Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/09/01. The contractual start date was in October 2013. The draft report began editorial review in March 2014 and was accepted for publication in June 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
SR Vallabhaneni is the chief investigator of the GLOBALSTAR project, which received an unrestricted research grant from COOK Medical. Rob Riemsma is a member of the National Institute for Health Research Health Technology Assessment and Efficacy and Mechanism Evaluation editorial boards.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2014. This work was produced by Armstrong et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
An aneurysm is a permanent, localised (i.e. focal) dilatation of an artery having at least a 50% increase in diameter compared with the expected normal diameter of the artery in question. 1 The normal aorta is shaped like a walking cane, following a course through the chest (thoracic aorta) and abdomen (abdominal aorta). Abdominal aortic aneurysms (AAAs) account for 75% of all aortic aneurysms (AAs) and are located, by definition of the abdomen, below the diaphragm,2 most below the renal arteries. 3 Those that do not involve, but are close to the origin of, the renal arteries [juxtarenal AAAs (JRAAAs)] account for about 16% of AAAs. 4 Thoracic aortic aneurysms (TAAs) account for the other 25% of AAs. Of these, 15% extend into the abdomen and are therefore referred to as thoracoabdominal aortic aneurysms (TAAAs). 5
Thoracoabdominal aortic aneurysms are stratified using the Crawford classification system based on their distribution within the aorta. 6 The Crawford classification system subdivides TAAAs into:
-
Type I: descending thoracic aorta (distal to the left subclavian artery) to the abdominal aorta above the renal arteries.
-
Type II: descending thoracic aorta (distal to the left subclavian artery) to the renal arteries and may continue on to the bifurcation.
-
Type III: mid to distal descending thoracic aorta and involves most of the abdominal aorta as far as the bifurcation.
-
Type IV: this includes the upper abdominal aorta and all or none of the infrarenal aorta, thus including the origins of the visceral arteries (renal, superior mesenteric and coeliac).
-
Type V: this extends from the distal thoracic aorta including the coeliac and superior mesenteric origins, but not the renal arteries (modified by Safi’s group). 6
Because most aneurysms are asymptomatic it is difficult to estimate their prevalence, but screening studies in the UK have estimated a prevalence of AAAs of 1.3–12.7% depending on the age group studied and the definition of the AAA. The incidence of symptomatic AAAs in men is approximately 25 per 100,000 patient-years at age 50 years, increasing to 78 per 100,000 in those older than 70 years of age. 3 For TAAAs, the incidence estimate varies from under one to about three per 100,000 patient-years. 7 In Scotland, over 7 years (2001–7), the breakdown of TAAAs by type is shown in Table 1. Note that suprarenal implies inclusion of at least one renal artery origin, with no extension to the coeliac or superior mesenteric arteries above.
| Measure | Type I | Type II | Type III | Type IV | Type V | Suprarenal |
|---|---|---|---|---|---|---|
| Number | 7 | 11 | 12 | 38 | 1 | 7 |
| Percentage | 9 | 14 | 16 | 50 | 1 | 9 |
True aneurysm formation is a distinct aetiopathological process, with some similarities to atherosclerosis and an increasingly understood genetic basis. Other causes include trauma, vasculitis, cystic medial necrosis and postsurgical anastomotic disruption. 5 AAAs are about three times more common in men than in women. 3
Abdominal aortic aneurysms enlarge at varying rates and symptoms (although very rare) that can occur as an aneurysm enlarges include a pulsating sensation in the abdomen, back pain and abdominal pain that may spread to the back. Patients with a symptomatic TAAA or AAA need rapid medical attention given that the risk of rupture increases with the size of the aneurysm, and those aneurysms larger than 6 cm in diameter have an annual risk of rupture of 25%. Among patients with a ruptured AAA, the mortality rate is about 80%; even when they undergo emergency surgery, only about half survive beyond 30 days. Several studies indicate that, without surgery, the 5-year survival rate for patients with aneurysms larger than 5 cm is about 20%. For TAAAs, the 5-year survival rate without operation has been reported between 7% and 20%. 7 The national screening programme care pathway recommends screening men from age 65 years and that those aneurysms of at least 5.5 cm should be referred for consideration for elective surgery. 8
As well as concomitant medical treatment for any other cardiovascular disease, there are two main methods of repair: open surgical repair (OSR) and endovascular repair of an abdominal aortic aneurysm (EVAR). In the most recent appraisal by the National Institute for Health and Care Excellence (NICE), in 2009,3 EVAR was recommended as an option for an unruptured AAA that was infrarenal, i.e. below the renal arteries, although the decision was recommended to be based on:
-
aneurysm size and morphology
-
patient age, general life expectancy and fitness for OSR
-
the short- and long-term benefits and risks of the procedures including aneurysm-related mortality and operative mortality.
It was recommended that EVAR for ruptured aneurysms be reserved for research only. For TAAAs there is no NICE guidance, although National Services Scotland stated that ‘. . . endovascular stent repair is a relatively new procedure, but is becoming accepted practice within vascular medicine for treatment of certain types of aneurysm and is recommended by the National Institute for Health and Clinical Effectiveness [NICE].’7 Also, the Joint Working Group to produce guidance on delivering an EVAR Service has produced guidance on all aspects of EVAR delivery, which covers both TAAs and AAAs, and is currently available from the Medicines and Healthcare products Regulatory Agency (MHRA) website. 9
The latest American Heart Association TAA guidelines state that ‘. . . endovascular stent grafting should be strongly considered when feasible . . . for patients with degenerative or traumatic aneurysms . . . exceeding 5.5 cm, saccular aneurysms, or postoperative pseudoaneurysms . . .’. 10 They do not specifically recommend EVAR for TAAAs except to refer to previous guidance, which includes a recommendation for its use. 11 Indeed, for patients with chronic dissection, particularly if associated with a connective tissue disorder, but without significant comorbid disease, and a descending thoracic aortic diameter exceeding 5.5 cm, OSR is recommended. Therefore, there is clearly uncertainty about how to treat TAAAs.
In addition, there is uncertainty about how to treat JRAAAs. This is because the normal EVAR procedure requires that the endograft extends to the healthy parts of the running aorta above and below the aneurysm to attach the stent graft. A JRAAA implies that the aneurysm, although it does not include the origin of the renal artery, is so close to the origin that there is insufficient space to attach the stent graft to the aorta without overlapping the ostia of renal arteries. A solution that has been developed for this situation is fenestrated EVAR (fEVAR), where the stent graft fabric extends over the renal arteries, but perfusion to these arteries is preserved via accurately placed windows (fenestrations) within the stent graft fabric. Aortic branches such as renal arteries that would lose perfusion if not for such fenestrations are called target vessels. Customised fenestrated stent grafts with up to four openings to the target vessels (two renal arteries, superior mesenteric artery and coeliac artery) have thus been developed to allow perfusion of the target vessels. 12,13 fEVAR technology is enabling an expansion of the patient population, increasing the number that may be eligible for EVAR. 10
Thoracoabdominal aortic aneurysms, by definition, extend through the diaphragm from the thoracic to the abdominal cavity, thus including the origin of at least one of the visceral arteries [from top to bottom, coeliac, superior mesenteric artery (SMA) and renal arteries]. The fact that a target vessel origin is included means that the target vessel arises from the aneurysm, thus implying that a gap must be bridged from any fenestra in the stent graft within the aorta to the ostia of the target vessel. The procedure required in this population is then referred to as branched EVAR (bEVAR). The term branched refers to the need to bridge the gap (created by increased diameter of aorta) between the main body of aortic stent graft and target vessels and not to any actual branch from the graft itself. 13 Where the aneurysm is suprarenal, it involves at least one renal artery and it does not extend high enough to reach the diaphragm, thus falling short of a type IV TAAA. In this case, it is strictly neither a JRAAA nor a TAAA, but given that it requires bEVAR it can be included within the TAAA population.
The implantation of standard EVAR is a relatively simpler procedure, requiring accurate longitudinal placement of the graft. The fEVAR and bEVAR procedures are more challenging, as graft positioning requires both longitudinal and rotational alignment of the fenestrations with the target vessels. Any misalignment can lead to partial or total covering of the ostia of the target vessel (shuttering), resulting in reduced blood flow or occlusion. Furthermore, although Field et al. concluded that there was a case for providing a UK service, it should be confined to highly specialised centres and that this was in the context of ‘no data published on survival with and without intervention in the UK’. 14 This lack of evidence is compounded by potentially many comparators given variations in the basic procedure. 15
Commissioners are receiving increasing requests for fEVAR and bEVAR, but it is not clear whether or not the extra cost of fEVAR or bEVAR compared with the alternative of OSR is justified by advantages for patients. 14 In the absence of recent UK evidence-based guidance on JRAAA and TAAAs, evidence synthesis with economic modelling is needed to answer these questions.
Current evidence
A health technology assessment (HTA) review in 2009 assessed the outcomes of treatment with fEVAR compared with the outcomes after OSR in patients with JRAAAs. 4 This review included five single-centre, single-arm prospective cohort studies on fEVAR and seven studies on OSR (one prospective and six retrospective). The authors concluded that results up to 2 years’ follow-up in fEVAR studies compare favourably with the outcomes in the OSR studies. fEVAR had lower 30-day mortality than OSR (1.8% vs. 3.1%) and a lower late mortality over the period of time that patients were followed (12.8% vs. 23.7%). However, there was uncertainty regarding long-term outcomes and all of these observations were based on low-quality evidence.
Two further systematic reviews, one in JRAAAs16 and the other in TAAAs,17 have attempted to make a comparison between OSR, and fEVAR and bEVAR, respectively. Bakoyiannis et al. 17 found seven case series of EVAR of TAAA in a total of 155 patients. They compared results from these studies with those from a selected set of studies on OSR (including one using a hybrid approach) and generally found similarity in outcomes with, for example, 30-day mortality of 7.1% for bEVAR compared with a range of 5% to 19% for OSR. However, given that no comparative studies were found, their only conclusions were that bEVAR has ‘. . . encouraging results for patients considered unfit for conventional open repair’. 17
In JRAAAs, Nordon et al. 16 also compared ‘cohorts’ from one set of studies of fEVAR with those from another set of studies of OSR. They did find a higher 30-day mortality with OSR (relative risk 1.03, 95% confidence interval 1.01 to 1.04). However, given again a lack of comparative studies, their only conclusions were that ‘. . . selectivity within the study groups and lack of a rigorous classification prohibit more robust comparison’. 16
More recently, in 2012 a review by the College voor Zorgverzekeringen (CvZ) in the Netherlands of both fEVAR and bEVAR also found no comparative studies and, thus, concluded that there was insufficient evidence to recommend either procedure:
From this review, it appears that there is insufficient evidence of good methodological quality to conclude that the endovascular treatment of aneurysms of the complex aorta can be regarded as effective. The endovascular treatment with fenestrated and/or branched devices does not meet the required level of knowledge and practice and cannot be considered to ensure medical care performance under the Health Insurance and related regulations.
De Groot et al. 18 [translated from the summary]
However, to date, no similar assessment has been carried out for the UK setting. The current assessment will therefore attempt to evaluate the clinical effectiveness, safety and cost-effectiveness of fEVAR or bEVAR in comparison with conventional treatment for JRAAAs or TAAAs in the UK.
Chapter 2 Aims and objectives
Aim
The aim of this project was to assess the impact of fEVAR and bEVAR on patient outcomes and NHS resources, to propose possible changes in patient management and make recommendations for further research.
Objectives
-
To assess the clinical effectiveness and safety of fEVAR and bEVAR in comparison with conventional treatment, i.e. no surgery or OSR (including any hybrid alternatives) for two populations – JRAAA and TAAA.
-
To assess, from a NHS perspective, the cost-effectiveness of fEVAR and bEVAR in comparison with conventional treatment, i.e. no surgery, or OSR or any hybrid alternatives for two populations – JRAAA and TAAA.
Chapter 3 Decision problem
Population
The target patient population for fEVAR and bEVAR is those adult patients (aged ≥ 18 years) who are at risk of rupture of JRAAAs or TAAAs (distal to left subclavian, i.e. excluding aortic arch) which involve, or are close enough to, at least one aortic branch such that a standard EVAR would occlude the branch. This would include patients who are unsuitable for OSR because of the presence of significant comorbid conditions and those who are unsuitable for treatment with a standard EVAR as a result of the presence of a short proximal neck at those positions.
The decision to repair AAAs using fEVAR and bEVAR requires careful patient selection to identify those patients who will most likely benefit from the repair as the decision depends on consideration of three important risk factors such as:
-
estimated risk of aneurysm ruptures if the patient is not treated
-
estimated risk of death due to surgery, either EVAR or OSR, which depends on the fitness of the patient and also on the success rate of the treating physician’s institution
-
estimated longevity of the patient if not for the aneurysm.
Subgroups of interest are as follows:
-
elective asymptomatic [detected mainly incidentally (majority) or by screening]
-
elective symptomatic (uncommon)
-
number of target vessels [one, two, three or four (two renal arteries plus SMA and coeliac)]
-
patients who are on dialysis (as renal artery perfusion not needed).
Intervention
Fenestrated EVAR and bEVAR are specially manufactured stent grafts with openings to allow blood to reach branches of the aorta.
Endovascular repair of AAAs allows the exclusion of the dilated aneurysmal segment of the aorta from the systematic circulation. The procedure requires, however, that the endograft extends to the healthy parts of the aorta above and below the aneurysm. However, the neck of a JRAAA is too short for a standard EVAR. fEVAR provides a solution to overcome this problem by enabling the continuation of blood flow to the renal and visceral arteries through holes or fenestrations in the fabric of the stent graft. These fenestrations are designed to match the ostium of the renal and visceral arteries. There are three varieties of fenestration (small, large and scallop) and their location needs to be customised to fit the anatomy of the patient. 4 If the device is not properly designed, if the alignment is inaccurate, or if the catheterisation of the visceral arteries is not possible, the procedure may fail.
Branched EVAR provides a solution when the target vessels arise from a segment of the aneurysmal aorta. A branched stent graft is deployed in a dilated aorta and, therefore, there is a gap created between the target vessels and stent graft, which requires bridging. Any branches coming off the stent graft are called cuffs (not branches). Branches in the form of covered stents between the aortic stent graft and the target vessels are indicated for both fEVAR and bEVAR in order to anchor the stent graft (i.e. to prevent migration), although for bEVAR they have the additional purpose of bridging the gap. Some aneurysms may require a combination of one or more fenestrations and one or more branches. In this case, a combination of fEVAR and bEVAR may be required. 13
It is essential to realise that there is a close correspondence between each of the two populations and each of the two interventions. JRAAA implies that the aneurysm is close to, but does not involve, the opening to the branches, which are the renal arteries. Therefore, the term ‘branched’ is not appropriate and, thus, JRAAA patients are eligible only for fEVAR. A TAAA implies that the aneurysm extends from the abdominal into the thoracic cavity and, thus, given the proximity of the renal branches to the diaphragm, is very likely to involve the branches, therefore implying the term ‘branched’. Therefore, TAAA patients are eligible only for bEVAR. Both patients with JRAAAs and those with TAAAs are also eligible for OSR or no surgery depending on the general fitness of the patient.
Once a graft is placed in a patient, life-long follow-up at regular intervals is necessary to ensure that the graft remains in its intended location, that the components have adequate overlap and the aneurysm remains excluded from systemic blood flow and blood pressure. Should the need arise, routine follow-up allows the performance of timely and appropriate intervention through detection of events that could affect the long-term outcomes.
Comparators
The comparators considered relevant for this assessment are:
-
OSR is the historic treatment method for JRAAAs or TAAAs
-
no surgery (best medical therapy only).
No surgery (and thus optimal conservative care) is a valid comparator in those patients considered unfit for OSR. For OSR, it is important to distinguish between the populations for which fEVAR and bEVAR are indicated as the morbidity and mortality of the ‘open surgical equivalent’ is greater. This is because the higher the aneurysm, the higher the aortic clamp must be placed and, thus, the greater the stress on the heart and the more extensive the potential ischaemia (e.g. to viscera and spinal cord). Also, OSR for a TAAA usually requires left-heart bypass or hypothermic circulatory arrest, which increases the risk. 19 As no aortic clamp is needed for EVAR, this differential risk does not apply between fEVAR and bEVAR. Hence, any study assessing OSR performed in a population eligible for fEVAR cannot be compared with one eligible for bEVAR.
In 2008, Greenberg and Lytle reported that the patients selected to undergo OSR for a TAAA are faced with a mortality risk ranging from 3% to 17%, with an incidence of perioperative death at centres with extensive experience < 10%. 15 Moreover, the risk of spinal cord ischaemia remains between 4% and 11% and the postoperative renal complications, such as worsening of renal function, are also considerable, with an incidence ranging between 17% and 25%; up to 15% of patients ultimately might require haemodialysis. 15 In addition, cardiopulmonary complications after aortic surgery are common. 15
Chapter 4 Clinical effectiveness review
The systematic review was conducted using methods as recommended in the Centre for Reviews and Dissemination (CRD) guidance for undertaking reviews in health care20 and the Cochrane Handbook for reviews of intervention studies. 21
Methods of clinical effectiveness review
Inclusion and exclusion criteria
Population
Studies including adult patients who were eligible for fEVAR (aged ≥ 18 years) with JRAAAs or eligible for bEVAR with TAAAs, i.e. with proximity to, or involvement of, target vessels such that EVAR was unsuitable.
Setting
Secondary care.
Intervention
Fenestrated EVAR and bEVAR.
Comparator (clinical effectiveness studies only)
Open surgical repair (including hybrid repair) or no surgery for patients considered unsuitable for OSR.
Outcomes
-
Risk of death: all-cause mortality and aneurysm-related mortality – primary outcome.
-
Probability of technical success (target vessel perfusion).
-
Durability (risk of relapse).
