Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 07/32/05. The contractual start date was in November 2008. The draft report began editorial review in June 2013 and was accepted for publication in December 2013. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Professor Sarah Lamb is the chairperson of the National Institute of Health Research Health Technology Assessment programme Clinical Evaluation and Trials Board.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Williams et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Background
Epidemiology of rheumatoid arthritis
Rheumatoid arthritis (RA) is the most common inflammatory polyarthritis. 1 It is a chronic unpredictable disorder that can cause persistent joint pain, joint damage and long-term disability (especially in the hands and feet). The prevalence of RA is 1.16% in women and 0.44% in men, increasing with age to 5% in those aged over 55 years. 1 It affects approximately 1% of the UK adult population. Five years after diagnosis, 40% of people with RA have relatively normal function (13% in remission), 44% have mild to moderate disability and 16% have marked functional disability. 2 The cause of the disease is unknown but both environmental and genetic factors are believed to contribute.
Rheumatoid arthritis is a whole-body disorder with greater mortality and multisystemic effects. The condition usually starts in the small joints of the hands and feet and later spreads to involve the larger joints. T-cells infiltrate the synovium, resulting in hypertrophy and inflammation of the local area and supporting ligaments. Deformities arise as a result of joint cartilage being eroded, which can then extend into the bone cortex. There are common forms of deformities at the wrist (dorsal ulnar head subluxation),3 metacarpophalangeal (MCP) joints (volar subluxation of proximal phalanges and ulnar drift of fingers), fingers (swan-neck and boutonnière deformities) and thumb [instability at the MCP and interphalangeal (IP) joints]. Tendon rupture can occur as a result of weakening by synovial invasion or abrasion over an irregular bony prominence.
Alongside this process of inflammation and deformity, other common associated problems for the hands and wrists are pain, weakness and restricted mobility resulting in loss of function and social participation. 4–6 RA patients report hand function to be important in their daily lives,5 with at least 70% of patients reporting hand and wrist dysfunction. 7
Pharmacological management of rheumatoid arthritis
Although there are increasingly effective drug treatments,8,9 the condition has no known cure. Thus, the goals of management are to prevent or control joint damage, loss of function and decrease pain. 10 In order to achieve these goals, combinations of pharmacological, non-pharmacological and surgical treatments are used.
The chief categories of drugs used are analgesics, non-steroidal anti-inflammatory drugs (NSAIDs), disease-modifying anti-rheumatic drugs (DMARDs) (non-biological and biological) and corticosteroids. NSAIDs offer a purely symptomatic treatment, commonly used when the disease flares up. There are a multitude of DMARDs licensed for use and it is now agreed that these should be used early on in the disease process for improved control. 11 They are commonly used in combinations to achieve greater benefit but with no extra harm. Corticosteroids offer a fast-acting solution and are frequently used between changes in DMARD regimes. They can be used in oral, intravenous (IV) and intra-articular forms, depending on how widespread the scope of the problem. The side effects limit the long-term use of these drugs. Biological DMARDs, or biologics, are a relatively recent development in the management of RA. Owing to their greater financial cost, their use is restricted by National Institute for Health and Care Excellence (NICE) guidelines to patients whose disease is not controlled by conventional DMARDs. 12 The main classes of biologics in common use at present are tumour necrosis factor (TNF) inhibitors or ‘anti-TNF’ (including infliximab, etanercept, adalimumab and certolizumab pegol) and rituximab, which works by depleting B lymphocytes. Newer biologics such as abatacept and tocilizumab are used where anti-TNF and rituximab do not work or cause adverse effects.
Non-pharmacological management of rheumatoid arthritis
Current UK clinical guidelines for the management of RA recommend the use of physiotherapy and occupational therapy as adjuncts to drug treatment. 13,14 The three most common components of physiotherapy/occupational therapy for RA hands are exercise therapy, joint protection (JP) advice and provision of functional splinting and assistive devices,15 although we are not aware of any research describing current clinical practice in the NHS.
The use of exercise treatment for RA primarily aims to increase strength, stability and range of motion by tackling rheumatoid cachexia (loss of body cell mass and muscle architecture), disuse atrophy and joint/soft tissue restriction. 16 Additional benefits may include reducing pain and increasing sensory–motor function. 17 A systematic review18 of six randomised controlled trials (RCTs) of the effectiveness of dynamic general exercise programmes in RA concluded that dynamic exercise was effective in improving muscular endurance and strength, without having detrimental effects on disease activity or pain. The number of RCTs that have specifically investigated the effects of exercise on RA hands and wrists is limited to five small studies with short-term follow-up limited to a few months (n = 44, 50, 52, 44 and 67 individuals). 19–23 Each of these studies demonstrated small improvements in hand impairment and/or function with no increase in joint swelling, pain or disease activity.
Adherence to any exercise programme is crucial, as it is suggested that there is a dose–response relationship in both healthy and RA populations for strength and pain. 24 Adherence with short-term supervised exercise programmes is generally high. 25 However longer-term and home-based exercise programmes do not have the same response,26 although data are sparse. There is some evidence to show that a programme incorporating a behaviour-change framework based on the Health Belief Model27 is effective in maintaining long-term adherence in RA patients. 28
Joint protection advice includes pain management advice, planning and pacing activities, regular rest, altering patterns of joint movement and assistive device use in order to minimise pain and fatigue and make tasks easier. This advice may be provided in the form of information leaflets (e.g. Looking After Your Joints When You Have Arthritis29), one-to-one sessions, group interventions or a combination of these. Evidence suggests that, provided appropriate education methods are applied, JP improves function and reduces pain in the short and long term. 28,30
Provision of splinting is widespread in UK clinical practice,31 with the objectives of reducing hand and wrist pain, improving hand function and reduction or prevention of deformity and soft tissue contractures, although evidence of efficacy is unclear. 32 Types of splinting may be categorised into resting or functional depending on the exact requirements of the patient.
Costs of rheumatoid arthritis
The economic cost of RA is substantial for both the individual patient and society as a whole. 33 Patients with poor and declining function from their diagnosis of RA experience much higher costs of care overall. 34 A report by the National Rheumatoid Arthritis Society in 2010 found that the overall cost of RA to the UK economy was almost £8B per annum, with NHS expenditure totalling approximately £700M. 35 To date, no studies evaluating exercise in hand and wrist RA have detailed costs involved or included a cost-effectiveness analysis.
Rationale for Strengthening And stretching for Rheumatoid Arthritis of the Hand trial
Wrist and hand dysfunction as a result of pain, loss of movement and weakness is a common problem in RA. To address this, exercises are currently recommended as part of clinical management of people living with RA with an increasing shift towards more active treatments at an earlier stage. These recommendations are not supported by high-quality evidence.
With previous small-scale studies showing some promise over the short term, there is a clear need for long-term evaluation of an optimised hand exercise programme in a large group of people living with RA. As part of this evaluation, it is important that strategies to maximise programme adherence are incorporated and evaluated. A mixed-methods approach provides rich data that should facilitate understanding of why such a programme does or does not work.
In the Strengthening And stretching for Rheumatoid Arthritis of the Hand (SARAH) trial we evaluate a hand exercise programme that will be acceptable to NHS physiotherapists and occupational therapists based on current available evidence. Such a programme is over and above what is currently provided in the UK NHS. A parallel economic evaluation will enable conclusions to be made about cost-effectiveness.
Research objectives
-
To estimate the clinical effectiveness of adding an optimised exercise programme for hands and upper limbs in addition to usual care in the reduction of hand dysfunction and pain for patients with RA.
-
To estimate the cost-effectiveness of adding this programme to usual care.
-
To describe, qualitatively, the experience of participants in the trial with a particular emphasis on acceptability of the intervention, exercise behaviours and reasons for adherence/non-adherence.
The null hypotheses of the study were that there would be no difference in either the clinical and cost outcomes between the two treatment arms.
Chapter 2 Methods
Trial design
The SARAH trial was a pragmatic, multicentre RCT. The control arm (usual care) consisted of JP education, advice on simple mobility exercises for the whole body and, if appropriate, functional splinting. This was compared with an experimental intervention consisting of the same regimen supplemented with an optimised exercise programme. This programme consisted of strengthening and stretching exercises for the hand, wrist and upper limb delivered over six sessions with a therapist over a 12-week period.
Participants
Inclusion criteria
-
RA meeting the American College of Rheumatology (ACR) clinical and immunological criteria (Box 1). 10
-
Pain and dysfunction of the hands and/or wrist joints.
-
No treatment with a DMARD or having been on a stable DMARD regimen for 3 months or more.
Patients must meet four of the following seven criteria:
-
morning stiffness in and around joints lasting at least 1 houra
-
swelling in three or more jointsa
-
swelling in hand or wrist jointsa
-
symmetrical joint swellinga
-
erosions or decalcification on radiographs of hand and/or wrist
-
rheumatoid nodules
-
abnormal serum rheumatoid factor.
Must be present for at least 6 weeks.
Exclusion criteria
-
Age less than 18 years.
-
Upper limb joint surgery, or fracture, in the previous 6 months.
-
Being on a waiting list for upper limb orthopaedic surgery.
-
Pregnancy.
Screening and recruitment
Patients were approached primarily by clinical staff during routine clinic visits. They were provided with a patient information sheet (see Appendix 1) and asked if they would consider participating. If they agreed, a research clinician (physiotherapist, occupational therapist or nurse) then contacted them to book an appointment at a research clinic. At this appointment, the trial information was discussed, the patient had an opportunity to ask questions, eligibility was rechecked and, if appropriate, patients were consented (see Appendix 2). Patients were asked to give written informed consent according to principles of Good Clinical Practice and the Declaration of Helsinki. 36 At the time of consent, outcome assessors collected baseline measures.
In addition, patients on rheumatology consultant or therapy review lists without planned clinic appointments were approached using postal questionnaires. They were sent an information sheet and a brief questionnaire to return with a pre-paid envelope by the research team. The questionnaire asked whether or not they were currently experiencing problems with their hands and/or wrists and about other eligibility criteria. Patients who returned the questionnaire were contacted by the research clinician and, if appropriate, had a research clinic appointment made.
Settings and locations
The trial was run in the hospitals of 17 NHS trusts (names at time of participation):
-
Basingstoke and North Hampshire Hospitals NHS Foundation Trust (North Hampshire Hospital)
-
Derby Hospitals NHS Foundation Trust (Royal Derby Hospital)
-
Dorset Primary Care Trust (Victoria Hospital)
-
George Eliot Hospital NHS Trust (George Eliot Hospital)
-
Heart of England NHS Foundation Trust (Solihull Hospital)
-
Nuffield Orthopaedic Centre NHS Trust (Nuffield Orthopaedic Centre)
-
Poole Hospital NHS Foundation Trust (Poole General Hospital)
-
Portsmouth Hospitals NHS Trust (Queen Alexandra Hospital)
-
Royal Bournemouth NHS Foundation Trust (Christchurch Hospital)
-
Royal National Hospital for Rheumatic Diseases NHS Foundation Trust (Bath Royal National Hospital for Rheumatic Diseases)
-
South Warwickshire General Hospitals NHS Trust (Warwick Hospital and Stratford-Upon-Avon Hospital)
-
Sussex Community NHS Trust (Bognor Regis War Memorial Hospital)
-
Winchester and Eastleigh Health Care Trust (Royal Hampshire County Hospital)
-
Worcestershire Acute Hospitals NHS Trust (Worcestershire Royal Hospital; Alexandra Hospital, Redditch; Kidderminster Hospital and Treatment Centre)
-
Wrightington, Wigan and Leigh NHS Foundation Trust (Wrightington Hospital)
-
University Hospitals Coventry and Warwickshire [University Hospital (Walsgrave site) and Rugby St Cross Hospital]
-
University Hospitals of Leicester NHS Trust (Leicester Royal Infirmary and Leicester General Hospital).
Interventions
Full details of the interventions are provided in Chapter 3, but the interventions are described briefly here for continuity.
All interventions were delivered by physiotherapists or occupational therapists experienced in the treatment of hand and rheumatology conditions. They were independent of the recruitment and randomisation procedures, attended a training session delivered by the trial team and received ongoing support and guidance regarding the intervention to ensure quality standards were met. Therapists were trained to deliver both the experimental and control interventions. Contamination was minimised through a variety of quality control measures that included monitoring the treatment logs completed at each session, visiting therapists at the beginning of their time delivering interventions on the trial for quality assurance purposes and providing additional materials sufficient to cover only participants randomised to the experimental arm of the study. The rationale and protocol for the interventions were described in a training and reference manual. Both control and experimental interventions were developed using focus group meetings with stakeholder clinicians from across the UK.
Participants randomised to the control intervention (usual care) had, depending on the clinical need, between one and three sessions of outpatient therapy with a maximum total contact time of 1.5 hours. Treatments in these sessions consisted of JP advice, general mobility exercises and, if appropriate, functional splinting. Participants were provided with information sheets 29,37,38 and were encouraged to remain active. The participant was not reviewed again by the treating therapist after the 1.5 hours unless there were additional splinting requirements.
