Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/114/02. The contractual start date was in November 2011. The draft report began editorial review in August 2013 and was accepted for publication in February 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Crossan et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Liver fibrosis is scarring of the liver. 1 Subsequently, areas of regenerating hepatocytes surrounded by fibrosis tissue develop, resulting in the development of liver cirrhosis. 1 Fibrosis and cirrhosis form chronic liver disease. Every year, around 6000 to 7000 people in the UK die from chronic liver disease2,3 and about 600 adults have to have a liver transplant to survive. 4 In 2000, cirrhosis accounted for nearly 500 deaths in men aged 25 to 44 years and nearly 300 deaths in women of this age group, a seven- to eightfold increase in the deaths compared with the rate in 1970. 2 The age-standardised death rates from cirrhosis tripled from 2 per 100,000 population to 6 per 100,000 population between 1970 and 2000 in England,2 and doubled from 9 per 100,000 population to 19 per 100,000 population between 1979 and 2007 in Scotland. 5
Diagnostic testing for fibrosis and cirrhosis
Liver biopsy
Currently, histological examination of a tiny piece of liver tissue (liver biopsy) is considered the reference standard for the diagnosis and monitoring of liver fibrosis and cirrhosis. This is usually performed through the skin under the guidance of ultrasound6–8 and involves taking a small section of the lesion using a sharp hollow needle. This can usually be performed under local anaesthesia. 6–8 The main risks of percutaneous biopsy are clinically significant bleeding (1.1–1.6%),6,7 which can be fatal. 7
Histological examination provides a spectrum of information, including liver architecture, presence and extent of steatosis, presence and grade of necroinflammation and presence and extent of liver fibrosis. It can also provide a diagnosis in cases of unexplained liver function test abnormalities. This amount of information is not provided by any non-invasive test, as they are mainly confined in the assessment of liver fibrosis. Therefore, liver biopsy will remain essential in many cases, whereas non-invasive liver function tests will be used in cases where the aetiology of liver disease is known and the clinical question is the extent of fibrosis.
Liver fibrosis is assessed in liver histological scoring systems using various staging systems that assess liver architecture and fibrosis. Such systems include Ishak, Knodell, Sheuer and METAVIR. 9,10 The METAVIR scoring system stages fibrosis in five categories, from 0 to 4, while the Ishak system stages fibrosis in seven categories, 0 to 6. Cirrhosis always represents the end stage of the spectrum and is characterised by bridging fibrosis and regenerative nodules.
It should be stressed that histological stages are descriptive semiquantitative categories that assess both liver architecture and liver fibrosis and do not provide a quantitative assessment of liver fibrosis. 10,11 The numbers that have been assigned to histological stages have no quantitative relationship between them, i.e. METAVIR fibrosis stage 2 does not mean twice the amount of fibrosis of stage 1. 12 Therefore, non-invasive fibrosis markers, which assess fibrosis quantitatively, should be ideally developed and validated with reference to a histological quantitative assessment of liver fibrosis. 13,14 Such histological methods have indeed been developed and quantify fibrosis by measuring liver collagen using digital image analysis. 15–17
As liver biopsy assesses only a tiny amount of the liver, sample variability could potentially misclassify the extent of fibrosis. In addition, histological staging is also prone to intra- and interobserver variability, even when senior liver histopathologists are involved. A French study found that, in patients with chronic hepatitis C (HCV), 35% of biopsies 15 mm in length were not categorised correctly. 18 The study suggested that a sample at least 25 mm in length is necessary to evaluate fibrosis accurately with a semiquantitative score, with the possible exception of cirrhosis. Biopsies of such length are not always feasible with one needle pass in a percutaneous biopsy and, therefore, the patient’s discomfort and also the complication rate might increase. The misclassification rate (percentage of incorrect staging of fibrosis) of liver biopsy is the source of the myth that non-invasive fibrosis tests cannot achieve a high concordance with histological stages. This is only true for non-invasive tests for which their development was independent from liver histology, such as transient elastography (TE), although the diagnostic test accuracies of such tests are also evaluated using histology; it could be argued that in certain cases the false positive or false negative of such a test compared with the result of a liver biopsy is a fault of the biopsy rather than the test itself, i.e. the test diagnosed correctly what was missed by the biopsy. However, serum non-invasive fibrosis markers have been developed and calibrated with direct reference to a set of liver biopsies. Therefore, the perfect serum marker in this case would replicate the ‘golden’ histological standard and could theoretically reach an area under the receiver operator curve (AUROC) of 1, replicating even the misclassifications of a liver biopsy. 19
Non-invasive fibrosis tests
During the last few years, there has been an explosive development and use of non-invasive fibrosis tests. 20,21 These tests in many cases have replaced liver biopsy in clinical practice in the staging of fibrosis and follow-up of patients with established chronic liver disease, especially in patients with chronic HCV. The non-invasive liver tests (NILTs) can be broadly divided into three categories: simple or indirect serum markers, direct serum markers and imaging modalities.
Indirect serum markers or class II biomarkers consist of the combination of routine biochemical or haematological tests, such as transaminases, platelet count and albumin, and patient demographics that are associated with fibrosis, such as age or the presence of diabetes. 20 These tests usually have dual cut-offs: a high cut-off with high specificity and a low cut-off with high sensitivity. Depending on the clinical scenario and the disease prevalence, the low or high cut-off is used at the expense of increased false positives and false negatives, respectively. If these cut-offs are combined, then the number of false positive and false negatives are minimised; however, a number of patients will fall in the indeterminate range of fibrosis (i.e. their score will be between the low and the high cut-off) and will need either further non-invasive testing or a liver biopsy. Commonly used indirect serum markers are FIB-4, aspartate aminotransferase (AST)-to-alanine aminotransferase (ALT) ratio and APRI (AST to platelet ratio index).
Direct serum non-invasive tests or class I biomarkers are intended to detect extracellular matrix turnover and/or fibrogenic cell changes. The most common markers used in current assays involve measuring products of extracellular matrix synthesis or degradation, and the enzymes that regulate their production or modification, such as hyaluronic acid, serum collagenases and their inhibitors and profibrogenic cytokines. It should be noted that these markers are not exclusively found in liver tissue; therefore, they reflect fibrogenic processes in various other organs. For instance, the enhanced liver fibrosis (ELF) biomarker is influenced by age and sex. 22 Moreover, their sensitivity is low in the initial stages of fibrosis.
Various direct and indirect tests have been combined in patented commercial algorithms that improve the diagnostic accuracy of tests when used singly. These are ELF, Fibrotest, Fibrospect, Fibroindex and Fibrometer. Of the tests, Fibrotest (Fibrosure in the USA) is the most widely validated panel; it consists of five parameters, namely total bilirubin, haptoglobin, gamma-glutamyl-transpeptidase, α2-macroglobulin and apolipoprotein A1, and has been studied in viral hepatitis, non-alcoholic fatty liver disease (NAFLD) and alcoholic liver disease (ALD). 23 The Fibroindex was developed for patients with chronic HCV and uses platelet count, AST and g-globulin levels. 24 Fibrospect includes hyaluronate, tissue metalloproteinase (TIMP)-1 and α2-macroglobulin, and is validated in chronic HCV. 25 Fibrometers are a family of six blood tests: one for staging and one for quantifying liver fibrosis in each of the three main causes of liver disease (chronic viral hepatitis, ALD and NAFLD). 26 The ELF biomarker is a panel of direct noninvasive markers that includes hyaluronic acid, type III collagen and TIMP-1. 27 It has been used in patients with chronic HCV and NAFLD.
New imaging modalities offer better sensitivity and specificity than conventional techniques, such as ultrasound, computed tomography (CT) and magnetic resonance imaging (MRI). The last of these can only identify cirrhosis, based on imaging findings of coarse echo-texture, collaterals suggestive of portal hypertension and nodularity. These new modalities measure liver elasticity or liver stiffness based on ultrasound or magnetic resonance (MR) techniques. The most widely used imaging modality is TE or Fibroscan (Echosens, Paris). 28 Briefly, vibrations of mild amplitude and low frequency are transmitted by an ultrasound transducer, inducing an elastic shear wave that propagates within the liver. Pulse-echo ultrasonic acquisitions are performed to follow the shear wave and measure its speed, which is directly related to the tissue stiffness (the harder the tissue, the faster the shear propagates). Results are expressed in kilopascals (kPa) and correspond to the median value of 10 validated measurements ranging from 2.5 to 75 kPa, with 5.5 kPa reported to define normality. The volume of liver tissue evaluated by TE approximates a cylinder 4 × 1 cm which is at least 100 times bigger than a liver biopsy. Moreover, TE is painless and rapid (< 5 minutes) and thus highly acceptable for patients.
Other modalities include acoustic radiation force impulse (ARFI)29 and MR elastography. 30 ARFI allows the evaluation of liver stiffness in a region of interest (ROI) involving mechanical excitation of tissue by the use of short-duration (≈262 μs) acoustic pulses while performing a real-time B-mode conventional hepatic ultrasound. Results are expressed in m/s. Although the volume of liver explored is smaller than that for TE (10 mm long × 6 mm wide), a critical advantage is the possibility to choose the representative area of interest, thereby avoiding large vessels and ribs. An advantage over TE is that it can be easily incorporated into a modified ultrasound machine. MR elastography uses a modified phase-contrast method to evaluate the propagation of the shear waves within the liver. It is a very promising technique but is not yet widely available and cost might be an important limiting factor.
Finally, algorithms of sequential or contemporary use of NILTs have been used mainly in chronic HCV, to improve the diagnostic accuracy of single tests. 31 These are typically based on an agree–disagree scenario or the sequential use of a second test if the result of the first test falls in the grey zone of an indeterminate result.
A major limitation of all the above NILTs is the absence of uniformly established and validated cut-offs for specific aetiologies of liver disease and fibrosis stages and the poor methodological quality of many of the published studies. In a recent meta-analysis on TE, only 6 of 41 included studies had both histological evaluation and Fibroscan measurements optimally performed, while all studies had a high risk of bias based on quality assessment by the Quality Assessment of Diagnostic Accuracy Studies (QUADAS) tool. 32
Aetiologies assessed
The study population comprised all patients with chronic liver disease (irrespective of the aetiology for chronic liver disease, age and clinical presentation). The aetiologies modelled and assessed were hepatitis B (HBV), HCV, ALD and NAFLD. We modelled and analysed these four aetiologies of liver disease, as the staging of fibrosis is pertinent in their prognosis and management. In all other causes of chronic liver disease, only the diagnosis of cirrhosis is important and liver biopsy is seldom performed for staging of fibrosis. Therefore, patients with primary biliary cirrhosis, autoimmune hepatitis, haemochromatosis and Wilson’s disease are treated irrespective of fibrosis stage, whereas fibrosis evaluation is not pertinent in primary sclerosing cholangitis. 33–35 We also modelled liver cirrhosis irrespective of aetiology, as this diagnosis is important for every patient with chronic liver disease and heralds screening for oesophageal varices and hepatocellular carcinoma. 36
Chronic HCV is a major cause of liver-related morbidity and mortality worldwide, and it is estimated that around 200 million people are infected worldwide. 37 The diagnosis of chronic HCV is based on serological testing and does not require a liver biopsy. The natural history of chronic HCV is variable; it is estimated that one-third of the infected patients will progress to cirrhosis. 38 Factors that are associated with fibrosis progression are age at infection > 40 years, male sex, obesity, alcohol abuse, presence of diabetes and coinfection with human immunodeficiency virus (HIV). 37,38 Current therapeutic options include dual therapy with pegylated interferon and ribavirin in patients with genotypes 2, 3 and 4, and triple therapy with the addition of boceprevir or telaprevir in patients with genotype 1. The sustained virological response (SVR) rate in previously untreated patients is approximately 70% in genotype 4 and 80% in genotypes 1, 2 and 3. 37,39,40 Treatment is less successful in patients who were previously unsuccessfully treated, in obesity and in more advanced fibrosis. 37 Currently, antiviral treatment is recommended for all patients with chronic HCV irrespective of stage of fibrosis.
However, the decision to treat or not to treat is not always straightforward. 41,42 Many patients cannot tolerate the side effects of antiviral treatment, have unfavourable treatment response factors and in fact might never progress to severe fibrosis. Moreover, new treatment options with better efficacy and fewer side effects are rapidly emerging. 43,44 Non-invasive fibrosis tests offer the option not only of baseline fibrosis staging but also of follow-up measurements to determine the rate of fibrosis progression. Therefore, an alternative option would be to use an effective non-invasive fibrosis test for staging and treat only those patients with F2 and above, which represents clinically significant fibrosis. The test could be repeated in order to capture the false negatives and also potential fibrosis progression.
Chronic HBV is highly prevalent worldwide and it estimated that 350–400 million people are HBsAg carriers. 45 The natural history of the disease is variable; the virus itself is hepatotropic but not hepatotoxic, and liver damage is caused when the immune system attacks the hepatocytes that are infected by the virus. The natural history of the disease can be divided in four distinct phases. 46
-
(1) The ‘immune tolerant’ phase is characterised by hepatitis B e antigen (HBeAg) positivity and high levels of virus replication but normal transaminases and no or minimal necroinflammation and progression of fibrosis. The virus, although in high concentrations, is not recognised by the immune system at that phase. This phase usually occurs in patients with perinatal infection in the first years of their lives.
-
(2) The ‘immune reactive HBeAg(+)’ phase is characterised by immune reaction, which leads to decreased HBV replication but also to destruction of hepatocyte, elevated transaminases, necroinflammation and fibrosis. This phase may last for several years and leads to HBeAg seroconversion to anti-HBe.
-
(3) The ‘inactive HBC carrier state’ phase is characterised by low or undetectable HBV deoxyribonucleic acid (DNA) and normal transaminases. This phase is characterised by immunological control of the infection and is associated with low risk of cirrhosis.
-
(4) The ‘HBeAg(–) chronic hepatitis B’ phase may follow immediately after the HBeAg sero-conversion or after years in the inactive carrier state. It is characterised by periodic virus reactivation with a pattern of fluctuating levels of HBV DNA and aminotransferases, active necroinflammation and progression of fibrosis.
Patients in the ‘immune tolerant’ and the ‘inactive carrier’ phase do not need antiviral treatment as they are not at imminent risk of fibrosis progression, but require regular follow-up with determination of viral load and transaminases. 45 Available treatment options include nucleoside or nucleotides analogues indefinitely or pegylated interferon alfa-2b for a finite period of 12 months. Treatment indications are based on the combination of criteria that take into account the HBV DNA levels, ALT levels and severity of liver disease based on histology. Current treatment guidelines advocate liver biopsy before initiating treatment in the majority of cases. The only exception is patients with obviously active chronic HBV, i.e. those with ALT > 2 upper limit of normal (ULN) and HBV DNA > 20,000 IU/ml, who may start treatment without a biopsy. 45 Patients with abnormal transaminases, HBV DNA > 2000 IU/ml and a biopsy showing moderate to severe active necroinflammation and/or at least moderate fibrosis using a standardised scoring system should be started on antiviral treatment. 45 Non-invasive fibrosis tests could potentially substitute liver biopsy in such patients, i.e. those with a non-invasive diagnosis of ≥ F2. A minority of patients with moderate necroinflammation but < F2 fibrosis, who would need treatment according to guidelines, would not be captured with a non-invasive fibrosis test, and would only be treated once they progressed to F2.
Non-alcoholic fatty liver disease affects approximately 20% of the general population and encompasses a wide range of liver disease, from simple steatosis to necroinflammation, fibrosis and cirrhosis. 47 It is associated with obesity and is considered the hepatic manifestation of metabolic syndrome. 48 Non-alcoholic steatohepatitis (NASH) is the progressive form of NAFLD and affects 15–20% of patients with NAFLD. 49 Only patients with steatohepatitis have increased liver-related mortality. 47
Data on natural history of NAFLD are still scarce; in a meta-analysis of 10 studies comprising 221 patients, 37.6% had progressive fibrosis, 41.6% had no change and 20.8% had improvement in fibrosis over a mean follow-up of 5.3 years. 50 Age and initial necroinflammation grade were the only factors associated with progression of fibrosis. 50 Even in patients with NASH, the primary cause of death was cardiovascular disease, with liver disease being only the third cause. 51 Compensated cirrhosis due to NASH is associated with a lower mortality rate than that due to HCV, and also with lower rates of development of ascites, hyperbilirubinemia and hepatocellular carcinoma. 52
Treatment strategies for NAFLD/NASH are mainly based on lifestyle changes, including weight loss and exercise, and treatment of the individual components of the metabolic syndrome, such as diabetes, hypertension and hyperlipidaemia. 47 Vitamin E in non-diabetic patients and pioglitazone may improve steatosis and necroinflammation but not fibrosis, as shown in randomised controlled trials (RCTs). 47
Currently, no validated non-invasive tests are available to differentiate NAFLD from NASH. 47 Diagnosis of patients with advanced fibrosis (≥ F3) is of significance, as such patients could benefit from multidisciplinary treatment of metabolic syndrome components, targeted intervention for weight loss and specific treatment (vitamin E or pioglitazone) in selected cases.
Alcoholic liver disease encompasses a spectrum of injury that ranges from simple steatosis to cirrhosis. 53 The amount of ingested alcohol is the most important risk factor for the development of ALD. 54 Suggested safe limits are 21 units per week in men and 14 units per week in women. 53 Development of ALD is not dose dependent, as ALD is found in only a subset of patients. Women are more susceptible to alcohol-mediated liver injury than men. 55 Binge drinking and consumption of alcohol outside meal times are both associated with a higher risk of ALD. 55 The risk of developing cirrhosis is increased with ingestion of > 60–80 g/day of alcohol for > 10 years in men and > 20 g/day in women. 53
The only effective treatment in patients with ALD is abstinence. 53 Prognosis is determined both by the degree of liver fibrosis and by the subsequent drinking behaviour. Interestingly, 5-year mortality in patients with well-compensated ALD cirrhosis was 10% in those who abstained and 30% in those who continued drinking. 56 Abstinence improves the histological features of ALD and may reverse fibrosis or decompensated cirrhosis to compensated cirrhosis. Diagnosis of patients with advanced fibrosis (≥ F3) is of significance, as it will allow the timely provision of interventions to induce and maintain abstinence before cirrhosis occurs.
Decision problem to be addressed
As liver biopsy is an invasive procedure and is associated with morbidity and mortality risk, it is important (1) to assess the diagnostic accuracy of the different non-invasive fibrosis tests available and (2) to determine the most cost-effective approach in the clinical management of patients with chronic liver disease using either biopsy or non-invasive fibrosis tests for clinical decisions.
A range of non-invasive tests have become available and offer potential alternatives to liver biopsy. In order to assess the most appropriate use of the tests within a NHS setting, the relative accuracy and cost-effectiveness of the tests need to be evaluated. Furthermore, as liver biopsy is costly, and associated with morbidity and a small risk of mortality, the non-invasive tests may offer cost-effective alternatives.
Our analysis aims to assess the diagnostic accuracy and cost-effectiveness of the non-invasive tests in people with suspected liver fibrosis or cirrhosis. The tests are compared with each other, liver biopsy and strategies without testing. Fully incremental analyses are conducted wherever possible.
When assessing the cost-effectiveness of a test, it is important to consisder the consequences of the test result. A positive test result is likely to lead to a different course of treatment or action than a negative result; therefore, the consequences of an incorrect positive diagnosis are likely to differ from the consequences of an incorrect negative diagnosis. In order to reflect this, and a range of mobidity outcomes and mortality, our analyses are conducted using the quality-adjusted life-year (QALY) as the measure of outcome where possible. Where this has not been possible, analyses have been conducted to reflect potential differences between positive and negative diagnoses.
Structure of report
The rest of this report is structured as follows. The methods of the systematic review and overall methodological approach to the cost-effectiveness analysis are described in Chapter 3. Chapter 4 presents results of the systematic review and meta-analysis. Chapters 5–9 present the aetiology-specific methods and results of the cost-effectiveness analyses for HBV, HCV, ALD, NAFLD and cirrhosis, respectively. Chapter 10 is a discussion of the findings from the study is provided and Chapter 11 presents our conclusions.
Chapter 2 Objectives
There were two related objectives for the study:
-
To compare the diagnostic accuracy of different non-invasive tests in the diagnosis and monitoring of liver fibrosis and cirrhosis in patients with various aetiologies for chronic liver disease.
-
To estimate the incremental cost-effectiveness of the non-invasive tests in patients with various aetiologies for chronic liver disease.
Chapter 3 Methods of systematic review and economic evaluation modelling
Section 1 outlines the systematic review and meta-analysis methodology used in the study. Section 2 outlines the modelling methodology employed for the five aetiologies; HBV, HCV, NAFLD, ALD and cirrhosis.
Section 1: overview of systematic review methodology
Criteria for considering studies for review
The aim of the systematic review was to identify papers comparing the diagnostic accuracy of different non-invasive tests in the diagnosis and monitoring of liver fibrosis and cirrhosis with liver biopsy, and to synthesise the outcomes where possible. We included studies providing cross-sectional information of the index test(s) and reference test. In other words, we included all studies that reported staging of fibrosis by index test(s) and reference standard so that it is possible to know how many patients had a certain stage of fibrosis by index test and reference test (true positive), how many had that stage by index test but not on the reference test (false positive), how many did not have that particular stage by index test but were found to have that stage by reference test (false negative), and how many patients did not have a certain stage of fibrosis by index test or reference test (true negative) in the appropriate patient population, irrespective of language or publication status, or whether data were collected prospectively or retrospectively.
We also included comparative studies in which the different index tests were performed in the same study population, or studies in which different individuals in the study population received different index tests, and the choice of tests that the different individuals received were determined in a random manner or if all the patients underwent both the index tests that were assessed. We excluded diagnostic case–control studies from the analysis if there were at least four cross-sectional or comparative studies for that test. We also excluded studies where the maximum interval between the reference standard (liver biopsy) and the non-invasive fibrosis test (index test) was > 6 months.
Participants
Adult patients with chronic liver disease (irrespective of the aetiology and clinical presentation). Studies reporting on paediatric patients were excluded.
Index tests
Ultrasound, CT scan, MRI, elastography (TE by ultrasound or MR elastography), and direct and indirect serum markers (such as AST–ALT ratio, APRI, ELF test, Fibrotest, etc.).
Target condition
Liver fibrosis and cirrhosis.
Reference standards
Histopathological examination of liver tissue (percutaneous or transjugular or laparoscopic biopsy). The staging and grading of liver biopsy can be performed by various histological scoring systems such as Ishak, METAVIR, Knodell and others. 57 We included studies irrespective of the histological scoring system used. For data synthesis and analysis we transformed the histological scores used in individual studies to METAVIR for HBV, HCV and alcohol and to Kleiner for NAFLD/NASH as these are the most commonly used histological scores. Conversion of various histological stages to METAVIR is shown in Table 1.
| Ishak | Knodell | Scheuer | METAVIR |
|---|---|---|---|
| 0 | 0 | 0 | 0 |
| 1 | 1 | 1 | 1 |
| 2, 3 | 1 | 2 | 2 |
| 4, 5 | 3 | 3 | 3 |
| 6 | 4 | 4 | 4 |
Search methods for identification of studies
Electronic searches
The following databases were searched from 1988 until April 2012: MEDLINE (PubMed), EMBASE, Science Citation Index Expanded, Bioscience Information Service (BIOSIS), Cochrane Central Register of Controlled Trials (CENTRAL), Latin American and Caribbean Health Sciences Literature (LILACS) and Cumulative Index to Nursing and Allied Health Literature (CINAHL). 58,59
The search strategies for the different databases are provided in Appendix 1.
Initially, we did not use any filter; however, this yielded 200,000 references and a compromise had to be arranged, as it would not be possible to complete the analysis within the time scale allowed for this study. Therefore, a methodological filter is included but does not act as a filter for all search results (see Appendix 1). This represents a potential limitation in our search strategy.
Searching other sources
Reference lists of identified studies and reviews, and conference proceedings from the recent hepatobiliary conferences (last 2 years), were hand-searched to identify further studies.
Data collection and analysis
Selection of studies
The references were searched by two researchers independently for identification of relevant studies. No restrictions were placed on the language or the publication status (full text vs. abstract from conference proceedings). However, studies which reported on a total of fewer than 10 patients with fibrosis or cirrhosis were excluded. Full texts were obtained for the references that at least one of the reviewers considered relevant. Full-text articles were then used to include or exclude studies for the review.
Data extraction and management
Data were extracted by two reviewers independently. Any differences in the data extraction were resolved by the lead applicant, Professor Burroughs, and Dr Gurusamy. Data necessary to calculate the true positive, false positive, true negative and false negative diagnostic test results were extracted using the reference standard of liver biopsy. If the information on true positive, false positive, false negative and true negative diagnostic test results were not available directly, these were calculated from information available in the study. Data were entered into a Microsoft Excel (Microsoft Corporation, Redmond, WA, USA) file created for the purpose.
The following data were extracted:
-
year of publication
-
country/ethnicity of included patients
-
inclusion criteria
-
exclusion criteria
-
total number of patients
-
patients included in the analysis
-
mean age
-
mean body mass index (BMI)
-
sex
-
mean ALT
-
aetiology of liver disease
-
technical failure in undertaking liver biopsy or non-invasive tests
-
non-invasive test used
-
fibrosis histological scoring system used
-
non-invasive test cut-off for diagnosing specific fibrosis stages
-
distribution of patients across histological stages
-
sensitivity, specificity, true positive, false positive, false negative, true negative of non-invasive test for diagnosing different histological stages
-
number of patients with uninterpretable liver biopsies or index tests
-
number of patients with indeterminate non-invasive test for a specific fibrosis stage
-
methodological quality using the QUADAS-2 assessment tool.
Assessment of methodological quality
The quality of the studies was assessed independently by two reviewers using the QUADAS-2 assessment tool. 60–62 This tool comprises four domains: patient selection, index test, reference standard, and flow and timing. Each domain is assessed in terms of risk of bias, and the first three domains are also assessed in terms of concerns regarding applicability. Signalling questions are included to help judge the risk of bias. The quality criteria that were derived from the QUADAS-2 tool and were assessed are shown in Table 2.
| Quality assessed | Description | Choice | Comment |
|---|---|---|---|
| Domain 1: patient sampling | Was a consecutive or random sample of patients enrolled? | Yes/no/unclear | |
| Was a case–control design avoided? | Yes/no/unclear | ||
| Did the study avoid inappropriate exclusions? | Yes/no/unclear | For example exclusion of patients with severe or low fibrosis, obese, etc. | |
| Risk of bias | Could the selection of patients have introduced bias? | Low risk/high risk/unclear | Summarises previous questions: if any has no as answer then high risk, if any has unclear then unclear |
| Concerns about applicability | Are there concerns that the included patients and setting do not match the review question? | High/low concern/unclear | Tertiary centres, selected difficult cases |
| Domain 2: index test | Were the index test results interpreted without knowledge of the results of the reference standard? | Yes/no/unclear | Relevant only in US, CT, MRI |
| If a threshold was used, was it prespecified? | Yes/no/unclear | ||
| Risk of bias | Could the conduct or interpretation of the index test have introduced bias? | Low risk/high risk/unclear | Summarises previous questions: if any has no as answer then high risk, if any has unclear then unclear |
| Concerns about applicability | Are there concerns that the index test, its conduct, or interpretation differs from the review question? | High/low concern/unclear | Index test not conducted according to manufacturer recommendations |
| Domain 3: reference standard | Is the reference standard likely to correctly classify the target condition? | Yes/no/unclear | Yes if biopsy length > 15 mm and/or > 6 portal tracts |
| Was the reference standard results interpreted without knowledge of the results of the index tests? | Yes/no/unclear | ||
| Risk of bias | Could the reference standard, its conduct, or its interpretation have introduced bias? | Low risk/high risk/unclear | Summarises previous questions: if any has no as answer then high risk, if any has unclear then unclear |
| Concerns about applicability | Are there concerns that the target condition as defined by the reference standard does not match the question? | High/low concern/unclear | Always low concern |
| Domain 4: flow and timing | Was there an appropriate interval between index test and reference standard? | Yes/no/unclear | Yes if interval between biopsy and index test < 3 months, no if interval > 3 but < 6 months, excluded study if interval > 6 months |
| Did all patients receive the same reference standard? | Yes/no/unclear | ||
| Were all patients included in the analysis? | Yes/no/unclear | No if patients with uninterpretable results were not included in the analysis or if there were patients with indeterminate results | |
| Risk of bias | Could the patient flow have introduced bias? | Low risk/high risk/unclear | Summarises previous questions: if any has no as answer then high risk, if any has unclear then unclear |
Statistical analysis and data synthesis
The data obtained from the various studies are presented in the form of summary sensitivity and specificity with their corresponding 95% confidence intervals (CIs). The data were combined using the bivariate random-effects model with correlation between sensitivity and specificity63 using the METADAS macro developed by the Systematic Review Diagnostic Test Accuracy Group64 in the SAS 9.2 statistical software (SAS Institute Inc., Cary, NC, USA). We calculated the summary sensitivity and specificity at specific thresholds for tests with explicit thresholds such as serum markers and calculated the overall summary sensitivity and specificity for tests that do not have an explicit threshold (such as ultrasound).
The bivariate model allows for meta-analysis of diagnostic test accuracy studies to be conducted in which both the index test under study and the reference test (gold standard) are dichotomous. Bivariate analysis involves statistical distributions at two levels. At the lower level, it models the cell counts in the 2 × 2 tables extracted from each study using binomial distributions and logistic (log-odds) transformations of proportions. At the higher level, random study effects are assumed to account for heterogeneity in diagnostic test accuracy between studies beyond that accounted for by sampling variability at the lower level. 65
If the results did not converge using the above random-effects model with correlation between sensitivity and specificity, we performed the meta-analysis with variations of bivariate analysis. The variations included different assumptions such as no correlation between the sensitivity and specificity in the studies; random-effects model for sensitivity but fixed-effect model for specificity; fixed-effect model for sensitivity but random-effects model for specificity; and fixed-effect models for both sensitivity and specificity (Takwoingi, University of Birmingham, March 2013, personal communication).
It must, however, be pointed out that the assumptions used to perform the above analysis (e.g. if one assumes that there is no correlation between the sensitivity and specificity, one has to ensure this from a scatterplot and correlation coefficient, and when one assumes a fixed-effect model, the values should be relatively close to each other) were not always met and the summary values of a model that converged was used. This could have resulted in a biased effect estimate. The alternative was not to conduct a meta-analysis for those tests which would have meant that the information could not be used in the cost-effectiveness analysis.
We also calculated the median, the lowest and the highest prevalence for the specific stages of fibrosis in the studies included.
Investigations of heterogeneity
The following sources of heterogeneity were explored.
-
Studies of high methodological quality versus low methodological quality.
-
Different stages of fibrosis (different scoring systems were converted to comparable stages in METAVIR in viral diseases and alcohol, and to Kleiner scoring system in NAFLD).
-
Different reference histological scoring systems (e.g. Ishak scoring, METAVIR, Knodell score, etc.). 57
-
Different aetiological diagnosis (e.g. ALD, HCV infection, etc.).
-
Different threshold levels for classification of positive and negative results. We performed a meta-analysis for every possible cut-off in each fibrosis stage of the reference standard.
-
Studies not published in full text were compared with studies published in full text.
-
Different ranges of transaminases (normal, between normal and up to three times the normal level, and more than three times the normal level).
Section 2: overview of economic modelling methodology
The population of interest is patients who are suspected of having liver fibrosis or cirrhosis (patients who a hepatologist would wish to biopsy to inform treatment decisions). Owing to differences in treatment and natural history of disease, the analysis is conducted separately for subgroups defined according to aetiology. Five subgroups are defined for the analysis: patients with HBV, HCV, ALD, NAFLD and cirrhosis. More details are given in the dedicated chapters according to disease aetiology (see Chapter 5 for HBV, Chapter 6 for HCV, Chapter 7 for ALD and Chapter 8 for NAFLD).
The overall aim of the health economic analysis was to assess the incremental cost-effectiveness of the NILTs. Wherever possible, the analyses take a lifetime perspective. Health outcomes were measured using QALYs. A NHS perspective was taken for the estimation of costs. Both costs and QALYs were discounted at 3.5% in accordance with current National Institute for Health and Care Excellence (NICE) guidelines. 66
The consequences following diagnosis are estimated and included in the analyses. In most cases the test diagnoses are expected to potentially affect decisions about future treatments (HBV, HCV and cirrhosis) or behaviour change (ALD). The long-term costs and health outcomes as a result of these treatments/behaviour changes are taken into account in the analysis (including the potential impacts of correct and incorrect diagnoses). Where this has not been possible, due to insufficient evidence or lack of treatments specifically aimed at fibrosis, the analysis has been restricted to an incremental cost per correct diagnoses, supplemented by exploratory analyses (NAFLD). In the cost per correct diagnoses, correct positive diagnoses have been presented separately from correct negative diagnoses as the consequences of each are likely to be very different.
Comparators
Where a large number of applicable NILTs were located by the systematic literature review (HBV and HCV), a two-stage approach to the analysis was conducted. The first stage compared each NILT identified from the systematic review (per aetiology) with each other and with liver biopsy. Where analyses involved treatment (HBV, HCV and cirrhosis), two additional testing approaches were included: a ‘treat all’ approach, where everyone is treated, and a ‘no treatment’ approach, where no diagnostic tests or treatments are administered.
The second stage of the analysis evaluated comparisons of sequential testing strategies, again compared with each other, biopsy and the treat-all and treat-no-one strategy. For this, combinations of the two most cost-effective tests within each category were chosen based on an incremental analysis using a cost-effectiveness threshold value of £20,000. 66 We assumed a decision rule whereby the two most cost-effective tests from each category were combined with tests from the other categories (reflecting combinations which would happen in actual current practice or potential future practice). Some of the NILTs evaluated have defined ‘low’ or ‘high’ cut-off thresholds and were analysed as separate test options. Combinations of tests considered to be clinically implausible were excluded; for example, a NILT with a low cut-off diagnostic threshold would not be followed by a second NILT with a low cut-off diagnostic threshold in practice but could be followed by a test with a high cut-off threshold. The following assumptions were made when combining the tests:
-
If the first NILT used was an indirect serum marker, a patented or direct serum marker or an imaging modality or liver biopsy could be administered as a second test.
-
If the first NILT used was a direct or patented serum marker, an imaging modality or liver biopsy could be administered as a second test.
-
If the first test used was an imaging modality, a liver biopsy could be administered as a second test.
The analysis also assumed that the sensitivity and specificity of each test were independent of each other, i.e. there was no correlation of sensitivities and specificities of the tests used in the first stage and the second stage. The combinations were assumed to take four possible sequential testing strategies (Table 3).
| Strategy number | First NILT result | Second NILT result | ||
|---|---|---|---|---|
| Positive | Negative | Positive | Negative | |
| Strategy 1 | Treat patients | Liver biopsy | ||
| Strategy 2 | Do second test | Watchful waiting | Treat patients | Liver biopsy |
| Strategy 3 | Do second test | Liver biopsy | Treat patients | Liver biopsy |
| Perform two NILTs regardless of test outcome | ||||
| Strategy 4 | Agree (+): treat | Disagree: liver biopsy | ||
| Agree (–): watchful waiting | Positive: treatment | |||
| Negative: watchful waiting | ||||
The probabilities of having each of the four possible diagnoses (true negative, true positive, false negative, false positive) for the four sequential testing strategies were determined by multiplying the probabilities (i.e. using decision tree calculation methodology: multiplying probabilities along pathways from left to right to estimate the probability of each pathway).
Each of the sequential tests were compared with each other; liver biopsy alone; ‘treat all’ and ‘no treatment’ approaches; each cost-effective test singly; reported tests which used a combined cut-off; and any reported tests whose efficacy was estimated using a published algorithm derived from two or more tests used sequentially.
Synthesis of economic evidence
A decision tree model was constructed to estimate the cost-effectiveness of all comparators. Sensitivity and specificity data included in the decision tree were extracted from the meta-analysis (see Chapter 4).
Long-term costs and QALYs were taken from the literature if estimates specifically matching the decision tree pathways were available. Where this was not possible, long-term costs and outcomes were estimated using a series of Markov models. Figure 1 depicts the flow of data between the different modelling elements for the models estimating incremental cost per QALY.
FIGURE 1.
Illustration of data flow (input into decision tree from other modelling elements). FN, false negative; FP, false positive; TN, true negative; TP, true positive.
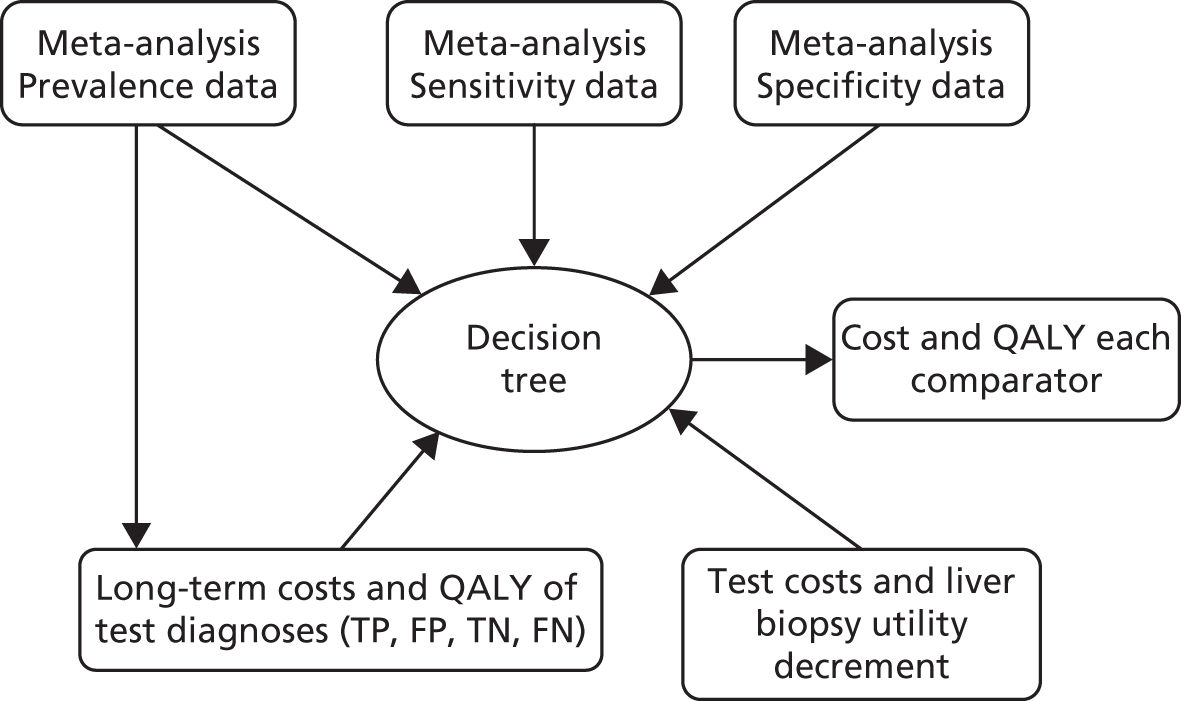
The watchful waiting strategy incorporated a retest every 2 years. We assumed that the retest would have perfect sensitivity and specificity in the base case for modelling practicality due to the large number of applicable NILTs identified.
Literature review
Literature searches were undertaken to identify incremental-cost-per-QALY analyses of the non-invasive tests for each aetiology. Titles and abstracts were reviewed and full papers were retrieved if deemed relevant. If existing systematic reviews were available, these were reviewed and the searches updated and/or amended as required.
Studies were excluded if not in the English language due to resource limitations. We gave preference to UK-based studies for cost data as there may be transferability issues using data from other populations due to underlying differences between the populations.
Literature searching was undertaken to populate input parameters for the models (for natural history, costs and QALY inputs). Titles and abstracts were reviewed and full papers were retrieved if deemed relevant. We started by identifying existing recent reviews. The papers identified in these were reviewed. The searches were updated, amended if needed, and rerun.
For data on natural history, inclusion criteria related to the population of interest. Judgements about the relevance of studies also took into account the country of origin (preference for UK data), high-quality and recent studies. For cost studies, those reporting data from a NHS perspective were preferred. For studies reporting health-related utility inclusion criteria requiring data from the population of interest (depending on aetiology), information on health had to be collected directly from patients and the method of preference elicitation had to be a choice-based method (e.g. time trade-off) in a UK population. As per standard NICE methods guidance,66 data obtained through the EQ-5D measure were preferred.
Further details of the search results are described in the cost-effectiveness chapters (see Chapters 5–9) and the individual search strategies are listed in Appendix 2.
Costs
All unit costs reported in the analysis for health states and liver biopsy are priced for the year 2012. Where required, costs were inflated to 2012 prices using NHS inflation indices. 67 Test costs for the NILTs are costed for the year 2012–13 as costs for some of the components for the NILTs were sourced in early January 2013.
Incremental-cost-per-quality-adjusted-life-year analyses
All analyses were fully incremental. In the incremental analyses, test strategies were ordered according to the least effective and test strategies which were found to be more costly and less effective (‘dominated’) than another strategy were ruled out of the analysis. Incremental cost-effectiveness ratios (ICERs) were calculated for the tests that were not dominated and test strategies with an ICER greater than that of a more effective intervention (‘extendedly dominated’) were also ruled out; the ICER was calculated using the formula
where C1 equals the cost of strategy 1, C0 equals the cost of (the next best) strategy 0, E1 equals QALYs from strategy 1 and E0 equals QALYs from (the next best) strategy.
The cost-effectiveness results for the remaining strategies which were not ruled out (not ‘dominated’ or ‘extendedly dominated’) were presented as ICERs. 68
Probabilistic sensitivity analysis (PSA) was conducted. With a PSA, rather than using the average values for each parameter input, the value is instead drawn from a distribution. The probability distribution for each input variable (natural history data, mortality rates, costs, QALYs, treatment effectiveness and test effectiveness) was constructed using estimates of the mean value and standard error (if required for probability distribution) and Monte Carlo simulation was used to randomly sample from each input distribution simultaneously for 1000 runs of the models. For each of the decision tree model outputs (1000 simulation runs), an average total lifetime cost and QALY was calculated for each testing strategy.
To summarise the uncertainty around the cost-effectiveness result, we constructed cost-effectiveness acceptability curves (CEACs) which are derived from a joint distribution of the costs and effects (QALYs) to represent the probability that a testing option is cost-effective (had the highest net monetary benefit) at different levels of a cost-effectiveness threshold (varied from £0 to £60,000 in analysis). Net benefit is calculated using the formula
where E is equivalent to the health outcome for a testing strategy, CR equals the ceiling ratio which is the cost-effectiveness threshold (range between £0 to £60,000) and C equals the cost of the testing strategy. 69
The CEAC represents the probability that a testing option has the highest probability of being cost-effective over a range of threshold values. However, as Fenwick et al. 69 have shown, the testing option with the highest probability of being cost-effective may not necessarily have the highest expected net benefit. In this case, the CEAC should not be used to identify the optimal option; instead, the cost-effectiveness acceptability frontier (CEAF) which plots the uncertainty associated with the optimal testing option (option with highest expected net benefit) for different cost-effectiveness threshold values may be more applicable.
We also present the CEAFs to illustrate the probability of any testing strategy being optimal (has the highest expected net benefit) compared with each other over a range of different cost-effectiveness thresholds (threshold value range varied from £0 to £60,000). 69,70
Cost per correct diagnosis (alcoholic liver diseases and non-alcoholic fatty liver disease)
The cost-per-correct-diagnoses analyses are presented incrementally. We carried out a probabilistic analysis where we estimated the number of correct true responses for each tests (positive and negative responses). We then compared the results of each test incrementally using the cost for each test to rule out tests which were more costly and provided less correct results. Liver biopsy was included as a comparator in the cost-per-correct-diagnosis analyses.
Chapter 4 Results of systematic review and meta-analysis
Systematic review results
Description of studies
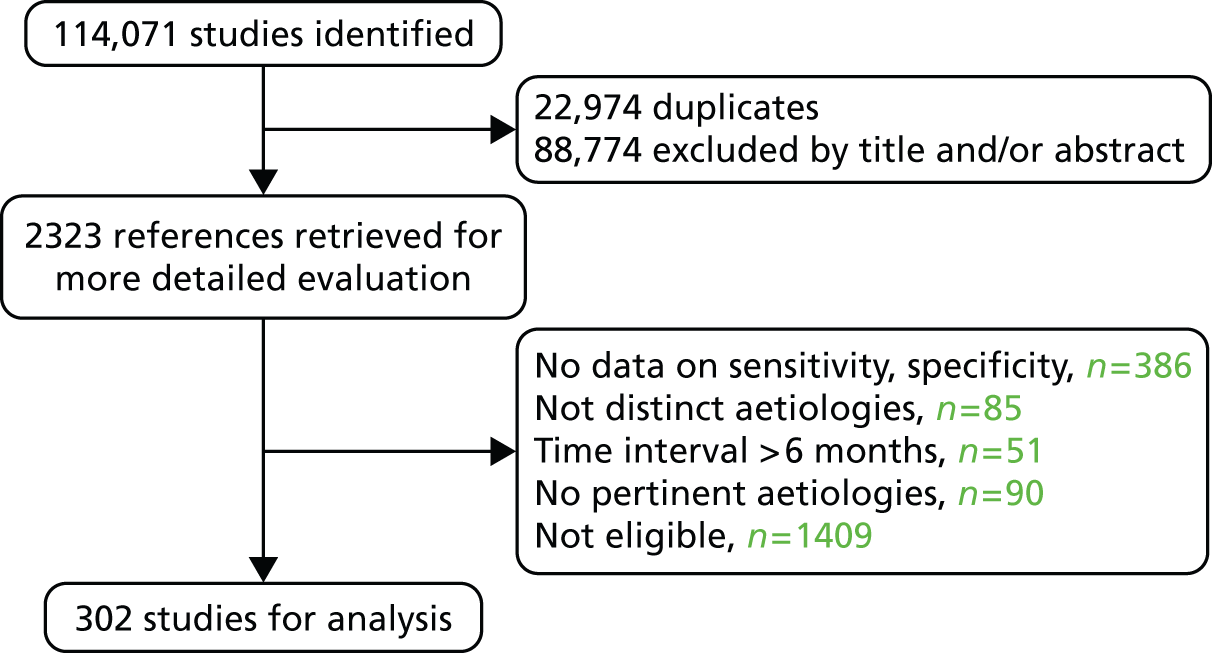
The search strategy initially retrieved 114,071 studies, or after duplicate exclusion, 91,097 studies. The flow chart is shown in Figure 2. Finally, data from 302 studies were analysed (HCV n = 162, HBV n = 52, NAFLD n = 48, radiology n = 60, ALD n = 12). 23–31,71–363 All but five of the included studies were captured by the search strategy79,256,276,332,334 These five studies were retrieved by manually searching the reference lists of included studies and published meta-analyses.
FIGURE 2.
Flow chart of literature review search.
Meta-analysis results
Data analysis was performed separately according to disease aetiology (HCV, HBV, NAFLD and ALD) as there are distinct patterns of fibrosis development in different aetiologies of chronic liver disease. For example, fibrosis in chronic viral hepatitis is characterised by portal-central septa and interface hepatitis, whereas capillarisation of sinusoids and intercellular fibrosis (chicken-wire fibrosis) are typical of alcoholic and non-alcoholic steatohepatitis. 364 This results in a statistically different amount of fibrosis as measured by liver collagen in patients with different aetiologies of liver disease but the same histological stage. 365 This is reflected in disease-specific cut-offs of non-invasive markers for the same histological stage, for example the cut-offs using Fibroscan for F2 fibrosis differ in HBV and HCV,32 but also in differences in diagnostic accuracy depending on the aetiology of liver disease. 20
Data from radiological methods of fibrosis assessment were pooled and analysed together irrespective of aetiology, as these methods are based on size and contour of the liver, echotexture and signs of portal hypertension rather than on disease-specific fibrotic patterns. Data on Fibroscan, ARFI and real-time elastography were analysed according to the aetiology of liver disease.
Non-invasive test cut-offs for the diagnosis of specific histological stages were not always predetermined, and consequently, varied in the included studies. This probably resulted in higher diagnostic accuracies of the non-invasive tests assessed when the cut-off was not predetermined, as such cut-offs were statistically determined to correlate in the best way with the biopsy results. We opted not to perform a separate meta-analysis for each stage-specific cut-off of a non-invasive test, but to group together cut-offs if the range was reasonable. Therefore, all reported sensitivities and specificities of a non-invasive test, when a range of cut-offs is mentioned in the results tables, are probably overestimated.
A number of NILTs, mainly indirect non-invasive fibrosis tests, report sensitivities and specificities at dual cut-offs, a high cut-off with high specificity and a low cut-off with high sensitivity. The low and high cut-off is usually set at 90–95% of sensitivity and specificity, respectively. Depending on the clinical scenario and the disease prevalence, the low or high cut-off is used at the expense of increased false positives and false negatives respectively. We performed separate meta-analyses for low and high cut-offs whenever such cut-offs were reported and were similar across studies. Patients who have test results greater than the higher cut-off are considered to be test positive and those with test results lower than the lower cut-off are considered to be test negative. If these cut-offs are combined, then false positives and false negatives are minimised but a number of patients will fall in the indeterminate range of fibrosis (i.e. their score will be between the low and the high cut-off) and will need either further non-invasive testing or a liver biopsy. Such patients with intermediate results were considered to have undergone a second test.
Table 4 provides a list of NILTs found, applicable aetiologies and a list of the components.
| Test | Components | Comments |
|---|---|---|
| Indirect serum non-invasive fibrosis tests | ||
| APGA | AST, platelet count, GGT, α-fetoprotein | HBV |
| APRI | AST, platelet count | HBV, HCV, NAFLD, ALD |
| Age–Platelet Index | Age, platelet count | HBV, HCV, NAFLD |
| AST–ALT ratio | AST, ALT | HBV, HCV, NAFLD |
| BARD | BMI, AST, ALT, presence of diabetes | |
| CDS | AST, ALT, platelet count, INR | HBV, HCV, NAFLD |
| FIB-4 | Age, AST, ALT, platelet count | HBV, HCV, NAFLD |
| Forns index | Age, γ-GT, cholesterol, platelet count | HBV, HCV, ALD |
| FibroQ | Age, AST, ALT, INR, platelet count | HCV |
| Fibrosis probability index | Age, past alcohol intake, AST, cholesterol, HOMA-IR | HCV |
| GUCI | AST, ALT, platelet count | HBV, HCV |
| Hui index | BMI, total bilirubin, platelet count, albumin | HBV |
| King’s | Age, AST, INR, platelet count | HCV |
| Lok’s index | AST, ALT, platelet count, INR | HBV, HCV, NAFLD |
| NAFLD fibrosis score | Age, BMI, presence of diabetes or IFG, AST, ALT, platelet count, albumin | NAFLD |
| NIHCED | Age, prothrombin time, platelet count, AST, ALT, splenomegaly, caudate lobe hypertrophy, right liver lobe atrophy | HCV |
| PAPAS | Platelet count, age, ALP, α-fetoprotein, AST | HBV |
| PGAA | Prothrombin time, GGT, apolipoprotein A1, α2-macroglobulin | ALD |
| Platelet count | Platelets count | HCV, NAFLD |
| Pohl index | AST, ALT, platelets | HCV |
| Direct non-invasive fibrosis tests | ||
| 13C-caffeine breath test | HBV, HCV, NAFLD | |
| Amino-breath test | Aminopyrine breath test | HCV |
| CTGF | Connective tissue growth factor | HBV |
| Fontana | Hyaluronic acid, TIMP-1, platelet count | HCV |
| Hyaluronic acid | Hyaluronic acid | HBV, HCV, NAFLD |
| Hepascore | Age, sex, α2-macroglobulin, hyaluronate, bilirubin, γ-GT | HBV, HCV, NAFLD |
| NAFIC | Ferritin, fasting insulin, type IV collagen | NAFLD |
| NAFLD diagnostic panel – advanced fibrosis | Presence of diabetes, AST, triglycerides, TIMP-1 | NAFLD |
| NAFLD diagnostic panel – any fibrosis | Presence of diabetes, sex, BMI, triglycerides, M30, M65-M30 | NAFLD |
| PIIINP | Amino-terminal propeptide of type III procollagen | HCV |
| PIIINP/MMP1 index | PIIINP, MMP1 | HCV |
| Type IV collagen | Type IV collagen | HBV, HCV, NAFLD |
| YKL-40 | YKL-40 | HCV, ALD |
| Commercial non-invasive serum fibrosis tests | ||
| ELF | PIIINP, hyaluronate, TIMP-1 | HCV, NAFLD |
| Fibroindex | Platelet count, AST, γ-globulin | HCV |
| Fibrometer | Platelets, prothrombin time, macroglobulin, AST, hyaluronate, age, urea | HCV |
| FibrospectII | α2-macroglobulin, hyaluronate and TIMP-1 | HCV |
| Fibrotest | γ-GT, haptoglobin, bilirubin, A1 apolipopotein, α2-macroglobulin | HBV, HCV, NAFLD, ALD |
| Imaging modalities | ||
| ARFI | Acoustic radiation force impulse imaging | HBV, HCV, NAFLD |
| Platelet–spleen ratio | Platelet count, spleen diameter | HCV |
| Real-time elastography | Real-time elastography | HBV, HCV, NAFLD |
| Fibroscan | Transient elastography | HBV, HCV, NAFLD, ALD |
| CT | Computed tomography scan | All aetiologies |
| MRI | Magnetic resonance imaging | All aetiologies |
| DW-MRI | Diffusion-weighted magnetic resonance imaging | All aetiologies |
| MR elastography | Liver stiffness measured with MRI | All aetiologies |
| US | Conventional ultrasound | All aetiologies |
| Contrast-enhanced ultrasound | Ultrasound after the intravenous injection of specific contrast material | All aetiologies |
| US SAPI | Splenic artery pulsatile index measured with ultrasound | All aetiologies |
| Algorithms of non-invasive fibrosis assessment | ||
| Bordeaux | Synchronous Fibrotest and Fibroscan | HCV |
| Fibropaca | Synchronous Fibrotest, APRI and Forns index | HCV |
| Leroy | Synchronous Fibrotest and APRI | HCV |
| SAFE | APRI and Fibrotest sequentially | HCV |
Results: hepatitis C virus
Data on patients with HCV were extracted from 162 studies. 23–29,31,71–224 Meta-analysis was performed separately for each non-invasive test which had been assessed at each METAVIR stage (F1–F4). Summary sensitivity and specificity for F2 and F4 are shown in Tables 5 and 6, while the sensitivity and specificity estimates for F1 and F3 are reported in Appendix 3. Individual study characteristics are shown in Appendix 4. The median prevalence (minimum–maximum) of fibrosis stages F1–F4 in included studies was for F1 0.875 (0.157–0.968), F2 0.522 (0.063–0.893), F3 0.291 (0.051–0.778) and F4 0.17 (0.026–0.681). Forest plots and summary receiver operating characteristic (SROC) plots of different NILTs across fibrosis stages are presented in Appendices 5 and 6, respectively.
| Test | Number of studies | Cut-off | Summary sensitivity (95% CI) | Summary specificity (95% CI) | Statistics |
|---|---|---|---|---|---|
| Indirect non-invasive serum tests | |||||
| APRI (low cut-off) | 47 | 0.4–0.7 | 0.82 (0.77 to 0.86) | 0.57 (0.49 to 0.65) | Bivariate random-effects model with correlation between sensitivity and specificity |
| APRI (high cut-off) | 36 | 1.5 | 0.39 (0.32 to 0.47) | 0.92 (0.89 to 0.95) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Age–Platelet Index | 1 | 3 | 0.58 (0.46 to 0.70) | 0.70 (0.64 to 0.84) | Single study |
| AST–ALT ratio | 7 | 0.6–1 | 0.44 (0.27 to 0.63) | 0.71 (0.62 to 0.78) | Bivariate random-effects model with correlation between sensitivity and specificity |
| CDS | 1 | 6 | 0.66 (0.59 to 0.73) | 0.49 (0.34 to 0.64) | Single study |
| FIB-4 (low cut-off) | 11 | 0.6–1.45 | 0.89 (0.79 to 0.95) | 0.42 (0.25 to 0.61) | Random-effects model for sensitivity and specificity without correlation |
| FIB-4 (high cut-off) | 9 | 1–3.25 | 0.59 (0.43 to 0.73) | 0.74 (0.56 to 0.87) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Forns index (low cut-off) | 18 | 4.2–4.5 | 0.88 (0.83 to 0.91) | 0.40 (0.33 to 0.48) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Forns index (high cut-off) | 15 | 6.9–8.7 | 0.35 (0.29 to 0.41) | 0.96 (0.92 to 0.98) | Bivariate random-effects model with correlation between sensitivity and specificity |
| FibroQ | 1 | 1.6 | 0.78 (0.71 to 0.83) | 0.66 (0.51 to 0.78) | Single study |
| Fibrosis probability index (low cut-off) | 2 | 0.2 | 0.91 (0.83 to 0.96) | 0.45 (0.34 to 0.57) | Fixed-effects model for sensitivity and specificity without correlation |
| Fibrosis probability index (high cut-off) | 2 | 0.8 | 0.42 (0.32 to 0.54) | 0.95 (0.87 to 0.98) | Fixed-effects model for sensitivity and specificity without correlation |
| GUCI | 3 | 0.33–1.1 | 0.65 (0.1 to 1.00) | 0.79 (0.03 to 1.00) | Bivariate random-effects model with correlation between sensitivity and specificity |
| King’s | 1 | 9.87 | 0.84 (0.75 to 0.9) | 0.70 (0.61 to 0.79) | Single study |
| King’s (low cut-off) | 1 | 4.46 | 0.62 (0.55 to 0.69) | 0.81 (0.76 to 0.86) | Single study |
| King’s (high cut-off) | 1 | 12.3 | 0.58 (0.51 to 0.65) | 0.79 (0.73 to 0.83) | Single study |
| Lok’s index | 4 | 0.2–1.67 | 0.67 (0.55 to 0.77) | 0.55 (0.29 to 0.78) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Platelets | 10 | 48–182 | 0.50 (0.41 to 0.59) | 0.89 (0.83 to 0.93) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Pohl index | 2 | Positive | 0.06 (0.04 to 0.1) | 0.99 (0.93 to 1.00) | Fixed-effects model for sensitivity and specificity without correlation |
| Direct serum non-invasive serum tests | |||||
| Aminopyrine breath test | 1 | 8.1 | 0.73 (0.57 to 0.85) | 0.74 (0.58 to 0.85) | Single study |
| Hyaluronic acid | 8 | 34–110 ng/ml | 0.75 (0.64 to 0.83) | 0.75 (0.68 to 0.82) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Hepascore | 10 | 0.31–0.5 | 0.73 (0.66 to 0.79) | 0.73 (0.65 to 0.79) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Hepascore (high cut-off) | 1 | 0.84 | 0.33 (0.24 to 0.43) | 0.92 (0.85 to 0.96) | Single study |
| MP3 | 1 | 0.3 | 0.82 (0.73 to 0.89) | 0.73 (0.63 to 0.81) | Single study |
| PIIINP | 2 | 8.3–9.1 | 0.78 (0.63 to 0.87) | 0.76 (0.54 to 0.90) | Fixed-effects model for sensitivity and specificity without correlation |
| PIIINP/MMP1 index | 1 | 0.3 | 0.65 (0.55 to 0.75) | 0.85 (0.77 to 0.90) | Single study |
| Type IV collagen | 5 | 110–298 | 0.88 (0.71 to 0.96) | 0.73 (0.63 to 0.82) | Random-effects model for sensitivity and specificity without correlation |
| YKL-40 (low cut-off) | 1 | 290 | 0.80 (0.66 to 0.89) | 0.33 (0.26 to 0.41) | Single study |
| YKL-40 (high cut-off) | 1 | 540 | 0.33 (0.21 to 0.48) | 0.80 (0.73 to 0.86) | Single study |
| Commercial non-invasive serum tests | |||||
| ELF | 1 | 8.75 | 0.84 (0.69 to 0.92) | 0.70 (0.52 to 0.83) | Single study |
| ELF (low cut-off) | 1 | 9.55 | 0.90 (0.85 to 0.93) | 0.52 (0.43 to 0.61) | Single study |
| ELF (high cut-off) | 1 | 11.07 | 0.47 (0.41 to 0.54) | 0.90 (0.83 to 0.94) | Single study |
| Fibroindex (low cut-off) | 4 | 1.25 | 0.83 (0.15 to 0.99) | 0.57 (0.22 to 0.86) | Random-effects model for sensitivity and specificity without correlation |
| Fibroindex (high cut-off) | 4 | 2.25 | 0.24 (0.11 to 0.43) | 0.98 (0.93 to 1.00) | Random-effects model for sensitivity and fixed-effect model for specificity without correlation |
| Fibrometer | 4 | 0.42–0.57 | 0.79 (0.69 to 0.86) | 0.73 (0.63 to 0.81) | Bivariate random-effects model with correlation between sensitivity and specificity |
| FibrospectII | 5 | 42–72 | 0.78 (0.49 to 0.93) | 0.71 (0.59 to 0.80) | Random-effects model for sensitivity and specificity without correlation |
| Fibrotest | 17 | 0.32–0.53 | 0.68 (0.58 to 0.77) | 0.72 (0.70 to 0.77) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Fibrotest (low cut-off) | 7 | 0.1–0.3 | 0.91 (0.86 to 0.94) | 0.41 (0.37 to 0.46) | Random-effects model for sensitivity and specificity without correlation |
| Fibrotest (high cut-off) | 10 | 0.6–0.7 | 0.57 (0.46 to 0.67) | 0.85 (0.74 to 0.92) | Bivariate random-effects model with correlation between sensitivity and specificity |
| ARFI | 3 | 1.21–1.34 | 0.79 (0.75 to 0.83) | 0.89 (0.84 to 0.93) | Fixed-effects model for sensitivity and specificity without correlation |
| PLT–Spleen ratio | 3 | 1750–2200 | 0.88 (0.62 to 0.99) | 0.73 (0.41 to 0.99) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Real-time elastography | 1 | 2.73 | 0.83 (0.73 to 0.90) | 0.92 (0.65 to 0.99) | Single study |
| Fibroscan | 37 | 5.2–10.1 | 0.79 (0.74 to 0.84) | 0.83 (0.77 to 0.88) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Combination of fibrosis non-invasive tests algorithms | |||||
| Bordeaux | 1 | – | 0.88 (0.85 to 0.91) | 0.89 (0.85 to 0.92) | Single study |
| Fibropaca | 1 | – | 0.85 (0.81 to 0.89) | 0.90 (0.86 to 0.93) | Single study |
| Leroy | 1 | – | 0.90 (0.79 to 0.96) | 0.98 (0.95 to 0.99) | Single study |
| SAFE | 4 | – | 1.00 (1.00 to 1.00) | 0.81 (0.80 to 0.83) | Fixed-effects model for sensitivity and specificity without correlation |
| Test | Number of studies | Cut-off | Summary sensitivity (95% CI) | Summary specificity (95% CI) | Statistics |
|---|---|---|---|---|---|
| Indirect non-invasive serum tests | |||||
| APRI (low cut-off) | 24 | 0.75–1 | 0.77 (0.73 to 0.81) | 0.78 (0.74 to 0.81) | Bivariate random-effects model with correlation between sensitivity and specificity |
| APRI (high cut-off) | 19 | 2 | 0.48 (0.41 to 0.56) | 0.94 (0.91 to 0.95) | Bivariate random-effects model with correlation between sensitivity and specificity |
| AST–ALT ratio | 13 | 1 | 0.49 (0.39 to 0.59) | 0.87 (0.75 to 0.94) | Bivariate random-effects model with correlation between sensitivity and specificity |
| CDS | 1 | 8 | 0.88 (0.66 to 0.97) | 0.67 (0.57 to 0.77) | Single study |
| FIB-4 (low cut-off) | 2 | 1.45 | 0.87 (0.74 to 0.94) | 0.61 (0.53 to 0.69) | Fixed-effects model for sensitivity and specificity without correlation |
| FIB-4 (high cut-off) | 3 | 3.25–4.44 | 0.51 (0.39 to 0.63) | 0.86 (0.81 to 0.90) | Fixed-effects model for sensitivity and specificity without correlation |
| Forns index (low cut-off) | 2 | 3.9–4.2 | 0.88 (0.60 to 1.00) | 0.43 (0.1 to 1.00) | Fixed-effect model for sensitivity and random-effects model for specificity without correlation |
| Forns index (high cut-off) | 1 | 6.9 | 0.67 (0.53 to 0.78) | 0.91 (0.84 to 0.95) | Single study |
| GUCI | 3 | Positive | 0.76 (0.07 to 0.99) | 0.85 (0.78 to 0.90) | Random-effects model for sensitivity and fixed-effect model for specificity without correlation |
| Lok’s index (low cut-off) | 2 | 0.2–0.26 | 0.84 (0.88 to 1.00) | 0.66 (0.01 to 100) | Fixed-effect model for sensitivity and random-effects model for specificity without correlation |
| Lok’s index (high cut-off) | 1 | 0.5 | 0.40 (0.29 to 0.52) | 0.95 (0.91 to 0.97) | Single study |
| Platelets | 10 | 130–196 | 0.68 (0.59 to 0.76) | 0.86 (0.72 to 0.94) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Direct serum non-invasive serum tests | |||||
| 13C-caffeine breath test | 2 | 0.01–1.7 | 0.88 (0.22 to 0.99) | 0.73 (0.18 to 0.97) | Random-effects model for sensitivity and fixed-effect model for specificity without correlation |
| Fontana | 1 | 0.3 | 0.79 (0.72 to 0.84) | 0.66 (0.61 to 0.71) | Single study |
| Hyaluronic acid | 7 | 78–237 ng/ml | 0.80 (0.61 to 0.91) | 0.88 (0.78 to 0.94) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Hepascore | 7 | 0.84 | 0.80 (0.68 to 0.88) | 0.83 (0.76 to 0.89) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Hepascore (low cut-off) | 1 | 0.58 | 0.80 (0.72 to 0.86) | 0.83 (0.80 to 0.85) | Single study |
| Hepascore (high cut-off) | 1 | 1.159 | 0.39 (0.31 to 0.48) | 0.99 (0.98 to 0.99) | Single study |
| PIIINP | 3 | 0.8–1 | 0.70 (0.42 to 0.89) | 0.84 (0.74 to 0.90) | Random-effects model for sensitivity and fixed-effect model for specificity without correlation |
| Type IV collagen | 1 | 190 | 0.78 (0.65 to 0.86) | 0.72 (0.61 to 0.81) | Single study |
| Commercial non-invasive serum tests | |||||
| ELF | 1 | 9.4 | 0.93 (0.69 to 0.99) | 0.79 (0.67 to 0.88) | Single study |
| ELF (low cut-off) | 1 | 10.06 | 0.90 (0.84 to 0.94) | 0.53 (0.46 to 0.59) | Single study |
| ELF (high cut-off) | 1 | 11.73 | 0.52 (0.43 to 0.60) | 0.90 (0.85 to 0.93) | Single study |
| Fibroindex | 1 | 1.82 | 0.70 (0.52 to 0.84) | 0.91 (0.82 to 0.96) | Single study |
| Fibrometer | 2 | 0.88 | 0.72 (0.36 to 0.92) | 0.88 (0.60 to 0.97) | Random-effects model for sensitivity and fixed-effect model for specificity without correlation |
| Fibrometer (low cut-off) | 1 | 0.63 | 0.96 (0.90 to 0.98) | 0.71 (0.68 to 0.74) | Single study |
| Fibrometer (high cut-off) | 1 | 0.98 | 0.36 (0.28 to 0.45) | 0.98 (0.97 to 0.99) | Single study |
| Fibrotest | 8 | 0.56–0.74 | 0.60 (0.43 to 0.76) | 0.86 (0.81 to 0.91) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Fibrotest (low cut-off) | 1 | 0.66 | 0.82 (0.74 to 0.88) | 0.77 (0.74 to 0.80) | Single study |
| Fibrotest (high cut-off) | 1 | 0.86 | 0.42 (0.34 to 0.51) | 0.96 (0.94 to 0.97) | Single study |
| Imaging modalities | |||||
| ARFI | 4 | 1.6–2.3 | 0.84 (0.72 to 0.91) | 0.77 (0.50 to 0.92) | Random-effects model for sensitivity and specificity without correlation |
| PLT–Spleen ratio | 1 | Spleen > 120, PLT < 140 | 0.85 (0.76 to 0.91) | 0.82 (0.80 to 0.84) | Single study |
| Real-time elastography | 1 | 3.93 | 0.91 (0.73 to 0.98) | 0.91 (0.80 to 0.97) | Single study |
| Fibroscan | 36 | 9.2–17.3 | 0.89 (0.84 to 0.92) | 0.91 (0.89 to 0.93) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Combination of fibrosis non-invasive tests algorithms | |||||
| Bordeaux | 1 | – | 0.87 (0.80 to 0.92) | 0.95 (0.93 to 0.96) | Single study |
| Fibropaca | 1 | – | 0.73 (0.62 to 0.81) | 0.97 (0.95 to 0.98) | Single study |
| SAFE | 4 | – | 0.74 (0.42 to 0.92) | 0.93 (0.91 to 0.94) | Random-effects model for sensitivity and fixed-effect model for specificity without correlation |
For the diagnosis of fibrosis stage ≥ F2, which was the one mainly used in economic modelling, 19 non-invasive tests were evaluated in single studies. Of 47 different evaluated tests, only 18 converged with the bivariate random-effects model [APRI low and high cut-offs, AST–ALT ratio, FIB-4 low and high cut offs, Forns index low and high cut-off, Göteborg University Cirrhosis Index (GUCI), Lok’s index, platelet count, hyaluronic acid, Hepascore, Fibrometer, Fibrotest standard, low and high cut-offs, platelet-to-spleen-diameter ratio and Fibroscan]. The most commonly evaluated non-invasive tests were APRI (low cut-off), which was evaluated in 47 studies,24,31,73,74,79,81,84,85,89,90,91,94,97,98,103,107,109,121,126,127,130,131,134,137,140,143,144,146,150,152–154,156–158,163,164,168,182,185,187,189,194,195,210,218,220,223 followed by TE in 37 studies28,29,75,76,86–88,91,95,98–100,102,105,106,110,116,119,130,137,141,147,153,155,159,161,164,170,172,173,194,199–201,211,223,224 and APRI (high cut-off) in 37 studies. 24,31,72–74,79,81,89,90,91,94,97,100,103,121,123,126,131,134,140,143,146,150,152–154,156–158,182,187,195,209,210,218,220,223
For the diagnosis of cirrhosis, there were 37 different evaluated tests; however, only nine converged with the bivariate random-effects model (APRI low and high cut-offs, AST–ALT ratio, platelet count, hyaluronic acid, Hepascore, Fibrotest and Fibroscan).
For the diagnosis of fibrosis stage ≥ F1, there were only five tests that reported diagnostic accuracy; however, none converged with the bivariate random-effects model.
For the diagnosis of fibrosis stage ≥ F3, there were 37 different evaluated tests, of which six converged with the bivariate random-effects model (APRI high cut-off, FIB-4 low and high cut-offs, Hepascore, Fibrotest and Fibroscan).
Uninterpretable NILT results were very rare in serum markers (< 1%) and were more frequently encountered in patients who were undergoing Fibroscan examination. The rate of uninterpretable results with Fibroscan (due to < 10 valid measurements, success rate < 60% and interquartile range > 30%) was 8.5%; however, this could be underestimated due to under-reporting.
Cut-offs of non-invasive tests for specific disease stages varied among studies and were predetermined in only 51 studies (31.4%). 25,31,72–74,83,85–87,90–92,94,95,97,99,100,106,109,117,119,127,132–134,143,144,146,150,154,156,157,167,169,171,172,175,186,187,192–194,203,213–216,220,222,223,366 We did not include data on APRI for cirrhosis from some studies in the meta-analysis because some cut-offs differed significantly from what is used in the literature. 82,137,168,194 Liver biopsy was of acceptable quality (≥ 15 cm in length with ≥ 6 portal tracks) in only 20 studies (12.3%),75,86,110,112,115–117,119,124,127,129,137,141,143,153,186,188,194,222,223 while minimum sample requirements were not reported in 84 (51.8%) studies. 26,27,72–74,77–80,84,85,88,91–93,96,100,101,103,104,106,107,111,114,118,121–123,125,126,130,131,133,135,138,140,147,148,151,152,159–163,165,167–169,172–175,177,178,180,181,183–185,190–192,196–204,208–215,218,221 Overall, only three studies86,143,222 had a low risk of bias in all of the domains of the QUADAS-2 tool; therefore, all our estimates may be biased. Quality assessment of included studies based on QUADAS-262 is shown in Table 7. Studies that were judged as low risk of bias or unknown in the three most important QUADAS domains, namely patient selection, index test and reference standard, were still a modest fraction of the total number of studies (29 out of 152; 19%). 72,73,79,80,86,91,92,106,110,133,135,143,148,165,167,173,197–199,201,203,206–208,211,214,215,221,222
| Study ID | Domain 1: patient sampling | Domain 2: index test | Domain 3: reference standard | Domain 4: flow and timing | |||
|---|---|---|---|---|---|---|---|
| Risk of bias | Concerns about applicability | Risk of bias | Concerns about applicability | Risk of bias | Concerns about applicability | Risk of bias | |
| Adams 200571 | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ |
| Ahmad 201172 | ? | ✓ | ✓ | ✓ | ? | ✓ | ✓ |
| Al Mohri73 | ? | ? | ✓ | ✓ | ? | ✓ | ✓ |
| Anaparthy 200974 | ✗ | ✗ | ✓ | ✓ | ? | ✓ | ? |
| Arena 200875 | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ |
| Beckebaum 201076 | ✗ | ✗ | ✗ | ✓ | ✗ | ✓ | ✓ |
| Bejarano 200977 | ✓ | ✓ | ✗ | ✓ | ? | ✓ | ✓ |
| Berg 200479 | ? | ? | ? | ? | ? | ✓ | ? |
| Borroni 200280 | ? | ? | ? | ? | ? | ✓ | ? |
| Bourliere 200681 | ✓ | ✗ | ✗ | ✓ | ✗ | ✓ | ✗ |
| Boursier 200982 | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ |
| Boursier 201283 | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ |
| Burton 201084 | ✗ | ✗ | ✓ | ✓ | ? | ✓ | ? |
| Cales 201085 | ✗ | ✗ | ✓ | ✓ | ? | ✓ | ✗ |
| Cales 201026 | ? | ? | ✗ | ✓ | ? | ✓ | ✓ |
| Calvaruso 201086 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Cardoso 201287 | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ |
| Carrion 200688 | ✓ | ✓ | ✗ | ✗ | ? | ✓ | ✓ |
| Carvalho 200889 | ✗ | ✗ | ✗ | ✓ | ✗ | ✓ | ✓ |
| Castera 200528 | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ |
| Castera 200791 | ? | ? | ✓ | ✓ | ? | ✓ | ? |
| Castera 200990 | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ |
| Ceriani 200192 | ? | ? | ✓ | ? | ? | ✓ | ? |
| Chen 200893 | ? | ? | ✗ | ✓ | ? | ✓ | ? |
| Cheung 200894 | ✗ | ✗ | ✓ | ✗ | ✗ | ✓ | ✓ |
| Cho 201195 | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ |
| Christensen 200696 | ? | ✗ | ✗ | ✓ | ? | ✓ | ✗ |
| Chrysanthos 200697 | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ |
| Cobbold 201098 | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ |
| Colletta 200599 | ✗ | ✗ | ✓ | ? | ✗ | ✓ | ✗ |
| Corradi 2009100 | ✗ | ✗ | ✓ | ✓ | ? | ✓ | ✓ |
| Crespo 2010101 | ? | ? | ✗ | ✓ | ? | ✓ | ✓ |
| Cross 2010102 | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ | ✗ |
| da Silva 2008103 | ? | ✗ | ✗ | ✓ | ? | ✓ | ✓ |
| Danila 2011104 | ? | ? | ✗ | ? | ? | ✓ | ✓ |
| De Ledinghen 2006105 | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ | ✓ |
| Degos 2010106 | ✓ | ✓ | ✓ | ✓ | ? | ✓ | ✓ |
| Dinesen 2008107 | ✗ | ✗ | ✗ | ✓ | ? | ✓ | ? |
| Esmat 2007108 | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ |
| Fabris 2006109 | ✗ | ✗ | ✓ | ✓ | ✗ | ✓ | ✓ |
| Fahmy 2011110 | ✓ | ✓ | ? | ✓ | ✓ | ✓ | ✓ |
| Fontaine 2009111 | ✗ | ✗ | ✗ | ✓ | ? | ✓ | ? |
| Fontana 2008112 | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ? |
| Fontanges 2008113 | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ |
| Forestier 2010114 | ✗ | ✗ | ✗ | ✗ | ? | ? | ✗ |
| Forns 2002115 | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ |
| Fraquelli 2011116 | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ✗ |
| Fujii 2009117 | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Fujimoto 2011118 | ✗ | ✓ | ✗ | ✓ | ? | ✓ | ✓ |
| Gaia 2011119 | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ |
| Ganne-Carrie 2006120 | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ | ✓ |
| Gara 2011121 | ✗ | ✗ | ✗ | ✓ | ? | ✓ | ✗ |
| Giannini 2006122 | ✓ | ✓ | ✗ | ✓ | ? | ✓ | ✓ |
| Gobel 2006123 | ✗ | ✗ | ✗ | ✓ | ? | ✓ | ✓ |
| Guechot 2010124 | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ |
| Guechot 1996125 | ✓ | ✓ | ✗ | ✗ | ? | ✓ | ✓ |
| Guzelbulut 2011126 | ✓ | ✓ | ✗ | ✓ | ? | ✓ | ✓ |
| Halfon 2006128 | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ |
| Halfon 2005129 | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ |
| Halfon 2007127 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Harada 2008130 | ✗ | ✗ | ✗ | ✓ | ? | ✓ | ✗ |
| Hsieh 2012131 | ✗ | ✗ | ✗ | ✓ | ? | ✓ | ✓ |
| Iacobellis 2005132 | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ |
| Imbert-Bismut 200123 | ✗ | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ |
| Imperiale 2000133 | ✓ | ✓ | ✓ | ✓ | ? | ✓ | ✗ |
| Islam 2005134 | ✗ | ✗ | ✓ | ✓ | ✗ | ✓ | ✗ |
| Iushchuk 2005135 | ? | ? | ? | ? | ? | ? | ? |
| Jazia 200978 | ✗ | ✗ | ✗ | ✓ | ? | ✓ | ✓ |
| Kalantari 2011136 | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ |
| Kamphues 2010137 | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ✗ |
| Kandemir 2009138 | ✗ | ✗ | ✗ | ✓ | ? | ✓ | ✓ |
| Kelleher 2005139 | ✗ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ |
| Khan 2008140 | ? | ✓ | ✗ | ✓ | ? | ✓ | ✓ |
| Kim 2011141 | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ |
| Koda 200724 | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ |
| Koizumi 2011142 | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ |
| Lackner 2005143 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Ladero 2010144 | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ |
| Lee 2011145 | ✓ | ✗ | ✗ | ✓ | ✗ | ✓ | ✓ |
| Leroy 2004147 | ✓ | ✓ | ✗ | ✓ | ? | ✓ | ✓ |
| Leroy 2007146 | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ |
| Leroy 2011148 | ? | ? | ? | ✓ | ? | ✓ | ✓ |
| Lewin 2007149 | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ |
| Lieber 2006150 | ✗ | ✗ | ✓ | ✓ | ✗ | ✓ | ✗ |
| Liu 2006151 | ✓ | ✓ | ✗ | ✓ | ? | ✓ | ✓ |
| Liu 2011153 | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ |
| Liu 2007152 | ✓ | ✓ | ✗ | ✓ | ? | ✓ | ✓ |
| Loko 2008154 | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ |
| Lupsor 2008155 | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ |
| Lupsor 200929 | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ |
| Macias 2006156 | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ |
| Macias 2011157 | ? | ? | ✓ | ✓ | ✗ | ✓ | ? |
| Martinez 2011158 | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ? |
| Morikawa 2011159 | ? | ✓ | ✗ | ✓ | ? | ✓ | ✓ |
| Murawaki 2001160 | ✗ | ✗ | ✗ | ✓ | ? | ✓ | ? |
| Myers 2002366 | ? | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ |
| Nitta 2009161 | ✓ | ✓ | ✗ | ✓ | ? | ✓ | ✓ |
| Nojiri 2010162 | ? | ✓ | ✗ | ✓ | ? | ✓ | ? |
| Nunes 2005163 | ✓ | ✓ | ✗ | ✓ | ? | ✓ | ✗ |
| Obara 2008164 | ✗ | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ |
| Oliveira 2005165 | ? | ? | ? | ✓ | ? | ✓ | ? |
| Orrlachio 2011166 | ✗ | ? | ✗ | ✓ | ✗ | ✓ | ✗ |
| Paggi 2008167 | ✓ | ✓ | ✓ | ✓ | ? | ✓ | ✗ |
| Parise 2006168 | ✓ | ✓ | ✗ | ✓ | ? | ✓ | ✓ |
| Park 2000169 | ✗ | ✓ | ✓ | ✓ | ? | ✓ | ✗ |
| Parkes 201127 | ✗ | ✓ | ✗ | ✓ | ? | ✓ | ✗ |
| Patel 200925 | ? | ✓ | ✓ | ✓ | ✗ | ✓ | ? |
| Patel 2011170 | ? | ✓ | ✗ | ✓ | ✗ | ✓ | ? |
| Pohl 2001171 | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ? |
| Poynard 2012172 | ✗ | ✓ | ✓ | ✓ | ? | ✓ | ✗ |
| Prati 2011173 | ? | ✓ | ? | ✓ | ? | ✓ | ✗ |
| Qiu 2004174 | ✓ | ✓ | ✗ | ✓ | ? | ✓ | ✓ |
| Reedy 1998175 | ✗ | ✓ | ✓ | ✓ | ? | ✓ | |
| Ronot 2010176 | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | |
| Rossi 2003177 | ✓ | ✓ | ✗ | ✓ | ? | ? | |
| Rossini 2010178 | ? | ? | ✗ | ✓ | ✓ | ? | |
| Said 2010179 | ✓ | ✓ | ✗ | ✓ | ✓ | ? | |
| Saitou 2005180 | ? | ✗ | ✗ | ✓ | ✓ | ? | |
| Sanvisens 2009181 | ✓ | ✓ | ✗ | ✓ | ✓ | ✗ | |
| Schiavon 2007182 | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ |
| Schiavon 2008183 | ✗ | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ |
| Schneider 2006185 | ? | ? | ✗ | ✓ | ✓ | ✓ | ? |
| Scneider 2005184 | ✓ | ✓ | ✗ | ✓ | ? | ✓ | ✓ |
| Sebastiani 201231 | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ |
| Sebastiani 2009187 | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ |
| Sebastiani 2006188 | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ |
| Sebastiani 2008186 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Sene 2006189 | ✗ | ✗ | ✗ | ✓ | ✗ | ✓ | ? |
| Sharabash 2009190 | ✗ | ✓ | ? | ✓ | ? | ✓ | ? |
| Shastry 2007191 | ? | ? | ✗ | ✓ | ? | ✓ | ✓ |
| Sheth 1997192 | ✗ | ✓ | ✓ | ✓ | ? | ✓ | ✗ |
| Singal 2011193 | ✓ | ✓ | ✓ | ✓ | ✗ | ✓ | ✗ |
| Sirli 2010194 | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Snyder 2007196 | ✓ | ✓ | ✗ | ✓ | ? | ✓ | ✓ |
| Snyder 2006195 | ✗ | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ |
| Sohn 2010197 | ? | ✓ | ? | ✓ | ? | ✓ | ? |
| Sporea 2008199 | ✓ | ✓ | ? | ✓ | ? | ✓ | ✗ |
| Sporea 2010200 | ✓ | ✓ | ✗ | ✓ | ? | ✓ | ✓ |
| Sporea 2011201 | ? | ✓ | ? | ✓ | ? | ✓ | ✓ |
| Sporea 2011198 | ? | ✓ | ? | ✓ | ? | ✓ | ? |
| Sterling 2006202 | ✓ | ✗ | ✗ | ✓ | ? | ✓ | ✗ |
| Stibbe 2011203 | ? | ✓ | ✓ | ✓ | ? | ✓ | ✗ |
| Sud 2009204 | ✓ | ✓ | ✗ | ✓ | ? | ✓ | ✓ |
| Testa 2006205 | ? | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ |
| Thompson 2009208 | ? | ✓ | ? | ✓ | ? | ✓ | ? |
| Thompson 2009206 | ? | ✓ | ? | ✓ | ? | ✓ | ? |
| Thompson 2010207 | ? | ✓ | ? | ✓ | ? | ✓ | ? |
| Toniutto 2007209 | ✗ | ✗ | ✗ | ✓ | ? | ✓ | ✓ |
| Trang 2008210 | ? | ✓ | ✗ | ✓ | ? | ✓ | ✗ |
| Trifan 2009211 | ✓ | ✓ | ? | ✓ | ? | ✓ | ✓ |
| Trocme 2006212 | ? | ✓ | ✗ | ✓ | ? | ✓ | ✗ |
| Vallet-Pichard 2007214 | ✓ | ✓ | ✓ | ✓ | ? | ✓ | ✓ |
| Tural 2007213 | ✓ | ✗ | ✓ | ✓ | ? | ✓ | ✓ |
| Valva 2011215 | ? | ✓ | ✓ | ✓ | ? | ✓ | ✓ |
| Varaut 2005216 | ✗ | ✗ | ✓ | ✓ | ✗ | ✓ | ✗ |
| Wai 2003218 | ✓ | ✗ | ✗ | ✓ | ? | ✓ | ✗ |
| Westin 2008219 | ✓ | ✓ | ? | ✓ | ✗ | ✓ | ? |
| Wilson 2006220 | ✗ | ✗ | ✓ | ✓ | ✗ | ✓ | ? |
| Wong 1998221 | ✓ | ✓ | ? | ✓ | ? | ✓ | ? |
| Zaman 2004222 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Zarski 2012223 | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ | ✗ |
| Ziol 2005224 | ✗ | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ |
We explored potential sources of heterogeneity as outlined in the methods section. As all but five studies were of low methodological quality,86,127,143,186,222 this potential source of heterogeneity could not be assessed. Significant heterogeneity was found mainly in relation to the transaminases level, without, however, a particular pattern in relation to the transaminase elevation. More specifically and according to source of heterogeneity, details of significant associations are:
-
Full text versus abstract publication: Fibrotest for ≥ F2 (significantly higher specificity if published in full text) and borderline for ≥ F3 (likelihood ratio test, p = 0.059) and platelet count for F4 (significantly lower specificity if published in full text).
-
Transaminases levels (comparator is normal transaminases): for ≥ F2 Fibrotest, hyaluronic acid, Hepascore, Lok’s index, Fibroscan; for ≥ F3 FIB-4 low and high cut-off, TE; and for F4 AST–ALT ratio. There was no specific pattern of influence; therefore, the test could have improved or worse diagnostic accuracy if the transaminases levels were high.
-
Histological score used (comparator is the METAVIR system): for Hepascore in ≥ F3, use of Ludwig scoring system in one study76 resulted in significantly lower sensitivity. This might reflect the particular study rather than the histological score used; for AST–ALT ratio in F4, use of Ludwig scoring system resulted in significantly higher sensitivity; and for platelet count in F4, use of Scheuer or Knodell resulted in significantly higher sensitivity and specificity, respectively.
Results: hepatitis B virus
Data on patients with HBV were extracted from 52 studies. 116,119,120,200,225–272 Meta-analyses were performed separately for each non-invasive test assessed at each METAVIR stage (F1–F4). Summary sensitivity and specificity for fibrosis stages F2 and F4 are shown in Tables 8 and 9, whereas summary sensitivity and specificity for fibrosis stages F1 and F3 are reported in Appendix 3. The median prevalence (minimum–maximum) of fibrosis stages F1–F4 in included studies was for F1 0.617 (0.416–0.884), F2 0.528 (0.269–0.915), F3 0.370 (0.171–0.780) and F4 0.209 (0–0.604). Individual study characteristics, and forest plots and SROC plots of the different NILTs across fibrosis stages, are presented in Appendices 4, 5 and 6, respectively.
| Test | Number of studies | Cut-off | Summary sensitivity (95% CI) | Summary specificity (95% CI) | Statistics |
|---|---|---|---|---|---|
| Indirect non-invasive serum tests | |||||
| APGA | 1 | 6.7 | 0.17 (0.10 to 0.27) | 0.98 (0.95 to 0.99) | Single study |
| APRI (low cut-off) | 8 | 0.4–0.6 | 0.80 (0.68 to 0.88) | 0.65 (0.52 to 0.77) | Bivariate random-effects model with correlation between sensitivity and specificity |
| APRI (high cut-off) | 6 | 1.5 | 0.37 (0.22 to 0.55) | 0.93 (0.85 to 0.97) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Age–Platelet Index | 1 | 3 | 0.68 (0.61 to 0.74) | 0.62 (0.57 to 0.67) | Single study |
| AST–ALT ratio | 1 | 0.67 | 0.57 (0.51 to 0.64) | 0.59 (0.54 to 0.63) | Single study |
| FIB-4 (low cut-off) | 4 | 1.1–1.7 | 0.68 (0.60 to 0.75) | 0.73 (0.67 to 0.79) | Bivariate random-effects model with correlation between sensitivity and specificity |
| FIB-4 (high cut-off) | 1 | 3.25 | 0.58 (0.04 to 0.17) | 0.99 (0.96 to 1.00) | Single study |
| Forns index (low cut-off) | 1 | 4.2 | 0.58 (0.47 to 0.68) | 0.77 (0.61 to 0.88) | Single study |
| Forns index (high cut-off) | 1 | 6.9 | 0.15 (0.08 to 0.24) | 1.00 (0.90 to 1.00) | Single study |
| GUCI | 1 | 0.2 | 0.67 (0.55 to 0.76) | 0.97 (0.85 to 0.99) | Single study |
| Hui index | 1 | 0.15 | 0.50 (0.39 to 0.61) | 0.91 (0.78 to 0.97) | Single study |
| PAPAS | 1 | 1.67 | 0.73 (0.62 to 0.81) | 0.78 (0.71 to 0.84) | Single study |
| Direct serum non-invasive serum tests | |||||
| Hyaluronic acid | 1 | 185.3 | 0.84 (0.73 to 0.91) | 0.83 (0.66 to 0.93) | Single study |
| Hepascore | 1 | 0.5 | 0.79 (0.68 to 0.86) | 0.74 (0.65 to 0.81) | Single study |
| Commercial non-invasive serum tests | |||||
| Fibrotest | 6 | 0.40–0.48 | 0.66 (0.57 to 0.75) | 0.80 (0.72 to 0.86) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Imaging modalities | |||||
| ARFI | 1 | 1.33 | 0.71 (0.59 to 0.80) | 0.67 (0.30 to 0.90) | Single study |
| Real-time elastography | 1 | 55.3 | 0.82 (0.67 to 0.91) | 0.65 (0.49 to 0.78) | Single study |
| Fibroscan | 13 | 6.3–8.9 | 0.71 (0.62 to 0.78) | 0.84 (0.74 to 0.91) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Test | Number of studies | Cut-off | Summary sensitivity (95% CI) | Summary specificity (95% CI) | Statistics |
|---|---|---|---|---|---|
| Indirect non-invasive serum tests | |||||
| APRI (low cut-off) | 4 | 1 | 0.58 (0.49 to 0.66) | 0.76 (0.70 to 0.81) | Fixed-effect model for sensitivity and random-effects model for specificity without correlation |
| APRI (high cut-off) | 3 | 2 | 0.24 (0.08 to 0.52) | 0.91 (0.83 to 0.96) | Random-effects model for sensitivity and fixed-effect model for specificity without correlation |
| Age–Platelet Index | 2 | 4–4.5 | 0.83 (0.72 to 0.90) | 0.74 (0.66 to 0.80) | Fixed-effects model for sensitivity and specificity without correlation |
| AST–ALT ratio | 3 | 1 | 0.33 (0.04 to 0.83) | 0.77 (0.69 to 0.84) | Random-effects model for sensitivity and fixed-effect model for specificity without correlation |
| FIB-4 (low cut-off) | 2 | 1.6–1.9 | 0.86 (0.79 to 0.91) | 0.82 (0.77 to 0.86) | Fixed-effects model for sensitivity and specificity without correlation |
| FIB-4 (high cut-off) | 1 | 3.6 | 0.30 (0.24 to 0.36) | 0.98 (0.97 to 0.99) | Single study |
| GUCI | 1 | 1 | 0.23 (0.10 to 0.43) | 0.91 (0.83 to 0.95) | Single study |
| Direct serum non-invasive serum tests | |||||
| Hyaluronic acid | 1 | 77 | 0.82 (0.52 to 0.95) | 0.88 (0.79 to 0.93) | Single study |
| Hepascore | 1 | 0.87 | 0.87 (0.62 to 0.96) | 0.85 (0.78 to 0.89) | Single study |
| Type IV collagen | 1 | 6.3 | 0.64 (0.35 to 0.85) | 0.89 (0.81 to 0.94) | Single study |
| Commercial non-invasive serum tests | |||||
| Fibrotest | 4 | 0.58–0.74 | 0.74 (0.25 to 0.96) | 0.90 (0.83 to 0.94) | Random-effects model for sensitivity and fixed-effect model for specificity without correlation |
| Imaging modalities | |||||
| Platelet–spleen ratio | 2 | 6 | 0.83 (0.74 to 0.90) | 0.77 (0.70 to 0.83) | Random-effects model for sensitivity and specificity without correlation |
| Real-time elastography | 1 | 90.3 | 0.71 (0.50 to 0.86) | 0.80 (0.69 to 0.88) | Single study |
| Fibroscan | 19 | 9.4–16.0 | 0.86 (0.79 to 0.91) | 0.85 (0.78 to 0.89) | Bivariate random-effects model with correlation between sensitivity and specificity |
Overall, there were 18 different non-invasive tests reported for the diagnosis of ≥ F2, of which 13 (72%) were reported in single studies. Of 18 different evaluated tests, only five converged with the bivariate random-effects model (APRI low and high cut-off, FIB-4 low cut-off, Fibrotest and Fibroscan). The most commonly evaluated non-invasive tests were Fibroscan (13 studies),116,200,225,230,241,242,246,247,250,251,263,264,272 APRI (low cut-off, eight studies),225,233,256–259,269,272 APRI (high cut-off, six studies)225,256–259,270 and Fibrotest (six studies). 225,247,249,255–257
For the diagnosis of cirrhosis, there were 14 different evaluated tests; however, only Fibroscan converged with the bivariate random-effects model.
For the diagnosis of fibrosis stage ≥ F1, there were eight tests that reported on diagnostic accuracy; however, none converged with the bivariate random-effects model.
For the diagnosis of fibrosis stage ≥ F3, there were 10 different evaluated tests, of which only Fibroscan converged with the bivariate random-effects model.
We explored potential sources of heterogeneity as outlined in the methods section. As all but one study200 were of low methodological quality, this potential source of heterogeneity could not be assessed. Significant heterogeneity was only found in relation to the transaminases level (comparator is normal transaminases): lower specificity for FIB-4 low cut-off in F2, and borderline lower specificity for Fibrotest in F2 (p = 0.055).
Cut-offs for specific histological stages were predetermined in 11 studies (21%). 119,200,225,230,232,252,253,255,258,264,271 Liver biopsy was of acceptable quality in 12 studies (23%). 116,119,200,225,227,230,234,251,263,266,271,272 We did not include data from some studies on APRI for F2 and F3,242 on Forns index for F2269 and on AST–ALT ratio for cirrhosis254 in the meta-analysis because of cut-offs that differed significantly from what is used in the literature. Only one study257 had low risk of bias in all of the domains of the QUADAS-2 tool; therefore, all our estimates may be biased and should be assessed with caution. Studies that were judged as low risk of bias or unknown in the three most important QUADAS domains, namely patient selection, index test and reference standard, were still a fraction of the total number of studies (11 out of 52; 23%). 225,235,236,241,242,244,246,257,263,265,271 Quality assessment of included studies based on QUADAS-262 is shown in Table 10.
| Study ID | Domain 1: patient sampling | Domain 2: index test | Domain 3: reference standard | Domain 4: flow and timing | |||
|---|---|---|---|---|---|---|---|
| Risk of bias | Concerns about applicability | Risk of bias | Concerns about applicability | Risk of bias | Concerns about applicability | Risk of bias | |
| Castera 2011225 | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ |
| Chan 2009226 | ✗ | ✗ | ✗ | ✓ | ? | ✓ | ✓ |
| Chen 2008227 | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ? |
| Chen 2012228 | ✗ | ✗ | ? | ✓ | ? | ✓ | ✗ |
| Fraquelli 2011116 | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ? |
| Fung 2011230 | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Gaia 2011119 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ |
| Ganne-Carrie 2006120 | ✓ | ✗ | ? | ✓ | ? | ✓ | ✓ |
| Gui 2010231 | ✗ | ✗ | ✗ | ✓ | ? | ✓ | ✓ |
| Guo-Qiu 2010232 | ✗ | ✗ | ✓ | ✓ | ? | ✓ | ? |
| Hongbo 2007233 | ✓ | ✓ | ✗ | ✓ | ? | ✓ | ✓ |
| Hu 2010234 | ? | ✓ | ✗ | ✓ | ✓ | ✓ | ? |
| Hui 2005235 | ? | ? | ? | ? | ? | ? | ? |
| Kim 2009238 | ✓ | ✓ | ✗ | ✓ | ? | ✓ | ✓ |
| Kim 2010236 | ? | ? | ✗ | ? | ? | ✓ | ✗ |
| Kim 2009239 | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ |
| Kim 2007237 | ✓ | ✓ | ✗ | ✓ | ? | ✓ | ? |
| Kim 2010236 | ? | ? | ? | ? | ? | ? | ? |
| Kwok 2009240 | ✓ | ✓ | ✗ | ✓ | ? | ✓ | ✗ |
| Lee 2011241 | ✓ | ✓ | ? | ✓ | ? | ✓ | ✓ |
| Lesmana242 | ? | ? | ? | ? | ? | ? | ? |
| Li 2012243 | ✗ | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ |
| Liu 2011244 | ? | ? | ? | ✓ | ? | ✓ | ? |
| Mallet 2009245 | ✗ | ✓ | ✗ | ✓ | ✗ | ✓ | ? |
| Marcellin 2009246 | ? | ✓ | ? | ? | ? | ✓ | ✗ |
| Miailhes 2011247 | ✗ | ✓ | ✗ | ✓ | ? | ✓ | ✗ |
| Mohamadnejad 2006248 | ? | ? | ✗ | ? | ✗ | ✓ | ? |
| Myers 2003249 | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ |
| Ogawa 2011250 | ? | ? | ✗ | ✓ | ? | ✓ | ? |
| Osakabe 2011251 | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ |
| Park 2003253 | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ |
| Park 2004254 | ✓ | ✓ | ✗ | ✓ | ? | ✓ | ✓ |
| Park 2005252 | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ |
| Poynard 2009255 | ✗ | ✓ | ✓ | ✓ | ? | ✓ | ? |
| Raftopoulos 2012256 | ? | ✓ | ✗ | ✓ | ✗ | ✓ | ? |
| Sebastiani 2007257 | ✓ | ✓ | ✗ | ✓ | ? | ✓ | ✓ |
| Seto 2011258 | ✗ | ✓ | ✓ | ✓ | ? | ✓ | ✓ |
| Shin 2008259 | ? | ✓ | ✗ | ✓ | ? | ✓ | ? |
| Sinakos 2011260 | ? | ✓ | ✗ | ✓ | ? | ✓ | ? |
| Sohn 2011261 | ? | ✓ | ✗ | ✓ | ? | ✓ | ? |
| Sokucu 2010262 | ✓ | ✓ | ✗ | ✓ | ? | ✓ | ? |
| Sporea 2010263 | ? | ✓ | ? | ✓ | ✓ | ✓ | ? |
| Sporea 2010200 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Vigano 2011264 | ? | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ |
| Wang 2012265 | ✓ | ✓ | ? | ✓ | ? | ✓ | ✓ |
| Wong 2008267 | ✓ | ✓ | ✗ | ✓ | ? | ✓ | ✗ |
| Wong 2010266 | ? | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ |
| Wong 2011268 | ? | ✓ | ✗ | ✓ | ? | ✓ | ? |
| Wu 2012269 | ? | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ |
| Zhang 2008271 | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ |
| Zhang 2011270 | ✗ | ✗ | ✗ | ✓ | ? | ✓ | ✓ |
| Zhu 2011272 | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ? |
Results: non-alcoholic fatty liver disease
Data on patients with NAFLD were extracted from 48 studies. 117,119,165,229,284–327 Meta-analysis was performed separately for each non-invasive test assessed at each Kleiner stage (F1–F4). Summary sensitivity and specificity for F3 and F4 are shown in Tables 11 and 12, while summary sensitivity and specificity for F1 and F2 are reported in Appendix 3. The median prevalence (minimum–maximum) of fibrosis stages F1–F4 in included studies was for F1 0.588 (0.367–0.814), F2 0.319 (0.119–0.526), F3 0.186 (0.050–0.440) and F4 0.128 (0.039–0.907). Individual study characteristics are presented in Appendix 4. The prevalence of F1–F4 in NAFLD is lower than the prevalence of such stages in the other evaluated aetiologies of liver disease; this is probably due to the relatively low prevalence of the progressive steatohepatitis among patients with NAFLD. 47 Forest plots and SROC plots of different NILTs across fibrosis stages are presented in Appendices 5 and 6, respectively.
| Test | Number of studies | Cut-off | Summary sensitivity (95% CI) | Summary specificity (95% CI) | Statistics |
|---|---|---|---|---|---|
| Indirect non-invasive serum tests | |||||
| APRI | 4 | 0.5–1.0 | 0.40 (0.07 to 0.86) | 0.82 (0.78 to 0.6) | Random-effects model for sensitivity and fixed-effect model for specificity without correlation |
| Age–Platelet Index | 1 | 6 | 0.66 (0.53 to 0.76) | 0.78 (0.74 to 0.81) | Single study |
| AST–ALT ratio (low cut-off) | 4 | 0.8 | 0.79 (0.51 to 0.91) | 0.70 (0.55 to 0.82) | Bivariate random-effects model with correlation between sensitivity and specificity |
| AST–ALT ratio (high cut-off) | 3 | 1.0 | 0.46 (0.29 to 0.65) | 0.91 (0.85 to 0.95) | Random-effects model for sensitivity and fixed-effect model for specificity without correlation |
| BARD | 7 | 2 | 0.84 (0.69 to 0.93) | 0.61 (0.47 to 0.73) | Bivariate random-effects model with correlation between sensitivity and specificity |
| FIB-4 (low cut-off) | 4 | 1.3–1.92 | 0.84 (0.75 to 0.90) | 0.74 (0.64 to 0.83) | Bivariate random-effects model with correlation between sensitivity and specificity |
| FIB-4 (high cut-off) | 2 | 3.25 | 0.38 (0.22 to 0.57) | 0.97 (0.92 to 0.99) | Bivariate random-effects model with correlation between sensitivity and specificity |
| NAFLD fibrosis score (low cut-off) | 10 | –1.455 | 0.80 (0.67 to 0.89) | 0.66 (0.57 to 0.74) | Bivariate random-effects model with correlation between sensitivity and specificity |
| NAFLD fibrosis score (high cut-off) | 9 | 0.676 | 0.40 (0.20 to 0.64) | 0.97 (0.94 to 0.98) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Platelets | 1 | 0.63 (0.57 to 0.69) | 0.76 (0.74 to 0.78) | Single study | |
| Direct serum non-invasive serum tests | |||||
| Hyaluronic acid | 4 | 46–50 | 0.88 (0.58 to 0.97) | 0.82 (0.75 to 0.87) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Hepascore | 1 | 0.37 | 0.75 (0.62 to 0.85) | 0.84 (0.78 to 0.89) | Single study |
| NAFIC (low cut-off) | 1 | 1 | 0.96 (0.88 to 0.98) | 0.67 (0.63 to 0.71) | Single study |
| NAFIC (high cut-off) | 1 | 3 | 0.84 (0.73 to 0.91) | 0.82 (0.79 to 0.85) | Single study |
| NDP: advanced fibrosis | 1 | 0.24 | 0.88 (0.64 to 0.96) | 0.70 (0.58 to 0.80) | Single study |
| Type IV collagen | 2 | 5 | 0.79 (0.69 to 0.87) | 0.80 (0.66 to 0.89) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Commercial non-invasive serum tests | |||||
| ELF | 1 | 10.35 | 0.80 (0.65 to 0.89) | 0.90 (0.84 to 0.94) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Fibrotest (low cut-off) | 3 | 0.3 | 0.88 (0.68 to 0.99) | 0.73 (0.56 to 0.85) | Random-effects model for sensitivity and specificity without correlation |
| Fibrotest (high cut-off) | 4 | 0.57–0.70 | 0.40 (0.24 to 0.58) | 0.96 (0.91 to 0.98) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Imaging modalities | |||||
| ARFI | 1 | 4.2 | 0.90 (0.77 to 0.96) | 0.90 (0.82 to 0.94) | Single study |
| Fibroscan | 8 | 7.5–10.4 | 0.82 (0.74 to 0.88) | 0.84 (0.78 to 0.89) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Combination of non-invasive test algorithms | |||||
| NAFLD fibrosis score and ELF (low cut-off) | 1 | 0.91 (0.79 to 0.96) | 0.96 (0.91 to 0.98) | Single study | |
| NAFLD fibrosis score and ELF (high cut-off) | 1 | 0.86 (0.73 to 0.94) | 0.99 (0.96 to 1.00) | Single study | |
| Fibroscan and Fibrotest | 1 | 0.39 (0.27 to 0.53) | 0.96 (0.92 to 0.98) | Single study | |
| Test | Number of studies | Cut-off | Summary sensitivity (95% CI) | Summary specificity (95% CI) | Statistics |
|---|---|---|---|---|---|
| Indirect non-invasive serum tests | |||||
| APRI | 2 | 0.54 and NA | 0.78 (0.71 to 0.99) | 0.71 (0.30 to 0.93) | Random-effects model for sensitivity and fixed-effect model for specificity without correlation |
| Age–Platelet Index | 1 | NA | 0.89 (0.56 to 0.98) | 0.83 (0.69 to 0.91) | Single study |
| AST–ALT ratio | 1 | 1 | 0.89 (0.56 to 0.98) | 0.73 (0.58 to 0.84) | Single study |
| BARD | 1 | 2 | 0.52 (0.33 to 0.71) | 0.84 (0.79 to 0.88) | Single study |
| CDS (low cut-off) | 1 | 3 | 0.89 (0.56 to 0.98) | 0.90 (0.87 to 0.96) | Single study |
| CDS (high cut-off) | 1 | 5 | 0.33 (0.12 to 0.65) | 1.00 (0.91 to 1.00) | Single study |
| FIB-4 (low cut-off) | 1 | 1.92 | 0.74 (0.54 to 0.87) | 0.71 (0.64 to 0.76) | Single study |
| Lok’s index (low cut-off) | 1 | 0.6 | 0.89 (0.56 to 0.98) | 0.68 (0.53 to 0.80) | Single study |
| Lok’s index (high cut-off) | 1 | 0.97 | 0.22 (0.06 to 0.55) | 1.00 (0.91 to 1.00) | Single study |
| Platelets | 2 | 160,000 | 0.96 (0.30 to 0.99) | 0.92 (0.85 to 0.96) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Direct serum non-invasive serum tests | |||||
| 13C-caffeine breath test | 1 | 1.27 | 0.90 (0.60 to 0.98) | 0.76 (0.61 to 0.87) | Single study |
| Hepascore | 1 | 0.7 | 0.87 (0.68 to 0.95) | 0.89 (0.84 to 0.93) | Single study |
| Commercial non-invasive serum tests | |||||
| Fibrotest | 1 | 0.57 | 0.74 (0.54 to 0.87) | 0.92 (0.88 to 0.95) | Single study |
| Imaging modalities | |||||
| Fibroscan | 4 | 10.3–17.5 | 0.96 (0.83 to 0.99) | 0.89 (0.85 to 0.92) | Bivariate random-effects model with correlation between sensitivity and specificity |
Overall, there were 24 different non-invasive tests reported for the diagnosis of ≥ F3, of which 11 (46%) were reported in single studies. 117,229,284,286,287,289,290,299,304,306,327 Of 24 different evaluated tests, 10 converged with the bivariate random-effects model [BARD (BMI, AST-ALT ratio, diabetes), AST–ALT ratio low cut-off, NAFLD fibrosis score low and high cut-offs, FIB-4 low and high cut-off, hyaluronic acid, type IV collagen, Fibrotest and Fibroscan]. The most commonly evaluated non-invasive tests were NAFLD fibrosis score (low cut-off, 10 studies), 290,300,301,309,311,315,320,322 NAFLD fibrosis score (high cut-off, nine studies),285,290,300,301,309,311,315,320,322 Fibroscan (eight studies)119,236,288,296,298,308,323,324 and BARD (seven studies). 284,291,300,301,312,315,319
For the diagnosis of cirrhosis, there were 14 different evaluated tests; however, only platelet count and Fibroscan converged with the bivariate random-effects model.
For the diagnosis of fibrosis stage ≥ F1, there were 12 tests that reported on diagnostic accuracy, and three of them converged with the bivariate random-effects model (NAFLD fibrosis score low and high cut-offs, TE).
For the diagnosis of fibrosis stage ≥ F2, there were 20 different evaluated tests, of which three converged with the bivariate random-effects model (NAFLD fibrosis score low and high cut-offs, Fibrotest).
Uninterpretable NILT results were very rare in serum markers (< 1%) and were more frequently encountered in patients who were undergoing Fibroscan examination. The rate of uninterpretable results with Fibroscan (due to < 10 valid measurements, success rate < 60% and interquartile range> 30%) was 9.6%; however, this could be underestimated due to under-reporting.
Cut-offs for specific histological stages were predetermined in 10 studies (21%). 117,119,284,291,309,311,312,315,321,322 Liver biopsy was of acceptable quality in 10 studies (21%). 117,119,229,284,286,293,308,319,321,326 We did not include data on APRI and NAFLD fibrosis scores from one study327 in the meta-analysis because of cut-offs that differed significantly from what is used in the literature. Only one study119 had low risk of bias in all of the domains of the QUADAS-2 tool; therefore, all our estimates may be biased. Studies that were judged as low risk of bias or unknown in the three most important QUADAS domains, namely patient selection, index test and reference standard, were 21% of the total number of studies (10 out of 48). 119,165,284,294,298,301,303,319,323 Quality assessment of included studies based on QUADAS-262 is shown in Table 13.
| Study ID | Domain 1: patient sampling | Domain 2: index test | Domain 3: reference standard | Domain 4: flow and timing | |||
|---|---|---|---|---|---|---|---|
| Risk of bias | Concerns about applicability | Risk of bias | Concerns about applicability | Risk of bias | Concerns about applicability | Risk of bias | |
| Adams 2011284 | ? | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ |
| Angulo 2007285 | ✗ | ✗ | ✗ | ✓ | ? | ✓ | ✓ |
| Blomme 2012286 | ✗ | ✗ | ✗ | ✓ | ✓ | ✓ | ? |
| Cales 2009287 | ✗ | ✗ | ? | ✓ | ? | ✓ | ✗ |
| Dixon 2001229 | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ? |
| Fujii 2009117 | ✗ | ✗ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Gaia 2011119 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ |
| Guajardo-Salinas 2010289 | ✓ | ✗ | ? | ✓ | ? | ✓ | ✓ |
| Guha 2008290 | ✗ | ✗ | ✗ | ✓ | ? | ✓ | ✓ |
| Harrison 2008291 | ✗ | ✗ | ✓ | ✓ | ? | ✓ | ? |
| Kaneda 2006292 | ✓ | ✓ | ✗ | ✓ | ? | ✓ | ✓ |
| Kayadibi 2009293 | ? | ✓ | ✗ | ✓ | ✓ | ✓ | ? |
| Kelleher 2006294 | ? | ? | ? | ? | ? | ? | ? |
| Khosravi 2011295 | ✓ | ✓ | ✗ | ✓ | ? | ✓ | ✓ |
| de Ledinghen 2009288 | ? | ? | ✗ | ? | ? | ✓ | ✗ |
| Lupsor 2010296 | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ |
| Lydatakis 2006297 | ✓ | ✓ | ✗ | ✓ | ? | ✓ | ? |
| Mahadeva 2010298 | ? | ? | ? | ? | ? | ? | ? |
| Manousou 2011299 | ✓ | ✓ | ✗ | ✓ | ? | ✓ | ✗ |
| McPherson 2010301 | ✓ | ✓ | ? | ✓ | ? | ✓ | ✓ |
| McPherson 2011300 | ? | ? | ? | ? | ? | ? | ? |
| Obara 2008164 | ✗ | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ |
| Oliveira 2005165 | ? | ? | ? | ✓ | ? | ✓ | ? |
| Orlacchio 2012302 | ✗ | ✓ | ✗ | ✓ | ✗ | ✓ | ? |
| Pais 2011303 | ? | ✓ | ? | ? | ? | ✓ | ✗ |
| Palmeri 2011304 | ✗ | ✓ | ✗ | ✓ | ? | ✓ | ✗ |
| Papalavrentios 2011305 | ? | ? | ✗ | ? | ✗ | ✓ | ? |
| Park 2011306 | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ |
| Pawitpok 2006307 | ? | ? | ✗ | ✓ | ? | ✓ | ? |
| Petta 2011308 | ✓ | ✓ | ✗ | ✓ | ✓ | ✓ | ✓ |
| Pimentel 2010309 | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ |
| Poynard 2006310 | ✓ | ✓ | ✗ | ✓ | ? | ✓ | ✓ |
| Qureshi 2008311 | ✗ | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ |
| Raszeja-Wyscomirska 2010312 | ✗ | ✓ | ✓ | ✓ | ? | ✓ | ? |
| Ratziu 2004313 | ? | ✓ | ✗ | ✓ | ✗ | ✓ | ? |
| Ratziu 2006314 | ✓ | ✓ | ✗ | ✓ | ? | ✓ | ✓ |
| Ruffilo 2011315 | ✗ | ✓ | ✓ | ✓ | ? | ✓ | ✓ |
| Sakugawa 2005316 | ? | ✓ | ✗ | ✓ | ? | ✓ | ? |
| Santos 2005317 | ? | ✓ | ✗ | ✓ | ? | ✓ | ? |
| Shimada 2007318 | ? | ✓ | ✗ | ✓ | ? | ✓ | ? |
| Sumida 2011320 | ✓ | ✓ | ✗ | ✓ | ? | ✓ | ? |
| Sumida 2012319 | ? | ✓ | ? | ✓ | ✓ | ✓ | ? |
| Suzuki 2005321 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Wong 2008322 | ? | ✓ | ✓ | ✓ | ✗ | ✓ | ✓ |
| Wong 2008323 | ✓ | ✓ | ? | ✓ | ? | ✓ | ✓ |
| Wong 2009324 | ✓ | ✓ | ✗ | ✓ | ? | ✓ | ✗ |
| Yoneda 2008326 | ? | ✓ | ✗ | ✓ | ✓ | ✓ | ✗ |
| Yoneda 2011325 | ? | ✓ | ✗ | ✓ | ? | ✓ | ? |
| Younossi 2011327 | ? | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ |
We explored potential sources of heterogeneity as outlined in the methods section. As all but one study were of low methodological quality, this potential source of heterogeneity could not be assessed. More specifically and according to source of heterogeneity, details of significant associations are:
-
Histological score used (comparator is the Kleiner system): for NAFLD fibrosis score low cut-off in F2 and low and high cut-offs in F3, use of the Brunt scoring system resulted in significantly lower sensitivity and higher specificity.
-
Full text versus abstract publication: for BARD score in F3, full-text publication was associated with significantly higher specificity.
-
There was no heterogeneity identified in relation to transaminases levels.
Results: alcoholic liver disease
Data on patients with ALD were extracted from 12 studies. 114,273–283 Meta-analysis was performed separately for each non-invasive test assessed at each METAVIR stage (F1–F4). Summary sensitivity and specificity of non-invasive tests for each fibrosis stage are shown in Table 14. The median prevalence (minimum–maximum) of fibrosis stages F1–F4 in included studies was for F1 0.923 (single study), F2 0.633 (0.500–0.837), F3 0.509 (0.404–0.748) and F4 0.448 (0.145–0.971). Individual study characteristics, and forest plots and SROC plots of the different NILTs across fibrosis stages, are presented in Appendices 4, 5 and 6, respectively.
| Test | Number of studies | Cut-off | Summary sensitivity (95% CI) | Summary specificity (95% CI) | Statistics |
|---|---|---|---|---|---|
| Non-invasive tests for diagnosis of F ≥ 1 | |||||
| Fibroscan | 1 | 5.9 | 0.83 (0.74 to 0.89) | 0.88 (0.53 to 0.98) | Single study |
| Non-invasive tests for diagnosis of F ≥ 2 | |||||
| Fibroscan | 1 | 7.8 | 0.81 (0.7 to 0.88) | 0.92 (0.76 to 0.98) | Single study |
| Fibrotest (high cut-off) | 1 | 0.7 | 0.55 (0.47 to 0.63) | 0.93 (0.85 to 0.97) | Single study |
| Fibrotest (low cut-off) | 1 | 0.3 | 0.84 (0.77 to 0.89) | 0.65 (0.55 to 0.75) | Single study |
| APRI (high cut-off) | 2 | 1.5 | 0.54 (0.42 to 0.66) | 0.78 (0.64 to 0.88) | Fixed-effects model for sensitivity and specificity without correlation |
| APRI (low cut-off) | 2 | 0.5 | 0.72 (0.6 to 0.82) | 0.46 (0.33 to 0.6) | Fixed-effects model for sensitivity and specificity without correlation |
| Non-invasive tests for diagnosis of F ≥ 3 | |||||
| CK18 | 1 | 0.84 (0.73 to 0.92) | 0.71 (0.6 to 0.79) | Single study | |
| Forns index (high cut-off) | 1 | 6.9 | 0.41 (0.23 to 0.61) | 0.88 (0.66 to 0.97) | Single study |
| YKL-40 | 1 | 330 | 0.51 (0.38 to 0.63) | 0.89 (0.8 to 0.94) | Single study |
| Fibroscan | 4 | 11.0–12.5 | 0.87 (0.64 to 0.96) | 0.82 (0.67 to 0.91) | Random-effects model for sensitivity and specificity without correlation |
| Non-invasive tests for diagnosis of F ≥ 4 | |||||
| APRI (high cut-off) | 1 | 2 | 0.40 (0.22 to 0.61) | 0.62 (0.41 to 0.79) | Single study |
| Fibrotest (high cut-off) | 1 | 0.7 | 0.91 (0.82 to 0.96) | 0.87 (0.81 to 0.91) | Single study |
| Fibrotest (low cut-off) | 1 | 0.3 | 1.00 (0.95 to 1.00) | 0.50 (0.42 to 0.58) | Single study |
| PGAA | 1 | 7 | 0.78 (0.64 to 0.88) | 0.89 (0.85 to 0.92) | Single study |
| Fibroscan | 6 | 11.4–25.8 | 0.86 (0.76 to 0.92) | 0.83 (0.74 to 0.89) | Random-effects model for sensitivity and specificity without correlation |
Overall, there were four different non-invasive tests reported for the diagnosis of ≥ F3, of which three (75%) were reported in single studies. 273,275,282 The most commonly evaluated non-invasive test was Fibroscan (four studies);273,277,278,281 however, the results did not converge with the bivariate random-effects model. There were one, five and five NILTs evaluated for F1, F2 and F4 fibrosis stages, respectively, none of which converged with the bivariate random-effects model. APRI (high and low cut-offs) in F2 were evaluated in two studies273,283 and Fibroscan in F4 was evaluated in six studies;114,273,274,276,278,281 all other tests were evaluated in single studies.
There were four different non-invasive tests reported for the diagnosis of F4, of which three (75%) were reported in single studies. 273,279,280 The most commonly evaluated non-invasive test was Fibroscan (six studies),114,273,274,276,278,281 however, with cut-offs that widely ranged from 11.4 to 25.8 kPa. The rest of the tests for F4 were PGAA [prothrombin time, gamma-glutamyl transpeptidase (GGT), apolipoprotein A1, α2-macroglobulin], Fibrotest (at a low and high cut-off) and APRI (results only reported at a high cut-off; no reason provided for not including a low cut-off).
Cut-offs for specific histological stages were predetermined in three studies (25%),276,277,282 whereas liver biopsy was of acceptable quality in one study (8%). 273 There was no study with low risk of bias in all the domains of the QUADAS-2 tool; therefore, all of our estimates may be biased. Studies that were judged as low risk of bias or unknown in the three most important QUADAS domains, namely patient selection, index test and reference standard, were 25% of the total number of studies (3 out of 12). 276,279,282 Quality assessment of included studies based on QUADAS-262 is shown in Table 15.
| Study ID | Domain 1: patient sampling | Domain 2: index test | Domain 3: reference standard | Domain 4: flow and timing | |||
|---|---|---|---|---|---|---|---|
| Risk of bias | Concerns about applicability | Risk of bias | Concerns about applicability | Risk of bias | Concerns about applicability | Risk of bias | |
| Forestier 2010114 | ✗ | ✗ | ✗ | ✗ | ? | ✓ | ✗ |
| Janssens 2010273 | ✗ | ✗ | ✗ | ✗ | ✓ | ✓ | ✗ |
| Kim 2009274 | ? | ? | ✗ | ✓ | ✗ | ✓ | ? |
| Lavallard 2011275 | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ? |
| Melin 2005276 | ✓ | ✓ | ✓ | ? | ? | ✓ | ✗ |
| Mueller 2010277 | ✗ | ✗ | ✓ | ✓ | ✗ | ✓ | ? |
| Nahon 2008278 | ✓ | ✓ | ✗ | ✗ | ✗ | ✓ | ✗ |
| Naveau 1994279 | ? | ✓ | ? | ✓ | ? | ✓ | ✓ |
| Naveau 2005280 | ✓ | ✓ | ✗ | ✓ | ? | ✓ | ✓ |
| Nguyen-Khac 2008281 | ✗ | ✓ | ✗ | ✓ | ✗ | ✓ | ✗ |
| Tran 2000282 | ✓ | ✓ | ✓ | ✓ | ? | ✓ | ? |
| Vanbiervliet 2005283 | ✗ | ✗ | ✗ | ✗ | ? | ✓ | ✗ |
Although investigation of potential sources of heterogeneity was planned, it could not be performed due to the small number of studies.
Results: liver cirrhosis
Diagnostic accuracy of NILTs for cirrhosis was also analysed irrespective of aetiology of liver disease and used for the cirrhosis economic model. Summary sensitivity and specificity for cirrhosis are shown in Table 16. Median prevalence of cirrhosis in the evaluated studies was 0.18 (range 0–0.97). Fibroscan was by far the most commonly evaluated non-invasive test (65 studies). 28,29,75,76,86–88,91,95,98–100,102,105,106,110,114,116,119,130,137,141,147,153,155,159,161,164,170,172,173,194,199–201,211,223,224,225,230,241,242,246,247,250,251,263,264,272–274,276,278,281,288,296,298,308,323,324,236 We do not include a table on quality assessment of included studies to avoid repetition, as this was given separately according to disease aetiology.
| Test | Number of studies | Cut-off | Summary sensitivity (95% CI) | Summary specificity (95% CI) | Statistics |
|---|---|---|---|---|---|
| Indirect non-invasive serum tests | |||||
| APRI (low cut-off) | 27 | 0.75–1 | 0.75 (0.71 to 0.8) | 0.78 (0.75 to 0.81) | Bivariate random-effects model with correlation between sensitivity and specificity |
| APRI (high cut-off) | 23 | 2 | 0.45 (0.37 to 0.52) | 0.93 (0.9 to 0.95) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Age–Platelet Index | 3 | 0.88 (0.08 to 1.00) | 0.73 (0.43 to 0.91) | Random-effects model for sensitivity and specificity without correlation | |
| AST–ALT ratio | 13 | 1 | 0.49 (0.39 to 0.59) | 0.87 (0.75 to 0.94) | Bivariate random-effects model with correlation between sensitivity and specificity |
| CDS | 1 | 0.88 (0.66 to 0.97) | 0.67 (0.57 to 0.77) | Single study | |
| FIB-4 (low cut-off) | 5 | 1.45–1.92 | 0.84 (0.76 to 0.89) | 0.71 (0.62 to 0.79) | Bivariate random-effects model with correlation between sensitivity and specificity |
| FIB-4 (high cut-off) | 4 | 3.25–4.44 | 0.42 (0.2 to 0.69) | 0.92 (0.58 to 0.99) | Random-effects model for sensitivity and specificity without correlation |
| Forns index (low cut-off) | 2 | 4.2 | 0.88 (0.73 to 0.96) | 0.37 (0.26 to 0.49) | Fixed-effects model for sensitivity and specificity without correlation |
| Forns index (high cut-off) | 1 | 6.9 | 0.67 (0.53 to 0.78) | 0.91 (0.84 to 0.95) | Single study |
| GUCI | 4 | 0.33–1.11 | 0.64 (0.11 to 0.96) | 0.86 (0.81 to 0.9) | Random-effects model for sensitivity and fixed-effect model for specificity without correlation |
| King’s | 1 | 24.3 | 0.74 (0.59 to 0.85) | 0.90 (0.84 to 0.94) | Single study |
| Lok’s index (low cut-off) | 2 | 0.2–0.26 | 0.84 (0.09 to 1) | 0.66 (0.00 to 1.00) | Fixed-effect model for sensitivity and random-effects model for specificity without correlation |
| Lok’s index (high cut-off) | 1 | 0.5 | 0.40 (0.29 to 0.52) | 0.95 (0.91 to 0.97) | Single study |
| Platelets | 12 | 134–196 | 0.72 (0.62 to 0.81) | 0.88 (0.77 to 0.94) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Direct serum non-invasive serum tests | |||||
| 13C-caffeine breath test | 1 | 1.27 | 0.93 (0.77 to 0.98) | 0.84 (0.74 to 0.91) | Single study |
| Fontana | 1 | 0.2 | 0.79 (0.72 to 0.84) | 0.66 (0.61 to 0.71) | Single study |
| Hyaluronic acid | 8 | 78–237 | 0.81 (0.65 to 0.9) | 0.88 (0.8 to 0.94) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Hepascore | 9 | 0.7–0.87 | 0.82 (0.72 to 0.88) | 0.84 (0.79 to 0.88) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Hepascore (low cut-off) | 1 | 0.58 | 0.80 (0.72 to 0.86) | 0.83 (0.8 to 0.85) | Single study |
| Hepascore (high cut-off) | 1 | 1.16 | 0.39 (0.31 to 0.48) | 0.99 (0.98 to 0.99) | Single study |
| PIIINP | 3 | 0.8–1 | 0.70 (0.48 to 0.86) | 0.79 (0.34 to 0.96) | Fixed-effect model for sensitivity and random-effects model for specificity without correlation |
| Type IV collagen | 3 | 65–190 | 0.71 (0.57 to 0.82) | 0.76 (0.59 to 0.87) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Commercial non-invasive serum tests | |||||
| ELF | 1 | 9.4 | 0.93 (0.69 to 0.99) | 0.79 (0.67 to 0.88) | Single study |
| ELF (low cut-off) | 1 | 0.90 (0.84 to 0.94) | 0.53 (0.46 to 0.59) | Single study | |
| ELF (high cut-off) | 1 | 0.52 (0.43 to 0.6) | 0.90 (0.85 to 0.93) | Single study | |
| Fibroindex | 1 | 1.82 | 0.70 (0.52 to 0.84) | 0.91 (0.82 to 0.96) | Single study |
| Fibrometer | 2 | 0.88 | 0.72 (0.36 to 0.92) | 0.88 (0.6 to 0.97) | Random-effects model for sensitivity and fixed-effect model for specificity without correlation |
| Fibrometer (low cut-off) | 1 | 0.63 | 0.96 (0.9 to 0.98) | 0.71 (0.68 to 0.74) | Single study |
| Fibrometer (high cut-off) | 1 | 0.98 | 0.36 (0.28 to 0.45) | 0.98 (0.97 to 0.99) | Single study |
| Fibrotest | 13 | 0.75 | 0.61 (0.47 to 0.74) | 0.87 (0.83 to 0.9) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Fibrotest (low cut-off) | 2 | 0.3 | 0.89 (0.29 to 0.99) | 0.65 (0.01 to 1.00) | Fixed-effect model for sensitivity and random-effects model for specificity without correlation |
| Fibrotest (high cut-off) | 3 | 0.86 | 0.73 (0.14 to 0.98) | 0.94 (0.91 to 0.96) | Random-effects model for sensitivity and fixed-effect model for specificity without correlation |
| Imaging modalities | |||||
| ARFI | 4 | 1.59–2 | 0.84 (0.72 to 0.91) | 0.77 (0.5 to 0.92) | Random-effects model for sensitivity and specificity without correlation |
| Platelet–Spleen Index | 2 | 0.83 (0.28 to 0.98) | 0.85 (0.00 to 1.00) | Fixed-effect model for sensitivity and random-effects model for specificity without correlation | |
| Real-time elastography | 1 | 3.93 | 0.71 (0.5 to 0.86) | 0.80 (0.67 to 0.88) | Single study |
| Fibroscan | 65 | 9.2–26.5 | 0.89 (0.86 to 0.91) | 0.89 (0.87 to 0.91) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Combination of fibrosis non-invasive tests algorithms | |||||
| Bordeaux | 1 | 0.87 (0.8 to 0.92) | 0.95 (0.93 to 0.96) | Single study | |
| Fibropaca | 1 | 0.79 (0.72 to 0.84) | 0.66 (0.61 to 0.71) | Single study | |
| SAFE | 4 | 0.74 (0.42 to 0.92) | 0.93 (0.91 to 0.94) | Random-effects model for sensitivity and fixed-effect model for specificity without correlation | |
Results: imaging modalities results that were used irrespective of liver disease aetiology
Data on diagnostic accuracy of imaging modalities for the non-invasive assessment of liver fibrosis were analysed irrespective of the aetiology of liver disease, with the exception of Fibroscan and ARFI. These two tests measure liver stiffness that is associated with the amount of fibrosis and there is evidence of different cut-offs according to disease aetiology. The summary sensitivity and specificity of these imaging modalities for METAVIR stages F1–F4 are shown in Table 17. 30,93,98,100,101,110,118,132,145,149,152,166,167,176,184,185,227,270,305,328–363
| Test | Number of studies | Summary sensitivity (95% CI) | Summary specificity (95% CI) | Statistics |
|---|---|---|---|---|
| Imaging modalities for diagnosis of F ≥ 1 | ||||
| MR elastography | 5 | 0.83 (0.72 to 0.9) | 0.83 (0.67 to 0.92) | Random-effects model for sensitivity and specificity without correlation |
| CEMRE | 1 | 0.95 (0.75 to 0.99) | 1.00 (0.57 to 1.00) | Single study |
| CEMRI | 1 | 0.87 (0.78 to 0.93) | 0.75 (0.51 to 0.9) | Single study |
| CEUS | 1 | 0.80 (0.7 to 0.88) | 0.90 (0.6 to 0.98) | Single study |
| CT | 1 | 0.70 (0.52 to 0.83) | 0.64 (0.43 to 0.8) | Single study |
| DW-MRI | 1 | 0.79 (0.65 to 0.89) | 0.83 (0.55 to 0.95) | Single study |
| US | 1 | 0.77 (0.6 to 0.89) | 0.89 (0.69 to 0.97) | Single study |
| US MARS | 1 | 0.82 (0.64 to 0.92) | 0.75 (0.41 to 0.93) | Single study |
| Imaging modalities for diagnosis of F ≥ 2 | ||||
| CEMRI | 2 | 0.80 (0.15 to 0.99) | 0.60 (0.03 to 0.99) | Fixed-effect model for sensitivity and random-effects model for specificity without correlation |
| CEUS | 3 | 0.88 (0.07 to 1.00) | 0.73 (0.11 to 0.98) | Random-effects model for sensitivity and specificity without correlation |
| DW-MRI | 5 | 0.78 (0.63 to 0.88) | 0.78 (0.51 to 0.93) | Random-effects model for sensitivity and specificity without correlation |
| MR elastography | 3 | 0.94 (0.13 to 1.00) | 0.92 (0.72 to 0.98) | Random-effects model for sensitivity and fixed-effect model for specificity without correlation |
| US | 3 | 0.35 (0.14 to 0.63) | 0.86 (0.59 to 0.96) | Bivariate random-effects model with correlation between sensitivity and specificity |
| US SAPI | 3 | 0.74 (0.69 to 0.79) | 0.79 (0.72 to 0.85) | Fixed-effects model for sensitivity and specificity without correlation |
| US SAPI (high cut-off) | 2 | 0.61 (0.54 to 0.68) | 0.96 (0.9 to 0.98) | Fixed-effects model for sensitivity and specificity without correlation |
| US SAPI F2 (low cut-off) | 2 | 0.94 (0.9 to 0.97) | 0.39 (0.31 to 0.49) | Fixed-effects model for sensitivity and specificity without correlation |
| nDW-MRI | 1 | 0.90 (0.74 to 0.97) | 0.75 (0.3 to 0.95) | Single study |
| SPECT | 1 | 0.86 (0.67 to 0.95) | 0.83 (0.64 to 0.93) | Single study |
| US MARS | 1 | 0.85 (0.64 to 0.95) | 0.56 (0.33 to 0.77) | Single study |
| Imaging modalities for diagnosis of F ≥ 3 | ||||
| CEUS | 3 | 0.78 (0.14 to 0.99) | 0.87 (0.24 to 0.99) | Random-effects model for sensitivity and specificity without correlation |
| DEMRI | 1 | 0.93 (0.85 to 0.97) | 0.86 (0.69 to 0.95) | Single study |
| DW-MRI | 3 | 0.88 (0.00 to 1.00) | 0.73 (0.07 to 0.99) | Random-effects model for sensitivity and specificity without correlation |
| MR elastography | 6 | 0.91 (0.85 to 0.95) | 0.88 (0.80 to 0.93) | Bivariate random-effects model with correlation between sensitivity and specificity |
| US | 4 | 0.57 (0.31 to 0.79) | 0.80 (0.67 to 0.88) | Bivariate random-effects model with correlation between sensitivity and specificity |
| CEMRI | 1 | 0.75 (0.63 to 0.85) | 0.50 (0.36 to 0.64) | Single study |
| MRI | 1 | 0.78 (0.61 to 0.89) | 0.75 (0.60 to 0.86) | Single study |
| nDW-MRI | 1 | 0.96 (0.80 to 0.99) | 0.67 (0.35 to 0.88) | Single study |
| Imaging modalities for diagnosis of F ≥ 4 | ||||
| CEUS | 3 | 0.84 (0.01 to 1.00) | 0.88 (0.27 to 0.99) | Random-effects model for sensitivity and specificity without correlation |
| DW-MRI | 2 | 0.88 (0.01 to 1.00) | 0.73 (0.09 to 0.99) | Fixed-effect model for sensitivity and random-effects model for specificity without correlation |
| LSPI | 4 | 0.91 (0.41 to 0.99) | 0.88 (0.3 to 0.99) | Random-effects model for sensitivity and specificity without correlation |
| MR elastography | 3 | 1.00 (0.03 to 1.00) | 0.93 (0.20 to 1.00) | Random-effects model for sensitivity and specificity without correlation |
| MRI | 2 | 0.75 (0.64 to 0.83) | 0.80 (0.69 to 0.88) | Fixed-effects model for sensitivity and specificity without correlation |
| US | 25 | 0.73 (0.66 to 0.79) | 0.88 (0.83 to 0.92) | Bivariate random-effects model with correlation between sensitivity and specificity |
| US SAPI | 2 | 0.73 (0.25 to 0.95) | 0.67 (0.43 to 0.84) | Fixed-effects model for sensitivity and specificity without correlation |
| CEMRI | 1 | 0.81 (0.65 to 0.90) | 0.48 (0.37 to 0.60) | Single study |
| HVRI US | 1 | 0.90 (0.74 to 0.97) | 0.86 (0.78 to 0.92) | Single study |
| nDW-MRI | 1 | 0.95 (0.77 to 0.99) | 0.69 (0.42 to 0.87) | Single study |
| US MARS | 1 | 1.00 (0.68 to 1.00) | 0.50 (0.33 to 0.67) | Single study |
Certain diagnostic modalities that are not routinely used, such as MR spectroscopy,367 double-contrast material-enhanced MRI,368 maximal accumulative respiratory strain using ultrasound,234 liver enhancement ratio of gadoxetic acid enhanced MRI369 and single-photon emission CT parameters370 and real-time elastography,142 are not presented and were not used in the economic analysis, as they are not widely available and require further validation.
Data on ultrasound techniques using injection of contrast material (contrast-enhanced ultrasound)344 or measuring the splenic artery pulsatility index (ultrasound SAPI)152 were included in the analysis and in the economic modelling for HBV and HCV. These are based on ultrasound; however, they require well-trained and experienced operators and use signs that are not well validated. Moreover, all data come from specialised centres. Therefore, reported diagnostic accuracies are most probably overestimated and not reproducible in most centres. In most centres, ultrasound, CT and MRI can only diagnose cirrhosis with acceptable specificity but lower sensitivity, as early cirrhosis and lesser fibrosis stages are often missed. MR elastography is a promising tool, but is of limited use at the moment due to cost and availability.
Discussion of overall results
In total, 302 studies were selected for the meta-analysis. 23–29,31,71–327 For the fibrosis stages of interest in our models (F2 for HBV and HCV, F3 for NAFLD and F4 for ALD), there were 33 NILTs assessed in HCV,23–29,31,71–224 18 in HBV,116,119,120,200,225–272 24 in NAFLD117,119,165,229,284–327 and four in ALD. 114,273–283 However, 19 out of 33 NILTs in HCV,31,83,131,142,146,147,183,189,204,205,210 13 out of 18 in HBV,243,244,256–258,263,265 11 out of 24 in NAFLD117,229,284,286,287,289,290,299,304,306,327 and three out of four in ALD273,275,282 were assessed in single studies. HCV was the disease aetiology with most studies identified, while ALD had the fewest studies assessed.
There were no data available for tests that converged using the bivariate model for ALD; therefore, the use of NILTs in such patients for treatment decisions is uncertain and requires further study. Very few tests converged using the bivariate model for HBV: only APRI (high and low cut-offs), FIB-4 (low cut-off), Fibrotest and Fibroscan converged for F2 and Fibroscan alone for F3 and F4. In patients with NAFLD there is a wider choice, as NAFLD fibrosis score, FIB-4, BARD, AST–ALT ratio, Fibrotest and Fibroscan all converged. HCV is the liver disease with the highest number of available data and, subsequently, choices on NILTs. For the diagnosis of F2, APRI low and high cut-off, AST–ALT ratio, FIB-4 low and high cut-offs, Forns index low and high cut-offs, GUCI, Lok’s index, platelet count, hyaluronic acid, Hepascore, Fibrometer, Fibrotest standard, low and high cut-offs, platelet-to-spleen-diameter ratio and Fibroscan all converged.
Summary sensitivity and specificity of tests that converged were as follows in HCV and HBV (≥ F2 and F4) and NAFLD (≥ F3):
-
For HCV in ≥ F2: APRI low cut-off 82% and 57%, high cut-off 39% and 92%; AST–ALT ratio 44% and 71%; FIB-4 high cut-off 59% and 74%; Forns index low cut-off 88% and 40%, high cut-off 35% and 96%; GUCI 65% and 79%; Lok’s index 67% and 55%; platelet count 50% and 89%; hyaluronic acid 75% and 75%; Hepascore 73% and 73%; Fibrometer 79% and 73%; Fibrotest 68% and 72%; Fibroscan 79% and 83%.
-
For HCV in F4: APRI low cut-off 77% and 78%, high cut-off 48% and 94%; AST–ALT ratio 49% and 87%; platelet count 68% and 86%; hyaluronic acid 80% and 88%; Hepascore 80% and 83%; Fibrotest 60% and 86%; Fibroscan 89% and 91%.
-
For HBV in ≥ F2: APRI low cut-off 80% and 65%, high cut-off 37% and 93%; FIB-4 low cut-off 68% and 73%; Fibrotest 66% and 80%; Fibroscan 71% and 84%.
-
For HBV in F4: Fibroscan 86% and 85%.
-
For NAFLD in ≥ F3: AST–ALT ratio 79% and 70%, BARD 84% and 61%, FIB-4 low cut-off 84% and 74%, high cut-off 38% and 97%, NAFLD fibrosis score low cut-off 80% and 66%, high cut-off 40% and 97%, hyaluronic acid 88% and 82%, Fibrotest high cut-off 40% and 96%, Fibroscan 82% and 84%.
Regarding the choice of tests, these should be based on disease aetiology, local resources and availability, cost-effectiveness and disease prevalence or pretest probability. Indirect NILTs can be applied at the point of care and can differentiate patients in low risk, high risk and indeterminate for significant fibrosis or cirrhosis. Particularly in NAFLD, they could be used to rule out patients with low risk of fibrosis, thanks to their high negative predictive value given the low prevalence of advance fibrosis in the general population of patients with steatosis/NAFLD. Direct serum tests or Fibroscan can be used either as second tier test following an indeterminate result with an indirect marker, or as a one-off test to inform further decisions on treatment or as a rule-in/rule-out test for liver biopsy. Patients who test ‘negative’ (true negative or false negative) with a non-invasive test should be subsequently retested in order to capture disease progression. The optimal time interval for such retesting is unknown; however, a period of 1–2 years would be safe and reasonable. There are no data on the monitoring of liver fibrosis using sequential testing with NILTs.
Cut-offs of NILTs for specific fibrosis stages were not always predetermined or sufficiently validated and this happened more often in direct serum biomarkers and Fibroscan; this represents a significant limitation in the interpretation of their results. Among all NILTs, APRI (low and high cut-offs) was the one where the established cut-offs were almost universally used in published studies. Although there are established cut-offs for Forns index, FIB-4, AST–ALT ratio and Fibrotest, these were not consistently used in all studies. NAFLD fibrosis score and BARD in NAFLD (10285,290,300,301,309,311,315,320,322 and seven studies,284,291,300,301,312,315,319 respectively) had consistent cut-offs used across all studies.
Fibroscan was the NILT assessed in most studies across diseases aetiologies (37 studies in HCV, 13 in HBV, eight in NAFLD and six in ALD). 28,29,75,76,86–88,91,95,98–100,102,105,106,110,116,119,130,131,141,147,153,155,159,161,164,170,172,173,194,199–201,211,223–225,230,236,241,242,246,247,250,251,263,264,272,288,296,298,308,323,324,273,277,278,281 However, there are no established and validated cut-offs for specific fibrosis stages across disease aetiologies. This represents a limitation in the use of Fibroscan; therefore, all reported sensitivities and specificities are probably overestimated. APRI was also widely assessed in HCV and HBV (47 and eight studies, respectively) but not in NAFLD or ALD. 24,31,72–74,79,81,84,85,89–91,94,97,98,100,103,107,109,121,123,126,127,130,131,134,137,140,143,144,146,150,152–154,156–158,163,164,168,182,185,187,189,194,195,209,210,218,220,223
All non-commercial direct serum non-invasive tests assessed [hyaluronic acid, YKL-40, PIIINP (amino-terminal propeptide of type III procollagen), type IV collagen] did not have predetermined cut-offs; moreover, different enzyme-linked immunosorbent assay (ELISA) kits were used across studies. Hepascore, which is a non-commercial test that consists, among other indices, of hyaluronic acid, has predetermined cut-offs, which were not consistently used in all included studies.
Of the commercial NILTs, Fibrotest was the most widely assessed (23 studies in HCV,23,25,31,81,85,99,111,113,127,129,146,172,177,179,186,189,206,208,211,216,220,223,366 six in HBV,231,247,249,255–257 four in NAFLD284,303,313,314 and one in ALD280); however, the predetermined cut-offs used were not always used in included studies. Fibroindex,24,107,186 Fibrometer82,127,223 and FibrospectII25,96,145,170,190,196 were only evaluated in HCV for the stages of interest in our models. ELF was evaluated in three studies in HCV27,98,158 and in a single study in NAFLD. 290
Failures of the index test (e.g. due to high BMI for Fibroscan or haemolysis for serum tests) are not incorporated in the reported sensitivities and specificities of the NILTs. Moreover, instances where the reference standard was not adequate for analysis (insufficient sampling) is also not captured in the analysis (applicability of index test and reference standard).
Investigations of heterogeneity revealed an influence of the level of transaminases on diagnostic accuracy in some tests; however, the direction of this effect was not consistent. There was no significant heterogeneity with regard to type of publication (abstract or full text) and histological scoring systems in most tests and diseases.
Of the imaging modalities, MR elastography was assessed in three studies, with summary sensitivity and specificity of 0.94 and 0.92, respectively. Although these are very promising results, further validation of the technique and determination of disease and stage specific cut-offs is needed. Moreover, this technique is not yet widely available.
The methodological quality of included studies as assessed by the QUADAS-2 tool was poor; only 6 of the 302 studies (1.6%) were of high methodological quality. 86,127,143,186,200,222 Most common areas of high risk of bias was the conduct of the index test (cut-offs were not predetermined) and of the reference standard (liver biopsy samples were not of adequate length or did not have sufficient number of portal tracts for reliable staging). Therefore, all reported results are likely biased.
As will become apparent in the cost-effectiveness analysis, NILTs with the most robust data were not the most cost-effective. As mentioned, there is the risk of overestimating sensitivity and specificity in tests with few available data.
Chapter 5 Cost-effectiveness analysis: hepatitis B
This chapter describes the assessment of cost-effectiveness of non-invasive tests of fibrosis and cirrhosis in patients with HBV. The population of interest were HBeAg-positive and HBeAg-negative patients with suspected fibrosis or cirrhosis who would normally have a liver biopsy in order to assess eligibility for antiviral treatment, i.e. patients with increased viral load and/or elevated transaminases.
Evaluation approach for hepatitis B
Twenty-five relevant NILTs were evaluated in the first stage of the analysis, which compared the NILTs with liver biopsy alone and a ‘treat all’ and ‘no treatment’ approach. The NILTs evaluated are listed in Table 18 and are grouped according to test categories: indirect serum makers, direct and patented serum markers and imaging modalities.
| Indirect | Direct and patented | Imaging |
|---|---|---|
| AAR | Fibrotest | ARFI |
| APGA | Hyaluronic acid | CEUS |
| Age–Platelet Index | Hepascore | DW-MRI |
| APRI (high cut-off) | MR elastography | |
| APRI (low cut-off) | CT | |
| FIB-4 (high cut-off) | Fibroscan | |
| FIB-4 (low cut-off) | US | |
| Forns index (high cut-off) | US SAPI | |
| Forns index (low cut-off) | US SAPI (high cut-off) | |
| GUCI | US SAPI (low cut-off) | |
| Hui index | ||
| PAPAS |
The second stage of the analysis compared a selection of tests using alternative sequential testing strategies. The criteria for selecting these tests and the assumptions used regarding combinations of tests and sequential testing strategies are detailed in Chapter 3.
Three of the tests evaluated in the second stage of the analysis used a combined diagnostic cut-off threshold for staging fibrosis; the test outcomes reported a number of indeterminate responses which are listed in Table 19. The percentage of indeterminate results was estimated using the meta-analysis data and is an aggregated value estimated from the studies for each combined test. We allowed for patients who had an indeterminate response to receive a retest with a commonly used imaging modality Fibroscan (TE). We did not choose an indirect test as the combined tests were from the indirect test category and a subsequent indirect test would not enhance the diagnostic accuracy. Of the direct tests and imaging modalities, we chose Fibroscan based on availability and current clinical practice. Overall, 56 testing strategies were compared in the second stage of the analysis.
| Tests with a combined cut-off | % of persons with inconclusive result |
|---|---|
| APRI | 41 |
| FIB-4 | 31 |
| Forns index | 36 |
Model structure and parameters
Decision tree structure
A decision-tree model was constructed to evaluate the cost-effectiveness of the NILTs, liver biopsy, and the ‘treat all’ and ‘no treatment’ strategies. As per the schematic diagram depicting the flow of data in Chapter 3 (see Figure 1), the decision tree was populated with test sensitivity, specificity and average disease prevalence from the meta-analysis (see Chapter 4 for details), long-term costs and health outcomes from a series of Markov models, individual test costs sourced from published literature and hospital finance departments, and a measure of adverse effects associated with liver biopsy.
As discussed in Chapter 3, there are two stages to the decision tree analysis: the first stage where all tests are compared singly and the second stage where combinations of tests are compared using four different testing strategies. Schematic illustrations and descriptions of the sequential testing pathways are provided in Chapter 3. To estimate a cost and QALY for each testing strategy the long-term costs (and test costs) and QALY estimates (including disutility from liver biopsy if applicable) associated with each potential test outcome (true positive, false positive, true negative, false negative) were multiplied by the probability of each NILT returning a true positive, false positive, true negative or false negative result to give a cost and QALY estimate for each testing option.
Markov model structure
A series of Markov models were constructed to estimate the long-term costs and health outcomes associated with a correct (true positive and true negative) and incorrect (false positive and false negative) diagnosis for a hypothetical cohort of 1000 patients with HBV and suspected liver fibrosis or cirrhosis. An additional two Markov models were constructed to estimate the costs and outcomes associated with the ‘treat all’ and ‘no treatment’ approaches. Separate models were constructed for the HBeAg-positive and HBeAg-negative patient cohorts, as their natural history differs and, therefore, starting age, transition probabilities and relative risks (RRs) from treatment differed for both groups. The structural assumptions underlying the state transition models applied to both groups of patients. The models were evaluated over a lifetime period with a cycle length of 1 year. All costs were considered from the perspective of the NHS and health outcomes were measured in terms of QALYs. Costs and utilities were discounted using a rate of 3.5%. A threshold value for incremental cost-effectiveness was assumed to be £20,000–30,000 per additional QALY gained, based on UK guidelines. 66
Figure 3 shows a schematic representation of the patient pathway, which is a modified version of previously published models of liver fibrosis and cirrhosis in patients with HBV. 371,372 Fibrosis and cirrhosis health states were defined in the Markov model according to METAVIR score: mild fibrosis (F0–1), moderate fibrosis (F2–3) and compensated cirrhosis (F4). There were five other potential health states in the Markov model: decompensated cirrhosis, hepatocellular cancer (HCC), liver transplant, post liver transplant and a health state representing ‘death’, which the cohort could enter from all other health states at any time. The cohort progressed through the model as per the arrows in the illustration; the circular arrows leading back into the same health state indicate that patients could remain within that health state for longer than one cycle (cycle length set at 1 year) except for the liver transplant state, where patients could only progress to a post liver transplant health state or to death. The liver transplant state comprised two events (1 month’s duration for liver transplant and 11 months’ duration for post-liver transplantation care). Patients could progress to the HCC health state from the moderate, compensated cirrhosis and decompensated cirrhosis health states. Patients could not regress to an earlier health state. We note that although some recent studies show that fibrosis and even cirrhosis can regress with antiviral therapy,373 we did not allow for this in our model.
FIGURE 3.
Illustration of Markov model.
We assumed that a METAVIR test score of ≥ F2 equated to a positive test outcome (true positive and false positive) and treatment with antiviral agents would commence at this stage. Conversely, a METAVIR test score of < F2 indicated a negative test outcome. We incorporated treatment with peginterferon alfa-2a for 1 year for 10% of those who tested negative to reflect that a proportion of patients would receive treatment for necroinflammation. 45 The remaining 90% would undergo a policy of watchful waiting without immediate treatment.
The watchful waiting policy in the model incorporated a retest with a NILT every 2 years; the retest NILT was the same as the previous test used for the initial assessment. To allow for modelling, we assumed that the retest had perfect sensitivity and specificity and correctly diagnosed all patients. If a patient was diagnosed as positive, immediate treatment with antiviral agents would commence. We tested the robustness of this assumption in a sensitivity analysis by varying the retest sensitivity and specificity to mirror that of three commonly used NILTs: an indirect serum marker, APRI; a patented serum marker, Fibrotest; and an imaging modality, Fibroscan.
The initial starting health state for the population cohort in the models depended on the test outcome being modelled (true positive, false positive, true negative and false negative). For example, given a false positive or true negative test result, the population cohort entered the model in a F0–1 health state (no fibrosis), whereas for a true positive or false negative test result, the population cohort had an initial starting health state of F2–4, where the cohort (with fibrosis or cirrhosis) was then distributed among the health states F2–3 and F4 based on the prevalence data from the systematic review (56% and 44%, respectively).
Input parameters
The average disease prevalence used in the model (54%) was estimated as the proportion of patients with a METAVIR score ≥ F2, calculated from the meta-analysis results reported in Chapter 4. We used the sensitivity and specificity estimates for each NILT and the average prevalence estimate to calculate the probability of each test returning a true positive, false positive, true negative and false negative result. The probability of each NILT reporting a particular diagnostic test outcome is reported in Appendix 7.
Cohort data
We identified cohort characteristics (age and sex ratio) for use in the model from published NICE guidance on treatments for HBV: adefovir dipivoxil and pegylated interferon. After reviewing the sources of evidence for this guidance,374,375 we identified a Health Technology Assessment report for both treatments published in 2006 by Shepherd et al. 371 This report included an economic analysis. Shepherd et al. 371 sourced cohort data from a published study undertaken by Fattovich et al. ,376 which reported a median age and a male-to-female ratio based on 10 published studies for HBeAg-positive chronic HBV377–387 and four published studies for HBeAg-negative chronic HBV. 388–391 We employed the same assumptions as those used by Shepherd et al. :
-
HBeAg-positive: starting age of 31 years and percentage of males set at 70%.
-
HBeAg-negative: starting age of 40 years and percentage of males set at 90%.
Natural history: baseline transition probabilities used in model
The rate of disease progression in the models is regulated by transition probabilities. The systematic review and economic evaluation by Shepherd et al. 392 included a systematic review of epidemiological data for HBV. 371 We also reviewed a submission to NICE by Bristol Myers Squibb (BMS) for a single technology appraisal of entecavir treatment for HBV. Upon review, the Southampton Health Technology Assessment Centre (SHTAC) epidemiological search strategy was considered to be appropriate for our analysis. The papers used by the authors were reviewed and the search was rerun to carry out an updated search for natural history data. The submission report by BMS sourced data from the same literature sources as the SHTAC study.
Our updated epidemiological search strategy was undertaken to search from the year 2004 to 2012 (as SHTAC search was undertaken to 2004). The search was carried out in the MEDLINE database using the Ovid platform, and the search strategy is outlined in Appendix 2 (searched on 11 May 2012). The updated search located 597 papers, whose titles were reviewed to determine if they were relevant; 24 were retrieved for abstract review. None was relevant for our study.
As none of the papers located from the updated literature search was relevant, we reviewed the studies which informed the transition probabilities in the model constructed by Shepherd et al. 372,376,392–401
Some of the papers referenced by Shepherd et al. 371 were older published papers. 393,394 Others were published for other aetiologies of liver disease395 and other models did not provide separate transition probabilities for the mild and moderate health states. 399 The study by Wong et al. 393 was identified as relevant as the authors had reported separate transition probabilities for HBeAg-positive and HBeAg-negative. As the paper by Wong et al. 393 was published in 1995, we conducted a search for recently published studies which had cited this paper. This search located a 2010 paper by Dakin et al. ,372 which assessed the cost-effectiveness of various drug treatments for HBV.
The 2010 paper by Dakin et al. 372 sourced transition probability data from natural history studies, economic evaluations or the placebo arms of meta-analyses or RCTs. 393,395,396,398–400,402–410 The authors used a similar set of studies to those identified by Shepherd et al. 371 and many of the transition probabilities elicited were either identical or similar. The progression from compensated cirrhosis to decompensated cirrhosis was the same for both studies, as Dakin et al. 372 used a synthesis of the same papers396,398,399 used by Shepherd et al. 371
Dakin et al. 372 sourced aetiology-specific data for the probability of undergoing a liver transplant while in a decompensated cirrhosis or HCC health state, using data from the UK transplant registry, specifically for chronic HBV. This was felt to be a more relevant estimate to use in our model than the value used by Shepherd et al. ,371 who had used a value based on data from a 1997 study by Bennett et al. 395 This study had sourced a value from a paper on HCV411 and papers published in 1993412 and 1996. 413
We used the transition probabilities estimated by Dakin et al. 372 as the main source of transition probability data for the cirrhotic and post-cirrhotic health states (HCC, liver transplant and post liver transplant).
However, none of the studies reviewed elicited separate transition probability data for the precirrhotic health states (mild and moderate fibrosis). In the absence of transition probability data for these health states, we used data from a study in patients with mild chronic HCV. 414 This paper was identified in our review of data for our analysis of HCV but was also referenced as a source of cost data in the paper by Dakin et al. 372 We adopted this approach with regard to early transition probabilities and this was confirmed as appropriate with clinical colleagues.
Wright et al. 414 were unable to locate separate estimates of probabilities of progression from mild to moderate fibrosis disease stages health state from existing studies as they found that most data were based on retrospective natural history studies which do not use liver biopsy to stage fibrosis and progression. As the disease progression may not be linear, it may not be realistic to assume a constant rate of progression from mild fibrosis to cirrhosis, and so they estimated transition probabilities for mild and moderate health states using data from a trial of treatments for mild HCV undertaken in a number of London hospitals,415 during which the liver fibrosis stage was determined by liver biopsy. Transition probabilities are listed in Table 20.
| Health state–health state | Transition probability | PSA distribution | Source |
|---|---|---|---|
| Mild–moderate | 0.025 | Dirichlet | Wright et al.414 |
| Moderate–cirrhosis (HBeAg-positive) | 0.037 | ||
| Moderate–cirrhosis (HBeAg-negative) | 0.09 | Dakin et al.372 | |
| Moderate–HCC | 0.048 | ||
| Excess mortality–moderate fibrosis | 0.0035 | ||
| Compensated cirrhosis–decompensated cirrhosis | 0.05 | ||
| Compensated cirrhosis–HCC | 0.024 | ||
| Excess mortality–compensated cirrhosis | 0.051 | ||
| Decompensated cirrhosis–HCC | 0.024 | ||
| Decompensated cirrhosis–liver transplant | 0.016 | ||
| Decompensated cirrhosis–death | 0.30 | ||
| HCC–liver transplant | 0.0155 | ||
| Excess mortality–HCC | 0.56 | ||
| Liver transplant–death | 0.21 | ||
| Post liver transplant–death | 0.057 |
Mortality data
An all-cause mortality rate was calculated using the Interim Life tables for England and Wales 2008–10. 416 The risk of death increased each year according to age and the rate was weighted to allow for sex mix. The all-cause mortality rate was added to an excess mortality value (identified from study by Dakin et al. 372) associated with the moderate fibrosis, compensated cirrhosis and HCC health states to provide a total risk of death per year. The all-cause mortality rate was not applied to the decompensated cirrhosis, liver transplant and post-liver-transplant health states; instead, a total mortality rate identified from the study by Dakin et al. 372 was applied. Mortality rates in the HBeAg-positive model ranged from 0.0007 to 0.337, and from 0.002 to 0.34 in the HBeAg-negative model. Excess mortality rates and total mortality rates employed within the models are listed in Table 20.
Antiviral treatment for hepatitis B: type and duration
The NICE website was reviewed to source national guidance on drug treatment for HBV.
We located guidelines for licensed drugs which included peginterferon alfa-2a (Pegasys®, Roche), entecavir (Baraclude®, BMS) and tenofovir disoproxil (Viread®, Gilead). Entecavir and tenofovir have marketing authorisation within the UK for the treatment of chronic HBV infection in adults with compensated liver disease and evidence of active viral replication, persistently elevated serum ALT levels and histological evidence of active inflammation and/or fibrosis. Peginterferon alfa-2a has UK marketing authorisation for the treatment of HBeAg-positive and HBeAg-negative chronic HBV infection in adults with compensated liver disease and evidence of viral replication, increased ALT and histologically verified liver inflammation and/or fibrosis. Interferon antiviral agents are contraindicated in chronic hepatitis patients with decompensated cirrhosis. 375,417,418
The following treatment assumptions were employed in the model: only patients in the moderate fibrosis (F2–3), compensated cirrhosis (F4) and decompensated cirrhosis health states received treatment with antiviral agents; patients in the HCC, liver transplant and post liver transplant health states received usual standard of care.
Treatment with either entecavir or tenofovir (lifetime duration) was administered if patients tested positive (true positive or false positive); half of the eligible patients received entecavir and the other half received tenofovir.
Ten per cent of the patients who tested negative (true negative or false negative) received peginterferon alfa-2a for 1 year only, and if treatment was unsuccessful (we assumed that 30% of treated true negative patients would successfully respond to treatment and would no longer progress to any further health states except the death health state due to all-cause mortality), they would receive subsequent treatment with either entecavir or tenofovir for lifetime duration if diagnosed as positive (≥ F2) at retest. The remaining 90% of true negative and false negative patients would undergo ‘watchful waiting’, where they would then receive treatment with either entecavir or tenofovir for lifetime duration (if diagnosed as positive during a retest).
The dosage of treatment was based on national recommendations sourced from the British National Formulary (BNF) 64419 (see Table 24).
Treatment effectiveness
A meta-analysis by Woo et al. 420 which evaluated the relative efficacies of the first 12 months of treatment for chronic HBV was identified from a general search carried out using Google Scholar (http://scholar.google.com) and the search terms ‘treatment effectiveness’, and ‘tenofovir and entecavir’ (search date 12 May 2012). The paper evaluated a number of drugs – lamivudine, pegylated interferon, adefovir dipivoxil, entecavir, telbivudine and tenofovir – for use as monotherapy or in combination therapy in treatment-naive individuals.
Woo et al. 420 conducted a systematic literature review to locate studies of published RCTs of drugs used to treat chronic HBV as either monotherapies or combination therapies. They included studies that examined the impact of treatment in both HBeAg-positive and HBeAg-negative patients. The studies included in the review were required have examined the use of the drug using randomised, phase 3, controlled trials comparing new drug treatments with either a placebo or a licensed drug. The methodological quality of each paper was assessed independently by two reviewers using the Cochrane risk of bias tool.
The data were analysed using a Bayesian mixed-treatment comparison (MTC) analysis which allowed the authors to combine direct and indirect comparisons of the treatments. Our model used the treatment efficacy elicited from the Bayesian MTC for pegylated interferon, entecavir and tenofovir. The study did not provide separate efficacy data for peginterferon for HBeAg-negative chronic HBV, and we assumed the same RR as for HBeAg-positive. Treatment effectiveness represented by RRs and their associated CIs are displayed in Table 21.
| Drug | RR | 95% CI |
|---|---|---|
| HBeAg (+ve) | ||
| Entecavir | 0.56 | 0.12 to 0.94 |
| Tenofovir | 0.53 | 0.06 to 0.95 |
| Peginterferon alfa-2a | 0.52 | 0.06 to 0.95 |
| HBeAg (–ve) | ||
| Entecavir | 0.64 | 0.01 to 1.00 |
| Tenofovir | 0.65 | 0.01 to 1.00 |
| Peginterferon alfa-2a | 0.52 | 0.06 to 0.95 |
We assumed that patients who tested false positive and who were in a mild state would receive the same treatment benefit from antiviral treatment as those in a moderate or cirrhotic health state. We also assumed that patients who test false negative and receive peginterferon alfa-2a for 1 year would not receive treatment benefit but would incur the costs and disutility associated with peginterferon alfa-2a treatment.
Cost data
To populate our model, we undertook a search for other relevant cost-effectiveness literature that would provide data on the costs associated with treating the different levels of fibrosis and cirrhosis in patients with HBV. To do this, we employed the search strategy devised by Shepherd et al. 371 and undertook an updated search using the MEDLINE database (Ovid platform, search date 10 May 2012, searched 2004–12). The search strategy is listed in Appendix 2. Additional search terms were added to locate papers related to other relevant treatment (entecavir, tenofovir disoproxil, and their brand names, baraclude and viread). Nine hundred and seventy-one papers were located and their titles were reviewed to determine if they were eligible. Twenty-three were retrieved for abstract review. Of these, 17 were excluded and six were retrieved for full review (Table 22). Chapter 3 contains further details of the literature review inclusion criteria used when reviewing and assessing the applicability of cost data literature.
| Authors | Title | Journal | Source cost data used in study |
|---|---|---|---|
| Brown et al.421 2004 | Hepatitis B management costs in France, Italy, Spain, and the United Kingdom | Journal of Clinical Gastroenterology | Questionnaire used to collect data from specialist clinicians in UK, Spain, Italy and France |
| Dakin et al.372 2010 | Cost–utility analysis of tenofovir disoproxil fumarate in the treatment of chronic hepatitis B | Value in Health | Published literature: Wright et al. 2006414 |
| Jones et al.422 2010 | Tenofovir disoproxil fumarate for the treatment of chronic hepatitis B | SHTAC | Published literature: Wright et al. 2006414 |
| Jones et al.423 2009 | Adefovir dipivoxil and pegylated interferon alfa for the treatment of chronic hepatitis B: an updated systematic review and economic evaluation | Health Technology Assessment | Published literature: Wright et al. 2006,414 Longworth 2003424 |
| Takeda et al.425 2007 | A systematic review and economic evaluation of adefovir dipivoxil and pegylated interferon-alfa-2a for the treatment of chronic hepatitis B | Journal of Viral Hepatitis | Expert opinion and published literature: Wong 1995,393 Longworth 2003424 |
| Veenstra et al.426 2007 | Cost-effectiveness of peginterferon alfa-2a compared with lamivudine treatment in patients with HBe-antigen-positive chronic hepatitis B in the United Kingdom | European Journal of Gastroenterology and Hepatology | Expert opinion and published literature: Wright et al. 2006,414 Longworth 2003,424 Bennett et al. 1997395 |
Of the six papers reviewed, four papers included cost data from a study on mild HCV by Wright et al. 414 This study collected resource use and cost data alongside a RCT for mild HCV. 414,415 Detailed cost data were collected from three centres based in London, Newcastle and Southampton. Resource use information collected covered inpatient and outpatient care, investigations, procedures, drug use and other services including psychiatric services.
One of the studies, by Veenstra et al. ,426 sourced additional costs from a study by Bennett et al. ;395 however, this study collected costs for the health states using a US population which, due to the difference in the health-care systems, would not be transferable to a UK population.
The study by Brown et al. 421 collected data on resource use from specialists based in four countries including the UK. The authors used the data collected to identify resource use and associated costs for the management of fibrosis and cirrhosis.
The study by Takeda et al. 425 also used the 1995 Wong paper393 as a source; as this paper is an older published paper, we felt there would be more up-to-date costs available.
Three of the studies used data from the Cost-Effectiveness of Liver Transplantation (CELT) study424 to elicit a cost for the liver transplant health states. The CELT study collected costs on adult patients listed for an isolated liver transplant (aged 16 years and over) between December 1995 and December 1996. The costs were collected and split into phases, which were determined according to when the resource use took place and according to disease aetiology (HBV, HCV, alcoholic cirrhosis). Data were split into an assessment phase, candidacy phase, transplant phase and post-transplant phases. The assessment phase started at the date of admission for assessment of suitability for liver transplantation to the date of listing for transplantation (for patients who were not listed for transplantation, the discharge date was used as the end date). The candidacy phase started at the date of listing to the date the patient was admitted for the transplant operation. The transplant phase started at the date of admission for the transplant operation to the date of discharge following the operation and the post-transplant phase started at the date of discharge following the operation onwards for a period of 2 years. Resource use data on blood products used, number of dietitian sessions, drugs used, inpatient stay, nutritional support received, outpatient visits, physiotherapy sessions, tests, length of transplant operation and key treatments and investigations were collected.
Based on the review, we concluded that the data on costs for treating HCV from the study by Wright et al. 414 for the mild, moderate and cirrhotic health states would be comparable with the costs for treating patients with HBV in these health states, as the resource use identified and collected (inpatient, outpatient care, procedures) should be similar. The health state costs sourced from this study did not contain the cost of antiviral treatment; therefore, they were suitable to use and we added the cost of treatment separately in the model.
From the review, we decided to source information for the decompensated cirrhosis, HCC, liver transplant and post-liver-transplant health states from the CELT study. 424 Using the raw data collected in this study, we calculated the cost for all patients with HBV who had received a transplant (sample size 24). The average length of admission for the liver transplant operation was 28 days. We approximated this to 1 month and calculated the yearly cost of a liver transplant as 11 months of post-transplant care (estimated from the average monthly cost in the first year following transplantation) plus the month the transplant operation took place. We also calculated a cost for the post-transplant health state, estimated as the average monthly cost of the second year of post-transplant care (sample size 24).
To calculate a cost for the decompensated cirrhosis and HCC health states, we used the average cost for patients with HBV awaiting liver transplant (patients in the ‘assessment’ and ‘candidacy’ disease stages were used; sample size 25).
Costs were inflated to 2012 levels using NHS inflation indices. 67 Health state costs did not include the costs associated with antiviral treatment; these were included separately. Costs are shown in Table 23.
| Health state | Cost per year, £ | Standard error | PSA distribution | Source |
|---|---|---|---|---|
| Mild fibrosis | 185 | 36.39 | Gamma | Wright et al. 2006414 |
| Moderate fibrosis | 986 | 101.69 | Gamma | |
| Compensated cirrhosis | 1521 | 309.05 | Gamma | |
| Decompensated cirrhosis | 36,194 | 9967.19 | Gamma | Longworth et al.424 |
| HCC | 36,194 | 9967.19 | Gamma | |
| Liver transplant | 64,122 | 5584.70 | Gamma | |
| Post liver transplant | 16,321 | 7932.51 | Gamma |
As we employed two different sources of data to populate our model, there is a sizable difference between the cost for the compensated and decompensated cirrhosis health states. We conducted a sensitivity analysis where we populated our model with the cost for the decompensated cirrhosis health state (£9121) and HCC health state (£8127) from the study by Wright et al. 414 The main component of the costs for these two health states in the Wright et al. 414 model was inpatient-days. We inflated the costs to 2012 prices using NHS inflation indices. 67
Test costs
Non-invasive liver tests using imaging modalities were sourced from published Department of Health reference costs. 427 Costs of direct and indirect serum markers were obtained from communication with finance departments based at the Royal Free Hospital. Costs for patented serum markers were sourced directly from manufacturers and via communication with finance departments based at the Royal Free Hospital (see Appendix 5, Table 68).
The two most commonly performed liver biopsy tests are percutaneous and transjugular liver biopsy. We assumed that, for our purposes, patients received a percutaneous liver biopsy, as this tends to be associated with less severe adverse effects. We estimated that a diagnostic liver biopsy would cost £956.61. 428 Where required, costs were inflated to 2012 prices using NHS inflation indices. 67 Tests costs and sources are listed in Appendix 9. All NILT tests costs are based on incremental costs and exclude the capital costs of the equipment.
Medication costs
Treatment costs and recommended dosages were sourced from the BNF 64419 and are listed in Table 24.
Utility data
An initial literature search for existing published utility data for HBV was undertaken using the MEDLINE database (Ovid platform, searched 11 May 2012, coverage 2004 to 2012). We supplemented this search using both the cost-effectiness analysis (CEA) Registry (searched 11 May 2012, all dates) and EuroQol website (searched 11 May 2012, all dates).
We searched the MEDLINE database using the search strategy for quality of life devised by Shepherd et al. 371 We updated this search to include papers from 2004 onwards. The full search strategy is outlined in Appendix 2. This search returned 121 papers.
We searched the EuroQol using the general search term ‘hepatitis B’. This search returned 16 papers. We also searched the CEA Registry using the search term ‘hepatitis B’, which returned 39 studies.
The title and abstract of each paper was reviewed and full papers were retrieved for review if they met our inclusion criteria. Chapter 3 outlines the inclusion criteria that applied when reviewing studies for quality of life data. After excluding three duplicate papers, eight were retrieved for full review. 372,414,425,429–432
Four of the studies retrieved372,423,425,429 used the same study by Levy et al. 430 as a source for utility values.
Levy et al. 430 employed the standard gamble technique to collect health-related utility data for six HBV-related health states (from both infected and uninfected respondents). Hypothetical health states were developed using expert opinion and the Liver Disease Quality of Life Instrument. Data were elicited from respondents from the USA, Canada, the UK, Spain, Hong Kong and China. The study analysed 1134 respondents, of whom 100 were from the uninfected population and 93 from the infected population were from the UK.
One study used clinical judgement to elicit health-related quality of life (HRQoL) values. 431 The authors noted that the weights were arbitrary and better sources could be used.
A 2008 study by McLernon et al. 432 conducted a systematic review of published literature. 414,433–436 They obtained values for the compensated cirrhosis, decompensated cirrhosis and HCC health states using these data; however, the studies had mainly been carried out in HCV and the search conducted looked for quality of life data for all aetiologies, not specifically for HBV.
The search also identified the 2006 study on treatment in mild HCV by Wright et al. 414 As none of the other studies reviewed identified separate utility values for mild and moderate health states, we adopted the same approach to sourcing data as that used for costs. We employed the same utility values used by Wright et al. 414 for the HCV mild, moderate and compensated cirrhosis health states. As both HBV and HCV lead to cirrhosis and related complications, it is assumed that cirrhosis would impact on the quality of life similarly for patients with HBV and HCV.
We decided to source utility values for the decompensated cirrhosis, decompensated cirrhosis, HCC, liver transplant and post liver transplant health states from the CELT study for HBV patients. 424 We used this source rather than one of the studies reviewed, as the only study reviewed which collected data directly used a standard gamble technique430 rather than a generic preference-based measure such as the EQ-5D. As we had data available for patients with HBV (sourced from CELT study), it was felt this would be a more accurate source of data to use rather than the data elicited from the study by Levy et al. 430 or the systematic review by McLernon et al. ,432 which were applicable to all liver disease aetiologies.
Additionally, we concluded that both the Wright et al. 414 and the CELT study424 would be suitable sources of data as they had both used the EQ-5D preference measure to elicit utility values within a UK population (see Chapter 3 for inclusion criteria for quality of life data).
During the mild HCV RCT,414 questionnaires were self-administered at baseline and treatment weeks 12, 24 and 48, and at follow-up weeks 12, 24 and 48. For the moderate and cirrhotic health states, 302 patients were sent an EQ-5D (of whom 60% of those with diagnosed as being in a moderate health state responded, and 54% of those diagnosed as having cirrhosis responded).
The CELT study424 collected HRQoL data using the EQ-5D questionnaire. We analysed the data collected for HBV patients at the time patients were placed on the waiting list for a transplant, and at 3 months, 6 months, 12 months and 24 months post transplant.
It was assumed that utility values for the decompensated cirrhosis and HCC health states would be the same (sample size 25). This was assumed to be equivalent to the average utility value at ‘listing’ stage.
A utility value for the liver transplant health state was estimated using an area under the curve approach and the average utility values collected at 3, 6 and 12 months post transplant (sample size 24). A utility value for the post liver transplant disease stage was estimated from the average utility for HBV patients collected at 24 months post transplant (sample size 24). A PSA was carried out using a utility decrement approach. A beta distribution is often employed for utility values; however, this may not be appropriate for states close to death, where values of less than one are possible. For the HBV model, we performed a simple transformation of the data (D = 1 – U, where D is the utility decrement and U is the utility value). The decrement was constrained on the interval ‘0 to positive infinity’ and a gamma distribution was then applied. 437 Table 25 lists the utility data used in the model.
| Health state | Utility value | SE | PSA distribution | Source |
|---|---|---|---|---|
| Mild fibrosis | 0.77 | 0.035 | Gamma | Wright et al.414 |
| Moderate fibrosis | 0.66 | 0.018 | ||
| Compensated cirrhosis | 0.55 | 0.032 | ||
| Decompensated cirrhosis | 0.57 | 0.076 | Longworth et al.424 | |
| HCC | 0.57 | 0.076 | ||
| Liver transplant | 0.73 | 0.016 | ||
| Post liver transplant | 0.78 | 0.064 | ||
| Mild: during treatment | 0.65 | 0.035 | ||
| Moderate: during treatment | 0.55 | 0.018 | ||
| Compensated cirrhosis: during treatment | 0.44 | 0.040 | ||
| Death | 0 | 0 | Assumption |
As we sourced utility value data from two different studies, the utility values used in the study for the decompensated cirrhosis and HCC health states were slightly higher than those used for the compensated cirrhosis health state. We undertook a sensitivity analysis where we set the utility values for the decompensated cirrhosis and HCC health states to those used in the HCV model (see Chapter 6) – as these were lower values than those used in the HBV model – to test if this had any effect on the robustness of the results.
Adverse effects associated with antiviral treatment
As peginterferon alfa-2a has associated side effects (such as influenza-like symptoms, depression and anxiety), we allowed for the disutility associated with antiviral treatment to be reflected in the model. We modelled a disutility decrement that was applicable during treatment, using data identified from the Wright et al. 414 study. This study reported HRQoL data using the EQ-5D instrument for 144 patients. They used the data from weeks 12 and 24 from the baseline date to estimate utility decrement values of 0.11. The decrement was applied to all patients in the mild and moderate fibrosis and compensated cirrhosis health states as a multiplicative disutility for the duration of treatment with peginterferon alfa-2a. It was conservatively assumed that any side effects associated with treatment with entecavir or tenofovir would have no impact on quality of life.
Disutility from non-invasive liver treatment and liver biopsy
We have assumed that any adverse events associated with the NILTs would not have a significant impact on health-related utility. However, as liver biopsy is associated with morbidity and mortality risks and patient discomfort, we incorporated a utility decrement to represent expected adverse events. In the absence of identified data, this was modelled by applying a utility decrement of 0.2 based on data employed in a previous study,428 where the authors had conducted a literature review for data on liver biopsy associated adverse events and mortality. We undertook a number of sensitivity analyses to test the robustness of the results to changes in the utility decrement.
Estimated long-term costs and quality-adjusted life-years
The estimated long-term costs and QALYs for HBeAg-positive and HBeAg-negative chronic HBV (output from Markov models) are shown in Table 26.
| Diagnostic test outcome | Cost (£ 2012 per person) | QALY (per person) |
|---|---|---|
| HBeAg-positive | ||
| TP | 105,126 | 7.99 |
| TN | 39,201 | 15.61 |
| FP | 97,859 | 17.06 |
| FN | 99,997 | 7.46 |
| Treat all persons | 101,794 | 12.15 |
| Treat no one | 37,966 | 9.64 |
| HBeAg-negative | ||
| TP | 98,145 | 6.65 |
| TN | 32,772 | 13.12 |
| FP | 90,877 | 15.31 |
| FN | 92,571 | 6.18 |
| Treat all persons | 94,815 | 10.62 |
| Treat no one | 37,518 | 8.82 |
Analysis
As discussed previously in Chapter 3, a PSA was conducted, and an incremental analysis was carried out to estimate the most cost-effective testing option. A CEAC and CEAF were constructed to summarise the uncertainty around the results. Several one-way sensitivity analyses were also undertaken.
Treatment benefit
Our base-case analysis assumed that patients who are in a mild health state (F0–1) receive the same treatment benefit (same effectiveness) as patients in a moderate or cirrhotic health state, despite being incorrectly diagnosed and treated. 373 We tested the robustness of this assumption in a sensitivity analysis by setting the treatment benefit for patients who incorrectly receive treatment while in a mild health state to zero.
Robust test accuracy data
In the base-case analysis, all tests were included despite there being limited data available for some tests. A sensitivity analysis including only tests where the standard bivariate random-effects model used for the meta-analysis63 converged was conducted. We conducted an analysis with the five tests where the bivariate model had converged – APRI high cut-off, Fibroscan, Fibrotest, FIB-4 low cut-off and APRI low cut-off – and compared with liver biopsy, ‘treat all’ and ‘treat no one’ strategies.
Utility value amendment
We carried out an analysis where we amended the utility values within the model for the decompensated cirrhosis, HCC, liver transplant and post liver transplant. The sensitivity analysis used the lower values from the HCV model (see Chapter 6 for values).
Change in liver biopsy decrement
We also carried out analyses using different utility decrement values to measure the adverse effects from liver biopsy. The base-case analysis set the utility decrement value to 0.2 based on a previous analysis which had conducted a literature search for data relating to adverse events and mortality associated with liver biopsy. 428 In the sensitivity analysis we set the utility decrement at 0 and 0.3 to test the impact on the robustness of the results.
Change to health state costs
We carried out an analysis where we changed the costs for the decompensated cirrhosis and HCC health states to the costs used in the Wright et al. 414 study.
Average disease prevalence
Prevalence within the model is based on studies that may have been carried out largely in tertiary care centres, and the prevalence of liver fibrosis in this population may be an overestimate. To test the impact of this we undertook a sensitivity analyses using the minimum prevalence (disease prevalence modelled as ≥ F2 at 27%), maximum prevalence (92%) and the 25th and 75th quartile values (43% and 65%, respectively), estimated from the meta-analysis of the systematic review data. In this sensitivity analysis, it is assumed that the sensitivity and specificity of the tests would be the same in populations with high and low prevalence as observed in the studies included in the review.
Sensitivity and specificity of retest
Our base-case analysis assumes that the retest (from the meta-analysis of the systematic review data in the watchful waiting strategy for patients with a negative test result) has perfect sensitivity and specificity. This is likely to overestimate the accuracy of the retest procedure. We tested the impact of this assumption by applying the sensitivity and specificity of three commonly used tests: APRI low cut-off (estimated sensitivity of 80% and specificity of 65%), Fibrotest (estimated sensitivity of 66% and specificity of 80%) and Fibroscan (estimated sensitivity of 71% and specificity of 84%).
Choice of tests for second stage of analysis
For the second stage of the analysis, the two most cost-effective tests when assessed within each specific test category singly (with and without a defined threshold) were used in the analysis of sequential testing. To test if changing the method used to choose tests for the second stage of the analysis had an effect on the overall result, we carried out an analysis where the most effective NILT from within each NILT category and the least costly NILT from each category were included in the analysis of sequential testing.
Change to hepatitis B e antigen-negative model (age and sex ratio)
We also undertook a sensitivity analysis, where we set the age-and-sex-mix data in the HBeAg-negative model equivalent to the age and sex mix in the HBeAg-positive model (base age set to 31 years and the proportion of cohort who were male set at 70%) to check if the results from both models would be similar. The only remaining differences between the HBeAg-positive and HBeAg-negative models related to the different efficacy of treatment and the increased probability of moving to a cirrhotic state from a moderate health state.
Reduction in length of time of successful response rate to peginterferon alfa-2a treatment
Our base-case analysis for true-negative patients assumes that 30% of those who receive treatment with peginterferon alfa-2a have a successful response and no longer progress to further health states in the model retaining a risk of all-cause mortality only. We tested this assumption in the model by assuming that the successful response lasts for 15 years only.
Change to non-invasive liver test costs
We carried out a sensitivity analysis where we changed the cost of the NILTs [we set the watchful waiting retest cost and all NILT costs within the model to the same cost; for comparison, we assumed an indirect serum marker test cost (we chose a commonly used test, APRI, as our comparator)]. By changing the cost of a NILT, we aimed to determine if changing the test cost (marginal cost) had an impact on the robustness of the results.
Results
Hepatitis B e antigen-positive chronic hepatitis B
At a cost-effectiveness threshold of £20,000, the cost-effective strategy is to employ a sequential testing strategy where a direct serum marker, hyaluronic acid, is combined with an imaging modality, MR elastography, with an ICER of £19,612. With this testing strategy, positive test diagnoses are confirmed with a second NILT and if the results disagree, a liver biopsy is administered to confirm the result; negative test responses undergo a process of watchful waiting.
Using a higher cost-effectiveness threshold value of £30,000, an imaging modality, MR elastography, used singly, becomes the most cost-effective option, with an ICER of £28,585. When we assessed all tests singly in the first stage of the analysis, the most cost-effective test given a threshold value of £20,000 was GUCI, with an ICER of £19,716.
As the CEAF is more representative of the uncertainty around the optimal testing option, we present the CEAF (Figure 4) in the main analysis and the CEAC in Appendix 6.
FIGURE 4.
Cost-effectiveness acceptability frontier for HBeAg-positive second stage of the analysis. HA, hyaluronic acid; MRE, MR elastography.

