Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 07/43/01. The contractual start date was in January 2010. The draft report began editorial review in June 2013 and was accepted for publication in November 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Cassell et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Sexually transmitted infections in the UK
Since 1998 there has been a substantial increase in reported cases of sexually transmitted infection (STI), most strikingly in the 16–24 years age group. 1 Across genitourinary medicine (GUM) clinics in the UK in 2007, young people accounted for 65% of chlamydia cases, 50% of cases of genital warts and 50% of gonorrhoea infections. 1 Chlamydia is the most common STI in under-25s. Since 1998, the rate of diagnosed chlamydia has more than doubled in the 16–24 years age group (from 447 per 100,000 in 1998 to 1102 per 100,000 in 2007). This may be because of a combination of a higher proportion of young people testing, improved diagnostic methods and increased risk behaviour. 1 Chlamydia infection can frequently go undetected, particularly in women, as it is often asymptomatic. 1 If left untreated, chlamydia can lead to pelvic inflammatory disease and infertility in women. This highlights the importance of testing this higher-risk age group to ensure prompt diagnosis and treatment.
It is estimated that 11–12% of 16- to 19-year-olds presenting at a GUM clinic with an acute STI will become reinfected within a year. 2 In order to minimise reinfection, preventative measures are required, including effective methods of notifying partners to ensure rapid diagnosis and treatment and reduce the likelihood of index patients being reinfected from the same source.
Current partner notification practice
Partner notification (PN) is an essential element of STI control. It supports patients by enabling diagnosis and treatment for their sexual partners and is an effective way of identifying at-risk and infected persons. PN has been defined as the spectrum of ‘public health activities in which sexual partners of individuals with STD are notified, informed of their exposure and offered treatment and support services’. 3
Treatment of partners remains important for three reasons:
-
to protect the original patient from reinfection and its health consequences
-
to prevent the further spread of infection by infected partner(s)
-
to reduce transmission of STIs (at a population level) by shortening the duration of infection, which is a key determinant of onward transmission rates. 4,5
In the UK, PN has been supported mainly by specialist health advisers (HAs) based in GUM clinics. However, as a result of the growth of the National Chlamydia Screening Programme (NCSP) in England, this role has also increasingly been taken on by local chlamydia screening offices (CSOs) in the community (including general practice surgery settings). 6
Patient referral, in which the patient contacts their sexual partner(s) to arrange testing and/or treatment, supported by the HA, is the most commonly used method of referral, as most partners prefer to contact their partners themselves. Moreover, this additional support is not available in some settings. 7 However, provider and contract referral are also used. 3 In provider referral, the HA offers to contact one or more of the index’s partners on their behalf; in contract referral, patients are asked to agree to a specialist HA informing their partner(s) if this has not been done after a verbally agreed period of time. These are important services to reach partners who might not otherwise be informed, for example casual or ex-partners. The extent to which patient referral is supplemented by health providers contacting partners on the patient’s behalf, and with their agreement (provider or contract referral), is variable. 8 There is, to date, no three-way comparison between patient referral alone, provider referral and contract referral. One trial, situated in a service with very high PN rates, suggested no advantage of contract referral over patient referral. In this study, contract referral achieved 1.15 partners tested per index case, versus 1.27 for patient referral. 9 However, in a setting with lower rates of successful PN, the offer of contract referral if partners did not present within 3 days achieved 0.62 partners tested per index case of gonorrhoea compared with 0.37 both for simple and for enhanced patient referral. 10 Most relevant to this trial is a study by Katz et al.,11 which achieved partner treatment of rates of 0.72 per index case for provider referral, and 0.22 and 0.18 for two differing forms of patient referral.
Partner notification in the primary care setting
Sexually transmitted infections are increasingly diagnosed and treated within the primary care setting,12,13 and around a third of patients presenting to GUM clinics first seek care from their general practice surgery. 14 Maximising the quality of care for patients seen in general practice with STIs has considerable potential for public health gain. However, clinical structure and process standards for PN remain poorly implemented in primary care. 15
Partner notification has been shown to present particular challenges to primary care practitioners. 16 There is historical evidence that only 30% of UK general practitioners would treat a partner,17 and as few as 13% of index patients have a documented attendance at a GUM clinic. 18 General practitioners (GPs) may overestimate how much PN they do,19 while patients treated by a GP are more likely to require retreatment than those treated in a GUM clinic at the outset. 20 This may be because of a lack of established processes for PN within many general practices.
The GP or practice nurse faces several specific challenges in PN compared with sexual health services. The sexual partners of index patients are often not registered at the practice, and general practice has no mechanism for enabling STI treatment in this situation, or for following up compliance. Even if partners are registered at the practice, the duty of confidentiality to individual patients presents difficulties in PN if the index patient declines to discuss their infection with the partner. Staff in general practice may not be confident in handling common issues with PN, which can be time-consuming and require training or support from a HA. 21 Patients diagnosed with a STI may be less willing to give frank information on number of partners, particularly casual or concurrent partners, to familiar staff than to a specialist STI service,21,22 although this has not been shown consistently. 7,23
Recognising these difficulties, guidance from the National Institute for Health and Care Excellence recommended that all patients with a STI, regardless of the setting of diagnosis, should be offered support in PN, which may be within the primary care setting or through referral to a PN specialist. 24 It did not, however, specify standards for content or delivery of this support. A high-quality randomised controlled trial (RCT) has demonstrated that specifically trained practice nurses can achieve PN outcomes equivalent to those achieved by referral to attend a GUM clinic. 7 This trial provided important evidence that PN in the form of patient referral can be undertaken within a highly motivated and specifically trained primary care setting. However, it does not provide an adequate model for a comparison between patient, provider and contract referral in a primary care setting, as it is unlikely that provider or contract referral could become routine work among all general practice surgeries in the foreseeable future, especially in those with no particular interest in sexual health.
The National Chlamydia Screening Programme
Evidence from a Department of Health-funded chlamydia screening pilot study of opportunistic screening in primary and secondary health-care settings demonstrated that it was feasible and acceptable to test women for chlamydia using urine samples using these services. 25 In a separate Health Technology Assessment (HTA)-funded programme, the Chlamydia Screening Studies (ClaSS) project,23 a cross-sectional study of 19,773 women and men aged 16–39 years, selected from general practice, invited participants to collect urine and vulvovaginal swab (for women) specimens at home and post to the laboratory for testing. These studies confirmed that the prevalence of chlamydia was highest amongst those aged under 25 years and was similar in both men and women. The urine and swab tests were also shown to be suitable samples for diagnostic testing with nucleic acid amplification tests (NAATs).
The NCSP in England, which was established in 2003, is an opportunistic testing programme which has been rolled out over a number of years. The key objectives of the programme are to ‘prevent and control chlamydia through early detection and treatment of infection; and reduce onward transmission to sexual partners and prevent the consequences of untreated infection’. 26 Screening has come to contribute an increasing proportion of primary care STI diagnoses and must be considered as part of the relevant population when conducting any trial of PN in primary care.
There has been marked geographical variation in the mix of services contributing NCSP tests, and in positivity by setting, with positivity in educational settings as low as 3%. 27 By 2010, primary care was increasingly identified by NCSP as a key setting. In 2007/8, the highest percentage of tests was conducted within the community contraceptive services, at 25.9%; a further 17.9% were tested in youth services, 13.4% in education and 12.6% in general practice. 28 Coverage of the target 16- to 24-year-old population varied between 0% and 14% in different primary care trusts (PCTs), with local NHS targets set at 15% coverage. 29 In 2007, over 270,729 screens were performed, with 9.5% females and 8.4% of males testing positive. 1
The Department of Health Public Health Outcomes Framework 2013–16, published in 2012, now specifies the number of chlamydia diagnoses per unit population as a public health outcome target for England, replacing coverage targets. 30
The NCSP targets under-25s who are sexually active to test for chlamydia and offers sexual health promotion advice. Although the programme has a national co-ordination team, the organisation of each geographical area is determined at local level. In its early years, the NCSP encouraged the development of varying models of service provision including testing in outreach settings such as colleges, prisons, youth services and even shopping centres. Possible location categories for the treatment of index patients (as specified by the NCSP) are GUM, family planning, CSO, general practice, pharmacy and other.
The organisation of PN varies markedly, with GUM clinics providing this service in some areas, and community-based local chlamydia co-ordinators in others, while some high-volume areas have not made specified provision to support PN in primary care.
The commissioned research and its implementation
Given the increase in reported STIs over the last 10 years1 and variable management of PN in the UK,31 there was an evident need for further robust, evidence-based research on the effectiveness of different methods of PN. The National Institute for Health Research (NIHR) HTA programme therefore sought to commission a RCT to evaluate the clinical effectiveness and cost-effectiveness of different existing approaches to PN, specifically in primary care. The HTA tendered for a RCT comparing patient referral, provider referral and contract referral for patients diagnosed with a bacterial STI in the primary care setting. This study was commissioned as a full-scale RCT, and included health economics, mathematical modelling and patient factor components. It also included the building of a web-based referral tool that could both facilitate referral from primary care to specialist GUM services where HAs were based and collect summary outcome data on PN. It was envisaged that this web tool could subsequently have a role in NHS clinical care.
At the outset of the research, we undertook initial pilot work (phase 1) on the assumption that the RCT would scale up in the form in which it had been commissioned. However, it became clear that this was not likely to be feasible because of recruitment problems. We then undertook additional work aimed at improving recruitment. This included exploring potential for improvement in our existing recruitment strategy through literature review and consultation (phase 2), implementing the new recruitment strategy (phase 3) and, finally, implementing an additional novel recruitment strategy being used in a related study elsewhere (phase 4). Unfortunately, these efforts were ultimately unsuccessful, but they provide some interesting lessons relevant to the future planning of certain types of trial in primary care.
Phase 1 also identified some interesting conceptual and operational challenges to the planned comparison between the three proposed arms of this trial. As we sought to develop standardised approaches to provider referral and contract referral in consultation with practitioners, it emerged that practitioners found clear-cut distinctions between these approaches problematic. We explored and addressed this using qualitative methods.
Because of recruitment failure, we were unable to compare the costs of achieving our proposed outcomes. We are, however, able to present the costs of the intervention arms used in this study, and also of the intensive recruitment strategy used in phase 4, which may inform recruitment plans for other studies.
Unfortunately, because of insufficient recruitment, the patient factors and modelling work were not possible and they are not reported.
Outline of report
This report presents the various phases of recruitment, focusing on findings of relevance to other future studies in the fields of PN, sexual health in primary care or recruitment challenges. The four phases of recruitment mentioned above are presented, alongside methodological chapters exploring the practice of PN and the economic findings, and a chapter setting out the health landscape of chlamydia screening and its implications for research in sexual health.
Chapter 2 Summary and protocol for the randomised controlled trial as originally planned
Overview of the proposed randomised controlled trial
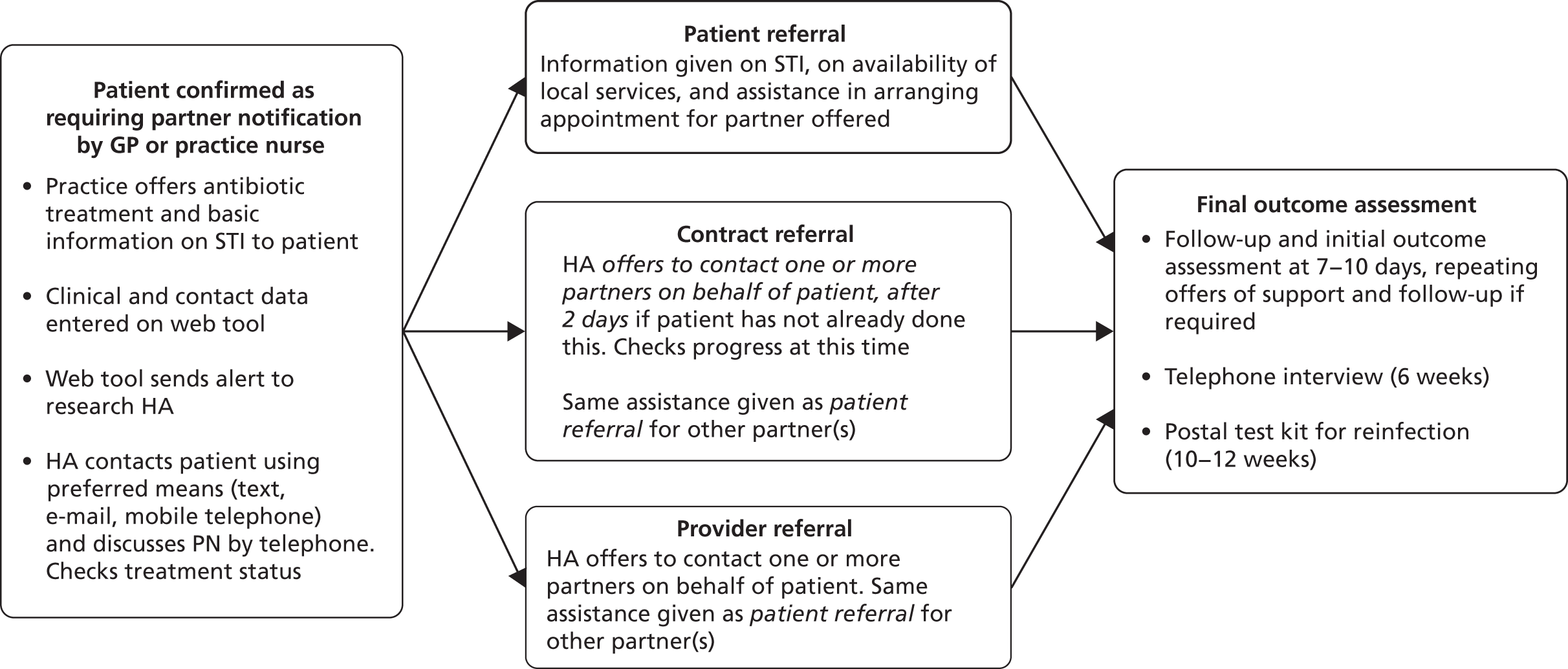
The technologies compared were three different interventions in PN (Figure 1):
-
PATIENT REFERRAL, where patients are given information about their infection and asked to tell their partner about the problem and the need to be treated.
-
PROVIDER REFERRAL, where, in addition to (i), patients are asked to agree to a specialist HA (contact tracing expert) contacting one or more of their partner(s) at the time of diagnosis.
-
CONTRACT REFERRAL, where, in addition to (i), patients are asked to agree to a specialist HA informing partner(s) if this has not been done after a verbally agreed period of time (usually no more than 7 days).
FIGURE 1.
Summary of PN interventions and outcome assessment.
The null hypothesis
Provider referral and contract referral offer no advantage over patient referral alone in PN for curable STIs in the primary care setting.
In order to answer the research question for the main trial, the following objectives and outcomes were established.
Objectives
-
To standardise, appropriate for the primary care setting, three contemporary and evidence-based models of PN for STIs (patient referral, provider referral and contract referral).
-
To compare the clinical effectiveness of these three models.
-
To compare the cost-effectiveness of these three models.
-
To determine the acceptability to patients of each approach to PN, and to identify means of improving PN rates for ‘highly connected’ partnerships.
-
To enhance the efficiency of the trial through mathematical modelling of the potential impact of each modality of PN on outcomes for different types of partner (main, casual and ex-partners) and for men who have sex with men (MSM).
-
To provide comprehensive, definitive evidence for policy-makers and public health practitioners on the implementation of clinically effective and cost-effective PN for patients diagnosed with STIs in the primary care setting.
Outcomes
Primary outcomes of the randomised controlled trial
-
Number of partners per index patient treated for chlamydia and/or gonorrhoea/non-specific urethritis/pelvic inflammatory disease.
-
Proportion of index patients testing negative for the relevant STI at 3 months.
Secondary outcomes of the randomised controlled trial
-
Number of partners per index patient presenting for treatment.
-
Proportion of index patients having at least one partner treated.
-
Number of main, casual and ex-partners per index patient tested for the relevant STI.
-
Number of main, casual and ex-partners testing positive for the relevant STI.
-
Number of current partners tested for a human immunodeficiency virus (HIV) infection by 3 months.
-
Number of index patients tested for a HIV infection by 3 months.
-
Time to definitive treatment of index patient for the relevant STI.
-
Time to definitive treatment of current partner for the relevant STI.
-
Uptake by index patients of ‘contract’ and ‘provider’ referral for one or more partners, within the relevant randomised groups.
-
Patient-related factors impacting on PN or STI disclosure to main, casual and ex-partners.
Identification and recruitment of eligible individuals
Target population
The target population was as follows:
-
all 16- to 24-year-olds testing positive for chlamydia after chlamydia screening
-
all patients (aged ≥ 16 years) diagnosed with a curable STI following clinical presentation
-
all patients (aged ≥ 16 years) diagnosed elsewhere and attending the practice for antibiotic treatment.
Exclusion criteria
-
Any patient aged < 16 years.
-
Any patient unable to fully understand the trial information.
-
Any patient who required translation of trial information.
-
Any patient who required advocacy.
Setting
The setting of the trial was a diverse sample of primary care practices, both in specific localities [primary care research network (PCRN) practices in the South East region of England] and nationally [recruited via the Medical Research Council (MRC) General Practice Research Framework and the PCRN].
Patient and public involvement
A focus group of six sixth-form college students were consulted regarding their opinion on the participant information resources and recruitment methods. In addition, two University College London medical students who were members of the ‘Sexpression’ group (a network of student-led projects that teaches about relationships and sex education in local communities across the UK) agreed to sit on our Trial Steering Committee and advise on participant information resources and recruitment methods.
Approach to recruitment
Participants were to be recruited through letters of invitation, and personal invitation on attending the practice as follows:
-
Practices were asked to mail out letters to 300 randomly selected 16- to 24-year-olds at the beginning of the pilot (taken from the practice list), inviting them for a chlamydia test (as part of the NCSP). The invitation letter also mentioned the trial.
-
Clinical staff (usually practice nurses) approached potential participants, either (a) at the time of first attending the practice either with symptoms of a suspected or presumptive STI (symptomatic patients) or (b) at the time of chlamydia testing (asymptomatic patients, most of whom are tested as part of the NCSP) (Figure 2). The clinic staff were asked to explain that the practice was taking part in a study, as part of which additional assistance might be given to patients needing PN for a STI. It was explained that the practice had been randomly allocated to a group which would help either with providing care for STI patients up to the recommended national standard or with additional options for support. Patients were told the allocation of the practice. It was also explained that, should the patient be diagnosed with a STI, participation in the trial would mean, in all cases, that they would be contacted by an experienced HA. This HA would, depending on the trial allocation of the practice, assist them in their plans and actions to inform and obtain care for both current and ex-partners. Patients agreeing to be recruited at this stage provided personal contact details through which the study HA could communicate with them.
FIGURE 2.
Process of enrolling a participant.

Randomisation
Cluster randomisation at practice level was chosen for two reasons. First, there was a strong likelihood that clinical practice would be influenced by participation in the trial – with randomisation at patient level, practitioners who considered that ‘provider referral’ or ‘contract referral’ had advantages might more readily suggest that patients randomised to ‘patient referral’ attended a specialist clinic. Second, randomisation of patients who attend unpredictably in the middle of a busy surgery is more challenging than randomisation of patients with chronic disorders, while practice randomisation reduces this difficulty.
Consent at time of test versus consent at time of diagnosis
Two approaches to consent were piloted: consent at time of test (CAT) and consent at time of positive diagnosis (CAD). It was anticipated that CAD would reduce the workload for the health professional. However, a previous failed trial demonstrated the difficulty of achieving recruitment at the same time as a positive diagnosis is communicated to the patient. 32
We obtained written informed consent from all adults (aged ≥ 16 years). Where written informed consent was not possible (i.e. the patient had been tested for chlamydia, but no researcher was immediately available to take consent), consent was taken over the telephone soon after the patient had taken the test. In this case, potential participants were asked to agree to a researcher calling them to take consent. A ‘reason-for-test’ form included information on why patients were testing, whether or not they were interested in participating in the trial, contact information and signature (if they were happy for consent to be taken over the telephone).
Partner notification process
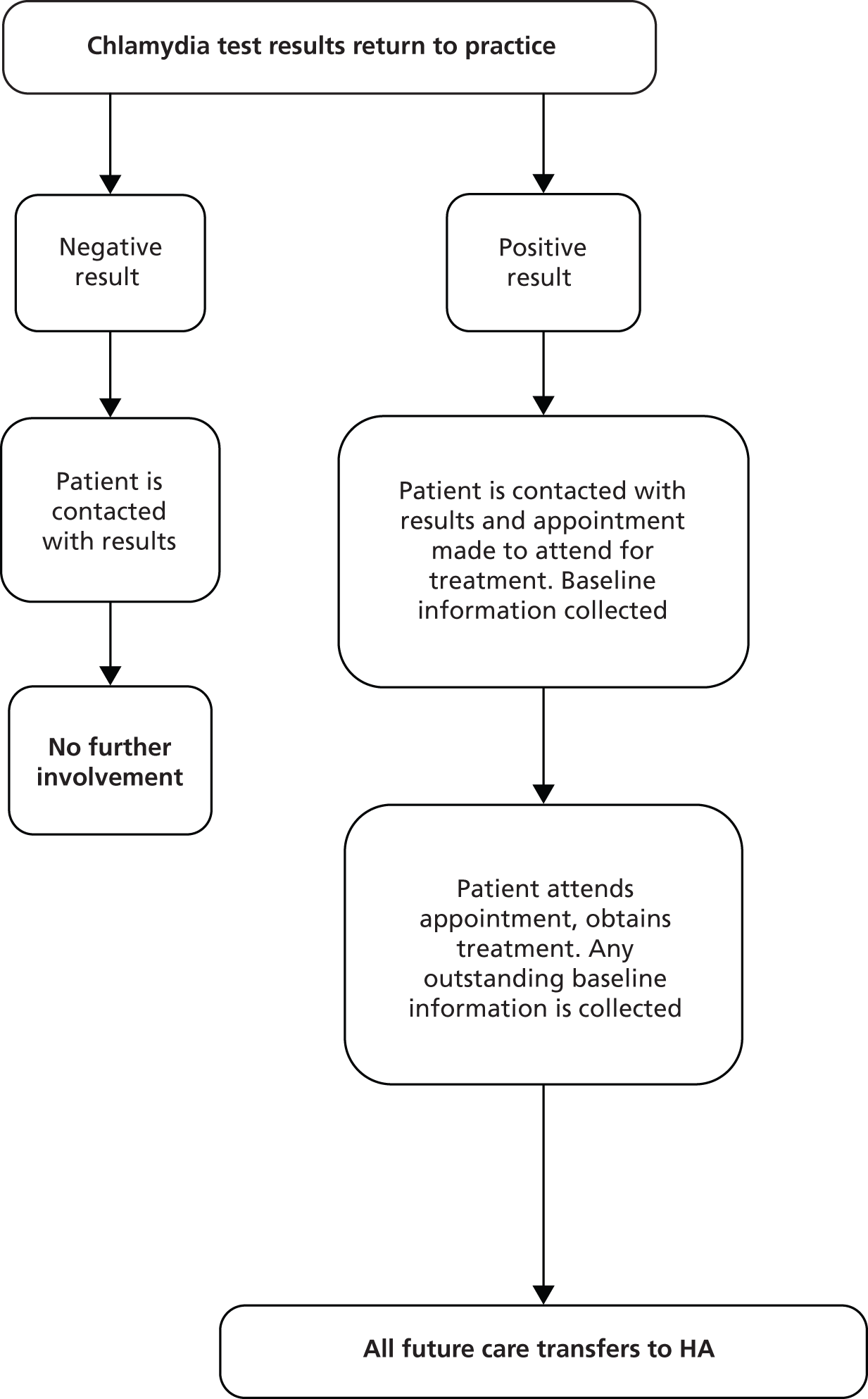
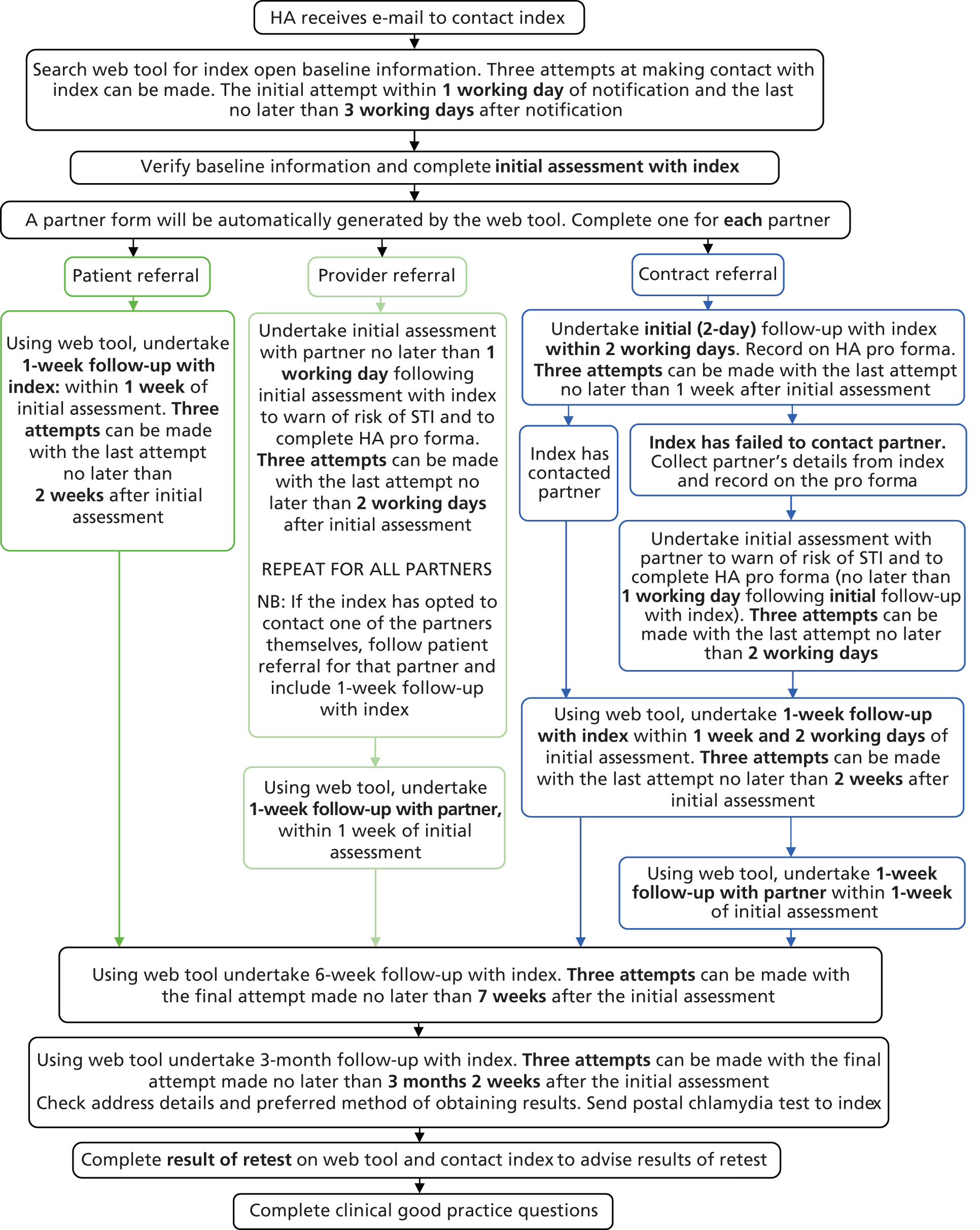
Following the diagnosis of a STI in general practice surgeries, patients were identified as in need of antibiotic treatment and PN (see Figure 1). The practice offered antibiotic treatment and basic information on STIs to the patient. Minimal clinical and contact data were then recorded on the web tool (see Chapter 8 for details). If the patient received a definite STI diagnosis, practice staff entered this information on the web tool, which automatically sent the research study HAs the basic contact information needed to contact and manage the patient, and information on the randomisation status of the practice (Figure 3). The HA then contacted the patient using their preferred means of contact (e.g. text, e-mail or mobile phone call). During the initial consultation the HA discussed PN with the patient and checked their treatment status. The patient was then followed up at 1 week, 6 weeks and 3 months for their PN outcome assessment (Box 1). The HA sent test kits for patients to be tested for reinfection at the 3-month period (testing with a NAAT). The patient then returned the tests to the HA based at Barts Health NHS Trust Sexual Health Services at The Royal London Hospital (Barts) for them to send to the laboratory, which subsequently returned the results to the HA (Figure 4).
FIGURE 3.
Initial management of infection and referral to HA.
HA records additional index details and baseline information for partner(s).
Additional assessment for provider/contract referral patients:
HA advises partner of contact with STI, and the need for testing and treatment.
Index can remain anonymous or be identifiable to partner.
1-week follow-upHA verifies via index: whether partner(s) have been notified, screened and treated.
Additional assessment for provider/contract referral patients:
HA verifies via partner(s): outcomes of partner(s) testing and treatment.
Targeted STI risk reduction with partner.
6-week follow-upHA verifies via index: adherence to treatment for both index and partner.
3-month follow-up (all arms)HA prompts index to test for reinfection using a self-taken postal chlamydia testing kit.
HA checks for additional STI/HIV screening and gives sexual health risk reduction information.
FIGURE 4.
Health adviser flow chart.
