Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 08/13/24. The contractual start date was in July 2010. The draft report began editorial review in November 2013 and was accepted for publication in April 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Stephen Allen has carried out research in probiotics supported by Cultech, UK, has been an invited guest at the Yakult Probiotic Symposium, has received a speaker’s fee from Astellas, UK, and has received research funding from Yakult, UK.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Hood et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Overall introduction to the Probiotics for Antibiotic-Associated Diarrhoea study
The Probiotics for Antibiotic-Associated Diarrhoea (PAAD) study aimed to develop the platform for, and possibly implement, a randomised controlled trial (RCT) of probiotics administered with antibiotics to prevent antibiotic-associated diarrhoea (AAD). To justify a trial, we first needed an estimate of the magnitude of the AAD problem in care homes. We therefore set out to do a two-stage study. The purpose of PAAD stage 1 was to determine the prevalence of antibiotic prescribing and indication, the prevalence of AAD and to provide an indication of the prevalence of antibiotic-resistant organisms in the stool of care home residents. This would provide useful information in its own right to help guide care in this vulnerable population, in which infections are frequent and antibiotic prescribing is common and often not evidence based. It would also provide the basis for determining whether or not a RCT of probiotics to prevent AAD was justified and, if so, to generate data required for a sample size calculation for such a trial. The trial component of the study formed PAAD stage 2.
Antibiotic use in care homes
At least 4% of UK and US populations aged 65 years or over live in care homes. 1–3 Demand for long-term care in the UK is estimated to rise by up to 150% over the next 50 years. 4
Although data on infection prevalence in care homes are limited, point prevalence studies suggest that between 5% and 10% of residents in care homes will be prescribed antibiotics for a presumed infection at any one time. 5,6 This antibiotic use has consequences for residents’ quality of life (QoL) from both benefits and harms associated with antibiotic use, costs of care and increased risk that subsequent infections will be antibiotic resistant. 7–9 However, up to 40% of antibiotics prescribed in care homes might be inappropriate. 8–10 Accurate estimates of prescription rates by antibiotic class and indication are lacking.
Antibiotic-associated diarrhoea
Antibiotic treatment disrupts the normal flora of the gut, sometimes causing diarrhoea. 11 The primary mechanism is thought to be disturbance in the metabolism of carbohydrates, short-chain fatty acids and bile acids, resulting in impaired resistance to pathogens. 12 While any antibiotic can cause AAD, clindamycin, cephalosporins, aminopenicillins and, more recently, fluoroquinolones have been directly linked with AAD, particularly in hospitalised patients. 13 Older patients with frequent hospitalisations and high comorbidity are at greatest risk. 13 Little is known about the frequency and type of antibiotics prescribed in care homes, or about the incidence and aetiology of AAD. AAD varies in incidence, is especially common in winter, and can occur in up to 39% of hospitalised patients receiving antibiotics. 14 A challenge to clinicians is to identify cases of AAD due to Clostridium difficile (Hall and O’Toole 1935) Prévot 1938 infection since this is the most commonly identified and treatable pathogen responsible and is also implicated in more severe cases of AAD. 11 C. difficile is implicated in 20–30% of AAD cases. 15
Clostridium difficile
Clostridium difficile is a Gram-positive, anaerobic, spore-forming bacteria that was identified in the late 1970s and has recently been highlighted as a potential deadly threat to hospitalised patients and residents of care homes. 13,16 The spores can survive for lengthy periods in the environment and gut; therefore, there is a high risk of cross-infection through direct patient-to-patient contact, via health-care staff or via a contaminated environment. The Health Protection Agency’s (HPA) data from voluntary surveillance of C. difficile in England, Wales and Northern Ireland in 2006 described an overall increase in incidence, from 2005 to 2006, of 8% in England and 15% in Wales, with the highest incidence in the elderly. 17 Although there were some HPA data for positive stool samples originating in primary care, there are no data to indicate whether or not these are follow-up samples from hospitalised patients. We were not able to identify prospective UK data on C. difficile outside hospitals, nor were we able to identify studies that involved prospective, systematic sampling. The second study of Infectious Intestinal Disease (IID2) included the incidence of C. difficile among people living in the community but not in care homes. 18
Clostridium difficile-associated diarrhoea
Clostridium difficile-associated diarrhoea (CDAD) is the most commonly identified cause of AAD, and is responsible for most cases of pseudomembranous colitis; it typically occurs in care homes, among other settings. 19 CDAD occurs most often as a consequence of disruption of the indigenous colonic microflora following broad-spectrum antibiotic treatment. C. difficile accounts for 20–30% of AAD,15 although some estimates are more conservative. 11 Although the majority of individuals recover fully, elderly and frail individuals in particular may suffer loss of dignity or become seriously ill with dehydration (as a consequence of the diarrhoea), and some may go on to develop pseudomembranous colitis.
Exposure to antibiotics within the previous 2 months is the most important risk factor for developing CDAD. 20 Cumulative antibiotic exposure appears to be associated with an increased risk of CDAD. 21 Other well-recognised risk factors include age [hospital patients aged over 65 years are four times more likely than general medical patients to develop CDAD (73.6 vs. 16 per 1000 admissions)], hospitalisation, severity of underlying illness, nasogastric tube and use of proton pump inhibitors or H2-agonists. 22–24
Clostridium difficile-associated diarrhoea and antibiotic-associated diarrhoea infection in care homes
There are few data regarding antibiotic use, AAD and CDAD in UK care homes. Most of the research to date has been carried out in hospital settings or in the USA. However, residents in care homes in the UK have many of the risk factors associated with developing AAD and CDAD (e.g. age over 65 years, frailty, multiple comorbidities and frequent antibiotic treatment).
Antibiotic use in US residential homes is common: estimations of single time point prevalence range from 8% to 17%, and in one study between 50% and 75% of residents received at least one antibiotic prescription over a 12-month period. 20 We conducted a prescribing audit of care homes in one health authority and found that 134 (7%) of 1901 residents were on an antibiotic in a single day. A study in care homes in Sweden found that 25% of residents were prescribed an antibiotic during a 3-month observation period. 25 However, considerably fewer antibiotics are prescribed in Sweden than in the UK. 26 It is not known how many of these residents developed AAD or how many had C. difficile in these studies. Providing reasonable estimates of these outcomes for the UK relate to the scientific importance of our study to the NHS. Diarrhoea within long-term care facilities (LTCFs) can cause fatalities and could become endemic.
Laffan et al. 27 retrospectively reviewed CDAD incidence and prevalence in a single 200-bed LTCF in Baltimore in the USA between July 2001 and December 2003. The incidence of CDAD ranged from 0 to 2.62 cases per 1000 resident-days. They found that CDAD in this LTCF occurred most often in patients who had recently been admitted to hospital. 27 US studies by Kutty et al. and Chang et al. found that over 90% of post-hospitalisation cases of CDAD occur within 30 days of discharge. 28,29
Riggs et al. , in the USA, found that over 50% of patients admitted to a LTCF during an outbreak were asymptomatic carriers of C. difficile (stool culture positive, but no diarrhoea). 30
A review of the diagnosis, management and prevention of C. difficile in LTCFs concluded that the epidemiology, risks and outcomes of C. difficile infection in older residents of LTCFs need to be better understood. 31 Better treatment modalities to reduce the risk of recurrent disease need to be developed and assessed, and measures such as antimicrobial stewardship should be introduced.
Probiotics to prevent antibiotic-associated diarrhoea
Probiotics are dietary supplements containing a single culture or mixed culture of live microorganisms such as bacteria or yeast which, when administered in adequate amounts, confer a health benefit on the host by improving the properties of the indigenous microflora. 32 Probiotics have been proposed as a preventative intervention for AAD, including CDAD, as they reinforce the human intestinal barrier. 14,33–35 Probiotics’ likely mechanism of action is through secretion of antimicrobial factors, such as bacteriocins, and competition for adherence to the binding sites on mucins and epithelial cells, thereby preventing detrimental colonisation and contributing to barrier function. 36 Certain probiotic strains are resistant to digestion by enteric or pancreatic enzymes, gastric acid and bile, and prevent the adherence, establishment and/or replication of pathogens in the gastrointestinal tract. Probiotics are generally well tolerated and free of adverse effects although, theoretically, the introduction of live bacteria may carry the risk of introducing resistance genes and causing septicaemia. 37
A previous Cochrane review of probiotics as a treatment for presumed infectious diarrhoea included 23 studies with a total of 1917 participants and found that probiotics reduced the risk of diarrhoea at 3 days [relative risk (RR) 0.66, 95% confidence interval (CI) 0.55 to 0.77, random-effects model; 15 studies] and the mean duration of diarrhoea by 30.48 hours (95% CI 18.51 to 42.46 hours, random-effects model; 12 studies). 35 A Cochrane review of probiotics for the prevention and treatment of AAD38 included 63 studies with a total of 11,811 participants and indicated a statistically significant association of probiotic administration with reduction in AAD [RR 0.58, 95% CI 0.50 to 0.68; p < 0.001; I2 = 54%; (risk difference –0.07, 95% CI –0.10 to –0.05) (number needed to treat 13, 95% CI 10.3 to 19.1)] in trials reporting on the number of patients with AAD. However, there is significant heterogeneity in the pooled results and the evidence is insufficient to determine whether this association varies systematically by population, antibiotic characteristic or probiotic preparation. The review concluded that probiotics are associated with a reduction in AAD; however, more research is required to determine which probiotics, patients and antibiotics are associated with the greater efficacy.
A recent Cochrane review of probiotics for the prevention of CDAD in adults and children included 23 studies with a total of 4213 participants and found that probiotics significantly reduce the risk of CDAD by 64%. 39 The incidence of CDAD was 2.0% in the probiotic group, compared with 5.5% in the placebo, no treatment, control group (RR 0.36, 95% CI 0.26 to 0.51). The review concluded that the moderate-quality evidence suggested that probiotics are both safe and effective for preventing CDAD.
A systematic review of six studies of paediatric patients found significant benefit to patients from using probiotics in per-protocol analyses compared with control patients (RR 0.43, 95% CI 0.25 to 0.75). However, no evidence for a difference was found in intention-to-treat analyses (RR 1.01, 95% CI 0.64 to 1.61). 40 Hickson et al. 41 randomised patients to receive either antibiotics and placebo (n = 66) or antibiotics and probiotics (n = 69) and found that 7 of 57 patients in the probiotic group, compared with 19 patients from 56 in the control group, developed AAD. However, only 135 of 1760 hospitalised patients taking antibiotics were randomised in this trial (randomisation rate 7.6%), which may limit the applicability of the findings. 41
Database searches
We searched MEDLINE and EMBASE prior to starting this study, and our findings indicated that the evidence base for the use of probiotics is incomplete. In the light of our searches we have made recommendations about what should be done taking into consideration this uncertainty and about future research. 42 More specifically, we were not able to identify evidence for or against using probiotics to prevent AAD in people admitted to LTCF/care homes or in primary care. It is essential that research is carried out in the setting in which the evidence will be applied, the findings of hospital-based studies may not apply to the care home setting, as antibiotics, routes of administration and patient profiles differ between hospitals and care homes.
Conducting studies in care homes
There are 1203 care homes in Wales, of which 25% provide nursing care (300 homes). There are a total of 2607 care homes and 570 nursing homes in south-west England. Within these homes, there are approximately 427,000 beds across England and Wales, and it is understood that the number of beds will need to be increased in line with the projected rise in demand for long-term care. 1,2
Conducting studies in care homes, especially nursing homes, poses unique challenges. Care home research participants are more likely to be older, physically frail and cognitively impaired compared with other research settings. 43 Recruitment, consent, retention and data collection can be time-consuming and difficult and extra time and help should be provided to ensure that the staff in care homes have the ability to carry out the research procedures. 44 Care home staff are also more likely to move after short periods of employment.
Rationale for the Probiotics for Antibiotic-Associated Diarrhoea study
There are (incomplete) surveillance data from the USA and the UK, and UK clinical experience to suggest that AAD including CDAD is an important problem in care homes in the UK. There are strong grounds for evaluating probiotics in conjunction with antibiotic treatment to prevent AAD in care homes, but this strategy has never been properly evaluated in a clinical trial. However, before a trial is justified, the importance of the problem to the independent care home sector and NHS, a firm basis for sample size calculation and trial planning, needs to be more clearly established.
The introduction of probiotics in conjunction with antibiotic treatment in care homes could lead to a significant reduction in AAD, as antibiotic treatment is common in this group; spread is a particular risk, and patient frailty increases risk of acquisition and of complications. Diarrhoea in this group can lead to serious illness resulting in hospital admissions, cause illness in fellow care home residents, increase vulnerability through reduced nutrition and dehydration, result in ongoing incontinence, cause urinary tract infections (UTIs) and have a profoundly negative impact on dignity. Therefore, we proposed a two-stage study.
The PAAD study stage 1 would establish the descriptive data we needed to confirm both the magnitude of the problem and that our sample size calculation assumptions were correct. Knowing the amount and nature of antibiotics prescribed in care homes and describing AAD and CDAD would be useful in its own right and would help to guide future antibiotic prescribing decisions in this setting. To account for the known difficulties in conducting research in this setting, we also planned to pilot study procedures, model consent procedures, and develop training material to train care home staff in how to conduct the RCT and determine if cascading of the training to new colleagues would be possible.
If PAAD stage 1 confirmed a trial was justified, PAAD stage 2 would be a RCT to generate robust evidence that would fill an important gap in the evidence base about the use of probiotics, in conjunction with antibiotic treatment, in older people in care homes.
Probiotics for Antibiotic-Associated Diarrhoea study objectives
Probiotics for Antibiotic-Associated Diarrhoea stage 1: primary objectives
-
To conduct prospective systematic ascertainment of the incidence of AAD in care homes.
-
To allow an appraisal of the estimated sample size for a RCT in PAAD stage 2.
Probiotics for Antibiotic-Associated Diarrhoea stage 1: secondary objectives and auxiliary study objectives
-
To conduct prospective systematic ascertainment of antibiotic use in care homes.
-
To estimate the risk of AAD overall and from particular antibiotics in care home settings.
-
To identify barriers and implementation issues in conducting a trial of AAD prevention/amelioration in a care home setting.
-
To determine the prevalence of asymptomatic C. difficile carriage in residents within selected care homes.
The probiotic feasibility and acceptability study
-
To test the acceptability and feasibility of administering probiotic in a small number of care home residents.
-
To pilot and develop trial procedures including modelling consent procedures.
-
To develop a training package for care home personnel to implement the trial.
The qualitative study
-
To conduct focus groups and individual qualitative interviews with care home residents, their family, care home staff and general practitioners (GPs).
-
To explore the ethical and practical issues of consent and assent, particularly the topic of advanced consent, for elderly residents who may/may not have capacity to consent.
Probiotics for Antibiotic-Associated Diarrhoea stage 2 objectives
The primary objective was to:
-
assess the effectiveness of probiotics taken in conjunction with antibiotic treatment in reducing the incidence of AAD.
The secondary objectives were to:
-
assess the effectiveness of probiotics taken in conjunction with antibiotic treatment in reducing the incidence of CDAD
-
evaluate the impact of probiotics in conjunction with antibiotic treatment on the functional status and QoL
-
evaluate the cost-effectiveness of probiotics in conjunction with antibiotic treatment in reducing AAD.
Changes to objectives
The originally planned feasibility study, to test the acceptability and practicability of administering probiotic in a small number of residents, was not undertaken for two reasons. First, on discussion with the pharmaceutical company (Actiel), it was confirmed that the VSL#3 powder could be dissolved in as little as 25–50 ml of liquid or sprinkled on food; therefore, the initial concern over drinking a large volume of liquid (100 ml) was appeased. Second, it was considered resource and time intensive to conduct alongside the main observation study.
Summary
In summary, PAAD stage 1 aimed to identify the rates of antibiotic prescribing and AAD in care homes, to determine the prevalence of C. difficile in baseline stool samples and to provide reliable incidence data and confirm the basis of our sample size calculation for the RCT in PAAD stage 2.
If PAAD stage 1 indicated that AAD is a rare, unimportant problem, then, based on explicit stopping rules, we would not progress to PAAD stage 2.
In addition, work was planned in PAAD stage 1 to allow us anticipate and address challenges in the PAAD stage 2 trial design and implementation. In particular, we were keen to explore the acceptability of advanced consent procedures in a possible trial of probiotics to prevent AAD.
Chapter 2 Probiotics for Antibiotic-Associated Diarrhoea stage 1: a prospective observational study of antibiotic prescribing and antibiotic-associated diarrhoea in care home residents
Methods
Study design
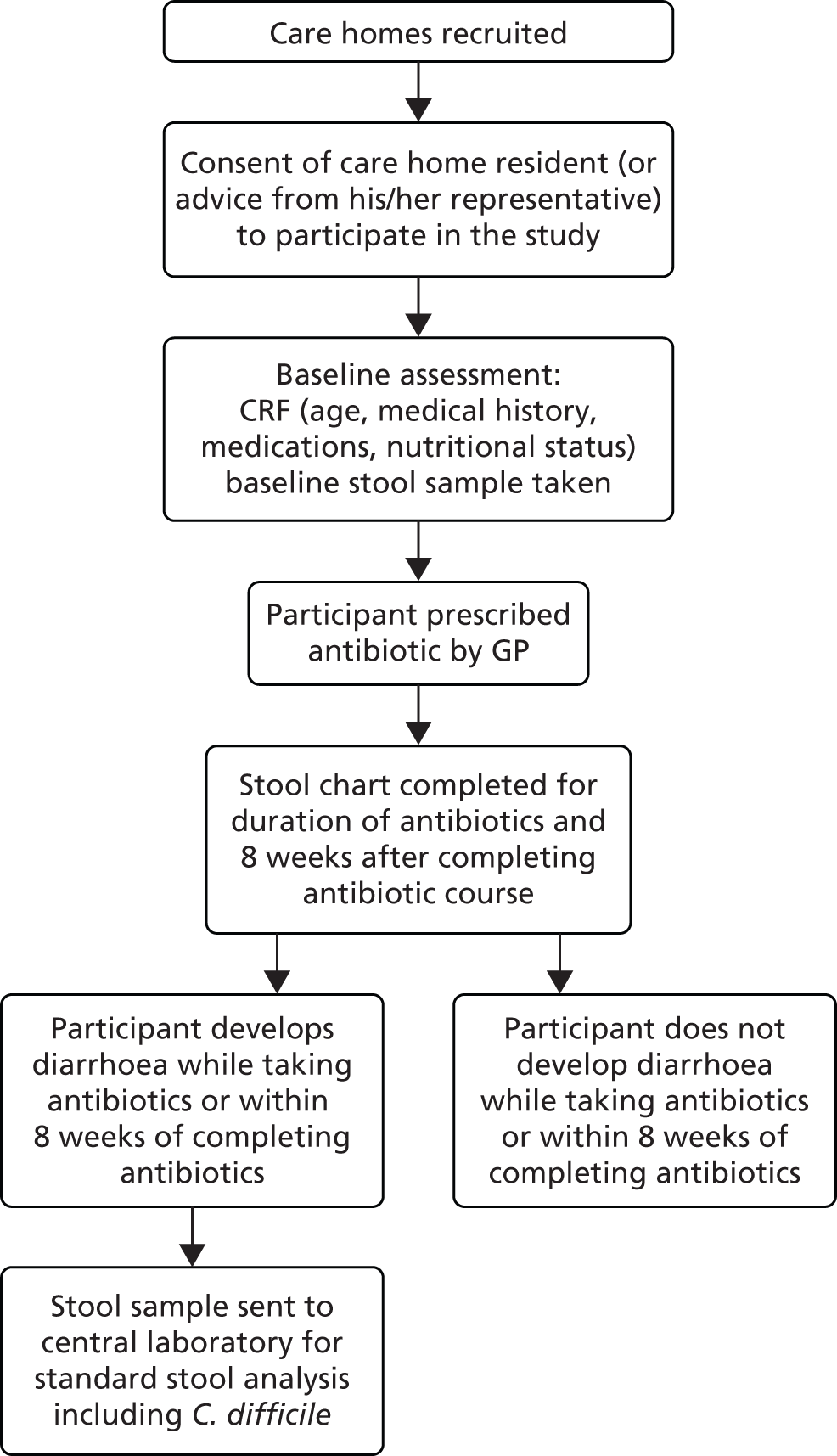
The PAAD study stage 1 was a prospective observational cohort study of antibiotic prescribing and associated AAD in care home residents (Figure 1). The study was conducted between November 2010 and March 2012 in care homes in South Wales. We aimed to recruit a total of 270 care home residents and follow up each resident for 12 months. The South East Wales Research Ethics Committee (REC) approved the study (10/WSE03/31). Agreement to conduct the study at the care homes was either given by the care home manager, regional manager or care homeowner (if privately owned) or given by the local authority (LA).
FIGURE 1.
Study design. CRF, case report form.
Study objectives
Primary objectives
-
To conduct prospective systematic ascertainment of the incidence of AAD in care homes.
-
To allow an appraisal of the estimated sample size for a RCT in stage 2.
The aim was to use this information to identify the scale of the AAD problem in care homes, provide reliable incidence data and confirm the basis of our sample size calculation for a RCT of probiotics in PAAD stage 2.
Secondary objectives
-
To conduct prospective systematic ascertainment of antibiotic use in care homes.
-
To estimate the risk of AAD overall and from particular antibiotics in care home settings.
-
To identify barriers and implementation issues in conducting a trial of AAD prevention/amelioration in a care home setting.
-
To determine the prevalence of asymptomatic C. difficile carriage in residents within selected care homes.
The aim was to use this information, in conjunction with AAD incidence, to estimate the risk of AAD overall, from antibiotics overall, and from particular antibiotics in care home settings. Additionally, the observational study would serve as an opportunity to ascertain the practicalities of conducting a RCT in a care home environment.
We planned an interim analysis at 8 months after commencing recruitment of residents, which would include follow-up data from residents recruited within the first 6 months, to assess the scale of the AAD problem in care homes. The results of the interim analysis, in relation to the explicit stopping rules defined by the research team, would determine whether or not progression to the PAAD stage 2 RCT was warranted.
Participants and recruitment
Care home recruitment
The research team identified all care homes in South Wales, stratified them based on the type of care they provided (nursing, residential or dual registered, i.e. providing both nursing and residential care), randomly ordered them within their stratum and approached the care homes sequentially by telephone to arrange a meeting with the manager of the care home to discuss the study further.
During this meeting, the aims and objectives of the study were explained in more detail and an informal questionnaire was used to prompt the team to gather data about the home in order to assess the feasibility and practicality of carrying out the study at that care home.
Care homes were recruited when the relevant manager and care home owner agreed for PAAD stage 1 to go ahead at their site and at least three staff at the site were willing to take responsibility for conducting the study in their care home. Reimbursement was offered for the time staff dedicated to the study or additional time spent over their contracted hours and additional research nurse or research officer support was provided by the National Institute for Social Care and Health Research – Clinical Research Centre (NISCHR CRC) where needed.
Once the essential governance documents were completed, signed and returned to the research team, the care home was provided with all the information, training and equipment necessary to carry out resident recruitment and study procedures.
Participant recruitment
Residents were eligible for the study if they met all of the PAAD stage 1 inclusion criteria (Table 1).
| Inclusion criteria | Exclusion criteria |
|---|---|
| Resident admitted to the care home for > 24 hours | There were no exclusion criteria for the PAAD stage 1 observational study |
| Planned admission to care home of 1 month or more (excludes short term respite care) | |
| Written confirmed consent/assent provided |
There were two categories of eligible residents: those who had capacity to consent and those unable to consent for themselves. Therefore, consent procedures differed depending on whether or not residents had capacity at the time the resident was approached and recruited into the study.
Senior care home/nursing staff were asked to identify residents who were eligible to join the study. Where staff members were unsure of the mental capacity status of an eligible resident, mental capacity was assessed during the consent procedure to determine capacity to consent. Study information was given using written information available in several forms (either the full information sheet or a more visually accessible information sheet in large print and a pictorial information sheet) to ensure that residents were given a full opportunity to consent for themselves. Residents with mental capacity and willing to participate were asked to provide written consent or verbal consent (for those unable to write). This was witnessed by two senior staff members. Where residents lacked capacity, senior care home/nursing staff were asked to identify representatives, herein referred to as ‘consultee’, for each resident (e.g. next of kin, those who visit most regularly). Consultees were provided with the study information sheet accompanied with a verbal explanation when they came into the care home or, if this was not possible, by post and then followed up with a telephone call. The consultees of the residents were explicitly asked, either in writing or verbally, to consider whether or not they believed the resident would want to join the study. Consultees who believed their relative would have wanted to participate in the study were asked to sign a consent form to document their decision.
For residents who did not have an available consultee, the plan was to contact Age UK for advice or request that the residents’ GP nominate an appropriate person or act on the resident’s behalf. Both of these options are suggested in the 2005 Mental Capacity Act (MCA). 45 However, this situation did not arise during the course of the study.
During the recruitment process, residents and their consultees were given as much time as needed to consider the information and the opportunity to question the care home staff, their GP or other independent parties to decide whether or not they were willing to participate in the study. All consent, written or verbal, and assessment of mental capacity was undertaken by study-trained senior care home staff/nursing staff. All residents and consultees were advised that they could withdraw participation in the study at any time without it affecting their care.
Study procedures
Study training
The research team aimed to deliver bespoke study training to staff at each individual care home because of the heterogeneity of the recruited care homes, in terms of both size and type of resident care. Core standardised training modules included the fundamentals of good clinical practice (GCP) as well as study-specific training on how to approach residents/consultees about the study, assessing capacity, taking informed consent, interpreting and using the Malnutrition Universal Assessment Tool (MUST), reporting serious adverse events (SAEs), interpreting the Bristol Stool Chart (BSC), taking and sending stool samples and data collection.
Initial training was provided before the start of the study, before recruitment, once the study began and continuously after that point with specific staff groups to refresh their memory. As much flexibility as possible was given to the time (i.e. evenings, weekends) and number of training sessions in order to provide training to as many senior staff members and registered nurses as possible without disrupting the usual routines in the homes. Owing to the need to retain adequate staffing levels at the homes, it was often not possible for all of the care assistants to attend training; therefore, we requested that the training be cascaded from those who had attended training to more junior members of staff.
Monthly teleconference calls were set up with key staff in each of the care homes to be used as a forum to update care home status, share challenges, find solutions and generally maintain engagement and enthusiasm for the study. Incentives, in terms of both monetary (vouchers) and non-monetary (certificates, specialised training courses, cake for site of the month, etc.) value were also provided.
Throughout the course of the study the research team organised three workshops with the aim of promoting engagement with the study and to provide ongoing training. Workshops were held in Cardiff city centre and were designed to help care home staff discuss procedures to obtain consent or maximise accurate case report form (CRF) completion.
Data collection
Prior to the recruitment of residents, care home staff were asked to complete a care home information CRF. The purpose of this CRF was to elicit summary information about the care home characteristics and, to that end, it incorporated questions asking about the number of beds, number of residents, staffing levels and whether or not there was an infection control policy in place. A further CRF was completed at the end of the study to provide a measure of change in number of residents and staffing levels over the study period.