-
Risk of adverse events:
-
graft infection
-
device migration
-
endoleaks (early/late, type I/type III)
-
target vessel stenosis/occlusion
-
aneurysm growth
-
structural disintegration
-
modular separation
-
kinking leading to thrombosis
-
stent comes off or breaks
-
aortic rupture
-
stroke
-
paraplegia/spinal cord ischaemia.
-
-
Reintervention (any reason, for a stated adverse event).
-
Annual radiation dosage.
Study design
Step 1
Randomised and non-randomised trials in which participants are assigned to the intervention group or comparator group, and which report at least one of the listed outcomes.
Step 2
As no controlled trials were likely to be found, studies with a cohort design were included if they make a comparison with a control group. These studies were referred to as comparative.
Step 2 is included given the likely lack of controlled trials and in order to permit the review of evidence that makes a comparison even if the evidence is of low quality, in particular a high risk of selection bias. Note that, to qualify as comparative, studies had to demonstrate that there had been some attempt to make the populations receiving each of the treatments comparable and that any evidence of intent to select based on some prognostic factor would imply exclusion.
The following study/publication types were excluded:
-
case series/case reports
-
pre-clinical and animal studies
-
reviews, editorials and opinion pieces.
Search strategy
Search strategies were based on the target conditions, i.e. JRAAAs and TAAAs. Searches were not limited by language or publication status (unpublished or published).
Rapid appraisal for existing systematic reviews on juxtarenal abdominal aortic aneurysms and thoracoabdominal aortic aneurysms
In order to identify existing and on-going systematic reviews, guidelines and guidance on JRAAAs and TAAAs, a rapid appraisal of appropriate sources was undertaken on the following resources:
-
Cochrane Database of Systematic Reviews (CDSR) (Wiley). Searched issue 7: 2013.
-
Database of Abstracts of Reviews of Effects (DARE) (Wiley). Searched issue 3: 2013.
-
HTA Database (Wiley). Searched issue 3: 2013.
-
NHS Economic Evaluation Database (NHS EED) (Wiley). Searched issue 3: 2013.
-
International Guidelines Network Library (internet). Searched up to 19 August 2013. URL: www.g-i-n.net/.
-
National Guideline Clearinghouse (internet). Searched up to 19 August 2013. URL: www.guideline.gov/.
-
NICE Guidance (internet). Searched up to 19 August 2013. URL: http://guidance.nice.org.uk/.
-
National Institute for Health Research (NIHR) HTA (internet). Searched up to 19 August 2013. URL: www.nets.nihr.ac.uk/programmes/hta.
-
International Prospective Register of Systematic Reviews (PROSPERO) (internet). Searched up to 19 August 2013. URL: www.crd.york.ac.uk/prospero/.
-
US Food and Drug Administration (internet). Searched up to 20 August 2013. URL: www.fda.gov/.
-
MHRA (internet). Searched up to 20 August 2013. URL: www.mhra.gov.uk/.
Clinical effectiveness
Searches were undertaken to identify studies investigating treatment of JRAAAs and TAAAs. Full strategies for the clinical effectiveness searching are presented in Appendix 1. The following resources were searched from inception:
-
MEDLINE (OvidSP). Searched 1946 to week 3 September 2013.
-
MEDLINE In-Process & Other Non-Indexed Citations (OvidSP). Searched up to 30 September 2013.
-
MEDLINE Daily Update (OvidSP). Searched up to 30 September 2013.
-
EMBASE (OvidSP). Searched 1974 to 4 September 2013.
-
Cochrane Central Register of Controlled Trials (CENTRAL) (Wiley). Searched issue 8: 2013.
Conference searches
The following conference proceedings were searched:
-
VEITHsymposium™ (internet): 2011–13.
-
Charing Cross Symposium (internet): 2011–13.
-
Vascular Annual Meeting (internet): 2011–13.
Handling of citations
Identified references were downloaded into EndNote (Thomson Reuters, CA, USA) software for further assessment and handling. Rigorous records were maintained as part of the searching process. Individual records within the EndNote reference libraries were tagged with searching information, such as searcher, date searched, database host, database searched, strategy name and iteration, theme or search question. This enabled the information specialist to track the origin of each individual database record, and its progress through the screening and review process.
Quality assurance within the search process
For all searches undertaken by Kleijnen Systematic Reviews Information Team, the main EMBASE strategy for each set of searches were independently peer reviewed by a second information specialist, using the Canadian Agency for Drugs and Technologies in Health peer-review checklist. 22
Screening strategy
Two reviewers independently screened titles and abstracts of all reports identified by searches and discrepancies were discussed. Full copies of all studies deemed potentially relevant, after discussion, were obtained and two reviewers independently assessed these for inclusion; any disagreements were resolved by consensus or discussion with a third reviewer.
Methods of analysis/synthesis
Given that no studies met all of the inclusion criteria, a narrative account of the number of studies screened for inclusion and reasons for excluding studies was given.
Results of clinical effectiveness review
Summary of inclusion/exclusion
The literature searches retrieved 5253 records before deduplication. Owing to overlap between the databases, 1985 duplicate records were removed. The remaining 3268 records were screened at title and abstract stage. Based on titles and abstracts, 3244 records were excluded, leaving 24 publications to be ordered. All 24 studies were excluded as none of the ordered studies satisfied our inclusion criteria. Figure 1 shows the flow of studies through the review process and Appendix 2 provides details, with reasons for exclusion, of all publications excluded at the full-paper screening stage.
FIGURE 1.
Flow of studies through the screening process: clinical effectiveness review.
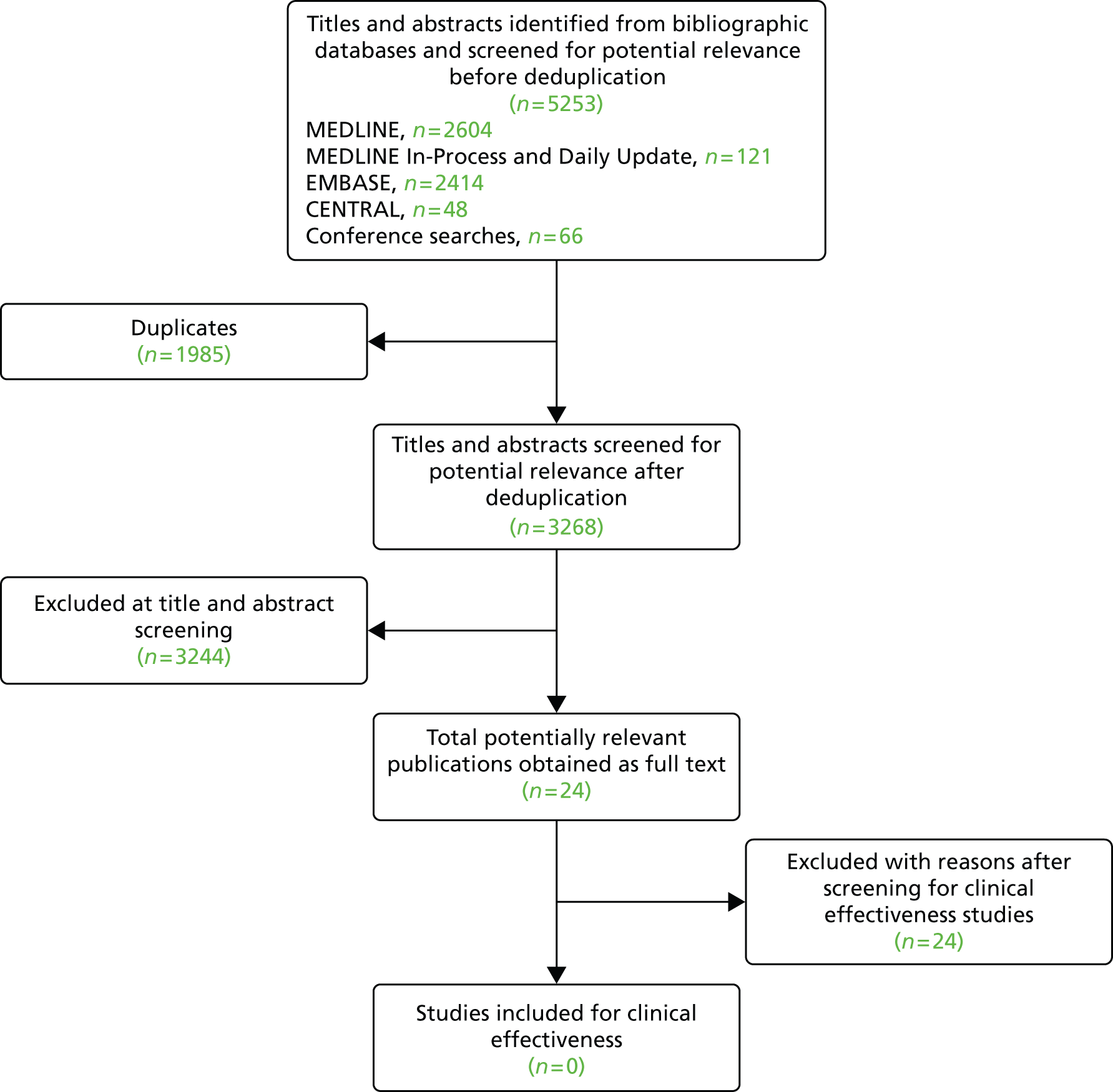
Summary of reasons for exclusion
Sixteen studies were excluded on study design, six studies were excluded on intervention and two on comparator (chimney grafts). Among the six studies which were excluded on intervention, three studies were excluded because they compared EVAR with OSR, with no indication of fEVAR or bEVAR being used;23–25 one used EVAR with suprarenal fixation;26 and the other two were excluded because they reported results from surgeon-modified fenestrated–branched stent grafts, which are not considered as appropriate interventions for this evaluation. 27,28
Five out of 16 studies that were excluded on study design reported a comparison. However, all of the studies acknowledged that they had groups which were not comparable at baseline given that they had selectively assigned patients to the groups being compared, i.e. not the same population. Therefore, these studies were considered to be ‘non-comparative studies’. Below are examples and quotes from the five studies.
In all studies patients were selected by the clinicians based on their characteristics and, generally, patients who were deemed younger and physiologically fitter underwent OSR29 whereas, in contrast, older and high-risk patients were chosen for EVAR. One study reported that ‘All [EVAR] patients were not considered suitable for [OSR].’30 One study quoted:
The policy for implanting a fenestrated stent-graft was based on high risk for open surgery (old age, severe comorbidities, previous aortic reconstruction, or need for suprarenal clamping) and whenever the quality of the neck was too poor to provide a seal with a standard stent-graft that would be durable for the expected lifespan of the patient.
Chisci et al. 31
One study appeared to be comparative at first instance, because the study explicitly stated that: ‘In all patients who underwent OSR, anatomic suitability for fEVAR was assessed through CT [computerised tomography] review.’32 However, it was excluded because of the following quote:
When f-EVAR is technically feasible, the physicians usually recommended younger and generally fit patients to have an open repair, as was the case for patients with very large aneurysms. Older patients and those with multiple comorbidities were recommended to have fEVAR. Exceptions occurred due to patient preference and availability of funding. Patients selected by the clinicians based on their characteristics.
Canavati et al. 32
According to Greenberg et al. 33:
Patients treated with ER [endovascular repair] were on average 8.6 years older (71.3 ± 12 versus 62.7 ± 13 years, p < 0.001) than those treated with an SR [surgical repair], but no gender differences between repair types were found. Our bias has been to treat patients who are younger or have fewer comorbid factors with conventional surgery, a tendency that is readily reflected in the preoperative patient characteristics (Table 3).
The above quote clearly demonstrates that selecting patients based on their clinical characteristics can cause a significant baseline imbalance between two groups, making them non-comparable. This evidence further supports our decision to exclude studies that were non-comparative.
Chapter 5 Cost-effectiveness review
Methods of cost-effectiveness review
The systematic review was conducted using methods in line with those recommended in the CRD guidance for undertaking reviews in health care20 and the Cochrane Handbook for reviews of intervention studies. 21
Inclusion and exclusion criteria
Population
Studies including adult patients who were eligible for fEVAR (aged ≥ 18 years) with JRAAAs or eligible for bEVAR with TAAAs, i.e. with proximity to, or involvement of, target vessels such that EVAR was unsuitable.
Setting
Secondary care.
Intervention
fEVAR and bEVAR.
Comparator (clinical effectiveness studies only)
OSR or no surgery for patients considered unsuitable for OSR.
Outcomes
-
Cost-effectiveness.
-
Health-care cost.
-
Social care costs.
Study design
-
Cost-effectiveness analysis (CEA).
-
Cost study.
Search strategy
Search strategies were based on the target conditions, JRAAAs and TAAAs. Searches were not limited by language or publication status (unpublished or published). Resources were searched to identify cost-effectiveness studies of treatments for JRAAAs and TAAAs. Where appropriate, strategies incorporated a cost-effectiveness search filter. Full strategies for the cost-effectiveness searching are presented in Appendix 1. The following resources were searched from inception:
-
MEDLINE (OvidSP). Searched 1946 to week 3 September 2013.
-
MEDLINE In-Process & Other Non-Indexed Citations (OvidSP). Searched up to 1 October 2013.
-
MEDLINE Daily Update (OvidSP). Searched up to 1 October 2013.
-
EMBASE (OvidSP). Searched 1974 to 27 September 2013.
-
NHS EED (Wiley). Searched issue 3: 2013.
-
EconLit (EBSCOhost). Searched up to 30 September 2013.
Screening strategy
Titles and abstracts of all reports identified by the searches were screened by one reviewer and ambiguities were resolved by consulting a second reviewer. Full copies of all studies deemed potentially relevant were obtained and were assessed by one reviewer for inclusion; any ambiguities were resolved by consulting a second reviewer.
Methods of analysis/synthesis
Given that no studies met all of the inclusion criteria, a narrative account of the number of studies screened for inclusion and reasons for excluding studies was given.
Results of cost-effectiveness review
Summary of inclusion/exclusion
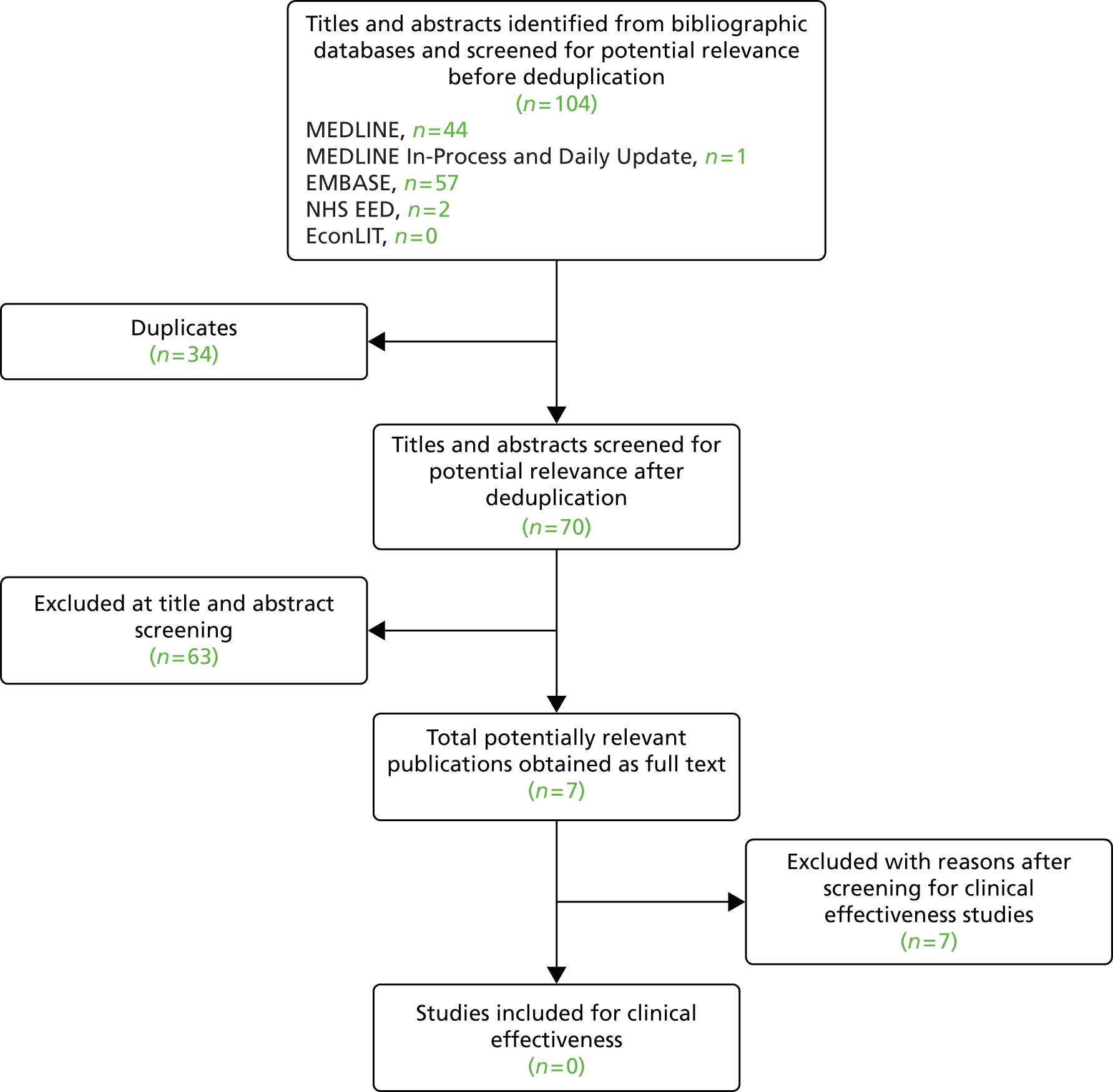
The health economics search identified 104 references before deduplication. Owing to overlap between the databases, 34 duplicate records were removed. The remaining 70 records were screened. Of these, seven were included for the full assessment based on initial screening. After full-text review no studies were included, as presented in Figure 2. Six of these seven studies estimated the cost-effectiveness of EVAR compared with OSR for patients with a pararenal AAA who were defined as unsuitable for EVAR25,34–38 and, thus, these studies were excluded based on the population. Furthermore, five of them,34–38 were presented at conferences, and these abstracts were not presented in such a way that data could be extracted from it. The other study39 reported costs of fEVAR, but this was reported without sufficient details and in Canadian dollars.
