the parameters are based upon expert opinion. This demonstrates the limited evidence available in this field, making construction of an economic model in this topic difficult. There is also the consideration of the link between PTA hearing loss and QALYs. The mapping of hearing loss to QALYs was based upon unpublished work conducted by Simon Dixon, and correspondence with the author allowed access to the data. The ordinary least squares regression used to develop the mapping estimate was deemed acceptable up to a hearing loss of 40 dB; however, SAs showed that robustness to the mapping could not be assumed.
Another consideration was the possibility of a microcosting analysis of the HA intervention. This was the method used in the NICE guidance. 17 The cost of HAs in the NICE guidance17 is slightly larger, and hence this model could potentially be underestimating the costs of the HA intervention; however, the costs used are the current NHS reference costs (see Table 32), which represent the current reimbursement of HAs, which could potentially be seen as more relevant.
Despite these limitations, to the authors’ knowledge this is the first economic evaluation that has been conducted on interventions for OME in children with Down syndrome. In comparison with the economic evaluation conducted for the NICE guidance17 on OME, the clinical pathways model is more flexible, allowing the child to be managed with different treatments over time, implying that the model is more representative of the clinical situation.
Finally, both models assume a greater degree of hearing loss in a child with Down syndrome than a child in the general population.
The VOI analysis in the clinical care pathways model identifies greatest value in improving surgery recovery rates, with a population EVPI of £357,000. There is no capacity for benefit in research directed towards reducing surgery complication rates. The surgery-focused trial model also identifies a capacity to derive value from research into improving surgical recovery rates, provided that research costs do not exceed approximately £650,000. Finally, symptom management with BAHA appears the least preferred option, with a very small EVPI derived.
Recommendations
These recommendations are suggested, and should be considered, based on a relatively low response rate, which, nonetheless, is considered to be sufficiently representative of the populations surveyed.
-
In order to maximise recruitment and retention, future research of the cost effectiveness and clinical-effectiveness of interventions for glue ear in children with Down syndrome should be based on an observational cohort study design.
-
There is a possible role for small in-depth studies in particular subgroups of children, as it is unlikely, with the diversity of experience in this population, that one approach will address all issues.
-
If a RCT design is proposed, all of the professionals concerned must be fully trained in the methodology involved and be able to communicate that to parents who are approached for participation.
-
If a RCT design is proposed, all of the professionals involved must be confident in their explanation to parents about clinical equipoise surrounding all interventions including, if appropriate, WW.
-
If a RCT design is proposed, researchers should be aware of parental concerns expressed in this report and design any trial to maximise participation.
-
If comparing only two-treatment options, these should be softband BAHA and grommets.
-
Future research should consider within-subject measures of developmental outcomes. If a standardised assessment tool is not available, initial work will need to be undertaken to develop appropriate tools.
-
Although improved hearing levels might be seen as the primary outcome measure owing to the ease of measurement and obvious link with intervention, speech, language and communication are considered to be equally, if not more, important domains by both parents and professionals.
-
If question-based outcome measures are to be used, resources should be available to support all parents to access and complete them.
-
In order to be cost-effective, research costs should be < £650,000.
Acknowledgements
We thank the following:
The parents who completed questionnaires, and participated in interviews and focus groups.
The clinicians and teachers who completed questionnaires and took part in the Delphi review.
The paediatricians who identified appropriate families for inclusion, and distributed questionnaires and reminders.
Sue Thurman and all members of the steering group who have given considerable amounts of time to advising the research team on the development and reporting of the project.
Helen White, Administrator, NIHR Nottingham Hearing Biomedical Research Unit, for support throughout the project and for considerable work in formatting the report.
Sam Catterick, Research Assistant, NIHR Nottingham Hearing Biomedical Research Unit, for support with the literature review and formatting of the report.
Mark Edmondson-Jones, Senior Research Fellow, NIHR Nottingham Hearing Biomedical Research Unit, for statistical analyses and interpretation.
Professor Mark Haggard for support and advice concerning outcome measurements.
Helen Spencer for statistical analyses and interpretation of the Cambridge OME data.
Casey Quinn, Lecturer in Health Economics, University of Nottingham, for development of the VOI analysis plan in the original application.
Sarah Allen, Specialist Advisor for Hearing Impairment, Nottingham Children’s Speech and Language Therapy Service, for her contribution to the development of the original outline application.
The referees engaged by the NIHR HTA programme for helpful comments and suggestions on this report.
Contributions of authors
The authors of this report are listed in the section below.
The following authors were involved in the construction of the original bid including the research design: Heather Fortnum, Paul Leighton, Claire Benton, Elizabeth Marder and Andrew Marshall.
All authors were part of the project management team that met monthly throughout the course of the project. All contributed to the development of the methodology, interpretation of the results, and writing up and dissemination of the findings. All approved the final version of the report.
Specific contributions of each author
Dr Heather Fortnum (Associate Professor and Reader in Hearing Research) was the principal investigator and led all elements of the project. Major contribution to the design of all survey instruments, co-ordination of the Delphi review, analyses of all elements of the parent and professional data sets, wrote the first draft of Chapters 1–3 and 6 of the final report, and led the revision and submission of the final report.
Dr Paul Leighton (Senior Research Fellow in Qualitative Methods) supervised and led the design, analyses and reporting of the qualitative elements of the project, designed the final version of the Delphi review, facilitated the focus groups, and wrote the first draft of Chapter 4 of the report.
Dr Murray D Smith (Associate Professor in Health Economics) led the design, analyses and reporting of the economic modelling element of the project, and wrote Chapter 5 of the report.
Dr Lisa Brown (research fellow) contributed to the searching and drafting of the literature review, and the design of data collection tools and databases, coordinated data collection for the project (including distributing questionnaires, conducting interviews with parents and facilitating focus groups), co-ordinated distribution of the professionals’ survey and developed initial versions of the Delphi review.
Matthew Jones (research assistant and health economist) contributed to the searching and drafting of the literature review, and the development and analyses of the economic modelling, and contributed to Chapter 5 of the report.
Claire Benton (clinical lead for paediatric audiology), co-ordinated and advised on the audiology elements of the project, and contributed to identification and recruitment of families.
Dr Elizabeth Marder (consultant paediatrician) co-ordinated and advised on the clinical paediatric elements of the project, led the identification and recruitment of families and acted as the contact point for the paediatricians involved in the study.
Mr Andrew Marshall (consultant otolaryngologist) co-ordinated and advised on the clinical ENT elements of the project.
Kate Sutton (speech and language therapist) co-ordinated and advised on the SLT aspects of the research and contributed to the identification of families.
Project steering group
The project steering group was chaired by Sue Thurman and included representatives of the relevant professional disciplines, professional organisations, parent support associations, parents and the Down syndrome population (see Appendix 1 for the list of members).
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Massa H, Cripps A, Lehmann D. Otitis media: viruses, bacteria, biofilms and vaccines. Med J Aust 2009;191:S44-9.
