Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 12/73/01. The contractual start date was in January 2013. The draft report began editorial review in May 2013 and was accepted for publication in January 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Long et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Description of the health problem
Definition
Epicondylitis occurs when tendons in the elbow develop microscopic tears. This degeneration is sometimes referred to as tendinopathy. 1,2 Epicondylitis is characterised by pain and tenderness in the lateral (tennis elbow) or medial (golfer’s elbow) humeral epicondyle. 1 Lateral epicondylitis is more common than medial epicondylitis;3 a demographic study (n = 4783) found the overall prevalence of lateral epicondylitis to be 1.3%, compared with 0.4% for medial epicondylitis. 4 For this reason, this review focuses on lateral epicondylitis.
Lateral epicondylitis has been defined as ‘a painful condition affecting the tendinous tissue of the origins of the wrist extensor muscles at the lateral epicondyle of the humerus, leading to a loss of function of the affected limb . . . it can have a major impact on an individual’s social and professional life’. 5
Pain in this area is referred to as ‘tennis elbow’, ‘lateral elbow pain’, ‘lateral epicondylitis’, ‘lateral epicondylalgia’, ‘rowing elbow’, ‘tendonitis of the common extensor origin’ and ‘peritendinitis of the elbow’. 5–7 The condition is referred to throughout this report as ‘lateral elbow tendinopathy’.
Epidemiology
Lateral elbow tendinopathy (LET) is a common complaint causing characteristic pain in the lateral elbow and upper forearm, and tenderness of the forearm extensor muscles. It is associated with pain over the lateral epicondyle when gripping and manipulating the hand. 8 It is thought to be an overuse injury, caused by repetitive loading of the extensor tendons of the forearm where they attach to the lateral epicondyle. 2 Consistent absence of inflammatory cells has resulted in the consensus that the process is non-inflammatory in nature, although neurogenic inflammation may play a role. 8 If symptoms prevail for more than 3 months, the condition is labelled chronic9 and, at this stage of disease, inflammatory cells are absent and replaced by degenerative signs in the tissue. 10,11 The patient’s pain experience in the chronic phase is thought to culminate from changes in both the peripheral and central nervous systems. 8
The prevalence of LET is between 1% and 3%, with an incidence in UK general practice of four to seven consultations per thousand in 2006 and 2012. 2,5 Onset for LET peaks during early middle age, at approximately 40–50 years. 2,7 Men and women are equally affected;5 however, among women aged 42–46 years, the incidence is as high as 10%. 12,13 In 75% of patients it is the dominant arm that is affected. 14
Lateral elbow tendinopathy is brought on by occupational activities and sports that involve a repetitive wrist extension or a power grip. 2 The condition is most commonly associated with work-related activities requiring repetitive wrist flexion and extension,15 such as cutting meat, plumbing and working on cars. 2 Racquet sports, golf and throwing are also known causes. Although the condition is referred to as ‘tennis elbow’, tennis accounts for only 5% of cases of LET. 2,5,6
The condition is recognised as challenging to treat and is prone to recurrent episodes. 8 The average duration of a typical episode ranges from 6 to 24 months, with most patients (89%) reporting recovery by 1 year. 8
Aetiology
Lateral elbow tendinopathy is an overload injury that occurs after minor or unrecognised trauma to the forearm extensor muscles. 2,7 It is considered a cumulative trauma injury that occurs over time from repeated use of the muscles of the arm and forearm. 2,7 Patients often present with a clear history of a likely cause of repetitive strain or possibly a history of acute injury; however, this is not always the case. 2 Although the clinical presentation of LET is reasonably straightforward and easy to recognise, underlying pathophysiology is more complex (the multifactorial pathophysiology is shown in Figure 1). 8 Overuse of the extensor muscles causes microtears around the origin of the extensor muscle at the lateral epicondyle of the humerus, leading to fibrosis and granulation tissue. 2 Microscopic and histological analyses of affected tendons have identified four key changes: (1) increased cell numbers and ground substance; (2) vascular hyperplasia or neovascularisation; (3) increased concentration of neurochemicals; and (4) disorganised and immature collagen. Consistent absence of inflammatory cells has resulted in the consensus that the process is non-inflammatory in nature, although neurogenic inflammation may play a role. The presence of typical inflammatory symptoms, such as night pain, early-morning stiffness and stiffness after a period of inactivity, suggests that there may be an inflammatory component in the acute phase. Increased vascularity in the region of the extensor origin has been seen on colour Doppler ultrasonography, and investigators have suggested that this may be the source of pain in patients with LET. 16
FIGURE 1.
Multifactorial pathology of LET (adapted by permission from BMJ Publishing Group Ltd. A new integrative model of lateral epicondylalgia. Coombes BK, Bisset L, Vicenzino B. Vol. 43, pp. 252–8, Br J Sports Med 20098).
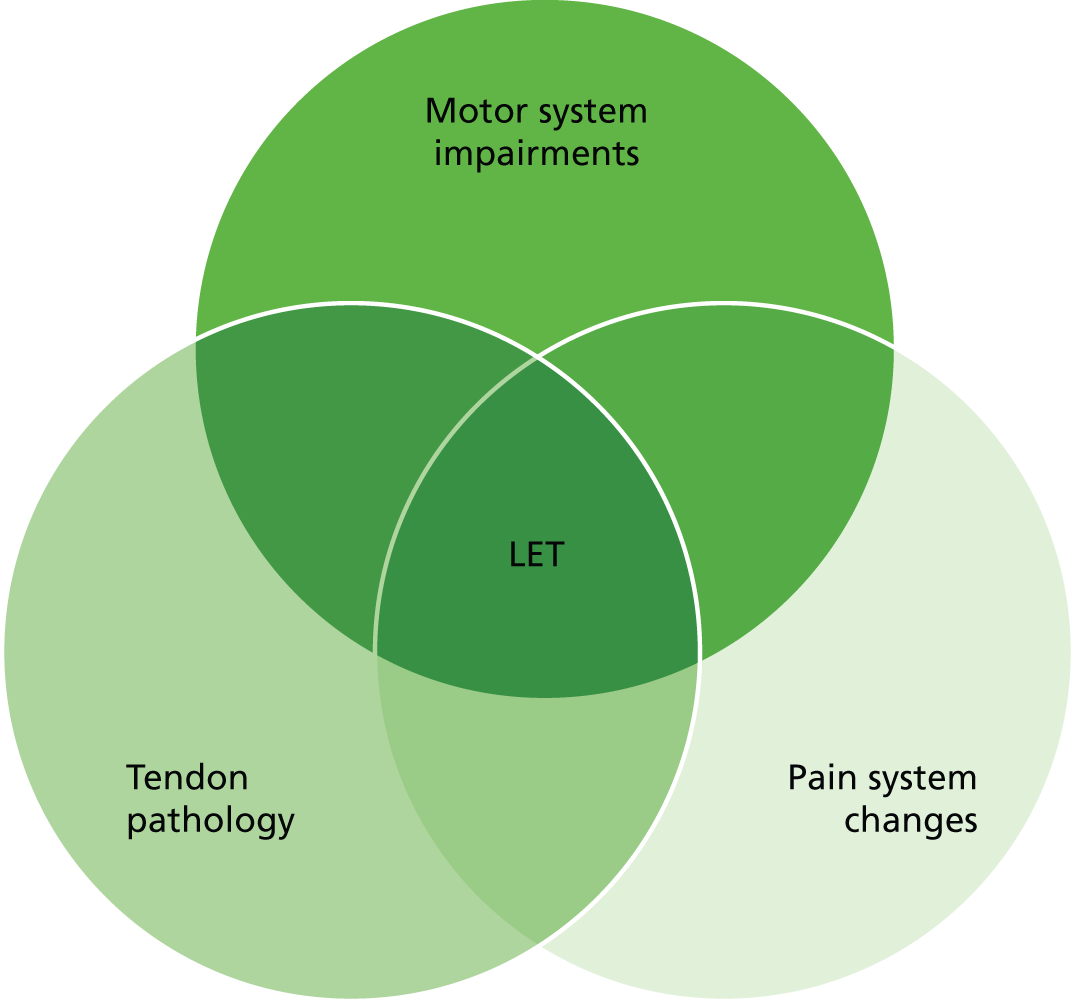
If symptoms prevail for more than 3 months, the condition is labelled chronic. 9 At this stage of disease, inflammatory cells are essentially absent, replaced by degenerative signs in the tissue,10,11 hence the suggested term epicondylosis or tendinosis. 10,17 The aetiology of pain in the chronic stage is as yet unknown, although the patient’s pain experience may culminate from changes in both the peripheral and central nervous systems. 8 This has been linked to an increase in neural transmitters in the affected tissue, which may be responsible for activating or sensitising peripheral nociceptors. 8 Uncertainty about the aetiology may explain why there is no clearly effective treatment in the chronic stage of the disease. 18
Significance for patients including quality of life
Lateral elbow tendinopathy is a painful condition affecting the tendinous tissue of the origins of the wrist extensor muscles at the lateral epicondyle of the humerus, leading to loss of function of the affected limb. Although the prognosis for many is positive, with full recovery within 3–6 months, some patients still report symptoms after 1 year. LET restricts the ability of workers to do their job, resulting in reduced wages caused by days lost at work or slowed work, and also restricts the ability to pursue chosen leisure activities. 19 At its extreme, it can become a handicap to those who are prevented from performing certain activities required as part of daily roles. 19
Measurement of health
A variety of measures are used to monitor the progress of LET and to measure the effectiveness of interventions. Often a combination of measures is commonly employed, addressing physical variables such as pain and strength, functional and psychosocial limitations. 20
Pain intensity is a quantitative estimate of the severity of pain and is commonly measured by verbal rating, visual analogue or numerical rating scales. Several questionnaires are available that assess multiple aspects of pain. Developed specifically for use with LET, the Patient-Rated Tennis Elbow Evaluation (PRTEE) [formerly the Patient-Rated Forearm Evaluation Questionnaire (PRFEQ)] has a pain subscale. It is a 15-item questionnaire designed to measure forearm pain and disability in patients with LET. 21 Patients can rate their level of pain (five items) on a numerical scale (0–10). 21,22 In addition to the individual subscale scores for pain and function, a total score can be calculated on a scale of 100 (0 = no disability) for which pain and function are weighted equally. 21 Another expression of pain commonly used in the assessment of LET is tenderness. This may be indicated via a yes/no response, but can also be quantified using the pressure pain threshold, defined as the minimum amount of pressure that produces pain, and it is typically measured using an algometer. 23
Function is defined as a capacity or body characteristic, such as strength or range of joint movement. Maximum grip strength and pain-free grip strength are common measures providing an objective index of upper extremity function. 24 The wrist extensors, some of which attach to the lateral epicondyle via the common extensor tendon, stabilise the wrist during gripping activities;24 therefore, gripping can stress the damaged tendon and generate pain. Grip strength is usually measured with a hand dynamometer. 24 For maximum grip strength, the subject squeezes the dynamometer as tightly as possible. For pain-free grip strength, the trigger is gripped increasingly tightly until the pain threshold in the elbow is just reached. In addition, there are many scoring systems used to evaluate elbow function; the PRTEE, for example, has a function subscale for a range of specific (six items) and usual activities (four items). 21
Impairment and activity limitation is typically measured using standardised questionnaires. 20,25 The PRTEE, for example, has two sections relating to disability (11-point scale on which respondent’s estimate the difficulty experienced in carrying out named activities over the previous week). 20 Other questionnaires include the disabilities of the arm, shoulder and hand (DASH),26,27 and DASH-Quick (DASH-Q),25 as well as elbow-specific measures, for example the Liverpool Elbow Score25,28 and the Mayo Elbow Performance Index. 25 The impact on activities of daily living (ADLs) and thus quality of life (QoL) is also measured using, for example, Short Form questionnaire-36 items (SF-36), Short Form questionnaire-12 items and European Quality of Life-5 Dimensions (EQ-5D) as well as absence from or resumption of work statistics.
Patient-rated Likert scales are also commonly used as an indicator of global status or change. 20 The Likert scale is a 6-point scale varying between –2 (much worse) and +3 (completely recovered). 20 Global improvement was not considered in this review.
Current service provision
National guidelines
The following guidance relating to the treatment of LET has been issued by the National Institute for Health and Care Excellence (NICE):
-
Autologous Blood Injection for Tendinopathy: Guidance (IPG 438). 29
-
Extracorporeal Shockwave Therapy for Refractory Tennis Elbow (IPG 313). 30
-
NHS Evidence – Clinical Knowledge Summaries: Tennis Elbow. 31
Similar databases in Scotland, for example Scottish Medicines Consortium and the Scottish Intercollegiate Guidelines Network, were searched; however, no additional guidance for the treatment of LET was identified.
Current management
The initial management of lateral epicondylitis aims to treat symptoms of pain and inflammation, promote healing, increase work and leisure activities and reduce risk of aggravating the condition or developing a new injury. Pharmacotherapy, electrophysical therapy, exercise and multimodal therapy tend to be the main conservative management strategies for LET. 8
Treatment options on initial diagnosis include general measures (defined as activity modification, heat and cold therapy and rest), non-steroidal anti-inflammatory drugs (NSAIDs), orthoses [devices to control, guide, limit and/or immobilise an extremity, joint or body segment (e.g. reduce weight bearing or restrict/assist movement)], acupuncture, exercise (general and eccentric exercise) and physiotherapy [often includes different treatment modalities, e.g. exercise, joint mobilisation, friction massage, electrotherapy, low-level laser therapy (LLLT) and therapeutic ultrasound]. Conservative measures are effective in about 80% of cases. In the event that patients do not respond to initial treatment measures, glucocorticoid injection (GCI) is usually considered. Although extracorporeal shock wave therapy (ESWT) is recognised by NICE as a potentially beneficial treatment for refractory LET, until further evidence becomes available it is available for use only in certain circumstances. 30 Surgical intervention for refractory LET is considered after 6–12 months of inadequate non-surgical management; however, this remains the last option because of morbidity and inconsistent outcomes.
Current service provision is summarised in Figure 2.
FIGURE 2.
Management of LET, UK (adapted from Map of Medicine: Lateral Epicondylitis). 2 a, Physiotherapy combines a range of treatment options; b, the definition of conservative interventions for this review was any non-surgical treatment and, as such, covers both first- and second-line treatments outlined above; and c, ESWT, although recognised as a promising intervention evidence, is inconsistent and can only be used with specific arrangements for clinical governance, consent and audit or research.
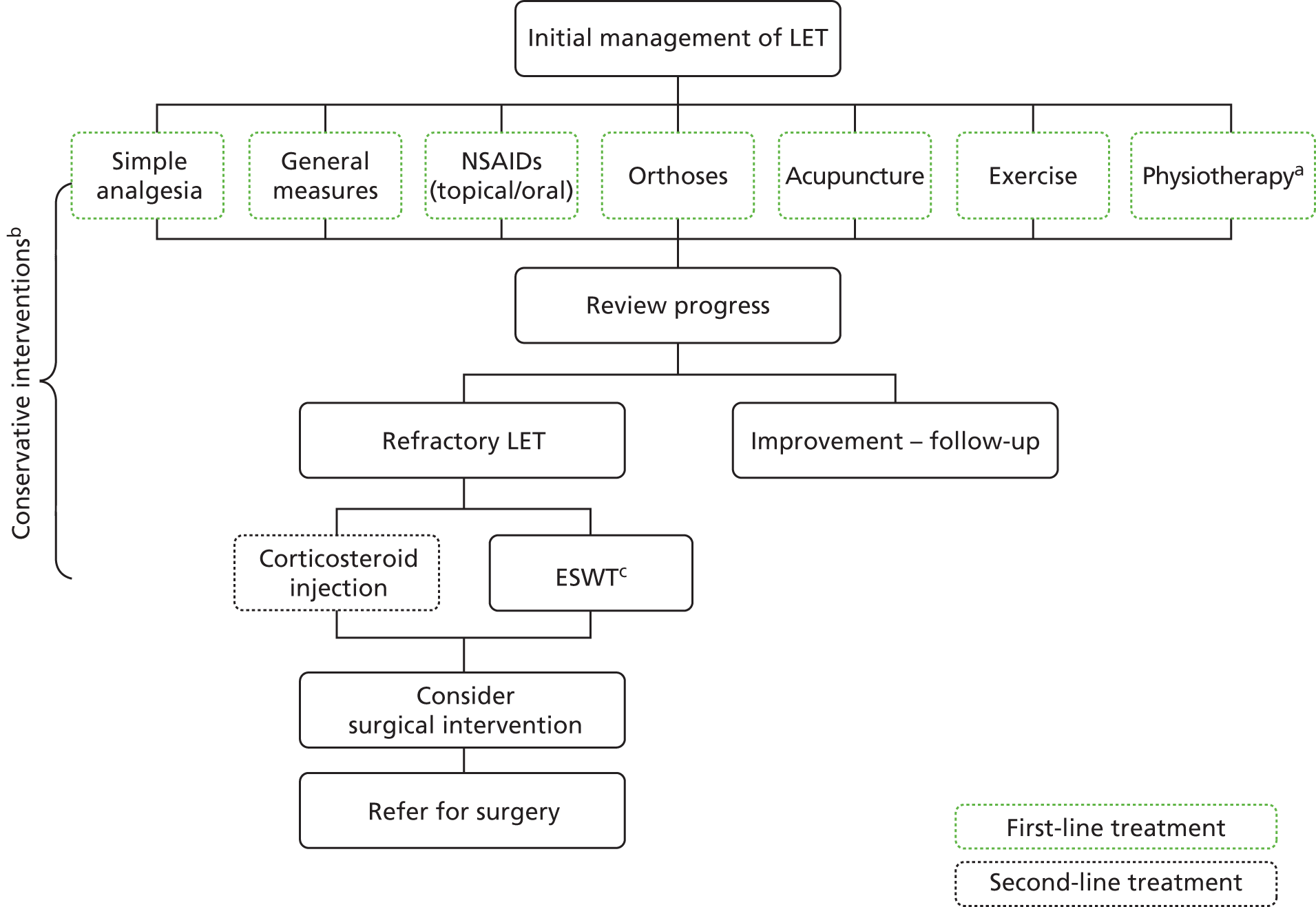
Other treatments include iontophoresis (topical introduction of ionised drugs into the skin using electrical current), phonophoresis (ultrasonography-enhanced delivery of topical drugs), LLLT; autologous whole-blood injections, platelet-rich plasma (PRP) injection and botulinum toxin type A injections.
This review considers all non-surgical treatments.
Description of interventions and current evidence
There are a number of medical and non-medical interventions available for the treatment of LET. Pharmacotherapy, electrophysical therapy, exercise and multimodal therapy tend to be the main conservative management strategies for LET. A brief description of the interventions used is given in Table 1; the list is set out by intervention and in this case is distinct from the person(s) administering the interventions (e.g. physiotherapy incorporates a number of the treatment modalities listed separately).
| Intervention | Current evidence |
|---|---|
| Acupuncture | A collection of procedures that involves the stimulation of points on the body using a variety of techniques, such as penetrating the skin with needles that are then manipulated manually or by electrical stimulation |
| ABI | Blood is taken from the patient and reinjected around the affected tendon. The aim is to supply the tendon with growth factors that start the healing process |
| Botulinum toxin injection | A neurotoxin that acts by inhibiting the release of the neurotransmitter acetylcholine at neuromuscular junctions, reducing muscle contractions. Delivered via intramuscular or subcutaneous injection |
| GCI | A type of medication that contains man-made versions of the hormone cortisol and is used to reduce the inflammation. A minimum 6-week interval between injections with a maximum of three injections at the same site |
| ESWT | A non-invasive treatment in which a device is used to pass acoustic shockwaves through the skin to the affected area |
| Exercise | General exercise and strengthening exercises performed by slowly letting out the muscle, i.e. controlled lengthening of muscle fibres (eccentric exercise) |
| General measures | Modification of activities that cause the symptoms, for example avoiding lifting, gripping, pronation |
| Iontophoresis | A technique using a small electric charge to deliver a medicine or other chemical through the skin (an injection without the needle) |
| LLLT | Low-level lasers or light-emitting diodes to alter cellular function |
| NSAIDs | Oral (ibuprofen) and topical (gels and creams) NSAIDs have long been the first line of treatment for all sites of tendinitis |
| Orthoses | Orthotic devices in the form of a brace, splint, cast, band, or strap to support the affected limb |
| Other injection therapies | Glycosaminoglycan polysulphate injection and sodium hyaluronic therapies |
| PRP therapy | PRP is an autologous blood-derived product; the application of PRP enhances wound, tendon and bone healing |
| Physiotherapy | Physiotherapy is the therapeutic use of physical agents or means, such as massage and exercise (general and eccentric), to relieve pain and stiffness. Physiotherapists administer treatments such as therapeutic ultrasound, LLLT and ESWT (defined elsewhere in the table). The definition of physiotherapy varies between studies |
| Prolotherapy (also known as proliferative injection therapy) | An injection-based treatment (non-pharmacological and non-active irritant solution into the body in the region of tendons or ligaments for the purpose of strengthening weakened connective tissue and alleviating musculoskeletal pain) |
| Pulsed electromagnetic field | Uses electrical energy to direct a series of magnetic pulses through injured tissue |
| Therapeutic ultrasound | Ultrasound therapy (thermal and mechanical) uses sound waves generated through a transducer head to penetrate soft tissues |
| Watch and wait/wait and see | An approach that allows time to pass before medical intervention or therapy is used |
Current evidence
A background search has identified that, although there are already systematic reviews of randomised controlled trials (RCTs), including Cochrane reviews, on many common interventions for LET, many of these are out of date by 10 years or more. In the process of developing the protocol and search strategy for this review, the Cochrane systematic reviews by Struijs et al. 32 and Green et al. 33,34 were identified.
A 2002 Cochrane review by Struijs et al. 32 assessed the clinical effectiveness of orthotic devices for the treatment of tennis elbow. Five RCTs were included. 35–39 The limited number of included trials presented few outcome measures and limited long-term results. Pooling was not possible because of the large heterogeneity among trials. The authors concluded that the effectiveness of orthotic devices for LET could not be made, and that more well-designed and well-conducted RCTs of sufficient power were needed. 32
Another Cochrane review reported in the same year, by Green et al. ,33 assessed the effectiveness of NSAIDs for the treatment of tennis elbow. Fourteen trials were included in the review. 35,37,40–52 The sample size of the included studies was generally small, with a median follow-up of 2 weeks (range 1–12 weeks). 33 The authors concluded that there is some support for the use of topical NSAIDs to relieve lateral elbow pain at least in the short term [weighted mean difference (WMD) = –1.88, 95% confidence interval (CI) –2.54 to –1.21]. 33 There remains insufficient evidence to recommend or discourage the use of oral NSAIDs, although it appears injection may be more effective than oral NSAIDs in the short term. No evidence of a direct comparison between topical and oral NSAIDs was identified.
A Cochrane review published in the same year, and by the same authors (Green et al. 34), assessed the effectiveness of acupuncture in the treatment of adults with lateral elbow pain with respect to pain reduction, improvement in function, grip strength and adverse effects. The authors concluded that there is insufficient evidence to either support or refute the use of acupuncture (either needle or laser) in the treatment of lateral elbow pain. This review has demonstrated needle acupuncture to be of short-term benefit with respect to pain, but this finding is based on the results of two small trials, the results of which were not able to be combined in meta-analysis. No benefit lasting more than 24 hours following treatment has been demonstrated. No trial assessed or commented on potential adverse effect. Further trials, utilising appropriate methods and adequate sample sizes, are needed before conclusions can be drawn regarding the effect of acupuncture on tennis elbow.
The main focus of this review was, therefore, current reviews and studies, i.e. those that have been published in the last 10 years. Given the publication dates of the identified reviews, the eligible date range for the inclusion of RCTs or systematic reviews in this review was 2003–13 (see Chapter 2, Study selection). Thus, we rely on existing systematic reviews within the eligible date range to capture and synthesise RCT evidence published before 2003.
Research methods
The aim of this review was to:
-
provide an overview of systematic reviews of the current evidence for the clinical effectiveness of conservative interventions for the treatment of LET; summarise the results and assess study quality
-
identify the number of RCTs meeting the specified inclusion criteria not included in the most valid and up-to-date systematic reviews included in the overview
-
identify which RCTs could contribute further evidence to existing systematic reviews (included in the overview) and where there may be a need for a systematic review, to synthesise evidence for newer treatments
-
conduct a systematic review of cost-effectiveness studies.
This evidence is sought in comparison with current practice with other conservative interventions. For the purposes of this review, ‘conservative’ is defined as any treatment except surgery. The clinical effectiveness and cost-effectiveness of the interventions are measured objectively by health outcomes, QoL and cost and cost-effectiveness.
A review protocol was developed and set out the methods used in the review (PROSPERO registration number: CRD42013003593). 53 The review was undertaken following the principles published by the NHS Centre for Reviews and Dissemination. 54
The methods for the review of clinical effectiveness studies are described in Chapter 2, Methods of reviewing clinical effectiveness and for cost-effectiveness see Chapter 3, Methods for reviewing cost-effectiveness.
Research question
The question addressed by this review was: what is the evidence for the clinical effectiveness and cost-effectiveness for conservative interventions for the treatment of elbow tendinopathy?
Chapter 2 Clinical effectiveness
Methods of reviewing clinical effectiveness
The aim of the clinical effectiveness review was to provide an overview of systematic reviews of the evidence for the clinical effectiveness of conservative interventions for the treatment of LET and to quantify the number of RCTs meeting the specified inclusion criteria not included in the most valid and up-to-date systematic reviews included in the overview.
Search strategy
The search strategy was developed in MEDLINE (via Ovid) and adapted for use in other databases; the search strategies for each database are detailed in Appendix 1. The search strategy combines terms for ‘tendinopathy’ with ‘elbow’ and uses a RCT/systematic review filter and a cost-effectiveness filter to identify the methodologically relevant studies. An information specialist identified the search terms by consulting the literature and with assistance from the review team. An iterative search process was used to ensure an appropriate balance of sensitivity and specificity. Medical subject heading (MeSH) terms used in the original MEDLINE search were translated for use in other databases as necessary.
Electronic databases were searched in January 2013 and the searches were run from inception to January 2013. The following databases were searched: MEDLINE (via Ovid); MEDLINE In-Process & Other Non-Indexed Citations (via Ovid); EMBASE (via Ovid); Allied and Complementary Medicine Database (AMED; via Ovid); Cumulative Index to Nursing and Allied Health Literature (CINAHL; via EBSCOhost); Web of Science (via Thomson Reuters); Cochrane Database of Systematic Reviews; Cochrane Central Register of Controlled Trials (via CENTRAL); Database of Abstracts of Reviews of Effects (DARE; via Cochrane); Health Technology Assessment (HTA; via Cochrane); Physiotherapy Evidence Database (PEDro); and ClinicalTrials.gov. NHS Economic Evaluation Database (NHS EED; via Cochrane) was also searched for cost-effectiveness studies. All database searching was conducted by an information specialist. Further searching was carried out by checking the references of retrieved studies and contacting experts. The internet was also searched for background information.
The database search results were exported to EndNote (X5; Thomson Reuters, CA, USA) and deduplicated using the software and manual checking. This is with the exception of PEDro and ClinicalTrials.gov, which were screened separately. The final number of references screened and the number retrieved per database are detailed in Appendix 1.
Study selection
Relevant studies were identified in two stages using predefined eligibility criteria. Titles and abstracts were examined independently by two researchers and screened for possible inclusion. Disagreements were resolved by discussion. Full texts of the identified studies were obtained. Two researchers examined these independently for inclusion or exclusion and disagreements were resolved by discussion. A third reviewer was available if necessary.
Inclusion and exclusion criteria
Population
The population for this assessment are adults aged ≥ 16 years with lateral tendinopathy of the elbow.
Interventions
The interventions considered are conservative interventions for the treatment of tennis elbow. For the purposes of this review, ‘conservative’ treatment was classified as any non-surgical treatment (see Chapter 1, Current management).
Comparators
The comparator(s) will include placebo or other conservative interventions (i.e. any non-operative treatments).
Outcomes
The main outcomes are pain, function, QoL measured using a validated QoL tool, recurrence, remain/return to work, sport activity and harms of intervention.
Study design
For the review of clinical effectiveness, systematic reviews of RCTs and RCTs were included.
For the purpose of this review, a systematic review was defined as one that has a focused research question; explicit search criteria that are available to review, either in the document or on application; explicit inclusion/exclusion criteria; definitions of the population(s), intervention(s), comparator(s) and outcome(s) of interest; a critical appraisal of included studies, including consideration of internal and external validity of the research; and a synthesis of the included evidence, whether narrative or quantitative.
The following study designs were excluded: uncontrolled studies; animal models; narrative reviews, editorials, opinions; non-English-language papers; and reports published as meeting abstracts only, or for which insufficient methodological details were reported to allow critical appraisal of study quality.
Other
The eligible date range for the inclusion of studies in this overview of systematic reviews was 2003–13. Thus, we rely on existing systematic reviews within the eligible date range to capture and synthesise evidence published before 2003.
Critical appraisal and data extraction
Data extraction
Data were extracted from included studies by one reviewer and checked by another reviewer. Authors of studies were contacted to provide missing information, as necessary.
Assessment of Multiple Systematic Reviews
Two reviewers (LC and LL) read the full text of relevant reviews and assessed the methodological quality of included reviews using the Assessment of Multiple Systematic Reviews (AMSTAR) (a measurement tool to assess systematic reviews) checklist. The 11 criteria were rated as ‘met’ or ‘unclear’/‘not met’. Systematic reviews were excluded if the review was of low quality (rating of fewer than 4 of a possible 11 points as assessed using AMSTAR). All items on the AMSTAR measurement tool were given equal weighting. Studies scoring 8 points or higher were then analysed using a Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach (see Grading of Recommendations Assessment, Development, and Evaluation).
Methods of data synthesis
Grading of Recommendations Assessment, Development, and Evaluation
Principles from GRADE were used for an overall assessment of the quality of evidence for each intervention. The GRADE concept is based on an assessment of the following criteria: quality of primary studies, design of primary studies, consistency and directness. An overall assessment of the quality of evidence was based on a summary of these four criteria, as presented in Table 2.
| Level of quality of evidencea | Classification of evidence |
|---|---|
| High-quality evidence | One or more updated, high-quality systematic reviews based on at least: |
|
|
|
|
| Moderate-quality evidence | One or more updated systematic reviews of high or moderate quality based on at least: |
|
|
|
|
| Low-quality evidence | One or more systematic reviews of variable quality based on: |
|
|
|
|
|
|
| No evidence from systematic reviews | There is no systematic review identified on this topic |
The GRADE approach addresses many of the perceived shortcomings of existing models of evidence evaluation. 55 Evidence is rated across studies for specific clinical outcomes. 55 The GRADE approach specifically assesses methodological flaws within the component studies, consistency of results across different studies, generalisability of research results to the wider patient base and how effective the treatments have been shown to be. 55 Evidence based on RCTs begins as high-quality evidence, but confidence in the evidence may be decreased for several reasons including study limitations, inconsistency of results, indirectness of evidence, imprecision and reporting bias. 55
Grading of Recommendations Assessment, Development, and Evaluation data synthesis
For each intervention, data were extracted for all the outcomes judged to be important (pain, function, QoL, recurrence, remain/return to work, sport activity, harms of intervention). Evidence profiles were created for a range of time points [short term (0–6 weeks), intermediate term (7–26 weeks) and long term (> 26–52 weeks)] using the GRADE approach. Assessments of the quality of evidence for each important outcome takes into account the study design, limitations of the studies, consistency of the evidence across studies, the directness of the evidence and the precision of the estimate. The evidence included in the review was based on RCTs and, as such, under the GRADE approach, begins as high-quality evidence, but confidence can be decreased for several reasons. We chose to be liberal in our assessment of study limitations and did not rate the quality of evidence down because of limitations tied to poor reporting, such as not clearly reporting whether or not there was concealment of allocation in trials. Three main criteria were used for assessing trial limitations: concealment of allocation, blinding and follow-up.
One reviewer (LL) extracted data from the reviews and prepared evidence profiles using GRADEpro software (version 3.6 for Windows; Jan Brozek, Andrew Oxman, Holger Schünemann, McMaster University; 2008), with detailed footnotes explaining the judgments that were made. The evidence profiles were checked by one other member of the team (CH).
After grading the quality of evidence for each outcome in each comparison in each systematic review, the overall level of quality of the combined evidence was considered as detailed in Table 2. In the table of overall level of quality, the following statements were used to indicate direction of effect: ‘improves’, ‘reduces’, ‘no difference’ and ‘unclear’. ‘Unclear’ also includes inconsistent evidence.
Data summary
As pain and function are usually continuous outcomes, data were summarised using the:
-
standardised mean difference (SMD) [summary statistic used when studies assess the same outcome but measure it in a variety of ways, difference in mean outcome between groups/standard deviation (SD) of outcomes among participants] with 95% CI as reported in the included reviews
-
WMD (weighted mean calculated for groups before and after an intervention and the WMD would be the difference between start and finish values. Usually calculated as the sum of the differences in the individual studies, weighted by the individual variances for each study) with 95% CI as reported in the included reviews. In Cochrane reviews this is now referred to as ‘mean difference’; although the meta-analysis computes a weighted average of the differences in means, no weighting is involved in the calculation of a statistical summary of a single study.
For dichotomous outcomes, relative risk and 95% CI are presented when possible. Pooled effect estimates were presented according to the model used in the review.
We note the potential for some confusion with respect to the interpretation of the direction of effect. We found that in some cases it was not clear if the values reported were based on the difference in pre–post change (i.e. the difference between the pre–post, within-subject, differences in the treatment and control groups) or the difference in post-intervention value (the difference in an outcome between the treatment and control groups). Other potential sources of confusion when interpreting the direction of effect included whether or not the outcome was desired (a decrease in pain is desirable, whereas a decrease in function is not and vice versa) and the direction of any scale (a high value might indicate high levels of pain/function or it may indicate a high level of benefit in terms of pain relief or improved function). Another potential for confusion concerns whether or not the convention of intervention control is adhered to. This is particularly likely to be a problem when active interventions or different doses of the same intervention are being compared. Given that our study is an overview of systematic reviews, our general approach was to accept the interpretation of the direction of effect as defined in each systematic review. We checked the original source papers for only one of the interventions, sodium hyaluronate.
Results
Quantity of research available
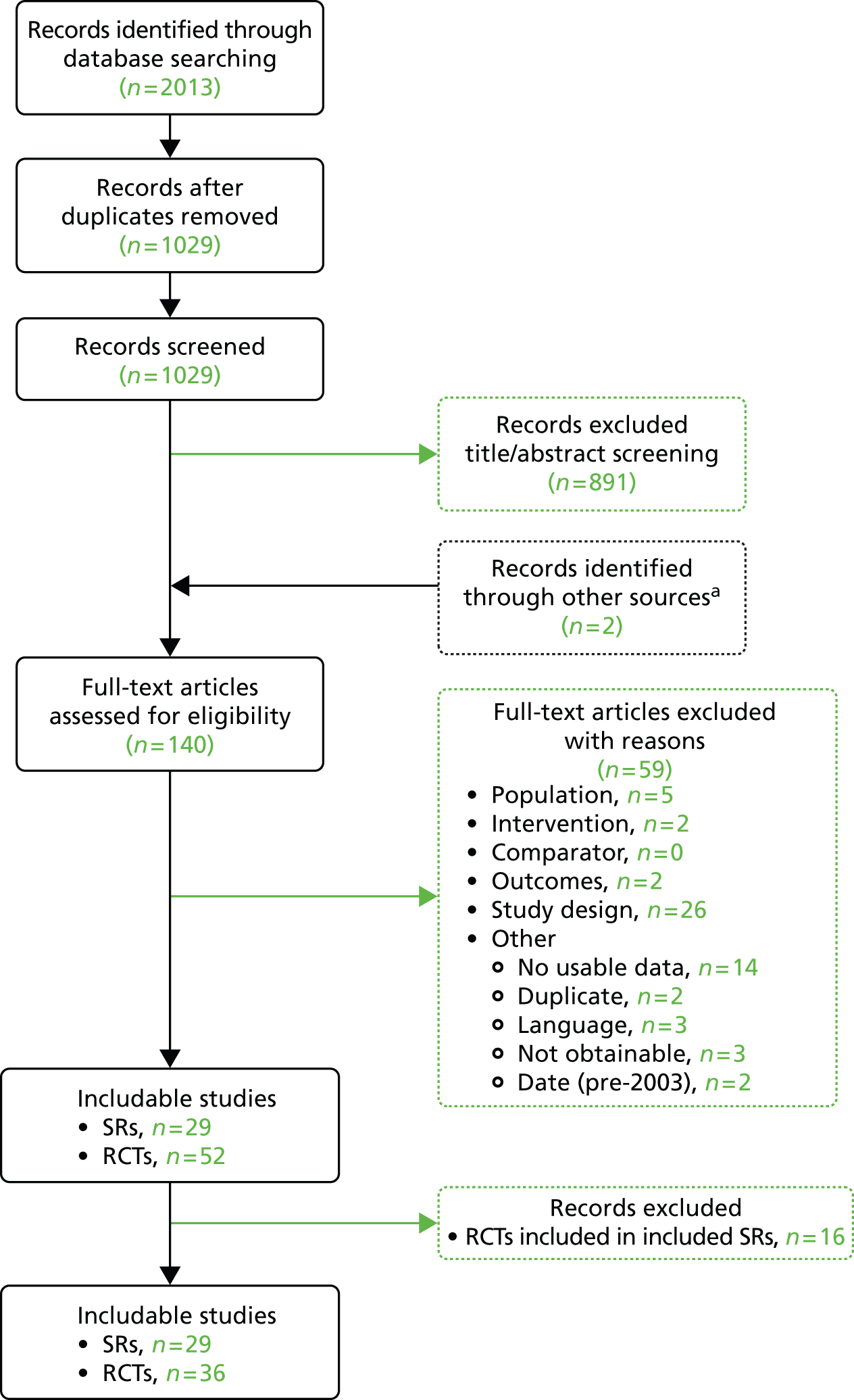
The systematic review of electronic databases for clinical effectiveness studies produced 1029 titles and abstracts, of which 891 were judged not to meet our inclusion criteria and were excluded. An additional two studies relevant to the effectiveness overview were identified when screening the cost searches. In total, 1031 unique titles and abstracts were screened.
