Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 08/116/97. The contractual start date was in July 2010. The draft report began editorial review in January 2014 and was accepted for publication in September 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Amar Rangan reports grants and personal fees from DePuy Ltd and grants from JRI Ltd; both are outside the submitted work. In addition, Professor Rangan has UK and European patent applications pending. Sarah Lamb is chairperson of the Health Technology Assessment Clinical Trials and Evaluation Board.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Costa et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
Fractures of the distal radius are extremely common injuries. In the Western world, 6% of women will have sustained such a fracture by the age of 80 years and 9% by the age of 90 years. 1 As the population continues to age, these figures are likely to increase further. The optimal management of fractures of the distal radius in adults remains controversial. There is a bimodal distribution in terms of age. Younger patients frequently sustain complicated high-energy injuries involving the wrist joint. However, fractures of the distal radius are also common in older patients, who are more likely to sustain low-energy fractures, often related to osteoporosis. 2 This study is designed to address both groups of patients, as the key management issues pertain to all patients with a fracture of the distal radius.
In general, fractures of the distal radius are treated non-operatively if the bone fragments are not displaced or the fragments can be held in a good anatomical alignment (reduction) by a plaster cast or orthotic. However, if this is not possible then operative fixation is required. This carries inherent risks for the patient and considerable cost implications for the NHS; much of this cost is related to the choice of fixation. 3
There are several operative options but the two most common in the UK are percutaneous Kirschner-wire (K-wire) fixation and volar locking-plate fixation using fixed-angle screws (locking plates). Each surgical method has its own advantages and disadvantages.
Kirschner-wire fixation is a long-standing and widely practised technique. During this procedure, smooth metal wires with a sharp point are passed across the fracture site through the skin. This is a relatively simple, quick and minimally invasive technique, which is cheap and requires limited operative hardware. However, as the fixation is not ‘rigid’ (the wires are inherently flexible) the wrist has to be immobilised in plaster cast; normally for 6 weeks or until the wires are removed. There is a risk of infection where the wires enter the skin. There is also a risk that the fracture will ‘collapse’ when the wires are removed, leading to deformity and loss of function. 4
Locking-plate fixation, in the distal radius and for other fractures, has been facilitated by recent advances in implant technology, which allow the screws to be ‘locked’ into the plate. This produces a ‘fixed-angle’ bone–plate construct, (previously, plate-and-screw constructs relied on friction alone to maintain their position on the bone). Although originally designed for use in osteoporotic bone specifically, the theoretical advantages of the locking plates may equally be applied to high-energy (often multifragmentary) fractures in younger patients. The technique has become increasingly popular in the UK and across the developed world over the last 5 years. The procedure requires an incision over the volar (palm) side of the wrist. The plate and screws are then applied to the bone fragments under direct vision. This produces a rigid construct,5 and, therefore, the patients can be permitted to mobilise their wrist more quickly, potentially reducing future stiffness. As the plate and screws can remain inside the patient permanently, the risk of later collapse of the fracture is also smaller. However, this technique takes longer than a K-wire fixation and there is a greater risk of serious intraoperative complications such as injury to a nerve or blood vessel. 5 There is also a risk of flexor and/or extensor tendon irritation and rupture. 6 The locking-plate hardware itself is specialised and considerably more expensive; a cost-analysis of the total cost of each intervention (Plant et al., University Hospital Coventry and Warwickshire NHS Trust, 2009, unpublished data) suggested that the use of a locking plate increased the cost of the operation itself (total operating department cost) threefold.
In 2003, Handoll and Madhok7 summarised the results of a series of Cochrane reviews of randomised controlled trials of the treatment of fractures of the distal radius and ‘exposed the serious deficiency in the available evidence’. However, they were able to identify key areas for future research, including the area of ‘when and what type of surgery is indicated’. An influential group of academic trauma surgeons (AO UK Research Committee chaired by Professor Keith Willett) recently identified our specific research question as a ‘top priority’ for urgent investigation. 8
Relevance of project
The clinical management of any fracture depends on several factors including the severity of the fracture and the personal circumstances and comorbidity of the patient. These variables are generally out of the control of the treating surgeon. However, the type of operative intervention can be determined after the event. Although there will always be some variability in clinical practice, patients with a fracture of the distal radius may expect a reasonably consistent approach to the operative treatment of such a common fracture. However, for this particular injury there seems to be no consensus. 2 For fractures of the distal radius, perhaps more than any other fracture, the operative intervention varies enormously depending on the preference, training and experience of the treating surgeon and the policy, inventory and budget of the treating institution.
We recently performed a pilot study where the clinical details and radiological images of five patients with a displaced fracture of the distal radius were presented independently to 33 experienced trauma surgeons in the UK. The cases included younger patients with high-energy injuries and fragility fractures in older patients. The surgeons were asked to provide a treatment plan for each patient. There was < 50% agreement in the treatment regime for any individual patient. Across all of the cases, 44% of surgeons would use volar locking plates, 28% would use K-wire fixation, 7% would use volar non-locking plates, 5% would use external fixators and 15% would treat the patient with manipulation and a plaster cast alone. 9
We intend to provide high-level evidence to inform the future management of adult patients with a displaced fracture of the distal radius by directly comparing the results of K-wire fixation and locking-plate fixation in a multicentre randomised controlled clinical trial.
Null hypothesis
There is no difference in the Patient-Rated Wrist Evaluation© (PRWE) questionnaire score 12 months post injury between adult patients with a dorsally displaced fracture of the distal radius treated with locking-plate fixation versus K-wire fixation.
Objectives
The primary objective is to quantify and draw inferences on observed differences in the PRWE questionnaire score (a validated assessment of wrist function) between the trial treatment groups at 12 months post injury.
The secondary objectives are to quantify and draw inferences on observed differences in the Disabilities of Arm, Shoulder and Hand (DASH) questionnaire score (a validated assessment of general upper-limb function) between the trial treatment groups at 12 months post injury; to determine the complication rate of K-wire fixation versus locking-plate fixation at 12 months post injury; and to investigate, using appropriate statistical and economic analysis methods, the resource use, and comparative cost-effectiveness, of K-wire fixation versus locking-plate fixation.
Chapter 2 Methods
Trial design
This was a multicentre, stratified (by centre, intra-articular extension of the fracture and age of the patient, with balanced randomisation 1 : 1) double-blind, controlled trial.
Participants
Inclusion criteria
Patients were eligible for this study if:
-
They sustained a dorsally displaced fracture of the distal radius, which was defined as a fracture within 3 cm of the radiocarpal joint.
-
The treating consultant surgeon believed that they would benefit from operative fixation of the fracture.
-
They were over the age of 18 years and able to give informed consent.
-
The injury was < 2 weeks old.
Exclusion criteria
Patients were excluded from participation in this study if:
-
The fracture extended > 3 cm from radiocarpal joint.
-
The fracture was open with a Gustilo grading greater than 1. 10
-
The articular surface of the fracture could not be reduced by indirect techniques. (In a small number of fractures, the joint surface is so badly disrupted that the surgeon will have to open up the fracture in order to restore the anatomy under ‘direct’ vision.)
-
There were contraindications to anaesthetic.
-
There was evidence that the patient would be unable to adhere to trial procedures or complete questionnaires, such as cognitive impairment or intravenous drug abuse.
Patients who sustained other injuries, which could potentially affect the primary outcome measure at 1 year post injury, for example disruption of the carpal ligaments, were documented but included in the analysis.
Screening and recruitment
Patients were recruited from the emergency departments and fracture clinics at the trial centres. Any patient with a fracture of the distal radius who, in the opinion of the treating surgeon, required an operative intervention was referred to the local research associate for screening. The research associate then presented the eligible patient with the participant information sheet. Patients were given the opportunity to discuss any issues related to the trial with the research associate, as well as with members of their family and friends.
Consent
Informed consent was obtained by the local research associate. In general, patients who were admitted with a fracture of the distal radius had their surgery on the following day, so there was sufficient time for the patients to consider taking part in the trial. Any new information that arose during the trial that affected participants’ willingness to take part was reviewed by the Trial Steering Committee (TSC); if necessary this was communicated to all participants by the trial co-ordinator. A revised consent form was completed if necessary.
Settings and locations
The trial was run in 18 trauma centres across the UK (Box 1).
-
Addenbrooke’s Hospital.
-
Frenchay Hospital.
-
Ipswich Hospital.
-
James Cook University Hospital.
-
John Radcliffe Hospital.
-
Leicester General Hospital.
-
North Tyneside General Hospital.
-
Northampton General Hospital.
-
Peterborough City Hospital.
-
Poole Hospital.
-
Royal Blackburn Hospital.
-
Royal Victoria Infirmary.
-
St Thomas’ Hospital.
-
University Hospital Coventry & Warwickshire.
-
University Hospital North Staffordshire.
-
University Hospital of North Tees.
-
Wansbeck General Hospital.
-
Wexham Park Hospital.
Sample size
We performed an audit of the patients who had an operative fixation of their distal radius fracture at the University Hospital Coventry and Warwickshire (UHCW) over a 4-month period in 2007. 11 This revealed that 32 patients had either a K-wire fixation or a locking-plate fixation at our hospital during this period. Therefore, we anticipated that there would be 90–100 eligible patients per year. As each of the recruiting centres served a similar catchment area (c. 500,000 patients) we expected that there would be approximately 900–1000 eligible patients within the trial centres each year. All of the centres chosen to take part in this study regularly performed both locking-plate fixation and K-wire fixation for patients with a fracture of the distal radius.
In previous trials performed at the lead centre, recruitment rates of between 70% and 90% were achieved for trials comparing different surgical treatments (both elective and trauma procedures). At a conservative estimate of 35–40% recruitment for a multicentre study, we were, therefore, confident of achieving the recruitment target of 390 patients in 12–18 months.
Randomisation
Those patients who consented to take part in the trial had their method of fixation allocated using a secure, centralised telephone randomisation service. The allocated treatment was then reported back to the research associate, who informed the patient and the treating surgeon. The surgeon then arranged the allocated surgery on the next available trauma operating list, as per standard practice at that institution. This ensured the integrity of the randomisation process.
Sequence generation
Randomisation was on a 1 : 1 basis, stratified by centre, intra-articular extension of the fracture and age of the patient (above or below 50 years).
Stratification by centre helped to ensure that any clustering effect related to the centre itself was equally distributed in the trial arms. The catchment area (the local population served by the hospital) was similar for all of the hospitals (c. 500,000), each hospital being a major orthopaedic unit dealing with these fractures on a daily basis. While it is possible that the surgeons at one centre may be more expert in one or other treatment than those at another centre, all of the recruiting hospitals were chosen on the basis that both techniques are currently routinely available at the centre, that is theatre staff and surgeons were already equally familiar with both forms of fixation. This could not eliminate the surgeon-specific effect of an individual at any one centre. 12 However, as the fixation of a fracture of the distal radius is a common procedure performed on routine trauma operating lists, many surgeons would be involved in the management of this group of patients: between 10 and 30 surgeons at each centre, including both consultants and trainees. Therefore, we expected that each individual surgeon would operate on only two or three patients enrolled in the trial, greatly reducing the risk of a surgeon-specific effect on the outcome in any one centre.
Stratification on the basis of intra-articular extension of the fracture (specifically involvement of the articular surface of the radiocarpal joint) eliminated a major potential confounder, since disruption of this articular surface may predispose to secondary osteoarthritis of the wrist. 13 Recent evidence14 suggests that other associated features of fractures of the distal radius which commonly appear in the classification systems, such as involvement of the ulna styloid process and distal radio-ulnar joint involvement, do not actually affect the functional outcome of the injury. Therefore, we did not include any other variables in the stratification of the randomisation sequence.
Stratification on the basis of age was used to discriminate between younger patients with normal bone quality sustaining high-energy fractures and older patients with low-energy (fragility) fractures related to osteoporosis. Empirically, both of these groups of patients could benefit from the fixed-angle stability provided by locking-plate fixation. However, the stratification would help to identify any effect related to the quality of the patient’s bone. The use of dual-energy X-ray absorptiometry (DEXA) is widely regarded as the gold standard for the assessment of bone density. However, such an investigation may be expensive and not routinely available at all centres. Therefore, we used age as a surrogate for bone density. In a large study in Norway involving 7600 participants, it was demonstrated that forearm bone mineral density remains stable up until the age of 50 years. After the age of 50 years, bone mineral density in males decreased steadily, while in females there was an initial decline between the ages of 50 and 65, with a further decline in the age groups thereafter. 15 A recent study by Court-Brown and Caeser16 assessed over 1000 patients with a fracture of the distal radius. This study confirmed that there is a clear bimodal distribution for this type of fracture according to the age of the patient. The crossover of the two peaks of incidence was around 50 years of age. These studies provide strong evidence that patients over the age of 50 years become increasingly vulnerable to fragility fractures of the distal radius. Therefore, we chose the age of 50 years as the cut-off for this trial. Furthermore, the study by Court-Brown and Caeser16 demonstrated that in the UK approximately 60% of patients sustaining a fracture of the distal radius are over 50 years, while 40% are younger than 50 years. The number of patients above and below this stratification age would, therefore, be similar (see Statistical analysis).
Blinding
In this surgical trial, the patients could not be blinded to their treatment. The treating surgeons were, of course, not blind to the treatment, but took no part in the postoperative assessment of the patients. The statistical analyses were not performed blind.
Postrandomisation withdrawals
Participants could withdraw from the trial at any time without prejudice. If patients decided to have the treatment to which they were not randomised, they were followed up wherever possible and data collected as per the protocol until the end of the trial. The primary analysis was on an intention-to-treat basis with a secondary per-protocol analysis.
Interventions
All of the hospitals involved in this trial used both of the methods of fixation at the time of the trial and all of the surgeons involved were familiar with both techniques. Operative fixation of fractures of the distal radius usually took place under a general anaesthetic, but this decision was made by the attending anaesthetist. Patients undergoing surgical fixation for a fracture of the distal radius are placed in a supine (lying on their back) position with the affected arm on an ‘arm-board’ extension to the operating table, but the exact details of the positioning of the patient were left to the discretion of the treating surgeon. A copy of the ‘operating record’ formed part of the trial data set.
Each patient underwent the allocated surgery according to the preferred technique of the operating surgeon. Although the basic principles of percutaneous K-wire fixation and locking-plate fixation are inherent in the technique (see Kirschner-wire fixation and Volar locking plate), there are several different implant systems and several different options for the positioning of wires and screws. Similarly, each surgeon made minor modifications to their surgical technique according to preference and the specific pattern of each fracture. In this trial, the details of the surgery were left entirely to the discretion of the surgeon to ensure that the results of the trial could be generalised to as wide a group of patients as possible.
Although all of the surgeons in the trial were familiar with both techniques, it is possible that an individual surgeon may have had more experience with one technique than the other. We expect that the proficiency of an individual surgeon to perform the procedure may change over time, as the surgeon gains experience and expertise. The term ‘learning curve’ is often used to describe this process. It was important to monitor the learning curve for all surgeons throughout the trial. The operating time recorded on the operative record for each surgery was used as a proxy to measure the task efficiency of the surgeons (quality assurance of the clinical process), and the number of complications (e.g. infections) at 6 weeks after surgery was also recorded as a patient-based outcome. Given the number of centres and surgeons taking part in this trial, no individual surgeon was likely to perform more than a small number of the procedures. However, where data were available for individual surgeons, temporal variations in operation times and complications at 1 week were modelled for each surgeon using a power curve,12 for the trend with appropriate adjustment for confounding factors such as the age of the patients. In addition, as this study involved multiple surgeons, we expected that a more complex hierarchical model12 that fully accounted for the structure of the data might prove to be useful; therefore, we expected fitting models of this class in addition to the simpler models. Results from the learning curve analysis for each surgeon would inform inferences regarding overall treatment differences and if necessary guide recommendations for implementation and training.
Kirschner-wire fixation
The wires are passed through the skin over the dorsal aspect of the distal radius and into the bone in order to hold the fracture in the correct (anatomical) position. The size and number of wires, the insertion technique and the configuration of wires were left entirely to the discretion of the surgeon. A plaster cast was applied at the end of the procedure to supplement the wire fixation as per standard surgical practice. This cast holds the wrist still and is left on until the wires are removed at the 6-week follow-up appointment.
Volar locking plate
The locking plate is applied through an incision over the volar (palm) aspect of the wrist. Again, the details of the surgical approach, the type of plate, and the number and configuration of screws were left to the discretion of the surgeon. The screws in the distal portion of the bone were ‘fixed-angle’, that is screwed into the plate, but this is standard technique for the use of these plates. The type of proximal screw was left to the discretion of the surgeon; these may be locking or non-locking screws, as the bone in this area provides a much better purchase for the screws. Some surgeons used a temporary plaster cast to hold the patients’ wrist still but the fixed-angle stability provided by the locking plate was generally sufficient to allow early controlled range-of-movement exercises. The use of a cast or otherwise was again left to the discretion of the surgeon as per usual practice.
Radiographic evaluation
Patients were evaluated radiographically at presentation, and then at 6 weeks and 12 months post injury. The degree of palmar tilt, the ulnar variance and the presence of metaphyseal comminution were determined from the calibrated digital images of the posteroanterior and lateral radiographs using the OsiriX DICOM (Digital Imaging and Communications in Medicine) viewer (www.osirix-viewer.com).
The palmar tilt was measured as the angle between lines drawn perpendicular to the long axis and along the distal joint surface of the radius as described by Goldfarb et al. 17 and Mann et al. 18 (Figure 1). Dorsal angulation was denoted by a positive value and volar angulation by a negative value. 19
FIGURE 1.
Ulnar variance (the distance between the radial and ulnar articular surfaces).
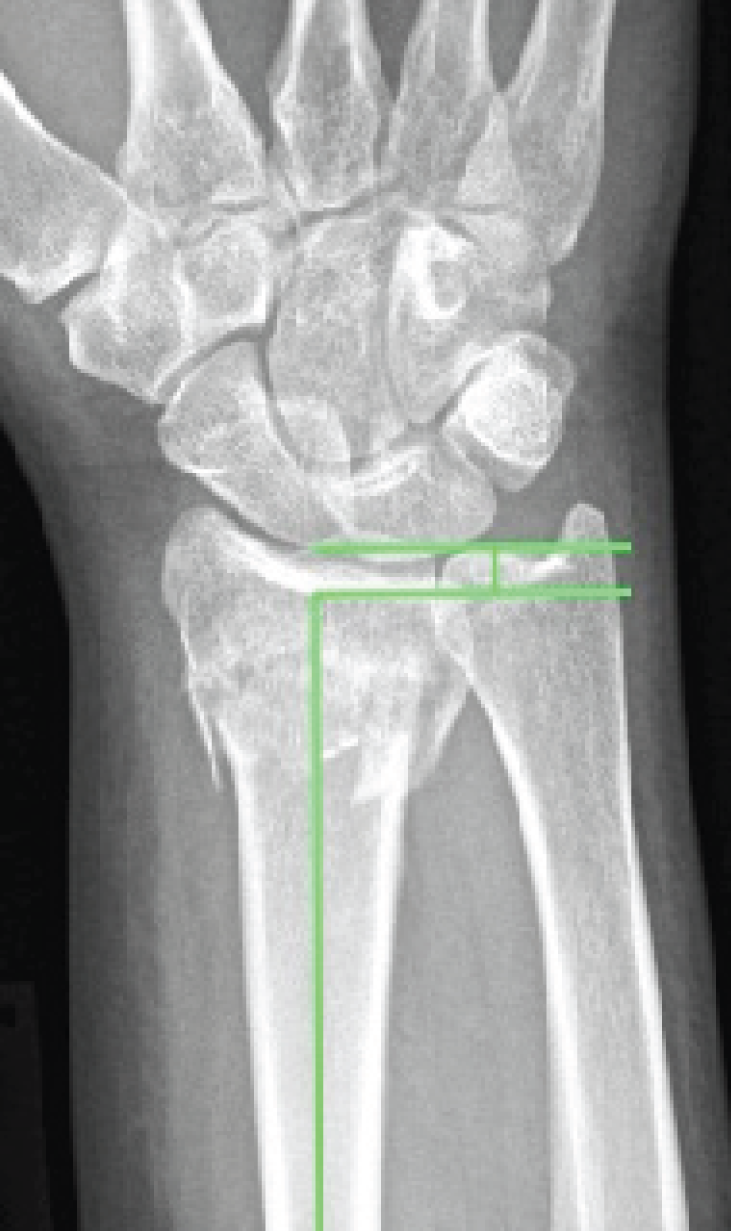
The ulnar variance was determined using the method of perpendiculars described by Steyers and Blair20 Lines were drawn along the distal ulnar aspect of the radius and the distal cortical rim of the ulnar parallel to the perpendicular of the long axis of the radius20–22 (Figure 2). The distance between these lines measures the ulnar variance. 20
FIGURE 2.
Palmar tilt (the angle between the articular surface of the radius and the perpendicular of the long axis of the radius).

Rehabilitation
We ensured that all patients randomised into the two groups received standardised written physiotherapy advice detailing the exercises they needed to perform for rehabilitation following their injury. All of the patients in both groups were advised to move their shoulder, elbow and finger joints fully within the limits of their comfort. Those patients in the K-wire group were encouraged to perform range-of-movement exercises at the wrist as soon as their plaster cast was removed at the 6-week follow-up appointment. Those patients in the locking-plate group could begin the exercises immediately if they did not have a plaster cast or as soon as the cast was removed. In this pragmatic trial, any other rehabilitation input beyond the written information sheet (including a formal referral to physiotherapy) was left to the discretion of the treating surgeon. However, a record of any additional rehabilitation input (type of input and number of additional appointments) together with a record of any other investigations/interventions was requested as part of the 3-month, 6-month and 12-month postal follow-ups and this formed part of the trial data set.
Outcomes
The primary outcome measure for this study was the PRWE questionnaire score. 23 The PRWE is a validated questionnaire which is self-reported (filled out by the patient). It consists of 15 items specifically related to the function of the wrist. These data were collected at baseline and 3, 6 and 12 months postoperatively. The PRWE questionnaire score is the most sensitive outcome measure for patients sustaining this specific injury. 24
The secondary outcome measures in this trial were:
-
DASH questionnaire score: the DASH outcome measure is a 30-item self-report questionnaire designed to provide a more general measure of physical function and symptoms in people with musculoskeletal disorders of the upper limb. 25
-
EuroQol – Five Dimensions (EQ-5D): the EQ-5D is a validated, generic, quality of life questionnaire consisting of five domains related to daily activities with a three-level answer possibility. The combination of answers leads to a health profile of five digits that can then be converted into a utility that represents the overall quality of life of the patient. 26
Complications: all complications were recorded.
Resource use was monitored for the economic analysis. Patients retrospectively reported use of primary, secondary and community health-care use, medications and aids/instruments as well as social care, informal care, out-of-pocket expenses and lost productivity via a short questionnaire which was administered at 3, 6 and 12 months post surgery. Patient self-reported information on service use has been shown to be accurate in terms of the intensity of use of different services. 27,28
Follow-up measures
We used techniques common in long-term cohort studies to ensure minimum loss to follow-up, such as collection of multiple contact addresses and telephone numbers, mobile telephone numbers and e-mail addresses. Considerable efforts were made by the trial team to keep in touch with patients throughout the trial by means of newsletters, etc. Data were collected at baseline and at 6 weeks, 3 months, 6 months and 12 months post surgery (Table 1).
| Time point | Data collection |
|---|---|
| Baseline | PRWE and DASH pre injury, EQ-5D pre injury and contemporary, radiographs |
| 6 weeks | Complication records, radiographs and operative record |
| 3 months | PRWE, DASH, EQ-5D, record of complications/rehabilitation or other interventions and economics questionnaire |
| 6 months | PRWE, DASH, EQ-5D, record of complications/rehabilitation or other interventions and economics questionnaire |
| 12 months | PRWE, DASH, EQ-5D, radiographs, record of complications/rehabilitation or other interventions and economics questionnaire |
The PRWE23 is a 15-item questionnaire, designed specifically for assessment of distal radial fractures and wrist injuries, that rates wrist function using a range of questions in two (equally weighted) sections concerning the patient’s experience of pain and disability. Scoring for all the questions is via a 10-point ordered categorical scale ranging from ‘no pain’ or ‘no difficulty’ (0) to ‘worst possible pain’ or ‘unable to do’ (10). Five questions relate to a patient’s experience of pain and 10 relate to function and disability; the scores for the 10 function items are summed and divided by 2 and added to the scores of the five pain items to give a total score out of 100 (best score = 0 and worst score = 100).
We performed a retrospective pilot study29 that obtained PRWE questionnaire scores for 84 patients (42 each receiving either a volar locking-plate fixation or a manipulation under anaesthetic and K-wire fixation) at 12 months after surgery. The mean PRWE questionnaire scores for the K-wire and locking-plate groups at 12 months were 33.2 and 27.5, respectively, with a pooled standard deviation (SD) of 19. The SD for the PRWE questionnaire score is similar to that reported for other studies of pain and disability after fracture of the distal radius,30 which was in the range of 17–23 points.
We assumed a normal distribution for the PRWE questionnaire scores, which seemed appropriate given that our pilot study indicated that the 12-month group mean PRWE questionnaire score would be expected to be approximately 30 with a SD of 20. An appropriate sample size for detecting a six-point difference between groups at the 5% level with 80% power was, therefore, 175 patients in each group (power and sample size software, available at http://medipe.psu.ac.th/episoft/pssamplesize/). A six-point difference between groups equates to a standardised effect size of 0.3, for an assumed SD of 20 points. MacDermid et al. 24 found that the PRWE questionnaire is sensitive enough to detect subtle but clinically relevant changes in wrist function of this order of magnitude in patients sustaining a fracture of the distal radius, for example changes between 3 and 6 months (effect size 0.5). At the individual level, a change in the PRWE questionnaire score of 6 points reflects the difference between turning a doorknob or cutting a loaf of bread with mild pain versus no pain. We believed that such an improvement would be important to patients on an individual level and at population level and could lead to a change in clinical practice in the UK.