- Barr E, Dungworth J, Hunter K, McFarlane M, Kubba H. The prevalence of ear, nose and throat disorders in preschool children with Down’s syndrome in Glasgow. Scott Med J 2011;56:98-103. http://dx.doi.org/10.1258/smj.2011.011036.
- Wu J, Morris J. Trends in maternal age distribution and the live birth prevalence of Down’s syndrome in England and Wales: 1938–2010. Eur J Hum Genet 2013;21:943-7. http://dx.doi.org/10.1038/ejhg.2012.288.
- Balkany T, Mischke R, Downs M, Jafek B. Ossicular abnormalities in Down’s syndrome. Arch Otolarngol Head Neck Surg 1979;87:372-84.
- Brown P, Lewis G, Parker A, Maw A. The skull base and nasopharynx in Down’s syndrome in relation to hearing impairment. Clin Otolaryngol Allied Sci 1989;14:241-6. http://dx.doi.org/10.1111/j.1365-2273.1989.tb00368.x.
- Strome M. Down’s syndrome: a modern otorhinolaryngological perspective. Laryngoscope 1981;91:1581-94.
- Bluestone C. Studies in otitis media: Children’s Hospital of Pittsburgh, University of Pittsburgh progress report – 2004. Laryngoscope 2004;114:1-26. http://dx.doi.org/10.1097/01.mlg.0000148223.45374.ec.
- Kanamori G, Witter M, Brown J, Williams-Smith L. Otolaryngologic manifestations of Down syndrome. Otolaryngol Clin North Am 2000;33:1285-92. http://dx.doi.org/10.1016/S0030-6665(05)70281-4.
- Venail F, Gardiner Q, Mondain M. ENT and speech disorders in children with Down’s syndrome: an overview of pathophysiology, clinical features, treatments, and current management. Clin Pediatr 2004;43:783-91. http://dx.doi.org/10.1177/000992280404300902.
- Harigai S, Nagai K, Nakamura K, Iino Y, Tanaka Y. Hearing Impairment and Otitis Media with Effusion in Down’s Syndrome. Recent Advances in Otitis Media. Amsterdam/New York: Kugler; 1994.
- Schwartz D, Schwartz R. Acoustic impedance and otoscopic findings in young children with Down’s syndrome. Arch Otolaryngol 1978;104:652-6. http://dx.doi.org/10.1001/archotol.1978.00790110042011.
- Selikowitz M. Health problems and health checks in school-aged children with Down syndrome. J Paediatr Child Health 1992;28:383-6. http://dx.doi.org/10.1111/j.1440-1754.1992.tb02697.x.
- Tomasevic P. Management of hearing impairment in children with Down’s syndrome. Aust J Otolaryngol 1998;3:25-8.
- van Gameren-Oosterom H, Fekkes M, Buitendijk S, Mohangoo A, Bruil J, Van Wouwe J. Development, problem behavior, and quality of life in a population based sample of eight-year-old children with Down syndrome. PLOS ONE 2011;6. http://dx.doi.org/10.1371/journal.pone.0021879.
- Cleland J, Wood S, Hardcastle W, Wishart J, Timmins C. Relationship between speech, oromotor, language and cognitive abilities in children with Down’s syndrome. Int J Lang Commun Disord 2010;45:83-95. http://dx.doi.org/10.3109/13682820902745453.
- Shott S, Joseph A, Heithaus D. Hearing loss in children with Down syndrome. Int J Pediatr Otorhinolaryngol 2001;61:199-205. http://dx.doi.org/10.1016/S0165-5876(01)00572-9.
- Surgical Management of Otitis Media with Effusion in Children. London: NICE; 2008.
- Selikowitz M. Short-term efficacy of tympanostomy tube for secretory otitis media in children with Down syndrome. Dev Med Child Neurol 1993;35:511-15. http://dx.doi.org/10.1111/j.1469-8749.1993.tb11681.x.
- Iino Y, Imamura Y, Harigai S, Tanaka Y. Efficacy of tympanostomy tube insertion for otitis media with effusion in children with Down syndrome. Int J Pediatr Otorhinolaryngol 1999;49:143-9. http://dx.doi.org/10.1016/S0165-5876(99)00117-2.
- Rovers M, Black N, Browning G, Maw R, Zielhuis G, Haggard M. Grommets in otitis media with effusion: an individual patient data meta-analysis. Arch Dis Child 2005;90:480-5. http://dx.doi.org/10.1136/adc.2004.059444.
- Browning G, Rovers M, Williamson I, Lous J, Burton M. Grommets (ventilation tubes) for hearing loss associated with otitis media with effusion in children. Cochrane Database Syst Rev 2010;10. http://dx.doi.org/10.1002/14651858.CD001801.pub3.
- Martin G, Klusek J, Estigarribia B, Roberts J. Language characteristics of individuals with Down syndrome. Top Lang Disord 2009;29:112-32. http://dx.doi.org/10.1097/TLD.0b013e3181a71fe1.
- Karkanevatos A, Lesser T. Grommet insertion in children: a survey of parental perceptions. J Laryngol Otol 1998;112:732-41. http://dx.doi.org/10.1017/S002221510014157X.
- Howell P. Signs of developmental stuttering up to age eight and at 12 plus. Clin Psychol Rev 2007;27:287-306. http://dx.doi.org/10.1016/j.cpr.2006.08.005.
- Feagans L, Kipp E, Blood I. The effects of otitis media on the attention skills of day-care-attending toddlers. Dev Psychol 1994;30:701-8. http://dx.doi.org/10.1037/0012-1649.30.5.701.
- McGee R, Silva P, Stewart I. Behaviour problems and otitis media with effusion: a report from the Dunedin Multidisciplinary Child Development Study. New Zeal Med J 1982;95:655-7.
- Bennett K, Haggard M, Silva P, Stewart I. Behaviour and developmental effects of otitis media with effusion into the teens. Arch Dis Child 2001;85:91-5. http://dx.doi.org/10.1136/adc.85.2.91.
- Higson J, Haggard M. Parent versus professional views of the developmental impact of a multi-faceted condition at school age: otitis media with effusion (‘glue ear’). Br J Educ Psychol 2005;75:623-43. http://dx.doi.org/10.1348/000709905X41906.
- Berman S, Roark R, Luckey D. Theoretical cost effectiveness of management options for children with persisting middle ear effusions. Pediatrics 1994;93:353-63.
- Williamson I. Otitis media with effusion in children. Clin Evid 2011.
- Hellstrom S, Groth A, Jorgensen F, Pettersson A, Ryding M, Uhlen I, et al. Ventilation tube treatment: a systematic review of the literature. Otolaryngol Head Neck Surg 2011;145:383-95. http://dx.doi.org/10.1177/0194599811409862.
- Van Den Aardweg M, Schilder A, Herkert E, Boonacker C, Rovers M. Adenoidectomy for otitis media in children. Cochrane Database Syst Rev 2010;1. http://dx.doi.org/10.1002/14651858.CD007810.pub2.
- Perera R, Haynes J, Glasziou P, Heneghan C. Autoinflation for hearing loss associated with otitis media with effusion. Cochrane Database Syst Rev 2006;4.