Development and implementation of a web tool for clinical referral and outcome measurements
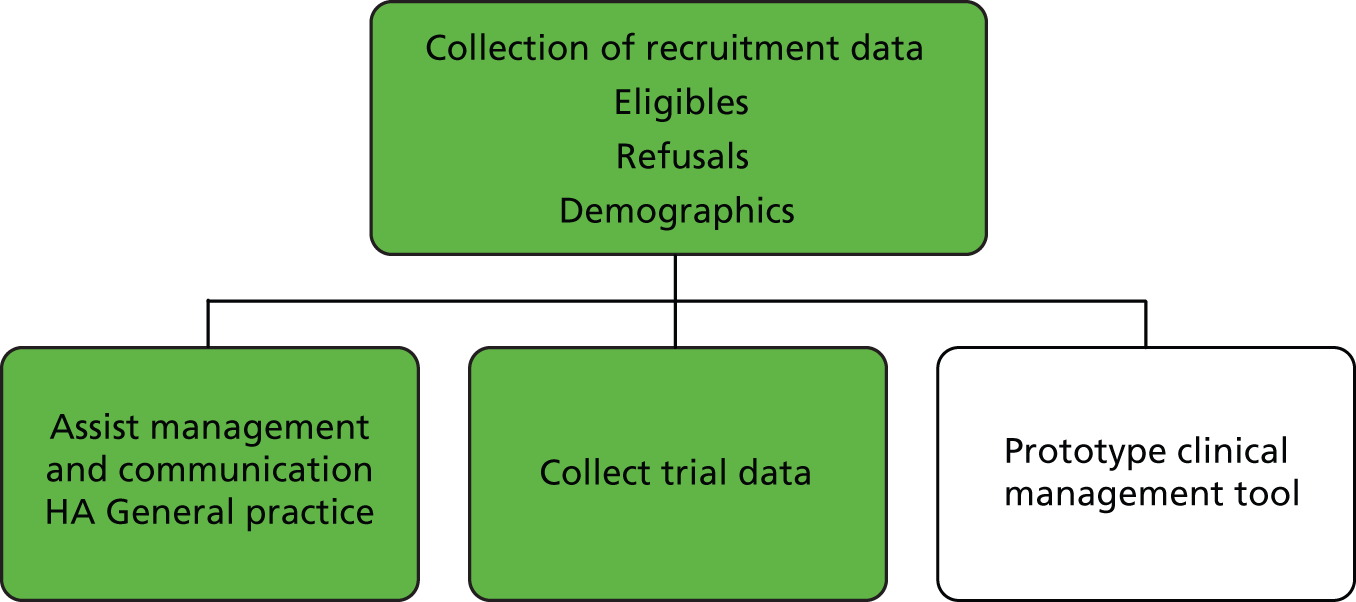
A key aspect of the trial was the development of a web-based referral system, the ‘web tool’. This system enabled us to collect the recruitment and trial data and assist with the management and communication between the HA and general practice. It was also envisaged that this referral tool could be used in a number of settings where PN can be carried out remotely by a professional (Figure 5). We report further on the development of this system in Chapter 8.
FIGURE 5.
Web tool functions.
Data collection
There were a number of data collection points and Table 1 summarises the data captured from each stage of the research process. The data specification was adapted from the accelerated partner therapy (APT) trial specification in order to allow for direct comparison between trials. 33
| Study component | Purpose |
|---|---|
| Tests/diagnosis at practice | Test dates and results |
| Baseline data collected by nurse at practice | Additional information on patients diagnosed with a STI (treatment information and contact details) |
| Initial assessment by HA | Number of partners and individual partner details (can be anonymous) |
| 1-week follow-up with index or contact (by HA) | Partner tested/treated/diagnosed |
| 6-week follow-up with index (by HA) | Follow-up of partners, new partners, treatment adherence |
| 3-month follow-up with index (by HA) | Test for a HIV infection (index and current partner), details of previously undisclosed partners, test for reinfection |
Substudies
Three substudies were included in the PN programme to enhance the trial. We proposed to explore patient-related determinants of PN with a view to advising on targeting different approaches of PN. The economic evaluation proposed to determine the cost-effectiveness of alternative methods of PN, and the mathematical modelling study proposed to address the problem of the right skew in the number (and nature) of sexual partnerships.
Chapter 3 Phase 1: pilot of randomised controlled trial as originally planned
Introduction
As there was no existing or required commissioned pilot, we proposed to run a pilot before scaling up to a full-scale trial. The aim of the first phase of this research was to ascertain the feasibility and acceptability of the proposed trial. 34
Phase 1 was initially planned to run for 3 months but was extended to 4 months from November 2010 to February 2011. We also addressed emerging challenges in operationalising different approaches to PN, which are separately reported in Chapter 9.
We used an evaluation framework recommended by Thabane et al. 35 to assess the key elements of phase 1: process, resources, management and scientific assessment. Key performance indicators, results and actions are summarised in Table 2.
| Evaluation | Key assessment criteria | Results |
|---|---|---|
| Process: assessment of the feasibility and acceptability of the processes key to the success of the trial | ||
|
|
|
| Action | Investigate barriers to screening and establish new strategies to improve recruitment. Revise and improve trial materials | |
| Resources: assessment of the time and resource problems | ||
|
|
|
| Action | Investigate NCSP lab processes. Establish strategies for initial approach to patients to be made before appointment | |
| Management: assessment of potential human and data management problems | ||
|
|
|
| Action | Interrogate NCSP screening and diagnosis rates. Establish attendance rates of 16- to 24-year-olds. Use trial posters instead of NCSP posters Establish strategies to promote the trial to the wider practice team and improve practice engagement with the trial |
|
| Scientific: assessment of the intervention as intended | ||
|
|
|
| Action | Make a request to funders that the trial be reduced to two arms, and recalculate sample size accordingly Organise a dummy run of patients through the PN pathway |
|
Objectives of phase 1
-
To establish the current processes for chlamydia testing, treatment and PN in general practice.
-
To monitor the impact of recruiting at time of test versus time of diagnosis.
-
To assess the feasibility of our proposed approach to recruitment.
-
To monitor the numbers of eligible, tested, consented and diagnosed patients.
-
To assess any time and resource problems.
-
To assess potential human and data management problems.
-
To assess the intervention as intended.
Setting
Six pilot practices, accounting for approximately 10% of the sites required for the main trial, were recruited to take part in the pilot. These practices were identified through the MRC General Practice Research Framework register and PCRN South East as having a sufficient population of young people in the target population. Three practices were from the South East, one was from Yorkshire and Humberside, one was from the East Midlands and one was from the South West. The three practices piloting each approach were randomised to patient, provider and contract referral arms. In addition, two approaches to consent were piloted: CAT and CAD.
Target recruitment
Based on the original sample size calculations for the main trial we aimed to recruit 25 patients per year per practice. It was, therefore, estimated that it should be feasible to recruit 5–10 patients per practice over 3 months. This would require writing invitations to 300 patients in each practice. Each practice sent invitations to approximately 300 16- to 24-year-olds, who were selected at random from the practice register.
Patients were invited by letter to take a chlamydia test at the practice and the trial was mentioned in the invitation letter. Sixteen- to 24-year olds who were attending the practices for other reasons were to be invited to test opportunistically, as were patients who were aged ≥ 16 years who presented with a symptomatic STI or had been diagnosed elsewhere and were attending the practice for treatment.
Results of the evaluation
Process
Current process in practice
During the trial, practices were asked to ensure that all test results came back to the practice and that all results and treatment of patients diagnosed with a STI be dealt with by the practice. Before the trial started we established the practice’s current processes. The pre-trial testing and treatment procedures in the pilot practices were as follows:
-
Three practices offered in-house STI services ‘quite often’ and three practices offered them ‘occasionally’.
-
All the practices were registered as NCSP practices.
-
In five of the six practices the chlamydia test results went directly to the local NCSP CSO.
-
In five practices antibiotics for positive chlamydia patients aged 15–24 years were given by the local NCSP chlamydia co-ordinator.
-
One practice gave antibiotics directly to chlamydia patients diagnosed with a STI.
-
In five practices patients paid for their prescription if they were diagnosed with a STI or STI symptoms; in the other practice patients did not pay for this treatment.
Approaches to recruitment: letter versus opportunistic recruitment
The response to invitation letters was exceptionally poor, with only 11 out of 2218 of 16- to 24-year-olds having a test in response to the letter (0.5%). Nine tests were generated from CAD practices and two tests from CAT practices. Overall, during the 4-month period (November 2010 to February 2011) 30 tests were conducted in total (Table 3), 11 generated by letter and 19 through opportunistic recruitment in the six pilot practices. This amounted to less than two tests per month per practice.
| Recruitment data | Phase 1 |
|---|---|
| Dates (inclusive) | November 2010–February 2011 |
| Total number of chlamydia testsa | 31 |
| Number of positive chlamydia tests | 1 |
| Total number of active practicesb | 6 |
| Phase 1 practices activeb | 6 |
| Phase 3 practices activeb | – |
| Phase 4 practices activeb | – |
Feedback from practices showed that they had focused effort exclusively on sending out the letters and waiting for a response, and had not proactively approached 16- to 24-year-olds when they came into the surgery for another reason.
Approaches to consent: consent at test versus consent at diagnosis
Consent at test practices
All patients who took a chlamydia test were asked to take part in the trial and agreed (Table 4).
| Patient referral practice | Provider referral practice | Contract referral practice |
|---|---|---|
| No patients tested (this practice was unable to recruit patients opportunistically or symptomatically) | Two patients tested, results negative | Five patients tested, results negative |
Consent at diagnosis practices
Recruitment at the time of diagnosis was problematic (Table 5).
| Patient referral practice | Provider referral practice | Contract referral practice |
|---|---|---|
| One patient tested, result negative. One patient recruited when presented with a symptomatic STI | Five patients tested, results negative (an additional 440 letters, which were different from the trial letters, were sent to fulfil the practice's contract with NCSP) | 17 patients tested, all results negative. Six patients presented to the practice with a symptomatic STI, three were not eligible and three could not be contacted for consent |
Trial processes
Trial materials were reported by practice staff to be of a high standard and well designed and the trial name ‘Spread the Word’ was popular. However, using separate patient information leaflets and reason-for-test forms for opportunistic versus symptomatic patients was found confusing.
Practices reported that the NCSP testing and laboratory processes were different from the everyday process used in practice for general laboratory requests, and organising for NCSP results to be returned to the practice was time-consuming and not straightforward. In one practice this had demoralised the practice nurse to the extent that she did not want to continue in the trial.
Staff did not feel comfortable approaching patients to screen opportunistically and believed that patients would be embarrassed if they were asked to take a chlamydia test.
The web tool processes were straightforward and the web tool was considered to be simple and easy to use.
Partner notification pathway
One patient was diagnosed and consented into the trial. The HA initially spoke to the patient and was asked to call back; however, the HA was unable to make contact with this patient again.
Resources
Practices reported that the training session was well organised and training materials were comprehensive and easy to use. The web tool was felt to be simple and easy to use, and we did not experience any problems with incorrect data input from practices. However, staff did report that they often forgot to invite patients to screen and that there was insufficient time to approach patients during appointments, in addition to the reluctance described above.
Management
Five out of six practices reported very little interaction with the local NCSP staff and office. They were not aware of NCSP targets and there was some misapprehension that the trial team were part of the NCSP.
Practice nurses reported that it was difficult to engage their colleagues in the trial and to promote chlamydia screening throughout the practice and that often they were working alone. They reported that very few 16- to 24-year-old patients were seen in their practices, and that they did not see many chlamydia cases.
We were unable to assess the web tool as a PN management tool, as we did not have sufficient numbers of diagnosed patients to be managed through the pathway.
Scientific
In developing manuals in consultation with the HAs who would provide PN, it became apparent that it was hard to define a process of contract referral in a way that was clearly distinct from provider referral. HAs described their practice as a process of negotiation with the patient, leading towards the choice of a mode of PN.
Because insufficient numbers of patients were diagnosed with chlamydia we were unable to assess the PN pathway.
Summary
The small number of tests conducted coupled with the low positivity rate meant we did not have a sufficient number of patients diagnosed with a STI come through the trial to test all trial procedures.
It was agreed by the trial team and Trial Steering Committee that we needed to explore the barriers to testing and identify improved recruitment strategies in practice in order to boost recruitment if the trial was to be scaled up successfully.
Chapter 4 Phase 2: identifying improvements
Introduction
From our experience in phase 1 (see Chapter 1), it was evident that the major challenge we faced was to identify and implement effective means of increasing the number of STI cases diagnosed in each practice, in order to recruit an adequate number of patients into the trial.
Phase 2 of the pilot ran from March 2011 to June 2011. Our overall aim was to identify solutions to our recruitment challenges. We also addressed challenges in operationalising different approaches to PN, which are separately reported in Chapter 9.
The objectives for phase 2 were:
-
to undertake a review of literature relevant to improving uptake of chlamydia testing within general practice
-
to explore in detail existing chlamydia testing rates in general practice, with a view to optimising recruitment of additional practices
-
to explore the potential for use of incentives to boost recruitment
-
to interview primary care practice staff involved in phase 1 to identify barriers to recruitment, potential facilitators and challenges in managing the trial
-
to maintain and enhance recruitment in existing practices.
Literature review
Methods
Since our application for funding, the NCSP had moved on in its policy and practice. Given the degree of local variation in its implementation, we were keen to identify novel and effective approaches to increasing testing rates. We therefore undertook a literature review, aimed at identifying alternative approaches to increasing STI testing in general practice and generally optimising our approach to recruitment.
Based on our experiences in phase 1, the review focused particularly on interventions that could improve chlamydia testing/screening rates for young people up to 24 years old in general practice or similar settings; the use of incentives (for patients or health-care staff) to improve testing rates and/or recruitment in the field of sexual health; clinical pathways used to organise the testing of young people for STIs in general practice or similar settings; general practice staff experiences, behaviour and attitudes to STI testing; and the use of general practice by young people.
Three broad sources of evidence were reviewed, of which the findings are presented in Table 6. The review was targeted, but did not take the form of a formal systematic review.
First, we studied a review published in 2006, which summarised evidence for various elements of chlamydia screening and testing programmes relevant to the UK health system. 5 Second, we searched PubMed for peer-reviewed publications since 2005 on chlamydia testing and screening, using relevant search terms derived from the review in order to identify recently published settings. We reviewed full-text versions of original research relating to the UK and settings where general practice has a broadly similar role (e.g. Australia). Third, we reviewed policy documents, newsletters and conference abstracts focused on England, which described the implementation of chlamydia screening and testing in the general practice setting and included evaluation of effectiveness. The studies we identified are summarised in Table 6.
Findings
| Author | Chlamydia screening intervention | Conclusion | Relevance and implications for the PN trial |
|---|---|---|---|
| Campbell et al., 200636 | Opportunistic sampling of general practice surgery lists in Birmingham and Bristol, 16- to 24-year-olds. Postal invitation to collect urine or swab sample at home | Proactive postal screening may have more impact than opportunistic. Convenience and privacy of home sampling welcomed by those who did respond. Non-responders did not understand purpose, felt low personal risk (especially men), did not want responsibility | Perception of low risk and lack of awareness about infection are barriers. Find methods to increase awareness amongst target population, plus need for regular checks |
| Pimenta et al., 200325 | Women opportunistically invited in variety of health-care settings | Study may overestimate enthusiasm for home sampling. No examples of negative responses. Reported some dissatisfaction with being offered information leaflets by reception staff (too indiscreet). Respondents attending ‘sexually related services’ were most comfortable with offer | Focus attention towards approaches at clinics in general practice surgeries that are sexually related, e.g. contraception clinics Ensure opportunistic approaches are discreet |
| Santer et al., 200337 | Sampling at eight Edinburgh general practice surgeries. Women < 20 years having contraceptive consultations or pregnancy testing; women < 35 years having smear tests. Offered urine testing. Purposive sampling based on test result, age, reason for attendance | Age range 15–31 years Knowledge: women showed lack of awareness of chlamydia in general, and understanding that asymptomatic infections can cause problems. Experience of screening: all glad to have been screened; recognised risk of infertility, benefits of treatment; appreciated ease of testing; would advise friends to be tested; felt all sexually active women should be offered testing but given choice |
Raise awareness among both genders through information on asymptomatic nature and possible long-term risks in leaflet on chlamydia Ensure testing and study recruitment is a simple process Ensure patients are aware of the support available afterwards |
| Perkins et al., 200338 | General practice surgery staff, GUM and family planning clinics in Wirral and Portsmouth. Opportunistic of all women 16–24 years attending regardless of reason. Self-collected urine samples | HCP views of receptionists: waiting room inappropriate to offer leaflet; offer of test causes embarrassment and offence; too busy to offer to all women; problem of multiple offers of tests to same person Of GPs and nurses: impact on workload (extra 2–3 minutes on consultation if information leaflet already given, 10 minutes if not); reluctance to manage positive results in practice; problem targeting women only; remuneration important Conclusion: chlamydia screening in general practice should be by call–recall |
Education and feedback to practice staff on the nature of the study and screening benefit to younger patients Consider boosting opportunistic offers with research staff days Consider offers are made in private where possible Maintain regular feedback on progress and where practice is placed within league of screening practices in study Emphasise support available to all staff from research team during study Consider buying out some receptionist time to help target when reminders should pop up for relevant staff |
| Bilardi et al., 201041 | Cluster randomised trial testing the effect of a small financial incentive on chlamydia testing rates in Australian general practice | A small financial incentive did not increase testing. Staff described other barriers to testing | Financial incentives to GPs are unlikely alone to suffice to increase testing |
| McNulty et al., 201039 | HCW opportunistic screening invitation behaviour. High, medium and low screening practices invited to focus groups | In all screening programme areas the majority of practices screened fewer than 5% of 16- to 24-year-old patients Men tend not to be asked QOF measure for chlamydia screening would help with auditing offers made |
Support and pro-screening attitudes of practice staff as a whole team are essential in raising screening rates Willingness to approach patients regardless of reason for visit is essential |
| Walker et al., 201140 | Longitudinal strategies to maintain participants in study | Patients recruited by non-clinic staff in private. Every female in target age group approached in general practice surgeries. Small incentives, regular contact with research team and contact detail-checking helped sustain high level of engagement in 12-month study | Recruitment face-to-face in surgeries worked well Incentive to stay in the study to the end may be necessary for participants |
Potential for testing young people through postal and in-practice approaches
In a study of consultations by young adults in general practice, Salisbury et al. 42 compared opportunistic and systematic postal chlamydia screening methods using 27 general practice surgeries around Bristol and Birmingham, reporting on patients aged 16 to 24 years. They estimated that, on their own, each method (face-to-face through the general practice surgery when attending at least once over a 12-month period, or being sent a test kit via post) would fail to contact a substantial minority of the target group. Of nearly 13,000 eligible patients, an estimated 21% of patients would not attend their practice during the 12-month period but would be reached by postal screening, 9% would not receive a postal invitation (e.g. because the wrong address was held by their general practice surgery) but would attend their general practice surgery, and 11% would be missed by both methods.
Andersen et al. 43 compared in-home screening with usual care practices for chlamydial infection with nearly 30,500 21- to 23-year-olds in Aarhus County, Denmark, between October 1997 and February 1998. The authors had previously shown this age group to have the highest infection rates, of 23.7% (men aged 21 years) and 8.7% (women aged 22 years). Patients were randomly assigned to one of three groups. Group 1 (n = 4500) were sent a home sampling kit to their centrally registered home address; group 2 (n = 4500) were sent a reply card (pre-stamped and addressed to the study centre) to their home address with which a home sampling kit could be ordered; and group 3 (n = 21,439), as well as groups 1 and 2, had access to their usual care of swab sampling at their general practice surgery. The invitation contained a letter describing the outreach programme and a leaflet about the infection. The test was a urine capture for men, and a saline pipette vaginal flush for women, which were posted directly back to the test lab at Aarhus hospital (pre-paid postal packaging was included in the kit). Results could be sent to any address or their doctor. Although only 1.4% of men and 19.4% of women in the usual care-only control group received any test during the year, this was substantially increased by the two mailing options. For males, options 1 and 2 were equal, with overall coverage around 17%. In females, 39% in group 1 and 33% in group 2 received a test. Although in women an equal number of infections was detected in groups 1 and 2, among men more infections were detected when the kit was posted to them than when they were invited to request a test kit. 43 In all three arms, about 9% of women and only 0.5–1.4% of males received a conventional test in the practice.
Recruitment and retention of young people in sexual health intervention studies
A longitudinal investigation was conducted in Australia (CIRIS – Chlamydia Incidence and Reinfection Rates Study) by Walker et al. 40 to investigate retention in a chlamydia screening study in women between 16 and 25 years old recruited to primary health-care clinics. Follow-up was by post at 3-monthly intervals plus the return of questionnaires and self-collected vaginal swabs. To maximise retention, the team used recruiting staff who were independent of clinic staff, patients were recruited in private, patients regularly communicated with study staff and follow-up was made as easy as possible including incentives and small gifts to patients (of gift vouchers – AUS$10 voucher at 3 months, AUS$25 at 6 and 9 months, and AUS$50 at 12 months plus small gifts with each follow-up test kit, e.g. tampons, cosmetics, confectionery, as agreed by the local ethics committee) to maintain good will. At the start, information on the study was presented with STI information, some condoms and lubricant. Female research assistants were placed in each clinic for up to 6 months and approached every 16- to 25-year-old woman presenting for a consultation. They recruited 66% of eligible women, 79% of whom were retained to the end of the 12-month study. Sixty-six per cent of the total were recruited from general practice clinics.
Walker et al. 40 reported that loss to follow-up was associated with lower education level, recruitment from a sexual health centre rather than a general practice and previously testing positive for chlamydia. Other factors, including age and number of sexual partners, were not associated with loss to follow-up. They believe face-to-face recruitment was a strong factor in its success. Intensive communication strategies with patients through a variety of means and prompting for contact detail updates before sending out the next follow-up pack were associated with good retention in this and other studies (Atherton et al. , 2010, cited by Walker et al. 40).
The experiences, attitudes and behaviours of general practice staff
In a recent study, McNulty et al. 39 interviewed practice staff to explore attitudes and testing behaviours within the NCSP. The focus was on the opportunistic screening habits of practice staff with 16- to 24-year-olds who attended their practice for any reason. Twenty-five focus groups were held from late 2005 to early 2007, comprising 72 GPs, 46 nurses, 23 administrators and receptionists, eight practice managers and seven other staff. Interviews with two low-screening practices were conducted later.
The range of practice chlamydia screening rates was 0% to > 30%, and in all screening programme areas the majority of practices had screened fewer than 5% of their 16- to 24-year-old patients.
McNulty and colleagues found stark differences between staff attitudes to opportunistic screening for chlamydia in low- and high-screening practices. Low-screeners tended to have low belief in the success of screening in this way, and tended to only have a single champion within the practice. In contrast, high-screening practices’ staff held strong beliefs in the utility of opportunistic screening of the target group. All practices reported low numbers of offers to men and felt motivation would be increased with regular reminders at practice meetings, screening training and feedback to practices on successful detection rates from NCSP. In all practices, clinicians’ (GPs, nurses and health-care assistants) self-belief in raising the screening regardless of the patient’s reason for attending was a key factor to their frequency of approach. A whole-team positive view on the value of chlamydia screening was identified as being crucial to the practice’s screening rates. There was no Quality and Outcomes Framework measure associated with chlamydia screening, which also had the effect of decreasing the perceived priority of the NCSP’s campaign. Auditing of offers and acceptance rates is problematic whilst there is no Read code to record this information. The authors conclude that their results are likely to be generalisable across England, but raise the issue of a lack of short- and long-term intervention studies to assess whether or not the methods used by high-screening practices can transform attitudes about opportunistic screening and offers within the teams of low-screening practices.
The authors noted that high screening practices had:
-
a screening champion (not necessarily the GP)
-
normalised screening, so all at-risk patients were offered opportunistic screening whenever they attended
-
facilitated screening using a variety of time-saving methods including computer prompts, test kits in the reception area, youth clinics and receptionist involvement
-
sustained screening through frequent reminders to practices via newsletters from the CSO with feedback on their performance and those of their neighbours
-
advertised screening to the ‘at-risk’ population
-
undertaken training prior to registration as a screening site. 44
The guidance, during the time of our phase 1 pilot, on chlamydia screening to providers and commissioners from NCSP was:
that PCTs build their programmes around the existing core services of Reproductive and Sexual Health (RSH) Services, community pharmacy, general practice, and termination of pregnancy services – and then consider other measures to increase access and target specific ‘at risk’ groups such as websites, outreach etc. 45
Incentives for practices to test young people for chlamydia
In England’s pilot chlamydia screening studies, where general practice surgeries were paid to test, they achieved a 33% screening rate for women in the 16–24 years age group who were invited while attending their general practice surgery for any reason. An average of 80% of women in the 16–24 years age group accepted screening at their general practice surgery when asked, with acceptance lower in younger women. 25 In this first large-scale opportunistic chlamydia screening study in England, the research covered settings including general practice, family planning, GUM clinics, adolescent sexual health clinics, termination of pregnancy clinics and women’s services in hospitals (antenatal, colposcopy, gynaecology and infertility clinics) in two health authorities (Wirral, and Portsmouth and South East Hampshire). Pimenta et al. 25 also reported in this study that major factors influencing women’s decision to accept screening were the non-invasive nature of the urine test method and treatment, desire to protect future fertility and the experimental nature of the screening programme.
Investigators on the Australian Chlamydia Control Effectiveness Pilot (ACCEPt) research study, currently ongoing, explored in a pilot stage the use of incentives to GPs for opportunistic chlamydia testing of young women between 16 and 24 years. 41 Of 145 general practices approached, 12 practices were recruited and assigned to receive either a small payment (AUS$5) or no payment for testing a patient for chlamydia. Forty-five GPs participated between May 2008 and January 2009. GPs were advised to use first-pass urine, self-collected vaginal swabs or endocervical swabs. Practices were provided with screening posters and leaflets for waiting rooms, and a DVD of the education session for GPs unable to attend their training session. Chlamydia testing increased from 6.2% to 8.8% in the control group and from 11.5% to 13.4% in the intervention group, a non-significant increase. The GPs reported that the major barriers to increased chlamydia testing included lack of time, difficulty in remembering to offer a test and lack of patient awareness around testing. The authors believe the lack of test-rate feedback and payment made at the end of the 6-month period to the intervention group GPs were both limiting factors, as many GPs appeared to forget they were in a trial. They also believe the control group’s testing increase was partly due to the educational components of the study. They note there is insufficient research on the use of reminders and incentives to determine what an appropriate level is to raise screening rates by GPs (Professor J Hocking, Melbourne School of Population and Global Health, 2011, personal communication).
We also reviewed a recent example from a chlamydia screening programme which was local to one of the pilot practices (Jason May and Suzy Dion, Northamptonshire Healthcare NHS Foundation Trust, 2011, personal communication and unpublished report). This district had used incentives for GPs to encourage each practice to meet a 10% screening target, as recommended by the NCSP. For every month that a practice opportunistically screened 10% of their 16- to 24-year-old cohort, a £50 gift voucher was paid. We were advised that this had resulted in an increase in screening rates, although only 27% reached their 10% target at least once over the period, but we were not able to identify a formal evaluation.
Incentives
Expert advice from a marketing specialist
As a result of the literature review and poor uptake of testing in phase 1 of the trial, we felt we should explore the possibility of using incentives for the trial. In addition to the literature review summarised above, we spoke to a marketing specialist with substantial experience in running national campaigns, marketing and public relation projects. He suggested that we consider incentives for both the patients and practice staff as described in the sections following (Harvey Atkinson, University of Brighton Students’ Union, 2011, personal communication).
Patient incentives
We were advised that it was felt that for our target age group (16–24 years) we would need to have incentives and that those incentives would need to have an immediate gain for the individual. It was suggested that this could be an inexpensive incentive which could be reinforced by a substantial deferred reward, such as a raffle for an iPod. The marketing specialist also speculated that this age group might be more likely to engage if approached by someone of their own age group, by using volunteers to approach individuals.
Practice incentives
For the practice, incentives could be given to the group and/or the individual. For individual incentives, the team would need to ascertain how each individual could be motivated. The motivations of the reception staff, for example, might be different from the motivation of nurses. It was suggested that there needed to be a practice champion who would act as the change agent and have ownership of the study. The team would need to consider by whom the different staff types are influenced, and whoever this is would need to deliver the message of why screening is important. The marketing specialist also emphasised that screening needed to be embedded into practice behaviour, making it normal practice, and that all parties needed to be engaged.
For group incentives at practice level, a deferred reward must be attractive to all involved (there should be a consensus amongst the group). It was suggested that it could be beneficial for the team to attend a practice meeting, along with the contraception and sexual health nurse and local chlamydia screening officers. This meeting would include explaining why screening is so important, clearly stating how much money can be earned by improving screening numbers and what the incentives are for individuals at the practice.
It was recommended that an outline for a poster could be provided to the practice for the practice manager to tailor to practice requirements. Furthermore, weekly and/or monthly updates should be sent to the practice on screening rates and highlighting numbers screened. It was proposed that competitions could be set up between practices (e.g. practice of the month), encouraging increased testing.
The marketing specialist suggested we discover what works best for the practice and give them various options for running the screening, allowing them to tailor the screening process to fit in with their own procedures (e.g. manual or electronic check-in system).
Survey of young people
We also conducted a short survey over a 2-week period by snowball recruitment using a social networking site, members of a youth group, and members of a sexual health-oriented group (Sexpression) to explore incentives for young people. A short questionnaire, consisting of nine questions, was posted online for young people aged 16–24 years to access and respond to anonymously (a hard copy was also made available).
One hundred and seventy-three young people took part, the majority of whom were in full-time education (74%, n = 128). Twenty per cent were not in any form of education or training (n = 34) and 6% were in part-time education (n = 11).
A large majority of respondents (83%, n = 144) said that they would take a chlamydia test if a doctor or nurse asked them to during a routine appointment. This was more than those who said they would if the receptionist asked them to test (61%, n = 105) or if self-test kits were available in the reception (66%, n = 115). Of the 17% (n = 29) who would not take a test, 38% (n = 11) said they would change their mind if offered an incentive. One participant who would not take the test or take part in the study was concerned that sexual health issues would appear on their medical record and they also expressed concern that ‘you don’t get paid to see a doctor so why get paid for this?’ This response demonstrated potential barriers to using incentives.
We asked all participants which incentives would make them more likely to take a test and participate in the study. One hundred and seventy-one participants replied to this question, of whom 42% (n = 72) said that incentives would not make a difference. Of the remaining 99 participants, 88 (89%) said that a cash incentive would make them more likely to take a chlamydia test and be part of a study, 64 (65%) said that shopping vouchers would, and only 13 (13%) said that mobile phone credits would make a difference to their participation (respondents were able to select more than one option).