Participants randomised to the exercise programme received the usual care package plus an additional five sessions with a therapist over a 12-week period. The aim was to increase hand function using exercises to stretch and strengthen the muscles and tendons, as well as mobilise the joints of the hand and wrist and improve dexterity. This was supported by a home exercise programme (reinforced by a behaviour change approach and exercise diary) to be performed daily.
Monitoring the intervention delivery
Attendance rates and content of treatment appointments were recorded by the therapist using treatment logs. Logs were completed for each session for all participants and returned to the study co-ordinating centre. Close contact was maintained between the clinical research fellow and the therapy departments to address any problems that were highlighted by the treatment logs.
All sites were visited to ensure smooth implementation of the interventions within the trial. This quality control process involved the same clinical research fellow auditing treatment logs and notes and observing experimental arm intervention sessions.
Outcomes
Follow-up data collection was by face-to-face clinical assessments at 4 and 12 months following randomisation. Where face-to-face assessment was not possible, postal and telephone data collection methods were used to obtain core data. The outcome measures are detailed in Table 1 and Appendix 3.
| Domain | Data source | Measures: instrument (scale/units – high value is better score unless specified) | Time points (months) |
|---|---|---|---|
| Function | Research clinic questionnaire (participant reported) | MHQ – overall hand function score (0–100) MHQ – overall score (0–100) |
0, 4, 12 |
| Pain | Research clinic questionnaire | Pain sub-scale of MHQ (0–100; high score is worse) ‘Troublesomeness’ rating (0–20; high score is worse) |
0, 4, 12 |
| Impairment | Research clinic examination (performed by outcome assessor) | Joint deformity (MCP joint only) – goniometer (degrees; high score is worse) Wrist range of motion (flexion/extension) – goniometer (degrees) Finger range of motion (combined flexion and combined extension) – ruler (mm; high score is worse for combined flexion) Thumb opposition range of motion – observation (0–10) Dexterity – timed nine-hole peg test (seconds; high score is worse) Grip and pinch strength – dynamometer (N) |
0, 4, 12 |
| Disease Activity | Medical records Research clinic examination |
ESR (mm/hour) and/or CRP (mg/l) blood test Hand and wrist joint tenderness and swelling count – examination (0–22; high score is worse) |
0, 4, 12 |
| HRQoL | Research clinic questionnaire | SF-12 (0–100) EQ-5D-3L (health utility) (0–1) |
0, 4, 12 |
| Self-efficacy | Research clinic questionnaire | Confidence to manage their condition (seven item questionnaire) | 0, 4, 12 |
| Satisfaction | Research clinic questionnaire | Treatment satisfaction item Satisfaction subscale of MHQ (0–100) |
4, 12 |
| Global Change | Research clinic questionnaire | Participant-rated improvement in their condition question (7-point Likert scale) Perceived benefit/harm from trial treatments (5-point Likert scale) |
4, 12 |
| Adherence | Research clinic questionnaire | Adherence with treatment (5-item questionnaire) Patient reported current exercise behaviour |
0, 4, 12 |
| Economics | Research clinic questionnaire | Resource use questionnaire | 4, 12 |
The primary outcome measure was the overall hand function subscale of the Michigan Hand Outcome Questionnaire (MHQ), which has shown to be a reliable, valid and responsive measure for a RA population. 39–41 The overall MHQ contains six domains:
-
overall hand function
-
activities of daily living (ADLs)
-
pain
-
work performance
-
aesthetics
-
patient satisfaction with hand function.
Scores range from 0 to 100, with higher scores indicating better performance, except for the pain scale. For the pain scale, a higher score indicates more pain.
In the initial version of the protocol, the Arthritis Impact Measurement Scale 2 (AIMS2) finger and hand function subscale was documented as the primary outcome measure. However, prior to the full trial commencing, we conducted a pilot study involving 16 participants, and as part of this evaluated both the AIMS2 and MHQ. Based on this the MHQ was substituted as the primary outcome measure. This substantial amendment was approved by the Multicentre Research Ethics Committee and full trial protocol subsequently published. 42
Secondary outcome measures consisted of measures of pain troublesomeness,43 self-efficacy,44 the European Quality of Life-5 Dimensions-3 Levels (EQ-5D-3L),45 the Short Form questionnaire-12 items (SF-12),46 socioeconomic questions (employment status, sick days in past month due to RA in wrists/hands, benefits, highest educational qualification, household income), treatment preference and healthcare use and costs. Blood test results [C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), serum rheumatoid factor] and current medication (prescribed and as required in last 7 days) were taken from hospital and prescription records. The outcome assessor was present to answer any questions regarding the measures but was trained not to influence the participant’s responses. Following completion of the case report form, a physical assessment was performed in a standardised order and standardised positions. This included the measurement of joint deformity (MCP ulnar/radial deviation in maximum pronation, where ulnar deviation is recorded as a positive value) and active range of motion (wrist flexion and extension from the neutral position with a goniometer,47 combined finger flexion according to Ellis and Bruton,48 combined finger extension and thumb opposition according to Kapandji). 49 A modified swollen and tender joint count (22 joints of hand and wrist)50 was taken, along with a test of upper limb dexterity (nine-hole peg test according to Mathiowetz et al. ). 51 Finally, two forms of grip strength (full-hand and tripod pinch) were measured using the MIE Digital Grip Analyser (MIE Medical Research Ltd, Leeds, UK). 52 The standard test position recommended by the American Society of Hand Therapists was used. 53 Patients were sat in a straight-backed chair without arm rests, feet flat on floor, the shoulder of the assessed limb relaxed by the side, the elbow flexed to 90°, the wrist extended and in ulnar deviation between 0° and 15° and the forearm rotated to neutral pronation/supination. The mean of three maximal 3-second grips was calculated for each hand, with 60-second rests between repetitions.
Harms/adverse events and reasons for withdrawals are tabulated in Chapter 4.
While aware of the Outcome Measures in Rheumatology (OMERACT) core set of disease activity measures, we prioritised outcomes that were likely to be sufficiently sensitive to detect any effect resulting from the interventions.
Sample size
A standardised mean difference of 0.3 is reported to represent a clinically important difference in hand function in this group. 54 A previous small study using a similar intervention found a mean benefit of 0.7 in the AIMS2 with a standard deviation of 1.81 and a standardised effect size of 0.39. 23 This suggested that in this larger, more pragmatic, multicentre trial, a standardised effect size of 0.3 in the similar function score using the MHQ would be realistic and meaningful. To show this difference with 80% power at the 5% significance level, we required data on at least 352 participants [using Statistical Analysis Software (V9.2, SAS Institute Inc., Cary, NC, USA) procedure GLMPOWER]. Assuming a 25% loss to follow-up would require at least 469 participants. Over 15 months we expected 1200 people with hand RA to be referred at our participating centres. If half of these were assessed for study entry and 80% of them joined the study, we would have 480 participants (1200 × 0.5 × 0.8). This was our target sample size. The assumptions underlying the sample size calculation were monitored by the Data Monitoring and Ethics Committee (DMEC) throughout the study.
Randomisation
Randomisation to the exercise programme or usual care was via a central telephone randomisation service at Warwick Clinical Trials Unit, University of Warwick.
Sequence generation
The variable block randomisation schedule was prepared by the trial statistician (CM). Randomisation was stratified by centre to control for any confounding factors evident at local recruitment sites, such as therapist effects or local contamination of intervention.
Allocation concealment
The research clinician telephoned the randomisation service, and only once the participant was registered in the trial was the random allocation generated. Hence allocation was concealed. Allocation was faxed or emailed direct to the therapist, and the participant was told either when their first appointment was made or at the appointment itself.
Blinding
The outcome assessor was blind to the group allocation of the participant and was independent of intervention delivery. Participants were requested not to disclose group allocation to the outcome assessor. If an outcome assessor was unblinded, this was recorded. If they remained unblinded, the assessors were asked to guess which allocation they thought the participant was given. The participants and therapists could not be blinded to the group allocation.
Statistical methods
Participants were analysed according to the treatment group to which they were randomised, regardless of the treatment that they actually received (intention-to-treat analysis). 55 Analyses were guided by an analysis plan prepared before data were available. Demographic, clinical characteristics and baseline measurements are presented to evaluate the comparability of intervention groups and generalisability to clinical settings (see Chapter 4, Baseline data). A Consolidated Standards of Reporting Trials (CONSORT) diagram was produced (see Figure 4). 56
The difference between the intervention groups in mean score of MHQ overall hand function score from baseline to 4 and 12 months was analysed by a linear model, adjusted for the baseline score. Means and 95% confidence intervals (CIs) are given.
The secondary outcome measures of change in pain, impairment measures, disease activity, quality of life, self-efficacy, satisfaction and global change scores were analysed in a similar manner to the primary outcome measure.
Analyses also took account of age, sex and current drug regimens by including baseline medication covariates on outcome analyses. The therapy groups used were biologic DMARD, combination non-biologic DMARDs, single non-biologic DMARD and no DMARD. Patient-rated improvement was compared using the Wilcoxon test. Other ordinal testing for patient-rated benefit/harm and treatment satisfaction used the Mantel–Haenszel chi-squared test for linear association and tests for differences in other categorical data including medication and performing exercises used Pearson’s chi-squared. Standardised mean differences are calculated as the unadjusted mean difference between treatments divided by the pooled baseline standard deviation.
A pre-specified complier average causal effect (CACE) analysis was conducted to determine if the level of compliance with the exercise programme affected participant outcome as measured by the overall hand function subscale of the MHQ (primary outcome). The CACE estimates were calculated using the instrumental variable method described in Dunn et al. 57 The analysis was performed using a threshold of six or more sessions (i.e. attended all sessions) as defining compliance. An additional analysis investigated a threshold of three or more sessions.
The impact of therapist effects was evaluated by including a random therapist effect nested within centre in the main outcome model. The effect of missing data was investigated using a Markov chain Monte Carlo multiple imputation analysis.
In the original protocol, the primary analysis was stated as a repeated measures mixed model, but this was changed to an adjusted linear model to make the results more interpretable by a wide audience. It was also decided that survival analysis methods applied to surgical and serious adverse (SAE) event data were not appropriate. These changes were approved by the DMEC and Multicentre Research Ethics Committee (MREC) in the statistical analysis plan and Version 2 of the protocol which was subsequently published. 42
Statistical analyses were performed using SAS V9.2 software.
Database and data processing
The database was held on Warwick Clinical Trial Unit’s Microsoft SQL Server system (Microsoft Inc., Bellevue, WA, USA) and imposed rules for data entry which include valid range for responses, linked dates and patient identification numbers.
Data were single entered into the database by study personnel. The trial statistician carried out checks of plausibility of values, missing data and form return rates to enable further queries to be resolved prior to freezing data for scheduled DMEC reports and analysis.
For data quality assessment, 10% of all forms at all time points were randomly selected for data checking on a 2-monthly basis. All disagreements found when checking were corrected and any systematic faults found as a result of the checks were also corrected. The levels of accuracy specified were 1% for primary end point data and 5% for secondary end point data. Five time periods were covered and disagreement levels were always below 1% apart from one occasion when, in one 2-month period, a 2% level was found for primary data. Corrections were made and the problem did not recur.
Scoring and missing items
Michigan Hand Outcome Questionnaire scales were scored as described by Chung et al. 58 Individual item scores are summed to give a raw score for each scale. These are converted to a score between 0 and 100 using the MHQ scoring algorithms. Averages of right and left hand are used for those scales where both hands are measured. Scales were calculated if at least 50% of their items were completed. An overall score can be obtained by averaging the score for all scales (with pain scale reversed). At least four scales need to be completed to calculate the overall MHQ score.
The SF-12 was scored as described by Ware et al. 59 We analysed mean component scores for the Mental (Mental Component Score; MCS) and Physical (Physical Component Score; PCS) subscales. Missing responses were dealt with using an imputation algorithm. 60
European Quality of Life-5 Dimensions was used mainly for economic analyses evaluation; methods and results for the economic analyses evaluations are described in Chapter 6.
Further details on the scoring used for impairment measures is given in Appendix 4.
Subgroup analyses
Statistical tests of interaction were used to perform pre-specified subgroup analysis on baseline drug regimen (no DMARD, single DMARD, combination DMARD or biologic DMARD) and disease duration (< 5 years or ≥ 5 years). Post hoc exploratory analyses were also conducted for baseline MHQ overall hand function (< 52.5 points or ≥ 52.5 points), age (< 60 years or ≥ 60 years), type of referral (clinic or review list mail out), gender, baseline ESR (< 16 mm/hour or ≥ 16 mm/hour), baseline CRP (< 6 mg/l or ≥ 6 mg/l), SF-12 PCS (< 34 points or ≥ 34 points) and SF-12 MCS (< 50 points or ≥ 50 points).