FIGURE 2.
Flow of studies through the screening process: cost-effectiveness review.
Chapter 6 Cost-effectiveness analysis
This chapter will focus on the economic impact of fEVAR and bEVAR. Our objective had been to perform an economic evaluation of these interventions for patients with JRAAAs and TAAAs. However, for several reasons it was decided that a CEA evaluating fEVAR and bEVAR was not possible. First, the systematic review assessing the clinical effectiveness (see Chapter 4) showed that no comparative study had been done that could provide clinical effectiveness data. Besides the clinical effectiveness, it was also not feasible to obtain valid estimates for the other input parameters. In particular, the stent grafts used vary widely between patients and between centres. The stent grafts used in fEVAR/bEVAR are not a homogeneous product as they are custom made and, therefore, it is difficult to obtain reliable cost estimates for the graft itself. Furthermore, different manufacturers also make these stent grafts and hospitals receive discounts from these manufacturers, leading to even more uncertainty in the costs. The large variation observed also makes it difficult to estimate resource use (besides the stent grafts) based on the opinion of only a few experts. To have any validity, expert opinion should be sought from a large group of experts with fEVAR and bEVAR experience, and this was not feasible within the constraints of this short report. Finally, according to the experts consulted (SV and PH), this intervention is a rapidly changing area and, therefore, an economic evaluation will project the cost-effectiveness of fEVAR and bEVAR only at this moment, and these results will not be valid for the near future. Consequently, this chapter will describe how an economic evaluation could be performed if more data were available. Furthermore, we will provide suggestions for data collection (see Analyses).
Design
An economic evaluation performed for the UK health-care setting usually adopts the NHS perspective, including only direct costs and consequences. Outcomes should be measured in quality-adjusted life-years (QALYs) and life-years (LYs). Costs and outcomes need to be discounted at 3.5% per year40 and are preferably estimated for a lifetime horizon.
Population
The population is patients with JRAAAs and TAAAs and, thus, are considered unsuitable for EVAR. In fact, there would be two main subgroups: those fit for OSR and those unfit for OSR. Without the existence of the interventions fEVAR or bEVAR, patients would be treated with an OSR if considered to be fit enough. Patients who are not fit for OSR would be treated with no surgery, i.e. medication only. Fitness of patients for an OSR can be determined based on the fitness criteria that were used for the EVAR trials,41 which evaluated EVAR with either OSR (EVAR 1 trial) or medication (EVAR 2 trial) for the treatment of AAAs depending on the fitness of the patient. Patients were considered unfit for OSR if a patient had (1) an American Society of Anesthesiologists (ASA) grade of IV, (2) cardiac symptoms, (3) renal symptoms or (4) respiratory symptoms. However, it seems that the guidelines for assessing the fitness for OSR are subject to treatment variation. Aneurysm size was also important for determining the fitness of a patient, as patients who were earlier described as ‘unfit for open repair’ and later developed a larger aneurysm were suddenly deemed ‘fit for the procedure’. 42
Interventions and comparators
Based on the fitness for OSR, two treatment comparisons for this population should be evaluated: (1) fEVAR/bEVAR compared with OSR and (2) fEVAR/bEVAR compared with no surgery. The surgical procedures are described in Chapter 3, Intervention and Chapter 3, Comparators. The no-surgery strategy can be defined as optimal conservative care, i.e. patients should receive statins, beta-blockers, antihypertensive agents, antiplatelet drugs, and/or lifestyle advice. Ideally, the cost-effectiveness of fEVAR and bEVAR must be evaluated separately.
Model structure
To estimate the lifetime cost-effectiveness of fEVAR and bEVAR it is essential that a decision model should be constructed to include all sources of evidence and to extrapolate short-term results. It could be useful to create two separate models, one for short-term outcomes and one for long-term outcomes. The short-term model can be a simple decision tree estimating costs and effects preoperatively (including the effect of waiting time), intraoperatively and postoperatively up to 30 days. A long-term model should be used to estimate the lifetime costs and effects for the individuals who have survived the initial procedure and the 30-day postoperative period. The long-term model structure can be adopted from previously published HTA reports for the evaluation of EVAR by Brown et al. 42 and Chambers et al. ,43 as it is likely that the treatment and disease pathways of EVAR and fEVAR or bEVAR are comparable, even if the input parameters are not. See Appendix 3 for the quality of these studies based on the Drummond and Jefferson checklist. 44
Short-term model (fit for open surgical repair)
It is likely that patients have to wait until the actual surgery can be performed. In particular, patients who will be treated with fEVAR or bEVAR have to wait as a large proportion of the patients will have to wait for custom-made devices while the inventory of off-the-shelf devices is expanding. The inclusion of waiting time in the model is deemed to be important as a difference in waiting time could lead to differences in mortality. It might also lead to a reduction in quality of life during this time period, although this might be difficult to measure.
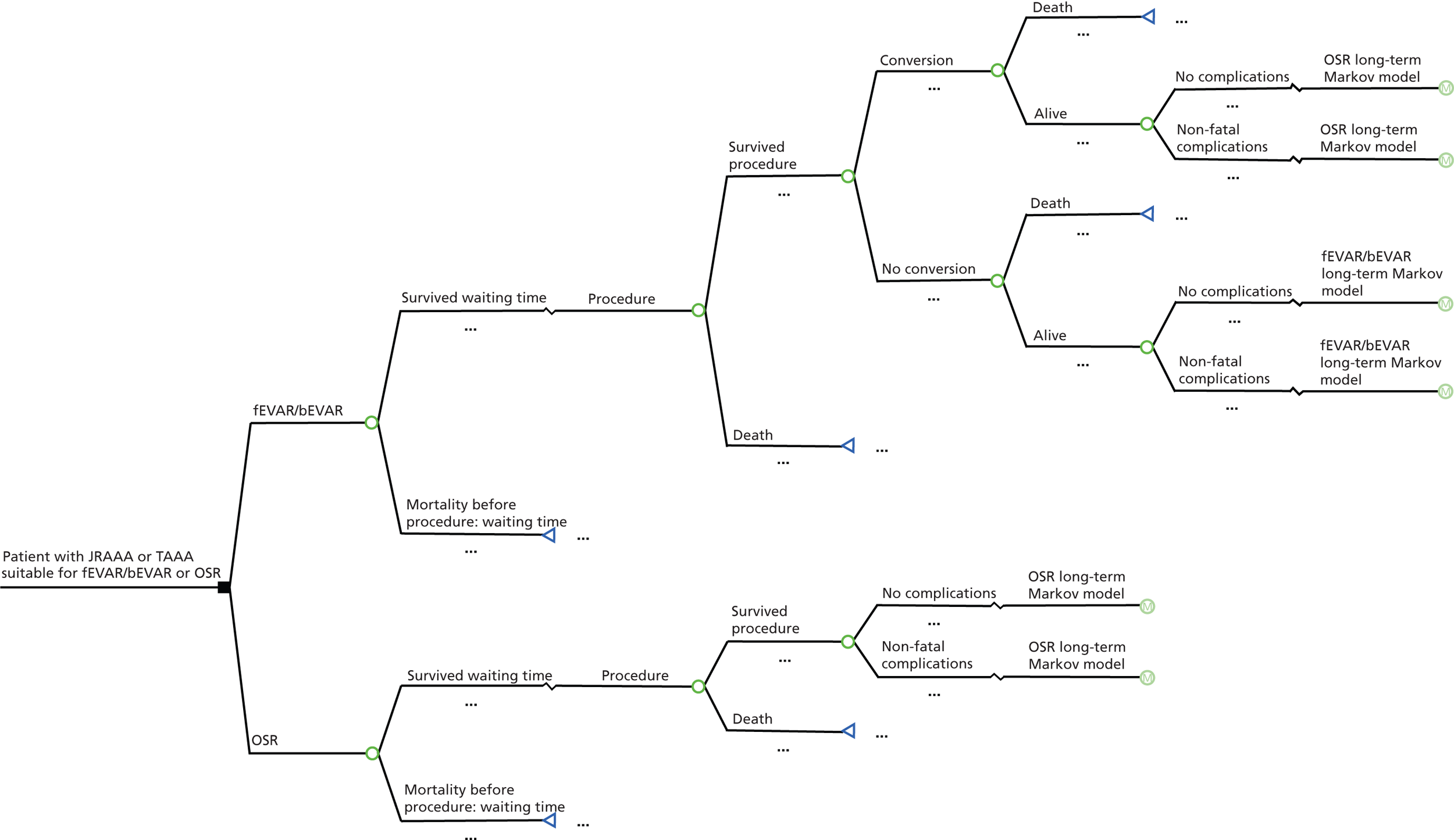
Patients who actually receive fEVAR or bEVAR may be converted to an OSR because of, for example, an endoleak or stent graft migration. It is important that these conversions are incorporated into the model. Chambers et al. 43 assumed that a conversion takes place during the time span of the short-term model. In their model, conversion from OSR to EVAR was not included as it is unlikely and the EVAR 1 trial showed that none of the OSR patients were converted. 45
Furthermore, mortality (all-cause and aneurysm related) and complications during the first 30 days need to be incorporated in the short-term model, if a difference between interventions exists. Complications can be categorised into four types: cardiac complications [e.g. myocardial infarction (MI)], pulmonary complications (e.g. respiratory failure), renal complications (e.g. renal failure) and neurological complications (e.g. stroke). Furthermore, it is important that reinterventions are also incorporated into the model because EVAR is associated with more reinterventions than OSR46 for standard EVAR and, thus, it is possible that the same may be true for fEVAR/bEVAR. A reintervention for patients who underwent fEVAR or bEVAR could either be an OSR or, again, an endovascular procedure.
Figure 3 shows a possible structure for a short-term decision model for this comparison. Reinterventions are included as complications.
FIGURE 3.
Short-term decision model: fit for surgery.
The disease progression of patients who have survived the initial preoperative and postoperative days can be simulated with the long-term model. Furthermore, for patients who have suffered from complications such as (disabling) stroke it could be useful to use an additional model or disease state to estimate their lifetime costs and QALYs as those patients often generate more costs and have a lower quality of life than the rest of the population.
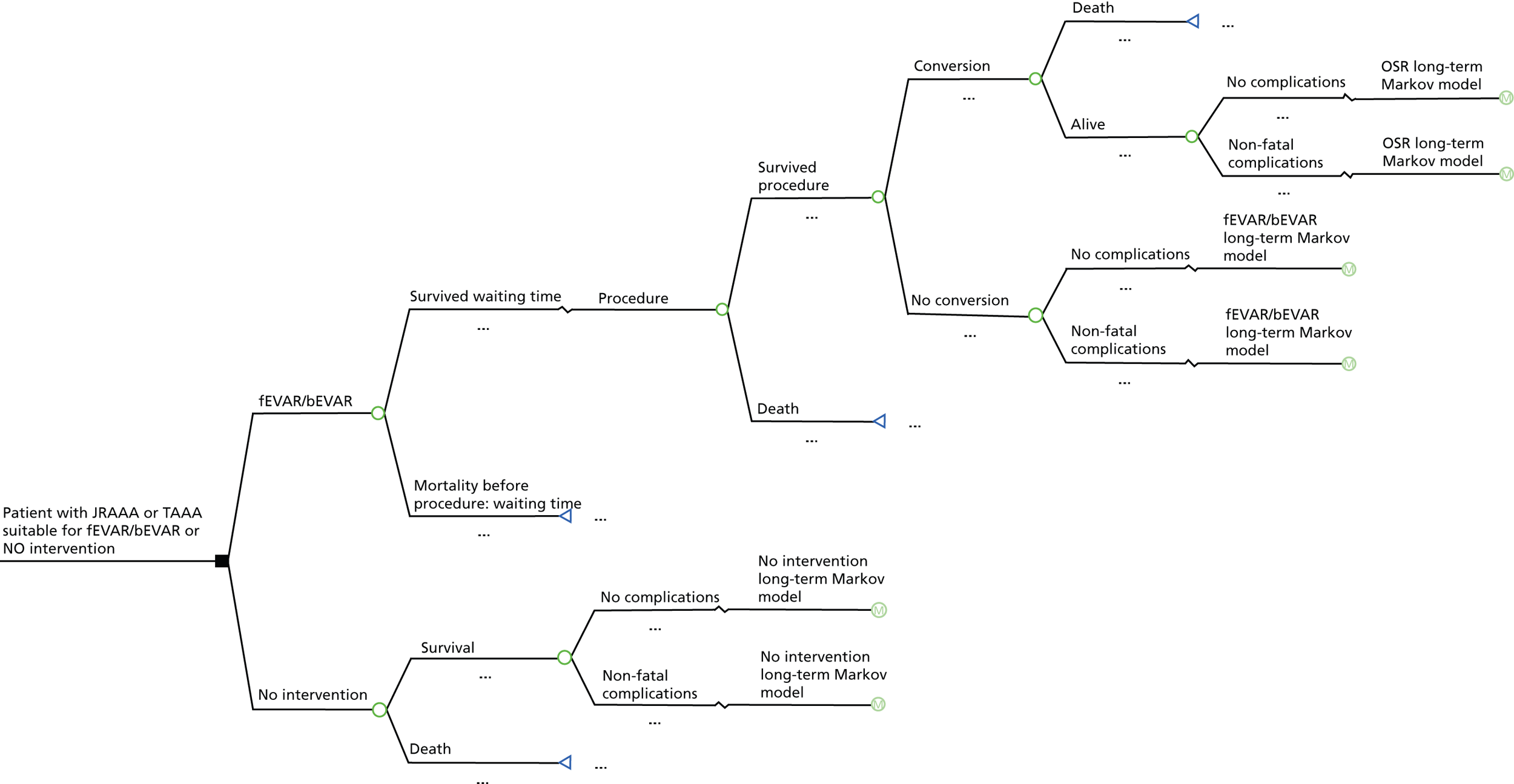
Short-term model (unfit for OSR)
The same suggestions as described above for those fit for OSR apply for this comparison, although waiting time is not an issue for patients treated with medication only and conversion from fEVAR or bEVAR to OSR might not be possible as patients are considered not fit for OSR. However, conversions to OSR could be possible when serious complications occur during fEVAR/bEVAR or the aneurysm has been ruptured before the planned fEVAR/bEVAR takes place. Figure 4 shows a possible structure for a short-term decision model for this comparison.
FIGURE 4.
Short-term decision model: unfit for surgery.
Long-term model
The long-term model should estimate lifetime costs and effects of patients who have survived the waiting time before the procedure and the 30-day postoperative period. For such a long-term model, a Markov model would be most appropriate. This model could consist of two chronic health states: (1) event-free and (2) (non-)aneurysm-related death and this structure is similar to the economic evaluation of EVAR compared with OSR presented by Chambers et al. 43 Patients start out in the event-free state and are exposed to the risk of experiencing a non-fatal event. Patients could be readmitted to the hospital if, for example, a reintervention is required. Note that the readmission state is a temporary health state: after the readmission, patients move back to the event-free state. Eventually, all patients will die from aneurysm-related causes or from non-aneurysm-related causes. Figure 5 shows this structure. It could be useful to include an additional state if there is a difference in non-fatal complications (e.g. stroke) that have a substantial impact on the quality of life of patients. Furthermore, the impact of radiation of the CT scans used during follow-up and of the fluoroscopy used during the interventions could be incorporated in the model if this is deemed important. However, the mean age of the patients included in the EVAR 1 trial46 is relatively high (74.1 ± 6.1 years) and, thus, the impact of radiation on the incremental costs and effects will probably be negligible.
FIGURE 5.
Long-term Markov model.
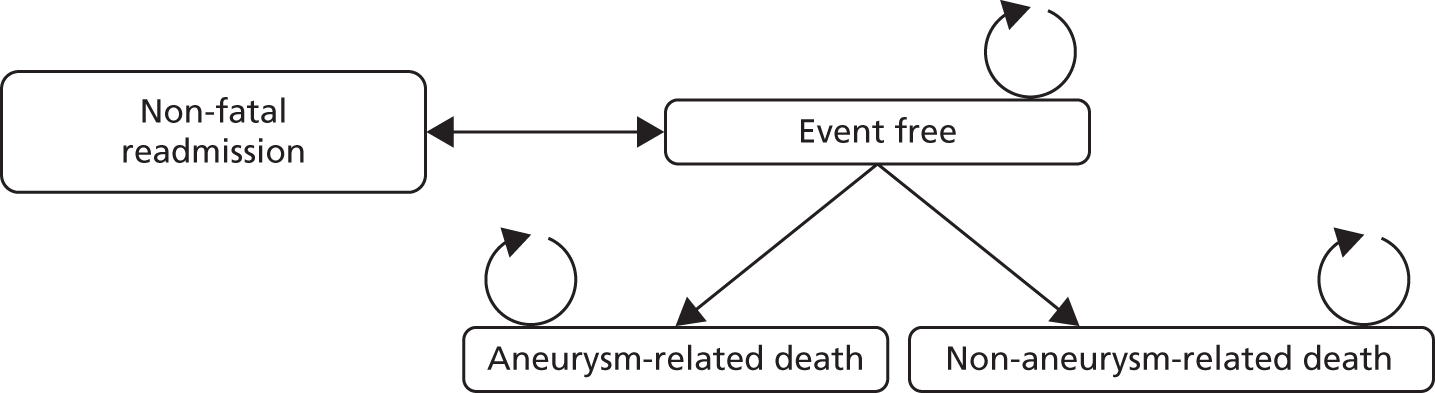
The cycle length needs to be short enough so that an event occurs at most once per cycle. 47 A cycle length of 6 months could be considered as appropriate according to the timing of the events as used by Brown et al. 42 A half-cycle correction needs to be incorporated when it is unknown when in the cycle a transition occurs. 47
Assumptions regarding model structure
The assumptions detailed below can be made when data are lacking.
