Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 08/14/06. The contractual start date was in September 2009. The draft report began editorial review in December 2013 and was accepted for publication in April 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Livingston et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Scientific background
The frequency of dementia will rise dramatically over the next 20 years because of increased longevity. In the UK, 700,000 people currently have dementia (> 1% of the entire UK population), and this figure is projected to reach over 1 million by 2020 and double again in the 20 years after that. 1,2 Dementia affects the person with the illness, his or her family and society through increasing dependence and challenging behaviour. In the UK, dementia care is currently estimated to cost £23B per year and costs are projected to treble in the next 30 years as the number of older people increases;2,3 for comparison, the entire NHS budget is currently £96B per year. England’s National Dementia Strategy4 emphasises that ‘Family carers are the most important resource available for people with dementia’ (p. 12). Families and individuals bear the biggest financial burden; two-thirds of people with dementia live at home, receiving most of their care from family carers, who save the public purse more than £6B per year. 2
About 40% of family carers of people with dementia have clinically significant depression or anxiety, while others have significant psychological symptoms. 5,6 These symptoms are more common when the family carer is older, a woman, living with the person with dementia and reports greater carer burden and behavioural symptoms of dementia. 5,6 This impacts on the NHS as well as on patients and families, as carer psychological morbidity, in particular depression, predicts care breakdown and, therefore, institutionalisation,7,8 as well as elder abuse. 9 However, a recent report shows that levels of services and support for people with dementia and families are inadequate. 2 Specialist, individually tailored, psychological support to people with dementia and their family carers has been shown to reduce the rate of, although not necessarily the time to, care home admission in the USA, although there is no evidence in the UK. 10,11 Only multicomponent interventions have been shown to be effective in preventing institutionalisation. 11,12 Nationally, a reduction in care home placements would have huge benefits for society, because most older people want to continue living at home, and those who do live at home report higher quality of life (QoL) than those placed in care homes. 13 There are also economic benefits, for example, in reduced use of health services by carers suffering from psychological symptoms and, in particular, in reduced use of care homes, which are a very expensive resource.
Evidence for a coping-based psychological therapy for carers
In our team’s earlier systematic review of interventions to improve the mental health of carers, we identified, prior to starting this study, 62 references which met our inclusion criteria. 10 We found that behavioural management and coping strategy-based interventions had been efficacious, that interventions targeting individuals tended to work better than group interventions and that the minimum number of sessions of individual behaviour management which had been shown to be efficacious was six. Education about dementia by itself, group behavioural therapy and supportive therapy were not effective carer interventions. An earlier review had suggested that interventions which required active participation of the carers were more effective. 14 In a further systematic review of evidence about treating dementia carer anxiety, we found that cognitive–behavioural therapy (CBT) and other therapies developed primarily to target depression did not effectively treat anxiety. 5 Preliminary evidence suggested that an intervention might be effective if it included relaxation techniques and other strategies to help carers actively find ways to manage caring demands rather than reducing demands by reducing contact and avoidance. Overall, while evidence is promising, it is scant and of low quality, and further randomised controlled trials (RCTs) of interventions for carer psychological health in dementia are required. 15
The Coping with Caregiving programme
The Coping with Caregiving programme16,17 was developed in the USA as a group intervention. It is a manual-based, psychological intervention delivered in weekly group sessions. It was comprehensibly evaluated in the Resources for Enhancing Alzheimer’s Caregiver Health project, which recruited carers from a range of clinical and community sources in the USA; depression scores were significantly decreased in treatment groups compared with controls in all of the studies16–19 and self-efficacy scores were increased. 18 Although the impact of this therapy programme on rates of institutionalisation has not been tested, a recent systematic review finds that there is some evidence that carer support can reduce institutionalisation and that therapies such as the Coping with Caregiving programme, which include problem-solving strategies and offer carers a choice of support strategies which can be tailored to their individual needs, are most effective. 11
Delivery of therapy
The National Institute for Health and Care Excellence (NICE) recognises that psychological therapy for dementia family carers should be a key component of high-quality dementia care:20 ‘Carers of people with dementia who experience psychological distress and negative psychological impact should be offered psychological therapy, including cognitive–behavioural therapy, conducted by a specialist practitioner’ (p. 40). However, the report noted the paucity of evidence in this area, and recommended that research is needed to address the question: ‘For carers of people with dementia, is a psychological intervention cost-effective when compared with usual care?’ (p. 45). Our team’s systematic review found only one study of the cost-effectiveness of a therapeutic approach [similar to the StrAtegies for RelaTives (START) intervention] for supporting carers. 20,21 It examined the cost-effectiveness of a modular, multicomponent intervention delivered in carers’ homes, in three sessions by telephone, supplemented by five group sessions (five or six carers in each) delivered by telephone. Focusing on hours of caregiving, the authors found a significant difference over the 6-month study period, with carers in the intervention group having more time to dedicate to activities unrelated to caregiving, which has potentially positive impacts on emotional well-being and QoL. 22,23 Therefore, more evidence is needed of the effectiveness and cost-effectiveness of interventions for supporting caregivers.
Rationale for new therapy in the NHS
Although the efficacy trials discussed above have shown promising reductions in family carer morbidity, there are no manual-based therapies currently available for dementia carers in the NHS, nor is there an evidence base to demonstrate whether or not such standardised psychological interventions can be realistically, effectively and economically delivered to family carers within NHS services. A therapy which is needed by many NHS consumers that can be effectively implemented only by clinical psychologists is unlikely to be economically viable. However, too little professional training is unlikely to be helpful; a recent unstructured, non-manual-based befriending programme delivered by ex-carers was ineffective in reducing anxiety or depression. 24
The UK national agenda is to have a stepped care approach to improve access to psychological therapies in which less intensive therapy is delivered by psychology graduates supervised by clinical psychologists. 25 In this study, we used similar delivery infrastructure; we anticipated that a psychological therapy specifically tailored to the emotional, practical and information needs of carers could have significant population benefits, including greater carer and care recipient well-being and decreased statutory care costs. Our therapy was delivered by psychology graduates, trained and supervised by the coapplicant experts in psychology, carer involvement, nursing and psychiatry, all of whom work in the NHS. Our intervention was as suggested by the evidence: individual, manualised, with different elements, required active participation, incorporated relaxation and was based on the US Coping with Caregiving programme.
Through our clinical and personal involvement in caring for people with dementia, we are aware that it can be difficult for carers to attend groups held outside their home at one specific time because of their caring commitments. There is evidence from systematic reviews that therapies individualised to the carer receiving them are most effective in delaying institutionalisation,11 and that individual behavioural therapies are more effective than group interventions in reducing carer morbidity. 10 Therefore, with the authors’ agreement, we adapted Coping with Caregiving for NHS use as an individual therapy. As a result, sessions were quicker to deliver because, in groups, time is needed for all group members’ problems to be discussed; thus, the number of weekly sessions required decreased from 13 to 8 after piloting.
Chapter 2 Objectives
We carried out this RCT to test the clinical effectiveness and cost-effectiveness of the manual-based individual therapy for dementia carers delivered by psychology graduates compared with treatment as usual (TAU) in the short (up to 8 months)26,27 and longer term (up to 2 years).
Chapter 3 Methods
Trial registration
This trial is registered as ISCTRN70017938. The full protocol is available online.
Trial design
The study is a randomised, parallel-group superiority trial, with blinded outcome assessment, recruiting participants 2 : 1 to intervention and TAU to allow for therapist clustering.
Patient and public involvement
Shirley Nurock, a former family carer working with the Alzheimer’s Society, contributed to the design of the study at the application stage and commented on the study as part of the Trial Management Group throughout, including the information sheet. Lynne Ramsey, a family carer, who was then in a Dementias and Neurodegenerative Disease Research Network (DeNDRoN) group, was a member of the steering group and helped to make decisions about the study. U Hla Htay, a family carer, who we initially met as a University College London MSc student, contributed to the Data Monitoring Committee.
Participants
Eligibility criteria for participants
We included family carers of patients with dementia referred in the previous year who:
-
provided emotional or practical support at least weekly
-
identified themselves as the primary carer of someone with dementia not living in 24-hour care.
We excluded family carers who:
-
were unable to give informed consent to the trial
-
were currently taking part in a RCT in their capacity as a family carer (not just as an informant)
-
lived more than 1.5 hours’ travelling time from the researchers’ base.
To help identify these potential participants, we completed the Mini Mental State Examination28 in carers aged ≥ 60 years. Participants who scored < 24 out of 30 were discussed with the investigators GL or CC to determine if the low score was related to cognition, mood or education. If carers were judged to have dementia, they were not included in the study and the referring clinician was informed. This occurred only once in the study.
Settings and locations where the data were collected
Participants were recruited through three mental health trusts: Camden and Islington NHS Foundation Trust (CIFT), North Essex Partnership Foundation Trust (NEPFT) and North East London and Essex Foundation Trust (NELFT) Admiral Nurse Service. We also recruited from a neurology clinic at University College London (UCL) called the Dementia Research Centre (DRC), a tertiary service whose referrals include a high rate of people with young-onset dementia. Although we had planned to recruit from Barnet, Enfield and Haringey Mental Health Trust, the trust refused to provide extra treatment costs and we did not recruit from there. The sampling frame encompassed urban, suburban and rural areas and ethnic and social class diversity. Recruitment was assisted by North Thames DeNDRoN.
Referrals and recruitment procedure
Prospective participants were initially approached by a clinician they knew and given or sent an information sheet. Those interested in participating were then referred to the research team. The referral gave the name, sex and relationship of the carer to the patient, as well as the patient’s sex. The researchers were divided into two teams (A and B). The team that was to carry out blinded assessments for this client (e.g. A) telephoned the client 24 hours or more after they received the information sheet. The team member answered any questions and then arranged to meet those who thought they wished to take part, to obtain their informed consent and complete the baseline assessment before randomisation. Recruitment of carers to the trial commenced on 4 November 2009 and finished on 8 June 2011. The first 4-month follow-up took place on 4 March 2010, with the final 8-month follow-up on 7 February 2012 and the final 24-month follow-up on 7 June 2013.
Randomisation
The assessing team member gave the details of those enrolled to the trial manager, who entered them into the online randomisation system. Randomisation to intervention or TAU was carried out using an online computer-generated randomisation system. Randomisation was stratified by trust using random permuted blocks. An allocation ratio of 2 : 1 (intervention to TAU) was used to allow for potential clustering effects by therapist in the intervention arm. 29 It was set up and maintained by an independent Clinical Trials Unit at the Institute of Psychiatry, King’s College London, and accessed only by the START trial manager and supervising clinical psychologist.
Blinding
Outcome assessors were blinded to randomisation status, but it was not possible to blind study participants. The researchers worked in two teams, each assessing outcomes for approximately half the participants and providing therapy to those allocated to treatment in the half of participants they were not assessing. These teams were in different rooms and had supervision separately so as to remain blinded. Assessors asked participants at the beginning of each interview not to disclose their allocation group.
The trial manager told the intervention team (B if the assessment team was A or vice versa) the result, but the assessment team was not informed of the outcome of randomisation. The participant was then telephoned by a member of the intervention team and informed that they had been allocated either to TAU and would be contacted for a 4-month follow-up (by the assessment team A, if the therapy team was A or vice versa) or to the intervention. If allocation was to therapy, an appointment was made for the first session of the therapy to start. Allocation within the individual team was according to workload.
Intervention
See Appendix 3 for the START manual.
Location of interventions
Participants were usually seen for assessment and for intervention in their own homes, although, if participants so requested, they were seen in other settings. In the intervention group, most participants were seen at home, but a minority were seen at UCL (n = 7) or the NHS trust’s offices (n = 13). One carer was seen both at work and at a local restaurant and another both at home and in the trust.
Development of the intervention manual
Dolores Gallagher-Thompson gave us permission to adapt the ‘Coping with Caregiving’ manual, a 13-session group therapy. We therefore used it to develop the START manual, an eight-session manual-based individual therapy programme adapted for UK use with individual family carers. The therapy was adapted by the project team, GL, PR, CC and DL, by considering UK usage of language and culture, brevity, and the fact that we were addressing individuals rather than a group.
We began by familiarising ourselves with the structure and content of the manual, which made use of the stress appraisal and coping response model. In this, stress is seen as the mismatch between primary appraisal (perceived demand) and secondary appraisal (perceived ability to cope and available resources and options, and principles from CBT).
Why change from group to individual intervention?
Carers face difficulties in attending a group intervention, as it can be impossible to make alternative care arrangements for their relative and to be available at a prespecified time. Individual therapy also has the advantage that it can be tailored to the specific problems faced by the carer, and our previous systematic review found that therapies worked better with individuals rather than groups. Individual therapies are quicker to deliver, as, in groups, time is needed for all group members’ problems to be discussed and, so, the number of sessions was decreased.
Reducing the number of sessions
We identified the key components of the intervention and began reducing the 13 sessions to eight shorter sessions. This was a collaborative process. At this early stage, care was taken to adapt the language and tone of the American manual to ensure that the language of the revised manual was suitable for its target audience and to ensure that it was written in a clear and accessible style. Although the content of sessions varied, each session followed a broadly similar structure: an introduction, a review of a homework task from the previous session (from session 2 onwards), one or more specific topics including worked examples and space for carers to identify their own examples, a stress reduction technique, a session summary and a homework task (e.g. keeping a diary of challenging behaviours for the following week).
In the original manual, the sessions were (1) stress and well-being, (2) target behaviours, (3) strategies for changing behaviour, (4) refining our behaviour plans, (5) behaviours and thoughts, (6) changing unhelpful thoughts, (7) communication styles, (8) communication and memory problems, (9) planning for the future, (10) more planning for the future, (11) pleasant events, (12) refining your pleasant events and (13) review and conclusion.
Having initially produced the eight-session manual, the team revised each session to ensure that the content was written in appropriate UK English, was without jargon, was comprehensive and included both theoretical components and exercises for the participants to work through their own examples and experiences. Attention was given to ensuring a balance between information provision and interactive exercises inviting the carer to reflect on their own resources and strategies for coping, as well as relaxation exercises. In the original manual, ‘planning for the future’ emphasised end-of-life care, but, as most of our participants were expected to be seen soon after diagnosis, ‘planning for the future’ also encompassed getting support from other relatives, increasing community services, considering power of attorney and planning for a care home. The carer could then identify priorities and work through the information which they felt would be most useful. We piloted the manual by the research assistants trying it with PR, GL, CC, DL and each other, and altered it whenever it did not flow, or was unclear or repetitive. The research assistants met in groups, practised delivering the sessions and provided oral and written feedback on how to improve the sessions and increase the accessibility and clarity of the session content.
This process was repeated until the researchers and therapists agreed that the sessions were ready for use. Sessions were practised with each other to ensure that all researchers had the same understanding of how to deliver the therapy. Two separate versions of the manual were written: one for the carer to keep and one for the therapist to use, which included additional prompts and guidance to the therapist. In addition to the text, pictures and images were included to make the manual more user-friendly.
At this point, the sessions were piloted by PR with a carer who would have met the criteria for inclusion in the study. After each session, the clinical psychologist provided feedback to the team on how both the process and content was received by the carer, including the ease of delivery and timing of the sessions and adjustments were made accordingly. Final versions of both the therapist and carer versions of the manual were then produced for use within the study.
The therapy took place in the carers’ preferred location, usually their home, without the patient in the room and at a time convenient to them. It was individually tailored to address the particular problems the carer was experiencing with the person for whom they cared. The therapy was carried out with an interpreter if the carer did not speak English fluently. Four participants needed translation, three of whom were randomised to the therapy sessions. The languages were Bengali (two carers), Farsi and Turkish (one each).
The eight sessions covered:
1. Psychoeducation about dementia, carer stress and understanding behaviours of the person cared for. This session discussed what dementia was and identified the difficulties faced by the carer. If the carer did not have any, we had examples of common problems. It introduced the idea of stress and how carers could recognise when they felt stressed.
2–5. Discussion of behaviours or situations that the carer finds difficult, incorporating behavioural management techniques including functional analysis; skills to take better care of themselves, including identifying and changing unhelpful thoughts; assertive communication; effective communication with people with dementia and promoting emotion-focused coping strategies and acceptance; and where to get emotional support and positive reframing.
6. Future needs of the patient, with information about care and legal planning specifically adapted to the UK. We discussed what they wanted to plan and who else they wanted to consult. We gave the carers information leaflets about making common decisions as individually appropriate. 30
7. Planning pleasant activities. This incorporated aspects of behavioural activation and reflected that many previous pleasant activities were impractical but that there may be many small enjoyable activities which still could be incorporated and the carer could add them to the day for either themselves or the person with dementia.
8. Maintaining the skills learned over time. This encompassed discussing which techniques and ideas the carer had found useful and making a written plan of what they would use in the future.
Each session ended with a different stress reduction technique session. Carers were given homework tasks to complete between sessions, including relaxation, identifying triggers and reactions to challenging behaviours, and identifying and challenging negative thoughts. The therapist and the family carer both had a manual, and carers filled in and kept their own manual. Our group audio-recorded relaxation exercises for use in these sessions and gave them to carers on a compact disc (CD). The relaxation techniques used were signal breath, focused breathing, physical grounding, guided imagery (three different versions), meditation and stretching.
We defined adherence to therapy after consulting with our clinical psychologist supervisor, on clinical grounds, as participating in five or more sessions.
Training and delivery
We employed and trained psychology graduates with no clinical training to deliver the intervention. Researchers in the two groups carried out assessments on carers having received training in taking informed consent, good clinical practice and all the assessment tools being administered. There was a strong practical focus in the training programme on how to deliver the therapy, potential clinical dilemmas, working with interpreters, empathic listening skills, effective use of supervision, safe working practice and when to ask for help. Throughout the training, a strong emphasis was placed upon the researchers guiding carers to where they themselves could get answers to their concerns or questions rather than feeling the need to provide specialised advice themselves within the session. We trained therapists to adhere to the manual and required them to demonstrate, by role-play, competence in delivering each session of the intervention.
Monitoring fidelity to the manualised intervention
Therapists recorded one therapy session per participant. We (PR, CC, DL and GL) devised a fidelity checklist for each session by considering the most important components of the session. The session to be recorded was selected at random by the trial manager using a Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) formula before any interventions were carried out. This session was rated for fidelity to the manual by a therapist in the same team who was not involved in that participant’s intervention using the standard checklist. An overall fidelity score for each session was then given by the rater considering whether or not the therapist was ‘keeping the carer focused on the manual’. Possible scores ranged from 1, meaning ‘not at all’, to 5, meaning ‘very focused’. We planned to have two researchers rate independently initially while inter-rater reliability was established. Ratings were out of a possible five points. If fidelity scores were not high, the supervising clinical psychologist addressed this in supervision. This was the case for 7 out of the 131 recorded sessions, and often it was because carers had refused to participate in a particular aspect of a session.
Supervision
Following the recruitment and training of the psychology graduates the process of formal clinical supervision began. Our clinical psychologist, PR, met with each of the two teams (of three or four psychology assistants) for 1.5 hours of group supervision a fortnight. In addition to this group supervision, she was available for individual supervision, which either was requested by the psychology graduates on an ad hoc basis or, on occasion, was initiated by the investigators. Additionally, weekly team meetings with DL, GL and PR were held for the entire team, during which both procedural and clinical issues were addressed. The psychology graduates could approach one of the research team at any time if they had concerns or questions relating to their clients, for example if risk issues arose during a session.
A group supervision format was adopted as it was seen as the most effective use of available resources, with psychology graduates benefiting from both the professional expertise of their supervisor and the clinical experiences of their peers. It was hoped that the group format would maximise peer support both within and outside the supervision sessions and facilitate effective teamworking. The supervision format was tailored to reflect the specific needs of the START research project. During the course of the project, supervision performed a number of functions including case management, clinical skills development, monitoring the fidelity to the manualised intervention and ensuring safe practice with clients and staff support. Each of these functions is explored in turn below.
Case management
Once recruitment was under way and psychology graduates were fully trained, they held a caseload of approximately 4–6 clients for intervention. An important function of supervision was to ensure that all of these interventions were being managed effectively and appropriately. Therefore, in every group supervision session, each psychology graduate provided a brief update of his or her caseload, ensuring that clients and any related issues or concerns did not get overlooked, for example if there was a repeated pattern of cancellation or non-attendance that the psychology graduate had not identified. This aspect of supervision was extremely important as the psychology graduates had varied levels of experience and skill in managing their own caseloads and they were juggling delivering the intervention with offering baseline and follow-up assessments. This process also encouraged psychology graduates to be transparent about their work and to recognise when apparently simple or straightforward cases were more complex than initially perceived. As the cases for intervention were allocated and managed within the team, it was useful for psychology graduates to be aware of who their colleagues were seeing and who had space to take on new clients, developing a sense of shared responsibility.
Clinical skills development
Group and individual supervision sessions provided psychology graduates with the opportunity to develop their clinical skills via a range of approaches. In addition to a brief summary of their caseloads, at the start of every supervision session the group would agree an agenda and psychology graduates would identify a clinical challenge or dilemma that they wished to explore in more detail. Generally, each psychology graduate would talk in detail about one client; however, this very much depended on their present caseload. If there was a large agenda, psychology graduates were encouraged to prioritise and negotiate in terms of urgency among themselves. Although the intervention was manualised and psychology graduates were expected to strictly adhere to the manual, there was great variety in the dilemmas that they encountered in delivering the intervention. Often, the challenge for the psychology graduates was how to respond to their clients and overcome difficulties continuing to be empathic while sticking to the prescribed intervention, for example when carers would repeatedly ask them for solutions or direct advice.
For the more in-depth clinical discussions, PR encouraged the psychology graduates to identify a particular focus or question which they wanted to address rather than simply talking about a client in an unfocused way. These clinical discussions encompassed what the psychology graduates thought had worked well and what they had already tried, as well as what they felt that they could have done differently. In addition to offering advice and potential solutions to the psychology graduates, she encouraged them to share ideas and experiences with their peers and to make connections and identify themes across cases so that supervision was a positive learning experience regardless of whether or not their own cases were being discussed. As many of the psychology graduates hoped to develop a career in either clinical or academic psychology, they were keen within supervision sessions to link their practice and experiences to wider psychological theory. Various themes tended to emerge within their discussions of clinical cases, for example around grief and loss, or managing the therapeutic relationship, and the group would choose academic papers and book chapters to read for supervision sessions to extend their learning and understanding and to connect back to their cases.
In addition to using therapy tapes to monitor the fidelity to the intervention manual, listening to tapes of sessions provided the opportunity to reflect upon and develop clinical skills. All of the psychology graduates had the opportunity to play extracts from their taped sessions focusing on both general skills and the specific challenges that they faced in delivering the intervention to carers, for example how to manage sessions where the carer was extremely talkative, or how to respond when carers were unable to identify examples of challenging behaviours or possible coping strategies. Listening to the tapes was often combined with role-playing sections of intervention sessions to experiment with different ways to deliver the manual. Although role-play and listening to tapes can be anxiety provoking, because the psychology graduates were used to listening to and rating each other’s tapes outside supervision, they were more comfortable using these methods within supervision.
Ensuring safe practice with clients
An important aspect of supervision was to identify and respond to any risks identified during the course of assessment or intervention. The psychology graduates were provided with specific training in how to respond to any risks disclosed by carers, in relation to harm to both themselves and the person they were caring for. Psychology graduates would speak to one of the lead investigators if the abuse Modified Conflict Tactic Score (MCTS) was in the possibly abusive range (≥ 2) or if they had any concerns about potential abuse. Similarly, if carers reported high levels of depression or anxiety, this was discussed with the lead investigators. If concerns were raised, a plan was made with one of the lead investigators about how to manage the risk and information was shared with the local clinical teams.
Within supervision, psychology graduates were encouraged to identify any concerns about risk and prioritise these for discussion. As noted above, asking the psychology graduates to talk about their entire caseload at each session was an important way of ensuring that any concerns were identified. By talking about examples of good practice as well as difficulties and ensuring that there was an opportunity for individual supervision on request and a senior member of the team was always available, it was hoped that a culture of transparency developed and that the psychology graduates would always raise concerns about their clients. Time was also taken within supervision to highlight the importance of behaving ethically and safely in all aspects of clinical work, for example reflecting on maintaining clear boundaries clinically and how to practise safely when working alone in people’s homes.
Staff support
In offering therapeutic interventions to carers of people with dementia, the psychology graduates, who had limited direct clinical experience, were frequently faced with the often distressing day-to-day lives of the carers and their relatives with dementia. An important dimension of clinical supervision was to provide a supportive context for the psychology graduates to develop self-reflexivity, exploring and making sense of their own responses to the people and situations that they were working with clinically. The combination of group and individual supervision meant that the psychology graduates benefited from the support of their peers and felt that their experiences were validated by their shared experiences.
Treatment as usual
As several teaching trusts were involved, we expected the TAU to be similar to good TAU throughout the UK and based on the NICE guidelines. 31 Services are based around the person with dementia. Treatment is medical, psychological and social. Thus, it consisted of assessment, diagnosis and information, drug treatment, cognitive stimulation therapy, practical support, treatment of neuropsychiatric and cognitive symptoms and carer support. We have described TAU by trust below.
Camden and Islington NHS Foundation Trust
Patients and carers in this service were referred by a general practitioner (GP) to the Memory Service for assessment and diagnosis, assessed at home with family carer and, after investigations, offered a diagnostic appointment. Medication was prescribed as was appropriate and, if on anti-dementia medication, followed up by memory clinic nurses. Information and education in dementia was offered initially by nurses but then by dementia advisors situated in offices in and managed by the Alzheimer’s Society. Patients were offered cognitive stimulation therapy. Risk assessments were carried out and risk plans put in place, for example telecare, driving information to the Driver and Vehicle Registry Agency (DVLA), medical identification (ID) bracelets, advice regarding power of attorney and capacity assessment, and social services referral for personal care, day centre and financial advice. Neuropsychiatric symptoms in patients were assessed and managed.
North East London Foundation Trust
We recruited from NELFT’s Admiral Nurse Service. Admiral Nurses are mental health nurses who specialise in dementia and work with family carers and people with dementia in the community and other settings. Family carers are referred to the Admiral Nursing Service through self-referral, directly from the GP, or from community mental health teams and the Memory Service. They assess the needs of both the carer and the person with dementia, and provide information, education and advice about caring for someone with dementia. Admiral Nurses focus on the needs of the family carer and provide emotional and psychological support to families through the transitional phases of the illness, such as diagnosis, when the condition advances, care home placement and end-of-life care. Referrals may be made to other services and they liaise with other health and social care professionals on behalf of the family.
North Essex Partnership University NHS Foundation Trust
Patients and carers in this service were referred by a GP to the Memory Service for assessment and diagnosis, assessed at home with family carer and, after investigations, offered a diagnostic appointment. The diagnosis was given in the clinic with a doctor or at home with nurses. Other interventions included medication as appropriate and, if on antidementia medication, follow-up by memory clinic nurses; risk assessment with a risk plan put in place, for example telecare and driving information to the DVLA; medical ID bracelet; and social services referral for personal care or day centre. Information and education in dementia was offered initially by nurses but thereafter by dementia advisors. Neuropsychiatric symptoms were assessed and managed. Patients were referred to dementia advisors situated in the Alzheimer’s Society and carers were offered support groups.
University College London Hospitals NHS Foundation Trust
Patients were referred by their local GP, memory clinic or specialist to this cognitive disorders clinic. They were assessed in the clinic, with investigations completed during the visit. They were admitted as day case for cerebrospinal fluid examination or for other investigations. A few weeks later they had a diagnostic appointment with a neurologist, when they received the results of any tests, and on the same day saw a nurse consultant for discussion. In this discussion they were given information, education around legal and financial implications and a risk assessment was carried out. All patients were offered the opportunity to take part in clinical trials and other research. Available patient support groups were highlighted to them. This was followed up by telephone consultations. If appropriate, patients returned to the nurse-led therapeutics clinic for medication. Complex cases were followed up in the clinic and all others signposted back to their local services.
Barnet, Enfield and Haringey Mental Health Trust
We did not recruit participants from this trust as they did not agree to extra treatment costs.
Chapter 4 Outcomes
Assessments
Carers were interviewed at baseline and 4, 8, 12 and 24 months after randomisation, usually in their own home, unless they preferred to come to the research team base in UCL. We continued to follow up carers, asking them to remain in the study for 2 years, even if the person they were looking after went into a care home or died. They continued to give us data about themselves and their own use of services and, if the care recipient was in a care home, about the recipient’s use of services. Information collected at baseline consisted of sociodemographic details about the carer and the person with dementia, and clinical and resource use items. Collection of clinical and resource use information was repeated at all follow-up data collection time points.
Sociodemographic details obtained at baseline included age, sex, ethnicity, relationship to the patient (e.g. spouse or child), level of education, last occupation and living situation.