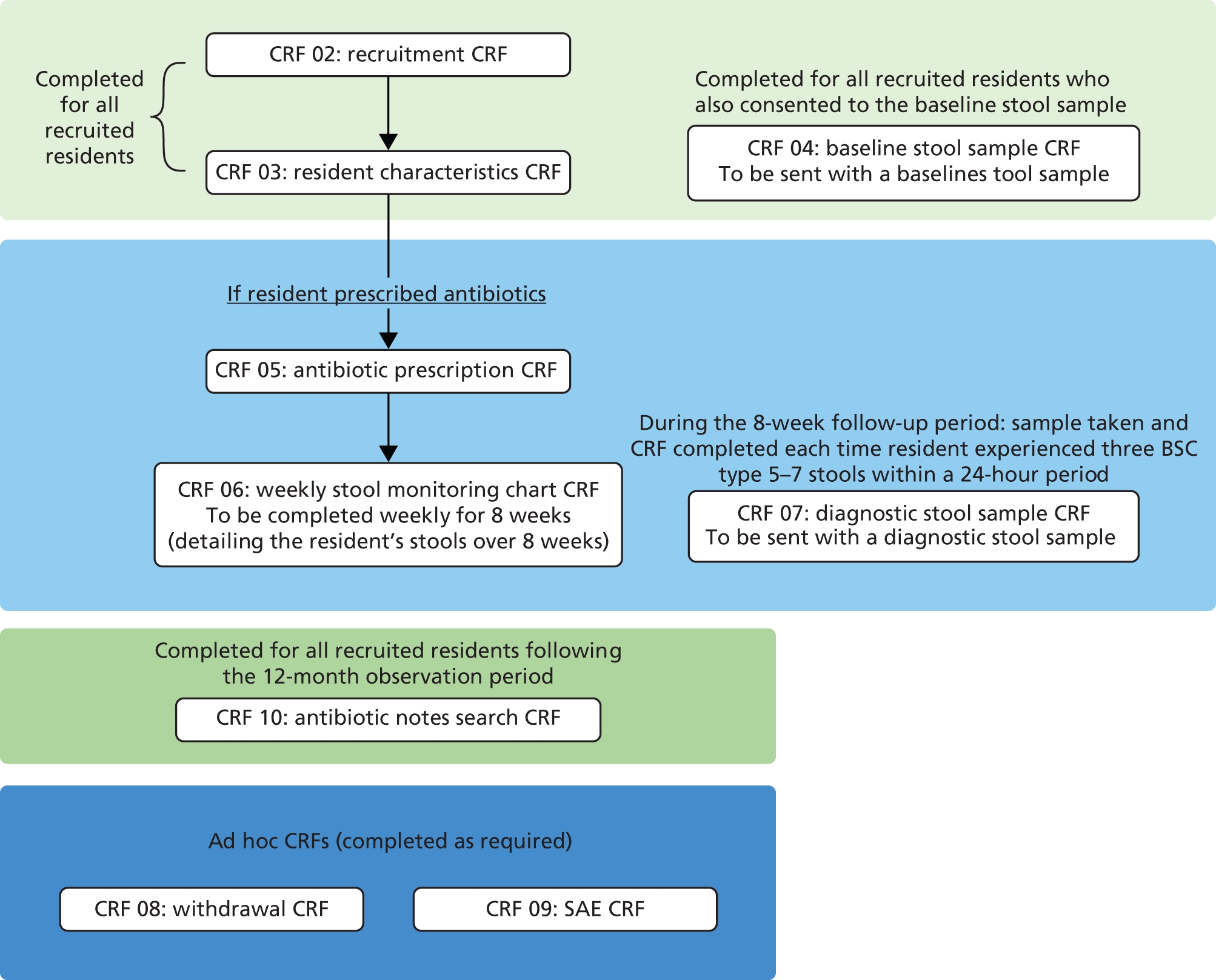
Data about residents participating in the observational study were collected in two phases (1) ‘baseline’ data were collected as soon as possible following consent and (2) ‘follow-up’ data were collected when a resident recruited into the study was prescribed antibiotics at any point during the 12 months following recruitment (Figure 2).
FIGURE 2.
Data flow of recruited residents.
Serious adverse event reporting was undertaken for all recruited residents regardless of whether or not they were prescribed antibiotics during the 12-month observational period. The CRFs included standard screening and assessment tools (among other questions devised by the research team) (Table 2).
| Tool | Description of tool | When recorded |
|---|---|---|
| MUST | MUST is a five-step screening tool used to assess nutritional risk. The algorithm requires information about current body mass index, weight loss and current health status in order to provide the health professional with a score used to guide nutritional management of the patient46 | When recruited into the study (CRF 03) Each time the resident was prescribed an antibiotic (CRF 05) |
| Clinical Frailty Scale | The Clinical Frailty Scale is a visual aid used to assess a person’s perceived frailty. It has been used as a predictor of death or need for entry into care facility. The tool uses nine pictures, each with brief descriptions, on a Likert-type scale in which options vary between very fit and very severely frail or terminally ill47 | When recruited into the study (CRF 03) |
| BSC | The BSC is a visual aid used by health professionals as a diagnostic marker of digestive health. The scale depicts seven pictures of human faeces in various forms, ranging from faeces described as type 1 (separate hard lumps, like nuts; hard to pass) to type 7 (watery, no solid pieces, i.e. entirely liquid) | On the CRFs that accompanied the baseline stool sample (CRF 04) When a stool bowel movement was recorded following an antibiotic prescription (CRF 06) and/or diagnostic stool sample(s) (CRF 07) |
Table 3 details, for each PAAD study CRF, time point for data collection, a brief description of the type of data collected and who had overall responsibility for collecting the data. The research team requested that the original CRF should be sent to the PAAD study trial manager and a copy kept in the site file in each care home.
| CRF | Data collected | Time point | Collected by |
|---|---|---|---|
| CRF 01 – care home information | Type, size, occupancy of home. Staffing levels and infection control training | Prior to commencing recruitment | Care home staff |
| CRF 02 – recruitment | Eligibility confirmation, age, gender, capacity status | Immediately post consent | Care home staff |
| CRF 03 – resident characteristics | Medical history, recent hospitalisation, recent antibiotic use | Immediately post consent | Care home staff |
| CRF 04 – baseline stool sample | Date and time of stool collection. Consistency of stool | Within 1 week from consent | Care home staff |
| CRF 05 – antibiotic prescription | Name, dose, route, frequency, duration and indication of antibiotic | Day of commencement of antibiotic treatment until duration end date | Care home staff/NISCHR CRC research officers |
| CRF 06 – weekly stool monitoring chart | Time and consistency of bowel movements. Whether or not a stool sample was taken | Day of commencement of antibiotic treatment, every day for duration of antibiotic + 8 weeks | Care home staff/NISCHR CRC research officers |
| CRF 07 – diagnostic stool sample | Date and time of stool collection. Consistency of stool | When participant develops diarrhoea within the 8-week follow-up period following antibiotics | Care home staff |
| CRF 08 – withdrawal | Date and reason for withdrawal | Following a withdrawal | Care home staff/NISCHR CRC research officers |
| CRF 09 – SAE reporting | Details of adverse event (outcome, description, seriousness, expectedness and causality) | Following any SAE | Care home staff/NISCHR CRC research officers |
| CRF 10 – antibiotic notes search | Name, dose, route, frequency, duration and indication of antibiotics prescribed in the 3 months prior to study entry | At the end of the recruitment period at the care home | Care home staff/NISCHR CRC research officers |
Data collected following antibiotic prescription
For each antibiotic prescribed during the study period, care home staff recorded the medical indication, name, route, dose, day and duration of prescription on the antibiotic CRF (05). Immediately following an antibiotic prescription for the duration of and up until 8 weeks after the end of the antibiotic course, the care home staff collected data on the resident’s daily stools (including the time the stool was passed and the BSC type of the stool), using the stool monitoring CRF (06), reported on a weekly basis.
If care home staff observed loose stools (BSC score 5–7) in a participating resident during the stool monitoring follow-up period, a stool sample was taken on the second episode of loose stools to ensure that samples were collected whenever an episode of AAD might have occurred. This sample was sent to the laboratory along with a diagnostic stool sample CRF (07) which stated the stool’s consistency and time of sample collection.
Samples were sent to a central reference laboratory specialist antimicrobial chemotherapy unit for C. difficile culture and screening for the carriage of antibiotic-resistant organisms. Staff at care homes were also encouraged to also send a stool sample to their local laboratory as per their routine procedure.
Medication data
The care home staff or NISCHR CRC research officers were also asked to take a photocopy of the medication administration record (MAR) from the care plan of each consented resident. To maintain anonymity, residents’ names were replaced by their participant identifier (PID). The photocopy of the MAR was requested as soon as possible following consent, each time an antibiotic was prescribed and for the entire month of March 2012. The intention of the 1-month review was to inform an audit of the effectiveness of care home reporting of antibiotics. The purpose of the request following consent and following an antibiotic prescription was to determine whether or not there were any trends between certain medications and AAD or CDAD.
Serious adverse event reporting, withdrawals and loss to follow-up
When care home staff became aware that a participating resident had experienced a SAE, they were asked to complete a SAE CRF within 24 hours on becoming aware of the event.
The research team was notified of study withdrawals and deaths via the withdrawal CRF. Participating residents who were admitted to hospital were not considered lost to follow-up unless they stayed in hospital for the remainder of their time in the study.
Data management
Participant tracking
Residents were ‘tracked’ throughout their time in the study using the completed CRFs (and the dates on the CRFs) that were sent from care homes. Data were added on to the PAAD stage 1 database when these CRFs were received at the South East Wales Trials Unit (SEWTU). The data manager was able to search for each recruited resident in the database using the resident’s PID to determine which CRFs had been completed for each individual and, hence, what stage the resident was at in the study.
Clinical data
Receipt of CRFs was logged in the database. CRFs were first visually checked, then processed using Cardiff TeleForm, an optical mark recognition system, and stored in Statistical Product and Service Solutions (SPSS) data sets (SPSS version 20; IBM Corporation, Armonk, NY, USA). Missing/invalid data, identified during any of the above points and using data validation checks in SPSS, were queried at site using source data from care notes. Data corrections were undertaken prior to scanning in TeleForm, or via syntax in SPSS.
Stool data
Stool sample results were collated and stored electronically at the Public Health Wales laboratory. Care home staff were asked to fax or send the research team at SEWTU a copy of the completed diagnostic stool sample CRF (07) so that the research team could maintain a record of when stools samples had been sent to the laboratory.
Stool sampling
All care homes were provided with a protocol on the stool sampling procedure in order to promote standardisation when collecting and sending stool samples. The research team provided all care homes with disposable bowl inserts for commode pots, sample tubes, adhesive tube labels and spatulas as well as a fridge for storing samples that could not be immediately sent to the laboratory. Stool samples were taken either from stool passed into commode inserts or from the resident’s incontinence pad and placed into a sample tube. The research team requested that the sample [with the diagnostic stool sample CRF (07)] should be labelled with the resident’s PID and initials and sent to the central reference laboratory using Post OfficeTM-approved SafeboxesTM.
Stool sample analysis
The stool samples were cultured, including for C. difficile, and then stored at −70 °C. Cultured C. difficile isolates were then ribotyped, toxin tested and subjected to antimicrobial sensitivity testing as well as screened for carriage of antibiotic-resistant bacteria. As the results of the loose stool study samples were used for research purposes only and were not fully analysed in real time, they were not sent back to the care homes or GPs.
Statistical methods
Care home sampling frame
Care homes in South-East Wales were split into three strata, based on the type of care home: nursing, residential or dual registered. The team intended to purposively recruit three care homes from each stratum, making a total sample of nine care homes. The aim of this method of sampling was to gain an insight into the amount of variability between and within different types of care homes (in terms of both the homes themselves and their residents).
Resident sample size justification
We predicted that nine care homes would generate a sample of approximately 270 residents. Previous literature indicated that 40% of care home residents would be likely to be prescribed antibiotics over a 12-month period. This figure would enable the research team to fit a 95% CI to an AAD rate of 25% ± 10%.
Interim analysis
A planned interim analysis was carried out evaluating data collected during the initial 3 months of the study, together with 2 additional months of data for AAD follow-up. The interim analysis would provide estimates of recruitment, antibiotic prescriptions and episodes of AAD. The duration and severity of AAD were also assessed. These estimates would provide a scientific and medical justification for a trial of probiotics to prevent/ameliorate AAD in care home residents, allow an understanding of the feasibility of recruiting in this setting and give a firm basis for sample size calculation for the RCT.
Owing to the descriptive nature of the interim analysis, no adjustments were made to the final analyses to correct for the fact that an interim analysis had been performed.
Progression/stopping criteria
Specific criteria related to recruitment, antibiotic prescribing and AAD were defined a priori. Specifically, it was deemed infeasible to continue to a RCT if the percentage of residents recruited from those approached was less than 60%. To justify a scientific and medical need for a RCT of probiotics in care homes to reduce AAD, we felt that at least 27% of residents needed to have been prescribed at least one course of antibiotics at the interim time point and at least 18% of prescriptions would have to result in at least one episode of AAD. Should our AAD progression criteria not be met, we planned to also take the severity of AAD into account when determining whether or not to progress to a trial.
Data analysis
Descriptive statistics were calculated for each care home type and overall using means (standard deviations), medians [interquartile ranges (IQRs)] and proportions, as appropriate.
All incidence rates were calculated as per care home resident-year. Clustering of antibiotic prescriptions and AAD by resident was explored and estimates were appropriately inflated.
The probability of residents being prescribed antibiotics was estimated by fitting a logistic regression model, with results presented as odds ratios (ORs), 95% CIs, and p-values.
We used logistic regression models to compare samples with and without antibiotic-resistant bacteria for identified risk factors. Results are presented as ORs, 95% CIs and p-values.
To estimate the risk of developing AAD, a two-level logistic regression model was fitted with stool-monitoring periods nested within care home residents. Results are presented as ORs, 95% CIs and p-values. The time from antibiotic prescription to first episode of AAD was similarly estimated by fitting a two-level Cox proportional hazards model. Results are presented as hazard ratios (HRs) with associated 95% CIs and p-values.
All regression models were entered in the following blocks: stool monitoring characteristics (for the AAD models only), resident characteristics and care home characteristics. Explanatory variables were included if they were associated with their outcome at the 20% level in a univariable analysis, with variables removed from the final multivariable regression models if they were not significant at the 5% level and were of marginal significance (p-value > 0.1) in the univariable analysis. All relevant modelling assumptions were checked prior to reporting. Estimates from the regression models are presented from each stage of the multivariable model, with corresponding univariable estimates.
The two-level Cox proportional hazards model was implemented using Stata version 10.0.18 (StataCorp LP, College Station, TX, USA). All other analyses were performed using IBM SPSS.
Substantial changes to study protocol
Over the course of the study a number of changes were made to the PAAD stage 1 protocol. Initially, the intention was to include a sample of care homes of differing sizes from those with 20 beds or fewer to those with 100 beds or more. However, there were very few nursing and dual-registered care homes with 20 beds or fewer in the South-East Wales region. As the most important factor in the care home sample was care home type (nursing, residential and dual registered) it was decided to recruit three care homes of each type regardless of size.
Several changes were made to improve recruitment and data collection. These included allowing verbal consent (with witnesses present) to be provided if the resident or consultee was unable to sign the consent form and enabling the research team to take copies of recruited residents’ MAR sheets at specific time points to ensure the collection of valid concomitant medication data. It also came to light that many of the residents and their consultees were frustrated that they were unable to give consent at the time they were approached (i.e. directly after reading and digesting the information on the participant information sheet) because the protocol stated a mandatory 24-hour ‘consideration’ time before they could join the study. As a result of this the protocol was amended (with REC approval) to allow residents/consultees an opportunity to provide consent at the time of their choosing.
Antibiotic prescribing and associated diarrhoea: findings from a prospective observational cohort study of care home residents
Care homes
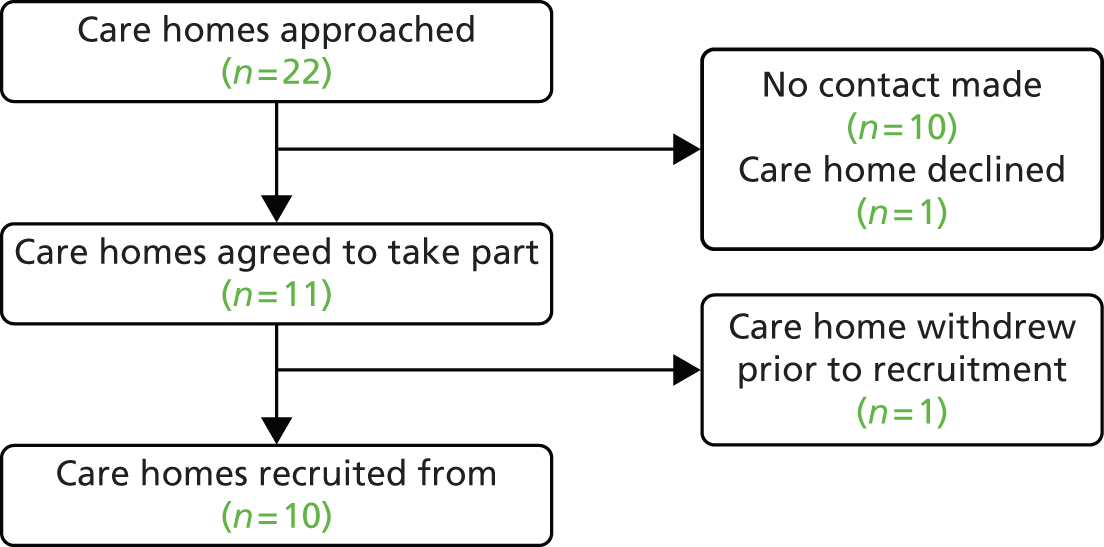
Eleven care homes were recruited to the study. However, one withdrew before any residents were recruited. Residents were therefore recruited from 10 care homes: four nursing, four residential and two dual-registered homes. Nine homes were privately managed and one was managed by a LA. The median number of beds was 39.5 (IQR 31.0–50.0) and the median number of residents at the time of recruitment was 33.0 (IQR 28.0–50.0). The median number of staff working in a care home over a typical 24-hour period was 16.0 (IQR 14.0–25.0), with 20.5% (n = 41) categorised as short-term staff members (i.e. employed for less than 12 months) (Table 4 and Figure 3).
FIGURE 3.
Care home flow diagram.
| Care home type | Care home information | Accommodation details | Staffing details | |||
|---|---|---|---|---|---|---|
| Number of care homes | Privately managed, n (%) | Total number of beds, median (IQR) | Total number of residents, median (IQR) | Total number of staff working in the last 24 hours, median (IQR) | Short-term staff working in the last 24 hours,a median (IQR) | |
| Nursing | 4 | 4 (100.0) | 36.0 (30.5–45.5) | 30.0 (27.5– 41.0) | 15.0 (13.0–20.5) | 3.0 (1.5–4.5) |
| Residential | 4 | 3 (75.0) | 35.5 (28.0–39.5) | 31.5 (26.5–37.0) | 15.5 (13.0–16.5) | 2.5 (1.0–3.5) |
| Dual registered | 2 | 2 (100.0) | 70.0 (54.0–86.0) | 66.5 (50.0–83.0) | 37.0 (29.0–45.0) | 10.0 (0.0–20.0) |
| Overall | 10 | 9 (90.0) | 39.5 (31.0–50.0) | 33.0 (28.0–50.0) | 16.0 (14.0–25.0) | 2.5 (1.0–4.0) |
Participants
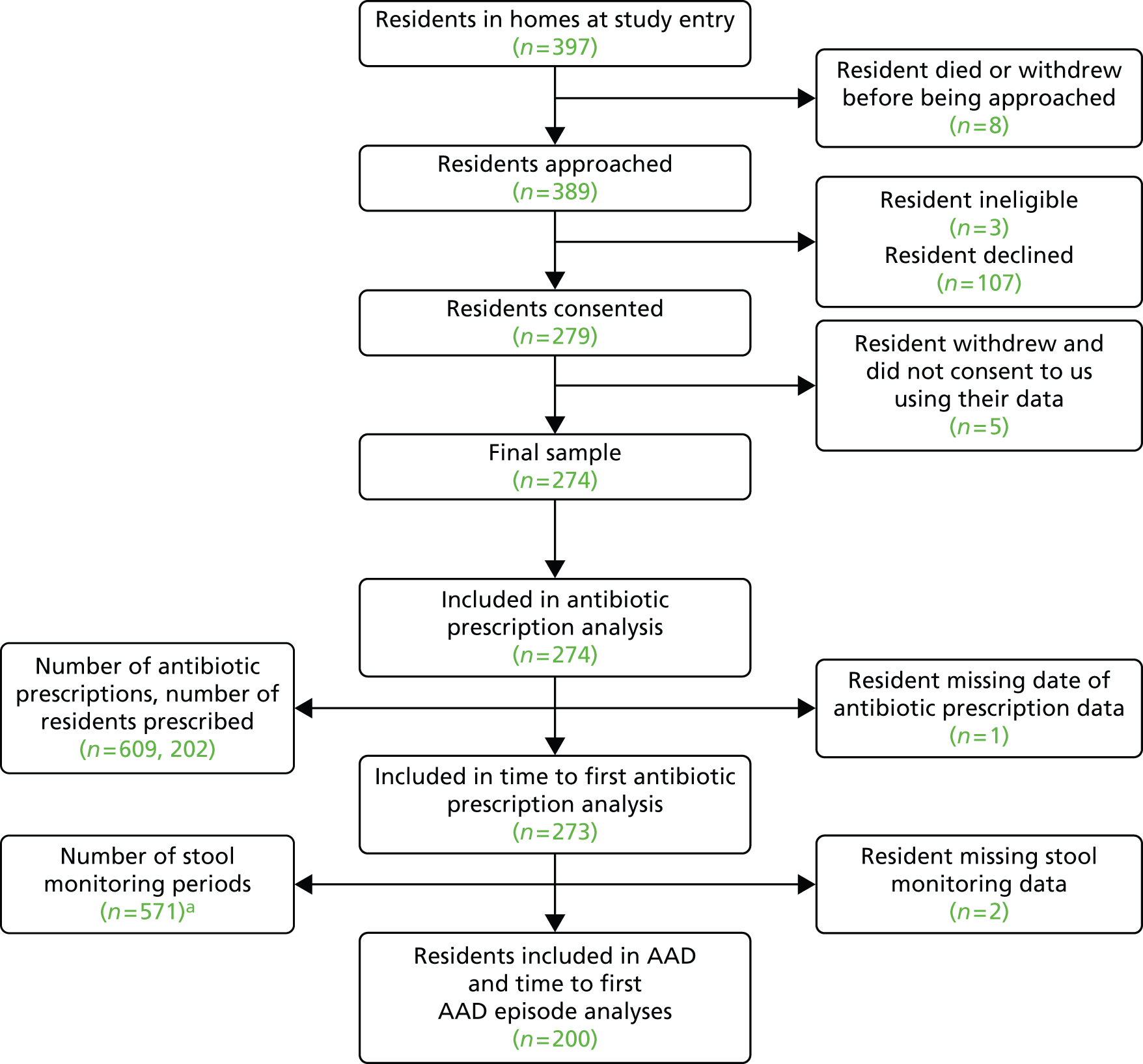
Three of the 389 residents (or consultees) approached were ineligible and 107 declined participation. A total of 279 residents were therefore recruited (71.7%). Of those recruited, 19 withdrew, 16 (84%) because they moved to a non-participating care home. Five of the 19 residents who withdrew from the study also withdrew permission for data already collected to be used; therefore, our analyses are based on a maximum of 274 residents (Figure 4). There were 81 hospitalisations reported during the study period, with at least one hospitalisation reported for 58 (21.2%) residents (incidence rate of 0.14 hospitalisations per resident-year, 95% CI 0.09 to 0.20 hospitalisations). In total, 64 residents died during the study period. No deaths were deemed study related. Residents were observed for a median of 310 days (IQR 230–364 days).
FIGURE 4.
Resident flow diagram. a, Some prescriptions overlapped in time and so did the stool monitoring periods in these instances.
Descriptive data
Residents had a median age of 86 years (IQR 82–90 years), with 20.4% (n = 56) < 80 years old, 57.7% (n = 158) between 80 and 90 years old and 21.9% (n = 60) older than 90 years. The majority of residents (75.9%) were female. Overall, 28.5% (n = 78) had capacity to provide informed consent for themselves. Few residents had any of the prespecified relevant serious medical conditions. At baseline, 7.7% (n = 21) had faecal incontinence with loose stools, 1.8% (n = 5) had diarrhoea and 66.8% (n = 183) routinely used incontinence pads. A ‘very fit to managing well’ classification was attributed to 13.5% (n = 37) of residents, 51.1% (n = 140) were classed as ‘vulnerable to moderately frail’ and 35.4% (n = 97) as ‘severely frail to terminally ill’. Nursing homes had more frail residents than residential homes. At baseline, 63.0% (n = 172) were classified as having a low nutritional risk status, 14.3% (n = 39) as medium risk and 22.7% (n = 62) as high risk. In the 4 weeks prior to recruitment, 6.9% (n = 19) had been admitted to hospital and 20.8% (n = 57) of residents were prescribed antibiotics (Table 5).
| Care home type (number of residents) | Age, median (IQR) | Gender (male), n (%) | Capacity to provide informed consent for study, n (%) | Admitted to hospital in last 4 weeks, n (%) | Prescribed antibiotics in last 4 weeks, n (%) | Routinely wear incontinence pads, n (%)a | MUST: low risk, n (%)b | MUST: medium risk, n (%)b | MUST: high risk, n (%)b | Clinical Frailty Score of 1–3, n (%)c | Clinical Frailty Score of 4–6, n (%)c | Clinical Frailty Score of 7–9, n (%)c |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nursing (n = 87) | 87.0 (83.0–91.5) | 26 (29.9) | 25 (28.7) | 11 (12.6) | 17 (19.5) | 66 (75.9) | 55 (64.0) | 9 (10.5) | 22 (25.6) | 2 (2.3) | 42 (48.3) | 43 (49.4) |
| Residential (n = 87) | 85.0 (76.5–89.0) | 19 (21.8) | 33 (37.9) | 6 (6.9) | 18 (20.7) | 39 (44.8) | 58 (66.7) | 14 (16.1) | 15 (17.2) | 22 (25.3) | 47 (54.0) | 18 (20.7) |
| Dual registered (n = 100) | 85.0 (82.0–90.0) | 21 (21.0) | 20 (20.0) | 2 (2.0) | 22 (22.0) | 78 (78.0) | 59 (59.0) | 16 (16.0) | 25 (25.0) | 13 (13.0) | 51 (51.0) | 36 (36.0) |
| Overall (n = 274) | 86.0 (82.0–90.0) | 66 (24.1) | 78 (28.5) | 19 (6.9) | 57 (20.8) | 183 (66.8) | 172 (63.0) | 39 (14.3) | 62 (22.7) | 37 (13.5) | 140 (51.1) | 97 (35.4) |
Interim analysis
At the interim time point, we had approached 363 care home residents and recruited 260, giving a recruitment rate of 72% (95% CI 67% to 77%). We recorded at least one antibiotic prescription for 119 residents, giving an antibiotic prescribing rate of 46% (95% CI 40% to 52%). There were 152 antibiotic prescriptions recorded at the interim time point, 51 of which with a corresponding episode of AAD, giving an AAD rate of 34% (95% CI 25% to 42%). As all criteria had been met, we were permitted to begin designing a RCT of probiotics to prevent/ameliorate AAD in care home residents (Table 6).
| Outcome | Stop if | Estimate | Result (proceed/stop) |
|---|---|---|---|
| Recruitment | The proportion of residents recruited is less than 60% of those approached | 260/363 = 72% (95% CI 67% to 77%) | Proceed |
| Antibiotic prescribing | The proportion of recruited residents prescribed at least one course of antibiotics is < 27% | 119/260 = 46% (95% CI 40% to 52%) | Proceed |
| AAD | The proportion of antibiotic prescriptions (with follow-up data) resulting in at least one episode of AAD is < 18% | 51/152 = 34% (95% CI 25% to 42%)a | Proceed |
| Severe AADb | The proportion of antibiotic prescriptions resulting in at least one episode of AAD < 18% and the proportion of AAD episodes classed as severe is low | There were no episodes of AAD lasting longer than 2 weeks. Of the 34 diagnostic stool samples received and analysed, eight (24%) were found to contain C. difficile. There were no episodes of AAD that resulted in hospitalisation or death | Proceed |
Antibiotic prescriptions
There were 609 antibiotic prescriptions recorded over the study period, with 73.7% (n = 202) of residents being prescribed at least one antibiotic course. We found an incidence of 2.16 antibiotic prescriptions per care home resident-year (95% CI 1.90 to 2.46 prescriptions).
Antibiotics were prescribed for a median of 7.0 days (IQR 6.0–7.0 days), with 14.7% (n = 88) prescribed for less than 5 days, 74.9% (n = 447) prescribed for between 5 and 7 days, 6.7% (n = 40) prescribed for between 8 and 10 days and 3.7% (n = 22) prescribed for more than 10 days.