Sensitivity analyses
Sensitivity analyses were planned to explore effects of results of adjustment for age, sex, site and any imbalance in baseline characteristics. A repeated measures analysis was also performed.
Ethical approval and monitoring
Ethics committee approval
The SARAH trial was approved by the Oxford C Multicentre Research Ethics Committee in June 2008 (Research Ethics Committee reference 08/H0606/47) and by the research and development department of each participating centre. The final approved study protocol has been published. 42
Trial Steering Committee
A Trial Steering Committee (TSC) was responsible for monitoring and supervising the progress of the SARAH trial. The TSC consisted of three independent experts, a lay member and leading members of the Trial Management Group (TMG). Membership of the TSC is given in Acknowledgements.
Data Monitoring and Ethics Committee
The DMEC was independent of the trial and was tasked with monitoring ethical, safety and data integrity. The trial statistician provided data and analyses requested by the DMEC at each of the meetings. Membership of the DMEC is given in Acknowledgements.
Trial Management Group
A TMG was responsible for the day-to-day management of the trial, consisting of the chief investigator, research fellows, statistician, trial co-ordinator, research nurse and data-entry clerk. The role of the group was to monitor all aspects of the conduct and progress of the trial, to ensure that the protocol was adhered to and to take appropriate action to safeguard participants and the quality of the trial itself.
Chapter 3 Intervention description and rationale
Introduction
The commissioning brief requested a large-scale pragmatic RCT to investigate the clinical effectiveness and cost-effectiveness of an exercise programme for the management of RA of the hand. The brief stipulated a trial of conventional care plus a defined package of hand exercise therapy incorporating the following elements:
-
a defined package of hand exercise therapy with instruction from an appropriate therapist
-
the setting should be an out-patient clinic or therapy department
-
the control group would receive ‘conventional care’ only.
A multifaceted approach was undertaken to design an intervention in response to these requirements. This chapter explores the development of the interventions for the experimental and control arms of the SARAH trial. Defining conventional or usual care within the NHS as well as designing an exercise programme specifically for the hand constituted the major elements of the initial development stages.
Development of the Strengthening And stretching for Rheumatoid Arthritis of the Hand intervention
The initial design of the SARAH intervention was based on a small study61 which was modified after drawing together several strands of evidence including current guidelines, evidence from the literature, expert and patient opinion, and physiological and theoretical considerations. Subsequent testing took place during a pilot study at two hospitals in the West Midlands in which 16 subjects received a specific hand exercise programme in addition to usual NHS care.
Clinical guidelines
Current UK guidelines for the management of RA62–64 recommend that access to physiotherapists and occupational therapists should be offered to all people with RA to assess the impact and treat the consequences of the condition. Specifically, the stated aims of therapy include:
-
facilitating ADLs (e.g. washing, using the toilet, dressing, cooking, eating, working and leisure)
-
improving general health-related fitness and encouraging regular exercise
-
providing instruction on a range of strategies to reduce pain and stress on joints while carrying out everyday activities
-
providing instruction on specific exercises aimed at enhancing joint flexibility and muscle strength and reducing other functional impairments
-
providing information and advice about short-term pain relief provided by electrophysical interventions (e.g. transcutaneous electrical nerve stimulation and wax baths).
Treatment provided by occupational therapists and/or physiotherapists can involve a variety of modalities to achieve these aims. According to surveys of current practice, the three most common components of therapy currently provided for people with RA of the hands are exercise therapy, JP advice and functional splinting. 15,65–67 Hence, current treatment is a balance between the provision of strategies to support and protect joints (including symptomatic relief) and exercise to improve or maintain strength, flexibility and functional ability. However, little guidance is available as to the specifics of any exercise programme.
Evidence base
There is evidence suggesting that exercise improves general muscular endurance and strength without detrimental effects on disease activity or pain in RA. 68 However, few studies have investigated the effect of exercises specifically for the rheumatoid hand. Some improvements in strength, mobility and/or function with no negative effects have been reported,19,20,22,61,69,70 although the long-term effectiveness has not been established.
A systematic review of the literature was performed to establish the evidence base for exercise in RA. This encompassed general exercise programmes designed for the whole body as well as those specifically addressing the hand and upper limb. In particular, exercise programmes from those studies that described the actual intervention in detail were evaluated as part of designing the final SARAH intervention protocol.
General exercise
Several studies have investigated the effects of various types of exercise on different aspects of the patient experience, the majority reporting beneficial responses. 18 Almost all have involved general or ‘whole-body’ programmes focusing on aerobic fitness, strengthening and/or active range-of-movement (ROM) exercises.
In the past, issues of disease activity and potential irritation of symptoms led to a degree of caution in exercise prescription for sufferers of RA. Current evidence suggests that exercise does not appear to have a negative impact on the disease process and may in fact be beneficial,68 including for the small joints of the hands and feet. 71 It should be noted that these studies were not specific to the hands; rather the hands were used during general upper limb exercise activity.
Specific hand exercise
Very few studies have specifically investigated the effect of hand exercises for patients with RA. A systematic review in 200472 found nine studies of sufficient quality from which no definite conclusions could be reached owing to the different designs, outcome measures and exercises utilised. These findings were reinforced in a similar review73 which concluded that evidence was lacking as to the effectiveness of shoulder- and hand-strengthening exercises.
An updated systematic review was undertaken by members of the SARAH trial team, including only RCTs or controlled clinical trials that used quasi-randomised methods of treatment. 74 Five studies were included19–22,61 (Table 2). None of the studies described any significant detrimental effect resulting from exercise – if anything there was a tendency for some measures of disease activity to improve even with intensive exercise. Reported gains in strength following exercise were variable and of unknown clinical significance.
| Study | Number of subjects, follow-up duration | Exercise type | Outcomes | ||
|---|---|---|---|---|---|
| Strength | ROM | Function | |||
| Dellhag et al., 199221 | n = 52, 4 weeks | Strength and/or ROM | No change | ↑ Some measures | No change |
| Brighton et al., 199319 | n = 44, 4 years | ROM and functional | ↑ Gross grip and pincer grip | ↓ Loss of ROM | Not measured |
| Hoeing et al., 199322 | n = 41, 12 weeks | Strength or ROM or combined ROM/strength | ↑ Some measures | No change | Not measured |
| Buljina et al., 200120 | n = 100, 3 weeks | Strength and ROM (+ heat) | ↑ Strength (all) | ↑ | ↑ |
| O’Brien et al., 200623 | n = 67, 6 months | Strength and/or ROM | ↑ Some grip strength measures | No change | ↑ |
Eight studies reported hand exercise programmes in sufficient detail to enable reproduction (Table 3). They all used various combinations of strength and/or stretching exercises, with only two attempting to differentiate between them in subsequent analyses. 22,61 Three of the studies named the same source for the exercise intervention used,76,77 while a fourth designed their programme following a survey of hand therapists. 61 None of the remaining studies provided any rationale for the exercises used.
| Author | Frequency | Sets/reps | Type | Exercise |
|---|---|---|---|---|
| McLaughlin and Reynolds, 197375 | × 2/day for 4 weeks | Start 1 × 5; progress to 1 × 10 | Flexion of PIP and DIP joints | |
| Finger extension | ||||
| Radial deviation of fingers against gravity | ||||
| Flexion, extension and radial deviation of wrist | ||||
| Pronation and supination | ||||
| Flexion of thumb IP joint | ||||
| Thumb opposition | ||||
| Intrinsic muscle exercise | ||||
| Dellhag et al., 199221 | × 3/week for 4 weeks | 1 × 5 | Flatt’s programmea | Finger flexion, extension and radial deviation |
| Wrist flexion, extension and ulnar deviation | ||||
| Thumb opposition and abduction | ||||
| Shoulder rotation, flex and abduction | ||||
| Brighton et al., 199319 | × 1/day for 4 years | 1 × 5 |
|
|
| Hoenig et al., 199322 | × 2/day for 12 weeks | 1 × 10 | ROM | Tendon gliding exercises |
| Strength | Balanced resistive hand exercises including finger abduction and adduction with MCP extended and gross grip (with therapy putty – plasticity rating 85) | |||
| Buljina et al., 200120 | × 1/day for 3 weeks | 1 × 5 | Flatt’s programmea | Finger flexion, extension and radial deviation |
| Wrist flexion, extension and ulnar deviation | ||||
| Thumb opposition and abduction | ||||
| Shoulder rotation, flexion and abduction | ||||
| Strength | Balanced resistive hand exercises including finger abduction and adduction with MCP extended and gross grip (with therapy putty – plasticity rating 85) | |||
| O’Brien et al., 200623 | × 2/day for 6 months | Start 1 × 5; progress to 1 × 20 | Strength and ROM |
|
| ROM only | Stretches including:
|
|||
| Rønningen and Kjeken, 200869 | × 5/week minimum for 14 weeks | 1 × 3 | Conservative | Gentle exercises performed against resistance of a soft dough:
|
| 1 × 10 | Intensive | As above except: thumb opposition performed against resistance; touching the tip of each finger with the thumb and rolling a ‘ball’ with the palm on the table were removed; each exercise repeated ×10 except radial ‘finger walking’ (× 5); finger flexion and extension exercises repeated three times during the training session | ||
| Brorsson et al., 200970 | × 5/week | 1 × 10 | Gross grip, pinch grip, finger ‘clawing’ and rolling putty with flat hand (with therapy putty – soft, medium or firm) |
Theoretical and physiological considerations
Strength
Weakness associated with RA is well documented, with reports of patients having an average of 40% of normal power and pinch grip within 6 months of diagnosis. 78 The mechanisms behind this are thought to be due in part to ‘rheumatoid cachexia’ (loss of muscle cell mass and destruction of muscle architecture because of the autoimmune, catabolic nature of the condition) as well as disuse atrophy of muscle. 79 This loss of muscle tissue is of particular concern considering the importance of muscle strength and power to provide movement as well as joint stability and protection.
Accepted principles of exercise physiology conclude that an increase in strength requires a sufficient training stimulus, in the form of volume and intensity, in accordance with the principle of overload. This can be altered by manipulation of frequency, load, number of sets and repetitions, and rest intervals. 80 If the effects of muscle atrophy are to be countered, then muscles have to work at an appropriate intensity and with sufficient volume79 to induce muscle hypertrophy. Other important aspects include duration of programme, specificity of exercises and individualisation (i.e. adjusting the programme to suit each participant). Depending on response and duration of the programme, progression is required to maintain improvement and prevent plateauing or potential reversal of training effects.
Unfortunately, the literature provides little detail concerning exercise protocols, especially with regard to strengthening. Of the studies examining exercise in rheumatoid hands, only five (see Table 3) described the loads used, and no mention was made of how initial load was selected or progressed by these or any other trial. The volume of exercise varied according to the different regimes, ranging from one set of three repetitions up to a maximum of one set of 20 repetitions. These were performed anywhere between three times a week and twice a day for periods lasting from 3 weeks to 4 years.
Mobility
Although deficits in upper limb ROM are commonly described as a consequence of RA, there is very little quantitative information in the literature. One study reported mean reductions of up to 17% in hand ROM in the early stages of the disease (i.e. within 7–12 months of diagnosis) compared with age- and gender-matched healthy individuals. 81 A cross-sectional study reported that 28% of RA patients had wrist and finger ROM deficits in the dominant hand of greater than 15 degrees compared with healthy volunteers after 2–4 years, rising to 35% and 49% for the wrist and fingers, respectively, after 8 years. 6 Other upper limb joints have also been implicated, including the shoulder, making it difficult to place the hand into positions for efficient function. 82 Decreased finger ROM has been linked to reduced hand function. 17,83 In accordance with this limited evidence, we considered it important to maintain or improve ROM in all the upper limb joints.
The tendon sheaths of RA patients have also been reported as prone to adhesions. Specific ‘tendon gliding’ exercises for the hand have been developed that target combined movements of the fingers to maintain full mobility of the flexor and extensor tendons. 84
Evidence to support ROM exercises is mixed, with some studies finding no change22,61,69 while others have reported some improvement or decline in the speed at which movement is lost. 19,20 A study looking at finger stretching exercises for sufferers of systemic sclerosis concluded that passive ROM was significantly improved in each finger after 1 month and this was further improved or maintained after 1 year. This involved one to three repetitions of each exercise with 10-second holds performed once a day. 85
Given the importance of sufficient flexibility in satisfactory functioning of the hand and the lack of evidence that ROM exercise causes harm, we included specific exercises in the experimental intervention developed for the SARAH trial in an attempt to improve, or at least maintain, mobility of the upper limb.
Expert opinion
A crucial part of the development programme was the advice received from clinicians and other experts, including patient groups. Most notably, a consensus meeting was held with specialist hand therapists from across England to gain further understanding of normal practice within typical NHS clinics and to assist in the design of the exercise intervention. The result of this discussion was a standardised protocol for usual care. Agreement was also reached as to what would constitute an effective and practical exercise programme for the hand and upper limb. It needed to be feasible to perform within NHS hand therapy clinics taking into account normal appointment duration and commonly available rehabilitation materials.