A total of 140 full-text papers were reviewed to assess if they met the inclusion criteria. From these, 59 papers were excluded; details of these papers, with reasons for their exclusion, can be found in Appendix 2. This left 81 articles included in this systematic review, of which 29 were systematic reviews or meta-analyses and 52 were reports of RCTs.
The included RCTs (n = 52) were then screened to identify those incorporated in the identified systematic reviews; this led to the exclusion of a further 16 studies. In total, we identified 36 RCTs not already incorporated into a systematic review (see Summary of randomised controlled trials).
The study selection process is summarised in Figure 3.
FIGURE 3.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart for the clinical effectiveness review. SRs, systematic reviews. a, Identified in the cost-effectiveness systematic review.
Assessment of quality and effectiveness: systematic reviews
A total of 29 systematic reviews were included in the review.
The systematic reviews were graded according to overall point score using the AMSTAR measurement tool to assess the methodological quality of systematic reviews (see Appendix 3). All items on the AMSTAR measurement tool were given equal weighting. Systematic reviews were considered of low quality if their rating was less than 4 of a possible 11 points, intermediate quality if they had a rating of between 4 and 7 of a possible 11 points and high quality if they had a rating of between 8 and 11 points. Five systematic reviews had a rating of less than 4 points, 19 systematic reviews were considered of intermediate quality (scoring between 4 and 7 points) and five systematic reviews had a rating of 8 points and were considered to be of high quality.
A summary is provided in Table 3, and a more detailed overview of these studies together with quality assessment (AMSTAR score) is provided in Appendices 3 and 4. Only studies scoring 8 points or more in the AMSTAR assessment were analysed using the GRADE principles. Studies scoring 1 to 7 points on the AMSTAR measurement tool were not analysed further or considered in the recommendations made.
| Author, year (study) | Number of included studiesa (number of participants) | Methodological quality (QR/QPS) |
|---|---|---|
| High quality (scoring 8–11 AMSTAR points) | ||
| Barr et al., 200956 | 5 RCTs (n = 597) | QR = high (AMSTAR, 8 points); QPS: mean = 6.8 points, range = 4–8 points; (PEDro scale, 11 points) |
| Trudel et al., 200457 | 5 RCTs (n = 215) | QR = high (AMSTAR, 8 points); QPS: range 34–44 points (out of 48 points); (MacDermidb quality score) |
| Buchbinder et al., 200658 | 10 RCTs (n = 1099) | QR = high (AMSTAR, 8 points); QPS: no validated scale used |
| Smidt et al., 200359 | 23 RCTs (n = NR) | QR = high (AMSTAR, 8 points); QPS: mean = 6.7 points, range 1–11 points; (Amsterdam–Maastricht Consensus list, 12 points) |
| Coombes et al., 201060 | 17 RCTs (n = 1687) | QR = high (AMSTAR, 8 points); QPS: mean = 9.8 points, range 7–12 points; (modified PEDro scale range, 13 points) |
| Intermediate quality (scoring 4–7 AMSTAR points) | ||
| Woodley et al., 200761 | 3 RCTs (n = 184) | QR = high (AMSTAR, 7 points); QPS: mean = 6.3 points, range 5–8 points; (PEDro scale 1–11); QPS mean = 7.3 points, range 6–8 points; (van Tulder scale 0–11) |
| Bjordal et al., 200862 | 13 RCTs (n = 730) | QR = moderate (AMSTAR, 7 points); QPS: mean = 6.5 points, range 4–8 points; (Delphi/PEDro checklist) |
| Kalichman et al., 201163 | 4 RCTs (n = 273) | QR = moderate (AMSTAR, 7 points); QPS: no validated scale used |
| Raman et al., 201264 | 6 RCTs (n = 283) | QR = moderate (AMSTAR, 7 points); QPS: mean score = 35 points, range 32–40 points; (MacDermid quality score) |
| Rabago et al., 200965 | 3 RCTs (n = 68) | QR = moderate (AMSTAR, 7 points); QPS: mean = 7 points, range 5–9 points; (Delphi score, 0–9) |
| Gaujoux-Viala et al., 200966 | 8 RCTs (n = 887) | QR = moderate (AMSTAR, 7 points); QPS: mean = 3 points, range 2–5 points; (Jadad scale, 1–5 points) |
| Zhang et al., 201167 | 3 RCTs (n = 232) | QR = moderate (AMSTAR, 7 points); QPS: mean = 5 points, range 4–5 points; (Jadad score, 5 points) |
| Bisset et al., 200568 | 28 RCTs (n = NR) | QR = moderate (AMSTAR, 7 points); QPS: mean = 9.4 points, range 8–13 points; (modified PEDro rating scale, 1–15 points) |
| Borkholder et al., 200469 | 11 RCTs n = 312) | QR = moderate (AMSTAR, 6 points); QPS: mean = 26.3 points, range 44.5–16.5 points; [MacDermid quality score, Sackett’s level 1b (n = 1), Level 2b (n = 10)] |
| Trinh et al., 200470 | 6 RCTs (n = 282) | QR = moderate (AMSTAR, 6 points); QPS: mean = 4 points, range 3–5 points; (Jadad scale, 1–5 points) |
| Taylor et al., 201171 | 4c RCTs (n = 286) | QR = moderate (AMSTAR, 6 points); QPS: no quality appraisal conducted |
| aTumilty et al., 201072 | 13 RCTs (n = 472) | QR = moderate (AMSTAR, 6 points); QPS: mean = 6.5 points, range 5–8 points; (PEDro rating scale, 11 points) |
| Zacher et al., 200873 | 4 RCTs (n = 286) | QR = moderate (AMSTAR 6 points); QPS: no validated quality appraisal tool though some consideration for quality reported |
| Herd and Meserve et al., 200874 | 13 RCTs (n = 639) | QR = moderate (AMSTAR 5 points); QPS: mean = 5 points, range 1–8 points; (PEDro rating scale, points 1–8) |
| cJoseph et al., 201275 | 3 RCTs (n = 196) | QR = moderate (AMSTAR, 5 points); QPS: mean = 7 points, range 7 points;c (PEDro rating scale, points 1–8) |
| dTumilty et al., 201076 | 11 RCTs (n = NR) | QR = moderate (AMSTAR, 5 points); QPS: mean = 7 points, range 5–8 points; (PEDro rating scale, 8 points) |
| Baxter et al., 200877 | 3 RCTs (n = 166) | QR = moderate (AMSTAR, 4 points); QPS: mean 6 points, range 5–7 points; (van Tulder scale, 11 points) |
| Farren, 201278 | 3 RCTs (n = 175) | QR = moderate (AMSTAR, 4 points); QPS: mean = 4 points, range 4–5 points; (Jadad score, 5 points) |
| Kohia et al., 200879 | 16 RCTs (n = 1814) | QR = moderate (AMSTAR, 4 points); QPS: no quality assessment tool used |
| Low quality (scoring 1–3 AMSTAR points) | ||
| Bisset et al., 201180 | 56 RCTs + 18 SRs of RCTs (n = NR) | QR = low (AMSTAR, 3 points); QPS: NR |
| Chang et al., 201081 | 10 RCTs (n = 449) | QR = low (AMSTAR, 3 points); QPS: mean = 5 points, range 3–8 points; (PEDro rating scale, 11 points) |
| Snyder and Evans, 201282 | 4 RCTs (n = 470) | QR = low (AMSTAR, 3 points); QPS: mean = 7 points, range 6–8 points; (PEDro rating scale, 8 points) |
| Pagorek, 200983 | 2 RCTs (n = 48) | QR = low (AMSTAR, 3 points); QPS: no quality assessment tool used |
| Crawford and Laiou, 200784 | 14 RCTs (n = NR) | QR = low (AMSTAR, 1 points); QPS: quality assessed but no validated tool used |
Summary of high-quality systematic review findings
Five of the included systematic reviews had a rating of 8 points and were considered of high quality. 56–60 Data for all important outcome measures were extracted from three of these high-quality reviews and analysed using the GRADE principles (see Methods of data synthesis). 58–60 Two of the reviews are referred to in the write-up but, because of the lack of reported data, were not analysed using the GRADE principles. 56,57 A summary of systematic review findings for the five high-quality reviews is given in the following sections.
Electrocorporeal shock wave therapy
One high-quality review, by Buchbinder et al. ,58 examined the effect of shock wave therapy on lateral epicondylitis. Neither severity of LET nor details of co-interventions were reported in any of the studies. Buchbinder et al. 58 performed searches up to and including February 2005. A total of 10 RCTs were included in their review,84–93 with nine RCTs85–93 (1006 participants) comparing ESWT with placebo and one94 comparing ESWT with a steroid injection (93 participants). Data from six trials were pooled. 85–87,89,90 Pooled analysis for pain and function outcomes were performed using data from four of the placebo-controlled studies. 87,89,90,95 Results from two placebo-controlled trials could not be pooled because of inadequate reporting of results. 91,93 Further information is available in the Cochrane review of ESWT for LET (published online 2005). 96
The nine placebo-controlled trials85–93 reported conflicting results, with three trials85–87 reporting significant differences in favour of ESWT for pain and function, whereas four trials reported no benefit of ESWT over placebo for these outcomes. 88–91 However, when the available data were pooled, the authors found that most benefits observed in the positive trials were no longer statistically significant. Two pooled analysis, both containing three trials, showed that ESWT is not more effective than placebo at reducing pain in the short term (4–6 weeks)85,89,90 or intermediate term (12 weeks). 86,87,90 The evidence pertaining to this outcome was considered of moderate quality when we assessed using the GRADE principles (see GRADE profiles in Appendix 4). Pooled analysis of three trials86,87,90 showed no benefit for ESWT over placebo for function in the intermediate term (12 weeks), as measured by grip strength. The evidence for this outcome was considered of moderate quality when we assessed using the GRADE principles (Table 4 and see GRADE profiles in Appendix 4).
| Outcomes | Number of participants; (number of studies); follow-up period | Quality of the evidence (as assessed by GRADE) | Relative effect (95% CI) | Overall results |
|---|---|---|---|---|
| Pain (short term), VAS (100 mm) | 446; three studies; 4–6 weeks | ⊕⊕⊕⊝ moderatea because of inconsistency | – | The mean pain (short term) in the intervention groups was 9.42 (20.7 lower to 1.86 higher) |
| Pain (intermediate term), resisted wrist extension (Thomsen test) | 455; three studies; 12 weeks | ⊕⊕⊕⊝ moderatea because of inconsistency | – | The mean pain (intermediate term) in the intervention groups was 9.04 lower (19.37 lower to 1.28 higher) |
| Function (intermediate term), mean grip strength | 448; three studies; 12 weeks | ⊕⊕⊕⊝ moderateb because of inconsistency | – | The mean function (intermediate term) in the intervention groups was 0.05 SDs higher (0.13 lower to 0.24 higher) |
| QoL | Outcome NR | Outcome NR | – | – |
| Remain/return to work | Outcome NR | Outcome NR | – | – |
| Sport activity | Outcome NR | Outcome NR | – | – |
| Recurrence | Outcome NR | Outcome NR | – | – |
| Adverse events (mild) | 60; one study; 5 weeks | ⊕⊕⊕⊝ moderatec because of inconsistency | – | Tingling during therapy (five in placebo group), aching after therapy (one in placebo group), soreness after therapy (four in placebo group) and increased pain symptoms after therapy (three in placebo group) |
| Adverse events (general) | 542; one study; 52 weeks | ⊕⊕⊕⊝ moderatec,d because of inconsistency | OR 4.3 (2.9 to 6.3)e | – |
One RCT in the review by Crowther et al. 94 reported that steroid injection was more effective than ESWT at 3 months after the end of treatment, assessed by a reduction in pain of 50% from baseline as the criterion of success. The evidence for this outcome was considered of moderate quality when we assessed it using the GRADE principles (Table 5; and see GRADE profiles in Appendix 4). This reported pain relief with GCIs is consistent with findings from one other systematic review97 and a subsequent RCT of GCI for lateral elbow pain which found limited evidence of a short-term improvement in symptoms with steroid injections compared with placebo, a local anaesthetic, orthoses, physiotherapy or NSAIDs. 98 However, long-term benefits of steroid injection were not considered in these reviews.
| Outcomes | Number of participants; (number of studies); follow-up period | Quality of the evidence (as assessed by GRADE) | Relative effect (95% CI) | Overall results |
|---|---|---|---|---|
| Pain reduction of 50% from baseline as a criterion of success | 73; one study; 3 months | ⊕⊕⊕⊝ moderatea because of risk of bias | – | – |
Laser therapy
Two high-quality systematic reviews were found, containing 14 RCTs in total.
One high-quality review, by Smidt et al. ,59 examined the effect of laser therapy on lateral epicondylitis. Neither the severity of tennis elbow nor the duration of symptoms was mentioned for any of the included studies and no co-interventions were mentioned.
The search was performed from database inception up to and including January 1999. A total of eight RCTs99–106 (six with acceptable validity100–103,105,106) comparing the effects of laser with placebo were included in the review. One trial compared the effects of laser with therapeutic ultrasound (plus friction massage). 66 No pooling of data was possible because of insufficient data or clinical or statistical heterogeneity.
One high-quality systematic review, by Trudel et al. ,57 examined the effect of laser therapy on lateral epicondylitis compared with placebo. The search was performed from January 1983 up to and including March 2003. A total of six RCTs of variable quality (294 participants) comparing the effects of laser with placebo laser therapy were included in the review. 100–103,105,106 Neither severity of lateral epicondylitis nor details of co-interventions were reported in any of the studies. No numerical data for any outcome were reported and no pooling of data was performed.
Laser therapy compared with placebo
Smidt et al. 59 assessed eight studies comparing the effects of laser with placebo. 99–106 One RCT showed no statistically significant effects on pain in the short term (3 weeks),106 but contradictory results were reported for intermediate (6 weeks to 6 months) assessments for mean pain (Table 6). 104,106 The evidence for no effect of laser on pain relief compared with placebo in the short term (one RCT106) was considered of moderate quality when we assessed it using the GRADE principles (see Table 6 and GRADE profiles in Appendix 4). The evidence for pain relief with laser therapy in the intermediate and long term (two RCTs104,106) was considered to be of low quality when we assessed it using the GRADE principles (see Table 6 and GRADE profiles in Appendix 4).
| Outcomes | Number of participants; studies; period of follow-up period | Quality of the evidence (as assessed by GRADE) | Relative effect (95% CI) | Overall results |
|---|---|---|---|---|
| Pain (0–6 weeks), VAS | NR; one study; 3 weeks | ⊕⊕⊕⊝ moderatea because of imprecision | – | The mean pain (0–6 weeks) in the intervention groups was 0.25 SDs lower (0.96 lower to 0.47 higher) |
| Pain (7 weeks), VAS | NR; one study; 7 weeks | ⊕⊕⊝⊝ lowa,b because of inconsistency, imprecision | – | The mean pain (7 weeks) in the intervention groups was 0.46 SDs lower (1.19 lower to 0.27 higher) |
| Pain (13 weeks), VAS | NR; one study; 13 weeks | ⊕⊕⊝⊝ lowa,b because of indirectness, imprecision | – | The mean pain (13 weeks) in the intervention groups was 2 SDs lower (2.77 to 1.22 lower) |
| Function | O/C; NR | O/C; NR | O/C; NR | – |
| QoL | O/C; NR | O/C; NR | O/C; NR | – |
| Remain/return to work | O/C; NR | O/C; NR | O/C; NR | – |
| Sport activity | O/C; NR | O/C; NR | O/C; NR | – |
| Recurrence | O/C; NR | O/C; NR | O/C; NR | – |
| Adverse events | O/C; NR | O/C; NR | O/C; NR | – |
One high-quality systematic review, Trudel et al. ,57 found six RCTs100,101,103–105,107 (294 subjects) which collectively investigated the effects of laser therapy compared with placebo laser therapy in the treatment of lateral epicondylitis. 57 The findings of all six studies (a combination of high- and low-quality RCTs) suggest that laser is not significantly better than placebo laser for function (grip strength) and pain severity in the short term. 28,100,101,104,105,107 However, no numerical data were reported in this systematic review and so the results of these primary studies could not contribute to our assessment of the evidence using the GRADE principles.
Laser therapy compared with physiotherapy/physiotherapeutic modalities
Smidt et al. 59 compared therapeutic ultrasound and friction massage108,109 and reported no benefit of laser therapy for pain relief in either the short (3 weeks) or intermediate (7 weeks) term. 108 However, the evidence for this outcome was considered of low quality when we assessed it using the GRADE principles (Table 7; and see GRADE profiles in Appendix 4).
| Outcomes | Number of participants; studies; follow-up period | Quality of the evidence (as assessed by GRADE) | Relative effect (95% CI) | Overall results |
|---|---|---|---|---|
| Pain (short term), VAS | NR; one study; 3 weeks | ⊕⊕⊝⊝ lowa,b because of risk of bias, imprecision | – | The mean pain (short term) in the intervention groups was 0.92 SDs higher (0.17 to 1.67 higher) |
| Pain (7 weeks), VAS | NR; one study; 7 weeks | ⊕⊕⊝⊝ lowb,c because of risk of bias, imprecision | – | The mean pain (7 weeks) in the intervention groups was 0.84 SDs higher (0.09 to 1.58 higher) |
Within the review by Trudel et al. ,57 one low-quality RCT of 30 participants found that, when used in combination with traditional physiotherapy (therapeutic ultrasound and friction massage), laser provided no great benefit for pain and grip strength. 106 However, contradictory results were found in two low-quality RCTs with a total of 93 participants. 101,106 They found significant short- and long-term improvements in pain and function (grip strength). No numerical data were provided and the results of these studies could not contribute to our assessment of the evidence using the GRADE principles.
Therapeutic ultrasound
Two high-quality systematic reviews were found, containing 15 RCTs in total. 58,60
One high-quality review59 examined the effect of therapeutic ultrasound on lateral epicondylitis. The review included nine RCTs39,101,104,106,110–114 comparing therapeutic ultrasound with placebo (three RCTs102,109,110), laser therapy (one RCT108), exercise and mobilisation (one RCT112) and other physiotherapy modalities and conservative treatments (seven RCTs39,102,109–112,114). Neither the severity of tennis elbow nor the duration of symptoms was mentioned for any of the included studies. No co-interventions were mentioned.
The search was performed up to and including January 1999. Pooled analysis was not performed for most studies because of the lack of data. Two studies comparing therapeutic ultrasound with placebo were pooled for pain outcomes in the intermediate term. 109,110
One high-quality systematic review57 examined the effect of therapeutic ultrasound (alone and in combination with other therapies) on lateral epicondylitis compared with placebo. The search was performed up to and including March 2003. A total of six RCTs of variable quality (294 participants) were included in the review. 109–112,114,115 Only one RCT was judged to be of sufficient quality to be considered in this overview. Neither severity of lateral epicondylitis nor details of co-interventions were reported in any of the studies. No numerical data for any outcome were reported and no pooling of data was performed.
Therapeutic ultrasound compared with placebo
In one high-quality systematic review (Smidt et al. 59), three studies compared the effectiveness of therapeutic ultrasound with placebo. 102,109,110 Two of the studies reported beneficial effects for therapeutic ultrasound in the short term (4 weeks) as well as the intermediate term (8 and 13 weeks). 109,110 Smidt et al. 59 report that pooling of two RCTs for the intermediate-term outcomes109,110 resulted in a large effect size for pain relief in favour of therapeutic ultrasound (SMD –0.98, 95% CI –1.64 to –0.33). The consistent evidence from all three RCTs reporting increased pain relief in both the short and intermediate term was considered to be of moderate quality102,109,110 (Table 8 and see GRADE profiles in Appendix 4).
| Outcomes | Number of participants; studies; follow-up period | Quality of the evidence (as assessed by GRADE) | Relative effect (95% CI) | Overall results |
|---|---|---|---|---|
| Pain (short term), VAS | NR; one study; 6 weeks | ⊕⊕⊕⊝ moderatea because of imprecision | – | The mean pain (short term) in the intervention groups was 0.61 SDs lower (1.07 to 0.15 lower) |
| Pain (8 weeks), VAS | NR; (one study) | ⊕⊕⊕⊝ moderatea because of imprecision | – | The mean pain (8 weeks) in the intervention groups was 0.66 SDs lower (1.13 to 0.20 lower) |
| Pain (13 weeks), VAS | NR; one study; 13 weeks | ⊕⊕⊕⊝ moderatea because of imprecision | – | The mean pain (13 weeks) in the intervention groups was 1.33 SDs lower (1.87 to 0.80 lower) |
| Function | O/C; NR | O/C; NR | – | – |
| QoL | O/C; NR | O/C; NR | – | – |
| Remain/return to work | O/C; NR | O/C; NR | – | – |
| Sport activity | O/C; NR | O/C; NR | – | – |
| Recurrence | O/C; NR | O/C; NR | – | – |
| Adverse events | O/C; NR | O/C; NR | – | – |
The benefits of both therapeutic ultrasound and therapeutic ultrasound plus friction massage for pain relief were confirmed in a high-quality systematic review by Trudel et al. 57 One high-quality RCT, by Stratford et al. ,111 reported significant pain relief using therapeutic ultrasound alone compared with placebo in the short term. Stratford et al. 111 also examined therapeutic ultrasound in combination with friction massage, phonophoresis alone and phonophoresis with frictional massage, and found all treatments to be beneficial for pain relief; however, no one treatment was superior to another.
Therapeutic ultrasound compared with laser
There was one included study in the Smidt et al. 59 review comparing therapeutic ultrasound (plus friction massage) with laser therapy. 106 Therapeutic ultrasound (plus friction massage) was reported to be superior to laser for pain relief in both the short term (SMD pain –0.92, 95% CI –1.67 to –0.17) and the intermediate term (SMD pain –0.84, 95% CI –1.58 to –0.09). The evidence was considered to be of moderate quality (Table 9; and see GRADE profiles in Appendix 4).
| Outcomes | Number of participants; studies; follow-up period | Quality of the evidence (as assessed by GRADE) | Relative effect (95% CI) | Overall results |
|---|---|---|---|---|
| Pain (short term), VAS | NR; one study; 3 weeks | ⊕⊕⊕⊝ moderatea because of risk of bias | – | The mean pain (short term) in the intervention groups was 0.92 SDs lower (1.67 to 0.17 lower) |
| Pain (intermediate term), VAS | NR; one study; 7 weeks | ⊕⊕⊕⊝ moderatea because of risk of bias | – | The mean pain (intermediate) in the intervention groups was 0.84 SDs lower (1.58 to 0.09 lower) |
Therapeutic ultrasound compared with exercises
One study in the Smidt et al. 59 review, i.e. that by Pienimaki et al. ,112 found therapeutic ultrasound (plus friction massage) to be inferior to exercises for pain relief in the intermediate term (SMD pain 0.95, 95% CI 0.26 to 1.64). The evidence was considered to be of moderate quality (Table 10; and see GRADE profiles in Appendix 4).
| Outcomes | No of participants (studies); follow-up period | Quality of the evidence (as assessed by GRADE) | Relative effect (95% CI) | Overall results |
|---|---|---|---|---|
| Pain (intermediate term), VAS | NR; one study; 8 weeks | ⊕⊕⊕⊝ moderatea because of risk of bias | – | The mean pain (intermediate term) in the intervention groups was 0.95 SDs higher (0.26 to 1.64 higher) |
Exercises
Two high-quality systematic reviews were found, containing nine RCTs in total. 57,59
One high-quality review59 examined the effect of exercises and mobilisation techniques on lateral epicondylitis. No definition of exercises and mobilisation techniques was given. Neither the severity of tennis elbow nor the duration of symptoms was mentioned for any of the included studies. No co-interventions were mentioned.
The search was performed up to and including January 1999. Five RCTs comparing the effects of therapeutic ultrasound (plus friction massage) with exercises and mobilisation techniques were included in the review,36,112,113,116,117 with only one trial of acceptable quality. No pooling of data was possible because of insufficient data or clinical or statistical heterogeneity.
One high-quality systematic review57 examined the effect of exercises on lateral epicondylitis compared with placebo. The search was performed up to and including March 2003. A total of four RCTs of variable quality (125 participants) were included in the review. 112,115,118,119 Only two RCTs were judged to be of sufficient quality to be considered in this overview. 112,118 Neither severity of lateral epicondylitis nor details of co-interventions were reported in any of the studies. No numerical data for any outcome were reported and no pooling of data was performed.
Exercise compared with therapeutic ultrasound (plus friction massage)
In one high-quality review,59 one RCT demonstrated a large effect on pain relief from exercises compared with therapeutic ultrasound plus friction massage in the intermediate term (8 weeks) (SMD –0.95, 95% CI –1.64 to –0.26). 112 Evidence for this outcome was considered moderate quality (Table 11; and see GRADE profiles in Appendix 4). Four other relevant RCTs included in this review were either of poor validity or provided insufficient data on relevant outcome measures,36,113,117,120 leading the authors to conclude that there is insufficient evidence to demonstrate either benefit or lack of effect of exercises and mobilisation techniques for LET.
| Outcomes | Number of participants; studies; follow-up period | Quality of the evidence (as assessed by GRADE) | Relative effect (95% CI) | Overall results |
|---|---|---|---|---|
| Pain (intermediate term) VAS | NR; one study; 8 weeks | ⊕⊕⊕⊝ moderatea because of risk of bias | – | The mean pain (intermediate) in the intervention groups was 0.95 SDs lower (1.64 to 0.26 lower) |
| Function | O/C; NR | O/C; NR | – | – |
| QoL | O/C; NR | O/C; NR | – | – |
| Remain/return to work | O/C; NR | O/C; NR | – | – |
| Sport activity | O/C; NR | O/C; NR | – | – |
| Recurrence | O/C; NR | O/C; NR | – | – |
| Adverse events | O/C; NR | O/C; NR | – | – |
However, in a high-quality systematic review, Trudel et al. 57 reported on four RCTs that found that progressive strengthening and stretching programmes resulted in significantly greater reductions in pain than the alternative treatment state. 110,111,115,119 Two of these RCTs112,118 found significant benefits in function (as determined by grip strength) in those who participated in the strengthening and stretching programmes. However, no data were reported in the systematic review and, hence, it was not possible to independently assess the quality of the evidence.
Glucocorticoid injections
Two high-quality systematic reviews were found, containing 17 RCTs in total. 56,60
One high-quality review60 included 12 RCTs (1171 participants) examining the effect of GCIs on lateral epicondylitis. 38,40,50,116,118,120–126 Severity of tennis elbow (mean pain score before treatment) was reported for six of the included studies and ranged from 49 to 83 on a visual analogue scale (VAS) score (0–100). Co-interventions were not mentioned. The search was performed up to and including March 2010. Pooled analysis was not performed for most studies because of heterogeneity.
One high-quality systematic review56 included five RCTs examining the effect of GCIs on lateral epicondylitis compared with physiotherapeutic interventions. 116,120–122,127 The search was performed up to and including March 2009. Pooled analysis was performed for two studies, with the remainder being unsuitable because of heterogeneity. Co-interventions administered to injection participants were fairly comparable between studies. However, 21% of physiotherapy participants in one study122 received additional treatment, compared with 81% in the comparable study. Severity of lateral epicondylitis in participants prior to treatment was not mentioned.
One high-quality systematic review, by Coombes et al. ,60 found consistent findings from eight RCTs that GCIs reduced pain and increased function40,116,120–125 (as measured by pain-free grip strength) in the short term compared with other interventions (watch and wait,120–122 physiotherapy,40,116,121,122 NSAIDs,40 placebo123,124 and PRP injections125), but this effect was reversed in the intermediate and long term. These negative effects remained significant at 1 year, apart from for GCIs compared with NSAIDs for pain relief, which did not differ. The evidence for no effect on pain and no improvement in function in the intermediate and long term from GCIs was considered of moderate quality when we assessed it using the GRADE principles (Table 12 and see GRADE profiles in Appendix 4).
| Outcomes | Number of participants; studies; follow-up period | Quality of the evidence (as assessed by GRADE) | Relative effect (95% CI) | Overall results |
|---|---|---|---|---|
| Pain (intermediate term), VAS (0–100) | 241; three studies; 26 weeks | ⊕⊕⊝⊝ lowa,b because of risk of bias, inconsistency | – | The mean pain (intermediate term) in the intervention groups was 0.07 SDs higher (0.50 lower to 0.63 higher) |
| Function (short term), DASH | 64; one study; 4 weeks | ⊕⊕⊕⊝ moderatec because of risk of bias | – | The mean function (short term) in the intervention groups was 0.14 SDs higher (0.42 lower to 0.69 higher) |
| Function (intermediate term), DASH | 64; one study; 26 weeks | ⊕⊕⊕⊝ moderatec because of risk of bias | – | The mean function (intermediate term) in the intervention groups was 0.25 SDs lower (0.82 lower to 0.32 higher) |
| QoL | O/C; NR | O/C; NR | O/C; NR | – |
| Remain/return to work | O/C; NR | O/C; NR | O/C; NR | – |
| Sport activity | O/C; NR | O/C; NR | O/C; NR | – |
| Recurrence | O/C; NR | O/C; NR | O/C; NR | – |
| Adverse event (pain), post-injection pain | 88; one study; 24 weeks | ⊕⊕⊝⊝ lowd,e because of risk of bias, inconsistency | RR 1.64 (0.90 to 2.98) | – |
| Adverse event (atrophy) | 88; one study; 24 weeks | ⊕⊕⊝⊝ lowd,e because of risk of bias, inconsistency | RR 1.77 (0.73 to 4.29) | – |
| Adverse event (depigmentation) | 64; one study; 26 weeks | ⊕⊕⊝⊝ lowc,e because of risk of bias, inconsistency | RR 0.53 (0.05 to 5.58) | – |
Glucocorticoid injections compared with placebo
Three RCTs118,123,128 comparing GCIs with placebo had conflicting results, with two RCTs GCI having a significant effect on reduction of pain in the short term. 123,128 Pooled analysis of all three RCTs found placebo to be favoured for pain relief in the intermediate term. Evidence for this outcome was considered of low quality when we assessed it using the GRADE principles (see Table 12 and GRADE profiles in Appendix 4).
Corticosteroid injections compared with no intervention (or watch and wait)
In a pooled analysis of three RCTs,120–122 GCIs were found to have a large effect (defined as SMD > 0.8) on short-term pain relief compared with no intervention (observation or watch and wait). The evidence for this outcome was considered low quality when we assessed it using the GRADE principles (Table 13; and see GRADE profiles in Appendix 4). A pooled analysis of two RCTs121,122 found pain relief after receiving no intervention in both the intermediate and long term. Evidence for both of these outcomes was considered of moderate quality when we assessed it using the GRADE principles (see Table 13 and GRADE profiles in Appendix 4).
| Outcomes | Number of participants; studies; follow-up period | Quality of the evidence (as assessed by GRADE) | Relative effect (95% CI) | Overall results |
|---|---|---|---|---|
| Pain (short term), VAS/NRS/PRFEQ pain subscale | 277; three studies; 4 weeks | ⊕⊕⊝⊝ lowa,b because of risk of bias, imprecision | – | The mean pain (short term) in the intervention groups was 1.44 SDs lower (1.17 to 1.71 lower) |
| Pain (intermediate term), VAS | 253; two studies; 26 weeks | ⊕⊕⊕⊝ moderatea because of risk of bias | – | The mean pain (intermediate term) in the intervention groups was 0.40 SDs higher (0.67 to 0.14 higher) |
| Pain (long term), VAS | 253; two studies; 52 weeks | ⊕⊕⊕⊝ moderatea because of risk of bias | – | The mean pain (long term) in the intervention groups was 0.31 SDs higher (0.61 to 0.01 higher) |
| Function (short term), pain-free function scale/PRFEQ function subscale | 277; three studies; 4 weeks | ⊕⊕⊕⊝ moderatea because of risk of bias | – | The mean function (short term) in the intervention groups was 1.50 SDs higher (1.22 to 1.77 higher) |
| Function (intermediate term), pain-free function scale/PRFEQ function subscale | 253; three studies; 26 weeks | ⊕⊕⊕⊝ moderatea because of risk of bias | – | The mean function (intermediate term) in the intervention groups was 0.51 SDs lower (0.76 to 0.25 lower) |
| Function (long term), pain-free function scale/PRFEQ function subscale | 253; three studies; 52 weeks | ⊕⊕⊕⊝ moderatea because of risk of bias | – | The mean function (long term) in the intervention groups was 0.32 SDs lower (0.57 to 0.06 lower) |
Glucocorticoid injections compared with physiotherapy
In the systematic review by Coombes et al. ,60 three RCTs comparing GCIs with physiotherapy had conflicting results, with two RCTs120,121 showing GCIs to have a large effect on reduction of pain in the short term. 120–122 The authors suggest that this heterogeneity is because of different physiotherapy protocols between studies. 60 Pooled analysis found physiotherapy to be favoured in the intermediate term and long term. Evidence for both these outcomes was considered moderate quality when we assessed it using the GRADE principles (Table 14 and see GRADE profiles in Appendix 4).
| Outcomes | Number of participants; studies; follow-up period | Quality of the evidence (as assessed by GRADE) | Relative effect (95% CI) | Overall results |
|---|---|---|---|---|
| Pain (intermediate term), VAS/NRS | 257; two studies; 26 weeks | ⊕⊕⊕⊝ moderatea because of risk of bias | – | The mean pain (intermediate term) in the intervention groups was 0.56 SDs higher (0.82 to 0.31 higher) |
| Pain (long term), VAS/NRS | 257; two studies; 52 weeks | ⊕⊕⊕⊝ moderatea because of risk of bias | – | The mean pain (long term) in the intervention groups was 0.48 SDs higher (0.73 to 0.23 higher) |
| Function (short term), pain-free function scale/PRFEQ function subscale | 281; three studies; 4 weeks | ⊕⊕⊕⊝ moderateb because of risk of bias | – | The mean function (short term) in the intervention groups was 1.29 SDs higher (1.03 to 1.55 higher) |
| Function (intermediate term), pain-free function scale/PRFEQ function subscale | 257; two studies; 26 weeks | ⊕⊕⊕⊝ moderatea because of risk of bias | – | The mean function (intermediate term) in the intervention groups was 0.64 SDs lower (0.90 to 0.39 lower) |
| Function (long term), pain-free function scale/PRFEQ function subscale | 257; two studies; 52 weeks | ⊕⊕⊕⊝ moderatea because of risk of bias | – | The mean function (long term) in the intervention groups was 0.57 SDs lower (0.82 to 0.32 lower) |
| Recurrencec | 281; three studies; 52 weeks | ⊕⊕⊝⊝ lowb,d because of risk of bias, imprecision | – | – |
All of the included studies in a high-quality systematic review by Barr and Blanchard56 found that GCIs were significantly more effective than physiotherapeutic interventions for outcome measurements at short-term follow-up. In the intermediate term, three of the studies found that physiotherapeutic interventions were significantly more effective than GCIs. 121,122,127 Their main conclusion was that GCIs are effective at short-term follow-up for functional improvement (measured by pain-free grip strength) and physiotherapeutic interventions are effective at intermediate- and long-term follow-up.
However, despite GCIs being found to be more effective in the short term than physiotherapeutic interventions, Barr and Blanchard56 note that reported recurrence rates varied from 34% to 74% in three of the included studies. 116,121,122
Glucocorticoid injections compared with non-steroidal anti-inflammatory drugs
In one RCT,40 GCIs were found to have a large effect on reduction of pain in the short term compared with a NSAID (naproxen). The evidence for this outcome was considered moderate quality when we assessed it using the GRADE principles (Table 15 and see GRADE profiles in Appendix 4).
| Outcomes | Number of participants; studies; follow-up period | Quality of the evidence (as assessed by GRADE) | Relative effect (95% CI) | Overall results |
|---|---|---|---|---|
| Pain (short term), NRS (0–9) | 106; one study; 4 weeks | ⊕⊕⊕⊝ moderatea because of risk of bias | – | The mean pain (short term) in the intervention groups was 1.02 SDs lower (0.61 to 1.43 lower) |
| Pain (intermediate term), NRS (0–9) | 106; one study; 26 weeks | ⊕⊕⊕⊝ moderatea because of risk of bias | – | The mean pain (intermediate term) in the intervention groups was 0.52 SDs higher (0.92 to 0.13 higher) |
| Pain (long term), impairment of function (NRS) | 106; one study; 52 weeks | ⊕⊕⊕⊝ moderatea because of risk of bias | – | The mean pain (long term) in the intervention groups was 0.19 SDs higher (0.58 higher to 0.19 lower) |
| Function (short term), impairment of function (NRS 0–9) | 106; one study; 4 weeks | ⊕⊕⊕⊝ moderatea because of risk of bias | – | The mean function (short term) in the intervention groups was 0.92 SDs higher (0.51 to 1.32 higher) |
| Function (intermediate term), impairment of function (NRS 0–9) | 106; one study; 26 weeks | ⊕⊕⊕⊝ moderatea because of risk of bias | – | The mean function (intermediate term) in the intervention groups was 0.29 SDs lower (0.68 lower to 0.10 higher) |
| Function (long term), impairment of function (NRS 0–9) | 106; one study; 52 weeks | ⊕⊕⊕⊝ moderatea because of risk of bias | – | The mean function (long term) in the intervention groups was 0.19 SDs lower (0.58 lower to 0.19 higher) |
Glucocorticoid injections compared with platelet-rich plasma injections
In one RCT,125 GCIs were found to result in a reduction in pain in the short term compared with PRP injections. The evidence for this outcome was considered of moderate quality when we assessed it using the GRADE principles (Table 16; and see GRADE profiles in Appendix 4).
| Outcomes | Number of participants; studies; follow-up period | Quality of the evidence (as assessed by GRADE) | Relative effect (95% CI) | Overall results |
|---|---|---|---|---|
| Pain (short term), VAS (0–100) | 100; one study; 4 weeks | ⊕⊕⊕⊝ moderatea because of risk of bias | – | The mean pain (short term) in the intervention groups was 0.44 SDs lower (0.04 to 0.84 lower) |
| Pain (intermediate term), VAS (0–100) | 100; one study; 26 weeks | ⊕⊕⊕⊝ moderatea because of risk of bias | – | The mean pain (intermediate term) in the intervention groups was 0.86 SDs higher (1.27 to 0.45 higher) |
| Pain (long term), VAS (0–100) | 100; one study; 52 weeks | ⊕⊕⊕⊝ moderatea because of risk of bias | – | The mean pain (long term) in the intervention groups was 0.83 SDs higher (1.24 to 0.42 higher) |
| Function (short term), DASH scale | 100; one study; 4 weeks | ⊕⊕⊕⊝ moderatea because of risk of bias | – | The mean function (short term) in the intervention groups was 0.52 SDs higher (0.12 to 0.92 higher) |
| Function (intermediate term), DASH scale | 100; one study; 26 weeks | ⊕⊕⊕⊝ moderatea because of risk of bias | – | The mean function (intermediate term) in the intervention groups was 0.48 SDs lower (0.88 to 0.08 lower) |
| Function (long term), DASH scale | 100; one study; 52 weeks | ⊕⊕⊕⊝ moderatea because of risk of bias | – | The mean function (long term) in the intervention groups was 0.69 SDs lower (1.09 to 0.28 lower) |
Sodium hyaluronate injections
One high-quality review, by Coombes et al. ,60 included one RCT129 (331 participants) examining the effect of sodium hyaluronate injections on lateral epicondylitis. Severity of tennis elbow (mean pain score before treatment) was reported to be 8.5 out of 10 on a VAS score prior to treatment. Co-interventions were not mentioned. The search was performed up to and including March 2010.