However, it is also helpful to consider the effect of choosing two alternative minimal clinically important difference (MCID) values of 5 and 7 points on the PRWE questionnaire score scale on the required sample size for 80% power at the 5% level (Table 2).
| MCID | Standardised effect size | Sample size per group |
|---|---|---|
| 5 | 0.25 | 252 |
| 6 | 0.30 | 175 |
| 7 | 0.35 | 129 |
In summary, this study used the PRWE questionnaire score at 12 months after surgery as the primary outcome measure. The total number of patients required to obtain a power of 80% to detect a six-point difference between groups for the primary outcome measure was 350, that is 175 patients were required in each treatment group. In trials run previously at our institution comparing two different surgical techniques, we experienced a ≈5% loss to follow-up. With an allowance for a conservative 10% loss to follow-up, we planned to recruit a minimum of 390 patients.
If recruitment proceeded at a faster rate than expected, for example if there were particularly adverse winter weather, which is known to be a primary causative factor for distal radius fractures, then there was the possibility of increasing the overall trial sample size within the defined recruitment period. Increasing the sample size would provide an increase in the power to detect a six-point difference between groups for the primary outcome measure above the set level of 80%. Recruitment was monitored and discussed at monthly Trial Management Group (TMG) meetings and, where appropriate, recruitment projections were undertaken to assess this possibility. See Appendix 1 for details of recruitment projections and new sample sizes for increasing power.
Adverse event management
Adverse events are defined as any untoward medical occurrence in a clinical trial subject and which do not necessarily have a causal relationship with the treatment. All adverse events were listed on the appropriate case report form for routine return to the Distal Radius Acute Fracture Fixation Trial (DRAFFT) central office.
Serious adverse events (SAEs) were defined as any untoward and unexpected medical occurrence that:
-
results in death
-
is life-threatening
-
requires hospitalisation or prolongation of existing inpatients’ hospitalisation
-
results in persistent or significant disability or incapacity
-
is a congenital anomaly or birth defect
or any other important medical condition which, although not included in the above, may require medical or surgical intervention to prevent one of the outcomes listed.
All SAEs were entered onto the serious adverse event reporting form and given to the DRAFFT central office within 24 hours of the investigator becoming aware of them. Once this was received, causality and expectedness were determined by the chief investigator. SAEs that were deemed to be unexpected and related to the trial were notified to the Research Ethics Committee (REC) within 15 days for a non-life-threatening event and within 7 days for a life-threatening event. All such events were reported to the TSC and Data Monitoring Committee (DMC) at their next meetings. SAEs that were expected as part of the surgical interventions, and that did not need to be reported to the main REC, were complications of anaesthesia or surgery (e.g. wound infection, delayed wound healing and thromboembolic events). All participants experiencing SAEs were followed up as per protocol until the end of the trial.
Risks and benefits
The risks associated with this study were predominantly the risks associated with the surgery: infection, bleeding and damage to the adjacent structures such as nerves, blood vessels and tendons. Participants in both groups underwent surgery and were potentially at risk from any/all of these complications. The application of the locking plates requires a surgical incision over the volar aspect of the wrist, which, empirically, may lead to a higher risk of injury to the adjacent structures. However, the evidence available to quantify this risk was limited. 13 Late attrition and rupture of the extensor tendons have been associated with volar locking plates,31 but, equally, late collapse and secondary deformity have been associated with K-wire fixation. 19 The risk of secondary (as a result of the injury) osteoarthritis of the wrist joint, particularly in those patients with intra-articular extensions of the fracture, is well recognised,13 but there were no data to suggest what that risk is or that the risk is greater in one group or another. We believed that the overall risk profile was similar for the two interventions but an assessment of the number of complications in each group was a secondary objective of this trial.
Statistical analysis
Analysis plan
The statistical analysis plan was agreed with the DMC at the start of the study. Any subsequent amendments to this initial plan are clearly stated and justified. Interim analyses were performed only where directed by the DMC. The trial registration details were updated soon after the start of the trial; this was not a change to the statistical analysis plan, but merely the inclusion of extra detail in the registration document, which was added at the suggestion of the DMC.
Software
The routine statistical analysis was carried out using R version 3.0.1 (The R Foundation for Statistical Computing, Vienna, Austria, www.r-project.org/). When any analyses were required, data were retrieved from the trial database by the trial statistician. The statistician imported data directly into the statistical package R for analysis and reporting. The version numbers of all software used, data files and all R scripts were made available to the DMC on request at any stage of the trial. Statistical results were reported in accordance with Consolidated Standards of Reporting Trials (CONSORT) guidelines (www.consort-statement.org/).
Data validation
Prior to formal analysis, data were checked for outliers and missing values and validated using the defined score ranges for all outcome measures. Queries were reported to the trial co-ordinator and investigated. Standard statistical summaries (e.g. medians and ranges or means and variances, dependent on the distribution of the outcome) and graphical plots showing correlations were presented for the primary outcome measure and all secondary outcome measures. Baseline data were summarised to check comparability between treatment arms, and to highlight any characteristic differences between those individuals in the study, those ineligible for the study, and those eligible but withholding consent.
Missing data
As seemed likely at the outset, some data were not available because of the voluntary withdrawal of patients, lack of completion of individual data items or general loss to follow-up. Where possible, the reasons for data ‘missingness’ were ascertained and reported. Although missing data were not expected to be a problem for this study, the nature and pattern of the missingness were carefully considered – including, in particular, if data could be treated as missing completely at random (MCAR). If judged appropriate, we planned to impute missing data using the multiple imputation facilities (mice package) available in R. Any imputation methods used for scores and other derived variables were carefully considered and justified. We planned that if the degree of missingness was relatively low, as expected, the primary analysis would be based on complete cases only (complete case analysis), with analysis of imputed data sets used to assess the sensitivity of the analysis to the missing data. Reasons for ineligibility, non-compliance, withdrawal or other protocol violations would be stated and any patterns summarised. More formal analysis, for example using logistic regression with ‘protocol violation’ as a response, was also considered if appropriate and if it aided interpretation.
Interim analyses
Interim analyses were performed only where directed by the DMC. Any interim analyses would follow the same procedure as the final analyses.
Final statistical analyses
Null hypothesis
We considered that, while it may be of interest to the patient to know the time from surgery to returning to ‘normal’ function, our previous experience of collecting data related to this outcome has been very difficult. In addition, there is no clear formal definition of when a fracture can be considered to have fully healed and the patient is able to return to normal activity. This would be necessary if the time to this event were potentially to be used as the primary outcome measure in this trial. Therefore, for these reasons and as the practical difficulties and cost implications for a time-to-event analysis would be considerable, the main analysis investigated differences in the primary outcome measure, the PRWE questionnaire score at 12 months after surgery, between the two treatment groups (K-wire fixation and locking-plate fixation) on an intention-to-treat basis.
Null hypothesis: there is no difference in the PRWE questionnaire score 12 months post injury between adult patients with a dorsally displaced fracture of the distal radius treated with locking-plate fixation and those treated with K-wire fixation.
In addition to the analysis at 12 months post injury, early functional status was also assessed and reported at 3 months and 6 months.
Multilevel model
Differences between treatment groups were assessed, based on a normal approximation for the PRWE questionnaire score, at 12 months postoperatively, and at interim occasions. Tests were two-sided and considered to provide evidence for a significant difference if p-values are < 0.05 (5% significance level). Estimates of treatment effects were presented with 95% confidence intervals (CIs).
The stratified randomisation procedure should have ensured a balance in age, intra-articular extension and the recruiting centre between test treatments. Although generally we had no reason to expect that the clustering effects would be important for this study, in reality the data were likely to be hierarchical in nature, with patients naturally clustered into groups by recruiting centre and surgeon. Therefore, we proposed to account for this by generalising the conventional linear (fixed-effects) regression approach to a more general multilevel modelling approach; where patients are naturally grouped by surgeons and, likewise, surgeons are grouped by recruiting centres. This model would formally incorporate terms that allowed for possible heterogeneity in responses for patients owing to the recruiting centre and the surgeon, in addition to the fixed effects of the treatment groups, patient age and intra-articular extension. As discussed earlier, we expected that each individual surgeon would operate on only a small number (two or three) of the patients enrolled in the trial, which would greatly reduce the likelihood of a surgeon-specific effect on the outcome. Therefore, although we considered formally constructing a model with two levels of clustering (recruiting centre and surgeon), we expected that, if there were sparse data on individual surgeon effects, the final model would be simplified and consist of single random effect accounting for the recruiting centre only.
The main analyses were conducted using specialist multilevel modelling functions available in the software package R. PRWE questionnaire data were assumed to be normally distributed, although we considered the possibility of appropriate variance-stabilising transformation. The primary focus was the comparison of the two treatment groups of patients, and this was reflected in the analysis, which was reported together with appropriate diagnostic plots that check the underlying model assumptions. Although baseline PRWE questionnaire scores were recorded using a retrospective assessment by each patient post injury, these were not considered to be sufficiently reliable for formal baseline adjustment in the multilevel model. Mean baseline PRWE questionnaire scores together with other patient demographic data (e.g. age and gender) were reported for each treatment group to assess population comparability post randomisation.
The interactions between the stratifying variables (age and intra-articular extension) and the main treatment effect were not expected to be large; therefore, for practical reasons, the study was not powered with subgroup analysis in mind, as this would require a substantial and unrealistic increase in overall sample size. However, analyses were planned for each of the stratifying variables, particularly for participants aged over 50 years, who constitute a sizeable and important subgroup within the full study population. Formal tests of interaction between each stratifying variable and the treatment factor were reported together with appropriate 95% CIs and p-values. Significant results from this analysis were interpreted with due caution and reported as such, in line with recommendations for subgroup analysis made elsewhere. 32
Complications
The temporal patterns of any complications were presented graphically and if appropriate a time-to-event analysis (Kaplan–Meier survival analysis) was considered to assess the overall risk and risk within individual classes of complications (e.g. infection). This was to be the primary analysis for complications. However, if multiple complications proved to be widely reported then a Poisson regression model (or zero-inflated Poisson regression model) was planned to assess overall differences in counts of events between groups, adjusting for potential confounding factors such as age and gender. Multiple complications were defined as two or more independent events, that is, not continuations of a previous complication, in the same patient and were identified only after discussion with the clinical team.
Reporting
Wherever possible, the results of all analyses were presented in a simple and easy-to-follow manner and related any observed differences to their clinical importance, so that they could be clearly understood by those with only rudimentary statistical knowledge. Open and confidential reports of the statistical analyses were planned, as required, by the trial statistician and, where appropriate, results were to be disseminated through peer-reviewed journals, conference presentations and local mechanisms.
Health economic analysis plan
Objectives
The economic evaluation was designed to estimate costs of distal radial fractures treated by locking-plate fixation and by K-wire fixation. If appropriate, the primary objective was to evaluate the incremental cost-effectiveness of distal radial fractures treated by locking-plate fixation versus K-wire fixation.
A within-trial analysis comparing the outcomes and costs up to 12 months’ follow-up using trial data was undertaken for two age groups: (1) patients < 50 years of age and (2) patients ≥ 50 years of age.
The evaluation followed the reference case guidance for technology appraisals set out by the National Institute for Health and Care Excellence (NICE). 33
In the longer term, we will develop a cost-effectiveness analysis modelling outcomes and costs up to 10 years post surgery. The methods for this second analysis will be presented in due course. However, the results for this analysis are not presented in the present report; this will require longer follow-up data, which are not currently available.
Measurement of outcomes
The economic analysis used quality-adjusted life-years (QALYs) to measure health outcomes. Health-related quality of life was estimated using responses from the EuroQol – Five Dimensions 3 Level. 26 Patients completed the EQ-5D questionnaire at baseline, 3 months, 6 months and 12 months after randomisation as secondary outcomes of the trial. Standard UK tariff values were applied to these responses at each time point to obtain utility. QALYs were calculated as an ‘area under the curve’ and formed the main outcome measure of the study. In addition, patients self-completed EQ-5D assessing their pre-injury quality of life retrospectively.
Measurement of costs
Patient-reported data on resource usage were collected within the trial at 3 months, 6 months and 12 months. For the 3-month data, the recall period was since discharge from hospital. For the other cases, it was since the last questionnaire was due to be completed. The questionnaires included number of further inpatient stays following the initial operation (including specialty and length of stay), number of outpatient, primary and community care visits, use of aids and adaptations, and medication use. Patients also reported the use of personal social services related to their treatment, as well as private costs such as treatments within private settings and time off work. Personal social services included number of weeks of frozen/hot meals on wheels, laundry services and number of visits of carers and social workers.
Costs were estimated combining patient-reported resource usage with unit cost data obtained from national databases such as the British National Formulary (BNF) and Personal Social Services Research Unit (PSSRU) Costs of Health and Social Care. 34 Any unit cost that was not available was estimated in consultation with the UHCW finance department.
The analyses initially took the perspective of the NHS including the costs of health and social care. Subsequent analyses adopted a societal perspective taking into account productivity costs (time away from work) and out-of-pocket expenditures incurred by patients in relation with their treatment. The currency used is pounds sterling (£).
Missing data
Although missing data were not expected to be a problem in this study, the economic analyses followed the nature and pattern of the missingness reported by the statisticians. This was illustrated as MCAR, missing at random or not missing at random.
A base-case analysis of the complete data set was conducted including only patients with complete cost and QALY data.
We then explored the use of the last observation carried forward (LOCF) approach to deal with missing values. Costs and EQ-5D values were carried forward for only one successive missing observation.
Finally, we imputed missing data through multiple imputations. 35,36 This method, assuming that data are missing at random, replaces the missing values with plausible substitutes based on the distribution of observed data and, by using iterative multivariable regression techniques, includes randomness to reflect uncertainty. All baseline variables were used to impute missing data. This approach is recommended for economic analyses alongside clinical trials, as it reflects the uncertainty inherent in replacing missing data. 12 We considered the impact on the cost-effectiveness results using both imputation methods in the sensitivity analyses.
Within-trial analysis
Main characteristics of the analysis
The within-trial analysis aimed to determine the intervention that would maximise health outcomes (1) within the limited NHS budget and (2) within a societal perspective over the 12-month trial follow-up.
It adopted an intention-to-treat perspective and consisted of a cost–utility analysis over 12 months examining the cost per QALY gained for all patients. As the analysis uses a 1-year time horizon, discounting for the future cost and health outcome is not necessary in this analysis. We calculated the incremental cost-effectiveness ratios by dividing the average difference in cost between the two arms by the average difference in QALYs between the two arms as follows:
The incremental cost-effectiveness ratio (ICER) represents the additional cost per unit of outcome gained. This indicates the trade-off between total cost and effectiveness when choosing between volar locking-plate fixation and K-wire fixation. When compared against the marginal trade-off for the NHS as a whole – the cost-effectiveness threshold – this gives an indication of whether or not spending additional money on volar locking-plate fixation appears efficient. As a guideline rule, we used the NICE implicit cost per QALY threshold of £20,000–30,000 per QALY to determine cost-effectiveness. NICE accepts an intervention with an ICER of less than £20,000 per QALY as cost-effective and generally states that an intervention costing more than £30,000 per QALY is not considered cost-effective. The more expensive intervention was considered cost-effective as long as its ICER is within or below the £20,000–30,000 per QALY range.
Uncertainty
The non-parametric bootstrapping approach was used to determine the level of sampling uncertainty around the ICER by generating 10,000 estimates of incremental costs and benefits. We planned to present cost-effectiveness scatter plots illustrating the uncertainty surrounding the cost-effectiveness estimates. The cost-effectiveness acceptability curves (CEACs) were derived by plotting the bootstrapped estimates. The bootstrap approach is a non-parametric method that considers the original sample as though it were the population and draws multiple random samples from the original sample. The CEACs illustrated the probability that a treatment is cost-effective in relation to a comparator, as a function of the threshold willingness to pay (WTP). 12 These were constructed using the net benefit approach. The standard non-parametric method for constructing CEACs is to bootstrap 10,000 samples from the original data, in order to plot the proportion of times each treatment represents the maximum average net benefit for a range of values of WTP values. Net monetary benefit (NMB) is calculated for each bootstrap estimate for a range of ceiling ratios as follows:
where λ is the value a decision-maker would be willing to pay.
The intervention with NMB > 0 should be adopted taking into account the NHS WTP threshold. A series of net benefits were calculated for relevant λ values ranging from £0 to £50,000 (£5000 increments) to incorporate the £20,000–30,000 per QALY gain threshold range currently specified for NICE decision-making in England and Wales. 32
Mean incremental net benefits between the two arms were reported, with 95% bootstrap CIs calculated using the bias-corrected method. 37
Sensitivity
We planned to undertake univariate sensitivity analyses to explore key uncertainties in the scenarios considered.
The results for complete cost and quality of life data (i.e. those with no missing data) as well as a strict per-protocol analysis of the data were provided to identify the impact of missing data on the analysis and any sensitivity to protocol violations.
As patients might also recover function within the first 3 months (rather than continuously to 3 months), a quicker initial recovery was explored in QALY calculations, where each patient’s quality of life was assumed to reach its observed 3-month level at 6 weeks postoperatively.
The cost assumptions in the analysis were modified if relevant (e.g. least expensive implant).
Sensitivity analyses were also planned to consider stratified analyses by factors such as age, gender, trial centre, baseline PRWE and DASH questionnaire scores, and intra-articular extension, if this was found to affect quality of life within the trial period.
Longer-term analysis
For the longer term, we will use decision-analytic modelling to compare locking-plate fixation and K-wire fixation for distal radial fractures over a longer time frame. We will use a 10-year Markov decision model based upon within clinical trial results to estimate the long-term effectiveness of fixations and the proportional hazards of complications and also revision in both people < 50 years of age and people ≥ 50 years of age.
The model will include four possible states: (1) successful fixation; (2) complications; (3) revised fixation/surgery; and (4) death and its structure (Figure 3).
FIGURE 3.
States and allowable state transitions corresponding to use of a distal radius acute fracture fixation.
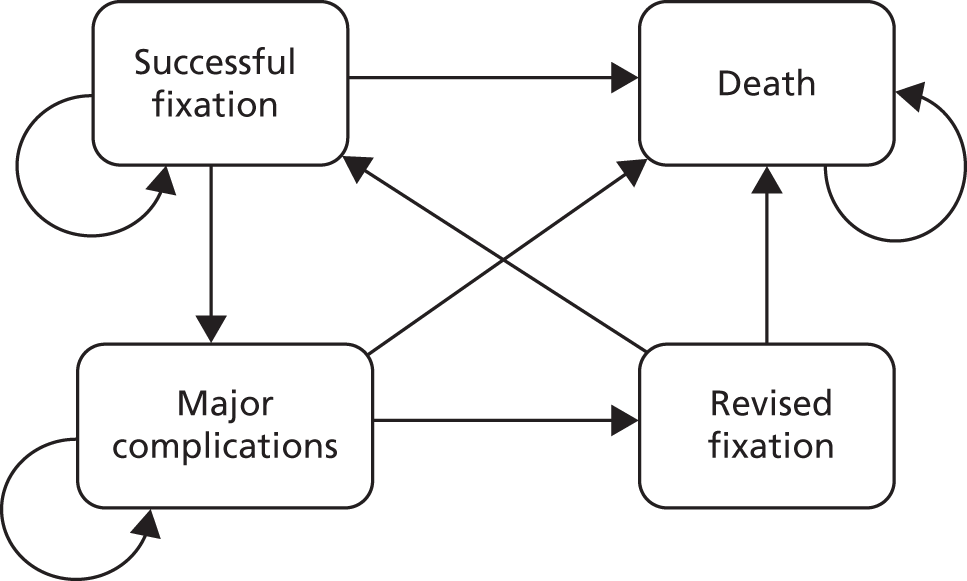
The impact of complications and subsequent revision surgery will be assessed using information from various sources: the extrapolation of within-trial data and the longer complication follow-up data which is currently ongoing, the published literature and/or expert opinions to create the most accurate model to compare locking-plate fixation and K-wire fixation for distal radial fractures. We will conduct a systematic review of cost-effectiveness models in the treatment distal radius fracture. This will act as the main resource of transition probabilities for the model. Where possible, transition probabilities will be based on the latest UK studies reflecting current care pathways and associated expectations relating to long-term patient functioning.
Two different base case scenarios will be considered, < 50 years of age cohort and ≥ 50 years of age cohort, both followed for 10 years.
Following the NICE reference case,33 costs and benefits will be discounted at 3.5% and a NHS perspective adopted.
Deterministic one-way sensitivity analysis will be performed to check the results over a plausible range of prior distributions placed on the time-varying model parameters. Multiway deterministic sensitivity analyses will be undertaken by modelling optimistic (‘best case’) and pessimistic (‘worse case’) scenarios. 38
Reporting
The results of the within-trial analyses are presented such that people with basic cost-effectiveness analysis knowledge can understand it.
It was planned that results would be disseminated through peer-reviewed journals, conference presentations and other types of research work dissemination. We expected to provide the within-trial analysis results for the trial main paper and we aimed at producing one extra paper focusing on the full health economics analysis with more mature data in the longer term.
Ethical approval and monitoring
Standard NHS cover for negligent harm was in place. There was no cover for non-negligent harm.
Ethics committee approval
The DRAFFT trial was approved by the Coventry Research Ethics Committee in February 2010 (REC reference 10/H1210/10) and by the research and development department of each participating centre. The final approved study protocol has been published.
Trial management group
The day-to-day management of the trial was the responsibility of the trial co-ordinator, based at Warwick Clinical Trials Unit (CTU) and supported by the CTU administrative staff. This was overseen by the TMG, which met monthly to assess progress. It was also the responsibility of the trial co-ordinator to undertake training of the research associates at each of the trial centres. The trial statistician and health economist were closely involved in the setting up of data capture systems and the design of databases and clinical reporting forms.
Trial Steering Committee
A TSC was responsible for monitoring and supervising the progress of the DRAFFT trial. The TSC consisted of four independent experts, a lay member and leading members of the TMG. Membership of the TSC is given in Acknowledgements.
Data Monitoring Committee
The DMC was independent of the trial and was tasked with monitoring ethical, safety and data integrity. The trial statistician provided data and analyses requested by the DMC at each of the meetings. Membership of the DMC is given in Acknowledgements.
Chapter 3 Results
Screening
Overall
The overall flow of patients in the trial is shown in Figure 4. The total number of patients screened was 12,162; Table 3 shows the age and gender split.
FIGURE 4.
Consolidated Standards of Reporting Trials (CONSORT) plot: overall flow of patients in the study. MUA, manipulation under anaesthetic.
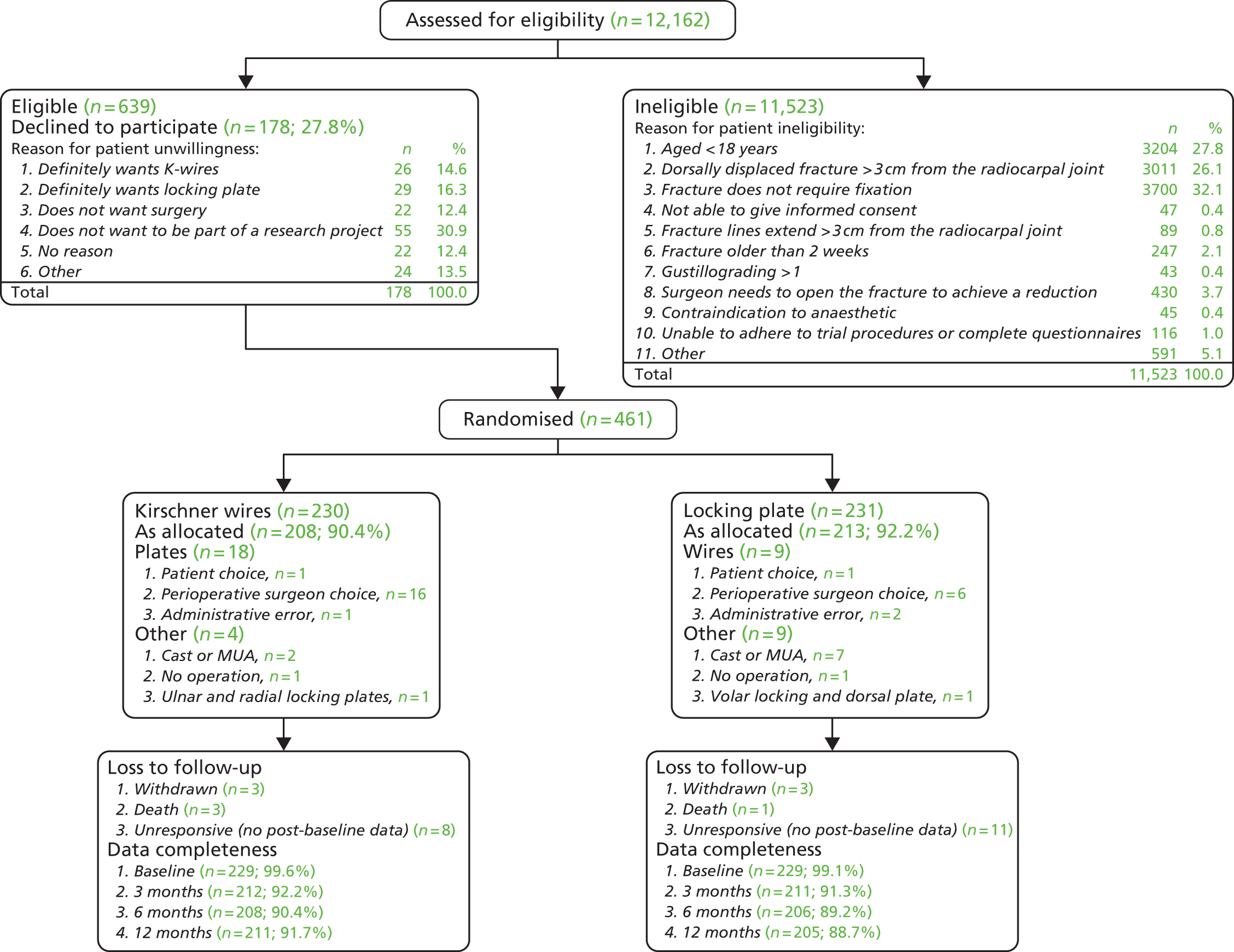
| Gender | Age group (years) | Total, n (%)a | |
|---|---|---|---|
| < 50, n (%) | ≥ 50, n (%) | ||
| Female | 2317 (21.6) | 4806 (44.7) | 7123 (66.3) |
| Male | 2798 (26.0) | 830 (7.7) | 3628 (33.7) |
| Total | 5115 (47.6) | 5636 (52.4) | 10,751 (100.0) |
The overall median age of screened patients, where data were available, was 53 years [interquartile range (IQR) 18–72 years]; for females it was 63 years (IQR 39–77 years) and for males 23 years (IQR 12–48 years).