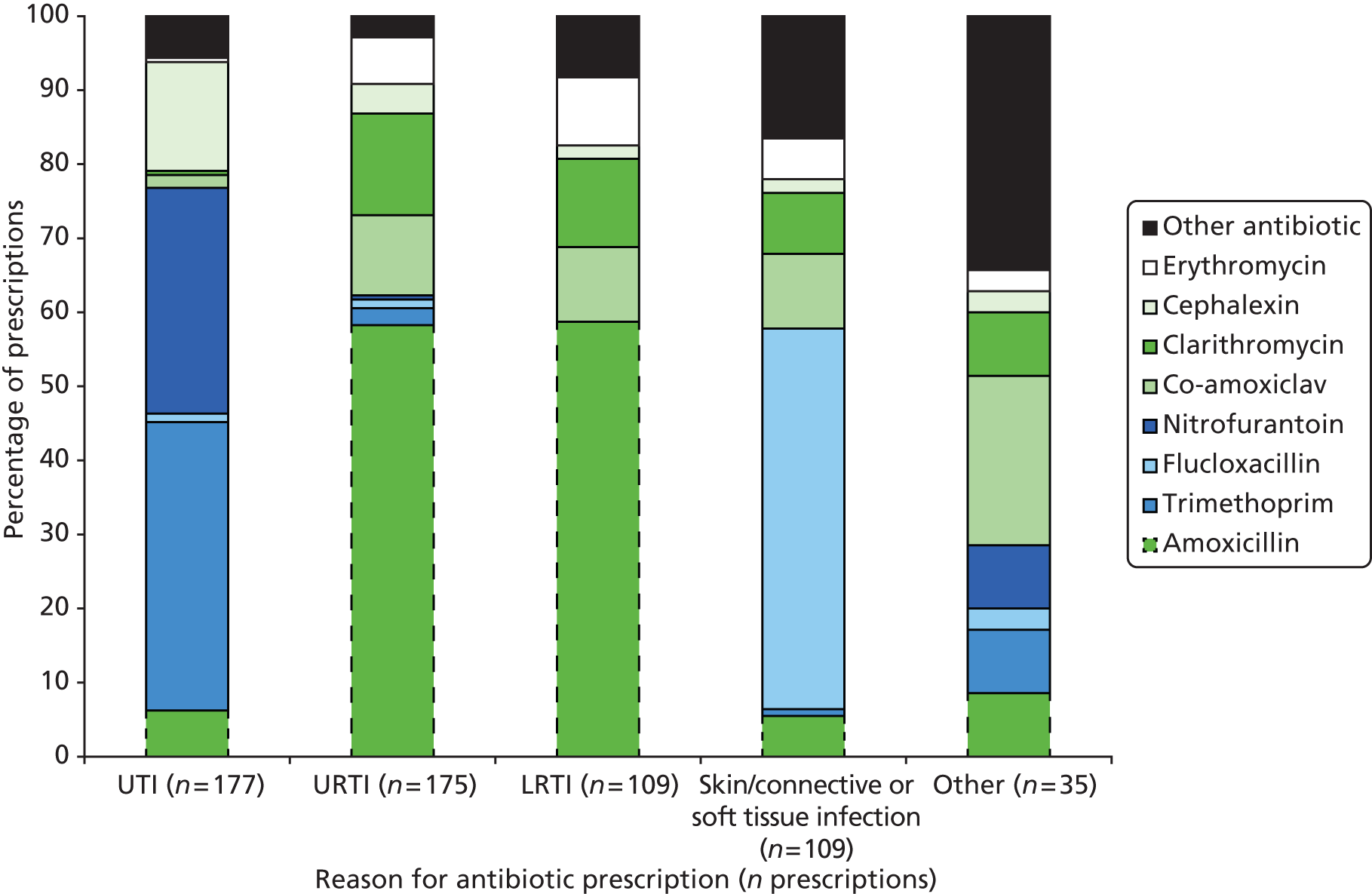
Antibiotics were most commonly prescribed for UTIs [29.3% (n = 177) of all antibiotic prescriptions], followed by URTIs [28.8% (n = 174)], skin/connective/soft tissue infections [18.2% (n = 110)] and lower respiratory tract infections (LRTIs) [18.0% (n = 109)]. The proportion of antibiotics prescribed for each indication varied by care home type. For example, prescriptions for UTIs accounted for 25.4% (n = 71) of all prescriptions in nursing homes, 43.5% (n = 70) in residential homes and 21.8% (n = 36) in dual-registered homes (Figure 5). The five most commonly prescribed antibiotics were amoxicillin [30.6% (n = 186) of all prescriptions], trimethoprim [12.7% (n = 77)], flucloxacillin [10.4% (n = 63)], nitrofurantoin [9.5% (n = 58)] and co-amoxiclav [8.7% (n = 53)]. A wide range of antibiotics were prescribed for each indication (Figure 6). The fluoroquinolone ciprofloxacin, previously implicated in CDAD, was prescribed relatively uncommonly, accounting for 3.0% (n = 18) of all antibiotic prescriptions recorded during the study period. Prescriptions of ciprofloxacin were primarily given for UTIs [44.4% (n = 8) of ciprofloxacin prescriptions] and skin/connective or soft tissue infections [27.8% (n = 5)]. Ciprofloxacin was also prescribed for LRTIs in two cases, for one UTI, for one gastrointestinal infection and also in one instance as prophylaxis.
FIGURE 5.
Reason for antibiotic prescriptions by care home type.
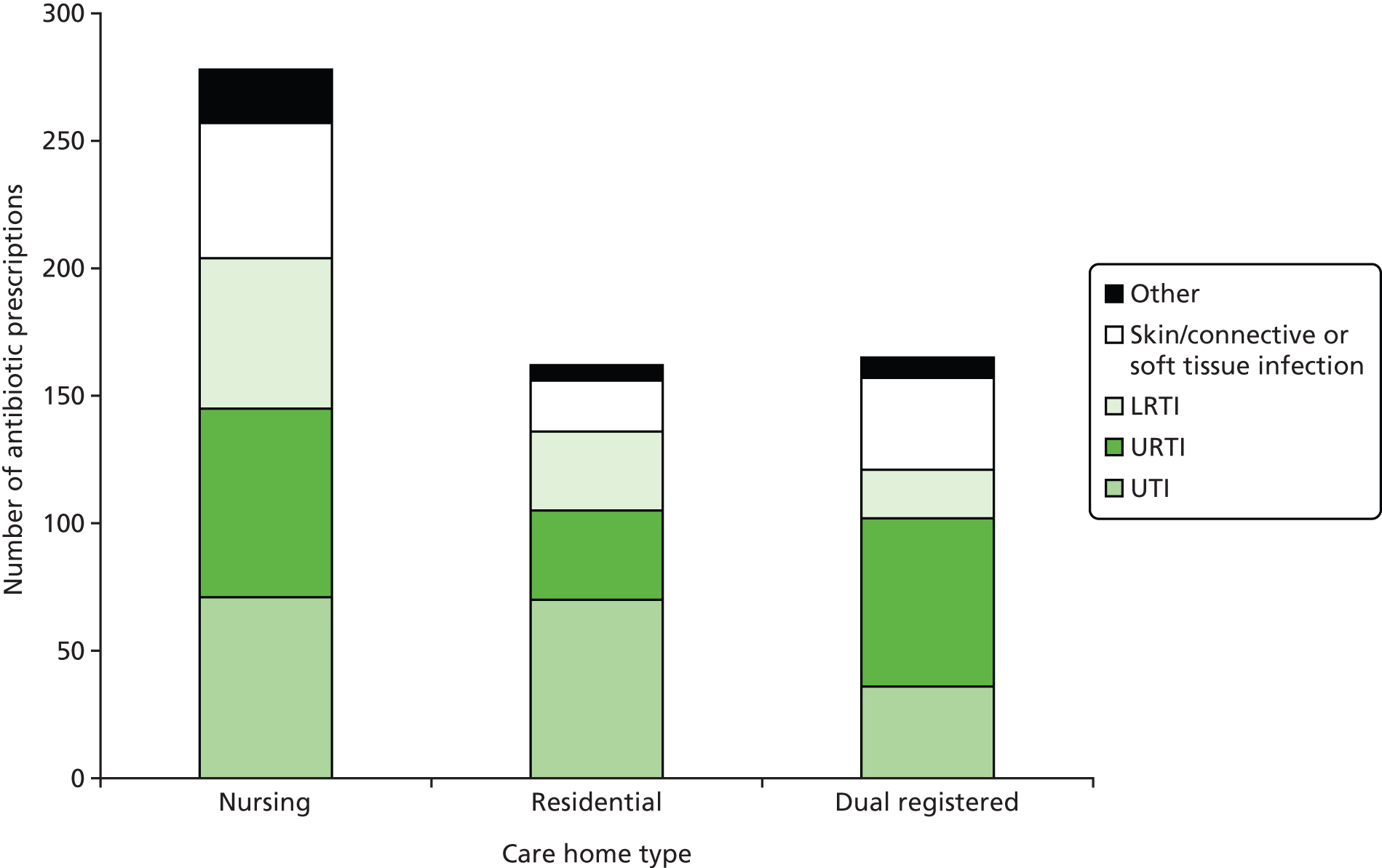
FIGURE 6.
Type of antibiotic prescribed by indication.
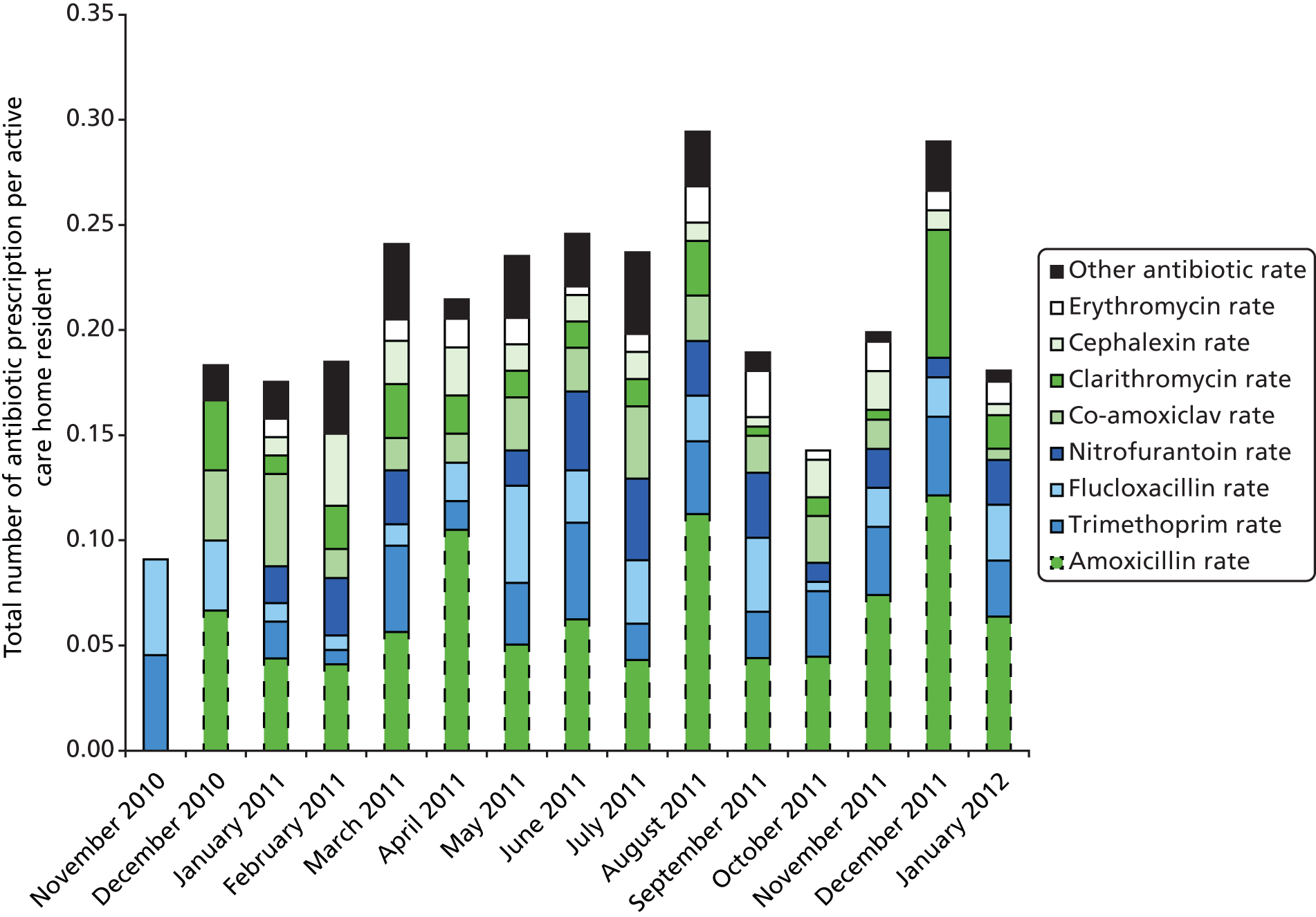
While the total number of antibiotic prescriptions each month varied considerably (range 0.09–0.29 prescriptions and average 0.21 prescriptions per resident), there was no obvious marked seasonal variation (Figure 7).
FIGURE 7.
Antibiotic prescription rate by study month (stacked by antibiotic type). The height of each bar represents the total antibiotic prescribing rate per resident for the corresponding study month.
Compared with nursing home residents, those residents from dual-registered care homes had a significantly lower chance of being prescribed antibiotics during the study period (OR 0.38, 95% CI 0.18 to 0.79; p = 0.009). The odds of being prescribed an antibiotic during the study period were 2.64 times higher for residents who had been prescribed antibiotics in the 4 weeks before study entry (95% CI 1.17 to 5.99; p = 0.020). Exposure to antibiotics was similar in residents who were vulnerable to moderately frail or severely frail to terminally ill and those who were very fit to managing well (Table 7).
| Variable | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| Resident characteristics | With care home characteristics | |||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Clinical frailty: very fit to managing well | Reference category for clinical frailty | |||||
| Clinical frailty: vulnerable to moderately frail | 2.14 (0.99 to 4.65) | 0.054 | 2.15 (0.98 to 4.71) | 0.057 | 1.88 (0.84 to 4.23) | 0.127 |
| Clinical frailty: severely frail to terminally ill | 1.58 (0.71 to 3.51) | 0.263 | 1.67 (0.74 to 3.75) | 0.218 | 1.33 (0.56 to 3.16) | 0.525 |
| Prescribed antibiotics 4 weeks prior to study entry | 2.56 (1.15 to 5.72) | 0.022 | 2.55 (1.14 to 5.72) | 0.023 | 2.64 (1.17 to 5.99) | 0.020 |
| Care home type: nursing | Reference category for care home type | |||||
| Care home type: residential | 0.48 (0.23 to 0.99) | 0.048 | – | – | 0.49 (0.22 to 1.09) | 0.080 |
| Care home type: dual registered | 0.39 (0.19 to 0.79) | 0.009 | – | – | 0.38 (0.18 to 0.79) | 0.009 |
Antibiotic-associated diarrhoea
Three antibiotic prescriptions (from two residents) provided no corresponding stool monitoring data. From the remaining 606 antibiotic prescriptions there were 571 stool monitoring periods, ranging between 1 and 11 weeks. The discrepancy between the number of prescriptions and monitoring periods arose because residents could be prescribed multiple antibiotics in the same week (hence, there was only one ongoing monitoring period). There were 447 unique episodes of AAD reported, with 43.5% (n = 87) of residents who were prescribed antibiotics experiencing at least one episode of AAD during the study period. There were 0.57 episodes of AAD per care home resident-year for those prescribed antibiotics (95% CI 0.41 to 0.81 episodes).
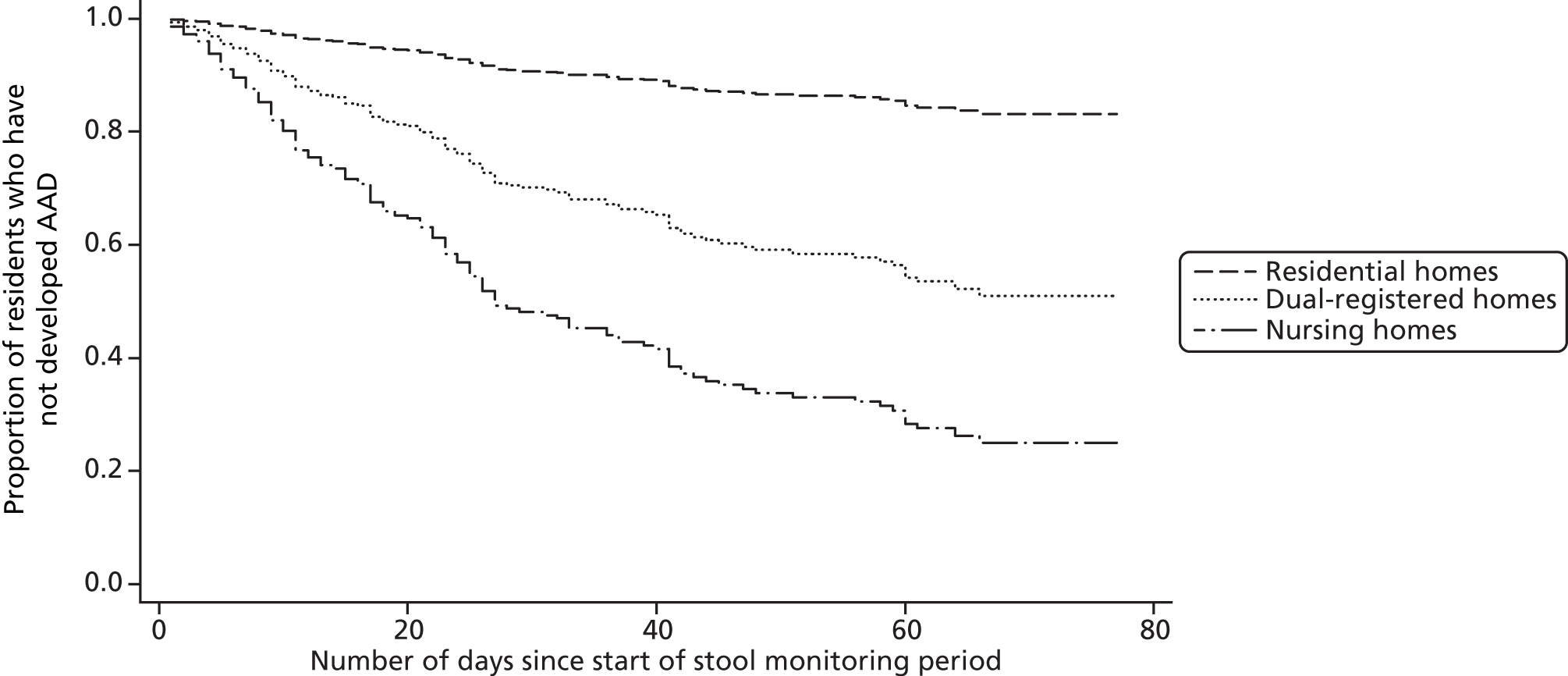
After controlling for length of stool-monitoring period, the odds of developing AAD during a stool monitoring period were more than twice as high in residents who were prescribed co-amoxiclav (OR compared with no co-amoxiclav prescription 2.19, 95% CI 1.06 to 4.52; p = 0.033), with the first AAD episode also occurring sooner (median time to first AAD episode for residents who were and were not prescribed co-amoxiclav was 23 days compared with 28 days, respectively; HR 2.08, 95% CI 1.18 to 3.66; p = 0.011). Time to first AAD episode was also shorter for residents who routinely used incontinence pads than for residents who did not (median 26 vs. 42 days, respectively; HR 2.54, 95% CI 1.26 to 5.13; p = 0.009). Compared with residents in nursing homes, those in residential homes were significantly less likely to develop AAD during the study period (OR 0.12, 95% CI 0.05 to 0.27; p < 0.001) (Table 8) and experienced a slower time to first AAD episode (median for residents in residential homes 56 days, median for residents in nursing homes 21 days; HR 0.14, 95% CI 0.06 to 0.32; p < 0.001) (Table 9 and Figure 8).
| Variable | Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|---|
| Stool monitoring period characteristics | With resident characteristics | With care home characteristics | ||||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Stools monitored for ≤ 4 weeks | Reference category for length of stool-monitoring period | |||||||
| Stools monitored for 5–8 weeks | 3.59 (2.01 to 6.41) | < 0.001 | 3.48 (1.95 to 6.23) | < 0.001 | 3.61 (1.99 to 6.55) | < 0.001 | 3.65 (1.96 to 6.82) | < 0.001 |
| Stools monitored for > 8 weeks | 2.99 (1.80 to 4.97) | < 0.001 | 3.04 (1.82 to 5.08) | < 0.001 | 3.27 (1.94 to 5.52) | < 0.001 | 3.40 (1.96 to 5.89) | < 0.001 |
| Prescribed co-amoxiclav | 2.31 (1.23 to 4.31) | 0.009 | 2.30 (1.19 to 4.46) | 0.014 | 2.05 (1.05 to 4.01) | 0.035 | 2.19 (1.06 to 4.52) | 0.033 |
| Resident had capacity to provide informed consent for study | 0.56 (0.29 to 1.08) | 0.084 | 0.72 (0.35 to 1.49) | 0.374 | 0.76 (0.36 to 1.62) | 0.472 | ||
| Resident frequently used incontinence pads at study entry | 2.84 (1.52 to 5.30) | 0.001 | 2.59 (1.30 to 5.15) | 0.007 | 1.90 (0.94 to 3.82) | 0.073 | ||
| Clinical frailty: very fit to managing well | Reference category for clinical frailty | |||||||
| Clinical frailty: vulnerable to moderately frail | 2.38 (0.95 to 6.01) | 0.066 | 2.43 (0.94 to 6.28) | 0.067 | 1.69 (0.59 to 0.45) | 0.328 | ||
| Clinical frailty: severely frail to terminally ill | 2.87 (1.10 to 7.44) | 0.031 | 2.30 (0.83 to 6.37) | 0.108 | 1.40 (0.43 to 4.56) | 0.575 | ||
| Care home type: nursing | Reference category for care home type | |||||||
| Care home type: residential | 0.11 (0.05 to 0.24) | < 0.001 | 0.12 (0.05 to 0.27) | < 0.001 | ||||
| Care home type: dual registered | 0.69 (0.36 to 1.35) | 0.278 | 0.60 (0.27 to 1.32) | 0.199 | ||||
| Variable | Univariable | Multivariable | ||||||
|---|---|---|---|---|---|---|---|---|
| Stool monitoring period characteristics | With resident characteristics | With care home characteristics | ||||||
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Prescribed co-amoxiclav | 2.13 (1.20 to 3.78) | 0.010 | 2.13 (1.20 to 3.78) | 0.010 | 1.96 (1.11 to 3.47) | 0.020 | 2.08 (1.18 to 3.66) | 0.011 |
| Resident frequently used incontinence pads at study entry | 4.04 (2.04 to 7.99) | < 0.001 | – | – | 3.80 (1.87 to 7.70) | < 0.001 | 2.54 (1.26 to 5.13) | 0.009 |
| Clinical frailty: very fit to managing well | Reference category for clinical frailty | |||||||
| Clinical frailty: vulnerable to moderately frail | 4.60 (1.50 to 14.07) | 0.007 | – | – | 3.76 (1.19 to 11.85) | 0.024 | 2.49 (0.78 to 7.94) | 0.124 |
| Clinical frailty: severely frail to terminally ill | 4.58 (1.44 to 14.50) | 0.010 | – | – | 2.98 (0.91 to 9.78) | 0.071 | 1.75 (0.50 to 6.09) | 0.381 |
| Care home type: nursing | Reference category for care home type | |||||||
| Care home type: residential | 0.10 (0.05 to 0.23) | < 0.001 | – | – | – | – | 0.14 (0.06 to 0.32) | < 0.001 |
| Care home type: dual registered | 0.80 (0.44 to 1.47) | 0.476 | – | – | – | – | 0.82 (0.43 to 1.57) | 0.547 |
FIGURE 8.
Survival curves illustrating the association between care home type and time to first AAD episode.
Clostridium difficile-associated diarrhoea
Of the 447 unique episodes of AAD, corresponding microbiological data from stool samples were available for only 55. C. difficile was cultured from eight of the sample. C. difficile was also detected in a further five stool samples taken after residents experienced loose stools (i.e. BSC type 5–7 stools) although the frequency of stools did not meet our definition of AAD. The 13 samples were obtained from nine residents in the same care home. In total, 12 samples were toxin B positive and there were nine different ribotypes (005, 010, 014, 020, 026, 027, 106, 160 and 193). No ribotype was found in more than one resident (Table 10).
| Anonymised unique participant ID | Date sample taken | Ribotype | Toxin B | CDAD according to our definition |
|---|---|---|---|---|
| 1 | 2 March 2011 | 193 | Positive | Yes |
| 1 | 8 May 2011 | 193 | Positive | Yes |
| 2 | 10 May 2011 | 160 | Positive | Yes |
| 3 | 24 April 2011 | 010 | Negative | No |
| 4 | 4 January 2012 | 020 | Positive | Yes |
| 5 | 12 April 2011 | 106 | Positive | Yes |
| 5 | 24 April 2011 | 106 | Positive | Yes |
| 6 | 7 June 2011 | 014 | Positive | No |
| 6 | 20 July 2011 | 014 | Positive | No |
| 7 | 23 April 2011 | 026 | Positive | Yes |
| 7 | 23 April 2011 | 026 | Positive | Yes |
| 8 | 30 April 2011 | 027 | Positive | No |
| 9 | 12 June 2011 | 005 | Positive | No |
Prevalence and risk factors for bowel carriage of antibiotic-resistant bacteria and Clostridium difficile in care home residents
Participant flow and recruitment
Of the 274 residents recruited, 80.7% (n = 221) provided a stool sample at study entry. Samples were collected from all 10 participating care homes, with collection rates between care homes varying from 66.7% to 96.4% (Table 11). Participants who provided samples had a median age of 86.0 years (IQR 82.0–90.0 years) and 78.3% (n = 173) were women. Over one-fifth (n = 49) had been prescribed antibiotics in the 4 weeks prior to study entry and 7.2% (n = 16) of participants had been admitted to hospital in this time frame. There were no statistically significant differences between participants who did and did not provide samples (Table 12).
| Care home type | Care home | Number of participants recruited | Stool sample collection rate, % (n) |
|---|---|---|---|
| Nursing | A | 21 | 61.9 (13) |
| B | 42 | 83.3 (35) | |
| D | 11 | 90.9 (10) | |
| I | 13 | 69.2 (9) | |
| Residential/EMI | E | 18 | 66.7 (12) |
| F | 17 | 82.4 (14) | |
| H | 24 | 83.3 (20) | |
| J | 28 | 96.4 (27) | |
| Dual registered | C | 59 | 74.6 (44) |
| G | 41 | 90.2 (37) | |
| Overall | 274 | 80.7 (221) | |
| Variable | Provided stool sample data (N = 221) | Did not provide stool sample data (N = 53) | p-value |
|---|---|---|---|
| Age of resident (years), median (IQR) | 86.0 (82.0–90.0) | 85.0 (82.0–90.0) | 0.915 |
| Age of resident < 80 years, % (n/N) | 21.7 (48/221) | 15.1 (8/53) | 0.283 |
| Age of resident ≥ 80 years, % (n/N) | 78.3 (173/221) | 84.9 (45/53) | |
| Gender (female), % (n/N) | 78.3 (173/221) | 66.0 (35/53) | 0.061 |
| Capacity to provide informed consent for study, % (n/N) | 28.1 (62/221) | 30.2 (16/53) | 0.757 |
| Clinical frailty: very fit to managing well, % (n/N) | 12.2 (27/221) | 18.9 (10/53) | 0.216 |
| Clinical frailty: vulnerable to moderately frail, % (n/N) | 50.2 (111/221) | 54.7 (29/53) | |
| Clinical frailty: severely frail to terminally ill, % (n/N) | 37.6 (83/221) | 26.4 (14/53) | |
| MUST: low risk, % (n/N) | 62.3 (137/220) | 66.0 (35/53) | 0.528 |
| MUST medium risk, % (n/N) | 15.5 (34/220) | 9.4 (5/53) | |
| MUST: high risk, % (n/N) | 22.3 (49/220) | 24.5 (13/53) | |
| Prescribed antibiotics in last 4 weeks, % (n/N) | 22.2 (49/221) | 15.1 (8/53) | 0.254 |
| Admitted to hospital in last 4 weeks, % (n/N) | 7.2 (16/221) | 5.7 (3/53) | 0.684 |
Baseline data
Stool type
There was wide variation in the consistency of stool samples. Samples were most often described as a BSC type 4, but over one-quarter of samples (n = 58) were described as a types 5–7 (Table 13).