- van Zon A, van der Heijen G, van Dongen T, Burton M, Schilder A. Antibiotics for otitis media with effusion in children. Cochrane Database Syst Rev 2012;9. http://dx.doi.org/10.1002/14651858.CD009163.pub2.
- Griffin G, Flynn C. Antihistamines and/or decongestants for otitis media with effusion (OME) in children. Cochrane Database Syst Rev 2011;9. http://dx.doi.org/10.1002/14651858.CD003423.pub3.
- Simpson S, Lewis R, van der Voort J, Butler C. Oral or topical nasal steroids for hearing loss associated with otitis media with effusion in children. Cochrane Database Syst Rev 2011;5.
- Gulani A, Sachdev H. Zinc supplements for preventing otitis media. Cochrane Database Syst Rev 2012;4.
- Davies B. Auditory disorders in Down’s syndrome. Scand Audiol Suppl 1988;30:65-8.
- Mitchell R, Call E, Kelly J. Ear, nose and throat disorders in children with Down syndrome. Laryngoscope 2003;113:259-63. http://dx.doi.org/10.1097/00005537-200302000-00012.
- MRC Multicentre Otitis Media Study Group . Adjuvant adenoidectomy inpersistent bilateral otitis media with effucion: hearing and revision surgery outcomes through 2 years in the TARGET randomised trial. Clin Otolaryngol 2012;37:107-16. http://dx.doi.org/10.1111/j.1749-4486.2012.02469.x.
- Casselbrant M, Mandel E, Rockette H, Kurs-Lasky M, Fall P, Bluestone C. Adenoidectomy for otitis media with effusion in 2–3-year-old children. Int J Pediatr Otorhinolaryngol 2009;73:1718-24. http://dx.doi.org/10.1016/j.ijporl.2009.09.007.
- Price D, Orvidas L, Weaver A, Farmer S. Efficacy of adenoidectomy in the treatment of nasal and middle ear symptoms in children with Down syndrome. Int J Pediatr Otorhinolaryngol 2004;68:7-13. http://dx.doi.org/10.1016/j.ijporl.2003.08.053.
- Ramakrishnan Y, Marley S, Leese D, Davison T, Johnson I. Bone-anchored hearing aids in children and young adults: the Freeman Hospital experience. J Laryngol Otol 2011;125:153-7. http://dx.doi.org/10.1017/S002221511000188X.
- Kunst S, Hol M, Cremers C, Mylanus E. Bone-anchored hearing aid in patients with moderate mental retardations: impact and benefit assessment. Otol Neurotol 2007;28:793-7. http://dx.doi.org/10.1097/MAO.0b013e31809ed93a.
- McDermott A, Williams J, Huo M, Reid A, Proops D. The role of bone anchored hearing aids in children with Down syndrome. Int J Pediatr Otorhinolaryngol 2008;72:751-7. http://dx.doi.org/10.1016/j.ijporl.2008.01.035.
- Sheehan P, Hans P. UK and Ireland experience of bone anchored hearing aids (BAHA) in individuals with Down syndrome. Int J Pediatr Otorhinolaryngol 2006;70:981-6. http://dx.doi.org/10.1016/j.ijporl.2005.10.008.
- The Clinical and Cost Effectiveness of Surgical Insertion of Grommets for Otitis Media with Effusion (Glue Ear) in Children. Glasgow: NHS Quality Improvement Scotland; 2008.
- Reidpath D, Glasziou P, Del Mar C. Systematic review of autoinflation for treatment of glue ear in children. BMJ 1999;318:1177-8. http://dx.doi.org/10.1136/bmj.318.7192.1177.
- O’Brien M, Prosser L, Paradise J, Ray G, Kulldorff M, Kurs-Lasky M, et al. New vaccines against otitis media: projected benefits and cost-effectiveness. Pediatrics 2009;123:1452-63. http://dx.doi.org/10.1542/peds.2008-1482.
- Fletcher M, Fritzell B. Pneumococcal conjugate vaccines and otitis media: an appraisal of the clinical trials. Int J Otolaryngol 2012. http://dx.doi.org/10.1155/2012/312935.
- Taylor S, Marchiosop P, Vergison A, Harriague J, Hausdorff W, Haggard M. Impact of pneumococcal conjugate vaccination on otitis media: a systematic review. Clin Infect Dis 2012;54:1765-73. http://dx.doi.org/10.1093/cid/cis292.
- Petrou S, Dakin H, Abangma G, Benge S, Williamson I. Cost-utility analysis of topical intranasal steroids for otitis media with effusion based on evidence from the GNOME trial. Value Health 2010;13:543-51. http://dx.doi.org/10.1111/j.1524-4733.2010.00711.x.
- Robotham D, Hassiotis A. Randomised controlled trials in learning disabilities: a review of participant experiences. Adv Ment Health Learn Disabil 2009;3:42-6.
- Prescott R, Counsell C, Gillespie W, Grant A, Russell I, Kiauka S, et al. Factors that limit the quality, number and progress of randomised controlled trials. Health Technol Assess 1999;3.
- Ross S, Grant A, Counsell C, Gillespie W, Russell I, Prescott R. Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol 1999;52:1143-56. http://dx.doi.org/10.1016/S0895-4356(99)00141-9.
- Spaar A, Frey M, Turk A, Karrer W, Puhan M. Recruitment barriers in a randomized controlled trial from the physicians’ perspective – a postal survey. BMC Med Res Methodol 2009;9. http://dx.doi.org/10.1186/1471-2288-9-14.
- Caldwell P, Butow P, Craig J. Parents’ attitudes to children’s participation in randomised controlled trials. J Pediatr 2003;142:554-9. http://dx.doi.org/10.1067/mpd.2003.192.
- Shilling V, Young B. How do parents experience being asked to enter a child in a randomised controlled trial?. BMC Med Ethics 2009;10. http://dx.doi.org/10.1186/1472-6939-10-1.
- Nabulsi M, Khalil Y, Makhoul J. Parental attitudes towards and perceptions of their children’s participation in clinical research: a developing-country perspective. J Med Ethics 2011;37:420-3. http://dx.doi.org/10.1136/jme.2010.035899.
- Ackerman SJ, Duff S, Dennehy P, Mafilios M, Krilov L. Economic impact of an infection control education program in a specialized preschool setting. Pediatrics 2001;108. http://dx.doi.org/10.1542/peds.108.6.e102.
- Hellstrom S, Axelsson S, Bostrom K, Eckerlund I, Groth A, Hakanson K, et al. Tympanostomy Tube Insertion for Otitis Media in Children. Stockholm, Sweden: Swedish Council on Technology Assessment in Health Care (SBU); 2008.
- Timmerman A, Meesters M, Speyer R, Anteunis L. Psychometric qualities of questionnaires for the assessment of otitis media impact. Clin Otolaryngol 2007;32:429-39. http://dx.doi.org/10.1111/j.1749-4486.2007.01570.x.