There was clear bimodality in the age distributions for each gender (Figure 5), particularly for females. A Gamma mixture model, gammamixEM function in R package mixtools, was fitted to data from each gender to estimate the proportionate split and median ages in each group; bootstrapping (n = 100) was used to estimate 95% CIs for these statistics.
FIGURE 5.
Age distribution of screened patient bars and fitted distributions for (a) females (n = 7123) and (b) males (n = 3628).
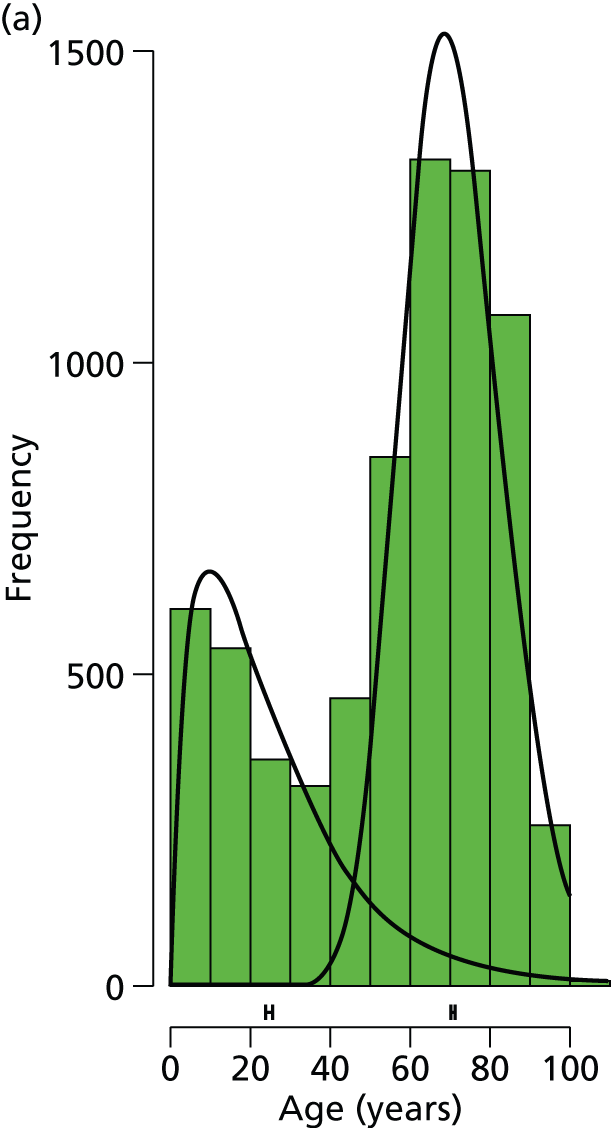
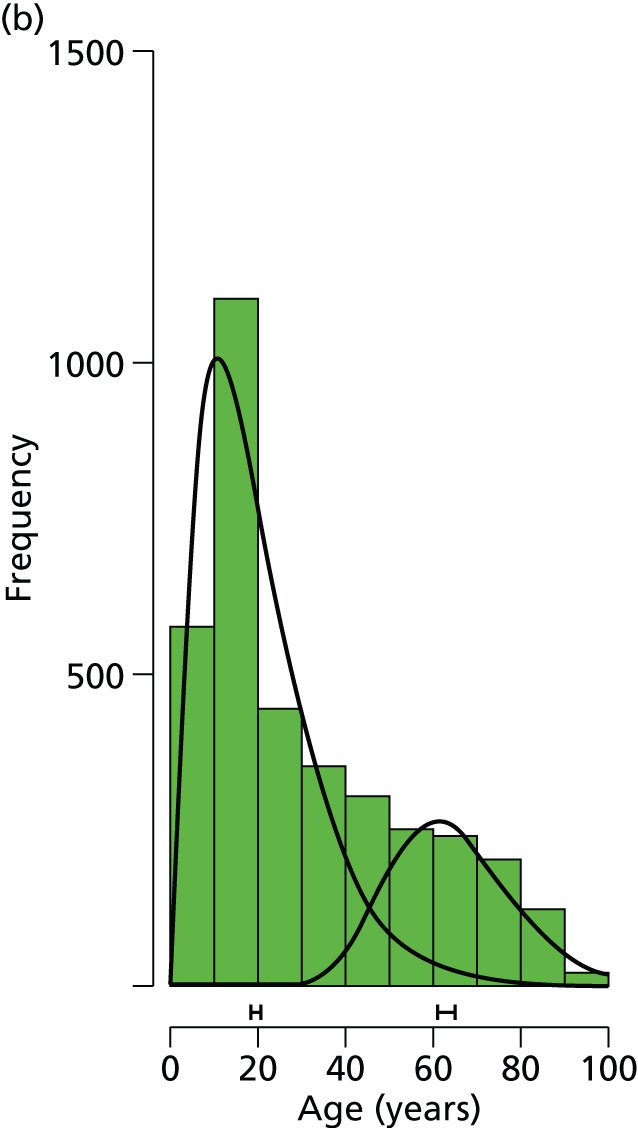
The modelling suggests that, for females, the ‘young’ group has a median age of 25.1 years (95% CI 24.1 to 25.8 years) and the ‘old’ group has a median age of 71.1 years (95% CI 70.6 to 71.5 years), with the split by group (young : old) given by 32.3% : 67.7% (95% CI 30.8 to 33.5). For males, the ‘young’ group has a median age of 19.7 years (95% CI 18.3 to 20.6 years) and the ‘old’ group has a median age of 63.7 years (95% CI 61.3 to 65.1 years), with the split by group (young : old) given by 73.5% : 26.5% (95% CI 69.3 to 76.0).
A recent study by Court-Brown and Caeser16 assessed over 1000 patients with a fracture of the distal radius. This study suggested that there is a clear bimodal distribution for this type of fracture according to the age of the patient; with a crossover of the two peaks of incidence at around 50 years of age. This was also the case for our data, for both males and females, with Figure 5 showing that the approximate division between the two age groups was also at around 50 years. Stratification on the basis of age was used to discriminate between younger patients with normal bone quality sustaining high-energy fractures and older patients with low-energy (fragility) fractures related to osteoporosis.
Eligibility
Figure 4 shows that the total number eligible for the study was 639, that is the total number screened (12,162) minus the total number ineligible (11,523). The main reasons for patient ineligibility were that the fracture did not require fixation (32.1%), the fracture was dorsally displaced > 3 cm from the radiocarpal joint (26.1%) and the patient was aged < 18 years (27.8%).
Willingness
Of the 639 eligible patients, 461 (72.1%) were willing and 178 were unwilling to take part in the trial. The main reason for being unwilling to participate was a simple unwillingness to be part of a research project (55 patients; 30.9%). Of those patients who expressed a preference, there was a balance between the treatment groups, with 26 patients stating a preference for wires (14.6%) and 29 stating a preference for a plate (16.3%).
In total, 461 patients were willing to participate in the DRAFFT study. The gender and age of the individuals who were willing and unwilling to participate in the study are shown in Table 4 and the distribution of ages by gender and group in Figure 6.
| Characteristic | Willing (n = 461) | Unwilling (n = 178) |
|---|---|---|
| Age in years, mean (SD) | 58.8 (15.9) | 56.1 (18.8) |
| Gender (% female) | 83.0 | 79.1 |
FIGURE 6.
Box plot showing age distribution by gender and willingness to participate in trial. F, female; M, male.
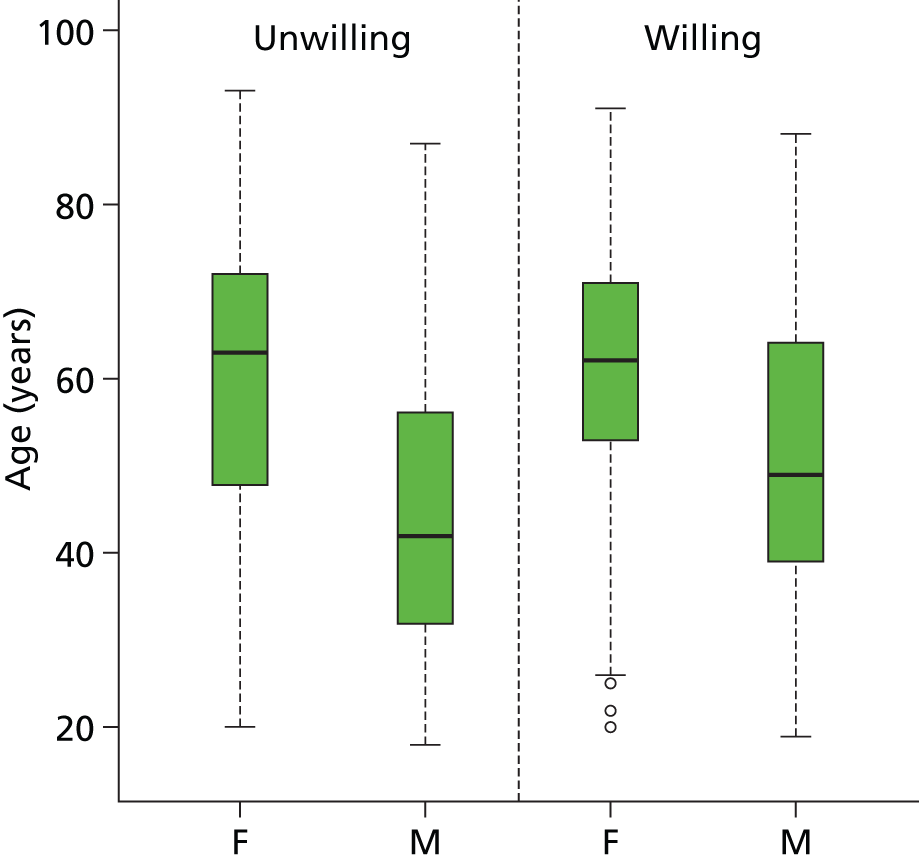
A t-test showed that ages did not differ significantly between groups (p = 0.088) and a chi-squared test showed no difference in gender split between groups (p = 0.301).
Recruitment
The trial planned to recruit as a minimum 390 patients in total. Formal recruitment started on 1 January 2011 and finished on 30 June 2012. Recruitment took place at 18 centres (Table 5).
| Centre | Code |
|---|---|
| 1. Addenbrookes Hospital | ADH |
| 2. Frenchay Hospital | FRH |
| 3. Ipswich Hospital | IPH |
| 4. James Cook University Hospital | JCH |
| 5. John Radcliffe Hospital | JRH |
| 6. Leicester General Hospital | LGH |
| 7. North Tyneside General Hospital | NTG |
| 8. Northampton General Hospital | NGH |
| 9. Peterborough City Hospital | PCH |
| 10. Poole Hospital | POH |
| 11. Royal Blackburn Hospital | BBH |
| 12. Royal Victoria Infirmary | RVI |
| 13. St Thomas’ Hospital | STH |
| 14. University Hospital Coventry and Warwickshire | UHC |
| 15. University Hospital North Staffordshire | UNS |
| 16. University Hospital of North Tees | NTH |
| 17. Wansbeck General Hospital | WGH |
| 18. Wexham Park Hospital | WPH |
Overall
Overall trends in recruitment are shown in Figure 7; the final number of participants recruited to the study was 461 (Table 6).
FIGURE 7.
Overall DRAFFT recruitment (thick black line) and recruitment by centre (January 2011 to June 2012); logarithmic scale used to enhance detail for individual centres. Original target = 390 patients.

| Hospital | Treatment group | Total | Months open | Rate (per month) | |
|---|---|---|---|---|---|
| K-wire | Locking plate | ||||
| UHC | 50 | 50 | 100 | 23 | 4.51 |
| RVI | 30 | 30 | 60 | 19 | 3.21 |
| WGH | 15 | 16 | 31 | 12 | 2.58 |
| FRH | 15 | 14 | 29 | 18 | 1.62 |
| BBH | 13 | 13 | 26 | 11 | 2.53 |
| ADH | 12 | 12 | 24 | 17 | 1.50 |
| JCH | 13 | 11 | 24 | 17 | 1.47 |
| IPH | 11 | 12 | 23 | 18 | 1.32 |
| JRH | 11 | 12 | 23 | 13 | 1.78 |
| UNS | 10 | 11 | 21 | 15 | 1.48 |
| NGH | 11 | 9 | 20 | 11 | 1.86 |
| POH | 9 | 11 | 20 | 13 | 1.58 |
| STH | 8 | 10 | 18 | 14 | 1.30 |
| LGH | 6 | 5 | 11 | 11 | 1.09 |
| PCH | 6 | 5 | 11 | 10 | 1.15 |
| WPH | 5 | 5 | 10 | 7 | 1.64 |
| NTH | 4 | 4 | 8 | 5 | 1.78 |
| NTG | 1 | 1 | 2 | 1 | 4.68 |
| Average | – | – | – | 13.05 | 1.96 |
| Total | 230 | 231 | 461 | 235 | – |
The final number of recruited participants was above the initial target of 390.
Recruitment proceeded at a faster rate than expected; therefore, the decision was taken to allow the sample size to increase above the target of 390 patients.
Population characteristics
The randomisation was stratified by hospital, age group and intra-articular extension. Table 7 shows recruitment numbers by treatment group, age group and intra-articular extension.
| Age group, years | Intra-articular extension | Treatment | Total | |
|---|---|---|---|---|
| K-wire | Locking plate | |||
| < 50 | No | 31 | 33 | 64 |
| Yes | 29 | 27 | 56 | |
| ≥ 50 | No | 91 | 89 | 180 |
| Yes | 79 | 82 | 161 | |
| Total | 230 | 231 | 461 | |
The breakdown by age, gender and treatment group is shown in Table 8.
| Age group, years | Gender | Treatment | Total | |
|---|---|---|---|---|
| K-wire | Locking plate | |||
| < 50 | Female | 35 | 46 | 81 |
| Male | 25 | 14 | 39 | |
| ≥ 50 | Female | 156 | 148 | 304 |
| Male | 14 | 23 | 37 | |
| Total | 230 | 231 | 461 | |
The majority (304 out of 461) of patients recruited to the trial were female and ≥ 50 years of age, as expected.
Surgeons and operations
Before proceeding to analyse the outcomes, it is informative to look at the details of the surgical procedures.
Surgeons
Treatments were undertaken by 244 different surgeons; the median number of operations per surgeon was 1 (IQR 1–2). Figure 8 shows a histogram of the number of procedures undertaken by each surgeon.
FIGURE 8.
Histogram of numbers of operation for each surgeon.
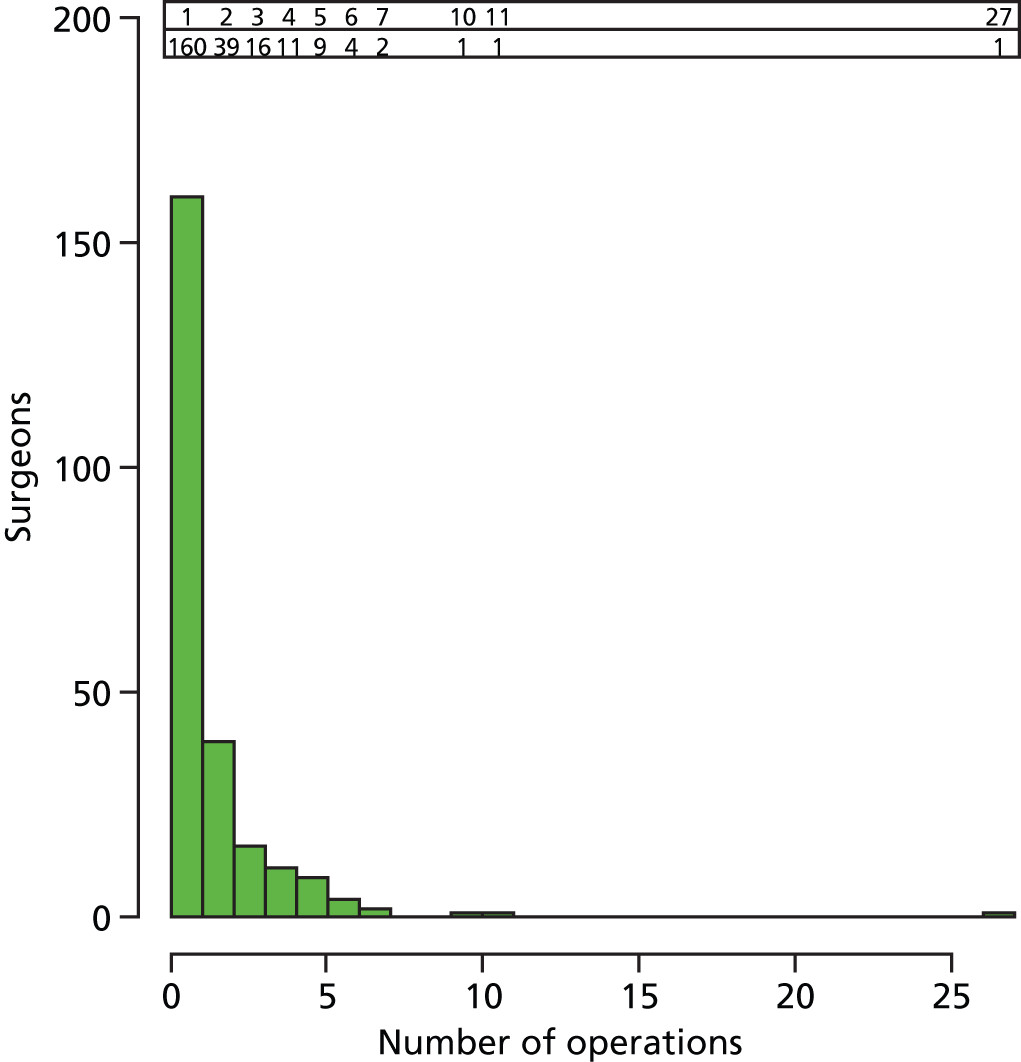
As expected, any individual surgeon operated only on a small number of patients (n = 2 or 3) enrolled in the study; 88% of surgeons (215 out of 244) treated fewer than three study participants. This greatly reduces the likelihood of a surgeon-specific effect on the outcome at any one centre, that is one particularly good or bad surgeon dominating the other surgeons in the study.
Operations
Details of the surgeon grade and experience and methods employed for each procedure are shown in Table 9 and operation times by surgeon grade are shown in Figure 9.
| Details of surgeons and surgery | K-wire (n = 230) | Locking plate (n = 231) |
|---|---|---|
| Perioperative antibiotic (no : yes) | 58 : 164 (71%) | 19 : 191 (83%) |
| Operated wrist (right : left) | 101 : 24 (54%) | 102 : 124 (54%) |
| Intraoperative problems (no : yes) | 222 : 4 (2%) | 219 : 4 (2%) |
| Surgeon grade, n (%)a | ||
| Consultant | 60 (26) | 71 (31) |
| Specialist trainee | 102 (44) | 106 (46) |
| Staff grade/associate specialist | 30 (13) | 29 (13) |
| Other | 34 (15) | 20 (9) |
| Surgeon experience (number of prior operations), n (%)a | ||
| 0 | 0 (0) | 1 (0) |
| < 5 | 11 (5) | 8 (3) |
| 5–10 | 16 (7) | 23 (10) |
| 11–20 | 25 (11) | 30 (13) |
| > 20 | 171 (74) | 158 (68) |
| Wires, n (%) | ||
| Number of wires useda | ||
| 1 | 1 (0) | – |
| 2 | 96 (42) | – |
| 3 | 105 (46) | – |
| > 3 | 5 (2) | – |
| Wire sizea | ||
| 1.6 mm | 187 (81) | – |
| 1.1 mm | 1 (0) | – |
| Other | 12 (5) | – |
| Techniquea | ||
| Kapandji | 54 (23) | – |
| Interfragmentary | 78 (34) | – |
| Mixed technique | 71 (31) | – |
| Plates, n (%) | ||
| Number of screws useda | ||
| 3 | – | 20 (9) |
| 4 | – | 62 (27) |
| 5 | – | 42 (18) |
| > 5 | – | 88 (38) |
| Proximal screwa | ||
| Locking | – | 103 (45) |
| Non-locking | – | 110 (48) |
| Operation time | ||
| Minutes | 31 | 66 |
| Median (IQR) | 24 (45) | 50 (85) |
| Mean (SD) | 37.2 (19.8) | 69.9 (27.7) |
| Length of stay | ||
| Days | 1 | 1 |
| Median (IQR) | 0 (1) | 0 (1) |
FIGURE 9.
Operation times by surgeon grade and procedure.

There was no evidence to suggest that operation times differed between surgeon grades.
Outcomes
Treatment allocation
Table 10 shows the treatment allocation and the treatments received by treatment group. In the K-wire group 90.4% of participants received their allocated intervention and in the locking-plate group 92.2% received their allocated intervention.
| Received | Allocated | ||
|---|---|---|---|
| K-wire, n | Locking plate, n | Total, n | |
| K-wire | 208 | 9 | 217 |
| Locking plate | 18 | 213 | 231 |
| Other | 4 | 9 | 13 |
| Total | 230 | 231 | 461 |
Unless stated otherwise, all analyses reported here will be on an intention-to-treat basis, that is by allocated treatment.
Baseline characteristics
The baseline characteristics are well balanced between treatment groups (Table 11).
| Baseline characteristics | K-wire (n = 230) | Locking plate (n = 231) |
|---|---|---|
| Sex (female : male) | 191 : 39 (17%) | 194 : 37 (15%) |
| Intra-articular extension (no : yes) | 122 : 108 (47%) | 122 : 109 (47%) |
| Age (years) | 59.7 (16.4) | 58.3 (14.9) |
| Injury side (right : left) | 101 : 123 (53%) | 101 : 124 (54%) |
| Handedness (right : left) | 196 : 32 (14%) | 202 : 26 (11%) |
| Previous problem on injured side (no : yes) | 197 : 33 (14%) | 191 : 39 (17%) |
| BMI (kg/m2) | 24.8 (4.0) | 26.5 (5.3) |
| Osteoporosis (no : yes) | 208 : 22 (10%) | 211 : 20 (9%) |
| Regular analgesia (no : yes) | 160 : 70 (30%) | 169 : 61 (26%) |
| Smoker (no : yes) | 180 : 50 (22%) | 189 : 42 (18%) |
| Mechanism of injurya | ||
| Low-energy fall | 190 (83%) | 189 (82%) |
| High-energy fall | 36 (16%) | 36 (16%) |
| Road traffic collision | 1 (0%) | 4 (2%) |
| Crush | 1 (0%) | 0 (0%) |
| Other | 2 (1%) | 2 (1%) |
| Fracture classificationa,b | ||
| A1 : A2 : A3 | 0 (0%) : 73 (32%) : 84 (37%) | 0 (0%) : 71 (31%) : 78 (34%) |
| B1 : B2 : B3 | 1 (0%) : 1 (0%) : 1 (0%) | 4 (2%) : 1 (0%) : 0 (0%) |
| C1 : C2 : C3 | 33 (14%) : 26 (11%) : 7 (3%) | 30 (13%) : 34 (15%) : 11 (5%) |
| Alcohol consumption (per week)a | ||
| 0–7 units | 164 (71%) | 164 (71%) |
| 8–14 units | 39 (17%) | 45 (19%) |
| 15–21 units | 16 (7%) | 14 (6%) |
| > 21 units | 11 (5%) | 8 (3%) |
| Pre-injury scores (retrospectively) | ||
| PRWE | 2.6 (8.4) | 2.8 (8.7) |
| DASH | 5.4 (12.7) | 4.6 (10.8) |
| EQ-5D | 0.92 (0.17) | 0.94 (0.15) |
| Patient preference (after randomisation) | ||
| K-wire | 38 (17%) | 39 (17%) |
| Locking plate | 35 (15%) | 42 (18%) |
| No preference | 156 (68%) | 150 (65%) |
Imaging outcomes
Dorsal angle (°) and ulnar variance (mm) data were extracted from available images from all study participants before their operation (pre-op), and 6 weeks and 12 months postoperatively. In addition to the principal clinician extracting these images (assessor 1), an additional clinician (assessor 2) also assessed and extracted data independently. Table 12 shows estimates of intraclass correlation coefficients (ICCs), with 95% CIs based on 1000 bootstrapped samples, for dorsal angle and ulnar variance data at the preoperative assessment, and at 6 weeks and 12 months postoperatively. The ICC provides a measure of agreement between the two assessors and can be interpreted as follows: 0 to 0.2 poor, 0.2 to 0.4 fair, 0.4 to 0.6 moderate, 0.6 to 0.8 substantial and 0.8 to 1.0 almost perfect.
| Occasion | Measure | n | ICC | 95% CI |
|---|---|---|---|---|
| Preoperative | Dorsal angle (°) | 279 | 0.94 | 0.91 to 0.96 |
| Ulnar variance (mm) | 277 | 0.87 | 0.78 to 0.94 | |
| Week 6 | Dorsal angle (°) | 266 | 0.84 | 0.77 to 0.90 |
| Ulnar variance (mm) | 266 | 0.82 | 0.70 to 0.92 | |
| 12 months | Dorsal angle (°) | 208 | 0.87 | 0.81 to 0.91 |
| Ulnar variance (mm) | 209 | 0.89 | 0.82 to 0.93 |
It is clear from Table 12 that for both measures there was almost perfect agreement between both assessors. This is displayed visually by the Bland–Altman plots in Figure 10, where the difference between data from the two assessors is plotted against the mean with 95% CIs. We conclude from the analysis presented in Table 12 and Figure 10 that there was excellent agreement between assessors, indicating a high level of confidence in the measurements made by the principal assessor (assessor 1). The following comparative analysis (between intervention groups) is based purely on the data extracted from the images by this assessor.
