Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 09/22/136. The contractual start date was in January 2011. The draft report began editorial review in October 2013 and was accepted for publication in August 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
The authors have no current personal financial interests; the following non-financial interests are declared: Dr Hilton was a member of the National Institute for Health and Care Excellence (NICE) Interventional Procedures Advisory Committee (2002–7); a member of the National Institute for Health Research (NIHR) Evaluation, Trials and Studies Co-ordinating Centre (NETSCC) Health Technology Assessment (HTA) Therapeutic Procedures Panel (2007–8) and the NETSCC-HTA Clinical Evaluations and Trials Prioritisation Group (2008–10); chair of the NICE development group for clinical guideline on urinary incontinence in women (2004–7); and a member of the James Lind Alliance Working Partnership on research priorities on urinary incontinence (2007–9). These last two groups identified the research question underlying this report as an important area for further research. Dr Lucas currently chairs the European Association of Urology Guidelines panel on Urinary Incontinence which has produced guidance on the use of urodynamics in clinical practice based on existing evidence. Professor McColl is a member of the NIHR Journals Library Editorial Group although she was not involved in the editorial processes for this report.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Hilton et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction and background
Prevalence of urinary incontinence
Urinary incontinence (UI), while rarely life-threatening, may seriously influence the physical, psychological and social well-being of affected individuals. 1–4 The impact on the families and carers may be profound and the resource implications for the health service considerable. 5 Prevalence figures for UI range from 5% to 69% in women aged 15 years and older, with most studies in the range 25–45%. 6 More severe UI is reported in 4–7% of women aged under 65 years, and around 5 million women over 20 years of age in England and Wales may be affected. 7
Although absolute prevalence rates vary widely, the distribution of UI subtypes appears more consistent, with stress UI (SUI) or mixed UI (MUI) accounting for 65–85% of cases. 8 Isolated SUI accounts for approximately half of all incontinence, with most studies reporting 10–39% prevalence; MUI is the next most common, with prevalence figures of 7.5–25.0%; isolated urgency UI appears to be relatively uncommon, with 1–7% prevalence. 6
Costs of urinary incontinence and investigation
A study of UI across 14 European countries reported the mean annual per capita UI-related costs to range from €359 in the UK/Ireland (for patients predominantly treated in primary care) to €515 in Germany and €655 in Spain (for patients treated by specialists). 9 A systematic review of the costs associated with UI and overactive bladder (OAB) similarly found the annual per capita costs to vary considerably between individual studies and countries, with the highest reported being in institutionalised individuals in the USA at US$9872. 10 A UK study using 1999/2000 prices estimated the annual cost to the NHS in England of treating clinically significant UI in women at £233M, with total annual service costs (including costs borne by individuals) of £411M. 11
Several methods are used in the assessment of UI to guide management decisions, and invasive urodynamic tests (IUTs) may form part of this. Essentially these investigations evaluate functional aspects of the lower urinary tract; cystometry, the most commonly used IUT, looks at the pressure/volume relationships during bladder filling, storage and emptying, with a view to defining a functional diagnosis as distinct from a purely symptomatic one. The costing report associated with the National Institute for Health and Clinical Excellence [now, the National Institute for Health and Care Excellence (NICE)] clinical guideline on UI used an estimated charge of £176 for each IUT (2006/7 English national tariff), and calculated the annual national cost of urodynamic investigations as over £22M. 12 From this, the potential saving from not undertaking urodynamic investigations before conservative treatment was estimated at approximately £3M. 12
Changes in available operative techniques and, in particular, the introduction of less invasive approaches such as mid-urethral tapes, have resulted in dramatic alterations to surgical practice in recent years. 13 Hospital Episode Statistics (HES) demonstrate a 50% increase in surgery for SUI in the 10 years following the introduction of mid-urethral tapes in 1997, with numbers apparently plateauing at 11,000–13,000 procedures annually in England between 2006/7 and 2012/13. 14 The NICE costing report estimated further savings of £321,000 from more rational use of IUTs before surgery, although this is perhaps a conservative estimate being based on ‘current use’ of 70% (the actual figure is probably closer to 100%) and ‘future use’ of 50%. 12 A more realistic estimate of annual savings based on 2012/13 national tariff costs (£403 per procedure for Healthcare Resource Group LB42Z)15 and HES activity data would be approximately £3.3M. There would also be an additional ‘opportunity cost’ saving from the alternative use of staff and equipment currently devoted to invasive urodynamic testing. It remains to be demonstrated, but should be recognised, that this saving may come at no detriment to health and with the avoidance of what some women undoubtedly see as unpleasant and embarrassing procedures.
Existing literature on clinical utility of invasive urodynamic tests prior to surgery
Urodynamic tests comprise a group of investigations used to evaluate function of the lower urinary tract; some of these are invasive (requiring catheterisation) and some are non-invasive. The tests are most often used for diagnosis, planning of appropriate intervention and prediction of treatment outcome, although they can also be used repeatedly to monitor the progress of disease over time or as outcome measures in clinical research. While cystometry is the most commonly used IUT, videocystometry and ambulatory bladder pressure monitoring are used by some. The current position of invasive urodynamic testing in the diagnostic pathway is not agreed and practices vary considerably: in a UK survey in 2002, only half of the units surveyed had guidelines on indications for the tests and 85% carried out cystometry in all women with incontinence. 16 Current guidance from NICE suggests that cystometry is not required prior to conservative treatments for UI, or prior to surgery where the diagnosis of SUI is clear on clinical grounds [i.e. where there are no symptoms of OAB or voiding dysfunction (VD), no anterior compartment prolapse and no previous surgery for SUI]. 17,18
The National Institute for Health and Care Excellence, National Institute for Health Research (NIHR) Health Technology Assessment (HTA), The Cochrane Collaboration and the International Consultation on Incontinence (ICI) have each recently undertaken systematic reviews on the subject of urodynamics and called for further high-quality primary research confirming clinical utility. 17,19–23 The specific aim of the current study is to assess the feasibility of a future large randomised controlled trial (RCT) to address a key research recommendation of the NICE and Cochrane reviews of the subject. The clinical utility of invasive urodynamic testing was also among the top prioritised uncertainties identified within the James Lind Alliance Urinary Incontinence Priority Setting Partnership in 2008. 24,25
A decision-analysis study from the USA failed to find support for invasive urodynamics before surgery in women likely to have SUI. 26 A similar economic assessment within the NICE report on UI, using assumptions more applicable to current NHS practice, found that for every 10,000 patients assessed there would be approximately 13 additional cures using invasive urodynamics, at an additional cost per cure of £26,125. With a ‘willingness-to-pay’ threshold of £20,000 per quality-adjusted life-year (QALY), each cure would have to generate 1.3 QALYs for invasive urodynamics to be considered cost-effective. 17 Based on a gain of QALYs of 0.07 per annum for a woman cured compared with a woman not cured,27,28 this would require each cured woman to survive 19 years post treatment (assuming QALYs are not discounted); given that typical women undergoing surgical treatment for SUI are in their mid-40s (range 20s to 70s), their average life expectancy would be much greater than this, suggesting that invasive urodynamic testing may be cost-effective.
One small RCT showed no significant benefit from cystometry prior to conservative treatment, although interpretation is difficult, given that the control (not investigated) group in this study received both bladder retraining and pelvic floor muscle training (PFMT), whereas the intervention (cystometry) group received either bladder retraining or PFMT. 29 In a cohort study from the North Thames region, women were no more likely to benefit from incontinence surgery if they had undergone preoperative urodynamic testing,30 and a study of Medicare patients in the USA found that those who had preoperative testing appeared more likely to develop urge incontinence after their surgery. 31 A secondary analysis of data from a US randomised surgical trial found that preoperative investigation did not predict failure32 or postoperative VD. 33
Other studies ongoing during protocol development
Post funding, but during the refinement of the protocol for INVasive Evaluation before Surgical Treatment of Incontinence Gives Added Theraputic Effect? (INVESTIGATE-I), the investigators became aware of two other trials looking at the clinical utility of urodynamics in similar patient groups. One was from a multicentre group in the Netherlands [Value of Urodynamics prior to Stress Incontinence Surgery (VUSIS-1); www.controlled-trials.com/mrct/trial/385179/urodynamic], the other from the US Urinary Incontinence Treatment Network [Value of Urodynamic Evaluation (ValUE); www.controlled-trials.com/mrct/trial/472073/urodynamic]. 34 Both of these were full trials using a non-inferiority design. VUSIS-1 did not specifically define a non-inferiority margin, although the sample size was determined from a power of 70% using less than 5% difference between groups; this trial was terminated prematurely due to slow recruitment after achieving only 23% (59/260) of its planned accrual. 35 ValUE defined a non-inferiority margin of 11% (equivalent to a standardised difference of < 0.8), which we consider too high, that is we would look on a difference in outcome between groups of 11% as being clinically quite important and one that might potentially influence the decisions of both clinicians and patients. 36
In the ValUE study, women with a clinical diagnosis of SUI or stress predominant MUI, who also have clinically demonstrable stress leakage (i.e. a slightly different patient group from INVESTIGATE-I), were randomised to either no further assessment or to undergo urodynamic investigation (as in INVESTIGATE-I). In view of the recruitment difficulties with VUSIS-1, the Netherlands group proceeded to a further study of alternative design (VUSIS-2; www.controlled-trials.com/mrct/trial/474127/vierhout),37 in which all women underwent invasive urodynamic testing, and only those with discordant clinical and urodynamic findings were randomised between surgical treatment (as dictated by their clinical assessment) and individual treatment (dictated by the combination of clinical and urodynamic results); neither participants nor health-care professionals involved were blinded to the urodynamic results in either group.
The primary outcome in ValUE and both VUSIS studies was based on the Urogenital Distress Inventory (UDI) score at 12 months (ValUE used a 70% reduction in UDI along with a Patient Global Impression of Improvement score of ‘very much better’ or ‘much better’ as indicative of treatment success). Although we preferred the use of international standard outcomes as intended by the ICI Modular Questionnaire (ICIQ) as our primary outcome, we subsequently chose to include the UDI as an additional secondary outcome38 to facilitate easier comparison of results between these various studies and the incorporation of our results, even from this feasibility study, into a meta-analysis.
Each of these studies has been published during the period of recruitment and follow-up in INVESTIGATE-I;35,36,39 their results are discussed later in this report. How much they have already influenced clinical opinion and practice or will do so in the future is unclear, although a ‘point-counterpoint’ debate published after these studies (in 2013) makes it clear that there is still a question to be answered. 40,41 The most recent update of the Cochrane review of urodynamics for the management of UI in children and adults continues to emphasise the need for larger definitive trials, in which people are randomly allocated to management according to urodynamic findings or to standard management based on history and clinical examination. 42
Rationale for an initial feasibility study and pilot trial
Although NICE, NIHR HTA, The Cochrane Collaboration and the ICI have all called for large high-quality primary research to establish the clinical utility of invasive urodynamic investigations, there were several reasons to conduct a pilot trial and feasibility assessment before undertaking a definitive trial.
First, the sample size for a definitive trial was considered using estimates and assumptions from the modelling exercises cited above,17,26 and from a previous surgical trial. 43,44 However, such calculations are very sensitive to parameter values such as the proportion of recruits with SUI,26 the proportions of poor outcomes in the two arms and the effect size (target difference) of interest; currently available information is insufficient to plan a study that could be expected with reasonable certainty to produce robust results. Our own very preliminary sample size calculations gave figures between 1100 and 6700 per treatment arm. Since designing the feasibility study, the most recent Cochrane review of urodynamics in adults and children indicates that a similarly large sample size would be required to address this question. 42
Given the possible size of a definitive trial on this question therefore, a feasibility study was considered crucial to test assumptions made, give relevant estimates of key parameters and inform power calculations for the definitive trial.
Second, invasive urodynamic testing has been widely used in clinical practice over the last 30 years and, despite the lack of evidence of clinical utility, many clinicians look on cystometry as a mandatory part of the investigation of patients with UI, particularly prior to surgical treatment. 45–47 A survey of members of the British Society of Urogynaecology (BSUG) has shown a high level of disagreement with the NICE guidance in this respect,48 and others have questioned the safety of the recommendations. 49 We were aware that, although the ValUE study completed recruitment,36 the investigators encountered initial problems with lack of clinician equipoise (Peggy Norton, University of Utah Health Care, 2010, personal communication). Hence we needed to establish whether or not sufficient clinicians were in equipoise and willing to enrol and randomise patients within a definitive trial.
Finally, patients may not so easily see the importance of ‘testing a test’ in the same way as they might view testing a treatment. Indeed, they are willing and often keen to undergo investigation (even when this is invasive),50 in the belief that this will inevitably guide them and their clinicians towards appropriate treatment and away from inappropriate and possibly harmful interventions. Two HTA-funded trials of radiography for low-back pain were only able to recruit 23% and 51% of patients who were approached to enter the randomised arms. 51,52 The VUSIS-1 study was terminated prematurely when it had achieved only 23% of its planned recruitment. 35 Hence it was necessary to investigate patients’ willingness to take part in a RCT of this particular diagnostic test and to identify barriers to, and facilitators of, participation.
Overall, therefore, while we were encouraged that other researchers have similarly seen this topic as an important clinical uncertainty and have sought to undertake trials of similar design to that proposed in INVESTIGATE, we remained of the opinion that a feasibility study was an important step before embarking on a definitive trial using public funds.
It was recognised that a pilot RCT alone was probably inadequate to address the complexities of the determination of feasibility for a definitive trial in this aspect of health care. While most mixed-methods studies to date have been limited to combining qualitative methods and RCTs,53 we developed a protocol comprising a national survey of relevant clinicians, qualitative interviews with both trial participants (face to face) and clinicians (telephone), a randomised external pilot trial and a nested health economic analysis. Post hoc additions to the protocol included an update to the original clinician survey and a questionnaire to those identifying potential trial participants [research nurses and principal investigators (PIs)] regarding issues of screening sensitivity.
Chapter 2 Study components
Specific objectives
The objective of the proposed future definitive trial is to address the question of whether or not invasive urodynamic testing compared with basic clinical assessment with non-invasive testing alters treatment decisions and outcomes in women suitable for surgical treatment of SUI or stress predominant MUI. The outcome measures proposed would include the quantification of post-treatment urinary leakage, impact on general health and condition-specific quality of life (QoL), adverse effects from investigation or treatment and health economic outcomes. Thus, in a possible future definitive trial, it might be established whether or not invasive urodynamic testing should indeed be offered to all women prior to surgery.
The objective of the current feasibility study (INVESTIGATE-I) was to inform the decision whether or not to proceed to such a definitive RCT and whether or not any refinements to the design or conduct of that trial are warranted.
Study components
A mixed-methods approach was chosen to assess the feasibility of a future definitive RCT. There were five components to the study, each addressing different aspects of the overall determination of feasibility:
-
A pragmatic multicentre randomised pilot (external or rehearsal pilot) trial (see Chapter 3). This was designed to rehearse the methods and processes of a future definitive randomised trial. As such, it evaluated patient identification strategies, recruitment numbers and patients’ willingness to be randomised. The rate of retention within the study and the effectiveness of outcome measures in terms of response and completion rates were also evaluated. The pilot was also designed to provide outcome data to inform sample size calculations for a future definitive trial.
-
A full economic evaluation undertaken within the above pilot RCT (see Chapter 4). The pilot study rehearsed the data collection for the economic evaluation, which included health state utilities and costs to the NHS and patients. To inform the definitive economic analysis, the pilot study assessed consistency of resource use in administration of the IUT and other tests, surgical and non-surgical treatments, and the ease of access to information from hospital databases about resource use. It also piloted the use of data collection instruments.
-
National online surveys of clinicians’ views about urodynamics (see Chapter 5). In order to assess the extent of ‘buy-in’ to a future definitive trial, the survey questionnaires explored surgeons’ views about the necessity for urodynamic investigations in a range of clinical scenarios and also their opinion about the importance of the research question underlying the INVESTIGATE studies. Since it was anticipated that a future robust trial would require a sample size very much larger than previous studies and seemed likely to need the involvement of a large number of units, clinicians’ workload in incontinence surgery and their willingness to randomise their own patients in a definitive trial was also assessed. A brief second survey was undertaken towards the end of the study to assess changes in clinical opinion over time as a result of other publications in the area. 35,36,39
-
Qualitative interviews with a subset of surgeons (see Chapter 6). The interview topic guide used here sought to illuminate the questionnaire responses from component 3 above. This complemented the results of the survey and explored further how clinicians use the results of IUTs to inform their decisions. The interview data were used to explore the differences between personal and community equipoise and the effect these may have on willingness to randomise patients into a future trial; they were also used to investigate some of the sociological aspects of diagnostic tests and, in particular, how clinicians approach a test that is widely used but lacking evidence of clinical utility.
-
Qualitative interviews with a subset of women eligible for the pilot trial to assess their experiences of the study (see Chapter 7). By approaching those who did and did not agree to participate, we sought to define the reasons behind these decisions. The interview topic guide was designed to facilitate exploration of patients’ experiences of being approached to take part in the trial, their perceptions of the study information sheets and the burden associated with study outcome questionnaires.
The methods employed, results obtained and key messages from these different study components are described separately in Chapters 3–7; discussion is combined in Chapter 8, and the overall consideration of feasibility is presented in Chapter 9. The latest version of the protocol is available on the NIHR Journals Library website. The report is made in line with the Consolidated Standards of Reporting Trials (CONSORT) statement;54 the CONSORT diagram for the randomised pilot trial is given as Figure 5; the CONSORT checklist is shown in Appendix 1.
Chapter 3 Randomised external pilot trial
Methods
This was a pragmatic multicentre randomised external (rehearsal) pilot trial to assess patient recruitment and willingness to be randomised, rehearse trial methods and processes, and provide outcome data to inform sample size calculations for a future definitive trial. All of these were considered important elements of the determination of feasibility.
Units recruiting to the trial
Recruitment to the pilot trial was initially limited to six specified units; these were a mix of specialist urogynaecology (Newcastle upon Tyne and Leicester) and female urology (Sheffield and Swansea) departments in university teaching hospitals providing secondary- and tertiary-level care, and general gynaecology units in district general hospitals providing secondary care services (Wansbeck Hospital, Northumberland and Queen Elizabeth Hospital, Gateshead).
In order to improve adherence with recruitment targets and to test the processes for possible future use, two Patient Identification Centre (PIC) sites (Sunderland Royal Hospital and South Tyneside District General Hospital) and one additional full recruiting site (South Tees Hospitals NHS Foundation Trust) were introduced during 2012.
Inclusion and exclusion criteria
Inclusion criteria
Inclusion criteria for the pilot trial (and currently anticipated inclusion criteria for the future definitive trial) were as follows:
Women were required to fulfil ALL criteria to be eligible:
-
Clinical diagnosis of SUI or stress predominant MUI.
-
Women must state that their family is complete.
-
Women should have undergone a course of PFMT (± other non-surgical treatments for their urge symptoms) with inadequate resolution of their symptoms.
-
Both the woman herself and her treating clinician should agree that surgery is an appropriate and acceptable next line of treatment.
Exclusion criteria
For the pilot trial (and currently anticipated for a future definitive trial), the following situations excluded eligibility:
-
Symptomatic uterovaginal prolapse requiring treatment.
-
Previous surgery for UI or pelvic organ prolapse (POP).
-
Urodynamic investigation within the last 3 years.
-
Neurological disease causing UI.
-
Current involvement in competing research studies (e.g. studies of investigation or treatment of UI).
-
Unable to give competent informed consent.
Withdrawal options
There were two trial withdrawal options:
-
Withdrawing completely, that is withdrawal from the allocated investigation protocol and provision of follow-up data. Consent would be sought to retain data collected up to the point of withdrawal and to complete an ‘end of study’ visit at the time of withdrawal.
-
Withdrawing partially, that is withdrawal from the allocated investigation protocol (including a request to move to the alternative investigation arm) but continuing to provide follow-up data by attending clinic and completing questionnaires.
Participants’ reasons for withdrawal were recorded where possible, as the information might be relevant to the protocol for a future definitive study.
Recruitment
Potential trial recruits were identified by the study research nurses prior to attending new or follow-up appointments for SUI or MUI in the clinics run by the unit clinical leads. The Patient Information Sheet (PIS) was available in two forms: a short (one-page) introduction to the study and a more detailed (six-page) description of the trial and the implications of involvement (see Appendix 5 and 6). The short PIS was sent out along with a letter of invitation (see Appendix 2), with new appointments or with a reminder letter to attend follow-up appointments; this allowed any questions that the woman may have about the study to be addressed at the one visit; the full PIS was provided on request. Those declining to take part underwent further investigation and/or treatment as appropriate at the same visit. Those agreeing to take part signed a study consent form (see Appendix 10); with the patient’s agreement, the general practitioner (GP) was notified of their involvement in the trial (see Appendix 3).
Where other potential recruits became apparent only at the time of a clinic visit, they were invited to take part in the study and given verbal and written information. After a period of at least 24 hours to read, consider and discuss the information with family and/or friends, the research nurse contacted the patient by telephone to respond to any further outstanding questions and review their decision regarding involvement.
Patient and public involvement
In order to ensure that issues of importance to women undergoing IUTs would be addressed by a future definitive trial, advice and opinions were sought from patients and patient advocates at all stages of the INVESTIGATE-I study, particularly at the time of its conception, design and initiation. One of the trial grant holders (BSB), a clinical researcher, was the past chair of the Bladder and Bowel Foundation (B&BF), a patient-led support and advocacy organisation. B&BF members, staff and trustees were involved at the early stages of trial development in co-ordinating the involvement of patients in reviewing the protocol, materials and grant applications. The B&BF was also involved in identifying patient members for the Trial Steering Committee (TSC). Incontinence is a sensitive issue that is seldom discussed or acknowledged in public, so identifying women who were willing to participate in this capacity was less straightforward than may be the case in other areas of health care.
A particular challenge for the trial was the design of materials such as the PISs. In addition to explaining clearly the trial’s purpose and what involvement would mean for participants, these had to address two issues specific to the trial that are not common to many studies.
First, a diagnostic test that is routinely used, even an invasive test, is often accepted without question by patients in the belief that it will serve to inform treatment decisions. In this context, explaining the absence of good evidence of its value and the equipoise that exists between a diagnostic test and no test is more challenging than explaining equipoise between two treatments. It was important that participants understood that they were not being denied an effective element of the care process.
Second, a feasibility study may not be perceived to be as important as a definitive trial by potential participants. The PIS had to outline the potential importance of a feasibility study in making best use of public funds by informing the design of a definitive trial that could ultimately result in less invasive but equally effective patient care pathways.
Lay members of the TSC and a previous service user (trial participant) were involved in reviewing the plain English summary.
In a future definitive trial, a broader spread of patient and public representation could be sought. This might include, women’s network members from professional organisations or research support structures (e.g. Royal College of Obstetricians and Gynaecologists and Research Design Services); ex-patients; and, ex-trial participants. A Patient Advisory Group facilitated by one of the research team could serve to increase the level of engagement from patient and public representatives. As a result of our experiences in these feasibility studies, it would be intended to extend patient and public involvement (PPI) throughout the whole development and implementation of a definitive trial, including, design of the research (through contribution to proposal and protocol development); formulation of patient information materials (through consultation with PPI representatives); and, trial management (through membership of TSC), reporting and dissemination (through contribution to trial publication and presentation to lay audiences).
Randomisation
To ensure concealment of allocation, randomisation was undertaken by an internet-accessed computer randomisation system held by the Newcastle Clinical Trials Unit (NCTU); randomisation between intervention and control was 1 : 1 and was stratified by centre using random block length. The recruiter logged into the system by password and site identification code and then entered the date of birth and initials of the patient they were randomising. The system responded with a unique randomisation number and the trial arm to which the patient had been randomised. This was viewed on the screen and backed up with an e-mail confirmation to the individual carrying out the randomisation and also copied to the central trial office.
Sample size
The sample size for the external pilot trial was determined pragmatically, using the recommended minimum of 30 participants per arm. 55 We aimed to recruit 60 participants per trial arm to investigate both the distribution and key parameters of the outcome measures. Previous trials in the area of pelvic floor dysfunction, including investigation,29 surgical44,56,57 and non-surgical treatments58 suggested average attrition rates of 13% (7–20%) between identification and randomisation, 16% (6–20%) between randomisation and treatment, and 13% (9–20%) between treatment and follow-up at 6 months. Taking the more pessimistic figure in each case, we estimated that a total of 240 eligible patients should be approached allowing for 50% overall attrition. The recruiting units collectively undertook 540 relevant procedures per year; therefore, identifying 240 eligible women within the originally planned 9-month recruitment period should not have presented undue difficulty.
Blinding
It was neither feasible nor appropriate to blind participants or clinicians (investigating and operating) as to the allocation of investigation strategy.
Interventions
Patients were randomised [documented on case report form (CRF) – ‘visit 1′ – see Appendix 19c] to receive either:
-
no IUT: basic clinical assessment supplemented by non-invasive tests as directed by the clinician; these included frequency/volume charting or bladder diary, mid-stream urine culture, urine flow rate and residual urine volume measurement (by ultrasound), or
-
IUT: basic clinical and non-invasive tests as above, plus invasive urodynamic testing. Dual-channel subtracted cystometry with simultaneous pressure/flow voiding studies is the most commonly applied technique in the evaluation of patients prior to surgery for SUI in most centres. Videourodynamics and ambulatory bladder pressure monitoring are used as alternative or additional invasive tests in some units; these tests were also permissible within the pilot trial, at the discretion of the clinician.
Given the pragmatic nature of the pilot trial, we were not prescriptive about which tests were carried out, nor indeed about exactly how they were carried out, save for the expectation that they would conform to good urodynamic practices. 59,60 For this reason, we do not feel it appropriate to give a detailed description of the interventions in accordance with the TIDieR guidelines. 61 Readers wishing to understand more about the interventions might refer to standard texts,62,63 or to standardisation documents. 59,60
Further investigation was undertaken, where appropriate, at the same visit or a later one, as per local custom, and the treatment plan formulated.
Outcome measures
In INVESTIGATE-I, we were primarily concerned with determining the number of eligible patients in each unit, and the rates of patient recruitment, randomisation, retention and response. We also piloted the collection of the outcome measures for a future definitive trial, to assess data yield (e.g. percentage of recruited participants returning completed questionnaires) and quality (e.g. completeness and consistency of responses within returned questionnaires). This information was collected to guide the choice and mode of administration of questionnaires and data collection tools in a future definitive trial.
In a definitive trial, we would intend to use patient reported outcome measures as opposed to the more traditional methods for the quantification of leakage as the primary outcome. Our preferred primary outcome, rehearsed in the pilot trial, was:
-
the combined symptom score of the ICIQ Female Lower Urinary Tract Symptoms (ICIQ-FLUTS) questionnaire at 6 months after treatment. 43
Secondary outcomes for the future trial, also rehearsed in the pilot, comprise:
-
general health questionnaire [Short Form 12 version 2 (SF-12v2) © Health Survey 1994, 2002; QualityMetric Incorporated and Medical Outcomes Trust]. 64
-
quantification of urinary leakage [3-day bladder diary and ICIQ Urinary Incontinence Short Form (ICIQ-UI SF)]. 65
-
prevalence of symptomatic ‘de novo’ functional abnormalities including VD and detrusor overactivity (DO) (using subscales in ICIQ-FLUTS,43 with cystometric investigation in symptomatic patients).
-
the impact of urinary symptoms on QoL [ICIQ Lower Urinary Tract Symptoms Quality of Life (ICIQ-LUTSqol) questionnaire and UDI]. 38,66
-
EuroQol-5D (EQ-5D)-3 Level (EQ-5D-3L). 67
-
utility values from the EQ-5D-3L and from Short Form 6D (SF-6D) [the latter derived from responses to the Short Form 12 (SF-12)]. 68
-
costs to the NHS.
-
QALYs derived from both EQ-5D-3L and the SF-6D.
-
incremental cost per QALY with QALYs based on both EQ-5D-3L and SF-6D data.
Further details of the scoring systems applied to the ICIQs and UDI are given in Appendix 16.
Thus, within INVESTIGATE-I, we piloted the collection of the above outcome measures, to assess data yield (e.g. percentage of recruited participants returning completed questionnaires) and quality (e.g. completeness and consistency of responses within returned questionnaires). This information can then be used to guide the choice and mode of administration of questionnaires and data collection tools in a future definitive trial.
Baseline assessment of study outcomes
Following consent and randomisation, patients were given a pack of baseline study outcome questionnaires; these were presented in the order ICIQ-FLUTS, ICIQ-LUTSqol, ICIQ-UI SF, UDI, EQ-5D and SF-12 (see Appendix 17). Participants were asked to complete the questionnaires at home, within 2 weeks of receipt, and to post their responses, using the addressed prepaid envelope provided, to the Trial Manager at the NCTU.
Subsequent treatment within the trial
Following investigation, it would be expected that women randomised to the control (no IUT) arm of the study, i.e. those treated on the basis of clinical assessment and non-invasive tests (documented on CRF – ‘visit 2’ – see Appendix 19e), would undergo surgical treatment (documented on CRF – ‘visit 4′ – see Appendix 19g) (Figure 1). Given the pragmatic nature of the study, the choice of operation was left to the individual surgeon and patient; as only primary cases were included, it was anticipated that this would be either a retropubic or transobturator foramen mid-urethral tape procedure in most cases. Those randomised to the intervention (IUT) arm, i.e. undergoing invasive urodynamic testing (documented on CRF – ‘visit 3′ – see Appendix 19f), had similar surgical treatment when urodynamic stress incontinence (USI) was confirmed (documented on CRF – ‘visit 4’). Where other diagnoses were identified following investigation, alternative treatments might be offered (documented on CRF – ‘visit 5′ – see Appendix 19i); these included bladder retraining, anti-muscarinic drug treatments, neuromodulation, botulinum toxin injections (where DO was diagnosed), or clean intermittent self-catheterisation (where a VD was identified). Exactly which of these interventions was chosen depended on what conservative treatments had been used before entry into the trial; for example, if a woman had tried PFMT plus bladder retraining before entry, she was likely to be offered anti-muscarinic drug treatment if DO was shown on invasive urodynamic testing. In all centres the treatment algorithm employed was in keeping with the then current NICE recommendations (2006). 17 In some cases where mixed abnormalities were reported, women would first undergo one or more of these interventions (to stabilise bladder overactivity, or improve voiding efficiency) and then proceed to surgery for SUI. After the participant entered the study the clinician remained free to recommend alternative investigation or treatment to that specified in the protocol at any stage if they felt it to be in the participant’s best interest. In these cases the participant remained in the study for the purposes of follow-up and data analysis.
FIGURE 1.
Diagram of the study design and the flow of participants. a, The choice of non-surgical treatments is left to the clinician and patient, but may include bladder retraining, drugs, neuromodulation, botulinum toxin injections, and clean intermittent catheterisation, depending on IUT results, local protocols and previous trials of therapy. NAD, no abnormality detected; USI, urodynamic stress incontinence.
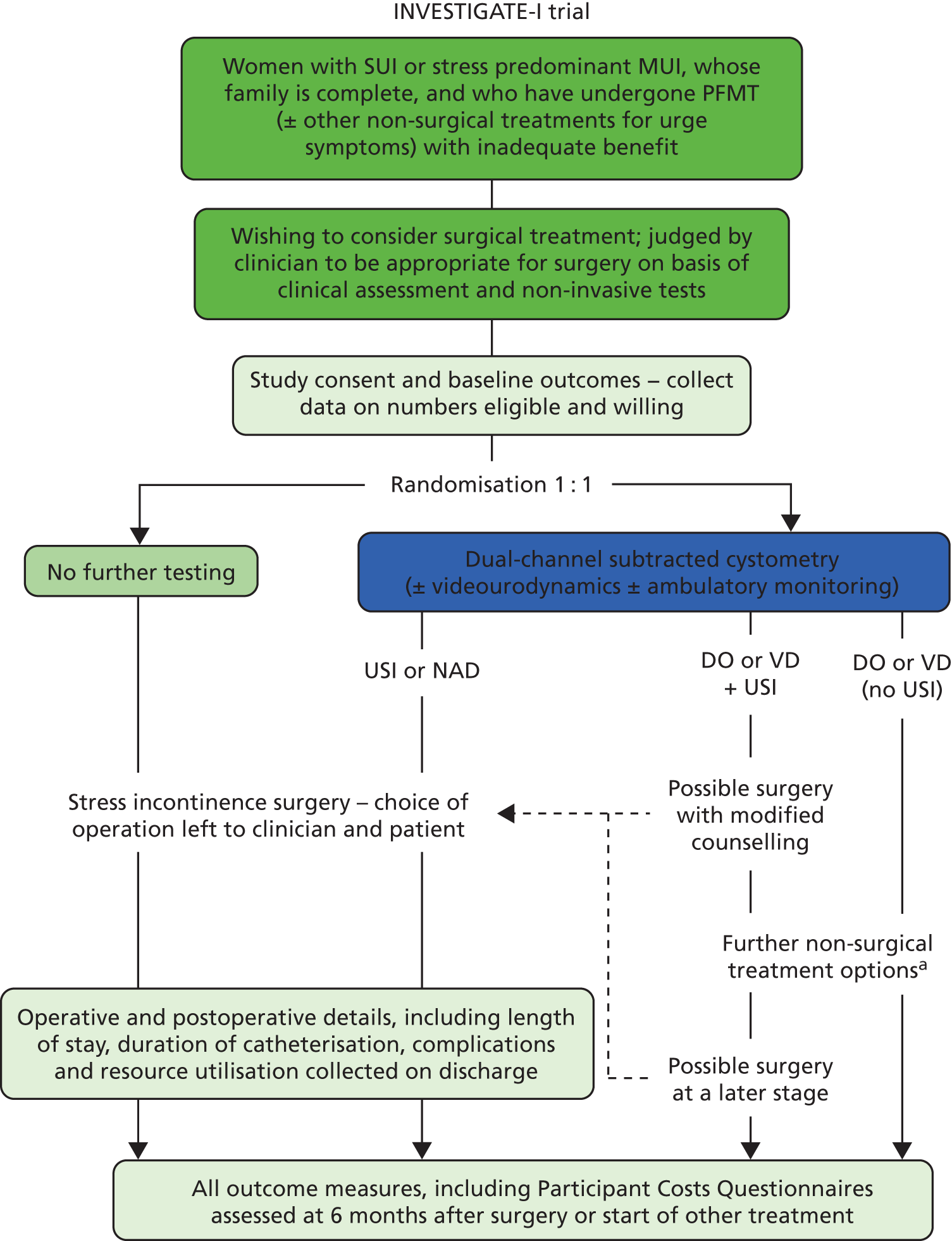
Any adverse events (AEs) or serious adverse events (SAEs) were documented in the CRF (see Appendices 20 and 21); SAE notification was faxed to the NCTU within 24 hours.
Follow-up
Clinicians arranged postoperative follow-up or other outpatient review, as per their normal practice and timing (documented on CRF – ‘visit 6’ – see Appendix 19h). Patients were sent a pack of follow-up study outcome questionnaires along with a prepaid envelope by the NCTU at 6 months after the start of treatment (i.e. 6 months after the date of surgery, or the start of any non-surgical intervention, or period of ‘watchful waiting’). This applied in all cases, even where surgery was undertaken as a secondary intervention in those women initially treated non-surgically. They were asked to complete the questionnaires at home and return them to the NCTU. Those failing to return questionnaires within 1 month of the initial request were contacted by the appropriate research nurse by telephone, to encourage responses. In the last 9 months of the study, the option of completing the questionnaire over the telephone with the research nurse was also given to participants during the reminder telephone call. If the questionnaires were not returned after the telephone reminder, a further copy of the questionnaires was mailed to the participant with a reminder letter. The patients withdrawal or completion of study follow-up was documented on CRF – ‘visit 7’ (see Appendix 19k).
Governance and regulatory arrangements
Ethics and research and development approval
The conduct of this study was in accordance with the ethical principles set out in the Declaration of Helsinki (2008)69 and the Research Governance Framework for Health and Social Care (second edition, 2005). 70 Application for ethical approval was made through the Integrated Research Application System, and a letter of favourable ethical opinion was obtained from Newcastle & North Tyneside 1 Research Ethics Committee (REC) on 6 January 2011 – reference number 10/H0906/76. Application for research and development (R&D) approval was made via the NIHR Co-ordinated System for gaining NHS Permissions (CSP) – reference number 62776. Global sign-off for R&D approval was received on 15 March 2011, with local R&D approvals of the protocol between 28 March 2011 and 9 August 2011.
Changes to the original protocol
Two amendments were made to the original protocol. The first (v1.1; dated 1 July 2011) added detail to the protocol on the collection of health economics outcomes from the study, and included the Participant Costs Questionnaire (PCQ) and the trial management plan as appendices. The second (v1.2; dated 12 September 2012) related to a change in the method for follow-up reminders; as in the original protocol, a telephone reminder would be undertaken by the local site research nurse if the questionnaire had not been returned after 4 weeks; in addition, if after the telephone reminder, the questionnaires were not returned within a further 2 weeks, a further copy of the questionnaires would be mailed to the participant with a reminder letter. Both amendments were approved by the study sponsor and by Newcastle & North Tyneside 1 REC.
Clinical trials agreements
Clinical trials agreements (CTAs), using the model for non-commercial research within the health service, were established for the various study sites with sponsor Newcastle upon Tyne Hospitals NHS Foundation Trust (NuTH) between 25 May and 15 August 2011. Site initiation visits took place between 30 March and 17 June 2011, with the start to recruitment permitted (‘green light’ to proceed) only after completion of all regulatory approvals and site initiation, between 14 June and 15 August 2011 (for the primary sites) (Table 1).
| Site | Type | R&D approval | CTA | Site initiation | Site open to recruitment | |
|---|---|---|---|---|---|---|
| Newcastle | Primary | Full | 28 March 2011 | 25 May 2011 | 17 June 2011 | 18 June 2011 |
| Gateshead | Primary | Full | 29 March 2011 | 14 June 2011 | 13 April 2011 | 15 June 2011 |
| Wansbeck | Primary | Full | 25 July 2011 | 28 July 2011 | 21 April 2011 | 29 July 2011 |
| Sheffield | Primary | Full | 7 July 2011 | 29 June 2011 | 28 April 2011 | 8 July 2011 |
| Swansea | Primary | Full | 23 June 2011 | 30 June 2011 | 8 April 2011 | 1 July 2011 |
| Leicester | Primary | Full | 9 August 2011 | 15 August 2011 | 30 March 2011 | 16 August 2011 |
| South Tees | Secondary | Full | 9 July 2012 | 17 July 2012 | 2 August 2012 | 3 August 2012 |
| South Tyneside | Secondary | PIC | 17 September 2012 | 23 August 2012 | 18 September 2012 | |
| Sunderland | Secondary | PIC | 30 May 2012 | 30 May 2012 | 31 May 2012 | |
Following approval of an extension to recruitment, one additional recruiting site and two PIC sites were approved.
Consent
Women were informed about the detail of the study with the brief and more detailed PIS, and by discussion with the local research nurse independently of the clinician responsible for ongoing care, and of staff undertaking investigations. Patients provided written informed consent. Separate written consent to take part in the qualitative patient interview substudy was sought, and it was made clear to trial participants that they were under no obligation to take part in the qualitative substudy (see Chapter 7).
To inform the design of a future definitive trial, those who declined to participate in the trial or who withdrew prematurely were asked for their reasons for withdrawal, but the right to refuse to participate without giving reasons was also respected.
Other regulatory arrangements
Other regulatory arrangements for the study, relating to confidentiality, indemnity, on-site monitoring and internal audit, day-to-day management by the Trial Management Group (TMG), and oversight by the TSC and Data Monitoring and Ethics Committee (DMEC) are described in detail in the study protocol, the latest version of which is available on the NIHR Journals Library website.
Encouraging participant recruitment
It is unclear why some trials appear to recruit more easily to target than others. 71 Factors related to the research question itself (e.g. being a cancer or drug trial), related to trial organisation (e.g. having a dedicated trial manager) and related to treatment access (e.g. involving a treatment only available within the trial) have been shown to be associated with more successful recruitment. Other strategies have been employed to encourage recruitment for example, newsletters and mailshots, although it has not been shown unequivocally that these are causally linked to changes in recruitment. 72,73 One of the aims of a feasibility study is to investigate how well units are able to identify eligible trial participants and recruit them. A number of additional strategies were employed within INVESTIGATE-I, partly to encourage recruitment in the pilot itself, but more particularly to rehearse them as possible strategies within a future definitive trial. These included the establishment of additional study sites, and strategies to facilitate communication and staff engagement.
Additional study sites
Following approval by HTA of a 9-month extension to recruitment (initially 2 months, then a further 7 months), one additional full recruiting site (South Tees Hospitals NHS Foundation Trust) and two PIC sites (Sunderland Royal Hospital and South Tyneside District General Hospital) were established.
Communication and staff engagement
Study acronym and logo
The full study title incorporated the underlying clinical question addressed, the overall study methodology, and identified the trial element as having a randomised design. The short title (INVasive Evaluation before Surgical Treatment of Incontinence Gives Added Therapeutic Effect?), study acronym (INVESTIGATE-I), and logo (incorporating a graphic image of dripping and calmed water) did not simply provide a random selection of letters from the full title to give a snappier sound bite. They each serve to complement and ‘stand for’ the full title, add to the effectiveness and understanding of the message, by a representational name and image. They were used in all communications to trial staff, regulatory authorities, other clinicians, patients (other than when site specific stationery was appropriate) and the trial website, and as such provided a constant identity for the INVESTIGATE studies. The importance of such study ‘branding’ is emphasised in the STEPS study. 72
Basecamp
Basecamp© (developed by www.37signals.com, Chicago, IL, USA) is a web-based project management application; this was used for communication and document sharing between members of the TMG and between the TMG and other members of the research team, particularly those based outwith Newcastle.
Trial website
A trial website (www.investigate-trial.com) was developed early during the project as a means of increasing awareness of the INVESTIGATE studies within the research team, for other staff at the various study sites, for clinical colleagues who might be interested to learn more and perhaps to collaborate in a future trial, and for the general public. It includes information about the current study (INVESTIGATE-I), including the justification, methodology, and recruitment progress; reference is also made to a possible future definitive study; trial governance arrangements are included, with appropriate links; PISs and study newsletters (v.i.) are available for download, and there are links to open-access publications from the INVESTIGATE studies; contact details for the research team and site clinical staff are also provided. All sections are updated as necessary, and a ‘latest news’ section on the home page gives topical issues regarding trial progress and staff development (see Appendix 22).
Study newsletters
Study newsletters were circulated to the research team every 2 to 3 months during the trial period. These covered, information about the study, including protocol amendments; progress with the trial and interview studies; feedback from the TSC and DMEC meetings, and from the trial funder; details of study presentations and publications; personal news from the trial team (see Appendix 23). Progress with recruitment against target was included using the ‘Recruitment to Target’ (RtT) thermometer (©Newcastle upon Tyne Hospitals NHS Foundation Trust and Newcastle University) (v.i.).
Recruitment updates
At times when recruitment was a particularly acute concern, a weekly progress update was distributed to the research team. These were employed in particular during the 2-month provisional extension (during which 50% recruitment had to be completed in order to secure a further extension) and in the final weeks of recruitment. These updates were limited to information on recruitment, but showed this by centre, with a competitive edge to encourage peer rivalry; progress was illustrated in a variety of ways [e.g. using a ‘league table’; the RtT thermometer; black, red, amber, green (‘BRAG’) flag status (black = zero recruits, red = > 24% off target, amber = 15–24% off target, green = 0–14% off target); and countdown clock and filmstrip graphics (see Appendix 24)].
‘Recruitment to Target’ thermometer
During the construction of the study website, a graphic device described as the ‘RtT (Recruitment to Target) thermometer’ was developed to help trial staff visualise progress against recruitment target numbers and timing. This was initially formatted in Microsoft PowerPoint (Microsoft Corporation, Redmond, WA, USA), as a simple graphic image illustrating actual recruitment against recruitment target (including a BRAG status pennant, colour-coded as above), and time expired of the available study recruiting time, in the form of a ‘maximum and minimum thermometer’. It was then converted into hypertext markup language (HTML) code that can easily be adapted for use in any trial, and added into a website (see Appendix 25). The use of the device was subsequently disseminated for use in other studies via the NCTU trial managers and Comprehensive Local Research Network (CLRN).
Statistical analysis
Given that this was a pilot trial, the statistical analysis was largely descriptive in nature and provided estimates of key trial parameters to inform the design of the future definitive trial. Screening and recruitment numbers were summarised in a CONSORT diagram. In addition, screening numbers were summarised by centre and recruitment numbers were summarised by month and centre. Results were reported at baseline and 6-month follow-up time points. Data analysis was by intention to treat.
Categorical variables were summarised as percentages per category by treatment arm. Questionnaire scale and subscale totals and continuous variables were summarised by mean and standard deviation (SD) and 5-number summaries [median, interquartile range (IQR) and range] by treatment arm and time point. The burden of missing data were summarised by response rates for each variable. No data imputation was attempted for any outcome [other than in the economic evaluation (see Chapter 4)]. The summary statistics for the primary outcome measure were combined with the target/minimum clinically important difference (MCID) and recruitment, retention and response rates to inform the sample size for a future definitive trial.
Results
Screening
Overall, 771 patients were identified by research nurses from clinic notes and correspondence as being potential recruits into the study, and were sent the PISs. Of those screened, 284 were deemed eligible for the trial, giving a ‘screen positive’ rate of 37%. The reasons for non-eligibility of screened patients are shown in Table 2; most commonly these were patients not having undergone supervised PFMT prior to referral (14%), urgency or urgency predominant MUI (12%), failure to attend clinic appointments (11%), patients not wishing to participate (8%), patients with prolapse requiring treatment (5%), or clinicians feeling that surgery was not appropriate (5%). Although the reasons for non-eligibility varied between centres, the overall figures were obviously heavily weighted by the centre screening the highest number of patients. In some units, patients not wishing to participate made up a larger proportion of screening failures; overall however, 78% of eligible women identified were recruited into the study.
| Code | Description | Newcastle | Gateshead | Wansbeck | Leicester | Swansea | South Tees | Sheffield | Total | Per cent |
|---|---|---|---|---|---|---|---|---|---|---|
| 11 | Patient has not undergone a course of PFMT | 74 | 10 | 15 | 4 | 2 | 0 | 0 | 105 | 14 |
| 14 | Urge incontinence | 85 | 2 | 5 | 0 | 0 | 0 | 0 | 92 | 12 |
| 13 | Other (give details) | 52 | 5 | 17 | 8 | 2 | 0 | 2 | 86 | 11 |
| 15 | Did not attend clinic | 54 | 24 | 2 | 0 | 1 | 0 | 0 | 81 | 11 |
| 7 | Patient does not wish to participate, include reason if offered | 13 | 9 | 10 | 10 | 14 | 0 | 3 | 59 | 8 |
| 1 | Symptomatic uterovaginal prolapse requiring treatment | 22 | 0 | 8 | 4 | 1 | 5 | 0 | 40 | 5 |
| 8 | Clinician feels surgery inappropriate | 1 | 26 | 1 | 2 | 1 | 8 | 0 | 39 | 5 |
| 9 | Patient does not wish surgery | 5 | 11 | 4 | 0 | 1 | 0 | 0 | 21 | 3 |
| 2 | Previous surgery for UI or POP | 7 | 0 | 1 | 0 | 0 | 0 | 1 | 9 | 1 |
| 3 | Urodynamic investigation within the last 3 days | 5 | 1 | 1 | 0 | 0 | 0 | 0 | 7 | 1 |
| 10 | Patient does not consider her family is complete | 4 | 2 | 0 | 0 | 0 | 0 | 0 | 6 | 1 |
| 4 | Neurological disease causing UI | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| 5 | Current involvement in a conflicting research study | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | Unable to give competent informed consent | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 12 | Study not discussed at clinic visit (please give reason) | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 3 | 0 |
| Recruited | 75 | 50 | 37 | 20 | 17 | 15 | 8 | 222 | 29 | |
| Total screened | 399 | 140 | 103 | 48 | 39 | 28 | 14 | 771 | 100 | |
| Per cent of screened women recruited | 19 | 36 | 36 | 42 | 44 | 54 | 57 | 29 | ||
| Per cent of eligible women recruited | 84 | 85 | 76 | 67 | 55 | 100 | 73 | 78 |
The numbers screened at individual centres varied between 14 and 399; the percentage of eligible women recruited varied between 55% and 100%, but did not show an obvious trend with the number screened (see Table 2). Although a single code was assigned to each patient, it is possible that codes were used variably in the different centres, and that there may have been some inconsistency or overlap in the use of codes. For example, it is possible that ‘patient does not wish to participate’ could overlap with ‘patient does not wish surgery’. While the centres screening larger numbers of women also recruited larger numbers (Figure 2), the conversion from screening to recruitment decreased as the screening number increased (Figure 3).
FIGURE 2.
Numbers screened and recruited at individual centres.