- Dakin H, Petrou S, Haggard M, Benge S, Williamson I. Mapping analyses to estimate health utilities based on responses to the OM8–30 otitis media questionnaire. Qual Life Res 2010;19:65-8. http://dx.doi.org/10.1007/s11136-009-9558-z.
- Ritchie J, Lewis J. Qualitative Research Practice: A Guide for Social Science Students and Researchers. London: Sage; 2003.
- Pope C, Ziebland S, Mays N. Analysing qualitative data. BMJ 2000;320:114-16. http://dx.doi.org/10.1136/bmj.320.7227.114.
- Ritchie J, Spencer L, Bryman A, Burgess R. Analysing Qualitative Data. London: Routledge; 1993.
- Labour Force Survey. London: ONS; 2004.
- Murphy M, Black N, Lamping D. Consensus development methods, and their use in clinical guideline development. Health Technol Assess 1998;2.
- Vernon W. The Delphi technique: a review. Int J Ther Rehabil 2009;16:69-76. http://dx.doi.org/10.12968/ijtr.2009.16.2.38892.
- Graham B, Regehr G, Wright J. Delphi as a method to establish consensus for diagnostic criteria. J Clin Epidemiol 2003;56:1150-6. http://dx.doi.org/10.1016/S0895-4356(03)00211-7.
- Du Plessis E, Human S. The Art of the Delphi Technique: highlighting its scientific merit. Health SA Gesondheid 2007;12:13-24. http://dx.doi.org/10.4102/hsag.v12i4.268.
- Hsu C, Sandford B. The Delphi Technique: making sense of consensus. Practi Assess Res Eval 2007;12:1-8.
- Births and Deaths in England and Wales, 2011. London: ONS; 2012.
- Levels and Trends in Child Mortality: Report 2012. New York: United Nations Inter-agency Group for Child Mortality Estimation; 2012.
- Dahle A, McCollister F. Hearing and otologic disorders in children with Down syndrome. Am J Ment Defic 1986;90:636-42.
- Curtis L. Unit Costs of Health and Social Care 2012. Personal Social Services Research Unit, University of Kent; 2011.
- Kay DJ, Nelson M, Rosenfeld RM. Meta-analysis of tympanostomy tube sequelae. Otolaryngol Head Neck Surg 2001;124:374-80. http://dx.doi.org/10.1067/mhn.2001.113941.
- Randall DA, Hoffer ME. Complications of tonsillectomy and adenoidectomy. Otolaryngol Head Neck Surg 1998;118:61-8. http://dx.doi.org/10.1016/S0194-5998(98)70376-6.
- Down’s Syndrome Association . Key Facts 2013. www.downs-syndrome.org.uk/about-us/key-facts-and-faqs.html (accessed 25 January 2013).
- Indicator Area: Learning Disability; Health Economic Report. York: York Health Economic Consortium/National Primary Care Research and Development Centre; 2009.
- Pillay D, Girdler S, Collins M, Leonard H. ‘Its not what you were expecting, but it’s still a beautiful journey’: the experience of mothers of children with Down syndrome. Disabil Rehabil 2012;34:1501-10. http://dx.doi.org/10.3109/09638288.2011.650313.
- Sterling A, Warren SF. Maternal responsivity in mothers of young children with Down syndrome [published online ahead of print 19 July 2013]. Dev Neurorehabil 2013. http://dx.doi.org/10.3109/17518423.2013.772671.
Appendix 1 Membership of the steering group
Mrs Sue Thurman, chairperson of SLT and registered intermediary.
Mrs Tara Hall, parent of a child with Down syndrome.
Mrs Alison Curtis, parent of a child with Down syndrome.
Professor Anne Schilder, Professor of Paediatric Otorhinolaryngology, Director ENT Clinical Trials Programme.
Mr David Stewart OBE DL, head teacher.
Michelle McCafferkey, self-advocate and adult with Down syndrome.
Dr Jill Ellis, medical representative, Down Syndrome Medical Interest Group.
Mr Stuart Mills, information officer, Down’s Syndrome Association.
Appendix 2 Parent questionnaire
Appendix 3 Discussion areas for interviews
Ice breaker – set tone that it is about them – make them feel they have something to say (less interrogative):
-
There are many different health issues and experiences that parents and carers of children with Down syndrome might face. What health issues or experiences stand out for you?
-
We know that Down syndrome can be associated with hearing issues. Has your child had any difficulties in this regard? How has this affected them? How has this affected you? Prompt for . . . glue ear and most significant issue associated with it.
Treatment
There are a number of treatment options for glue ear; I’d like to talk about those . . .
-
Has your child required treatment for glue ear? If so, what treatments have they received?
-
Were these effective? Were there any difficulties with them?
-
Were there any other treatments that you would have liked to have received? Any that you refused. (Why?)
-
A preferred treatment.
Research in health and health care
We propose to carry out research to improve treatment of OME, I’d like to talk about research . . .
-
What do you know about health research?
-
Prompt – on TV, in the news, ‘breakthrough’
-
Prompt – What do you think about health research?
-
-
Would you be willing to take part in a health research study, say where a new treatment was being tested?
-
Would you be willing for your child to be involved in a health research study, where existing potential treatments are tested and compared?
-
Prompt – to explore differing responses
-
Prompt – benefits and difficulties of a child being in a study; benefits and difficulties of a child with Down syndrome being in a study.
-
Research in detail
There are different types of research. Here are some which might be used. We would like your opinion on each.
-
Have you heard of clinical trials? What do you understand by this?
-
Prompt – explain what it is and myths about clinical trials
-
Prompt – real benefits and difficulties
-
-
Randomisation is often something that people are unfamiliar with or consider to be ‘scary’. What do you understand by this?
-
Have you heard of observational research? What do you understand by this?
-
Prompt – explain what it is and myths about observational studies
-
Prompt – real benefits and difficulties
-
-
Follow-up? Issues of attrition? Outcomes?
-
Would you be willing for your child to be involved in a clinical trial . . . in an observational study . . . why?
Our study processes
Research studies can be difficult to run. It is important to get the small details right. Can you help with this?
-
Why would you get involved in a study about Down syndrome and OME? What might prevent you from getting involved?
-
Prompt – disruption to routine; flexibility
-
Prompt – concerns about clinical treatment/no treatment
-
Prompt – study burden; clinical burden
-
Prompt – immediate vs. future benefits and improvements in service.
-
-
If we approached you about being involved what would help you to make a decision . . . Before getting involved in any study, all the details and information is explained to you . . . how would you like this to be done, and by whom . . .?
-
From your view as a parent, what sort of improvement should we be looking for as professionals . . . [improved . . .] [less interventions . . .]?
-
Prompt – Which of these do you think is most important?
-
Appendix 4 Discussion areas for focus groups
Outline the purpose of the session. Discuss consent and confidentiality. Describe intention to consider two broad topics:
-
value of more research
-
nature and form of future research.
Outline the ground rules for interaction and highlight expectations.
Ice breaker . . . Say your name for tape.
Tell us the name and age of your child and your experience of glue ear/hearing issues. Has it been a problem?
What treatment options have you had?
Question 1 :
Do you think that more research in this area would be worthwhile?