When asked the lowest value of incentive that would make them more likely to participate, 39% (n = 58) of 172 participants still responded that no amount would make a difference, although this was less than for the previous question. A £5 incentive was the most popular option among those who said that an incentive would make them more likely to participate, with 34% of participants choosing this response (n = 58). Seventeen per cent of participants replied that £10 would be the minimum they would accept (n = 29), 6% (n = 11) chose £2, and 4% chose £1 (n = 7). The majority of respondents (76% of 167, n = 127) stated that they would prefer all participants to receive the same incentive, rather than a small incentive to take part and the chance to win a much larger prize (lottery incentives).
In conclusion, over 40% of the sample stated that incentives would not make a difference to their decision whether or not to participate in the study, and more than 80% of respondents would participate if asked by their doctor or nurse. This suggests that incentives for patients may not be an effective way to boost testing and recruitment. It should be noted, however, that this group included a high percentage of students still in full-time education, which may have biased the results, as they may not be representative of all 16- to 24-year-olds.
Practice staff experiences and views on barriers and attitudes to recruitment
Practice interviews
During phase 2 of the pilot, members of the trial team visited every practice in the pilot to establish the practice staff’s experiences and views on barriers and attitudes to recruitment. The practice staff found the trial materials and web tool easy to use. However, there appeared to be a number of myths held by the practice staff which affected their ability to test for chlamydia. Most stated that they saw very few young people in their practice, and it was generally believed that chlamydia was not prevalent in their area. There also appeared to be no systematic way of identifying potential participants within the practice and some staff were unclear about the best way to approach young people to test for chlamydia and enter the trial.
Staff also reported having no time to recruit patients, and communication difficulties in approaching patients to test for chlamydia when they were attending for a completely different health matter.
Treatment issues
How patients diagnosed with a STI received their treatment varied considerably by practice and proved to be an additional barrier to the trial. The practices were told that they needed to have a structure in place to administer the treatment, either managing this under a Patient Group Direction/Patient Specific Direction (PGD/PSD) or using their normal processes. The drugs required for treatment had to be obtained through a suitable supply chain. It was agreed that the practices should be referred to a guideline established by the study team and should manage this process themselves. Treatment was administered via the practice, through the NCSP or via prescription.
Testing in practice and lab processes
It was also highlighted during the practice interviews that there was confusion over the laboratory processes. All practices were part of the NCSP and in the majority of practices’ results were previously being sent directly to the local CSO rather than coming back to the practice. However, during the time of the trial all test results were required to come back to the practice in order for patients diagnosed with a STI to be managed under the trial. As many practices were using the NCSP kits, we provided them with labels to place on the NCSP laboratory forms to redirect back to the practice, rather than on to the local CSO (the local CSO was made aware of this process). Test results requested on normal laboratory forms came back to the practice as normal. A system also had to be put in place for each practice to ensure that the research nurse received all the test results, since tests could be conducted by other members of the clinical team due to NCSP using a different laboratory from practice tests.
In some areas there was concern over who paid for the laboratory tests. We discovered that most PCTs had block contracts with laboratories covering a number of diagnostic tests (not just chlamydia). Some PCTs, however, used block contracts for NCSP tests or the CSOs negotiated separate contracts with the laboratories depending on their arrangements. This variation was an additional hurdle when setting up the practices.
Regional training nurse focus group
The research co-ordinator also ran a focus group with the General Practice Research Framework’s regional training nurses (RTNs) to discuss their views on the trial and recruitment. The key findings were that the RTNs also believed the myths (that people would not want to test and young people do not attend the practice). They were confused over the NCSP and how this could run in parallel with the trial. They also suggested asking young people what would encourage them to test.
Implications
Results of this focus group and the practice interviews suggested that the myths in practice needed to be addressed, that the NCSP involvement be clarified, that a systematic way to identify patients be established in each practice in the most effective and time-efficient way and that staff were clear on the best way to approach young people to screen and invite them into the trial.
Maintaining and enhancing ongoing recruitment
Recruitment report
This study had been funded on the assumption that a large number of chlamydia tests would be taken within general practice, in accordance with national data showing a large and growing proportion of all chlamydia tests taking place in this setting. However, the low number of tests we were experiencing was surprising. We therefore collaborated with the Health Protection Agency (HPA) to explore the distribution of chlamydia testing within practices in more detail, since these data were not publicly available.
The Chlamydia Vital Signs Indicator 2010/11 measures the proportion of the 15- to 24-year-old total population tested for chlamydia outside GUM clinics. The target set by the Department of Health was 35% for testing undertaken during the period 1 April 2010 to 31 March 2011. Data for monitoring the vital signs indicator are based on NCSP data returns and testing outside GUM not reported to the NCSP. In 2010–11, 25.2% of the population aged 15–24 years were tested for chlamydia (1,733,220 tests) based on data reported to the NCSP. 46
Data received from the NCSP reported 6319 participating NCSP practices across England, of which around one-third (n = 2895) registered no positive chlamydia test results between April and December 2010. The range of tests performed by these practices over this period was 1–176, with a mean of nine tests per practice. Only eight practices performed between 332 and 2336 tests, averaging 797, and obtained over 30 positive results.
This indicates that there were very few NCSP practices testing for chlamydia at the rate the trial required to conduct the planned study (Table 7). Positivity varied little by practice, and stood at 6% at the time of the study.
| Number of positive tests | Number of practices | Average number of tests (range) |
|---|---|---|
| 0 | 2895 | 9 (1–176) |
| 1–5 | 3128 | 38 (1–474) |
| 6–10 | 236 | 102 (18–200) |
| 11–20 | 43 | 191 (52–1118) |
| 21–30 | 9 | 360 (172–1175) |
| > 30 | 8 | 797 (332–2336) |
What are the general practice consultation rates of young people?
Table 8 reports data from a QRESEARCH® (University of Nottingham, Nottingham, UK) report showing the patient consultation rates with GPs and nurses of 15- to 19-year-olds and 20- to 24-year-olds over the period 1998–2009 in England. 47
| Incidence of consultations in general practice per registered person per year (2008/9) | ||
|---|---|---|
| Gender | Age (years) | Incidence |
| Male | 15–19 | 2.2 |
| 20–24 | 2.2 | |
| Female | 15–19 | 4.2 |
| 20–24 | 5.6 | |
Although measures of dispersion are not given in this report, the data indicate that a substantial majority of people within the 15–24 years age group are visiting their registered practice for a GP or nurse appointment at least once per year.
Summary of findings relevant to improving testing rates and recruitment through modification of our processes
Despite a wide range of innovative approaches in the NCSP, including incentives both for young people and for staff, we had identified little robust evaluation that could reliably inform our strategies to improve recruitment.
However, recently emerging peer-reviewed literature suggested that addressing practice organisation and culture could be useful. A team approach to recruitment, along with an identified ‘champion’ within a practice, appeared to be important, and a good understanding across all staff of the benefits of chlamydia testing appeared to be key. Newsletters and feedback on practice-level performance were also seen to be helpful and relevant to our study.
Embarrassment of practice staff about offering a chlamydia test in an unrelated consultation was consistently reported to be a challenge, and emerged in our practice interviews as a barrier. The peer-reviewed literature emphasised the need to normalise the offer of testing, an approach which was likely to be best addressed through training and a whole-practice approach. Published research reported that widespread advertising of chlamydia testing as a standard offer within the practice through posters and leaflets was widely used in practices with high testing rates. It also suggested that a highly organised and strategic approach using computer prompts and similar aides-memoires was necessary to identify young people. We concluded that it would be helpful to support practices in developing focused plans aimed at approaching, offering tests to and recruiting young people to the study (the latter particularly in ‘consent at test’ practices). We also planned to use newsletters and feedback on testing rates more proactively as a way of improving engagement.
The peer-reviewed literature (notably from the CLaSS study) suggested that mailed offers of chlamydia testing kits could yield uptake of up to a third of young people. However, in studies using this approach, the patient’s first encounter with the general practice was at the time of treatment. Our approach of inviting young people by letter, and additionally asking the practice to undertake opportunistic testing, had not yielded useful numbers of people attending the practice in order to have a test. Moreover, it seemed to have encouraged practices to ‘take their eye off the ball’ in taking advantage of young people’s attendance as an opportunity to test. Our commission was to evaluate different approaches to PN within primary care (taken to mean general practice for the purposes of this study), and we also noted that the current direction of Department of Health policy was to encourage chlamydia testing within general practice, as the major complement to sexual health settings. We therefore concluded that in order to focus the intervention on primary care we needed to focus wholly on the opportunistic approach which had been successful in the initial English pilots of chlamydia testing. 25,48
Our practice interviews were consonant with the peer-reviewed literature on barriers to testing. However, we identified three myths which emerged in many interviews and which were discouraging primary care staff from enthusiastic or strategic approaches to opportunistic testing. First, there was a widespread belief that very few young people attended the practice; second, it was generally thought that, even if common nationally, chlamydia was not common in their area; and, finally, many staff believed that young people would be unwilling to test in a practice setting. These findings suggested that we could use high-quality data from a number of domains to address these beliefs. The high-quality data on attendance rates in general practice that we consulted suggest that attendance – particularly of young men – is much higher than generally thought, while epidemiological data show that variation in chlamydia prevalence by geography is much lower than in many other STIs. Previous studies show high levels of acceptability for testing despite the initial concerns of staff. We concluded that active information campaigns could be used to counter these myths within participating practices.
The question of incentivisation was complex and challenging. Our opportunities for personal incentivisation of practice staff were limited by the internal structures of each practice, regulations on use of our research funding, and NHS research ethics approvals (including their likely view of any proposal to incentivise staff either for testing or for recruitment). This is likely to explain the paucity of evaluations of staff incentivisation. We did identify one NCSP location where vouchers had been offered to staff, but the evaluation was not sufficiently robust to ascribe effect to cause. On balance, we felt we were not in a position to offer direct staff recruitment. Nevertheless, we were able to pay NHS service support costs that fully covered research-related staff costs (including initial testing), and it was not clear to us how or whether the information on this practice-level incentive was being used. This had potential for improving practice engagement, through a ‘top-down’ approach, and we concluded that information on potential income ‘lost’ should be reported to practices in order to encourage effective planning for chlamydia testing.
The question of whether or not young people should be incentivised was very interesting. At this time, many NCSP locations were using incentives ranging from key rings and boxer shorts to prize draws for (for example) iPads. However, there was little evidence of evaluation and no evidence on which to choose between the range of incentives on offer. The marketing expert we consulted was insistent that monetary or voucher incentives would be necessary for recruitment of young people. However, we remained concerned as a research group to maintain the distinction between retention in research (really required only after a positive diagnosis) and the testing situation in which an intervention was being offered in a situation of relative trust for the benefit of the young person’s own health. Interestingly, our survey of young people suggested that such interventions offered by a health-care professional were not much more likely to be taken up if an incentive were offered. This may not apply where an offer is made in non-health-care settings (e.g. peer-led sessions in schools or universities), where no relationship of trust is in play. Based on this, and the finding above that practice staff themselves had a number of beliefs and attitudes that made them reluctant to offer tests, we concluded that incentivisation of young people was unlikely to produce any important increase in testing rates.
Finally, there was clearly great variability in the processes of treatment and testing. The NCSP had, by and large, set up systems in which local chlamydia co-ordinators, generally employed by the PCT, undertook to inform young people of their results, treat positives and organise PN. This was different from other tests for infection undertaken by the practice, and also from the proportion of chlamydia tests which were taken from people who were not eligible for the NCSP on grounds of age or for whom the ‘wrong’ (i.e. non-NCSP) form was used. We concluded that it would be necessary to have a customised pathway to address this within each practice.
Phase 2 recruitment rates
During the second phase of the pilot (March 2011 to June 2011) testing increased by 100% (from 31 to 62 tests). However, this accounted for only 11% of the 16- to 24-year-olds attending the practices over that period (Table 9).
| Recruitment data | Phase 1 | Phase 2 |
|---|---|---|
| Dates (inclusive) | November 2010–February 2011 | March 2011–June 2011 |
| Total number of chlamydia testsa | 31 | 62 |
| Number of positive chlamydia tests | 1 | 3 |
| Total number of active practicesb | 6 | 5 |
| Phase 1 practices activeb | 6 | 5 |
| Phase 3 practices activeb | – | – |
| Phase 4 practices activeb | – | – |
Chapter 5 Phase 3: implementing improvements identified in phase 2
Introduction
The overall aims of phase 3 were to implement the processes developed in phase 2 in two new pilot practices and continue to recruit in and monitor existing practices.
The objectives for phase 3 were:
-
to continue to develop recruitment in and monitor phase 1 pilot practices based on the findings of phase 2
-
to recruit two new practices and induct them using the findings from phase 2.
Methods
Based on the findings of phase 2, we identified and implemented within our existing practices the following specific changes.
Practice champion
A member of staff in the practice was identified as the practice champion to motivate and be a point of contact for other practice staff. Practices were encouraged to have champions for each staff group (e.g. nurse champion, GP champion).
Additional trial materials
We provided additional trial materials for the practice team. Information leaflets were designed specifically for the GPs and primary care team. They included information about the trial and benefits to the practice and challenged some of the myths we found within general practice (i.e. that they do not see many people of the target age group, that young people do not want to be tested for chlamydia in their general practice surgery and that their practice population do not get chlamydia). We previously used the NCSP posters in practices to encourage young people to test; however, we introduced trial posters designed to be eye-catching for the practice waiting and consulting rooms. The poster invited 16- to 24-year-olds to take part in the study and to ask a GP or nurse about the Spread the Word health study.
Development and implementation of practice ‘action plans’
Practice action plans were developed and tailored to each practice. Specific objectives were identified in consultation with each practice to help improve recruitment. The action plans covered objectives, actions required, implementation dates and means of verification. Practice action plans were finalised after additional training (if required) and practice visit. Objectives within each action plan included:
-
establish a visible practice champion(s)
-
encourage team building: ensure the trial is and remains a high priority in the practice; ensure feedback to the whole practice on progress; ensure that enough trial materials are kept in consulting rooms; answer the queries of other members of staff about the trial; motivate and encourage staff (which could include using practice targets)
-
display trial materials prominently and make them easy for young people to see and pick up
-
highlight all patients aged 16–24 years coming in for appointments and alert the relevant staff to offer a test during the appointment (this could include computer alerts on the practice system or a note at time of booking)
-
reinforce that all chlamydia testing was to be carried out by the practice and not through the NCSP process, to avoid confusion (confirming the laboratory process to be used)
-
have practice champion remind all clinic staff weekly to give out trial information and ask all 16- to 24-year-olds to take a test and refer to research nurse for consenting (and considering the most effective way to do this, e.g. e-mails, face to face, practice meetings)
-
use a banner at self check-in screen to invite all 16- to 24-year-olds to take part in the trial
-
upload information about the trial on the practice website
-
communicate earnings per patient to the practice staff (practice champion to cascade to colleagues)
-
give monthly feedback on performance indicators and discuss any barriers the practice staff may have (research nurse to ensure that information is fed back)
-
educate the wider team and engage all practice staff about the trial (practices were offered a visit by a member of the trial team to present to the practice staff, slides were also provided if the practice champion wanted to inform staff at a practice meeting, the general practice surgery/primary care information leaflet was distributed to practice staff)
-
have reception give out test kits (or kits including trial material placed on counter to return to reception or nurse/GP)
-
have receptionists hand out patient information leaflets and trial material
-
have practice staff use scripts to help explain the trial (to be used at their own discretion)
-
include a chlamydia test on registration to new patients aged 16–24 years.
Development of educational materials for practice staff
Scripts were prepared for each staff type to help explain the trial. Additional information, sourced from NCSP, was also provided on how to talk to young people, young people’s attitudes towards sex and relationships, and normalising testing.
Feedback of testing rates and footfall
Feedback was provided to practices on testing rates and footfall (i.e. the number of people in the target age group attending that practice). We collated individual pilot practice footfall and testing data, which demonstrated to each practice that a large number of young people were attending the practices taking part in the pilot, but only a small number were being tested (data on footfall and positivity rates were reported in the primary care team information sheet).
Practice meetings
The trial team offered to talk to the practice as a whole about the trial to dispel the myths around chlamydia testing and discuss specific practice barriers. Two of the practices requested a meeting.
Feedback on payment structure
Payment details of all screening and trial recruitment activity to date were sent to the practice manager, nurses and lead GPs in the five currently active pilot practices, excluding one other practice that dropped out of the study as a result of staffing issues. Practices’ letters included what they could have earned if they had reached their target. This was based on their estimated attendance (i.e. if screening 20% of the attendees had been achieved, this is what you could have earned). They were given information on how many participants the practice had recruited in the previous month and how many could have been recruited, based on their attendance figures. The difference in actual earnings and potential earnings through service support costs was considerable in some cases (e.g. actual £445.95 compared with potential £16,243).
Additional training of staff
Additional nurses were trained in one practice to help boost recruitment by increasing the number of nurses who could obtain consent.
Newsletter
A newsletter was sent to all participating practices in August 2011 showing that testing had increased but the proportion testing positive was low. The newsletter stated this was to be expected in general practice, as we know practices would (on average) have to test 20 people to get one positive test (there had been concern by some practices that they were not seeing many patients diagnosed with a STI). The newsletter encouraged practices to continue to screen all 16- to 24-year-olds opportunistically. It also highlighted that data show that the positivity rate is highest in slightly older men, 20- to 24-year-old males (8.6%),27 and that national figures show an average of two to three general practice visits per year from this age group. 49 We also presented the 20% footfall targets.
Recruitment of new practices
We recruited two new practices and inducted them using the processes identified in phase 2. The focus was on testing people opportunistically and recruiting patients who presented with a symptomatic STI – letter invitations were not to be sent. Of the two new practices, one provided level 2 (intermediate) sexual health service provision and a top recruiting NCSP site, the other a NCSP site with very little chlamydia testing in practice.
It was decided that new practices would be randomised only into the ‘patient’ or ‘provider’ referral arms, following the decision to drop ‘contract’ referral as a third arm (see Chapter 9).
Retention of phase 1 practices
Over the period July 2011 to January 2012, phase 3 of the pilot, testing increased; however, three practices dropped out of the trial. One was a CAD practice and was receiving very few positive results. It reported being disheartened by the results and finding the laboratory processes confusing and, therefore, it did not want to continue. Another practice had only sent out the initial invitation letters and had not attempted to recruit anyone opportunistically. This practice was undergoing a practice move at the time, so felt unable to continue with the trial. In the third practice the nurse did not want the support of other practice staff to test and recruit, which made the recruitment of patients untenable. Additionally, a nurse in another practice went on maternity leave during phase 3, which led to a break in recruitment whilst a new nurse was trained.
Phase 3 recruitment rates
Table 10 sets out recruitment data for phase 3, with previous phases also presented for comparison. By the end of phase 3 the percentage of those attending general practice surgeries who were tested had increased (to 12%), but it did not reach the target of 20%.
| Recruitment data | Phase 1 | Phase 2 | Phase 3 |
|---|---|---|---|
| Dates (inclusive) | November 2010–February 2011 | March 2011–June 2011 | July 2011–January 2012 |
| Total number of chlamydia testsa | 31 | 62 | 120 |
| Number of positive chlamydia tests | 1 | 3 | 7 |
| Total number of active practicesb | 6 | 5 | 6 |
| Phase 1 practices activeb | 6 | 5 | 4 |
| Phase 3 practices activeb | – | – | 2 |
| Phase 4 practices activeb | – | – | – |
The practice action plans allowed practices to develop a systematic way of identifying potential participants and recruiting them into the trial. However, the feedback to practices on potential earnings suggested that the use of monetary incentives had little impact on the practices. The aim of the newsletter was to motivate staff, particularly disheartened staff who had seen few or no patients diagnosed with a STI. Whilst appreciated by staff, this also appeared to have no effect on testing rates. The sharp decline in the testing rates after 3 months may have been due to practice fatigue; however, one of the new practices recruited in phase 3 reported that they were beginning to see the same individuals coming through the practice and they believed they had already approached most people within that age group.
Chapter 6 Phase 4: intensive recruitment by external researchers
Introduction
As reported above, despite additional evidence-based interventions, we had not been able to deliver acceptable opportunistic testing rates needed to underpin this PN trial either in initially recruited practices or in the phase 3 newly recruited practices.
Following advice from our Trial Steering Committee, we considered an alternative approach currently being used in the pilot phase of the ACCEPt primary care chlamydia screening study currently taking place in Australia.
Methods
We had a teleconference with investigators from this study, including a member of our Trial Steering Committee. The ACCEPt team was undertaking a prevalence study in Australian general practice, prior to the implementation of a randomised trial of chlamydia screening based on primary care. The ACCEPt team advised us that for this prevalence study they were using a system in which researchers external to the general practice surgery (although occasionally an in-practice nurse) would undertake a 2- to 3-week period of intensive recruitment. This would involve approaching every young person in the waiting room and seeking their consent for chlamydia testing. The team had found that beyond 2–3 weeks practices found the presence of a recruiter burdensome, and that the proportion of reattenders rose, causing concerns about repeated approaches. Australian colleagues had also experienced similar recruitment issues for chlamydia testing in primary care. Australian practice staff believed they did not see many young people, yet data showed that young people do attend general practice surgery at least once a year. They also reported similar barriers to testing (e.g. not enough time, discomfort about raising sexual health testing) (Professor J Hocking, Melbourne School of Population and Global Health, 2011, personal communication).
A review of the literature found that this approach had been successful in a UK study undertaken by Grun et al. 50 in a London general practice surgery prior to the implementation of the NCSP. There were also informal reports of similar approaches within the NCSP in England [NCSP regional facilitators (RFs), 2011, personal communication].
With the support of the Trial Steering Committee and our funders we moved to phase 4 of the trial, the final phase, using external researchers for an intensive recruitment period in each practice. It was expected that this would potentially boost recruitment into the trial. We sought to assess the feasibility and effectiveness of this approach, which has implications for the feasibility in general of intervention studies targeting young people attending general practice.
The overall aim for phase 4 of the trial was to recruit patients using a trained external researcher for a brief intensive recruitment period in each practice.
The objectives for phase 4 of the trial were:
-
to recruit eight new practices and retain two already involved for this phase
-
to assemble and manage a team of external researchers
-
to set up practices for intensive recruitment
-
to boost recruitment into the trial.
Practice selection and recruitment
Ten practices were involved in the intensive recruitment phase. Two practices from the previous pilot phase agreed to participate in the intensive recruitment phase: one from Yorkshire and Humberside (a new practice from phase 3) and one from the South East (an original pilot practice from phase 1). Eight additional new practices were recruited: four from the South East, one from Yorkshire and Humberside, two from London and one from the South West.
We sought to identify practices capable of large screening numbers that were capable of hosting research, using the sample frame of the NCSP data set on number of tests and positives by practice and supported by PCRN data on practice characteristics and research capability. In selecting practices, we prioritised the following desirable characteristics:
-
capacity to conduct research (Are they able to conduct research? Do they have the space personnel and time?)
-
number of potential NCSP cases on the basis of the number of positive tests per year from NCSP data set
-
number of 16- to 24-year-olds seen in the practice over the last 6 months (estimated footfall based on practice size and age composition)
-
research experience of the practice (number of years, number of studies, types of studies)
-
specific sexual health sessions run in the practice including contraception (if ‘yes’, details of experience) which are associated with concentration of large numbers of young people in a single session and which can be targeted for recruitment
-
level of enthusiasm of practice (as reported by PCRN or trial team)
-
adoption of a practice team-based approach
-
number of GPs/nurses/health-care workers able to consent.
Practices were approached by the trial team or a member of the PCRN. Each practice was asked to complete an eligibility criteria form. If the initial contact was from the PCRN, the trial team contacted the practice following an expression of interest if they fitted at least two or more of the eligibility criteria. The trial team was looking for a mixture of practices (practices seeing a high number of patients diagnosed with a STI and those with high enthusiasm for the trial and a whole-team approach). Once selected, the practice was asked to complete a pre-trial questionnaire.
An additional practice was also recruited at its request because of the keen interest of the practice GP, who was previously a national NCSP GP champion (the GP champion’s role was to provide peer support to other local GPs and motivate practices to take on and offer chlamydia screening to their patients). The GP in this practice wished to do all the consenting personally and requested that it be a CAD practice.
Overview of intensive recruitment processes within practices
Figure 6 summarises the processes of recruitment within practices. In order to achieve these, we needed to develop operational guidelines and training procedures, recruit and train a cohort of external researchers, and ensure that all governance requirements were met prior to commencing recruitment. These processes are also described below.
FIGURE 6.
Intensive recruitment flow chart. CT, chlamydia testing; RA, research assistant; RN, research nurse.

Recruitment and training of external researchers
We engaged a total of five researchers. Two were members of the trial team and had non-clinical research backgrounds, and three were employed on sessional contracts. One of these researchers was from a general nursing background and unfamiliar with research, while two were from a community sexual health background and experienced in research and working with young people. In addition, a PCRN chose to provide (unremunerated) recruitment in one of its practices, and this was done by three individuals.
A researcher was placed in-house (at the practice) for a maximum of 3 weeks. In the case of two practices the researcher was required to stay in accommodation near the site to ensure that clinics would be covered in full. An additional three practices required 2–4.45 hours’ travel each day.
Research passports and Criminal Records Bureau checks were undertaken as necessary. These were required for most individuals.
The research team conducted training sessions for the researchers. Box 2 shows the content covered in these sessions. Researchers also undertook a role-play session where the researcher acted out approaching a patient in the waiting room using different scenarios. Researchers were also given a training manual which included the topics covered on the training day.
An introduction to the trial (explanation of chlamydia and the importance of timely treatment of the index and their partners).
Review of pilot to date (all phases).
Explanation of PN.
Summary of PN interventions and outcome assessment.
Web-based data collection tool (access levels and security).
Outcome measures.
Consent process.
Reason for intensive recruitment.
The optimum recruitment process.
The role of the external researcher.
The role of the practice team.
Chlamydia testing facts for the area.
How the researcher could help in the practice.
Potential barriers and how to deal with them.
When recruiting in practices, the external researcher wore a T-shirt bearing the word ‘researcher’ and their first name on the front; this made it easy for patients to identify them. The trained researcher was situated in the waiting room (in some cases moving around waiting rooms, depending on the number of waiting areas) and gained consent in a private area. The researcher could use the trial posters or message screen as an aid when approaching patients. The researcher asked the patients if they had seen the posters and explained that the practice was taking part in an important research study and that all young people between the ages of 16 and 24 years were being asked to take part, minimising any concerns that they were being chosen specifically to take part.
The aim was to recruit with minimum disruption to the practice and achieve high participation. Once the researcher had made initial contact in the waiting room, the patient was taken into a private room to check eligibility and go through the consent process. The process could be interrupted if the GP or nurse was ready to see the patient. The researcher reassured the patient that they would not miss their appointment and that they could complete the consenting process after their appointment if they were called in. When gaining consent from patients the researcher took the patient through the patient information leaflet, taking them through each point and highlighting why the trial was important (i.e. to find the best way of providing PN, that the NHS recommends a yearly test and a test after a change of partner). Once consented, the patient went to the toilet to collect a sample and returned it to the researcher (or reception/drop box/GP/nurse if the researcher was busy with another patient).
When gaining consent from patients the researcher took the patient through the patient information leaflet, taking them through each point and highlighting why the trial was important (i.e. to find the best way of providing PN, that the NHS recommends a yearly test and a test after a change of partner). All chlamydia tests were documented on a chlamydia test list to be uploaded onto the web tool by a trained practice member of staff (normally at the end of each day) (see Figure 6).
For patients using self check-in it was recommended that patient information leaflets be highly visible by the check-in screen. This message on the check-in screen duplicated the message on the posters asking all 16- to 24-year-olds to pick up a leaflet and help with the study.
Each researcher was required to complete a tracker form, to track the recruitment of patients. Data included individuals approached, age, gender, consent, if the study was mentioned by clinical staff, whether consent took place before or after the appointment (or both), if the patient refused to take part and the reason for refusal.
Practice set-up and training
Each practice was set up by a member of the trial team and the external researcher. The trial team member was present for the first 1 or 2 days of the trial. The lead GP, research nurse and practice manager were required to liaise directly with the researcher and trial team member to establish the most effective way of identifying and approaching 16- to 24-year-old patients whilst in the practice waiting room and how best to manage the chlamydia testing and laboratory process within the practice.
Any GPs or nurses who might take consent or follow up patients diagnosed with a STI were trained on site by the trial member. Where possible, the trial team also attended a practice meeting to highlight the trial to all practice staff.
Role of the practice staff in intensive recruitment
A revised information leaflet for the GP and primary care team was provided. This included a background to the trial, trial objectives, myths in practice, what practice participation would involve (including details of intensive recruitment and encouraging all staff to direct eligible patients to the researcher), benefits for the practice, ethics, feedback and trial team contact information.
All clinicians were instructed to ask their patients if they had been approached by the in-house researcher. If they had already been approached and consented, the clinician was asked to reinforce the importance of the study. If their patient had not yet been approached, clinicians were asked to mention the importance of the trial and, if agreeable, encourage the patient to take a chlamydia test. If the patient took a test at this point they were directed to contact the researcher directly after their appointment. If the researcher was not available they were asked to leave their completed test at reception or in a clearly marked deposit box along with a signed reason-for-test form. The researcher was then able to obtain consent over the telephone. Once the result was sent back to the surgery, the research nurse or GP dealt with the patient’s treatment and referral to the HA.
Results in intensive recruitment practices during phase 4
A total of 1444 potential participants were identified through the intensive recruitment phase (Figure 7) Of these, 88 were not approached; some because the researcher recalled they had been approached previously (30.7%), 15.9% were reported to be too ill or with someone too ill and 11.4% were not approached as they were with children (Table 11).
FIGURE 7.
Intensive recruitment in 10 practices (data recorded by intensive recruitment researchers). a, Two missing data.