FIGURE 3.
Number and percentage recruited to trial by number screened (shown on log scale) at each centre. The graph plots the percentage and number of participants recruited. The number of participants is indicated by black filled circles and the percentage of participants by green open circles.

Quality assurance of screening processes
In view of the variations seen in screening and recruitment between centres, a quality assurance check was made with PIs and recruiting staff in each unit, confirming that all employed a similar practice in relation to screening; this was stated in the study protocol as follows:
Potential trial recruits will be identified by the study research nurses prior to attending new or follow-up appointments for SUI or MUI in the clinics run by the unit clinical leads. The Patient Information Sheet (PIS) will be sent out with new appointments or with a reminder letter to attend follow-up appointments; this will allow any questions that the woman may have about the study to be addressed at the one visit. Those declining to take part would undergo further investigation and or treatment as appropriate at the same visit. Those agreeing to take part will sign a study consent form.
If other potential recruits become apparent only at the time of a clinic visit, they will be invited to take part in the study, and will be given verbal and written information. After a period of at least 24 hours to read, consider and discuss the information with family and/or friends, the research nurse will contact the patient by telephone to respond to any further outstanding questions, and review their decision regarding involvement.
It is possible that women referred to the various centres were in some way different, although the workload and nature of the units would have made this unlikely. The number of women screened in individual centres would therefore be expected to be determined by the ease with which PIs or research nurses were able to identify eligible women from referral letters or hospital notes. It might also be a reflection of their individual position on the spectrum of sensitivity versus specificity, that is whether they perceived the priority as being only to screen those women who were very likely to be eligible, or saw the importance of ‘broadening the net’ to include all potential recruits. In view of the pragmatic intention of the pilot trial, we did not give a strict definition to the term ‘stress predominant MUI’, preferring to leave it to clinicians to determine this within their own practices. It is possible that individual screeners or PIs may have interpreted the term variably, such that this also could have contributed to variation in recruitment rates.
In order to explore these issues further, a series of 20 identical vignettes were distributed to screeners via the trial Basecamp site. These were mainly based on actual GP referral letters, although in some cases with modifications to cover the range of inclusion and exclusion criteria. Sixteen vignettes mentioned one or more definite inclusion criteria (SUI, stress predominant MUI, PFMT, family complete); the other four had possible inclusions (UI but not specified as to stress or urgency related; ‘wet all the time’; PFMT mentioned but level of supervision not specified). Four had definite exclusions (previous pelvic floor surgery; neurological disease; urgency predominant MUI) and 15 contained possible exclusions (unsupervised PFMT). The vignettes are shown in Appendix 26.
Each of the 11 screeners from the seven full recruiting units graded the vignettes independently, on the basis of the following instructions:
What we want to know is whether you would have considered each of the women described in the letters to be a potential recruit for the INVESTIGATE-I trial. In other words, if you had reviewed the letter at the time that we were looking for recruits into the trial would you, or would you not, have sent out a Patient Information Leaflet (PIL) to the woman described (please tick either ‘Yes’ or ‘No’ in the blue boxes on the score sheet). It would also be helpful to know whether you feel the decision is clear-cut, or borderline (by ticking in the appropriate green box), and something of why you made that decision (by ticking the orange boxes and adding comments as appropriate), on the score sheet provided.
The possible responses were, therefore, clear cut ‘Yes’ (Y); borderline ‘Yes’ (?Y); borderline ‘No’ (?N), or clear cut ‘No’ (N). Each screener’s grading for the various vignettes is shown in Table 3. For six vignettes, everyone agreed that the patient was eligible; for one, all agreed that the patient was not eligible; the grade breakdown for the remainder was mixed.
| Vignette no. | Centre and screener | % Yes | Grade breakdown | Majority grade | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WGH | RVI | LE | SHE | RVI | WGH | ST | SW | QEH | LE | SW | ||||||||
| 1 | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 2 | 2 | Y | ?Y | ?N | N | ||||
| 8 | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 100 | 11 | 0 | 0 | 0 | Y | Y |
| 14 | Y | Y | Y | Y | Y | Y | Y | Y | ?Y | Y | Y | 100 | 10 | 1 | 0 | 0 | Y | Y |
| 17 | Y | Y | Y | ?Y | Y | Y | ?Y | Y | Y | Y | Y | 100 | 9 | 2 | 0 | 0 | Y | Y |
| 4 | Y | Y | ?Y | ?Y | ?Y | Y | Y | Y | Y | Y | Y | 100 | 8 | 3 | 0 | 0 | Y | Y |
| 7 | Y | Y | Y | ?Y | Y | ?Y | ?Y | Y | Y | ?Y | ?Y | 100 | 6 | 5 | 0 | 0 | Y | Y |
| 1 | ?Y | Y | ?Y | ?Y | ?Y | Y | ?Y | ?Y | ?Y | Y | Y | 100 | 4 | 7 | 0 | 0 | ?Y | Y |
| 3 | Y | Y | ?Y | Y | Y | Y | Y | Y | Y | ?Y | ?N | 91 | 8 | 2 | 1 | 0 | Y | Y |
| 20 | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | 91 | 10 | 0 | 0 | 1 | Y | Y |
| 6 | ?Y | ?Y | ?Y | ?Y | ?Y | Y | ?Y | ?Y | ?Y | Y | ?N | 91 | 2 | 8 | 1 | 0 | ?Y | Y |
| 12 | ?Y | ?Y | Y | ?Y | ?Y | ?Y | N | ?Y | ?Y | ?Y | N | 82 | 1 | 8 | 0 | 2 | ?Y | Y |
| 16 | Y | ?Y | Y | ?Y | ?Y | N | ?N | ?Y | Y | N | Y | 73 | 4 | 4 | 1 | 2 | Y/?Y | Y |
| 9 | ?Y | ?Y | ?Y | ?Y | ?N | Y | ?Y | ?N | N | ?Y | ?Y | 73 | 1 | 7 | 2 | 1 | ?Y | Y |
| 2 | Y | ?Y | ?Y | ?Y | Y | Y | N | N | Y | N | ?N | 64 | 4 | 3 | 1 | 3 | Y | Y |
| 11 | ?Y | ?Y | N | ?Y | ?Y | Y | N | ?Y | N | N | N | 55 | 1 | 5 | 0 | 5 | ?Y/N | Y |
| 5 | ?Y | N | ?Y | ?Y | ?N | ?N | Y | N | N | N | ?N | 36 | 1 | 3 | 3 | 4 | N | N |
| 18 | N | ?Y | N | N | N | ?N | ?Y | ?Y | N | ?Y | N | 36 | 0 | 4 | 1 | 6 | N | N |
| 19 | N | ?Y | N | ?N | ?Y | Y | Y | N | N | ?N | N | 36 | 2 | 2 | 2 | 5 | N | N |
| 10 | ?N | N | ?Y | ?N | ?N | N | N | N | N | ?N | ?N | 9 | 0 | 1 | 5 | 5 | ?N/N | N |
| 13 | ?Y | N | N | ?N | ?N | ?N | N | N | N | N | N | 9 | 0 | 1 | 3 | 7 | N | N |
| 15 | N | N | N | N | N | N | N | N | N | N | N | 0 | 0 | 0 | 0 | 11 | N | N |
| % Yes (Y or ?Y) | 80 | 80 | 75 | 75 | 70 | 65 | 65 | 65 | 60 | 60 | 45 | |||||||
| ‘Yes’ when majority ‘No’ | 2 | 2 | 2 | 1 | 1 | 1 | 3 | 1 | 0 | 1 | 0 | |||||||
| ‘No’ when majority ‘Yes’ | 0 | 0 | 1 | 0 | 1 | 2 | 4 | 2 | 2 | 3 | 5 | |||||||
| Total ‘disagreements’ | 2 | 2 | 3 | 1 | 2 | 3 | 7 | 3 | 2 | 4 | 5 | |||||||
Assuming a majority decision was one in which the ‘%Yes’ grading was above or below 50% (irrespective of whether the decisions were considered to be clear-cut or borderline), in other words that the majority felt that the patient described in the vignette was (or was not) eligible for screening, then there were 34 occasions on which one or more individual screeners ‘disagreed’ with the majority. The number of ‘disagreements’ varied across the 11 screeners; this ranged from one screener who dissented from the majority decision for 1/20 vignettes to another who dissented in 7/20 vignettes. Table 3 reports these separately as occasions on which the screener said ‘Yes’ when the majority said ‘No’, and those on which the screener said ‘No’ when the majority said ‘Yes’. The former judgement would lead some patients being deemed eligible and sent the PIS when they might be found to be ineligible at a later appointment (i.e. erring on the side of over-inclusiveness at the screening stage). The latter judgement would lead to some potential recruits not being invited to take part in the trial when they would have been eligible. Given the difficulty in recruiting patients in some centres, it is the latter judgement that should be minimised within trials.
Free-text comments were sought to help clarify the screeners’ decisions. These included:
Vignette 2 (majority view – clear-cut ‘yes’) Four comments, all along the same line,that is the letter did not specifically mention PFMT; they appeared, therefore, to have taken the view that it had not been done rather than ‘might have been done’.
Vignette 3 (majority view – clear-cut ‘yes’) One comment: ‘Need to check notes and if documented that pt [patient] has stress incontinence and received PFMT then would be eligible but if it is only on patient’s say so then further investigations would be beneficial to give a diagnosis.’ The vignette did specifically state: ‘Complaining of stress incontinence. She denies any urgency and says that when she coughs and laughs she passes small amounts of urine.’ As well as: ‘She has tried pelvic floor exercises including an internal pelvic toner to no avail’.
Vignette 5 (majority view – borderline ‘yes’) Five comments, essentially taking the view that the vaginal laxity was the greater problem and the incontinence less of an issue. Physiotherapy report (included with referral) states: ‘She has attended on 3 occasions in total and reports that her continence symptoms have become more manageable but not completely resolved’ and ‘on examination there was no significant vaginal or uterine vaginal or uterine descent’.
Vignette 6 (majority view – borderline ‘yes’) One comment, essentially same as vignette 3 (same screener).
Vignette 9 (majority view – borderline ‘yes’) Three comments, all along the same lines – no supervised physiotherapy, and best assess later.
Vignette 10 (majority view – borderline ‘no’) One comment, highlighted the patient had previous surgery and may not have done PFMT, but indicated ‘yes’ to screening.
Vignette 11 (majority view – borderline ‘yes’) Four comments, indicating need for PFMT (this was not mentioned in the letter, although it did state that the patient wished to consider surgery); two also referred to young age and therefore uncertainty of family plans.
Vignette 12 (majority view – borderline ‘yes’) Two comments, one relating to complaint of ‘dragging sensation’, one to need for PFMT (not mentioned in letter).
Vignette 13 (majority view – clear-cut ‘no’) One comment on definition of ‘repair operation’.
Vignette 16 (majority view – borderline ‘yes’) Three comments both relating to the history of OAB. Letter states:
She has been treated in the past for urinary problems, and has had a number of medications, and says that she even had Botox injections to her bladder. Since these latter interventions her symptoms have changed somewhat; previously she reported both urge and stress incontinence, but now she is left with only the stress element, with leakage occurring particularly on coughing or sneezing, or when she is at the gym.
Vignette 18 (majority view – clear-cut ‘no’) Most referred to lack of supervised PFMT specifically indicated in letter. One commented that ‘Patient may feel she has done 6 months physio and it may be agreed that surgery is an appropriate treatment now’.
Vignette 19 (majority view – clear-cut ‘yes) One comment related to treatment for rectal (not uterovaginal) prolapse.
Vignette 20 (majority view – borderline ‘yes’) One comment referred to need for pad at night and that this could represent OAB or fistula.
Hence the majority of the explanatory comments related to missing information, most commonly whether or not PFMT had been undertaken at all, or whether or not it had been supervised. A number also related to reports of vaginal laxity or dragging sensation, although information about clinical findings in relation to POP either was not present or was negative. There were also uncertainties or misinterpretations of the significance of descriptions of rectal prolapse and repair surgery.
Differences between units were not clearly apparent and the relationship between disparity in screening categorisation in this exercise and screening to recruitment ratios in the trial itself was also not obvious.
In a future trial it would be appropriate to:
-
ensure that definitions in inclusion and exclusion criteria are clarified (e.g. prolapse symptoms vs. clinical findings vs. need for treatment; rectal vs. uterovaginal prolapse, etc.)
-
suggest that where information is missing from referral letters it is assumed the patient might be eligible, and therefore that the default action should be to send out the PIS, unless obvious exclusions are specified
-
arrange group training/standards setting sessions for PIs and research nurses to agree a consistent approach to the screening and recruitment process across sites.
Recruitment
Monthly recruitment by centre is shown in Table 4 and Figure 4, for the initial recruitment period (up to the end of March 2012) and for the period of extension (from April to December 2012). Regulatory requirements took approximately 3 months longer than anticipated and, as a result, recruitment targets were revised. Even once all approvals were in place, and all sites in a position to start recruitment, the rate of accrual was significantly less than required with some sites unable to identify any patients for some weeks after opening to recruitment. Although proposed in 2011,74 the NIHR 70-day benchmark for recruitment was not published until 2012 and was not a required of CLRNs until after 2013. 75 Nevertheless, several steps were introduced to improve recruitment, including the incorporation of additional clinicians on two of the existing sites and the establishment of an additional full recruiting site and two PIC sites. A request was made for a 9-month unfunded extension to the recruitment period.
| Year.Quarter | Period | Date | Predicted recruitment | Actual end of month recruitment by site | Totals | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Original | First revised | Second revised | Newcastle | Gateshead | Wansbeck | South Tees | PIC sites | Leicester | Swansea | Sheffield | Monthly | Cumulative | |||
| 1.2 | Initial recruitment period | 1 April 2011 | 0 | ||||||||||||
| 1.2 | 1 May 2011 | 20 | |||||||||||||
| 1.2 | 1 June 2011 | 40 | |||||||||||||
| 1.3 | 1 July 2011 | 60 | 0 | 0 | 1 | 1 | 1 | ||||||||
| 1.3 | 1 August 2011 | 90 | 20 | 1 | 6 | 0 | 0 | 6 | 7 | ||||||
| 1.3 | 1 September 2011 | 120 | 40 | 11 | 7 | 1 | 0 | 0 | 0 | 12 | 19 | ||||
| 1.4 | 1 October 2011 | 150 | 60 | 12 | 8 | 1 | 0 | 0 | 0 | 2 | 21 | ||||
| 1.4 | 1 November 2011 | 180 | 90 | 14 | 8 | 1 | 0 | 0 | 0 | 2 | 23 | ||||
| 1.4 | 1 December 2011 | 210 | 120 | 20 | 8 | 2 | 2 | 1 | 0 | 10 | 33 | ||||
| 2.1 | 1 January 2012 | 240 | 150 | 38 | 20 | 8 | 4 | 3 | 3 | 0 | 5 | 38 | |||
| 2.1 | 1 February 2012 | 180 | 54 | 23 | 13 | 8 | 10 | 3 | 0 | 19 | 57 | ||||
| 2.1 | 1 March 2012 | 210 | 70 | 26 | 18 | 12 | 11 | 5 | 2 | 17 | 74 | ||||
| 2.2 | 1 April 2012 | 240 | 87 | 26 | 24 | 16 | 13 | 6 | 2 | 13 | 87 | ||||
| 2.2 | +2 months | 1 May 2012 | 104 | 34 | 27 | 18 | 13 | 9 | 4 | 18 | 105 | ||||
| 2.2 | 1 June 2012 | 121 | 41 | 35 | 20 | 0 | 16 | 10 | 4 | 21 | 126 | ||||
| 2.3 | Extension period +7 months | 1 July 2012 | 138 | 47 | 35 | 21 | 0 | 18 | 10 | 4 | 9 | 135 | |||
| 2.3 | 1 August 2012 | 155 | 51 | 40 | 28 | 0 | 0 | 19 | 11 | 4 | 18 | 153 | |||
| 2.3 | 1 September 2012 | 172 | 55 | 41 | 28 | 0 | 0 | 19 | 13 | 4 | 7 | 160 | |||
| 2.4 | 1 October 2012 | 189 | 59 | 43 | 31 | 5 | 0 | 19 | 14 | 6 | 17 | 177 | |||
| 2.4 | 1 November 2012 | 206 | 62 | 45 | 31 | 10 | 0 | 20 | 14 | 6 | 11 | 188 | |||
| 2.4 | 1 December 2012 | 223 | 66 | 47 | 33 | 11 | 0 | 20 | 16 | 7 | 12 | 200 | |||
| 3.1 | 1 January 2013 | 240 | 75 | 50 | 37 | 15 | 0 | 20 | 17 | 8 | 22 | 222 | |||
FIGURE 4.
Monthly total recruitment numbers. The original and revised predictions of overall recruitment are shown as continuous and dashed lines, and actual recruitment in histogram; the overall ‘BRAG’ flag or ‘RtT’ status is also illustrated.

The number of participants recruited per recruiting month (i.e. between the completion of all site-specific regulatory requirements and the end of the study) varied between 0.4 and 3.9 per month at the original sites (mean 1.9); at the additional full recruiting site this figure was 2.5 per month; the PICs did not identify any potentially eligible patients for referral to a recruiting site in the 8 months that they were active.
Randomisation
Of the 284 women screened positive, 222 agreed to randomisation into the trial, giving a trial consent rate of 78%. This recruitment total (222) represented 93% of the planned sample size (240) for the pilot trial. Overall, 110 women were randomised to the control or no IUT arm and 112 to the intervention or IUT arm. Immediately after randomisation it became apparent that one woman in the no arm was ineligible for the trial and she was withdrawn leaving a total of 221 eligible patients randomised (109 in the no IUT arm and 112 in the IUT arm).
The screening, recruitment, randomisation and trial follow-up are summarised in the CONSORT diagram shown as Figure 5.
FIGURE 5.
Trial CONSORT flow diagram. DNA, did not attend.
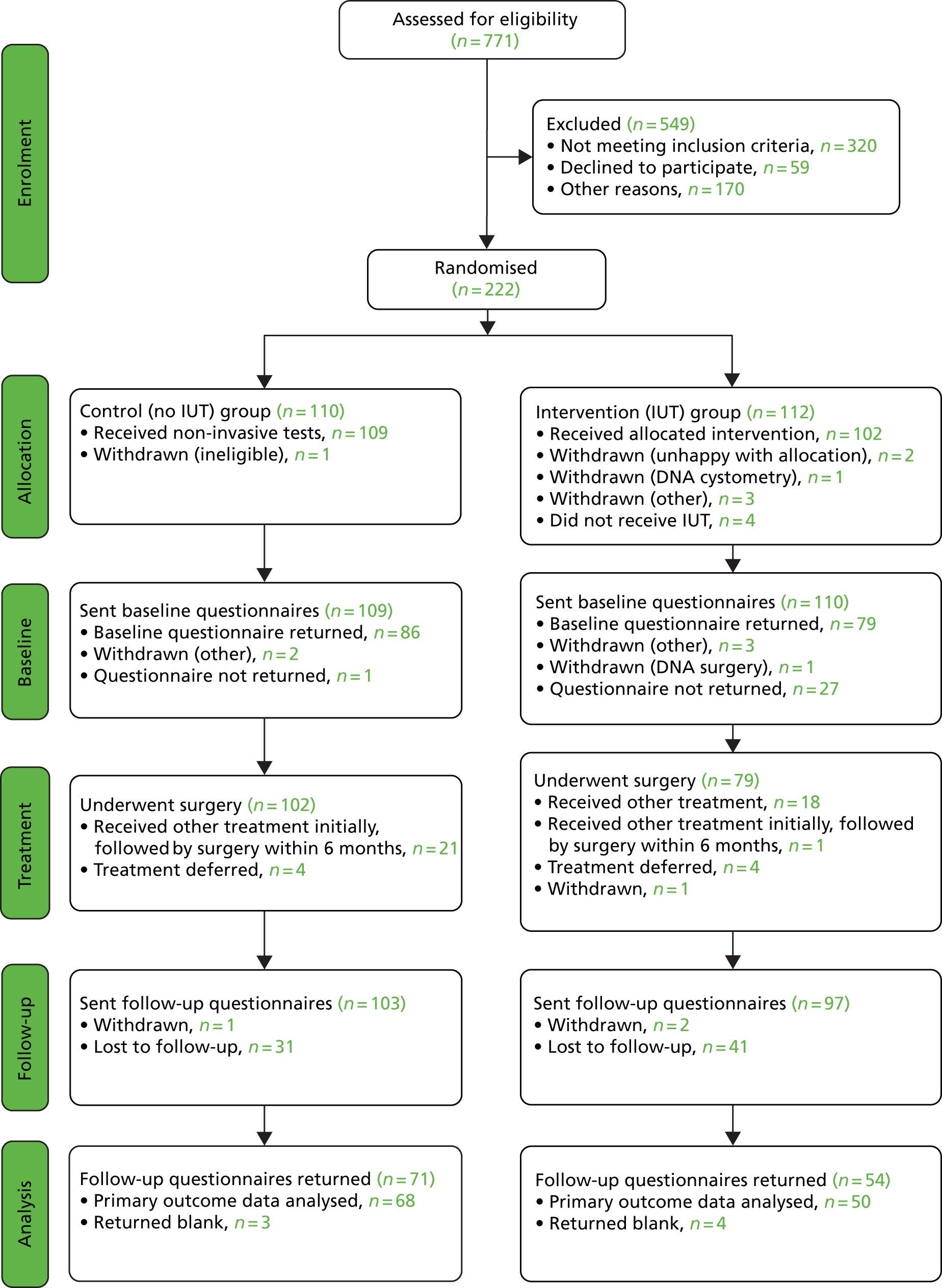
Retention
Demographic data and details of any subsequent treatment for incontinence were collected from hospital notes and CRFs (see Appendices 19a–k), and women were asked to complete questionnaires on clinical outcomes (see Appendix 17) and a 3-day bladder diary (see Appendix 18) at baseline and 6 months after the start of treatment (i.e. 6 months after the date of surgery, or the start of any non-surgical intervention, or period of ‘watchful waiting’). Baseline questionnaires were sent to 219 women and returned by 165; this represented a response rate of 75% overall, 72% in the IUT arm and 79% in the no IUT arm. At 6 months after treatment, questionnaires were returned by 63% (125/200) of those who were sent questionnaires at follow-up; 56% (54/97) in the IUT arm and 69% (71/103) in the no IUT arm.
Six women returned a completely blank questionnaire booklet (three in each study arm); one further woman in the IUT arm completed only the EQ-5D and SF-12 questionnaires, but for the purpose of return of primary outcomes this was categorised as returning a blank questionnaire, as the ICIQ-FLUTS was not completed. This information is summarised in the trial CONSORT diagram Figure 5. Six of the seven women who returned blank questionnaires reported ‘no significant urinary symptoms’ on the follow-up CRF (visit 6). The same six either annotated the front of their questionnaire or bladder diary, or in one case telephoned the NCTU indicating that they had not had urinary problems since their surgery. One of the women who returned a blank questionnaire reported ‘significant urinary symptoms’ on the follow-up CRF; she also annotated her diary to indicate that there had been little change in her urinary symptoms following her surgery, although she improved slightly with subsequent drug treatment.
The progress of recruitment and follow-up is shown in Figure 6. It also shows the anticipated follow-up at 6 months, although these predictions do not make allowance for individual centre waiting times for investigation and surgery; this was certainly an error that would require attention in planning a future definitive trial. The actual times at which follow-up questionnaires were posted out to participants (at 6 months after surgery or start of treatment) do reflect these waits, and illustrate an average additional delay to follow-up of approximately 4 months. Figure 6 also illustrates the actual rate at which follow-up questionnaires were received back at the NCTU. At the time of closure of the database for final analysis, 125 follow-up questionnaires had been received (exceeding the target of 120), although as per the CONSORT diagram, seven of these omitted primary outcome data – ICIQ-FLUTS total score.
FIGURE 6.
Actual recruitment with anticipated and actual receipt of follow-up questionnaires. Lines illustrate actual monthly recruitments, anticipated follow-up (based on 6 months after recruitment), anticipated follow-up (based on questionnaires posted – 6 months after treatment), and actual follow-up (outcome questionnaires received back).