Other assessments include:
-
The Hospital Anxiety and Depression Scale (HADS)32 is a self-complete scale, and is validated for all age groups and settings, in people who are physically well or ill, and in Asian and African ethnic groups. 33 The scale is summarised as HADS-depression (HADS-D) and HADS-anxiety (HADS-A), with scores ranging from 0 to 21, and as a HADS-total score (HADS-T), with scores ranging from 0 to 42 (higher scores indicating more symptoms). The HADS-T is our chosen primary outcome as it has better sensitivity and positive predictive value than either of the individual scales in identifying depression when compared with International Classification of Diseases (ICD) criteria. 34 The anxiety and depression scores were also dichotomised as ‘case’ and ‘non-case’, with a cut-off point of 8 or 9. 33
-
The Zarit Burden Interview is a 22-item self-report questionnaire and is the most consistently used measure of carer burden;35 scores range from 0 to 88, with higher scores indicating more burden. We used it to adjust for the baseline burden on carers as those who have more burden may be expected to be more stressed.
-
The MCTS is a self-completed measure of potentially abusive behaviour by the carers towards those for whom they care. 36 Ten behaviours are scored on whether or not, during the previous 3 months, these have occurred never (0), almost never (1), sometimes (2), most of the time (3) or all of the time (4), and these items can be added to make a score. These behaviours range from shouting, through threatening, to shaking or slapping. A score of 2 or more on any one of the items is classified as an abusive behaviour. If participants scored this on any item, the score was discussed with a supervising clinician and, if it was judged that the person with dementia was at risk, permission was asked to inform the clinical team so that the carer and patient could have appropriate help. This scale has been validated for use in family carers of people with dementia. 37
-
The Health Status Questionnaire (HSQ)38 mental health domain measures health-related QoL throughout the age range, is sensitive to change and has been validated in older and younger people. It is summarised as a continuous score, ranging from 0 to 100, with higher scores indicating better outcome.
-
European Quality of Life-5 Dimensions (EQ-5D)39,40 is a standardised measure of health status which provides a simple descriptive profile and a single index value for health status. The EQ-5D descriptive system comprises five dimensions: mobility, self-care, usual activities, pain or discomfort, and anxiety or depression. Each dimension has three levels: no problems, some problems and severe problems. The respondent is asked to indicate his or her health state by ticking (or placing a cross) in the box against the most appropriate statement in each of the five dimensions. The digits for the five dimensions can be combined in a five-digit number describing the respondent’s health state.
-
The Client Service Receipt Inventory (CSRI)41 comprehensively covers services, including (but not limited to) inpatient stays, outpatient attendances, day hospital treatment, visits to social clubs, meals at lunch clubs, day care visits, and hours spent in contact with community-based professionals such as community teams for older people, community psychologists, community psychiatrists, GPs, nurses (either practice, district or community psychiatric), social workers, occupational therapists, paid home help or care workers, and physiotherapists.
At all time points, carers were also asked for information about the person with dementia using:
-
The Neuropsychiatric Inventory (NPI). 42 The NPI is a validated instrument with 12 symptom domains that are scored for their severity and frequency and summarised as a single continuous score (higher scores indicating worse symptoms). This was included as neuropsychiatric symptoms have been shown to be associated with carer psychological morbidity. Possible scores range from 0 to 144, with higher scores indicating more severe symptoms.
-
Quality of Life-Alzheimer’s disease (QoL-AD). 43 The QoL-AD is an instrument used to rate the QoL of people with dementia and can be observer rated. It was rated by the family carer to assess the patient’s overall QoL. The total score ranges from 13 to 52, with higher scores indicating better outcome.
-
Clinical Dementia Rating (CDR). The CDR, which we used as an informant instrument, grades the level of impairment of someone with dementia [categories: healthy (0), very mild (0.5), mild (1), moderate (2), severe (3)]. 44
Changes to trial outcome after trial commenced
After the trial commenced, the team began to think further about the clinical relevance of this trial and concluded that the appropriate clinical questions were ‘does the intervention work?’ and, if it works, ‘does it keep working and show more effect over time or does the effect wear off?’. As the intervention targets coping strategies, in theory, over time there may be a larger separation between those who use ‘good’ strategies and those who do not when dealing with the intractable problem of somebody with dementia. On the other hand, people may revert to their previous tactics and usefulness may be lost over time. In the last case, we might consider whether or not a ‘top up’ would be appropriate. We therefore proposed to our steering group, data management group and the National Institute of Health Research Health Technology Assessment programme that we split the follow-up period into short- and long-term follow-up.
We agreed with all of them that our primary short-term outcome would be repeated measures of the HADS at 4 and 8 months and our long-term outcome would be repeated measures of the HADS at 1 and 2 years. Our original proposal was inconsistent whether we would use the HADS-A or HADS-D score as a primary outcome. There are three validation studies in community samples (as opposed to solely, for example, people with cancer). The largest of these included 6163 participants, more than five times the sum of the other validation studies and includes random samples of adults throughout the age group and groups of people with psychiatric and physical illnesses. 34 Although the HADS is usually used to generate scores and caseness for the two subscales of clinically significant anxiety and depression separately, this study found that HADS-T had better sensitivity and positive predictive value than either of the individual scales in identifying cases when validated against standardised clinical criteria. The authors comment that their results are in line with those of previous smaller studies. We therefore concluded that the HADS-T was the best measure to use.
In addition, we requested and received a 5-year no-cost extension to the trial. We wished to follow up the participants every 6 months to examine carer mental health and care recipient admission to 24-hour care settings (homes and continuing care beds) over the longer term or until death at home. This is because changes in the rate of admissions may become more apparent over a longer time.
We submitted all of these agreed changes as major amendments to National Research Ethics Committee and appropriate research and development departments and they were agreed.
Short-term primary outcome (up to 8 months’ follow-up)
Long-term main outcomes (12 to 24 months’ follow-up)
-
Carer HADS-T score.
-
Cost-effectiveness: costs using the CSRI alongside the carer QALYs calculated from the EQ-5D by applying societal weights.
Seven-year follow-ups
Time to entry to 24-hour care (this was added after funding and is not in this report, which covers the 2 years post recruitment).
Sample size
This was calculated to test our main hypotheses that HADS-T score will be significantly lower in the intervention group than in the TAU group.
This study was originally powered for a primary outcome of HADS-A score based on data from a cross-sectional pilot study of family carers. Mean HADS-A scores for this group were 7.2 with a standard deviation (SD) of 4. A decrease of 2 points in mean score and a 0.5 change in SD was considered to be clinically significant (expert consensus). To detect such a difference, with 90% power at 5% significance level, 75 participants per group were required. To account for therapist clustering, a design effect of 1.87 was used for the intervention group, assuming an average of 30 carers per therapist and an intracluster correlation coefficient (ICC) of 0.03. 46 Based on these calculations and inflating for 20% attrition, we planned to recruit 90 participants in the TAU group (no clustering) and 168 participants in the intervention group (clustering).
For the reasons given above, it was agreed that the primary outcome should be changed to HADS-T score. As recruitment was complete, the sample size available for analysis was fixed with achieved numbers of 87 in the TAU group and 173 in the intervention group. The following power calculation justified that this achieved sample size was adequate to address the new primary objectives based on the HADS-T outcomes at 4 and 8 months.
This power calculation considers the short-term primary analysis of HADS-T score using repeated measurements at 4 and 8 months, with an adjustment (using analysis of covariance) for baseline score. We calculated that the sample size available (87 carers in the control group and 173 in the intervention group) would be sufficient to detect a clinically important difference of at least 2.4 points on the HADS-T.
The calculations assumed a HADS-T SD of 7.4 (as given from our cross-sectional pilot study data), correlation between baseline and follow-up scores of 0.5 and a correlation between repeated follow-up measurements of 0.7 (both chosen as conservative estimates). Based on these values, with the available sample size we have 80% power to detect a difference of 2.4 points on the HADS-T and 90% power to detect a 2.7-point difference, both consistent with differences considered to be clinically important. This calculation has factored in adjustments for 10% dropout and a design effect of 1.4 for clustering in the intervention arm (calculated using the known average cluster size, 15 carers per therapist and an assumed ICC of 0.03). 46
Statistical methods
Scoring questionnaires
The relevant outcome scores were calculated from the individual items for each instrument according to standard algorithms. Where individual items were missing, standard procedures (where available) for the calculation of these outcome scores were used. Single missing data items on the HADS were imputed using the subscale mean, but cases with more than one missing item in any given subscale were excluded as invalid. 47 Missing cells on the Brief COPE scale were imputed using the carer’s own mean score for that particular subscale if there was one item missing (Charles Carver, University of Miami, 2011, personal communication). For the QoL-AD, if one or two items were missing, the mean of the completed items was used to impute the missing items. For all other outcomes, scores were calculated only where all relevant items had been completed.
Clinical outcomes
Separate regression analyses were used to estimate group differences in HADS-T score over the short term (using 4- and 8-month follow-ups) and the longer term (using 12- and 24-month follow-ups). In both cases, random-effects models accounted for repeated measurements and therapist clustering in the intervention arm. Adjustments were made for baseline HADS-T scores and centre (on which randomisation was stratified), and also on factors believed from the literature to affect affective symptoms (carer age, sex, carer burden and care recipient neuropsychiatric symptoms). All analyses were carried out by intention to treat, but we excluded. in the short term. carers who had data missing at both 4- and 8-month follow-up and, in the longer term, those with data missing at the 12- and 24-month follow-up. If an individual’s data were available at 4 months but not at 8 months, or vice versa, their partial data were used in the short-term analysis, and similarly with the 12- and 24-month data for the long-term modelling.
Similar approaches were taken for analyses of the secondary outcomes. Random-effects logistic regression was used for binary outcomes. In the short term, entry of the person with dementia to 24-hour care was compared between groups using a simple comparison of proportions (not allowing for clustering) because of small numbers. For the long-term analyses, the effect of the intervention on the time until institutionalisation was examined using parametric shared frailty models to allow for clustering in the intervention arm and adjustment for baseline factors. (The models fitted had a gamma distribution for the shared frailty and a Weibull survival distribution for time until institutionalisation.)
Model assumptions were examined by checking the normality of the residuals and also plotting the residuals compared with the fitted values both at the individual and therapist cluster level. To quantify therapist clustering, unadjusted ICCs at each follow-up within the intervention group were estimated with 95% confidence intervals (CIs).
Sensitivity analyses were used to reanalyse the outcomes in various ways to assess robustness of our conclusions. These analyses considered adjustment for imbalances in baseline characteristics between the randomised groups and the differential effects of treatment over time (treatment by time interaction). In considering missing data, we examined the extent to which missing outcome varied by baseline characteristics using logistic regression. In these analyses, for each outcome a binary variable was created according to whether or not it was missing (1 = missing score, 0 = valid score). This binary outcome was used in logistic regression to determine associations with baseline factors. In a sensitivity analysis, the main analyses for each outcome were repeated adjusting for those factors found to be associated with missingness, thus making missing at random assumptions more plausible. In the longer-term analyses, we also fitted models incorporating all repeated measurements (at 4, 8, 12 and 24 months) and examined the interaction between treatment group and long-/short-term follow-up.
Cost-effectiveness
The primary economic evaluation was a cost-effectiveness analysis comparing differences in treatment costs for carers receiving the START intervention with QALYs computed from the EQ-5D40 and societal weights48 over 1–8 months’ and then 1–24 months’ follow-up. In a secondary analysis, differences in treatment costs were compared with changes in in HADS-T score over the same time periods. Both the primary and secondary economic evaluations were undertaken from the perspective of health and social care agencies.
Data on services used and support received by the carer and the person with dementia were collected using an adapted version of the CSRI as above at baseline (randomisation) and at 4, 8, 12 and 24 months. On each occasion, the carer was asked to report service use over the previous 4 months. For the primary economic analyses over 1–8 months and then 1–24 months, our focus was on service use by the carer.
Total carer-related costs were derived by combining health and social care service use data with estimated unit costs. Costs were calculated for the periods 1–8 months and 1–24 months. We did not collect service use data during the period between the 12- and 21-month data collection time points (on average 8 months). Costs during this period were interpolated from the 12- and 24-month data.
Unit costs were obtained from publicly available sources and set at 2009–10 prices: Department of Health National Schedule of Reference Costs 2008–949 for inpatient (accident and emergency) and outpatient attendances; the Personal Social Services Research Unit (PSSRU) unit cost compendium50 for some inpatient services such as acute adult inpatient services; most other community-based health and social care professional services; and voluntary organisation services for a small number of services used by a few carers. Unit costs of services are listed in Appendix 1.
The cost of the START intervention was calculated using data on time spent by therapists in training and supervision with a clinical psychologist and contacts that therapists had with carers in delivering the intervention. Cost per hour of contact for therapists and supervising clinical psychologist were based on figures in the PSSRU compendium,50 taking the mid-point of the relevant scales and including employer costs (national insurance and superannuation contributions) and appropriate overheads (capital, administration and managerial, including recruitment costs). We added costs for the relaxation CDs based on the market rates for copying and delivering.
We analysed HADS-T and QALY differences between START and TAU interventions using a multilevel, mixed-effects model to account for therapist clustering in the intervention arm and repeated measures at 4, 8, 12 and 24 months. For the HADS-T analysis, we adjusted for baseline HADS-T score, centre and carer age and sex, carer burden (Zarit) and neuropsychiatric symptoms (NPI) of the person with dementia. For the QALY analyses, we adjusted for the same baseline variables, except substituting QALY for HADS-T.
Differences in total health and social care cost between the START and TAU interventions were regressed on treatment allocation, baseline costs, centre, carer age and sex, carer burden (Zarit) and care recipient neuropsychiatric symptoms (NPI). We used a linear multilevel regression model to account for therapist clustering in the intervention arm and repeated measures for each individual. Non-parametric bootstrapping was used to estimate 95% CIs for mean costs. Significance (p < 0.05) was judged where the bias-corrected 95% CIs of between-group change score excluded zero.
All analyses were conducted on an intention-to-treat basis; carers were excluded if data were missing at both 4 and 8 months for the 1–8-month economic analysis, and, for the 1–24-month economic analysis, carers were excluded if data were missing at 4, 8 and 24 months. Because the estimation of QALYs requires data at each time point, only complete cases were included in the cost-effectiveness analysis. No imputation was conducted.
Each incremental cost-effectiveness ratio (ICER) was calculated as the difference in the cost of the START and TAU interventions divided by the difference in outcome (measured by HADS-T score or QALYs). Cost-effectiveness acceptability curves (CEACs) were plotted to locate the findings of the economic evaluation in their wider decision-making context. The CEAC illustrates the probability that the START intervention would be seen as cost-effective compared with TAU across a range of hypothesised values placed on incremental outcome improvements (willingness to pay by health and social care system decision-makers). Each CEAC was derived using a net benefit approach. Monetary values of incremental effects and incremental costs for each case were combined and net monetary benefit (NMB) derived as:
where NMB was net monetary benefit, λ is willingness to pay for a 1-point difference in the outcome measure (HADS-T score or QALYs) and subscripts a and b denote the TAU and START interventions, respectively. We explored a range of λ values for each outcome. We were able to account for sampling uncertainty and make adjustments as necessary in the primary analyses and sensitivity analyses.
For the cost-effectiveness analyses carried out at 24 months’ follow-up, costs and outcomes in the second year were discounted at a rate of 3.5%, as recommended by NICE. 51 The discount rate was varied from 0% to 6% to assess the impact changing the NICE-recommended rate had on costs, outcome and the ICER.
Two sensitivity analyses were conducted to assess the robustness of our results. The first sensitivity analysis adjusted for those variables which predicted missing outcomes, HADS-T score and QALYs, and was investigated separately for each outcome using logistic regression. The first step was to model a binary variable (missing vs. not missing) in bivariate logistic regression with each baseline demographic variable. Those variables identified as significantly associated with missing were then used in multivariate logistic regression to determine which remained significant. The main analyses were then repeated, adjusting for those factors which were found to be associated with ‘missingness’ on each outcome. For the analysis of HADS-T scores, the variables found to be associated with ‘missingness’ were patient living with carer, relationship to carer, carer having children at home, patient ethnicity and COPE dysfunction score. For the QALY outcome, the carer’s work situation (employed vs. unemployed) and ethnicity were associated with ‘missingness’.
A second sensitivity analysis adjusted for imbalances in baseline characteristics between the treatment groups that occurred despite randomisation [i.e. adjusting for carers’ work situation, relationship to patient, and patients’ and carers’ education and living situation (coresident or living separately)]. These analyses were chosen so as to be consistent with those used in the effectiveness analysis. 41
We also plotted the CIs around the NMB to estimate the impact of uncertainty.
All economic analyses were carried out using Stata, version 12 (StataCorp LP, Chicago, IL, USA).
Chapter 5 Results
Participant flow and recruitment
Two hundred and sixty (55%) of the 472 carers referred were randomised (CIFT = 182, NELFT = 16, DRC = 35, NEPFT = 27) to the trial. The others declined to participate (n = 181; 38%), did not meet inclusion criteria (n = 22; 5%) or were uncontactable (n = 9; 2%).
Short-term clinical results (over 8 months)
The flow through the study to the short-term (8-month) outcome is shown in the Consolidated Standards of Reporting Trials (CONSORT) diagram (Figure 1).
FIGURE 1.
The CONSORT flow diagram (short-term analysis). a, To be included in the primary short-term analysis, the individual must have at least one score available for the HADS at 4 or 8 months. Those excluded have no measurements for either.
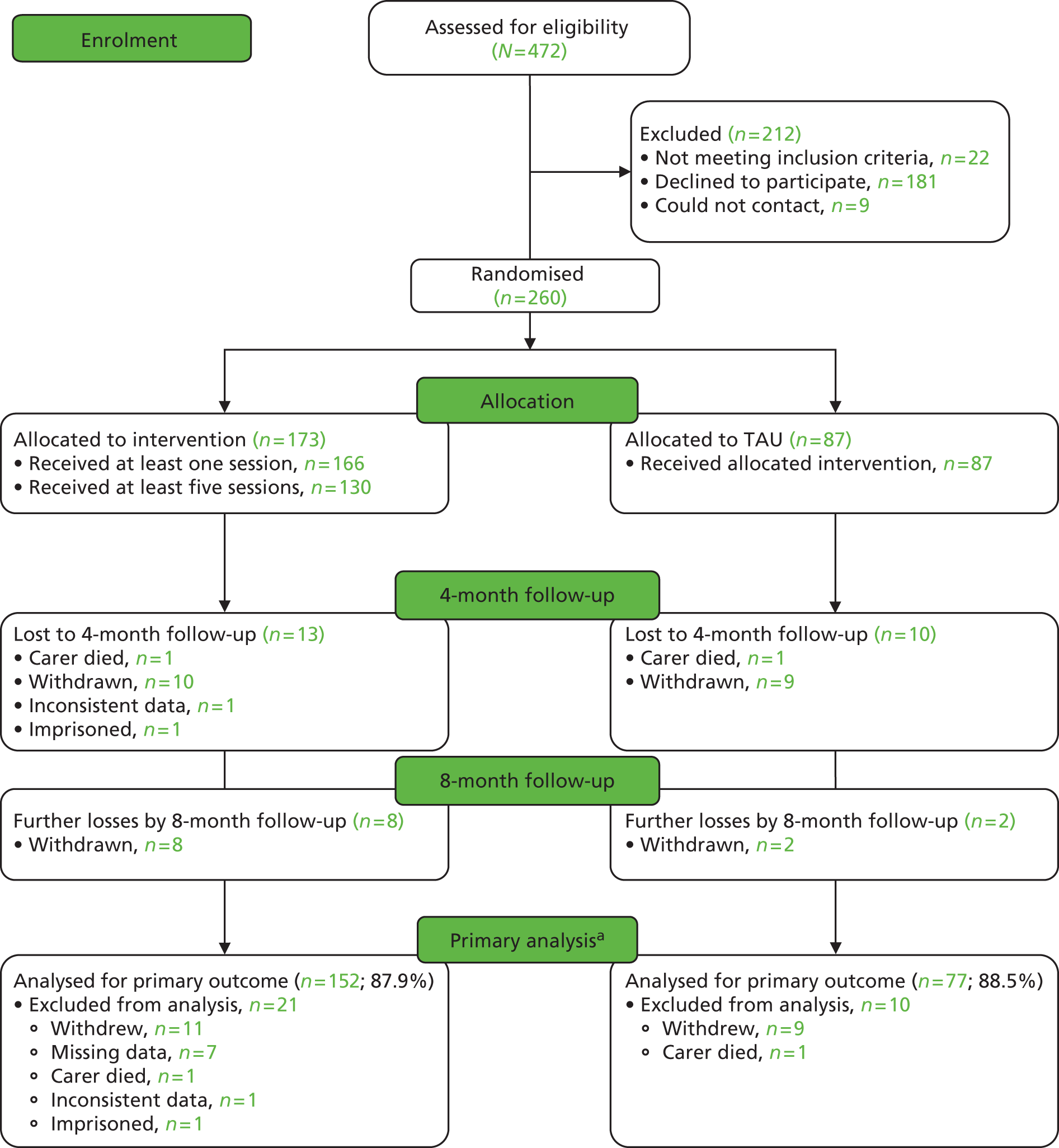
Participant flow through study
Over the 8-month follow-up, 12 carers from the control group and 21 from the intervention group withdrew or were lost to follow-up (see Figure 1). These included two who died (one from each group). In the intervention group, one carer gave inconsistent data and was withdrawn by the team and one was in prison. The participants gave the following reasons for withdrawal: wanted treatment but not allocated to it (four in TAU group), did not feel the intervention was for them (three in intervention group), too busy (four in intervention group, one in TAU group), disliked talking about care recipient when they were not there (one in TAU group, one in intervention group), other family member wanted them to withdraw (one in TAU group), unwell (one in intervention group), care recipient died (one in TAU group) and trial too upsetting (one in intervention group). Six gave no reason (five in intervention group, one in TAU group). Three others did not participate and were not contactable at the 4- or 8-month follow-up, but have since come back to the study.
External validity
Table 1 compares the known demographic details of those who consented and those who did not and shows that the study sample had good external validity. Those who consented were, however, slightly more likely to be married to the care recipient than those who did not consent.
| Demographics | Carers eligible but not randomised (n = 190) | Carers randomised (n = 260) |
|---|---|---|
| Carer sex | ||
| Male | 56 (29%) | 82 (32%) |
| Patient sex | ||
| Male | 75 (39%) | 108 (42%) |
| Patient relationship to carer | ||
| Spouse/partner | 65 (34%) | 109 (42%) |
| Child | 90 (47%) | 113 (43%) |
| Friend | 8 (4%) | 6 (2%) |
| Daughter’s/son’s partner | 4 (2%) | 12 (5%) |
| Nephew/niece | 8 (4%) | 8 (3%) |
| Grandchild | 4 (2%) | 6 (2%) |
| Sibling | 5 (3%) | 4 (2%) |
| Other | 6 (3%) | 2 (1%) |
Baseline data
One hundred and seventy-three (66.5%) participants were randomised to the intervention group and 87 were randomised to TAU. Details are shown in Table 2. In general, randomisation achieved good balance for patient and baseline carer demographic and clinical characteristics between the randomised groups (Tables 2 and 3).
| Demographics | Carer | Patient | ||
|---|---|---|---|---|
| TAU | Intervention | TAU | Intervention | |
| Mean (SD), minimum, maximum | Mean (SD), minimum, maximum | |||
| Age (years) | 56.1 (12.3), 27, 89 (n = 87) | 62.0 (14.6), 18, 88 (n = 172) | 78.0 (9.9), 53, 96 (n = 87) | 79.9 (8.3), 55, 95 (n = 173) |
| n (%) | n (%) | |||
| Sex | ||||
| Female | 62 (71.3%) | 116 (67.1%) | 50 (57.5%) | 102 (59.0%) |
| Male | 25 (28.7%) | 57 (32.9%) | 37 (42.5%) | 71 (41.0%) |
| Total | 87 | 173 | 87 | 173 |
| Ethnicity | ||||
| White UK | 65 (74.7%) | 131 (76.2%) | 61 (70.1%) | 126 (72.8%) |
| White other | 5 (5.7%) | 10 (5.8%) | 6 (6.9%) | 14 (8.1%) |
| Black and minority | 17 (19.5%) | 31 (18.0%) | 20 (23.0%) | 33 (19.1%) |
| Total | 87 | 172a | 87 | 173 |
| Marital status | ||||
| Not currently married | 25 (28.7%) | 61 (35.3%) | 47 (54.0%) | 92 (53.2%) |
| Married/common law | 62 (71.3%) | 112 (64.7%) | 40 (46.0%) | 81 (46.8%) |
| Total | 87 | 173 | 87 | 173 |
| Education | ||||
| No qualifications | 18 (20.7%) | 45 (26.0%) | 44 (51.2%) | 73 (44.5%) |
| School-level qualification | 33 (37.9%) | 51 (29.5%) | 16 (18.6%) | 28 (17.1%) |
| Further education | 36 (41.4%) | 77 (44.5%) | 26 (30.2%) | 63 (38.4%) |
| Total | 87 | 173 | 86 | 164 |
| Work situation | ||||
| Full-time | 28 (32.2%) | 36 (20.8%) | N/A | N/A |
| Part-time | 20 (23.0%) | 27 (15.6%) | N/A | N/A |
| Retired | 23 (26.4%) | 80 (46.2%) | N/A | N/A |
| Not working | 16 (18.4%) | 30 (17.3%) | N/A | N/A |
| Total | 87 | 173 | N/A | N/A |
| Living with carer | ||||
| Yes | N/A | N/A | 50 (57.5%) | 113 (65.3%) |
| Total | N/A | N/A | 87 | 173 |
| Scale | Carer | Patient | ||
|---|---|---|---|---|
| TAU | Intervention | TAU | Intervention | |
| HADS-T scoresa | 14.8 (7.4) (n = 87) | 13.5 (7.3) (n = 172) | N/A | N/A |
| HADS-A scoresa | 9.3 (4.3) (n = 87) | 8.1 (4.4) (n = 172) | N/A | N/A |
| HADS-D scoresa | 5.5 (3.9) (n = 87) | 5.4 (3.8) (n = 172) | N/A | N/A |
| QoL-AD scoresa | N/A | N/A | 29.9 (6.9) (n = 87) | 30.2 (6.9) (n = 170) |
| HSQ mental health scoresa | 58.2 (21.7) (n = 87) | 58.3 (22.4) (n=171) | N/A | N/A |
| MCTS total scoresa | 2.7 (3.1) (n = 87) | 2.5 (2.9) (n=172) | N/A | N/A |
| Zarit total scoresa | 38.1 (17.0) (n = 84) | 35.3 (18.4) (n = 165) | N/A | N/A |
| NPI total scoresa | N/A | N/A | 26.6 (20.1) (n = 86) | 24.0 (19.0) (n = 171) |
| CDR overall scoreb | N/A | N/A | (n = 87) | (n = 171) |
| Score 0.5 | N/A | N/A | 12 (13.8%) | 30 (17.5%) |
| Score 1 | N/A | N/A | 43 (49.4%) | 91 (53.2%) |
| Score 2 | N/A | N/A | 30 (34.5%) | 48 (28.1%) |
| Score 3 | N/A | N/A | 2 (2.3%) | 2 (1.2%) |
| HADS-A case (score of ≥ 9)b | 48 (55.2%) (n = 87) | 85 (49.4%) (n = 172) | N/A | N/A |
| HADS-D case (score of ≥ 9)b | 17 (19.5%) (n = 87) | 36 (20.9%) (n = 172) | N/A | N/A |
| MCTS (at least one item with score of ≥ 2)b | 38 (43.7%) (n = 87) | 82 (47.7%) (n = 172) | N/A | N/A |
Intervention
Therapists
We trained 10 psychology graduates with no further clinical training – seven women and three men – who were ethnically white British (eight) and black and minority ethnic (two) in the age range 23–33 years. They worked with between 11 and 32 participants each (mean 17.3 participants) (Table 4).
| Therapist | Number of carers (%) |
|---|---|
| 1 | 11 (6.4) |
| 2 | 21 (12.1) |
| 3 | 12 (6.9) |
| 4 | 19 (11.0) |
| 5 | 12 (6.9) |
| 6 | 11 (6.4) |
| 7 | 32 (18.5) |
| 8 | 21 (12.1) |
| 9 | 17 (9.8) |
| 10 | 17 (9.8) |
| Total | 173 (100.0) |
Adherence with therapy
Of the eight therapy sessions offered, five or more were attended by 130 (75.1%) carers in the intervention group (Figure 2 shows details of number of sessions attended). Seven (4.0%) of those in the intervention group withdrew before taking part in any therapy sessions. Adherence (attending five or more sessions) was better in those of white ethnicity [110 (78.0%) vs. 19 (61.3%)] than in those of other ethnicity and slightly better for male carers [46 (80.7%) vs. 84 (72.4%) of female carers] and those with at least A-level education [56 (80.0%) vs. 74 (71.8%)]. Adherence was similar by age group [aged < 60–75 years (77.3%) vs. 55 years (73.3%)] and work situation [in paid work 49 (77.8%) vs. other 81 (73.6%)].
FIGURE 2.
Number of sessions completed by participants.