A list of upper limb exercises described in the literature, along with others proposed by various hand therapists, was examined in detail and decisions made as to which of these exercises were the most important to include in a programme specifically designed for RA. The selection was based on clinical relevance, a desire to include all functionally relevant movements/muscle actions of the hand and wrist, avoidance of replication and convenience/duration issues, especially with regard to the home exercise component. Initial load and volume, as well as progression and regression strategies, were also agreed.
Pilot study
The acceptability and feasibility of trial procedures and the exercise intervention were tested in a pilot study that ran from June 2009 to February 2010 and involved two senior therapists at two sites. The therapists received a half-day training session and a manual describing the trial and interventions. Sixteen participants were recruited and five were interviewed following their treatment to provide detailed feedback. Patient materials, exercise instructions and some trial procedures were modified following recommendations from therapists and patients as well as observations made by the trial team. Specifically, the assessment form, the Borg scale of perceived exertion of exercises86 and other forms used to document intervention delivery were altered along with instructions for the pinch grip and resisted wrist extension exercises. Information provided to patients at discharge from treatment was also developed following therapist recommendations.
The Strengthening And stretching for Rheumatoid Arthritis of the Hand intervention
The intervention was delivered by UK-registered physiotherapists and/or occupational therapists with expertise in rheumatology and hand rehabilitation. It occurred within individual sessions at typical NHS therapy outpatient clinics. All therapists were NHS employees who treated trial participants alongside their normal caseload. In most centres, therapists delivered both the usual care components and the exercise programme to participants. Other centres split this role so that one therapist provided usual care while another conducted the exercise sessions (Table 4).
| Treatment component | Therapists, n (%) (N = 48) | |
|---|---|---|
| Delivered both components | 34 (71) | |
| Delivered usual care component only | To usual care arm participants only | 1 (2) |
| To exercise arm participants only | 2 (4) | |
| To participants in both arms | 5 (10) | |
| Delivered exercise component only | 6 (13) | |
All sessions for both arms of the trial were to be completed within 12 weeks, after which time the patient was discharged (Figure 1). Patients receiving the experimental intervention were encouraged to continue with their programmes at home following discharge.
FIGURE 1.
Strengthening And stretching for Rheumatoid Arthritis of the Hand intervention flow chart.

Control arm: usual care only
The design of the usual care intervention was based on evidence from the literature and discussions with hand therapists. Clinical guidelines on the management of RA agree that the goals of management are symptom control, prevention or control of joint damage, reduction of disability, and maintenance or improvement of quality of life. 10,63,64
According to a Cochrane review in 2004, therapy for RA can include a variety of interventions including training of motor function, instruction on JP, advice and instruction in the use of assistive devices and provision of splints. 87 The most commonly provided treatments appear to be prescriptions of assistive devices, orthoses, hand-training instructions and patient education. 88 Evidence of effectiveness varies with relatively strong support for exercise and self-management interventions and modest support for JP programmes, orthoses and comprehensive care interventions. 89
The control arm treatment for the SARAH trial consisted of an initial assessment and advice session with the option of a further two follow-up sessions as necessary. Treatment included the provision of JP information, functional splinting, assistive devices and other general advice as required. In order to evaluate the effects of exercise, participants in the control arm were not prescribed any specific exercises for the upper limb. Apart from this, the content of the control arm was consistent with what is considered usual care according to discussions with specialist hand therapists, unpublished surveys of current practice and clinical guidelines. 31,66,67,87
Joint protection education
Joint protection is a self-management technique widely taught to people living with RA. The aim of JP in RA is to reduce pain, inflammation, joint stress and deformity through using assistive devices and alternative movement patterns of affected joints to perform everyday activities. Conventional JP strategies include pain management advice, planning and pacing activities, regular rest, altering patterns of joint movement and assistive device use. 28,90
Commonly available booklets from Arthritis Research UK were provided to reinforce information. 29,37,38 These included Rheumatoid Arthritis,38 a booklet providing general information about the disease and its management; Looking After Your Joints When You Have Arthritis,29 describing various self-management techniques and JP advice; and Keep Moving – How a few Simple Exercises can Make You Feel Better About Yourself and Your Arthritis,37 a booklet providing general exercise information along with suggestions as to specific exercises that could be performed for all parts of the body.
In 2004, Hammond and Freeman28 reported that an educational, behaviourally based patient education programme providing JP advice of 8–12 hours’ duration has a small positive effect in improving function. It is unclear how widespread this approach is in the UK, therefore the SARAH trial used more conventional forms of advice provision which involved individual advice provided within a normal treatment session with reinforcement at subsequent sessions if required.
Splinting
A survey of members of the British Association of Hand Therapists found that 100% of respondents reported regularly prescribing orthotics in the management of RA, usually in an effort to decrease hand and wrist pain and improve hand function. 66 There are two approaches to splinting. Resting wrist splints are prescribed mainly to reduce pain and other signs of inflammation and, to a lesser extent, to preserve function, although the evidence for their effectiveness is limited. 91 Functional wrist splints are used intermittently during functional activities in which resistance, weight or protracted positioning are likely to stress the hand and wrist. This type of ‘intermittent support’ splinting has more evidence of effectiveness than the ‘immobilisation’ resting splint approach. 92,93
The provision of functional wrist splints is common practice in the UK15 and, therefore, was not restricted in the SARAH trial as we expected their use to be similar in both arms of the study. In contrast, resting splints were not included as a treatment modality owing to some evidence suggesting little benefit from their use.
Summary
The control arm can be summarised as follows:
-
individual appointment(s) with a therapist (number of sessions dependent on clinical need up to a maximum of three sessions or 1.5 hours in total)
-
JP advice
-
provision of Arthritis Research Campaign (ARC) booklets containing further advice and exercise information
-
functional splinting as deemed necessary by the therapist
-
assistive devices as required
-
no resting splints provided
-
no explicit exercise prescription
-
no manual therapy (i.e. joint mobilisations) or electrotherapy
-
assessment and treatment documented using a standardised log.
Experimental arm: hand exercise plus usual care
The experimental arm consisted of the usual care described above plus a hand and upper limb exercise programme (Figure 2), which included seven mobility exercises and four strength exercises against resistance (i.e. therapy putty, Theraband or hand exerciser balls).
FIGURE 2.
Strengthening And stretching for Rheumatoid Arthritis of the Hand exercises.

The experimental intervention involved a total of six sessions, of which the last five were exercise sessions (Figure 3). Participants were provided with an exercise booklet with pictures and instructions describing the programme as well as the resistance materials required. They were asked to perform the programme daily at home between clinic sessions for a period of approximately 12 weeks.
FIGURE 3.
Content of SARAH interventions.

A modified Borg scale was used to set the load (resistance) for the strength exercises based on self-perception of effort. This 10-point version of the original Rate of Perceived Exertion scale86 has been validated for use in regulating the intensity of resistance exercise. 94 For each strength exercise, the level of resistance was determined by the subjects’ rating of perceived effort using the weaker hand in order to avoid overloading the more affected side. The load was purposefully set at a moderate level initially (3–4 on the scale) to permit subsequent progression, enhance motivation and adherence and reduce the possibility of increasing patient symptoms.
Although initial guidelines as to sets and repetitions (volume) of exercise were provided (Table 5), the programme was tailored to the abilities of the participant by progressing the load and volume of each exercise according to each patient’s capabilities. If necessary, the manner of executing a particular exercise could also be modified. For example, joint restrictions may prevent the degree of movement described in a particular exercise, in which case the participant achieved as much movement as possible within their available ROM. The overriding goal was for the participant to get as close as possible to performing the exercise in an ideal manner at a volume and load that was achievable while still providing a stimulus for physiological change. A defined protocol for both strength and ROM exercise progression (or regression) was provided for subsequent sessions according to both patient capability and therapist judgement (see Table 5).
| Exercise type | Exercise | Frequency | Sets | Repetitions | Initial hold | Initial load | Progression |
|---|---|---|---|---|---|---|---|
| Mobility | MCP flexion | Daily | 1 | × 5 | 5 seconds (where required) | – | Step 1: increase up to 10 repetitions Step 2: increase up to 10-second holds |
| Tendon gliding | |||||||
| Finger radial walking | |||||||
| Wrist circumduction | |||||||
| Finger abduction | |||||||
| Hand-behind-head | |||||||
| Hand-behind-back | |||||||
| Strength | Eccentric wrist extension | Daily | 1 | × 10 (minimum 8 repetitions; maximum 12 repetitions) | – | Between 3 and 4 on modified 10-point Borg scale | Step 1: 2 × 10 repetitions Step 2: 4–5 on Borg scale Step 3: 5–6 on Borg scale Step 4: 3 × 10 repetitions |
| Gross grip | |||||||
| Finger adduction | |||||||
| Pinch grip |
Adherence
Adherence to any exercise programme is vital to ensure that the dosage required to strengthen muscle and improve flexibility is achieved. Previous studies have found a dose–response relationship between prescribed exercise and improvement in strength and pain among patients with arthritis who adhere to the exercise programe. 24 It is especially important in the context of the SARAH trial as participants were required to perform the programme at home between sessions in order to provide a sufficient dose for physiological change to occur.
Unfortunately, patient adherence to home treatment programmes is typically low. 26 We aimed to maximise adherence to the prescribed exercise regimen by incorporating evidence-based strategies recommended for routine use by health professionals to promote patient behaviour change. 95 This involved a two-stage mechanism aimed at increasing the intention to adhere to the exercise regimen, along with enabling the translation of this behavioural intention into actual behaviour.
In collaboration with the therapist, participants set a hand-related functional goal that they hoped to achieve by carrying out the prescribed exercise regime, in accordance with SMART (specific, measurable, attainable, realistic and timely) principles. Therapists then went on to assess the patient’s confidence in successfully carrying out the exercise programme (self-efficacy) on a 10-point visual analogue scale (VAS) (0 = no confidence, 10 = highly confident). 95 A minimum level of self-efficacy for the exercise regimen was set (7/10) and, if necessary, we developed collaborative activities to identify barriers to, and facilitators of, exercise behaviour in order to boost confidence. The guiding philosophy was that behaviour is more likely to be performed if people believe they are able to perform it, and if it is perceived as relating to a personally relevant goal. 96
The intention to perform a behaviour does not always result in the actual behaviour being carried out. 97 In order to translate behavioural intention into action, Gollwitzer’s concept of implementation intentions98 was used. Implementation intentions link situational cues (i.e. good opportunities to act) with behavioural responses (in this case performing the prescribed exercise programme) that are effective in attaining desired outcomes (i.e. functional goal patient wishes to achieve). Participants were asked to specify when and where (i.e. situation) they would perform the prescribed exercise regimen, and to put this in writing, such that the implementation intention could be formed as ‘when situation Y is encountered, then I will initiate behaviour Z in order to reach goal X’. In other words, when the participant is in the designated place at the designated time, this will serve as a cue to perform the exercise programme in order to achieve their pre-determined functional goal. Implementation intentions have been shown to be effective in promoting the initiation of goal-related behaviour, and in sustaining such behaviour through the shielding of ongoing goal pursuit from unwanted influences. 99
Adherence was further supported through the use of exercise diaries, in which participants recorded their performance of prescribed exercises. In addition to assisting therapists in deciding on the need to modify the prescribed exercises, diaries promote important behaviour change techniques, including immediate feedback and self-monitoring. 100
Exercise diary
At each exercise session, participants were provided with a diary sheet (see Appendix 5) to record completion of the exercise programme during the appointment and for subsequent days until the next session. This served various purposes:
-
a reminder to perform the exercise programme daily, especially as the participant was aware that the therapist would review it at the next session
-
a means of gauging adherence to the programme
-
a method of initiating discussion regarding success (or lack of) in the performance of the programme since the last appointment
-
an aid in deciding on progression/regression of exercise programme.
There is evidence to suggest that the use of an exercise diary improves adherence to a home exercise programme, especially when participants are aware that programme performance will be monitored. 101
Goal setting and patient contract
At the end of the first exercise session, the goal-setting exercise was undertaken, with the participant stating both what they aimed to achieve as well as how they planned to achieve it. The aim was to increase compliance by attempting to strengthen the intention and motivation to perform the exercise programme and using action plans to convert this into actual behaviour. Goals and action plans were recorded and signed-off by both the therapist and participant. Both participant and therapist kept a copy of the form (see Appendix 6) which was reviewed, along with the exercise diary (see Appendix 5), at each exercise session.
Summary
The experimental intervention can be summarised as follows:
-
assessment and advice session plus five 30- to 45-minute exercise sessions spread over 12 weeks
-
content of usual care arm treatment.
-
an exercise programme aiming to improve strength, mobility and dexterity (including four strength exercises for the hand and seven mobility exercises of all the upper limb joints)
-
a home exercise plan with exercises performed daily
-
a standardised protocol for progression or regression
-
strategies to improve programme adherence including exercise diaries
-
no resting splints
-
no manual therapy or electrotherapy
-
assessment and treatment documented using a standardised log.