Demographic data
Table 5 provides the demographic data by trial arm; the consistency of these variables between IUT and no IUT arms confirms the validity of the randomisation process.
| Characteristic | Combined | IUT | No IUT | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||||||
| Ethnicity | ||||||||||||
| Caucasian | 216 | 98 | 110 | 99 | 106 | 97 | ||||||
| Black | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Asian | 4 | 2 | 1 | 1 | 3 | 3 | ||||||
| Other | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Characteristic | Combined | IUT | No IUT | |||||||||
| n | Mean (SD) | Median (IQR) | Range | n | Mean (SD) | Median (IQR) | Range | n | Mean (SD) | Median (IQR) | Range | |
| Age (years) | 222 | 47.0 (9.7) | 46.5 (40–52) | 24–77 | 112 | 47.1 (9.5) | 46.5 (40.0–52.0) | 29–75 | 110 | 46.8 (10.0) | 46.5 (40.0–52.0) | 24–77 |
| BMI (kg/m2) | 208 | 28.4 (5.9) | 27.5 (24.1–31.5) | 18–55 | 106 | 29.3 (6.5) | 28.3 (24.4–33.7) | 20–55 | 102 | 27.4 (5.0) | 26.8 (23.9–30.7) | 18–45 |
Completeness of data collection
The questionnaire packs contained four condition-specific scales (ICIQ-FLUTS, ICIQ-UI SF, ICIQ –LUTSqol and UDI), two general health scales (EQ-5D and SF-12) and a 3-day bladder diary. When the questionnaire packs were reviewed, it was evident that not all patients had completed all scales in their entirety, although missing values within individual scales were few. The columns to the right-hand side of Table 6 show the proportion of each questionnaire or subscale that could be calculated from the data provided.
| Questionnaire | IUT | No IUT | Overall completion ratea | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6 months | Baseline | 6 months | Baseline | 6 monthsb | ||||||||||||||||
| n | Mean (SD) | Median (IQR) | Range | n | Mean (SD) | Median (IQR) | Range | n | Mean (SD) | Median (IQR) | Range | n | Mean (SD) | Median (IQR) | Range | Partial n (%) | Complete n (%) | Partial n (%) | Complete n (%) | ||
| ICIQ-FLUTS overall score | 77 | 16.9 (5.7) | 17 (13–21) | 4–37 | 47 | 9.2 (7.5) | 8 (4–12) | 0–38 | 85 | 16.4 (6.3) | 16 (11–21) | 3–34 | 66 | 6.9 (5.0) | 6 (3–9) | 0–26 | 3 (2) | 162 (98) | 5 (4) | 113 (90) | |
| Subscales | Filling | 78 | 4.4 (2.3) | 4 (3–6) | 0–11 | 48 | 3.0 (2.3) | 3 (1–4) | 0–11 | 85 | 4.0 (2.6) | 3 (2–6) | 0–10 | 66 | 2.4 (1.8) | 2 (1–3) | 0–8 | 2 (1) | 163 (99) | 3 (3) | 114 (91) |
| Voiding | 79 | 1.8 (2.0) | 1 (0–3) | 0–9 | 49 | 2.0 (2.0) | 2 (0–3) | 0–9 | 86 | 1.5 (1.7) | 1 (0–2) | 0–9 | 68 | 2.3 (2.1) | 2 (0–4) | 0–8 | 0 (0) | 165 (100) | 1 (1) | 117 (94) | |
| Incontinence | 78 | 10.8 (3.3) | 11 (8–13) | 2–19 | 49 | 4.0 (4.9) | 3 (1–5) | 0–20 | 86 | 10.8 (3.6) | 11 (8–13) | 2–19 | 68 | 2.3 (3.1) | 2 (0–3) | 0–16 | 1 (1) | 164 (99) | 1 (1) | 117 (94) | |
| ICIQ-UI SF | 78 | 14.0 (3.7) | 14 (12–16) | 4–21 | 49 | 5.3 (6.0) | 3 (0–8) | 0–21 | 85 | 14.1 (3.8) | 15 (12–17) | 4–21 | 65 | 3.3 (4.5) | 1 (0–4) | 0–18 | 2 (1) | 163 (99) | 3 (3) | 114 (91) | |
| ICIQ-LUTSqol | 73 | 46.8 (10.9) | 47 (40–52) | 26–74 | 44 | 26.7 (12.3) | 22 (20–28) | 19–76 | 84 | 48.5 (11.7) | 46 (39–58) | 30–72 | 65 | 25.3 (9.6) | 21 (20–28) | 19–65 | 8 (5) | 157 (95) | 9 (7) | 109 (87) | |
| UDI overall score | 64 | 133.3 (43.5) | 133.5 (109–159) | 25–245 | 42 | 49.1 (44.1) | 37.1 (17–69) | 0–191 | 74 | 130.1 (43.8) | 125.8 (96–162) | 50–227 | 59 | 33.9 (39.7) | 24.2 (4–46) | 0–150 | 27 (16) | 138 (84) | 17 (14) | 101 (81) | |
| Subscales | Stress | 76 | 82. 9 (21.0) | 87.5 (75–100) | 25–100 | 50 | 24.5 (26.1) | 25 (0–38) | 0–100 | 80 | 80.2 (21.2) | 87.5 (63–100) | 38–100 | 65 | 18.1 (27.0) | 0 (0–25) | 0–100 | 6 (4) | 156 (95) | 2 (2) | 115 (92) |
| Irritative | 71 | 38.4 (25.4) | 33.3 (17–54) | 0–100 | 48 | 16.5 (20.5) | 8.3 (0–25) | 0–100 | 80 | 33.7 (24.3) | 31.3 (17–50) | 0–92 | 64 | 10.0 (13.3) | 4.2 (0–17) | 0–54 | 13 (8) | 151 (91) | 6 (5) | 112 (90) | |
| Obstructive/discomfort | 68 | 17.6 (17.6) | 13.6 (6–23) | 0–73 | 43 | 10.9 (15.1) | 4.6 (0–18) | 0–64 | 80 | 14.8 (14.2) | 13.6 (3–20) | 0–61 | 64 | 8.9 (12.4) | 2.3 (0–14) | 0–57 | 17 (10) | 148 (90) | 11 (9) | 107 (86) | |
At baseline, the ICIQ-FLUTS overall score could be calculated for 98% of subjects who had returned the questionnaire pack and was partially completed by only 2%. No patients provided an incomplete submission for all subscales of this instrument, and the completion rates were therefore slightly higher for individual ICIQ-FLUTS subscales than for the overall score. The completion rates for the ICIQ-UI SF and ICIQ-LUTSqol scales were 99% and 95%, respectively, and for the UDI scale was 84%. For the latter three scales, there were occasional questionnaire packs in which the whole scale had not been completed at baseline.
At 6 months after treatment for incontinence, the ICIQ-FLUTS overall score could be calculated for 90% subjects who had returned the questionnaire pack and was only partially completed for 4%. The completion rates for the ICIQ-UI SF and ICIQ-LUTSqol scales were 91% and 87%, respectively, and for the overall UDI scale was 81%. For all four scales, there were occasional questionnaire packs in which the whole scale had not been completed at 6 months. The distribution of missing data on these scales and subscales is described in Table 7. It was found that 6% of all items making up the ICIQ-FLUTS overall score were missing. Most women had no missing items, but there were seven women who failed to complete any item in this scale. There were similar low percentages of missing items in the other three scales, and the numbers of women who failed to complete any item on a scale were two for ICIQ-UI SF, four for ICIQ-LUTSqol and one for UDI. These high completion rates suggest that there were few problems with individual items on a scale for women in the pilot trial.
| Questionnaire | Items in scale | Missinga scale items,b n (%) | Missing items per woman median (IQR)c | Rangec | |
|---|---|---|---|---|---|
| ICIQ-FLUTS overall score | 12 | 95 (6.3) | 0 (0–0) | 0–12 | |
| Subscales | Filling | 4 | 36 (7.2) | 0 (0–0) | 0–4 |
| Voiding | 3 | 23 (6.1) | 0 (0–0) | 0–3 | |
| Incontinence | 5 | 36 (5.8) | 0 (0–0) | 0–5 | |
| ICIQ-UI SF | 3 | 28 (7.5) | 0 (0–0) | 0–3 | |
| ICIQ-LUTSqol | 19 | 153 (6.4) | 0 (0–0) | 0–19 | |
| UDI overall score | 19 | 163 (6.9) | 0 (0–0) | 0–19 | |
| Subscales | Stress | 2 | 18 (7.2) | 0 (0–0) | 0–2 |
| Irritative | 6 | 51 (6.8) | 0 (0–0) | 0–6 | |
| Obstructive/discomfort | 11 | 94 (6.8) | 0 (0–0) | 0–11 | |
The right-hand columns of Table 8 show how many items on the 3-day bladder diary were available. Only 148 women returned the diary at baseline (68% of those women sent baseline questionnaires). Data were available in 99% of the returned diaries to compute the average number of visits to the bathroom during the day and night, although the average number of pads used in 24 hours was only available on 65% of returned diaries. This latter variable was not completed at all in 30% of diaries and was partially available in 5%.
| 3-day diary | IUT | No IUT | Overall completion ratea | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6 months | Baseline | 6 months | Baseline | 6 months | ||||||||||||||||
| n | Mean (SD) | Median (IQR) | Range | n | Mean (SD) | Median (IQR) | Range | n | Mean (SD) | Median (IQR) | Range | n | Mean (SD) | Median (IQR) | Range | Partial n (%) | Complete n (%) | Partial n (%) | Complete n (%) | ||
| Average visits to bathroom | Day time | 69 | 7.4 (2.2) | 7.3 (5.7–8.3) | 3–14 | 44 | 6.8 (24.5) | 5.8 (5.0–7.3) | 3–34 | 79 | 7.6 (3.0) | 6.7 (5.7–8.3) | 3–20 | 61 | 6.2 (1.3) | 6.3 (5.3–7) | 4–9 | 2 (1) | 146 (99) | 0 (0) | 105 (53) |
| Night time | 69 | 0.9 (0.7) | 0.7 (0.3–1.3) | 0–4 | 32 | 1.3 (1.0) | 1 (1.0–1.7) | 0–4 | 79 | 0.8 (0.7) | 0.7 (0.0–1.3) | 0–3 | 41 | 1.1 (0.6) | 1 (1.0–1.3) | 0–3 | 2 (1) | 146 (99) | 0 (0) | 105 (53) | |
| Average pads used in 24 hours | 45 | 2.8 (2.0) | 2.3 (1.7–4.0) | 0–10 | 21 | 1.7 (4.9) | 0 (0–1) | 0–22 | 59 | 2.7 (1.9) | 2.7 (1.0–3.7) | 0–8 | 26 | 0.5 (1.0) | 0.0 (0.0–0.7) | 0–4 | 8 (5) | 96 (65) | 13 (7) | 42 (21) | |
At 6 months after treatment, 105 diaries were returned (53% of those sent the 6-month questionnaire pack). Data were available on the average number of visits to the bathroom on all of these, but only 40% of the 105 diaries that were returned completed the diary for the number of pads used; 12% partially completed it and 48% provided no data on pad use at all. Additionally, 10 women returned blank bladder diaries, five in each study arm. Five of these women annotated the diaries to indicate that they did not have current symptoms (four in the no IUT arm and one in the IUT arm).
The response rate at both time points for the bladder diary was low, and data on the number of pads used was a particular problem using this diary format. It should be noted that ‘pad use’ was recorded in a single box at the bottom of the diary sheets (see Appendix 18) and may have been more easily overlooked by patients than other items on the diary.
Comparison of responders and non-responders to the six-month questionnaire
In view of the unexpectedly high rate of non-response to the 6-month questionnaires, a limited comparison of responders and non-responders was made on the basis of their clinical follow-up. A total of 135 women had a postoperative follow-up visit documented on the study database; 93 actually attended an outpatient clinic and 42 had a review by telephone (routine practice in three of the centres).
During clinical follow-up, 17 women reported significant urinary symptoms, and 13 had significant clinical findings on examination (including four tape extrusions); none of those with positive examination findings reported symptoms. The symptoms specified by 13 of these 17 women included, one to three episodes of UTI (three women); OAB symptoms (five women); other incontinence symptoms (three women); suprapubic pain (one woman); and only one woman reported persistence of SUI.
Of the 125 women who returned follow-up questionnaires at 6 months after treatment, 83 had clinical follow-up, of whom 12/83 (14.5%) described significant urinary symptoms, and 9/83 (10.8%) had significant examination findings, at the clinical review. Of the 81 who failed to return follow-up questionnaires at 6 months following treatment, 52 had clinical follow-up, of whom 5/52 (9.6%) described significant urinary symptoms, and 4/52 (7.7%) had significant examination findings. While those women returning the 6-month questionnaires somewhat more often had significant symptoms or examination findings at earlier clinical review than those failing to do so, the numbers do not allow meaningful statistical comparison.
Questionnaire data
Baseline
Table 6 shows the distribution of the questionnaire scales at baseline by trial arm. The ICIQ-FLUTS total score has a possible range of 0–48. The distribution of ICIQ-FLUTS total score at baseline was fairly symmetrical with a mean of 16.9 (SD 5.7) in the IUT arm and 16.4 (SD 6.3) in the no IUT arm. The distributions of the other scales and subscales were similarly well matched between the IUT and no IUT arms and were fairly symmetrical.
Six-month follow-up
Table 6 also shows the distribution of the questionnaire scales at 6-month follow-up by trial arm. The distribution of ICIQ-FLUTS total score at follow-up had a mean of 9.2 in the IUT arm and 6.9 in the no IUT arm. The distribution of ICIQ-UI SF (possible values 0–21) had a mean of 5.3 in the IUT arm and 3.3 in the no IUT arm. The distribution of ICIQ-LUTSqol (possible values 19–76) had a mean of 26.7 in the IUT arm and 25.3 in the no IUT arm. The distribution of UDI overall score (possible values 0–300) had a mean of 49.1 in the IUT arm and 33.9 in the no IUT arm. For all scales, typical scores were much lower than at baseline. The distribution of the ICIQ-FLUTS total scores at 6-month follow-up by trial arm is shown in the upper part of Figure 7. The shape of these distributions at 6 months was generally positively skewed, which reflects the fact that many women had experienced considerable relief from their initial symptoms, but some had not.
FIGURE 7.
Distribution of ICIQ-FLUTS total score at 6 months and changes between baseline and 6 months by trial arm. Graphs by randomisation group.
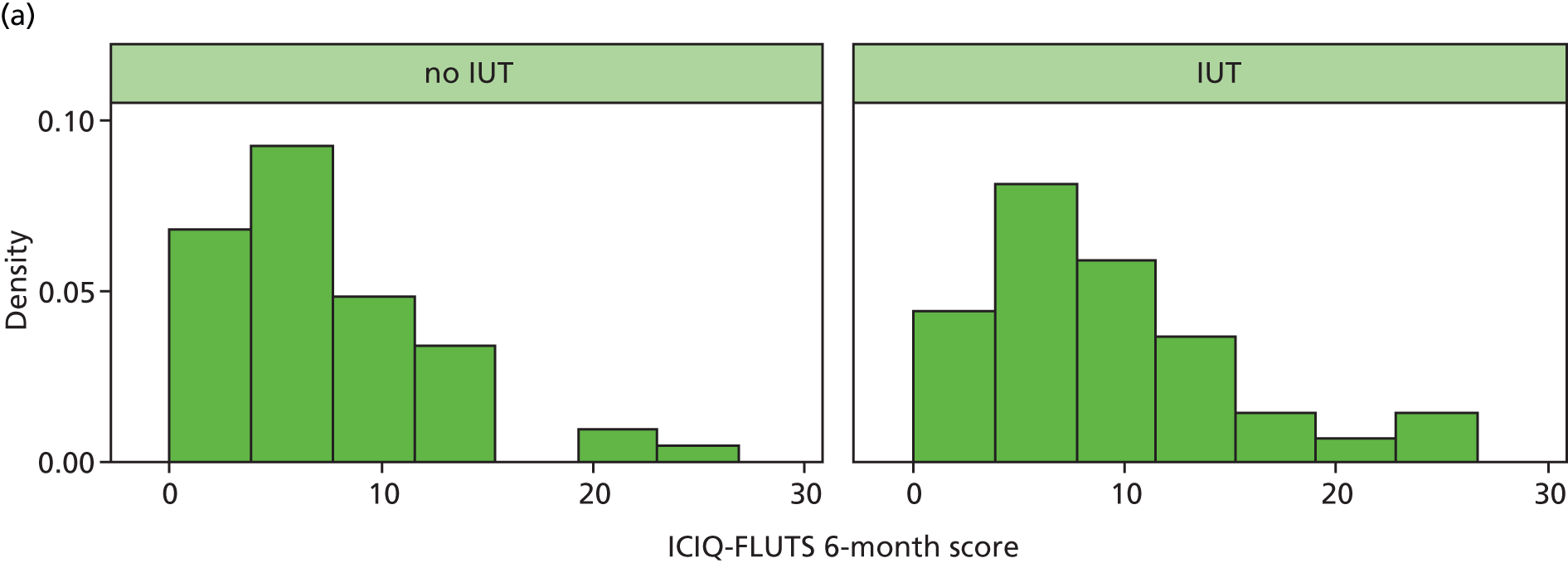
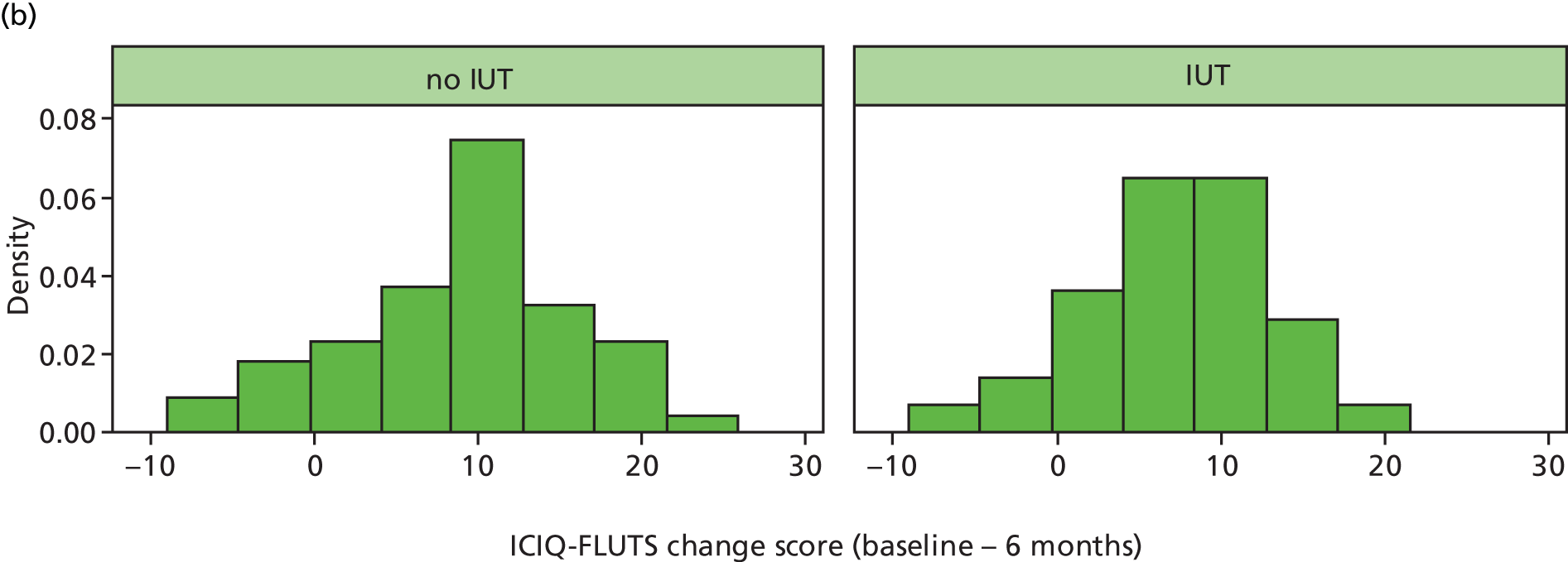
It is difficult to interpret any difference in mean scores between baseline and 6 months follow-up from Table 6, because many of the women who provided baseline data failed to do so at 6 months. Table 9 shows the distribution of the paired changes in scale scores for those women who had completed both questionnaires. It can be seen that the mean change in ICIQ-FLUTS total score was 7.8 in the IUT arm and 9.3 in the no IUT arm. The distribution of the change scores for the ICIQ-FLUTS total scores is shown in the lower part of Figure 7. Typically, there was a marked drop in these scores over 6 months, but little difference in the mean changes between the trial arms. This pattern was also seen in the other four scales. However, no formal comparison between arms is appropriate in a pilot study.
| Questionnaire | n | Mean (SD) | Median (IQR) | Range |
|---|---|---|---|---|
| IUT arm | ||||
| ICIQ-FLUTS – overall score | 31 | 7.8 (5.9) | 7 (4–15) | –5–18 |
| ICIQ-UI SF | 34 | 8.9 (6.0) | 11 (4–13) | –3–16 |
| ICIQ-LUTSqol | 29 | 20.0 (11.4) | 23 (12–28) | –5–41 |
| UDI – overall score | 27 | 79.5 (45.5) | 75 (51–122) | –21–161 |
| No IUT arm | ||||
| ICIQ-FLUTS – overall score | 48 | 9.3 (7.3) | 10.5 (5.5–15.0) | –9–22 |
| ICIQ-UI SF | 49 | 10.2 (5.8) | 11 (6–15) | –4–21 |
| ICIQ-LUTSqol | 47 | 23.7 (13.9) | 23 (14–35) | –3–50 |
| UDI – overall score | 41 | 94.1 (55.3) | 92 (70–117) | –66–221 |
Bladder-diary data
Table 8 shows the results from the 3-day bladder diaries by trial arm.
Baseline
The mean number of daytime bathroom visits was 7.4 in the IUT arm and 7.6 in the no IUT arm. The average number of night-time bathroom visits was 0.9 in the IUT arm and 0.8 in the no IUT arm. The average number of pads used in 24 hours was 2.8 in the IUT arm and 2.7 in the no IUT arm. The two arms were well balanced at baseline.
Six-month follow-up
The mean number of daytime bathroom visits was 6.8 in the IUT arm and 6.2 in the no IUT arm. The average number of night-time bathroom visits was 1.3 in the IUT arm and 1.1 in the no IUT arm. The average number of pads used in 24 hours was 1.7 in the IUT arm and 0.5 in the no IUT arm. The two arms at had similar distributions at this time point.
Treatment data
Table 10 gives details of the surgical treatment received by the trial subjects for their UI. In the IUT arm, 80% received surgery, compared with 95% in the no IUT arm. For those undergoing surgery, additional details are given further down the table. It can be seen that the distributions of operation type, grade of surgeon, anaesthetic technique and use of antibiotic prophylaxis were similar between the trial arms.
| Item | Combined arms, n (%) | IUT arm, n (%) | No IUT arm, n (%) |
|---|---|---|---|
| Operation carried out | 185 (88) | 82 (80) | 103 (95) |
| Grade of surgeon | |||
| Consultant | 147 (79) | 66 (82) | 81 (78) |
| ST6–7 | 14 (7.5) | 6 (7) | 8 (8) |
| ST3–5 | 3 (2) | 2 (2) | 1 (1) |
| ST1–2 | 1 (0.5) | 1 (1) | 0 (0) |
| Other | 17 (9) | 6 (7) | 11 (11) |
| Unknown | 3 (2) | 1 (1) | 2 (2) |
| Operation undertakena | |||
| Retropubic tape | 159 (86) | 70 (86) | 89 (87) |
| Transobturator tape | 24 (13) | 11 (14) | 13 (13) |
| Single-incision tape | 0 (0) | 0 (0) | 0 (0) |
| Colposuspension | 1 (1) | 0 (0) | 1 (1) |
| Fascial sling | 0 (0) | 0 (0) | 0 (0) |
| Periurethral injection | 6 (3) | 3 (4) | 3 (3) |
| Other | 1 (1) | 1 (1) | 0 (0) |
| Type of anaesthetic | |||
| General | 53 (29) | 23 (28.5) | 30 (29.5) |
| Spinal | 9 (5) | 6 (7.5) | 3 (3) |
| Epidural | 0 (0) | 0 (0) | 0 (0) |
| Local or local + sedation | 121 (66) | 52 (64) | 69 (67.5) |
| Unknown | 2 (1) | 1 (1) | 1 (1) |
| Antibiotic prophylaxis given | 168 (92) | 74 (92.5) | 94 (92) |
Details of the non-surgical treatments are given in Tables 11 and 12. One woman in the no IUT arm and four (4%) in the IUT arm decided to defer any treatment initially (designated as ‘watchful waiting’). A further 15 women (15%) in the IUT arm underwent lifestyle changes or other non-surgical treatments. As routine incontinence management, more than one lifestyle change was commonly documented, and other non-surgical treatments were often used in combination; 28 treatments were applied in these 15 women. Despite (unsuccessful) completion of a course of supervised PFMT being an inclusion criterion for the trial, six women underwent further PFMT alone (two) or in combination with other non-surgical treatments (four).
| Treatment | Number | |
|---|---|---|
| Bladder retraining | 8 | |
| Lifestyle changes | 9 | |
| Reduce caffeine | 4 | |
| Weight reduction | 2 | |
| Double voiding | 1 | |
| Increase fluids | 1 | |
| Unspecified | 1 | |
| Antimuscarinic drugs | 8 | |
| Solifenacin | 7 | |
| Extended-release oxybutynin | 1 | |
| PFMT | 6 | |
| Watchful waiting | 5 | |
| No. | Study arm | Bladder retraining | Lifestyle changes | Antimuscarinic drugs | PFMT | Watchful waiting |
|---|---|---|---|---|---|---|
| 1 | IUT | ✓ | ✓ | ✓ | ||
| 2 | IUT | ✓ | ✓ | ✓ | ||
| 3 | IUT | ✓ | ||||
| 4 | IUT | ✓ | ✓ | ✓ | ||
| 5 | IUT | ✓ | ✓ | ✓ | ✓ | |
| 6 | IUT | ✓ | ✓ | |||
| 7 | No IUT | ✓ | ||||
| 8 | IUT | ✓ | ||||
| 9 | IUT | ✓ | ||||
| 10 | IUT | ✓ | ||||
| 11 | IUT | ✓ | ✓ | |||
| 12 | IUT | ✓ | ||||
| 13 | IUT | ✓ | ||||
| 14 | IUT | ✓ | ||||
| 15 | IUT | ✓ | ||||
| 16 | IUT | ✓ | ✓ | |||
| 17 | IUT | ✓ | ✓ | |||
| 18 | IUT | ✓ | ||||
| 19 | IUT | ✓ | ||||
| 20 | IUT | ✓ |
Adverse events and serious adverse events
Only two SAEs were reported. One woman in the IUT arm experienced bleeding from suburethral incision 12 days after surgery; she required readmission and vaginal packing. An operative vaginal injury had been identified and repaired primarily in the same woman (reported separately as an AE). One woman in the control arm developed breast cancer shortly after the operation and subsequently underwent a mastectomy. Both women had received their allocated treatment prior to the SAE; while one clearly related to the incontinence treatment, neither event was categorised as being related to the trial intervention (invasive urodynamic testing).
In addition, 23 AEs in 22 women were reported to the NCTU; these included three operative bladder injuries (3/185 = 1.6% perforation rate) and two vaginal injuries. Six episodes of urinary tract infection (UTI) were reported, two in the IUT arm, and four in the no IUT arm; all occurred following surgery, and none immediately after invasive urodynamic testing. Of the 22 patients in whom events were reported, 12 were randomised to the IUT arm and 10 to the no IUT arm; while most or all of these AEs could have been related to surgery, none were categorised as relating to the trial intervention itself (invasive urodynamic testing).
Key messages
-
All the proposed trial processes and outcome measures likely to be required in a future definitive RCT of invasive urodynamic testing versus clinical assessment and non-invasive testing were effectively rehearsed within the pilot study.
-
Greater clarity in the inclusion and exclusion criteria and an ‘assume eligibility’ approach might assist trial staff to identify potential recruits more appropriately.
-
Thirty-seven per cent of women screened were eligible for inclusion in the trial and 78% of eligible women identified in each centre were recruited.
-
Regulatory requirements took longer than anticipated. In addition, waiting times between initial assessment, trial recruitment, invasive urodynamic testing and admission for surgery varied between units. These delays would need to be more adequately addressed within the management plan.
-
The recruitment numbers at individual centres ranged from 12% to 225% of the original planned centre targets, which will need to be considered in the definitive trial planning. The start up of an additional recruitment site improved recruitment, but establishment of PICs was not helpful in this particular study
-
Regular communication through a range of media appears to have a positive effect on trial staff engagement, although the impact of this on recruitment is difficult to evaluate.
-
Baseline questionnaires were completed by 75% of participants, although only 63% of those sent follow-up questionnaires (56% of those recruited) returned them at 6 months after start of treatment.
-
A small number of participants returned blank follow-up questionnaires, although most of these included some annotation to indicate that the respondent was free from symptoms. Changes to the design of the booklets might obviate this problem in a future trial.
-
Although the rate of return of questionnaires was lower than expected, missing data within the returned booklets were few. The ICIQ-FLUTS overall score could be calculated for 98% of subjects at baseline; ICIQ-UI SF, ICIQ-LUTSqol and overall UDI score could be calculated for 99%, 95% and 84%, respectively. At 6 months, not only were fewer questionnaires returned, but the completion rates were also slightly lower at 90%, 91%, 87% and 81%, respectively. We would rationalise the questionnaires used in a future trial.
-
Bladder diaries were less often completed than questionnaire booklets; only 68% of the baseline diaries and 53% of those sent follow-up diaries at 6 months were returned. Although patterns of voiding could be ascertained from all diaries returned, only 65% at baseline and 40% at 6 months provided information on pad use. If bladder diaries were to be used in a future trial, modification to the recording of pad use should be considered.
-
A small number of women elected to defer treatment, although 95% of women in the control arm underwent surgical treatment, compared with 80% in the IUT arm, reflecting changes in the management plan and the use of further non-surgical treatments following invasive urodynamic testing.
-
Few AEs were recorded during the study; these were evenly spread across the study arms. Most were expected AEs related to treatment, and none were related to the trial intervention itself. The effectiveness of our detection of UTI following invasive urodynamic testing might be questioned, and should be modified for a future definitive trial.
-
The pilot trial was a crucial element of the package of feasibility studies, and identified several important issues for the planning of a future definitive trial.
Chapter 4 Economic evaluation
Methods
The economic evaluation rehearsed data collection and analyses to inform a definitive trial. In terms of data collection, we assessed the ease of collecting information and consistency of resource use in administration of the IUTs, other tests, surgical and non-surgical treatments, and piloted the use of economic data collection instruments. In terms of data analysis, a cost–utility analysis was performed where health state utilities for each participant were based on data obtained using self-administered SF-12 and EQ-5D-3L questionnaires completed by participants at baseline and at 6-month follow-up. Stochastic and deterministic sensitivity analyses were used to assess the importance of statistical and other uncertainties.
Cost data collection
We considered costs to both the NHS and the patients. The main components of the costs to the NHS were the intervention (IUTs) and subsequent surgery, which included staff costs and equipment, consumables and overhead costs associated with these tests and surgeries. Other relevant costs to the NHS included the cost of non-invasive diagnostic tests, other treatment costs and the cost of subsequent care. The costs borne by the patients and their families in terms of out-of-pocket expenses and the time and travel costs of accessing services were also collected through patient self-completed questionnaires (see Appendix 28).
Cost of invasive urodynamic testing and non-invasive diagnostic tests
A micro-costing exercise,76 where a detailed service delivery process was identified with all the relevant resource items measured separately, was used to generate the unit cost of the IUT. This cost was derived from resources used to perform the procedure, including consumables, reusable items, staffing and the use of the consulting room.
Lists of individual consumable and reusable items were obtained from (NuTH) (Liz Dixon, NuTH, 2013, personal communication) and it was assumed for the purposes of this study that the use of these items was the same across all sites (the same simplifying assumption would not be made for the definitive trial). Information on the type and grade of staff present in the consulting room was obtained from the CRF (visit 3). In order to derive the staff and consulting room costs of the IUT, relevant information was recorded in the CRF for every participant in the IUT arm. Within the feasibility study we assessed the completeness of data recorded on the CRF. The specific information needed for economic analysis included:
-
time of patient entry into and leaving the consulting room
-
grade and type of operator present
-
grade of other staff present
-
postinvestigation complications.
These data were combined with the unit costs of the resources to estimate an average cost of an IUT per patient. Unit cost data came from the following sources: the cost per unit of time for each grade of staff involved came from the Unit Costs of Health and Social Care;77 consumables and reusable item unit costs were derived from manufacturers’ and suppliers’ price lists.
Three types of IUT might be performed in this patient group: dual-channel subtracted cystometry, videourodynamics and ambulatory urodynamics. The standard IUT is dual-channel subtracted cystometry, which is the most commonly performed procedure, and the other two tests are used at the discretion of the clinician as an alternative or additional invasive test. For the feasibility study, micro-costing was only undertaken for dual-channel subtracted cystometry and this unit cost was applied to the other IUTs. For a future definitive trial, a micro-costing technique will be applied to all three IUTs, to identify potential variations in the cost of an IUT depending on the chosen procedure.
Costs were also derived for a number of non-invasive tests that may also be performed for patients in both IUT and no IUT arms, and these were:
-
frequency/volume charting or bladder diary
-
mid-stream urine culture
-
urine flow rate
-
residual urine volume measurement (ultrasound).
Information on the use of these non-invasive tests was collected via the CRF (visit 2) for all patients. In this feasibility study, these costs have been omitted but in a definitive study the cost of each test will be based on the staff time, consumables and equipment used; it has already been ascertained that these data are available (Liz Dixon, personal communication).
Cost of surgical treatment
The costs associated with surgery were also an important cost driver. In a definitive trial, a micro-costing exercise will be conducted (or alternatively data would be taken from a published costing exercise should a high-quality, UK-relevant study be available at the time when data analysis is conducted). For the feasibility study, the NHS Reference Costs for the surgery were adopted,78 where the unit cost of a tension-free vaginal tape (TVT) surgery was used as the surgery cost. The feasibility study assessed the completeness of data recorded in the CRF. The following information will be needed for the economic analysis in a future definitive trial on the use of surgery and was recorded in the CRF (visit 4) for every participant in the feasibility study:
-
grade of anaesthetist present at operation
-
type of anaesthesia (general, regional, local ± sedation)
-
time of patient entry into and leaving operating room
-
time of patient entry into and leaving recovery room (if applicable)
-
grade of surgeon present
-
grade of other staff present
-
date of admission
-
date of discharge (if date of discharge was the same as admission it will be assumed that the procedure was performed as a day case)
-
postoperative complications.
Costs of other treatments
The inclusion criteria for the feasibility study included the requirement that both patient and clinician felt that surgical treatment was an appropriate next option for their SUI or stress predominant MUI that had failed to resolve following PFMT; hence, surgery was the anticipated treatment for women in the no IUT arm. Other treatments could, however, be provided to women in the intervention arm, where alternative or additional diagnoses were made following IUT. These treatments included bladder retraining, antimuscarinic drug treatments, neuromodulation or botulinum toxin injections (where DO was diagnosed), and clean intermittent self-catheterisation (where a VD was identified).
Information on the use of these treatments was collected from the CRF (visit 5) only for women in the IUT arm of the study not undergoing surgery. The cost of these treatments were estimated from one study site (Liz Dixon, personal communication) and from a HTA report. 79 In a definitive study, the cost of each treatment will be based on the staff time, consumables and equipment used from each of the study sites.
Costs collected from Participant Costs Questionnaires
A PCQ was designed to collect information on the use of NHS health services (primary and secondary) and patients’ out-of-pocket expenses during the follow-up period. The responses to this questionnaire were analysed in terms of response rates and completeness of data. The patients’ and caregivers’ costs were excluded from the economic analyses reported here but would be included in the economic evaluation conducted as part of the definitive study.
The PCQ was designed to be as comprehensive as possible but, at the same time, not to overburden the participants. The PCQ consisted of two parts: part A recorded information on the level of usage of the health services and the costs of any other self-purchased health care required to manage the condition; part B collected information on the time and travel costs of the participants attending each possible type of NHS services. The role of part B was to inform the calculation of unit costs of the participants attending each type of health service, and this would then be combined with the information obtained from part A to derive total costs to the NHS and the patients. As part B is lengthy compared with part A, within the feasibility study part A was administered with the 6-month symptom outcomes questionnaires, and part B was posted separately 2 weeks later, so as to be perhaps less burdensome than completion of both questionnaires at the same time. This practice might also have an impact on the response rates for part A and part B, which was assessed in the feasibility study.
Part A of the PCQ collected information on patients’ use of NHS resources related to the patient’s UI in both secondary and primary care. The use of secondary care services included non-protocol outpatient visits and readmissions relating to UI (protocol visits being those scheduled for the purposes of data collection, which were excluded). The use of primary care services included prescription medications relevant to the management of incontinence, contacts with primary care practitioners (e.g. GPs, practice nurses), continence nurses and physiotherapists. The unit costs for secondary care resources were obtained from NHS Reference Costs. 78 The unit costs for primary care resources were derived from the Unit Costs of Health and Social Care. 77 Prescription medication costs were based on the actual cost per GP prescription as provided by the Unit Costs of Health and Social Care,77 as medication details were not collected in the feasibility study beyond the CRF closure at the time of first clinical follow-up.
Participant out-of-pocket costs comprised three elements: (1) data collected in part B of the PCQ on travel costs for accessing NHS primary and secondary care; (2) data collected in part B of the PCQ on time costs of travelling to and attending NHS primary and secondary care; and, (3) data collected in part A of the PCQ on self-purchased health-care and related management costs. The estimation of travel costs required information from participants about the number of visits to health-care services (collected in part A), and the unit cost of making a single journey to each type of health-care provider (derived from information in part B). The participants were asked, in part B of the PCQ, for each type of visit, the mode of transport they used and the one-way fare if they travelled by bus, taxi or train, or the number of miles they travelled and parking fees if they used a private car. Participants’ time costs were collected in a similar manner. Participants were asked how long on average they spent travelling to and attending each type of health-care provider. They were also asked what activity they would have been undertaking [e.g. paid work, leisure, housework (in the case of parents or carers)] had they not attended the health-care provider. These data were presented in their natural units (e.g. hours and minutes) and attached monetary value using standard economic conventions (e.g. the Department of Transport estimates for the value of leisure time). 80 These unit time costs, measured in terms of their natural and monetary terms were then combined with estimates of number of health-care contacts to calculate patients’ time costs. If someone accompanied them, the same questions were asked for the accompanying person. Self-purchased health care includes over-the-counter medications and containment products, such as incontinence pads. Private health insurance costs were included if the insurance was purchased for UI-related conditions. Management costs of UI-related conditions, such as the costs of doing extra laundry, were also included. This included the time cost of doing the extra laundry and money spent for using a launderette or laundry service if applicable.
Completeness of data
Information on the type of IUT, type of surgery, grade of staff present and the length of time for each procedure was recorded on the CRF. The feasibility study assessed the completeness of the data collection to inform on any issue encountered that would need to be addressed in a full trial.
The response rates and completeness of the PCQ and self-assessed health questionnaires (EQ-5D-3L and SF-12) were also assessed. Response rates were analysed to identify any potential issues affecting patients completing the questionnaires to inform the practice in the future definitive trial.
Data analysis
Cost–utility analysis
As set out in the study protocol, we rehearsed the cost–utility analysis from a NHS perspective using available data collected in the trial. Utility scores were based on QALY values derived from SF-12 and EQ-5D-3L at baseline and at the 6-month follow-up. The primary analysis was the incremental cost per QALY at 6 months, where QALYs were based on the responses to the EQ-5D-3L converted into QALYs using the area under the curve method. 81 The results were presented as point estimates of mean incremental costs, QALYs, and incremental cost per QALY. Cost–utility analysis was also conducted where QALYs were based on SF-6D scores derived from responses to the SF-12. 68
The analysis should not be thought of as providing answers to the study question but as an exercise to inform the development of the definitive study and to identify potential issues and strategies that might be used to overcome them when undertaking the analysis of the cost-effectiveness data. Thus, the analyses presented in the results section are an example of the form of analysis that might be conducted, but do not provide a sufficient evidence base for informing changes to current policy. Nevertheless, they would have value as part of any subsequent evidence synthesis exercise.
Sensitivity analysis
Deterministic and stochastic sensitivity analyses were both performed. Deterministic sensitivity analyses were carried out to test for the effect of assumptions and variability, such as an exploration of alternative unit costs applied to the different resources used. In the sensitivity analysis, the cost of containment products provided by the NHS was assigned to patients who had not received surgery. It was assumed that patients who had not received surgery were still incontinent and the inclusion of this cost was explored in the sensitivity analysis only.
A stochastic sensitivity analysis, which explores the impact of the statistical imprecision surrounding estimates of costs, effects and cost-effectiveness, was undertaken to allow presentation of the level of variance around outcome measures included in the cost–utility analysis. Uncertainty surrounding the cost-effectiveness ratio was addressed using the bootstrapping technique. The results of the bootstrapping simulation were presented on the ‘cost-effectiveness plane’, which highlights the preferred investigation strategy. If the results lie in the north-west or south-east quadrants the preferred investigation strategy is clear, as one option dominates the other (i.e. is less costly and more effective). If the results lie in the north-east or south-west quadrants the decision as to which is the preferred investigation strategy is less clear (i.e. one option may be less costly but also less effective, or more effective but at greater cost); the incremental cost-effectiveness ratio (ICER) may aid this decision. The bootstrapping was also used to estimate confidence intervals for both costs and effects from the IUT and no IUT arms of the pilot trial. A cost-effectiveness acceptability curve was also used to present the probability of the IUT being cost-effective based on a range of values for society’s willingness to pay.
Results
There were 222 patients initially randomised to the pilot trial comparing the IUT arm and the no IUT arm. From these 222 patients, information on 218 patients was used in the economic analysis; 110 in the IUT arm and 108 in the no IUT arm.
Completeness of data
Analysis of the information collected on the CRF and the PCQ part A and part B allowed us to identify any issues with data collection that would be relevant to a future definitive trial.
A total of 125 part A questionnaires were returned by patients of which 8 were returned blank; in some cases, the participant simply annotated the front of the leaflet indicating ‘no additional costs’; in others, no annotation was made. The overall response rate was 56.3% (52.7% when blank responses were omitted). One of the completed questionnaires had missing information on the randomisation group, and therefore, was not included in the analysis. Of those who had responded, the majority completed all questions in the questionnaire and there were few missing responses.
A total of 119 part B questionnaires were returned. Eighteen were returned blank, so the response rate was 53.6% (45.5% when blank responses were excluded). The larger number of blank questionnaires for part B compared to part A was likely to be due to the length of the part B questionnaire (5 pages in part A compared with 15 pages in part B). As with part A, those who returned the questionnaire completed the majority of questions. We identified some questions that seemed to cause confusion, in particular those relating to caregivers’ time at inpatient visits; these questions could be amended for a definitive trial. If patients find the questionnaire too burdensome, assumptions can also be made to reduce the length of the questionnaire. We can assume that patients found part B more burdensome as a higher number of participants returned blank questionnaires (8.1%) compared with the number of blank responses returned for part A (3.6%). We might then, for example, omit the practice nurse section, assuming that the time and travel spent at a GP visit is the same for a practice nurse visit. The CRFs were used to collect information on the IUT (visit 3) and surgery (visit 4). With regards to the IUT, some of the information needed to calculate costs was missing. The cost of the IUT was based only on dual-channel cystometry as it was anticipated to be the most frequently used: 101 out of 110 patients had this procedure, 4 women had videocystometry and the type of test was not reported for the remaining 5 women. The ‘time into the consulting room’ was not reported for 16 patients and the ‘time out of the consulting room’ was not recorded for 21 patients. The ‘type of operator’ was missing for 9 patients and the ‘grade of operator’ was missing for 17 patients.
A total of 182 patients underwent surgery. There was some missing information but overall most CRFs were fully completed. All patients had a ‘date of admission’ recorded but two patients had no ‘date of discharge’ recorded. In the analysis, their ‘date of discharge’ was assumed to be the same as their ‘date of admission’. It was assumed that the patients were only admitted for surgery as a day case; this was explored in the sensitivity analysis. Two ‘date of discharge’ records were illogical (i.e. discharge date was before the date of admission). For one participant, it was changed from 2012 to 2013 and for the other it was changed from 2011 to 2013 to match the rest of the patient’s information. Time ‘out of recovery unit’ was the most commonly omitted item with information not available for 15 patients; all the other entry and exit times for the surgery had between one and three missing responses. Documentation of the staff present in theatre was usually fully completed with between one and seven missing responses and the most commonly missing item was ‘other staff present’. Overall, the CRF was completed for most patients with information missing for only a few.
Resource use and costs
The two study arms incurred different initial health-care costs: the IUT arm incurred the cost of the IUT, surgery and other treatments. The no IUT arm incurred the surgery cost alone. It was expected that both arms would experience similar follow-up health-care costs. The unit costs for each of the health-care resources are presented in Table 13.
| Resource use | Cost per unit (£) | Source/note |
|---|---|---|
| Cost of the intervention (IUT) | ||
| Consumable items | 24.99 | In-study micro costing |
| Capital resources | 10.26 | In-study micro costing |
| Per minute of consulting room | 0.42 | In-study micro costing |
| Per minute of staff – grade 3 | 0.18 | Pay scales 201382 |
| Per minute of staff – grade 5 | 0.25 | Pay scales 201382 |
| Per minute of staff – grade 6 | 0.31 | Pay scales 201382 |
| Per minute of staff – grade 8 | 0.44 | Pay scales 201382 |
| Per minute of staff – consultant | 2.45 | PSSRU 201277 |
| Per minute of staff – SpR/SST | 0.97 | PSSRU 201277 |
| Cost of surgery | ||
| TVT surgery | 1393.00 | NHS Reference Costs 2011–1278 |
| Admission – day | 312.00 | NHS Reference Costs 2011–12 – urinary incontinence and other urinary problems without CC78 |
| Admission – night | 585.00 | NHS Reference Costs 2011–1278 |
| Follow-up – secondary care | ||
| Inpatient visits | 585.00 | NHS Reference Costs 2011–1278 |
| Outpatient visits | 103.00 | NHS Reference Costs 2011–1278 – urology department |
| Follow-up – primary care | ||
| GP practice visits | 36.00 | PSSRU 201277 |
| GP home visits | 92.00 | PSSRU 201277 |
| GP telephone consultation | 22.00 | PSSRU 201277 |
| Practice nurse visit | 11.63 | PSSRU 201277 |
| Continence nurse visit | 22.00 | NHS Reference Costs 2011–1278 |
| Physiotherapist visit | 17.00 | PSSRU 201277 |
| Prescription | 8.31 | PSSRU 201277 |
| Travel | ||
| Hospital car | 9.19 | ISD 2012 – Table R91083 |
| Ambulance | 263.00 | NHS Reference Costs 2011–12 – emergency transfers78 |
| Cost of other treatments | ||
| Bladder retraininga | 283.00 | NHS Reference Costs 2011–1278; Liz Dixon, personal communication |
| PFMTb | 108.50 | PSSRU 201277 |
| Alternative behaviour modificationc | 21.00 | NHS Reference Costs 2011–1278 |
| Watchful waiting (containment products) | 42.00 | Imamura et al.79 |
| Antimuscarinic drugs (6-month dosage) | ||
| Solifenacin 5 mg | 167.56 | BNF84 |
| Solifenacin 10 mg | 217.85 | BNF84 |
| Oxybutynin extended release | 83.54 | BNF84 |
Table 14 presents details on the resource use for the IUT and no IUT arms of the feasibility trial, including the average length of time patients spent in hospital after their surgery, the average length of time of the IUT, the average use of primary care and secondary care health resources. The average resource use is based on the average contacts of patients in each arm who used health-care resources during the follow-up period. This information was collected from part A of the PCQ. On average, the IUT arm used more health-care resources in the follow-up period with the exception of GP practice visits and outpatient visits. The average resource use needs to be analysed with caution as extreme responses may skew the data.
| Item | No IUT (N = 108) | IUT (N = 110) | ||
|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |
| Duration of IUT | – | – | 89 | 40.040 (11.028) |
| Proportion receiving surgery | 101 | – | 81 | – |
| Proportion having surgery as a day case | 100 | – | 80 | – |
| Length of admission (days) for surgery if as an inpatienta | 1 | 1.000 (0.000) | 1 | 1.000 (0.000) |
| Number of patients who completed the PCQ part A | 66 | – | 50 | – |
| GP practice visit | 10b | 2.500 (1.581)c | 10 | 2.400 (1.776) |
| GP home visit | 10 | 0.000 (0.000) | 9 | 0.000 (0.000) |
| GP telephone consultation | 10 | 1.100 (2.025) | 9 | 1.440 (3.245) |
| Practice nurse visit | 6 | 2.000 (0.894) | 3 | 2.670 (1.155) |
| Continence nurse visit | 3 | 1.330 (0.577) | 4 | 2.000 (1.414) |
| Physiotherapist visit | 2 | 1.500 (0.707) | 3 | 5.330 (4.041) |
| Outpatient visit | 14 | 2.140 (0.949) | 14 | 1.860 (0.663) |
| Inpatient visit | 8 | 0.500 (0.926) | 6 | 1.000 (1.549) |
| Prescription | 3 | 1.670 (1.155) | 8 | 3.500 (2.726) |
Table 15 presents the average cost per patient based on complete cases only; these are cases where we had complete QALY information (i.e. both baseline and 6-month EQ-5D-3L questionnaires were completed), complete CRF information and complete PCQ part A. It is apparent from these data that the IUT arm has a higher average total cost than the no IUT arm; however, these results need to be interpreted with caution due to the small numbers of participants contributing data in each trial arm. The reason that the IUT arm has a higher average cost per patient in the complete case analysis is because a high proportion of patients in the IUT arm with complete information have had surgery.
| Investigation strategy | n | Mean (£) | SD (£) | Range (£) | IQR (£) | ||
|---|---|---|---|---|---|---|---|
| Min. | Max. | p25 | p75 | ||||
| IUT | 30 | 1815.26 | 455.38 | 276.05 | 2839.52 | 1769.65 | 1897.60 |
| No IUT | 51 | 1775.37 | 210.39 | 1705.00 | 2608.94 | 1705.00 | 1705.00 |
Quality-adjusted life-years
At baseline, there were 58 (26.6%) missing observations for EQ-5D-3L and 68 (31.2%) missing observations for SF-12. At 6 months, there were 104 (47.7%) missing observations for EQ-5D-3L and 112 (48.6%) for SF-12. In total, 105 patients had complete information at both baseline and 6-month follow-up to generate QALY values using the EQ-5D-3L (45 in the IUT arm and 60 in the no IUT arm). A total of 97 patients had complete information at both baseline and 6-month follow-up to generate SF-6D QALY scores (39 in the IUT arm and 58 in the no IUT arm). Overall, there was a higher percentage of complete data from the no IUT arm than the IUT arm.
The average QALY values for the IUT arm were slightly higher for the SF-6D. An independent sample t-test was performed and found no evidence of a statistically significant difference in mean QALY scores between the two study arms, as might be expected given the small sample size (Table 16).
| Questionnaire | No IUT | n | IUT | n | Mean difference (95% CI) | Significance |
|---|---|---|---|---|---|---|
| EQ-5D | ||||||
| Baseline | 0.8614 | 85 | 0.8384 | 75 | ||
| 6 months | 0.9060 | 65 | 0.8843 | 49 | ||
| QALY | 0.4452 | 60 | 0.4421 | 45 | 0.00305a (–0.02580 to 0.01330) | 0.869 |
| SF-6D | ||||||
| Baseline | 0.7469 | 81 | 0.7523 | 69 | ||
| 6 months | 0.7805 | 65 | 0.7846 | 47 | ||
| QALY | 0.3804 | 58 | 0.3912 | 39 | –0.01080a (–0.00710 to 0.01140) | 0.401 |
Cost–utility analysis
As noted earlier, only an illustrative example of the cost–utility analysis is presented, which is meant to be exploratory and should be interpreted with caution for the following reasons:
-
The study sample size was not powered for the results of analysis to be definitive.
-
Information on non-invasive tests was collected for the feasibility study but was not used in the economic analysis. These tests can be performed on patients in both the IUT and no IUT arms so it is difficult to determine the bias, if any, that the exclusion of these tests has on the results of the economic analysis.
-
Information on participants’ out-of-pocket costs was not included in the analysis.
-
Micro-costing for the IUT was conducted based on information from one site, therefore, could be under/overestimating the costs of the IUT arm.
Further details below describing the extent of data available for the analyses illustrate why analyses based on these data are not sufficient to inform changes in practice.
There were 54.5% missing data on health-care resource use during the follow-up period in the IUT arm and 38.9% missing data in the no IUT arm. There was also incomplete information in the CRFs with regards to the IUT and surgery. QALY values were generated using the responses to the EQ-5D-3L questionnaire but there were only complete data for 40.9% of the IUT arm and 55.6% of the no IUT arm. The missing data leads to an underestimation of average costs, whereas the direction of the effect on QALYs is uncertain. The patients’ and caregivers’ costs, despite being collected, were not included in the economic analysis presented below. The reason for this was due to the low available number of completed responses to the PCQ part B. We assumed therefore that both arms would incur similar time and travel costs if they required follow-up treatment. The cost–utility analyses conducted were a rehearsal for a future definitive trial and hence NHS costs were felt to be sufficient for this purpose.
An illustrative analysis was conducted from a NHS perspective only using all patient records collected during the feasibility study where missing information on the CRFs was imputed. QALY values used in this example were based on EQ-5D-3L scores, missing QALY values were estimated using multiple imputation. This analysis was chosen as it was considered to be less biased than other analyses. This was because major NHS costs information was collected on the CRFs, especially the IUT and surgery were the main cost drivers in treating the condition of SUI, and by imputing cost values on this information, we could minimise potential bias on costs; whereas we could not be certain about the direction of impact due to missing QALY data. In Appendix 27, a further set of analyses are reported that explore the implications of missing data using alternative assumptions.
Illustrative example of the cost–utility analysis: imputed values used for missing case report form data
In this analysis, imputation was adopted for missing CRF data on resource use related to the IUT and surgery, which were the key cost drivers affecting the cost-effectiveness of the IUT. The imputed values included using the median length of time (39 minutes) of an IUT, using a consultant as the main operator of an IUT and using a day case as the length of admission after surgery (as only a minority of patients were admitted overnight after the surgery). Table 17 presents the cost–utility results using imputation for missing CRF data. The IUT arm has a lower average cost per patient and has a lower average QALY value than the no IUT arm. The cost per QALY for the IUT compared with surgery alone is £8090. These results need to be interpreted with caution as it is argued that there needs to be a difference of 0.075 in EQ-5D values for there to be a significant impact on cost per QALY ratios. 85 The probability of the IUT being cost-effective decreases as the society’s willingness to pay for a QALY threshold increases.
| Investigation strategy | Cost (£) | QALY | ICER (£) | Probability that the IUT is cost-effective for different threshold values for society’s willingness to pay for a QALY | ||||
|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||
| IUT | 1507 | 0.3857 | 8090 | 96% | 80% | 56% | 45% | 37% |
| no IUT | 1661 | 0.4047 | 4% | 20% | 44% | 55% | 63% | |
Figure 8 presents the results of the bootstrapping simulation, which addresses the statistical uncertainty around costs and effects. As the majority of the iterations generated from the bootstrapping simulation were generally in the south-west quadrants, it suggested that the IUT arm tended to incur less cost than the no IUT arm but provided lower QALY values. The location of the average of the incremental cost and QALY pair simulations on the cost-effectiveness plane supports this, the average mean QALY difference is –0.006. This highlights the uncertainty around the cost–utility results. The cost-effectiveness of the IUT will depend on the threshold chosen to evaluate cost per QALY. This is further supported by the cost-effectiveness acceptability curve seen in Figure 9, which demonstrates that if society had zero willingness to pay for an additional QALY then IUT was 96% likely to be cost-effective; as society’s willingness to pay for a QALY increased, the likelihood of IUT being cost-effective decreased. Further economic analyses including complete case analysis, sensitivity analysis and base-case analysis with SF-6D used to calculate QALY values can be found in Appendix 27.
FIGURE 8.
Incremental cost-utility scatterplot with imputation of CRF data: IUT vs. no IUT.
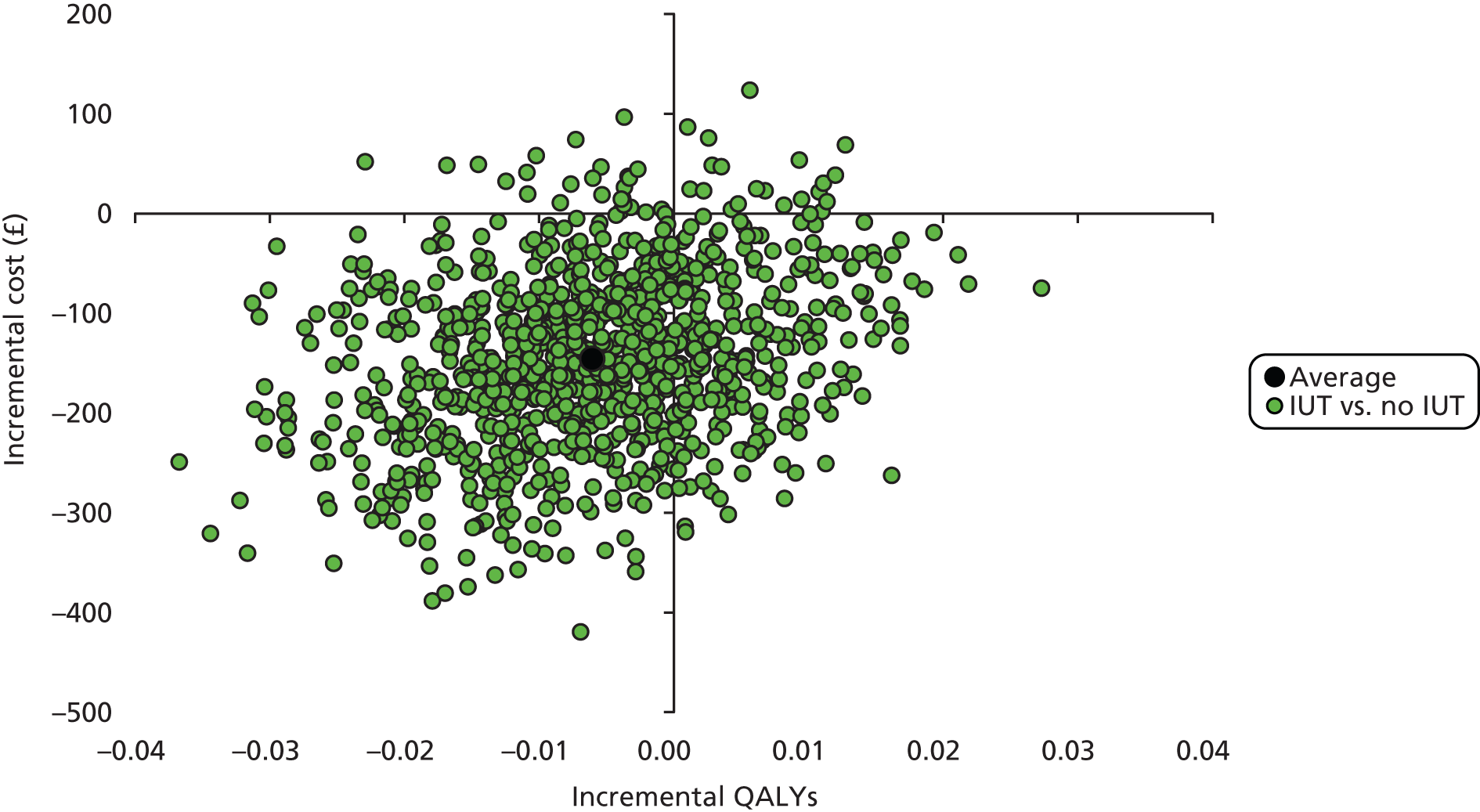
FIGURE 9.
Cost-effectiveness acceptability curve with imputation of CRF data: IUT vs. no IUT.
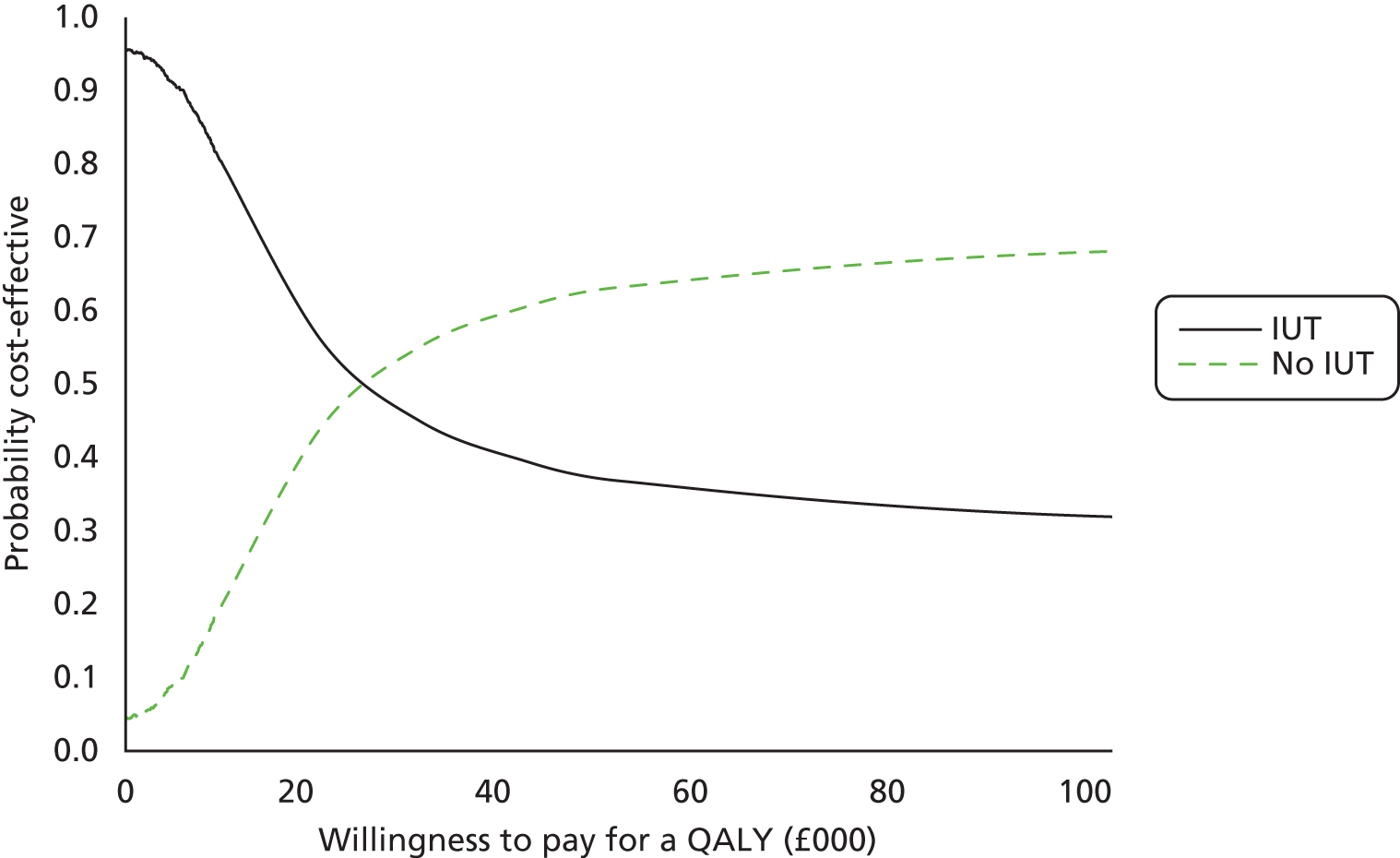
Key messages
-
The economic evaluation rehearsed data collection and analysis to inform the definitive trial.
-
The ease of data collection and the consistency of resource use in administration of relevant investigations and treatments were evaluated, and the use of economic data collection instruments was piloted.
-
The response rates for PCQs were 53% for part A and 46% for part B excluding blank responses, and of those returned, the majority were completed appropriately. Modifications to part B in particular should be considered in a definitive trial to make the PCQ questionnaires less burdensome for patients.
-
A cost–utility analysis was performed with QALY values from EQ-5D-3L and SF-12. The cost calculation included NHS resource use collected in the CRF. Complete data to undertake the cost–utility analysis were available in only 41% of the IUT arm and 56% of the no IUT arm; sensitivity analyses were adopted to assess the significance of statistical and other uncertainties.
-
The IUT arm had a marginally lower average total cost than the no IUT arm; however, a wider spread of costs of the IUT arm was observed. This may reflect the fact that surgery was not the chosen treatment for some patients in the IUT arm. All of the economic analyses found that the IUT arm had a lower average cost per patient than the no IUT arm in their incremental results except the complete case analysis (see Appendix 27). However, when a bootstrapping technique was performed on the incremental results to present the uncertainty around the cost-effectiveness ratio, the majority of iterations from the bootstrapping simulation were in the southern quadrants for all of the economic analyses. This highlights the potential cost savings experienced by the IUT arm.
-
Quality-adjusted life-years determined from the SF-6D were slightly higher in the IUT arm, though the difference in QALYs calculated from the EQ-5D-3L and the SF-6D was not statistically significant. In the economic analyses in Appendix 27, the average result from the bootstrapping technique was positioned in the southern quadrants but on the y-axis. These analyses supported our original findings from the t-test; there is no significant difference in QALY values between the IUT and no IUT arms.
-
Several limitations to this evaluation were recognised. While there were few missing data points within questionnaires, the number of completed questionnaires was low. The costs of non-invasive tests were omitted from the cost–utility analysis. Only NHS costs have been included in the analysis. The micro-costing of IUT was based on the cost of resources at one site; this might lead to an over/underestimation of the cost. Finally, costs and QALYs were only estimated over 6 months; this might be too short a time horizon for the full consideration of costs and QALYs. The impact of extending a time horizon is unclear at this stage but would need assessing as part of a modelling exercise informed by the results of the definitive trial.
-
While we would propose that these limitations be modified in a future definitive trial, they meant that the present analysis must be interpreted with caution.
Chapter 5 Clinician survey
The initial survey results from August 2011 have previously been published as Hilton P, Bryant A, Howel D, McColl E, Buckley BS, Lucas M, et al. Assessing professional equipoise and views about a future clinical trial of invasive urodynamics prior to surgery for stress urinary incontinence in women: a survey within a mixed methods feasibility study. Neurourol Urodyn 2012;31:1223–30.
Methods
The intended recipients of the survey were those clinicians likely to be undertaking surgical treatment for women with SUI; members of the BSUG and the British Association of Urological Surgeons (BAUS) Section of Female, Neurological and Urodynamic Urology (BAUS-SFNUU) were chosen. The survey was designed to be distributed and completed electronically. An introductory e-mail was drafted that included:
-
a description of the INVESTIGATE studies
-
links to further information on the NIHR-HTA (www.hta.ac.uk/project/2272.asp) and trial (www.investigate-trial.com) websites
-
a link to the SurveyMonkey site where the questionnaire was hosted
-
contact details should potential respondents prefer to obtain a paper-based questionnaire and a reply-paid envelope (none did).
A copy of the paper-based questionnaire is included as Appendix 14.
The questionnaire itself sought categorised demographic data regarding respondents’ grade or rank, role (specialty and extent of specialisation), gender, time since graduation, access to and current use of IUTs, and their current workload in surgery for SUI. In order to assess current use of urodynamics in the patient group of interest, respondents were asked:
Do you currently arrange invasive urodynamic tests for most (say 75%) of your female patients with stress or stress predominant mixed incontinence?
Respondents were then presented with the following clinical scenario:
A 45-year old woman with two children, who has been sterilised; she has previously undergone pelvic floor muscle training and possibly other conservative treatments (in some scenarios), without benefit; she has not had any previous continence surgery.
They were then given six urinary symptom descriptions of varying complexity:
-
Complains of stress incontinence, but no frequency, nocturia, urgency or urgency incontinence; no symptoms of voiding difficulty; stress incontinence IS demonstrated on clinical examination.
-
Complains of stress incontinence, but no frequency, nocturia, urgency or urgency incontinence; no symptoms of voiding difficulty; stress incontinence IS NOT demonstrated on clinical examination.
-
Complains of stress incontinence, mild frequency, urgency and urgency incontinence, but describes the stress as the more significant problem; no symptoms of voiding difficulty.
-
Complains of stress incontinence, frequency (× 10 per day), nocturia (× 2 per night), urgency and urgency incontinence, with stress and urge of similar magnitude; no symptoms of voiding difficulty.
-
Complains of stress incontinence, frequency (× 15 per day), nocturia (× 2 per night), urgency and urgency incontinence, but describes the urge as the more significant problem; no symptoms of voiding difficulty.
-
Complains of stress incontinence, but no frequency, nocturia, urgency or urgency incontinence; also reports hesitancy, poor flow, and feeling of incomplete emptying.
Using a modified version of a bidirectional scale developed for measuring clinician and patient preferences in surgery,86 respondents were asked to rate the strength of their views about the necessity for IUTs before undertaking surgical treatment on an 11-point Likert scale from ‘unnecessary’ (+5) through ‘undecided’ (0) to ‘essential’ (–5) (Figure 10). They were specifically asked to respond on the basis of their own opinion, regardless of their current practices, and regardless of what they might have read in recent literature or current guidelines.
FIGURE 10.
Modified bidirectional scale developed for measuring clinician and patient preferences in surgery, after Young et al. 86