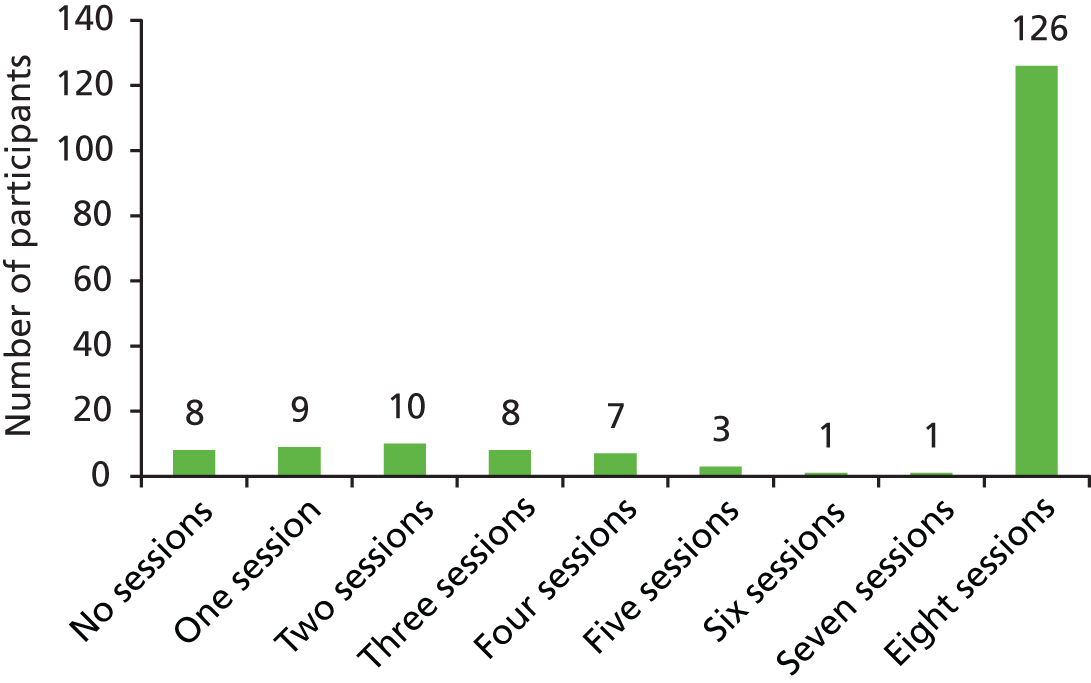
Fidelity
Double rating was carried out for the first 13 participants; these had a Cohen’s kappa value of 0.77, which is substantial agreement. 52 After this, we judged it was necessary to have only a single rating for the remaining participants. Fidelity rating was carried out for 128 (78%) of the 165 participants who received one or more intervention(s). The remaining 38 either refused to be audiotaped (10) or withdrew before carrying out the session which had been randomly selected for assessment (n = 28). For 100 (78%) participants fidelity was rated as 5, for 20 (16%) it was rated as 4, for five it was rated as 3 and for three it was rated as 2. Overall mean fidelity was calculated by adding each average rating (rating 1 plus rating 2, divided by 2) of the first 13 participants to the ratings of the remaining 105 participants, and dividing by the overall number of participants with available fidelity ratings. The mean fidelity score was 4.7 (SD 0.66).
Languages, translation and interpreters
Four eligible participants used translators at the recruitment stage, in Turkish (one), Bengali (two) and Farsi (one), and all four consented and were randomised: three to intervention and one to TAU. One participant allocated to intervention changed his or her mind and withdrew from intervention before session 1 but not follow-ups; one completed five sessions and one completed eight sessions.
Missing outcome data
The proportion of missing data for the main trial outcomes is given in Table 5 by group and follow-up time. The primary outcome (HADS-T score) was missing for 35 (13.5%) patients at 4 months and for 56 (21.5%) patients at 8 months, with a slightly higher proportion missing in the intervention group: 40 (23.0%) in the intervention group and 16 (18.0%) in the TAU group. Two hundred and twenty-nine (88.1%) had HADS-T data for at least one of the 4- and 8-month points and, so, could be included in the primary analyses. Using logistic regression models, we identified baseline factors that were associated with missing outcome data.
| Scale | Baseline | 4 months | 8 months | |||
|---|---|---|---|---|---|---|
| TAU, % missing | Intervention, % missing | TAU, % missing | Intervention, % missing | TAU, % missing | Intervention, % missing | |
| HADS-A | 0.0 | 0.6 | 13.8 | 13.3 | 18.4 | 23.1 |
| HADS-D | 0.0 | 0.6 | 13.8 | 13.3 | 18.4 | 23.1 |
| HADS-T | 0.0 | 0.6 | 13.8 | 13.3 | 18.4 | 23.1 |
| Zarit total | 3.4 | 4.6 | 26.4 | 20.2 | 31.0 | 32.4 |
| MCTS total | 0.0 | 0.6 | 20.7 | 20.2 | 26.4 | 30.6 |
| HSQ mental health | 0.0 | 1.2 | 17.2 | 16.8 | 24.1 | 29.5 |
| QoL-AD total | 0.0 | 1.7 | 24.1 | 20.8 | 29.9 | 30.6 |
Missingness of HADS-T score at 4 months was associated with the patient living with carer (p = 0.007) and for the 8-month outcome with having dependent children at home (p = 0.003), patient ethnicity (p = 0.013), patient living with carer (p = 0.002), patient relationship to carer (p = 0.011) and the COPE dysfunction score (p = 0.004).
Clinical outcomes
Primary outcome
Analysis of HADS-T, adjusting for trust and baseline score and for factors related to outcome (carer age and sex, NPI and Zarit scores), showed a mean difference of –1.80 points (95% CI –3.29 to –0.31 points; p = 0.02) in favour of the intervention (Table 6). If the model did not include factors relating to outcome, then the results were similar, with an average decrease in score of –1.46 points (95% CI –2.89 to –0.03 points; p = 0.05). There was little therapist clustering: ICC at 4 months was 0.02 (95% CI 0.00 to 0.09) and at 8 months was 0.00 (95% CI 0.00 to 0.08).
| Measure | TAU | TAU | Intervention | Intervention | Adjusted for baseline score and centre, difference (95% CI) | Adjusted also for carers age, sex, NPI and Zarit, difference (95% CI) |
|---|---|---|---|---|---|---|
| 4 months, mean (SD) | 8 months, mean (SD) | 4 months, mean (SD) | 8 months, mean (SD) | |||
| HADS-T scores | 14.3 (7.4) (n = 75) | 14.9 (8.0) (n = 71) | 12.4 (7.4) (n = 150) | 12.9 (7.9) (n = 133) | –1.46 (–2.89 to –0.03; p-value = 0.05) (n = 229) | –1.80 (–3.29 to –0.31; p-value = 0.02) (n = 220) |
| QoL-AD scores | 29.8 (5.8) (n = 66) | 29.7 (6.3) (n = 61) | 30.6 (6.4) (n = 136) | 30.2 (7.2) (n = 119) | 0.80 (–0.45 to 2.05) (n = 205) | 0.59 (–0.72 to 1.89) (n = 197) |
| HSQ mental health scores | 58.4 (18.0) (n = 72) | 58.2 (19.2) (n = 66) | 62.7 (20.8) (n = 144) | 58.6 (22.0) (n = 122) | 4.55 (0.92 to 8.17) (n = 219) | 4.09 (0.34 to 7.83) (n = 211) |
| HADS-A scores | 8.6 (4.2) (n = 75) | 8.8 (4.4) (n = 71) | 7.5 (4.2) (n = 150) | 7.6 (4.4) (n = 133) | –0.62 (–1.43 to 0.19) (n = 229) | –0.91 (–1.76 to –0.07) (n = 220) |
| HADS-D scores | 5.7 (4.0) (n = 75) | 6.1 (4.2) (n = 71) | 4.9 (3.9) (n = 150) | 5.3 (4.0) (n = 133) | –0.88 (–1.68 to –0.09) (n = 229) | –0.91 (–1.71 to –0.10) (n = 220) |
| HADS-A case (score of ≥ 9) | 36 (48.0%) (n = 75)a | 33 (46.5%) (n = 71)a | 54 (36.0%) (n = 150)a | 53 (39.9%) (n = 133)a | 0.35 (0.11 to 1.18) (n = 229)b | 0.30 (0.08 to 1.05) (n = 220)b |
| HADS-D case (score of ≥ 9) | 18 (24.0%) (n = 75)a | 23 (32.4%) (n = 71)a | 25 (16.7%) (n = 150)a | 28 (21.1%) (n = 133)a | 0.25 (0.08 to 0.81) (n = 229)b | 0.24 (0.07 to 0.76) (n = 220)b |
| MCTS (at least one item with score of ≥ 2) | 28 (40.6%) (n = 69)a | 23 (35.9%) (n = 64)a | 50 (36.0%) (n = 139)a | 40 (33.3%) (n = 120)a | 0.47 (0.18 to 1.23) (n = 214)b | 0.48 (0.18 to 1.27) (n = 206)b |
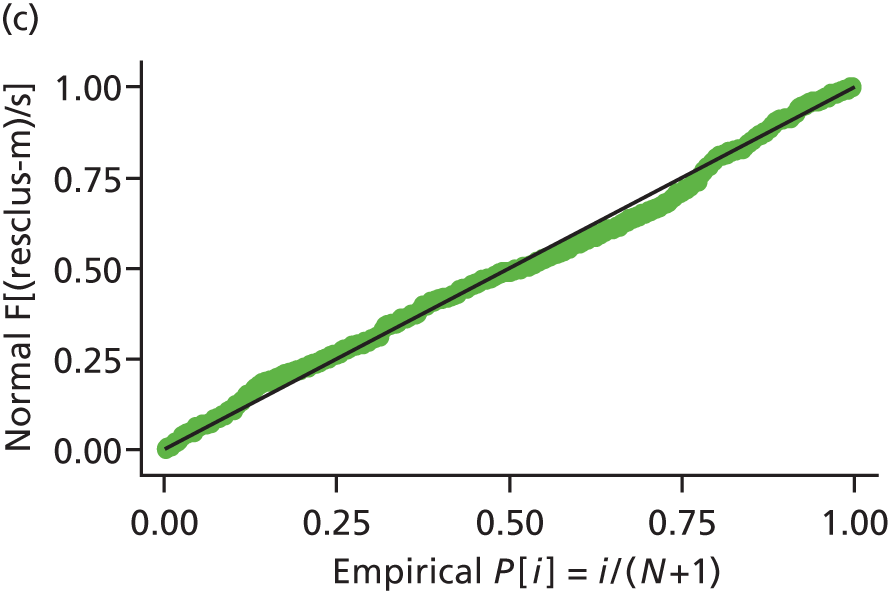
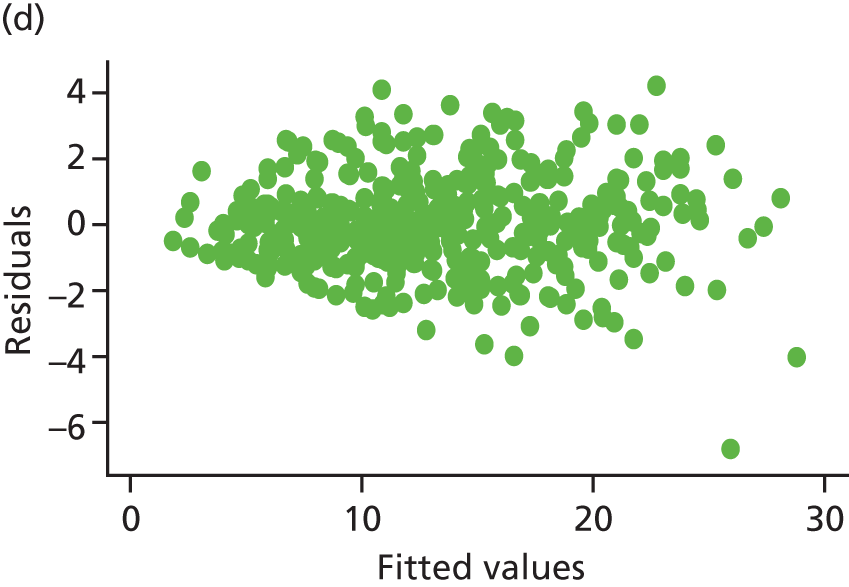
Model assumptions were examined by checking the normality of the residuals and also plotting the residuals compared with the fitted values. These were done at the individual and therapist cluster level. These plots are shown in Figure 3 and do not indicate concerns about the model fit.
FIGURE 3.
Checking model assumptions: residuals and fitted values from the models for HADS-T (based on the model-adjusted only for centre and baseline score). (a) Normality of residuals (individual level); (b) residuals vs. fitted values (individual level); (c) normality of residuals (cluster level); and (d) residuals vs. fitted values (cluster level).
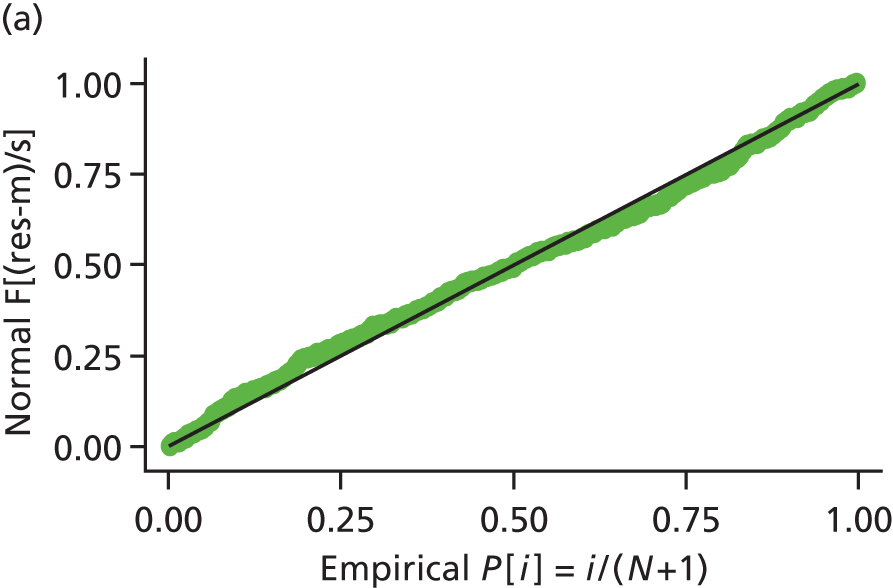
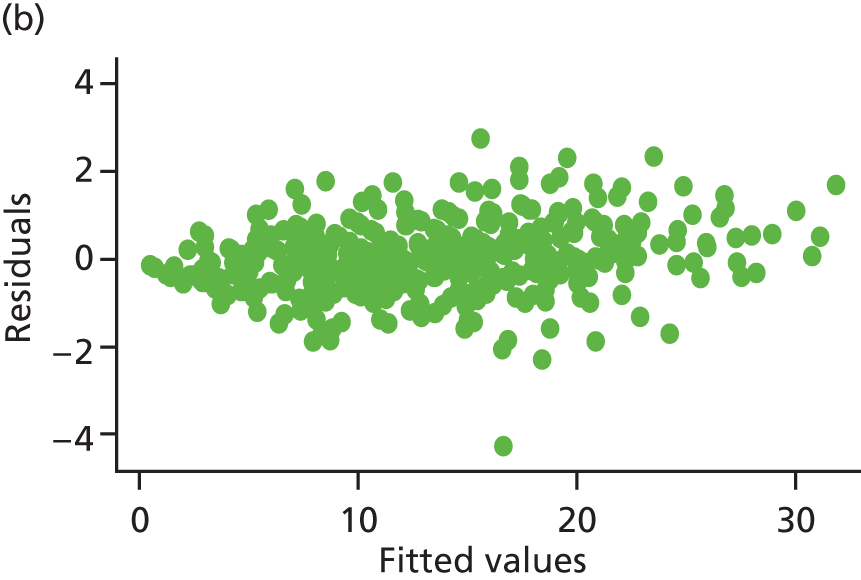
Sensitivity analysis
For the sensitivity analysis we adjusted for baseline factors associated with missingness of the HADS-T outcome. Missingness of the 4-month HADS-T was associated with patient living with carer (p = 0.007) and for the 8-month outcome with having dependent children at home (p = 0.003), patient ethnicity (p = 0.01), patient living with carer (p = 0.002), patient relationship to carer (p = 0.010) and the COPE dysfunction score (p = 0.004). Refitting the main models adjusting for these significant predictors of missing values did not have a significant impact on the results (mean difference –1.53, 95% CI –2.96 to –0.10). We also carried out a sensitivity analysis to adjust for baseline imbalances, namely carer’s work situation, relationship to carer and patient and carer education and living situation, which again gave similar conclusions (mean difference –1.78, 95% CI –3.30 to –0.27). Models including an interaction with time showed no evidence of a differential effect of the intervention between the 4- and 8-month time points (p = 0.90).
Secondary outcomes
Depression and anxiety caseness on the HADS
There was a significant reduction in the odds of HADS-D cases in the intervention group compared with TAU, with an odds four times higher for the TAU group [adjusted odds ratio (OR) 0.24, 95% CI 0.07 to 0.76]. Similarly, there was some evidence for a reduction in odds of HADS-A caseness (OR 0.30, 95% CI 0.08 to 1.05).
Anxiety and depression symptoms
Adjusted models for the individual HADS-A and HADS-D continuous scales indicated significant beneficial effects of the intervention over 8 months, with average decreases in scores of –0.910 (95% CI –1.763 to –0.070) and –0.91 (95% CI –1.71 to –0.10), respectively.
For HADS-A, sensitivity analyses adjusting for significant demographic and clinical predictors of missing values, namely patient living with carer, relationship to carer, carer having dependent children at home, patient ethnicity and COPE dysfunction score, gave a result with no significant difference between groups (mean difference –0.68, 95% CI –1.49 to 0.11; n = 229).
The same analysis of HADS-D showed a significant difference between groups (mean difference –0.90, 95% CI –1.70 to –0.11; n = 229).
Models including an interaction with time showed no evidence of a differential effect of the intervention between the 4- and 8-month time points for either HADS-A or HADS-D.
Carer (Health Status Questionnaire) and care recipient (Quality of Life-Alzheimer’s disease) quality of life
There was no significant difference between groups in the person with dementia’s overall QoL (QoL-AD). The HSQ mental health scale for the carer did, however, indicate significantly higher average scores and, hence, improved mental health (mean difference 4.09, 95% CI 0.34 to 7.83).
Models including an interaction with time showed no evidence of a differential effect of the intervention between the 4- and 8-month time points for either the HSQ mental health or the QoL-AD.
Modified Conflict Tactics Scale for significant abuse
There was some evidence of a decrease in abusive behaviour (OR 0.48, 95% CI 0.18 to 1.27). There was no evidence of an interaction between the intervention and the outcome at the 4- and 8-month follow-ups.
Time to care home admission
Fourteen patients were admitted to a care home during the 8-month follow-up period (not included in Table 7): three (3.6%) in the TAU group and 11 (6.4%) in the intervention group. Simple analyses indicate no evidence of a statistically significant difference between the groups (Fisher’s exact test, p = 0.56). This outcome will be considered more extensively in analyses of longer-term follow-up.
Health economic analysis: short term over 8 months
Service use and costs
Carers used a wide range of health and social care services over the 8-month period, as can be seen in Table 7. We did not impute individual items of service use and means are presented for non-missing cases only. Outpatient hospital and GP services were used by quite high proportions of participants.
| Service | Baseline, % (n) | 4 months, % (n) | 8 months, % (n) | |||
|---|---|---|---|---|---|---|
| TAU (N = 87) | Intervention (N = 173) | TAU (N = 75) | Intervention (N = 150) | TAU (N = 71) | Intervention (N = 134) | |
| Outpatient services | ||||||
| Outpatient hospital services | 33.3 (29) | 37.0 (64) | 32.0 (24) | 38.7 (58) | 28.2 (20) | 37.3 (50) |
| Community-based services | ||||||
| Admiral Nurse | 5.7 (5) | 3.5 (6) | 2.6 (2) | 6.0 (9) | 2.8 (2) | 3.7 (5) |
| Chiropodist | 5.7 (5) | 13.9 (24) | 9.3 (7) | 11.3 (17) | 12.7 (9) | 17.2 (23) |
| Counsellor | 8.0 (7) | 2.3 (4) | 9.3 (7) | 2.7 (4) | 11.3 (8) | 1.5 (2) |
| Dentist | 27.6 (24) | 30.6 (53) | 29.3 (22) | 30.7 (46) | 36.6 (26) | 33.6 (45) |
| GP | 54.0 (47) | 54.3 (94) | 53.3 (40) | 50.0 (75) | 47.9 (34) | 48.5 (65) |
| NHS Direct | 1.1 (1) | 1.2 (2) | 1.3 (1) | 0.0 (0) | 0.0 (0) | 1.5 (2) |
| Optician | 17.2 (15) | 16.8 (29) | 8.0 (6) | 15.3 (23) | 21.1 (15) | 18.7 (25) |
| Outreach worker | 1.1 (1) | 0.6 (1) | 0.0 (0) | 0.7 (1) | 1.4 (1) | 0.0 (0) |
| Home care worker/care attendant | 2.3 (2) | 1.7 (3) | 4.0 (3) | 1.3 (2) | 1.4 (1) | 1.5 (2) |
| Physiotherapist | 0.0 (0) | 0.6 (1) | 1.3 (1) | 0.0 (0) | 0.0 (0) | 2.2 (3) |
| Hygienist | 0.0 (0) | 0.6 (1) | 0.0 (0) | 0.7 (1) | 1.4 (1) | 0.7 (1) |
| Company medical check-up | 0.0 (0) | 0.6 (1) | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.0 (0) |
| Nurse (advanced) | 1.1 (1) | 0.6 (1) | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.0 (0) |
| Occupational therapist | 0.0 (0) | 0.6 (1) | 0.0 (0) | 0.7 (1) | 0.0 (0) | 0.0 (0) |
| Community psychiatrist | 0.0 (0) | 0.6 (1) | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.0 (0) |
| Practice nurse | 6.9 (6) | 2.3 (4) | 2.6 (2) | 4.7 (7) | 0.0 (0) | 5.2 (7) |
| Ambulance transport | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.7 (1) | 0.0 (0) | 0.0 (0) |
| Dietitian | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.7 (1) | 0.0 (0) | 0.0 (0) |
| Other services | 16.1 (14) | 9.8 (17) | 22.7 (17) | 14.0 (21) | 12.7 (9) | 13.4 (18) |
The patterns of service use were weighted by their unit costs. Mean costs – grouped into outpatient, community and other services – are given in Table 8. The table distinguishes between the START and TAU groups, and reports figures for two time periods: the 4-month period between baseline and the 4-month assessment, and the 4-month period between the 4- and 8-month assessments. Although the number of users of outpatient services was higher in the intervention group (see Table 8), their average outpatient service cost was lower as they used outpatient services less frequently than people in the TAU group.
| Cost categories and outcomes used in the economic evaluation | TAU, mean (SD) | Intervention, mean (SD) | Intervention vs. TAU over 1–8 months, difference (95% CI) adjusted for baseline variables | ||
|---|---|---|---|---|---|
| 1–4 months | 5–8 months | 1–4 months | 5–8 months | ||
| Costs (£, 2009–10) | |||||
| Outpatient | 140 (428) | 125 (385) | 99 (183) | 99 (237) | –42 (–118 to 34) |
| Community | 107 (148) | 110 (153) | 101 (180) | 96 (153) | –7 (–34 to 19) |
| Other | 134 (1028) | 9 (36) | 96 (845) | 68 (461) | 27 (–151 to 205) |
| Total | 381 (1102) | 244 (450) | 296 (1006) | 262 (598) | –14 (–239 to 211) ( n = 193) |
| Outcomes used in the economic evaluation | |||||
| HADS-T scoresa | 14.3 (7.4) | 14.9 (8.0) | 12.4 (7.4) | 12.9 (7.9) | –1.79 (–3.22 to –0.37) (n = 220) |
| EQ-5D | 0.77 (0.23) | 0.79 (0.14) | 0.77 (0.22) | 0.76 (0.24) | 0.03 (–0.01 to 0.08) (n = 212) |
The right-hand column of Table 8 shows the difference in costs between the START and TAU groups across the whole evaluation period of 8 months. Excluding the direct cost of the intervention itself, mean costs over the study period (1–8 months) were £558 in the START group and £625 in the TAU group. After adjustment for baseline characteristics (see above) the standardised difference was £14, with the 95% CI (–£239 to £211) suggesting that there was not a significant difference in costs between the two groups. For purposes of comparison, scores on the two outcome measures used in the economic evaluation are included towards the bottom of Table 8.
The calculation of cost of the therapy is based on the time spent by the 10 therapists in delivering one-to-one therapy to carers, their own training sessions (40 sessions of 2.5 hours over a 6-week period), time spent making telephone calls to participants, time spent writing up notes and supervision of the therapists by the clinical psychologist (1.5 hours each per week for 8 weeks). Looking at the average time spent in each session with the carer, excluding those who did not have any sessions and those who used translators (n = 163), the median time per session was 1 hour 16 minutes (76 minutes). The mean time per session was 1 hour 17 minutes (77 minutes, SD 20 minutes). For those who used translators, the mean time per session was 3 hours 47 minutes (SD 1 hour 36 minutes), with a range of 2 hours 40 minutes to 4 hours 55 minutes. We calculated that the mean cost per session per carer was £36. Adding in the cost of the relaxation CDs (which totalled £284), the overall mean direct intervention cost averaged £232.15 per carer.
Including the cost of intervention itself in the comparison between the groups and adjusting for baseline variables as we did in the clinical effectiveness calculations, costs for the START group were slightly but not significantly higher than for the TAU group. The mean cost difference was £252 (95% CI –£28 to £565) for sample members on the EQ-5D, and £247 (95% CI £0 to £569) for sample members on the HADS-T measure (Table 9).
| Outcome | Incremental differences (START minus TAU) and ICERs over 8-month evaluation period |
|---|---|
| With QALY as outcome (n = 177)a | |
| Incremental health and social care costs (£, 2009–10), mean (95% CI) | £252 (–£28 to £565) |
| Incremental QALY gain, mean (95% CI) | 0.042 (0.015 to 0.071) |
| ICER (£ per QALY) | 6000 |
| With HADS-T as outcome (n = 191)b | |
| Incremental health and social care costs (£, 2009–10), mean (95% CI) | £247 (£0 to £569) |
| Incremental HADS-T score change, mean (95% CI) | 2.10 (0.51 to 3.75) |
| ICER (£ per unit change on HADS-T) | £118 |
Cost-effectiveness
Results from the net benefit regression using the two outcomes examined in the economic evaluation (QALYs and HADS-T score) are summarised and the ICERs reported in Table 10. The cost and outcome differences are obtained after adjustment for baseline characteristics and are influenced slightly by size of sample with complete data for each outcome.
| Outcome | Incremental differences and ICERs over 8-month evaluation period | |
|---|---|---|
| Adjusting for significant demographic and clinical predictors of missing values | Adjusting for baseline imbalances | |
| With QALY as outcome (n = 177)a | ||
| Incremental health and social care costs (£, 2009–10), mean (95% CI) | £229 (–£94 to £552) | £236 (–£101 to £617) |
| Incremental QALY gain, mean (95% CI) | 0.042 (0.014 to 0.070) | 0.041 (0.012 to 0.071) |
| ICER (£ per QALY) | £5452 | £5756 |
| With HADS-T as outcome (n = 191)b | ||
| Incremental health and social care costs (£, 2009–10), mean (95% CI) | £226 (–£74 to £525) | £231 (–£46 to £583) |
| Incremental HADS-T score change, mean (95% CI) | 2.11 (0.41 to 3.81) | 2.07 (0.44 to 3.90) |
| ICER (£ per unit change on HADS-T) | £107 | £112 |
Health and social care system costs were slightly higher for carers who received the START intervention (in addition to TAU) but the difference was not statistically significant, and carers in this group enjoyed significantly better outcomes, whether measured in terms of health-related QoL (QALY) or affective symptoms (HADS-T). Whether or not these results imply that START is cost-effective compared with TAU depends on the decision-maker’s willingness to pay for these gains in QoL and affective symptoms. To aid discussion of willingness to pay, we computed the ICERs. We also plotted the associated CEACs and examined the CIs around NMB.
Looking first at QALY as the outcome, the mean cost per QALY gained was £6000. The CEAC is shown in Figure 4, illustrating the probability of cost-effectiveness for each of a number of different hypothesised values of willingness to pay. At the £20,000 per QALY threshold associated with NICE recommendations, the probability that the START intervention would be seen as cost-effective was 93% and at the higher NICE threshold of £30,000 it was 99%. 53
FIGURE 4.
Cost-effectiveness acceptability curve: START intervention (manual-based coping strategy therapy) vs. TAU; health and social care perspective, with effectiveness measured in QALY gain over 8 months.