| Stool typea | Samples, % (n) |
|---|---|
| 1 | 5.4 (12) |
| 2 | 14.0 (31) |
| 3 | 14.9 (33) |
| 4 | 37.6 (83) |
| 5 | 15.4 (34) |
| 6 | 8.6 (19) |
| 7 | 2.3 (5) |
| Missing stool type | 1.8 (4) |
| Overall | 100.0 (221) |
Outcomes and estimates
Isolates cultured
In total, 478 isolates were cultured from the 221 collected stool samples (approximately 2.2 isolates cultured per sample) (Table 14). The three most commonly cultured isolates were E. coli [88.2% (n = 195) of samples], Enterococcus spp. (ex Thiercelin and Jouhaud 1903) Schleifer and Kilpper-Bälz 1984 [54.3% (n = 120)] and Pseudomonas spp. Migula 1894 [25.3% (n = 56)]. The number of isolates cultured varied among care homes, as did the number of different isolates found in their stool samples (Table 15). In terms of total bacterial load, the total Columbia blood agar count was most commonly in the region of 107–108 per 10-μl loop of faeces.
| Care home type | Care home | Number of samples/participants | Total number of isolates cultured | Average number isolates/participant | Average number isolates/participant |
|---|---|---|---|---|---|
| Nursing | A | 13 | 28 | 2.2 | 2.2 |
| B | 35 | 75 | 2.1 | ||
| D | 10 | 23 | 2.3 | ||
| I | 9 | 21 | 2.3 | ||
| Residential/EMI | E | 12 | 24 | 2.0 | 2.1 |
| F | 14 | 27 | 1.9 | ||
| H | 20 | 44 | 2.2 | ||
| J | 27 | 55 | 2.0 | ||
| Dual registered | C | 44 | 91 | 2.1 | 2.2 |
| G | 37 | 90 | 2.4 | ||
| Overall | 221 | 478 | 2.2 | 2.2 | |
| Isolate | Care home type | Overall | ||||||
|---|---|---|---|---|---|---|---|---|
| Nursing | Residential/EMI | Dual registered | ||||||
| % (n) | Range (%)a | % (n) | Range (%)a | % (n) | Range (%)a | % (n) | Range (%)a | |
| E. coli | 89.6 (60) | 85.7–100.0 | 90.4 (66) | 83.3–96.3 | 85.2 (69) | 84.1–86.5 | 88.2 (195) | 83.3–100.0 |
| Enterococcus spp. | 59.7 (40) | 48.6–90.0 | 50.7 (37) | 40.7–65.0 | 53.1 (43) | 45.5–62.2 | 54.3 (120) | 40.7–90.0 |
| Pseudomonas spp. | 22.4 (15) | 10.0–44.4 | 23.3 (17) | 10.0–33.3 | 29.6 (24) | 22.7–37.8 | 25.3 (56) | 10.0–44.4 |
| Klebsiella b /Enterobacter c /Serratia d | 16.4 (11) | 7.7–20.0 | 17.8 (13) | 0.0–25.0 | 17.3 (14) | 16.2–18.2 | 17.2 (38) | 0.0–25.0 |
| Other | 16.4 (11) | 7.7–22.9 | 12.3 (9) | 0.0–25.0 | 18.5 (15) | 16.2–20.5 | 15.8 (35) | 0.0–25.0 |
| Proteus spp. | 14.9 (10) | 0.0–23.1 | 11.0 (8) | 7.1–20.0 | 19.8 (16) | 15.9–24.3 | 15.4 (34) | 0.0–24.3 |
| Number of samples/participants | 100.0 (67) | 100.0 (73) | 100.0 (81) | 100.0 (221) | ||||
Antibiotic resistance
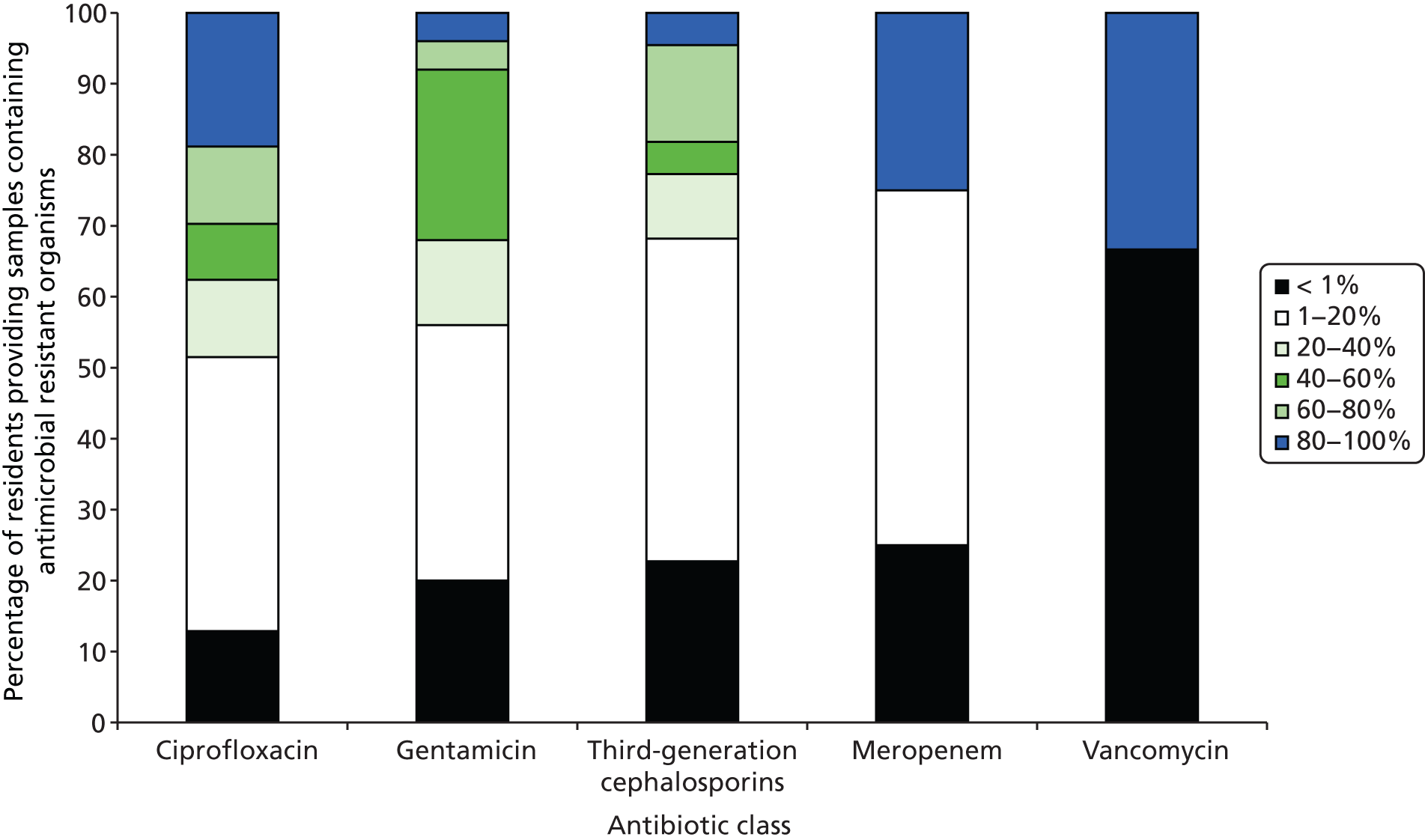
Organisms which were resistant to one or more of the tested antibiotics were found in 51.6% (n = 114) of participants. Enterobacteriaceae resistant to ciprofloxacin were found in 47.1% (n = 104) of participants, to gentamicin in 12.2% (n = 27), to third-generation cephalosporins in 10.9% (n = 24), to meropenem in 1.9% (n = 4) and to vancomycin in 1.4% (n = 3). There was wide variation in the proportion of participants providing stool samples containing bacteria resistant to various antibiotics in different types of care homes, with care home-level intracluster correlations (ICCs) ranging from 0.00 for meropenem and vancomycin to 0.16 for gentamicin (Table 16). Of the four residents who provided samples containing meropenem-resistant isolates, one was prescribed an antibiotic (amoxicillin) and none had been hospitalised in the 4 weeks prior to study entry. Although resistance to ciprofloxacin, gentamicin and third-generation cephalosporins was mainly found in E. coli [95.2% (n = 99), 63.0% (n = 17) and 73.9% (n = 17), respectively], resistance to meropenem was exclusively found in Pseudomonas spp. (Figure 9). Resistant bacterial load, expressed as a percentage of total bacterial load, varied between antibiotic classes (Figure 10). For example, where isolates resistant to ciprofloxacin were found in stool samples, ciprofloxacin-resistant isolates accounted for less than 1% of the total quantity of isolates cultured in samples 12.8% of the time.
| Care home type | Antibiotic/antibiotic class | Number of samples | ||||
|---|---|---|---|---|---|---|
| Ciprofloxacin | Gentamicin | Ceftazidime/cefotaxime (third-generation cephalosporins) | Meropenem | Vancomycin | ||
| Nursing | 62.7 (42) | 16.4 (11) | 10.4 (7) | 0.0 (0) | 0.0 (0) | 67 |
| Residential | 35.6 (26) | 6.8 (5) | 8.2 (6) | 2.7 (2) | 2.7 (2) | 73 |
| Dual registered | 44.4 (36) | 13.6 (11) | 13.6 (11) | 2.5 (2) | 1.2 (1) | 81 |
| Overall | 47.1 (104) | 12.2 (27) | 10.9 (24) | 1.8 (4) | 1.4 (3) | 221 |
| Care home ICCa | 0.06 | 0.16 | 0.10 | 0.0 | 0.0 | – |
FIGURE 9.
Cultured isolates and their resistance to antibiotics.
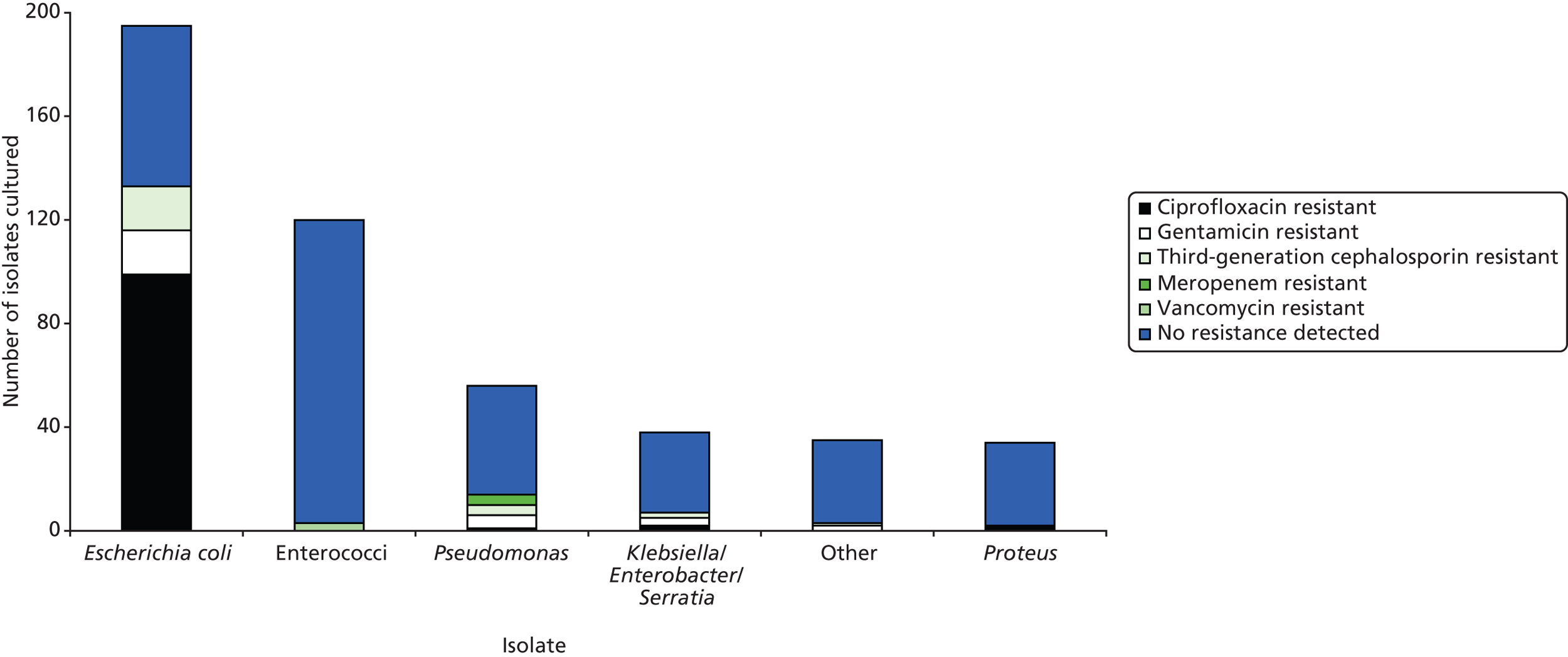
FIGURE 10.
Percentage of resistant bacterial load by antibiotic class.
Factors associated with carriage of antibiotic-resistant bacteria
The odds of participants carrying antibiotic-resistant bacteria in their stools significantly increased with age (OR for 80 years or older 2.54, 95% CI 1.23 to 5.26; p = 0.012) and previous antibiotic use in the 4 weeks prior to study entry (OR 2.21, 95% CI 1.11 to 4.43; p = 0.025). The odds of carrying antibiotic-resistant bacteria were significantly lower for participants in residential homes than for those in nursing homes (OR 0.44, 95% CI 0.21 to 0.91; p = 0.028). After controlling for the age of the resident, clinical frailty status was not significantly associated with carriage of antibiotic-resistant bacteria (Table 17).
| Variable | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| Resident characteristics | With care home characteristics | |||||
| OR (95% CI) | p-value | OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age of resident < 80 years | Reference category for age of resident | |||||
| Age of resident ≥ 80 years | 2.94 (1.49 to 5.81) | 0.002 | 2.67 (1.30 to 5.49) | 0.007 | 2.54 (1.23 to 5.26) | 0.012 |
| Clinical frailty: very fit to managing well | Reference category for clinical frailty | |||||
| Clinical frailty: vulnerable to moderately frail | 2.48 (1.03 to 6.00) | 0.044 | 2.11 (0.82 to 5.40) | 0.122 | 1.78 (0.68 to 4.65) | 0.243 |
| Clinical frailty: severely frail to terminally ill | 2.21 (0.89 to 5.48) | 0.089 | 1.74 (0.64 to 4.69) | 0.275 | 1.35 (0.46 to 3.78) | 0.614 |
| Prescribed antibiotics 4 weeks prior to study entry | 2.07 (1.07 to 4.00) | 0.031 | 2.10 (1.06 to 4.17) | 0.034 | 2.13 (1.06 to 4.26) | 0.033 |
| Care home type: nursing | Reference category for care home type | |||||
| Care home type: residential | 0.39 (0.20 to 0.77) | 0.007 | – | – | 0.44 (0.21 to 0.93) | 0.030 |
| Care home type: dual registered | 0.57 (0.30 to 1.11) | 0.099 | – | – | 0.58 (0.29 to 1.16) | 0.123 |
Prevalence of Clostridium difficile
Clostridium difficile was cultured in 7.2% (n = 16) of stool samples. The prevalence varied between care homes, from no C. difficile detected in four care homes to C. difficile being detected in 19.4% (n = 7) of samples from a single home.
One of the 16 samples from which C. difficile was cultured was toxin B negative, with the remaining 15 being toxin B positive. There were 11 different ribotypes (001, 002, 005, 015, 021, 027, 062, 103, 106, 160 and 193). In the majority of cases, ribotypes were unique to each home, with one instance of a ribotype occurring within the same home twice (n = 106). The care home level ICC was 0.29 (Table 18).
| Care home type | Care home | Number of samples | C. difficile cultured, % (n) | Ribotypesa |
|---|---|---|---|---|
| Nursing | A | 13 | 15.4 (2) | 015, 027 |
| B | 36 | 19.4 (7) | 005, 027, 103, 106 (×2), 160, 193 | |
| D | 10 | 0.0 (0) | N/A | |
| I | 9 | 22.2 (2) | 001, 005 | |
| Residential/EMI | E | 12 | 0.0 (0) | N/A |
| F | 14 | 0.0 (0) | N/A | |
| H | 20 | 15.0 (3) | 002, 005, 027 | |
| J | 27 | 0.0 (0) | N/A | |
| Dual registered | C | 43 | 2.3 (1) | 021 |
| G | 37 | 2.7 (1) | 062 | |
| Overall | 221 | 7.2 (16) | 001, 002, 005 (×3), 015, 021, 027 (×3), 062, 103, 106 (×2), 160, 193 | |
Barriers and implementation issues identified and lessons learned
Research experience
Very few of the 10 sites had any experience of participating in research. Care homes are a unique research environment since 24-hour care is provided to residents and, within any 1 day, care home staff have to deal with many unpredictable daily events that need an immediate response. Careful consideration and a flexible approach were needed to embed a study into this busy environment. We worked closely with our lay representative and care home staff ahead of study commencement and throughout the study to ensure implementation of PAAD stage 1 went as smoothly as possible and that all valuable and essential lessons learned from PAAD stage 1 could be applied to PAAD stage 2. The most important factors we identified for successfully delivering research of this kind in care home included the following.
Timing of approach to care homes
The time of year for approaching the care homes was critical, i.e. care homes are significantly affected by winter pressures. Thus, training, embedding study procedures and commencing recruitment proved difficult and, as a result, many care homes did not start recruitment until late winter or spring. Approaching care homes and ensuring the study was up and running well ahead of the winter period was pivotal when it came to setting up PAAD stage 2.
Care home set-up and training
A significant amount of time at the beginning was spent engaging with the care home staff and building up their understanding, as well as setting up the processes, documentation and training for all staff. Initial half-day training sessions were delivered and short (1 hour), tailored training sessions concentrating on each staff member’s role and responsibility for the shift (day, night and weekend) were also designed. Realistic time frames need to be set for approaching and bringing on board care homes. In addition, we found that staggering care home set-up and ensuring recruitment has commenced before focusing on another care home was likely to increase compliance with the Protocol and dedication of care home staff to study procedures.
Staff engagement and motivation
Care home staff had little understanding of what it meant to carry out research; as a result they felt overwhelmed by the tasks required of them, and not all staff understood the protocol. Half-day workshops were also offered to care home staff to meet the study team and principal investigators (PIs) to discuss issues and successes for the site, as well as focusing on topics such as how to improve recruitment or resolve quality of data. Additional monetary and non-monetary incentives, such as providing opportunities for continuing professional development (CPD) (e.g. GCP training) or personal incentives for staff working on specific aspects of the protocol (e.g. gift vouchers), increased the level of interest in the study by junior staff and return of completed stool-monitoring CRFs.
Communication
The shift patterns of care home staff meant that not all handover information was communicated well. Key members of staff covering all shift patterns were required to discuss the PAAD study at each shift change, and prompts were added to handover sheets as a reminder of this. Posters were provided for care home notice boards to let relatives and residents know about the study and to ensure that the PAAD study remained at the forefront of their staff members’ minds. Newsletters were also used to share information between sites and to remind staff of key study procedures.
Additional support for care homes
To ensure compliance with all study procedures, care homes required a lot of additional support. Within PAAD stage 1, we introduced a research nurse in two care homes for 2.5 days per week and saw a huge improvement in all areas of study procedures. We saw a dramatic increase in recruitment rates (all residents were recruited within 3 weeks of site initiation), data collection quality and confidence, and dedication of care home staff in delivering study procedures.
Summary
In summary, over 12 months the study team found that care homes are very different to other environments in which general clinical research is carried out and that some of the issues raised are the result of researchers’ lack of understanding in this new research environment rather than any naivety on the part of care home staff. The study team has identified better ways of undertaking research in care homes. Recommendations for future research conducted in these unique environments have been made and include:
-
At the design stage of the study, consult an ‘expert’ with experience of working in a care home.
-
Allow plenty of time to initially approach care homes, set up the sites, recruit residents and undertake the study.
-
Bolster the confidence of care home staff.
-
Ensure that processes are easy for staff to complete and where possible follow their own processes, so as to not add to their workload.
-
Identify study research leads among those working all shift patterns (weekdays, nights and weekends).
-
Provide training to care home staff several times: 2–3 weeks before start of the study, before recruitment, once the study begins and continuously after that point, with specific staff groups to identify what is required of them.
-
Embed own staff or employ research nurses in the care homes to collect information from residents and carry out any sampling.
Summary of main findings
This prospective observational cohort study of 274 residents from 10 care homes across South Wales found that antibiotics were prescribed at a rate of 2.16 per resident-year, with almost three-quarters of residents prescribed at least one antibiotic course during the 16-month study period (median length of follow-up 310 days). Residents were over two and a half times more likely to be prescribed antibiotics during the study period if they had been prescribed antibiotics in the 4 weeks prior to study entry. Residents were prescribed a wide range of antibiotics for each indication. The incidence of AAD in those prescribed antibiotics was 0.57 episodes per resident-year, with 43.5% of residents prescribed antibiotics experiencing at least one episode of AAD. Not all episodes of diarrhoea following antibiotic use can be ascribed to antibiotics, and our study does not seek to demonstrate causality, merely association. CDAD occurred in less than 15% of residents who developed AAD and for whom a stool sample was sent for microbiological analysis. Although all CDAD episodes were found in residents from the same care home, the unique ribotypes suggest that the episodes were not associated with an outbreak. Residents in nursing homes were more likely to be prescribed antibiotics and experience AAD. Residents were more likely to experience AAD if they had been prescribed co-amoxiclav or if they routinely used incontinence pads. The majority of residents (80.7%) provided a stool sample at baseline. Analysis of these samples demonstrated that there were high levels of carriage of antibiotic-resistant organisms in care home residents, particularly of ciprofloxacin-resistant organisms. Although recent hospitalisation was not associated with carriage of resistant organisms, recent antibiotic use was. Carriage of resistant organisms was significantly higher in nursing homes than it was in residential homes, even after controlling for clinical frailty. C. difficile was more common in nursing homes, but there was little suggestion of clustering by care home type.
Chapter 3 Exploring ethical and practical challenges of conducting research in care home settings
Background
Both stages of the PAAD study were governed by laws and regulations concerning mental capacity to provide consent to participate in research; PAAD stage 1 was covered by the MCA (2005)45 and, for those residents who lacked capacity, personal consultees were able to provide agreement on behalf of the resident for participation. PAAD stage 2 was covered by the Medicines for Human Use (Clinical Trials): Regulations (2004)48 and consent would need to be given by a personal legal representative or a professional legal representative of the participant.
The PAAD study presented two main challenges relating to consent. First, there were two categories of residents who were eligible to join the PAAD study: those who had capacity and were able to consent to participate for themselves and those who were unable to consent for themselves [lacked capacity under the MCA (2005)45]. Trained senior care home staff/nursing staff or trained PAAD study research nurses were responsible for assessing mental capacity by periodically checking that the information the resident was given was understood by the resident. In addition, PAAD stage 2 would present novel challenges in relation to advanced consent both for individuals who have capacity and for those who lack capacity. Researchers49 have previously advocated an advanced consent procedure for individuals who might not be able to give their consent to participate in a clinical trial at the time of randomisation. Advanced consent may be particularly useful if the study intervention is administered in an emergency setting. 50 For PAAD stage 2, ‘advanced consent’ refers to a situation in which residents would be recruited into the study for a 12-month period, but randomised to receive a probiotic or placebo only if they were to be prescribed an antibiotic (which may be anything from the next day to 365 days after giving consent). Indeed, it was possible that some residents could enter PAAD stage 2 having capacity to consent, and then subsequently lose capacity (either temporarily or permanently) because of an illness or deterioration in their health during the 12 months that their consent to be part of PAAD stage 2 applied. The MCA (2005)45 makes provisions for a personal consultee to advise on the continued participation of research subjects should they lose capacity during a study,51,52 but this is in contrast to clinical trials of investigational medicinal products (CTIMPs), when consent from an adult to participate in a trial remains valid, even after loss of capacity, provided that the trial is not changed in any material way.
In PAAD stage 1, when residents lacked capacity, the resident’s consultee, typically a friend or relative who visited the resident most often, was given information about the study, and asked for advice about whether or not, in his or her view, the resident would have wanted to join the study.
In PAAD stage 2 (a CTIMP), in the event of residents lacking capacity, a legal representative would have been required to give consent for the resident to participate in the trial. For the majority of residents who lack capacity, a legal representative would typically be a friend or relative (a personal legal representative), but could have been a professional legal representative. A professional legal representative should not be connected with the conduct of the trial.
Owing to the PAAD study presenting novel challenges in relation to consent, we aimed to explore some of the ethical and practical challenges of conducting these studies within the care home setting using qualitative methods. The aim was to optimise an acceptable informed consent process in a vulnerable population in preparation for PAAD stage 2, but also to inform the design of other similar studies.
Design and aims
Specific aims of the qualitative exploration of practical and ethical issues
-
To collect data on the various merits and problems associated with a number of models of consent for both PAAD stage 1 and PAAD stage 2 for residents who either have capacity or lack capacity.
-
To establish the views of residents, relatives, care home staff and GPs regarding participation in two study designs (observational vs. CTIMP).
-
To establish the feasibility and acceptability of taking advance consent for research trial procedures (as would be required in PAAD stage 2) in care home residents.
-
To establish views of relatives, residents and care home staff on participating in PAAD stage 1.
-
To establish care home staff views about the study training for staff, the requirements of the study and its impact on the care home including the implications of potentially finding C. difficile in the care home population.
We used qualitative research methods because this allowed us to explore in depth respondents’ views and experiences, including topics that we were unable to predict in advance. We thought it important to understand the views of a range of stakeholders and, so, our respondents included residents, relatives, care home staff and GPs who have a responsibility for the general medical care of residents and who may be asked to assess eligibility for research studies. We recruited through 10 care homes in South Wales, which were participating in PAAD stage 1. Data collection was undertaken through a combination of face-to face interviews with residents, relatives and GPs to facilitate in-depth reflection of respondents’ own involvement in the study and focus groups among care home staff to facilitate discussion about their collective involvement in research.
A shorter version of this exploration of ethical and practical challenges of conducting research in care home settings has previously appeared in the journal Trials. 53
Participant recruitment
Recruitment of residents
Care home staff approached residents whom they felt had the mental capacity to consent to the qualitative study. Residents were considered eligible to participate in the qualitative study even if they had not given consent to participate in PAAD stage 1. A total of 14 residents consented to the study, all of whom had consented to take part in PAAD stage 1. A research nurse conducted interviews with informants in a private room in the care home, usually the resident’s bedroom. Interviews lasted between 9 and 54 minutes, with an average of 23 minutes. Participating residents were offered £25 in recognition of their time.
Recruitment of relatives
Relatives of residents, four partners and 10 sons or daughters, were also invited by the care home staff to be interviewed (all of whom had given advice as a personal consultee that their relative should be part of PAAD stage 1) and all consented. Once again, interviews were conducted in a private room within the care home by a research nurse. Interviews lasted between 12 and 31 minutes, with an average of 19 minutes. Participating relatives were offered £25 in recognition of their time.
Recruitment of care home staff
Each care home was asked to nominate three members of staff who were most closely involved with the PAAD study. Staff were invited to participate in a focus group that took place at a city-centre hotel. A total of 19 staff from 10 care homes participated in the focus groups. Two focus groups were held, one with senior staff (10 participants) and the other with junior staff (nine participants). Each group discussion lasted approximately 90 minutes. The focus groups were facilitated by a research nurse and a qualitative researcher, both of whom were experienced in running focus groups. Neither had had prior contact with the care home staff regarding the PAAD study. As care home staff were participating ‘off duty’, £25 was offered to each participant. Lunch was provided, and travel expenses were reimbursed.