Prompt to all: yes/no/unsure
Discussion points:
-
Why do you feel this?
-
What would the value of more research be? Current treatment – are options poor? Options are limited. Research will lead to advancements.
How important is it to do research into this condition?
How important is glue ear?
Are other issues more pressing?
Our research is showing us that this is a complex condition both for families and for medical professionals and that it might be difficult to do research.
Question 2:
Do you think that it would be possible to do more research in this area?
Prompts to all: Do you have any concerns about research?
Discussion points:
-
What problems could you imagine that might make involvement in a research study difficult for you and your family? Study burden, clash with routine, disruption and change to treatment
Would you be willing to take part in future research?
What could encourage you to take part in future research?
What benefits do you think that you might get from taking part in a research study?
If more research is to take place, it’s our job to make suggestions as to what form that research should take.
An important comparison to make is to get your perspectives upon RCT research and cohort study research:
-
What do you think about randomisation? Would it put you off being involved?
-
If surgery was included in a study would this put you off?
-
If WW was included in a study would this put you off?
-
Is there any treatment that you either definitely would or definitely would not want to be included in a research study?
-
In research it can feel as if choice and control are being removed from you, and from medical professionals. Do you think that this would be a problem for you?
Because this is a complex condition, which can have a wide range of consequences for a child or for a family, it is unclear what changes or improvements research should seek. Your perspective on this would be helpful.
-
What outcomes should research consider?
-
Improved hearing, improved speech and communication, fewer ear Infections, improved behaviour, progress at school.
-
-
Medical professionals have indicated that they think improved speech and language is the most important outcome to investigate (more so than hearing alone). Do you agree with this?
As part of our job we need to make recommendations about how to make this research as attractive as possible to give it the best chance of being successful.
-
Would it matter who contacted you about a future research study?
-
Would it help if you knew them?
-
Is there a particular medical professional to whom you would respond?
-
Would it help if a senior member of staff/a surgeon/someone you knew contacted you?
-
What sort of information would you need to know before you would say ‘yes’ to getting involved?
-
Information about the research processes? Information about the researchers? Information about the treatment options?
-
-
Is there a limit to how many research visits you would be willing to make? Is there a limit to how long you would want to stay involved?
-
Is there anything that we could do to encourage your participation?
-
Make it easier for you to participate, money, co-ordinate with routine appointments, visit you at home.
-
To close:
Thank you for your contribution.
Are there any questions that you would like to ask?
Reiterate issue of confidentiality and respecting the privacy of other contributors.
Appendix 5 Summary table for framework analysis of parent interviews and focus groups
| 1. The challenges of Down syndrome and OME | ||||
|---|---|---|---|---|
| 1.1 Symptoms | 1.2 Consequences | 1.3 Priorities for improvement | 1.4 Other uncategorised comments | |
|
|
|
|
|
| 2. Diagnosis and treatment pathway | ||||
| 2.1 Diagnosis | 2.2 Treatment pathways | 2.3 Healthcare professionals | 2.4 Other uncategorised comments | |
|
|
|
|
|
| 3. Treatment – experiences and opinions | ||||
| 3.1 Surgery – experiences and opinions | 3.2 Surgery – efficacy of grommets | 3.3 Experience/impact of non-surgery | 3.4 Commentary about non-surgery | |
|
|
|
|
|
| 4. AHR | ||||
| 4.1 Purpose of AHR | 4.2. Understanding of AHR | 4.3 Researching OME in Down syndrome population | 4.4 Motivators to participating in OME research | 4.5 Barriers to participating in OME research |
|
|
|
|
|
| 5. Study design detail | ||||
| 5.1 Experiences and opinions of RCT | 5.2 Randomisation | 5.3 Experiences and opinions of observational research | ||
|
|
|
||
Appendix 6 OMQ-14 instrument
Appendix 7 Text version of the online questionnaire available to professionals
Appendix 8 Delphi review: development of the statements and of the consensus
| Effective treatment and management of glue ear in children who have Down syndrome is particularly important because of the high prevalence of glue ear in this population | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 27 | 21 (78%) | 6 (22%) | 0 | 0 | 0 |
| Effective treatment and management of glue ear in children who have Down syndrome is particularly important because difficulties with hearing can contribute to other developmental and/or behavioural problems | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 27 | 18 (67%) | 9 (33%) | 0 | 0 | 0 |
| The treatment and management of glue ear in a child who has Down syndrome poses distinctive challenges compared with other children | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 27 | 16 (59%) | 9 (33%) | 2 (7%) | 0 | 0 |
| It is difficult to be entirely confident of effective treatment and management of glue ear in a child who has Down Syndrome because of the variety of symptoms that may be indicative of glue ear (i.e. no single certain indicator to respond to) | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 27 | 5 (18%) | 14 (52%) | 4 (15%) | 4 (15%) | 0 |
| It is difficult to be entirely confident of effective treatment and management of glue ear in a child who has Down syndrome because of the fluctuation in symptoms and severity of glue ear (e.g. the extent of hearing loss may change from one week to the next) | ||||||
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 26 | 4 (15%) | 12 (46%) | 4 (15%) | 6 (23%) | 0 |
| It is difficult to be entirely confident of effective treatment and management of glue ear in a child who has Down syndrome because of the differing ways in which individual patients respond to treatment | ||||||
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 25 | 3 (12%) | 12 (48%) | 5 (20%) | 5 (20%) | 0 |
| Shortened statement combining previous three statements It is difficult to be entirely confident of effective treatment and management of glue ear in a child who has Down Syndrome | ||||||
| Round 2 | 25 | 0 | 20 (80%) | 2 (8%) | 3 (12%) | 0 |
| Evidence derived from a well-founded RCT study could improve current guidelines for clinical practice in the treatment and management of glue ear in children who have Down syndrome | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 27 | 12 (44%) | 14 (52%) | 1 (4%) | 0 | 0 |
| Evidence derived from a well-founded observational study could improve current guidelines for clinical practice in the management of glue ear in children who have Down syndrome | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 27 | 6 (22%) | 21 (78%) | 0 | 0 | 0 |
| Current clinical guidelines are adequate and do not require improvement | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 27 | 0 | 1 (4%) | 11 (41%) | 13 (48%) | 2 (7%) |
| 2 | 25 | 0 | 0 | 8 (32%) | 16 (64%) | 1 (4%) |