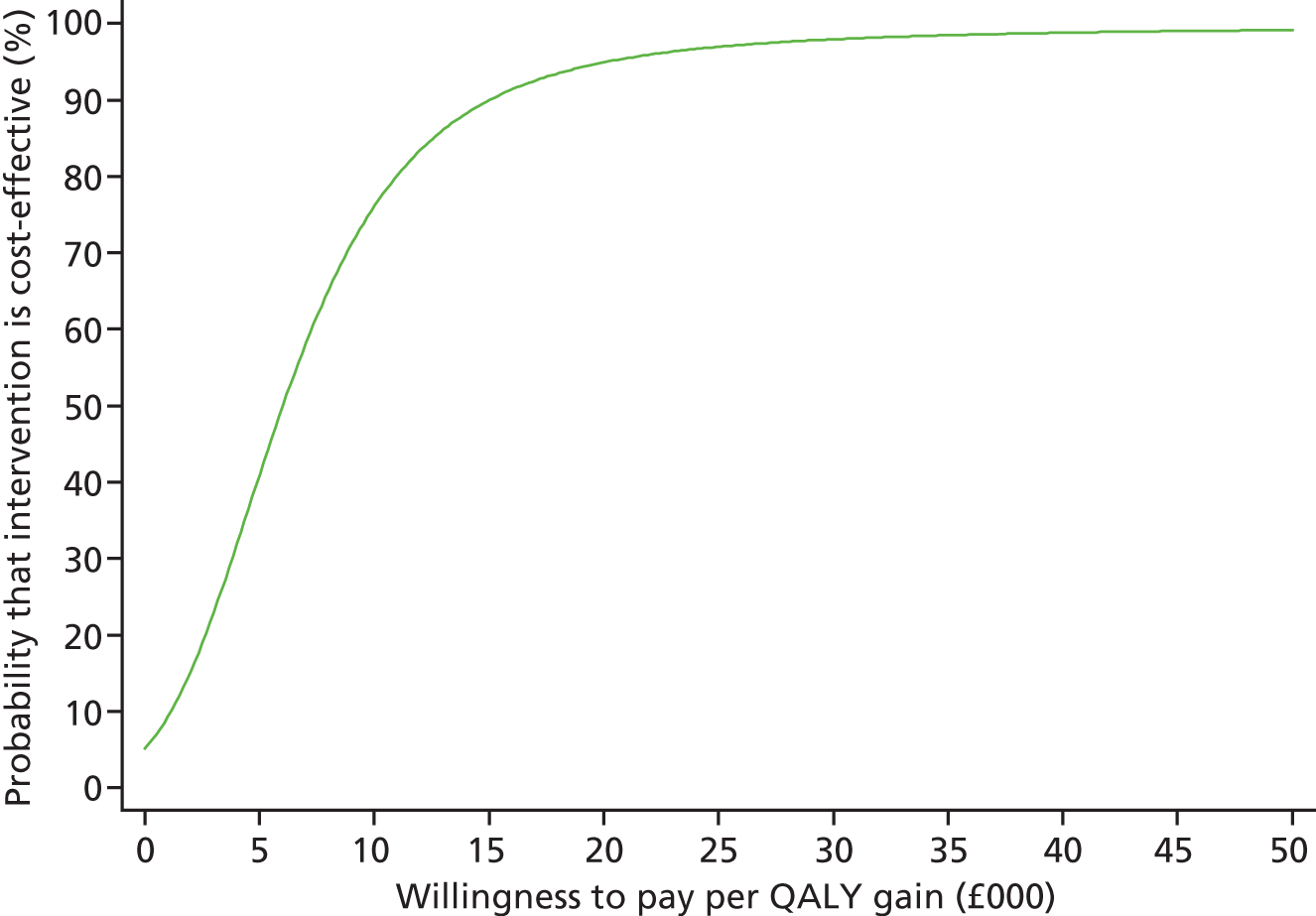
The 95% CIs around NMB suggest that there is a strong likelihood that the START intervention is cost-effective at the £30,000 threshold.
For the other outcome measure, the HADS-T measure of affective symptoms, the mean cost per 1-point difference on the HADS-T was £118. The CEAC for this outcome measure is shown in Figure 5. We are not aware of any previously suggested monetary thresholds for gauging cost-effectiveness on the HADS. However, if we assumed a willingness to pay of £500, the probability that the START intervention would be seen as cost-effective would be 95%. We can also refer to a previous suggestion that a minimally important clinical difference on the HADS-T is 1.6. The mean cost of achieving such a change with the START intervention would be £189.
FIGURE 5.
Cost-effectiveness acceptability curve: START intervention (manual-based coping strategy therapy) vs. TAU; health and social care perspective, with effectiveness measured in HADS gain over 8 months.
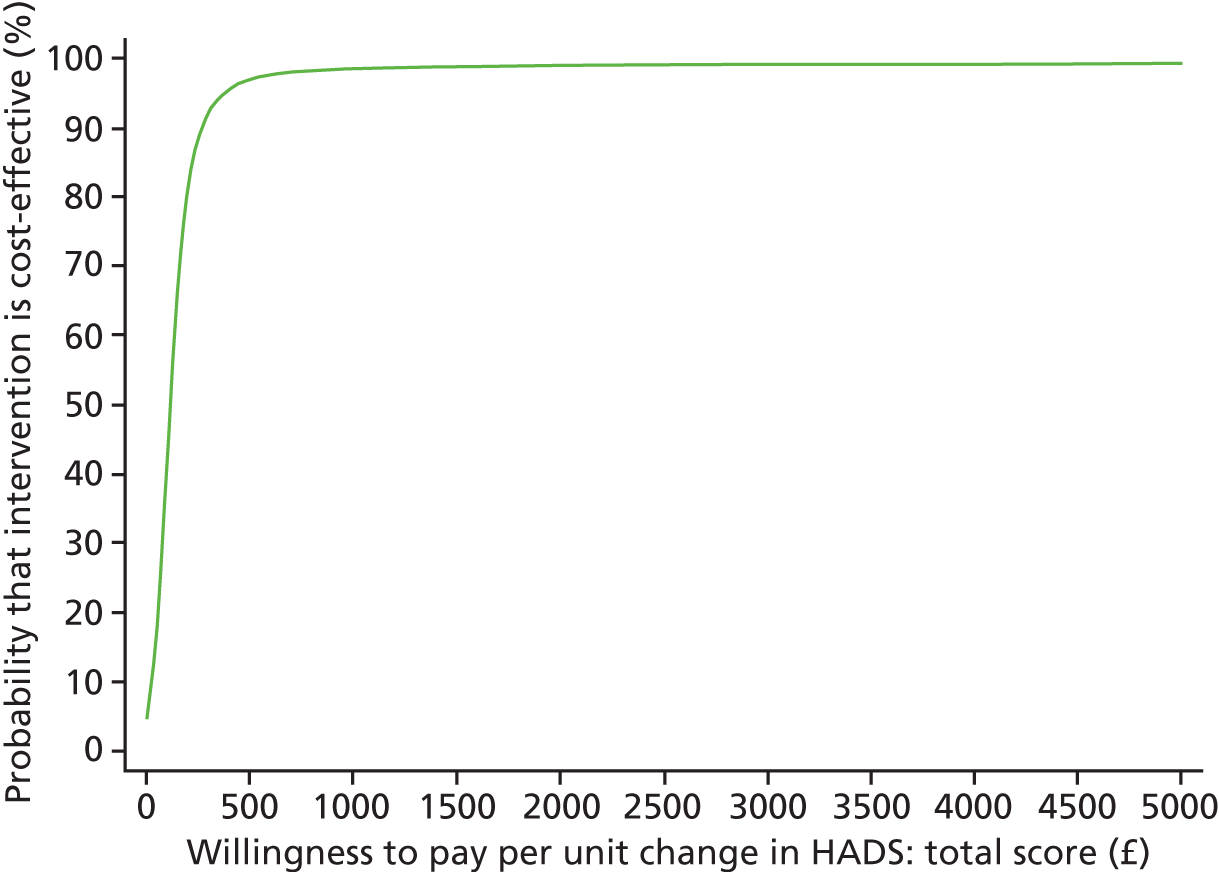
Sensitivity analyses
The first sensitivity analysis adjusted for significant baseline differences on demographic and clinical predictors of missing values. The results were similar to those from the primary analyses and are summarised in the first column of figures in Table 10.
The mean ICER values are now £5452 per additional QALY and £107 per 1-point difference in HADS-T score. Figure 6 shows the CEAC with QALY as the outcome measure; at the lower-bound NICE threshold of £20,000, the START intervention has an approximately 95% likelihood of being seen as cost-effective, rising to 98% at the £30,000 threshold.
FIGURE 6.
Sensitivity analysis of CEAC with QALY as the outcome measure over 8 months.
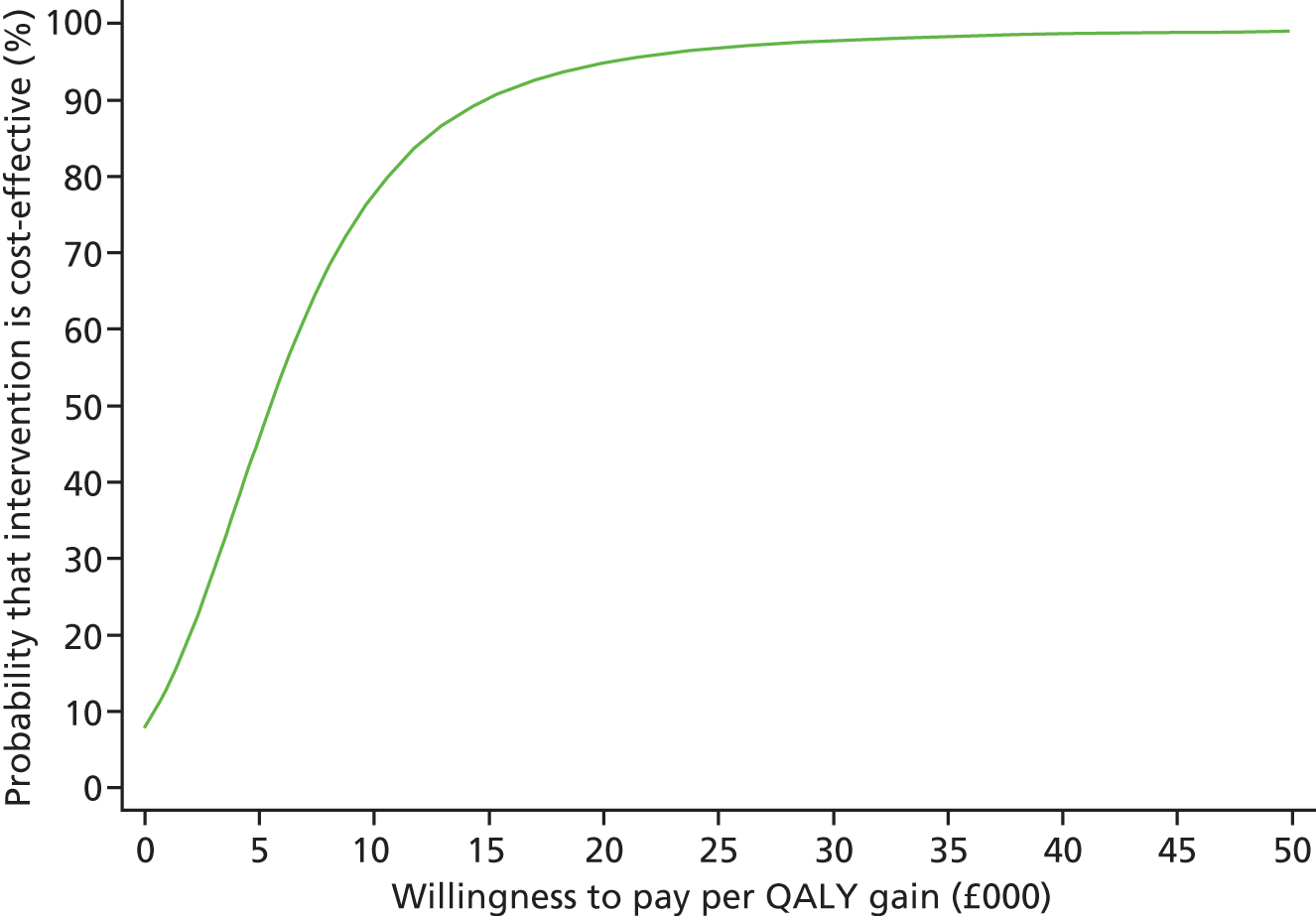
The second sensitivity analysis adjusted for imbalances in baseline characteristics. The results were again quite similar to those from the primary analyses and are summarised in the second column of figures in Table 11. The mean ICER values from this further analysis are £5756 per additional QALY, and £112 per 1-point difference in HADS-T score.
| Measure | Missing at 12 months, n (%) | Missing at 24 months, n (%) | ||
|---|---|---|---|---|
| Randomised group | Randomised group | |||
| TAU | Intervention | TAU | Intervention | |
| HADS-A | 20 (23) | 35 (20) | 23 (26) | 41 (24) |
| HADS-D | 20 (23) | 35 (20) | 23 (26) | 41 (24) |
| HADS-T | 20 (23) | 35 (20) | 23 (26) | 41 (24) |
| MCTS total | 32 (37) | 59 (34) | 40 (46) | 78 (45) |
| HSQ mental health | 26 (30) | 52 (30) | 32 (37) | 60 (35) |
| QoL-AD total | 34 (39) | 58 (34) | 38 (44) | 77 (45) |
The CEAC with QALY as the outcome measure is shown in Figure 6. At the lower-bound NICE threshold of £20,000, the START intervention has a 93% likelihood of being seen as cost-effective, rising to 98% at the £30,000 threshold.
The CIs around NMB for these two sensitivity analyses are shown in Appendix 2. Taking into account uncertainty in the estimation suggests that a degree of caution should be exercised in concluding that START is necessarily cost-effective.
Long-term outcomes (12 and 24 months)
The CONSORT diagram (Figure 7) shows the flow of participants through the study up to the 2-year follow-up. The primary outcome (HADS-T score) was missing for 55 (21.2%) patients at 12 months and for 64 (24.6%) patients at 24 months. Two hundred and nine (80.4%) patients had HADS-T data for at least one of the long-term follow-up points, and so could be included in the primary analyses.
FIGURE 7.
The CONSORT flow diagram for long-term outcomes (12 and 24 months). a, To be included in the primary long-term analysis, the individual must have at least one score available for the HADS at 12 or 24 months. Those excluded have no measurements at 12 or 24 months.
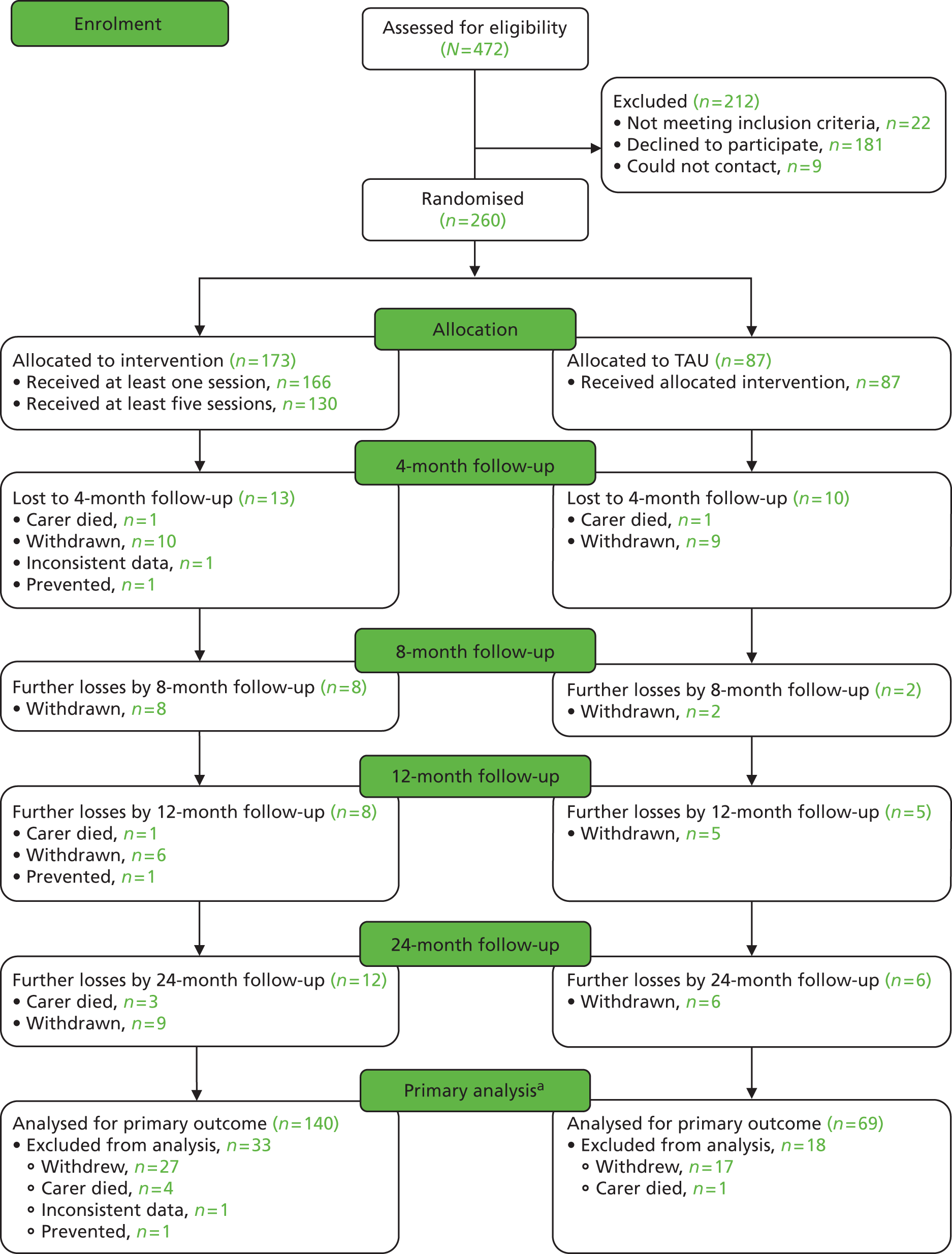
Missing outcomes
The proportion of missing data for the main trial outcomes is given in Table 11 by group and follow-up time.
Clinical outcomes
Primary and secondary clinical outcomes are shown in Table 12. This summarises average scores at months 12 and 24 and gives the estimated effect of therapy compared with TAU for primary and secondary outcomes.
| Measure | TAU | Intervention | Adjusted for baseline score and centre, difference (95% CI) | Adjusted also for carer’s age, sex, NPI and Zarit, difference (95% CI) | ||
|---|---|---|---|---|---|---|
| 12 months, mean (SD) | 24 months, mean (SD) | 12 months, mean (SD) | 24 months, mean (SD) | |||
| HADS-T scores | 14.6 (8.9) (n = 64) |
15.5 (9.5) (n = 64) |
12.5 (7.9) (n = 138) |
13.6 (8.3) (n = 132) |
–1.84 (–3.50 to –0.17)b (n = 209) |
–2.58 (–4.26 to –0.90)b (n = 200) |
| QoL-AD scores | 30.0 (6.4) (n = 53) |
29.4 (7.0) (n = 49) |
30.5 (6.7) (n = 114) |
29.9 (6.7) (n = 95) |
0.16 (–1.30 to 1.63)b (n = 174) |
0.17 (–1.37 to 1.70)b (n = 168) |
| HSQ mental health scores | 56.2 (22.5) (n = 61) |
55.0 (21.2) (n = 55) |
61.9 (20.6) (n = 121) |
60.2 (19.8) (n = 113) |
7.16 (2.72 to 11.60) (n = 189) |
7.47 (2.87 to 12.08)b (n = 183) |
| HADS-A scores | 8.8 (5.1) (n = 67) |
9.2 (5.3) (n = 64) |
7.5 (4.4) (n = 138) |
8.1 (4.9) (n = 132) |
–0.75 (–1.75 to 0.25)b (n = 209) |
–1.16 (–2.15 to –0.18)b (n = 200) |
| HADS-D scores | 5.9 (4.3) (n = 67) |
6.3 (4.9) (n = 64) |
5.0 (4.2) (n = 138) |
5.5 (4.2) (n = 132) |
–1.14 (–2.00 to –0.28)b (n = 209) |
–1.45 (–2.32 to –0.57)b (n = 200) |
| HADS-A case (score of ≥ 9) | 33 (49.3)a (n = 67) |
32 (50.0)a (n = 64) |
54 (39.1)a (n = 138) |
57 (43.2)a (n = 132) |
0.53 (0.24 to 1.16)c (n = 209) |
0.57 (0.26 to 1.24)c (n = 200) |
| HADS-D case (score of ≥ 9) | 18 (26.9)a (n = 67) |
19 (29.7)a (n = 64) |
24 (17.4)a (n = 138) |
30 (22.7)a (n = 132) |
0.22 (0.05 to 0.96)c (n = 209) |
0.14 (0.04 to 0.53)c (n = 192) |
| MCTS (at least one item with score of ≥ 2) | 21 (38.2)a (n = 55) |
11 (23.4)a (n = 47) |
41 (36.0)a (n = 114) |
28 (29.5)a (n = 95) |
0.96 (0.42 to 2.19)c (n = 176) |
0.83 (0.36 to 1.93)c (n = 171) |
Analysis of HADS-T scores, adjusting for trust and baseline score and for factors related to outcome (carer age and sex, NPI and Zarit scores), showed a mean difference of –2.58 points (95% CI –4.26 to –0.90 points; p = 0.003) in favour of the intervention. If the model did not include factors relating to outcome then the results were similar, with an average decrease in score of –1.84 (95% CI –3.50 to –0.17; p = 0.03). The therapist ICC at 12 months was 0.00 (95% CI 0.00 to 0.07) and at 24 months was 0.00 (95% CI 0.00 to 0.07).
Sensitivity analyses adjusting for significant demographic and clinical predictors of missing values, namely patient living with carer and COPE dysfunction score, still gave a result with significant difference between groups (difference in means –2.69, 95% CI –4.39 to –0.98; p = 0.002; n = 200), as did sensitivity analyses adjusting for factors imbalanced at baseline (carer work, carer education, patient education, relationship with carer, lives with carer; difference in means –2.37, 95% CI –4.11 to –0.63, p = 0.008).
Models including an interaction with time showed no evidence of a differential effect of the intervention between the 4- and 8-month time points or between the 12- and 24-month time points for HADS-T score (p = 0.92) and indicated a similar long-term treatment effect.
Model assumptions were examined by checking plots assessing the normality of the residuals and also plotting residuals versus fitted values. These were done for residuals at both the individual and the therapist cluster level. These plots did not indicate concerns about the model fit.
Secondary outcomes
Results of models considering the long-term effect of intervention on secondary outcomes are also given in Table 12.
Depression and anxiety caseness on the HADS
From the adjusted models there was a significant reduction in the odds of HADS-D cases in the intervention group compared with TAU, with an odds seven times higher for the TAU group (OR 0.14, 95% CI 0.04 to 0.53). Similarly, there was some evidence for a reduction in odds of HADS-A caseness (OR 0.57, 95% CI 0.26 to 1.24).
Anxiety and depression symptoms
Adjusted models for the individual HADS-A and HADS-D continuous scales indicated significant beneficial effects of the intervention at 24 months, with average decreases in scores of –1.16 (–2.15 to –0.18; n = 200) and –1.45 (95% CI –2.32 to –0.57), respectively.
Models including an interaction with time showed no evidence of a differential effect of the intervention between the 4- and 8-month time points or between the 12- and 24-month time points for HADS-A or HADS-D (p = 0.99 and p = 0.86, respectively).
Carer (Health Status Questionnaire) and care recipient (Quality of Life-Alzheimer’s disease) quality of life
There was no significant difference between groups for the person with dementia’s overall QoL (QoL-AD). The HSQ mental health scale for the carer did, however, indicate significantly higher average scores and hence improved mental health (mean difference 7.47, 95% CI 2.87 to 12.08; n = 183).
Models including an interaction with time showed no evidence of a differential effect of the intervention between the 4- and 8-month time points or between the 12- and 24-month time points for either QoL-AD or HSQ mental health (p = 0.24 and p = 0.14, respectively).
Time to care home admission
Two hundred and fifty-nine (87 in TAU and 172 in the intervention group) individuals were included in the analysis. We found that one person with dementia was included twice as two different carers (spouse and daughter) had been included and gave different dates of birth of the patient. The individual was included only once and from the first recruitment date (spouse carer’s recruitment). Both carers had been in the intervention group.
For four people (three in TAU and one in the intervention group), the baseline date and the last known at home date were the same; thus, they were censored at time = 0 days.
Of the remaining 84 in the TAU group, 17 (20.2%) were admitted to a care home, and in the intervention group, 32 (18.7%) of the remaining 171 were admitted, within the 24-month follow-up period.
Figure 8 shows a Kaplan–Meier plot for time until admission to a care home for each randomised group.
FIGURE 8.
Time until admission to a care home for each randomised group over 24 months’ follow-up.
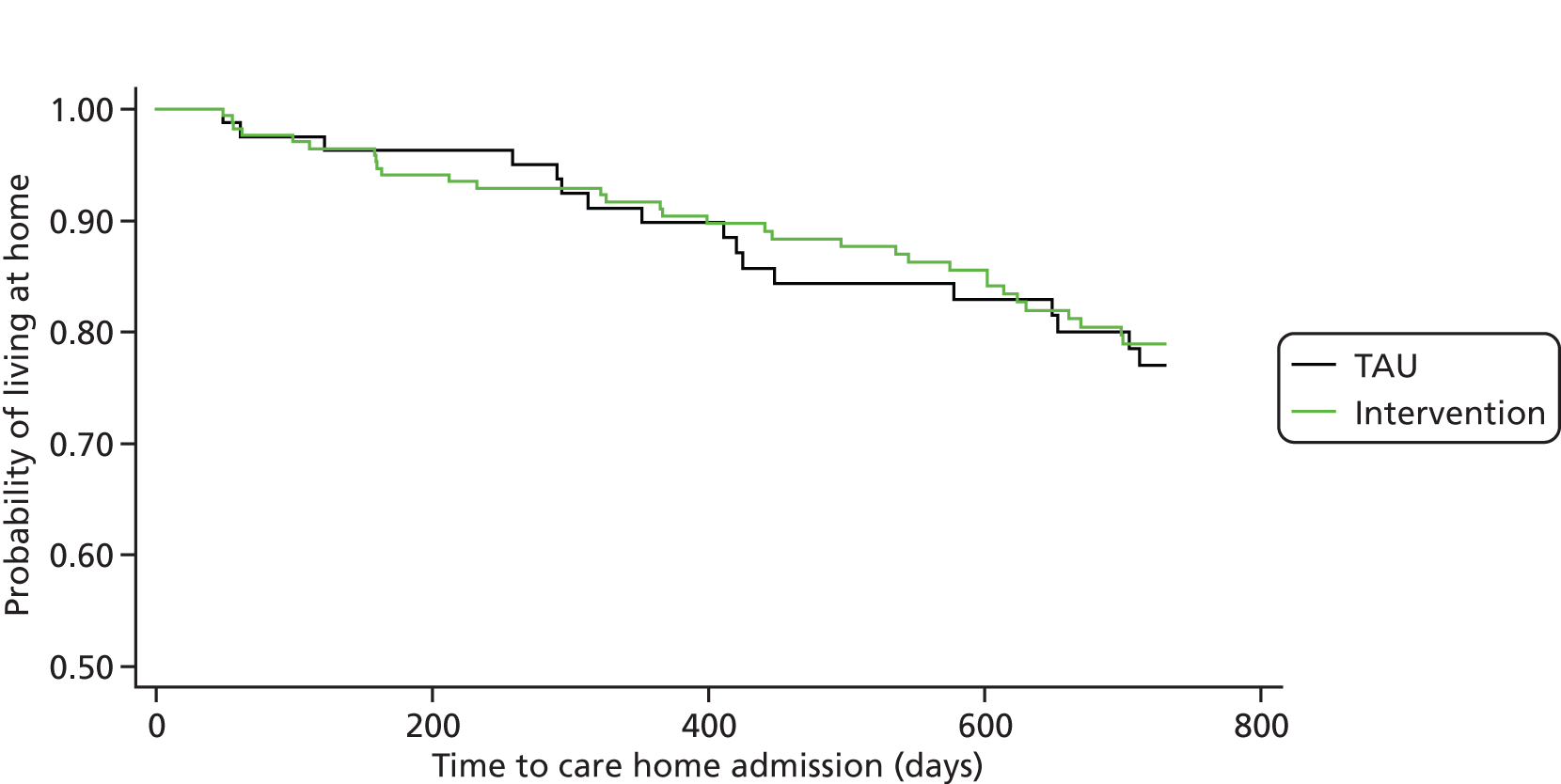
A parametric shared frailty model assuming a gamma distribution for the shared frailty with a Weibull survival distribution was fitted for the time to institutionalisation. The model included trust and randomisation group and additionally adjusted for carer age, carer sex, baseline NPI total and baseline Zarit total. This model included 242 people with dementia. The estimated hazard ratio (HR) was 0.83 with 95% CI 0.44 to 1.56 (p = 0.56), showing no significant evidence of an effect of the intervention compared with TAU. Sensitivity analyses fitted the same model with addition adjustment for baseline imbalances (carer work, carer education, patient education, relationship with carer, lives with carer). This gave HR 0.62 (95% CI 0.31 to 1.23; p = 0.17; n = 232).