| Reason not approached | n | % |
|---|---|---|
| Already approached | 27 | 30.7 |
| Too ill/with someone too ill | 14 | 15.9 |
| With child(ren) | 10 | 11.4 |
| With parent | 6 | 6.8 |
| Mobility problems | 6 | 6.8 |
| Already in study | 6 | 6.8 |
| Misjudged age | 5 | 5.7 |
| Missed patient/did not show up | 5 | 5.7 |
| On mobile phone | 3 | 3.4 |
| Pregnant | 2 | 2.3 |
| Deaf | 1 | 1.1 |
| Not sexually active (informed by another patient) | 1 | 1.1 |
| Patient travelling | 1 | 1.1 |
| Advised not to approach | 1 | 1.1 |
Researchers did not approach some patients accompanied by their parents because they considered it inappropriate to do so. For example, some patients accompanied by their parents would not make eye contact with the researcher or appeared uncomfortable in the waiting room. There was no difference between gender and approach by researcher [93.4% (806/863) of females were approached compared with 92.6% (339/366) males, χ2 = 0.241; p = 0.62].
In total, 1356 patients were approached, of whom 209 (15%) were outside the relevant age group. Of the remaining patients, 492 were tested and consented (43%) and 653 refused to participate (57%) (missing data n = 2).
The largest proportion of patients who refused to participate (36.6%) did so because they had already been tested elsewhere, while a smaller proportion of patients said that they did not want to participate (21.6%). Other reasons specified included not being sexually active, no time, too unwell, did not need a test, parent refused, they were already in a trial and they had recently passed urine (Table 12).
| Refusal reason | n | % |
|---|---|---|
| Tested elsewhere | 239 | 36.6 |
| Did not want to do | 140 | 21.6 |
| Not sexually active | 46 | 7.0 |
| No time/too busy | 46 | 7.0 |
| Too stressed/upset/unwell/not practical | 27 | 3.9 |
| Called into appointment (and did not return) | 21 | 3.2 |
| Feels did not need to test | 20 | 3.1 |
| Othera | 98 | 15 |
| Not known | 16 | 2.5 |
There was no difference in recruitment rates by the type of researcher recruiting. Externally employed researchers recruited 42.9% (237/553) of those approached, the trial team recruited 44.6% (150/336) and the PCRN team recruited 41.0% (105/256) (PCRN staff recruited in one practice based in Yorkshire and Humberside). Recruitment was more successful when the trial had been mentioned to the patient by a member of the practice staff (Table 13), with 68% (98/144) of patients consenting when practice staff mentioned the trial compared with 39% (394/1001) consenting when they did not. There was no difference in consent rate by gender [41.7% (335/804) of females consented compared with 46.3% (157/339) of males, χ2 = 2.100; p = 0.15].
| Staff type | % consented |
|---|---|
| GP | 66.7 (28/42) |
| HCA | 81.8 (9/11) |
| Nurse | 66.3 (59/89) |
| Reception | 100 (2/2) |
| Other | 30.8 (4/13) |
| No mention | 75 (3/4) |
| Not knowna | 39.5 (371/940) |
| Not applicableb | 36.4 (16/44) |
Summary of all recruitment during phase 4
Table 14 summarises all recruitment during phase 4, with data from previous phases for comparison.
| Item | Phase 1 | Phase 2 | Phase 3 | Phase 4 | Total |
|---|---|---|---|---|---|
| Dates (inclusive) | November 2010–February 2011 | March 2011–June 2011 | July 2011–January 2012 | February 2012–August 2012 | November 2010–August 2012 |
| Total number of chlamydia testsa | 31 | 62 | 120 | 570 | 783 |
| Number of positive chlamydia tests | 1 | 3 | 7 | 14 | 25 |
| Total number of active practicesb | 6 | 5 | 6 | 11 | 28 |
| Phase 1 practices activeb | 6 | 5 | 4 | 1 | – |
| Phase 3 practices activeb | – | – | 2 | 1 | – |
| Phase 4 practices activeb | – | – | – | 9 | – |
Chapter 7 The National Chlamydia Screening Programme as a research infrastructure: implications for research into chlamydia control
Introduction
This research was commissioned in response to a need for evidence on best management of individuals diagnosed with a STI or HIV infection as diagnosis and management extended further into non-specialist settings, including primary care. A partnership with the NCSP was seen as crucial to providing ‘real-world’ evidence of relevance to the NHS. The specific characteristics of the NCSP at the time of this research had important implications for the conduct of our research, which we present.
This chapter addresses the relationship between the structure and processes of the NCSP during the period of our research, during which they were already undergoing some evolution. It should be noted that this model has changed very considerably since then. These changes are described at the end of this chapter and their implications for future research addressed.
The policy landscape and the structures of the National Chlamydia Screening Programme
The NCSP was implemented at a time when localism was a political priority for the NHS. There was an emphasis on devolution of decision-making to the local level; priorities and accountability were managed at PCT level with performance managed by strategic health authorities. 51 The consequent cost and manpower implications of localism for the NCSP were explored in a 2009 National Audit Office report52 which describes the degree of variation and resulting challenges for achieving economies of scale.
The NCSP did, however, have national structures for oversight. At the time of the study, the former HPA, now part of Public Health England (PHE), was responsible for the national co-ordination and monitoring of the programme through a small central NCSP team based at the Centre for Infections. Local screening offices submitted core data electronically to this central team on a quarterly basis.
Primary care trusts, which commissioned services, had managerial control of chlamydia screening locally, while the HPA’s role was to provide standards, guidance and support. National targets for chlamydia coverage encouraged an emphasis on increasing the number of tests, regardless of positivity.
The Local and Regional Service of the HPA hosted a network of RFs. Their remit was to communicate the national strategy, support local programmes in achieving screening targets, and assist in commissioning laboratory contracts. The National Chlamydia Screening Steering Group provided external scientific and technical advice to the Department of Health and produced a set of mandatory guidelines on structure, process and outcome monitoring for local programmes. These were contained in the core requirements. 52
Multidisciplinary local chlamydia screening steering groups at PCT level were responsible for the local strategy, adapting screening priorities to reflect local need and overseeing implementation of their programme. Local programmes developed various aspects of screening strategies and processes. These included testing, treatment and PN processes, priority of locations for testing, types of testing kits used, laboratory contracts, laboratory processes and management of results, and data reporting to the NCSP. Programmes also developed their own materials to complement screening resources available nationally. Local administrative structures varied but usually included a CSO and co-ordinator, programme lead and clinical staffing, in partnership with PCTs, local laboratories and health-care providers. 6 These CSOs were generally self-contained services dedicated to the processing of NCSP test results and the management of patients diagnosed with chlamydia.
The interface between the NCSP and local CSOs at the time of our pilot is shown in Figure 8, reproduced from the NCSP Core Requirements, fifth edition. 53 [This figure no longer represents the current NCSP structure, which is set out in the NCSP Standards (sixth edition).]26
FIGURE 8.
National Chlamydia Study Programme (NCSP) structure. Reproduced with permission from NCSP Core Requirements, fifth edition, 2010. 53

It is important to note that CSOs’ role in communicating results to patients and managing those diagnosed was an anomaly in NHS services. It was modelled on structures used in the 2000 pilots of opportunistic chlamydia screening. 25,48 With few exceptions, when a NHS service provides a test for a patient (e.g. a full blood count, radiography or a HIV test) that service is responsible for providing a result to the patient and – at least initially – managing further care. The NCSP was unusual in that, with a small number of exceptions, results of chlamydia tests in the target age group were processed by CSOs.
This had three important consequences for clinical care from the perspective of general practice. First, clinical responsibility for following through the result of a test was different from usual in the case of NCSP chlamydia tests. Second, no matter whether they took few or many NCSP tests, practices were likely to perceive that they had very few chlamydia cases, simply because the ones they did diagnose were managed elsewhere and the practice was not informed of this. Third, practices were meant to use different testing forms and treatment strategies for individuals tested for chlamydia within the NCSP from those tested outside the screening programme. The latter could include people outside the age range, or those tested for clinical reasons (such as pelvic inflammatory disease) who also had other tests taken.
Discussions revealed that some CSOs appeared to have an impression that general practice was not competent to manage chlamydia. They also sometimes experienced a potential conflict of interest with any study focusing on chlamydia interventions within general practice, in that their role could disappear if chlamydia patients were to be treated and provided with PN services via general practice without the need for a CSO. For example, at one meeting of the researchers with a local CSO, together with its local screening group, the following concerns were raised about the study:
-
The study was likely to have an adverse impact on NCSP data, care pathway and on quality outcomes (e.g. time to treat partners).
-
The quality of care that could be provided within general practice might be suboptimal, with variation in the provision of care within a locality if GPs treat with no NCSP control over this.
-
Objections were raised to randomisation by PN modality.
-
Some participants were concerned that it would be unacceptable to patients for their chlamydia test results to be recorded on their general practice surgery record, and felt that individual informed consent should be sought for this (despite NCSP guidance confirming that this can be appropriate on a routine basis).
-
Concerns were expressed about the competence of staff in general practice surgery to manage chlamydia patients, to implement child protection for under-fifteens, or to prescribe appropriately.
The breadth of these concerns is interesting, given the fact that general practices already undertake a significant amount of sexual health work, particularly contraception, and are experienced in a wide range of child protection issues.
The wider structures of the NCSP presented challenges for the setup of this research programme, which we explore in this chapter.
Engagement with national and regional National Chlamydia Screening Programme leadership
The trial was intended to take advantage of the chlamydia screening undertaken in primary care by the NCSP to optimise screening rates and recruitment to the trial. The director of the NCSP was, before her departure, a co-investigator, with a view to support in recruiting practices, avoiding conflicting priorities and generally facilitating collaboration with the NCSP.
An essential element of our relationship with NCSP leadership was the recognition of mutual benefit to both parties: we would have access to individuals screening through the NCSP while NSCP would gain from an increase in testing due to enhanced chlamydia screening strategies in participating practices adopted by the trial team.
Together we developed a communications strategy which utilised the network of NCSP RFs to cascade information to the local CSOs around the country. We provided briefing materials and frequently asked questions for this purpose. Our communications plan included placing information and regular updates on the NCSP website with links to our own website, and preparing information for GP champions within NCSP, which would be cascaded via the NCSP GP network.
We briefed the national team of RFs in their June 2010 monthly meeting, and concerns about data reporting and the provision of patient care were addressed by the trial team. The director of the NCSP reinforced the programme’s support of the trial, the mutual benefit and the key role of the RF in communicating the objectives and importance of the trial to local CSOs. We also attempted to engage with the network of GP champions via the national GP lead, until this network was disbanded in late 2010, and liaised with the NCSP communications team.
As a trial team we were reliant on RFs to communicate and promote our trial to the local CSOs in their regional areas. The success of communication using this route was variable. The trial team contacted the local CSO which covered any practice being considered for recruitment at any time in the planning and implementation of the trial. On occasions when we approached local CSOs they did not recall being briefed and were concerned that they did not know anything about the trial.
Local CSOs were particularly concerned about reporting of tests and the accuracy of testing data for their areas. This was because they were performance managed on the number of tests, which contributed to coverage targets, as well as various quality aspects of the care provided. We stressed through all our communications with practices that the trial was not part of the NCSP but that we were reporting the screening data back to the NCSP during the trial. In an attempt to reinforce our close relationship with the NCSP we had initially used NCSP posters to promote screening. However, this may have helped to foster a general misunderstanding across practices, identifying us with the NCSP. To avoid further confusion, separate trial posters were designed and all NCSP materials were removed from the practice during the trial period.
Estimates of National Chlamydia Screening Programme and other chlamydia testing in general practice
At the time of the study, 16.1% of all chlamydia tests were reported to be taken in general practice. 54 Moreover, NCSP tests were understood to be complemented by additional ‘non-NCSP, non-GUM’ tests, which had been shown to contribute a large number of additional tests based on analysis of disaggregated laboratory data sets. These non-NCSP, non-GUM tests were chlamydia tests in the target age group that were not routed through NCSP. This might be because the practice was not registered with the NCSP, or a non-NCSP form was used for any reason. It was believed that these tests could add up to an extra 50% to the number of NCSP tests in general, and of course these patients would be managed through practices (the model proposed in this study) and not by CSOs. However, these data were not available at practice level to the central NCSP team.
Once we commenced the pilot and experienced unexpectedly low testing rates, additional data and surveillance expertise was recruited onto the Trial Steering Committee. The results of these analyses are discussed in Chapter 4.
National Chlamydia Screening Programme data sharing
In order that patients could be managed by the practice in accordance with the trial protocol, results needed to be returned directly to the practice instead of the local CSO. To ensure that there would be no negative impact on NCSP coverage targets, it was essential that all tests would be counted towards the local coverage tally. We therefore arranged for all testing and PN data to be reported directly to the NCSP using our custom-built web tool. The web tool specification was developed in close collaboration with the NCSP so that the data we collected fulfilled NCSP reporting requirements and were compatible with NCSP data sets. Because of the anonymity of testing data on the trial web tool, these data were reported back as aggregate data via the non-NCSP, non-GUM data set.
Impact of National Chlamydia Screening Programme operating outside normal practice
As noted above, it is likely that the management of screening, diagnosis and treatment of patients by the CSOs has encouraged in practices the impression that they do not see much chlamydial infection.
This suggestion was reinforced by an exception to the rule, in the form of a practice which took part in phase 4. This practice, which was recruited in the intensive recruitment phase because of its enthusiasm and high screening rates, was aware of the prevalence of chlamydia at the practice. This can be explained by the different processes already adopted by this practice. All test results for patients were received directly from the laboratory. The treatment of its patients was managed in-house unless there was a clinical requirement to attend the GUM clinic for further tests or the patient desired to be treated elsewhere. This process had been negotiated by a senior partner who had been involved in the setting up of chlamydia services in the locality and was influential in shaping the service to ensure that the practice requirement was able to monitor its own chlamydia incidence.
It may be that the operation of the NSCP outside primary care services and usual NHS pathways of care demotivated GPs and therefore lowered testing rates, alongside the widespread availability of chlamydia testing in other settings, including educational settings and family planning services.
Operational issues arising from localism
Engagement of practices with chlamydia screening office
The engagement of practices taking part in our trial with their local CSO varied greatly. One practice did not know that it was registered as participating in the chlamydia screening programme, while others did not know who their local co-ordinator was, and sometimes there had been no engagement prior to or during the trial. By contrast, in another case the local CSO was already closely engaged with the practice and attended the practice meeting when the proposed trial was presented to the wider primary care team. In this practice the programme manager and the screening promotions manager were very supportive, offering to manage testing and reporting processes differently to ensure that the results were directed back to the practice where the patient would be managed. In addition, they shared the results of a marketing strategy they had adopted locally to encourage testing.
Variation in process
Screening processes
Local CSO processes were not standardised. In each locality NCSP laboratory forms were custom printed and collected the same information for mandatory reporting to the NCSP. As detailed previously, results were required to come back to the practice during the trial in order that patients diagnosed with a STI could be managed in the practice.
A number of options were available to each practice, depending in part on what forms they used for their non-NCSP laboratory tests:
-
to process the tests as per normal practice for other (non-NCSP) patients having a chlamydia test, generating a computer-driven laboratory form for each patient
-
to process chlamydia tests on a generic laboratory form used for other tests
-
to use the NCSP laboratory forms with a trial label redirecting results back to the practice.
No option was unproblematic and these processes had to be resolved on a practice-by-practice basis. This involved liaising directly with the laboratory managing the chlamydia screening for the local programme to establish the best process for each individual practice. In some cases, a different laboratory was used for NCSP versus non-NCSP chlamydia tests, which were sent on different forms, raising concerns in the practice about payment for tests.
Processing tests according to normal practice for non-National Chlamydia Screening Programme tests
The normal testing process for chlamydia outside the NCSP varied by practice and was dependent on the laboratory used. In most practices where a non-NCSP chlamydia test is required, a laboratory form and specimen label would be autogenerated from the individual’s computerised record.
In order to promote opportunistic screening, practices in the trial were encouraged to ask patients to take a test whilst at the practice. Testing kits were, therefore, prepared with trial materials and were readily available so that patients could take a test whilst waiting for their appointment. The need to generate a computer-driven form for each patient made this difficult, as kits could not be prepared in advance.
In some cases, the local practice laboratory used for general diagnostics was not the same as the laboratory used by the NCSP. This caused issues regarding both the testing kits and testing processes. At one practice, the local laboratory would accept only a urine sample, which meant that the practice was not able to use the self-test vaginal swabs from the NCSP kits. The practice was concerned that it would incur costs for using its own specimen pots for chlamydia screening. In some cases a standard urine sample pot was allowed; in others a specific sample pot requiring urine to be pipetted into it was mandatory.
In one case it emerged that an endocervical swab used for vaginal examinations, which could not be administered as a self-test, was mistakenly being used for NCSP self-tests.
Using a generic lab form
One practice used a pre-labelled urine specimen pot and a generic laboratory form, but generic laboratory forms did not appear to be readily available at other practices.
Using a redirect label
In most cases, when NCSP kits were used, the agreed solution was to include a label redirecting results directly to the practice. This was because these practices were used to using the NCSP forms and were reluctant to change their process. In all cases the interface between the practice, laboratory and local CSO was different and support from the trial team was required to ensure that the laboratory redirected results and the testing figures were not double counted. This solution, however, raised issues concerning patient confidentiality. NCSP tests are not generally recorded on an individual patient’s record. However, any patient agreeing to test during the trial would have this recorded on their general practice surgery record. Whilst in theory this appeared problematic, in practice each patient who agreed to test during the trial, regardless of whether or not they consented into the study, was informed that their results would come back to the practice and all agreed to this process.
Consequences of complexity due to National Chlamydia Screening Programme-specific laboratory forms
In one practice from pilot phase 1, the requirement to liaise with the laboratory to establish the best process had taken so much of the research nurse’s time that she became disengaged and subsequently the practice withdrew from the trial. We recognised that we would need to understand how practices currently manage their laboratory processes and chlamydia screening both within and outside the NCSP. This would enable us to allocate time for the appropriate system to be set up between all parties before the practice was able to start recruitment. Relevant questions were subsequently incorporated into the pre-trial questionnaire to collect these data. The resource intensity of this activity highlighted a real concern when considering the time that would be required to set up each practice when scaling up the trial to over 66 practices across England.
In another practice recruited for the intensive recruitment there were historical problems with the laboratory co-operating with any changes in process. The local NCSP lead agreed to extract trial results from their data and forward them to the practice. This arrangement was delayed and compromised when it transpired that the commissioning organisation for the chlamydia screening services was not part of the local PCT detailed on our research governance approval. This caused a delay in returning test results during the intensive recruitment phase.
Laboratory contracts
Contract arrangements between laboratories and local CSOs were found to be unclear and varied. In some cases, while the trial team was setting up processes, contracts were under review and being renegotiated. In general, however, laboratory managers were more than willing to facilitate trial requirements.
Where practices opted not to redirect results from NCSP laboratory forms, the cost of the chlamydia test was not always covered by the block contract with NCSP and was charged to the practice. One laboratory insisted on a confirmation letter from the local CSO, as contractually it was obliged to provide results directly to the local CSO; another required the trial team to provide it with the actual number of tests processed during the trial so that it could charge the local CSO directly. In another practice, the trial team was requested to meet the testing costs, as the laboratories would not allocate costs to the local CSO or the individual practice.
Prescribing and dispensing
Whilst the NCSP directive is to provide free treatment to all 15- to 24-year-olds, in practice this was not always the case. In three out of 17 practices medication was administered free of charge directly to the patient. These were practices with a locally enhanced service contract where a nurse prescriber administered medication during appointments where patients returned for treatment.
In other practices this was not the case. The trial team advised that medication should be prescribed under a patient-specific directive and administered free of charge. We provided information on how to manage a patient-specific directive, although this proved challenging, as practices were not familiar with using them. Practices were reluctant to store medication on site and did not have suitably trained staff available to dispense medication to individuals.
Clinical governance at the interface between practices and local chlamydia screening offices
The stand-alone character of CSOs on occasion made it challenging to organise an effective way of working with practices. Earlier in this chapter, we described the concerns expressed by local CSO staff about the study. In one locality, these proved unresolvable, and a general practice surgery choosing to take part in the study was not permitted to use NCSP forms.
In relation to this locality, we had a high-level meeting with the director of the NCSP. It was confirmed on all sides that it was not in the power of the NCSP team to direct any local CSO to approve the study. Nevertheless, with appropriate NHS Research Ethics and Research and Development approvals in place there was nothing to stop the practice from taking part in the study, but the tests and positives would not count within the NCSP targets. This put us in the uncomfortable position of appearing to undermine the local NCSP targets by working in parallel. In a pilot stage this was not a major problem, but had we been in a position to scale up this could have presented substantial conflicts between NIHR-sponsored research and the NHS delivery settings it aims to support.
Patients under 16 years
There was general concern across the local chlamydia screening areas regarding the management of patients under 16 years if we were managing the testing and care on their behalf. This was in fact easily remedied, as patients below this age group were not eligible for the trial and would be directed through the local CSO in the normal way, as they would not have been consented.
A way forward for future research on chlamydia interventions for primary care
As evidenced in this chapter, the anomalous stand-alone status of the majority of CSOs at the time of our study had complex consequences for our ability to deliver the research in an efficient, standardised and orderly manner, because of the complex and idiosyncratic nature of laboratory processes and care pathways. There were also potential conflicts of interest and cultural separation, which made it hard to achieve synergy between general practice and the CSOs. The main reason for the failure of the trial to scale up was the low number of tests in general practice, despite targeted interventions. However, the challenging interface with the NCSP was time-consuming and hard to resolve even for the small number of practices we worked with at any given time.
Recent policy shifts have changed the sexual health landscape, and future researchers in this field are likely to experience different challenges. First, the Public Health Outcomes Framework of 2012 has replaced targets for chlamydia testing coverage with a target for achieving a certain number of chlamydia diagnoses. 30 This is likely to focus commissioners on testing in settings with relatively high positivity, such as general practice in contrast with, for example, educational settings. 27 The NCSP is currently developing a GP engagement strategy that aims to promote and support chlamydia testing in general practice surgery.
Recent policy has focused on integrating the NCSP into local sexual health services, with the Department of Health supporting the integration of CSOs into sexual health and primary care services. 55 The NHS Guidance on integrating the NCSP56 suggests that the role of the CSO and local co-ordinator was essential when setting up the programme, but that this model is outdated by recent developments in service provision. Current guidance suggests that chlamydia screening should, wherever possible, be delivered within existing primary care, sexual and reproductive health and GUM services, and recommends avoidance of stand-alone CSOs. 26
There has also been a seismic shift in the commissioning arrangements for sexual health services in England. STI and HIV infection prevention services have from 1 April 2013 been commissioned by local authorities,57 while general practice-based contraceptive services are commissioned via NHS England, through the General Medical Services contract. The HPA has been incorporated into PHE, which has wider responsibilities.
These changes are likely to streamline structures for chlamydia testing and management, by encouraging care pathways that treat the local configuration of services as a single health economy. We are likely to lose the potential conflicts of interest between those managing chlamydia only and other services diagnosing and managing the range of STIs. However, the commissioning of STI testing within general practice is likely to go through a period of flux.
Research into STIs in primary care does appear to have some specific challenges,58 and it will be important in the future for commissioners to consider how to ensure that such services are fit for evaluation and for research. In this context, it is worth noting that the NCSP is not, by many criteria, a true screening programme59 but instead a chlamydia control activity. 60 The UK National Screening Committee describes it as a disease management programme. 61 Screening programmes are generally highly standardised, with fixed points of intervention that can be utilised for further intervention and evaluation, and, as we have seen, NCSP structures at the time of the study were variable.
If we are to answer complex questions in STI-related public health – such as how to provide PN, prevention counselling or support for related risks such as alcohol misuse – these will need to be tested on a relatively homogeneous background of provider services. Research funders need to work with commissioners, whilst professional groups need to consider the planning of future infrastructure in which the evaluation of complex interventions such as PN can be fitted and tested.
Chapter 8 Development of a referral web tool
Introduction
A key development of the trial was the web tool referral system. The trial referral web tool was developed in collaboration with the University of Sheffield by the Clinical Trials Research Unit (CTRU) in partnership with epiGenesys Limited. It resided on an existing web-based clinical management system at Sheffield (Prospect). We developed a web-based data collection system to capture, store and transfer data from the general practice surgeries to Barts Health NHS Trust Sexual Health Services at The Royal London Hospital. Data were captured using a highly secure web database system. The trial database was accessed via Prospect, the CTRU’s centralised and secure web-based data management system, compatible with a number of different interfaces (both PC and Mac).
A non-disclosure agreement was put into place between the MRC and Sheffield CTRU before a detailed web specification could be sent to the web developers. This agreement was agreed and signed off and the web specification sent to the developers in the week commencing 21 June 2010 (finalisation of this agreement took approximately 1 month). A demonstration site for user acceptance tests was available from 11 August 2010. Sign-off of the service level agreement by both parties took approximately 4 months (from July 2010 to October 2010).
User testing
The web tool system was tested by the trial team and reviewed by the Interact Lab at the University of Sussex. Feedback was also given by health-care professionals (including research nurses, GPs and HAs). Users reported the system as easy to use; some suggestions were made to improve the database.
System access
Only the system administrator (trial manager) was able to add new users to the system and had the responsibility for assigning logins to authorise users. The system administrator did not have access to any patient-identifying information. The trial statistician was also able to download anonymised data. Each user was assigned a level of access appropriate for their role. General practice surgery staff could access only their own practice information. Practice staff could view only the data that were entered by the general practice surgery. They were unable to view the HA consultations of their patients.
Data security and system support
Data were stored on Prospect, housed in the CTRU’s secure web server Corporate Information and Computing Services (CiCS). A local port-based software firewall was maintained on the Prospect servers, restricting access remotely. There was secure web access via HTTPS (Hyper Text Transfer Protocol Secure) and remote access to the server was assigned through a secure shell (SSH) using unique usernames and passwords. All data were encrypted using a minimum of 128-bit encryption.
There was managed backup of the data with backup copies saved hourly and retained for 24 hours. Daily copies were retained for 7 days, weekly copies for 5 weeks, monthly copies for 12 months and annual copies indefinitely. These backups were mirrored across two virtual servers. Copies of the backups were encrypted and transferred to an alternative site each evening. The data centre had an uninterruptable power supply and backup generator. Twin Janet network communications enabled a backup communications link. Servers were protected by a local port-based software firewall and the University of Sheffield firewall. Access to the systems was permitted only to authorised staff. The system was hosted on a virtual server that holds two physical servers in two different locations managed by CiCS. Only authorised staff could access these facilities and they required key locks and swipe card locks to access the site.
The development manager and software development manager provided maintenance and support for the system for the duration of the trial. It was agreed that major faults would be responded to within 1 working day and minor within 3 working days. Any critical system failure would be automatically notified to the development team (there were no critical system failures during the period of the trial).
Data
Anonymous data were collected on all chlamydia test results carried out in the practice during the time of the trial. Once a patient tested positive, additional information was collected by the GP/nurse in the practice. Data were entered directly onto the web tool. The web tool allowed for real-time monitoring of testing, recruitment, results and PN. For each potential participant added onto the web tool, a unique identifier was generated automatically. Any edits made to the data required the user to provide a reason for the change, allowing for a full audit trail of the data. The system logged all user interactions with the system and input of and modification of records (date- and time-stamped). The web tool complied with the requirements of the E6 Guideline for Good Clinical Practice (1996)62 developed by the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use and the Data Protection Act 1998. 63
Consistency checks were employed at data entry stage to avoid inconsistencies and improve the integrity of the data. Regular checks of the database by the trial team ensured that any missing data were entered in a timely manner.
Reports
Each practice had access to a report to allow for easy follow-up of all tests awaiting results. The HAs were able to access a number of reports (Box 3). Anonymous data could be exported as a comma-separated values (CSV) file by the trial manager or statistician at any time during the trial. The trial manager was able to monitor real-time recruitment.
New notifications (of new positives from practices requiring action).
Site list (details of all practices and staff participating).
Study reportsList of participants without contact information.
Address labels for retest.
List of all patients by name.
Feedback
We obtained feedback from GPs and nurses involved in the trial. Overall, few respondents commented at any length about the web tool used in the trial; most comments simply acknowledged its ease of use. The small numbers of patients and the limited period over which the web tool was used at most practices may have been insufficient to fully explore the application or effectiveness of the web tool.
Implementation into practice
Implementing the web tool into practices in the trial was straightforward and did not present us with any major challenges. During the intensive recruitment (phase 4), some practices requested that a member of the practice administrative team be trained to enter patient details onto the web tool at the end of each day rather than have this task being managed by a more expensive time-poor research nurse.
Feasibility of implementation to NHS settings outside trial
Improving PN rates for patients seen in general practice with a STI has considerable potential for transmission prevention. We have demonstrated that it is feasible to develop a web-based referral system to facilitate PN for general practice patients. The web tool enables the maintenance of data quality, access controls, security and real-time monitoring.
We are currently undertaking a clinical evaluation of the implementation and effectiveness of the web tool system in NHS practices in the Bristol area. This will be reported at a later stage, as agreed with the NIHR HTA.
Chapter 9 Standardisation of provider and contract referral
Introduction
Our original commissioning brief specified a three-way comparison of methods of offering PN to individuals diagnosed with common bacterial STIs (mainly Chlamydia trachomatis but also Neisseria gonorrhoeae) in UK primary care. The proposed comparison of patient referral, provider referral and contract referral reflected existing UK guidance for PN,64 and a wider international literature, which treats these three approaches as established and distinct. 63,65
We consulted with PN practitioners in order to standardise these three approaches for our planned RCT, and observed a lack of clarity about the definition and role of contract referral in current practice, as well as a degree of ambiguity in the UK guidance. A summary of definitions is contained in Table 15.
| Method of partner notification | Commissioning brief | Our RCT protocol | SSHA manual64 |
|---|---|---|---|
| Patient referral | Patient referral when health service personnel encourage index patients to notify their partners | Patient referral where (i) patients are given information about their infection, and asked to tell their partner about the problem and the need to be treated. Information given on STI and on availability of local services, and assistance in arranging appointment for partner offered | Patient, partner, passive or self-referral Denotes when the index patient with the infection informs sexual partners. They are encouraged to notify partner(s) of their possible infection without the direct involvement of a HA. The patient may:
|
| Provider referral | Provider referral when third parties, usually health service personnel, notify partners | Provider referral where, in addition to (i), patients will be asked to agree to a specialist HA contacting one or more of their partner(s) at the time of diagnosis. HA offers to contact one or more partners on behalf of patient Same assistance given as patient referral for other partner(s) |
Provider or active referral A health-care worker notifies a patient’s partner(s). In the UK HAs in GUM clinics almost exclusively perform this. The index patient provides information on partner(s) to a HA, who then confidentially traces and notifies the partner(s) directly |
| Contract referral | Contract (or ‘conditional’) referral when health service personnel contract with index patients to notify their partners, with the understanding that the health service personnel will notify those partners who do not visit the health service by an agreed date | Contract referral where, in addition to (i), patients will be asked to agree to a specialist HA (contact tracing expert) to inform partner(s) if this has not been done after a verbally agreed period of time (usually no more than 7 days) Same assistance given as patient referral for other partner(s) |
Conditional, contract or negotiated referral A hybrid approach may be employed where an initial patient referral is followed up by a provider referral after an agreed period of time, if the contact has not attended |
We recognised that our research needed to accurately reflect current practice and developed a 1-day workshop to address the following questions:
-
Are the three PN methods as described in the Society of Sexual Health Advisers (SSHA) manual clear to PN practitioners?62
-
In practice, are the three methods distinct and feasible to deliver?