Sodium hyaluronate injections compared with placebo
One RCT reported reductions in pain after injections of sodium hyaluronate compared with placebo (short term, 3.91, 95% CI 3.54 to 4.28; p < 0.0001; intermediate term, 2.89, 95% CI 2.58 to 3.20; p < 0.0001; and long term, 3.91, 95% CI 3.55 to 4.28; p < 0.0001). 129 Evidence for this outcome was considered of moderate quality when we assessed it using the GRADE principles (Table 17; and see GRADE profiles in Appendix 4).
| Outcomes | Number of participants; studies; follow-up period | Quality of the evidence (as assessed by GRADE) | Relative effect (95% CI) | Overall results |
|---|---|---|---|---|
| Pain (short term), VAS | 331; one study; 4 weeks | ⊕⊕⊕⊝ moderatea because of risk of bias | – | The mean pain (short term) in the intervention groups was 3.91 SDs lower (3.54 to 4.28 lower) |
| Pain (intermediate term), VAS | 331; one study; 26 weeks | ⊕⊕⊕⊝ moderatea because of risk of bias | – | The mean pain (intermediate term) in the intervention groups was 2.89 SDs lower (2.58 to 3.2 lower) |
| Pain (long term), VAS | 331; one study; 1 year | ⊕⊕⊕⊝ moderatea because of risk of bias | – | The mean pain (long term) in the intervention groups was 3.91 SDs lower (3.55 to 4.28 lower) |
| Function | O/C; NR | O/C; NR | – | – |
| QoL | O/C; NR | O/C; NR | – | – |
| Remain/return to work | O/C; NR | O/C; NR | – | – |
| Sport activity | O/C; NR | O/C; NR | – | – |
| Recurrence | O/C; NR | O/C; NR | – | – |
| Adverse events (pain) | 331; one study; 52 weeks | ⊕⊕⊝⊝ lowb because of risk of bias | RR 0.6 (0.15 to 2.48) | – |
Therapeutic ultrasound-guided injection of sclerosing solution
One high-quality review60 included one RCT (36 participants) examining the effect of therapeutic ultrasound-guided injection of sclerosing solution on lateral epicondylitis. 130 Severity of tennis elbow (mean pain score before treatment) was reported to be 69 out of 100 on a VAS score prior to treatment. Co-interventions were not mentioned. The search was performed up to and including March 2010.
Therapeutic ultrasound-guided injection of sclerosing solution compared with placebo
Therapeutic ultrasound-guided injection of lauromacrogol, a sclerosing solution, was compared with saline injection in one RCT. 131 No effect on pain or function was found. The evidence for this outcome was considered to be of high quality when we assessed it using the GRADE principles (Table 18; and see GRADE profiles in Appendix 4).
| Outcomes | Number of participants; studies; follow-up period | Quality of the evidence (as assessed by GRADE) | Relative effect (95% CI) | Overall results |
|---|---|---|---|---|
| Pain (short term) | 36; one study; 4 weeks | ⊕⊕⊕⊝ moderatea because of imprecision | – | The mean pain (short term) in the intervention groups was 0.20 SDs higher (0.47 lower to 0.88 higher) |
| Function | O/C; NR | O/C; NR | – | – |
| QoL | O/C; NR | O/C; NR | – | – |
| Remain/return to work | O/C; NR | O/C; NR | – | – |
| Sport activity | O/C; NR | O/C; NR | – | – |
| Recurrence | O/C; NR | O/C; NR | – | – |
| Adverse events (overall)b | 87; one study; 12 weeks | ⊕⊕⊕⊝ moderatea because of imprecision | – | – |
Glycosaminoglycan polysulphate injections
One high-quality review60 included one RCT132 (65 participants) examining the effect of glycosaminoglycan polysulphate injections on lateral epicondylitis. Severity of tennis elbow (mean pain score before treatment) was reported to be 60 out of 100 on a VAS score prior to treatment. Co-interventions were not mentioned. The search was performed up to and including March 2010.
Glycosaminoglycan polysulphate injections compared with placebo
Arteparon (glycosaminoglycan polysulphate), administered as a series of five injections once a week, was compared with placebo injection in one RCT. 132 No short- or intermediate-term effects on pain relief were reported. The evidence for this outcome was considered of moderate quality when we assessed it using the GRADE principles (Table 19; and see GRADE profiles in Appendix 4).
| Outcomes | Number of participants; studies; follow-up period | Quality of the evidence (as assessed by GRADE) | Relative effect (95% CI) | Overall results |
|---|---|---|---|---|
| Pain (short term), VAS (0–100) | 65; one study; 4 weeks | ⊕⊕⊕⊝ moderatea because of risk of bias | – | The mean pain (short term) in the intervention groups was 0.21 SDs lower (0.72 lower to 0.30 higher) |
| Pain (intermediate term), VAS (0–100) | 65; one study; 26 weeks | ⊕⊕⊕⊝ moderatea because of risk of bias | – | The mean pain (intermediate) in the intervention groups was 0.38 SDs lower (0.89 lower to 0.13 higher) |
| Function | O/C; NR | O/C; NR | – | – |
| QoL | O/C; NR | O/C; NR | – | – |
| Remain/return to work | O/C; NR | O/C; NR | – | – |
| Sport activity | O/C; NR | O/C; NR | – | – |
| Recurrence | O/C; NR | O/C; NR | – | – |
| Adverse events (pain), local pain | 60; one study; 26 weeks | ⊕⊕⊕⊝ moderatea because of risk of bias | RR 2.27 (0.93 to 5.58) | – |
| Adverse events (haematoma) | 60; one study; 26 weeks | ⊕⊕⊕⊝ moderatea because of risk of bias | RR 4.39 (0.22 to 87.82) | – |
Botulinum toxin
One high-quality review60 included one RCT133 (60 participants) examining the effect of botulinum toxin injections on lateral epicondylitis. Severity of tennis elbow (mean pain score before treatment) was reported to be 66 out of 100 on a VAS score prior to treatment. The search was performed up to and including March 2010. Co-interventions were not mentioned. The most common adverse events recorded following treatment with botulinum toxin were weakness of finger extension and paresis of digits, with one patient reporting paresis that persisted for 3 months. Although the potential for paresis may call into question the use of botulinum toxin for this condition, it may offer an explanation for its mechanism of action, i.e. that the paralytic effect of botulinum toxin forces the extensor group of muscles to rest for a period of 2–4 months, thereby allowing the tendon fibres close to the lateral epicondyle time to repair.
Botulinum toxin compared with placebo
One RCT investigated peritendinous injection of botulinum toxin in chronic lateral epicondylalgia. 133 Compared with the placebo, the RCT reported a large reduction in pain after injections of botulinum toxin in the short term [mean pair measured using the VAS (1–100) 1.23, 95% CI 0.67 to 1.78; p < 0.0001]. The evidence for this outcome was considered moderate quality when we assessed it using the GRADE principles (Table 20 and see GRADE profiles in Appendix 4).
| Outcomes | Number of participants; studies; follow-up period | Quality of the evidence (as assessed by GRADE) | Relative effect (95% CI) | Overall results |
|---|---|---|---|---|
| Pain (short-term), VAS (0–100) | 60; one study; 4 weeks | ⊕⊕⊕⊝ moderatea because of imprecision | – | The mean pain (short term) in the intervention groups was 1.23 SDs lower (0.67 to 1.78 lower) |
| Function | O/C; NR | O/C; NR | – | – |
| QoL | O/C; NR | O/C; NR | – | – |
| Remain/return to work | O/C; NR | O/C; NR | – | – |
| Sport activity | O/C; NR | O/C; NR | – | – |
| Recurrence | O/C; NR | O/C; NR | – | – |
| Adverse events (overall) | 60; one study; 4 weeks | ⊕⊕⊕⊝ moderatea because of imprecision | RR 2.11 (1.15 to 3.89) | – |
| Adverse event (post-injection pain) | 60; one study; 4 weeks | ⊕⊕⊕⊝ moderatea because of imprecision | RR 2.00 (0.19 to 20.90) | – |
| Adverse event (nausea) | 60; one study; 4 weeks | ⊕⊕⊕⊝ moderatea because of imprecision | RR 0.33 (0.01 to 7.87) | – |
| Adverse event (finger weakness) | 60; one study; 4 weeks | ⊕⊕⊕⊝ moderatea because of imprecision | RR 1.67 (0.69 to 4.00) | – |
| Adverse event (paresis) | 60; one study; 4 weeks | ⊕⊕⊕⊝ moderatea because of imprecision | RR 9.00 (0.51 to 160.17) | – |
Prolotherapy
One high-quality review60 included one RCT134 (24 participants) examining the effect of prolotherapy (also known as proliferative injection therapy) on lateral epicondylitis. Severity of tennis elbow (mean pain score before treatment) was reported to be 4.8 out of 10 on a numerical rating scale (NRS) score prior to treatment. Co-interventions were not mentioned. The search was performed up to and including March 2010.
Prolotherapy compared with placebo
Compared with placebo, one RCT reported a large reduction in pain after prolotherapy in the intermediate term [mean pair score (NRS) 2.62, 95% CI 1.36 to 3.88; p < 0.0001]. 134 The prolotherapy intervention consisted of a series of three prolotherapy injections (solution of hypertonic glucose and local anaesthetic) over an 8-week period. The evidence for this outcome was considered low quality when we assessed it using the GRADE principles (Table 21; and see GRADE profiles in Appendix 4).
| Outcomes | Number of participants; studies; follow-up period | Quality of the evidence (as assessed by GRADE) | Relative effect (95% CI) | Overall results |
|---|---|---|---|---|
| Pain (short term),resting pain (NRS) | 24; one study; 4 weeks | ⊕⊕⊝⊝ lowa,b because of risk of bias, imprecision | – | The mean pain (short term) in the intervention groups was 0.27 SDs lower (1.15 lower to 0.61 higher) |
| Pain (intermediate term), resting pain (NRS) | 24; one study; 26 weeks | ⊕⊕⊝⊝ lowa–c because of risk of bias, imprecision | – | The mean pain (intermediate term) in the intervention groups was 2.62 SDs lower (1.36 to 3.88 lower) |
| Function | O/C; NR | O/C; NR | – | – |
| QoL | O/C; NR | O/C; NR | – | – |
| Remain/return to work | O/C; NR | O/C; NR | – | – |
| Sport activity | O/C; NR | O/C; NR | – | – |
| Recurrence | O/C; NR | O/C; NR | – | – |
| Adverse events (pain) | 20; one study; 16 weeks | ⊕⊕⊝⊝ lowa–c because of risk of bias, imprecision | – | – |
| Adverse event (irritation), local irritation | 20; one study; 16 weeks | ⊕⊕⊝⊝ lowa–c because of risk of bias, imprecision | RR 5.00 (0.27 to 92.62) | – |
Summary of randomised controlled trials
Randomised controlled trials evaluated in an intermediate-/low-quality systematic review
We identified 24 systematic reviews61–84 that were considered of intermediate (scoring four to seven AMSTAR points) or low (scoring 1 to 3 points) quality (see Table 3). Between them, these reviews included 40 unique RCTs [full papers published in English language between 2003 and January 2013 (the period of interest for this review)]; of these, 11 were included in the high-quality reviews. Thus, as we evaluated evidence only from included high-quality reviews, evidence from 29 of these RCTs was not taken into account. Of these 29, the majority were placebo-controlled trials. The sample sizes varied from 10 to 199 participants and the majority of studies (48%) had fewer than 50 participants. These studies are summarised (sample size and interventions evaluated) in Table 22 and we indicate where they could contribute to evidence in Table 23. Detailed quality appraisal of RCTs was not conducted, as stated in the protocol.
| Author, year | n | Interventions evaluated |
|---|---|---|
| Baskurt et al., 2003135 | 61 | Naproxen (gel) + phonophoresis vs. naproxen (gel) + iontophoresis |
| Chan and Ng, 2003136 | 15 | No brace vs. brace with minimal tension vs. brace with 3.5 kg of force tension vs. brace with 5 kg of force tension |
| Langen-Pieters et al., 2003137 | 13 | Manipulation + exercise vs. US |
| Nirschl et al., 2003138 | 199 | Iontophoresis with dexamethasone sodium phosphate vs. placebo |
| Paoloni et al., 2003139 | 86 | Topical GTN patch vs. placebo patch |
| Paungmali et al., 2003140 | 24 | Mobilisation with movement vs. placebo |
| Selvanetti et al., 2003141 | 60 | Exercise + stretching + counselling vs. sham US + exercise |
| Struijs et al., 2003142 | 31 | Manipulation vs. US + friction massage + stretching + strengthening |
| Vicenzino et al., 2003143 | 16 | Taping vs. placebo |
| Struijs et al., 2004144 | 180 | PT vs. brace only vs. brace + US |
| Cleland et al., 2005145 | 10 | C spine + local treatment vs. local treatment alone |
| Spacca et al., 2005146 | 155 | 1.3% diclofenac gel vs. placebo |
| Hayton et al., 2005147 | 40 | 50 units [botulinum toxin A (Botox®, Allergan, Buckinghamshire, UK)] of botulinum toxin injection vs. placebo |
| Lewis et al., 2005148 | 164 | Naproxen vs. GCI vs. placebo |
| Martinez-Silvestrini et al., 2005149 | 94 | Stretching vs. eccentric exercise vs. concentric exercise |
| Faes et al., 2006150 | 63 | Brace vs. no brace |
| Stasinopoulos and Stasinopoulos, 2006151 | 75 | Cyriax physiotherapy vs. supervised exercise (EE + static stretching) |
| D’Vaz et al., 2006152 | 55 | Pulsed low-intensity therapeutic ultrasound vs. placebo |
| Lam and Cheing, 2007153 | 39 | Active laser with an energy dose of 0.275 J per tender point vs. placebo (sham laser) |
| Placzek et al., 2007154 | 132 | 60 U [botulinum toxin A (Dysport®, Ipsen UK)] of botulinum toxin injection vs. placebo |
| Vicenzino et al., 2007155 | 24 | Mobilisation with movement vs. placebo vs. no intervention |
| Stergioulas 2007156 | 50 | LLLT gallium-arsenide (Ga-As) infrared laser with a wavelength of 904 nm (class IIIb Laser Product, Frank Line IR 30, Fysiomed, Edegem, Belgium), frequency of 50 Hz, intensity of 40 mW and energy density of 2.4 J/cm2, plus plyometric exercises vs. placebo laser plus the same plyometric exercises |
| Luginbuhl et al., 2008157 | 29 | Isometric grip strength exercise with tennis ball + isometric resisted wrist extension exercise vs. forearm support band/combined treatment with forearm support band + strengthening |
| Oken et al., 2008158 | 58 | LLLT vs. brace vs. US |
| Staples et al., 2008159 | 68 | ESWT (dose: 2000 shock waves per weeks set at maximum level tolerated by patient, frequency 240 pulses per minute); n = 36 vs. placebo ESWT (subtherapeutic dose: 100 shock waves per week, 0.03 mJ/mm2 frequency, 90 pulses per minute); n = 32 |
| Espandar et al., 2010160 | 48 | 60 U (Dysport) of botulinum toxin injection vs. placebo |
| Nagrale et al., 2009161 | 60 | Deep-friction massage vs. phonophoresis with gel |
| Park et al., 2010162 | 31 | Isometric strengthening exercises + medication for first 4 weeks vs. isometric strengthening exercises |
| Tyler et al., 2010163 | 21 | EE + stretching + US + cross-friction massage + heat + ice vs. isotonic strengthening + US + cross-friction massage + heat + ice |
| Authors, year | n | Interventions evaluated |
|---|---|---|
| Viswas et al., 2012164 | 20 | Cyriax physiotherapya (three treatment sessions per week for 4 weeks) vs. supervised exercise programme (three treatment sessions per week for 4 weeks) |
| Stefanou et al., 2012165 | 86 | 10 mg of dexamethasone via iontophoresis self-contained path with a 24-hour battery vs. 10 mg of dexamethasone vs. 10 mg of triamcinolone injection |
| Soderberg et al., 2012166 | 37 | 6-week home exercise regimen (eccentric training for wrist extensors and a forearm band) vs. forearm band only; n = 19 |
| Skorupska et al., 2012167 | 80 | LLLT; n = 40 [second randomisation – conservative treatment of LLLT (1 J/cm2) (n = 20) or myofascial pain physiotherapy treatment of LLLT (5 J/cm2) (n = 20)] (10-day therapy) vs. US; n = 40 [second randomisation – conservative treatment of US (0.5 W/cm2 3 MHz) (n = 20) or myofascial pain physiotherapy treatment of US (0.7 W/cm2 1 MHz) (n = 20)] (10-day therapy) |
| Omar et al., 2012168 | 30g | Steroid injection vs. PRP injection |
| Gunduz et al., 2012169 | 59 | Physical therapy (hot pack, US therapy and friction massage) 10 sessions vs. single corticosteroid injection (methylprednisolone acetate and 1 ml of prilocaine) vs. ESWT 10 sessions |
| Forogh et al., 2012170 | 24 | New-designed orthosis (4 weeks) vs. standard counterforce orthosis (4 weeks) |
| Ajimsha et al., 2012171 | 65 | Myofascial release vs. sham US therapy |
| Agostinucci et al., 2012172 | 70 | Gel cold pack + exercise (twice daily, four times per week for 6 weeks) vs. Cryo-MAXb + exercise (twice daily, four times per week for 6 weeks) vs. Cryo-MAX only (twice daily, four times per week for 6 weeks) vs. exercise only (twice daily, four times per week for 6 weeks) |
| Wolf et al., 2011173 | 28 | Corticosteroid + lidocaine vs. autologous blood + lidocaine vs. 3 ml of injection saline + lidocaine |
| Thanasas et al., 2011174 | 28 | ABI 3 ml (single injection) + eccentric muscle strengthening vs. PRP 3 ml (therapeutic ultrasound guidance) + eccentric muscle strengthening |
| Polat et al., 2011175 | 55 | 48 mg/day of betahistine dihydrochloride for 10 days vs. 750 mg/day of naproxen sodium for 10 days |
| Peterson et al., 201118 | 81 | Exercise (daily with weekly load increase; 3 months) vs. wait list |
| Gosens et al., 2011176 | 100 | Leucocyte-enriched PRP vs. corticosteroid |
| Fernandez-Carnero et al., 2011177 | 18 | Cervical spine thrust manipulation vs. thoracic spine thrust manipulation |
| Creaney et al., 2011178 | 150 | PRP injection vs. ABI |
| Collins et al., 2011179 | 183 | ESWT (1500 shocks at 18 kV) vs. placebo [ESWT with Styrofoam™ (The Dow Chemical Company, Midland, MI, USA) block against the coupling membrane and fluid-filled bag] |
| Blanchette and Normand, 2011180 | 27 | ASTM twice daily for 5 weeks vs. advice on natural evolution of LET, computer ergonomics, stretching exercises |
| Bellapianta et al., 2011181 | 31 (elbows) | GCI; single-injection technique vs. GCI; peppered-injection technique (elbows) |
| Backer et al., 2011182 | 40 | 2–4 locally applied medicinal leeches vs. 30-day course topical diclofenac (gel, 300 g) |
| Ozturan et al., 2010183 | 57 | Corticosteroid injection vs. ABI vs. ESWT |
| Kazemi et al., 2010184 | 60 | Methylprednisolone (20 mg of methylprednisolone with 1 ml of 2% lidocaine) vs. ABI (2 ml of arteria brachialis distal region of the ipsilateral upper limb + 1 ml of 2% lidocaine) |
| Garg et al., 2010185 | 44 (elbows) | Wrist extension splint (elbows) vs. counterforce forearm strap (brace) (elbows) |
| Emanet et al., 2010186 | 47 (elbows) | Laser (1 J/cm2 for 2 minutes, 5 days per week for 3 weeks) vs. placebo laser [(laser deactivated) for 2 minutes, 5 days per week for 3 weeks] |
| Akin et al., 2010187 | 60 | US (15 sessions) + epicondylitis bandage vs. placebo US (15 sessions) + epicondylitis bandage |
| Paoloni et al., 2009188 | 136 | Topical glyceryl trinitrate patch 0.03 mg/hour (0.72 mg/24 hours), 0.06 mg/hour (1.44 mg/24 hours); 0.15 mg/hour (3.6 mg/24 hours) (OrthoDerm, Cure Therapeutics, NY, USA) vs. placebo patch |
| McCallum et al., 2011189 | 58 | Glyceryl trinitrate transdermal patch (one-quarter of a 5-mg/24-hour Nitro-dur patch) vs. placebo patch (one-quarter of a 5-mg/24-hour Nitro-dur demonstration patch) |
| Jafarian et al., 2009190 | 52 | Elbow strap orthosis vs. elbow sleeve orthosis vs. wrist splint vs. placebo orthosis |
| Dogramaci et al., 2009191 | 75 | Lidocaine (1 ml) + peppering vs. triamcinolone (1 ml) + lidocaine (1 ml) peppering injection vs. triamcinolone (1 ml) + lidocaine (1 ml) injection |
| Coff et al., 2009192 | 26 | InterX + soft-tissue massage, stretching, US and exercise vs. soft-tissue massage, stretching, US and exercise |
| Toker et al., 2008193 | 21 | Oral and topical anti-inflammatory drugs vs. single local injection of a corticosteroid and anaesthetic mixture |
| Sabeti et al., 2008194 | 20 | ESWT 1000 shocks (three sessions) vs. ESWT 2000 shocks (three sessions) |
| Radwan et al., 2008195 | 56 | ESWT [1500 shocks at 18 kV (0.22 mJ/mm2)] vs. percutaneous tenotomy of the common extensor origin |
| Nourbakhsh and Fearon, 2008196 | 18 | Low-frequency electrical stimulation (intensity as tolerated) (six sessions); n = 10 vs. low-frequency electrical stimulation (intensity set at 0) (six sessions) |
| Nourbakhsh and Fearon, 2008197 | 23 | OEMT (oscillating energy focused on tender point) (six sessions) vs. OEMT (oscillating energy directed above or below tender points) (six sessions) |
| Ho et al., 2007198 | 16 | Microcurrent therapy + exercise (10 sessions) vs. exercise only |
Randomised controlled trials not included in an existing systematic review
Thirty-six RCTs were identified that were not included in the systematic reviews included in the overview. A summary is given in Table 23, and a detailed summary of study characteristics is available in Appendix 5. A detailed quality appraisal of these studies was not conducted, as stated in the protocol.
Four studies had a placebo or sham control171,186,187,189 and the remainder (n = 32) were head-to-head studies. 18,167–170,172–185,188–198 The majority of studies had small sample sizes (≤ 50 participants, n = 18; 51–100 participants, n = 15; > 100 participants, n = 3).
Evidence summary
This section provides a summary of the evidence based on the GRADE analysis of the high-quality systematic reviews, highlights RCTs that were included in an intermediate-/low-quality systematic review identified in our searches and highlights where subsequently published RCTs were identified; an overview is provided in Table 24.
| Intervention | Comparison | Results (combined)a | Quality of evidence (based on the GRADE principles)b | Notes | RCTs included in a low-/intermediate-quality review for which evidence was not evaluated with the GRADE principles | Subsequently published relevant primary studiesb |
|---|---|---|---|---|---|---|
| ESWT | Placebo | No difference in pain | Low | Evidence from one high-quality review in need of updating. Inconsistent results in primary studies for pain and function | – | Six RCTs169,179,183,194,195,199 |
| GCI | No difference in function | Low | ||||
| Laser therapy | Placebo | Unclear | Low | Evidence from one high-quality review in need of updating. Inconsistent results in primary studies for pain and function | Three153,156,158 | One RCT186 |
| Other PT modalities | ||||||
| Therapeutic ultrasound | Placebo | Improves pain relief | Low | Based on primary studies of moderate quality in a high-quality systematic review in need of updating | Five137,142,152,158,163 | Three RCTs169,187,192 |
| LLLT | ||||||
| Exercises | ||||||
| Exercises | Exercises | Improves pain relief | Low | Based on one primary study of moderate quality in a high-quality systematic review in need of updating | Seven137,141,149,151,157,162,163 | Four RCTs18,164,166,172 |
| US + friction massage | ||||||
| GCI | WS | Improves pain relief in the short term | Low | Based on at least two primary studies of moderate quality, with consistent results in a high-quality systematic review in need of updating | One148 | 10 RCTs165,168,169,173,176,181,183,184,191,193 |
| Placebo | No difference for pain relief in the intermediate and long term | Low | ||||
| PT | Improves function in the short term | Low | – | – | – | |
| NSAID | No difference in function in the long term | Low | – | – | – | |
| PRP | ||||||
| Sodium hyaluronate | Placebo | Improves pain relief | Low | Based on one moderate-quality primary study in a high-quality systematic review in need of updating | – | – |
| Therapeutic ultrasound (sonographically)-guided injection of sclerosing solution | Placebo | No difference in pain | Low | Based on at least one high-quality primary study in an up-to-date high-quality systematic review | – | – |
| Glycosaminoglycan polysulphate injections | Placebo | No difference in pain | Low | Based on one moderate-quality primary study in an up-to-date high-quality systematic review | – | – |
| Botulinum toxin | Placebo | Beneficial for pain relief | Low | Based on at least one high-quality primary study in an up-to-date high-quality systematic review | Three147,154,160 | |
| Prolotherapy | Placebo | Improves pain relief | Low | Based on one low-quality primary study in an up-to-date high-quality systematic review | – | – |
| Manipulation/manual therapy | NA | NA | NA | There is no high-quality systematic review identified on this topic | Five137,140,142,145,155 | Two RCTs177,197 |
| Acupuncture | NA | NA | NA | There is no high-quality systematic review identified on this topic | – | – |
| Orthotics | NA | NA | NA | There is no high-quality systematic review identified on this topic | Four136,144,150,158 | Four RCTs158,170,185,190 |
| ABI | NA | NA | NA | There is no high-quality systematic review identified on this topic | – | Five RCTs173,178,183,184,174 |
| Soft-tissue mobilisation | NA | NA | NA | There is no high-quality systematic review identified on this topic | – | One RCT180 |
| PRP injections | NA | NA | NA | There is no high-quality systematic review identified on this topic | – | Five RCTs125,168,174,176,178 |
| Iontophoresis | NA | NA | NA | There is no high-quality systematic review identified on this topic | – | One RCT165 |
| NSAIDs (topical and oral) | NA | NA | NA | There is no high-quality systematic review identified on this topic | Two146,148 | One RCT193 |
| Cryotherapy | NA | NA | NA | There is currently no systematic review identified on this topic | – | One RCT172 |
| Leech therapy | NA | NA | NA | There is currently no systematic review identified on this topic | – | One RCT182 |
| Myofascial release | NA | NA | NA | There is currently no systematic review identified on this topic | – | Two RCTs167,171 |
| Topical glyceryl trinitrate patch | NA | NA | NA | There is currently no systematic review identified on this topic | One139 | One RCT188 |
| Electrical stimulation/electric devices, for example InterX (Neuro resource Group, Inc.; Plano, TX, USA) | NA | NA | NA | There is currently no systematic review identified on this topic | – | Three RCTs192,196,198 |
| Betahistine dihydrochloride | NA | NA | NA | There is currently no systematic review identified on this topic | – | One RCT175 |
| Iontophoresis | NA | NA | NA | There is currently no systematic review identified on this topic | One138 | – |
| Phonophoresis + PT | NA | NA | NA | There is currently no systematic review identified on this topic | One135 | – |
| Rest (WS) | NA | NA | NA | There is currently no systematic review identified on this topic | One200 | One RCT18 |
| Cyriax physiotherapy (friction massage + Mill’s manipulation) | NA | NA | NA | There is currently no systematic review identified on this topic | One151 | One RCT164 |
Extracorporeal shock wave therapy
The evidence reviewed to date suggests little or no benefit for pain relief or function from ESWT compared with placebo or steroid injections in the short and intermediate term. However, given the inconsistencies in results in the primary studies58 and the overall evidence as determined using the GRADE principles was low (see Table 24). Five subsequent RCTs were identified169,179,183,194,195 Of these, four were head-to-head studies. 169,183,194,195 The mean sample size of these studies was 48 (SD 18.7) participants. For this reason we recommend that, although a systematic review could be beneficial focusing on conducting good-quality RCTs with clearly described patient selection and treatment protocols, validated outcome measures and a minimum of 1-year follow-up, as recommended by NICE guidance,30 may be more beneficial.
Laser therapy
The evidence reviewed to date suggests some benefit for pain relief in the intermediate term using laser therapy compared with placebo, yet no benefit for pain relief in the short term. No benefits for laser therapy in either the short or intermediate term were observed compared with other physiotherapeutic modalities (therapeutic ultrasound plus friction massage). There were inconsistencies in results in the primary studies and overall low level of evidence as determined using the GRADE principles (see Table 24). We identified one relevant RCT not currently included in a systematic review;186 this was a placebo-controlled study and had a sample size of 47 participants, thus, on its own, it may have limited impact on the existing recommendations regarding this intervention. 156,158,186 In addition, we identified three RCTs included in intermediate-/low-quality reviews. 153,156,158 As there is insufficient evidence to demonstrate either benefit or lack of effect of laser for LET, and given there are recent RCTs (2003–13) we recommend that an updated systematic review may be of benefit. However, some consideration should also be given to conducting good-quality RCTs.
Therapeutic ultrasound
Given the moderate quality and consistency in results in the primary studies for pain relief, the evidence for the benefit of therapeutic ultrasound in the short and intermediate term compared with placebo and laser therapy is promising. However, the systematic reviews on which these findings are based need updating, and the overall level of evidence, as determined using the GRADE principles, is low (see Table 24). 57,59 We identified five RCTs that were included an intermediate-/low-quality systematic review. 137,142,152,158,163 Three additional relevant RCTs published subsequent to the most up-to-date systematic review were also identified. 169,187,192 Of the three RCTs identified, one is placebo controlled187 and two are head-to-head comparisons;169,192 all studies have small sample sizes [the mean sample size of these studies was 48 (SD 19.3) participants]. Although the evidence for pain relief in the short and intermediate term using therapeutic ultrasound is promising, an updated systematic review is needed before a recommendation can be made. And, given the small sample sizes of the RCTs identified some consideration should also be given to conducting good-quality, larger-scale RCTs.
Exercises
Given the paucity of the available data (one RCT with moderate-quality evidence for pain relief in the intermediate term112), the overall low level of evidence as determined using the GRADE principles (see Table 24) and the subsequent publication of four relevant RCTs,18,164,166,172 we conclude that there is insufficient evidence at present to demonstrate either benefit or lack of effect of exercises for LET. All of the subsequent RCTs identified are recent publications18,164,166,172 In addition, seven RCTs were identified that were included in an intermediate-/low-quality systematic review. 137,141,149,151,157,162,163 An updated, good-quality systematic review of exercises for LET is needed before stronger recommendations can be made; however, we suggest that some consideration should also be given to conducting large-scale, good-quality RCTs of clearly defined exercise modalities with sufficient follow-up periods (to 1 year).
Glucocorticoid injections
Given the largely moderate quality of the evidence and the consistency in results in the primary studies for pain relief and improved function, there is evidence for the benefit of GCIs in the short term; however, the evidence for benefit in terms of pain relief or improved function in the intermediate and long term is inconclusive. However, given the need to update the systematic reviews on which these findings are based,56,60 and the subsequent publication of 10 new RCTs,165,168,169,173,176,181,183,184,191,193 the overall level of evidence, as determined using the GRADE principles, is low (see Table 24). All of the subsequent RCTs were head-to-head comparisons, and all but one study (Gosens et al. ,176 n = 100) had a sample size of < 100. In addition, one RCT was identified that was evaluated in an intermediate-/low-quality systematic review. 148 Although the evidence that GCIs elicit pain relief and functional improvement in the short term is promising, these effects do not appear to continue into the intermediate and long term. Subsequent RCTs were identified so an updated systematic review may be of benefit. Given the inconclusiveness of evidence regarding the potential harms of injection over the long term, we recommend conducting good-quality, larger-scale RCTs considering core outcomes for the short, intermediate, and long term with appropriate follow-up (1 year). We also recommend subgroup analysis of existing RCT data with the aim of ascertaining whether or not certain groups of patients are more likely to benefit from GCI than others; this should also be a consideration in the design of new trials.
Sodium hyaluronate injections
Although there is only one RCT129 showing benefits in pain relief in the short, intermediate and long term, the trial has 331 participants and is of moderate quality. Because of the overall low level of evidence as determined using the GRADE principles (see Table 24), and no subsequent RCTs, we conclude that there is only low-level evidence for sodium hyaluronate for pain relief in the short, intermediate and long term. An updated systematic review of sodium hyaluronate for LET is needed before stronger recommendations can be made. Given the paucity of RCT evidence identified in this review the priority should be placed on conducting good-quality RCTs; systematic review evidence may be useful for informing this.
Therapeutic ultrasound-guided injection of sclerosing solution
Given the paucity of the available data (one RCT) showing no benefits of therapeutic ultrasound-guided injection of sclerosing solution on pain relief in the short term, the quality of the trial is moderate and current as there are no new RCTs published for this intervention. Hence, the overall level of evidence for the lack of pain relief in the short term is judged to be of overall low quality as determined using the GRADE principles (see Table 24). We conclude that there is insufficient evidence at present to demonstrate either benefit or lack of effect. No systematic reviews focusing specifically on this intervention were identified and therefore we recommend conducting a systematic review.
Glycosaminoglycan polysulphate injections
Although there is only one RCT132 examining the effect of glycosaminoglycan polysulphate on LET, it is of moderate quality and current, as there are no more recent RCTs of this intervention. Hence, the overall level of evidence for the lack of pain relief in the short and intermediate term is judged to be of low quality, as determined using the GRADE principles. We conclude that the evidence that injections of glycosaminoglycan polysulphate fail to provide pain relief in the short and intermediate term is of low quality. No systematic reviews focusing specifically on this intervention were identified and therefore we recommend conducting a systematic review. We also recommend further good-quality RCTs evaluating this intervention.
Botulinum toxin injection
Although there is only one RCT133 comparing the effect of botulinum toxin on LET, the trial is of moderate quality and current. Although the evidence suggests potential for a large reduction in pain in the short term, this needs to be considered against the adverse events; we consider current evidence to be of low quality as determined using the GRADE principles (see Table 24). There are three more recent placebo-controlled RCTs,133,147,154 but these were incorporated in two recent systematic reviews identified in our searches (see Table 3) that were not considered high quality and therefore were not analysed in our GRADE analysis. We recommend that a high-quality systematic review is conducted. We suggest that some consideration should be given to conducting good-quality, large-scale RCTs with sufficient sample size and including an active control with appropriate follow-up to capture potential adverse events. Similar to our recommendation for GCI we would also suggest conducting subgroup analysis of existing RCT data to ascertain whether or not certain patient groups are more likely to respond to this intervention; this should also be a consideration for newly designed trials.
Prolotherapy
Although there is only one RCT134 comparing the effect of prolotherapy toxin on LET and the quality of the trial is low, the evidence is current as there are no new RCTs published for this intervention. Hence, the overall level of evidence for a large reduction in pain in the intermediate term is judged to be of low quality as determined using the GRADE principles (see Table 24). No systematic reviews focusing specifically on this intervention were identified, and we therefore recommend conducting a systematic review. We also suggest that further good-quality RCTs evaluating this intervention are needed.
Chapter 3 Cost-effectiveness
Methods of reviewing cost-effectiveness
Search strategy
Full details of the search strategies can be found in Appendix 1.
Inclusion and exclusion criteria
The inclusion and exclusion criteria for economic evaluations were identical to those for the systematic review of clinical effectiveness except that:
-
non-randomised studies were included (e.g. decision model-based analysis or analysis of person-level cost and clinical effectiveness data alongside observational studies) and
-
full cost-effectiveness analyses, cost–utility analyses, cost–benefit analyses and cost-consequence analyses will be included. Stand-alone UK cost analyses were also sought and appraised.
Titles and abstracts returned by the search strategy were examined independently by two researchers (LC and LL) and screened for possible inclusion.
Data extraction
Two independent reviewers (LC and LL) selected eligible publications initially based on titles and abstracts. Potentially relevant articles were scrutinised and their data extracted using a standardised data extraction form. This form was also used for data synthesis. Data extraction forms were checked by a third reviewer (CH). Any disagreement between the reviewers was resolved by consultation with a third reviewer (CH).