The CEAF shows that the probability of hyaluronic acid combined with MR elastography (strategy 2) being the optimal testing option, given a cost-effectiveness threshold value of £20,000, is 4%. The CEAF also shows that MR elastography, used singly, has a 13% probability of being cost-effective for cost-effectiveness thresholds starting at £28,585, which reduces to a 3.5% probability when the cost-effectiveness threshold increases to £43,946. For cost-effectiveness threshold values greater than £43,946, the ‘treat all’ strategy has a 35% probability of being cost-effective.
What is noticeable about the CEAF is that two of the strategies (testing with MR elastography alone or a combination of hyaluronic acid and MR elastography) have a very low probability of being the optimal testing option, compared with a testing strategy of treating everyone.
For reasons of clarity, the CEAC presented in Appendix 10 displays only those testing options which had a ≥ 4% probability of being cost-effective. The CEAC also displays some tests that are not picked up by the CEAF, including the Forns (high cut-off) serum marker. The reasons for this include a skew in cost data and also the fact that those tests which have the highest probability of being cost-effective may not be the optimal testing option.
Liver biopsy was a comparator for both stages of the analysis; however, this testing option is dominated by other less costly but more effective options. Table 27 presents incremental results for the first stage of the analysis and Table 28 incremental results for the second stage where a number of combined tests are compared using a number of different sequential testing strategies (see Chapter 3 for details of testing strategies).
| Test strategy | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Treat no one | 37,831 | 9.64 | – | – | – |
| FIB-4 (high cut-off) | 73,028 | 11.33 | – | – | Extendedly dominated |
| Forns index (high cut-off) | 73,115 | 11.35 | – | – | Extendedly dominated |
| APGA | 73,373 | 11.36 | – | – | Extendedly dominated |
| Liver biopsy | 75,957 | 11.41 | – | – | Dominated |
| APRI (high cut-off) | 75,139 | 11.45 | – | – | Dominated |
| US | 77,657 | 11.48 | – | – | Dominated |
| Hui index | 76,047 | 11.50 | – | – | Dominated |
| US SAPI (high cut-off) | 75,610 | 11.51 | – | – | Extendedly dominated |
| GUCI | 74,921 | 11.52 | 37,090 | 1.88 | 19,716 |
| Fibroscan | 79,004 | 11.61 | – | – | Dominated |
| Forns index (low cut-off) | 80,008 | 11.61 | – | – | Dominated |
| Fibrotest | 79,519 | 11.62 | – | – | Dominated |
| MR elastography | 77,657 | 11.64 | 2,737 | 0.12 | 23,468 |
| US SAPI | 80,442 | 11.66 | Extendedly dominated | ||
| PAPAS | 80,223 | 11.65 | – | – | Dominated |
| Hyaluronic acid | 79,084 | 11.66 | – | – | Extendedly dominated |
| DW-MRI | 80,751 | 11.67 | – | – | Extendedly dominated |
| FIB-4 (low cut-off) | 81,347 | 11.66 | – | – | Extendedly dominated |
| Hepascore | 81,399 | 11.69 | – | – | Extendedly dominated |
| ARFI | 83,487 | 11.71 | – | – | Dominated |
| AAR | 84,951 | 11.72 | – | – | Dominated |
| CEUS | 82,377 | 11.74 | – | – | Extendedly dominated |
| CT | 84,097 | 11.73 | – | – | Dominated |
| Age–Platelet Index | 84,289 | 11.73 | – | – | Dominated |
| APRI (low cut-off) | 83,770 | 11.75 | – | – | Extendedly dominated |
| US SAPI (low cut-off) | 91,287 | 11.95 | – | – | Extendedly dominated |
| Treat all | 101,484 | 12.18 | 23,827 | 0.54 | 44,256 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Treat no one | 37,831 | 9.64 | – | – | – |
| (S1) Forns index (high cut-off) | 73,960 | 11.16 | – | – | Dominated |
| Forns index (high cut-off) | 73,084 | 11.35 | – | – | Dominated |
| (S2) Forns index (high cut-off) and MR elastography | 73,062 | 11.35 | – | – | Dominated |
| (S2) Forns index (high cut-off) and Fibrotest | 73,037 | 11.35 | – | – | Dominated |
| (S2) Forns index (high cut-off) and hyaluronic acid | 73,011 | 11.35 | – | – | Extendedly dominated |
| (S1) US SAPI (high cut-off) | 76,238 | 11.38 | – | – | Dominated |
| (S1) GUCI | 75,539 | 11.40 | – | – | Dominated |
| Liver biopsy | 75,957 | 11.41 | – | – | Dominated |
| (S3) Forns index (high cut-off) and Fibrotest | 75,965 | 11.42 | – | – | Dominated |
| (S3) Forns index (high cut-off) and hyaluronic acid | 75,939 | 11.43 | – | – | Dominated |
| (S3) Forns index (high cut-off) and MR elastography | 75,927 | 11.43 | – | – | Dominated |
| FIB-4 (combined cut-off) and Fibroscan | 74,911 | 11.43 | – | – | Dominated |
| (S4) Forns index (high cut-off) and Fibrotest | 74,953 | 11.45 | – | – | Dominated |
| (S3) GUCI and US SAPI (high cut-off) | 75,805 | 11.46 | – | – | Dominated |
| (S3) Fibrotest and US SAPI (high cut-off) | 76,025 | 11.46 | – | – | Dominated |
| (S3) GUCI and Fibrotest | 75,904 | 11.46 | – | – | Dominated |
| Forns index (combined cut-off) and Fibroscan | 75,253 | 11.47 | – | – | Dominated |
| (S2) Fibrotest and US SAPI (high cut-off) | 74,851 | 11.47 | – | – | Dominated |
| (S3) Hyaluronic acid and US SAPI (high cut-off) | 75,908 | 11.47 | – | – | Dominated |
| (S3) GUCI and hyaluronic acid | 75,803 | 11.48 | – | – | Dominated |
| (S2) GUCI and US SAPI (high cut-off) | 74,431 | 11.48 | – | – | Dominated |
| (S4) Forns index (high cut-off) and hyaluronic acid | 75,342 | 11.48 | – | – | Dominated |
| (S3) GUCI and MR elastography | 75,778 | 11.48 | – | – | Dominated |
| (S2) GUCI and Fibrotest | 74,517 | 11.49 | – | – | Dominated |
| (S3) Fibrotest and MR elastography | 76,198 | 11.49 | – | – | Dominated |
| (S4) Forns index (high cut-off) and MR elastography | 75,937 | 11.50 | – | – | Dominated |
| (S2) Fibrotest and MR elastography | 75,023 | 11.50 | – | – | Dominated |
| (S2) GUCI and hyaluronic acid | 74,416 | 11.50 | – | – | Dominated |
| (S4) Fibrotest and US SAPI (high cut-off) | 75,667 | 11.50 | – | – | Dominated |
| (S2) GUCI and MR elastography | 74,404 | 11.50 | – | – | Extendedly dominated |
| (S3) Hyaluronic acid and MR elastography | 76,028 | 11.51 | – | – | Dominated |
| US SAPI (high cut-off) | 75,618 | 11.51 | – | – | Dominated |
| (S4) GUCI and US SAPI (high cut-off) | 75,450 | 11.51 | – | – | Dominated |
| (S4) GUCI and Fibrotest | 75,400 | 11.52 | – | – | Dominated |
| (S1) Fibrotest | 80,207 | 11.52 | – | – | Dominated |
| (S2) Hyaluronic acid and US SAPI (high cut-off) | 75,265 | 11.52 | – | – | Dominated |
| GUCI | 74,942 | 11.52 | – | – | Extendedly dominated |
| (S4) Hyaluronic acid and US SAPI (high cut-off) | 75,751 | 11.53 | – | – | Dominated |
| (S4) GUCI and hyaluronic acid | 75,440 | 11.54 | – | – | Dominated |
| (S1) MR elastography | 78,061 | 11.55 | – | – | Dominated |
| (S4) Fibrotest and MR elastography | 76,226 | 11.55 | – | – | Dominated |
| (S4) GUCI and MR elastography | 75,814 | 11.56 | – | – | Extendedly dominated |
| (S2) Hyaluronic acid and MR elastography | 75,386 | 11.56 | 37,555 | 1.9 | 19,612 |
| (S4) Hyaluronic acid and MR elastography | 76,069 | 11.57 | – | – | Extendedly dominated |
| (S1) Hyaluronic acid | 79,752 | 11.58 | – | – | Dominated |
| APRI (combined cut-off) and Fibroscan | 77,596 | 11.59 | – | – | Extendedly dominated |
| Fibrotest | 79,495 | 11.62 | – | – | Dominated |
| MR elastography | 77,627 | 11.64 | 2241 | 0.1 | 28,585 |
| Hyaluronic acid | 79,148 | 11.67 | – | – | Extendedly dominated |
| Treat all | 101,484 | 12.18 | 23,857 | 0.5 | 43,946 |
A scatterplot illustrating the position of each testing strategy on the CEAC compared with the testing strategy ‘treat no one’ can be found in Appendix 12.
Hepatits B e antigen-negative chronic hepatitis B
The base-case analysis result indicates that given a cost-effectiveness threshold of £30,000, the cost-effective strategy is to adopt a strategy of ‘treat all’ with an ICER of £28,137. All other testing strategies are dominated by the ‘treat all’ strategy. As all other tests were dominated, the ICER presented represents the ‘treat all’ strategy versus a ‘treat no one’ strategy. Given a UK threshold range of £20,000, the ‘treat no one’ strategy was the most cost-effective option to adopt.
The CEAF (Figure 5) for the HBeAg-negative model shows that the probability of ‘treat all’ being the optimal testing option (highest expected net benefit), given a cost-effectiveness threshold value of £30,000, is 38%. The CEAF also shows that ‘treat all’ has a 35% probability of being cost-effective for cost-effectiveness thresholds starting at £28,137.
FIGURE 5.
Cost-effectiveness acceptability frontier for HBeAg-negative: second stage of the analysis.

Appendix 10 displays the CEAC for the overall base-case analysis for HBeAg-negative model. For reasons of clarity, only those strategies that have on average a ≥ 4% probability of being the most cost-effective option have been included. The CEAC demonstrates that, given the data, there is a 38% chance that the additional cost of the ‘treat all’ strategy, compared with all other test strategies, is at or below £30,000 per life-year gained.
Liver biopsy was a comparator for both stages of the analysis; however, this testing option is dominated by other less costly but more effective options.
Table 29 presents incremental results for the first stage of the analysis and Table 30 presents the incremental results for the second stage where a number of combined tests are compared using a number of different sequential testing strategies (see Chapter 3 for details of the four testing strategies presented in the tables as S1, S2, S3 and S4).
| Test strategy | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Treat no one | 37,579 | 8.83 | – | – | – |
| FIB-4 (high cut-off) | 67,267 | 9.56 | – | – | Extendedly dominated |
| Forns index (high cut-off) | 67,348 | 9.58 | – | – | Extendedly dominated |
| APGA | 67,653 | 9.60 | – | – | Extendedly dominated |
| Liver biopsy | 70,274 | 9.64 | – | – | Dominated |
| APRI (high cut-off) | 69,428 | 9.70 | – | – | Dominated |
| GUCI | 69,196 | 9.76 | – | – | Extendedly dominated |
| Hui index | 70,308 | 9.77 | – | – | Dominated |
| US SAPI (high cut-off) | 69,635 | 9.78 | – | – | Extendedly dominated |
| US | 71,710 | 9.81 | – | – | Extendedly dominated |
| MR elastography | 71,791 | 9.93 | – | – | Extendedly dominated |
| Fibroscan | 73,007 | 9.93 | – | – | Extendedly dominated |
| Fibrotest | 73,739 | 9.93 | – | – | Dominated |
| Forns index (low cut-off) | 74,317 | 9.94 | – | – | Dominated |
| Hyaluronic acid | 73,448 | 9.96 | – | – | Extendedly dominated |
| PAPAS | 74,493 | 9.98 | – | – | Extendedly dominated |
| US SAPI | 74,508 | 9.99 | – | – | Extendedly dominated |
| DW-MRI | 74,774 | 10.01 | – | – | Extendedly dominated |
| FIB-4 (low cut-off) | 75,648 | 10.01 | – | – | Dominated |
| Hepascore | 75,624 | 10.03 | – | – | Extendedly dominated |
| CEUS | 76,577 | 10.09 | – | – | Extendedly dominated |
| ARFI | 77,512 | 10.10 | – | – | Extendedly dominated |
| Age–Platelet Index | 78,647 | 10.13 | – | – | Dominated |
| CT | 78,129 | 10.13 | – | – | Dominated |
| AAR | 79,321 | 10.13 | – | – | Dominated |
| APRI (low cut-off) | 78,083 | 10.13 | – | – | Extendedly dominated |
| US SAPI (low cut-off) | 85,587 | 10.45 | – | – | Extendedly dominated |
| Treat all | 96,525 | 10.92 | 58,947 | 2.09 | 28,137 |
| Strategies | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Treat no one | 37,579 | 8.83 | – | – | – |
| (S1) Forns index (high cut-off) | 68,237 | 9.40 | – | – | Dominated |
| (S2) Forns index (high cut-off) and Fibrotest | 66,908 | 9.58 | – | – | Dominated |
| (S2) Forns index (high cut-off) and MR elastography | 66,892 | 9.58 | – | – | Dominated |
| (S2) Forns index (high cut-off) and hyaluronic acid | 66,885 | 9.58 | – | – | Extendedly dominated |
| Forns index (high cut-off) | 67,361 | 9.58 | – | – | Extendedly dominated |
| (S1) GUCI | 69,766 | 9.64 | – | – | Dominated |
| Liver biopsy | 70,274 | 9.64 | – | – | Dominated |
| (S3) Forns index (high cut-off) and Fibrotest | 70,285 | 9.65 | – | – | Dominated |
| (S3) Forns index (high cut-off) and hyaluronic acid | 70,261 | 9.65 | – | – | Dominated |
| (S1) US SAPI (high cut-off) | 70,230 | 9.65 | – | – | Dominated |
| (S3) Forns index (high cut-off) and MR elastography | 70,242 | 9.65 | – | – | Dominated |
| (S4) Forns index (high cut-off) and Fibrotest | 69,015 | 9.68 | – | – | Dominated |
| (S3) GUCI and US SAPI (high cut-off) | 70,122 | 9.68 | – | – | Dominated |
| FIB-4 (combined cut-off) and Fibroscan | 69,189 | 9.69 | – | – | Dominated |
| (S3) Fibrotest and US SAPI (high cut-off) | 70,345 | 9.69 | – | – | Dominated |
| (S3) GUCI and Fibrotest | 70,212 | 9.69 | – | – | Dominated |
| (S3) Hyaluronic acid and US SAPI (high cut-off) | 70,226 | 9.70 | – | – | Dominated |
| (S3) GUCI and hyaluronic acid | 70,115 | 9.70 | – | – | Dominated |
| (S3) GUCI and MR elastography | 70,090 | 9.71 | – | – | Dominated |
| (S2) GUCI and US SAPI (high cut-off) | 68,562 | 9.71 | – | – | Dominated |
| (S2) Fibrotest and US SAPI (high cut-off) | 68,870 | 9.71 | – | – | Dominated |
| (S4) Forns index (high cut-off) and hyaluronic acid | 69,463 | 9.72 | – | – | Dominated |
| (S2) GUCI and Fibrotest | 68,637 | 9.72 | – | – | Dominated |
| Forns index (combined cut-off) and Fibroscan | 69,559 | 9.72 | – | – | Dominated |
| (S3) Fibrotest and MR elastography | 70,497 | 9.72 | – | – | Dominated |
| (S2) GUCI and hyaluronic acid | 68,541 | 9.73 | – | – | Dominated |
| (S2) GUCI and MR elastography | 68,529 | 9.73 | – | – | Extendedly dominated |
| (S3) Hyaluronic acid and MR elastography | 70,332 | 9.74 | – | – | Dominated |
| (S2) Fibrotest and MR elastography | 69,023 | 9.74 | – | – | Extendedly dominated |
| (S4) GUCI and Fibrotest | 69,537 | 9.75 | – | – | Dominated |
| (S4) Fibrotest and US SAPI (high cut-off) | 69,827 | 9.76 | – | – | Dominated |
| (S4) Forns index (high cut-off) and MR elastography | 70,115 | 9.76 | – | – | Dominated |
| (S2) Hyaluronic acid and US SAPI (high cut-off) | 69,332 | 9.76 | – | – | Dominated |
| GUCI | 69,167 | 9.76 | – | – | Extendedly dominated |
| (S4) GUCI and US SAPI (high cut-off) | 69,590 | 9.77 | – | – | Dominated |
| (S4) GUCI and hyaluronic acid | 69,610 | 9.78 | – | – | Dominated |
| (S4) Hyaluronic acid and US SAPI (high cut-off) | 69,928 | 9.78 | – | – | Dominated |
| US SAPI (high cut-off) | 69,609 | 9.78 | – | – | Dominated |
| (S2) Hyaluronic acid and MR elastography | 69,438 | 9.80 | – | – | Extendedly dominated |
| (S4) Fibrotest and MR elastography | 70,429 | 9.81 | – | – | Dominated |
| (S4) GUCI and MR elastography | 70,011 | 9.81 | – | – | Extendedly dominated |
| (S4) Hyaluronic acid and MR elastography | 70,277 | 9.83 | – | – | Extendedly dominated |
| (S1) MR elastography | 72,171 | 9.84 | – | – | Dominated |
| (S1) Fibrotest | 74,242 | 9.84 | – | – | Dominated |
| APRI (combined cut-off) and Fibroscan | 71,769 | 9.87 | – | – | Dominated |
| (S1) Hyaluronic acid | 73,861 | 9.88 | – | – | Dominated |
| MR elastography | 71,737 | 9.93 | – | – | Extendedly dominated |
| Fibrotest | 73,812 | 9.94 | – | – | Dominated |
| Hyaluronic acid | 73,426 | 9.96 | – | – | Extendedly dominated |
| Treat all | 96,525 | 10.92 | 58,947 | 2.09 | 28,137 |
A scatterplot illustrating the position of each testing strategy on the cost-effectiveness acceptability curve compared with the testing strategy ‘treat no one’ can be found in Appendix 12.
Sensitivity analysis results
Hepatits B e antigen-positive
The base results remained robust to the majority of the sensitivity analyses, including changes to utility values, health state costs, liver biopsy disutility decrement and changes to NILT test costs; however, they were sensitive to some of the analyses. These are detailed as follows.
Removing tests from the analysis (where the studies did not converge using the bivariate model) changed the result so that the most cost-effective option, when all tests were compared singly, was to use APRI with a high cut-off; however, with an ICER of £20,673, if using a strict cost-effectiveness threshold of £20,000 this would not be adopted. If the cost-effectiveness threshold was set at £30,000, Fibroscan would be the most cost-effective test with an ICER of £23,345. The ‘treat all’ approach had the highest effectiveness (similar to base case) but with an ICER of £39,747 was not cost-effective at the standard UK threshold range of £20,000–30,000.
Setting treatment benefit to zero for patients who were incorrectly treated (patients in a mild health state who were diagnosed as false positive) significantly increased the ICER for ‘treat all’ to £550,668. The ICER for MR elastography increased to £32,220, which given a strict cost-effectiveness threshold of £20,000–30,000, would not be cost-effective. The most cost-effective option to adopt, assuming that the cost-effectiveness threshold is £20,000, is to use an indirect serum marker, GUCI, which has an ICER value of £19,934.
Changing the prevalence to the maximum prevalence estimated from the meta-analysis changed the result when all tests were compared singly; FIB-4 with a high cut-off became the most cost-effective test, with an ICER of £17,871. When the 75th quartile was used, the results changed so that treating everyone with a prior diagnostic test was the most cost-effective strategy if the cost-effectiveness threshold was at the upper bound of the recommended range (threshold £30,000, ICER £26,718).
Hepatits B e antigen-negative
The base results remained robust to the majority of sensitivity analyses; however, they were sensitive to some of the analyses which are detailed below.
Amending the sex ratio and starting age to reflect the HBeAg-negative model affected the results. Assuming a cost-effectiveness threshold of £30,000, the most cost-effective option would be to use MR elastography singly with an ICER of £25,546. However, if a strict cost-effectiveness threshold of £20,000 was employed, none of the options would be cost-effective.
Amending the average disease prevalence value used within the model changes the result; using a maximum prevalence returns a result where, given a cost-effectiveness threshold of £30,000, using MR elastography singly becomes cost-effective, with an ICER of £21,489. When the minimum disease prevalence is used, ‘treat all’ remains the most cost-effective given a threshold value of £30,000, but the ICER value decreases to £22,871.
Allowing for zero treatment benefit for patients who are treated incorrectly while in a mild health state (false-positive patients) changes the overall result, where MR elastography becomes the most cost-effective; however, the ICER of £32,194 is above the standard cost-effectiveness threshold value of £20,000–30,000. ‘Treat all’ also becomes cost-ineffective as a strategy, with an ICER of £53,660.
The base-case analysis assumes that the retest has perfect sensitivity and specificity (for modelling feasibility due to large number of tests and to ensure comparability across tests). When we amended this and set the retest sensitivity to that of three NILTs, APRI, Fibrotest and Fibroscan, the results did change for HBeAg-negative. Overall, the costs and QALYs for some of the NILTs increased, as the retest meant that some persons in mild health state (F0–1) started to receive treatment incorrectly (false-positive diagnosis), thereby incurring the associated costs and benefit (increased QALY outcome). ‘Treat all’ remains the most effective strategy, but is no longer the most cost-effective at standard cost-effectiveness threshold ranges. The most cost-effective test is now MR elastography, with an ICER in the upper band of the threshold range (£). MR elastography has a sensitivity of 94% of and specificity of 92%, implying that it will identify most patient correctly. This test is promising; however, as mentioned in Chapter 1, it is of limited use at present due to its cost and availability. Further studies to assess its diagnostic accuracy are required, as this was not one of the studies where the bivariate model converged and so the results may not be robust.
Short tables of results for the sensitivity analyses for the HBeAg-positive and HBeAg-negative models (excluded are ‘dominated’ and ‘extendedly dominated’ test strategies) are located in Appendix 11 (full tables are available on request).
Discussion
We have estimated the cost-effectiveness of 56 testing strategies of sequential testing using 25 NILTs in patients with chronic HBV. The results differed for the HBeAg-positive and HBeAg-negative populations.
The analysis found that for HBeAg-positive patients, adopting a sequential testing strategy where patients who tested positive using hyaluronic acid were retested with a second NILT, MR elastography, to confirm results was the most cost-effective approach given a cost-effectiveness threshold of £20,000. However, though hyaluronic acid combined with MR elastography is the most cost-effective test strategy, there is considerable uncertainty around this result and the probability of it being the optimal choice (having the highest expected net benefit) is very low (4%). If the upper bound of the standard cost-effectiveness range, £30,000, was considered to be appropriate, the most cost-effective strategy would be with MR elastography singly, but again this result was associated with substantial uncertainty (13% probability of being the optimal choice). Within the £20,000–30,000 threshold range, the difference between the expected QALYs and costs was similar for many of the test combinations, and taking into account the uncertainty around the input parameters results in high levels of uncertainty.
When NILTs were assessed singly, testing with an indirect serum marker, GUCI, was the most cost-effective option at a cost-effectiveness threshold of £20,000 with a mean ICER of £19,716, but again associated with considerable uncertainty. For a threshold of £30,000, MR elastography was most cost-effective, with an ICER of £23,486.
For patients with HBeAg-negative disease, the strategy of treating all patients without testing and regardless of the degree of fibrosis offered the largest QALY gain. This is, however, at a substantial additional cost and would be cost-effective only if considered towards the upper bound of the NICE cost-effectiveness threshold range,66 as it had an ICER of £28,317. All of the NILTs, either alone or sequentially followed by treatment, provided a QALY gain compared with a strategy of no testing and no treatment, but the gain was less than for the ‘treat all’ strategy, and they were dominated or extendedly dominated by the ‘treat all (no prior test)’ treatment strategy as their costs or ICERs were higher. There was less uncertainty in these results at the £30,000 threshold value where the probability of the ‘treat all’ strategy being optimum was 38%.
Similar findings for treatment in HBeAg-negative patients have been reported in assessments of the treatments conducted to inform national guidelines; the evidence review group which looked at the evidence for entecavir conducted an analysis where they analysed lifetime treatment duration for HBeAg-negative patients. This returned an ICER of £27,124 per QALY gained, similar to our base-case analysis result. 417
This difference in results between both models highlights that treatment is more cost-effective in patients with positive disease as the HBeAg-negative population tend to be older with a higher risk of progressing from a moderate health state to a cirrhotic health state than the HBeAg-positive cohort. The impact of treatment is also more modest in this group than for the HBeAg-negative population modelled.
We conducted a sensitivity analysis to confirm that age and sex ratio drive the difference. The sensitivity analysis results showed that when all tests were compared singly, using the indirect serum marker, GUCI, had an ICER of £23,065, similar to the base-case result for the HBeAg-positive model. As the age and sex ratio had been changed, this implies that the RRs (treatment effect) drives the remaining difference between both cohorts and, indeed, is the driver behind a NILT not being cost-effective for the HBeAg-negative cohort given a strict cost-effectiveness threshold of £20,000.
Another thing to note about the results for both models is that the differences between test outcomes are very small, especially in relation to the health outcomes (QALYs), which implies that the long-term costs resulting from a particular test diagnosis (true positive, false positive, false negative or true negative) are the driving factor behind the analysis for HBeAg-positive and -negative chronic HBV.
An issue also arises regarding using the systematic literature review data to calculate the disease prevalence of liver fibrosis within a population, as most studies may have been set within a tertiary care centre rather than within a screening programme for the general population, and so disease prevalence may be overestimated. When we used the minimum prevalence for the HBeAg-positive model, this did affect the results; GUCI remained the most cost-effective test when all NILTs were compared singly, but the ICER for MR elastography increased significantly to £37,348.
Data on diagnostic accuracy of NILTs in patients with chronic HBV were limited and of low quality; therefore, our results should be interpreted with caution. Data on hyaluronic acid came from a single study, while MR elastography is not widely available and needs further validation as a diagnostic test.
Although Fibroscan is widely used in most centres, it was not the most cost-effective option, even in the sensitivity analysis when only studies that converged using the bivariate model were considered (given a strict £20,000 cost-effectiveness threshold). Our results are, therefore, indicative that sufficiently validated non-invasive testing strategies are more cost-effective in patients with HBeAg-positive chronic HBV than liver biopsy or treatment decisions based on viral load and transaminases alone, but no robust specific recommendations on the use of specific non-invasive tests can be made. Future research should further explore these possibilities.
Chapter 6 Cost-effectiveness analysis: hepatitis C
This chapter details the analysis of the cost-effectiveness of the diagnostic tests for staging fibrosis in patients with HCV. The NILTs considered for evaluation in this chapter were those considered applicable for use in people with HCV, for which data on sensitivity and specificity were available.
Evaluation approach for hepatitis C
Fifty-seven relevant NILTs were evaluated in the first stage of the analysis, which compared the NILTs with liver biopsy, ‘treat all’ and ‘no treatment’ strategies. The NILTs evaluated are listed in Table 31, and are grouped according to defined test categories: indirect serum makers, direct and patented serum markers and imaging modalities.
| Indirect | Direct and patented | Imaging |
|---|---|---|
| APRI | ELF | ARFI |
| Age-Platelet Index | ELF (high cut-off) | CT |
| APRI (high cut-off) | ELF (low cut-off) | EOB-MRI |
| APRI (low cut-off) | Fibroindex (high cut-off) | MRI |
| AST–ALT ratio | Fibroindex (low cut-off) | PLT–Spleen ratio |
| CDS | Fibrometer | Fibroscan (TE) |
| Fibrosis Index | Fibrospect | US |
| FIB-4 | Fibrotest | US SAPI |
| FIB-4 (high cut-off) | Fibrotest (high cut-off) | US SAPI (high cut-off) |
| FIB-4 (low cut-off) | Fibrotest (low cut-off) | US SAPI (low cut-off) |
| FibroQ | Hyaluronic acid | CEUS |
| Forns index | Hyaluronic acid (high cut-off) | DW-MRI |
| Forns index (high cut-off) | Hyaluronic acid (low cut-off) | MR elastography |
| Forns index (low cut-off) | Hepascore | |
| FPI (high cut-off) | Hepascore (high cut-off) | |
| FPI (low cut-off) | MP3 | |
| GUCI | PIINP/MMP-1 index | |
| King’s | PIINP | |
| King’s (high cut-off) | PLT | |
| King’s (low cut-off) | Type IV collagen | |
| Lok’s index | YKL-40 (high cut-off) | |
| Pohl index | YKL-40 (low cut-off) |
The second stage of the analysis compared a selection of tests using alternative sequential testing strategies. The criteria for selecting these tests and the assumptions used regarding combinations of tests and sequential testing strategies are detailed in Chapter 3. The systematic review and subsequent meta-analysis of the data provided test outcome results for a number of published algorithms used for staging fibrosis [sequential algoritm for fibrosis evaluation (SAFE), Leroy, Fibropaca and Bordeaux], which were evaluated in the second stage of the analysis.
Ten tests evaluated in the second stage of the analysis used a combined diagnostic cut-off threshold for staging fibrosis; the test outcomes reported a number of indeterminate responses, which are listed in Table 32. We allowed for patients who had an indeterminate response to receive a retest with a commonly used imaging modality Fibroscan (TE). We did not choose an indirect test as the majority of the 10 tests with a combined diagnostic cut-off were from the indirect test category and a subsequent indirect test would not enhance the diagnostic accuracy. Of the direct tests and imaging modalities, we chose Fibroscan based on availability and current clinical practice. Overall, 56 testing strategies were compared in the second stage of the analysis.
| Combined diagnostic threshold test | Persons with indeterminate result, % |
|---|---|
| APRI | 43 |
| ELF | 41 |
| FIB-4 | 29 |
| Fibroindex | 35 |
| Fibrospect | 53 |
| Forns index | 45 |
| Fibrotest | 38 |
| Hyaluronic acid | 44 |
| Hepascore | 33 |
| YKL-40 | 47 |
Model structure and parameters
Decision tree structure
A decision tree model was constructed to evaluate the cost-effectiveness of the NILTs, liver biopsy, the ‘treat all’ and ‘no treatment’ strategies. As per the schematic diagram depicting the flow of data in Chapter 3 (see Figure 1), the decision tree was populated with sensitivity, specificity and prevalence data from the meta-analysis of the systematic review data, cost and QALY outputs from a series of Markov models, individual test costs sourced from published literature and hospital finance departments and a measure of adverse effects associated with liver biopsy.
As discussed previously, there are two stages to the decision tree analysis: the first stage where all tests are compared singly and the second stage where combinations of tests are compared using four different strategies. Schematic illustrations and descriptions of the sequential testing pathways are provided in Chapter 3. To estimate costs and QALYs for each testing option, the long-term costs (and test costs) and QALY estimates (including disutility from liver biopsy if applicable) associated with each potential test outcome (true positive, false positive, true negative and false negative) were multiplied by the probability of each NILT returning a true positive, false positive, true negative or false negative result.
Markov model structure
A series of Markov models were constructed to estimate the long-term costs and outcomes associated with a correct (true positive and true negative) and an incorrect (false positive and false negative) diagnosis for a hypothetical cohort of 1000 HCV patients with suspected liver fibrosis or cirrhosis. An additional two Markov models were constructed to estimate the costs and outcomes associated with the ‘treat all and ‘treat no one’ approaches.
Figure 6 shows a schematic representation of the patient pathway. The structure is a modified version of previously published models of liver fibrosis in HCV. 414,438 The starting health states in the Markov models were representative of the METAVIR categorisations for staging fibrosis. There were eight potential health states in the model: mild fibrosis (F0–1), moderate/significant fibrosis (F2–3), compensated cirrhosis (F4), decompensated cirrhosis, HCC, liver transplant, post liver transplant and death. The patient cohort progressed through the model as per the arrows in the illustration; the circular arrows leading back into the same health state indicate that patients could remain within health states for longer than one cycle, except in the liver transplant state where patients could only progress to a post liver transplant health state or death. Patients could not regress back to an earlier health state. Patients could progress from all health states to the ‘death’ health state at any time.
FIGURE 6.
Illustration of Markov model.

On the basis of clinical advice, we assumed that a METAVIR test score of ≥ F2 equated to a positive test outcome (true positive and false positive) and treatment with antiviral agents would commence at this stage; conversely, a METAVIR test score of < F2 indicated a negative test outcome and a policy of watchful waiting without immediate antiviral treatment would commence (see Chapter 5 for description of the watchful waiting policy assumed in the model).
As for the HBV Markov models, the initial starting health state for the population cohort in the models depended on the test outcome being modelled [e.g. for a false positive test result, the population cohort entered the model in a mild health state (F0–1), and for a test result of true positive the population cohort entered the model in a F2–4 health state, where the patient cohort was then distributed according to the estimated disease prevalence (F2–3 62%; F4 38%)].
Input parameters
Average disease prevalence was based on the systematic review of test accuracy (53%) using the same methods used for the HBV model (see Chapter 5). We used the sensitivity and specificity estimates for each NILT and the average prevalence estimate to calculate the probability of each test returning a true positive, false positive, true negative and false negative result (see Appendix 7).
Cohort characteristics
The characteristics of the cohort (age, sex and weight) were sourced from published literature. 414,439 Various studies have determined that treatment will have different effects according to patient genotype;37,439 therefore, our model cohort is split to mirror the distribution of chronic HCV genotype 1, 2 and 3, and 4 infections,439 allowing us to model the effectiveness of treatment per genotype. These are listed in Table 33.
Natural history: baseline transition probabilities used in model
The recent systematic review by Shepherd et al. 371 was reviewed and the epidemiological search strategy (see Chapter 5 and Appendix 2 for details) updated for HCV and rerun using the MEDLINE database (Ovid platform, searched 1 December 2012) for relevant papers related to disease progression in HCV. The search located 2343 papers, none of which was deemed relevant for our purposes. The rate of disease progression assumed in the models was based on transition probabilities sourced from a published cost-effectiveness study by Wright et al. ,414 which was identified in the literature search for quality of life and cost data. This study estimated transition probabilities for the mild and moderate HCV health states from a trial of treatments for mild HCV undertaken in a number of London hospitals,414 during which the liver fibrosis stage was determined by liver biopsy. We deemed this a suitable study to use as it was a UK-based study, and noted that it does not assume that the progression between early health stages is linear. 414
Transition probabilities for the compensated cirrhosis, decompensated cirrhosis, HCC, liver transplant and post liver transplant health states were also sourced from this study. For these health states, the authors sourced the probabilities from published literature. Data from a study by Fattovich et al. 409 were used for the compensated cirrhosis, decompensated cirrhosis and HCC health states, and for mortality-specific data relating to each health state. The study by Fattovich et al. 409 was conducted in seven European centres, including a UK tertiary centre. The study was a retrospective study looking at a cohort of 384 compensated cirrhotic patients who were assessed annually for a mean of 5 years.
Wright et al. 414 sourced transition probabilities relating to the liver transplant and post liver transplant health state from a study by Siebert et al. ,435 where the rate of liver transplants was estimated for HCV patients in the USA and revised downwards by 2% based on European transplant registry data and estimated the probability of death following a liver transplant from a survival analysis of the UK liver transplant registry data conducted by the Royal College of Surgeons. 440 Transition probabilities used in the model are listed in Table 34.
| Health state–health state | Transition probability | PSA distribution | Source |
|---|---|---|---|
| Mild–moderate | 0.025 | Dirichlet | Wright et al.414 |
| Moderate–cirrhosis | 0.037 | ||
| Cirrhosis–decompensated cirrhosis | 0.04 | ||
| Cirrhosis/decompensated cirrhosis–HCC | 0.14 | ||
| Decompensated cirrhosis/HCC–liver transplant | 0.02 | ||
| Decompensated cirrhosis–death | 0.13 | ||
| HCC–death | 0.43 | ||
| Liver transplant–death (year 1) | 0.15 | ||
| Post liver transplant–death | 0.03 | ||
| All-cause mortality | 0.0014–0.335 | Interim Life Tables, 2008–10416 |
Mortality data
An all-cause mortality rate applied to all patients within the model; this was calculated using the Interim Life Tables for England and Wales, 2008–10. 416 The risk of death increased each year according to age and the rate was weighted to allow for sex mix.
Antiviral treatment: type, dosage, duration and effectiveness
In the model, patients received treatment with antiviral agents if they tested positive (true positive or false positive with a test score of ≥ F2).
If patients had a successful response to treatment (modelled using a SVR rate), they no longer progressed through the health states pathway in the model and retained a risk of all-cause mortality only. This is similar to the assumption employed by Wright et al. 414
Antiviral treatment for the management of liver fibrosis and cirrhosis in patients with HCV was based on NICE guidance. 441–443 Treatment type, duration and dosage in the model varied according to HCV genotype. In accordance with NICE guidelines, our model assumed that HCV genotype 1 patients received treatment with a combination of peginterferon alfa-2a or alfa-2b, ribavirin, and either telaprevir or boceprevir. 441,442 Patients with HCV genotype 2, 3 and 4 were assumed to receive dual therapy with a combination of peginterferon alfa-2a or alfa-2b and ribavirin. 443
Different branded products are marketed and available in the UK for ribavirin (BNF 64419); the summary of product characteristics for one of the brands [Rebetol®, Merck Sharpe & Dohme (MSD)] advises that it should only be used in combination with peginterferon alfa-2b (ViraferonPeg®, MSD). 444 To allow for this, we modelled two different combinations of antiviral agents in the model (combined as per their summary of product characteristics) with half of eligible patients receiving one combination and the other half of eligible patients receiving the other.
-
HCV genotype 1: peginterferon alfa-2a (Pegasys®, Roche) combined with ribavirin (Copegus®, Roche) and telaprevir (Incivo®, Janssen) or peginterferon alfa-2b (ViraferonPeg®, MSD) combined with ribavirin (Rebetol®, MSD) and boceprevir (Victrelis®, MSD)
-
HCV genotype 2, 3 and 4: peginterferon alfa-2a (Pegasys®, Roche) combined with ribavirin (Copegus®, Roche) or peginterferon alfa-2b (ViraferonPeg®, MSD) combined with ribavirin (Rebetol®, MSD).
Patients in the mild, moderate and compensated cirrhosis health states would receive treatment with antiviral agents if they tested positive (true positive or false positive). Patients in the decompensated cirrhosis, HCC, liver transplant and post liver transplant health states were assumed to receive no antiviral treatment, but instead received usual standard of care. 37
Treatment dosage and treatment duration was based on those recommended in the summary of product characteristics for each drug (www.medicines.org.uk/emc); drug dosage depended on the weight of the patient; for modelling purposes we assumed that the drug dosage would be administered as per the assumed average weight in the model (79.8 kg). 439 Treatment effectiveness, represented in the model by the SVR rate, was sourced from published literature. 445 Patients within the model were assumed to be treatment naive (had not received previous treatment with antiviral agents). Different SVR rates were employed in the model and varied according to genotype and drug therapy administered. We assumed that treatment benefit would occur during the treatment year.
Different SVR rates for the mild, moderate and cirrhotic health states were not employed within the model as separate rates were not reported within the source literature,441,445 except for HCV genotype 1 patients treated with peginterferon alfa-2b, ribavirin and boceprevir,442 where a different SVR rate was reported for HCV patients in a cirrhotic health state. As it has been noted that the SVR rates with pegylated IFN-α and ribavirin are lower in patients with advanced fibrosis and cirrhosis than in patients with mild or moderate fibrosis,37 we carried out a sensitivity analysis where we reduced the SVR rate for the patient cohort receiving treatment in a cirrhotic health state. The SVR rates, recommended dosage and specific duration employed in the model as part of dual or triple therapy are listed in Table 35.
| Drug combination | Dosagea | Treatment duration | SVR rate applied | Source of SVR rate |
|---|---|---|---|---|
| Genotype 1: treatment-naive patients | ||||
| Peginterferon alfa-2a, ribavirin, telaprevir | 180 mg, 1200 mg and 2250 mg | PR and Telaprevir TW 12, PR TW 48 | 75% | NICE technology appraisal TA252441 |
| Peginterferon alfa-2b, ribavirin, boceprevir | 120 mg, 1000 mg and 2400 mg | PR for 4 weeks, PR and boceprevir TW 36, PR TW 48 | 66.1% | NICE technology appraisal TA253442 |
| Genotype 1: cirrhotic patients (treatment naive) | ||||
| Peginterferon alfa-2b, ribavirin and boceprevir | 120 mg, 1000 mg and 2400 mg | PR for 4 weeks, PR and boceprevir TW 48 | 41.7% | NICE technology appraisal TA253442 |
| Genotype 2 and 3: treatment-naive patients | ||||
| Peginterferon alfa-2a, ribavirin | 180 mg, 1200 mg | PR for 24 weeks | 76% | Fried et al.439 |
| Peginterferon alfa-2b, ribavirin | 1200 mg, 1000 mg | PR for 24 weeks | 82% | Manns et al.445 |
| Genotype 4: treatment-naive patients | ||||
| Peginterferon alfa-2a, ribavirin | 180 mg, 1200 mg | PR for 48 weeks | 77% | Fried et al.439 |
| Peginterferon alfa-2b, ribavirin | 120 mg, 1000 mg | PR for 48 weeks | 69% | Kamal et al.446 |
Costs
Health states
We updated the search strategy for costs devised by Shepherd et al. 371 for HCV (see Appendix 2 for search strategy). Using the MEDLINE database (via Ovid platform, searched 1 December 2012), the search located 21 papers, eight of which were retrieved for full review;447–454 however, six of these papers were based outside the UK,447–449,453,454 one contained no costs451 and two were guidelines papers. 450,452 As none of the papers found was applicable, the costs of treating patients with mild, moderate and compensated cirrhosis were sourced from a published cost-effectiveness study by Wright et al. 414 identified from the literature review for quality-of-life data for HCV. This study looked at the effectiveness of antiviral agents for mild HCV, costs for which were collected during a RCT. The cost data from this study have been widely used in other recently published papers. 455–457
The authors collected resource use and cost data alongside a mild HCV RCT. Detailed cost data were collected from three centres based in London, Newcastle and Southampton. Resource use information collected covered inpatient and outpatient care, investigations, procedures, drug use and other services including psychiatric services.
The costs associated with treating the decompensated cirrhosis, HCC, liver transplant and post liver transplant health states were sourced from a UK cost-effectiveness study of liver transplantation424 (CELT study) which collected costs on adult patients listed for an isolated liver transplant (aged 16 years and over) between December 1995 and December 1996. Data collection was conducted between 1995 and 1999 and split into four phases: assessment, candidacy, transplant and post-transplant phases (see Chapter 5 for description) and collected according to disease aetiology (HBV, HCV, ALD). Resource-use data on blood products used, number of dietitian sessions, drugs used, inpatient stay, nutritional support received, outpatient visits, physiotherapy sessions, tests, length of transplant operation and key treatments and investigations were collected.
We calculated the cost for HCV patients who had received a transplant (sample size 67). The average length of time for a liver transplant procedure (from admission to discharge) was 28 days. We approximated this to 1 month and calculated the yearly cost of a liver transplant as 11 months of post-transplant care (estimated from the average monthly cost in the first year following transplantation) plus the month in which the transplant operation took place. We also calculated a cost for the post liver transplant health state, estimated from the average monthly cost for the last 12 months of post-transplant care (sample size 40).
The data were also used to estimate a cost for the decompensated cirrhosis and HCC health states. For this, the average costs of treating patients with decompensated cirrhosis were assumed to be equivalent to patients considered for a transplant. Resource-use data of patients with HCV during the ‘assessment’ and ‘candidacy’ stages of transplantation were calculated (sample size 56).
Costs were inflated to 2011–12 levels using standard NHS inflation indices. 67 Health state costs did not include the costs associated with antiviral treatment; these were included separately in the model. Health state costs are listed in Table 36.
| Health state | Annual cost, £ | Standard error | PSA distribution | Source |
|---|---|---|---|---|
| Mild fibrosis | 185 | 36.39 | Gamma | Wright et al.414 |
| Moderate fibrosis | 959 | 101.69 | ||
| Compensated cirrhosis | 1521 | 309.05 | ||
| Decompensated cirrhosis | 38,871 | 9410.46 | Longworth et al.424 | |
| Hepatocellular cancer | 38,871 | 9410.46 | ||
| Liver transplant | 69,174 | 7054.86 | ||
| Post liver transplant | 4356 | 861.57 | ||
| Death | 0 | 0 | Assumed |
Test costs
Costs of imaging modalities were sourced from published Department of Health reference costs. 427 Costs of direct and indirect serum markers were obtained from communication with finance departments based at the Royal Free Hospital. Costs for patented serum markers were sourced directly from manufacturers and via communication with finance departments based at the Royal Free Hospital. The cost of a percutaneous liver biopsy (see Chapter 5 for further details on choice of liver biopsy) was sourced from published literature428 (see Chapter 5). Test costs including sources are listed in Appendix 9.
Medication costs
For HCV genotype 1 and 4 infection, a cost of 48 weeks of treatment was applied. For HCV genotype 2 and 3 infection, a cost approximate to 24 weeks of treatment was applied (Table 37). Treatment costs were sourced using the BNF 64419 and a total cost of treatment was calculated according to the recommended dosage and duration detailed in Table 35.
| Treatment | Genotype 1 | Genotype 2 and 3 | Genotype 4 |
|---|---|---|---|
| Combination therapy | |||
| Peginterferon alfa-2a and ribavirin | 4446 | 10,411 | |
| Peginterferon alfa-2b and ribavirin | 5435 | 10,870 | |
| Triple therapy | |||
| Peginterferon alfa-2a and ribavirin and telaprevir | 32,809 | ||
| Peginterferon alfa-2b and ribavirin and boceprevir (treatment naive) | 33,270 | ||
| Peginterferon alfa-2b and ribavirin and boceprevir (compensated cirrhosis) | 41,670 | ||
Utility values
A search was carried out using the MEDLINE database (via Ovid platform, searched 1 December 2012) for quality of life data for use in the HCV model (see Appendix 2 for search strategy). The search returned 459 papers; seven were retrieved for full review. 371,414,432,455,457–459 From these, the most relevant data identified were found in the published study on the Mild Hepatitis C trial414 as it was from a UK population using the EQ-5D. As this study was also used to identify data for the mild, moderate and cirrhotic health states in the HBV model, details regarding the elicitation of utility values for the mild, moderate and compensated cirrhosis health states are reported in the chapter for HBV (see Chapter 5) and will not be duplicated here.
As for the HBV model, we sourced health-related utility data for the decompensated cirrhosis, HCC, liver transplant and post liver transplant health states from the CELT study. 424 The transplantation study included patients with a range of conditions that warranted liver transplantation including HCV. HRQoL data were collected using the EQ-5D.
It was assumed that utility values for the decompensated cirrhosis and HCC health states would be the same (sample size 56). This was assumed to be equivalent to the average utility value at the time patients were placed on the waiting list for a transplant.
A utility value for the liver transplant health state was estimated using an area under the curve approach and the utility values collected at 3, 6 and 12 months post transplantation (sample size 67). A utility value for the post-transplant health state was estimated using the average utility for patients with HCV patients collected at 24 months post transplant (sample size 40).
Utility values during treatment and after sustained virological response
Treatment with peginterferon alfa-2a or -2b and ribavirin is associated with a number of adverse effects such as severe fatigue, depression, irritability, sleeping disorders, skin reactions, dyspnoea, neutropenia, anaemia, thrombocytopenia and ALT flares and more severe side effects such as seizures, bacterial infections, autoimmune reactions, interstitial lung disease, a neuroentinitis, bone marrow aplasia or idiopathic thrombocytopenia. 37 To reflect this in the model, we assumed a disutility value that was applicable during treatment using data identified from the Wright et al. 414 study. The authors collected HRQoL data using the EQ-5D (sample number: 144 patients), using the data from weeks 12 and 24 when most people were still taking treatment.
It was conservatively assumed that any adverse events associated with boceprevir or telaprevir treatment (skin rash and exacerbation of anaemia) would have no effect on HRQoL in the base-case analysis. We tested this assumption in a sensitivity analysis by applying a utility decrement value of 0.05 applicable during treatment with boceprevir and telaprevir for HCV genotype 1 patients.
This study identified a number of previously published studies460–462 which reported that the HRQoL score for patients significantly improved after successful treatment with interferon and, therefore, assumed that therapy for mild HCV may benefit patients for non-hepatological reasons. They measured the improvement in HRQoL after a successful response to treatment by collecting data using the EQ-5D from 21 patients with mild fibrosis who had a SVR. As there were insufficient data to estimate an EQ-5D value for patients in a post-SVR moderate health state, the authors estimated a post-SVR EQ-5D value by substituting the mean estimated HRQoL for moderate disease as the baseline value into the analysis of covariance (ANCOVA) model which had been used to estimate the treatment effect for patients with mild disease. We used the same assumption as Wright et al. 414 and allowed for an increased EQ-5D value post SVR.
The study by Wright et al. 414 did not report a separate HRQoL value for patients in a cirrhotic health state receiving treatment and post SVR. For this, we used the same assumption as that used in a study by Grishchenko et al. ,455 who assumed that patients with cirrhosis had the same absolute gain in HRQoL as patients with mild disease.
Using the identified data, we applied a 0.11 utility decrement from baseline during treatment and a 0.05 utility increment from baseline if they had a SVR after treatment.
We tested the use of differing HRQoL values applicable during antiviral treatment and post SVR by carrying out a sensitivity analysis where we assumed that HRQoL values did not change from baseline during or after treatment. Utility values are listed in Table 38.
| Health stage | Utility value | SE | PSA distribution | Source |
|---|---|---|---|---|
| Mild fibrosis | 0.77 | 0.035 | Beta | Wright et al.414 |
| Moderate fibrosis | 0.66 | 0.018 | ||
| Compensated cirrhosis | 0.55 | 0.032 | ||
| Decompensated cirrhosis | 0.49 | 0.056 | Longworth et al.421 | |
| HCC | 0.49 | 0.056 | ||
| Liver transplant | 0.51 | 0.053 | ||
| Post liver transplant | 0.52 | 0.061 | ||
| Death | 0 | 0 | Assumed | |
| Mild fibrosis (during treatment) | 0.65 | 0.035 | Wright et al.414 | |
| Moderate fibrosis (during treatment) | 0.55 | 0.018 | ||
| Compensated cirrhosis (during treatment) | 0.44 | 0.04 | Grishchenko et al.455 | |
| Mild fibrosis (SVR, after treatment) | 0.82 | 0.04 | Wright et al.414 | |
| Moderate fibrosis (SVR, after treatment) | 0.71 | 0.05 | ||
| Compensated cirrhosis (SVR, after treatment) | 0.60 | 0.04 | Grishchenko et al.455 |
Disutility from non-invasive liver test and liver biopsy
We have assumed that no NILT has associated adverse events that would impact on HRQoL. However, as liver biopsy is associated with morbidity and mortality risks and patient discomfort, a measure of adverse effects resulting from liver biopsy was modelled by applying a utility decrement of 0.2 where applicable. 428 As the decrement value was arbitrary, we undertook a number of sensitivity analyses to test the robustness of the results to changes in the utility decrement.
Estimated long-term costs and quality-adjusted life-years
Table 39 presents the costs and QALYs from the Markov models for each potential diagnostic testing outcome.
| Diagnostic test outcome | Cost (£ 2012 per person) | QALY (per person) |
|---|---|---|
| True positive | 68,667 | 12.89 |
| True negative | 21,812 | 15.84 |
| False positive | 32,318 | 16.86 |
| False negative | 71,818 | 12.48 |
| Treat all persons | 51,374 | 14.77 |
| Treat no one | 55,173 | 12.47 |
Analysis
Probabilistic and one-way sensitivity analyses were undertaken. We also conducted threshold analyses around the assumptions of treatment benefit and the impact of changes to the costs and effectiveness of treatment.
Robust test accuracy data
In the base-case analysis, all tests were included despite there being limited data available for some tests. A sensitivity analysis was conducted including only tests where the bivariate model used for the meta-analysis converged (for 14 NILTs).
Changes to utility values
As mentioned previously, we carried out an analysis where we set utility values constant at baseline utility values before, during and after treatment.
We also carried out two other analyses regarding the utility values used; we set the utility values equivalent to those used in a study by Shepherd et al. 392 We also carried out an analysis where we increased the utility value of all health states by 0.1 to determine if this had any effect on the robustness of the results.
Average disease prevalence
Prevalence within the model is based on studies that may have been carried out largely in tertiary care centres, and the prevalence of liver fibrosis in this population may be an overestimate. To test the impact of this, we undertook sensitivity analyses using the minimum prevalence (17%) and the maximum prevalence (83%) estimated from the meta-analysis of the systematic review data.
Change to progression rates after sustained virological response while in cirrhotic health state
The base-case analysis assumes that the risk of death of patients who respond successfully to treatment (experience a SVR) is equivalent to that for the general population. We tested this assumption for patients in a compensated cirrhosis health state as it has been noted that they may retain a small risk for progressing to these health states. 37,463 We modelled this by allowing patients in the compensated cirrhosis health state to retain a small risk of decompensated cirrhosis (0.004) and HCC (0.002) after a successful response to treatment (SVR). 464
Lower sustained virological response rate
As mentioned previously, it may be the case that patients in a cirrhotic health state have a lower SVR rate; to test this assumption, we carried out an analysis where we reduced the SVR rate by 20% (assuming the same estimate used in the study by Liu et al. 438) for patients who received treatment in a cirrhotic health state.
Change in cost of non-invasive liver tests
We carried out a sensitivity analysis where we changed the cost of the NILTs (we set the watchful waiting retest cost and all NILT costs within the model to the same cost); for comparison, we assumed an indirect serum marker test cost (we chose a commonly used test, APRI, as our comparator). By changing the cost of a NILT (in some cases reducing the test cost significantly, e.g. reducing cost of ELF from £108 to £4.50) we aimed to determine if changing the test cost (marginal cost) had an impact on the robustness of the results.
Sensitivity and specificity of retest
Our base-case analysis assumes that the retest (from the meta-analysis of the systematic review data in the watchful waiting strategy for patients with a negative test result) has perfect sensitivity and specificity. We tested this assumption by applying the sensitivity and specificity of three commonly used tests: APRI (estimated sensitivity of 77% and specificity of 81%), Fibrotest (estimated sensitivity of 68% and specificity of 75%) and Fibroscan (estimated sensitivity of 79% and specificity of 83%).
Choice of tests for second stage of the analysis
For the second stage of the analysis, the two most cost-effective tests when assessed within each specific test category singly (with and without a defined threshold) were used in the analysis of sequential testing. To test if changing the method used to choose tests for the second stage of the analysis had an effect on the overall result, we carried out an analysis where we chose the most effective NILT from within each NILT category and the least costly NILT from each category.
Change in genotype distribution
We carried out an analysis where we amended the distribution of the population cohort per genotype in the model to determine if this had an impact on the results (HCV genotype 1 set at 50%, HCV genotype 2 and 3 set at 41% and HCV genotype 4 set at 9%).
Change to utility values
We also carried out analyses using different utility decrement values to represent the adverse effects from liver biopsy. The base-case analysis set the utility decrement value to 0.2; in the analyses we set the utility decrement at 0 and 0.3 to test the impact on the robustness of the results.
Adverse events
Both telaprevir and boceprevir carry a risk of adverse events. We tested the assumption that this would not have a significant impact on health-related utility by assuming a disutility decrement value of 0.05, which was applicable to all patients who received triple therapy with peginterferon alfa-2a or -2b, ribavirin and either telaprevir or boceprevir.
Threshold sensitivity analyses
Treatment benefit for patients who are incorrectly diagnosed
The base-case analysis reflects that HCV patients who are diagnosed incorrectly (‘false positive’) receive benefit from treatment despite only having mild disease. Treatment benefit in the model is reflected by the probability of a SVR and patients in a mild health state receive the same treatment benefit (same successful response rate measured using SVR rate) as patients who are in a moderate or cirrhotic health state. We tested the robustness of this assumption in a sensitivity analysis by undertaking a threshold sensitivity analysis where we reduced the SVR rate for patients in a mild health state (F0–1) by decrements of 10%.
Sensitivity analysis on drug costs and sustained virological response rates
We are aware that new drugs are in development for the treatment in people with HCV; for example, a new protease inhibitor has recently been investigated in phase 3 trials. 43,44 With this in mind, we conducted sensitivity analyses exploring the impact of the costs and effectiveness of treatments of fibrosis in HCV on the conclusions regarding the cost-effectiveness of the test strategies.
We increased the SVR rate for genotype 1 and 4 HCV infections to reflect the potential efficacy of new drugs suitable for treatment in HCV. Based on early results from two phase 3 studies,43 we assumed an increased SVR rate of 90% for genotype 1 HCV infection and genotype 4 patients. No amendments were made to the SVR rates for genotypes 2 and 3 HCV infections from those in the base-case analysis based on the results of a published non-inferiority study of the same new treatment. 43 We then also increased the cost of drug treatment assuming an additional £20,000 and £40,000 cost per patient for 12 weeks of treatment with the new drug (this was added to the existing cost for peginterferon alfa-2a and alfa-2b and ribavirin used in the base-case analysis). We did not allow for different SVR rates per health state.
Results
Base case
At a standard UK threshold range, the cost-effective strategy is to adopt a ‘treat all’ approach with an ICER of £9204. For values below this, a patented serum maker, Fibrospect (combined cut-off), where indeterminate responses are retested with an imaging modality, Fibroscan, is the most cost-effective option.
The CEAF (Figure 7) shows that the probability of ‘treat all’ having the highest expected net benefit, given a cost-effectiveness threshold value of £20,000, is 45%. For lower threshold ranges, the CEAF also shows that there is considerable uncertainty around which test has the highest expected net benefit. For cost-effectiveness thresholds lower than £9200 [using Fibrospect (with a combined cut-off) where the inconclusive responses are retested with Fibroscan] is most likely to have the highest expected net benefit; however, there is considerable uncertainty around this result and the probability of it being optimal is < 4%. Liver biopsy was a comparator for both stages of the analysis; however, this testing option is dominated by other less costly but more effective options.
FIGURE 7.
Cost-effectiveness acceptability frontier for the decision concerning most the cost-effective testing strategy to employ for HCV. PLT, platelet.
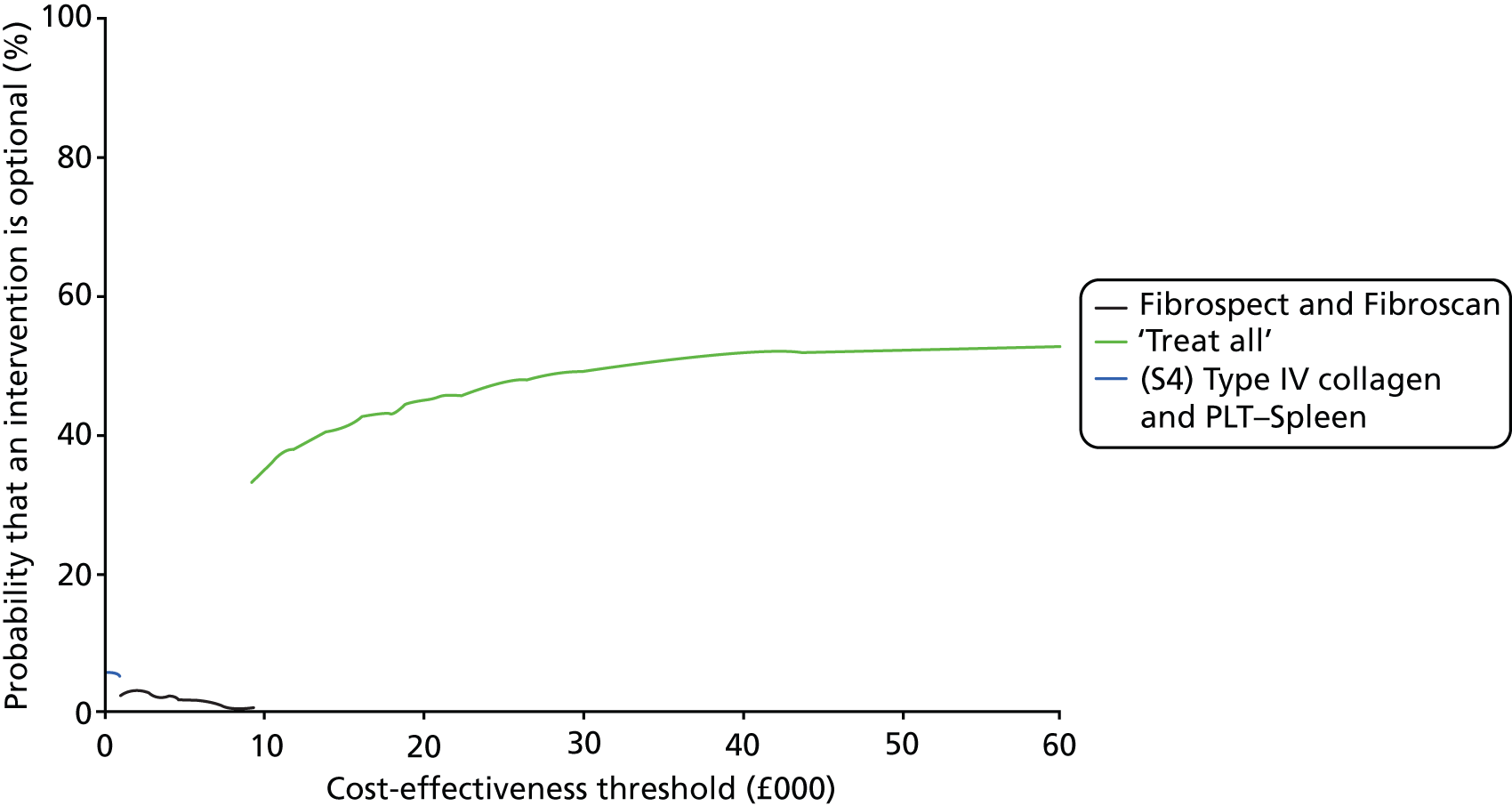
Appendix 10 displays the CEAC for the overall base-case analysis. For reasons of clarity, only those strategies that have a ≥ 5% or greater probability of being optimal have been included. The CEAC demonstrates that, given a cost-effectiveness threshold value of £20,000, the probability that the ‘treat all’ strategy is cost-effective compared with the other testing strategies is 0.449. This indicates that, given the data, there is a 45% chance that the additional cost of the ‘treat all’ strategy, compared with all other test strategies, is at or below £20,000 per life-year gained. The CEAC also displays that liver biopsy has a high probability of being cost-effective for thresholds values below approximately £3500. This does not translate into the CEAF due to skewed data on the costs of the tests and a high level of uncertainty on differences in costs between the alternative test strategies. Often, the testing option with a high probability of being cost-effective may not be the optimal choice, which is what the CEAF represents (Fenwick et al. ). 69
Table 40 presents incremental results for the first stage of the analysis and Table 41 presents incremental results for the second stage where a number of combined tests are compared using a number of different sequential testing strategies (see Chapter 3 for details of testing strategies, S1, S2, S3 and S4). A scatterplot illustrating the position of each testing strategy on the cost-effectiveness acceptability curve compared with the testing strategy ‘treat no one’ can be found in Appendix 12.
| Test strategy | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Treat none | 54,878 | 12.45 | – | – | Dominated |
| Liver biopsy | 48,710 | 14.03 | – | – | Dominated |
| Pohl | 47,727 | 14.04 | – | – | Dominated |
| Fibroindex (high cut-off) | 47,769 | 14.08 | – | – | Dominated |
| Forns Index (high cut-off) | 47,426 | 14.12 | – | – | Dominated |
| Hepascore (high cut-off) | 47,897 | 14.13 | – | – | Dominated |
| FPI (high cut-off) | 47,335 | 14.14 | – | – | Dominated |
| APRI (high cut-off) | 47,525 | 14.14 | – | – | Dominated |
| FIB-4 | 47,900 | 14.15 | – | – | Dominated |
| US | 48,090 | 14.17 | – | – | Dominated |
| ELF (high cut-off) | 47,846 | 14.17 | – | – | Dominated |
| Hyaluronic acid (high cut-off) | 48,969 | 14.18 | – | – | Dominated |
| PLT | 47,742 | 14.18 | – | – | Dominated |
| US SAPI (high cut-off) | 47,073 | 14.18 | – | – | Dominated |
| YKL-40 (high cut-off) | 48,536 | 14.19 | – | – | Dominated |
| Fibrotest (high cut-off) | 47,896 | 14.22 | – | – | Dominated |
| PIIINP/MMP-1 index | 47,724 | 14.24 | – | – | Dominated |
| King’s (low cut-off) | 47,743 | 14.24 | – | – | Dominated |
| King’s (high cut-off) | 47,963 | 14.25 | – | – | Dominated |
| Fibrosis Index | 47,423 | 14.25 | – | – | Dominated |
| ARFI | 47,126 | 14.25 | – | – | Dominated |
| GUCI | 47,791 | 14.25 | – | – | Dominated |
| AST–ALT | 48,629 | 14.26 | – | – | Dominated |
| Age–Platelet Index | 47,847 | 14.26 | – | – | Dominated |
| MR | 47,101 | 14.26 | – | – | Dominated |
| EOB-MRI | 48,054 | 14.26 | – | – | Dominated |
| MR elastography | 46,896 | 14.27 | – | – | – |
| FIB-4 (high cut-off) | 48,158 | 14.27 | – | – | Dominated |
| CEUS | 47,215 | 14.28 | – | – | Extendedly dominated |
| APRI | 47,522 | 14.28 | – | – | Extendedly dominated |
| Fibroscan | 47,449 | 14.28 | – | – | Extendedly dominated |
| US SAPI | 47,763 | 14.29 | – | – | Extendedly dominated |
| DW-MRI | 47,890 | 14.30 | – | – | Dominated |
| Fibrotest | 48,327 | 14.30 | – | – | Dominated |
| Hyaluronic acid | 48,013 | 14.30 | – | – | Dominated |
| PIINP | 47,921 | 14.30 | – | – | Dominated |
| Hepascore | 48,189 | 14.31 | – | – | Dominated |
| Fibrometer | 48,104 | 14.32 | – | – | Dominated |
| MP3 | 48,008 | 14.33 | – | – | Dominated |
| Fibrospect | 48,210 | 14.33 | – | – | Dominated |
| Type IV collagen | 47,888 | 14.34 | – | – | Dominated |
| Hyaluronic acid low | 48,824 | 14.34 | – | – | Dominated |
| King’s | 47,990 | 14.34 | – | – | Dominated |
| ELF | 48,232 | 14.34 | – | – | Dominated |
| CT | 48,727 | 14.35 | – | – | Dominated |
| FibroQ | 48,372 | 14.35 | – | – | Dominated |
| PLT–Spleen | 47,803 | 14.35 | – | – | Extendedly dominated |
| Forns index | 50,555 | 14.37 | – | – | Dominated |
| Lok’s index | 49,077 | 14.38 | – | – | Dominated |
| APRI (low cut-off) | 48,713 | 14.40 | – | – | Extendedly dominated |
| CDS | 49,429 | 14.40 | – | – | Dominated |
| Fibroindex (low cut-off) | 48,872 | 14.40 | – | – | Extendedly dominated |
| ELF (low cut-off) | 49,041 | 14.44 | – | – | Extendedly dominated |
| FPI (low cut-off) | 49,232 | 14.47 | – | – | Extendedly dominated |
| FIB-4 (low cut-off) | 49,407 | 14.48 | – | – | Extendedly dominated |
| Forns index (low cut-off) | 49,571 | 14.49 | – | – | Dominated |
| Fibrotest (low cut-off) | 49,534 | 14.49 | – | – | Extendedly dominated |
| YKL-40 (low cut-off) | 50,156 | 14.50 | – | – | Dominated |
| US SAPI (low cut-off) | 49,561 | 14.51 | – | – | Extendedly dominated |
| Treat all | 51,241 | 14.73 | 4,345 | 0.46 | 9,351 |
| Test strategy | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Treat none | 54,878 | 12.45 | – | – | Dominated |
| Liver biopsy | 48,710 | 14.03 | – | – | Dominated |
| (S3) Type IV collagen and PLT–Spleen | 47,099 | 14.16 | – | – | Dominated |
| (S3) King’s and PLT–Spleen | 47,139 | 14.16 | – | – | Dominated |
| (S3) King’s and type IV collagen | 47,113 | 14.16 | – | – | Dominated |
| Hepascore (combined cut-off) and Fibroscan | 47,675 | 14.19 | – | – | Dominated |
| (S3) FPI (low cut-off) and type IV collagen | 47,417 | 14.21 | – | – | Dominated |
| (S3) FPI (low cut-off) and PLT–Spleen | 47,461 | 14.21 | – | – | Dominated |
| (S2) King’s and type IV collagen | 47,001 | 14.21 | – | – | Dominated |
| (S2) King’s and PLT–Spleen | 46,999 | 14.21 | – | – | Dominated |
| Fibroindex (combined cut-off) and Fibroscan | 47,368 | 14.21 | – | – | Dominated |
| (S3) Fibrotest (low cut-off) and PLT–Spleen | 47,548 | 14.21 | – | – | Dominated |
| (S4) King’s and type IV collagen | 46,978 | 14.21 | – | – | Dominated |
| (S4) King’s and PLT–Spleen | 46,965 | 14.22 | – | – | Dominated |
| (S3) King’s and Fibrotest (low cut-off) | 47,579 | 14.22 | – | – | Dominated |
| (S3) Type IV collagen and US SAPI (low cut-off) | 47,516 | 14.22 | – | – | Dominated |
| (S4) Type IV collagen and PLT–Spleen | 46,911 | 14.22 | – | – | – |
| (S2) Type IV collagen and PLT–Spleen | 46,994 | 14.22 | – | – | Dominated |
| (S3) King’s and US SAPI (low cut-off) | 47,606 | 14.22 | – | – | Dominated |
| Leroy | 47,248 | 14.23 | – | – | Dominated |
| (S4) FPI (low cut-off) and type IV collagen | 47,328 | 14.24 | – | – | Dominated |
| (S2) FPI (low cut-off) and type IV collagen | 47,328 | 14.24 | – | – | Dominated |
| (S2) FPI (low cut-off) and PLT–Spleen | 47,359 | 14.24 | – | – | Dominated |
| (S4) FPI (low cut-off) and PLT–Spleen | 47,347 | 14.24 | – | – | Dominated |
| (S2) Fibrotest (low cut-off) and PLT–Spleen | 47,519 | 14.24 | – | – | Dominated |
| (S4) Fibrotest (low cut-off) and PLT–Spleen | 47,446 | 14.24 | – | – | Dominated |
| (S4) King’s and Fibrotest (low cut-off) | 47,521 | 14.25 | – | – | Dominated |
| (S4) Type IV collagen and US SAPI (low cut-off) | 47,427 | 14.25 | – | – | Dominated |
| Forns index (combined cut-off) and Fibroscan | 47,233 | 14.25 | – | – | Dominated |
| (S4) King’s and US SAPI (low cut-off) | 47,525 | 14.25 | – | – | Dominated |
| (S1) Type IV collagen | 48,155 | 14.25 | – | – | Dominated |
| (S1) PLT–Spleen | 48,233 | 14.26 | – | – | Dominated |
| (S1) King’s | 48,375 | 14.26 | – | – | Dominated |
| Fibropaca | 47,545 | 14.26 | – | – | Dominated |
| APRI (combined cut-off) and Fibroscan | 47,355 | 14.26 | – | – | Dominated |
| (S2) King’s and Fibrotest (low cut-off) | 47,467 | 14.26 | – | – | Dominated |
| Fibrospect (combined cut-off) and Fibroscan | 46,954 | 14.27 | 43.55 | 0.05 | 928 |
| (S2) King’s and US SAPI (low cut-off) | 47,466 | 14.27 | – | – | Dominated |
| Bordeaux | 47,026 | 14.27 | – | – | Extendedly dominated |
| ELF (combined cut-off) and Fibroscan | 47,533 | 14.28 | – | – | Dominated |
| (S2) Type IV collagen and US SAPI (low cut-off) | 47,411 | 14.28 | – | – | Extendedly dominated |
| Hyaluronic acid and Fibroscan | 47,770 | 14.29 | – | – | Dominated |
| YKL-40 (combined cut-off) and Fibroscan | 48,251 | 14.31 | – | – | Dominated |
| FIB-4 (combined cut-off) and Fibroscan | 47,739 | 14.31 | – | – | Dominated |
| Fibrotest (combined cut-off) and Fibroscan | 47,748 | 14.31 | – | – | Extendedly dominated |
| SAFE | 47,985 | 14.33 | – | – | Dominated |
| Type IV collagen | 47,882 | 14.34 | – | – | Dominated |
| King’s | 47,976 | 14.34 | – | – | Dominated |
| PLT–Spleen | 47,874 | 14.35 | – | – | Extendedly dominated |
| (S1) FPI (low cut-off) | 49,467 | 14.42 | – | – | Dominated |
| (S1) Fibrotest (low cut-off) | 49,699 | 14.44 | – | – | Dominated |
| (S1) US SAPI (low cut-off) | 49,746 | 14.46 | – | – | Dominated |
| FPI (low cut-off) | 49,218 | 14.47 | – | – | Extendedly dominated |
| Fibrotest (low cut-off) | 49,536 | 14.49 | – | – | Dominated |
| US SAPI (low cut-off) | 49,549 | 14.51 | – | – | Extendedly dominated |
| Treat all | 51,241 | 14.73 | 4,287 | 0.47 | 9204 |
Sensitivity analyses
The base-case analysis result remained robust to the majority of the sensitivity analyses. One of the analyses, where we held the utility values constant at baseline values (values did not differ before, during or post treatment), did increase the ICER for the ‘treat all’ scenario to £16,727; however, assuming a cost-effectiveness threshold of £20,000, this would still be an acceptable ICER. All other results returned a similar ICER value to the base-case analysis.
Appendix 11 contains the short tables of incremental analysis results for all sensitivity analyses (excluding ‘dominated’ and ‘extendedly dominated’ test strategies; full tables are available on request).
Threshold sensitivity analysis
Treatment benefit varied for incorrectly diagnosed patients in mild health state.
Varying the effectiveness of treatment in patients with mild fibrosis (F0–1) affects the results of the analysis. Assuming that no patients in a mild health state (F0–1) receive benefit from treatment returns a result where the ‘treat all’ strategy is dominated (i.e. it is more costly and less effective than other strategies). We then reduced the effectiveness of the treatment in patients with mild fibrosis (modelled using the SVR rate) by decrements of 10%.
Reducing the SVR rates in the model by 90%, 80% and 70% returned a result where ‘treat all’ remained dominated by other strategies. When we reduced the SVR rate by 60%, the ‘treat all’ strategy was the most effective strategy but with an ICER of £723,503. The most cost-effective testing strategy to use for these stages was to test with an imaging modality (MR elastography).
When we reduced the SVR rate by 50%, ‘treat all’ became the most effective strategy but not the most cost-effective with an ICER of £92,995; testing with MR elastography was the most cost-effective strategy. Given a cost-effectiveness threshold of £20,000, the ‘treat all’ strategy is no longer cost-effective when the SVR rate is reduced by approximately 23% or more. Figure 8 plots the decreasing ICER value for the ‘treat all’ strategy relative to the probability of an increased successful response rate to treatment (SVR rate) for patients in a mild health state.
FIGURE 8.
Illustration of the reduction in the ‘treat all’ ICER when the SVR rate for F0–1 patients is varied.
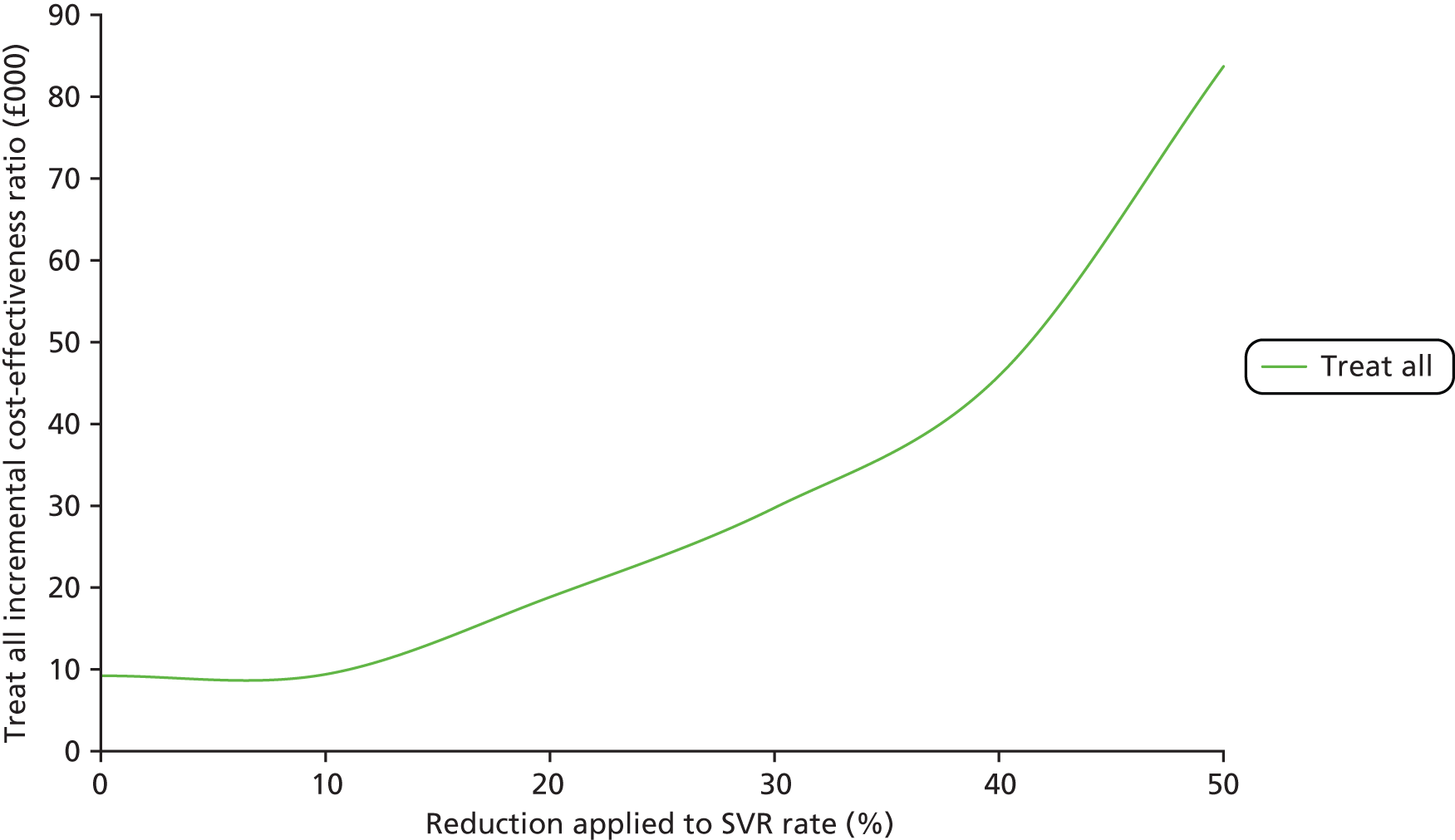
Increased effectiveness of cost and antiviral treatment
Changing both the SVR rate and increasing the cost by £20,000 (to reflect the new drug treatment) does not change the base-case results, with ‘treat all’ remaining the most cost-effective strategy with an ICER of £10,009.
Increasing the additional cost by £40,000 (with increased SVR rates) does change the results where the ICER for treat all is now £21,174, which would be not cost-effective given a £20,000 threshold. The most cost-effective option to adopt would be MR elastography, with an ICER of £9189.
Illustration of the reduction in the ‘treat all’ ICER when the SVR rate for F0–1 patients is varied.
Discussion
The analysis found that the most cost-effective option to adopt is one where all patients are treated irrespective of fibrosis stage. This result held when compared with individual tests and strategies incorporating combinations of multiple tests, and for all cost-effectiveness threshold ranges above approximately £10,000. It was also robust to most of the amendments in the sensitivity analysis. When we compared all tests singly, all other tests were either dominated or extendedly dominated, except for MR elastography which, for these very low cost-effective threshold values, was the most cost-effective strategy.
National Institute for Health and Care Excellence guidance for peginterferon alfa and ribavirin treatment in patients with HCV recommends two options for treatment in patients with mild chronic HCV: treat immediately or adopt a watchful waiting approach. The guidelines noted that initiating treatment at an earlier stage bypasses the need for an invasive liver biopsy tests. However, with the introduction of NILTs such as MR elastography and Fibroscan, less invasive testing to stage fibrosis is possible. 443
A key driver in the cost-effectiveness results is that patients with milder degrees of fibrosis (e.g. F0–1) gain benefit from treatment, albeit at an increased cost. Therefore, the ‘treat all’ strategy and the tests with highest sensitivity tend to have better results. The assumption that the efficacy of antiviral therapy is similar for patients with mild HCV compared with those with histologically more advanced HCV457,465 was tested in a threshold sensitivity analysis which found that for the ‘treat all’ strategy to cease to be cost-effective, the SVR rate following treatment for patients with mild fibrosis would need to be reduced by 23%. Once we reduced the SVR rate by 23%, MR elastography became the most cost-effective strategy; this was the NILT with the highest sensitivity and specificity (94% and 92%, respectively).
A 2006 study by Grieve et al. 457 analysed if it was cost-effective to treat patients at a mild stage compared with waiting till patients progressed to a moderate disease stage; they found that it was generally more effective to provide antiviral treatment at a mild rather than a moderate disease stage and this strategy would gain improved outcomes (QALYs) rather than treating at a later stage.
However, treating patients with mild chronic HCV (who may not necessarily require treatment) exposes this patient cohort to the risk of side effects associated with peginterferon alfa and the direct antiviral agents, boceprevir and telaprevir. Newer drugs may provide more effective treatment with fewer side effects, so waiting to treat this patient cohort may be a better option. 466 If we were to adopt this strategy, then testing all patients with MR elastography would be the most cost-effective option (when using a NILT singly).
New antiviral treatments for HCV are currently undergoing trials which indicate that these drugs may be more effective in genotype 1 and 4 HCV infections than current triple therapy. 43 However, the cost-effectiveness of such drugs will depend on the price of the new drug and robust data on effectiveness. Currently evidence is promising but limited to uncontrolled studies and a non-inferiority study demonstrating equivalence. If the SVR rates observed in the studies are borne out in practice, then the ‘treat all’ strategy could still be cost-effective if the overall impact on treatment costs is an increase of £20,000, but not for an increase of £40,000.
When all tests were compared singly, the tests with low threshold values for classification of fibrosis tended to have relatively higher health outcomes than tests without threshold or with high cut-off thresholds. These tests tended to have high sensitivity values and low specificity values; when we conducted the threshold sensitivity analysis around treatment benefit, NILTs with lower cut-off thresholds no longer yielded the highest health outcomes. Rather, tests with both a high sensitivity and specificity such as MR elastography had the higher QALY gain, indicating that when patients in a mild health state are treated, tests which are more likely to identify more persons as positive (i.e. treat more persons) will be more effective.
As some of the tests analysed have few studies on which their diagnostic accuracy is based, this means that some tests may have results which overestimate their effectiveness. Reducing the number of tests to those where only the bivariate model converged did not change the overall result; however, only 14 NILTs were compared at this stage, illustrating that for the majority of tests the results of the meta-analysis may not be very robust. When we removed ‘treat all’ from this analysis, Fibroscan was the most cost-effective testing option to use.
We have considered the SVR a good surrogate marker of treatment efficacy. This is similar to current NICE guidance for boceprevir and telaprevir; however, our results are applicable only if the SVR rate is a good surrogate marker and may change if other methods of determining treatment efficacy are developed.
The results indicate that treating all patients is cost-effective; however, this result is sensitive to changes in treatment response rates for those with mild fibrosis.
Chapter 7 Cost-effectiveness analysis: alcoholic liver disease
This chapter details the analysis approach for ALD and includes details of the model structure, inputs and results. The population considered for analysis were people with ALD who were suspected of having developed alcoholic steatohepatitis (ASH) with cirrhosis.
Background
Five NILTs were identified in the systematic review for staging liver fibrosis and cirrhosis in ALD: APRI with a (high cut-off) diagnostic threshold, Fibrotest (high cut-off), Fibrotest (low cut-off), PGAA and Fibroscan.
Current clinical management of alcoholic cirrhosis focuses on alcohol abstinence, aggressive nutritional therapy rich in calories and proteins, and primary and secondary prophylaxis of cirrhosis complications. 35 If required and available, addiction specialists, motivational therapy and anticraving drugs are also recommended. 35
The European Association for the Study of the Liver (EASL) guidelines note that patients with ASH and cirrhosis are at risk of developing clinical decompensation, liver failure and HCC. However, prolonged abstinence can reverse previously decompensated cirrhosis to a compensated state. As the incidence of HCC among patients with alcoholic cirrhosis ranges from 7% to 16% after 5 years, the guidelines recommend that screening for HCC should be performed with ultrasound every 6 months, as recommended for any patient with cirrhosis. Screening for alcohol-induced damage in other organs (heart, kidneys) should also take place.
The guidelines do note that specific therapies have been tested in patients with alcoholic cirrhosis (including S-adenosyl-l-methionine, anabolic-androgenic steroids); however, none has any consistent beneficial effects. 35
Liver transplantation is recommended for patients who remain abstinent for a 6-month period before being added to the waiting list for liver transplantation. 35 The 6-month period is recommended to capture those patients who may recover from their liver disease and also to identify the subset of patients who may remain abstinent after a liver transplant. NICE guidance recommends that patients who still have decompensated liver disease after 3 months of best management and abstinence from alcohol and who are suitable candidates for liver transplantation should be referred to assessment for liver transplantation and considered this to be a cost-effective treatment option. 467
The EASL and NICE guidelines do not recommend a specific treatment applicable after diagnosis with fibrosis or cirrhosis; rather, they recommend that, regardless of the severity, abstinence and early management of alcohol abuse or dependence is warranted in all patients with ASH. Therefore, we were unable to use the same approach for a cost-effectiveness analysis as for HBV and HCV. Instead, we focus on the health economic impact of diagnosis as a result of abstinence, assuming that abstinence may increase as a result of diagnosis of fibrosis.
Literature review results
We identified a recent systematic review and economic analysis of non-invasive diagnostic assessment tools for the detection of liver fibrosis in patients with suspected alcohol-related liver disease;428 the non-invasive tests analysed in this report were ELF, Fibrotest, Fibroscan (TE) and Fibromax.
The tests considered in the analysis by Stevenson et al. 428 differed from the tests found during our literature review. However, this study did not find any data on diagnostic accuracy for one of the NILTs, Fibromax. With regard to the diagnostic accuracy of the ELF test, the authors located one study that looked at the European liver fibrosis test (ELF and age)468 which had reported findings for diagnosis of moderate to severe fibrosis; however, the diagnostic accuracy for identifying cirrhosis was not reported. The authors concluded that the data with regard to the diagnostic accuracy of the ELF test in relation to cirrhosis are not robust.
The review was considered highly relevant to our decision problem and population of interest. We carried out a literature search for cost-effectiveness studies of non-invasive tests in patients with ALD using the search strategy detailed in the Health Technology Asessment (HTA) report by Stevenson et al. 428 We updated the search to include the five NILTs analysed for ALD. We conducted the search using the MEDLINE database (via Ovid platform, searched 25 June 2013, all years searched). The search strategy is detailed in Appendix 2.
The search located 264 papers; titles were reviewed and the only relevant study found was the economic analysis detailed in the HTA report by Stevenson et al. 428
The report by Stevenson et al. 428 estimated the incremental costs and incremental QALYs for 10 strategies which were based on using a NILT alone to confirm cirrhosis or a combination of a NILT and liver biopsy. The authors assumed that all patients in the model would receive lifestyle and abstinence advice. If the test outcome was positive, the treatment strategy would be expanded to include monitoring for HCC, hepatic encephalopathy and oesophageal varices.
Cost-effectiveness analysis
The analysis conducted by Stevenson et al. 428 was considered relevant, as they also considered the use of non-invasive diagnostic testing in patients with ALD. They conducted their analysis from a UK perspective and their considered population was deemed to be similar (patients with ALD suspected of having cirrhosis). The model structure employed in this study seemed appropriate given current clinical practice and the data inputs for costs and QALYs were disease specific (based on data for ALD).
We replicated the model to include the tests relevant to our decision problem and also conducted some sensitivity analyses where we assessed the impact of alternative inputs and adjustments to the model structure. To replicate the model, we constructed a decision tree model to assess the cost-effectiveness of the non-invasive tests in patients with ALD.
Decision tree model
The following section (section 1) provides a brief description of the Stevenson et al. 428 model, which we replicated. This includes a summary of the inputs which we also employed in our model. We then detail (see section 2) the changes we made to the model for our analysis (updated inputs, different non-invasive tests and diagnostic accuracy).
Section 1: model structure
The relevant patient population to be assessed with either a NILT or liver biopsy would be those patients suspected of having liver cirrhosis (F4).
The care pathway reflected in the model is that a positive test result (true positive or false positive) indicated that the patient would receive monitoring for HCC, varices, ascites and hepatic encephalopathy; lifestyle advice would also be given. Given a negative test result (true negative or false negative), the patient would receive lifestyle advice only, including the recommendation to become abstinent or to reduce alcohol consumption.
Abstinence rates following diagnosis with liver biopsy
Only one study had been identified regarding abstinence rates following diagnosis, which was a small sample (n = 96) and had only been published in abstract form (we conducted an updated search on 26 June 2013 and were unable to find a published paper for the study). This study reported that after diagnosis with liver biopsy, 31% of those with a negative test result for cirrhosis became abstinent, whereas 62% of those with a positive test result for cirrhosis became abstinent. No specific data were found on abstinence rates following a NILT.
Probability of developing cirrhosis following diagnosis
There is a probability that patients who continue to drink after diagnosis may develop cirrhosis (false positive and true negative patients). This was set at 20% following clinical advice.
Quality-adjusted life-years
Quality-adjusted life-year estimates were sourced from a HTA report on HCC screening by Thompson Coon et al. 469 The QALY value reported in the Thompson Coon et al. study for ALD patients with cirrhosis who undergo annual serum α-fetoprotein with 6-monthly ultrasound scans was used as an estimate for patients who test true positive and stop drinking. For true-positive patients who continue to drink, QALY value was based on survival rates for cirrhotic patients who continued to drink derived from a study by Verrill et al. 470
For true-negative patients who abstain from drinking, the QALY value was estimated using the average age from the study by Thompson Coon et al. 469 (53 years) and EQ-5D population norms (assuming that this population would live for a further 20 years). In the absence of further data, it was assumed that the QALY for false positives who abstain from drinking would be equivalent to that of true negative patients who abstain. For false-positive patients who continue to drink, the study conservatively assumed the same QALY; however, it was assumed that the QALY for the proportion of false-negative patients and true-negative patients who continue to drink and subsequently develop cirrhosis would be the same as that for true positives who continue to drink.
A QALY value for false-negative patients who abstain from drinking was sourced from the Thompson Coon et al. 469 study. For false-negative patients who continue to drink, the QALY value was set at 40% of the value of false-negative patients who abstain.
Estimates (where applicable) were adjusted for differing survival rates (abstainers vs. non abstainers) and for oesophageal bleeds. QALY estimates are detailed in Table 42.
| QALY end points | QALY |
|---|---|
| TP compensated cirrhosis (abstain drinking) | 9.679 |
| TP compensated cirrhosis (continue drinking) | 4.399 |
| FP compensated cirrhosis (abstain drinking) | 11.066 |
| FP compensated cirrhosis (continue drinking) | 11.066 |
| TN compensated cirrhosis (abstain drinking) | 11.066 |
| TN compensated cirrhosis (continue drinking) | 11.066 |
| FN compensated cirrhosis (abstain drinking) | 9.359 |
| FN compensated cirrhosis (continue drinking) | 3.744 |
Adverse events and mortality risk
The risk for adverse events (0.72%) and mortality (0.09%) associated with liver biopsy was estimated following a systematic review.
Liver biopsy adverse events costs and quality-adjusted life-years
A cost of £1000 (reflect hospital stay) and a QALY decrement of 0.2 associated with a serious adverse effect resulting from liver biopsy applied in the model.
Section 2: amendments to model
Test strategies assessed
We analysed 10 potential testing strategies incorporating the applicable NILTs. The strategies analysed the use of all tests either alone (biopsy or a NILT) or in combination. We also included a comparator where all patients receive treatment for cirrhosis (HCC screening) without testing (similar to ‘treat all’ strategies from the HBV and HCV models).
-
Biopsy all patients.
-
Test all patients with APRI high cut-off and biopsy those in whom cirrhosis is indicated.
-
Test all patients with Fibrotest high cut-off and biopsy those in whom cirrhosis is indicated.
-
Test all patients with Fibrotest low cut-off and biopsy those in whom cirrhosis is indicated.
-
Test all patients with PGAA and biopsy those in whom cirrhosis is indicated.
-
Test all patients with Fibroscan and biopsy those in whom cirrhosis is indicated.
-
Use APRI high cut-off and assume result is definite.
-
Use Fibrotest high cut-off and assume result is definite.
-
Use Fibrotest low cut-off and assume result is definite.
-
Use PGAA and assume result is definite.
-
Use Fibroscan and assume result is definite.
-
Diagnose all patients as having cirrhosis.
Abstinence rates following diagnosis with a non-invasive liver test
As abstinence rates are based on clinical practice and experience, it is plausible to assume that biopsy as an invasive procedure which may include hospitalisation and the risk of side effects such as fatal bleeding may serve as a larger warning for patients (patients may be more likely to comply after a positive biopsy result than after a positive result with a NILT). So, we assumed a lower rate of abstinence for patients who were tested with a NILT. In the base case, we assumed the rate for abstinence after diagnosis with a NILT would be 10% lower than the abstinence rates after biopsy. As the abstinence figures after a NILT are not known with certainty and these figures may potentially have a large impact on the decision tree outcomes, we undertook a number of sensitivity analyses where we decreased and increased the abstinence values applicable after diagnosis with a NILT.
The EASL position paper for ALD55 notes that 40% of patients with ALD who have already developed fibrosis and continue to drink will develop cirrhosis. We received clinical advice on this and in the general population; the consensus is that 10–30% of patients will develop cirrhosis if they continue to drink. However, as some persons may not develop fibrosis irrespective of drinking patterns (as developing fibrosis is genetically determined), the figure may be slightly higher (40%) in those who have developed some degree of fibrosis, making them more prone to developing cirrhosis. We used the base-case value of 20% in the main analysis and conducted sensitivity analyses around this parameter.
Costs
We inflated the costs used in the study by Stevenson et al. 428 from 2008–9 to 2012 prices using NHS inflation indices. The costs used in the decision tree are displayed in Table 43.
| End point decision tree costs 2012 | Cost, £ |
|---|---|
| True positive compensated cirrhosis (abstain drinking) | 32,080 |
| True positive compensated cirrhosis (continue drinking) | 42,239 |
| False positive compensated cirrhosis (abstain drinking) | 26,916 |
| False positive compensated cirrhosis (continue drinking) | 26,916 |
| True negative compensated cirrhosis (abstain drinking) | 1070 |
| True negative compensated cirrhosis (continue drinking) | 1070 |
| False negative compensated cirrhosis (abstain drinking) | 27,928 |
| False negative compensated cirrhosis (continue drinking) | 38,628 |
The costs had been sourced from a HTA report which looked at the costs associated with screening persons with ALD for HCC. 469 The authors also used the costs for mild and moderate fibrosis health states for HCV based on the mild HCV trial414 described in Chapter 6 to modify the costs of true-positive patients progressing to a more severe state (decompensated cirrhosis) if they continued to drink and for false-positive patients who abstained from further drinking. Estimates for the true-negative end points were assumed based on clinical advice. The costs of monitoring and treatment of oesophageal bleeding and the cost of detecting a hepatic encephalopathy were included.
Estimation of probabilities of a non-invasive liver test returning a true-positive, false-positive, true-negative or false-negative result
The average prevalence of disease (defined as METAVIR score of F4) was taken from the average prevalence reported in papers included in the meta-analysis (see Chapter 4). We used the sensitivity and specificity estimates for each NILT (see Chapter 4 for estimates) and the average prevalence estimate to calculate the probability of each test returning a true-positive, false-positive, true-negative and false-negative result (see Appendix 7).
Test costs
Costs for APRI and PGAA indirect serum markers were obtained from communication with finance departments based at the Royal Free Hospital. Costs for patented serum markers (Fibrotest) were sourced directly from manufacturers and via communication with finance departments based at the Royal Free Hospital. A test cost for Fibroscan was sourced as per clinical advice; we used the 2011–12 Department of Health reference costs for an ultrasound with duration of < 20 minutes. The cost of a percutaneous liver biopsy (see Chapter 5 for further details on choice of liver biopsy) was sourced from published literature428 (see Chapter 5). Test costs including sources are listed in Appendix 9.
Analysis
A PSA was undertaken and we conducted an incremental analysis of the results. A CEAC and CEAF were constructed. We also carried out univariate sensitivity analyses and conducted two analyses where we amended the structure of the ALD model. A scatterplot illustrating the position of each testing strategy on the cost-effectiveness acceptability curve compared with the least costly testing strategy can be found in Appendix 12.
Sensitivity analyses
Amendments to model inputs
Abstinence rates
We amended the abstinence rates assumed in the model following diagnoses with a NILT. We set the rates equivalent to those for liver biopsy and then increased the rates in increments of 10% to determine when the base-case result changed.
Probability of developing cirrhosis
We set the rate to five different values (10%, 30%, 40%, 50% and 60%) to test the impact on results. The rates chosen reflected the fact that we do not know the exact fibrosis level of patients who test true negative or false positive, and so this range allows us to test the impact of a change in this parameter on results.
Mortality rates and adverse events
We varied these in the model using a range of lower and upper estimates.
Amendments to model structure
Additional health states
We conducted an analysis where we allowed for progression to HCC and subsequent liver transplant. This is not included as an option in the Stevenson et al. 428 model as they argued that current evidence shows that it is of borderline cost-effectiveness and would not have an impact on the cost-effectiveness of a NILT. However, as the NILT may affect the care pathway (including decision to continue drinking which may indicate further progression to more severe health states), and as ALD is one of the main reasons for liver transplantation in the UK, we included liver transplant as an option in the care pathway. Furthermore, in the CELT study of transplantation424 it was found that the larger incremental cost-per-QALY ratio for ALD patients was in part due to a larger proportion of ALD patients being considered unsuitable for transplantation after undergoing the assessment process. Since this study was conducted, clear guidelines have been agreed by the six liver transplant centres within the UK and endorsed by the UK Liver Advisory Group to include careful assessment of psychosocial and substance use factors for patients with a diagnosis of ALD. 471
Cost and QALY estimates were sourced from the CELT study. 424 Upon clinical advice, we set the estimate for the percentage of patients with ALD who have a liver transplant to 4%, to reflect the number of patients with alcoholic cirrhosis who may potentially develop a tumour while abstinent.
Different starting population-advanced fibrosis (≥ F3)
An additional analysis was undertaken where we used a different section of the data as our starting population cohort: our base-case population cohort consists of patients who are suspected of having cirrhosis; the sensitivity analysis analysed the impact of substituting different data, representing patients who are suspected of having advanced fibrosis (F3), from the meta-analysis.
Results
Base-case results imply that the most cost-effective strategy is to use liver biopsy only to diagnose cirrhosis in patients with ALD, with an ICER of £822 (Table 44). The strategy producing the highest QALY gain is where all patients suspected of having cirrhosis receive monitoring; however, this is not the most cost-effective with an ICER of £70,861, which is above the standard UK cost-effectiveness threshold range. 66
| Strategy | Tests | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|---|
| Strategy 2 | APRI (high cut-off) and liver biopsy | 17,415 | 8.79 | – | – | – |
| Strategy 7 | APRI (high cut-off) | 22,463 | 8.87 | – | – | Dominated |
| Strategy 10 | PGAA | 19,061 | 8.98 | – | – | Dominated |
| Strategy 11 | Fibroscan | 20,009 | 9.02 | – | – | Dominated |
| Strategy 8 | Fibrotest (high cut-off) | 19,504 | 9.03 | – | – | Dominated |
| Strategy 5 | PGAA and liver biopsy | 17,613 | 9.07 | 198 | 0.28 | 701 |
| Strategy 9 | Fibrotest (low cut-off) | 24,671 | 9.13 | – | – | Dominated |
| Strategy 6 | Fibroscan and liver biopsy | 17,702 | 9.14 | – | – | Extendedly dominated |
| Strategy 3 | Fibrotest (high cut-off) and liver biopsy | 17,724 | 9.17 | – | – | Extendedly dominated |
| Strategy 4 | Fibrotest (low cut-off) and liver biopsy | 17,801 | 9.26 | – | – | Extendedly dominated |
| Strategy 1 | Liver biopsy | 17,812 | 9.31 | 199 | 0.24 | 822 |
| Strategy 12 | All patients treated as having cirrhosis (receive HCC screening) | 31,004 | 9.50 | 13,193 | 0.19 | 70,861 |
We constructed a CEAC (see Appendix 10) and CEAF (Figure 9). The CEAF shows that strategy 2 (liver biopsy) has the highest probability of being the optimal choice (highest expected net benefit) for threshold values of £822 and above.
FIGURE 9.
Cost-effectiveness acceptability frontier for ALD.
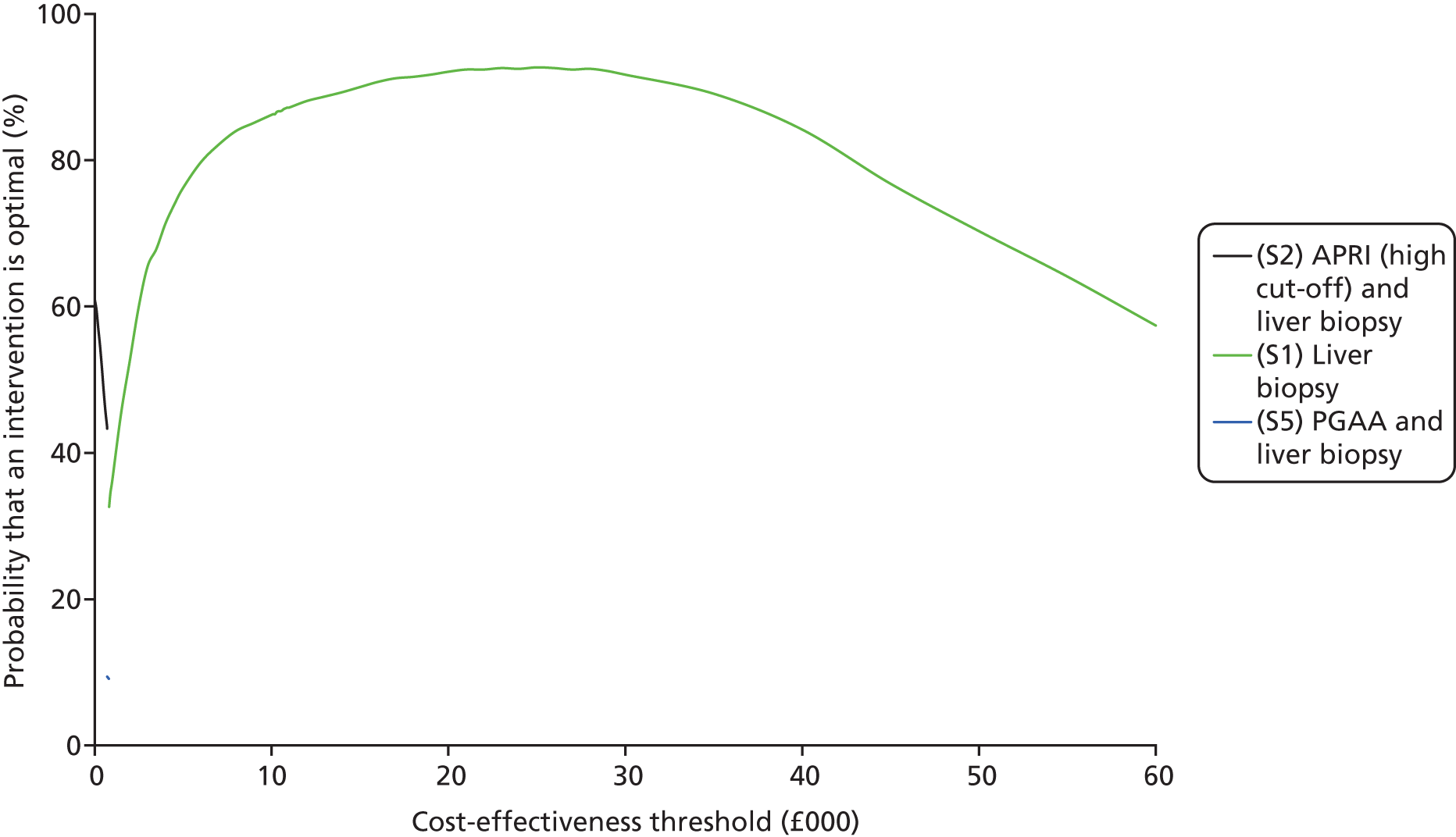
The CEAC presented in Appendix 10 shows that strategy 1, liver biopsy, has the highest probability of being cost-effective (92%) given a cost-effectiveness threshold of £20,000 and this probability falls as the cost-effectiveness threshold increases (for clarity of illustration, only strategies which have a ≥ 10% probability of being the optimal strategy are shown in the CEAC).
Sensitivity analysis results
Univariate sensitivity analyses
A number of one-way sensitivity analyses were conducted around parameters in the model for which the data were assumed or advised by clinical opinion (abstinence rates, probability of developing cirrhosis if patient continues to drink). Table 45 lists the ranges over which the values were varied and whether or not this had any impact on the base-case result.
| Parameter | Base-case value | Extreme values tested | Impact on cost-effectiveness |
|---|---|---|---|
| Probability of developing cirrhosis (continue drinking FP and TN patients) | 20% | 10–50% | No change |
| Probability of developing cirrhosis (continue drinking FP and TN patients) | 20% | 60% | All treated as cirrhotic (receive HCC screening) becomes cost-effective option |
| Mortality rate following liver biopsy | 0.09% | 0.05–0.20% | No change |
| Adverse event rate following liver biopsy | 0.72% | 0.05–0.90% | No change |
Change in abstinence rate following diagnosis with a non-invasive liver test (diagnosis of cirrhosis and no cirrhosis)
The base-case values used in the model for abstinence rates after diagnosis with a NILT were 52% following a diagnosis of cirrhosis and 31% following a diagnosis of no cirrhosis. We conducted a number of analyses varying these rates (Table 46).
| Change in abstinence rate | Rate following diagnosis: cirrhosis | Rate following diagnosis: no cirrhosis | Impact cost-effectiveness |
|---|---|---|---|
| Increase 10% | 62% | 41% | Fibrotest (low cut-off) and liver biopsy becomes most cost-effective testing option |
| Increase 15% | 67% | 46% | Fibrotest (high cut-off) becomes most cost-effective test |
| Increase 20% | 72% | 51% | Fibrotest (high cut-off) becomes most cost-effective test |
When the abstinence rates after diagnosis with a NILT were set equivalent to the abstinence rates after a liver biopsy (increase of 10%), the most cost-effective testing option was strategy 4, a combination of Fibrotest (low cut-off) as an initial test and liver biopsy as a second test to confirm positive diagnoses, with an ICER of £6366.
However, when we increased the abstinence rates following diagnosis with a NILT by 15% and 20%, this impacted the results where Fibrotest (high cut-off) became the most cost-effective test with an ICER of £13,115 and £5896, respectively. This test has the highest sensitivity and specificity (91% and 87%).
Increase in abstinence rate after diagnosis of no cirrhosis with a non-invasive liver test
We conducted an analysis where we only increased the probability that patients would become abstinent after a diagnosis of no cirrhosis (using a NILT), leaving all other base-case values constant. We increased the base-case value of 31% by 10% and 20%. Increasing the abstinence rate by 10% changed the result and strategy 4 [Fibrotest (low cut off) with liver biopsy] used as a second test to confirm results became the most cost-effective with an ICER of £6833. When we increased the abstinence rate by 20%, using strategy 3 [Fibrotest (high cut off) as an initial test and liver biopsy used as a second test to confirm positive results] became the most cost-effective strategy with an ICER of £4730.
Amendments to model structure
Different starting population: advanced fibrosis (≥ F3)
An analysis where we incorporated a different starting population (F3) did not change the overall result: liver biopsy remained the most cost-effective testing option. Table 47 displays the results of the analysis.
| Strategy | Test strategy | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|---|
| Strategy 3 | Forns index (high cut-off) and liver biopsy | 21,135 | 8.30 | – | – | – |
| Strategy 8 | Forns index (high cut-off) | 22,085 | 8.36 | – | – | Dominated |
| Strategy 5 | YKL-40 and liver biopsy | 21,224 | 8.40 | – | – | Extendedly dominated |
| Strategy 10 | YKL-40 | 22,224 | 8.42 | – | – | Extendedly dominated |
| Strategy 9 | Fibroscan | 23,456 | 8.62 | – | – | Extendedly dominated |
| Strategy 7 | CK18 | 24,646 | 8.62 | – | – | Extendedly dominated |
| Strategy 2 | CK18 and liver biopsy | 21,486 | 8.78 | 351 | 0.49 | 722 |
| Strategy 4 | Fibroscan and liver biopsy | 21,547 | 8.80 | – | – | Extendedly dominated |
| Strategy 1 | Liver biopsy | 21,652 | 8.99 | 166 | 0.21 | 800 |
| Strategy 12 | All treated as cirrhotic (HCC screening) | 31,963 | 9.14 | 10,311 | 0.15 | 69,522 |
Additional health states
We allowed for persons with cirrhosis (who remained abstinent within the model) to have a small probability of undergoing a liver transplant (4%, based on clinical advice). We incorporated cost and QALY estimates sourced from the CELT study421 specifically for patients with ALD (cost estimate of £11,202 and QALY value of 0.58); we inflated the historic cost to 2012 prices (£17,741) using NHS inflation indices.
The results are presented in Table 48. Liver biopsy remains the most cost-effective testing option, with an ICER of £688. However, similar to the base-case analysis, this result is dependent on the abstinence rates used in the analysis. When we amend the abstinence rate after diagnosis with a NILT so that it became equivalent to the abstinence rate after diagnosis with a liver biopsy, the results change, so that strategy 4 [Fibrotest (low cut off with liver biopsy to confirm positive results)] becomes the most cost-effective, with an ICER of £4756.
| Strategy | Test strategy | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|---|
| Strategy 2 | APRI (high cut-off) and liver biopsy | 17,637 | 8.80 | – | – | – |
| Strategy 7 | APRI (high cut-off) | 22,538 | 8.88 | – | – | Dominated |
| Strategy 10 | PGAA | 19,213 | 8.99 | – | – | Dominated |
| Strategy 11 | Fibroscan | 20,148 | 9.03 | – | – | Dominated |
| Strategy 8 | Fibrotest (high cut-off) | 19,638 | 9.04 | – | – | Dominated |
| Strategy 5 | PGAA and liver biopsy | 17,928 | 9.09 | – | – | Extendedly dominated |
| Strategy 9 | Fibrotest (low cut-off) | 24,845 | 9.14 | – | – | Dominated |
| Strategy 6 | Fibroscan and liver biopsy | 18,027 | 9.16 | – | – | Dominated |
| Strategy 3 | Fibrotest (high cut-off) and liver biopsy | 18,055 | 9.19 | – | – | Dominated |
| Strategy 4 | Fibrotest (low cut-off) and liver biopsy | 18,077 | 9.28 | – | – | Dominated |
| Strategy 1 | Liver biopsy | 18,000 | 9.33 | 363 | 0.53 | 688 |
| Strategy 12 | All treated as cirrhotic (receive HCC screening) | 30,705 | 9.42 | 12,705 | 0.10 | 133,479 |
Cost per correct diagnosis analysis
We also constructed a simple probabilistic decision model to assess the cost per correct diagnosis of the applicable NILTs compared with liver biopsy. As for the decision tree analysis, fibrosis prevalence in the model was estimated using a section of the meta-analysis equivalent to the proportion of the population with a test score of ≥ F4, measured using the METAVIR score. Based on the diagnostic accuracy of each test, the patient was classified as true positive, true negative, false positive or false negative. True positives and true negatives were considered to be correct diagnoses.
Test costs
We employed the same test costs for the NILTs and liver biopsy as used in the decision tree model.
Analysis
We assumed that liver biopsy as the reference standard had perfect sensitivity and specificity, implying that biopsy accurately stages liver fibrosis. Using a hypothetical cohort of 1000 ALD patients suspected of having liver fibrosis, we estimated the incremental cost associated with each test compared with the next best alternative. Tests which had fewer numbers of true-positive or true-negative outcomes, with a higher test cost than other tests which had higher correct diagnoses, were ruled out of the analysis (dominated). We present the results separately for positive and negative diagnoses as the consequences are different for each. Results are shown in Tables 49 and 50.
| Test | Number of TPs using NILT | Test cost, £ | Number of incremental correct diagnoses | Incremental cost (test only), £ | Incremental cost per correct diagnosis (TP): each test compared with next best alternative (£/correct diagnosis gained) |
|---|---|---|---|---|---|
| APRI (high cut-off) | 144 | 4.05 | – | – | – |
| PGAA | 285 | 7.69 | 141 | 3.64 | Extendedly dominated |
| Fibroscan | 315 | 51.00 | – | – | Dominated |
| Fibrotest (high cut-off) | 334 | 43.60 | 190 | 39.55 | Extendedly dominated |
| Fibrotest (low cut-off) | 363 | 43.60 | 219 | 39.55 | 0.18 |
| Liver biopsy | 366 | 956.61 | 4 | 593.92 | 168 |
Liver biopsy also returns the highest correct number of negative responses (n = 634) (see Table 50); however, the incremental cost per correct positive diagnosis for using liver biopsy was £13.62, compared with an incremental cost per correct diagnosis of £0.02 associated with using the next best alternative, PGAA, as the diagnostic test.
| Test | Number of TNs using NILT | Test cost, £ | Number of incremental correct diagnoses | Incremental cost (test only), £ | Incremental cost per correct diagnosis (TN): each test compared with next best alternative (£/correct diagnosis gained) |
|---|---|---|---|---|---|
| Fibrotest F4 (low cut-off) | 317 | 43.60 | – | – | Dominated |
| APRI F4 (high cut-off) | 392 | 4.05 | – | – | – |
| Fibroscan F4 | 526 | 51.00 | – | – | Dominated |
| Fibrotest F4 (high cut-off) | 551 | 43.60 | – | – | Dominated |
| PGAA F4 | 564 | 7.69 | 172 | 3.64 | 0.02 |
| Liver biopsy | 634 | 956.61 | 70 | 948.92 | 13.62 |
Results of cost per correct diagnosis analysis
Column 2 in Table 49 shows the number of correct positive diagnoses using a NILT or liver biopsy. The highest number of correct positive diagnoses is given using a liver biopsy,366 which is estimated from the disease prevalence from the meta-analysis. Using Fibrotest (low cut-off) returns the next highest number of true positives. 363 The tests are compared incrementally with tests which provide fewer true-positive results at a higher test cost ruled out (dominated) of the analysis. Liver biopsy returned the greatest number of correct diagnoses; however, the incremental cost per correct positive diagnosis for using liver biopsy was £168 compared with an incremental cost per correct diagnosis of £0.18 associated with using the next best alternative, Fibrotest (low cut-off), as the diagnostic test.
Discussion
Liver biopsy is the most cost-effective testing option to adopt given our model at the standard UK cost-effectiveness threshold range. This result is sensitive to assumptions about the abstinence rates after diagnosis, difference in abstinence between people tested with biopsy and NILT, and rates of progression to cirrhosis rates in patients who continue to drink. More accurate data on these parameters are needed in order to reduce uncertainty in these results.
Our results were particularly sensitive to changes in the abstinence rate after diagnosis with a NILT (holding the rates for liver biopsy constant). When we raise the abstinence rates after diagnosis with a NILT, the test with the highest sensitivity and specificity becomes the most cost-effective (the summary sensitivity and specificity would be comparable with liver biopsy).
When we increased the abstinence rate after diagnosis with a NILT by 20%, tests which have high summary sensitivity and specificities tend to be the most clinically effective: Fibroscan and Fibrotest (low cut-off) [Fibroscan 86% and 83% and Fibrotest (low cut-off) 100% and 50%]. This implies that when we increase the abstinence rates following diagnosis with a NILT compared with liver biopsy, tests which have a similar diagnostic accuracy to liver biopsy tend to be more effective.
Liver biopsy also appears to be the most cost-effective option, even when we amend the modelling structure to allow for a different patient cohort (F3) or additional health states (liver transplant). However, this result is also sensitive to changes in the abstinence rates applicable after a NILT; as there are few data on this, the results are uncertain.
The lack of data available regarding cessation rates after diagnosis with either a NILT or liver biopsy are a limitation in the study. One is based on a small study, the other on an assumption, and both are key drivers of the analysis which leave the results very uncertain.
The HTA report by Stevenson et al. 428 did not report an incremental analysis. However, they did carry out a number of threshold analyses where they decreased the abstinence rate and increased it to see at which point biopsy became cost-effective compared with a test. They found that the lower the abstinence rate after a NILT, the more likely it is that liver biopsy is cost-effective.
Liver biopsy was also a less costly testing option as we assumed that it had perfect sensitivity and specificity and could accurately identify false-positive responses from true-negative outcomes. A true-negative outcome had a lower cost than a false-positive outcome in the model. Any test that returned a high false-positive result was more costly; for example, using Fibrotest (low cut-off) was more costly and less effective than using Fibrotest to initially identify patients followed by liver biopsy to confirm diagnosis. A reduction in all-cause mortality due to abstinence is not captured in the model. This would further strengthen the robustness of using liver biopsy, if we still assumed higher abstinence rates following a liver biopsy than following a NILT.
Therefore, despite any mortality or morbidity risks associated with liver biopsy, the increased cost associated with false positives and the fact that the utility value for these patients was the same as if true negative (in other words there was no assumed treatment benefit associated with being diagnosed as false positive in the model) meant that any test which returned a high number of false-positive outcomes would be the least cost-effective.
The incremental cost of using liver biopsy compared with a NILT is high when we look at the cost per correct true negative. PGAA, which has the highest specificity, returns the highest number of true negatives which will be picked up by a NILT. However, for tests with a lower specificity, Fibrotest (low cut-off), the cost per correct diagnosis (true negative) using a liver biopsy is the cheapest option compared with using liver biopsy to diagnose true negatives for other tests.
Chapter 8 Cost-effectiveness analysis: non-alcoholic fatty liver disease
This chapter details the approach used to conduct an analysis of non-invasive liver tests for use in patients with NAFLD. The population considered for analysis were persons with NAFLD who were suspected of having developed NASH with fibrosis or cirrhosis.
Literature search
Cost-effectiveness of non-invasive liver tests in non-alcoholic fatty liver disease
We conducted a literature review using a modified version of the search strategy used for ALD to locate published cost-effectiveness studies which had analysed the use of the NILTs in a NAFLD population. We used the MEDLINE database (via Ovid platform, searched 24 June 2013, all years searched). The search strategy used is detailed in Appendix 2 and inclusion criteria are listed in Chapter 3. The search returned 14 papers whose titles and abstract were reviewed. No cost-effectiveness studies were found.
Treatment in non-alcoholic fatty liver disease and effectiveness
We identified a position paper on the treatment of NAFLD and NASH published by EASL in 2010472 which recommends that an initial treatment strategy for patients with NASH would involve treating insulin resistance and reducing body weight (particularly visceral adiposity). Potential treatment options mentioned in the guideline include weight loss and physical exercise interventions, insulin-sensitising agents, vitamin E therapy and antiobesity surgery; however, the guidelines recommend that all pharmacological medications for treatment of NASH should be considered as experimental. Additionally, treatment and monitoring of metabolic and cardiovascular comorbidities in patients with NAFLD is recommended by the EASL guidelines.
We searched the reference list of the EASL position paper and the American Association for the Study of the Liver (AASLD) practice guidelines47 for papers providing evidence of relative treatment effects on the potential treatment strategies outlined in the guidelines and supplemented this using a general literature search conducted using Google Scholar (search terms included ‘NAFLD’, ‘NASH’ ‘vitamin E therapy’ and ‘pioglitazone’, searched 26 June 2013). We also sought clinical advice to identify up-to-date, relevant studies on potential treatments. Using this triple approach, we identified relevant papers on insulin-sensitising agents,473–475 weight loss and exercise interventions,476,477 behavioural interventions478 and bariatric surgery. 479
The EASL position paper472 noted that no studies analysing the impact of glitazones and their effect on the cessation or decrease in progression of fibrosis/cirrhosis have shown a convincing benefit. We identified a 2010 systematic review and random-effects meta-analysis Rakoski et al. 473 of eight published studies480–488 which analysed the use of insulin sensitisers [thiazolidineodiones (glitazones)] and metformin in patients with NAFLD and/or NASH. The primary outcome of interest for each paper related to histological response to treatment. When the authors looked at all nine studies together, insulin-sensitising agents showed a significant improvement in fibrosis; however, a subgroup analysis looking only at studies which investigated the use of glitazones (six studies) found that glitazones (rosiglitazone, pioglitazone) did not result in a significant improvement in fibrosis. A sensitivity analysis conducted by the authors excluding patients with diabetes from the analysis found that, when this subset of patients was removed, pioglitazone did result in a significant decrease in fibrosis. The study did not, however, provide any applicable data to use in an economic model, such as the reduced probability of patients progressing to a more severe NASH state as a result of treatment.
A 2012 cost–utility study by Mahady et al. 475 derived a treatment effect (RR) for histological improvement with pioglitazone from a 2011 meta-analysis of randomised trials conducted by Mahady et al. 489 The authors estimated a RR of 1.40 for pioglitazone versus placebo using three published studies of randomised trials where pioglitazone was used as add-on therapy to standard lifestyle advice. 481–483 However, the 2012 meta-analysis by Rakoski et al. 473 (reviewed above) had also analysed these three studies in their subgroup meta-analysis of glitazones and found that glitazones did not result in an improvement in fibrosis. In addition, the 2010 paper by Sanyal et al. ,481 which was analysed by Mahady et al. 489 in their meta-analysis, noted that no significant improvement was seen in fibrosis scores as a result of pioglitazone treatment. Indeed, in their paper, Mahady et al. 489 themselves note that the degree of improvements with thiazoidinediones (TZDs) is modest, estimated at approximately one-quarter of a grade per year for fibrosis.
Rakoski et al. 473 also noted that the glitazones have potential serious side effects. This point was emphasised, too, by the 2012 AASLD Practice Guidelines, which recommend that pioglitazone can be used to treat steatohepatitis in patients with NASH; however, the long-term safety and efficacy of the drug in patients with NASH is not yet established.
There are no RCTs published analysing treatment for metabolic conditions and the resulting impact on fibrosis in patients with NASH. 47
The use of metformin was not recommended for use as specific treatment for liver disease in adults with NASH47 and the EASL position paper472 notes that controlled studies of metformin show no benefit resulting from this treatment. 487,488 Lavine et al. 490 conducted a randomised, double-blind, double-dummy, placebo-controlled clinical trial of 173 patients (aged 8–17 years) with biopsy-confirmed NALFD. 490 The study analysed whether children with NALFD would improve with use of vitamin E or metformin, and concluded that, compared with a placebo, neither therapy demonstrated significant improvements in histological features.
The 2012 AASLD guidelines491 recommend that vitamin E (α-tocopherol) administered at daily dose of 800 IU/day for non-diabetic adults with biopsy-proven NASH may be considered as first-line pharmacotherapy. The recommendations are partially based on the results of the pioglitazone versus vitamin E versus placebo for the treatment of nondiabetic patients with non-alcoholic steatohepatitis (PIVENS) trial,481 which found that although there was an improvement in hepatic steatosis and lobular inflammation, fibrosis scores were not significantly improved by use of vitamin E. The AASLD guidelines do not give much detail around the background to this decision; rather, they note that the primary end point of the PIVENS trial was an improvement in NAS ≥ 2 points, with at least 1-point improvement in hepatocellular ballooning and 1-point improvement in either the lobular inflammation or the steatosis score, and no increase in the fibrosis score. It seems that the recommendation was based on the improvement in liver histology seen during the PIVENS trial (but not necessarily an improvement in the fibrosis score).
The 2012 cost–utility study by Mahady et al. 475 also derived a treatment effect (RR) for vitamin E treatment. This estimate was derived from the PIVENS trial; however, as noted above, the paper for the PIVENS trial481 notes that fibrosis scores were not significantly improved with vitamin E treatment. The RR employed in the cost–utility study (1.35) indicates that very little benefit results from treatment.
A RCT by Promrat et al. 492 assessed the effect of weight loss on NASH and found that weight reduction achieved through lifestyle interventions (diet and exercise programme and behavioural change) led to an improvement in steatosis, lobular inflammation and ballooning injury (measured using the NASH histological activity score); however, there was no significant change in hepatic fibrosis after 1 year of the study intervention. The study included patients with well-characterised NASH (histologically and clinically), and used a standardised, protocol-based lifestyle intervention. However, this was a small study (30 persons completed the study), a post-liver biopsy was carried out for only 90% of the participants and none of the participants had cirrhosis (16% had bridging fibrosis). 492 As the study was conducted for only 48 weeks, it may not be possible to confirm if lifestyle interventions are effective, as long-term data suggest that only 15% of participants lose > 10% of their body weight; in addition, adherence to weight-loss programmes drops after the first few months and most people regain weight. 472
Bariatric surgery is indicated to be effective in patients with NASH and fibrosis. 479 NICE guidelines for obesity493 recommend the use of bariatric surgery as a treatment option if patients have a BMI of ≥ 40 kg/m2 or for patients with a BMI between 35 kg/m2 and 40 kg/m2 with other significant disease such as type 2 diabetes or high blood pressure. The guidelines also recommend this surgery as a first-line treatment option for adults with a BMI > 50 kg/m2. However, the guidelines do not specifically recommend bariatric surgery for use in patients with NAFLD. In addition, the recent AASLD guidelines noted that it is premature to consider bariatric surgery as an established option to specifically treat NASH rather than obesity in general. A systematic review and meta-analysis by Mummadi et al. 479 concluded that fibrosis appears to improve after surgery-induced weight loss. Another 2008 study by De Freitas et al. 494 found that there is good evidence that bariatric surgery is associated with NAFLD regression in morbidly obese patients. However, the limitation of these studies is that they are retrospective or prospective studies. A 2010 Cochrane review495 of bariatric surgery in obese patients with NASH did not locate any RCTs or quasi-RCTs that evaluated a bariatric procedure compared with another intervention in patients with NASH, and the authors concluded that this lack of RCT studies meant that they could not assess the benefits and harms of bariatric surgery as a potential treatment in patients with NASH.
Health-related quality of life in non-alcoholic fatty liver disease patients
The search for papers on treatment effectiveness also identified three studies that contained information on HRQoL in patients with NAFLD, two of which had reported HRQoL values.
The cost–utility study by Mahady et al. 475 could not locate any sources for HRQoL for patients with NASH, as no prior studies have been conducted. They used utilities from studies based on other causes of liver disease432,435 and assumed that cirrhosis, decompensated cirrhosis and HCC represent a common pathway for liver disease and that the decrement in quality of life associated with these conditions is similar irrespective of the initial cause.
We located two papers which estimated HRQoL values in patients with NASH and NALFD. One was a study by David et al. 496 which assessed HRQoL of patients with diagnosed NAFLD and NASH. Using the SF-36, they found that patients with NALFD had lower reported scores and greater degrees of physical limitations than patients with HBV or HCV. They noted that the physical component summary score was similar to that of patients with HBV in a decompensated cirrhosis health state. Participants were mainly non-Hispanic white (76%) with a college education (71%), and over half (58%) had an income over $50,000. HRQoL was lower in the respondents with NASH and the authors also found that scores were worse for persons with cirrhosis than without cirrhosis. The authors reported the median SF-36 physical component score by fibrosis level, but insufficient information was provided for the other components required to enable mapping to the preference-based SF-6D in order to calculate QALYs.
The second was a published study497 which reported HRQoL data for liver disease health states for all aetiologies including NASH. However, this was based on clinical opinion rather than empirical data reported by patients. The authors surveyed 18 general practitioners (GPs) and 12 hepatologists (Scotland and England) using a questionnaire and a Delphi approach. In addition, this report did not detail on what scale the values were estimated, and so it was not possible to interpret the reported estimates.
Summary
Currently, no pharmacological treatments or surgical interventions are explicitly recommended for use in patients with NASH by UK guidelines. The main limitation with the current published studies of potential effective interventions for NALFD/NASH is that none of the studies reviewed collected robust data on effective treatments for patients with NASH and fibrosis. Some of the recommended treatment interventions also include physical exercise and weight-loss programmes; these are also recommended for the treatment of obesity irrespective of the degree of fibrosis progression, and so it is difficult to identify the accurate impact on fibrosis regression as any impact may be incidental and may not be explicitly captured in the programme outcomes.
The lack of published studies with relevant data on treatment options administered specifically for patients with NASH with fibrosis limited the modelling approach for NASH. As we could not identify robust cost and QALY estimates or data on treatment effectiveness, we were unable to model the long-term treatment pathway if diagnosed as true positive, false positive, true negative or false negative and were, therefore, unable to use the same modelling approach used for the HBV and HCV analyses.
Approach to analysis
We constructed a probabilistic decision model to assess the cost per correct diagnosis of the applicable NILTs compared with liver biopsy. We also conducted an exploratory analysis to assess the possible implications of using the NILTs in a primary care setting to inform the referral pathway to tertiary care for patients with NASH.
Cost per correct diagnosis
The systematic review identified data for 35 NILTs for use in NASH (using a section of the meta-analysis equivalent to the proportion of the population with a METAVIR score of ≥ F3). The number of true-positive, false-positive, true-negative or false-negative outcomes reported for each NILT was extracted from the meta-analysis data (see Chapter 4). The average disease prevalence estimated from the meta-analysis data was 19%. We calculated the probability of each test returning a true-positive, false-positive, true-negative and false-negative result using the average disease prevalence and sensitivity and specificity estimates (see Appendix 7).
Five of the tests evaluated in the second stage of the analysis used a combined diagnostic cut-off threshold (low and high cut-offs) for staging fibrosis; the use of a combined threshold results in a number of indeterminate responses. We assumed that patients who had an indeterminate response were retested with a commonly used imaging modality, Fibroscan, based on availability and clinical practice (choice based on clinical advice). We did not choose patented, direct or indirect tests as several of the tests with a combined diagnostic cut-off were also in these categories; our clinical advisor advised us that the same type of test would not be repeated in practice.
Test costs
Costs of imaging modalities were sourced from published Department of Health reference costs. 427 Costs of direct and indirect serum marker were obtained from communication with finance departments based at the Royal Free Hospital and costs for patented serum markers were sourced directly from manufacturers and via communication with finance departments based at the Royal Free Hospital (see Appendix 9, Table 78). The cost of a percutaneous liver biopsy (see Chapter 5 for further details on choice of liver biopsy) was sourced from published literature. 428 Where applicable, costs were inflated to 2012 prices using NHS inflation indices. 67 All NILT test costs are based on incremental costs and exclude the capital costs of the equipment. Test costs and sources are listed in Appendix 9.
Analysis
We assumed that liver biopsy as the reference standard had perfect sensitivity and specificity, implying that biopsy accurately diagnoses all healthy and unhealthy patients. Using a hypothetical cohort of 1000 patients with NAFLD and suspected of having liver fibrosis, we estimated the incremental cost associated with each test compared with the next best alternative. Prevalence was based on mean prevalence data from the systematic review, which found that 19% of people tested had fibrosis level 3 disease or greater. Tests which had fewer numbers of true-positive or true-negative outcomes, with a higher test cost than other tests which had higher correct diagnoses, were ruled out of the analysis (dominated). We present the results separately for positive and negative diagnoses as the consequences are different for each. Results are shown in Tables 51 and 52.
| Test | Number of TPs using NILT | Test cost, £ | Number of incremental correct diagnoses | Incremental cost (test only), £ | Incremental cost per correct diagnosis (TP): each test compared with next best alternative (£/correct diagnosis gained) |
|---|---|---|---|---|---|
| NFS TE | 15 | 55.95 | – | – | Dominated |
| FIB-4 (high cut-off) | 71 | 4.40 | – | – | Dominated |
| Fibrotest TE | 74 | 94.60 | – | – | Dominated |
| NFS (high cut-off) | 75 | 4.95 | – | – | Dominated |
| APRI | 76 | 4.05 | – | – | Dominated |
| Fibrotest (high cut-off) | 76 | 43.60 | – | – | Dominated |
| AST–ALT (high cut-off) | 88 | 0.90 | – | – | Dominated |
| PLT | 119 | 3.50 | – | – | Dominated |
| Age–PLT Index | 124 | 3.50 | – | – | Dominated |
| NFS all | 134 | 20.85 | – | – | Dominated |
| Hepascore | 143 | 16.24 | – | – | Dominated |
| AST–ALT (low cut-off) | 149 | 0.90 | – | – | Dominated |
| FIB-4 all | 149 | 21.09 | – | – | Dominated |
| Type IV collagen | 150 | 20.00 | – | – | Dominated |
| ELF | 151 | 108.00 | – | – | Dominated |
| NFS (low cut-off) | 151 | 4.95 | – | – | Dominated |
| TE | 155 | 51.00 | – | – | Dominated |
| NAFIC (high cut-off) | 158 | 28.17 | – | – | Dominated |
| Fibrotest all | 158 | 59.31 | – | – | Dominated |
| FIB-4 (low cut-off) | 159 | 4.40 | – | – | Dominated |
| BARD | 160 | 0.90 | – | – | – |
| NFS ELF (high cut-off) | 164 | 112.95 | – | – | Dominated |
| Hyaluronic acid | 165 | 8.00 | 6 | 7.10 | 1.27 |
| NDP | 166 | 21.18 | – | – | Extendedly dominated |
| Fibrotest (low cut-off) | 169 | 43.60 | – | – | Dominated |
| ARFI | 170 | 51.00 | – | – | Dominated |
| NFS ELF all | 171 | 114.81 | – | – | Dominated |
| MR elastography | 172 | 199.00 | – | – | Dominated |
| NFS ELF (low cut-off) | 172 | 112.95 | – | – | Dominated |
| NAFIC all | 180 | 35.59 | – | – | Dominated |
| NAFIC (low cut-off) | 181 | 28.17 | 16 | 20.17 | 1.29 |
| Liver biopsy | 189 | 956.61 | 8 | 928.44 | 112.30 |
| Test | Number of TNs using NILT | Test cost, £ | Number of incremental correct diagnoses | Incremental cost (test only), £ | Incremental cost per correct diagnosis (TN): each test compared with next best alternative (£/correct diagnosis gained) |
|---|---|---|---|---|---|
| BARD | 491 | 0.90 | – | – | Dominated |
| NFS (low cut-off) | 535 | 4.95 | – | – | Dominated |
| NAFIC (low cut-off) | 545 | 28.17 | – | – | Dominated |
| NDP | 566 | 21.18 | – | – | Dominated |
| AST–ALT (low cut-off) | 568 | 0.90 | – | – | Dominated |
| Fibrotest (low cut-off) | 593 | 43.60 | – | – | Dominated |
| FIB-4 (low cut-off) | 603 | 4.40 | – | – | Dominated |
| PLT | 617 | 3.50 | – | – | Dominated |
| Age–PLT Index | 632 | 3.50 | – | – | Dominated |
| NAFIC all | 640 | 35.59 | – | – | Dominated |
| Type IV collagen | 650 | 20.00 | – | – | Dominated |
| NAFIC (high cut-off) | 666 | 28.17 | – | – | Dominated |
| Hyaluronic acid | 666 | 8.00 | – | – | Dominated |
| APRI | 668 | 4.05 | – | – | Dominated |
| TE | 681 | 51.00 | – | – | Dominated |
| Hepascore | 682 | 16.24 | – | – | Dominated |
| MR elastography | 715 | 199.00 | – | – | Dominated |
| ARFI | 726 | 51.00 | – | – | Dominated |
| ELF | 730 | 108.00 | – | – | Dominated |
| AST–ALT (high cut-off) | 740 | 0.90 | – | – | – |
| FIB-4 all | 754 | 21.09 | – | – | Dominated |
| NFS ELF (low cut-off) | 778 | 112.95 | – | – | Dominated |
| Fibrotest (high cut-off) | 779 | 43.60 | – | – | Dominated |
| NFS all | 780 | 20.85 | – | – | Dominated |
| Fibrotest TE | 780 | 94.60 | – | – | Dominated |
| Fibrotest all | 783 | 59.31 | – | – | Dominated |
| FIB-4 (high cut-off) | 783 | 4.40 | 44 | 3.50 | 0.08 |
| NFS (high cut-off) | 786 | 4.95 | 3 | 0.55 | 0.21 |
| NFS TE | 795 | 55.95 | 9 | 51.00 | 5.53 |
| NFS ELF all | 805 | 114.81 | – | – | Dominated |
| NFS ELF (high cut-off) | 805 | 112.95 | 10 | 57.00 | 5.72 |
| Liver biopsy | 811 | 956.61 | 6 | 843.66 | 145.39 |
Results of cost per correct diagnosis
Cost per correct diagnosis (positive): see Table 51
Column 2 in the table shows the number of correct positive diagnoses using a NILT or liver biopsy. The highest number of correct diagnoses is given using a liver biopsy (n = 189), and is assumed to be 100% accurate for all of those tested and which is estimated from the disease prevalence from the meta-analysis. An indirect serum marker, NAFIC (ferritin, fasting insulin, type IV collagen), using a combined cut-off threshold or a low cut-off threshold also returns a high number of true positives (n = 180 and n = 181, respectively). One test, an indirect serum marker, was extendedly dominated, as the incremental cost per correct diagnosis was higher for this test (£26.65) than for another indirect serum marker, NAFIC with a low diagnostic cut-off threshold, when compared with the next best alternative, hyaluronic acid. The incremental cost per correct positive diagnosis for liver biopsy was £112.30, compared with an incremental cost per correct diagnosis of £1.29 associated with using the next best alternative, NAFIC (low cut-off), as the diagnostic test.
Cost per correct diagnosis (negative): see Table 52
Liver biopsy returns the highest number of correct negative diagnoses (811) estimated from the disease prevalence from the meta-analysis. The majority of tests were dominated by other tests which had lower tests costs and returned higher numbers of true-negative results. A combination of NAFLD fibrosis score (NFS)–ELF test using a combined cut-off threshold returned 805 negative diagnoses at an additional cost of £5.72 per correct negative diagnosis compared with the next best alternative, NFS combined with Fibroscan. Although liver biopsy returned the highest amount of negative responses, the cost per correct negative diagnosis was £145.39 compared with the next best alternative, NFS–ELF (high cut-off). The least expensive cost per correct diagnosis compared with the next best alternative was FIB-4 (high cut-off) at £0.08 per correct true-negative response identified.
Exploratory analysis
We conducted an exploratory analysis to assess the possible costs and benefits of using the NILTs in a primary care setting to inform referral of patients to tertiary care. We constructed a simple decision model analysing the referral pathway for patients with NAFLD who have suspected fibrosis or cirrhosis.
Given the lack of data available, this analysis can only be considered as exploratory. We estimate the potential impact of the strategies on the costs of treatment based on the assumptions below. Robust data were not available to estimate the impact of the alternative strategies on health outcomes; however, we also present a sensitivity analysis exploring a range of alternative assumptions about possible impacts using a range of QALY estimates.
Scenarios assessed
Our considered population was a hypothetical cohort of 1000 patients with NAFLD with suspected fibrosis. Our analysis considered a number of scenarios, which were determined using clinical advice.
-
Immediate referral of all patients having NAFLD (irrespective of fibrosis level) to a tertiary care centre for testing, treatment and management.
-
At primary care level, test all patients with NAFLD with a NILT. If the Kleiner score is ≥ F3 (advanced fibrosis), refer patients to tertiary care centre for treatment and management. If the NILT score indicated low risk for advance fibrosis (Kleiner < F3), treat and manage care in primary care setting.
-
Biopsy all patients, treat and manage all patients with a Kleiner score of ≥ F3 at a tertiary care centre. Refer those with a Kleiner score of < F3 for treatment and management in primary care.
Strategy 1 was considered to most closely reflect current UK practice. We assumed that scenarios 1 and 2 would incorporate an initial diagnosis with a NILT. Following clinical advice, we modelled the use of an indirect serum marker test (FIB-4 with a combined diagnostic threshold cut-off). As this test may return a number of indeterminate results (from the meta-analysis data of the studies for FIB-4 combined, 33% of the test results would be inconclusive), we assumed that this proportion of patients would receive a second test with an imaging modality, Fibroscan, to confirm a diagnosis. Using these two tests returned an outcome where 866 patients were diagnosed as low risk and 134 patients were deemed high risk: true negative (86%), false negative (1%), true positive (4%) and false positive (9%).
Input parameters
Prevalence of population with fibrosis level of ≥ F3
Following clinical advice we set the average disease prevalence to 5%. We assumed that the sensitivity and specificity of the tests would be same as observed in the studies included in the systematic review in this population.
Resource use and cost
We did not identify any published studies in the literature review that provided long-term specific cost data related to treatment and management of care in patients with NASH and fibrosis. Based on the recommended management of NASH (and potential pharmacological or surgical treatment listed in the EASL guidelines),472 and using clinical advice, we identified resource use items for patients with NASH.
Assumptions regarding resource use
The time frame adopted in the analysis was 5 years and a discount rate of 3.5% was applied. 66
High risk (≥ F3) treated in tertiary care
We assumed that this patient group would initially be tested with a NILT, FIB-4 (with inconclusive results retested with Fibroscan), in either a tertiary care setting (strategy 1) or a primary care setting (strategy 2). Using strategy 3, we assumed that patients would be tested with a liver biopsy in a primary care setting. Following clinical advice we assumed that patients with a METAVIR score of ≥ F3 would have two assessments per year with a consultant hepatologist. Exercise and diet programmes would be initiated and monitored by specialists (hepatologist, dietitian, physiotherapist and psychologist), with an assessment every 6 months. Treatment would involve aggressive management of metabolic syndrome components with statins, pioglitazones and antihypertensives. We modelled that a proportion of this cohort who test true positive (4%) would have a 0.04% probability of progressing to a cirrhotic health state per year. 475 Twenty per cent of patients who progressed to a cirrhotic health state would receive screening for HCC twice per year (using a combination of ultrasound and monitoring of α-fetoprotein levels). For those patients who progressed to HCC each year, we allowed for a 30-minute assessment by the hepatologist to feed back results (assuming a full assessment by a hepatologist would normally last 30 minutes, approximately). For the proportion who did not develop HCC, we allowed for 30 minutes of a hospital-based nurse’s time to feed back results via letter.
We assumed that 3.5% of the patients who developed HCC would undergo liver transplantation (0.04% progression rate to HCC health state). 475
National Institute for Health and Care Excellence guidance for obesity (CG43) recommends that persons with a BMI ≥ 40 kg/m2 or with a BMI between 35 kg/m2 and 40 kg/m2 with other significant disease, who fill the following criteria, may be considered for bariatric surgery:
-
individual has a BMI of ≥ 40 kg/m2 OR individual has a BMI between 35 kg/m2 and 40 kg/m2 in addition to another significant disease (e.g. type 2 diabetes or high blood pressure) that could be improved if they lost weight
-
all appropriate non-surgical measures have been tried but have failed to achieve or maintain adequate, clinically beneficial weight loss for at least 6 months
-
the person has been receiving or will receive intensive management in a specialist obesity service
-
the person is generally fit for anaesthesia and surgery
-
the person commits to the need for long-term follow-up.
The guidelines also recommend that persons with a BMI > 50 kg/m2 may be recommended for bariatric surgery as a first-line treatment. 493 In the absence of data on the percentage of persons who may be offered surgery as additional treatment if all other options have failed, we built into our model that a small proportion of the high-risk cohort will have bariatric surgery. We sourced this proportion using the costing report for the NICE clinical guidelines for obesity. 493 The costing report for the obesity guidelines assumed that of all adults who are obese nationally, 1% of these would have a BMI of 50 kg/m2, and of those adults who meet the criteria for bariatric surgery as a first-line intervention, 80% of cases would be considered appropriate for surgical intervention. The costing report assumed that for those cases in which bariatric surgery is indicated and appropriate, 100% would choose to have the surgical procedure. We employed the same assumption as that used by the costing report in or analysis.
Low risk (< F3) treated in tertiary care
We assumed that this patient group would receive an initial test with FIB-4 (with inconclusive results retested with Fibroscan) in a tertiary care setting (strategy 1). Following clinical advice we assumed that patients with a METAVIR score of < F3 would receive an initial assessment with a consultant hepatologist and thereafter would receive one assessment per year. Exercise and diet programmes would be initiated and monitored by a hepatologist and 20% of this population cohort would see a dietitian (initial assessment and once per year thereafter). Treatment would also involve aggressive management of metabolic syndrome components with statins, pioglitazones and antihypertensives. This patient cohort would also have annual liver function tests administered by the clinical hepatologist at the yearly assessment. We assumed that an arbitrary proportion of this group (5%) would have an additional assessment by the hepatologist to feed back results. The remaining 95% would have results fed back via letter (we identified the resource use for this as 30 minutes of a hospital-based nurse’s time). This group would also receive a retest with a NILT to check progression of fibrosis at 5 years. We conservatively assumed that for the annual liver function tests and the 5-year NILT retest, a hospital-based nurse would spend 30 minutes’ administration time (non-face-to-face time) for each contact episode organising tests, sending samples to laboratories for analysis and compiling results.
Low risk (< F3) treated in primary care
We assumed that this patient group either would be tested with a NILT, FIB-4 (with inconclusive results retested with Fibroscan) in a primary care setting (strategy 2), or would initially be tested with a liver biopsy in a primary care setting (strategy 3). Following clinical advice we assumed that patients who have a METAVIR score of < F3 would receive one 30-minute assessment per year with a GP who would advise on and monitor diet and exercise programmes. As in tertiary care, treatment would also involve aggressive management of metabolic syndrome components with statins, pioglitazones and antihypertensives. This patient cohort would also have annual liver function tests (administered by a practice nurse during a 30-minute assessment) and would receive a retest with a NILT to check progression of fibrosis at 5 years. We conservatively assumed that for the annual liver function tests and the 5-year NILT retest, a nurse based at a GP practice would spend 30 minutes’ administration time for each contact episode (non-face-to-face time) organising tests, sending samples to laboratories for analysis and compiling results.
Proportion of patients receiving combination of drugs or drugs alone (for treatment of metabolic conditions)
Following clinical advice, we modelled that patients could receive more than one drug: pioglitazone, vitamin E, statins or antihypertensives. The proportion or combination would remain the same if patients were treated in a tertiary care or a primary care setting. Simvastatin was chosen as the drug of choice for statins as this is the most commonly prescribed statin. 498 Lisinopril was chosen as an antihypernsive drug as per NICE guidance CG137, which advised that the first-line drug of choice for hypertension should be an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin ll receptor blocker. 499 We assumed that the drug would be an ACE inhibitor. We sourced the dosage for pioglitazone and vitamin E suspension (alpha tocopheryl acetate) from the BNF. 419 It was assumed in this exploratory analysis that the formulation of the drugs prescribed would be the cheapest non-proprietary drug available. Estimates of the proportions taking each type of medication are described in Table 53; estimates were based on clinical opinion.
| Drug(s) | Tertiary care and primary care high risk (≥ F3), % | Tertiary care and primary care low risk (< F3), % |
|---|---|---|
| Statins only | 25 | 40 |
| Statins and pioglitazone | 15 | 10 |
| Statins and vitamin E | 20 | 0 |
| Antihypertensive only | 5 | 20 |
| Antihypertensive and pioglitazone | 15 | 10 |
| Antihypertensive and vitamin E | 20 | 0 |
Costs associated with resource use
National published sources of unit cost data were applied to the estimates of resource use: BNF for costs of medication;419 unit costs of health and social care published by the Personal Social Services Research Unit (PSSRU) for NHS staff costs;67 and Department of Health reference costs427 for costs of surgical interventions such as bariatric surgery. The cost of a liver transplant was taken from the CELT study421 described in Chapter 5, using the incremental cost of transplantation for ALD patients from date of transplant followed up over 2 years. Test costs for FIB-4, Fibroscan and liver biopsy are listed in Appendix 9. We assumed that test costs would be the same regardless of administration setting (primary or tertiary). Table 54 provides a list of all identified resource use and associated costs. Where required, costs were inflated to 2012 prices using NHS inflation indices. 67
| Resource use | Unit cost | Lower cost | Upper cost | Source |
|---|---|---|---|---|
| Tertiary care staff: first assessment | ||||
| Consultant hepatologist | 216.00 | 143.00 | 251.00 | Department of Health reference costs 2011–12 (Code 306)427 |
| Dietitian | 91.00 | 15.00 | 148.00 | Department of Health reference costs 2011–12 (Code 654)427 |
| Exercise: physiotherapist | 49.00 | 37.00 | 64.00 | Department of Health reference costs 2011–12 (Code 650)427 |
| Behavioural treatment: psychologist | 89.00 | 66.00 | 66.00 | Department of Health reference costs 2011–12 (Code 656)427 |
| Tertiary care staff: follow-up assessment | ||||
| Consultant hepatologist | 187.00 | 98.00 | 271.00 | Department of Health reference costs 2011–12 (Code 306)427 |
| Dietitian | 93.00 | 16.00 | 119.00 | Department of Health reference costs 2011–12 (Code 654)427 |
| Exercise: physiotherapist | 43.00 | 24.00 | 24.00 | Department of Health reference costs 2011–12 (Code 650)427 |
| Behavioural treatment: psychologist | 313.00 | 51.00 | 204.00 | Department of Health reference costs 2011–12 (Code 656)427 |
| Nurse: hospital based (hour, non-face-to-face contact) | 41.00 | 35.00 | PSSRU 2012 Table 14.367 | |
| Primary care staff | ||||
| GP (per minute patient contact) | 3.70 | 3.10 | – | PSSRU 2012 Table 10.8B67 |
| Practice nurse (hour, face-to-face contact) | 53.00 | 45.00 | – | PSSRU 2012 Table 10.667 |
| Practice nurse (hour, non-face-to-face contact) | 41.00 | 35.00 | – | PSSRU 2012 Table 10.667 |
| Medication cost (per year) | ||||
| Statin (simvastatin) | 11.86 | – | – | BNF (accessed 24 June 2013)419 |
| Antihypertensive (lisinopril) | 14.47 | – | – | BNF (accessed 24 June 2013)419 |
| Diabetes medication (pioglitazone) | 515.5 | – | – | BNF (accessed 24 June 2013)419 |
| Vitamin E | 797.60 | – | – | BNF (accessed 24 June 2013)419 |
| Test costs | ||||
| US | 51.00 | 32.00 | 62.00 | Department of Health reference costs 2011–12 (Code RA23Z)427 |
| Monitoring of α-fetoprotein levels | 1.38 | – | – | Royal Free, 8 July 2013, personal communicationa |
| Screening for HCC | 52.38 | – | – | Assumed to be cost of US and monitoring of α-fetoprotein levels |
| Liver function tests (AST–ALT) | 0.90 | – | – | AST, ALT (Royal Free, 8 July 2013, personal communication)a |
| Surgical interventions | – | – | ||
| Bariatric surgery | 6,479 | – | – | NICE guideline CG43: costing report493 |
| Liver transplant surgery and post care | 17,741 | – | – | CELT study424 |
Analysis exploring impact on resource use
We carried out an initial analysis where we estimated a cost per person for each scenario over for a 5-year period. We discounted the costs and QALYs using the recommended UK discount rate of 3.5%. 66 The costs were based on the resource use identified and included assessment costs, drug costs, screening costs, surgical costs and test costs. The results have been disaggregated to show the cost of treatment for those who test low risk (negative test response, true negative or false negative) or high risk (positive test response, true positive or false positive) for each group. Table 55 presents the results of the cost analysis for the three scenarios.
| Year | Scenario 1 | Scenario 2 | Scenario 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cost low risk | Cost high risk | Cost total | Cost low risk | Cost high risk | Cost total | Cost low risk | Cost high risk | Cost total | |
| Year 1 | 329 | 218 | 546 | 235 | 218 | 453 | 1148 | 129 | 1277 |
| Year 2 | 277 | 227 | 504 | 211 | 227 | 438 | 182 | 85 | 267 |
| Year 3 | 267 | 220 | 487 | 204 | 220 | 423 | 176 | 82 | 258 |
| Year 4 | 258 | 212 | 471 | 197 | 212 | 409 | 170 | 79 | 250 |
| Year 5 | 266 | 205 | 471 | 206 | 205 | 411 | 1000 | 77 | 1077 |
| Total | 2479 | 2135 | 3130 | ||||||
Scenario 1 (refer all patients to tertiary care centre) and scenario 2 (refer only those who test ≥ F3 to tertiary care) result in similar resource use costs per person. The cost for the high-risk scenario is the same for both groups, as those diagnosed as positive in the primary care setting would be referred to a tertiary care setting. The cost for the low-risk group differs, with the cost of being treated in a primary care setting being slightly lower than that of being treated in a tertiary care setting for patients deemed low risk.
Allowing for a prevalence rate of 5% means that liver biopsy would accurately diagnose 95% of the cohort as true negative; in this scenario, fewer patients (n = 50) would be diagnosed and treated as high risk within a tertiary care centre than in scenario 1 or 2 (n = 134). However, liver biopsy has the most expensive resource cost per scenario; this was to be expected, as though we allowed for liver biopsy to have perfect sensitivity and specificity in the analysis, it also had the most expensive test costs (£957). We conducted a sensitivity analysis where we reduced of liver biopsy to that of Fibroscan and FIB-4 (£55.40) to determine the impact of a change in liver biopsy cost. This changed the results so that liver biopsy became the least costly scenario (£1482 per person).
The prevalence rate used in the exploratory analysis is 5% (as per clinical advice); if we amended this to 20% (as per systematic review data) the cost per arm would increase (£3170 per person for scenario 1, £2868 per person for scenario 2 and £4063 per person for scenario 3).
Analysis exploring impact on health outcomes
No data were available to accurately assess the impact of the referral pathway on the health outcomes of patients. We conducted a further tentative exploratory analysis to assess possible impacts on health outcomes. We estimated hypothetical QALYs for each test outcome (true negative, false negative, true positive and false positive) based on assumptions about possible health outcomes in patients with NASH.
We did not identify data on the expected lifetime QALY estimates of people with NAFLD. In the absence of available data, we assumed illustrative QALY values for patients with NAFLD based on the average lifetime QALYs of patients with HBV. The expected mean QALYs for each of the four type of diagnosis (true positive, false positive, true negative, false negative) from the HBeAg-negative model was assumed to represent the average QALYs for patients with NAFLD and corresponding diagnoses. It is recognised that the HRQoL and prognoses of patients with NAFLD may be lower in practice; however, data were not available to include in the analysis.
We assumed that the expected lifetime QALYs of patients diagnosed as true or false positive would be similar in a primary care setting as for patients diagnosed in tertiary care. A positive diagnosis in primary care would involve immediate referral to a tertiary centre for further investigation and so would be unlikely to lead to substantially different health outcomes. A difference in the health outcomes may be more likely for patients whose test results are negative. We assumed that the estimates of lifetime QALYs for people with true negative diagnoses treated in a tertiary care centre would be slightly higher than for those treated in a primary care setting. It is hypothesised that patients treated in tertiary centres may benefit from additional treatment such as access to behavioural therapists, physiotherapists and advice from dieticians, even if their disease has not reached a more advanced stage of progression. We hypothesise that the impact of management in primary care may be greater for patients with false-negative diagnoses, and that the health outcomes of these patients may be less in primary care than in tertiary care due to less intensive monitoring. We conducted an illustrative analysis where we assumed that the QALYs gained for the group of patients treated in primary care would either be 90% or 75% of the QALYs gained of comparable patients treated in the tertiary care setting (Table 56).
| Diagnostic test outcome | Tertiary care | Primary care | |
|---|---|---|---|
| Lifetime QALY | Varied at 90% | Varied at 75% | |
| FN | 6.18 | 5.56 | 4.64 |
| TN | 13.12 | 11.81 | 9.84 |
Using the assumed range of QALY values, we can estimate the lifetime QALY that may apply for scenarios 1 and 2, assuming that the NILTs used are FIB-4 combined, followed by Fibroscan as a retest for indeterminate results. The QALY gain for scenario 1 will remain constant at 13.00 as all patients will be treated within tertiary care. The results are shown in Table 57.
| Diagnostic test outcome | Expected mean QALYs | |
|---|---|---|
| Scenario 2 | Scenario 3 | |
| High impact on FN (75%) and TN (90%) | 11.86 | 11.55 |
| High impact on FN (75%) and low impact on TN (100%) | 12.98 | 12.80 |
| Low impact on FN (90%) and low impact on TN (100%) | 12.99 | 12.80 |
| Low impact on FN (90%) and high impact on TN (90%) | 11.87 | 11.55 |
The largest decrease in QALYs for both scenarios 2 and 3 is when we assume that the quality of life is 25% lower in false negative patients and 10% lower in true-negative patients when treated in primary care as opposed to tertiary care. However, if we assume that true-negative patients have the same QALY gain regardless of setting but false-negative patients retain a QALY which is 25% less than in tertiary care, the difference in QALY gain between scenarios 1 and 2 is marginal. This implies that the true-negative response has a higher impact on the overall QALY gain. This is due to the fact that the testing strategy used (FIB-4, followed by Fibroscan retest for indeterminate patients) only has a 1% combined probability of predicting a false-negative result. Tests which have a higher probability of false-negative outcomes and a lower probability of true-negative outcomes may have different result when the values are varied.
Discussion
The lack of treatments specifically for the treatment of fibrosis progression in people with NAFLD meant that a different approach to modelling was required. We conducted an incremental cost per case detected to assess the relative cost-effectiveness of the tests. The results were analysed according to positive and negative diagnoses as the potential consequences of each are likely to be very different. The analysis of the incremental cost per correct positive diagnosis found that most of the tests were dominated or extendedly dominated by liver biopsy; however, hyaluronic acid had an ICER of £1.27 and NAFIC (low cut-off) had an ICER of £1.29. The ICER for liver biopsy was £112.30, which means that it costs an additional £112.30 to obtain an additional correctly diagnosed positive result compared with the next best alternative. The analysis of the incremental cost per correct negative diagnosis found that FIB-4 (high cut-off) and NFS (high cut-off) had ICERs of below £1, the ICERs for NFS ELF was £5.72 and for biopsy was £145.39. Whether or not the ICERs for the biopsy represent good value for money is difficult to judge as there are no established cost-effectiveness thresholds for this measure. In addition, they do not take into account the potential negative impacts of biopsy on morbidity and mortality.
We conducted an exploratory analysis to tentatively assess the potential use of the tests to determine referral to tertiary care. A lack of data meant that as some of our inputs are arbitrary (frequency of appointments, exact resource use that would be incurred in primary care including access to specialists such as dieticians or behavioural therapists), we would expect the results to be sensitive to changes in assumptions or costs. The fact that they are confirms the need for well-designed long-term studies of patients with NASH, part of which would include collecting good-quality resource use data for use in economic models and further analysis.
The QALY analysis is based on assumptions as to date there have been no studies that have collected HRQoL using a comparable measure such as an EQ-5D in patients with NAFLD. The lack of robust QALY data is also a limitation. In the absence of viable data, we have based the analysis on results for people with HBV. This assumption may have underestimated the decrement in HRQoL for patients with NASH.
One of the main limitations with regards to modelling NASH is the lack of effectiveness data relating to the impact of treatment on fibrosis in patients with NASH. Well-designed prospective studies of patients with NAFLD who are followed for long periods are lacking. 472 This lack of data does not allow us to model the long-term care pathway for NASH patients.
Treatment is often indicated for other conditions (diabetes, obesity, cardiovascular diseases) and, indeed, most people die earlier from cardiovascular disease-related comorbidities than liver-related disease. 500 It is also hard to separate out why treatment has sometimes been given (e.g. bariatric surgery, liver biopsies are performed during surgery but surgery is not necessarily carried out for a liver disease-related cause).
Although we did identify a recently published cost–utility analysis for treatment in patients with NASH, we did not feel that the existing published data of potential treatments and their effect on liver fibrosis in patients with NASH were of sufficient quality or quantity for use in a detailed economic model. Indeed, the data derived by Mahady et al. 489 indicate that both treatments analysed (pioglitazone and vitamin E) had only a modest effect on liver fibrosis progression.
Well-designed long-term prospective RCTs are required in patients with NASH to capture the impact of treatment and its progression on disease, to obtain valid estimates of quality of life and to obtain good-quality long-term costing estimates for treatment and management of patients with NASH.
Chapter 9 Cost-effectiveness analysis: cirrhosis
This chapter reports the cost-effectiveness analysis for cirrhosis and contains details of the literature review, modelling approach and results. The population of interest are those patients suspected of having cirrhosis, irrespective of aetiology.
Literature review
Background
Cirrhosis is the end stage of every chronic liver disease. The development of HCC may accelerate the course of the disease at any stage. Cirrhosis comprises two health states: compensated and decompensated. Decompensated cirrhosis is defined by the presence of ascites, variceal bleeding, encephalopathy and/or jaundice. HCC develops at a rate of approximately 3% per year in people with cirrhosis. 501
We searched the NICE guidelines website for national guidance on the management and treatment of cirrhosis (applicable for all aetiologies). NICE guidelines for cirrhosis are currently in development. We also searched for recent EASL guidelines for best-practice recommendations for the treatment of cirrhosis. EASL guidance and position papers for HBV, HCV and ALD37,45 recommend long-term monitoring for HCC in patients with cirrhosis.
Literature search
A search for relevant literature pertaining to costs and quality-of-life data for cirrhosis was conducted using the MEDLINE database (via Ovid platform, search date 26 July 2013). To search for literature related to cost data for cirrhosis we used the search terms ‘cirrhosis’ and ‘costs’ or ‘Costs and Cost Analysis’, and for literature relating to quality-of-life data we used the search terms ‘cirrhosis’ and ‘Quality of Life’. The search for cost data returned 413 papers and the search for quality-of-life data returned 739 papers. We identified four papers that contained relevant data. 456,502–504
The study by Thompson Coon et al. 456 reported the clinical effectiveness, cost-effectiveness and cost–utility of surveillance of HCC in patients with cirrhosis, using serum α-fetoprotein testing and/or ultrasound examination. They allowed for treatment with liver transplantation or resection. The authors456 conducted an analysis which analysed three aetiologies (ALD, HBV and HCV) separately but also produced a mixed cohort weighted according to proportions of persons with ALD (57.6%), HBV (7.3%) and HCV (35.1%), and, using these models, estimated lifetime costs for various HCC surveillance strategies. Thompson Coon et al. 456 concluded that the most cost-effective strategy to adopt for screening for HCC in patients with cirrhosis (mixed aetiology) was to conduct screening using ultrasound and serum α-fetoprotein testing on a 6-monthly basis.
The 2008 paper identified by Andersson et al. 502 looked at a similar screening programme to Thompson Coon et al. 456 within a US setting. They found that semiannual ultrasound surveillance was a cost-effective strategy that improved outcomes at a reasonable cost. Bolondi et al. 503 reported details of an Italian cost-effectiveness analysis of a programme of monitoring HCC using ultrasound and α-fetoprotein testing. The analysis was conducted alongside a study in which a cohort of patients with liver cirrhosis (n = 313) received monitoring and a cohort of similar patients who acted as a control (n = 104). The authors concluded that the surveillance programme was not a good use of health-care resources. 503 Saab et al. 504 reported a decision-analytic model to compare the cost-effectiveness of ultrasound, α-fetoprotein with ultrasound and CT from a US (Medicare) perspective. The authors concluded that ultrasound is the most cost-effective strategy.
Of all the studies identified, the analysis by Thompson Coon et al. 456 was most relevant to NHS practice; however, none examined the use of the NILTs to determine which patients enter programmes of monitoring or screening for HCC. We therefore conducted our own analysis of the cost-effective use of the NILTs in this setting.
Cost-effectiveness analysis approach
We constructed a decision tree model to estimate the cost-effectiveness of NILTs in diagnosing cirrhosis. The population were persons who were suspected of having cirrhosis. The analysis adopted a time horizon of 4 years. The systematic review for NILTs for use in patients with cirrhosis identified 59 applicable tests (see Chapter 4).
Model structure
The decision tree was based on the recommended management for patients with cirrhosis, which includes screening for oesophageal varices (and ascites) and HCC. Patients received an initial NILT to diagnose the presence of cirrhosis. Screening would occur only if the test returned a positive outcome (true positive or false positive).
Model inputs
Average disease prevalence and test outcome
We estimated the average disease prevalence based on the data from the meta-analysis of the NILTs (20%). We calculated the probability of each NILT returning a true positive, true negative, false negative or false positive outcome using the sensitivity and specificity estimates from the meta-analysis data (see Chapter 4) and the estimated average disease prevalence. The probability of each NILT returning a specific diagnostic test outcome is listed in Appendix 7.
Screening tests and frequency
Following clinical advice, a large study of a surveillance programme for HCC in cirrhosis patients was identified. 505 This was a RCT analysing screening for HCC (using α-fetoprotein testing and ultrasound examination every 6 months). Patients were allocated either to screening with an α-fetoprotein test and ultrasound examination every 6 months or to no screening. The sample size was large (9373 in screening group and 9443 in control group). The authors found that biannual screening with ultrasound examination and α-fetoprotein testing was associated with a reduction in mortality (rate ratio 0.63; 95% CI 0.41 to 0.98), compared with no screening. We used this study as the source for effectiveness data related to screening (as a reduction in HCC mortality of 37%). As our clinical effectiveness data were based on the combination of the two tests used 6-monthly, we used the same screening strategy in our model. We sourced costs for the screening tests from national sources,427 and via personal communication with finance departments at the Royal Free Hospital (see Appendix 9, Table 78). We sourced additional costs of screening from the study by Thompson Coon et al. 456
Mortality from hepatocellular cancer: adjusted for screening and no screening
The study by Zhang et al. 505 found that, for patients who were screened for HCC, there was a 37% reduction in mortality from HCC compared with the non-screened group. We factored this into our model; we estimated that a diagnostic test outcome of false negative (no screening programme) would have a QALY value which was 37% lower than a diagnostic test outcome of true positive (screening programme).
Test costs
The cost of the NILTs were sourced from Department of Health reference costs 2011–12,427 personal communication with finance departments based at the Royal Free Hospital and communication with manufactures of patented serum markers (see Appendix 9, Table 78). The cost for liver biopsy was sourced from published literature. 428 Where required, costs were inflated to 2012 prices using NHS inflation indices. 67
Costs and quality-adjusted life-year end points of decision tree
We used the average costs and QALY values over a 4-year period sourced from the model developed for HBeAg-positive disease. We used this cohort as we sourced the reduction in mortality rate with screening from the study by Zhang et al. 505 who had conducted the RCT in a population with HBV. The time period was also defined by the Zhang et al. 505 study, which recruited patients over a 2-year period and with a follow-up period of up to 5 years (average time period of follow-up was 3–5 years).
We used the true-positive Markov model for HBeAg-positive as we assumed that patients would receive usual standard of care (including antiviral agents if applicable) alongside screening or no-screening programmes. Costs and QALYs values were estimated from the model for a 4-year period. These estimates also captured the progression through health states (including progression from moderate or cirrhotic health states to more advanced liver disease states including decompensated cirrhosis). The costs and QALYs also captured the probability of a patient moving from a compensated cirrhosis health state to a decompensated cirrhosis health state. The costs and screening for oesophageal varices are included within the costs and transition probabilities for the compensated cirrhosis health state. This treatment pathway also captures treatment for HCC with liver transplant, including the post liver transplant state.
We assumed that patients who tested true negative or false positive would incur the costs and QALYs of patients in a moderate to advanced fibrosis health state (F2–3). As patients who test false positive also receive screening, we included the additional cost of screening every 6 months with ultrasound examination and α-fetoprotein testing over a 4-year period in this cost.
We assumed that patients who tested true positive or false negative would incur the costs and QALYs of patients in a cirrhotic health state (F4). Patients who tested false negative were assumed to not receive screening, and, therefore, the cost of 6-monthly screening with ultrasound examination and α-fetoprotein testing was excluded from their 4-year costs.
The cost and QALY end points used in the model are detailed in Table 58.
| Diagnostic test outcome | Cost | QALY |
|---|---|---|
| TP | 29,913 | 1.91 |
| FN | 29,703 | 1.19 |
| TN | 23,665 | 2.25 |
| FP | 23,875 | 2.25 |
Analysis
We conducted a probabilistic analysis and compared the results of each non-invasive test and liver biopsy incrementally to determine the cost-effective testing approach. We also constructed a CEAC and CEAF of the results.
We conducted a sensitivity analysis where we allowed for an increase of 10% (arbitrary value) to apply to the QALY value for false positive patients, to reflect the treatment benefit that may apply from receiving screening (although not necessarily indicated in this patient cohort, there may be some benefit due to earlier diagnosis of HCC). We also conducted a second sensitivity analysis where we removed the 6-monthly cost of testing with α-fetoprotein.
Results
The results of the incremental analysis are listed in Table 59. The results show that the most cost-effective test to use would be Forns index (serum marker with a sensitivity of 100% and specificity of 74% in patients with cirrhosis), with an ICER of £1926.
| Diagnostic test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| CDS (high cut-off) | 24,908 | 2.082 | – | – | Dominated |
| Fibrometer (high cut-off) | 24,947 | 2.085 | – | – | Dominated |
| Fibrosis Index | 24,907 | 2.089 | – | – | – |
| Hepascore (high cut-off) | 24,920 | 2.090 | – | – | Dominated |
| Lok’s index (high cut-off) | 24,918 | 2.091 | – | – | Extendedly dominated |
| FIB-4 (high cut-off) | 24,921 | 2.096 | – | – | Dominated |
| APRI (high cut-off) | 24,920 | 2.098 | – | – | Extendedly dominated |
| AST–ALT ratio | 24,929 | 2.105 | – | – | Extendedly dominated |
| ELF (high cut-off) | 25,024 | 2.108 | – | – | Dominated |
| BARD | 24,935 | 2.109 | – | – | Extendedly dominated |
| Fibrotest | 24,972 | 2.122 | – | – | Dominated |
| GUCI | 24,942 | 2.126 | – | – | Dominated |
| Forns index (high cut-off) | 24,933 | 2.130 | – | – | Extendedly dominated |
| APRI (combined cut-off) and Fibroscan | 24,942 | 2.133 | – | – | Dominated |
| Hepascore (combined cut-off) and Fibroscan | 24,945 | 2.135 | – | – | Dominated |
| PIIINP | 24,976 | 2.135 | – | – | Dominated |
| Fibroindex | 24,974 | 2.136 | – | – | Dominated |
| Type IV collagen | 24,973 | 2.136 | – | – | Dominated |
| US SAPI | 25,019 | 2.138 | – | – | Dominated |
| Fibrotest (high cut-off) | 24,967 | 2.138 | – | – | Dominated |
| PLT | 24,940 | 2.138 | – | – | Dominated |
| Fibropaca | 25,419 | 2.139 | – | – | Dominated |
| Fibrometer | 24,976 | 2.139 | – | – | Dominated |
| US | 24,982 | 2.139 | – | – | Extendedly dominated |
| SAFE | 25,628 | 2.140 | – | – | Dominated |
| Fibrotest (combined cut-off) and Fibroscan | 24,979 | 2.141 | – | – | Dominated |
| King’s | 24,940 | 2.141 | – | – | Extendedly dominated |
| MR | 25,119 | 2.141 | – | – | Dominated |
| MRI | 25,118 | 2.142 | – | – | Dominated |
| APRI (low cut-off) | 24,958 | 2.143 | – | – | Dominated |
| PGAA | 24,945 | 2.147 | – | – | Extendedly dominated |
| Fontana | 24,999 | 2.147 | – | – | Dominated |
| FIB-4 (combined cut-off) and Fibroscan | 24,951 | 2.148 | – | – | Dominated |
| APRI | 24,973 | 2.148 | – | – | Dominated |
| Hepascore (low cut-off) | 24,962 | 2.149 | – | – | Dominated |
| FIB-4 | 24,960 | 2.149 | – | – | Dominated |
| Hyaluronic acid | 24,946 | 2.150 | – | – | Dominated |
| Hepascore | 24,961 | 2.151 | – | – | Dominated |
| FIB-4 (low cut-off) | 24,972 | 2.154 | – | – | Dominated |
| Lok’s index (low cut-off) | 24,982 | 2.155 | – | – | Dominated |
| ARFI | 25,001 | 2.155 | – | – | Dominated |
| PLT–Spleen ratio | 24,998 | 2.157 | – | – | Dominated |
| ELF (combined cut-off) and Fibroscan | 25,059 | 2.158 | – | – | Dominated |
| Bordeaux | 25,021 | 2.159 | – | – | Dominated |
| CEUS | 25,046 | 2.161 | – | – | Dominated |
| Fibrotest (low cut-off) | 25,012 | 2.161 | – | – | Dominated |
| Forns index (low cut-off) | 25,031 | 2.161 | – | – | Dominated |
| Age–Platelet Index | 24,971 | 2.161 | – | – | Dominated |
| CDS | 24,983 | 2.162 | – | – | Dominated |
| Fibroscan | 24,988 | 2.162 | – | – | Dominated |
| Fibrometer (combined cut-off) and Fibroscan | 24,988 | 2.162 | – | – | Dominated |
| DW-MRI | 25,125 | 2.162 | – | – | Dominated |
| CDS (low cut-off) | 24,946 | 2.162 | 39 | 0.07 | 527 |
| ELF (low cut-off) | 25,070 | 2.164 | – | – | Dominated |
| Forns index (combined cut-off) and Fibroscan | 24,982 | 2.167 | – | – | Dominated |
| ELF | 25,050 | 2.168 | – | – | Dominated |
| Fibrometer (low cut-off) | 25,009 | 2.172 | – | – | Dominated |
| MR elastography | 25,127 | 2.177 | – | – | Dominated |
| Forns index | 24,975 | 2.177 | 29 | 0.01 | 1926 |
The CEAF (Figure 10) shows that given a cost-effectiveness threshold value of £20,000, Forns index used alone has a 50% probability of being the optimal test (highest expected net benefit).The CEAC (see Appendix 10) shows that Forns index has the highest probability of being the most cost-effective test (for illustrative clarity, only tests which have a ≥ 5% probability of being cost-effective are shown in the CEAC).
FIGURE 10.
Cost-effectiveness acceptability frontier for cirrhosis. CDS, Cirrhosis Discriminant Score.