| New statement There is a need for new and improved clinical guidelines for the treatment and management of glue ear in children who have Down syndrome | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 2 | 24 | 8 (33%) | 13 (54%) | 2 (8%) | 0 | 1 (4%) |
| I believe that children who have glue ear who participate in research could receive immediate clinical benefits (e.g. less ear infection and improved hearing, speech and language) | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 27 | 1 (4%) | 13 (48%) | 10 (37%) | 2 (7%) | 1 (4%) |
| Amended statement I believe that children with Down syndrome, who have glue ear, who participate in research, could receive immediate additional clinical benefits (e.g. less ear infection and improved hearing, speech and language) (We have amended this statement to be more specific to children who have Down syndrome and added the word ‘additional’.) |
||||||
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 2 | 24 | 1 (4%) | 11 (46%) | 9 (37%) | 3 (12%) | 0 |
| Amended statement Participation in research will bring ADDITIONAL clinical benefits (e.g. less ear infection, improved hearing, speech and language) to a child with Down syndrome and glue ear (We have amended this statement to clarify that the additional benefits would be due to taking part in research.) |
||||||
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 3 | 24 | 2 (8%) | 12 (50%) | 9 (37%) | 1 (4%) | |
| I believe that children who have glue ear who participate in research could receive immediate non-clinical benefits (e.g. improved social interaction, progress at school) | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 27 | 1 (4%) | 12 (44%) | 11 (41%) | 2 (7%) | 1 (4%) |
| Amended statement I believe that children with Down syndrome, who have glue ear, who participate in research, could receive immediate additional non-clinical benefits (e.g. improved social interaction, progress at school) (We have amended this statement to be more specific to children who have Down syndrome and added the word ‘additional’.) |
||||||
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 2 | 24 | 1 (4%) | 12 (50%) | 9 (37%) | 2 (8%) | 0 |
| Amended statement Participation in research will bring ADDITIONAL non-clinical benefits (e.g. improved social interaction, progress at school) to a child with Down syndrome and glue ear (We have amended this statement to clarify that the additional benefits would be due to taking part in research.) |
||||||
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 3 | 24 | 1 (4%) | 13 (54%) | 9 (37%) | 1 (4%) | |
| I believe that, irrespective of immediate outcomes (i.e. whether results are positive or negative), further research in the treatment and management of glue ear in children who have Down syndrome will contribute to our understanding of how best to manage this condition, i.e. benefits are not immediate but will improve the development of treatment in the future | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 27 | 13 (48%) | 14 (52%) | 0 | 0 | 0 |
| Further research in this field provides an opportunity for personal professional development that I would find attractive | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 27 | 7 (26%) | 13 (48%) | 7 (26%) | 0 | 0 |
| 2 | 25 | 4 (16%) | 17 (68%) | 4 (16%) | 0 | 0 |
| I would support the need for further research in this field | ||||||
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 27 | 18 (67%) | 9 (33%) | 0 | 0 | 0 |
| For a future study of the treatment and management of glue ear in children who have Down syndrome, please indicate whether you would favour RCT or observational research, or whether you feel that study design is irrelevant | ||||
|---|---|---|---|---|
| Round | n | RCT | Observational | Irrelevant |
| 1 | 25 | 10 (40%) | 13 (52%) | 2 (8%) |
| A RCT in this field (and the random allocation of treatment) is justified because we are unsure about which treatment option offers the best outcomes | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 26 | 6 (23%) | 7 (27%) | 8 (31%) | 4 (15%) | 1 (4%) |
| 2 | 25 | 4 (16%) | 9 (36%) | 7 (28%) | 5 (20%) | 0 |
| Amended statement Because there is uncertainty about which treatment option offers the best outcomes a RCT in this field (and the random allocation of treatment) is justified | ||||||
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 3 | 23 | 4 (17%) | 14 (61%) | 5 (22%) | 0 | |
| The necessity for individual tailored treatment for glue ear in children who have Down Syndrome means that an observational study (with treatment recommended by their clinician is most appropriate) | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 26 | 1 (4%) | 15 (58%) | 7 (27%) | 2 (8%) | 1 (4%) |
| 2 | 25 | 1 (4%) | 18 (72%) | 5 (20%) | 1 (4%) | 0 |
| 3 | 23 | 1 (4%) | 15 (65%) | 7 (30%) | 0 | |
| A study of glue ear in children who have Down Syndrome should include WW as a treatment option | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 26 | 8 (31%) | 8 (31%) | 7 (27%) | 2 (8%) | 1 (4%) |
| 2 | 25 | 7 (28%) | 14 (56%) | 1 (4%) | 1 (4%) | 2 (8%) |
| A study of glue ear in children who have Down Syndrome should include grommets as a treatment option | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 25 | 8 (32%) | 14 (56%) | 2 (8%) | 1 (4%) | 0 |
| A study of glue ear in children who have Down syndrome should include grommets and adenoidectomy as a treatment option | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 26 | 4 (15%) | 12 (46%) | 8 (31%) | 1 (4%) | 1 (4%) |
| 2 | 25 | 2 (8%) | 17 (68%) | 5 (20%) | 1 (4%) | 0 |
| 3 | 23 | 1 (4%) | 20 (87%) | 2 (9%) | 0 | |
| A study of glue ear in children who have Down syndrome should include softband BAHA as a treatment option | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 26 | 7 (27%) | 17 (65%) | 2 (8%) | 0 | 0 |
| A study of glue ear in children who have Down syndrome should include a conventional hearing aid as a treatment option | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 26 | 10 (38%) | 13 (50%) | 3 (12%) | 0 | 0 |
| Please indicate which two of the above treatment options you would prioritise for inclusion in a research study | ||||||
|---|---|---|---|---|---|---|
| Round | n | WW | Grommets | Grommets and adenoidectomy | Softband BAHA | Conventional HA |
| 1 | 25 | 5 (10%) | 14 (28%) | 6 (12%) | 16 (32%) | 9 (18%) |
| 2 | 24 | 1 (2%) | 17 (34%) | 4 (8%) | 21 (42%) | 5 (10%) |
| Please indicate which of the treatment options (if any) should NOT be included in a study. Please tick as many as you want | ||||||
|---|---|---|---|---|---|---|
| Round | n | WW | Grommets | Grommets and adenoidectomy | Softband BAHA | Conventional HA |
| 1 | 7 | 2 (29%) | 0 | 5 (71%) | 0 | 1 (14%) |
| Expanded statement Please indicate which of the treatment options (if any) should NOT be included in a study. Please tick as many as you want (Note: In Round 1 this statement achieved a low response. Any future studies are unlikely to be able to include all treatment options. It would therefore be helpful if you could indicate which options would be at the bottom of your list.) |
||||||
| Round | n | WW | Grommets | Grommets and adenoidectomy | Softband BAHA | Conventional HA |
| 2 | 22 | 7 (26%) | 1 (4%) | 14 (52%) | 1 (4%) | 4 (15%) |
| Is there is any other treatment option you would like to be studied? | |
|---|---|
| Round 1 | Two comments supporting body-worn or hardband BAHAs |
| A study of glue ear in children who have Down syndrome should seek improvements in a child’s hearing to be meaningful | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 26 | 15 (58%) | 11 (42%) | 0 | 0 | 0 |