Conversion
In the economic evaluation by Chambers et al. ,43 it was assumed that patients who convert from EVAR to OSR during the primary admission have the same long-term prognosis as patients initially undergoing OSR. We would suggest using the same assumption if follow-up data are not available. Furthermore, they assumed that patients treated initially with EVAR could only convert to OSR before entering the long-term model and, thus, assumed that conversions take place in the first 30 days post procedure. A recent systematic review showed that 1.5% of the patients treated with EVAR will undergo a conversion to OSR in the first 30 days. 48 For late conversion, this is estimated at 2% and, ideally, both late and early conversions are incorporated in the models.
Complications
Brown et al. 42 did not incorporate clinical events such as MI or stroke in the model as there was no significant difference between the treatment strategies (OSR vs. EVAR) in the randomised controlled trial (RCT). This assumption might be valid for the evaluation of EVAR. However, the results of clinical studies will show if this assumption also holds for an economic evaluation of fEVAR or bEVAR compared with OSR or no surgery. Moreover, a RCT is likely to be underpowered to find significant effects for these events. Generally, when these events are considered clinically relevant, they should be included in the long-term model.
Extrapolation of treatment clinical effectiveness
Clinical studies showed that EVAR was associated with lower perioperative mortality than OSR, but the risk of late AAA mortality and reinterventions was higher in patients treated with EVAR. 46 However, how the difference in mortality between treatments develops over time is uncertain. Brown et al. 42 assumed in the base-case analysis that there is no difference in the rate of AAA deaths between treatments after 8 years, because the risk of AAA death diminishes over time. Chambers et al. 43 used a constant hazard ratio (1.072) for non-aneurysm-related death for EVAR compared with OSR until the non-aneurysm-related survival curves converged.
Input parameters
Transition probabilities
For the short-term model (see Figures 3 and 4) it is important to collect data in the period before the treatment and up to 30 days after the procedure. Data concerning mortality, conversions, reinterventions and complications (cardiac, pulmonary, renal, neurological) are important for this model. The structure of the long-term model (see Figure 5) requires three time-dependent transition probabilities: (1) non-aneurysm-related mortality, (2) aneurysm-related mortality and (3) non-fatal readmissions. These transition probabilities need to be based on general population mortality estimates (non-aneurysm-related mortality) and on clinical effectiveness data from comparative studies.
Chapter 4, evaluating the clinical effectiveness, showed that there were no comparative studies of fEVAR/bEVAR in either those fit or unfit for OSR. Treatment allocation was often not based on chance and, therefore, patient groups were not comparable (i.e. age and comorbidities). 31 Thus, we have no source of information on the efficacy of fEVAR/bEVAR in preventing mortality and serious complications.
An additional literature search was performed to identify studies that may assist in developing a cost-effectiveness model for fEVAR and bEVAR, i.e. studies that provide information on the many input parameters of a model (see Appendix 4).
Ideally, a multicentre RCT of UK patients anatomically unsuitable for EVAR should be conducted to have an unbiased estimate of the clinical effectiveness, while simultaneously providing data on costs and quality of life of those patients treated with fEVAR/bEVAR/OSR or no surgery. Several authors have suggested that a RCT could be necessary to obtain robust estimates for the clinical effectiveness. 32,33,49 However, if a RCT is not possible then a prospective controlled study would be necessary. As in the EVAR trials (EVAR 1 and EVAR 2) it would probably be necessary to conduct two separate trials evaluating two comparisons based on the fitness for OSR: (1) fEVAR or bEVAR compared with OSR and (2) fEVAR or bEVAR compared with no surgery. At least a follow-up of 60 months might be necessary as it seems that the survival of patients treated with EVAR or OSR is comparable after 60 months (see figure 71 in Chambers et al. 43). Mortality should also be measured during any waiting time as this could have an impact on the clinical effectiveness of fEVAR and bEVAR. For the model, important baseline characteristics of the patients that (at least) should be collected in such a trial are age, sex, aneurysm size, comorbidities, ASA grade, fitness for surgery, smoking status, body mass index, diabetes, systolic blood pressure, diastolic blood pressure, medication history, serum creatinine and serum cholesterol. These baseline characteristics can be used in regression analyses to estimate transition probabilities and long-term costs. As institutional characteristics may be of predictive value as well, these should also be collected.
Extrapolation of the trial results will be necessary because the time horizon of the model will be longer than that of the follow-up of the RCT to capture all differences in costs and effects between treatments. Crucial assumptions concerning survival have to be made when data are lacking. The impact of these assumptions on the cost-effectiveness needs to be explored through sensitivity analyses.
Costs
When the cost-effectiveness of fEVAR or bEVAR is evaluated for the UK, it is preferred to use the NHS perspective, i.e. direct medical costs. Alongside any prospective controlled study, resource use of patients should be collected. For the short-term model, is it important that valid resource use estimates (e.g. theatre time or blood products) of the procedures are estimated. When a societal perspective is used, it is important that indirect costs (productivity costs, travel costs) are also estimated using diaries.
For unit costs routine, NHS sources (e.g. NHS reference cost, Personal Social Services Research Unit, British National Formulary) should be used and this could be supplemented by expert opinion. Table 2 shows the resource use and unit costs that need to be collected.
| Category | Resource use | Unit costs |
|---|---|---|
| Procedurea | ||
| Preoperative embolisation | – | Per procedure |
| Cardiopulmonary bypass and reperfusion | – | Per procedure |
| Theatre time | Minutes | Per minute |
| Preoperative stay | Days | Per day |
| ICU stay | Days | Per day |
| High-dependency unit stay/coronary care unit | Days | Per day |
| Ward stay | Days | Per day |
| Red blood cell | Units | Per unit |
| Platelets | Units | Per unit |
| Fresh-frozen plasma | Units | Per unit |
| Fluoroscopic time | Minutes | Per minute |
| Contrast | ml | Per ml |
| Device and consumables | – | Actual and list prices |
| Medication use | ||
| Statins | mg | Per mg |
| Antiplatelet | mg | Per mg |
| Anticoagulant | mg | Per mg |
| Beta-blocker | mg | Per mg |
| Antihypertensive | mg | Per mg |
| Follow-upb | ||
| Outpatient visits | Number of visits | Per visit |
| CT scan | Number of scans | Per scan |
| Indirect costsc | ||
| Productivity costs | – | – |
| Travel costs | – | – |
| Complicationsd | ||
| ICU stay | Days | Per day |
| High-dependency unit stay | Days | Per day |
| Ward stay | Days | Per day |
| Outpatient visit | Visits | Per visit |
| Emergency room visit | Visits | Per visit |
| Procedurese | – | Per procedure |
Tables 3 and 4 present estimates of some of the key parameters identified in Table 2. This overview is based on the five clinical studies that were identified in the clinical effectiveness review (see Chapter 4, Summary of reasons for exclusions) that reported these resource use items. Note that these studies were excluded from the clinical effectiveness review because of selective treatment assignment. Thus, it is crucial to realise that these values should not be used for an economic model without adjustments; instead, they should be seen as indicative for which parameters might differ between treatments.
| Parameter | Min. | Max. | Mean | Source |
|---|---|---|---|---|
| Mean theatre time (minutes) | 89 | 150 | 120 | Donas et al.,29 Chisci et al.31 |
| Median theatre time (minutes) | – | – | 235 | Canavati et al.32 |
| Mean blood loss (ml) | – | – | 3436 | Vallabhaneni et al.30 |
| Median blood loss (ml) | 1550 | 2000 | 1775 | Chisci et al.,31 Canavati et al.32 |
| Mean stay (days) | 7.2 | 21.7 | 14.0 | Donas et al.,29 Vallabhaneni et al.,30 Chisci, et al.31 |
| Median stay (days) | – | – | 12 | Canavati et al.32 |
| Mean ICU stay (days) | 7.4 | 29.3 | 18.3 | Vallabhaneni et al.,30 Canavati et al.32 |
| Median ICU stay (days) | – | – | 28 | Canavati et al.32 |
| Parameter | Min. | Max. | Mean | Source |
|---|---|---|---|---|
| Mean theatre time (minutes) | 266 | 290 | 278 | Donas et al.,29 Chisci et al.31 |
| Median theatre time (minutes) | – | – | 300 | Canavati et al.32 |
| Mean blood loss (ml) | – | – | 370 | Vallabhaneni et al.30 |
| Median blood loss (ml) | 500 | 1250 | 875 | Chisci et al.,31 Canavati, et al.32 |
| Mean fluoroscopy time (minutes) | 54 | 88 | 71 | Donas et al.,29 Chisci et al.31 |
| Mean contrast (ml) | 156 | 288 | 222 | Donas et al.,29 Chisci et al.31 |
| Mean stay (days) | 3.5 | 10.5 | 7.2 | Donas et al.,29 Vallabhaneni et al.,30 Chisci et al.31 |
| Median stay (days) | – | – | 7 | Canavati et al.32 |
| Mean ICU stay (days) | 0.9 | 6.2 | 3.6 | Vallabhaneni et al.,30 Canavati et al.32 |
| Median ICU stay (days) | – | – | 4 | Canavati et al.32 |
Outcomes
The outcomes of interest for the model are LYs and QALYs. LYs are directly estimated in the model using the number of years that patients live after each intervention. Then LYs can be combined with quality-of-life estimates to obtain the number of QALYs for each intervention. It is important to obtain quality-of-life data alongside a prospective controlled study in order to assess differences in QALYs between a minimally invasive procedure (fEVAR/bEVAR) compared with an invasive procedure (OSR) and also to compare fEVAR/bEVAR with no procedure. Quality of life was measured with the European Quality of Life-5 Dimensions (EQ-5D™) alongside the EVAR 1 and 2 trials (Tables 5 and 6). These values might also be appropriate for patients with JRAAAs or TAAAs as it is likely that these patients have the same quality of life as patients with AAAs. However, ideally quality-of-life measurements should be performed alongside any prospectively controlled study, preferably with the EQ-5D or another instrument that allows valuation of health. 40 Importantly, these patients will receive at least the questionnaire at randomisation (preoperative) and at several time points in the first postoperative year (e.g. 30 days, 6 months, 12 months) depending on the cycle length of the model. Furthermore, it would be useful if the quality of life was also measured after the first postoperative year.
| Time point | EVAR (N = 543) | OSR (N = 539) | ||||
|---|---|---|---|---|---|---|
| Mean | SD | n | Mean | SD | n | |
| Baseline | 0.75 | 0.22 | 541 | 0.74 | 0.23 | 531 |
| 0–3 monthsa | 0.73 | 0.21 | 238 | 0.67 | 0.25 | 245 |
| 3–12 monthsa | 0.71 | 0.25 | 476 | 0.73 | 0.23 | 414 |
| 12–24 monthsa | 0.74 | 0.24 | 398 | 0.75 | 0.25 | 371 |
| Time point | EVAR (N = 166) | No surgery (N = 172) | ||||
|---|---|---|---|---|---|---|
| Mean | SD | n | Mean | SD | n | |
| Baseline | 0.58 | 0.31 | 164 | 0.63 | 0.28 | 171 |
| 0–3 monthsa | 0.57 | 0.28 | 48 | 0.56 | 0.29 | 92 |
| 3–12 monthsa | 0.64 | 0.28 | 122 | 0.60 | 0.26 | 120 |
| 12–24 monthsa | 0.65 | 0.24 | 88 | 0.60 | 0.30 | 68 |
If a societal perspective is adopted it is important to estimate care-related quality of life in informal caregivers based on, for example, the CarerQol questionnaire. 50
Analyses
Base case
The base-case analysis should report the (incremental) cost-effectiveness of fEVAR/bEVAR compared with OSR and fEVAR/bEVAR compared with no surgery in both disaggregated and aggregated forms.
Subgroup analyses
Important subgroups can be determined by age, sex, whether or not dialysis dependent, aneurysm size, fitness, number of target vessels, whether elective or emergency and symptomatic compared with non-symptomatic as these characteristics may have a substantial impact on the cost-effectiveness. Furthermore, the type of OSR could also be used to determine subgroups as these differ in perioperative mortality. 32
Sensitivity analyses
The impact of parameters such as the costs of the endovascular procedures and 30-day mortality on the cost-effectiveness of fEVAR or bEVAR should be evaluated in a univariate sensitivity analysis. Scenario analyses estimating the influence of late non-aneurysm mortality estimates and late aneurysm mortality on the cost-effectiveness should be performed. Furthermore, it could be useful to conduct best- and worse-case scenario analyses and to produce tornado diagrams. Probabilistic sensitivity analysis should be performed to estimate parameter uncertainty and cost-effectiveness acceptability curves could be very informative. Lastly, structural uncertainty should be addressed by varying structural assumptions (e.g. disease progression of patients who are converted form fEVAR/bEVAR to OSR).
Chapter 7 Discussion
Clinical effectiveness findings
The question regarding the clinical effectiveness and safety of fEVAR and bEVAR in comparison with conventional treatment was the primary aim of this project and, given that no studies met all of the inclusion criteria, it still remains unanswered. In the past, other systematic reviews have compared the clinical effectiveness and safety of fEVAR technology with OSR. However, none of them found any RCTs or any comparative studies to answer this question. A systematic review by Nordon et al. 51 reported that non-RCTs were identified and they included eight cohort studies reporting 368 fEVAR cases and 12 cohort studies reporting 1164 OSRs of JRAAs. One systematic review by Cross et al. 52 identified 11 fEVAR studies describing 660 procedures with no comparison. However, this study also acknowledged in its conclusions that ‘there are currently no controlled trials comparing fEVAR with OSR, and current evidence is weak with many unanswered questions’. 52
Another systematic review included five single-arm studies on fEVAR and seven studies on OSR (one prospective and six retrospective). 4 They also identified and included a study in the form of an abstract that compared the results of fEVAR with those of OSR. However, this study was not included in our systematic review because it was clear that the study was non-comparative. First, the groups were not comparable at baseline in that patients in the fEVAR group were older than patients in the OSR group (mean age 77.6 ± 6.0 years vs. 69.6 ± 8.0 years) and had a significantly greater incidence of severe cardiac and pulmonary comorbidities and diabetes. 4 Second, all of the other studies that we screened at full paper stage that were described as comparative turned out to be non-comparative in that they selected based on prognostic characteristics, i.e. they assigned younger, fitter patients to OSR and older, high-risk patients to fEVAR. This was also supported by the baseline characteristics in those excluded studies (see Chapter 6, Design). This was not evident from the title and abstract and could be ascertained only by going through the full article in detail.
A Dutch CvZ report, published in 2012, described a systematic review on the endovascular treatment of complex aneurysms of the aorta. 18 The CvZ report concluded that evidence in the form of comparative research, not necessarily randomised, is feasible and required to make a judgement about the clinical effectiveness and safety of interventions such as fEVAR and bEVAR. This was in line with our understanding of the topic that at least a well-conducted comparative study wherein participants are not assigned to a certain intervention by clinicians based on prognostic characteristics (age, fitness and comorbidities) is essential to answer this question. Neither this systematic review nor the CvZ review identified any relevant comparative studies.
Interestingly, all of the apparent comparative studies used the same method of selection, i.e. to allocate those patients with the poorest prognosis to fEVAR or bEVAR. This is unsurprising as publications to date largely reflect the introduction and early evolution of the techniques, which are applicable only to a relatively small proportion of all aneurysm patients and in a much smaller number of centres than OSR is available. It would have been negligent in the early days of fEVAR and bEVAR to offer the then new and unproven technique to patients for whom a proven technique, viz. OSR, is an option with acceptable operative risk. Given that these patients are probably not suitable for OSR, OSR is not the correct comparator, i.e. no surgery would be appropriate. Of course, it might be considered unethical to allocate such people to no surgery and, therefore, the most useful comparison would be between fEVAR or bEVAR and OSR, but in the younger fitter population.
Cost-effectiveness findings
The systematic review of cost-effectiveness found no economic studies of fEVAR or bEVAR. In addition, because of the lack of clinical effectiveness data and the difficulty in obtaining reliable cost data given huge variation in the nature of procedures between centres and the likely rapid change in technology, it was decided not to perform a CEA. Instead, the methods for performing the analysis as well as the data that would be required have been described. This includes those data that ideally would come from a RCT in each of two populations (those fit enough and those not fit enough for OSR) that would inform two distinct models.
Strengths and limitations of the clinical effectiveness review
Strengths
Extensive literature searches were conducted in an attempt to maximise retrieval of relevant studies. These included electronic searches of a variety of bibliographic databases, as well as screening of clinical trials registers and conference abstracts to identify unpublished studies. Because of the known difficulties in identifying studies in this topic no study design search filters were used and search strategies were developed to maximise sensitivity at the expense of reduced specificity. Thus, large numbers of citations were identified and screened. Despite this, none of them met the inclusion criteria of the review.
Clear inclusion criteria were specified in the protocol for this review. Eligibility of studies for inclusion is therefore transparent. In addition, we have provided specific reasons for excluding any of the studies considered potentially relevant at initial citation screening. The review process followed recommended methods to minimise the potential for error and/or bias; two reviewers independently screened studies for inclusion and any disagreements were resolved by consensus. Moreover, no previous reviews identified any relevant studies either.
Limitations
Despite efforts to include evidence, it should be noted that no RCTs were identified and the studies reporting comparative data did not have comparative groups at baseline because all of them selectively assigned patients to the intervention and comparator groups.