Respondents were then asked to use a Likert-type categorical scale graded ‘not at all important’, ‘somewhat important’, ‘very important’ or ‘extremely important’ to express their views about the importance of the research question:
Does invasive urodynamic testing prior to surgical treatment of stress or stress predominant mixed urinary incontinence improve the clinical- and cost-effectiveness of treatment compared to clinical assessment with non-invasive testing?
A vignette of the design of a proposed definitive trial was described, as:
The design of such a study is anticipated to be similar to that of our pilot study, i.e. a pragmatic multicentre RCT, randomising women with stress or stress predominant mixed incontinence, who fail to respond to pelvic floor muscle training, to receive either:
-
no further assessment prior to surgical treatment (over and above the basic clinical assessment and non-invasive tests that they would have previously undergone)
or
-
invasive urodynamic tests (conventional cystometry, video urodynamics or ambulatory urodynamics), with subsequent treatment dictated by the investigation results.
Respondents were asked about their willingness to participate and to randomise patients within such a trial. For those unwilling to randomise, open questions with free-text responses were asked about their reasons for their view and about acceptable alternative trial designs. Finally, the questionnaire asked about respondents’ willingness to participate in a short telephone, qualitative interview to explore further whether or not and how they use the results of urodynamic investigations to inform their clinical decisions and to contextualise the questionnaire responses; if willing, respondents were asked to provide preferred contact details and optimum time for contact.
Initial draft versions of the survey materials were piloted for content validity and functionality of the online system by a small group of gynaecologists and urologists, who were neither members of the BSUG nor the BAUS-SFNUU and therefore who would not be recipients of the finalised questionnaire. Eighteen invitations were distributed and 12 responses obtained. Following minor alterations, survey information materials and data collection instruments were submitted for approval by the research committees of the BSUG and the BAUS-SFNUU. In order to maintain confidentiality of e-mail addresses, the organisations themselves then circulated study information and invitations to participate to their respective memberships in August 2011 (see Appendix 4).
Reminder e-mails were sent at 3 and 6 weeks after the initial circulation to all potential respondents, as it was not possible to target the reminders to non-respondents. The survey site was closed 12 weeks after the initial invitations. There are very few individuals who are members of both organisations, but a footnote was appended to the invitation letter apologising for dual circulation and requesting that individuals make only a single response.
Survey update
Given the length of time between the circulation of the initial survey (August 2011) and the conclusion of this study, and the known emerging publications from other studies,35,36,39 it was felt appropriate to undertake a further brief survey before publication of this report. An e-mail (BSUG) or newsletter (BAUS-SFNUU) invitation to complete an abbreviated questionnaire was again circulated to members of the BSUG and the BAUS-SFNUU by their respective secretariats in June 2013; this contained a link to the SurveyMonkey site where the questionnaire was hosted. This was much briefer than the initial questionnaire and included only six questions regarding respondents’ current clinical role, their view as to the importance of the research question and their willingness to randomise patients into a definitive trial. They were also asked to comment on the proposed primary trial outcome (ICIQ-FLUTS) and possible alternatives, and asked for their opinion about the MCID for this outcome (see Appendix 15). This was done, recognising the lack of existing data on the MCID, and the proposal from the ‘DELTA study’ that expert opinion might be one approach to establishing the target difference. 87 Bearing in mind the rate and speed of response seen in the original survey (v.i.), a single reminder e-mail was sent to members of both specialist societies 2 weeks after the initial circulation, and the survey site closed for analysis after 4 weeks.
Statistical analysis
The analyses of survey responses were carried out on the data sets following closure of the survey websites. Basic descriptive statistics including response rates, percentages in categories and summary statistics were used for all relevant outcomes. No attempt was made to impute missing data for any of the outcomes. ‘equipoise ratios’ (ERs) were calculated after Young et al. 86 to report the three proportions for each scenario: those clinicians who regarded IUTs as essential (to a greater or lesser extent), i.e. gave scores of –5 to –1; those who had no preference between using IUTs or not, i.e. gave a score of 0; and, those who regarded it as unnecessary (to a greater or lesser extent), i.e. gave scores of +1 to +5.
Results
Original survey responses
The BSUG and BAUS-SFNUU membership databases are fluid, with new members joining and others leaving continuously throughout the year; hence the numbers sent reminder letters were slightly different from the number of initial invitations. For the first survey, initial invitations went to 332 BSUG members and 185 BAUS-SFNUU members, with most of these, plus a small number of new members, being sent reminder e-mails to follow up the initial invitation. In calculating response rates, we used as the denominator the number receiving the initial invitation. A total of 176/517 (34%) responded to the survey, with the majority answering most of the questionnaire. The response rate was not significantly different between urologists (36%: 67/185) and gynaecologists/urogynaecologists (32%: 106/332), with three responses coming from individuals who did not report their specialty.
Of those responding, 55% did so after the initial circulation, 36% after the first reminder letter and 9% after the second reminder. Following each circulation, the majority of responses were received within the first week (97%, 63% and 100%, respectively), with 97%, 79% and 100% being within 2 weeks (Figure 11).
FIGURE 11.
Original survey responses by week after invitation.