Study quality assessment
The methodological quality of economic evaluations were assessed according to internationally accepted criteria such as the Consensus on Health Economic Criteria list questions developed by Evers et al. 201
Results
Summary of cost-effectiveness studies
The flow of papers is summarised in Figure 4. In brief, 183 unique citations were identified, 16 of which were ordered in full. Of these articles, 13 did not meet the study design criterion for inclusion and were excluded. Of the remaining three, one was an abstract for which more information was requested but not received and two were formally included. Further details and references for these excluded papers are available in Appendix 6.
FIGURE 4.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart for the economic evaluations review. a, Thirteen studies were not cost-effectiveness evaluations but were considered appropriate for the clinical effectiveness systematic review. After deduplication against the clinical effectiveness search results, two studies were considered suitable for inclusion in the review (see Chapter 2, Quantity of research available).

Summary: study characteristics
Two published full economic evaluations addressing the cost-effectiveness of interventions for the treatment of LET met the inclusion criteria for the review: Korthals-de Bos et al. 200 and Struijs et al. 202 One abstract was also identified which met the specified inclusion criteria,203 for which additional information was requested from the corresponding author; however, at the time of writing no response had been received. The abstract is referred to in the discussion in this section but was not formally included in the cost-effectiveness review. An overview of identified cost-effectiveness studies is given in Table 25 and summary characteristics are given for the included full papers in Table 26.
| Study ID | Comparison | Location | Notes | ||||
|---|---|---|---|---|---|---|---|
| B | GCI | PRP | PT | WS | |||
| Abstract | |||||||
| Peerbooms et al., 2012203 | ✗ | ✗ | Norway | CEA (Markov model); abstract only | |||
| Full papers | |||||||
| Struijs et al., 2006202 | ✗a | ✗a | The Netherlands | CEA (trial based); clinical effectiveness data published in Smidt et al.121 | |||
| Korthals-de-Bos et al., 2004200 | ✗ | ✗ | ✗ | The Netherlands | CEA, CUA (trial based) | ||
| Study ID | Setting, country, perspective | Population | Study purpose | Study approach | Comparators | Outcomes measured; time points | Source of funding |
|---|---|---|---|---|---|---|---|
| Korthals-de Bos et al., 2004200 | Primary care, the Netherlands, societal | Patients aged 18–70 years with pain at the lateral side of the elbow for at least 6 weeks (n = 185) | To assess cost-effectiveness and cost–utility of brace only, physiotherapy and the combination of brace and physiotherapy for patients with tennis elbow | Trial-based cost-effectiveness analysis; economic evaluation alongside a RCT | GCI (n = 62), WS (n = 59), PT (n = 64) | General improvement (6-point scale), pain during the day (11-point scale translated to a 100-point scale), elbow disability [PFFQ (100-point scale)], QoL (EQ-5D); self-reported questionnaires at baseline, 3, 6, 12, 26 and 52 weeks | Health Insurance Council Fund for Investigative Medicine and The Netherlands Organisation for Scientific Research |
| Struijs et al., 2006202 | Primary care, the Netherlands, societal | Patients with elbow complaints for at least 6 weeks and clinically diagnosed LET, which aggravated with both pressure on the lateral epicondyle of the humerus and resisted dorsiflexion of the wrist (n = 180) | To evaluate the cost-effectiveness of GCIs, physiotherapy and a WS policy for primary care patients with LET | Trial-based cost-effectiveness analysis; economic evaluation alongside a RCT | B (n = 68), PT (n = 56), PT + B (n = 56) | Global measure of improvement (6-point scale), severity of complaint (11-point scale), pain intensity of most severe complaint (11-point scale): QoL (EQ-5D); blinded assessor at baseline, 6 and 52 weeks | Financed by Bauerfeind AG (Zeulenroda-Triebes, Germany) (manufacturer of orthotic devices) |
Summary: results
Mean effects reported as mean improvement from baseline to 1 year and costs (direct, indirect and total) over 1 year are presented in Table 27. Incremental cost-effectiveness and cost–utility ratios are presented in Table 28. Cost–utility ratios [cost/quality-adjusted life-year (QALY) gained] in the included studies are based on total costs.
| Mean effect/costs to both columns 1 and 2 | Korthals-de Bos et al.200 | Struijs et al.202 | |||||
|---|---|---|---|---|---|---|---|
| WS (n = 59) | GCI (n = 62) | PT (n = 64) | PT (n = 56) | B (n = 68) | B + PT (n = 56) | ||
| Mean effects | Success, n (%)a | NA | NA | NA | 47 (89) | 86 (54) | 47 (87) |
| Severity of complaintb | NA | NA | NA | 19 (28) | 31 (20) | 21 (32) | |
| Pain most important complaintb | NA | NA | NA | 27 (60) | 60 (28) | 27 (58) | |
| Pain during the dayc | 39 (26) | 35 (26) | 45 (28)d | NA | NA | NA | |
| PFFQe | 35 (21) | 27 (23) | 40 (22)d | 37 (16) | 40 (18) | 42 (20) | |
| Utilities (EQ-5D)f | 0.81 (0.12)g | 0.78 (0.14)g | 0.82 (0.14)g | 0.12 (0.16)h | 0.17 (0.29)h | 0.18 (0.30)h | |
| Mean (SD) costs (€) | Direct health-care cost total | 56 (100) | 143 (187) | 214 (92) | 237 (149) | 190 (342) | 309 (225) |
| Direct non-health-care cost total | 57 (182) | 125 (379) | 96 (101) | 179 (298) | 374 (1042) | 204 (613) | |
| Direct cost total | 113 (241) | 268 (467) | 309 (163) | 417 (386) | 564 (173) | 518 (802) | |
| Indirect cost total | 518 (1549) | 164 (507) | 612 (2456) | 557 (1851) | 1416 (2890) | 739 (2072) | |
| Total cost | 631 (1627) | 430 (872) | 921 (2648) | 975 (1989) | 1980 (3673) | 1258 (2403) | |
| Outcome measure | Korthals-de Bos et al.200 | Struijs et al.202 | ||||
|---|---|---|---|---|---|---|
| WS–GCI | PT–WS | PT–GCI | B–PT | PT–B + PT | B–B + PT | |
| ICER as reported in the published paper | ||||||
| Success rate (%) | NA | NA | NA | B €33,641 (95% CI €7363 to €2,263,232) | €5625 (95% CI €–6679 to €597,372) | €68,423 (95% CI €31,827 to €989,986) |
| General improvement (6-point scale) | WS €2035 | PT €4675 | PT €3089 | NA | NA | NA |
| Severity of complainta | NA | NA | NA | B €405 (95% CI €37 to €101,453) | €26 (95% CI €–522 to €922) | €–835 (95% CI €–53,181 to €–229) |
| Pain most serious complaint | NA | NA | NA | B €3142 (95% CI €2765 to €537,918) | €–42 (95% CI €–9836 to €155) | €356 (95% CI €64 to €47,910) |
| Pain during the day (0–100 scale) | WS €43 | PT €64 | PT €53 | NA | NA | NA |
| PFFQ | WS €29 | PT €72 | PT €46 | B €324 (95% CI €–249 to €33,774) | €17 (95% CI €–374 to €510) | €–392 (95% CI €–52,981 to €42) |
| ICUR as reported in the published paper (total costs), reported as cost/QALY gain | ||||||
| Utility (EQ-5D, 0–1) | WS €6807 | PT €34,461 | PT €12,158 | €23,517 | €1588 | €–71,897 |
In the Korthals-de Bos et al. 200 study, direct health-care costs and indirect costs were the main determinants of the total costs. Direct health-care costs were lower for the wait-and-see policy (€56) than for physiotherapy (€214) and lower for GCIs (€143) than for physiotherapy (€214). Indirect costs were higher in the physiotherapy group (€612) and the wait-and-see group (€518) than in the injection group (€164). Over the study period (1 year) GCIs were less costly but also less effective than physiotherapy; the incremental cost–utility ratio (ICUR) for physiotherapy compared with GCIs was approximately €12,000 per utility gain (total costs), and €1800 per utility gain (direct health-care costs). The ICUR for physiotherapy compared with the wait-and-see policy was more than €34,000 per utility gain (total costs) and approximately €16,000 per utility gain (direct health-care costs). The wait-and-see policy produced slightly better clinical results (Table 27) at an increased cost compared with GCIs, resulting in an ICUR of approximately €7000 per utility gained (total costs). The ICUR for this comparison based on direct health-care costs alone yielded an ICUR of –€2900; less costly than GCIs. The cost-effectiveness ratios (general improvement, pain during the day and disability) indicated that no intervention was less costly and more effective.
In the study by Struijs et al. ,202 over the study period (1 year), no statistically significant differences were identified for any of the effectiveness measures between the three interventions. Direct health-care costs were lower for the brace group (€190) than for physiotherapy (€237) or brace and physiotherapy in combination (€309). Costs were suggested to be higher in the brace and physiotherapy group because of costs incurred during the intervention period. Indirect costs were higher in the brace-only group (€1416) than in the groups treated with a brace and physiotherapy in combination (€739) or physiotherapy alone (€557). For brace only compared with physiotherapy, the cost-effectiveness ratios for the outcome measures success rate (€34,000), severity of complaint (€405) and pain for the most serious complaint (€3100) differed significantly; all favoured physiotherapy. However, the 95% CIs around these estimates were wide, €7000 to €2,263,200, €37 to €101,500 and €2800 to €537,900, respectively, and, therefore, drawing a definitive conclusion from these data is not recommended. Comparing brace and combination treatment ratios for success rate (€68,000), pain for most important complaint (€356) and score on EQ-5D (–€72,000) all favoured combination treatment. When comparing cost-effectiveness ratios for physiotherapy and combination treatment statistically, no significant differences were identified and no difference was reported for either cost or effect. Over the study period (1 year), brace only was less costly but more effective than physiotherapy; the ICUR for this comparison was approximately €23,500 per utility gain (total costs) and approximately –€900 (direct health-care costs). Combination treatment produced slightly better clinical results than both brace only and physiotherapy, resulting in an ICUR of only –€71,897 and €1588, respectively (total costs). The ICUR for these comparisons based on direct health costs alone yielded ICURs of €1200 and €11,900 respectively.
The analysis conducted by Struijs et al. 202 used sensitivity analyses to evaluate the influence of true income on the outcome of costs compared with the mean income of the Dutch population to account for the effect of individuals with a high income and the influence of job type on sick leave given that patients doing jobs involving heavy labour are likely to be on sick leave for longer (this was separated on the basis of whether or not lifting was a major part of paid employment). Neither sensitivity analysis led to different conclusions from the results of the primary analysis. No sensitivity analyses were conducted in the Korthals-de Bos et al. 200 study.
Quality appraisal
A quality appraisal was carried out on the two studies using the Evers et al. checklist. 201 A summary of the results is provided in Table 29.
| Item number | Checklist item | Korthals-de Bos et al.200 | Struijs et al.202 |
|---|---|---|---|
| 1 | Is the study population clearly described? | Y | Y |
| 2 | Are competing alternatives clearly described? | Y | Y |
| 3 | Is a well-defined research question posed in answerable form? | Y | Y |
| 4 | Is the economic study design appropriate to the stated objective? | Y | Y |
| 5 | Is the chosen time horizon appropriate to include relevant costs and consequences? | Y | Y |
| 6 | Is the actual perspective chosen appropriate? | Y | Y |
| 7 | Are all important and relevant costs for each alternative identified? | Y | Y |
| 8 | Are all costs measured appropriately in physical units? | Y | Y |
| 9 | Are costs valued appropriately? | Y | Y |
| 10 | Are all important and relevant outcomes for each alternative identified? | Y | Y |
| 11 | Are all outcomes measured appropriately? | Y | Y |
| 12 | Are outcomes valued appropriately? | Y | Y |
| 13 | Is an incremental analysis of costs and outcomes of alternatives performed? | Y | Y |
| 14 | Are all future costs and outcomes discounted appropriately? | Na | Na |
| 15 | Are all important variables, whose values are uncertain, appropriately subjected to sensitivity analysis? | N | Y |
| 16 | Do the conclusions follow from the data reported? | Y | Y |
| 17 | Does the study discuss the generalisability of the results to other settings and patient/client groups? | N | Y |
| 18 | Does the article indicate that there is no potential conflict of interest of study researcher(s) and funder(s)? | Y | Y |
| 19 | Are ethical and distributional issues discussed appropriately? | N | Pb |
Study design
Both included studies were prospectively conducted, trial-based, cost-effectiveness studies set in primary care in the Netherlands. Both economic evaluations were carried out alongside a RCT and are conducted from a societal perspective. In both studies, baseline characteristics of the study populations were considered comparable. Only the study by Struijs et al. 202 acknowledged the limited generalisability of its findings with respect to patient groups together with possible distributional implications, for example suggesting that identification of subgroups that may favour certain specific interventions maybe an area for research. Neither study discussed ethical issues.
Data
Both studies considered similar clinical outcomes. These were (1) global measure of improvement (6-point scale) – this measure was dichotomised in both studies, i.e. patients who reported being completely recovered or much improved; (2) pain – severity (Struijs et al. 202), intensity (Struijs et al. 202) and during the day (Korthals-de Bos et al. 200) all measured on an 11-point scale; (3) functional (elbow) disability as measured using the pain-free function questionnaire (PFFQ); and (4) QoL measured with the EQ-5D and expressed as utility values ranging from 0 to 1, where 1 represents perfect health. The study by Struijs et al. 202 also considered other outcomes, but these are not reported in the economic evaluation. 121,202 Both studies measured outcomes at baseline, 6, 12, 26 weeks and 1 year after randomisation; Korthals-de Bos et al. 200 additionally measured outcomes at 3 weeks after randomisation. Both studies translated all outcome values for the pain scale and PFFQ into a 100-point scale to facilitate interpretation and allow comparison across outcome measures. Both papers tabulated effects and QoL as mean improvement from baseline to 1 year, although comparison of cost-effectiveness at other time points, for example short, intermediate and long term, would also be useful in comparing these interventions.
As discussed in Chapter 2, the time points at which outcomes are measured in LET is an important consideration as some treatments may be more effective in the short term (6–26 weeks) with effects wearing off after more than 1 year. For example, GCIs may offer short-term benefits; however, effects may have worn off after 1 year so comparison of effect with physiotherapy at 1 year is questionable. Considering this, the suggestion to define a core outcome set for defined time points (short, intermediate and long term) is considered a research priority as this deficiency inhibits the ability to compare the results of different studies and inform decision-making.
Details of methods of patient recruitment were given and if more details were available elsewhere, cross-reference was made to the relevant publication (Struijs et al. 202). Both studies reported methods of collecting health-care resource quantity data and applying unit costs to them. The study by Struijs et al. 202 used standard forms for physiotherapists and questionnaires filled out by patients at 6 weeks’, 26 weeks’ and 1 year’s follow-up, and the study by Korthals-de Bos et al. 200 collected data by means of five cost diaries per patient (patient completed) for the 1-year period. Both studies reported unit costs and quantities separately and provided explanation as to the estimation of unit costs (Table 30).
| Cost inputs | Type of costs |
|---|---|
| Direct health-care costsa |
|
|
|
|
|
|
|
|
|
|
|
| Direct non-health-care costsa |
|
|
|
| Indirect costsb |
|
|
Both studies stated the date of the unit costs used and provided details when price and currency conversion adjustments were made. Korthals-de Bos et al. 200 reported 1999 values with no adjustment made to account for the study year (2004). Similarly, the study by Struijs et al. 202 used costs from 2004; with no adjustment made to allow for the fact that the study was conducted in 2006.
Analysis and interpretation of results
Neither study analysed outcomes beyond 1 year and, therefore, did not require the use of a discount rate. The analysis conducted by Struijs et al. 202 used sensitivity analyses to evaluate the influence of true income compared with the mean income of Dutch population and the influence of job type on sick leave given that individuals doing jobs involving heavy labour are likely to be on sick leave for longer (this was separated on the basis of whether or not lifting was a major part of paid employment). However, as previously noted, neither analysis found different conclusions from the results of the primary analysis. No sensitivity analyses was provided in the Korthals-de Bos et al. 200 study and, therefore, the degree to which cost differences were true differences as opposed to the results of chance alone or estimated precisely cannot be established.
Both studies were powered to detect differences in clinical outcomes rather than costs. Neither study found clear differences in effect between the treatments reviewed at 1 year. However, differences in total costs were apparent, but it was not possible to determine whether or not these differences were statistically significant because of a lack of power.
It was unclear to what extent the results from the studies may be generalisable across countries or patient populations.
Abstract
One abstract (Peerbooms et al. 203) analysed the cost-effectiveness of PRP compared with corticosteroids in the treatment of LET in a Norwegian setting. This was a model-based cost–utility analysis, based on clinical data from two papers reporting results from a RCT comparing the effect of PRP (n = 49) with corticosteroids (n = 51) as treatment of lateral epicondylitis; both RCTs were identified in the review of clinical effectiveness. 125,176 VAS pain scores were mapped to the EQ-5D using established methodology to enable a cost–utility analysis. The authors report that results show an incremental cost-effectiveness ratio of €5000 per QALY. The probabilistic analysis demonstrates that the probability of leucocyte-enriched PRP being the cost-effective alternative is as high as 99% even when the willingness to pay for additional QALY is as low as €13,000. The authors concluded that, compared with corticosteroids, treating LET with leucocyte-enriched PRP represents the cost-effective treatment strategy in Norway. We requested more information on this abstract from the authors, but none was received to allow a more detailed assessment of the study for inclusion.
Discussion and conclusions
The aim of this review of economic evaluations was to identify studies assessing the cost-effectiveness of conservative interventions for the treatment of LET. As discussed in Chapter 2, Interventions, ‘conservative intervention’ was defined for the purposes of this review as any non-surgical treatment. We identified two includable studies: one considered brace compared with physiotherapy (and in combination)202 and the other considered GCIs compared with physiotherapy compared with wait-and-see approach. 200 One further abstract was identified203 that evaluated the cost-effectiveness of PRP compared with GCIs; however, more detailed information was not available to allow critical analysis.
Looking at the methods of economic evaluations used in the full papers, we observed that the authors used both cost-effectiveness analysis with a clinical outcome, such as pain or disability measure, or global improvement, and cost–utility analysis with cost per utility gain as the benefit measure. Both studies met most of the criteria for quality when considered against the Evers checklist and, for this reason, were considered to be well-conducted cost-effectiveness analyses. Omissions identified in the study by Korthals-de Bos et al. 200 included the absence of sensitivity analysis and lack of consideration of the generalisability of results to other settings or patient groups or ethical distribution issues. The study by Struijs et al. 202 checked most of the criteria on the checklist; however, only limited consideration was given to the generalisability of results with respect to different patient groups. Of additional note, the study by Korthals-de Bos et al. 200 was independently conducted and funded by a research grant. The study was conducted in 2004 and was more than likely used as the basis for the analysis by Struijs et al. ;202 two of the authors of the 2004 study were involved in the 2006 analysis. The study by Struijs et al. 202 was supported by an industry grant from the manufacturer of the orthotic device used in the study. 144,200
Effectiveness estimates in the economic evaluation of GCIs compared with physiotherapy compared with wait-and-see approach (Korthals-de Bos et al. 200) favoured GCI over physiotherapy or wait-and-see options for short-term treatment for all outcomes; however, longer-term follow-up (1 year) suggests that physiotherapy is the best option, followed by a wait-and-see approach. GCIs were likely to be the most cost-effective option in the short term, from a societal perspective, as this therapy facilitated earlier return to work. Struijs et al. 202 found physiotherapy to be superior to brace only at 6 weeks for pain, disability and satisfaction; however, brace-only treatment was superior on ability to conduct daily activities. Combination treatment was superior to brace on severity of complaints, disability and satisfaction. However, at 26 weeks and 1 year, no significant differences were identified. 144 The estimates of cost-effectiveness in both studies relied on the accompanying trials, which were too small to overcome uncertainty about the size of effects. Of additional comment, the comparison between interventions and time points needs to be considered when designing future evaluations, as comparing physiotherapy with GCIs at the 1-year time point has arguably little value when it is more likely that the effects of injections are short term.
Both studies incorporated EQ-5D estimates of utility. Korthals-de Bos et al. 200 incorporated utility estimates at 1 year; however, there were no significant differences between the reported means for the three treatment groups, i.e. 0.81, 0.78, 0.82 for wait-and-see policy, GCIs and physiotherapy respectively. 200 Similarly, Struijs et al. 202 report utility estimates at 1-year follow-up as mean improvement from baseline 0.12, 0.17 and 0.18 for physiotherapy, brace and combination therapy respectively. 202 Both studies report no significant differences between the interventions reviewed in respect of QoL.
In the Korthals-de Bos et al. 200 study, the ICURs (total costs) were (approximately) €7000 per utility gain for the wait-and-see policy compared with corticosteroid injections; €12,000 per utility gain for physiotherapy compared with corticosteroid injections; and €34,500 for physiotherapy compared with the wait-and-see policy. Longer-term physiotherapy appeared to be more cost-effective. In the Struijs et al. 202 study, cost-effectiveness ratios and cost–utility ratios showed physiotherapy to be the most cost-effective, although none of the findings were statistically significant. The ICURs (total costs) were (approximately) €23,500 per utility gain for brace only compared with physiotherapy only; –€71,900 for the brace only compared with combination therapy; and €1600 for physiotherapy only compared with combination therapy.
The included studies are well-conducted economic evaluations. However, the studies report little difference in effectiveness between interventions in terms of the outcomes measured at 1 year. The study by Korthals-de Bos et al. 200 reported that GCI was likely to be the most cost-effective option in the short term, from a societal perspective, as it facilitated earlier return to work. Longer-term physiotherapy appeared to be more cost-effective. However, the estimates of effectiveness relied on the accompanying trials, which were too small to overcome uncertainty about the size of effects. Both studies report differences in costs between interventions (in some cases seemingly significant differences); however, wide CIs and a lack of power to test for statistical significance in this respect meant that robust conclusions could not be made.
Given the complexity of treatment because of the complex pathology of the condition, the existing evidence on economic outcomes is considered to be insufficient to inform decision-making in the context of the research question specified in this review.
Chapter 4 Discussion
Statement of principal findings
Clinical effectiveness
The objectives of this review were to provide an overview of systematic reviews of the evidence for the clinical effectiveness of conservative interventions for the treatment of LET; quantify the number of RCTs meeting the specified inclusion criteria not included in the most relevant and up-to-date systematic reviews included in the overview; suggest which RCTs could contribute further evidence to existing systematic reviews (included in the overview); and determine where a systematic review to synthesise evidence for newer treatments may be of benefit.
Background searches identified that although there are already systematic reviews of RCTs, including Cochrane reviews, evaluating interventions for the treatment of LET many of these are out of date by 10 years. Therefore, we included systematic reviews of RCTs and RCTs from 2003 to 2013. Twenty-nine systematic reviews and 36 RCTs were identified that met prespecified inclusion criteria.
Systematic reviews
Twenty-nine systematic reviews were included in the review. 56–84 These reviews focused on the following interventions: topical drug treatment (diclofenac); local injections [botulinum toxin injection, GCIs, autologous blood injection (ABI) and PRP]; and non-drug treatments (LLLT, ESWT, exercise, massage, manipulation, orthoses, and acupuncture). These studies were assessed using the AMSTAR measurement tool and overall considered to be of intermediate quality (mean score 5.7 points; range 1–8 points). Only five of the 29 studies were considered to be high quality;56–60 of these, three were subjected to full GRADE analysis58–60 and two were referred to in the write-up but, because of a lack of reported data, were not analysed using the GRADE principles. 56,57 It is worth noting that in the review by Coombes et al. 60 the population considered was broad, i.e. the population with all musculoskeletal conditions. This study was included in the current review as results data were accessible by condition.
In the remaining 24 systematic reviews considered of intermediate or low quality, 40 unique RCTs (published 2003–13) were identified from the bibliographies of the publications. Eleven of these RCTs had been included and evaluated in one of the high-quality reviews; the remaining 29 studies have been recorded in our review and were taken into account in the research recommendations made.
Bisset et al.80
From our searches we identified one review80 among the 29 studies published in Clinical Evidence in 2011 that provided an overview of the clinical effectiveness of treatments for tennis elbow. The searches for the Bisset et al. 80 review were conducted in November 2009 [search dates from either 1966 (MEDLINE and Cochrane) or 1980 (EMBASE)] and found a total of 80 systematic reviews, RCTs and observational studies. Inclusion criteria for the review conducted by Bisset et al. 80 were slightly broader than those used in the current review in that they allowed for the consideration of evidence from observational studies and considered global improvement in addition to the outcomes pain relief, functional improvement and QoL. The review by Bisset et al. 80 was not included in our GRADE analysis as it scored low on the AMSTAR measurement; we did not take into account the underlying principles of the Clinical Evidence reviews. We have, however, considered our findings in the context of the review by Bisset et al. 80 (see Chapter 4, Current clinical effectiveness evidence in context).
Ten57–59,61,62,65,68,70,79,84 of the 29 studies identified in the current review were also included in the review by Bisset et al. 80 Evidence from these 10 studies was evaluated in the overview by Bisset et al. ;80 a summary of recommendations from the overview is given in Table 31. We compare our results against these recommendations in Chapter 4, Current clinical effectiveness evidence in context.
| Effect | Treatment |
|---|---|
| Unlikely to be beneficial |
|
|
|
| Unknown effectiveness |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Likely to be beneficial |
|
|
|
|
|
|
|
|
|
|
Randomised controlled trials
We identified a number of RCTs that had been evaluated in an intermediate-/low-quality systematic review (n = 29) and (because of the low-quality score) were not considered in the GRADE analysis. In addition, we identified 36 RCTs not evaluated in a systematic review. Study characteristics are reported in detail in Appendix 5 and a summary is given in Table 23. As the aim of this overview was to quantify the RCT evidence, we did not quality appraise the identified RCTs against a validated checklist.
When reviewing the evidence, we highlighted a number of issues (see Chapter 4, Other issues), for example a lack of a standard set of outcome measures by time point (short, intermediate and long term) hindering interpretation and synthesis of results. This, alongside differences in the definitions of interventions as well as treatment protocols (dosing) between the studies, also makes it difficult to compare results.
Current clinical effectiveness evidence in context
We considered five of the systematic reviews identified to be of high quality. Our results are summarised in Chapter 2, Results, and Table 24. Comparing our results with the recommendations made by Bisset et al. 80 in their 2011 overview, we did not find any additional evidence to contradict any of the recommendations made. However, the following revisions/additions were noted:
-
The effectiveness of LLLT was considered unclear based on the evidence reviewed in the current review. Benefit was seen in the intermediate rather than the short term; however, the quality of evidence this finding was based on was considered low.
-
A potential benefit in terms of the short-term reduction of pain was found for botulinum toxin injection (not reviewed in the Bisset et al. 80 overview); however, given the overall low quality of evidence (assessed using the GRADE principles, together with the reported incidence of known adverse effects) further research is needed before a firm recommendation can be made.
-
A potential benefit for sodium hyaluronate injection in short, intermediate and long term; however, the overall low quality of evidence (assessed using the GRADE principles) means that further research is required before a firm recommendation can be made.
-
Overall evidence for the reduction of pain with therapeutic ultrasound-guided injection of sclerosing solution (short term), glycosaminoglycan polysulphate injections (short and intermediate term) and prolotherapy was considered low and evidence was considered insufficient to make firm recommendations in respect of these treatments.
Other issues
In conducting this overview of systematic reviews, we identified a number of issues that need to be taken into account when interpreting results from either reviews or RCTs.
-
Definition of interventions
-
Inconsistent definitions between studies, for example physiotherapy which was often made up of multiple treatments that differ between studies; exercise regimens, etc. make it difficult to compare results between studies.
-
-
Dosing
-
Variation in dosages between studies also poses a problem when combining studies of therapies involving very different doses in that it can dilute the effect size of the effective dose. The review by Bjordal et al. 62 highlights this.
-
-
Outcomes
-
Lack of a standard set of outcome measures in clinical trials for LET hinders interpretation and synthesis of results. A core set of outcome measures including overall pain with or without provocation, a dichotomous measure of pain, a measure of upper extremity function (Upper Extremity Function Scale or DASH) and the ability to carry out usual activities, work and/or sport and a measure of QoL would ease interpretation of results.
-
Inclusion of short-, intermediate- and long-term outcomes to cover fact that some people recover within 3–6 months and some still report symptoms after 1 year.
-
Cost-effectiveness
A systematic review identified two economic evaluations – Korthals-de Bos et al. 200 and Struijs et al. 202
The included evaluations considered GCIs compared with physiotherapy compared with wait-and-see approach,200 and brace compared with physiotherapy and in combination (i.e. brace or physiotherapy alone compared with combination). 202 Results from the Korthals-de Bos et al. 200 study suggest that, from a societal perspective, GCIs may be cost-effective by facilitating an earlier return to work than the other interventions. However, over longer term (52 weeks) physiotherapy was shown to have a greater effect. In the study by Struijs et al. 202 physiotherapy was found to be superior to brace in the short term (6 weeks), but no difference between treatments was identified at either 26 weeks or 1 year. Similarly, no significant difference was identified between the treatments in either of the studies in terms of QoL. However, the estimates of effectiveness in both evaluations rely on accompanying trials that were too small to overcome uncertainty about the size of effects. Similarly, neither evaluation was sufficiently powered to determine whether or not the differences in costs identified were significant.
Only one health economic model was identified but reported in abstract only,203 so was not considered in full as part of the cost-effectiveness evaluation.
The existing evidence on economic outcomes is considered insufficient to inform decision-making in respect of the research question for this review.
Further research
Based on the evidence identified in this overview of clinical and cost-effectiveness, and taking into account the fact that Cochrane reviews are in progress for autologous blood and PRP injections and an update of the earlier Cochrane review on NSAIDs (topical and oral), we recommend that future research should primarily focus on:
-
The areas for which recent reviews have been inconclusive and unevaluated or subsequent RCTs; consider conducting larger-scale, good-quality RCTs (sufficient sample size, core set of outcomes for defined time points and appropriate follow-up) before conducting/updating systematic reviews
-
LLLT, ESWT, therapeutic ultrasound, combination physiotherapy, orthotics and manipulation.
-
-
The areas for which recent reviews are of moderate quality (and suggest a likely benefit) and unevaluated or subsequent RCTs; conduct a high-quality systematic review and use the findings to inform study design for larger-scale, good-quality RCTs. In addition, consider subgroup analysis of existing RCT data to ascertain whether or not certain patient groups are more likely to benefit from the intervention under review
-
glucocorticoid injections, botulinum toxin injections and exercise.
-
-
The areas for which no recent systematic reviews were identified and few or no subsequent RCTs were identified; we suggest considering conducting a full systematic review of existing evidence and using the findings to inform the study design for larger-scale, good-quality RCTs:
-
acupuncture (we recommend an update of the 2002 Cochrane review34), wait-and-see/watch-and-wait approach, orthotics, manipulation/manual therapy, Cyriax physiotherapy, soft-tissue therapy, iontophoresis, cryotherapy, myofascial release and electrical stimulation
-
assuming there is a clinical rationale for the use of the indication, phonophoresis, sodium hyaluronate, therapeutic ultrasound (sonographically)-guided injection of sclerosing solution and glycosaminoglycan polysulphate injections.
-
-
Set-up larger-scale, good-quality effectiveness studies giving consideration to the following issues:
-
establish a core set of outcomes for defined time points (short, intermediate and long term) against which the clinical effectiveness of interventions can be measured allowing for more accurate comparison of results between studies
-
establish the effectiveness of interventions for given time points; short, intermediate and long term to enable relevant comparisons, for example injection therapies are likely to offer more benefit in the short term than physiotherapy, which may have greater benefits over longer term
-
consider that treatment often comprises more than one intervention; assessment of the effectiveness of combination treatments
-
consider the effectiveness of different interventions for different subgroups of patients (as suggested in the paper by Struijs et al. 202).
-
-
Subgroup analysis of existing RCT data to ascertain whether or not different patient groups respond differently to interventions. Use the findings when considering study design for newly conducted RCTs.
-
A network meta-analysis to compare multiple treatments (three or more) using both direct comparisons of interventions within RCTs and indirect comparisons across trials based on a common comparator. In this case there are many placebo-controlled trials; however, caution would be required given the varying nature of placebo comparators used.
-
Incorporate economic evaluation alongside the clinical trials to collate unit costs and resource use data; however, the accompanying trial must be of good quality and sufficient to generate robust evidence on clinical effectiveness and reduce uncertainty about the size of the effect.
-
Use clinical effectiveness data to construct a decision model to evaluate the most cost-effective treatment method.
Strengths and limitations
The overview of clinical effectiveness systematic reviews and systematic review of cost-effectiveness studies were conducted by an independent research team using the latest evidence and to a prespecified protocol (PROSPERO CRD42013003593). 53
Limitations were identified as follows:
-
The searches were limited to English language because of resource limitations, which may have led us to exclude important studies.
-
The focus of the review is on LET rather than ‘elbow tendinopathies’; LET is the predominant condition.
-
We did not consider uncontrolled studies or systematic reviews of uncontrolled studies to assure high quality with minimum risk of bias.
-
We did not consider dosing studies; however, it is unclear whether or not intervention-effective studies looking at different doses would add to the study.
-
We did not consider global improvement or other dichotomous outcomes, which has been shown to add value.
-
The summary of findings is based on evidence from three of five high-quality systematic reviews.
-
Few studies report the cost-effectiveness of conservative interventions for the treatment of LET; however, if clinical effectiveness data show no benefit the intervention is unlikely to be cost-effective.
Conclusions
The clinical effectiveness evidence from the high-quality systematic reviews identified in this overview continues to show uncertainty as to the effectiveness of many conservative interventions for the treatment of LET. Although there is some evidence to suggest potential benefits for some treatments, for example botulinum toxin injection (short term) and sodium hyaluronate injection, the quality of evidence this is based on is low (as per the GRADE principles) and as such further research is needed before any recommendation is made. Although new RCT evidence has been identified with both active and placebo control comparisons, these studies are, largely, made up of small sample sizes and, therefore, give rise to uncertainty as to the size of reported effects within them.
Conclusions concerning cost-effectiveness are also unclear. Although the two economic evaluations identified were considered good quality, the accompanying trials on which they are based are too small to overcome uncertainty about the size of effects reported. Similarly, although both studies reported difference in costs, neither study was set up to detect a statistically significant difference in this respect. One health economic model was identified, but this was available only in abstract format and for this reason was not included in our review.
Therefore, we conclude that further research is needed. This is in respect of conducting good-quality, up-to-date systematic reviews where indicated, but, primarily, focusing on conducting larger-scale, good-quality clinical trials with a core set of outcome measures (for defined time points) and appropriate follow-up to facilitate both synthesis and interpretation of evidence. In addition, we also consider that subgroup analysis of existing RCT data may be beneficial to ascertain whether or not certain patient groups are more likely to respond to treatments.
Acknowledgements
About Peninsula Technology Assessment Group
The Peninsula Technology Assessment Group (PenTAG) is part of the Evidence Synthesis and Modelling for Health Improvement (ESMI) group at the University of Exeter Medical School. PenTAG was established in 2000 and carries out independent HTAs for the UK HTA Programme, systematic reviews and economic analyses for the NICE Centre for Public Health Excellence, as well as for other local and national decision-makers. The group is multidisciplinary and draws on individuals’ backgrounds in public health, health services research, computing and decision analysis, systematic reviewing, statistics, and health economics. The Institute of Health Research is made up of discrete but methodologically related research groups, among which HTA is a strong and recurring theme.
A list of our recent projects can be found on our website: http://medicine.exeter.ac.uk/pentag/workstreams/healthtechnologyassessment/ (last accessed 3 September 2014).
We would like to acknowledge the help of Ian Goodwin (Ramsay Healthcare, Mount Stuart Hospital, Torbay, Devon, UK) in the preparation of this document. We would particularly like to thank our expert advisors (Professor Rachelle Buchbinder, Dr Victoria Goodwin, Dr Leon Poltawski and Dr Nynke Smidt) for their help in reviewing and providing comments on the report.
Professor Rachelle Buchbinder, National Health and Medical Research Council Practitioner Fellow Director, Monash Department of Clinical Epidemiology, Cabrini Hospital; Department of Epidemiology and Preventive Medicine, School of Public Health and Preventive Medicine, Monash University; and joint co-ordinating editor, Cochrane Musculoskeletal Group.
Dr Victoria Goodwin, Peninsula Collaboration for Leadership in Applied Health Research and Care, University of Exeter Medical School.
Dr Leon Poltawski, University of Exeter Medical School.
Dr Nynke Smidt, Assistant Professor in Epidemiology, Department of Epidemiology, University Medical Center Groningen, University of Groningen, the Netherlands.
Contribution of authors
Linda Long assessed abstracts and titles and papers for inclusion and exclusion in both systematic reviews, led the effectiveness review for the overview of clinical effectiveness studies, wrote the clinical effectiveness section and related appendices (Chapter 2 and Appendices 2, 3, 4 and 7) and contributed to the summary, background and discussion sections of the report (Chapters 1 and 4). She also contributed to the editing of the report.
Simon Briscoe compiled and ran the search strategies for clinical effectiveness and cost-effectiveness.
Chris Cooper contributed to the search strategy for clinical effectiveness and cost-effectiveness.
Chris Hyde developed the protocol, contributed to both systematic reviews, interpretation of data and to the writing and editing of the report. He is director of the Technology Appraisal Reports group at PenTAG and guarantor of the report.
Louise Crathorne provided overall project management, assessed abstracts and titles and papers for inclusion and exclusion in both systematic reviews. She led the cost-effectiveness systematic review, wrote the cost-effectiveness section (and related appendices) of the report (Chapter 3 and Appendix 6), contributed to Appendices 2 and 3, wrote Appendix 5, contributed to the summary, background and discussion sections (Chapters 1 and 4), and collated and formatted the report and conducted a final consistency check.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Shiri R, Viikari-Juntura E. Lateral and medial epicondylitis: role of occupational factors. Best Prac Res Clin Rheumatol 2011;25:43-57. http://dx.doi.org/10.1016/j.berh.2011.01.013.