Modified Conflict Tactics Scale for significant abuse
There was no evidence for a decrease in abusive behaviour (adjusted OR 0.83, 95% CI 0.36 to 1.93) (Figure 9).
FIGURE 9.
Modified Conflict Tactic Scale for significant abuse over 24 months in intervention and control groups.
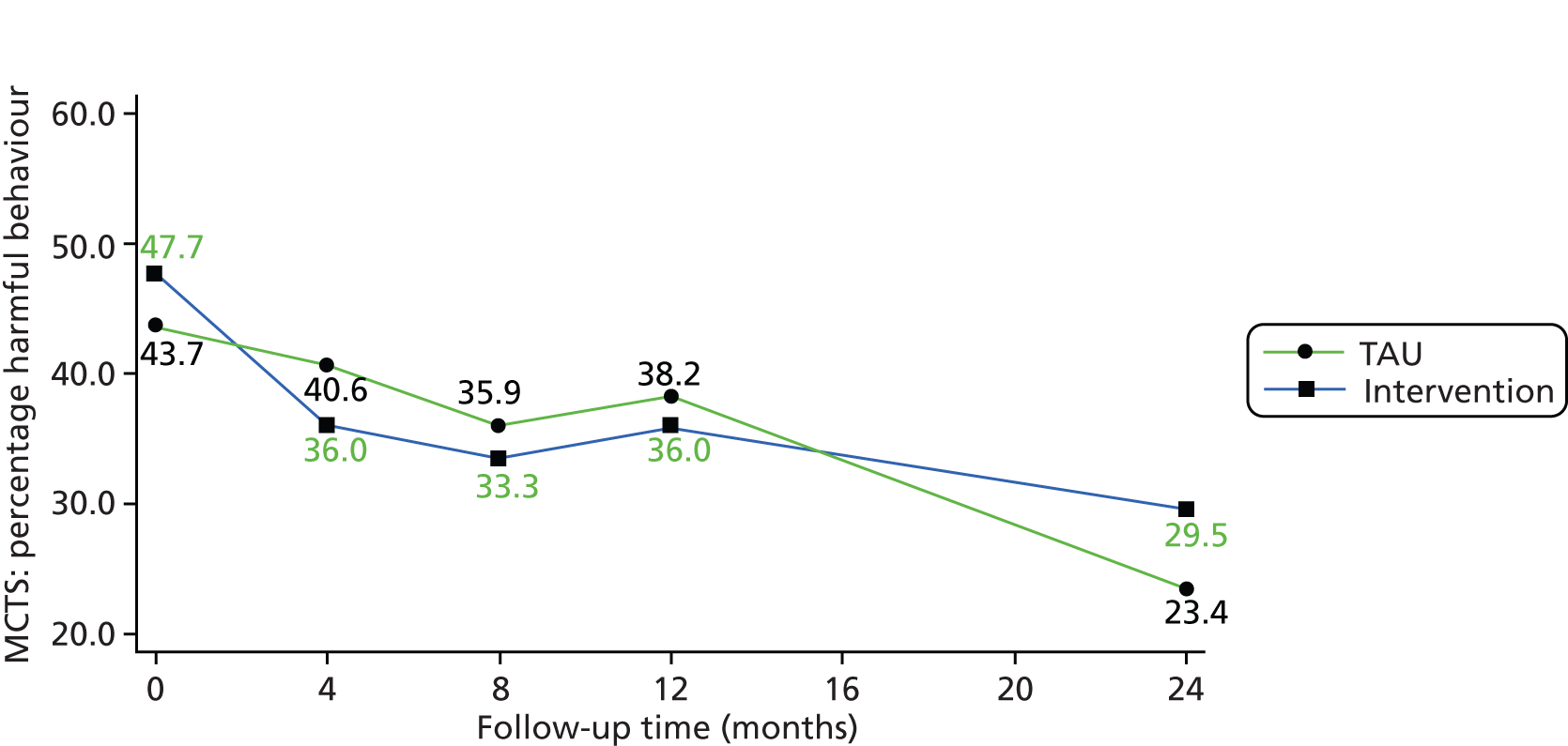
Cost-effectiveness (over 24 months)
Service use and costs
Economic data for the 24-month follow-up period were available for 196 carers (75%): 64 in the TAU group and 132 in the intervention group. The number of carers using services in the 24-month follow-up period is detailed in Table 13. As can be seen in Table 13, carers used a wide range of health and social care services. GP services were used by a high proportion of participants.
| Services | 12 months | 24 months | ||
|---|---|---|---|---|
| TAU (N = 67), % (n) using service | Intervention (N = 138), % (n) using service | TAU (N = 64), % (n) using service | Intervention (N = 132), % (n) using service | |
| Outpatient services | ||||
| Outpatient hospital services | 24.7 (19) | 32.7 (50) | 27.6 (24) | 32.9 (53) |
| Community-based services | ||||
| Admiral Nurse | 3.9 (3) | 3.3 (5) | 1.3 (1) | 1.9 (3) |
| Chiropodist | 6.5 (5) | 11.1 (17) | 6.5 (5) | 8.5 (13) |
| Counsellor | 3.9 (3) | 3.3 (5) | 2.6 (2) | 1.9 (3) |
| Dentist | 28.6 (22) | 27.0 (40) | 23.4 (18) | 31.3 (48) |
| GP | 42.9 (33) | 35.2 (53) | 48.1 (37) | 44.4 (68) |
| Optician | 10.4 (8) | 8.5 (13) | 16.4 (25) | 16.4 (25) |
| Outreach worker | 1.3 (1) | 0.0 (0) | 0.0 (0) | 0.0 (0) |
| Home care worker/care attendant | 0.0 (0) | 0.7 (1) | 3.9 (6) | 0.0 (0) |
| Physiotherapist | 1.3 (1) | 2.1 (3) | 1.4 (2) | 1.4 (2) |
| District nurse | 2.6 (2) | 0.7 (1) | 0.7 (1) | 0.7 (1) |
| Support worker | 0.0 (0) | 0.7 (1) | 0.7 (1) | 0.7 (1) |
| Psychotherapist | 0.0 (0) | 0.7 (1) | 0.0 (0) | 0.7 (1) |
| Occupational therapist | 0.0 (0) | 0.7 (1) | 0.7 (1) | 0.7 (1) |
| Speech and language therapist | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.7 (1) |
| Practice nurse | 1.3 (1) | 0.7 (1) | 0.0 (0) | 3.9 (6) |
| Cardiac nurse | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.7 (1) |
| Ambulance transport | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.7 (1) |
| Social worker | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.7 (1) |
| Other services | 2.6 (2) | 0.7 (1) | 0.0 (0) | 2.7 (3) |
Mean health and social care costs are reported in Table 14. The table distinguishes the START and TAU groups and reports figures for the 4-month period from 9 to 12 months and the 4-month period from 21 to 24 months. In contrast to the outpatient service costs over the 4-month period between baseline and the 4-month assessment and the 4-month period between the 4- and 8-month assessments, outpatient service costs over 9–12 months and 21–24 months were higher in the intervention group as participants in this group used outpatient services more frequently than those in the TAU group.
| Cost categories and outcomes used in the economic evaluationa | TAU, mean (SD) | Intervention, mean (SD) | Intervention vs. TAU over 1–24 months, difference (95% CI) adjusted for baseline variables | ||
|---|---|---|---|---|---|
| 9–12 months | 21–24 months | 9–12 months | 21–24 months | ||
| Costs (£, 2009–10)a | |||||
| Outpatient | 88 (190) | 102 (289) | 90 (154) | 111 (213) | 26 (–117 to 170; n = 176) |
| Community | 143 (336) | 94 (170) | 132 (329) | 108 (144) | –44 (–172 to 84; n = 176) |
| Other | 5 (31) | 0 (0) | 2 (19) | 67 (699) | 67 (–162 to 296; n = 176) |
| Total | 236 (390) | 196 (332) | 224 (373) | 286 (765) | 173 (–115 to 460; n = 175) |
| Outcomes used in the economic evaluationb | |||||
| EQ-5D | 0.81 (0.19) | 0.75 (0.18) | 0.79 (0.23) | 0.72 (0.24) | 0.05 (–0.03 to 0.13; n = 184) |
| HADS-T scoresa | 14.6 (8.9) | 14.4 (8.9) | 12.5 (7.9) | 12.7 (7.7) | –2.45 (–4.01 to –0.90; n = 200) |
Table 14 shows the difference in costs between the START and TAU groups across the whole evaluation period of 24 months. To calculate costs over 24 months, we summed costs over the study periods 1–8 months, 9–12 months, 13–20 months and 21–24 months. Costs during the period between the 12- and 21-month data collection time points (on average 8 months) were interpolated from 12- and 24-month data. Mean costs over the study period (1–24 months) were £1492 in the START group and £1578 in the TAU group. After adjustment of baseline characteristics, the standardised difference was £173, with the 95% CI (–£115 to £460) suggesting that there was no significant difference in costs between the two groups. The scores on the two outcome measures used in the economic evaluation are included at the bottom of Table 14.
Mean costs (including the direct costs of intervention and health and social care system costs) for the 24-month period for carers in the START group were higher, although not significantly so, than for carers in the TAU group. The mean cost difference was £336 (95% CI –£223 to £895) for sample members on the EQ-5D and £303 (95% CI –£206 to £812) for sample members on the HADS-T measure (Table 15).
| Measure | Incremental differences (START minus TAU) and ICERs 24-month evaluation time point |
|---|---|
| With QALY as outcome (n = 144)a | |
| Incremental health and social care costs (£, 2009–10), mean (95% CI) | £336 (–£223 to £895) |
| Incremental QALY gain, mean (95% CI) | 0.03 (–0.01 to 0.06) |
| ICER (£ per QALY) | £11,200 |
| With HADS-T as outcome (n = 156)b | |
| Incremental health and social care costs (£, 2009–10), mean (95% CI) | £303 (–£206 to £812) |
| Incremental HADS-T score change (reversed so higher scores show better outcomes), mean (95% CI) | 1.52 (–0.57 to 3.62) |
| ICER (£ per unit change on HADS-T) | £199 |
Cost-effectiveness ratio
Results from the net benefit regression using the two outcomes examined in the economic evaluation (QALYs and HADS-T score) over 1–24 months are summarised and the ICERs reported in Table 15. Similar to the analyses conducted over the 8-month time period, the costs and outcome differences are obtained after adjustment for baseline characteristics and are influenced by the sample with complete data for each outcome.
Over the 24 months, carers who received the START intervention had slightly, but not statistically significantly, higher total costs and better, although not statistically significant, differences in outcomes whether measured in terms of health-related QoL (QALY) or affective symptoms (HADS-T). We explored the decision-maker’s willingness to pay for gains in QoL and affective symptoms to assess whether or not these results imply that the START intervention is cost-effective. We computed the ICERs and plotted the associated CEACs, and examined the CIs around NMB.
Looking at QALY as the outcome, the mean cost per QALY gain was £11,200. The CEAC is shown in Figure 10. At the £20,000 per QALY threshold associated with NICE recommendations, the probability that the START intervention would be seen as cost-effective was 65%, and at the higher NICE threshold of £30,000 it was 75%.
FIGURE 10.
Cost-effectiveness acceptability curve: START intervention (manual-based coping strategy therapy) vs. TAU; health and social care perspective, with effectiveness measured in QALY gain over 24 months.
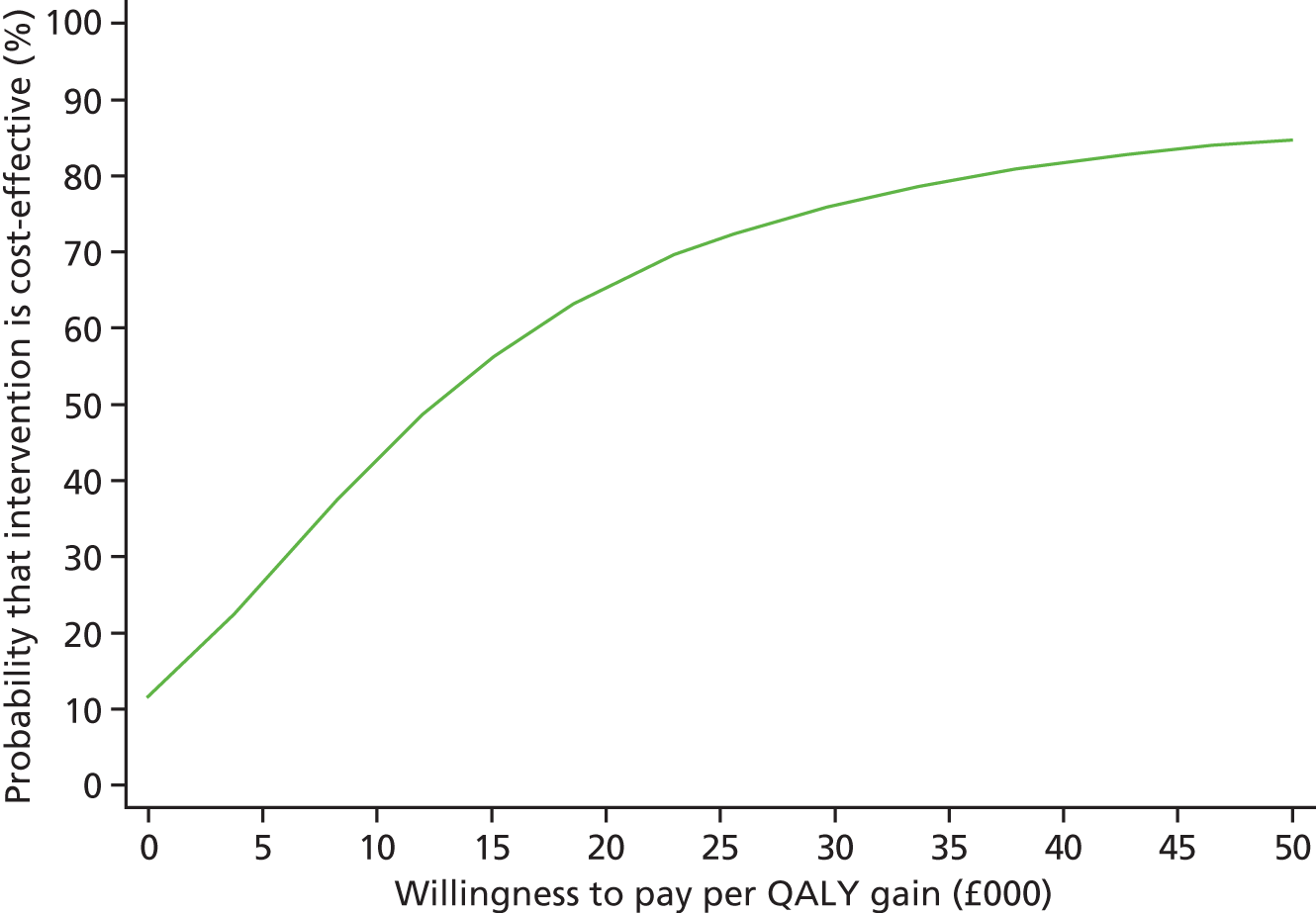
For the other outcome measure, the HADS-T measure of affective symptoms, mean cost per 1-point difference on the HADS-T was £199. The CEAC threshold for this outcome measure is shown in Figure 11. Assuming a willingness to pay of £500, the probability that the START intervention would be seen as cost-effective would be 78%.
FIGURE 11.
Cost-effectiveness acceptability curve: START intervention (manual-based coping strategy therapy) vs. TAU; health and social care perspective, with effectiveness measured in HADS gain over 24 months.
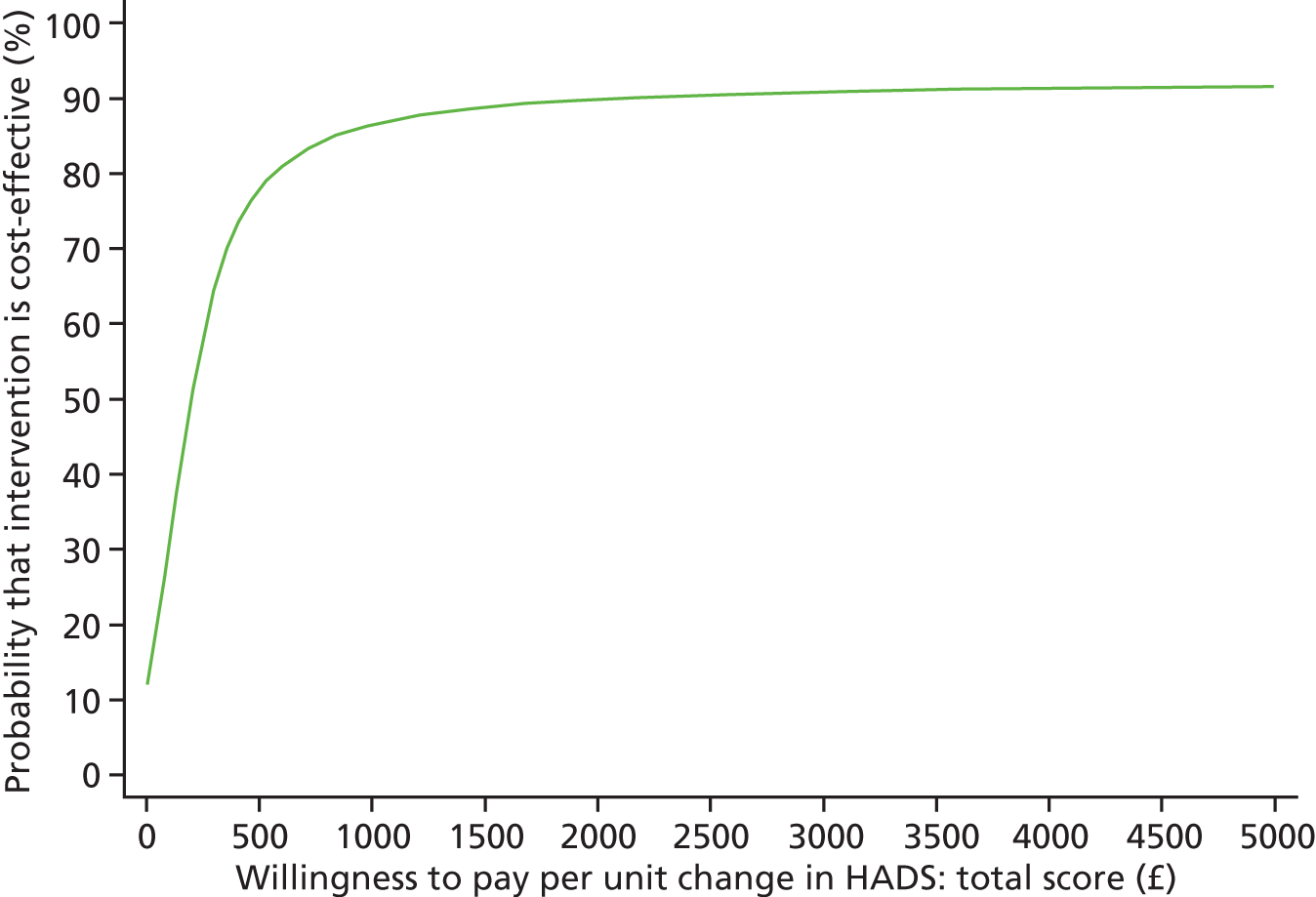
The discounted incremental costs and outcomes at 24 months, at rates of 0% and 6%, are shown in Appendix 1. Discounting the incremental cost and outcomes by 0% and 6% did not greatly alter the findings.
Sensitivity analysis
We followed the same approach as in the 8-month analysis to assess the robustness of the findings of the cost-effectiveness analysis at 24 months. In the first sensitivity analysis, we adjusted for significant differences on demographic and clinical predictors of missing values. This analysis did not alter the results and is summarised in Table 16. The mean ICER values from this analysis are £9767 per additional QALY and £125 per 1-point difference in HADS-T score.
| Measure | Incremental differences (START minus TAU) and ICERs over 24-month evaluation time period | |
|---|---|---|
| Adjusting for significant demographic and clinical predictors of missing values | Adjusting for baseline imbalances | |
| With QALY as outcome (n = 144)a | ||
| Incremental health and social care costs (£, 2009–10), mean (95% CI) | £293 (–£277 to £862) | £257 (–£333 to £847) |
| Incremental QALY gain, mean (95% CI) | 0.03 (–0.01 to 0.06) | 0.03 (–0.01 to 0.06) |
| ICER (£ per QALY) | £9767 | £8567 |
| With HADS-T as outcome (n = 156)b | ||
| Incremental health and social care costs (£, 2009–10), mean (95% CI) | £252 (–£294 to 799) | £282 (–£265 to £828) |
| Incremental HADS-T score change, mean (95% CI) | 2.02 (–0.06 to 4.08) | 1.35 (–0.95 to 3.64) |
| ICER (£ per unit change on HADS-T) | £125 | £209 |
Figure 12 shows the CEAC with QALY as the measure of outcome. The START intervention has an approximately 67% likelihood of being seen as cost-effective at the lower bound of NICE threshold of £20,000, rising to 75% at the £30,000 threshold.
FIGURE 12.
Cost-effectiveness acceptability curve for START with QALY as the outcome measure over 24 months.
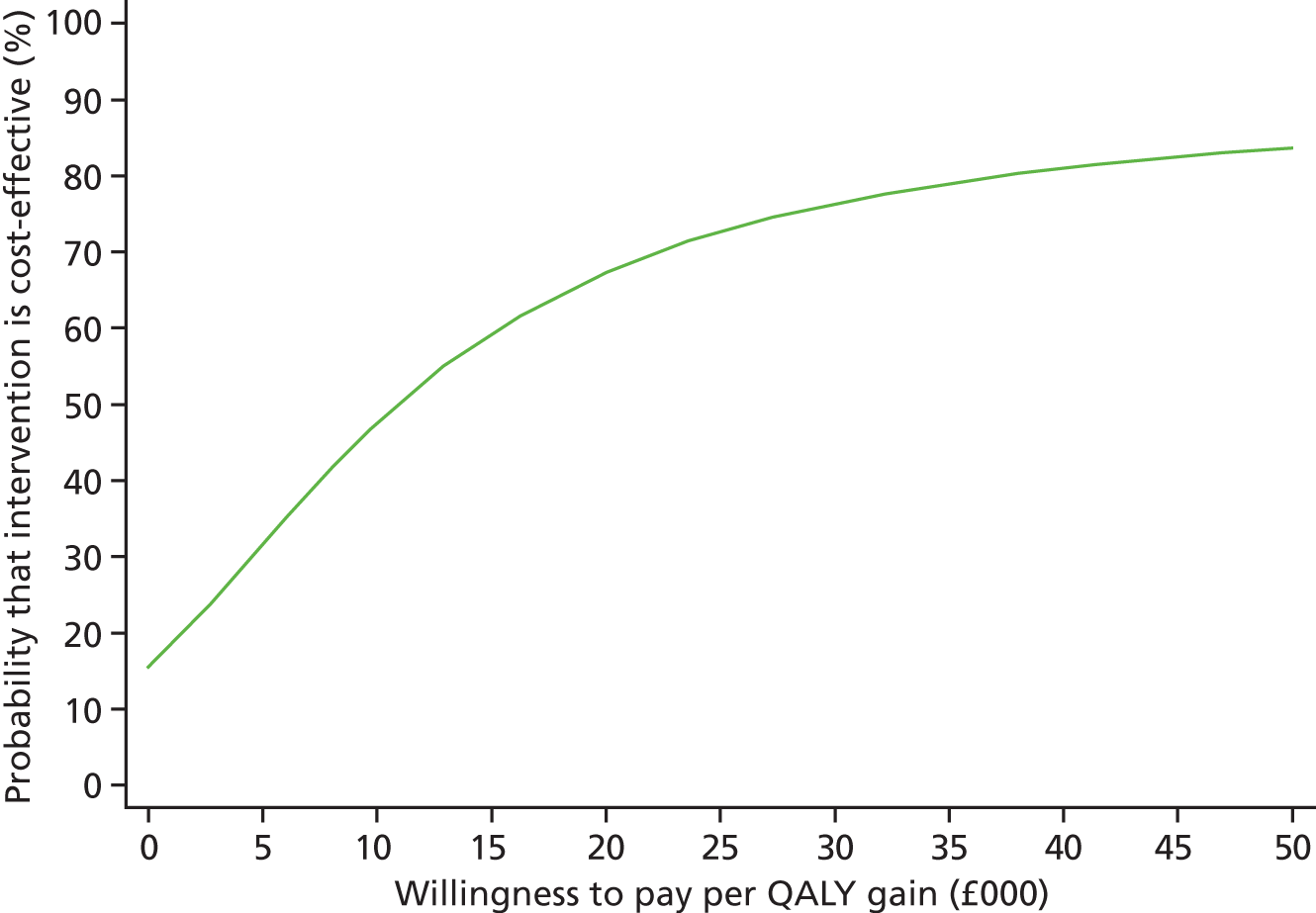
Figure 13 shows the CEAC with HADS-T score as the measure of outcome. The START intervention has an approximately 89% likelihood of being seen as cost-effective at £500. The likelihood of cost-effectiveness is above 95% if the willingness to pay for a 1-point improvement in outcome is > £1000.
FIGURE 13.
Cost-effectiveness acceptability curve for START with HADS-T as the outcome measure over 24 months.
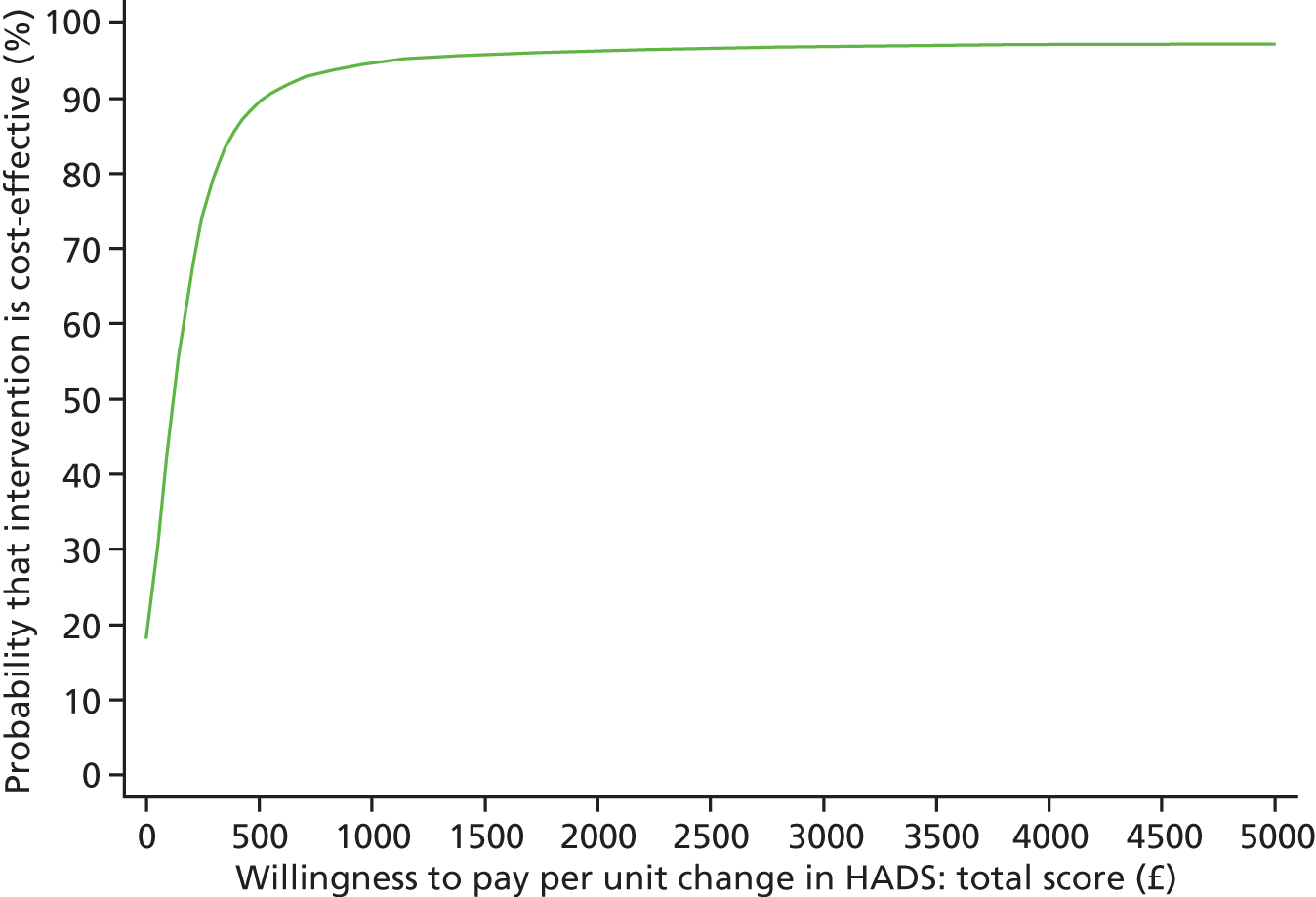
In the second sensitivity analysis adjusted for imbalances in baseline characteristics, the findings were similar to those from the primary analyses. The results are shown alongside those of the first sensitivity analysis in Table 16. The mean ICER values from this second sensitivity analysis are £8567 per additional QALY and £209 per 1-point difference in HADS-T score.