Demographics
Table 18 provides baseline characteristics of those who responded to the initial survey. The specialist societies were able to provide only limited information about the demographic of their respective membership. Of the 332 BSUG members, 76% were full (consultant) members, 23% associate (non-consultant) members and 1% emeritus (retired) members. The BAUS-SFNUU had 185 full members who were all consultants.
| Variable | n (N = 158) | % |
|---|---|---|
| Current clinical role | ||
| Special interest | 101 | 64 |
| Subspecialist | 49 | 31 |
| Other/missing | 8 | 5 |
| Specialty | ||
| Gynaecologist | 90 | 57 |
| Urologist | 66 | 42 |
| Other | 2 | 1 |
| Sex | ||
| Male | 110 | 70 |
| Years since graduation from medical school | ||
| 0–5 | 1 | 1 |
| 6–10 | 1 | 1 |
| 11–15 | 11 | 7 |
| 16–20 | 38 | 24 |
| 21–30 | 77 | 49 |
| 31–40 | 30 | 19 |
| Undertake urodynamic investigations | 125 | 79 |
| If not, have access to urodynamics | 33 | 21 |
| Volume of SUI operations per year | ||
| 0–10 | 15 | 9 |
| 11–50 | 84 | 53 |
| 51–100 | 43 | 27 |
| 101–200 | 15 | 9 |
| > 200 | 1 | 1 |
| Arrange cystometry for > 75% of patients | 139 | 88 |
The response rates were similar between specialties (BSUG 32.9%; BAUS-SFNUU 36.2%); among the BSUG members, consultants were more likely to respond than non-consultants, and, among the BAUS members, women were more likely to respond than men. One hundred and fifty-eight of the 176 responses (90%) were from consultants (as opposed to trainees or specialty doctors), and the results are only presented from this subgroup, as we were interested in the views of clinicians who could potentially decide whether or not a clinic could be used to recruit patients to a future trial.
Urodynamic access and use
All consultant respondents reported having access to urodynamic facilities for their patients, and 79% undertook urodynamic investigations themselves; 88% indicated that they currently arrange IUTs in most of their female patients with SUI or stress predominant MUI (see Table 18).
Clinical scenarios
Responses in terms of the necessity for IUTs in the six clinical scenarios are given in Figure 12 and Table 19. For each of these scenarios, only between 2% and 7% of respondents were undecided, with most reporting highly polarised opinions, i.e. towards the left or right ends of the Likert scale. For the three more complex symptom descriptions, over 90% responded –5 to –1 (i.e. IUT ‘essential’ to a greater or lesser extent); between 2.0% and 4.5% responded 0 (i.e. ‘undecided’); and, less than 7% responded +1 to +5 (i.e. IUT ‘unnecessary’ to a greater or lesser extent.) For the three simpler symptom descriptions, which might be summarised as ‘SUI or stress predominant MUI’ and comprise the patients intended as eligible in the pilot and future definitive trials, a greater range of opinions was expressed. However, even in scenario 1 (pure SUI with clinically demonstrable leakage on coughing), two-thirds thought IUTs necessary to a greater or lesser extent (i.e. gave scores of –1 to –5), with over one-third of respondents considering IUTs essential (i.e. gave a score of –5): the ER was 66 : 1 : 33.
FIGURE 12.
Responses to the necessity for IUTs in the six clinical scenarios. (a) Scenario 1: pure SUI – stress leak IS demonstrable (ER = 66 : 1 : 34); (b) scenario 2: pure SUI – stress leak is NOT demonstrable (ER = 83 : 6 : 12); (c) scenario 3: STRESS predominant MUI (ER = 89 : 5 : 6); (d) scenario 4: EQUAL severity MUI (ER = 96 : 2 : 3); (e) scenario 5: URGE predominant MUI (ER = 92 : 2 : 6); and, (f) scenario 6: pure SUI – symptoms of VOIDING difficulty (ER = 93 : 4 : 3).
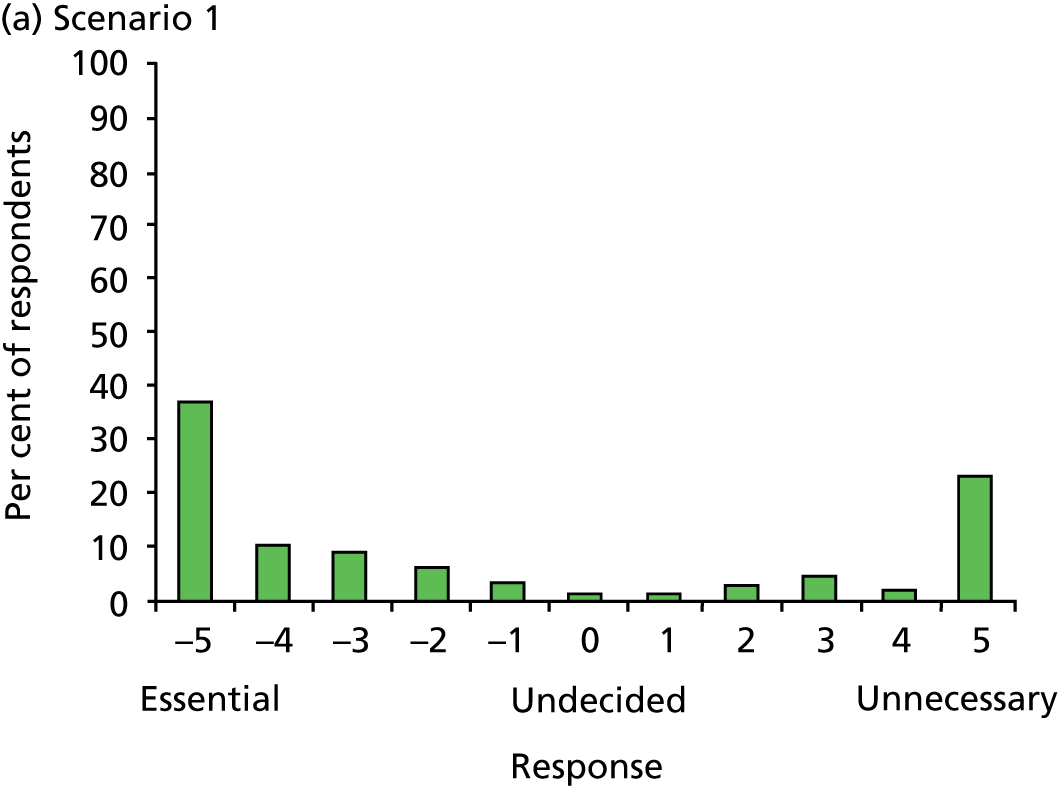

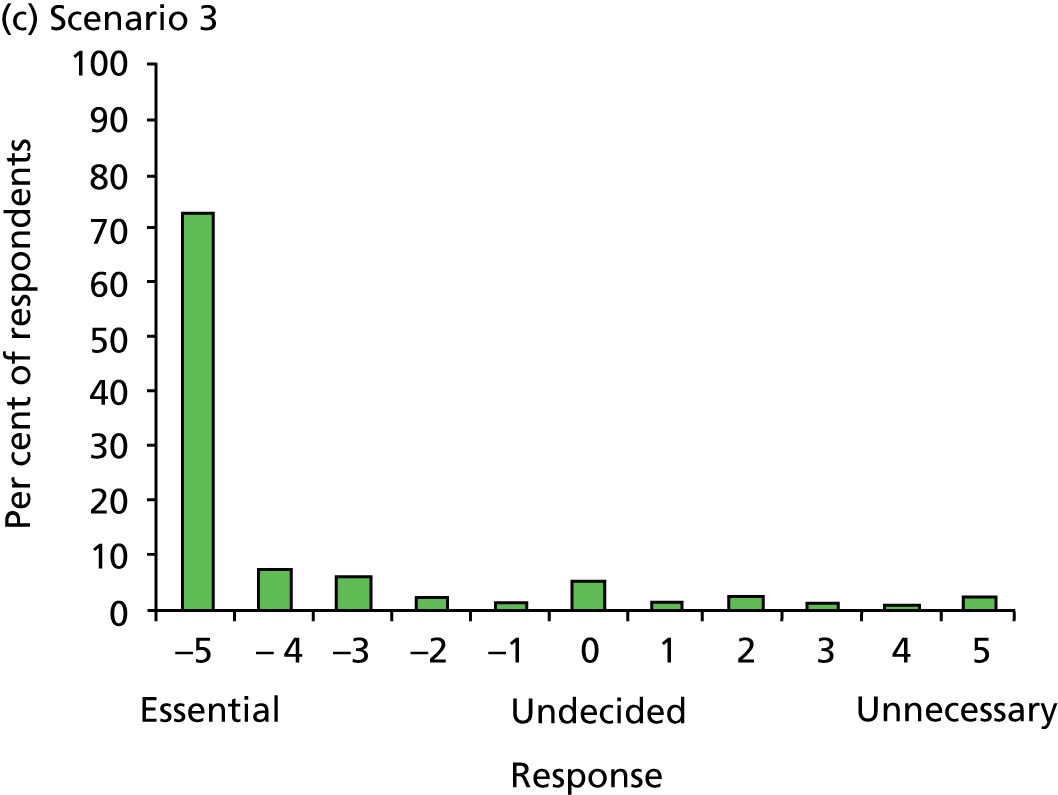

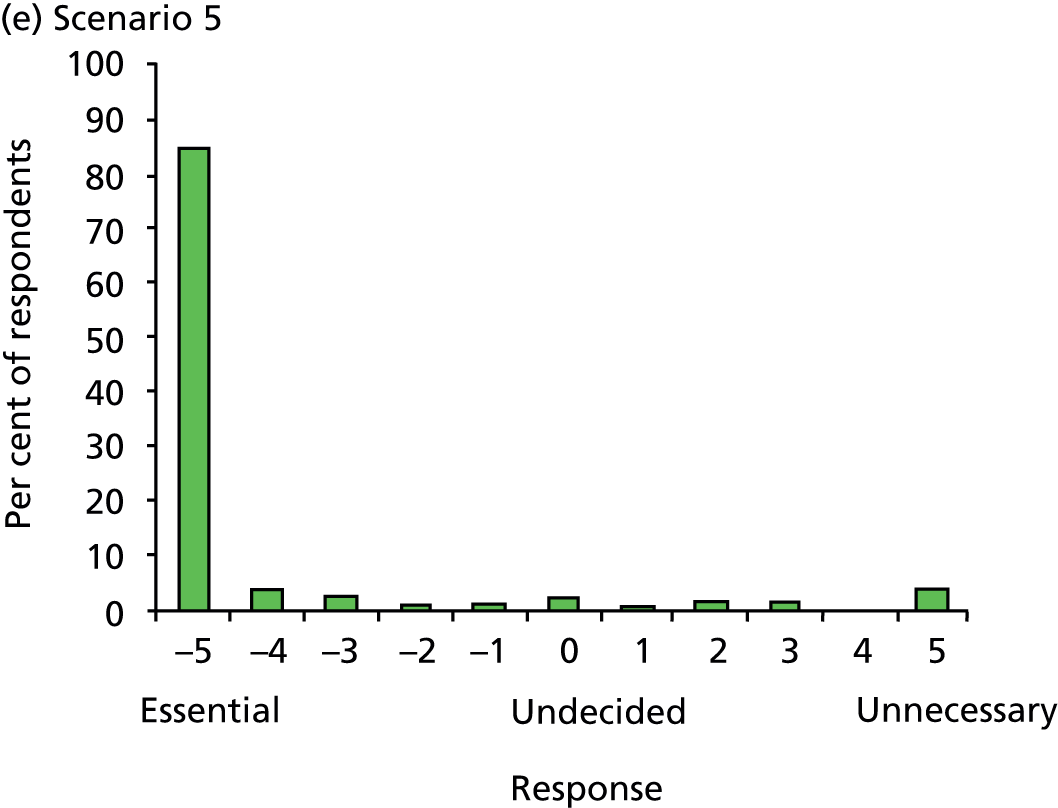

| 11-point scale | Essential | Undecided | Unnecessary | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| –5 | –4 | –3 | –2 | –1 | 0 | 1 | 2 | 3 | 4 | 5 | |
| Scenario 1 (n = 154) | Complains of stress incontinence, but no frequency, nocturia, urgency or urgency incontinence; no voiding difficulty; stress incontinence IS demonstrated on clinical examination. Pure SUI; stress leak IS demonstrable | ||||||||||
| Opinion (%) | 38.5 | 10.5 | 9 | 5 | 2.5 | 1.5 | 1.5 | 3 | 5 | 2 | 21.5 |
| ER (%) | 66 | 1 | 34 | ||||||||
| Scenario 2 (n = 154) | Complains of stress incontinence, but no frequency, nocturia, urgency or urgency incontinence; no voiding difficulty; stress incontinence NOT demonstrated on clinical examination. Pure SUI; stress leak NOT demonstrable | ||||||||||
| Opinion (%) | 58.5 | 13.5 | 6 | 2.5 | 1 | 6.5 | 1 | 3 | 3 | 1 | 4 |
| ER (%) | 82 | 6.5 | 11.5 | ||||||||
| Scenario 3 (n = 152) | Complains of stress incontinence, mild frequency, urgency and urgency incontinence, but describes more significant problem; no symptoms of voiding difficulty. STRESS predominant MUI | ||||||||||
| Opinion (%) | 75 | 7 | 5 | 2 | 0 | 5 | 1 | 2 | 1 | 0 | 2 |
| ER (%) | 89 | 4.5 | 6.5 | ||||||||
| Scenario 4 (n = 153) | Complains of stress incontinence, frequency × 10, nocturia × 2, urgency and urgency incontinence, of similar magnitude; no symptoms of voiding difficulty. EQUAL severity MUI | ||||||||||
| Opinion (%) | 86 | 4.5 | 3 | 2 | 0 | 2 | 0.5 | 0 | 0 | 0 | 2 |
| ER (%) | 95.5 | 2 | 2.5 | ||||||||
| Scenario 5 (n = 154) | Complains of stress incontinence, frequency × 15, nocturia × 2, urgency and urgency incontinence, urge as the more significant problem; no symptoms of voiding difficulty. URGE predominant MUI | ||||||||||
| Opinion (%) | 84 | 4 | 2.5 | 0.5 | 0.5 | 2 | 0.5 | 1.5 | 0.5 | 0 | 4 |
| ER (%) | 91.5 | 2 | 6.5 | ||||||||
| Scenario 6 (n = 154) | Complains of stress incontinence, but no frequency, nocturia, urgency or urgency incontinence; also poor flow, and feeling of incomplete emptying. Pure SUI; symptoms of VOIDING difficulty | ||||||||||
| Opinion (%) | 83 | 4 | 3 | 2 | 0 | 4.5 | 2 | 0 | 0.5 | 0 | 0.5 |
| ER (%) | 93 | 4.5 | 2.5 | ||||||||
Views about a future definitive trial
The results above could be interpreted as indicating that clinicians had little doubt about the value of IUTs and would be unlikely to be interested in a future clinical trial. However, when asked to rate the importance of the research question, 24% rated it ‘extremely important’, 45% ‘very important’, 26% ‘somewhat important’, and only 5% thought it ‘not at all important’ (Figure 13).
FIGURE 13.
The importance of the research question.

Inevitably, the number of generalists included in the survey was small, and responses did not differ markedly between gynaecologists and urologists, although generalists (n = 6) were somewhat less likely to look on the question as being ‘extremely important’ or ‘very important’ (33%), than consultants with a special interest (69%) or subspecialists (74%) (Figure 14).
FIGURE 14.
Importance of the research question by level of consultant specialisation within gynaecology and urology. Six general urology and gynaecology consultants are excluded from the figure.

On the 10-point Likert scale of ‘willingness to randomise’, 64.6% gave a score of seven or over. The breakdown of scores by degree of specialisation showed that 61.3% of consultants with a special interest gave a score of ≥ 7 compared with 81.9% of subspecialists (Figure 15).
FIGURE 15.
Likert scale of consultant ‘willingness to randomise’ by level of specialisation. Six general urology and gynaecology consultants are excluded from the figure.

Survey update responses
There were 145/498 (29%) responses to the survey update. Allowing for slight differences in the timing of distribution of the initial request and reminder by the BSUG and the BAUS-SFNUU, 49% of responses were returned in the first week after distribution, 55% within 2 weeks, and 96% within 3 weeks (1 week after the reminder e-mail).
On this occasion, we specifically sought responses from consultants/specialists only, so as to capture the views of those surgeons who might potentially wish to collaborate in a future definitive trial. Of all the responses, 4.1% came from general obstetricians and gynaecologists/urologists, 60.7% from consultants with an interest in urogynaecology/female urology, and 30.3% from subspecialists in urogynaecology/female urology. The number and timing of responses and the demographics of respondents were therefore, perhaps unsurprisingly, very similar between our initial survey and the more recent update.
Of all respondents, 68% still rated our research question ‘very important’ or ‘extremely important’ (Figure 16).
FIGURE 16.
The importance of the research question as reported by consultants in the initial survey (August 2011) and in the update (June 2013).

On the Likert scale of ‘willingness to randomise’, 61% recorded a score ≥ 7/10 (Figure 17). A total of 102/145 (70%) of respondents provided e-mail addresses, indicating their interest in contributing to a possible future definitive trial. Although a number of these came from e-mail servers external to the NHS (e.g. www.doctors.org.uk and www.yahoo.co.uk) and 20 came via the generic server nhs.net, comparison of e-mail addresses suggested that these 102 respondents represented 89 separate hospitals or NHS Trusts in England (79), Scotland (4), Wales (3), Northern Ireland (2) and the Republic of Ireland (1).
FIGURE 17.
Likert scale of ‘willingness to randomise’ as reported by consultants in the initial survey (August 2011) and in the update (June 2013).

When asked about the appropriateness of ICIQ-FLUTS as a primary outcome measure, 15% either had no opinion or omitted the question; 77% (91% of those expressing an opinion) felt this was an appropriate outcome. In response to an open question, 10 respondents suggested alternative primary outcomes as shown in Box 1.
Q11 (SUI) from ICIQ-UI.
ICIQ-UI SF (two respondents).
Electronic Personal Assessment Questionnaire.
Composite outcome of complications and failure.
Complication rates (e.g. failure, retention, OAB) (two respondents).
Combined subjective and objective evaluation.
Simple measure of incontinence (yes/no).
Respondents were asked to categorise what they considered the MCID in ICIQ-FLUTS score, given a maximum score of 48, and bands 1–4, 5–8, etc. Of all respondents, 50% either had no opinion or omitted the question. Of those responding, the modal response was in the range 9–12 (Figure 18).
FIGURE 18.
Respondents views on MCID for ICIQ-FLUTS.