-
Under what circumstances do practitioners offer specific PN strategies?
-
If there are conflicts between guidance and practice, how might these be resolved?
Method
Overview
We used a qualitative and participatory approach, as we considered the analysis of simulated clinical practice with reflective discussion the best available approach. 66 During a 1-day workshop, experienced PN practitioners were observed while contributing to focus groups, actor-assisted role plays and a plenary discussion. All discussions and field notes were recorded and used to undertake a thematic analysis.
Development of workshop
We developed the workshop as a multidisciplinary research team. Participants completed a short online survey to establish their views of current practice. This helped us to refine focus group topic guides and establish areas requiring clarification. We designed three role plays of commonly encountered, uncomplicated PN scenarios for Chlamydia trachomatis based on existing guidance, discussion with practitioners and informal review of recent literature.
Selection and recruitment of participants
Ten participants, a major part of whose job was PN, were purposively selected via the network of the study’s lead HA.
Data collection: focus groups, role play and plenary discussion
The first focus group session established the participants’ perceptions of PN, which were further explored in actor-assisted role plays. These were followed by researcher-led discussion using topic guides and subject matter created dynamically in-session. A second focus group session specifically explored the practitioners’ and actors’ (as patients) perceptions of PN exemplified in the role play and implications for practice. In the final plenary session, an emerging view on how to deal with any ambiguity was established.
The focus groups, role plays and plenary session were digitally recorded and transcribed. Contributing materials included the online questionnaire, observer field notes and flipchart sheet notes.
Format of workshop
The 1-day workshop took place at a central London university venue. The format of the day and rationale for each activity are summarised in Table 16.
| Activity | Format | Purpose |
|---|---|---|
| Introduction | Plenary | Introduction and welcome, familiarity with objectives of the day and with research team |
| Discussion of current practice and choice of PN strategy | Focus group: participants divided into two groups, facilitated by a clinically active member of research team | Explore PN views and practices prior to role play. Engage with others to reduce self-consciousness in role play |
| Introduction to role play | Two members of research team (MS, CE) demonstrated a role play, facilitated a discussion of the trial scenarios on which we needed guidance, and introduced the role-play tasks | Understand where the research team required input and advice. Reduce self-consciousness about role play by observing demonstration |
| Role plays with patient role played by an actor: patient referral, provider referral, contract referral | The practitioners were divided into three groups of three or four. Each group rotated through role-play sessions on patient referral, provider referral and contract referral. Each actor played the patient and worked with a single scenario. Practitioners acted the practitioner role in turn. In some groups a greater proportion of the time was spent observing the first or second role play and discussing implications for practice | Explore practice and views about the three approaches to PN, and their consistency across the three different groups of practitioners taking part The use of actors aimed to standardise the role play and limit potential drift away from topics, given limited time |
| Discussion of role play | Practitioners and actors discussed their observations, experiences and views of doing the role plays using the scenarios, and the implications in relation to their normal practice. They were divided into two focus groups, facilitated by a researcher | Reflection on relationship of role play to usual practice, and what this might mean for the researchers in seeking to standardise the three approaches to PN for research purposes |
| Plenary discussion | General discussion, including actors | Explore degree of consensus across the whole group for an emerging view on how we should deal with the provider/contract referral ambiguity |
| Debrief for researchers | Discussion with project team | Identify next steps for the project team |
Results
Background of partner notification practitioners
Ten out of twelve invited practitioners attended the workshop: HAs (n = 8) and senior nurses (n = 2), working in GUM clinics (n = 6), young people’s services (n = 2) or community outreach clinics (n = 2). The locations ranged from large urban areas to smaller towns across England, in London, the South East, the South West, and Yorkshire and the Humber. Practitioners’ experience ranged from 2 to > 20 years, with equal male–female representation.
Data collected
Over 7 hours of data were collected and transcribed, including 15 role plays (five for each of the three PN strategies). The following themes were identified.
Theme 1: partner notification in practice
Partner notification
All practitioners experienced PN practice as highly individualised, negotiating and adapting their strategy to individual patient needs. Patient-related factors such as age, sexual orientation, nature and length of relationship, and willingness to contact partners were considered. Practitioners described using their instinct to guide their PN offer, although they found this difficult to articulate.
Instinct
You get a feel, and I think that’s always the trouble that my profession has had is to try and quantify what the ‘getting a bit of a feel’ is when you’re in an interaction with a patient and actually then trying to actually explain to someone else.
HA5, male, London, GUM clinic
I suppose on the first offering, [it’s about] reading how they feel about letting someone know, really, and getting a feel.
HA8, female, large southern town, GUM clinic
Highly individualised
It really depends on the patients and the story or understanding of their partners that you get.
HA2, female, London, GUM clinic
But it’s very much their decision because I think the outcomes will be much better.
HA1, female, northern city, community clinics
You usually know quite soon if they are happy to let an individual know. Or they might be happy to let one know and not quite sure about another one. So for me, it’s one to one with that individual. And also you kind of know if they are telling the truth.
HA8, female, large southern town, GUM clinic
Sexual orientation
[I have] done the gay men’s clinic for quite some time in various long spells, and that’s where the vast majority of provider referrals come from. You’re talking 20 to 30 people per patient. And it’s me. I also get a kick out of that. So I’m really giving them that. Yeah, it becomes a mission, actually. I start making charts of them all.
HA2, female, London, GUM clinic
Age
Young people have a specific pro forma which goes into all the issues around young people; not just the sexual health content. So it’s social, education, job protection; all that sort of stuff.
SN2, male, London, young people’s clinic
Nature and length of relationship
If they are in a primary relationship with somebody then I talk about the cycles of reinfection, it’s important for the partner. But if it’s an ex-partner or multiple or casual, then we just say, ‘You do not have any symptoms. It’s really important that we get these people in for at least a test.’
HA7, male, south coast city, GUM and community clinic
In discussions about how clinic staff manage PN, the amount of HA effort available because of patient case load was mentioned. There was a perception that not all clinics pursue PN with equal thoroughness:
I do not think we are quite as rabid as some of the clinics about getting every single name. I think we are much more inclined to be patient focused, but we then do check later on what the outcome is. So we do not just do it and then leave it, but we check with the index [patient].
SN1, female, southern town, GUM clinic
Partner notification aims
Health advisers reported the following aims when undertaking PN and used a variety of techniques to achieve them:
-
review the current status of the patient and recent past with respect to the STI
-
facilitate the health and STI status of the patient, preventing reinfection
-
emphasise the ‘here to help you’ role: advise and encourage rather than order action; remain impartial at all times
-
‘read the patient’ by building up a view on whether or not they believe the patient will do what they are saying they will do
-
identify all appropriate partners and priority partner(s) with the patient (e.g. current partner)
-
minimise the onward transmission of the STI through unwitting partners.
Motivational interviewing
Some aspects of the negotiation process adopted by HAs fit well within a motivational interviewing framework, particularly the following methods:
-
Be collaborative, not authoritarian. For example, reinforce support available, maintain and reinforce the patient’s control in the PN process, formulate plans, ensure they are committed to agreed outcomes.
-
Engage the patient’s own motivation. For example, ask what their main priorities and concerns are, discover intentions to notify a partner, work through examples and consequences.
-
Reinforce the patient’s autonomy. For example, give extra time to absorb and think about courses of action, explain how provider referral works in an anonymous setting.
Techniques used by health advisers to achieve patient notification aims
-
Patient choice: maintain and reinforce the patient’s control in the situation, ‘what would you like to achieve?’
-
Education: give the patient facts about their STI, its transmission and symptoms, treatment and complications if left untreated; explain how the HA will proceed on the patient’s behalf with reassurance of anonymity if contacting partners; correct any myths about the infection (e.g. only women can become infertile if chlamydia is left untreated, or men do not get symptoms).
-
Build scenarios: point towards consequences that could be faced later in response to a decision made now; question potentially flawed thinking, for example noting how suspicions and concern will be aroused (possibly magnified) if the partner realises a delay has occurred unnecessarily.
-
Check for and identify risks: was protection used in the relationship? Was sex consensual? Is the patient at risk of violence? Did the relationship end badly? Will the patient be reinfected?
-
Support: give ideas for ways to broach the subject with a partner; offer to ring back after a short period to allow the patient time to consider their options; offer ways to help the patient.
-
Build rapport through reassurance, for example ‘Due to lack of symptoms it’s impossible to say where it came from’; empathy, for example ‘Puts you in a difficult situation, we realise that, we can help you’; normalising the STI, for example ‘All girls know about chlamydia, it’s in all the mags now’; jokes, for example ‘You have got better things to do than come here . . .’
-
Use of hooks: use information given by the patient to help the patient navigate their choices, for example, if the patient reveals they had chlamydia 6 months ago, ‘We can call that a failed treatment [and then there’s no blame here within the couple]’.
-
Overcome challenges: respond rapidly to a patient’s block or fear, and try to free up the dialogue to continue the conversation in a positive direction.
-
Use the patient’s moral conscience to encourage disclosure: ‘I think the right thing to do would be’, ‘He needs to have a choice about what to do’, ‘They both need to know’.
-
Review, check back and ‘park’: throughout the conversation review and check which PN process has been agreed for each partner. If the conversation moves away from a partner, ‘park’ and return to discuss at a later point in the conversation.
Support tools
Pro formas and contact slips were commonly used as support tools to facilitate PN.
Pro formas
Eight out of 10 HAs reported that they use a pro forma or guidelines to capture details from a patient and recorded the following details:
-
diagnosis
-
name of HA who saw them
-
date of treatment
-
number of partners
-
details about partners: whether regular or casual sex/type of sex/where the sex took place/nature of the relationship/time length of relationship
-
outcome of PN conversation: untraceable/patient to inform/method used to inform contact
-
additional details (e.g. the last sexual encounter, whether protection was used and risk factors such as smoking or alcohol) were recorded when working with student populations.
Contact slips
Most HAs have access to contact slips which are used by patients to inform partners about the need for screening and the location of their nearest clinics. When the partner attends for testing/treatment, the slip is used to close the PN loop. If the partner attends a different clinic, the slips are returned to the originating clinic. One HA reported that recently revamped and colourful contact slips were having a positive effect on return rates.
New initiatives
One HA reported a new initiative where HAs are now responsible for dispensing medication, which brings forward contact with the HA before the patient has a chance to disengage with the service:
One link we use into engaging the patient is to dispense medication . . . And that was used as a sort of way of actually trying to just really have an opening link with the patient, rather than health advisers being seen as an add-on and policing their sexual behaviour.
HA5, male, London, GUM clinic
Another HA reported strong enthusiasm by young patients and MSM for an anonymous web-based PN tool during a recent straw poll of patients. Other online strategies are being considered by HAs (i.e. dating sites such as Gaydar or Plenty of Fish), although issues with patient confidentiality need to be worked through before HAs can use websites to facilitate their work.
Complex cases
A number of difficult situations were mentioned where HAs were uncertain about progressing with PN. These included sex workers and their clients; sauna users; patients with a history of repeated reinfection; and patients who say that they have no way of contacting a partner they met online.
Patient notification timings
The general timeline for PN involved five stages over a 2-week period:
-
Day 0: test result is positive.
-
Day 0–1: initial contact with patient to discuss the STI and next steps in managing the infection. PN is discussed and a plan of action on who will perform PN is agreed. Contact slips may be used for patients to pass information to their partners.
-
Day 1–14: PN undertaken by patient or HA.
-
Day 2–14: follow-up call by HA to check on PN progress and medicine compliance. The time delay varies depending on whether a patient referral had been agreed or if the patient wanted more time to consider their options.
-
Day 2 onwards: when partners have presented, the HAs attempt loop closure on PN, contributing to service auditing (Table 17).
| HA telephone call | Patient referral arm | Provider referral arm | |
|---|---|---|---|
| Immediate | Delayed | ||
| 1 | Agree patient to make calls to partner(s) to notify. If no agreement reached, patient referred to GUM clinic for provider support | Offer provider referral, patient agrees, HA performs this immediately once contact details are available | Offer provider referral, patient not sure for one or more partners. Time-to-think delay agreed between patient and HA |
| 2 | – | – | Check on progress for patients who were unsure and needed more time to consider their options. If required HA agrees with patient to take on notification of one or more partners |
| 3 | Follow up patient referrals | Follow up on progress with any agreed patient referrals | Follow up on progress with any agreed patient referrals |
Patient referral
Patient referral was the preferred first approach for most practitioners, who were concerned about the long-term impact if patients did not disclose themselves. Where clients were in a relationship, practitioners used motivational interviewing techniques to encourage patients to notify partners themselves.
But you’re responsible, it’s your body, it’s your relationship, it’s your partner, we are just trying to facilitate . . . you’re treated, we do not want you to get reinfected but we also want your partner to get the screening and treated because he might have something else.
SN2, male, London, young people’s clinic
I would think I like to give people the opportunity to refer themselves [perform patient referral]. I think the outcome is better for them.
HA8, female, large southern town, GUM clinic
I think they normally find that interaction [with the HA] quite helpful, to have that input, because that’s what they want for that situation [patient referral] but they might not necessarily have the skills.
HA7, male, south coast city, GUM and community clinic
Provider referral
All practitioners reported using provider referral to some extent, with some using it by choice and experience rather than as a clinic-endorsed practice. This was typically seen as more appropriate for casual or unknown partners, or for young patients who did not want to reveal their identity to partners within their social group. Greater uptake of provider referral was reported for students, women and particularly gay men, and it was seen as more suited to certain population contexts and types of infections, particularly the blood-borne viruses HIV and hepatitis B and C.
Some practitioners chose to use provider referral to achieve a perceived better outcome rather than negotiating with an unwilling patient to undertake notification themselves, although some felt uncomfortable that provider referral took away patients’ autonomy and choice.
Mostly we favour a provider referral just to take on ourselves and hopefully it’s going to take a better outcome as a result.
HA7, male, south coast city, GUM and community clinics
I would not say that the culture of my clinic was necessarily provider referral. That’s for me . . . because I know that I can get everything done.
HA2, female, London, GUM clinic
I know the main aim is that the partner is notified, but I tend to give people the benefit of the doubt and hope they will do a patient referral. And I find it made me a little bit uncomfortable from just taking away someone’s independence and choice.
HA8, female, large southern town, GUM clinic
Different population contexts and infection types
The provider referrals, I think, are by far . . . this is also an urban [city] clinic, so by far the preferred. I tend to do those [provider referral] more for the blood-borne infections, particularly with MSM.
HA2, female, London, GUM clinic
We have a high student population so we get quite good outcomes from provider referral.
HA1, female, northern city, community clinics
You can sometimes get [women] to look at the bigger picture and say, ‘But he might be infecting other women and you do not want other women to be in the same situation you are.’
HA3, female, London, GUM clinic
Contract referral
Practitioners reported using contract referral most often for patients with HIV infection or in other circumstances where protection of vulnerable patients was seen as a priority.
We do [offer contract referral] and it’s usually with HIV-positive patients. That’s the primary focus.
HA5, male, London, GUM clinic
Contract referrals, the only time that I know that it’s been used in my clinic has been around HIV patients and usually that process has begun as generated by the HIV team staff.
HA2, female, London, GUM clinic
[Contract for] HIV I think and ones with syphilis, geriatric with syphilis. We have used it a few times with HIV, with people who will not disclose their status but continuously put their partner at risk.
SN1, female, southern town, GUM clinic
Sometimes there’s vulnerability issues with the young person. There’s some child protection stuff. So quite often [we are] contracting with them that they need to do it by this amount of time or legally we would want to step in.
SN2, male, London, young people’s clinic
Theme 2: scenario building
Practitioners invested considerable time and effort in helping patients deal with difficult questions that disclosure might raise within the partnership, such as infidelity. Practitioners used motivational interviewing and challenged patients’ blocking strategies, helping patients to build scenarios and enabling them to project into the future and identify any anticipated regret. Practitioners also suggested creative solutions to introduce the subject to partners or elicit telephone numbers for previous partners.
When you do those patient referrals, sometimes [the patients] want to do them but they do not necessarily know the best ways. So I always say, ‘Have you thought about how you might bring the subject up?’ or ‘You might want to say . . .’
HA7, male, south coast city, GUM and community clinics
It’s our job to help you so it’s not about forcing you to do anything; it’s just working with you really. Obviously you can choose to tell them yourself but obviously you have been a bit worried about that. What we can do is we can let them know on your behalf if it helps?
HA4, female, London, GUM clinic
Is it possible to say, ‘Look, if we are going to be starting to think about trying for a baby maybe I’d better go for a sexual health check up, and check that I have not got anything before we start because I’ve had sex with people before you?’
HA3, female, London, GUM clinic
The first thing people often say is ‘Hang on a moment, why did you not tell me?’ And that creates a whole other dynamic for you.
HA5, male, London, GUM clinic
Approach it very calmly, say that in your history, ’cos it’s a new relationship, as someone that you like to consider your sexual well-being so actually while you were away you decided to have a check up. And then you can decide what you want to tell him the outcome of that was . . . would you rather he found out from a third party or from you?
HA5, male, London, GUM clinic
And it’s a fairly new relationship, so I do not think that it’s without . . . I do not think it’s outside the expected realm that this is a possibility in a new relationship that an infection could be there. You would not actually have to disclose that . . . he knew you had a previous partner just before him, you would not really have a reason to have to tell him there was any overlap at all. He would not gain anything from knowing that.
HA2 female, London, GUM clinic
You can just maybe talk to some work guys, just to get his phone number. Just say he owes you £5, track him down like that.
HA4, female, London, GUM clinic
Creative solutions
What do you think about maybe telling him you have been to the GP, you had thrush or a urinary infection; something like that? And your GP has suggested that you both either go to your GP or both go to the local clinic and have a full check up?
HA3, female, London, GUM clinic
Theme 3: movement between strategies
Although practitioners were able to define the three distinct PN methods, it emerged through role play that there was often a fluid movement between methods as a response to individual patient need. This movement between strategies was enacted through either a follow-up call to check on progress (usually 2 weeks) or a shorter check-back call (2 days) for patients who needed time to consider their options. A time frame for the call was agreed with the patient at the initial consultation.
I can give you a little while to think about it, a couple of days, and then give you a ring back and see how you are feeling about it.
HA1, female, northern city, community clinic
Delayed provider referral
Practitioners often held the offer of provider referral in reserve at the initial consultation and proposed it only during the agreed follow-up call if it became clear that the patient had been unable or unwilling to broach PN with a partner. Practitioners considered this delayed provider referral as normal practice and did not distinguish it from an immediate offer of provider referral. However, they acknowledged that delayed provider referral was sometimes clearly inappropriate, for example when the patient was unwilling to attempt a patient referral first.
Everyone would be followed up and it’s on that follow-up call for, say, chlamydia, 2 weeks down the line, to actually talk to them and reassess the situation. If then they have had problems you do provider referral.
HA8, female, large southern town, GUM clinic
In some cases, practitioners suggested that patients who were initially undecided about provider referral took extra time to decide. Practitioners negotiated a time frame to check back with the patient, allowing them time to absorb information and consider implications already discussed.
We do when there’s a fragility, a psychological vulnerability around the impact of that diagnosis. But I can sense that actually it’s important that they do tell their partner, they want to tell their partner but, actually, they just cannot quite work it out at this point and they need more time to absorb it and think about it. So often I will say to them, ‘Well, why don’t we set a timeframe here . . . If I follow you up, maybe by that point you’ll have got to this stage,’ and then I follow them up.
HA5, male, London, GUM clinic
Theme 4: contract or delayed provider referral?
Participants did not regard contract referral as normal practice for common bacterial STIs. Discussions across the day demonstrated some ambiguity and inconsistency around what constituted a contract referral. Some practitioners described contract referral as an agreement made with the patient that they would follow them up within a set time frame to check their progress, and that this agreement constituted a contract.
So they are expecting a call, so in effect that’s a kind of contract that we will [follow up]. I’m not saying you have got to do it by this time, but I’m definitely going to follow you up.
HA8, female, large southern town, GUM clinic
We tell all our patients that we will call them in a couple of weeks’ time just to see how they are and we wrap it round saying, ‘Did you have any problems after the tablets? Were you okay?’ But we always tell them [in advance] and say, ‘And then we can see how you’re getting on with telling your partners.’ So, in a way, the contract referrals are implicit in the normal way that we work because of the checking we do at 2 weeks. If they have not been able to do it then, then we’ll [offer provider referral].
HA3, female, London, GUM clinic
However, this is not contract referral as previously defined (see Table 15). The practitioner has not agreed with the patient that their partner will be notified directly by the practitioner if they have not attended by a certain time. Instead they have agreed a follow-up call to check on progress. The key difference is that the patient still must agree to provider referral, rather than this having already been agreed or contracted. An indication of whether contract referral has taken place is whether the practitioner collected partner details during the initial conversation for subsequent use if needed. Three of ten practitioners said they would initially request partners’ names so that they could refer to them clearly in conversation with the patient, but none would ask for further partner contact details until an offer of provider referral had been accepted.
Findings in relation to published literature
We found conflicts between practice and guidance. Contract referral as presented in the SSHA manual states:
A hybrid approach may be employed where an initial patient referral is followed up by a provider referral after an agreed period of time, if the contact has not attended.
(p. 20)64
This implies that a contract is made with the patient during the initial consultation that the practitioner will contact a partner directly if they have not attended by an agreed period of time – no questions asked.
However, guidance for patient referral states:
It is important to negotiate a back-up plan during the first interview, if possible (for example, ‘If he’s not been within x days/weeks should I contact him directly, or speak to you again? . . . Is it ok to ring you? . . .’)
(p. 32)64
If the patient agrees to the practitioner contacting the partner directly as a back-up plan, this would constitute a contract referral as defined earlier in the manual. If the patient agrees to be called back and subsequently takes up the offer of provider referral, this would fall under ‘provider referral’ in the guidance, despite differing from a provider referral agreed at the initial consultation. Confusingly, some participants described this incorrectly as ‘contract referral’, even though there was no initial agreement for the practitioner to contact the partner directly.
If a separate category of delayed provider referral was introduced into the guidance this could clarify between a provider referral offered initially and one offered as part of a back-up plan, as is common practice. This would help practitioners to accurately categorise this type of PN offer as a ‘delayed provider referral’ and not as a ‘contract referral’.
Guidance also recommends that for patient referral:
A follow-up interview may be necessary if there is no record of the contact having attended. The purpose of this is to check progress, gather any additional data and repeat the offer of provider referral if the index patient is having difficulty. There is evidence that many patients who initially opt to inform their own partners subsequently agree to provider referral at follow-up interviews.
(p. 32)64
The guidance to ‘repeat the offer of provider referral’ at the follow-up call suggests that an offer of provider referral should have already been made at the initial planned patient referral consultation.
These ambiguities seem to occur as a result of attempting to use distinct definitions for PN methods in the guidance to describe the fluid PN strategy adopted by many practitioners in practice. Some practitioners said they found the term ‘contract’ unhelpful, as its meaning overlaps with negotiating a provider referral, which was closer to how they saw their work.
Other findings
Professionals in contact with patients
The lack of face-to-face contact with the research HA concerned some HAs. They thought it would be harder to build up rapport and invite confidential answers about sexual partners to be revealed to an unknown person over the telephone. There is a change in health-care professional: the patient is transferred from general practice surgery staff to the PN research HA, and the research HA is not available for face-to-face contact because of geographical constraints.
-
It is important that the patient knows who will follow up (the name of the research HA) if follow-up is necessary, since it will not be the person they are seeing now in their general practice surgery.
-
In one northern city it is normal practice for the patient to be asked to collect their prescription from reception with a health-care professional who could explain the study to them. This implies a need to gain the patient’s consent at time of test.
Patient care
-
For continuation of patients’ clinical care, what is the process if retreatment of index must be initiated?
-
The research HA will not necessarily know the local information and clinic specifics for the patient’s location. Can relevant information be provided in advance to the HA? How?
-
Consider providing an opportunity for patient feedback.
Implications for the health adviser call to a patient
-
A short list of four questions that can be worked through in 2 minutes should be considered.
-
The first scenario presented by the research team was felt to be too wordy and complex for a patient to process. A short introduction is necessary in order to reduce the risk of losing the patient early on. The telephone conversation should also adopt the ‘chunking and checking’ methods used in communication skills so as to not encourage the patient to switch off.
-
Evening telephone calls to patients must be planned for in cases where the patient is unable to take a call during the day.
-
A decision should be made whether the patient is able to call the HA back at a convenient time on a set number. HA were concerned that a Withheld Number call to a patient does not set the right tone and does not eliminate the possibility of it being interpreted as a hoax call. There is a preference for revealing the caller number.
-
Faced with reluctance to allocate time to talk to the HA, it was unclear how many times the HA should attempt to contact index patient?
-
All patients should receive a follow-up call from a HA after the PN discussion (Figure 9).
FIGURE 9.
Telephone call timeline for patient referral (calls 1 and 3) and provider referral (calls 1, 2 and 3).

Conclusions
The discussions captured from the 1-day event contributed widely to the design of the main study. In addition, the data provide a set of rich conversations about HA work and the typical behaviour of patients during the PN process. This includes the complications that can occur, how HAs dynamically manage the combined aims of transferring knowledge about the STI, how they ensure that patient health is protected, and how they maintain or reinforce the patient’s control in how partners can be notified of their screening needs and how they help preserve the integrity of the current relationship.
Strengths and weaknesses
The 1-day workshop allowed us to collect novel observational data on the working practices of PN professionals in a UK setting. These findings describe the current practice of experienced PN practitioners for the first time, and have implications for both clinical and research communities.
A limitation of the study is that we were unable to observe real-life clinical practice. The logistical and ethical barriers to obtaining consent from patients for the taping of initial conversations about sexual partnerships following a STI diagnosis are very challenging. We considered an analysis of simulated clinical practice with reflective discussion the best available approach. A further limitation is that the group was small, and may not have sampled the full range of organisational cultures in clinics offering PN.
Implications for the main study
The questions the workshop aimed to answer were:
-
Are the three PN methods as described in the SSHA manual clear to PN practitioners?64
-
In theory, practitioners understood the definitions of the three distinct PN methods. However, there was considerable ambiguity about the precise meaning of contract referral in the context of common bacterial STIs. Practitioners used only two methods: patient and provider referral. Practitioners did not use either method exclusively but moved between strategies responding to patient need, often delaying an immediate offer of provider referral and negotiating a delayed provider referral with patients to optimise PN.
-
-
In practice, are the three methods distinct and feasible to deliver?
-
The two methods used of patient and provider referral were found to be feasible and operational. In the latter case there is an issue with the time delay until the check-back call, which is agreed with the patient on an individual basis. This delay ranged between 2 days and 14 days. A final timeline for HA calls was agreed, as shown in Figure 9.
-
-
Under what circumstances do practitioners offer specific PN strategies?
-
While approaches to individual clients varied, the overall approach to negotiation of PN in practice was remarkably consistent across the group. Adopted PN methods varied depending on the STI diagnosed and patient-related factors. All practitioners tailored their offer of PN to individual patient need and were often guided by their instincts. Patient referral was the preferred method, with practitioners using motivational interviewing techniques to help patients make their own decisions to preserve their current relationships and protect their own health.
-
Provider referral was more often used for clients such as gay men and young people, for whom it was seen as more effective. Some practitioners, however, found provider referrals difficult to manage because they believed that it took away patient choice and autonomy.
-
Contract referral (as defined in guidance) was regularly used for chronic and serious STIs including HIV, hepatitis B and C, and sometimes syphilis, but not often for common STIs.
-
-
If there are conflicts between guidance and practice, how might these be resolved?
-
Existing guidance may need to be modified to reflect our findings and we propose consultation on the following advice and recategorisation of PN methods:
-
Patient referral: no change.
-
Immediate provider referral: patient agrees from the outset that the HA may contact a partner immediately.
-
Delayed provider referral: patient needs some time to consider the offer of provider referral and agrees to a call back (2 days) to discuss this or, if during a routine follow-up call (2 weeks) the patient has not managed PN themselves, provider referral is offered.
-
Contract referral is reserved for blood-borne viruses and syphilis and removed from the guidance for bacterial STIs.
-
-
This will have implications for training and assessment of competencies for all the professional groups with an interest in PN.
Unanswered questions and further research
There remains a marked lack of qualitative or operational research on PN with HAs or other specialists in PN, and we are therefore unable to provide a detailed comparison with similar studies. Interestingly, there remains no published three-way comparison between provider, contract and patient referral, although provider and contract referral have separately been compared with patient referral. 9–11 This absence suggests that there may be operational overlap between contract and provider referral not reported previously, which has implications for both clinical practice and research evaluation of different approaches to PN.
Chapter 10 Cost analyses and preliminary economic evaluation
Scope of the economic evaluation
As a result of recruitment failure, we were unable to complete the economic evaluation originally planned. It was possible only to carry out a limited cost analysis of the pilot study and preliminary economic evaluation of the intensive recruitment phase.
The economic assessment presented in this chapter focuses on two main topics: (1) the costs of the PN model presented here; and (2) the costs of intensive recruitment as undertaken in phase 4.