| A study of glue ear in children who have Down syndrome should seek improvements in a child’s speech, language and communication to be meaningful | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 26 | 7 (27%) | 14 (54%) | 4 (15%) | 1 (4%) | 0 |
| A study of glue ear in children who have Down syndrome should seek improvements in a child’s social interaction with others to be meaningful | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 26 | 7 (27%) | 10 (38%) | 6 (23%) | 3 (12%) | 0 |
| 2 | 25 | 3 (12%) | 14 (56%) | 5 (20%) | 3 (12%) | 0 |
| 3 | 23 | 1 (4%) | 18 (78%) | 4 (17%) | 0 | |
| A study of glue ear in children who have Down syndrome should seek improvements in a child’s progress at school/nursery to be meaningful | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 26 | 6 (23%) | 9 (35%) | 9 (35%) | 2 (8%) | 0 |
| 2 | 25 | 4 (16%) | 13 (52%) | 5 (20%) | 3 (12%) | 0 |
| 3 | 23 | 0 | 21 (91%) | 2 (9%) | 0 | |
| A study of glue ear in children who have Down syndrome should focus exclusively upon clarifying organisational matters and refining clinical pathways rather than clinical/non-clinical outcomes for the child | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 26 | 0 | 1 (4%) | 6 (23%) | 16 (62%) | 3 (11%) |
| Amended statement A study of glue ear in children who have Down syndrome should focus upon clarifying organisational matters and refining clinical pathways rather than clinical/non-clinical outcomes for the child (Note: we have edited this question to remove the ‘exclusive’ focus.) |
||||||
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 2 | 25 | 0 | 1 (4%) | 2 (8%) | 20 (80%) | 2 (8%) |
| Please indicate which of these five outcomes should be the primary outcome of a future research study | ||||||
|---|---|---|---|---|---|---|
| Round | n | Improvement in hearing | Improvement in speech language and communication | Improvement in social interaction | Improvement in progress in school/nursery | Clarifying organisational matters and refining clinical pathways |
| 1 | 26 | 17 (65%) | 7 (27%) | 2 (8%) | 0 | 0 |
| 2 | 25 | 21 (84%) | 4 (16%) | 0 | 0 | 0 |
| New statement Please indicate which of these five outcomes should be the secondary outcome of a future research study | ||||||
| Round | n | Improvement in hearing | Improvement in speech language and communication | Improvement in social interaction | Improvement in progress in school/nursery | Clarifying organisational matters and refining clinical pathways |
| 2 | 24 | 1 (4%) | 17 (71%) | 3 (12%) | 1 (4%) | 2 (8%) |
| I would be willing to recruit families to a RCT study in this field | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 26 | 8 (31%) | 7 (27%) | 10 (38%) | 0 | 1 (4%) |
| 2 | 25 | 6 (24%) | 15 (60%) | 4 (16%) | 0 | 0 |
| I would be willing to recruit families to an observational study in this field | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 26 | 9 (35%) | 15 (58%) | 2 (8%) | 0 | 0 |
| The clinical and developmental difficulties associated with children who have Down syndrome make it inappropriate to undertake research with this population | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 25 | 0 | 0 | 1 (4%) | 16 (64%) | 8 (32%) |
| The clinical and developmental difficulties associated with children who have Down syndrome make it impossible to objectively capture data on hearing or other outcomes | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 25 | 0 | 1 (4%) | 0 | 15 (60%) | 9 (36%) |
| The clinical and developmental difficulties associated with children who have Down syndrome make it impossible to capture the impact of glue ear independently of other health or behavioural traits | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 25 | 1 (4%) | 5 (20%) | 7 (28%) | 9 (36%) | 3 (12%) |
| 2 | 25 | 0 | 3 (12%) | 7 (28%) | 14 (56%) | 1 (4%) |
| 3 | 23 | 0 | 0 | 21 (91%) | 2 (9%) | |
| The range and variety of symptoms associated with glue ear in children who have Down syndrome limit the impact/value of further research in this field, i.e. each case is different so treatment will remain individualised irrespective of research findings | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 25 | 0 | 3 (12%) | 5 (20%) | 15 (60%) | 2 (8%) |
| The complexity of treating (multidisciplinary and varied) glue ear in a child who has Down Syndrome limits the potential value of further research in this field, i.e. each case is different so treatment will remain individualised irrespective of research findings | ||||||
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 25 | 0 | 3 (12%) | 5 (20%) | 14 (56%) | 3 (12%) |
| Amended statement (combining the previous two statements) The complexity of symptoms and/or treatment of glue ear in a child who has Down syndrome limits the potential value of further research in this field, i.e. each case is different so treatment will remain individualised irrespective of research findings | ||||||
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 2 | 25 | 0 | 5 (20%) | 4 (16%) | 13 (52%) | 3 (12%) |
| 3 | 24 | 0 | 0 | 24 (100%) | 0 | |
| I would find it difficult to describe the potential benefits of involvement in a research study to families who might be recruited to a study | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 25 | 1 (4%) | 0 | 5 (20%) | 15 (60%) | 4 (16%) |
| 2 | 23 | 0 | 1 (4%) | 1 (4%) | 19 (83%) | 2 (9%) |
| I would find it difficult to describe to parents the rationale and processes associated with a RCT | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 24 | 1 (4%) | 3 (12%) | 4 (17%) | 12 (50%) | 4 (17%) |
| 2 | 25 | 0 | 1 (4%) | 3 (12%) | 19 (76%) | 2 (8%) |
| I would find it difficult to describe to parents the rationale and processes associated with an observational study | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 25 | 0 | 0 | 3 (12%) | 16 (64%) | 6 (24%) |
| I would be concerned about losing professional autonomy in a research study for which treatment is randomly allocated | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 25 | 0 | 7 (28%) | 4 (16%) | 8 (32%) | 6 (24%) |
| 2 | 24 | 1 (4%) | 6 (25%) | 2 (8%) | 12 (50%) | 3 (12%) |
| 3 | 24 | 0 | 3 (12%) | 18 (75%) | 3 (13%) | |
| I would be concerned about the needs of the research study overtaking the needs of the child and/or family in a research study | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 25 | 1 (4%) | 9 (36%) | 3 (12%) | 9 (36%) | 3 (12%) |
| 2 | 24 | 0 | 10 (42%) | 1 (4%) | 11 (46%) | 2 (8%) |
| 3 | 24 | 0 | 6 (25%) | 15 (63%) | 3 (12%) | |
| I would be concerned that the processes of a research study would adversely affect my relationship with the child and their family | ||||||
|---|---|---|---|---|---|---|
| Round | n | Strongly agree | Agree | Neutral | Disagree | Strongly disagree |
| 1 | 25 | 0 | 3 (12%) | 5 (20%) | 14 (56%) | 3 (12%) |
| 2 | 25 | 0 | 0 | 2 (8%) | 19 (76%) | 4 (16%) |
Delphi review: final consensus
The closing bank of statements included 42 statements. [From the original 43 in round one, five statements were revised to form two new statements and two entirely new statements were created (43 – 5 + 4 = 42)]. Of these, consensus was established in 31 statements, five remain unresolved, and six were one-off questions which did not seek consensus. Consensus was defined as ≥ 80% of responses being strongly agree or agree, or ≥ 80% of responses being strongly disagree or disagree. In those statements where consensus was established 21 concluded in agreement, and ten concluded in disagreement.