Key messages
-
The response rates to the initial survey (34%) and update (29%) were disappointingly low given that the circulation was to members of relevant specialist professional societies. This raises the question as to just how representative of the totality of surgical practice in incontinence the responses are, although it is likely that the respondents were those with a particular interest in the subject matter or in clinical research.
-
The surgical workloads reported by respondents amounted to a total of approximately 8300 procedures per year; HES for England reported approximately 12,500 procedures for SUI in 2009–10 and 2010–11. While informal, self-reported surgical activity is notoriously unreliable, the respondents clearly embrace a significant proportion of incontinence surgery.
-
All respondents reported having access to IUTs, with 79% undertaking investigations themselves, confirming the relevance of their opinions to the survey questions.
-
Following the initial survey, the majority of responses were received after the first circulation, and over 90% were received after a first reminder. The majority of responses were received within 2 weeks of original distribution or reminders. This would suggest that with similar e-mail/online surveys no more than one reminder is necessary, and the time between initial distribution and reminder, and between reminder and survey website closure can be limited with minimal loss to responses. The initial survey sought responses from all grades of society membership, whereas the update sought responses from consultant/specialist grades only. There were 158 consultant responses to the initial survey, and 145 responses to the update.
-
The majority (88%) of consultant respondents reported undertaking IUTs on most of their patients with SUI or stress predominant MUI. When asked to rate the necessity for IUTs in a range of clinical scenarios of varying symptom complexity, few respondents were undecided, with most reporting highly polarised opinions. For the three more complex symptom descriptions, 83%–86% looked on IUTs as essential (i.e. graded –5 on a scale of –5 to +5). For the three simpler symptom descriptions, a greater range of opinions was expressed. However, even in the situation of pure SUI that is clinically demonstrable, two-thirds thought IUTs necessary to a greater or lesser extent (i.e. graded –1 to –5), with over one-third of respondents considering IUTs essential (i.e. graded –5).
-
Despite the apparent strength of professional opinion favouring IUTs in women with SUI or stress predominant MUI, when asked to rate the importance of the research question underlying INVESTIGATE, 69% rated it ‘extremely important’ or ‘very important’ and 65% gave a ‘willingness to randomise’ score of seven or over (on a scale of 0–10).
-
Although the number of general gynaecologists and urologists responding to the survey was small, they were somewhat less likely to look on the question as being important, and unlikely to be willing to recruit patients into a definitive trial. This should be borne in mind when selecting possible sites for a future trial.
-
Despite the publication of two other trials addressing the issue of the clinical utility of urodynamics in female SUI (both of which concluded that clinical assessment was not inferior to invasive urodynamic testing prior to surgery for SUI or stress predominant MUI), these latter opinions persisted largely unchanged two years after the initial survey. Over 100 respondents, representing approximately 88 NHS Trusts across the UK, indicated a willingness to become involved in a future definitive multicentre trial.
-
The majority of respondents (77% overall, or 91% of those expressing an opinion) felt ICIQ-FLUTS was an appropriate outcome; 50% ventured an opinion as to the MCID for this scale.
Chapter 6 Clinician interview study
Methods
As indicated above, this was an ‘opt-in’ follow-up from the initial survey responses. A purposively selected subsample was drawn from those respondents indicating a willingness to take part in the interview study, who provided contact details. Interviews continued until a point of saturation was reached (i.e. that no new material was emerging from the interviews).
An information sheet was provided (see Appendix 9), and while return of a completed questionnaire was taken as indicative of implied consent to participate in the clinician survey, written consent to take part in the interview was sought (see Appendix 13).
Telephone interviews were undertaken by an experienced qualitative researcher (see Acknowledgements) using a topic guide based on the survey and developed through discussion within the project team. The topic guide ensured all areas of interest were covered, but was used flexibly with the aim of allowing interviews to flow as freely and naturally as possible and to allow participants to discuss issues that were important to them. The interviewer prompted as appropriate to ensure that all views were fully explained, and the meaning of participants’ responses were clear. All interviews were audio-recorded and transcribed verbatim.
Qualitative analysis
Analysis of the interview data were based on the constant comparative method. 88 Transcripts were read three to four times and open codes initially applied line by line to the data to represent the meaning or significance of each sentence or group of sentences by the data analyst. Generation of the open codes proceeded sequentially, with no attempt at this stage to impose any framework on the data. The open codes were then incrementally grouped into organising categories or themes, by the analyst and study lead together. These categories were modified and checked constantly as further open codes were incorporated as analysis proceeded. When categories had been created to express all of the open codes, explicit specifications were written for each of the categories to assist in determining under what circumstances data should be assigned to any given category. The categories and their specifications (the coding scheme) were then programmed into the NVivo 10 (QSR International, Warrington, UK) qualitative software. The coding scheme was then used to process the data set systematically by assigning each section of text to a category, according to the category specifications.
Results
Of the 176 survey respondents, 87 (49%) agreed to being approached for interview and provided contact details. A diverse sample was recruited purposively to include, gynaecologists and urologists; those who did/did not routinely use IUTs; those with different approaches to when invasive urodynamic testing was needed; those with different perspectives on both the planned RCT, and their willingness to randomise patients. A total of 18 interviews were carried out, by which point data saturation was attained.
As would be expected from the quantitative results, and given the nature of the purposive sampling method, interview participants tended to be polarised in their view and regarded invasive urodynamic testing as either essential or of limited use.
For those interviewees who undertook invasive urodynamic testing regularly, the tests seemed to have a range of functions that clinicians regarded as valuable. The first of these was to add to the overall clinical picture and help inform the best course of action.
Well it helps with someone who has a history of stress incontinence and you have not been able to demonstrate it. Then you want to try and quantify the leakage and urodynamic testing can help you do that sometimes.
Participant 05
A second function was for invasive urodynamic testing to act as a ‘safety net’ to prevent unnecessary or inappropriate surgery. The fact that many of these patients would be offered surgery was important, and several participants very clearly framed future surgery as further underpinning the need to be as sure as they possibly could be about the diagnosis.
I would use urodynamic tests on anyone that I was going to operate on, it’s very easy to operate, but it’s not very easy to un-operate, so if you have a complication that arises as a result of your surgery, you can’t go back and say ‘well I would have liked that information, if I’d known that beforehand, I would have done something different’.
Participant 02
A third function of invasive urodynamic testing was to facilitate the appropriate counselling of patients. The clinician’s job was understood as being to gather all the available information that could then be presented to the patient along with treatment options and likely outcomes.
It gives you reasonable scientific evidence to sit with the patient and say ‘that is what you have got, that is what we are going to do, and that is the outcome’.
Participant 14
Interestingly, those who reported using IUTs routinely did not always do so because they perceived value in the tests. For a minority, there was an element of ‘fitting in’ with what colleagues did and adopting local customs and practices.
I have just moved to a new trust, my colleague investigates all patients who have stress incontinence before surgery. In my previous post I didn’t actually undertake urodynamics in patients who had pure stress incontinence symptoms so at the moment I, I suppose you could say that I’m doing it because it’s, it’s sort of departmental protocol really.
Participant 09
For those interviewees who used IUTs relatively rarely, this position was underpinned by a range of factors. The first of these was the understanding that the use of invasive urodynamic testing is not currently recommended by NICE prior to conservative treatments, and that, while it may be needed in more complex clinical scenarios, there is no evidence to support its use prior to surgery where the diagnosis of SUI is likely based on clinical assessment alone.
It [his/her current practice] is based on the NICE guidance which suggests you don’t have to do it in every woman.
Participant 03
Those clinicians who did not use IUTs routinely were much more attuned to the potentially unnecessary time and cost implications and weighed these against the likely ‘added value’ of IUTs. Unless a case was complicated, they believed that IUTs would not alter the treatment plan and that the information that could be obtained from other sources (such as patient history, examination and bladder diary) was sufficient.
We have things like flow meters which, you know, in the clinic, we have bladder scans, we can measure residuals, patients are quite good at filling in frequency volume chart [...] and a good physical examination combined with these non-invasive tests that I’ve just mentioned, I think gives you more information than urodynamics.
Participant 01
As in the overall survey responses, about two-thirds of the clinicians interviewed thought the basic research question of the INVESTIGATE studies to be an important one. For some, this was because they believed there was genuine uncertainty about the benefit of IUTs.
I think it is worth doing because as well as telling us whether urodynamics is useful, there will be a lot of information which will tell us in what ways it can be useful, it will say these are the things you should be looking at.
Participant 16
However, for others, the desire for a definitive trial was because they believed they knew the answer to the question but felt the need for ‘harder evidence’ to support their practice and encourage others to change theirs. Within the sample, there were examples of both interviewees who believed a trial would show IUTs should be used, and those who believed a trial would show the opposite.
I still think it is important that we answer this question because you know my certainty up to now is based on what I have been taught and what I have observed but that is not based on research particularly so I still think it is a very important research question.
When we asked you whether or not you thought the question that investigate is addressing is important, you said ‘very important’.
Well on the basis of what the NICE guidelines said, if we stopped doing it in the large number of cases that they suggest we should stop, then it would free up funding for something else.
As a deliberate outcome of the purposive sampling, we interviewed fairly even numbers of both those who would be willing to randomise into a definitive trial and those who would not. Unsurprisingly, those who always undertook invasive urodynamic testing and regarded it as essential were least likely to be willing to randomise, even if they had indicated they thought it an important research question. In these cases, they wanted the question answered in order to provide hard evidence that invasive urodynamic testing is necessary, but, because they were not personally in equipoise, they were not prepared to allow their patients to be part of producing that evidence.
I wouldn’t be happy [to randomise patients], no. That’s in keeping with my belief that it is an important test.
Participant 12
In contrast, those who appeared genuinely uncertain about invasive urodynamic testing, or at least were happy not doing it, were the ones most prepared to randomise.
I don’t have a problem not doing the urodynamics . . . so it makes perfect sense to put our patients into the trial . . . I wouldn’t see a problem with that at all . . . and I think the, you know, if we can answer the question it would be very worthwhile.
Participant 09
Key messages
-
The interviews facilitated a more detailed understanding of whether or not and how participants used the results of IUTs within their practice and the relative value that they attached to these.
-
The majority of those using invasive urodynamic testing routinely were convinced of its clinical utility in terms of helping to decide the best course of action and helping to counsel patients, although a small number reported that their practice in relation to invasive urodynamic testing was influenced more by local norms around its use rather than any personal commitment to it on their part.
-
In contrast, those who used invasive urodynamic testing relatively rarely saw little additional benefit from its use (the information that could be obtained from other sources such as patient history, examination and bladder diary was sufficient) but significant potential costs (e.g. in terms of time, financial implications).
-
The analysis of the interview study data also gave some insight into the apparent inconsistency in survey responses between lack of personal equipoise over the value of invasive urodynamic testing on the one hand, and the majority view that the basic research question was important and associated with a high degree of willingness to randomise patients into a definitive RCT on the other hand.
-
While some clinicians’ views were shaped by genuine uncertainty about the value of IUTs, more commonly the research question was regarded as important because clinicians believed they personally knew the answer and wanted research in order to change others’ practice and bring it in line with their own.
-
This could introduce a significant bias to randomisation, if clinicians who regarded invasive urodynamic testing as essential were unwilling to have some of their patients denied it; or alternatively if those who use invasive urodynamic testing relatively infrequently were unwilling to risk their patients being exposed to what they see as unnecessary tests. While recognition of a degree of community equipoise may allow many to ‘suspend’ their lack of personal equipoise and agree to randomise their patients into a future definitive trial, it is likely that some will find this unacceptable.
Chapter 7 Patient interview study
Methods
Interviews were carried out to explore women’s understanding and their experiences of the study, the consent processes and their decision to participate. Purposive sampling was used to include women from a range of ages, trial participation status (did not agree to randomisation; randomised and retained to final follow-up; randomised but did not provide full follow-up data), allocation status (IUT or basic assessment), treatment received (surgery or conservative management) and study site.
The PIS included a description of this part of the study and an indication that women might be approached for interview. Those women who did not agree to being randomised within the trial were approached as soon as possible thereafter for interview. Women who did agree to randomisation were approached at the end of the trial, so as to capture both their reasons for agreeing to participate and their overall experience of taking part in the study.
A specific PIS was provided for the interview study (see Appendices 7 and 8) and written consent was obtained from all interviewees (see Appendices 11 and 12). The interviews were carried out by an expert qualitative interviewer (see Acknowledgements) and, with permission of interviewees, were audio recorded and transcribed verbatim. The vast majority of interviews were carried out face to face but a small number were completed by telephone due to participants’ availability and preferences. The interviews were semistructured using a prompt guide with broad topic areas but the emphasis was on encouraging women to discuss their own perspectives freely and allowing them to discuss issues that were important to them. The interviewer prompted as appropriate to ensure that all views were fully explained and the meaning of participants’ responses were clear. The prompt guide was developed from a literature review and discussions within the project team and was modified as the interviews progressed to incorporate issues raised by earlier interviewees. The purpose of the interviews was to explore women’s understanding and experience of the study, their decisions around participation and their perceived barriers to and facilitators of participation in a RCT. This information will inform the decision of whether or not to proceed to a definitive trial (i.e. whether or not women are likely to participate) and enable us to refine the content of the information given to women and the recruitment and data collection procedures used.
Data collection and analysis was iterative, using the constant comparative method. 88 Data collection continued until saturation of themes was reached, that is the point at which interviews no longer generated new concepts. As for the clinician interviews, transcripts were read three to four times and open codes were initially applied line by line to the data to represent the meaning or significance of each sentence or group of sentences by the data analyst. Generation of the open codes proceeded sequentially, with no attempt to impose any framework on the data. The open codes were then incrementally grouped into organising categories or themes, by the analyst and study lead together.
These categories were modified and checked constantly as further open codes were incorporated as analysis proceeded. When categories had been created to express all of the open codes, explicit specifications were written for each of the categories to assist in determining under what circumstances data should be assigned to any given category. The categories and their specifications (the coding scheme) were then programmed into NVivo 10 qualitative sofware. The coding scheme was used to process the data set systematically by assigning each section of text to a category, according to the category specifications.
Results
All women who declined to participate in the pilot study were invited to take part in an interview. A total of 51 were approached but, unfortunately, none were willing to be interviewed.
A total of 111 pilot study participants were invited to take part in an interview. A diverse sample was approached in order to include, those from different study sites; those from the two study arms; those who did and did not complete all follow-up; and a wide range of ages. A total of 36 women indicated they were willing to be interviewed. Of these, 29 were interviewed; two withdrew from the interview study before the interview could be arranged; one had moved and so was no longer covered by our research governance approvals; and four were not interviewed as they were from groups already well represented in the sample (all were contacted to explain this). Details of the final interview sample are shown in Table 20.
| Detail | Number |
|---|---|
| Study site | |
| Newcastle | 10 |
| Gateshead | 8 |
| Wansbeck | 3 |
| Leicester | 6 |
| Swansea | 2 |
| Total | 29 |
| Study arm | |
| Urodynamics | 13 |
| No further testing | 16 |
| Total | 29 |
| Trial status | |
| Completed follow-up | 17 |
| Incomplete follow-up | 12 |
| Total | 29 |
| Age (by year of birth) | |
| 1935–39 | 2 |
| 1940–44 | 0 |
| 1945–49 | 4 |
| 1950–54 | 1 |
| 1955–59 | 4 |
| 1960–64 | 6 |
| 1965–69 | 3 |
| 1970–74 | 5 |
| 1975–79 | 4 |
| Total | 29 |
The invitation to participate and reasons for agreeing
Women’s first reactions to receiving the invitation to participate in the pilot study were almost exclusively positive. The decision to take part was commonly made quickly and easily, and very few reported feeling the need to talk with family or friends as part of the decision-making process.
I didn’t really think about it at all I was, once it was explained to me, I was quite happy to do it.
Participant 11
As is commonly found in other studies,89–91 many women’s reasons for participation were altruistic and included wanting to help research, to help others with the same condition, and to make some form of repayment for the help and treatment they were receiving.
I felt like they were doing me a favour in trying to make my body work better, so the only thing I could do was to try and repay that, try and help them to improve the service and help improve it for other people.
Participant 03
Participating in the pilot did not seem to require a lot from them and so no particular participation burden was perceived.
She explained it very clearly and said all it is basically is just to monitor how many times you go to the toilet, and how much you drink, and roughly how much your output was. And to me I thought that wasn’t a big problem. Only a few minutes of your time in your day, just to keep track.
Participant 04
The specific nature of the study and the intervention being assessed was an important factor for many women. The possibility of having invasive tests performed prior to any surgical treatment was something that many were aware of and were worried about.
I had spoken to other people who had had the same operation as I was going to have and they had told me that the worst part about the operation, apart from being in hospital and having the operation and the discomfort afterwards, was having the tests beforehand and they said it just felt like there was a lot of discomfort and you know it’s just not a very nice experience.
Participant 08
Participants generally understood that by taking part in the study they might be able to avoid having these invasive tests, and for some this was an important motivating factor for participation.
There was a 50 : 50 chance I wouldn’t have to have urodynamics which I really didn’t want to have.
Participant 01
What really worried me [about pursuing treatment for her condition] was having all the bladder tests beforehand. Because I felt quite stressed about things like that and I was told there was a chance if I entered the trial I might still have to have them but there was a chance I might not have to have them which was quite a good incentive.
Participant 05
It is worth noting that of 11 participants withdrawing from the study, 5 did so within 6 weeks of randomisation (between 0 and 39 days); one was randomised in error; the other 4 had been randomised to receive invasive urodynamic testing; one withdrew because she did not wish to complete the study questionnaires, and 3 because they wished no further investigation.
Those who were subsequently randomised to the ‘no further testing’ arm reported being very pleased with this outcome.
They went and put my name in whatever it was, the random selection, and I came out with the non-diagnostic one which I was really pleased about.
Participant 14
This was discussed both in terms of wanting to avoid the tests per se, but also the possibility that this might shorten the time they had to wait for treatment.
I had heard that it was quite long winded and a slow process through various different tests and there was one of the tests that I had heard was quite horrible as well [. . . ] so when they said, you know we are doing this trial because we are not sure whether that’s actually necessary and if you are chosen for the [no further testing arm] you bypass all that I just thought great [laughs] because obviously it’s not something you want to keep having a problem with, you want to get it sorted as quickly as possible don’t you?
Participant 10
The information provided about the study
Reactions to the written information were mostly positive – it was regarded as clear and informative and there was enough information for women to be able to make a decision about taking part. The short version contained enough information for some people and the flow diagram was popular. Others liked to have the fuller detail in the longer version. Overall, most people found it helpful, describing it as easy to read, informative, and pitched at the right level.
So everything was really well explained you know, so yeah I mean I can’t fault it really, no I was well impressed with it all.
That one had that flow chart at the back as well do you remember?
Oh yes that’s right yes. This is very clear I thought.
I like to read everything. I feel more confident that I’m in the know, I know what procedure, what the procedure would take, how long it would take. I like to know everything about everything.
The use participants made of the material varied – some read it once only or just skimmed it, others read it more than once and a small number did additional research about the study on the internet.
I think I just read it, I didn’t take too much in I think, I think I was just so looking forward to getting my operation that is all I was really erm . . . really bothered about. I don’t think I read too much about the ins and outs of the study.
Participant 20
Basically I just went on-line and looked at the various things and just erm . . . just looked at the study.
Participant 15
Some were happy with the verbal information at the time of their consultation and paid little attention to the written material, particularly the longer version.
Personally I wouldn’t bother with the big one, I think that there is enough information, and if you get good medical staff to start with like I did, who actually took the time to go through it with you and say this is what this says, now read it on there, erm . . . so I think if you get that then you certainly don’t need the bigger one.
Participant 07
Several women commented on their wish for further information about their planned surgery, although this was not directly relevant to the study. Suggestions for how the study information itself might be improved were limited but included keeping it as short and concise as possible and sending it out prior to the consultation as some women reported they felt anxious at the consultation and so did not initially pay much attention to the information. Given that some women valued the verbal information they received from clinical staff more than the written information, being able to go to the consultation with questions prepared may have been helpful.
Understanding of the study
Participants’ understanding of the study was broadly good, although there were some cases in which people appeared confused about the overall aim. Overall, there was a generally good understanding that the study was assessing the value of a particular diagnostic test rather than the treatment they would ultimately receive. Many talked explicitly about how, while participation in the study could influence the route they took to treatment, it was ultimately unlikely to change the final outcome. Establishing this was often important to securing their participation.
I remember asking him ‘so if I don’t have the test will it have any effect on any treatment I have, and will it have any effect on you deciding what I need?’ No he said, it was purely for this investigation.
Participant 22
I knew it wasn’t going to make any difference to my care really other than whether or not I would have to have urodynamics.
Participant 01
Not all participants understood the study in this way, though. A small number, when asked to explain what they thought the study was about, did focus on the subsequent treatment rather than the invasive testing.
I think it’s about finding the right appropriate erm . . . ways forward to treat people with urinary problems. Erm . . . whether surgery or invasive treatment is appropriate or whether there is another kind of treatment that might be more beneficial.
Participant 17
I think it’s sort of, collecting information to see the difference in someone’s life after having the operation I think and how they felt the process had gone really I suppose.
Participant 20
The principle of random allocation to one of two possible groups was generally well understood.
That is where the flow chart was very clear about the two groups of people, that it would be totally random, whether you were selected for group A or group B.
Participant 12
I was either going to be chosen . . . I think 50% were chosen for a test and 50% weren’t. And I was probably [one of] the lucky ones because I didn’t have to have the test.
Participant 04
There were, however, a small number of participants who appeared to think that participation in the study automatically meant they would avoid the invasive tests.
Did you think there was a possibility that you might have the invasive tests?
Erm . . . no I think the registrar said to me if I signed up for the study I wouldn’t have them.
Experiences of study participation
The first set of questionnaires participants were asked to complete at baseline was generally described as simple to fill in, easy to understand and straightforward.
No problems at all [. . .] I found them easy to understand.
Participant 04
Very easy to understand . . . not things that you really had to think about.
Participant 07
A few minor issues were raised: there wasn’t always a box to tick that was applicable to them; some questions were hard to answer (e.g. when asked to work out costs or where judgement was called for); and some thought the questions were a little repetitive.
Sometimes there wasn’t, you know how there were tick boxes kind of thing, it . . . none of those were really the answer that I wanted to give.
Participant 11
A little bit repetitive but that’s how they are, and thinking I have already answered that.
Participant 15
There were also some comments on the practical challenges associated with measuring urine output for the bladder diary.
It was difficult, I couldn’t always get a correct amount of urine that I was passing because you can’t carry a jug around in work [laughs], so it was, basically some of it was a little bit of guesswork.
Participant 17
The second set of questionnaires sent out 6 months after treatment were similarly felt to be relatively simple to complete. However, given that many had had successful treatment and now had few, if any, symptoms to report, there was quite a lot of discussion about the relevance of the questions. Indeed, one participant reported having called the study office to check she had been sent the right questionnaires to complete, and others were a little concerned that it might appear that they had not completed the questionnaires at all because so much was not now applicable to them.
In fact I rang up about the second questionnaire because it seemed to be totally unsuitable. It was the same, it seemed to me to be the same or virtually the same [one] after the operation, as before.
Participant 12
I actually sent it back with absolutely nothing on it at all because it said ‘have you been to visit the doctor in 6 months’, and I hadn’t and it said go to the next section, and go to the next section and so by the end of it, there was nothing on it and I sent it back completely blank and I thought they will think I have not bothered filling this in.
Participant 14
While some actually found completing the 6-month questionnaires quite enjoyable (as it underlined for them how successful the treatment had been), others reported finding them burdensome and irrelevant now they had few or no symptoms to report.
Not relevant at all, not to me anyway. Yes, because I mean the problem was solved then so, why harp on about how many pads am I wearing now because I don’t wear them, simple as that, nothing.
Participant 09
This seemed particularly to apply to the bladder diaries.
It did want another bladder diary I think afterwards and I have not completed the bladder diary because I just didn’t get round to it to be honest with you. I had it in my bag to take to work with me and I just didn’t get round to doing it.
Participant 21
It was the sheer amount of them, because I had already done them in the past and then there was a whole other lot to do and then the final one was completely irrelevant.
Participant 01
Key messages
-
Women were, in general, very positive about the study and found the decision to participate straightforward; this is in keeping with the finding that the majority of eligible women agreed to randomisation within the trial.
-
We had hoped to interview some women who had declined randomisation, feeling that their views may be crucial to successful planning of a definitive trial. While it was regrettable that no ‘decliners’ agreed to interview, the fact that so few eligible women in fact declined randomisation mitigates the impact of this gap in our knowledge.
-
In addition to the ‘altruistic’ factors motivating participation, the potential to avoid having IUTs was an important factor for some women specific to this study.
-
Trial PISs (both short and full versions) were appreciated by interviewees. Supplementary information from trial and clinic staff was also seen as important, emphasising the need for appropriate training of all staff involved.
-
The importance of having information about the trial prior to consultation was emphasised; this triangulates with the need to have effective screening processes in all centres.
-
Questionnaires used at baseline and 6 months after the start of treatment were generally seen as being easy to complete, if a little repetitive (especially at 6 months); this is in keeping with the low rate of missing information from those questionnaires that were returned.
-
Repeating questionnaires at 6 months when many women had few, if any, symptoms to report was sometimes felt to be burdensome and irrelevant; this is in keeping with the number of blank follow-up questionnaires returned. In a future definitive trial, it would be important to emphasise the need to complete and return questionnaires even if there are few symptoms, but also to modify questionnaires to allow ‘short-cutting’ of irrelevant areas.
Chapter 8 Discussion
The randomised external pilot trial
The pilot trial can be considered a success. Although recruitment was initially slow, and was more successful in some centres than others throughout, we were able to recruit patients from all our study centres in sufficient numbers to confirm that recruitment was feasible, and that women were happy to engage with the study objectives and be randomised. The study procedures were seen to be adequate and functional in most areas, and we have gained important insights to inform the design and efficient conduct of a future definitive trial. These include, allowing a realistic time frame for regulatory approval and site start-up; employing a range of strategies to retain trial centre engagement (e.g. website, newsletters, recruitment updates, RtT thermometer); and modifying screening instructions and procedures to ensure that an ‘assume eligibility’ approach is employed. The potential for running standard-setting screening exercises for centres is an important consideration.
The pilot trial clearly demonstrated that there remains a need for a definitive study. We identified a change in planned treatment for 19% of the women randomised to the invasive urodynamic testing arm (compared with 4% in the no IUT arm). This confirms that undergoing invasive urodynamic testing does influence practice, and is in keeping with some other studies in this area,39,42,92 albeit the results have not been consistent across all studies. 36 Based on the outcome measures reported for the women at 6 months, the pilot trial suggests that there is a small difference in outcome as a result of this change in practice. Whether or not this difference is statistically, clinically and economically significant remains unproven, and will require a larger trial. Given the uncertainty around the cost-effectiveness outcomes (see below), the occurrence of a measurable change in practice with a limited difference in outcome and uncertain cost–benefit leads us to conclude that a definitive study is necessary.
Knowing the completion rates for the various questionnaire outcomes we have piloted is useful and will help to inform a future trial. Completion rates were high for all questionnaires with a similar rate and spread of missing items. It is however recognised that the completion of questionnaires can be burdensome for participants. 93 This may be particularly the case for those with few or no symptoms; this may account for the number of blank questionnaires returned at 6 months, and was apparent from the patient interview study. Accepting that the UDI was the fourth instrument in a booklet of 6 questionnaires in total, it had a slightly lower completion rate at both baseline and 6 months. The questions in ICIQ-UI SF overlap considerably with those in the longer ICIQ-FLUTS and so we recommend omitting both UDI and ICIQ-UI SF from the definitive trial to reduce respondent burden. We anticipate that this may improve completion of the remaining items. Greater emphasis needs to be placed on the importance of returning a completed questionnaire even in the absence of any remaining symptoms. Cost questionnaires could be modified to allow ‘short-cutting’ of irrelevant areas.
Bladder-diary data and pad-test use were poorly completed in our pilot. This may be because many of the women would have completed similar diaries or frequency/volume charts earlier in their continence assessment; it may be seen as rather more intrusive than simple questionnaire responses; it is possible that the diary design mitigated against consistent completion of pad-use data. The trial recruitment process enrolled only women with SUI or stress predominant MUI, and the diary data did not show any evidence of abnormal urinary frequency or nocturia and there appeared to be no change at 6 months in either arm (other than in pad-use). We recommend consideration of omitting or modifying diary data and pad use in the definitive trial, so as to focus on incontinence episodes in order to increase the completion rate of these data.
Few AEs were recorded during the study; these were evenly spread across the study arms; most were expected AEs related to treatment, and none were related to the trial intervention (IUT) itself. The most common anticipated AE following the trial intervention (IUT) is UTI, with reported incidences between 3% and 20%. 94 Information about the occurrence of UTI or additional GP visits was sought at the postoperative clinical review (CRF – visit 6). Patients would conventionally be advised to report persistent symptoms of increased frequency of micturition, dysuria or haematuria following IUTs. It is quite likely however that most episodes of UTI occurring following IUTs would have been reported to and treated by GPs rather than trial staff. Such episodes may not have been documented on CRFs nor reported to the NCTU, and their incidence may therefore be significantly under-reported here. We recommend introduction of a system for more effective capture of this information in a future definitive trial, for instance by giving patients a contact telephone number, or an event diary to note such events during follow-up.
There was a high rate of loss to follow-up after treatment. Although 75% of women had either face-to-face or telephone follow-up (typically at 2 to 3 months) after surgical treatment, only 56% (63% of those circulated) returned follow-up questionnaires at 6 months. The lack of follow-up questionnaires being returned may reflect the fact that most were happy with the outcome of their treatment, and we found some evidence to support this. Nevertheless, in a future definitive trial it would be necessary to ensure a much higher rate of response to the primary outcome. As suggested above, it would be desirable to rationalise the number of instruments and hence burden associated with questionnaire completion. Alternative modes of completion for follow-up questionnaires (e.g. telephone or web based), and providing incentives to return questionnaires,95 are further evidence-based strategies that might enhance retention rates for data collection. A further possibility is to link questionnaire completion at follow-up to the face-to-face clinic review, thereby allowing a check by a research nurse or trial co-ordinator of item completion before patients leave the clinic area; this would, however, require a change to the current practice of some units, and risk some of the pragmatic nature of the trial.
Screening and recruitment
It was evident during the pilot trial that, despite trial staff following a consistent protocol for patient screening, there was wide variation in the number of women identified and in the conversion rate from screening to recruitment between centres.
When trial staff involved in screening were presented with a standard series of vignettes of patient information, the rate of screening varied between 45% and 80%; however, the correlation between disparities in screening categorisation in this exercise and screening to recruitment ratios in the trial itself was not strong.
From the screening vignette exercise, it appeared that greater clarity in the definition of terms used within the inclusion and exclusion criteria might assist trial staff to identify more appropriately potential recruits in a future definitive trial.
The frequency with which information relevant to recruitment is omitted from GP referral letters suggests that in relevant patient groups it should be assumed that women are eligible, and study information should therefore be sent out by default, ecxept when obvious exclusions are specified.
Economic evaluation
There are a number of limitations within the economic evaluation presented. Despite the small amount of missing data within questionnaires that were returned, the relatively small number of completed questionnaires that were returned leaves the analysis open to non-response bias. The costs of non-invasive tests and other treatments were omitted from the cost–utility analysis; such data should of course be included in an economic evaluation conducted as part of a definitive study. Non-invasive tests might be performed on patients in both no IUT and IUT arms, and therefore, we could assume that these costs would be evenly distributed across the two groups and would not affect the overall difference in costs between study arms. Invasive urodynamic testing however was only performed on one (intervention) arm and hence the inclusion of this cost was crucial to identifying the differential costs of diagnostic tests between the IUT and no IUT arms.
Surgery was the expected treatment in the control arm and the majority (94%) of women in the no IUT arm underwent this procedure. Other treatments were available to the IUT arm where other diagnoses with or without USI were made on the basis of invasive urodynamic testing. Excluding these treatment costs from the economic analysis may have resulted in a considerable underestimate of the average treatment costs for those in the IUT arm (only 74% of whom underwent surgery).
For the pilot study, only NHS costs were considered in the cost–utility analysis; patient and caregiver costs were collected but not included in the current analysis. It is possible that both travel and out-of-pocket expenses including self-purchased health-care and related management costs might be different between trial arms. This information would be included in a future definitive trial.
The micro-costing of the IUT was based on the cost of resources at only one trial site. This could lead to an over/underestimation of the average cost of an IUT. In a definitive trial, this micro-costing should be conducted across a number of sites to generate the most accurate unit cost. Only one type of IUT was micro-costed in the pilot trial despite there being three types of IUTs in use. The majority of patients (92%) received dual-channel cystometry as their IUT; it needs to be determined for a definitive trial if costing this IUT is sufficient or if video- and ambulatory urodynamics should also be costed. The cost of surgery should also be micro-costed or alternatively data taken from a published costing exercise should a high-quality, UK-relevant study be available at the time when data analysis is conducted. The use of the standard NHS cost for a TVT surgery in this pilot could have meant that the costs of surgery were over/underestimated for patients in the pilot trial. Since this was done for both randomised arms it should not affect the overall cost-effectiveness of an IUT.
Notwithstanding these limitations, the economic analysis was successful as a component of the feasibility study. We have demonstrated that meaningful and usable data were collected using the instruments we designed for this purpose. The CRF pages where hospital-based costs were identified functioned well, with low rates of missing data (10% or less), most often in relation to additional personnel in the operating theatre or the urodynamic assessment. The CRF pages would need to be reviewed and reminders of the importance of completing all data fields would need to be included in a definitive trial. Revised standard operating procedures for the conduct of a definitive trial would make the data query pathway more robust and auditable.
The two-part PCQ appeared to perform reasonably well and most returned part A questionnaires having few items of missing data. Part B had a similar overall response rate although the item completion rate was slightly lower. Questions that appeared confusing and some areas that could be removed or combined together without loss of meaningful data have been identified. A piloting exercise for the revised PCQ forms with some patients in the early phase of the definitive trial would be advisable to ensure maximum ease of use of the final instrument.
In terms of the analysis, the feasibility study data demonstrated that costs, QALYs and cost-effectiveness can be derived from the data we have collected, although given the response rates, and limitations identified, any conclusions drawn from the current data can only be tentative. This preliminary analysis demonstrated invasive urodynamic testing to dominate the cost-effectiveness analysis, both in the full analysis and the complete case analysis. The dominant effect was, however, statistically uncertain, as demonstrated by the wide confidence intervals. The reasons for this may include the large number of missing data, and also the decision we took to omit non-surgical cost data in the women whose treatment decision was altered in the IUT arm. In actual fact, the cost-saving observed by 15% of this group not having immediate surgery will be partly offset by the additional costs of physiotherapy, behavioural modification and drug prescriptions which we have not analysed at this point. In a future definitive trial an economic model is recommended that would extrapolate from the short-term trial follow-up in to the longer term. Such a model would be informed by the results of the trial but also include effects and costs that persist over time. It is perhaps worthy of note that the small effect apparent in the economic analysis was in the opposite direction to the changes seen in patient reported outcomes in the pilot trial itself (albeit they were also small); this may simply be a reflection of sample size, or an indication that the two analyses measure fundamentally different things.
Despite the large number of missing questionnaires, it was reassuring to see that the sensitivity analyses (imputation, best- and worst-case analysis, see Appendix 27) produced entirely similar results which gives us confidence in the methods and assumptions our analyses required. In any future trial, the use of these sensitivity analyses should be adopted to ensure a robust and reliable final cost-effectiveness analysis including all costed elements in patients receiving both surgical and non-surgical treatments in each randomisation group.
Finally, it is noteworthy that the considerable uncertainty in the cost-effectiveness analysis provides further justification for a definitive trial.
Clinicians’ views
The clinician survey and qualitative interviews were directed at identifying professional opinion within a specialist group interested in the management of female UI. The overall response rate to the initial survey (34%) and subsequent update (29%) must leave any conclusions open to question, due to the potential for non-response bias. A number of previous surveys of similar national and international professional groups have been published, with response rates between 21% and 67%. 48,96–98 None of these studies employed incentives to take part, and indeed none used reminder letters or e-mails. 95,99 Clearly the level of interest or excitement generated by the topic in potential respondents is of importance in encouraging responses. It is, however, difficult to explain why surveys on such similar topics should achieve such varying response rates in different countries (21% to 57%)96,97 or why a survey on a clinical guideline48 should achieve a different response rate from one on a major recommendation from the same guidance (i.e. this study) (64% vs. 34%).
The limited information on the specialty group membership makes comparison of respondents and non-respondents difficult. It is possible that those who did respond may hold systematically different views on the use of invasive urodynamic testing and on the research question than those who did not participate in the survey. While this cannot be entirely refuted, the following findings would argue against this possibility. The surgical workloads reported by respondents to the initial (2011) survey amounted to a total of approximately 8300 procedures per year; HES for England reported approximately 12,500 procedures for SUI in 2009–10 and 2010–11. 14 While informal, self-reported surgical activity is notoriously unreliable, the respondents clearly embrace a significant proportion of incontinence surgery. All respondents reported having access to invasive urodynamic testing, with 79% undertaking investigations themselves, confirming the relevance of their opinions to the survey questions.
We found that the majority of respondents to the survey considered invasive urodynamic testing to be necessary to a greater or lesser degree before surgical intervention in SUI, whether or not patients have additional symptoms suggestive of OAB or VD. Not only were few clinicians apparently undecided on the issue (i.e. were in personal equipoise), but there was little evidence of professional community equipoise. Only when clinicians are in equipoise on an issue or recognise it to be an area of genuine uncertainty are they likely to feel comfortable to randomise their patients between treatments or, as in this case, investigation strategies. Hence, measuring surgeon preference is a crucial component of trial feasibility.
Despite this lack of personal equipoise and the fact that invasive urodynamic testing was considered necessary across all scenarios, the majority of respondents regarded the basic research question as being important (70%), and most would be prepared to randomise patients into a definitive RCT to address this (60%). These views persisted over the 2 years between our initial survey and the update, despite publication of two other trials addressing a similar research question. 36,39 Analysis of the interview study data gives some insight into the reasons for this apparent inconsistency. It might be anticipated that clinicians would only regard a research question as important and be prepared to randomise their patients in a study where they themselves were uncertain of the best course of action and are looking to the study as a means of resolving that uncertainty. However, discussion of these issues in interviews revealed a more complex picture. While some clinicians’ views were shaped by genuine uncertainty about the value of invasive urodynamic testing, more commonly the research question was regarded as important because clinicians believed they knew the answer and wanted research in order to change others’ practice and bring it in line with their own. This could introduce an important complicating factor to whether or not they would be prepared to randomise patients because clinicians who regarded invasive urodynamic testing as essential may not be willing to have some of their patients be denied it. However, in contrast, those who appeared genuinely uncertain about invasive urodynamic testing, or were happy not doing it, were the ones that seemed happiest to randomise. While recognition of a degree of community equipoise may allow many to ‘suspend’ their lack of personal equipoise and agree to randomise their patients into a future definitive trial,100 it is likely that some will find this unacceptable. We are not aware of any evidence regarding whether or not these different stances may affect willingness to fully engage with the trial or pursue it to completion. From the survey update, however, there is an indication from a majority of our target group of their willingness to recruit patients into a future definitive study.
Survey reminders and response times
Following the initial survey, the majority of responses were received after the first circulation and over 90% were received after a first reminder. The majority of responses were received within 2 weeks of original distribution or reminders.
Previous systematic reviews on the subject of postal surveys suggest that the more reminders are undertaken, the better the response rate;95,99 our experience would suggest that with similar e-mail/online surveys, no more than one reminder is necessary, and the time between initial distribution and reminder, and between reminder and site closure can be limited with minimal loss to responses.
In the update survey, albeit a briefer enquiry using a single reminder e-mail and a shorter response time, we obtained a very similar response rate from specialists to that seen from the initial survey. This would seem to validate this accelerated approach in online surveys.
Patients’ views
The patient interview study showed our patients to be generally very positive towards all aspects of the trial and found the process of approach, screening, consent and recruitment to be accessible, straightforward and easy to understand. The trial processes before investigation and during follow-up were not burdensome, although we obtained some helpful comments in relation to the completion of long questionnaires in the absence of residual symptoms. It was interesting to learn that a number of respondents had a previously undeclared preference for avoiding invasive urodynamic testing and, while willing to be randomised, expressed relief at being allocated to no further testing. This finding certainly resonates with clinical experience that many women find the urodynamic assessment (or the anticipation of it) to be worrying or slightly distressing. 101,102 This is in marked contrast to the overwhelming view of the majority of clinicians responding to the survey,103 for whom invasive urodynamic testing is seen as essential in most clinical situations prior to surgical treatment; it also contrasts with the perception that invasive testing may be ‘what women want’. 50 This dichotomy of views stresses the importance of this research question to define more clearly in what situations invasive urodynamic testing can be avoided, and therefore provides further support for proceeding to a definitive study.
Determining the sample size for a future definitive trial
As recommended in a forthcoming monograph on ways of specifying a target difference for a trial, we tried to determine estimates from more than one approach. 87 The first approach was to try to elicit information for a future trial by a survey of consultant members of the BSUG and the BAUS-SFNUU (see Chapter 5). Among other things, the update survey in June 2013 asked these clinicians the following question:
The ICIQ-FLUTS questionnaire is scored between 0 and 48. What do you consider is the minimum difference in ICIQ-FLUTS combined symptom score that you would consider to be clinically important (as opposed to statistically significant)?
The ICIQ-FLUTS scale has not been used in many published studies to date, and, perhaps unsurprisingly, only 50% of consultants responding expressed an opinion. They were given a choice of seven ranges of the scale to define the clinically important difference (from 1–4 to > 24) and all these ranges were chosen by at least one clinician, with the modal range being 9–12 (see Figure 18). It is not known how strong and informed their views were. However, in separate discussions, members of the study team did not find it easy to choose a target difference based on the limited use of the scale so far.
Another approach to setting the target difference was to use data from the external pilot trial: the SD of the primary outcome would inform the sample size calculation and allow any target difference to be expressed as a standardised effect size. When the pilot trial results became available, it became apparent that the distribution of the ICIQ-FLUTS total score at 6 months, and the difference between scores at baseline and 6 months, typically had low values. The mean score (SD) at 6 months in the ‘no-IUT’ arm was 7.3 (5.3) and the mean change between baseline and 6 months was 9.3 (7.3). It was therefore not realistic to see differences in mean outcomes between trial arms in the order of 9–12 units. Given the trial results, the study team then decided that differences of 2, 3 or 4 units would be a realistic and meaningful difference that might be achieved in any comparison of an intervention for women eligible for a future trial. It was felt that a difference of around three units would also be of clinical interest since a decrease of this level would equate to complete recovery for one of the symptoms assessed in the ICIQ-FLUTS score. Given the observed SDs, these target differences of 2, 3 or 4 units are equivalent to standardised effect sizes of 0.33, 0.50 and 0.67 when comparing mean scores at 6 months, or 0.29, 0.43 and 0.57 when comparing mean changes in score over 6 months. In contrast, a difference of 9–12 units would equate to a standardised effect size of 1.5–2.0, which is a very large difference; many trials are planned on a standardised effect size of around 0.5. Cohen has suggested that standardised differences of 0.2, 0.5 and 0.8 correspond to ‘small’, ‘medium’ and ‘large’ effect sizes. 104
If a study is planned on the basis of a ‘realistic’ value for the target difference, then consideration has to be made of whether or not this is also a ‘clinically important’ difference. If it is clear that this is not a ‘clinically important’ difference, then there are real doubts whether or not the trial should take place. In this case, the modal estimate of a ‘clinically important’ difference from the clinician survey was much higher than our estimate of ‘realistic’ target differences having seen the pilot trial results. However, these ‘realistic’ differences correspond to small or medium standardised effect sizes and recovery in one of the symptoms investigated. In addition, the current lack of data from published trials using ICIQ-FLUTS, and therefore evidence on which to base expert judgement, casts some doubt of the usefulness of a survey of experts in this situation. We have therefore used the pilot trial results to derive target differences on which to plan a future definitive trial.
The key parameters necessary to calculate the sample size are shown below:
-
type 1 error: 5%
-
power: 90%
-
eligibility rate among those screened: 37%
-
recruitment (consent) rate of those found eligible: 78%
-
response rate for ICIQ-FLUTS at 6 months for those recruited (i.e. retention rate for primary outcome): 56%
-
SD of ICIQ-FLUTS at 6 months: 6
-
SD of change in ICIQ-FLUTS from baseline: 7
-
correlation between ICIQ-FLUTS at baseline and 6 months: 0.25
-
smallest difference between mean ICIQ-FLUTS scores in trial arms that is of clinical interest – as chosen: 2, 3 and 4.
There are two possible approaches to analysis, and hence sample size calculations, when data are available at baseline and follow-up:
-
comparing mean changes between baseline and follow-up, or
-
comparing means at follow-up adjusting for baseline.
Tables 21 and 22 show the necessary numbers that would have to be screened, approached for recruitment to trial and provide response data at follow-up. Table 21 shows that, if the minimum difference of interest in change scores was two units, then a total of 516 responses on the primary outcome (258 per arm) would be needed. This would require 3194 women to be screened, of whom 1182 would be eligible and asked to take part in the trial and 922 would need to be recruited.
| Requirement | Difference to be detected | ||
|---|---|---|---|
| 2 | 3 | 4 | |
| Number of RESPONSES to primary outcome | 516 | 230 | 130 |
| Number of RECRUITED patients | 922 | 410 | 232 |
| Number of eligible women APPROACHED | 1182 | 526 | 298 |
| Number of women SCREENED for eligibility | 3194 | 1422 | 806 |
| Requirement | Difference to be detected | ||
|---|---|---|---|
| 2 | 3 | 4 | |
| Number of RESPONSES to primary outcome | 356 | 158 | 90 |
| Number of RECRUITED patients | 636 | 282 | 162 |
| Number of eligible women APPROACHED | 816 | 362 | 208 |
| Number of women SCREENED for eligibility | 2206 | 978 | 562 |
The numbers required for a trial comparing mean changes are greater than those comparing means at follow-up adjusting for baseline. However, as shown in Figure 7, the distribution of the primary outcome at follow-up was very positively skewed, so the sample size calculations based on the SDs of this variable are potentially misleading. Those based on the change scores are therefore more appropriate. If a future definitive trial was designed on the more conservative basis of the sample size necessary for a change score analysis, this should also provide at least 90% power for a comparison of means at follow-up adjusted for baseline.
Results integrated into Cochrane meta-analysis
The Cochrane review on urodynamic investigation for the management of UI in adults and children was first reported in 2002, with new citations added in 2006, 2011 and 2012. The most recent review was undertaken during the course of the INVESTIGATE-I study, and included two new trials. 34–36,39 A pre-publication version of this review was shared with the current authors,42 and one of the authors of the Cochrane review, a member of the INVESTIGATE-I TSC, agreed to incorporate outcomes from the pilot trial into appropriate meta-analyses. The following outcomes were incorporated:
-
number with incontinence within first year (subjective) (ICIQ-FLUTS Q10a; response = ‘> never’) – see Figure 19
-
number reporting SUI at clinic visit within first year (from subjective reports on CRF – ‘visit 6’) – see Figure 20
-
number treated conservatively (from non-surgical treatments on CRF – ‘visit 5’) – see Figure 21
-
number treated with drugs (from non-surgical treatments on CRF – ‘visit 5’) – see Figure 22
-
number treated with surgery (from surgical treatments on CRF – ‘visit 4’) – see Figure 23
-
number whose treatment was changed after urodynamics (from non-surgical treatments on CRF – ‘visit 5’) – see Figure 24
-
number with urgency symptoms or urgency incontinence after treatment from subjective reports on CRF – ‘visit 6’) – see Figure 25
-
number of AEs/complications after treatment (from AE and SAE reports to NCTU) – see Figure 26.
FIGURE 19.
Forest plot: number with any incontinence within first year (subjective). (Updated from graph 1.1 in Cochrane review. 42)
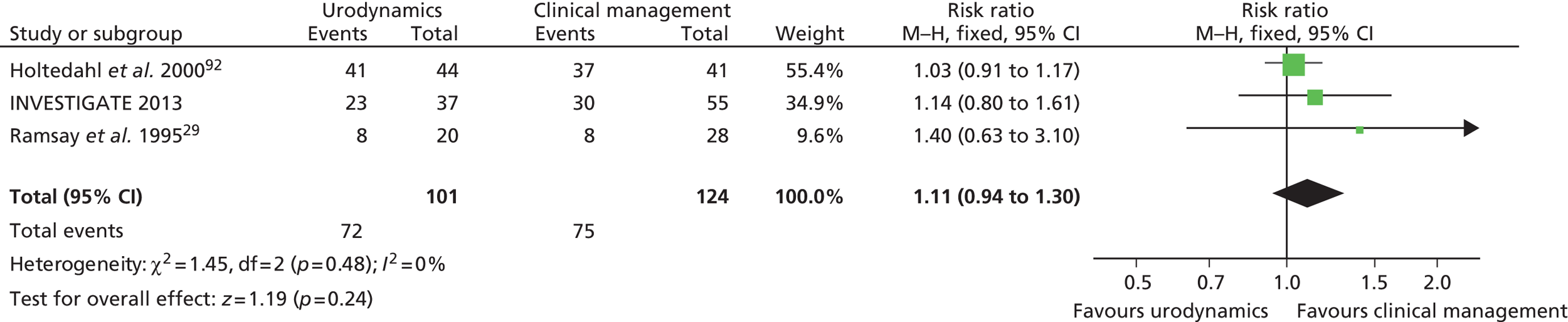
FIGURE 20.
Forest plot: number reporting SUI at clinic visit within first year. (Updated from graph 1.16 in Cochrane review. 42)
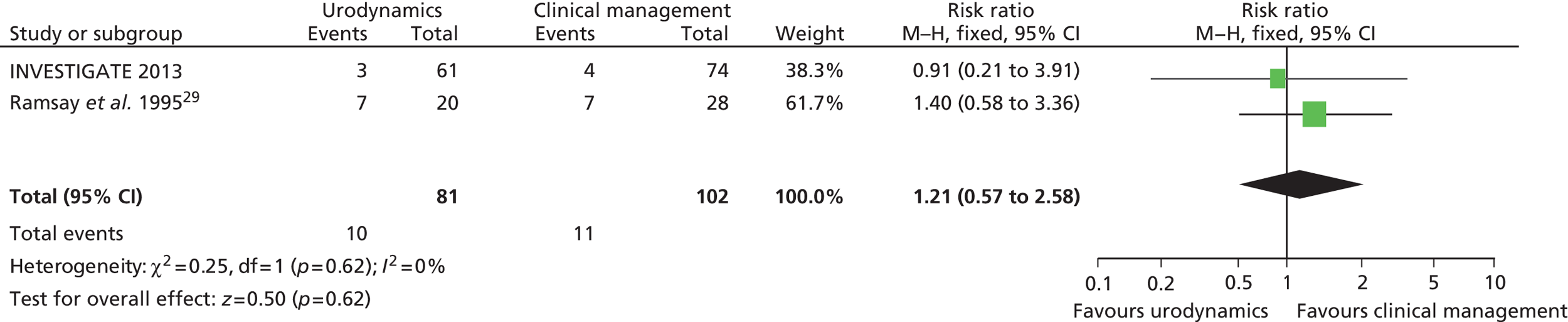
FIGURE 21.
Forest plot: number treated conservatively. (Updated from graph 1.5 in Cochrane review. 42)
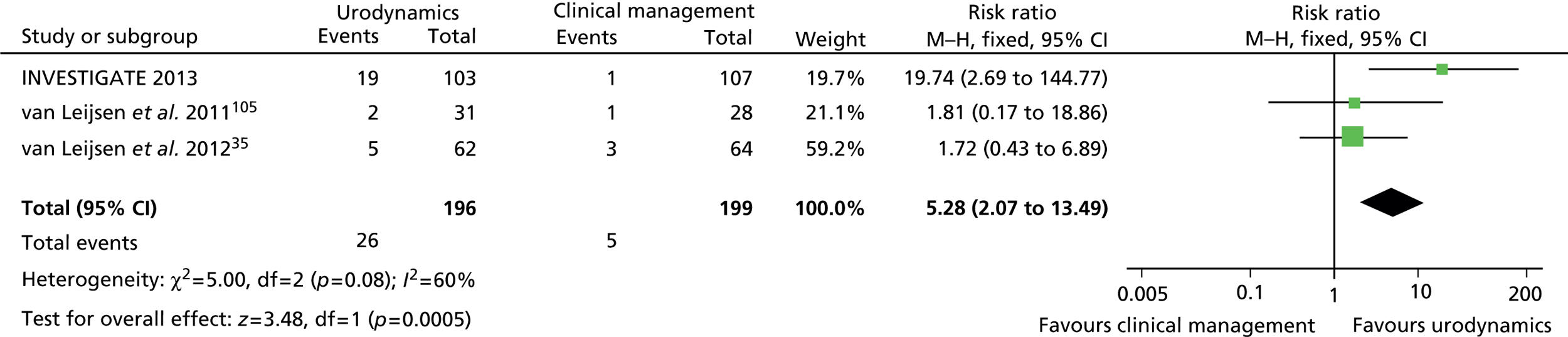
FIGURE 23.
Forest plot: number treated with surgery. (Updated from graph 1.7 in Cochrane review. 42)
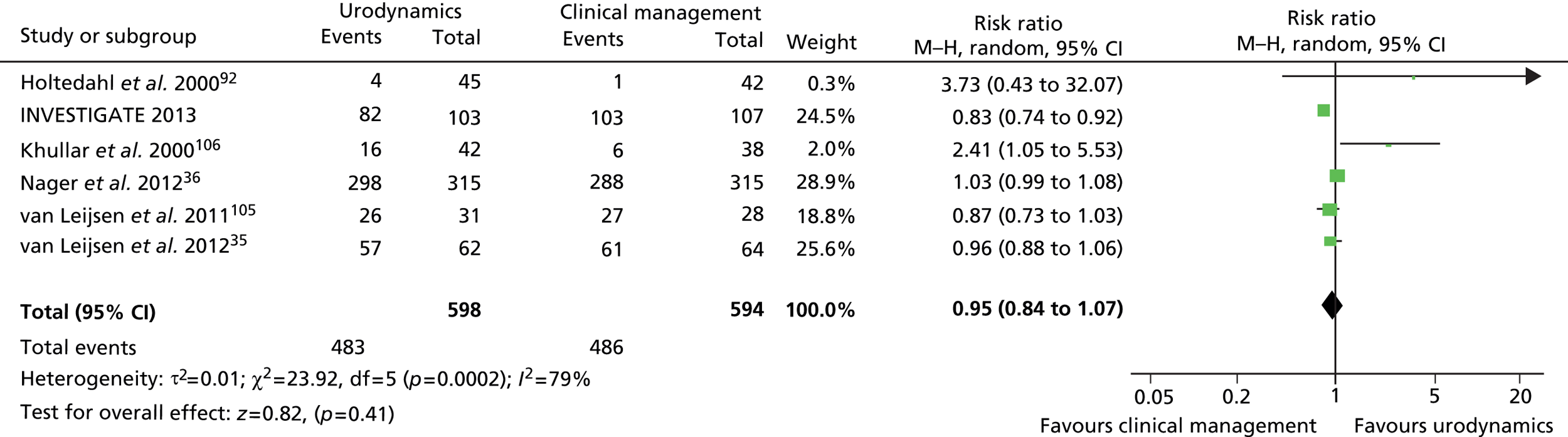
FIGURE 24.
Forest plot: number whose treatment was changed after urodynamics. (Updated from graph 1.8 in Cochrane review. 42)
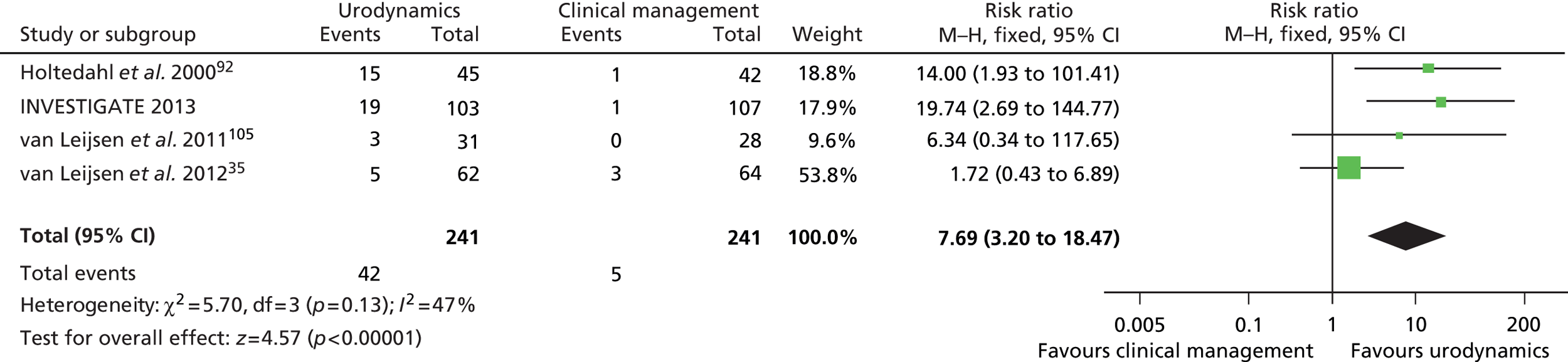
FIGURE 25.
Forest plot: number with urgency or urgency incontinence after treatment. (Updated from graph 1.11 in Cochrane review. 42)