- Map of Medicine . Map of Medicine: Lateral Epicondylitis 2011. http://healthguides.mapofmedicine.com/choices/map/epicondylitis1.html (accessed 3 September 2014).
- Hamilton PG. The prevalence of humeral epicondylitis: a survey in general practice. J R Coll Gen Pract 1986;36:464-5.
- Shiri R, Viikari-Juntura E, Varonen H, Heliovaara M. Prevalence and determinants of lateral and medial epicondylitis: a population study. Am J Epidemiol 2006;164:1065-74. http://dx.doi.org/10.1093/aje/kwj325.
- De Smedt T, de Jong A, Van Leemput W, Lieven D, Van Glabbeek F. Lateral epicondylitis in tennis: update on aetiology, biomechanics and treatment. Br J Sports Med 2007;41:816-19. http://dx.doi.org/10.1136/bjsm.2007.036723.
- Murtagh JE. Tennis elbow. Aust Fam Phys 1988;17:90-1.
- Buchbinder R, Green SE, Struijs P. Tennis elbow. Clin Evid 2008.
- Coombes BK, Bisset L, Vicenzino B. A new integrative model of lateral epicondylalgia. Br J Sports Med 2009;43:252-8. http://dx.doi.org/10.1136/bjsm.2008.052738.
- Classification of chronic pain . Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl 1986;3:S1-226.
- Nirschl RP, Ashman ES. Elbow tendinopathy: tennis elbow. Clin Sports Med 2003;22:813-36. http://dx.doi.org/10.1016/S0278-5919(03)00051-6.
- Regan W, Wold LE, Coonrad R, Morrey BF. Microscopic histopathology of chronic refractory lateral epicondylitis. Am J Sports Med 1992;20:746-9. http://dx.doi.org/10.1177/036354659202000618.
- Chard MD, Hazleman BL. Tennis elbow – a reappraisal. Br J Rheumatol 1989;28:186-90. http://dx.doi.org/10.1093/rheumatology/28.3.186.
- Verhaar JA. Tennis elbow. Anatomical, epidemiological and therapeutic aspects. Int Orthop 1994;18:263-7.
- Nirschl RP, Pettrone FA. Tennis elbow. The surgical treatment of lateral epicondylitis. J Bone Joint Surg Am 1979;61:832-9.
- Thorson EP, Szabo RM. Tendonitis of the wrist and elbow. Occup Med 1989;4:419-31.
- Hodgson RJ, O’Connor PJ, Grainger AJ. Tendon and ligament imaging. Br J Radiol 2012;85:1157-72. http://dx.doi.org/10.1259/bjr/34786470.
- Khan KM, Cook JL, Kannus P, Maffulli N, Bonar SF. Time to abandon the ‘tendinitis’ myth. BMJ 2002;324:626-7. http://dx.doi.org/10.1136/bmj.324.7338.626.
- Peterson M, Butler S, Eriksson M, Svardsudd K. A randomized controlled trial of exercise versus wait-list in chronic tennis elbow (lateral epicondylosis). Uppsala J Med Sci 2011;116:269-79. http://dx.doi.org/10.3109/03009734.2011.600476.
- Clements LG, Chow S. Effectiveness of a custom-made below elbow lateral counterforce splint in the treatment of lateral epicondylitis (tennis elbow). Can J Occup Ther 1993;60:137-44. http://dx.doi.org/10.1177/000841749306000305.
- Watson T, Poltawski L. Measuring clinically important change with the patient-rated tennis elbow evaluation. Hand Ther 2011;16:52-7. http://dx.doi.org/10.1258/ht.2011.011013.
- MacDermid JC. The Patient-Rated Tennis Elbow Evaluation (PRTEE) User Manual. Hamilton, ON: McMaster University; 2007.
- Rompe JD, Overend TJ, MacDermid JC. Validation of the patient-rated tennis elbow evaluation questionnaire. J Hand Ther 2007;20:3-10. http://dx.doi.org/10.1197/j.jht.2006.10.003.
- Ylinen J. Pressure algometry. Aust J Physiother 2007;53. http://dx.doi.org/10.1016/S0004-9514(07)70032-6.
- Bhargava AS, Eapen C, Kumar SP. Grip strength measurements at two different wrist extension positions in chronic lateral epicondylitis-comparison of involved vs. uninvolved side in athletes and non athletes: a case-control study. Sports Med Arthrosc Rehabil Ther Technol 2010;2. http://dx.doi.org/10.1186/1758-2555-2-22.
- Longo UG, Franceschi F, Loppini M, Maffulli N, Denaro V. Rating systems for evaluation of the elbow. Br Med Bull 2008;87:131-61. http://dx.doi.org/10.1093/bmb/ldn023.
- Beaton DE, Katz JN, Fossel AH, Wright JG, Tarasuk V, Bombardier C. Measuring the whole or the parts? Validity, reliability, and responsiveness of the disabilities of the arm, shoulder and hand outcome measure in different regions of the upper extremity. J Hand Ther 2001;14:128-46. http://dx.doi.org/10.1016/S0894-1130(01)80043-0.
- Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG). Am J Ind Med 1996;29:602-8. http://dx.doi.org/10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L.
- Sathyamoorthy P, Kemp GJ, Rawal A, Rayner V, Frostick SP. Development and validation of an elbow score. Rheumatology 2004;43:1434-40. http://dx.doi.org/10.1093/rheumatology/keh367.
- Autologous Blood Injection for Tendinopathy: Guidance (IPG 438). London: NICE; 2013.
- Extracorporeal Shockwave Therapy for Refractory Tennis Elbow (IPG313). London: NICE; 2009.
- Clinical Knowledge Summaries: Tennis Elbow. London: NICE; 2009.
- Struijs PA, Smidt N, Arola H, Dijk CN, Buchbinder R, Assendelft WJ. Orthotic devices for the treatment of tennis elbow. Cochrane Database Syst Rev 2002;1.
- Green S, Buchbinder R, Barnsley L, Hall S, White M, Smidt N, et al. Non-steroidal anti-inflammatory drugs (NSAIDs) for treating lateral elbow pain in adults. Cochrane Database Syst Rev 2002;2.
- Green S, Buchbinder R, Barnsley L, Hall S, White M, Smidt N, et al. Acupuncture for lateral elbow pain. Cochrane Database Syst Rev 2002;1.
- Burton AK. A comparative trial of forearm strap and topical anti-inflammatory as adjuncts to manipulative therapy in tennis elbow. Man Med 1988;3:141-3.
- Dwars BJ, Feiter de P, Parka P, Haarman HKTHM. Functional treatment of tennis elbow: a comparative study between an elbow support and physical therapy. Sp Med Health 1990;4:237-41.
- Erturk H, Celiker R, Sivri A, Cetin A, Cindas A. The efficacy of different treatment regimens that are commonly used in tennis elbow. J Rheumatol Med Rehab 1997;8:298-301.
- Haker E, Lundberg T. Elbow band, splintage and steroids in lateral epicondylalgia (tennis elbow). Pain Clin 1993;6:103-12.
- Holdsworth LK, Anderson DM. Effectiveness of ultrasound used with a hydrocortisone coupling medium or epicondylitis clasp to treat lateral epicondylitis: pilot study. Physiotherapy 1993;79:19-25. http://dx.doi.org/10.1016/S0031-9406(10)60535-4.
- Hay EM, Paterson SM, Lewis M, Hosie G, Croft P. Pragmatic randomised controlled trial of local corticosteroid injection and naproxen for treatment of lateral epicondylitis of elbow in primary care. BMJ 1999;319:964-8. http://dx.doi.org/10.1136/bmj.319.7215.964.
- Adelaar R, Maddy L, Emroch L. Diflunisal vs naproxen in the management of mild to moderate pain associated with epicondylitis. Adv Ther 1987;4:317-27.
- Burnham R, Gregg R, Healy P, Steadward R. The effectiveness of topical diclofenac for lateral epicondylitis. Clin J Sport Med 1998;8:78-81. http://dx.doi.org/10.1097/00042752-199804000-00002.
- Demirtas RN, Oner C. The treatment of lateral epicondylitis by iontophoresis of sodium salicylate and sodium diclofenac. Clin Rehab 1998;12:23-9. http://dx.doi.org/10.1191/026921598672378032.
- Forster K, Schmid K, Reichelt A. Acute epicondylitis of the elbow induced by sporting activities and its conservative treatment. Sportverl Sportschaden 1997;11:16-20.
- Jenoure P, Rostan A, Gremion G, Meier J, Grossen R, Bielinki R, et al. Multi-centre, double-blind, controlled clinical study on the efficacy of diclofenac epolamine Tissugel plaster in patients with epicondylitis. Med Sport 1997;50:285-92.
- Labelle H, Guibert R. Efficacy of diclofenac in lateral epicondylitis of the elbow also treated with immobilization. Arch Fam Med 1997;6:257-62. http://dx.doi.org/10.1001/archfami.6.3.257.
- Labelle H, Guibert R. Efficacy of an oral NSAID in the treatment of tennis elbow: a double blind randomised and controlled trial [abstract]. J Bone Joint Surg (BR) 1994;75-b.
- Percy EC, Carson JD. The use of DMSO in tennis elbow and rotator cuff tendinitis: a double-blind study. Med Sci Sport Exerc 1981;13:215-19. http://dx.doi.org/10.1249/00005768-198104000-00001.
- Primbs P, Tomasi M. Results of a double-blind study with Amuno gelo vs. placebo. Fortschr Med Orig 1983;101:242-44.
- Saartok T, Eriksson E. Randomized trial of oral naproxen or local injection of betamethasone in lateral epicondylitis of the humerus. Orthopaedics 1986;2:191-4.
- Schapira D, Linn S, Scharf Y. A placebo-controlled evaluation of diclofenac diethylamine salt in the treatment of lateral epicondylitis of the elbow. Curr Ther Res 1991;49:162-8.
- Stull P, Jokl P. Comparison of difunisal and naproxen in the treatment of tennis elbow. Clin Ther 1986;9:62-6.
- Conservative Interventions for Elbow Tendinopathy (HTA No 12/73). London: NICE; 2012.
- Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Healthcare. York: CRD, University of York; 2009.
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. http://dx.doi.org/10.1136/bmj.39489.470347.AD.
- Barr SC, Cerisola FL, Blanchard V. Effectiveness of corticosteroid injections compared with physiotherapeutic interventions for lateral epicondylitis: a systematic review. Physiotherapy 2009;4:251-65. http://dx.doi.org/10.1016/j.physio.2009.05.002.
- Trudel D, Duley J, Zastrow I, Kerr EW, Davidson R, MacDermid JC. Rehabilitation for patients with lateral epicondylitis: a systematic review. J Hand Ther 2004;17:243-66. http://dx.doi.org/10.1197/j.jht.2004.02.011.
- Buchbinder R, Green SE, Youd JM, Assendelft WJJ, Barnsley L, Smidt N. Systematic review of the efficacy and safety of shock wave therapy for lateral elbow pain. J Rheumatol 2006;33:1351-63.
- Smidt N, Assendelft WJ, Arola H, Malmivaara A, Green S, Buchbinder R, et al. Effectiveness of physiotherapy for lateral epicondylitis: a systematic review. Ann Med 2003;35:51-62. http://dx.doi.org/10.1080/07853890310004138.
- Coombes BK, Bisset L, Vicenzino B. Efficacy and safety of corticosteroid injections and other injections for management of tendinopathy: a systematic review of randomised controlled trials. Lancet 2010;376:1751-67. http://dx.doi.org/10.1016/S0140-6736(10)61160-9.
- Woodley BI, Newsham-West RJ, Baxter GD. Chronic tendinopathy: effectiveness of eccentric exercise. Br J Sports Med 2007;41:188-99. http://dx.doi.org/10.1136/bjsm.2006.029769.
- Bjordal JM, Lopes-Martins RA, Joensen J, Couppe C, Ljunggren AE, Stergioulas A, et al. A systematic review with procedural assessments and meta-analysis of low level laser therapy in lateral elbow tendinopathy (tennis elbow). BMC Musculoskel Disord 2008;9. http://dx.doi.org/10.1186/1471-2474-9-75.
- Kalichman L, Bannuru RR, Severin M, Harvey W. Injection of botulinum toxin for treatment of chronic lateral epicondylitis: systematic review and meta-analysis (Provisional abstract). Semin Arthrit Rheum 2011;6:532-8. http://dx.doi.org/10.1016/j.semarthrit.2010.07.002.
- Raman J, MacDermid JC, Grewal R. Effectiveness of different methods of resistance exercises in lateral epicondylosis--a systematic review. J Hand Ther 2012;25:5-25. http://dx.doi.org/10.1016/j.jht.2011.09.001.
- Rabago D, Best TM, Zgierska AE, Zeisig E, Ryan M, Crane D. A systematic review of four injection therapies for lateral epicondylosis: prolotherapy, polidocanol, whole blood and platelet-rich plasma. Br J Sports Med 2009;43:471-81. http://dx.doi.org/10.1136/bjsm.2008.052761.
- Gaujoux-Viala C, Dougados M, Gossec L. Efficacy and safety of steroid injections for shoulder and elbow tendonitis: a meta-analysis of randomised controlled trials. Ann Rheum Dis 2009;68:1843-9. http://dx.doi.org/10.1136/ard.2008.099572.
- Zhang T, Adatia A, Zarin W, Moitri M, Vijenthira A, Chu R, et al. The efficacy of botulinum toxin type A in managing chronic musculoskeletal pain: a systematic review and meta analysis. Inflammopharmacology 2011;19:21-34. http://dx.doi.org/10.1007/s10787-010-0069-x.
- Bisset LP, Paungmali A, Vicenzino B, Beller E. A systematic review and meta-analysis of clinical trials on physical interventions for lateral epicondylalgia. Br J Sports Med 2005;39:411-22. http://dx.doi.org/10.1136/bjsm.2004.016170.
- Borkholder CD, Hill VA, Fess EE. The efficacy of splinting for lateral epicondylitis: a systematic review. J Hand Ther 2004;17:181-99. http://dx.doi.org/10.1197/j.jht.2004.02.007.
- Trinh KV, Phillips SD, Ho E, Damsma K. Acupuncture for the alleviation of lateral epicondyle pain: a systematic review. Rheumatology 2004;43:1085-90. http://dx.doi.org/10.1093/rheumatology/keh247.
- Taylor RS, Fotopoulos G, Maibach H. Safety profile of topical diclofenac: a meta-analysis of blinded, randomized, controlled trials in musculoskeletal conditions. Curr Med Res Opin 2011;27:605-22. http://dx.doi.org/10.1185/03007995.2010.550606.
- Tumilty S, Munn J, McDonough S, Hurley DA, Basford JR, Baxter GD. Low level laser treatment of tendinopathy: a systematic review with meta-analysis. Photomed Laser Surg 2010;28:3-16. http://dx.doi.org/10.1089/pho.2008.2470.
- Zacher J, Altman R, Bellamy N, Bruehlmann P, Da Silva J, Huskisson E, et al. Topical diclofenac and its role in pain and inflammation: an evidence-based review. Curr Med Res Opin 2008;24:925-50. http://dx.doi.org/10.1185/030079908X273066.
- Herd CR, Meserve BB. A systematic review of the effectiveness of manipulative therapy in treating lateral epicondylalgia. J Manual Manipulative Ther 2008;16:225-37. http://dx.doi.org/10.1179/106698108790818288.
- Joseph MF, Taft K, Moskwa Kathryn, Denegar Maria, Craig R. Deep friction massage to treat tendinopathy: a systematic review of a classic treatment in the face of a new paradigm of understanding. J Sport Rehab 2012;21:343-53.
- Tumilty S, Munn J, McDonough S, Hurley DA, Basford JR, Baxter GD, et al. Laser Florence 2009: A Gallery through the Laser Medicine World. AIP Conference Proceedings. Red Hook, NY: Curran Associates Inc. Proceedings.com; 2010.
- Baxter GD, Bleakley C, McDonough S. Clinical effectiveness of laser acupuncture: a systematic review. J Acupuncture Meridian Stud 2008;1:65-82. http://dx.doi.org/10.1016/S2005-2901(09)60026-1.
- Farren E. Needle acupuncture in the treatment of lateral epicondylitis. J Acupuncture Assoc Chartered Physiother 2012;2012:25-31.
- Kohia M, Brackle J, Byrd K, Jennings A, Murray W, Wilfong E. Effectiveness of physical therapy treatments on lateral epicondylitis. J Sport Rehabil 2008;17:119-36.
- Bisset L, Coombes B, Vicenzino B. Tennis elbow. Clin Evid 2011:ii-1117.
- Chang WD, Wu JH, Yang WJ, Jiang JA. Therapeutic effects of low-level laser on lateral epicondylitis from differential interventions of Chinese-Western medicine: systematic review. Photomed Laser Surg 2010;28:327-36. http://dx.doi.org/10.1089/pho.2009.2558.
- Snyder KR, Evans TA. Effectiveness of corticosteroids in the treatment of lateral epicondylosis. J Sport Rehabil 2012;21:83-8.
- Pagorek S. Effect of manual mobilization with movement on pain and strength in adults with chronic lateral epicondylitis. J Sport Rehabil 2009;18:448-57.
- Crawford JO, Laiou E. Conservative treatment of work-related upper limb disorders – a review. Occup Med 2007;57:4-17. http://dx.doi.org/10.1093/occmed/kql084.
- Rompe JD, Hope C, Kullmer K, Heine J, Burger R. Analgesic effect of extracorporeal shock-wave therapy on chronic tennis elbow. J Bone Joint Surg 1996;78:233-7.
- Rompe JD, Decking J, Schoellner C, Theis C. Repetitive low-energy shock wave treatment for chronic lateral epicondylitis in tennis players. Am J Sports Med 2004;32:734-43. http://dx.doi.org/10.1177/0363546503261697.
- Pettrone FA, McCall BR, Brian R. Extracorporeal shock wave therapy without local anesthesia for chronic lateral epicondylitis. J Bone Joint Surg 2005;87:1297-304. http://dx.doi.org/10.2106/JBJS.C.01356.
- Chung B, Wiley JP. Effectiveness of extracorporeal shock wave therapy in the treatment of previously untreated lateral epicondylitis – a randomized controlled trial. Am J Sports Med 2004;32:1660-7. http://dx.doi.org/10.1177/0363546503262806.
- Speed CA, Nichols D, Richards C, Humphreys H, Wies JT, Burnet S, et al. Extracorporeal shock wave therapy for lateral epicondylitis – a double blind randomised controlled trial. J Orthopaed Res 2002;20:895-8. http://dx.doi.org/10.1016/S0736-0266(02)00013-X.
- Haake M, Konig IR, Decker T, Riedel C, Buch M, Muller HH. Extracorporeal shock wave therapy in the treatment of lateral epicondylitis – a randomized multicenter trial. J Bone Joint Surg 2002;84A:1982-91.
- Melikyan EY, Shahin E, Miles J, Bainbridge LC. Extracorporeal shock-wave treatment for tennis elbow. A randomised double-blind study. J Bone Joint Surg 2003;85:852-5.
- Levitt R, Selemick H, Ogden J. Extracorporeal Shock Wave Therapy for Chronic Lateral Epicondylitis – An FDA Study n.d.
- Mehra A, Zaman T, Jenkin A. The use of a mobile lithotripter in the treatment of tennis elbow and plantar fasciitis. Surg J Royal Coll Surg Edin Ireland 2003;1:290-2.
- Crowther MA, Bannister GC, Huma H, Rooker GD. A prospective, randomised study to compare extracorporeal shock-wave therapy and injection of steroid for the treatment of tennis elbow. J Bone Joint Surg 2002;84:678-9. http://dx.doi.org/10.1302/0301-620X.84B5.12741.
- Rompe JD, Hopf C, Kullmer K, Heine J, Burger R, Nafe B. Low-energy extracorporal shock wave therapy for persistent tennis elbow. Int Orthopaed 1996;20:23-7. http://dx.doi.org/10.1007/s002640050021.
- Buchbinder R, Green S, Youd JM, Assendelft WJJ, Barnsley L, Smidt N. Shock wave therapy for lateral elbow pain. Cochrane Database Syst Rev 2005;1.
- Assendelft WJ, Hay EM, Adshead R, Bouter LM. Corticosteroid injections for lateral epicondylitis: a systematic overview. Br J Gen Prac 1996;46:209-16.
- Assendelft WJJ, Green SB, Struijs P, Smidt N. Tennis elbow (lateral epicondylitis). Clin Evidence 2003;9:1388-98.
- Gudmundsen J, Vikne J. [Laser treatment of epicondylitis humeri and rotator cuff syndrome. Double blind study – 200 patients.]. Nor Tidskr Idrettsmed 1987;2:6-15.
- Haker E, Lundeberg T. Laser treatment applied to acupuncture points in lateral humeral epicondylalgia. A double-blind study. Pain 1990;43:243-7. http://dx.doi.org/10.1016/0304-3959(90)91078-W.
- Haker E, Lundeberg T. Is low-energy laser treatment effective in lateral epicondylalgia?. J Pain Symptom Manag 1991;6:241-6. http://dx.doi.org/10.1016/0885-3924(91)90014-U.
- Haker E, Lundeberg T. Pulsed ultrasound treatment in lateral epicondylalgia. Scand J Rehabil Med 1991;23:115-18.
- Krasheninnikoff M, Ellitsgaard N, Rogvi-Hansen B, Zeuthen A, Harder K, Larsen R, et al. No effect of low power laser in lateral epicondylitis. Scand J Rheumatol 1994;23:260-3. http://dx.doi.org/10.3109/03009749409103726.
- Lundeberg T, Haker E, Thomas M. Effect of laser versus placebo in tennis elbow. Scand J Rehabil Med 1987;19:135-8.
- Papadopoulos ES, Smith RW, Cawley MI, Mani R. Low-level laser therapy does not aid the management of tennis elbow. Clin Rehabil 1996;10:9-11. http://dx.doi.org/10.1177/026921559601000103.
- Vasseljen O Jr, Hoeg N, Kjeldstad B, Johnsson A, Larsen S. Low level laser versus placebo in the treatment of tennis elbow. Scand J Rehabil Med 1992;24:37-42.
- Basford JR, Sheffield CG, Cieslak KR. Laser therapy: a randomized, controlled trial of the effects of low intensity Nd:YAG laser irradiation on lateral epicondylitis. Arch Phys Med Rehabil 2000;81:1504-10. http://dx.doi.org/10.1053/apmr.2000.17812.
- Vasseljen O. Low-level laser versus traditional physiotherapy in the treatment of tennis elbow. Physiotherapy 1992;78:329-34. http://dx.doi.org/10.1016/S0031-9406(10)61481-2.
- Lundeberg T, Abrahamsson P, Haker E. A comparative study of continuous ultrasound, placebo ultrasound, and rest in epicondylalgia. Scand J Rehabil Med 1988;20:99-101.
- Binder A, Hodge G, Greenwood AM, Hazleman BL, Page Thomas DP. Is therapeutic ultrasound effective in treating soft tissue lesions?. Br Med J 1985;290:512-14. http://dx.doi.org/10.1136/bmj.290.6467.512.
- Stratford PW, Levy DR, Gauldie S, Miseferi D, Levy K. The evaluation of phonophoresis and friction massage as treatments for extensor carpi radialis tendinitis: a randomized controlled trial. Physiother Can 1989;41:93-9.
- Pienimaki TT, Tarvainen TK, Siira PT, Vanharanta H. Progressive strengthening and stretching exercises and ultrasound for chronic lateral epicondylitis. Physiotherapy 1996;82:522-30. http://dx.doi.org/10.1016/S0031-9406(05)66275-X.
- Drechsler WI, Knarr JF, Snyder-Mackler L. A comparison of two treatment regimes for lateral epicondylitis: a randomized trial of clinical interventions. J Sport Rehabil 1997;6:226-34.
- Halle JS, Franklin RJ, Karalfa BL. Comparison of four treatment approaches for lateral epicondylitis of the elbow. J Orthop Sport Phys Med 1986;8:62-9. http://dx.doi.org/10.2519/jospt.1986.8.2.62.
- Pienimaki T, Karinen P, Kemila T, Koivukangas P, Vanharanta H. Long-term follow-up of conservatively treated chronic tennis elbow. A prospective and retrospective analysis. Scand J Rehab Med 1998;30:159-66. http://dx.doi.org/10.1080/003655098444093.
- Verhaar JA, Walenkamp GH, van Mameren H, Kester AD, van der Linden AJ. Local corticosteroid injection versus Cyriax-type physiotherapy for tennis elbow. J Bone Joint Surg 1996;1:128-32.
- Vicenzino B, Collins D, Wright A. The initial effects of a cervical spine manipulative physiotherapy treatment on the pain and dysfunction of lateral epicondylalgia. Pain 1996;68:69-74. http://dx.doi.org/10.1016/S0304-3959(96)03221-6.
- Newcomer KL, Laskowski GR, Idank DM, McLean TJ, Egan KS. Corticosteroid injection in early treatment of lateral epicondylitis. Clin J Sport Med 2001;11:214-22. http://dx.doi.org/10.1097/00042752-200110000-00002.
- Svernlov B, Adolfsson L. Non-operative treatment regime including eccentric training for lateral humeral epicondylalgia. Scand J Med Sci Sports 2001;11:328-34. http://dx.doi.org/10.1034/j.1600-0838.2001.110603.x.
- Tonks JH, Pai SK, Murali SR. Steroid injection therapy is the best conservative treatment for lateral epicondylitis: a prospective randomised controlled trial. Int J Clin Prac 2007;61:240-6. http://dx.doi.org/10.1111/j.1742-1241.2006.01140.x.
- Smidt N, van der Windt DA, Assendelft WJ, Devillé WL, Korthals-de Bos IB, Boulter LM. Corticosteroid injections, physiotherapy, or a wait-and-see policy for lateral epicondylitis: a randomised controlled trial. Lancet 2002;359:657-62. http://dx.doi.org/10.1016/S0140-6736(02)07811-X.
- Bisset LB, Beller E, Jull G, Brooks P, Darnell R, Vicenzino B. Mobilisation with movement and exercise, corticosteroid injection, or wait and see for tennis elbow: randomised trial. BMJ 2006;333. http://dx.doi.org/10.1136/bmj.38961.584653.AE.
- Lindenhovius A, Henket M, Gilligan BP, Lozano-Calderon S, Jupiter JB, Ring D. Injection of dexamethasone versus placebo for lateral elbow pain: a prospective, double-blind, randomized clinical trial. J Hand Surg 2008;33:909-19. http://dx.doi.org/10.1016/j.jhsa.2008.02.004.
- Price R, Sinclair H, Heinrich I, Gibson T. Local injection treatment of tennis elbow – hydrocortisone, triamcinolone and lignocaine compared. Br J Rheumatol 1991;30:39-44. http://dx.doi.org/10.1093/rheumatology/30.1.39.
- Peerbooms JC, Sluimer J, Bruijn DJ, Gosens T. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med 2010;38:255-62. http://dx.doi.org/10.1177/0363546509355445.
- Okcu G, Yercan H, Ozic U. The comparison of single dose versus multi-dose local corticosteroid injections for tennis elbow. Clin Res 2002;13:158-63.
- Uzunca K, Birtane M, Tastekin N. Effectiveness of pulsed electromagnetic field therapy in lateral epicondylitis. Clin Rheumatol 2007;26:69-74. http://dx.doi.org/10.1007/s10067-006-0247-9.
- Poltawski L, Johnson M, Watson T. Microcurrent therapy in the management of chronic tennis elbow: pilot studies to optimize parameters. Physiother Res Int 2012;17:157-66. http://dx.doi.org/10.1002/pri.526.
- Petrella RJ, Cogliano A, Decaria J, Mohamed N, Lee R. Management of tennis elbow with sodium hyaluronate periarticular injections. Sports Med Arthrosc Rehabil Ther Technol 2010;2. http://dx.doi.org/10.1186/1758-2555-2-4.
- Zeiseg E, Fahlstrom M, Ohberg L, Alfredson H. Pain relief after intratendinous injections in patients with tennis elbow: results of a RCT. Br J Sports Med 2008;42:267-71. http://dx.doi.org/10.1136/bjsm.2007.042762.
- Zeisig E, Fahlstrom M, Ohberg L, Alfredson H. Pain relief after intratendinous injections in patients with tennis elbow: results of a randomised study. Br J Sports Med 2008;42:267-71. http://dx.doi.org/10.1136/bjsm.2007.042762.
- Akermark C, Crone H, Elsasser U, Forsskahl B. Glycosaminoglycan polysulfate injections in lateral humeral epicondylalgia: a placebo-controlled double-blind trial. Int J Sports Med 1995;16:196-200. http://dx.doi.org/10.1055/s-2007-972991.
- Wong SM, Hui AC, Tong P-Y, Poon DWF, Yu E, Wong LKS. Treatment of lateral epicondylitis with botulinum toxin: a randomized, double-blind, placebo-controlled trial. Ann Intern Med 2005;143:793-7. http://dx.doi.org/10.7326/0003-4819-143-11-200512060-00007.
- Scarpone M, Rabago DP, Zgierska A, Arbogast G, Snell E. The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clin J Sport Med 2008;18:248-54. http://dx.doi.org/10.1097/JSM.0b013e318170fc87.
- Baskurt F, Ozcan A, Algun C. Comparison of effects of phonophoresis and iontophoresis of naproxen in the treatment of lateral epicondylitis. Clin Rehabil 2003;17:96-100. http://dx.doi.org/10.1191/0269215503cr588oa.
- Chan HL, Ng GY. Effect of counterforce forearm bracing on wrist extensor muscles performance. Am J Phys Med Rehabil 2003;82:290-5. http://dx.doi.org/10.1097/01.PHM.0000057223.04648.39.
- Langen-Pieters P, Weston P, Brantingham JW. A randomized, prospective pilot study comparing chiropractic care and ultrasound for the treatment of lateral epicondylitis. Eur J Chiroprac 2003;50:211-18.
- Nirschl RP, Rodin DM, Ochiai DH, Maartmann-Moe C. Iontophoretic administration of dexamethasone sodium phosphate for acute epicondylitis: a randomized, double-blinded, placebo-controlled study. Am J Sports Med 2003;31:189-95.
- Paoloni JA, Appleyard RC, Nelson J, Murrell GA. Topical nitric oxide application in the treatment of chronic extensor tendinosis at the elbow: a randomized, double-blinded, placebo-controlled clinical trial. Am J Sports Med 2003;6:915-20.
- Paungmali A, O’Leary S, Souvlis T, Vicenzino B. Hypoalgesic and sympathoexcitatory effects of mobilization with movement for lateral epicondylalgia. Phys Ther 2003;83:374-83.
- Selvanetti A, Barrucci A, Antonaci A, Martinez P, Marra S, Necozione S. Role of the eccentric exercise in the functional reeducation of lateral epicondylitis: a randomised controlled clinical trial. Med Sport 2003;56:103-13.
- Struijs PA, Damen PJ, Bakker EW, Blankevoort L, Assendelft WJ, van Dijk CN. Manipulation of the wrist for management of lateral epicondylitis: a randomized pilot study. Phys Ther 2003;7:608-16.
- Vicenzino B, Brooksbank J, Minto J, Offord S, Paungmali A. Initial effects of elbow taping on pain-free grip strength and pressure pain threshold. J Orthopaed Sports Phys Ther 2003;33:400-7. http://dx.doi.org/10.2519/jospt.2003.33.7.400.
- Struijs PA, Kerkhoffs GM, Assendelft WJ, Van Dijk CN. Conservative treatment of lateral epicondylitis: brace versus physical therapy or a combination of both-a randomized clinical trial. Am J Sports Med 2004;2:462-9. http://dx.doi.org/10.1177/0095399703258714.
- Cleland JA, Flynn TW, Palmer JA. Incorporation of manual therapy directed at the cervicothoracic spine in patients with lateral epicondylalgia: A pilot clinical trial. J Manu Manip Ther 2005;13:143-51. http://dx.doi.org/10.1179/106698105790824932.
- Spacca G, Cacchio A, Forgacs A, Monteforte P, Rovetta G. Analgesic efficacy of a lecithin-vehiculated diclofenac epolamine gel in shoulder periarthritis and lateral epicondylitis: a placebo-controlled, multicenter, randomized, double-blind clinical trial. Drugs Under Exp Clin Res 2005;31:147-54.
- Hayton MJ, Santini AJ, Hughes PJ, Frostick SP, Trail IA, Stanley JK. Botulinum toxin injection in the treatment of tennis elbow. A double-blind, randomized, controlled, pilot study. J Bone Joint Surg 2005;3:503-7.
- Lewis M, Hay EM, Elaine M, Paterson SM, Croft P. Local steroid injections for tennis elbow: does the pain get worse before it gets better? Results from a randomized controlled trial. Clin J Pain 2005;21:330-4. http://dx.doi.org/10.1097/01.ajp.0000125268.40304.b3.
- Martinez-Silvestrini JA, Newcomer KL, Gay RE, Schaefer MP, Kortebein P, Arendt KW. Chronic lateral epicondylitis: comparative effectiveness of a home exercise program including stretching alone versus stretching supplemented with eccentric or concentric strengthening. J Hand Ther 2005;18:411-19. http://dx.doi.org/10.1197/j.jht.2005.07.007.
- Faes M, van der Akker, Lint JA, Kooloos JG, Hopman MT. Dynamic extensor brace for lateral epicondylitis. Clin Orthopaed Relat Res n.d.:149-57.
- Stasinopoulos D, Stasinopoulos I. Comparison of effects of Cyriax physiotherapy, a supervised exercise programme and polarized polychromatic non-coherent light (Bioptron light) for the treatment of lateral epicondylitis. Clin Rehabil 2006;20:12-23. http://dx.doi.org/10.1191/0269215506cr921oa.
- D’Vaz AP, Ostor AJ, Speed CA, Jenner JR, Bradley M, Prevost AT, et al. Pulsed low-intensity ultrasound therapy for chronic lateral epicondylitis: a randomized controlled trial. Rheumatology 2006;5:566-70. http://dx.doi.org/10.1093/rheumatology/kei210.
- Lam LK, Cheing GL. Effects of 904-nm low-level laser therapy in the management of lateral epicondylitis: a randomized controlled trial. Photomed Laser Surg 2007;2:65-71. http://dx.doi.org/10.1089/pho.2006.2047.
- Placzek R, Drescher W, Deuretzbacher G, Hempfing A, Meiss AL. Treatment of chronic radial epicondylitis with botulinum toxin A. A double-blind, placebo-controlled, randomized multicenter study. J Bone Joint Surg 2007;89:255-60. http://dx.doi.org/10.2106/JBJS.F.00401.
- Vicenzino B, Paungmali A, Teys P. Mulligan’s mobilization-with-movement, positional faults and pain relief: current concepts from a critical review of literature. Man Ther 2007;12:98-108. http://dx.doi.org/10.1016/j.math.2006.07.012.
- Stergioulas A. Effects of low-level laser and plyometric exercises in the treatment of lateral epicondylitis. Photomed Laser Surg 2007;25:205-13. http://dx.doi.org/10.1089/pho.2007.2041.
- Luginbuhl R, Brunner F, Schneeberger AG. No effect of forearm band and extensor strengthening exercises for the treatment of tennis elbow: a prospective randomised study. Chir Organi Mov 2008;91:35-40. http://dx.doi.org/10.1007/s12306-007-0006-3.
- Oken OK, Ayhan F, Canpolat S, Yorgancioglu ZR, Oken OF. The short-term efficacy of laser, brace, and ultrasound treatment in lateral epicondylitis: a prospective, randomized, controlled trial. J Hand Ther 2008;21:63-7. http://dx.doi.org/10.1197/j.jht.2007.09.003.
- Staples MP, Forbes A, Ptasznik R, Gordon J, Buchbinder R. A randomized controlled trial of extracorporeal shock wave therapy for lateral epicondylitis (tennis elbow). J Rheumatol 2008;35:2038-46.
- Espandar R, Heidari P, Rasouli MR, Saadat S, Farzan M, Rostami M, et al. Use of anatomic measurement to guide injection of botulinum toxin for the management of chronic lateral epicondylitis: a randomized controlled trial. Can Med Assoc J 2010;182:768-73. http://dx.doi.org/10.1503/cmaj.090906.
- Nagrale AV, Herd CR, Ganvir S, Ramteke G. Cyriax physiotherapy versus phonophoresis with supervised exercise in subjects with lateral epicondylalgia: a randomized clinical trial. J Manual Manip Ther 2009;17:171-8. http://dx.doi.org/10.1179/jmt.2009.17.3.171.
- Park JY, Park HK, Choi JH, Moon ES, Kim BS, Kim WS, et al. Prospective evaluation of the effectiveness of a home-based program of isometric strengthening exercises: 12-month follow-up. Clin Orthop Surg 2010;2:173-8. http://dx.doi.org/10.4055/cios.2010.2.3.173.
- Tyler TF, Thomas GC, Nicholas SJ, McHugh MP. Addition of isolated wrist extensor eccentric exercise to standard treatment for chronic lateral epicondylosis: a prospective randomized trial. J Shoulder Elbow Surg 2010;19:917-22. http://dx.doi.org/10.1016/j.jse.2010.04.041.
- Viswas R, Ramachandran R, Anantkumar PK. Comparison of effectiveness of supervised exercise program and cyriax physiotherapy in patients with tennis elbow (lateral epicondylitis): a randomized clinical trial. Sci World J 2012;2012. http://dx.doi.org/10.1100/2012/939645.
- Stefanou A, Marshall N, Holdan W, Siddiqui A. A randomized study comparing corticosteroid injection to corticosteroid iontophoresis for lateral epicondylitis. J Hand Surg 2012;37:104-9. http://dx.doi.org/10.1016/j.jhsa.2011.10.005.
- Soderberg J, Grooten WJ, Ang BO. Effects of eccentric training on hand strength in subjects with lateral epicondylalgia: a randomized-controlled trial. Scand J Med Sci Sports 2012;22:797-803. http://dx.doi.org/10.1111/j.1600-0838.2011.01317.x.