Recruitment of general practitioners
Senior care home staff were asked to name the main GP who attended residents within the care home. Letters of invitation were sent to all 11 GPs named and two GPs responded. In view of this, the researchers directly contacted 69 other GPs in the health board area to request an interview, which resulted in 10 GPs agreeing to take part in face-to-face interviews. Three of these GPs were aligned to a care home participating in PAAD stage 1, while seven were not but nevertheless attended residents in care homes. GP interviews lasted between 20 and 35 minutes with an average of 27 minutes. GPs were offered £50 for their time.
Ethical permissions
South East Wales REC gave ethical approval for the qualitative study on 10 March 2011. All respondents were provided with an information sheet about the purpose of the qualitative study and what was being asked of them. All respondents signed a consent form immediately prior to the interview or focus group.
Data collection
An interview topic guide defined the main topics while allowing flexibility to pursue issues in more depth as they emerged from the interviews and focus groups. Broad subject areas included participants’ views of the consent processes that had been used for PAAD stage 1 and their experiences of participating in PAAD stage 1, where appropriate. We collected data on the various merits and problems associated with a number of models of consent that could be used for a trial that lasts a reasonably long period such as 12 months. Discussion also covered how consent discussions should take place, for example with a witness, over the telephone, in person or by post. Respondents were asked what time frame they felt advanced consent should cover. In addition, the researcher presented the respondent with a range of hypothetical scenarios about taking advanced consent and asked the respondents to reflect on the potential advantages and disadvantages (Box 1). They were also asked for their opinion on what should happen should the resident lose (and potentially regain) capacity during a research trial. Data from the focus groups and interviews were audio-recorded and transcribed verbatim.
Mrs Jones is an elderly resident in a care home participating in PAAD stage 2. Mrs Jones has been assessed by one of the care home staff as not having capacity to consent to the PAAD study for herself. Staff in the care home have approached her daughter, who lives in London and visits her mother at the home about once a month, to ask if she would act as a personal legal representative on her mother’s behalf to give consent for her to participate in a RCT of a probiotic versus a placebo.
What do you think might be some of the concerns of Mrs Jones’s daughter?
Mr Edwards is a resident in a care home and has been assessed as having capacity to consent himself for the PAAD study stage 2. However, 6 months later he loses capacity. There is still a likelihood that he will need antibiotics in the future.
Do you feel that Mr Edwards should still be part of the study?
What would your concerns be?
Data analysis
Data were analysed using thematic analysis with an abductive approach (incorporating themes that had been identified in advance and themes that were derived from the data). 54 This approach involves systematically coding data according to a thematic framework, which is developed iteratively. Researchers met regularly to compare coding, discussing evidence for themes, and came to a consensus on the final framework. The thematic framework was applied to the data using the coding software package NVivo 8 (QSR International Pty Ltd, Doncaster, VIC, Australia). Interpretations were discussed between members of the study team.
Interviews with residents
A total of 14 residents were interviewed. There were no major objections to being involved in research generally and the PAAD study specifically, although some of the residents who were interviewed did not remember that they had consented to the PAAD study or what it involved.
When asked why they agreed to take part in PAAD stage 1, residents gave a variety of reasons, including wanting to help science, wanting to help others including their nursing home as diarrhoea is a problem for them, because it was new and interesting and because they had no objections to participating. One resident stated that he could not remember why he took part.
With regard to being in a study which used a method of advanced consent, the majority of residents stated that they would be happy to be consented just once and it was not necessary to keep checking to see if they still wanted to be involved:
Once at the beginning, I think that would be sufficient.
Resident 9
One resident stated that she thought that the researcher should check consent every 3 months to ensure that she was well enough to participate. Moreover, another stated that there was no need to reconsent somebody as long as there was an option to withdraw from the study.
I think there should be an escape route if you like.
Resident 12
Only two residents wanted someone to check more than once to see if they were still happy to be part of the study. In this respect, residents seemed to be confusing giving their consent to participate with a health professional checking their eligibility to be randomised.
Opinions on asking relatives to consent on behalf of residents were divided. The majority felt that the relative would know the wishes of the resident and that it would be better to ask a relative than a professional legal representative.
Oh yes, a relative, because I think they know you better and you understand them better.
Resident 10
However, one resident stated that relatives should only be chosen if they were trustworthy and close to the resident.
You don’t know, you see, whether they’re [the family] all that trustworthy.
Resident 9
Other residents felt that some relatives may not want to be bothered and may be too busy to help. Other potential problems included the relative not agreeing with the research, difficulties with relatives who do not live in the vicinity of the care home and some relatives being elderly and confused.
When discussing if a resident should continue to participate in the study if he or she lost mental capacity, some suggested that the resident should be withdrawn from the study as the research would be too demanding. Others, however, felt that the resident should continue to participate. Opinions were divided on whether or not to give this decision to the relative.
I would say exceptionally, I would want to proceed with the survey [research study] because it’s so important.
Resident 12
The people doing the research, I think they should really ask whoever is acting on their behalf.
Resident 13
Interviews with relatives
We interviewed 14 relatives. All 14 relatives we interviewed were happy for their resident relative to participate in both PAAD stages 1 and 2. Many felt that, compared with other medical studies involving invasive treatments, the PAAD study was comparatively harmless and they could see the potential benefits to medicine and society. No major ethical concerns were spontaneously raised by relatives.
No relatives raised any concerns about C. difficile circulating within the home. A few relatives did raise some queries about the probiotic including:
-
Have the probiotics been tested on healthy populations and what was the outcome?
-
How might the probiotics affect the resident if they are normally prone to constipation?
-
Could the probiotics ‘cancel out’ the effect of the antibiotic?
-
Could the probiotic affect any other medication that the resident takes?
-
Would it matter if the resident had problems with swallowing?
Some relatives said they wanted more detail in the information sheet, while others reported that they were sometimes overwhelmed with paperwork, and wanted the information in a simple and short format.
The relatives included in this sample were typically regular visitors at the homes. Many reported that they found this helpful when being asked to be a personal consultee or personal legal representative. They felt they knew the residents’ wishes well. They felt that relatives who visit irregularly, or who have little contact with the home, would feel more anxious about being asked to take on the roles of personal consultee or personal legal representative, partly because they would not be able to personally monitor the resident’s condition and partly because there may be less trust established between care home staff and relatives.
I think they [residents] trust their relative to act on their behalf. I think a stranger coming in and trying to talk them into doing something, they would be on their guard.
Relative 12
I would say ‘no’ (to acting as a personal legal representative) if I were only here once a month to be honest with you.
Relative 8
None of the 14 relatives we interviewed expressed any major concerns about PAAD stage 2 lasting 12 months. Generally, they understood why obtaining advanced consent was required. Two relatives commented that 12 months can be an important time span with some residents’ health considerably deteriorating in this time.
The sample of relatives we interviewed was split in terms of their views about whether or not the research team needed to reconsent during the 12 months of PAAD stage 2. Three relatives felt that consent needed to be checked at regular intervals during the study (typically every 3–6 months), although none of these relatives stated that reconsent needed to be established in writing. Four relatives felt that it was not necessary to reconsent but that the care home staff should continue to remind relatives informally that the resident was still part of the PAAD study.
Well, if you’ve given consent, it might be nice to be reminded of it, you know.
Relative 13
Seven relatives stated that reconsenting during the 12 months was not required, as long as it was made clear that residents and relatives had the right to withdraw from the study.
The sample of relatives was also split in relation to the question of what should happen if a resident has given consent but then loses mental capacity during the course of the study. Five relatives felt that a relative should be consulted about the resident’s continued participation.
You are going to want somebody to act on his behalf.
Relative 1
Four relatives felt that a relative should be informed as a matter of courtesy that the resident had given their consent to participate in the study.
You know, maybe it would just be like an act of common courtesy.
Relative 14
Five relatives felt that the resident should continue in the study regardless of whether a relative is informed or has given his or her consent. Many respondents acknowledged that this was a difficult issue. None speculated about what might happen should a relative disagree with a resident’s continued participation.
Focus groups with care home staff
We conducted two focus groups with care home staff (one with senior staff and one with more junior staff) with both groups comprising 19 care home staff.
Care home staff were generally very positive about the value of the PAAD study and their participation therein. They reported that their original willingness to participate in the PAAD study arose from a belief that older people were generally neglected in research, that antibiotics and diarrhoea were important issues to address in this client group, that there were professional benefits for staff through increased training and links to Cardiff University and that they were providing benefit to society.
. . . because there isn’t a lot of research in elderly care, we just felt that it’d be nice to do something that would be research based.
Focus group, junior staff
You need something to bring you up, bring you out, make sure that you’re still doing something with your career, if you like, and we did, we sat down at one of our meetings for the qualified, sat down talked about it, and felt it would be good for us, stimulate us.
Focus group, senior staff
However, having started the PAAD study most care home staff were surprised at the intensity and the scale of the paperwork involved.
It’s also finding the time to do the consenting, and spend that extra bit of time to talk through: ‘cos we’re getting them interested in it, you know, um well we did find that a bit hard, just getting the time and really sit down, ‘cos with 54 residents, and getting the relatives in, a lot of them would come after tea-time, which is a really busy time, you know around tea-time, after tea-time, a really busy time, only two qualified on, quite hard then.
Focus group, senior staff
This situation was eased by NISCHR CRC research nurses preparing ‘resident packs’ which contained all the relevant documents. NISCHR CRC research nurses had developed good relationships with care home staff and their increasing involvement was considered to be extremely useful.
Many senior staff reported that consenting for PAAD stage 1 had been quite straightforward. However, junior staff reported that taking consent was often difficult and very time-consuming. Senior staff were considered to be better able to obtain consent from relatives than junior staff. There were specific difficulties in obtaining consent in relation to (1) communicating with relatives who visited infrequently, (2) locating a nominated consultee when no personal consultee (relative) could be found and (3) explaining the study to residents with capacity, only for the resident to forget the purpose of the study shortly afterwards. Some staff reported that relatives wanted simple explanations and were slightly put off by the length of the information sheet. Consequently, staff reported that they had used the simplified information sheets, which had been designed for residents, for relatives.
I think the way it will be written down will make a lot of difference whether people consent or not because, you know, a lot of words can sometimes put people off, it’s a lot of terminology.
Focus group, junior staff
Staff reported that research procedures for PAAD stage 1 had been reasonable. Problems were identified with some staff forgetting to complete CRF forms (particularly CRF 6), collecting stool samples (particularly for residents who were semi-independent or from those who had loose stool and wore pads). The BSC had not been used in all care homes prior to the PAAD study and it took some time to train all carers about the detail of data collection required for the study.
Communication between staff was reported to be an issue. Although the PAAD study was sometimes discussed during staff handover, it was frequently dropped from discussions because of competing priorities. Staff stated that information about the PAAD study was not always appropriately cascaded down to junior staff. Care homes took different approaches to reminding staff about the study, including putting stickers on residents’ notes in the bedroom and putting BSCs on toilet doors.
Care home staff felt that PAAD stage 2 may give rise to more concerns among relatives who were approached to act as personal legal representatives. During the focus groups, staff expressed confusion over exactly what a ‘legal representative’ may mean (e.g. whether it meant that a power of attorney was required), and how they should explain this to relatives. PAAD stage 2 was considered to be more complicated than PAAD stage 1 and more support was considered necessary for care home staff. Once the concept of advanced consent was clearly explained, care home staff did not see advanced consent as an issue. This was a model they were familiar with in relation to gaining advanced consent for photographs or taking residents out on trips. However, a couple of staff raised the point that personal legal representatives may themselves die or lose capacity over the period of 12 months. It was considered necessary to obtain written consent only once, but staff thought that residents and relatives acting as personal legal representatives should be reminded of their participation in the PAAD study a few times during the year. It was felt that this could be adequately done through newsletters. Care home staff thought it reasonable that they also remind residents and their relatives of their participation in the PAAD study at the point at which the resident was randomised, as they would normally telephone the relative to inform them that the resident had been seen by a GP and had been prescribed antibiotics. Should a personal legal representative not be found, care home staff thought that social workers and or members of the community psychiatric team could be approached to undertake the role of a nominated legal representative. It was felt that solicitors would not get involved in health-related matters and advocates were difficult to get hold of and tended not to get involved in research issues.
With the exception of only a couple of members of staff, all staff felt that it if a resident lost capacity during PAAD stage 2, then a legal representative would need to be approached and consent taken. When it was explained that (legally) this was not the case, some care home staff still insisted that they would want to do this to ‘cover themselves’. At an absolute minimum they felt that they should inform the relatives.
Well I think you’d have to reconsent with somebody that could give, well I feel I’d have to ask the next of kin, if we could carry on. I’d really feel I’d have to do that.
Focus group, senior staff
There were only a few reports from care home staff that relatives had asked questions about C. difficile circulating in the home. There were very few cases being reported of relatives bringing in probiotics for residents. Care home staff did not feel that GPs were very aware of the PAAD study, despite having received letters regarding their patients’ involvement in the study.
Interviews with general practitioners
We interviewed 10 GPs. Overall, no major ethical concerns were raised by GPs regarding PAAD stage 1 or stage 2. The study was considered to have no harms, but it was felt that there was the potential for the individual residents and the care home population to benefit.
Rates of C. difficile or harm that comes from it . . . I think you have to see it as something clinically useful, as something that is going to make a clinical difference in the end.
GP 8
Some ethical tensions were raised, for example there may be a conflict of interest if GPs prescribe antibiotics and are paid to enter a resident into the trial (in which case GPs may be more likely to prescribe an antibiotic); the study may increase residents’ anxiety about taking antibiotics (being alerted that antibiotics have side-effects); and the amount of information given to residents or relatives should not be disproportionate to the harmless nature of the investigational medicinal product (IMP). One GP commented that he had been informed by letter that a resident had given consent to the PAAD study. This caused the GP concern because, in his view, this patient lacked the mental capacity to give consent. Another commented that older adults often require time to consider a request to participate in a study, but, when given time, they often forget what they were considering. One GP felt uncomfortable including patients without capacity in a trial on the basis of another’s advice.
I don’t feel comfortable about it at all because it, partly because I, I do feel that the patient should be able to give informed consent if they are receiving something that is out of the ordinary from their usual treatment.
GP 5
General practitioners felt that, although obtaining consent could be tricky at a practical level, most relatives would be happy to be a personal consultee/personal legal representative. Some GPs also wished to remind us that some relatives themselves are elderly and easily confused. A few GPs felt that some relatives would not want to take on this responsibility.
All GPs thought that the advance consent arrangements for PAAD stage 2 were appropriate. Reconsenting throughout the 12 months of PAAD stage 2 was generally not considered necessary and was regarded as unnecessary paperwork. Reconsenting was considered even less necessary when it was explained that residents would be randomised only once during the trial. However, some GPs did comment that it would be appropriate, and a matter of courtesy, to verbally remind residents (or relatives, in cases where residents do not have capacity) at 3- or 4-month intervals that they are still part of the PAAD study, and are free to withdraw at any time. Other GPs suggested that at the time of randomisation the nurse/matron could verbally check with the resident (or relative) that they are happy to be part of the study and this should be recorded by the nurse in the resident’s notes.
One GP also raised the issue of how reconsenting during a 12-month period could be managed if trial participants were to lose capacity during the study period.
I suppose the issue would be for someone who went from having capacity to losing capacity, and if you’re going to reconsent, you’re going to have to reconsent everybody. Because it ought to be consistent.
GP 1
When asked to reflect on their role in PAAD stage 2, GPs commented that they would want the process to be as streamlined as possible, that they would want the research nurses/matrons to assess as many of the eligibility criteria as possible, and they would want a clear reminder that the resident was consented into the PAAD study. Many considered that out-of-hours cover would lead to problems with the system, and being contacted by the care home/research nurse on a Monday morning to confirm eligibility following an out-of-hours antibiotic prescription over the weekend would be considered a rather irritating request that would receive a fairly short-shrift response compared with other priorities. They explained that they would not want to undertake a separate care home visit that is not clinically indicated just to confirm eligibility to participate in a research study.
Although PAAD stage 2 was generally felt to be a low-risk study, some GPs did reflect on the need to ensure that systems were appropriately in place as (1) other researchers may follow similar systems for other IMPs and (2) GPs were prescribing an IMP that was not available on the NHS.
When asked what would encourage them to participate in PAAD stage 2, GPs suggested money (although note previously mentioned ethical tension regarding being paid for a study and for prescribing antibiotics), having evidence of CPD for revalidation/appraisal, and being provided with some data for their practice (or care home population) on AAD, outcomes, etc.
All GPs, with one exception, felt that a resident who lost mental capacity during PAAD stage 2 should be allowed to continue in the study. Most GPs said that it would be appropriate to inform the next of kin that the resident had consented to the study, but, with the exception of the one GP previously mentioned, no GP suggested that a relative could override a resident’s wishes. The fact that residents could lose (and regain) capacity during the study gave further weight to their arguments that residents did not need to be reconsented during the 12 months of PAAD stage 2, as it would be impossible to reconsent those who had originally given consent for themselves and then lost capacity. They argued that, if the research team were to reconsent residents, we would need to do that consistently across the study population, regardless of whether or not they had lost capacity.
Summary of results
Residents, relatives, care home staff and GPs are generally supportive of older adults in care homes participating in research studies. However, respondents were concerned about the best way of facilitating this, and the amount of detail that participants can reasonably understand and retain from consent discussions. Stakeholders were generally accepting of a model of advanced consent for studies including drug trials and, although the majority did not believe that formal written reconsenting was required through the trial period, they felt that participants should be reminded verbally of their involvement throughout the trial. Some participants (particularly care home staff and relatives) felt that, if a care home resident loses capacity during a trial, then his or her legal representative should be asked to give consent for the resident’s continued participation.
A paper arising from this qualitative study has previously been published in the journal Trials. 53
Chapter 4 Stage 2: a randomised controlled trial of probiotics to prevent antibiotic-associated diarrhoea – study set-up and lessons learned
Introduction
Background
Results from PAAD stage 1 demonstrated sufficient grounds for progressing to stage 2: a double-blind RCT to assess the clinical effectiveness of probiotics taken in conjunction with antibiotic treatment in reducing the incidence of AAD. The process required to set up the RCT was lengthy and resource intensive, largely because the study involved a CTIMP. The Medicines for Human Use (Clinical Trials): Regulations 200448 requires a favourable opinion from an ethics committee, and authorisation from the competent authority, before a CTIMP can begin. The challenges encountered in obtaining statutory approvals, and in the process of research governance in care homes, both unique to establishing a RCT in this research-naive environment, resulted in significant delay. A number of strategies were employed to minimise the impact of these issues. However, during this period of delay, new evidence emerged from the probiotic lactobacilli and bifidobacteria in AAD and C. difficile diarrhoea in the elderly (PLACIDE) trial55 regarding the effectiveness of probiotics in reducing the incidence of AAD and CDAD in older hospital inpatients. As a result, discussion took place with members of the Trial Management Group (TMG), Trial Steering Committee (TSC) and the National Institute for Health Research (NIHR) Health Technology Assessment (HTA) funding programme (our funders), and the decision was made not to progress to the recruitment phase of PAAD stage 2. Therefore, the planned objectives, to evaluate the clinical effectiveness and cost-effectiveness of probiotics in conjunction with antibiotic treatment in reducing AAD, could not be delivered.
Objectives
The primary objective was to assess the effect of probiotics, taken in conjunction with antibiotics, on the incidence of AAD. This would be ascertained by comparing the proportion of residents in each arm who experienced at least one episode of AAD during the 8 weeks following randomisation.
The PAAD study stage 2 also had a secondary objective: to assess the cost-effectiveness of probiotics for AAD in care home residents. Total costs would be determined by multiplying the recorded data on health-care resource use (including the cost of the probiotic) by relevant unit costs and assessing these against quality-adjusted life-years generated from the European Quality of Life-5 Dimensions (EQ-5D) data. 56 Standard economic evaluation methods57 for dealing with skewed cost data, parameter uncertainty and joint uncertainty in the cost-effectiveness ratio were to be applied.
Summary of research methods
Design and aims
The PAAD study stage 2 was planned to be a multicentre, double-blinded, placebo-controlled, two-arm, individually randomised trial of the effectiveness of probiotics taken in conjunction with antibiotic treatment in reducing incidence of AAD in care home residents.
The study design involved obtaining consent for the resident to be randomised to receive a probiotic or matched placebo taken in conjunction with an antibiotic, should an oral antibiotic be prescribed by a responsible clinician in the usual course of care at any point during the subsequent 12 months. Residents and their relatives (where applicable) would be fully informed of the study using written materials and posters in care homes, supplemented with verbal explanations. Consent was to be taken from residents who were able and willing to provide this. In the event of cognitive impairment that limited ability to provide fully informed consent, relatives and/or personal legal representatives would be approached to provide consent on their behalf.
Consented, enrolled residents who were prescribed an oral antibiotic in the course of usual care at any time during the 12 months after recruitment would be assessed by the GP to ensure that they remained eligible to participate in the trial. If the resident was eligible, he or she would be individually randomised to receive probiotics (VSL#3), or placebo, in addition to the antibiotic prescription. Residents would be followed up by a research nurse for 8 weeks following randomisation (see Appendix 1).
The aim was to randomise 400 residents (200 per arm) from approximately 24 care homes. We planned to recruit from care homes that had at least 50 residents. Smaller care homes would be considered if the recruitment potential in these care homes was considered adequate. GPs serving the recruited care homes would have played a pivotal role in recruitment and it was therefore essential to secure their commitment to the study at the same time as the commitment of the care home (see Appendix 2).
Participants
Residents would be eligible for the study if they met all of the inclusion criteria and none of the exclusion criteria. Inclusion criteria at consent were resident in a care home for 24 hours or more, with a minimum planned period of residence of 1 month, and able to provide informed consent or have a representative who could provide consent for inclusion. Residents met the exclusion criteria if they were severely immunocompromised, had an artificial heart valve in situ, had a medical history of acute pancreatitis, required nasojejunal feeding/nasogastric feeding, currently had a colostomy or did not have capacity and were already receiving a probiotic.
Residents who had provided advanced consent and remained eligible for study medication would be randomised to receive either the probiotic or the placebo once an antibiotic for an acute infection had been prescribed by selection of the next sequentially numbered study medication pack.
Intervention
There were two planned intervention arms. The active arm was the nutritional supplement VSL#3 probiotic, which contained approximately 450 billion live lactic acid bacteria and bifidobacteria, together with maltose and silicon dioxide. There were eight different strains of potentially beneficial bacteria: Streptococcus thermophilus Orla-Jensen 1919, Bifidobacterium breve Reuter 1963, Bifidobacterium longum Reuter 1963, Bifidobacterium infantis Reuter 1963, Lactobacillus acidophilus (Moro 1900) Hansen and Mocquot 1970, Lactobacillus plantarum (Orla-Jensen 1919) Bergey et al. 1923, Lactobacillus paracasei Collins et al. 1989 and Lactobacillus delbrueckii ssp. bulgaricus (Orla-Jensen 1919) Weiss et al. 1984.
The matching placebo arm of the study consisted of freeze-dried powder (4.4 g), matched for taste, consistency, odour and colour.
The resident was to be given one sachet twice a day for 21 days. The sachet would be opened and the contents stirred into 25–50 ml of cold water or any non-fizzy drink or sprinkled onto cold food and consumed immediately. The study medication would be given in between the antibiotic therapy and not in conjunction with the antibiotic. The study medication would be commenced within 72 hours of the resident being prescribed an antibiotic.
Outcomes
The primary outcome was the occurrence of at least one episode of AAD during the 8 weeks following randomisation. AAD was defined as three or more loose stools (defined as 5–7 on the BSC) in a 24-hour period following a period of normal stool consistency.
Secondary outcomes included the occurrence of CDAD. This was defined as having a diagnostic stool sample (following the occurrence of AAD) containing C. difficile toxin A or B. The duration, frequency and recurrence of AAD were also to be measured, where the duration of AAD was defined as the total number of consecutive 24-hour periods that a resident had AAD. The frequency of AAD was defined as the number of episodes of AAD a resident experienced during the 8-week follow-up period. AAD episodes were considered unique only if they were separated by a period of at least 3 days of ‘normal’ stool consistency (defined as 1–4 on the BSC).
Other secondary outcome measures included:
-
Recovery from the illness that triggered the prescription of antibiotic treatment.
-
Adherence to the study intervention and antibiotic treatment.
-
Health-care resource use and unplanned hospitalisations, including all-cause and AAD related, during the 8-week follow-up period.
Health-related QoL was also to be measured using the EQ-5D56 as a self-reported or proxy measure. Adverse events included reported symptoms such as vomiting, abdominal pain, excessive flatulence, bloating and skin rashes. All-cause mortality during the 8-week follow-up period would also be measured.
Sample size
A total of 400 residents (200 per arm) needed to be randomised in order to achieve 80% power, at the 5% level, to detect a 50% relative reduction in the incidence of AAD in those given probiotic alongside antibiotic treatment, compared with placebo alongside antibiotic treatment. The sample size was calculated using the interim data analysis from PAAD stage 1 assuming an AAD incidence of 25% in the placebo arm. The sample size was adjusted for a 20% rate of withdrawal and loss to follow-up (including death) using data from PAAD stage 1.
Taking into consideration the results from the interim analysis performed in PAAD stage 1 and a more recent interim assessment of antibiotic prescribing and AAD (from first antibiotic prescription), assuming an AAD incidence of 25% in the placebo arm, in order to randomise 400 residents, advanced consent was required from at least 607 residents (assuming that 66% would be prescribed at least one course of antibiotics during the 12-month monitoring period and subsequently randomised). Approximately 1214 residents would be approached in order to achieve this consent rate (assuming that only 50% will provide consent). With an average of 60 residents per home, we needed to recruit from a minimum of 21 care homes. In order to allow for care home drop-out, more than anticipated withdrawals/drop-out/declines and fewer than anticipated care homes, we aimed to recruit from 24 care homes.
Data collection
Data would be collected for 8 weeks following randomisation, to include a daily diary which records symptoms and medications used and side-effects, a stool chart and QoL questionnaire (EQ-5D).
Data collection would be undertaken by care home staff, following training in completion of study-specific data collection tools, supported by research nurses who would visit care homes regularly to assist with recruitment and randomisation and study-specific procedures.
Analysis
For the primary analysis, a logistic regression model would be constructed with ‘AAD during the 8-week follow-up’ (YES/NO) as the dependent variable and ‘study arm’ (probiotic/placebo) as an explanatory variable. The analysis would be adjusted for the potential clustering of residents within care homes via multilevel analysis, and potential, prespecified confounding/risk factors as specified in the statistical analysis plan. The primary analysis was to be based on the intention-to-treat principle. In a similar way to the primary analysis, a logistic regression model would be constructed for the secondary analysis, with ‘returned positive stool sample for C. difficile toxin A and B following AAD’ (YES/NO) as the dependent variable and ‘study arm’ (probiotic /placebo) as an explanatory variable, in order to investigate the effect of probiotics on the development of CDAD during the 8-week follow-up period. In an analogous way to the primary analysis, this analysis was to be adjusted for clustering (if present) and confounding factors.
Challenges encountered and strategies used
Ethics approval
The REC provides an independent review of research involving humans, and is required to ensure that the research reaches the required ethical standards. 58 The Medicines for Human Use (Clinical Trials): Regulations 200448 require that those incapable of giving consent should be given special protection. The REC must consider whether or not there is justification provided by the research team for involving adults lacking capacity in the trial. An application for ethics approval was submitted to the South East Wales REC Panel D. Ethics approval for the study was not granted initially, as the REC requested further clarification from the research team on a number of issues including the remuneration to care homes for taking part in the research, the potential recruitment of residents without mental capacity and also the assessment of mental capacity.
Data obtained from PAAD stage 1 showed that the majority of eligible residents (69%) lacked capacity. This evidence, together with the need for a representative sample population without bias, was provided as support for the inclusion of residents without capacity to the REC. The MCA (2005)45 states that there is a presumption that a person has mental capacity, unless there is concern to the contrary. If concerns were raised about a resident’s capacity to consent to participate, an assessment of mental capacity would be conducted initially by senior care home staff familiar with the resident. If the care home staff or researchers were uncertain about a resident’s mental capacity, the research nurses trained and experienced in assessment of mental capacity would conduct an assessment using a standardised template and record the findings.