Statements for which agreement was reached:
| Statement | % score | Round |
|---|---|---|
| Effective treatment and management of glue ear in children who have Down Syndrome is particularly important because of the high prevalence of glue ear in this population | 100% | 1 |
| Effective treatment and management of glue ear in children who have Down Syndrome is particularly important because difficulties with hearing can contribute to other developmental and/or behavioural problems. | 100% | 1 |
| The treatment and management of glue ear in a child who has Down syndrome poses distinctive challenges compared with other children | 92% | 1 |
| Evidence from a well-founded RCT study could improve current guidelines for clinical practice in the treatment and management of glue ear in children who have Down syndrome | 96%a | 1 |
| Evidence from a well-founded observational study could improve current guidelines for clinical practice in the treatment and management of glue ear in children who have Down syndrome | 100%a | 1 |
| I believe that, irrespective of immediate outcomes (i.e. whether results are positive or negative), further research in the treatment and management of glue ear in children who have Down syndrome will contribute to our understanding of how best to manage this condition, i.e. benefits are not immediate but will improve the development of treatment in the future | 100% | 1 |
| I would support the need for further research in this field | 100% | 1 |
| A study of glue ear in children who have Down syndrome should include grommets as a treatment option | 88% | 1 |
| A study of glue ear in children who have Down syndrome should include softband BAHA as a treatment option | 92% | 1 |
| A study of glue ear in children who have Down syndrome should include a conventional HA as a treatment option | 88% | 1 |
| A study of glue ear in children who have Down syndrome should seek improvements in a child’s hearing to be meaningful | 100% | 1 |
| A study of glue ear in children who have Down syndrome should seek improvements in a child’s speech, language and communication to be meaningful | 81% | 1 |
| I would be willing to recruit families to an observational study in this field | 92% | 1 |
| It is difficult to be entirely confident of effective treatment and management of glue ear in a child who has Down syndrome | 80%b | 2 |
| There is a need for new and improved clinical guidelines for the treatment and management of glue ear in children who have Down syndrome | 87% | 2 |
| Further research in this field provides an opportunity for personal professional development, which I would find attractive | 84% | 2 |
| A study of glue ear in children who have Down syndrome should include WW as a treatment option | 84% | 2 |
| I would be willing to recruit families to a RCT study in this field | 84% | 2 |
| A study of glue ear in children who have Down syndrome should include grommets and adenoidectomy as a treatment option | 91% | 3 |
| A study of glue ear in children who have Down syndrome should seek improvements in the child’s social interaction with others to be meaningful | 82% | 3 |
| A study of glue ear in children who have Down syndrome should seek improvements in the child’s progress at school/nursery to be meaningful | 91% | 3 |
Statements where disagreement was reached:
| Statement | % score | Round |
|---|---|---|
| The clinical and developmental difficulties associated with children who have Down syndrome make it inappropriate to undertake research with this population | 96% | 1 |
| The clinical and developmental difficulties associated with children who have Down syndrome make it impossible to objectively capture data on hearing or other outcomes | 96% | 1 |
| I would find it difficult to describe to parents the rationale and processes associated with an observational study | 88% | 1 |
| A study of glue ear in children who have Down syndrome should focus upon clarifying organisational matters and refining clinical pathways rather than clinical/non-clinical outcomes for the child | 88% | 2 |
| I would find it difficult to describe the potential benefits of involvement in a research study to families who might be recruited to a study | 91% | 2 |
| I would find it difficult to describe to parents the rationale and processes associated with a RCT | 84% | 2 |
| I would be concerned that the processes of a research study would adversely affect my relationship with the child and their family | 92% | 2 |
| The clinical and developmental difficulties associated with children who have Down syndrome make it impossible to capture the impact of glue ear independently of other health or behavioural traits | 100% | 3 |
| The complexity of symptoms and/or treatment of glue ear in children who have Down syndrome limits the potential value of further research in this field, i.e. each case is different so treatment will remain individualised irrespective of research findings | 100%a | 3 |
| I would be concerned about losing professional autonomy in a research study in which treatment is randomly allocated | 88% | 3 |
Statements where consensus was not reached after three rounds:
| Statement | % score |
|---|---|
| Participation in research will bring additional clinical benefits (e.g. less ear infection, improved hearing, speech and language) to a child with Down syndrome and glue ear | 58% agree |
| 42% disagree | |
| Participation in research will bring additional non-clinical benefits (e.g. improved social interaction, progress at school) to a child with Down syndrome and glue ear | 58% agree |
| 42% disagree | |
| Because there is uncertainty about which treatment option offers the best outcomes a RCT in this field (and the random allocation of treatment) is justified | 78% agree |
| 22% disagree | |
| The necessity for individually tailored treatment for glue ear in children who have Down syndrome means that an observational study (with treatment recommended by their clinician) is most appropriate | 70% agree |
| 30% disagree | |
| I would be concerned about the needs of the research study overtaking the needs of the child and/or family in a research study | 25% agree |
| 75% disagree |
Appendix 9 Explanation of methodology for randomised controlled trials and observational health studies
Observational health research
Over a period of time, researchers monitor a cohort of patients who have received different interventions and compare outcomes (e.g. hearing loss).
For example, a group of children with glue ear could be identified and then followed up over a period of months to see if the children who had grommets had better or worse outcomes than those who used HAs or those who had received no intervention.
Health research using a randomised controlled trial
The effect of different treatments for a particular condition is studied in two or more groups of patients who are selected at random to receive each treatment. By using random selection, each group is similar in terms of age, gender, etc. allowing doctors and researchers to decide with more certainty whether one treatment option is more effective than the other.
For example, one group of children with glue ear could be randomly selected to receive grommets, whereas the other groups could receive HAs or no treatment at all (i.e. active observation). They would be followed up by researchers at standard times. The outcomes would then be compared to show which group had the most benefit.
List of abbreviations
- AHR
- applied health research
- AOM
- acute otitis media
- BAHA/softband BAHA
- bone-anchored hearing aid (note: softband BAHA refers to a bone conduction hearing aid but the acronym BAHA is commonly used)
- CDI
- communicative development inventory
- CI
- confidence interval
- CPPI
- clinical care pathways perfect information
- dB HL
- decibel hearing level
- ENT
- ear, nose and throat
- EVPI
- expected value of perfect information
- GCBI
- Glasgow Children’s Benefit Inventory
- HA
- hearing aid
- HUI
- Health Utility Index
- ICER
- incremental cost-effectiveness ratio
- INB
- incremental net benefit
- IPD
- individual patient data
- NICE
- National Institute for Health and Care Excellence
- NRC
- NHS reference costs
- OME
- otitis media with effusion
- pa
- per annum
- PE
- pressure equalisation
- pop-EVPI
- population expected value of perfect information
- PSS
- Personal Social Services
- PTA
- pure tone average
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SA
- sensitivity analysis
- SLT
- speech and language therapist
- SOM
- secretory otitis media
- T-tube
- tympanostomy tube
- TARGET
- Trial of Alternative Regimens in Glue Ear Treatment
- ToD
- teacher of the deaf
- TPI
- trial perfect information
- VOI
- value of information
- WW
- watchful waiting