FIGURE 10.
Bland–Altman plots showing agreement between assessors 1 and 2 for dorsal angle (°) preoperatively (a), 6 weeks (c) and 1 year (e); and for ulnar variance (mm) preoperatively (b), 6 weeks (d) and 1 year (f). Plots show differences plotted against mean data, with 95% CIs indicated by dashed lines.
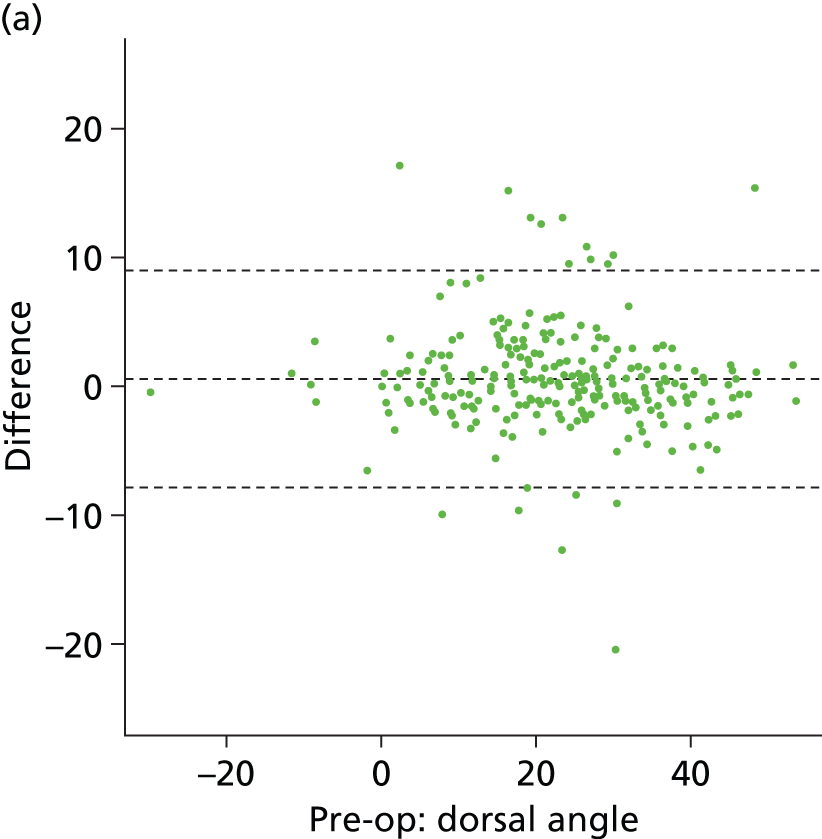


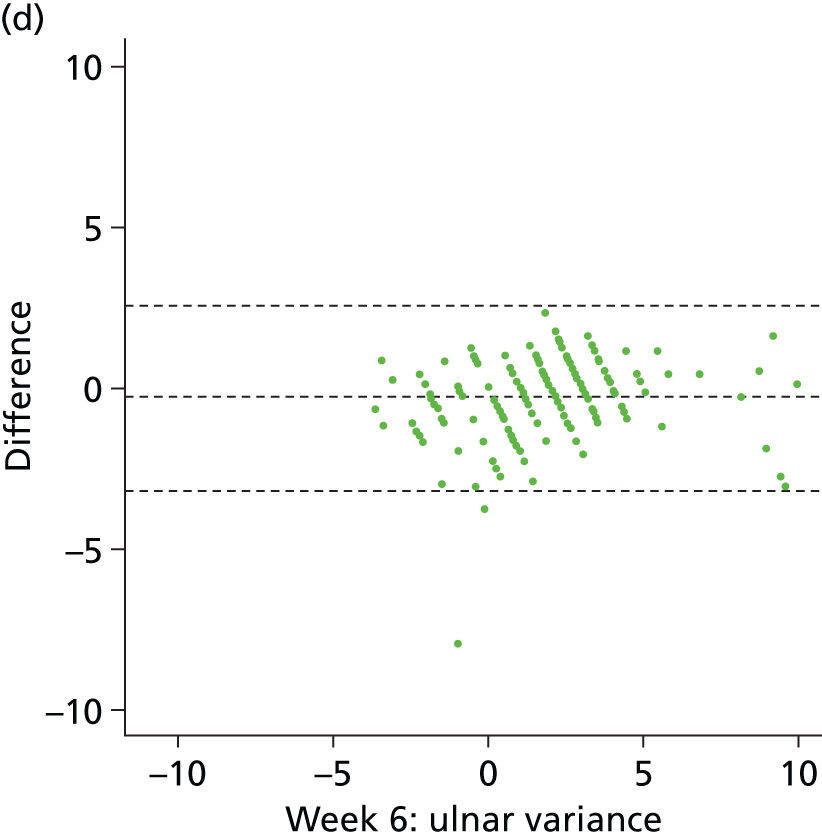


Image data were available from all 461 study participants before their operation (pre-op), and 6 weeks and 12 months postoperatively. However, some data were not available for analysis because of lack of collection, corrupted data files (which could not be opened) and poor-quality images that could not be analysed. In summary, only six study participants (1%) had no image data available at any occasion.
Metaphyseal comminution was reported at the preoperative assessment, in addition to dorsal angle and ulnar variance, and appeared to be well balanced across intervention groups, with 70.0% (152 out of 217) and 70.1% (155 out of 221) of study participants being assessed affirmatively for this characteristic for the K-wire and locking-plate groups respectively. Table 13 shows the mean and SD for imaging outcomes at baseline, 6 weeks and 12 months postoperatively, and estimated treatment effects after adjustment at 6 weeks and 12 months. Adjusted effects were from mixed-effects regression analysis based on complete cases with treatment group, age group, sex and intra-articular extension as covariates (fixed effects) and recruiting centre as a random effect.
| Imaging outcome | K-wire | Locking plate | Difference (95% CI) | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | n | Mean (SD) | n | Raw | Adjusteda | |||
| Dorsal angle (°) | Baseline | 23.10 (12.57) | 220 | 22.05 (13.85) | 225 | –1.05 | – | – |
| 6 weeks | –1.16 (11.00) | 215 | –4.31 (9.39) | 215 | –3.15 | –3.18 (–5.02 to –1.33) | < 0.001 | |
| 12 months | –0.49 (12.35) | 178 | –5.20 (8.24) | 173 | –4.70 | –4.64 (–6.76 to –2.53) | < 0.001 | |
| Ulnar variance (mm) | Baseline | 3.26 (2.62) | 219 | 3.55 (3.24) | 222 | 0.29 | – | – |
| 6 weeks | 1.95 (2.11) | 215 | 1.20 (2.18) | 215 | –0.75 | –0.75 (–1.15 to –0.35) | < 0.001 | |
| 12 months | 2.43 (2.31) | 179 | 1.32 (2.02) | 173 | –1.11 | –1.08 (–1.53 to –0.63) | < 0.001 | |
In summary, these results indicate that there was a significantly larger (more positive) dorsal angle in the K-wire group than the locking-plate group, at both 6 weeks and 12 months post operation. In addition, the variability in dorsal angle was significantly less in the locking-plate group than in the K-wire group at both 6 weeks and 12 months (F-test to compare the variances of two samples from normal populations; p = 0.020 and p < 0.001 and ratio of variances 0.73 and 0.45, at 6 weeks and 12 months, respectively). There was a significantly larger (more positive) ulnar variance in the K-wire group than in the locking-plate group, at both 6 weeks and 12 months post operation. However, there was no difference in variability between groups for ulnar variance at 6 weeks and 12 months (p = 0.615 and p = 0.081).
The image data are summarised further in the box plots of Figure 11, which clearly show the temporal trends in data and the difference in variance between dorsal angle data between groups at 12 months post operation.
FIGURE 11.
Box plots of baseline and postoperative scores and trends in means (filled circles) with 95% CIs for (a) dorsal angle and (b) ulnar variance.


Functional and quality of life outcomes
The primary outcome for this study is the PRWE questionnaire score23 at 12 months after surgery. Early functional status was also assessed and reported at 3 months and 6 months. The DASH questionnaire25 was used as a more general measure of physical function and symptoms in people with musculoskeletal disorders of the upper limb. Quality of life was assessed using EQ-5D. Data are summarised for each outcome measure, at each assessment occasion, in Tables 14–16. Baseline data are retrospective measurements made by study participants to assess pre-injury function and quality of life at recruitment. For EQ-5D, participants also made a post-injury assessment.
| Treatment | Measure | Baseline | 3 months | 6 months | 12 months |
|---|---|---|---|---|---|
| K-wire | n | 229 | 212 | 208 | 211 |
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 | |
| Mean | 2.6 | 33.9 | 22.3 | 15.3 | |
| Median | 0.0 | 30.5 | 18.3 | 9.0 | |
| Maximum | 62.5 | 97.0 | 76.0 | 79.0 | |
| SD | 8.4 | 22.3 | 18.6 | 15.8 | |
| Locking plate | n | 229 | 211 | 206 | 204 |
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 | |
| Mean | 2.8 | 31.5 | 20.6 | 13.9 | |
| Median | 0.0 | 27.5 | 16.0 | 7.5 | |
| Maximum | 75.5 | 97.5 | 91.0 | 79.5 | |
| SD | 8.7 | 22.4 | 17.7 | 17.1 |
| Treatment | Measure | Baseline | 3 months | 6 months | 12 months |
|---|---|---|---|---|---|
| K-wire | n | 229 | 203 | 195 | 201 |
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 | |
| Mean | 5.4 | 31.1 | 21.1 | 16.2 | |
| Median | 0.8 | 27.6 | 15.8 | 8.3 | |
| Maximum | 80.0 | 84.2 | 87.5 | 86.1 | |
| SD | 12.7 | 20.6 | 18.5 | 17.9 | |
| Locking plate | n | 227 | 207 | 199 | 195 |
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 | |
| Mean | 4.6 | 28.9 | 19.2 | 13.0 | |
| Median | 0.0 | 22.5 | 14.2 | 6.7 | |
| Maximum | 65.8 | 96.7 | 81.9 | 90.8 | |
| SD | 10.8 | 21.1 | 17.5 | 15.6 |
| Treatment | Measure | Baseline | Post-injury | 3 months | 6 months | 12 months |
|---|---|---|---|---|---|---|
| K-wire | n | 229 | 223 | 205 | 200 | 204 |
| Minimum | 0.1 | –0.4 | –0.3 | –0.2 | 0.1 | |
| Mean | 0.9 | 0.4 | 0.7 | 0.8 | 0.8 | |
| Median | 1.0 | 0.3 | 0.8 | 0.8 | 0.9 | |
| Maximum | 1.0 | 0.8 | 1.0 | 1.0 | 1.0 | |
| SD | 0.2 | 0.3 | 0.2 | 0.2 | 0.2 | |
| Locking plate | n | 229 | 225 | 207 | 194 | 194 |
| Minimum | 0.0 | –0.6 | –0.2 | 0.1 | –0.1 | |
| Mean | 0.9 | 0.4 | 0.7 | 0.8 | 0.9 | |
| Median | 1.0 | 0.3 | 0.8 | 0.8 | 0.9 | |
| Maximum | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | |
| SD | 0.2 | 0.3 | 0.2 | 0.2 | 0.2 |
Figures 12–14 show trends in means and box plots of distribution of scores for PRWE, DASH and EQ-5D by treatment group.
FIGURE 12.
(a) Box plots of postoperative scores and (b) trends in means with 95% CIs for the PRWE questionnaire scores.


FIGURE 13.
(a) Box plots of postoperative scores and (b) trends in means with 95% CIs for the DASH questionnaire scores.
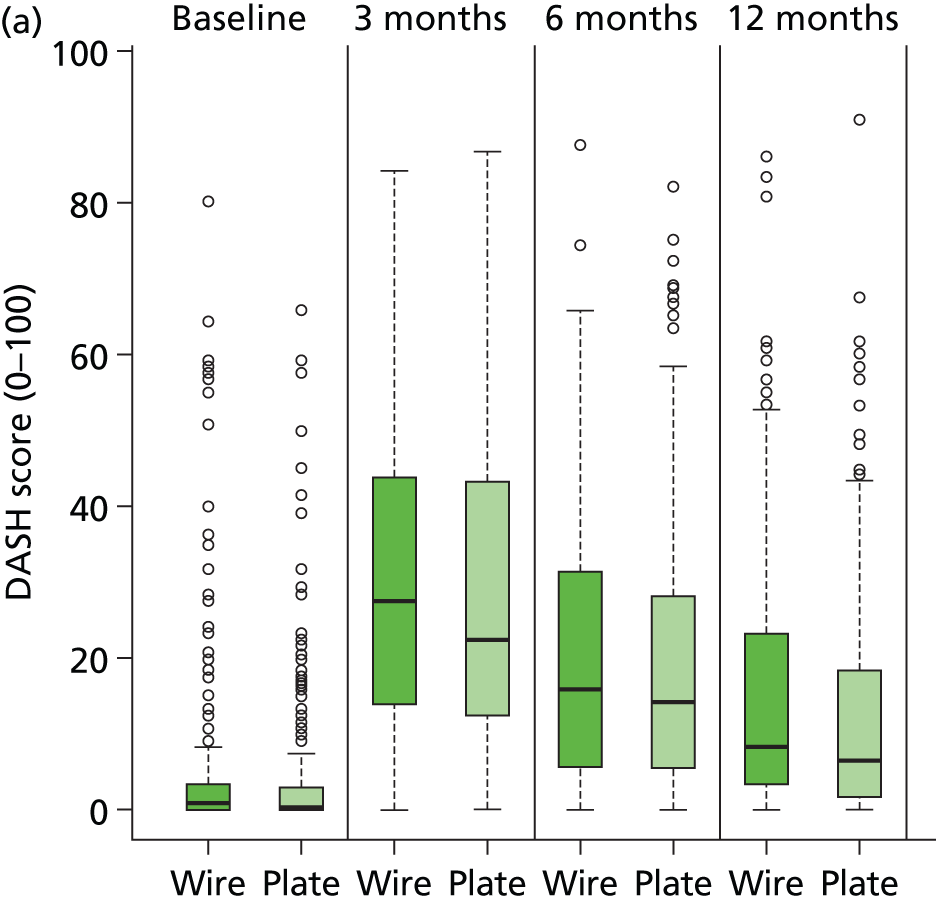

FIGURE 14.
(a) Box plots of postoperative scores and (b) trends in means with 95% CIs for EQ-5D scores.
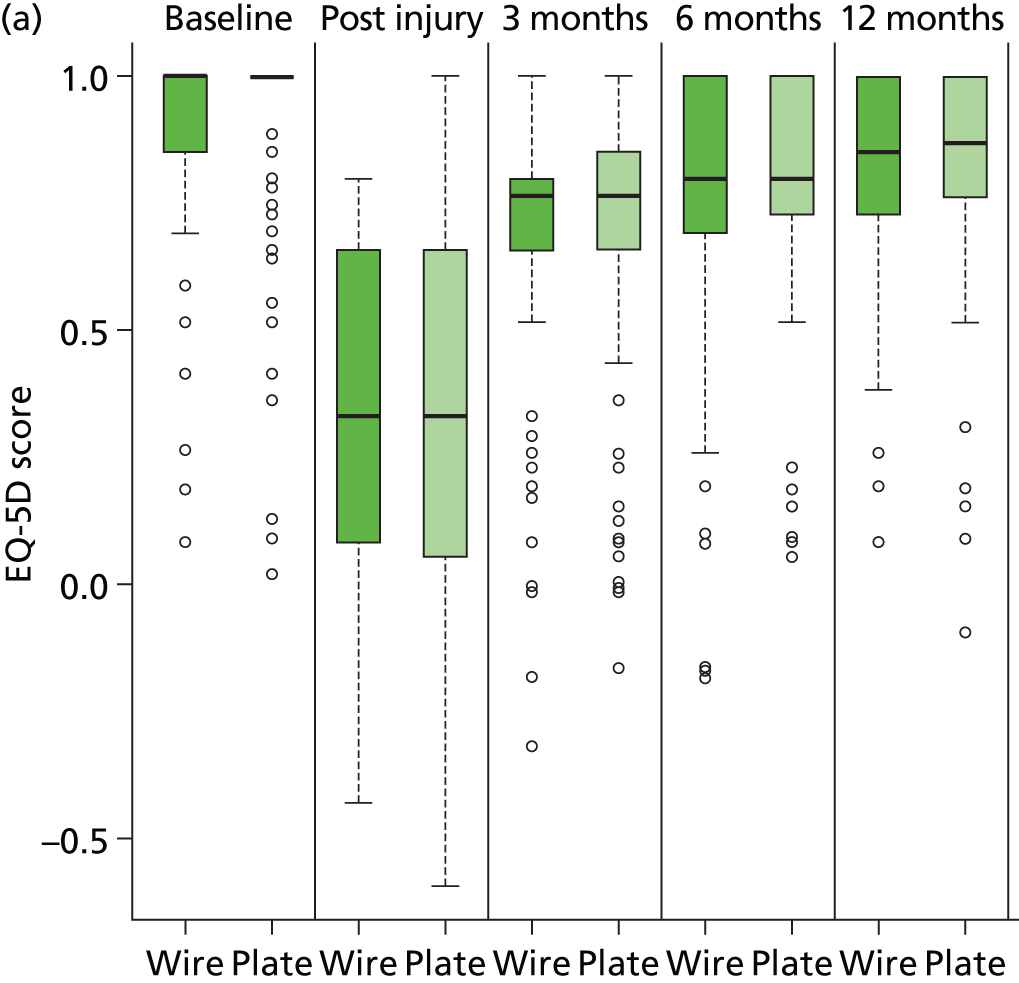

There appears to be, from a visual inspection of the plots, very little evidence for treatment group difference for the PRWE questionnaire score but some evidence for marginally better function in the locking-plate group than the K-wire group for the DASH questionnaire scores. Scores recover in the postoperative period in both groups but function is still worse than before the injury (based on retrospective assessment). Both PRWE and DASH questionnaire scores are left-skewed at the 12-month assessment, as they approach the score minimum. There is also very little evidence for treatment group difference for the EQ-5D score.
Table 17 shows means and SDs by treatment group. A mixed-effects linear regression model is used to estimate treatment differences (and 95% CIs). The full (planned) model incorporated terms that allowed for possible heterogeneity in responses for patients owing to the recruiting centre and the surgeon, in addition to the fixed effects of the treatment groups, sex, patient age and intra-articular extension. However, as we foresaw in the study protocol, the overwhelming majority of surgeons operated on fewer than three patients (see Chapter 3, Surgeons and operations), so a simplified model is used that implements a single random effect to account for the recruiting centre. Although baseline PRWE questionnaire scores were recorded using a retrospective assessment by each patient post injury, these were not considered sufficiently reliable for formal baseline adjustment in the multilevel model. The interactions between the stratifying variables (age and intra-articular extension) and the main treatment effect were not expected to be large; therefore, for practical reasons, the study was not powered with subgroup analysis in mind. However, formal tests of interaction between each stratifying variable and the treatment factor are reported together with appropriate 95% CIs and p-values in Table 17.
| Outcome | K-wire | Locking plate | Difference (95% CI) | p-value | |||
|---|---|---|---|---|---|---|---|
| Mean (SD) | n | Mean (SD) | n | Raw | Adjusteda | ||
| Primary outcome PRWE questionnaire score at 12 months in all participants | |||||||
| All participants | 15.3 (15.8) | 211 | 13.9 (17.1) | 204 | –1.4 | –1.3 (–4.5 to 1.8) | 0.398 |
| Primary outcome PRWE questionnaire score at 12 months in subgroups | |||||||
| Aged < 50 years | 13.2 (13.0) | 52 | 15.3 (16.3) | 49 | 2.0 | 1.4 (–5.0 to 7.8) | 0.338 |
| Aged ≥ 50 years | 16.0 (16.5) | 159 | 13.4 (17.4) | 155 | –2.6 | –2.2 (–5.8 to 1.4) | |
| Intra-articular extension = no | 16.7 (16.5) | 110 | 13.2 (16.1) | 105 | –3.5 | –3.3 (–7.6 to 1.1) | 0.211 |
| Intra-articular extension = yes | 13.8 (14.9) | 101 | 14.7(18.2) | 99 | 0.9 | 0.7 (–3.8 to 5.2) | |
| Secondary outcomes at 12 months | |||||||
| DASH | 16.2 (17.9) | 201 | 13.0 (15.6) | 195 | –3.3 | –3.2 (–6.5 to 0.0) | 0.051 |
| EQ-5D | 0.83 (0.19) | 204 | 0.85 (0.19) | 194 | 0.02 | 0.02 (–0.02 to 0.06) | 0.353 |
The adjusted estimate of the treatment effect for the PRWE questionnaire score for the full population is –1.3 (95% CI –4.5 to 1.8) in favour of the locking-plate group; the p-value of 0.398 indicates that there is no evidence for a statistically significant difference in PRWE questionnaire scores between the treatment groups at 12 months post operation. The MCID for the PRWE questionnaire score is 6 points; therefore, we conclude, based on our estimated CIs, that if there is a difference in functional scores between treatment groups it is likely to be sufficiently small as to be clinically unimportant.
The ≥ 50 years age group formed a sizeable subgroup of patients; therefore, this was identified a priori as being of particular interest. The adjusted estimate of the treatment effect for the full population is –2.2 (95% CI –5.8 to 1.4) in favour of the locking-plate group; the p-value of 0.338 indicates there is no evidence for a significant interaction term between age group and treatment group.
There was some evidence for a small and marginally significant (p = 0.051) treatment effect for the DASH score at 12 months in favour of the locking-plate group, although the effect size was small, at –3.2 (95% CI –6.5 to 0.0). As for the PRWE questionnaire scores, an interaction test provided no evidence that the treatment effect for DASH differed between age groups (p = 0.309) and the small improvement in function for the plate group is evident in the over-50-years age group only. EQ-5D failed to show any significant differences between treatment groups.
A likelihood ratio test provided no strong evidence to support the inclusion of a random-effect to model hospital centre (p = 0.119; chi-squared test) for the primary outcome measure, PRWE questionnaire score. In addition, the effects of age group, sex and intra-articular extension were not significant (at the 5% level) in the fitted model for PRWE questionnaire scores [p-values 0.764, 0.090 and 0.462; F-tests from analysis of variance (ANOVA)]. Therefore, the inferences for the PRWE questionnaire scores would be approximately equivalent to the above if a simple t-test had been used to compare treatment groups (t-test; p = 0.396 and 95% CI for raw difference –4.5 to 1.8). The above analyses are based on assumed approximate normality of the residuals for the PRWE questionnaire score at 12 months; the box plots in Figure 12 suggest that this approximation is poor. The median 12-month PRWE questionnaire scores were 9.0 for the K-wire group and 7.5 for the locking-plate group, and a Mann–Whitney test also provided no evidence to suggest that the treatment groups differed significantly (0.127). Inferences for the DASH questionnaire score were also equivalent between unadjusted and adjusted analysis (t-test; p = 0.055 and 95% CI for raw difference –6.6 to 0.1); median DASH questionnaire scores were 8.3 for the K-wire group and 6.7 for the locking-plate group, and a Mann–Whitney test also provided some evidence to suggest that the treatment groups differed significantly (0.071).
An adjusted per-protocol analysis of the PRWE questionnaire gave an adjusted treatment effect estimate of –1.0 (95% CI –4.2 to 2.2) and p-value equal to 0.530. An adjusted per-protocol analysis of the DASH questionnaire gave an adjusted treatment effect estimate of –3.1 (95% CI –6.3 to 0.2) and p-value equal to 0.066.