- Skorupska E, Lisinski P, Samborski W. The effectiveness of the conservative versus myofascial pain physiotherapy in tennis elbow patients: double-blind randomized trial of 80 patients. J Musculoskel Pain 2012;20:41-50. http://dx.doi.org/10.3109/10582452.2011.635846.
- Omar AS, Ibrahim ME, Ahmed AS, Said M. Local injection of autologous platelet rich plasma and corticosteroid in treatment of lateral epicondylitis and plantar fasciitis: randomized clinical trial. Egypt Rheumatol 2012;34:43-9. http://dx.doi.org/10.1016/j.ejr.2011.12.001.
- Gunduz R, Malas FU, Fevziye U, Borman P, Kocaoglu S, Ozcakar L. Physical therapy, corticosteroid injection, and extracorporeal shock wave treatment in lateral epicondylitis. Clinical and ultrasonographical comparison. Clin Rheumatol 2012;31:807-12. http://dx.doi.org/10.1007/s10067-012-1939-y.
- Forogh B, Khalighi M, Javanshir MA, Ghoseiri K, Kamali M, Raissi G. The effects of a new designed forearm orthosis in treatment of lateral epicondylitis. Disabil Rehabil Assist Technol 2012;7:336-9. http://dx.doi.org/10.3109/17483107.2011.635330.
- Ajimsha MS, Chithra S, Thulasyammal RP. Effectiveness of myofascial release in the management of lateral epicondylitis in computer professionals. Arch Phys Med Rehabil 2012;93:604-9. http://dx.doi.org/10.1016/j.apmr.2011.10.012.
- Cherry E, Agostinucci J, McLinden J. The effect of cryotherapy and exercise on lateral epicondylitis: a controlled randomised study. Int J Ther Rehabil 2012;19:641-50. http://dx.doi.org/10.12968/ijtr.2012.19.11.641.
- Wolf JM, Ozer K, Scott F, Gordon MJ, Williams AE. Comparison of autologous blood, corticosteroid, and saline injection in the treatment of lateral epicondylitis: a prospective, randomized, controlled multicenter study. J Hand Surg 2011;8:1269-72. http://dx.doi.org/10.1016/j.jhsa.2011.05.014.
- Thanasas C, Papadimitriov G, Charalambidis C, Paraskevopoulos I, Papanikolaou A. Platelet-rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: a randomized controlled clinical trial. Am J Sports Med 2011;39:2130-4. http://dx.doi.org/10.1177/0363546511417113.
- Polat A, Ekinci O, Terzioglu B, Canbora MK, Muftuoglu T, Gorgec M. Treatment of lateral epicondylitis using betahistine dihydrochloride. J Musculoskel Pain 2011;19:201-6. http://dx.doi.org/10.3109/10582452.2011.609643.
- Gosens T, Peerbooms JC, van Laar W, den Oudsten BL. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. Am J Sports Med 2011;6:1200-8. http://dx.doi.org/10.1177/0363546510397173.
- Fernandez-Camero J, Cleland JA, Arbizu RL. Examination of motor and hypoalgesic effects of cervical vs. thoracic spine manipulation in patients with lateral epicondylalgia: a clinical trial. J Manip Physiol Therap 2011;34:432-40. http://dx.doi.org/10.1016/j.jmpt.2011.05.019.
- Creaney L, Wallace A, Curtis M, Connell D. Growth factor-based therapies provide additional benefit beyond physical therapy in resistant elbow tendinopathy: a prospective, double-blind, randomised trial of autologous blood injections versus platelet-rich plasma injections. Br J Sports Med 2011;45:966-71. http://dx.doi.org/10.1136/bjsm.2010.082503.
- Collins ED, Hildreth DH, Jafarnia KK. A clinical study of extracorporeal shock waves (ESW) for treatment of chronic lateral epicondylitis. Curr Orthopaed Pract 2011;22:185-92. http://dx.doi.org/10.1097/BCO.0b013e31820d830e.
- Blanchette MA, Normand MC. Augmented soft tissue mobilization vs natural history in the treatment of lateral epicondylitis: A pilot study. J Manip Physiol Therap 2011;34:123-30. http://dx.doi.org/10.1016/j.jmpt.2010.12.001.
- Bellapianta J, Swartz F, Lisella J, Czajka J, Neff R, Uhl R. Randomized prospective evaluation of injection techniques for the treatment of lateral epicondylitis. Orthopedics 2011;34:e708-12. http://dx.doi.org/10.3928/01477447-20110922-13.
- Backer M, Ludtke R, Afra D, Cesur O, Langhorst J, Fink M, et al. Effectiveness of leech therapy in chronic lateral epicondylitis: a randomized controlled trial. Clin J Pain 2011;27:442-7. http://dx.doi.org/10.1097/AJP.0b013e318208c95b.
- Ozturan KE, Yucel I, Cakici H, Guven M, Sungur I. Autologous blood and corticosteroid injection and extracorporeal shock wave therapy in the treatment of lateral epicondylitis. Orthopedics 2010;33:84-91. http://dx.doi.org/10.3928/01477447-20100104-09.
- Kazemi M, Azma K, Tavana B, Moghaddam FR, Panahi A. Autologous blood versus corticosteroid local injection in the short-term treatment of lateral elbow tendinopathy. Am J Phys Med Rehabil 2010;89:660-7. http://dx.doi.org/10.1097/PHM.0b013e3181ddcb31.
- Garg R, Adamson GJ, Dawson PA, Shankwiler JA, Pink MM. A prospective randomized study comparing a forearm strap brace versus a wrist splint for the treatment of lateral epicondylitis. J Shoulder Elbow Surg 2010;19:508-12. http://dx.doi.org/10.1016/j.jse.2009.12.015.
- Emanet SK, Altan LI, Yurtkuran M. Investigation of the effect of GaAs laser therapy on lateral epicondylitis. Photomed Laser Surg 2010;28:397-403. http://dx.doi.org/10.1089/pho.2009.2555.
- Akin C, Öken Ö, Koseoglu BF. Short-term effectiveness of ultrasound treatment in patients with lateral epicondylitis: randomized, single-blind, placebo-controlled, prospective study. Turk J Rheumatol 2010;25:50-5. http://dx.doi.org/10.5152/tjr.2010.01.
- Paoloni JA, Murrell GA, Burch RM, Ang RY. Randomised, double-blind, placebo-controlled clinical trial of a new topical glyceryl trinitrate patch for chronic lateral epicondylosis. Br J Sports Med 2009;43:299-302. http://dx.doi.org/10.1136/bjsm.2008.053108.
- McCallum SD, Paoloni JA, Murrell GAC. Five-year prospective comparison study of topical glyceryl trinitrate treatment of chronic lateral epicondylosis at the elbow. Br J Sports Med 2011;45:416-20. http://dx.doi.org/10.1136/bjsm.2009.061002.
- Jafarian FS, Demneh ES, Tyson SF. The immediate effect of orthotic management on grip strength of patients with lateral epicondylosis. J Orthopaed Sports Phys Ther 2009;39:484-9. http://dx.doi.org/10.2519/jospt.2009.2988.
- Dogramaci Y, Kalaci A, Sava N, Duman IG, Yanat AN. Treatment of lateral epicondylitis using three different local injection modalities: a randomized prospective clinical trial. Arch Orthopaed Trauma Surg 2009;10:1409-14. http://dx.doi.org/10.1007/s00402-009-0832-x.
- Coff L, Massy-Westropp N, Caragianis S. Randomized controlled trial of a new electrical modality (InterX) and soft tissue massage, stretching, ultrasound and exercise for treating lateral epicondylitis. Hand Ther 2009;14:46-52. http://dx.doi.org/10.1258/ht.2009.009008.
- Toker S, Kilinçoğlu V, Aksakalli E, Gulcan E, Ozkan K. [Short-term results of treatment of tennis elbow with anti-inflammatory drugs alone or in combination with local injection of a corticosteroid and anesthetic mixture. Acta Orthop Traumatol Turc 2008;42:184-7. http://dx.doi.org/10.3944/AOTT.2008.184.
- Sabeti M, Dorotka R, Goll A, Funovics PT, Schmidt M, Trieb K, et al. Focussed extracorporeal shockwave therapy for tennis elbow – a prospective, randomised, single blind pilot study trial. Phys Med Rehab Kuror 2008;18:83-6. http://dx.doi.org/10.1055/s-2007-991133.
- Radwan YA, ElSobhi G, Badawy WS, Reda A, Khalid S. Resistant tennis elbow: shock-wave therapy versus percutaneous tenotomy. Int Orthopaed 2008;32:671-7. http://dx.doi.org/10.1007/s00264-007-0379-9.
- Nourbakhsh MR, Fearon FJ. An alternative approach to treating lateral epicondylitis. A randomized, placebo-controlled, double-blinded study. Clin Rehabil 2008;22:601-9. http://dx.doi.org/10.1177/0269215507088447.
- Nourbakhsh MR, Fearon FJ. The effect of oscillating-energy manual therapy on lateral epicondylitis: a randomized, placebo-control, double-blinded study. J Hand Ther 2008;21:4-13. http://dx.doi.org/10.1197/j.jht.2007.09.005.
- Ho LOL, Kwong WL, Cheing GL. Effectiveness of microcurrent therapy in the management of lateral epicondylitis: a pilot study. Hong Kong Physiother J 2007;25:14-20. http://dx.doi.org/10.1016/S1013-7025(08)70004-6.
- Staples MP, Forbes A, Ptasznik R, Gordon J, Buchbinder R. A randomized controlled trial of extracorporeal shock wave therapy for lateral epicondylitis (tennis elbow). J Rheumatol 2008;35:2038-46.
- Korthals-de Bos IB, Smidt N, Tulder MW, Rutten-van Mölken MP, Adèr HJ, Windt DA, et al. Cost effectiveness of interventions for lateral epicondylitis: results from a randomised controlled trial in primary care. Pharmacoeconomics 2004;22:185-95. http://dx.doi.org/10.2165/00019053-200422030-00004.
- Evers S, Goossens M, de Vet H, van Tulder M, Ament A. Criteria list for assessment of methodological quality of economic evaluations: Consensus on Health Economic Criteria. Int J Technol Assess Health Care 2005;21:240-5. http://dx.doi.org/10.1136/bjsm.2006.026187.
- Struijs PAA, Korthals-de Bos, van Tulder MW, van Dijk CN, Bouter LM, Assendelft WJ. Cost effectiveness of brace, physiotherapy, or both for treatment of tennis elbow . . . Including commentary by Pluim BM. Br J Sports Med 2006;40:637-43.
- Peerbooms JCG, Poole C, Jorgensen E. The cost effectiveness of platelet rich plasma versus corticosteroids in the treatment of lateral epicondylitis. Value Health 2012;15.
- Haker E. Lateral Epicondylalgia (Tennis Elbow). A Diagnostic and Therapeutic Challenge. Stockholm: Karolinska Institutet; 1991.
- Sackett DL, Straus SE, Richardson WS, Rosenberg WM, Haynes RB. Evidence-Based Medicine: How to Practice and Teach EBM. Toronto: Churchill Livingstone; 2000.
Appendix 1 Literature search strategies
Search strategies: clinical effectiveness
MEDLINE
Host: Ovid.
Data parameters: 1946 to November week 3 2012.
Date searched: 4 January 2013.
Searcher: SB.
Hits: 285.
Search strategy
-
(tend?nopath* or paratend?nopath*).tw.
-
(tend?n?s?s or tend?nitis or p?r?ten???itis).tw.
-
tendinopathy/
-
bursitis.tw.
-
bursitis/
-
or/1-5
-
(elbow? or “common extensor origin”).tw.
-
elbow/
-
elbow joint/
-
or/7-9
-
6 and 10
-
(“lateral epicondylitis” or “medial epicondylitis” or “elbow pain?”).tw.
-
((tennis or golfer* or row* or shooter* or archer*) adj1 elbow?).tw.
-
tennis elbow/
-
or/11-14
-
(random* or “controlled trial?” or “clinical trial?” or rct?).tw.
-
Randomized controlled trial.pt.
-
(“systematic review?” or “meta-analys?s” or “meta analys?s” or metaanalys?s).tw.
-
meta-analysis.pt.
-
or/16-19
-
15 and 20
-
limit 21 to (english language and yr=“1990 -Current”)
MEDLINE In-Process and Other Non-Indexed Citations
Host: Ovid.
Data parameters: 3 January 2013.
Date searched: 4 January 2013.
Searcher: SB.
Hits: 15.
Search strategy
-
(tend?nopath* or paratend?nopath*).tw.
-
(tend?n?s?s or tend?nitis or p?r?ten???itis).tw.
-
bursitis.tw.
-
or/1-3
-
(elbow? or “common extensor origin”).tw.
-
4 and 5
-
(“lateral epicondylitis” or “medial epicondylitis” or “elbow pain?”).tw.
-
((tennis or golfer* or row* or shooter* or archer*) adj1 elbow?).tw.
-
or/6-8
-
(random* or “controlled trial?” or “clinical trial?” or rct?).tw.
-
(“systematic review?” or “meta-analys?s” or “meta analys?s” or metaanalys?s).tw.
-
10 or 11
-
9 and 12
-
limit 13 to english language
EMBASE
Host: Ovid.
Data parameters: 1980 to 2013 week 1.
Date searched: 7 January 2013.
Searcher: SB.
Hits: 361.
Search strategy
-
(tend?nopath* or paratend?nopath*).tw.
-
(tend?n?s?s or tend?nitis or p?r?ten???itis).tw.
-
tendinitis/
-
tendon injury/
-
bursitis.tw.
-
bursitis/
-
or/1-6
-
(elbow? or “common extensor origin”).tw.
-
elbow/
-
elbow joint/
-
or/8-10
-
7 and 11
-
(“lateral epicondylitis” or “medial epicondylitis” or “elbow pain?”).tw.
-
epicondylitis/
-
((tennis or golfer* or row* or shooter* or archer*) adj1 elbow?).tw.
-
tennis elbow/
-
or/13-16
-
(random* or “controlled trial?” or “clinical trial?” or rct?).tw.
-
(“systematic review?” or “meta-analys?s” or “meta analys?s” or metaanalys?s).tw.
-
18 or 19
-
17 and 20
-
limit 21 to (english language and yr=“1990 -Current”)
Allied and Complementary Medicine Database (AMED)
Host: Ovid.
Data parameters: 1985 to December 2012.
Date searched: 8 January 2013.
Searcher: SB.
Hits: 72.
Search strategy
-
(tend?nopath* or paratend?nopath*).tw.
-
(tend?n?s?s or tend?nitis or p?r?ten???itis).tw.
-
tendinopathy/
-
bursitis.tw.
-
bursitis/
-
or/1-5
-
(elbow? or “common extensor origin”).tw.
-
elbow/
-
elbow joint/
-
or/7-9
-
6 and 10
-
(“lateral epicondylitis” or “medial epicondylitis” or “elbow pain?”).tw.
-
((tennis or golfer* or row* or shooter* or archer*) adj1 elbow?).tw.
-
tennis elbow/
-
or/11-14
-
(random* or “controlled trial?” or “clinical trial?” or rct?).tw.
-
Randomized controlled trial.pt.
-
(“systematic review?” or “meta-analys?s” or “meta analys?s” or metaanalys?s).tw.
-
meta analysis.pt.
-
or/16-19
-
15 and 20
-
limit 21 to (english language and yr=“1990 -current”)
Cumulative Index to Nursing and Allied Health Literature (CINAHL)
Host: EBSCOhost.
Data parameters: not applicable.
Date searched: 4 January 2013.
Searcher: SB.
Hits: 535.
Search strategy
-
TI (tend?nopath* OR paratend?nopath*) OR AB (tend?nopath* OR paratend?nopath*)
-
TI (tend?n?s?s or tend?nitis or p?r?ten???itis or p?r?ten??itis) OR AB (tend?n?s?s or tend?nitis or p?r?ten???itis or p?r?ten??itis)
-
(MH “Tendinopathy”)
-
TI (bursitis) OR AB (bursitis)
-
(MH “Bursitis”)
-
S1 OR S2 OR S3 OR S4 OR S5
-
TI (elbow* OR “common extensor origin”) OR AB (elbow* OR “common extensor origin”)
-
(MH “Elbow”)
-
(MH “Elbow Joint”)
-
(MH “Elbow Pain”)
-
S7 OR S8 OR S9 OR S10
-
S6 AND S11
-
TI (“lateral epicondylitis” OR “medial epicondylitis” OR “elbow pain*”) OR AB (“lateral epicondylitis” OR “medial epicondylitis” OR “elbow pain*”)
-
TI ((tennis OR golfer* OR row* OR shooter* OR archer*) N1 elbow*) OR AB ((tennis OR golfer* OR row* OR shooter* OR archer*) N1 elbow*)
-
(MH “Tennis Elbow”)
-
S11 OR S12 OR S13 OR S14 OR S15
-
TI (random* or “controlled trial*” or “clinical trial*” or rct*) OR AB (random* or “controlled trial*” or “clinical trial*” or rct*)
-
PT randomized controlled trial
-
TI (“systematic review*” or “meta-analys?s” or “meta analys?s” or metaanalys?s) OR AB (“systematic review*” or “meta-analys?s” or “meta analys?s” or metaanalys?s)
-
PT systematic review
-
S17 OR S18 OR S19 OR S20
-
S16 AND S21
-
S16 AND S21 Limiters - Published Date from: 19900101-20121231; English Language
Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Database of Abstracts of Reviews of Effects, Health Technology Assessment (CDSR, CENTRAL, DARE and HTA)
Host: the Cochrane Collaboration.
Data parameters: Cochrane Database of Systematic Reviews (CDSR) and CENTRAL: Issue 12 of 12, December 2012; DARE and HTA: Issue 4 of 4, October 2012.
Date searched: 4 January 2013.
Searcher: SB.
Hits: CDSR = 9; CENTRAL = 188; DARE = 20; HTA = 0.
Search strategy
-
(tend?nopath* or paratend?nopath*):ti or (tend?nopath* or paratend?nopath*):ab
-
(tend?n?s?s or tend?nitis or p?r?ten???itis):ti or (tend?n?s?s or tend?nitis or p?r?ten???itis):ab
-
MeSH descriptor: [Tendinopathy] this term only
-
bursitis:ti or bursitis:ab
-
MeSH descriptor: [Bursitis] this term only
-
#1 or #2 or #3 or #4 or #5
-
(elbow* or “common extensor origin”):ti or (elbow* or “common extensor origin”):ab
-
MeSH descriptor: [Elbow] this term only
-
MeSH descriptor: [Elbow Joint] this term only
-
MeSH descriptor: [Elbow Joint] this term only
-
#7 or #8 or #9 or #10
-
#6 and #11
-
(“lateral epicondylitis” or “medial epicondylitis” or “elbow pain*”):ti or (“lateral epicondylitis” or “medial epicondylitis” or “elbow pain*”):ab
-
((tennis or golfer* or row* or shooter* or archer*) near/1 elbow*):ti or ((tennis or golfer* or row* or shooter* or archer*) near/1 elbow*):ab
-
MeSH descriptor: [Tennis Elbow] this term only
-
#12 or #13 or #14 or #15
-
(random* or “controlled trial*” or “clinical trial*” or rct*):ti or (random* or “controlled trial*” or “clinical trial*” or rct*):ab
-
(“systematic review*” or “meta-analys?s” or “meta analys?s” or metaanalys?s):ti or (“systematic review*” or “meta-analys?s” or “meta analys?s” or metaanalys?s): #17 or #18
-
#16 and #19 from 1990, in Cochrane Reviews (Reviews and Protocols), Other Reviews, Trials and Technology Assessments
Web of Science (Scientific Citation Index Expanded, Social Sciences Citation Index, Arts and Humanities Citation Index, Conference Proceedings Citation Index – Science, Conference Proceedings Citation Index – Social Science and Humanities)
Host: Thomson Reuters.
Data parameters: not applicable.
Date searched: 4 January 2013.
Searcher: SB.
Hits: 440.
Search strategy
-
Topic=(tend?nopath* or paratend?nopath*) OR Topic=(tend?n?s?s or tend?nitis or p?r?ten???itis or p?r?ten??itis) OR Topic=(bursitis)
-
Lemmatization=Off
-
-
Topic=(elbow* or “common extensor origin”)
-
Lemmatization=Off
-
-
#1 AND #2
-
Lemmatization=Off
-
-
Topic=(“lateral epicondylitis” or “medial epicondylitis” or “elbow pain*”) OR Topic=((tennis or golfer* or row* or shooter* or archer*) near/1 elbow*)
-
Lemmatization=Off
-
-
#3 OR #4
-
Lemmatization=Off
-
-
Topic=(random* or “controlled trial*” or “clinical trial*” or rct*) OR Topic=(“systematic review*” or “meta-analys?s” or “meta analys?s” or metaanalys?s)
-
Lemmatization=Off
-
-
#5 AND #6
-
Lemmatization=Off
Physiotherapy Evidence Database (PEDro)
Host: Centre for Evidence-Based Physiotherapy at the George Institute for Global Health.
Data parameters: not applicable.
Date searched: 7 January 2013.
Searcher: SB.
Hits: 39.
Search strategy
Select ‘Advanced Search’
Abstract and title: elbow
Problem: pain
Published since: 1990
Combine search fields using AND
Notes: search includes cost-effectiveness studies
ClinicalTrials.gov
Host: US National Institutes of Health.
Data parameters: not applicable.
Date searched: 8 January 2013.
Searcher: SB.
Hits: 49.
Search strategy
(elbow AND (tennis OR tendinopathy OR tendonopathy OR tendinitis OR tendonitis OR tendinosis OR tendonosis OR bursitis)) OR “lateral epicondylitis” OR “medial epicondylitis”
Note that search includes cost-effectiveness studies.
Numbers of references retrieved
| Database | Hits |
|---|---|
| MEDLINE | 285 |
| MEDLINE In-Process & Other Non-Indexed Citations | 15 |
| EMBASE | 361 |
| AMED | 72 |
| CINAHL | 535 |
| CDSR | 9 |
| CENTRAL | 20 |
| DARE | 188 |
| HTA | 0 |
| Web of Science | 440 |
| PEDro | 39 |
| Clinical trials.gov | 49 |
| Total | 2013 |
| Duplicates | 896 |
| Total records to screen | 1117 |
| Total records in EndNote filea | 1029 |
Search strategies: cost-effectiveness
Database: MEDLINE
Host: Ovid.
Data parameters: 1946 to November week 3 2012.
Date searched: 7 January 2013.
Searcher: SB.
Hits: 48.
Search strategy
Strategy as MEDLINE above with costs filter below from line 16:
-
exp “Costs and Cost Analysis”/
-
exp Economics/
-
exp models, economic/
-
(pharmacoeconomic* or economic* or price* or cost* or cba or cea or cua or “health utilit*”).tw.
-
ec.fs.
-
or/16-20
-
15 and 21
-
limit 22 to (english language and yr=“1990 -Current”)
MEDLINE In-Process & Other Non-Indexed Citations
Host: Ovid.
Data parameters: 4 January 2013.
Date searched: 7 January 2013.
Searcher: SB.
Hits: 3.
Search strategy
Strategy as MEDLINE In-Process & Other Non-Indexed Citations above with costs filter below from line 10:
-
(pharmacoeconomic* or economic* or price* or cost* or cba or cea or cua or “health utilit*”).tw.
-
9 and 10
-
limit 12 to english language
EMBASE
Host: Ovid.
Data parameters: 1980 to 2013 week 1.
Date searched: 8 January 2013.
Searcher: SB.
Hits: 92.
Search strategy
-
Strategy as EMBASE above with costs filter below from line 16:
-
exp “Costs and Cost Analysis”/
-
exp Economics/
-
models, economic/
-
exp health economics/
-
(pharmacoeconomic* or economic* or price* or cost* or cba or cea or cua or “health utilit*”).tw.
-
pe.fs.
-
or/16-21
-
15 and 22
-
limit 23 to (english language and yr=“1990 -Current”)
Allied and Complementary Medicine Database (AMED)
Host: Ovid.
Data parameters: 1985 to December 2012.
Date searched: 8 January 2013.
Searcher: SB.
Hits: 3.
Search strategy
Strategy as AMED above with costs filter below from line 15:
-
exp Economics/
-
(pharmacoeconomic* or economic* or price* or cost* or cba or cea or cua or “health utilit*”).tw.
-
16 or 17
-
15 and 18
Cumulative Index to Nursing and Allied Health Literature (CINAHL)
Host: EBSCOhost.
Data parameters: not applicable.
Date searched: 7 January 2013.
Searcher: SB.
Hits: 75.
Search strategy
Strategy as CINAHL above with costs filter below from line 17:
-
MH “Costs and Cost Analysis+”
-
MH “Fees and Charges+”
-
MH “Resource Allocation+”
-
MH “Economics, Pharmaceutical”
-
TI (pharmacoeconomic* or economic* or price* or cost* or cba or cea or cua or “health utilit*”) OR AB (pharmacoeconomic* or economic* or price* or cost* or cba or cea or cua or “health utilit*”)
-
S17 OR S18 OR S19 OR S20 OR S21
-
S16 AND S22 Limiters - Published Date from: 19900101-20121231; English Language
Cochrane (Cochrane Database of Systematic Reviews, Cochrane Central Register of Controlled Trials, Database of Abstracts of Reviews of Effects, Health Technology Assessment and NHS Economic Evaluation Database)
Host: the Cochrane Collaboration.
Data parameters: CDSR and CENTRAL: Issue 12 of 12, December 2012; DARE, HTA and NHS EED: Issue 4 of 4, October 2012.
Date searched: 7 January 2013.
Searcher: SB.
Hits: CDSR = 0; CENTRAL = 10; DARE = 0; HTA = 0; and NHS EED = 2.
Search strategy
Strategy as Cochrane above with costs filter below from line 17:
-
MeSH descriptor: [Economics] explode all trees
-
MeSH descriptor: [Models, Economic] 4 tree(s) exploded
-
(pharmacoeconomic* or economic* or price* or cost* or cba or cea or cua or “health utilit*”):ti or (pharmacoeconomic* or economic* or price* or cost* or cba or cea or cua or “health utilit*”):ab from 1990, in Cochrane Reviews (Reviews and Protocols), Other Reviews, Trials, Technology Assessments and Economic Evaluations
-
#17 or #18 or #19
-
#16 and #20
Web of Science (Scientific Citation Index Expanded, Social Sciences Citation Index, Arts and Humanities Citation Index, Conference Proceedings Citation Index – Science, Conference Proceedings Citation Index – Social Science and Humanities)
Host: Thomson Reuters.
Data parameters: not applicable.
Date searched: 7 January 2013.
Searcher: SB.
Hits: 42.
Search strategy
Strategy as Web of Science above with costs filter below on line 6:
-
TS=(pharmacoeconomic* or economic* or price* or cost* or cba or cea or cua or “health utilit*”)
Numbers of references retrieved
| Database | Hits |
|---|---|
| MEDLINE | 48 |
| MEDLINE In-Process & Other Non-Indexed Citations | 3 |
| EMBASE | 92 |
| AMED | 3 |
| CINAHL | 75 |
| CDSR | 0 |
| CENTRAL | 10 |
| DARE | 0 |
| HTA | 0 |
| NHS EED | 2 |
| Web of Science | 42 |
| Total | 275 |
| Duplicates | 91 |
| Total records to screen | 184 |
Appendix 2 Clinical effectiveness excluded studies
| Papers excluded | Reason for exclusion |
|---|---|
| de Vos RJ, van Veldhoven PL, Moen MH, Weir A, Tol JL, Maffulli N. Autologous growth factor injections in chronic tendinopathy: a systematic review. Br Med Bull 2010;95:63–77 | Population |
| Weitoft T, Forsberg C. Importance of immobilization after intraarticular glucocorticoid treatment for elbow synovitis: a randomized controlled study. Arthrit Care Res 2010;62:735–7 | Population |
| Ellis RF, Hing WA. Neural mobilization: a systematic review of randomized controlled trials with an analysis of therapeutic efficacy. J Manual Manip Ther 2008;16:8–22 | Population |
| Bohr PC. Systematic review and analysis of work-related injuries to and conditions of the elbow. Am J Occup Ther 2011;65:24–8 | Population |
| Jabbari B, Machado D. Treatment of refractory pain with botulinum toxins – an evidence-based review. Pain Med 2011;12:1594–606 | Population |
| Malliaras P, Maffulli N, Garau G. Eccentric training programmes in the management of lateral elbow tendinopathy. Disabil Rehabil 2008;30:1590–6 | Intervention |
| Genc H, Nacir B, Duyur Cakit B, Saracoglu M, Erdem HR. The effects of coexisting fibromyalgia syndrome on pain intensity, disability, and treatment outcome in patients with chronic lateral epicondylitis. Pain Med 2012;13:270–80 | Outcome |
| Massey T, Derry S, Moore RA, McQuay HJ. Topical NSAIDs for acute pain in adults. Cochrane Database Syst Rev 2010;6:CD007402 | Outcome |
| Ernst E, Lee MS, Myeong S. Acupuncture for rheumatic conditions: an overview of systematic reviews. Rheumatology 2010;49:1957–61 | Study design |
| Kazeami M, Azma K, Tavana B, Moghaddam FR, Panahi A. Autologous blood versus corticosteroid local injection in the short-term treatment of lateral elbow tendinopathy: a randomized clinical trial of efficacy. Am J Phys Med Rehabil 2010; 89:660–7 | Study design |
| Ott OJ, Hertel S, Gaipl US, Frey B, Schmidt M, Fietkau R. Benign painful elbow syndrome First results of a single center prospective randomized radiotherapy dose optimization trial. Strahlenther Onkol 2012;188:873–7 | Study design |
| McHardy A, Hoskins W, Pollard H, Onley R, Windsham R. Chiropractic Treatment of Upper Extremity Conditions: A Systematic Review. J Manip Physiol Therap 2008;31:146–59 | Study design |
| Radpasand M, Owens E. Combined multimodal therapies for chronic tennis elbow: pilot study to test protocols for a randomized clinical trial. J Manip Physiol Therap 2009;32:571–85. [Erratum published in J Manipulative Physiol Ther 2009;32:701] | Study design |
| Stasinopoulos D, Stasinopoulos I, Pantelis M, Stasinopoulou K. Comparison of effects of a home exercise programme and a supervised exercise programme for the management of lateral elbow tendinopathy. Br J Sports Med 2010;44:579–83 | Study design |
| Bisset L, Smidt N, Van der Windt DA, Bouter LM, Jull G, et al. Conservative treatments for tennis elbow do subgroups of patients respond differently? Rheumatology 2007;46:1601–5 | Study design |
| Hart L. Corticosteroid and other injections in the management of tendinopathies: a review. Clin J Sport Med 2011;21:540–1 | Study design |
| Maffulli N, Longo UG, Loppini M, Denaro V. Current treatment options for tendinopathy. Exp Opin Pharmacother 2010;11:2177–86 | Study design |
| Raman J, MacDermid JC, Grewal R. Effectiveness of different methods of strengthening exercises in lateral epicondylosis: a systematic review. J Hand Ther 2011;24:388–9 | Study design |
| Rabago D, Ryan M, Lee K, Chourasia A, Sesto M, Zgierska A, et al. The efficacy of prolotherapy using dextrose-morrhuate for lateral epicondylosis: A pilot randomized controlled trial. BMC Complement Alt Med (International Research Congress on Integrative Medicine and Health 2012 Portland, OR, USA) | Study design |
| Fernandez-Camero J, Fernández-de-las-Peñas C, Cleland JA. Immediate hypoalgesic and motor effects after a single cervical spine manipulation in subjects with lateral epiconylalgia. J Manip Physiol Therap 2008;31:675–81 | Study design |
| Galvin R, Callaghan C, Chan WS, Dimitrov BD, Fahey T. Injection of botulinum toxin for treatment of chronic lateral epicondylitis: systematic review and meta-analysis. Semin Arthrit Rheum 2011;40:585–7 | Study design |
| Torro J, Brunetti L, Patel MK. Iontophoretic administration of dexamethasone for musculoskeletal pain. J Musculoskel Med 2011;28:410–21 | Study design |
| Posadzki P. Is spinal manipulation effective for pain? An overview of systematic reviews. Pain Med 2012;13:754–61 | Study design |
| Scher DL, Wolf JM, Owens BD. Lateral epicondylitis. Orthopedics 2009;32:276–82 | Study design |
| Orchard J, Kountouris A. The management of tennis elbow. BMJ 2011;342:1199–202 | Study design |
| Fulop AM, Dhimmer S, Deluca JR, Johanson DD, Lenz RV, Patel KB, et al. A meta-analysis of the efficacy of laser phototherapy on pain relief. Clin J Pain 2010;26:729–36 | Study design |
| Scudeller L, Del Fante C, Perotti C, Pavesi CF, Coscia D, Scotti V, et al. N of 1, two contemporary arm, randomised controlled clinical trial for bilateral epicondylitis: a new study design. BMJ 2011;343:d7653 | Study design |
| Olaussen M, Holmedal Ø, Lindbæk M, Brage S. Physiotherapy alone or in combination with corticosteroid injection for acute lateral epicondylitis in general practice: a protocol for a randomised, placebo-controlled study. BMC Musculoskel Disord 2009;10:152 | Study design |
| Bokhari AR, Murrell GAC. The role of nitric oxide in tendon healing. J Shoulder Elbow Surg 2012;21:238–44 | Study design |
| Unlu Z, Tarhan S, Ovali GY, Pabuscu Y. Sonographic-guided injection of corticosteroid in the treatment of lateral epicondylitis. J Musculoskel Pain 2009;17:48–58 | Study design |
| Szabo RM. Steroid Injection for lateral epicondylitis. J Hand Surg 2009;34A:326–30 | Study design |
| Chesterton LS, van der Windt DA, Sim J, Lewis M, Mallen CD, Mason EE, et al. Transcutaneous electrical nerve stimulation for the management of tennis elbow: a pragmatic randomized controlled trial: the TATE trial (ISRCTN 87141084). BMC Musculoskel Disord 2009;10:156 | Study design |
| Krogh T, Fredberg U, Stengaard-Pedersen K, Jensen P, Christensen R, Ellingsen T. Treatment of lateral epicondylitis with injection of platelet-rich plasma or corticosteroid versus saline: a randomized, double-blind, placebo-controlled trial. Arthrit Rheum 2012;64:S415–16 | Study design |
| Yim ES, Corrado G. Gianmichael. Ultrasound in sports medicine: relevance of emerging techniques to clinical care of athletes. Sports Med 2012;42:665–80 | Study design |
| Radpasand M, Owens E. Combined multimodal therapies for chronic tennis elbow: pilot study to test protocols for a randomized clinical trial. J Manip Physiol Therap 2009;32:571–85 | No usable data |
| Callaghan C, Galvin R, Chan W-S, Dimitrov BD, Fahey T. The effectiveness of botulinum toxin injection in the management of lateral epicondylitis: a systematic review. Irish Society of Chartered Physiotherapists (ISCP) Conference 2010. Physiother Ir 2011;32:33–4 | No usable data |
| Huang D, Gu Y-H, Liao Q, Yan X-B, Zhu S-H, Gao C-Q. Effects of linear-polarized near-infrared light irradiation on chronic pain. Sci World J 2012;2012:567496 | No usable data |
| Oken O, Kahraman Y, Ayhan F, Canpolat S, Yorgancioglu ZR, Oken OF. The short-term efficacy of laser, brace, and ultrasound treatment in lateral epicondylitis: a prospective, randomized, controlled trial. J Hand Ther 2008;21:63–8. [Erratum published in J Hand Ther 2008;21:303] | No usable data |
| Goldman RH, Stason WB, Park SK, Kim R, Mudgal S, Davis RB, et al. Low-dose amitriptyline for treatment of persistent arm pain due to repetitive use. Pain 2010;149:117–23 | No usable data |
| Dick FD, Graveling RA, Munro W, Walker-Bone K. Workplace management of upper limb disorders: a systematic review. Occup Med 2011;61:19–25 | No usable data |
| Clijsen R, Taeymans J, Baeyens JP, Barel AO, Clarys P. The effects of iontophoresis in the treatment of musculoskeletal disorders – a systematic review and meta-analysis. Drug Deliv Lett 2012;2:180–94 | No usable data |
| Hoksrud AF, Bahr R. Injectable agents derived from or targeting vascularity: has clinical acceptance in managing tendon disorders superseded scientific evidence? J Musculoskel Neuronal Interact 2011;11:174–84 | No usable data |
| Im SH. Effects of an Autologous Platelet-Rich Plasma (PRP) and Electrical Shock Wave Therapy (ESWT) in lateral epicondylitis. Double-blind randomized controlled trial. Am Acad Phys Med Rehabil 2012;4:S271–2. (Annual Assembly Atlanta: Atlanta, GA, USA) | No usable data (conference abstract) |
| Creuze A, Petit H, De Seze M. Efficacy of botulinum A toxin injections for epicondylitis unresponsive to medical treatment: 38 cases. Ann Phys Rehabil 2010;53:e100. (25e Congres de Medecine Physique et de Readaptation. Marseille, France) | No usable data (conference abstract) |
| Ferrero G, Orlandi D, Fabbro E, Sconfienza LM, Silvestri E. One-year survey of two different ultrasound (US)-guided percutaneous treatments of lateral epicondylitis: Results of a randomised controlled trial. Cardiovascular and Interventional Radiology. Conference: Cardiovascular and Interventional Radiological Society of Europe, (CIRSE) Munich, Germany; 2011 | No usable data (conference abstract) |
| Petrella RJ, Decaria J, Petrella M. Randomized, double-blind control trial of peri-articular hyaluronic acid: botulinus toxin injection in lateral epicondylosis. Osteoarthritis Research Society International World Congress (OARSI): Barcelona, Spain; 2012 | No usable data (conference abstract) |
| Kirillova EK, Khabirov R. Treatment of epicondylitis of the elbow joint with chondroprotectors. Scandinavian Journal of Rheumatology 2012;41:S126 [abstracts of the 34th Scandinavian Congress of Rheumatology (51PP31), Copenhagen Denmark Conference] | No usable data (conference abstract) |
| Bovaira MT, Calvo A, Jimenez A, Palacios L, Lopez A, March R. Treatment of lateral epicondylitis with pulsed radiofrequency. Comparative study between two different procedures. 29th Annual European Society of Regional Anaesthesia, ESRA Congress 2010 Porto Portugal. Conference; 2010 | No usable data (conference abstract) |
| Gaujoux-Viala CG, Dougados, M. Efficacy and safety of steroid injections for shoulder and elbow tendonitis: A meta-analysis of randomized controlled trials. Arthrit Rheum 2008;58:S390 | Duplicate data |
| Oken O, Kahraman Y, Ayhan F, Canpolat S, Yorgancioglu ZR, Oken OF. The short-term efficacy of laser, brace, and ultrasound treatment in lateral epicondylitis: a prospective, randomized, controlled trial. J Hand Ther 2008;21: 63–8. [Erratum published in J Hand Ther 2008;21: 303] | Duplicate of erratum (record #417) |
| Olmez N, Memis A. [Evidence based data for management of lateral epicondylitis: review]. Turk Klin Tip Bilim 2010;30:303–11 | Language |
| Schüller BK, Neugebauer EA. [Evidence for laser acupuncture in cases of orthopedic diseases. A systematic review]. Schmerz 2008;22:9–15 | Language |
| Venditto T, Tognolo L, Lucrezia, Saracino F, Pagnotta L, Santilli V. [Repetitive low-energy shock wave therapy for chronic lateral epicondylitis]. Sci Riabil 2012;14:14–21 | Language |
| Barr S, Cerisola FL, Blanchard V. Effectiveness of corticosteroid injections compared with physiotherapeutic interventions for lateral epicondylitis: a systematic review. Physiotherapy 2009;95:251–65 | Not obtainable |
| Okcu G, Erkan S, Entürk M, Ozalp RT, Yercan HS. Evaluation of injection techniques in the treatment of lateral epicondylitis: A prospective randomized clinical trial. Acta Orthop Traumatol Turc 2012;46:26–9 | Not obtainable |
| Redler LH, Thompson SA, Hsu SH, Ahmad CS, Levine WN. Platelet-rich plasma therapy: a systematic literature review and evidence for clinical use. Phys Sportsmed 2011;39:42–51 | Not obtainable |
| Struijs P, Smidt N, Arola H, van Dijk CN, Buchbinder R, Assendelft WJ. Orthotic devices for tennis elbow: a systematic review. Br J Gen Prac 2001;51:924–9 | Publication date pre-2003 (cut-off for inclusion in review) |
| Smidt N, Assendelft WJ, van der Windt DA, Hay EM, Buchbinder R, Bouter LM. Corticosteroid injections for lateral epicondylitis: a systematic review. Pain 2002;96:23–40 | Publication date pre-2003 (cut-off for inclusion in review) |
| Tyler TF, Thomas GC, Nicholas SJ, McHugh MP. Addition of isolated wrist extensor eccentric exercise to standard treatment for chronic lateral epicondylosis: a prospective randomized trial. J Shoulder Elbow Surg 2010;19:917–22 | Included in an included SR |
| Lin YC, Tu YK, Chen S, Lin I, Chen S, Guo HR. Comparison between botulinum toxin and corticosteroid injection in the treatment of acute and subacute tennis elbow: a prospective, randomized, double-blind, active drug-controlled pilot study. Am J Phys Med Rehabil 2010;89:653–9 | Included in an included SR |
| Nagrale AV, Herd CR, Ganvir S, Ramteke G. Cyriax physiotherapy versus phonophoresis with supervised exercise in subjects with lateral epicondylalgia: a randomized clinical trial. J Manual Manip Ther 2009;17:171–8 | Included in an included SR |
| Uzunca K, Birtane M, Taştekin N. Effectiveness of pulsed electromagnetic field therapy in lateral epicondylitis. Clin Rheumatol 2007;1:69–74 | Included in an included SR |
| Lam LK, Cheing JL. Effects of 904-nm low-level laser therapy in the management of lateral epicondylitis: a randomized controlled trial. Photomed Laser Surg 2007;2:65–71 | Included in an included SR |
| Scarpone M, Rabago DP, Zgierska A, Arbogast G, Snell E. The efficacy of prolotherapy for lateral epicondylosis: a pilot study. Clin J Sport Med 2008;18:248–54 | Included in an included SR |
| Lindenhovius A, Henket M, Gilligan BP, Lozano-Calderon S, Jupiter JB, Ring D. Injection of dexamethasone versus placebo for lateral elbow pain: a prospective, double-blind, randomized clinical trial. J Hand Surg 2008;33:909–19 | Included in an included SR |
| Luginbuhl R, Brunner F, Schneeberger AG. No effect of forearm band and extensor strengthening exercises for the treatment of tennis elbow: a prospective randomised study. Chir Organi Mov 2008;91:35–40 | Included in an included SR |
| Zeisig E, Fahlström M, Ohberg L, Alfredson H. Pain relief after intratendinous injections in patients with tennis elbow: results of a randomised study. Br J Sports Med 2008;42:267–71 | Included in an included SR |
| Oken O, Kahraman Y, Ayhan F, Canpolat S, Yorgancioglu ZR, Oken OF. The short-term efficacy of laser, brace and ultrasound treatment in lateral epicondylitis: A prospective, randomised, controlled trial. J Hand Ther 2008;21:63–7 | Included in an included SR |
| Park J-Y, Park H-K, Choi J-H, Moon E-S, Kim B-S, Kim W-S, et al. Prospective evaluation of the effectiveness of a home-based program of isometric strengthening exercises: 12-month follow-up. Clin Orthoped Surg 2010;2:173–8 | Included in an included SR |
| Peerbooms J, Sluimer J, Bruijn DJ, Gosens T. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med 2010;38:255–62 | Included in an included SR |
| Petrella R, Cogliano A, Decaria J, Mohamed N, Lee R. Management of tennis elbow with sodium hyaluronate periarticular injections. Sports Med Arthros Rehabil Ther Technol 2010;2:4 | Included in an included SR |
| Stergioulas A. Effects of low-level laser and plyometric exercises in the treatment of lateral epicondylitis. Photomed Laser Surg 2007;25:205–13 | Included in an included SR |
| Staples M, Forbes A, Ptasznik R, Gordon J, Buchbinder R. A randomized controlled trial of extracorporeal shock wave therapy for lateral epicondylitis (tennis elbow). J Rheumatol 2008;35:2038–46 | Included in an included SR |
| Tonks JH, Pai SK, Murali SR. Steroid injection therapy is the best conservative treatment for lateral epicondylitis: a prospective randomised controlled trial. Int J Clin Prac 2007;61:240–6 | Included in an included SR |
Appendix 3 Clinical effectiveness review Assessment of Multiple Systematic Reviews grading
| The AMSTAR checklist for methodological assessment | Study reference numbera | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Item | 56 | 57 | 58 | 59 | 60 | 61 | 62 | 63 | 64 | 65 | 66 | 67 | 69 | 68 | 70 | 71 |
| 1 | Was an ‘a priori’ design provided? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 2 | Was there duplicate study selection and data extraction? | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 |
| 3 | Was a comprehensive literature search performed? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 |
| 4 | Was the status of publication (that is, ‘grey’ literature) used as an inclusion criterion? | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 |
| 5 | Was a list of studies (included and excluded) provided? | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | Were the characteristics of the included studies provided? | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 |
| 7 | Was the scientific quality of the included studies assessed and documented? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 |
| 8 | Was the scientific quality of the included studies used appropriately in formulating conclusions? | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 0 |
| 9 | Were the methods used to combine the findings of studies appropriate? | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 1 | 0 | 1 |
| 10 | Was the likelihood of publication bias assessed? | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| 11 | Were potential conflicts of interest included? | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Total AMSTAR score (points) | 8 | 8 | 8 | 8 | 8 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 6 | 6 | 6 | |
| The AMSTAR checklist for methodological assessment | Study reference numbera | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number | Item | 72 | 73 | 74 | 75 | 76 | 77 | 78 | 79 | 80 | 81 | 82 | 83 | 84 |
| 1 | Was an ‘a priori’ design provided? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 |
| 2 | Was there duplicate study selection and data extraction? | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | Was a comprehensive literature search performed? | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| 4 | Was the status of publication (that is, ‘grey’ literature) used as an inclusion criterion? | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 |
| 5 | Was a list of studies (included and excluded) provided? | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | Were the characteristics of the included studies provided? | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 |
| 7 | Was the scientific quality of the included studies assessed and documented? | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 |
| 8 | Was the scientific quality of the included studies used appropriately in formulating conclusions? | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| 9 | Were the methods used to combine the findings of studies appropriate? | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | Was the likelihood of publication bias assessed? | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 11 | Were potential conflicts of interest included? | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 |
| Total AMSTAR score (points) | 6 | 6 | 5 | 5 | 5 | 4 | 4 | 4 | 3 | 3 | 3 | 3 | 1 | |
Appendix 4 Grading of Recommendations, Assessment, Development and Evaluation profiles
This section details the GRADE profiles for each of the included high-quality studies.