A scatterplot illustrating the position of each testing strategy on the cost-effectiveness acceptability curve compared with the least costly testing strategy can be found in Appendix 12.
Sensitivity analysis
Allowing for a 10% increase to the QALY value for patients who are false positive does change the result so that using Forns index (low cut-off) becomes the most cost-effective with an ICER of £1106.
A test with a low diagnostic cut-off is more likely to pick up more positive results, as we are increasing the QALY outcome for patients who are false positive; it is more likely that tests with a low cut-off will have a higher QALY gain, as evidenced by results (see Appendix 11).
Removing the cost of α-fetoprotein from the analysis did not change the base-case results.
Discussion
The results imply that using an indirect serum marker, Forns index, which has a high sensitivity (100%) and a specificity of 74%, is the most cost-effective test, although this testing option is sensitive to changes in assumptions around benefit for those who receive treatment when diagnosed as false positive. It has been necessary to include several assumptions in order to conduct the analysis. These include that, in the absence of data on the impact of surveillance on HRQoL, the reduction in mortality from screening would translate directly into an equivalent reduction in QALYs.
The current analysis only takes a 4-year time horizon. Unfortunately, it was not possible to extrapolate the data reported in the RCT by Zhang et al. over a longer period. 505 If the health outcomes associated with positive diagnoses were estimated over a longer period, we would expect these to have a greater impact on the analysis, although this would be tempered by increased costs of surveillance. There is very little difference in the mid-term outcomes from the tests (cost and QALY values), implying that the difference between each test given a 4-year horizon is slight; extrapolation to lifetime would give a clearer picture as it would include the long-term impact of each test (long-term impact based on test diagnoses).
The analysis evaluates screening for HCC using α-fetoprotein testing and ultrasound examination as the clinical effectiveness data were based on a study which used this screening strategy; however, current practice may be to use ultrasound examination only. We examined the impact of removing the cost of α-fetoprotein testing in the sensitivity analysis; however, it may be the case that effectiveness is reduced with the exclusion of α-fetoprotein testing (likewise, less frequent screening would also reduce the clinical effectiveness).
Chapter 10 Discussion
We have comprehensively reviewed the accuracy and cost-effectiveness of NILTs of fibrosis and cirrhosis. We identified a substantial amount of evidence for the NILTs as a whole (114,071 potential papers identified, 302 relevant papers), and this is the first study to systematically evaluate and synthesise this evidence base. The NILTs vary substantially in terms of how they diagnose fibrosis severity, and in their cost. Some NILTs, such as the AST–ALT ratio, have been available in the NHS for a long time; however, recently there has been a rapid expansion in the introduction and use of new NILTs, which could put pressure on NHS resources.
The systematic review identified 41 NILTs for use in HCV, 21 NILTs for use in HBV, seven NILTs for use in ALD and 28 NILTs for use in NAFLD. Thirty-six NILTs were found for cirrhosis (irrespective of aetiology) and 14 imaging modalities were found that were applicable for all aetiologies. The highest number of studies identified was for HCV (n = 162). ALD had the lowest number of studies identified (n = 12). Fibroscan was the NILT assessed in most studies across disease aetiologies: 37 in HCV, 13 in HBV, eight in NAFLD and six studies in ALD.
Given limited health-care resources, the importance of considering the costs and benefits of competing interventions is now widely recognised in order to make best use of available resources. The consideration of cost-effectiveness is just as important for diagnostic tests as it is for treatments; in doing so it is necessary to consider the health and cost consequences as a result of the test. In relation to this decision problem, considering a cost per case detected would not give a complete picture as it would not reflect important differences in the consequences of an incorrect positive test compared with the consequences of an incorrect negative test. This does create a significant challenge for the cost-effectiveness analysis as the clinical studies typically focus on the accuracy of the tests rather than on effectiveness and health outcomes. We have attempted to overcome this challenge through the use of decision-analytic models and the synthesis of a range of data.
Our analyses have reflected the different causes of fibrosis and cirrhosis. Disease progression, care pathways and patient characteristics vary substantially between aetiologies; these have been analysed separately, and different modelling approaches have been required to account for these differences. For aetiologies HBV and HCV, active treatments are available which could (potentially) be instigated depending on the presence of a specific level of fibrosis, and there is a reasonable evidence base for these treatments. In these cases it is possible to reflect the potential consequences of the outcome of the fibrosis test within the analysis (i.e. the start of treatment or not). The situation for ALD and NAFLD is somewhat different as most interventions are aimed at behaviour change and would not depend on the outcome of the test (e.g. a patient presenting with a BMI of 30 kg/m2 would likely be given dietary and exercise advice regardless of whether the NILT indicates level 1 or 3 fibrosis). There has been investigation of some pharmacological treatments of fibrosis in these patients, but generally the evidence is considered to be weak and they have not so far been included in the standard guidelines for treatment (e.g. EASL position paper for the treatment of patients with NAFLD). In practice, the pathway of care for these patients diagnosed with significant fibrosis or cirrhosis may include increased monitoring, screening for HCC and treatment of complications; however, evidence on the effectiveness of these overall packages of care is not available. The cost-effectiveness analyses of the NILTs in ALD is based on their potential impact on abstinence rates and draws heavily on a previously published HTA. 428 The analysis for NAFLD is limited to an incremental analysis of the cost per correct positive/negative diagnoses and exploratory analyses around the cost-effectiveness including longer-term outcomes. The cost-effective testing strategy for each aetiology is presented in Table 60.
| Aetiology | Cost-effective test for a threshold of £20,000 per QALY gained | Cost-effective test for a threshold of £30,000 per QALY gained | |
|---|---|---|---|
| Incremental analysis | |||
| HBeAg-positive | |||
| Single test | GUCI | MR elastography | |
| Sequential tests | Strategy 2: first NILT, hyaluronic acid; second NILT, MR elastography | MR elastography used singly | |
| HBeAg-negative | |||
| Single and sequential testing | No test or treatment | Treat all (no prior test) | |
| Hepatitis C | |||
| Single test | Treat all (no prior test) | Treat all (no prior test) | |
| Sequential tests | Treat all (no prior test) | Treat all (no prior test) | |
| ALD | |||
| Liver biopsy | Liver biopsy | ||
| Cirrhosis | |||
| Forns index | Forns index | ||
| Cost-effective test for a threshold of £2 per correct diagnosis | Cost-effective test for a threshold of £10 per correct diagnosis | Cost-effective test for a threshold of £150 per correct diagnosis | |
| Cost per correct diagnosis | |||
| NAFLD | |||
| True positive | NAFIC (low cut-off) | NAFIC (low cut-off) | Liver biopsy |
| True negative | NFS (high cut-off) | NFS ELF (high cut-off) | Liver biopsy |
The results for HCV show that a strategy of treating all those suspected of fibrosis without testing is cost-effective. For conclusions regarding the population with HBV, the results were less clear. For patients with HBeAg-negative disease, the conclusion regarding cost-effectiveness depends on the specific cost-effectiveness threshold employed. In the UK, NICE specifies a cost-effectiveness threshold range of £20,000–30,000 per additional QALY, below which technologies are usually considered cost-effective and within which specific additional factors must be considered important. It is unclear which threshold is appropriate in this circumstance: we note that NICE has previously approved treatment for people with HBV with an ICER above the £20,000 lower bound;417 however, we also acknowledge that recent research has suggested that the threshold for the NHS should be lower than that specified by NICE. 506
Current NICE guidance for HCV recommends that all patients with mild HCV should receive treatment with antiviral agents. 443 Our analysis reinforces this recommendation; our findings indicate that that treating all patients irrespective of fibrosis stage without prior testing is the most cost-effective option. However, recent clinical practice guidelines published by EASL state that all treatment-naive patients with compensated chronic liver disease related to HCV should receive treatment (if willing). 507 The guidelines also argue that the timing of treatment in patients with minimal or no fibrosis is debatable and could be deferred pending the development and availability of new treatment (a strategy which should include regular assessment). One reason to defer would be due to the potential side effects of current triple therapy (boceprevir and telaprevir). We conducted a sensitivity analysis which incorporated a HRQoL decrement to represent potential side effects of current treatment; however, this had no effect on the overall conclusions (see Chapter 6). We also conducted an exploratory analysis to evaluate potential new antiviral treatment (see Chapter 6), the results of which depended on the increase in treatment price. With this analysis, if treatment cost was increased by more than approximately £37,500 (with a corresponding increase in SVR rate: see Chapter 6 for details), the strategy ‘treat all’ was not cost-effective; however, this analysis was exploratory and not based on actual data. The issue of whether or not treatment should be deferred until the arrival of new antiviral drugs cannot be answered conclusively given current data on efficacy and treatment cost.
Current NICE guidelines for HBV374 recommend that antiviral treatment is considered for people with evidence of fibrosis following a liver biopsy, or following either a biopsy or diagnosis using Fibroscan for adults aged < 30 years. Our results for HBeAg-positive patients (though highly uncertain: see Chapter 5) found that the use of a NILT with treatment initiation if the patient tested positive was the most cost-effective option without the need for biopsy. The analysis focusing on only those tests where the bivariate model converged found that testing with Fibroscan to assess fibrosis level prior to treatment was the cost-effective option. This finding applied to all patients in the analysis and not just to young adults as in the NICE guidance. For HBeAg-negative patients, our findings were somewhat different from the current NICE guidelines. These found that treatment without prior testing was cost-effective if the upper bound of the NICE threshold was accepted, and no treatment if the lower cost-effectiveness threshold was considered to apply. Current EASL clinical practice guidelines for HBV recommend that all patients should receive treatment if they have HBV DNA levels > 2000 IU/ml and/or serum ALT levels above the ULN for the laboratory, and results from a liver biopsy or a non-invasive marker showing moderate to severe necroinflammation and/or fibrosis using a standardised scoring system (e.g. at least grade A2 or stage F2 by METAVIR scoring). 45 However, as noted above, our analysis shows that for HBeAg-negative patients, strategies without prior testing were the most cost-effective options.
The assessment of cost-effectiveness of the NILTs in HCV found that results were driven by the estimates of treatment effectiveness in this particular group. Treatments for fibrosis in these populations, as for people with HBV, have marketing authorisations for treatment of fibrosis regardless of METAVIR score, and patients with only mild fibrosis may benefit from early treatment. 443 The absolute benefit in terms of health outcomes may, however, not be as great as for patients with more severe levels of fibrosis, and the cost-effectiveness was uncertain. We assessed whether or not using the NILTs to target treatment to those with more severe fibrosis would be a cost-effective use of resources compared with a strategy of treating all those people with HBV or HCV and suspected fibrosis. The base-case analysis found that a scenario where everyone received early treatment was the most effective and cost-effective option, compared with using NILTs to target treatment for those with more severe fibrosis. However, when we conducted a sensitivity analysis around the assumption of treatment benefit in patients with mild fibrosis, treating everyone without a prior diagnostic test stopped being the most cost-effective option when we reduced treatment benefit by approximately 23%, after which it became cost-effective to use a NILT, MR elastography, which had high summary sensitivity and specificity in this population (94% and 92%). If the absolute benefit from treatment is not as high in patients with mild fibrosis as it is for patients with more advanced fibrosis, then it would be more advisable to target treatment using a NILT to identify those with advanced disease.
Given that increasingly sedentary lifestyles and changing dietary patterns mean that NAFLD poses a significant health problem, we strongly recommend that these evidence gaps are addressed. Currently, fatty liver accounts for 0.1% of all deaths in England (648 deaths annually). Fatty liver is also an underlying contributory cause for 1801 deaths per year, which is higher than for any other cause of liver disease. The prevalence of NALFD is also increasing in children, with 10–77% among those who are obese,508 and its presence is associated with progressive liver disease including cirrhosis, which could lead to the need for liver transplantation. The implications of the increasing extent of NAFLD within the population, and in particular within the paediatric population, suggest that this will place an ever-increasing burden on the NHS.
Long-term prospective studies of target-based interventions are required in this population (both adult and paediatric) to determine effective treatments to halt the progression of fibrosis and subsequently limit the encumbrance of this disease on the health-care system.
Additionally, in NAFLD, non-invasive tests differentiate significant fibrosis but not steatohepatitis from simple steatosis. Whereas simple steatosis is usually benign, steatohepatitis could potentially progress to end-stage liver disease. Steatohepatitis is not necessarily characterised by fibrosis, meaning that it could potentially be missed and, therefore, patients and doctors could be falsely reassured. Non-invasive assays that differentiate steatohepatitis from steatosis are based in apoptosis rather than fibrosis509 and have not been adequately validated. Subsequent development and validation of such markers is warranted given the increasing prevalence of NAFLD in the general population. Their assessment was beyond the scope of this review.
Strengths of analysis
Our meta-analysis of NILTs has been the most detailed and extensive to date, including all described serum tests and imaging modalities with no language restrictions and using state-of-the-art statistical and reporting methods. A similar recent study by Chou et al. 510 conducted a systematic review evaluating the diagnostic accuracy of blood tests to identify fibrosis or cirrhosis in patients with HCV infection. However, this study restricted its analysis to blood tests for fibrosis, searched fewer databases and excluded studies not in the English language. The study included serum NILTs but did not include imaging modalities such as Fibroscan in HCV. In addition, the study did not attempt to estimate the cost-effectiveness of these tests for use in HCV. One of the main strengths of our analysis is that we have analysed the cost-effectiveness of these tests in both HBV and HCV and we have based this cost-effectiveness on the long-term impact resulting from a diagnostic test outcome rather than just basing it on the cost of the test itself.
Limitations of analysis
Although the totality of the evidence was substantial for the NILTs, there was considerable variation in the amount and quality of the evidence for individual tests. A great number of NILTs for specific fibrosis stages either were assessed in single studies or had results that did not converge using the random-effects model with correlation between sensitivity and specificity. We assessed the impact of this on the conclusions from the cost-effectiveness analyses through sensitivity analyses including only those where the most robust models of test accuracy were possible. This reduced the number of tests analysed for HBV by 20 and for HCV by 43. The number of tests excluded emphasises how many NILTs had diagnostic accuracy data that were not robust. When we removed the tests, the results changed for HBeAg-positive model only.
Furthermore, only five of the included studies or 1.6% were of high methodological quality; therefore, all results are likely to be biased. This implies that further studies with better design are warranted to increase the robustness of the results.
Finally, cut-offs of NILTs for specific fibrosis stages varied in published studies and were not always predetermined or sufficiently validated. This is similar to measuring renal function with serum creatinine but not knowing the exact ULN. Apart from the practical issue of applying the NILTs in clinical practice with uncertain cut-offs, this resulted in overestimation of diagnostic accuracy of NILTs in the meta-analysis in all cases where there is a range of cut-offs.
The number of data available varied considerably between aetiologies. For example, for NAFLD we identified a position paper by EASL472 and a practice guideline by AASLD. 47 Current treatment recommended in both papers included weight-loss programmes and treatment to ameliorate the metabolic conditions associated with NAFLD. However, we found insufficient evidence around either lifestyle interventions or effective pharmacological treatments directed at the liver to prevent fibrosis progression specifically in patients with NASH.
Uninterpretable and indeterminate results were reported in < 50% of studies; however, they were infrequent in serum non-invasive tests (<1%) and could be considered negligible. In the case of Fibroscan, uninterpretable results were prevalent in 8–10% of examinations; this is probably an underestimated failure rate, as not all studies reported on failures while others included failures in the exclusion criteria. In a prospective study of over 10,000 Fibroscan examinations, unreliable results were reported in 15.8% of cases and were associated with obesity, age > 52 years, operator experience and presence of diabetes. 511 This significant failure rate is an important caveat in the use of Fibroscan. This NILT was the most cost-effective option when tests which did not converge (using bivariate model) were excluded from the analysis for HCV and HBeAg (positive), where the cost-effectiveness threshold was £20,000 and £30,000, respectively. However, as we did not account for indeterminate Fibroscan results in our economic analysis, its cost-effectiveness is likely to have been overestimated.
The searches for the systematic review were conducted in April 2012; as the research took place over a 2-year time period, it was not possible to conduct an updated search. This may mean that we have missed some diagnostic accuracy studies published since April 2012. However, given the robust findings for some of the aetiologies, for example that the ‘treat all’ strategy remained robust to the majority of sensitivity analyses for patients with HCV, any additional studies may not have a substantial impact on our analysis.
Currently, most studies report the results for diagnostic test accuracy using a 2 × 2 classification matrix, which restricts test results to either positive or negative. 512 Using these data, we can predict the summary sensitivity and specificity data required to summarise the diagnostic accuracy for each NILT. We initially attempted to construct the models for HBV and HCV using a 3 × 3 classification matrix to allow for the multiple categories of the METAVIR staging system. The restriction of studies reporting in a 2 × 2 format did not allow us to estimate with precision the proportion of people who tested incorrectly who may have had a higher or lower degree of disease. For example, if a non-invasive test returns a certain number of false-positive outcomes, and we used a section of the data which represented a by METAVIR score of F3, it is not possible from the data provided in the studies to determine the number of persons who actually have mild (F1) or moderate (F2) fibrosis. Likewise, if we use a F2-by-METAVIR section of the data, although we can estimate the probability of a test reporting a false-negative result, we do not know the split within this false-negative result that is applicable to either advanced disease (F3) or cirrhosis (F4).
A 2012 paper by Schuetz et al. 513 examined whether a 3 × 2 classification matrix is better than the 2 × 2 classification matrix when assessing diagnostic accuracy. They found that the parameters for diagnostic accuracy (summary and sensitivity estimates) decrease significantly if a 3 × 2 table is used. As there was a lack of studies reporting data using a 3 × 2 classification matrices, we used a F2 by METAVIR section of the data for our HBV and HCV models. Using this meant that we could not model the population cohorts who are diagnosed as F2 or F3 separately. Although we could identify the prevalence of patients with a METAVIR score of F4 from the meta-analysis data, we could not with accuracy identify the same for F3 as the reported data for F3 also included F4 scores (i.e. this section of the data represented diagnostic accuracy from the study for METAVIR scores which were ≥ F3).
Our findings, though UK based, should be applicable to health systems where the treatment pathway for patients with liver disease is similar. However, our results may not be generalisable to other settings, particularly resource-poor countries especially where the finding is to treat all patients irrespective of disease level.
A transferability issue may also arise over the estimated prevalence used in the analysis. The prevalence was estimated from the studies found during the systematic review; however, these were conducted in tertiary care settings, mainly in countries where the populations might be very different from those in countries with a lower level of health care.
Liver biopsy, although the reference standard for fibrosis assessment, is not 100% sensitive and specific, due to sample and intra- and interobserver variability. Moreover, it assesses fibrosis semiquantitatively and histological scores include both description of fibrosis and architecture. The misclassification rate of liver biopsy is the source of the myth that non-invasive fibrosis tests cannot achieve a high concordance with histological stages. However, serum non-invasive fibrosis markers have been developed and calibrated with direct reference to a set of liver biopsies. Therefore, the perfect serum marker in this case would replicate the ‘golden’ histological standard and could theoretically reach an AUROC of 1, replicating even the misclassifications of a liver biopsy. Imaging modalities, such as Fibroscan, that have been developed independently of liver biopsy, could potentially be affected by the misclassification rate of a liver biopsy. A potential solution would be to validate NILTs against clinical outcomes; however, this would take time and would require large cohorts of patients. Another solution would be for non-invasive fibrosis markers, which assess fibrosis quantitatively, to be ideally developed and validated with reference to a pure histological quantitative assessment of liver fibrosis. Such histological methods that quantify fibrosis by measuring liver collagen using digital image analysis have indeed been developed and could be used in future studies.
Chapter 11 Conclusion
The evidence suggests that, for HCV, treating all patients without prior diagnostic testing is the most cost-effective option. For HBV, the results differed for patients with HBeAg-positive disease and HBeAg-negative disease. The results suggests that if the upper bound of the standard UK cost-effectiveness threshold range is accepted for patients with HBeAg-negative disease, a strategy where all patients are treated regardless of fibrosis level is cost-effective.
For patients with HBeAg-positive disease, at standard UK cost-effectiveness thresholds the results are highly uncertain, with several test strategies having similar expected outcomes and costs. Based on our results, using two NILTs sequentially (hyaluronic acid combined with MR elastography using the second sequential testing strategy outlined in Chapter 2) is most likely to be the optimal strategy at a threshold of £20,000; however, there is only a 4% probability of this being optimal.
Liver fibrosis and cirrhosis from HBV and HCV are significant health problems worldwide. The findings from the models may not be transferable to a resource setting where funds are limited and the ability to treat all patients is not a realistic option.
Abstinence is recommended for patients with ALD. There was a lack of data to allow robust modelling of the impact of testing on abstinence rates and whether or not these are affected by the degree of invasiveness of the tests. If abstinence is likely to increase following diagnosis and if it is likely to be higher following an invasive test, then biopsy is very likely to be cost-effective. If there is no differential impact of the invasiveness of the test on abstinence, then Fibrotest is likely to be cost-effective (with either a high or low test threshold depending on the overall impact of fibrosis diagnosis on abstinence rates).
For NAFLD, most interventions are aimed at behavioural change and are not necessarily recommended specifically to reduce or halt fibrosis progression (e.g. weight-loss programmes for obesity). We located some studies for pharmacological interventions which had looked at the impact on fibrosis in NASH, but found that they had not conclusively demonstrated significant impact; this implies that the current potential pharmacological treatments such as pioglitazones are not effective in patients with NASH and fibrosis.
Implications for research
Further research could examine if the model is applicable to other settings, particularly resource-poor settings. Hepatitis is a global health problem and the pathways of care and expected treatment outcomes are likely to differ between settings. As such, the consequences of a false-negative test and a false-positive test may have different levels of importance according to locality. The cost-effectiveness of the non-invasive tests could be evaluated in these specific local settings, taking into account availability of treatments, local cost data and HRQoL values.
The impact of new therapies on cost-effectiveness (higher costs but fewer side effects and better efficacy) for HCV also warrant further investigation.
We were limited in our modelling approach as diagnostic studies do not report data using 3 × 2 tables, which would have allowed us to model the diagnostic test outcomes with precision. Future studies need to report all outcomes from tests rather than dichotomising into 2 × 2 tables in order to make the results more applicable for cost-effectiveness studies of diagnostic tests.
With alcoholic steatohepatitis, as abstinence is recommended at any stage of liver disease, we have assumed that the diagnosis of fibrosis impacts on abstinence rates and, furthermore, that abstinence may be further increased through more invasive tests. This was based on weak evidence, and further research could be conducted to assess the impact of testing on abstinence. It may be the case that interventions that include monitoring and support may be more effective in patients with lower degrees of fibrosis than non-invasive tests or biopsy. Further research needs to be conducted in this area.
With NAFLD, the lack of data was a significant limitation to our modelling. Considering the growing burden of the related complications with this disease on the NHS, long-term prospective studies are required that collect data on the impact of treatments on patients with NASH and fibrosis, long-term resource use and associated HRQoL using a comparable measure such as the EQ-5D. Data are also required on the relative effectiveness of management and treatment in primary care rather than secondary referrals.
Additionally, NILTs cannot differentiate simple steatosis from steatohepatitis in patients with NAFLD. Therefore, there is a need to develop reliable non-invasive tests for this, as simple steatosis is usually non-progressive, whereas steatohepatitis could potentially progress to significant fibrosis and cirrhosis.
High-quality studies with a low risk of bias for NILTs are required to allow for sufficient validation of specific cut-offs to stage fibrosis in different disease aetiologies. These require the use of predetermined cut-offs for the NILTs, adequate biopsy samples, selection of consecutive patients with no inappropriate exclusions and adequate reporting of patient flow and indeterminate results.
The potential use of NILTs to predict liver-related complications rather than to stage fibrosis should be further explored. This would provide a hard end point and overcome the need for liver biopsy.
Acknowledgements
Contributions of authors
Catriona Crossan (Research Fellow, Health Economics) was responsible for the economic modelling and review of data inputs for the economic modelling. She contributed to the analysis and interpretation of results and led on drafting and revision of the final report.
Dr Emmanuel A Tsochatzis (Senior Clinical Lecturer and Honorary Consultant in Hepatology) was responsible for the systematic review and provided oversight to whole project (March 2013 to July 2013). He also contributed to the analysis and interpretation of results, and drafting and revision of the final report.
Dr Louise Longworth (Reader, Health Economics) oversaw the economic analysis. She also contributed to the analysis and interpretation of results, and drafting and revision of the final report.
Dr Kurinchi Gurusamy (Lecturer in Surgery) conducted the meta-analysis of results from the systematic review. He also contributed to the analysis and interpretation of results, and drafting and revision of the final report.
Professor Brian Davidson (Professor of Surgery) contributed to the analysis and interpretation of results, and drafting and revision of the final report.
Dr Manuel Rodríguez-Perálvarez (MD and PhD in Hepatology), Dr Konstantinos Mantzoukis (Research Fellow), Dr Julia O’Brien (Consultant Gastroenterologist & Hepatologist), Dr Evangelos Thalassinos (Clinical Research Fellow) and Dr Vassilios Papastergiou (Research Fellow in Hepatology) reviewed and extracted data identified in the systematic review.
Professor Andrew Burroughs (Professor of Hepatology) provided oversight to the whole project (November 2010 to March 2013), contributed to the analysis and interpretation of results, and drafting and revision of the final report.
Andrew Burroughs, Emmanuel A Tsochatzis, Kurinchi Gurusamy and Brian Davidson developed the project proposal and secured funding for the project.
We would like to thank Eszter Nagy for providing administrative support in the preparation and formatting of the report.
We would like to thank Ailish Higgins for reviewing the economic models.
We would like to thank Anna Noel-Storr for her contribution in developing the search strategy for the non-invasive tests in the PubMed and EMBASE databases.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Bataller R, Brenner DA. Liver fibrosis. J Clin Invest 2005;115:209-18. http://dx.doi.org/10.1172/JCI24282.
- Department of Health . Annual Report of the Chief Medical Officer 2001 2001. www.dh.gov.uk/en/Publicationsandstatistics/Publications/AnnualReports/DH_4005607 (accessed 8 March 2009).
- Office for National Statistics . Population Estimates. UK Population Grows to 60,975,000 2008. www.statistics.gov.uk/cci/nuggetasp?id=6 (accessed 8 March 2009).
- NHS Blood and Transplant . Transplant Activity in the UK 2007–2008 n.d. www.uktransplant.org.uk/ukt/statistics/transplant_activity_report/current_activity_reports/ukt/transplant_activity_uk_2007–2008.pdf (accessed 8 March 2009).
- The Scottish Government publications . Health in Scotland 2007: Annual Report of the Chief Medical Officer. The Chief Medical Officer’s Report to First Minister on the Health of the Nation 2008. www.scotland.gov.uk/Publications/2008/11/26155748/8 (accessed 8 March 2009).
- Denzer U, Arnoldy A, Kanzler S, Galle PR, Dienes HP, Lohse AW. Prospective randomized comparison of minilaparoscopy and percutaneous liver biopsy: diagnosis of cirrhosis and complications. J Clin Gastroenterol 2007;41:103-10. http://dx.doi.org/10.1097/01.mcg.0000225612.86846.82.
- Terjung B, Lemnitzer I, Dumoulin FL, Effenberger W, Brackmann HH, Sauerbruch T, et al. Bleeding complications after percutaneous liver biopsy. An analysis of risk factors. Digestion 2003;67:138-45. http://dx.doi.org/10.1159/000071293.
- Cholongitas E, Senzolo M, Standish R, Marelli L, Quaglia A, Patch D, et al. A systematic review of the quality of liver biopsy specimens. Am J Clin Pathol 2006;125:710-21. http://dx.doi.org/10.1309/W3XCNT4HKFBN2G0B.
- The French METAVIR Cooperative Study Group . Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology 1994;20:15-20.
- Germani G, Hytiroglou P, Fotiadu A, Burroughs AK, Dhillon AP. Assessment of fibrosis and cirrhosis in liver biopsies: an update. Semin Liver Dis 2011;31:82-90. http://dx.doi.org/10.1055/s-0031-1272836.
- Standish RA, Cholongitas E, Dhillon A, Burroughs AK, Dhillon AP. An appraisal of the histopathological assessment of liver fibrosis. Gut 2006;55:569-78. http://dx.doi.org/10.1136/gut.2005.084475.
- Germani G, Burroughs AK, Dhillon AP. The relationship between liver disease stage and liver fibrosis: a tangled web. Histopathology 2010;57:773-84. http://dx.doi.org/10.1111/j.1365-2559.2010.03609.x.
- Tsochatzis EA, Manousou P, Fede G, Dhillon AP, Burroughs AK. Validating non-invasive markers of fibrosis: the need for a new histological reference standard. Gut 2011;60:1442-3. http://dx.doi.org/10.1136/gut.2010.229484.
- Isgro G, Calvaruso V, Andreana L, Luong TV, Garcovich M, Manousou P, et al. The relationship between transient elastography and histological collagen proportionate area for assessing fibrosis in chronic viral hepatitis. J Gastroenterol 2012;48:921-9. http://dx.doi.org/10.1007/s00535-012-0694-9.
- Manousou P, Dhillon AP, Isgro G, Calvaruso V, Luong TV, Tsochatzis E, et al. Digital image analysis of liver collagen predicts clinical outcome of recurrent hepatitis C virus 1 year after liver transplantation. Liver Transpl 2011;17:178-88. http://dx.doi.org/10.1002/lt.22209.
- Calvaruso V, Burroughs AK, Standish R, Manousou P, Grillo F, Leandro G, et al. Computer-assisted image analysis of liver collagen: relationship to Ishak scoring and hepatic venous pressure gradient. Hepatology 2009;49:1236-44. http://dx.doi.org/10.1002/hep.22745.
- Calvaruso V, Dhillon AP, Tsochatzis E, Manousou P, Grillo F, Germani G, et al. Liver collagen proportionate area predicts decompensation in patients with recurrent hepatitis C virus cirrhosis after liver transplantation. J Gastroenterol Hepatol 2012;27:1227-32. http://dx.doi.org/10.1111/j.1440-1746.2012.07136.x.
- Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology 2003;38:1449-57. http://dx.doi.org/10.1016/j.hep.2003.09.022.
- Tsochatzis EA, Germani G, Hall A, Anousou PM, Dhillon AP, Burroughs AK. Noninvasive assessment of liver fibrosis: the need for better validation. Hepatology 2011;53:1781-2. http://dx.doi.org/10.1002/hep.24271.
- Martinez SM, Crespo G, Navasa M, Forns X. Noninvasive assessment of liver fibrosis. Hepatology 2011;53:325-35. http://dx.doi.org/10.1002/hep.24013.
- Castera L. Noninvasive methods to assess liver disease in patients with hepatitis B or C. Gastroenterology 2012;142:1293-302.
- Lichtinghagen R, Pietsch D, Bantel H, Manns MP, Brand K, Bahr MJ. The Enhanced Liver Fibrosis (ELF) score: normal values, influence factors and proposed cut-off values. J Hepatol 2013;59:236-42. http://dx.doi.org/10.1016/j.jhep.2013.03.016.
- Imbert-Bismut F, Ratziu V, Pieroni L, Charlotte F, Benhamou Y, Poynard T, et al. Biochemical markers of liver fibrosis in patients with hepatitis C virus infection: a prospective study. Lancet 2001;357:1069-75. http://dx.doi.org/10.1016/S0140-6736(00)04258-6.
- Koda M, Matunaga Y, Kawakami M, Kishimoto Y, Suou T, Murawaki Y. Fibrolndex, a practical index for predicting significant fibrosis in patients with chronic hepatitis C. Hepatology 2007;45:297-306. http://dx.doi.org/10.1002/hep.21520.
- Patel K, Benhamou Y, Yoshida EM, Kaita KD, Zeuzem S, Torbenson M, et al. An independent and prospective comparison of two commercial fibrosis marker panels (HCV FibroSURE and FIBROSpect II) during albinterferon alfa-2b combination therapy for chronic hepatitis C. J Viral Hepatol 2009;16:178-86. http://dx.doi.org/10.1111/j.1365-2893.2008.01062.x.
- Cales P, Halfon P, Batisse D, Carrat F, Perre P, Penaranda G, et al. Comparison of liver fibrosis blood tests developed for HCV with new specific tests in HIV/HCV co-infection. J Hepatol 2010;52. http://dx.doi.org/10.1016/S0168-8278(10)61048-3.
- Parkes J, Guha IN, Roderick P, Harris S, Cross R, Manos MM, et al. Enhanced Liver Fibrosis (ELF) test accurately identifies liver fibrosis in patients with chronic hepatitis C. J Viral Hepatol 2011;18:23-31. http://dx.doi.org/10.1111/j.1365-2893.2009.01263.x.
- Castera L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, et al. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 2005;128:343-50. http://dx.doi.org/10.1053/j.gastro.2004.11.018.
- Lupsor M, Badea R, Stefanescu H, Sparchez Z, Branda H, Serban A, et al. Performance of a new elastographic method (ARFI technology) compared to unidimensional transient elastography in the noninvasive assessment of chronic hepatitis C. Preliminary results. J Gastrointestin Liv Dis 2009;18:303-10.
- Huwart L, Sempoux C, Vicaut E, Salameh N, Annet L, Danse E, et al. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology 2008;135:32-40. http://dx.doi.org/10.1053/j.gastro.2008.03.076.
- Sebastiani G, Halfon P, Castera L, Mangia A, Di Marco V, Pirisi M, et al. Comparison of three algorithms of non-invasive markers of fibrosis in chronic hepatitis C. Aliment Pharmacol Ther 2012;35:92-104. http://dx.doi.org/10.1111/j.1365-2036.2011.04897.x.
- Tsochatzis EA, Gurusamy KS, Ntaoula S, Cholongitas E, Davidson BR, Burroughs AK. Elastography for the diagnosis of severity of fibrosis in chronic liver disease: a meta-analysis of diagnostic accuracy. J Hepatol 2011;54:650-9. http://dx.doi.org/10.1016/j.jhep.2010.07.033.
- European Association for the Study of the Liver . EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol 2009;51:237-67. http://dx.doi.org/10.1016/j.jhep.2009.04.009.
- European Association for the Study of the Liver . EASL clinical practice guidelines for HFE hemochromatosis. J Hepatol 2010;53:3-22. http://dx.doi.org/10.1016/j.jhep.2010.03.001.
- European Association for the Study of the Liver . EASL Clinical Practice Guidelines: Wilson’s disease. J Hepatol 2012;56:671-85. http://dx.doi.org/10.1016/j.jhep.2011.11.007.
- Tsochatzis EA, Bosch J, Burroughs AK. New therapeutic paradigm for patients with cirrhosis. Hepatology 2012;56:1983-92. http://dx.doi.org/10.1002/hep.25915.
- European Association for the Study of the Liver . EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol 2011;55:245-64. http://dx.doi.org/10.1016/j.jhep.2011.02.023.
- Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet 1997;349:825-32. http://dx.doi.org/10.1016/S0140-6736(96)07642-8.
- Jacobson IM, McHutchison JG, Dusheiko G, Di Bisceglie AM, Reddy KR, Bzowej NH, et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N Engl J Med 2011;364:2405-16. http://dx.doi.org/10.1056/NEJMoa1012912.
- Poordad F, McCone J, Bacon BR, Bruno S, Manns MP, Sulkowski MS, et al. Boceprevir for untreated chronic HCV genotype 1 infection. N Engl J Med 2011;364:1195-206. http://dx.doi.org/10.1056/NEJMoa1010494.
- Wilck MB, Hamel MB, Baden LR. Clinical decisions. Management of incidental hepatitis C virus infection--polling results. N Engl J Med 2009;360. http://dx.doi.org/10.1056/NEJMclde0902765.
- Afdhal NH, Lok AS, Di Bisceglie AM. Clinical decisions. Management of incidental hepatitis C virus infection. N Engl J Med 2009;360:1902-6. http://dx.doi.org/10.1056/NEJMclde0900131.
- Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med 2013;368:1878-87. http://dx.doi.org/10.1056/NEJMoa1214853.
- Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med 2013;368:1867-77. http://dx.doi.org/10.1056/NEJMoa1214854.
- European Association for the Study of the Liver . EASL clinical practice guidelines: management of chronic hepatitis B virus infection. J Hepatol 2012;57:167-85. http://dx.doi.org/10.1016/j.jhep.2012.02.010.
- Hadziyannis SJ, Papatheodoridis GV. Hepatitis B e antigen-negative chronic hepatitis B: natural history and treatment. Sem Liv Dis 2006;26:130-41. http://dx.doi.org/10.1055/s-2006-939751.
- Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Gastroenterology 2012;142:1592-609. http://dx.doi.org/10.1053/j.gastro.2012.04.001.
- Tsochatzis E, Papatheodoridis GV, Manesis EK, Kafiri G, Tiniakos DG, Archimandritis AJ. Metabolic syndrome is associated with severe fibrosis in chronic viral hepatitis and non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2008;27:80-9. http://dx.doi.org/10.1111/j.1365-2036.2007.03538.x.
- Qian MY, Yuwei JR, Angus P, Schelleman T, Johnson L, Gow P. Efficacy and cost of a hepatocellular carcinoma screening program at an Australian teaching hospital. J Gastroenterol Hepatol 2010;25:951-6. http://dx.doi.org/10.1111/j.1440-1746.2009.06203.x.
- Argo CK, Northup PG, Al-Osaimi AM, Caldwell SH. Systematic review of risk factors for fibrosis progression in non-alcoholic steatohepatitis. J Hepatol 2009;51:371-9. http://dx.doi.org/10.1016/j.jhep.2009.03.019.
- Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology 2006;44:865-73. http://dx.doi.org/10.1002/hep.21327.
- Sanyal AJ, Banas C, Sargeant C, Luketic VA, Sterling RK, Stravitz RT, et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology 2006;43:682-9. http://dx.doi.org/10.1002/hep.21103.
- O’Shea RS, Dasarathy S, McCullough AJ. Alcoholic liver disease. Hepatology 2010;51:307-28. http://dx.doi.org/10.1002/hep.23258.
- Colombo M. Screening and diagnosis of hepatocellular carcinoma. Liver Int 2009;29:143-7. http://dx.doi.org/10.1111/j.1478-3231.2008.01938.x.
- European Association for the Study of the Liver . EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol 2012;57:399-420. http://dx.doi.org/10.1016/j.jhep.2012.04.004.
- Tome S, Lucey MR. Review article: current management of alcoholic liver disease. Aliment Pharmacol Ther 2004;19:707-14. http://dx.doi.org/10.1111/j.1365-2036.2004.01881.x.
- Goodman ZD. Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol 2007;47:598-607. http://dx.doi.org/10.1016/j.jhep.2007.07.006.
- Buntinx F, Aertgeerts B, Macaskill P, Knottnerus J, Buntinx F. The Evidence Base of Clinical Diagnosis. Oxford and Hoboken, NJ: BMJ Books; 2009.
- Whiting P, Westwood M, Burke M, Sterne J, Glanville J. Systematic reviews of test accuracy should search a range of databases to identify primary studies. J Clin Epidemiol 2008;61:357-64. http://dx.doi.org/10.1016/j.jclinepi.2007.05.013.
- Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3. http://dx.doi.org/10.1186/1471-2288-3-25.
- Whiting PF, Weswood ME, Rutjes AW, Reitsma JB, Bossuyt PN, Kleijnen J. Evaluation of QUADAS, a tool for the quality assessment of diagnostic accuracy studies. BMC Med Res Methodol 2006;6. http://dx.doi.org/10.1186/1471-2288-6-9.
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529-36. http://dx.doi.org/10.7326/0003-4819-155-8-201110180-00009.
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982-90. http://dx.doi.org/10.1016/j.jclinepi.2005.02.022.
- Takwoingi Y, Deeks J. MetaDAS: A SAS Macro for Meta-Analysis of Diagnostic Accuracy Studies – User Guide Version 1.3 2010. http://srdta.cochrane.org/sites/srdta.cochrane.org/files/uploads/MetaDAS%20Readme%20v13%20May%202012.pdf (accessed 25 October 2012).
- Macaskill P, Gatsonis C, Deeks J, Harbord R, Takwoingi Y, Deeks JJ, et al. Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy: Version 10. The Cochrane Collaboration; 2010.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Curtis L. Unit Costs of Health and Social Care 2012. Canterbury: PSSRU, University of Kent; 2012.
- Drummond M, O’Brien BJ, Stoddart GL, Torrance GW. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 1997.
- Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of cost-effectiveness acceptability curves. Health Econ 2001;10:779-87. http://dx.doi.org/10.1002/hec.635.
- Barton GR, Briggs AH, Fenwick EAL. Optimal cost-effectiveness decisions: the role of the cost-effectiveness acceptability curve (CEAC), the cost-effectiveness acceptability frontier (CEAF), and the expected value of perfection information (EVPI). Value Health 2008;11:886-97. http://dx.doi.org/10.1111/j.1524-4733.2008.00358.x.
- Adams LA, Bulsara M, Rossi E, DeBoer B, Speers D, George J, et al. Hepascore: an accurate validated predictor of liver fibrosis in chronic hepatitis C infection. Clin Chem 2005;51:1867-73. http://dx.doi.org/10.1373/clinchem.2005.048389.
- Ahmad W, Ijaz B, Javed FT, Gull S, Kausar H, Sarwar MT, et al. A comparison of four fibrosis indexes in chronic HCV: development of new fibrosis-cirrhosis index (FCI). BMC Gastroenterol 2011;11. http://dx.doi.org/10.1186/1471-230X-11-44.
- Al-Mohri H, Cooper C, Murphy T, Klein MB. Validation of a simple model for predicting liver fibrosis in HIV/hepatitis C virus-coinfected patients. HIV Med 2005;6:375-8. http://dx.doi.org/10.1111/j.1468-1293.2005.00330.x.
- Anaparthy R, Guturu P, Snyder N, Sood G. Diagnostic utility of APRI for staging of liver disease in hepatitis C patients with ESRD on hemodialysis. Gastroenterology 2009;136:A833-4. http://dx.doi.org/10.1016/S0016-5085(09)63842-7.
- Arena U, Vizzutti F, Abraldes JG, Corti G, Stasi C, Moscarella S, et al. Reliability of transient elastography for the diagnosis of advanced fibrosis in chronic hepatitis C. Gut 2008;57:1288-93. http://dx.doi.org/10.1136/gut.2008.149708.
- Beckebaum S, Iacob S, Klein CG, Dechene A, Varghese J, Baba HA, et al. Assessment of allograft fibrosis by transient elastography and noninvasive biomarker scoring systems in liver transplant patients. Transplantation 2010;89:983-93. http://dx.doi.org/10.1097/TP.0b013e3181cc66ca.
- Bejarano G, Vergara M, Dalmau B, Puig J, Bella MR, Suarez D, et al. Prospective evaluation of liver fibrosis in chronic viral hepatitis C infection using the Sabadell NIHCED (Non-Invasive Hepatitis-C-Related Cirrhosis Early Detection) index. Revista Espanola De Enfermedades Digestivas 2009;101:325-35. http://dx.doi.org/10.4321/S1130-01082009000500004.
- Ben Jazia E, Kaabia N, Benabdelkader A, Khalifa M, Harrabi I, Braham A, et al. Noninvasive fibrosis markers for the prediction of significant fibrosis in patients with chronic hepatitis C virus infection in Tunisia. Infect Dis Clin Pract 2009;17:385-7. http://dx.doi.org/10.1097/IPC.0b013e3181bf60d3.
- Berg T, Sarrazin C, Hinrichsen H, Buggisch P, Gerlach T, Zachoval R, et al. Does noninvasive staging of fibrosis challenge liver biopsy as a gold standard in chronic hepatitis C?. Hepatology 2004;39:1456-7. http://dx.doi.org/10.1002/hep.20226.
- Borroni G, Ceriani R, Tommasini MA, Maltempo C, Felline C, Contu L, et al. Platelet count: a simple marker of liver cirrhosis in chronic hepatitis C (CHC) infection. J Hepatol 2002;36. http://dx.doi.org/10.1016/S0168-8278(02)80146-5.
- Bourliere M, Penaranda G, Renou C, Botta-Fridlund D, Tran A, Portal I, et al. Validation and comparison of indexes for fibrosis and cirrhosis prediction in chronic hepatitis C patients: proposal for a pragmatic approach classification without liver biopsies. J Viral Hepat 2006;13:659-70. http://dx.doi.org/10.1111/j.1365-2893.2006.00736.x.
- Boursier J, Bacq Y, Halfon P, Leroy V, De Ledinghen V, De Muret A, et al. Improved diagnostic accuracy of blood tests for severe fibrosis and cirrhosis in chronic hepatitis C. Eur J Gastroenterol Hepatol 2009;21:28-3. http://dx.doi.org/10.1097/MEG.0b013e32830cebd7.
- Boursier J, de Ledinghen V, Zarski J-P, Fouchard-Hubert I, Gallois Y, Oberti F, et al. Comparison of eight diagnostic algorithms for liver fibrosis in hepatitis C: new algorithms are more precise and entirely noninvasive. Hepatology 2012;55:58-67. http://dx.doi.org/10.1002/hep.24654.
- Burton MJ, Pham H, Sunesara I, McGloster N, Oliver N, McGuire BM. Performance of the APRI score in African Americans with chronic hepatitis C. Hepatology 2010;52:1236A-7A.
- Cales P, Boursier J, Bertrais S, Oberti F, Gallois Y, Fouchard-Hubert I, et al. Optimization and robustness of blood tests for liver fibrosis and cirrhosis. Clin Biochem 2010;43:1315-22. http://dx.doi.org/10.1016/j.clinbiochem.2010.08.010.
- Calvaruso V, Camma C, Di Marco V, Maimone S, Bronte F, Enea M, et al. Fibrosis staging in chronic hepatitis C: analysis of discordance between transient elastography and liver biopsy. J Viral Hepat 2010;17:469-74. http://dx.doi.org/10.1111/j.1365-2893.2009.01199.x.
- Cardoso A-C, Carvalho-Filho RJ, Stern C, Dipumpo A, Giuily N, Ripault M-P, et al. Direct comparison of diagnostic performance of transient elastography in patients with chronic hepatitis B and chronic hepatitis C. Liver Int 2012;32:612-21. http://dx.doi.org/10.1111/j.1478-3231.2011.02660.x.
- Carrion JA, Navasa M, Bosch J, Bruguera M, Gilabert R, Forns X. Transient elastography for diagnosis of advanced fibrosis and portal hypertension in patients with hepatitis C recurrence after liver transplantation. Liver Transpl 2006;12:1791-8. http://dx.doi.org/10.1002/lt.20857.
- Carvalho-Filho RJ, Schiavon LL, Narciso-schiavon JL, Sampaio JP, Lanzoni VP, Ferraz MLG, et al. Optimized cutoffs improve performance of the aspartate aminotransferase to platelet ratio index for predicting significant liver fibrosis in human immunodeficiency virus/hepatitis C virus co-infection. Liver Int 2008;28:486-93. http://dx.doi.org/10.1111/j.1478-3231.2008.01675.x.
- Castera L, Bail BL, Roudot-Thoraval F, Bernard PH, Foucher J, Merrouche W, et al. Early detection in routine clinical practice of cirrhosis and oesophageal varices in chronic hepatitis C: comparison of transient elastography (FibroScan) with standard laboratory tests and non-invasive scores. J Hepatol 2009;50:59-68. http://dx.doi.org/10.1016/j.jhep.2008.08.018.
- Castera L, Loko MA, Dabis F, Le Bail B, Winnock M, Coureau G, et al. Validation of simple indexes (FIB-4, APRI, forns index) and platelet count for non invasive prediction of liver fibrosis in HIV-HCV coinfected French patients. Hepatology 2007;46.
- Ceriani R, Borroni G, Tommasini MA, Maltempo C, Felline C, Contu L, et al. Platelet count and AST/ALT ratio can predict liver cirrhosis in chronic HCV infection. J Hepatol 2001;34. http://dx.doi.org/10.1016/S0168-8278(01)81119-3.
- Chen CH, Lin ST, Yang CC, Yeh YH, Kuo CL, Nien CK. The accuracy of sonography in predicting steatosis and fibrosis in chronic hepatitis C. Dig Dis Sci 2008;53:1699-706. http://dx.doi.org/10.1007/s10620-007-0048-2.
- Cheung RC, Currie S, Shen H, Bini EJ, Ho SB, Anand BS, et al. Can we predict the degree of fibrosis in chronic hepatitis C patients using routine blood tests in our daily practice?. J Clin Gastroenterol 2008;42:827-34. http://dx.doi.org/10.1097/MCG.0b013e318046ea9a.
- Cho HJ, Seo YS, Lee KG, Hyun JJ, An H, Keum B, et al. Serum aminotransferase levels instead of etiology affects the accuracy of transient elastography in chronic viral hepatitis patients. J Gastroenterol Hepatol 2011;26:492-500. http://dx.doi.org/10.1111/j.1440-1746.2010.06419.x.
- Christensen C, Bruden D, Livingston S, Deubner H, Homan C, Smith K, et al. Diagnostic accuracy of a fibrosis serum panel (FIBROSpect II) compared with Knodell and Ishak liver biopsy scores in chronic hepatitis C patients. J Viral Hepatol 2006;13:652-8. http://dx.doi.org/10.1111/j.1365-2893.2006.00743.x.
- Chrysanthos NV, Papatheodoridis GV, Savvas S, Kafiri G, Petraki K, Manesis EK, et al. Aspartate aminotransferase to platelet ratio index for fibrosis evaluation in chronic viral hepatitis. Eur J Gastroenterol Hepatol 2006;18:389-96. http://dx.doi.org/10.1097/00042737-200604000-00012.
- Cobbold JFL, Crossey MME, Colman P, Goldin RD, Murphy PS, Patel N, et al. Optimal combinations of ultrasound-based and serum markers of disease severity in patients with chronic hepatitis C. J Viral Hepatol 2010;17:537-45. http://dx.doi.org/10.1111/j.1365-2893.2009.01209.x.
- Colletta C, Smirne C, Fabris C, Toniutto P, Rapetti R, Minisini R, et al. Value of two noninvasive methods to detect progression of fibrosis among HCV carriers with normal aminotransferases. Hepatology 2005;42:838-45. http://dx.doi.org/10.1002/hep.20814.
- Corradi F, Piscaglia F, Flori S, D’Errico-Grigioni A, Vasuri F, Tame MR, et al. Assessment of liver fibrosis in transplant recipients with recurrent HCV infection: usefulness of transient elastography. Dig Liver Dis 2009;41:217-25. http://dx.doi.org/10.1016/j.dld.2008.06.009.
- Crespo SM, Bridges MD, Keaveny AP, Nakhleh RE, McPhail A, Pungpapong S. Magnetic resonance elastography is superior to the HULF index and APRI in evaluating fibrosis due to recurrent HCV after liver transplantation. Hepatology 2010;52:843A-4A.
- Cross TJS, Calvaruso V, Maimone S, Carey I, Chang TP, Pleguezuelo M, et al. Prospective comparison of Fibroscan, King’s score and liver biopsy for the assessment of cirrhosis in chronic hepatitis C infection. J Viral Hepatol 2010;17:546-54. http://dx.doi.org/10.1111/j.1365-2893.2009.01210.x.
- da Silva RG, Fakhouri R, do Nascimento TVB, Santos IM, Barbosa LMM. Aspartate aminotransferase-to-platelet ratio index for fibrosis and cirrhosis prediction in chronic hepatitis C patients. Braz J Infect Dis 2008;12:15-9.
- Danila M, Sporea I, Sirli R, Bota S, Braticevici CF, Petrisor A, et al. Acoustic radiation force impulse elastography (ARFI) for predicting the severity of fibrosis in patients with chronic HCV hepatitis – a bicentric Romanian study. Gastroenterology 2011;140.
- de Ledinghen V, Douvin C, Kettaneh A, Ziol M, Roulot D, Marcellin P, et al. Diagnosis of hepatic fibrosis and cirrhosis by transient elastography in HIV/hepatitis C virus-coinfected patients. J Acquir Immune Defic Syndr 2006;41:175-9. http://dx.doi.org/10.1097/01.qai.0000194238.15831.c7.
- Degos F, Perez P, Roche B, Mahmoudi A, Asselineau J, Voitot H, et al. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study). J Hepatol 2010;53:1013-21. http://dx.doi.org/10.1016/j.jhep.2010.05.035.
- Dinesen L, Caspary WF, Chapman RW, Dietrich CF, Sarrazin C, Braden B. C-13-methacetin-breath test compared to also noninvasive biochemical blood tests in predicting hepatic fibrosis and cirrhosis in chronic hepatitis C. Dig Liver Dis 2008;40:743-8. http://dx.doi.org/10.1016/j.dld.2008.01.013.
- Esmat G, Metwally M, Zalata KR, Gadalla S, Abdel-Hamid M, Abouzied A, et al. Evaluation of serum biomarkers of fibrosis and injury in Egyptian patients with chronic hepatitis C. J Hepatol 2007;46:620-7. http://dx.doi.org/10.1016/j.jhep.2006.12.010.
- Fabris C, Smirne C, Toniutto P, Colletta C, Rapetti R, Minisini R, et al. Assessment of liver fibrosis progression in patients with chronic hepatitis C and normal alanine aminotransferase values: the role of AST to the platelet ratio index. Clin Biochem 2006;39:339-43. http://dx.doi.org/10.1016/j.clinbiochem.2006.01.011.
- Fahmy MI, Badran HM. Comparison of transient elastography to Doppler indices in prediction of hepatitis C induced liver fibrosis and cirrhosis. Egypt J Radiol Nucl Med 2011;42:111-17. http://dx.doi.org/10.1016/j.ejrnm.2011.05.001.
- Fontaine H, Nalpas B, Vallet-Pichard A, Mallet V, Pol S. Low diagnosis accuracy of fibrotest and Fib-4 in HCV-infected hemodialysis and kidney recipients. Hepatology 2009;50.
- Fontana RJ, Goodman ZD, Dienstag JL, Bonkovsky HL, Naishadham D, Sterling RK, et al. Relationship of serum fibrosis markers with liver fibrosis stage and collagen content in patients with advanced chronic hepatitis C. Hepatology 2008;47:789-98. http://dx.doi.org/10.1002/hep.22099.
- Fontanges T, Bailly F, Trepo E, Chevallier M, Maynard-Muet M, Nalet B, et al. Discordance between biochemical markers of liver activity and fibrosis (Actitest-Fibrotest) and liver biopsy in patients with chronic hepatitis C. Gastroenterol Clin Biol 2008;32:858-65. http://dx.doi.org/10.1016/j.gcb.2008.05.019.
- Forestier J, Dumortier J, Guillaud O, Ecochard M, Roman S, Boillot O, et al. Noninvasive diagnosis and prognosis of liver cirrhosis: a comparison of biological scores, elastometry, and metabolic liver function tests. Eur J Gastroenterol Hepatol 2010;22:532-40. http://dx.doi.org/10.1097/MEG.0b013e3283343f58.
- Forns X, Ampurdanes S, Llovet JM, Aponte J, Quinto L, Martinez-Bauer E, et al. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology 2002;36:986-92. http://dx.doi.org/10.1053/jhep.2002.36128.
- Fraquelli M, Rigamonti C, Casazza G, Donato MF, Ronchi G, Conte D, et al. Etiology-related determinants of liver stiffness values in chronic viral hepatitis B or C. J Hepatol 2011;54:621-8. http://dx.doi.org/10.1016/j.jhep.2010.07.017.
- Fujii H, Enomoto M, Fukushima W, Ohfuji S, Mori M, Kobayashi S, et al. Noninvasive laboratory tests proposed for predicting cirrhosis in patients with chronic hepatitis C are also useful in patients with non-alcoholic steatohepatitis. J Gastroenterology 2009;44:608-14. http://dx.doi.org/10.1007/s00535-009-0046-6.
- Fujimoto K, Tonan T, Azuma S, Kage M, Nakashima O, Johkoh T, et al. Evaluation of the mean and entropy of apparent diffusion coefficient values in chronic hepatitis C: correlation with pathologic fibrosis stage and inflammatory activity grade. Radiology 2011;258:739-48. http://dx.doi.org/10.1148/radiol.10100853.
- Gaia S, Carenzi S, Barilli AL, Bugianesi E, Smedile A, Brunello F, et al. Reliability of transient elastography for the detection of fibrosis in non-alcoholic fatty liver disease and chronic viral hepatitis. J Hepatol 2011;54:64-71. http://dx.doi.org/10.1016/j.jhep.2010.06.022.
- Ganne-Carrie N, Ziol M, De Ledinghen V, Douvin C, Marcellin P, Castera L, et al. Accuracy of liver stiffness measurement for the diagnosis of cirrhosis in patients with chronic liver diseases. Hepatology 2006;44:1511-17. http://dx.doi.org/10.1002/hep.21420.
- Gara N, Kleiner DE, Zhao X, Koh C, Rotman Y, Heller T, et al. Performance of transient elastography and APRI for prediction of advanced liver disease in a north American population with chronic hepatitis C. Hepatology 2011;54.
- Giannini EG, Zaman A, Ceppa P, Mastracci L, Risso D, Testa R. A simple approach to noninvasively identifying significant fibrosis in chronic hepatitis C patients in clinical practice. J Clin Gastroenterol 2006;40:521-7. http://dx.doi.org/10.1097/00004836-200607000-00011.
- Gobel T, Vordenwulbecke S, Hauck K, Fey H, Haussinger D, Erhardt A. New multi protein patterns differentiative liver fibrosis stages and hepatocellular carcinoma in chronic hepatitis C serum samples. World J Gastroenterol 2006;12:7604-12.
- Guechot J, Lasnier E, Sturm N, Paris A, Zarski J-P. AHEFs . Automation of the Hepascore and validation as a biochemical index of liver fibrosis in patients with chronic hepatitis C from the ANRS HC EP 23 Fibrostar cohort. Clin Chim Acta 2010;411:86-91. http://dx.doi.org/10.1016/j.cca.2009.10.011.
- Guechot J, Laudat A, Loria A, Serfaty L, Poupon R, Giboudeau J. Diagnostic accuracy of hyaluronan and type III procollagen amino-terminal peptide serum assays as markers of liver fibrosis in chronic viral hepatitis C evaluated by ROC curve analysis. Clin Chem 1996;42:558-63.
- Guzelbulut F, Cetinkaya ZA, Sezikli M, Yasar B, Ozkara S, Ovunc AOK. AST–platelet ratio index, Forns index and FIB-4 in the prediction of significant fibrosis and cirrhosis in patients with chronic hepatitis C. Turk J Gastroenterol 2011;22:279-85.
- Halfon P, Bacq Y, De Muret A, Penaranda G, Bourliere M, Ouzan D, et al. Comparison of test performance profile for blood tests of liver fibrosis in chronic hepatitis C. J Hepatol 2007;46:395-402. http://dx.doi.org/10.1016/j.jhep.2006.09.020.
- Halfon P, Bourliere M, Deydier R, Botta-Fridlund D, Renou C, Tran A, et al. Independent prospective multicenter validation of biochemical markers (fibrotest-actitest) for the prediction of liver fibrosis and activity in patients with chronic hepatitis C: the fibropaca study. Am J Gastroenterol 2006;101:547-55. http://dx.doi.org/10.1111/j.1572-0241.2006.00411.x.
- Halfon P, Bourliere M, Penaranda G, Deydier R, Renou C, Botta-Fridlund D, et al. Accuracy of hyaluronic acid level for predicting liver fibrosis stages in patients with hepatitis C virus. Comp Hepatol 2005;4. http://dx.doi.org/10.1186/1476-5926-4-6.
- Harada N, Soejima Y, Taketomi A, Yoshizumi T, Ikegami T, Yamashita Y-i, et al. Assessment of graft fibrosis by transient elastography in patients with recurrent hepatitis C after living donor liver transplantation. Transplantation 2008;85:69-74. http://dx.doi.org/10.1097/01.tp.0000297248.18483.16.
- Hsieh YY, Tung SY, Lee K, Wu CS, Wei KL, Shen CH, et al. Routine blood tests to predict liver fibrosis in chronic hepatitis C. World J Gastroenterol 2012;18:746-53. http://dx.doi.org/10.3748/wjg.v18.i8.746.
- Iacobellis A, Fusilli S, Mangia A, Clemente R, Festa V, Giacobbe A, et al. Ultrasonographic and biochemical parameters in the non-invasive evaluation of liver fibrosis in hepatitis C virus chronic hepatitis. Aliment Pharmacol Ther 2005;22:769-74. http://dx.doi.org/10.1111/j.1365-2036.2005.02633.x.
- Imperiale TF, Said AT, Cummings OW, Born LJ. Need for validation of clinical decision aids: use of the AST/ALT ratio in predicting cirrhosis in chronic hepatitis C. Am J Gastroenterol 2000;95:2328-32. http://dx.doi.org/10.1111/j.1572-0241.2000.02322.x.
- Islam S, Antonsson L, Westin J, Lagging M. Cirrhosis in hepatitis C virus-infected patients can be excluded using an index of standard biochemical serum markers. Scand J Gastroenterol 2005;40:867-72. http://dx.doi.org/10.1080/00365520510015674.
- Iushchuk ND, Znoiko OO, Safiullina NK, Dudina KR, Kelli EI, Klimova EA, et al. Diagnostic significance of type IV collagen and hyaluronic acid in the serum of patients with chronic hepatitis C for staging hepatic fibrosis. Ter Arkh 2005;77:50-5.
- Kalantari H, Hoseini H, Babak A, Yaran M. Validation of hepascore as a predictor of liver fibrosis in patients with chronic hepatitis C infection. Hepat Res Treat 2011;2011. http://dx.doi.org/10.1155/2011/972759.
- Kamphues C, Lotz K, Rocken C, Berg T, Eurich D, Pratschke J, et al. Chances and limitations of non-invasive tests in the assessment of liver fibrosis in liver transplant patients. Clin Transplant 2010;24:652-9. http://dx.doi.org/10.1111/j.1399-0012.2009.01152.x.
- Kandemir O, Polat G, Saracoglu G, Tasdelen B. The predictive role of AST level, prothrombin time, and platelet count in the detection of liver fibrosis in patients with chronic hepatitis C. Turk J Med Sci 2009;39:857-62.
- Kelleher TB, Mehta SH, Bhaskar R, Sulkowski M, Astemborski J, Thomas DL, et al. Prediction of hepatic fibrosis in HIV/HCV co-infected patients using serum fibrosis markers: the SHASTA index. J Hepatol 2005;43:78-84. http://dx.doi.org/10.1016/j.jhep.2005.02.025.
- Khan DA, Fatima Tuz Z, Khan FA, Mubarak A. Evaluation of diagnostic accuracy of APRI for prediction of fibrosis in hepatitis C patients. J Ayub Med Coll Abbottabad 2008;20:122-6.
- Kim SU, Jang HW, Cheong JY, Kim JK, Lee MH, Kim DJ, et al. The usefulness of liver stiffness measurement using FibroScan in chronic hepatitis C in South Korea: a multicenter, prospective study. J Gastroenterol Hepatol 2011;26:171-8. http://dx.doi.org/10.1111/j.1440-1746.2010.06385.x.
- Koizumi Y, Hirooka M, Kisaka Y, Konishi I, Abe M, Murakami H, et al. Liver fibrosis in patients with chronic hepatitis C: noninvasive diagnosis by means of real-time tissue elastography – establishment of the method for measurement. Radiology 2011;258:610-17. http://dx.doi.org/10.1148/radiol.10100319.
- Lackner C, Struber G, Liegl B, Leibl S, Ofner P, Bankuti C, et al. Comparison and validation of simple noninvasive tests for prediction of fibrosis in chronic hepatitis C. Hepatology 2005;41:1376-82. http://dx.doi.org/10.1002/hep.20717.
- Ladero JM, Delkader J, Ortega L, Fernandez C, Devesa MJ, Lopez-Alonso G, et al. Non-invasive evaluation of the fibrosis stage in chronic hepatitis C: a comparative analysis of nine scoring methods. Scand J Gastroenterol 2010;45:51-9. http://dx.doi.org/10.3109/00365520903305544.
- Lee VS, Miller FH, Omary RA, Wang Y, Ganger DR, Wang E, et al. Magnetic resonance elastography and biomarkers to assess fibrosis from recurrent hepatitis C in liver transplant recipients. Transplantation 2011;92:581-6. http://dx.doi.org/10.1097/TP.0b013e31822805fa.
- Leroy V, Hilleret M-N, Sturm N, Trocme C, Renversez J-C, Faure P, et al. Prospective comparison of six non-invasive scores for the diagnosis of liver fibrosis in chronic hepatitis C. J Hepatol 2007;46:775-82. http://dx.doi.org/10.1016/j.jhep.2006.12.013.
- Leroy V, Monier F, Bottari S, Trocme C, Sturm N, Hilleret M-N, et al. Circulating matrix metalloproteinases 1, 2, 9 and their inhibitors TIMP-1 and TIMP-2 as serum markers of liver fibrosis in patients with chronic hepatitis C: comparison with PIIINP and hyaluronic acid. Am J Gastroenterol 2004;99:271-9. http://dx.doi.org/10.1111/j.1572-0241.2004.04055.x.
- Leroy V, Sturm N, Guechot J, Zafrani ES, Paris A, Bosson JL, et al. Steato-hepatitis has a strong impact on liver stiffness measured by transient elastography in chronic hepatitis C patients. J Hepatol 2011;54. http://dx.doi.org/10.1016/S0168-8278(11)60340-1.
- Lewin M, Poujol-Robert A, Boelle P-Y, Wendum D, Lasnier E, Viallon M, et al. Diffusion-weighted magnetic resonance imaging for the assessment of fibrosis in chronic hepatitis C. Hepatology 2007;46:658-65. http://dx.doi.org/10.1002/hep.21747.
- Lieber CS, Weiss DG, Morgan TR, Paronetto F. Aspartate aminotransferase to platelet ratio index in patients with alcoholic liver fibrosis. Am J Gastroenterol 2006;101:1500-8. http://dx.doi.org/10.1111/j.1572-0241.2006.00610.x.
- Liu C-H, Lin J-W, Tsai F-C, Yang P-M, Lai M-Y, Chen J-H, et al. Noninvasive tests for the prediction of significant hepatic fibrosis in hepatitis C virus carriers with persistently normal alanine aminotransferases. Liver Int 2006;26:1087-94. http://dx.doi.org/10.1111/j.1478-3231.2006.01355.x.
- Liu CH, Hsu SJ, Lin JW, Hwang JJ, Liu CJ, Yang PM, et al. Noninvasive diagnosis of hepatic fibrosis in patients with chronic hepatitis C by splenic Doppler impedance index. Clin Gastroenterol Hepatol 2007;5:1199-206. http://dx.doi.org/10.1016/j.cgh.2007.07.017.
- Liu CH, Liang CC, Huang KW, Liu CJ, Chen SI, Lin JW, et al. Transient elastography to assess hepatic fibrosis in hemodialysis chronic hepatitis C patients. Clin J Am Soc Nephrol 2011;6:1057-64. http://dx.doi.org/10.2215/CJN.04320510.
- Loko MA, Castera L, Dabis F, Le Bail B, Winnock M, Coureau G, et al. Validation and comparison of simple noninvasive indexes for predicting liver fibrosis in HIV-HCV-coinfected patients: ANRS CO3 aquitaine cohort. Am J Gastroenterol 2008;103:1973-80. http://dx.doi.org/10.1111/j.1572-0241.2008.01954.x.
- Lupsor M, Badea R, Stefanescu H, Grigorescu M, Sparchez Z, Serban A, et al. Analysis of histopathological changes that influence liver stiffness in chronic hepatitis C: results from a cohort of 324 patients. J Gastrointestin Liver Dis 2008;17:155-63.
- Macias J, Giron-Gonzalez JA, Gonzalez-Serrano M, Merino D, Cano P, Mira JA, et al. Prediction of liver fibrosis in human immunodeficiency virus/hepatitis C virus coinfected patients by simple non-invasive indexes. Gut 2006;55:409-14. http://dx.doi.org/10.1136/gut.2005.065904.
- Macias J, Mira J, Gilabert I, Neukam K, Roldan C, Viloria M, et al. Combined use of aspartate aminotransferase, platelet count and matrix metalloproteinase 2 measurements to predict liver fibrosis in HIV/hepatitis C virus-coinfected patients. HIV Med 2011;12:14-21. http://dx.doi.org/10.1111/j.1468-1293.2010.00836.x.
- Martinez SM, Fernandez-Varo G, Gonzalez P, Sampson E, Bruguera M, Navasa M, et al. Assessment of liver fibrosis before and after antiviral therapy by different serum marker panels in patients with chronic hepatitis C. Aliment Pharmacol Ther 2011;33:138-48. http://dx.doi.org/10.1111/j.1365-2036.2010.04500.x.
- Morikawa H, Fukuda K, Kobayashi S, Fujii H, Iwai S, Enomoto M, et al. Real-time tissue elastography as a tool for the noninvasive assessment of liver stiffness in patients with chronic hepatitis C. J Gastroenterology 2011;46:350-8. http://dx.doi.org/10.1007/s00535-010-0301-x.
- Murawaki Y, Koda M, Okamoto K, Mimura K, Kawasaki H. Diagnostic value of serum type IV collagen test in comparison with platelet count for predicting the fibrotic stage in patients with chronic hepatitis C. J Gastroenterol Hepatol 2001;16:777-81. http://dx.doi.org/10.1046/j.1440-1746.2001.02515.x.
- Nitta Y, Kawabe N, Hashimoto S, Harata M, Komura N, Kobayashi K, et al. Liver stiffness measured by transient elastography correlates with fibrosis area in liver biopsy in patients with chronic hepatitis C. Hepatol Res 2009;39:675-84. http://dx.doi.org/10.1111/j.1872-034X.2009.00500.x.
- Nojiri S. Noninvasive evaluation of hepatic fibrosis in HCV-infected patients using EOB-MR imaging. J Hepatol 2010;52:S169-70. http://dx.doi.org/10.1016/S0168-8278(10)60417-5.
- Nunes D, Fleming C, Offner G, O’Brien M, Tumilty S, Fix O, et al. HIV infection does not affect the performance of noninvasive markers of fibrosis for the diagnosis of hepatitis C virus-related liver disease. J Acquir Immune Defic Syndr 2005;40:538-44. http://dx.doi.org/10.1097/01.qai.0000184856.31695.bf.
- Obara N, Ueno Y, Fukushima K, Nakagome Y, Kakazu E, Kimura O, et al. Transient elastography for measurement of liver stiffness measurement can detect early significant hepatic fibrosis in Japanese patients with viral and nonviral liver diseases. J Gastroenterol 2008;43:720-8. http://dx.doi.org/10.1007/s00535-008-2225-2.
- Oliveira AC, Santos VN, Salgado AL, Lanzoni VP, Nogueira MD, Martins JR, et al. Serum hyaluronic acid and the AST/ALT ratio in the diagnosis of advanced fibrosis in patients with non alcoholic liver disease and chronic hepatitis C. J Hepatol 2005;42. http://dx.doi.org/10.1016/S0168-8278(05)82096-3.
- Orlacchio A, Bolacchi F, Petrella MC, Pastorelli D, Bazzocchi G, Angelico M, et al. Liver contrast enhanced ultrasound perfusion imaging in the evaluation of chronic hepatitis C fibrosis: preliminary results. Ultrasound Med Biol 2011;37:1-6. http://dx.doi.org/10.1016/j.ultrasmedbio.2010.10.012.
- Paggi S, Colli A, Fraquelli M, Vigano M, Del Poggio P, Facciotto C, et al. A non-invasive algorithm accurately predicts advanced fibrosis in hepatitis C: a comparison using histology with internal-external validation. J Hepatol 2008;49:564-71. http://dx.doi.org/10.1016/j.jhep.2008.07.007.
- Parise ER, Oliveira AC, Figueiredo-Mendes C, Lanzoni V, Martins J, Nader H, et al. Noninvasive serum markers in the diagnosis of structural liver damage in chronic hepatitis C virus infection. Liver Int 2006;26:1095-9. http://dx.doi.org/10.1111/j.1478-3231.2006.01356.x.
- Park GJ, Lin BP, Ngu MC, Jones DB, Katelaris PH. Aspartate aminotransferase: alanine aminotransferase ratio in chronic hepatitis C infection: is it a useful predictor of cirrhosis?. J Gastroenterol Hepatol 2000;15:386-90. http://dx.doi.org/10.1046/j.1440-1746.2000.02172.x.
- Patel K, Friedrich-Rust M, Lurie Y, Grigorescu M, Stanciu C, Lee C-M, et al. FibroSURE and FibroScan in relation to treatment response in chronic hepatitis C virus. World J Gastroenterol 2011;17:4581-9. http://dx.doi.org/10.3748/wjg.v17.i41.4581.
- Pohl A, Behling C, Oliver D, Kilani M, Monson P, Hassanein T. Serum aminotransferase levels and platelet counts as predictors of degree of fibrosis in chronic hepatitis C virus infection. Am J Gastroenterol 2001;96:3142-6. http://dx.doi.org/10.1111/j.1572-0241.2001.05268.x.
- Poynard T, De Ledinghen V, Zarski JP, Stanciu C, Munteanu M, Vergniol J, et al. Relative performances of FibroTest, Fibroscan, and biopsy for the assessment of the stage of liver fibrosis in patients with chronic hepatitis C: a step toward the truth in the absence of a gold standard. J Hepatol 2012;56:541-8. http://dx.doi.org/10.1016/j.jhep.2011.08.007.
- Prati GM, D’Ambrosio R, Fraquelli M, Aghemo A, Rumi MG, Ronchi G, et al. The diagnostic accuracy of fibroscan for cirrhosis is influenced by liver morphometry in HCV patients with an SVR. Hepatology 2011;54.
- Qiu Y, Hoshida YJ, Kato N, Moriyama M, Otsuka M, Taniguchi H, et al. A simple combination of serum type IV collagen and prothrombin time to diagnose cirrhosis in patients with chronic active hepatitis C. Hepatol Res 2004;30:214-20. http://dx.doi.org/10.1016/j.hepres.2004.10.006.
- Reedy DW, Loo AT, Levine RA. AST/ALT ratio <=1 is not diagnostic of cirrhosis in patients with chronic hepatitis C. Dig Dis Sci 1998;43:2156-9. http://dx.doi.org/10.1023/A:1018888021118.
- Ronot M, Asselah T, Paradis V, Michoux N, Dorvillius M, Baron G, et al. Liver fibrosis in chronic hepatitis C virus infection: differentiating minimal from intermediate fibrosis with perfusion CT. Radiology 2010;256:135-42. http://dx.doi.org/10.1148/radiol.10091295.
- Rossi E, Adams L, Prins A, Bulsara M, De Boer B, Garas G, et al. Validation of the FibroTest biochemical markers score in assessing liver fibrosis in hepatitis C patients. Clin Chem 2003;49:450-4. http://dx.doi.org/10.1373/49.3.450.
- Rossini A, Cabassa P, Gatti E, Contessi GB, Morone M, Maroldi R. Assessment of liver fibrosis by acoustic radiation force elastography in healthy subjects and patients with chronic hepatitis C. Hepatology 2010;52.
- Said Y, Salem M, Mouelhi L, Mekki H, Houissa F, Ben Rejeb M, et al. Correlation between liver biopsy and fibrotest in the evaluation of hepatic fibrosis in patients with chronic hepatitis C. Tunis Med 2010;88:573-8.
- Saitou Y, Shiraki K, Yamanaka Y, Yamaguchi Y, Kawakita T, Yamamoto N, et al. Noninvasive estimation of liver fibrosis and response to interferon therapy by a serum fibrogenesis marker, YKL-40, in patients with HCV-associated liver disease. World J Gastroenterol 2005;11:476-81.
- Sanvisens A, Serra I, Tural C, Tor J, Ojanguren I, Barluenga E, et al. Hyaluronic acid, transforming growth factor-beta1 and hepatic fibrosis in patients with chronic hepatitis C virus and human immunodeficiency virus co-infection. J Viral Hepat 2009;16:513-18. http://dx.doi.org/10.1111/j.1365-2893.2009.01103.x.
- Schiavon LL, Filho RJC, Narciso JL, Sampaio JP, Lanzoni VP, Ferraz MLG, et al. Expanding the applicability of noninvasive fibrosis markers in HIV/HCV co-infected patients. Hepatology 2007;45:257-8. http://dx.doi.org/10.1002/hep.21507.
- Schiavon LL, Narciso-Schiavon JL, Carvalho Filho RJ, Sampaio JP, Medina-Pestana JO, Lanzoni VP, et al. Serum levels of YKL-40 and hyaluronic acid as noninvasive markers of liver fibrosis in haemodialysis patients with chronic hepatitis C virus infection. J Viral Hepatol 2008;15:666-74. http://dx.doi.org/10.1111/j.1365-2893.2008.00992.x.
- Schneider ARJ, Teuber G, Kriener S, Caspary WF. Noninvasive assessment of liver steatosis, fibrosis and inflammation in chronic hepatitis C virus infection. Liver Int 2005;25:1150-5. http://dx.doi.org/10.1111/j.1478-3231.2005.01164.x.
- Schneider ARJ, Teuber G, Paul K, Nikodem A, Duesterhoeft M, Caspary WF, et al. Patient age is a strong independent predictor of 13C-aminopyrine breath test results: a comparative study with histology, duplex-Doppler and a laboratory index in patients with chronic hepatitis C virus infection. Clin Exp Pharmacol Physiol 2006;33:300-4. http://dx.doi.org/10.1111/j.1440-1681.2006.04365.x.
- Sebastian G, Vario A, Guido M, Alberti A. Performance of noninvasive markers for liver fibrosis is reduced in chronic hepatitis C with normal transaminases. J Viral Hepatol 2008;15:212-18. http://dx.doi.org/10.1111/j.1365-2893.2007.00932.x.
- Sebastiani G, Halfon P, Castera L, Pol S, Thomas DL, Mangia A, et al. SAFE biopsy: a validated method for large-scale staging of liver fibrosis in chronic hepatitis C. Hepatology 2009;49:1821-7. http://dx.doi.org/10.1002/hep.22859.
- Sebastiani G, Vario A, Guido M, Noventa F, Plebani M, Pistis R, et al. Stepwise combination algorithms of non-invasive markers to diagnose significant fibrosis in chronic hepatitis C. J Hepatol 2006;44:686-93. http://dx.doi.org/10.1016/j.jhep.2006.01.007.
- Sene D, Limal N, Messous D, Ghillani-Dalbin P, Charlotte F, Thiolliere JM, et al. Biological markers of liver fibrosis and activity as non-invasive alternatives to liver biopsy in patients with chronic hepatitis C and associated mixed cryoglobulinemia vasculitis. Clin Biochem 2006;39:715-21. http://dx.doi.org/10.1016/j.clinbiochem.2006.04.019.
- Sharabash NM, Jensen DM, Cao D, Reau N, Mohanty SR, Satoskar RS, et al. Fibrospect II (FS II) score overestimates the degree of fibrosis in chronic hepatitis C (CHC) patients with significant renal dysfunction. Hepatology 2009;50.
- Shastry L, Wilson T, Lascher S, Nord JA. The utility of aspartate aminotransferase/platelet ratio index in HIV/hepatitis C-co-infected patients. AIDS 2007;21:2541-3. http://dx.doi.org/10.1097/QAD.0b013e3282f1fe59.
- Sheth SG, Flamm SL, Gordon FD, Chopra S. AST/ALT ratio predicts cirrhosis in patients with chronic hepatitis C virus infection. Am J Gastroenterol 1998;93:44-8. http://dx.doi.org/10.1111/j.1572-0241.1998.044_c.x.
- Singal AG, Thomassen LV, Gretch DR, Shuhart MC. Use of the AST to platelet ratio index in HCV/HIV co-infected patients. Aliment Pharmacol Ther 2011;33:566-77. http://dx.doi.org/10.1111/j.1365-2036.2010.04560.x.
- Sirli R, Sporea I, Bota S, Popescu A, Cornianu M. A comparative study of non-invasive methods for fibrosis assessment in chronic HCV infection. Hepat Mon 2010;10:88-94.
- Snyder N, Gajula L, Xiao S-Y, Grady J, Luxon B, Lau DTY, et al. APRI: an easy and validated predictor of hepatic fibrosis in chronic hepatitis C. J Clin Gastroenterol 2006;40:535-42. http://dx.doi.org/10.1097/00004836-200607000-00013.
- Snyder N, Nguyen A, Gajula L, Soloway R, Xiao SY, Lau DTY, et al. The APRI may be enhanced by the use of the FIBROSpect II in the estimation of fibrosis in chronic hepatitis C. Clin Chim Acta 2007;381:119-23. http://dx.doi.org/10.1016/j.cca.2007.02.046.
- Sohn JH, Kim TY, Jun DW, Eun CS, Jeon YC, Han DS. Platelet count/spleen diameter ratio is more accurate for prediction of significant and extensive fibrosis than AST-to-platelet ratio index (APRI) in patients with chronic hepatitis C. J Hepatol 2010;52. http://dx.doi.org/10.1016/S0168-8278(10)60426-6.
- Sporea I, Sirli R, Bota S, Tanaka H, Iijima H, Badea RI, et al. Is ARFI elastography useful for fibrosis evaluation in patients with chronic HCV hepatitis in our clinical practice? – A multicenter international study. Hepatology 2011;54:559A-60A. http://dx.doi.org/10.1016/j.ejrad.2012.08.018.
- Sporea I, Sirli R, Deleanu A, Tudora A, Curescu M, Cornianu M, et al. Comparison of the liver stiffness measurement by transient elastography with the liver biopsy. World J Gastroenterol 2008;14:6513-17. http://dx.doi.org/10.3748/wjg.14.6513.
- Sporea I, Sirli R, Deleanu A, Tudora A, Popescu A, Curescu M, et al. Liver stiffness measurements in patients with HBV vs HCV chronic hepatitis: a comparative study. World J Gastroenterol 2010;16:4832-7. http://dx.doi.org/10.3748/wjg.v16.i38.4832.
- Sporea I, Sirli R, Popescu A, Badea R, Lupsor M, Focsa M, et al. Is it better to use together transient elastography (TE) and acoustic radiation force impulse elastography (ARFI) for fibrosis evaluation in patients with chronic HCV hepatitis?. Gastroenterology 2011;1.
- Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006;43:1317-25. http://dx.doi.org/10.1002/hep.21178.
- Stibbe KJM, Verveer C, Francke J, Hansen BE, Zondervan PE, Kuipers EJ, et al. Comparison of non-invasive assessment to diagnose liver fibrosis in chronic hepatitis B and C patients. Scand J Gastroenterol 2011;46:962-72. http://dx.doi.org/10.3109/00365521.2011.574725.
- Sud A, Hui JM, Farrell GC, Bandara P, Kench JG, Fung C, et al. Improved prediction of fibrosis in chronic hepatitis C using measures of insulin resistance in a probability index. Hepatology 2004;39:1239-47. http://dx.doi.org/10.1002/hep.20207.
- Testa R, Testa E, Giannini E, Borro P, Milazzo S, Isola L, et al. Noninvasive ratio indexes to evaluate fibrosis staging in chronic hepatitis C: role of platelet count/spleen diameter ratio index. J Intern Med 2006;260:142-50. http://dx.doi.org/10.1111/j.1365-2796.2006.01673.x.
- Thompson A, Elliott L, Tillmann H, McHutchison J, Patel K. Paired biopsy comparison of two liver fibrosis marker panels (HCV fibrotest, hepascore) in following histological response to antiviral therapy for chronic hepatitis C (CHC). Hepatology 2009;50. http://dx.doi.org/10.1016/S0168-8278(09)60425-6.
- Thompson AJ, Clark PJ, Tillmann HL, Torbenson M, Pulkstenis E, Subramanian M, et al. Independent validation of the safe biopsy algorithm: analysis of the achieve cohorts. Gastroenterology 2010;1.
- Thompson AJ, Elliott L, Tillmann HL, McHutchison J, Patel K. A large independent, single center comparison of two commercial fibrosis marker panels (HCV FibroSure and HepaScore) for moderate-to-advanced stage fibrosis in CHC patients. Gastroenterology 2009;136. http://dx.doi.org/10.1016/S0016-5085(09)63844-0.
- Toniutto P, Fabris C, Bitetto D, Falleti E, Avellini C, Rossi E, et al. Role of AST to platelet ratio index in the detection of liver fibrosis in patients with recurrent hepatitis C after liver transplantation. J Gastroenterol Hepatol 2007;22:1904-8. http://dx.doi.org/10.1111/j.1440-1746.2006.04628.x.
- Trang T, Petersen JR, Snyder N. Non-invasive markers of hepatic fibrosis in patients co-infected with HCV and HIV: comparison of the APRI and FIB-4 index. Clin Chim Acta 2008;397:51-4. http://dx.doi.org/10.1016/j.cca.2008.07.009.
- Trifan A, Cojocariu C, Sfarti C, Stanciu C. Prospective clinical trial on the accuracy of non-invasive tests to predict liver fibrosis in chronic HCV patients. Hepatology 2009;50:1074A-5A.
- Trocme C, Leroy V, Sturm N, Hilleret MN, Bottari S, Morel F, et al. Longitudinal evaluation of a fibrosis index combining MMP-1 and PIIINP compared with MMP-9, TIMP-1 and hyaluronic acid in patients with chronic hepatitis C treated by interferon-alpha and ribavirin. J Viral Hepat 2006;13:643-51. http://dx.doi.org/10.1111/j.1365-2893.2006.00730.x.
- Tural C, Tor J, Sanvisens A, Perez-Alvarez N, Martinez E, Ojanguren I, et al. Accuracy of simple biochemical tests in identifying liver fibrosis in patients co-infected with human immunodeficiency virus and hepatitis C virus. Clin Gastroenterol Hepatol 2009;7:339-45. http://dx.doi.org/10.1016/j.cgh.2008.11.019.
- Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. Comparison with liver biopsy and FibroTest. Hepatology 2007;46:32-6. http://dx.doi.org/10.1002/hep.21669.
- Valva P, Casciato P, Diaz Carrasco JM, Gadano A, Galdame O, Galoppo MC, et al. The role of serum biomarkers in predicting fibrosis progression in pediatric and adult hepatitis C virus chronic infection. PLOS One 2011;6. http://dx.doi.org/10.1371/journal.pone.0023218.
- Varaut A, Fontaine H, Serpaggi J, Verkarre V, Vallet-Pichard A, Nalpas B, et al. Diagnostic accuracy of the fibrotest in hemodialysis and renal transplant patients with chronic hepatitis C virus. Transplantation 2005;80:1550-5. http://dx.doi.org/10.1097/01.tp.0000183399.85804.02.
- Viana MSVB, Takei K, Yamaguti DCC, Guz B, Strauss E. Use of AST platelet ratio index (APRI Score) as an alternative to liver biopsy for treatment indication in chronic hepatitis C. Ann Hepatol 2009;8:26-31.
- Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38:518-26. http://dx.doi.org/10.1053/jhep.2003.50346.
- Westin J, Ydreborg M, Islam S, Alsio A, Dhillon AP, Pawlotsky JM, et al. A non-invasive fibrosis score predicts treatment outcome in chronic hepatitis C virus infection. Scand J Gastroenterol 2008;43:73-80. http://dx.doi.org/10.1080/00365520701514461.
- Wilson LE, Torbenson M, Astemborski J, Faruki H, Spoler C, Rai R, et al. Progression of liver fibrosis among injection drug users with chronic hepatitis C. Hepatology 2006;43:788-95. http://dx.doi.org/10.1002/hep.21091.
- Wong VS, Hughes V, Trull A, Wight DG, Petrik J, Alexander GJ. Serum hyaluronic acid is a useful marker of liver fibrosis in chronic hepatitis C virus infection. J Viral Hepatol 1998;5:187-92. http://dx.doi.org/10.1046/j.1365-2893.1998.00100.x.
- Zaman A, Rosen H, Oh E, Ingram K, Smith K. Prospective assessment of FIBROSpect II hepatic to detect fibrosis in patients with chronic Hepatitis C. Am J Gastroenterol 2004;99.
- Zarski JP, Sturm N, Guechot J, Paris A, Zafrani ES, Asselah T, et al. Comparison of nine blood tests and transient elastography for liver fibrosis in chronic hepatitis C: the ANRS HCEP-23 study. J Hepatol 2012;56:55-62. http://dx.doi.org/10.1016/j.jhep.2011.05.024.
- Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology 2005;41:48-54. http://dx.doi.org/10.1002/hep.20506.
- Castera L, Bernard PH, Le Bail B, Foucher J, Trimoulet P, Merrouche W, et al. Transient elastography and biomarkers for liver fibrosis assessment and follow-up of inactive hepatitis B carriers. Aliment Pharmacol Ther 2011;33:455-65. http://dx.doi.org/10.1111/j.1365-2036.2010.04547.x.
- Chan HLY, Wong GLH, Choi PCL, Chan AWH, Chim AML, Yiu KKL, et al. Alanine aminotransferase-based algorithms of liver stiffness measurement by transient elastography (Fibroscan) for liver fibrosis in chronic hepatitis B. J Viral Hepatol 2009;16:36-44. http://dx.doi.org/10.1111/j.1365-2893.2008.01037.x.
- Chen YP, Dai L, Wang JL, Zhu YF, Feng XR, Hou JL. Model consisting of ultrasonographic and simple blood indexes accurately identify compensated hepatitis B cirrhosis. J Gastroenterol Hepatol 2008;23:1228-34. http://dx.doi.org/10.1111/j.1440-1746.2008.05421.x.
- Chen YP, Liang XE, Dai L, Zhang Q, Peng J, Zhu YF, et al. Improving transient elastography performance for detecting hepatitis B cirrhosis. Dig Liver Dis 2012;44:61-6. http://dx.doi.org/10.1016/j.dld.2011.08.004.
- Dixon JB, Bhathal PS, O’Brien PE. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology 2001;121:91-100. http://dx.doi.org/10.1053/gast.2001.25540.
- Fung J, Lai CL, Cheng C, Wu R, Wong DKH, Yuen MF. Mild-to-moderate elevation of alanine aminotransferase increases liver stiffness measurement by transient elastography in patients with chronic hepatitis B. Am J Gastroenterol 2011;106:492-6. http://dx.doi.org/10.1038/ajg.2010.463.
- Gui H-l, Gao C-f, Wang H, Liu X-e, Xie Q, Dewaele S, et al. Altered serum N-glycomics in chronic hepatitis B patients. Liver Int 2010;30:259-67. http://dx.doi.org/10.1111/j.1478-3231.2009.02170.x.
- Guo-Qiu W, Nai-Feng L, Xiao-Bo V, Linxian L, Chen Z, Lixia G, et al. The level of connective tissue growth factor in sera of patients with hepatitis B virus strongly correlates with stage of hepatic fibrosis. Viral Immunol 2010;23:71-8. http://dx.doi.org/10.1089/vim.2009.0067.
- Hongbo L, Xiaohui L, Hong K, Wei W, Yong Z. Assessing routine and serum markers of liver fibrosis in CHB patients using parallel and serial interpretation. Clin Biochem 2007;40:562-6. http://dx.doi.org/10.1016/j.clinbiochem.2007.01.022.
- Hu X, Shao J, Bai J, Wang J, Qian L. New noninvasive assessment of liver fibrosis in chronic hepatitis B: maximal accumulative respiration strain. J Ultrasound Med 2010;29:1213-21.
- Hui AY, Chan HL-Y, Wong VW-S, Liew C-T, Chim AM-L, Chan FK-L, et al. Identification of chronic hepatitis B patients without significant liver fibrosis by a simple noninvasive predictive model. Am J Gastroenterol 2005;100:616-23. http://dx.doi.org/10.1111/j.1572-0241.2005.41289.x.
- Kim BK, Kim DY, Park JY, Ahn SH, Chon CY, Kim JK, et al. Validation of FIB-4 and comparison with other simple noninvasive indices for predicting liver fibrosis and cirrhosis in hepatitis B virus-infected patients. Liver Int 2010;30:546-53. http://dx.doi.org/10.1111/j.1478-3231.2009.02192.x.
- Kim BK, Kim SA, Park YN, Cheong JY, Kim HS, Park JY, et al. Noninvasive models to predict liver cirrhosis in patients with chronic hepatitis B. Liver Int 2007;27:969-76. http://dx.doi.org/10.1111/j.1478-3231.2007.01519.x.
- Kim DY, Kim SU, Ahn SH, Park JY, Lee JM, Park YN, et al. Usefulness of FibroScan for detection of early compensated liver cirrhosis in chronic hepatitis B. Dig Dis Sci 2009;54:1758-63. http://dx.doi.org/10.1007/s10620-008-0541-2.
- Kim SU, Ahn SH, Park JY, Kang W, Kim DY, Park YN, et al. Liver stiffness measurement in combination with noninvasive markers for the improved diagnosis of B-viral liver cirrhosis. J Clin Gastroenterol 2009;43:267-71. http://dx.doi.org/10.1097/MCG.0b013e31816f212e.
- Kwok R, Gonzalez-Arce V, Kim A, Ngu MC, Lee AU. Evaluation of hepatic fibrosis in chronic hepatitis B using transient elastography. J Gastroenterol Hepatol 2009;24.
- Lee IC, Chan C-C, Huang Y-H, Huo T-I, Chu C-J, Lai C-R, et al. Comparative analysis of noninvasive models to predict early liver fibrosis in hepatitis B e antigen-negative chronic hepatitis B. J Clin Gastroenterol 2011;45:278-85. http://dx.doi.org/10.1097/MCG.0b013e3181dd5357.
- Lesmana CRA, Salim S, Hasan I, Sulaiman AS, Gani RA, Pakasi LS, et al. Diagnostic accuracy of transient elastography (FibroScan) versus the aspartate transaminase to platelet ratio index in assessing liver fibrosis in chronic hepatitis B: the role in primary care setting. J Clin Pathol 2011;64:916-20. http://dx.doi.org/10.1136/jclinpath-2011-200044.
- Li F, Zhu C-L, Zhang H, Huang H, Wei Q, Zhu X, et al. Role of hyaluronic acid and laminin as serum markers for predicting significant fibrosis in patients with chronic hepatitis B. Braz J Infect Dis 2012;16:9-14. http://dx.doi.org/10.1016/S1413-8670(12)70267-2.
- Liu H-B, Zhou J-P, Zhang Y, Lv X-H, Wang W. Prediction on liver fibrosis using different APRI thresholds when patient age is a categorical marker in patients with chronic hepatitis B. Clin Chim Acta 2011;412:33-7. http://dx.doi.org/10.1016/j.cca.2010.08.032.
- Mallet V, Dhalluin-Venier V, Roussin C, Bourliere M, Pettinelli ME, Giry C, et al. The accuracy of the FIB-4 index for the diagnosis of mild fibrosis in chronic hepatitis B. Aliment Pharmacol Ther 2009;29:409-15. http://dx.doi.org/10.1111/j.1365-2036.2008.03895.x.
- Marcellin P, Ziol M, Bedossa P, Douvin C, Poupon R, De Ledinghen V, et al. Non-invasive assessment of liver fibrosis by stiffness measurement in patients with chronic hepatitis B. Liver Int 2009;29:242-7. http://dx.doi.org/10.1111/j.1478-3231.2008.01802.x.
- Miailhes P, Pradat P, Chevallier M, Lacombe K, Bailly F, Cotte L, et al. Proficiency of transient elastography compared to liver biopsy for the assessment of fibrosis in HIV/HBV-coinfected patients. J Viral Hepat 2011;18:61-9. http://dx.doi.org/10.1111/j.1365-2893.2010.01275.x.
- Mohamadnejad M, Montazeri G, Fazlollahi A, Zamani F, Nasiri J, Nobakht H, et al. Noninvasive markers of liver fibrosis and inflammation in chronic hepatitis B-virus related liver disease. Am J Gastroenterol 2006;101:2537-45. http://dx.doi.org/10.1111/j.1572-0241.2006.00788.x.
- Myers RP, Tainturier MH, Ratziu V, Piton A, Thibault V, Imbert-Bismut F, et al. Prediction of liver histological lesions with biochemical markers in patients with chronic hepatitis B. J Hepatol 2003;39:222-30. http://dx.doi.org/10.1016/S0168-8278(03)00171-5.
- Ogawa E, Furusyo N, Murata M, Ohnishi H, Toyoda K, Taniai H, et al. Longitudinal assessment of liver stiffness by transient elastography for chronic hepatitis B patients treated with nucleoside analog. Hepatol Res 2011;41:1178-88. http://dx.doi.org/10.1111/j.1872-034X.2011.00869.x.
- Osakabe K, Ichino N, Nishikawa T, Sugiyama H, Kato M, Kitahara S, et al. Reduction of liver stiffness by antiviral therapy in chronic hepatitis B. J Gastroenterol 2011;46:1324-34. http://dx.doi.org/10.1007/s00535-011-0444-4.
- Park GJ, Katelaris PH, Jones DB, Seow F, Lin BP, Le Couteur DG, et al. The C-caffeine breath test distinguishes significant fibrosis in chronic hepatitis B and reflects response to lamivudine therapy. Aliment Pharmacol Ther 2005;22:395-403. http://dx.doi.org/10.1111/j.1365-2036.2005.02623.x.
- Park JH, Park CK, Kim ES, Park SY, Jo CM, Tak WY, et al. The diagnostic value of serum hyaluronic acid, 7S domain of type IV collagen and AST/ALT ratio as markers of hepatic fibrosis in chronic hepatitis B and cirrhosis patients. Taehan Kan Hakhoe Chi 2003;9:79-88.
- Park SY, Kang KH, Park JH, Lee JH, Cho CM, Tak WY, et al. Clinical efficacy of AST/ALT ratio and platelet counts as predictors of degree of fibrosis in HBV infected patients without clinically evident liver cirrhosis. Korean J Gastroenterol 2004;43:246-51.
- Poynard T, Ngo Y, Marcellin P, Hadziyannis S, Ratziu V, Benhamou Y. Impact of adefovir dipivoxil on liver fibrosis and activity assessed with biochemical markers (FibroTest-ActiTest) in patients infected by hepatitis B virus. J Viral Hepat 2009;16:203-13. http://dx.doi.org/10.1111/j.1365-2893.2008.01065.x.
- Raftopoulos SC, George J, Bourliere M, Rossi E, de Boer WB, Jeffrey GP, et al. Comparison of noninvasive models of fibrosis in chronic hepatitis B. Hepatol Int 2012;6:457-67. http://dx.doi.org/10.1007/s12072-011-9296-5.
- Sebastiani G, Vario A, Guido M, Alberti A. Sequential algorithms combining non-invasive markers and biopsy for the assessment of liver fibrosis in chronic hepatitis B. World J Gastroenterol 2007;13:525-31. http://dx.doi.org/10.3748/wjg.v13.i4.525.
- Seto W-K, Lee C-F, Lai C-L, Ip PPC, Fong DY-T, Fung J, et al. A new model using routinely available clinical parameters to predict significant liver fibrosis in chronic hepatitis B. PLOS One 2011;6. http://dx.doi.org/10.1371/journal.pone.0023077.
- Shin WG, Park SH, Jang MK, Hahn TH, Kim JB, Lee MS, et al. Aspartate aminotransferase to platelet ratio index (APRI) can predict liver fibrosis in chronic hepatitis B. Dig Liver Dis 2008;40:267-74. http://dx.doi.org/10.1016/j.dld.2007.10.011.
- Sinakos E, Manolakopoulos S, Papatheodoridis G, Papalavrentios L, Papageorgiou MV, Papachrysos N, et al. Transient elastography (FibroScan) in patients with chronic hepatitis B in everyday clinical practice. J Hepatol 2011;54:S140-S1. http://dx.doi.org/10.1016/S0168-8278(11)60352-8.
- Sohn JH, Kim TY, Jeong JY, Jun DW, Eun CS, Jeon YC, et al. Insulin resistance correlates with the severity of fibrosis in chronic hepatitis B. Hepatol Int 2011;5.
- Sokucu S, Gokce S, Gulluoglu M, Aydogan A, Celtik C, Durmaz O. The role of the non-invasive serum marker FibroTestActiTest in the prediction of histological stage of fibrosis and activity in children with nave chronic hepatitis B infection. Scand J Infect Dis 2010;42:699-703. http://dx.doi.org/10.3109/00365541003774616.
- Sporea I, Sirli R, Popescu A, Danila M. Acoustic Radiation Force Impulse (ARFI) – a new modality for the evaluation of liver fibrosis. Med Ultrason 2010;12:26-31.
- Vigano M, Paggi S, Lampertico P, Fraquelli M, Massironi S, Ronchi G, et al. Dual cut-off transient elastography to assess liver fibrosis in chronic hepatitis B: a cohort study with internal validation. Aliment Pharmacol Ther 2011;34:353-62. http://dx.doi.org/10.1111/j.1365-2036.2011.04722.x.
- Wang J, Guo L, Shi XY, Pan WQ, Bai YF, Ai H. Real-time elastography with a novel quantitative technology for assessment of liver fibrosis in chronic hepatitis B. Eur J Radiol 2012;81:E31-E6. http://dx.doi.org/10.1016/j.ejrad.2010.12.013.
- Wong GLH, Wong VWS, Choi PCL, Chan AWH, Chan HLY. Development of a non-invasive algorithm with transient elastography (Fibroscan) and serum test formula for advanced liver fibrosis in chronic hepatitis B. Aliment Pharmacol Ther 2010;31:1095-103. http://dx.doi.org/10.1111/j.1365-2036.2010.04276.x.
- Wong GLH, Wong VWS, Choi PCL, Chan AWH, Chim AML, Yiu KKL, et al. Evaluation of alanine transaminase and hepatitis B Virus DNA to predict liver cirrhosis in hepatitis B e antigen-negative chronic hepatitis B using transient elastography. Am J Gastroenterol 2008;103:3071-81. http://dx.doi.org/10.1111/j.1572-0241.2008.02157.x.
- Wong GLH, Wong VWS, Choi PCL, Chan AWH, Chim AML, Yiu KKL, et al. On-treatment monitoring of liver fibrosis with transient elastography in chronic hepatitis B patients. Antivir Ther 2011;16:165-72. http://dx.doi.org/10.3851/IMP1726.
- Wu SD, Ni YJ, Liu LL, Li H, Lu LG, Wang JY. Establishment and validation of a simple noninvasive model to predict significant liver fibrosis in patients with chronic hepatitis B. Hepatol Int 2012;6:360-8. http://dx.doi.org/10.1007/s12072-011-9328-1.
- Zhang MB, Qu EZ, Liu JB, Wang JR. Quantitative assessment of hepatic fibrosis by contrast-enhanced ultrasonography. Chin Med Sci J 2011;26:208-15. http://dx.doi.org/10.1016/S1001-9294(12)60002-9.
- Zhang Y-X, Wu W-J, Zhang Y-Z, Feng Y-L, Zhou X-X, Pan Q. Noninvasive assessment of liver fibrosis with combined serum aminotransferase/platelet ratio index and hyaluronic acid in patients with chronic hepatitis B. World J Gastroenterol 2008;14:7117-21. http://dx.doi.org/10.3748/wjg.14.7117.
- Zhu X, Wang LC, Chen EQ, Chen XB, Chen LY, Liu L, et al. Prospective evaluation of fibroscan for the diagnosis of hepatic fibrosis in patients with chronic hepatitis B virus infection. Hepatol Int 2011;5.
- Janssens F, De Suray N, Piessevaux H, Horsmans Y, De Timary P, Starkel P. Can transient elastography replace liver histology for determination of advanced fibrosis in alcoholic patients: a real-life study. J Clin Gastroenterol 2010;44:575-82. http://dx.doi.org/10.1097/MCG.0b013e3181cb4216.
- Kim SG, Kim YS, Jung SW, Kim HK, Jang JY, Moon JH, et al. The usefulness of transient elastography to diagnose cirrhosis in patients with alcoholic liver disease. Korean J Hepatol 2009;15:42-51. http://dx.doi.org/10.3350/kjhep.2009.15.1.42.
- Lavallard VJ, Bonnafous S, Patouraux S, Saint-Paul MC, Rousseau D, Anty R, et al. Serum markers of hepatocyte death and apoptosis are non invasive biomarkers of severe fibrosis in patients with alcoholic liver disease. PLOS One 2011;6. http://dx.doi.org/10.1371/journal.pone.0017599.
- Melin PDA, Gauchet A, Schoeny M, Fournier C, Sandrin L, Diebold MD. Cirrhosis screening in alcoholism consultation using fibroscan. Hepatology 2005;42.
- Mueller S, Millonig G, Sarovska L, Friedrich S, Reimann FM, Pritsch M, et al. Increased liver stiffness in alcoholic liver disease: differentiating fibrosis from steatohepatitis. World J Gastroenterol 2010;16:966-72. http://dx.doi.org/10.3748/wjg.v16.i8.966.
- Nahon P, Kettaneh A, Tengher-Barna I, Ziol M, de Ledinghen V, Douvin C, et al. Assessment of liver fibrosis using transient elastography in patients with alcoholic liver disease. J Hepatol 2008;49:1062-8. http://dx.doi.org/10.1016/j.jhep.2008.08.011.
- Naveau S, Poynard T, Benattar C, Bedossa P, Chaput JC. Alpha-2-macroglobulin and hepatic fibrosis. Diagnostic interest. Dig Dis Sci 1994;39:2426-32. http://dx.doi.org/10.1007/BF02087661.
- Naveau S, Raynard B, Ratziu V, Abella A, Imbert-Bismut F, Messous D, et al. Biomarkers for the prediction of liver fibrosis in patients with chronic alcoholic liver disease. Clin Gastroenterol Hepatol 2005;3:167-74. http://dx.doi.org/10.1016/S1542-3565(04)00625-1.
- Nguyen-Khac E, Chatelain D, Tramier B, Decrombecque C, Robert B, Joly JP, et al. Assessment of asymptomatic liver fibrosis in alcoholic patients using fibroscan: prospective comparison with seven non-invasive laboratory tests. Aliment Pharmacol Ther 2008;28:1188-98. http://dx.doi.org/10.1111/j.1365-2036.2008.03831.x.
- Tran A, Benzaken S, Saint-Paul MC, Guzman-Granier E, Hastier P, Pradier C, et al. Chondrex (YKL-40), a potential new serum fibrosis marker in patients with alcoholic liver disease. Eur J Gastroenterol Hepatol 2000;12:989-93. http://dx.doi.org/10.1097/00042737-200012090-00004.
- Vanbiervliet G, Marine-Barjoan E, Gelsi E, Anty R, Piche T, Benzaken S, et al. Evaluation of liver fibrosis with APRI score in patients with chronic alcoholic liver disease. J Hepatol 2005;42. http://dx.doi.org/10.1016/S0168-8278(05)82109-9.
- Adams LA, George J, Bugianesi E, Rossi E, De Boer WB, van der Poorten D, et al. Complex non-invasive fibrosis models are more accurate than simple models in non-alcoholic fatty liver disease. J Gastroenterol Hepatol 2011;26:1536-43. http://dx.doi.org/10.1111/j.1440-1746.2011.06774.x.
- Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology 2007;45:846-54. http://dx.doi.org/10.1002/hep.21496.
- Blomme B, Francque S, Trepo E, Libbrecht L, Vanderschaeghe D, Verrijken A, et al. N-glycan based biomarker distinguishing non-alcoholic steatohepatitis from steatosis independently of fibrosis. Dig Liver Dis 2012;44:315-22. http://dx.doi.org/10.1016/j.dld.2011.10.015.
- Cales P, Laine F, Boursier J, Deugnier Y, Moal V, Oberti F, et al. Comparison of blood tests for liver fibrosis specific or not to NAFLD. J Hepatol 2009;50:165-73. http://dx.doi.org/10.1016/j.jhep.2008.07.035.
- De Ledinghen V, Wong VW, Vergniol J, Wong G, Bail BL, Chim AML, et al. Prediction of fibrosis in patients with NAFLD using transient elastography: a prospective multicentre study. Hepatology 2009;50:767A-8A.
- Guajardo-Salinas GE, Hilmy A. Prevalence of nonalcoholic fatty liver disease (NAFLD) and utility of FIBROspect II to detect liver fibrosis in morbidly obese hispano-american patients undergoing gastric bypass. Obes Surg 2010;20:1647-53. http://dx.doi.org/10.1007/s11695-009-0027-0.
- Guha IN, Parkes J, Roderick P, Chattopadhyay D, Cross R, Harris S, et al. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: validating the European liver fibrosis panel and exploring simple markers. Hepatology 2008;47:455-60. http://dx.doi.org/10.1002/hep.21984.
- Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut 2008;57:1441-7. http://dx.doi.org/10.1136/gut.2007.146019.
- Kaneda H, Hashimoto E, Yatsuji S, Tokushige K, Shiratori K. Hyaluronic acid levels can predict severe fibrosis and platelet counts can predict cirrhosis in patients with nonalcoholic fatty liver disease. J Gastroenterol Hepatol 2006;21:1459-65.
- Kayadibi H, Gultepe M, Yasar B, Ince AT, Ozcan O, Ipcioglu OM, et al. Diagnostic value of serum prolidase enzyme activity to predict the liver histological lesions in non-alcoholic fatty liver disease: a surrogate marker to distinguish steatohepatitis from simple steatosis. Dig Dis Sci 2009;54:1764-71. http://dx.doi.org/10.1007/s10620-008-0535-0.
- Kelleher TB, MacFarlane C, de Ledinghen V, Beaugrand M, Foucher J, Castera L, et al. Risk factors and hepatic elastography (fibroscan) in the prediction of hepatic fibrosis in non alcoholic steatohepatitis. Gastroenterology 2006;130.
- Khosravi S, Alavian SM, Zare A, Daryani NE, Fereshtehnejad SM, Daryani NE, et al. Non-alcoholic fatty liver disease and correlation of serum alanin aminotransferase level with histopathologic findings. Hepat Mon 2011;11:452-8.
- Lupsor M, Badea R, Stefanescu H, Grigorescu M, Serban A, Radu C, et al. Performance of unidimensional transient elastography in staging non-alcoholic steatohepatitis. J Gastrointestin Liver Dis 2010;19:53-60.
- Lydatakis H, Hager IP, Kostadelou E, Mpousmpoulas S, Pappas S, Diamantis I. Non-invasive markers to predict the liver fibrosis in non-alcoholic fatty liver disease. Liver Int 2006;26:864-71. http://dx.doi.org/10.1111/j.1478-3231.2006.01312.x.
- Mahadeva S, Mahfudz A, Vijayanathan A, Goh KL, Arumugam K, Cheah PL. Accuracy of liver stiffness measurement in an Asian population with non-alcoholic fatty liver diseasea preliminary report. J Gastroenterol Hepatol 2010;25.
- Manousou P, Kalambokis G, Grillo F, Watkins J, Xirouchakis E, Pleguezuelo M, et al. Serum ferritin is a discriminant marker for both fibrosis and inflammation in histologically proven non-alcoholic fatty liver disease patients. Liver Int 2011;31:730-9. http://dx.doi.org/10.1111/j.1478-3231.2011.02488.x.
- McPherson S, Anstee QM, Henderson E, Burt AD, Day CP. Are simple non-invasive scoring systems for fibrosis reliable in patients with nafld and normal ALT levels?. Hepatology 2011;54.
- McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010;59:1265-9. http://dx.doi.org/10.1136/gut.2010.216077.
- Orlacchio A, Bolacchi F, Antonicoli M, Coco I, Costanzo E, Tosti D, et al. Liver elasticity in NASH patients evaluated with real-time elastography (RTE). Ultrasound Med Biol 2012;38:537-44. http://dx.doi.org/10.1016/j.ultrasmedbio.2011.12.023.
- Pais R, Lebray P, Fedchuk L, Charlotte F, Poynard T, Ratziu V. Validation of a simple algorithm combining serum markers and elastometry for the diagnosis of fibrosis in NAFLD. Hepatology 2011;54:1127A-8A.
- Palmeri ML, Wang MH, Rouze NC, Abdelmalek MF, Guy CD, Moser B, et al. Noninvasive evaluation of hepatic fibrosis using acoustic radiation force-based shear stiffness in patients with nonalcoholic fatty liver disease. J Hepatol 2011;55:666-72. http://dx.doi.org/10.1016/j.jhep.2010.12.019.
- Papalavrentios L, Sinakos E, Hytiroglou P, Drevelegas K, Drevelegas A, Akriviadis E. 3 tesla diffusion-weighted MRI for assessing liver fibrosis in non alcoholic fatty liver disease. Hepatology 2011;54:1120A-1A.
- Park GJH, Wiseman E, George J, Katelaris PH, Seow F, Fung C, et al. Non-invasive estimation of liver fibrosis in non-alcoholic fatty liver disease using the 13C-caffeine breath test. J Gastroenterol Hepatol 2011;26:1411-16. http://dx.doi.org/10.1111/j.1440-1746.2011.06760.x.
- Pawitpok C, Kongtawelert P, Ua-Arayaporn S, Punyarit P, Chutaputti A. Efficacy of serum hyaluronic acid level in predicting liver fibrosis in patients with nonalcoholic fatty liver disease. J Gastroenterol Hepatol 2006;21.
- Petta S, Di Marco V, Camma C, Butera G, Cabibi D, Craxi A. Reliability of liver stiffness measurement in non-alcoholic fatty liver disease: the effects of body mass index. Aliment Pharmacol Ther 2011;33:1350-60. http://dx.doi.org/10.1111/j.1365-2036.2011.04668.x.
- Pimentel SK, Strobel R, Goncalves CG, Sakamoto DG, Ivano FH, Coelho JCU. Evaluation of the nonalcoholic fat liver disease fibrosis score for patients undergoing bariatric surgery. Arg Gastroenterol 2010;47:170-3. http://dx.doi.org/10.1590/S0004-28032010000200010.
- Poynard T, Ratziu V, Charlotte F, Messous D, Munteanu M, Imbert-Bismut F, et al. Diagnostic value of biochemical markers (NashTest) for the prediction of non alcoholo steato hepatitis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol 2006;6. http://dx.doi.org/10.1186/1471-230X-6-34.
- Qureshi K, Clements RH, Abrams GA. The utility of the ‘NAFLD fibrosis score’ in morbidly obese subjects with NAFLD. Obes Surg 2008;18:264-70. http://dx.doi.org/10.1007/s11695-007-9295-8.
- Raszeja-Wyszomirska J, Szymanik B, Lawniczak M, Kajor M, Chwist A, Milkiewicz P, et al. Validation of the BARD scoring system in Polish patients with nonalcoholic fatty liver disease (NAFLD). BMC Gastroenterol 2010;10. http://dx.doi.org/10.1186/1471-230X-10-67.
- Ratziu V, Le Calvez S, Messous D, Charlotte F, Bonhay L, Munteanu M, et al. Diagnostic value of biochemical markers (Fibrotest) for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease (NAFLD). J Hepatol 2004;40. http://dx.doi.org/10.1016/S0168-8278(04)90596-X.
- Ratziu V, Massard J, Charlotte F, Messous D, Imbert-Bismut F, Bonyhay L, et al. Diagnostic value of biochemical markers (FibroTest-FibroSURE) for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol 2006;6. http://dx.doi.org/10.1186/1471-230X-6-6.
- Ruffillo G, Fassio E, Alvarez E, Landeira G, Longo C, Dominguez N, et al. Comparison of NAFLD fibrosis score and BARD score in predicting fibrosis in nonalcoholic fatty liver disease. J Hepatol 2011;54:160-3. http://dx.doi.org/10.1016/j.jhep.2010.06.028.
- Sakugawa H, Kobashigawa K, Yamashiro T, Maeshiro T, Miyagi S, Shiroma J, et al. Clinical usefulness of biochemical markers of liver fibrosis in patients with nonalcoholic fatty liver disease. World J Gastroenterol 2005;11:255-9. http://dx.doi.org/10.3748/wjg.v11.i2.255.
- Santos VND, Leite-Mor MMB, Kondo M, Martins JR, Nader H, Lanzoni VP, et al. Serum laminin, type IV collagen and hyaluronan as fibrosis markers in non-alcoholic fatty liver disease. Braz J Med Biol Res 2005;38:747-53. http://dx.doi.org/10.1590/S0100-879X2005000500012.
- Shimada M, Kawahara H, Ozaki K, Fukura M, Yano H, Tsuchishima M, et al. Usefulness of a combined evaluation of the serum adiponectin level, HOMA-IR, and serum type IV collagen 7S level to predict the early stage of nonalcoholic steatohepatitis. Am J Gastroenterol 2007;102:1931-8. http://dx.doi.org/10.1111/j.1572-0241.2007.01322.x.
- Sumida Y, Yoneda M, Hyogo H, Itoh Y, Ono M, Fujii H, et al. Validation of the FIB4 index in a Japanese nonalcoholic fatty liver disease population. BMC Gastroenterol 2012;12. http://dx.doi.org/10.1186/1471-230X-12-2.
- Sumida Y, Yoneda M, Hyogo H, Yamaguchi K, Ono M, Fujii H, et al. A simple clinical scoring system using ferritin, fasting insulin, and type IV collagen 7S for predicting steatohepatitis in nonalcoholic fatty liver disease. J Gastroenterol 2011;46:257-68. http://dx.doi.org/10.1007/s00535-010-0305-6.
- Suzuki A, Angulo P, Lymp J, Li D, Satomura S, Lindor K. Hyaluronic acid, an accurate serum marker for severe hepatic fibrosis in patients with non-alcoholic fatty liver disease. Liver Int 2005;25:779-86. http://dx.doi.org/10.1111/j.1478-3231.2005.01064.x.
- Wong VW, Wong GL, Chim AM, Tse AM, Tsang SW, Hui AY, et al. Validation of the NAFLD fibrosis score in a Chinese population with low prevalence of advanced fibrosis. Am J Gastroenterol 2008;103:1682-8. http://dx.doi.org/10.1111/j.1572-0241.2008.01933.x.
- Wong VW, Wong GL, Chim AM, Yiu K, Chan AW, Choi PC, et al. Combination of the median and variability of liver stiffness measurements by transient elastography improves the accuracy of diagnosing advanced fibrosis in nonalcoholic fatty liver disease. J Hepatol 2008;48.
- Wong VWS, Vergniol J, Chan HLY, de Ledinghen V. Diagnostic power of Fibroscan in predicting liver fibrosis in nonalcoholic fatty liver disease reply. Hepatology 2009;50:2049-50. http://dx.doi.org/10.1002/hep.23364.
- Yoneda M, Fujii H, Sumida Y, Hyogo H, Itoh Y, Ono M, et al. Platelet count for predicting fibrosis in nonalcoholic fatty liver disease. J Gastroenterol 2011;46:1300-6. http://dx.doi.org/10.1007/s00535-011-0436-4.
- Yoneda M, Yoneda M, Mawatari H, Fujita K, Endo H, Iida H, et al. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with nonalcoholic fatty liver disease (NAFLD). Dig Liver Dis 2008;40:371-8. http://dx.doi.org/10.1016/j.dld.2007.10.019.
- Younossi ZM, Page S, Rafiq N, Birerdinc A, Stepanova M, Hossain N, et al. A biomarker panel for non-alcoholic steatohepatitis (NASH) and NASH-related fibrosis. Obes Surg 2011;21:431-9. http://dx.doi.org/10.1007/s11695-010-0204-1.
- Asbach P, Klatt D, Schlosser B, Biermer M, Muche M, Rieger A, et al. Viscoelasticity-based staging of hepatic fibrosis with multifrequency MR elastography. Radiology 2010;257:80-6. http://dx.doi.org/10.1148/radiol.10092489.
- Aube C, Oberti F, Korali N, Namour MA, Loisel D, Tanguy JY, et al. Ultrasonographic diagnosis of hepatic fibrosis or cirrhosis. J Hepatol 1999;30:472-8. http://dx.doi.org/10.1016/S0168-8278(99)80107-X.
- Aube C, Winkfield B, Oberti F, Vuillemin E, Rousselet MC, Caron C, et al. New Doppler ultrasound signs improve the non-invasive diagnosis of cirrhosis or severe liver fibrosis. Eur J Gastroenterol Hepatol 2004;16:743-51. http://dx.doi.org/10.1097/01.meg.0000108357.41221.e5.
- Awaya H, Mitchell DG, Kamishima T, Holland G, Ito K, Matsumoto T. Cirrhosis: modified caudate-right lobe ratio. Radiology 2002;224:769-74. http://dx.doi.org/10.1148/radiol.2243011495.
- Cardi M, Muttillo IA, Amadori L, Petroni R, Mingazzini P, Barillari P, et al. Superiority of laparoscopy compared to ultrasonography in diagnosis of widespread liver diseases. Dig Dis Sci 1997;42:546-8. http://dx.doi.org/10.1023/A:1018895009305.
- Cioni G, Dalimonte P, Zerbinati F, Ventura P, Cristani A, Vignoli A, et al. Duplex-Doppler ultrasonography in the evaluation of cirrhotic patients with portal-hypertension and in the analysis of their response to drugs. J Gastroenterol Hepatol 1992;7:388-92. http://dx.doi.org/10.1111/j.1440-1746.1992.tb01005.x.
- Colli A, Cocciolo M, Riva C, Martinez E, Prisco A, Pirola M, et al. Abnormalities of Doppler waveform of the hepatic veins in patients with chronic liver disease: correlation with histologic findings. AJR Am J Roentgenol 1994;162:833-7. http://dx.doi.org/10.2214/ajr.162.4.8141001.
- Colli A, Fraquelli M, Andreoletti M, Marino B, Zuccoli E, Conte D. Severe liver fibrosis or cirrhosis: accuracy of US for detection--analysis of 300 cases. Radiology 2003;227:89-94. http://dx.doi.org/10.1148/radiol.2272020193.
- D’Onofrio M, Martone E, Brunelli S, Faccioli N, Zamboni G, Zagni I, et al. Accuracy of ultrasound in the detection of liver fibrosis in chronic viral hepatitis. Radiol Med 2005;110:341-8.
- Do RKG, Chandanara H, Felker E, Hajdu CH, Babb JS, Kim D, et al. Diagnosis of liver fibrosis and cirrhosis with diffusion-weighted imaging: value of normalized apparent diffusion coefficient using the spleen as reference organ. AJR Am J Roentgenol 2010;195:671-6. http://dx.doi.org/10.2214/AJR.09.3448.
- Ferral H, Male R, Cardiel M, Munoz L, Ferrari FQY. Cirrhosis – diagnosis by liver surface-analysis with high-frequency ultrasound. Gastroint Radiol 1992;17:74-8. http://dx.doi.org/10.1007/BF01888512.
- Gaia S, Carucci P, Spandre M, Cosso L, Evangelista A, Bugianesi E, et al. Ultrasound evaluation, liver stiffness measurement and biopsy for staging of hepatic fibrosis in patients with chronic liver disease: a comparative study. Hepatology 2011;54.
- Gaiani S, Gramantieri L, Venturoli N, Piscaglia F, Siringo S, D’Errico A, et al. What is the criterion for differentiating chronic hepatitis from compensated cirrhosis? A prospective study comparing ultrasonography and percutaneous liver biopsy. J Hepatol 1997;27:979-85. http://dx.doi.org/10.1016/S0168-8278(97)80140-7.
- Gierblinski I, Przelaskowski A, Wocial T, Kazubek M, Zych W, Walewska-Zielecka B. Measurement of liver stiffness by color-coded ultrasound elastography: a preliminary clinical feasibility study. Gastroenterologia Polska 2008;15:151-5.
- Goyal AK, Pokharna DS, Sharma SK. Ultrasonic diagnosis of cirrhosis: reference to quantitative measurements of hepatic dimensions. Gastrointest Radiol 1990;15:32-4. http://dx.doi.org/10.1007/BF01888729.
- Ibrahim HR, El-Hamid AA, Tohamy A, Habba MR. Diagnostic value of apparent diffusion coefficient calculated with diffusion-weighted MRI for quantification of liver fibrosis. Egypt J Radiol Nucl Med 2011;42:119-31. http://dx.doi.org/10.1016/j.ejrnm.2011.05.003.
- Ishibashi H, Maruyama H, Takahashi M, Shimada T, Kamesaki H, Fujiwara K, et al. Demonstration of intrahepatic accumulated microbubble on ultrasound represents the grade of hepatic fibrosis. Eur Radiol 2012;22:1083-90. http://dx.doi.org/10.1007/s00330-011-2346-5.
- Joseph AE, Saverymuttu SH, al-Sam S, Cook MG, Maxwell JD. Comparison of liver histology with ultrasonography in assessing diffuse parenchymal liver disease. Clin Radiol 1991;43:26-31. http://dx.doi.org/10.1016/S0009-9260(05)80350-2.
- Kim BH, Lee JM, Lee YJ, Lee KB, Suh KS, Han JK, et al. MR elastography for noninvasive assessment of hepatic fibrosis: experience from a tertiary center in Asia. J Magn Reson Imaging 2011;34:1110-16. http://dx.doi.org/10.1002/jmri.22723.
- Kim BK, Han K-H, Park JY, Ahn SH, Chon CY, Kim JK, et al. A novel liver stiffness measurement-based prediction model for cirrhosis in hepatitis B patients. Liver Int 2010;30:1073-81. http://dx.doi.org/10.1111/j.1478-3231.2010.02269.x.
- Ladenheim JA, Luba DG, Yao F, Gregory PB, Jeffrey RB, Garcia G. Limitations of liver surface US in the diagnosis of cirrhosis. Radiology 1992;185:21-3. http://dx.doi.org/10.1148/radiology.185.1.1523310.
- Lee HS, Kim JK, Cheong JY, Han EJ, An SY, Song JH, et al. Prediction of compensated liver cirrhosis by ultrasonography and routine blood tests in patients with chronic viral hepatitis. Korean J Hepatol 2010;16:369-75. http://dx.doi.org/10.3350/kjhep.2010.16.4.369.
- Liu CH, Lin JW, Tsai FC, Yang PM, Lai MY, Chen JH, et al. Noninvasive tests for the prediction of significant hepatic fibrosis in hepatitis C virus carriers with persistently normal alanine aminotransferases. Liver Int 2006;26:1087-94. http://dx.doi.org/10.1111/j.1478-3231.2006.01355.x.
- Lutz HH, Gassler N, Tischendorf FW, Trautwein C, Tischendorf JJ. Doppler ultrasound of hepatic blood flow for noninvasive evaluation of liver fibrosis compared with liver biopsy and transient elastography. Dig Dis Sci 2012;57:2222-30. http://dx.doi.org/10.1007/s10620-012-2153-0.
- Nagata N, Miyachi H, Nakano A, Nanri K, Kobayashi H, Matsuzaki S. Sonographic evaluation of the anterior liver surface in chronic liver diseases using a 7.5-MHz annular-array transducer: correlation with laparoscopic and histopathologic findings. J Clin Ultrasound 2003;31:393-400. http://dx.doi.org/10.1002/jcu.10195.
- Nishiura T, Watanabe H, Ito M, Matsuoka Y, Yano K, Daikoku M, et al. Ultrasound evaluation of the fibrosis stage in chronic liver disease by the simultaneous use of low and high frequency probes. Br J Radiol 2005;78:189-97. http://dx.doi.org/10.1259/bjr/75208448.
- Numminen K, Tervahartiala P, Halavaara J, Isoniemi H, Hockerstedt K. Non-invasive diagnosis of liver cirrhosis: magnetic resonance imaging presents special features. Scand J Gastroenterol 2005;40:76-82. http://dx.doi.org/10.1080/00365520410009384.
- Ong TZ, Tan HJ. Ultrasonography is not reliable in diagnosing liver cirrhosis in clinical practice. Singapore Med J 2003;44:293-5.
- Rustogi R, Ganger D, Horowitz J, Harmath C, Wang Y, Chen ZE, et al. Accuracy of MR Elastography (MRE) vs. imaging features in the diagnosis of severe fibrosis and cirrhosis. Hepatology 2011;54.
- Sandrasegaran K, Akisik FM, Lin C, Tahir B, Rajan J, Saxena R, et al. Value of diffusion-weighted MRI for assessing liver fibrosis and cirrhosis. AJR Am J Roentgenol 2009;193:1556-60. http://dx.doi.org/10.2214/AJR.09.2436.
- Shen L, Li JQ, Zeng MD, Lu LG, Fan ST, Bao H. Correlation between ultrasonographic and pathologic diagnosis of liver fibrosis due to chronic virus hepatitis. World J Gastroenterol 2006;12:1292-5.
- Venkatesh S, Teo L, Ang B, Ehman R. Detection of liver fibrosis: comparison of magnetic resonance elastography and diffusion-weighted MRI. AJR Am J Roentgenol 2010;194.
- Viganò M, Visentin S, Aghemo A, Rumi MG, Ronchi G. US features of liver surface nodularity as a predictor of severe fibrosis in chronic hepatitis C. Radiology 2005;234. http://dx.doi.org/10.1148/radiol.2342041267.
- Wang J-H, Changchien C-S, Hung C-H, Eng H-L, Tung W-C, Kee K-M, et al. FibroScan and ultrasonography in the prediction of hepatic fibrosis in patients with chronic viral hepatitis. J Gastroenterol 2009;44:439-46. http://dx.doi.org/10.1007/s00535-009-0017-y.
- Xu Y, Wang B, Cao H. An ultrasound scoring system for the diagnosis of liver fibrosis and cirrhosis. Chin Med J 1999;112:1125-8.
- Zhu NY, Chen KM, Chai WM, Li WX, Du LJ. Feasibility of diagnosing and staging liver fibrosis with diffusion weighted imaging. Chin Med Sci J 2008;23:183-6. http://dx.doi.org/10.1016/S1001-9294(09)60036-5.
- Carlson JJ, Kowdley KV, Sullivan SD, Ramsey SD, Veenstra DL. An evaluation of the potential cost-effectiveness of non-invasive testing strategies in the diagnosis of significant liver fibrosis. J Gastroenterol Hepatol 2009;24:786-91. http://dx.doi.org/10.1111/j.1440-1746.2009.05778.x.
- Hall A, Germani G, Isgro G, Burroughs AK, Dhillon AP. Fibrosis distribution in explanted cirrhotic livers. Histopathology 2012;60:270-7. http://dx.doi.org/10.1111/j.1365-2559.2011.04094.x.
- Myers RP, Ratziu V, Imbert-Bismut F, Charlotte F, Poynard T. C MGGdEMslPLaV . Biochemical markers of liver fibrosis: a comparison with historical features in patients with chronic hepatitis C. Am J Gastroenterol 2002;97:2419-25. http://dx.doi.org/10.1111/j.1572-0241.2002.05997.x.
- Friedrich-Rust M, Muller C, Winckler A, Kriener S, Herrmann E, Holtmeier J, et al. Assessment of liver fibrosis and steatosis in PBC with fibroscan, MRI, MR-spectroscopy, and serum markers. J Clin Gastroenterol 2010;44:58-65. http://dx.doi.org/10.1097/MCG.0b013e3181a84b8d.
- Aguirre DA, Behling CA, Alpert E, Hassanein TI, Sirlin CB. Liver fibrosis: noninvasive diagnosis with double contrast material-enhanced MR imaging. Radiology 2006;239:425-37. http://dx.doi.org/10.1148/radiol.2392050505.
- Motosugi U, Ichikawa T, Oguri M, Sano K, Sou H, Muhi A, et al. Staging liver fibrosis by using liver–enhancement ratio of gadoxetic acid-enhanced MR imaging: comparison with aspartate aminotransferase-to-platelet ratio index. Magn Reson Imaging 2011;29:1047-52. http://dx.doi.org/10.1016/j.mri.2011.05.007.
- Shiramizu B, Theodore T, Bassett R, Coel M, Sherman KE, Glesby IJ, et al. Correlation of single photon emission computed tomography parameters as a noninvasive alternative to liver biopsies in assessing liver involvement in the setting of HIV and hepatitis C virus coinfection: a multicenter trial of the adult AIDS clinical trials group. J Acquir Immune Defic Syndr 2003;33:329-35. http://dx.doi.org/10.1097/00126334-200307010-00006.
- Shepherd J, Jones J, Takeda A, Davidson P, Price A. Adefovir dipivoxil and pegylated interferon alfa-2a for the treatment of chronic hepatitis B: a systematic review and economic evaluation. Health Technol Assess 2006;10. http://dx.doi.org/10.3310/hta10280.
- Dakin H, Bentley A, Dusheiko G. Cost-utility analysis of tenofovir disoproxil fumarate in the treatment of chronic hepatitis B. Value Health 2010;13:922-33. http://dx.doi.org/10.1111/j.1524-4733.2010.00782.x.
- Marcellin P, Asselah T. Long-term therapy for chronic hepatitis B: hepatitis B virus DNA suppression leading to cirrhosis reversal. J Gastroenterol Hepatol 2013;28:912-23. http://dx.doi.org/10.1111/jgh.12213.
- Diagnosis and Managment of Chronic Hepatitis B in Children, Young People and Adults. London: NICE; 2013.
- Adefovir Dipivoxil and Peginteferon Alfa-2a for the Treatment of Chronic Hepatitis B. London: NICE; 2006.
- Fattovich G. Natural history and prognosis of hepatitis B. Sem Liv Dis 2003;23:47-58. http://dx.doi.org/10.1055/s-2003-37590.
- Realdi G, Alberti A, Rugge M, Bortolotti F, Rigoli AM, Tremolada F, et al. Seroconversion from hepatitis B e antigen to anti-HBe in chronic hepatitis B virus infection. Gastroenterology 1980;79:195-9.
- Hoofnagle JH, Dusheiko GM, Seeff LB, Jones EA, Waggoner JG, Bales ZB. Seroconversion from hepatitis B e antigen to antibody in chronic type B hepatitis. Ann Intern Med 1981;94:744-8. http://dx.doi.org/10.7326/0003-4819-94-6-744.
- Fattovich G, Rugge M, Brollo L, Pontisso P, Noventa F, Guido M, et al. Clinical, virologic and histologic outcome following seroconversion from HBeAg to anti-HBe in chronic hepatitis type B. Hepatology 1986;6:167-72. http://dx.doi.org/10.1002/hep.1840060203.
- Moreno-Otero R, Garcia-Monzon C, Garcia-Sanchez A, Buey LG, Pajares JM, Di Bisceglie AM. Development of cirrhosis after chronic type B hepatitis: a clinicopathologic and follow-up study of 46 HBeAg-positive asymptomatic patients. Am J Gastroenterol 1991;86:560-4.
- Zarski JP, Marcellin P, Cohard M, Lutz JM, Bouche C, Rais A. Comparison of anti-HBe-positive and HBe-antigen-positive chronic hepatitis B in France. J Hepatol 1994;20:636-40. http://dx.doi.org/10.1016/S0168-8278(05)80352-6.
- Di Marco V, Lo Iacono O, Cammà C, Vaccaro A, Giunta M, Martorana G, et al. The long-term course of chronic hepatitis B. Hepatology 1999;30:257-64. http://dx.doi.org/10.1002/hep.510300109.
- Lin SM, Sheen IS, Chien RN, Chu CM, Liaw YF. Long-term beneficial effect of interferon therapy in patients with chronic hepatitis B virus infection. Hepatology 1999;29:971-5. http://dx.doi.org/10.1002/hep.510290312.
- Yuen MF, Hui CK, Cheng CC, Wu CH, Lai YP, Lai CL. Long-term follow-up of interferon alfa treatment in Chinese patients with chronic hepatitis B infection: the effect on hepatitis B e antigen seroconversion and the development of cirrhosis-related complications. Hepatology 2001;34:139-45. http://dx.doi.org/10.1053/jhep.2001.25273.
- Chang MH, Hsu HY, Hsu HC, Ni YH, Chen JS, Chen DS. The significance of spontaneous hepatitis B e antigen seroconversion in childhood: with special emphasis on the clearance of hepatitis B e antigen before 3 years of age. Hepatology 1995;22:1387-92.
- Sancheztapias JM, Costa J, Mas A, Pares A, Bruguera M, Rodes J. Analysis of factors predicting early seroconversion to anti-Hbe in Hbeag-positive chronic hepatitis B. J Hepatol 1988;6:15-22. http://dx.doi.org/10.1016/S0168-8278(88)80458-6.
- Liaw YF, Tai DI, Chu CM, Chen TJ. The development of cirrhosis in patients with chronic type-B hepatitis – a prospective study. Hepatology 1988;8:493-6. http://dx.doi.org/10.1002/hep.1840080310.
- Fattovich G, Farci P, Rugge M, Brollo L, Mandas A, Pontisso P, et al. A randomized controlled trial of lymphoblastoid interferon-α in patients with chronic hepatitis B lacking HBeAg. Hepatology 1992;15:584-9. http://dx.doi.org/10.1002/hep.1840150405.
- Lampertico P, Del Ninno E, Manzin A, Donato MF, Rumi MG, Lunghi G, et al. A randomized, controlled trial of a 24-month course of interferon alfa 2b in patients with chronic hepatitis B who had hepatitis B virus DNA without hepatitis B e antigen in serum. Hepatology 1997;26:1621-5. http://dx.doi.org/10.1002/hep.510260634.
- Tassopoulos NC, Volpes R, Pasture G, Heathcote J, Buti M, Goldin RD, et al. Efficacy of lamivudine in patients with hepatitis B e antigen- negative/hepatitis B virus DNA-positive (precore mutant) chronic hepatitis B. Hepatology 1999;29:889-96. http://dx.doi.org/10.1002/hep.510290321.
- Brunetto MR, Oliveri F, Coco B, Leandro G, Colombatto P, Gorin JM, et al. Outcome of anti-HBe positive chronic hepatitis B in alpha-interferon treated and untreated patients: a long term cohort study. J Hepatol 2002;36:263-70. http://dx.doi.org/10.1016/S0168-8278(01)00266-5.
- Shepherd J, Brodin H, Cave C, Waugh N, Price A, Gabbay J. Pegylated interferon α–2a and -2b in combination with ribavirin in the treatment of chronic hepatitis C: a systematic review and economic evaluation. Health Technol Assess 2004;8. http://dx.doi.org/10.3310/hta8390.
- Wong JB, Koff RS, Tine F, Pauker SG. Cost-effectiveness of interferon-α2b treatment for hepatitis B e antigen-positive chronic hepatitis B. Ann Intern Med 1995;122:664-75. http://dx.doi.org/10.7326/0003-4819-122-9-199505010-00004.
- Di Bisceglie AM, Rustgi VK, Hoofnagle JH, Dusheiko GM, Lotze MT. Hepatocellular carcinoma. Ann Intern Med 1988;108:390-401. http://dx.doi.org/10.7326/0003-4819-108-3-390.
- Bennett WG, Inoue Y, Beck JR, Wong JB, Pauker SG, Davis GL. Estimates of the cost-effectiveness of a single course of interferon-α2b in patients with histologically mild chronic hepatitis C. Ann Intern Med 1997;127:855-65. http://dx.doi.org/10.7326/0003-4819-127-10-199711150-00001.
- Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, arid current and emerging prevention and control measures. J Viral Hepat 2004;11:97-107. http://dx.doi.org/10.1046/j.1365-2893.2003.00487.x.
- Lau DTY, Everhart J, Kleiner DE, Park Y, Vergalla J, Schmid P, et al. Long-term follow-up of patients with chronic hepatitis B treated with interferon alfa. Gastroenterology 1997;113:1660-7. http://dx.doi.org/10.1053/gast.1997.v113.pm9352870.
- Crowley S, Tognarini D, Desmond P, Lees M, Saal G. Introduction of lamivudine for the treatment of chronic hepatitis B: expected clinical and economic outcomes based on 4-year clinical trial data. J Gastroenterol Hepatol 2002;17:153-64. http://dx.doi.org/10.1046/j.1440-1746.2002.02673.x.
- Crowley SJ, Tognarini D, Desmond PV, Lees M. Cost-effectiveness analysis of lamivudine for the treatment of chronic hepatitis B. Pharmacoeconomics 2000;17:409-27. http://dx.doi.org/10.2165/00019053-200017050-00001.
- de Franchis R, Hadengue A, Lau G, Lavanchy D, Lok A, McIntyre N. EASL International Consensus Conference on Hepatitis B. 13–14 September, 2002 Geneva, Switzerland. Consensus statement (long version). J Hepatol 2003;39:S3-25.
- Lai CL, Dienstag J, Schiff E, Leung NW, Atkins M, Hunt C, et al. Prevalence and clinical correlates of YMDD variants during lamviudine therapy for patients with chronic hepatitis B. Clin Infect Dis 2003;36. http://dx.doi.org/10.1086/368083.
- Fattovich G, Brollo L, Giustina G, Noventa F, Pontisso P, Alberti A, et al. Natural history and prognostic factors for chronic hepatitis type B. Gut 1991;32:294-8. http://dx.doi.org/10.1136/gut.32.3.294.
- Fattovich G, Pantalena M, Zagni I, Realdi G, Schalm SW, Christensen E. Effect of hepatitis B and C virus infections on the natural history of compensated cirrhosis: a cohort study of 297 patients. Am J Gastroenterol 2002;97:2886-95. http://dx.doi.org/10.1111/j.1572-0241.2002.07057.x.
- Liaw YF, Lin DY, Chen TJ, Chu CM. Natural course after the development of cirrhosis in patients with chronic type B hepatitis: a prospective study. Liver 1989;9:235-41. http://dx.doi.org/10.1111/j.1600-0676.1989.tb00405.x.
- De Jongh FE, Janssen HLA, De Man RA, Hop WCJ, Schalm SW, Van Blankenstein M. Survival and prognostic indicators in hepatitis B surface antigen-positive cirrhosis of the liver. Gastroenterology 1992;103:1630-5.
- Boring CC, Squires TS, Tong T. Cancer statistics, 1993. CA Cancer J Clin 1993;43:7-26. http://dx.doi.org/10.3322/canjclin.43.1.7.
- Pereira A. Health and economic consequences of HCV lookback. Transfusion 2001;41:832-9. http://dx.doi.org/10.1046/j.1537-2995.2001.41060832.x.
- Sonnenberg FA, Gregory P, Yomtovian R, Russell LB, Tierney W, Kosmin M, et al. The cost-effectiveness of autologous transfusion revisited: Implications of an increased risk of bacterial infection with allogeneic transfusion. Transfusion 1999;39:808-17. http://dx.doi.org/10.1046/j.1537-2995.1999.39080808.x.
- Fattovich G, Giustina G, Degos F, Diodati G, Tremolada F, Nevens F. Effectiveness of interferon alfa on incidence of hepatocellular carcinoma and decompensation in cirrhosis type C. J Hepatol 1997;27. http://dx.doi.org/10.1016/S0168-8278(97)80302-9.
- Veenstra D, Sullivan SD, Iloeje UH. Estimating future hepatitis B virus (HBV) disease burden in the United States using a disease simulation model. Value Health 2003;6. http://dx.doi.org/10.1016/S1098-3015(10)64012-0.
- Ascher NL, Lake JR, Emond J, Roberts J. Liver transplantation for hepatitis C virus-related cirrhosis. Hepatology 1994;20:24S-7S. http://dx.doi.org/10.1002/hep.1840200708.
- Kilpe VE, Krakauer H, Wren RE. An analysis of liver transplant experience from 37 transplant centers as reported to medicare. Transplantation 1993;56:554-61. http://dx.doi.org/10.1097/00007890-199309000-00012.
- Detre KM, Belie SH, Lombardero M. Liver transplantation for chronic viral hepatitis. Viral Hepat Rev 1996;2.
- Wright M, Grieve R, Roberts J, Main J, Thomas HC, Alexander G, et al. Health benefits of antiviral therapy for mild chronic hepatitis C: randomized controlled trial and economic evaluation. Health Technol Assess 2006;10. http://dx.doi.org/10.3310/hta10210.
- Sweeting MJ, De Angelis D, Neal KR, Ramsay ME, Irving WL, Wright M, et al. Estimated progression rates in three United Kingdom hepatitis C cohorts differed according to method of recruitment. J Clin Epidemiol 2006;59:144-52. http://dx.doi.org/10.1016/j.jclinepi.2005.06.008.
- Office for National Statistics . Mortality Statistics. Deaths Registered in 2010 2011. www.ons.gov.uk/ons/taxonomy/index.html?nscl=Interim+Life+Tables (accessed 5 December 2013).
- Entecavir for the Treatment of Chronic Hepatitis B. London: NICE; 2009.
- Tenofovir Disoproxil for the Treatment of Chronic Hepatitis B. London: NICE; 2009.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2012.
- Woo G, Tomlinson G, Nishikawa Y, Kowgier M, Sherman M, Wong DKH, et al. Tenofovir and entecavir are the most effective antiviral agents for chronic hepatitis B: a systematic review and Bayesian meta-analyses. Gastroenterology 2010;139:1218-29. http://dx.doi.org/10.1053/j.gastro.2010.06.042.
- Brown RE, De Cock E, Colin X, Antoñanzas F, Iloeje UH. Hepatitis B management costs in France, Italy, Spain, and the United Kingdom. J Clin Gastroenterol 2004;38:S169-74. http://dx.doi.org/10.1097/00004836-200411003-00009.
- Jones J, Colquitt J, Shephard J, Harris P, Cooker K. Tenofovir disoproxil fumarate for the treatment of chronic hepatitis B infection. Health Technol Assess 2010;14:23-9. http://dx.doi.org/10.3310/hta14Suppl1/04.
- Jones J, Shepherd J, Baxter L, Gospodarevskaya E, Hartwell D, Harris P, et al. Adefovir dipivoxil and pegylated interferon alpha for the treatment of chronic hepatitis B: an updated systematic review and economic evaluation. Health Technol Assess 2009;13. http://dx.doi.org/10.3310/hta13350.
- Longworth L, Young T, Buxton MJ, Ratcliffe J, Neuberger J, Burroughs A, et al. Midterm cost-effectiveness of the liver transplantation program of England and Wales for three disease groups. Liver Transpl 2003;9:1295-307. http://dx.doi.org/10.1016/j.lts.2003.09.012.
- Takeda A, Jones J, Shepherd J, Davidson P, Price A. A systematic review and economic evaluation of adefovir dipivoxil and pegylated interferon-alpha-2a for the treatment of chronic hepatitis B. J Viral Hepat 2007;14:75-88. http://dx.doi.org/10.1111/j.1365-2893.2006.00808.x.
- Veenstra DL, Sullivan SD, Dusheiko GM, Jacobs M, Aledort JE, Lewis G, et al. Cost-effectiveness of peginterferon α–2a compared with lamivudine treatment in patients with HBe-antigen-positive chronic hepatitis B in the United Kingdom. Eur J Gastroenterol Hepatol 2007;19:631-8. http://dx.doi.org/10.1097/MEG.0b013e3281108079.
- NHS Reference Costs 2011–12. London: Department of Health; 2012.
- Stevenson M, Lloyd-Jones M, Morgan MY, Wong R. Non-invasive diagnostic assessment tools for the detection of liver fibrosis in patients with suspected alcohol-related liver disease: a systematic review and economic evaluation. Health Technol Assess 2012;16. http://dx.doi.org/10.3310/hta16040.
- Shepherd J, Gospodarevskaya E, Frampton G, Cooper K. Entecavir for the treatment of chronic hepatitis B infection. Health Technol Assess 2009;13:31-6. http://dx.doi.org/10.3310/hta13suppl3/05.
- Levy AR, Kowdley KV, Iloeje U, Tafesse E, Mukherjee J, Gish R, et al. The impact of chronic hepatitis B on quality of life: a multinational study of utilities from infected and uninfected persons. Value Health 2008;11:527-38. http://dx.doi.org/10.1111/j.1524-4733.2007.00297.x.
- Dusheiko GM, Roberts JA. Treatment of chronic type B and C hepatitis with interferon alfa: an economic appraisal. Hepatology 1995;22:1863-73.
- McLernon DJ, Dillon J, Donnan PT. Health-state utilities in liver disease: a systematic review. Med Decis Making 2008;28:582-92. http://dx.doi.org/10.1177/0272989X08315240.
- Chong CAKY, Gulamhussein A, Jenny Heathcote E, Lilly L, Sherman M, Naglie G, et al. Health-state utilities and quality of life in hepatitis C patients. Am J Gastroenterol 2003;98:630-8. http://dx.doi.org/10.1111/j.1572-0241.2003.07332.x.
- Sherman KE, Sherman SN, Chenier T, Tsevat J. Health values of patients with chronic hepatitis C infection. Arch Intern Med 2004;164:2377-82. http://dx.doi.org/10.1001/archinte.164.21.2377.
- Siebert U, Sroczynski G, Rossol S, Wasem J, Ravens-Sieberer U, Kurth BM, et al. Cost effectiveness of peginterferon α–2b plus ribavirin versus interferon α–2b plus ribavirin for initial treatment of chronic hepatitis C. Gut 2003;52:425-32. http://dx.doi.org/10.1136/gut.52.3.425.
- Younossi ZM, Boparai N, McCormick M, Price LL, Guyatt G. Assessment of utilities and health-related quality of life in patients with chronic liver disease. Am J Gastroenterol 2001;96:579-83. http://dx.doi.org/10.1111/j.1572-0241.2001.03537.x.
- Briggs A, Claxton K, Sculpher M. Decision Modelling for Health Economic Evaluation. Oxford: Oxford University Press; 2006.
- Liu S, Schwarzinger M, Carrat F, Goldhaber-Fiebert JD. Cost effectiveness of fibrosis assessment prior to treatment for chronic hepatitis C patients. PLOS One 2011;6. http://dx.doi.org/10.1371/journal.pone.0026783.
- Fried MW, Shiffman ML, Rajender Reddy K, Smith C, Marinos G, Gonçales FL, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med 2002;347:975-82. http://dx.doi.org/10.1056/NEJMoa020047.
- UK & Ireland Liver Transplant Audit, 2003. London: Royal College of Surgeons; 2003.
- Telaprevir for the Treatment of Genotype 1 Chronic Hepatitis C. London: NICE; 2012.
- Boceprevir for the Treatment of Genotype 1 Chronic Hepatitis C. London: NICE; 2012.
- Peginterferon Alfa and Ribavirin for the Treatment of Mild Chronic Hepatitis C. London: NICE; 2007.
- Datapharm . Electronic Medicines Compendium (eMC) n.d. www.medicines.org.uk/emc (accessed 2 December 2012).
- Manns MP, Wedemeyer H, Cornberg M. Treating viral hepatitis C: efficacy, side effects, and complications. Gut 2006;55:1350-9. http://dx.doi.org/10.1136/gut.2005.076646.
- Kamal SM, El Tawil AA, Nakano T, He Q, Rasenack J, Hakam SA, et al. Peginterferon α-2b and ribavirin therapy in chronic hepatitis C genotype 4: impact of treatment duration and viral kinetics on sustained virological response. Gut 2005;54:858-66. http://dx.doi.org/10.1136/gut.2004.057182.
- Camma C, Petta S, Enea M, Bruno R, Bronte F, Capursi V, et al. Cost-effectiveness of boceprevir or telaprevir for untreated patients with genotype 1 chronic hepatitis C. Hepatology 2012;56:850-60. http://dx.doi.org/10.1002/hep.25734.
- Esteban R, Buti M. Triple therapy with boceprevir or telaprevir for treatment naive HCV patients. Best Pract Res Clin Gastroenterol 2012;26:445-53. http://dx.doi.org/10.1016/j.bpg.2012.09.001.
- Jacobson IM, Marcellin P, Zeuzem S, Sulkowski MS, Esteban R, Poordad F, et al. Refinement of stopping rules during treatment of hepatitis C genotype 1 infection with boceprevir and peginterferon/ribavirin. Hepatology 2012;56:567-75. http://dx.doi.org/10.1002/hep.25865.
- Ramachandran P, Fraser A, Agarwal K, Austin A, Brown A, Foster GR, et al. UK consensus guidelines for the use of the protease inhibitors boceprevir and telaprevir in genotype 1 chronic hepatitis C infected patients. Aliment Pharmacol Ther 2012;35:647-62. http://dx.doi.org/10.1111/j.1365-2036.2012.04992.x.
- Shiffman ML, Esteban R. Triple therapy for HCV genotype 1 infection: telaprevir or boceprevir?. Liver Int 2012;32:54-60. http://dx.doi.org/10.1111/j.1478-3231.2011.02718.x.
- Swiss Association for the Study of the Liver . Treatment of chronic hepatitis C genotype 1 with triple therapy comprising telaprevir or boceprevir. Swiss Med Wkly 2012;142.
- Wilby KJ, Partovi N, Ford JA, Greanya E, Yoshida EM. Review of boceprevir and telaprevir for the treatment of chronic hepatitis C. Can J Gastroenterol 2012;26:205-10.
- Fried MW, Hadziyannis SJ, Shiffman ML, Messinger D, Zeuzem S. Rapid virological response is the most important predictor of sustained virological response across genotypes in patients with chronic hepatitis C virus infection. J Hepatol 2011;55:69-75. http://dx.doi.org/10.1016/j.jhep.2010.10.032.
- Grishchenko M, Grieve RD, Sweeting MJ, De Angelis D, Thomson BJ, Ryder SD, et al. Cost-effectiveness of pegylated interferon and ribavirin for patients with chronic hepatitis C treated in routine clinical practice. Int J Technol Assess Health Care 2009;25:171-80. http://dx.doi.org/10.1017/S0266462309090229.
- Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Cramp M, et al. Surveillance of cirrhosis for hepatocellular carcinoma: systematic review and economic analysis. Health Technol Assess 2007;11. http://dx.doi.org/10.3310/hta11340.
- Grieve R, Roberts J, Wright M, Sweeting M, DeAngelis D, Rosenberg W, et al. Cost effectiveness of interferon alpha or peginterferon alpha with ribavirin for histologically mild chronic hepatitis C. Gut 2006;55:1332-8. http://dx.doi.org/10.1136/gut.2005.064774.
- John-Baptiste AA, Tomlinson G, Hsu PC, Krajden M, Heathcote EJ, Laporte A, et al. Sustained responders have better quality of life and productivity compared with treatment failures long after antiviral therapy for hepatitis C. Am J Gastroenterol 2009;104:2439-48. http://dx.doi.org/10.1038/ajg.2009.346.
- Marcellin P, Chousterman M, Fontanges T, Ouzan D, Rotily M, Varastet M, et al. Adherence to treatment and quality of life during hepatitis C therapy: a prospective, real-life, observational study. Liver Int 2011;31:516-24. http://dx.doi.org/10.1111/j.1478-3231.2011.02461.x.
- Ware JE, Bayliss MS, Mannocchia M, Davis GL. Health-related quality of life in chronic hepatitis C: impact of disease and treatment response. Hepatology 1999;30:550-5. http://dx.doi.org/10.1002/hep.510300203.
- Bonkovsky HL, Wolley M. Reduction of health-related quality of life in chronic hepatitis C and improvement with interferon therapy. Hepatology 1999;29:264-70. http://dx.doi.org/10.1002/hep.510290124.
- Davis GL, Balart LA, Schiff ER, Lindsay K, Bodenheimer HC, Perrillo RP, et al. Assessing health-related quality of life in chronic hepatitis C using the sickness impact profile. Clin Ther 1994;16:334-43.
- Cheinquer N, Cheinquer H, Wolff FH, Coelho-Borges S. Effect of sustained virologic response on the incidence of hepatocellular carcinoma in patients with HCV cirrhosis. Braz J Infect Dis 2010;14:457-61. http://dx.doi.org/10.1016/S1413-8670(10)70093-3.
- Morgan TR, Ghany MG, Kim HY, Snow KK, Shiffman ML, De Santo JL, et al. Outcome of sustained virological responders with histologically advanced chronic hepatitis C. Hepatology 2010;52:833-44. http://dx.doi.org/10.1002/hep.23744.
- Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, et al. Peginterferon alfa-2b plus ribavirin compared with interferonalfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 2001;358:958-65. http://dx.doi.org/10.1016/S0140-6736(01)06102-5.
- Tillmann HL. Hepatitis C infection and presence of advanced fibrosis: wait or treat? Why wait? There is no time to lose, is there?. J Hepatol 2013;58:412-14. http://dx.doi.org/10.1016/j.jhep.2012.12.007.
- Alcohol Use Disorders: Physical Complications. London: NICE; 2010.
- Rosenberg WMC, Voelker M, Thiel R, Becka M, Burt A, Schuppan D, et al. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology 2004;127:1704-13. http://dx.doi.org/10.1053/j.gastro.2004.08.052.
- Thompson Coon J, Rogers G, Hewson P, Wright D, Anderson R, Jackson S, et al. Surveillance of cirrhosis for hepatocellular carcinoma: a cost-utility analysis. Br J Cancer 2008;98:1166-75. http://dx.doi.org/10.1038/sj.bjc.6604301.
- Verrill C, Markham H, Templeton A, Carr NJ, Sheron N. Alcohol-related cirrhosis – early abstinence is a key factor in prognosis, even in the most severe cases. Addiction 2009;104:768-74. http://dx.doi.org/10.1111/j.1360-0443.2009.02521.x.
- National Health Service (NHS) . NHS Blood and Transplant – About Transplants n.d. www.organdonation.nhs.uk/about_transplants/organ_allocation.
- Ratziua V, Bellentanib S, Cortez-Pintoc H, Dayd C, Marchesinie G. A position statement on NAFLD/NASH based on the EASL 2009 special conference. J Hepatol 2010;53:372-84. http://dx.doi.org/10.1016/j.jhep.2010.04.008.
- Rakoski MO, Singal AG, Rogers MAM, Conjeevaram H. Meta-analysis: insulin sensitizers for the treatment of non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2010;32:1211-21. http://dx.doi.org/10.1111/j.1365-2036.2010.04467.x.
- Musso G, Cassader M, Rosina F, Gambino R. Impact of current treatments on liver disease, glucose metabolism and cardiovascular risk in non-alcoholic fatty liver disease (NAFLD): a systematic review and meta-analysis of randomised trials. Diabetologia 2012;55:885-904. http://dx.doi.org/10.1007/s00125-011-2446-4.
- Mahady SE, Wong G, Craig JC, George J. Pioglitazone and vitamin E for nonalcoholic steatohepatitis: a cost utility analysis. Hepatology 2012;56:2172-9. http://dx.doi.org/10.1002/hep.25887.
- Dixon JB, Bhathal PS, Hughes NR, O’Brien PE. Nonalcoholic fatty liver disease: improvement in liver histological analysis with weight loss. Hepatology 2004;39:1647-54. http://dx.doi.org/10.1002/hep.20251.
- Huang MA, Greenson JK, Chao C, Anderson L, Peterman D, Jacobson J, et al. One-year intense nutritional counseling results in histological improvement in patients with nonalcoholic steatohepatitis: a pilot study. Am J Gastroenterol 2005;100:1072-81. http://dx.doi.org/10.1111/j.1572-0241.2005.41334.x.
- Bellentani S, Grave RD, Suppini A, Marchesini G, Bedogni G, Bugianesi E, et al. Behavior therapy for nonalcoholic fatty liver disease: the need for a multidisciplinary approach. Hepatology 2008;47:746-54. http://dx.doi.org/10.1002/hep.22009.
- Mummadi RR, Kasturi KS, Chennareddygari S, Sood GK. Effect of bariatric surgery on nonalcoholic fatty liver disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol 2008;6:1396-402. http://dx.doi.org/10.1016/j.cgh.2008.08.012.
- Sanyal AJ, Mofrad PS, Contos MJ, Sargeant C, Luketic VA, Sterling RK, et al. A pilot study of vitamin E versus vitamin E and pioglitazone for the treatment of nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol 2004;2:1107-15. http://dx.doi.org/10.1016/S1542-3565(04)00457-4.
- Sanyal AJ, Chalasani N, Kowdley KV, McCullough A, Diehl AM, Bass NM, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010;362:1675-85. http://dx.doi.org/10.1056/NEJMoa0907929.
- Aithal GP, Thomas JA, Kaye PV, Lawson A, Ryder SD, Spendlove I, et al. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology 2008;135:1176-84. http://dx.doi.org/10.1053/j.gastro.2008.06.047.
- Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 2006;355:2297-307. http://dx.doi.org/10.1056/NEJMoa060326.
- Ratziu V, Giral P, Jacqueminet S, Charlotte F, Hartemann-Heurtier A, Serfaty L, et al. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement With Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology 2008;135:100-10. http://dx.doi.org/10.1053/j.gastro.2008.03.078.
- Idilman R, Mizrak D, Corapcioglu D, Bektas M, Doganay B, Sayki M, et al. Clinical trial: insulin-sensitizing agents may reduce consequences of insulin resistance in individuals with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2008;28:200-8. http://dx.doi.org/10.1111/j.1365-2036.2008.03723.x.
- Shields WW, Thompson KE, Grice GA, Harrison SA, Coyle WJ. The effect of metformin and standard therapy versus standard therapy alone in nondiabetic patients with insulin resistance and nonalcoholic steatohepatitis (NASH): a pilot trial. Ther Adv Gastroenterol 2009;2:157-63. http://dx.doi.org/10.1177/1756283X09105462.
- Uygun A, Kadayifci A, Isik AT, Ozgurtas T, Deveci S, Tuzun A, et al. Metformin in the treatment of patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther 2004;19:537-44. http://dx.doi.org/10.1111/j.1365-2036.2004.01888.x.
- Haukeland JW, Konopski Z, Linnestad P, Azimy S, Loberg EM, Haaland T, et al. Abnormal glucose tolerance is a predictor of steatohepatitis and fibrosis in patients with non-alcoholic fatty liver disease. Scand J Gastroenterol 2005;40:1469-77. http://dx.doi.org/10.1080/00365520500264953.
- Mahady SE, Webster AC, Walker S, Sanyal A, George J. The role of thiazolidinediones in non-alcoholic steatohepatitis – a systematic review and meta analysis. J Hepatol 2011;55:1383-90. http://dx.doi.org/10.1016/j.jhep.2011.03.016.
- Lavine JE, Schwimmer JB, Van Natta ML, Molleston JP, Murray KF, Rosenthal P, et al. Effect of Vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents the tonic randomized controlled trial. JAMA 2011;305:1659-68. http://dx.doi.org/10.1001/jama.2011.520.
- Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012;55:2005-23. http://dx.doi.org/10.1002/hep.25762.
- Promrat K, Kleiner DE, Niemeier HM, Jackvony E, Kearns M, Wands JR, et al. Randomized controlled trial testing the effects of weight loss on nonalcoholic steatohepatitis. Hepatology 2010;51:121-9. http://dx.doi.org/10.1002/hep.23276.
- Obesity Guidance on the Prevention, Identification, Assessment and Managment of Overweight and Obesity in Adults and Children. London: NICE; 2006.
- De Freitas ACT, Campos ACL, Coelho JCU. The impact of bariatric surgery on nonalcoholic fatty liver disease. Curr Opin Clin Nutr Metab Care 2008;11:267-74. http://dx.doi.org/10.1097/MCO.0b013e3282fbd33f.