Missing data
There are 46 study participants with missing data at the 12-month study end point; the data are 90.0% (415 out of 461) complete. A summary of missing values, by age group and sex, for the PRWE questionnaire at 12 months is shown in Table 18 and the full pattern of missing values is shown in Tables 19 and 20 for K-wire and locking-plate groups, respectively. Baseline data, age group, hospital, treatment allocation, intra-articular extension and sex were all complete.
| Age (years) | Sex | Data, n | Missing, n | Total, n | % data missing |
|---|---|---|---|---|---|
| < 50 | Female | 73 | 8 | 81 | 9.9 |
| Male | 28 | 11 | 39 | 28.2 | |
| ≥ 50 | Female | 281 | 23 | 304 | 7.6 |
| Male | 33 | 4 | 37 | 10.8 | |
| Total | 415 | 46 | 461 | 10.0 | |
| Treatment | n | Baseline | Injury | 3 months | 6 months | 12 months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRWE | DASH | EQ-5D | EQ-5D | PRWE | DASH | EQ-5D | PRWE | DASH | EQ-5D | PRWE | DASH | EQ-5D | ||
| K-wire | 168 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 3 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 4 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | |
| 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | |
| 6 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | |
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | |
| 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | |
| 4 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | |
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | |
| 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 4 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | |
| 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | |
| 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | |
| 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | |
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | |
| 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | |
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | |
| 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | |
| 6 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | |
| 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | |
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | |
| 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 8 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total | 230 | 1 | 1 | 1 | 7 | 18 | 27 | 25 | 22 | 35 | 30 | 19 | 29 | 26 |
| Treatment | n | Baseline | Injury | 3 months | 6 months | 12 months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRWE | DASH | EQ-5D | EQ-5D | PRWE | DASH | EQ-5D | PRWE | DASH | EQ-5D | PRWE | DASH | EQ-5D | ||
| Locking plate | 168 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 2 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | |
| 2 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 4 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | |
| 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | |
| 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | |
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | |
| 5 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | |
| 4 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | |
| 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | |
| 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | |
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | |
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | |
| 1 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | |
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | |
| 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | |
| 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 | 0 | |
| 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | |
| 7 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | |
| 2 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 10 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Total | 231 | 2 | 4 | 2 | 6 | 20 | 24 | 24 | 25 | 32 | 37 | 27 | 36 | 37 |
Tables 19 and 20 show that 336 study participants (n = 168 + n = 168) had complete score data on all occasions. There is no evidence that missingness patterns differed between treatment groups. Forty-five study participants did not provide PRWE questionnaire score data at 12 months: 19 in the K-wire group and 26 in the locking-plate group. The data were 90% complete for the primary outcome measure. In total, only 19 study participants had no post-baseline data: eight in the wire group and 11 in the plate group.
Logistic regression, with missing data coded as 1 and complete data as 0, indicated that age group and sex were predictive of the PRWE questionnaire score missingness at 12 months; p-values from chi-squared tests for including age and sex in the logistic regression model were 0.017 and 0.022 respectively. Males were more likely not to complete questionnaires than females, and those < 50 years were less likely to complete questionnaires than those ≥ 50 years of age. This is clearly apparent from an inspection of Table 19.
Study participants aged < 50 years and males were less likely to provide complete data; experience suggests this is not an unexpected result. Assuming that missingness is fully explained by these variables (for which we have complete data), that is assuming that data are missing at random, our approach to the analysis in which we adjust for the variables that are predictive of missingness is sensible and should yield unbiased estimates of the treatment effect.
Imputing the missing data and rerunning the analysis on fully complete data provides a useful sensitivity analysis. Missing data were imputed using the mice package in R (multiple imputation by chained equations; http://cran.r-project.org/web/packages/mice/) and pooled estimates35 of model parameters based on 50 data imputations were obtained for the mixed-effects regression models reported in Table 9. The pooled estimates of the treatment group effect were –1.2 (95% CI –4.2 to 1.9) for the PRWE and –3.2 (95% CI –6.2 to –0.1) for the DASH questionnaire scores, with the percentage of the variability attributable to the uncertainty caused by the missing data estimated at 5.8% and 5.5% for the two outcome measures respectively. In summary, the inferences based on the complete data, after imputation, are not markedly different from those reported from the complete case analysis in Table 18.
Complications
Complications were reported by participants at the 6-week follow-up assessment, and also at any point during the first 12 months of follow-up using the study adverse event reporting procedures. Table 21 shows numbers of patients reporting complications by treatment group.
| Complication | K-wire (n = 230) | Locking plate (n = 231) | p-valuea | ||
|---|---|---|---|---|---|
| No, n | Yes, n | No, n | Yes, n | ||
| Cast and dressing | |||||
| Cast after operation | 5 | 224 | 57 | 171 | < 0.001 |
| Dressing change | 215 | 12 | 219 | 11 | 0.834 |
| Plaster change | 187 | 42 | 211 | 17 | 0.001 |
| Postoperative complications | |||||
| Refracture | 225 | 2 | 226 | 2 | 1.000 |
| Neurological injury | 215 | 14 | 210 | 20 | 0.373 |
| Vascular injury | 229 | 0 | 228 | 0 | 1.000 |
| Tendon injury | 225 | 4 | 223 | 6 | 0.751 |
| Superficial wound infection | 209 | 18 | 216 | 12 | 0.264 |
| Deep wound infection | 226 | 1 | 227 | 1 | 1.000 |
| Treatment | |||||
| Antibiotics | 217 | 13 | 220 | 11 | 0.682 |
| Removal of metalwork | 223 | 7 | 220 | 11 | 0.472 |
| Debridement | 228 | 2 | 230 | 1 | 0.623 |
| Revision | 222 | 5 | 226 | 2 | 0.285 |
There was no evidence to suggest that rates for any of the reported complications (e.g. wound infections) differed between treatment groups, based on comparing counts in groups using Fisher’s exact test. The proportion of participants given a cast after the operation differed between treatment groups, as expected, as did, consequently, the number of plaster changes required.
Chapter 4 Results of economic analysis
Introduction
The health economics analysis was designed to provide an economic evaluation of distal radial fractures treated by volar locking-plate fixation compared with K-wire fixation. The aim was to assess the cost-effectiveness of distal radial fractures treated by volar locking-plate fixation versus K-wire fixation from the NHS perspective. A societal perspective for costs was adopted for sensitivity analysis.
Unit cost data
Unit cost of resource use
Individual-level resource use was combined with unit costs to calculate the total health-care use cost for each patient. In order to convert resource usage figures into costs, unit cost figures were assigned from national databases such as the PSSRU Costs of Health and Social Care 201234 and the Department of Health’s National Schedule of Reference Costs. 39 Further inpatient care following the initial operation was costed as Minimal Elbow and Lower Arm Procedures for Non-Trauma (HRG codes HB79Z and HB73Z) except if surgical hand complications such as metal removal or debridement were reported at 6-week follow-up, where inpatient stay was costed as Minor Elbow and Lower Arm Procedures for Trauma (HRG codes HA73B and HAB73C). All costs were adjusted to 2012 prices using the Campbell and Cochrane Economics Methods Group and Evidence for Policy and Practice Information and Coordinating Centre Cost Converter. 40 Table 22 presents the summary of unit costs.
| Item | Unit cost (£) | Source |
|---|---|---|
| Initial operation | ||
| Cost for average distal fracture surgery (≥ 19 years) | 1983.39 | National schedule of reference costs year 2011–2012 – Minor Elbow and Lower Arm Procedures for Trauma – HA73C39 |
| Day case | 1375.34 | National schedule of reference costs year 2011–2012 – Minor Elbow and Lower Arm Procedures for Trauma – HA73C39 |
| Adjustment per day ± average LOS (≥ 19 years) | 278.07 | National schedule of reference costs year 2011–2012 – Elective Inpatient Excess day – HA73C39 |
| Average LOS for distal fracture | 0.95 days | Trial data |
| VLP: average LOS | 0.98 days | Trial data, t-test, p = 0.696 |
| K-wire: average LOS | 0.92 days | Trial data |
| Average surgery time | 53.57 minutes | Trial data |
| VLP: average surgery time | 70.00 minutes | Trial data, t-test, p = 0.000 |
| K-wire: average surgery time | 37.18 minutes | Trial data |
| VLP: implant + consumables | 818.26 | UHCW |
| K-wires: implant + consumables | 54.23 | UHCW |
| Subsequent inpatient care | ||
| Inpatient (orthopaedics – wrist/arm) | ||
| Cost for average LOS | 2064.71 | National schedule of reference costs year 2011–2012 – HB79Z39 |
| Day case | 692.04 | National schedule of reference costs year 2011–2012 – HB79Z39 |
| Adjustment per day ± average LOS | 302.01 | National schedule of reference costs year 2011–2012 – HB73Z39 |
| Inpatient (orthopaedics – other bones) | ||
| Cost for average LOS | 3556.04 | National schedule of reference costs year 2011–2012 – HB99Z39 |
| Day case | 928.36 | National schedule of reference costs year 2011–2012 – HB99Z39 |
| Adjustment per day ± average LOS | 293.08 | National schedule of reference costs year 2011–2012 – HB99Z39 |
| Inpatient (other non-surgery) | ||
| Cost for average LOS | 2688.10 | National schedule of reference costs year 2011–2012 – HB91Z39 |
| Day case | 602.61 | National schedule of reference costs year 2011–2012 – HB91Z39 |
| Adjustment per day ± average LOS | 261.21 | National schedule of reference costs year 2011–2012 – HB91Z39 |
| Inpatient (other) | ||
| Rehabilitation unit | 985.00 | PSSRU 2012, p. 114, weekly service costs per bed34 |
| Outpatient care | ||
| Orthopaedicsa | 103.72 | National schedule of reference costs year 2011–2012 – 110T39 |
| Pathologyb (for blood tests) | 60.74 | National schedule of reference costs year 2011–2012 – 82239 |
| Radiology (for radiographs, per event) | 153.21 | National schedule of reference costs year 2011–2012 – 81139 |
| Physiotherapy (per session) | 40.70 | National schedule of reference costs year 2011–2012 – 65039 |
| Primary and community care | ||
| GP surgery visit | 40.00 | PSSRU 2012, pp. 182–3, £3.40 per minute, average 11.7 minutes per visit34 |
| GP clinic visit | 58.00 | PSSRU 2012, pp. 182–3, £4.3 per minute, 17.2 minutes per visit34 |
| GP home visit | 101.00 | PSSRU 2012, pp. 182–3, £258 per hour, average 23.4 minutes per visit 34 |
| GP phone contact | 24.00 | PSSRU 2012, pp. 182–3, 7.1 minutes per contact34 |
| Practice nurse surgery visit | 11.63 | PSSRU 2012, pp. 180–1, £45 per hour, average 15.5 minutes per visit34 |
| Practice nurse home visit | 18.75 | PSSRU 2012, pp. 180–1, £45 per hour, average 25 minutes per visit34 |
| Practice nurse phone contact | 3.50 | PSSRU 2012, pp. 180–1, £35 per hour, average 6 minutes per visit34 |
| District nurse surgery visit | 10.85 | PSSRU 2012, p. 175, £42 per hour, average 15.5 minutes per visit34 |
| District nurse home visit | 21.83 | PSSRU 2012, p. 175, £61 per hour, average 20 minutes per visit34 |
| District nurse phone contact | 4.20 | PSSRU 2012, p. 175, £42 per hour, average 6 minutes per visit34 |
| Physiotherapist surgery visit | 15.00 | PSSRU 2012, p. 167, £30 per hour, average 30 minutes per visit34 |
| Physiotherapist home visit | 44.16 | PSSRU 2010, p. 151, £39 per hour, average 60 minutes per visit, inflated |
| Occupational therapist surgery visit | 15.00 | PSSRU 2012, p. 167, £30 per hour, average 30 minutes per visit34 |
| Occupational therapist home visit | 44.16 | PSSRU 2010, p. 151, £39 per hour, average 60 minutes per visit34 |
Unit cost of medications
Unit costs for medications were obtained from the BNF No. 67 (September 2013)41 and the NHS Electronic Drug Tariff (October 2013). 42 Patients reported details for medications that were taken within the three budgetary periods (discharge to 3 months, 3–6 months, 6–12 months), including the quantity taken per day and the number of days. We calculated the total medication costs using the average cost per dose for each product and the mean quantity taken per day during the reported number of days. Where a dose range reported ‘as required’, we included the cost of one box of the drug. We assumed that all medications were in tablet form unless stated. If the dose of the drug was not recorded, we assumed the patient received the same dosage as other patients who reported the same drugs. If the quantity was not recorded, we applied the average quantity for that drug as reported in the data. Table 23 shows all the unit costs for the drugs that were reported in the trial.
| Item | Unit cost (£)a | Source |
|---|---|---|
| Alendronic acid | 1.44 | 10 mg (from pack of 28) |
| Alendronic acid | 1.10 | 70 mg (from pack of 4) |
| Amitriptyline | 0.84 | 10 mg (from pack of 28) |
| Amitriptyline | 0.83 | 25 mg (from pack of 28) |
| Amoxicillin | 0.95 | 250 mg (from pack of 21) |
| Aspirin | 0.76 | 75 mg (from pack of 28) |
| Atenolol | 0.83 | 100 mg (from pack of 28) |
| Atorvastatin | 1.89 | 10 ml (from pack of 28) |
| Bendroflumethiazide | 0.81 | 2.5 mg (from pack of 1) |
| Bisoprolol | 1.02 | 5 mg (from pack of 28) |
| Calcium carbonate chewable | 9.33 | (From pack of 100) |
| Calcium salts (ADCAL®, ProStrakan) | 7.25 | (From pack of 100) |
| Calcium salts (Cacit®, Warner Chilcott) | 11.81 | (From pack of 76) |
| Citalopram | 1.31 | 10 mg (from pack of 28) |
| Clarithromycin | 2.52 | 250 mg (from pack of 14) |
| Co-amoxiclav | 2.34 | 250 mg (from pack) |
| Cocodamol | 3.54 | 30 mg (from pack of 100) |
| Cocodamol (Solphadol®, Sanofi-Aventis) | 6.74 | (From pack of 100) |
| Codeine | 2.26 | 30 mg (from pack of 30) |
| Co-dydramol | 1.06 | 500 mg (from pack of 30) |
| Cholecalciferol (ADCAL-D3®, ProStrakan) | 3.84 | 1500 mg (from pack of 56) |
| Cholecalciferol (Calceos®, Innotech International) | 3.62 | (From pack of 60) |
| Cholecalciferol (Calcichew-D3®, Takeda) | 4.24 | (From pack of 60) |
| Cholecalciferol (Calfovit D3®, Menarini) | 4.32 | (From pack of 30) |
| Cholecalciferol (Fultium®, Internis) | 8.44 | (From pack of 84) |
| Diclofenac | 1.42 | 50 mg (from pack of 84) |
| Diclofenac (Voltarol®, Novartis) | 3.46 | 25 mg (from pack of 30) |
| Dihydrocodeine | 5.18 | 60 mg (from pack of 56) |
| Enalapril maleate | 0.95 | 10 mg (from pack of 28) |
| Ergocalciferol | 30.34 | 1.25 mg (from pack of 50,000) |
| Esomeprazole (Nextum®, AstraZeneca) | 25.19 | 40 mg (from pack of 28) |
| Eye drops | 2.80 | (From pack of 10) |
| Ferrous sulphate | 1.01 | (From pack of 28) |
| Flucloxacillin | 1.77 | 250 mg (from pack of 1) |
| Flucloxacillin | 2.89 | 500 mg (from pack of 28) |
| Furosemide | 0.80 | 20 mg (from pack of 28) |
| Ibuprofen | 1.44 | 200 mg (from pack of 84) |
| Ibuprofen | 1.73 | 400 mg (from pack of 84) |
| Ibuprofen (gel) | 2.13 | 50 g (from pack of 1) |
| Lansoprazole (Prevacid®, Takeda) | 1.20 | 15 mg (from pack of 28) |
| Lercanidipine | 4.98 | 10 mg (from pack of 28) |
| Meloxicam | 1.54 | 15 mg (from pack of 30) |
| Meptazinol (Meptid®, Almirall Ltd) | 22.11 | 200 mg (from pack of 112) |
| Mirtazapine | 3.08 | 15 mg (from pack of 28) |
| Morphine | 11.21 | 10 mg (from pack of 1) |
| Morphine salts (Oramorph®, Boehringer Ingelheim) | 1.78 | 10 mg (from pack of 100) |
| Naproxen | 1.25 | 250 mg (from pack of 28) |
| Naproxen | 1.65 | 500 mg (from pack of 28) |
| Nefopam | 10.54 | 30 mg (from pack of 90) |
| Nefopam hydrochloride (Acupan®, iNova Pharmaceuticals) | 10.54 | 30 mg (from pack of 90) |
| Nifedipine (Adalat®, Bayer) | 7.23 | 5 mg (from pack of 84) |
| Olanzapine | 1.17 | 2.5 mg (from pack of 28) |
| Omeprazole | 1.62 | 10 mg/20 mg (from pack of 28) |
| Oxycodone | 8.70 | 5 ml (from pack of 50) |
| Oxycodone hydrochloride (OxyContin®, Purdue Pharma) | 49.82 | 20 mg (from pack of 56) |
| Oxycodone hydrochloride (OxyContin®, Purdue Pharma) | 74.81 | 30 mg (from pack of 56) |
| Paracetamol | 0.16 | 500 mg (from pack of 16) |
| Polymyxins (Polyfax®, TEVA UK) | 3.26 | 4 g (from pack of 1) |
| Pregabalin | 2.77 | 400 mg (from pack of 84) |
| Risedronate | 19.12 | 35 mg (from pack of 4) |
| Ramipril (capsules) | 1.39 | 10 mg (from pack of 28) |
| Strontium ranelate (Protelos®, Servier) | 25.60 | 2 g (from pack of 28) |
| Tibolone (Livial®, Merck Sharp and Dohme) | 10.36 | 2.5 mg (from pack of 28) |
| Tamoxifen | 5.71 | 10 mg (from pack of 30) |
| Tramadol | 1.14 | 50 mg (from pack of 30) |
| Venlafaxine (Efexor®, Effexor® and Trevilor®, Pfizer) | 2.62 | 37.5 mg (from pack of 56) |
| Topical NSAIDs (Voltarol Emulgel®, Novartis) | 1.55 | (From pack of 20) |
| Zopiclone | 1.30 | (From pack of 28) |
Unit cost of aids and adaptations
Patients reported any equipment that they had used to protect their injury or make their daily life easier to manage and the number of those. A number of aids and adaption tools were suggested and patients could add other equipment that was not in the list. Unit costs for aids and adaptations were taken from the website MobilitySmart (www.mobilitysmart.cc/), accessed in October 2013, which supplies the NHS and the PSSRU Costs of Health and Social Care 2012. 34 We calculated the total aids costs using the number of aids and the cost for each product. If the quantity was not recorded, we applied the average quantity for that aid as reported in the data. Reported aids and adaptations and unit prices are available in Table 24. Those costs were incurred by the NHS.
| Item | Unit cost (£) | Source |
|---|---|---|
| Wrist brace/splint | 10.00 | MobilitySmart |
| Grab rail | 95.00 | PSSRU 2012, p. 111, average total cost34 |
| Dressing aids | 5.95 | MobilitySmart |
| Long-handle shoe horns | 3.98 | MobilitySmart |
| Bathing aids | 23.35 | MobilitySmart |
| Kitchen aids (jar/tin openers, etc.) | 21.44 | MobilitySmart |
Intervention costs
The initial fixation surgery cost was based on the initial hospital stay and the operative cost (see Table 22). The cost of the initial distal radial fracture fixation surgery was assessed using NHS reference costs and HRG code HA73B (Minor Elbow and Lower Arm Procedures for Trauma). Costs for the initial operative period were identified for each patient using the average length of stay as reported in the patient records for the primary surgery. They were assumed to be £1375.34 for a day case or £1983.39 for overnight admission. We used excess bed-day costs when patient experience a length of stay beyond the average length of stay reported. For example, the cost to NHS was £1375.34 if a patient was discharged the same day, £1983.39 if the patient was referred to overnight hospital care at least one night but no more than the average length of stay; extra bed-day costs (based on a bed-day cost of £278.07) were added if a patient stayed more than the average length of stay. This cost was taken to include all expenditures incurred prior to discharge, including any items provided to patients before departure.
The operative costs for both volar locking-plate and K-wire fixations were provided by UHCW finance department; these included implant costs and consumables and are reported in Table 25.
| Description of item | Code of item | Quantity used | Unit price (£) | Cost (£) |
|---|---|---|---|---|
| K-wire | ||||
| Reinforced gown XL | 95224 | 2 | 3.74 | 7.48 |
| Surgical visor masks | 48247 | 2 | 1.01 | 2.03 |
| Theatre masks | 48100 | 2 | 0.06 | 0.11 |
| Gammex gloves | 351143 | 1 | 0.68 | 0.68 |
| Biogel gloves | 96180 | 2 | 1.14 | 2.28 |
| Non-sterile gloves | 8801 | 5 | 0.02 | 0.11 |
| Image intensifier cover | 39.00.02 | 3 | 2.49 | 7.47 |
| Laceration pack | RMT9150 | 1 | 13.24 | 13.24 |
| Surface wipes | CMW01X | 6 | 0.03 | 0.18 |
| Black bag | 1 | 0.06 | 0.06 | |
| Yellow bag | UN3291 | 2 | 0.05 | 0.11 |
| Polythene bag for extras | 1 | 0.02 | 0.02 | |
| Inco pad | 200995 | 2 | 0.13 | 0.26 |
| K-wire | OS292160 | 3 | 2.94 | 8.82 |
| Sterilisation of drill | TSU | 1 | 10.00 | 10.00 |
| Ethicon sutures | w319 | 1 | 1.22 | 1.22 |
| Mepore 9 × 10 cm | r33334 | 1 | 0.16 | 0.16 |
| Total for equipment used for K-wire surgery | 54.23 | |||
| Volar locking plate | ||||
| Surgical visor masks | 48247 | 3 | 1.01 | 3.04 |
| Theatre masks | 48100 | 2 | 0.06 | 0.11 |
| Gammex gloves | 351143 | 1 | 0.68 | 0.68 |
| Biogel gloves | 96180 | 3 | 1.14 | 3.42 |
| Non-sterile gloves | 8801 | 6 | 0.02 | 0.14 |
| Image intensifier cover | 39.00.02 | 3 | 2.49 | 7.47 |
| Upper limb pack | RMT9150 | 1 | 12.40 | 12.40 |
| Surface wipes | CMW01X | 6 | 0.03 | 0.18 |
| Black bag | 1 | 0.06 | 0.06 | |
| Yellow bag | UN3291 | 2 | 0.05 | 0.11 |
| Polythene bag for extras | 1 | 0.02 | 0.02 | |
| Inco pad | 200995 | 2 | 0.13 | 0.26 |
| Distal volar radius plate | DVRAW(L/R) | 1 | 384.00 | 384.00 |
| Shaft screws | CS(14) | 3 | 27.00 | 81.00 |
| Bar screws | FP(12) | 6 | 44.00 | 264.00 |
| Ethicon sutures | w319 | 1 | 1.22 | 1.22 |
| Mepore 9 × 10 cm | r33334 | 1 | 0.16 | 0.16 |
| Sterilisation of basic set | TSU | 1 | 20.00 | 20.00 |
| Sterilisation of drill | TSU | 2 | 10.00 | 20.00 |
| Sterilisation of distal volar radius set | TSU | 1 | 20.00 | 20.00 |
| Total for equipment used for volar locking plate surgery | 818.26 | |||
The total cost of each arm of the trial was calculated combining the resource use and unit cost data along with the initial surgery cost.
Societal costs
Costs from the societal perspective were calculated by combining productivity loss and loss of earnings due to work absence, private costs such as treatments within private settings and out-of-pocket expenses incurred as a result of the wrist surgery, and reported use of Personal Social Services related to the treatment. We assumed that the Personal Social Services costs were unlikely to be directly supported by the NHS and this is why they were included in the societal perspective sensitivity analysis.
Personal social services included number of weeks of frozen/hot meals on wheels, laundry services per load, and number of visits of carers and social workers. Unit costs were assigned using PSSRU and Information Centre of Personal Social Services in councils; they are reported in Table 26.
| Item | Unit cost (£) | Source |
|---|---|---|
| Personal social services | ||
| Frozen meals on wheels (weekly) | 41.40 | Lewisham council, adult social care |
| Hot meals on wheels (weekly) | 46.00 | PSSRU 2012, p. 12534 |
| Laundry services (per load) | 4.30 | North Yorkshire social care |
| Care workers/help at home (per visit) | 18.00 | PSSRU 2012, p. 193 per weekday hour34 |
| Social workers (per visit) | 39.00 | PSSRU 2012, p. 190 per hour34 |
| Cost of lost productivity | ||
| Days off work (per day) | 101.20 | Office for National Statistics – April 2012, median grossly week earning for full-time employees £50644 |
Patients reported their time off work in days or in terms of lost income because of their wrist fracture at every collection point. We employed a human capital approach using the gross median weekly pay rate for full-time employees from the Office for National Statistics (£506, April 2012)44 and divided this by 5 to generate the cost of lost productivity per day.
Utility and quality-adjusted life-years
Patient health-related quality of life was assessed using the EQ-5D,43 which was included along with the patient resource-use questionnaires. Changes in EQ-5D scores at baseline, 3 months, 6 months and 12 months were evaluated using two-sample t-tests to explore any important differences in these end points within the time frame of the trial. They are useful to understand the impact of the length of the follow-up on repeated measurements of the health outcome in which insufficiently long follow-up periods may potentially introduce biases in the subsequent cost-effectiveness analysis.
In line with the NICE reference case,33 the primary outcome for the economic evaluation was QALY. Patient responses to the EQ-5D questionnaire at each time point were converted to QALYs using the standard UK tariff values45 and an area under the curve approach. QALYs were calculated by multiplying these values with the time spent in each state, with quality of life linearly interpolated for the periods between the four observations provided in the trial data. QALYs represent a quality-weighted survival value in which 1 QALY is the equivalent of 1 year of full health. Average QALYs between adjacent time points were calculated to generate smoothed estimates between those time points.
Missing data
We calculated the mean total costs per patient from a NHS perspective adding the cost of inpatient stay, outpatient visit, consultations, medication, equipment, and applicable intervention costs for all patients where response data were available. Respondents who fail to complete individual items of the EQ-5D are not allocated a utility index score. From the overall sample, missing data represented 7.07%. Importantly, the health economics criteria for inclusion were slightly more restrictive than those for the statistical analysis. The complete data analysis was based on 278 patients.
For those cases in which neither resource usage nor quality-of-life data were available, these figures could not be calculated. We addressed missingness using two alternative methods.