| Question | Should ESWT vs. placebo be used for LET? | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Buchbinder et al.58 | ||||||||||
| Quality assessment | Patients (n) | Effect | Quality | ||||||||
| Studies (n) | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | ESWT | Placebo | Relative (95% CI) | Absolute | |
| Pain (short term) [follow-up 4–6 weeks; measured with VAS (100 mm); range of scores –3.6 to 19] | |||||||||||
| 3 | RCT | No serious risk of bias | Seriousa | No serious indirectness | No serious imprecisions | None | 224 | 222 | NR | MD 9.42 lower (20.70 lower to 1.86 higher) | ⊕⊕⊕⊝ moderate |
| Pain (intermediate term) [follow-up 12 weeks; measured with resisted wrist extension (Thomsen Test)] | |||||||||||
| 3 | RCT | No serious risk of bias | Seriousa | No serious indirectness | No serious imprecisions | None | 226 | 229 | NR | MD 9.04 lower (19.37 lower to 1.28 higher) | ⊕⊕⊕⊝ moderate |
| Function (intermediate term) (follow-up 12 weeks; measured with mean grip strength) | |||||||||||
| 3 | RCT | No serious risk of bias | Seriousb | No serious indirectness | No serious imprecisions | None | 221 | 227 | NR | SMD 0.05 higher (0.13 lower to 0.24 higher) | ⊕⊕⊕⊝ moderate |
| QoL | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Remain/return to work | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Sport activity | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Recurrence | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Adverse events (mild) (follow-up 5 weeks) | |||||||||||
| 1 | RCT | No serious risk of bias | Seriousc | No serious indirectness | No serious imprecisions | None | 11/31 (35.5%)d | 13/29 (44.8%)e | NR | 448 fewer per 1000 (from 448 fewer to 448 fewer) | ⊕⊕⊕⊝ moderate |
| Adverse events (general) (follow-up 52 weeks) | |||||||||||
| 1 | RCT | No serious risk of bias | Seriousc,f | No serious indirectness | No serious imprecisions | None | 134/271 (49.4%)g | 137/271 (50.6%) | OR 4.3 (2.9 to 6.3)g | 309 more per 1000 (from 242 more to 360 more) | ⊕⊕⊕⊝ moderate |
| Question | Should ESWT vs. GCI be used for LET? | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Buchbinder et al.58 | ||||||||||
| Quality assessment | Patients (n) | Effect | Quality | ||||||||
| Studies (n) | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | ESWT | GCI | Relative (95% CI) | Absolute | |
| Pain (follow-up 3 months; assessed with reduction of pain 50% from baseline as criterion of success) | |||||||||||
| 1 | RCT | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | 21/25 (84.0%) | 29/48 (60.4%) | NR | 604 fewer per 1000 (from 604 fewer to 604 fewer) | ⊕⊕⊕⊝ moderate |
| Question | Should laser therapy vs. placebo be used for LET? | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Smidt et al.59 | ||||||||||
| Quality assessment | Patients (n) | Effect | Quality | ||||||||
| Studies (n) | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Laser | Placebo | Relative (95% CI) | Absolute | |
| Pain (0–6 weeks) (follow-up 3 weeks; measured with VAS) | |||||||||||
| 1 | RCT | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousa | None | NR | NR | NR | SMD 0.25 lower (0.96 lower to 0.47 higher) | ⊕⊕⊕⊝ moderate |
| Pain (7 weeks) (follow-up 7 weeks; measured with VAS; range of scores = –0.27) | |||||||||||
| 1 | RCT | No serious risk of bias | Seriousb | No serious indirectness | Seriousa | None | NR | NR | NR | SMD 0.46 lower (1.19 lower to 0.27 higher) | ⊕⊕⊝⊝ low |
| Pain (13 weeks) (follow-up 13 weeks; measured with VAS) | |||||||||||
| 1 | RCT | No serious risk of bias | No serious inconsistency | Seriousb | Seriousb | None | NR | NR | NR | SMD 2.00 lower (2.77 higher to 1.22 lower) | ⊕⊕⊝⊝ low |
| Function | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| QoL | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Remain/return to work | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Sport activity | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Recurrence | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Adverse events | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Question | Should laser therapy vs. friction massage be used for LET? | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Smidt et al.59 | ||||||||||
| Quality assessment | Patients (n) | Effect | Quality | ||||||||
| Studies (n) | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Laser | Friction massage | Relative (95% CI) | Absolute | |
| Pain (short term) (follow-up 3 weeks; measured with VAS) | |||||||||||
| 1 | RCT | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | NR | NR | NR | SMD 0.92 higher (0.17 lower to 1.67 higher) | ⊕⊕⊝⊝ low |
| Pain (7 weeks) (follow-up 7 weeks; measured with VAS) | |||||||||||
| 1 | RCT | Seriousc | No serious inconsistency | No serious indirectness | Seriousb | None | NR | NR | NR | SMD 0.84 higher (0.09 lower to 1.58 higher) | ⊕⊕⊝⊝ low |
| Question | Should ultrasound vs. placebo be used for LET? | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Smidt et al.59 | ||||||||||
| Quality assessment | Patients (n) | Effect | Quality | ||||||||
| Studies (n) | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Ultrasound | Placebo | Relative (95% CI) | Absolute | |
| Pain (short term) (follow-up 6 weeks; measured with VAS) | |||||||||||
| 1 | RCT | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousa | None | NR | NR | NR | SMD 0.61 lower (1.07 higher to 0.15 lower) | ⊕⊕⊕⊝ moderate |
| Pain (8 weeks) | |||||||||||
| 1 | RCT | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousa | None | NR | NR | NR | SMD 0.66 lower (1.13 higher to 0.20 lower) | ⊕⊕⊕⊝ moderate |
| Pain (13 weeks) (follow-up 13 weeks; measured with VAS) | |||||||||||
| 1 | RCT | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousa | None | NR | NR | NR | SMD 1.33 lower (1.87 higher to 0.80 lower) | ⊕⊕⊕⊝ moderate |
| Function | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| QoL | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Remain/return to work | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Sport activity | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Recurrence | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Adverse events | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Question | Should ultrasound (+ friction massage) vs. laser therapy be used for LET? | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Smidt et al.59 | ||||||||||
| Quality assessment | Patients (n) | Effect | Quality | ||||||||
| Studies (n) | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Ultrasound + friction massage | Laser | Relative (95% CI) | Absolute | |
| Pain (short-term) (follow-up 3 weeks; measured with VAS) | |||||||||||
| 1 | RCT | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | NR | NR | NR | SMD 0.92 lower (1.67 higher to 0.17 lower) | ⊕⊕⊕⊝ moderate |
| Pain (intermediate) (follow-up 7 weeks; measured with VAS) | |||||||||||
| 1 | RCT | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | NR | NR | NR | SMD 0.84 lower (1.58 higher to 0.09 lower) | ⊕⊕⊕⊝ moderate |
| Question | Should ultrasound vs. exercises be used for LET? | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Smidt et al.59 | ||||||||||
| Quality assessment | Patients (n) | Effect | Quality | ||||||||
| Studies (n) | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Ultrasound | Laser | Relative (95% CI) | Absolute | |
| Pain (intermediate) (follow-up 8 weeks; measured with VAS) | |||||||||||
| 1 | RCT | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | NR | NR | NR | SMD 0.95 higher (0.26 lower to 1.64 higher) | ⊕⊕⊕⊝ moderate |
| Question | Should exercises vs. ultrasound (+ friction massage) be used for LET? | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Smidt et al.59 | ||||||||||
| Quality assessment | Patients (n) | Effect | Quality | ||||||||
| Studies (n) | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Exercises | Ultrasound + friction massage | Relative (95% CI) | Absolute | |
| Pain (intermediate) (follow-up 8 weeks; measured with VAS) | |||||||||||
| 1 | RCT | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | NR | NR | NR | SMD 0.95 lower (1.64 higher to 0.26 lower) | ⊕⊕⊕⊝ moderate |
| Function | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| QoL | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Remain/return to work | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Sport activity | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Recurrence | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Adverse events | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Question | Should GCI vs. placebo be used for LET? | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Coombes et al.60 | ||||||||||
| Quality assessment | Patients (n) | Effect | Quality | ||||||||
| Studies (n) | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | GCI | Placebo | Relative (95% CI) | Absolute | |
| Pain (intermediate) [follow-up 26 weeks; measured with VAS (0–100)] | |||||||||||
| 3 | RCTs | Seriousa | Seriousb | No serious indirectness | No serious imprecision | None | 160 | 81 | NR | SMD 0.07 higher (0.50 lower to 0.63 higher) | ⊕⊕⊝⊝ low |
| Function (short term) (follow-up 4 weeks; measured with DASH) | |||||||||||
| 1 | RCT | Serious | No serious inconsistency | No serious indirectness | No serious imprecision | None | 31 | 33 | NR | SMD 0.14 higher (0.42 lower to 0.69 higher) | ⊕⊕⊕⊝ moderate |
| Function (intermediate term) (follow-up 4 weeks; measured with DASH) | |||||||||||
| 1 | RCT | Serious | No serious inconsistency | No serious indirectness | No serious imprecision | None | 31 | 33 | NR | SMD 0.25 lower (0.82 lower to 0.32 higher) | ⊕⊕⊕⊝ moderate |
| QoL | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Remain/return to work | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Sport activity | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Recurrence | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| AEs (pain) (follow-up 24 weeks; assessed with post-injection pain) | |||||||||||
| 1 | RCT | Seriousc | Seriousd | No serious indirectness | No serious imprecision | None | 30/59 (50.8%) | 9/29 (31.0%) | RR 1.64 (0.90 to 2.98) | 199 more per 1000 (from 31 fewer to 614 more) | ⊕⊕⊝⊝ low |
| AE (atrophy) (follow-up 24 weeks) | |||||||||||
| 1 | RCT | Seriousc | Seriousd | No serious indirectness | No serious imprecision | None | 18/59 (30.5%) | 5/29 (17.2%) | RR 1.77 (0.73 to 4.29) | 133 more per 1000 (from 47 fewer to 567 more) | ⊕⊕⊝⊝ low |
| AE (depigmentation) (follow-up 26 weeks) | |||||||||||
| 1 | RCT | Seriousc | Seriouse | No serious indirectness | No serious imprecision | None | 1/31 (3.2%) | 2/33 (6.1%) | RR 0.53 (0.05 to 5.58) | 28 fewer per 1000 (from 58 fewer to 278 more) | ⊕⊕⊝⊝ low |
| Question | Should GCI vs. no intervention (observation or wait-and-see) be used for LET? | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Coombes et al.60 | ||||||||||
| Quality assessment | Patients (n) | Effect | Quality | ||||||||
| Studies (n) | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | GCI | No intervention (observation or wait-and-see) | Relative (95% CI) | Absolute | |
| Pain (short term) (follow-up 4 weeks; measured with VAS/NRS/PRFEQ pain subscale) | |||||||||||
| 3 | RCTs | Seriousa | No serious inconsistency | No serious indirectness | Seriousb | None | 139 | 138 | NR | SMD 1.44 lower (1.17 higher to 1.71 lower) | ⊕⊕⊝⊝ low |
| Pain (intermediate term) (follow-up 26 weeks; measured with VAS) | |||||||||||
| 2 | RCTs | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | 139 | 138 | NR | SMD 0.40 higher (0.67 lower to 0.14 higher) | ⊕⊕⊕⊝ moderate |
| Pain (long term) (follow-up 52 weeks; measured with VAS) | |||||||||||
| 2 | RCTs | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | 139 | 138 | NR | SMD 0.31 higher (0.61 lower to 0.01 higher) | ⊕⊕⊕⊝ moderate |
| Function (short term) (follow-up 4 weeks; measured with pain-free function scale/PRFEQ function subscale) | |||||||||||
| 3 | RCTs | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | 139 | 138 | NR | SMD 1.50 higher (1.22 lower to 1.77 higher) | ⊕⊕⊕⊝ moderate |
| Function (intermediate term) (follow-up 26 weeks; measured with pain-free function scale/PRFEQ function subscale) | |||||||||||
| 3 | RCTs | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | 139 | 138 | NR | SMD 0.51 lower (0.76 higher to 0.25 lower) | ⊕⊕⊕⊝ moderate |
| Function (long term) (follow-up 52 weeks; measured with pain-free function scale/PRFEQ function subscale) | |||||||||||
| 3 | RCTs | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | 139 | 138 | NR | SMD 0.32 lower (0.57 higher to 0.06 lower) | ⊕⊕⊕⊝ moderate |
| Question | Should GCI vs. NSAIDs be used for LET? | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Coombes et al.60 | ||||||||||
| Quality assessment | Patients (n) | Effect | Quality | ||||||||
| Studies (n) | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | GCI | NSAIDs | Relative (95% CI) | Absolute | |
| Pain (short term) [follow-up 4 weeks; measured with NRS (0–9)] | |||||||||||
| 1 | RCT | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | NR | NR | NR | SMD 1.02 lower (0.61 higher to 1.43 lower) | ⊕⊕⊕⊝ moderate |
| Pain (intermediate term) [follow-up 26 weeks; measured with NRS (0–9)] | |||||||||||
| 1 | RCT | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | NR | NR | NR | SMD 0.52 higher (0.92 lower to 0.13 higher) | ⊕⊕⊕⊝ moderate |
| Pain (long term) [follow-up 52 weeks; measured with impairment of function (NRS)] | |||||||||||
| 1 | RCT | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | NR | NR | NR | SMD 0.19 higher (0.58 higher to 0.19 lower) | ⊕⊕⊕⊝ moderate |
| Function (short term) [follow-up 4 weeks; measured with impairment of function (NRS)] | |||||||||||
| 1 | RCT | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | NR | NR | NR | SMD 0.92 higher (0.51 lower to 1.32 higher) | ⊕⊕⊕⊝ moderate |
| Function (intermediate term) [follow-up 26 weeks; measured with impairment of function (NRS)] | |||||||||||
| 1 | RCT | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | NR | NR | NR | SMD 0.29 lower (0.68 lower to 0.10 higher) | ⊕⊕⊕⊝ moderate |
| Function (long term) [follow-up 52 weeks; measured with impairment of function (NRS)] | |||||||||||
| 1 | RCT | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | NR | NR | NR | SMD 0.19 lower (0.58 lower to 0.19 higher) | ⊕⊕⊕⊝ moderate |
| Question | Should GCI vs. physiotherapy be used for LET? | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Coombes et al.60 | ||||||||||
| Quality assessment | Patients (n) | Effect | Quality | ||||||||
| Studies (n) | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | GCI | PT | Relative (95% CI) | Absolute | |
| Pain (intermediate term) (follow-up 26 weeks; measured with VAS/NRS; better indicated by lower values) | |||||||||||
| 2 | RCTs | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | 127 | 130 | NR | SMD 0.56 higher (0.82 lower to 0.31 higher) | ⊕⊕⊕⊝ moderate |
| Pain (long term) (follow-up 52 weeks; measured with VAS/NRS; better indicated by lower values) | |||||||||||
| 2 | RCTs | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | 127 | 130 | NR | SMD 0.48 higher (0.73 lower to 0.23 higher) | ⊕⊕⊕⊝ moderate |
| Function (short term) (follow-up 4 weeks; measured with pain-free function scale/PRFEQ function subscale) | |||||||||||
| 3 | RCTs | Seriousa,b | No serious inconsistency | No serious indirectness | No serious imprecision | None | 139 | 142 | NR | SMD 1.29 higher (1.03 lower to 1.55 higher) | ⊕⊕⊕⊝ moderate |
| Function (intermediate term) (follow-up 26 weeks; measured with pain-free function scale/PRFEQ function subscale) | |||||||||||
| 2 | RCTs | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | 127 | 130 | NR | SMD 0.64 lower (0.90 higher to 0.39 lower) | ⊕⊕⊕⊝ moderate |
| Function (long term) (follow-up 52 weeks; measured with pain-free function scale/PRFEQ function subscale) | |||||||||||
| 2 | RCTs | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | 127 | 130 | NR | SMD 0.57 lower (0.82 higher to 0.32 lower) | ⊕⊕⊕⊝ moderate |
| Recurrencec | |||||||||||
| 3 | RCTs | Seriousb | NR | NR | Seriousc | NR | 127 | 130 | NR | NR | ⊕⊕⊝⊝ low |
| Question | Should GCI vs. PRP injections be used for LET? | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Coombes et al.60 | ||||||||||
| Quality assessment | Patients (n) | Effect | Quality | ||||||||
| Studies (n) | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | GCI | PRP | Relative (95% CI) | Absolute | |
| Pain (short term) [follow-up 4 weeks; measured with VAS (0–100)] | |||||||||||
| 1 | RCT | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | 51 | 49 | NR | SMD 0.44 lower (0.04 higher to 0.84 lower) | ⊕⊕⊕⊝ moderate |
| Pain (intermediate term) [follow-up 26 weeks; measured with VAS (0–100)] | |||||||||||
| 1 | RCT | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | 51 | 49 | NR | SMD 0.86 higher (1.27 lower to 0.45 higher) | ⊕⊕⊕⊝ moderate |
| Pain (long term) [follow-up 52 weeks; measured with VAS (0–100)] | |||||||||||
| 1 | RCT | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | 51 | 49 | NR | SMD 0.83 higher (1.24 lower to 0.42 higher) | ⊕⊕⊕⊝ moderate |
| Function (short term) (follow-up 4 weeks; measured with DASH scale) | |||||||||||
| 1 | RCT | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | 51 | 49 | NR | SMD 0.52 higher (0.12 lower to 0.92 higher) | ⊕⊕⊕⊝ moderate |
| Function (intermediate term) (follow-up 26 weeks; measured with DASH scale) | |||||||||||
| 1 | RCT | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | 51 | 49 | NR | SMD 0.48 lower (0.88 higher to 0.08 lower) | ⊕⊕⊕⊝ moderate |
| Function (long term) (follow-up 52 weeks; measured with DASH scale) | |||||||||||
| 1 | RCT | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | 51 | 49 | NR | SMD 0.69 lower (1.09 higher to 0.28 lower) | ⊕⊕⊕⊝ moderate |
| Question | Should sodium hyaluronate vs. placebo be used for LET? | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Coombes et al.60 | ||||||||||
| Quality assessment | Patients (n) | Effect | Quality | ||||||||
| Studies (n) | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Sodium hyaluronate | Placebo | Relative (95% CI) | Absolute | |
| Pain (short term) (follow-up 4 weeks; measured with VAS) | |||||||||||
| 1 | RCT | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | 165 | 166 | NR | SMD 3.91 lower (3.54 higher to 4.28 lower) | ⊕⊕⊕⊝ moderate |
| Pain (intermediate term) (follow-up 26 weeks; measured with VAS) | |||||||||||
| 1 | RCT | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | 165 | 166 | NR | SMD 2.89 lower (2.58 higher to 3.20 lower) | ⊕⊕⊕⊝ moderate |
| Pain (long term) (follow-up 52 weeks; measured with VAS) | |||||||||||
| 1 | RCT | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | 165 | 166 | NR | SMD 3.91 lower (3.55 higher to 4.28 lower) | ⊕⊕⊕⊝ moderate |
| Function | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| QoL | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Remain/return to work | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Sport activity | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Recurrence | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| AEs (pain) (follow-up 24 weeks; assessed with post-injection pain) | |||||||||||
| 1 | RCT | Very seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | 3/165 (1.8%) | 5/166 (3.0%) | RR 0.60 (0.15 to 2.48) | 12 fewer per 1000 (from 26 fewer to 45 more) | ⊕⊕⊝⊝ low |
| Question | Should botulinum toxin injection vs. placebo be used for LET? | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Coombes et al.60 | ||||||||||
| Quality assessment | Patients (n) | Effect | Quality | ||||||||
| Studies (n) | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Botulinum toxin A injection (Botox®) | Placebo | Relative (95% CI) | Absolute | |
| Pain (short term) [follow-up 4 weeks; measured with VAS (0–100)] | |||||||||||
| 1 | RCT | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousa | None | 30 | 30 | NR | SMD 1.23 lower (0.67 higher to 1.78 lower) | ⊕⊕⊕⊝ moderate |
| Function | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| QoL | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Remain/return to work | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Sport activity | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Recurrence | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Adverse events (overall) (follow-up 4 weeks) | |||||||||||
| 1 | RCT | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousa | None | 19/30 (63.3%) | 9/30 (30.0%) | RR 2.11 (1.15 to 3.89) | 333 more per 1000 (from 45 more to 867 more) | ⊕⊕⊕⊝ moderate |
| Adverse events (post-injection pain) (follow-up 4 weeks) | |||||||||||
| 1 | RCT | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousa | None | 2/30 (6.7%) | 1/30 (3.3%) | RR 2.00 (0.19 to 20.90) | 33 more 1000 (from 23 fewer to 663 more) | ⊕⊕⊕⊝ moderate |
| Adverse events (nausea) (follow-up 4 weeks) | |||||||||||
| 1 | RCT | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousa | None | 0/30 (0.0%) | 1/30 (3.3%) | RR 0.33 (0.01 to 7.87) | 22 fewer per 1000 (from 33 fewer to 229 more) | ⊕⊕⊕⊝ moderate |
| AEs (finger weakness) (follow-up 4 weeks) | |||||||||||
| 1 | RCT | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousa | None | 10/30 (33.3%) | 6/30 (20.0%) | RR 1.67 (0.69 to 4.00) | 134 more per 1000 (from 62 fewer to 600 more) | ⊕⊕⊕⊝ moderate |
| AEs (paresis) (follow-up 4 weeks) | |||||||||||
| 1 | RCT | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousa | None | 4/30 (13.3%) | 0/30 (0.0%) | RR 9.00 (0.51 to 160.17) | NR | ⊕⊕⊕⊝ moderate |
| Question | Should prolotherapy vs. placebo be used for LET? | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Coombes et al.60 | ||||||||||
| Quality assessment | Patients (n) | Effect | Quality | ||||||||
| Studies (n) | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Prolotherapy | Placebo | Relative (95% CI) | Absolute | |
| Pain (short term) [follow-up 4 weeks; measured with NRS (resting pain)] | |||||||||||
| 1 | RCT | Serious | No serious inconsistency | No serious indirectness | Seriousa,b | None | 12 | 12 | NR | SMD 0.27 lower (1.15 lower to 0.61 higher) | ⊕⊕⊝⊝ low |
| Pain (intermediate term) [follow-up 26 weeks; measured with NRS (resting pain)] | |||||||||||
| 1 | RCT | Seriousc | No serious inconsistency | No serious indirectness | Seriousa,b | None | 12 | 12 | NR | SMD 2.62 lower (1.36 higher to 3.88 lower) | ⊕⊕⊝⊝ low |
| Function | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| QoL | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Remain/return to work | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Sport activity | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Recurrence | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| AEs (pain) (follow-up 16 weeks) | |||||||||||
| 1 | RCT | Seriousc | No serious inconsistency | No serious indirectness | Seriousa,b | None | 10/10 (100%) | 10/10 (100%) | NR | 1000 fewer per 1000 (from 1000 fewer to 1000 fewer) | ⊕⊕⊝⊝ low |
| AEs (irritation) (follow-up 16 weeks; assessed with local irritation) | |||||||||||
| 1 | RCT | Seriousc | No serious inconsistency | No serious indirectness | Seriousa,b | None | 2/10 (20%) | 0/10 (0%) | RR 5.00 (0.27 to 92.62) | NR | ⊕⊕⊝⊝ low |
| Question | Should therapeutic ultrasound-guided injection of sclerosing solution vs. placebo be used for LET? | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Coombes et al.60 | ||||||||||
| Quality assessment | Patients (n) | Effect | Quality | ||||||||
| Studies (n) | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Ultrasonography-guided injection of sclerosing solution | Placebo | Relative (95% CI) | Absolute | |
| Pain (short term) (follow-up 4 weeks) | |||||||||||
| 1 | RCT | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousa | None | 18 | 18 | NR | SMD 0.20 higher (0.47 lower to 0.88 higher) | ⊕⊕⊕⊝ moderate |
| Function | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| QoL | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Remain/return to work | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Sport activity | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Recurrence | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| AEs (overall) (follow-up 12 weeks) | |||||||||||
| 1 | RCT | No serious risk of bias | No serious inconsistency | No serious indirectness | Seriousa | None | 0/45 (0%)b | 0/42 (0%)b | NR | NR | ⊕⊕⊕⊝ moderate |
| Question | Should glycosaminoglycan polysulphate (arteparon) injections vs. placebo be used for LET? | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Coombes et al.60 | ||||||||||
| Quality assessment | Patients (n) | Effect | Quality | ||||||||
| Studies (n) | Design | Risk of bias | Inconsistency | Indirectness | Imprecision | Other considerations | Glycosaminoglycan polysulphate (arteparon) injections | Placebo | Relative (95% CI) | Absolute | |
| Pain (short term) [follow-up 4 weeks; measured with VAS (0–100)] | |||||||||||
| 1 | RCT | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | 34 | 31 | NR | SMD 0.21 lower (0.72 lower to 0.30 higher) | ⊕⊕⊕⊝ moderate |
| Pain (intermediate term) [follow-up 26 weeks; measured with VAS (0–100); range of scores –0.13–0.89] | |||||||||||
| 1 | RCT | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | 34 | 31 | NR | SMD 0.38 lower (0.89 lower to 0.13 higher) | ⊕⊕⊕⊝ moderate |
| Function | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| QoL | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Remain/Return to work | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Sport activity | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Recurrence | |||||||||||
| NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| AEs (pain) (follow-up 26 weeks; assessed with local pain) | |||||||||||
| 1 | RCT | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | 13/32 (40.6%) | 5/28 (17.9%) | RR 2.27 (0.93 to 5.58) | 227 more per 1000 (from 12 fewer to 818 more) | ⊕⊕⊕⊝ moderate |
| AEs (haematoma) (follow-up 26 weeks) | |||||||||||
| 1 | RCT | Seriousa | No serious inconsistency | No serious indirectness | No serious imprecision | None | 2/32 (6.3%) | 0/28 (0.0%) | RR 4.39 (0.22 to 87.82) | NR | ⊕⊕⊕⊝ moderate |
Appendix 5 Randomised controlled trials, study characteristics
| Study ID (funding) | Design | n | Participants | Intervention group | Control group | Outcomes | Length of follow-up |
|---|---|---|---|---|---|---|---|
| Viswas et al., 2012164 (not reported) | Randomised | 20 | Adults aged 30–45 years with LET with symptoms for 8–10 weeks | Cyriax physiotherapya (three treatment sessions per week for 4 weeks); n = 10 | Supervised exercise programme (three treatment sessions per week for 4 weeks); n = 10 | Pain intensity (VAS 10 cm), and functional status (TEFS) | None |
| Stefanou et al., 2012165 (Travanti Pharma Inc., Mendota Heights, MN, USA) | Randomised | 86 | Adults aged 18–70 years with LE made by local tenderness to palpation just distal and anterior to the lateral epicondyle | 10 mg of dexamethasone via iontophoresis self-contained path with a 24-hour battery; n = 31 10 mg of dexamethasone; n = 27 10 mg of triamcinolone injection; n = 28 | NA | Grip strength (change in, flexion vs. extension using dynamometer); pain (PRTEE); function (PRTEE) | 6 months |
| Soderberg et al., 2012166 (Rehband, Stockholm, Sweden) | Randomised, controlled, single blind | 37 | Adults with positive diagnostic criteria according to bHaker204 | 6-week home exercise regimen (eccentric training for wrist extensors and a forearm band); n = 18 | Forearm band only; n=19 | Pain-free hand grip strength; pain-free wrist extensor strength; change in proportion of cases with epicondylalgia; ratings of perceived pain (VAS) | 6 weeks |
| Skorupska et al., 2012167 (State Committee for Scientific Research, Warsaw, Poland (project N404 169534) | Randomised, double blind | 80 | Adults aged ≥ 18 years diagnosed with LET, epicondylitis, forearm extensor enthesopathy or inflammation, or acute state LET | LLLT; n = 40 [2nd randomisation – conservative treatment of LLLT (1 J/cm2) (n = 20) or myofascial pain physiotherapy treatment of LLLT (5 J/cm2) (n = 20) (10-day therapy)] | US; n = 40 [2nd randomisation – conservative treatment of US (0.5 W/cm2 3 MHz) (n = 20) or myofascial pain physiotherapy treatment of US (0.7 W/cm2 1 MHz) (n = 20) (10-day therapy)] | Presence and sensitivity of TrPs (algometer); pain (VAS); DASH; grip strength (dynamometer) | 12 months |
| Omar et al., 2012168 (not reported) | Randomised | 30c | Adults aged 18 years-plus with LET | Steroid injection; n = 15 | PRP injection; n = 15 | Pain (VAS); function (DASH) | 6 weeks |
| Gunduz et al., 2012169 (not reported) | Randomised | 59 | Pain on the lateral side of the elbow severe enough to interfere with daily living (≤ 3 months), tenderness over lateral epicondyle compared with that of normal elbow; pain during extension of wrist and fingers against resistance | Physical therapy (hot pack, ultrasound therapy, and friction massage) 10 sessions; n = 19 Single corticosteroid injection (methylprednisolone acetate and 1 ml prilocaine); n = 20 ESWT 10 sessions; n = 20 | NA | Pain (VAS); function [grip strength and pinch strength (dynamometer)] | 1, 3, and 6 months |
| Forogh et al., 2012170 (not reported) | Randomised, single blind | 24 | Adults aged 30–50 years with LET | New-designed orthosis; n = 12 (4 weeks) | Standard counterforce orthosis; n = 12 (4 weeks) | Pain and function (PRTEE); pain threshold (algometer); and, grip strength (dynamometer) | None |
| Ajimsha et al., 2012171 (Kerala State Government Grant/Mahatma Gandhi University, Muttom, Kerala, India) | Randomised, controlled, single blind | 65 | Adult computer professionals aged 20–40 years with a diagnosis of LET on the mouse-operating arm | Myofascial release; n = 33 | Sham US therapy; n = 32 | Pain severity and functional disability (PRTEE scale) | 4, 12 weeks |
| Agostinucci et al., 2012172 (Modular Thermal Technologies and College of Human Science and Services, University of Rhode Island, North Kingstown, RI, USA) | Randomised | 70 | Adults aged ≥ 18 years with pain localised to the lateral elbow for a minimum of 3 months | Gel cold pack + exercise; n = 21 Cryo-MAXd + exercise; n = 22 Cryo-MAX only; n = 19 (all twice daily, 4 times per week for 6 weeks) | Exercise only; n = 9 (twice daily, 4 times per week for 6 weeks) | Grip strength; pain (during single arm chair pick-up); DASH (all assessed pre- and post-treatment) | None |
| Wolf et al., 2011173 (American Society for Surgery of the Hand, Chicago, IL, USA) | Randomised, controlled, single blind | 28 | Adults aged ≥ 18 years with LET for a minimum of 6 months | Corticosteroid + lidocaine; n = 9. Autologous blood + lidocaine; n = 9 | 3 ml injection saline + lidocaine; n = 10 | Pain (VAS), DASH, PRFEQ (pre-, 2 weeks, 2 months and 6 months) | 2 weeks, 2 and 6 months |
| Thanasas et al., 2011174 (not reported) | Randomised, controlled | 28 | Adults aged ≥ 18 years with clinically diagnosed LET | ABI 3 ml (single injection) + eccentric muscle strengthening; n = 14 | PRP 3 ml (ultrasound guidance) + eccentric muscle strengthening; n = 14 | Pain (VAS); Liverpool Elbow Score | 6 weeks, 3 and 6 months |
| Polat et al., 2011175 (not reported) | Randomised, double blind | 55 | Adults aged ≥ 18 years with chronic pain over the lateral epicondyle with a mean duration of pain ≥ 3 months | 48 mg/day of betahistine dihydrochloride for 10 days; n = 33 | 750 mg/day of naproxen sodium for 10 days; n = 32 | VAS and Verhaar Criteria | Day 10, 3- and 6-months |
| Peterson et al., 201118 [Swedish Research Council, The Amersham Fund (Uppsala University, Uppsala, Sweden), The Research Fund at Uppsala County Council, The Family Medicine Foundation, and Uppsala University (Uppsala, Sweden)] | Randomised, controlled | 81 | Adults aged 20–75 years with symptoms of LET ≥ 3 months; and, verified diagnosis | Exercise (daily with weekly load increase; 3 months); n = 40 | Wait list; n = 41 | Pain (Cozen’s teste and modified empty can testf); muscle strength (dynamometer); DASH; Gothenburg QoL | 3 months |
| Gosens et al., 2011176 (BioMet Inc. Warsaw IN, USA) | Randomised, double blind, controlled | 100 | LET for ≥ 6 months and pain of < 50 on a VAS for pain with symptoms for ≥ 6 months and previously treated with cast immobilisation, corticosteroid injection, or physiotherapy | Leucocyte-enriched PRP; n = 51 | Corticosteroid; n = 49 | Pain (VAS); function (DASH) | 12 and 24 months (12 months reported in Peerbooms et al., 201095) |