Strengths, limitations and uncertainties of the cost-effectiveness analysis
As for the review of clinical effectiveness, extensive searches were undertaken. Given that no de novo CEA could be conducted, whether fEVAR or bEVAR are cost-effective remains uncertain. However, the current report gives a clear model structure that can be used to inform decisions about which data should be collected, and can be a first step in the development of a final model once data collection has taken place.
Conclusions
The systematic review of clinical effectiveness studies showed that no comparative study has been done that could provide reliable clinical effectiveness data. All studies that compared either fEVAR or bEVAR with either OSR or no surgery explicitly selected patients based on prognosis, i.e. essentially the populations for each comparator were not the same. Therefore, it was decided that a CEA evaluating fEVAR and bEVAR was not possible. Consequently, we decided to describe how an economic evaluation could be performed if more data were available. This includes those data that ideally would come from a RCT in each of two populations, those fit enough and those not fit enough for OSR, which would inform two distinct models.
Suggested research priorities
Given that no studies met the inclusion criteria, there is clearly much uncertainty in all outcomes, including probability of technical success (target vessel perfusion), risk of death, durability (risk of relapse), risk of adverse events and quality of life. Therefore, there is clearly a need for a RCT, or at least a well-conducted prospective cohort study with proper comparability at baseline and statistical techniques to adjust for potential confounding. Also, we recommend that this should be at least in the population of younger, fitter patients, i.e. those for whom OSR is suitable, in order answer the question whether fEVAR or bEVAR is clinically effective or cost-effective in comparison with OSR. Ideally, there should also be one RCT for each of fEVAR and bEVAR and consideration should also be given for separate trials in the non-OSR eligible population. Ideally such a trial will be long enough to identify any patients who develop longer-term problems such as renal failure.
When considering a RCT evaluating fEVAR/bEVAR, Canavati et al. 32 has identified that it can be a challenge to include a sufficient number of patients so that subgroup analyses with enough statistical power can be performed. Subgroup analyses are important as there will be a large variation in both procedures but especially in open procedures for this indication leading to variation in operative risk. Greenberg et al. 33 has also raised the challenge of including sufficient patients. We therefore recommend a multicentre study. However, Greenberg et al. 33 also recognised the challenge of disseminating endovascular skills and the rapid technology improvements that occur for branched devices. It is important to keep in mind that a learning effect can exist and this can negatively influence the perioperative mortality of these procedures. Verhoeven et al. 49 have recognised this problem and suggested that a learning curve has to be taken into account because half of the occlusions in non-stented fenestrations or scallops occurred in fEVAR interventions that were performed in the early stage. Second, therefore, we recommend strict criteria in terms of surgeon experience, centre experience (e.g. 50 fEVAR and/or 25 bEVAR procedures in the last 3 years), type of stent graft and OSR technique. Also, a ‘tracker trial’, i.e. one where the intervention is allowed to change with improvements in technology, might be considered. 53 Ideally, subgroup analysis could also be undertaken to investigate the effect of risks such as aneurysm size and presence of comorbidities.
In addition, given the inability to conduct a CEA, due largely to the lack of clinical effectiveness data, we recommend that one be conducted alongside any RCT or other prospective cohort study. Ideally, in order to take into account both the short- and long-term economic consequences, this ought to be constructed as a model as described in Chapter 6. Furthermore, in designing the prospective study, there is the opportunity to collect primary data to inform such a model, such as resource use and utilities, as specified in detail in Chapter 6.
Acknowledgements
Diana Hilmer devised and performed the literature searches and provided information support to the project.
Contributions of authors
Nigel Armstrong and Sohan Deshpande planned and performed the systematic review and the interpretation of evidence.
Laura Burgers and Maiwenn Al planned and performed the cost-effectiveness review, and researched and reported the CEA methods.
Rob Riemsma, Johan Severens and Jos Kleijnen provided senior advice and support to the systematic review and CEA sections, respectively.
SR Vallabhaneni and Peter Holt provided expert clinical advice throughout the project.
All parties were involved in drafting and/or commenting on the report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Johnston KW, Rutherford RB, Tilson MD, Shah DM, Hollier L, Stanley JC. Suggested standards for reporting on arterial aneurysms. Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery. J Vasc Surg 1991;13:452-8. http://dx.doi.org/10.1067/mva.1991.26737.
- National Heart Lung and Blood Institute . What Is an Aneurysm? 2011. www.nhlbi.nih.gov/health/health-topics/topics/arm/ (accessed 11 September 2013).
- Endovascular Stent–Grafts for the Treatment of Abdominal Aortic Aneurysms. NICE Technology Appraisal Guidance 167. NICE; 2009.
- Medical Advisory Secretariat . Fenestrated endovascular grafts for the repair of juxtarenal aortic aneurysms: an evidence-based analysis. Ont Health Technol Assess Ser 2009;9:1-51.
- Merck . Aortic Aneurysms 2012. www.merckmanuals.com/professional/cardiovascular_disorders/diseases_of_the_aorta_and_its_branches/aortic_aneurysms.html (accessed 30 August 2013).
- Safi HJ, Miller CC. Spinal cord protection in descending thoracic and thoracoabdominal aortic repair. Ann Thorac Surg 1999;67:1937-9.
- National Services Devision . Review of Thoraco-Abdominal Aortic Aneurysm Services Within NHS Scotland 2007. www.nsd.scot.nhs.uk/publications/servicereviews/Review%20of%20Thoraco-Abdominal%20Aortic%20Aneurysm%20Services%202007.pdf (accessed 30 August 2013).
- NHS Institute for Innovation and Improvement . Abdominal Aortic Aneurysm Screening: Map of Medicine Internet 2010. http://aaa.screening.nhs.uk/getdata.php?id=611 (accessed 30 September 2013).
- MHRA Committee on the Safety of Devices . Delivering an Endovascular Aneurysm Repair (EVAR) Service 2011. www.mhra.gov.uk/home/groups/clin/documents/news/con103000.pdf (accessed 30 August 2013).
- American Heart Association . Guidelines for the Diagnosis and Management of Patients With Thoracic Aortic Disease: ACC AHA Pocket Guideline; Based on the 2010 ACCF AHA AATS ACR ASA SCA SCAI SIR STS SVM. 2010. http://my.americanheart.org/idc/groups/ahaecc-internal/@wcm/@sop/documents/downloadable/ucm_423806.pdf (accessed 29 August 2013).
- Svensson LG, Kouchoukos NT, Miller DC, Bavaria JE, Coselli JS, Curi MA, et al. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent–grafts. Ann Thorac Surg 2008;85:S1-4. http://dx.doi.org/10.1016/j.athoracsur.2007.10.099.
- Anderson JL, Berce M, Hartley DE. Endoluminal aortic grafting with renal and superior mesenteric artery incorporation by graft fenestration. J Endovasc Ther 2001;8:3-15. http://dx.doi.org/10.1583/1545-1550(2001)008<0003:EAGWRA>2.0.CO;2.
- Hobbs SD, England A, Rao Vallabhaneni S. Consultation Document. Liverpool: University of Liverpool; 2013.
- Field ML, Harrington D, Bashir M, Kuduvalli M, Oo A. Intervention on thoracic and thoracoabdominal aortic aneurysms: can the UK offer a service?. J R Soc Med 2012;105:457-63. http://dx.doi.org/10.1258/jrsm.2012.110354.
- Greenberg RK, Lytle B. Endovascular repair of thoracoabdominal aneurysms. Circulation 2008;117:2288-96. http://dx.doi.org/10.1161/CIRCULATIONAHA.107.716134.
- Nordon IM, Hinchliffe RJ, Holt PJ, Loftus IM, Thompson MM. Modern treatment of juxtarenal abdominal aortic aneurysms with fenestrated endografting and open repair: a systematic review. Eur J Vasc Endovasc Surg 2009;38:35-41. http://dx.doi.org/10.1016/j.ejvs.2009.02.012.
- Bakoyiannis CN, Economopoulos KP, Georgopoulos S, Klonaris C, Shialarou M, Kafeza M, et al. Fenestrated and branched endografts for the treatment of thoracoabdominal aortic aneurysms: a systematic review. J Endovasc Ther 2010;17:201-9. http://dx.doi.org/10.1583/09-2964.1.
- de Groot IB, Ligtenberg G, Vijgen S. Endovasculaire Behandeling Van Complexe Aneurysmata Van De Aorta 2012. www.cvz.nl/binaries/content/documents/zinl-www/documenten/publicaties/rapporten-en-standpunten/2012/1208-endovasculaire-behandeling-van-complexe-aneurysmata-van-de-aorta/Endovasculaire+behandeling+van+complexe+aneurysmata+van+de+aorta.pdf (accessed 10 September 2013).
- Frederick JR, Woo YJ. Thoracoabdominal aortic aneurysm. Ann Cardiothorac Surg 2012;1:277-85. http://dx.doi.org/10.3978/j.issn.2225-319X.2012.09.01.
- Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. CRD; 2009.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011.
- Canadian Agency for Drugs and Technologies in Health . CADTH Peer Review Checklist for Search Strategies 2013. www.cadth.ca/en/resources/finding-evidence-is (accessed 17 July 2013).
- Salata K, Katznelson R, Beattie WS, Carroll J, Lindsay TF, Djaiani G. Endovascular versus open approach to aortic aneurysm repair surgery: rates of postoperative delirium. Can J Anaesth 2012;59:556-61. http://dx.doi.org/10.1007/s12630-012-9695-7.
- Bhamidipati CM, LaPar DJ, Mehta GS, Kern JA, Kron IL, Upchurch GR, et al. Have thoracic endografting outcomes improved since US Food and Drug Administration approval?. Ann Thorac Surg 2011;91:1314-22. http://dx.doi.org/10.1016/j.athoracsur.2011.01.037.
- Sultan S, Hynes N. Clinical efficacy and cost per quality-adjusted life years of pararenal endovascular aortic aneurysm repair compared with open surgical repair. J Endovasc Ther 2011;18:181-96. http://dx.doi.org/10.1583/10-3072.1.
- Greenberg RK, Chuter TAM, Lawrence-Brown M, Haulon S, Nolte L, Zenith I. Analysis of renal function after aneurysm repair with a device using suprarenal fixation (Zenith AAA Endovascular Graft) in contrast to open surgical repair. J Vasc Surg 2004;39:1219-28. http://dx.doi.org/10.1016/j.jvs.2004.02.033.
- Tsilimparis N, Perez S, Dayama A, Ricotta JJ. Endovascular repair with fenestrated–branched stent grafts improves 30-day outcomes for complex aortic aneurysms compared with open repair. Ann Vasc Surg 2013;27:267-73. http://dx.doi.org/10.1016/j.avsg.2012.05.022.
- Tsilimparis N, Ricotta JJ, De Freitas DJ, Dayama A, Reeves JG, Brewster LP, et al. A comparison of endovascular and hybrid strategies to treat high-risk patients with complex aortic aneurysms. Paper presented at 2012 Southern Association for Vascular Surgery Annual Meeting, Scottsdale, AZ, 18–21 January 2012. J Vasc Surg 2011;54. http://dx.doi.org/10.1016/j.jvs.2011.10.055.
- Donas KP, Eisenack M, Panuccio G, Austermann M, Osada N, Torsello G. The role of open and endovascular treatment with fenestrated and chimney endografts for patients with juxtarenal aortic aneurysms. J Vasc Surg 2012;56:285-90. http://dx.doi.org/10.1016/j.jvs.2012.01.043.
- Vallabhaneni R, Caputo F, Duwayri Y, Rubin BG, Geraghty PJ, Raman KG, et al. Midterm comparison of open versus endovascular repair of pararenal aneurysms. Paper presented at 2011 Vascular Annual Meeting of the Society for Vascular Surgery, Chicago, IL, 16–18 June 2011. J Vasc Surg 2011;53. http://dx.doi.org/10.1016/j.jvs.2011.03.069.
- Chisci E, Kristmundsson T, de Donato G, Resch T, Setacci F, Sonesson B, et al. The AAA with a challenging neck: outcome of open versus endovascular repair with standard and fenestrated stent–grafts. J Endovasc Ther 2009;16:137-46. http://dx.doi.org/10.1583/08-2531.1.
- Canavati R, Millen A, Brennan J, Fisher RK, McWilliams RG, Naik JB, et al. Comparison of fenestrated endovascular and open repair of abdominal aortic aneurysms not suitable for standard endovascular repair. J Vasc Surg 2013;57:362-7. http://dx.doi.org/10.1016/j.jvs.2012.08.040.
- Greenberg RK, Lu Q, Roselli EE, Svensson LG, Moon MC, Hernandez AV, et al. Contemporary analysis of descending thoracic and thoracoabdominal aneurysm repair: a comparison of endovascular and open techniques. Circulation 2008;118:808-17. http://dx.doi.org/10.1161/CIRCULATIONAHA.108.769695.
- Sultan S. Pararenal endovascular aortic repair is the superior to open repair and affords the most clinically efficacious and cost-effective outcome. Paper presented at 8th Western Vascular Institute Symposium (WVI), Galway, Ireland, 24–25 June 2010. Vascular 2010;18.
- Sultan S, Hynes N. A mid- to long-term experience of clinical efficacy and cost per quality-adjusted-life years with pararenal endovascular aortic repair (PEVAR) without fenestration for pararenal AAA compared with open surgical repair. Paper presented at Cardiovascular and Interventional Radiological Society of Europe (CIRSE), Munich, Germany, 14 September 2011. Cardiovasc Intervent Radiol 2011;34.
- Sultan S, Hynes N. Clinical efficacy and cost per quality-adjusted-life years with pararenal endovascular aortic repair (PEVAR) for para-renal AAA compared with open surgical repair. Paper presented at 24th Annual Symposium of the Transcatheter Cardiovascular Therapeutics (TCT), Miami, FL, 22–26 October 2012. J Am Coll Cardiol 2012;60:B38-9.
- Sultan S, Hynes N. Five-year experience with endovascular aneurysm repair without fenestration for pararenal aortic aneurysms: clinical efficacy, reintervention rates, and cost-effectiveness compared with open repair in high deliberate-practice volume centers. Paper presented at 38th Annual Symposium of the Society for Clinical Vascular Surgery (SCVS), Scottsdale, AZ, 7–10 April 2010. J Vasc Surg 2010;51:1068-9. http://dx.doi.org/10.1016/j.jvs.2010.01.004.
- Hynes N, Sultan S. Five-year experience with evar without fenestration for juxtarenal. Clinical efficacy, reintervention rates & cost-effectiveness compared with open repair in high deliberare practice volume centres. Paper presented at Transcatheter Cardiovascular Therapeutics Symposium, San Francisco, CA, 21–25 September 2009. Am J Cardiol 2009;104.
- Health Quality Ontario . Fenestrated endovascular grafts for the repair of juxtarenal aortic aneurysms: an evidence-based analysis. Ont Health Technol Assess Ser 2009;9:1-51.
- Guide to the Methods of Technology Appraisal 2013. London: NICE; 2013.
- Brown LC, Epstein D, Manca A, Beard JD, Powell JT, Greenhalgh RM. The UK Endovascular Aneurysm Repair (EVAR) trials: design, methodology and progress. Eur J Vasc Endovasc Surg 2004;27:372-81. http://dx.doi.org/10.1016/j.ejvs.2003.12.019.
- Brown LC, Powell JT, Thompson SG, Epstein DM, Sculpher MJ, Greenhalgh RM. The UK EndoVascular Aneurysm Repair (EVAR) trials: randomised trials of EVAR versus standard therapy. Health Technol Assess 2012;16.
- Chambers D, Epstein D, Walker S, Fayter D, Paton F, Wright K, et al. Endovascular stents for abdominal aortic aneurysms: A systematic review and economic model. Health Technol Assess 2009;13.
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. The BMJ Economic Evaluation Working Party. BMJ 1996;313:275-83. http://dx.doi.org/10.1136/bmj.313.7052.275.
- Epstein DM, Sculpher MJ, Manca A, Michaels J, Thompson SG, Brown LC, et al. Modelling the long-term cost-effectiveness of endovascular or open repair for abdominal aortic aneurysm. Br J Surg 2008;95:183-90. http://dx.doi.org/10.1002/bjs.5911.
- Greenhalgh RM, Brown LC, Powell JT, Thompson SG, Epstein D, Sculpher MJ. Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med 2010;362:1863-71. http://dx.doi.org/10.1056/NEJMoa0909305.
- Siebert U, Alagoz O, Bayoumi AM, Jahn B, Owens DK, Cohen DJ, et al. State-transition modeling: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-3. Value Health 2012;15:812-20. http://dx.doi.org/10.1016/j.jval.2012.06.014.
- Moulakakis KG, Dalainas I, Mylonas S, Giannakopoulos TG, Avgerinos ED, Liapis CD. Conversion to open repair after endografting for abdominal aortic aneurysm: a review of causes, incidence, results, and surgical techniques of reconstruction. J Endovasc Ther 2010;17:694-702. http://dx.doi.org/10.1583/1545-1550-17.6.694.
- Verhoeven ELG, Vourliotakis G, Bos WTGJ, Tielliu IFJ, Zeebregts CJ, Prins TR, et al. Fenestrated stent grafting for short-necked and juxtarenal abdominal aortic aneurysm: an 8-year single-centre experience. Eur J Vasc Endovasc Surg 2010;39:529-36. http://dx.doi.org/10.1016/j.ejvs.2010.01.004.
- Brouwer WB, van Exel NJ, van Gorp B, Redekop WK. The CarerQol instrument: a new instrument to measure care-related quality of life of informal caregivers for use in economic evaluations. Qual Life Res 2006;15:1005-21. http://dx.doi.org/10.1007/s11136-005-5994-6.
- Nordon IM, Hinchliffe RJ, Holt PJ, Loftus IM, Thompson MM. Modern treatment of juxtarenal abdominal aortic aneurysms with fenestrated endografting and open repair: a systematic review. Eur J Vasc Endovasc Surg 2009;38:35-41. http://dx.doi.org/10.1016/j.ejvs.2009.02.012.