We initially used the LOCF approach. Costs and EQ-5D values were carried forward for only one successive missing observation. Patients with more than one consecutive missing observation were omitted from the analyses. Observations were not carried backwards. The LOCF imputation analysis was then conducted on a sample of 367 patients. The advantage of the LOCF method is that it minimises the number of subjects who are eliminated from the analysis. However, the LOCF method has also been found to give a biased estimate of the treatment effect and underestimate the variability of the estimated result. 46,47
We then used multiple imputations via chained equations35,36 to complete missing data assuming missing at random and using Stata 12 (StataCorp LP, College Station, TX, USA). Missing cost data were predicted in terms of QALYs, treatment received, length of stay, age, gender, job status, patient’s self-reported health status, PRWE questionnaire score and DASH questionnaire score. Missing QALY data were predicted in terms of this same list (excluding QALYs), plus each of the cost items. In order to remove implausible data, missing cost data were constrained to be positive. A total of 10 imputations were created to stabilise the result. The reported cost-effectiveness results were synthesised based on all imputed data sets.
Cost-effectiveness analysis
Our analysis was a cost–utility analysis over 12 months examining the cost per QALY gained for all patients. We initially undertook descriptive statistics of costs and EQ-5D scores and conducted parametric tests (Students’ t-tests and ANOVA tests) to evaluate any important differences in the end points within the time frame of the trial. We then calculated ICERs by dividing the average difference in cost between the two arms by the average difference in QALYs between the two arms.
We also present cost-effectiveness scatter plots illustrating the uncertainty surrounding the cost-effectiveness estimates. The cost-effectiveness planes were derived using bootstrapping with replacement.
Sensitivity analyses were conducted to explore the impact of costs and missing data on the study results. First, the influence of outliers was evaluated; for this, participants with very large NHS health-care expenses were excluded from the analysis. These outliers may have a large effect on the mean cost and lead to different cost-effectiveness analysis results. Therefore, it is important, especially for policy-makers, to produce extreme scenarios to capture the worse and best cases. To evaluate the impact of missing data, the complete case analysis was conducted including only those participants with no missing data. The results of this analysis were compared with the results obtained through imputation using LOCF and then multiple imputations. We undertook the same analysis using costs from the societal perspective; this perspective includes the costs of use of personal social services, cost of lost productivity and private expenses in addition to the NHS costs. Finally, we carried out an adjusted analysis. To be consistent with the clinical analysis, we evaluated the cost-effectiveness by adjusting a set of characteristics at baseline such as gender, age, PRWE questionnaire score and DASH questionnaire score. Baseline EQ-5D score was also added to account for any possible baseline imbalance and to improve the precision of the estimates.
Cost-effectiveness results
Resource use and QALY data were available for 278 patients, with 137 patients receiving volar locking-plate fixation and 141 patients receiving K-wire fixation.
Health-care resource use
Table 27 presents the details of health-care resource used over the 12 months after discharge for complete case data. Resource use is broadly comparable between the two treatments. Patients were frequent users of physiotherapy outpatient visits (6.9 visits over the 12 months) and reported on average 3.6 radiology visits and 3.6 visits in orthopaedics outpatient visits. Use of general practitioner (GP) visits and nurse visits was infrequent. Patients reported using a wrist brace or splint. In terms of medications, we note that paracetamol tablets were the most reported medications in the questionnaires. No significant differences were observed between the two groups. Patients receiving K-wires appear to have used more bathing aids than patients receiving volar locking plates at a 10% significance level (p = 0.087).
| Item | Dose | Mean usage (SD) | Difference: p-value of t-test | |
|---|---|---|---|---|
| K-wire (n = 141) | Volar locking plate (n = 137) | |||
| Subsequent inpatient care | ||||
| Orthopaedics (wrist, arm, other bones) | – | 0.383 (1.252) | 0.343 (1.320) | 0.796 |
| Rehabilitation unit | – | 0.007 (0.084) | 0 | 0.325 |
| Other surgery | – | 0.170 (0.845) | 0.117 (0.385) | 0.500 |
| Outpatient care | ||||
| Orthopaedics | – | 3.340 (4.063) | 3.788 (4.633) | 0.392 |
| Pathology | – | 0.298 (1.423) | 0.358 (1.464) | 0.730 |
| Radiology | – | 1.801 (2.723) | 2.197 (2.695) | 0.225 |
| Physiotherapy | – | 6.128 (8.255) | 7.584 (11.212) | 0.218 |
| Primary and community care | ||||
| In surgery | ||||
| GP | – | 0.766 (2.144) | 0.555 (1.212) | 0.315 |
| Practice nurse | – | 0.113 (0.549) | 0.080 (0.322) | 0.541 |
| Physiotherapist | – | 0.879 (2.126) | 0.854 (4.420) | 0.951 |
| Occupational therapist | – | 0.071 (0.408) | 0.088 (1.025) | 0.858 |
| In clinic | ||||
| GP | – | 0 | 0.029 (0.241) | 0.151 |
| Practice nurse | – | 0 | 0.007 (0.085) | 0.311 |
| District nurse | – | 0 | 0 | |
| Physiotherapist | – | 0.241 (0.940) | 0.409 (2.102) | 0.389 |
| Occupational therapist | 0.014 (0.168) | 0.051 (0.426) | 0.340 | |
| At home | ||||
| Practice nurse | – | 0.007 (0.084) | 0 | 0.325 |
| Physiotherapist | – | 0.014 (0.168) | 0 | 0.325 |
| Occupational therapist | – | 0.071 (0.842) | 0 | 0.325 |
| Aids and adaptation | ||||
| Wrist brace/splint | – | 0.780 (1.076) | 0.964 (1.239) | 0.188 |
| Grab rail | – | 0.078 (0.464) | 0.029 (0.270) | 0.286 |
| Dressing aids | – | 0.071 (0.425) | 0.109 (0.649) | 0.557 |
| Long-handle shoe horns | – | 0.014 (0.168) | 0.007 (0.085) | 0.669 |
| Bathing aids | – | 0.021 (0.145) | 0 | 0.087 |
| Kitchen aids (jar/tin openers, etc.) | – | 0.078 (0.687) | 0.066 (0.406) | 0.856 |
| Medications | ||||
| Alendronic acid | 10 mg | 0.043 (0.235) | 0.022 (0.147) | 0.382 |
| Alendronic acid | 70 mg | 0.220 (1.043) | 0.0451 (0.3900) | 0.077 |
| Amitriptyline | 10 mg | 0.064 (0.600) | 0.058 (0.683) | 0.944 |
| Amitriptyline | 25 mg | 0 | 0.029 (0.241) | 0.151 |
| Amoxicillin | 250 mg | 0 | 0.022 (0.256) | 0.311 |
| Aspirin | 75 mg | 0.028 (0.337) | 0.073 (0.703) | 0.499 |
| Atenolol | 100 mg | 0 | 0.029 (0.342) | 0.311 |
| Atorvastatin | 10 ml | 0.028 (0.337) | 0 | 0.325 |
| Bendroflumethiazide | 2.5 mg | 0 | 0.657 (7.689) | 0.311 |
| Bisoprolol | 5 mg | 0.028 (0.337) | 0 | 0.325 |
| Calcium carbonate chewable | – | 0.028 (0.205) | 0.015 (0.171) | 0.544 |
| Calcium salts (ADCAL®, ProStrakan) | – | 0.028 (0.205) | 0.007 (0.085) | 0.267 |
| Calcium salts (Cacit®, Warner Chilcott) | – | 0 | 0.007 (0.085) | 0.311 |
| Cholecalciferol (ADCAL-D3®, ProStrakan) | 1500 mg | 0.064 (0.418) | 0.058 (0.482) | 0.920 |
| Cholecalciferol (Calceos®, Innotech International) | – | 0.043 (0.376) | 0.036 (0.307) | 0.883 |
| Cholecalciferol (Calcichew-D3®, Takeda) | – | 0.035 (0.421) | 0.066 (0.571) | 0.615 |
| Cholecalciferol (Calfovit D3®, Menarini) | – | 0.007 (0.084) | 0 | 0.325 |
| Cholecalciferol (Fultium®, Internis) | – | 0.014 (0.168) | 0 | 0.325 |
| Citalopram | 10 mg | 0.028 (0.337) | 0 | 0.325 |
| Clarithromycin | 250 mg | 0.007 (0.084) | 0 | 0.325 |
| Co-amoxiclav | 250 mg | 0 | 0.022 (0.256) | 0.311 |
| Cocodamol | 30 mg | 0.142 (0.661) | 0.095 (0.541) | 0.518 |
| Cocodamol (Solphadol®, Sanofi-Aventis) | – | 0 | 0.022 (0.256) | 0.311 |
| Codeine | 30 mg | 0.652 (3.408) | 0.234 (1.208) | 0.176 |
| Co-dydramol | 500 mg | 0 | 0.044 (0.361) | 0.151 |
| Diclofenac | 50 mg | 0.043 (0.313) | 0.051 (0.390) | 0.840 |
| Diclofenac (Voltarol®, Novartis) | 25 mg | 0 | 0.029 (0.208) | 0.097 |
| Dihydrocodeine | 60 mg | 0.121 (1.143) | 0.095 (1.111) | 0.850 |
| Enalapril maleate | 10 mg | 0.007 (0.084) | 0 | 0.325 |
| Ergocalciferol | 1.25 mg | 0.007 (0.084) | 0 | 0.325 |
| Esomeprazole (Nextum®, AstraZeneca) | 40 mg | 0 | 0.029 (0.342) | 0.311 |
| Eye drops | – | 0.128 (1.516) | 0 | 0.325 |
| Ferrous sulphate | – | 0 | 1.020 (0.851) | 0.155 |
| Flucloxacillin | 250 mg | 0.794 (9.432) | 0 | 0.325 |
| Flucloxacillin | 500 mg | 0.014 (0.168) | 0.015 (0.171) | 0.984 |
| Furosemide | 20 mg | 0.028 (0.337) | 0 | 0.325 |
| Ibuprofen | 200 mg | 0.092 (0.477) | 0.102 (0.633) | 0.882 |
| Ibuprofen | 400 mg | 0.007 (0.084) | 0.022 (0.190) | 0.400 |
| Ibuprofen (gel) | 50 g | 0.007 (0.084) | 0 | 0.325 |
| Lactulose | 300 ml | 0 | 1.314 (15.378) | 0.311 |
| Lansoprazole (Prevacid®, Takeda) | 15 mg | 0 | 0.029 (0.342) | 0.311 |
| Lercanidipine | 10 mg | 0 | 0.029 (0.342) | 0.311 |
| Meloxicam | 15 mg | 0 | 0.022 (0.256) | 0.311 |
| Meptazinol (Meptid®, Almirall Ltd) | 200 mg | 0 | 0.007 (0.085) | 0.311 |
| Mirtazapine | 15 mg | 0.014 (0.168) | 0.029 (0.342) | 0.641 |
| Morphine | 10 mg | 0.397 (4.716) | 0.044 (0.513) | 0.384 |
| Morphine salts (Oramorph®, Boehringer Ingelheim) | 10 mg | 0 | 0.007 (0.085) | 0.311 |
| Naproxen | 250 mg | 0.014 (0.168) | 0.015 (0.171) | 0.984 |
| Naproxen | 500 mg | 0 | 0.015 (0.171) | 0.311 |
| Nefopam | 30 mg | 0 | 0.015 (0.171) | 0.311 |
| Nefopam hydrochloride (Acupan®, iNova Pharmaceuticals) | 30 mg | 0.007 (0.084) | 0 | 0.325 |
| Olanzapine | 2.5 mg | 0 | 0.029 (0.342) | 0.311 |
| Omeprazole | 10 mg | 0.007 (0.084) | 0.029 (0.342) | 0.457 |
| Omeprazole | 20 mg | 0.035 (0.347) | 0.029 (0.342) | 0.880 |
| Oxycodone | 5 ml | 0 | 0.007 (0.085) | 0.311 |
| Oxycodone hydrochloride (OxyContin®, Purdue Pharma) | 20 mg | 0.028 (0.337) | 0 | 0.325 |
| Oxycodone hydrochloride (OxyContin®, Purdue Pharma) | 30 mg | 0.028 (0.337) | 0 | 0.325 |
| Paracetamol | 500 mg | 2.702 (10.11) | 1.285 (4.618) | 0.136 |
| Polymyxins (Polyfax®, TEVA UK) | 4 g | 0.298 (3.537) | 0 | 0.325 |
| Risedronate | 35 mg | 0.028 (0.337) | 0.058 (0.482) | 0.546 |
| Ramipril (capsules) | 10 mg | 0 | 0.029 (0.240) | 0.311 |
| Strontium ranelate (Protelos®, Servier) | 2 g | 0 | 0.029 (0.342) | 0.311 |
| Tibolone (Livial®, Merck Sharp and Dohme) | 2.5 mg | 0.043 (0.505) | 0 | 0.311 |
| Tamoxifen | 10 mg | 0 | 0.022 (0.180) | 0.311 |
| Tramadol | 50 mg | 0.262 (1.850) | 0.212 (1.160) | 0.785 |
| Zopiclone | – | 0 | 0.007 (0.085) | 0.311 |
Costs
The total costs associated with resource use during the trial among complete case data are shown in Table 28. The mean total NHS resource use costs are £3385 for K-wires and £4288 for volar locking plates and were significantly higher for volar locking plates (by £903). Lost earnings and productivity losses to employers through sickness absences appeared higher in the K-wire arm, but the difference does not appear to be significant. Overall, societal costs are on average £48 higher in the K-wire arm. However, there were no significant differences in NHS resource use costs and societal costs between the treatment arms according to the t-tests.
| Item | Parameter | K-wire (n = 141) | Volar locking plate (n = 137) | Difference: p-value of t-test |
|---|---|---|---|---|
| NHS resource use costs (£)a | Mean (SD) | 3384.78 (2097.09) | 4287.89 (2244.30) | |
| Incremental (SE) | 903.41 (260.43) | < 0.001 | ||
| Social care costs (£) | Mean (SD) | 13.01 (105.37) | 8.18 (57.97) | |
| Incremental (SE) | –4.83 (10.24) | 0.637 | ||
| Private cost (£) | Mean (SD) | 22.74 (123.57) | 30.22 (158.78) | |
| Incremental (SE) | 7.48 (17.04) | 0.372 | ||
| Cost of lost productivity (£) | Mean (SD) | 436.51 (1876.50) | 316.06 (1481.78) | |
| Incremental (SE) | –120.44 (203.16) | 0.405 | ||
| Societal costs (£, includes all the previous cost categories) | Mean (SD) | 1984.10 (2679.43) | 1936.48 (3299.80) | |
| Incremental (SE) | –47.63 (360.04) | 0.828 | ||
Health outcomes
Table 29 details the EQ-5D scores at pre injury, baseline, 3 months, 6 months and 12 months in complete case data and when missing values are imputed. Both groups showed increasing EQ-5D scores from baseline to the last follow-up point; the most important increase is observed between baseline and 3-month follow-up point for both treatments, doubling the baseline EQ-5D score. It is noted that patients at 12 months have not recovered an EQ-5D score equivalent to their pre-injury, retrospectively reported, EQ-5D score.
| Time point | Parameter | K-wire (n = 141) | Volar locking plate (n = 137) | Difference: p-value of t-test |
|---|---|---|---|---|
| Retrospective pre injury | Mean (SD) | 0.928 (0.170) | 0.947 (0.149) | |
| Incremental (SE) | 0.019 (0.019) | 0.320 | ||
| Baseline | Mean (SD) | 0.354 (0.303) | 0.373 (0.317) | |
| Incremental (SE) | 0.019 (0.037) | 0.611 | ||
| 3 months | Mean (SD) | 0.725 (0.210) | 0.739 (0.224) | |
| Incremental (SE) | 0.014 (0.026) | 0.591 | ||
| 6 months | Mean (SD) | 0.787 (0.193) | 0.807 (0.183) | |
| Incremental (SE) | 0.020 (0.023) | 0.366 | ||
| 12 months | Mean (SD) | 0.842 (0.188) | 0.845 (0.184) | |
| Incremental (SE) | 0.002 (0.022) | 0.918 | ||
| Total QALYs | Mean (SD) | 0.731 (0.173) | 0.745 (0.162) | |
| Incremental (SE) | 0.014 (0.020) | 0.484 | ||
On average, patients receiving a volar locking plate had higher EQ-5D index scores at baseline and at each follow-up point, but independent-sample t-tests indicated that the changes in EQ-5D score over time were not statistically significant. The average total number of QALYs over the 12 months was marginally higher in the volar locking-plate arm (0.745) than in the K-wire arm (0.731). Tables 30 and 31 provide the mean EQ-5D change scores between pre-injury and the follow-up points for the LOCF imputed analysis and multiple imputed data respectively. The same comments applied to the EQ-5D scores. The statistical tests indicated that there were no significant differences between EQ-5D scores at baseline for the two treatment arms, and, for this reason, adjustments on EQ-5D at baseline were not required.
| Time point | Parameter | K-wire (n = 187) | Volar locking plate (n = 180) | Difference: p-value of t-test |
|---|---|---|---|---|
| Retrospective pre injury | Mean (SD) | 0.918 (0.176) | 0.942 (0.142) | |
| Incremental (SE) | 0.024 (0.009) | 0.149 | ||
| Baseline | Mean (SD) | 0.358 (0.307) | 0.373 (0.323) | |
| Incremental (SE) | 0.015 (0.033) | 0.641 | ||
| 3 months | Mean (SD) | 0.714 (0.219) | 0.722 (0.242) | |
| Incremental (SE) | 0.008 (0.024) | 0.747 | ||
| 6 months | Mean (SD) | 0.768 (0.216) | 0.794 (0.195) | |
| Incremental (SE) | 0.026 (0.022) | 0.225 | ||
| 12 months | Mean (SD) | 0.824 (0.209) | 0.841 (0.189) | |
| Incremental (SE) | 0.017 (0.021) | 0.411 | ||
| Total QALYs | Mean (SD) | 0.717 (0.187) | 0.735 (0.177) | |
| Incremental (SE) | 0.018 (0.019) | 0.345 | ||
| Time point | Parameter | K-wire (n = 230) | Volar locking plate (n = 230) | Difference: p-value of t-testa |
|---|---|---|---|---|
| Retrospective pre injury | Mean (SD) | 0.919 (0.169) | 0.936 (0.154) | |
| Incremental (SE) | 0.075 (0.005) | 0.259 | ||
| Baseline | Mean (SD) | 0.358 (0.305) | 0.363 (0.323) | |
| Incremental (SE) | 0.070 (0.009) | 0.856 | ||
| Total QALYs | Mean (SD) | 0.734 (0.167) | 0.742 (0.160) | |
| Incremental (SE) | 0.013 (0.005) | 0.649 | ||
Cost-effectiveness results within the NHS perspective
Table 32 shows the total costs and QALY gains for each of the treatment arms. The differences in QALY gains between groups, 0.008 QALYs, were minimal and suggested only small health benefits of volar locking plates over K-wires. The volar locking-plate group had the highest QALY gains over the trial period. The mean total costs were higher for the volar locking-plate group; the high SD for the cost estimates indicates the presence of a few outlying individuals who incurred significant health service costs.
| Parameter | K-wires | Volar locking plate |
|---|---|---|
| n | 230 | 230a |
| Total QALY gain (SD) | 0.734 (0.166) | 0.742 (0.159) |
| Total cost (£) (SD) | 3440.07 (2539.27) | 4145.25 (2203.16) |
Table 33 provides the cost-effectiveness results, showing the incremental costs and benefits as well as the ICER. Interpretation should be tempered by considering the small between-group differences in QALY gains and the high level of uncertainty surrounding the QALY estimates. The results suggest that volar locking plates were more expensive than K-wires, with an incremental cost of £726.46, and had higher, albeit small, QALY gains.
| Treatment comparison | Incremental cost (£) (95% CI) | Incremental QALY gain (95% CI) | ICER (£) |
|---|---|---|---|
| Volar locking plate vs. K-wires | 726.46 (588.44 to 864.48) | 0.008 (–0.001 to 0.018) | 89,322 |
We graphically represented the uncertainty of these cost-effectiveness estimates in a cost-effectiveness plane (Figure 15) using bootstrapping with 10,000 iterations. We sampled, at random with replacement, each of our 10 imputed data sets, producing 10,000 incremental cost and incremental QALY estimates. Figure 15 shows that all the points are above the x-axis, indicating that the volar locking plate was more costly than K-wires, and more points are to the right of the y-axis, indicating that the volar locking plate produced more QALYs than K-wires.
FIGURE 15.
Cost-effectiveness plane generated from bootstrapped mean cost and QALY differences over 12 months (NHS perspective, all patients).
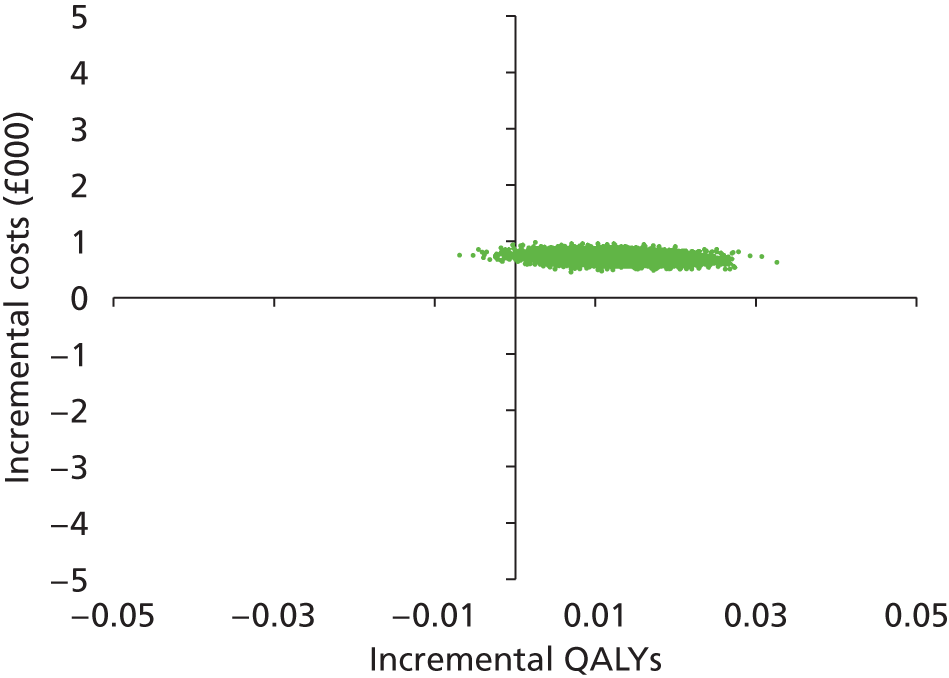
The points lay in the north-east quadrant of the cost-effectiveness plane. In this area there is a trade-off between effect and cost: additional health benefit can be obtained but at a higher cost. The question that then arises is whether or not the trade-off is acceptable, that is if the health gain is worth the additional cost. 48 This decision is based on the ICER and what the decision-makers are willing to pay for the additional benefit. The guideline rule is the NICE threshold of £20,000–30,000 per QALY, and the ICER of volar locking plates versus K-wires equals £89,322 per QALY. Under these circumstances, volar locking plates cannot be considered cost-effective, as its ICER is above the £20,000–30,000 per QALY range.
The CEAC showing the probability that volar locking-plate fixation is cost-effective is presented in Figure 16 with a range of cost-effectiveness WTP threshold values. The probability of being cost-effective at a WTP threshold of £20,000 per QALYs was nil and at a threshold of £30,000 was 3%.
FIGURE 16.
Cost-effectiveness acceptability curves at 12 months (NHS perspective, all patients).