Therapist training and support
The intervention and rationale were documented in a manual in accordance with the principles of the Medical Research Council guidance for complex interventions. 102
To standardise the treatment provided, all therapists attended a training session (maximum of 4 hours depending on group size), including a practical element, at which they were instructed in how to treat participants according to the trial protocol. Therapists were provided with the treatment manuals which comprehensively described the interventions, including a session-by-session guide. None of the proposed interventions were beyond the scope of normal therapy practice.
The SARAH team was in contact with the treating therapists throughout the duration of the trial. Three update events were held during the intervention phase of the study to provide a forum for therapists to discuss any problems that had arisen. These were attended by therapists from 15 out of 17 trusts that were recruiting. The therapists unable to attend were visited to provide an update on the trial.
Monthly newsletters were also circulated among all involved therapists giving further advice and information regarding the trial, as required.
Quality control
All treatments provided during each session were recorded in a detailed log by therapists and returned to the trial centre once treatment had finished. These were reviewed, along with other trial paperwork, by the research fellow responsible for the design of the intervention (PH). Any digressions from protocol or queries about treatments provided were directed back to the therapists with advice, reinforcement or reminders regarding treatment protocols, as necessary. Information from the treatment logs was entered onto the trial database.
Quality control visits were performed to ensure adherence with intervention protocols. This involved the research fellow (PH) observing each therapist delivering a treatment session with a trial participant to ensure compliance with the protocols. Treatment logs and other paperwork associated with treatment of the participants were also reviewed. As well as ensuring consistency between therapists across all centres, it also served as an avenue for further support.
Chapter 4 Results
Trial sites
Centre characteristics
Seventeen UK NHS trusts (21 individual departments) participated in the study (Table 6). The recruitment period at each trust varied from 2 to 19 months with resulting variation in the number of participants recruited at each site.
| Trust name | Start date | Months of recruitment | Number of therapists |
|---|---|---|---|
| University Hospitals Coventry and Warwickshire NHS Trust | October 2009 | 19 | 6 |
| South Warwickshire NHS Foundation Trust | October 2009 | 19 | 2 |
| Basingstoke and North Hampshire Hospital NHS Foundation Trust | December 2009 | 15 | 3 |
| Wrightington, Wigan and Leigh NHS Trust | December 2009 | 17 | 3 |
| Royal National Hospital for Rheumatic Diseases NHS Foundation Trust | January 2010 | 17 | 2 |
| Winchester and Eastleigh Healthcare NHS Trust | January 2010 | 16 | 3 |
| Poole Hospital NHS Foundation Trust | March 2010 | 14 | 3 |
| Portsmouth Hospitals NHS Trust | March 2010 | 14 | 4 |
| Royal Bournemouth and Christchurch Hospitals NHS Trust | March 2010 | 14 | 1 |
| Dorset Primary Care Trust | April 2010 | 3a | 1 |
| Worcestershire Acute Hospitals NHS Trust | April 2010 | 13 | 5 |
| Nuffield Orthopaedic Centre NHS Trust | June 2010 | 11 | 5 |
| George Eliot NHS Trust | June 2010 | 11 | 2 |
| Heart of England NHS Trust | February 2011 | 3 | 1 |
| Sussex Community NHS Trust | March 2011 | 2 | 1 |
| University Hospitals of Leicester NHS Trust | March 2011 | 3 | 2 |
| Derby Hospitals NHS Foundation Trust | April 2011 | 2 | 4 |
Participating therapists
Forty-eight therapists were involved in providing treatment for both arms of the study (Table 7). Almost two-thirds were occupational therapists and the remainder physiotherapists. All were working in specialist hand therapy departments. The number of therapists involved at each site varied from one to six (see Table 6). The level of experience of each therapist (as determined by Agenda for Change pay banding103) is described in Table 7. The vast majority of therapists were on either band 6 or 7 and the median level of experience was 13 years.
| Agenda for Change band | Occupational therapists (n) | Physiotherapists (n) |
|---|---|---|
| 5 | 3 | 0 |
| 6 | 18 | 8 |
| 7 | 10 | 9 |
| Total a | 31 | 17 |
Participant flow
The overall flow of participants through the study is described in the CONSORT diagram (Figure 4). Further detail for each stage is provided in following sections.
FIGURE 4.
Consolidated Standards of Reporting Trials flow diagram.

Recruitment
Screening
Screening and recruitment took place between October 2009 and May 2011. A total of 1606 patients were screened in rheumatology clinics and on review lists (Table 8), of whom 512 (32%) were eligible and willing to attend a research clinic appointment (Table 9), with a further 564 (35%) not eligible – Table 10 displays, where known, the reasons for ineligibility. Of those eligible but not willing, no reason was given in the majority of cases (73%) (Table 11).
| Trust name | Screened | Recruited |
|---|---|---|
| University Hospitals Coventry and Warwickshire NHS Trust | 179 | 59 |
| South Warwickshire NHS Foundation Trust | 150 | 61 |
| Basingstoke and North Hampshire Hospital NHS Foundation Trust | 60 | 40 |
| Wrightington, Wigan and Leigh NHS Trust | 132 | 37 |
| Royal National Hospital for Rheumatic Diseases NHS Foundation Trust | 126 | 40 |
| Winchester and Eastleigh Healthcare NHS Trust | 239 | 37 |
| Poole Hospital NHS Foundation Trust | 202 | 37 |
| Portsmouth Hospitals NHS Trust | 114 | 31 |
| Royal Bournemouth and Christchurch Hospitals NHS Trust | 50 | 19 |
| Dorset Primary Care Trust | 10 | 5 |
| Worcestershire Acute Hospitals NHS Trust | 125 | 34 |
| Nuffield Orthopaedic Centre NHS Trust | 59 | 24 |
| George Eliot NHS Trust | 95 | 27 |
| Heart of England NHS Trust | 36 | 13 |
| Sussex Community NHS Trust | 10 | 7 |
| University Hospitals of Leicester NHS Trust | 11 | 11 |
| Derby Hospitals NHS Foundation Trust | 8 | 8 |
| Total | 1606 | 490 |
| Category | n (%) |
|---|---|
| Eligible, willing and research clinics appointment booked | 512 (32) |
| Eligible but not willing | 530 (33) |
| Not eligible (reason known) | 292 (18) |
| Not eligible (reason unknown) | 272 (17) |
| Total | 1606 |
| Reason | n (%) |
|---|---|
| Not RA | 49 (17) |
| Medicine change | 129 (44) |
| No hand/wrist problems | 34 (12) |
| Upper limb/surgery/waiting list | 21 (7) |
| Other reason | 59 (20) |
| Total | 292 |
| Reason | n (%) |
|---|---|
| Lack of time | 85 (16) |
| Travel | 38 (7) |
| Feel well | 23 (4) |
| No reason | 384 (73) |
| Total | 530 |
Recruitment
Of the 512 patients initially given appointments for baseline assessment, six were found to be subsequently ineligible, six did not attend or cancelled and 10 declined to participate, resulting in 490 patients being randomised (see Figure 4). Although the recruitment target of 480 was achieved in April 2011, a further 10 participants were recruited in early May 2011 as bookings made in the later stages before the target was reached were all honoured. The final recruitment total was 490 patients over a 20-month period. The proportion of participants in each arm was equivalent across all centres.
During the study, two participants recruited and allocated to the usual care arm withdrew from the study and also withdrew consent for their data to be used. Therefore, totals for subsequent analysis use a potential maximum of 488.
Baseline data
Baseline characteristics of participants by trial arm
The baseline characteristics of patients recruited to the trial are summarised in Table 12. The randomisation process appears to have been successful, with both study arms well matched in terms of demographic data, primary outcome measure and clinical assessment findings. Baseline RA status was similar in both groups with respect to disease activity (ESR and CRP levels) and disease duration (average ≈ 10 years), with both markers indicating a population with ‘stable’ RA (see Table 12).
| Baseline characteristic | Exercise programme | Usual care |
|---|---|---|
| Referral source, n (%) | ||
| N | 246 | 242 |
| Clinic referral | 198 (81) | 199 (82) |
| Mail out from review list | 48 (20) | 43 (18) |
| Age (years) | ||
| N | 246 | 242 |
| Mean (SD) | 61.3 (12) | 63.5 (11) |
| Median (IQR) | 63 (53–70) | 64 (57–72) |
| Minimum, maximum | 27, 94 | 24, 86 |
| Sex, n (%) | ||
| N | 246 | 242 |
| Male | 58 (24) | 58 (24) |
| Female | 188 (76) | 186 (76) |
| Ethnic group, n (%) | ||
| N | 246 | 240 |
| White | 238 (97) | 235 (98) |
| Indian | 3 (1) | 2 (1) |
| Pakistani | 0 (0) | 1 (< 1) |
| Mixed | 3 (1) | 1 (< 1) |
| Other | 2 (1) | 1 (< 1) |
| Marital status, n (%) | ||
| N | 246 | 241 |
| Single | 24 (10) | 10 (4) |
| Married | 157 (64) | 155 (64) |
| Separated | 4 (2) | 5 (2) |
| Divorced | 19 (8) | 24 (10) |
| Widowed | 28 (11) | 36 (15) |
| Cohabiting | 14 (6) | 11 (5) |
| Employment status, n (%) | ||
| N | 246 | 242 |
| Full-time employed | 29 (12) | 22 (9) |
| Part-time employed | 26 (11) | 30 (12) |
| Self-employed | 11 (5) | 10 (4) |
| Unpaid work | 3 (1) | 2 (1) |
| Unemployed | 12 (5) | 6 (3) |
| Full-time student | – | 1 (< 1) |
| Looking after home | 24 (10) | 20 (8) |
| Retired/economically inactive | 141 (57) | 151 (62) |
| Receiving any state benefits, n (%) | ||
| N | 243 | 240 |
| No | 110 (45) | 111 (46) |
| Yes | 133 (55) | 129 (54) |
| Educational level, n (%) | ||
| N | 239 | 237 |
| Higher degree | 26 (11) | 26 (11) |
| NVQ4/NVQ5/degree or equivalent | 18 (8) | 14 (6) |
| Higher education below degree | 23 (10) | 25 (11) |
| NVQ3/GCE A-level equivalent | 18 (8) | 25 (11) |
| NVQ2/GCE O-level equivalent | 41 (17) | 39 (17) |
| NVQ1/CSE other grade equivalent | 24 (10) | 10 (4) |
| Foreign/other | 9 (4) | 11 (5) |
| No qualification | 80 (34) | 87 (37) |
| Right/left hand dominant, n (%) | ||
| N | 246 | 240 |
| Right | 226 (92) | 215 (90) |
| Left | 18 (7) | 23 (9) |
| Not clearly one or the other | 2 (1) | 2 (1) |
| Gross annual household income, n (%) | ||
| N | 218 | 221 |
| < £10,000 | 59 (27) | 45 (20) |
| £10,000 to < £20,000 | 62 (28) | 82 (37) |
| £20,000 to < £30,0000 | 49 (23) | 41 (19) |
| £30,000 to < £40,000 | 18 (8) | 23 (10) |
| £40,000 to < £50,000 | 16 (7) | 15 (7) |
| ≥ £50,000 | 14 (6) | 15 (7) |
| Patient treatment preference, n (%) | ||
| N | 244 | 241 |
| Usual care | 11 (5) | 6 (3) |
| Usual care plus exercise | 100 (41) | 95 (39) |
| No preference | 133 (55) | 140 (58) |
| Years since RA diagnosis, estimated by participant | ||
| N | 218 | 218 |
| Median (IQR) | 10 (4–21) | 10 (4–22) |
| Baseline ESR | ||
| N | 188 | 185 |
| Median (IQR) | 15 (7–28) | 16 (8–28) |
| Baseline CRP | ||
| N | 219 | 210 |
| Median (IQR) | 5 (3–12) | 6 (3–12) |
| Previous fracture or surgery for hand, wrist or upper limb | ||
| n/N (%) | 75/212 (35) | 69/218 (32) |
Over 80% of participants were recruited from direct clinic referrals, the majority of them white females with a median age of 63 years (range 24–94 years).
Treatment preference was also well matched, with just over half stating no preference in both groups and another 40% preferring the exercise arm.
As part of the initial session with the therapist, participants were asked whether or not they had ever undergone surgery for the upper limb. Of the 430 responses, 144 (33%) replied ‘yes’, with similar proportions in both arms of the trial.