- Chavez-Tapia NC, Tellez-Avila FI, Barrientos-Gutierrez T, Mendez-Sanchez N, Lizardi-Cervera J, Uribe M. Bariatric surgery for non-alcoholic steatohepatitis in obese patients. Cochrane Database Syst Rev 2010;1.
- David K, Kowdley KV, Unalp A, Kanwal F, Brunt EM, Schwimmer JB, et al. Quality of life in adults with nonalcoholic fatty liver disease: baseline data from the nonalcoholic steatohepatitis clinical research network. Hepatology 2009;49:1904-12. http://dx.doi.org/10.1002/hep.22868.
- Donnan PT, McLernon D, Dillon JF, Ryder S, Roderick P, Sullivan F, et al. Development of a decision support tool for primary care management of patients with abnormal liver function tests without clinically apparent liver disease: a record-linkage population cohort study and decision analysis (ALFIE). Health Technol Assess 2009;13. http://dx.doi.org/10.3310/hta13250.
- NHS Business Service Authority . Prescribing Analysis Charts – Cardiovascular System n.d. www.nhsbsa.nhs.uk/PrescriptionServices/2582.aspx.
- Hypertension: Clinical Management of Primary Hypertension in Adults. London: NICE; 2011.
- Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther 2011;34:274-85. http://dx.doi.org/10.1111/j.1365-2036.2011.04724.x.
- D’Amico G, Garcia-Tsao G, Pagliaro L. Natural history and prognostic indicators of survival in cirrhosis: a systematic review of 118 studies. J Hepatol 2006;44:217-31. http://dx.doi.org/10.1016/j.jhep.2005.10.013.
- Andersson KL, Salomon JA, Goldie SJ, Chung RT. Cost effectiveness of alternative surveillance strategies for hepatocellular carcinoma in patients with cirrhosis. Clin Gastroenterol Hepatol 2008;6:1418-24. http://dx.doi.org/10.1016/j.cgh.2008.08.005.
- Bolondi L, Sofia S, Siringo S, Gaiani S, Casali A, Zironi G, et al. Surveillance programme of cirrhotic patients for early diagnosis and treatment of hepatocellular carcinoma: a cost effectiveness analysis. Gut 2001;48:251-9. http://dx.doi.org/10.1136/gut.48.2.251.
- Saab S, Ly D, Nieto J, Kanwal F, Lu D, Raman S, et al. Hepatocellular carcinoma screening in patients waiting for liver transplantation: a decision analytic model. Liver Transpl 2003;9:672-81. http://dx.doi.org/10.1053/jlts.2003.50120.
- Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol 2004;130:417-22. http://dx.doi.org/10.1007/s00432-004-0552-0.
- Claxton K, Martin S, Soares M, Rice N, Spackman E, Hinde S, et al. Methods for the Estimation of the NICE Cost Effectiveness Threshold 2013.
- European Association for the Study of the Liver . EASL Clinical Practice Guidelines: managment of hepatitis C virus infection. J Hepatol 2014;60:392-420. http://dx.doi.org/10.1016/j.jhep.2013.11.003.
- Barshop NJ, Francis CS, Schwimmer JB, Lavine JE. Nonalcoholic fatty liver disease as a comorbidity of childhood obesity. Ped Health 2009;3:271-81. http://dx.doi.org/10.2217/phe.09.21.
- Feldstein AE, Wieckowska A, Lopez AR, Liu YC, Zein NN, McCullough AJ. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology 2009;50:1072-8. http://dx.doi.org/10.1002/hep.23050.
- Chou R, Wasson N. Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection: a systematic review. Ann Intern Med 2013;158:807-20. http://dx.doi.org/10.7326/0003-4819-158-11-201306040-00005.
- Castera L, Foucher J, Bernard P-H, Carvalho F, Allaix D, Merrouche W, et al. Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology 2010;51:828-35. http://dx.doi.org/10.1002/hep.23425.
- Shinkins B, Thompson M, Mallett S, Perera R. Diagnostic accuracy studies: how to report and analyse inconclusive test results. BMJ 2013;346. http://dx.doi.org/10.1136/bmj.f2778.
- Schuetz GM, Schlattmann P, Dewey M. Use of 3 × 2 tables with an intention to diagnose approach to assess clinical performance of diagnostic tests: meta-analytical evaluation of coronary CT angiography studies. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e6717.
Appendix 1 Literature review: diagnostic test accuracy data
Search strategy
Date of search: April 2012.
-
CT.ti,ab.
-
tomodensitometry.ti,ab.
-
PET.ti,ab.
-
MRI.ti,ab.
-
NMRI.ti,ab.
-
zeugmatogra*.ti,ab.
-
((computed or computeri?ed or magneti* or proton or “Acoustic Radiation Force Impulse” or ARF) adj5 (tomogra* or scan or scans or imaging)).ti,ab.
-
Tomography, X-Ray Computed/
-
Magnetic Resonance Imaging/
-
elastography.ti,ab.
-
elastographies.ti,ab.
-
sonoelastography.ti,ab.
-
sonoelastographies.ti,ab.
-
sono-elastography.ti,ab.
-
sono-elastographies.ti,ab.
-
elastogram.ti,ab.
-
elastograms.ti,ab.
-
vibroacoustography.ti,ab.
-
vibroacoustographies.ti,ab.
-
vibro-acoustography.ti,ab.
-
vibro-acoustographies.ti,ab.
-
fibroscan.ti,ab.
-
elastometry.ti,ab.
-
elasticity.ti,ab.
-
“liver stiffness”.ti,ab.
-
elastogra*.ti,ab.
-
echogra*.ti,ab.
-
ultrason*.ti,ab.
-
ultrasound.ti,ab.
-
Ultrasonography/
-
Elasticity Imaging Techniques/
-
Elasticity.ti,ab.
-
((alanine* or aspartate* or glutamic*) and (transaminase or aminotransferase*)).ti,ab.
-
SGOT.ti,ab.
-
SGPT.ti,ab.
-
AST.ti,ab.
-
ALT.ti,ab.
-
Aspartate Aminotransferases/
-
Alanine Transaminase/
-
platelet.ti,ab.
-
platelets.ti,ab.
-
thrombocyte.ti,ab.
-
thrombocytes.ti,ab.
-
APRI.ti,ab.
-
Blood Platelets/
-
46. Biological Markers/
-
ELF.ti,ab.
-
“enhanced liver fibrosis”.ti,ab.
-
Fibrotest.ti,ab.
-
Fibrosure.ti,ab.
-
Fibrometer.ti,ab.
-
FIB4.ti,ab.
-
FIB-4.ti,ab.
-
BARD.ti,ab.
-
Fibrospect.ti,ab.
-
Hepascore.ti,ab.
-
“Hyaluronic acid”.ti,ab.
-
hyaluronate.ti,ab.
-
Hyaluronic Acid/
-
“Forns index”.ti,ab.
-
laminin.ti,ab.
-
Laminin/
-
YKL-40.ti,ab.
-
“YKL 40”.ti,ab.
-
“Type IV collagen”.ti,ab.
-
Collagen Type IV/
-
“Procollagen III N-peptide”.ti,ab.
-
“Lok index”.ti,ab.
-
MP3.ti,ab.
-
MP-3.ti,ab.
-
“Fibrosis probability index”.mp. or “sydney index”.ti,ab.
-
FPI.ti,ab.
-
Fibroindex.ti,ab.
-
“Virahep-C index”.ti,ab.
-
“Virahep C index”.ti,ab.
-
“Göteborg University Cirrhosis Index”.ti,ab.
-
GUCI.ti,ab.
-
SHASTA.ti,ab.
-
Glycocirrhotest.ti,ab.
-
Glycofibrotest.ti,ab.
-
BAAT.ti,ab.
-
“NAFLD fibrosis score”.ti,ab.
-
Cytokeratin-18.ti,ab.
-
“Cytokeratin 18”.ti,ab.
-
M30.ti,ab.
-
M-30.ti,ab.
-
“NASJH test”.ti,ab.
-
“NAFIC score”.ti,ab.
-
PGA.ti,ab.
-
“PGAA index”.ti,ab.
-
“Bonancini score”.ti,ab.
-
“Pohl score”.ti,ab.
-
“Cirrhosis discriminant score”.ti,ab.
-
“Age-platelet index”.ti,ab.
-
TIMP-1.ti,ab.
-
“tissue inhibitory metalloprotease”.ti,ab.
-
MBT.ti,ab.
-
“C-methacetin breath test”.ti,ab.
-
“Phosphoproteomic biomarker”.ti,ab.
-
“Phosphoproteomic biomarkers”.ti,ab.
-
PICP.ti,ab.
-
PIIINP.ti,ab.
-
PON-I.ti,ab.
-
“paraoxonase I”.ti,ab.
-
MFAP-4.ti,ab.
-
“MFAP 4”.ti,ab.
-
MFAP4.ti,ab.
-
“microfibril associated glycoprotein 4”.ti,ab.
-
or/1-9,27-30,33-39
-
limit 109 to yr=“1988 -Current”
-
or/10-26,30-32,40-108
-
limit 111 to yr=“2001 -Current”
-
110 or 112
-
cirrhosis.ti,ab.
-
cirrhoses.ti,ab.
-
fibrosis.ti,ab.
-
fibroses.ti,ab.
-
“liver disease”.ti,ab.
-
(hepatitis or hepatic).ti,ab.
-
steatohepatitis.ti,ab.
-
Liver Cirrhosis/
-
Fibrosis/
-
Liver Diseases/
-
Hepatitis/
-
or/114-124
-
113 and 125
-
exp “sensitivity and specificity”/
-
“reproducibility of results”/
-
diagnos*.ti. or diagnostic.ab.
-
di.fs.
-
sensitivit*.ab.
-
specificit*.ab.
-
(ROC or “receiver operat*”).ab.
-
Area under curve/
-
(“Area under curve” or AUC).ab.
-
(sROC or “optimal cut-off”).ab.
-
(accura* or ((gold* or reference) adj2 standard)).ti,ab.
-
(likelihood adj3 (ratio* or function*)).ab.
-
((true or false) adj3 (positive* or negative*)).ab.
-
((positive* or negative* or false or true) adj3 (rate* or predictive)).ti,ab.
-
or/127-140
-
126 and 141
-
*liver cirrhosis/di
-
*hepatitis/
-
*fibrosis/
-
(liver or hepatitis or hepatic or fibrosis).ab.
-
di.fs.
-
146 and 147
-
or/143-145,148
-
113 and 149
-
113 and 149
-
142 or 151
Search narrative
This search strategy has been kept deliberately very broad – utilising only two main search concepts: index test(s) (concept A) – lines 1–108 – and the disease of interest (concept B)/location of disease of interest (concept B) – lines 110–120. A methodological filter (concept C) is included but does not act as a filter to all search results [it is used in parallel: (A AND B AND C) OR (A AND B-focused)].
Potential studies for inclusion were initially identified from published non-Cochrane reviews and background literature. This generated a reference set of 70 potential (and probable) studies for inclusion to use to test the search strategy detailed above. The strategy was designed without knowledge of the 70 potential studies or of the search strategies used to identify the 70 from their original publications. All 70 studies were identified by the above strategy.
The yield from the above strategy was high. However, due the large number of tests within the scope of the review, a large yield could not be avoided.
Validation string
(“19196449” or “18448567” or “16823833” or “15685546” or “19013661” or “18673426” or “18410556” or “18672413” or “16394849” or “16020491” or “17255218” or “18192914” or “17258346” or “17530363” or “18987556” or “19413672” or “17663420” or “18568136” or “18637064” or “18818788” or “18930329” or “18705692” or “19261000” or “21904476” or “17608672” or “18218676” or “19030204” or “19104699” or “18544945” or “19308312” or “18832522” or “18083083” or “12883497” or “20493576” or “20180868” or “19060630” or “19013661” or “18672413” or “19758273” or “19171202” or “18339075” or “18285716” or “18339592” or “19999223” or “18796094” or “18706734” or “18482283” or “18553008” or “18692034” or “17156890” or “17321634” or “17634962” or “17914968” or “16970597” or “16538110” or “16487951” or “16863553” or “17032409” or “16118349” or “17032410” or “16737415” or “16620291” or “16825937” or “16268817” or “16109665” or “15894397” or “15915455” or “16284529” or “15122779” or “17393509” or “18390575” or “19291784”).ui.)
EMBASE
Date of search: April 2012.
-
exp *Liver Cirrhosis/ or exp *Liver Fibrosis/ or exp *Liver Disease/ or exp *Hepatitis/
-
(liver or hepatic).ti,ab.
-
exp *Liver/
-
3 or 2
-
(cirrhosis or cirrhoses or fibrosis or fibroses or liver disease or hepatitis or steatohepatitis).ti,ab.
-
4 and 5
-
1 or 6
-
(CT or tomodensitometry or MRI or NMRI or zeugmatogra*).ti,ab.
-
((computed or computerised or computerized or CT or magneti* or MR or NMR or proton) and (tomogra* or scan or scans or imaging)).ti,ab.
-
exp *computer assisted tomography/
-
exp *nuclear magnetic resonance imaging/
-
(elastography or elastographies or sonoelastography or sonoelastographies or sono-elastography or sono-elastographies or elastogram or elastograms or vibroacoustography or vibroacoustographies or vibro-acoustography or vibro-acoustographies or fibroscan or elastometry or elasticity or liver stiffness or echogra* or ultrason* or ultrasound).ti,ab.
-
exp *ultrasound/
-
exp *elastography/
-
((alanine* or aspartate* or glutamic*) and (transaminase or aminotransferase*)).ti,ab.
-
(platelet or platelets or thrombocyte or thrombocytes or APRI or ELF or enhanced liver fibrosis or Fibrotest or Fibrosure or Fibrometer or FIB4 or FIB-4 or BARD or Fibrospect or Hepascore or Hyaluronic acid or hyaluronate or Forns index or laminin or YKL-40 or YKL 40 or Type IV collagen or Procollagen III N-peptide or Lok index or MP3 or MP-3 or Fibrosis probability index or FPI or Fibroindex or Virahep-C index or Virahep C index or Göteborg University Cirrhosis Index or GUCI or SHASTA or Glycocirrhotest or Glycofibrotest or BAAT or NAFLD fibrosis score or Cytokeratin-18 or Cytokeratin 18 or M30 or M-30 or NASJH test or NAFIC score or PGA or PGAA index or Bonancini score or Pohl score or Cirrhosis discriminant score or Age-platelet index or TIMP-1 or tissue inhibitory metalloprotease or MBT or C-methacetin breath test or Phosphoproteomic biomarker or Phosphoproteomic biomarkers or PICP or PIIINP or PON-I or paraoxonase I or MFAP-4 or MFAP 4 or MFAP4 or microfibril associated glycoprotein 4).ti,ab.
-
(SGOT or SGPT or AST or ALT).ti,ab.
-
exp *alanine aminotransferase/
-
exp *aspartate aminotransferase/
-
exp *thrombocyte/
-
exp *biological marker/
-
(2001* or 2002* or 2003* or 2004* or 2005* or 2006* or 2007* or 2008* or 2009* or 2010* or 2011* or 2012*).em.
-
12 or 14 or 16 or 20 or 21
-
22 and 23
-
8 or 9 or 10 or 11 or 13 or 15 or 17 or 18 or 19
-
limit 25 to yr=“1988 -Current”
-
24 or 26
-
7 and 27
Science Citation Index expanded
Date of search: 1988 to April 2012.
#1 TS=(cirrhosis OR cirrhoses OR fibrosis OR fibroses or liver disease or hepatitis or steatohepatitis)
#2 TS=(liver or hepatic)
#3 TS=(CT OR tomodensitometry OR PET OR MRI OR NMRI OR zeugmatogra*)
#4 TS=((Acoustic Radiation Force Impulse or ARFI OR computed OR computerised OR computerized OR CT OR magneti* OR MR OR NMR OR proton) AND (tomogra* OR scan OR scans OR imaging))
#5 TS=(elastography or elastographies or sonoelastography or sonoelastographies or sono-elastography or sono-elastographies or elastogram or elastograms or vibroacoustography or vibroacoustographies or vibro-acoustography or vibro-acoustographies or fibroscan or elastometry or elasticity or liver stiffness OR echogra* OR ultrason* OR ultrasound)
#6 TS=( (alanine* OR aspartate* OR glutamic*) AND (transaminase OR aminotransferase*))
#7 TS=(SGOT OR SGPT OR AST OR ALT OR platelet OR platelets OR thrombocyte OR thrombocytes OR APRI OR ELF OR enhanced liver fibrosis OR Fibrotest OR Fibrosure OR Fibrometer OR FIB4 OR FIB-4 OR BARD OR Fibrospect OR Hepascore OR Hyaluronic acid OR hyaluronate OR Forns index OR laminin OR YKL-40 OR YKL 40 OR Type IV collagen OR Procollagen III N-peptide OR Lok index OR MP3 OR MP-3 OR Fibrosis probability index OR FPI OR Fibroindex OR Virahep-C index OR Virahep C index OR Göteborg University Cirrhosis Index OR GUCI OR SHASTA OR Glycocirrhotest OR Glycofibrotest OR BAAT OR NAFLD fibrosis score OR Cytokeratin-18 OR Cytokeratin 18 OR M30 OR M-30 OR NASJH test OR NAFIC score OR PGA OR PGAA index OR Bonancini score OR Pohl score OR Cirrhosis discriminant score OR Age-platelet index OR TIMP-1 OR tissue inhibitory metalloprotease OR MBT OR C-methacetin breath test OR Phosphoproteomic biomarker OR Phosphoproteomic biomarkers OR PICP OR PIIINP OR PON-I OR paraoxonase I OR MFAP-4 OR MFAP 4 OR MFAP4 OR microfibril associated glycoprotein 4)
#8 (#3 OR #4 OR #5 OR #6 OR #7)
#9 (#1 AND #2 AND #8)
Appendix 2 Literature review: cost-effectiveness analyses (hepatitis B, hepatitis C, alcoholic liver disease, non-alcoholic liver disease, cirrhosis)
Hepatitis B
Database, platform: MEDLINE (via Ovid)
Search strategy: Natural History
Date of search: 10 May 2012.
-
*EPIDEMIOLOGY/
-
*INCIDENCE/
-
*PREVALENCE/
-
incidence.ti.
-
prevalence.ti.
-
epidemiol$.ti.
-
(etiolog$ or aetiolog$).ti.
-
or/1-7
-
exp *Hepatitis B/
-
8 and 9
-
limit 10 to (english language and humans)
-
limit 11 to yr=“2004 -Current”
Search strategy: costs
Date of search: 11 May 2012.
-
exp Hepatitis B/ or Hepatitis B, Chronic/
-
exp Hepatitis B Virus/ or exp Hepatitis B Antibodies/
-
(hbv or hepatitis-B or hepatitis B or HBeAg negative or HBeAg positive or HBsAG).mp.
-
1 or 2 or 3
-
(pegylat$ adj3interferon$ or peg-ifn or peginterferon$ or pegasys or pegintron or viraferonpeg).mp.
-
(interferon alpha 2a or interferon alfa 2a or interferon alpha 2b or interferon alfa 2b or alpha interferon or intron$ or viraferon or roferon).mp.
-
exp interferon-alpha/
-
6 or 7
-
exp Polyethylene Glycols/
-
polyethylene glycol$.mp. or peg$.tw.
-
9 or 10
-
8 and 11
-
5 or 12
-
13 and 4
-
limit 14 to english language
-
(adefovir dipivoxil or adefovir$ or hepsera).mp.
-
16 and 4
-
17
-
limit 18 to english language
-
(tenofovir disoproxil or tenofovir$ or viread).mp.
-
20 and 4
-
limit 21 to english language
-
(entecavir or entecavir$ or baraclude).mp.
-
23 and 4
-
limit 24 to english language
-
exp ECONOMICS/
-
exp ECONOMICS, HOSPITAL/
-
exp ECONOMICS, PHARMACEUTICAL/
-
exp ECONOMICS, NURSING/
-
exp ECONOMICS, DENTAL/
-
exp ECONOMICS, MEDICAL/
-
exp “Costs and Cost Analysis”/
-
Cost-Benefit Analysis/
-
VALUE OF LIFE/
-
exp MODELS, ECONOMIC/
-
exp FEES/ and CHARGES/
-
exp BUDGETS/
-
(economic$ or price$ or pricing or financ$ or fee$ or pharmacoeconomic$ or pharma economic$).tw.
-
(cost$ or costly or costing$ or costed).tw.
-
(cost$ adj2 (benefits$ or utilit$ or minim$ or effective$)).tw.
-
(expenditure$ not energy).tw.
-
(value adj2 (money or monetary)).tw.
-
budget$.tw.
-
(economic adj2 burden).tw.
-
“resource use”.ti,ab.
-
or/26-45
-
news.pt.
-
letter.pt.
-
editorial.pt.
-
comment.pt.
-
or/47-50
-
46 not 51
-
52 and 4
-
52 and 15
-
52 and 19
-
52 and 22
-
52 and 25
-
53
-
58 and 54 and 55 and 56 and 57
-
limit 59 to english language
-
limit 60 to yr=“2004 -Current”
Search strategy: quality of life
Date of search: 11 May 2012.
-
value of life/
-
quality adjusted life year/
-
quality adjusted life.ti,ab.
-
(qaly$ or qald$ or qale$ or qtime$).ti,ab.
-
disability adjusted life.ti,ab.
-
daly$.ti,ab.
-
health status indicators/
-
(sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).ti,ab.
-
(sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six or short form six).ti,ab.
-
(sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).ti,ab.
-
(sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).ti,ab.
-
(sf20 or sf 20 or short form 20 or shortform or sf twenty or sf twenty or sftwenty or shortform twenty or short form twenty).ti,ab.
-
(euroqol or euro qol or eq5d or eq 5d).ti,ab.
-
(hql or hqol or h qol or hrqol or hr qol).ti,ab.
-
(hye or hyes).ti,ab.
-
health$ year$ equivalent$.ti,ab.
-
health utilit$.ab.
-
(hui or hui1 or hui2 or hui3).ti,ab.
-
disutil$.ti,ab.
-
rosser.ti,ab.
-
quality of well being.ti,ab.
-
quality of wellbeing.ti,ab.
-
qwb.ti,ab.
-
willingess to pay.ti,ab.
-
standard gamble$.ti,ab.
-
time trade off.ti,ab.
-
time tradeoff.ti,ab.
-
tto.ti,ab.
-
(index adj2 well being).mp.
-
(quality adj2 well being).mp.
-
(health adj3 utilit$ ind$).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
-
((multiattribute$ or multi attribute$) adj3 (health ind$ or theor$ or health state$ or utilit$ or analys$)).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
-
quality adjusted life year$.mp.
-
(15D or 15 dimension$).mp.
-
(12D or 12 dimension$).mp.
-
rating scale$.mp.
-
linear scal$.mp.
-
linear analog$.mp.
-
visual analog$.mp.
-
(categor$ adj2 scal$).mp.
-
or/1-40
-
(letter or editorial or comment).pt.
-
41 not 42
-
exp Hepatitis B/ or Hepatitis B, Chronic/
-
exp Hepatitis B Virus/ or exp Hepatitis B Antibodies/
-
(hbv or hepatitis-B or hepatitis B or HBeAg negative or HBeAg positive or HBsAG).mp.
-
44 or 45 or 46
-
43 and 47
-
limit 48 to english language
-
limit 49 to yr=”2004 -Current
Non-alcoholic fatty liver disease
Database, platform: MEDLINE (via Ovid)
Search strategy 1
Date of search: 24 June 2013.
-
(enhanced adj liver adj fibrosis).tw.
-
(elf adj test$).tw.
-
(elf and diagnos$).tw.
-
(elf and (fibros*s or cirrhos*s)).tw.
-
elf.tw.
-
exp liver cirrhosis/ or exp liver diseases/
-
Fatty Liver/di, dh, de, dt, ec [Diagnosis, Diet Therapy, Drug Effects, Drug Therapy, Economics]
-
6 and 7
-
5 and 8
-
1 or 2 or 3 or 4 or 9
-
age-plt index.tw.
-
api index.tw.
-
apri.tw.
-
arfi.tw.
-
astalt.tw.
-
ast-alt.tw.
-
bard.tw.
-
coll4.tw.
-
typeIVcollagen.tw.
-
type IV Collagen.tw.
-
FIB 4.tw.
-
Fibrotest.tw.
-
Hyaluronic Acid.tw.
-
Hepascore.tw.
-
NAFIC.tw.
-
nafic score.tw.
-
ndp.tw.
-
nedaplatin.tw.
-
nfs.tw.
-
nafld fibrosis score.tw.
-
plt.tw.
-
magentic resonance elastography.tw.
-
mre.tw.
-
(transient adj elsatograph$).tw.
-
(elastograph$ and liver).tw.
-
or/11-35
-
exp liver cirrhosis/ or exp liver diseases/
-
Fatty Liver/di, dh, de, dt, ec [Diagnosis, Diet Therapy, Drug Effects, Drug Therapy, Economics]
-
(fibros*s or chirrhos*s).tw.
-
37 or 38 or 39
-
Biological Markers/
-
(biomarker$ or bio-marker$).tw.
-
(marker$ and (biologic$ or biochemical or serum or direct or indirect)).tw.
-
Algorithms/
-
algorithm$.tw.
-
(composite and blood).tw.
-
or/41-46
-
36 and 47
-
Hyaluronic Acid/
-
((hyaluronic adj acid) or (hyalauronate or hyaluronan)).tw.
-
49 or 50
-
(procollagen or piinp or p3np or ppcp).tw.
-
((tissue and inhibitor and metalloproteinase$) or timps).tw.
-
51 and 52 and 53
-
52 or 53 or 54
-
36 and 55
-
Alpha-Macroglobulins/
-
((alpha and macroglobulin$) or (alpha adj 2m)).tw.
-
57 or 58
-
((apolipoprotein$ adj a 1) or apoa 1).tw.
-
Haptoglobins/
-
haptoglobin$.tw.
-
61 or 62
-
(bilrubin$ or hematoidin$).tw.
-
(gamma adj glutamyl adj transpeptidase$).tw.
-
(gamma adj glutamyltransferase$).tw.
-
64 or 65 or 66
-
59 and 60 and 63 and 64 and 67
-
59 or 60 or 63 or 64 or 67
-
36 and 69
-
(alanine adj (aminotransferase$ or aminotransaminase$)).tw.
-
(serum adj glutamic adj oxaloacetic adj transaminase$).tw.
-
sgpt.tw.
-
71 or 72 or 73
-
(asparate adj (aminotransferase$ or aminotransaminase$)).tw.
-
(serum adj glutamic adj oxaloacetic adj transaminase$).tw.
-
sgot.tw.
-
75 or 76 or 77
-
59 and 60 and 63 and 64 and 67 and 74 and 78
-
59 or 60 or 63 or 64 or 67 or 74 or 78
-
36 and 80
-
exp “Sensitivity and Specificity”/
-
sensitivity.tw.
-
specificity.tw.
-
((pre-test or pretest) adj probability).tw.
-
post-test probability.tw.
-
predictive value$.tw.
-
likelihood ratio$.tw.
-
or/82-88
-
48 and 89
-
56 and 89
-
70 and 91
-
81 and 89
-
90 or 91 or 92 or 93
-
iqur.tw.
-
biopredictive.tw.
-
echosens.tw.
-
95 or 96 or 97
-
10 or 36 or 54 or 68 or 79 or 94 or 98
-
exp “Costs and Cost Analysis”/
-
Economics/
-
exp Economics, Hospital/
-
exp Economics, Medical/
-
Economics, Nursing/
-
exp models,economic/
-
Economics, Pharamceutical/
-
exp “Fees and Charges”/
-
exp Budgets/
-
budget$.tw.
-
ec.fs.
-
cost$.ti.
-
(cost$ adj2 (effective$ or utilit$ or benefit$or minimi$)).ab.
-
(economic$ or pharmaceconomic$ or pharmaco-economic$).ti.
-
(price$ or pricing$).tw.
-
(financial or finance or finances or financed).tw.
-
(fee or fees).tw.
-
(value adj2 (money or monetary)).tw.
-
quality-adjusted life years/
-
(qaly or qalys).af.
-
(quality adjusted life year or quality adjusted life years).af.
-
101 or 120
-
99 and 121
-
Liver Cirrhosis/ or Middle Aged/ or Aged/ or Liver Diseases, Alcoholic/ or Hepatitis/ or Fatty Liver/ or Adult/ or Liver Diseases/ or non alcoholic liver disease.mp. or Liver/
-
122 and 123
Search strategy 2
Date of search: 26 July 2013.
-
cost effectiveness.mp. or Cost-Benefit Analysis/
-
Hepatitis/ or Fatty Liver/ or non alcoholic steatohepatitis.mp. or Liver/
-
1 and 2
Alcoholic liver disease
Database, platform: MEDLINE (via Ovid)
Search strategy
Date of search: 21 June 2013.
-
(enhanced adj liver adj fibrosis).tw.
-
(elf adj tests$).tw.
-
(elf and diagnos$).tw.
-
(elf and (fibros* or cirrhos*s)).tw.
-
elf.tw.
-
exp liver cirrhosis/ or exp liver diseases, alcoholic/
-
5 and 6
-
1 or 2 or 3 or 4 or 7
-
Cytokeratin-18.tw.
-
Forns.tw.
-
Fibroscan.tw.
-
YKL-40.tw.
-
(transient adj elastograph$).tw.
-
(elastograph$ and liver).tw.
-
or/9-14
-
exp liver cirrhosis/ or exp liver diseases, alcoholic/
-
(fibros* or cirrhos*s).tw.
-
16 or 17
-
Biological Markers/
-
(biomarker$ or bio-markers$).tw.
-
(marker$ and (biologic$ or biochemical or serum or direct or indirect)).tw.
-
Algorithms/
-
algorithm$.tw.
-
(composite and blood).tw.
-
or/19-24
-
18 and 25
-
Hyaluronic Acid/
-
((hyaluronic adj acid) or (hyaluronate or hyaluronan)).tw.
-
27 or 28
-
(procollagen or piinp or p3np or ppcp).tw.
-
((tissue and inhibitor and metalloproteinase$) or timps).tw.
-
29 and 30 and 31
-
30 or 31 or 32
-
18 or 33
-
Alpha-Macroglobulins/
-
((apha and macroglobulin$) or (alpha adj 2m)).tw.
-
35 or 36
-
((apolipoprotein$ adj a 1) or apoa 1).tw.
-
Haptoglobins/
-
haptoglobin$.tw.
-
39 or 40
-
(bilirubin$ or hematoidin$).tw.
-
(gamma adj glutamyl adj transpeptidase$).tw.
-
(gamma adj glutamyltransferase$).tw.
-
((gamma adj gt) or ggt or ggtp).tw.
-
43 or 44 or 45
-
37 and 38 and 41 and 42 and 46
-
37 or 38 or 41 or 42 or 46
-
18 and 48
-
(alanine adj (aminotransferase$ or aminotransaminase$)).tw.
-
(serum adj glutamic adj pyruvic adj transaminase$).tw.
-
sgpt.tw.
-
50 or 51 or 52
-
(aspartate adj (aminotransferase$ or aminotransaminase$)).tw.
-
(serum adj glutamic adj oxaloacetic adj transaminase$).tw.
-
sgot.tw.
-
54 or 55 or 56
-
37 and 38 and 41 and 42 and 46 and 53 and 57
-
37 or 38 or 41 or 42 or 46 or 53 or 57
-
18 and 59
-
exp “Sensitivity and Specificity”/
-
sensitivity.tw.
-
specificity.tw.
-
((pre-test or pretest) adj probability).tw.
-
post-test probability.tw.
-
predictive value$.tw.
-
likelihood ratio$.tw.
-
or/61-67
-
26 and 68
-
34 and 68
-
49 and 68
-
60 and 68
-
69 or 70 or 71 or 72
-
iqur.tw.
-
biopredictive.tw.
-
echosens.tw.
-
74 or 75 or 76
-
7 or 15 or 32 or 47 or 58 or 73 or 77
-
exp “Costs and Cost Analysis”/
-
Economics/
-
exp Economics, Hospital/
-
exp Economics, Medical/
-
Economics, Nursing/
-
exp models, economic/
-
Economoics, Pharmaceutical/
-
exp “Fees and Charges”/
-
exp Budgets/
-
budget$.tw.
-
ec.fs.
-
cost$.ti.
-
(cost$ adj2 (effective$ or utilit$ or benefit$ or minimi$)).ab.
-
(economic$ or pharmacoeconomic$ or pharmaco-economic$).ti.
-
(prices$ or pricing$).tw.
-
(financial or finance or finances or financed).tw.
-
(fee or fees).tw.
-
(value adj2 (money or monetary)).tw.
-
quality-adjusted life years/
-
(qaly or qalys).af.
-
(quality adjusted life year or quality adjusted life years).af.
-
or/79-99
-
78 and 100
-
limit 101 to english language
Hepatitis C
Database, platform: MEDLINE (via Ovid)
Natural history
Search strategy
Date of search: 1 December 2012.
-
*EPIDEMIOLOGY/
-
*INCIDENCE/
-
*PREVALENCE/
-
incidence.ti.
-
prevalence.ti.
-
epidemiol$.ti.
-
(etiolog$ or aetiolog$).ti.
-
or/1-7
-
exp *Hepatitis C/
-
8 and 9
-
limit 10 to (human and english language)
-
limit 11 to yr=“2004 -Current”
Costs
Search strategy
Date of search: 1 December 2012.
-
exp Hepatitis C/ or Hepatitsi C, Chronic.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
-
exp Hepatitis C Virus/ or exp Hepatitis C Antibodies/
-
(hcv or hepatitis-C or heptatitis C).mp.
-
1 or 2 or 3
-
((pegylat$ adj3 interferon$) or peg-ifn or peginterferon$ or peg-interferon$ or pegasys or pegintron or viraferonpeg).mp.
-
(interferon alpha 2a or interferon alfa 2a or interferon alpha 2b or interferon alfa 2b or alpha interferon or intron$ or viraferon or roferon).mp.
-
exp interferon-alpha/
-
6 or 7
-
exp Polyethylene Glycols/
-
polyethylene glycol$.mp. or peg$.tw.
-
9 or 10
-
8 and 11
-
5 or 12
-
4 and 13
-
limit 14 to english language
-
(Ribavirin or ribavirin$ or copegus or rebetol).mp.
-
4 and 16
-
17
-
limit 18 to english language
-
(Telaprevir or telaprevir$ or incivo).mp.
-
4 and 20
-
limit 21 to english language
-
(Boceprevir or Boceprevir$ or victrelis).mp.
-
4 and 23
-
limit 24 to english language
-
exp ECONOMICS/
-
exp ECONOMICS, HOSPITAL/
-
exp ECONOMICS, PHARMACEUTICAL/
-
exp ECONOMICS, NURSING/
-
exp ECONOMICS, DENTAL/
-
exp ECONOMICS, MEDICAL/
-
exp “Costs and Cost Analysis”/
-
Cost-Benefit Analysis/
-
VALUE OF LIFE/
-
exp MODELS, ECONOMIC/
-
exp FEES/ and CHARGES/
-
exp BUDGETS/
-
(economics$ or price$ or pricing or fianc$ or fee$ or pharamacoenomics$ or pharma economics$).tw.
-
(cost$ or costly or costing$ or costed).tw.
-
(cost$ adj2 (benefit$ or utilit$ or minim$ or effective$)).tw.
-
(expenditure$ not energy).tw.
-
(value adj (money or monetary)).tw.
-
budget$.tw.
-
(economic adj2 burden).tw.
-
“resource use”.ti,ab.
-
or/26-45
-
news.pt.
-
editorial.pt.
-
comment.pt.
-
letter.pt.
-
or/47-50
-
46 not 51
-
52 and 4
-
52 and 15
-
52 and 19
-
52 and 23
-
52 and 25
-
53 and 54 and 55 and 56 and 57
-
limit 58 to english language
Quality of life
Search strategy
Date of search: 1 December 2012.
-
value of life/
-
quality adjusted life year/
-
quality adjusted life.ti,ab.
-
(qaly$ or qald$ or qale$ or qtime$).ti,ab.
-
disability adjusted life.ti,ab.
-
daly$.ti,ab.
-
health status indicators/
-
(sf36 or sf 36 or short form 36 or shortform 36 or sf thirtysix or sf thirty six or shortform thirtysix or shortform thirty six or short form thirtysix or short form thirty six).ti,ab.
-
(sf6 or sf 6 or short form 6 or shortform 6 or sf six or sfsix or shortform six or short form six or short form six).ti,ab.
-
(sf12 or sf 12 or short form 12 or shortform 12 or sf twelve or sftwelve or shortform twelve or short form twelve).ti,ab.
-
(sf16 or sf 16 or short form 16 or shortform 16 or sf sixteen or sfsixteen or shortform sixteen or short form sixteen).ti,ab.
-
(sf20 or sf 20 or short form 20 or shortform or sf twenty or sf twenty or sftwenty or shortform twenty or short form twenty).ti,ab.
-
(euroqol or euro qol or eq5d or eq 5d).ti,ab.
-
(hql or hqol or h qol or hrqol or hr qol).ti,ab.
-
(hye or hyes).ti,ab.
-
health$ year$ equivalent$.ti,ab.
-
health utilit$.ab.
-
(hui or hui1 or hui2 or hui3).ti,ab.
-
disutil$.ti,ab.
-
rosser.ti,ab.
-
quality of well being.ti,ab.
-
quality of wellbeing.ti,ab.
-
qwb.ti,ab.
-
willingess to pay.ti,ab.
-
standard gamble$.ti,ab.
-
time trade off.ti,ab.
-
time tradeoff.ti,ab.
-
tto.ti,ab.
-
(index adj2 well being).mp.
-
(quality adj2 well being).mp.
-
(health adj3 utilit$ ind$).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
-
((multiattribute$ or multi attribute$) adj3 (health ind$ or theor$ or health state$ or utilit$ or analys$)).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
-
quality adjusted life year$.mp.
-
(15D or 15 dimension$).mp.
-
(12D or 12 dimension$).mp.
-
rating scale$.mp.
-
linear scal$.mp.
-
linear analog$.mp.
-
visual analog$.mp.
-
(categor$ adj2 scal$).mp.
-
or/1-40
-
(letter or editorial or comment).pt.
-
41 not 42
-
exp Hepatitis C/ or Hepatitis C, Chronic/
-
exp Hepatitis B Virus/ or exp Hepatitis B Antibodies/
-
(hbv or hepatitis-C or hepatitis C).mp.
-
44 or 45 or 46
-
43 and 47
-
limit 48 to english language
Cirrhosis
Platform: MEDLINE (via Ovid)
Date of search: 26 July 2013.
Search strategy
Costs
-
cirrhosis.mp.
-
costs.mp. or “Costs and Cost Analysis”/
-
1 and 2
-
limit 3 to english language
Quality of life
-
cirrhosis.mp.
-
quality of life.mp. or “Quality of Life”/
-
1 and 4
-
limit 5 to english language
Appendix 3 Results: meta-analysis data
| Test | Number of studies | Cut-off | Summary sensitivity (95% CI) | Summary specificity (95% CI) | Statistics |
|---|---|---|---|---|---|
| Indirect serum non-invasive tests | |||||
| King’s | 1 | 24.3 | 0.80 (0.74 to 0.85) | 0.67 (0.30 to 0.90) | Single study |
| Direct serum non-invasive serum tests | |||||
| Hyaluronic acid | 1 | 16 | 0.36 (0.28 to 0.45) | 0.92 (0.87 to 0.95) | Single study |
| Commercial non-invasive serum tests | |||||
| FibrospectII | 1 | 42 | 0.86 (0.49 to 0.97) | 0.69 (0.50 to 0.83) | Single study |
| Imaging modalities | |||||
| ARFI | 3 | 1.04–1.19 | 0.71 (0.65 to 0.77) | 0.86 (0.70 to 0.94) | Fixed-effect model for sensitivity and random-effects model for specificity without correlation |
| Fibroscan | 8 | 4.5–8.8 | 0.85 (0.75 to 0.91) | 0.87 (0.75 to 0.91) | Random-effects model for sensitivity and specificity without correlation |
| Test | Number of studies | Cut-off | Summary sensitivity (95% CI) | Summary specificity (95% CI) | Statistics |
|---|---|---|---|---|---|
| Indirect non-invasive serum tests | |||||
| APRI (low cut-off) | 18 | 0.5–1.0 | 0.84 (0.82 to 0.86) | 0.56 (0.44 to 0.68) | Fixed-effect model for sensitivity and random-effects model for specificity without correlation |
| APRI (high cut-off) | 15 | 1.5–2.0 | 0.53 (0.43 to 0.62) | 0.86 (0.79 to 0.91) | Bivariate random-effects model with correlation between sensitivity and specificity |
| CDS | 2 | 8 | 0.54 (0.43 to 0.65) | 0.74 (0.66 to 0.81) | Fixed-effects model for sensitivity and specificity without correlation |
| FIB-4 (low cut-off) | 11 | 1.45 | 0.80 (0.72 to 0.86) | 0.64 (0.56 to 0.72) | Bivariate random-effects model with correlation between sensitivity and specificity |
| FIB-4 (high cut-off) | 11 | 3.25 | 0.37 (0.28 to 0.46) | 0.94 (0.90 to 0.97) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Forns index (low cut-off) | 1 | 4.2 | 0.92 (0.81 to 0.97) | 0.34 (0.26 to 0.43) | Single study |
| Forns index (high cut-off) | 1 | 6.9 | 0.55 (0.41 to 0.68) | 0.87 (0.80 to 0.92) | Single study |
| FibroQ | 1 | 1.6 | 0.86 (0.78 to 0.91) | 0.44 (0.35 to 0.52) | Single study |
| GUCI | 1 | 0.26 | 0.58 (0.39 to 0.76) | 0.73 (0.58 to 0.84) | Single study |
| King’s | 1 | 24.3 | 0.74 (0.59 to 0.85) | 0.90 (0.84 to 0.94) | Single study |
| Lok’s index (low cut-off) | 2 | 0.2 | 0.90 (0.85 to 0.95) | 0.33 (0.27 to 0.39) | Fixed-effects model for sensitivity and specificity without correlation |
| Lok’s index (high cut-off) | 2 | 0.5–0.58 | 0.50 (0.40 to 0.59) | 0.84 (0.77 to 0.89) | Fixed-effects model for sensitivity and specificity without correlation |
| NIHCED | 1 | 6 | 0.72 (0.65 to 0.78) | 0.75 (0.67 to 0.81) | Single study |
| Platelets | 2 | 140–150 | 0.53 (0.43 to 0.99) | 0.88 (0.47 to 0.98) | Random-effects model for sensitivity and fixed-effect model for specificity without correlation |
| Pohl index | 3 | Positive | 0.15 (0.04 to 0.42) | 0.98 (0.96 to 0.99) | Random-effects model for sensitivity and fixed-effect model for specificity without correlation |
| Direct serum non-invasive serum tests | |||||
| 13C-caffeine breath test | 1 | 0.021 | 0.75 (0.63 to 0.85) | 0.79 (0.64 to 0.89) | Single study |
| Hyaluronic acid | 4 | 20–85 | 0.79 (0.52 to 0.93) | 0.72 (0.65 to 0.78) | Random-effects model for sensitivity and fixed-effect model for specificity without correlation |
| Hyaluronic acid (low cut-off) | 1 | 48 | 0.77 (0.50 to 0.92) | 1.00 (0.98 to 1.00) | Single study |
| Hyaluronic acid (high cut-off) | 1 | 160 | 0.22 (0.13 to 0.37) | 1.00 (0.98 to 1.00) | Single study |
| Hepascore | 7 | 0.5–0.83 | 0.81 (0.71 to 0.87) | 0.76 (0.68 to 0.83) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Hepascore (low cut-off) | 2 | 0.49 | 0.81 (0.41 to 0.96) | 0.75 (0.18 to 0.98) | Fixed-effect model for sensitivity and random-effects model for specificity without correlation |
| Hepascore (high cut-off) | 2 | 0.84–0.90 | 0.48 (0.40 to 0.56) | 0.93 (0.90 to 0.95) | Fixed-effects model for sensitivity and specificity without correlation |
| PIIINP | 2 | 8–9.1 | 0.71 (0.58 to 0.81) | 0.63 (0.54 to 0.71) | Fixed-effects model for sensitivity and specificity without correlation |
| PIIINP/MMP1 index | 1 | 0.3 | 0.86 (0.71 to 0.94) | 0.74 (0.67 to 0.80) | Single study |
| Type IV collagen | 1 | 130 | 0.67 (0.53 to 0.78) | 0.76 (0.60 to 0.87) | Single study |
| YKL-40 | 1 | 100 | 0.82 (0.70 to 0.90) | 0.57 (0.49 to 0.65) | Single study |
| Commercial non-invasive serum tests | |||||
| ELF (low cut-off) | 1 | 9.59 | 0.85 (0.77 to 0.90) | 0.63 (0.57 to 0.70) | Single study |
| ELF (high cut-off) | 1 | 10.22 | 0.70 (0.61 to 0.78) | 0.85 (0.80 to 0.89) | Single study |
| Fibroindex | 1 | 1.35 | 0.52 (0.33 to 0.70) | 0.92 (0.75 to 0.98) | Single study |
| Fibrometer | 2 | 0.63–0.67 | 0.84 (0.77 to 0.89) | 0.78 (0.75 to 0.81) | Fixed-effects model for sensitivity and specificity without correlation |
| FibrospectII | 1 | 0.5 | 0.85 (0.72 to 0.92) | 0.72 (0.62 to 0.80) | Single study |
| Fibrotest | 9 | 0.32–0.67 | 0.73 (0.56 to 0.85) | 0.69 (0.55 to 0.80) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Fibrotest (low cut-off) | 2 | 0.22 | 0.85 (0.44 to 0.98) | 0.58 (0.54 to 0.99) | Fixed-effect model for sensitivity and random-effects model for specificity without correlation |
| Fibrotest (high cut-off) | 2 | 0.59–0.63 | 0.69 (0.59 to 0.74) | 0.84 (0.81 to 0.87) | Fixed-effects model for sensitivity and specificity without correlation |
| Imaging modalities | |||||
| ARFI | 4 | 1.49–2.11 | 0.85 (0.69 to 0.94) | 0.89 (0.72 to 0.97) | Random-effects model for sensitivity and specificity without correlation |
| Real-time elastography | 1 | 3.25 | 0.86 (0.72 to 0.93) | 0.96 (0.82 to 0.99) | Single study |
| Fibroscan | 19 | 8.6–15.4 | 0.88 (0.82 to 0.92) | 0.90 (0.85 to 0.93) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Test | Number of studies | Cut-off | Summary sensitivity (95% CI) | Summary specificity (95% CI) | Statistics |
|---|---|---|---|---|---|
| Indirect non-invasive serum tests | |||||
| APRI (low cut-off) | 1 | 0.4 | 0.70 (0.59 to 0.78) | 0.83 (0.68 to 0.92) | Single study |
| APRI (high cut-off) | 1 | 1.5 | 0.37 (0.26 to 0.50) | 0.82 (0.73 to 0.89) | Single study |
| CDS | 1 | 4 | 0.28 (0.19 to 0.41) | 0.90 (0.80 to 0.95) | Single study |
| Lok’s index | 1 | 0.87 | 0.48 (0.36 to 0.61) | 0.90 (0.80 to 0.95) | Single study |
| Direct serum non-invasive tests | |||||
| CTGF | 1 | 125.6 | 0.61 (0.49 to 0.71) | 0.71 (0.47 to 0.87) | Single study |
| Commercial non-invasive serum tests | |||||
| Fibrotest | 1 | – | 0.72 (0.57 to 0.83) | 0.64 (0.49 to 0.76) | Single study |
| Imaging modalities | |||||
| Real-time elastography | 1 | – | 0.87 (0.76 to 0.94) | 0.85 (0.64 to 0.95) | Single study |
| Fibroscan | 2 | 6.1 | 0.69 (0.53 to 0.82) | 0.62 (0.39 to 0.80) | Fixed-effects model for sensitivity and specificity without correlation |
| Test | Number of studies | Cut-off | Summary sensitivity (95% CI) | Summary specificity (95% CI) | Statistics |
|---|---|---|---|---|---|
| Indirect non-invasive serum tests | |||||
| FIB-4 (low cut-off) | 2 | 0.67–1.00 | 0.89 (0.84 to 0.93) | 0.76 (0.69 to 0.81) | Fixed-effects model for sensitivity and specificity without correlation |
| FIB-4 (high cut-off) | 1 | 2.65 | 0.38 (0.33 to 0.44) | 0.98 (0.96 to 0.99) | Single study |
| Forns index (low cut-off) | 2 | 5.2 | 0.99 (0.85 to 1.00) | 0.20 (0.12 to 0.32) | Fixed-effects model for sensitivity and specificity without correlation |
| Forns index (high cut-off) | 2 | 8.4 | 0.32 (0.20 to 0.46) | 0.92 (0.83 to 0.97) | Fixed-effects model for sensitivity and specificity without correlation |
| Hui index | 2 | 0.15 | 0.88 (0.76 to 0.94) | 0.51 (0.37 to 0.65) | Fixed-effects model for sensitivity and specificity without correlation |
| Direct serum non-invasive tests | |||||
| 13C-caffeine breath test | 1 | 1.49 | 1.00 (0.76 to 1.00) | 0.72 (0.56 to 0.84) | Single study |
| CTGF | 1 | 141 | 0.69 (0.44 to 0.86) | 0.85 (0.75 to 0.92) | Single study |
| Commercial non-invasive serum tests | |||||
| Fibrotest | 3 | 0.31–0.42 | 0.49 (0.01 to 0.99) | 0.71 (0.53 to 0.84) | Random-effects model for sensitivity and fixed-effect model for specificity without correlation |
| Imaging modalities | |||||
| Real-time elastography | 1 | 80.7 | 0.73 (0.54 to 0.86) | 0.76 (0.62 to 0.85) | Single study |
| Fibroscan | 13 | 7.3–10.7 | 0.69 (0.58 to 0.78) | 0.84 (0.79 to 0.89) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Test | Number of studies | Cut-off | Summary sensitivity (95% CI) | Summary specificity (95% CI) | Statistics |
|---|---|---|---|---|---|
| Indirect non-invasive serum tests | |||||
| APRI | 1 | 0.5 | 0.05 (0.01 to 0.17) | 0.97 (0.87 to 0.99) | Single study |
| NAFLD fibrosis score (low cut-off) | 3 | –0.1657 to –1.456 | 0.82 (0.77 to 0.87) | 0.48 (0.40 to 0.56) | Bivariate random-effects model with correlation between sensitivity and specificity |
| NAFLD fibrosis score (high cut-off) | 2 | 0.676 | 0.29 (0.22 to 0.36) | 0.92 (0.85 to 0.96) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Direct serum non-invasive serum tests | |||||
| Hyaluronic acid | 1 | 24.6 | 0.82 (0.52 to 0.95) | 0.68 (0.46 to 0.85) | Single study |
| Laminin | 1 | 282 | 0.82 (0.52 to 0.95) | 0.89 (0.69 to 0.97) | Single study |
| NAFLD diagnostic panel | 1 | 0.42 | 0.61 (0.46 to 0.75) | 0.72 (0.57 to 0.84) | Single study |
| Type IV collagen | 1 | 145 | 0.64 (0.35 to 0.85) | 0.89 (0.69 to 0.97) | Single study |
| Commercial non-invasive serum tests | |||||
| ELF | 1 | 9.8 | 0.61 (0.52 to 0.69) | 0.80 (0.70 to 0.87) | Single study |
| Imaging modalities | |||||
| Real-time elastography | 1 | 102 | 0.79 (0.65 to 0.88) | 0.90 (0.60 to 0.98) | Single study |
| Fibroscan | 3 | 5.3–5.9 | 0.87 (0.81 to 0.92) | 0.76 (0.57 to 0.88) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Combination of non-invasive test algorithms | |||||
| NAFLD fibrosis score and ELF (low cut-off) | 1 | 0.92 (0.86 to 0.96) | 0.52 (0.41 to 0.63) | Single study | |
| NAFLD fibrosis score and ELF (high cut-off) | 1 | 0.60 (0.51 to 0.69) | 0.91 (0.83 to 0.96) | Single study | |
| Test | Number of studies | Cut-off | Summary sensitivity (95% CI) | Summary specificity (95% CI) | Statistics |
|---|---|---|---|---|---|
| Indirect non-invasive serum tests | |||||
| APRI | 2 | 0.43–0.5 | 0.69 (0.21 to 0.95) | 0.82 (0.07 to 0.97) | Fixed-effect model for sensitivity and random-effects model for specificity without correlation |
| BARD | 1 | 2 | 0.44 (0.35 to 0.54) | 0.70 (0.62 to 0.77) | Single study |
| FIB-4 | 1 | 1.45 | 0.55 (0.45 to 0.64) | 0.87 (0.81 to 0.92) | Single study |
| NAFLD fibrosis score (low cut-off) | 4 | –1.455 | 0.79 (0.56 to 0.92) | 0.65 (0.46 to 0.80) | Bivariate random-effects model with correlation between sensitivity and specificity |
| NAFLD fibrosis score (high cut-off) | 5 | 0.676 | 0.29 (0.07 to 0.68) | 0.95 (0.87 to 0.98) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Direct serum non-invasive serum tests | |||||
| Hepascore | 1 | 0.44 | 0.51 (0.41 to 0.60) | 0.88 (0.82 to 0.93) | Single study |
| Hyaluronic acid | 1 | 218 | 0.78 (0.45 to 0.94) | 0.89 (0.67 to 0.97) | Single study |
| NAFIC (low cut-off) | 1 | 0 | 0.95 (0.91 to 0.98) | 0.33 (0.29 to 0.37) | Single study |
| NAFIC (high cut-off) | 1 | 2 | 0.84 (0.77 to 0.89) | 0.75 (0.71 to 0.78) | Single study |
| Commercial non-invasive serum tests | |||||
| ELF | 1 | 9.9 | 0.70 (0.59 to 0.79) | 0.80 (0.72 to 0.86) | Single study |
| Fibrometer | 1 | 0.490 | 0.78 (0.67 to 0.87) | 0.96 (0.92 to 0.98) | Single study |
| FibrospectII | 1 | 20 | 1.00 (0.95 to 1.00) | 0.42 (0.32 to 0.52) | Single study |
| Fibrotest (low cut-off) | 3 | 0.30–0.34 | 0.70 (0.56 to 0.81) | 0.75 (0.70 to 0.80) | Bivariate random-effects model with correlation between sensitivity and specificity |
| Fibrotest (high cut-off) | 2 | 0.7 | 0.15 (0.03 to 0.90) | 0.98 (0.90 to 0.99) | Random-effects model for sensitivity and fixed-effect model for specificity without correlation |
| Imaging modalities | |||||
| Real-time elastography | 1 | 94 | 0.84 (0.65 to 0.94) | 1.00 (0.87 to 1.00) | Single study |
| Fibroscan | 7 | 6.8–10.0 | 0.79 (0.72 to 0.85) | 0.76 (0.71 to 0.80) | Random-effects model for sensitivity and fixed-effect model for specificity without correlation – the studies were clustered; fixed effects model for both sensitivity and specificity did not alter the mean but altered the CI by about 2% |
| Combination of non-invasive test algorithms | |||||
| NAFLD fibrosis score and ELF (low cut-off) | 1 | 0.90 (0.81 to 0.95) | 0.86 (0.78 to 0.91) | Single study | |
| NAFLD fibrosis score and ELF (high cut-off) | 1 | 0.79 (0.69 to 0.87) | 0.91 (0.85 to 0.95) | Single study | |
| NAFLD fibrosis score and Fibroscan | 1 | 0.65 (0.51 to 0.76) | 0.64 (0.56 to 0.71) | Single study | |
| Fibrotest and Fibroscan | 1 | 0.71 (0.57 to 0.81) | 0.76 (0.68 to 0.82) | Single study | |
Appendix 4 Diagnostic test accuracy of non-invasive fibrosis tests in individual studies
| Study ID | Test | Index test assessed | Fibrosis stage assessed | Index test cut-off | Sens. | Spec. | TP | FP | FN | TN |
|---|---|---|---|---|---|---|---|---|---|---|
| Adams 2005 training71 | Hepascore_F2 | Hepascore | F2 | 0.5 | 67 | 92 | 34 | 5 | 17 | 61 |
| Adams 2005 training71 | Hepascore_F3 | Hepascore | F3 | 0.5 | 95 | 81 | 21 | 18 | 1 | 77 |
| Adams 2005 training71 | Hepascore_F4 | Hepascore | F4 | 0.84 | 71 | 84 | 5 | 18 | 2 | 92 |
| Adams 2005 validation71 | Hepascore_F2 | Hepascore | F2 | 0.5 | 63 | 89 | 37 | 5 | 22 | 40 |
| Adams 2005 validation71 | Hepascore_F3 | Hepascore | F3 | 0.5 | 88 | 74 | 40 | 15 | 5 | 44 |
| Adams 2005 validation71 | Hepascore_F4 | Hepascore | F4 | 0.84 | 71 | 89 | 12 | 10 | 5 | 77 |
| Ahmad 201172 | APRI_F2_combined | APRI | F2 | 0.5 and 1.5 | 31 | 22 | 2 | 13 | ||
| Ahmad 201172 | APRI_F2_high | APRI | F2 | 1.5 | 43 | 68 | 31 | 22 | 58 | 46 |
| Ahmad 201172 | APRI_F2_low | APRI | F2 | 0.5 | 87 | 55 | 2 | 13 | ||
| Ahmad 201172 | AST_ALT_ratio_F4 | AST–ALT ratio | F4 | 1 | 35 | 68 | 9 | 44 | 12 | 92 |
| Ahmad 201172 | FI_F2 | Fibrosis Index | F2 | 2.1 | 100 | 58 | 52 | 0 | 37 | 68 |
| Ahmad 201172 | FI_F4 | Fibrosis Index | F4 | 3.3 | 38 | 100 | 8 | 0 | 13 | 136 |
| Ahmad 201172 | FIB4_F3_combined | FIB-4 | F3 | 1.45 and 3.25 | 32 | 18 | 8 | 52 | ||
| Ahmad 201172 | FIB4_F3_high | FIB-4 | F3 | 3.25 | 59 | 82 | 32 | 18 | 23 | 84 |
| Ahmad 201172 | FIB4_F3_low | FIB-4 | F3 | 1.45 | 47 | 50 | 8 | 52 | ||
| Al Mohri 200573 | APRI_F2_combined | APRI | F2 | 0.5, 1.5 | 17 | 0 | 6 | 6 | ||
| Al Mohri 200573 | APRI_F2_high | APRI | F2 | 1.5 | 52 | 100 | 17 | 0 | 16 | 13 |
| Al Mohri 200573 | APRI_F2_low | APRI | F2 | 0.5 | 82 | 46 | 27 | 7 | 6 | 6 |
| Al Mohri 200573 | FIB4_F2_combined | FIB-4 | F2 | 1.45 and 3.25 | 11 | 0 | 7 | 11 | ||
| Al Mohri 200573 | FIB4_F2_high | FIB-4 | F2 | 3.25 | 33 | 100 | 11 | 0 | 22 | 13 |
| Al Mohri 200573 | FIB4_F2_low | FIB-4 | F2 | 1.45 | 79 | 85 | 26 | 2 | 7 | 11 |
| Anaparthy 200974 | APRI_F2_combined | APRI | F2 | 0.5 and 1.5 | 4 | 2 | 7 | 17 | ||
| Anaparthy 200974 | APRI_F2_high | APRI | F2 | 1.5 | 20 | 93 | 4 | 2 | 16 | 28 |
| Anaparthy 200974 | APRI_F2_low | APRI | F2 | 0.5 | 65 | 57 | 13 | 13 | 7 | 17 |
| Arena 200875 | TE_F2 | Fibroscan | F2 | 7.8 | 83 | 82 | 70 | 12 | 14 | 54 |
| Arena 200875 | TE_F3 | Fibroscan | F3 | 10.8 | 91 | 94 | 51 | 6 | 5 | 88 |
| Arena 200875 | TE_F4 | Fibroscan | F4 | 14.8 | 94 | 92 | 27 | 10 | 2 | 111 |
| Beckebaum 201076 | APRI_F3_high | APRI | F3 | 1.47 | 13 | 2 | 12 | 23 | ||
| Beckebaum 201076 | FIB4_F3_high | FIB-4 | F3 | 3.54 | 8 | 2 | 17 | 23 | ||
| Beckebaum 201076 | FinroIndex_F3 | Fibroindex | F3 | 1.85 | 13 | 2 | 12 | 23 | ||
| Beckebaum 201076 | FT_F3 | Fibrotest | F3 | 0.67 | 10 | 1 | 15 | 24 | ||
| Beckebaum 201076 | Hepascore_F3 | Hepascore | F3 | 0.83 | 13 | 2 | 12 | 23 | ||
| Beckebaum 201076 | Lok_F3_high | Lok’s index | F3 | 0.58 | 10 | 1 | 15 | 24 | ||
| Beckebaum 201076 | TE_F1 | TE | F1 | 4.7 | 89.10 | 100.00 | 43 | 0 | 5 | 2 |
| Beckebaum 201076 | TE_F2 | Fibroscan | F2 | 7.1 | 73.00 | 100.00 | 27 | 0 | 10 | 13 |
| Beckebaum 201076 | TE_F3 | Fibroscan | F3 | 10.9 | 75.00 | 95.80 | 19 | 1 | 6 | 24 |
| Beckebaum 201076 | TE_F4 | Fibroscan | F4 | 17.3 | 100.00 | 97.30 | 4 | 1 | 0 | 45 |
| Bejarano 200977 | NIHCED_F3 | NIHCED | F3 | 6 | 72 | 75 | 137 | 33 | 53 | 98 |
| Berg 200479 | APRI_F2_high | APRI | F2 | 1.5 | 37 | 93 | 93 | 16 | 160 | 215 |
| Berg 200479 | APRI_F2_low | APRI | F2 | 0.5 | 82 | 53 | 207 | 109 | 46 | 122 |
| Berg 200479 | APRI_F3_combined | APRI | F3 | 93 | 16 | 46 | 122 | |||
| Berg 200479 | APRI_F3_combined | APRI | F3 | 50 | 25 | 39 | 286 | |||
| Berg 200479 | APRI_F3_high | APRI | F3 | 2 | 39 | 93 | 50 | 25 | 77 | 332 |
| Berg 200479 | APRI_F3_low | APRI | F3 | 1 | 69 | 80 | 88 | 71 | 39 | 286 |
| Berg 200479 | APRI_F4_combined | APRI | F4 | 30 | 45 | 13 | 310 | |||
| Berg 200479 | APRI_F4_high | APRI | F4 | 2 | 48 | 89 | 30 | 45 | 32 | 377 |
| Berg 200479 | APRI_F4_low | APRI | F4 | 1 | 76 | 74 | 47 | 112 | 15 | 310 |
| Boroni 200280 | PLT_F4 | Platelet count | F4 | 134 | 59 | 100 | 19 | 0 | 13 | 188 |
| Bourliere 200681 | APRI_F2_combined | APRI | F2 | 0.5 and 1.5 | 22 | 5 | 30 | 75 | ||
| Bourliere 200681 | APRI_F2_high | APRI | F2 | 1.5 | 22 | 95 | 22 | 5 | 77 | 131 |
| Bourliere 200681 | APRI_F2_Low | APRI | F2 | 0.5 | 70 | 55 | 69 | 61 | 30 | 75 |
| Bourliere 200681 | APRI_F4_combined | APRI | F4 | 1 and 2 | 6 | 9 | 5 | 180 | ||
| Bourliere 200681 | APRI_F4_high | APRI | F4 | 2 | 38 | 96 | 6 | 9 | 10 | 210 |
| Bourliere 200681 | APRI_F4_low | APRI | F4 | 1 | 69 | 82 | 11 | 39 | 5 | 180 |
| Bourliere 200681 | Forns_F2_combined | Forns index | F2 | 4.2 and 6.9 | 30 | 5 | 20 | 73 | ||
| Bourliere 200681 | Forns_F2_high | Forns index | F2 | 6.9 | 30 | 96 | 30 | 5 | 69 | 131 |
| Bourliere 200681 | Forns_F2_low | Forns index | F2 | 4.2 | 80 | 54 | 79 | 63 | 20 | 73 |
| Bourliere 200681 | FT_F2_combined | Fibrotest | F2 | 54 | 14 | 3 | 27 | |||
| Bourliere 200681 | FT_F2_high | Fibrotest | F2 | 0.6 | 55 | 90 | 54 | 14 | 45 | 122 |
| Bourliere 200681 | FT_F2_low | Fibrotest | F2 | 0.1 | 97 | 20 | 96 | 109 | 3 | 27 |
| Boursier 200982 | APRI_F3_low | APRI | F3 | 0.581 | 78 | 75 | 207 | 198 | 59 | 593 |
| Boursier 200982 | Fibrometer_F3 | FibroMeter | F3 | 0.628 | 84 | 79 | 223 | 166 | 43 | 625 |
| Boursier 200982 | Fibrometer_F4_combined | FibroMeter | F4 | 42 | 19 | 5 | 667 | |||
| Boursier 200982 | Fibrometer_F4_high | FibroMeter | F4 | 0.979 | 36 | 98 | 42 | 19 | 76 | 920 |
| Boursier 200982 | Fibrometer_F4_low | FibroMeter | F4 | 0.628 | 96 | 71 | 113 | 272 | 5 | 667 |
| Boursier 200982 | FT_F3_combined | Fibrotest | F3 | 0.44 and 0.63 | 178 | 127 | 43 | 562 | ||
| Boursier 200982 | FT_F3_high | Fibrotest | F3 | 0.631 | 67 | 84 | 178 | 127 | 88 | 664 |
| Boursier 200982 | FT_F3_low | Fibrotest | F3 | 0.448 | 84 | 71 | 223 | 229 | 43 | 562 |
| Boursier 200982 | FT_F4_combined | Fibrotest | F4 | 50 | 38 | 21 | 723 | |||
| Boursier 200982 | FT_F4_high | Fibrotest | F4 | 0.862 | 42 | 96 | 50 | 38 | 68 | 901 |
| Boursier 200982 | FT_F4_low | Fibrotest | F4 | 0.66 | 82 | 77 | 97 | 216 | 21 | 723 |
| Boursier 200982 | Hepascore_F3_combined | Hepascore | F3 | 0.497 and 0.904 | 128 | 55 | 48 | 562 | ||
| Boursier 200982 | Hepascore_F3_high | Hepascore | F3 | 0.904 | 48 | 93 | 128 | 55 | 138 | 736 |
| Boursier 200982 | Hepascore_F3_low | Hepascore | F3 | 0.497 | 82 | 71 | 218 | 229 | 48 | 562 |
| Boursier 200982 | Hepascore_F4_combined | 46 | 9 | 24 | 779 | |||||
| Boursier 200982 | Hepascore_F4_high | Hepascore | F4 | 1.159 | 39 | 99 | 46 | 9 | 72 | 930 |
| Boursier 200982 | Hepascore_F4_low | Hepascore | F4 | 0.581 | 80 | 83 | 94 | 160 | 24 | 779 |
| Boursier 201283 | Bordeaux_F2 | Bordeaux algorithm | F2 | 88 | 89 | 374 | 33 | 51 | 271 | |
| Boursier 201283 | Bordeaux_F4 | Bordeaux algorithm | F4 | 87 | 95 | 95 | 31 | 14 | 589 | |
| Boursier 201283 | SAFE_F2 | SAFE algorithm | F2 | 100 | 88 | 977 | 97 | 0 | 712 | |
| Boursier 201283 | SAFE_F4 | SAFE algorithm | F4 | 61 | 93 | 138 | 109 | 89 | 1450 | |
| Burton 201084 | APRI_F2_low | APRI | F2 | 0.5 | 71 | 69 | 23 | 15 | 9 | 33 |
| Cales 201085 | Fibrometer_F2 | FibroMeter | F2 | 0.419 | 80 | 76 | 441 | 121 | 110 | 384 |
| Cales 201026 | APRI_F2_low | APRI | F2 | 0.7 | 62 | 78 | 74 | 14 | 46 | 49 |
| Cales 201026 | FIB4_F2_low | FIB-4 | F2 | 1.28 | 72 | 67 | 74 | 14 | 46 | 49 |
| Cales 201026 | Fibrometer_F2 | FibroMeter | F2 | 0.48 | 76 | 72 | 91 | 18 | 29 | 45 |
| Cales 201026 | FT_F2_high | Fibrotest | F2 | 0.65 | 61 | 83 | 73 | 11 | 47 | 52 |
| Cales 201026 | Hepascore_F2 | F2 | 0.31 | 90 | 59 | 108 | 26 | 12 | 37 | |
| Calvaruso 201086 | TE_F2 | Fibroscan | F2 | 7.1 | 113 | 21 | 76 | 21 | ||
| Cardoso 201287 | TE_F2 | Fibroscan | F2 | 7.1 | 68 | 88 | 133 | 20 | 63 | 147 |
| Cardoso 201287 | TE_F3 | Fibroscan | F3 | 9.5 | 67 | 92 | 58 | 22 | 29 | 254 |
| Cardoso 201287 | TE_F4 | Fibroscan | F4 | 12.5 | 84 | 94 | 26 | 20 | 5 | 312 |
| Carrion 200688 | TE_F2 | Fibroscan | F2 | 8.5 | 90 | 81 | 66 | 18 | 7 | 78 |
| Carrion 200688 | TE_F4 | Fibroscan | F4 | 12.5 | 100 | 87 | 19 | 20 | 0 | 131 |
| Carvalho 200889 | APRI_F2_combined | APRI | F2 | 0.5, 1.5 | 23 | 12 | 3 | 21 | ||
| Carvalho 200889 | APRI_F2_high | APRI | F2 | 1.5 | 51 | 82 | 23 | 12 | 22 | 54 |
| Carvalho 200889 | APRI_F2_low | APRI | F2 | 0.5 | 94 | 32 | 42 | 45 | 3 | 21 |
| Carvalho 200889 | APRI_F4_combined | APRI | F4 | 1, 2 | 11 | 9 | 2 | 61 | ||
| Carvalho 200889 | APRI_F4_high | APRI | F4 | 2 | 55 | 90 | 11 | 9 | 9 | 82 |
| Carvalho 200889 | APRI_F4_low | APRI | F4 | 1 | 90 | 67 | 18 | 30 | 2 | 61 |
| Carvalho 200889 | FIB4_F2_low | FIB-4 | F2 | 1 | 91 | 33 | 41 | 44 | 4 | 22 |
| Castera 200528 | TE_F2 | Fibroscan | F2 | 7.1 | 67 | 89 | 91 | 5 | 45 | 42 |
| Castera 200528 | TE_F3 | Fibroscan | F3 | 9.5 | 73 | 91 | 61 | 9 | 22 | 91 |
| Castera 200528 | TE_F4 | Fibroscan | F4 | 12.5 | 87 | 91 | 40 | 12 | 6 | 125 |
| Castera 200791 | APRI_F2_combined | APRI | F2 | 0.5, 1.5 | 44 | 1 | 35 | 28 | ||
| Castera 200791 | APRI_F2_high | APRI | F2 | 1.5 | 28 | 98 | 44 | 1 | 113 | 42 |
| Castera 200791 | APRI_F2_low | APRI | F2 | 0.5 | 78 | 65 | 122 | 15 | 35 | 28 |
| Castera 200791 | APRI_F4_combined | APRI | F4 | 1, 2 | 16 | 14 | 9 | 114 | ||
| Castera 200791 | APRI_F4_high | APRI | F4 | 2 | 41 | 91 | 16 | 14 | 23 | 147 |
| Castera 200791 | APRI_F4_low | APRI | F4 | 1 | 77 | 71 | 30 | 47 | 9 | 114 |
| Castera 200791 | FIB4_F2_combined | FIB-4 | F2 | 1.45, 3.25 | 49 | 3 | 42 | 30 | ||
| Castera 200791 | FIB4_F2_high | FIB-4 | F2 | 3.25 | 31 | 93 | 49 | 3 | 108 | 40 |
| Castera 200791 | FIB4_F2_low | FIB-4 | F2 | 1.45 | 73 | 70 | 115 | 13 | 42 | 30 |
| Castera 200791 | Forns_F2_combined | Forns index | F2 | 4.2, 6.9 | 36 | 0 | 27 | 15 | ||
| Castera 200791 | Forns_F2_high | Forns index | F2 | 6.9 | 35 | 100 | 36 | 0 | 121 | 43 |
| Castera 200791 | Forns_F2_low | Forns index | F2 | 4.2 | 83 | 35 | 130 | 28 | 27 | 15 |
| Castera 200791 | PLT_F4 | Platelet count | F4 | 130 | 59 | 91 | 23 | 14 | 16 | 147 |
| Castera 200990 | APRI_F4_combined | APRI | F4 | 21 | 13 | 25 | 186 | |||
| Castera 200990 | APRI_F4_high | APRI | F4 | 2 | 30 | 94 | 21 | 13 | 49 | 215 |
| Castera 200990 | APRI_F4_low | APRI | F4 | 1 | 64 | 81 | 45 | 42 | 25 | 186 |
| Castera 200990 | AST_ALT_ratio_F4 | AST/ALT ratio | F4 | 1 | 31 | 89 | 22 | 25 | 48 | 204 |
| Castera 200990 | FT_F4 | Fibrotest | F4 | 0.75 | 55 | 86 | 39 | 31 | 31 | 217 |
| Castera 200990 | Lok_F4_combined | Lok’s index | F4 | 28 | 13 | 10 | 105 | |||
| Castera 200990 | Lok_F4_high | Lok’s index | F4 | 0.5 | 28 | 13 | 42 | 225 | ||
| Castera 200990 | Lok_F4_low | Lok’s index | F4 | 0.2 | 86 | 46 | 50 | 123 | 10 | 105 |
| Castera 200990 | PLT_F4 | Platelet count | F4 | 150 | 41 | 94 | 29 | 14 | 41 | 214 |
| Castera 200990 | TE_F4 | Fibroscan | F4 | 12.6 | 83 | 95 | 55 | 10 | 11 | 212 |
| Ceriani 200192 | AST_ALT_ratio_F4 | AST/ALT ratio | F4 | 1 | 30 | 98 | 6 | 3 | 14 | 119 |
| Cheung 200894 | APRI_F2_combined | APRI | F2 | < 0.5 and > 1.5 | 84 | 7 | 67 | 68 | ||
| Cheung 200894 | APRI_F2_high | APRI | F2 | 1.5 | 26 | 96 | 84 | 7 | 239 | 160 |
| Cheung 200894 | APRI_F2_low | APRI | F2 | 0.5 | 21 | 60 | 100 | 255 | 67 | 68 |
| Cheung 200894 | APRI_F3_combined | APRI | F3 | 69 | 21 | 21 | 113 | |||
| Cheung 200894 | APRI_F3_high | APRI | F3 | 1.5 | 37 | 93 | 69 | 21 | 118 | 282 |
| Cheung 200894 | APRI_F3_low | APRI | F3 | 0.5 | 11 | 63 | 166 | 190 | 21 | 113 |
| Cheung 200894 | AST_ALT_ratio_F2 | AST–ALT ratio | F2 | 1 | 20 | 82 | 65 | 30 | 258 | 137 |
| Cheung 200894 | AST_ALT_ratio_F3 | AST–ALT ratio | F3 | 1 | 21 | 82 | 39 | 55 | 148 | 248 |
| Cheung 200894 | Lok_F3_combined | Lok’s index | F3 | 95 | 52 | 13 | 95 | |||
| Cheung 200894 | Lok_F3_high | Lok’s index | F3 | 0.5 | 51 | 83 | 95 | 52 | 92 | 251 |
| Cheung 200894 | Lok_F3_low | Lok’s index | F3 | 0.2 | 93 | 31 | 174 | 209 | 13 | 94 |
| Cheung 200894 | PLT_F2 | Platelet count | F2 | 150 | 28 | 92 | 90 | 13 | 233 | 154 |
| Cheung 200894 | PLT_F3 | Platelet count | F3 | 150 | 39 | 90 | 73 | 30 | 114 | 273 |
| Cheung 200894 | Pohl_F2 | Pohl score | F2 | Positive | 7 | 98 | 23 | 3 | 300 | 164 |
| Cheung 200894 | Pohl_F3 | Pohl score | F3 | Positive | 9 | 98 | 17 | 6 | 170 | 297 |
| Cho 201195 | TE_F2 | Fibroscan | F2 | 7.4 | 88 | 90 | 49 | 3 | 7 | 27 |
| Cho 201195 | TE_F3 | Fibroscan | F3 | 9.7 | 90 | 87 | 28 | 7 | 3 | 48 |
| Cho 201195 | TE_F4 | Fibroscan | F4 | 14.7 | 100 | 89 | 6 | 9 | 0 | 71 |
| Christensen 200696 | FibrospectII_F3 | Fibrospect II | F3 | 0.5 | 85.2 | 72.7 | 44 | 25 | 8 | 65 |
| Chrysanthos 200697 | APRI_F2_combined | APRI | F2 | < 0.5 and > 1.5 | 44 | 16 | 31 | 64 | ||
| Chrysanthos 200697 | APRI_F2_high | APRI | F2 | 1.5 | 30 | 88 | 44 | 16 | 102 | 122 |
| Chrysanthos 200697 | APRI_F2_low | APRI | F2 | 0.5 | 79 | 46 | 115 | 74 | 31 | 64 |
| Chrysanthos 200697 | APRI_F4_combined | APRI | F4 | 22 | 20 | 23 | 162 | |||
| Chrysanthos 200697 | APRI_F4_high | APRI | F4 | 2 | 27 | 81 | 22 | 20 | 36 | 206 |
| Chrysanthos 200697 | APRI_F4_low | APRI | F4 | 1 | 60 | 72 | 35 | 64 | 23 | 162 |
| Cobbold 201098 | APRI_F2_low | APRI | F2 | 0.66 | 83 | 78 | 31 | 7 | 6 | 23 |
| Cobbold 201098 | APRI_F4_low | APRI | F4 | 0.92 | 86 | 77 | 12 | 12 | 2 | 41 |
| Cobbold 201098 | ELF_F2 | ELF | F2 | 8.75 | 84 | 70 | 31 | 9 | 6 | 21 |
| Cobbold 201098 | ELF_F4 | ELF | F4 | 9.4 | 93 | 79 | 13 | 11 | 1 | 42 |
| Cobbold 201098 | TE_F2 | Fibroscan | F2 | 8 | 90 | 65 | 33 | 11 | 4 | 20 |
| Cobbold 2010 98 | TE_F4 | Fibroscan | F4 | 10 | 79 | 87 | 11 | 7 | 3 | 46 |
| Colletta 200599 | FT_F2 | Fibrotest | 0.48 | 21 | 81 | 3 | 5 | 11 | 21 | |
| Colletta 200599 | TE_F2 | Fibroscan | F2 | 8.74 | 100 | 100 | 14 | 0 | 0 | 26 |
| Corradi 2009100 | APRI_F2_high | APRI | F2 | 1.5 | 59 | 74 | 8 | 6 | 5 | 17 |
| Corradi 2009100 | Forns_F2_low | Forns index | 4.2 | 100 | 8 | 13 | 21 | 0 | 2 | |
| Corradi 2009100 | FT_F2_high | Fibrotest | F2 | 0.6 | 67 | 30 | 9 | 16 | 4 | 7 |
| Corradi 2009100 | TE_F2 | Fibroscan | F2 | 8.7 | 71 | 61 | 11 | 15 | 5 | 23 |
| Crespo 2010101 | APRI_F3_high | APRI | F3 | 1.5 | 40 | 61 | 4 | 16 | 7 | 24 |
| Cross 2010102 | Kings_F1 | King’s Score | F1 | 7.6 | 80 | 69 | 145 | 2 | 36 | 4 |
| Cross 2010102 | Kings_F2 | King’s Score | F2 | 9.87 | 84 | 70 | 75 | 29 | 14 | 69 |
| Cross 2010102 | Kings_F3 | King’s Score | F4 | 24.3 | 74 | 90 | 29 | 15 | 10 | 133 |
| Cross 2010102 | TE_F1 | Fibroscan | F1 | 6.75 | 68 | 91 | 123 | 1 | 58 | 5 |
| Cross 2010102 | TE_F2 | Fibroscan | F2 | 8.85 | 74 | 88 | 66 | 12 | 23 | 86 |
| Cross 2010102 | TE_F4 | Fibroscan | F4 | 10.05 | 93 | 88 | 36 | 18 | 3 | 130 |
| da Silva 2008103 | APRI_F2_combined | APRI | F2 | < 0.5 and > 1.5 | 13 | 0 | 2 | 11 | ||
| da Silva 2008103 | APRI_F2_high | APRI | F2 | 1.5 | 46.4 | 100 | 13 | 0 | 15 | 22 |
| da Silva 2008103 | APRI_F2_low | APRI | F2 | 0.5 | 92.9 | 50 | 26 | 11 | 2 | 11 |
| da Silva 2008103 | APRI_F4_combined | APRI | F4 | 1 and 2 | 6 | 1 | 1 | 27 | ||
| da Silva 2008103 | APRI_F4_high | APRI | F4 | ≥ 2.0 | 46.2 | 97.3 | 6 | 1 | 7 | 36 |
| da Silva 2008103 | APRI_F4_low | APRI | F4 | ≤ 1.0 | 92.3 | 73 | 12 | 10 | 1 | 27 |
| Danila 2011104 | ARFI_F1 | ARFI | F1 | 1.04 | 89.8 | 93.3 | 153 | 1 | 17 | 14 |
| Danila 2011104 | ARFI_F2 | ARFI | F2 | 1.21 | 82.5 | 93.9 | 125 | 2 | 27 | 31 |
| Danila 2011104 | ARFI_F3 | ARFI | F3 | 1.49 | 81.8 | 90.4 | 73 | 9 | 16 | 87 |
| Danila 2011104 | ARFI_F4 | ARFI | F4 | 1.82 | 82.8 | 87.1 | 28 | 19 | 6 | 132 |
| Degos 2010106 | TE_F2 | Fibroscan | F2 | 5.2 | 90 | 32 | 506 | 239 | 56 | 112 |
| Degos 2010106 | TE_F4 | Fibroscan | F4 | 12.9 | 72 | 89 | 91 | 87 | 35 | 700 |
| DeLedinghen 2006105 | TE_F2 | Fibroscan | F2 | 4.5 | 93 | 18 | 41 | 23 | 3 | 5 |
| DeLedinghen 2006105 | TE_F4 | Fibroscan | F4 | 11.8 | 100 | 93 | 17 | 4 | 0 | 51 |
| Dinesen 2008107 | 13CBT_F3 | 13Cmethacetin breath test | F3 | 0.021 | 75.4 | 79.5 | 43 | 8 | 14 | 31 |
| Dinesen 2008107 | 13CBT_F4 | 13Cmethacetin breath test | F4 | 0.0146 | 92.5 | 84.1 | 25 | 11 | 2 | 58 |
| Dinesen 2008107 | APRI_F3_low | APRI | F3 | 0.75 | 64.9 | 84.6 | 37 | 6 | 20 | 33 |
| Dinesen 2008107 | APRI_F4_low | APRI | F4 | 1 | 66.7 | 75.4 | 18 | 17 | 9 | 52 |
| Dinesen 2008107 | AST_ALT_ratio_F3 | AST–ALT ratio | F3 | 0.85 | 70.2 | 48.7 | 40 | 20 | 17 | 19 |
| Dinesen 2008107 | AST_ALT_ratio_F4 | AST–ALT ratio | F4 | 1 | 63 | 59.4 | 17 | 28 | 10 | 41 |
| Dinesen 2008107 | Fibroindex_F3 | Fibroindex | F3 | 1.35 | 66.7 | 84.6 | 38 | 6 | 19 | 33 |
| Dinesen 2008107 | Fibroindex_F4 | Fibroindex | F4 | 1.82 | 70.4 | 91.3 | 19 | 6 | 8 | 63 |
| Esmat 2007108 | HA_F3 | Hyaluronic acid | F3 | 20 | 88 | 68 | 45 | 48 | 6 | 101 |
| Esmat 2007108 | YKL_40_F3 | YKL-40 | F3 | 100 | 82 | 57 | 42 | 64 | 9 | 85 |
| Fabris 2006109 | APRI_F2_low | APRI | F2 | 0.4 | 55 | 83 | 6 | 5 | 5 | 24 |
| Fahmy 2011110 | TE_F2 | Fibroscan | F2 | 7 kPa | 87 | 86 | 58 | 6 | 9 | 37 |
| Fahmy 2011110 | TE_F4 | Fibroscan | F4 | 16.5 kPa | 87 | 91 | 19 | 8 | 3 | 80 |
| Fontaine 2009111 | FIB4_F3_low | FIB-4 | F3 | 53 | 65 | 13 | 30 | 12 | 55 | |
| Fontaine 2009111 | FT_F2 | Fibrotest | F2 | None given | 56 | 63 | 26 | 23 | 21 | 40 |
| Fontaine 2009111 | FT_F3 | Fibrotest | F3 | 42 | 84 | 11 | 14 | 15 | 71 | |
| Fontaine 2009111 | FT_F4 | Fibrotest | F4 | 25 | 94 | 2 | 6 | 6 | 96 | |
| Fontana 2008112 | Fontana_F4 | Fontana | F4 | 0.2–0.3 | 79 | 66 | 152 | 108 | 41 | 211 |
| Fontanges 2008113 | FT_F2 | Fibrotest | F2 | 0.385 | 74 | 65 | 37 | 16 | 13 | 30 |
| Fontanges 2008113 | FT_F3 | Fibrotest | F3 | 0.455 | 86 | 68 | 24 | 3 | 4 | 5 |
| Forestier 2010114 | TE_F4 | Fibroscan | F4 | 12.6 kPa | 85 | 93 | 25 | 4 | 4 | 54 |
| Forns 2002 training115 | Forns_F2_combined | Forns index | F2 | 37 | 11 | 5 | 120 | |||
| Forns 2002 training115 | Forns_F2_high | Forns index | F2 | 6.9 | 44 | 96 | 37 | 11 | 48 | 255 |
| Forns 2002 training115 | Forns_F2_low | Forns index | F2 | 4.2 | 94 | 45 | 80 | 146 | 5 | 120 |
| Forns 2002 validation115 | Forns_F2_combined | Forns index | F2 | 10 | 5 | 2 | 47 | |||
| Forns 2002 validation115 | Forns_F2_high | Forns index | F2 | 6.9 | 30 | 95 | 10 | 5 | 23 | 87 |
| Forns 2002 validation115 | Forns_F2_low | Forns index | F2 | 4.2 | 94 | 51 | 31 | 45 | 2 | 47 |
| Fraquelli 2011116 | TE_F2 | Fibroscan | F2 | 8.8 kPa | 81 | 77 | 197 | 49 | 47 | 160 |
| Fraquelli 2011116 | TE_F4 | Fibroscan | F4 | 14.6 kPa | 100 | 88 | 44 | 49 | 0 | 360 |
| Fuji 2009117 | AST_ALT_ratio_F4 | AST–ALT ratio | F4 | 1 | 65 | 56 | 11 | 37 | 6 | 46 |
| Fuji 2009117 | APRI_F4 | APRI | F4 | 82 | 70 | 14 | 25 | 3 | 58 | |
| Fuji 2009117 | CDS_F4 | CDS | F4 | 88 | 67 | 15 | 27 | 2 | 56 | |
| Gaia 2011119 | TE_F1 | Fibroscan | F1 | 4.5 kPa | 90 | 33 | 67 | 2 | 7 | 1 |
| Gaia 2011119 | TE_F2 | Fibroscan | F2 | 7.5 kPa | 74 | 79 | 29 | 8 | 10 | 30 |
| Gaia 2011119 | TE_F3 | Fibroscan | F3 | 10.1 kPa | 77 | 90 | 13 | 6 | 4 | 54 |
| Gaia 2011119 | TE_F4 | Fibroscan | F4 | 11.5 kPa | 69 | 93 | 9 | 4 | 4 | 60 |
| Ganne-Carrie 2006120 | TE_F4 | Fibroscan | F4 | 10.4 | 88 | 85 | 26 | 40 | 4 | 228 |
| Gara 2011121 | APRI_F4_low | APRI | F4 | 1.0 | 79 | 78 | 12 | 23 | 3 | 81 |
| Gara 2011121 | TE_F4 | Fibroscan | F4 | 13.1 kPa | 100 | 89 | 15 | 11 | 0 | 93 |
| Giannini 2006122 | AST_ALT_ratio_F2 | AST–ALT ratio | F2 | 0.66 | 73.7 | 65 | 129 | 82 | 46 | 152 |
| Giannini 2006122 | PLT_F2 | Platelet count | F2 | 163,000 | 61.7 | 80.8 | 108 | 45 | 67 | 189 |
| Gobel 2006123 | APRI_F2_high | APRI | F2 | 1.5 | 75 | 87 | 33 | 5 | 11 | 34 |
| Guechot 2010124 | Hepascore_F2 | Hepascore | F2 | 0.5 | 77 | 70 | 190 | 80 | 57 | 186 |
| Guechot 2010124 | Hepascore_F3 | Hepascore | F3 | 0.6 | 80 | 70 | 124 | 107 | 31 | 250 |
| Guechot 2010124 | Hepascore_F4 | Hepascore | F4 | 0.84 | 84 | 73 | 64 | 118 | 12 | 318 |
| Guechot 2010124 | HA_F3 | Hyaluronic acid | F3 | 85 μg/l | 60 | 74 | 66 | 59 | 44 | 167 |
| Guechot 2010124 | HA_F4 | Hyaluronic acid | F4 | 110 μg/l | 79.2 | 89.4 | 42 | 29 | 11 | 244 |
| Guechot 2010124 | PIIINP_F3 | PIIINP | F3 | 0.8 kU/l | 70 | 63.4 | 77 | 83 | 33 | 143 |
| Guechot 2010124 | PIIINP_F4 | PIIINP | F4 | 1.0 kU/l | 64.5 | 91.2 | 34 | 24 | 19 | 249 |
| Guzelbulut 2011126 | APRI_F2_combined | APRI | F2 | < 0.5 and > 1.5 | 36 | 6 | 13 | 30 | ||
| Guzelbulut 2011126 | APRI_F2_high | APRI | F2 | 1.5 | 43.37 | 91.04 | 36 | 6 | 47 | 61 |
| Guzelbulut 2011126 | APRI_F2_low | APRI | F2 | 0.5 | 84.34 | 44.78 | 70 | 37 | 13 | 30 |
| Guzelbulut 2011126 | APRI_F4_combined | APRI | F4 | 22 | 5 | 14 | 80 | |||
| Guzelbulut 2011126 | APRI_F4_high | APRI | F4 | 2 | 43.14 | 94.95 | 22 | 5 | 29 | 94 |
| Guzelbulut 2011126 | APRI_F4_low | APRI | F4 | 1 | 72.55 | 80.81 | 37 | 19 | 14 | 80 |
| Guzelbulut 2011126 | FIB4_F2_combined | FIB-4 | F2 | 76 | 47 | 0 | 7 | |||
| Guzelbulut 2011126 | FIB4_F4_combined | FIB-4 | F4 | 28 | 8 | 5 | 57 | |||
| Guzelbulut 2011126 | Forns_F2_combined | Forns index | F2 | 39 | 4 | 5 | 23 | |||
| Guzelbulut 2011126 | Forns_F4_combined | Forns index | F4 | 34 | 9 | 1 | 27 | |||
| Guzelbulut 2011126 | FIB4_F2_high | FIB-4 | F2 | 1 | 91.57 | 29.85 | 76 | 47 | 7 | 20 |
| Guzelbulut 2011126 | FIB4_F2_low | FIB-4 | F2 | 0.6 | 100 | 10.45 | 83 | 60 | 0 | 7 |
| Guzelbulut 2011126 | FIB4_F4_high | FIB-4 | F4 | 3.25 | 54.9 | 91.92 | 28 | 8 | 23 | 91 |
| Guzelbulut 2011126 | FIB4_F4_low | FIB-4 | F4 | 1.45 | 90.2 | 57.58 | 46 | 42 | 5 | 57 |
| Guzelbulut 2011126 | Forns_F2_high | Forns index | F2 | 6.9 | 46.99 | 94.03 | 39 | 4 | 44 | 63 |
| Guzelbulut 2011126 | Forns_F2_low | Forns index | F2 | 4.2 | 93.98 | 34.33 | 78 | 44 | 5 | 23 |
| Guzelbulut 2011126 | Forns_F4_high | Forns index | F4 | 6.9 | 66.67 | 90.91 | 34 | 9 | 17 | 90 |
| Guzelbulut 2011126 | Forns_F4_low | Forns index | F4 | 4.2 | 98.04 | 27.27 | 50 | 72 | 1 | 27 |
| Halfon 2006128 | FT_F2 | Fibrotest | F2 | 0.36 | 73 | 72 | 168 | 77 | 62 | 197 |
| Halfon 2005 validation129 | HA_F1 | Hyaluronic acid | F1 | 16 | 91 | 36 | 42 | 12 | 76 | 142 |
| Halfon 2005 validation129 | HA_F2_high | Hyaluronic acid | F2 | 121 | 14 | 99 | 17 | 1 | 101 | 135 |
| Halfon 2005 validation129 | HA_F2_low | Hyaluronic acid | F2 | 25 | 78 | 53 | 32 | 43 | 28 | 151 |
| Halfon 2005 validation129 | HA_F3_high | Hyaluronic acid | F3 | 160 | 22 | 100 | 13 | 0 | 47 | 194 |
| Halfon 2005 validation129 | HA_F3_low | Hyaluronic acid | F3 | 50 | 100 | 79 | 10 | 0 | 3 | 241 |
| Halfon 2005 validation129 | HA_F4 | Hyaluronic acid | F4 | 237 | 31 | 99 | 4 | 2 | 9 | 239 |
| Halfon 2007127 | APRI_F2_low | APRI | F2 | 0.39 | 77 | 66 | 112 | 71 | 34 | 139 |
| Halfon 2007127 | APRI_F3_low | APRI | F3 | 0.58 | 75 | 76 | 38 | 73 | 13 | 232 |
| Halfon 2007127 | APRI_F4_low | APRI | F4 | 0.83 | 100 | 83 | 13 | 58 | 0 | 285 |
| Halfon 2007127 | Fibrometer_F2 | FibroMeter | F2 | 0.57 | 64 | 81 | 93 | 40 | 53 | 170 |
| Halfon 2007127 | Fibrometer_F3 | FibroMeter | F3 | 0.667 | 82 | 76 | 42 | 73 | 9 | 232 |
| Halfon 2007127 | Fibrometer_F4 | FibroMeter | F4 | 0.88 | 92 | 87 | 12 | 45 | 1 | 298 |
| Halfon 2007127 | FT_F2 | Fibrotest | F2 | 0.44 | 67 | 80 | 98 | 42 | 48 | 168 |
| Halfon 2007127 | FT_F3 | Fibrotest | F3 | 0.45 | 84 | 69 | 43 | 95 | 8 | 210 |
| Halfon 2007127 | FT_F4 | Fibrotest | F4 | 0.56 | 85 | 74 | 11 | 89 | 2 | 254 |
| Halfon 2007127 | Hepascore_F2 | Hepascore | F2 | 0.32 | 77 | 63 | 112 | 78 | 34 | 132 |
| Halfon 2007127 | Hepascore_F3 | Hepascore | F3 | 0.53 | 78 | 72 | 40 | 85 | 11 | 220 |
| Halfon 2007127 | Hepascore_F4 | Hepascore | F4 | 0.61 | 92 | 72 | 12 | 96 | 1 | 247 |
| Harada 2008130 | APRI_F2_low | APRI | F2 | 0.84 | 73 | 91 | 15 | 3 | 6 | 32 |
| Harada 2008130 | TE_F1 | Fibroscan | F1 | 8.8 kPa | 68 | 100 | 23 | 0 | 11 | 22 |
| Harada 2008130 | TE_F2 | Fibroscan | F2 | 9.9 kPa | 90 | 91 | 19 | 3 | 2 | 32 |
| Harada 2008130 | TE_F3 | Fibroscan | F3 | 15.4 kPa | 75 | 95 | 9 | 2 | 3 | 42 |
| Harada 2008130 | TE_F4 | Fibroscan | F4 | 26.5 kPa | 100 | 98 | 5 | 1 | 0 | 50 |
| Harada 2008130 | HA_F2 | Hyaluronic acid | F2 | 103 ng/ml | 38 | 83 | 8 | 6 | 13 | 29 |
| Harada 2008130 | PLT_F2 | Platelet count | F2 | 48 U/l | 38 | 89 | 8 | 4 | 13 | 31 |
| Harada 2008130 | typeIVcoll_F2 | Type IV collagen | F2 | 298 ng/ml | 52 | 83 | 11 | 6 | 10 | 29 |
| Hsieh 2012131 | AST_ALT_ratio_F2 | AST–ALT ratio | F2 | 0.6 | 67.9 | 70.7 | 133 | 12 | 63 | 29 |
| Hsieh 2012131 | AST_ALT_ratio_F3 | AST–ALT ratio | F3 | 0.6 | 77.5 | 53.2 | 86 | 59 | 25 | 67 |
| Hsieh 2012131 | APRI_F2_combined | APRI | F2 | < 0.5 and > 1.5 | 111 | 17 | 6 | 4 | ||
| Hsieh 2012131 | APRI_F2_high | APRI | F2 | 1.5 | 56.6 | 58.5 | 111 | 17 | 85 | 24 |
| Hsieh 2012131 | APRI_F2_low | APRI | F2 | 0.5 | 96.9 | 9.7 | 190 | 37 | 6 | 4 |
| Hsieh 2012131 | APRI_F3_combined | APRI | F3 | 77 | 51 | 1 | 9 | |||
| Hsieh 2012131 | APRI_F3_high | APRI | F3 | 1.5 | 69.4 | 59.5 | 77 | 51 | 34 | 75 |
| Hsieh 2012131 | APRI_F3_low | APRI | F3 | 0.5 | 99.1 | 7.1 | 110 | 117 | 1 | 9 |
| Hsieh 2012131 | CDS_F2 | CDS | F2 | 6 | 66.3 | 48.8 | 130 | 21 | 66 | 20 |
| Hsieh 2012131 | CDS_F3 | CDS | F3 | 6 | 73.9 | 45.2 | 82 | 69 | 29 | 57 |
| Hsieh 2012131 | FIB4_F2_combined | FIB-4 | F2 | 93 | 4 | 36 | 22 | |||
| Hsieh 2012131 | FIB4_F2_high | FIB-4 | F2 | 3.25 | 47.4 | 90.2 | 93 | 4 | 103 | 37 |
| Hsieh 2012131 | FIB4_F2_low | FIB-4 | F2 | 1.45 | 81.6 | 53.7 | 160 | 19 | 36 | 22 |
| Hsieh 2012131 | FIB4_F3_combined | FIB-4 | F3 | 68 | 29 | 14 | 44 | |||
| Hsieh 2012131 | FIB4_F3_high | FIB-4 | F3 | 3.25 | 61.3 | 77 | 68 | 29 | 43 | 97 |
| Hsieh 2012131 | FIB4_F3_low | FIB-4 | F3 | 1.45 | 87.4 | 34.9 | 97 | 82 | 14 | 44 |
| Hsieh 2012131 | FibroQ_F2 | FibroQ | F2 | 1.6 | 77.6 | 65.9 | 152 | 14 | 44 | 27 |
| Hsieh 2012131 | FibroQ_F3 | FibroQ | F3 | 1.6 | 85.66 | 43.7 | 95 | 71 | 16 | 55 |
| Hsieh 2012131 | Lok_F2 | Lok’s index | F2 | 0.2 | 81.6 | 58.8 | 160 | 17 | 36 | 24 |
| Hsieh 2012131 | Lok_F3_low | Lok’s index | F3 | 0.2 | 88.3 | 37.3 | 98 | 79 | 13 | 47 |
| Hsieh 2012131 | Pohl_F2 | Pohl score | F2 | 3.8 | 4.59 | 100 | 9 | 0 | 187 | 41 |
| Hsieh 2012131 | Pohl_F3 | F3 | 3.8 | 7.2 | 99.2 | 8 | 1 | 103 | 125 | |
| Iacobellis 2005132 | PLT_F2 | Platelet count | F2 | 140.000/µl | 51 | 90 | 330 | 50 | 318 | 446 |
| Iacobellis 2005132 | PLT_F4 | Platelet count | F4 | 82 | 87 | 67 | 138 | 15 | 923 | |
| Iacobellis 2005132 | PLTspleen_F2 | Platelet count–spleen diameter ratio | F2 | 33 | 92 | 214 | 40 | 434 | 455 | |
| Iacobellis 2005132 | PLTspleen_F4 | Platelet count–spleen diameter ratio | F4 | 85 | 82 | 70 | 191 | 12 | 870 | |
| Imbert-Bismut 200123 | FT_F2 | F2 | 40 | 78 | 69 | 47 | 23 | 13 | 51 | |
| Imbert-Bismut 200123 | FT_F2_high | Fibrotest | F2 | 70 | 62 | 95 | 37 | 4 | 23 | 70 |
| Imbert-Bismut 200123 | FT_F2_low | Fibrotest | F2 | 20 | 92 | 46 | 55 | 40 | 5 | 34 |
| Imperiale 2000133 | AST_ALT_ratio_F4 | AST–ALT ratio | F4 | 1 | 52 | 91 | 15 | 12 | 14 | 116 |
| Islam 2005134 | APRI_F4_low | APRI | F4 | 1 | 78 | 75 | 16 | 40 | 5 | 119 |
| Islam 2005134 | GUCI_F4 | GUCI | F4 | 1 | 80 | 78 | 17 | 35 | 4 | 123 |
| Islam 2005134 | PLT_F4 | Platelet count | F4 | 190 | 80 | 77 | 17 | 36 | 4 | 122 |
| Iushchuk 2005135 | HA_F4 | Hyaluronic acid | F4 | 100 ng/ml | 100 | 84.6 | 28 | 18 | 0 | 102 |
| Jazia 200978 | APRI_F2 | APRI | F2 | 0.72 | 93 | 58 | 25 | 3 | 2 | 5 |
| Kalantari 2011136 | Hepascore_F2 | Hepascore | F2 | 0.34 | 67 | 56 | 29 | 16 | 14 | 21 |
| Kalantari 2011136 | Hepascore_F3 | Hepascore | F3 | 0.61 | 82 | 86 | 19 | 8 | 4 | 49 |
| Kalantari 2011136 | Hepascore_F4 | Hepascore | F4 | 0.84 | 100 | 97 | 16 | 2 | 0 | 62 |
| Kamphues 2010137 | APRI_F2_low | APRI | F2 | 0.4845 | 70 | 63 | 45 | 11 | 19 | 19 |
| Kamphues 2010137 | FIB4_F2_high | FIB-4 | F2 | 2.8 | 44 | 87 | 28 | 4 | 36 | 26 |
| Kamphues 2010137 | FIB4_F4_high | FIB-4 | F4 | 4.44 | 44 | 84 | 4 | 14 | 5 | 71 |
| Kamphues 2010137 | TE_F2 | Fibroscan | F2 | 8.5 kPa | 72 | 83 | 46 | 5 | 18 | 25 |
| Kamphues 2010137 | TE_F4 | Fibroscan | F4 | 10.5 kPa | 100 | 65 | 9 | 30 | 0 | 55 |
| Kandemir 2009138 | GUCI__F3 | GUCI | F3 | 0.261 | 58.33 | 72.73 | 14 | 12 | 10 | 32 |
| Khan 2008140 | APRI_F2_combined | APRI | F2 | < 0.5 and > 1.5 | 26 | 3 | 11 | 32 | ||
| Khan 2008140 | APRI_F2_high | APRI | F2 | 1.5 | 41 | 95 | 26 | 3 | 38 | 53 |
| Khan 2008140 | APRI_F2_low | APRI | F2 | 0.5 | 83 | 66 | 53 | 24 | 11 | 32 |
| Khan 2008140 | APRI_F3_combined | APRI | F3 | 0.9 and 1.75 | 17 | 5 | 4 | 63 | ||
| Khan 2008140 | APRI_F3_high | APRI | F3 | 1.75 | 56 | 94 | 17 | 5 | 13 | 85 |
| Khan 2008140 | APRI_F3_low | APRI | F3 | 0.9 | 90 | 70 | 26 | 27 | 4 | 63 |
| Kim 2011141 | TE_F2 | Fibroscan | F2 | 6.2 | 76 | 97.5 | 38 | 1 | 12 | 40 |
| Kim 2011141 | TE_F3 | Fibroscan | F3 | 7.7 | 100 | 95.7 | 21 | 3 | 0 | 67 |
| Kim 2011141 | TE_F4 | Fibroscan | F4 | 11 | 77.8 | 93.5 | 7 | 5 | 2 | 77 |
| Koda 2007 training24 | APRI_F2_low | APRI | F2 | 0.36 | 26.5 | 95.1 | 117 | 86 | 6 | 31 |
| Koda 2007 training24 | APRI_F2_combined | APRI | F2 | < 0.36 and > 0.85 | 19 | 5 | 1 | 19 | ||
| Koda 2007 training24 | APRI_F2_high | APRI | F2 | 0.85 | 31.6 | 91.7 | 19 | 5 | 41 | 55 |
| Koda 2007 training24 | APRI_F2_low | APRI | F2 | 0.36 | 31.6 | 98.3 | 59 | 41 | 1 | 19 |
| Koda 2007 training24 | Fibroindex_F2_combined | Fibroindex | F2 | 1.25 and 2.25 | 18 | 2 | 2 | 24 | ||
| Koda 2007 training24 | Fibroindex_F2_high | Fibroindex | F2 | 2.25 | 30 | 96.7 | 18 | 2 | 42 | 58 |
| Koda 2007 training24 | Fibroindex_F2_low | Fibroindex | F2 | 1.25 | 40 | 96.3 | 58 | 36 | 2 | 24 |
| Koda 2007 training24 | Forns_F2_combined | Forns index | F2 | 4.5 and 8.7 | 13 | 1 | 4 | 15 | ||
| Koda 2007 training24 | Forns_F2_high | Forns index | F2 | 8.7 | 21.7 | 98.3 | 13 | 1 | 47 | 59 |
| Koda 2007 training24 | Forns_F2_low | Forns index | F2 | 4.5 | 25.6 | 93.3 | 56 | 45 | 4 | 15 |
| Koda 2007 training24 | APRI_F2_combined | APRI | F2 | 0.36 and 0.85 | 42 | 5 | 6 | 31 | ||
| Koda 2007 training24 | APRI_F2_high | APRI | F2 | 0.85 | 34.1 | 95.7 | 42 | 5 | 81 | 112 |
| Koda 2007 training24 | Fibroindex_F2_combined | Fibroindex | F2 | 1.25 and 2.25 | 44 | 3 | 7 | 47 | ||
| Koda 2007 training24 | Fibroindex_F2_high | Fibroindex | F2 | 2.25 | 35.8 | 97.4 | 44 | 3 | 79 | 114 |
| Koda 2007 training24 | Fibroindex_F2_low | Fibroindex | F2 | 1.25 | 40.2 | 94.3 | 116 | 70 | 7 | 47 |
| Koda 2007 training24 | Forns_F2_combined | Forns index | F2 | 4.5 and 8.7 | 30 | 4 | 6 | 31 | ||
| Koda 2007 training24 | Forns_F2_high | Forns index | F2 | 8.7 | 24.3 | 96.6 | 30 | 4 | 93 | 113 |
| Koda 2007 training24 | Forns_F2_low | Forns index | F2 | 4.5 | 25.6 | 97.6 | 120 | 87 | 3 | 30 |
| Lackner 2005143 | APRI_F2_high | APRI | F2 | 1.5 | 44 | 96 | 45 | 60 | 6 | 69 |
| Lackner 2005143 | APRI_F2_low | APRI | F2 | 0.5 | 88 | 44 | 48 | 101 | 3 | 28 |
| Lackner 2005143 | APRI_F4_high | APRI | F4 | 2 | 55 | 93 | 38 | 21 | 13 | 108 |
| Lackner 2005143 | APRI_F4_low | APRI | F5–F6 | 1 | 93 | 70 | 44 | 33 | 7 | 96 |
| Lackner 2005143 | AST_ALT_ratio_F4 | AST–ALT ratio | F5–F6 | 1 | 36 | 90 | 7 | 18 | 12 | 158 |
| Lackner 2005143 | CDS_F3 | CDS | F3 | 8 | 10 | 100 | 5 | 0 | 45 | 144 |
| Lackner 2005143 | PLT_F2 | Platelet count | Ishak F3 | 150 | 42 | 97 | 41 | 3 | 56 | 94 |
| Lackner 2005143 | PLT_F4 | Platelet count | F4 | 150 | 77 | 88 | 25 | 19 | 7 | 143 |
| Lackner 2005143 | Pohl_F3 | Pohl score | F3 | Positive | 18 | 98 | 9 | 3 | 41 | 141 |
| Ladero 2010144 | APRI_F2_low | APRI | F2 | 0.5 | 54.7 | 80.6 | 102 | 47 | 84 | 196 |
| Ladero 2010144 | FIB4_F2_low | FIB-4 | F2 | 1.35 | 78.6 | 58.1 | 168 | 171 | 18 | 72 |
| Ladero 2010144 | Forns_F2_low | Forns index | F2 | 4.2 | 50.6 | 75.8 | 141 | 120 | 45 | 123 |
| Ladero 2010144 | GUCI_F2 | GUCI | F2 | 0.3 | 29.6 | 90.3 | 168 | 171 | 18 | 72 |
| Ladero 2010144 | Kings_F2_high | King’s Score | F2 | 4.46 | 22.8 | 93.5 | 108 | 52 | 78 | 191 |
| Ladero 2010144 | Kings_F2_low | King’s Score | F2 | 12.3 | 81.5 | 62.4 | 116 | 45 | 70 | 198 |
| Lee 2011145 | FibrospectII_F1 | FibroSpect II | F1 | 42 | 87.5 | 70 | 6 | 8 | 1 | 18 |
| Leroy 2004147 | PIIINP/MMP-1 index_F2 | PIIINP/MMP-1 index | F2 | 0.3 | 65 | 85 | 55 | 17 | 29 | 94 |
| Leroy 2004147 | PIIINP/MMP-1 index_F3 | PIIINP/MMP-1 index | F3 | 0.3 | 85 | 74 | 31 | 41 | 5 | 117 |
| Leroy 2007146 | APRI_F2_combined | APRI | F2 | < 0.5 and > 1.5 | 66 | 11 | 8 | 24 | ||
| Leroy 2007146 | APRI_F2_high | APRI | F2 | 1.5 | 72.3 | 87.8 | 66 | 11 | 25 | 78 |
| Leroy 2007146 | APRI_F2_low | APRI | F2 | 0.5 | 91.6 | 26.8 | 83 | 65 | 8 | 24 |
| Leroy 2007146 | APRI_F3_combined | APRI | F3 | 1 and 2 | 38 | 21 | 6 | 69 | ||
| Leroy 2007146 | APRI_F3_high | APRI | F3 | 2 | 73.9 | 84 | 38 | 21 | 13 | 108 |
| Leroy 2007146 | APRI_F3_low | APRI | F3 | 1 | 89.1 | 53.8 | 45 | 60 | 6 | 69 |
| Leroy 2007146 | Forns_F2_combined | Forns index | F2 | 4.2 and 6.9 | 38 | 6 | 11 | 38 | ||
| Leroy 2007146 | Forns_F2_high | Forns index | F2 | 6.9 | 41.9 | 92.9 | 38 | 6 | 53 | 83 |
| Leroy 2007146 | Forns_F2_low | Forns index | F2 | 4.2 | 88.4 | 42.4 | 80 | 51 | 11 | 38 |
| Leroy 2007146 | Forns_F3_combined | Forns index | F3 | 4.2 and 6.9 | 28 | 17 | 4 | 44 | ||
| Leroy 2007146 | Forns_F3_high | Forns index | F3 | 6.9 | 54.2 | 87 | 28 | 17 | 23 | 112 |
| Leroy 2007146 | Forns_F3_Low | Forns index | F3 | 4.2 | 91.7 | 34.1 | 47 | 85 | 4 | 44 |
| Leroy 2007146 | FT_F2 | Fibrotest | F2 | 0.32 | 75.8 | 74.2 | 69 | 23 | 22 | 66 |
| Leroy 2007146 | FT_F2_high | Fibrotest | F2 | 0.59 | 45.1 | 89.9 | 41 | 9 | 50 | 80 |
| Leroy 2007146 | FT_F2_low | Fibrotest | F2 | 0.22 | 89 | 52.8 | 81 | 42 | 10 | 47 |
| Leroy 2007146 | FT_F3 | Fibrotest | F3 | 0.32 | 90.2 | 64.3 | 46 | 46 | 5 | 83 |
| Leroy 2007146 | FT_F3_high | Fibrotest | F3 | 0.59 | 66.7 | 87.6 | 34 | 16 | 17 | 113 |
| Leroy 2007146 | FT_F3_low | Fibrotest | F3 | 0.22 | 94.1 | 41.9 | 48 | 75 | 3 | 54 |
| Leroy 2007146 | Hepascore_F2 | Hepascore | F2 | 0.5 | 53.8 | 83.9 | 49 | 14 | 42 | 75 |
| Leroy 2007146 | Hepascore_F2_combined | Hepascore | F2 | 0.5 and 0.84 | 30 | 7 | 42 | 75 | ||
| Leroy 2007146 | Hepascore_F2_high | Hepascore | F2 | 0.84 | 33 | 92 | 30 | 7 | 61 | 82 |
| Leroy 2007146 | Hepascore_F3_combined | Hepascore | F3 | 0.5 and 0.84 | 24 | 13 | 12 | 105 | ||
| Leroy 2007146 | Hepascore_F3_high | Hepascore | F3 | 0.84 | 47.1 | 89.8 | 24 | 13 | 27 | 116 |
| Leroy 2007146 | Hepascore_F3_low | Hepascore | F3 | 0.5 | 76.5 | 81.1 | 39 | 24 | 12 | 105 |
| Leroy 2007146 | MP3_F2 | MP3 | F2 | 0.3 | 82.4 | 72.7 | 75 | 24 | 16 | 65 |
| Leroy 2007146 | MP3_F3 | MP3 | F3 | 0.3 | 92.2 | 59.4 | 47 | 52 | 4 | 77 |
| Leroy 2011148 | TE_F2 | Fibroscan | F2 | 7.6 | 80 | 80 | 142 | 45 | 45 | 184 |
| Lieber 2006150 | APRI_F2_combined | APRI | F2 | < 0.5 and > 1.5 | 21 | 9 | 12 | 22 | ||
| Lieber 2006150 | APRI_F2_high | APRI | F2 | 1.5 | 21 | 9 | 38 | 65 | ||
| Lieber 2006150 | APRI_F2_low | F2 | 0.5 | 47 | 52 | 12 | 22 | |||
| Liu 2006151 | APRI_F2_high | APRI | F2 | 1.5 | 0 | 100 | 0 | 0 | 21 | 58 |
| Liu 2006151 | APRI_F2_low | APRI | F2 | 0.5 | 28.6 | 94 | 6 | 3 | 15 | 55 |
| Liu 2006151 | AST_ALT_ratio_F2 | AST–ALT ratio | F2 | 1 | 45.3 | 62.1 | 10 | 22 | 11 | 36 |
| Liu 2011153 | APRI_F2_high | APRI | F2 | 1.5 | 3 | 100 | 3 | 0 | 98 | 183 |
| Liu 2011153 | APRI_F2_low | APRI | F2 | 0.5 | 79 | 70 | 80 | 55 | 21 | 128 |
| Liu 2011153 | APRI_F3 | APRI | F3 | 0.75 | 93 | 90 | 37 | 24 | 3 | 220 |
| Liu 2011153 | APRI_F4_high | APRI | F4 | 2 | 0 | 99 | 0 | 3 | 14 | 267 |
| Liu 2011153 | APRI_F4_low | APRI | F4 | 1 | 43 | 92 | 6 | 22 | 8 | 248 |
| Liu 2011153 | TE_F2 | Fibroscan | F2 | 5.3 | 93 | 88 | 94 | 22 | 7 | 161 |
| Liu 2011153 | TE_F3 | Fibroscan | F3 | 8.3 | 95 | 99 | 38 | 2 | 2 | 242 |
| Liu 2011153 | TE_F4 | Fibroscan | F4 | 9.2 | 100 | 96 | 14 | 11 | 0 | 259 |
| Loko 2008154 | APRI_F2_combined | APRI | F2 | < 0.5 and > 1.5 | 57 | 2 | 19 | 21 | ||
| Loko 2008154 | APRI_F2_high | APRI | F2 | 1.5 | 36.1 | 95.4 | 57 | 2 | 100 | 41 |
| Loko 2008154 | APRI_F2_low | APRI | F2 | 0.5 | 87.9 | 48.8 | 138 | 22 | 19 | 21 |
| Loko 2008154 | APRI_F4_combined | APRI | F4 | 1 and 2 | 19 | 25 | 6 | 100 | ||
| Loko 2008154 | APRI_F4_high | APRI | F4 | 2 | 47.5 | 84.4 | 19 | 25 | 21 | 135 |
| Loko 2008154 | APRI_F4_low | APRI | F4 | 1 | 85 | 62.5 | 34 | 6 | 6 | 100 |
| Loko 2008154 | FIB4_F2_high | FIB-4 | F2 | 1 | 83.4 | 53.5 | 131 | 20 | 26 | 23 |
| Loko 2008154 | FIB4_F2_low | FIB-4 | F2 | 0.6 | 98.1 | 20.9 | 154 | 34 | 3 | 9 |
| Loko 2008154 | FIB4_F3_combined | FIB-4 | F3 | 1.45 and 3.25 | 22 | 9 | 19 | 90 | ||
| Loko 2008154 | FIB4_F3_high | FIB-4 | F3 | 3.25 | 31 | 93 | 22 | 9 | 49 | 120 |
| Loko 2008154 | FIB4_F3_low | FIB-4 | F3 | 1.45 | 73.2 | 69.8 | 52 | 39 | 19 | 90 |
| Loko 2008154 | FIB4_F4_combined | FIB-4 | F4 | 1.45 and 3.25 | 19 | 25 | 7 | 102 | ||
| Loko 2008154 | FIB4_F4_high | FIB-4 | F4 | 3.25 | 40 | 90.6 | 19 | 25 | 21 | 135 |
| Loko 2008154 | FIB4_F4_low | FIB-4 | F4 | 1.45 | 82.5 | 63.7 | 33 | 58 | 7 | 102 |
| Loko 2008154 | Forns_F2_combined | Forns index | F2 | 4.2 and 6.9 | 29 | 0 | 20 | 9 | ||
| Loko 2008154 | Forns_F2_high | Forns index | F2 | 6.9 | 23 | 100 | 29 | 0 | 97 | 26 |
| Loko 2008154 | Forns_F2_low | Forns index | F2 | 4.2 | 84.1 | 34.6 | 106 | 17 | 20 | 9 |
| Loko 2008154 | PLT_F4 | Platelet count | F4 | 150 | 67.5 | 77.5 | 27 | 26 | 13 | 91 |
| Lupsor 2008155 | TE_F1 | Fibroscan | F1 | 4.9 | 87.5 | 92.3 | 272 | 1 | 39 | 12 |
| Lupsor 2008155 | TE_F2 | Fibroscan | F2 | 7.4 | 75.9 | 83.6 | 159 | 19 | 51 | 95 |
| Lupsor 2008155 | TE_F3 | Fibroscan | F3 | 9.1 | 86.8 | 83.9 | 92 | 35 | 14 | 183 |
| Lupsor 2008155 | TE_F4 | Fibroscan | F4 | 11.85 | 86.9 | 90.7 | 60 | 24 | 9 | 231 |
| Lupsor 200929 | ARFI_F1 | ARFI | F1 | 1.19 | 62 | 86 | 61 | 2 | 37 | 12 |
| Lupsor 200929 | ARFI_F2 | ARFI | F2 | 1.34 | 68 | 93 | 46 | 3 | 22 | 41 |
| Lupsor 200929 | ARFI_F3 | ARFI | F3 | 1.61 | 79 | 95 | 40 | 3 | 11 | 59 |
| Lupsor 200929 | ARFI_F4 | ARFI | F4 | 2 | 80 | 95 | 34 | 4 | 8 | 67 |
| Lupsor 200929 | TE_F1 | Fibroscan | F1 | 5.2 | 85 | 93 | 83 | 1 | 15 | 13 |
| Lupsor 200929 | TE_F2 | Fibroscan | F2 | 8.1 | 85 | 95 | 58 | 2 | 10 | 42 |
| Lupsor 200929 | TE_F3 | Fibroscan | F3 | 9.6 | 96 | 87 | 48 | 8 | 2 | 54 |
| Lupsor 200929 | TE_F4 | Fibroscan | F4 | 13.1 | 95 | 89 | 40 | 8 | 2 | 62 |
| Macias 2006156 | APRI_F2_combined | APRI | F2 | < 0.5 and > 1.5 | 78 | 10 | 12 | 36 | ||
| Macias 2006156 | APRI_F2_high | APRI | F2 | 1.5 | 51 | 91 | 78 | 10 | 75 | 100 |
| Macias 2006156 | APRI_F2_low | APRI | F2 | 0.5 | 92 | 33 | 141 | 74 | 12 | 36 |
| Macias 2006156 | APRI_F4_combined | APRI | F4 | 21 | 25 | 9 | 127 | |||
| Macias 2006156 | APRI_F4_high | APRI | F4 | 2 | 53 | 89 | 21 | 25 | 19 | 198 |
| Macias 2006156 | APRI_F4_low | APRI | F4 | 1 | 78 | 57 | 31 | 96 | 9 | 127 |
| Macias 2006156 | AST_ALT_RATIO_F4 | AST–ALT ratio | F4 | 1 | 38 | 77 | 15 | 51 | 25 | 172 |
| Macias 2006156 | Bonacini_F4 | BONACINI MODEL | F4 | 7 | 43 | 83 | 17 | 38 | 23 | 185 |
| Macias 2006156 | Forns_F2_combined | Forns index | F2 | 4.2 and 6.9 | 66 | 4 | 34 | 42 | ||
| Macias 2006156 | Forns_F2_high | Forns index | F2 | 6.9 | 43 | 96 | 66 | 4 | 87 | 106 |
| Macias 2006156 | Forns_F2_low | Forns index | F2 | 4.2 | 78 | 38 | 119 | 68 | 34 | 42 |
| Macias 2006156 | PLT_F4 | Platelet count | F4 | 150 | 63 | 37 | 25 | 140 | 15 | 83 |
| Macias 2011157 | APRI_F2_combined | APRI | F2 | < 0.5 and > 1.5 | 75 | 20 | 57 | 111 | ||
| Macias 2011157 | APRI_F2_high | APRI | F2 | 1.5 | 22 | 92 | 75 | 20 | 189 | 235 |
| Macias 2011157 | APRI_F2_low | F2 | 0.5 | 78 | 44 | 207 | 144 | 57 | 111 | |
| Macias 2011157 | Forns_F2_combined | Forns index | F2 | 84 | 31 | 37 | 66 | |||
| Macias 2011157 | Forns_F2_high | Forns index | F2 | 6.9 | 32 | 88 | 84 | 31 | 180 | 224 |
| Macias 2011157 | Forns_F2_low | Forns index | F2 | 4.2 | 86 | 26 | 227 | 189 | 37 | 66 |
| Martinez 2011158 | APRI_F2_combined | APRI | F2 | < 0.5 and > 1.5 | 108 | 8 | 21 | 57 | ||
| Martinez 2011158 | APRI_F2_high | APRI | F2 | 1.5 | 47 | 93 | 108 | 8 | 121 | 103 |
| Martinez 2011158 | APRI_F2_low | APRI | F2 | 0.5 | 91 | 51 | 208 | 54 | 21 | 57 |
| Martinez 2011158 | APRI_F4_combined | APRI | F4 | 1 and 2 | 61 | 19 | 22 | 160 | ||
| Martinez 2011158 | APRI_F4_high | APRI | F4 | 2 | 49 | 91 | 61 | 19 | 63 | 197 |
| Martinez 2011158 | APRI_F4_low | APRI | F4 | 1 | 82 | 74 | 102 | 56 | 22 | 160 |
| Martinez 2011158 | ELF_F2_combined | ELF | F2 | –0.45 and 1.07 | 108 | 11 | 23 | 58 | ||
| Martinez 2011158 | ELF_F2_high | ELF | F2 | 1.07 | 47 | 90 | 108 | 11 | 121 | 100 |
| Martinez 2011158 | ELF_F2_low | ELF | F2 | –0.45 | 90 | 52 | 206 | 53 | 23 | 58 |
| Martinez 2011158 | ELF_F4_combined | ELF | F4 | 0.06 and 1.73 | 64 | 22 | 12 | 114 | ||
| Martinez 2011158 | ELF_F4_high | ELF | F4 | 1.73 | 52 | 90 | 64 | 22 | 60 | 194 |
| Martinez 2011158 | ELF_F4_low | ELF | F4 | 0.06 | 90 | 53 | 112 | 102 | 12 | 114 |
| Martinez 2011158 | FIB4_F3_combined | FIB-4 | F3 | 1.45 and 3.25 | 84 | 17 | 12 | 118 | ||
| Martinez 2011158 | FIB4_F3_high | FIB-4 | F3 | 3.25 | 54 | 91 | 84 | 17 | 71 | 168 |
| Martinez 2011158 | FIB4_F3_low | FIB-4 | F3 | 1.45 | 92 | 64 | 143 | 67 | 12 | 118 |
| Martinez 2011158 | Forns_F2_combined | Forns index | F2 | 101 | 8 | 25 | 64 | |||
| Martinez 2011158 | Forns_F2_high | Forns index | F2 | 6.9 | 44 | 93 | 101 | 8 | 128 | 103 |
| Martinez 2011158 | Forns_F2_low | Forns index | F2 | 4.2 | 89 | 58 | 204 | 47 | 25 | 64 |
| Morikawa 2011159 | TE_F2 | Fibroscan | F2 | 10.1 | 88.6 | 86.5 | 42 | 7 | 5 | 47 |
| Morikawa 2011159 | TE_F3 | Fibroscan | F3 | 13.3 | 89.7 | 86.6 | 29 | 9 | 3 | 60 |
| Morikawa 2011159 | TE_F4 | Fibroscan | F4 | 16.3 | 85.7 | 85.4 | 14 | 12 | 2 | 73 |
| Murawaki 2001160 | PLT_F2 | Platelet count | F2 | 160 | 68 | 71 | 60 | 22 | 28 | 55 |
| Murawaki 2001160 | PLT_F3 | Platelet count | F3 | 140 | 68 | 74 | 33 | 10 | 15 | 27 |
| Murawaki 2001160 | typeIVcoll_F2 | Type IV collagen | F2 | 110 | 77 | 73 | 68 | 21 | 20 | 56 |
| Murawaki 2001160 | typeIVcoll_F3 | Type IV collagen | F3 | 130 | 66 | 75 | 32 | 9 | 16 | 28 |
| Myers 2002366 | FT_F2_combined | Fibrotest | F2 | 42 | 11 | 10 | 67 | |||
| Myers 2002366 | FT_F2_high | Fibrotest | F2 | 0.6 | 50 | 91 | 42 | 11 | 42 | 116 |
| Myers 2002366 | FT_F2_low | Fibrotest | F2 | 0.2 | 88 | 53 | 74 | 60 | 10 | 67 |
| Nitta 2009161 | TE_F2 | Fibroscan | F2 | 7.1 | 81 | 80 | 80 | 13 | 19 | 53 |
| Nitta 2009161 | TE_F3 | Fibroscan | F3 | 9.6 | 88 | 82 | 50 | 19 | 7 | 89 |
| Nitta 2009161 | TE_F4 | Fibroscan | F4 | 11.6 | 92 | 78 | 22 | 31 | 2 | 110 |
| Nunes 2005163 | APRI_F4_low | APRI | F4 | 0.75 | 74 | 68 | 13 | 12 | 5 | 27 |
| Nunes 2005163 | Forns_F4_low | Forns index | F4 | 3.9 | 61 | 62 | 11 | 15 | 7 | 24 |
| Nunes 2005163 | HA_F4 | Hyaluronic acid | F4 | 107 | 61 | 73 | 11 | 11 | 7 | 28 |
| Nunes 2005163 | PIIINP_F4 | PIIINP | F4 | 0.8 | 79 | 69 | 14 | 12 | 4 | 27 |
| Nunes 2005163 | PLT_F4 | Platelet count | F4 | 196 | 63 | 66 | 11 | 13 | 7 | 26 |
| Obara 2008164 | APRI_F2_low | APRI | F2 | 0.70 | 89 | 87 | 25 | 3 | 3 | 20 |
| Obara 2008164 | TE_F1 | Fibroscan | F1 | 5.6 | 93 | 78 | 7 | 9 | 1 | 34 |
| Obara 2008164 | TE_F2 | Fibroscan | F2 | 9.5 | 89 | 83 | 25 | 4 | 3 | 19 |
| Obara 2008164 | TE_F3 | Fibroscan | F3 | 10.3 | 94 | 69 | 15 | 11 | 1 | 24 |
| Obara 2008164 | TE_F4 | Fibroscan | F4 | 17.2 | 80 | 88 | 8 | 5 | 2 | 36 |
| Obara 2008164 | HA_F2 | Hyaluronic acid | F2 | 96.6 | 82 | 78 | 23 | 5 | 5 | 18 |
| Obara 2008164 | PLT_F2 | Platelet count | F2 | 122.0 | 64 | 96 | 18 | 1 | 10 | 22 |
| Obara 2008164 | typeIVcoll_F2 | Type IV collagen | F2 | 128.0 | 89 | 74 | 25 | 6 | 3 | 17 |
| Oliveira 2005165 | AST_ALT_ratio_F3 | AST–ALT ratio | F3 | 1 | 27 | 78 | 6 | 13 | 16 | 48 |
| Oliveira 2005165 | HA_F3 | Hyaluronic acid | F3 | 77 | 77 | 17 | 14 | 5 | 47 | |
| Paggi 2008167 | APRI_F3_combined | APRI | F3 | 1 and 2 | 58 | 22 | 34 | 189 | ||
| Paggi 2008167 | APRI_F3_high | APRI | F3 | 2 | 36 | 92 | 58 | 22 | 102 | 248 |
| Paggi 2008167 | APRI_F3_low | APRI | F3 | 1 | 70 | 79 | 137 | 81 | 34 | 189 |
| Parise 2006168 | APRI_F2_low | APRI | F2 | 0.7 | 85 | 66 | 73 | 41 | 13 | 79 |
| Parise 2006168 | AST_ALT_ratio_F2 | AST–ALT ratio | F2 | 0.8 | 52 | 61 | 45 | 47 | 41 | 73 |
| Parise 2006168 | AST_ALT_ratio_F4 | AST–ALT ratio | F4 | 1 | 36 | 82 | 16 | 29 | 28 | 133 |
| Parise 2006168 | HA_F2 | Hyaluronic acid | F2 | 34.2 | 85 | 71 | 73 | 35 | 13 | 85 |
| Parise 2006168 | HA_F4 | Hyaluronic acid | F4 | 78.6 | 91 | 81 | 40 | 31 | 4 | 131 |
| Park 2000169 | AST_ALT_ratio_F4 | AST–ALT ratio | F4 | 1 | 47 | 96 | 14 | 5 | 16 | 118 |
| Parkes 201127 | ELF_F3_combined | ELF | F3 | 78 | 35 | 17 | 149 | |||
| Parkes 201127 | ELF_F3_high | ELF | F3 | 10.22 | 70 | 85 | 78 | 35 | 33 | 201 |
| Parkes 201127 | ELF_F3_low | ELF | F3 | 9.59 | 85 | 63 | 94 | 87 | 17 | 149 |
| Patel 200925 | FibrospectII_F2 | FibroSpect II | F2 | 71 | 65 | 138 | 20 | 56 | 38 | |
| Patel 200925 | APRI_F2_combined | APRI | F2 | < 0.5 and > 1.5 | 90 | 98 | 9 | 1 | 1 | 46 |
| Patel 200925 | FIB4_F3_combined | FIB-4 | F3 | 1.45 and 3.25 | 75 | 95 | 6 | 3 | 2 | 54 |
| Patel 200925 | FibrospectII_F2 | FibroSpect II | F2 | 95 | 66 | 21 | 25 | 1 | 48 | |
| Patel 200925 | Forns_F2_combined | Forns index | F2 | 4.21 and 6.9 | 84 | 88 | 11 | 5 | 2 | 38 |
| Patel 200925 | FT_F2 | Fibrotest | F2 | 100 | 61 | 18 | 26 | 0 | 40 | |
| Patel 2011170 | TE_F2 | Fibroscan | F2 | 10.1 | 77 | 88 | 33 | 21 | 10 | 150 |
| Patel 2011170 | TE_F4 | Fibroscan | F4 | 11.7 | 94 | 88 | 17 | 24 | 1 | 172 |
| Pohl 2001171 | AST_ALT_ratio_F3 | AST–ALT ratio | F3 | 1 | 47 | 82 | 17 | 21 | 19 | 96 |
| Pohl 2001171 | Pohl_F3 | Pohl score | F3 | 41 | 99 | 16 | 1 | 22 | 116 | |
| Poynard 2012172 | FT_F2 | Fibrotest | F2 | 0.48 | 66 | 85 | 520 | 75 | 268 | 426 |
| Poynard 2012172 | FT_F4 | Fibrotest | F4 | 0.74 | 68 | 89 | 135 | 120 | 64 | 970 |
| Poynard 2012172 | TE_F2 | Fibroscan | F2 | 8.8 | 48 | 93 | 378 | 35 | 410 | 466 |
| Poynard 2012172 | TE_F4 | Fibroscan | F4 | 14.5 | 65 | 95 | 129 | 55 | 70 | 1036 |
| Prati 2011173 | TE_F4 | Fibroscan | F4 | 12 | 54 | 94 | 6 | 1 | 5 | 16 |
| Qui 2004174 | typeIVcpll_F4 | Type IV collagen | F4 | 190 | 77 | 72 | 45 | 23 | 13 | 59 |
| Reedy 1998175 | AST_ALT_ratio_F4 | AST–ALT ratio | F4 | 1 | 44 | 94 | 10 | 3 | 13 | 51 |
| Rossi 2003177 | FT_F2_combined | Fibrotest | F2 | 20 | 5 | 4 | 22 | |||
| Rossi 2003177 | FT_F2_high | Fibrotest | F2 | 0.6 | 42 | 94 | 20 | 5 | 28 | 72 |
| Rossi 2003177 | FT_F2_low | Fibrotest | F2 | 0.1 | 92 | 29 | 44 | 55 | 4 | 22 |
| Rossini 2009178 | APRI_F3_low | APRI | F3 | 0.6 | 14 | 3 | 4 | 19 | ||
| Rossini 2009178 | ARFI_F3 | ARFI | F3 | 2.11 | 15 | 2 | 3 | 20 | ||
| Rossini 2009178 | ARFI_F4 | ARFI | F4 | 2.33 | 9 | 7 | 1 | 23 | ||
| Said 2010179 | FT_F2 | Fibrotest | F2 | 0.5 | 85.1 | 72.2 | 40 | 5 | 7 | 13 |
| Said 2010179 | FT_F3 | Fibrotest | F3 | 0.52 | 92.5 | 53.8 | 24 | 18 | 2 | 21 |
| Said 2010179 | FT_F4 | Fibrotest | F4 | 0.75 | 85.7 | 70.7 | 6 | 17 | 1 | 41 |
| Saitu 2005180 | HA_F2 | Hyaluronic acid | F2 | 75.7 ng/ml | 75 | 81 | 58 | 6 | 19 | 26 |
| Saitu 2005180 | HA_F4 | Hyaluronic acid | F4 | 183.5 ng/ml | 80 | 80 | 24 | 16 | 6 | 63 |
| Saitu 2005180 | PIIINP_F2 | PIIINP | F2 | 0.835 U/ml | 78 | 75 | 60 | 8 | 17 | 24 |
| Saitu 2005180 | PIIINP_F4 | PIIINP | F4 | 0.995 U/ml | 77 | 66 | 23 | 27 | 7 | 52 |
| Saitu 2005180 | typeIVcoll_F2 | Type IV collagen | F2 | 5.75 ng/ml | 65 | 69 | 50 | 10 | 27 | 22 |
| Saitu 2005180 | typeIVcoll_F4 | Type IV collagen | F4 | 6.55 ng/ml | 60 | 61 | 18 | 31 | 12 | 48 |
| Sanvisens 2009181 | HA_F3 | Hyaluronic acid | F3 | 48 µg/l | 87 | 70 | 20 | 14 | 3 | 32 |
| Schiavon 2007182 | APRI_F2_combined | APRI | F2 | < 0.5 and > 1.5 | 21 | 11 | 6 | 84 | ||
| Schiavon 2007182 | APRI_F2_high | APRI | F2 | 0.95 | 44 | 93 | 21 | 11 | 27 | 144 |
| Schiavon 2007182 | APRI_F2_low | APRI | F2 | 0.4 | 88 | 54 | 42 | 71 | 6 | 84 |
| Schiavon 2007182 | APRI_F3_combined | APRI | F3 | 0.55 and 1.0 | 8 | 20 | 1 | 127 | ||
| Schiavon 2007182 | APRI_F3_high | APRI | F3 | 1 | 42 | 89 | 8 | 20 | 11 | 164 |
| Schiavon 2007182 | APRI_F3_low | APRI | F3 | 0.55 | 95 | 69 | 18 | 57 | 1 | 127 |
| Schiavon 2008183 | HA_F2_combined | Hyaluronic acid | F2 | 16 | 22 | 9 | 57 | |||
| Schiavon 2008183 | HA_F2_high | Hyaluronic acid | F2 | 36 | 84 | 16 | 22 | 29 | 118 | |
| Schiavon 2008183 | HA_F2_low | Hyaluronic acid | F2 | 80 | 41 | 36 | 83 | 9 | 57 | |
| Schiavon 2008183 | YKL_40_F2_combined | YKL-40 | F2 | 15 | 28 | 9 | 46 | |||
| Schiavon 2008183 | YKL_40_F2_high | YKL-40 | F2 | 33 | 80 | 15 | 28 | 30 | 112 | |
| Schiavon 2008183 | YKL-40_F2_low | YKL-40 | F2 | 80 | 33 | 36 | 94 | 9 | 46 | |
| Schneider 2006185 | 13CBT_F4 | 13Cmethacetin breath test | F4 | 13C50 1.7 | 83 | 63 | 16 | 24 | 3 | 40 |
| Schneider 2006185 | APRI_F2_low | APRI | F2 | 0.75 | 81 | 65 | 38 | 13 | 9 | 23 |
| Schneider 2006185 | APRI_F4_low | APRI | F4 | 1 | 77 | 63 | 15 | 24 | 4 | 40 |
| Sebastiani 201231 | APRI_F2_combined | APRI | F2 | < 0.5 and > 1.5 | 160 | 23 | 171 | 337 | ||
| Sebastiani 201231 | APRI_F2_high | APRI | F2 | 1.5 | 29 | 95 | 160 | 23 | 392 | 438 |
| Sebastiani 201231 | APRI_F2_low | APRI | F2 | 0.5 | 69 | 73 | 381 | 124 | 171 | 337 |
| Sebastiani 201231 | APRI_F4_combined | APRI | F4 | 1 and 2 | 46 | 63 | 29 | 711 | ||
| Sebastiani 201231 | APRI_F4_high | APRI | F4 | 2 | 41 | 93 | 46 | 63 | 67 | 837 |
| Sebastiani 201231 | APRI_F4_low | APRI | F4 | 1 | 74 | 79 | 84 | 189 | 29 | 711 |
| Sebastiani 201231 | Fibropaca_F2 | Fibropaca algorithm | F2 | 86 | 90 | 238 | 25 | 41 | 225 | |
| Sebastiani 201231 | Fibropaca_F4 | Fibropaca algorithm | F4 | 73 | 97 | 56 | 21 | 21 | 672 | |
| Sebastiani 201231 | Forns_F2_combined | Forns index | F2 | 4.2 and 6.9 | 337 | 157 | 33 | 92 | ||
| Sebastiani 201231 | Forns_F2_high | Forns index | F2 | 6.9 | 61 | 66 | 337 | 157 | 215 | 304 |
| Sebastiani 201231 | Forns_F2_low | Forns index | F2 | 4.2 | 94 | 20 | 519 | 369 | 33 | 92 |
| Sebastiani 201231 | FT_F2 | Fibrotest | F2 | 0.49 | 62 | 81 | 342 | 88 | 210 | 373 |
| Sebastiani 201231 | FT_F4 | Fibrotest | F4 | 0.75 | 30 | 89 | 34 | 99 | 79 | 801 |
| Sebastiani 201231 | Leroy_F2 | Leroy algorithm | F2 | 90 | 98 | 45 | 5 | 5 | 245 | |
| Sebastiani 201231 | SAFE_F2 | SAFE algorithm | F2 | 100 | 78 | 236 | 45 | 0 | 159 | |
| Sebastiani 201231 | SAFE_F4 | SAFE algorithm | F4 | 82 | 92 | 75 | 57 | 16 | 655 | |
| Sebastiani 2009187 | APRI_F2_combined | APRI | F2 | < 0.5 and > 1.5 | 255 | 40 | 306 | 810 | ||
| Sebastiani 2009187 | APRI_F2_high | APRI | F2 | 1.5 | 27 | 96 | 255 | 40 | 676 | 1064 |
| Sebastiani 2009187 | APRI_F2_low | APRI | F2 | 0.5 | 67 | 73 | 625 | 294 | 306 | 810 |
| Sebastiani 2009187 | APRI_F4_combined | APRI | F4 | 90 | 101 | 42 | 1542 | |||
| Sebastiani 2009187 | APRI_F4_high | APRI | F4 | 2 | 47 | 94 | 90 | 101 | 101 | 1743 |
| Sebastiani 2009187 | APRI_F4_low | APRI | F4 | 1 | 78 | 84 | 149 | 302 | 42 | 1542 |
| Sebastiani 2009187 | SAFE_F2 | SAFE algorithm | F2 | 100 | 77 | 517 | 99 | 0 | 330 | |
| Sebastiani 2009187 | SAFE_F4 | SAFE algorithm | F4 | 90 | 93 | 129 | 100 | 14 | 1415 | |
| Sebastiani 2006188 | FT_F4 | Fibrotest | F4 | 50 | 93 | 15 | 11 | 15 | 150 | |
| Sebastiani 2008 EALT186 | AST_ALT_ratio_F2 | AST–ALT ratio | F2 | 1 | 43 | 58 | 49 | 21 | 66 | 28 |
| Sebastiani 2008 EALT186 | Fibroindex_F2_combined | Fibroindex | F2 | 1.25 and 2.25 | 22 | 0 | 37 | 34 | ||
| Sebastiani 2008 EALT186 | Fibroindex_F2_high | Fibroindex | F2 | 2.25 | 19 | 100 | 22 | 0 | 93 | 49 |
| Sebastiani 2008 EALT186 | Fibroindex_F2_low | Fibroindex | F2 | 1.25 | 68 | 69 | 78 | 15 | 37 | 34 |
| Sebastiani 2008 EALT186 | Forns_F2_combined | Forns index | F2 | 4.2 and 6.9 | 24 | 1 | 17 | 24 | ||
| Sebastiani 2008 EALT186 | Forns_F2_high | Forns index | F2 | 6.9 | 21 | 98 | 24 | 1 | 91 | 48 |
| Sebastiani 2008 EALT186 | Forns_F2_low | Forns index | F2 | 4.2 | 85 | 49 | 98 | 25 | 17 | 24 |
| Sebastiani 2008 EALT186 | FT_F2 | Fibrotest | F2 | 0.49 | 82 | 72 | 94 | 14 | 21 | 35 |
| Sebastiani 2008 NALT186 | AST_ALT_ratio_F2 | AST–ALT ratio | F2 | 1 | 12 | 88 | 4 | 6 | 28 | 42 |
| Sebastiani 2008 NALT186 | Fibroindex_F2_combined | Fibroindex | F2 | 1.25 and 2.25 | 3 | 0 | 19 | 37 | ||
| Sebastiani 2008 NALT186 | Fibroindex_F2_high | Fibroindex | F2 | 2.25 | 10 | 100 | 3 | 0 | 29 | 48 |
| Sebastiani 2008 NALT186 | Fibroindex_F2_low | Fibroindex | F2 | 1.25 | 41 | 77 | 13 | 11 | 19 | 37 |
| Sebastiani 2008 NALT186 | Forns_F2_combined | Forns index | F2 | 4.2 and 6.9 | 16 | 0 | 14 | 33 | ||
| Sebastiani 2008 NALT186 | Forns_F2_high | Forns index | F2 | 6.9 | 50 | 100 | 16 | 0 | 16 | 48 |
| Sebastiani 2008 NALT186 | Forns_F2_low | Forns index | F2 | 4.2 | 57 | 67 | 18 | 15 | 14 | 33 |
| Sebastiani 2008 NALT186 | FT_F2 | Fibrotest | F2 | 0.49 | 67 | 85 | 21 | 7 | 11 | 41 |
| Sene 2006189 | APRI_F2_low | APRI | F2 | 0.38 | 72 | 75 | 38 | 18 | 27 | 55 |
| Sene 2006189 | FT_F2 | Fibrotest | F2 | 0.37 | 85 | 75 | 55 | 18 | 10 | 55 |
| Sene 2006189 | HA_F2 | Hyaluronic acid | F2 | 45 | 67 | 64 | 44 | 26 | 21 | 47 |
| Sene 2006189 | PLT_F2 | Platelet count | F2 | 182 | 57 | 79 | 37 | 15 | 28 | 58 |
| Sharabash 2009190 | FibrospectII_F2 | Fibrospect II | F2 | 72 | 83 | 86 | 5 | 2 | 1 | 12 |
| Shastry 2007191 | APRI_F3_low | APRI | F3 | 0.6 | 12 | 19 | 0 | 17 | ||
| Sheth 1997192 | AST_ALT_ratio_F4 | AST–ALT ratio | F4 | 1 | 53 | 100 | 25 | 0 | 22 | 92 |
| Singal 2011193 | APRI_F3_combined | APRI | F3 | 12 | 5 | 1 | 37 | |||
| Singal 2011193 | APRI_F3_combined | APRI | F3 | 7 | 6 | 3 | 36 | |||
| Singal 2011193 | APRI_F3_high | APRI | F3 | 1.5 | 48 | 94 | 12 | 5 | 13 | 76 |
| Singal 2011193 | APRI_F3_low | APRI | F3 | 0.5 | 96 | 46 | 24 | 44 | 1 | 37 |
| Singal 2011193 | APRI_F3_high | APRI | F3 | 1.5 | 29 | 93 | 7 | 6 | 17 | 75 |
| Singal 2011193 | APRI_F3_low | APRI | F3 | 0.5 | 88 | 44 | 21 | 45 | 3 | 36 |
| Sirli 2010194 | APRI_F2_low | APRI | F2 | 0.52 | 70 | 81 | 94 | 3 | 40 | 13 |
| Sirli 2010194 | FIB4_F2 | FIB-4 | F2 | 2.14 | 36 | 100 | 48 | 0 | 86 | 16 |
| Sirli 2010194 | FIB4_F4 | FIB-4 | F4 | 2.31 | 80 | 78 | 12 | 30 | 3 | 105 |
| Sirli 2010194 | Forns_F2_low | Forns index | F2 | 4.57 | 72 | 68 | 96 | 5 | 38 | 11 |
| Sirli 2010194 | Forns_F4 | Forns index | F4 | 5.93 | 100 | 74 | 15 | 35 | 0 | 100 |
| Sirli 2010194 | Lok_F2 | Lok’s index | F2 | 0.17 | 58 | 81 | 78 | 3 | 56 | 13 |
| Sirli 2010194 | Lok_F4_low | Lok’s index | F4 | 0.26 | 87 | 82 | 13 | 24 | 2 | 111 |
| Sirli 2010194 | PLT_F2 | Platelet count | F2 | 176 | 37 | 100 | 50 | 0 | 84 | 16 |
| Sirli 2010194 | PLT_F4 | Platelet count | F4 | 155 | 87 | 84 | 13 | 22 | 2 | 113 |
| Sirli 2010194 | TE_F2 | Fibroscan | F2 | 6.8 | 61 | 73 | 82 | 4 | 52 | 12 |
| Sirli 2010194 | TE_F4 | Fibroscan | F4 | 13.3 | 93 | 96 | 14 | 5 | 1 | 130 |
| Snyder 2007196 | FibrospectII_F2 | Fibrospect II | F2 | 55 | 82 | 77 | 41 | 10 | 9 | 33 |
| Snyder 2007196 | FibrospectII_F2_combined | Fibrospect II | F2 | 25 and 85 | 26 | 0 | 0 | 18 | ||
| Snyder 2006 prosp195 | APRI_F2_combined | APRI | F2 | < 0.5 and > 1.5 | 34 | 4 | 10 | 45 | ||
| Snyder 2006 prosp195 | APRI_F2_high | APRI | F2 | 1.5 | 44 | 94 | 34 | 4 | 44 | 68 |
| Snyder 2006 prosp195 | APRI_F2_low | APRI | F2 | 0.5 | 87 | 62 | 68 | 27 | 10 | 45 |
| Snyder 2006 prosp195 | APRI_F3_combined | APRI | F3 | 53 | 137 | 4 | 117 | |||
| Snyder 2006 prosp195 | APRI_F3_high | APRI | F3 | 1.2 | 83 | 73 | 41 | 28 | 8 | 74 |
| Snyder 2006 prosp195 | APRI_F3_low | APRI | F3 | 0.5 | 96 | 48 | 47 | 53 | 2 | 49 |
| Snyder 2006 prosp195 | APRI_F4_high | APRI | F4 | 2 | 50 | 94 | 13 | 7 | 13 | 118 |
| Snyder 2006 prosp195 | AST_ALT_ratio_F4 | AST–ALT ratio | F4 | 1 | 88 | 41 | 23 | 74 | 3 | 51 |
| Snyder 2006 retro195 | APRI_F2_combined | APRI | F2 | < 0.5 and > 1.5 | 54 | 7 | 28 | 94 | ||
| Snyder 2006 retro195 | APRI_F2_high | APRI | F2 | 1.5 | 31 | 96 | 54 | 7 | 120 | 169 |
| Snyder 2006 retro195 | APRI_F2_low | APRI | F2 | 0.5 | 83 | 54 | 148 | 80 | 28 | 94 |
| Snyder 2006 retro195 | APRI_F3_combined | APRI | F3 | 41 | 28 | 2 | 49 | |||
| Snyder 2006 retro195 | APRI_F3_high | APRI | F3 | 1.2 | 81 | 50 | 53 | 137 | 13 | 137 |
| Snyder 2006 retro195 | APRI_F3_low | APRI | F3 | 0.5 | 94 | 43 | 62 | 156 | 4 | 117 |
| Sohn 2010197 | PLTspleen_F2 | Platelet count–spleen diameter ratio | F2 | 2200 | 81.5 | 71.4 | 28 | 4 | 6 | 10 |
| Sporea 2008199 | TE_F2 | Fibroscan | F2 | 6.8 | 59.6 | 93.3 | 97 | 2 | 64 | 28 |
| Sporea 2010200 | TE_F2 | Fibroscan | F2 | 6.8 | 60 | 88 | 167 | 5 | 111 | 34 |
| Sporea 2010200 | TE_F3 | Fibroscan | F3 | 8.6 | 62 | 81 | 82 | 35 | 50 | 150 |
| Sporea 2010200 | TE_F4 | Fibroscan | F4 | 13.3 | 77 | 93 | 30 | 19 | 9 | 259 |
| Sporea 2011201 | TE_F2 | Fibroscan | F2 | 6.7 | 77.5 | 86.7 | 117 | 6 | 35 | 39 |
| Sporea 2011201 | TE_F4 | Fibroscan | F4 | 12.2 | 96.2 | 89.6 | 96 | 10 | 4 | 87 |
| Sporea 2011198 | ARFI_F1 | ARFI | F1 | 1.19 | 68.5 | 83.3 | 433 | 9 | 204 | 45 |
| Sporea 2011198 | ARFI_F2 | ARFI | F2 | 1.29 | 79.7 | 87.5 | 367 | 28 | 92 | 204 |
| Sporea 2011198 | ARFI_F3 | ARFI | F3 | 1.57 | 90.2 | 85.3 | 280 | 57 | 31 | 323 |
| Sporea 2011198 | ARFI_F4 | ARFI | F4 | 1.59 | 83.7 | 80 | 144 | 104 | 28 | 415 |
| Sterling 2006 Total cohort202 | FIB4_F2_combined | FIB-4 | F2 | 366 | 126 | 43 | 71 | |||
| Sterling 2006 Total cohort202 | FIB4_F2_high | FIB-4 | F2 | 1 | 69.4 | 58.4 | 366 | 126 | 161 | 177 |
| Sterling 2006 Total cohort202 | FIB4_F2_low | FIB-4 | F2 | 0.6 | 91.8 | 23.4 | 484 | 232 | 43 | 71 |
| Sterling 2006 Total cohort202 | FIB4_F3_combined | FIB-4 | F3 | 40 | 22 | 58 | 467 | |||
| Sterling 2006 Total cohort202 | FIB4_F3_high | FIB-4 | F3 | 3.25 | 23 | 96.6 | 40 | 22 | 134 | 634 |
| Sterling 2006 Total cohort202 | FIB4_F3_low | FIB-4 | F3 | 1.45 | 66.7 | 71.2 | 116 | 189 | 58 | 467 |
| Sterling 2006 Validation202 | FIB4_F2_high | FIB-4 | F2 | 1 | 64.5 | 57.1 | 110 | 45 | 62 | 60 |
| Sterling 2006 Validation202 | FIB4_F2_low | FIB-4 | F2 | 0.6 | 89.5 | 23.8 | 153 | 80 | 19 | 25 |
| Sterling 2006 Validation202 | FIB4_F3_high | FIB-4 | F3 | 3.25 | 21.7 | 96.8 | 13 | 6 | 48 | 210 |
| Sterling 2006 Validation202 | FIB4_F3_low | FIB-4 | F3 | 1.45 | 70 | 73.7 | 43 | 56 | 18 | 160 |
| Stibbe 2011203 | FIB4_F3_combined | FIB-4 | F3 | 5 | 0 | 5 | 16 | |||
| Stibbe 2011203 | FIB4_F3_high | FIB-4 | F3 | 3.25 | 28 | 100 | 5 | 0 | 13 | 23 |
| Stibbe 2011203 | FIB4_F3_low | FIB-4 | F3 | 1.45 | 72 | 70 | 13 | 7 | 5 | 16 |
| Sud 2009204 | FPI_F2_high | FPI | F2 | 0.8 | 43 | 94 | 36 | 6 | 48 | 86 |
| Sud 2009204 | FPI_F2_low | FPI | F2 | 0.2 | 96 | 44 | 81 | 52 | 3 | 40 |
| Sud 2009204 | FPI_F2_high | FPI | F2 | 0.8 | 42 | 98 | 31 | 1 | 43 | 51 |
| Sud 2009204 | FPI_F2_low | FPI | F2 | 0.2 | 85 | 48 | 63 | 27 | 11 | 25 |
| Testa 2006205 | Aminobreathtest_F2 | Aminobreath test | Ishak F2 | 8.1 | 73 | 73.7 | 27 | 10 | 10 | 28 |
| Testa 2006205 | PLTspleen_F2 | Platelet count–spleen diameter ratio | Ishak F2 | 1750 | 78.4 | 78.9 | 29 | 8 | 8 | 30 |
| Thompson 2009208 | FT_F2 | Fibrotest | F2 | 65 | 63 | 70 | 102 | 37 | 175 | |
| Thompson 2009208 | FT_F3 | Fibrotest | F3 | 67 | 38 | 57 | 185 | 28 | 114 | |
| Thompson 2009208 | FT_F3 | Fibrotest | F3 | 35 | 84 | 13 | 56 | 24 | 291 | |
| Thompson 2009208 | Hepascore_F2 | Hepascore | F2 | 73 | 78 | 86 | 29 | 191 | ||
| Thompson 2009208 | Hepascore_F3 | Hepascore | F3 | 74 | 105 | 11 | 194 | |||
| Thompson 2009208 | Hepascore_F4 | Hepascore | F4 | 22 | 56 | 15 | 291 | |||
| Thompson 2009208 | FT_F2 | Fibrotest | F2 | 62 | 60 | 29 | 35 | 18 | 52 | |
| Thompson 2009208 | Hepascore_F2 | Hepascore | F2 | 71 | 62 | 33 | 33 | 14 | 54 | |
| Thompson 2010207 | SAFE_F2 | SAFE algorithm | F2 | 100 | 80 | 422 | 360 | 0 | 1439 | |
| Thompson 2010207 | SAFE_F4 | SAFE algorithm | F4 | 52 | 92 | 64 | 168 | 60 | 1929 | |
| Toniutto 2007209 | APRI_F2_high | APRI | F2 | 1.4 | 76 | 77 | 25 | 16 | 8 | 53 |
| Trang 2008210 | APRI_F2_combined | APRI | F2 | < 0.5 and > 1.5 | 18 | 4 | 3 | 9 | ||
| Trang 2008210 | APRI_F2_high | APRI | F2 | 1.5 | 36 | 87.1 | 18 | 4 | 32 | 27 |
| Trang 2008210 | APRI_F2_low | APRI | F2 | 0.42 | 94 | 29 | 47 | 22 | 3 | 9 |
| Trang 2008210 | APRI_F3_combined | APRI | F3 | 8 | 5 | 3 | 24 | |||
| Trang 2008210 | APRI_F3_high | APRI | F3 | 1.85 | 28 | 90.3 | 8 | 5 | 20 | 48 |
| Trang 2008210 | APRI_F3_low | APRI | F3 | 0.71 | 89.3 | 45.3 | 25 | 29 | 3 | 24 |
| Trang 2008210 | FIB4_F2_combined | FIB-4 | F2 | 25 | 6 | 8 | 18 | |||
| Trang 2008210 | FIB4_F2_high | FIB-4 | F2 | 2.05 | 50 | 81.3 | 25 | 6 | 25 | 25 |
| Trang 2008210 | FIB4_F2_low | FIB-4 | F2 | 1.39 | 84 | 58.1 | 42 | 13 | 8 | 18 |
| Trang 2008210 | FIB4_F3_combined | FIB-4 | F3 | 9 | 5 | 1 | 25 | |||
| Trang 2008210 | FIB4_F3_high | FIB-4 | F3 | 3.25 | 33.3 | 90.7 | 9 | 5 | 19 | 48 |
| Trang 2008210 | FIB4_F3_low | FIB-4 | F3 | 1.45 | 96.4 | 47.2 | 27 | 28 | 1 | 25 |
| Trifan 2009211 | Lok_F2 | Lok’s index | F2 | –1.67 | 80.7 | 32.2 | 121 | 109 | 57 | 25 |
| Trifan 2009211 | Lok_F2 | Lok’s index | F2 | –0.63 | 55 | 66.8 | 29 | 85 | 24 | 174 |
| Trifan 2009211 | APRI_F2 | APRI | F2 | 0.75 | 61 | 71.3 | 52 | 82 | 126 | 52 |
| Trifan 2009211 | APRI_F3 | APRI | F3 | 0.76 | 72 | 59 | 38 | 106 | 15 | 153 |
| Trifan 2009211 | FIB4_F2 | FIB-4 | F2 | 1.66 | 52.5 | 75 | 45 | 70 | 134 | 64 |
| Trifan 2009211 | FIB4_F3 | FIB-4 | F3 | 1.36 | 83 | 53 | 44 | 122 | 9 | 137 |
| Trifan 2009211 | FT_F2 | Fibrotest | F2 | 0.53 | 49.1 | 81.6 | 32 | 66 | 146 | 68 |
| Trifan 2009211 | FT_F3 | Fibrotest | F3 | 0.45 | 86 | 56.3 | 46 | 114 | 7 | 145 |
| Trifan 2009211 | Forns_F2 | Forns index | F2 | 5.35 | 61.2 | 70.5 | 53 | 82 | 125 | 52 |
| Trifan 2009211 | Forns_F3 | Forns index | F3 | 6.38 | 56 | 80.3 | 30 | 52 | 23 | 207 |
| Trifan 2009211 | TE_F2 | Fibroscan | F2 | 8.65 | 61 | 74.3 | 46 | 82 | 132 | 52 |
| Trifan 2009211 | TE_F3 | Fibroscan | F3 | 12.5 | 94 | 84.2 | 50 | 41 | 3 | 218 |
| Trocme 2006212 | FI_F2 | Fibrosis Index | F2 | 82 | 70 | 43 | 22 | 9 | 50 | |
| Trocme 2006212 | FI_F3 | Fibrosis Index | F3 | 82 | 80 | 21 | 11 | 5 | 42 | |
| Tural 2009213 | APRI_F3_combined | APRI | F3 | 0.5, 1.5 | 47 | 35 | 6 | 64 | ||
| Tural 2009213 | APRI_F3_high | APRI | F3 | 1.5 | 47 | 35 | 47 | 195 | ||
| Tural 2009213 | APRI_F3_low | APRI | F3 | 0.5 | 88 | 166 | 6 | 64 | ||
| Tural 2009213 | FIB4_F3_combined | FIB-4 | F3 | 1.45, 3.25 | 22 | 8 | 20 | 156 | ||
| Tural 2009213 | FIB4_F3_high | F3 | 3.25 | 22 | 8 | 72 | 222 | |||
| Tural 2009213 | FIB4_F3_low | 1.45 | 74 | 74 | 20 | 156 | ||||
| Tural 2009213 | Forns_F3_combined | Forns index | F3 | 4.2, 6.9 | 37 | 22 | 10 | 70 | ||
| Tural 2009213 | Forns_F3_high | Forns index | F3 | 6.9 | 37 | 22 | 57 | 208 | ||
| Tural 2009213 | Forns_F3_low | Forns index | F3 | 4.2 | 84 | 160 | 10 | 70 | ||
| Vallet-Pichard 2007214 | FIB4_F3_combined | FIB-4 | F3 | 55 | 14 | 38 | 561 | |||
| Vallet-Pichard 2007214 | FIB4_F3_high | FIB-4 | F3 | 3.25 | 37.6 | 98.2 | 55 | 14 | 91 | 687 |
| Vallet-Pichard 2007214 | FIB4_F3_low | FIB-4 | F3 | 1.45 | 74.3 | 80.1 | 108 | 140 | 38 | 561 |
| Valva 2011215 | HA_F2 | Hyaluronic acid | F2 | 103.1 | 66.7 | 90 | 8 | 1 | 4 | 9 |
| Valva 2011215 | HA_F2 | Hyaluronic acid | F2 | 109.7 | 100 | 82.3 | 5 | 3 | 0 | 14 |
| Valva 2011215 | PIIINP_F2 | PIIINP | F2 | 9.1 | 75 | 80 | 9 | 2 | 3 | 8 |
| Valva 2011215 | PIIINP_F3 | PIIINP | F3 | 9.1 | 100 | 64.7 | 5 | 6 | 0 | 11 |
| Varaut 2005216 | FT_F2_high | Fibrotest | F2 | 0.6 | 24 | 92 | 12 | 5 | 38 | 55 |
| Varaut 2005216 | FT_F2_low | Fibrotest | F2 | 0.2 | 84 | 45 | 42 | 33 | 8 | 27 |
| Wai 2003 training218 | APRI_F2_combined | APRI | F2 | < 0.5 and > 1.5 | 37 | 5 | 8 | 47 | ||
| Wai 2003 training218 | APRI_F2_high | APRI | Ishak F3 | 1.5 | 41 | 95 | 37 | 5 | 54 | 96 |
| Wai 2003 training218 | APRI_F2_low | APRI | Ishak F3 | 0.5 | 91 | 47 | 83 | 54 | 8 | 47 |
| Wai 2003 training218 | APRI_F4_combined | APRI | Ishak F5 | 16 | 11 | 3 | 123 | |||
| Wai 2003 training218 | APRI_F4_high | APRI | Ishak F5 | 2 | 57 | 93 | 16 | 11 | 12 | 153 |
| Wai 2003 training218 | APRI_F4_low | APRI | Ishak F5 | 1 | 89 | 75 | 25 | 41 | 3 | 123 |
| Wai 2003 validation218 | APRI_F2_combined | APRI | Ishak F3 | < 0.5 and > 1.5 | 35 | 4 | 4 | 35 | ||
| Wai 2003 validation218 | APRI_F2_high | APRI | Ishak F3 | 1.5 | 71 | 86 | 35 | 4 | 14 | 25 |
| Wai 2003 validation218 | APRI_F2_low | APRI | Ishak F3 | 0.5 | 86 | 71 | 25 | 14 | 4 | 35 |
| Wai 2003 validation218 | APRI_F4_combined | APRI | Ishak F5 | 8 | 5 | 0 | 65 | |||
| Wai 2003 validation218 | APRI_F4_high | APRI | Ishak F5 | 2 | 73 | 92 | 8 | 5 | 3 | 62 |
| Wai 2003 validation218 | APRI_F4_low | APRI | Ishak F5 | 1 | 100 | 88 | 4 | 9 | 0 | 65 |
| Westin 2008219 | GUCI_F2 | GUCI | Ishak F3 | 0.33 | 68 | 72 | 36 | 49 | 17 | 128 |
| Westin 2008219 | GUCI_F2 | GUCI | Ishak F3 | 1.11 | 59 | 80 | 60 | 25 | 42 | 103 |
| Westin 2008219 | GUCI_F4 | GUCI | Ishak F5 | 0.33 | 100 | 87 | 6 | 29 | 0 | 195 |
| Westin 2008219 | GUCI_F4 | GUCI | Ishak F5 | 1.11 | 44 | 89 | 14 | 21 | 18 | 177 |
| Wilson 2006220 | APRI_F2_combined | APRI | Ishak F3 | < 0.5 and > 1.5 | 2 | 7 | 3 | 68 | ||
| Wilson 2006220 | APRI_F2_high | APRI | Ishak F3 | 1.5 | 18 | 94 | 2 | 7 | 9 | 109 |
| Wilson 2006220 | APRI_F2_low | APRI | Ishak F3 | 0.5 | 73 | 59 | 8 | 48 | 3 | 68 |
| Wilson 2006220 | FT_F2_combined | Fibrotest | Ishak F3 | 6 | 41 | 1 | 57 | |||
| Wilson 2006220 | FT_F2_high | Fibrotest | Ishak F3 | 0.48 | 56 | 65 | 6 | 41 | 5 | 75 |
| Wilson 2006220 | FT_F2_low | Fibrotest | Ishak F3 | 0.31 | 89 | 49 | 9 | 59 | 1 | 57 |
| Wong 1998221 | HA_F4 | Hyaluronic acid | F4 | 86 | 88 | 18 | 13 | 3 | 96 | |
| Zaman 2004222 | FibrospectII_F2 | Fibrospect II | F2 | 71.8 | 73.9 | 28 | 18 | 11 | 51 | |
| Zarski 2012223 | APRI_F2_low | APRI | F2 | 0.5 | 33.1 | 96.6 | 59 | 6 | 119 | 198 |
| Zarski 2012223 | APRI_F4_high | APRI | F4 | 2 | 77.1 | 99.7 | 93 | 0 | 28 | 261 |
| Zarski 2012223 | Fibrometer_F2 | FibroMeter | F2 | 0.411 | 87.6 | 56.4 | 157 | 90 | 21 | 114 |
| Zarski 2012223 | Fibrometer_F4 | FibroMeter | F4 | 0.88 | 69.6 | 88.7 | 85 | 29 | 36 | 232 |
| Zarski 2012223 | FT_F2_high | Fibrotest | F2 | 0.48 | 75.8 | 66.2 | 135 | 69 | 43 | 135 |
| Zarski 2012223 | FT_F4 | Fibrotest | F4 | 0.74 | 71.4 | 81 | 86 | 50 | 35 | 211 |
| Zarski 2012223 | Hepascore_F2 | Hepascore | F2 | 0.5 | 74.7 | 72.5 | 134 | 57 | 45 | 147 |
| Zarski 2012223 | Hepascore_F4 | Hepascore | F4 | 0.84 | 76.8 | 81.3 | 93 | 50 | 28 | 211 |
| Zarski 2012223 | TE_F2 | Fibroscan | F2 | 5.2 | 96.6 | 34.8 | 173 | 133 | 5 | 71 |
| Zarski 2012223 | TE_F4 | Fibroscan | F4 | 12.9 | 76.8 | 89.6 | 93 | 26 | 28 | 235 |
| Ziol 2005224 | TE_F2 | Fibroscan | F2 | 8.8 | 56 | 91 | 91 | 8 | 72 | 80 |
| Ziol 2005224 | TE_F3 | Fibroscan | F3 | 9.6 | 86 | 85 | 65 | 26 | 11 | 149 |
| Ziol 2005224 | TE_F4 | Fibroscan | F4 | 14.6 | 86 | 96 | 42 | 8 | 7 | 194 |
| Study ID | Test | Index test assessed | Fibrosis stage assessed | Cut-off | Sens. | Spec. | TP | FP | FN | TN |
|---|---|---|---|---|---|---|---|---|---|---|
| Castera 2011225 | APRI_F2_combined | APRI | F2 | 62 | 100 | 6 | 0 | 17 | 10 | |
| Castera 2011225 | APRI_F2_high | APRI | F2 | > 1.5 | 14 | 100 | 6 | 0 | 38 | 16 |
| Castera 2011225 | APRI_F2_low | APRI | F2 | < 0.5 | 62 | 64 | 27 | 6 | 17 | 10 |
| Castera 2011225 | APRI_F4_combined | APRI | F4 | 47 | 96 | 2 | 2 | 8 | 36 | |
| Castera 2011225 | APRI_F4_high | APRI | F4 | > 2 | 13 | 96 | 2 | 2 | 13 | 43 |
| Castera 2011225 | APRI_F4_low | APRI | F4 | < 1 | 47 | 80 | 7 | 9 | 8 | 36 |
| Castera 2011225 | Fibrotest_F2 | Fibrotest | F2 | 0.48 | 62 | 81 | 27 | 3 | 17 | 13 |
| Castera 2011225 | Fibrotest_F4 | Fibrotest | F4 | 0.74 | 47 | 91 | 7 | 4 | 8 | 41 |
| Castera 2011225 | TE_F2 | Fibroscan | F2 | 7.1 | 68 | 63 | 30 | 6 | 14 | 10 |
| Castera 2011225 | TE_F4 | Fibroscan | F3 | 9.6 | 87 | 80 | 13 | 9 | 2 | 36 |
| Chan 2009226 | TE_F1 | Fibroscan | F1 | 72 | 80 | 6 | 30 | 3 | 122 | |
| Chan 2009226 | TE_F3 | Fibroscan | F3 | 84 | 76 | 32 | 30 | 6 | 93 | |
| Chan 2009226 | TE_F4 | Fibroscan | F3 | 60 | 93 | 24 | 8 | 16 | 113 | |
| Chen 2012227 | TE_F4 | Fibroscan | F4 | 10.4 | 93 | 71 | 69 | 70 | 5 | 171 |
| Fraquelli 2011116 | TE_F2 | Fibroscan | F2 | 77 | 77 | 42 | 11 | 12 | 38 | |
| Fraquelli 2011116 | TE_F4 | Fibroscan | F4 | 1 | 83 | 16 | 14 | 0 | 73 | |
| Fung 2011230 | TE_F2 | Fibroscan | F2 | 83 | 63 | 9 | 10 | 2 | 17 | |
| Fung 2011230 | TE_F4 | Fibroscan | F4 | 100 | 68 | 0 | 12 | 0 | 26 | |
| Gaia 2011119 | TE_F3 | Fibroscan | F3 | 64 | 84 | 22 | 16 | 4 | 28 | |
| Gaia 2011119 | TE_F4 | Fibroscan | F4 | 48 | 87 | 19 | 25 | 3 | 23 | |
| Gaia 2011119 | TE_F1 | Fibroscan | F1 | 61 | 72 | 27 | 13 | 10 | 20 | |
| Ganne-Carrie 2006120 | TE_F4 | Fibroscan | F4 | 10.4 | 82 | 85 | 13 | 16 | 3 | 90 |
| Gui 2010231 | Fibrotest_F1 | Fibrotest | F1 | 70 | 63 | 31 | 16 | 12 | 28 | |
| Gui 2010231 | Fibrotest_F3 | Fibrotest | F3 | 72 | 67 | 62 | 29 | 24 | 58 | |
| Hongbo 2007233 | APRI_F2_low | APRI | F2 | 0.4 | 72 | 74.56 | 68 | 58 | 26 | 170 |
| Hongbo 2007233 | APRI_F1_low | APRI | F1 | 0.4 | 70 | 82.93 | 60 | 6 | 26 | 30 |
| Hui 2005235 | Hui_F3 | Hui index | F3 | 93 | 49 | 86 | 28 | 6 | 27 | |
| Hui 2005 – validation235 | Hui_F3 | Hui index | F3 | 75 | 53 | 30 | 16 | 10 | 19 | |
| Kim 2009238 | TE_F4 | Fibroscan | F4 | 64 | 75 | 25 | 13 | 14 | 39 | |
| Kim 2009239 | API_F4 | Age-Platelet Index | F4 | 4.5 | 76.1 | 71.4 | 51 | 18 | 16 | 45 |
| Kim 2010236 | TE_F4 | Fibroscan | F4 | 10.1 | 76.1 | 81 | 51 | 12 | 16 | 51 |
| Kim 2007237 | AAR_F4 | AST–ALT ratio | F4 | 1 | 52 | 72 | 41 | 75 | 38 | 192 |
| Kim 2007237 | API_F4 | Age-Platelet Index | F4 | 4 | 88.6 | 74.1 | 70 | 69 | 9 | 198 |
| Kim 2007237 | APRI_F4_combined | APRI | F4 | 16 | 20 | 34 | 210 | |||
| Kim 2007237 | APRI_F4_high | APRI | F4 | 2 | 20.3 | 92.5 | 16 | 20 | 63 | 247 |
| Kim 2007237 | APRI_F4_low | APRI | F4 | 1 | 57 | 78.6 | 45 | 57 | 34 | 210 |
| Kim 2010236 | FIB4_F3_combined | FIB-4 | F3 | 127 | 7 | 29 | 260 | |||
| Kim 2010236 | FIB4_F3_high | FIB-4 | F3 | > 2.65 | 38.5 | 97.9 | 127 | 7 | 203 | 331 |
| Kim 2010236 | FIB4_F3_low | FIB-4 | F3 | < 1 | 91.2 | 72.8 | 301 | 78 | 29 | 260 |
| Kim 2010236 | FIB4_F4_combined | FIB-4 | F4 | 69 | 7 | 27 | 368 | |||
| Kim 2010236 | FIB4_F4_high | FIB-4 | F4 | 3.6 | 30 | 98.4 | 69 | 7 | 161 | 431 |
| Kim 2010236 | FIB4_F4_low | FIB-4 | F4 | 1.6 | 88.2 | 84 | 203 | 70 | 27 | 368 |
| Kwok 2009240 | TE_F3 | Fibroscan | F3 | 80 | 88.6 | 5 | 3 | 1 | 26 | |
| Lee 2011241 | CDS_F1 | CDS | F1 | 4 | 28 | 90.2 | 17 | 6 | 43 | 55 |
| Lee 2011241 | Lok’s model_F1 | Lok’s index | F1 | 0.87 | 48.3 | 90.2 | 29 | 6 | 31 | 55 |
| Lee 2011241 | TE_F2 | Fibroscan | F2 | 44.9 | 100 | 66 | 0 | 81 | 61 | |
| Lee 2011241 | TE_F4 | Fibroscan | F4 | 100 | 58.7 | 24 | 76 | 0 | 108 | |
| Lesmana 2011242 | TE_F2 | Fibroscan | F2 | 60.3 | 63.6 | 44 | 16 | 29 | 28 | |
| Lesmana 2011242 | TE_F3 | Fibroscan | F3 | 65.5 | 80.7 | 18 | 17 | 10 | 72 | |
| Li 2012243 | HA_F2 | Hyaluronic acid | F2 | 185.3 | 84 | 83 | 48 | 5 | 9 | 25 |
| Liu 2011244 | AAR_F2 | AST–ALT ratio | F2 | 0.67 | 57.2 | 58.7 | 123 | 169 | 92 | 239 |
| Liu 2011244 | API_F2 | Age–Platelet Index | F2 | 3 | 67.9 | 62 | 146 | 155 | 69 | 253 |
| Liu 2011244 | FIB4_F2_low | FIB-4 | F2 | 1.1 | 73.5 | 68.1 | 158 | 130 | 57 | 278 |
| Mallet 2009245 | FIB4_F3_low | FIB-4 | F3 | 0.67 | 0.71 | 0.73 | 30 | 28 | 11 | 69 |
| Marcellin 2009246 | TE_F2 | Fibroscan | F2 | 70 | 83 | 61 | 15 | 26 | 71 | |
| Marcellin 2009246 | TE_F3 | Fibroscan | F3 | 86 | 85 | 37 | 20 | 6 | 111 | |
| Marcellin 2009246 | TE_F4 | Fibroscan | F4 | 93 | 87 | 13 | 21 | 1 | 138 | |
| Miailhes 2011247 | Fibrotest_F2 | Fibrotest | F2 | 0.38 | 77 | 86 | 28 | 3 | 8 | 18 |
| Miailhes 2011247 | Fibrotest_F3 | Fibrotest | F3 | 0.42 | 94 | 77 | 19 | 9 | 1 | 28 |
| Miailhes 2011247 | Fibrotest_F4 | Fibrotest | F4 | 0.58 | 100 | 81 | 12 | 9 | 0 | 36 |
| Miailhes 2011247 | TE_F2 | Fibroscan | F2 | 81 | 87 | 29 | 3 | 7 | 18 | |
| Miailhes 2011247 | TE_F3 | Fibroscan | F3 | 85 | 87 | 17 | 5 | 3 | 32 | |
| Miailhes 2011247 | TE_F4 | Fibroscan | F4 | 92 | 94 | 11 | 3 | 1 | 42 | |
| Myers 2003249 | Fibrotest_F2 | Fibrotest | F2 | 0.4 | 54 | 80 | 33 | 30 | 28 | 118 |
| Ogawa 2011250 | TE_F1 | Fibroscan | F1 | 66 | 71 | 25 | 2 | 13 | 4 | |
| Ogawa 2011250 | TE_F2 | Fibroscan | F2 | 95 | 74 | 19 | 6 | 1 | 18 | |
| Ogawa 2011250 | TE_F3 | Fibroscan | F3 | 87 | 75 | 7 | 9 | 1 | 27 | |
| Ogawa 2011250 | TE_F4 | Fibroscan | F4 | 75 | 89 | 3 | 4 | 1 | 36 | |
| Osakabe 2011251 | TE_F2 | Fibroscan | F2 | 73 | 100 | 33 | 0 | 12 | 6 | |
| Osakabe 2011251 | TE_F3 | Fibroscan | F3 | 70 | 87 | 19 | 3 | 8 | 21 | |
| Osakabe 2011251 | TE_F4 | Fibroscan | F4 | 79 | 92 | 11 | 3 | 3 | 34 | |
| Park 2003253 | collIV_F4 | Type IV collagen | F4 | 6.3 | 63.6 | 88.6 | 7 | 10 | 4 | 79 |
| Park 2003253 | HA_F4 | Hyaluronic acid | F4 | 77 | 81.8 | 87.3 | 9 | 11 | 2 | 78 |
| Park 2004254 | AAR_F4 | AST–ALT ratio | F4 | 1 | 39 | 76 | 32 | 58 | 50 | 183 |
| Park 2005252 | Caffeine Breath test_F3 | Caffeine breath test | F3 | 100 | 72 | 12 | 10 | 0 | 26 | |
| Poynard 2009255 | Fibrotest_F2 | Fibrotest | F2 | 0.48 | 66 | 69 | 112 | 90 | 58 | 202 |
| Raftopoulos 2012256 | APRI_F2_combined | APRI | F2 | 21 | 2 | 16 | 68 | |||
| Raftopoulos 2012256 | APRI_F2_high | APRI | F2 | 1.5 | 28 | 98 | 21 | 2 | 54 | 102 |
| Raftopoulos 2012256 | APRI_F2_low | APRI | F2 | 0.5 | 79 | 65 | 59 | 36 | 16 | 68 |
| Raftopoulos 2012256 | APRI_F4_low | APRI | F4 | 1.0 | 10 | 31 | 5 | 133 | ||
| Raftopoulos 2012256 | Fibrotest_F2 | Fibrotest | F2 | 0.48 | 54 | 82 | 41 | 19 | 35 | 85 |
| Raftopoulos 2012256 | Fibrotest_F4 | Fibrotest | F4 | 0.73 | 12 | 18 | 3 | 146 | ||
| Raftopoulos 2012256 | Hepascore_F2 | Hepascore | F2 | 0.5 | 79 | 94 | 59 | 27 | 16 | 77 |
| Raftopoulos 2012256 | Hepascore_F4 | Hepascore | F4 | 0.87 | 13 | 25 | 2 | 139 | ||
| Sebastiani 2007257 | AAR_F4 | AST–ALT ratio | F4 | 1 | 7 | 95 | 2 | 4 | 20 | 84 |
| Sebastiani 2007257 | APRI_F2_high | APRI | F2 | 1.5 | 27 | 96 | 20 | 1 | 55 | 34 |
| Sebastiani 2007257 | APRI_F2_low | APRI | F2 | 0.5 | 71 | 87 | 53 | 5 | 22 | 30 |
| Sebastiani 2007257 | APRI_F4_high | APRI | F4 | 2 | 43 | 85 | 9 | 13 | 13 | 75 |
| Sebastiani 2007257 | Fibrotest_F2 | Fibrotest | F2 | 81 | 90 | 61 | 4 | 14 | 32 | |
| Sebastiani 2007257 | Fibrotest_F4 | Fibrotest | F4 | 56 | 96 | 12 | 4 | 10 | 84 | |
| Sebastiani 2007257 | Forns_F2_combined | Forns index | F2 | 11 | 0 | 32 | 27 | |||
| Sebastiani 2007257 | Forns_F2_high | Forns index | F2 | 6.9 | 15 | 100 | 11 | 0 | 64 | 35 |
| Sebastiani 2007257 | Forns_F2_low | Forns index | F2 | 4.2 | 58 | 78 | 44 | 8 | 32 | 27 |
| Sebastiani 2007257 | GUCI_F2 | GUCI | F2 | 0.2 | 67 | 96 | 50 | 1 | 25 | 34 |
| Sebastiani 2007257 | GUCI_F4 | GUCI | F4 | 1 | 21 | 91 | 5 | 8 | 17 | 80 |
| Sebastiani 2007257 | Hui_F2 | Hui index | F2 | 0.15 | 50 | 91 | 38 | 3 | 38 | 32 |
| Seto 2011258 | APGA_F2 | APGA | F2 | 6.7 | 17 | 98 | 13 | 3 | 64 | 157 |
| Seto 2011258 | APRI_F2_combined | APRI | F2 | 30 | 19 | 8 | 64 | |||
| Seto 2011258 | APRI_F2_high | APRI | F2 | 1.5 | 39 | 88 | 30 | 19 | 47 | 141 |
| Seto 2011258 | APRI_F2_low | APRI | F2 | 0.5 | 89 | 40 | 69 | 96 | 8 | 64 |
| Seto 2011258 | FIB4_F2_combined | FIB-4 | F2 | 7 | 2 | 37 | 118 | |||
| Seto 2011258 | FIB4_F2_high | FIB-4 | F2 | 3.25 | 9 | 99 | 7 | 2 | 70 | 158 |
| Seto 2011258 | FIB4_F2_low | FIB-4 | F2 | 1.45 | 52 | 74 | 40 | 42 | 37 | 118 |
| Seto 2011258 | PAPAS_F2 | PAPAS | F2 | 1.67 | 73 | 78 | 56 | 35 | 21 | 125 |
| Shin 2008259 | APRI_F2_combined | APRI | F2 | 106 | 21 | 4 | 42 | |||
| Shin 2008259 | APRI_F2_high | APRI | F2 | 1.5 | 75 | 83 | 106 | 21 | 35 | 102 |
| Shin 2008259 | APRI_F2_low | APRI | F2 | 0.5 | 97 | 34 | 137 | 81 | 4 | 42 |
| Sinakos 2011260 | TE_F4 | Fibroscan | F4 | 5 | 14 | 0 | 40 | |||
| Sokucu 2010262 | Fibrotest_F3 | Fibrotest | F3 | 0 | 2 | 9 | 14 | |||
| Sporea 2010263 | ARFI_F2 | ARFI | F2 | 1.33 | 71 | 66 | 46 | 2 | 19 | 4 |
| Sporea 2010263 | TE_F2 | Fibroscan | F2 | 7.6 | 60 | 83 | 39 | 1 | 26 | 5 |
| Sporea 2010200 | TE_F2 | Fibroscan | F2 | 59 | 70 | 40 | 22 | 27 | 51 | |
| Sporea 2010200 | TE_F3 | Fibroscan | F3 | 53 | 85 | 17 | 16 | 16 | 91 | |
| Sporea 2010200 | TE_F4 | Fibroscan | F4 | 86 | 99 | 6 | 1 | 1 | 132 | |
| Vigano 2011264 | TE_F2 | Fibroscan | F2 | 55 | 95 | 36 | 3 | 30 | 56 | |
| Vigano 2011264 | TE_F4 | Fibroscan | F4 | 75 | 93 | 15 | 7 | 5 | 98 | |
| Wong 2008267 | TE_F4 | Fibroscan | F4 | 90 | 79 | 18 | 17 | 2 | 63 | |
| Wong 2010 training266 | Forns_F3_combined | Forns index | F3 | 21 | 7 | 1 | 21 | |||
| Wong 2010 training266 | Forns_F3_high | Forns index | F3 | 8.4 | 28 | 91 | 21 | 7 | 53 | 75 |
| Wong 2010 training266 | Forns_F3_low | Forns index | F3 | 5.2 | 99 | 26 | 73 | 61 | 1 | 21 |
| Wong 2010 training266 | TE_F3 | Fibroscan | F3 | 95 | 58 | 48 | 4 | 34 | 70 | |
| Wong 2010 validation266 | Forns_F3_combined | Forns index | F3 | 9 | 4 | 0 | 8 | |||
| Wong 2010 validation266 | Forns_F3_high | Forns index | F3 | 8.4 | 43 | 93 | 9 | 4 | 12 | 57 |
| Wong 2010 validation266 | Forns_F3_low | Forns index | F3 | 5.2 | 100 | 13 | 21 | 53 | 0 | 8 |
| Wong 2010 validation266 | TE_F3 | Fibroscan | F3 | 81 | 61 | 37 | 4 | 24 | 17 | |
| Wong 2011268 | TE_F3 | Fibroscan | F3 | 75 | 47 | 26 | 4 | 29 | 12 | |
| Wong 2011 after Tx268 | TE_F3 | Fibroscan | F3 | 100 | 47 | 24 | 0 | 47 | 20 | |
| Wu 2012269 | APRI_F2_low | APRI | F2 | 0.6 | 62 | 65 | 167 | 74 | 103 | 138 |
| Wu 2012269 | FIB4_F2_low | FIB-4 | F2 | 1.57 | 70 | 69 | 189 | 66 | 81 | 146 |
| Zhang 2008271 | APRI_F1_high | APRI | F1 | 1.5 | 35.7 | 81.6 | 21 | 14 | 36 | 66 |
| Zhang 2008271 | APRI_F2_high | APRI | F2 | 1.5 | 44.7 | 84.3 | 36 | 9 | 44 | 48 |
| Zhu 2011272 | APRI_F2_low | APRI | F2 | 0.5 | 82 | 83.3 | 65 | 16 | 14 | 80 |
| Zhu 2011272 | APRI_F4_low | APRI | F4 | 1 | 75.9 | 69.2 | 22 | 45 | 7 | 101 |
| Zhu 2011272 | FIB4_F2_low | FIB-4 | F2 | 1.7 | 74 | 84.4 | 58 | 15 | 21 | 81 |
| Zhu 2011272 | FIB4_F4_low | FIB-4 | F4 | 1.9 | 69 | 75.3 | 20 | 37 | 9 | 110 |
| Zhu 2011272 | TE_F2 | Fibroscan | F2 | 88 | 90.6 | 70 | 9 | 9 | 87 | |
| Zhu 2011272 | TE_F4 | Fibroscan | F4 | 93.1 | 91.1 | 27 | 13 | 2 | 133 |
| Study ID | Test | Index test assessed | Fibrosis stage assessed | Index test cut-off | Sens. | Spec. | TP | FP | FN | TN |
|---|---|---|---|---|---|---|---|---|---|---|
| Forestier 2010114 | TE_F4 | Fibroscan | F4 | 11.4 kPa | 84 | 91 | 66 | 1 | 13 | 10 |
| Janssens 2010273 | APRI_F2_combined | APRI | F2 | 18 | 1 | 15 | 6 | |||
| Janssens 2010273 | APRI_F2_high | APRI | F2 | 1.5 | 63 | 71 | 26 | 2 | 15 | 6 |
| Janssens 2010273 | APRI_F2_low | APRI | F2 | 0.5 | 43 | 88 | 18 | 1 | 23 | 7 |
| Janssens 2010273 | APRI_F4_high | APRI | F4 | 2 | 40 | 61 | 8 | 8 | 12 | 13 |
| Janssens 2010273 | TE_F3 | Fibroscan | F3 | 17 kPa | 72 | 76.5 | 23 | 4 | 9 | 13 |
| Janssens 2010273 | TE_F4 | Fibroscan | F4 | 21.1 kPa | 75 | 80 | 15 | 6 | 5 | 23 |
| Janssens 2010273 | Forns_F3_high | Forns index | F3 | 6.9 | 42.1 | 85.7 | 9 | 2 | 13 | 15 |
| Kim 200974 | TE_F4 | Fibroscan | F4 | 25.8 | 90 | 87 | 26 | 2 | 3 | 14 |
| Lavallard 2011275 | CK18_F3 | CK18-Total | F3 | 84 | 71 | 49 | 25 | 9 | 60 | |
| Melin 2005276 | TE_F4 | Fibroscan | F4 | 13 | 34 | 1 | 0 | 0 | ||
| Mueller 2010277 | TE_F3 | Fibroscan | F3 | 12.5 | 96 | 80 | 43 | 11 | 2 | 45 |
| Nahon 2008278 | TE_F3 | Fibroscan | F3 | 11.6 | 87 | 89 | 96 | 4 | 14 | 33 |
| Nahon 2008278 | TE_F4 | Fibroscan | F4 | 22.7 | 84 | 83 | 66 | 12 | 13 | 56 |
| Naveau 2005280 | FT_F2_high | Fibrotest | F2 | 0.7 | 55 | 93 | 77 | 6 | 63 | 75 |
| Naveau 2005280 | FT_F2_low | Fibrotest | F2 | 0.3 | 84 | 66 | 118 | 28 | 22 | 53 |
| Naveau 2005280 | FT_F4_high | Fibrotest | F4 | 0.7 | 91 | 87 | 62 | 20 | 6 | 133 |
| Naveau 2005280 | FT_F4_low | Fibrotest | F4 | 0.3 | 100 | 50 | 68 | 77 | 0 | 77 |
| Naveau 1994279 | PGAA_F4 | PGAA | F4 | 7 | 79 | 89 | 36 | 30 | 10 | 241 |
| Nguyen-Khac 2008281 | TE_F1 | Fibroscan | F1 | 5.9 | 83 | 86 | 79 | 1 | 16 | 7 |
| Nguyen-Khac 2008281 | TE_F2 | Fibroscan | F2 | 7.8 | 80 | 91 | 62 | 2 | 15 | 24 |
| Nguyen-Khac 2008281 | TE_F3 | Fibroscan | F3 | 11 | 87 | 81 | 46 | 10 | 7 | 41 |
| Nguyen-Khac 2008281 | TE_F4 | Fibroscan | F4 | 19.5 | 86 | 84 | 28 | 11 | 5 | 59 |
| Tran 2000282 | YKL40_F3 | YKL-40 | F3 | 330 µg/l | 50.8 | 88.5 | 30 | 10 | 29 | 77 |
| Vanbiervliet 2005283 | APRI_F2_combined | APRI | F2 | 44 | 19 | 13 | 37 | |||
| Vanbiervliet 2005283 | APRI_F2_high | APRI | F2 | 1.5 | 50 | 78 | 44 | 19 | 44 | 68 |
| Vanbiervliet 2005283 | APRI_F2_low | APRI | F2 | 0.5 | 85 | 43 | 74 | 50 | 13 | 37 |
| Study ID | Test | Index test assessed | Fibrosis stage assessed | Index test cut-off | Sens. | Spec. | TP | FP | FN | TN |
|---|---|---|---|---|---|---|---|---|---|---|
| Adams 2011284 | APRI_F2 | APRI | F2 | 0.43 | 71.1 | 69.6 | 69 | 44 | 28 | 101 |
| Adams 2011284 | APRI_F3 | APRI | F3 | 0.54 | 72 | 77.1 | 38 | 43 | 15 | 146 |
| Adams 2011284 | APRI_F4 | APRI | F4 | 0.54 | 77.3 | 70.9 | 18 | 64 | 5 | 155 |
| Adams 2011284 | BARD_F2 | BARD | F2 | 2 | 44.3 | 70.4 | 43 | 43 | 54 | 102 |
| Adams 2011284 | BARD_F3 | BARD | F3 | 2 | 60.4 | 71.5 | 32 | 54 | 21 | 135 |
| Adams 2011284 | BARD_F4 | BARD | F4 | 3 | 52.2 | 83.8 | 12 | 35 | 11 | 184 |
| Adams 2011284 | FIB4_F2_low | FIB-4 | F2 | 1.45 | 54.4 | 87.5 | 53 | 18 | 44 | 127 |
| Adams 2011284 | FIB4_F3_low | FIB-4 | F3 | 1.54 | 74 | 86.9 | 39 | 25 | 14 | 164 |
| Adams 2011284 | FIB4_F4_low | FIB-4 | F4 | 1.92 | 72.7 | 70.9 | 17 | 64 | 6 | 155 |
| Adams 2011284 | FT_F2_low | Fibrotest | F2 | 0.34 | 58.9 | 73.2 | 57 | 39 | 40 | 106 |
| Adams 2011284 | FT_F3_high | Fibrotest | F3 | 0.47 | 60.8 | 89.8 | 32 | 19 | 21 | 170 |
| Adams 2011284 | FT_F4_high | Fibrotest | F4 | 0.57 | 72.7 | 92.1 | 17 | 17 | 6 | 202 |
| Adams 2011284 | Hepascore_F2 | Hepascore | F2 | 0.44 | 50.5 | 88.3 | 49 | 17 | 48 | 128 |
| Adams 2011284 | Hepascore_F3 | Hepascore | F3 | 0.37 | 75.5 | 84.1 | 40 | 30 | 13 | 159 |
| Adams 2011284 | Hepascore_F4 | Hepascore | F4 | 0.7 | 87.0 | 89.0 | 20 | 24 | 3 | 196 |
| Angulo 2007 – validation285 | NFS_F3_all | NFS | F3 | 1.455 and 0.676 | 32 | 7 | 17 | 127 | ||
| Angulo 2007 – estimation285 | NFS_F3_all | NFS | F3 | 1.455 and 0.676 | 64 | 7 | 23 | 273 | ||
| Angulo 2007 – estimation285 | NFS_F3_high | NFS | F3 | > 0.676 | 51 | 98 | 64 | 7 | 61 | 319 |
| Angulo 2007 – estimation285 | NFS_F3_low | NFS | F3 | < –1.455 | 82 | 77 | 103 | 82 | 23 | 273 |
| Angulo 2007 – validation285 | NFS_F3_high | NFS | F3 | > 0.676 | 43 | 96 | 32 | 7 | 42 | 172 |
| Angulo 2007 – validation285 | NFS_F3_low | NFS | F3 | < –1.455 | 77 | 71 | 57 | 52 | 17 | 127 |
| Cales 2009287 | APRI_F2 | APRI | F2 | 0.5 | 66.1 | 90.6 | 43 | 16 | 22 | 154 |
| Cales 2009287 | Fibrometer_F2 | FibroMeter | F2 | 78.5 | 95.9 | 51 | 7 | 14 | 163 | |
| Cales 2009287 | NFS_F2_high | NFS | F2 | 60.9 | 96.3 | 40 | 6 | 25 | 164 | |
| Dixon 2001229 | HAIR_NASH | HAIR | NASH | 2 | 80 | 89 | 21 | 9 | 5 | 70 |
| Fujii 2009117 | AAR_F4 | AST–ALT ratio | F4 | 88 | 72 | 8 | 11 | 1 | 30 | |
| Fujii 2009117 | AP_F4 | Age–Platelet Index | F4 | 88 | 84 | 8 | 7 | 1 | 34 | |
| Fujii 2009117 | APRI_F4 | APRI | F4 | 76 | 70 | 7 | 12 | 2 | 29 | |
| Fujii 2009117 | CDS_F4_high | CDS | F4 | 33 | 100 | 3 | 0 | 6 | 41 | |
| Fujii 2009117 | CDS_F4_low | CDS | F4 | 89 | 90 | 8 | 4 | 1 | 37 | |
| Fujii 2009117 | HALT-C_F4_high | HALT-C | F4 | 22 | 100 | 2 | 0 | 7 | 41 | |
| Fujii 2009 17 | HALT-C_F4_low | HALT-C | F4 | 89 | 68 | 8 | 13 | 1 | 28 | |
| Gaia 2011119 | TE F1 | Fibroscan | F1 | 84 | 57 | 41 | 10 | 8 | 13 | |
| Gaia 2011119 | TE_F1 | Fibroscan | F2 | 76 | 80 | 25 | 8 | 8 | 31 | |
| Gaia 2011119 | TE_F2 | Fibroscan | F3 | 65 | 80 | 11 | 11 | 6 | 44 | |
| Gaia 2011119 | TE_F3 | Fibroscan | F4 | 78 | 96 | 7 | 3 | 2 | 60 | |
| Guajardo-Salinas 2010289 | TE_F4 | FibroSpect II | F2 | 100 | 42 | 80 | 49 | 0 | 35 | |
| Guha 2008290 | FIBROSPECT_F2 | ELF | F1 | 9.8 | 61 | 80 | 69 | 16 | 44 | 63 |
| Guha 2008290 | ELF_F1 | ELF | F2 | 9.9 | 70 | 80 | 54 | 23 | 23 | 92 |
| Guha 2008290 | ELF_F2 | ELF | F3 | 10.35 | 80 | 90 | 35 | 15 | 9 | 133 |
| Guha 2008290 | ELF_F3 | Combined panel (NFS + ELF) | 68 | 7 | 9 | 41 | ||||
| Guha 2008290 | NFS_ELF_F1_all | Combined panel (NFS + ELF) | F1 | 60 | 91 | 68 | 7 | 45 | 72 | |
| Guha 2008290 | NFS_ELF_F1_high | Combined panel (NFS + ELF) | F1 | 92 | 52 | 104 | 38 | 9 | 41 | |
| Guha 2008290 | NFS_ELF_F1_low | Combined panel (NFS + ELF) | F2 | 61 | 10 | 8 | 99 | |||
| Guha 2008290 | NFS_ELF_F2_all | Combined panel (NFS + ELF) | F2 | 79 | 91 | 61 | 10 | 16 | 105 | |
| Guha 2008290 | NFS_ELF_F2_high | Combined panel (NFS + ELF) | F2 | 89 | 86 | 69 | 16 | 8 | 99 | |
| Guha 2008290 | NFS_ELF_F2_low | Combined panel (NFS + ELF) | F3 | 38 | 1 | 4 | 142 | |||
| Guha 2008290 | NFS_ELF_F3_all | Combined panel (NFS + ELF) | F3 | 86 | 99 | 38 | 1 | 6 | 147 | |
| Guha 2008290 | NFS_ELF_F3_high | Combined panel (NFS + ELF) | F3 | 91 | 96 | 40 | 6 | 4 | 142 | |
| Guha 2008290 | NFS_ELF_F3_low | NFS | F1 | 36 | 7 | 9 | 40 | |||
| Guha 2008290 | NFS_F1_all | NFS | F1 | 32 | 91 | 36 | 7 | 77 | 72 | |
| Guha 2008290 | NFS_F1_high | NFS | F1 | 92 | 50 | 104 | 40 | 9 | 40 | |
| Guha 2008290 | NFS_F1_low | NFS | F2 | 52 | 13 | 8 | 66 | |||
| Guha 2008290 | NFS_F2_all | NFS | F2 | 68 | 89 | 52 | 13 | 25 | 102 | |
| Guha 2008290 | NFS_F2_high | NFS | F2 | 89 | 57 | 69 | 49 | 8 | 66 | |
| Guha 2008290 | NFS_F2_low | NFS | F3 | 34 | 10 | 4 | 87 | |||
| Guha 2008290 | NFS_F3_all | NFS | F3 | 77 | 93 | 34 | 10 | 10 | 138 | |
| Guha 2008290 | NFS_F3_high | NFS | F3 | 91 | 59 | 4 | 61 | 4 | 87 | |
| Harrison 2008291 | NFS_F3_low | BARD | F3 | 95 | 65 | 173 | 227 | 9 | 418 | |
| Kaneda 2006292 | BARD_F3 | Hyaluronic acid | F3 | 42 | 100 | 89 | 40 | 12 | 0 | 96 |
| Kaneda 2006292 | HA_F3 | Platelet count | F4 | 100 | 95 | 19 | 6 | 0 | 123 | |
| Kaneda 2006292 | PLT_F4 | Type IV collagen | F3 | 78 | 87 | 31 | 14 | 9 | 94 | |
| Kayadibi 2009293 | AST/ALT_NASH | AST–ALT ratio | NASH | 1.09 | 56 | 58 | 24 | 5 | 19 | 6 |
| Kelleger 2005294 | TE_F2 | Fibroscan | F2 | 10 | 88 | 72 | 57 | 18 | 8 | 46 |
| Khosravi 2011295 | AST/ALT_F3_low | AST–ALT ratio | F3 | 0.88 | 87.5 | 79.7 | 7 | 28 | 1 | 111 |
| Ledinghen 2009288 | TE_F2 | Fibroscan | F2 | 7 | 77 | 77 | 61 | 30 | 18 | 99 |
| Ledinghen 2009288 | TE_F3 | Fibroscan | F3 | 8.7 | 84 | 87 | 37 | 21 | 7 | 143 |
| Ledinghen 2009288 | TE_F4 | Fibroscan | F4 | 10.3 | 95 | 88 | 19 | 23 | 1 | 165 |
| Lupsor 2010296 | TE_F1 | Fibroscan | F1 | 5.3 | 93.5 | 78.2 | 44 | 5 | 3 | 20 |
| Lupsor 2010296 | TE_F2 | Fibroscan | F2 | 6.8 | 66.7 | 84.3 | 12 | 8 | 6 | 46 |
| Lupsor 2010296 | TE_F3 | Fibroscan | F3 | 10.4 | 100 | 96.8 | 5 | 2 | 0 | 65 |
| Lydatakis 2006297 | HA_NASH | Hyaluronic acid | F1 | 148.5 | 95.7 | 96.3 | 22 | 1 | 1 | 26 |
| Lydatakis 2006297 | Laminin_NASH | Laminin | F1 | 292.5 | 73.9 | 71.4 | 17 | 8 | 6 | 19 |
| Mahadeva 2010298 | TE_F3 | Fibroscan | F3 | 9.4 | 83 | 89 | 5 | 2 | 1 | 17 |
| Manousou 2011299 | Ferritin_NASH | Serum ferritin | NASH | 240 | 91 | 70 | 58 | 14 | 6 | 33 |
| McPherson 2010301 | APRI_F3 | APRI | F3 | 1 | 27 | 89 | 7 | 13 | 20 | 105 |
| McPherson 2010301 | AST/ALT_F3_high | AST–ALT ratio | F3 | 1 | 52 | 90 | 14 | 12 | 13 | 106 |
| McPherson 2010301 | AST/ALT_F3_low | AST–ALT ratio | F3 | 0.8 | 74 | 78 | 20 | 26 | 7 | 92 |
| McPherson 2010301 | BARD_F3 | BARD | F3 | 2 | 89 | 44 | 24 | 66 | 3 | 52 |
| McPherson 2010301 | FIB4_F3_all | FIB-4 | F3 | 7 | 2 | 4 | 77 | |||
| McPherson 2010301 | FIB4_F3_high | FIB-4 | F3 | 3.25 | 26 | 98 | 7 | 2 | 20 | 116 |
| McPherson 2010301 | FIB4_F3_low | FIB-4 | F3 | 1.3 | 85 | 65 | 23 | 41 | 4 | 77 |
| McPherson 2010301 | NFS_F3_all | NFS | F3 | 9 | 2 | 6 | 68 | |||
| McPherson 2010301 | NFS_F3_high | NFS | F3 | 0.676 | 33 | 98 | 9 | 2 | 18 | 116 |
| McPherson 2010301 | NFS_F3_low | NFS | F3 | –1.45 | 78 | 58 | 21 | 50 | 6 | 68 |
| McPherson 2011300 | AST/ALT_F3_low | AST–ALT ratio | F3 | 0.8 | 94 | 44 | 54 | 139 | 3 | 109 |
| McPherson 2011300 | BARD_F3 | BARD | F3 | 2 | 94 | 26 | 54 | 184 | 3 | 64 |
| McPherson 2011300 | FIB4_F3_low | FIB-4 | F3 | 1.3 | 82 | 77 | 47 | 57 | 10 | 191 |
| McPherson 2011300 | NFS_F3_low | NFS | F3 | –1.45 | 82 | 51 | 47 | 122 | 10 | 126 |
| Oliveira 2005165 | AST/ALT_F3_high | AST–ALT ratio | F3 | 25 | 86 | 4 | 10 | 11 | 64 | |
| Oliveira 2005165 | HA_F3 | Hyaluronic acid | F3 | 75 | 75 | 11 | 19 | 4 | 56 | |
| Pais 2011303 | FT_TE_F2 | Fibrotest + Fibroscan | F2 | < 0.48 and ≤ 9.6 | 76 | 71 | 36 | 38 | 15 | 119 |
| Pais 2011303 | FT_TE_F3 | Fibrotest + Fibroscan | F3 | > 0.48 and > 7.9 | 39 | 96 | 20 | 6 | 31 | 151 |
| Pais 2011 303 | NFS_TE_F2 | NFS + Fibroscan | F2 | < 1.445 and ≤ 9.6 | 64 | 65 | 33 | 57 | 18 | 100 |
| Pais 2011303 | NFS_TE_F3 | NFS + Fibroscan | F3 | > 0.66 and 7.9 | 7 | 98 | 4 | 3 | 47 | 154 |
| Palmeri 2011304 | ARFI_F3 | ARFI | F3 | 4.2 | 90 | 90 | 36 | 10 | 4 | 86 |
| Park 2011306 | CBT_F4 | Caffeine breath test | F4 | 1.27 | 90 | 76 | 9 | 9 | 1 | 29 |
| Pawitpok 2006307 | HA_F2 | Hyaluronic acid | F2 | 218.5 | 78 | 89 | 7 | 2 | 2 | 16 |
| Petta 2011308 | TE_F2 | Fibroscan | F2 | 7.25 | 69 | 70 | 47 | 23 | 21 | 55 |
| Petta 2011308 | TE_F3 | Fibroscan | F3 | 8.75 | 76 | 78 | 25 | 25 | 8 | 88 |
| Pimentel 2010309 | NFS_F3_high | NFS | F3 | 0.676 | 83 | 97 | 20 | 3 | 2 | 133 |
| Pimentel 2010309 | NFS_F3_all | NFS | F3 | 20 | 3 | 1 | 109 | |||
| Pimentel 2010309 | NFS_F3_low | NFS | F3 | –1.455 | 21 | 27 | 1 | 109 | ||
| Poynard 2006 Training310 | NASH_test_NASH | NASH test | NASH | 39 | 92 | 11 | 10 | 17 | 122 | |
| Poynard 2006 Valid310 | NASH_test_NASH | NASH test | NASH | 29 | 98 | 10 | 1 | 25 | 61 | |
| Qureshi 2010311 | NFS_F1_all | NFS | F1 | > 0.676 and < –1.455 | 59 | 8 | 49 | 61 | ||
| Qureshi 2010311 | NFS_F1_high | NFS | F1 | > 0.676 and < –1.457 | 59 | 8 | 158 | 106 | ||
| Qureshi 2010311 | NFS_F1_low | NFS | F1 | > 0.676 and < –1.456 | 164 | 57 | 49 | 61 | ||
| Qureshi 2010311 | NFS_F2_all | NFS | F2 | > 0.676 and < –1.455 | 38 | 29 | 14 | 96 | ||
| Qureshi 2010311 | NFS_F2_high | NFS | > 0.676 and < –1.459 | 38 | 29 | 51 | 213 | |||
| Qureshi 2010311 | NFS_F2_low | NFS | F2 | > 0.676 and < –1.458 | 79 | 142 | 14 | 96 | ||
| Qureshi 2010311 | NFS_F3_all | NFS | F3 | > 0.676 and < –1.455 | 22 | 45 | 2 | 108 | ||
| Qureshi 2010311 | NFS_F3_high | NFS | F3 | > 0.676 and <–1.461 | 22 | 45 | 28 | 236 | ||
| Qureshi 2010311 | NFS_F3_low | NFS | F3 | > 0.676 and < –1.460 | 48 | 173 | 2 | 108 | ||
| Raszeja-Wyscomirska 2010312 | BARD_F3 | BARD | F3 | 2 | 87 | 73 | 13 | 24 | 2 | 64 |
| Ratziu 2004313 | FT_F3_all | Fibrotest | F3 | 6 | 2 | 0 | 58 | |||
| Ratziu 2004313 | FT_F3_high | Fibrotest | F3 | 0.6 | 60 | 97 | 6 | 2 | 4 | 77 |
| Ratziu 2004313 | FT_F3_low | Fibrotest | F3 | 0.3 | 100 | 73 | 10 | 21 | 0 | 58 |
| Ratziu 2006 group1314 | FT_F2_all | Fibrotest | F2 | 7 | 3 | 7 | 101 | |||
| Ratziu 2006 group1314 | FT_F2_high | Fibrotest | F2 | 0.7 | 18 | 98 | 7 | 3 | 33 | 127 |
| Ratziu 2006 group1314 | FT_F2_low | Fibrotest | F2 | 0.3 | 83 | 78 | 33 | 29 | 7 | 101 |
| Ratziu 2006 group1314 | FT_F3_all | Fibrotest | F3 | 5 | 5 | 1 | 107 | |||
| Ratziu 2006 group1314 | FT_F3_high | Fibrotest | F3 | 0.7 | 25 | 97 | 5 | 5 | 15 | 146 |
| Ratziu 2006 group1314 | FT_F3_low | Fibrotest | F3 | 0.3 | 95 | 71 | 19 | 44 | 1 | 107 |
| Ratziu 2006 group2314 | FT_F2_all | Fibrotest | F2 | 4 | 1 | 9 | 49 | |||
| Ratziu 2006 group2314 | FT_F2_high | Fibrotest | F2 | 0.7 | 13 | 98 | 4 | 1 | 27 | 65 |
| Ratziu 2006 group2314 | FT_F2_low | Fibrotest | F2 | 0.3 | 71 | 74 | 22 | 17 | 9 | 49 |
| Ratziu 2006 group2314 | FT_F3_all | Fibrotest | F3 | 4 | 1 | 2 | 56 | |||
| Ratziu 2006 group2314 | FT_F3_high | Fibrotest | F3 | 0.7 | 25 | 99 | 4 | 1 | 12 | 80 |
| Ratziu 2006 group2314 | FT_F3_low | Fibrotest | F3 | 0.3 | 88 | 69 | 14 | 25 | 2 | 56 |
| Ruffilo 2011315 | BARD_F3 | BARD | F3 | ≥ 2 | 51 | 77 | 19 | 23 | 18 | 78 |
| Ruffilo 2011315 | NFS_F3_all | NFS | F3 | < –1.455 and > 0.676 | 23 | 100 | 5 | 0 | 17 | 74 |
| Ruffilo 2011315 | NFS_F3_high | NFS | F3 | > 0.676 | 5 | 0 | 32 | 101 | ||
| Ruffilo 2011315 | NFS_F3_low | NFS | F3 | > –1.455 | 20 | 27 | 17 | 74 | ||
| Sakugawa 2005316 | coll4_F3 | Type IV collagen | F3 | 5 | 81 | 71 | 39 | 19 | 9 | 45 |
| Sakugawa 2005316 | coll4_NASH | Type IV collagen | NASH | 5 | 70 | 81 | 49 | 8 | 21 | 34 |
| Sakugawa 2005316 | HA_F3 | Hyaluronic acid | F3 | 50 | 69 | 83 | 33 | 11 | 15 | 53 |
| Sakugawa 2005316 | HA_NASH | Hyaluronic acid | NASH | 50 | 66 | 90 | 46 | 4 | 24 | 38 |
| Santos 2005317 | coll4_F1 | Type IV collagen | F1 | 145 | 64 | 89 | 7 | 2 | 4 | 17 |
| Santos 2005317 | HA_F1 | Hyaluronic acid | F1 | 24.6 | 82 | 68 | 9 | 6 | 2 | 13 |
| Santos 2005317 | laminin_F1 | Laminin | F1 | 282 | 82 | 89 | 9 | 2 | 2 | 17 |
| Santos 2005317 | coll4_NASH | Type IV collagen | NASH | 5 | 41 | 95 | 27 | 1 | 39 | 18 |
| Sumida 2011 training320 | NAFIC_NASH_high | NAFIC score | NASH | ≥ 2 | 66 | 91 | 65 | 7 | 33 | 72 |
| Sumida 2011 training320 | NAFIC_NASH_low | NAFIC score | NASH | ≥ 1 | 94 | 48 | 92 | 41 | 6 | 38 |
| Sumida 2011 total320 | NAFIC_F2_all | NAFIC score | F2 | 0 and ≥ 2 | 127 | 118 | 7 | 153 | ||
| Sumida 2011 total320 | NAFIC_F2_high | NAFIC score | F2 | ≥ 2 | 84 | 74 | 127 | 118 | 25 | 349 |
| Sumida 2011 total320 | NAFIC_F2_low | NAFIC score | F2 | > 0 | 95 | 33 | 145 | 314 | 7 | 153 |
| Sumida 2011 total320 | NAFIC_F3_all | NAFIC score | F3 | < 1 and ≥ 3 | 56 | 99 | 3 | 371 | ||
| Sumida 2011 total320 | NAFIC_F3_high | NAFIC score | F3 | ≥ 3 | 84 | 82 | 56 | 99 | 11 | 453 |
| Sumida 2011 total320 | NAFIC_F3_low | NAFIC score | F3 | > 1 | 96 | 67 | 64 | 181 | 3 | 371 |
| Sumida 2011 total320 | NFS_F2_all | NFS | F2 | ≤ 1.145 and ≥ 0.676 | 33 | 16 | 25 | 305 | ||
| Sumida 2011 total320 | NFS_F2_high | NFS | F2 | ≥ 0.676 | 23 | 96 | 33 | 18 | 112 | 425 |
| Sumida 2011 total320 | NFS_F2_low | NFS | F2 | > –1.145 | 86 | 69 | 125 | 136 | 20 | 306 |
| Sumida 2011 total320 | NFS_F3_all | NFS | F3 | ≤ –1.455 and >0.676 | 28 | 21 | 5 | 325 | ||
| Sumida 2011 total320 | NFS_F3_high | NFS | F3 | > 0.676 | 33 | 95 | 21 | 28 | 43 | 496 |
| Sumida 2011 total320 | NFS_F3_low | NFS | F3 | ≥ –1455 | 92 | 62 | 59 | 199 | 5 | 325 |
| Sumida 2011 validation320 | NAFIC_NASH_high | NAFIC score | NASH | ≥ 2 | 60 | 87 | 146 | 26 | 98 | 172 |
| Sumida 2011 validation320 | NAFIC_NASH_low | NAFIC score | NASH | ≥ 1 | 88 | 43 | 215 | 113 | 29 | 85 |
| Sumida 2012319 | Age-PLT_index_F3 | Age–Platelet Index | F3 | 6 | 66 | 78 | 42 | 113 | 22 | 399 |
| Sumida 2012319 | APRI_F3 | APRI | F3 | 1 | 67 | 81 | 43 | 97 | 21 | 415 |
| Sumida 2012319 | AST/ALT_F3_high | AST–ALT ratio | F3 | 1 | 48 | 92 | 31 | 41 | 33 | 471 |
| Sumida 2012319 | AST/ALT_F3_low | AST–ALT ratio | F3 | 0.8 | 66 | 76 | 42 | 123 | 22 | 389 |
| Sumida 2012319 | BARD_F3 | BARD | F3 | 2 | 80 | 65 | 51 | 179 | 13 | 333 |
| Sumida 2012319 | FIB4_F3_all | FIB-4 | F3 | < 1.45 and > 3.25 | 31 | 28 | 6 | 330 | ||
| Sumida 2012319 | FIB4_F3_high | FIB-4 | F3 | 3.25 | 48 | 95 | 31 | 26 | 33 | 486 |
| Sumida 2012319 | FIB4_F3_low | FIB-4 | F3 | 1.45 | 90 | 64 | 58 | 184 | 6 | 328 |
| Suzuki 2005321 | HA_F3 | Hyaluronic acid | F3 | 46.1 | 85 | 79.7 | 17 | 12 | 3 | 47 |
| Wong 2008322 | NFS_F2_all | NFS | F2 | < –1.455 and > 0.676 | 0 | 2 | 26 | 102 | ||
| Wong 2008322 | NFS_F2_high | NFS | F2 | 0.676 | 0 | 98 | 0 | 2 | 41 | 119 |
| Wong 2008322 | NFS_F2_low | NFS | F2 | –1455 | 37 | 84 | 15 | 19 | 26 | 102 |
| Wong 2008322 | NFS_F3_all | NFS | F3 | < –1.455 and > 0.676 | 0 | 2 | 11 | 117 | ||
| Wong 2008322 | NFS_F3_high | NFS | F3 | 0.676 | 0 | 99 | 0 | 2 | 18 | 142 |
| Wong 2008322 | NFS_F3_low | NFS | F3 | –1455 | 39 | 81 | 7 | 27 | 11 | 117 |
| Wong 2008323 | TE_F3 | Fibroscan | F3 | 7.5 | 82 | 71 | 14 | 15 | 3 | 38 |
| Wong 2009324 | TE_F2 | Fibroscan | F2 | 7.0 | 79 | 76 | 80 | 35 | 21 | 110 |
| Wong 2009324 | TE_F3 | Fibroscan | F3 | 8.7 | 84 | 83 | 47 | 32 | 9 | 158 |
| Wong 2009324 | TE_F4 | Fibroscan | F4 | 10.3 | 92 | 88 | 23 | 27 | 2 | 194 |
| Yoneda 2008326 | TE_F1 | Fibroscan | F1 | 5.9 | 86.1 | 88.9 | 68 | 2 | 11 | 16 |
| Yoneda 2008326 | TE_F2 | Fibroscan | F2 | 6.65 | 88.2 | 73.9 | 45 | 12 | 6 | 34 |
| Yoneda 2008326 | TE_F3 | Fibroscan | F3 | 9.8 | 85.2 | 81.4 | 14 | 15 | 3 | 65 |
| Yoneda 2008326 | TE_F4 | Fibroscan | F4 | 17.5 | 100 | 96.6 | 88 | 0 | 0 | 9 |
| Yoneda 2011325 | PLT_F3 | Platelet count | F3 | 0.774 | 62.7 | 76.3 | 144 | 293 | 84 | 927 |
| Yoneda 2011325 | PLT_F4 | Platelet count | F4 | 0.918 | 80.5 | 88.8 | 33 | 111 | 8 | 896 |
| Younossi 2011327 | APRI_F1 | APRI | F1 | 0.5 | 56 | 97.4 | 2 | 1 | 37 | 39 |
| Younossi 2011327 | APRI_F3 | APRI | F3 | 0.5 | 71 | 96.7 | 1 | 2 | 15 | 61 |
| Younossi 2011327 | M30_NASH | M30 | NASH | 272.9 | 72.5 | 64.1 | 29 | 14 | 11 | 25 |
| Younossi 2011327 | NDP_F1 | NAFLD diagnostic panel: model predicting any fibrosis | F1 | 0.4242 | 60.6 | 71.8 | 24 | 11 | 15 | 29 |
| Younossi 2011327 | NDP_F3 | NAFLD diagnostic panel: model predicting severe fibrosis | F3 | 0.2442 | 86.7 | 70.4 | 14 | 19 | 2 | 44 |
| Younossi 2011327 | NDP_NASH | NAFLD diagnostic panel: NASH model | NASH | 0.36 | 79.4 | 73.7 | 32 | 11 | 8 | 28 |
| Younossi 2011327 | NFS_F1_low | NFS | F1 | –0.1657 | 84.8 | 34.2 | 33 | 26 | 6 | 14 |
| Study ID | Test | Index test assessed | Fibrosis stage assessed | Comment | Index test cut-off | Sens. | Spec. | TP | FP | FN | TN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Aguirre 2006368 | DEMRI | Double-enhanced MRI (gadolinium and SPIO) | F3 | 4.76 millisecond TE SPGR | 93 | 87 | 67 | 4 | 5 | 25 | |
| Asbach 2010328 | MRE_F1 | MR elastography | F1 | 2.84 | 80 | 81 | 58 | 3 | 14 | 13 | |
| Asbach 2010328 | MRE_F2 | MR elastography | F2 | 3.18 | 77 | 97 | 40 | 1 | 12 | 35 | |
| Asbach 2010328 | MRE_F3 | MR elastography | F3 | 3.32 | 97 | 87 | 34 | 7 | 1 | 46 | |
| Asbach 2010328 | MRE_F4 | MR elastography | F4 | 4.21 | 100 | 91 | 19 | 6 | 0 | 63 | |
| Aube 1999329 | US_F4 | US | F4 | 11 parameters | 38 | 23 | 10 | 119 | |||
| Aube 2004330 | US_F3 | US | F3 | Five parameters | 23 | 11 | 9 | 63 | |||
| Aube 2004330 | US_F4 | US | F4 | Five parameters | 22 | 4 | 4 | 76 | |||
| Awaya 2002331 | MRI_F4 | MRI | F4 | Modified caudate lobe ratio | C/RL-r > 0.9 | 72 | 77 | 87 | 26 | 34 | 89 |
| Cardi 1997332 | US_F4 | US | F4 | Echotexture, nodularity, spleen size | 79 | 10 | 15 | 81 | |||
| Chen 200893 | US_F2 | US | F2 | Echotexture, nodularity, irregular surface | 27 | 63 | 23 | 8 | 61 | 14 | |
| Chen 200893 | US_F3 | US | F3 | Echotexture, nodularity, irregular surface | 14 | 66 | 3 | 29 | 19 | 57 | |
| Chen 2008227 | US_F4 | US | F4 | 86 | 63 | 84 | 124 | 14 | 211 | ||
| Cioni 1992333 | US_F4 | US | F4 | PV flow | 39 | 1 | 20 | 57 | |||
| Cobbold 201098 | CEUS_F4 | CEUS | F4 | 8 | 71 | 91 | 10 | 5 | 4 | 48 | |
| Cobbold 201098 | CEUS_F2 | CEUS | F2 | 10.25 | 53 | 73 | 20 | 8 | 17 | 22 | |
| Colli 1994334 | US_F4 | US | F4 | Hepatic vein waveform | 12 | 8 | 4 | 28 | |||
| Colli 2003335 | US_F4 | US | F4 | Any one of nodularity, caudate lobe hypertropy, pattern of PV flow | 68 | 79 | 50 | 48 | 23 | 179 | |
| Corradi 2009100 | US_SAPI_F2 | Splenic arterial pulsatity index | F2 | 78 | 83 | 10 | 4 | 3 | 19 | ||
| Crespo 2010101 | MRE_F3 | MR elastography | F3 | 3.5 | 90 | 71 | 10 | 12 | 1 | 28 | |
| D’Onofrio 2005336 | US_F3 | US | F3 | PV, spleen size, parenchyma, liver margins | Any of the four parameters present | 68 | 68 | 19 | 25 | 9 | 52 |
| Do 2010337 | DWMRI_F2 | DW-MRI | F2 | b-values 0, 50, 500 | ADC 1.68 | 67 | 61 | 20 | 2 | 10 | 2 |
| Do 2010337 | DWMRI_F3 | DW-MRI | F3 | b-values 0, 50, 500 | ADC 1.53 | 56 | 71 | 14 | 3 | 11 | 6 |
| Do 2010337 | DWMRI_F4 | DW-MRI | F4 | b-values 0, 50, 500 | ADC 1.68 | 76 | 60 | 16 | 5 | 5 | 8 |
| Do 2010337 | DWMRI_F2 | DW-MRI | F2 | b-values 0, 50, 500 normalised ADC (liver/spleen) | 1.41 | 90 | 77 | 27 | 1 | 3 | 3 |
| Do 2010337 | DWMRI_F3 | DW-MRI | F3 | b-values 0, 50, 500 normalised ADC (liver/spleen) | 1.41 | 96 | 71 | 24 | 3 | 1 | 6 |
| Do 2010337 | DWMRI_F4 | DW-MRI | F4 | b-values 0, 50, 500 normalised ADC (liver/spleen) | 1.4 | 95 | 66 | 20 | 4 | 1 | 9 |
| Fahmy 2011110 | US_F2 | US | F2 | ≥ 0.64 | 69 | 74 | 46 | 11 | 21 | 32 | |
| Fahmy 2011110 | US_F4 | US | F4 | ≥ 0.71 | 87 | 65 | 19 | 31 | 3 | 57 | |
| Fahmy 2011110 | US_SAPI_F2 | Splenic arterial pulsatity index | F2 | ≥ 1 | 66 | 72 | 44 | 12 | 23 | 31 | |
| Fahmy 2011110 | US_SAPI_F4 | Splenic arterial pulsatity index | F4 | ≥ 1.06 | 74 | 67 | 16 | 29 | 6 | 59 | |
| Ferral 1992338 | US_F4 | US | F4 | Nodular surface | 28 | 7 | 4 | 31 | |||
| Friedrich-Rust 2010367 | CEMRI_F2 | MR elastography intravenoud gadolinium-enhanced sequences | F2 | Primovist | After 10 minutes | 90 | 76 | 31 | 2 | 3 | 8 |
| Fujimoto 2011118 | DWMRI_F1 | DW-MRI | F1 | 1.35 | 79 | 83 | 34 | 2 | 9 | 10 | |
| Fujimoto 2011118 | DWMRI_F2 | DW-MRI | F2 | 1.32 | 85 | 91 | 29 | 2 | 5 | 19 | |
| Fujimoto 2011118 | DWMRI_F3 | DW-MRI | F3 | 1.27 | 87 | 84 | 20 | 5 | 3 | 27 | |
| Fujimoto 2011118 | DWMRI_F4 | DW-MRI | F4 | 1.23 | 75 | 72 | 9 | 12 | 3 | 31 | |
| Gaia 2009339 | US_F4 | US | F4 | Nodularity | 63 | 86 | 12 | 6 | 7 | 36 | |
| Gaiani 1997340 | US_F4 | US | F4 | Portal velocity, nodularity | 37 | 32 | 10 | 133 | |||
| Gierblinski 2008341 | RTE_F1 | RTE | F1 | Colour coded | > 35.5% | 86 | 84 | 30 | 1 | 5 | 3 |
| Goyal 1990342 | US_F4 | US | F4 | R/L liver lobe ratio | 1.3 | 28 | 0 | 10 | 38 | ||
| Hu 2010234 | US_MARS_F1 | US MARS | F1 | MARS (a novel parameter from sonographic videos) | 22.49 | 82 | 75 | 23 | 2 | 5 | 6 |
| Hu 2010234 | US_MARS_F2 | US MARS | F2 | MARS (a novel parameter from sonographic videos) | 21.81% | 85 | 56 | 17 | 7 | 3 | 9 |
| Hu 2010234 | US_MARS_F4 | US MARS | F4 | MARS (a novel parameter from sonographic videos) | 20.32% | 100 | 50 | 8 | 14 | 0 | 14 |
| Huwart 200830 | MRE_F1 | MR elastography | F1 | 2.42 | 85 | 91 | 63 | 2 | 11 | 20 | |
| Huwart 200830 | MRE_F2 | MR elastography | F2 | 2.49 | 100 | 91 | 52 | 4 | 0 | 40 | |
| Huwart 200830 | MRE_F3 | MR elastography | F3 | 3.13 | 91 | 97 | 30 | 2 | 3 | 61 | |
| Huwart 200830 | MRE_F4 | MR elastography | F4 | 4.13 | 100 | 96 | 18 | 3 | 0 | 75 | |
| Iacobellis 2005132 | US_F2 | US | F2 | Nodular liver surface | Positive | 16 | 97 | 104 | 15 | 544 | 480 |
| Iacobellis 2005132 | US_F4 | US | F4 | Nodular liver surface | 46 | 93 | 38 | 74 | 44 | 987 | |
| Ibrahim 2011343 | DWMRI_F2 | DW-MRI | F2 | Mean hepatic ADC at 300 seconds/mm2 (b-value) | ≤ 1.89 | 90 | 89 | 16 | 2 | 2 | 18 |
| Ishibashi 2012344 | CEUS_F2 | CEUS | F2 | Sonazoid (second-generation microbubbles) | 15-minute phase intensity of difference | 88 | 72 | 69 | 10 | 9 | 25 |
| Ishibashi 2012344 | CEUS_F3 | CEUS | F3 | Sonazoid (second-generation microbubbles) | 85 | 91 | 44 | 5 | 8 | 56 | |
| Ishibashi 2012344 | CEUS_F4 | CEUS | F4 | Sonazoid (second-generation microbubbles) | 97 | 90 | 28 | 8 | 1 | 76 | |
| Joseph 1991345 | US_F1 | US | F1 | Parenchyma heterogeneity | 24 | 2 | 7 | 17 | |||
| Kim 2011346 | MRE_F1 | MR elastography | F1 | 2.87 | 80 | 90 | 32 | 2 | 8 | 18 | |
| Kim 2011346 | MRE_F2 | MR elastography | F2 | 3.05 | 89.7 | 87.1 | 26 | 4 | 3 | 27 | |
| Kim 2011346 | MRE_F3 | MR elastography | F3 | 3.57 | 94.7 | 90.2 | 18 | 4 | 1 | 37 | |
| Kim 2011346 | MRE_F4 | MR elastography | F4 | 5.32 | 100 | 92.2 | 9 | 4 | 0 | 47 | |
| Kim 2010 EALT347 | LSPI_F4 | Spleen diameter-to-platelet radio index | F4 | 42 | 96.3 | 67.4 | 77 | 28 | 3 | 58 | |
| Kim 2010 EALT347 | LSPI_F4 | Spleen diameter-to-platelet radio index | F4 | 94 | 67.5 | 97.7 | 54 | 2 | 26 | 84 | |
| Kim 2010 NALT347 | LSPI_F4 | Spleen diameter-to-platelet radio index | F4 | 38 | 98 | 69.2 | 97 | 20 | 2 | 45 | |
| Kim 2010 NALT347 | LSPI_F4 | Spleen diameter-to-platelet radio index | F4 | 62 | 85.9 | 93.8 | 85 | 4 | 14 | 61 | |
| Ladenheim 1992348 | US_F4 | US | F4 | Nodular surface | 1 | 5 | 7 | 37 | |||
| Lee 2011145 | MRE_F1 | MR elastography | F1 | 3.81 | 88 | 79 | 6 | 5 | 1 | 20 | |
| Lee 2010349 | US_F4 | US | F4 | NA | 38.9 | 87.4 | 14 | 21 | 22 | 146 | |
| Lewin 2007149 | MRE_F3 | MR elastography | F3 | 1.21 | 87 | 87 | 13 | 5 | 2 | 34 | |
| Liu 2006152 | US_SAPI_F2_high | SAPI | F2 | 1.05 | 66.7 | 89.7 | 14 | 6 | 7 | 52 | |
| Liu 2006152 | US_SAPI_F2_low | SAPI | F2 | 0.85 | 97.5 | 38.8 | 20 | 35 | 1 | 23 | |
| Liu 2006152 | US_SAPI_F2 | SAPI | F2 | 1 | 76 | 80 | 256 | 33 | 81 | 133 | |
| Liu 2006152 | US_SAPI_F2_high | SAPI | F2 | 1.1 | 61 | 98 | 206 | 3 | 131 | 163 | |
| Liu 2006152 | US_SAPI_F2_low | SAPI | F2 | 0.85 | 94 | 39 | 317 | 101 | 20 | 65 | |
| Liu 2006152 | US_SAPI_F4 | SAPI | F4 | 1.2 | 88 | 82 | 74 | 75 | 10 | 344 | |
| Lutz 2012351 | HVRI_US_F4 | US | F4 | HVRI | 1.185 | 90 | 86 | 27 | 13 | 3 | 82 |
| Motosugi 2011369 | CEMRI_F1 | MR elastography intravenoud gadolinium-enhanced sequences | F1 | Primovist (liver-specific contrast agent) | 1.91 | 87 | 75 | 73 | 4 | 11 | 12 |
| Motosugi 2011369 | CEMRI_F2 | MR elastography intravenoud gadolinium-enhanced sequences | F2 | 1.76 | 74.6 | 56 | 50 | 15 | 17 | 18 | |
| Motosugi 2011369 | CEMRI_F3 | MR elastography intravenoud gadolinium-enhanced sequences | F3 | 1.76 | 75.4 | 50 | 43 | 22 | 14 | 22 | |
| Motosugi 2011369 | CEMRI_F4 | MR elastography intravenoud gadolinium-enhanced sequences | F4 | 1.75 | 81.6 | 49.1 | 29 | 33 | 7 | 31 | |
| Nagata 2003352 | US_F4 | US | F4 | Liver nodularity assessed – 3.75 standard | 73 | 58 | 16 | 23 | 6 | 32 | |
| Nagata 2003352 | US_F4 | US | F4 | Liver nodularity assessed – 7.5 experimental | 68 | 82 | 15 | 10 | 7 | 45 | |
| Nishiura 2005353 | US_F4 | US | F4 | Score of three parameters | 6.5 | 100 | 100 | 22 | 0 | 0 | 81 |
| Numminen 2005354 | MRI_F4 | MRI | F4 | 1.5T MRI | 87 | 92 | 26 | 2 | 4 | 24 | |
| Ong 2003355 | US_F4 | US | F4 | 3.75 MHz US | 38 | 85 | 6 | 11 | 10 | 61 | |
| Orrlachio 2011166 | CEUS_F3 | Contrast-enhanced US | F3 | 16 | 77 | 83 | 15 | 5 | 4 | 25 | |
| Paggi 2008167 | US_F3 | US | F3 | 73 | 90 | 117 | 27 | 43 | 243 | ||
| Papalavrentios 2011305 | MRI_F3 | MRI | F3 | 1.16 × 10–3 | 8 | 1 | 0 | 9 | |||
| Ronot 2010176 | CT_F2 | Mean transit time | F2 | 13.4 | 71 | 65 | 21 | 8 | 9 | 14 | |
| Rustogi 2011356 | MRE_F3 | MR elastography | F3 | 5.9 | 85 | 88 | 27 | 5 | 5 | 35 | |
| Rustogi 2011356 | MRI_F3 | MRI | F3 | Morphological criteria | 78 | 75 | 25 | 10 | 7 | 30 | |
| Sandrasegaran 2009357 | US_F4 | DW-MRI | F2 | 103 | 73 | 59 | 37 | 11 | 14 | 16 | |
| Sandrasegaran 2009357 | US_F4 | DW-MRI | F3 | 98 | 52 | 71 | 21 | 11 | 20 | 26 | |
| Schneider 2005184 | US_F4 | US | F4 | Reduced PV undulations | 77 | 100 | 13 | 0 | 4 | 102 | |
| Schneider 2006185 | SPECT_F2 | US | F4 | PV flow < 12.5 cm/second | 87 | 69 | 17 | 20 | 2 | 44 | |
| Shen 2006 | CEMRE_F1 | US | F4 | Spleen length | 12.1 | 60 | 75 | 18 | 74 | 12 | 221 |
| Shiramizy 2006 | MRE_F1 | Single-photon emission CT | F2 | Minimum spleen pixel and right hepatic lobe | 19 | 4 | 3 | 20 | |||
| Venkatesh 2010359 | US_F4 | MR elastography intravenoud gadolinium-enhanced sequences | F1 | Contrast enhanced | 2.91 kPa | 95 | 100 | 18 | 0 | 1 | 5 |
| Venkatesh 2010359 | US_F4 | MR elastography | F1 | 2.83 kPa | 95 | 100 | 18 | 0 | 1 | 5 | |
| Vigano 2005360 | US_F4 | US | F4 | Nodularity | 53 | 91 | 20 | 6 | 17 | 65 | |
| Wang 2009361 | CEUS_F1 | US | F4 | Liver surface and parenchyma, hepatic vessels and spleen index | 74 | 86 | 45 | 36 | 16 | 223 | |
| Xu 2005362 | CEUS_F2 | US | F4 | Scoring system | 10 | 88 | 97 | 21 | 1 | 3 | 41 |
| Zhang 2011270 | CEUS_F3 | CEUS | F1 | 1.02 | 80 | 86 | 61 | 1 | 15 | 9 | |
| Zhang 2011270 | CEUS_F4 | CEUS | F2 | 0.96 | 75 | 86 | 50 | 3 | 17 | 17 | |
| Zhang 2011270 | DWMRI_F2 | CEUS | F3 | 0.83 | 71 | 84 | 31 | 10 | 13 | 52 | |
| Zhang 2011270 | US_F4 | CEUS | F4 | 0.72 | 76 | 80 | 27 | 10 | 8 | 41 | |
| Zhu 2008363 | US_F4 | DW-MRI | F2 | b-value = 500 s/mm2 | 84 | 80 | 21 | 4 | 4 | 14 |
Appendix 5 Forest plots
This appendix presents forest plots of sensitivity and specificity of non-invasive tests across all fibrosis stages in patients with chronic HBV, chronic HCV, NAFLD and ALD. Plots are presented when there are data available from at least two studies. Studies are represented by their reference number in the report.
FIGURE 11.
Alcoholic liver disease: APRI F2 (high cut-off) – sensitivity.
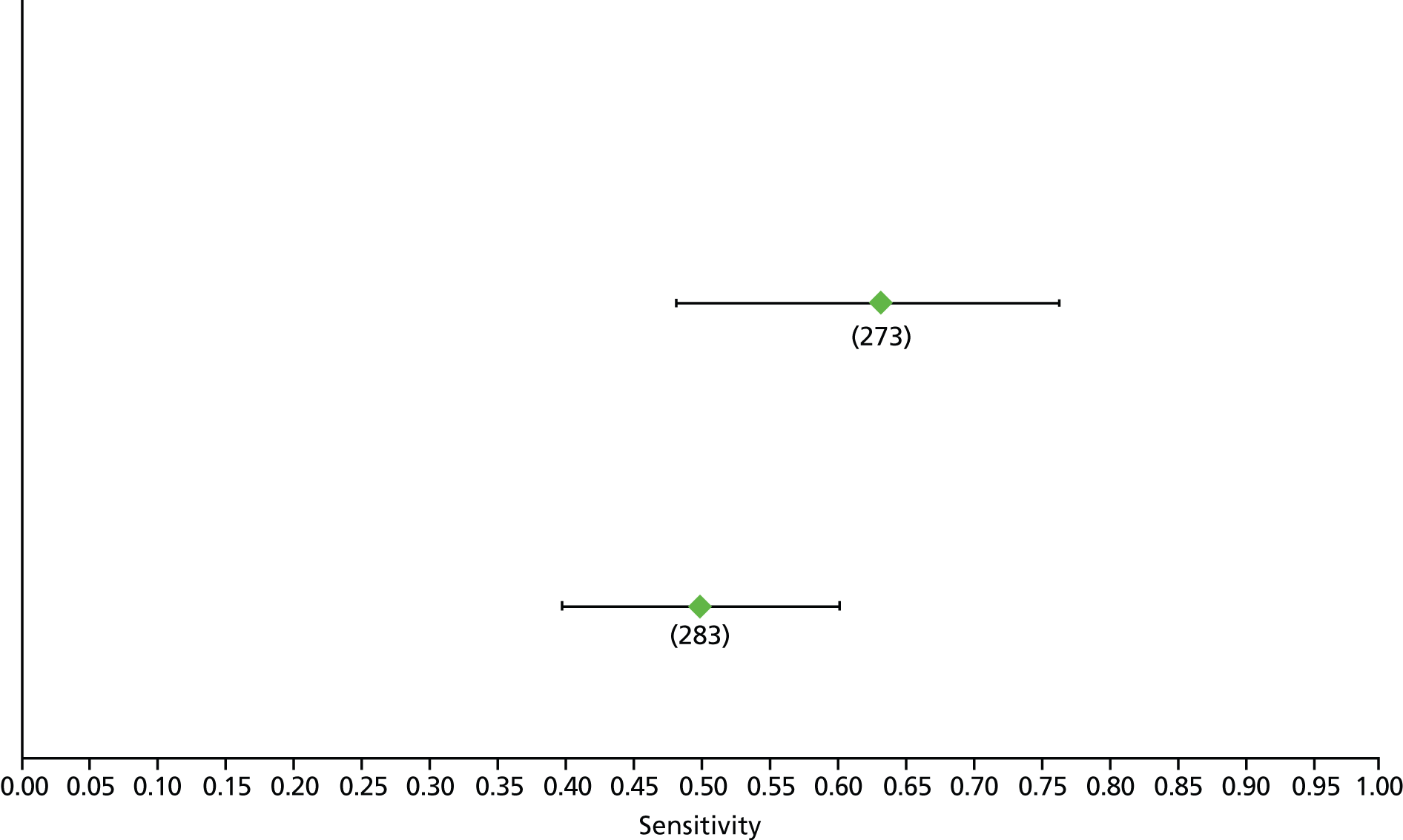
FIGURE 12.
Alcoholic liver disease: APRI F2 (high cut-off) – specificity.