The CEAC with QALY as the outcome measure is shown in Figure 14. At a threshold of £20,000, the START intervention has a likelihood of being seen as cost-effective of 70%, rising to 80% at the £30,000 threshold.
FIGURE 14.
Sensitivity analysis of CEAC with QALY as the outcome measure over 24 months.
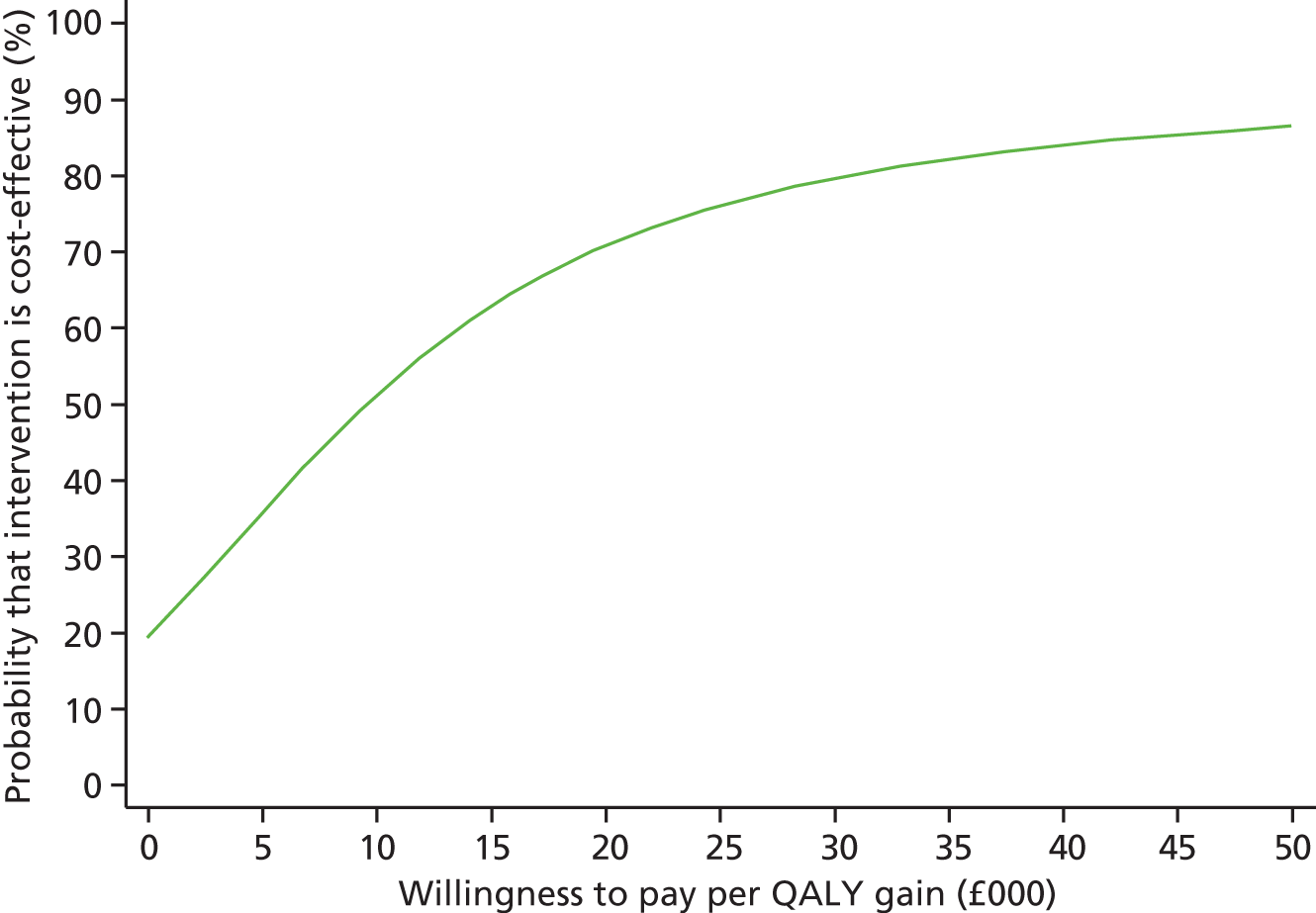
Figure 15 shows the CEAC with HADS-T score as the measure of outcome. This second sensitivity analysis adjusting for factors associated with imbalances in baseline characteristics slightly increased the likelihood that the START intervention would be seen as cost-effective. In this sensitivity analysis, the START intervention has an approximately 72% likelihood of being seen as cost-effective at £500 and remained above 82% if the willingness to pay for a 1-point improvement in outcome is over £1000.
FIGURE 15.
Sensitivity analysis of CEAC with HADS-T as the outcome measure over 24 months.
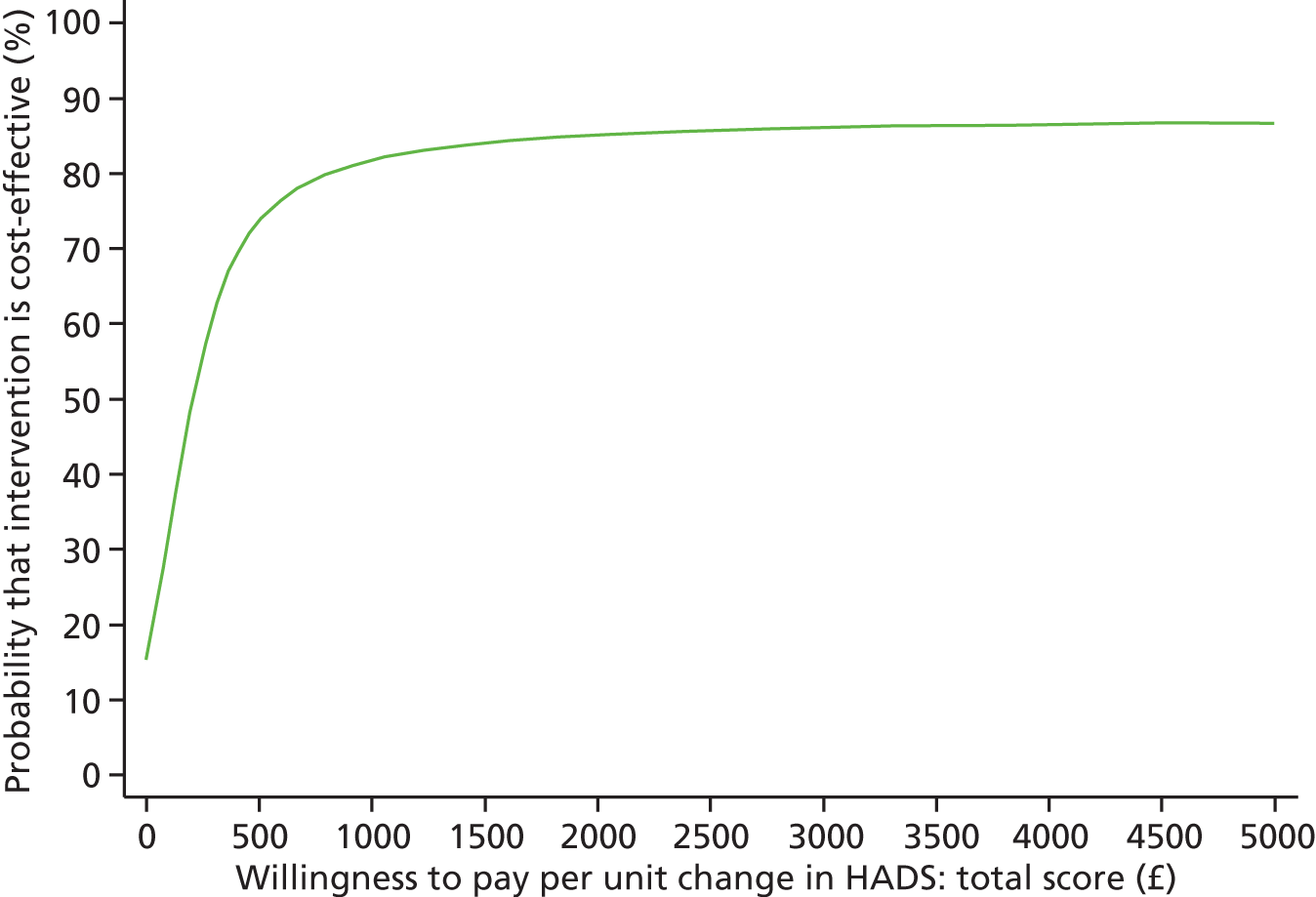
The CIs around NMB for these two sensitivity analyses are shown in Appendix 2.
Chapter 6 Discussion
Key findings
Primary and secondary outcome of clinical effectiveness
This study was a pragmatic RCT of a multicomponent intervention. It is the first study to show that family carers of people with dementia referred to secondary or tertiary care benefit from a structured psychological intervention delivered by psychology graduates and supervised by a clinical psychologist. This change was significant in terms of both depression and anxiety symptoms and also in QoL and depression caseness. The intervention was effective 8 months after randomisation and continued to work 2 years later. Abusive behaviour reduced in both groups and there was no significant difference between them. There was an indication of possible reduction in the chances of being admitted to a care home but this was not significant.
Primary and secondary outcome of cost-effectiveness
We examined whether or not eight sessions of manual-based coping strategy therapy delivered over 8–14 weeks by supervised psychology graduates to family carers of people with dementia added to TAU was cost-effective compared with TAU alone. Over the 8-month evaluation period, the START intervention was found to have a high probability of being seen as cost-effective by reference to both primary outcome measures examined, i.e. improvements in carers’ affective symptoms and gains in carers’ health-related QoL. The sensitivity analyses considering alternative approaches to the analysis suggest a more cautious conclusion as to the cost-effectiveness of the START intervention.
Interpretation
The effect size in terms of the total mean affective symptoms was small, but previous evidence from studies in which researchers set out to calculate what a clinically important difference in the HADS would be suggests that treatment effects are in the range that is important to patients. 54 This study, based on consideration of an emotional function and mastery domain from a scale administered at the same times as the HADS, found the minimally important difference in the HADS-T score to be 1.61 and 1.68 for the former and latter domain, respectively.
Incidence of clinical depression increased in the control group but not in the intervention group, and the ORs indicate that, at follow-up at 8 months, those in the TAU group were four times more likely, and at 24 months were five times more likely, to have clinically significant depression, suggesting that the intervention is clinically important. In keeping with this, carer QoL improved.
We thought that in the long term this intervention may also delay care home admission of the person with dementia and, therefore, increase his or her QoL. The short-term follow-up over 8 months did not show this but at 2 years there was an indication that it may tend to delay care home admission and we will reconsider this effect over the following 5 years. Previous studies of information and advice to people with dementia and their families have suggested this can improve the patient’s QoL but not the carer’s psychological symptoms and an intervention which is targeted at the carer may not help the person with dementia to the same extent. 55
This may be the explanation for the lack of effect on care recipients’ QoL. However, QoL is a complex phenomenon and the measure we used is a global one, thus considering finance, housing and physical health in addition to well-being, and this may have made it less likely to change.
The cost-effectiveness finding is driven more by the outcome differences between the groups than by the cost difference; for example, at the 8-month time point, carers in the control group were four times more likely to have clinically significant depression than carers receiving the START intervention.
Our study was effective over time and acceptable to most participants, who made time and space for it despite the commitments of being a carer and, frequently, also being employed or being unwell themselves. There is little evidence of harm with withdrawal from the treatment being at a similar rate to withdrawal from TAU arm, although one carer said that he found the therapy too upsetting and three felt that it was not for them.
There are currently no interventions which have been shown to reduce elder abuse. 56,57 Our study was not powered to find a significant change in abuse and for ethical reasons we made clinicians aware of clinically significant abusive behaviour in the control group; thus, abused carers in this group were often offered clinical and social support as well as monitoring of the behaviour and, if felt appropriate, adult protection measures were taken. Thus, we think it is unlikely that there would be a significant difference between groups. We were pleased that abusive behaviour went down over time in both groups and previous research has not suggested that this is what would happen without intervention. 58 We do not know whether those people who acted in an abusive way were more likely to refuse to participate in the study (although the high rates of abusive behaviour even in relation to prevalence studies make this less likely)59 or to leave the study or if the care recipient was more likely to be admitted to a care home.
Strengths and limitations
Study design
This was designed as a pragmatic study with broad inclusion criteria and followed closely the Medical Research Council guidance on the evaluation of a complex intervention. The clustering of therapists was accounted for by the analysis, and outcome measures were carefully considered for validity and to ensure the participants were not overburdened and an economic evaluation was conducted. The follow-up rates were satisfactory at > 80% and sensitivity analyses adjusting for missing outcome data showed similar results.
Sensitivity analyses
One of the strengths of the study is the sensitivity analyses, which showed the same results as for the primary analysis.
Bias
We took care to minimise bias in several ways. We used standardised measures validated for the population. Although randomisation was independent and follow-up raters were blinded to allocation to avoid bias in allocation or in rater assessment, the family carers inevitably knew which group they were in. Similarly, although the data were entered and cleaned while blind to intervention status, the statistical team were aware that the intervention group was the larger group.
Health economics
Each carer recruited to the study was scheduled to have eight sessions with the therapist but some carers had fewer sessions. Although this was taken into account in calculating the costs of the intervention, the impact that different numbers of sessions might have had on carers’ outcomes was not the focus of this study.
The evaluation was conducted from a health and social care perspective, and concentrated on outcomes experienced by carers. We did not, therefore, measure the costs of treatment and care services used by the individuals with dementia who were being supported by these carers, nor did we attach monetary values to the time spent by carers in providing support to their relatives.
Sample size for the study design was calculated on the basis of the power required to demonstrate differences in one of the effectiveness measures and not on the basis of costs or cost-effectiveness. Although it would have been preferable for the study also to have been powered on an economic variable, this would have required a considerable increase in sample size given that economic measures tend to be highly skewed. In turn this would have had implications for both the research budget and ethics, as it would have been necessary to recruit participants beyond the point at which clinical dominance has been determined. We used CEACs to represents the uncertainty in the estimation of the ICER.
Generalisability
We recruited a sample of carers with varying demographic and clinical characteristics from a range of services. People were only excluded if they had dementia or lived too far away for the therapy to be delivered. Further evidence of external validity is the demographic similarity between those who consented and those who did not. Participants were recruited from three mental health trusts and from a neurology centre for rare dementias and were from urban, suburban and rural areas. The trust differed in geographical location (Essex and London) and in referrers (psychiatrists, neurologists, Admiral Nurses and other mental health professionals).
Participants were in the main children (44%) or partners (42%) of the person with dementia but were also in-laws, nieces and nephews, grandchildren, siblings and friends. The participants were of either sex (31% male), throughout the adult age range (18–89 years) and from a range of ethnic (around three-quarters white UK) and socioeconomic backgrounds. We had thought that those carers in paid work might be unable to access the intervention, but this did not appear to be the case, and carers were working full-time or part-time, or were retired or not currently working.
The carers’ scores on the outcome measures and burden at baseline ranged widely. Most of the people with dementia had relatively mild dementia at baseline, with the mean clinical dementia scale rating being 1.5–1.6, with 1 meaning mild and 2 meaning moderate dementia. Thus, we think that the intervention is generally acceptable to and the results are generally applicable to the family carers around the time people present to secondary care with dementia in the UK. The levels of anxiety and depressive symptoms, case-level anxiety and depression, neuropsychiatric symptoms and carer abusive behaviour, however, were slightly higher than in a recent cohort study of newly referred people with dementia and so those with more problems may have been more likely to consent to the study. 9,60
We did, however, inform the clinical teams about abusive behaviour of the carers in the TAU group when there was no intervention in place and, thus, may have improved the outcome for the TAU.
Implementation of a complex intervention
The intervention was manual based, standardised and supervised, and the high-fidelity ratings and lack of clustering by therapist suggests that the intervention can be delivered consistently. However, our researchers saw most people at their homes and were more flexible in timing than most non-emergency services within the NHS, sometimes seeing people in the evening. The researchers saw 19 out of 173 participants in the evenings or out of hours for their intervention sessions. There was a very satisfactory follow-up rate of 88.1% overall at 8 months with similar rates in both arms. At 2 years, the follow-up rate was still over 80%. The instruments were validated and standardised. These results suggest that our findings are valid.
Adherence to the intervention
Over three-quarters of the participants fulfilled our definition of compliance to the intervention. We found it difficult to deliver the therapy to people who did not speak English, although there were only four of those carers in the study, three of whom were in the intervention group. In retrospect, we did not allow enough time and budget to translate the whole manual and deliver the therapy with translators. It is, thus, difficult to determine the effect of using the manual through a translator. As translating the manual is a one-off process, the cost should be less of an issue if our findings are implemented in the NHS.
We have not been able to consider how many sessions were necessary to effect change as those people who did not finish the sessions were different from those who did, often stopping through their own illness or that of a family member. In addition, most people did all the sessions, and so the numbers are small.
Overall evidence
Earlier studies of similar interventions in the USA have shown results consistent with ours. The study from which we derived START uses a similar intervention and it also alleviated depressive symptoms in carers, but we judge that our intervention is more practical for many carers as we did not require them all to come to a group at the same time. 17 There is no other rigorous study of an intervention delivered by psychology graduates without previous clinical training, a group who are relatively available and inexpensive but with a background in understanding the concepts. Within the USA, a similar therapy to ours delivered to individuals was found to be significantly cost-effective in completers compared with controls in terms of freeing up caring time. 21 We are not aware of any other interventions in this group for which health economic evaluations have been undertaken and we discuss this below.
Not all psychosocial interventions in family carers of people with dementia are effective. 15 Recent RCTs of psychosocial interventions in Europe (UK, Denmark and Norway) using different models have been ineffective in terms of carer psychological symptoms and QoL, thus showing that our findings were not explained solely by the offer of a therapist to spend time and attention. 24,61,62
The earliest study of the three used trained ex-carers in a voluntary sector befriending intervention and was not taken up by many carers. 24 This suggests that acceptability, as well as the theoretical background, needs to be a major focus of any intervention. Danish Alzheimer’s Intervention Study (DAISY) was a multifaceted intervention involving both the person with mild Alzheimer’s disease and the carer. 61 The intervention was individualised to the dyad, who were seen both individually and together by a nurse for counselling, based on validating the participants and focusing on retained and positive attributes and skills. It allowed patient and carer to tell their own life story and explain what mattered to them. Carers and those cared for also attended separate courses over a period of 1 year to educate them about dementia. Like our study, it did not improve the patient’s QoL. Unlike ours, it did not help the carer’s QoL. In contrast to the former study, 72% of participants completed the intervention according to the study’s definition (attending three or more of individual and educational sessions). This suggests that the content of the intervention, as well as its acceptability in terms of take-up, is also important. The intervention contrasts with our study in validating the carer’s and care recipient’s position. Ours was more focused on accepting difficulties, changing the carer’s thoughts and behaviour, and the carer finding ways to look after themselves, communicate their needs, increase pleasant events and find help. The most recent study was also aimed at the carer and the person with dementia. 62 It was delivered by trained and supervised nurses and occupational therapists and families and comprised education about dementia in two half-day sessions, five individual counselling sessions when the therapist worked with them using a problem-solving model, and six group meetings of 2 hours each, concentrating on problem-solving and increasing pleasant events. Again, while the pleasant events were similar to our model, the focus on problem-solving rather than acceptance differed.
Our earlier studies have found that family carers tend to become more anxious and depressed over time without intervention, and that this is associated with an increase in abusive behaviour, and thus we included carers who were not depressed at presentation to services. 63,64 The preventative effect that was found highlights that these carers can benefit from early intervention. This contrasts with a study of cognitive–behavioural interventions in patients with hip fracture which was unsuccessful in preventing depression. 65
Comparison of short- and long-term effects
The START intervention was effective in the short and long term (2 years); there was no difference between the clinical effects at either time period. There is a paucity of literature which considers how long it takes for a psychological intervention to work and the period for which it continues to work. Previous research in carers had shown that interventions continue to work for an average of 7–11 months. 66 One previous intervention with spouse carers of people with dementia with flexible content which included behavioural management of difficult behaviour, promoting communication among family members and staff availability to help with emergent behaviours and problems, found there was no significant effect at 4 months, but there was an effect at 1 year which was sustained for 3 years after the intervention. 67
We thought that it was possible either that the effect of the intervention might wear off over time as carers resorted to earlier coping strategies or that carers might continue to change their coping strategies and the difference between groups would increase over time. Neither of these seemed to be the case (at least alone) and there is no evidence of a ‘top-up’ being necessary to maintain the effect.
Structured psychological treatment of depression in other groups
A recent structured intervention in family carers of people with stroke with care competencies taught and assessed by nurses did not improve their mood, QoL or burden, or improve patient outcome. 15 This is further evidence that knowledge by itself is not efficacious.
Previous work has found that a RCT of telephone-administered psychotherapy for depressive symptoms worked over the 16 weeks it was administered, but was no longer significantly efficacious at 1 year. 68 Similarly, a RCT of a ‘coping with depression’ psychoeducational group programme, with booster session up to 4 months after the intervention had finished, was effective in reducing depression at 6 months but not at 12 months. 69
In contrast, a RCT of CBT as an adjuvant therapy for treatment-resistant depression found that it was effective at the 6- and 12-month follow-up. 70 Ten therapist-delivered sessions of CBT over the internet were effective in reducing depression at 4 and 8 months (equivalent to our short-term measures). 71 CBT treatment for health anxiety was effective at 6 months, 1 and 2 years with no significant increase in costs for anxiety and depression, but was ineffective in increasing health-related QoL. 72
We are unsure what the difference is between the therapies that continue to be efficacious for affective symptoms and those that do not, but our intervention adds to the evidence that therapies can continue to be effective over years.
Comparison with other health economics studies
There is little previous evidence on the cost-effectiveness of psychosocial interventions for carers of people with dementia. A recent review found some evidence that such interventions could lead to greater improvements in outcome and also generate cost savings. 20 However, only one of the studies covered by that review employed a similar therapeutic approach to the START intervention. 21 It examined the cost-effectiveness of a modular multicomponent intervention delivered in carers’ homes, with three sessions by telephone, supplemented by five group sessions (five or six carers in each) delivered by telephone. Focusing on hours of care-giving, the authors found a significant difference over the 6-month study period, with carers in the intervention group having more time to dedicate to activities unrelated to caregiving, which has potentially positive impacts on emotional well-being and QoL.
Chapter 7 Conclusions
Implications for clinical practice
The START intervention is clinically effective in terms of carer impacts in the short term (8 months) and long term (2 years) both for mood and for QoL.
Many countries, including the UK, face rapidly growing numbers of single people over the coming decades, while policy frameworks continue to assume that families will remain the frontline providers of (unpaid) care and support. Most people with dementia also prefer to receive support from family members. In these circumstances, an intervention that is cost neutral, even over a relatively short period, and which significantly improves carer mental health and QoL, should be made more widely available.
From these results, it would appear that the intervention is also likely to be perceived as cost-effective by reference to NICE thresholds; there is, therefore, both a clinical and an economic case for supporting carers of people with dementia using such an approach. This cost-effectiveness advantage arises because the intervention improved carer outcomes while not significantly increasing overall costs, with the additional cost of the intervention being partly counterbalanced by a reduction in service-related costs.
Our group has developed training and the manual is available online with the British Medical Journal papers from this study and so it would be possible to disseminate this further to other memory services to offer START as part of the routine management of dementia. In Camden and Islington, Memory Services and Improving Access to Psychological Therapies services are piloting it. 73 Further follow-up will consider longer-term effects on carer mood, QoL, abusive behaviour and cost-effectiveness, and whether or not, as in other longer-term studies, patient’s time to care home admission has been lengthened. 74
Unanswered questions and future research
This study reports carer outcomes and that there was no evidence of a change in the magnitude of effectiveness between 4 and 8 months, or at 12 months and 2 years, thus suggesting some lasting effect. We have no data after 2 years. However, what we have is more than in many antidepressant trials, and further analysis of the effectiveness of the intervention compared with antidepressants and the calculation, if possible, of standardised effect size would help to put it in context.
One previous study found therapy led to improvement in spouse carer mental health over many years and a reduction in nursing home admissions for patients. 74 Patients whose spouses received the intervention experienced a 28.3% reduction in the rate of nursing home placement compared with usual care controls, with a difference in model-predicted median time to placement of 557 days. Improvements in carers’ satisfaction with social support, response to patient behaviour problems and symptoms of depression collectively accounted for 61.2% of the intervention’s beneficial impact on placement. We do not yet know whether or not our short therapeutic intervention will lead to a long-term reduction in carer nursing home placement but are extending this study to find out. It may be that the inclusion of non-carers (and, therefore, more often non-resident carers) means these outcomes differ.
Further qualitative research is needed to consider whether or not all of the intervention components were valued by family carers, whether or not it was delivered at the appropriate time and how it could be improved; we have now done this and our findings suggest that different carers use different components and that a multimodel intervention is therefore appropriate. 75 Future analysis should consider mechanism of action, in terms of both coping strategies and freeing up carer time.
In addition, it is important to consider the effects on people with dementia in terms of clinical outcome (cognition, neuropsychiatric symptoms) and on health and social care costs. A cost-effectiveness analysis considering the costs for both carers and care recipients, and the clinical and QoL effectiveness for them both would be very informative and may add to the policy implications. In addition, we wish to analyse the effect on abuse further and in particular the interaction between abuse and care home admission as abuse may lead to admission and this may prevent further abuse.
Finally, the purpose of research is implementation of clinically effective and cost-effective interventions in clinical practice and we have successfully applied for an implementation grant from the Alzheimer’s Society, which includes assessing the implementation.
Acknowledgements
We would like to thank the participating carers, the CIFT, UCL Hospital, the NELFT, the NEPFT and, in particular, Vincent Kirchner and Lisa Gee for referring many patients. We would like to thank the members of the Steering Committee – Joanna Murray (chairperson), Thana Balamurali, Kate Maxmin, Lynne Ramsay, Mabel Saili, Lynis Lewis – and of the Data Monitoring Committee – Cornelius Katona (chairperson) and U Hla Htay. Shirley Nurock gave advice throughout as an expert family carer. The START research team acknowledges the support of the National Institute for Health Research through the DeNDRoN.
We would like to thank the researchers/therapists Monica Manela, Ryan Li, Ruth Shanley, Amy Waugh, Lynsey Kelly, Allana Austin, Peter Keohane, Shilpa Bavishi, Amanda Shulman and Jonathan Bradley collected and entered the data and implemented the manual. Barbara Schehl calculated the costs of services.
Contributions of authors
Gill Livingston is a professor of older people’s mental health; Julie Barber is a lecturer in medical statistics; Penny Rapaport is a principal clinical psychologist; Martin Knapp is a professor of social policy; Mark Griffin is a lecturer in medical statistics; Renee Romeo is a lecturer in health economics; Derek King is a research fellow; Debbie Livingston is a trial manager; Elanor Lewis-Holmes is a research assistant; Cath Mummery is a consultant neurologist and honorary senior lecturer; Zuzana Walker, is a reader in psychiatry of the elderly; Juanita Hoe is a senior clinical research associate; and Claudia Cooper is a clinical senior lecturer.
Gill Livingston, Claudia Cooper, Juanita Hoe, Zuzana Walker, Debbie Livingston, Cath Mummery, Martin Knapp, Elanor Lewis-Holmes and Penny Rapaport contributed to the conception and design of the study.
Gill Livingston, Claudia Cooper, Juanita Hoe, Zuzana Walker, Julie Barber and Mark Griffin contributed to the analytic plan.
Julie Barber and Mark Griffin analysed the clinical data.
Renee Romeo, Martin Knapp and Derek King analysed the health economic data.
Gill Livingston, Claudia Cooper, Zuzana Walker, Juanita Hoe and Cath Mummery led recruitment from their trusts.
Gill Livingston drafted most of the report.
Renee Romeo drafted the health economic section.
Elanor Lewis-Holmes drafted some of the method.
Julie Barber, Penny Rapaport, Martin Knapp, Mark Griffin, DK, Debbie Livingston, Cath Mummery, Zuzana Walker, Juanita Hoe, Elanor Lewis-Holmes and Claudia Cooper revised it critically for important intellectual content and gave final approval of the version to be published.
Gill Livingston will act as guarantor.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Ferri CP, Prince M, Brayne C, Brodaty H, Fratiflioni L, Ganguli M, et al. Global prevalence of dementia: a Delphi consensus study. Lancet 2005;366:2112-17. http://dx.doi.org/10.1016/S0140-6736(05)67889-0.