The REC required further justification for inclusion of residents without capacity from the research team in order to meet the conditions set out in the Medicines for Human Use (Clinical Trials) Regulations 2004 Informed Consent in Clinical Trials Guidance. 59 Preliminary findings available from the PAAD stage 1 showed that there were significant differences between the groups of participants with and without capacity in terms of prescription rates for antibiotics, frailty scores, nutritional risk and rates of AAD. These data were provided to the REC as evidence for the inclusion of residents without capacity as the population most at risk of antibiotic treatment and the associated adverse consequences and, therefore, most likely to benefit from the intervention. Ethics approval was granted following this evidence.
The process for gaining REC approval for this trial took 4 months. In contrast, PAAD stage 1 received full approval from a different REC within 1 month based on the initial application.
Understandably, the process required for ethics approval for a RCT in care home populations has been more complex than in a non-CTIMP study based in the same population. Gaining approval took a longer period of time, and significantly more evidence was required for the justification for including residents without capacity.
Competent authority approval
The intervention that was planned to be used in the trial was a probiotic preparation (VSL#3) that is commercially available to the general public as a food supplement. The research team presented evidence that the manufacturers class the probiotic as a food supplement that is commercially available without prescription and is low risk, and that its use within the trial was within its indication in the investigator brochure. The Medicines and Healthcare products Regulatory Agency (MHRA) subsequently confirmed that the trial was classified as a CTIMP and did require clinical trial authorisation (CTA).
Before granting CTA, the MHRA requires proof that the whole manufacturing process has been carried out in accordance with terms and conditions of a marketing authorisation (MA) for the product to ensure that strict quality assurance is maintained throughout the process. A qualified person (QP) provides certification for each batch of a medicinal product in accordance with good manufacturing practice. The product used in this trial had a complex manufacturing and importation process before reaching the trial site. The request for CTA was not initially accepted as the MHRA required further information regarding the MA; however, the status of the product as an IMP was difficult to reconcile with its licenced manufacture and production as a food supplement, despite being listed as a medicine in the British National Formulary.
The delays in MHRA approval and the contract impacted on labelling of the IMP and placebo and delayed commencement of randomisation. This in turn had a knock-on effect on the start of enrolling residents into the trial and obtaining consent, as it was undesirable to have a large gap between consent and beginning the process of randomising residents should they be prescribed an antibiotic.
The classification as a CTIMP had a significant impact on the time to commencement of the trial. The discrepancy between the statutory authorisations requirement for an IMP, and the absence of any such documentation for the product used in the trial, had a significant impact on the continuation of the trial.
Research governance
Research governance in care homes is complex owing to their status as non-NHS institutions, the ambiguous nature of the environment as both a public and a private space and the unique nature of the setting where the community of residents must be considered as a whole in addition to each individual. 60
The contractual and financial arrangements of the resident as self-funding, LA funded or a mix of both will have an impact on research governance. This is further complicated by whether the care provider is a commercial or non-commercial organisation (independent sector, voluntary sector or LA owned). This means that residents may be covered by different research governance processes, and may change from one process to another if their individual circumstances change.
As non-NHS institutions, there was a lack of clarity regarding where responsibility for research governance lay for the care homes involved in this trial in Wales. This was further clouded by the classification of the trial as a CTIMP and the fact that, although care homes would be the main sites, GP practices would also be sites and are NHS organisations. This resulted in delays with gaining site-specific assessment (SSA), as non-NHS SSAs are carried out by the REC; however, the responsibility for NHS SSAs lies with NHS research and development departments. The trial also covered a number of NHS trusts and LAs’ social service departments. Each of these organisations conducted their own approvals process.
The question of who would be acting as PI for the trial sites also raised difficulties. The structure varies between each individual care home; however, those with the most responsibility on a day-to-day basis are care home managers. Care home managers usually have a nursing background, but some are not registered health-care professionals and have a managerial background.
The regulations governing CTIMPs conducted at more than one site define ‘investigator’ as the ‘authorised health professional’ responsible for the conduct of that trial at a trial site and, if the trial is conducted by a team of health professionals at a site, the investigator is the leader responsible for that team. The regulations define health professional as a doctor, dentist, nurse or pharmacist. The MHRA advised that only care home managers who fulfilled these criteria would be eligible to act as investigator for the trial. It also highlighted that GCP guidelines state that each individual involved in conducting a trial shall be qualified by education, training and experience to perform his or her tasks. Although GCP training and extensive study-specific training was provided to care home managers acting as PI for the site, there was almost no prior research experience among this group. Extensive training and support is required by those with responsibility as PI in research-naive care homes.
Research governance in care homes lacks clarity. The process will differ between care homes, care home residents, LAs and regions. Where care homes are participating in trials, particularly those involving IMP and both NHS and non-NHS sites, the process for identifying responsibility for research governance is problematic and resource intensive.
General practitioner recruitment
There is no standardised model of health-care provision for residents in care homes. Individual care homes have individual policies regarding provision of GP services. This may encourage residents to register with a ‘preferred practice’ that provides health care for a large number of residents at the home, or encourage them to remain with their existing GP when moving into the home. A number of factors may influence this, including geographical location (rural or urban), relationship with local GP practices and financial arrangements.
The trial was designed to minimise the involvement of residents’ GPs because of experience from the observational study and feedback from GPs involved in a qualitative study that investigated the consent process for the RCT in PAAD stage 1. GPs had concerns about the impact on their workload if they were required to check eligibility for care home residents and preferred a streamlined approach which minimised their role. The study protocol stated that a research nurse would assess eligibility for the trial and the responsible clinician at the resident’s GP practice would provide final approval for the resident’s inclusion. However, recruiting GP practices whose patients were resident in participating care homes proved challenging because of difficulties in arranging meetings with the busy clinicians and their reluctance to commit to this responsibility. If care homes had residents registered with a number of GP practices, agreement to participate had to be sought from a number of partners from each practice.
The practical difficulties experienced when seeking agreement to participate from GP practices, and the requirements for each practice to be a trial site in terms of GCP training and study-specific training for each GP, proved to be a major barrier to conducting the trial. Advice was sought from the MHRA in an effort to further minimise the role of the resident’s GP. Clarification was sought as to whether or not a registered nurse could confirm eligibility by accessing care home records for the resident or, where required, their medical notes held by the GP. The MHRA’s response was explicit that the decision whether or not a subject is eligible for entry into a clinical trial is considered to be a medical decision and, therefore, must be made by a medically qualified doctor.
A strategy for communicating with GPs was developed to minimise the burden on them. This included the availability of online GCP training along with drawing up a letter of agreement in place of a more comprehensive practice agreement.
Care home recruitment
The RCT initially involved approaching 32 care homes. Five agreed to participate, with a number of other homes expressing interest awaiting further follow-up of the interest to participate. Twenty care homes declined to participate, four of these at a care home company group meeting. Reasons given for declining were involvement in other studies (n = 2), concerns regarding residents’ suitability for research as a result of their cognitive impairment (n = 1) and workload (n = 1). Twelve care homes gave no reason for declining. It is unclear if there are any differences between homes by category (nursing vs. residential) in willingness to participate. The dependency and care requirements of residents will vary between the two, as will staffing levels and skill mix within the homes. Future studies in this area may need to consider employing a GP clinical fellow to take this responsibility.
All participating care homes had a dual registration for nursing and residential care. There was a wide variation in the ratio of residential to nursing beds, from 1 : 3 to 15 : 3. It is also unknown whether or not the proportion of residents in the care home who have a cognitive disorder has an effect on agreement to participate. All participating care homes had residents with and without mental capacity residing in the home.
Contacting care homes was a very time-consuming process. Consent for the care home to participate is required from the care home manager and also, in some cases, the regional manager for the care home group. A consensus of agreement from the care home staff is also required for the care home manager to agree to participate. Consent in care homes has been described as a two-tiered process, where obtaining consent at institutional/community level is required before progressing to consent from individuals. 61 Care home managers were generally positive about becoming involved in a research study and welcomed the engagement with the researchers. The potential benefits of collaborating with a higher education institute, and the opportunity to gain additional training and skills, were viewed as incentives to participate in the study. Care home staff also welcomed the opportunity to examine current practice and the potential for changing practice, where there was evidence to support this. This was seen in one participating care home, with the adoption of BSC monitoring in a care home where there had previously been unsupported attempts to introduce it by some care home staff. The process for recruiting 24 care homes was originally expected to take 5 months but this was extended to 13 months as a result of the above difficulties.
Indemnity
Other issues that have arisen include the indemnity insurance requirements for care homes participating in research, particularly CTIMP studies, as non-NHS sites. The REC confirmed that care homes were required to provide evidence of adequate insurance indemnity. Indemnity is provided by the sponsor for research activity and, therefore, the sponsor would be liable for any non-negligent harm resulting from activity conducted in accordance with the protocol. However, any negligence on behalf of the site (for example harm caused by protocol deviations such as giving an incorrect dose) would not be covered by the sponsor’s insurance and, although the likelihood of a claim arising out of the trial is small, should be covered by the site insurance. The REC was concerned that harm resulting from activity carried out in contravention of the protocol would not be covered by existing policies as this would not form part of the care home staff’s usual activities. The REC received advice from the Health Research Authority that, if the activities did not form part of their routine professional practice, care homes should be asked to take out an extension to cover research activity under their existing insurance.
Advice from research networks with experience in research in this setting [NIHR ENRICH (Enabling Research in Care Homes) programme] suggested that, where a study involved an investigative product or an intervention, indemnity would need to be checked on a case-by-case basis. Discussion was sought with a participating care home’s broker whether research would be covered under existing insurance or whether additional insurance was required. The broker confirmed that additional conditions would be required to be met, such as evidence of study-specific training, prior to providing additional cover for research activity.
This requirement for each care home acting as a research site to provide indemnity for negligence on its behalf had not previously been encountered by the sponsor and, therefore, was not detailed in the existing contract with the care homes. A requirement for additional indemnity further impacted on the costs and time required to set up a RCT in the care home sector and may impact on care homes agreeing to participate.
Reasons for early termination of the trial
The extended time required for setting up the trial resulted in the anticipated date for first participant recruitment being significantly delayed. Staff in the first care home were trained and ready to begin recruitment 8 months after the initial anticipated date. However, CTA had not yet been granted and, therefore, recruitment could not commence.
During this period of delay, the TMG became aware of emerging evidence that there was no longer sufficient scientific evidence regarding the effectiveness of probiotics in reducing the incidence of AAD and CDAD in older hospital inpatients. 55 The PLACIDE study recommended that no further studies assessing probiotics for AAD should be undertaken until further evidence is generated regarding which strains maybe effective in reducing AAD (in vitro evidence).
The TMG considered that the PLACIDE study population is likely to be very similar in terms of age and frailty to a population recruited from care homes and that, although there were some differences in the strains of probiotic organisms used in the PLACIDE study IMP and in VSL#3, there was also some overlap (Box 2). The sample size of the PLACIDE study and the apparently tight CIs around their estimates suggested that PAAD stage 2 would be unlikely to come to a different finding about the effect of probiotics on the duration and severity of AAD.
Despite the differences between the two studies, the TMG considered that there was no longer equipoise regarding the study question. However, it was agreed that continuing to build a research base within a care home setting was vital.
This was considered to have a significant impact on the justification for commencing recruitment to PAAD stage 2. Following consultation with the appropriate steering committees, and presentation to the HTA, the decision was made that recruitment should not commence and the project should close.
-
Population:
-
older in PAAD
-
likely to be more frail in PAAD
-
more without capacity in PAAD
-
sicker in PLACIDE.
-
-
Antibiotics prescribed:
-
more intravenous antibiotics in PLACIDE (although many would have been started on intravenous antibiotics and switched to oral antibiotics), PAAD would be almost exclusively oral.
-
-
Probiotic used as intervention:
-
different way of giving probiotic – PAAD powder to dilute and drink or sprinkle on food, PLACIDE tablet
-
different strains of bacteria in each probiotic (both contain a strain of Lactobacillus).
-
During the close-down period, the study team continued the existing engagement with care homes to establish a research base in the care home sector. This process was intended to optimise the value of resources invested in research in this setting and aimed to support future research in the care homes sector. Part of this process involved care home research priority setting exercises with stakeholders.
Chapter 5 Stakeholder involvement
Introduction
The relationship between engagement of stakeholders in research planning and design, and the quality of the research and its subsequent utilisation and impact on outcomes, has been discussed. 62,63 The NIHR HTA programme advocates involving service users in research and has developed an evidence-based approach, which includes approaches to reducing barriers to meaningful participation. 64 Involving patients, carers and members of the public in the design and conduct of trials and studies improves the quality of the research undertaken. 65 There is a dearth of research conducted in care homes and with that comes a lack of knowledge regarding how to best carry out research. Care home staff can be hesitant about getting involved, as time and resources are sparse. Designing a research study that understood this busy and demanding environment and the pressures the care home staff are under was essential.
The stakeholders
Throughout the study’s lifetime, a number of different stakeholders provided invaluable advice or took part in interviews to enhance the research delivery. These included lay representatives; a director of seven care homes; a former care home nurse and care home manager; care home staff; residents and their relatives and professional representatives; NISCHR CRC research officers/nurses; and representatives from the NIHR ENRICH programme.
Methods of stakeholder involvement
During the study development stage
The PAAD research team worked closely with a director of seven care homes in Wales. A number of meetings were held with the primary aim of:
-
obtaining more information on the structure of care homes
-
gaining GP involvement and model of care delivery
-
recruiting care homes
-
estimating costs of conducting the research
-
determining who could provide approval of research in care homes and finally conducting the study
-
determining the additional resources required
-
resolving consent issues
-
determining prescription and treatment schedules
-
assessing the feasibility of administering the probiotic and taking stool samples.
Valuable advice was provided which enhanced the final project design.
During the study
Lay representative on the Trial Management Group
We recruited a lay representative onto our TMG through Involving People. He was a qualified nurse who had 40 years of activity in the voluntary/community sector, half of which was directly linked with elderly care in independent care homes. He had experience of multidisciplinary teamworking and committee activity. Most importantly, his stated passion was to bring evidence-based practice to care homes. During the planning stage and the initial trial period we were able to use his experience to create the documents required to obtain the research data in a format easily understood by care home staff and to provide a framework for inducting both NISCHR CRC research officers/nurses and care home staff into the task to be undertaken. He was also pivotal at providing insight into the general issues the care home staff and residents face every day, which over time became known as understanding ‘the rhythm of the care home’, making the integration of our research activities into the care home smoother.
Lay representative on the Trial Steering Committee
A care home manager formed part of the PAAD study TSC and, therefore, commented on the protocol and associated TSC documents and sent her approval ahead of study commencement. Unfortunately, owing to work-related time commitments she was unable to attend many of the TSC meetings and withdrew just before PAAD stage 2 was closed.
Workshops and regular teleconferences with care home staff
Two workshops were held with care home staff. These included breakout sessions to discuss challenges and find solutions to topics such as (1) training and site set-up, (2) recruitment and consent, (3) practical challenges and logistical issues and (4) ethical and practical issues of conducting research in care homes. These provided valuable insight into the problems care homes were facing regarding embedding research procedures into everyday running and provided solutions which could be rolled out in real time from which the both the study and the staff could benefit from. Staff found these workshops incredible useful and attendance was always very high.
Regular teleconferences were also set up to share experiences, discuss real-time issues and concerns and ensure that both the study and specific study procedures remained in the forefront of the minds of care home staff.
Focus groups with care home staff
As part of the qualitative study (described in Chapter 3), focus groups were conducted with all relevant care home staff, which brought a deeper insight and understanding into conducting research in care homes, the pressures care home staff are under in their everyday roles and, very importantly, their views on the feasibility of taking consent in both PAAD stage 1 and PAAD stage 2.
Interviews with residents and their relatives
As part of the qualitative study (described in Chapter 3), interviews were conducted with both residents and their relatives, who provided a useful insight into what it was like taking part in research, and in particular their views on advanced consent and what was appropriate in this population were sought.
Interviews with general practices
As part of the qualitative study (described in Chapter 3), interviews were conducted with GPs who regularly attended residents in care homes. These interviews provided valuable data about what GPs considered to be important issues in relation to conducting research in care homes and the PAAD study in particular.
National Institute for Social Care and Health Research – Clinical Research Centre research officers and nurses
The NISCHR CRC research officers and nurses played a pivotal role supporting the care homes to deliver the research. They therefore built up a unique understanding of the problems faced by the care home staff in undertaking research. The NISCHR CRC research officers/nurses were able to provide an expert link between the researchers and the care home staff. Throughout the course of the study and within the workshops, in a breakout session, the NISCHR CRC staff provided the team with vital information regarding how best to embed procedures into the care homes. Owing to the variation between care homes, they were also able to develop personalised documentation and systems for the care homes to use in order to maintain a record of who had been approached, recruited and followed up. They also provided insight into how effective the training sessions were, often identifying where more training was required, as well as proposing better ways in which to motivate and engage with staff regarding the study.
Enabling Research in Care Homes
Owing to the lack of knowledge regarding how best to carry out research in this research-naive environment, we attempted to bring together researchers working in this environment to share experiences and develop effective procedures. An e-mail was distributed to all HTA chief investigators working on a study in care homes and we were approached by the chief investigator of ENRICH. A number of teleconferences and meetings were held with ENRICH colleagues. As a result, we provided ENRICH with an article for its website on the challenges of conducting research in care homes and the lessons we learned. 66
Enabling Research in Care Homes provided us with advice regarding indemnity and research governance within care homes, since this is one particular issue that currently does not seem to be resolved, as well as a list of contacts for potential TSC members.
End of the study
Research priority setting by care homes
Background
There is a recognised need for the development of a more structured and evidence-based approach to health-care provision for care homes. 67 However, recruitment of frail, older people to research is a complex68 and resource- and time-intensive process. 44 This has resulted in widespread concern about under-representation of older people from clinical trials69 and development for strategies to increase participation of older people in clinical trials. 70,71
Given the wide range of topics that require further investigation, and limited resources, there is a need for stakeholders to participate in decisions regarding the prioritisation of topics for future research. The importance of patient and public involvement in research has been the focus of a number of initiatives in health and social care research,66,72 including the need for public involvement in identifying research priorities. 73 Research priority setting processes assist health-care researchers and policy-makers to effectively target research that has the greatest potential public health benefit. 74 Nominal group technique has been used as a method of combining quantitative and qualitative data collection in a group setting to assist with priority setting. 75 The priority setting project formed part of the PAAD study activities; however, it was also an integral part of the continued engagement with care homes and other groups.
Objectives
The primary aim of the project was to establish a set of priorities for research in health care in care homes within South-East Wales. It was anticipated that the project would focus on this locality, but would be of relevance to the wider care home and research community. The process was informed by review of existing evidence, the identification of emerging themes, and the involvement of relevant stakeholders to reach an agreement on elicitation of research topics, and ordering of priorities.
The secondary aim was to identify research questions that could be developed as proposals to research commissioning bodies to be considered for funding.
The tertiary aim was to establish a collaborative group to continue developing the platform for research in care homes established during the PAAD study. This would continue to support future research in care homes and maintain engagement of stakeholders.
Methods
Participatory research techniques were utilised to achieve the aims of the project. A working group was formed to develop and conduct the project. The process was supported by an information-gathering and validation exercise, including a literature review. Emerging themes were elicited from the participants and consensus methods were then used to prioritise these themes and develop research questions around them.
Stakeholders were identified and invited to participate in the process with regard to their interest and involvement in research in care homes. These included care home staff, both senior and junior grades, from care homes involved in PAAD stages 1 and 2 and other care homes who responded to information flyers distributed by post. Each member was asked to consult with the wider group of care home staff that he or she was representing. Participants were invited to attend a workshop event that was held in a conference facility and facilitated by members of the PAAD study team.
Following a general discussion of issues related to conducting research in care homes, individuals were invited to identify and write a list of three areas of their work in care homes that they felt would most be helped by research. The facilitators worked with the participants to form these ideas into research questions. These were shared with the group and collated into a list. A number of topics were also generated from discussion with the researchers and were included in the list of ideas elicited. The list of topics was discussed and the researchers checked with the participants that these represented their initial ideas.
A priority-setting exercise was conducted with participants to determine, weigh and rank the results generated. Agreement on priorities was achieved through nominal group technique work to order the priorities by means of scoring the research questions. Participants were asked to identify the six topics which were ranked as the most important to them and to allocate them scores from most important to the least important.
Owing to difficulties experienced by care homes in making staff available to attend a workshop, a number of participants who were unable to attend the workshop were subsequently invited to rank the topics that had been generated at the event via a postal survey. The survey was addressed to the named participant or manager of the care home. The topics remained in the order in which they had been generated and did not contain details of scores generated during the workshop. They were asked to score the six topics on the list that they felt were the most important from the most important to the least important of their chosen six topics. A stamped addressed envelope was provided and responses could be anonymous.
Results
Seven participants agreed to attend the workshop; however, owing to a number of unforeseen circumstances, five were subsequently unable to attend on the day of the workshop. Participants attending the event were two qualified nurses from dual-registered care homes in South Wales who were involved in the day-to-day care of residents and had responsibility for supervising a team of care assistants and nurses. One participant had been involved in PAAD stage 1 while employed at another care home and was currently employed by a care home that had agreed to participate in stage 2. The other participant had not previously participated in the PAAD study and did not have experience of participating in research, but was employed at a care home that had agreed to participate in stage 2.
A total of 23 topics were identified at the workshop event, which included a spectrum of service delivery themes and more specific health-related questions (see Appendix 3). Participants at the workshop experienced some difficulty deciding how to allocate scores to topics. Where they felt strongly about two topics, and were unable to choose which to allocate the score to, they were asked to score them by allocating the score to both.
Six completed postal surveys were returned and all returned completed postal surveys were anonymous. Three completed surveys had not been scored in accordance with the instructions: participants had either rated every topic on a scale of importance or had marked only the six most important topics and had not prioritised them. These were not included in the prioritisation scoring.
Three returned surveys were scored in accordance with the instructions and the method used at the workshop (most important topic was allocated 6 points, through to 1 point for the least important of their chosen topics). One of these participants, who had been unable to decide how to allocate scores, had also allocated scores jointly to more than one topic, although not explicitly invited to do so. Where scores had been jointly allocated, they were each given the same number of points. The scores allocated by survey were combined with scores allocated at the workshop (see Appendix 4).
The highest priorities for care home research in rank ordering were:
-
How can communication between staff and hospitals be improved when residents move into and out of hospitals? Can the prescribing and dispensing of medication process be improved to reduce wasted time and resources? (19)
-
How can care home staff, particularly nursing staff, best be kept up to date with staff development/evidence updates for nurses in care homes? (13)
-
What are the best methods of diagnosing UTIs and what methods can be used for collecting reliable urine samples in female residents with cognitive impairments? (12)
-
How can the current multiple and complex care referral pathways be improved so as to reduce the long waits and wasted resources? (Care appears to be organised around professional services rather than the patient, are there ways to make the process person-centred?) (11)
-
What improvements can be made in access to dental care for residents? And how effective are techniques to improve oral hygiene, such as use of suction/rinse toothbrushes? (11)
-
Are there ways UTIs can be prevented and how can UTIs be predicted? (10)
Discussion
There was some uniformity between scores allocated at the workshop and those subsequently allocated by postal survey. The topics that were rated as ‘most important’ were predominantly related to service provision and improvements, staff development and evidence-based practice and the diagnosis, prevention and treatment of UTIs. The topics scored as the most important topic and the third most important topic were also rated as important by those who incorrectly completed postal surveys, although none of these rated the second highest scoring topic as important.
Limitations
Owing to the time and resources available for the project, only care home staff were approached to participate in the priority setting. Participation was not invited from others stakeholders, such as GPs, geriatricians, charities or interest groups, or residents and their relatives on this occasion. Invitations were initially posted to care homes, and were subsequently followed up by a visit to the care home or a telephone call to the manager or, if unavailable, their deputy. This contact was intended to gain feedback from the care home regarding their participation in a research study and to discuss participation in the priority setting exercise. Care home staff were generally positive about their research involvement and expressed interest in setting research priorities in the care home sector.
Despite intensive attempts to encourage participation, care home staff experienced difficulty in attending an off-site event because of existing commitments within the care home and unexpected events requiring their presence. However, those that did attend valued the opportunity to have a voice in defining potential future research.
Generalisability
These results are from a small sample of nursing staff with managerial responsibilities from care homes in South Wales who had previously participated in, or agreed to participate in, the PAAD study, or had expressed interest in research in care homes. All those who participated had a pre-existing interest in research, and a number of those had experience in conducting research. All were from medium to large care homes, although they represented a range of care home providers (independently owned, national care home groups and LA managed).
Conclusion
Procedures from one environment are not necessarily transferable to another. In the case of the PAAD study, understanding the general rhythm of the care home and the pressures the staff were under on a day-to-day basis was essential to the success of the study. Stakeholders from a number of environments are required in order to fully gain a deep understanding of how to successfully design and conduct studies in care homes. The PAAD study stakeholders made a significant contribution to the design and conduct of a study being delivered in a research-naive environment. The care home staff valued the fact that they were able to contribute to the way in which the research was conducted and became enthused with the idea that research could become an everyday part of care home life and that evidence-based practice was not just a term associated with hospitals. Continuing to develop and maintain a network of research active care homes and researchers is imperative if care home research is going to succeed.
Chapter 6 Discussion
Stage 1: the observational study
Introduction
The PAAD stage 1 prospective observational cohort study of 274 residents from 10 care homes across South Wales found that antibiotics were prescribed at a rate of 2.16 per resident-year, with almost three-quarters of residents prescribed at least one antibiotic course during the 16-month study period (median length of follow-up 310 days). Residents were over 2.5 times more likely to be prescribed antibiotics during the study period if they had been prescribed antibiotics in the 4 weeks prior to study entry. Residents were prescribed a wide range of antibiotics for each indication. The incidence of AAD in those prescribed antibiotics was 0.57 episodes per resident-year, with 43.5% of residents prescribed antibiotics experiencing at least one episode of AAD. CDAD occurred in > 15% of residents who developed AAD and for whom a stool sample was sent for microbiological analysis. Although all CDAD episodes were found in residents from the same care home, the unique ribotypes suggest that the episodes were not associated with an outbreak. Residents in nursing homes were more likely to be prescribed antibiotics and experience AAD. The risk of AAD with prescriptions of co-amoxiclav was double that associated with other antibiotics. Risk of AAD was also increased in those who routinely used incontinence pads.
Analysis of stool samples taken at study entry showed that over half of residents carried organisms resistant to antibiotics, with resistance to ciprofloxacin particularly high. The proportion of participants carrying antibiotic-resistant organisms varied between different types of care homes. We found that, while recent hospitalisation was not associated with carriage of resistant organisms, recent antibiotic use was. We also found that carriage of resistant organisms was significantly more likely for residents who were prescribed antibiotics in the 4 weeks prior to study entry, and for those in nursing homes, even after controlling for clinical frailty. C. difficile was more common in nursing homes, but there was little suggestion of clustering of type by home.
Strengths and limitations of the prospective observational study
We conducted this study in a population and setting difficult to research,76,77 but we were able to estimate the rates of participation, antibiotic prescription and AAD. Despite needing to obtain advice about resident participation from consultees for the majority of participants in care homes, the recruitment target was achieved in PAAD stage 1. This study can be considered to have a low risk of bias because of relatively high inclusion rates in each care home and relatively complete follow-up data. 78 Although every effort was made to maintain the validity of the antibiotic and stool data, it is possible that this was not always achieved. Prescriptions during periods of hospitalisation were not transcribed onto study CRFs, so the number of antibiotic prescriptions may have been under-reported. Antibiotic prescriptions that were not recorded for the purpose of the study may also have resulted in a lack of stool data for the defined follow-up period. It is, therefore, possible that we have underestimated the incidence of AAD. Residents who were recorded as routinely experiencing three or more loose stools in a 24-hour period before observations were started were not classed as experiencing AAD during the study. We assumed that if stool data were missing, AAD was unlikely to have occurred, but there may have been some episodes of AAD that were not recorded. Our approach has, therefore, provided a conservative lower bound of estimated AAD in care homes. Although we found that residents in residential homes are significantly less likely to experience AAD than those in nursing homes, this may be in part because of the increased mobility, with associated less intense monitoring of residents’ stools. We also found that residents were more likely to experience AAD if they routinely used incontinence pads. This may be a real effect, but could reflect easier observation and reporting of loose stool types, or be an artefact as a result of stool and urine mixing in an incontinence pad. Not all episodes of diarrhoea following antibiotic use can be ascribed to antibiotics and our study does not seek to demonstrate causality, merely association.