FIGURE 13.
Alcoholic liver disease: APRI F2 (low cut-off) – sensitivity.

FIGURE 14.
Alcoholic liver disease: APRI F2 (low cut-off) – specificity.
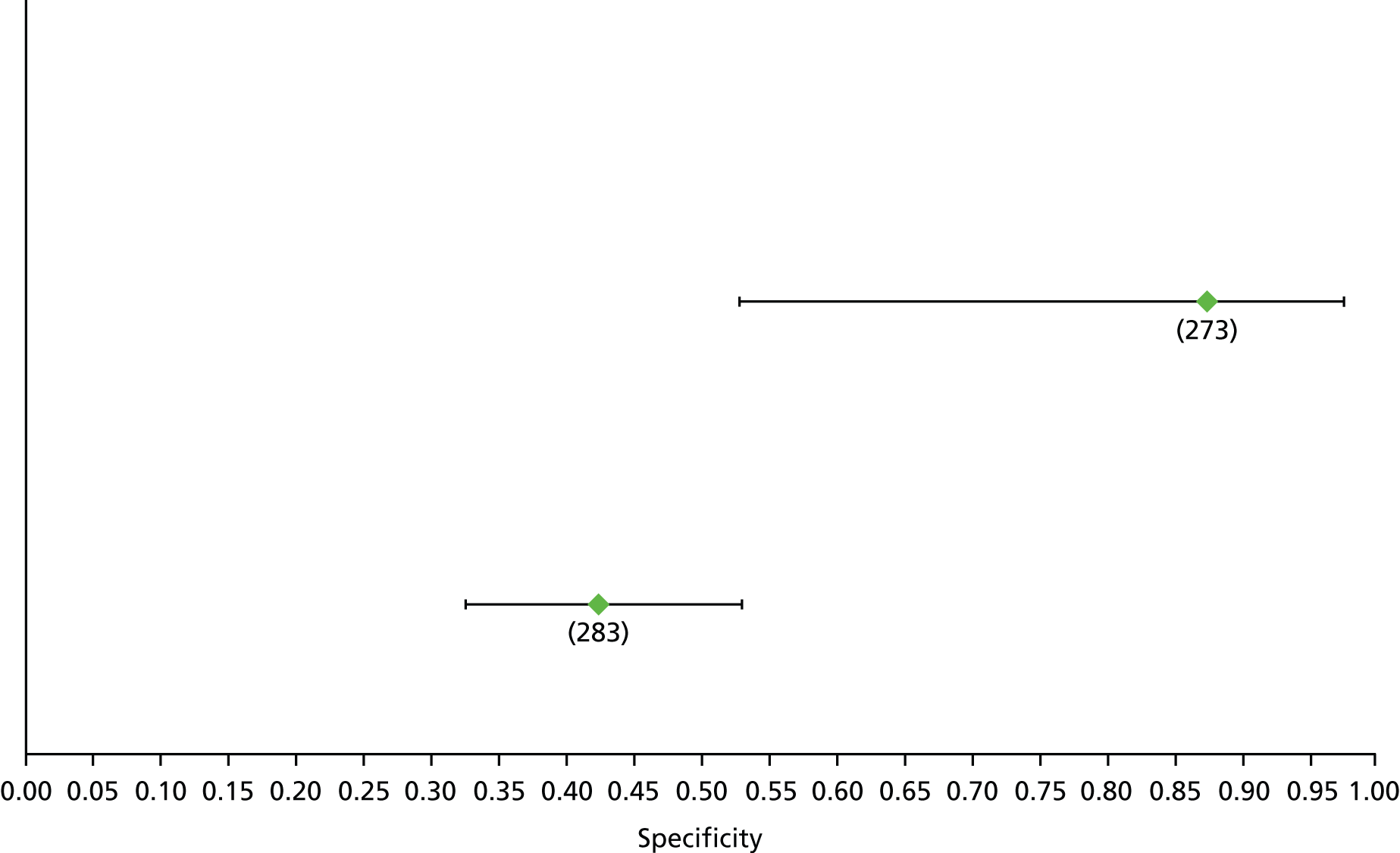
FIGURE 15.
Alcoholic liver disease: TE F3 – sensitivity.