Comparison of costs for alternative partner notification pathways
The success of any new strategy or intervention must be balanced by the resources required to achieve the intended outcome, and additional resources must be evaluated in terms of any additional benefit that can be attributed to them and whether or not the additional costs are justified given any additional benefit. Thus, the costs of achieving any PN success as a result of either a patient or provider referral pathway used in the pilot are integral to assessing the cost-effectiveness of each approach.
It was not possible to assess the success of the strategy properly in terms of outcomes compared with current practice owing to the recruitment issues in this trial. However, we can attribute costs to the patient and provider referral pathways used in the pilot and compare these with the costs that are likely to be incurred by other PN strategies that have been evaluated in the UK to date. Thus, the resulting analysis is not a full economic evaluation comparing costs and outcomes of two or more alternatives,67 but a partial evaluation which compares a number of alternative strategies in terms of costs only.
Economic objective
What are the costs of the patient and provider referral models of PN as used in the pilot and how do these costs compare with other proposed/existing pathways that might be used for the purpose of PN?
Methods
We compared the costs associated with the pilot PN pathways with two separate published studies that investigated the costs (and outcomes) associated with PN strategies compared with current practice in the UK.
The two studies with which we compared the pilot pathways are: (1) the NIHR-funded ClaSS project, which included a nested PN trial;23 and (2) the MRC-funded APT studies. 33,68 In both studies, resource use data were collected alongside a primary clinical study and unit costs were applied. The average cost per case of PN as reported in the published ClaSS and APT studies is naturally affected by the success in terms of relative effectiveness of any particular strategy. For example, if a particular PN strategy successfully reaches and treats more sexual partners than an alternative strategy at the same cost, the cost per partner treated will be lower than the alternative, as more partner(s) have been treated. In the current study, it is not appropriate to compare across these different strategies in terms of effectiveness because the approaches apply to different individuals in different geographical settings and such a direct comparison will cause bias.
Furthermore, the ClaSS project represented a randomised controlled trial while the APT study was an exploratory trial and was not randomised. Instead we attempted to consider the typical pathway for one index case and their respective partners. We therefore assumed the average resource use (e.g. average time of consultation with the health-care professional relevant to that pathway) and applied unit costs to the average resource use likely to be incurred by one individual and their partners on that pathway. In order to provide a consistent approach to the following comparative analysis of costs it was necessary to make some assumptions prior to conducting the analysis.
Assumptions
-
All pathways begin from the diagnosis of and discussion with the index case and include the costs and resources associated with the index case to ensure consistency across pathways. The inclusion of the index patient is required because some pathways include PN advice at this initial stage and fair disaggregation of associated resource use would not be possible.
-
Based on results from the ClaSS study, we have assumed that each index case generates 1.5 partners.
-
All index patients and their partners comply with all aspects of the pathway.
-
All index cases and partners receive the same treatment and tests where required, and so the costs of tests and treatment are not included in any of the pathways. We included only resource use associated with trying to deliver PN (that is, the associated staff costs).
-
The ClaSS project and APT study pathways assessed the success of PN in their respective studies by recording how many partners were consulted or treated as part of the outcome. The cost of follow-up telephone calls to the index to assess the outcome for the partner, if required, was assumed to be a cost of the research and not part of the intervention and, therefore, was not directly recorded. To achieve consistency with the current study, resource use and associated costs have been included for these studies to represent the required follow-up to index cases or partners to assess whether or not PN has been achieved. These costs are assumed to be the same as those incurred by the current study.
We present a brief summary of the ClaSS study, the APT study and the patient and provider pathways used in the pilot of the current study. For both pathways in each of the three studies we explain how we have determined resource use and applied unit costs.
Partner notification in the Chlamydia Screening Studies
The primary objective of the PN trial, which was an integral component of the ClaSS study, was to explore effectiveness of PN advice provided in a primary care setting compared with referral to genitourinary clinics. The latter was and still is the current practice.
The costs incurred by each PN strategy are presented from the perspective of the NHS. Costs were originally obtained in pounds sterling at 2003 prices and subsequently updated to 2005 prices for the ClaSS report. We have used reported resource use from the ClaSS study and applied wages and costs that apply for 201169 for the purpose of the current report. Practice nurses recorded the total duration of the consultation, which included the time taken to give results and treatment, explain the study, obtain consent, and conduct randomisation followed by either PN or referral. Published data on the duration of GUM clinic consultations for PN were used.
In the nested PN trial for the ClaSS project, nurse-led PN at the practice for the index patient while the patient is receiving their own result was estimated to add just a few minutes extra on to the consultation time. For individuals in the comparator arm, where the index was advised to seek PN advice at the GUM clinic, the appointment with the nurse at the general practice surgery was for the purpose of receiving treatment and the result only. The index patient then required an additional appointment at the GUM clinic for PN advice. Partner(s) were also required to attend the GUM clinic for treatment and advice.
A slight adjustment was made to the ClaSS project results which are presented here. The initial appointment in the ClaSS project was estimated to take almost 42 minutes for those individuals randomised to the nurse-led PN strategy and 38.8 minutes for those randomised to the GUM PN strategy, but both these timings included time for randomisation and consent to the study. In the current study, the explanation of the study and taking of consent were estimated to take between 10 and 15 minutes. We have assumed the mid-point of this range (12.5 minutes) and deducted this from all the timings.
Partner notification in the Accelerated Partner Therapy study
The objective of the APT study was to compare costs and outcomes associated with three alternative methods of expedited PN in the primary care setting. The cost analysis carried out alongside the exploratory trial was conducted from the perspective of the NHS and considered only direct health service costs.
Following the pre-determined criteria, eligible index patients who had given consent to participate in the study were offered one of three methods of PN: (1) APTHotline, telephone assessment of their sex partner by a clinic-based nurse-qualified HA; (2) APTPharmacy, assessment of their sex partner by a trained community pharmacist; or (3) routine clinic PN, patient referral which included infection-specific information, advice that the sex partner should attend the clinic for testing and treatment and, in one clinic, a standard letter detailing antibiotic treatment options for the sex partner to give to his/her GP if appropriate. Each index patient was asked to choose which method they preferred for each contactable sexual partner. The index was instructed to provide the partner with all relevant information. Once the partner engaged with the allocated PN method, the appropriate health-care professional explained the study and sought consent from the partner to participate. Any partner who did not like the method of PN chosen for them by the index patient could default to routine PN (PN at GUM clinic as per ClaSS study).
In the APTHotline group, either partners collected the treatment pack from the clinic reception or the index patient could take the pack to them, which occurred if the sex partner completed his/her telephone assessment before the index patient had left the clinic. Partners in the APTPharmacy group received their treatment packs from the trained community pharmacist at the time of their consultation.
Engagement in the APT process was apparent when the partner adhered to the method of PN chosen for them by the index case. In some cases it was necessary for the index case to be followed up for confirmation about where, if at all, the partner received APT or routine care. All resource use incurred as a result of the APT strategies was collected prospectively.
Routine partner notification
Resource use associated with routine PN in the APT study was based on the primary data collected in the ClaSS project, as described in the previous section.
-
APTHotline
The estimated cost of the APTHotline included the cost of the telephone equipment, the cost of the consultation and the cost of the receptionist’s time spent giving out the packs (for those partners who collected the APT pack themselves). In addition to the duration of the consultation, it was assumed that the nurse-qualified HA spent 10 minutes carrying out administrative work such as filling in forms and passing on relevant information to the receptionist.
-
APTPharmacy
The duration of the consultation with the community pharmacist was recorded in the primary study and the cost per hour applied is based on recent published sources. In addition to the duration of the consultation, it was assumed, based on direct reports from the study, that the pharmacist spent 10 minutes carrying out administrative work such as filling in forms. The APT pack was collected on the spot, at the end of the consultation with the community pharmacist. Appropriate costs from secondary sources for 201169 have been applied to the resource use.
Patient and provider referral pathways as used in the pilot study
Two alternative approaches to PN were compared in the pilot: the patient referral pathway and the provider referral pathway. In both pathways, the first step is to notify the index patient of their positive diagnosis and invite them to attend the practice for treatment. At the appointment the general practice surgery nurse treats the patient and collects baseline information which is entered onto a custom-built web tool that sends an automatic email alert to the HA.
Index patients are randomised at general practice surgery level and the appropriate pathway is offered to the patient by the HA. The HA attempts to contact the index patient by telephone and a maximum of three attempts are applied to this stage. If the patient is not successfully contacted within three attempts they are not pursued further. The duration of all relevant contact is recorded on the web tool as far as possible by the HA.
-
Patient referral pathway
-
Under patient referral, patients are given information about their infection and asked to tell their partner about the problem and the need to be treated. This is carried out by the HA by telephone. During the initial call, the HA checks the patient’s baseline information and records additional information about the index’s sexual history and details of sexual partner(s). One point of follow-up is pursued with a maximum of three attempts made to contact the index. Information is recorded by hand on pro formas and transferred to the web tool.
-
-
1-week follow-up for index
-
The HA calls the index to check their treatment and adherence. The HA asks if the index’s partner(s) has/have accessed testing or treatment and also asks about any new partners. Information is recorded by hand on pro formas and transferred to the web tool.
-
-
Provider referral pathway
-
Under the provider referral pathway, in addition to information and support given as per patient referral, the index patients are asked if they would prefer the HA to contact one or more of their partner(s) on their behalf, anonymously if required. Indexes may use patient referral, provider referral or a mixture of both referral methods. For instance, index patients might accept the offer of provider referral for their ex-partners and patient referral for their current partner.
-
If the provider option is taken up, in addition to the details collected for patient referral, the HA takes partners’ contact information: name, telephone number and when there was sexual contact. The HA will then contact the partners. A maximum of three attempts are applied to this stage. If the patient is not successfully contacted within three attempts they are not pursued further. Information is recorded by hand on pro formas and transferred to the web tool. All partners contacted by the HA are followed up at 1 week.
-
-
1-week follow-up for partner
-
The HA calls the partner to check attendance, diagnosis, treatment and adherence. Information is recorded by hand on pro formas and transferred to the web tool.
-
Where a mixture of both patient and provider referral methods are used, the HA would follow up the index at 1 week regarding any partners that the index has contacted themselves, as per patient referral.
Results
Pathways 1–6 present the pathways for each of the six strategies under comparison. Tables 18–20 present the resource use in terms of items such as length of appointment, number of telephone calls, etc. The unit costs applied to these resource items such as relevant staff salary or cost of call and equipment is appropriately applied to each item.
| Resource used | Strategy 1: PN at practice (£, 2011) | Strategy 2: PN at GUM clinic (£, 2011) | ||||
|---|---|---|---|---|---|---|
| Time (a) | Hourly rate (b) | Cost (a) × (b) | Time (a) | Hourly rate (b) | Cost (a) × (b) | |
| Nurse consultation at general practice surgery for index includes PN advice | 29.41a minutes | £43b/60 minutes = 0.71 | £20.88 | – | – | – |
| Nurse consultation at general practice surgery for index excludes PN advice | – | – | – | 26.3a minutes | £43b/60 minutes = 0.71 | £18.67 |
| Index at GUM clinic receiving PN advice | – | – | – | 12a minutes | £43b/60 minutes = 0.71 | £8.52 |
| Partner at GUM clinic receiving PN advice | 12a minutes | £43b/60 minutes = 0.71 | £8.52 | 12a minutes | £43b/60 minutes = 0.71 | £8.52 |
| Follow-up: time spent by HA/admin in follow-up telephone calls (10 minutes) to index plus 5 minutes’ admin time | 15 minutes | £24b/60 minutes = 0.40 | £6.00 | 15 minutes | £24b/60 minutes = 0.40 | £6.00 |
| Cost of telephone calls: assumed two failures and one successful | – | £1.84 (successful), £0.25 (failure) | £2.34 | – | £1.84 (successful), £0.25 (failure) | £2.34 |
| Assume all index patients have 1.5 partners | – | – | £4.26 | – | – | £4.26 |
| Total cost | – | – | £42.00 | – | – | £48.31 |
| Resource used | Strategy 3: hotline (£, 2011) | Strategy 4: pharmacy (£, 2011) | ||||
|---|---|---|---|---|---|---|
| Time (a) | Hourly rate (b) | Cost (a) × (b) | Time (a) | Hourly rate (b) | Cost (a) × (b) | |
| Index identified as positive in GUM clinic receives explanation and PN advice | 24a minutes | £43b/60 minutes = 0.71 | £17.04 | 24a minutes | £43b/60 minutes = 0.71 | £17.04 |
| Partner phones APTHotline and speaks to HA | 11c minutes | £43b/60 minutes = 0.71 | £7.81 | – | – | – |
| Nurse admin time completing paper work | 10 minutes | £43b/60 minutes = 0.71 | £7.10 | – | – | – |
| Receptionist time for providing pack to partner | 5 minutes | £24b/60 minutes = 0.40 | £2.00 | – | – | – |
| Partner consultation time with pharmacist | – | – | – | 14c minutes | £60/60 minutes = 1.0 | £14.00 |
| Administration time incurred by pharmacists | – | – | – | 10 minutes | £60/60 minutes = 1.0 | £10.00 |
| Follow-up: time spent by HA in follow-up telephone calls (10 minutes) to index plus 5 minutes’ admin time | 15 minutes | £24b/60 minutes = 0.40 | £6.00 | 15 minutes | £24b/60 minutes = 0.40 | £6.00 |
| Cost of telephone equipment (mobile and credit) | Per partner | – | £1.37 | – | – | – |
| Follow-up to index to clarify partner has received treatment: cost of telephone calls: assumed two failures and one successful | – | £1.84 (successful), £0.25 (failure) | £2.34 | – | £1.84 (successful), £0.25 (failure) | £2.34 |
| Assume all index patients have 1.5 partners | Partner-related costs × 0.5 | (£7.81 + £7.10 + £2.00 + £1.37) = £18.28 × 0.5 | £9.14 | – | £14.00 × 0.5 = £7 | £7.00 |
| Total cost | – | – | £52.80 | – | – | £56.38 |
| Resource used | Strategy 5: patient referral (£, 2011) | Strategy 6: provider referral (£, 2011) | ||||
|---|---|---|---|---|---|---|
| Time (a) | Hourly rate (b) | Cost (a) × (b) | Time (a) | Hourly rate (b) | Cost (a) × (b) | |
| Nurse consultation at general practice surgery for index excludes PN advice. Baseline information entered onto web tool | 26.3 minutes (assumption based on ClaSS when PN is carried out at GUM clinic – this is assumed to include time taken for nurse to add details to web tool) | £43a/60 minutes = 0.71 | £18.67 | 26.3 minutes (assumption based on ClaSS when PN is carried out at GUM clinic – this is assumed to include time taken for nurse to add details to web tool) | £43a/60 minutes = 0.71 | £18.67 |
| HA receives alert. Initial assessment with index: the HA calls index, verifies baseline information and takes details of partner(s). Data transferred to web tool | 12.5b (10–15) minutes | £43a/60 minutes = 0.71 | £8.87 | 12.5b (10–15) minutes | £43a/60 minutes = 0.71 | £8.87 |
| HA discusses the option of provider referral | – | – | – | Assume 2.5 minutes extra (ClaSS) | £43a/60 minutes = 0.71 | £1.78 |
| Cost of call (assuming three in total, one successful and two failures) | – | £1.84 (successful), £0.25 (failure) | £2.34 | £1.84 (successful), £0.25 (failure) | £2.34 | |
| Admin time for 1-week call | 5 minutes | £24a/60 minutes = 0.40 | £2.00 | 5 minutes | £24a/60 minutes = 0.40 | £2.00 |
| Provider referral requires an initial assessment with partner: the HA calls partner, verifies baseline information and takes details of partner(s). Data transferred to web tool | – | – | – | 12.5b (10–15) minutes | £43a/60 minutes = 0.71 | £8.87 |
| Cost of call (assuming three in total, one successful and two failures) | – | – | – | £1.84 (successful), £0.25 (failure) | £2.34 | |
| Admin time for 1-week call | – | – | – | 5 minutes | £24a/60 minutes = 0.40 | £2.00 |
| Follow-up with index: the HA calls index, checks adherence, whether partners have been advised and treated and if any additional partner(s). Data transferred to web tool | 5 minutes | £43a/60 minutes = 0.71 | £3.55 | 5 minutes | £43a/60 minutes = 0.71 | £3.55 |
| Follow-up of index: cost of call (assuming three in total, one successful and two failures) | – | £1.84 (successful), £0.25 (failure) | £2.34 | – | £1.84 (successful), £0.25 (failure) | £2.34 |
| Follow-up of index: admin time for 1-week call | 5 minutes | £24a/60 minutes = 0.40 | £2.00 | 5 minutes | £24a/60 minutes = 0.40 | £2.00 |
| Follow-up with partner: the HA calls partner, checks adherence, and treatment and if any additional partner(s). Data transferred to web tool | N/A | N/A | N/A | 5 minutes | £43a/60 minutes = 0.71 | £3.55 |
| Follow-up of partner: cost of call (assuming three in total, one successful and two failures) | N/A | N/A | N/A | – | £1.84 (successful), £0.25 (failure) | £2.34 |
| Follow-up of partner: admin time for 1-week call | N/A | N/A | N/A | 5 minutes | £24a/60 minutes = 0.40 | £2.00 |
| Partner at GUM clinic receiving treatment and PN advice | 12c minutes | £43a/60 minutes = 0.71 | £8.52 | 12c minutes | £43a/60 minutes = 0.71 | £8.52 |
| Assume provider referral takeup(?) | N/A | N/A | N/A | 100% | 100% | 100% |
| Assume all index patients have 1.5 partners | Partner-related costs × 0.5 | £8.52 × 0.5 = £4.26 | £4.26 | Partner-related costs × 0.5 | £29.62 × 0.5 = £14.81 | £14.81 |
| Total cost | – | – | £52.53 | – | – | £85.98d |
The cheapest strategy is the ClaSS project strategy of providing PN advice to the index at the practice when the index receives the result of their own test (strategy 1), with the partner(s) being assumed to attend the local GUM clinic to get their own PN advice and treatment. This strategy has an estimated cost of approximately £42.00 per index, assuming the index case has 1.5 partners.
The ClaSS project strategy of PN at the GUM clinic (strategy 2) is the next cheapest strategy. This strategy assumes that the index case is identified at the practice but that the index then attends the GUM clinic to receive PN advice and the partner also subsequently presents at the GUM clinic for assessment and treatment. Thus there are additional health service interactions required, adding to the costs for this strategy which are estimated as £48.31 per index (assuming 1.5 partners).
Strategy 5 (patient referral) and strategy 3 (the APT study strategy of PN via a hotline) are roughly equal in cost. In the patient referral strategy (strategy 5), the patient assumes the responsibility of contacting the partner and the average cost per index of this strategy is £52.53 (assuming 1.5 partners). In the APT strategy (strategy 3), the index is assumed to be identified as positive at the GUM clinic and the partner is requested (by the index) to telephone the study hotline for assessment and advice about how to get treatment (each index is assumed to have 1.5 partners). The average cost per index case for strategy 3 is approximately £52.80. In both these strategies the effort of contacting the partner is incurred by the index.
Strategy 4 (PN via pharmacy) assumes the index receives PN advice at the GUM clinic during diagnosis but requires the partner to attend the pharmacy for assessment and treatment. The additional cost of the pharmacist consultation contributes to this strategy being slightly more expensive than the preceding ones. This strategy costs approximately £56.38 per index case (assuming 1.5 partners).
Strategy 6 (provider referral) is the most costly strategy. Like strategy 2 (PN at GUM clinic), this strategy requires additional interactions with the health service in that after initial diagnosis of the index (irrespective of the setting) the index does not receive PN advice at that point, but a subsequent and entirely new interaction is required as the HA receives an alert to contact the index, provide them with an assessment and discuss PN advice. In the provider referral strategy all the costs of contacting the partner(s) are borne by the health service and not the patient.
Thus strategy 6 (provider referral) is the most costly strategy, with an estimated cost of approximately £85.98 per index (assuming 1.5 partners). For this reported strategy it is assumed that all partners are contacted by the provider at the wish of the index, although in the strategy index cases could potentially choose a mixture of patient and provider referral approaches for their partners. If a mixture was the preferred approach then it can be assumed the average cost for the strategy would be somewhere between the results of strategy 6, where it is assumed in this case that provider referral is applied to all partners, and the results of strategy 5, where patient referral is applied to all partners (Figures 10–12).
FIGURE 10.
Chlamydia Screening Studies pathways. (a) PN at general practice surgery; and (b) PN at GUM clinic.

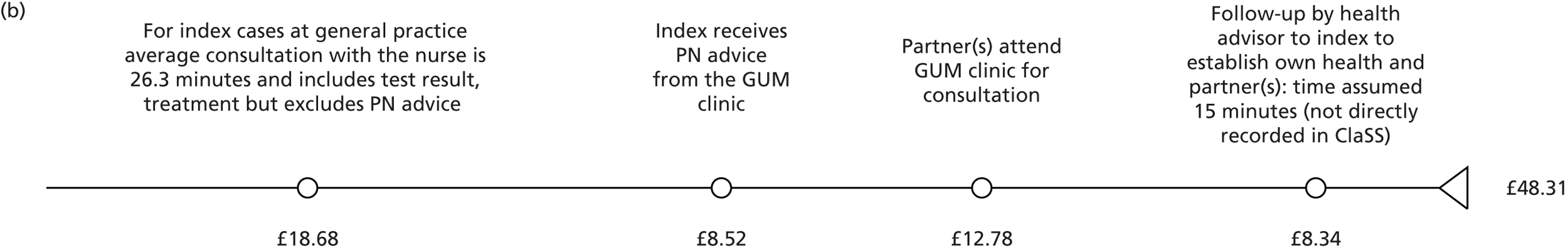
FIGURE 11.
Accelerated Partner Therapy pathways. (a) PN via hotline; and (b) PN via pharmacy.

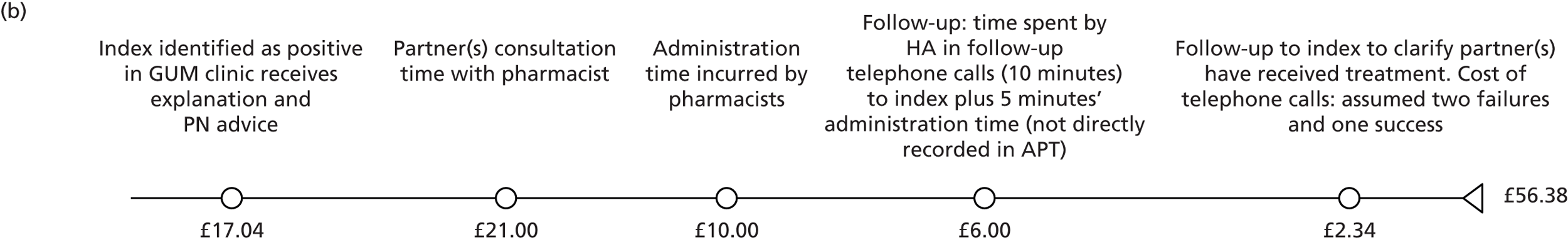
FIGURE 12.
Pilot pathways. (a) Patient referral; and (b) provider referral.


Discussion
The estimated costs of the alternative strategies of PN range from approximately £40 to £90 per index case, based on the assumptions that all index cases have 1.5 partners requiring PN and there is complete compliance of all index patients and their partners in adherence to whatever PN approach is indicated by the particular strategy, and excluding costs associated with the actual test and treatment for everyone involved.
The least costly strategy appears to be that of PN at the practice (strategy 1) as offered during the ClaSS project. This costs approximately £42.00 per index case. This strategy costs less than any other because the index receives PN advice at the same time as being diagnosed (i.e. receiving their own result) and the costs of contacting the potentially infected sexual partner(s) are borne entirely by the index, which is asked to inform their sexual partner that they should attend the GUM clinic for their own assessment and advice. There is no cost incurred by the health-care provider in this strategy related to contacting the partner, although the partner is ultimately assumed to attend the GUM clinic, and this cost is included.
Strategy 6 (provider referral) is the most costly strategy because the health service bears the costs of contacting the partners (or potentially some of them). If the index did not choose provider referral, even though offered, this strategy would cost no more than the patient referral strategy (strategy 5).
Strengths and weaknesses
The strength of this analysis is that it is the first to directly compare a full range of potentially feasible alternative strategies for PN advice to index patients and their partners, in terms of costs. The costs associated with some pathways were estimated as part of previous studies, and for the purpose of comparison some assumptions were required. However, in all cases, the assumptions were made in advance of any calculations and have not been adjusted in the light of the results.
A weakness in the analysis is that the comparison does not represent the costs associated with a ‘head to head’ comparison in a primary study. Furthermore, the costs presented are estimates and the detail of their differences should be interpreted with caution. The limited data available and the limitations imposed by the halting of the wider trial mean that the costs are estimates based on relatively small numbers and present an indication only of potential differences. Appropriate information with which to estimate confidence intervals around resource use, such as length of time for appointments, was available for some resources used but not others. Therefore, we have not presented confidence intervals for the estimated costs. Detailed scrutiny through in-depth sensitivity analysis would be inappropriate given the available data.
The major weakness is that very few positive index cases were identified to enable PN to be tested. We have compared the proposed pilot strategies with strategies from other studies, and it would not be appropriate to attempt to assess cost-effectiveness relative to other strategies, since the comparison was not head to head or randomised. Therefore, in order to facilitate the reporting of comparable costs for the pathways in this study, all strategies are assumed to be equally successful in achieving their objective. However, this is not likely to be the case and, as a result, costing studies alone cannot be helpful to decision-makers. Clearly the least costly strategies to administer will not necessarily save money if they are ineffective in achieving their desired objective. In contrast, the more costly strategies might be sufficiently more effective to justify the additional cost through a demonstrable increase in the success or effectiveness of the strategy. This has not been proved in this study. Indeed, the difficulty in trial recruitment and the failure to recruit enough infected individuals into the study was the reason that it is possible to present only an estimated comparison of costs. Thus, the major weakness of this study is that it has not been possible to assess the strategies in terms of their relative cost-effectiveness.
Conclusions
The results from this costing study suggest that alternative PN strategies are unlikely to differ much in terms of costs. However, the more responsibility is assumed by the health service to contact partners to facilitate PN strategies (and further follow index cases), the more costly the strategies will be.
Unanswered questions for future research
Whether or not the additional costs to the health service implied by strategies such as provider referral, which assume the most responsibility for contacting partners, are justified in terms of effectiveness and lead to a greater societal benefit by reducing the spread of the disease would need to be fully assessed in a cost-effectiveness study with appropriate population modelling.
Partner notification is an essential component of addressing and treating STIs. The conclusion from this study is that increasing the degree of responsibility assumed by the health service to reach partners will increase the cost. A justification for spending more of the health service’s limited resources to reach partners to achieve the outcome sought is required, which this study was unable to show.
Intensive recruitment to increase uptake of individuals being offered screening for chlamydia: is it effective and is it cost-effective?
Background
The planned economic data collection and analysis for this study was to be carried out during the main trial itself, and was not scheduled to have any role in the pilot trial. The objective of the pilot study was to explore the feasibility of recruiting individuals for chlamydia screening and PN in the general practice setting. When it became clear during the pilot that recruitment to the full trial may be problematic, the research team decided to explore a dedicated intensive recruitment approach.
Acknowledging that the full trial was unlikely to proceed without a better response to recruitment, and thus in an effort to salvage all possible information from the project’s pilot trial, the economic team considered it necessary to attempt to include a comparison to the intensive recruitment approach used in order to undertake an economic analysis. An economic evaluation is defined as a comparison of two or more interventions in terms of costs and outcomes. 67 Thus, the only comparison that could be considered which was pragmatic and would not add any additional burden to staff in practices (and to the research team who would recruit in the practices, and for whom recruitment was the main priority) was a simple and pragmatic ‘before and after’ (intensive recruitment) study.
In the pilot trial, a specific aim was to intensively recruit patients for chlamydia screening in general practices by introducing a suitably trained researcher into a practice for a 3-week period. The costs and outcomes associated with this approach could be measured and the success of intensive recruitment could potentially be evaluated for the purpose of a preliminary economic analysis, if it could be compared with the costs and resources used by, and the outcomes achieved, prior to the intensive recruitment phase.
Objective
What is the additional cost and success of intensively recruiting patients in general practices compared with the existing approach within any practice?
The data from ‘before-and-after’ comparisons are more appropriately presented as cost–consequence analyses, where costs and outcomes are presented in a disaggregated manner but in which no further analysis to achieve a cost-effectiveness result is undertaken. This is appropriate in this case because the data for the comparison are not derived from a controlled experiment.
Estimating costs and outcomes used in general practices in the pilot with regard to chlamydia screening and identifying positive cases
General practices in the UK manage a diverse range of primary care issues which might include antenatal care and advice, treating patients for minor illness, identifying infection and chronic illness and where necessary referring patients on to secondary care, to mention but a few. Screening and/or testing individuals for infection with chlamydia is one of many tests and services practices can provide. Although the practice readily provides this wide variety of services it must be realised that it is a finite resource: when a nurse or doctor provides an appointment for the purpose of screening and treating an individual who is positive for an infection such as chlamydia, it means that that particular appointment is not available to another patient who has a different need. Thus, providing a chlamydia test to someone who is not likely to be positive for chlamydia (a low-risk individual) means that not only is there a waste of the time and resources used in that particular appointment, there is also a forgone benefit for the individual who would have liked an appointment but did not get one and was given a place in the queue instead. In health economics, this is referred to as ‘opportunity cost’. Opportunity cost is the value of the consequences/outcome/benefits forgone by choosing to deploy a resource in one way rather than in its next best alternative use. 67 It is, therefore, appropriate in an economic analysis to estimate costs and outcomes associated with the intervention, even if the intervention is perceived to be a service that already exists and is already being paid for.
Methods
We provided practices with a short baseline questionnaire to explore their existing approach to chlamydia testing and PN. The questionnaire was developed in a relatively short time frame (when it was realised recruitment was poor) and was intended to be a pragmatic assessment of what the approach of a particular practice to chlamydia testing and PN was prior to the anticipated period of intensive recruitment. This was a one-off opportunity to assess the testing regime prior to the proposed intensive recruitment intervention, as the questionnaire was sent out just prior to planned intensive recruitment at the 11 practices. It was not possible given limited time and resources to monitor the exact throughput of testing and the result (positive or negative case of infection) and the questionnaire relied on the recall of the previous month of the member of staff completing the questionnaire. The data collection sheet used to assess the existing approach to testing is presented in Appendix 1.