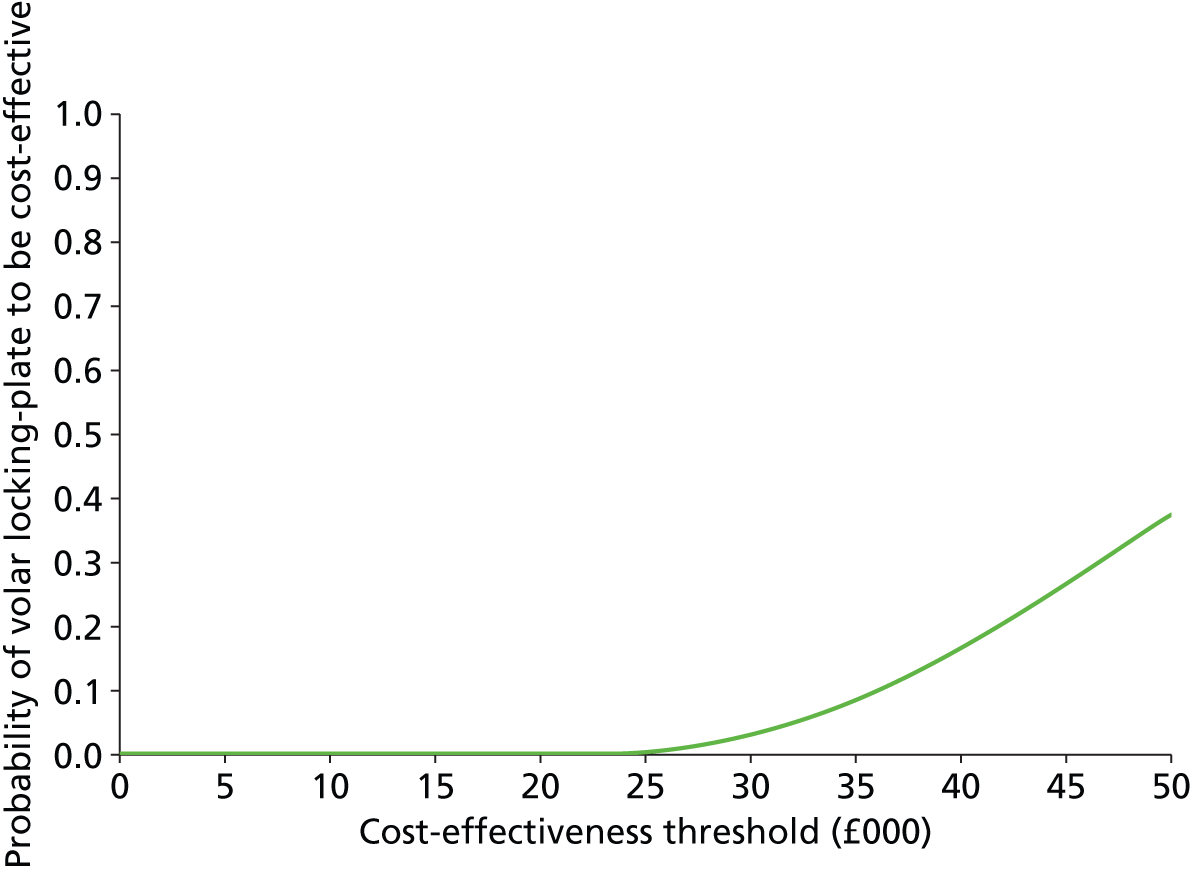
Sensitivity analyses
We undertook a number of sensitivity analyses to test the robustness of the base-case results, which are presented in Table 34.
| Sensitivity analysis | Incremental cost (£) (95% CI) | Incremental QALY gain (95% CI) | ICER (£) |
|---|---|---|---|
| Unadjusted | |||
| Complete-case data | |||
| Volar locking plate vs. K-wires | 903.41 (392.48 to 1414.34) | 0.014 (–0.025 to 0.053) | 64,026 |
| LOCF imputed data | |||
| Volar locking plate vs. K-wires | 799.17 (284.07 to 1314.27) | 0.018 (–0.019 to 0.055) | 44,468 |
| Societal perspective: multiple imputed data | |||
| Volar locking plate vs. K-wires | 581.00 (405.50 to 756.51) | 0.010 (0.001 to 0.019) | 58,852 |
| Societal perspective: complete-case data | |||
| Volar locking plate vs. K-wires | 790.45 (59.54 to 1521.36) | 0.014 (–0.025 to 0.053) | 56,020 |
| Adjusted | |||
| Multiple imputations | |||
| Volar locking plate vs. K-wires | 285.93 (171.41 to 400.44) | 0.009 (–0.015 to 0.032) | 32,730.50 |
| Complete-case data | |||
| Volar locking plate vs. K-wires | 907.44 (762.39 to 1052.50) | 0.014 (–0.018 to 0.046) | 64,662 |
| LOCF imputed data | |||
| Volar locking plate vs. K-wires | 799.17 (692.15 to 906.19) | 0.018 (–0.012 to 0.048) | 44,468 |
| Societal perspective: multiple imputed data | |||
| Volar locking plate vs. K-wires | 299.97 (157.68 to 442.26) | 0.010 (–0.013 to 0.033) | 30,385 |
| Societal perspective: complete-case data | |||
| Volar locking plate vs. K-wires | 791.85 (567.72 to 1015.99) | 0.014 (–0.018 to 0.046) | 56,425 |
| LOCF imputed data | |||
| Volar locking plate vs. K-wires | 799.17 (692.15 to 906.19) | 0.018 (–0.012 to 0.048) | 44,468 |
The first sensitivity analysis was based on complete-case data. This resulted in an incremental cost of £903 and an incremental QALY gain of 0.014, yielding an ICER of £64,026 per QALY.
The sensitivity analyses also expanded the cost-effectiveness analysis to the societal perspective. The incremental societal cost was £581 and the QALY gain was very comparable from the base-case analysis.
All the sensitivity analyses showed a higher mean cost and a higher but marginal QALY gain per patient receiving a volar locking plate. The ICERs of volar locking-plate fixation compared with K-wires were always more than £44,000 per QALY.
We also carried out cost-effectiveness analyses adjusting for the following baseline covariates including age, gender and EQ-5D scores. The adjusted analysis on the multiple imputations showed a lower incremental cost for a similar incremental QALYs gain both in the NHS and the societal perspectives; however, both ICERs remained above the cost-effectiveness threshold of £30,000 per QALY.
Subgroup analyses
The same analysis was then undertaken for patients < 50 years of age and patients ≥ 50 years of age, as the two age groups are assumed to present different types of fractures (high- and low-energy fractures) and so the impact of the fixation is expected to differ by age.
Table 35 shows the total costs and QALY gains for each of the treatment arms in each subgroup. Differences in QALY gains between groups were minimal in both age groups. The volar locking-plate group had the highest QALY gains in patients aged ≥ 50 years and K-wires had the highest QALY gains in younger patients. The mean total costs were higher for the volar locking-plate arm for both age subgroups. Older patients showed higher total costs than younger patients.
| Parameter | K-wires | Volar locking plate |
|---|---|---|
| n | 230 | 230 |
| Patients aged ≥ 50 years | ||
| Total QALY gain (SD) | 0.72 (0.14) | 0.74 (0.13) |
| Total cost (£) (SD) | 3759.9 (538.8) | 3972.8 (464.4) |
| Patients aged < 50 years | ||
| Total QALY gain (SD) | 0.77 (0.11) | 0.75 (0.10) |
| Total cost (£) (SD) | 2822.4 (499.9) | 3218.6 (461.4) |
Table 36 provides the cost-effectiveness results, showing the incremental costs and benefits as well as the ICER. Interpretation should be tempered by considering the small between-group differences in QALY gains and the high level of uncertainty surrounding the QALY estimates.
| Parameter | Incremental cost (£) (95% CI) | Incremental QALY gain (95% CI) | ICER (£ per QALY) |
|---|---|---|---|
| Patients aged ≥ 50 years | |||
| Volar locking plate vs. K-wires | 752.45 (538.88 to 921.03) | 0.014 (0.002 to 0.025) | 54,218 |
| Patients aged < 50 years | |||
| Volar locking plate vs. K-wires | 652.81 (425.26 to 880.37) | –0.008 (–0.024 to 0.008) | –81,601 (K-wires dominates) |
In the sample of patients aged ≥ 50 years, the evaluation suggests that patients treated with a volar locking-plate fixation gained 0.014 QALYs more than those treated with a K-wire fixation, at an increased cost of £752 per patient, yielding an ICER of £54,218 per QALY. Given a WTP of £20,000 for an additional QALY, the probability of volar locking plates being cost-effective is 46% based on the trial data, as shown in Figure 17.
FIGURE 17.
Cost-effectiveness acceptability curves by age subgroups at 12 months (NHS perspective).
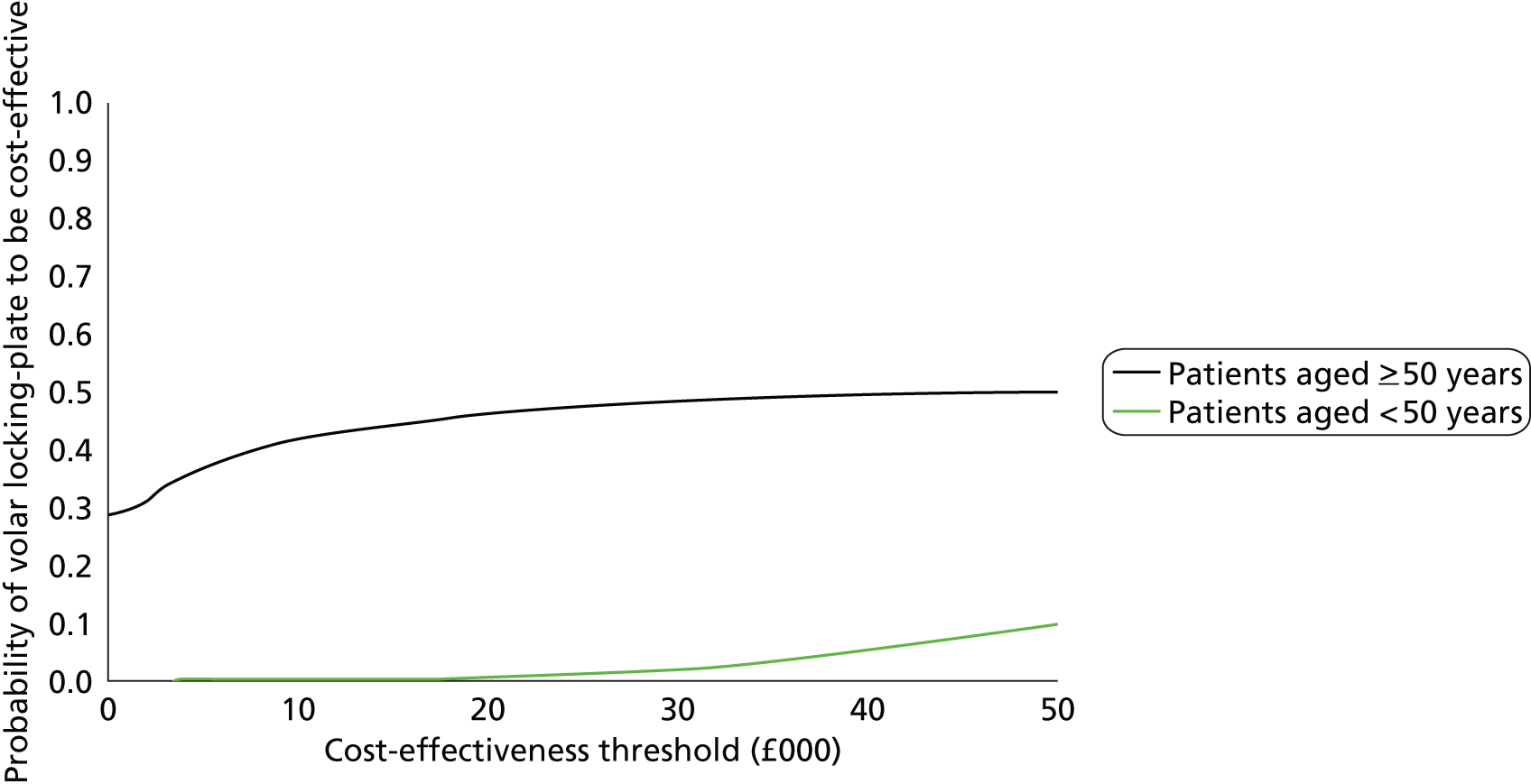
In the sample of patients aged < 50 years, the evaluation suggests that volar locking-plate fixation is associated with both lower benefits and higher costs than if they are treated with K-wires. The K-wire fixation appears to dominate for this group of individuals.
Sensitivity analyses
We undertook a number of sensitivity analyses to test the robustness of the subgroup results, which are presented in Table 37.
| Parameter | Incremental cost (£) (95% CI) | Incremental QALY gain (95% CI) | ICER (£ per QALY) |
|---|---|---|---|
| Patients aged ≥ 50 years | |||
| Complete-case data | |||
| Volar locking plate vs. K-wires | 944.93 (323.03 to 1566.83) | 0.019 (–0.030 to 0.067) | 50,959 |
| LOCF imputed data | |||
| Volar locking plate vs. K-wires | 858.60 (227.88 to 1489.32) | 0.021 (–0.025 to 0.067) | 41,018 |
| Societal perspective: multiple imputed data | |||
| Volar locking plate vs. K-wires | 628.57 (411.69 to 845.45) | 0.018 (0.007 to 0.029) | 35,323 |
| Patients aged < 50 years | |||
| Complete-case data | |||
| Volar locking plate vs. K-wires | 778.34 (–112.83 to 1669.52) | 0.001 (–0.065 to 0.068) | 590,973 |
| LOCF imputed data | |||
| Volar locking plate vs. K-wires | 621.14 (–221.54 to 1463.83) | 0.007 (–0.051 to 0.066) | 83,114 |
| Societal perspective: multiple imputed data | |||
| Volar locking plate vs. K-wires | 446.24 (172.32 to 720.15) | –0.013 (–0.028 to 0.003) | –34,326 (K-wires dominates) |
The results based on the complete cases are the same as the main analysis for patients aged ≥ 50 years, showing an incremental QALY gain of approximately 0.02 and an incremental cost between £629 and £945, yielding ICERs higher than £35,323 per QALY. With regard to younger patients, the QALY gain was close to nil for patients aged < 50 years with an additional cost of £778, and K-wires would be a preferred option for cost-minimisation reasons.
Summary
The results showed that patients with distal radius fractures did not show differences in QALY gain when treated with the volar locking-plate fixation or the K-wires. However, volar locking-plate fixation presented positive incremental costs. This difference was driven by the higher cost of delivery of the volar locking-plate fixation (£818.26 vs. £54.23); this cost was not offset through decreases in health-care resource use during the 12-month follow-up after discharge. The incremental cost of volar locking-plate fixation was £726 (95% CI £588.44 to £864.48) from an NHS perspective and £581 (95% CI £405.50 to £756.51) from a societal perspective.
The base-case analysis based on imputed data found the incremental QALY gain to favour volar locking-plate fixation; the difference was small (0.008; 95% CI –0.001 to 0.018). The limits of the CIs did not include high QALY gain values where the study could have concluded that volar locking plates were cost-effective; a back-of-the-envelope calculation indicates that a net QALY gain of at least 0.036 would be required for the incremental cost of £726 to provide cost-effectiveness at a WTP of £30,000 per QALY. The high ICER and the low probability of volar locking plates being cost-effective at a WTP between £20,000 and £30,000 demonstrate that volar locking-plate fixation is not cost-effective. This result is robust to sensitivity analysis and stochastic bootstrapping.
The subgroup analysis showed small fluctuations in effectiveness as measured in QALYs, differing with patients’ age group (≥ or < 50 years old). The incremental QALY gain of volar locking plates in patients aged ≥ 50 years was 0.014 (95% CI 0.002 to 0.025) whereas the incremental QALY gain of volar locking plates in patients < 50 years was –0.008 (95% CI –0.024 to 0.008). The right-hand limit of the CIs for QALY gains in younger patients shows that patients aged < 50 years are likely to experience an increased quality of life with K-wires. As volar locking-plate fixation is always associated with higher incremental costs than K-wires (£652.81; 95% CI £425.26 to £880.37), the evaluation suggests that K-wires fixation is preferred in younger patients. For patients aged ≥ 50 years, the limits of the CIs did not include QALY gain values higher than 0.018 and so volar locking-plate fixation is unlikely to be not cost-effective (a back-of-the-envelope calculation indicates that a net QALY gain of at least 0.038 would be required for the incremental cost of £752.50 to provide cost-effectiveness at a WTP of £30,000 per QALY).
The results showed that EQ-5D score was a suitable measure of health utility for this population and was sensitive to distal radius fractures and fixation, as there were important changes in the score values over the follow-up points.
Chapter 5 Summary and discussion
Screening
Over 12,000 patients were screened during the course of the trial. There was a bimodal distribution. The first peak was in younger men, although there was a smaller peak in women; both of these groups most commonly suffer high-energy injuries associated with sport, etc. The second peak was predominantly in women with lower-energy fractures most likely related to osteoporosis.
As expected, the most common reasons for patients being ineligible were ‘patient does not require fixation’, ‘patient is age less than 18 years’ and ‘fracture > 3 cm from the radiocarpal joint’, that is the fracture was not of the distal radius. These criteria accounted for the exclusion of roughly 10,000 patients. The other ineligible patients were spread around the remaining eligibility criteria.
Of note, 430 patients with intra-articular fractures were excluded on the basis that the ‘surgeon needed to open the fracture to achieve reduction of the joint surface’. Inevitably these fractures would be fixed with a plate, since the incision required to reduce the joint surface is essentially the same as that required for the insertion of the plate. There were 639 patients who fulfilled the eligibility criteria – some of whom did not wish to take part in the trial for the reasons described – so the majority of the patients who met the other eligibility criteria were considered by the surgeons to be eligible. Nonetheless, the number of patients excluded from the analysis on the basis of the ‘surgeon needed to open the fracture to achieve reduction of the joint surface’ eligibility criterion was perhaps greater than might have been expected given the proportion of complex intra-articular fractures, that is those who might be expected to fulfil this criteria, reported in the literature. Some surgeons may have taken a ‘cautious’ approach to the decision to include a patient in the trial, that is they excluded the patient if there was any doubt about their ability to subsequently reduce the joint surface without opening the joint. There was also some variability by trial centre in the number of patients excluded for this reason, but this was not unexpected and reflects variation in clinical practice and in particular the variation in surgeons’ willingness to open the joint surface. To some degree, variation in the surgeons’ preoperative assessment of their ability to reduce the fracture reflects such variation in clinical practice across the NHS.
Only a relatively small number of patients (n = 116) were excluded as being ‘unable to adhere to trial procedures or complete questionnaires’. This most likely reflects the fact that the questionnaires were completed by post and hence patients with no fixed address could not take part, and also that translation services could not be provided for patients whose first language was not English.
Declined to participate
Only 178 potentially eligible patients (28%) declined to take part. This is reassuring in terms of the trial paperwork and in particular the state of equipoise presented in the patient information sheet, but also in terms of the generalisability of the trial to the broader population. The most common reason (55 patients, 31% of those who declined to participate) was the ‘patient did not want to take part in a research project’.
Importantly, the number of patients declining to take part because they had a strong preference for one intervention was both small and equally divided between the treatment arms: 26 patients (15%) ‘definitely wants K-wires’ and 29 patients (16%) ‘definitely wants plate’. Therefore, patients had equipoise and patient preference should not have influenced the result of the trial.
Treatment according to allocation
This was clearly an important issue because either surgeon or patient preference could have led to crossover from one group to the other post randomisation. However, > 90% of both patient groups had their allocated treatment according to protocol, 208 out of 231 patients allocated to K-wires (90%) and 213 out of 230 patients allocated to locking plates (92%). This ensured that the primary intention-to-treat analysis was not different from the planned secondary per-treatment analysis. Interestingly, in the case of nine patients, the surgeon decided not to use either form of fixation, that is the patient had a manipulation under anaesthetic and application of plaster cast without ‘metalwork’.
Recruitment by centre
Each of the 18 centres successfully recruited to the trial. As expected, the centres that opened earliest in the trial recruited the most patients overall. Most centres achieved a recruitment rate of 1–2 patients per month, indicating that the pre-trial recruitment rate estimate was accurate.
The lead centre recruited the most patients, as may be expected. However, there was also a noticeable difference in the rate of recruitment in some other centres. This may reflect the size of the catchment area (number of patients seen) at that centre or the amount of snow and ice, and hence falls, in that area, for example Newcastle and North Tyneside, but may also reflect a different ‘clinical threshold’ for offering patients surgery following a fracture of the distal radius. This warrants further investigation.
Baseline characteristics of the two groups
The two groups were ‘beautifully balanced in terms of baseline characteristics’ (quote from the trial statistician).
In particular, the stratification by age, as a surrogate for bone density, and by intra-articular extension led to a good balance of these characteristics between treatment groups.
Patients
There were slightly more fractures of the left wrist than the right, but these were also evenly balanced between treatment groups, as were injuries to the ‘dominant hand’. This may reflect the fact that people generally carry things in their dominant hand, and, therefore, may be more likely to put out their non-dominant hand if they fall.
The number of patients with ‘pre-existing wrist problems’ was low (14% K-wires, 17% locking plates) and this was reflected in the pre-injury wrist scores, which were near ‘normal’ in both groups (PRWE questionnaire 3/100, where 0 is the best score).
As expected, the most common mechanism of injury was ‘low-energy fall’ at 82% in the K-wire group and 83% in the locking-plate group. All other patient characteristics were also evenly balanced between groups.
Fractures
As expected, the great majority of fractures were either displaced extra-articular (AO type A) or complete articular (AO type C) fractures. Partial articular (AO type B) fractures are most commonly palmar fractures or styloid fractures and would, therefore, not be eligible for this trial of dorsally displaced fractures of the distal radius. The type of fracture was evenly distributed between groups.
The other feature of note is that the proportion of AO type C3 fractures – the most severe intra-articular fractures – was low in both groups. This was also expected as these are the fractures where the surgeon would most commonly need ‘to open the fracture site to achieve reduction of the articular surface’ and were also, therefore, not eligible for the trial.
Surgeons
In this pragmatic trial, we expected that a large number of surgeons, reflecting the true breadth of expertise and experience provided within the NHS, would deliver the trial interventions. This was indeed the case, with 244 surgeons delivering a median of one operation each. This greatly reduces the likelihood of a surgeon-specific effect on the outcome at any one centre, that is one particularly good or bad surgeon dominating the other surgeons in the study.
However, it was also important to look at the balance of surgeons delivering the two different interventions. Interestingly, this was also well balanced between the groups.
Before the trial, some senior surgeons may have considered the K-wire intervention ‘simpler’ and therefore allowed more junior colleagues to perform this operation while the more experienced surgeons performed the more ‘complicated’ locking-plate fixation. This may have led to a ‘surgeon-effect’ within the trial. However, only marginally more of the plate fixations were performed by consultant (attending) surgeons: 31% for locking plates compared with 26% for K-wires. Similarly, the surgeon’s experience, as defined by the number of operations performed previously, was also well balanced between the groups. Therefore, a surgeon effect is very unlikely.
Just under half of the procedures in both groups (44% K-wires, 46% locking plates) were performed by ‘specialist registrars’, that is, trainees under supervision, which again reflects the reality of the delivery of trauma care in the NHS and is a testament to good training in this area.
Surgery
Although the principles of the fixation are inherent in the technique for both K-wires and locking plates, the details of the surgery were left to the discretion of the treating surgeon in this pragmatic trial.
Wires
As per traditional training, the great majority of wire fixations involved two or three wires. The most common size of wire used was 1.6 mm. The most common mode of insertion (34%) was ‘inter-fragmentary’, that is the wire was passed through the distal bone fragment and into the proximal. Twenty-four per cent of surgeons used the Kapandji technique alone, where the wire is inserted into the fracture site rather than the distal fragment and used as a buttress to hold the position of the bone. The remaining surgeries used a mixed technique, which may reflect a more recent trend.
Plates
The number of fixed-angle screws used in the distal bone fragment was variable but the largest single number used was ‘> 5’ in 38% of surgeries. This is in keeping with the use of modern locking-plate systems, which provide multiple options for gaining fixation in the distal metaphyseal bone, particularly in intra-articular fractures. Surgeons may have chosen to use fewer distal locking screws in the extra-articular fractures.
The bone in the proximal part of the radius is much thicker ‘cortical’ bone, such that traditional ‘non-locking’ screws are thought to achieve adequate fixation in this area. However, locking screws were also used in the proximal fragment in nearly half (44%) of the surgeries. This variation in practice has cost implications, as the locking screws are generally more expensive.
Duration of surgery
A key finding of this trial is that the K-wire surgery took less than half the time of the locking-plate fixation, 31 minutes (24 to 45 minutes) for K-wires compared with 66 minutes (50 to 85 minutes) for locking plates. There was no evidence that there was a difference in the duration of surgery according to the seniority of the lead surgeon.
As access to trauma operating theatre space is a key limiting factor in the delivery of trauma care across the NHS, and indeed has secondary effects on the delivery of elective (planned) surgery as well, this finding is of particular importance to policy-makers and NHS managers. It also has financial implications, as theatre operating time is an expensive resource. However, it is difficult to ‘cost’ this time accurately, as many of the costs of theatre time – staff, cleaning, maintenance – are present even if the theatre is not being used at 100% capacity. Theatre time was, therefore, not included in the health economics analysis, which may have led to some underestimation of the cost-effectiveness of K-wire fixation.
Follow-up rate
The follow-up rate was excellent, with 90% complete follow-up at each time point in both groups of patients. This suggests that the follow-up protocol was satisfactory, and also contributes to the external validity of the trial.
Outcomes
Primary outcome
The main finding of this trial is that there is no difference in the primary outcome PRWE questionnaire score at 3 months, 6 months or 12 months (difference in PRWE questionnaire score at 12 months –1.3, 95% CI –4.5 to 1.8; p = 0.398) between patients treated with K-wire fixation versus locking-plate fixation.
As the CIs exclude the MCID for the PRWE questionnaire, we conclude that any difference in functional scores between treatment groups is unlikely to be important to patients.
The size of the treatment effect seen in this trial is consistent with that seen in other studies of recovery following fracture of the distal radius. MacDermid et al. 30 conducted a prospective cohort study of 129 patients with a fracture of the distal radius; all patients completed the PRWE questionnaire at their baseline clinic visit and at 2 months, 3 months, 6 months and 12 months following their fracture. They reported a mean PRWE questionnaire score of 20.0 (SD 20.6) at 6 months and a score of 13.5 (SD 17.0) at 12 months. This is consistent with our reported values of 21.5 (SD 18.1) and 14.6 (SD 16.4) for the full population at 6 months and 12 months postoperatively.
Secondary outcomes
Patient-reported secondary outcomes
Furthermore, secondary clinical outcomes show that there is no difference between the groups in terms of health-related quality of life or the risk of complications. There was a borderline significant difference in the DASH questionnaire at one time point in favour of the locking plate, but this was well below the MCID. 49
Complications
There was no evidence to suggest that rates for any of the reported complications (e.g. wound infections) differed between treatment groups, based on comparing counts in groups. The number of complications was fortunately small and, again, it is important to note that the trial was not powered to look for differences in complications per se.
Although it is unlikely that there is a major difference in the overall rate of complications between K-wire fixation and locking-plate fixation, there may still be differences in the individual complications. This intuitively makes sense. One might expect the rate of superficial infection to be higher in the wire group (18 cases) versus the locking-plate group (12 cases), as the wires are generally applied directly through the skin, which is colonised by bacteria. Similarly, the number of neurological injuries in the locking-plate group (20 cases) versus the K-wire group (14 cases) may also be expected, since the plates require a surgical approach to the bone close to the major nerves of the wrist.
In terms of the requirement for further surgery, there was also little difference between the groups. Five patients in the K-wire group and two in the locking-plate group required revision surgery for loss of reduction, that is the bone was not held in an acceptable position during healing, as determined by the treating surgeon, whereas nine patients in the plate group required removal of symptomatic metalwork (four for screw penetration of the joint) and one patient had a buried K-wire which also required removal in theatre.