Baseline medications
The most common medications prescribed at baseline were non-biologic DMARDs. Approximately 20% of the cohort was on biologic DMARDs (Table 13). There was a small difference between arms regarding non-biologic DMARDs – the proportion of participants on combinations was slightly higher in the exercise programme arm than in the control arm while the proportion on single DMARDs was slightly lower (29.3% vs. 21.9% and 41.9% vs. 48.8% respectively).
| Prescribed current medications | Exercise programme | Usual care |
|---|---|---|
| Medication reported, N (%) | 246 (100) | 242 (100) |
| Treatment intensity medication group, n (%) | ||
| Biologic DMARDa | 52 (21) | 52 (22) |
| Combination non-biologic DMARDa | 72 (29) | 53 (22) |
| Single non-biologic DMARDa | 103 (42) | 118 (49) |
| No DMARD | 19 (8) | 19 (8) |
| Drug categories, n (%)b | ||
| Biologic DMARD | 52 (21) | 52 (22) |
| Single or combination non-biologic DMARD | 216 (88) | 208 (86) |
| Oral steroids | 49 (20) | 52 (22) |
| NSAIDs | 105 (43) | 100 (41) |
| Analgesics | 89 (36) | 84 (35) |
| Other prescribed medication | 91 (37) | 91 (38) |
| Additional ‘as required’ medication, n (%) | ||
| Extra NSAID(s) | 9 (4) | 15 (6) |
| Steroid tablet | 2 (1) | 5 (2) |
| Steroid injection into joint | 1 (< 1) | 0 (0) |
| Steroid injection into muscle | 2 (1) | 3 (1) |
Non-biologic DMARDs are older, less expensive immunosuppressant agents, such as methotrexate, which do not target individual cells or molecules specifically.
Baseline questionnaire scores
The scores for the questionnaires at baseline indicate that both arms of the study were similar (Table 14). The mean overall hand function score of the MHQ (primary outcome) was 52 for both groups out of a possible maximum of 100.
| Scales/measures | Exercise programme | Usual care | ||
|---|---|---|---|---|
| n | Mean (SD) | n | Mean (SD) | |
| MHQ (0–100; greater score = better function) | ||||
| MHQ overall hand function both | 246 | 52.1 (15.2) | 242 | 52.1 (16.4) |
| MHQ ADLs both | 244 | 54.5 (24.5) | 242 | 54.1 (25.0) |
| MHQ work | 246 | 48.2 (22.0) | 241 | 48.4 (22.0) |
| MHQ pain | 246 | 51.9 (21.9) | 242 | 51.4 (19.9) |
| MHQ aesthetics both | 245 | 56.9 (22.0) | 242 | 58.6 (22.1) |
| MHQ satisfaction both | 246 | 43.9 (19.7) | 241 | 43.5 (22.3) |
| MHQ overall score | 246 | 50.6 (16.4) | 242 | 50.9 (16.9) |
| SF-12 (population mean = 50, higher score is better HRQoL) | ||||
| SF-12 aggregate physical scale (PCS) | 246 | 33.8 (9.8) | 241 | 34.5 (9.5) |
| SF-12 aggregate mental scale (MCS) | 246 | 48.1 (10.7) | 242 | 48.9 (11.0) |
| SF-12 physical functioning | 245 | 34.6 (30.2) | 237 | 37.2 (30.4) |
| SF-12 role-physical | 243 | 45.0 (27.0) | 240 | 47.9 (24.7) |
| SF-12 bodily pain | 246 | 49.5 (26.8) | 242 | 50.6 (25.4) |
| SF-12 general health | 246 | 45.0 (27.2) | 242 | 46.4 (25.7) |
| SF-12 vitality | 246 | 38.2 (24.7) | 242 | 37.8 (26.1) |
| SF-12 social functioning | 246 | 69.5 (28.6) | 242 | 72.1 (27.4) |
| SF-12 role-emotional | 243 | 67.4 (30.1) | 240 | 70.5 (28.5) |
| SF-12 mental health | 245 | 62.9 (20.9) | 2239 | 64.2 (21.2) |
| EQ-5D-3L | ||||
| Health state (full health = 1.0) | 244 | 0.57 (0.29) | 240 | 0.59 (0.26) |
| VAS (your health today) | 246 | 66.1 (19.4) | 242 | 67.3 (18.1) |
| Other scores | ||||
| Pain troublesomeness overall score | 246 | 46.0 (22.2) | 242 | 48.5 (21.5) |
| Confidence in performing tasks overall score | 245 | 67.0 (20.3) | 242 | 68.7 (19.1) |
Baseline impairment measurements
Both study arms were well matched in terms of baseline impairment measures (Table 15). On average, participants had moderate deformity, reduced ROM and strength in the wrist and hand with some swollen and tender joints.
| Scores/counts | Exercise programme | Usual care | ||
|---|---|---|---|---|
| n a | Mean (SD) | n | Mean (SD) | |
| MCP joint deformity in degreesb | 245 | 6.8 (8.4) | 238 | 7.4 (9.4) |
| Active wrist ROM score in degreesc | 245 | 88.0 (29.6) | 239 | 90.1 (31.7) |
| Combined finger flexion in mmb | 245 | 13.0 (16.1) | 238 | 12.8 (16.1) |
| Composite finger extension in mmb | 244 | 21.3 (24.4) | 231 | 20.2 (25.2) |
| Thumb opposition scoreb | 246 | 8.2 (2.2) | 241 | 8.0 (2.1) |
| Swollen joint countd | 246 | 4.2 (4.8) | 241 | 4.1 (4.8) |
| Tender joint countd | 246 | 5.0 (5.4) | 241 | 4.8 (5.1) |
| Dexterity: nine-hole peg test in secondsb | 246 | 27.2 (8.2) | 240 | 27.3 (9.4) |
| Grip handle width used in mm | 245 | 22.3 (5.0) | 240 | 22.6 (4.3) |
| Maximum full hand grip force in newtonsb | 245 | 134.2 (83.3) | 240 | 130.3 (73.1) |
| Maximum pinch grip force in newtonsb | 243 | 40.2 (21.1) | 237 | 39.1 (19.6) |
Follow-up
Across the two arms, 452 (92%) and 438 (89%) participants were followed up at 4 and 12 months respectively (see Figure 4).
Table 16 displays the number of responses according to type of follow-up. The response rates were generally well balanced between the arms, although full response rate (i.e. came into clinic for follow-up) as were as total follow-up rate were slightly higher in the usual care arm than in the exercise programme arm at both 4 and 12 months.
| Type of questionnaire response | Baseline | 4-month follow-up | 12-month follow-up | |||
|---|---|---|---|---|---|---|
| Exercise programme, n (%) | Usual care, n (%) | Exercise programme, n (%) | Usual care, n (%) | Exercise programme, n (%) | Usual care, n (%) | |
| Questionnaires | ||||||
| Full response | 246 (100) | 242 (100) | 197 (80.1) | 211 (87.2) | 174 (70.7) | 190 (78.5) |
| Postal response | – | – | 20 (8.1) | 15 (6.2) | 33 (13.4) | 28 (11.6) |
| Telephone response | – | – | 7 (2.8) | 2 (0.8) | 9 (3.6) | 4 (1.7) |
| Total | 246 (100) | 242 (100) | 224 (91.1) | 228 (94.2) | 216 (87.8) | 222 (91.7) |
| Non-responder | – | – | 13 (5.3) | 10 (4.1) | 13 (5.3) | 10 (4.1) |
| Died | – | – | – | – | – | 2 (0.8) |
| Research clinic assessment formsa | ||||||
| Assessed | 246 (100) | 242 (100) | 197 (80.1) | 210 (86.8) | 174 (70.7) | 187 (77.3) |
| Medication detailsb | – | – | 15 (6.1) | 13 (5.4) | 37 (15.0) | 29 (12.0) |
| Total | 246 (100) | 242 (100) | 212 (86.2) | 223 (92.1) | 211 (85.8) | 216 (89.3) |
| Withdrawn | – | – | 9 (3.7) | 4 (1.7) | 8 (3.3) | 4 (1.7) |
Follow-up was completed in the time frames intended, with the median time from randomisation to follow-up being the same in the two groups at both 4 and 12 months (Table 17).
| Time period | Exercise programme, median (IQR) | Usual care, median (IQR) |
|---|---|---|
| Time to 4-month follow-up | 127 days (121–146 days) | 127 days (122–143 days) |
| Time to 12-month follow-up | 371 days (361–390 days) | 371 days (364–386 days) |
Withdrawals
In total, 25 participants (5%) withdrew from the trial during follow-up, with a greater proportion of these in the experimental arm (n = 17 vs. n = 8 for usual care). Table 18 presents the timings of the withdrawals from the trial by arm. In the exercise programme arm, the greatest proportion of participants withdrew from the trial at the time of their treatment. There was no dominant reason for withdrawal in either arm (Table 19). If participants withdrew from trial treatments, every effort was made to obtain follow-up data from these participants (withdrawal from treatments and withdrawal from trial were differentiated on the event notification form).
| Time of withdrawal from trial | Exercise programme, n (%) | Usual care, n (%) | Total, n (%) |
|---|---|---|---|
| Prior to treatment | 2 (0.8) | 1 (0.4) | 3 (0.6) |
| During treatment | 8 (3.3) | 2 (0.8) | 10 (2.0) |
| After treatment, before 4-month follow-up | 0 (0) | 1 (0.4) | 1 (0.2) |
| Between 4-month and 12-month follow-upa | 7 (2.8) | 4 (1.6) | 11 (2.3) |
| Total | 17 (6.9) | 8 (3.3) | 25 (5.1) |
| Reason | Exercise programme, n (%) | Usual care, n (%) | Total, n (%) |
|---|---|---|---|
| Travelling/time | 4 (24) | 2 (25) | 6 (24) |
| Poor health | 4 (24) | 2 (25) | 6 (24) |
| Unable to attend | 2 (12) | 1 (13) | 3 (12) |
| Does not want to/not committed | 3 (18) | 1 (13) | 4 (16) |
| Not in intervention arm | – | 2 (25) | 2 (8) |
| Reason not given | 4 (24) | – | 4 (16) |
| Total | 17 | 8 | 25 |
Comparisons of those retained compared with those lost to follow-up
There was no evidence of differences in sex or disease duration between those who did and did not complete follow-up at 12 months. Those with 12 months’ follow-up were, on average, 5 years older than those without and had higher baseline scores for MHQ hand function, SF-12 summary scores and EQ-5D-3L health state. These differences were greater in the exercise programme arm. Table 20 shows the characteristics of participants completing and not completing 12 months’ follow-up.
| Characteristic by arm | Participants completing 12-month follow-up | Participants not completing 12-month follow-up | p-value for difference |
|---|---|---|---|
| Exercise programme | |||
| Age at randomisation (years), mean (SD) | 62.0 (11.9) | 56.1 (14.6) | 0.0175 |
| Sex (percentage female) | 76.9 | 73.3 | 0.6705 |
| Disease duration (years), mean (SD) | 13.1 (11.0) | 13.2 (8.7) | 0.5461 |
| Baseline MHQ hand function, mean (SD) | 52.7 (15.2) | 47.3 (14.2) | 0.0678 |
| SF-12 PCS, mean (SD) | 34.3 (9.5) | 29.7 (10.4) | 0.0147 |
| SF-12 MCS, mean (SD) | 48.8 (10.6) | 43.0 (10.6) | 0.0049 |
| EQ-5D-3L health state, mean (SD) | 0.6 (0.3) | 0.4 (0.4) | 0.0116 |
| Usual care | |||
| Age (years), mean (SD) | 63.8 (11.1) | 60.5 (12.8) | 0.2785 |
| Sex (percentage female) | 76.6 | 70.0 | 0.5093 |
| Disease duration (years), mean (SD) | 13.9 (11.9) | 15.9 (13.3) | 0.6105 |
| Baseline MHQ hand function, mean (SD) | 52.8 (16.1) | 44.1 (18.0) | 0.0226 |
| SF-12 PCS, mean (SD) | 34.4 (9.6) | 35.2 (8.9) | 0.7063 |
| SF-12 MCS, mean (SD) | 49.2 (10.9) | 45.6 (12.1) | 0.1561 |
| EQ-5D-3L health state, mean (SD) | 0.6 (0.3) | 0.6 (0.3) | 0.7110 |
| Combined | |||
| Age, mean (SD) | 62.9 (11.5) | 57.8 (14.0) | 0.0080 |
| Sex (percentage female) | 76.7 | 72.0 | 0.4583 |
| Disease duration (years), mean (SD) | 13.5 (11.5) | 14.4 (10.8) | 0.4280 |
| Baseline MHQ hand function, mean (SD) | 52.8 (15.7) | 46.0 (15.7) | 0.0042 |
| SF-12 PCS, mean (SD) | 34.4 (9.5) | 31.9 (10.1) | 0.0888 |
| SF-12 MCS, mean (SD) | 49.0 (10.7) | 44.0 (11.2) | 0.0020 |
| EQ-5D-3L health state, mean (SD) | 0.6 (0.3) | 0.5 (0.4) | 0.0197 |
Blinding
Researchers measuring outcome were asked if they knew the allocation of participants or were able to guess; we received 278 responses to this question at 12 months (Table 21). Of these, 173/278 (62%) correctly identified the treatment received and 105/278 (38%) did not. The distribution was similar for usual care and exercise therapy, i.e. the probability that patients would correctly identify the treatment received was marginally higher than would be expected by chance (50%), but this probability was the same in both arms. The knowledge of treatment allocation had no effect in a sensitivity analysis of the primary outcome (p = 0.654 at 4 months, p = 0.407 at 12 months).