- Knapp M, Prince M, Albanese E, Banerjee S, Dhanasiri S, Fernández JL, et al. Dementia UK. London: Alzheimer’s Society; 2007.
- Living Well with Dementia: A National Dementia Strategy. London: Department of Health; 2009.
- Burns A, Robert P. The National Dementia strategy in England. BMJ 2009;338. http://dx.doi.org/10.1136/bmj.b931.
- Cooper C, Balamurali TBS, Selwood A, Livingston G. A systematic review of intervention studies about anxiety in caregivers of people with dementia. Int J Geriatr Psychiatry 2007;22:181-8. http://dx.doi.org/10.1002/gps.1656.
- Mahoney R, Regan C, Katona C, Livingston G. Anxiety and depression in family caregivers of people with Alzheimer disease: the LASER-AD study. Am J Geriatr Psychiatry 2005;13:795-801. http://dx.doi.org/10.1097/00019442-200509000-00008.
- Morris LW, Morris RG, Britton PG. The relationship between marital intimacy, perceived strain and depression in spouse caregivers of dementia sufferers. Br J Med Psychol 1988;61:231-6. http://dx.doi.org/10.1111/j.2044-8341.1988.tb02784.x.
- Gallagher D, Ni Mhaolain A, Crosby L, Ryan D, Lacey L, Coen RF, et al. Determinants of the desire to institutionalize in Alzheimer’s caregivers. Am J Alzheimers Dis Other Demen 2011;26:205-11. http://dx.doi.org/10.1177/1533317511400307.
- Cooper C, Selwood A, Blanchard M, Walker Z, Blizard R, Livingston G. The determinants of family carers’ abusive behaviour to people with dementia: results of the CARD study. J Affect Disord 2009;121:136-42. http://dx.doi.org/10.1016/j.jad.2009.05.001.
- Selwood A, Johnston K, Katona C, Lyketsos C, Livingston G. Systematic review of the effect of psychological interventions on family caregivers of people with dementia. J Affective Disord 2007;101:75-89. http://dx.doi.org/10.1016/j.jad.2006.10.025.
- Spijker A, Vernooij-Dassen M, Vasse E, Adang E, Wollersheim, Grol R, . Effectiveness of nonpharmacological interventions in delaying the institutionalization of patients with dementia: a meta-analysis. J Am Geriatr Soc 2008;56:1116-28. http://dx.doi.org/10.1111/j.1532-5415.2008.01705.x.
- Olazarán J, Reisberg B, Clare L, Cruz I, Peña-Casanova J, Del Ser T, et al. Nonpharmacological therapies in Alzheimer’s disease: a systematic review of efficacy. Dement Geriatr Cogn Disord 2010;30:161-78. http://dx.doi.org/10.1159/000316119.
- Hoe J, Katona C, Orrell M, Livingston G. Quality of life in dementia: care recipient and caregiver perceptions of quality of life in dementia: the LASER-AD study. Int J Geriatr Psychiatry 2007;22:1031-6. http://dx.doi.org/10.1002/gps.1786.
- Pinquart M, Sörensen S. Helping caregivers of persons with dementia: which interventions work and how large are their effects?. Int Psychogeriatr 2006;18:577-95. http://dx.doi.org/10.1017/S1041610206003462.
- Thompson CA, Spilsbury K, Hall J, Birks Y, Barnes C, Adamson J. Systematic review of information and support interventions for caregivers of people with dementia. BMC Geriatr 2007;7. http://dx.doi.org/10.1186/1471-2318-7-18.
- Coon DW, Thompson L, Steffen A, Sorocco K, Gallagher-Thompson D. Anger and depression management: psychoeducational skill training interventions for women caregivers of a relative with dementia. Gerontologist 2003;43:678-89. http://dx.doi.org/10.1093/geront/43.5.678.
- Gallagher-Thompson D, Arean P, Rivera P, Thompson LW. A psychoeducational intervention to reduce distress in Hispanic family caregivers. Clin Gerontol 2001;23:17-32. http://dx.doi.org/10.1300/J018v23n01_03.
- Steffen AM. Anger management for dementia caregivers: a preliminary study using video and telephone interventions. Behav Ther 2000;31:281-99. http://dx.doi.org/10.1016/S0005-7894(00)80016-7.
- Gallagher-Thompson D, Coon DW, Solano N, Ambler C, Rabinowitz Y, Thompson LW. Change in indices of distress among Latino and Anglo female caregivers of elderly relatives with dementia: site-specific results from the REACH national collaborative study. Gerontologist 2003;43:580-91. http://dx.doi.org/10.1093/geront/43.4.580.
- Knapp M, Iemmi V, Romeo R. Dementia care costs and outcomes: a systematic review. Int J Geriatr Psychiatry 2013;28:551-61. http://dx.doi.org/10.1002/gps.3864.
- Nichols LO, Chang C, Lummus A, Burns R, Matindale-Adams J, Graney MJ, et al. The cost-effectiveness of a behavior intervention with caregivers of patients with Alzheimer’s disease. J Am Geriatr Soc 2008;56:413-20. http://dx.doi.org/10.1111/j.1532-5415.2007.01569.x.
- Gonyea JG, O’Connor M, Carruth A, Boyle PA. Subjective appraisal of Alzheimer’s disease caregiving: the role of self-efficacy and depressive symptoms in the experience of burden. Am J Alzheimers Dis Other Demen 2005;20:273-80. http://dx.doi.org/10.1177/153331750502000505.
- Coen RF, O’Boyle CA, Coakley D, Lawlor BA. Individual quality of life factors distinguishing low-burden and high-burden caregivers of dementia patients. Dement Geriatr Cogn Disord 2002;13:164-70. http://dx.doi.org/10.1159/000048648.
- Charlesworth G, Shepstone L, Wilson E, Reynolds S, Mugford M, Price D, et al. Befriending carers of people with dementia: randomised controlled trial. BMJ 2008;336:1295-7. http://dx.doi.org/10.1136/bmj.39549.548831.AE.
- The Depression Report. A New Deal for Depression and Anxiety Disorders. London: London School of Economics; 2006.
- Livingston G, Barber J, Rapaport P, Griffin M, King D, Livingston D, et al. Clinical effectiveness of a manual based coping strategy programme (START, STrAtegies for RelaTives) in promoting the mental health of carers of family members with dementia: pragmatic randomised controlled trial. BMJ 2013;347. http://dx.doi.org/10.1136/bmj.f6276.
- Knapp M, King D, Romeo R, Schehl B, Barber J, Griffin M, et al. Cost-effectiveness of a manual based coping strategy programme in promoting the mental health of family carers of people with dementia (the START (STrAtegies for RelaTives) study): a pragmatic randomised controlled trial. BMJ 2013;347. http://dx.doi.org/10.1136/bmj.f6342.
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189-98. http://dx.doi.org/10.1016/0022-3956(75)90026-6.
- Roberts C, Roberts SA. Design and analysis of clinical trials with clustering effects due to treatment. Clin Trials 2005;2:152-62. http://dx.doi.org/10.1191/1740774505cn076oa.
- Livingston G, Leavey G, Manela M, Livingston D, Bavishi S, Shahriyarmolki K, et al. Making decisions for people with dementia who lack capacity: qualitative study of family carers in UK. BMJ 2010;341. http://dx.doi.org/10.1136/bmj.c4184.
- Dementia: Supporting People with Dementia and their Carers in Health and Social Care. London: NICE/SCIE; 2006.
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand 1983;67:361-70. http://dx.doi.org/10.1111/j.1600-0447.1983.tb09716.x.
- Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res 2002;52:69-77. http://dx.doi.org/10.1016/S0022-3999(01)00296-3.
- Spinhoven P, Ormel J, Sloekers PPA, Kempen GIJM, Speckens AEM, VanHemert AM. A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychol Med 1997;27:363-70. http://dx.doi.org/10.1017/S0033291796004382.
- Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist 1980;20:649-55. http://dx.doi.org/10.1093/geront/20.6.649.
- Beach SR, Schulz R, Williamson GM, Miller LS, Weiner MF, Lance CE. Risk factors for potentially harmful informal caregiver behavior. J Am Geriat Soc 2005;53:255-61. http://dx.doi.org/10.1111/j.1532-5415.2005.53111.x.
- Cooper C, Maxmin K, Selwood A, Blanchard M, Livingston G. The sensitivity and specificity of the Modified Conflict Tactics Scale for detecting clinically significant elder abuse. Int Psychogeriatr 2009;21:774-8. http://dx.doi.org/10.1017/S1041610209009387.
- Pettit T, Livingston G, Manela M, Kitchen G, Katona C, Bowling A. Validation and normative data of health status measures in older people: the Islington study. Int J Geriatr Psychiatry 2001;16:1061-70. http://dx.doi.org/10.1002/gps.479.
- Radosevich D, Pruitt M. Twelve-Item Health Status Questionnaire: HSQ-12 Version 2.0. Bloomington, IN: Health Outcomes Institute; 1995.
- EuroQoL Group . EuroQoL – a new facility for the measurement of health-related quality of life. Health Policy 1990;16:199-208. http://dx.doi.org/10.1016/0168-8510(90)90421-9.
- Beecham J, Knapp M, Thornicroft G. Measuring Mental Health Needs. London: Gaskell; 1992.
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308-14. http://dx.doi.org/10.1212/WNL.44.12.2308.
- Logsdon RG, Gibbons LE, McCurry SM, Teri L. Assessing quality of life in older adults with cognitive impairment. Psychosom Med 2002;64:510-9. http://dx.doi.org/10.1097/00006842-200205000-00016.
- Morris JC. Clinical dementia rating: a reliable and valid diagnostic and staging measure for dementia of the Alzheimer type. Int Psychogeriatr 1997;9:173-6. http://dx.doi.org/10.1017/S1041610297004870.
- Barry TL, Kaiser KL, Atwood JR. Reliability, validity, and scoring of the Health Status Questionnaire-12 version 2.0. J Nurs Meas 2007;15:24-35. http://dx.doi.org/10.1891/106137407780851778.
- Walwyn R, Roberts C. Therapist variation within randomised trials of psychotherapy: implications for precision, internal and external validity. Stat Methods Med Res 2010;19:291-315. http://dx.doi.org/10.1177/0962280209105017.
- Snaith RP. The Hospital Anxiety And Depression Scale. Health Qual Life Outcomes 2003;1. http://dx.doi.org/10.1186/1477-7525-1-29.
- Dolan P, Gudex C, Kind P, Williams A. A Social Tariff for EuroQol: Results from a UK Population Survey. Centre for Health Economics, University of York; 1995.
- National Schedule of Reference Costs 2008–09 for NHS Trusts and PCTs Combined. London: Department of Health; 2010.
- Curtis L. Unit Costs of Health and Social Care 2010. Canterbury: PSSRU, University of Kent; 2010.
- Guide to the Methods of Technology Appraisal. London: NICE; 2004.
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159-74. http://dx.doi.org/10.2307/2529310.
- Guide to the Methods of Technology Appraisal. London: NICE; 2008.
- Puhan MA, Frey M, Buchi S, Schunemann HJ. The minimal important difference of the hospital anxiety and depression scale in patients with chronic obstructive pulmonary disease. Health Qual Life Outcomes 2008;6. http://dx.doi.org/10.1186/1477-7525-6-46.
- Corbett A, Stevens J, Aarsland D, Day S, Moniz-Cook E, Woods R, et al. Systematic review of services providing information and/or advice to people with dementia and/or their caregivers. Int J Geriatr Psychiatry 2012;27:628-36. http://dx.doi.org/10.1002/gps.2762.
- Ploeg J, Hutchinson B, MacMillan H, Bolan G. A systematic review of interventions for elder abuse. J Elder Abuse Negl 2009;21:187-210. http://dx.doi.org/10.1080/08946560902997181.
- Cooper C, Selwood A, Livingston G. Knowledge, detection and reporting of abuse by health and social care professionals: a systematic review. Am J Geriatr Psychiatry 2009;17:826-38. http://dx.doi.org/10.1097/JGP.0b013e3181b0fa2e.
- Cooper C, Blanchard M, Selwood A, Walker Z, Livingston G. Family carers’ distress and abusive behaviour: longitudinal study. Br J Psychiatry 2010;196:480-5. http://dx.doi.org/10.1192/bjp.bp.109.071811.
- Cooper C, Selwood A, Blanchard M, Walker Z, Livingston G. The CARD study abuse of people with dementia by family carers. BMJ (Clinical Research Ed) 2009;22:583-6.
- Cooper C, Selwood A, Livingston G. The prevalence of elder abuse and neglect: a systematic review. Age Ageing 2008;37:151-60. http://dx.doi.org/10.1093/ageing/afm194.
- Waldorff FB, Buss DV, Eckermann A, Rasmussen MLH, Keiding N, Rishøj S, et al. Efficacy of psychosocial intervention in patients with mild Alzheimer’s disease: the multicentre, rater blinded, randomised Danish Alzheimer Intervention Study (DAISY). BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e4693.
- Bruvik FK, Allore HG, Ranhoff AH, Engedal K. The effect of psychosocial support intervention on depression in patients with dementia and their family caregivers: an assessor-blinded randomized controlled trial. Dement Geriatr Cogn Dis Extra 2013;3:386-97. http://dx.doi.org/10.1159/000355912.
- Cooper C, Katona C, Orrell M, Livingston G. Coping strategies, anxiety and depression in caregivers of people with Alzheimer’s disease. Int J Geriatr Psychiatry 2008;23:929-36. http://dx.doi.org/10.1002/gps.2007.
- Cooper C, Selwood A, Blanchard M, Walker Z, Blizard R, Livingston G. Abuse of people with dementia by family carers: representative cross sectional survey. BMJ 2009;338. http://dx.doi.org/10.1136/bmj.b155.
- Burns A, Banerjee S, Morris J, Woodward Y, Baldwin R, Proctor R, et al. Treatment and prevention of depression after surgery for hip fracture in older people: randomized, controlled trials. J Am Geriatr Soc 2007;55:75-80. http://dx.doi.org/10.1111/j.1532-5415.2007.01016.x.
- Sörensen S, Duberstein P, Gill D, Pinquart M. Dementia care: mental health effects, intervention strategies, and clinical implications. Lancet Neurol 2006;5:961-73. http://dx.doi.org/10.1016/S1474-4422(06)70599-3.
- Mittelman MS, Roth DL, Coon DW, Haley WE. Sustained benefit of supportive intervention for depressive symptoms in caregivers of patients with Alzheimer’s disease. Am J Psychiatry 2004;161:850-6. http://dx.doi.org/10.1176/appi.ajp.161.5.850.
- Mohr DC, Hart SL, Julian L, Catledge C, Honos-Webb L, Vella L, et al. Telephone-administered psychotherapy for depression. Arch Gen Psychiatry 2005;62:1007-14. http://dx.doi.org/10.1001/archpsyc.62.9.1007.
- Ellison JA, Greenberg LS, Goldman RN, Angus L. Maintenance of gains following experiential therapies for depression. J Consult Clin Psychol 2009;77:103-12. http://dx.doi.org/10.1037/a0014653.
- Wiles N, Thomas L, Abel A, Ridgway N, Turner N, Campbell J, et al. Cognitive–behavioural therapy as an adjunct to pharmacotherapy for primary care based patients with treatment resistant depression: results of the CoBalT randomised controlled trial. Lancet 2013;381:375-84. http://dx.doi.org/10.1016/S0140-6736(12)61552-9.
- Kessler D, Lewis G, Kaur S, Wiles N, King M, Weich S, et al. Therapist-delivered Internet psychotherapy for depression in primary care: a randomised controlled trial. Lancet 2009;374:628-34. http://dx.doi.org/10.1016/S0140-6736(09)61257-5.
- Tyrer P, Cooper S, Salkovskis P, Tyrer H, Crawford M, Byford S, et al. Clinical and cost-effectiveness of cognitive–behaviour therapy for health anxiety in medical patients: a multicentre randomised controlled trial. Lancet 2013;383:219-25. http://dx.doi.org/10.1016/S0140-6736(13)61905-4.
- Improving Access to Psychological Therapies Implementation Plan: National Guidelines for Regional Delivery. London: Department of Health; 2008.
- Mittelman MS, Haley WE, Clay OJ, Roth DL. Improving caregiver well-being delays nursing home placement of patients with Alzheimer disease. Neurology 2006;67:1592-9. http://dx.doi.org/10.1212/01.wnl.0000242727.81172.91.
- Sommerlad A, Manela M, Cooper C, Rapaport P, Livingston G. START (STrAtegies for RelaTives) coping strategy for family carers of adults with dementia: participants’ views about the intervention. BMJ Open 2014. http://dx.doi.org/10.1136/bmjopen-2014-005273.
Appendix 1 Unit cost of services
| Service | Unit cost (£) 2009–10a | Source |
|---|---|---|
| Anticoagulant service (per attendance) | 18 | Department of Health (2010)49 |
| Physiotherapy (per attendance) | 38 | Department of Health (2010)49 |
| Podiatry (per attendance) | 43 | Department of Health (2010)49 |
| Electrocardiography (per attendance) | 45 | Department of Health (2010)49 |
| Ophthalmology (per attendance) | 73 | Department of Health (2010)49 |
| Ear nose and throat (per attendance) | 85 | Department of Health (2010)49 |
| Other adult outpatient services (per attendance) | 90 | Department of Health (2010)49 |
| Dermatology (per attendance) | 93 | Department of Health (2010)49 |
| Diagnostic imaging – CT scan (brain) (per attendance) | 95 | Department of Health (2010)49 |
| Orthopaedics (per attendance) | 99 | Department of Health (2010)49 |
| Urology (per attendance) | 102 | Department of Health (2010)49 |
| Accident and emergency not-leading-to-admitted (per attendance) | 106 | Department of Health (2010)49 |
| Endocrinology (per attendance) | 110 | Department of Health (2010)49 |
| General surgery (per attendance) | 112 | Department of Health (2010)49 |
| Gynaecology (per attendance) | 118 | Department of Health (2010)49 |
| Clinical oncology (per attendance) | 126 | Department of Health (2010)49 |
| Medical gastroenterology (per attendance) | 128 | Department of Health (2010)49 |
| Diabetic medicine (per attendance) | 130 | Department of Health (2010)49 |
| Cardiology (per attendance) | 134 | Department of Health (2010)49 |
| Rheumatology (per attendance) | 138 | Department of Health (2010)49 |
| Respiratory medicine (per attendance) | 148 | Department of Health (2010)49 |
| Haematology (per attendance) | 152 | Department of Health (2010)49 |
| Nephrology (per attendance) | 156 | Department of Health (2010)49 |
| Neurology (per attendance) | 168 | Department of Health (2010)49 |
| Diagnostic imaging – MRI (per attendance) | 175 | Department of Health (2010)49 |
| Acute adult inpatient care (per bed-day) | 295 | Curtis (2010)50 |
| Elderly rehabilitation services (per bed-day) | 296 | Curtis (2010)50 |
| Admiral Nurse (per hour) | 31 | Curtis (2010)50 |
| Chiropodist (per hour) | 22 | Curtis (2010)50 |
| Counsellor (per hour) | 34 | Curtis (2010)50 |
| Dentist (per attendance)b | 87 | Curtis (2010)50 |
| GP (per consultation) | 28 | Curtis (2010)50 |
| NHS direct (per hour) | 3 | Curtis (2010)50 |
| Optician (per hour) | 29 | Curtis (2010)50 |
| Outreach worker (per hour) | 15 | Curtis (2010)50 |
| Home care worker/care attendant (per weekday hour) | 21 | Curtis (2010)50 |
| Physiotherapist (per hour) | 22 | Curtis (2010)50 |
| Psychotherapist (per hour) | 39 | Curtis (2010)50 |
| Hygienist (per hour) | 174 | Department of Health (2010)49 |
| Company medical check-up (per session)c | 175 | Marie Stopes International; www.mariestopes.org.uk/Womens_services/Well_Woman_screening/Company_health_screening.aspx |
| Nurse (advanced) (per hour) | 37 | Curtis (2010)50 |
| Occupational therapist (per hour) | 39 | Curtis (2010)50 |
| Community psychiatrist (per hour) | 22 | Curtis (2010)50 |
| Practice nurse (per hour) | 26 | Curtis (2010)50 |
| District nurse (per hour) | 31 | Curtis (2010)50 |
| Social worker (per hour) | 40 | Curtis (2010)50 |
| Speech and language therapist (per hour) | 22 | Curtis (2010)50 |
| Ambulance transport (per journey)d | 40–246 | Curtis (2010)50 |
| Dietician (per hour) | 22 | Curtis (2010)50 |
| Clinical support worker nursing (per hour) | 15 | Curtis (2010)50 |
| Support group (per hour) | 9 | Curtis (2010)50 |
| Community nurse (per hour) | 31 | Curtis (2010)50 |
| Orthodontist (per hour) | 174 | Curtis (2010)50 |
| Day care: NHS provision (per day) | 66 | Curtis (2010)50 |
| Day care: local authority provision (per day) | 43 | Curtis (2010)50 |
| Day care: voluntary provision (per day) | 42 | Curtis (2010)50 |
| Alzheimer’s café (per hour)e | 6 | Curtis (2010)50 |
| Dementia course (per hour)f | 17 | Dementia UK; http://dementiauk.org/what-we-do/learning-partnerships-and-training/opencources-2011/courses-by-huest-trainers |
| Alternative complementary therapies (per hour) | 40 | Professor Jennifer Beecham, PSSRU, London School of Economics and Political Science, 2012, personal communication |
| Yoga and pilates (per hour) | 7 | www.thetotalcareclinic.co.uk/yoga-pilates-classes-southampton.htm |
Discounted treatment and cost effects and incremental cost-effectiveness ratios over 24 months
| Outcome | Incremental differences and ICERs 24-month evaluation time point (discounted 0%) | Incremental differences and ICERs 24-month evaluation time point (discounted 6%) |
|---|---|---|
| With QALY as outcome (n = 144)a | ||
| Incremental health and social care costs (£, 2009–10), mean (95% CI) | £353 (–£223 to £929) | £325 (–£223 to £874) |
| Incremental QALY gain, mean (95% CI) | 0.03 (–0.01 to 0.06) | 0.03 (–0.01 to 0.06) |
| ICER (£ per QALY) | £11,767 | £10,833 |
| With HADS-T as outcome (n = 156)b | ||
| Incremental health and social care costs (£, 2009–10), mean (95% CI) | £315 (–£210 to £840) | £295 (–£204 to £794) |
| Incremental HADS-T change (reversed so higher scores show better outcomes), mean (95% CI) | 1.63 (–0.61 to 3.88) | 1.45 (–0.55 to 3.45) |
| ICER (£ per unit change on HADS-T) | £193 | £203 |
| Outcome | Incremental differences and ICERs 24-month evaluation time point (discounted 0%) | Incremental differences and ICERs 24-month evaluation time point (discounted 6%) |
|---|---|---|
| With QALY as outcome (n = 144)a | ||
| Incremental health and social care costs (£, 2009–10), mean (95% CI) | £308 (–£277 to £893) | £282 (–£277 to £842) |
| Incremental QALY gain, mean (95% CI) | 0.03 (–0.01 to 0.07) | 0.03 (–0.01 to 0.06) |
| ICER (£ per QALY) | £10,267 | £9400 |
| With HADS-T as outcome (n = 156)b | ||
| Incremental health and social care costs (£, 2009–10), mean (95% CI) | £264 (–£298 to £825) | £245 (–£292 to £782) |
| Incremental HADS-T change (reversed so higher scores show better outcomes), mean (95% CI) | 2.15 (–0.06 to 4.38) | 1.92 (–0.05 to 3.90) |
| ICER (£ per unit change on HADS-T) | £123 | £128 |
| Outcome | Incremental differences and ICERs 24-month evaluation time point (discounted 0%) | Incremental differences and ICERs 24-month evaluation time point (discounted 6%) |
|---|---|---|
| With QALY as outcome (n = 144)a | ||
| Incremental health and social care costs (£, 2009–10), mean (95% CI) | £271 (–£336 to £877) | £248 (–£332 to £828) |
| Incremental QALY gain, mean (95% CI) | 0.03 (–0.01 to 0.06) | 0.03 (–0.01 to 0.06) |
| ICER (£ per QALY) | £9033 | £8267 |
| With HADS-T as outcome (n = 156)b | ||
| Incremental health and social care costs (£, 2009–10), mean (95% CI) | £293 (–£269 to £855) | £274 (–£262 to £810) |
| Incremental HADS-T change (reversed so higher scores show better outcomes), mean (95% CI) | 1.44 (–1.01 to 3.90) | 1.28 (–0.90 to 3.45) |
| ICER (£ per unit change on HADS-T) | £203 | £214 |
Appendix 2 Net monetary benefit graphs
FIGURE 16.
Net monetary benefit line (with 95% CI lines): START intervention vs. TAU health and social care perspective, with effectiveness measured in QALY gain over 8 months.
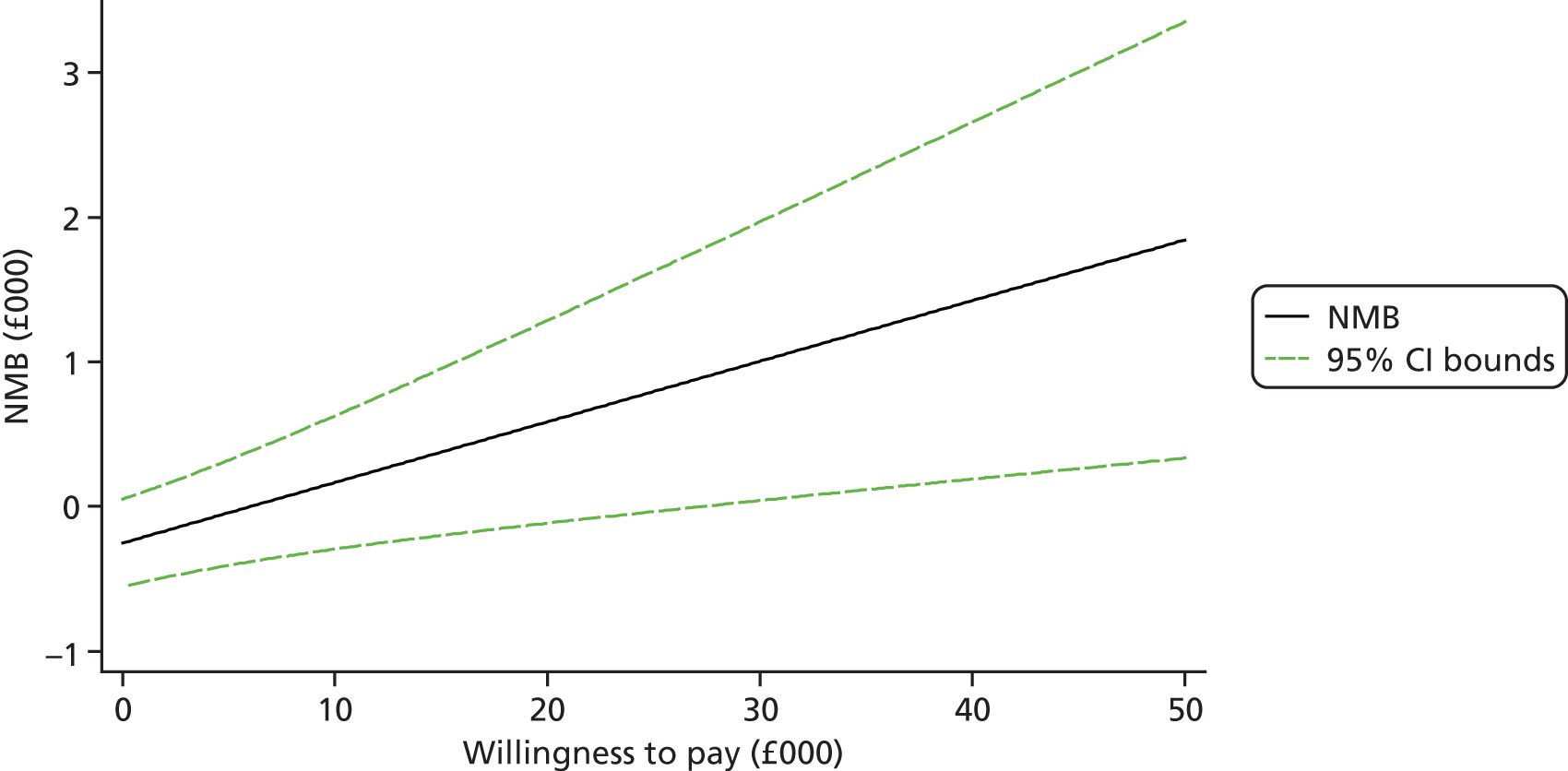
FIGURE 17.
Net monetary benefit line (with 95% CI lines): START intervention vs. TAU; health and social care perspective, with effectiveness measured on HADS-T score over 8 months.