Strengths of the stool sample study include its prospective nature and that we were also able to obtain a stool sample from residents who were asymptomatic or not acutely unwell.
Comparisons to existing research
Despite most residents lacking capacity to consent to inclusion in the study, a majority in each of the 10 homes were included and the population is representative of those now living in care homes in the UK. 79,80 Residents were predominantly aged 80 years and above, frail and at high nutritional risk. Levels of nutritional risk were similar to levels found in care homes in the 2011 Nutritional Screening Survey. 81 Residential, nursing and dual-registered homes were included; nearly all were privately managed, and some specialised in looking after the elderly mentally infirm. We found little seasonal variation in antibiotic prescribing and AAD, consistent with the mandatory and voluntary surveillance data. 17
Our estimate of antibiotic prescribing is consistent with an estimate obtained from a study conducted in North Wales,82 where 203 antibiotic prescriptions were recorded over a 9-week period in 15 nursing homes (giving an incidence rate of 2.3 antibiotic prescriptions per resident-year). A study conducted in nursing homes in Sweden83 found a rate of 0.51 antibiotic prescriptions per resident-year.
Estimates of AAD range from virtually zero in low-risk groups to over 40% in those at higher risk, but these estimates are derived from control groups in trials of interventions for AAD, and not prospective observational studies such as ours. 42
Carriage of third-generation cephalosporin resistance was higher than in published rates for urinary isolates in Wales (5%), despite the overall reduction in cephalosporin use that has been achieved between 2006–11,33 supporting arguments that the prevalence of extended-spectrum beta-lactamase-producing bacteria is rising in Europe. 34 Resistance to fluoroquinolones (ciprofloxacin) was also higher than previously published rates. Fluoroquinolone resistance in community urinary coliforms in 2011 in Wales among adults aged 80 years and over was 16.4%. Recent antibiotic use has been shown to be a key risk factor for carriage of antibiotic-resistant organisms in previous studies. 20,22–25,32,84,85 Previous antibiotic use has also been shown to be a risk factor for the presence of extended-spectrum beta-lactamase-producing strain of bacteria in long-term care. 86 A dose–response association has been proposed by one study27 and multidrug resistance has been linked to non-hospital antimicrobial consumption. 29 Nursing home residence has been previously shown to increase the risk of carriage of antibiotic-resistant organisms. 30,41,84 Although we found no association between recent hospitalisation and carriage of resistant organisms, an association was found in a recent study investigating risk factors for drug-resistant pathogens in community-acquired and health care-associated pneumonia. 84 The sample of care home residents recruited for this aspect of the research was representative of those recruited to the main cohort study. This group has been shown elsewhere to be representative of residents living in care homes across the UK. 79,80 The rate of C. difficile infection in England was approximately 366.9 per 100,000 in 2011 for people aged over 65 years, while the same rate was approximately 42.5 for those under 65 years of age. 7 This indicates that C. difficile is still an issue of concern for the specific age group, even though there was a 53% decrease in overall numbers of reported cases between 2008 and 2011. 8
The qualitative study on consent issues
Introduction
The qualitative study about consent and recruitment of care home residents to research found that residents, relatives, care home staff and GPs are generally supportive of older adults in care homes participating in research studies. However, respondents were concerned about the best way of facilitating this, and some subtle, but important, differences of opinion in how older adults should be recruited into studies were apparent.
Limitations of the qualitative study on consent issues
Although the qualitative study reported here incorporates views from a wide range of stakeholders, we acknowledge that our data may be biased. For example, we were able to interview only relatives who were regular visitors to the care homes, residents who had capacity and care home staff who were interested in participating in research. Although residents and relatives who had declined their consent to participate in PAAD stage 1 were invited to participate in the qualitative study, only those residents and relatives who had agreed to participate in PAAD stage 1 agreed to participate in the qualitative study. This could have resulted in a biased sample that included more people who were more accepting of research. We also do not know how many residents were approached by care home staff to participate and declined to be interviewed, which may be a further source of potential bias. Furthermore, the GPs who agreed to be interviewed may have been more knowledgeable of research ethics. We also acknowledge that our respondents may not have been so supportive of research had the IMP been perceived as more harmful.
Comparisons to existing research
Previous research has shown that an accurate and comprehensive patient information sheet is not enough to ensure comprehension of the main issues. 87 In our study this was evidenced by relatives struggling with long and detailed information sheets which care home staff sometimes supplemented, or replaced, with the short, graphically illustrated, information sheet which had been designed for residents. The data from our qualitative study therefore confirm the importance of researchers and ethics committees making every effort to improve the quality of consent discussions and consent documents, ensuring use of familiar words and ideas, short sentences and paragraphs and routinely computing the reading ease of information sheets. 88
One important issue relates to the comprehension and retention of study information to achieve an optimal level of informed consent. Residents and relatives were generally accepting of studies, which they believed to be low risk, and answered important questions that were in the interests of care home residents and the care home. Advanced consent was generally seen as acceptable in low-risk studies, and there was consensus that if someone lost capacity during a trial that they had consented to participate in, they should not necessarily be withdrawn from the trial unless the trial itself changed meaningfully.
The comprehension and retention of information by residents and their relatives becomes even more of a challenge in a study which lasts a reasonably long period of time (12 months in the case of PAAD stage 2), and in which some relatives, asked to act in the capacity of a personal consultee or a personal legal representative, are themselves frail and at risk of becoming cognitively impaired. However, these specific challenges, and challenges raised by other researchers, are not a reason to exclude older adults from research. Although it is important not to underestimate the difficulties, recruitment strategies need to be developed in order to maximise the involvement of frail older people while at the same time protecting their right to decline participation. As participant information sheets are highly standardised, those who recruit older adults to research studies will need training in methods of communication which promote collaborative decision making and enable them to convey complex principles such as equipoise and randomisation in appropriate language. Recruitment of older people, both with and without capacity, to research studies can be complex but the awareness and integrity of the researcher is fundamental to maintaining the principle of non-exploitation. 68 Particular attention should be given to assessing visual and auditory and mental status, compensating for communication and sensory deficits through the use of innovative consent procedures such as pictorial information sheets and interactive tools that help researchers to assess the potential participants’ understanding and retention of information relevant to the study. 89
The second main issue relates to the role of the personal consultee, or personal legal representative, particularly when a research subject loses mental capacity during a research study or trial. Some relatives expressed the view that the person to whom they were related was too elderly or frail and their participation in research was inappropriate, despite the resident themselves having had capacity to decide that they wanted to take part. If these relatives were to act as personal consultees or legal representatives it is uncertain whether their advice or decision on inclusion would reliably reflect the resident’s wish rather than their own. This was indeed raised as a concern by one resident. It is noteworthy also that 1 of the 10 GPs interviewed did not agree with including adults without capacity into any trial of a medicinal product. It is perhaps worth exploring the extent of this attitude among a larger and broader sample of GPs, health professionals, and care home staff, as if this belief is widely held it is likely that it will be a barrier to improving recruitment of this under-represented group into research trials, despite their heavy use of a wide range of health-care technologies.
In a study that lasts 12 months, it is likely that some care home residents who originally have capacity to give consent may lose mental capacity. Furthermore, it is not unusual in such an age group for capacity to be temporarily lost, for example as a result of delirium associated with a urinary tract or chest infection. 90 One important finding of our study was that many staff, relatives and residents felt that, if a resident loses capacity during a clinical trial, then a legal representative must be consulted about that resident’s continued participation in the study. This is in contrast to the majority of GPs, who believed that this would not need to be the case. The MCA (2005)45 makes provision for a personal consultee to advise on the continued participation of the research subject in studies, but this is in contrast to clinical trials of medicinal products, when consent from an adult to participate in the trial remains valid, even after loss of capacity, provided the trial is not significantly altered.
Although care home environments pose practical problems to researchers, our findings should inform other research teams that care home residents, relatives and staff may be generally very supportive of participating in research. Given that older people are the heaviest consumers of health and social care, challenging ageist practices and attitudes in research is important if they are to gain maximum benefit from advances in understanding and management. Ethics committees are also in a strong position to influence research practice and to reduce unethical discrimination on the grounds of age or social situation, by challenging researchers who continue to exclude these groups from research.
Research teams should consider using models of advanced consent for trials of interventions where there is a high likelihood that participants may not have capacity at the point of randomisation. We would also recommend that researchers who are conducting trials of medicinal products in care home settings ensure that care home staff and residents are made aware that residents who have consented to a study have the legal right to remain in the trial if they lose capacity during the study period, provided the person has not indicated otherwise. 48,49
Stage 2: the proposed randomised controlled trial of probiotics to prevent antibiotic-associated diarrhoea in care home residents
Main lessons learned from set up of Probiotics for Antibiotic-Associated Diarrhoea study stage 2
The process of setting up a RCT in a care home setting has been more complex than the process of setting up an observational study in the same setting and RCTs in other health-care settings. The additional statutory approvals required for a CTIMP resulted in a longer and more resource-intensive process than expected. Gaining ethics approval took a longer period of time than anticipated, and required significantly more evidence for the justification for including residents without capacity, than in the case of the observational study.
The classification of the study, which involved a widely available food supplement, as a CTIMP had a significant impact on the ability of the trial to proceed. The discrepancy between the statutory authorisations required for a CTIMP and those needed for an IMP, and the absence of any such documentation for a product generally characterised as a food supplement, had a significant impact on the continuation of the trial. The implementation by the MHRA of a risk-adaptive approach is intended to simplify the processes for initiating and conducting trials where a trial is considered to be in a low-risk category. This subsequent change in approach may have reduced the difficulties experienced during the setting up of the trial if it had been implemented prior to the initial application for approval.
There is a lack of clarity in research governance in care homes, particularly those involving IMP and both NHS and non-NHS sites. The process for identifying responsibility for research governance is problematic and resource intensive. Early consideration is required to ensure that sufficient indemnity is provided to cover research activity at non-NHS sites not previously familiar with conducting research.
Recruitment of care homes, and GPs whose patients are resident in participating care homes, is a very time-consuming process. Strategies can be developed to streamline the process and minimise the burden anticipated by those who raised initial concerns about participating. However, the multiple layers of permissions and agreement required at each level of the process before residents can be approached to participate, remains an arduous process.
The requirement for additional support by researchers to care homes that are research-naive settings had been identified during PAAD stage 1. However, the need to train care home staff conducting a CTIMP on issues such as randomisation and administration of IMP further increased the level of training required. The difficulties with care home staff availability for training required researchers to be flexible and imaginative when designing and delivering training.
The need for a more structured and evidence-based approach to delivery of health care to care home residents requires greater participation from this under-represented group. The challenges encountered during this study, and the strategies used to minimise their impact, can be used by researchers to inform the design and development of future research studies in care homes.
Priorities for future research in care homes
Those involved in the day-to-day care of residents in care homes consider, as a priority, research into methods to improve communication between care home staff and hospitals when a resident is admitted to or discharged from hospital. Care home staff described the problems associated with this; for example, the written handovers that accompany a care home resident requiring hospital admission were frequently mislaid and the discharge summary that is sent to the care home often did not contain adequate information. This often resulted in gaps in a resident’s care and unnecessary delays. Strategies to streamline the pharmacy processes are sought to avoid the duplication of medication dispensing, whereby discharge medication is dispensed despite prefilled monitored-dose devices being used in the care home. These issues impact on the care the resident receives; they may result in delayed treatment and in time and other resources being wasted following up by telephone and making pharmacy visits.
Research to evaluate strategies to enhance staff development and update staff in relation to evidence-based practice was also considered a priority. Care home staff, in particular nursing staff, highlighted their lack of opportunity, as non-NHS professionals, to access updated evidence and research findings. They considered that this was a major barrier to improving evidence-based care for this growing population that has increasingly complex health-care needs.
Care home staff also viewed as important questions relating to the best methods of diagnosing UTIs in this population, and reliable methods of urine sample collection in female residents with cognitive issues. They considered that UTIs had a significant impact on the physical, and often cognitive, well-being of care home residents, with difficulties associated making accurate diagnosis and treatment leading to delayed or inappropriate treatment. They also considered investigation into prevention and prediction of UTIs as important, although this was not considered as high a priority.
A number of other, lesser, priorities for care home research have also been identified (see Appendix 3). These included methods for improvements in care referral pathways for residents, improved access to dental care and techniques to improve oral hygiene, safe minimum staffing levels and how hospital care can be improved for residents.
Overall conclusion
Our study has demonstrated that residents of care homes are frequently prescribed antibiotics and frequently experience diarrhoea following their antibiotic prescription. Our estimate is generally higher than estimates derived from control groups of hospitalised patients in interventional studies. Stool samples were only collected for a small proportion of AAD. CDAD was detected in about 15% of episodes in which we had associated stool samples. Residents in nursing homes were most likely to be prescribed antibiotics and experience AAD, but the size of the differences may in part be the result of the intensity of monitoring of residents in these types of homes, rather than clinical differences in the residents. Clinicians should ensure that their antibiotic prescribing is evidence based in this setting wherever possible. There is a paucity of evidence to support antibiotic prescribing in care home residents. Further research is needed to investigate the benefits of antibiotic treatment, and the development and evaluation of preventative strategies for AAD in this setting.
Research staff should be mindful of research guidance and ensure that they have obtained an appropriate level of informed consent without overwhelming the participant with unnecessary detail. For research involving medicinal products, research staff should also be more explicit when recruiting that consent is still valid should an older person lose capacity during a trial unless the individual expresses beforehand a wish to be withdrawn in the event of loss of capacity and does not indicate objection or resistance after loss of capacity.
Stakeholder involvement in care home research is important throughout the research process from study design to recruitment of care homes and participants. Stakeholders’ insight into life in care homes and valuable contribution to the design and conduct of a study being delivered in a research-naive environment were essential to the success of the study.
Setting up and implementing research in care homes is more complex and time-consuming than may immediately be apparent. Our trial of probiotics to prevent AAD was classified as a CTIMP and this had far-reaching implications including the need for site-specific information forms for GPs, the logistical problems associated with the need for GPs to confirm care home residents’ eligibility to be randomised, even if the residents had already consented, and QP documentation for the probiotic. These problems were solved, but recruitment data published just as we were about to start the trial led the study team, the TSC and the funders to conclude that equipoise had shifted so far as to make continuing with the trial no longer justified. However, future studies of what some might consider food supplements should establish ahead of designing the study in detail whether or not the proposed trial will be classified as a CTIMP, as the requirement for GPs to confirm eligibility for randomisation makes trials of acutely unwell care home residents particularly challenging.
Acknowledgements
Contributions of authors
Professor Kerenza Hood (Professor of Medical Statistics, Director of the SEWTU) was a co-investigator, led the study design and supervised the statistical analysis, had overall responsibility for the study management and contributed to the study implementation and report submission.
Jacqui Nuttall (Senior Trial Manager) was a co-investigator, contributed to the study design, study management and study implementation, and assisted in drafting the report and report submission.
David Gillespie (Research Associate) contributed to the study design, carried out all statistical analyses and assisted in the drafting of the report and report submission.
Victoria Shepherd (Research Nurse) contributed to the study implementation and assisted in the drafting of the report and report submission.
Dr Fiona Wood (Senior Lecturer) was a co-investigator of PAAD stage 1, contributed to the design, data collection and analysis of qualitative data, and assisted in the drafting of the report and report submission.
Donna Duncan (Deputy Head of Nutrition and Dietetics) was a co-investigator, contributed to the study design, recruited and trained care homes and contributed to the study implementation and report submission.
Helen Stanton (Research Associate) contributed to the study design and acquisition of the data, and assisted in the drafting of the report and report submission.
Aude Espinasse (Research Assistant) contributed to the study design and acquisition of the data, and assisted in the drafting of the report and report submission.
Dr Mandy Wootton (Lead Scientist, Specialist Antimicrobial Chemotherapy Unit) contributed to the data analysis and report submission.
Dr Aruna Acharjya (Clinical Trial Monitor) contributed to data collection, trial management, study implementation and report submission.
Professor Stephen Allen (Professor of Paediatrics and International Health) was a co-investigator and contributed to the study design and report submission.
Professor Antony Bayer (Professor of Clinical Gerontology) was a co-investigator and contributed to the design and report submission.
Dr Ben Carter (Lecturer, Medical Statistics) was a co-investigator and contributed to the study design and report submission.
Professor David Cohen (Professor of Health Economics) was a co-investigator and contributed to the health economic evaluation and report submission.
Dr Nick Francis (Senior Clinical Research Fellow) contributed to the study design, study implementation and report submission.
Dr Robin Howe (Consultant Microbiologist) was a co-investigator, contributed to the study design, data analysis and report submission.
Dr Efi Mantzourani (Lecturer in Pharmacy Practice and Clinical Pharmacy) contributed to the study design and assisted in the drafting of the report and report submission.
Dr Emma Thomas-Jones (Research Fellow) contributed to the management of the study and report submission.
Alun Toghill (Involving People Representative) was a co-investigator and contributed to the study design and report submission.
Professor Christopher C Butler (Professor of Primary Care Medicine) was the chief investigator, lead applicant, study guarantor, and led the development of the research question, study design, study implementation and report submission.
Contribution of others
National Institute for Social Care and Health Research – Clinical Research Centre collected data and provided support to care homes.
Research support
Hayley Prout (research associate) recruited participants to the qualitative study and conducted all interviews and one of the focus groups.
Administrative support
Mrs Judith Evans (research administrator), Mrs Sian Dawes and Mr Rhys Thomas.
Members of the Trial Management Group
Dr Meirion Evans, Dr Anthony Johansen, Dr Neil Wigglesworth, Ms Julia Townson and all those who contributed to the study management.
Members of the Trial Steering Committee
Professor Peter Crome (chairperson), Dr Samuel Coenen, Mr Graham Tanner and Rachel Kemp.
Members of the independent Data Monitoring Committee
Dr Steven George, Professor Matthew Hickman, Dr Andrew Lovering and Professor John McLeod.
The Specialist Antimicrobial Chemotherapy Unit conducted the microbiological analysis.
Institutional support
We acknowledge with thanks the trial funders, the UK NIHR HTA. SEWTU is funded by the Welsh Government through NISCHR and the authors gratefully acknowledge SEWTU’s contribution to the study.
Services and participants
We acknowledge, with thanks, all of the services, the residents, their representatives and the care home staff who participated in the study.
We thank all those not otherwise mentioned above who have contributed to the PAAD study.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
Publications
Butler CC, Duncan D, Hood K. Does taking probiotics routinely with antibiotics prevent antibiotic-associated diarrhoea? BMJ 2012;344:e682.
Wood F, Prout H, Bayer A, Duncan D, Nuttall J, Hood K, et al. Consent, including advanced consent, of older adults to research in care homes: a qualitative study of stakeholders’ views. Trials 2013;4:247.
References
- Care of Elderly People: UK Market Survey 2012–13. London: Laing and Buisson; 2013.
- Bajekal M. Health Survey for England 2000: Care Homes and their Residents. London: The Stationery Office; 2002.
- Greenberg S, Fowles DG. A Profile of Older Americans: 2011. Washington, DC: US Department of Health and Human Services, Administration on Aging; 2011.
- Wittenberg R, Comas-Herrera A, Pickard L, Hancock R. Future demand for long-term care in the UK. A summary of projections of long-term care finance for older people to 2051. Newsletter of the Geneva Association of Insurance Economics 2004;11:2-4.
- ECDC . Healthcare Associated Infections in European Long Term Care Facilities. Bulletin 3. n.d. https://halt.wiv-isp.be/report/Reports/HALT-1/HALT%20Report%20Pilot%20Survey%20Nov%202009.pdf (accessed 16 July 2014).
- Williams D. Prevalence Survey of Healthcare Associated Infections in Long Term Care Facilities (Halt Study) 2012. www2.nphs.wales.nhs.uk/WHAIPDocs.nsf/Public/29B97D254270976280257A0D00426B0E/$file/Halt%20Report%20Wales.pdf?OpenElement (accessed 16 July 2014).
- van Buul L, van der Steen J, Veenhuizen R, Achterberg W, Schellevis F, Essink R, et al. Antibiotic use and resistance in long term care facilities. J Am Med Dir Assoc 2012;13.
- Warren J, Palumbo F, Fitterman L, Speedie S. Incidence and characteristics of antibiotic use in aged nursing home patients. J Am Geriatr Soc 1991;39:963-72.
- Nicolle L, Bentley D, Garibaldi R, Neuhaus E, Smith P. Antimicrobial use in long-term-care facilities. SHEA long-term-care committee. Infect Control Hosp Epidemiol 2000;21:537-45. http://dx.doi.org/10.1086/501798.
- Benoit SR, Nsa W, Richards CL, Bratzler DW, Shefer AM, Steele LM, et al. Factors associated with antimicrobial use in nursing homes: a multilevel model. J Am Geriatr Soc 2008;56:2039-44. http://dx.doi.org/10.1111/j.1532-5415.2008.01967.x.
- Bartlett JG. Clinical practice. Antibiotic-associated diarrhea. N Engl J Med 2002;346:334-9. http://dx.doi.org/10.1056/NEJMcp011603.
- Antunes LCM, Han J, Ferreira RBR, Lolic P, Borchers CH, Finlay BB. Effect of antibiotic treatment on the intestinal metabolome. Antimicrob Agents Chemother 2011;55:1494-503. http://dx.doi.org/10.1128/AAC.01664-10.
- Fletcher KR, Cinalli M. Identification, optimal management, and infection control measures for Clostridium difficile-associated disease in long-term care. Geriatr Nurs 2007;28:171-81. http://dx.doi.org/10.1016/j.gerinurse.2007.02.003.
- Surawicz CM. Probiotics, antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in humans. Best Pract Res Clin Gastroenterol 2003;17:775-83. http://dx.doi.org/10.1016/S1521-6918(03)00054-4.
- Nelson RL, Kelsey P, Leeman H, Meardon N, Patel H, Paul K, et al. Antibiotic treatment for Clostridium difficile-associated diarrhea in adults. Cochrane Database of Syst Rev 2011;9.
- Monaghan T, Boswell T, Mahida YR. Recent advances in Clostridium difficile-associated disease. Gut 2008;57:850-60.
- Health Protection Agency . Voluntary Surveillance of Clostridium Difficile Associated Disease in England, Wales and Northern Ireland 2012. www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1317138039648 (accessed 6 March 2013).
- Tam CT, Viviani L, Rodrigues LC, O’Brien SJ. The second study of Infectious Intestinal Disease (IID2): increased rates of recurrent diarrhoea in individuals aged 65 years and above. BMC Public Health 2013;13. http://dx.doi.org/10.1186/1471-2458-13-739.
- Berrington AW. National Clostridium difficile standards group: report to the Department of Health. J Hosp Infect 2004;56:1-38. http://dx.doi.org/10.1016/j.jhin.2003.10.016.
- Makris A, Gelone S. Clostridium difficile in the long-term care setting. J Am Med Dir Assoc 2007;8:290-9. http://dx.doi.org/10.1016/j.jamda.2007.01.098.
- Stevens V, Dumyati G, Fine LS, Fisher SG, van Wijngaarden E. Cummulative antibiotic exposures over time and the risk of Clostridium difficile infection. Clin Infect Dis 2011;53:42-8. http://dx.doi.org/10.1093/cid/cir301.
- Bignardi GE. Risk factors for Clostridium difficile infection. J Hosp Infect 1998;40:1-15. http://dx.doi.org/10.1016/S0195-6701(98)90019-6.
- Cunningham R, Dale B, Undy B, Gaunt N. Proton pump inhibitors as a risk factor for Clostridium difficile diarrhoea. J Hosp Infect 2003;54:243-5. http://dx.doi.org/10.1016/S0195-6701(03)00088-4.
- Starr JM, Martin H, McCoubrey J, Gibson G, Poxton IR. Risk factors for Clostridium difficile colonisation and toxin production. Age Ageing 2003;32:657-60. http://dx.doi.org/10.1093/ageing/afg112.
- Pettersson E, Vernby A, Mölstad S, Lundborg CS. Infections and antibiotic prescribing in Swedish nursing homes: a cross-sectional study. Scand J Infect Dis 2008;40:393-8. http://dx.doi.org/10.1080/00365540701745279.
- Goossens H, Ferech M, Vander Stichele R, Elseviers M. ESAC Project Group . Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 2005;365:579-87. http://dx.doi.org/10.1016/S0140-6736(05)17907-0.
- Laffan AM, Bellantoni MF, Greenough WB, Zenilman JM. Burden of Clostridium difficile-associated diarrhea in a long-term care facility. J Am Geriatr Soc 2006;54:1068-73. http://dx.doi.org/10.1111/j.1532-5415.2006.00768.x.
- Kutty PK, Benoit SR, Woods CW, Sena AC, Naggie S, Frederick J, et al. Assessment of Clostridium difficile-associated disease surveillance definitions, North Carolina, 2005. Infect Control Hosp Epidemiol 2008;29:197-202. http://dx.doi.org/10.1086/528813.
- Chang HT, Krezolek D, Johnson S, Parada JP, Evans CT, Gerding DN. Onset of symptoms and time to diagnosis of Clostridium difficile-associated disease following discharge from an acute care hospital. Infect Control Hosp Epidemiol 2007;28:926-31. http://dx.doi.org/10.1086/519178.
- Riggs MM, Sethi AK, Zabarsky TF, Eckstein EC, Jump RLP, Donskey CJ. Asymptomatic carriers are a potential source for transmission of epidemic and nonepidemic Clostridium difficile strains among long-term care facility residents. Clin Infect Dis 2007;45:992-8. http://dx.doi.org/10.1086/521854.
- Simor AE. Clostridium difficile diagnosis and management and prevention in long term care facilities: a review. J Am Geriatr Soc 2010;58:1556-64. http://dx.doi.org/10.1111/j.1532-5415.2010.02958.x.
- Havenaar R, Jos HJ, In’t Veld H, Wood BJB. The Lactic Acid Bacteria Vol. 1. The Lactic Acid Bacteria in Health and Disease. New York, NY: Elsevier; n.d.
- Gorbach SL, Chang TW, Goldin B. Successful treatment of relapsing Clostridium difficile colitis with Lactobacillus GG. Lancet 1987;2. http://dx.doi.org/10.1016/S0140-6736(87)92646-8.
- Sullivan Å, Nord CE. The place of probiotics in human intestinal infections. Int J Antimicrob Agents 2002;20:313-19. http://dx.doi.org/10.1016/S0924-8579(02)00199-1.
- Allen SJ, Okoko B, Martinez EG, Gregorio GV, Dans LF. Probiotics for treating infectious diarrhoea. Cochrane Database of Syst Rev 2004;2.
- Ohland CL, MacNaughton WK. Probiotic bacteria and intestinal epithelial barrier function. Am J Physiol Gastrointest Liver Physiol 2010;298:G807-19. http://dx.doi.org/10.1152/ajpgi.00243.2009.
- Venugoplan V, Shriner KA, Wong-Beringer A. Regulatory oversight and safety of probiotic use. Emerg Infect Dis 2010;16:1661-5. http://dx.doi.org/10.3201/eid1611.100574.
- Hempel S, Newberry SJ, Maher AR, Wang Z, Miles JN, Shanman R, et al. Probiotics for the prevention and treatment of antibiotic-associated diarrhoea: a systematic review and meta-analysis. JAMA 2012;307:1959-69. http://dx.doi.org/10.1001/jama.2012.3507.
- Goldenberg JZ, Ma SS, Saxton JD, Martzen MR, Vandvik PO, Thorlund K, et al. Probiotics for the prevention of Clostridium difficile associated diarrhoea in adults and children. Cochrane Database Syst Rev 2013;5.
- Johnston B, Supina A, Vohra S. Probiotics for paediatric antibiotics-associated diarrhea: a meta-analysis of randomized placebo-controlled trials. CMAJ 2006;175:377-83. http://dx.doi.org/10.1503/cmaj.051603.