| Actual group allocation | |||||
|---|---|---|---|---|---|
| Exercise programme | Usual care | ||||
| 4 months, n (%) | 12 months, n (%) | 4 months, n (%) | 12 months, n (%) | ||
| Clinician’s opinion | Usual care | 39 (25.5) | 51 (38.3) | 94 (63.9) | 91 (62.8) |
| Exercise programme | 114 (74.5) | 82 (61.7) | 53 (36.1) | 54 (37.2) | |
| Total | 153 | 133 | 147 | 145 | |
| Missing | 59 | 78 | 76 | 46 | |
Missing data
The proportion of missing data from the self-report questionnaires across all time points was generally low, with the rate exceeding 10% for only one item (diagnosis date at baseline). For the majority of measures, data were missing in less than 5% of cases, and both arms were relatively well-matched across all time points (Table 22).
| Missing items/scales questionnaires | Baseline | 4 month | 12 month | |||
|---|---|---|---|---|---|---|
| Exercise programme, n (%) (N = 246) | Usual care, n (%) (N = 242) | Exercise programme, n (%) (N = 224) | Usual care, n (%) (N = 228) | Exercise programme, n (%) (N = 216) | Usual care, n (%) (N = 222) | |
| Date of birth | 0 (0) | 0 (0) | NA | NA | NA | NA |
| RA diagnosis date | 28 (11.4) | 24 (9.9) | NA | NA | NA | NA |
| Date of completion | 0 (0) | 0 (0) | 2 (0.9) | 0 (0) | 2 (0.9) | 0 (0) |
| Days off work | 2 (0.8) | 4 (1.7) | 10 (4.5) | 4 (1.8) | 13 (6) | 7 (3.2) |
| Benefit information | 3 (1.2) | 3 (1.2) | 12 (5) | 13 (6) | 15 (7) | 11 (5%) |
| MHQ | ||||||
| MHQ: one or two missing (out of 57) | 14 (5.7) | 21 (8.7) | 10 (4.5) | 20 (9) | 13 (6.0) | 12 (5) |
| MHQ: more than two missing items | 3 (1.2) | 5 (2.1) | 18 (8) | 12 (5) | 3 (1.4) | 2 (0.9) |
| MHQ: overall hand function botha | 0 (0) | 0 (0) | 2 (0.9) | 1 (0.4) | 0 (0) | 0 (0) |
| MHQ: overall score both | 0 (0) | 0 (0) | 1 (0.4) | 0 (0) | 0 (0) | 0 (0) |
| Secondary outcomes | ||||||
| Pain troublesomeness: any missing | 0 (0) | 0 (0) | 9 (4.0) | 4 (1.8) | 11 (5) | 5 (2.3) |
| Confidence in tasks: any missing | 1 (0.4) | 2 (0.8) | 9 (4.0) | 3 (1.3) | 10 (5) | 6 (2.7) |
| Self-reported efficacy | 0 (0) | 0 (0) | 8 (3.6) | 4 (1.8) | 10 (5) | 8 (3.6) |
| EQ-5D-3L any items | 2 (0.8) | 2 (0.8) | 0 (0) | 0 (0) | 2 (0.9) | 3 (1.4) |
| SF-12 missing one or two items | 7 (2.8) | 8 (3.3) | 9 (4.0) | 5 (2.2) | 8 (3.7) | 4 (1.8) |
| SF-12 missing more than two items | 0 (0) | 1 (0.4) | 8 (3.6) | 5 (2.2) | 9 (4.2) | 5 (2.3) |
| SF-12 aggregate scales | 7 (2.8) | 9 (3.7) | 17 (8) | 10 (4.4) | 17 (8) | 9 (4.1) |
| SF-12 aggregate scales (with imputation) | 0 (0) | 1 (0.4) | 6 (2.7) | 2 (0.9) | 9 (4.2) | 5 (2.3) |
| Health resource usage sections | ||||||
| NHS services because of RA | NA | NA | 5 (2.2) | 5 (2.2) | 11 (5) | 10 (5) |
| Nights in NHS hospital (RA) | NA | NA | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Nights in NHS hospital (not RA) | NA | NA | 6 (2.7) | 1 (0.4) | 3 (1.4) | 2 (0.9) |
| NHS day case | NA | NA | 2 (0.9) | 1 (0.4) | 3 (1.4) | 3 (1.4) |
| NHS tests (RA) | NA | NA | 3 (1.3) | 7 (3.1) | 5 (2.3) | 9 (4.1) |
| NHS devices | NA | NA | 3 (1.3) | 4 (1.8) | 1 (0.5) | 5 (2.3) |
| Private health care (insurance) | NA | NA | 11 (5) | 12 (5) | 17 (8) | 12 (5) |
| Private hospital nights | NA | NA | 8 (3.6) | 7 (3.1) | 11 (5) | 13 (6) |
| Private hospital as a day case | NA | NA | 7 (3.1) | 9 (3.9) | 13 (6) | 8 (3.6) |
| Private hospital tests (RA) | NA | NA | 6 (2.7) | 4 (1.8) | 13 (6) | 11 (5) |
| Medication bought (RA) | NA | NA | 7 (3.1) | 6 (2.6) | 10 (5) | 7 (3.2) |
| Medical devices bought | NA | NA | 12 (5) | 16 (7) | 14 (6) | 10 (5) |
The pattern of low levels of missing data was repeated for the Research Clinic Assessment forms containing the impairment and disease activity measures (Table 23). Relatively higher levels of missing data for both arms were recorded for the 4- and 12-month follow-ups as compared with the questionnaires, which reflects the fact that these data were not collected for telephone or postal follow-ups.
| Missing items/scales clinical assessment forms | Baseline | 4-month follow-up | 12-month follow-up | |||
|---|---|---|---|---|---|---|
| Exercise programme, n (%) (N = 246) | Usual care, n (%) (N = 242) | Exercise programme, n (%) (N = 212) | Usual care, n (%) (N = 223) | Exercise programme, n (%) (N = 211) | Usual care, n (%) (N = 216) | |
| CRP and ESR | 10 (4%) | 10 (4%) | 25 (12%) | 41 (18%) | 51 (24%) | 40 (19%) |
| MCP joint deformity | 1 (< 1%) | 4 (2%) | 15 (7%) | 18 (8%) | 38 (18%) | 32 (15%) |
| Active wrist flexion/extension | 1 (< 1%) | 3 (1%) | 16 (8%) | 17 (8%) | 38 (18%) | 33 (15%) |
| Combined finger flexion | 1 (< 1%) | 4 (2%) | 17 (8%) | 17 (8%) | 38 (18%) | 31 (14%) |
| Composite finger extension | 3 (1%) | 11 (5%) | 16 (8%) | 24 (11%) | 39 (18%) | 35 (16%) |
| Thumb opposition | 5 (2%) | 3 (1%) | 16 (8%) | 18 (8%) | 39 (18%) | 31 (14%) |
| Swollen/tender joint count | – | 1 (< 1%) | 16 (8%) | 15 (7%) | 37 (18%) | 29 (13%) |
| Timed dexterity | – | 2 (1%) | 16 (8%) | 16 (7%) | 38 (18%) | 31 (14%) |
| Full and pinch grip strength | 5 (2%) | 6 (3%) | 20 (9%) | 23 (10%) | 47 (22%) | 38 (18%) |
Study treatments
Treatment attendance rates
Treatments were generally well attended, with 93% of participants completing the usual care treatment and 93% of participants partially or entirely completing the exercise programme (75% completed all six sessions). For the exercise programme, completion of treatment was defined as attendance at assessment and all five treatment sessions, and partial completion was between two and five exercise sessions. For usual care, completion of treatment was defined as attending all booked sessions (completion of treatment in usual care arm may include only attending for assessment session). The proportion of participants not attending any treatment sessions was roughly equal between the arms (Table 24). For the exercise programme there was a gradual attrition of patients as the sessions went on (Table 25). A quality control programme evaluated 38 of the 48 therapists, all of whom were following the protocol satisfactorily.
| Type of attendance | Exercise programme (maximum six sessions), n (%) | Usual care (maximum three sessions), n (%) |
|---|---|---|
| Median number of sessions | 6 | 1 |
| Failed to attend any appointment | 8 (3) | 7 (3) |
| Attended for assessment only | 8 (3) | 135 (56) |
| Partial completion of treatment | 46 (19) | 10 (4) |
| Completed treatment | 184 (75) | 225 (93) |
| Arm | Session 1, n (%) | Session 2, n (%) | Session 3, n (%) | Session 4, n (%) | Session 5, n (%) | Session 6, n (%) | Extra sessions, n (%) |
|---|---|---|---|---|---|---|---|
| Exercise programme | 238 (97) | 230 (94) | 219 (89) | 201 (82) | 188 (76) | 185 (75) | 2 (1) |
| Usual care | 236 (98) | 99 (41) | 27 (11) | – | – | – | 6 (3) |
Timing of delivery of interventions
Table 26 presents median times from randomisation to first and last appointment by arm. Interventions were largely delivered within 3 weeks of randomisation and completed within 3 months and 1 month for the experimental and control interventions respectively. Time to initial appointment was similar in both groups with the differences in treatment arm protocols reflected in the time to their final session.
| Period | Exercise programme, median (IQR) | Usual care, median (IQR) |
|---|---|---|
| Time from randomisation to first appointment attendance | 20 days (13–33 days) | 19 days (12–34 days) |
| Time from randomisation to last appointment attendance | 3.2 months (2.7–4.0 months) | 1.1 months (0.5–1.7 months) |
Type of treatment
Therapists completed treatment logs for each session detailing the types of treatment provided. Table 27 provides a summary of the treatment logs by arm and per session. Note that the usual care arm involved a maximum of three sessions only, although most participants in this arm received only one session. The figures indicate a high level of compliance with the treatment protocols with regard to provision of JP advice, information and completion of exercise arm paperwork.
| Treatment | Session 1 | Session 2 | Session 3 | Session 4 | Session 5 | Session 6 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment log data | Usual care, n (%) | Exercise programme, n (%) | Usual care, n (%) | Exercise programme, n (%) | Usual care, n (%) | Exercise programme, n (%) | Usual care, n (%) | Exercise programme, n (%) | Usual care, n (%) | Exercise programme, n (%) | Usual care, n (%) | Exercise programme, n (%) |
| Provided JP advice | 217 (92.3) | 220 (92.4) | 69 (69.7) | – | 8 (29.6) | – | – | – | – | – | – | – |
| Provided ARC booklets | 220 (93.6) | 222 (93.3) | – | – | – | – | – | – | – | – | – | – |
| Provided/modified/reviewed functional splinting? | 103 (43.8) | 98 (41.2) | 78 (78.8) | 89 (38.9) | 19 (70.4) | 68 (31.1) | – | 61 (30.3) | – | 50 (26.6) | – | 60 (32.4) |
| Helped patient complete exercise diary? | – | – | – | 210 (91.7) | – | 206 (94.1) | – | 181 (90.0) | – | 175 (93.1) | – | 172 (93.0) |
| Helped patient complete/reviewed Personal exercise guide? | – | – | – | 201 (87.8) | – | 187 (85.4) | – | 169 (84.1) | – | 163 (86.7) | – | 168 (90.8) |
| Ran through discharge advice? | – | – | – | – | – | – | – | – | – | – | – | 169 (91.4) |
| Discussed continuing with exercise programme? | – | – | – | – | – | – | – | – | – | – | – | 169 (91.4) |
A similar level of splinting was provided for participants in both arms of the trial. The higher percentages for subsequent sessions in the usual care arm reflect the fact that, for the majority of these participants, follow-up appointments were made with the express purpose of either providing a splint when identified as necessary in the initial session or reviewing and/or modifying those splints that were provided at the first session.