FIGURE 26.
Forest plot: number of AEs/complications after treatment. (Updated from graph 1.18 in Cochrane review. 42)
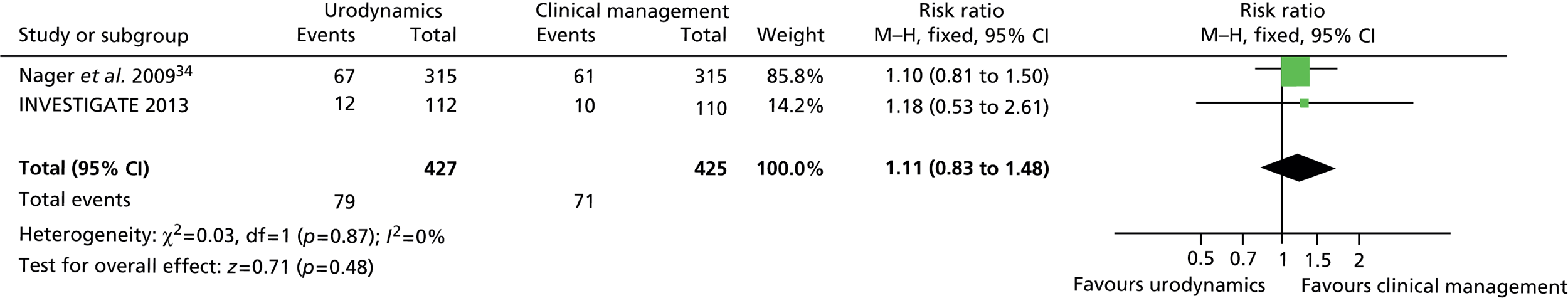
The authors’ conclusions from the Cochrane review included the following:42
When women with incontinence are assessed using urodynamics in addition to clinical methods, they are more likely to receive different treatment, and to have their management plan changed. However, the evidence was not conclusive in showing whether these differences in management resulted in differences in health outcomes, such as incontinence, quality of life or economic outcomes after treatment compared to women who did not have urodynamic tests.
Clement et al. ,42
The addition of the data from INVESTIGATE-I adds weight to the conclusion relating to changes in treatment (see Figure 24), since more women in the IUT arm received conservative and drug treatments than those in the control arm (see Figures 21 and 22). There was no significant difference in the proportion of women treated by surgery overall (see Figure 23); although fewer women in the IUT arm of INVESTIGATE-I received surgery, this analysis is dominated by one of the larger studies. 36
The review found no statistically significant differences in the rate of UI symptoms in the first year after treatment, and the addition of data from INVESTIGATE-I would not change this conclusion (see Figures 19 and 20). No other study had used our primary outcome ICIQ-FLUTS, so the meta-analysis cannot add to our pilot trial results. The Cochrane reviewers also indicate that:
in order to give a definitive answer to the question of whether urodynamic studies are no better than clinical assessment in significantly reducing incontinence in women at one year follow up, a trial of 3222 participants would be required. Assuming the incontinence event rate is similar to that of the four trials already included in this analysis, 1611 patients per arm would reduce the confidence interval of the risk ratio to ± 10% [RR would be 1.02 (95% CI 0.94 to 1.10)]
Clement et al. ,42
This calculation differs from that we produced based on the pilot trial; this is because the outcome used (incontinence or not, at 1 year) is binary and this usually requires a larger sample size than a numeric scale. In addition, the reviewers have chosen a precision of ± 10% around the risk ratio as the criteria for deriving the sample size; it is not clear why this would make such a trial definitive.
However, bearing in mind the anticipated size of a definitive trial to follow INVESTIGATE-I, and the inevitable narrowing of the uncertainty such a large study will provide, it would have a high likelihood of narrowing the uncertainties present in the majority of these comparisons and hence of answering the question of the role of invasive urodynamic testing in women with SUI or stress predominant MUI.
Chapter 9 Conclusions
Having considered the data presented in this report, the study team are confident that a definitive trial is feasible and remains necessary. We have successfully rehearsed the trial processes and, in doing so, have identified several ways in which the design and conduct of a future definitive trial can be improved. Our experience provides useful lessons in how to manage the time needed to bring multiple centres online through the UK regulatory process; we have produced an accurate and realistic estimate of the variation in recruitment likely from multiple centres and have rehearsed and refined effective methods of communication to keep staff engaged through the lifetime of a long study.
Refinements in the data collection process that will improve the quantity and quality of the data for a definitive trial have been identified, and we have also shown that a robust economic analysis is possible and produces consistent data.
Our interview studies produced some fascinating insights into the opinions of our clinical colleagues; most notably many expressed interest in supporting our work, and a definitive study in due course. The patients were very much of a positive mind about the study, and in particular allayed our fears about whether or not research to ‘test a test’ would be seen as important by them. The interviews also offered suggestions as to how the experience of participation could be improved and data collection maximised.
Clearly a definitive trial would be a challenge. Using the data from Table 21, our sample estimates fall between approximately 400 and 900 women recruited; with a recruitment rate of 78%, this would require between approximately 500 and 1200 eligible women to be approached; in turn, with a screen positive rate of 37%, this would mean between approximately 1400 and 3000 women would need to be identified for screening for eligibility; these ranges depend on the chosen outcome measure and effect size.
In this pilot trial, we identified 771 women for screening from seven centres over the course of 114 centre screening months (approximately 6.8 women/centre/screening month). Extrapolation of these figures would require 250–560 centre screening months to achieve the recruitment of 400–900 women. This would mean 8–20 centres recruiting for approximately 30 months or 15–30 centres recruiting over 18 months.
From our clinician survey update in 2013, we identified 102 individual consultant surgeons (representing approximately 90 separate hospitals or NHS trusts in the UK) who were willing to take part in a definitive trial.
Thus, while a multicentre study of this size is certainly challenging, these survey results suggest that there are sufficient centres expressing an interest in taking part to ensure that it can be delivered. Having a higher number of centres would have the advantage of a shorter recruitment window, which will reduce the risk of recruitment fatigue.
Why should further research in this area be commissioned?
The current position of invasive urodynamic testing in the diagnostic pathway for UI is not agreed and practices vary considerably. The existing evidence base to guide practice is limited, and several systematic reviews have concluded that there is a need for large clinical trials to establish clinical utility; patients and clinicians in a James Lind Alliance working partnership also identified this as an area of significant uncertainty and a research priority.
While currently the majority of clinicians managing patients with SUI in secondary care see invasive urodynamic testing as essential prior to surgical treatment, many also recognise the lack of evidence to support this view. The mismatch between clinicians’ and patients’ views over the application of invasive testing in this area justifies urgent attention.
We believe that this feasibility study and the lessons learned will facilitate the effective delivery of a definitive trial to address the continuing uncertainties regarding invasive urodynamic testing in women with SUI, which may therefore have a significant impact on the delivery and cost of continence services in the UK in the future.
Acknowledgements
The authors wish to express their gratitude to the following, who are considered members of the INVESTIGATE studies group:
Dr Mark Deverill was a co-applicant on the grant; due to a redistribution of duties within the health economics group in the Institute of Health & Society, he was replaced in the study team by LV. Professor Adrian Wagg was a co-applicant on the bid, but moved to take up a post in Canada prior to the finally accepted proposal and was replaced in the study team by CRC.
Principal investigators at other recruiting sites: Mr Andrew Beeby (Queen Elizabeth Hospital, Gateshead), Mr Richard Sill (Wansbeck Hospital, Northumberland) and Mr Paul Ballard (South Tees Hospitals).
Other consultant colleagues at recruiting sites: Dr Karen Brown, Mr Tahseen Hassan, Mr Chris Harding and Mr Andy Thorpe (Newcastle upon Tyne); Dr Mausumi De (Gateshead); Mr Chris Mayne and Mr Rod Teo (Leicester); Mr Steven Radley (Sheffield); Mr Simon Emery, Mr Jay Khastgir, Mr Robert Skyrme, Mr Andrew Allman, Miss Margaret Williams and Mr Arjun Nambiar (Swansea); Mr Athele Khunda (South Tees Hospitals).
Consultant colleagues at PIC sites: Mr James Nwabineli (South Tyneside) and Mr Jonathan Chamberlain (Sunderland).
Research associates: Dr Megan Murdoch (Newcastle upon Tyne); Mr Altaf Mangera and Dr Nadir Osman (Sheffield).
Colleagues contributing to trial management (at the NCTU): Mr Chris Speed (senior trial manager), Ms Shelley O’Rourke (trial secretary), Mrs Ruth Wood (database manager), Ms Julie Doughty (randomisation service) and Mrs Pauline Potts (assistant database manager) and the staff of NData UK Ltd.
Dr Janet Willars who conducted the qualitative interviews and Mrs Elizabeth Shaw who assisted with analysis of interview data.
Research and clinical nurses: Mrs Angela Black, Ms Liz Dixon and Mrs Wendy Robson (Newcastle upon Tyne); Ms Janet Rose (Gateshead); Mrs Helen Howlett and Mrs Corrine Farrell (Wansbeck); Mrs Lucy Barlow and Mrs Rebecca Thomas (Swansea); Ms Victoria Fowler and Miss Katie Behan (Leicester); Mrs Susannah Hulton (Sheffield); Mrs Julie Potts, Mrs Colette Anderson and Miss Victoria Phelps (South Tees).
In addition the authors wish to express their gratitude to the following:
Staff in R&D and finance departments of the trial sponsor, NuTH: Ms Amanda Tortice, Professor Gary Ford, Ms Manju Agarwal, Ms Wendy Mitson, Ms Christine Hughes, Ms Gill Chater, Ms Carolyn Robinson and Ms Vanessa Knowles.
Other members of the TSC: Professor Marcus Drake, Professor Cathryn Glazener, Dr Joy Adamson, Mrs Kate Partridge and Mrs Brenda Waller.
Members of the DMEC: Professor Steven Thornton, Dr Pamela Warner and Dr Fiona Reid.
Professor Cathryn Glazener, through her role with the Cochrane Incontinence Review Group, also helped with the formulation of forest plots setting our results in context of the latest Cochrane review on urodynamic investigation.
Dr Lindsay Marshall, Senior Lecturer in Computing Science, Newcastle University, for help with the RtT thermometer HTML code.
And finally, all patients and clinicians who contributed their time to take part in the trial, surveys and interview studies included in INVESTIGATE-I.
The authors also acknowledge the support of the NIHR through the Comprehensive Clinical Research Network.
Contributions of authors
Paul Hilton is the lead grant holder, he conceived the study, led on the protocol development, questionnaire design and writing the manuscript, and approved the final version for publication.
Natalie Armstrong, Denise Howel, Douglas G Tincello, Malcolm G Lucas, Brian S Buckley, Christopher R Chapple and Elaine McColl are coholders of the grant, and along with Luke Vale contributed to protocol development and to writing the manuscript, and approved the final version for publication.
Catherine Brennand was the trial manager, contributed to protocol development, questionnaire and database design, and to writing the manuscript, and approved the final version for publication.
Jing Shen, Andrew Bryant and Tara Homer contributed to statistical and economic analyses, as well as to writing the manuscript, and approved the final version for publication.
Natalie Armstrong additionally led on the interview studies.
Denise Howel additionally led on the statistical analysis.
Luke Vale additionally led on the health economic analysis.
Elaine McColl additionally led on the survey questionnaire design and formatting.
Paul Hilton, Natalie Armstrong, Douglas G Tincello, Malcolm G Lucas and Christopher R Chapple additionally contributed to data acquisition.
Study governance references
| Study full title | INVESTIGATE-I (INVasive Evaluation before Surgical Treatment of Incontinence Gives Added Therapeutic Effect?): a mixed-methods study to assess the feasibility of a future randomised controlled trial of invasive urodynamic testing prior to surgery for stress urinary incontinence in women | |
| Study short title | INVESTIGATE-I (INVasive Evaluation before Surgical Treatment of Incontinence Gives Added Therapeutic Effect?) | |
| Trial sponsor | NuTH | 5468 |
| Trial funder | NIHR Evaluation, Trials and Studies Co-ordinating Centre | 09/22/136 |
| Trial registration | International Standard Randomised Controlled Trials Register | ISRCTN71327395 |
| Ethical approval | Newcastle & North Tyneside 1 REC | 10/H0906/76 |
| NHS approvals | NIHR Co-ordinated System for gaining NHS Permissions | 62776 |
| Clinical Research Network portfolio | Reproductive Health/Urogenital (Surgery) | 10252 |
Study outputs
Publications
Murdoch M, McColl EM, Howel D, Deverill M, Buckley B, Lucas M, et al. INVESTIGATE-I (INVasive Evaluation before Surgical Treatment of Incontinence Gives Added Therapeutic Effect?): study protocol for a mixed methods study to assess the feasibility of a future randomised controlled trial of the clinical utility of invasive urodynamic testing. Trials 2011;12:169. URL: www.trialsjournal.com/content/pdf/1745-6215-12-169.pdf (accessed 19 December 2014).
Bosch JLHR, Cardozo LD, Hashim H, Hilton P, Oelke M, Robinson D. Constructing trials to show whether urodynamic studies are necessary in lower urinary tract disease. Neurourol Urodyn 2011;30:735–40. (This paper is not directly related to the INVESTIGATE studies, but includes reference to them and other related ongoing studies.)
Hilton P, Bryant A, Howel D, McColl E, Buckley B, Lucas M, et al. Assessing professional equipoise and views about a future clinical trial of invasive urodynamics prior to surgery for stress urinary incontinence in women: a survey within a mixed methods feasibility study. Neurourol Urodynam 2012;31:1223–30. URL: http://onlinelibrary.wiley.com/doi/10.1002/nau.22328/pdf (accessed 19 December 2014).
Hilton P, Armstrong N, Brennand C, Howel D, Shen J, Bryant A, et al. INVESTIGATE-I: a mixed methods study to assess the feasibility of a future randomised controlled trial of invasive urodynamic testing prior to surgery for stress urinary incontinence in women. Int Urogynecol J Pelvic Floor Dysfunct 2014;25(Suppl. 1):S182–3 (abstract).
Armstrong N, Hilton P. Doing diagnosis: whether and how clinicians use a diagnostic tool of uncertain clinical utility. Soc Sci Med 2014;120(2014):208–14. URL: http://ac.els-cdn.com/S0277953614005991/1-s2.0-S0277953614005991-main.pdf?_tid=e7929612-87a6-11e4-beab-00000aacb35d&acdnat=1419011344_a5bd0d72d34bc1d267a3d0eccb5059c8.
Presentations to learned societies
Hilton P. Constructing trials to show whether urodynamics are necessary in surgery for female stress urinary incontinence. International Consultation on Incontinence – Research Society, 2nd annual meeting, Bristol, June 2010.
Hilton P, Bryant A, Howel D, Armstrong N, McColl E on behalf of the INVESTIGATE Study Group. Assessing professional equipoise over urodynamics: a clinician survey within a mixed methods feasibility study. United Kingdom Continence Society 19th Annual Scientific Meeting, Liverpool, April 2012.
Armstrong N, Willars J, Hilton P. More than just a test: the social functions of invasive urodynamic tests. BSA Medical Sociology Annual Conference, Leicester, September 2012.
Hilton P, Armstrong N, Brennand C, Howel D, Shen J, Bryant A, et al. INVESTIGATE-I: a mixed methods study to assess the feasibility of a future randomised controlled trial of invasive urodynamic testing prior to surgery for stress urinary incontinence in women. United Kingdom Continence Society 21st Annual Scientific Meeting, London, April, 2014.
Hilton P, Armstrong N, Brennand C, Howel D, Shen J, Bryant A, et al. INVESTIGATE-I: a mixed methods study to assess the feasibility of a future randomised controlled trial of invasive urodynamic testing prior to surgery for stress urinary incontinence in women. Joint American Urogynecologic Society/ International Urogynecological Association Scientific Meeting, Washington DC, July 2014.
Homer T, Vale L, Shen J, Hilton P, on behalf of the INVESTIGATE studies group. Cost-utility and value of information analyses on the feasibility of a future randomised control trial of invasive urodynamic testing prior to surgery for stress urinary incontinence in women. International Society For Pharmacoeconomics and Outcomes Research, 17th Annual European Congress, Amsterdam, the Netherlands, November 2014.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Farage MA, Miller KW, Berardesca E, Maibach HI. Psychosocial and societal burden of incontinence in the aged population: a review. Arch Gynecol Obstet 2008;277:285-90. http://dx.doi.org/10.1007/s00404-007-0505-3.
- Hajjar RR. Psychosocial impact of urinary incontinence in the elderly population. Clin Geriat Med 2004;20:553-64. http://dx.doi.org/10.1016/j.cger.2004.04.009.
- Shaw C. A review of the psychosocial predictors of help-seeking behaviour and impact on quality of life in people with urinary incontinence. J Clin Nurs 2001;10:15-24. http://dx.doi.org/10.1046/j.1365-2702.2001.00443.x.
- Wyman JF, Harkins SW, Choi SC, Taylor JR, Fantl JA. Psychosocial impact of urinary incontinence in women. Obstet Gynecol 1987;70:378-81.
- Moore KH, Wagner TH, Subak L, de Wachter S, Dudding T, Hu TW, et al. Incontinence – 5th International Consultation on Incontinence. Paris, 2012. Arnhem, the Netherlands: ICUD-EAU; 2013.
- Milsom I, Altman D, Cartright R, Lapitan M, Nelson R, Sillen U, et al. Incontinence – 5th International Consultation on Incontinence. Paris, 2012. Arnhem, the Netherlands: ICUD-EAU; 2013.
- McGrother CW, Donaldson MM, Shaw C, Matthews RJ, Hayward TA, Dallosso HM, et al. Storage symptoms of the bladder: prevalence, incidence and need for services in the UK. BJU Int 2004;93:763-9. http://dx.doi.org/10.1111/j.1464-410X.2003.04721.x.
- Hannestad YS, Rortveit G, Sandvik H, Hunskaar S. A community-based epidemiological survey of female urinary incontinence: the Norwegian EPINCONT study. Epidemiology of Incontinence in the County of Nord-Trondelag. J Clin Epidemiol 2000;53:1150-7. http://dx.doi.org/10.1016/S0895-4356(00)00232-8.
- Papanicolaou S, Pons ME, Hampel C, Monz B, Quail D, Schulenburg MG, et al. Medical resource utilisation and cost of care for women seeking treatment for urinary incontinence in an outpatient setting. Examples from three countries participating in the PURE study. Maturitas 2005;52:S35-47. http://dx.doi.org/10.1016/j.maturitas.2005.09.004.
- Aguzzi G, Bartoli S, Tarricone R. Systematic Review of Urinary Incontinence and Overactive Bladder Cost-of-Illness Studies. Open Pharmacoecon &Amp; Health Econ J 2010;2:11-24.
- Turner DA, Shaw C, McGrother CW, Dallosso HM, Cooper NJ. MRC Incontinence Team . The cost of clinically significant urinary storage symptoms for community dwelling adults in the UK. BJU Int 2004;93:1246-52. http://dx.doi.org/10.1111/j.1464-410x.2004.04806.x.
- Urinary Incontinence: Costing Report – Implementing NICE Guidance in England. London: NICE; 2006.
- Hilton P. Long-term follow up studies in pelvic floor dysfunction: the Holy Grail or a realistic aim?. BJOG 2008;115:135-43. http://dx.doi.org/10.1111/j.1471-0528.2007.01557.x.
- Hospital Episode Statistics. London: DH; 2014.
- Payment by Results in the NHS: Tariff for 2012 to 2013. London: DH; 2013.
- Adekanmi OA, Edwards GJ, Barrington JW. The variation in urodynamic practice in the United Kingdom. J Obstet Gynecol 2002;22:48-50. http://dx.doi.org/10.1080/01443610120101727.
- Urinary Incontinence – The Management of Urinary Incontinence in Women. London: RCOG Press; 2006.
- Urinary Incontinence in Women – Update. London: RCOG Press; 2013.
- Glazener CMA, Lapitan MCM. Urodynamic studies for management of urinary incontinence in children and adults. Cochrane Database Syst Rev 2012;1.
- Griffiths D, Kondo A, Bauer S, Diamant N, Liao L, Schafer W, et al. Incontinence – 3rd International Consultation on Incontinence. Plymouth: Health Publications Ltd; 2005.
- Martin JL, Williams KS, Abrams KR, Turner DA, Sutton AJ, Chapple C, et al. Systematic review and evaluation of methods of assessing urinary incontinence. Health Technol Assess 2006;10. http://dx.doi.org/10.3310/hta10060.
- Martin JL, Williams KS, Sutton AJ, Abrams KR, Assassa RP. Systematic review and meta-analysis of methods of diagnostic assessment for urinary incontinence. Neurourol Urodyn 2006;25:674-83. http://dx.doi.org/10.1002/nau.20340.
- Rosier PFWM, Kuo H-C, de Gennaro M, Kakizaki H, Hashim H, van Meel TD, et al. Incontinence – 5th International Consultation on Incontinence. Arnhem, the Netherlands: ICUD-EAU; 2013.
- Buckley BS, Grant AM, Tincello DG, Wagg AS, Firkins L. Prioritising reseach: patients, carers and clinicians working together to identify and prioritise clinical uncertainties in urinary incontinence. Neurourol Urodyn 2010;29:708-14. http://dx.doi.org/10.1002/nau.20816.
- James Lind Alliance . Urinary Incontinence – Tackling Treatment Uncertainties Together 2009. www.lindalliance.org/pdfs/JLAUI Report18308_FINAL_amended.pdf (accessed 19 December 2014).
- Weber AM, Taylor RJ, Wei JT, Lemack G, Piedmonte MR, Walters MD. The cost-effectiveness of preoperative testing (basic office assessment vs. urodynamics) for stress urinary incontinence in women. BJU Int 2002;89:356-63. http://dx.doi.org/10.1046/j.1464-4096.2001.01687.x.
- Haywood KL, Garratt AM, Lall R, Smith JF, Lamb SE. EuroQol EQ-5D and condition-specific measures of health outcome in women with urinary incontinence: reliability, validity and responsiveness. Qual Life Res 2008;17:475-83. http://dx.doi.org/10.1007/s11136-008-9311-z.
- Manca A, Sculpher MJ, Ward K, Hilton P. A cost-utility analysis of tension-free vaginal tape versus colposuspension for primary urodynamic stress incontinence. BJOG 2003;110:255-62.
- Ramsay IN, Ali HM, Hunter M, Stark D, Donaldson K. A randomized controlled trial of urodynamic investigations prior to conservative treatment of urinary incontinence in the female. Int Urogynecol J Pelvic Floor Dysfunct 1995;6:277-81. http://dx.doi.org/10.1007/BF01901525.
- Black N, Griffiths J, Pope C, Bowling A, Abel P. Impact of surgery for stress incontinence on morbidity: cohort study. BMJ 1997;315:1493-8. http://dx.doi.org/10.1136/bmj.315.7121.1493.
- Anger JT, Rodriguez LV, Wang Q, Pashos CL, Litwin MS. The role of preoperative testing on outcomes after sling surgery for stress urinary incontinence. J Urol 2007;178:1364-8. http://dx.doi.org/10.1016/j.juro.2007.05.139.
- Nager CW, FitzGerald M, Kraus SR, Chai TC, Zyczynski H, Sirls L, et al. Urodynamic measures do not predict stress continence outcomes after surgery for stress urinary incontinence in selected women. J Urol 2008;179:1470-4. http://dx.doi.org/10.1016/j.juro.2007.11.077.
- Lemack GE, Krauss S, Litman H, FitzGerald MP, Chai T, Nager C, et al. Normal preoperative urodynamic testing does not predict voiding dysfunction after Burch colposuspension versus pubovaginal sling. J Urol 2008;180:2076-80. http://dx.doi.org/10.1016/j.juro.2008.07.027.
- Nager CW, Brubaker L, Daneshgari F, Litman HJ, Dandreo KJ, Sirls L, et al. Design of the Value of Urodynamic Evaluation (ValUE) trial: a non-inferiority randomized trial of preoperative urodynamic investigations. Contemp Clin Trials 2009;30:531-9. http://dx.doi.org/10.1016/j.cct.2009.07.001.
- van Leijsen SA, Kluivers KB, Mol BW, Broekhuis SR, Milani FL, Bongers MY, et al. Can preoperative urodynamic investigation be omitted in women with stress urinary incontinence? A non-inferiority randomized controlled trial. Neurourol Urodyn 2012;31:1118-23. http://dx.doi.org/10.1002/nau.22230.
- Nager CW, Brubaker L, Litman HJ, Zyczynski HM, Varner RE, Amundsen C, et al. A randomized trial of urodynamic testing before stress-incontinence surgery. N Engl J Med 2012;366:1987-97. http://dx.doi.org/10.1056/NEJMoa1113595.
- van Leijsen SA, Kluivers KB, Mol BW, Broekhuis SR, Milani FL, van der Vaart CH, et al. Protocol for the value of urodynamics prior to stress incontinence surgery (VUSIS) study: a multicenter randomized controlled trial to assess the cost effectiveness of urodynamics in women with symptoms of stress urinary incontinence in whom surgical treatment is considered. BMC Womens Health 2009;9. http://dx.doi.org/10.1186/1472-6874-9-22.
- Shumaker SA, Wyman JF, Uebersax JS, McClish D, Fanti JA. Health-related quality of life measures for women with urinary incontinence: the incontinence impact questionnaire and the urogenital distress inventory. Qual Life Res 1994;3:291-306. http://dx.doi.org/10.1007/BF00451721.
- van Leijsen SA, Kluivers KB, Mol BW, Hout J, Milani AL, Roovers JP, et al. Value of urodynamics before stress urinary incontinence surgery: a randomized controlled trial. Obstet Gynecol 2013;121:999-1008. http://dx.doi.org/10.1097/AOG.0b013e31828c68e3.
- Giarenis I, Cardozo LD. What is the value of urodynamic studies before stress incontinence surgery?. BJOG 2013;120:130-2. http://dx.doi.org/10.1111/1471-0528.12102.
- Brubaker L. An evidence-based approach to urodynamic testing. BJOG 2013;120:127-9. http://dx.doi.org/10.1111/1471-0528.12101.
- Clement KD, Lapitan MCM, Omar MI, Glazener CMA. Urodynamic studies for management of urinary incontinence in children and adults. Cochrane Database Syst Rev 2013;10.
- Brookes ST, Donovan JL, Wright M, Jackson S, Abrams P. A scored form of the Bristol Female Lower Urinary Tract Symptoms questionnaire: data from a randomized controlled trial of surgery for women with stress incontinence. Am J Obstet Gynecol 2004;191:73-82. http://dx.doi.org/10.1016/j.ajog.2003.12.027.
- Ward K, Hilton P. Multicentre randomised trial of TVT and colposuspension: 5 year follow-up. BJOG 2008;115:226-33. http://dx.doi.org/10.1111/j.1471-0528.2007.01548.x.
- MacLean AB, Cardozo L, MacLean AB, Cardozo L. Incontinence in Women. London: RCOG Press; 2002.
- Royal College of Obstetricians and Gynaecologists . Surgical Treatment of Urodynamic Stress Incontinence. Guideline No. 35 2003. www.rcog.org.uk/files/rcog-corp/uploaded-files/GT35UrodynamicStressIncontinence2003.pdf (accessed 26 July 2007).
- Fowler G, Richmond D. Urodynamics: a mandatory preoperative investigation?. Obstet Gynaecol 2006;8:86-90. http://dx.doi.org/10.1576/toag.8.2.086.27226.
- Basu M, Duckett JR, Moran P, Freeman R. Clinicians’ views on the NICE guideline on the management of female urinary incontinence. J Obstet Gynaecol 2009;29:529-32. http://dx.doi.org/10.1080/01443610903003167.
- Agur W, Housami F, Drake M, Abrams P. Could the National Institute for Health and Clinical Excellence guidelines on urodynamics in urinary incontinence put some women at risk of a bad outcome from stress incontinence surgery?. BJU Int 2009;103:635-9. http://dx.doi.org/10.1111/j.1464-410X.2008.08121.x.
- Majumdar A, Latthe P, Toozs-Hobson P. Urodynamics prior to treatment as an intervention: a pilot study. Neurourol Urodyn 2010;29:522-6. http://dx.doi.org/10.1002/nau.20810.
- Kendrick D, Fielding K, Bentley E, Miller P, Kerslake R, Pringle M. The role of radiography in primary care patients with low back pain of at least 6 weeks duration: a randomised (unblinded) controlled trial. Health Technol Assess 2001;5. http://dx.doi.org/10.3310/hta5300.
- Kerry S, Hilton S, Patel S, Dundas D, Rink E, Lord J. Routine referral for radiography of patients presenting with low back pain: is patients’ outcome influenced by GPs’ referral for plain radiography?. Health Technol Assess 2000;4.
- O’Cathain A, Murphy E, Nicholl J. Why, and how, mixed methods research is undertaken in Health Services Research in England: a mixed methods study. BMC Health Serv Res 2007;7. http://dx.doi.org/10.1186/1472-6963-7-85.
- Schulz KF, Altman DG, Moher D. Consort Group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c332.
- Lancaster GA, Dodd S, Williamson PR. Design and analysis of pilot studies: recommendations for good practice. J Eval Clin Pract 2004;10:307-12. http://dx.doi.org/10.1111/j. 2002.384.doc.x.
- Allahdin S, Bain C, Glazener C. Feasibility Study for a Randomised Controlled Trial Evaluating the Use of Absorbable Mesh, Polydioxanone and Polyglactin Sutures for Anterior and Posterior Vaginal Wall Prolapse Repairs. Christchurch, New Zealand: International Continence Society; 2006.
- Kitchener HC, Dunn G, Lawton V, Reid F, Nelson L, Smith AR. Laparoscopic versus open colposuspension--results of a prospective randomised controlled trial. BJOG 2006;113:1007-13. http://dx.doi.org/10.1111/j.1471-0528.2006.01035.x.
- Hagen S, Stark D, Glazener C, Sinclair L, Ramsay I. A randomized controlled trial of pelvic floor muscle training for stages I and II pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct 2009;20:45-51. http://dx.doi.org/10.1007/s00192-008-0726-4.
- Schafer W, Abrams P, Liao L, Mattiasson A, Pesce F, Spangberg A, et al. Good urodynamic practices: uroflowmetry, filling cystometry, and pressure-flow studies. Neurourol Urodyn 2002;21:261-74. http://dx.doi.org/10.1002/nau.10066.
- Singh G, Lucas M, Dolan L, Knight S, Rammage C, Toozs-Hobson P. Minimum standards for urodynamic practice in the UK. Neurourol Urodyn 2010;29:1365-72. http://dx.doi.org/10.1002/nau.20883.
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348. http://dx.doi.org/10.1136/bmj.g1687.
- Abrams P. Urodynamics. London: Springer-Verlag; 2005.
- Chapple CR, MacDiarmid SA, Patel A. Urodynamics Made Easy. Philadelphia, PA: Elsevier; 2009.
- Jenkinson C, Layte R. Development and testing of the UK SF-12 (short form health survey). J Health Serv Res Policy 1997;2:14-8.
- Avery K, Donovan J, Peters TJ, Shaw C, Gotoh M, Abrams P. ICIQ: a brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol Urodyn 2004;23:322-30. http://dx.doi.org/10.1002/nau.20041.
- Kelleher CJ, Cardozo LD, Khullar V, Salvatore S. A new questionnaire to assess the quality of life of urinary incontinent women. BJOG 1997;104:1374-9. http://dx.doi.org/10.1111/j.1471-0528.1997.tb11006.x.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. http://dx.doi.org/10.1016/0168-8510(96)00822-6.
- Brazier JE, Roberts JR. The estimation of a preference-based index from the SF-12. Med Care 2004;42:851-9. http://dx.doi.org/10.1097/01.mlr.0000135827.18610.0d.
- World Medical Association . Declaration of Helsinki 2008. www.wma.net/en/30publications/10policies/b3/17c.pdf (accessed 19 December 2014).
- Department of Health . NHS Research Governance Framework for Health and Social Care 2005. www.gov.uk/government/uploads/system/uploads/attachment_data/file/139565/dh_4122427.pdf (accessed 12 February 2015).
- Prescott RJ, Counsell CE, Gillespie WJ, Grant AM, Russell IT, Kiauka S, et al. Factors that limit the quality, number and progress of randomised controlled trials. Health Technol Assess 1999;3.
- Campbell MK, Snowdon C, Francis D, Elbourne D, McDonald AM, Knight R, et al. Recruitment to randomised trials: strategies for trial enrolment and participation study. The STEPS study. Health Technol Assess 2007;11. http://dx.doi.org/10.3310/hta11480.
- Treweek S, Mitchell E, Pitkethly M, Cook J, Kjeldstrøm M, Johansen M, et al. Cochrane Database Syst Rev 2010;4.
- HM Treasury, Department for Business Innovation & Skills . The Plan for Growth 2011. http://cdn.hm-treasury.gov.uk/2011budget_growth.pdf (accessed 19 December 2014).
- National Institute for Health Research Central Commissioning Facility . Performance in Initiating and Delivering Clinical Research: Information Submission Guidelines 2013 14 2013. www.nihr.ac.uk/policy-and-standards/Performance%20Guidelines%20Q2_1415%20Final%20FCTP.pdf (accessed 12 February 2015).
- Gray A, Clarke P, Wolstenholme J, Wordsworth S. Applied Methods of Cost-effectiveness Analysis in Health Care. Oxford: Oxford University Press; 2011.
- Curtis L. Unit Costs of Health and Social Care. Canterbury: Personal Social Services Research Unit, University of Kent; 2012.
- Department of Health (Payment by Results Team) . NHS Reference Costs 2011–12 2012. www.gov.uk/government/publications/nhs-reference-costs-financial-year-2011-to-2012 (accessed 19 December 2014).
- Imamura M, Abrams P, Bain C, Buckley B, Cardozo L, Cody J, et al. Systematic review and economic modelling of the effectiveness and cost-effectiveness of non-surgical treatments for women with stress urinary incontinence. Health Technol Assess 2010;14. http://dx.doi.org/10.3310/hta14400.
- Department of Transport . Transport Analysis Guidance: WebTAG 2013. www.dft.gov.uk/webtag/documents/expert/unit3.5.6.php (accessed 19 December 2014).
- Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ 1990;300:230-5. http://dx.doi.org/10.1136/bmj.300.6719.230.
- NHS Careers . Agenda for Change Pay Rates n.d. www.nhscareers.nhs.uk/working-in-the-nhs/pay-and-benefits/agenda-for-change-pay-rates/ (accessed 19 December 2014).
- Information Services Division Scotland . Scottish Ambulance Service, Expenditure and Statistics, by Board Area n.d. www.isdscotland.org/Health-Topics/Finance/Costs/File-Listings-2012.asp (accessed 19 December 2014).
- Joint Formulary Committee . British National Formulary (Online) n.d. www.medicinescomplete.com (accessed 19 December 2014).
- Brazier J, Ratcliffe J, Tsuchiya A, Salomon JA. Measuring and Valuing Health Benefits for Economic Evaluation. Oxford: Oxford University Press; 2007.
- Young JM, Solomon MJ, Harrison JD, Salkeld G, Butow P. Measuring patient preference and surgeon choice. Surgery 2008;143:582-8. http://dx.doi.org/10.1016/j.surg.2008.01.009.
- Cook JA, Hislop J, Adewuyi TE, Harrild K, Altman DG, Ramsay C, et al. Assessing methods to specify the target difference for a randomised controlled trial – DELTA (Difference ELicitation in TriAls) review. Health Technol Assess 2014;18. http://dx.doi.org/10.3310/hta18280.
- Glaser BG, Strauss AL. The Discovery of Grounded Theory: Strategies for Qualitative Research. Chicago: Aldine; 1967.
- McCann SK, Campbell MK, Entwistle VA. Reasons for participating in randomised controlled trials: conditional altruism and considerations for self. Trials 2010;11. http://dx.doi.org/10.1186/1745-6215-11-31.
- Rosenbaum JR, Wells CK, Viscoli CM, Brass LM, Kernan WN, Horwitz RI. Altruism as a reason for participation in clinical trials was independently associated with adherence. J Clin Epidemiol 2005;58:1109-14. http://dx.doi.org/10.1016/j.jclinepi.2005.03.014.
- Truong TH, Weeks JC, Cook EF, Joffe S. Altruism among participants in cancer clinical trials. Clin Trials 2011;8:616-23. http://dx.doi.org/10.1177/1740774511414444.
- Holtedahl K, Verelst M, Schiefloe A, Hunskaar S. Usefulness of urodynamic examination in female urinary incontinence--lessons from a population-based, randomized, controlled study of conservative treatment. Scand J Urol Nephrol 2000;34:169-74. http://dx.doi.org/10.1080/003655900750016544.
- Rolstad S, Adler J, Ryden A. Response burden and questionnaire length: is shorter better? A review and meta-analysis. Value Health 2011;14:1101-8. http://dx.doi.org/10.1016/j.jval.2011.06.003.
- Foon R, Toozs-Hobson P, Latthe P. Prophylactic antibiotics to reduce the risk of urinary tract infections after urodynamic studies. Cochrane Database Syst Rev 2012;10. http://dx.doi.org/10.1002/14651858.CD008224.pub2.
- Edwards PJ, Roberts I, Clarke MJ, DiGuiseppi C, Wentz R, Kwan I, et al. Methods to increase response to postal and electronic questionnaires. Cochrane Database Syst Rev 2009;3. http://dx.doi.org/10.1002/14651858.MR000008.pub4.
- Auwad W, Freeman RM, Swift S. Is the pelvic organ prolapse quantification system (POPQ) being used? A survey of members of the International Continence Society (ICS) and the American Urogynecologic Society (AUGS). Int Urogynecol J Pelvic Floor Dysfunct 2004;15:324-7.
- Cartwright R, Cardozo L. Usage of International Continence Society standardized terminology: a bibliometric and questionnaire study. Neurourol Urodyn 2010;29:1373-9. http://dx.doi.org/10.1002/nau.20894.
- Roos AM, Thakar R, Sultan AH, Scheer I. Female sexual dysfunction: are urogynecologists ready for it?. Int Urogynecol J Pelvic Floor Dysfunct 2009;20:89-101. http://dx.doi.org/10.1007/s00192-008-0735-3.
- McColl E, Jacoby A, Thomas L, Soutter J, Bamford C, Steen N, et al. Design and use of questionnaires: a review of best practice applicable to surveys of health service staff and patients. Health Technol Assess 2001;5. http://dx.doi.org/10.3310/hta5310.
- Freedman B. Equipoise and the ethics of clinical research. N Engl J Med 1987;317:141-5. http://dx.doi.org/10.1056/NEJM198707163170304.
- Bright E, Parsons BA, Swithinbank L. Increased patient information does not reduce patient anxiety regarding urodynamic studies. Urol Int 2011;87:314-8. http://dx.doi.org/10.1159/000331507.
- Doshani A, Mayne C, Tincello D. Anxiety among women attending urodynamic investigation: scoping study for a randomized trial of psychometric intervention. Neurourol Urodyn 2009;28:177-8. http://dx.doi.org/10.1002/nau.20660.
- Hilton P, Bryant A, Howel D, McColl E, Buckley BS, Lucas MG, et al. Assessing professional equipoise and views about a future clinical trial of invasive urodynamics prior to surgery for stress urinary incontinence in women: a survey within a mixed methods feasibility study. Neurourol Urodyn 2012;31:1223-30. http://dx.doi.org/10.1002/nau.22328.
- Cohen J. Statistical Power Analysis for the Behavioural Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988.
- van Leijsen SA, Kluivers KB, Mol BW, Broekhuis SR, Milani AL, Bongers MY, et al. A randomized controlled trial on the value of preoperative urodynamic investigation in women with stress incontinence. Int Urogynecol J Pelvic Floor Dysfunct 2011;22:S158-S60. http://dx.doi.org/10.1002/nau.22230.
- Khullar V, Salvatore S, Cardozo L, Howland E, Digesu A, Kelleher C, et al. Randomised Study of Ambulatory Urodynamics Versus Symptomatic Treatment of Symptomatic Women Without a Urodynamic Diagnosis n.d.
Appendix 1 Consolidated Standards of Reporting Trials checklist