| Fernandez-Carnero et al., 2011177 (not reported) | Randomised, single blind | 18 | Adults aged 18–60 years with LET; right-handed; dominant side affected | Cervical spine thrust manipulation; n = 9 | Thoracic spine thrust manipulation; n = 9 | Pain-free grip strength; pressure pain threshold | None |
| Creaney et al., 2011178 (not reported) | Randomised, double blind | 150 | Adult patients with LET ≥ 6 months; prior treatment failure (conservative measures including physical therapy exercises) | PRP injection; n = 80 | ABI; n = 70 | Pain and physical function (PRTEE) | 1, 3, 6-months |
| Collins et al., 2011179 [Health Tronics of Atlanta (GA, USA) and Baylor College of Medicine of Houston (TX, USA)] | Randomised, placebo controlled, double-blind | 183 | Adults aged ≥ 21 years with chronic LET ≥ 6 months; prior treatment failure;g pain at point of tenderness over the affected LE of ≥ 5.0 cm on a 10 cm VAS scale | ESWT (1500 shocks at 18 kV); n = 93 | Placebo (ESWT with Styrofoam block against the coupling membrane and fluid-filled bag); n = 90 | Pain (VAS 10 cm) and SF-36 (participant assessed). Pain (50% improvement over baseline and VAS of ≤ 4.0 (investigator and participant at 8 weeks) and no requirement for analgesics for elbow pain at 8 weeks | 4, 8, 12 weeks, 6 and 12 months |
| Blanchette and Normand 2011180 (Fondation de Recherché Chiropratique du Quebec, QB, Canada) | Randomised, controlled study | 27 | Adults aged ≥ 18 years with LET confirmed by the Cozen’se and Mill’sh test | ASTM twice daily for 5 weeks; n = 15 | Advice on natural evolution of LET, computer ergonomics, stretching exercises; n = 12 | Functional status [pain-free grip strength (baseline and 6 weeks)]; and, VAS and PRTEE [patient rated (baseline, 6 weeks, 3 months)] | 6 weeks, 3 months |
| Bellapianta et al., 2011181 (not reported) | Randomised | 31 | Adults aged ≥ 18 years with acute LET | Corticosteroid injection; single-injection technique; n = 15 (elbows) | Corticosteroid injection; peppered-injection technique; n = 18 (elbows) | VAS, DASH, grip strength | 10 weeks |
| Backer et al., 2011182 (Karl and Veronica Carstens Foundation, Essen, Germany) | Randomised, controlled, open | 40 | Adults aged 18–70 years with history of LET ≥ 3 months and presence of pain for 50% of last 30 days; pressure pain on radial epicondyle of the humerus; aggravation of pain during extension of the wrist against resistance; and positive middle finger test | 2–4 locally applied medicinal leeches; n = 20 | 30-day course topical diclofenac; gel (300 g) n = 20 | Pain (VAS – motion, grip and rest); DASH, SF-36, grip strength safety and use of rescue medication monitored using patients diaries and interview at days 7 and 45. Measured over 45 days | None |
| Ozturan et al., 2010183 (not reported) | Randomised | 57 | Adults aged ≥ 18 years with history of LET for ≥ 6 months, tenderness on palpation of the LET, > 40 mm on the VAS (Thomsen test) | Corticosteroid injection; n = 20. ABI; n = 20. ESWT; n = 20 | NA | Thomsen provocative testing, upper extremity functional scores, maximal grip strength | 4, 12, 26 and 52 weeks |
| Kazemi et al., 2010184 (not reported) | Randomised, single blind, controlled | 60 | Adults aged 27–64 years with a new episode of tennis elbow (within last year) | Methylprednisolone (20 mg of methylprednisolone with 1 ml of 2% lidocaine); n = 30 | ABI (2 ml of arteria brachialis distal region of the ipsilateral upper limb + 1 ml of 2% lidocaine); n = 30 | Pain (VAS, severity last 24 hours); function (ADLs measured by PFFQ; pain in maximum grip; DASH-Q; modified Nirschl questionnaire; maximum grip strength; pressure pain threshold) | 4 and 8 weeks |
| Garg et al., 2010185 (not reported) | Randomised | 44 | Adults lateral sided elbow pain, tenderness to palpation over the lateral extensor origin, pain with resisted wrist and long finger extension | Wrist extension splint; n = 24 (elbows) | Counterforce forearm strap (brace); n = 20 (elbows) | Pain and function (ASES Assessment Form and MEP) | 6 weeks |
| Emanet et al., 2010186 (not reported) | Randomised | 47 | Adult patients aged ≥ 18 years with LET for ≤ 3 months, and lack of serious systemic disease | Laser (1 J/cm2 2 minutes 5d per week for 3 weeks); n = 23 (elbows) | Placebo laser [(laser deactivated); 2 minutes 5 days per week for 3 weeks]; n = 24 (elbows) | Pain severity (VAS); tenderness (algometry); pain-free grip strength (dynamometer); Nottingham Health Profile: DASH, PRTEE | None |
| Akin et al., 2010187 (not reported) | Randomised, single blind, placebo controlled | 60 | Adults aged 25–62 years with LET | Ultrasound (15 sessions) + epicondylitis bandage; n = 30 | Placebo ultrasound (15 sessions) + epicondylitis bandage; n = 30 | Pain (VAS), hand grip strength (dynamometer; ADLs (DASH-Turkey); QoL (SF-36); patient satisfaction | 3 and 5 weeks |
| Paoloni et al., 2009188 (not reported) | Randomised, double blind, controlled | 136 | Adults patients aged 18–70 years with a diagnosis of chronic LET ≥ 3 months; ≥ 4 on a VAS with provocative elbow testing | OrthoDerm topical glyceryl trinitrate patch 0.03 mg/hour (0.72 mg/24 hours); n = 38 OrthoDerm topical glyceryl trinitrate patch 0.06 mg/hour (1.44 mg/24 hours); n = 30 OrthoDerm topical glyceryl trinitrate 15 mg/hour (3.6 mg/24 hours); n = 36 | Placebo patch; n = 32 | PRTEE; pain (VAS – at rest, with activity, intensity); function (grip strength, ORI-TETS); subjective global assessment of change in elbow symptoms | 8 weeks |
| McCallum et al., 2009189 (not reported) | Randomised, double blind, controlled (5-year follow-up data; trial data reported in Paoloni et al.106) | 58 | Adult patients with extensor tendinosis | Glyceryl trinitrate transdermal patch (one-quarter of a 5 mg/24-hour Nitro-dur patch); n = 27 | Placebo patch [one-quarter of a 5 mg/24-hour Nirto-dur (nitroglycerin) demonstration patch, (Merck, Sharp & Dohme, Whitehouse Station, NJ, USA)]; n = 31 | Pain (at rest, with activity, at night); local epicondylar and tendon tenderness; dynamometer-measured strength with Maudsley’s test; wrist extensor mean peak force; mean total work as measured by the ORI-TETS | None (5-year follow-up data) |
| Jafarian et al., 2009190 (not reported) | Randomised, crossover | 52 | Adults aged ≥ 18 years with LET ≥ 3 weeks | Elbow strap orthosis; n = 13 Elbow sleeve orthosis; n = 13. Wrist splint; n = 13 | Placebo orthosis; n = 13 | Maximum and pain-free grip strength (dynamometer) | None |
| Dogramaci et al., 2009191 (not reported) | Randomised, double blind | 75 | Adult patients with LET | Lidocaine (1 ml) + peppering; n = 25. Triamcinolone (1 ml) + lidocaine (1 ml) + peppering injection; n = 25 | Triamcinolone (1 ml) + lidocaine (1 ml) injection; n = 25 | Pain (patient assessed VAS 10 cm; satisfaction (Verhaar criteria) | 3 weeks, 6 months |
| Coff et al., 2009192 (not reported) | Randomised, controlled | 26 | Adults aged ≥ 18 years with LET (newly diagnosed or exacerbation of long-term LET); speak and understand English; and communicate perceived pain via VAS | InterX + soft-tissue massage, stretching, ultrasound and exercise; n = 13 | Soft-tissue massage, stretching, ultrasound and exercise; n = 13 | Pain (VAS 10 cm, patient rated); perceived difficulty in performing ADLs (VAS, patient rated); activities of personal care, household work, work, recreation/leisure, sleep (PRTEE); grip strength | 3 weeks, 9 months |
| Toker et al., 2008193 (not reported) | Randomised | 21 | Adults aged ≥ 18 years with LET | Oral and topical anti-inflammatory drugs; n = 10 | Single local injection of a corticosteroid and anaesthetic mixture; n = 11 | Pain (VAS 10 cm; activity) | 1 month |
| Sabeti et al., 2008194 (not reported) | Randomised, single blind | 20 | Adults with symptomatic LET > 6 months and failure on two different conservative therapies | ESWT 1000 shocks (three sessions); n = 10 | ESWT 2000 shocks (three sessions); n = 10 | Pain (VAS); force in maximum flexion of the fingers; subjective satisfaction and comfort | 12 weeks |
| Radwan et al., 2008195 (not reported) | Randomised, controlled | 56 | Adults aged ≥ 18 years with LET of elbow; failure of ≥ 6 months of conservative treatment (NSAIDs, corticosteroid injections, physical therapy, exercise programme, elbow brace) | ESWT (1500 shocks at 18 kV 0.22 mJ/mm2); n = 29 | Percutaneous tenotomy of the common extensor origin; n = 27 | Pain (VAS 100 mm); grip strength; residual pain (assessed at follow-up using Roles and Maudsley criteria) | 3, 6, 12 weeks, 12 months |
| Nourbakhsh and Fearon 2008196 (Department of Physical Therapy, North Georgia College and State University, Dahlonega, GA, USA) | Randomised, placebo controlled, double blind | 18 | Adults aged 24–72 years with chronic LET | Low-frequency electrical stimulation (intensity as tolerated) (six sessions); n = 10 | Low-frequency electrical stimulation (intensity set at zero) (six sessions); n = 8 | Grip strength; functional status; pain intensity; limited activity due to pain (assessed pre- and post-treatment) | 6 months (treatment group only) |
| Nourbakhsh et al., 2008197 (not reported) | Randomised, placebo controlled, double blind | 23 | Adults aged 24–72 years with chronic LET > 3 months | OEMT (oscillating energy focused on tender point) (six sessions); n = 11 | OEMT (oscillating energy directed above or below tender points) (six sessions); n = 12 | Grip strength (Jamar Dynamometer, PSFS and NRS); functional status; pain intensity; limited activity due to pain (assessed pre- and post-treatment) | 6 months (n=11) |
| Ho et al., 2007198 (not reported) | Randomised, controlled, single blind | 16 | Adults aged ≥ 18 years with LET > 3 months | Microcurrent therapy + exercise (10 sessions); n = 8 | Exercise only; n = 8 | Mechanical pain threshold; pain-free handgrip; maximum handgrip; pain aggravated by hand grip (VAS) (assessed baseline and end weeks 1 and 2, and follow-up) | 6 weeks |
Appendix 6 Cost-effectiveness review, excluded studies
| Papers excluded | Reason for exclusion |
|---|---|
| Buchbinder R, Richards BL. Is lateral epicondylitis a new indication for botulinum toxin? CMAJ 2010;182:749–50 | Study design; not CEA |
| Chesterton LS, van der Windt DA, Sim J, Lewis M, Mallen CD, Mason EE, et al. Transcutaneous electrical nerve stimulation for the management of tennis elbow: a pragmatic randomized controlled trial: the TATE trial (ISRCTN 87141084). BMC Musculoskel Disord 2009;10:156 | Study design; not CEA |
| Crowther MAA, Bannister GC, Huma H, Rooker GD. A prospective, randomised study to compare extracorporeal shock-wave therapy and injection of steroid for the treatment of tennis elbow. J Bone Joint Surg 2002;84:678–9 | Study design; not CEA |
| Derebery VJ, Devenport JN, Giang GM, Fogarty WT. The effects of splinting on outcomes for epicondylitis. Arch Phys Med Rehabil 2005;86:1081–8 | Study design; not CEA |
| Gosens T, Peerbooms JC, Laar W, Oudsten BL. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. Am J Sports Med 2011;39:1200–8 | Study design; not CEA |
| Jabbari B, Machado D. Treatment of refractory pain with botulinum toxins – an evidence-based review. PainMed 2011;12:1594–606 | Study design; not CEA |
| Kroslak M, Murrell GAC. Tennis elbow counterforce bracing. Tech Shoulder Elbow Surg 2007;8:75–9 | Study design; not CEA |
| Mishra A, Collado H, Fredericson M. Platelet-rich plasma compared with corticosteroid injection for chronic lateral elbow tendinosis. PM R 2009;1:366–70 | Study design; not CEA |
| Peerbooms JC, Sluimer J, Bruijn DJ, Gosens T. Positive effect of an autologous platelet concentrate in lateral epicondylitis in a double-blind randomized controlled trial: platelet-rich plasma versus corticosteroid injection with a 1-year follow-up. Am J Sports Med 2010;738:255–62 | Study design; not CEA |
| Smidt N, van der Windt DA, Assendelft WJ, Kreder HJ. Physiotherapy or a wait and see policy were the best options for lateral epicondylitis at 1 year. Evidence-Based Med 2002;7:153 | Study design; not CEA |
| Staples MP, Forbes A, Ptasznik R, Gordon J, Buchbinder R. A randomized controlled trial of extracorporeal shock wave therapy for lateral epicondylitis (tennis elbow). J Rheumatol 2008;35:2038–46 | Study design; not CEA |
| Thanasas C, Papadimitriou G, Charalambidis C, Paraskevopoulos I, Papanikolaou A. Platelet-rich plasma versus autologous whole blood for the treatment of chronic lateral elbow epicondylitis: a randomized controlled clinical trial. Am J Sports Med 2011;39:2130–4 | Study design; not CEA |
| Zacher J, Altman R, Bellamy N, Bruhlmann P, Da Silva J, Huskisson E, et al. Topical diclofenac and its role in pain and inflammation: an evidence-based review. Curr Med Res Opin 2008;24:925–50 | Study design; not CEA |
Appendix 7 Clinical effectiveness review, systematic reviews study characteristics and quality appraisal
| Date assessed as up-to-date review | Authors, year | Number of included studies (number of participants) | Methodological quality QR/QPS | Results | Notes |
|---|---|---|---|---|---|
| High quality (score ≥ 8 points on AMSTAR checklist) | |||||
| March 2009 | Barr et al., 200956 | Five RCTs (n = 597) | QR = high (AMSTAR 8) QPS: mean = 6.8 (range = 4–8) (PEDro scale; 11 points) | Physiotherapeutic interventions have a clinically significant effect on pain-free grip strength compared with the wait-and-see group at short-term follow-up (6 weeks), but only a small benefit at long-term follow-up (52 weeks) (two RCTs of adequate quality, pooled SE, not sufficient data). Corticosteroid injections are more effective than physiotherapeutic interventions for outcome measurements at short-term follow-up (between 3 and 7 weeks) (five RCTs: four adequate quality and one low quality). Pooled ES of pain-free grip strength (two RCTs of adequate quality), for short- (6 weeks), medium- (26 weeks) and long-term (52 weeks) follow-up. Forest plots presented, but no pooled data values stated. Despite corticosteroid injections being found to be more effective in the short-term compared with physiotherapeutic interventions, reported recurrence rates varied from 34% to 74% (three RCTs, adequate quality) | Corticosteroid injections are effective at short-term follow-up and physiotherapeutic interventions are effective at intermediate- and long-term follow-up [pooled ES for both outcome measures (pain free grip strength and rating of severity), not sufficient data]. At short-term follow-up there was no significant difference between the group receiving physiotherapeutic interventions and injections compared with injections alone (one RCT, low methodological quality). In the intermediate term, physiotherapeutic interventions were significantly more effective than corticosteroid injections (three RCTs, adequate quality) |
| 2004a | Trudel et al., 200457 | Five RCTs (n = 215) | QR: high (AMSTAR 8) QPS: range = 34–44 (out of 48) (MacDermid quality score) | No quantitative pooling, descriptive summary. Author’s conclusion: significant short-term effects in reducing pain using ultrasound. Similar reductions were seen with ultrasound in combination with friction massage, phonophoresis alone, phonophoresis with friction massage, and acupuncture. Significant increases in grip strength found with strengthening and stretching programs. No significant benefit was found for laser therapy. Progressive strengthening and stretching programmes demonstrated decreased pain compared with treatment alternatives. Strengthening and stretching programmes were also associated with an increase in grip strength (two RCTs; 78 participants). No significant difference was identified between laser and placebo (one RCT; 52 participants) | Evaluation of study (MacDermid quality score, 2004) and level of evidence (Sackett et al., 2000205) only grade 1b studies (n = 5) considered |
| February 2005 | Buchbinder et al., 200658 | 10 RCTs (n = 1099) | QR = high (AMSTAR 8) QPS: no validated scale used | ESWT is not more effective than placebo with respect to pain at rest at 4–6 weeks after the final treatment (three RCTs, including 446 participants), pooled WMD = –9.42 on a 100 VAS score (95% CI –20.70 to 1.86). ESWT is not more effective than placebo at 12 weeks after the final treatment with respect to pain provoked by resisted wrist extension (Thomsen test) (three RCTs, 455 participants), pooled WMD = –9.04 on a 100 VAS score (95% CI –19.37 to 1.28) and grip strength (SMD 0.05, 95% CI –0.13 to 0.24). Eleven of the 13 pooled analyses found no benefit of ESWT over placebo | – |
| January 1999 | Smidt et al., 200359 | 23 RCTs (n = NR) | QR = high (AMSTAR 8) QPS: mean = 6.7, range = 1–11 (Amsterdam–Maastricht Consensus list; 12 points) | There is weak evidence for the beneficial effects of ultrasound on pain in the intermediate term (two RCTs, SMD –0.98, 95% CI –1.64 to –0.33). There is no significant difference between laser therapy and placebo on pain in the short term (≤ 6 weeks) (eight RCTs). Exercise can significantly reduce pain (VAS) compared with ultrasound plus friction massage (SMD 0.95, 95% CI –1.64 to –0.26) (one RCT, adequate validity) | – |
| March 2010 | Coombes et al., 201060 | 17 RCTs (n = 1687) | QR = high (AMSTAR 8) QPS: mean = 9.8, range: 7–12 (modified PEDro scale range; 13 points) | Corticosteroid injections significantly reduces pain in the short term compared with no interventions (4 weeks, range 0–12) (SMD 1.44, 95% CI 1.17 to 1.71; p < 0.0001). Corticosteroid injections did not reduce pain in the intermediate term compared with no intervention (26 weeks, range 13–26) (SMD –0.40, 95% CI –0.67 to –0.14; p > 0.003) or long term (≥ 52 weeks) (SMD –0.31, 95% CI –0.61 to –0.01; p = 0.05). Sodium hyaluronate injections reduce pain compared with placebo in the short (SMD 3.91, 95% CI 3.54 to 4.28; p < 0.0001), intermediate (SMD 2.89, 95% CI 2.58 to 3.20; p < 0.0001) and long terms (SMD 3.91, 95% CI 3.55 to 4.28; p < 0.0001), botulinum toxin in the short term (SMD 1.23, 95% CI 0.67 to 1.78; p < 0.0001) and prolotherapy in the intermediate term (SMD 2.62, 95% CI 1.36 to 3.88; p < 0.0001) | – |
| Intermediate quality (score 4–7 points on AMSTAR checklist) | |||||
| January 2006 | Woodley et al., 200761 | Three RCTs (n = 184) | QR = high (AMSTAR 7) QPS: mean = 6.3, range = 5–8 (PEDro scale 1–11) mean = 7.3 range = 6–8 (van Tulder scale 0–11) | No quantitative pooling of data; descriptive summary. Author’s conclusions: there is insufficient quality evidence to suggest that eccentric exercise has a positive effect on clinical outcomes compared with concentric exercise, stretching, splinting, frictions and ultrasound | Mixed-patient population: tendinopathy of Achilles tendon, patella tendon and rotator cuff tendon (n = 11) |
| May 2008 | Bjordal et al., 200862 | 13 RCTs (n = 730) | QR = moderate (AMSTAR 7) QPS: mean = 6.5, range 4–8 (Delphi/PEDro checklist) | WMD for pain relief was 10.2 mm (95% CI 3.0 mm to 17.5 mm) and the RR for global improvement was 1.36 (95% CI 1.16 to 1.60). Trials that targeted acupuncture points reported negative results, as did trials with wavelengths 820 nm, 830 nm and 1064 nm. In a subgroup analysis using included studies (n = 5) with 904 nm lasers and one trial with 632 nm wavelength for which the LE tendon insertions were directly irradiated, WMD for pain relief was 17.2 mm (95% CI 8.5 mm to 25.9 mm) and 14.0 mm (95% CI 7.4 mm to 20.6 mm), respectively. RR for global pain improvement was only reported for 904 nm at 1.53 (95% CI 1.28 to 1.83); LLLT doses in this subgroup ranged between 0.5 and 7.2 J. Secondary outcome measures: pain-free grip strength improvement favouring LLLT (SMD 0.66, 95% CI 0.42 to 0.90; p < 0.0001). With subgroup analysis by application technique and wavelength, only trials with irradiation of tendons and wavelengths 632 nm or 904 nm showed positive results compared with controls (SMD 1.09, 95% CI 0.42 to 1.76 and SMD 1.30, 95% CI 0.91 to 1.68, respectively); and pressure pain threshold end of treatment (SMD 0.34, 95% CI 0.04 to 0.63); sick leave: relative risk for not being sick listed after treatment was significantly in favour of LLLT, RR 2.25 (95% CI 1.25 to 4.06; p = 0.0005) | – |
| 2011a | Kalichman et al., 201163 | Four RCTs (n = 273) | QR = moderate (AMSTAR 7) QPS: no validated scale used | Pooled results from a meta-analysis of the included RCTs show a moderate effect for pain favouring botulinum toxin: effect size –0.5; 95% CI –0.9 to –0.1, I2 = 56%) at 3 months. Effect size for pain also favoured botulinum toxin at 4 weeks: effect size –0.8, 95% CI –1.5 to –0.1 (based on three included studies). The pooled effect size for grip strength was 0.2 (95% CI –0.2 to 0.5). This is not statistically significant despite a trend towards favouring botulinum toxin | – |
| 2012a | Raman et al., 201264 | Six RCTs (n = 283) | QR = moderate (AMSTAR 7) QPS: mean score = 35 (range 32–40) (MacDermid quality score) | No quantitative pooling of data; descriptive summary. Author’s conclusions: included studies suggest that resistance exercise reduces pain and improves function for LE but optimal dose not defined | – |
| November 2008 | Rabago et al., 200965 | Three RCTs (n = 68) | QR = moderate (AMSTAR 7) QPS: mean = 7 (range 5–9) (Delphi score, 0–9) | No quantitative pooling of data; descriptive summary. Author’s conclusions: Results suggest each of the four therapies is effective for LE. Follow-up data (9–52 weeks) suggest sustained reduction in pain (relative effect sizes ranged from 51% to 68% Cohen’s d 1.4–6.68). Improvements were reported for isometric grip strength and grip strength | Mixed-study design: one RCT, one non-RCT and five prospective case series |
| November 2008 | Gaujoux-Viala et al., 200966 | Eight RCTs (n = 887) | QR = moderate (AMSTAR 7) QPS: mean = 3 (range 2–5) (Jadad scale; 1–5 points) | Quantitative pooling of data but mixed-patient population shoulder and elbow tendinitis; data not reported separately | Mixed-patient population: shoulder and elbow tendonitis (n = 16) |
| October 2010 | Zhang et al., 201167 | Three RCTs (n = 232) | QR = moderate (AMSTAR 7) QPS: mean = 5 (4–5) (Jadad score; 5 points) | Two studies included in meta-analysis. In results of subgroup meta-analysis pain relief was favoured versus control, SMD –0.27 (95% CI –0.86 to –0.01) | Mixed-patient population: shoulder pain, myofascial pain, whiplash, plantar fasciitis (n = 21 in SR; n = 15 in meta-analysis) |
| January 2005 | Bisset et al., 200568 | 28 RCTs (n = NR) | QR = moderate (AMSTAR 7) QPS: Mean = 9.4 (range 8–13) (modified PEDro rating scale; 1–15 points) | No quantitative pooling of data; descriptive summary. Author’s conclusions: evidence suggests no benefit with ESWT and insufficient evidence for the long-term benefit of physical interventions for the treatment of tennis elbow | |
| 2004a | Borkholder et al., 200470 | 11 RCTs (n = 312) | QR = moderate (AMSTAR 6) QPS: mean (adjusted) = 26.3 (range 44.5 to 16.5) (MacDermid quality score; Sackett’s Level 1b (n = 1), Level 2b (n = 10) | No quantitative pooling of data; descriptive summary | |
| April 2004 | Trinh et al., 200471 | Six RCTs (n = 282) | QR = moderate (AMSTAR 6) QPS: mean = 4 (range 3–5) (Jadad scale; 1–5 points) | No quantitative pooling of data due to heterogeneity; descriptive summary. Author’s conclusions: evidence from the included studies suggests acupuncture was successful for short-term LE pain relief than a control treatment (5/6 studies), and reduced pain compared with a form of sham acupuncture | |
| December 2010 | Taylor et al., 201171 | Three RCTs (n = 286) | QR = moderate (AMSTAR 6) QPS: no quality appraisal conducted | No quantitative pooling of data because of heterogeneity | Mixed-patient population: musculoskeletal soft-tissue injuries, rheumatologic diseases and osteoarthritis (n = 37) |
| 2010a | Tumilty et al., 201072 | 13 RCTs (n = 472) | QR = moderate (AMSTAR 6) QPS: mean = 6 (range 6–8) (PEDro rating scale; 11 points | Pooled results for studies with patient population with LE: grip strength (four studies) WMD 9.59 (95% CI 5.90 to 13.27) in favour of laser treatment (compared with control). Pooling of data was not valid for pain change score for LE | Mixed-patient population: LE, medial epicondylitis, shoulder tendinitis, suprasinitis tendinitis, Achilles tendinopathy, De Quervain’s tenosynovitis |
| 2008a | Zacher et al., 200873 | Four RCTs (n = 286) | QR = moderate (AMSTAR 6) QPS: no validated quality appraisal tool though some consideration for quality reported | No quantitative pooling of data; descriptive summary. Author’s conclusions: evidence from included studies suggests a reduction in pain and inflammation and improvement in patients’ functional capacity and mobility compared with placebo and comparable to other topical NSAIDs and some oral NSAIDs | Mixed-patient population: acute (blunt impact injuries, ankle sprain, rheumatic or traumatic conditions) and chronic conditions (knee osteoarthritis, osteoarthritis of the finger joint, LE periarticular states) (n = 19) |
| 2008a | Herd 200874 | 13 RCTs (n = 639) | QR = moderate (AMSTAR 5) QPS: mean = 5 (range 1–8) (PEDro rating scale; points 1–8) | No quantitative pooling of data; descriptive summary. Author’s conclusions: results support the use of Mulligan’s mobilisation with movement in providing immediate short- and long-term benefits. Although long-term effects were uncertain, results suggested a benefit of manipulative therapy directed at the cervical spine | – |
| 2012a | Joseph et al., 201275 | Three RCTs (n = 196) | QR = moderate (AMSTAR 5) QPS: mean = 7 (range 7) (PEDro rating scale; points 1–8) | No quantitative pooling of data because of heterogeneity; descriptive analysis. Author’s conclusions: evidence suggested a benefit of deep-friction massage in combination with a Mill’s manipulation for the treatment of elbow tendinopathy | Mixed-patient population (RCTs): LE and outlet impingement syndrome (n = 4), also includes non-randomised study designs (n = 5) |
| 2010a | Tumilty et al., 201076 | 11 RCTs (n = NR) | QR = moderate (AMSTAR 5) QPS: mean = 7 (range 5–8) (PEDro rating scale; 8 points) | Results reported in Tumilty et al., 201050 | Mixed-patient population: LET, rotator cuff tendinitis, Achilles tendinitis, various tendinopathies, medial epicondylitis (n = 25) |
| June 2008 | Baxter et al., 200877 | Three RCTs (N = 166) | QR = moderate (AMSTAR 4) QPS: mean 6 (range 5–7) (van Tulder scale; 11 points) | No quantitative pooling of data; descriptive analysis. Author’s conclusions: the clinical effect of laser acupuncture in the treatment of LE is uncertain because of limited evidence | Mixed population: soft-tissue injury, an acute or chronic pain condition or any systemic illness (e.g. myofascial pain, tension headache, post-operative nausea and vomiting) [18 RCTs (n = 1099) in total across all populations. Only three RCTs (n = 264) with lateral epicondylitis] |
| 2012a | Farren 201278 | 3 RCTs (n = 175) | QR = moderate (AMSTAR 4) QPS: mean = 4 (range 4–5) (Jadad score; 5 points) | No quantitative pooling of data; descriptive analysis. Author’s conclusions: mean pain relief reported as 55.8% (SD 2.95%) for acupuncture compared with 15.0% (SD 2.77%) for placebo suggesting a benefit associated with acupuncture for the treatment of lateral epicondylitis | – |
| 2008a | Kohia et al., 200879 | 16 RCTs (n = 1814) | QR = moderate (AMSTAR 4) QPS: no quality assessment tool used | No quantitative pooling of data; descriptive analysis. Author’s conclusions: in the long term (> 6 months), evidence did not suggest a difference between physical therapy, the wait-and-see method and corticosteroid injection. Acoustic shockwaves were not effective in the short (≤ 6 months) or long term (> 6 months) for decreasing pain or increasing grip strength. Evidence showed that physical therapy was more effective than either brace alone brace plus ultrasound in the short term. Corticosteroid injection was effective in both the short and long term and was more effective than Cyriax technique and elbow manipulation in the short term | Sackett’s level of evidence: Level I (n = 7); Level II (n = 9) |
| Low quality (score ≤ 4 points on AMSTAR checklist) | |||||
| 2009a | Bisset et al., 201180 | 56 RCTs + 18 SRs of RCTs | QR = low (AMSTAR 3) QPS: GRADE assessment | Overview of SRs; results summarised in main document, see Chapter 4, Bisset et al.,80 and Table 31 | – |
| 2009a | Chang et al., 201081 | 10 RCTs (n = 449) | QR = low (AMSTAR 3) QPS: mean = 5 (range 3–8) (PEDro rating scale; 11 points) | The effect of LLLT on pain relief after treatment was favourable (pooled estimate from three studies): ES (weighted) –0.71, 95% CI –0.82 to –0.60; p < 0.05. Similarly for LLLT on pain relief after follow-up the ES (weighted) –1.05, 95% CI –1.16 to –0.94; p < 0.05. The effect of LLLT on grasp force was favourable (pooled estimate from three studies): ES (weighted) 0.7, 95% CI 0.52 to 0.88; p < 0.05. Similar results were seen in favour of LLLT at follow-up: ES (weighted) 1.09, 95% CI 0.91 to 1.27; p < 0.05. The effect of LLLT on weight test (pooled estimate from two studies): ES (weighted) 0.58, 95% CI 0.37 to 0.81; p < 0.05. Similar results were seen at follow-up, ranging from 4 to 8 weeks: ES (weighted) 0.55, 95% CI 0.33 to 0.76; p < 0.050. The effect of LLLT on ROM (pooled estimate from two studies): ES (weighted) 1.27, 95% CI 0.37 to 0.81; NSD | – |
| 2012a | Snyder and Todd 201282 | Four RCTs (n = 470) | QR = Low (AMSTAR 3) QPS: mean = 7 (range 6–8) (PEDro rating scale; 8 points) | No quantitative pooling of data; descriptive analysis. Author’s conclusions: corticosteroid injections seem to be effective in the short-term relief of common wrist extensor pain; however, over the longer term they do not appear to be as effective and may have an adverse effect compared with other interventions (e.g. NSAIDs) or no treatment | – |
| 2009a | Pagorek 200983 | Two RCTs (n = 48) | QR = low (AMSTAR 3) QPS: no quality assessment tool used | No quantitative pooling of data; descriptive analysis. Author’s conclusions: there is some evidence to suggest that MWM treatment reduces pain and improves strength in adults with chronic LE | Mixed-study design: cohort, SRs (RCTs, cohort, case–control), case series, expert opinion (n = 9) |
| 2007a | Crawford and Laiou 200784 | 14 RCTs (n = NR) | QR = low (AMSTAR 1) QPS: quality assessed but no validated tool used | No quantitative pooling of data; descriptive analysis. Author’s conclusions: evidence in the included studies suggests a benefit of conservative treatments for LET management | Mixed-patient population: LE, medial epicondylitis, carpal tunnel syndrome, disorders of the shoulder, tension neck |
List of abbreviations
- ABI
- autologous blood injection
- ADL
- activity of daily living
- AMED
- Allied and Complementary Medicine Database
- AMSTAR
- Assessment of Multiple Systematic Reviews
- CDSR
- Cochrane Database of Systematic Reviews
- CENTRAL
- Cochrane Central Register of Controlled Trials
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- DARE
- Database of Abstracts of Reviews of Effects
- DASH
- disabilities of the arm, shoulder and hand questionnaire
- DASH-Q
- disabilities of the arm, shoulder and hand – quick questionnaire
- EQ-5D
- European Quality of Life-5 Dimensions
- ESWT
- extracorporeal shock wave therapy
- GCI
- glucocorticoid injection
- GRADE
- Grading of Recommendations, Assessment, Development and Evaluation
- HTA
- Health Technology Assessment
- ICUR
- incremental cost–utility ratio
- LET
- lateral elbow tendinopathy
- LLLT
- low-level laser therapy
- MeSH
- medical subject heading
- NHS EED
- NHS Economic Evaluation Database
- NICE
- National Institute for Health and Care Excellence
- NRS
- numerical rating scale
- NSAID
- non-steroidal anti-inflammatory drug
- PEDro
- Physiotherapy Evidence Database
- PenTAG
- Peninsula Technology Assessment Group
- PFFQ
- pain-free function questionnaire
- PRFEQ
- Patient-Rated Forearm Evaluation Questionnaire
- PRP
- platelet-rich plasma
- PRTEE
- Patient-Rated Tennis Elbow Evaluation questionnaire
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- RCT
- randomised controlled trial
- SD
- standard deviation
- SF-36
- Short Form questionnaire-36 items
- SMD
- standardised mean difference
- VAS
- visual analogue scale
- WMD
- weighted mean difference