FIGURE 16.
Alcoholic liver disease: TE F3 – specificity.

FIGURE 17.
Alcoholic liver disease: TE F4 – sensitivity.
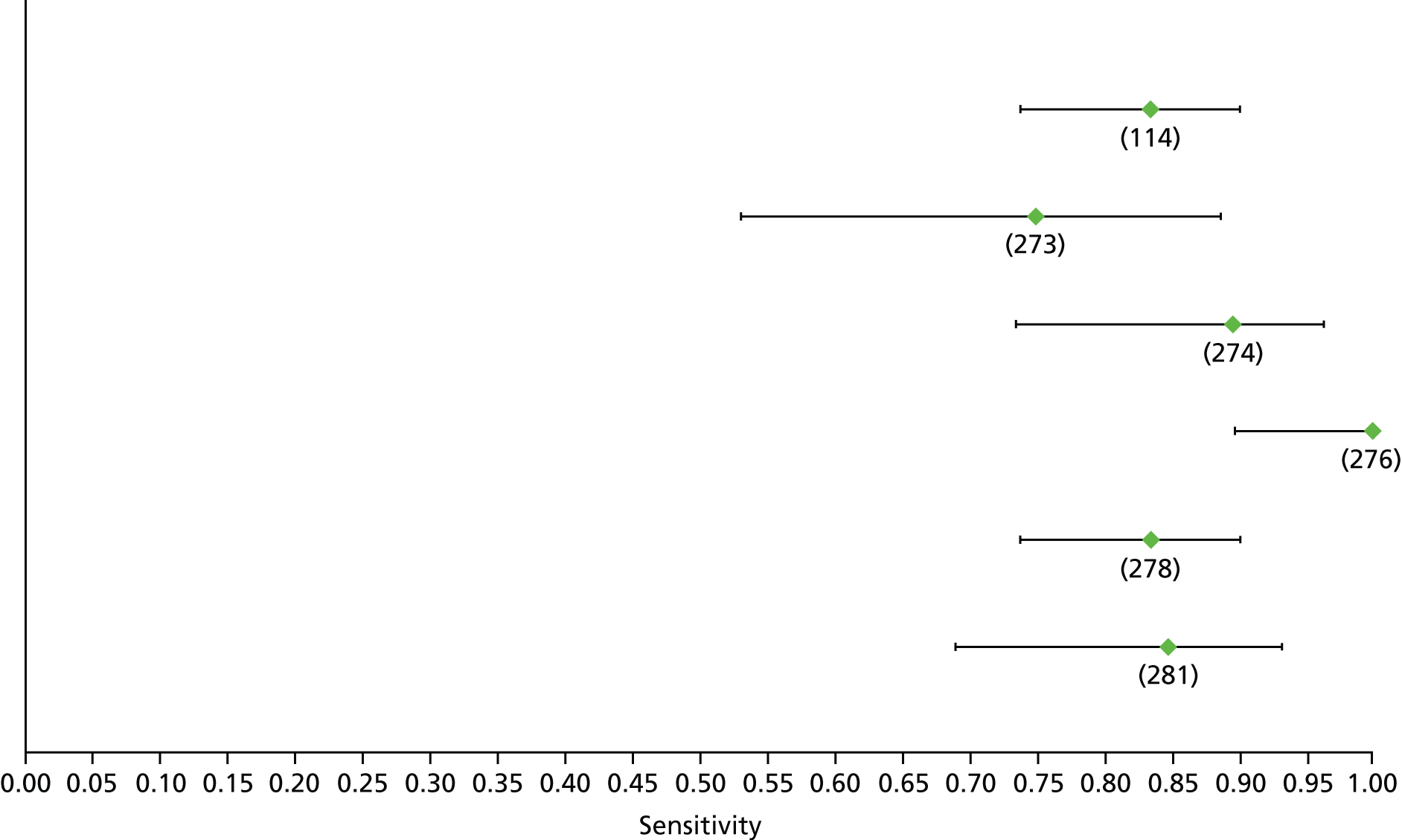
FIGURE 18.
Alcoholic liver disease: TE F4 – specificity.
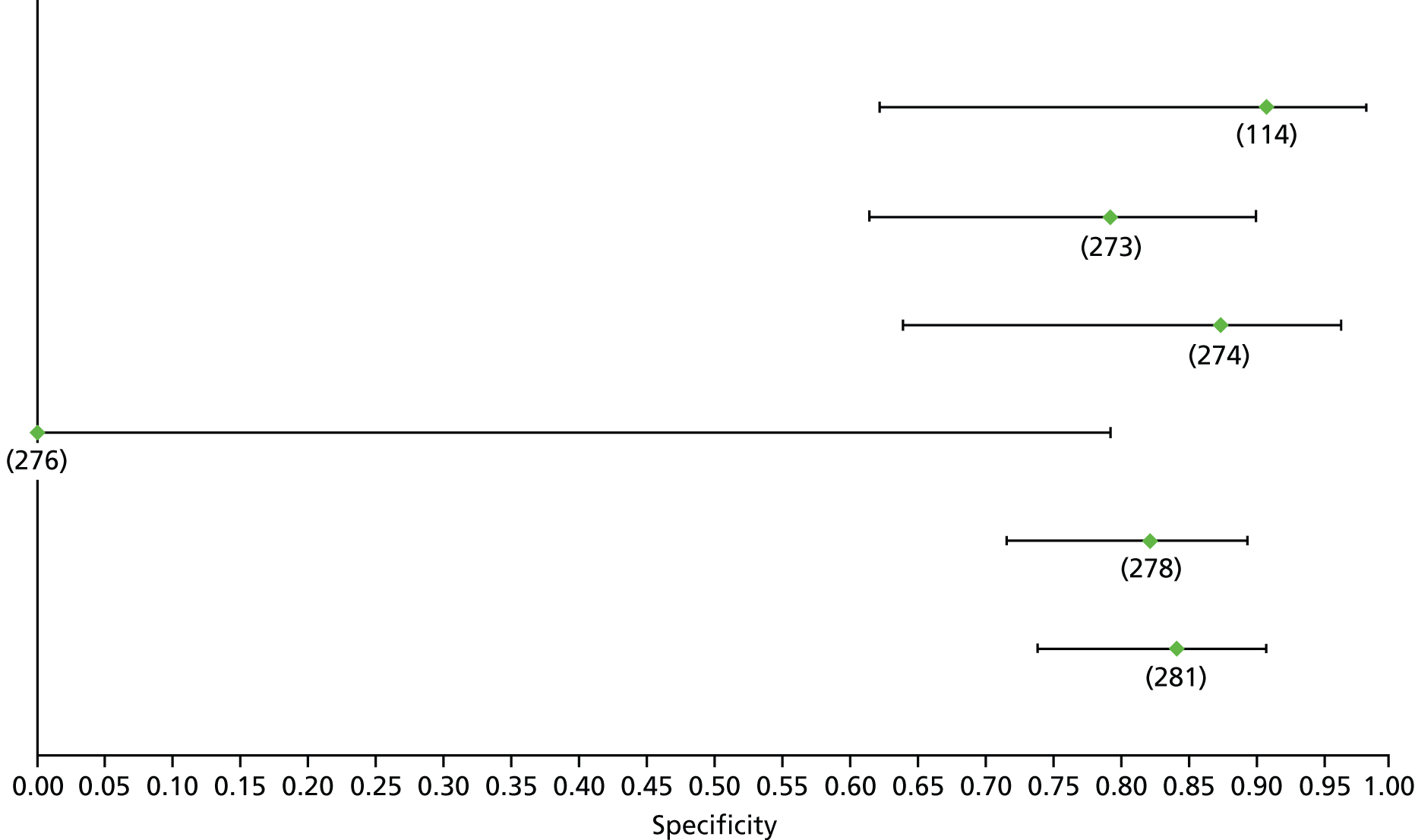
FIGURE 19.
Hepatitis B: APRI F2 (high cut-off) – sensitivity.

FIGURE 20.
Hepatitis B: APRI F2 (high cut-off) – specificity.

FIGURE 21.
Hepatitis B: APRI F2 (low cut-off) – sensitivity.
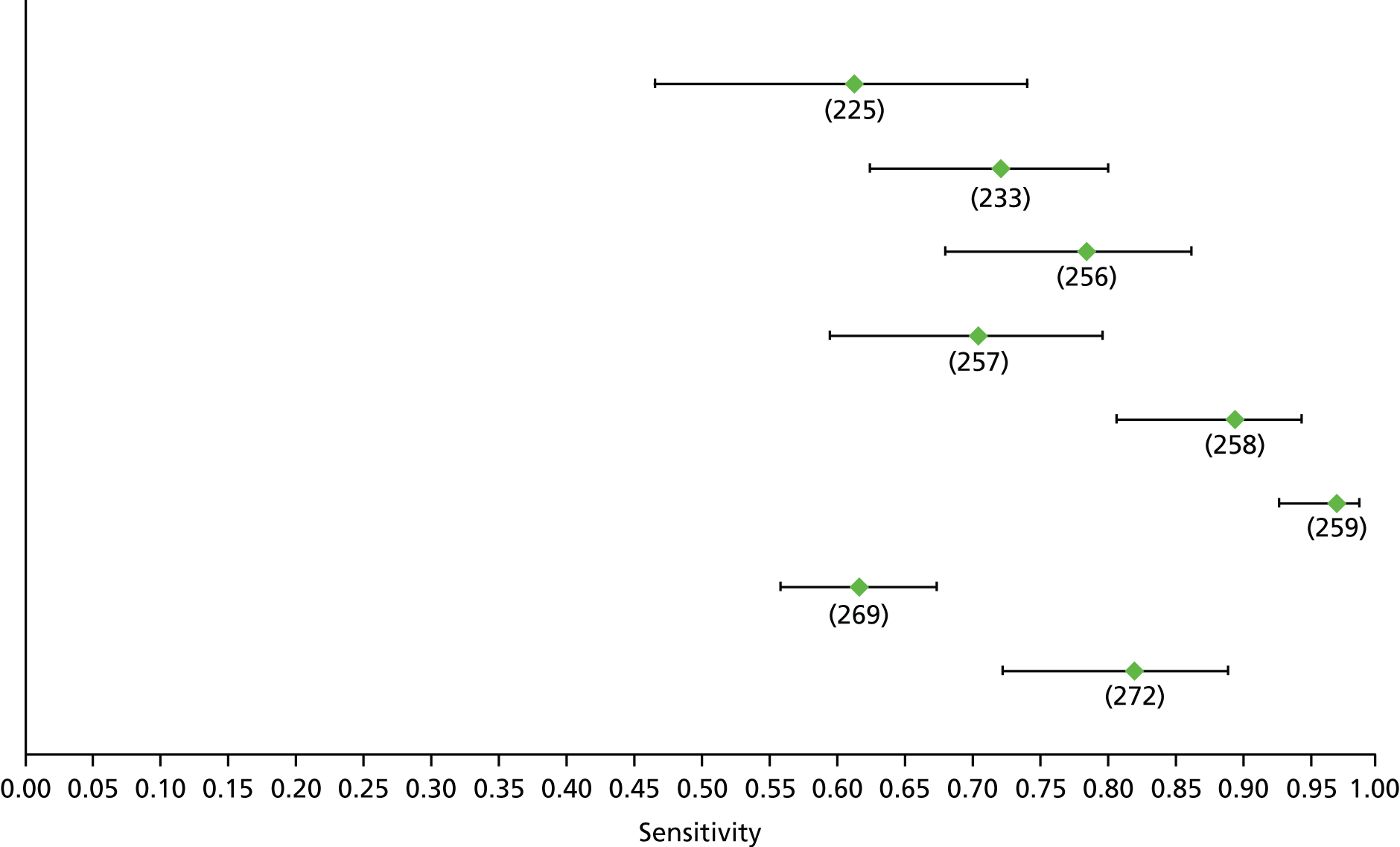
FIGURE 22.
Hepatitis B: APRI F2 (low cut-off) – specificity.
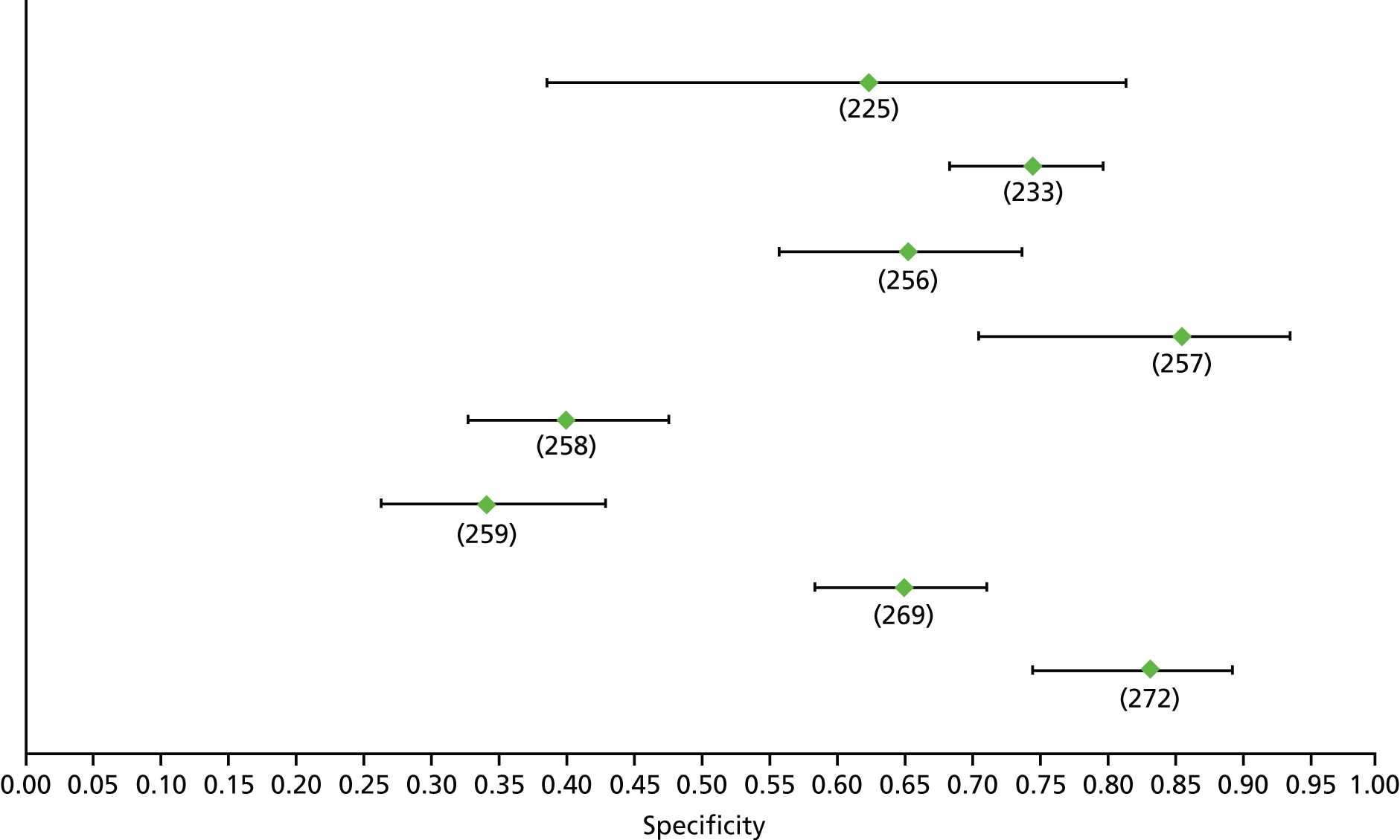
FIGURE 23.
Hepatitis B: FIB-4 F2 (low cut-off) – sensitivity.
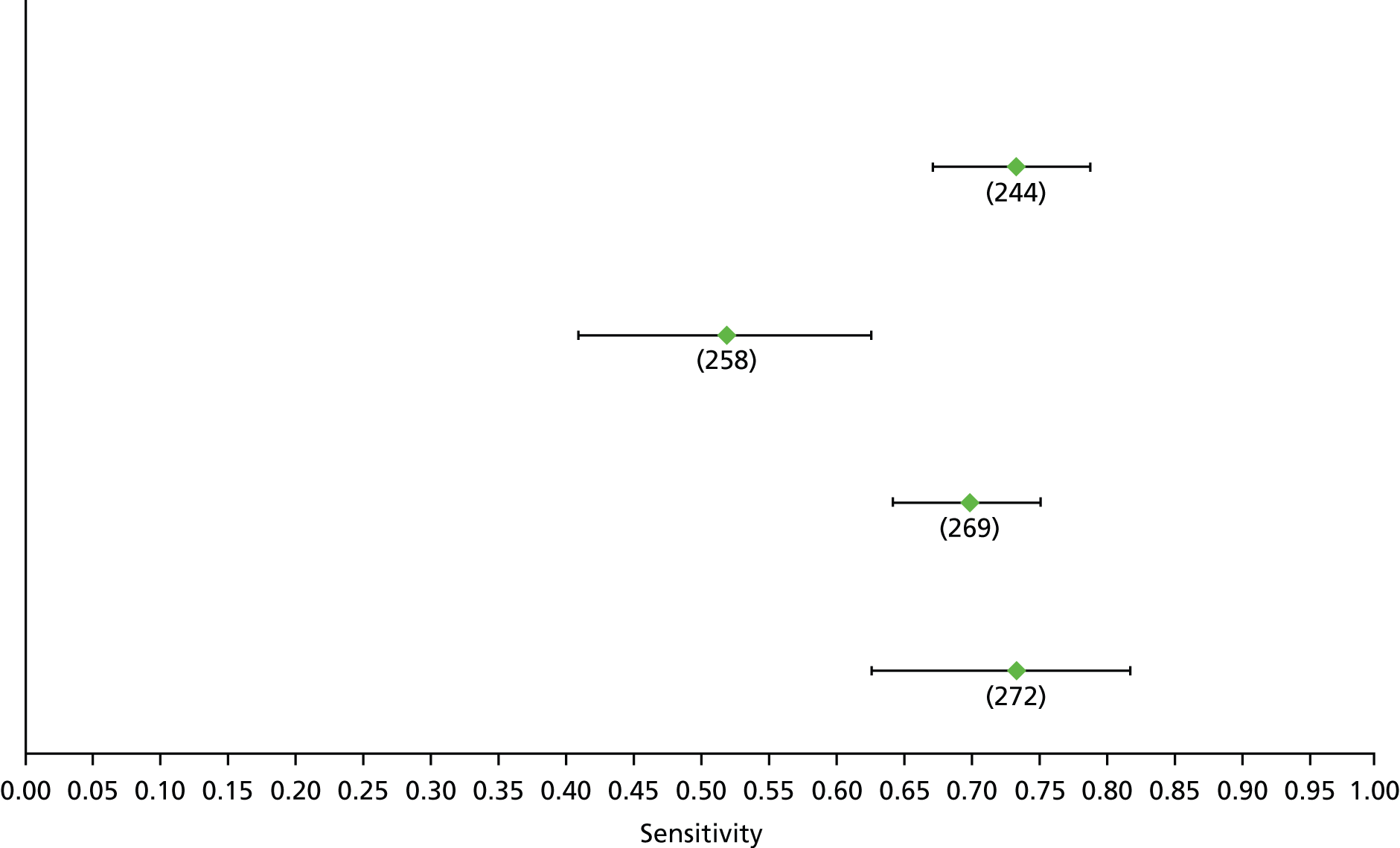
FIGURE 24.
Hepatitis B: FIB-4 F2 (low cut-off) – specificity.
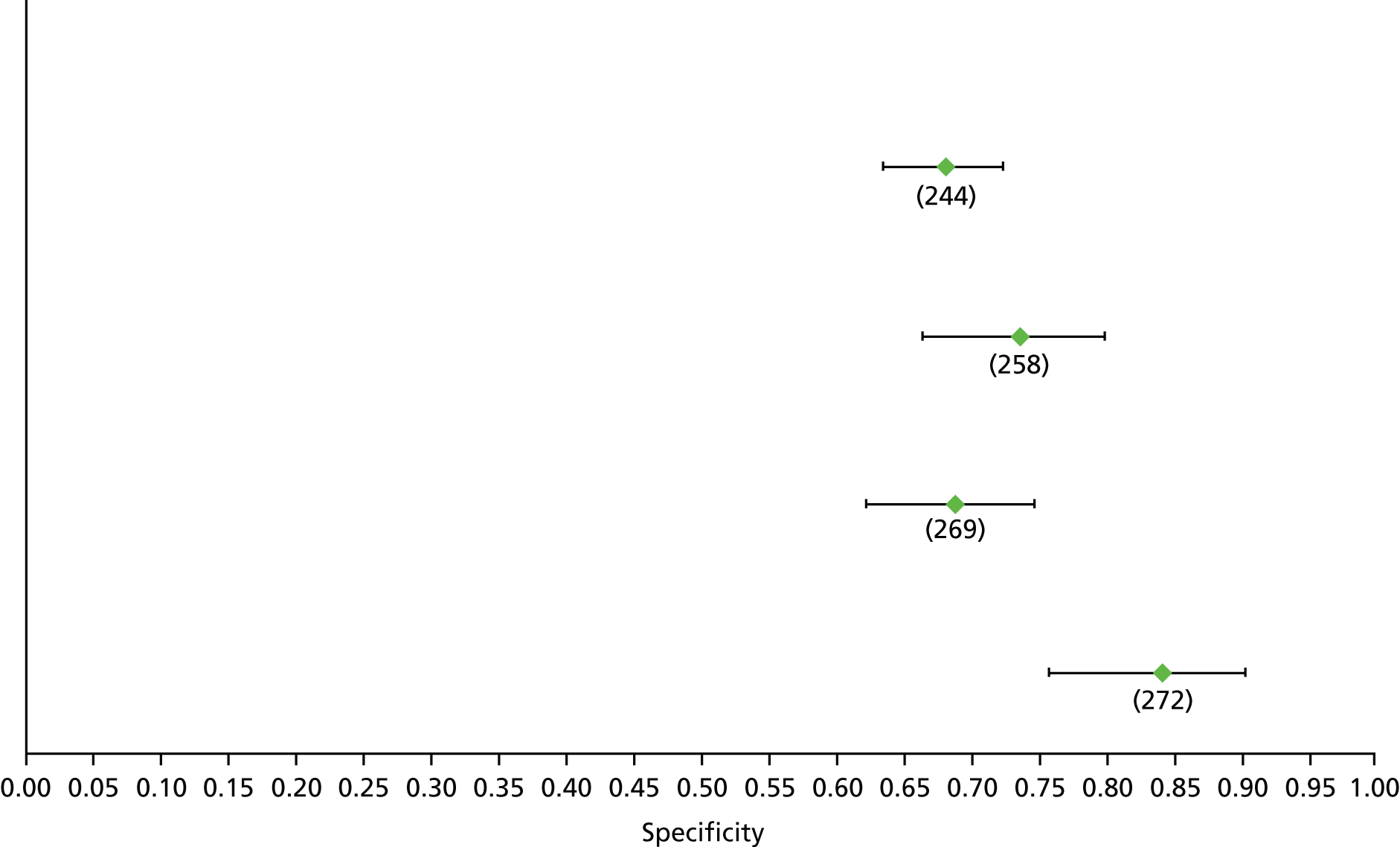
FIGURE 25.
Hepatitis B: Fibrotest F2 – sensitivity.

FIGURE 26.
Hepatitis B: Fibrotest F2 – specificity.

FIGURE 27.
Hepatitis B: TE F2 – sensitivity.

FIGURE 28.
Hepatitis B: TE F2 – specificity.
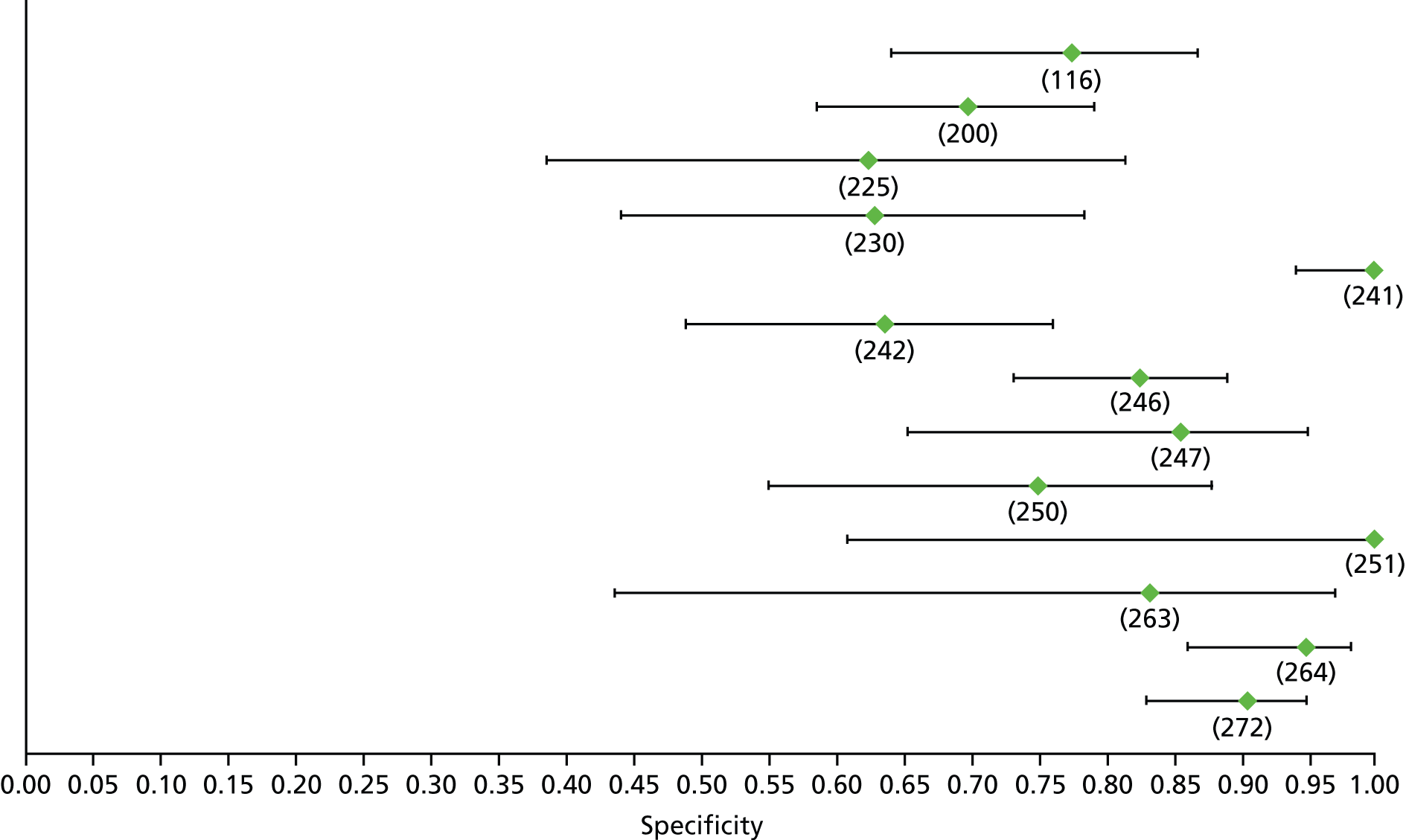
FIGURE 29.
Hepatitis B: FIB-4 F3 (low cut-off) – sensitivity.

FIGURE 30.
Hepatitis B: FIB-4 F3 (low cut-off) – specificity.
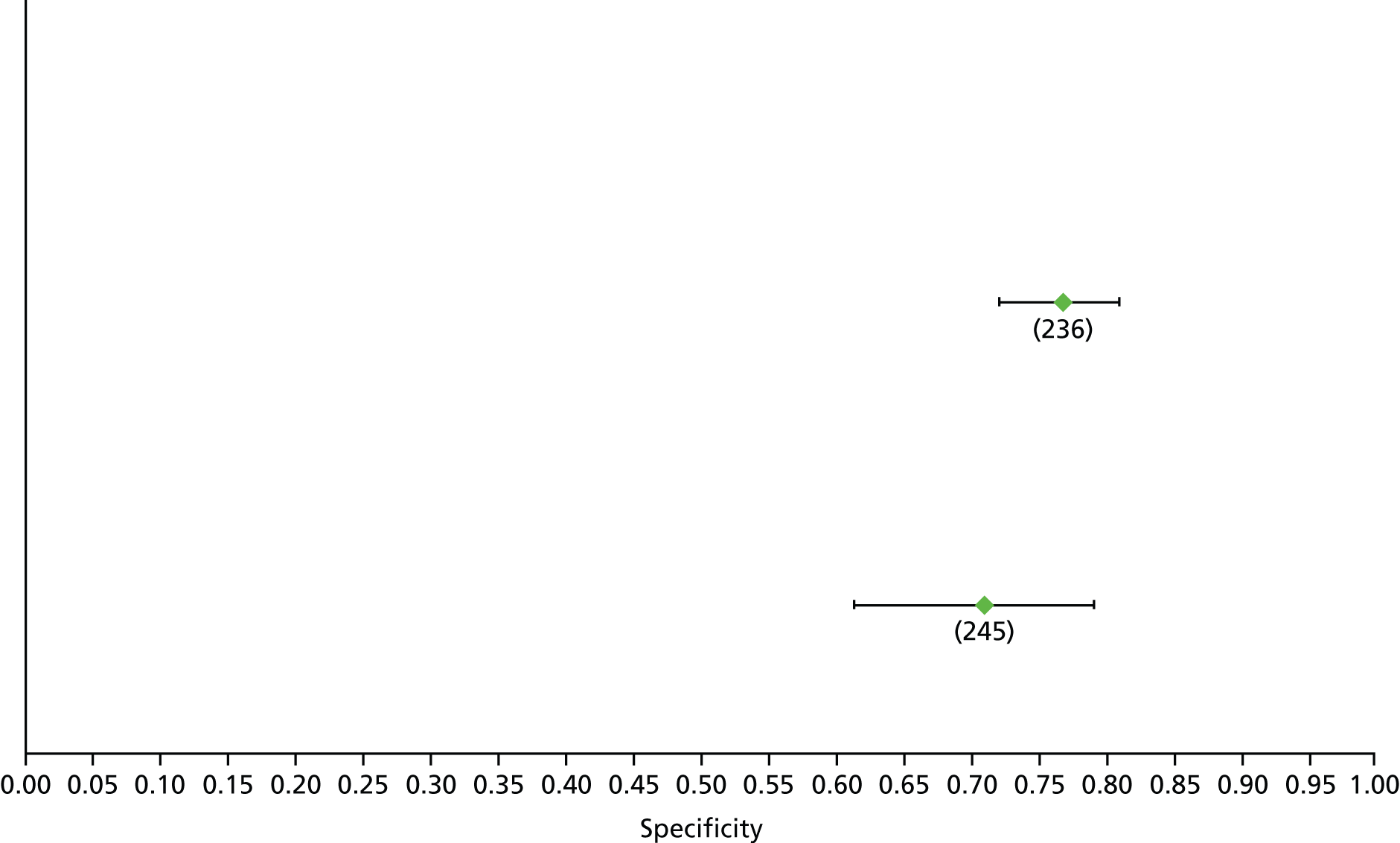
FIGURE 31.
Hepatitis B: Fibrotest F3 – sensitivity.
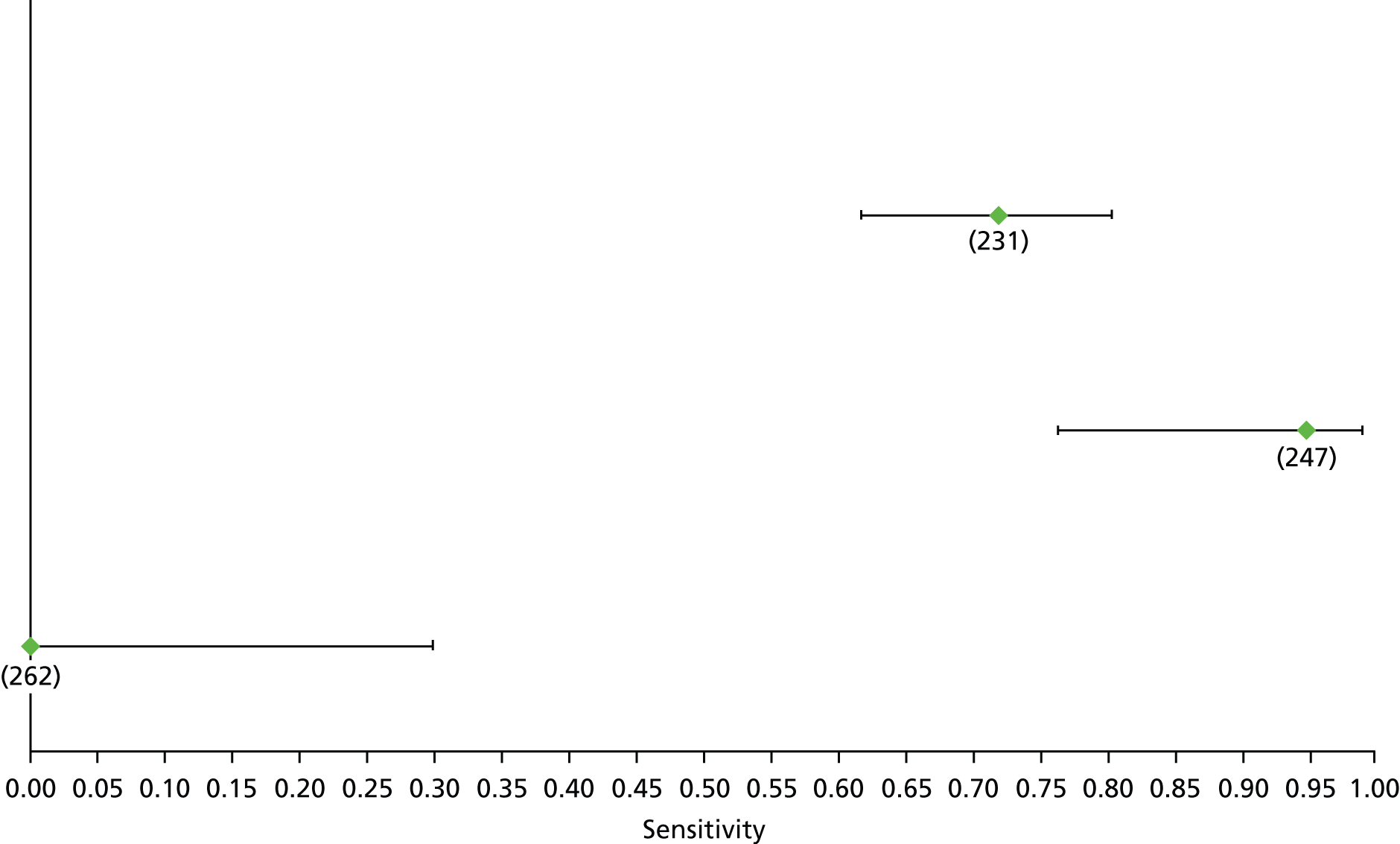
FIGURE 32.
Hepatitis B: Fibrotest F3 – specificity.
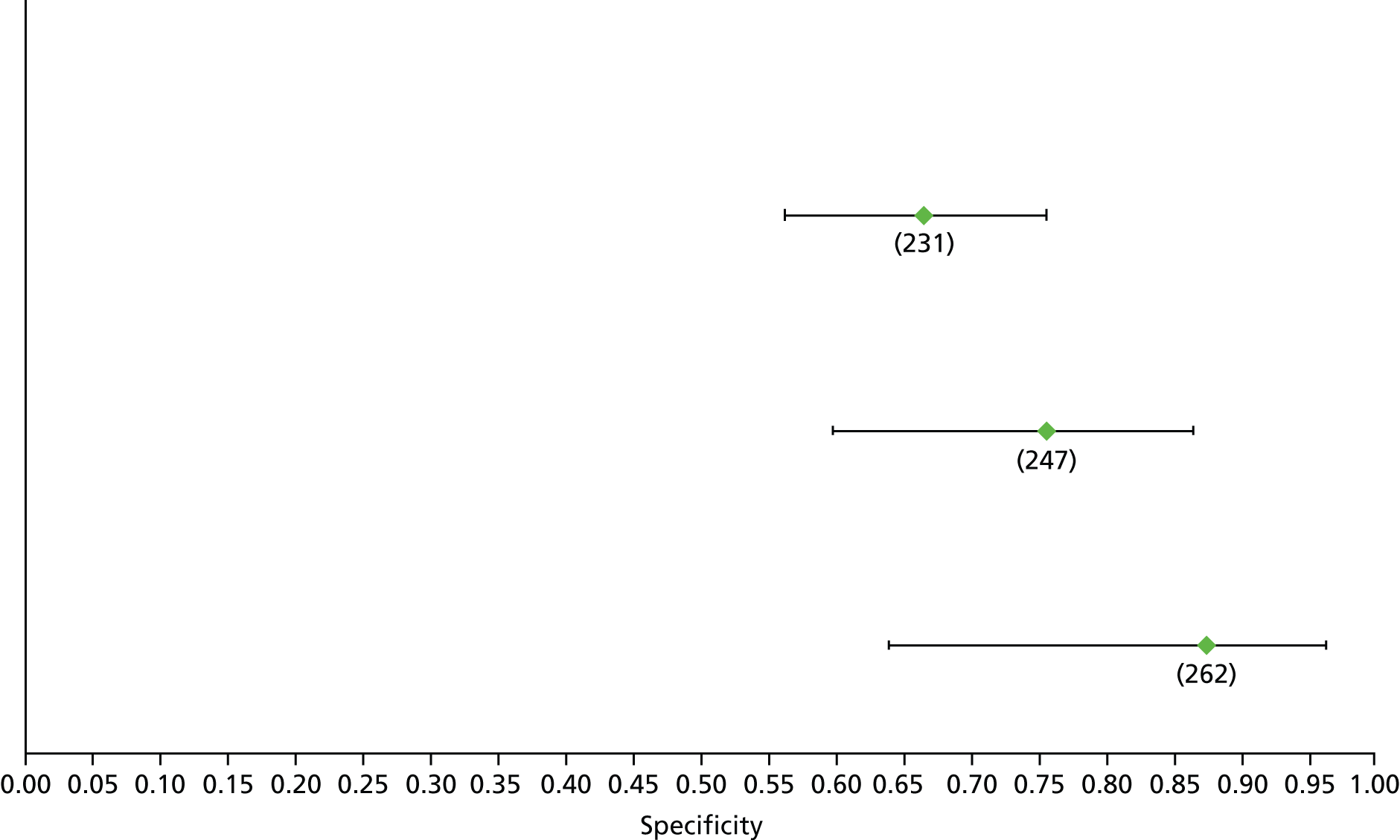
FIGURE 33.
Hepatitis B: Forns index F3 (high cut-off) – sensitivity. Val, validation cohort.
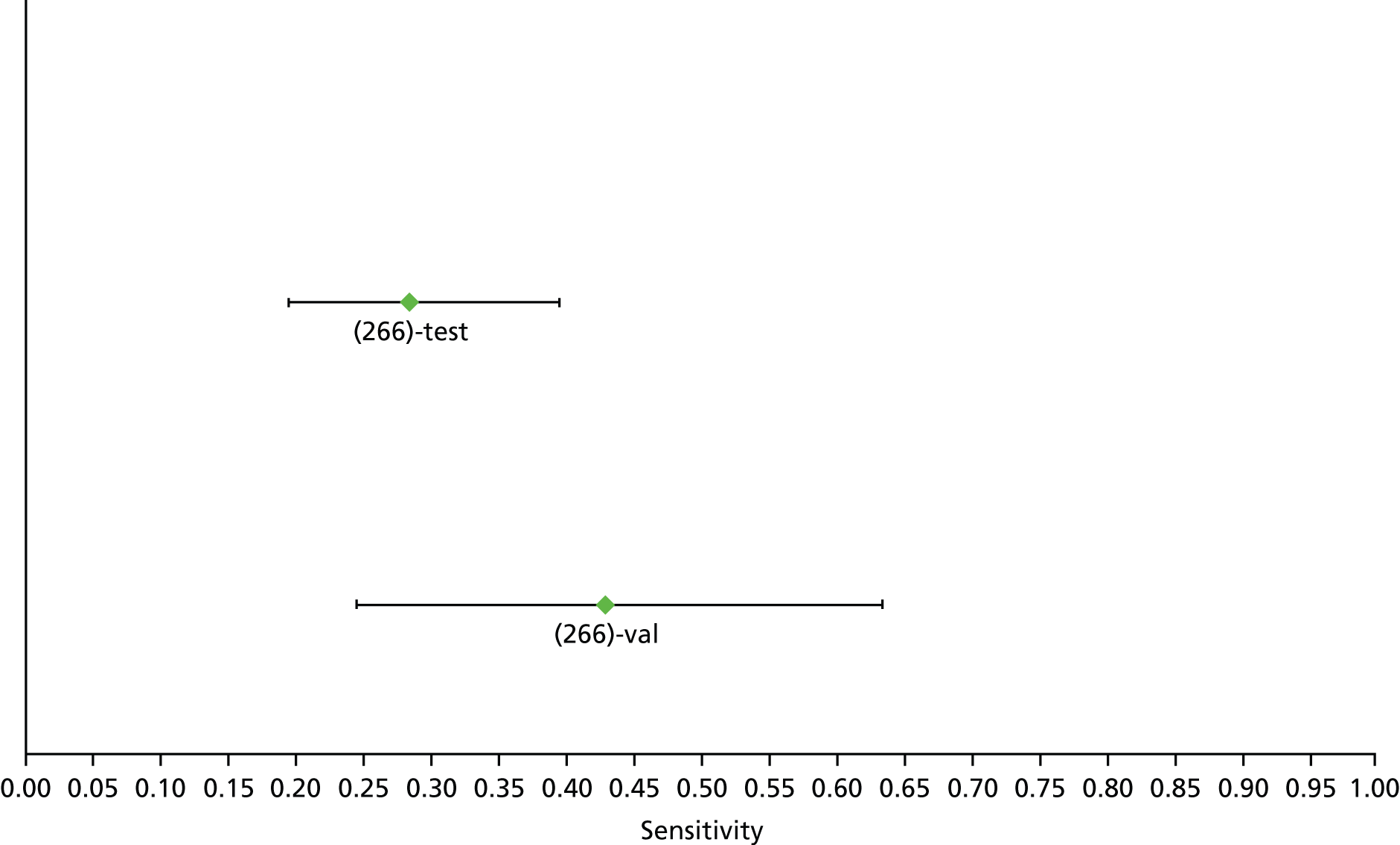
FIGURE 34.
Hepatitis B: Forns index F3 (high cut-off) – specificity. Val, validation cohort.
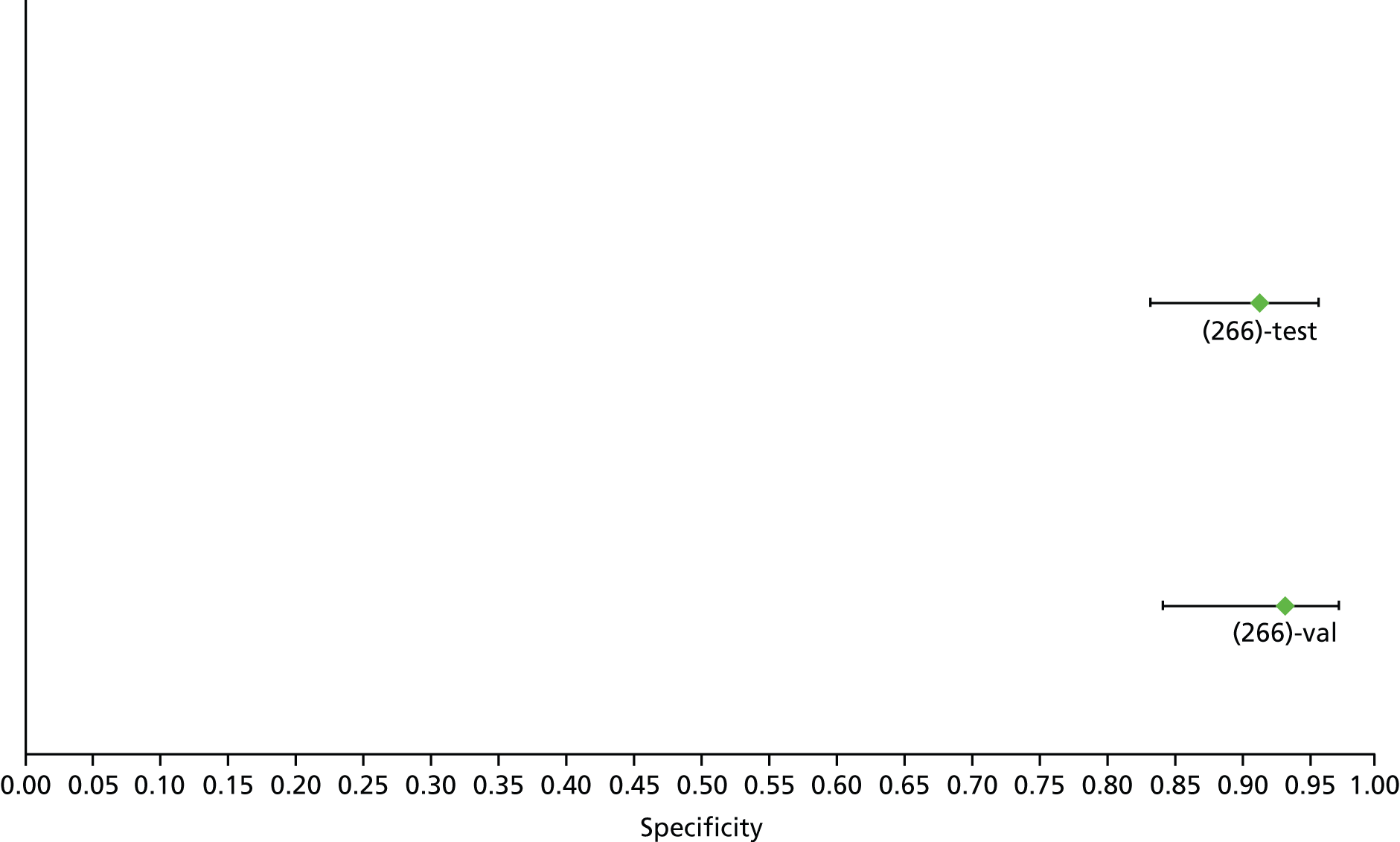
FIGURE 35.
Hepatitis B: Forns index F3 (low cut-off) – sensitivity. Val, validation cohort.
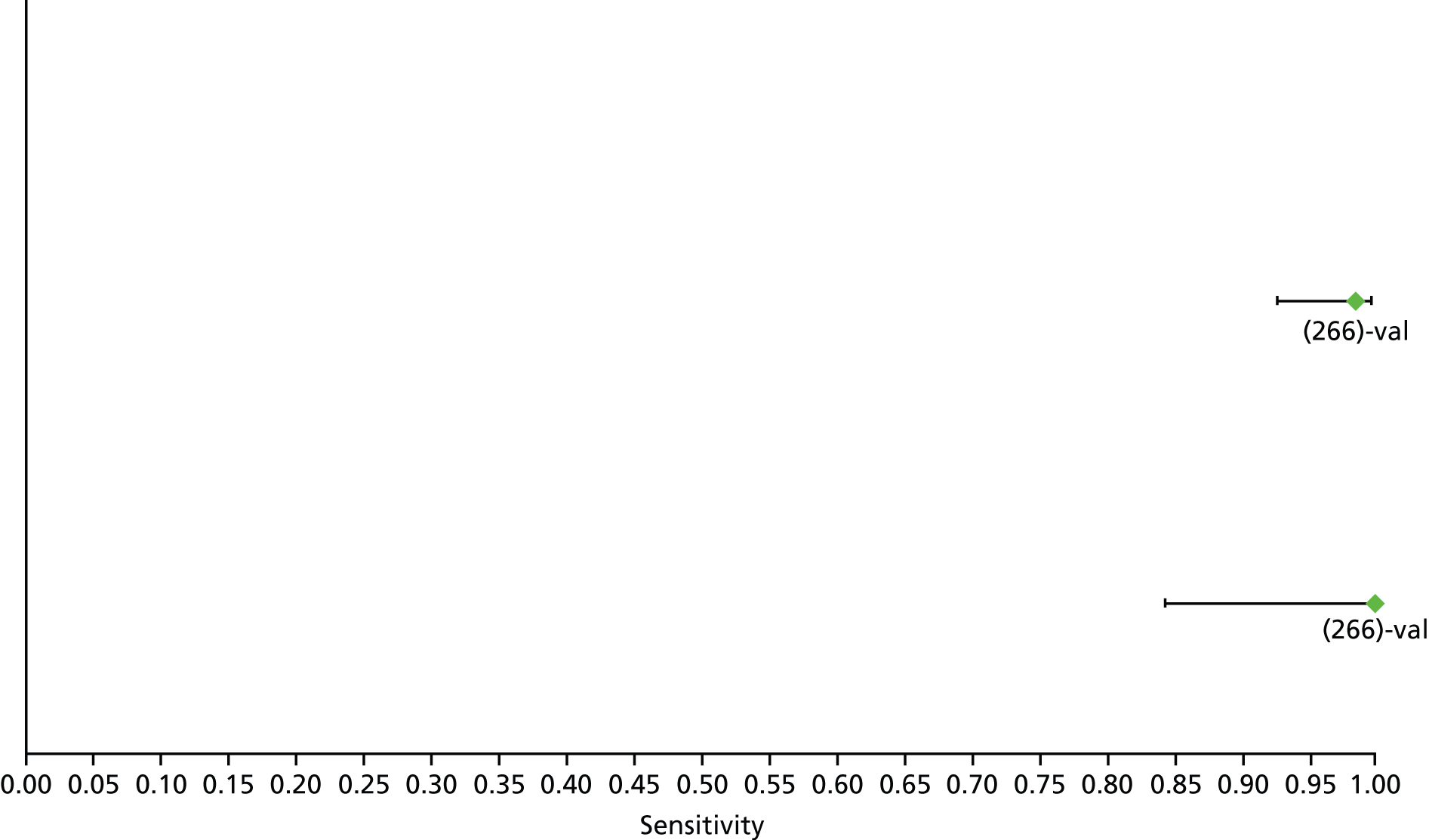
FIGURE 36.
Hepatitis B: Forns index F3 (low cut-off) – specificity. Val, validation cohort.

FIGURE 37.
Hepatitis B: Hui index F3 – sensitivity. Val, validation cohort.
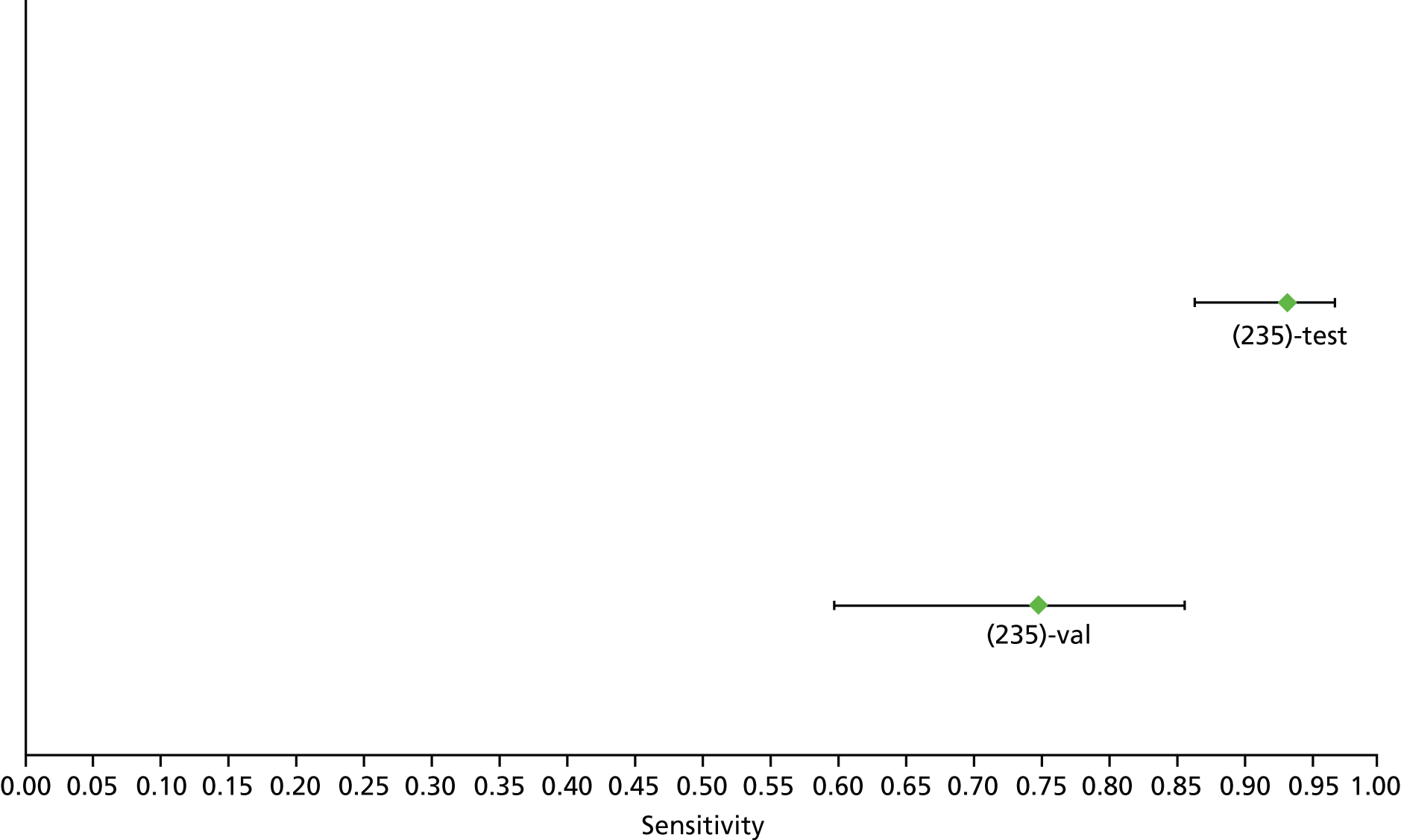
FIGURE 38.
Hepatitis B: Hui index F3 – specificity. Val, validation cohort.
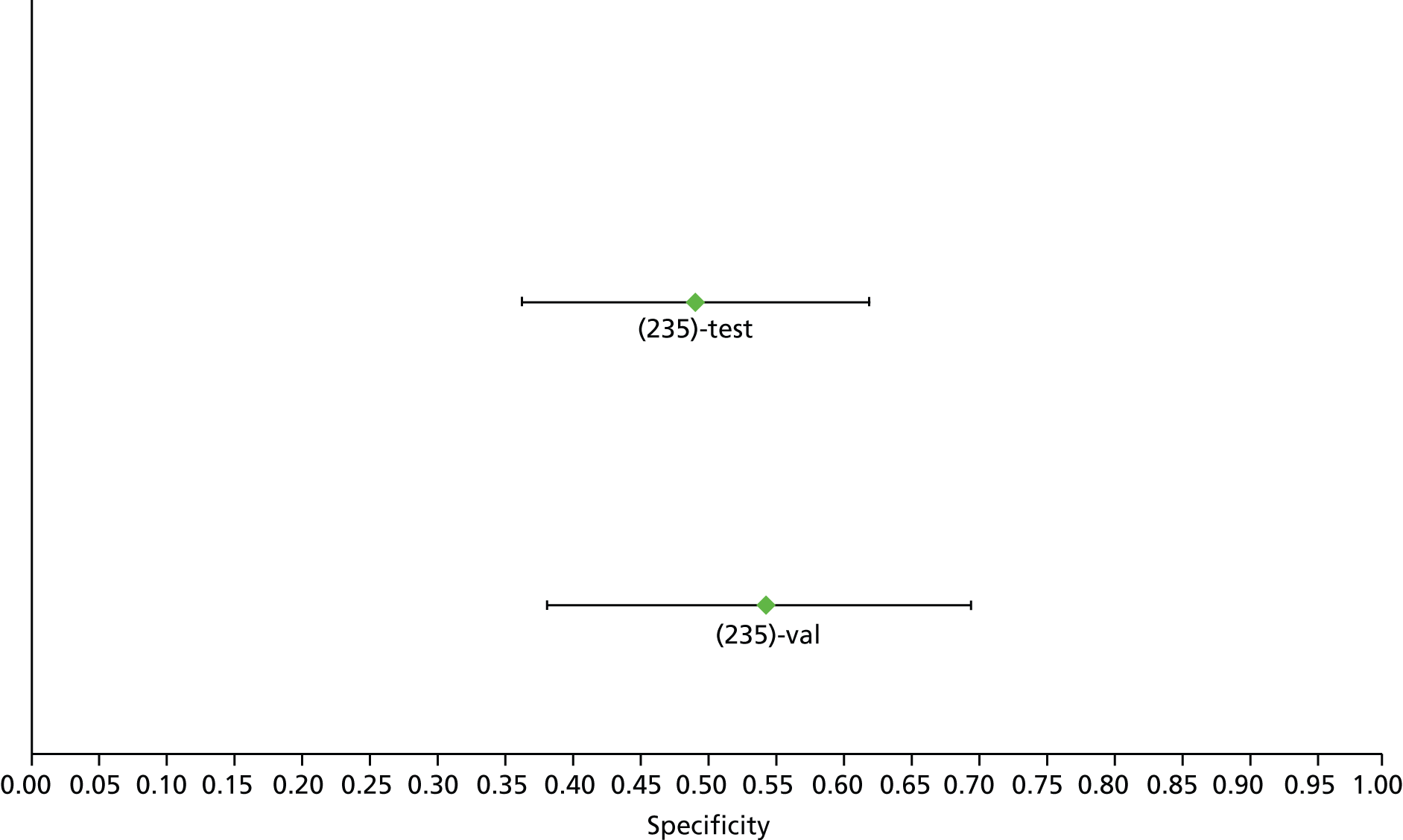
FIGURE 39.
Hepatitis B: TE F3 – sensitivity. Tx, treatment; val, validation cohort.
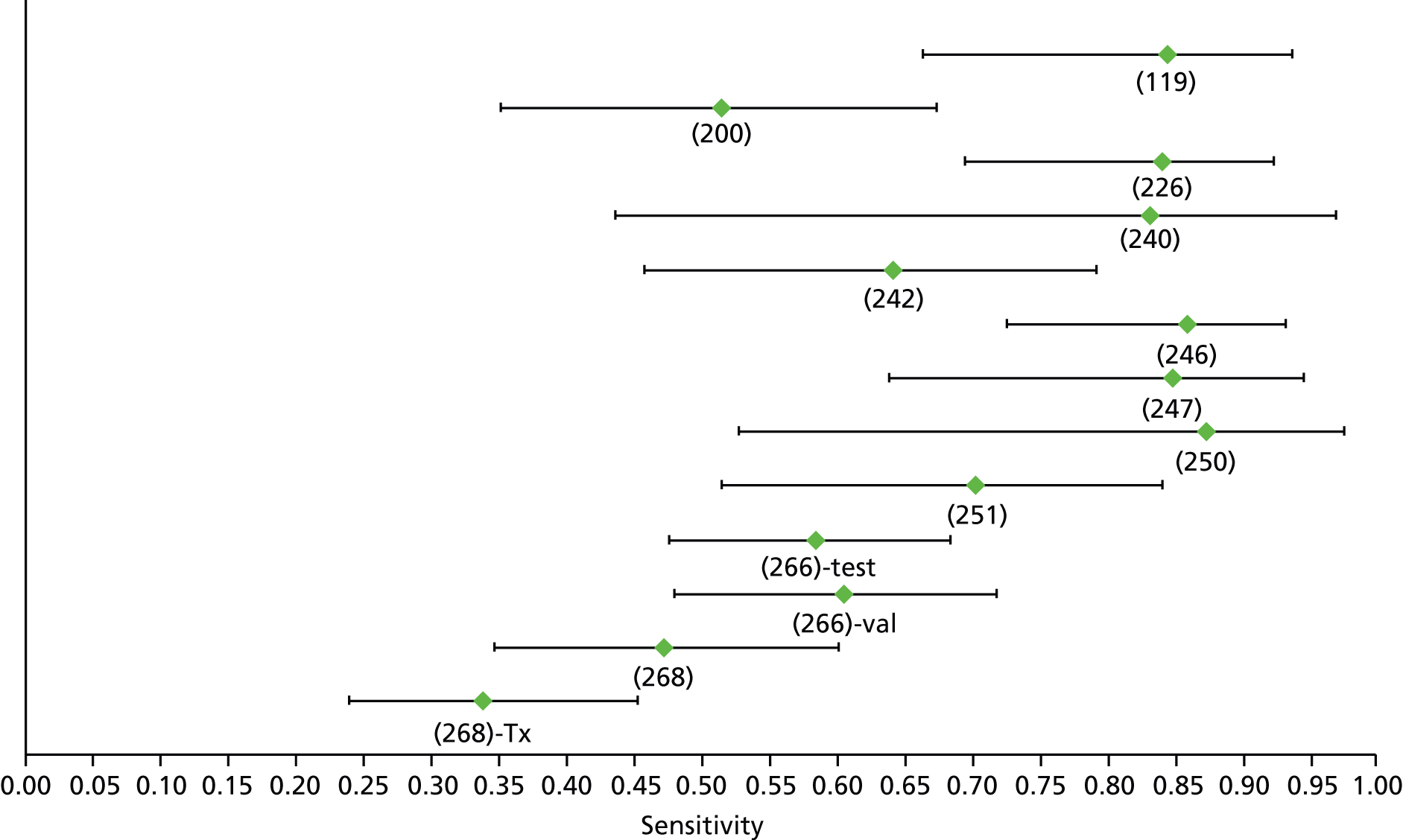
FIGURE 40.
Hepatitis B: TE F3 – specificity. Tx, treatment; val, validation cohort.

FIGURE 41.
Hepatitis B: AST–ALT ratio F4 – sensitivity.

FIGURE 42.
Hepatitis B: AST–ALT F4 – specificity.

FIGURE 43.
Hepatitis B: APRI F4 (high cut-off) – sensitivity.
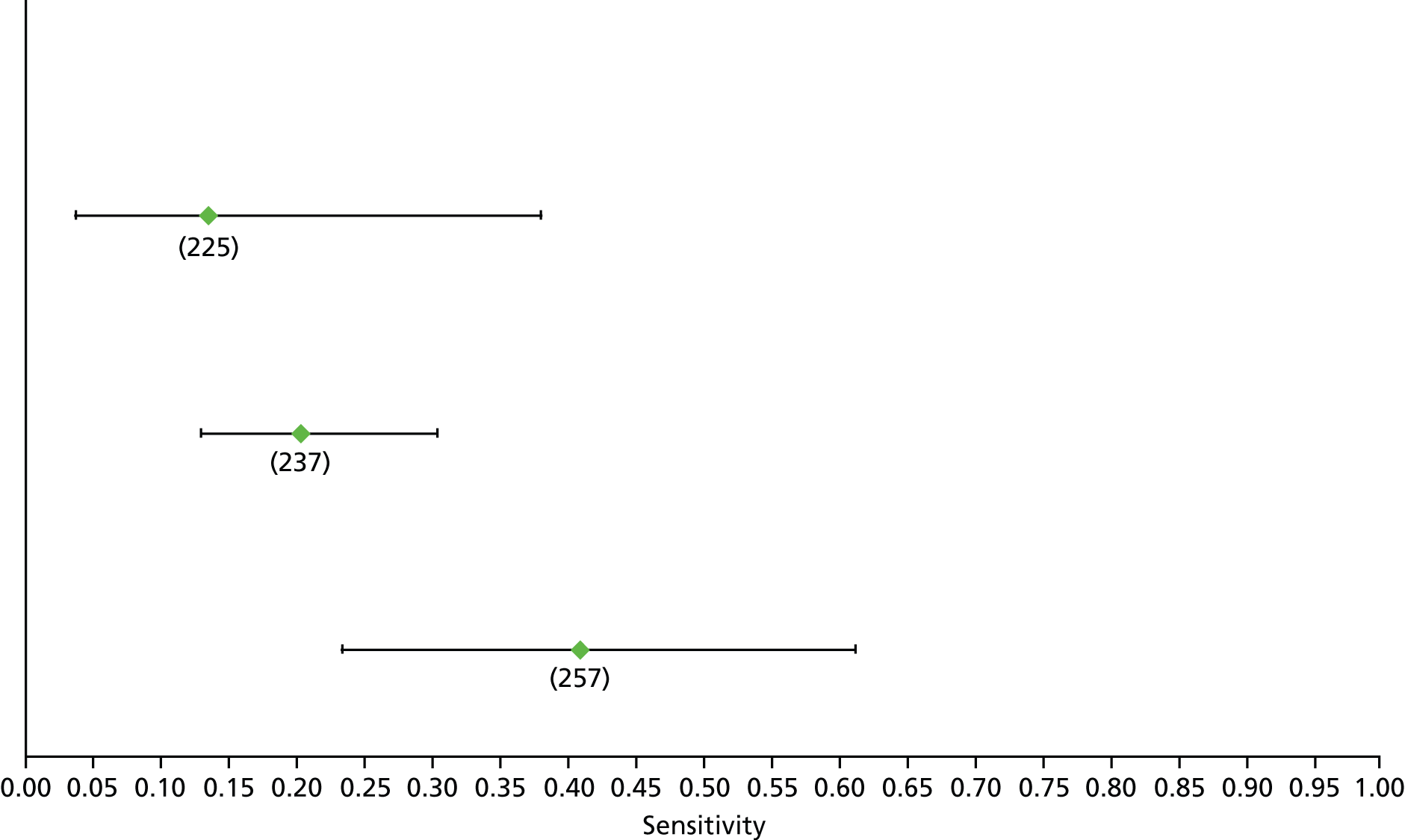
FIGURE 44.
Hepatitis B: APRI F4 (high cut-off) – specificity.

FIGURE 45.
Hepatitis B: APRI F4 (low cut-off) – sensitivity.
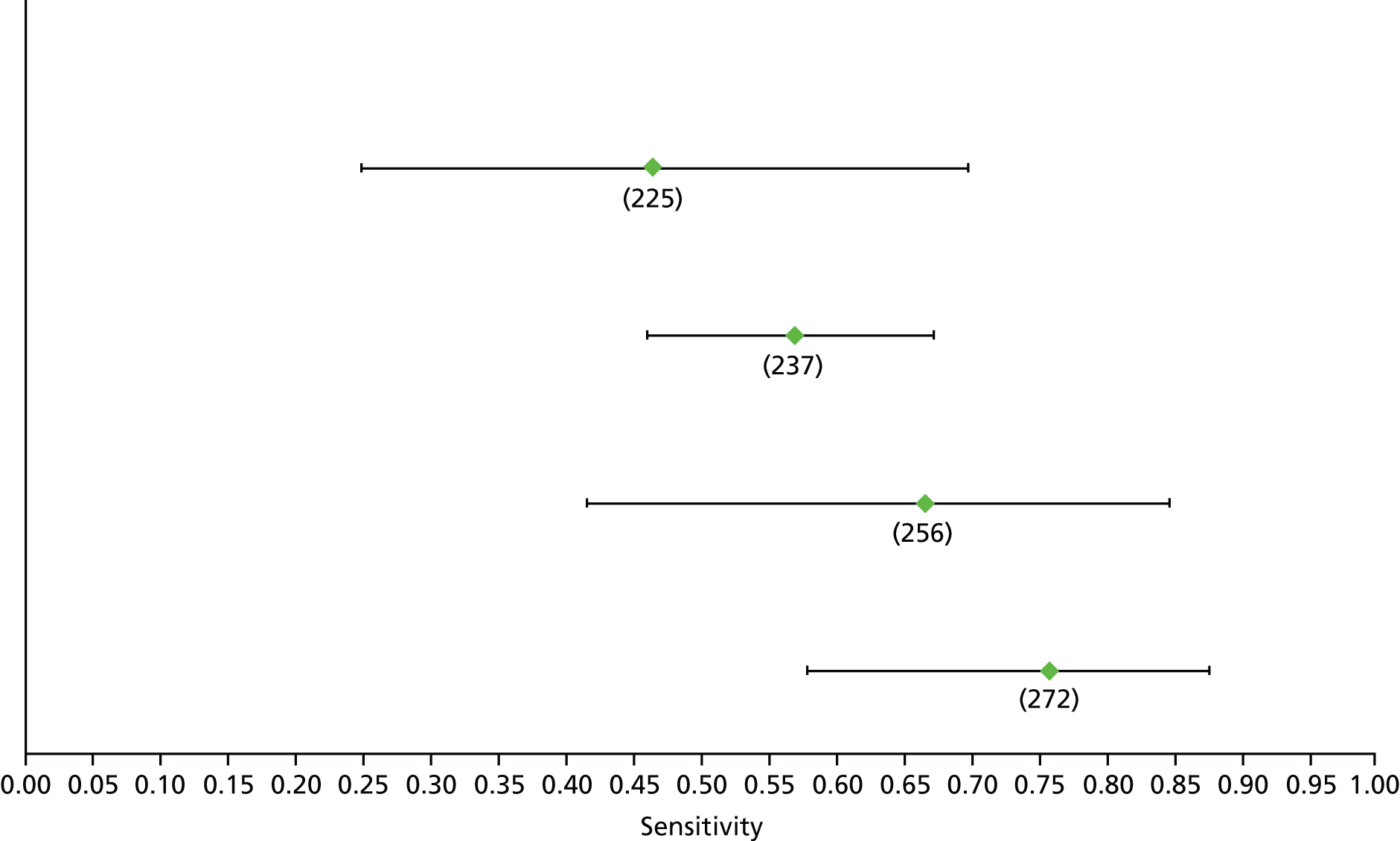
FIGURE 46.
Hepatitis B: APRI F4 (low cut-off) – specificity.
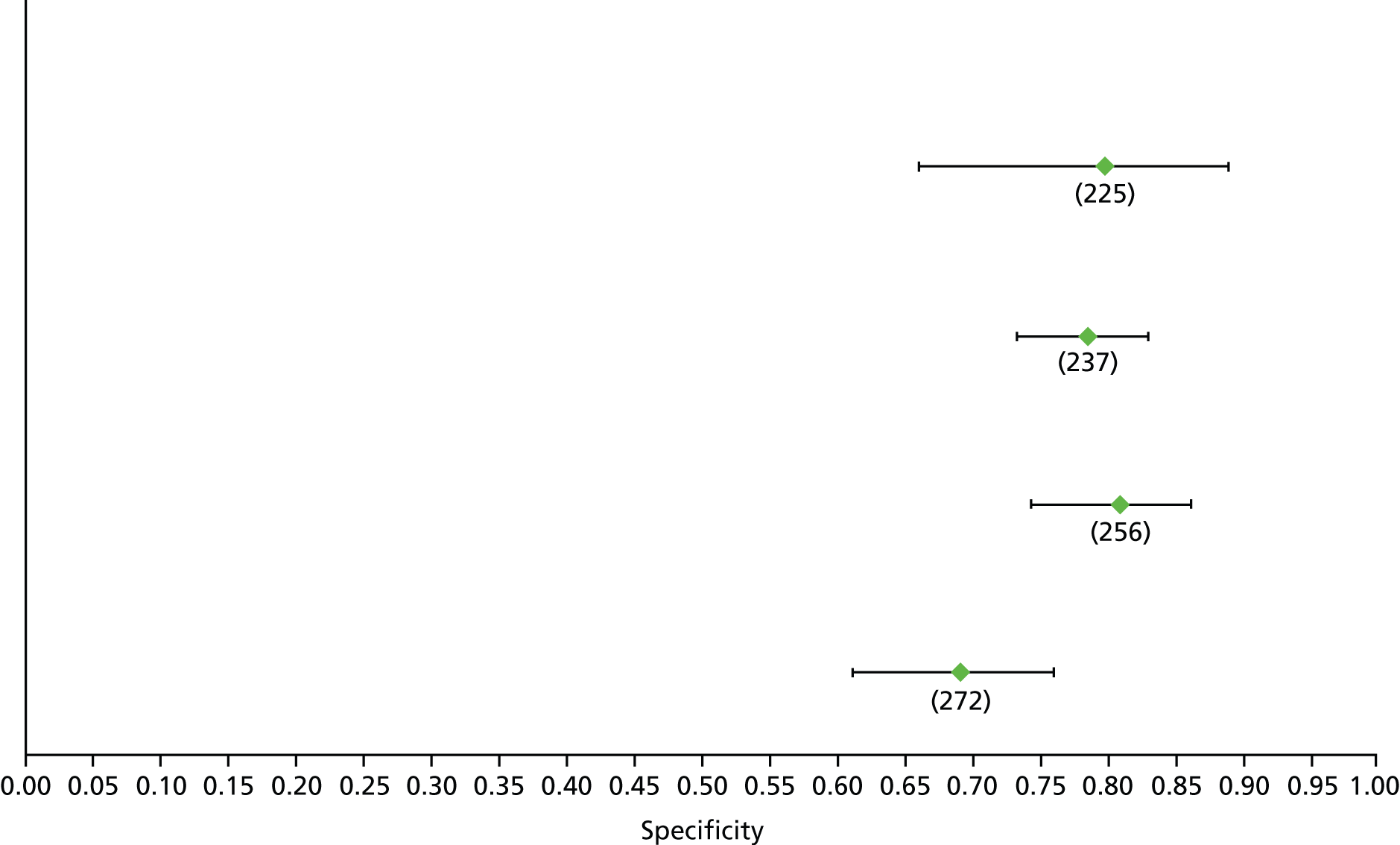
FIGURE 47.
Hepatitis B: FIB-4 F4 (low cut-off) – sensitivity.
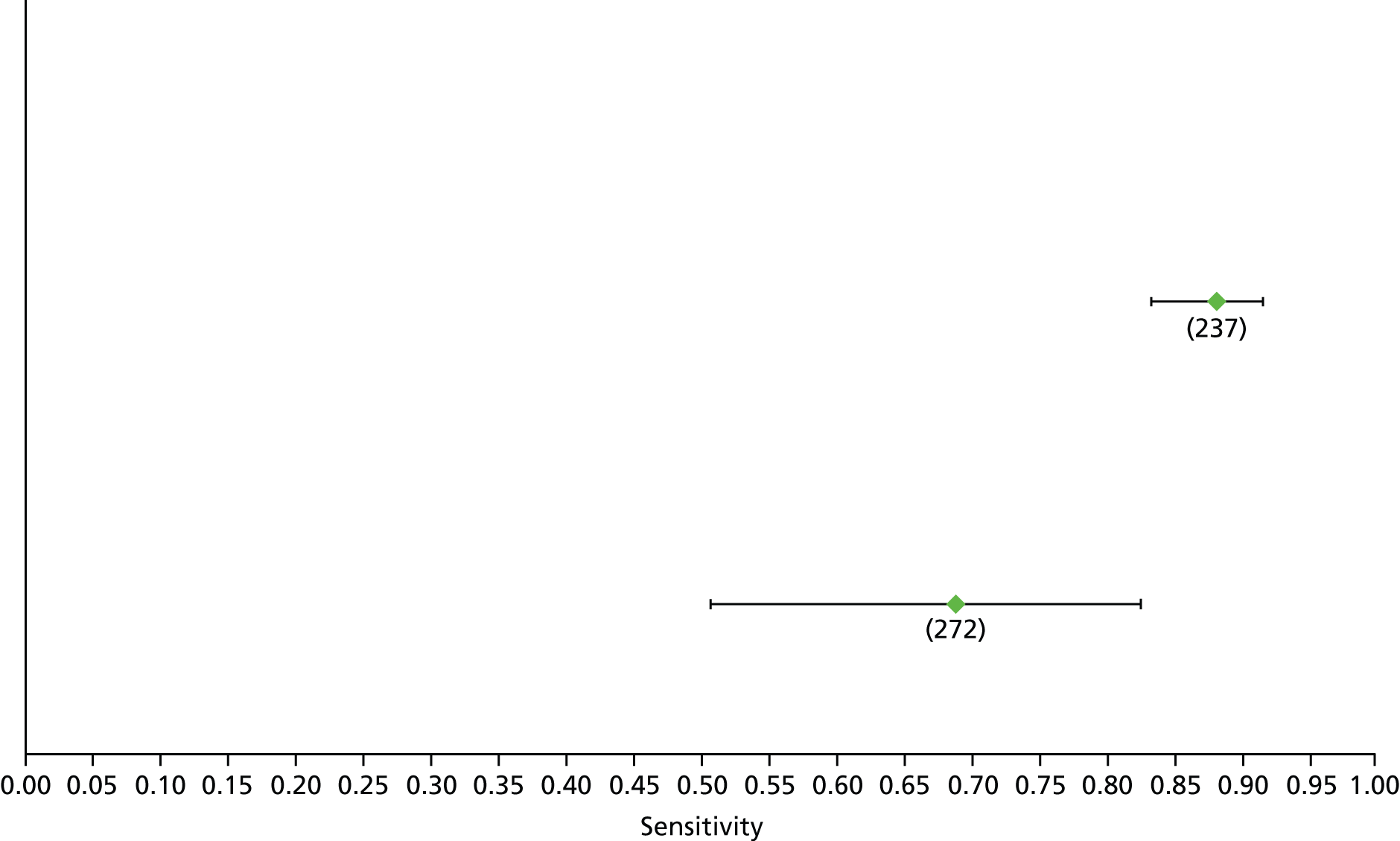
FIGURE 48.
Hepatitis B: FIB-4 F4 (low cut-off) – specificity.

FIGURE 49.
Hepatitis B: Fibrotest F4 – sensitivity.
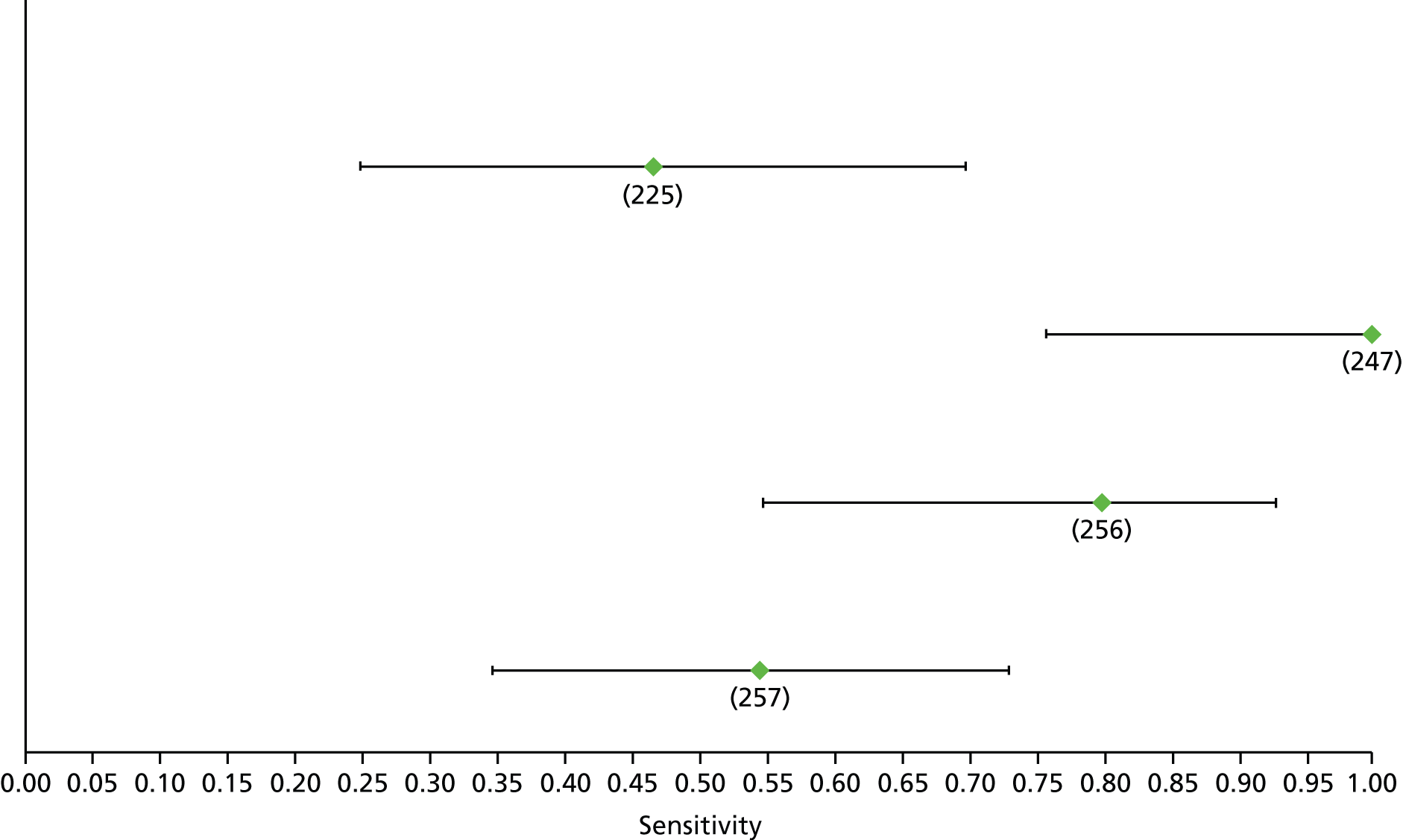
FIGURE 50.
Hepatitis B: Fibrotest F4 – specificity.
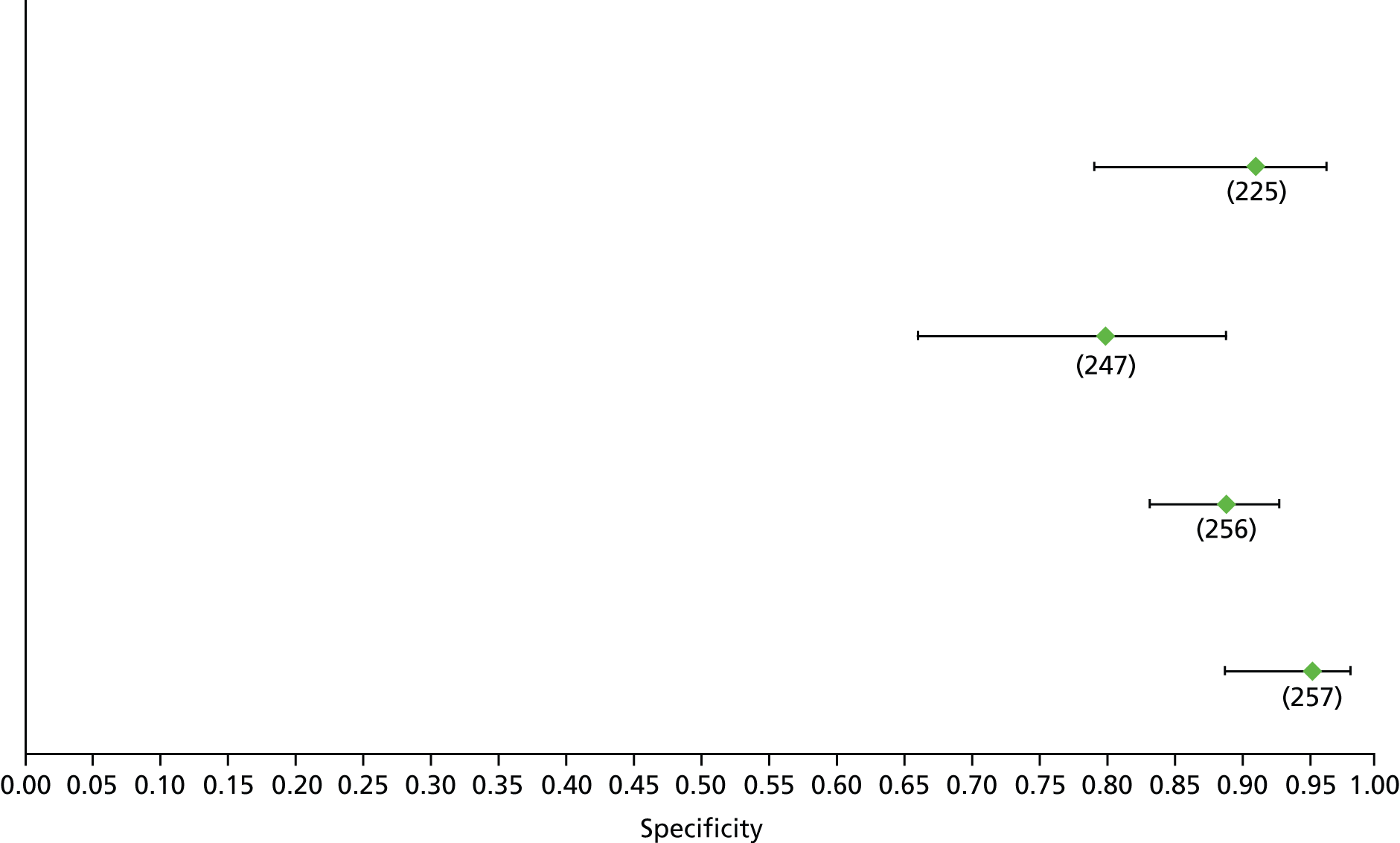
FIGURE 51.
Hepatitis B: TE F4 – sensitivity.

FIGURE 52.
Hepatitis B: TE F4 – specificity.
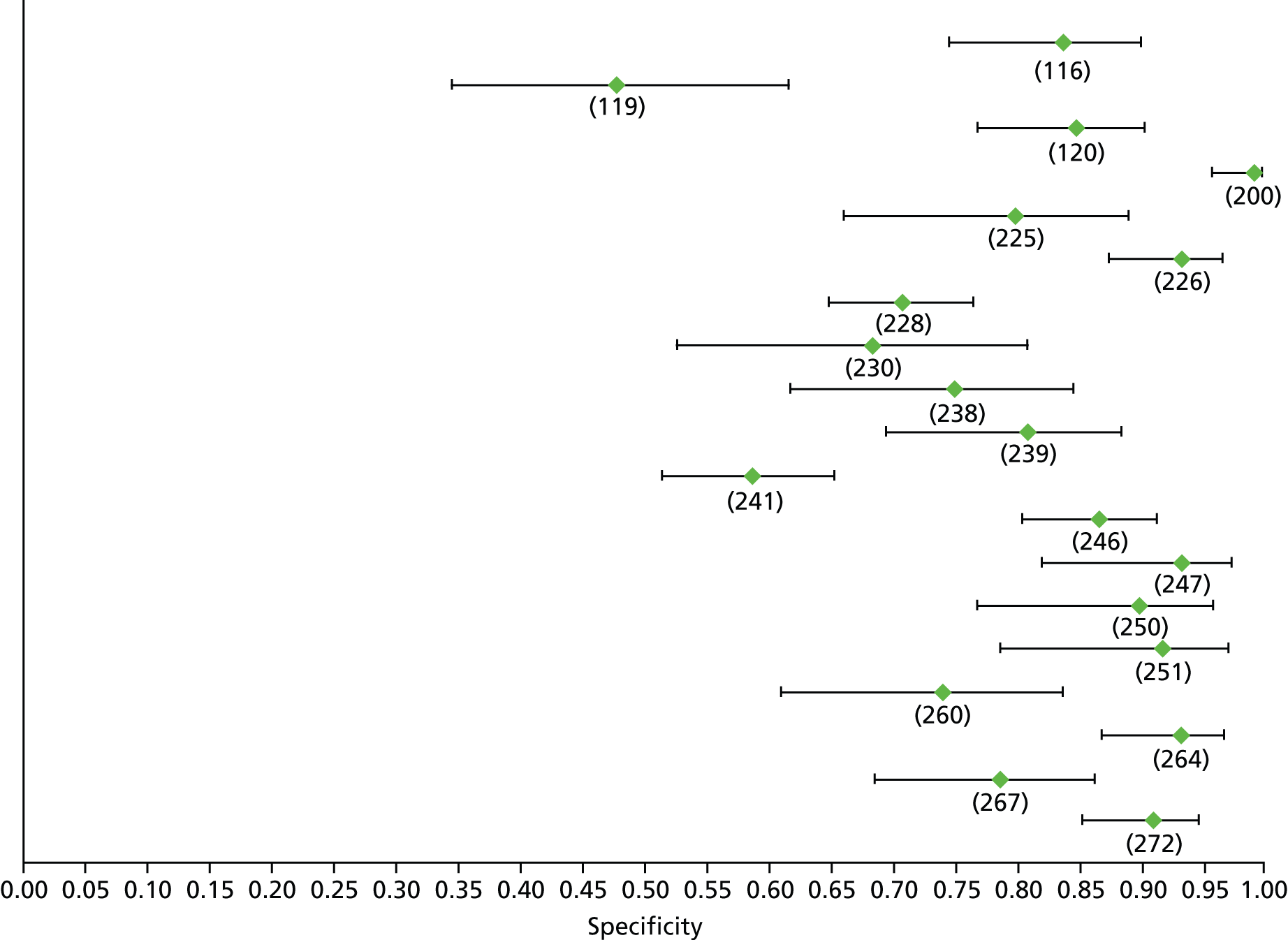
FIGURE 53.
Hepatitis C: TE F1 – sensitivity.

FIGURE 54.
Hepatitis C: TE F1 – specificity.
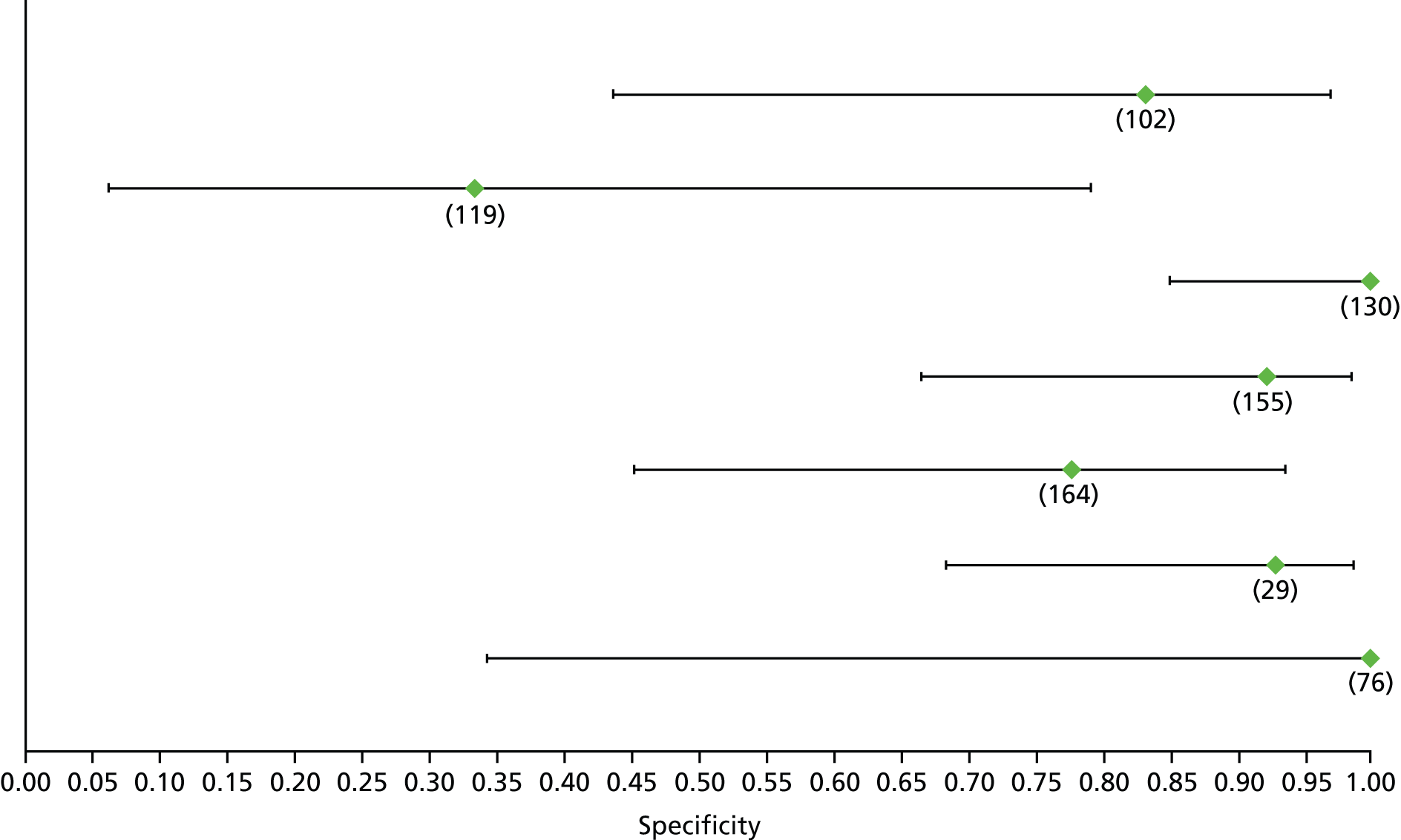
FIGURE 55.
Hepatitis C: APRI F2 (high cut-off) – sensitivity. Pros, prospective; val, validation cohort.

FIGURE 56.
Hepatitis C: APRI F2 (high cut-off) – specificity. Pros, prospective; val, validation cohort.

FIGURE 57.
Hepatitis C: APRI F2 (low cut-off) – sensitivity. Pros, prospective; retr, retrospective; val, validation cohort.
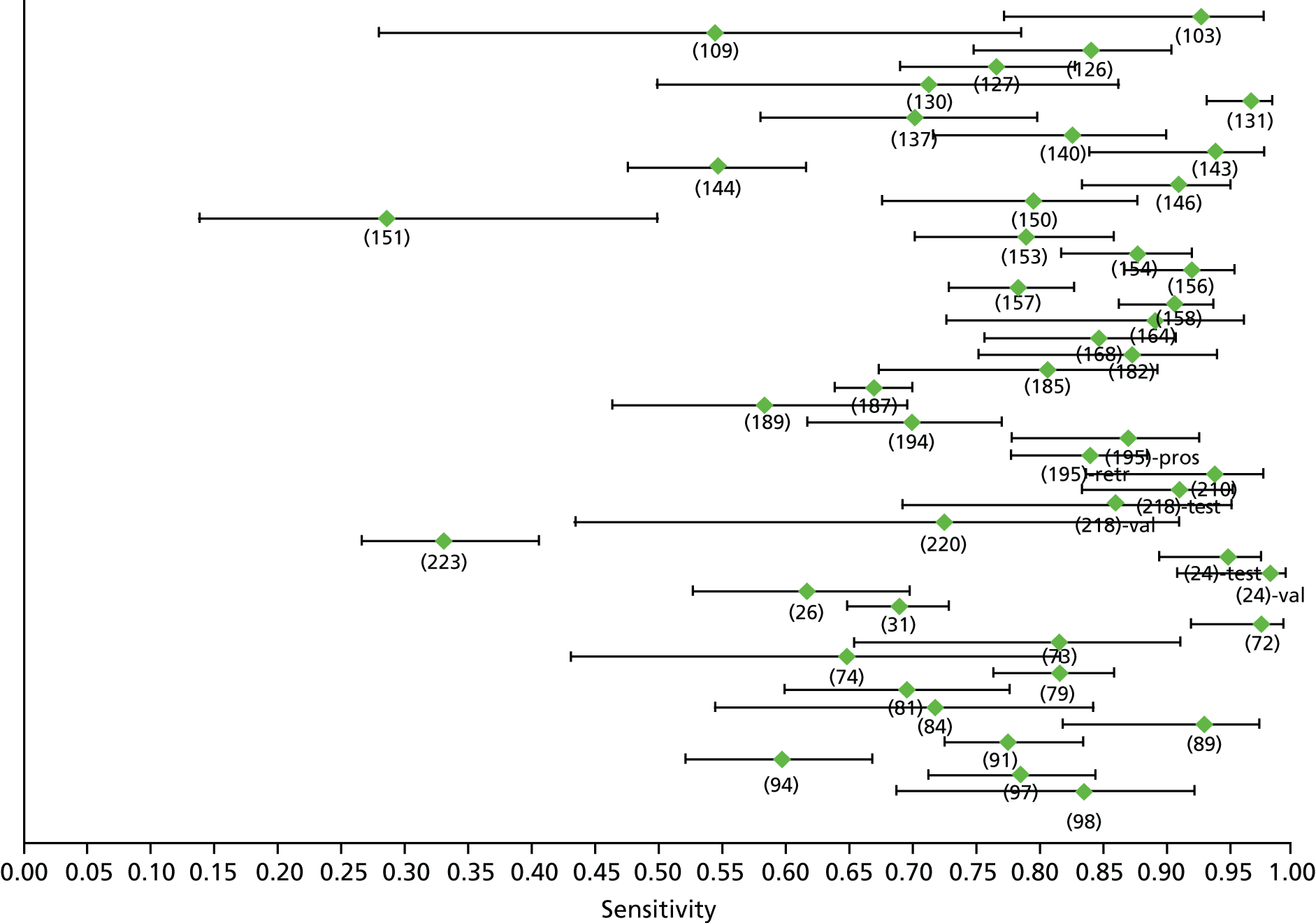
FIGURE 58.
Hepatitis C: APRI F2 (low cut-off) – specificity. Pros, prospective; retr, retrospective; val, validation cohort.

FIGURE 59.
Hepatitis C: AST–ALT (F2) – sensitivity. EALT, elevated ALT; NALT, normal ALT.

FIGURE 60.
Hepatitis C: AST–ALT (F2) – specificity. EALT, elevated ALT; NALT, normal ALT.
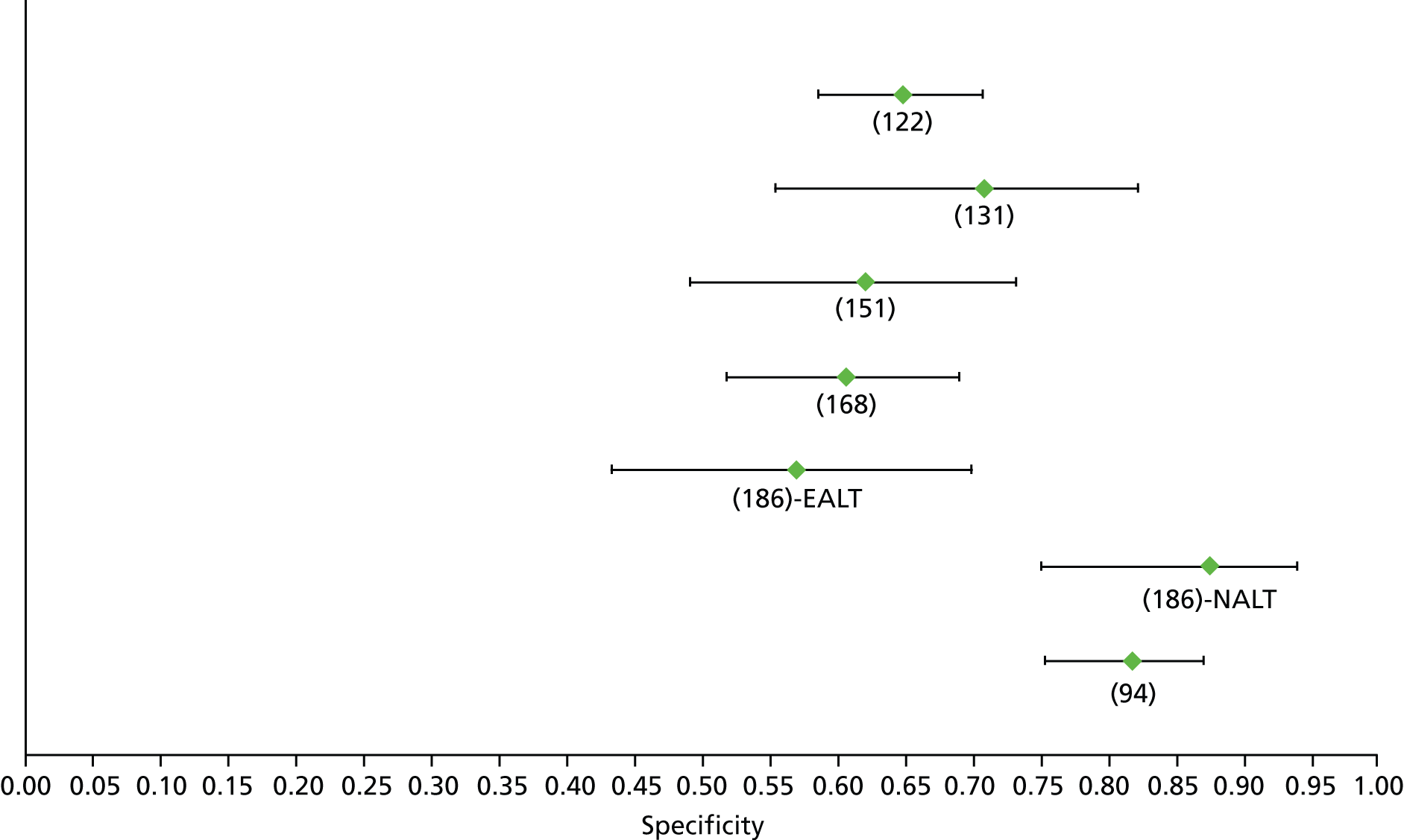
FIGURE 61.
Hepatitis C: FIB-4 F2 (high cut-off) – sensitivity. Tot, total; val, validation cohort.

FIGURE 62.
Hepatitis C: FIB-4 F2 (high cut-off) – specificity. Tot, total; val, validation cohort.

FIGURE 63.
Hepatitis C: FIB-4 F2 (low cut-off) – sensitivity. Tot, total; val, validation cohort.

FIGURE 64.
Hepatitis C: FIB-4 F2 (low cut-off) – specificity. Tot, total; val, validation cohort.
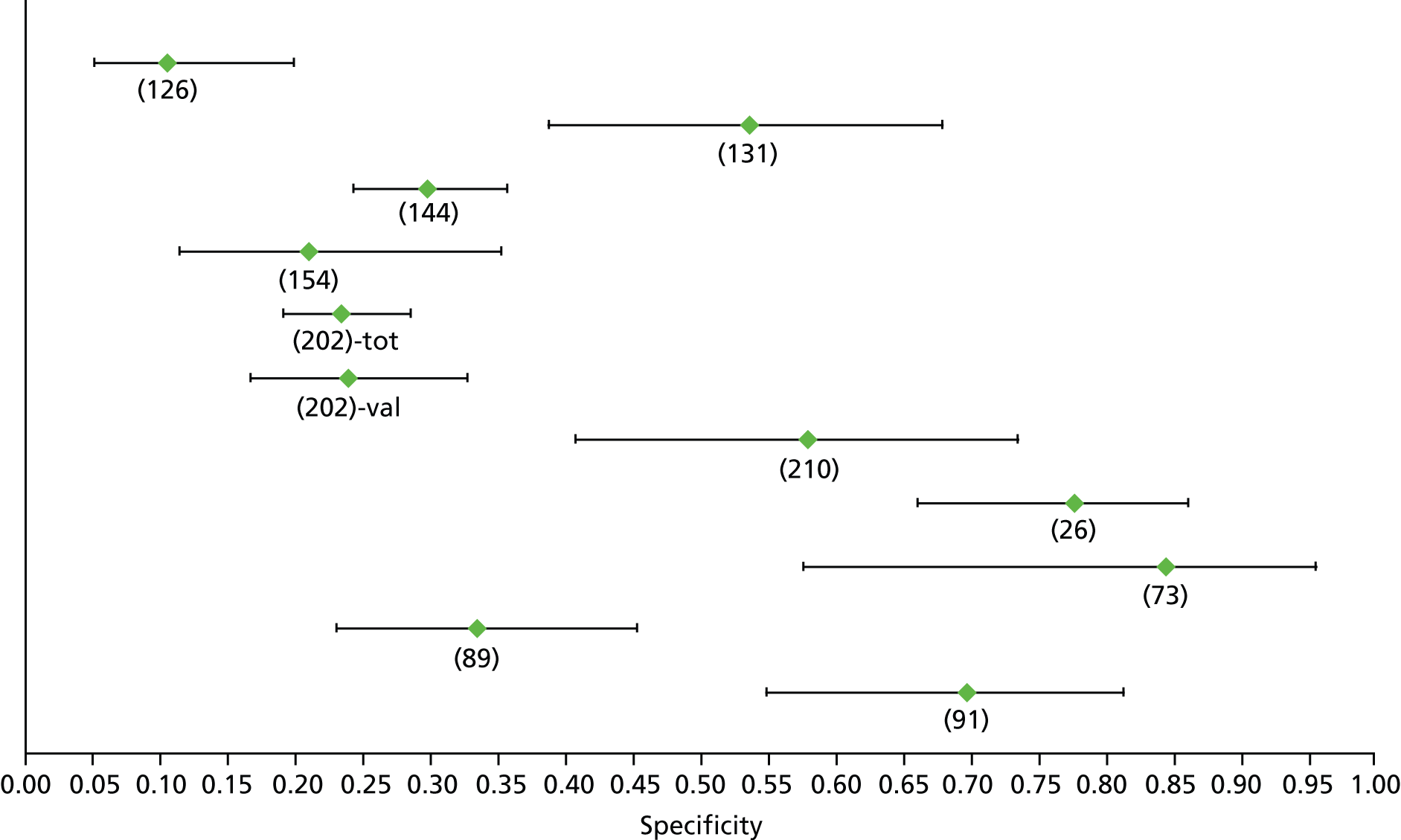
FIGURE 65.
Hepatitis C: Fibroindex F2 (high cut-off) – sensitivity. EALT, elevated ALT; NALT, normal ALT; test, derivation cohort; val, validation cohort.
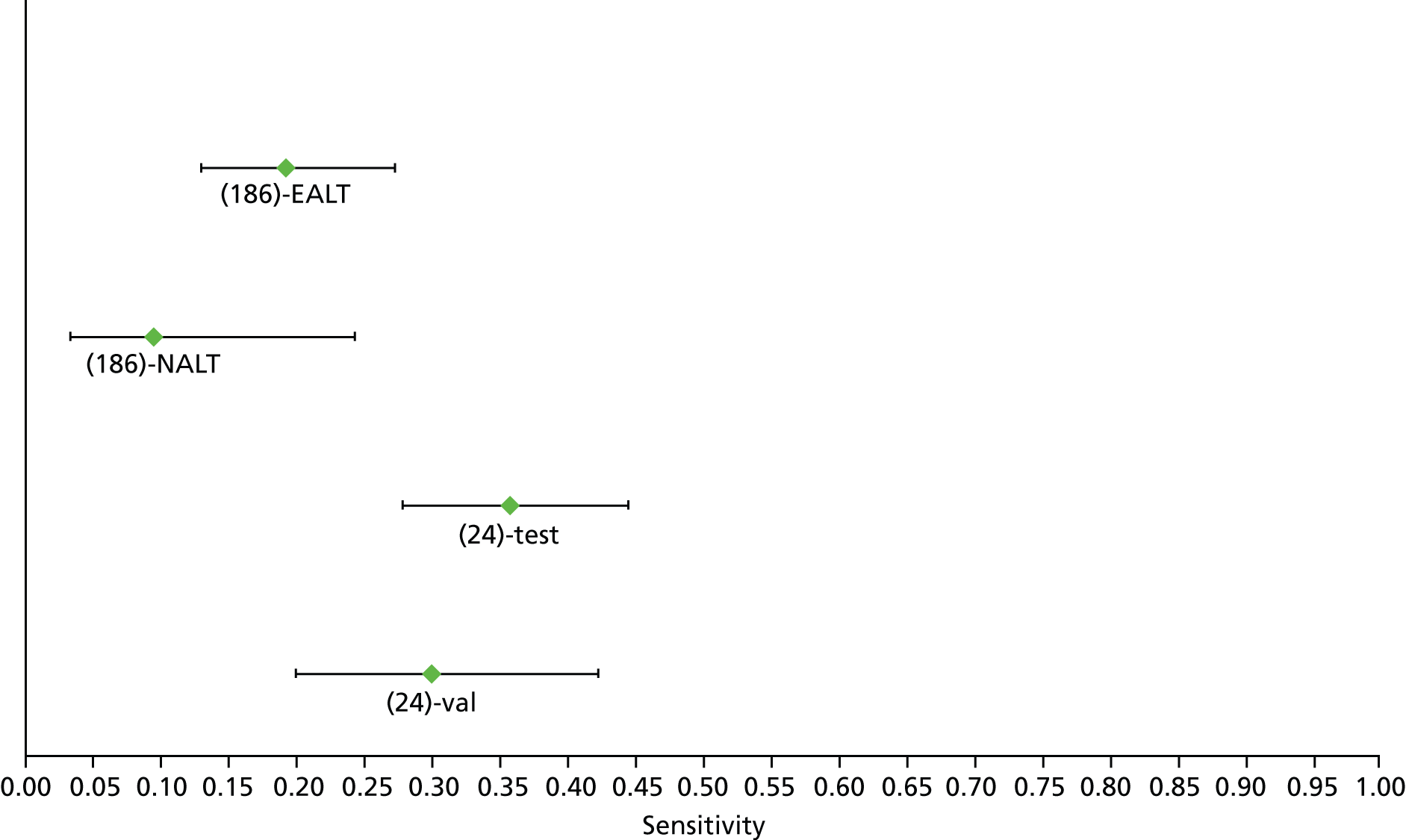
FIGURE 66.
Hepatitis C: Fibroindex F2 (high cut-off) – specificity. EALT, elevated ALT; NALT, normal ALT; test, derivation cohort; val, validation cohort.
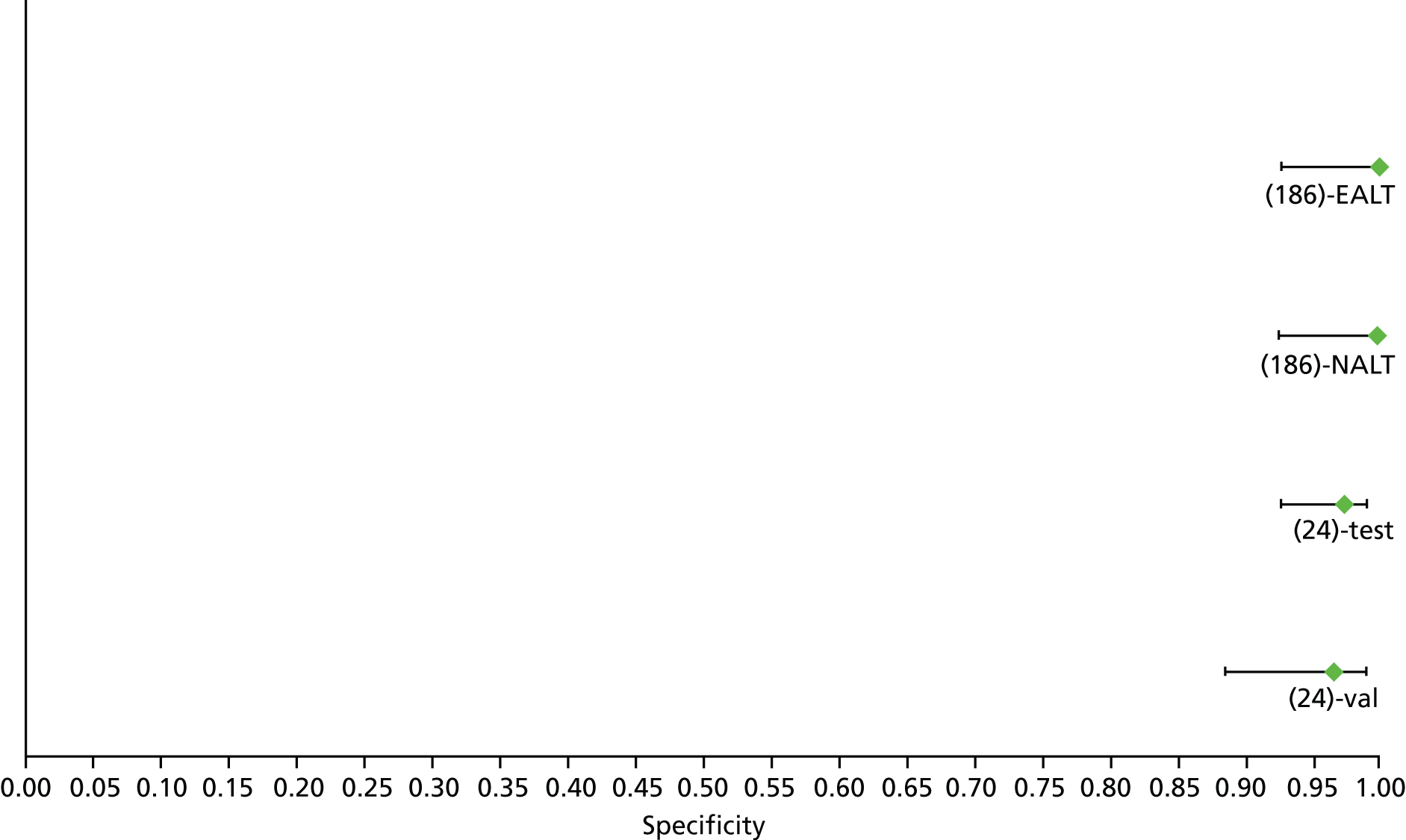
FIGURE 67.
Hepatitis C: Fibroindex F2 (low cut-off) – sensitivity. EALT, elevated ALT; NALT, normal ALT; test, derivation cohort; val, validation cohort.

FIGURE 68.
Hepatitis C: Fibroindex F2 (low cut-off) – specificity. EALT, elevated ALT; NALT, normal ALT; test, derivation cohort; val, validation cohort.
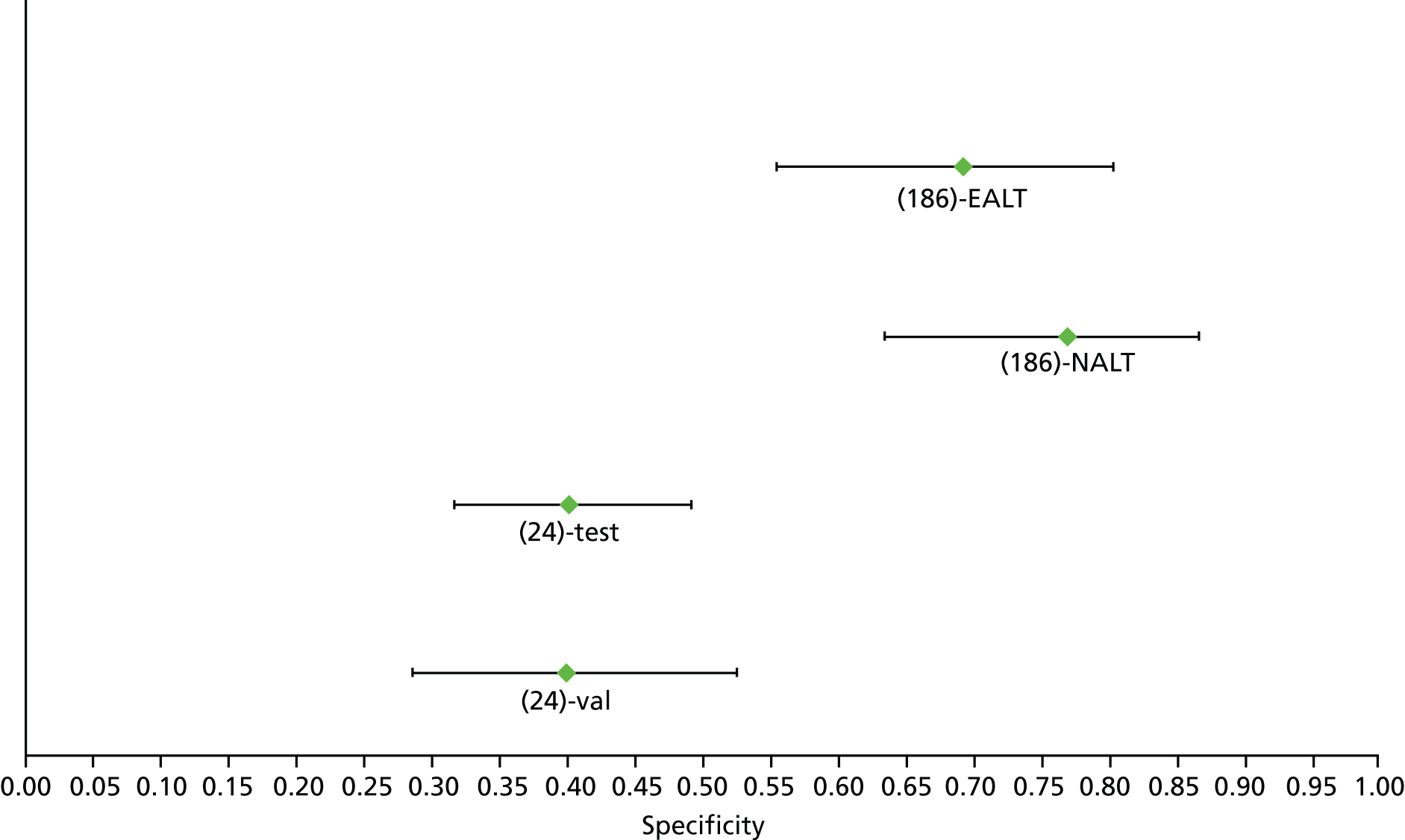
FIGURE 69.
Hepatitis C: Fibrometer F2 – sensitivity.

FIGURE 70.
Hepatitis C: Fibrometer F2 – specificity.
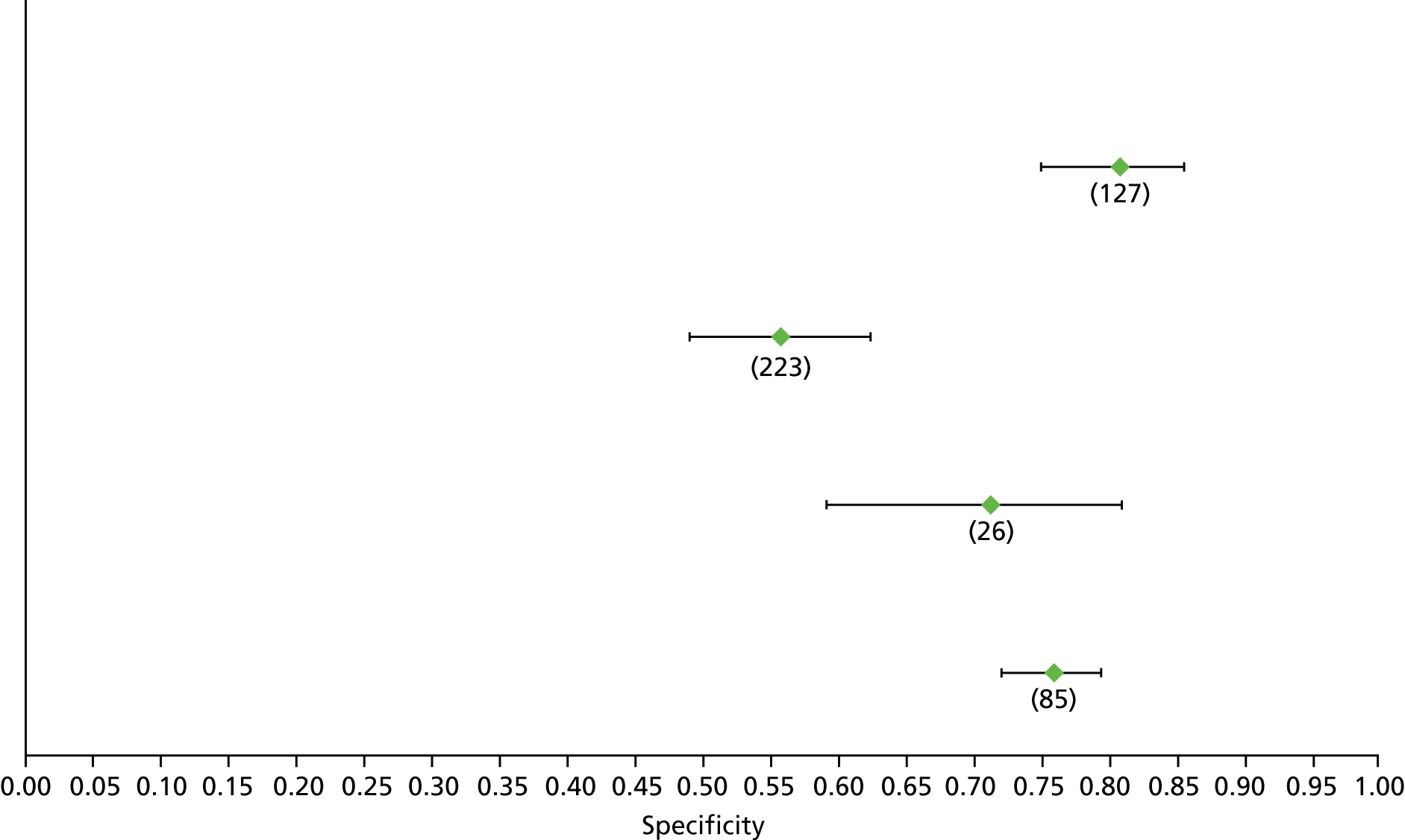
FIGURE 71.
Hepatitis C: FibroSpect II F2 – sensitivity.
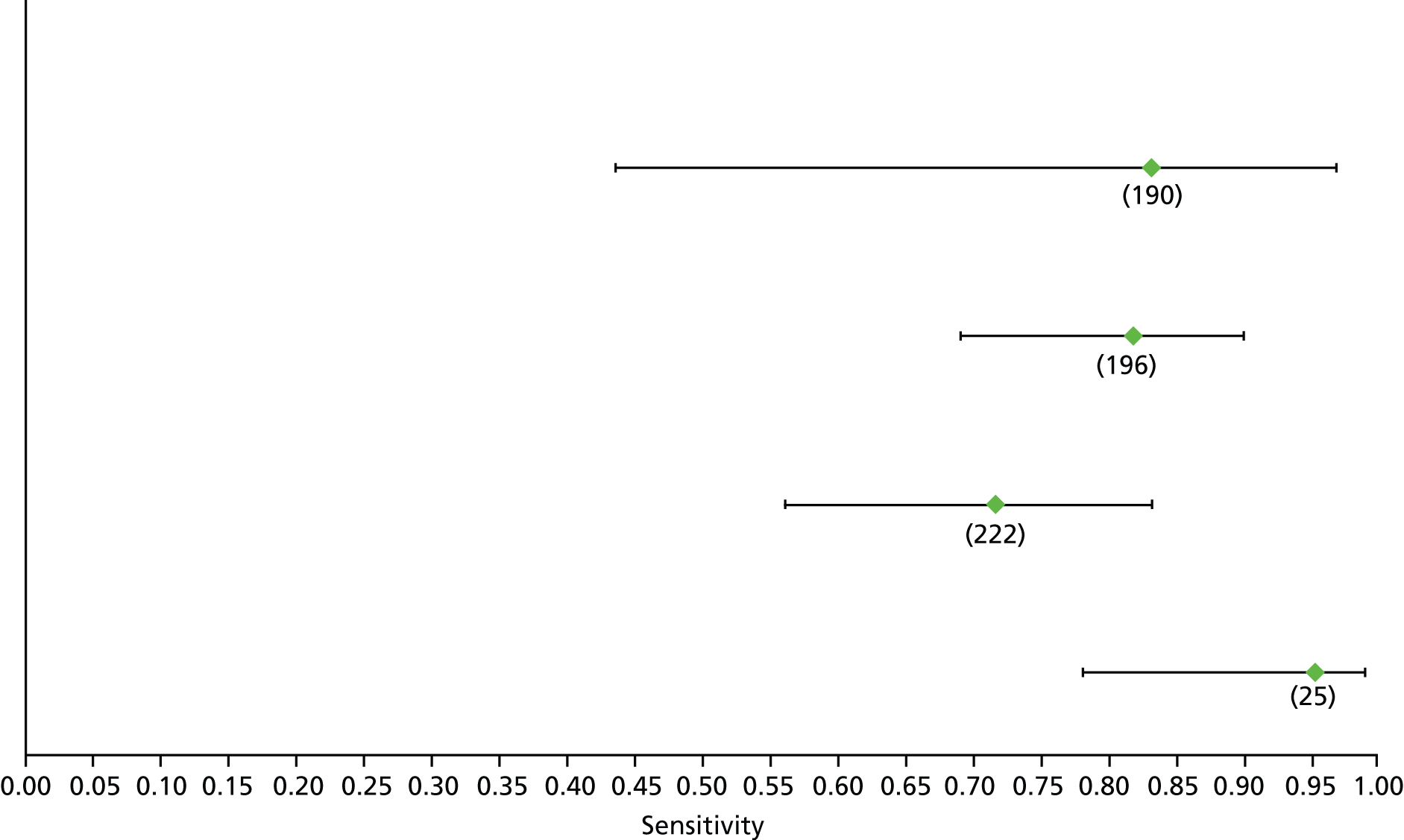
FIGURE 72.
Hepatitis C: FibroSpect II F2 – specificity.

FIGURE 73.
Hepatitis C: Forns index F2 (high cut-off) – sensitivity. EALT, elevated ALT; NALT, normal ALT; test, derivation cohort; val, validation cohort.
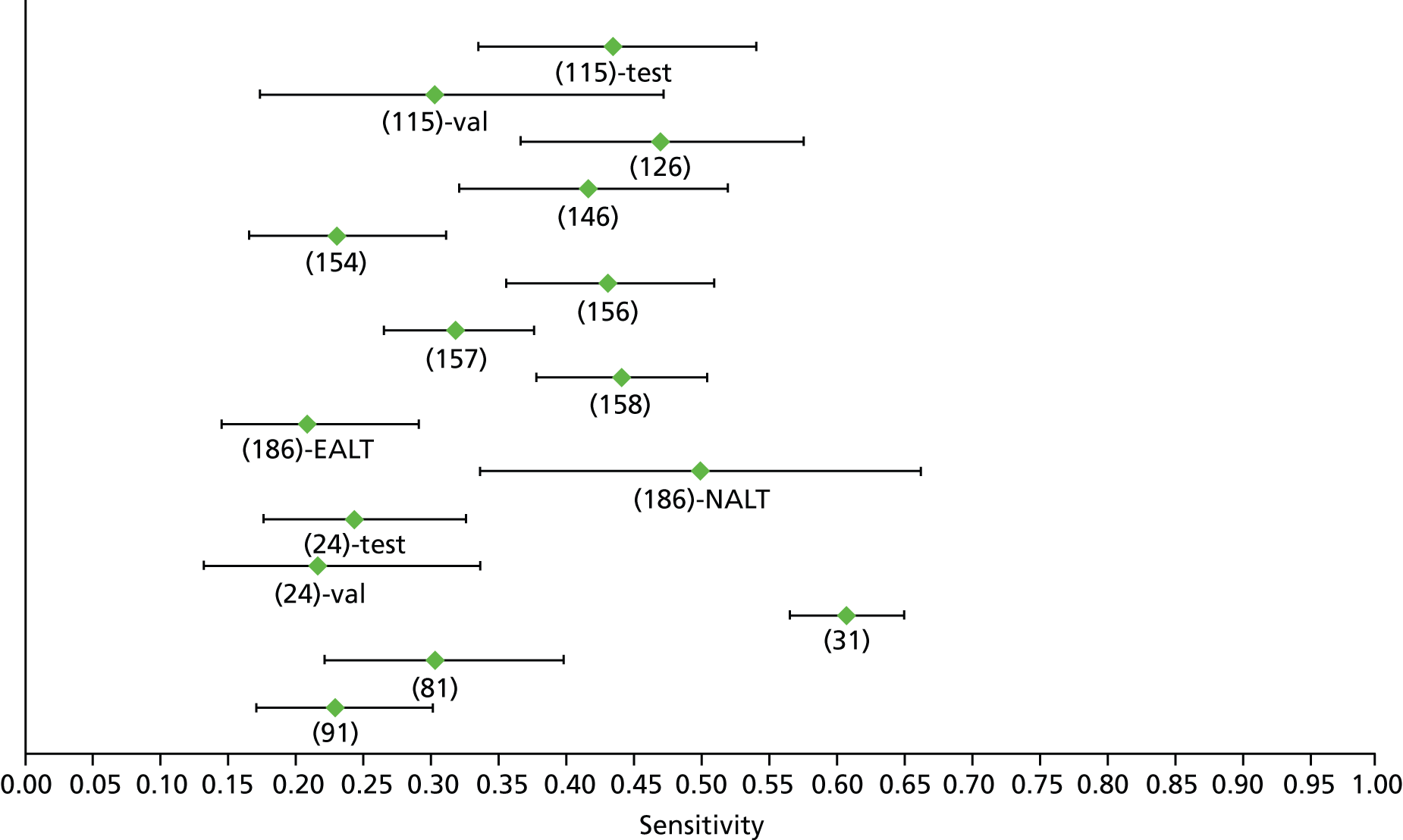
FIGURE 74.
Hepatitis C: Forns index F2 (high cut-off) – specificity. EALT, elevated ALT; NALT, normal ALT; test, derivation cohort; val, validation cohort.
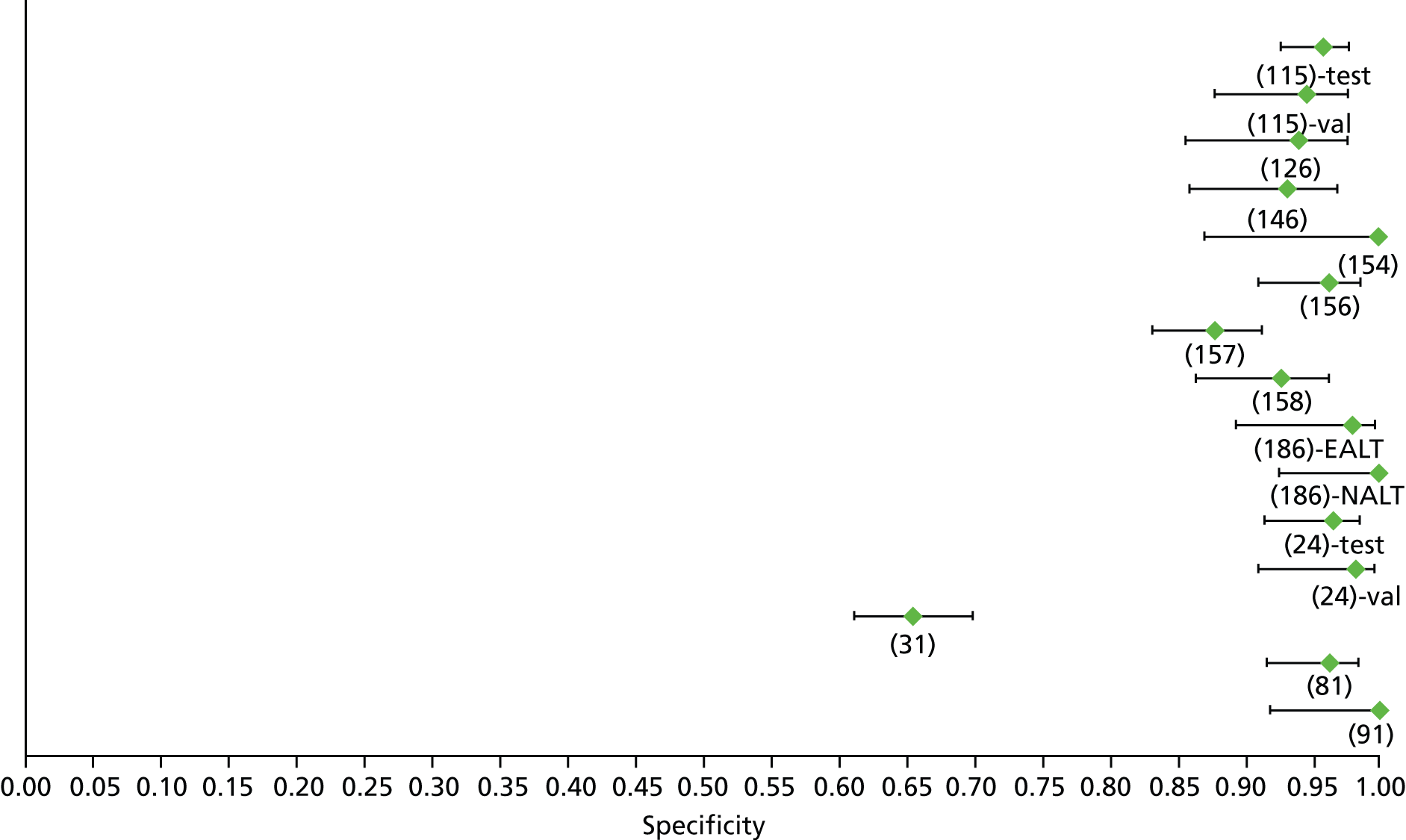
FIGURE 75.
Hepatitis C: Forns index F2 (low cut-off) – sensitivity. EALT, elevated ALT; NALT, normal ALT; test, derivation cohort; val, validation cohort.
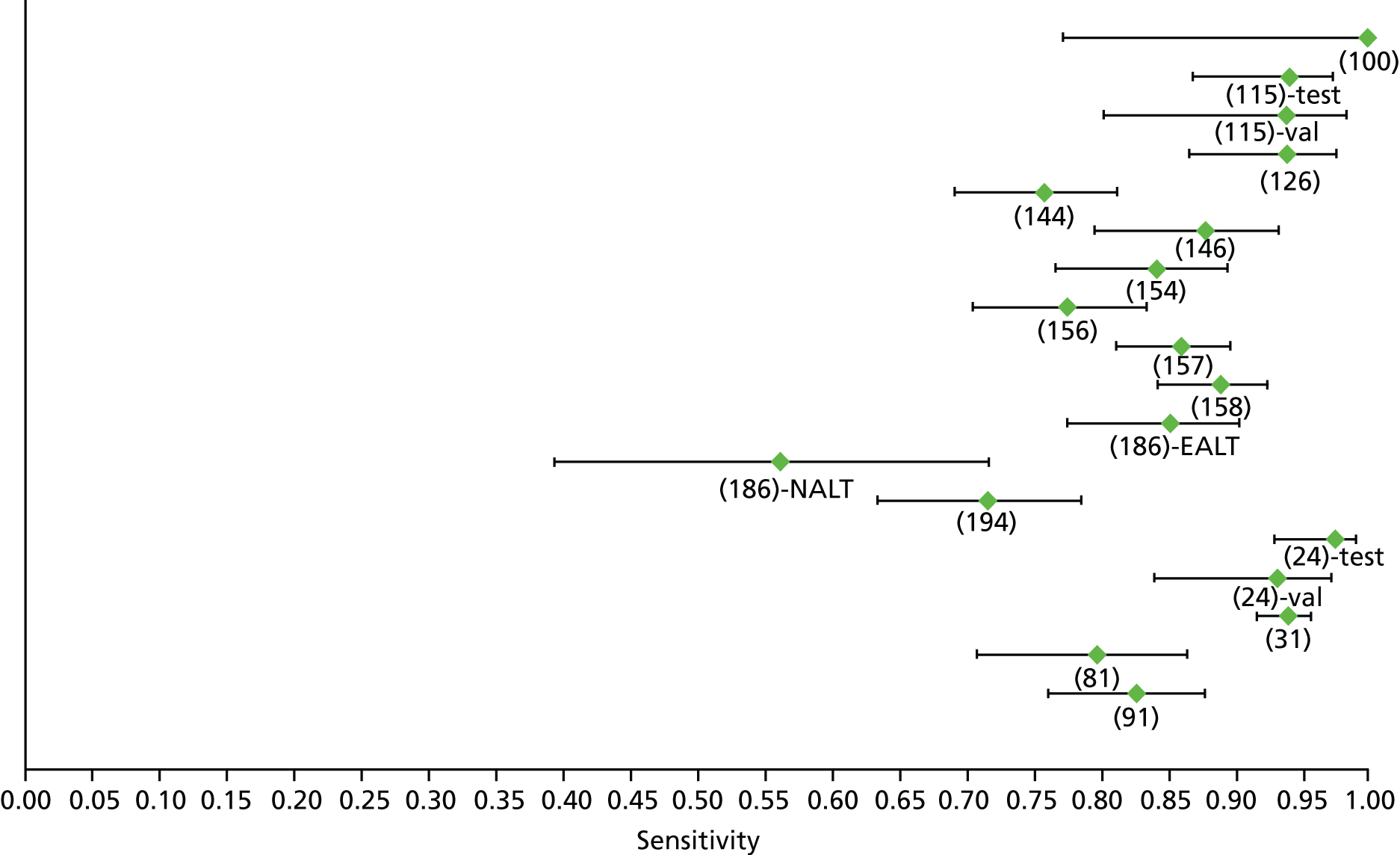
FIGURE 76.
Hepatitis C: Forns index F2 (low cut-off) – specificity. EALT, elevated ALT; NALT, normal ALT; test, derivation cohort; val, validation cohort.
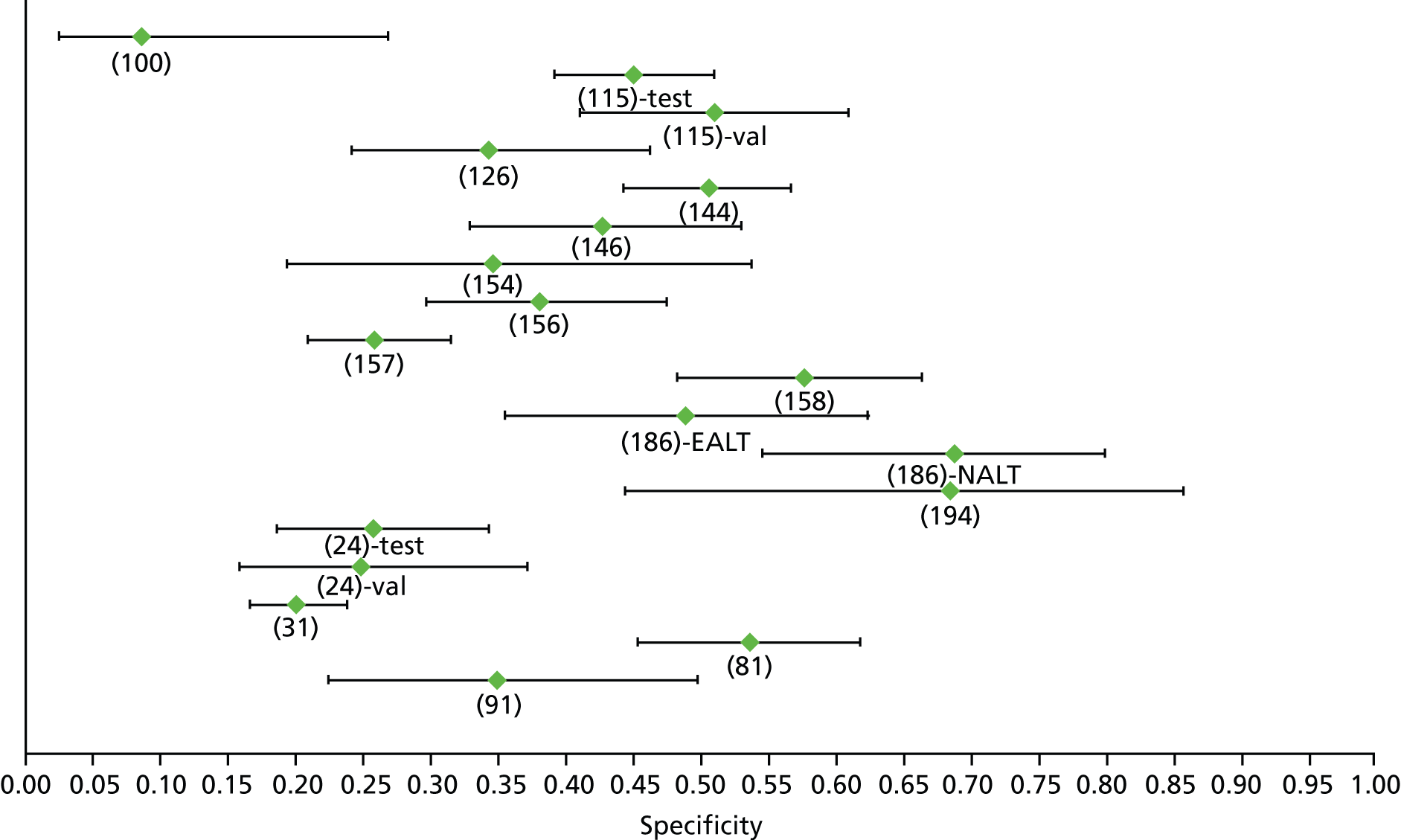
FIGURE 77.
Hepatitis C: Fibrotest F2 – sensitivity. EALT, elevated ALT; NALT, normal ALT.