- Cross J, Gurusamy K, Gadhvi V, Simring D, Harris P, Ivancev K, et al. Fenestrated endovascular aneurysm repair. Br J Surg 2012;99:152-9. http://dx.doi.org/10.1002/bjs.7804.
- Lilford RJ, Braunholtz DA, Greenhalgh R, Edwards SJL. Trials and fast changing technologies: the case for tracker studies. BMJ 2000;320:43-6. http://dx.doi.org/10.1136/bmj.320.7226.43.
- Fillinger M. Branched-fenestrated and chimney-type endovascular repairs for juxtarenal and thoracoabdominal aortic aneurysms. Paper presented at 2012 Vascular Annual Meeting of the Society for Vascular Surgery; National Harbor, MD, 7–9 June 2012. J Vasc Surg 2012;55:94S-95S. http://dx.doi.org/10.1016/j.jvs.2012.03.225.
- Thompson M. Fenestrated EVAR is Better than Open Surgery. Paper presented at Cardiovascular and Interventional Radiological Society of Europe (CIRSE), Valencia, Spain, 2–6 October 2010. Cardiovasc Intervent Radiol 2010;33.
- Nordon IM, Hinchliffe RJ, Holt PJ, Loftus IM, Thompson MM. Modern treatment of juxtarenal abdominal aortic aneurysms with fenestrated endografting and open repair: a systematic review. Paper presented at Cardiovascular and Interventional Radiological Society of Europe (CIRSE), Lisbon, Portugal, 19–23 September 2009. Cardiovasc Intervent Radiol 2009;32.
- Soltesz EG, Greenberg RK. Endovascular repair of thoracoabdominal aortic aneurysms with fenestrated–branched stent–grafts. Oper Tech Thorac Cardiovasc Surg 2010;15:86-99. http://dx.doi.org/10.1053/j.optechstcvs.2010.04.002.
- Greenberg RK, Lu Q, Roselli RR, Svensson LG, Moon MC, Hernandez AV, et al. Contemporary analysis of descending thoracic and thoracoabdominal aneurysm repair: a comparison of endovascular and open techniques. Circulation 2008;118:808-14. http://dx.doi.org/10.1161/CIRCULATIONAHA.108.769695.
- Greenberg R, Eagleton M, Mastracci T. Branched endografts for thoracoabdominal aneurysms. J Thorac Cardiovasc Surg 2010;140:S171-8. http://dx.doi.org/10.1016/j.jtcvs.2010.07.061.
- Panuccio G, Greenberg RK, Wunderle K, Mastracci TM, Eagleton MG, Davros W. Comparison of indirect radiation dose estimates with directly measured radiation dose for patients and operators during complex endovascular procedures. J Vasc Surg 2011;53:885-894.e1. http://dx.doi.org/10.1016/j.jvs.2010.10.106.
- Donas KP, Torsello G, Bisdas T, Osada N, Schonefeld E, Pitoulias GA. Early outcomes for fenestrated and chimney endografts in the treatment of pararenal aortic pathologies are not significantly different: a systematic review with pooled data analysis. J Endovasc Ther 2012;19:723-8. http://dx.doi.org/10.1583/JEVT-12-3952MR.1.
- Siegenthaler MP, Beyersdorf F. Endovascular repair of thoracoabdominal aortic aneurysms. Tex Heart Inst J 2009;36:446-8.
- Eide TMO, Romundstad P, Klepstad P, Myhre HO. Health-related quality of life in long term-survivors of thoracoabdominal aortic aneurysm repair. J Vasc Nurs 2005;23:88-94. http://dx.doi.org/10.1016/j.jvn.2005.06.002.
- Suominen V, Pimenoff G, Salenius J. Fenestrated and chimney endografts for juxtarenal aneurysms: early and midterm results. Scand J Surg 2013;102:182-8. http://dx.doi.org/10.1177/1457496913490464.
- Tambyraja AL, Fishwick NG, Bown MJ, Nasim A, McCarthy MJ, Sayers RD. Fenestrated aortic endografts for juxtarenal aortic aneurysm: Medium term outcomes. Eur J Vasc Endovasc Surg 2011;42:54-8. http://dx.doi.org/10.1016/j.ejvs.2011.03.033.
- Bruen KJ, Feezor RJ, Daniels MJ, Beck AW, Lee WA. Endovascular chimney technique versus open repair of juxtarenal and suprarenal aneurysms. J Vasc Surg 2011;53:895-90. http://dx.doi.org/10.1016/j.jvs.2010.10.068.
- Hayes PD, Sadat U, Walsh SR, Noorani A, Tang TY, Bowden DJ, et al. Cost-effectiveness analysis of endovascular versus open surgical repair of acute abdominal aortic aneurysms based on worldwide experience. J Endovasc Ther 2010;17:174-82. http://dx.doi.org/10.1583/09-2941.1.
- Sultan S, Hynes N. Five-year experience with pararenal endovascular aortic repair (PEVAR) without fenestration. Clinical efficacy reintervention rates & cost-effectiveness compared with Open Surgical Repair (OSR) in a high deliberare practice volume centre. In: J Vasc Surg. Conference: 2010 Vascular Annual Meeting of the Society for Vascular Surgery Boston, MA, 10–13 June 2010. Conference Publication 2010;51.
- Sultan S, Hynes N. Five-year experience with EVAR without fenestration for juxtarenal AAA repair: clinical efficacy, reintervention rates, and cost-effectiveness compared with open repair in high deliberate practice volume centers. Paper presented at 7th Annual Western Vascular Institute Symposium, Galway, Ireland, 21–22 May 2009. Vascular 2009;17.
- Michaels JA, Drury D, Thomas SM. Cost-effectiveness of endovascular abdominal aortic aneurysm repair. Br J Surg 2005;92:960-7. http://dx.doi.org/10.1002/bjs.5119.
- EVAR trial participants . Endovascular aneurysm repair and outcome in patients unfit for open repair of abdominal aortic aneurysm (EVAR trial 2): randomised controlled trial. Lancet 2005;365:2187-92. http://dx.doi.org/10.1016/S0140-6736(05)66628-7.
- EVAR trial participants . Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet 2005;365:2179-86. http://dx.doi.org/10.1016/S0140-6736(05)66627-5.
- Cambria RP, Clouse WD, Davison JK, Dunn PF, Corey M, Dorer D. Thoracoabdominal aneurysm repair: results with 337 operations performed over a 15-year interval. Ann Surg 2002;236:471-9. http://dx.doi.org/10.1097/00000658-200210000-00010.
Appendix 1 Search strategies
Rapid appraisal
Cochrane Database of Systematic Reviews (CDSR; Wiley)
Searched up to issue 7: 2013.
Database of Abstracts of Reviews of Effects (DARE; Wiley)
Searched up to issue 3: 2013.
Health Technology Assessment (HTA) Database (Wiley)
Searched up to issue 3: 2013.
Searched 19 August 2013.
#1 ((juxta-renal or juxtarenal or pararenal or para-renal or thoraco-abdominal or thoracoabdominal or thoracic abdominal) near/5 aneurysm*):ti,ab,kw (42)
#2 Medical subject heading (MeSH) descriptor: [Aortic Aneurysm, Thoracic] this term only (76)
#3 MeSH descriptor: [Aortic Aneurysm, Abdominal] this term only (554)
#4 (JRA or JRAs or PAAA or PAAAs or TAAA or TAAAs or JPAA or JPAAs):ti,ab (47)
#5 #1 or #2 or #3 or #4 (671)
CDSR search retrieved nine records.
Cochrane DARE search retrieved 80 records.
HTA Database search retrieved 50 records.
International Guidelines Network Library
URL: www.g-i-n.net
Searched up to 18 August 2013.
Searched 19 August 2013.
| Terms searched | Hits |
|---|---|
| Free text: juxtarenal AND aneurysm | 0 |
| Free text: juxta-renal AND aneurysm | 0 |
| Free text: pararenal AND aneurysm | 0 |
| Free text: para-renal AND aneurysm | 0 |
| Free text: thoraco-abdominal AND aneurysm | 0 |
| Free text: thoracoabdominal AND aneurysm | 0 |
| Free text: thoracic AND abdominal AND aneurysm | 0 |
| Free text: JRA | 0 |
| Free text: PAAA | 0 |
| Free text: TAAA | 0 |
| Free text: JPAA | 0 |
| Total (prior to deduplication) | 0 |
| Total (after to deduplication) | 0 |
National Guidelines Clearinghouse (internet)
URL: www.guideline.gov/
Searched up to 18 August 2013.
Searched 19 August 2013.
Advanced search
| Terms searched | Hits |
|---|---|
| ((juxta-renal or juxtarenal or pararenal or para-renal or thoraco-abdominal or thoracoabdominal or thoracic abdominal) and aneurysm*) | 17 |
| Total | 17 |
National Institute for Health Research Health Technology Assessment (internet)
URL: www.hta.ac.uk
Searched 19 August 2013.
Browsed by relevant terms found three references.
International Prospective Register of Systematic Reviews (PROSPERO)
URL: www.crd.york.ac.uk/prospero/
Searched up to 19 August 2013.
Searched 19 August 2013.
| Terms searched | Hits |
|---|---|
| Free text: juxta-renal | 0 |
| Free text: juxtarenal | 0 |
| Free text: aneurysm | 9 |
| Total | 9 |
Food and Drug Administration
URL: www.fda.gov/
Searched up to 20 August 2013.
Searched 20 August 2013.
Medical devices tab.
| Terms searched | Hits |
|---|---|
| Endovascular aneurysm | 36 |
| Total | 36 |
Medicines and Healthcare products Regulatory Agency
URL: www.mhra.gov.uk/
Searched up to 20 August 2013.
Searched 20 August 2013.
Full-text search: with all these words.
| Terms searched | Hits |
|---|---|
| Juxta-renal | 0 |
| Juxtarenal | 0 |
| Endovascular aneurysm | 9 |
| Total | 9 |
Clinical effectiveness
MEDLINE (OvidSP): 1946 to week 3 September 2013.
Searched 1 October 2013.
-
aortic aneurysm/ (17,850)
-
Aortic aneurysm, abdominal/ (13,424)
-
Aortic aneurysm, thoracic/ (8062)
-
or/1-3 (37,735)
-
(juxta-renal or juxtarenal or thoraco-abdominal or thoracoabdominal or thoracic abdominal or pararenal or para-renal or suprarenal or supra-renal or short-neck$ or shortneck$).ti,ab,ot,hw. (9228)
-
4 and 5 (2621)
-
((juxta-renal or juxtarenal) adj5 aneur?sm$).ti,ab,ot,hw. (194)
-
((thoraco-abdominal or thoracoabdominal or thoracic abdominal) adj5 aneur?sm$).ti,ab,ot,hw. (1620)
-
((pararenal or para-renal) adj5 aneur?sm$).ti,ab,ot,hw. (83)
-
((suprarenal or supra-renal) adj5 aneur?sm$).ti,ab,ot,hw. (227)
-
((short-neck$ or shortneck$) adj5 aneur?sm$).ti,ab,ot,hw. (15)
-
(visceral aortic segment$ adj5 aneur?sm$).ti,ab,ot,hw. (3)
-
or/7-12 (2001)
-
(JRAAA or JRAAAs or PAAA or PAAAs or TAAA or TAAAs or JPAA or JPAAs or SRA or SRAs or SRAA or SRAAs).ti,ab. (1327)
-
aneur?sm$.ti,ab. (82,158)
-
14 and 15 (285)
-
6 or 13 or 16 (2829)
-
animals/ not (animals/ and humans/) (3,941,632)
-
17 not 18 (2684)
-
letter.pt. (800,927)
-
editorial.pt. (333,488)
-
historical article.pt. (299,119)
-
or/20-22 (1,419,104)
-
19 not 23 (2604)
MEDLINE In-Process & Other Non-indexed Citations (OvidSP) and MEDLINE Daily Update (OvidSP): up to 30 September 2013.
Searched 1 October 2013.
-
aortic aneurysm/ (17)
-
Aortic aneurysm, abdominal/ (16)
-
Aortic aneurysm, thoracic/ (11)
-
or/1-3 (43)
-
(juxta-renal or juxtarenal or thoraco-abdominal or thoracoabdominal or thoracic abdominal or pararenal or para-renal or suprarenal or supra-renal or short-neck$ or shortneck$).ti,ab,ot,hw. (622)
-
4 and 5 (2)
-
((juxta-renal or juxtarenal) adj5 aneur?sm$).ti,ab,ot,hw. (23)
-
((thoraco-abdominal or thoracoabdominal or thoracic abdominal) adj5 aneur?sm$).ti,ab,ot,hw. (84)
-
((pararenal or para-renal) adj5 aneur?sm$).ti,ab,ot,hw. (5)
-
((suprarenal or supra-renal) adj5 aneur?sm$).ti,ab,ot,hw. (10)
-
((short-neck$ or shortneck$) adj5 aneur?sm$).ti,ab,ot,hw. (4)
-
(visceral aortic segment$ adj5 aneur?sm$).ti,ab,ot,hw. (0)
-
or/7-12 (120)
-
(JRAAA or JRAAAs or PAAA or PAAAs or TAAA or TAAAs or JPAA or JPAAs or SRA or SRAs or SRAA or SRAAs).ti,ab. (77)
-
aneur?sm$.ti,ab. (5216)
-
14 and 15 (17)
-
6 or 13 or 16 (122)
-
animals/ not (animals/ and humans/) (2696)
-
17 not 18 (122)
-
letter.pt. (24,401)
-
editorial.pt. (14,519)
-
historical article.pt. (132)
-
or/20-22 (39,033)
-
19 not 23 (121)
EMBASE (OvidSP): 1974 to 4 September 2013.
Searched 5 September 2013.
-
Juxtarenal aneurysm/ (14)
-
Juxtarenal aortic aneurysm/ (20)
-
Juxtarenal abdominal aortic aneurysm/ (8)
-
((juxta-renal or juxtarenal) adj5 aneur?sm$).ti,ab,ot,hw. (281)
-
thoracoabdominal aneurysm/ (55)
-
Thoracoabdominal aortic aneurysm/ (9)
-
Thoracoabdominal aorta aneurysm/ (254)
-
((thoraco-abdominal or thoracoabdominal or thoracic abdominal) adj5 aneur?sm$).ti,ab,ot,hw. (2127)
-
Pararenal aortic aneurysm/ (11)
-
((pararenal or para-renal) adj5 aneur?sm$).ti,ab,ot,hw. (120)
-
Suprarenal aortic aneurysm/ (2)
-
((suprarenal or supra-renal) adj5 aneur?sm$).ti,ab,ot,hw. (284)
-
((short-neck$ or shortneck$) adj5 aneur?sm$).ti,ab,ot,hw. (36)
-
(visceral aortic segment$ adj5 aneur?sm$).ti,ab,ot,hw. (5)
-
or/1-14 (2643)
-
(JRAAA or JRAAAs or PAAA or PAAAs or TAAA or TAAAs or JPAA or JPAAs or SRA or SRAs or SRAA or SRAAs).ti,ab. (1805)
-
aneur?sm$.ti,ab. (107,498)
-
16 and 17 (408)
-
15 or 18 (2669)
-
animal/ (1,886,529)
-
animal experiment/ (1,712,212)
-
(rat or rats or mouse or mice or murine or rodent or rodents or hamster or hamsters or pig or pigs or porcine or rabbit or rabbits or animal or animals or dogs or dog or cats or cow or bovine or sheep or ovine or monkey or monkeys).ti,ab,ot,hw. (5,802,448)
-
or/20-22 (5,802,448)
-
exp human/ (14,928,364)
-
human experiment/ (315,976)
-
or/24-25 (14,929,805)
-
23 not (23 and 26) (4,626,466)
-
19 not 27 (2564)
-
Letter.pt. (839,238)
-
Editorial.pt. (446,547)
-
Note.pt. (582,792)
-
or/29-31 (1,868,577)
-
28 not 32 (2414)
Cochrane Central Register of Controlled Trials (Wiley): up to Issue 8: 2013.
Searched 1 October 2013.
-
MeSH descriptor: [Aortic Aneurysm] this term only (118)
-
MeSH descriptor: [Aortic Aneurysm, Abdominal] this term only (556)
-
MeSH descriptor: [Aortic Aneurysm, Thoracic] this term only (77)
-
#1 or #2 or #3 (736)
-
(juxta renal or juxtarenal or thoraco abdominal or thoracoabdominal or thoracic abdominal or pararenal or para renal or suprarenal or supra renal or short neck* or shortneck*):ti,ab,kw (1231)
-
#4 and #5 (52)
-
((juxta renal or juxtarenal) near/5 aneur?sm*):ti,ab,kw (5)
-
((thoraco abdominal or thoracoabdominal or thoracic abdominal) near/5 aneur?sm*):ti,ab,kw (36)
-
((pararenal or para renal) near/5 aneur?sm*):ti,ab,kw (2)
-
((suprarenal or supra renal) near/5 aneur?sm*):ti,ab,kw (2)
-
((short neck* or shortneck*) near/5 aneur?sm*):ti,ab,kw (0)
-
(visceral aortic segment* near/5 aneur?sm*):ti,ab,kw (0)
-
#7 or #8 or #9 or #10 or #11 or #12 (44)
-
(JRAAA or JRAAAs or PAAA or PAAAs or TAAA or TAAAs or JPAA or JPAAs or SRA or SRAs or SRAA or SRAAs):ti,ab (45)
-
aneur?sm*:ti,ab (1851)
-
#14 and #15 (6)
-
#6 or #13 or #16 (63)
CENTRAL search retrieved 48 records.
Conference searches
VEITHsymposium: Annual Symposium on Vascular and Endovascular Issues: 2011–13
URL: www.veithsymposium.org/index.php?pg=home
Searched 9 October 2013.
No free search interface for abstracts existing. Search of programme only free available option.