Radiographic results
Dorsal angle (°) and ulnar variance (mm) data were extracted from available images from all study participants before their operation, and at 6 weeks and 12 months after their surgery. The dorsal angle is a measurement of how far the articulating surface of the distal radius is tilted away from the palm by the injury; a normal articular surface faces towards the palm by a few degrees. Ulnar variance is a measurement of how far the radius has shortened relative to the ulna; in the uninjured wrist they are usually the same length. The 6-week images were used to determine whether or not the initial reduction of the fracture was maintained, that is if the bone was held in the anatomical position by the fixation device. The 12-month images were used to look for ‘late collapse’ of the fracture, as discussed in the protocol.
Agreement between independent assessors (one radiologist and one orthopaedic surgeon), as determined by intraclass correlation, was above 0.8 for both measurements at each time point. This ‘near perfect’ agreement suggests that the measurements were reproducible, even if there was some error in individual measurements.
In terms of dorsal angulation, there was a mean 3° difference in the angulation in favour of the locking-plate group at 6 weeks. Similarly, there was a mean 0.75 mm less shortening of the radius in the locking-plate group.
These results are statistically significant. As noted in the introduction, most previous trials have suggested that locking-plate fixation provides improved radiographic outcomes, possibly because they can be used to aid the reduction of the bones into the correct (anatomic) position as well as holding that position when screwed to the bone surface. K-wires usually require the bone fragments to be reduced before the wires are inserted. However, the clinical relevance of such small differences is questionable. The long-term follow-up of the patients will hopefully provide some indication of whether or not radiographic measurements have a bearing on long-term clinical outcome.
At 12 months, the differences between the treatment groups persisted. The mean dorsal tilt in the wire group increased by 0.67° and the shortening by 0.33 mm between 6 weeks and 12 months. Therefore, ‘late collapse’ was minimal. This does not mean that late collapse did not occur in an individual patient but, on average, it was very unlikely to be clinically important.
It is difficult to explain why the mean dorsal tilt in the locking-plate group actually improved by 0.9° between 6 weeks and 12 months, other than measurement error.
Pre-planned subgroup and secondary analyses
At the beginning of the trial, three variables were identified as being of potential importance in terms of their influence on the recovery of patients following fracture of the distal radius. The randomisation sequence was therefore stratified on the basis of trial centre/hospital, age above and below 50 years as a surrogate for osteoporosis, and intra-articular extension of the fracture into the radiocarpal joint.
A single random effect was used to take account of the trial centre in the regression model used in the adjusted analysis; this made very little difference to the mean difference in PRWE questionnaire score between the groups (all participants: –1.3 adjusted, –1.4 raw).
The trial was not powered specifically for subgroup analysis. However, as three-quarters (314 out of 461) of the patients were in the ‘over 50 years’ subgroup, the estimate of the mean difference at 12 months is reasonably precise. The adjusted estimate of the mean difference between the treatments for the ‘over 50 subgroup’ is –2.2 points in favour of the plate fixation, but as the CIs exclude the MCID of 6 points on the PRWE questionnaire score (95% CI –5.8 to 1.4) this is unlikely to be clinically relevant.
The adjusted analysis for the ‘under 50 years’ subgroup was 1.4 in favour of the K-wire fixation. As the number of patients in this subgroup was smaller, the CIs are broader and do include the MCID (95% CI –5.0 to 7.8).
The number of patients analysed at 12 months with an intra-articular extension of the fracture (n = 200) was similar to those without (n = 215). Again, there was no evidence of a difference between the treatment groups. The mean difference was 0.7 (–3.8 to 5.2) for those patients with an intra-articular extension and –3.3 (–7.6 to 1.1) for those without.
Therefore, this trial provided no evidence of a clinically important difference between the effect of K-wire fixation and that of locking-plate fixation for patients under or over 50 years or for patients with and without an intra-articular extension of their distal radius fracture.
Finally, we also pre-planned a per-treatment (per protocol) secondary analysis of the data. As compliance with the trial was good, with > 90% of patients receiving their allocated treatment, the analysis by treatment given did not alter the result.
Health economic evaluation
Costs of initial surgery
The costs of the initial surgical interventions were markedly different; the total cost of the implants and theatre consumables was £54 for the K-wire fixation versus £818 for the locking-plate fixation. Therefore, K-wire fixation is a cost-saving intervention.
As noted above, this is a conservative estimate of the cost of initial surgery, as this does not take into account the extra theatre operating time required for the locking-plate fixation, which was 31 minutes for K-wires and 6 minutes for locking plates.
Other health resource use
The use of other health resource was broadly comparable between the treatment groups, as £1525 for K-wires and £1590 for volar locking plates. There was no statistically significant difference in the use of any individual service, orthotic or medication.
Both groups of patients were frequent users of physiotherapy services (seven visits over the 12 months) and reported on average 3.6 radiology visits for radiographs and 3.6 visits to the orthopaedic outpatient department. Use of GP visits and nurse visits was infrequent.
Societal costs
Cost from the societal perspective were calculated by combining productivity loss and loss of earnings due to work absence, private costs such as treatments within private settings and out-of-pocket expenses incurred as a result of the wrist surgery, and reported use of personal social services related to the treatment.
Again, there was very little difference between the treatment groups; overall societal costs were on average £70 higher for the patients having K-wire fixation.
Cost-effectiveness
There were no significant differences in health-related quality of life during the 12-month follow-up period and hence very little difference in average total QALY between the two groups: 0.08 difference, volar locking plate 0.745 versus K-wire 0.731.
As the locking-plate intervention was more expensive, with a total incremental cost of £726, the ICER of volar locking plates versus K-wires was £89,322 per QALY. Under these circumstances, volar locking plates cannot be considered cost-effective. The probability of being cost-effective at a WTP threshold of £20,000 per QALYs was nil and at a threshold of £30,000 was 3%.
Cost-effectiveness in pre-planned subgroups
There were small differences in QALY depending on the patients’ age group. Therefore, there were small differences in cost-effectiveness according to age.
As patients aged < 50 years were likely to experience a marginal increase in quality of life with the cheaper K-wires, the health economic evaluation suggests that K-wire fixation is always preferred in younger patients.
In patients aged ≥ 50 years, there was a marginal increase in the unadjusted QALY gain with locking-plate fixation. Given a WTP for an additional QALY of £20,000, the probability of locking-plate fixation being cost-effective is 46% based on the trial data. Therefore, it is unlikely that locking-plate fixation would be cost-effective in older patients.
Limitations
The main limitation of the trial is that it was not possible to blind either the surgeons or the patients to the study treatments. However, it could be argued that this is a positive feature in a pragmatic trial, as patients would know about their proposed treatment before surgery during routine care. 50
It should be noted that this study excluded patients whose fracture could not be reduced by indirect means, that is the results should not be generalised to the minority of patients whose fracture requires that the surgeon expose the individual bone fragments in order to restore the congruity of the wrist joint. The low number of patients in the trial with C3 fractures indicates that, as expected, the patients with the most complex intra-articular fractures were the ones who were excluded. None of the patients with extra-articular fractures should have been excluded, as this eligibility criterion refers only to the reduction of the ‘joint surface’. While it is possible that a small number of patients were excluded in error, we think this unlikely given the high quality of the screening data which we collected throughout the trial. Of course, we have no way of checking the radiographs of those patients whom the surgeon considered to be ineligible, but variation in the interpretation of fractures and fracture patterns is inherent in clinical practice and is reflected in this trial.
Compliance with the trial was good, with > 90% of patients receiving their allocated treatment. Some patients did cross over to have a different form of fixation, but an analysis by treatment given did not alter the result.
It is also important to underline that the health economics analysis used a conservative estimate of the cost of initial surgery. This did not take into account the extra theatre operating time required for the locking-plate fixation at 31 minutes for K-wires and 66 minutes for locking plates. It is likely that a microcosting analysis of the initial surgery in each arm could lead to even higher cost differences with regard to the cost of the initial surgery.
The long-term outcome of patients with a fracture of the distal radius is not known. Our data suggest that patients’ wrist function and quality of life improve over the 12 months following their surgery but do not return to pre-injury levels. The patients in this trial will be followed up annually, to determine the prevalence of late complications such as arthritis. In addition, this will provide us with data on the longer term that will allow a decision-analytic model where extrapolation will rely on more robust data on the benefits.
Chapter 6 Conclusion
This trial found no clinically relevant difference in PRWE questionnaire results in the 12 months following K-wire fixation versus volar locking-plate fixation. The health economic evaluation showed that K-wire fixation is cost saving.
The results of this trial will reverse the trend towards locking-plate fixation for this injury, and will, consequently, save time and money for health-care services around the world.
Acknowledgements
Contributions of authors
Matthew L Costa was chief investigator and led study conception and design, development of interventions, clinical responsibility, and the writing and reviewing of the report.
Juul Achten developed the protocol, is a member of the TMG and was involved in the writing and reviewing of the report.
Caroline Plant developed the protocol, is a member of the TMG and was involved in the writing and reviewing of the report.
Nick R Parsons developed the protocol, is a member of the TMG, was responsible for the statistical analysis of the trial and was involved in the writing and reviewing of the report.
Amar Rangan developed the protocol, was responsible for the recruitment of patients and was involved in the writing and reviewing of the report.
Sandy Tubeuf was responsible for the health economics analysis and was involved in the writing and reviewing of the report.
Ge Yu was responsible for empirical analyses for the health economic section.
Sarah E Lamb developed the protocol, is a member of the TMG and was involved in the writing and reviewing of the report.
Trial team
Trial management group
Professor Matthew L Costa, chief investigator; Dr Juul Achten, senior research fellow; Miss Jaclyn Brown, trial co-ordinator; Dr Nick R Parsons, trial statistician; Professor Sarah E Lamb, director of Clinical Trials Unit; Dr Caroline Plant, clinical input; Mrs Susie Hennings, senior project manager; Mrs Isabel Wall, trial administrator/acting trial co-ordinator; Miss Kate Packard, data clerk; and Mr Rhys Mant, data clerk.
Trial applicants
Professor Matthew L Costa, chief investigator; Professor Sarah E Lamb, co-applicant; Professor Damian Griffin, co-applicant; Dr Juul Achten, co-applicant; Dr Nick R Parsons, co-applicant; Dr Richard Edlin, co-applicant; and Mr Amar Rangan, co-applicant.
Trial Steering Committee
Professor David Torgerson, Director of York Trial Unit (chair); Jonathon Hill, Lecturer in Physiotherapy (independent member); Professor Ashley Blom, Professor of Orthopaedic Surgery (independent member); Professor Matthew L Costa, chief investigator; Professor Sarah E Lamb, director of Clinical Trials Unit; Professor Amar Rangan, principal investigator; Dr Juul Achten, senior research fellow; Miss Jaclyn Brown, trial co-ordinator; Dr Nick R Parsons, trial statistician; Dr Richard Edlin, trial health economist; Mrs Ceri Jones, manager, research and development, UHCW (sponsor representative); and Mrs Carole Beamish, lay member, quality assurance (independent member).
Data Monitoring Committee
Professor Lee Shepstone, Professor of Medical Statistics (chair); Mr Adrian Chojnowski, Consultant Orthopaedic Surgeon; and Mr David Ford, Consultant Orthopaedic Surgeon.
Statistician
Dr Nick Parsons.
Health economists
Dr Sandy Tubeuf and Dr Ge Yu.
Radiological evaluation
Dr Caroline Plant and Dr Salil Karkanis.
Research team: Distal Radius Acute Fracture Fixation Trial Study Group
Principal investigators
Mr Jaime Candal-Couto, Mr Tim Chesser, Mr Callum Clark, Professor Joe Dias, Miss Alison Edwards, Mr David Gidden, Mr Sameer Gidwani, Mr Andrew Gray, Mr Phillip Johnson, Mr Jonathon Jones, Mr Rajesh Nanda, Professor Amar Rangan, Mr Simon Richards, Mr Chris Robert, Mr Kevin Smith, Mr Srinivasan Makaram and Professor Keith Willett.
Research associates
Catherine Ashbrook-Raby, Ann-Marie Baker, Michelle Bardgett, Steven Barnfield, Wendy Bertram, Leslie Boulton, Carol Bowler, Lynsey Brown, Sue Brown, Zoe Buckingham, Jeanette Bunga, Alex Bush, Racquel Carpio, Sandra Carter, Caroline Clark, Rachel Clarkson, Jacqueline Claydon, Sue Cockburn, Edmund Cox, Louise Davies, Kate Dennison, Jane Dickson, Christine Dickson, Adam Dobson, Troy Douglin, Sue Drake, Lucy Dudgeon, Kate Eke, Zubeir Essat, Vivienne Fairclough, Jenny Finch, Abigail Ford, Rebecca Fox, Josey Garratt, Bridget Gray, Sarah Griffiths, Ruth Halliday, Birgit Hanusch, Nicola Hook, Suzanne Jerreat, Genessa Jones, Katrina Kennedy, Lucksy Kottam, Angela Larkin, Anna Lartey, Katie McGuiness, Maxine Middleton, Karthikeyan Muthamayandi, Bally Purewal, Simon Richards, Catherine Lawrence, Victoria Robinson, Ginny Rose, Harriet Savage, Alison Sears, Louise Spoors, Lunar Summers, Susanna Symonds, Julia Taylor, Claire Tricker, Kirstie Walker and Judith Wood.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
Publications
Costa ML, Achten J, Parsons NR, Rangan A, Edlin RP, Brown J, Lamb SE. UK DRAFFT: a randomised controlled trial of percutaneous fixation with Kirschner wires versus volar locking-plate fixation in the treatment of adult patients with a dorsally displaced fracture of the distal radius. BMC Musculoskelet Disord 2011;12:201.
Costa ML, Parsons NR, Rangan A, Griffin D, Tubeuf S, Lamb SE. Percutaneous fixation with Kirschner wires versus volar locking-plate fixation in adults with dorsally displaced fracture of distal radius: randomised controlled trial. BMJ 2014;349:g4807.
References
- Barrett JA, Baron JA, Karagas MR, Beach ML. Fracture risk in the U.S. Medicare population. J Clin Epidemiol 1999;52:243-9. http://dx.doi.org/10.1016/S0895-4356(98)00167-X.
- Chen NC, Jupiter JB. Management of distal radial fractures. J Bone Joint Surg Am 2007;89:2051-62. http://dx.doi.org/10.2106/JBJS.G.00020.
- Kakarlapudi TK, Santini A, Shahane SA, Douglas D. The cost of treatment of distal radial fractures. Injury 2000;31:229-32. http://dx.doi.org/10.1016/S0020-1383(99)00248-X.
- Mah ET, Atkinson RN. Percutaneous Kirschner wire stabilisation following closed reduction of Colles’ fractures. J Hand Surg 1992;17:55-62. http://dx.doi.org/10.1016/0266-7681(92)90012-Q.
- Willis AA, Kutsumi K, Zobitz ME, Cooney WP. Internal fixation of dorsally displaced fractures of the distal part of the radius: a biomechanical analysis of volar plate fracture stability. J Bone Joint Surg Am 2006;88:2411-17. http://dx.doi.org/10.2106/JBJS.E.00946.
- Arora R, Lutz M, Hennerbichler A, Krappinger D, Espen D, Gabl M. Complications following internal fixation of unstable distal radius fracture with a palmar locking-plate. J Orthop Trauma 2007;21. http://dx.doi.org/10.1097/BOT.0b013e318059b993.
- Handoll HH, Madhok R. From evidence to best practice in the management of fractures of the distal radius in adults: working towards a research agenda. BMC Musculoskelet Disord 2003;4. http://dx.doi.org/10.1186/1471-2474-4-27.
- Willett KM, Gray B, Moran CG, Giannoudis PV, Pallister I. Orthopaedic trauma research priority-setting exercise and development of a research network. Injury 2010;41:763-7. http://dx.doi.org/10.1016/j.injury.2010.03.026.
- Hull P, Baraza N, Whalley H, Brewster M, Costa M. Dorsally displaced fractures of the distal radius: a study of preferred treatment options among UK trauma and orthopaedic surgeons. Hand Surg 2010;15:185-91. http://dx.doi.org/10.1142/S0218810410004801.
- Gustilo RB, Anderson JT. Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am 1976;58:453-8.
- Hull P, Baraza N, Gohil M, Whalley H, Mauffrey C, Brewster M, et al. The Incidence of Dorsally Displaced Distal Radius Fractures n.d.
- Ramsay CR, Grant AM, Wallace SA, Garthwaite PH, Monk AF, Russell IT. Statistical assessment of the learning curves of health technologies. Health Technol Assess 2001;5. http://dx.doi.org/10.3310/hta5120.
- Downing ND, Karantana A. A revolution in the management of fractures of the distal radius?. J Bone Joint Surg Br 2008;90:1271-5. http://dx.doi.org/10.1302/0301-620X.90B10.21293.
- Zenke Y, Sakai A, Oshige T, Moritani S, Nakamura T. The effect of an associated ulnar styloid fracture on the outcome after fixation of a fracture of the distal radius. J Bone Joint Surg Br 2009;91:102-7. http://dx.doi.org/10.1302/0301-620X.91B1.21026.
- Berntsen GK, Fønnebø V, Tollan A, Søgaard AJ, Magnus JH. Forearm bone mineral density by age in 7,620 men and women: the Tromsø study, a population-based study. Am J Epidemiol 2001;153:465-73. http://dx.doi.org/10.1093/aje/153.5.465.
- Court-Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury 2006;37:691-7. http://dx.doi.org/10.1016/j.injury.2006.04.130.
- Goldfarb CA, Yin Y, Gilula LA, Fisher AJ, Boyer MI. Wrist fractures: what the clinician wants to know. Radiology 2001;219:11-28. http://dx.doi.org/10.1148/radiology.219.1.r01ap1311.
- Mann FA, Kang SW, Gilula LA. Normal palmar tilt: is dorsal tilting really normal?. J Hand Surg 1992;17:315-17. http://dx.doi.org/10.1016/0266-7681(92)90120-Q.
- Mackenney PJ, McQueen MM, Elton R. Prediction of instability in distal radial fractures. J Bone Joint Surg Am 2006;88:1944-51. http://dx.doi.org/10.2106/JBJS.D.02520.
- Steyers CM, Blair WF. Measuring ulnar variance: a comparison of techniques. J Hand Surg Am 1989;14:607-12. http://dx.doi.org/10.1016/0363-5023(89)90175-5.
- de Vries J, Souer JS, Zurakowski D, Ring D. Measurement of ulnar variance on uncalibrated digital radiographic images. Hand 2010;5:267-72. http://dx.doi.org/10.1007/s11552-009-9249-9.
- Mignemi ME, Byram IR, Wolfe CC, Fan KH, Koehler EA, Block JJ, et al. Radiographic outcomes of volar locked plating for distal radius fractures. J Hand Surg Am 2013;38:40-8. http://dx.doi.org/10.1016/j.jhsa.2012.10.007.
- MacDermid JC, Turgeon T, Richards RS, Beadle M, Roth JH. Patient rating of wrist pain and disability: a reliable and valid measurement tool. J Orthop Trauma 1998;12:577-86. http://dx.doi.org/10.1097/00005131-199811000-00009.
- MacDermid JC, Richards RS, Donner A, Bellamy N, Roth JH. Responsiveness of the short form-36, disability of the arm, shoulder, and hand questionnaire, patient-rated wrist evaluation, and physical impairment measurements in evaluating recovery after a distal radius fracture. J Hand Surg Am 2000;25:330-40. http://dx.doi.org/10.1053/jhsu.2000.jhsu25a0330.
- Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG). Am J Ind Med 1996;29:602-8. http://dx.doi.org/10.1002/(SICI)1097-0274(199606)29:6<602::AID-AJIM4>3.0.CO;2-L.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. http://dx.doi.org/10.1016/0168-8510(96)00822-6.
- Coole C, Drummond A, Sach TH, Watson PJ. Assessing the feasibility of collecting health care resource use data from general practices for use in an economic evaluation of vocational rehabilitation for back pain. J Eval Clin Pract 2011;17:204-7. http://dx.doi.org/10.1111/j.1365-2753.2010.01364.x.
- Ridyard CH, Hughes DA. Methods for the collection of resource use data within clinical trials: a systematic review of studies funded by the UK Health Technology Assessment programme. Value Health 2010;13:867-72. http://dx.doi.org/10.1111/j.1524-4733.2010.00788.x.
- Hull P, Baraza N, Gohil M, Whalley H, Mauffrey C, Brewster M, et al. Volar locking plates versus k-wire fixation of dorsally displaced distal radius fractures: a functional outcome study. J Trauma 2011;70:E125-8. http://dx.doi.org/10.1097/TA.0b013e3181e32714.
- MacDermid JC, Roth JH, Richards RS. Pain and disability reported in the year following a distal radius fracture: a cohort study. BMC Musculoskelet Disord 2003;4. http://dx.doi.org/10.1186/1471-2474-4-24.
- Benson EC, DeCarvalho A, Mikola EA, Veitch JM, Moneim MS. Two potential causes of EPL rupture after distal radius volar plate fixation. Clin Orthop Relat Res 2006;451:218-22. http://dx.doi.org/10.1097/01.blo.0000223998.02765.0d.
- Brookes ST, Whitley E, Peters TJ, Mulheran PA, Egger M, Davey Smith G. Subgroup analyses in randomised controlled trials: quantifying the risks of false-positives and false-negatives. Health Techol Assess 2001;5.
- Guide to the Methods of Technology Appraisal. London: NICE; 2008.
- Curtis L. Unit Costs of Health and Social Care 2012. Canterbury, Kent: Personal Social Services Research Unit; 2012.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: John Wiley and Sons; 1987.
- Little RJA, Rubin DB. Statistical Analysis with Missing Data. New York, NY: John Wiley and Sons; 2002.
- Efron B. Better bootstrap confidence-intervals. J Am Stat Assoc 1987;82:171-85. http://dx.doi.org/10.1080/01621459.1987.10478410.
- Weinstein MC, Siegel JE, Gold MR, Kamlet MS, Russell LB. Recommendations of the Panel on Cost-effectiveness in Health and Medicine. JAMA 1996;276:1253-8. http://dx.doi.org/10.1001/jama.1996.03540150055031.
- National Schedule of Reference Costs Year 2011–2012 NHS Trusts PCT Combined. London: DH; 2012.
- The Campbell and Cochrane Economics Methods Group (CCEMG) and the Evidence for Policy and Practice Information and Coordinating Centre (EPPI-Centre) . CCEMG – EPPI-Centre Cost Converter 2013. www.eppi.ioe.ac.uk/costconversion (accessed October 2013).
- British National Formulary. London: BMA and RPS; 2013.
- Electronic Drug Tariff. NHS Business Services Authority; 2013.
- EuroQol group . What Is EQ-5D? n.d. www.euroqol.org/ (accessed 22 November 2013).
- Statistical Bulletin: Annual Survey of Hours and Earnings, 2012 Provisional Results. London: Office for National Statistics; 2012.
- Dolan P. Modeling valuations for EuroQol health states. Med Care 1997;35:1095-108. http://dx.doi.org/10.1097/00005650-199711000-00002.
- Cook RJ, Zeng L, Yi GY. Marginal analysis of incomplete longitudinal binary data: a cautionary note on LOCF imputation. Biometrics 2004;60:820-8. http://dx.doi.org/10.1111/j.0006-341X.2004.00234.x.
- Jansen I, Beunckens C, Molenberghs G, Verbeke G, Mallinckrodt C. Analyzing incomplete discrete logitudinal clinical trial data. Stat Sci 2006;21:52-69. http://dx.doi.org/10.1214/088342305000000322.
- Gray A, Clarke P, Wolstenholme J, Wordsworth S. Applied Methods of Cost-effectiveness Analysis in Healthcare. Oxford: Oxford University Press; 2010.
- Beaton DE, van Eerd D, Smith P, van der Velde G, Cullen K, Kennedy CA, et al. Minimal change is sensitive, less specific to recovery: a diagnostic testing approach to interpretability. J Clin Epidemiol 2011;64:487-96. http://dx.doi.org/10.1016/j.jclinepi.2010.07.012.
- Schwartz D, Lellouch J. Explanatory and pragmatic attitudes in therapeutical trials. J Clin Epidemiol 2009;62:499-505. http://dx.doi.org/10.1016/j.jclinepi.2009.01.012.
Appendix 1 Consent form
Appendix 2 Patient information sheet
Appendix 3 Background information
Appendix 4 Baseline questionnaire
Appendix 5 Randomisation form
Appendix 6 Operation note
Appendix 7 Six-week follow-up clinic
Appendix 8 Example follow-up questionnaire
Appendix 9 Serious adverse event form
List of abbreviations
- ANOVA
- analysis of variance
- BNF
- British National Formulary
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CTU
- Clinical Trials Unit
- DASH
- Disabilities of Arm, Shoulder and Hand questionnaire
- DMC
- Data Monitoring Committee
- DRAFFT
- Distal Radius Acute Fracture Fixation Trial
- EQ-5D
- EuroQol – Five Dimensions
- GP
- general practitioner
- ICC
- intraclass correlation coefficient
- ICER
- incremental cost-effectiveness ratio
- IQR
- interquartile range
- K-wire
- Kirschner wire
- LOCF
- last observation carried forward
- MCAR
- missing completely at random
- MCID
- minimal clinically important difference
- NICE
- National Institute for Health and Care Excellence
- NMB
- net monetary benefit
- PRWE
- Patient-Rated Wrist Evaluation© questionnaire
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- REC
- Research Ethics Committee
- SAE
- serious adverse event
- SD
- standard deviation
- TMG
- Trial Management Group
- TSC
- Trial Steering Committee
- UHCW
- University Hospital Coventry and Warwickshire
- WTP
- willingness to pay