The cost data applied to the resource use estimated by each practice are explained and presented in Appendix 2.
Results
Outcome data for existing approach to chlamydia testing
Eleven answered questionnaires were returned, all relating to the previous month. The questionnaires required the respondent for each practice to highlight the approximate range of the number of tests taken from choice groups of 10. The possible options for the response were ≤ 10, ≤ 20, ≤ 30, and so on – although in some cases the practice did report an exact number. Unless otherwise indicated, if a practice reported that they tested ≤ 10 individuals in a month it was assumed (as shown in Table 21) that they had carried out 10 tests (although it was acknowledged that less than or equal to 10 could mean only one, and it is also possible and perhaps more realistic to assume an average of five). Where they reported the ≤ 20 range, it was assumed the number of tests was 20 – and given there was a lower band that could have been chosen, that ‘less than or equal to 20’ meant at least more than 10 and so on. It is acknowledged that 15 could have been an appropriate estimate for the less than or equal to 20 band but we assume that if the true number of tests was really less than 10 the responder would have chosen the ‘less than or equal to 10’ choice band.
| Practice identifier code | Total number of individuals screeneda | Total number of individuals testing positive |
|---|---|---|
| 1 | 60 | 5 |
| 2 | 40 | 2 |
| 3 | 30 | 1 |
| 4 | 30 | 2 |
| 5 | 30 | N/A |
| 6 | 10 | N/A |
| 7 | 246 | 131 |
| 8 | 10 | 2 |
| 9 | 50 | 3 |
| 10 | 10 | 1 |
| 11 | 30 | 0 |
This assumption, based on the uppermost limit of the choice band, was used to avoid introducing a favourable bias towards intensive recruitment. For instance, if the lowest estimate or the mid-point in the range for the number of tests carried out in the previous month was presented, it might suggest that intensive recruitment was more effective than would be appropriate. So the assumption was a pragmatic attempt to avoid any bias towards the intensive recruitment strategy.
The result for the number of positive cases of chlamydia detected in the month prior to the intensive recruitment phase was reported on the questionnaire as an exact number.
The number of tests reported at each practice and the number of positive cases identified are presented for each practice in Table 21.
The costs associated with the existing approach to chlamydia testing for each practice are presented in detail in Appendix 2.
Discussion of existing approach
The results suggest that general practices believed there was reasonable activity in testing individuals for chlamydia in the month prior to intensive recruitment. It had been planned to estimate costs associated with the reported testing activity. These costs are reported in Appendix 2 as a result of our concern with regard to their reliability. We believe to present results in terms of the average cost per test carried out or average cost per positive case identified would be misleading. The limitation here is likely to result from recall bias of the staff completing the questionnaire. It is also acknowledged that the pragmatic design of the questionnaire, in asking respondents to report a general band of activity, may have led to inaccurate reporting.
Concern over some of the assumptions necessary to estimate the costs associated with testing and the reliability of the data provided on testing by each practice suggests they should be viewed with some caution. This is discussed further in the Discussion section in reference to some other data recently made available.
Intensive recruitment testing
The principal objective for the intensive recruitment process was to encourage all 16- to 24-year-olds to test for chlamydia and consent to participate in the trial. The use of external researchers, trained in the offer of chlamydia testing and consent, has been successful in a number of contexts.
The hypothesis was that for disease areas such as STIs, where there is much stigma and embarrassment associated with the disease, parachuting an expert into an environment to recruit and stimulate motivation within the practice might perhaps be an appropriate approach which could be both effective and potentially cost-effective.
The additional resource use and costs associated with intensive recruitment were all collected by the research team whilst undertaking the process in each practice. The number of tests carried out and the number of positive cases identified are also directly calculated by the trial manager and the research team.
A number of assumptions were required for the cost analysis to be completed.
Assumptions and framework for intensive recruitment
There are three different types of costs:
-
General costs including training, recruitment and set-up. These are distributed across all participating practices as a lump sum cost. In addition, each practice would receive service support costs for participating in the research.
-
Labour costs are already provided directly from the intensive recruitment team and not routine sources. The cost estimates for salary of the staff involved are specific to the grade of the researcher, the duration spent at the practice, and any equipment or disposables used.
-
There are no PN costs included because no data are provided on PN or its success.
All costs are presented in pounds sterling in 2011/12 prices.
Data for intensive recruitment approach based on costs of research staff
Data from 10 clinics were available to assess the impact of intensive recruitment. The staff time involved and the costs associated with the process are presented in Table 22. The details of staff time and costs are presented in Appendix 3. The total costs are apportioned across the clinics. The average length of the intensive recruitment at each clinic is between 15 and 18 days (weekdays).
| Individual | Labour cost per hour (£) |
|---|---|
| Trial manager | 20.92 |
| Research co-ordinator | 18.39 |
| IR researcher | 21.96 |
| Research admin | 14.36 |
| General practitioner | 80.00 |
| Research nurse | 21.96 |
| Practice manager | 21.96 |
| Admin | 12.44 |
The average numbers of days and hours spent at each clinic to carry out the process is presented in Table 23.
| Task | Time taken | Average | Max. | Min. |
|---|---|---|---|---|
| Setting up | Days | 1.4 | 2 | 1 |
| Hours | 11.57 | 20 | 8 | |
| Recruitment | Days | 14.9 | 18 | 11 |
| Hours | 133.95 | 169.5 | 105.5 |
In Table 24, we present the costs for each clinic for the intensive recruitment period. In Appendix 3 we present the breakdown of these costs. The fixed costs of the process for set-up and training are apportioned equally and a fixed element applied to all practices. The time, resources and associated costs for each practice were recorded directly by the intensive recruitment team.
| Practice identifier code | Cost of intensive screening (£, 2011/12) | Number of cases screened | Number of positive cases identified |
|---|---|---|---|
| 1 | 3846 | 32 | 0 |
| 2 | 4604 | 54 | 3 |
| 3 | 6164 | 41 | 0 |
| 4 | 2548 | 27 | 0 |
| 5 | N/A | N/A | N/A |
| 6 | 4825 | 30 | 0 |
| 7 | 1578 | 103 | 3 |
| 8 | 6408 | 47 | 0 |
| 9 | 2200 | 25 | 2 |
| 10 | 5517 | 39 | 0 |
| 11 | 4356 | 51 | 2 |
The variance seen between practices (from £1578 to £6408) is a result of the travel and accommodation costs for practices at a longer distance from the researchers’ base. The costs for practice 7 are considerably lower, as recruitment was managed by the local PCRN.
In Table 24, outcome data for the intensive recruitment period showing number of cases screened and number of cases identified as positive are presented. Compared with the ‘existing’ activity data as reported by the prior to intensive recruitment survey, there is an overall increase in the number of screening tests in 6 of the 11 practices. In 4 out of 11 practices intensive recruitment testing was lower than what was reported prior to the intensive recruitment period. One practice did not, in the event, undergo a period of intensive recruitment.
In April 2013, new data became available from the chlamydia testing activity data set (CTAD), which is a relatively new (set up in 2011) universal disaggregate data set for the reporting of chlamydia testing data from all NHS and NHS-commissioned laboratories in England. We accessed the data relevant to the practices in our survey for the month prior to intensive recruitment. These data are presented alongside the results from the ‘before’ intensive recruitment survey and alongside the activity that resulted from intensive recruitment. These are presented in Table 25.
| Practice identifier code | Self-reported number of individuals screeneda | Reported number of individuals screenedb (CTAD) | Number of individuals screened (intensive recruitment) | Self-reported number of individuals testing positivea | Reported number of individuals testing positiveb (CTAD) | Number of positive cases identified (intensive recruitment) |
|---|---|---|---|---|---|---|
| 1 | 60 | 29 | 32 | 5 | 0 | 0 |
| 2 | 40 | 55 | 54 | 2 | 2 | 3 |
| 3 | 30 | 19 | 41 | 1 | 0 | 0 |
| 4 | 30 | 51 | 27 | 2 | 1 | 0 |
| 5 | 30 | No IR | N/A | N/A | No IR | N/A |
| 6 | 10 | No data | 30 | N/A | No data | 0 |
| 7 | 246 | No data | 103 | 131 | No data | 3 |
| 8 | 10 | 55 | 47 | 2 | 1 | 0 |
| 9 | 50 | 45 | 25 | 3 | 1 | 2 |
| 10 | 10 | 12 | 39 | 1 | 0 | 0 |
| 11 | 30 | 24 | 51 | 0 | 1 | 2 |
We compared CTAD figures for the month prior to intensive recruitment with the testing rates reported by practices. Three practices provided a reasonable recalled estimate of their activity (practices 9, 10, and 11). Although practices 9 and 11 might appear to have overestimated their screening rates, this is an artefact of the questionnaire in which the upper limit of the range offered was assumed.
Three results based on the questionnaire cannot be verified by the CTAD data. Practices 2, 4, 8 and 10 all underestimated their screening rates, and two practices overestimated their screening rates (practices 1 and 3) in the pre-intensive recruitment survey questionnaire.
All the results suggest that intensive recruitment is costly, but importantly it does not seem to produce a respectable number of positive cases of chlamydia identified, given the cost and effort.
The results of this study suggest that intensive recruitment helped to increase the number of tests carried out in some practices but not others, given the result of the survey carried out before intensive recruitment and the results of the CTAD figures. It is clear that the additional tests being carried out are not effective at identifying individuals who have the disease. The results suggest that in some practices there was an increase in the number of people who were tested but this did not lead to an increase in the number of positive cases identified.
Discussion
Although a comparison of activity before and after a period of intensive recruitment to screening was attempted, the results of our study suggest that a full economic evaluation was not required and a cost and consequence analysis was the most appropriate approach. The results show that the number of tests assumed carried out prior to intensive recruitment and reported through recall when completing the questionnaire was lower in the majority of practices (6 out of 11) before intensive recruitment took place. Thus, intensive recruitment for these six practices did improve the screening rate based on the questionnaire results. However, a comparison with the CTAD figures makes any inference from the effectiveness of intensive recruitment less clear. In 3 out of 11 practices it was implied that the screening rate was higher in the period before intensive recruitment than during intensive recruitment.
The results suggest that the intensive recruitment period may have been successful in a few practices in increasing the number of screening tests carried out in the general practice setting. The results suggest that the recalled number of positive cases detected in any practice in the month prior to intensive recruitment is likely to be an optimistic estimate.
However, it is clear that while intensive recruitment may have increased the number of tests carried out (although this is not convincing given the CTAD data), it did not lead to an improvement in the number of positive cases of infection being identified.
Costs associated with screening prior to the period of intensive recruitment were estimated and are reported in Appendix 2. The results of the costing exercise should be interpreted with caution, as a result of some of the assumptions relating to the number of tests carried out, who carried them out and the time devoted to them being carried out. However, these results suggest much higher costs associated with intensive recruitment.
The strength of this analysis is that it was the first primary study to collect cost and outcome data associated with active intensive recruitment of STI screening in a trial that was failing to recruit. All cost and resource use data, as well as all the clinical data on numbers of individuals who were screened and detected as positive and treated, have been collected in a primary research study. A limitation is that no robust comparator data exist with which to conduct an economic evaluation, and a further limitation is that these data were not collected as part of a controlled study. Furthermore, to estimate costs some assumptions were made about the time and resource use of the staff carrying out the tests.
The inferences of this study are that intensive recruitment was actually not effective at detecting positive cases. Intensive recruitment is shown to be costly and there is no clear improvement in terms of a resounding effect on numbers of positive chlamydia cases identified, as the positive cases identified remained zero in the majority of practices. The number of individuals undergoing a screening test may increase as a result of intensive recruitment, although the results of this study do not provide strong support for this. But it is clear that intensive recruitment is costly and does not achieve the desired objective of finding positive cases and treating them.
Chapter 11 Results of all phases
Partner notification outcomes
During all phases of the pilot a total of 25 (3.2%; n = 783) patients were diagnosed positive for a STI (24 for chlamydia and one for genital herpes) across eight practices. They are summarised in Figure 13. The majority of patients (n = 18) came through patient referral practices, five through provider and two through contract (Figures 14–17). The majority of patients diagnosed with a STI were female (22 females vs. 3 males). The HA was able to contact successfully 11 (44%) of the patients diagnosed with a STI, six (24%) of whom were followed up at 1 week and two (8%) subsequently followed up at the 6-week and 3-month periods.
FIGURE 13.
Follow-up of positive respondents.

FIGURE 14.
Consent at diagnosis practices recruited in phase 1 and continuing (n = 3) (8 November 2010–31 July 2012).
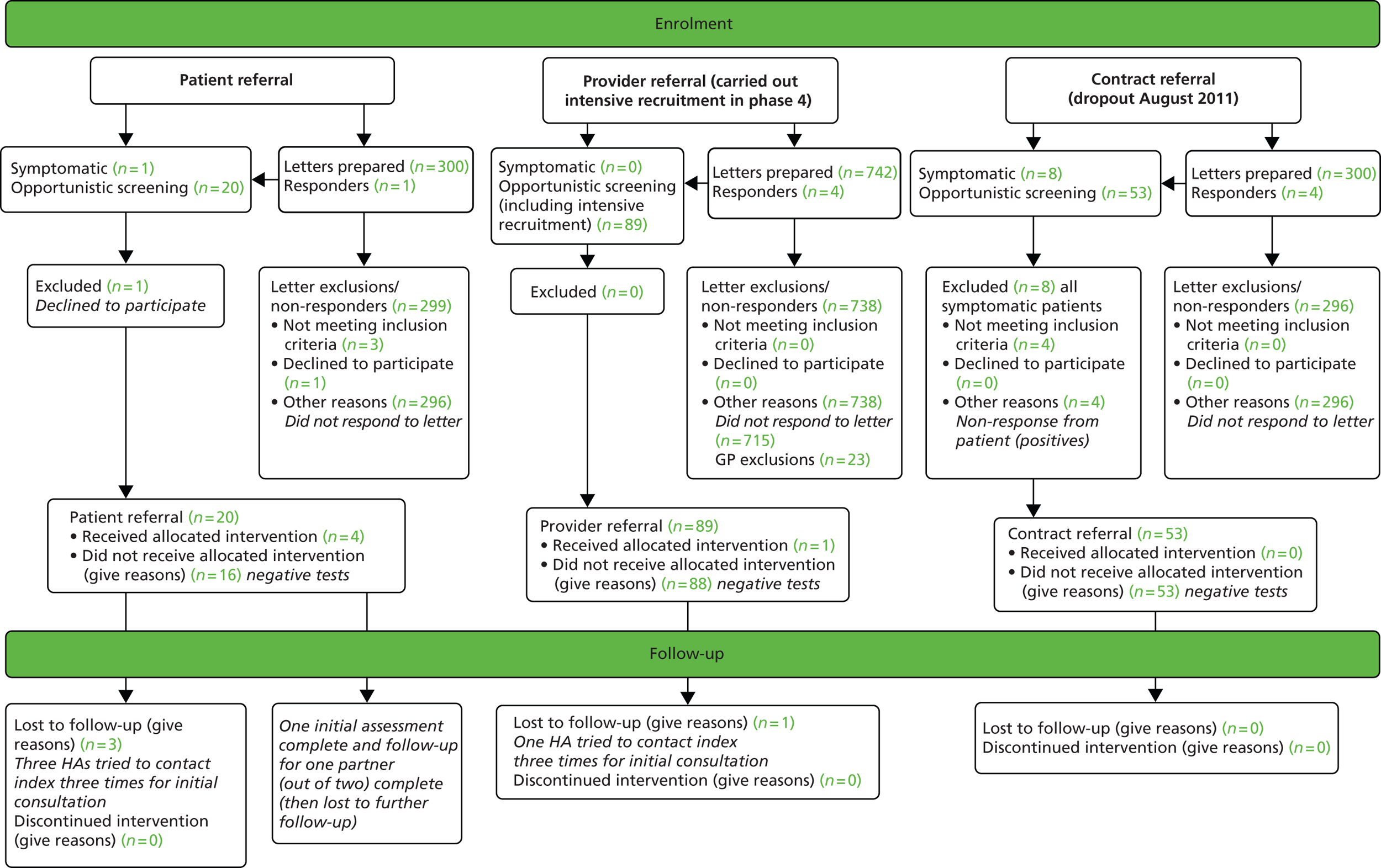
FIGURE 15.
Consent at test practices recruited in phase 1 and continuing (n = 3) (November 2010–31 July 2012).
FIGURE 16.
Consent at test practices newly recruited in phase 3 and continuing (n = 2) (11 July 2011–31 July 2012).

FIGURE 17.
Intensive recruitment practices recruited in phase 4 (n = 8) (2 February 2012–3 August 2012).

Partners were known to have been tested for four patients; three partners were diagnosed positive for chlamydia and for two of these the HA was told by the index patient that they had been treated. The two participants who were followed up at the 3-month period were retested for chlamydia, with both testing negative.
Given the small numbers, it is not possible to present outcome data for the range of primary and secondary outcomes.
Consolidated Standards of Reporting Trials diagrams
Summary recruitment data have been given at the end of each chapter describing a pilot phase, with a complete summary at the end of phase 4. Here we present for completeness the Consolidated Standards of Reporting Trials (CONSORT) flow diagrams for the various phases and categories of practice (Figures 14–18).
FIGURE 18.
Consent at diagnosis practice recruited in phase 4 (n = 1) (3 February 2012–31 July 2012).
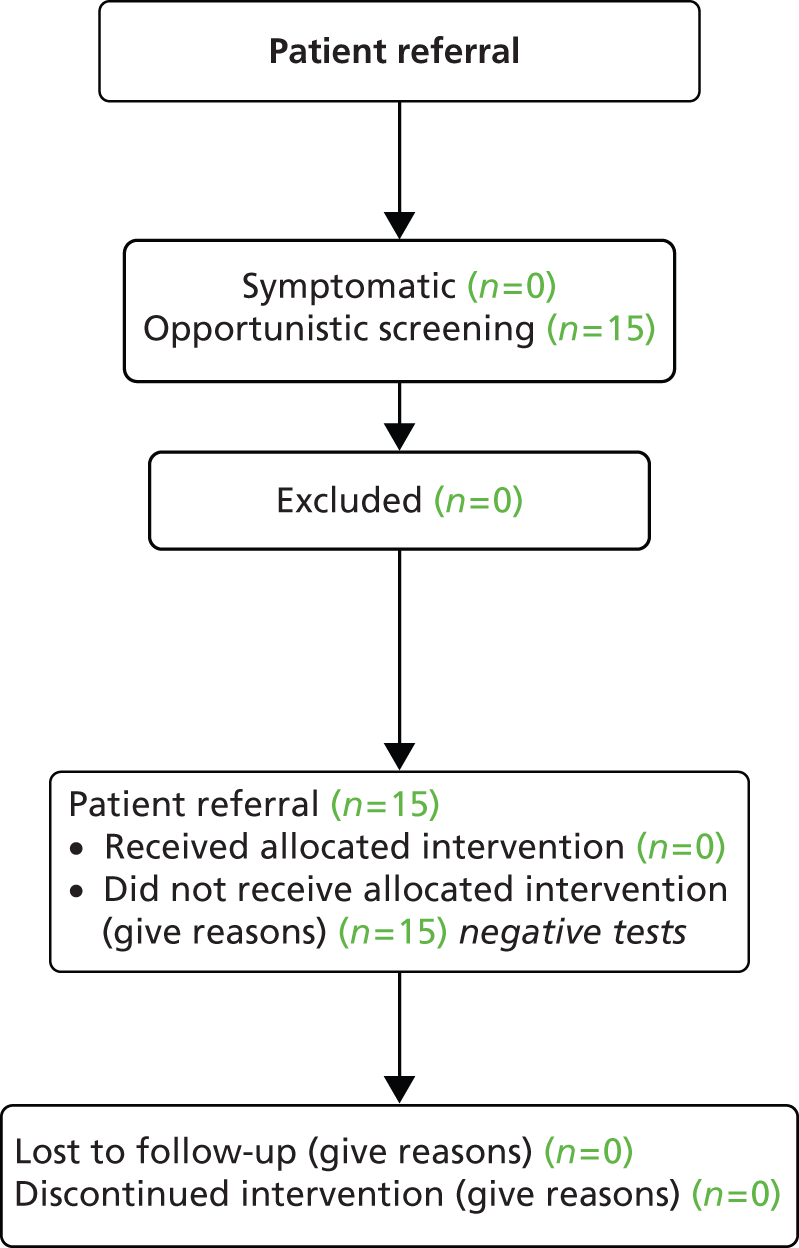
Chapter 12 Overall assessment
Summary evaluation
This was a PN trial which successfully recruited general practice surgeries in an initial pilot phase. However, despite being embedded within the NCSP, it foundered on a lack of chlamydia testing in recruited practices and we were not able to recruit sufficient individuals with chlamydia or other bacterial STI. Our initial strategy for recruitment was flawed. However, even with extensive use of existing and newly emerging evidence, consultation with stakeholders, and sufficient remuneration in the form of service support costs, we were unable to resolve the problem of recruitment failure.
In phase 4 we were able to demonstrate that recruitment by external researchers allocated to practices for periods of 2–3 weeks could provide additional tests, and this strategy may be of relevance to others seeking to recruit young people in primary care for other purposes. However, by this stage, it had been agreed that scale-up was unlikely to be feasible.
As a consequence, it was not possible to answer the research question.
As shown in the small number of data available on individuals diagnosed with chlamydia through the trial, there was also suboptimal retention. However, this was not of an extent that need have been fatal to the study given sufficient testing, and as a result of lack of numbers it remains unclear whether this could have been resolved by streamlining strategies for communication with diagnosed patients. This is being explored in an ongoing, post-trial evaluation of the web tool designed for this study with a view to future use in clinical service.
Reasons for recruitment failure and lessons learned
General practice
As previously described in Chapter 4, practice staff held a number of incorrect beliefs about young people’s attendance in practice, the acceptability to them of testing for chlamydia in this setting, and the prevalence of chlamydial infection. However, addressing these beliefs had little effect on recruitment.
A more fundamental barrier appeared to be the inability of nurses and doctors to prioritise, within a busy and highly reactive workplace culture, the promotion of asymptomatic testing for chlamydia. This was felt to be embarrassing for staff and potentially stigmatising to young people. Recruitment is easiest where patients are gathered in a designated clinic, which was not possible in this case.
We were able to demonstrate that use of an external researcher to recruit in the waiting room, as in a related Australian study, could enable opportunistic recruitment on a scale which did not appear to be possible within general practice itself, despite remuneration. Although this was not an inexpensive option, it should be considered by future researchers seeking to recruit young people opportunistically in primary care, especially on sensitive subjects.
The epidemiological basis for the study
We, our funders, and colleagues at the NCSP offices of the HPA had assumed that the considerable aggregate numbers of chlamydia tests that were being taken in primary care, and the growing proportion of all tests from that setting, would be sufficient to underpin this trial assuming careful selection of practices. However, none of us had realised the degree of dispersion of chlamydia tests within NCSP in a very large number of practices, and the very small number of practices that were currently doing sufficient tests to make a minimally useful contribution to recruitment. This finding presented a challenge that was to prove fatal to our study, given that we were not able to change testing behaviours substantially.
It also presents an interesting challenge for future studies in this area. The vast majority of practices continue to make tiny numbers of diagnoses (one to five per year), despite longstanding national policy commitments to developing STI care in general practice. This has implications for the appropriate orientation of future research in this field. Two service problems that need future research support are:
-
How can any care pathway be delivered on such an occasional basis, yet to ensure that people diagnosed with STI in primary care are offered an equivalent PN service to those diagnosed in sexual health-oriented services?
-
If general practice does not provide these tests, where, given current policy as discussed in Chapter 7, can they be accessed by the many young people who are not able to use a conveniently located sexual health service?
Partner notification and its evaluation in future trials
We were unable, in the event, to standardise or operationalise the proposed three arms of this trial. As previously noted, although several three-way trials have indeed been published,9–11 none to date has compared contract, provider and patient referral directly. We used an innovative participative methodology to explore HA practices. This work demonstrated that, in practice, provider and contract referral are subsumed together in a process of negotiation over time in the case of common bacterial STIs including chlamydia and gonorrhoea. This was close to a model of PN used in a trial by Cleveland in which provider referral was offered if a partner had not presented within 3 days. 10 In contrast, ‘contract referral’ in its standard meaning was used almost exclusively by these practitioners in managing infections with longstanding infectivity that were considered to have more serious health consequences, such as HIV, hepatitis B and C, and syphilis.
The structures of the National Chlamydia Screening Programme and their implications for future sexual health research in primary care
As previously noted, the majority of local CSOs managed patients testing positive from general practice without the practice taking part in this process. There was a culture of separation between these two services, which made it difficult for staff in CSOs to believe that a competent service could be provided from general practice for these patients, even though sexual health work takes place routinely in general practice. Structural features of the NCSP at the time of the study also meant that it was difficult for local chlamydia-screening staff not to see the study as a ‘competitor’ for their target numbers and – in the longer term – providing a model of service that undermined the future of the CSO in its form at the time of the study.
It will be important for commissioners of research to consider the need for a background of consistency and mutual support for future studies and to work with commissioners (now the local authorities) to facilitate research effectively. This will be needed to underpin best practice in all settings and also to enable audit, evaluation and research into complex interventions such as PN in community settings. Although research in this field is difficult, the absolute numbers and proportion of people diagnosed with STIs in primary care are substantial. 12,13,70 It is essential that continued research enable them to receive the high-quality care they deserve as the provision in primary care evolves. 55,57
Acknowledgements
We would like to acknowledge our Trial Steering Committee, chaired by Professor Paul Little; Professor Nicola Low, who contributed to the Trial Steering Committee and gave additional help; members of the ACCEPt team in Australia; Jason May and Suzy Dion; members of Sexpression; students at Brighton Hove and Sussex Sixth Form College; and all the practices, practice staff and patients who gave us their time and energy. We also thank Alireza Talebi, Bersabeh Sile and Dr Mary Macintosh of the HPA. We would also like to acknowledge the NIHR HTA programme for their ongoing support and advice before, during and beyond the study.
Contributions of authors
Professor Jackie A Cassell was the principal investigator and, together with Dr Greta Rait, took overall responsibility for the design, co-ordination and supervision of the study.
Dr Julie Dodds led and project managed the delivery of fieldwork, including the commissioning of the web tool, in close collaboration with Ms Stefania Lanza.
Dr Claudia Estcourt and Mr Merle Symonds contributed to the design and execution of the study, particularly on the specification and delivery of PN services and clinical issues in the management of STIs and PN.
Professor Tracy Roberts, Dr Hema Mistry and Mr Melcior Rossello-Roig designed and undertook the economic analysis.
Dr Carrie Llewellyn provided health psychology advice on the design of the intervention and on exploring PN practices.
Dr John Richens advised on digital data collection and the use of incentives in a sexual health setting.
Professor Helen Smith advised on all aspects of recruitment and care in general practice, as did Dr Kate Walters, who also provided maternity cover for Dr Greta Rait.
Dr Catherine Lowndes provided advice and expertise relating to the National Chlamydia Screening Programme.
Dr Andrew Copas provided expert statistical input and analysis, and Dr Peter White advised on the design of data collection with a view to modelling studies.
Dr Hilary Smith contributed to the design and analysis of the exploration of PN practice.
Dr Julie Dodds, Professor Tracy Roberts, Ms Stefania Lanza and Professor Jackie Cassell led the drafting of the report and all authors commented and contributed to redrafting.
Disclaimer
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Sexually Transmitted Infections and Young People in the United Kingdom: 2008 Report. London: Health Protection Agency; 2008.
- Hughes G, Nardone A. Sexual Health in Adolescents in the UK: What Do the Data Show?. London: Health Protection Agency; 2011.
- Arthur G, Lowndes CM, Blackham J, Fenton KA. European Surveillance of Sexually Transmitted Infections (ESSTI) Network . Divergent approaches to partner notification for sexually transmitted infections across the European Union. Sex Transm Dis 2005;32:734-41. http://dx.doi.org/10.1097/01.olq.0000175376.62297.73.
- Catchpole M. Sexually transmitted infections: control strategies. BMJ 2001;322:1135-6. http://dx.doi.org/10.1136/bmj.322.7295.1135.
- Low N, Bender N, Nartey L, Redmond S, Shang A, Stephenson J. Revised Rapid Review of Evidence for the Effectiveness of Screening for Genital Chlamydial Infection in Sexually Active Young Women and Men. London: National Institute for Health and Clinical Excellence; 2006.
- LaMontagne DS, Fenton KA, Randall S, Anderson S, Carter P. Establishing the National Chlamydia Screening Programme in England: results from the first full year of screening. Sex Transm Infect 2004;80:335-41. http://dx.doi.org/10.1136/sti.2004.012856.
- Low N, McCarthy A, Roberts TE, Huengsberg M, Sanford E, Sterne JA, et al. Partner notification of chlamydia infection in primary care: randomised controlled trial and analysis of resource use. BMJ 2006;332:14-9. http://dx.doi.org/10.1136/bmj.38678.405370.7C.
- Bell G, Ward H, Day S, Ghani AC, Goan U, Claydon E, et al. Partner notification for gonorrhoea: a comparative study with a provincial and a metropolitan UK clinic. Sex Transm Infect 1998;74:409-14. http://dx.doi.org/10.1136/sti.74.6.409.
- Potterat JJ, Rothenberg R. The case-finding effectiveness of self-referral system for gonorrhea: a preliminary report. Am J Public Health 1977;67:174-6. http://dx.doi.org/10.2105/AJPH.67.2.174.
- Cleveland J. A Cost-Effectiveness Study of Alternative Methods for Gonorrhea Contact Referral and Rescreening. Dade County, FL: Dade County Department of Public Health; 2011.
- Katz BP, Danos CS, Quinn TS, Caine V, Jones RB. Efficiency and cost-effectiveness of field follow-up for patients with Chlamydia trachomatis infection in a sexually transmitted diseases clinic. Sex Transm Dis 1988;15:11-6. http://dx.doi.org/10.1097/00007435-198801000-00003.