FIGURE 78.
Hepatitis C: Fibrotest F2 – specificity. EALT, elevated ALT; NALT, normal ALT;

FIGURE 79.
Hepatitis C: Fibrotest F2 (high cut-off) – sensitivity.
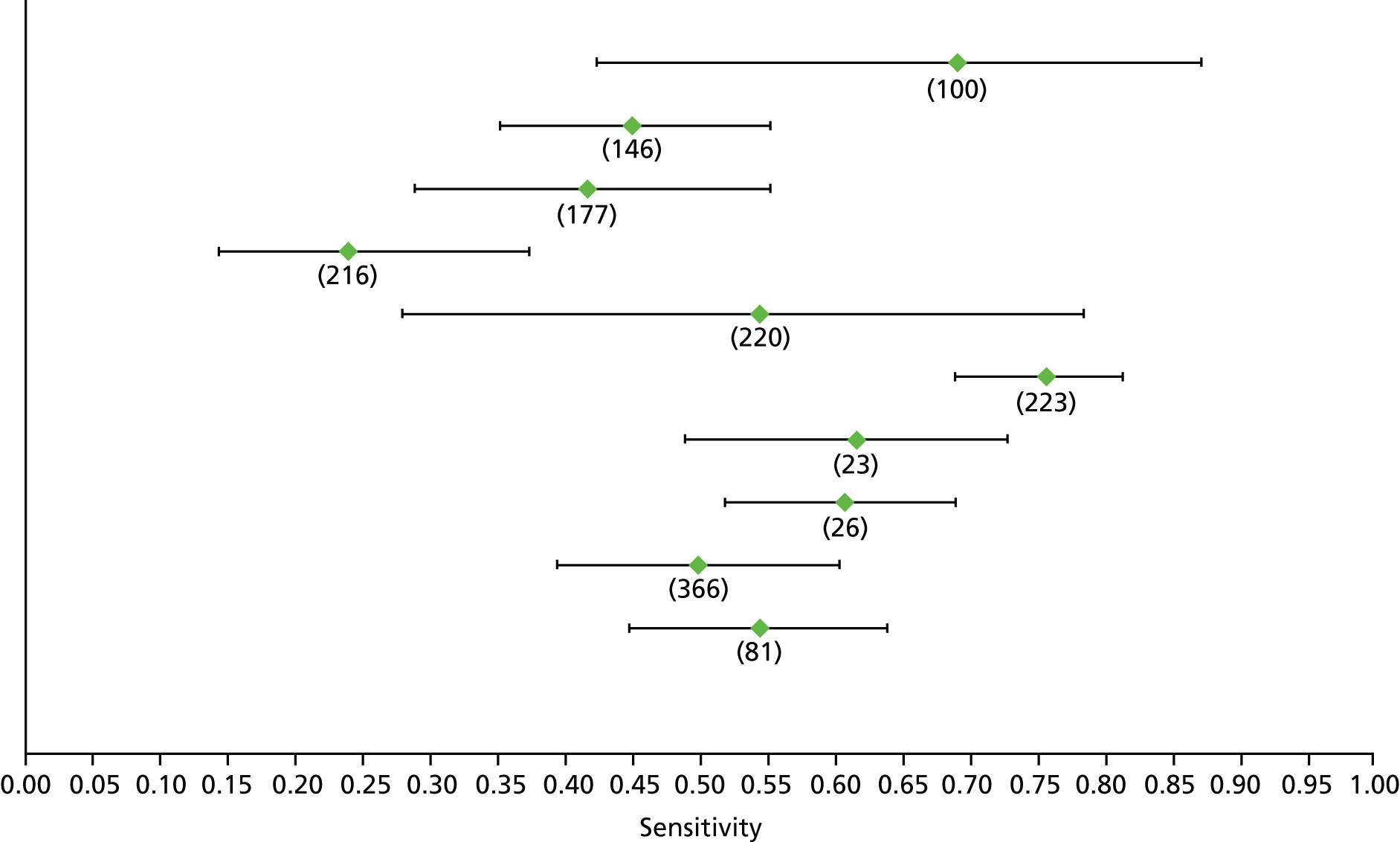
FIGURE 80.
Hepatitis C: Fibrotest F2 (high cut-off) – specificity.
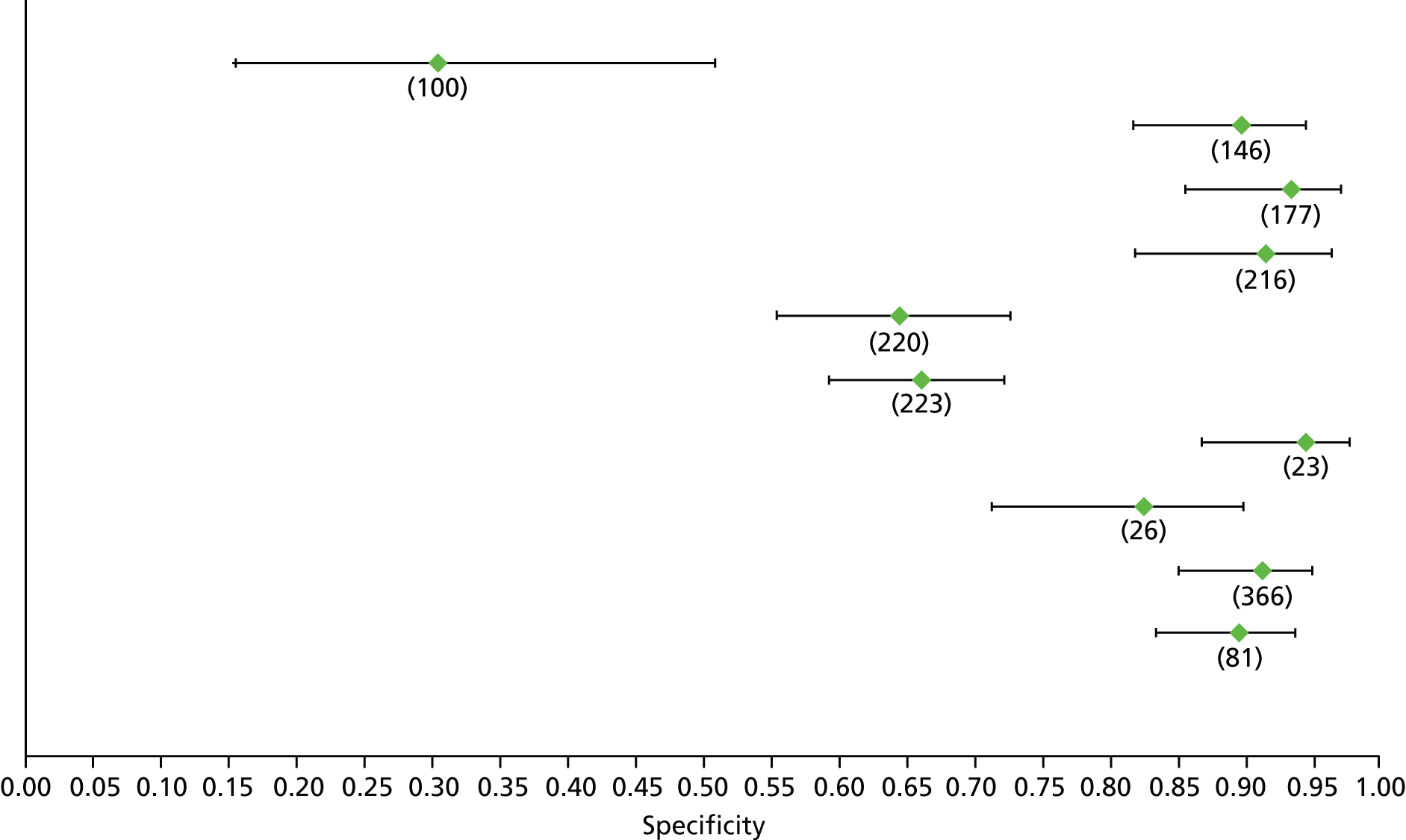
FIGURE 81.
Hepatitis C: Fibrotest F2 (low cut-off) – sensitivity.
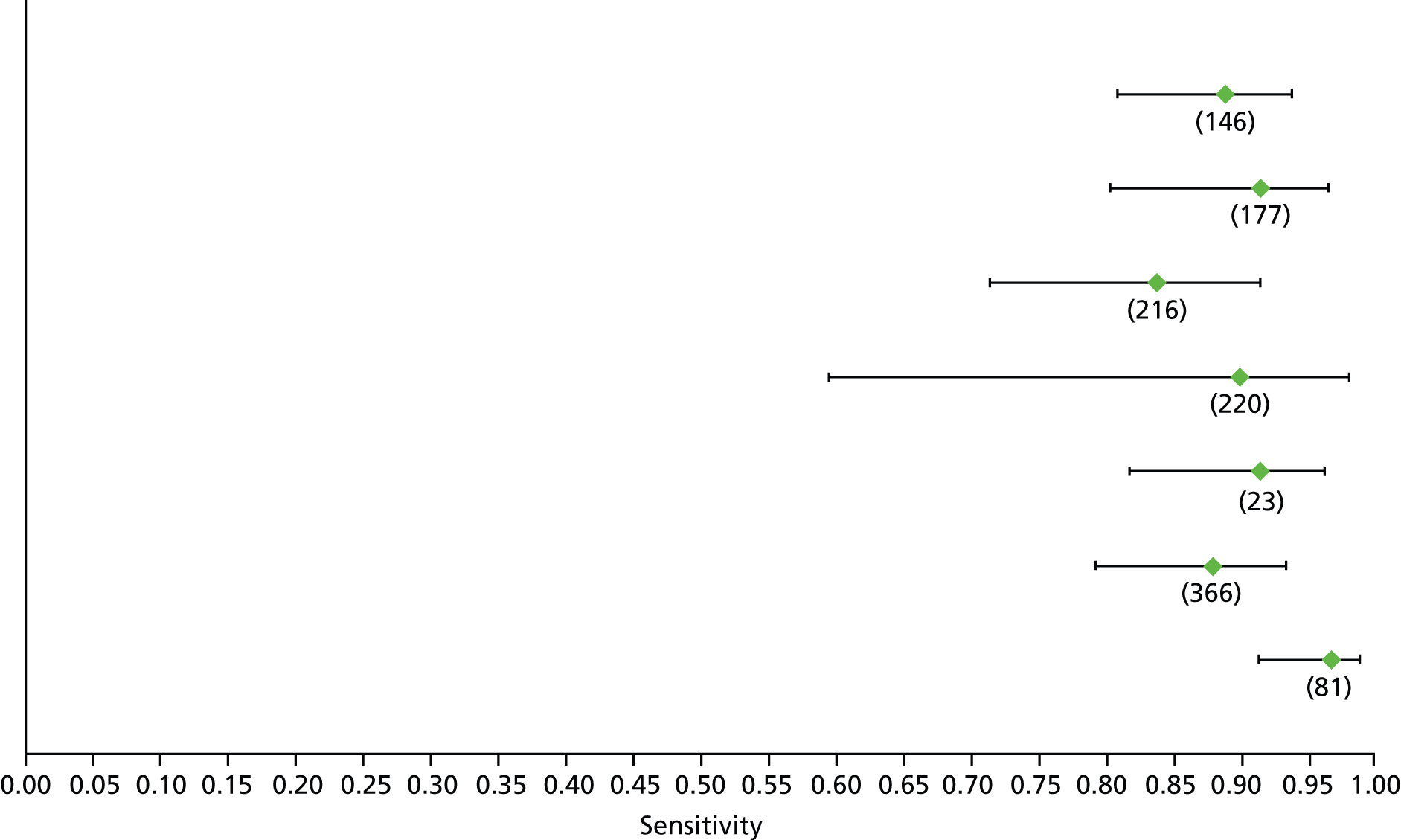
FIGURE 82.
Hepatitis C: Fibrotest F2 (low cut-off) – specificity.

FIGURE 83.
Hepatitis C: GUCI F2 – sensitivity.
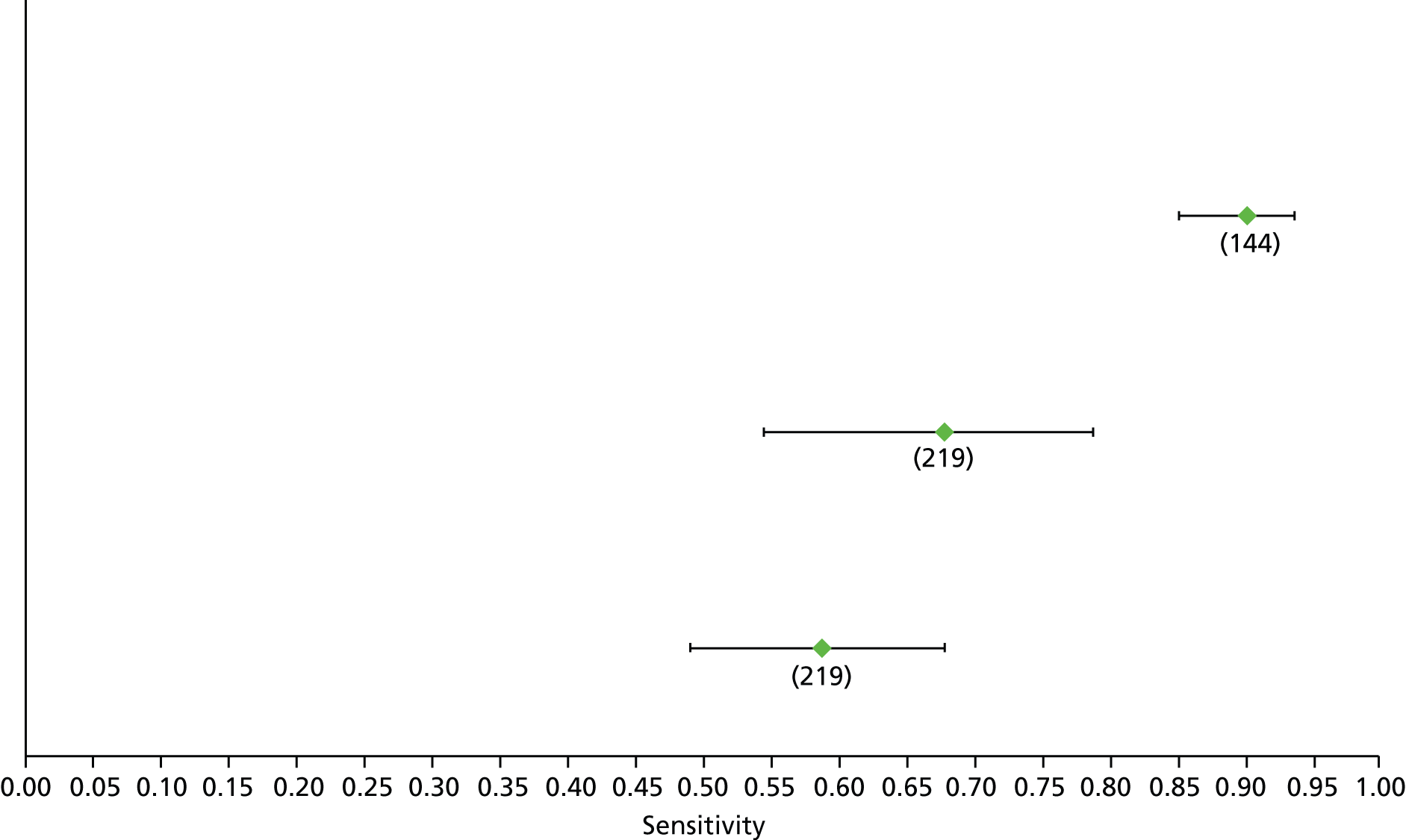
FIGURE 84.
Hepatitis C: GUCI F2 – specificity.

FIGURE 85.
Hepatitis C: hyaluronic acid F2 – sensitivity.
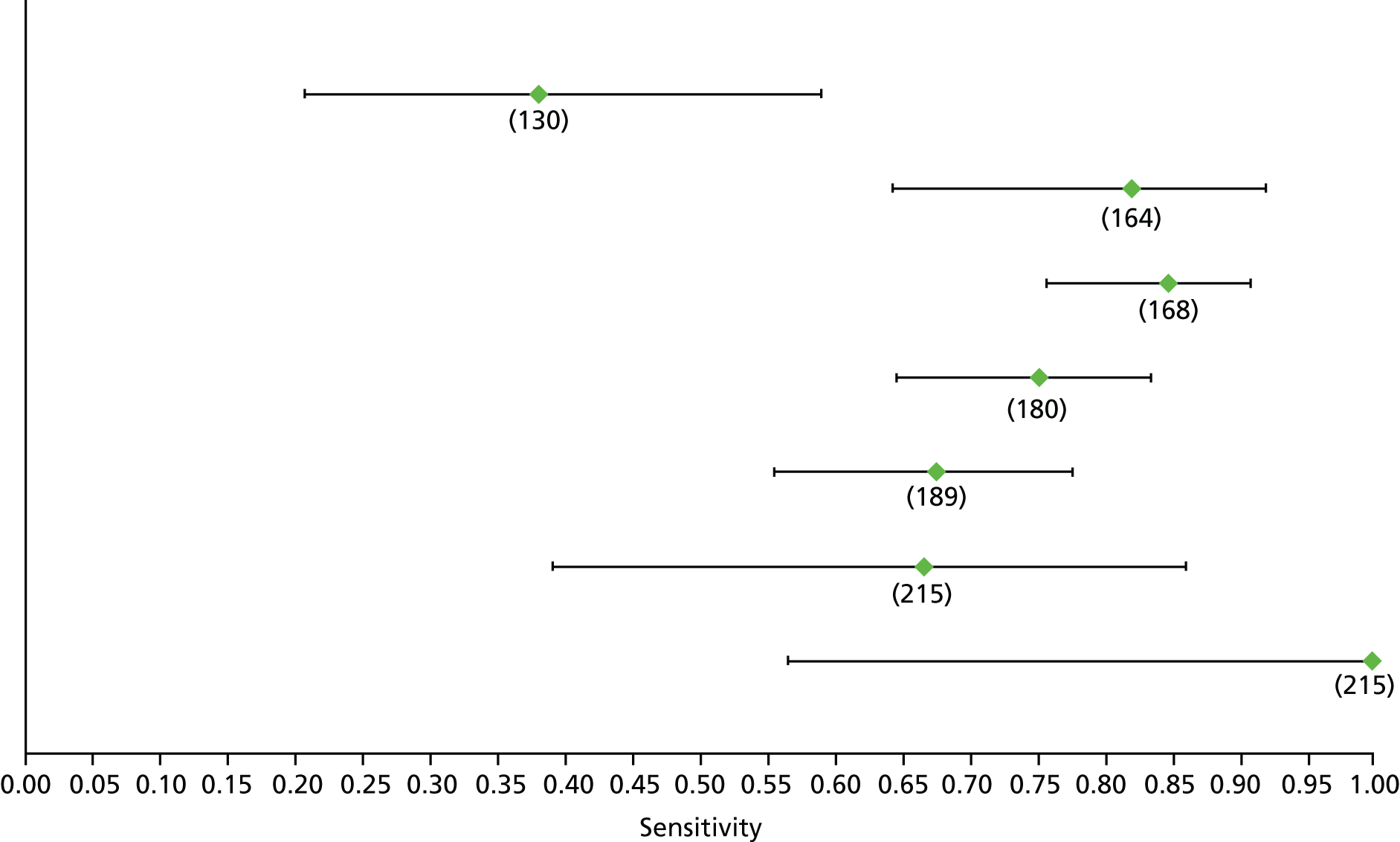
FIGURE 86.
Hepatitis C: hyaluronic acid F2 – specificity.
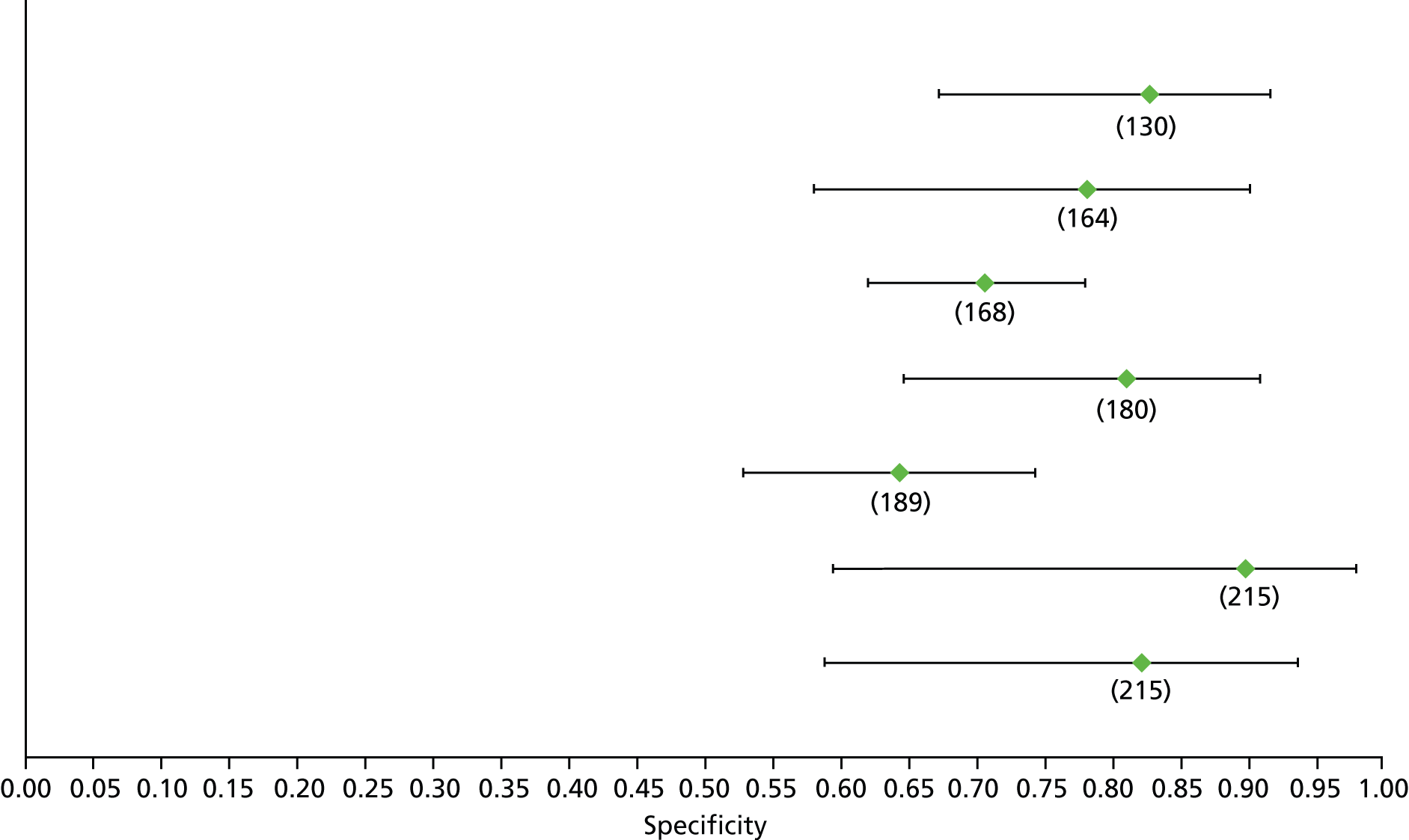
FIGURE 87.
Hepatitis C: Hepascore F2 – sensitivity.
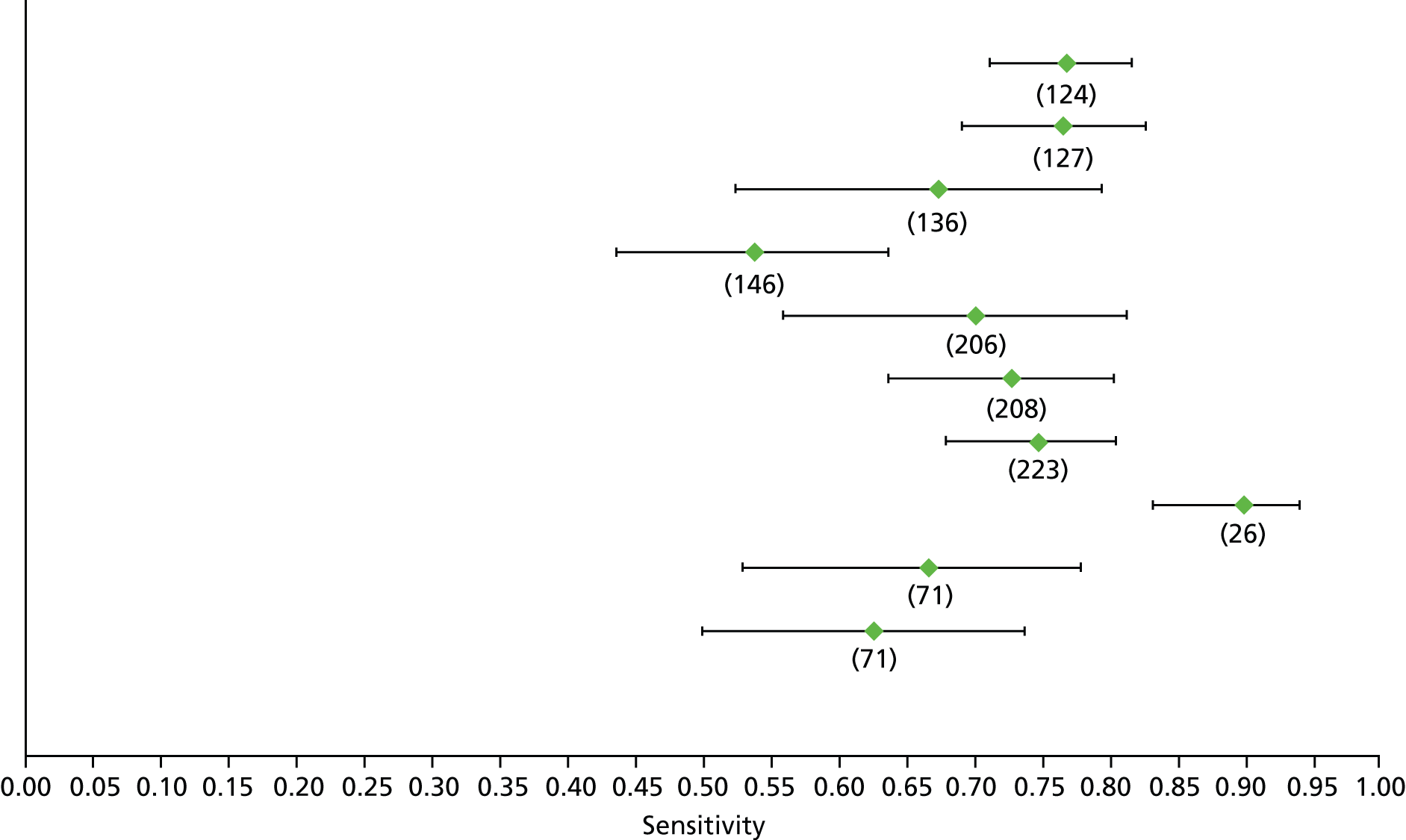
FIGURE 88.
Hepatitis C: Hepascore F2 – specificity.

FIGURE 89.
Hepatitis C: Lok’s index F2 – sensitivity.
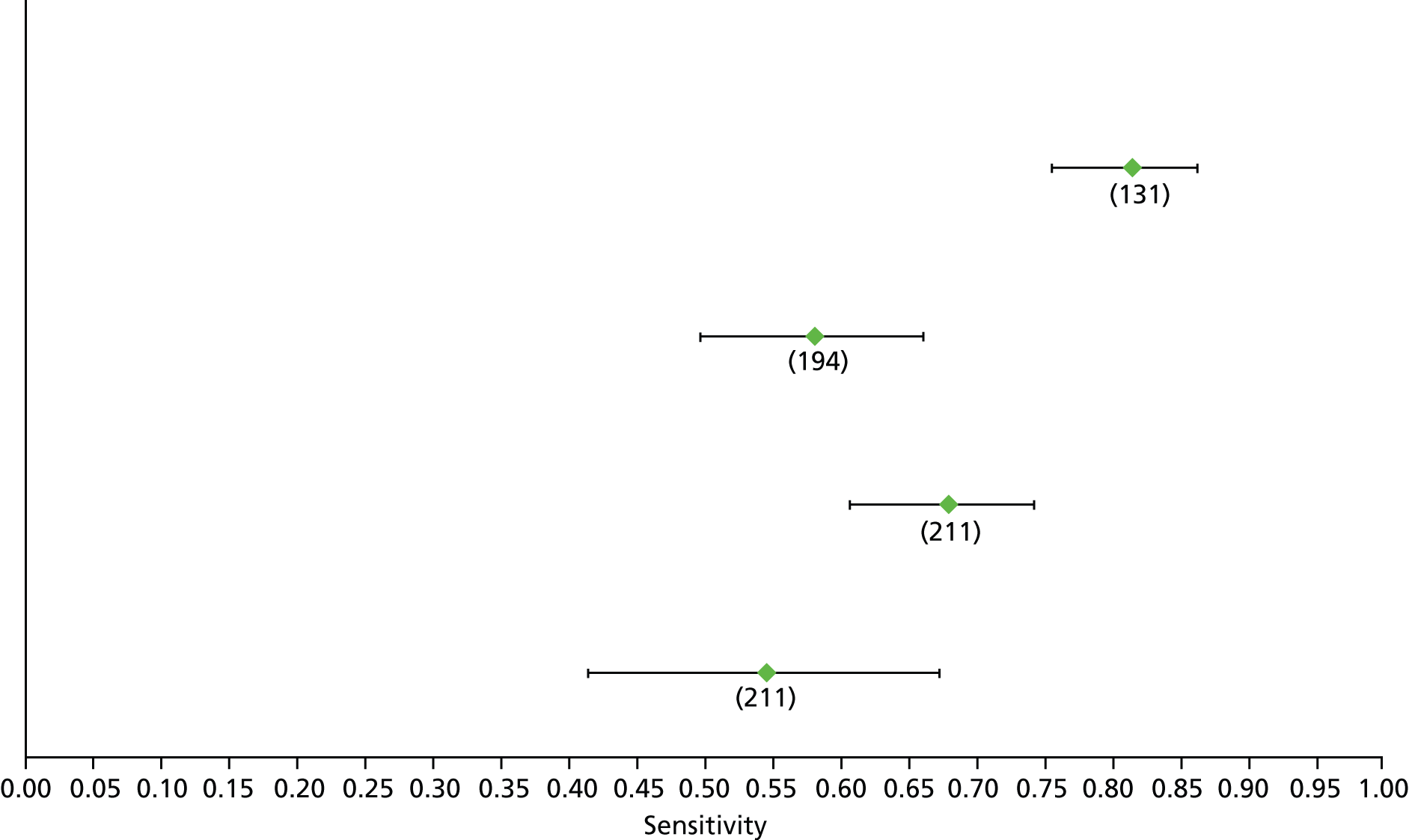
FIGURE 90.
Hepatitis C: Lok’s index F2 – specificity.
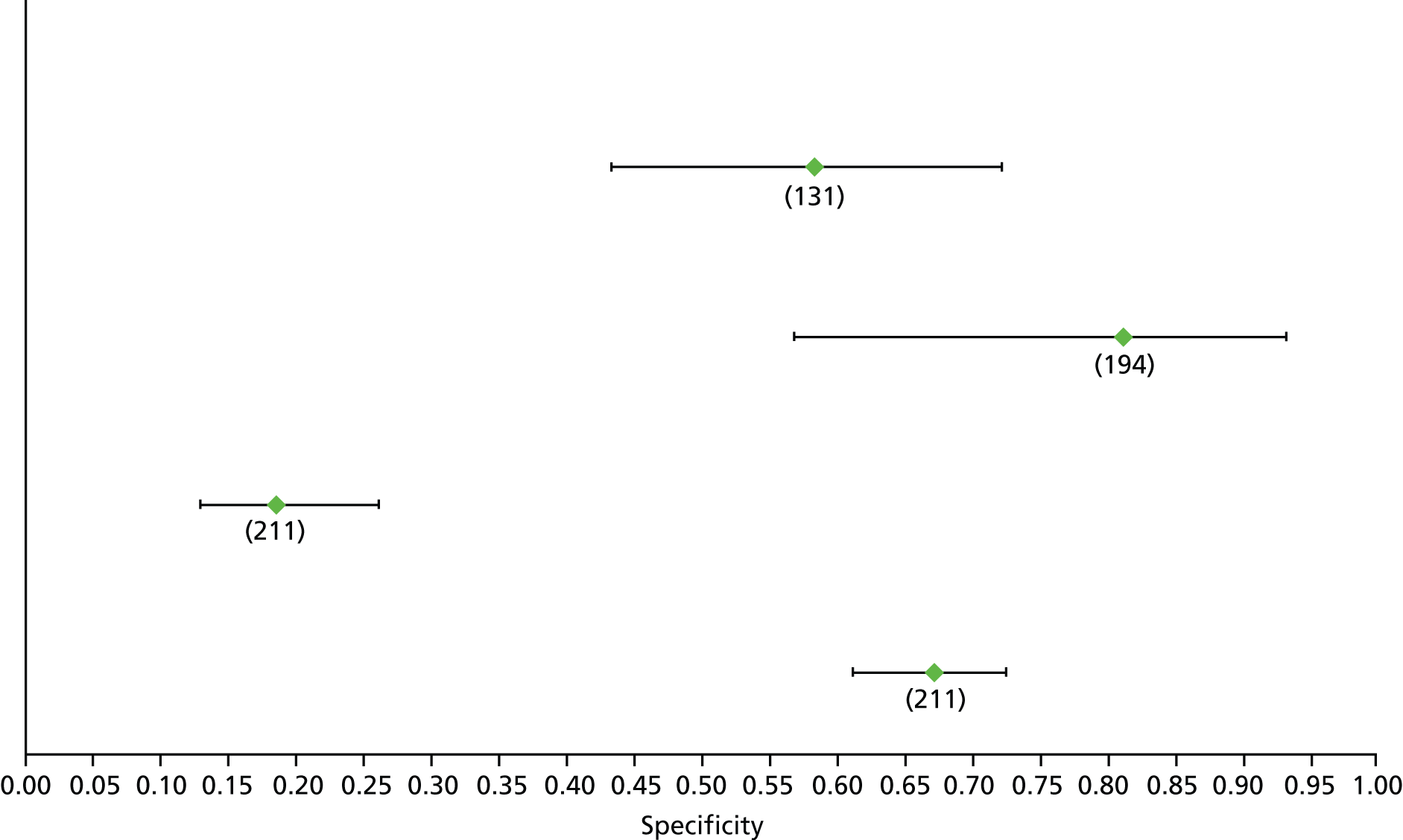
FIGURE 91.
Hepatitis C: platelet–spleen F2 – sensitivity.
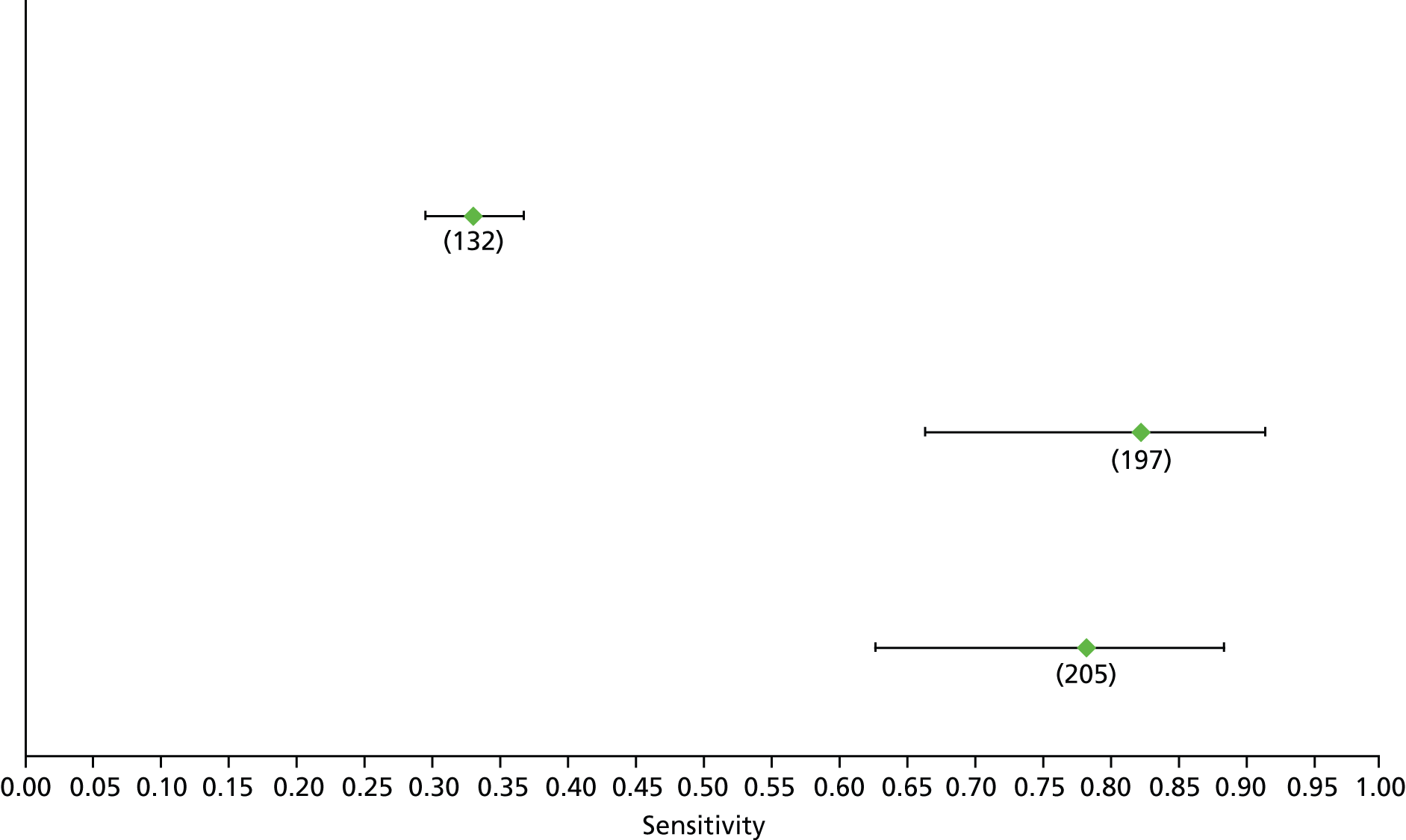
FIGURE 92.
Hepatitis C: platelet–spleen F2 – specificity.
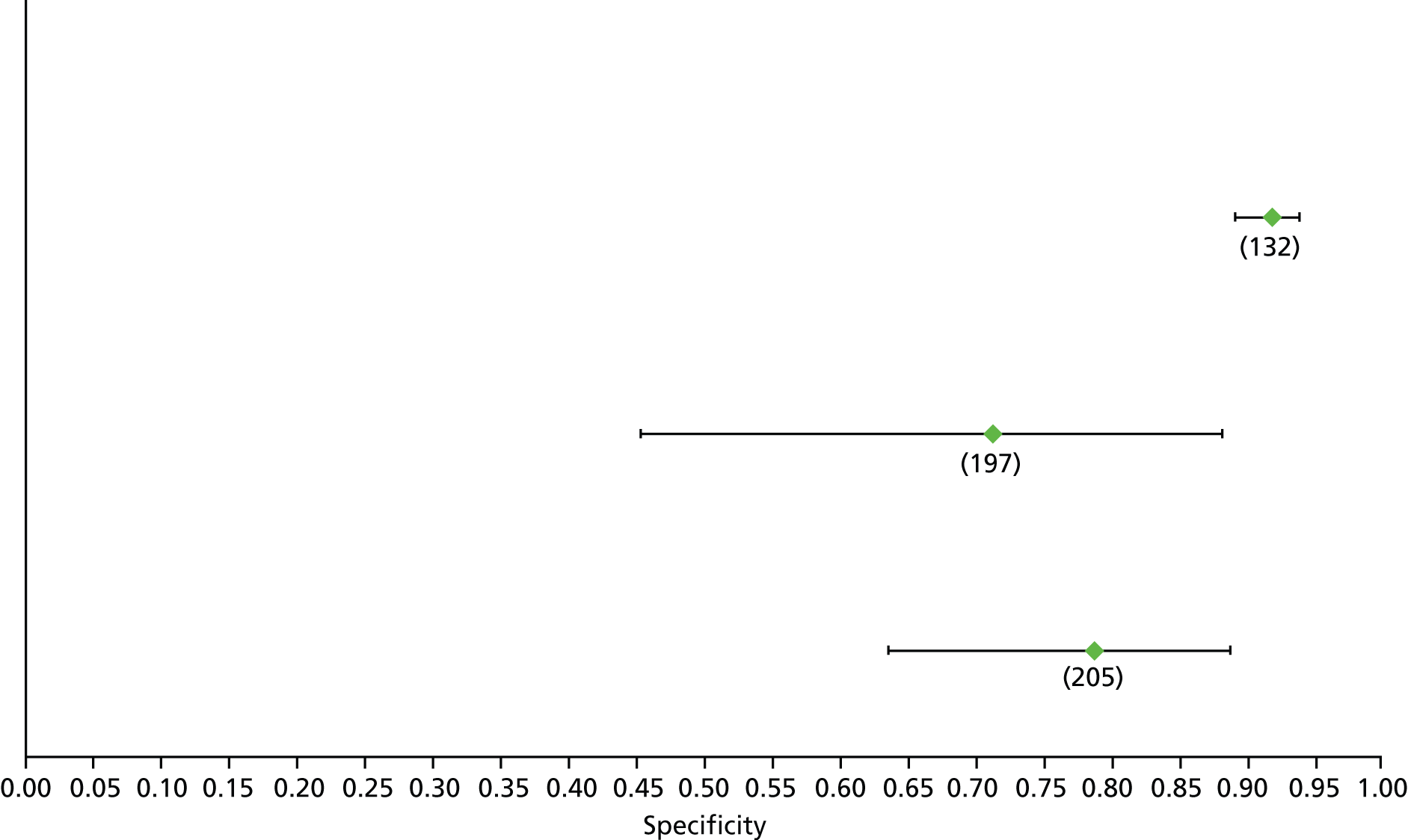
FIGURE 93.
Hepatitis C: platelet F2 – sensitivity.

FIGURE 94.
Hepatitis C: platelet F2 – specificity.
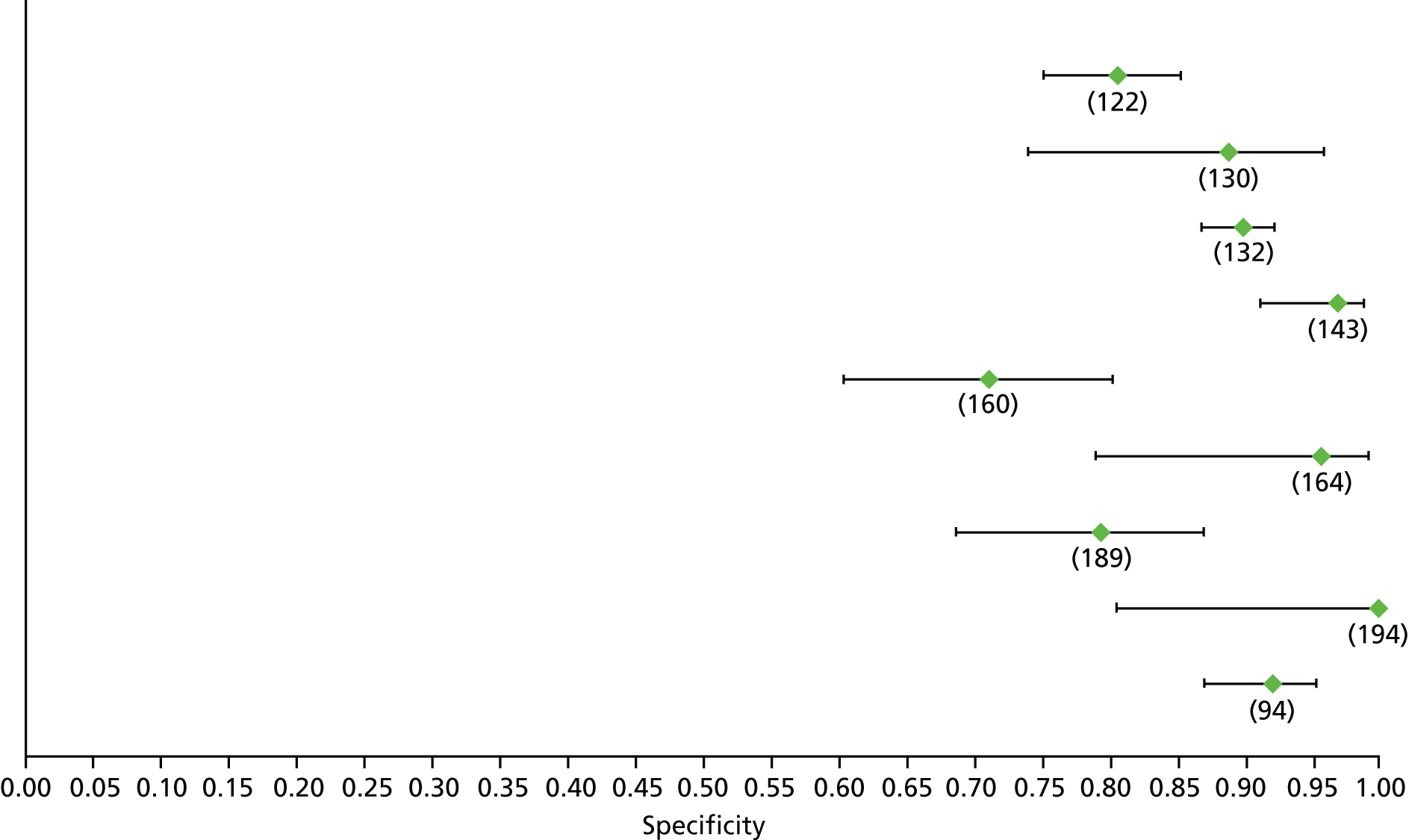
FIGURE 95.
Hepatitis C: Fibroscan F2 – sensitivity.
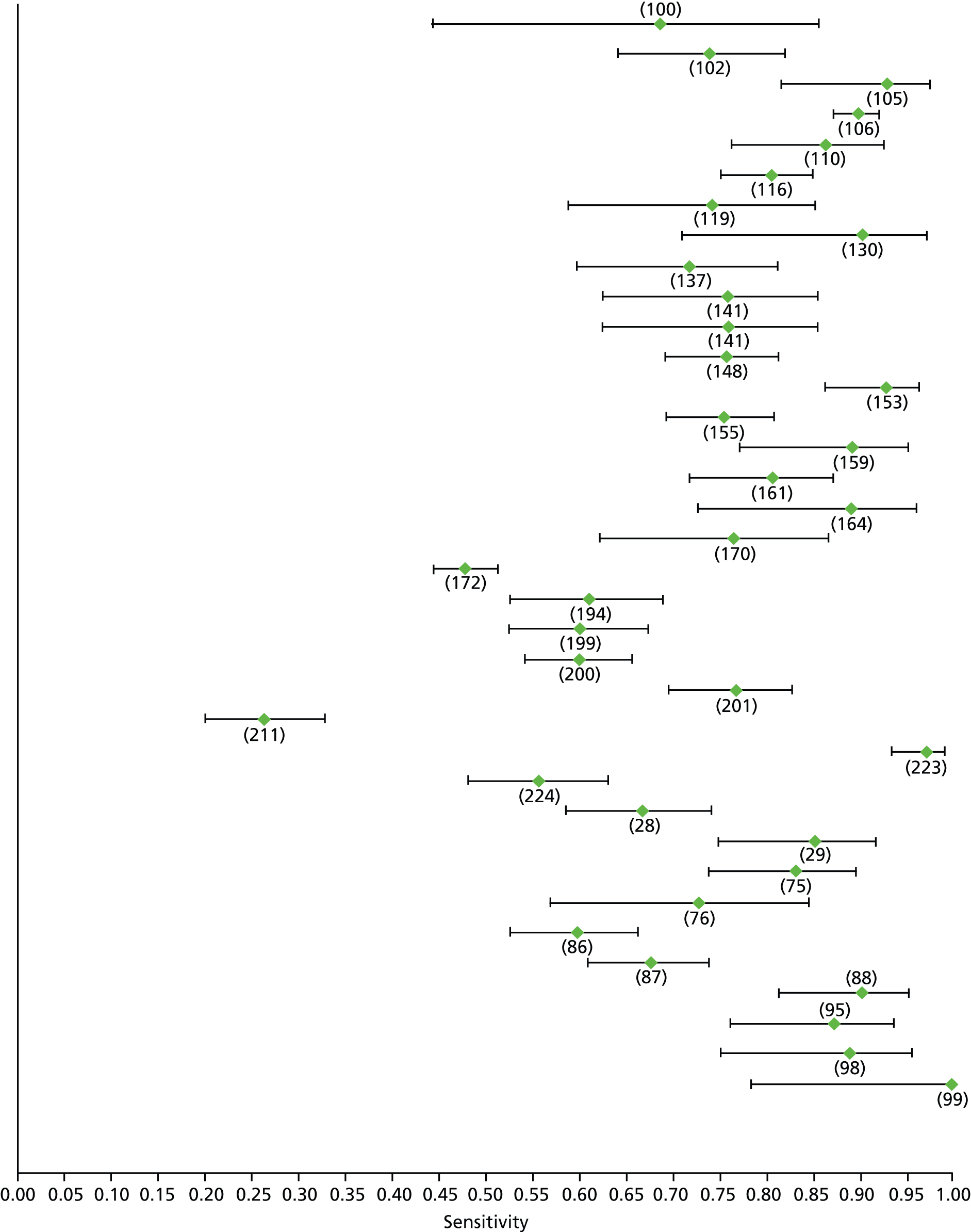
FIGURE 96.
Hepatitis C: Fibroscan F2 – specificity.
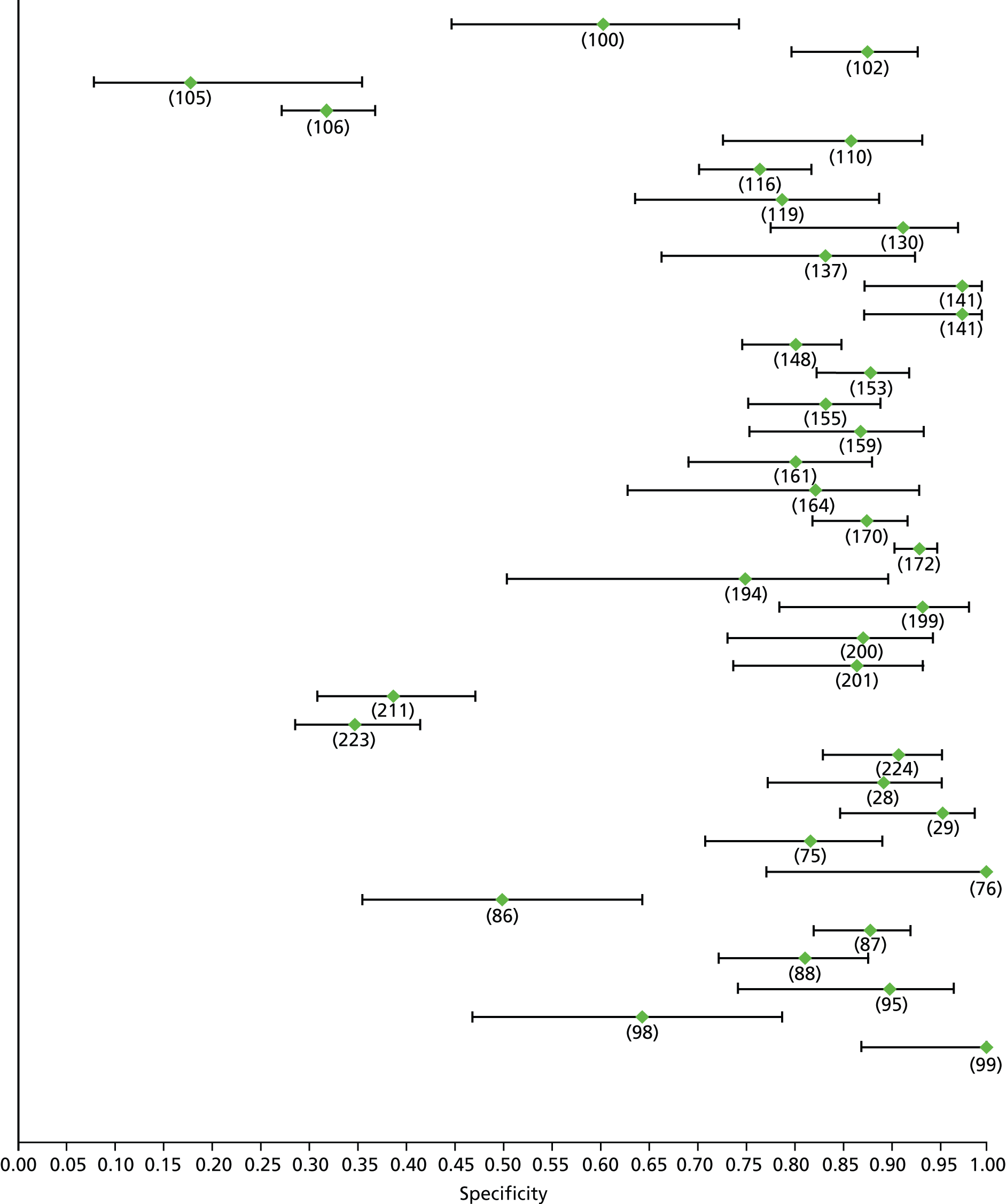
FIGURE 97.
Hepatitis C: type IV collagen F2 – sensitivity.

FIGURE 98.
Hepatitis C: type IV collagen F2 – specificity.

FIGURE 99.
Hepatitis C: APRI F3 (high cut-off) – sensitivity. Pros, prospective; retr, retrospective.

FIGURE 100.
Hepatitis C: APRI F3 (high cut-off) – specificity. Pros, prospective; retr, retrospective.

FIGURE 101.
Hepatitis C: APRI F3 (low cut-off) – sensitivity. Pros, prospective; retr, retrospective.

FIGURE 102.
Hepatitis C: APRI F3 (low cut-off) – specificity. Pros, prospective; retr, retrospective.

FIGURE 103.
Hepatitis C: FIB-4 F3 (high cut-off) – sensitivity. Tot, total; val, validation cohort.
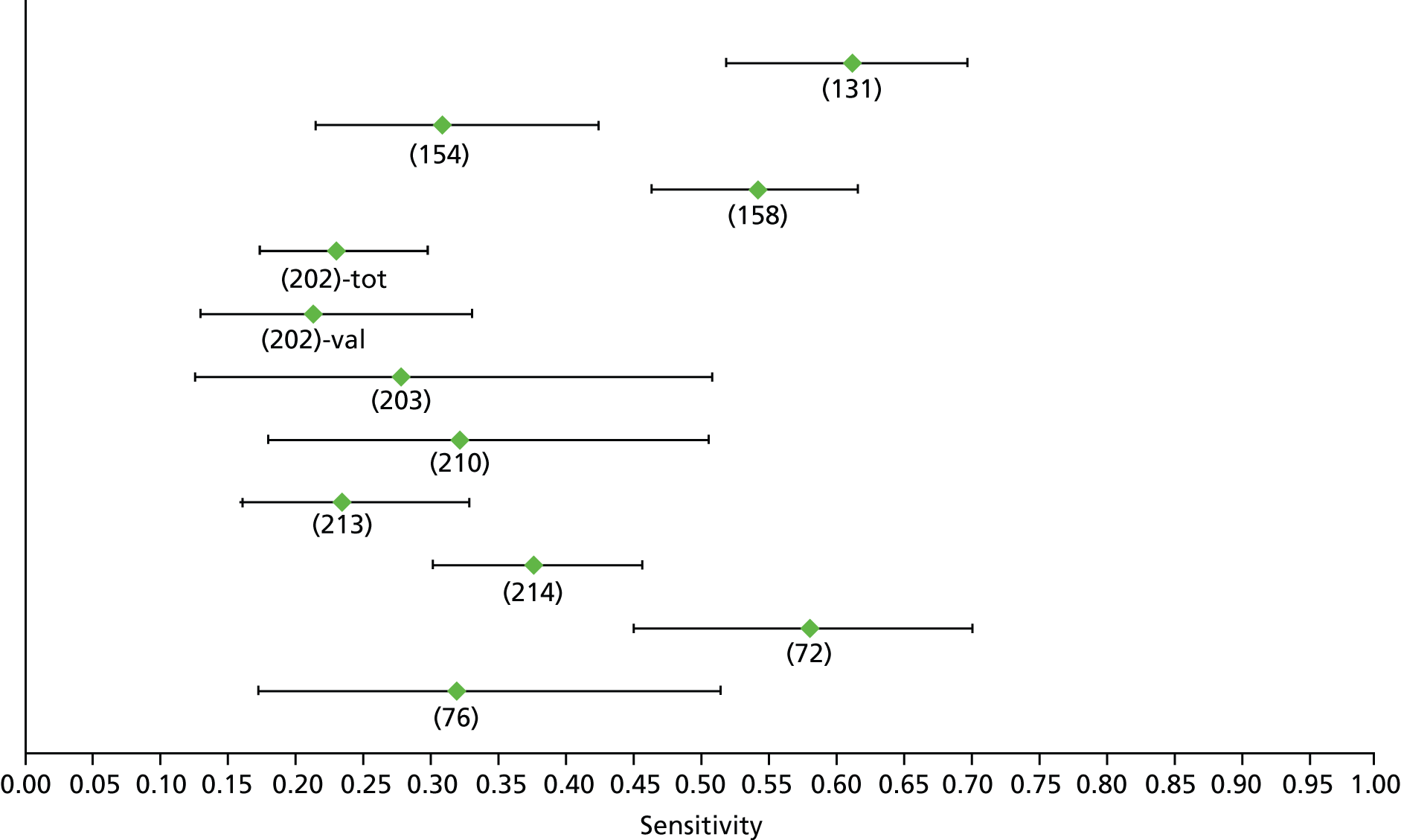
FIGURE 104.
Hepatitis C: FIB-4 F3 (high cut-off) – specificity. Tot, total; val, validation cohort.
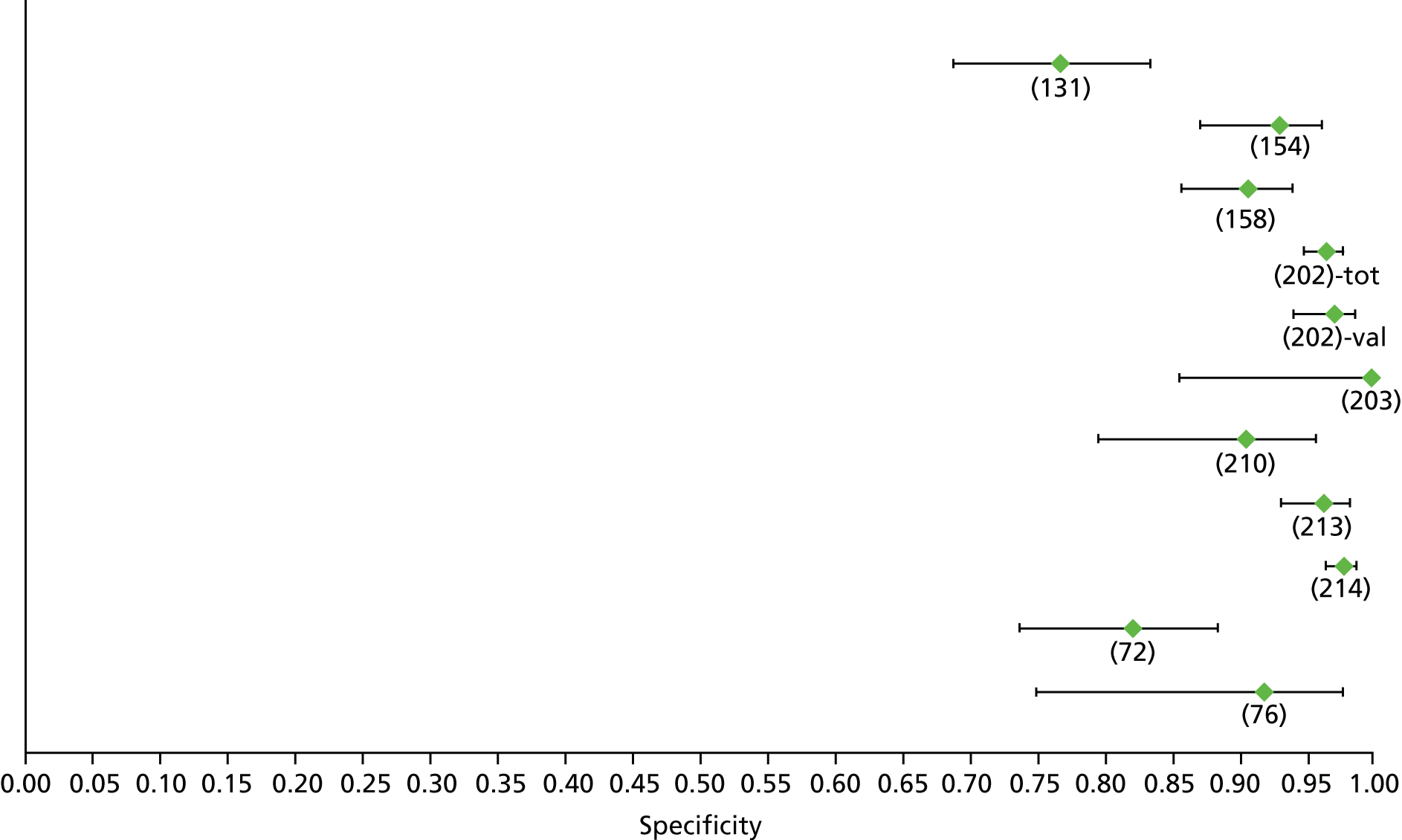
FIGURE 105.
Hepatitis C: FIB-4 F3 (low cut-off) – sensitivity. Tot, total; val, validation cohort.
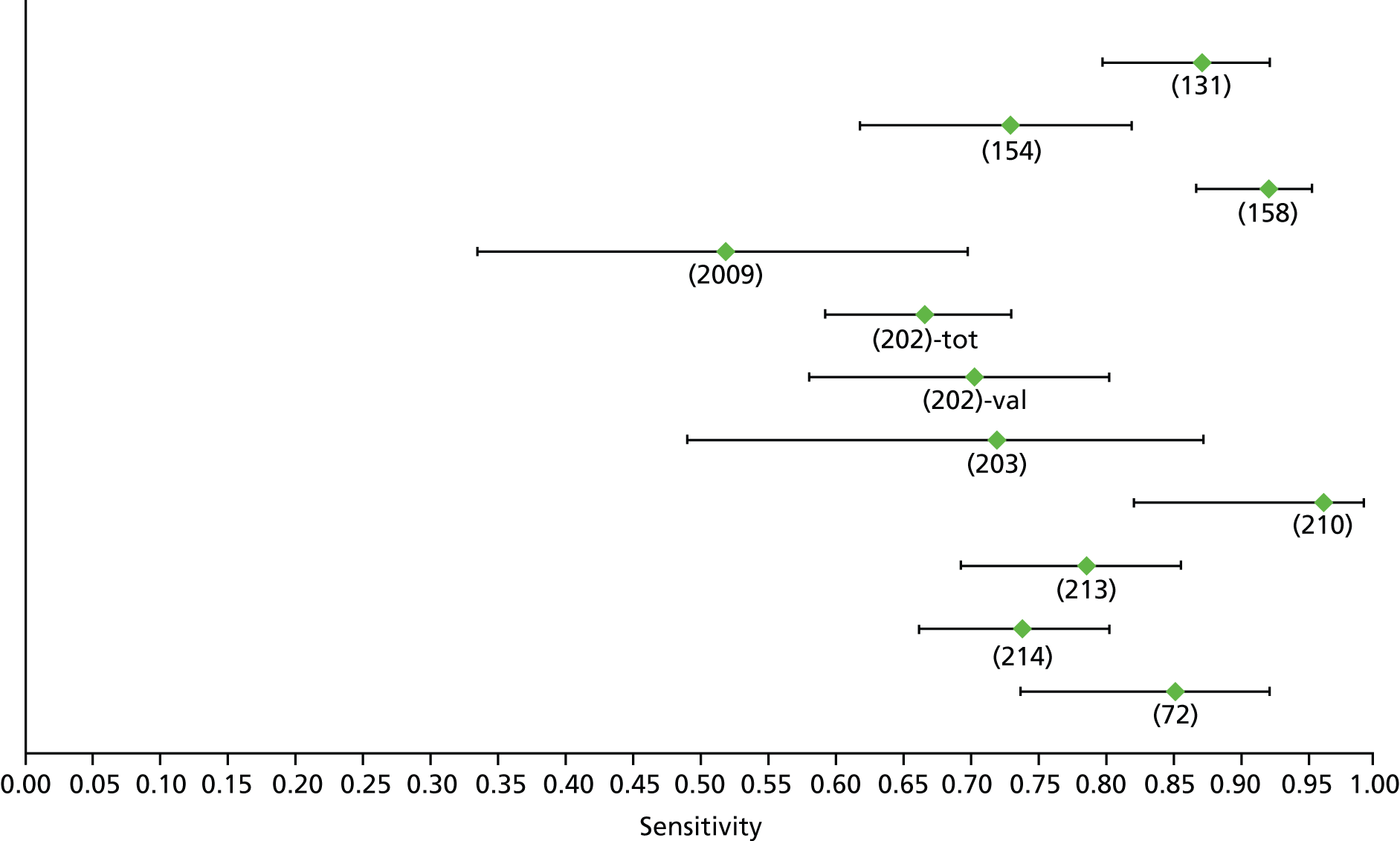
FIGURE 106.
Hepatitis C: FIB-4 F3 (low cut-off) – specificity. Tot, total; val, validation cohort.

FIGURE 107.
Hepatitis C: Fibrotest F3 – sensitivity.
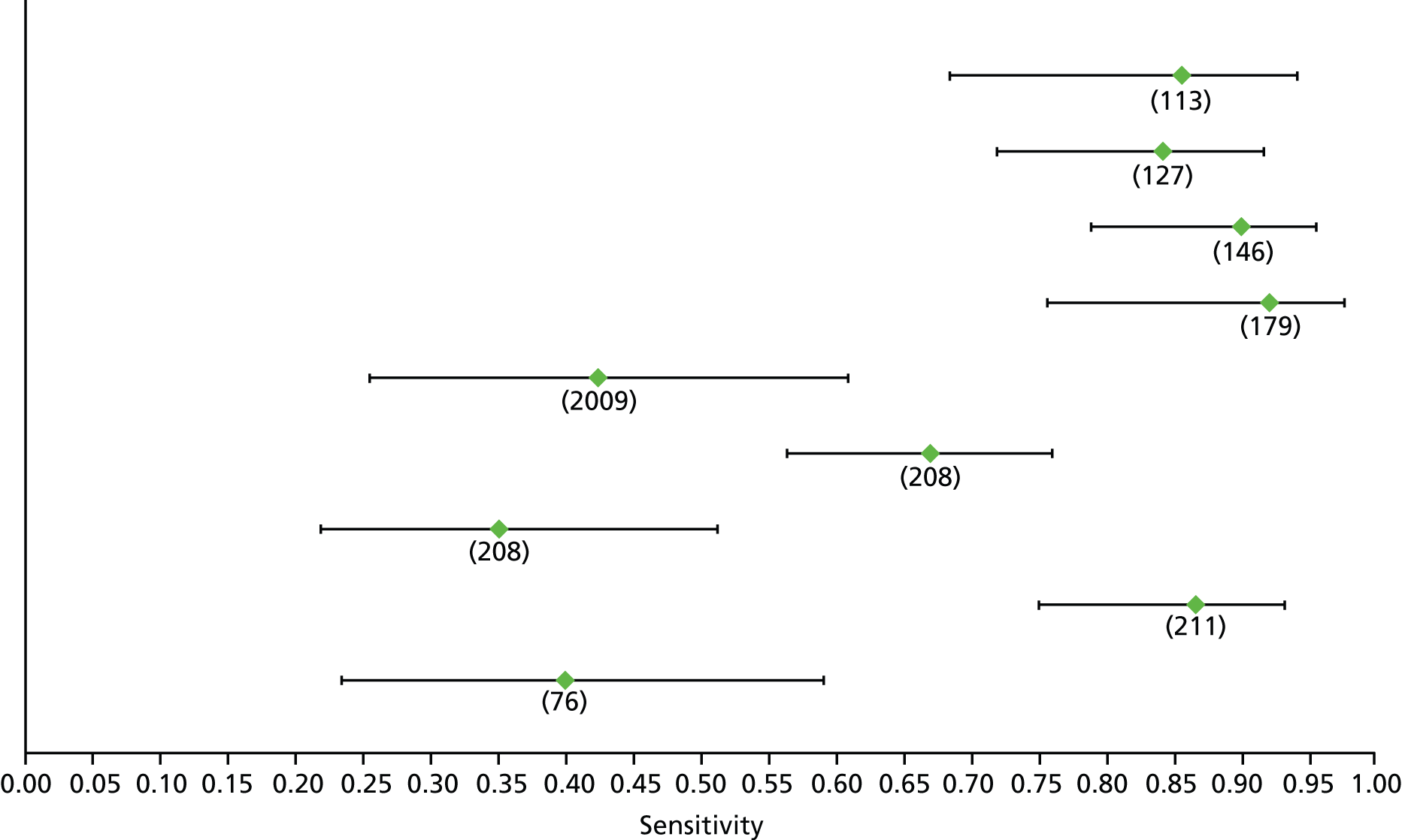
FIGURE 108.
Hepatitis C: Fibrotest F3 – specificity.
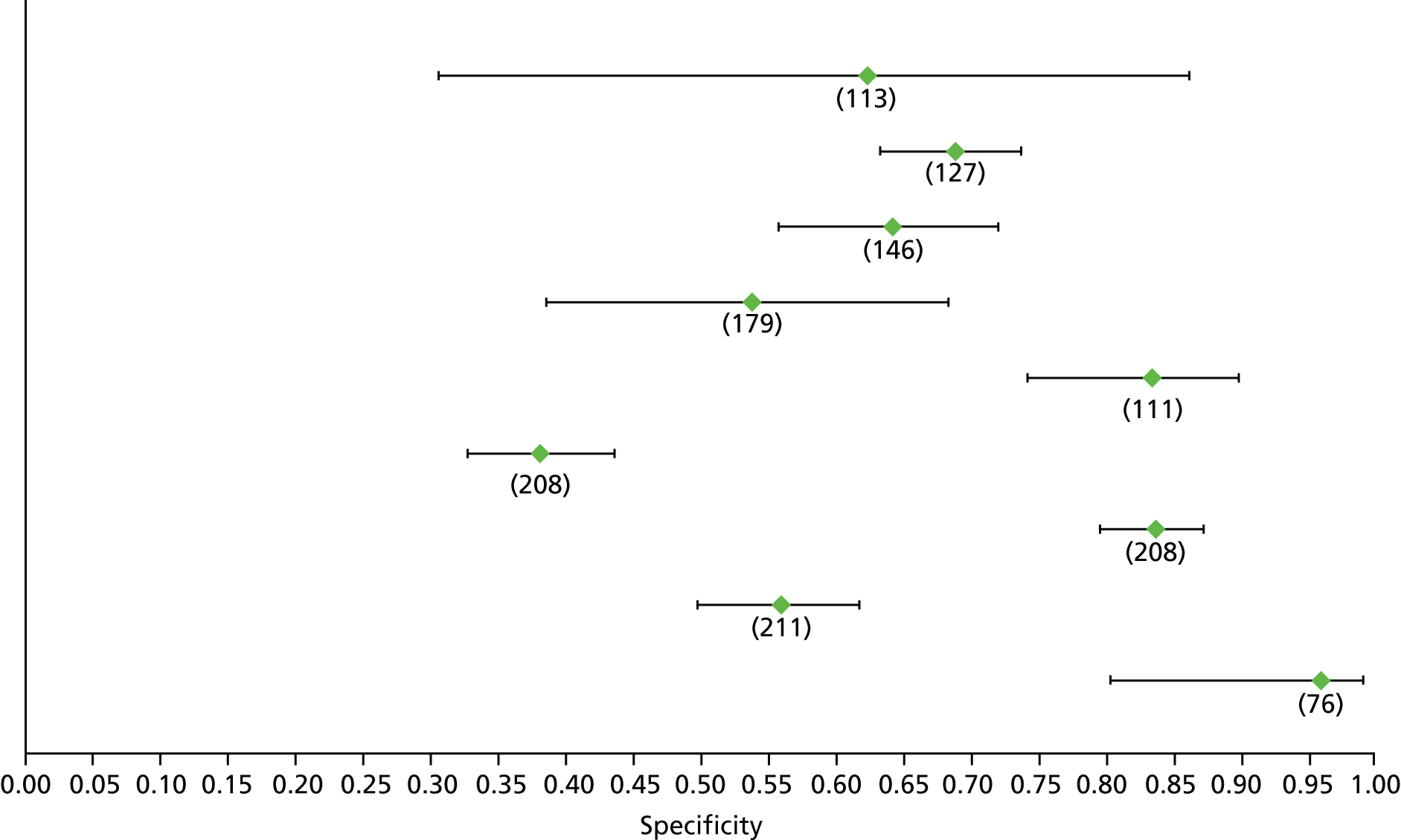
FIGURE 109.
Hepatitis C: hyaluronic acid F3 – sensitivity.

FIGURE 110.
Hepatitis C: hyaluronic acid F3 – specificity.

FIGURE 111.
Hepatitis C: Hepascore F3 – sensitivity.
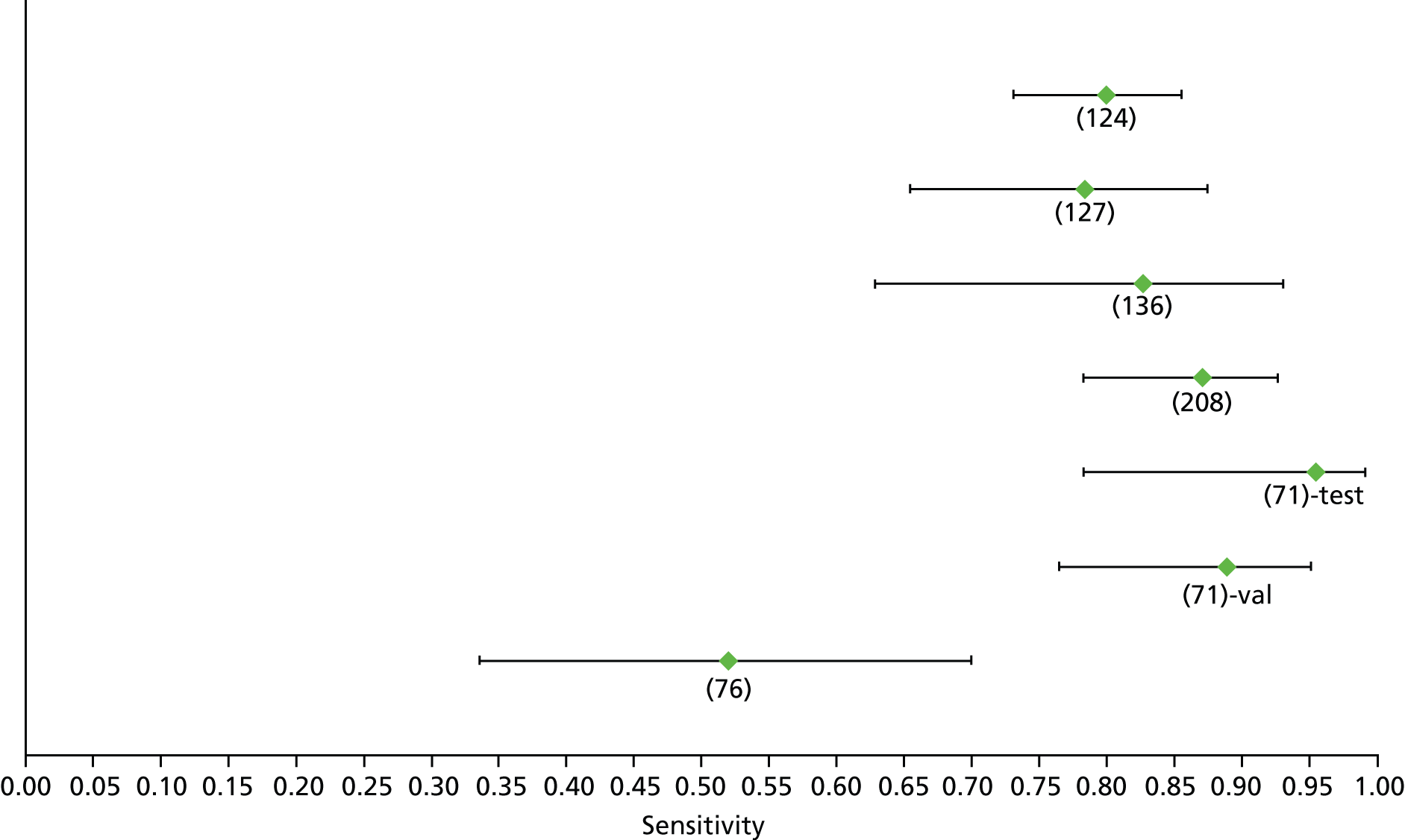
FIGURE 112.
Hepatitis C: Hepascore F3 – specificity.
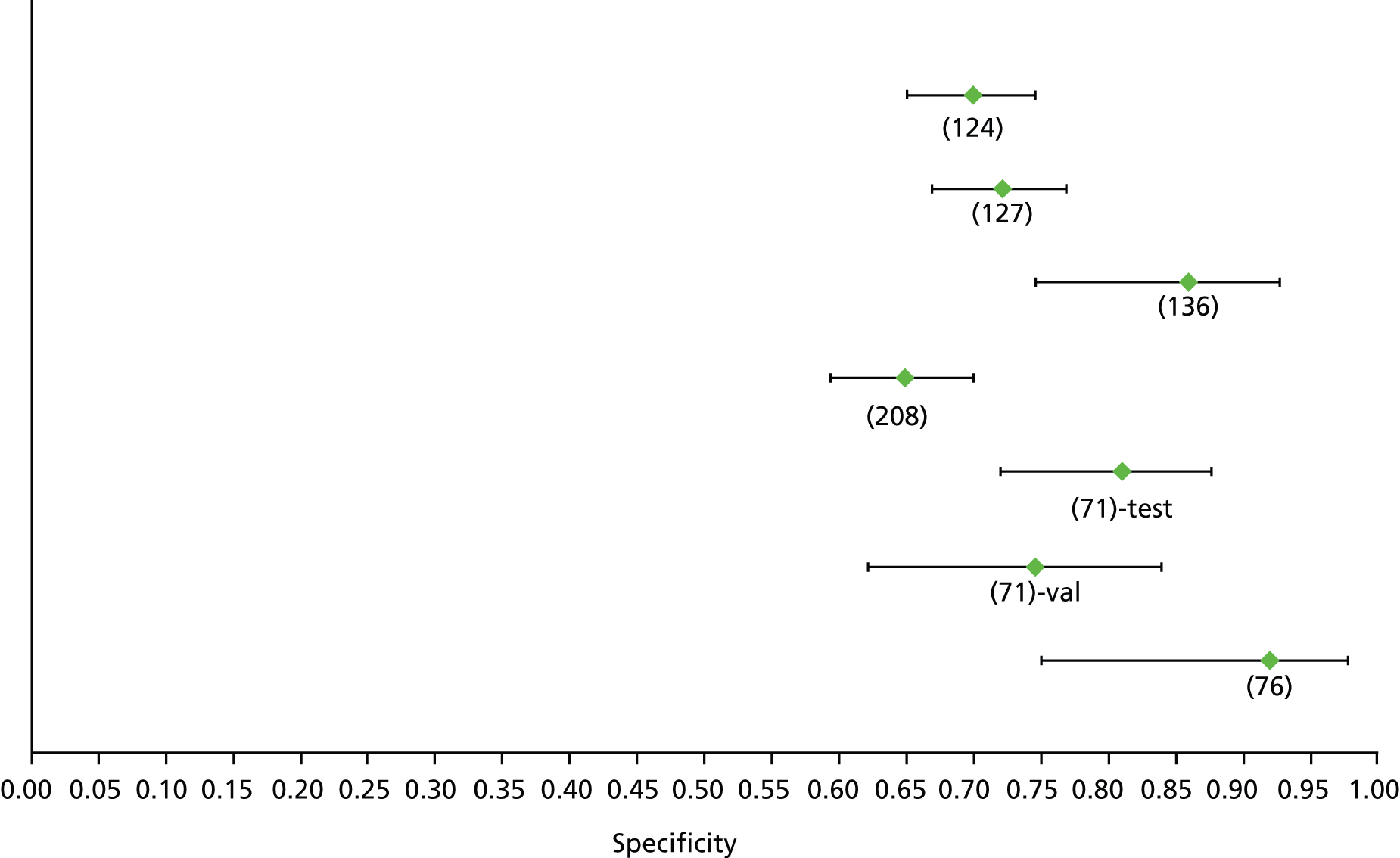
FIGURE 113.
Hepatitis C: 13C-caffeine breath test F4 – sensitivity.

FIGURE 114.
Hepatitis C: 13C-caffeine breath test F4 – specificity.

FIGURE 115.
Hepatitis C: APRI F4 (high cut-off) – sensitivity.

FIGURE 116.
Hepatitis C: APRI F4 (high cut-off) – specificity.
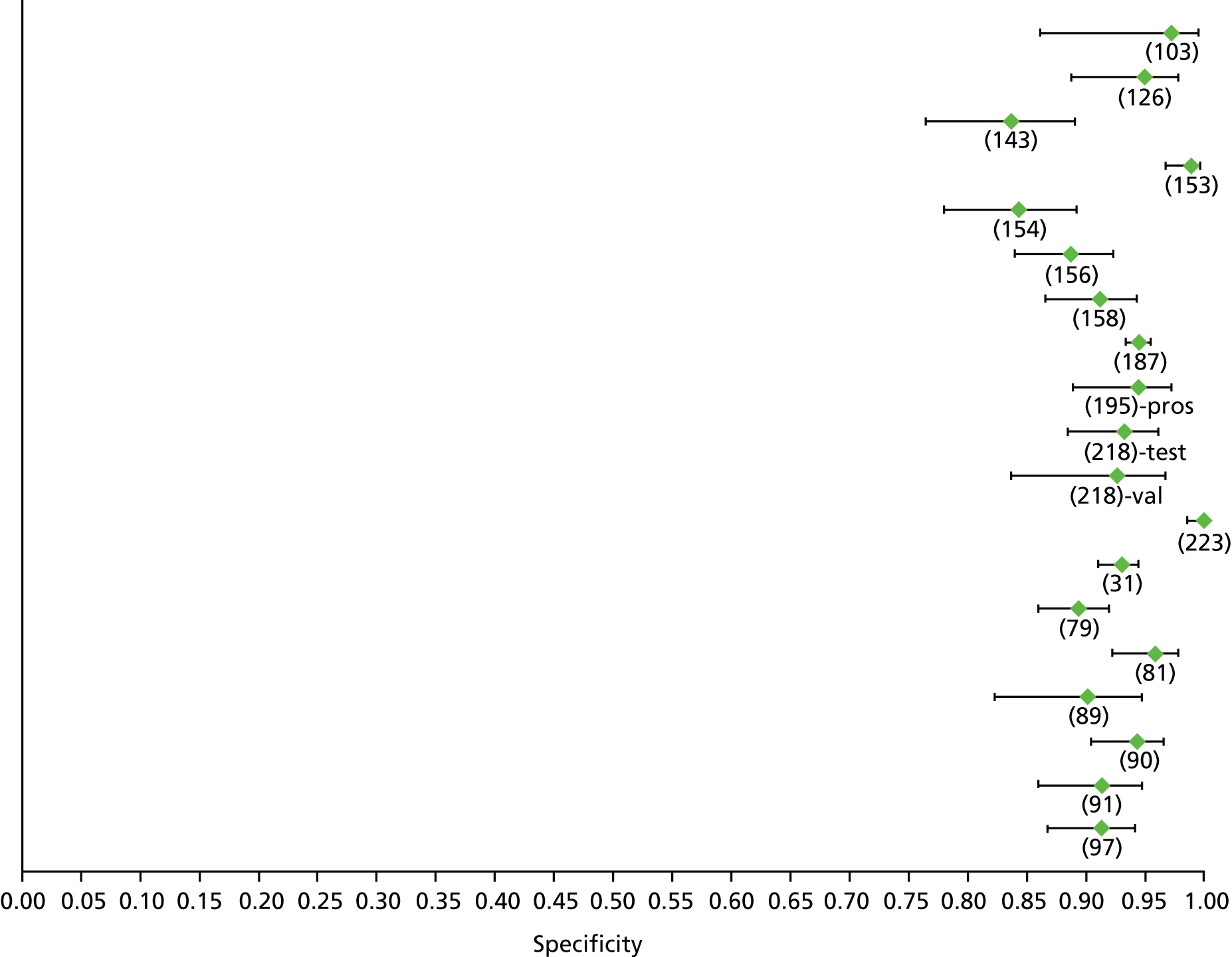
FIGURE 117.
Hepatitis C: APRI F4 (low cut-off) – sensitivity.

FIGURE 118.
Hepatitis C: APRI F4 (low cut-off) – specificity.
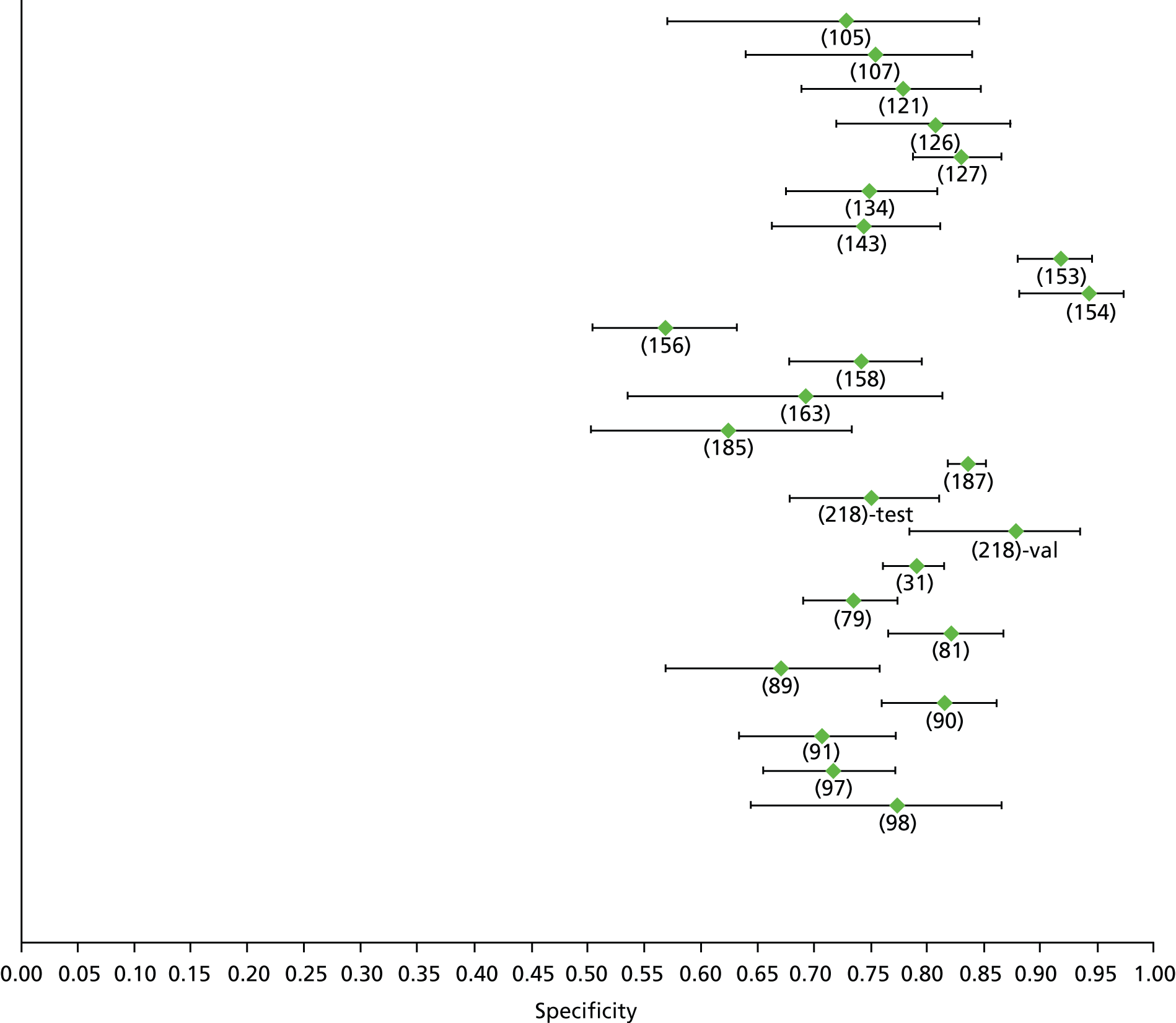
FIGURE 119.
Hepatitis C: AST–ALT ratio F4 – sensitivity.
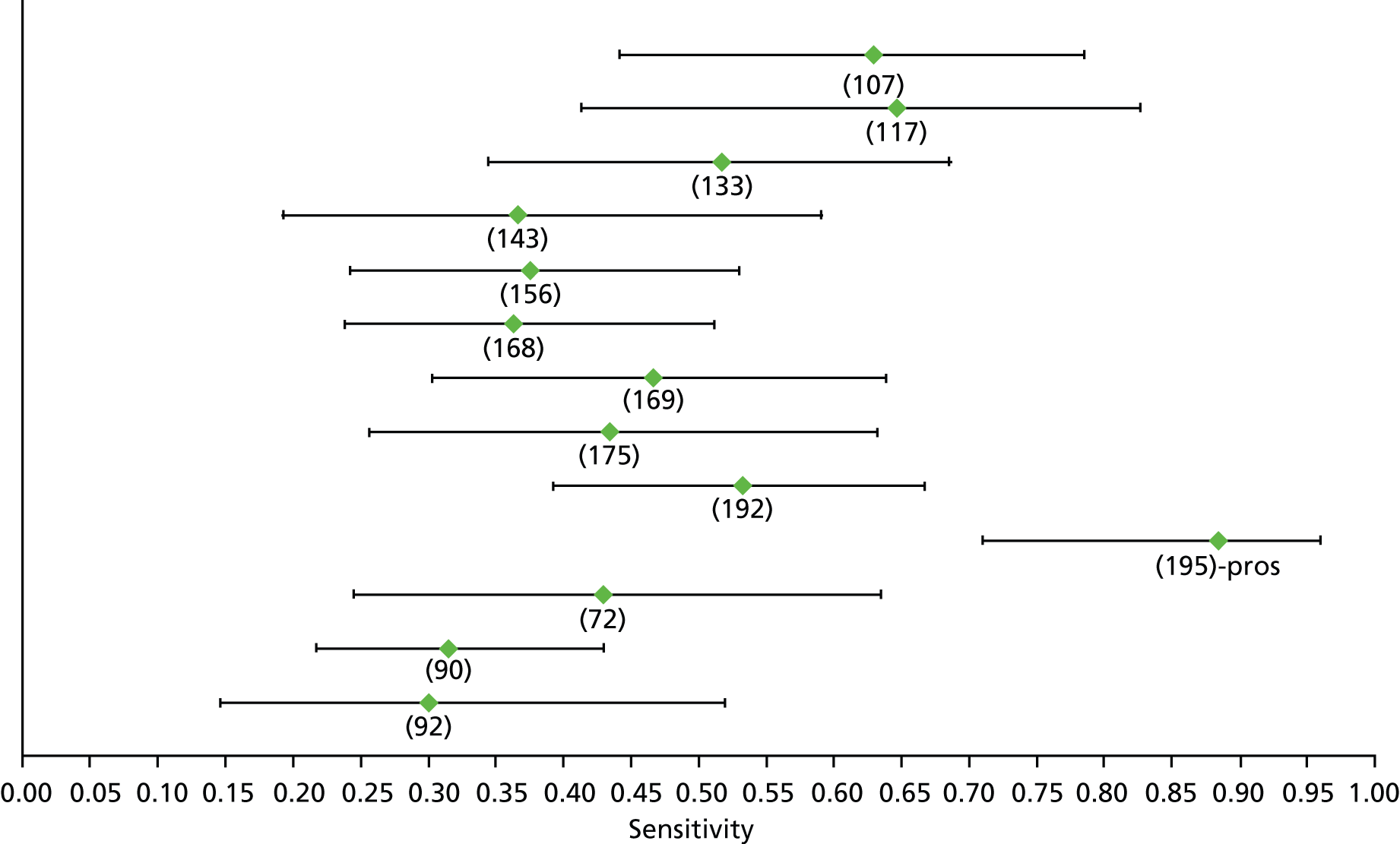
FIGURE 120.
Hepatitis C: AST–ALT ratio F4 – specificity.
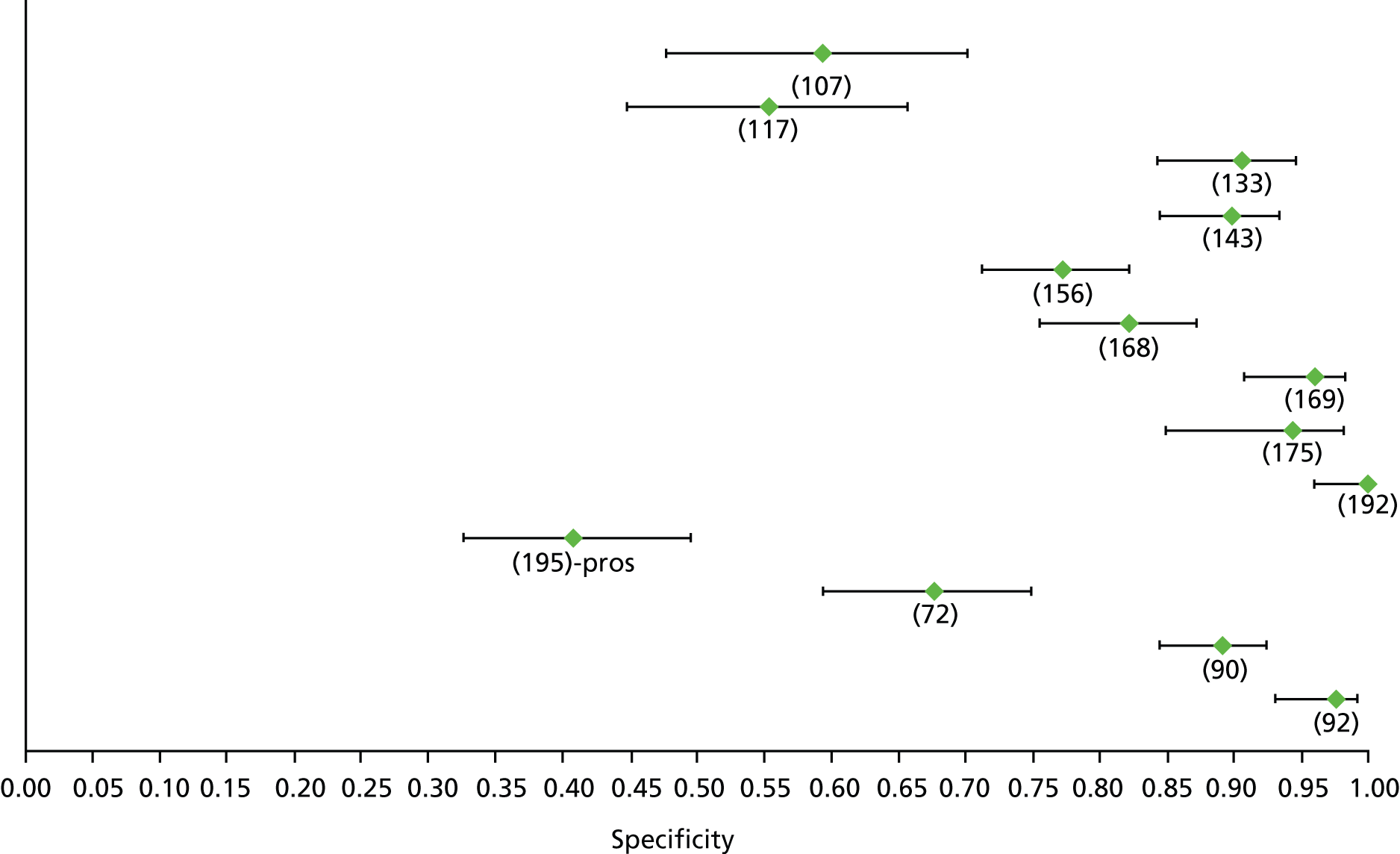
FIGURE 121.
Hepatitis C: FIB-4 F4 (high cut-off) – sensitivity.
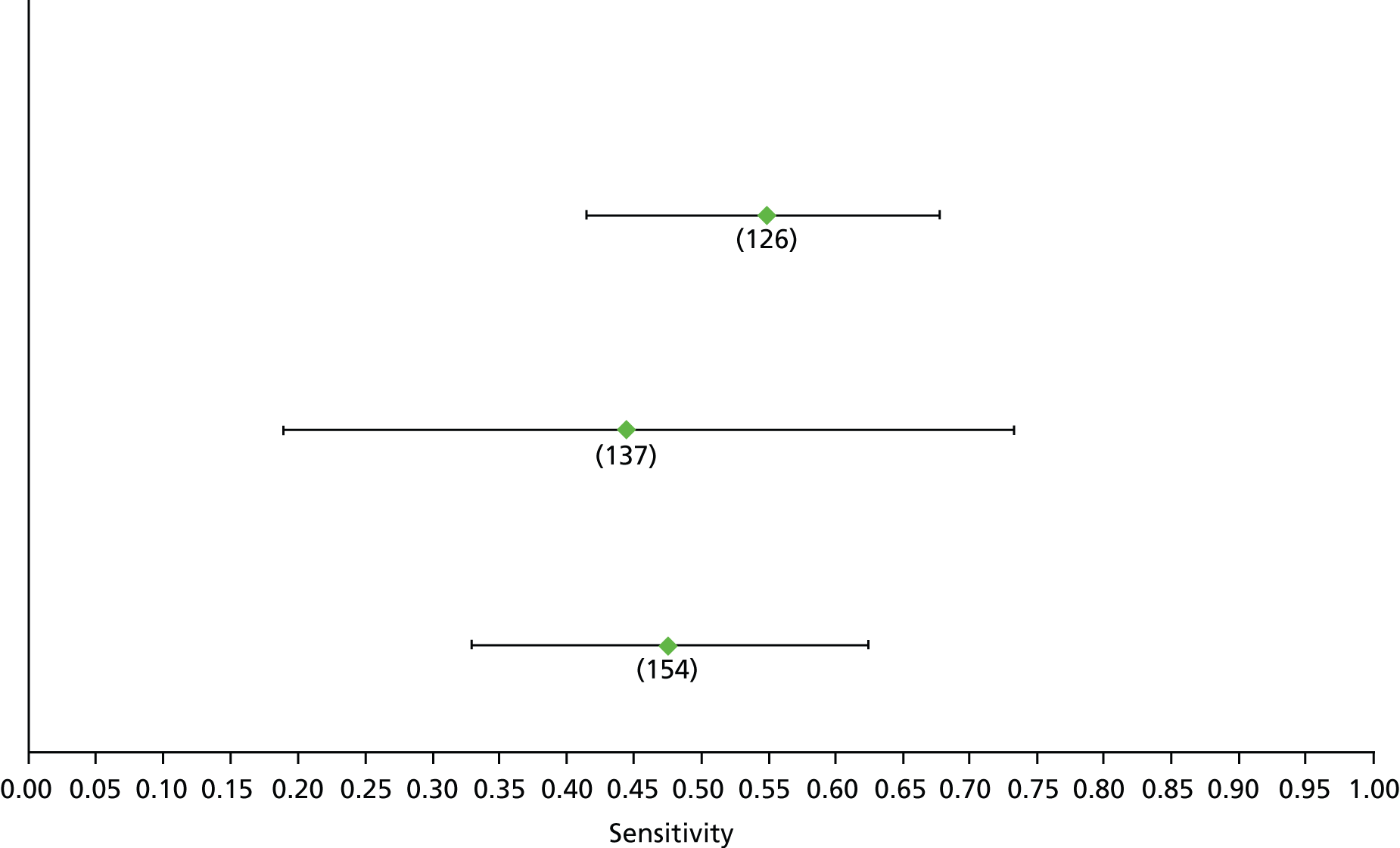
FIGURE 122.
Hepatitis C: FIB-4 F4 (high cut-off) – specificity.

FIGURE 123.
Hepatitis C: FIB-4 F4 (low cut-off) – sensitivity.
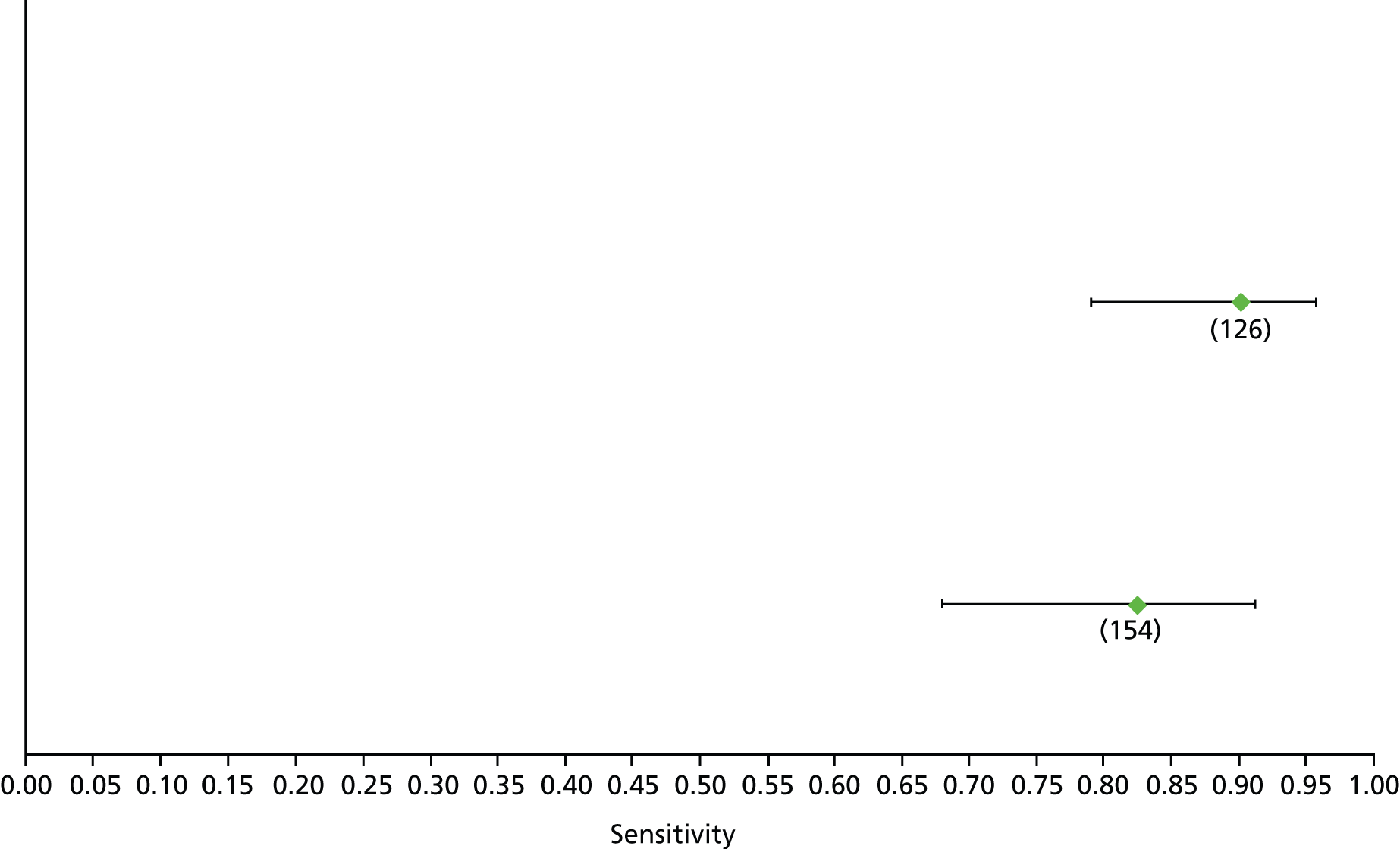
FIGURE 124.
Hepatitis C: FIB-4 F4 (low cut-off) – specificity.

FIGURE 125.
Hepatitis C: Fibrometer F4 – sensitivity.
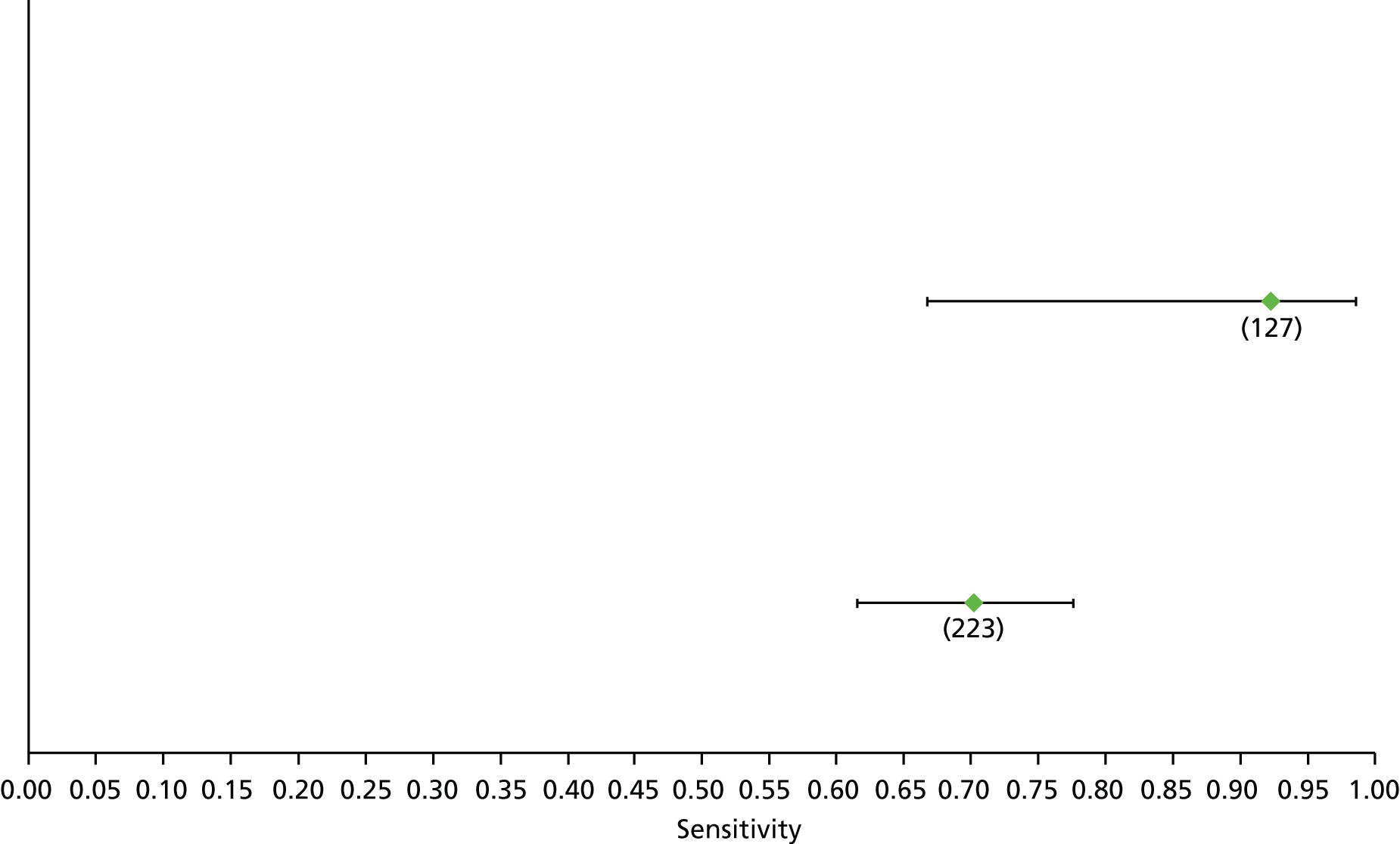
FIGURE 126.
Hepatitis C: Fibrometer F4 – specificity.

FIGURE 127.
Hepatitis C: Fibrotest F4 – sensitivity.
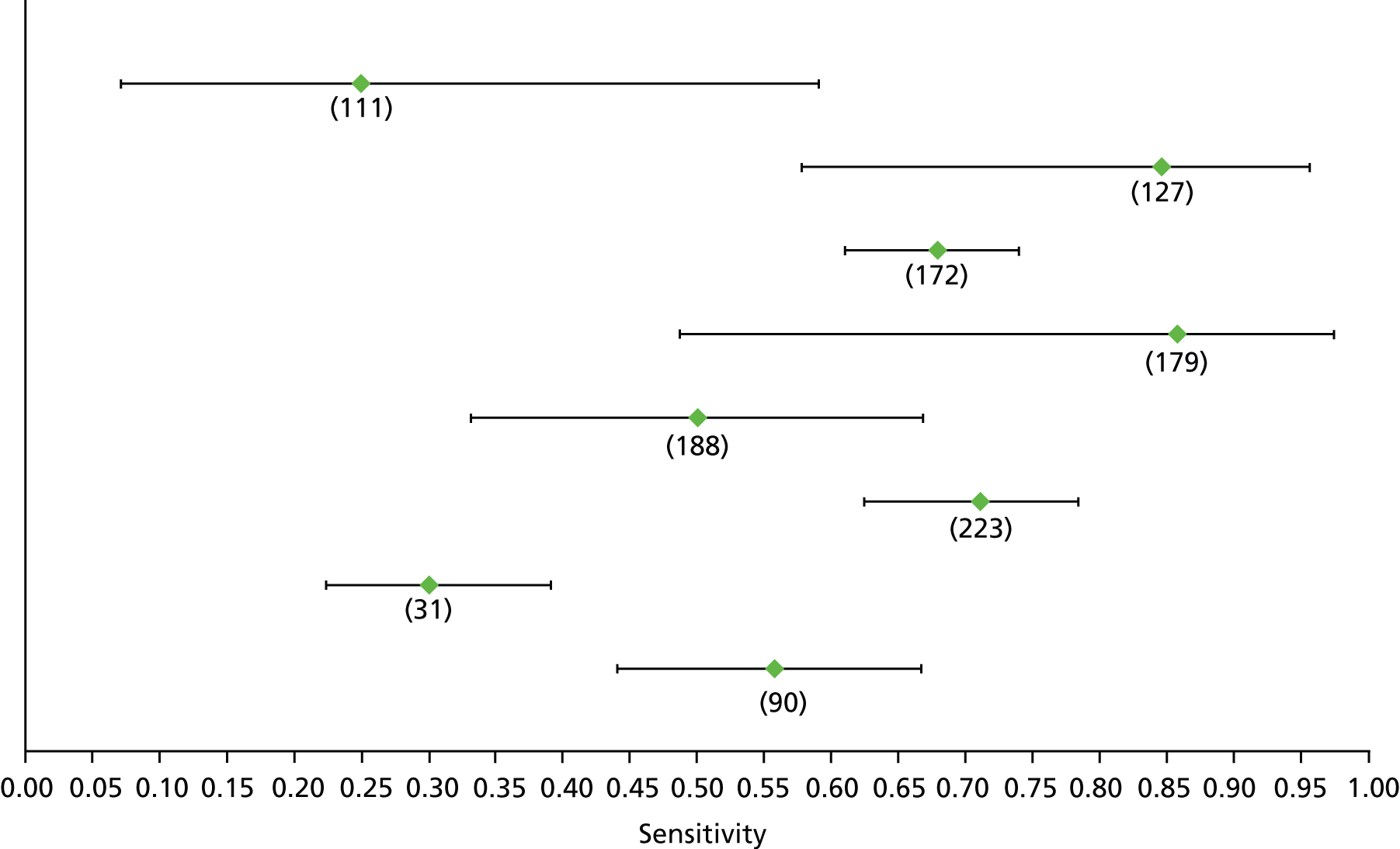
FIGURE 128.
Hepatitis C: Fibrotest F4 – specificity.
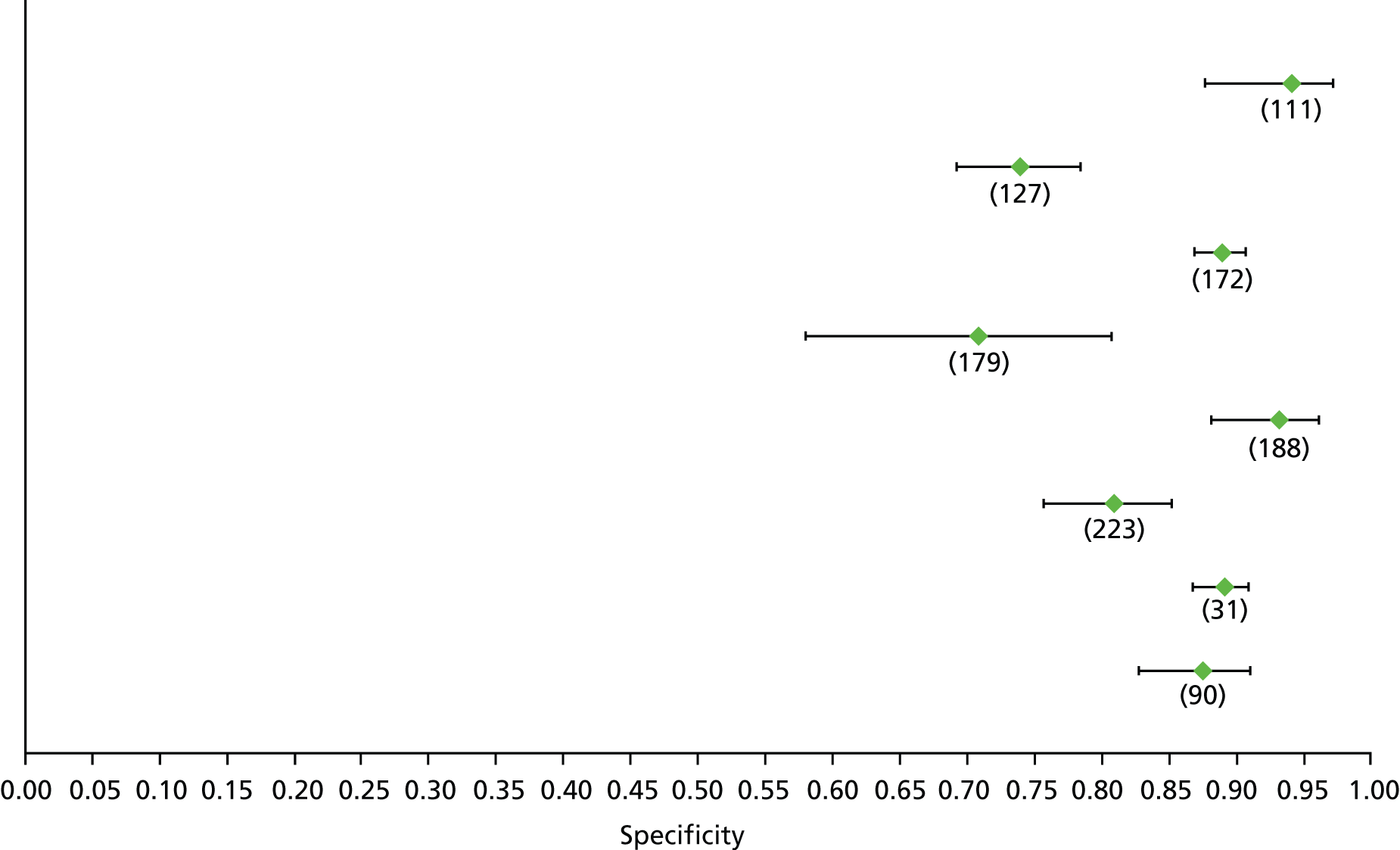
FIGURE 129.
Hepatitis C: GUCI F4 – sensitivity.

FIGURE 130.
Hepatitis C: GUCI F4 – specificity.

FIGURE 131.
Hepatitis C: hyaluronic acid F4 – sensitivity.
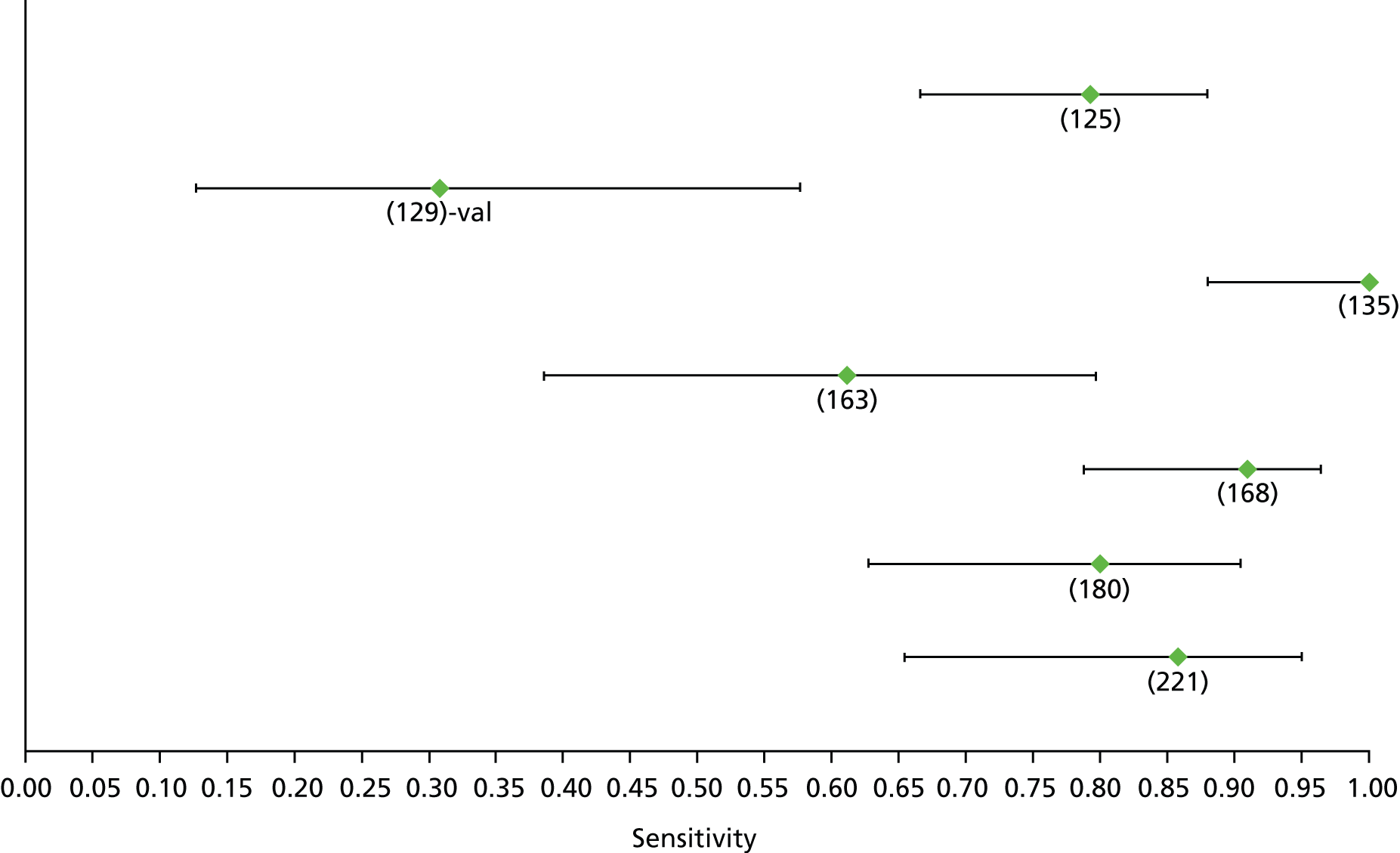
FIGURE 132.
Hepatitis C: hyaluronic acid F4 – specificity.

FIGURE 133.
Hepatitis C: Hepascore F4 – sensitivity. Val, validation cohort.
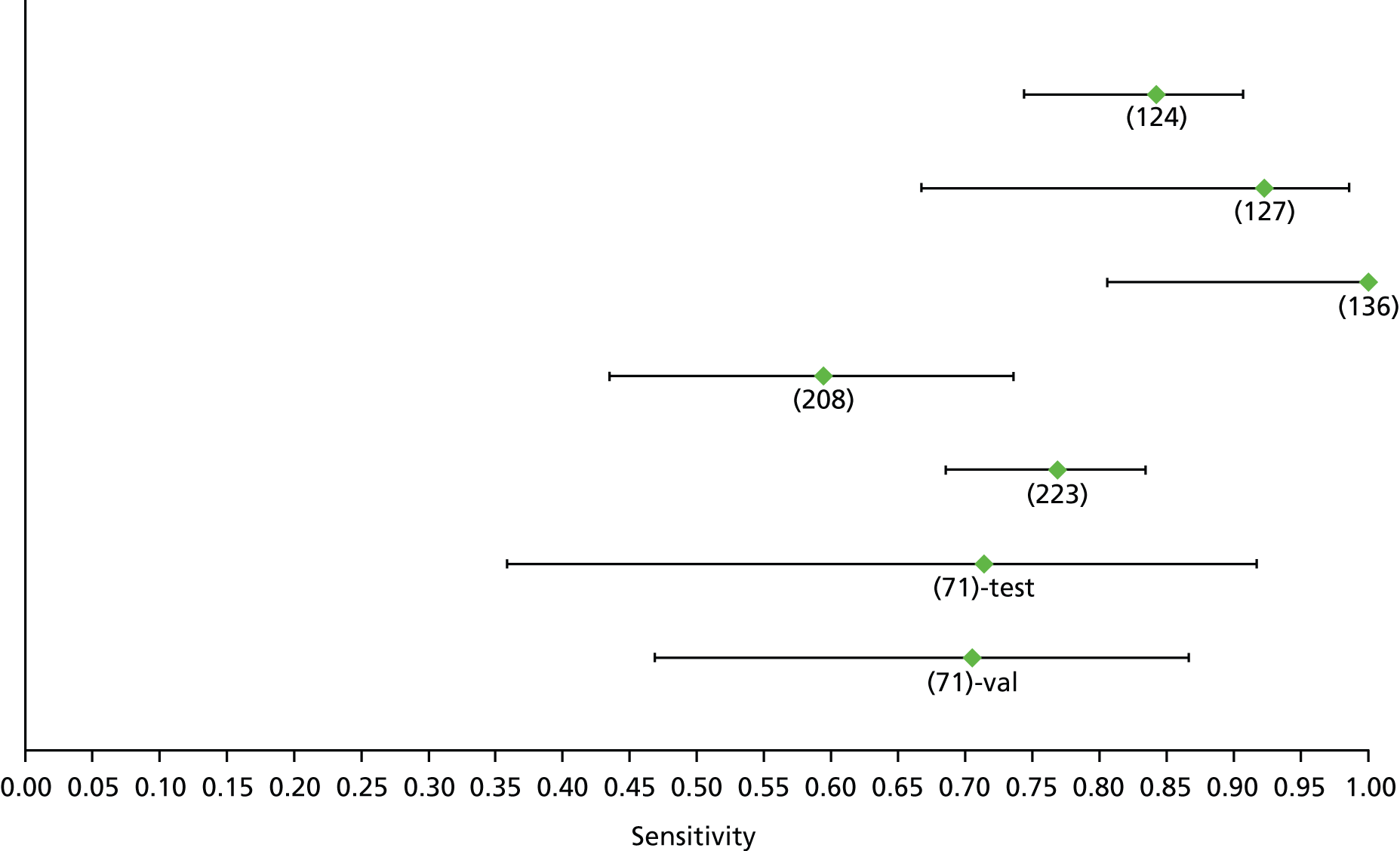
FIGURE 134.
Hepatitis C: Hepascore F4 – specificity. Val, validation cohort.

FIGURE 135.
Hepatitis C: platelet F4 – sensitivity.

FIGURE 136.
Hepatitis C: platelet F4 – specificity.
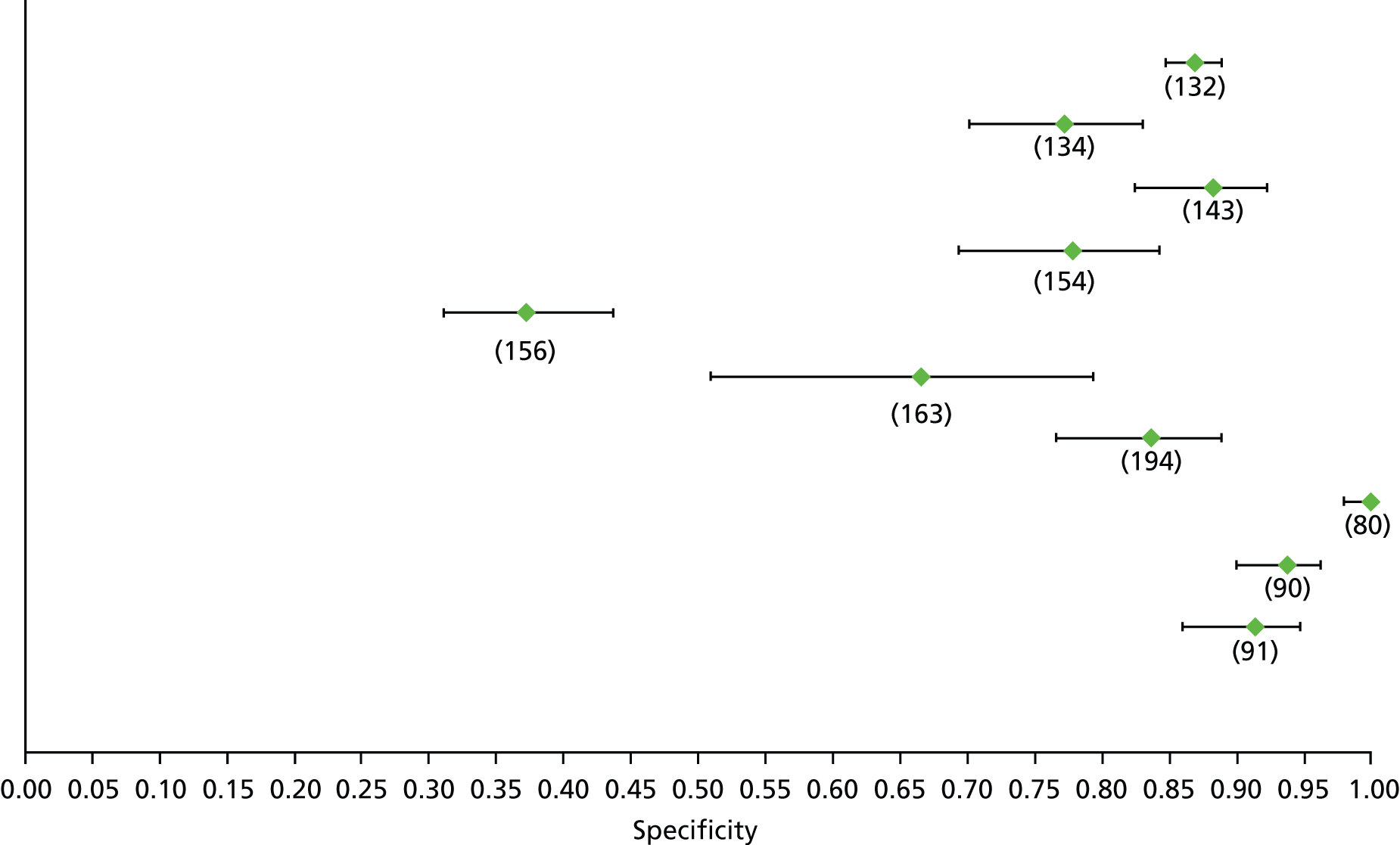
FIGURE 137.
Hepatitis C: TE F4 – sensitivity.
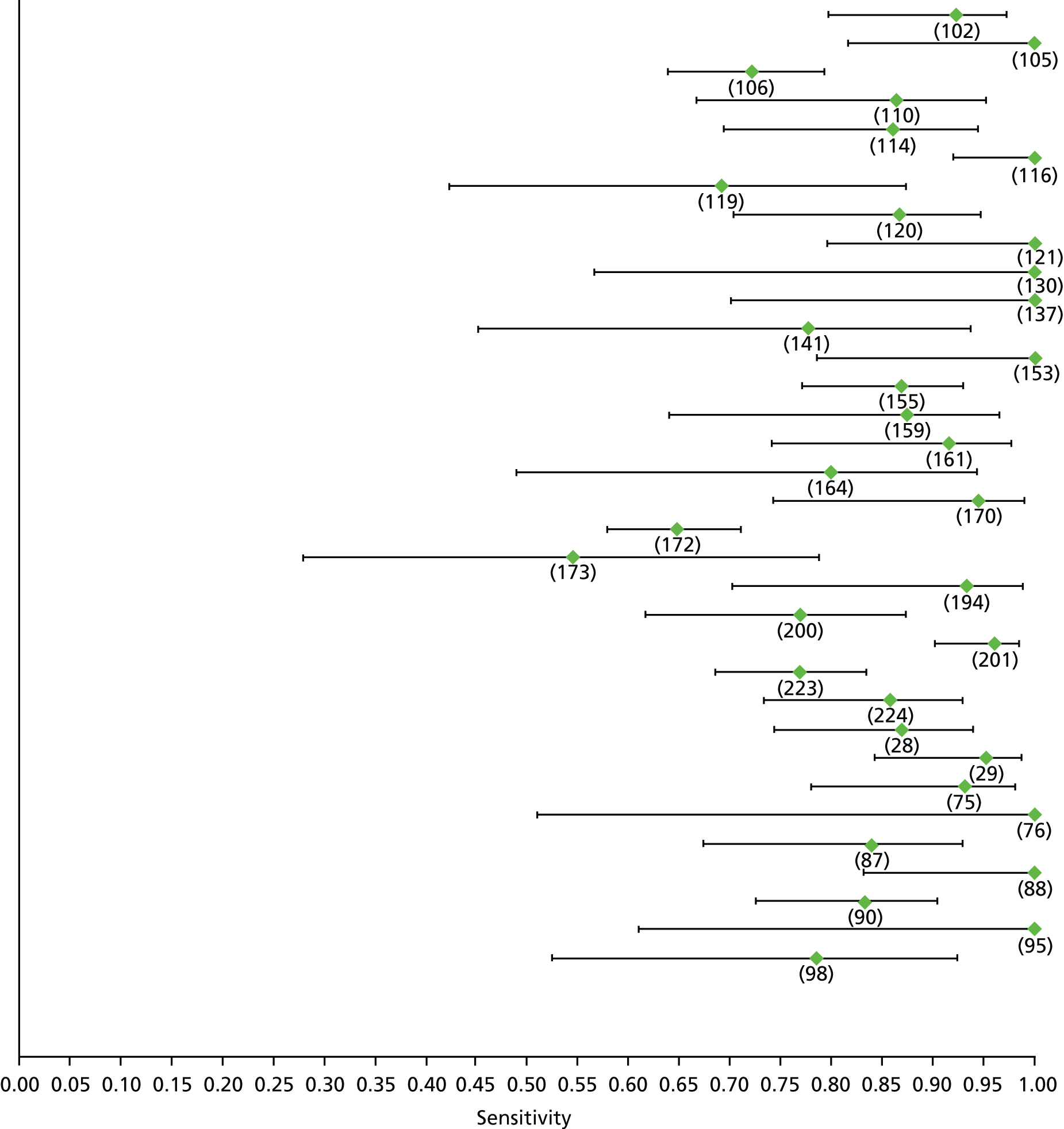
FIGURE 138.
Hepatitis C: TE F4 – specificity.

FIGURE 139.
Non-alcoholic fatty liver disease: NFS F1 (high cut-off) – sensitivity.

FIGURE 140.
Non-alcoholic fatty liver disease: NFS F1 (high cut-off) – specificity.

FIGURE 141.
Non-alcoholic fatty liver disease: NFS F1 (low cut-off) – sensitivity.

FIGURE 142.
Non-alcoholic fatty liver disease: NFS F1 (low cut-off) – specificity.

FIGURE 143.
Non-alcoholic fatty liver disease: TE F1 – sensitivity.

FIGURE 144.
Non-alcoholic fatty liver disease: TE F1 – specificity.

FIGURE 145.
Non-alcoholic fatty liver disease: APRI F2 – sensitivity.

FIGURE 146.
Non-alcoholic fatty liver disease: APRI F2 – specificity.

FIGURE 147.
Non-alcoholic fatty liver disease: Fibrotest F2 (high cut-off) – sensitivity. Val, validation cohort.
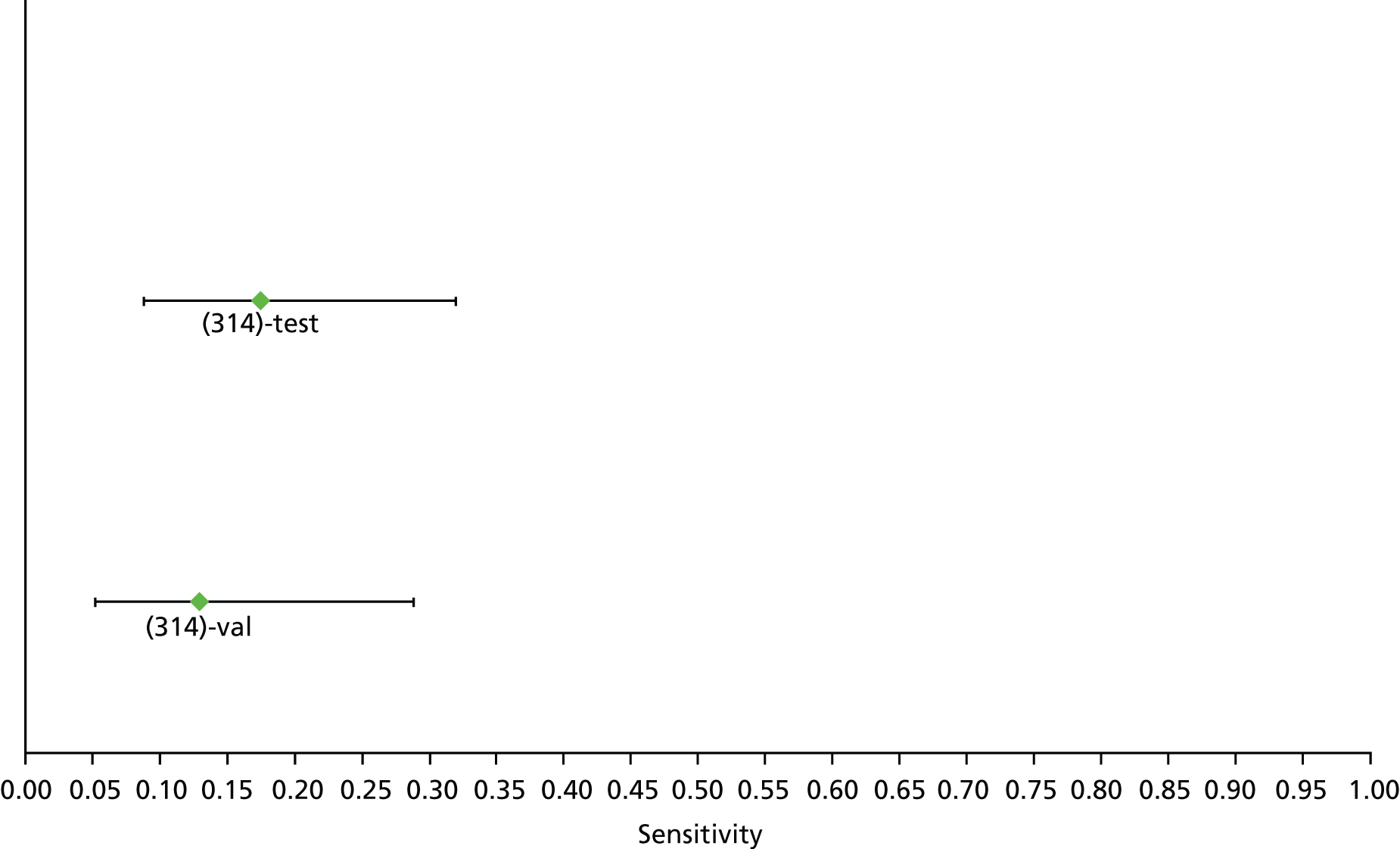
FIGURE 148.
Non-alcoholic fatty liver disease: Fibrotest F2 (high cut-off) – specificity. Val, validation cohort.

FIGURE 149.
Non-alcoholic fatty liver disease: Fibrotest F2 (low cut-off) – sensitivity. Val, validation cohort.

FIGURE 150.
Non-alcoholic fatty liver disease: Fibrotest F2 (low cut-off) – specificity. Val, validation cohort.

FIGURE 151.
Non-alcoholic fatty liver disease: NFS F2 (high cut-off) – sensitivity.
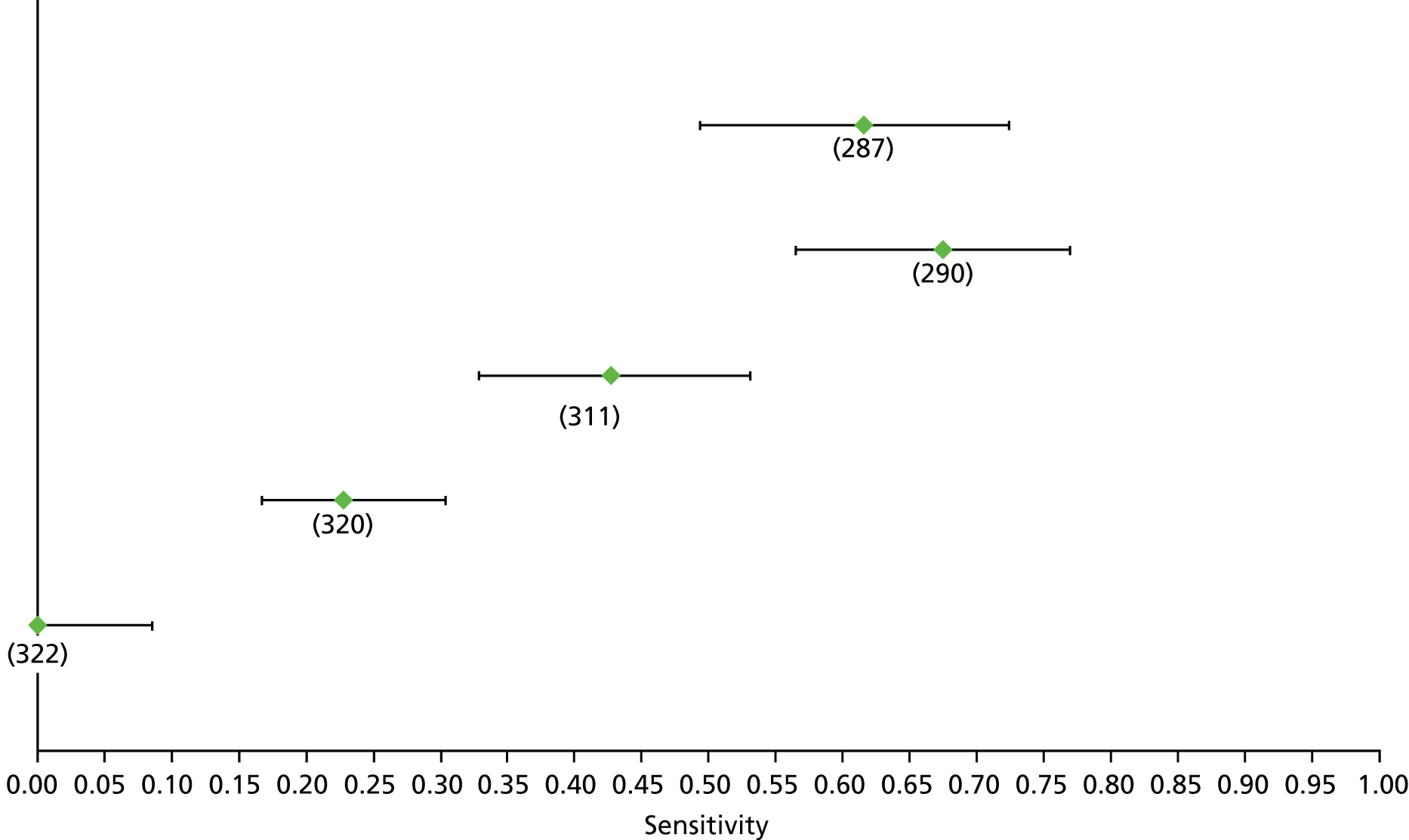
FIGURE 152.
Non-alcoholic fatty liver disease: NFS F2 (high cut-off) – specificity.
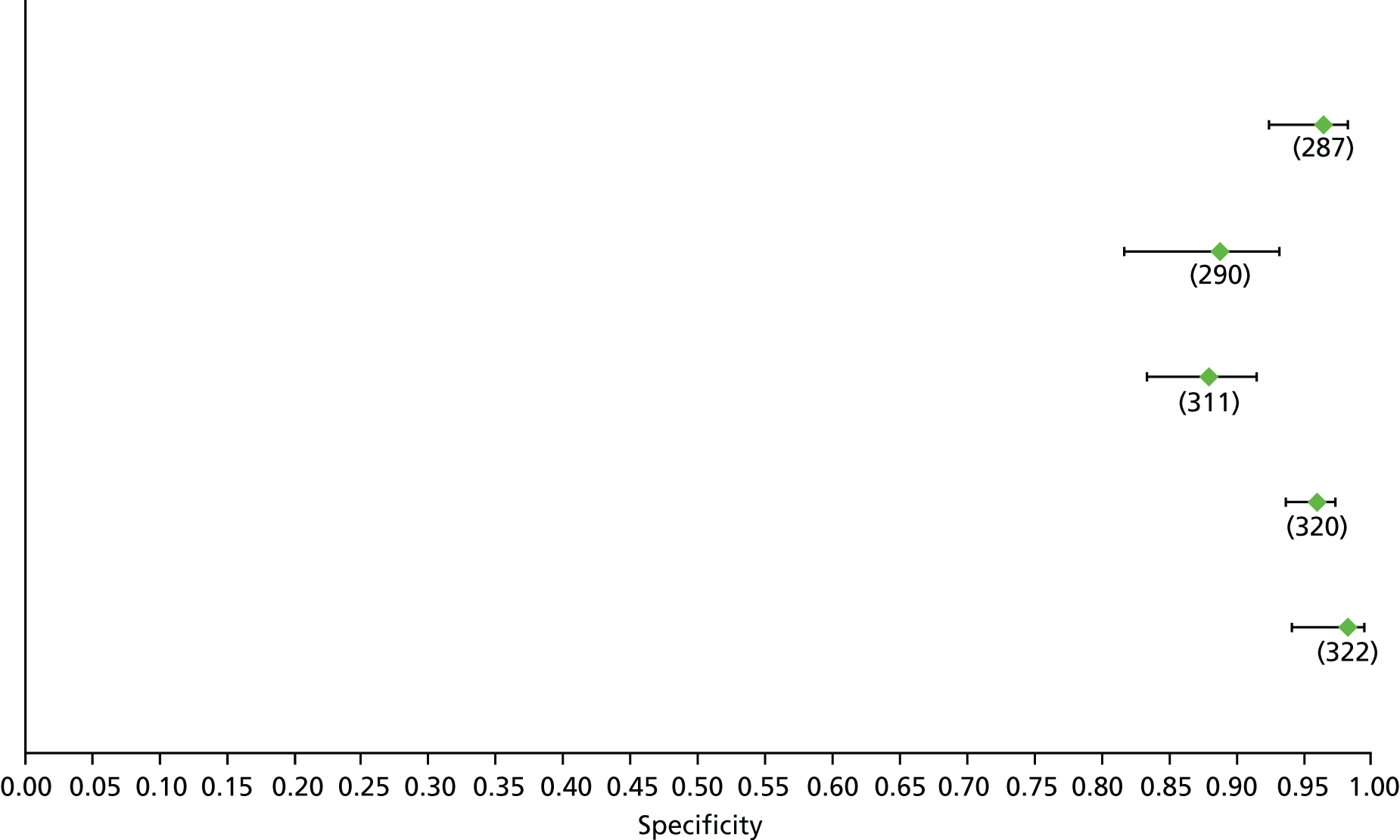
FIGURE 153.
Non-alcoholic fatty liver disease: NFS F2 (low cut-off) – sensitivity.
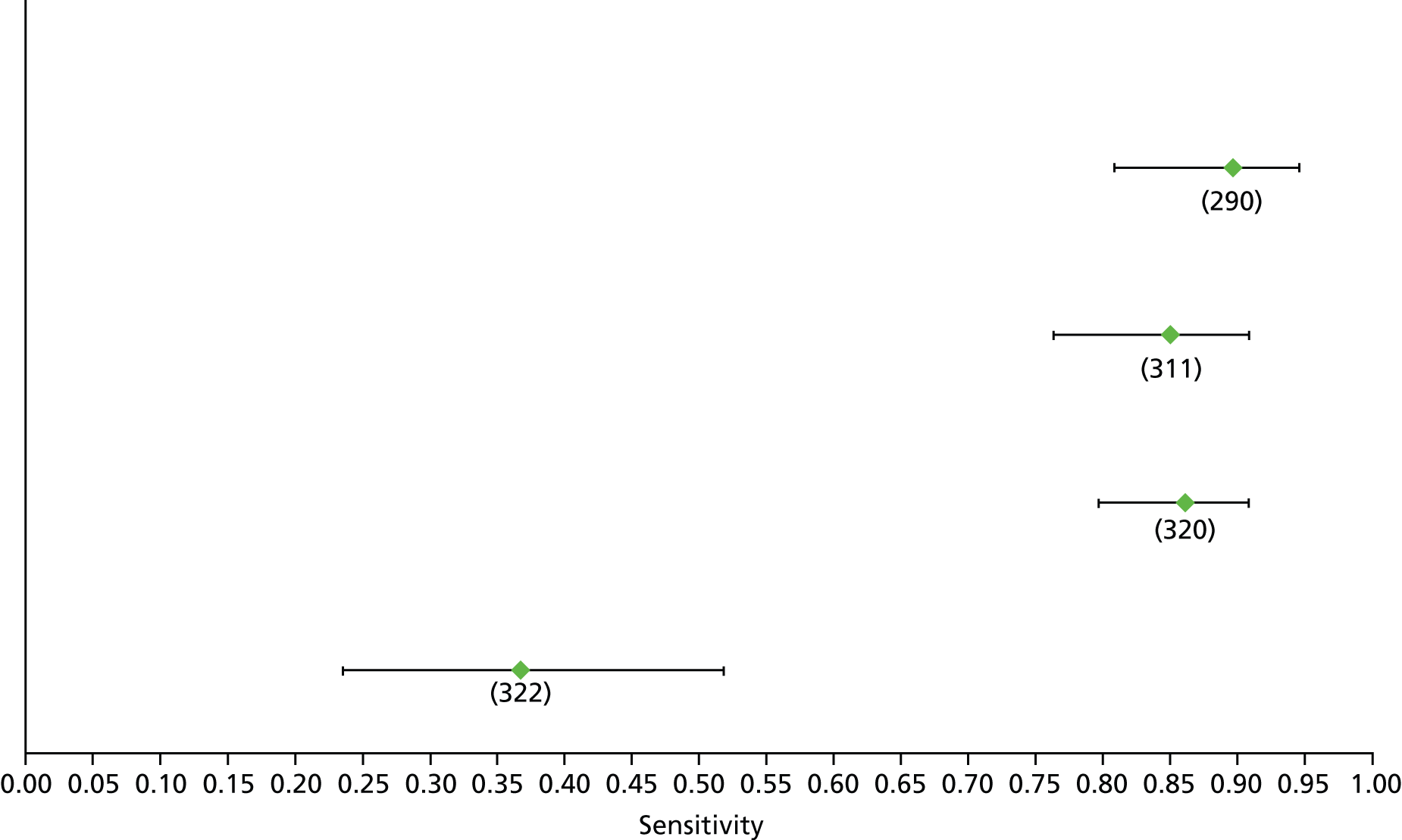
FIGURE 154.
Non-alcoholic fatty liver disease: NFS F2 (low cut-off) – specificity.
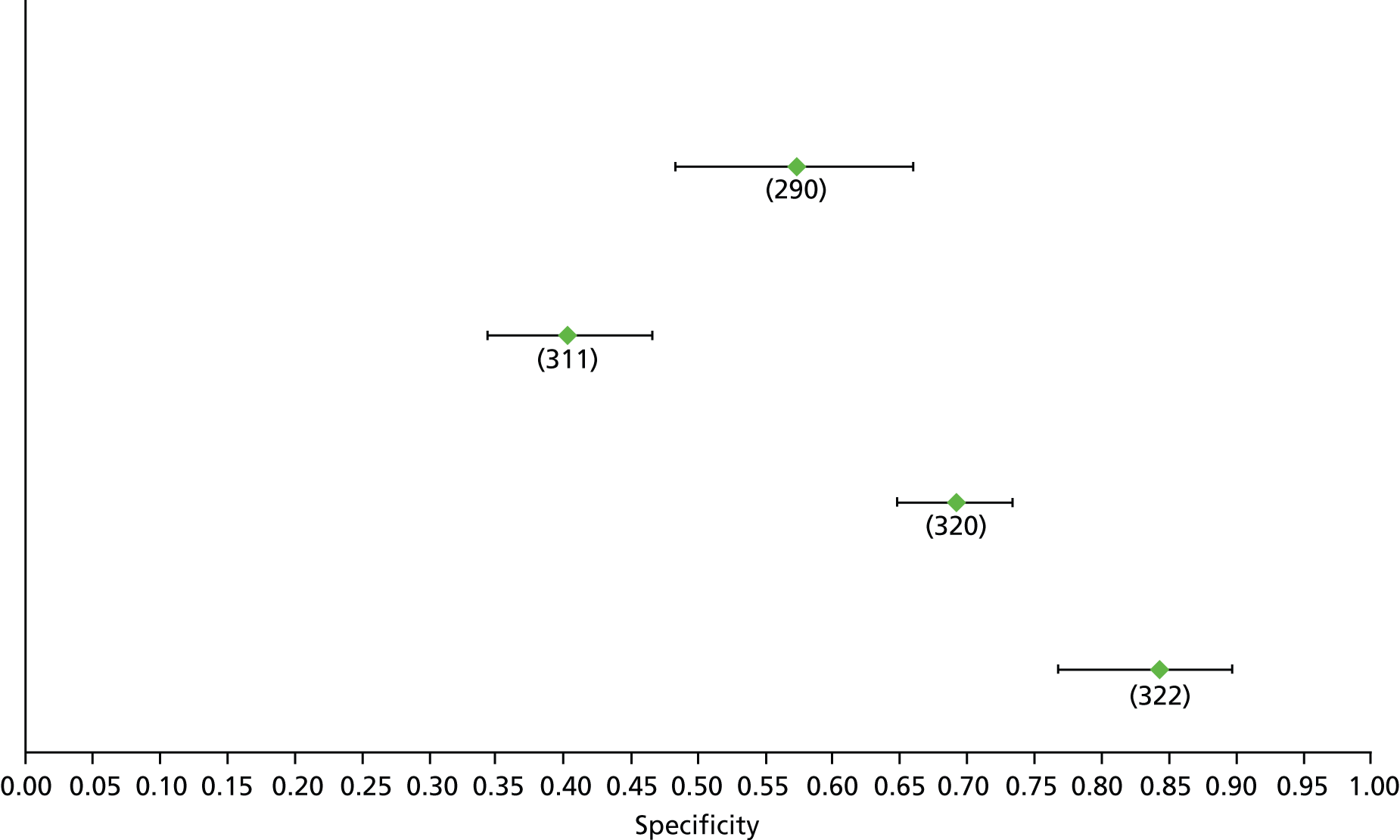
FIGURE 155.
Non-alcoholic fatty liver disease: TE F2 – sensitivity.
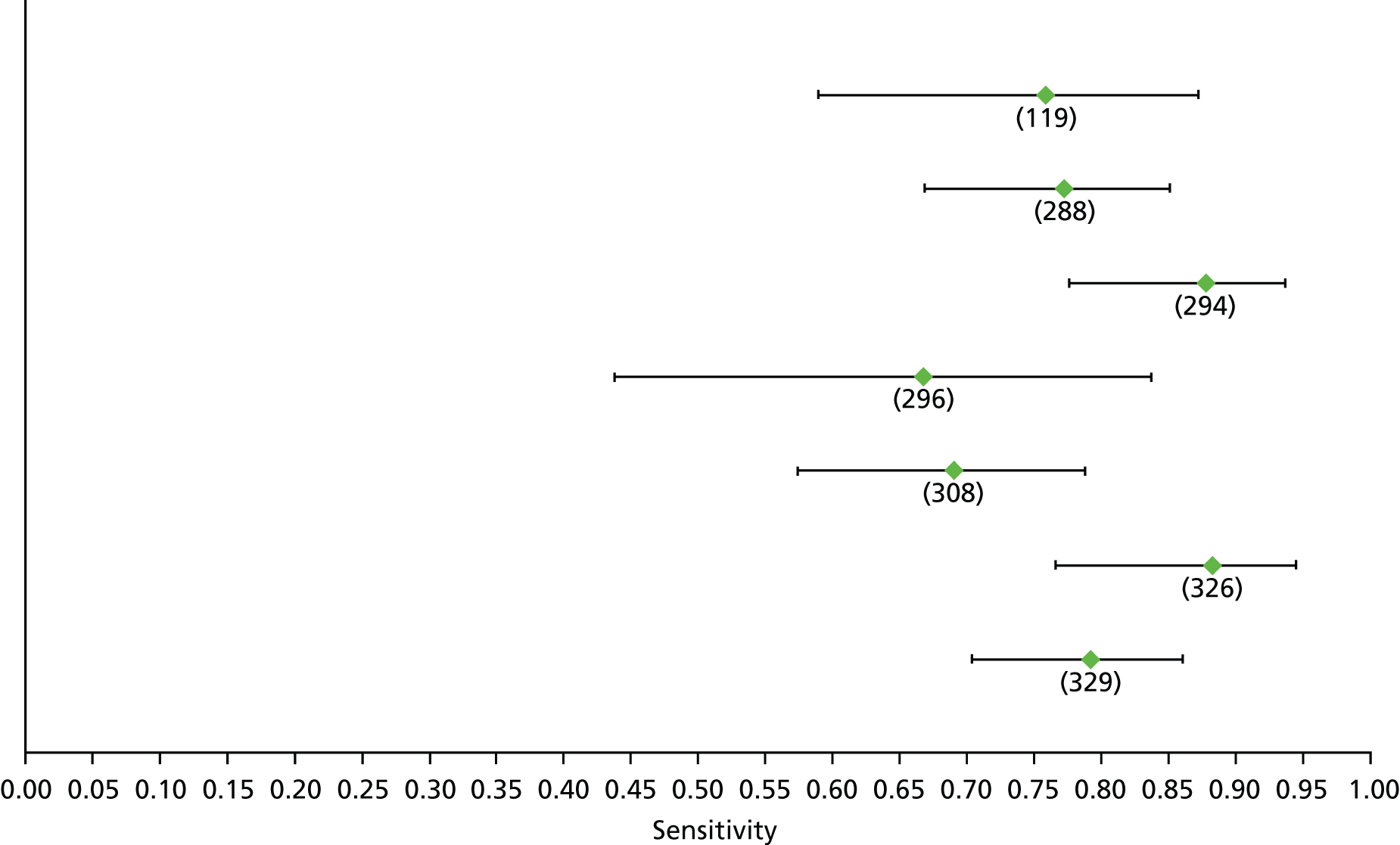
FIGURE 156.
Non-alcoholic fatty liver disease: TE F2 – specificity.