39th Veith Symposium, New York, NY, USA, 2012
38th Veith Symposium, New York, NY, USA, 2011
Charing Cross International Symposium: 2011–13
URL: www.cxvascular.com/cxsymposium
Searched 9 October 2013.
No search interface existing, no abstracts findable on webpage.
35th Charing Cross International Symposium, London 6–9 April, 2013.
34th Charing Cross International Symposium, London 14–17 April, 2012.
33th Charing Cross International Symposium, London 9–12 April, 2011.
Vascular Annual Meeting: 2011–13
URL: www.vascularweb.org/educationandmeetings/Pages/default.aspx
Searched 9 October 2013.
Searches for abstracts only possible per day on the website, no search interface for any of the years.
Vascular Annual Meeting, San Francisco, CA, USA, 2013
Searched in J Vasc Surg 2013,57(Suppl. 5).
Vascular Annual Meeting, National Harbor, MD, USA, 2012
Searched in J Vasc Surg 2012,55(Suppl. 6).
Vascular Annual Meeting, Chicago, IL, USA 2011
Searched in J Vasc Surg 2011,53(Suppl. 6).
| Condition | 2013 | 2012 | 2011 | Total |
|---|---|---|---|---|
| Juxta-renal | 0 | 0 | 0 | 0 |
| Juxtarenal | 2 | 1 | 3 | 6 |
| thoraco-abdominal | 2 | 1 | 0 | 3 |
| Thoracoabdominal | 9 | 7 | 7 | 16 |
| Thoracic abdominal | 0 | 0 | 0 | 0 |
| Pararenal | 1 | 1 | 2 | 4 |
| Para-renal | 0 | 0 | 0 | 0 |
| Suprarenal | 5 | 4 | 6 | 15 |
| Supra-renal | 1 | 0 | 1 | 2 |
| Short-neck | 1 | 1 | 0 | 2 |
| Shortneck | 0 | 0 | 0 | 0 |
| Short-necks | 0 | 0 | 0 | 0 |
| Shortnecks | 0 | 0 | 0 | 0 |
| JRAAA | 0 | 0 | 0 | 0 |
| JRAAAs | 0 | 0 | 0 | 0 |
| PAAA | 0 | 1 | 0 | 1 |
| PAAAs | 0 | 0 | 0 | 0 |
| TAAA | 2 | 5 | 4 | 11 |
| TAAAs | 1 | 4 | 0 | 5 |
| JPAA | 0 | 0 | 0 | 0 |
| JPAAs | 0 | 0 | 0 | 0 |
| SRA | 0 | 0 | 1 | 1 |
| SRAs | 0 | 0 | 0 | 0 |
| SRAA | 0 | 0 | 0 | 0 |
| SRAAs | 0 | 0 | 0 | 0 |
| Total (before deduplication) | 24 | 25 | 15 | 66 |
| Total (after deduplication) | 16 | 13 | 13 | 42 |
Cost-effectiveness
MEDLINE (OvidSP): 1946 to week 3 September 2013.
Searched 2 October 2013.
-
aortic aneurysm/ (17,850)
-
Aortic aneurysm, abdominal/ (13,424)
-
Aortic aneurysm, thoracic/ (8062)
-
or/1-3 (37,735)
-
(juxta-renal or juxtarenal or thoraco-abdominal or thoracoabdominal or thoracic abdominal or pararenal or para-renal or suprarenal or supra-renal or short-neck$ or shortneck$).ti,ab,ot,hw. (9228)
-
4 and 5 (2621)
-
((juxta-renal or juxtarenal) adj5 aneur?sm$).ti,ab,ot,hw. (194)
-
((thoraco-abdominal or thoracoabdominal or thoracic abdominal) adj5 aneur?sm$).ti,ab,ot,hw. (1620)
-
((pararenal or para-renal) adj5 aneur?sm$).ti,ab,ot,hw. (83)
-
((suprarenal or supra-renal) adj5 aneur?sm$).ti,ab,ot,hw. (227)
-
((short-neck$ or shortneck$) adj5 aneur?sm$).ti,ab,ot,hw. (15)
-
(visceral aortic segment$ adj5 aneur?sm$).ti,ab,ot,hw. (3)
-
or/7-12 (2001)
-
(JRAAA or JRAAAs or PAAA or PAAAs or TAAA or TAAAs or JPAA or JPAAs or SRA or SRAs or SRAA or SRAAs).ti,ab. (1327)
-
aneur?sm$.ti,ab. (82,158)
-
14 and 15 (285)
-
6 or 13 or 16 (2829)
-
economics/ (27101)
-
exp "costs and cost analysis"/ (182,173)
-
economics, dental/ (1866)
-
exp "economics, hospital"/ (19,372)
-
economics, medical/ (8566)
-
economics, nursing/ (3879)
-
economics, pharmaceutical/ (2599)
-
(economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (425,785)
-
(expenditure$ not energy).ti,ab. (17,493)
-
(value adj1 money).ti,ab. (22)
-
budget$.ti,ab. (17,166)
-
or/18-28 (549,953)
-
((energy or oxygen) adj cost).ti,ab. (2684)
-
(metabolic adj cost).ti,ab. (761)
-
((energy or oxygen) adj expenditure).ti,ab. (16,404)
-
or/30-32 (19,165)
-
29 not 33 (545,711)
-
animals/ not (animals/ and humans/) (3,941,632)
-
34 not 35 (512,158)
-
letter.pt. (800,927)
-
editorial.pt. (333,488)
-
historical article.pt. (299,119)
-
or/37-39 (1,419,104)
-
36 not 40 (484,528)
-
17 and 41 (44)
Costs filter
Centre for Reviews and Dissemination. NHS EED Economics Filter: Medline (Ovid) monthly search [Internet]. York: CRD; 2010.
URL: www.york.ac.uk/inst/crd/intertasc/nhs_eed_strategies.html (accessed 28 September 2010).
MEDLINE In-Process & Other Non-indexed Citations (OvidSP) and MEDLINE Daily Update (OvidSP): up to 1 October 2013.
Searched 2 October 2013.
-
aortic aneurysm/ (22)
-
Aortic aneurysm, abdominal/ (24)
-
Aortic aneurysm, thoracic/ (12)
-
or/1-3 (57)
-
(juxta-renal or juxtarenal or thoraco-abdominal or thoracoabdominal or thoracic abdominal or pararenal or para-renal or suprarenal or supra-renal or short-neck$ or shortneck$).ti,ab,ot,hw. (628)
-
4 and 5 (2)
-
((juxta-renal or juxtarenal) adj5 aneur?sm$).ti,ab,ot,hw. (23)
-
((thoraco-abdominal or thoracoabdominal or thoracic abdominal) adj5 aneur?sm$).ti,ab,ot,hw. (86)
-
((pararenal or para-renal) adj5 aneur?sm$).ti,ab,ot,hw. (5)
-
((suprarenal or supra-renal) adj5 aneur?sm$).ti,ab,ot,hw. (10)
-
((short-neck$ or shortneck$) adj5 aneur?sm$).ti,ab,ot,hw. (4)
-
(visceral aortic segment$ adj5 aneur?sm$).ti,ab,ot,hw. (0)
-
or/7-12 (122)
-
(JRAAA or JRAAAs or PAAA or PAAAs or TAAA or TAAAs or JPAA or JPAAs or SRA or SRAs or SRAA or SRAAs).ti,ab. (78)
-
aneur?sm$.ti,ab. (5279)
-
14 and 15 (18)
-
6 or 13 or 16 (124)
-
economics/ (4)
-
exp "costs and cost analysis"/ (227)
-
economics, dental/ (0)
-
exp "economics, hospital"/ (16)
-
economics, medical/ (5)
-
economics, nursing/ (0)
-
economics, pharmaceutical/ (2)
-
(economic$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (40,406)
-
(expenditure$ not energy).ti,ab. (1194)
-
(value adj1 money).ti,ab. (4)
-
budget$.ti,ab. (1841)
-
or/18-28 (42,342)
-
((energy or oxygen) adj cost).ti,ab. (220)
-
(metabolic adj cost).ti,ab. (68)
-
((energy or oxygen) adj expenditure).ti,ab. (934)
-
or/30-32 (1184)
-
29 not 33 (42,005)
-
animals/ not (animals/ and humans/) (3777)
-
34 not 35 (41,892)
-
letter.pt. (25,037)
-
editorial.pt. (14,927)
-
historical article.pt. (187)
-
or/37-39 (40,128)
-
36 not 40 (41,418)
-
17 and 41 (1)
Costs filter
Centre for Reviews and Dissemination. NHS EED Economics Filter: Medline (Ovid) monthly search [Internet]. York: CRD; 2010.
URL: www.york.ac.uk/inst/crd/intertasc/nhs_eed_strategies.html (accessed 28 September 2010).
EMBASE (OvidSP): 1974 to 27 September 2013.
Searched 30 September 2013.
-
Juxtarenal aneurysm/ (14)
-
Juxtarenal aortic aneurysm/ (20)
-
Juxtarenal abdominal aortic aneurysm/ (8)
-
((juxta-renal or juxtarenal) adj5 aneur?sm$).ti,ab,ot,hw. (282)
-
thoracoabdominal aneurysm/ (55)
-
Thoracoabdominal aortic aneurysm/ (10)
-
Thoracoabdominal aorta aneurysm/ (259)
-
((thoraco-abdominal or thoracoabdominal or thoracic abdominal) adj5 aneur?sm$).ti,ab,ot,hw. (2135)
-
Pararenal aortic aneurysm/ (11)
-
((pararenal or para-renal) adj5 aneur?sm$).ti,ab,ot,hw. (121)
-
Suprarenal aortic aneurysm/ (2)
-
((suprarenal or supra-renal) adj5 aneur?sm$).ti,ab,ot,hw. (284)
-
((short-neck$ or shortneck$) adj5 aneur?sm$).ti,ab,ot,hw. (36)
-
(visceral aortic segment$ adj5 aneur?sm$).ti,ab,ot,hw. (5)
-
or/1-14 (2653)
-
(JRAAA or JRAAAs or PAAA or PAAAs or TAAA or TAAAs or JPAA or JPAAs or SRA or SRAs or SRAA or SRAAs).ti,ab. (1817)
-
aneur?sm$.ti,ab. (107,843)
-
16 and 17 (409)
-
15 or 18 (2679)
-
health-economics/ (33,215)
-
exp economic-evaluation/ (205,196)
-
exp health-care-cost/ (196,808)
-
exp pharmacoeconomics/ (169,268)
-
or/20-23 (470,443)
-
(econom$ or cost or costs or costly or costing or price or prices or pricing or pharmacoeconomic$).ti,ab. (587,202)
-
(expenditure$ not energy).ti,ab. (23,264)
-
(value adj2 money).ti,ab. (1312)
-
budget$.ti,ab. (23,482)
-
or/25-28 (610,890)
-
24 or 29 (882,214)
-
letter.pt. (842,045)
-
editorial.pt. (448,232)
-
note.pt. (585,743)
-
or/31-33 (1,876,020)
-
30 not 34 (795,803)
-
(metabolic adj cost).ti,ab. (871)
-
((energy or oxygen) adj cost).ti,ab. (3154)
-
((energy or oxygen) adj expenditure).ti,ab. (19,870)
-
or/36-38 (23,086)
-
35 not 39 (790,755)
-
animal/ (1,889,845)
-
animal-experiment/ (1,717,671)
-
(rat or rats or mouse or mice or murine or rodent or rodents or hamster or hamsters or pig or pigs or porcine or rabbit or rabbits or animal or animals or dogs or dog or cats or cow or bovine or sheep or ovine or monkey or monkeys).ti,ab,ot,hw. (5,818,911)
-
or/41-43 (5,818,911)
-
exp human/ (14,981,558)
-
human-experiment/ (316,795)
-
45 or 46 (14,982,999)
-
44 not (44 and 47) (4,638,017)
-
40 not 48 (753,935)
-
19 and 49 (57)
Costs filter
Centre for Reviews and Dissemination. NHS EED Economics Filter: EMBASE (Ovid) weekly search [Internet]. York: CRD; 2010. URL: www.york.ac.uk/inst/crd/intertasc/nhs_eed_strategies.html (accessed 17 March 2011).
NHS Economic Evaluation Database (Wiley): up to Issue 3 July 2013.
Searched 2 October 2013.
#1 MeSH descriptor: [Aortic Aneurysm] this term only (118)
#2 MeSH descriptor: [Aortic Aneurysm, Abdominal] this term only (556)
#3 MeSH descriptor: [Aortic Aneurysm, Thoracic] this term only (77)
#4 #1 or #2 or #3 (736)
#5 (juxta renal or juxtarenal or thoraco abdominal or thoracoabdominal or thoracic abdominal or pararenal or para renal or suprarenal or supra renal or short neck* or shortneck*):ti,ab,kw (1231)
#6 #4 and #5 (52)
#7 ((juxta renal or juxtarenal) near/5 aneur?sm*):ti,ab,kw (5)
#8 ((thoraco abdominal or thoracoabdominal or thoracic abdominal) near/5 aneur?sm*):ti,ab,kw (36)
#9 ((pararenal or para renal) near/5 aneur?sm*):ti,ab,kw (2)
#10 ((suprarenal or supra renal) near/5 aneur?sm*):ti,ab,kw (2)
#11 ((short neck* or shortneck*) near/5 aneur?sm*):ti,ab,kw (0)
#12 (visceral aortic segment* near/5 aneur?sm*):ti,ab,kw (0)
#13 #7 or #8 or #9 or #10 or #11 or #12 (44)
#14 (JRAAA or JRAAAs or PAAA or PAAAs or TAAA or TAAAs or JPAA or JPAAs or SRA or SRAs or SRAA or SRAAs):ti,ab (45)
#15 aneur?sm*:ti,ab (1851)
#16 #14 and #15 (6)
#17 #6 or #13 or #16 (63)
The NHS EED search retrieved two records.
EconLit (EBSCOhost): up to 30 September 2013.
Searched 30 September 2013.
| S8 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 | Search modes – Boolean/phrase | Interface – EBSCOhost Research databases Search screen – advanced search Database – EconLit |
0 |
| S7 | TX ( ((JRAAA or JRAAAs or PAAA or PAAAs or TAAA or TAAAs or JPAA or JPAAs or SRA or SRAs or SRAA or SRAAs)) ) AND TX aneur?sm* | Search modes – Boolean/phrase | Interface – EBSCOhost Research databases Search screen – advanced search Database – EconLit |
0 |
| S6 | TX (visceral aortic segment* N5 aneur?sm*) | Search modes – Boolean/phrase | Interface – EBSCOhost Research databases Search screen – advanced search Database – EconLit |
0 |
| S5 | TX ((short-neck* or shortneck*) N5 aneur?sm*) | Search modes – Boolean/phrase | Interface – EBSCOhost Research databases Search screen – advanced search Database – EconLit |
0 |
| S4 | TX ((suprarenal or supra-renal) N5 aneur?sm*) | Search modes – Boolean/phrase | Interface – EBSCOhost Research databases Search screen – advanced search Database – EconLit |
0 |
| S3 | TX ((pararenal or para-renal) N5 aneur?sm*) | Search modes – Boolean/phrase | Interface – EBSCOhost Research databases Search screen – advanced search Database – EconLit |
0 |
| S2 | TX ((thoraco-abdominal or thoracoabdominal or thoracic abdominal) N5 aneur?sm*) | Search modes – Boolean/phrase | Interface – EBSCOhost Research databases Search screen – advanced search Database – EconLit |
0 |
| S1 | TX ((juxta-renal or juxtarenal) N5 aneur?sm*) | Search modes – Boolean/phrase | Interface – EBSCOhost Research databases Search screen – advanced search Database – EconLit |
0 |
Health-related quality of life
MEDLINE (OvidSP): 1946 to week 3 September 2013.
Searched 2 October 2013.
-
exp aortic aneurysm/ (40,779)
-
(aort$ adj5 aneur?sm$).ti,ab,ot,hw. (43,045)
-
1 or 2 (45,370)
-
(AAA or AAAs or JRAAA or JRAAAs or PAAA or PAAAs or TAAA or TAAAs or JPAA or JPAAs or SRA or SRAs or SRAA or SRAAs).ti,ab. (10,194)
-
aneur?sm$.ti,ab. (82,158)
-
4 and 5 (5172)
-
3 or 6 (45,377)
-
(sf36 or sf 36 or short form 36 or shortform 36).ti,ab. (15,461)
-
(sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).ti,ab. (1)
-
(sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).ti,ab. (992)
-
(euroqol or euro qol or eq5d or eq 5d).ti,ab. (3713)
-
(hql or hrql or hqol or hrqol or qol).ti,ab. (26,395)
-
(hye or hyes).ti,ab. (53)
-
health$ year$ equivalent$.ti,ab. (37)
-
(hui or hui1 or hui2 or hui3 or hui4 or hui-4 or hui-1 or hui-2 or hui-3).ti,ab. (872)
-
(quality of well being or quality of wellbeing or qwb).ti,ab. (381)
-
(Disability adjusted life year$ or Disability-adjusted life year$ or health adjusted life year$ or health-adjusted life year$ or years of healthy life or healthy years equivalent or years of potential life lost or years of health life lost or quality adjusted life year$).ti,ab. (7342)
-
(QALY$ or HRQOL or HRQL or DALY$ or HALY$ or YHL or YPLL or YHLL).ti,ab. (15,631)
-
(sf6D or sf 6D or short form 6D or shortform 6D or sf six D or sfsixD or shortform six D or short form six D).ti,ab. (406)
-
(timetradeoff or Time tradeoff or time trade off or tto).ti,ab. (1124)
-
(standard gamble or vas or visual analog$).ti,ab. (41,997)
-
(health state$ utilit$ or HSUV$ or health state$ value$ or health state$ preference$ or HSPV$).ti,ab. (476)
-
Disutilit$.ti,ab. (208)
-
Utilit$.ti,ab. (113,005)
-
life quality.ti,ab. (3335)
-
or/8-25 (199,191)
-
7 and 26 (382)
-
Animals/ not (animals/ and humans/) (3,941,632)
-
27 not 28 (373)
-
letter.pt. (800,927)
-
editorial.pt. (333,488)
-
historical article.pt. (299,119)
-
or/30-32 (1,419,104)
-
29 not 33 (371)
MEDLINE In-Process & Other Non-indexed Citations (OvidSP) and MEDLINE Daily Update (OvidSP): up to 1 October 2013.