- Cassell JA, Mercer CH, Sutcliffe L, Petersen I, Islam A, Brook MG, et al. Trends in sexually transmitted infections in general practice 1990–2000: population based study using data from the UK general practice research database. BMJ 2006;332:332-4. http://dx.doi.org/10.1136/bmj.38726.404120.7C.
- Hughes G, Williams T, Simms I, Mercer C, Fenton K, Cassell J. Use of a primary care database to determine trends in genital chlamydia testing, diagnostic episodes and management in UK general practice, 1990–2004. Sex Transm Infect 2007;83:310-13. http://dx.doi.org/10.1136/sti.2006.022673.
- Mercer CH, Sutcliffe L, Johnson AM, White PJ, Brook G, Ross JD, et al. How much do delayed healthcare seeking, delayed care provision, and diversion from primary care contribute to the transmission of STIs?. Sex Transm Infect 2007;83:400-5. http://dx.doi.org/10.1136/sti.2006.024554.
- Bailey AC, Johnson SA, Cassell JA. Are primary care-based sexually transmitted infection services in the UK delivering public health benefit?. Int J STD AIDS 2010;21:39-45. http://dx.doi.org/10.1258/ijsa.2009.008461.
- Cassell JA, Brook MG, Slack R, James N, Hayward A, Johnson AM. Partner notification in primary care. Sex Transm Infect 2003;79:264-5. http://dx.doi.org/10.1136/sti.79.3.264-a.
- Mason D, Kerry S, Oakeshott P. Postal survey of management of cervical Chlamydia trachomatis infection in English and Welsh general practices. BMJ 1996;313:1193-4. http://dx.doi.org/10.1136/bmj.313.7066.1193.
- Ross JD, Sutherland S, Coia J. Genital Chlamydia trachomatis infections in primary care. BMJ 1996;313:1192-3. http://dx.doi.org/10.1136/bmj.313.7066.1192a.
- Andersen B, Ostergaard L, Nygard B, Olesen F. Urogenital Chlamydia trachomatis infections in general practice: diagnosis, treatment, follow-up and contact tracing. Fam Pract 1998;15:223-8. http://dx.doi.org/10.1093/fampra/15.3.223.
- White C, Wardropper AG. Chlamydia in a district general hospital: an audit of treatment and contact tracing. Int J STD AIDS 1999;10:57-9. http://dx.doi.org/10.1258/0956462991912953.
- Alary M, Joly JR, Poulin C. Gonorrhea and chlamydial infection: comparison of contact tracing performed by physicians or by a specialized service. Can J Public Health 1991;82:132-4.
- Eitrem R, Erenius M, Meeuwisse A. Contact tracing for genital Chlamydia trachomatis in a Swedish county. Sex Transm Dis 1998;25:433-6. http://dx.doi.org/10.1097/00007435-199809000-00010.
- Low N, McCarthy A, Macleod J, Salisbury C, Campbell R, Roberts TE, et al. Epidemiological, social, diagnostic and economic evaluation of population screening for genital chlamydial infection. Health Technol Assess 2007;11.
- One to One Interventions to Reduce the Transmission of Sexually Transmitted Infections (STIs) including HIV, and to Reduce the Rate of Under 18 Conceptions, Especially among Vulnerable and at Risk Groups. London: NICE; 2007.
- Pimenta JM, Catchpole M, Rogers PA, Perkins E, Jackson N, Carlisle C, et al. Opportunistic screening for genital chlamydial infection. I: acceptability of urine testing in primary and secondary healthcare settings. Sex Transm Infect 2003;79:16-21. http://dx.doi.org/10.1136/sti.79.1.16.
- National Chlamydia Screening Programme Standards. London: Health Protection Agency; 2012.
- Johnson SA, Simms I, Sheringham J, Bickler G, Bennett CM, Hall R, et al. The implementation of chlamydia screening: a cross-sectional study in the south east of England. Sex Transm Infect 2010;86:217-21. http://dx.doi.org/10.1136/sti.2009.037283.
- NCSP: Five Years: The Fifth Annual Report of the National Chlamydia Screening Programme 2007/08. London: Health Protection Agency; 2008.
- New Frontiers: Annual Report of the National Chlamydia Screening Programme in England 2005/06. London: Health Protection Agency; 2006.
- Public Health Outcomes Framework 2013 to 2016. London: Department of Health; 2012.
- Trelle S, Shang A, Nartey L, Cassell J, Low N. Revised Rapid Review of Evidence for the Effectiveness of Partner Notification for Sexually Transmitted Infections including HIV. London: National Institute for Health and Clinical Excellence; 2009.
- Cassell JA. The Potential of Primary Care for Reducing the Transmission of Sexually Transmitted Infections. Cambridge: University of Cambridge; 2008.
- Estcourt C, Sutcliffe L, Cassell J, Mercer CH, Copas A, James L, et al. Can we improve partner notification rates through expedited partner therapy in the UK? Findings from an exploratory trial of Accelerated Partner Therapy (APT). Sex Transm Infect 2012;88:21-6. http://dx.doi.org/10.1136/sti.2010.047258.
- Developing and Evaluating Complex Interventions: New Guidance. London: Medical Research Council; 2008.
- Thabane L, Ma J, Chu R, Cheng J, Ismaila A, Rios LP, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol 2010;10. http://dx.doi.org/10.1186/1471-2288-10-1.
- Campbell R, Mills N, Sanford E, Graham A, Low N, Peters TJ. Does population screening for Chlamydia trachomatis raise anxiety among those tested? Findings from a population based chlamydia screening study. BMC Public Health 2006;6. http://dx.doi.org/10.1186/1471-2458-6-106.
- Santer M, Wyke S, Warner P. Women’s experiences of Chlamydia screening: qualitative interviews with women in primary care. Eur J Gen Pract 2003;9:56-61. http://dx.doi.org/10.3109/13814780309160403.
- Perkins E, Carlisle C, Jackson N. Opportunistic screening for chlamydia in general practice: the experience of health professionals. Health Soc Care Community 2003;11:314-20. http://dx.doi.org/10.1046/j.1365-2524.2003.00437.x.
- McNulty CA, Freeman E, Howell-Jones R, Hogan A, Randall S, Ford-Young W, et al. Overcoming the barriers to chlamydia screening in general practice – a qualitative study. Fam Pract 2010;27:291-302. http://dx.doi.org/10.1093/fampra/cmq004.
- Walker J, Fairley CK, Urban E, Chen MY, Bradshaw C, Walker SM, et al. Maximising retention in a longitudinal study of genital Chlamydia trachomatis among young women in Australia. BMC Public Health 2011;11. http://dx.doi.org/10.1186/1471-2458-11-156.
- Bilardi JE, Fairley CK, Temple-Smith MJ, Pirotta MV, McNamee KM, Bourke S, et al. Incentive payments to general practitioners aimed at increasing opportunistic testing of young women for chlamydia: a pilot cluster randomised controlled trial. BMC Public Health 2010;10.
- Salisbury C, Macleod J, Egger M, McCarthy A, Patel R, Holloway A, et al. Opportunistic and systematic screening for chlamydia: a study of consultations by young adults in general practice. Br J Gen Pract 2006;56:99-103.
- Andersen B, Olesen F, Moller JK, Ostergaard L. Population-based strategies for outreach screening of urogenital Chlamydia trachomatis infections: a randomized, controlled trial. J Infect Dis 2002;185:252-8. http://dx.doi.org/10.1086/338268.
- McNulty CA, Freeman E, Oliver I, Ford-Young W, Randall S. Strategies used to increase chlamydia screening in general practice: a qualitative study. Public Health 2008;122:845-56. http://dx.doi.org/10.1016/j.puhe.2007.10.009.
- Towards Best Practice for Chlamydia Screening in Reproductive and Sexual Health Services (RSH). London: Health Protection Agency; 2010.
- NHS Vital Signs 2010/11. London: Health Protection Agency; 2011.
- QRESEARCH, The Health and Social Care Information Centre . Trends in Consultation Rates in General Practice 1995 1996 to 2007 2008: Analysis of the QRESEARCH Database 2008.
- Pimenta JM, Catchpole M, Rogers PA, Hopwood J, Randall S, Mallinson H, et al. Opportunistic screening for genital chlamydial infection. II: prevalence among healthcare attenders, outcome, and evaluation of positive cases. Sex Transm Infect 2003;79:22-7. http://dx.doi.org/10.1136/sti.79.1.22.
- Hippisley-Cox J, Fenty J, Heaps M. Trends in Consultation Rates in General Practice 1995 to 2006: Analysis of the QRESEARCH Database. Leeds: The Information Centre for Health and Social Care; 2007.
- Grun L, Tassano-Smith J, Carder C, Johnson AM, Robinson A, Murray E, et al. Comparison of two methods of screening for genital chlamydial infection in women attending in general practice: cross sectional survey. BMJ 1997;315:226-30. http://dx.doi.org/10.1136/bmj.315.7102.226.
- Fenton KA, Ward H. National chlamydia screening programme in England: making progress. Sex Transm Infect 2004;80:331-3. http://dx.doi.org/10.1136/sti.2004.009787.
- Young People’s Sexual Health: The National Chlamydia Screening Programme. London: Department of Health; 2009.
- NCSP Core Requirements. London: Health Protection Agency; 2010.
- The Bigger Picture: The National Chlamydia Screening Programme 2008/09 Annual Report. London: Health Protection Agency; 2009.
- The Future Direction of the National Chlamydia Screening Programme. London: Department of Health; 2011.
- Integrating the National Chlamydia Screening Programme within Local Sexual Health Economies. London: Department of Health; 2012.
- A Framework for Sexual Health Improvement in England. London: Department of Health; 2013.
- Llewellyn C, Smith H, Pollard A. What is it about STI research that is unappealing? An experience of conducting sexual health research in general practice in England. Sex Transm Infect 2012;88. http://dx.doi.org/10.1136/sextrans-2012-050585.
- Low N, Geisler W, Stephenson J, Hook EW, Aral SO, Fenton KA, et al. The New Public Health and STD/HIV Prevention. New York, NY: Springer; 2013.
- Low N, Cassell JA, Spencer B, Bender N, Hilber AM, van Bergen J, et al. Chlamydia control activities in Europe: cross-sectional survey. Eur J Public Health 2011;22:556-61. http://dx.doi.org/10.1093/eurpub/ckr046.
- UK National Screening Committee . Related Programmes 2013. www.screening.nhs.uk/ (accessed 17 September 2014).
- E6 Guideline for Good Clinical Practice. Rockville, MD: U.S. Department of Health and Human Services, Food and Drug Administration; 1996.
- Data Protection Act 1998: Elizabeth II. London: The Stationery Office; 1998.
- The Manual for Sexual Health Advisers. London: Society of Sexual Health Advisers; 2004.
- Cowan FM, French R, Johnson AM. The role and effectiveness of partner notification in STD control: a review. Genitourin Med 1996;72:247-52.
- Kensing F, Blomberg J. Participatory design: issues and concerns. Comp Support Coop Work (CSCW) 1998;7:167-85. http://dx.doi.org/10.1023/A:1008689307411.
- Drummond MF, Sculpher MJ, Torrance GW, O’Brien BJ, Stoddart GL. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Roberts TE, Tsourapas A, Sutcliffe L, Cassell J, Estcourt C. Is Accelerated Partner Therapy (APT) a cost-effective alternative to routine patient referral partner notification in the UK? Preliminary cost–consequence analysis of an exploratory trial. Sex Transm Infect 2012;88:16-20. http://dx.doi.org/10.1136/sextrans-2011-050176.
- Curtis L. Unit Costs of Health and Social Care 2011. Canterbury: Personal Social Services Research Unit, University of Kent; 2011.
- Chlamydia Testing Data for 15–24 year olds in England, January to December 2012. London: Public Health England; 2013.
Appendix 1 Pre-intensive recruitment questionnaire
Chlamydia screening and the treatment of chlamydia infections in GP practices varies across the UK and may or may not utilise the National Chlamydia Screening Programme which is delivered in GP practices. This questionnaire will help us to understand how Chlamydia screening and treatment is currently managed in your practice.
Once you have completed this questionnaire, please fax or e-mail it back to XXX (trial manager contact details)
CLINIC NO: XXX DATE: XX.XX.XX
| Section A: general approach to chlamydia testing | ||||||
|---|---|---|---|---|---|---|
| Do you currently offer Chlamydia testing ‘in-house’? | Often | Never | Occasionally | |||
| Do you proactively identify 16- to 24-year olds for chlamydia testing? | Yes | No | If yes, please explain how and who does this? | |||
| How often do you proactively identify 16- to 24-year olds for chlamydia testing? | Daily | Weekly | Monthly | |||
| Are there any ways you can think of that could improve the identification of patients? | Yes | No | If yes, explain | |||
| How many chlamydia tests do you perform on average per month? | ≤ 10 | ≤ 20 | ≤ 30 | ≤ 40 | ≤ 50 | ≤ 60 |
| Who performs the chlamydia screening at your practice? | Nurse | GP | Both | Other | If both what percentage is performed by GP relative to nurse? | |
| Please describe the chlamydia testing procedure in your practice Please include any staff, equipment and disposables that might be used |
Description of activity and how long it takes: | |||||
| Staff | ||||||
| Equipment | ||||||
| Disposables | ||||||
| Other | ||||||
| What method of communication do you normally use to notify patients of their test results | Telephone call | Letter | Face-to-face consult | SMS message | Other, please specify? | |
| Who notifies the patient of their test result | Doctor | Nurse | Health advisor | Receptionist | Other, please specify? | |
| Who sees the patient when they come in for their treatment and how long is the appointment? | Doctor | Nurse | Health advisor | Receptionist | Other, please specify? | |
| Do you follow up partners of positive STI patients? | Never | Sometimes | Always | |||
| Describe (if relevant) how your practice follows up partners? | ||||||
| Do you refer patients for treatment elsewhere? | Never | Sometimes | Often | |||
| How many patients test positive on average per month for chlamydia in the practice? | ||||||
| Section B: testing for chlamydia with the National Chlamydia Screening Programme | ||||||
| Is this practice registered with the National Chlamydia Screening Programme to offer chlamydia testing to young people aged under 25 years? | Yes | No | Don’t know | |||
| If yes, please answer the following questions, if no go to section C | ||||||
| Do you use the screening packs provided by the NCSP? | Yes | No | If no, please specify what you do use | |||
| Are these tests sent to the same lab as other microbiology tests such as MSU? | Yes | No | If no, please specify where they are sent | |||
| Who receives the results for patients tested through the National Chlamydia Screening Programme? | The practice | Chlamydia screening office | Other, please specify | |||
| If received by the practice, who handles and acts on these results? | ||||||
| How do patients testing positive through the National Chlamydia Screening Programme normally get their antibiotic treatment? | Antibiotics given directly to patient in practice | Antibiotics prescribed for patient by practice, using normal prescription form | Antibiotics given by local Chlamydia screening office | |||
| If given by the practice, would these patients pay for their prescription (unless exempt)? | Yes | No | Comments | |||
| Section C: other chlamydia testing | ||||||
| Do you test for Chlamydia other than through the NCSP, i.e. for older people | Yes | No | Don’t know | |||
| If yes, how is this process managed? | ||||||
| Who handles and acts on the results of these tests? | ||||||
| If patient is positive how is their treatment provided? | Antibiotics prescribed for patient by practice, using normal prescription form | Antibiotics given directly to patient in practice | Patient referred elsewhere for treatment Please specify and give contact details | |||
| Would these patients pay for their prescription unless exempt? | Yes | No | It depends please specify | |||
| Is there anything else that you think would be helpful for us to know? | ||||||
Appendix 2 Costs of screening before intensive recruitment
The unit cost data applied to the estimates of resource use are taken from existing relevant studies where cost estimates for tests and treatment associated with chlamydia testing have already been analysed in detail, such as the ClaSS Project,23 or from established existing routine sources (e.g. Unit Costs of Health and Social Care 2011/12). 69 The data on number of tests carried out, the resources used, such as any equipment and disposables, and the number of positive cases detected were extracted directly from the completed questionnaires.
A number of assumptions were required to estimate the costs associated with testing and the outcome of testing.
Assumptions for existing approach to chlamydia testing
An interaction with either a doctor or a nurse at any practice takes approximately 10 minutes.
The hourly wage rate for the staff in any practice (doctor, nurse or administrative assistant) is taken from an established routine source, Unit Costs of Health and Social Care 2011. 69
In the most common pathway for a patient, the chlamydia test is performed by a doctor or nurse as indicated on the questionnaire. If the patient tests positive, they are contacted by telephone by a member of the administrative staff and then the patient returns for a consultation plus treatment. (As stated in assumption 1, this interaction is assumed to take approximately 10 minutes.)
Partner notification costs are not included because no data are provided on PN or its success.
Data for existing approach to chlamydia testing
Eleven answered questionnaires were returned, all relating to the previous month. The resource use for staff activity at the practice and the costs applied to other items are presented in Tables 26 and 27 respectively.
| Activity | Mean time (minutes) | Cost (£, 2011) |
|---|---|---|
| Surgery consultation time (nurse)69 | 10 | 7.17 |
| Surgery consultation time (doctor)69 | 11.7 | 36.00 |
| Administrative time69 | 5 | 2.00 |
| Resources | Cost (£, 2012) |
|---|---|
| Telephone callsa | 5.92 |
| Antibiotics (azithromycin)23 | 15.11 |
| Cost of a testb,23 | 8.95 |
Table 28 shows the costs incurred and the associated number of tests at each practice for both the existing approach to testing and intensive recruitment. Table 29 compares costs and positive cases identified for the existing approach and intensive recruitment.
| Practice identifier code | Cost (£, 2012) | Number of tests | ||
|---|---|---|---|---|
| Existing | Intensive | Existing | Intensive | |
| 1 | 3247 | 3846 | 60 | 32 |
| 2 | 1581 | 4604 | 40 | 51 |
| 3 | 1175 | 6164 | 30 | 41 |
| 4 | 1196 | 2548 | 30 | 27 |
| 5 | 976 | – | 30 | – |
| 6 | 325 | 4825 | 10 | 30 |
| 7 | 6853 | 1578 | 246 | 100 |
| 8 | 427 | 6408 | 10 | 47 |
| 9 | 2002 | 2200 | 50 | 23 |
| 10 | 352 | 5517 | 10 | 39 |
| 11 | 976 | 4356 | 30 | 51 |
| Practice identifier code | Cost (£, 2012) | Number of positives | ||
|---|---|---|---|---|
| Existing | Intensive | Existing | Intensive | |
| 1 | 3369 | 3846 | 5 | 0 |
| 2 | 1624 | 4604 | 2 | 3 |
| 3 | 1196 | 6164 | 1 | 0 |
| 4 | 1240 | 2548 | 2 | 0 |
| 5 | – | – | – | – |
| 6 | – | 4825 | – | 0 |
| 7 | 8615 | 1578 | 131 | 100 |
| 8 | 470 | 6407 | 2 | 0 |
| 9 | 2081 | 2200 | 3 | 2 |
| 10 | 374 | 5517 | 1 | 0 |
| 11 | 2233 | 4356 | 0 | 2 |
Appendix 3 Data for intensive recruitment
| Task | Time taken | Practice | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| Setting up | Days | 1 | 2 | 1 | 1 | – | 1 | 2 | 2 | 1 | 2 | 1 |
| Hours | 9 | 20 | 10 | 9.5 | – | 9 | 9 | 13 | 10.25 | 18 | 8 | |
| Recruitment | Days | 15 | 16 | 18 | 15 | – | 16 | 15 | 16 | 12 | 15 | 11 |
| Hours | 127 | 169.5 | 167.5 | 136.3 | – | 129 | 105.5 | 148.5 | 113 | 131.25 | 112 | |
| Number of tests and positives | Practice | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| Tests | 32 | 51 | 41 | 27 | – | 30 | 100 | 47 | 23 | 39 | 51 |
| Positives | 0 | 3 | 0 | 0 | – | 0 | 3 | 0 | 2 | 0 | 2 |
| Practice | Individual | Time (hours) | Labour cost per hour (£) | Other costs (£) | Total costs (£) |
|---|---|---|---|---|---|
| 1 | IR researcher | 127 | 21.96 | – | 3140.21 |
| 2 | IR researcher | 117 | 21.96 | 18.75 | 2588.07 |
| Research co-ordinator | 52.5 | 18.39 | 96.70 | 1062.18 | |
| 3 | IR researcher | 167.5 | 21.96 | 306.20 | 5304.98 |
| 4 | Research admin (RA2) | 136.33 | 14.36 | – | 1957.75 |
| 5 | – | – | – | – | – |
| 6 | IR researcher | 129 | 21.96 | 481.25 | 3995.29 |
| 7 | PCRN | 89.5 | – | – | – |
| 8 | IR researcher | 148.5 | 21.96 | 988.61 | 5371.03 |
| 9 | Research admin (RA2) | 113 | 14.36 | – | 1622.68 |
| 10 | IR researcher | 114.25 | 21.96 | 55.25 | 3997.60 |
| Research co-ordinator | 36 | 18.39 | 120.25 | 1025.75 | |
| 11 | Research co-ordinator | 112 | 18.39 | 1662.14 | 3860.94 |
| Practice | Activity | Individual | Time (hours) | Labour cost per hour (£) | Other costs (£) | Total costs (£) |
|---|---|---|---|---|---|---|
| 1 | Liaison with CSO | Research co-ordinator | 2 | 18.39 | – | 36.78 |
| Trial manager | 2 | 20.92 | – | 41.84 | ||
| Liaison with local lab | Research co-ordinator | 0 | – | – | – | |
| Trial manager | 0 | – | – | – | ||
| 2 | Liaison with CSO | Research co-ordinator | 5 | 18.39 | – | 91.95 |
| Trial manager | 0 | 20.92 | – | – | ||
| Liaison with local lab | Research co-ordinator | 0 | 18.39 | – | – | |
| Trial manager | 0 | 20.92 | – | – | ||
| 3 | Liaison with CSO | Research co-ordinator | 3 | 18.39 | – | – |
| Trial manager | 0 | 20.92 | – | – | ||
| Liaison with local lab | Research co-ordinator | 3 | 18.39 | – | 55.17 | |
| Trial manager | 0 | 20.92 | – | – | ||
| 4 | Liaison with CSO | Research co-ordinator | 0 | 18.39 | – | – |
| Trial manager | 0 | 20.92 | – | – | ||
| Liaison with local lab | Research co-ordinator | 3 | 18.39 | – | 55.17 | |
| Trial manager | 0 | 20.92 | – | – | ||
| 5 | Liaison with CSO | Research co-ordinator | – | – | – | |
| Trial manager | – | – | – | |||
| Liaison with local lab | Research co-ordinator | – | – | – | ||
| Trial manager | – | – | – | |||
| 6 | Liaison with CSO | Research co-ordinator | 2 | 18.39 | – | 36.78 |
| Trial manager | 0 | 20.92 | – | – | ||
| Liaison with local lab | Research co-ordinator | 2 | 18.39 | – | 36.78 | |
| Trial manager | 0 | 20.92 | – | – | ||
| 7 | Liaison with CSO | Research co-ordinator | 0 | 18.39 | – | – |
| Trial manager | 0 | 20.92 | – | – | ||
| Liaison with local lab | Research co-ordinator | 0 | 18.39 | – | – | |
| Trial manager | 0 | 20.92 | – | – | ||
| 8 | Liaison with CSO | Research co-ordinator | 0 | 18.39 | – | – |
| Trial manager | 0 | 20.92 | – | – | ||
| Liaison with local lab | Research co-ordinator | 0 | 18.39 | – | – | |
| Trial manager | 0 | 20.92 | – | – | ||
| 9 | Liaison with CSO | Research co-ordinator | 0 | 18.39 | – | – |
| Trial manager | 0 | 20.92 | – | – | ||
| Liaison with local lab | Research co-ordinator | 0 | 18.39 | – | – | |
| Trial manager | 0 | 20.92 | – | – | ||
| 10 | Liaison with CSO | Research co-ordinator | 0 | 18.39 | – | – |
| Trial manager | 0 | 20.92 | – | – | ||
| Liaison with local lab | Research co-ordinator | 0 | 18.39 | – | – | |
| Trial manager | 0 | 20.92 | – | – | ||
| 11 | Liaison with CSO | Research co-ordinator | 0 | 18.39 | – | – |
| Trial manager | 0.05 | 20.92 | 0.05 | 1.05 | ||
| Liaison with local lab | Research co-ordinator | 0 | 18.39 | – | – | |
| Trial manager | 0 | 20.92 | – | – |
| Practice | Team | Activity | Individual | Time (hours) | Labour cost per hour (£) | Other cost (£) | Total cost (£) | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 | PN trial team | Set-up: training of PC team | Research co-ordinator | 4 | 18.39 | 31.49 | 105.05 | ||
| Trial manager | 5 | – | 27.80 | 27.80 | |||||
| PC team (covered by SSCs) | PN training and IR set-up | General practitioner | 3 | 80.00 | 0.00 | 240.00 | |||
| Research nurse | 5 | 21.96 | 0.00 | 109.80 | |||||
| Practice manager | 2 | 21.96 | 0.00 | 43.92 | |||||
| Admin | 5 | 12.44 | 0.00 | 62.20 | |||||
| Additional training | – | – | 0.00 | – | |||||
| 2 | PN trial team | Set-up: training of PC team | Research co-ordinator | 20 | 18.39 | 0.00 | 367.80 | ||
| Trial manager | 0 | 20.92 | 0.00 | ||||||
| PC team (covered by SSCs) | PN training and IR set-up | General practitioner | 3 | 80.00 | 0.00 | 240.00 | |||
| Research nurse | 5 | 21.96 | 0.00 | 109.80 | |||||
| Practice manager | 3 | 21.96 | 0.00 | 65.88 | |||||
| Admin | 5 | 12.44 | 0.00 | 62.20 | |||||
| Additional training | – | – | 0.00 | – | |||||
| 3 | PN trial team | Set-up: training of PC team | Research co-ordinator | 10 | 18.39 | 125.86 | 309.76 | ||
| Trial manager | 0 | 20.92 | 0.00 | – | |||||
| PC team (covered by SSCs) | PN training and IR set-up | General practitioner | 3 | 80.00 | 0.00 | 240.00 | |||
| Research nurse | 5 | 21.96 | 0.00 | 109.80 | |||||
| Practice manager | 3 | 21.96 | 0.00 | 65.88 | |||||
| Admin | 5 | 12.44 | 0.00 | 62.20 | |||||
| Additional training | – | – | 0.00 | – | |||||
| 8 | PN trial team | Set-up: training of PC team including pre-meeting with team | Research co-ordinator | 5 | 18.39 | 260.84 | 352.79 | ||
| Trial manager | 1 | 20.92 | 169.10 | 190.02 | |||||
| PC team (covered by SSCs) | PN training and IR set-up | General practitioner | 2 | 80.00 | 0.00 | 160.00 | |||
| Research nurse | 5 | 21.96 | 0.00 | 109.80 | |||||
| Practice manager | 2 | 21.96 | 0.00 | 43.92 | |||||
| Admin | 5 | 12.44 | 0.00 | 62.20 | |||||
| Additional training | – | – | 0.00 | – | |||||
| 9 | PN trial team | Set-up: training of PC team plus team meeting | Research co-ordinator | 0 | 18.39 | 0.00 | – | ||
| Trial manager | 4 | 20.92 | 0.00 | 83.68 | |||||
| PC team (covered by SSCs) | PN training and IR set-up | General practitioner | 3 | 80.00 | 0.00 | 240.00 | |||
| Research nurse | 5 | 21.96 | 0.00 | 109.80 | |||||
| Practice manager | 2 | 21.96 | 0.00 | 43.92 | |||||
| Admin | 5 | 12.44 | 0.00 | 62.20 | |||||
| Additional training | – | – | 0.00 | – | |||||
| 10 | PN trial team | Set-up: training of PC team | Research co-ordinator | 18 (included in recruitment) | |||||
| Trial manager | 0 | 20.92 | 0.00 | – | |||||
| PC team (covered by SSCs) | PN training and IR set-up | General practitioner | 1 | 80.00 | 0.00 | 80.00 | |||
| Research nurse | 1 | 21.96 | 0.00 | 21.96 | |||||
| Practice manager | 3 | 21.96 | 0.00 | 65.88 | |||||
| Admin | 5 | 12.44 | 0.00 | 62.20 | |||||
| Additional training | – | – | 0.00 | – | |||||
| 11 | PN trial team | Set-up: training of PC team | Research co-ordinator | Included in recruitment costs | |||||
| Trial manager | 0 | 20.92 | 0.00 | – | |||||
| PC team (covered by SSCs) | PN training and IR set-up | General practitioner | 0 | 80.00 | 0.00 | – | |||
| Research nurse | 5 | 21.96 | 0.00 | 109.80 | |||||
| Practice manager | 0 | 21.96 | 0.00 | – | |||||
| Admin | 0 | 12.44 | 0.00 | – | |||||
| Additional training | – | – | 0.00 | – | |||||
List of abbreviations
- ACCEPt
- Australian chlamydia control effectiveness pilot
- APT
- accelerated partner therapy
- CAD
- consent at time of positive diagnosis
- CAT
- consent at time of test
- CiCS
- Corporate Information and Computing Services
- ClaSS
- Chlamydia Screening Studies
- CSO
- chlamydia screening office
- CTAD
- chlamydia testing activity data set
- CTRU
- Clinical Trials Research Unit
- GP
- general practitioner
- GUM
- genitourinary medicine
- HA
- health adviser
- HIV
- human immunodeficiency virus
- HPA
- Health Protection Agency
- HTA
- Health Technology Assessment
- MRC
- Medical Research Council
- MSM
- men who have sex with men
- NAAT
- nucleic acid amplification test
- NCSP
- National Chlamydia Screening Programme
- NIHR
- National Institute for Health Research
- PCRN
- primary care research network
- PCT
- primary care trust
- PHE
- Public Health England
- PN
- partner notification
- RCT
- randomised controlled trial
- RF
- regional facilitator
- RTN
- regional training nurse
- SSHA
- Society of Sexual Health Advisers
- STI
- sexually transmitted infection