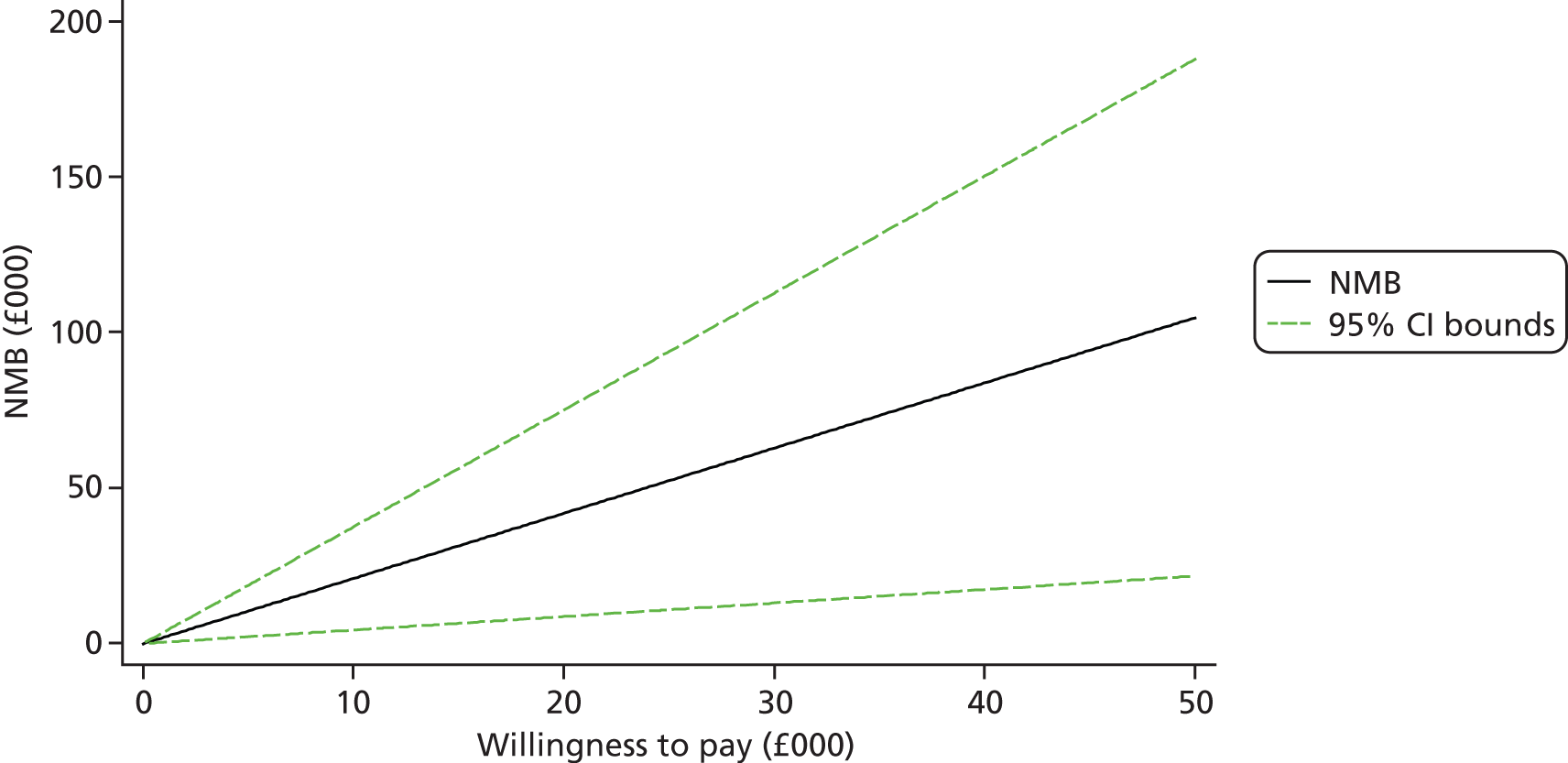
FIGURE 18.
Net monetary benefit line (with 95% CI lines): START intervention vs. TAU; health and social care perspective, with effectiveness measured in QALY gain over 8 months following sensitivity analysis adjusting for significant predictors of missing values.
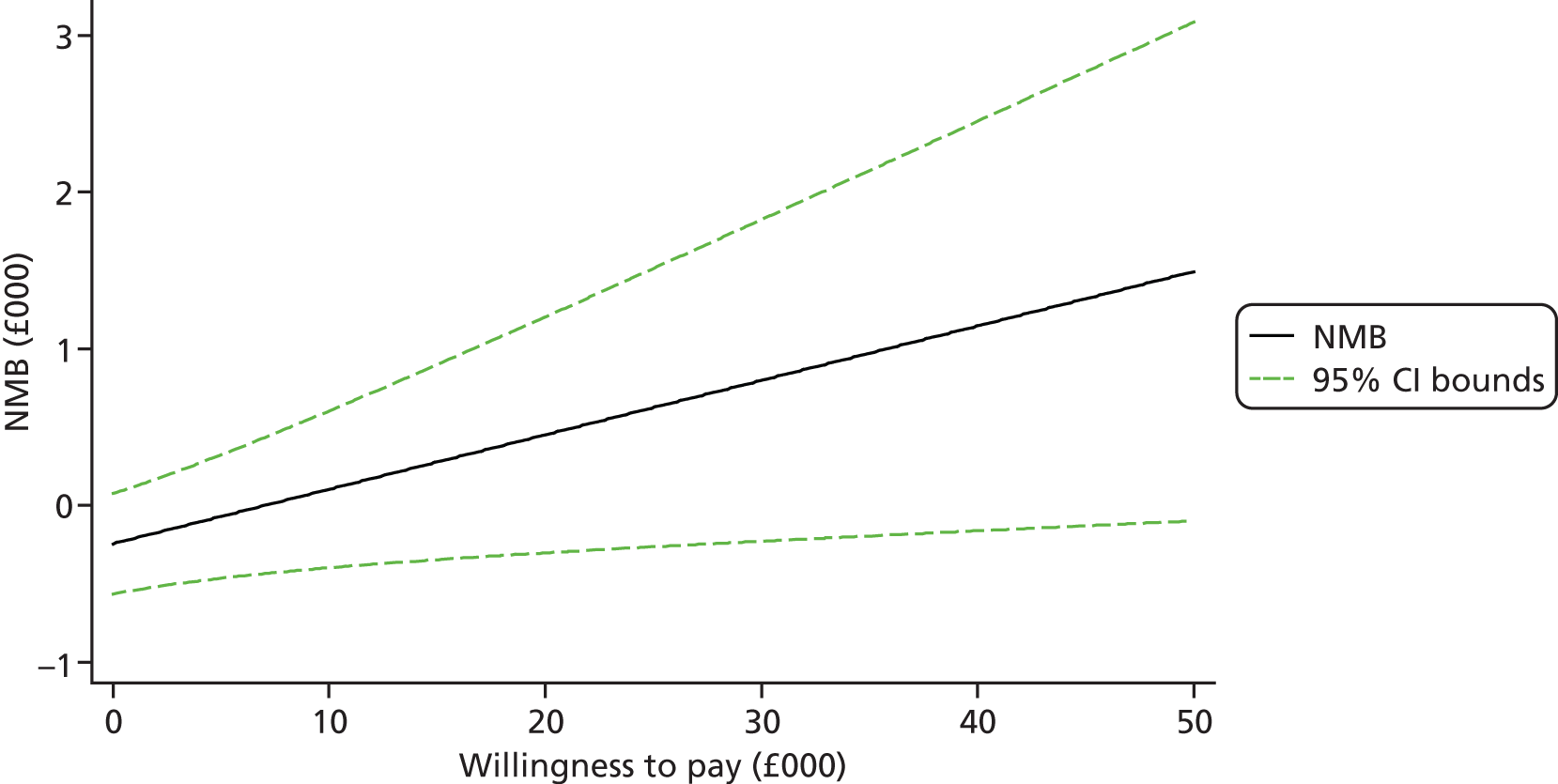
FIGURE 19.
Net monetary benefit line (with 95% CI lines): START intervention vs. TAU; health and social care perspective, with effectiveness measured in QALY gain over 8 months following sensitivity analysis adjusting for baseline imbalances.
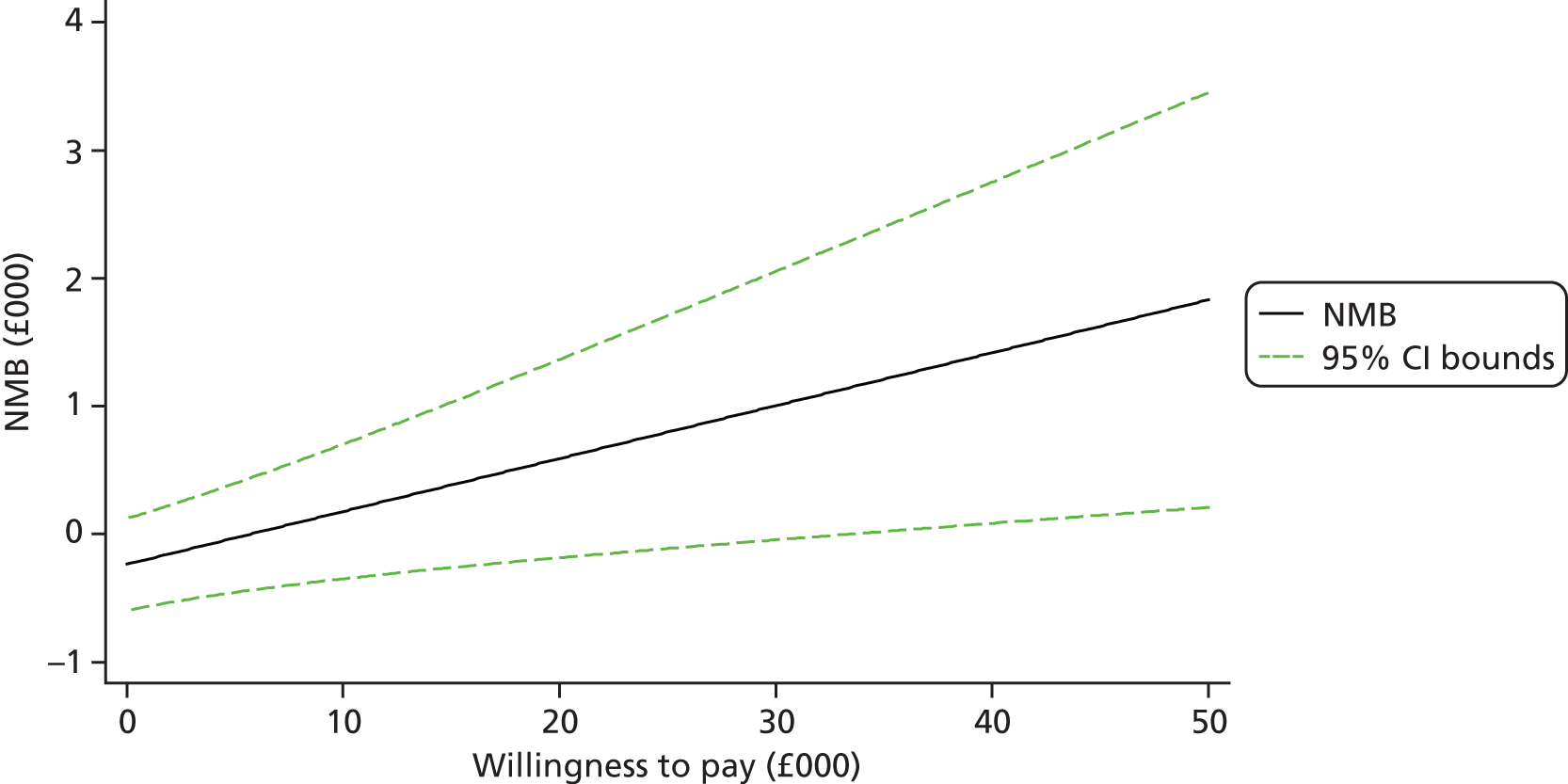
FIGURE 20.
Net monetary benefit line (with 95% CI lines): START intervention vs. TAU; health and social care perspective, with effectiveness measured in QALY gain over 24 months.
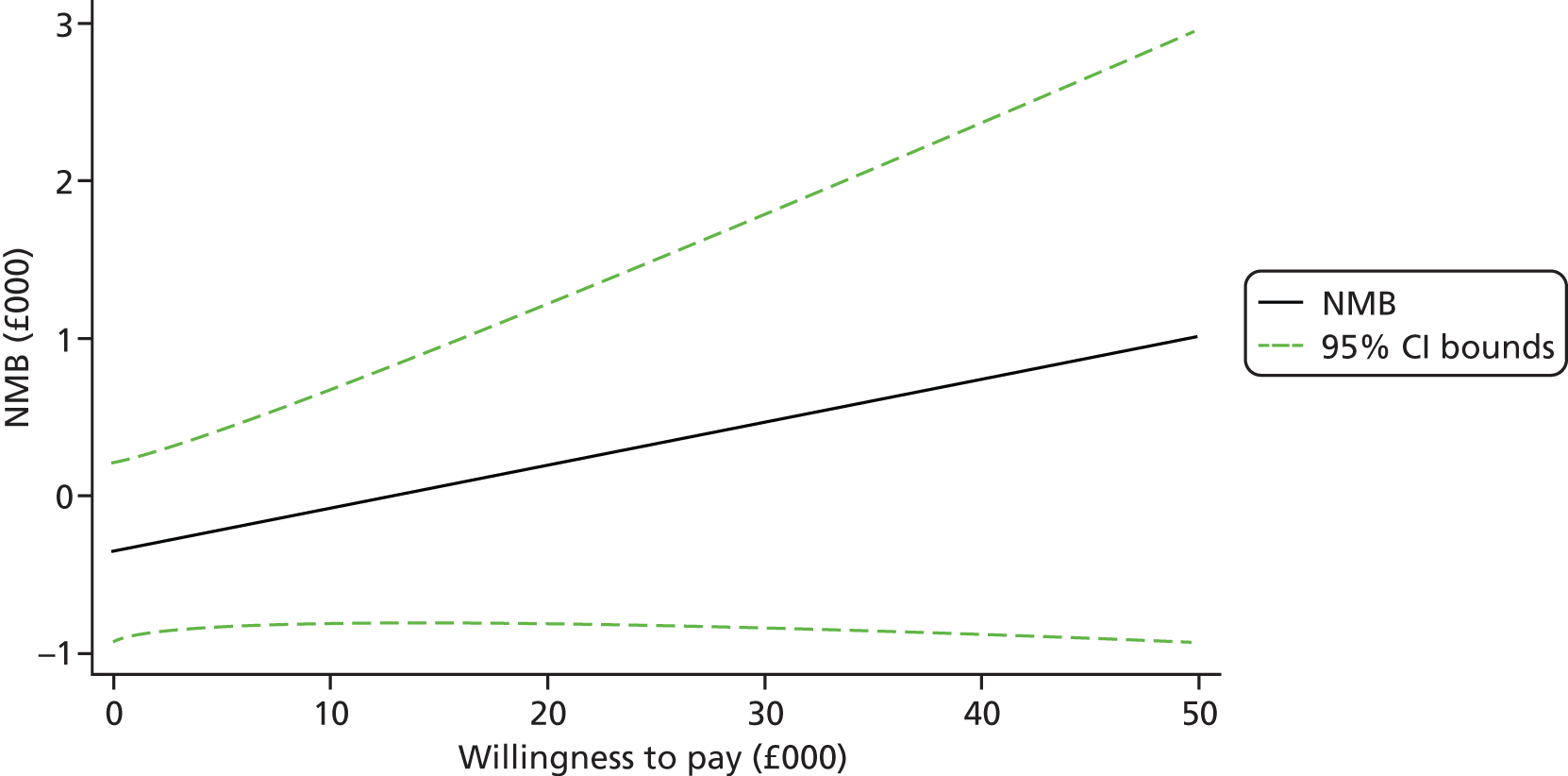
FIGURE 21.
Net monetary benefit line (with 95% CI lines): START intervention vs. TAU; health and social care perspective, with effectiveness measured on HADS-T score over 24 months.
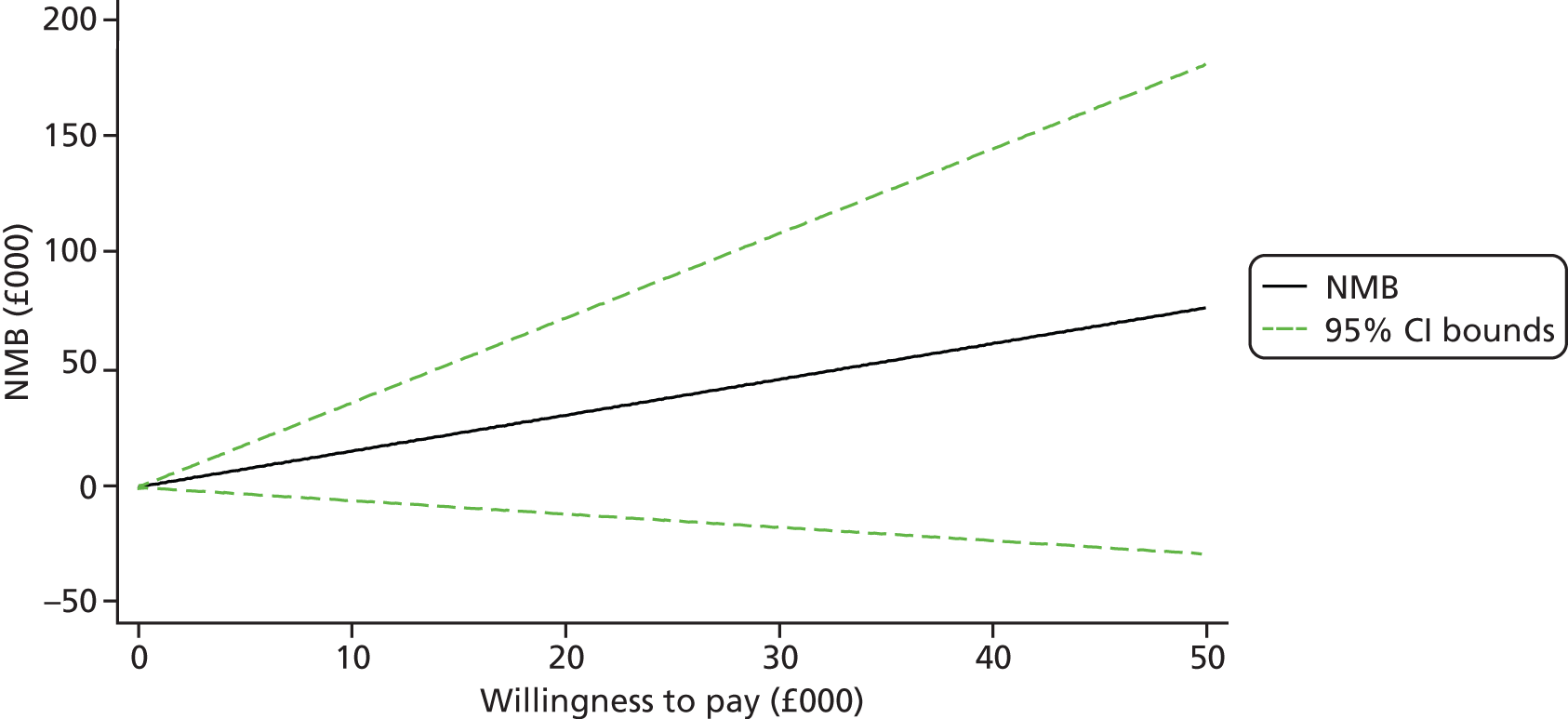
FIGURE 22.
Net monetary benefit line (with 95% CI lines): START intervention vs. TAU; health and social care perspective, with effectiveness measured in QALY gain over 24 months following sensitivity analysis adjusting for significant predictors of missing values.
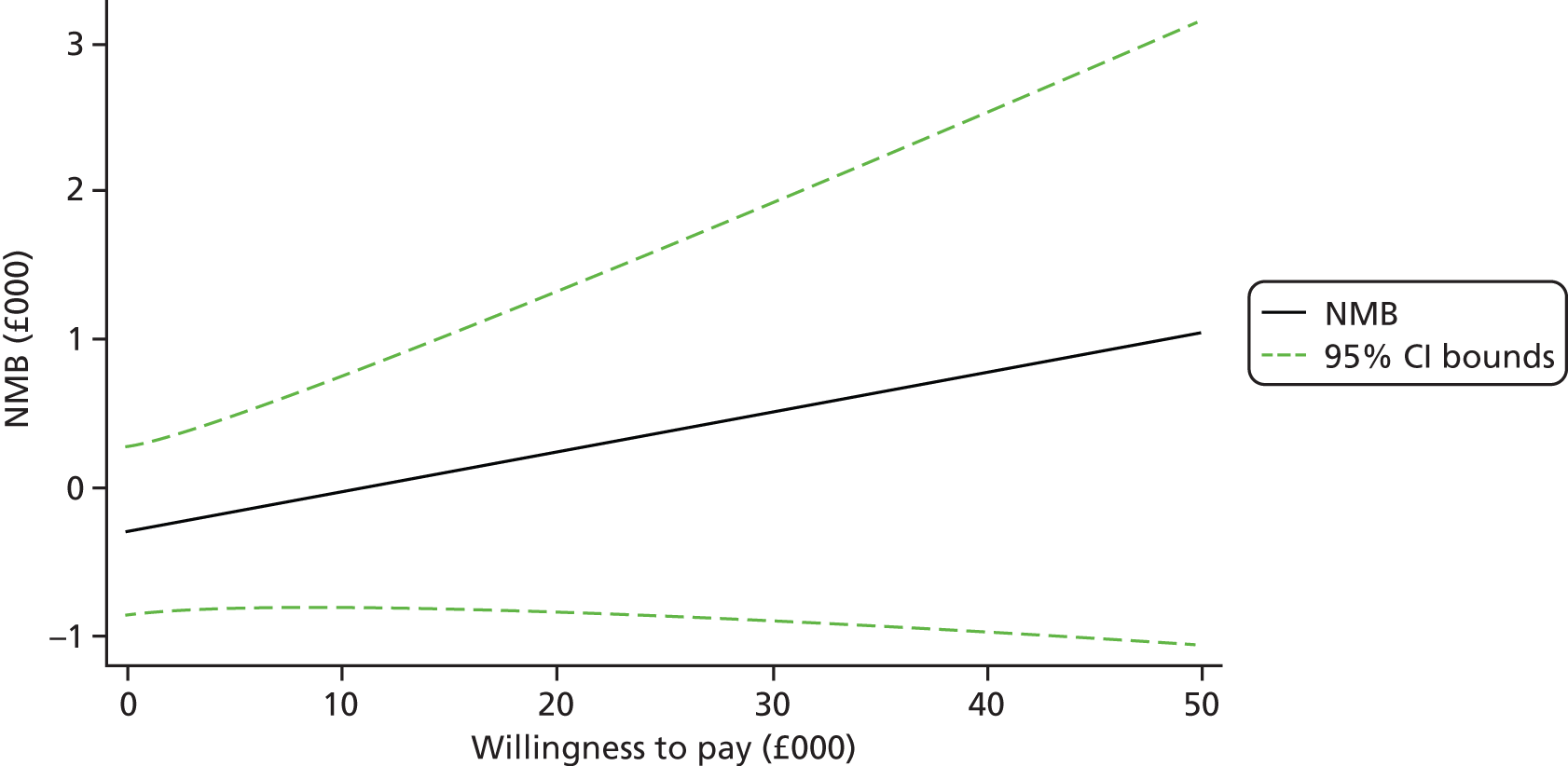
FIGURE 23.
Net monetary benefit line (with 95% CI lines): START intervention vs. TAU; health and social care perspective, with effectiveness measured in QALY gain over 24 months following sensitivity analysis adjusting for baseline imbalances.
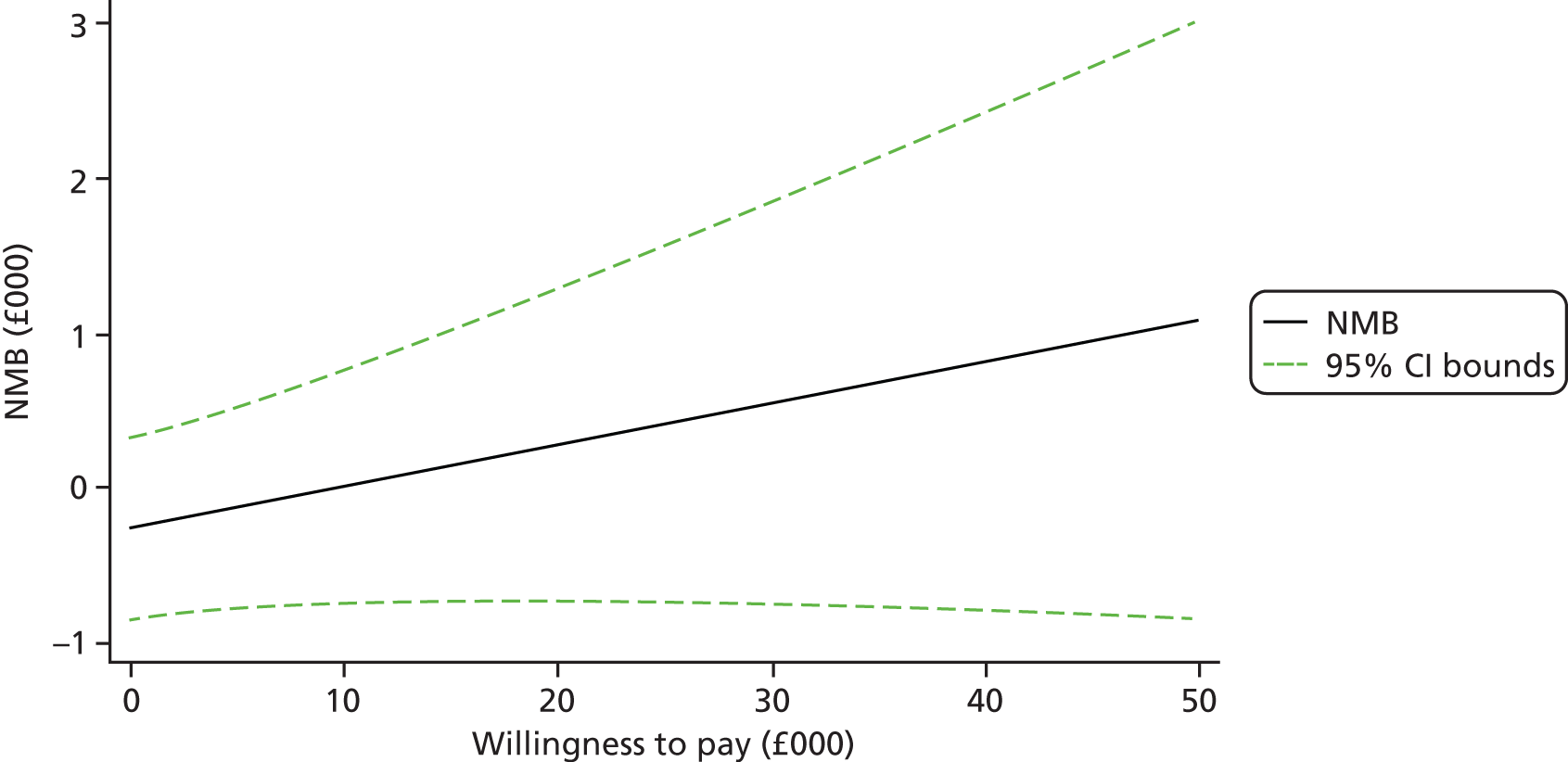
Appendix 3 The START manual
List of abbreviations
- CBT
- cognitive–behavioural therapy
- CD
- compact disc
- CDR
- Clinical Dementia Rating
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CIFT
- Camden and Islington NHS Foundation Trust
- CONSORT
- Consolidated Standards of Reporting Trials
- CSRI
- Client Service Receipt Inventory
- DeNDRoN
- Dementias and Neurodegenerative Disease Research Network
- DRC
- Dementia Research Centre
- DVLA
- Driver and Vehicle Licensing Agency
- EQ-5D
- European Quality of Life-5 Dimensions
- GP
- general practitioner
- HADS
- Hospital Anxiety and Depression Scale
- HADS-A
- Hospital Anxiety and Depression Scale-anxiety
- HADS-D
- Hospital Anxiety and Depression Scale-depression
- HADS-T
- Hospital Anxiety and Depression Scale-total
- HR
- hazard ratio
- HSQ
- Health Status Questionnaire
- ICC
- intracluster correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- ID
- identification
- MCTS
- Modified Conflict Tactics Scale
- NELFT
- North East London Foundation Trust
- NEPFT
- North Essex Partnership Foundation Trust
- NICE
- National Institute for Health and Care Excellence
- NMB
- net monetary benefit
- NPI
- Neuropsychiatric Inventory
- OR
- odds ratio
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- QoL-AD
- quality of life-Alzheimer’s disease
- RCT
- randomised controlled trial
- SD
- standard deviation
- START
- STrAtegies for RelaTives
- TAU
- treatment as usual
- UCL
- University College London