Searched 2 October 2013.
-
exp aortic aneurysm/ (62)
-
(aort$ adj5 aneur?sm$).ti,ab,ot,hw. (1479)
-
1 or 2 (1483)
-
(AAA or AAAs or JRAAA or JRAAAs or PAAA or PAAAs or TAAA or TAAAs or JPAA or JPAAs or SRA or SRAs or SRAA or SRAAs).ti,ab. (1153)
-
aneur?sm$.ti,ab. (5279)
-
4 and 5 (314)
-
3 or 6 (1484)
-
(sf36 or sf 36 or short form 36 or shortform 36).ti,ab. (1116)
-
(sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).ti,ab. (0)
-
(sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).ti,ab. (356)
-
(euroqol or euro qol or eq5d or eq 5d).ti,ab. (427)
-
(hql or hrql or hqol or hrqol or qol).ti,ab. (2421)
-
(hye or hyes).ti,ab. (2)
-
health$ year$ equivalent$.ti,ab. (1)
-
(hui or hui1 or hui2 or hui3 or hui4 or hui-4 or hui-1 or hui-2 or hui-3).ti,ab. (76)
-
(quality of well being or quality of wellbeing or qwb).ti,ab. (13)
-
(Disability adjusted life year$ or Disability-adjusted life year$ or health adjusted life year$ or health-adjusted life year$ or years of healthy life or healthy years equivalent or years of potential life lost or years of health life lost or quality adjusted life year$).ti,ab. (680)
-
(QALY$ or HRQOL or HRQL or DALY$ or HALY$ or YHL or YPLL or YHLL).ti,ab. (1392)
-
(sf6D or sf 6D or short form 6D or shortform 6D or sf six D or sfsixD or shortform six D or short form six D).ti,ab. (32)
-
(timetradeoff or Time tradeoff or time trade off or tto).ti,ab. (87)
-
(standard gamble or vas or visual analog$).ti,ab. (3338)
-
(health state$ utilit$ or HSUV$ or health state$ value$ or health state$ preference$ or HSPV$).ti,ab. (54)
-
Disutilit$.ti,ab. (23)
-
Utilit$.ti,ab. (10,198)
-
life quality.ti,ab. (299)
-
or/8-25 (17,678)
-
7 and 26 (13)
-
Animals/ not (animals/ and humans/) (3777)
-
27 not 28 (13)
-
letter.pt. (25,037)
-
editorial.pt. (14,927)
-
historical article.pt. (187)
-
or/30-32 (40,128)
-
29 not 33 (13)
EMBASE (OvidSP): 1974 to 27 September 2013.
Searched 30 September 2013.
-
exp aorta aneurysm/ (43,171)
-
(aort$ adj5 aneur?sm$).ti,ab,ot,hw. (50,328)
-
1 or 2 (50,328)
-
(AAA or AAAs or JRAAA or JRAAAs or PAAA or PAAAs or TAAA or TAAAs or JPAA or JPAAs or SRA or SRAs or SRAA or SRAAs).ti,ab. (12,813)
-
aneur?sm$.ti,ab. (107,843)
-
4 and 5 (6783)
-
3 or 6 (50,423)
-
(sf36 or sf 36 or short form 36 or shortform 36).ti,ab. (21,583)
-
(sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirty six or short form thirtysix or short form thirty six).ti,ab. (3)
-
(sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six).ti,ab. (1508)
-
(euroqol or euro qol or eq5d or eq 5d).ti,ab. (6003)
-
(hql or hrql or hqol or hrqol or qol).ti,ab. (41,749)
-
(hye or hyes).ti,ab. (83)
-
health$ year$ equivalent$.ti,ab. (42)
-
(hui or hui1 or hui2 or hui3 or hui4 or hui-4 or hui-1 or hui-2 or hui-3).ti,ab. (1164)
-
(quality of well being or quality of wellbeing or qwb).ti,ab. (415)
-
(Disability adjusted life year$ or Disability-adjusted life year$ or health adjusted life year$ or health-adjusted life year$ or years of healthy life or healthy years equivalent or years of potential life lost or years of health life lost or quality adjusted life year$).ti,ab. (9621)
-
(QALY$ or HRQOL or HRQL or DALY$ or HALY$ or YHL or YPLL or YHLL).ti,ab. (22,640)
-
(sf6D or sf 6D or short form 6D or shortform 6D or sf six D or sfsixD or shortform six D or short form six D).ti,ab. (611)
-
(timetradeoff or Time tradeoff or time trade off or tto).ti,ab. (1482)
-
(standard gamble or vas or visual analog$).ti,ab. (60,179)
-
(health state$ utilit$ or HSUV$ or health state$ value$ or health state$ preference$ or HSPV$).ti,ab. (692)
-
Disutilit$.ti,ab. (329)
-
Utilit$.ti,ab. (147,574)
-
life quality.ti,ab. (5991)
-
or/8-25 (273,757)
-
7 and 26 (467)
-
animal/ (1,889,845)
-
animal-experiment/ (1,717,671)
-
(rat or rats or mouse or mice or murine or rodent or rodents or hamster or hamsters or pig or pigs or porcine or rabbit or rabbits or animal or animals or dogs or dog or cats or cow or bovine or sheep or ovine or monkey or monkeys).ti,ab,ot,hw. (5,818,911)
-
or/28-30 (5,818,911)
-
exp human/ (14,981,558)
-
human-experiment/ (316,795)
-
32 or 33 (14,982,999)
-
31 not (31 and 34) (4,638,017)
-
27 not 35 (458)
-
letter.pt. (842,045)
-
editorial.pt. (448,232)
-
note.pt. (585,743)
-
or/37-39 (1,876,020)
-
36 not 40 (454)
Cost-effectiveness Analysis Registry (internet): 1990 to 9 October 2013.
URL: https://research.tufts-nemc.org/cear4/
Searched 9 October 2013.
| Terms searched | Articles | Ratios | Utility weights | Complete |
|---|---|---|---|---|
| Aorta aneurysm | 0 | 0 | 0 | 0 |
| Aorta aneurysms | 0 | 0 | 0 | 0 |
| Aortic aneurysm | 34 | 61 | 93 | 188 |
| Aortic aneurysms | 18 | 25 | 58 | 101 |
| Aorta aneurism | 0 | 0 | 0 | 0 |
| Aorta aneurisms | 0 | 0 | 0 | 0 |
| Aortic aneurism | 0 | 1 | 0 | 1 |
| Aortic aneurisms | 0 | 0 | 0 | 0 |
| AAA | 27 | 36 | 88 | 151 |
| AAAs | 12 | 18 | 42 | 72 |
| Total | 91 | 141 | 281 | 513 |
Appendix 2 Summary of studies excluded at full-paper, clinical effectiveness review
| Study | Final reason for exclusion | Comments |
|---|---|---|
| Fillinger, 201254 | Comparator | Chimney |
| Thompson, 201055 | Study design | Non-comparative |
| Nordon et al., 200956 | Study design | Abstract of systematic review |
| Soltesz and Greenberg, 201057 | Study design | Non-comparative study |
| Greenberg et al., 201058 | Study design | Erratum |
| Salata et al., 201223 | Intervention | EVAR |
| Bhamidipati et al., 201124 | Intervention | EVAR |
| Sultan and Hynes, 201125 | Intervention | EVAR |
| Greenberg et al., 201059 | Study design | Non-comparative study |
| Greenberg et al., 200426 | Intervention | EVAR with suprarenal fixation |
| Vallabhaneni et al., 201130 | Study design | Non-comparative |
| Canavati et al., 201332 | Study design | Non-comparative |
| Donas et al., 201229 | Study design | Non-comparative |
| Chisci et al., 200931 | Study design | Non-comparative |
| Tsilimparis et al., 201327 | Intervention | Surgeon-modified fenestrated–branched stent grafts |
| Tsilimparis et al., 201128 | Intervention | Surgeon-modified fenestrated–branched stent grafts |
| Panuccio et al., 201160 | Study design | Health economics |
| Donas et al., 201261 | Study design | Systematic review |
| Cross et al., 201252 | Study design | Systematic review |
| Siegenthaler and Beyersdorf, 200962 | Study design | Case series |
| Nordon et al., 200951 | Study design | Systematic review |
| Greenberg et al., 200833 | Study design | Non-comparative |
| Eide et al., 200563 | Study design | Health economics |
| Suominen et al., 201364 | Study design | Comparator chimney |
Appendix 3 Quality of cost-effectiveness studies of endovascular repair of abdominal aortic aneurysm
| Item | Checklist item (Drummond et al.44) | Brown et al.42 | Chambers et al.43 |
|---|---|---|---|
| 1 | The research question is stated | ✓ | ✓ |
| 2 | The economic importance of the research question is stated | ✗ | ✗ |
| 3 | The viewpoint of the analysis is clearly stated and justified | ✓ | ✓ |
| 4 | The rationale for choosing the alternative programmes or interventions compared is stated | ✓ | ✓ |
| 5 | The alternatives being compared are clearly described | ✓ | ✓ |
| 6 | The form of economic evaluation used is stated | ✓ | ✓ |
| 7 | The choice of form of economic evaluation is justified in relation to the question addressed | ✓ | ✓ |
| 8 | The source of effectiveness estimates used are stated | ✓ | ✓ |
| 9 | Details of the design and results of effectiveness study are given | ✓ | ✓ |
| 10 | Details of the method of synthesis or meta-analysis of estimates are given (if based on an overview of a number of effectiveness studies) | NA | ✓ |
| 11 | The primary outcome measure(s) for the economic evaluation are clearly stated | ✓ | ✓ |
| 12 | Methods to value health states and other benefits are stated | ✓ | ✓ |
| 13 | Details of the subjects from whom valuations are obtained are given | ✓ | ✗ |
| 14 | Productivity changes are reported separately | NA | NA |
| 15 | The relevance of productivity changes to the study question is discussed if included | NA | NA |
| 16 | Quantities of resources are reported separately from their unit costs | ✓ | ✓/✗a |
| 17 | Methods for the estimation of quantities and unit costs are described | ✓ | ✓ |
| 18 | Currency and price data are recorded | ✓ | ✓ |
| 19 | Details of currency of price adjustments for inflation or currency conversion are given | ✓ | ✗ |
| 20 | Details of any model are given | ✓ | ✓ |
| 21 | The choice of the model used and the key parameters on which it is based are justified | ✓ | ✗ |
| 22 | Time horizon of costs and benefits is stated | ✓ | ✓ |
| 23 | The discount rate is stated | ✓ | ✓ |
| 24 | The choice of rate is justified | ✗ | ✓ |
| 25 | An explanation is given if costs or benefits are not discounted | NA | NA |
| 26 | Details of statistical tests and confidence intervals are given for stochastic data | ✓ | ✓ |
| 27 | The approach to sensitivity analysis is given | ✓ | ✓ |
| 28 | The choice of variables for sensitivity analysis is justified | ✓ | ✓ |
| 29 | The ranges over which the variables are varied are stated | ✓ | ✓ |
| 30 | Relevant alternatives are compared | ✓ | ✓ |
| 31 | Incremental analysis is reported | ✓ | ✓ |
| 32 | Major outcomes are presented in both disaggregated and aggregated forms | ✓ | ✓ |
| 33 | The answer to the study question is given | ✓ | ✓ |
| 34 | Conclusions follow from the data reported | ✓ | ✓ |
| 35 | Conclusions are accompanied by the appropriate caveats | ✓ | ✓ |
Appendix 4 Summary of studies for recommended cost-effective analysis
| Author | Year | Cost-effectiveness | Health-care cost | Social care cost | Disease-specific outcomes | Generic utility | Incidence prevalence | Mortality | Model probability | Year of data | Setting | Time horizon | n |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brown et al.42 | 2012 | – | ✓ | – | – | – | – | – | ✓ | 1999–2009 | UK | 25 years | NA |
| Sultan and Hynes36 | 2012 | – | – | – | ✓ | – | – | ✓ | – | 2002–9 | NS | 3 years | 118 |
| Sultan and Hynes 35 | 2011 | – | – | – | ✓ | – | – | ✓ | – | 2002–9 | NS | 3 years | 118 |
| Sultan and Hynes25 | 2011 | – | – | – | ✓ | – | – | ✓ | – | 2001–9 | USA | 3 years | 118 |
| Tambyraja et al.65 | 2011 | – | – | – | ✓ | – | – | ✓ | – | 2005–10 | UK | > 6 months | 29 |
| Bruen et al.66 | 2011 | – | – | – | ✓ | – | – | ✓ | – | 2008–9 | USA | 30 days | 21 |
| Hayes et al.67 | 2010 | – | ✓ | – | – | – | – | – | – | NA | UK | Lifetime | NA |
| Sultan34 | 2010 | – | – | – | ✓ | – | – | ✓ | – | 2002–9 | NS | 3 years | 118 |
| Sultan and Hynes37 | 2010 | – | – | – | ✓ | – | – | ✓ | – | 2002–9 | NS | 5 years | 92 |
| Sultan and Hynes68 | 2010 | – | – | – | ✓ | – | – | ✓ | – | 2002–9 | NS | 5 years | 118 |
| Chambers et al.43 | 2009 | – | ✓ | – | – | – | – | – | ✓ | NA | UK | Lifetime | NA |
| Hynes and Sultan38 | 2009 | – | – | – | ✓ | – | – | ✓ | 2002–9 | NS | 5 years | 92 | |
| Sultan and Hynes69 | 2009 | – | – | – | ✓ | – | – | – | – | 2002–9 | NS | 5 years | 92 |
| Health Quality Ontario39 | 2009 | – | ✓ | – | – | – | ✓ | – | – | NA | Canada | NA | NA |
| Epstein et al.45 | 2008 | – | ✓ | – | – | – | – | – | – | NA | UK | Lifetime | NA |
| Michaels et al.70 | 2005 | – | ✓ | – | – | – | – | – | – | NA | UK | 5–15 years | NA |
| EVAR trial participants (EVAR 2)71 | 2005 | – | – | – | – | ✓ | – | – | – | 1999–2003 | UK | 24 months | 338 |
| EVAR trial participants (EVAR 1)72 | 2005 | – | – | – | – | ✓ | – | – | – | 1999–2003 | UK | 24 months | 1082 |
| Cambria et al.73 | 2002 | – | – | – | ✓ | – | – | – | – | 1987–2001 | USA | 24 months | 333 |
Appendix 5 PRISMA checklist
| Section/topic | # | Checklist item | Reported on page # |
|---|---|---|---|
| Title | |||
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both | i |
| Abstract | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number | xvii–xix |
| Introduction | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known | 1–4 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS) | 5 |
| Methods | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g. Web address), and, if available, provide registration information including registration number | xix |
| Eligibility criteria | 6 | Specify study characteristics (e.g. PICOS, length of follow-up) and report characteristics (e.g. years considered, language, publication status) used as criteria for eligibility, giving rationale | 9–10 |
| Information sources | 7 | Describe all information sources (e.g. databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched | 10–11 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated | Appendix 1 |
| Study selection | 9 | State the process for selecting studies (i.e. screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis) | 11 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g. piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators | NA |
| Data items | 11 | List and define all variables for which data were sought (e.g. PICOS, funding sources) and any assumptions and simplifications made | NA |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis | NA |
| Summary measures | 13 | State the principal summary measures (e.g. risk ratio, difference in means) | NA |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g. I2) for each meta-analysis | NA |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g. publication bias, selective reporting within studies) | NA |
| Additional analyses | 16 | Describe methods of additional analyses (e.g. sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified | NA |
| Results | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram | 11 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g. study size, PICOS, follow-up period) and provide the citations | NA |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12) | NA |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and confidence intervals, ideally with a forest plot | NA |
| Synthesis of results | 21 | Present results of each meta-analysis done, including confidence intervals and measures of consistency | NA |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see item 15) | NA |
| Additional analysis | 23 | Give results of additional analyses, if done [e.g. sensitivity or subgroup analyses, meta-regression (see item 16)] | NA |
| Discussion | |||
| Summary of evidence | 24 | Summarise the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g. health-care providers, users, and policy makers) | 46 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g. risk of bias), and at review level (e.g. incomplete retrieval of identified research, reporting bias) | 47 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research | 48 |
| Funding | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g. supply of data); role of funders for the systematic review | 2 |
List of abbreviations
- AA
- aortic aneurysm
- AAA
- abdominal aortic aneurysm
- ASA
- American Society of Anesthesiologists
- bEVAR
- branched endovascular repair of abdominal aortic aneurysm
- CDSR
- Cochrane Database of Systematic Reviews
- CEA
- cost-effectiveness analysis
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CRD
- Centre for Reviews and Dissemination
- CT
- computerised tomography
- CvZ
- College voor Zorgverzekeringen
- DARE
- Database of Abstracts of Reviews of Effects
- EQ-5D
- European Quality of Life-5 Dimensions
- EVAR
- endovascular repair of abdominal aortic aneurysm
- fEVAR
- fenestrated endovascular repair of an abdominal aortic aneurysm
- JRAAA
- juxtarenal abdominal aortic aneurysm
- LY
- life-year
- MeSH
- medical subject heading
- MHRA
- Medicines and Healthcare products Regulatory Agency
- MI
- myocardial infarction
- NHS EED
- National Health Service Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- OSR
- open surgical repair
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- SMA
- superior mesenteric artery
- TAA
- thoracic aortic aneurysm
- TAAA
- thoracoabdominal aortic aneurysm