- Hickson M, D’Souza AL, Muthu N, Rogers TR, Want S, Rajkumar C, et al. Use of probiotic Lactobacillus preparation to prevent diarrhoea associated with antibiotics: randomised double blind placebo controlled trial. BMJ 2007;335. http://dx.doi.org/10.1136/bmj.39231.599815.55.
- Butler CC, Duncan D, Hood K. Does taking probiotics routinely with antibiotics prevent antibiotic associated diarrhoea?. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.e682.
- Mentes JC, Tripp-Reimer T. Barriers and facilitators in nursing home intervention research. West J Nurs Res 2002;24:918-36. http://dx.doi.org/10.1177/019394502237702.
- Maas ML, Kelley LS, Park M, Specht JP. Issues in conducting research in nursing homes. West J Nurs Res 2002;24:373-89. http://dx.doi.org/10.1177/01945902024004006.
- Mental Capacity Act 2005: Elizabeth II. Chapter 9. London: The Stationery Office; 2005.
- Elia M. Screening for Malnutrition: A Multidisciplinary Responsibility. Development and Use of the ‘Malnutrition Universal Screening Tool’ (‘MUST’) for Adults 2003. www.bapen.org.uk/pdfs/must/must_exec_sum.pdf (accessed 15 July 2014).
- Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. CMAJ 2005;173:489-95. http://dx.doi.org/10.1503/cmaj.050051.
- Medicines for Human Use (Clinical Trials) . Regulations 2004 n.d. www.opsi.gov.uk/si/si2004/20041031.htm (accessed 22 July 2014).
- Rees E, Hardy J. Novel consent process for research in dying patients unable to give consent. BMJ 2003;327. http://dx.doi.org/10.1136/bmj.327.7408.198.
- Royal College of Obstetricians and Gynaecologists . Obtaining Valid Consent to Participate in Research While in Labour. Clinical Governance Advice No. 6a. n.d. www.rcog.org.uk/files/rcog-corp/CGAObtainingConsentResearchLabour0810.pdf (accessed 15 July 2014).
- The Mental Capacity Act 2005 (Loss of Capacity during Research Project) (England) Regulations 2007. London: The Stationery Office; 2007.
- Mental Capacity Act 2005 (Loss of Capacity during Research Project) (Wales) Regulations 2007. London: The Stationery Office; 2007.
- Wood F, Prout H, Bayer A, Duncan D, Nuttall J, Hood K, et al. Consent, including advanced consent, of older adults to research in care homes: a qualitative study of stakeholders’ views. Trials 2013;14. http://dx.doi.org/10.1186/1745-6215-14-247.
- Green JNT. Qualitative Methods for Health Research. London: Sage; 2004.
- Allen SJ, Wareham K, Wang D, Bradley C, Hutchings H, Harris W, et al. Lactobacilli and bifid bacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multicentre trial. Lancet 2013;382:1249-57. http://dx.doi.org/10.1016/S0140-6736(13)61218-0.
- Brooks RG. Euroqol: the current state of play. Health Policy 1996;37. http://dx.doi.org/10.1016/0168-8510(96)00822-6.
- Grey AM, Clarke PM, Wolstenhole JL, Wordsworth S. Applied Methods of Cost-effectiveness Analysis in Health Care. Oxford: Oxford University Press; 2011.
- Research Governance Framework for Health and Social Care. London: DH; 2005.
- National Research Ethics Service Medicines for Human Use (Clinical Trials) Regulations Informed Consent in Clinical Trials Guidance n.d. www.hra.nhs.uk/documents/2014/04/nres-guidance_informed-consent-ctimps_v3-1_2014-04-14.pdf (accessed 15 July 2014).
- Reed J, Cook G, Cook M. Research governance issues in the care home sector. J Res Nurs 2004;9:430-9. http://dx.doi.org/10.1177/136140960400900604.
- Lingler JH, Jablonski RA, Bourbonniere M, Kolanowski A. Informed consent to research in long-term care settings. Res Gerontol Nurs 2009;2:153-61. http://dx.doi.org/10.3928/19404921-20090428-03.
- Kaasalainen S, Williams J, Hadjistavropoulos T, Thorpe L, Whiting S, Neville S, et al. Creating bridges between researchers and long-term care homes to promote quality of life for residents. Qual Health Res 2010;20:1689-704. http://dx.doi.org/10.1177/1049732310377456.
- Washington AE, Lipstein SH. The patient centred outcomes research institute – promoting better information, outcomes and health. N Engl J Med 2011;365. http://dx.doi.org/10.1056/NEJMp1109407.
- Oliver S, Clarke-Jones L, Rees R, Milne R, Buchanan P, Gabbay J, et al. Involving consumers in research and development agenda setting for the NHS: developing an evidence-based approach. Health Technol Assess 2004;8.
- National Institute for Health Research . Patient and Public Involvement – Information for Researchers n.d. www.nets.nihr.ac.uk/data/assets/pdf_file/0015/70026/ppi-researchers.pdf (accessed 17 July 2014).
- National Institute for Health Research . Enabling Research in Care Homes (ENRICH) n.d. www.enrich.dendron.nihr.ac.uk (accessed 17 July 2014).
- British Geriatrics Society Joint Working Party . Quest for Quality: An Inquiry into the Quality of Healthcare Support for Older People in Care Homes: A Call for Leadership, Partnership and Improvement 2011. www.bgs.org.uk/campaigns/carehomes/quest_quality_care_homes.pdf (accessed 22 July 2014).
- Harris R, Dyson E. Recruitment of frail older people to research: lessons learnt through experience. J Adv Nurs 2001;35:643-51.
- Crome P, Lally F, Cherubini A, Oristrell J, Beswick AD, Clarfield AM, et al. Exclusion of older people from clinical trials. Drugs Aging 2011;28:667-77. http://dx.doi.org/10.2165/11591990-000000000-00000.
- Cherubini A. Increasing the Participation of the Elderly in Clincal Trials (PREDICT). Sheffield: Medical Economics and Research Centre; 2010.
- Luff R, Ferreira Z, Meyer J. Methods Review No. 8 Care Homes 2011. http://sscr.nihr.ac.uk/PDF/MR/MR8.pdf (accessed 17 July 2014).
- National Institute for Health Research . UK Clinical Research Network Patient and Public Involvement Strategy [11 11 2013] n.d. www.crn.nihr.ac.uk/can-help/healthcare-professionals/involving-patients-in-research/ (accessed 17 July 2014).
- National Institute for Health Research . INVOLVE: National Institute for Health Research n.d. www.invo.org.uk (accessed 11 November 2013).
- Viergever RF, Olifson S, Ghaffar A, Terry RF. A checklist for health research priority setting: nine common themes of good practice. Health Res Policy 2010;8.
- Gallagher M, Hares T, Spencer J, Bradshaw C, Webb I. The nominal group technique: a research tool for general practice?. Fam Pract 1993;10:76-81. http://dx.doi.org/10.1093/fampra/10.1.76.
- Hall S, Longhurst S, Higginson IJ. Challenges to conducting research with older people living in nursing homes. BMC Geriatr 2009;9. http://dx.doi.org/10.1186/1471-2318-9-38.
- Zermansky AG. Including care home residents in clinical research is fraught. BMJ 2005;331:1271-2. http://dx.doi.org/10.1136/bmj.331.7527.1271-c.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: Wiley-Blackwell; 2008.
- Lievesley N, Crosby G, Bowman C. The Changing Role of Care Homes. London: Centre for Policy on Ageing; 2011.
- Goodman C, Baron NL, Machen I, Stevenson E, Evans C, Davies SL, et al. Culture, consent, costs and care homes: enabling older people with dementia to participate in research. Aging Ment Health 2011;15:475-81. http://dx.doi.org/10.1080/13607863.2010.543659.
- Russell CA, Elia M. Nutrition Screening Survey in the UK and Republic of Ireland in 2010. A Report by the British Association for Parenteral and Enteral Nutrition (BAPEN). Redditch: BAPEN; 2011.
- Roberts C, Roberts J, Roberts RJ. Survey of healthcare-associated infection rates in a nursing home resident population. J Infect Prev 2010;11:82-6.
- Eklund Å, Hartvig P, Tverin I, Palm M, Sylvan S. Prevalence and management of infections in nursing homes in Uppsala County, Sweden. Open Longev Sci 2008;2:96-9. http://dx.doi.org/10.2174/1876326X00802010096.
- Lonnermark E, Friman V, Lappas G, Sandberg T, Berggren A, Adlerberth I. Intake of Lactobacillus plantarum reduces certain gastrointestinal symptoms during treatment with antibiotics. J Clin Gastroenterol 2010;44:106-12. http://dx.doi.org/10.1097/MCG.0b013e3181b2683f.
- Song HJ, Kim JY, Jung SA, Kim SE, Park HS, Jeong Y, et al. Effect of probiotic Lactobacillus (Lacidofil® cap) for the prevention of antibiotic-associated diarrhea: a prospective, randomized, double-blind, multicenter study. J Korean Med Sci 2010;25:1784-91. http://dx.doi.org/10.3346/jkms.2010.25.12.1784.
- Mendelson G, Hait V, Ben-Israel J, Gronich D, Granot E, Raz R. Prevalence and risk factors of extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae in an Israeli long-term care facility. Eur J Clin Microbiol Infect Dis 2005;24:17-22. http://dx.doi.org/10.1007/s10096-004-1264-8.
- Dixon-Woods M, Ashcroft RE, Jackson CJ, Tobin MD, Kivits J, Burton PR, et al. Beyond “misunderstanding”: written information and decisions about taking part in a genetic epidemiology study. Soc Sci Med 2007;65:2212-22. http://dx.doi.org/10.1016/j.socscimed.2007.08.010.
- Jefford M, Moore R. Improvement of informed consent and the quality of the consent document. Lancet Oncol 2008;9:485-93. http://dx.doi.org/10.1016/S1470-2045(08)70128-1.
- Bayer A, Fish M. The doctor’s duty to the elderly patient in clinical trials. Drugs Ageing 2003;20:1087-97. http://dx.doi.org/10.2165/00002512-200320150-00002.
- Young J, Inouye SK. Delirium in older people. BMJ 2007;334. http://dx.doi.org/10.1136/bmj.39169.706574.AD.
Appendix 1 Participant flow diagram
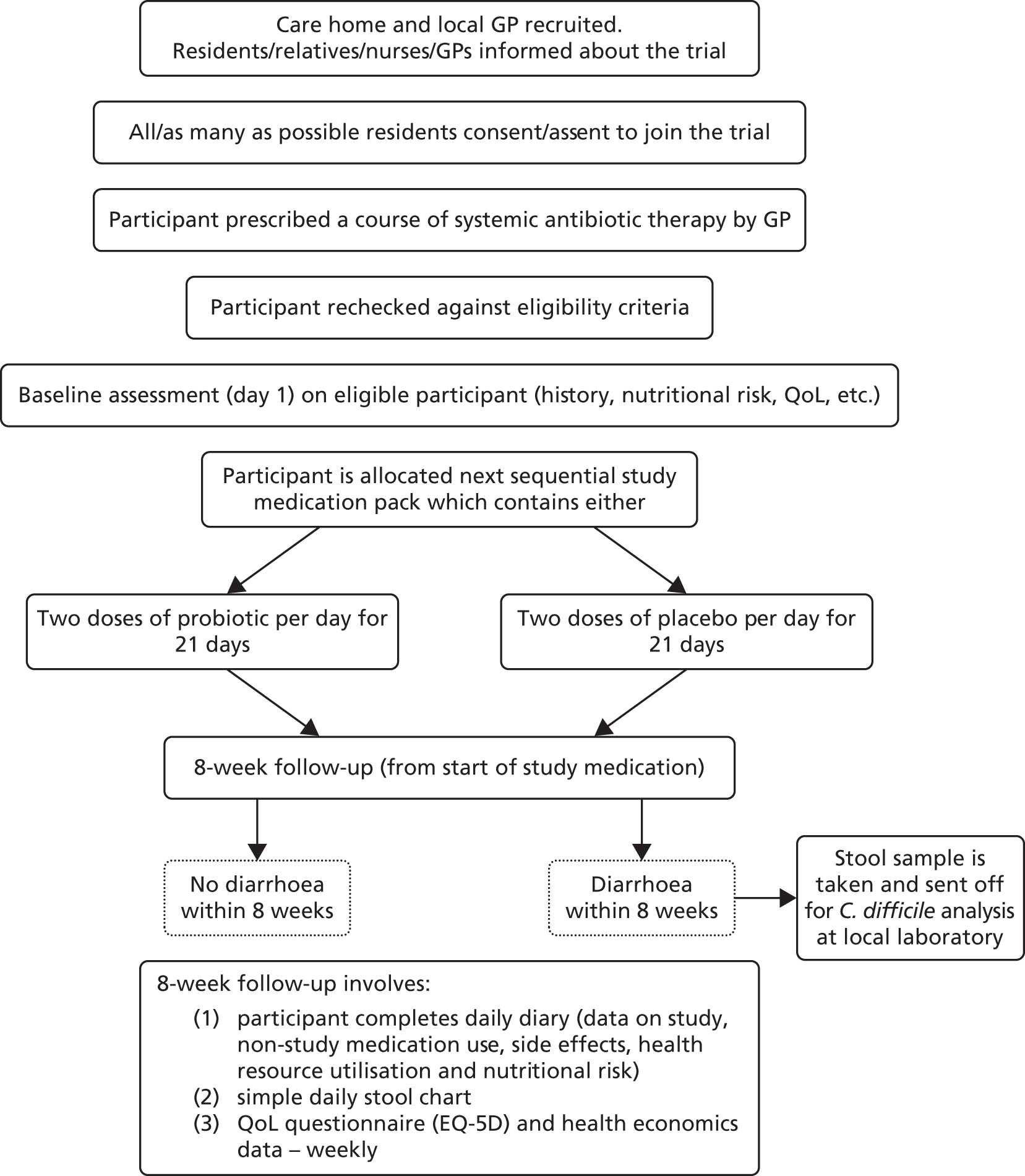
Appendix 2 Study schema
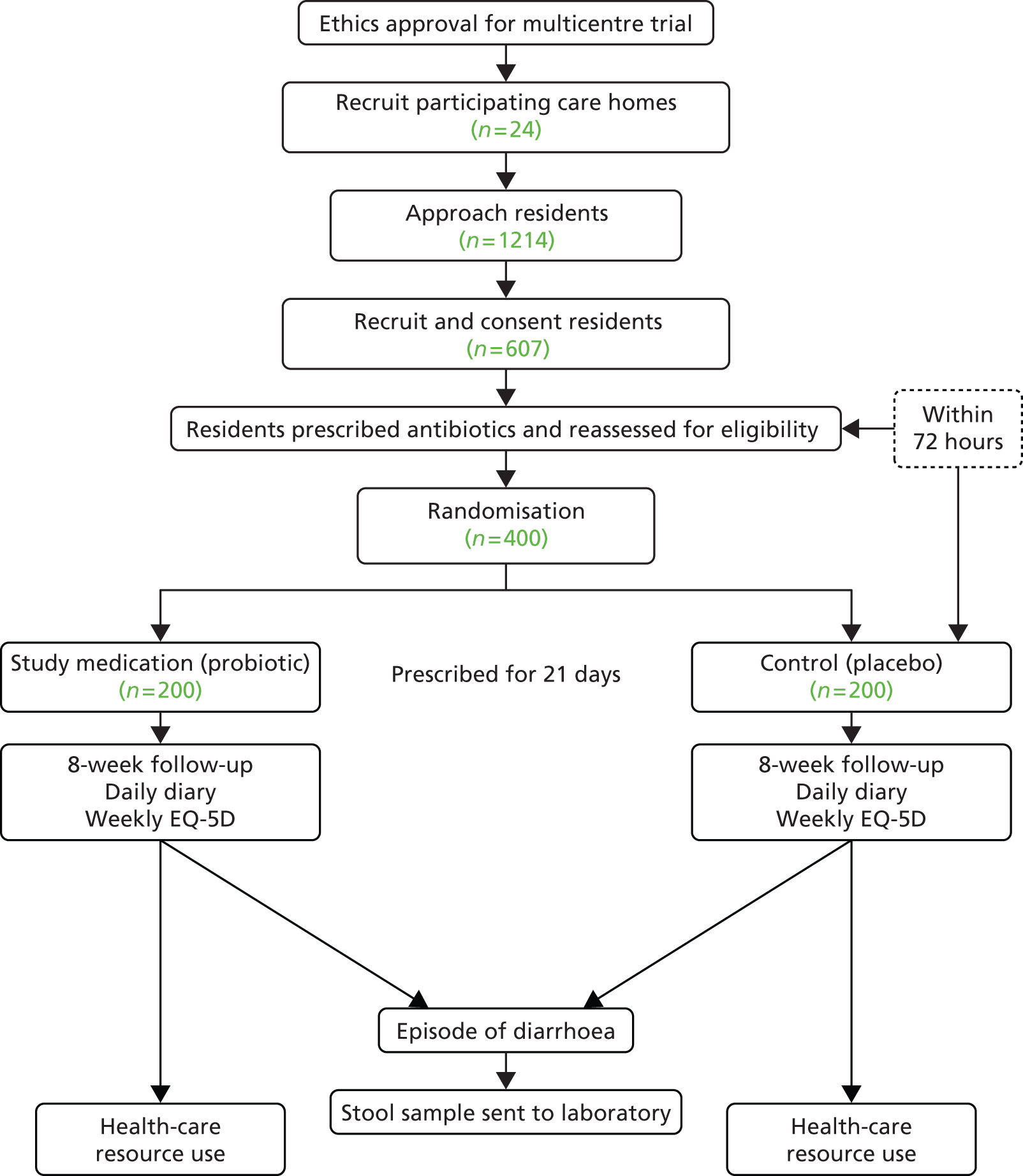
Appendix 3 Research priority topics
Please score the six topics you feel is the most important from 1 to 6 (1 is least important, 6 is the most important of your chosen six topics).
| Topic | Number |
|---|---|
| 1. How can care home staff best be kept up to date with staff development/evidence updates for nurses in care homes? | e.g. 3 |
| 2. How can hospital care for older people from care homes be improved? (Service configurations and interactions between care homes and hospital) | |
| 3. What are the best methods of diagnosing UTIs in elderly women and what methods can be used for collecting reliable urine samples? | |
| 4. Are there ways UTIs can be prevented and how can UTIs be predicted? | |
| 5. How can access to speech and language therapy be improved? (Is there potential to increase skill in care home staff to assess speech and language problems and support speech and language needs) | |
| 6. How can access to physiotherapy services be improved? | |
| 7. How can the current multiple and complex care referral pathways be improved so as to reduce the long waits and wasted resources? (Care appears to be organised around professional services rather than the patient, are there ways to make the process person centred) | |
| 8. Does participation in activities reduce levels of anxiety and depression levels in residents? | |
| 9. How can communication between staff and hospitals be improved when residents move into and out of hospitals? Can the prescribing and dispensing of medication process be improved to reduce wasted time and resources? | |
| 10. What is the evidence for the use of bedrails to reduce the risk of entrapment? (Particularly with the use of airflow mattresses) | |
| 11. What are safe procedures for the use of airflow mattresses for people weighing less than 50 kg? (Currently not suitable for those below 50 kg, but no currently recommended alternative available) | |
| 12. Do different models of interaction with GPs (how often doctors visit and whether it is the same doctor) impact on residents’ urgent care requirements or hospital admissions? | |
| 13. Is there evidence of an impact of changing nutritional patterns on moving in to a care home, compared with prior to moving in, on nutritional status? | |
| 14. How can the Butterfly scheme/This Is Me books improve transfers between care home and hospital? | |
| 15. Does the use of Bristol Stool Chart benefit care home residents, or result in best practice/improving standards? | |
| 16. What is the impact of use of ‘food first programme’, instead of prescribable dietary supplements, on residents’ nutrition? | |
| 17. Can we improve hydration by improving use of prethickened fluids instead of care home staff preparing drinks using thickening agents? | |
| 18. What are the benefits of participating in tai chi in wellbeing of care home residents? | |
| 19. Can the administration of i.v. fluids and antibiotics in care homes improve the health of residents and prevent hospital admissions? | |
| 20. What improvements can be made in access to dental care for residents? And how effective are techniques to improve oral hygiene, such as use of suction/rinse toothbrushes? | |
| 21. What are the benefits of different types of activity provision for care home residents, such as peer led groups? | |
| 22. What are the benefits of the use of sensory techniques in provision of activities for care home residents? | |
| 23. What are the safe minimum staffing levels and how do these relate to dependency scoring? How many staff do you actually need in care homes? |
Appendix 4 Research priority rank ordering
Priorities for research in care homes
| Topic | Priority ranking from workshop | Score from workshop | Priority ranking from postal survey | Score from postal survey | Total score | Rated as important, but not individually scored (information only) |
|---|---|---|---|---|---|---|
| 1. How can care home staff best be kept up to date with staff development/evidence updates for nurses in care homes? | 2, 4 | 8 | 2 | 5 | 13 | |
| 2. How can hospital care for older people from care homes be improved? (Service configurations and interactions between care homes and hospital) | Joint 1 | 6 | 3 | 4 | 10 | Marked as important twice |
| 3. What are the best methods of diagnosing UTIs in elderly women and what methods can be used for collecting reliable urine samples? | 1 | 6 | Joint 1 | 6 | 12 | Marked as important three times |
| 4. Are there ways UTIs can be prevented and how can UTIs be predicted? | Joint 1, 6, 4 | 10 | 10 | Marked as important twice | ||
| 5. How can access to speech and language therapy be improved? (Is there potential to increase skill in care home staff to assess speech and language problems and support speech and language needs?) | ||||||
| 6. How can access to physiotherapy services be improved? | ||||||
| 7. How can the current multiple and complex care referral pathways be improved so as to reduce the long waits and wasted resources? (Care appears to be organised around professional services rather than the patient, are there ways to make the process person centred) | 4, 1, 5 | 11 | 11 | Marked as important once | ||
| 8. Does participation in activities reduce levels of anxiety and depression levels in residents? | Marked as important once | |||||
| 9. How can communication between staff and hospitals be improved when residents move into and out of hospitals? Can the prescribing and dispensing of medication process be improved to reduce wasted time and resources? | Joint 1, 3 | 10 | 3, 2 | 9 | 19 | Marked as important twice |
| 10. What is the evidence for the use of bedrails to reduce the risk of entrapment? (Particularly with the use of airflow mattresses) | Joint 5 | 2 | Joint 5 | 2 | 4 | Marked as important once |
| 11. What are safe procedures for the use of airflow mattresses for people weighing less than 50 kg? (Currently not suitable for those below 50 kg, but no currently recommended alternative available) | Joint 5 | 2 | Joint 5 | 2 | 4 | |
| 12. Do different models of interaction with GPs (how often doctors visit and whether it is the same doctor) impact on residents urgent care requirements or hospital admissions? | 6, 5 | 3 | ||||
| 13. Is there evidence of an impact of changing nutritional patterns on moving in to a care home, compared with prior to moving in, on nutritional status? | ||||||
| 14. How can the Butterfly scheme/This Is Me books improve transfers between care home and hospital? | Marked as important once | |||||
| 15. Does the use of Bristol Stool Chart benefit care home residents, or result in best practice/improving standards? | Marked as important once | |||||
| 16. What is the impact of using the ‘food first programme’, instead of prescribable dietary supplements, on residents’ nutrition? | ||||||
| 17. Can we improve hydration by improving the use of prethickened fluids instead of care home staff preparing drinks using thickening agents? | ||||||
| 18. What are the benefits of participating in tai chi on the wellbeing of care home residents? | 6 | 1 | 1 | |||
| 19. Can the administration of i.v. fluids and antibiotics in care homes improve the health of residents and prevent hospital admissions? | Joint 6 | 1 | 1 | Marked as important once | ||
| 20. What improvements can be made in access to dental care for residents? And how effective are techniques to improve oral hygiene, such as use of suction/rinse toothbrushes? | 5 | 2 | 4, 1 | 9 | 11 | |
| 21. What are the benefits of different types of activity provision for care home residents, such as peer led groups? | ||||||
| 22. What are the benefits of the use of sensory techniques in provision of activities for care home residents? | Marked as important once | |||||
| 23. What are the safe minimum staffing levels and how do these relate to dependency scoring? How many staff do you actually need in care homes? | 4, 6 | 4 | 3, 2 | 6 | 10 | Marked as important once |
Priorities for research in care homes: rank ordering
| 1 | How can communication between staff and hospitals be improved when residents move into and out of hospitals? Can the prescribing and dispensing of medication process be improved to reduce wasted time and resources? |
| 2 | How can care home staff best be kept up to date with staff development/evidence updates for nurses in care homes? |
| 3 | What are the best methods of diagnosing UTIs in elderly women and what methods can be used for collecting reliable urine samples? |
| = 4 | How can the current multiple and complex care referral pathways be improved so as to reduce the long waits and wasted resources? (Care appears to be organised around professional services rather than the patient, are there ways to make the process person-centred) |
| = 4 | What improvements can be made in access to dental care for residents? And how effective are techniques to improve oral hygiene, such as use of suction/rinse toothbrushes? |
| = 6 | Are there ways UTIs can be prevented and how can UTIs be predicted? |
| = 6 | What are the safe minimum staffing levels and how do these relate to dependency scoring? How many staff do you actually need in care homes? |
| = 6 | How can hospital care for older people from care homes be improved? (Service configurations and interactions between care homes and hospital) |
| = 9 | What is the evidence for the use of bedrails to reduce the risk of entrapment? (Particularly with the use of airflow mattresses) |
| = 9 | What are safe procedures for the use of airflow mattresses for people weighing less than 50 kg? (Currently not suitable for those below 50 kg, but no currently recommended alternative available) |
| = 11 | What are the benefits of participating in tai chi on the wellbeing of care home residents? |
| = 11 | Can the administration of i.v. fluids and antibiotics in care homes improve the health of residents and prevent hospital admissions? |
Glossary
- Antibiotic-associated diarrhoea
- Three or more loose stools (defined as type 5–7 on the Bristol Stool Chart) in a 24-hour period following the prescription of an antibiotic, and for an additional 8 weeks.
- Clostridium difficile-associated diarrhoea
- An episode of antibiotic-associated diarrhoea attributable to C. difficile as a result of a culture-positive sample.
- Unique episodes of antibiotic-associated diarrhoea
- Episodes of antibiotic-associated diarrhoea separated by at least 3 days of no recorded diarrhoea.
- VSL#3
- A probiotic food supplement.
List of abbreviations
- AAD
- antibiotic-associated diarrhoea
- BSC
- Bristol Stool Chart
- CDAD
- Clostridium difficile-associated diarrhoea
- CI
- confidence interval
- CPD
- continuing professional development
- CRF
- case report form
- CTA
- clinical trial authorisation
- CTIMP
- clinical trial of an investigational medicinal product
- EQ-5D
- European Quality of Life-5 Dimensions
- GCP
- good clinical practice
- GP
- general practitioner
- HPA
- Health Protection Agency
- HR
- hazard ratio
- HTA
- Health Technology Assessment
- ICC
- intracluster correlation
- IMP
- investigational medicinal product
- IQR
- interquartile range
- LA
- local authority
- LRTI
- lower respiratory tract infection
- LTCF
- long-term care facility
- MA
- marketing authorisation
- MAR
- medication administration record
- MCA
- Mental Capacity Act
- MHRA
- Medicines and Healthcare products Regulatory Agency
- MUST
- Malnutrition Universal Screening Tool
- NIHR
- National Institute for Health Research
- NISCHR CRC
- National Institute for Social Care and Health Research – Clinical Research Centre
- OR
- odds ratio
- PAAD
- Probiotics for Antibiotic-Associated Diarrhoea study
- PI
- principal investigator
- PID
- participant identifier
- PLACIDE
- probiotic lactobacilli and bifidobacteria in antibiotic-associated diarrhoea and C. difficile diarrhoea in the elderly
- QoL
- quality of life
- QP
- qualified person
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- SACU
- specialist antimicrobial chemotherapy unit
- SAE
- serious adverse event
- SEWTU
- South East Wales Trials Unit
- SPSS
- Statistical Product and Service Solutions
- SSA
- site-specific assessment
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- URTI
- upper respiratory tract infection
- UTI
- urinary tract infection