FIGURE 157.
Non-alcoholic fatty liver disease: APRI F3 – sensitivity.

FIGURE 158.
Non-alcoholic fatty liver disease: APRI F3 – specificity.
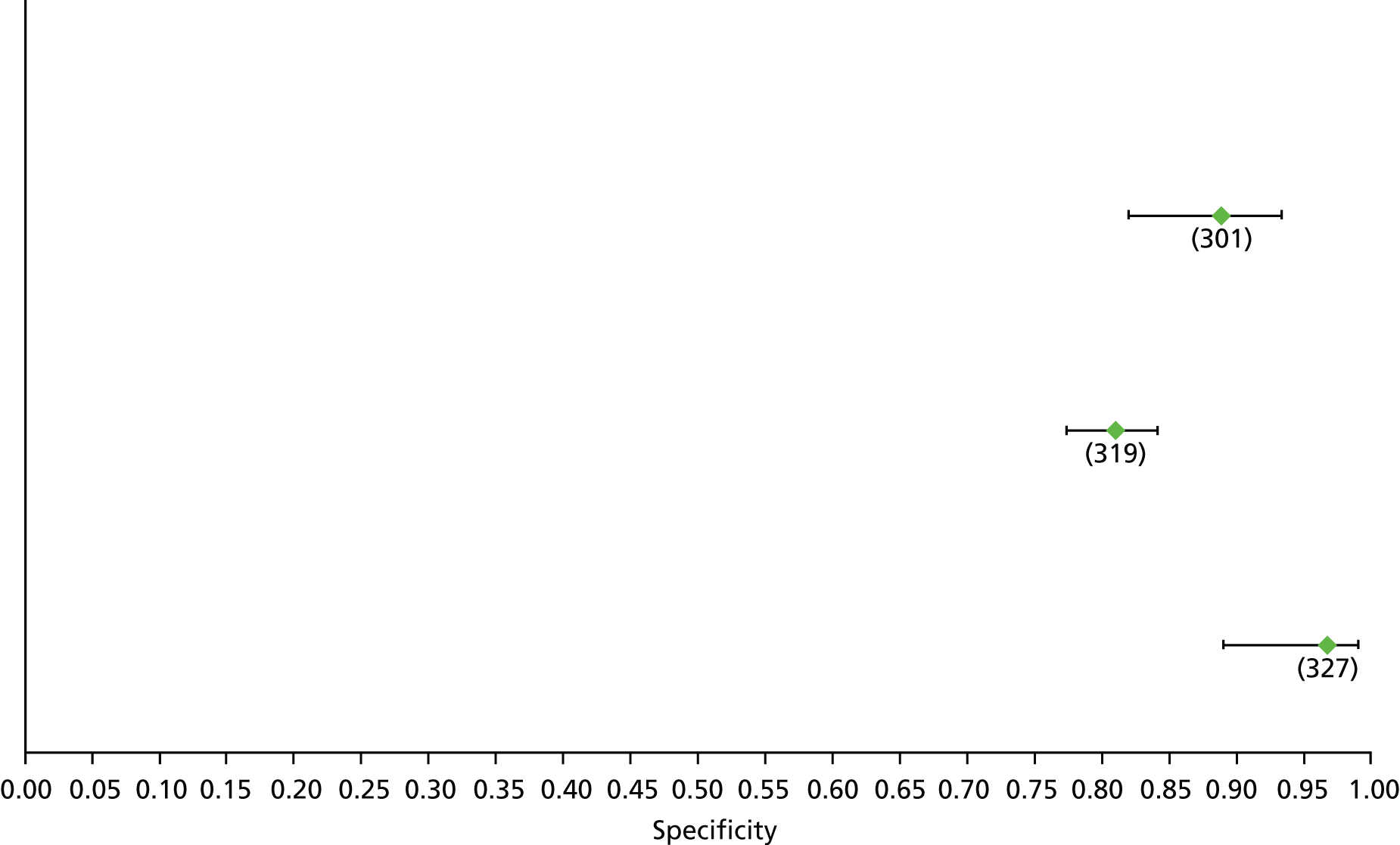
FIGURE 159.
Non-alcoholic fatty liver disease: BARD F3 – sensitivity.

FIGURE 160.
Non-alcoholic fatty liver disease: BARD F3 – specificity.

FIGURE 161.
Non-alcoholic fatty liver disease: FIB-4 F3 (high cut-off) – sensitivity.

FIGURE 162.
Non-alcoholic fatty liver disease: FIB-4 F3 (high cut-off) – specificity.
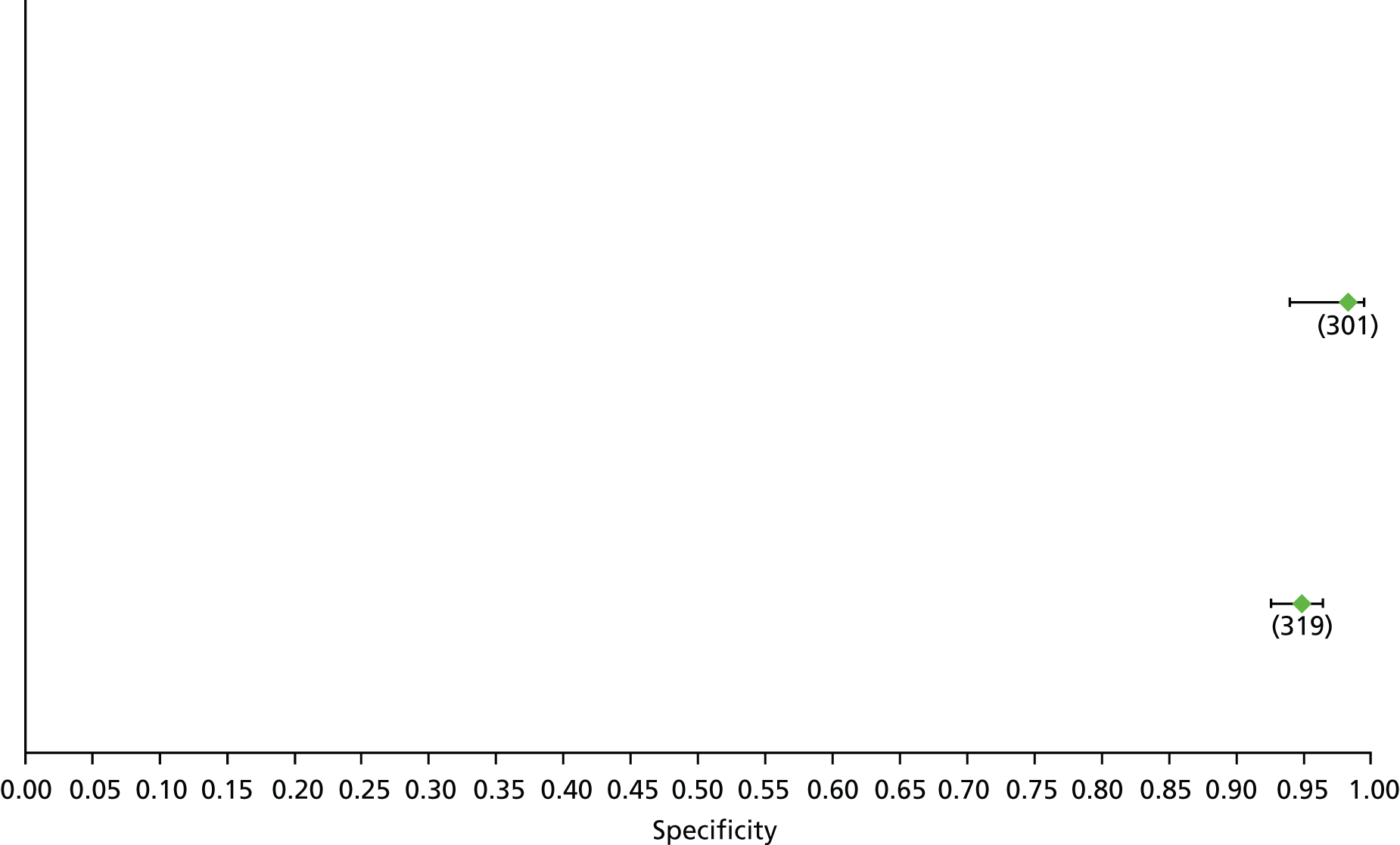
FIGURE 163.
Non-alcoholic fatty liver disease: FIB-4 F3 (low cut-off) – sensitivity.
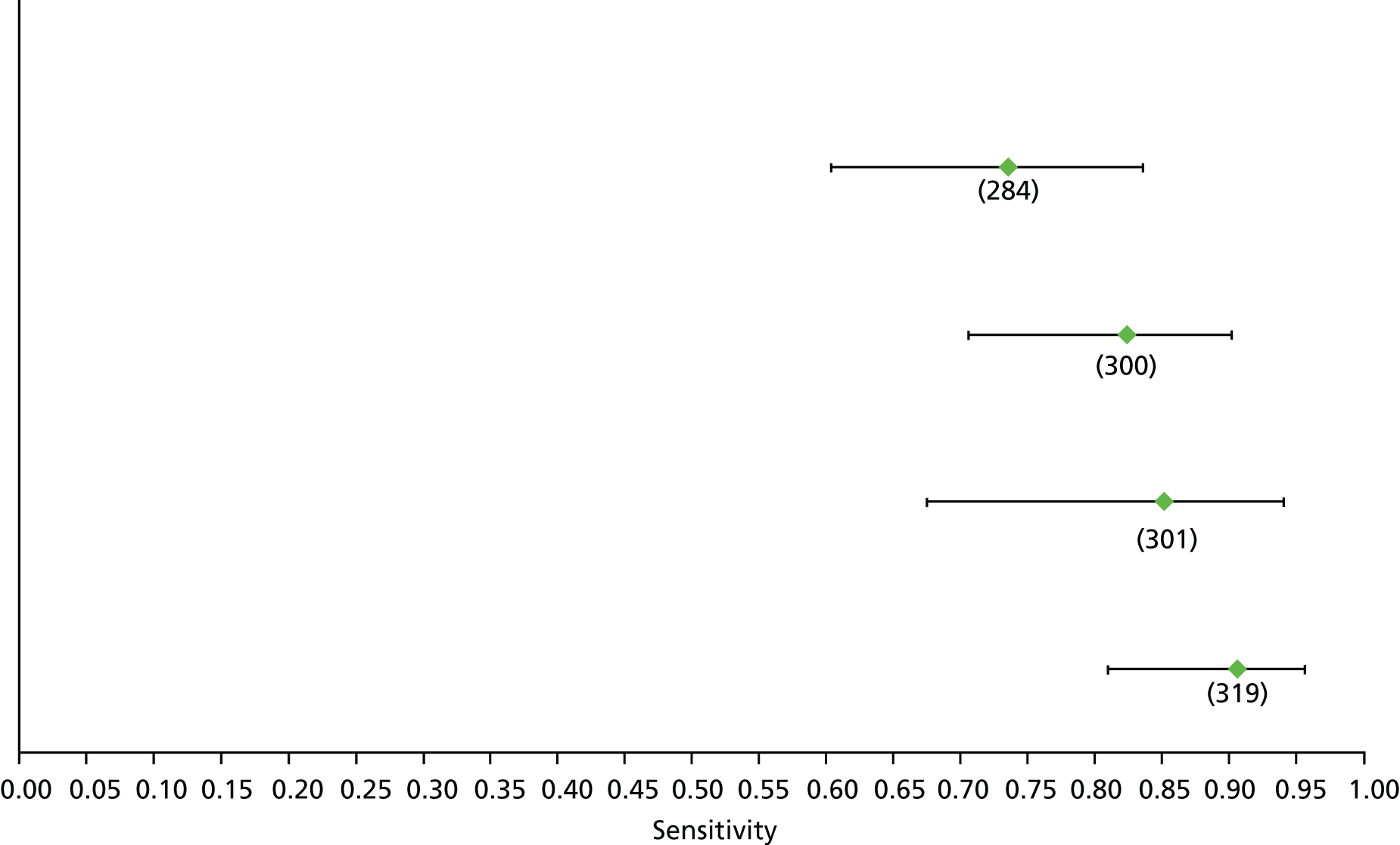
FIGURE 164.
Non-alcoholic fatty liver disease: FIB-4 F3 (low cut-off) – specificity.
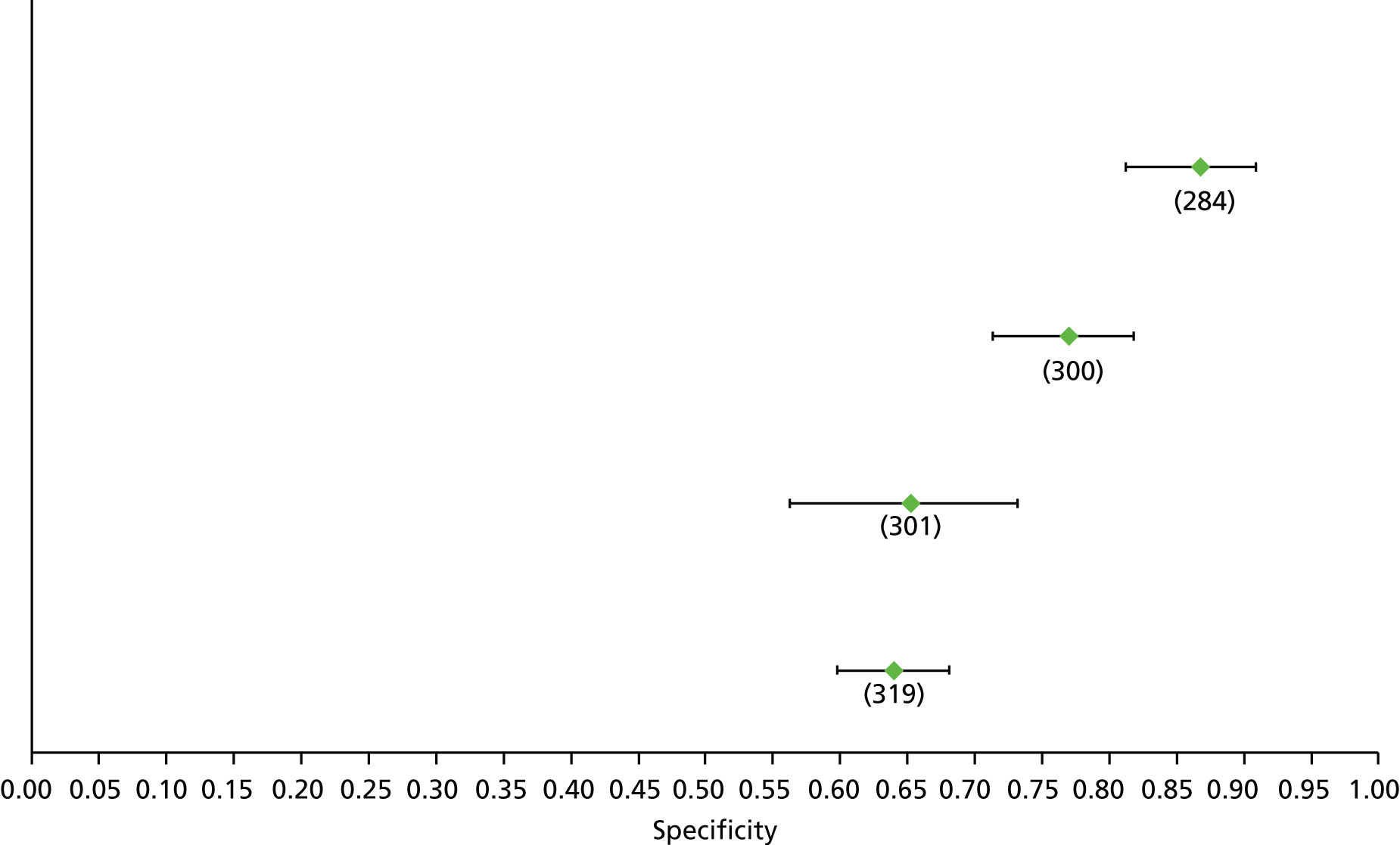
FIGURE 165.
Non-alcoholic fatty liver disease: hyaluronic acid F3 – sensitivity.

FIGURE 166.
Non-alcoholic fatty liver disease: hyaluronic acid F3 – specificity.
FIGURE 167.
Non-alcoholic fatty liver disease: NFS F3 (high cut-off) – sensitivity. Val, validation cohort.

FIGURE 168.
Non-alcoholic fatty liver disease: NFS F3 (high cut-off) – specificity. Val, validation cohort.

FIGURE 169.
Non-alcoholic fatty liver disease: NFS F3 (low cut-off) – sensitivity. Val, validation cohort.

FIGURE 170.
Non-alcoholic fatty liver disease: NFS F3 (low cut-off) – specificity. Val, validation cohort.
FIGURE 171.
Non-alcoholic fatty liver disease: TE F3 – sensitivity.
FIGURE 172.
Non-alcoholic fatty liver disease: TE F3 – specificity.
FIGURE 173.
Non-alcoholic fatty liver disease: APRI F4 – sensitivity.
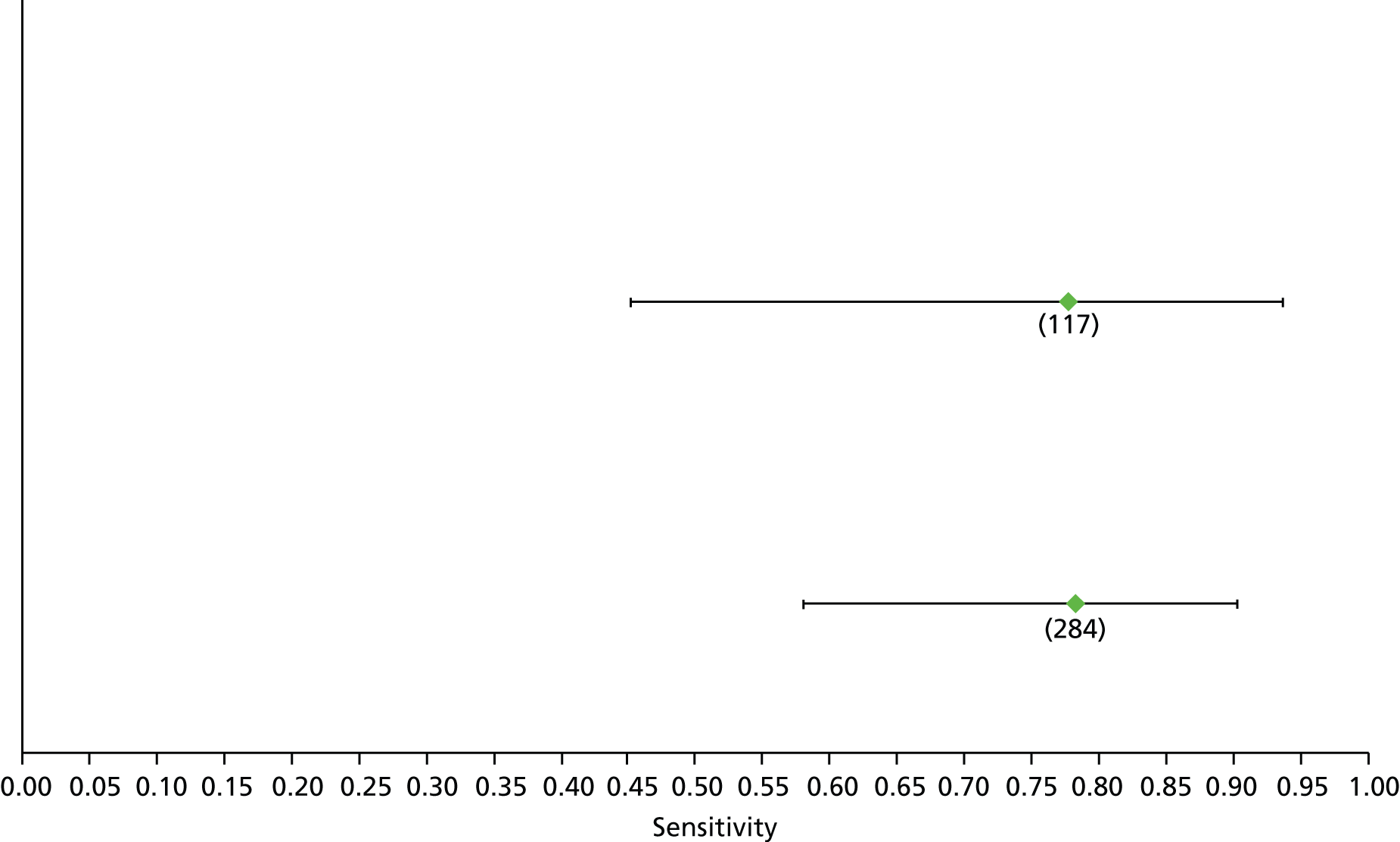
FIGURE 174.
Non-alcoholic fatty liver disease: APRI F4 – specificity.

FIGURE 175.
Non-alcoholic fatty liver disease: platelet F4 – sensitivity.

FIGURE 176.
Non-alcoholic fatty liver disease: platelet F4 – specificity.
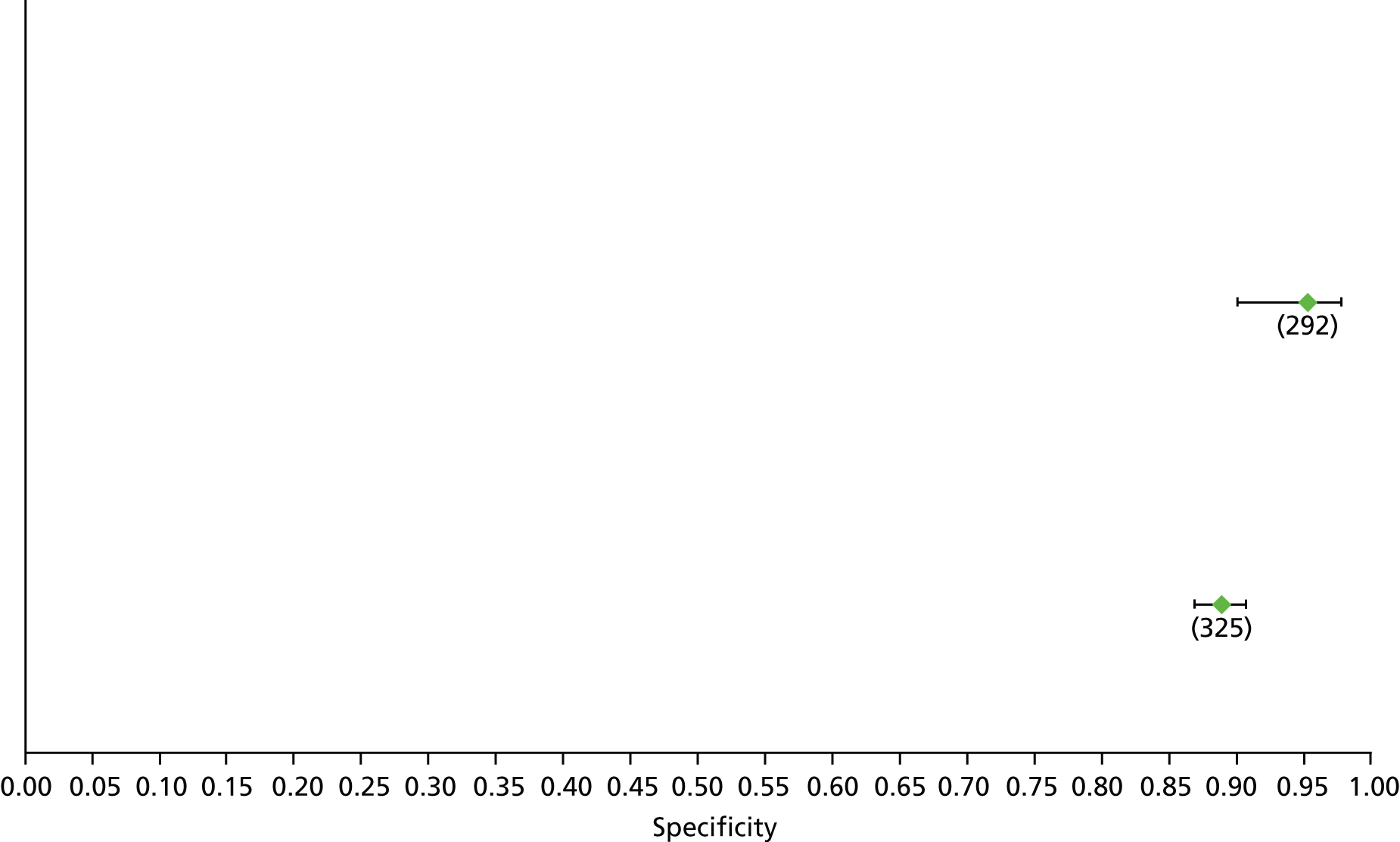
FIGURE 177.
Non-alcoholic fatty liver disease: TE F4 – sensitivity.
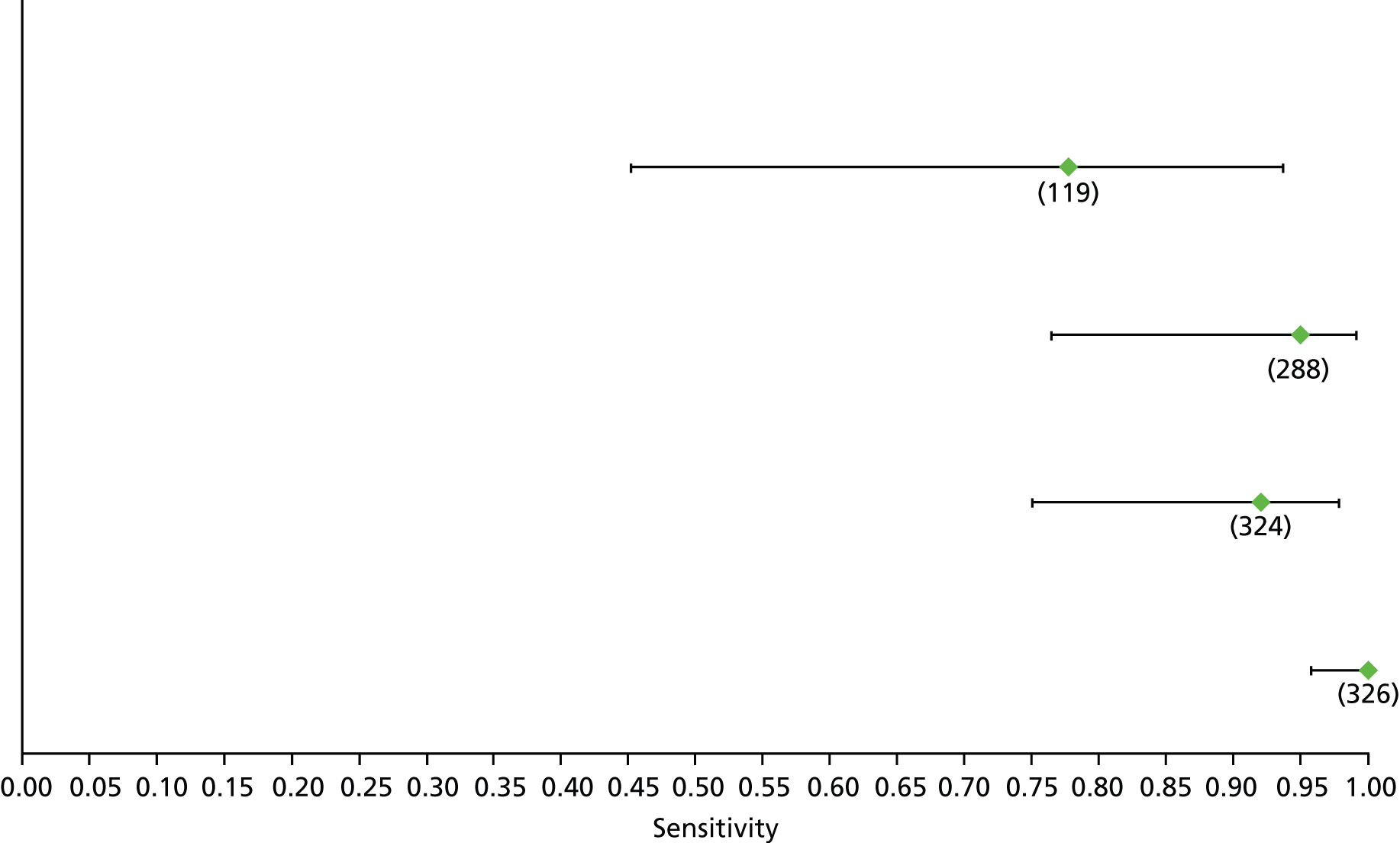
FIGURE 178.
Non-alcoholic fatty liver disease: TE F4 – specificity.
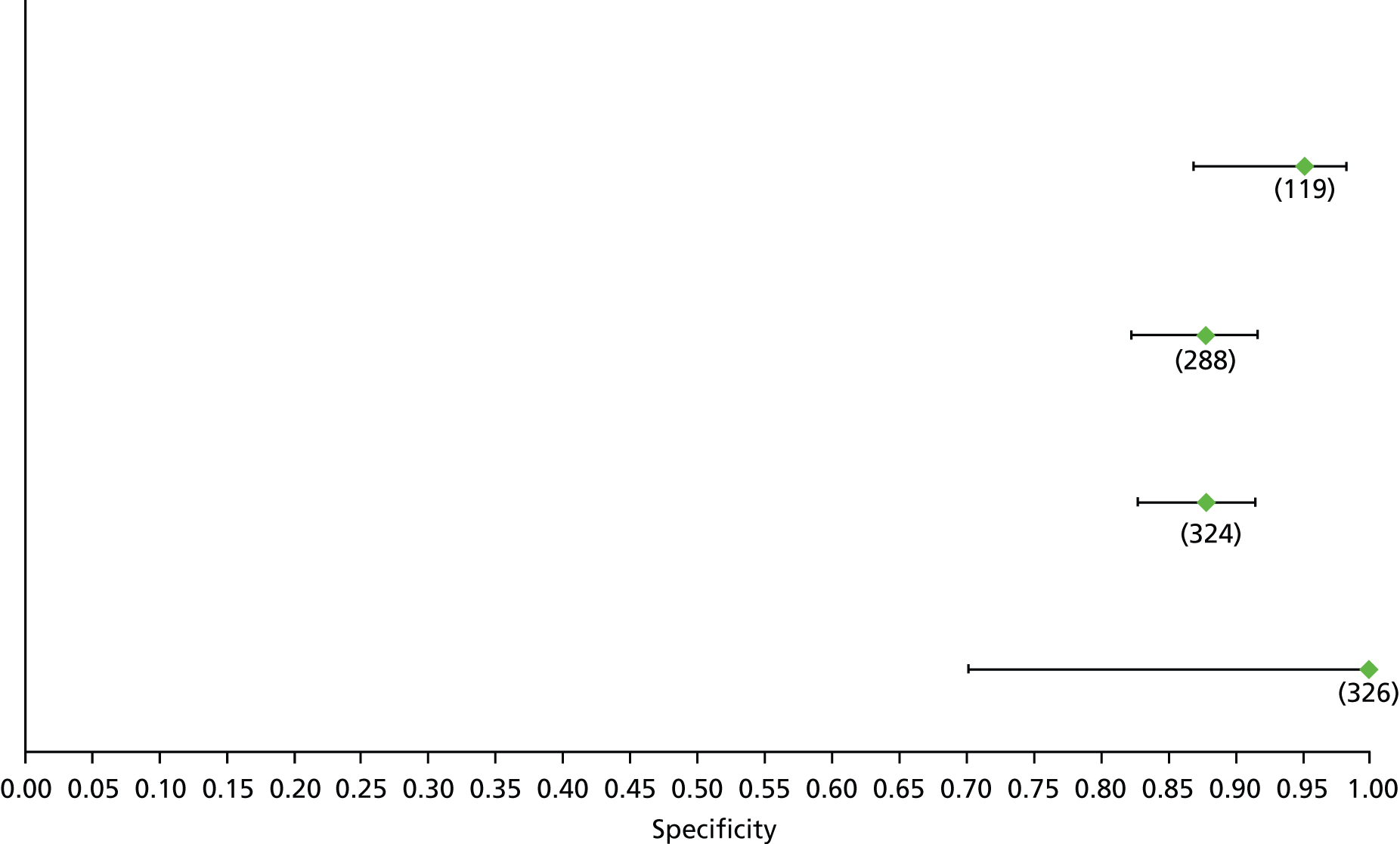
FIGURE 179.
Radiology: MR elastography F2 – sensitivity.

FIGURE 180.
Radiology: MR elastography F2 – specificity.
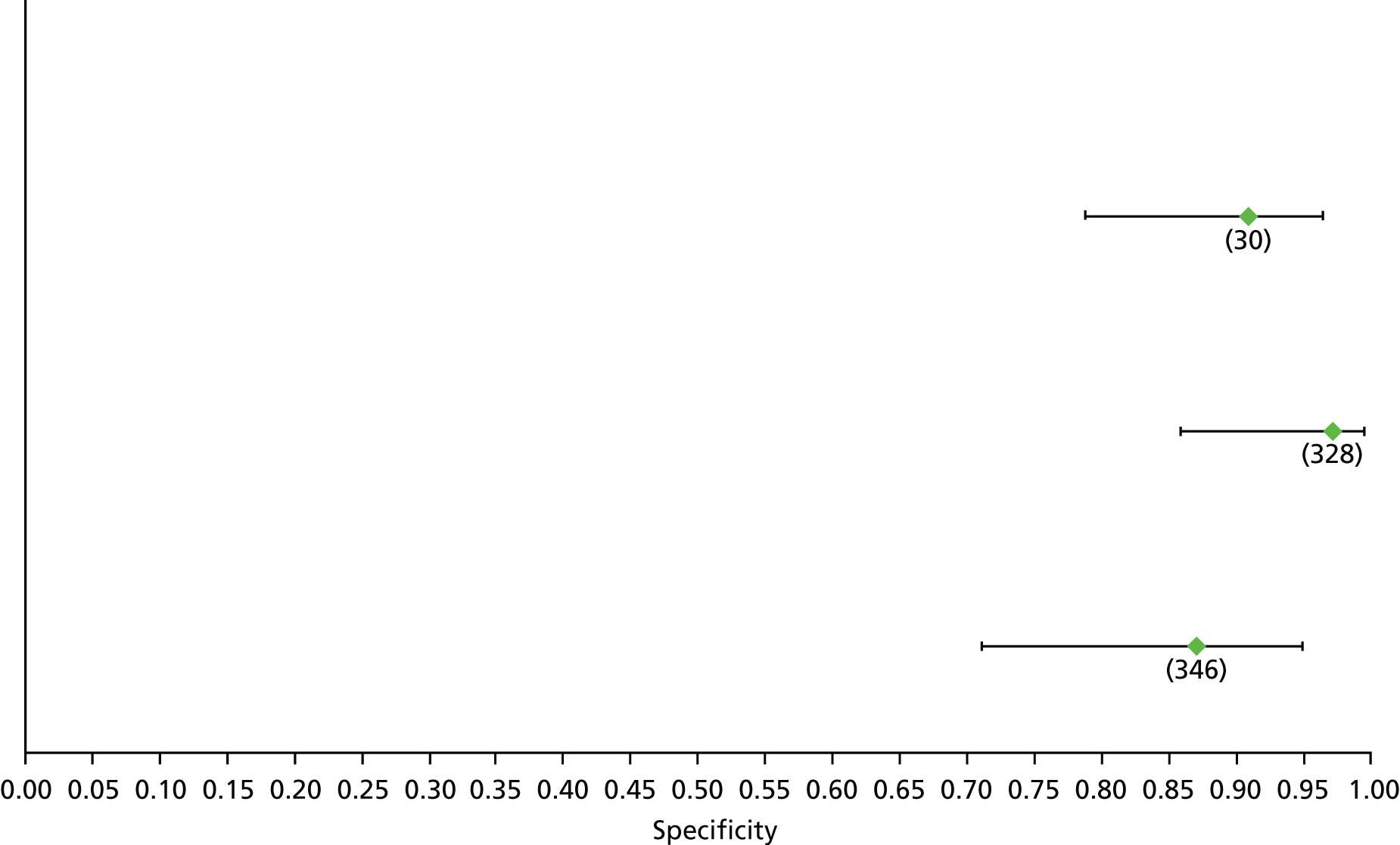
FIGURE 181.
Radiology: ultrasound F2 – sensitivity.

FIGURE 182.
Radiology: ultrasound F2 – specificity.

FIGURE 183.
Radiology: ultrasound SAPI F2 – sensitivity.
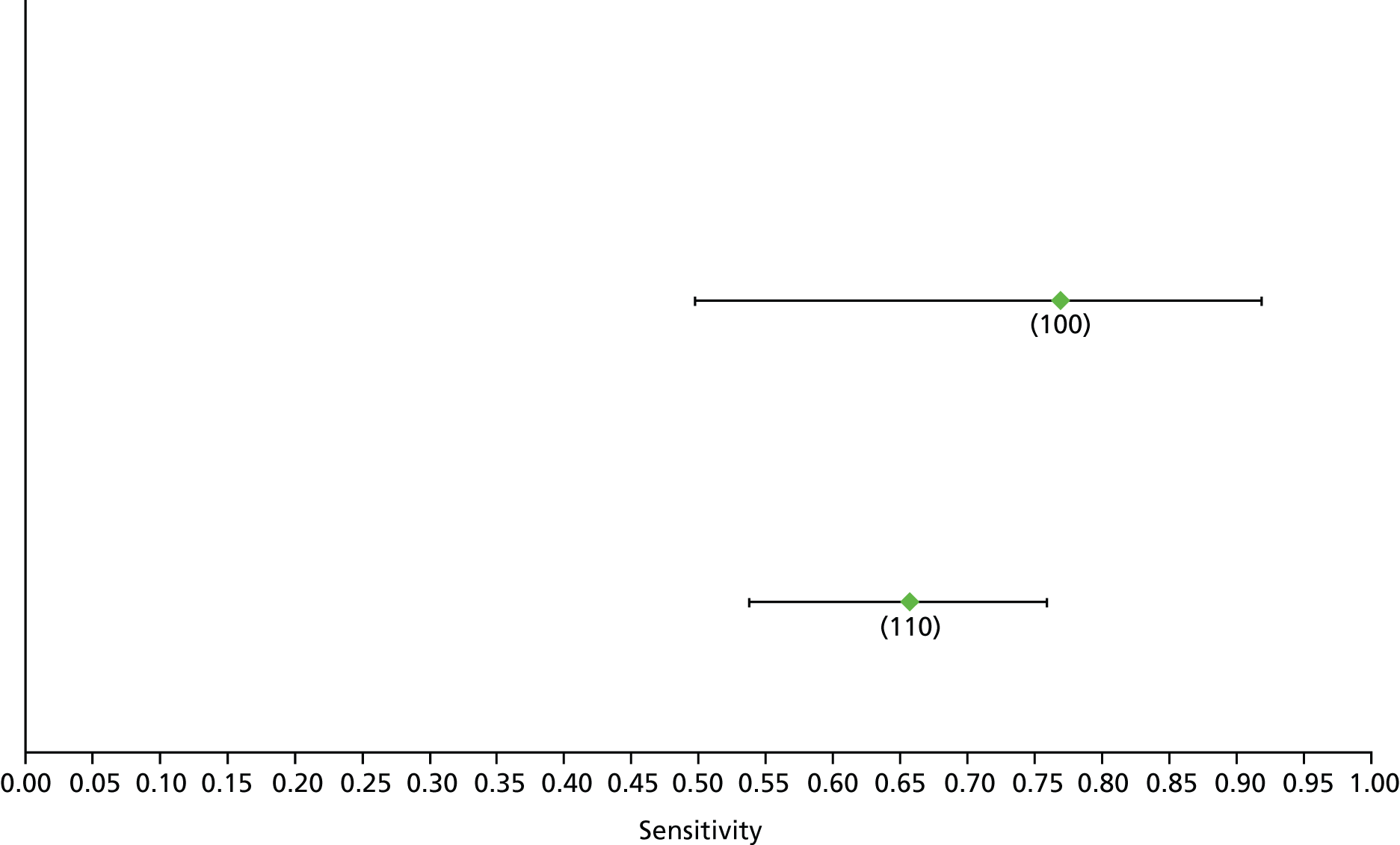
FIGURE 184.
Radiology: ultrasound SAPI F2 – specificity.

FIGURE 185.
Radiology: MR elastography F3 – sensitivity.

FIGURE 186.
Radiology: MR elastography F3 – specificity.

FIGURE 187.
Radiology: ultrasound F3 – sensitivity.

FIGURE 188.
Radiology: ultrasound F3 – specificity.

FIGURE 189.
Radiology: MR elastography F4 – sensitivity.
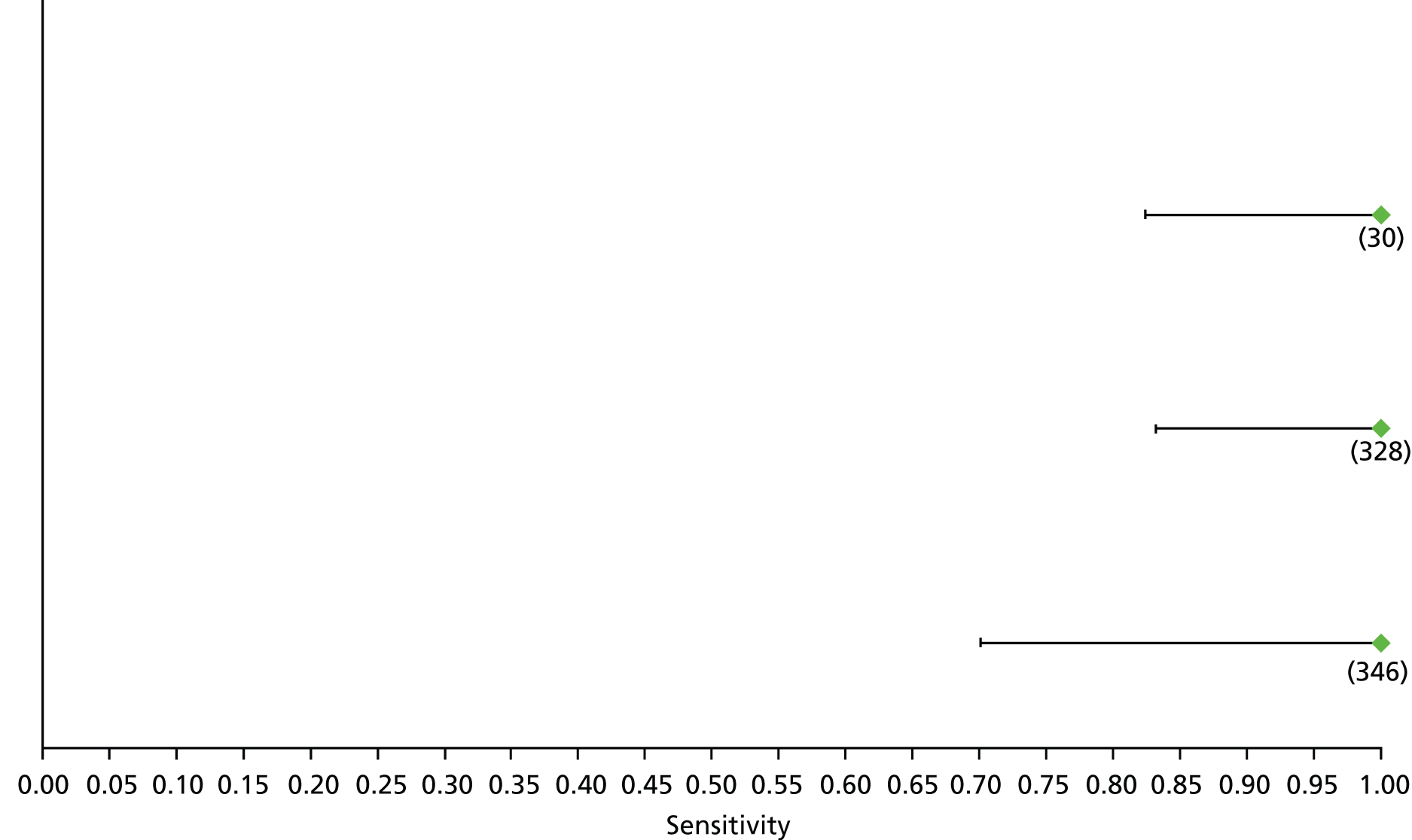
FIGURE 190.
Radiology: MR elastography F4 – specificity.

FIGURE 191.
Radiology: ultrasound F4 – sensitivity.
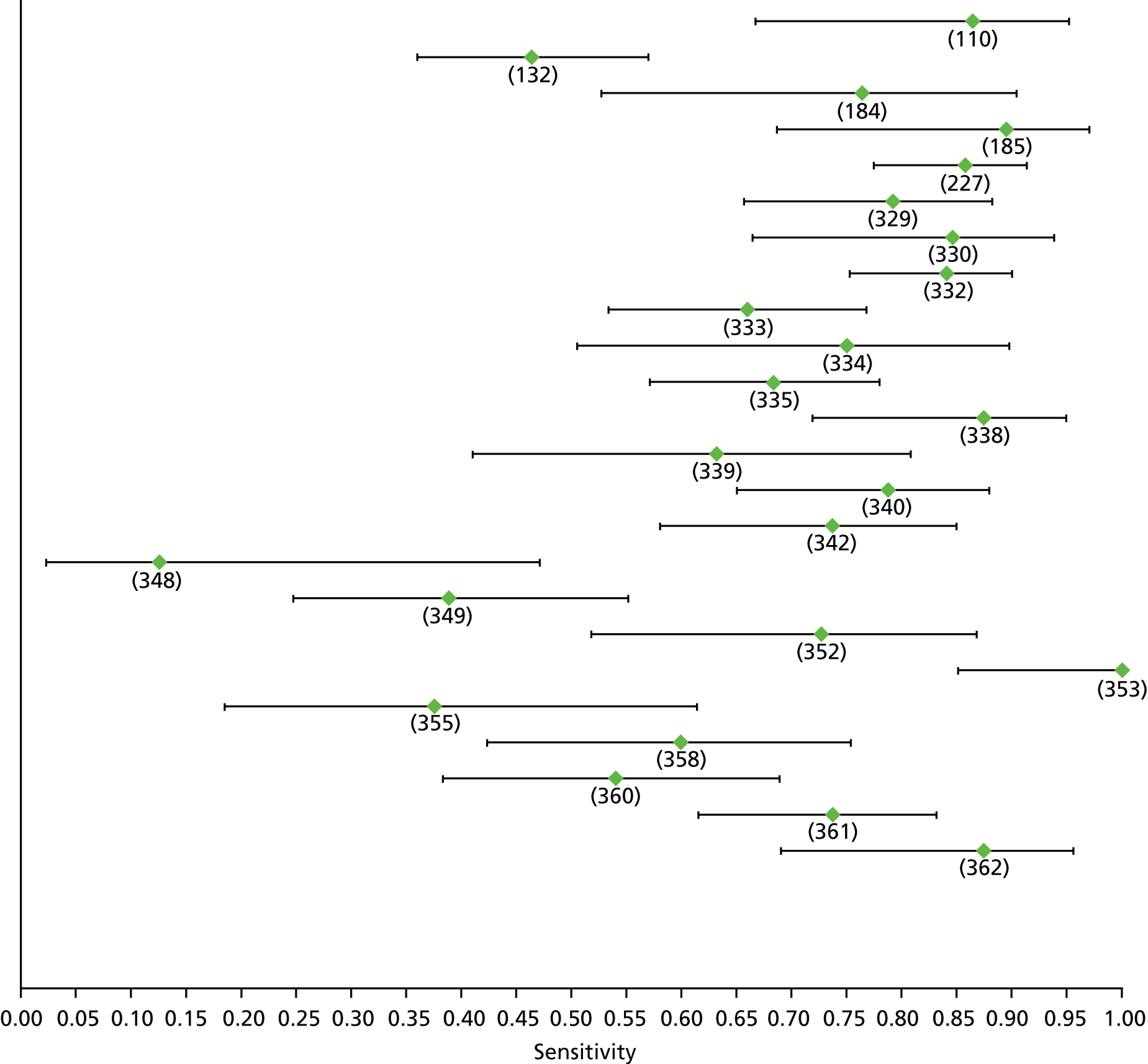
FIGURE 192.
Radiology: ultrasound F4 – specificity.
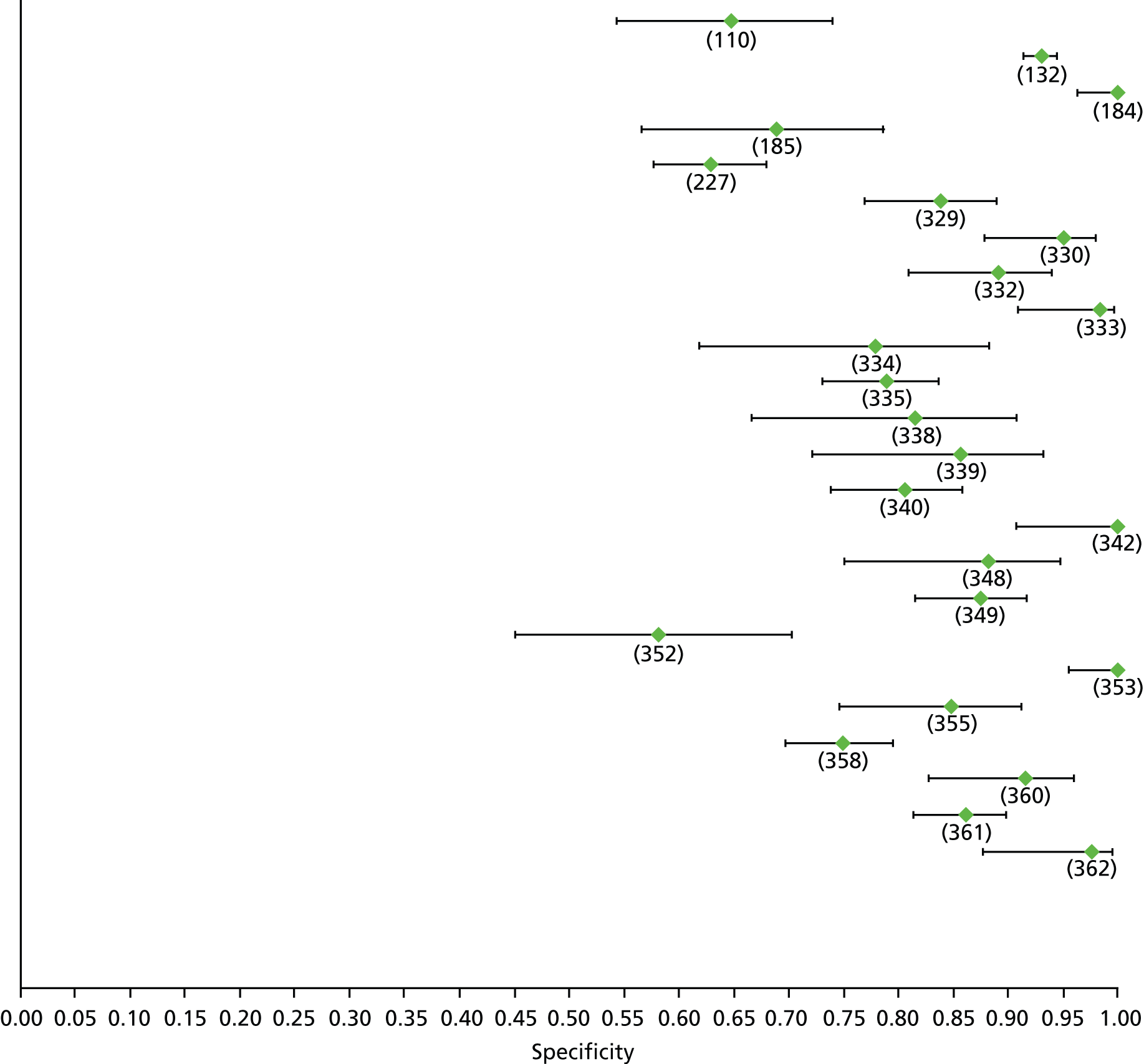
Appendix 6 Summary receiver operating characteristic curves
This appendix presents SROC curves of non-invasive tests across different disease aetiologies and fibrosis stages. We included only non-invasive tests that had available results from at least four studies with convergence by METADAS. The dot represents the relative sensitivity or specificity and the line represents the 95% CIs.
FIGURE 193.
APRI (high cut-off) in diagnosing ≥ F2 in patients with chronic HBV.

FIGURE 194.
APRI (low cut-off) in diagnosing ≥ F2 in patients with chronic HBV.
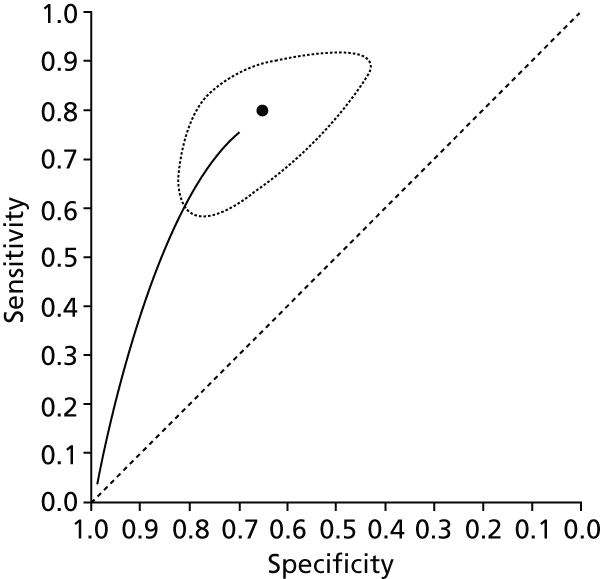
FIGURE 195.
FIB-4 (low cut-off) in diagnosing ≥ F2 in patients with chronic HBV.

FIGURE 196.
Fibrotest in diagnosing ≥ F2 in patients with chronic HBV.
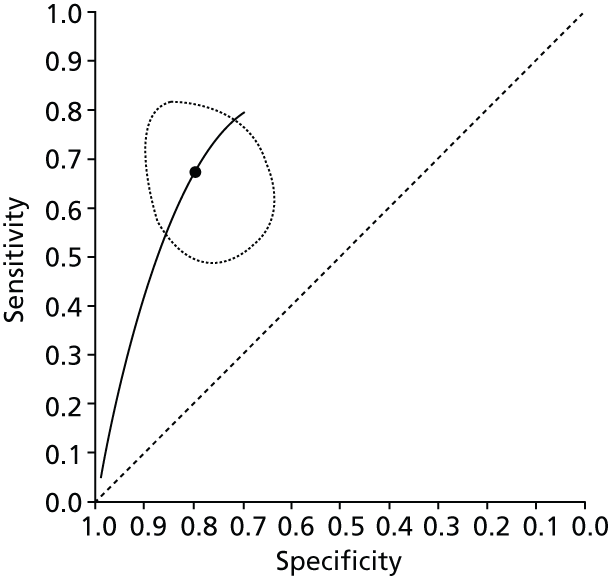
FIGURE 197.
Transient elastography (Fibroscan) in diagnosing ≥ F2 in patients with chronic HBV.

FIGURE 198.
Transient elastography (Fibroscan) in diagnosing ≥ F3 in patients with chronic HBV.

FIGURE 199.
Transient elastography (Fibroscan) in diagnosing F4 in patients with chronic HBV.
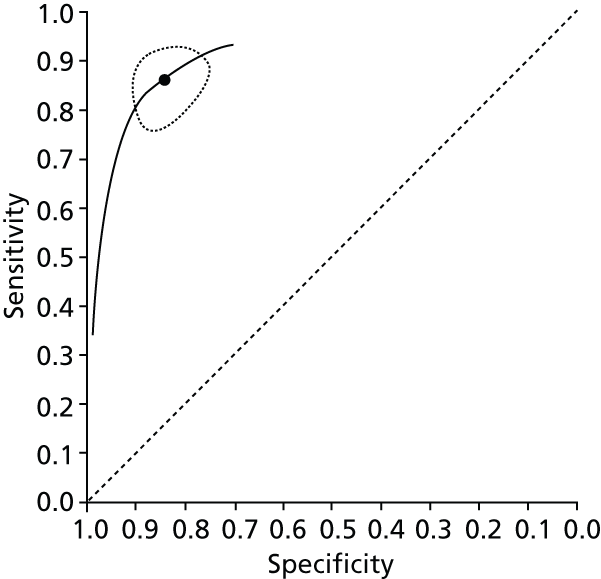
FIGURE 200.
APRI (high cut-off) in diagnosing ≥ F2 in patients with chronic HCV.

FIGURE 201.
APRI (low cut-off) in diagnosing ≥ F2 in patients with chronic HCV.

FIGURE 202.
FIB-4 (high cut-off) in diagnosing ≥ F2 in patients with chronic HCV.

FIGURE 203.
Lok’s index in diagnosing ≥ F2 in patients with chronic HCV.

FIGURE 204.
Forns index (high cut-off) diagnosing ≥ F2 in patients with chronic HCV.
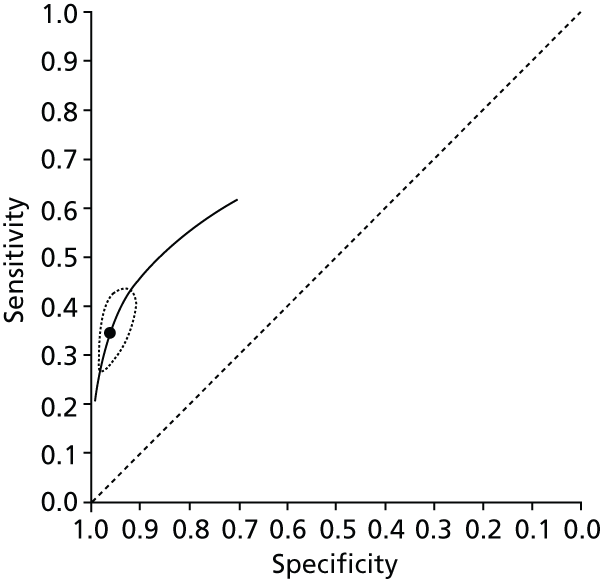
FIGURE 205.
Forns index (low cut-off) in diagnosing ≥ F2 in patients with chronic HCV.

FIGURE 206.
Fibrometer in diagnosing ≥ F2 in patients with chronic HCV.

FIGURE 207.
Fibrotest in diagnosing ≥ F2 in patients with chronic HCV.
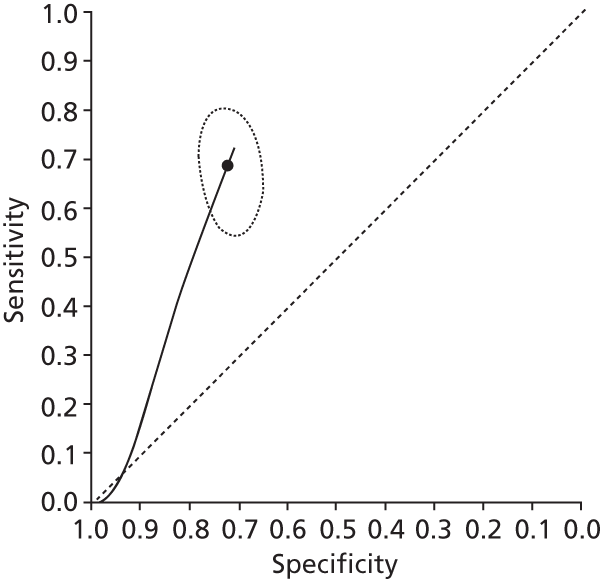
FIGURE 208.
Fibrotest (high cut-off) in diagnosing ≥ F2 in patients with chronic HCV.
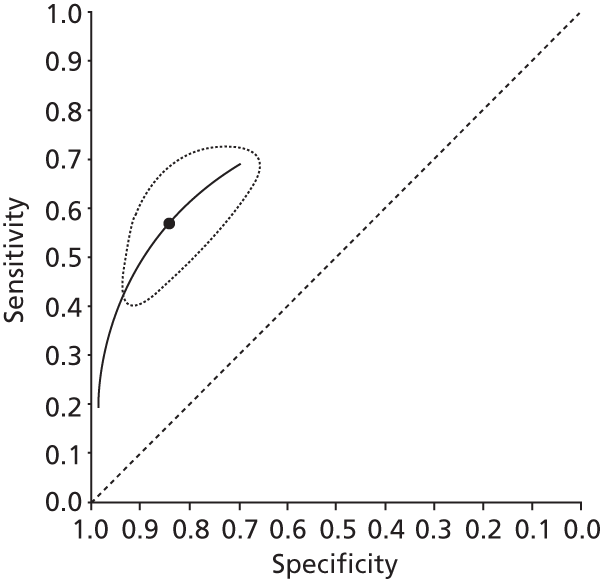
FIGURE 209.
Hyaluronic acid in diagnosing ≥ F2 in patients with chronic HCV.

FIGURE 210.
Hepascore in diagnosing ≥ F2 in patients with chronic HCV.
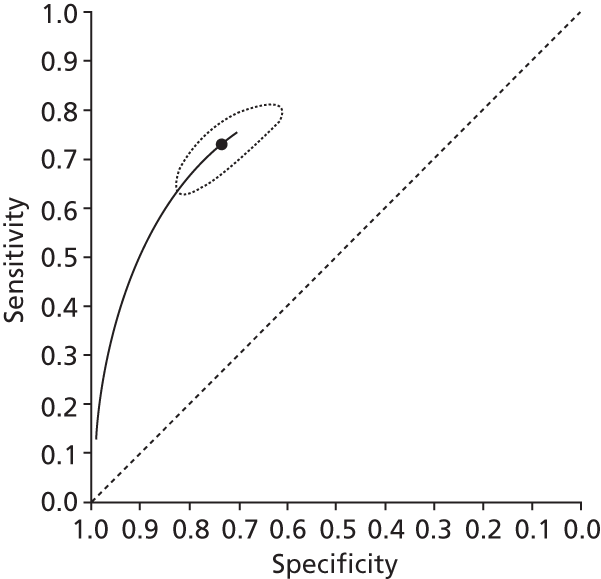
FIGURE 211.
Platelet count in diagnosing ≥ F2 in patients with chronic HCV.
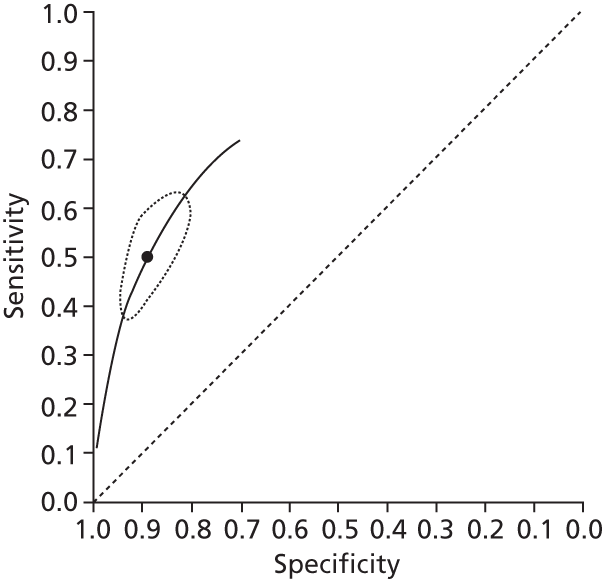
FIGURE 212.
Transient elastography (Fibroscan) in diagnosing ≥ F2 in patients with chronic HCV.

FIGURE 213.
Hepascore in diagnosing ≥ F3 in patients with chronic HCV.
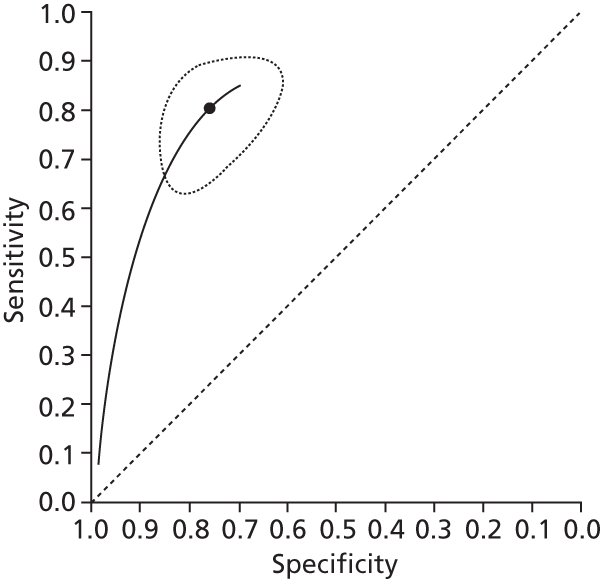
FIGURE 214.
FIB-4 (high cut-off) in diagnosing ≥ F3 in patients with chronic HCV.
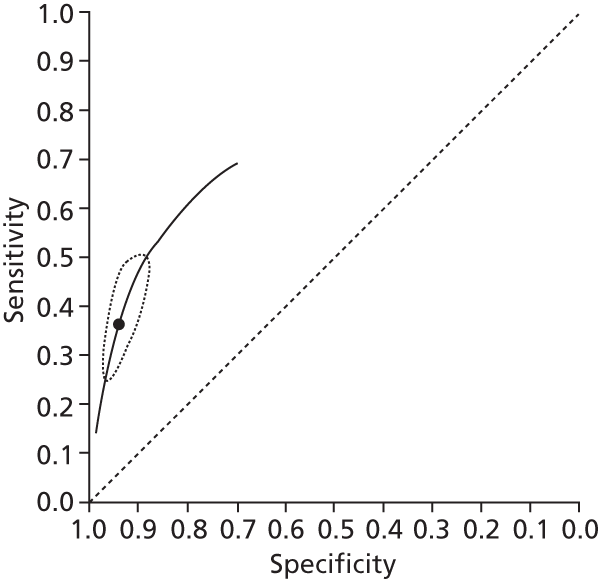
FIGURE 215.
FIB-4 (low cut-off) in diagnosing ≥ F3 in patients with chronic HCV.
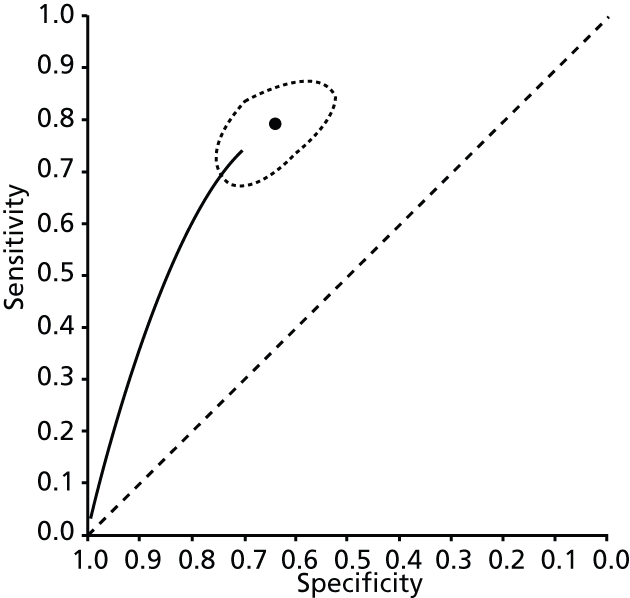
FIGURE 216.
APRI (high cut-off) in diagnosing ≥ F3 in patients with chronic HCV.

FIGURE 217.
Fibrotest in diagnosing ≥ F3 in patients with chronic HCV.

FIGURE 218.
Transient elastography (Fibroscan) in diagnosing ≥ F3 in patients with chronic HCV.
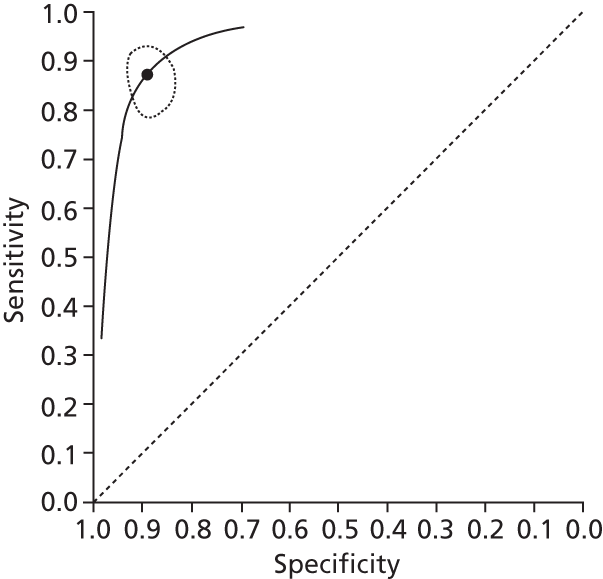
FIGURE 219.
APRI (high cut-off) in diagnosing F4 in patients with chronic HCV.
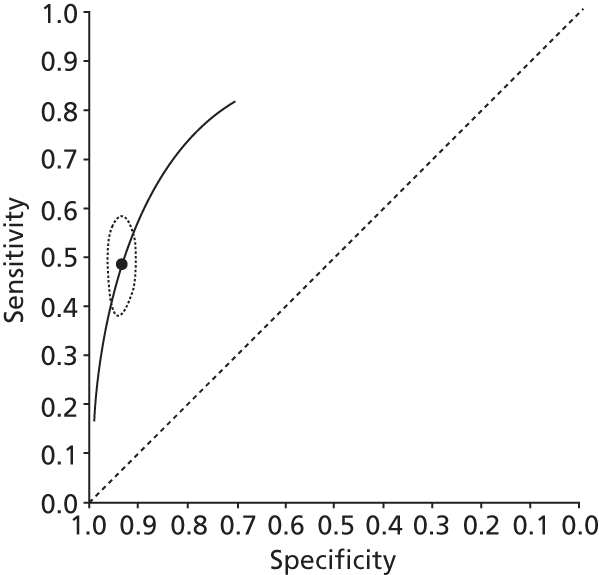
FIGURE 220.
APRI (low cut-off) in diagnosing F4 in patients with chronic HCV.
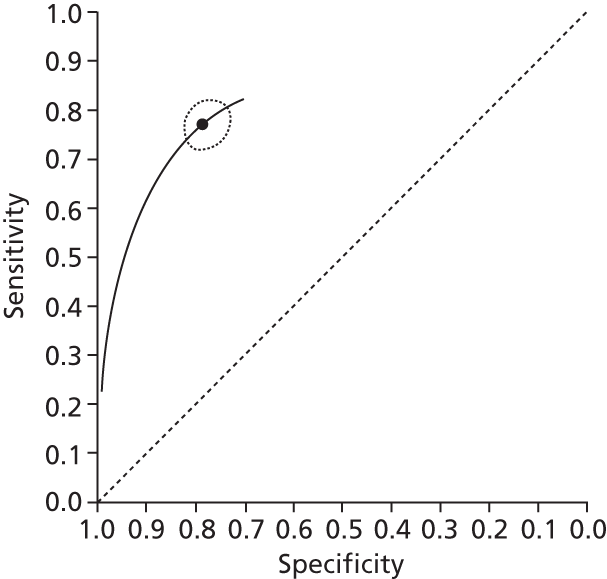
FIGURE 221.
AST–ALT ratio in diagnosing F4 in patients with chronic HCV.
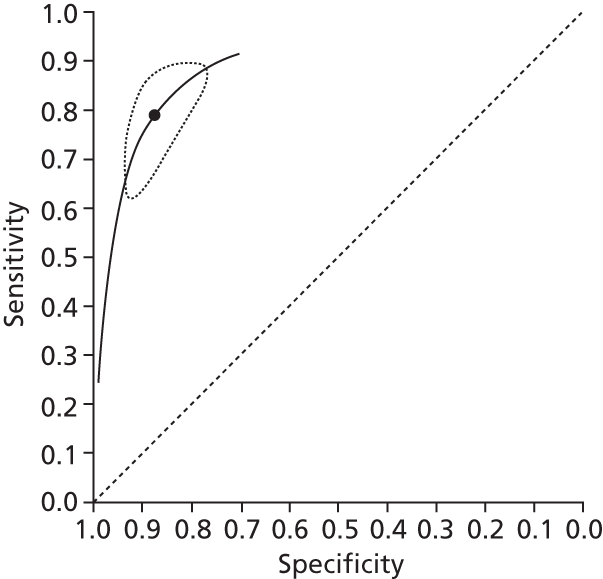
FIGURE 222.
Platelet count in diagnosing F4 in patients with chronic HCV.

FIGURE 223.
Hyaluronic acid in diagnosing F4 in patients with chronic HCV.
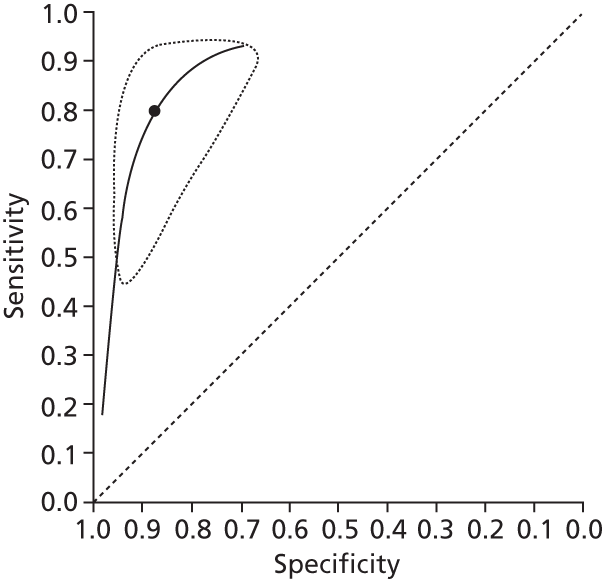
FIGURE 224.
Fibrotest in diagnosing F4 in patients with chronic HCV.
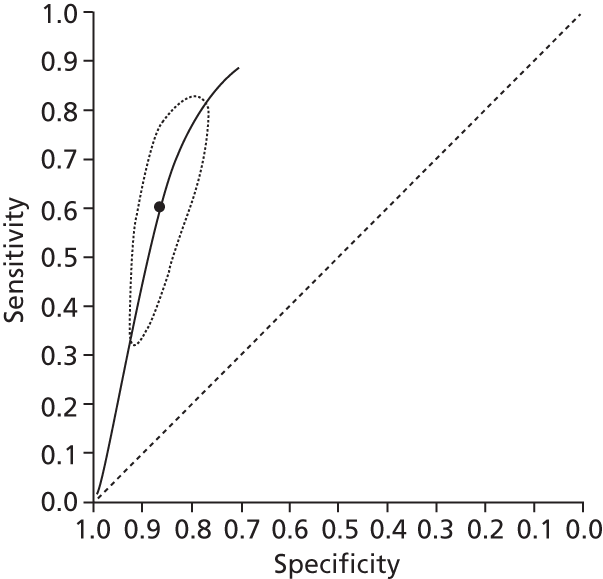
FIGURE 225.
Hepascore in diagnosing F4 in patients with chronic HCV.
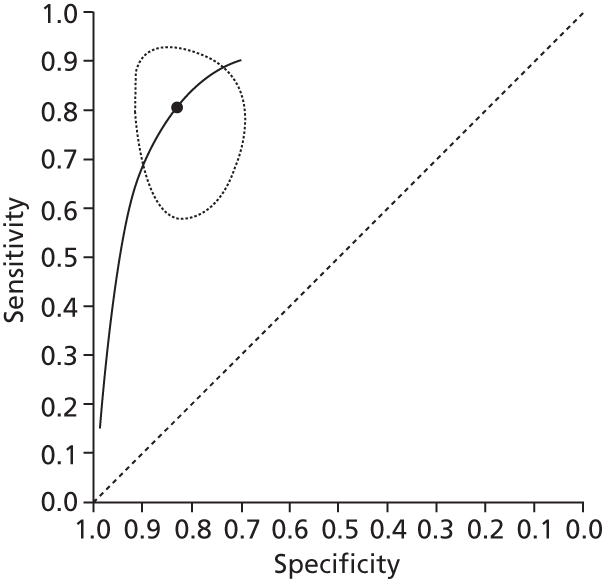
FIGURE 226.
Transient elastography (Fibroscan) in diagnosing F4 in patients with chronic HCV.

FIGURE 227.
Non-alcoholic fatty liver disease fibrosis score (low cut-off) in diagnosing ≥ F2 in patients with NASH.
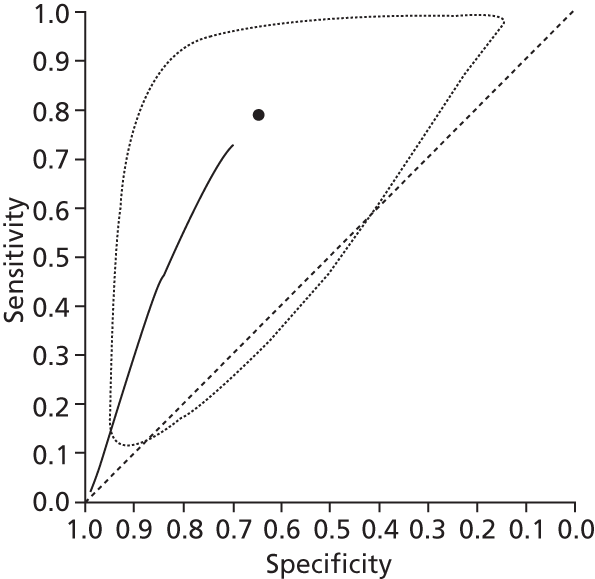
FIGURE 228.
Non-alcoholic fatty liver disease fibrosis score (high cut-off) in diagnosing ≥ F2 in patients with NASH.
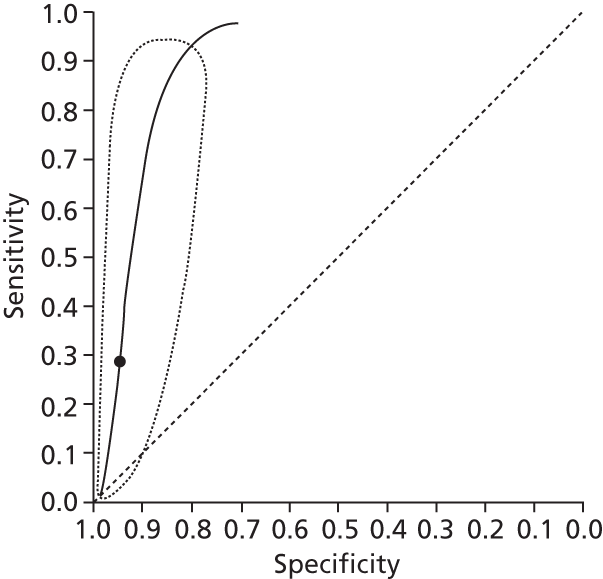
FIGURE 229.
Non-alcoholic fatty liver disease fibrosis score (low cut-off) in diagnosing ≥ F3 in patients with NASH.

FIGURE 230.
Non-alcoholic fatty liver disease fibrosis score (high cut-off) in diagnosing ≥ F3 in patients with NASH.

FIGURE 231.
BARD score in diagnosing ≥ F3 in patients with NASH.
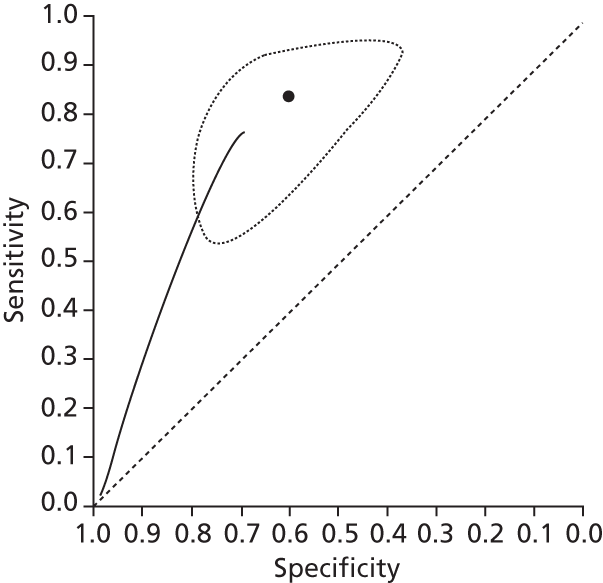
FIGURE 232.
Magnetic resonance elastography in diagnosing ≥ F3 in patients irrespective of liver disease aetiology.

FIGURE 233.
Ultrasound in diagnosing ≥ F3 in patients irrespective of liver disease aetiology.

FIGURE 234.
Ultrasound in diagnosing F4 in patients irrespective of liver disease aetiology.
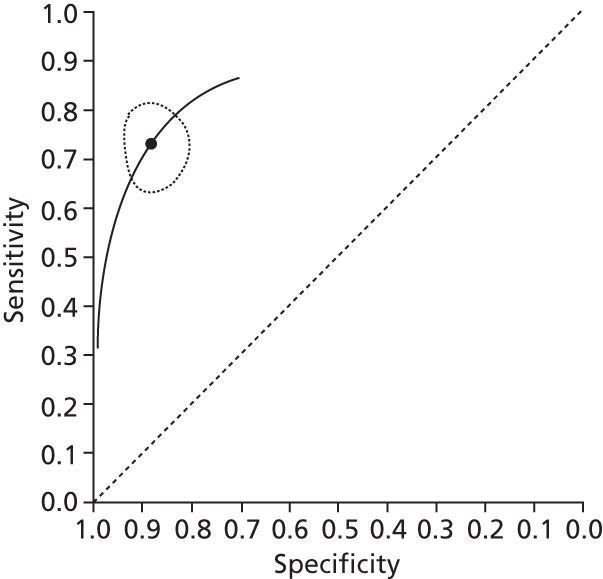
Appendix 7 Probability of non-invasive liver tests returning true-positive, false-negative, true-negative or false-positive results
| Test | TP, % | FN, % | TN, % | FP, % | Summary sensitivity | Summary specificity |
|---|---|---|---|---|---|---|
| Age–Platelet Index | 33 | 20 | 38 | 10 | 0.58 | 0.70 |
| AST–ALT | 23 | 29 | 33 | 14 | 0.44 | 0.71 |
| APRI | 40 | 12 | 38 | 9 | 0.77 | 0.81 |
| APRI (high cut-off) | 21 | 32 | 44 | 4 | 0.39 | 0.92 |
| APRI (low cut-off) | 43 | 10 | 27 | 20 | 0.82 | 0.57 |
| ARFI | 42 | 11 | 42 | 5 | 0.79 | 0.89 |
| CT | 37 | 16 | 30 | 17 | 0.70 | 0.64 |
| Bordeaux algorithm | 46 | 6 | 42 | 5 | 0.88 | 0.89 |
| CDS | 38 | 13 | 23 | 24 | 0.66 | 0.49 |
| ELF | 44 | 9 | 33 | 14 | 0.84 | 0.70 |
| ELF (high cut-off) | 25 | 28 | 43 | 5 | 0.47 | 0.90 |
| ELF (low cut-off) | 47 | 5 | 25 | 23 | 0.90 | 0.52 |
| EOB-MRI | 34 | 19 | 38 | 10 | 0.64 | 0.79 |
| FIB-4 | 18 | 35 | 41 | 6 | 0.34 | 0.86 |
| FIB-4 (high cut-off) | 31 | 22 | 35 | 12 | 0.59 | 0.74 |
| FIB-4 (low cut-off) | 47 | 6 | 20 | 28 | 0.89 | 0.42 |
| Fibrosis Index | 38 | 15 | 40 | 7 | 0.71 | 0.84 |
| Fibroindex (high cut-off) | 13 | 40 | 46 | 1 | 0.24 | 0.98 |
| Fibroindex (low cut-off) | 44 | 9 | 27 | 20 | 0.83 | 0.57 |
| Fibrometer | 42 | 11 | 34 | 14 | 0.79 | 0.73 |
| Fibropaca algorithm | 45 | 8 | 43 | 5 | 0.85 | 0.90 |
| FibroQ | 41 | 12 | 31 | 16 | 0.78 | 0.66 |
| FibroSpect | 41 | 11 | 33 | 14 | 0.78 | 0.71 |
| Forns index | 16 | 37 | 18 | 29 | 0.30 | 0.39 |
| Forns index (high cut-off) | 18 | 35 | 45 | 2 | 0.35 | 0.96 |
| Forns index (low cut-off) | 46 | 7 | 19 | 28 | 0.88 | 0.40 |
| Fibrosis Probability Index (high cut-off) | 22 | 30 | 45 | 2 | 0.42 | 0.95 |
| Fibrosis Probability Index (low cut-off) | 48 | 5 | 21 | 26 | 0.91 | 0.45 |
| Fibrotest | 36 | 17 | 34 | 13 | 0.68 | 0.72 |
| Fibrotest (high cut-off) | 30 | 23 | 14 | 7 | 0.57 | 0.85 |
| Fibrotest (low cut-off) | 48 | 5 | 20 | 28 | 0.91 | 0.41 |
| GUCI | 34 | 18 | 37 | 10 | 0.65 | 0.79 |
| Hyaluronic acid | 39 | 13 | 36 | 12 | 0.75 | 0.75 |
| Hyaluronic acid (high cut-off) | 13 | 41 | 43 | 4 | 0.23 | 0.92 |
| Hyaluronic acid (low cut-off) | 36 | 17 | 29 | 18 | 0.67 | 0.62 |
| Hepascore | 37 | 14 | 34 | 13 | 0.73 | 0.73 |
| Hepascore (high cut-off) | 17 | 35 | 44 | 4 | 0.33 | 0.92 |
| King’s | 44 | 8 | 33 | 14 | 0.84 | 0.70 |
| King’s (high cut-off) | 31 | 22 | 37 | 10 | 0.58 | 0.79 |
| King’s (low cut-off) | 33 | 20 | 38 | 9 | 0.62 | 0.81 |
| Lok’s index | 35 | 17 | 26 | 21 | 0.67 | 0.55 |
| MP3 | 43 | 9 | 35 | 13 | 0.85 | 0.73 |
| MR | 45 | 8 | 43 | 4 | 0.85 | 0.90 |
| PIINP/MMP-1 index | 38 | 18 | 40 | 7 | 0.65 | 0.85 |
| PIINP | 41 | 12 | 36 | 11 | 0.78 | 0.76 |
| PLT | 26 | 26 | 42 | 5 | 0.50 | 0.89 |
| PLT–Spleen | 46 | 6 | 5 | 13 | 0.88 | 0.73 |
| Pohl Index | 3 | 50 | 47 | 1 | 0.06 | 0.99 |
| Fibroscan | 42 | 11 | 39 | 8 | 0.79 | 0.83 |
| Type IV collagen | 46 | 6 | 35 | 13 | 0.88 | 0.73 |
| YKL-40 (high cut-off) | 18 | 35 | 38 | 9 | 0.33 | 0.80 |
| YKL-40 (low cut-off) | 42 | 11 | 16 | 32 | 0.80 | 0.33 |
| US | 18 | 34 | 40 | 7 | 0.35 | 0.86 |
| US SAPI | 39 | 14 | 37 | 10 | 0.74 | 0.79 |
| US SAPI (high cut-off) | 32 | 20 | 45 | 2 | 0.61 | 0.96 |
| US SAPI (low cut-off) | 50 | 3 | 19 | 29 | 0.94 | 0.39 |
| CEUS | 46 | 6 | 35 | 13 | 0.88 | 0.73 |
| DW-MRI | 41 | 11 | 37 | 10 | 0.78 | 0.78 |
| MR elastography | 50 | 3 | 43 | 4 | 0.94 | 0.92 |
| APRI (combined cut-off) | 40 | 13 | 41 | 7 | 0.75 | 0.86 |
| ELF (combined cut-off) | 43 | 9 | 40 | 8 | 0.82 | 0.84 |
| FIB-4 (combined cut-off) | 44 | 9 | 35 | 14 | 0.83 | 0.73 |
| Fibroindex (combined cut-off) | 30 | 22 | 45 | 2 | 0.58 | 0.95 |
| Fibrospect (combined cut-off) | 53 | 0 | 47 | 0 | 1.00 | 1.00 |
| Forns (combined cut-off) | 39 | 14 | 43 | 4 | 0.74 | 0.91 |
| Fibrotest (combined cut-off) | 47 | 7 | 35 | 12 | 0.87 | 0.74 |
| Hyaluronic acid (combined cut-off) | 34 | 19 | 34 | 13 | 0.64 | 0.72 |
| Hepascore (combined cut-off) | 22 | 31 | 43 | 4 | 0.42 | 0.91 |
| YKL-40 (combined cut-off) | 33 | 20 | 29 | 18 | 0.63 | 0.62 |
| Leroy algorithm | 47 | 5 | 46 | 1 | 0.90 | 0.98 |
| SAFE algorithm | 53 | 0 | 38 | 9 | 1.00 | 0.81 |
| Test | TP, % | FN, % | TN, % | FP, % | Summary sensitivity | Summary specificity |
|---|---|---|---|---|---|---|
| AAR | 31 | 23 | 27 | 19 | 0.57 | 0.59 |
| APGA | 9 | 45 | 8 | 38 | 0.17 | 0.98 |
| Age-Platelet Index | 4 | 51 | 28 | 17 | 0.07 | 0.62 |
| APRI (combined cut-off) | 39 | 15 | 41 | 4 | 0.73 | 0.91 |
| APRI (high cut-off) | 20 | 34 | 43 | 3 | 0.37 | 0.93 |
| APRI (low cut-off) | 43 | 11 | 30 | 16 | 0.80 | 0.65 |
| ARFI | 38 | 16 | 31 | 15 | 0.71 | 0.67 |
| FIB-4 (combined cut-off) | 9 | 46 | 45 | 1 | 0.16 | 0.98 |
| FIB-4 (high cut-off) | 5 | 49 | 45 | 1 | 0.09 | 0.99 |
| FIB-4 (low cut-off) | 37 | 17 | 33 | 12 | 0.68 | 0.73 |
| Fibrotest | 36 | 18 | 37 | 9 | 0.66 | 0.80 |
| Forns (combined cut-off) | 14 | 40 | 46 | 0 | 0.26 | 1.00 |
| Forns index (high cut-off) | 8 | 46 | 46 | 0 | 0.15 | 1.00 |
| Forns index (low cut-off) | 31 | 23 | 35 | 10 | 0.58 | 0.77 |
| GUCI | 36 | 18 | 44 | 1 | 0.67 | 0.97 |
| Hyaluronic acid | 46 | 9 | 38 | 8 | 0.84 | 0.83 |
| Hepascore | 43 | 12 | 34 | 12 | 0.79 | 0.74 |
| Hui index | 27 | 27 | 42 | 4 | 0.50 | 0.91 |
| PAPAS | 39 | 15 | 36 | 10 | 0.73 | 0.78 |
| Fibroscan | 38 | 16 | 38 | 7 | 0.71 | 0.84 |
| US | 19 | 35 | 39 | 7 | 0.35 | 0.86 |
| CEUS | 48 | 6 | 33 | 12 | 0.88 | 0.73 |
| DW-MRI | 42 | 12 | 36 | 10 | 0.78 | 0.78 |
| MR elastography | 51 | 3 | 42 | 4 | 0.94 | 0.92 |
| US SAPI | 40 | 14 | 36 | 10 | 0.74 | 0.79 |
| US SAPI (high cut-off) | 33 | 21 | 44 | 2 | 0.61 | 0.96 |
| US SAPI (low cut-off) | 51 | 3 | 18 | 28 | 0.94 | 0.39 |
| CT | 38 | 16 | 29 | 17 | 0.70 | 0.64 |
| Test | TP, % | FN, % | TN, % | FP, % | Summary sensitivity | Summary specificity |
|---|---|---|---|---|---|---|
| APRI (high cut-off) | 15 | 24 | 22 | 39 | 0.40 | 0.62 |
| Fibrotest (high cut-off) | 33 | 8 | 3 | 55 | 0.91 | 0.87 |
| Fibrotest (low cut-off) | 36 | 2 | 0 | 32 | 1.00 | 0.50 |
| 29 | 7 | 8 | 56 | 0.78 | 0.89 | |
| Fibroscan | 32 | 11 | 5 | 53 | 0.86 | 0.83 |
| Test | TP, % | FN, % | TN, % | FP, % | Summary sensitivity | Summary specificity |
|---|---|---|---|---|---|---|
| Age–Platelet Index | 14 | 7 | 61 | 17 | 0.66 | 0.78 |
| APRI | 9 | 13 | 65 | 14 | 0.40 | 0.82 |
| ARFI | 20 | 2 | 70 | 8 | 0.90 | 0.90 |
| AST–ALT (high cut off) | 10 | 12 | 71 | 7 | 0.46 | 0.91 |
| AST–ALT (low cut-off) | 17 | 5 | 55 | 23 | 0.79 | 0.70 |
| Bard | 18 | 3 | 48 | 31 | 0.84 | 0.61 |
| Type IV collagen | 17 | 4 | 63 | 16 | 0.79 | 0.80 |
| ELF | 17 | 4 | 70 | 8 | 0.80 | 0.90 |
| FIB-4 (high cut-off) | 8 | 13 | 76 | 3 | 0.98 | 0.97 |
| FIB-4 (low cut-off) | 18 | 3 | 58 | 20 | 0.84 | 0.74 |
| Fibrotest (high cut-off) | 9 | 13 | 75 | 3 | 0.40 | 0.96 |
| Fibrotest (low cut-off) | 19 | 3 | 57 | 21 | 0.88 | 0.73 |
| Fibrotest: Fibroscan | 9 | 13 | 75 | 3 | 0.39 | 0.96 |
| Hyaluronic acid | 19 | 3 | 64 | 13 | 0.88 | 0.82 |
| Hepascore | 16 | 5 | 66 | 12 | 0.75 | 0.84 |
| NAFIC (high cut-off) | 18 | 4 | 64 | 14 | 0.84 | 0.82 |
| NAFIC (low cut-off) | 21 | 1 | 53 | 26 | 0.96 | 0.67 |
| NDP: advanced fibrosis | 19 | 3 | 55 | 24 | 0.88 | 0.70 |
| NFS ELF (high cut-off) | 19 | 3 | 78 | 1 | 0.86 | 0.99 |
| NFS ELF (low cut-off) | 14 | 7 | 75 | 3 | 0.91 | 0.96 |
| NFS (high cut-off) | 9 | 13 | 76 | 2 | 0.40 | 0.97 |
| NFS (low cut-off) | 17 | 4 | 52 | 27 | 0.80 | 0.66 |
| NFS Fibroscan | 2 | 20 | 77 | 1 | 0.08 | 0.98 |
| PLT | 14 | 8 | 60 | 19 | 0.63 | 0.76 |
| Fibroscan (TE) | 18 | 4 | 66 | 13 | 0.82 | 0.84 |
| MR elastography | 20 | 2 | 69 | 9 | 0.91 | 0.88 |
| FIB-4 combined cut-off (inconclusive results retested with Fibroscan) | 17 | 5 | 73 | 5 | 0.79 | 0.93 |
| NFS (combined cut-off) (inconclusive results retested with Fibroscan) | 15 | 6 | 75 | 3 | 0.71 | 0.96 |
| NAFIC (combined cut-off) (inconclusive results retested with Fibroscan) | 21 | 1 | 62 | 16 | 0.95 | 0.79 |
| NFS ELF (combined cut-off) (inconclusive results retested with Fibroscan) | 20 | 2 | 78 | 1 | 0.90 | 0.99 |
| Fibrotest (combined cut-off) (inconclusive results retested with Fibroscan) | 18 | 4 | 76 | 3 | 0.83 | 0.87 |
| Test | TP, % | FP, % | FN, % | TN, % | Summary sensitivity | Summary specificity |
|---|---|---|---|---|---|---|
| Age–Platelet Index | 18 | 22 | 2 | 59 | 0.88 | 0.73 |
| APRI | 16 | 24 | 4 | 56 | 0.79 | 0.70 |
| APRI (combined cut-off) | 13 | 7 | 7 | 73 | 0.64 | 0.91 |
| APRI (high cut-off) | 9 | 6 | 11 | 74 | 0.45 | 0.93 |
| APRI (low cut-off) | 15 | 17 | 5 | 63 | 0.75 | 0.78 |
| ARFI | 17 | 18 | 3 | 62 | 0.84 | 0.87 |
| AST–ALT ratio | 10 | 10 | 10 | 70 | 0.49 | 0.87 |
| BARD | 10 | 13 | 10 | 67 | 0.52 | 0.84 |
| Bordeaux | 17 | 4 | 3 | 76 | 0.87 | 0.95 |
| CDS | 18 | 26 | 2 | 54 | 0.88 | 0.67 |
| CDS (high cut-off) | 7 | 1 | 13 | 79 | 0.33 | 1.00 |
| CDS (low cut-off) | 18 | 8 | 2 | 72 | 0.89 | 0.90 |
| ELF | 19 | 17 | 1 | 63 | 0.93 | 0.79 |
| ELF (combined cut-off) | 17 | 13 | 3 | 67 | 0.84 | 0.84 |
| ELF (high cut-off) | 10 | 8 | 10 | 72 | 0.52 | 0.90 |
| ELF (low cut-off) | 18 | 38 | 2 | 42 | 0.90 | 0.53 |
| Fibrosis Index (FI) | 8 | 1 | 12 | 79 | 0.38 | 1.00 |
| FIB-4 | 16 | 18 | 4 | 62 | 0.80 | 0.78 |
| FIB-4 (combined cut-off) | 15 | 6 | 5 | 74 | 0.75 | 0.93 |
| FIB-4 (high cut-off) | 8 | 6 | 12 | 74 | 0.42 | 0.92 |
| FIB-4 (low cut-off) | 17 | 23 | 3 | 57 | 0.84 | 0.71 |
| Fibroindex | 14 | 7 | 6 | 73 | 0.70 | 0.91 |
| Fibrometer | 14 | 10 | 6 | 70 | 0.72 | 0.88 |
| Fibrometer (combined cut-off) | 18 | 2 | 2 | 78 | 0.89 | 0.97 |
| Fibrometer (high cut-off) | 7 | 2 | 13 | 78 | 0.39 | 0.98 |
| Fibrometer (low cut-off) | 19 | 23 | 1 | 57 | 0.96 | 0.71 |
| Fibropaca | 15 | 2 | 5 | 78 | 0.73 | 0.97 |
| Fontana | 16 | 27 | 4 | 53 | 0.79 | 0.66 |
| Forns index | 20 | 21 | 0 | 59 | 1.00 | 0.74 |
| Forns index (combined cut-off) | 19 | 20 | 1 | 60 | 0.97 | 0.75 |
| Forns index (high cut-off) | 13 | 7 | 7 | 73 | 0.67 | 0.91 |
| Forns index (low cut-off) | 18 | 50 | 2 | 30 | 0.88 | 0.37 |
| Fibrotest | 12 | 11 | 8 | 70 | 0.61 | 0.87 |
| Fibrotest (combined cut-off) | 14 | 4 | 6 | 76 | 0.70 | 0.95 |
| Fibrotest (high cut-off) | 15 | 5 | 5 | 75 | 0.73 | 0.94 |
| Fibrotest (low cut-off) | 18 | 28 | 2 | 52 | 0.89 | 0.65 |
| GUCI | 13 | 11 | 7 | 69 | 0.64 | 0.86 |
| Hyaluronic acid | 16 | 9 | 4 | 71 | 0.81 | 0.88 |
| Hepascore | 16 | 13 | 4 | 67 | 0.82 | 0.84 |
| Hepascore (combined cut-off) | 13 | 1 | 7 | 79 | 0.66 | 0.99 |
| Hepascore (high cut-off) | 8 | 1 | 12 | 79 | 0.39 | 0.99 |
| Hepascore (low cut-off) | 16 | 14 | 4 | 66 | 0.80 | 0.83 |
| King’s | 15 | 8 | 5 | 72 | 0.74 | 0.90 |
| Lok’s index (high cut-off) | 8 | 4 | 12 | 76 | 0.40 | 0.95 |
| Lok’s index (low cut-off) | 17 | 27 | 3 | 53 | 0.84 | 0.66 |
| MR | 15 | 22 | 5 | 58 | 0.75 | 0.72 |
| PGAA | 16 | 9 | 4 | 71 | 0.78 | 0.89 |
| PIIINP | 14 | 17 | 6 | 63 | 0.70 | 0.79 |
| PLT | 14 | 10 | 6 | 70 | 0.72 | 0.88 |
| PLT–Spleen | 17 | 14 | 3 | 66 | 0.85 | 0.82 |
| SAFE | 15 | 6 | 5 | 74 | 0.74 | 0.93 |
| Fibroscan | 18 | 9 | 2 | 71 | 0.89 | 0.89 |
| Type IV collagen | 14 | 20 | 6 | 61 | 0.71 | 0.76 |
| CEUS | 17 | 10 | 3 | 70 | 0.84 | 0.88 |
| DW-MRI | 18 | 22 | 2 | 58 | 0.88 | 0.73 |
| MR elastography | 20 | 5 | 0 | 75 | 1.00 | 0.93 |
| MRI | 15 | 16 | 5 | 64 | 0.75 | 0.80 |
| US | 15 | 9 | 5 | 70 | 0.73 | 0.88 |
| US SAPI | 15 | 26 | 6 | 54 | 0.73 | 0.67 |
Appendix 8 Probabilistic sensitivity analysis parameters
| Parameter | Base-case value | Standard error | Distribution |
|---|---|---|---|
| HBV health-state costs (£ 2012) | |||
| Mild fibrosis | 185 | 36 | Gamma |
| Moderate fibrosis | 959 | 102 | |
| Compensated cirrhosis | 1521 | 309 | |
| Decompensated cirrhosis | 36,194 | 9967 | |
| HCC | 36,194 | 9967.190 | |
| Liver transplant | 64,122 | 5886 | |
| Post liver transplant | 16,321 | 7933 | |
| HBV utilities | |||
| Mild fibrosis | 0.77 | 0.035 | Gamma |
| Moderate fibrosis | 0.66 | 0.018 | |
| Compensated cirrhosis | 0.55 | 0.032 | |
| Decompensated cirrhosis | 0.57 | 0.076 | |
| HCC | 0.57 | 0.076 | |
| Liver transplant | 0.73 | 0.016 | |
| Post liver transplant | 0.78 | 0.064 | |
| HCV health-state costs (£ 2012) | |||
| Mild fibrosis | 185 | 36 | Gamma |
| Moderate fibrosis | 959 | 102 | |
| Compensated cirrhosis | 1521 | 309 | |
| Decompensated cirrhosis | 38,871 | 9410 | |
| HCC | 38,871 | 9410 | |
| Liver transplant | 69,174 | 7055 | |
| Post liver transplant | 4356 | 862 | |
| HCV utilities | |||
| Mild fibrosis | 0.77 | 0.035 | Beta |
| Moderate fibrosis | 0.66 | 0.018 | |
| Compensated cirrhosis | 0.55 | 0.032 | |
| Decompensated cirrhosis | 0.49 | 0.056 | |
| HCC | 0.49 | 0.056 | |
| Liver transplant | 0.51 | 0.053 | |
| Post liver transplant | 0.52 | 0.061 | |
| During treatment: mild fibrosis | 0.65 | 0.03 | |
| During treatment: moderate fibrosis | 0.55 | 0.018 | |
| During treatment: cirrhosis | 0.44 | 0.032 | |
| Following successful treatment: mild treatment | 0.82 | 0.04 | |
| Following successful treatment: moderate treatment | 0.71 | 0.05 | |
| Following successful treatment: compensated cirrhosis | 0.60 | 0.04 | |
| ALD | |||
| Probability cirrhosis if continue to drink | 20% | 0.02 | Beta |
| Abstinence rate after diagnosis of no cirrhosis with liver biopsy | 41% | 0.041 | |
| Abstinence rate after diagnosis of cirrhosis with liver biopsy | 62% | 0.0612 | |
| Abstinence rate after diagnosis of no cirrhosis with a NILT | 31% | 0.031 | |
| Abstinence rate after diagnosis of cirrhosis with a NILT | 52% | 0.052 | |
| Probability adverse event after liver biopsy | 0.72% | 0.00072 | |
| Mortality risk after liver biopsy | 0.09% | 0.00009 | |
| Cirrhosis | |||
| Reduction in mortality with HCC screening | 37% | 0.037 | Beta |
| HBV and HCV | |||
| Transition probabilities and all-cause mortality rate | Dirichlet | ||
| All aetiologies | |||
| Summary sensitivity and specificity estimates | Beta | ||
Appendix 9 Unit costs of non-invasive liver tests and liver biopsy
| Test | Unit cost, £ | Source |
|---|---|---|
| AAR | 0.90 | Royal Free, 12 December 2012, personal communication |
| AP (age–PLT ratio) (API?) | 3.50 | Royal Free, 12 December 2012, personal communication |
| APGA | 4.95 | Royal Free, 22 January 2013, personal communication |
| APRI | 4.05 | Royal Free, 12 December 2012, personal communication |
| ARFI | 51.00 | As per personal communication Royal Free: costed at same cost as Fibroscan |
| AST–ALT ratio (AAR) | 0.90 | Royal Free, 12 December 2012, personal communication |
| BARD | 0.90 | Royal Free, 29 May 2013, personal communication |
| Bordeaux | 94.60 | Costed as combination strategy (Fibrotest and Fibroscan) |
| CDS | 7.19 | Royal Free, 30 January 2013, personal communication |
| CEUS | 113.70 | Department of Health reference costs 2011–12427 (US > 20 minutes) plus contrast (SonoVue) cost: £48.70 (Royal Free personal communication) |
| CT | 105.00 | Department of Health reference costs 2011–12427 (CT with contrast pre and post scan): Diagnostic Imaging Outpatients |
| DW-MRI | 199.00 | Cost as per MRI with contrast (as per personal communication, Royal Free, 4 December 2012) |
| ELF | 108.00 | Wiktoria Jonasson, Royal Free, 9 May 2012, personal communication: cost of ELF is £90 + VAT |
| EOB-MRI | 199.00 | Cost as per MRI with contrast (as per Royal Free) |
| FIB-4 | 4.40 | Royal Free, 12 December 2012, personal communication |
| Fibroindex | 48.00 | Royal Free, 14 January 2013, personal communication |
| Fibrometer | 44.00 | Anne Laure Gilles, BioLiveScale, 22 May 2012, personal communication: quoted approximate price €50 (converted to UK cost using OECD indices) |
| Fibropaca-algorithm | 509.89 | Tests: APRI, Forns index, liver biopsy, Fibrotest: proportion calculated using Sebastiani et al.31 |
| FibroQ | 7.19 | Royal Free, 30 January 2013, personal communication |
| Fibroscan (TE) | 51.00 | Department of Health Reference Costs 2011–12:427 US < 20 minutes. From Diagnostic Imaging, Outpatients (DIAGIM-OP) code RA23Z. As per advice from Royal Free |
| Fibrosis Index (FI) | 4.40 | Royal Free, 12 December 2012, personal communication |
| Fibrospect | 35.34 | Royal Free, 14 January 2013, personal communication |
| Fibrotest | 43.60 | Jean Marie Castille, Directeur General (Biopredictive), 31 May 2012, personal communication: converted to GBP Sterling (OECD PPP and exchange rates data: rate 0.871929) |
| Fontana F4 | 31.50 | Royal Free, 24 July 2013, personal communication |
| Forns index | 4.26 | Royal Free, 22 January 2013, personal communication |
| FPI high | 8.58 | Royal Free, 22 January 2013, personal communication |
| FPI low | 8.58 | Royal Free, 22 January 2013, personal communication |
| Fibrotest Fibroscan | 94.60 | Costed as Fibrotest and Fibroscan |
| GUCI | 6.84 | Royal Free, 22 January 2013, personal communication |
| Hyaluronic acid | 8.00 | Royal Free, 22 January 2013, personal communication |
| Hepascore | 16.24 | Royal Free, 22 January 2013, personal communication |
| Hui index | 4.60 | Royal Free, 22 January 2013, personal communication |
| King’s | 6.84 | Royal Free, 22 January 2013, personal communication |
| Leroy algorithm | 724.74 | Tests: APRI, liver biopsy, Fibrotest: proportion calculated using Sebastiani et al.31 |
| Liver Biopsy | 956.61 | Stevenson et al.428 |
| Lok’s index (HALT-C) | 7.19 | Royal Free, 30 January 2013, personal communication |
| MP3 | 20.00 | Royal Free, 30 January 2013, personal communication |
| MR elastography | 199.00 | Department of Health Reference Costs 2011–12:427 Diagnostic Imaging Outpatients, MRI one area pre and post contrast (code RA23Z) |
| MRI | 199.00 | Cost as per MRI with contrast (as per Royal Free) |
| NAFIC | 28.17 | Royal Free, 29 May 2013, personal communication |
| NDP | 21.18 | Royal Free, 29 May 2013, personal communication |
| NFS high | 4.95 | Royal Free, 29 May 2013, personal communication |
| NFS ELF | 112.95 | Royal Free, 29 May 2013, personal communication |
| NFS TE | 55.95 | Sum of NFS and Fibroscan (TE) |
| PAPAS | 5.15 | Royal Free, 22 January 2013, personal communication |
| PGAA | 9.07 | Royal Free, 24 July 2013, personal communication |
| PIIINP/MMP-1 index | 48.00 | Royal Free, 14 January 2013, personal communication |
| PIINP | 28.00 | Royal Free, 12 December 2012, personal communication |
| PLT | 3.50 | Royal Free, 29 May 2013, personal communication |
| PLT–Spleen (SPRI) | 54.50 | Royal Free, 4 December 2012, personal communication |
| Pohl Index | 4.40 | Royal Free, 30 January 2013, personal communication |
| SAFE | 743.22 | Tests: APRI, liver biopsy, Fibrotest: proportion calculated using Sebastiani et al.31,187 |
| TE | 51.00 | Department of Health Reference Costs 2011–12:427 US < 20 minutes [from Diagnostic Imaging, Outpatients (DIAGIM-OP) code RA23Z as advised by Royal Free] |
| Type IV collagen | 20.00 | Royal Free, 30 January 2013, personal communication |
| US | 51.00 | Department of Health Reference Costs 2011–12:427 US < 20 minutes [from Diagnostic Imaging, Outpatients (DIAGIM-OP) code RA23Z] |
| US SAPI | 65.00 | Department of Health Reference Costs 2011–12:427 US > 20 minutes |
| YKL-40 | 20.00 | Royal Free, 8 February 2013, personal communication |
Appendix 10 Cost-effectiveness acceptability curves
FIGURE 235.
Cost-effectiveness acceptability curve for HBeAg-positive. HA, hyaluronic acid; MRE, MR elastography.
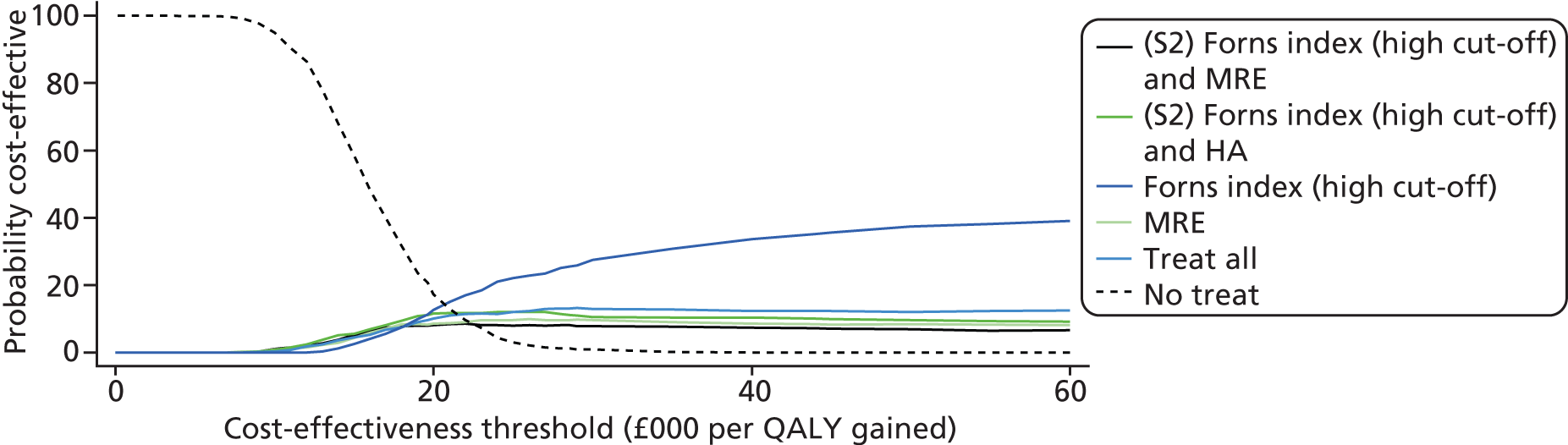
FIGURE 236.
Cost-effectiveness acceptability curve for HBeAg-negative. HA, hyaluronic acid; MRE, MR elastography.
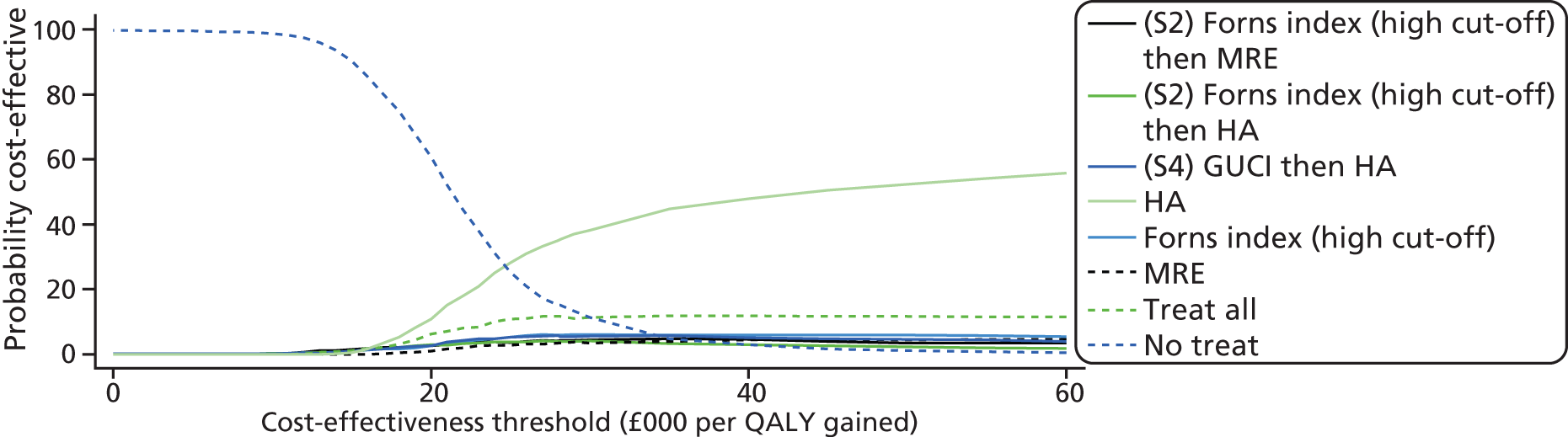
FIGURE 237.
Cost-effectiveness acceptability curve for HCV. FPI, Fibrosis Probability Index; US, ultrasound.
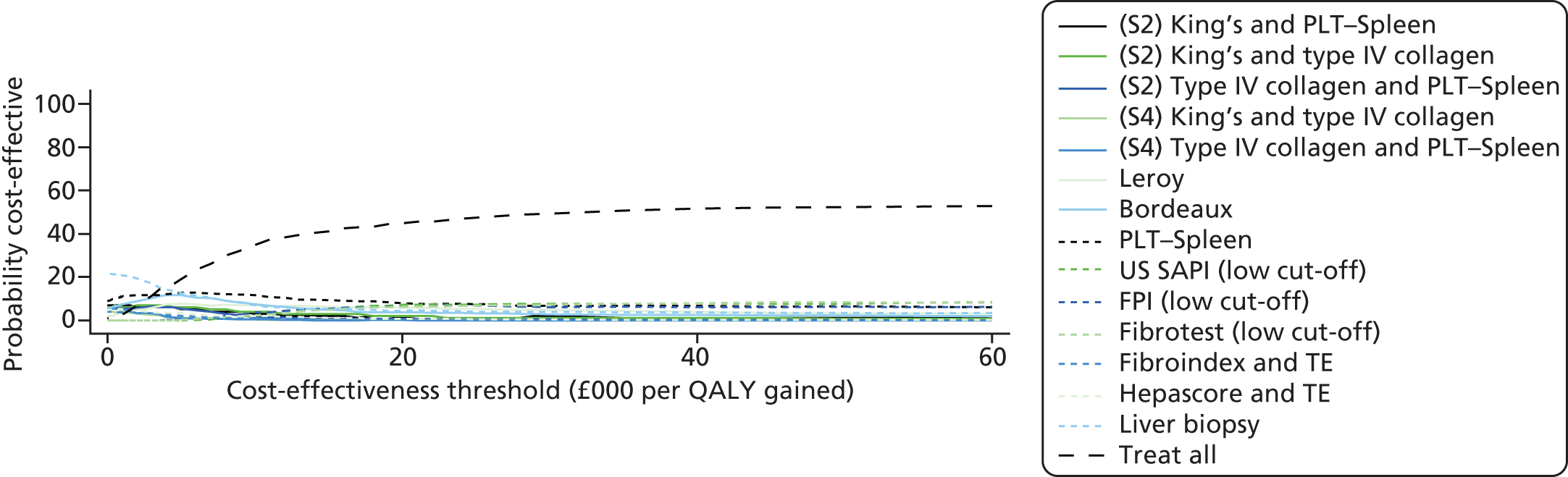
FIGURE 238.
Cost-effectiveness acceptability curve for ALD.
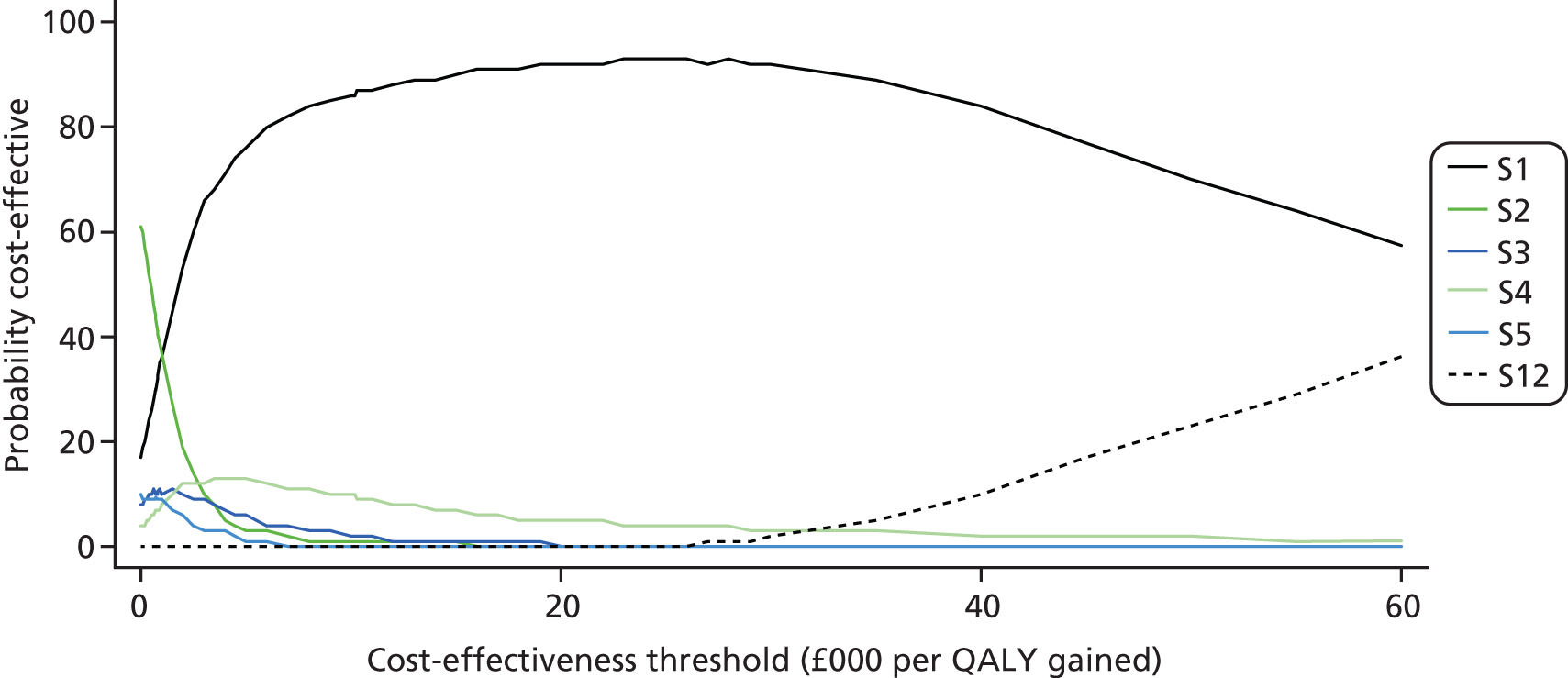
FIGURE 239.
Cost-effectiveness acceptability curve for cirrhosis. HA, hyaluronic acid.

Appendix 11 Sensitivity analysis
Results of sensitivity analyses: for clarity and presentation, all testing strategies which were ‘dominated’ or ‘extendedly dominated’ are excluded from the tables.
Sensitivity analyses tables for hepatitis B
HBeAg-positive
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Treat no one | 37,831 | 9.64 | – | – | – |
| APRI (high cut-off) | 75,210 | 11.45 | 37,380 | 1.81 | 20,673 |
| Fibroscan | 79,000 | 11.61 | 3790 | 0.16 | 23,345 |
| Treat all | 101,484 | 12.18 | 22,484 | 0.57 | 39,747 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Treat no one | 29,410 | 12.23 | – | – | – |
| GUCI | 57,054 | 13.73 | 27,644 | 1.50 | 18,486 |
| MR elastography | 60,233 | 13.81 | 3179 | 0.09 | 37,348 |
| Treat all | 99,263 | 14.76 | 39,030 | 0.95 | 41,177 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Treat no one | 49,392 | 6.30 | – | – | – |
| FIB-4 (high cut-off) | 108,296 | 9.59 | 58,904 | 3.30 | 17,871 |
| Test strategy | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| No treat | 34,436 | 10.709 | – | – | – |
| GUCI | 67,387 | 12.432 | 32,951 | 1.72 | 19,121 |
| MR elastography | 70,256 | 12.520 | 2869 | 0.09 | 32,618 |
| Hyaluronic acid | 72,298 | 12.570 | 2042 | 0.05 | 40,722 |
| Treat all | 100,896 | 13.264 | 28,598 | 0.69 | 41,229 |
| Test strategy | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| No treat | 41,192 | 8.68 | – | – | – |
| Forns index (high cut-off) | 79,060 | 10.45 | 37,868 | 1.77 | 21,387 |
| Treat all | 103,038 | 11.35 | 23,978 | 0.90 | 26,718 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| No treat | 37,831 | 9.64 | – | – | – |
| GUCI | 74,924 | 11.50 | 37,093 | 1.86 | 19,934 |
| MR elastography | 77,610 | 11.59 | 2686 | 0.08 | 32,200 |
| Treat all | 102,064 | 11.63 | 24,454 | 0.04 | 550,668 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Treat no one | 38,109 | 9.58 | – | – | – |
| GUCI | 75,108 | 11.48 | 36,999 | 1.90 | 19,476 |
| MR elastography | 77,641 | 11.57 | 2533 | 0.10 | 26,589 |
| US SAPI (low cut-off) | 91,418 | 11.91 | 13,777 | 0.34 | 41,083 |
| Treat all | 101,954 | 12.15 | 10,535 | 0.25 | 42,996 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Treat no one | 37,831 | 9.64 | – | – | – |
| GUCI | 74,925 | 11.52 | 37,095 | 1.88 | 19,733 |
| MR elastography | 77,585 | 11.64 | 2660 | 0.11 | 23,449 |
| Treat all | 101,484 | 12.18 | 23,899 | 0.54 | 44,019 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Treat no one | 37,831 | 9.64 | – | – | – |
| GUCI | 74,918 | 11.52 | 37,087 | 1.88 | 19,727 |
| MR elastography | 77,594 | 11.64 | 2676 | 0.11 | 23,846 |
| Treat all | 101,484 | 12.18 | 23,890 | 0.54 | 43,922 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Treat no one | 19,248 | 9.64 | – | – | – |
| GUCI | 56,277 | 11.52 | 37,029 | 1.88 | 19,694 |
| MR elastography | 59,473 | 11.66 | 3196 | 0.14 | 22,918 |
| Treat all | 84,468 | 12.24 | 24,994 | 0.58 | 43,095 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| No treat | 37,831 | 9.64 | – | – | – |
| GUCI | 74,915 | 11.52 | 37,084 | 1.88 | 19,725 |
| MR elastography | 77,013 | 11.64 | 2098 | 0.12 | 17,810 |
| Treat all | 101,484 | 12.18 | 24,471 | 0.54 | 45,456 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Treat no one | 37,831 | 9.64 | – | – | – |
| MR elastography | 95,175 | 12.02 | 57,345 | 2.38 | 24,077 |
| Treat all | 101,484 | 12.18 | 6309 | 0.15 | 40,836 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Treat no one | 37,831 | 9.64 | – | – | – |
| MR elastography | 90,312 | 11.94 | 52,481 | 2.29 | 22,868 |
| Treat all | 101,484 | 12.18 | 11,172 | 0.24 | 46,317 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Treat no one | 37,831 | 9.64 | – | – | – |
| MR elastography | 91,679 | 11.97 | 53,848 | 2.32 | 23,182 |
| Treat all | 101,484 | 12.18 | 9805 | 0.21 | 45,952 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Treat no one | 37,831 | 9.64 | – | – | – |
| (S2) Hyaluronic acid and US SAPI (high cut-off) | 75,246 | 11.52 | 37,414 | 1.88 | 19,903 |
| (S2) Hyaluronic acid and US SAPI (low cut-off) | 77,560 | 11.62 | 2315 | 0.09 | 24,883 |
| Hyaluronic acid | 79,005 | 11.66 | 1444 | 0.04 | 34,084 |
| Treat all | 101,484 | 12.18 | 22,480 | 0.52 | 43,150 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Treat no one | 37,831 | 9.64 | – | – | – |
| GUCI | 75,271 | 11.52 | 37,440 | 1.88 | 19,928 |
| MR elastography | 77,930 | 11.64 | 2659 | 0.12 | 22,601 |
| Treat all | 101,484 | 12.18 | 23,554 | 0.54 | 43,636 |
HBeAg-negative
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Treat no one | 37,579 | 8.83 | – | – | – |
| Treat all | 96,525 | 10.92 | 58,947 | 2.09 | 28,137 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Treat no one | 28,696 | 11.23 | – | – | – |
| Treat all | 120,532 | 15.24 | 91,836 | 4.02 | 22,871 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Treat no one | 49,584 | 5.68 | – | – | – |
| MR elastography | 96,726 | 7.87 | 47,142 | 2.18 | 21,581 |
| US SAPI (low cut-off) | 99,174 | 7.93 | 2448 | 0.07 | 36,897 |
| Test strategy | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| No treat | 33,514 | 9.837 | – | – | – |
| Treat all | 94,495 | 11.842 | 60,981 | 2.01 | 30,413 |
| Test strategy | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| No treat | 40,908 | 7.927 | – | – | – |
| Treat all | 97,007 | 9.971 | 56,099 | 2.04 | 27,447 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| No treat | 37,579 | 8.83 | – | – | – |
| Treat all | 96,525 | 10.92 | 58,947 | 2.09 | 28,137 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Treat no one | 37,579 | 8.83 | – | – | – |
| MR elastography | 71,699 | 9.87 | 34,120 | 1.04 | 32,694 |
| Treat all | 95,989 | 10.32 | 24,290 | 0.45 | 53,660 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Treat no one | 37,589 | 8.76 | – | – | – |
| Treat all | 96,314 | 10.82 | 58,724 | 2.05 | 28,603 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Treat no none | 37,579 | 8.83 | – | – | – |
| Treat all | 96,525 | 10.92 | 58,947 | 2.09 | 28,137 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Treat no one | 37,579 | 8.83 | – | – | – |
| Treat all | 96,525 | 10.92 | 58,947 | 2.09 | 28,137 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Treat no one | 18,894 | 8.83 | – | – | – |
| Treat all | 77,894 | 10.90 | 59,000 | 2.07 | 28,456 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Treat no one | 37,579 | 8.83 | – | – | – |
| MR elastography | 89,722 | 10.72 | 52,144 | 1.90 | 27,476 |
| Treat all | 96,525 | 10.92 | 6803 | 0.20 | 34,501 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Treat no one | 37,579 | 8.83 | – | – | – |
| MR elastography | 86,285 | 10.64 | 48,706 | 1.82 | 26,831 |
| Treat all | 96,525 | 10.92 | 10,241 | 0.28 | 36,615 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Treat no one | 37,579 | 8.83 | – | – | – |
| MR elastography | 85,041 | 10.63 | 47,462 | 1.81 | 26,260 |
| Treat all | 96,525 | 10.92 | 11,484 | 0.29 | 39,934 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| No treat | 37,579 | 8.83 | – | – | – |
| Treat all | 96,525 | 10.92 | 58,947 | 2.09 | 28,137 |
| Test | Cost £ | QALY | Incremental cost, £ | Incremental QALY | ICER £ |
|---|---|---|---|---|---|
| Treat no one | 37,579 | 8.83 | – | – | – |
| Treat all | 96,525 | 10.92 | 58,947 | 2.09 | 28,137 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Treat no one | 38,737 | 9.26 | – | – | – |
| GUCI | 73,824 | 10.78 | 35,088 | 1.52 | 23,065 |
| MR elastography | 76,631 | 10.89 | 2807 | 0.11 | 25,547 |
| US SAPI (low cut of) | 90,200 | 11.17 | 13,569 | 0.28 | 48,775 |
| Treat all | 99,905 | 11.36 | 9705 | 0.20 | 49,720 |
Sensitivity analysis tables for hepatitis C
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Forns index (high cut-off) | 47,426 | 14.119 | – | – | – |
| Fibroscan | 47,448 | 14.278 | 22 | 0.16 | 141 |
| Treat all | 51,241 | 14.732 | 3793 | 0.45 | 8370 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Pohl | 29,537 | 15.26 | – | – | – |
| US SAPI (high cut off) | 29,638 | 15.33 | 100 | 0.07 | 1424 |
| MR elastography | 29,974 | 15.39 | 337 | 0.06 | 5939 |
| CEUS | 30,269 | 15.42 | 294 | 0.03 | 9149 |
| Treat all | 38,159 | 16.18 | 7890 | 0.75 | 10,457 |
| Test strategy | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| (S4) Type IV collagen and MR elastography | 70,627 | 12.40 | – | – | – |
| MR elastography | 70,710 | 12.42 | 84 | 0.02 | 3893 |
| Treat all | 72,058 | 12.64 | 1348 | 0.22 | 6155 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| MR elastography | 47,212 | 19.52 | – | – | – |
| Treat all | 51,488 | 19.92 | 4276 | 0 | 10,813 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| MR elastography | 47,138 | 16.34 | – | – | – |
| Type IV collagen | 47,792 | 16.41 | 654 | 0.08 | 8615 |
| Treat all | 51,369 | 16.80 | 3577 | 0.39 | 9181 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| MR elastography | 49,455 | 14.16 | – | – | – |
| Treat all | 53,211 | 14.57 | 3757 | 0.41 | 9112 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| MR elastography | 49,668 | 14.12 | – | – | – |
| Treat all | 52,924 | 14.62 | 3256 | 0.50 | 6517 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| MR elastography | 46,603 | 14.26 | – | – | – |
| Treat all | 51,241 | 14.73 | 4638 | 0.47 | 9938 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Treat all | 51,241 | 14.73 | – | – | – |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Treat all | 51,241 | 14.73 | – | – | – |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Treat all | 51,241 | 14.73 | – | – | – |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| (S4) PIINP/MMP and MR elastography | 46,772 | 14.19 | – | – | – |
| MR elastography | 46,891 | 14.27 | 119 | 0.08 | 1452 |
| Treat all | 51,241 | 14.73 | 4350 | 0.46 | 9516 |
Hepatitis C: sensitivity analysis of genotype distribution used in analysis
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| MR elastography | 43,423 | 14.33 | – | – | – |
| Treat all | 46,182 | 14.76 | 1898 | 0.44 | 4352 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| MR elastography | 46,851 | 14.27 | – | – | – |
| Treat all | 51,241 | 14.73 | 4391 | 0.46 | 9468 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| MR elastography | 46,875 | 14.27 | – | – | – |
| Treat all | 51,241 | 14.73 | 4366 | 0.457 | 9546 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| MR elastography | 47,117 | 14.26 | – | – | – |
| Type IV collagen | 47,752 | 14.33 | 635 | 0.07 | 8824 |
| Treat all | 51,350 | 14.70 | 3598 | 0.37 | 9704 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| US SAPI (high cut-off) | 47,259.70 | 14.16 | – | – | – |
| MR elastography | 47,281.65 | 14.22 | 22 | 0.07 | 336 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| MR elastography | 47,283 | 14.23 | – | – | – |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| MR elastography | 47,142 | 14.23 | – | – | – |
| Treat all | 55,028 | 14.24 | 7886 | 0.011 | 723,503 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| MR elastography | 47,158 | 14.24 | – | – | – |
| Treat all | 54,153 | 14.32 | 6995 | 0.08 | 83,697 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| MR elastography | 47,125 | 14.25 | – | – | – |
| Treat all | 53,800 | 14.40 | 6675 | 0.15 | 45,877 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| MR elastography | 47,071 | 14.26 | – | – | – |
| US SAPI (low cut-off) | 50,623 | 14.38 | 3552 | 0.12 | 28,435 |
| Treat all | 53,193 | 14.47 | 2571 | 0.09 | 29,740 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| MR elastography | 46,951 | 14.26 | – | – | – |
| Treat all | 52,545 | 14.56 | 5594 | 0.30 | 18,830 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| MR elastography | 46,902 | 14.27 | – | – | – |
| Treat all | 51,241 | 14.73 | 4339 | 0.462 | 9384 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| MR elastography | 43,631 | 14.71 | – | – | – |
| TE | 44,188 | 14.72 | 557 | 0.01 | 56,413 |
| Treat all | 49,207 | 15.27 | 5576 | 0.56 | 10,009 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Treat no one | 54,940 | 12.46 | – | – | – |
| Pohl | 55,973 | 14.46 | 1033 | 2.00 | 517 |
| US SAPI (high cut-off) | 56,931 | 14.62 | 314 | 0.07 | 6083 |
| MR elastography | 57,913 | 14.73 | 982 | 0.11 | 9189 |
| Treat all | 69,108 | 15.26 | 11,195 | 0.53 | 21,174 |
Sensitivity analyses tables for alcoholic liver disease
| Strategy | Tests | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|---|
| Strategy 2 | APRI (high cut-off) and liver biopsy | 15,787 | 9.06 | – | – | – |
| Strategy 5 | PGAA and liver biopsy | 15,908 | 9.35 | 122 | 0.29 | 416 |
| Strategy 3 | Fibrotest (high cut-off) and liver biopsy | 16,016 | 9.46 | 108 | 0.10 | 1041 |
| Strategy 4 | Fibrotest (low cut-off) and liver biopsy | 16,187 | 9.53 | 171 | 0.08 | 2271 |
| Strategy 1 | Liver biopsy | 16,321 | 9.56 | 134 | 0.03 | 5199 |
| Strategy 12 | All patients treated as having cirrhosis (receive HCC screening) | 30,574 | 9.65 | 14,253 | 0.10 | 146,491 |
| Strategy | Tests | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|---|
| Strategy 2 | APRI (high cut-off) and liver biopsy | 19,199 | 8.52 | – | – | – |
| Strategy 1 | Liver biopsy | 19,409 | 9.08 | 210 | 0.56 | 377 |
| Strategy 12 | All patients treated as having cirrhosis (receive HCC screening) | 31,377 | 9.35 | 11,967 | 0.27 | 44,302 |
| Strategy | Tests | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|---|
| Strategy 1 | Liver biopsy | 20,971 | 8.82 | – | – | – |
| Strategy 12 | All patients treated as having cirrhosis (receive HCC screening) | 31,672 | 9.20 | 10,701 | 0.37 | 28,747 |
| Strategy | Tests | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|---|
| Strategy 1 | Liver biopsy | 22,352 | 8.58 | – | – | – |
| Strategy 12 | All patients treated as having cirrhosis (receive HCC screening) | 31,944 | 9.04 | 9591 | 0.46 | 20,835 |
| Strategy | Tests | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|---|
| Strategy 1 | Liver biopsy | 24,107 | 8.31 | – | – | – |
| Strategy 12 | All patients treated as having cirrhosis (receive HCC screening) | 32,461 | 8.85 | 8355 | 0.55 | 15,232 |
| Strategy | Tests | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|---|
| Strategy 2 | APRI (high cut-off) and liver biopsy | 17,480 | 8.80 | – | – | – |
| Strategy 5 | PGAA and liver biopsy | 17,703 | 9.08 | 223 | 0.28 | 795 |
| Strategy 1 | Liver biopsy | 17,913 | 9.32 | 209 | 0.24 | 854 |
| Strategy 12 | All patients treated as having cirrhosis (receive HCC screening) | 30,984 | 9.51 | 13,071 | 0.18 | 71,616 |
| Strategy | Tests | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|---|
| Strategy 2 | APRI (high cut-off) and liver biopsy | 17,486 | 8.79 | – | – | – |
| Strategy 5 | PGAA and liver biopsy | 17,674 | 9.06 | 188 | 0.27 | 693 |
| Strategy 1 | Liver biopsy only | 17,882 | 9.29 | 208 | 0.24 | 884 |
| Strategy 12 | All treated as having cirrhosis | 30,954 | 9.48 | 13,072 | 0.19 | 69,697 |
| Strategy | Tests | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|---|
| Strategy 2 | APRI (high cut-off) and liver biopsy | 17,480 | 8.80 | – | – | – |
| Strategy 5 | PGAA and liver biopsy | 17,703 | 9.08 | 223 | 0.28 | 795 |
| Strategy 1 | Liver biopsy | 17,913 | 9.32 | 209 | 0.24 | 854 |
| Strategy 12 | All patients treated as having cirrhosis (receive HCC screening) | 30,984 | 9.51 | 13,071 | 0.18 | 71,616 |
| Strategy | Tests | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|---|
| Strategy 2 | APRI (high cut-off) and liver biopsy | 17,488 | 8.78 | – | – | – |
| Strategy 1 | Liver biopsy | 17,840 | 9.29 | 169 | 0.23 | 721 |
| Strategy 12 | All patients treated as having cirrhosis (receive HCC screening) | 30,973 | 9.49 | 13,133 | 0.20 | 65,679 |
Cirrhosis sensitivity analysis
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Fibrosis Index | 24,908 | 2.09 | – | – | – |
| CDS (low cut-off) | 24,946 | 2.18 | 39 | 0.09 | 439 |
| Forns index | 24,974 | 2.22 | 28 | 0.04 | 653 |
| Forns index (low cut-off) | 25,032 | 2.27 | 58 | 0.05 | 1106 |
| Test | Cost, £ | QALY | Incremental cost, £ | Incremental QALY | ICER, £ |
|---|---|---|---|---|---|
| Fibrosis Index | 24,908 | 2.09 | – | – | – |
| CDS (low cut-off) | 24,946 | 2.16 | 38 | 0.07 | 526 |
| Forns index | 24,974 | 2.18 | 28 | 0.01 | 1884 |
Appendix 12 Scatterplots of cost-effectiveness analysis results
FIGURE 240.
Scatterplot of results: HBeAg (negative) – first stage of the analysis (all tests strategies compared with ‘treat no one’). US, ultrasound.
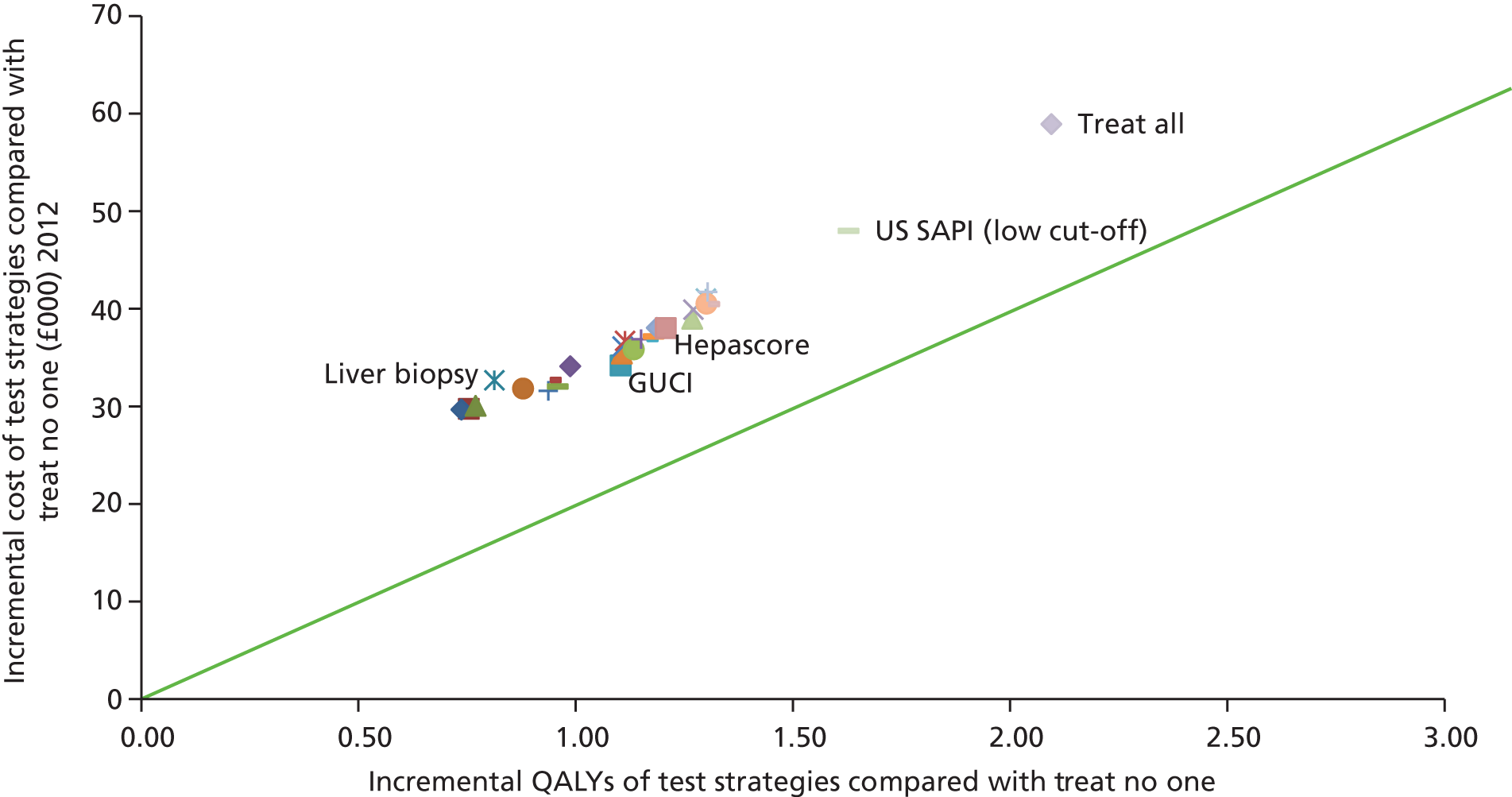
FIGURE 241.
Scatterplot of results: HBeAg (negative) – second stage of the analysis (all tests strategies compared with ‘treat no one’). HA, hyaluronic acid; MRE, MR elastography; S1, strategy 1.

FIGURE 242.
Scatterplot of results: HCV – first stage of the analysis (all tests strategies compared with ‘treat no one’). MRE, MR elastography; US, ultrasound.
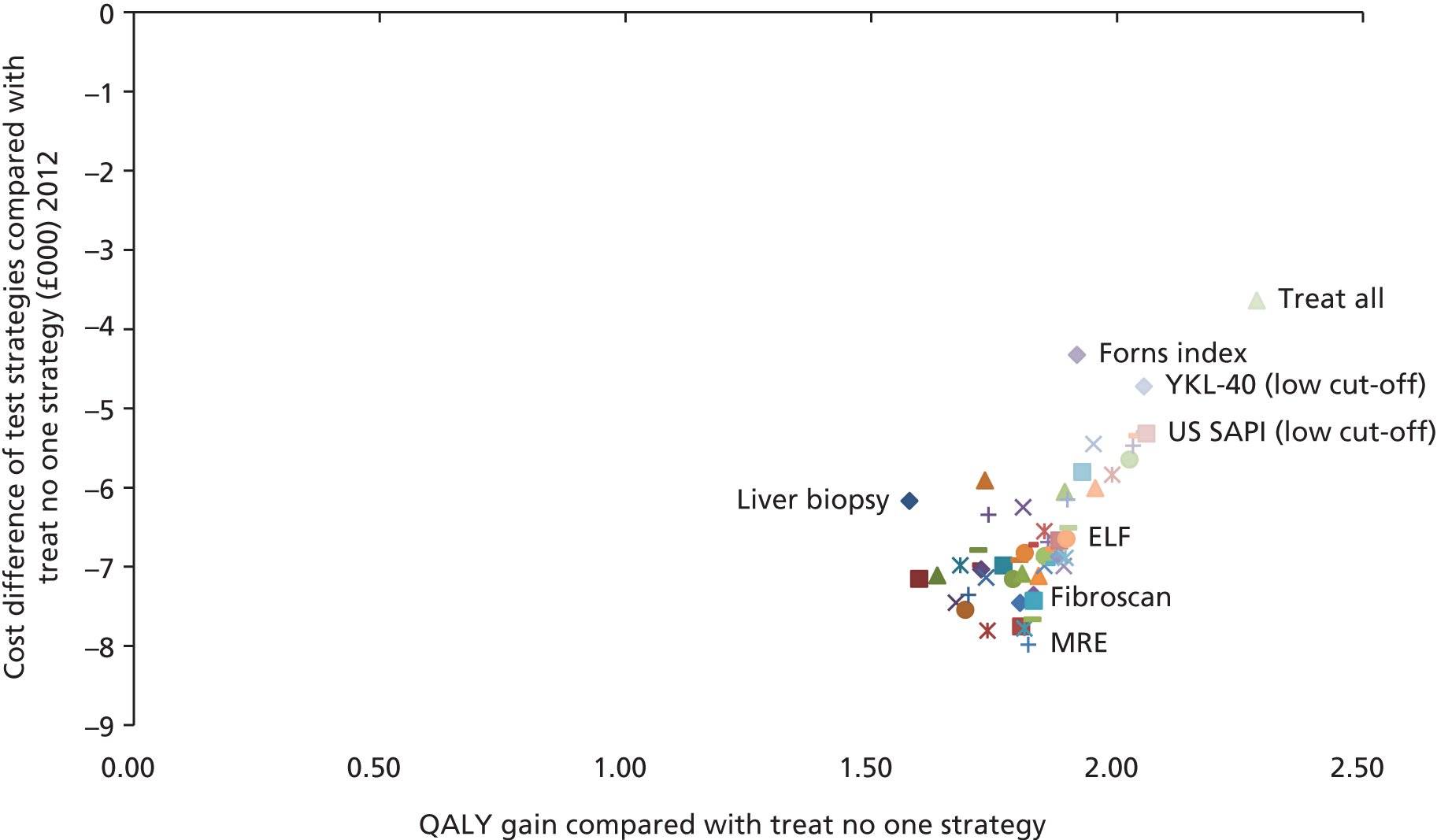
FIGURE 243.
Scatterplot of results: HCV – second stage of the analysis (all tests strategies compared with ‘treat no one’). HA, hyaluronic acid; MRE, MR elastography; S1, strategy 1.

FIGURE 244.
Scatterplot of results: ALD (all test strategies compared with least costly test strategy).

FIGURE 245.
Scatterplot of results: cirrhosis (all tests strategies compared with least costly test strategy). CDS, Cirrhosis Discriminant Score; US, ultrasound.

Appendix 13 Reporting patient and public involvement
Patient representatives were not present in the steering committee meetings, as these were dedicated to technical discussions around meta-analysis and disease-modelling data and assumptions.
Upon completion of our study, the British Liver Trust, which is the leading liver charity in the UK, was contacted in order to arrange the presentation and dissemination of the findings of our study in patients and their representatives.
There were no slots available in their latest meeting; however, these will be presented in a future meeting.
We further collaborated with Research Media and produced a flyer, which explains in simplified language our research on non-invasive tests, their effectiveness and their cost-effectiveness. This will be published online in the British Liver Trust website and also in International Innovation, which is considered one of the leading global dissemination resources (www.research-europe.com/index.php/digital_magazine/). Fifty hard copies of this publication will be sent to key stakeholders.
Glossary
List of abbreviations
- AASLD
- American Association for the Study of the Liver
- ACE
- angiotensin-converting enzyme
- ALD
- alcoholic liver disease
- ALT
- alanine aminotransferase
- APRI
- AST to platelet ratio index
- ARFI
- acoustic radiation force impulse
- AST
- aspartate aminotransferase
- AUROC
- area under the receiver operator curve
- BARD
- BMI, AST–ALT ratio, diabetes
- BMI
- body mass index
- BNF
- British National Formulary
- CEAC
- cost-effectiveness acceptability curve
- CEAF
- cost-effectiveness acceptability frontier
- CELT
- Cost-Effectiveness of Liver Transplantation
- CI
- confidence interval
- CT
- computed tomography
- DNA
- deoxyribonucleic acid
- EASL
- European Association for the Study of the Liver
- ELF
- enhanced liver fibrosis test
- GGT
- gamma-glutamyl transpeptidase
- GP
- general practitioner
- GUCI
- Göteborg University Cirrhosis Index
- HBeAg
- hepatitis B e antigen
- HBV
- hepatitis B
- HCC
- hepatocellular cancer
- HCV
- hepatitis C
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- MR
- magnetic resonance
- MRI
- magnetic resonance imaging
- MTC
- mixed-treatment comparison
- NAFIC
- ferritin, fasting insulin, type IV collagen
- NAFLD
- non-alcoholic fatty liver disease
- NASH
- non-alcoholic steatohepatitis
- NFS
- non-alcoholic fatty liver disease fibrosis score
- NICE
- National Institute for Health and Care Excellence
- NILT
- non-invasive liver test
- PGAA
- prothrombin time, GGT, apolipoprotein A1, α2-macroglobulin
- PIIINP
- amino-terminal propeptide of type III procollagen
- PSA
- probabilistic sensitivity analysis
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QUADAS
- Quality Assessment of Diagnostic Accuracy Studies
- RCT
- randomised controlled trial
- RR
- relative risk
- SAFE
- sequential algorithm for fibrosis evaluation
- SAPI
- splenic artery pulsatility index
- SHTAC
- Southampton Health Technology Assessment Centre
- SROC
- summary receiver operating characteristic
- SVR
- sustained virological response
- TE
- transient elastography (Fibroscan)
- TIMP
- tissue metalloproteinase
- ULN
- upper limit of normal