Consolidated Standards of Reporting Trials 2010 checklist of information to include when reporting a randomised trial
| Section/topic | Item no. | Checklist item | Reported on page no. |
|---|---|---|---|
| Title and abstract | |||
| 1a | Identification as a randomised trial in the title | i, vii | |
| 1b | Structured summary of trial design, methods, results, and conclusions (for specific guidance see CONSORT for abstracts) | xxiii–xxvii | |
| Introduction | |||
| Background and objectives | 2a | Scientific background and explanation of rationale | 1–4 |
| 2b | Specific objectives or hypotheses | 5 | |
| Methods | |||
| Trial design | 3a | Description of trial design (such as parallel, factorial) including allocation ratio | 5–6, 9 |
| 3b | Important changes to methods after trial commencement (such as eligibility criteria), with reasons | 12–13 | |
| Participants | 4a | Eligibility criteria for participants | 7–9 |
| 4b | Settings and locations where the data were collected | 7 | |
| Interventions | 5 | The interventions for each group with sufficient details to allow replication, including how and when they were actually administered | 9–10 |
| Outcomes | 6a | Completely defined pre-specified primary and secondary outcome measures, including how and when they were assessed | 10–12 |
| 6b | Any changes to trial outcomes after the trial commenced, with reasons | N/A | |
| Sample size | 7a | How sample size was determined | 9 |
| 7b | When applicable, explanation of any interim analyses and stopping guidelines | N/A | |
| Randomisation | |||
| Sequence generation | 8a | Method used to generate the random allocation sequence | 9 |
| 8b | Type of randomisation; details of any restriction (such as blocking and block size) | 9 | |
| Allocation concealment mechanism | 9 | Mechanism used to implement the random allocation sequence (such as sequentially numbered containers), describing any steps taken to conceal the sequence until interventions were assigned | 9 |
| Implementation | 10 | Who generated the random allocation sequence, who enrolled participants, and who assigned participants to interventions | 9 |
| Blinding | 11a | If done, who was blinded after assignment to interventions (for example, participants, care providers, those assessing outcomes) and how | N/A (9) |
| 11b | If relevant, description of the similarity of interventions | N/A | |
| Statistical methods | 12a | Statistical methods used to compare groups for primary and secondary outcomes | 15 |
| 12b | Methods for additional analyses, such as subgroup analyses and adjusted analyses | 41–44 (economic) | |
| Results | |||
| Participant flow (a diagram is strongly recommended) | 13a | For each group, the numbers of participants who were randomly assigned, received intended treatment, and were analysed for the primary outcome | 26 |
| 13b | For each group, losses and exclusions after randomisation, together with reasons | 26 | |
| Recruitment | 14a | Dates defining the periods of recruitment and follow-up | 23–28 |
| 14b | Why the trial ended or was stopped | N/A | |
| Baseline data | 15 | A table showing baseline demographic and clinical characteristics for each group | 29 |
| Numbers analysed | 16 | For each group, number of participants (denominator) included in each analysis and whether the analysis was by original assigned groups | 15, 30–37 |
| Outcomes and estimation | 17a | For each primary and secondary outcome, results for each group, and the estimated effect size and its precision (such as 95% confidence interval) | 30–31 |
| 17b | For binary outcomes, presentation of both absolute and relative effect sizes is recommended | N/A | |
| Ancillary analyses | 18 | Results of any other analyses performed, including subgroup analyses and adjusted analyses, distinguishing pre-specified from exploratory | 45–51 (economic) |
| Harms | 19 | All important harms or unintended effects in each group (for specific guidance see CONSORT for harms) | 37 |
| Discussion | |||
| Limitations | 20 | Trial limitations, addressing sources of potential bias, imprecision, and, if relevant, multiplicity of analyses | 52, 79–84 |
| Generalisability | 21 | Generalisability (external validity, applicability) of the trial findings | 7, 53 |
| Interpretation | 22 | Interpretation consistent with results, balancing benefits and harms, and considering other relevant evidence | 79–85 |
| Other information | |||
| Registration | 23 | Registration number and name of trial registry | viii, xxvii, 97 |
| Protocol | 24 | Where the full trial protocol can be accessed, if available | 13 |
| Funding | 25 | Sources of funding and other support (such as supply of drugs), role of funders | viii, xxvii, 96, 97 |
Appendix 2 Letter of invitation to potential trial participant
Appendix 3 General practitioner notification of recruitment
Appendix 4 E-mail invitation to take part in clinician survey
Appendix 5 Short patient information sheet for pilot randomised controlled trial
Appendix 6 Full patient information sheet for pilot randomised controlled trial
Appendix 7 Patient information for interview study (trial participants)
Appendix 8 Patient information for interview study (trial decliners)
Appendix 9 Surgeon information for interview study
Appendix 10 Consent form for pilot randomised controlled trial
Appendix 11 Consent form for patient interview study (trial participants)
Appendix 12 Consent form for patient interview study (trial decliners)
Appendix 13 Consent form for clinician interview study
Appendix 14 Clinician survey questionnaire
Appendix 15 Clinician survey questionnaire update
Appendix 16 Scoring systems for study questionnaires
| Questionnaire | Scale/subscale details | Question scoring | Overall score | Notes |
|---|---|---|---|---|
| aICIQ-FLUTS43 | Total of 12 questions; four questions on filling, three on voiding and five questions on incontinence | Each question is scored 1–4; thus, range of overall scores from 0 to 16, 12 and 20 for filling, voiding and incontinence scales, respectively | 0–48 where all subscale scores are added | Higher scores indicate greater impact of individual symptoms for the patient. Question 5 is different in the version used (08/04) from that of Brookes et al.43 |
| Patients completing the INVESTIGATE-I questionnaire chose responses to ‘How often do you pass urine during the day?’ as 1 to 6 times, 7 to 8 times, 9 to 10 times, 11 to 12 times, 13 or more. The previous version (Brookes et al.43) used, every 4 hours or more, every 3 hours, every 2 hours, hourly | ||||
| ICIQ-UI SF65 | three questions in total with no subscale | First question is scored 0–5, second one is scored either 0, 2, 4 or 6 and the final one is scored on a Likert scale from 0–10 | 0–21 where scores from each question are added | Higher scores indicate greater impact of symptoms |
| ICIQ-LUTSqol66 | 19 questions in total with no subscale | All questions are scored 1–4 | 19–76 where scores from each question are added | Greater values indicate increased impact on QoL. Three questions have a N/A option and until clarification we plan to classify as ‘not at all’ and score one so that the minimum score is 19 as required. Questions are 9a–11a, namely, ‘Does your urinary problem affect your “relationship with partner”, “sex life” and “family life” ?’ |
| UDI38 | two questions on stress, six questions on irritative symptoms and 11 questions on obstructive/discomfort symptoms | Each question is scored 0–3 and each subscale is scaled up so that the range becomes 0–100 | 0–300 where all subscale scores are added | Higher scores indicate greater impact of individual symptoms for the patient. Scores will be calculated using the method recommended by the scale authors; a score will be generated for each subscale and all subscales will be weighted equally and added |
Appendix 17 Baseline (and six-month) participant questionnaire pack
Appendix 18 3-day bladder diary
Appendix 19a Flow chart of case report form completion
Appendix 19b Participant contact details
Appendix 19c Randomisation
Appendix 19d Initial assessment
Appendix 19e Initial investigation
Appendix 19f Invasive urodynamic tests
Appendix 19g Surgery
Appendix 19h Postoperative follow-up
Appendix 19i Non-surgical treatment
Appendix 19j Medication and therapies
Appendix 19k End of study form
Appendix 20 Adverse event report form
Appendix 21 Serious adverse event report form
Appendix 22 INVESTIGATE studies website (www.investigate-trial.com)
Appendix 23 Trial newsletter example
Appendix 24 Recruitment update example
Appendix 25 Recruitment to Target thermometer

The ‘RtT’ thermometer features on our website home page. It was developed initially in Microsoft PowerPoint by PH, as a simple graphic image illustrating actual recruitment against recruitment target, and time expired of the available study recruiting time, in the form of a ‘maximum and minimum’ thermometer. It was then converted into HTML code that can easily be adapted for use in any trial, and added into a website. Although the basic parameters are easily modified by any user, the code author, Lindsay Marshall, Senior Lecturer in Computing Science at Newcastle University, is willing to provide free service for any person or organisation wishing to use this in other trial websites or documentation.
Appendix 26 Vignette general practitioner letters for evaluation of screening processes
We have had previous correspondence about the process of screening used in the INVESTIGATE-I study. The reason for this is that we had noticed quite marked differences between the various units not only in the numbers recruited, but also in the numbers they had to screen in order to achieve that recruitment. As far as we can tell, the hospitals themselves are broadly similar in workload, etc., the patients are pretty much the same, and the description of what you did to identify patients for screening also seems to be much the same.
We are keen to investigate this further and propose to do this by a series of ‘dummy GP letters’ or vignettes for you and all others involved in screening in your unit to assess. There are 20 numbered vignettes in the attached file. Each consists of one or more communications from GPs, clinic notes, or Physiotherapy reports. Some are genuine letters, some made up; some are quite short, others more detailed. I hope this will not take up too much of your time, but in order to get a better understanding of this issue, a full return from all staff involved in screening of patients in all our study sites is quite important. Your replies will of course be kept anonymous, although it is important that we can identify the centre at which you work.
What we want to know is whether you would have considered each of the women described in the letters to be a potential recruit for the INVESTIGATE-I trial. In other words, if you had reviewed the letter at the time that we were looking for recruits into the trial would you, or would you not, have sent out a Patient Information Leaflet (PIL) to the woman described (please tick either ‘Yes’ or ‘No’ in the blue boxes). It would also be helpful to know whether you feel the decision is clear-cut, or borderline (by ticking in the appropriate green box), and something of why you made that decision (by ticking the orange boxes and adding comments as appropriate). A score sheet is provided as a separate attachment with this e-mail; could you complete this for each patient and return to me by e-mail at your earliest convenience? Many thanks and best wishes.
Paul Hilton MD, FRCOG Consultant Gynaecologist & Urogynaecologist Royal Victoria Infirmary Newcastle upon Tyne, NE1 4LP. Tel: 0191–2825853; Fax: 0191–2825873; E-mail: paul.hilton@ncl.ac.uk or paul.hilton@nuth.nhs.uk.
Appendix 27 Economic analyses exploring alternative assumptions for missing data
Illustrative example of the cost–utility analysis: imputed values used for missing case report form data with Short Form 6D quality-adjusted life-year scores
Table 23 presents the cost–utility results using imputation for missing CRF data and the SF-6D to measure outcome data. The no IUT arm was dominated by the IUT arm as the no IUT arm had higher average cost but lower average QALY, however the probability of the IUT being cost-effective decreases as the society’s willingness to pay for a QALY threshold increases.
| Investigation strategy | Cost (£) | QALY | ICER (£) | Probability that the IUT is cost-effective for different threshold values for society’s willingness to pay for a QALY | ||||
|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||
| IUT | 1507.12 | 0.3855 | 96% | 96% | 95% | 92% | 88% | |
| No IUT | 1660.83 | 0.3770 | Dominated | 4% | 4% | 5% | 8% | 12% |
Figure 27 presents the results of the bootstrapping simulation, which addresses the uncertainty around costs and effects. As the majority of the iterations generated from the bootstrapping simulation were in the southern quadrants, it suggested that the IUT arm tended to incur less costs than the no IUT arm. The average of the cost and QALY pair simulations is situated close to the y-axis, indicating that there is not a significant difference in QALY values between the IUT arm and the no IUT arm. This supports the findings from the t-test conducted on the mean QALY differences between the two treatment groups in the main body of the monograph. This highlights the uncertainty around the cost–utility results and is further supported by the cost-effectiveness acceptability curve. Figure 28 demonstrates that if society had zero willingness to pay for an additional QALY then the IUT was 96% likely to be cost-effective; as society’s willingness to pay for a QALY increased, the likelihood of the IUT being cost-effective decreased.
FIGURE 27.
Incremental cost–utility scatterplot with imputation of CRF data with SF-6D QALY scores: IUT vs. no IUT.
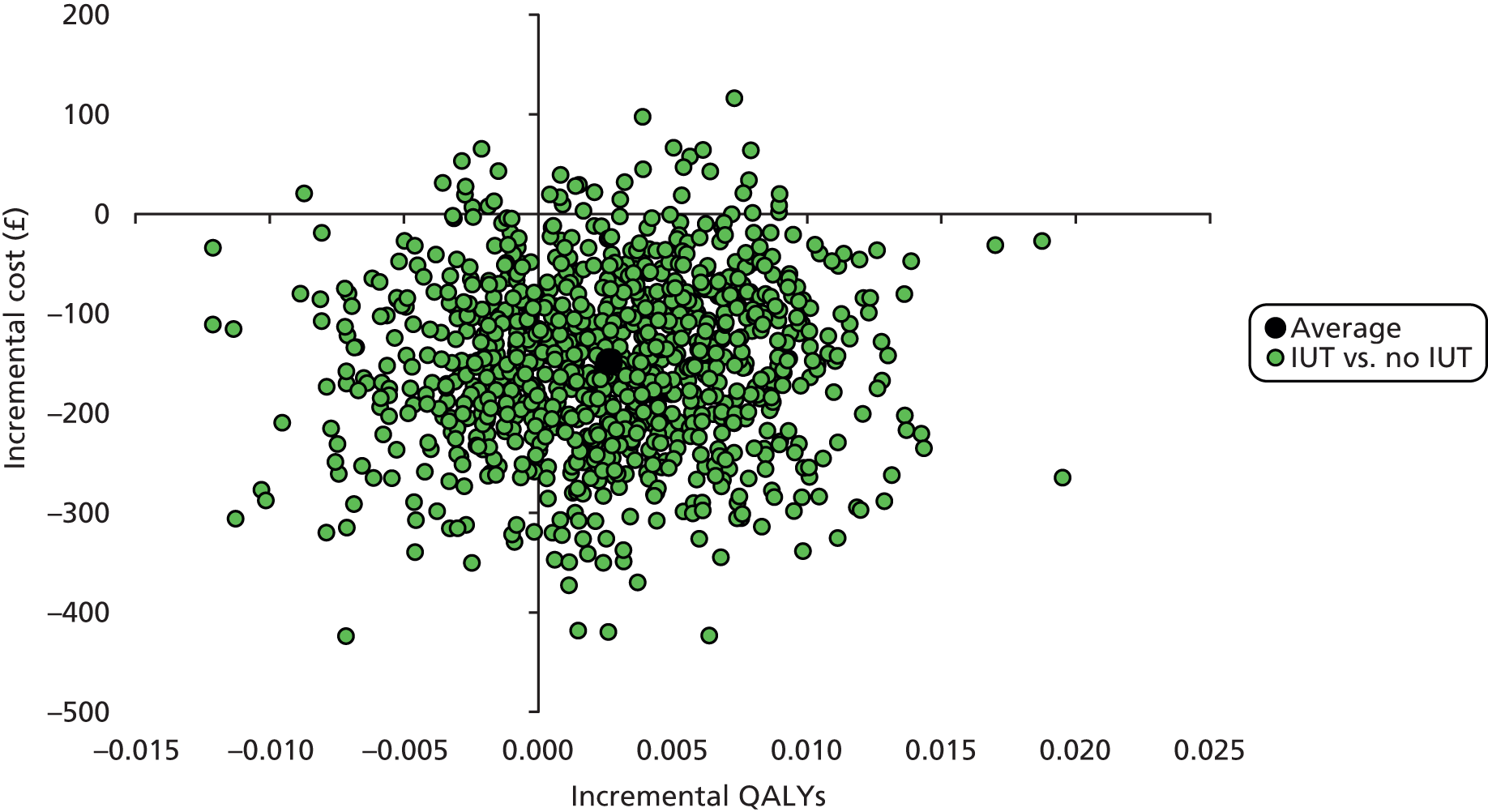
FIGURE 28.
Cost-effectiveness acceptability curve with imputation of CRF data: IUT vs. no IUT.
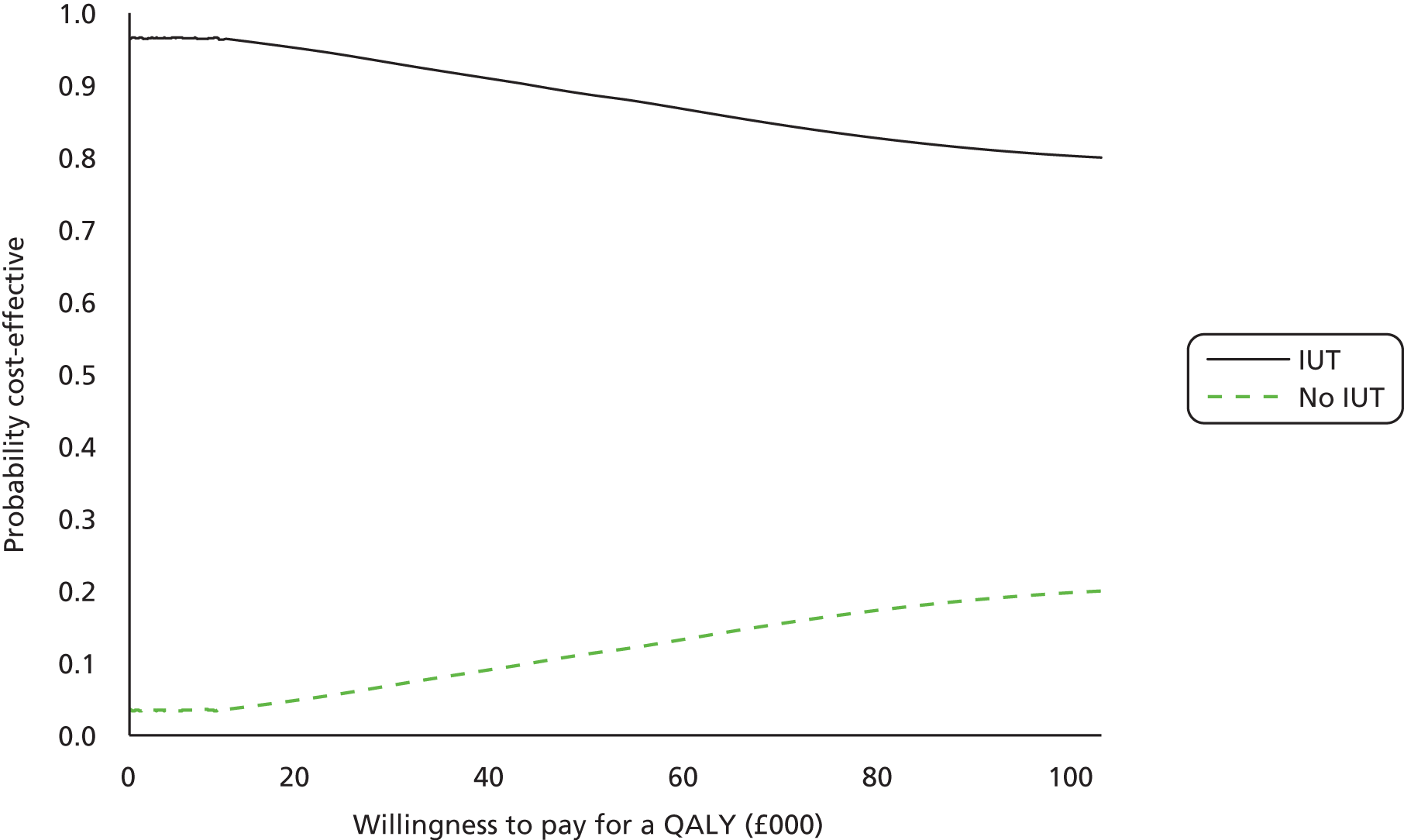
Illustrative example of the cost–utility analysis: complete case analysis
Table 24 presents the cost–utility results using complete case analysis. The results from this analysis need to be interpreted with caution as only 81 patients had complete information (IUT arm n = 30; no IUT arm n = 51), this means that the analysis is vulnerable to outliers in the data. The cost of an additional QALY gained from the IUT is £4944 when compared with no IUT. At zero willingness to pay, the IUT option is not cost-effective when compared with no IUT, however, the cost-effectiveness of the IUT increases as the willingness-to-pay threshold increases.
| Investigation strategy | Cost (£) | QALY | ICER (£) | Probability that the IUT is cost-effective for different threshold values for society’s willingness to pay for a QALY | ||||
|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||
| IUT | 1815 | 0.4479 | 4944 | 21% | 33% | 44% | 48% | 52% |
| No IUT | 1775 | 0.4398 | 79% | 67% | 56% | 51% | 48% | |
Figure 29 represents the bootstrapping results from the complete case analysis. Similarly to the previous analyses we can clear see the majority of the iterations are in the northern quadrants which suggests that the IUT is more expensive on average than no IUT. The complete case analysis has generated different incremental cost results because only two patients in the IUT group did not have surgery as a treatment option, the other 28 patients in the IUT group had the IUT and surgery and thus incurred a higher cost on average. Again there is uncertainty around the QALY difference between the two groups, with the average of the iterations positioned close to the y-axis suggesting there is no evidence of QALY difference between the two groups. Figure 30 represents the cost-effectiveness of the no IUT at a zero willingness-to-pay threshold which differs from previous analyses, in this analysis the IUT is only 21% cost-effective. However, as the willingness-to-pay threshold increases so does the cost-effectiveness of the IUT.
FIGURE 29.
Incremental cost–utility scatterplot for complete case analysis: IUT vs. no IUT.

FIGURE 30.
Cost-effectiveness acceptability curve for complete case analysis: IUT vs. no IUT.

Illustrative example of the cost–utility analysis: imputed values used for sensitivity analysis
Sensitivity analysis was conducted around the unit costs used in the base-case analysis; Table 25 presents the cost-utility results using the alternative costs. Within the sensitivity analysis the cost of containment products was included for patients in the IUT group who had not received surgery as a treatment option and were not classed as watchful waiting. (It was previously assumed that patients who were classed as watchful waiting would use containment products.) It was assumed that these patients would still be classed as incontinent and would need containment products. The analysis here used higher unit costs to assess the impact unit costs have on the cost-effectiveness of the IUT. The cost per QALY for the IUT compared with no IUT is £5046. At zero willingness to pay the IUT option is cost-effective when compared with no IUT however the cost-effectiveness of the IUT decreases as the willingness-to-pay threshold increases.
| Investigation strategy | Cost (£) | QALY | ICER (£) | Probability that the IUT is cost-effective for different threshold values for society’s willingness to pay for a QALY | ||||
|---|---|---|---|---|---|---|---|---|
| £0 | £10,000 | £20,000 | £30,000 | £50,000 | ||||
| IUT | 1686 | 0.4392 | 5046 | 89% | 56% | 36% | 31% | 26% |
| No IUT | 1791 | 0.4402 | 11% | 44% | 64% | 69% | 74% | |
Figure 31 represents the bootstrapping results from the sensitivity analysis. The majority of the bootstrapping iterations are positioned in the southern quadrants illustrating the cost savings for the IUT compared with no IUT. The position of the average result from the bootstrapping iterations suggests again that there is no significant difference in QALY values for the IUT compared with no IUT. This sensitivity analysis has resulted in the biggest QALY difference between the IUT and no IUT however the difference is less than 0.01. At zero willingness to pay the IUT is 89% cost-effective, the cost-effectiveness of the IUT decreases as the willingness-to-pay threshold increases, as highlighted in Figure 32.
FIGURE 31.
Incremental cost–utility scatterplot for sensitivity analysis: IUT vs. no IUT.

FIGURE 32.
Cost-effectiveness acceptability curve for sensitivity analysis: IUT vs. no IUT.

Appendix 28 Participant Costs Questionnaires
List of abbreviations
- AE
- adverse event
- B&BF
- Bladder and Bowel Foundation
- BAUS
- British Association of Urological Surgeons
- BAUS-SFNUU
- British Association of Urological Surgeons Section of Female, Neurological and Urodynamic Urology
- BRAG
- black, red, amber, green
- BSUG
- British Society of Urogynaecology
- CLRN
- Comprehensive Local Research Network
- CONSORT
- Consolidated Standards Of Reporting Trials
- CRF
- case report form
- CTA
- clinical trials agreement
- DMEC
- Data Monitoring and Ethics Committee
- DO
- detrusor overactivity
- EQ-5D
- EuroQol-5D
- EQ-5D-3L
- EuroQol-5D-3 Level
- ER
- equipoise ratio
- GP
- general practitioner
- HES
- Hospital Episode Statistics
- HTA
- Health Technology Assessment
- HTML
- hypertext markup language
- ICER
- incremental cost-effectiveness ratio
- ICI
- International Consultation on Incontinence
- ICIQ
- International Consultation on Incontinence Modular Questionnaire
- ICIQ-FLUTS
- International Consultation on Incontinence Modular Questionnaire Female Lower Urinary Tract Symptoms
- ICIQ-LUTSqol
- International Consultation on Incontinence Modular Questionnaire Lower Urinary Tract Symptoms Quality of Life
- ICIQ-UI SF
- International Consultation on Incontinence Modular Questionnaire Urinary Incontinence Short Form
- INVESTIGATE
- INVasive Evaluation before Surgical Treatment of Incontinence Gives Added Therapeutic Effect?
- IQR
- interquartile range
- IUT
- invasive urodynamic test
- MCID
- minimum clinically important difference
- MUI
- mixed urinary incontinence
- NCTU
- Newcastle Clinical Trials Unit
- NETSCC
- National Institute of Health Research Evaluation, Trials and Studies Co-ordinating Centre
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NuTH
- Newcastle upon Tyne Hospitals NHS Foundation Trust
- OAB
- overactive bladder
- PCQ
- Participant Costs Questionnaire
- PFMT
- pelvic floor muscle training
- PI
- principal investigator
- PIC
- Patient Identification Centre
- PIS
- Patient Information Sheet
- POP
- pelvic organ prolapse
- PPI
- patient and public involvement
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- R&D
- research and development
- RCT
- randomised controlled trial
- REC
- Research Ethics Committee
- RtT
- Recruitment to Target
- SAE
- serious adverse event
- SD
- standard deviation
- SF-12
- Short Form 12
- SF-12v2
- Short Form 12 version 2
- SF-6D
- Short Form 6D
- SUI
- stress urinary incontinence
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- TVT
- tension-free vaginal tape
- UDI
- Urogenital Distress Inventory
- UI
- urinary incontinence
- UTI
- urinary tract infection
- ValUE
- Value of Urodynamic Evaluation
- VD
- voiding dysfunction
- VUSIS
- Value of Urodynamics prior to Stress (urinary) Incontinence Surgery