Exercise programme progression
During the therapist training, emphasis was placed on the need to progress the exercise programmes as much as possible during the 3 months of treatment. A treatment log was completed for each session recording the details of the programme provided and allowing an estimation of treatment progression for those completing all six sessions. In this case, progression was defined as an increase in repetitions, sets or resistance between the first and last session. Maintained indicates no changes in exercise parameters between the first and last sessions, whereas regressed is a decrease in repetitions, sets or resistance between the first and last sessions. Table 28 describes the proportion of participants who were progressed, maintained or regressed for each exercise. This illustrates that the vast majority of participants progressed in at least one of the exercise variables (repetitions, sets, and/or resistance). Variation in the number of participants prescribed each exercise is because of the individualised nature of the programme.
| Exercise | Prescribed | Progressed, n (%) | Maintained, n (%) | Regressed, n (%) | Unknown, n (%) |
|---|---|---|---|---|---|
| MCP flexion | 180 | 146 (81.1) | 26 (14.4) | 2 (1.1) | 6 (3.3) |
| Tendon gliding | 180 | 136 (75.6) | 31 (17.2) | 6 (3.3) | 7 (3.9) |
| Radial walking | 177 | 145 (81.9) | 23 (13.0) | 3 (1.7) | 6 (3.4) |
| Wrist circumduction | 179 | 146 (81.6) | 22 (12.3) | 5 (2.8) | 6 (3.4) |
| Finger abduction | 179 | 149 (83.2) | 22 (12.3) | 2 (1.1) | 6 (3.4) |
| Hand behind head | 179 | 129 (72.1) | 39 (21.8) | 5 (2.8) | 6 (3.4) |
| Hand behind back | 179 | 128 (71.5) | 41 (22.9) | 3 (1.7) | 7 (3.9) |
| Wrist extension | 174 | 113 (64.9) | 41 (23.6) | 19 (10.9) | 1 (0.6) |
| Gross grip | 180 | 151 (83.9) | 21 (11.7) | 7 (3.9) | 1 (0.6) |
| Finger adduction | 177 | 114 (64.4) | 44 (24.9) | 18 (10.2) | 1 (0.6) |
| Pinch grip | 178 | 132 (74.2) | 34 (19.1) | 11 (6.2) | 1 (0.6) |
Outcomes and estimation
Numbers analysed
Data from a total of 488 participants were received at baseline (246 participants in exercise arm; 242 participants in usual care). At 4 months, this was reduced to 454 participants (222 participants in exercise arm; 227 participants in usual care) and at 12 months to 438 participants (216 participants in exercise arm; 222 participants in usual care).
Primary outcome: overall hand function
The primary outcome was the overall hand function subscale of the MHQ at 12 months. On average, both groups’ hand function scores improved over time although within group changes of the exercise programme group were approximately twice those of the usual care group (Figure 5 and Tables 29 and 30).
FIGURE 5.
Scores over time for MHQ overall hand function.
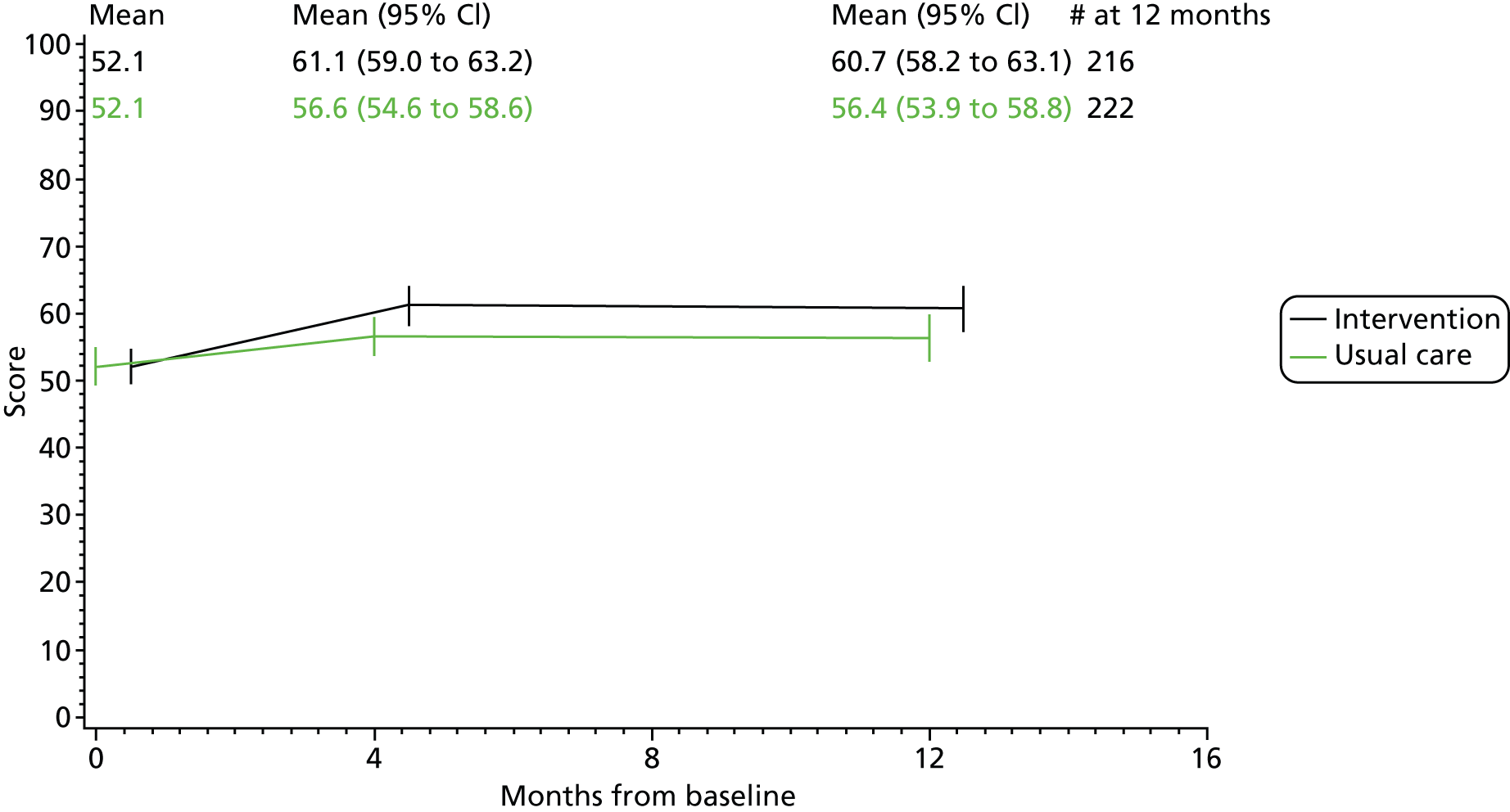
| Questionnaire | Exercise programme, mean (SD) | Usual care, mean (SD) | ||||
|---|---|---|---|---|---|---|
| Baseline | 4 months | 12 months | Baseline | 4 months | 12 months | |
| MHQ overall hand function (both hands)a | 52.1 (15.2) | 61.1 (16.0) | 60.7 (18.1) | 52.1 (16.4) | 56.6 (15.6) | 56.4 (18.6) |
| MHQ overall hand function (both hands)a | Number of patients | Mean change (95% CI) | Mean treatment difference (95% CI) | p-value | Standardised mean difference (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Exercise programme | Usual care | Exercise programme | Usual care | ||||
| 4 months | |||||||
| 222 | 227 | 8.73 (6.83 to 10.64) | 4.04 (2.17 to 5.91) | 4.60 (2.22 to 6.97) | 0.0002 | 0.30 (0.13 to 0.47) | |
| Adjusted for centre, sex and age | 4.91 (2.50 to 7.32) | 0.0001 | – | ||||
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 4.71 (2.32 to 7.11) | 0.0001 | – | ||||
| 12 months | |||||||
| 216 | 222 | 7.93 (5.98 to 9.88) | 3.56 (1.45 to 5.68) | 4.35 (1.60 to 7.10) | 0.0020 | 0.28 (0.09 to 0.46) | |
| Adjusted for centre, sex and age | 4.25 (1.46 to 7.03) | 0.0030 | – | ||||
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 4.28 (1.49 to 7.06) | 0.0028 | – | ||||
With regard to between group differences, participants allocated to the exercise programme had statistically significantly greater hand function compared with those receiving usual care at both 4 and 12 months. Adjustment for baseline differences between groups for centre, sex, age and medication usage resulted in a small increase in the mean treatment difference at 4 months and a small decrease at 12 months (Table 30).
Other self-report outcomes
Other Michigan Hand Outcome Questionnaire scores
The findings for the primary outcome were replicated for the ADLs subscale and the overall score of the MHQ at 4 and 12 months, and the work subscale at 12 months (Tables 31 and 32).
| Questionnaire | Exercise programme, mean (SD) | Usual care, mean (SD) | ||||
|---|---|---|---|---|---|---|
| Baseline | 4 months | 12 months | Baseline | 4 months | 12 months | |
| MHQ ADLs (both hands) | 54.5 (24.5) | 62.9 (24.1) | 61.3 (25.6) | 54.1 (25.0) | 56.9 (25.5) | 56.5 (27.5) |
| MHQ work | 48.2 (22.0) | 55.4 (24.3) | 57.8 (25.0) | 48.4 (22.0) | 54.0 (24.1) | 52.2 (24.9) |
| MHQ pain | 51.9 (21.9) | 43.3 (23.6) | 41.6 (24.3) | 51.4 (19.9) | 46.1 (21.1) | 45.3 (22.6) |
| MHQ aesthetics (both hands) | 56.9 (22.0) | 61.6 (23.1) | 63.2 (24.1) | 58.6 (22.1) | 61.7 (22.6) | 62.1 (22.7) |
| MHQ satisfaction (both hands) | 43.9 (19.7) | 54.1 (23.5) | 55.5 (23.6) | 43.5 (22.3) | 50.0 (22.6) | 50.9 (24.5) |
| MHQ overall score | 50.6 (16.4) | 58.6 (18.4) | 59.5 (19.4) | 50.9 (16.9) | 55.4 (17.9) | 55.5 (20.1) |
| MHQ | Number of patients | Mean change (95% CI) | Mean treatment difference (95% CI) | p-value | Standardised mean difference (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Exercise programme | Usual care | Exercise programme | Usual care | ||||
| MHQ ADLs (both hands) | |||||||
| 4 months | 220 | 228 | 7.86 (5.44 to 10.28) | 2.57 (–0.40 to 4.74) | 5.46 (2.41 to 8.51) | 0.0005a | 0.21 (0.08 to 0.35) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 5.66 (2.64 to 8.69) | 0.0003a | – | ||||
| 12 months | 214 | 222 | 5.89 (3.66 to 8.13) | 2.27 (–0.04 to 4.59) | 3.79 (0.64 to 6.93) | 0.0187a | 0.15 (0.02 to 0.28) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 3.48 (0.31 to 6.66) | 0.0321a | – | ||||
| MHQ work | |||||||
| 4 months | 220 | 225 | 6.12 (3.68 to 8.56) | 5.27 (2.62 to 7.92) | 1.00 (–2.43 to 4.43) | 0.5671 | 0.04 (–0.13 to 0.20) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 1.04 (–2.39 to 4.48) | 0.5518 | – | ||||
| 12 months | 215 | 221 | 8.12 (5.36 to 10.87) | 3.11 (0.23 to 5.98) | 5.21 (1.44 to 8.99) | 0.0071a | 0.23 (0.05 to 0.41) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 4.62 (0.82 to 8.42) | 0.0175a | – | ||||
| MHQ painb | |||||||
| 4 months | 219 | 226 | –7.60 (–9.94 to –5.26) | –5.11 (–7.58 to –2.63) | –2.61 (–5.79 to 0.58) | 0.1096 | –0.12 (–0.28 to 0.04) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | –3.30 (–6.50 to –0.11) | 0.0433a | – | ||||
| 12 months | 215 | 222 | –8.26 (–10.83 to –5.70) | –6.01 (–8.74 to –3.29) | –2.70 (–6.23 to 0.83) | 0.1341 | –0.11 (–0.29 to 0.07) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | –2.40 (–5.92 to 1.12) | 0.1814 | – | ||||
| MHQ aesthetics (both hands) | |||||||
| 4 months | 218 | 224 | 3.52 (0.89 to 6.14) | 2.84 (0.27 to 5.41) | 0.40 (–2.96 to 3.76) | 0.8152 | 0.03 (–0.14 to 0.20) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 0.39 (–2.96 to 3.74) | 0.8209 | – | ||||
| 12 months | 215 | 222 | 4.70 (1.81 to 7.59) | 3.37 (0.42 to 6.33) | 1.29 (–2.43 to 5.01) | 0.4967 | 0.06 (–0.13 to 0.25) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 1.01 (–2.70 to 4.72) | 0.5933 | – | ||||
| MHQ satisfaction (both hands) | |||||||
| 4 months | 221 | 224 | 9.59 (6.86 to 12.32) | 6.66 (4.01 to 9.31) | 3.29 (–0.22 to 6.80) | 0.0665 | 0.14 (–0.04 to 0.32) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 3.61 (0.12 to 7.09) | 0.0430a | – | ||||
| 12 months | 216 | 220 | 10.36 (7.53 to 13.18) | 7.06 (4.16 to 9.95) | 3.76 (–0.02 to 7.54) | 0.0517a | 0.16 (–0.04 to 0.35) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 3.38 (–0.37 to 7.13) | 0.0784 | – | ||||
| MHQ overall score | |||||||
| 4 months | 223 | 228 | 7.28 (5.65 to 8.91) | 4.34 (2.67 to 6.00) | 2.98 (0.73 to 5.24) | 0.0098a | 0.18 (0.04 to 0.32) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 3.17 (0.91 to 5.43) | 0.0063a | – | ||||
| 12 months | 216 | 222 | 7.59 (5.75 to 9.43) | 4.22 (2.23 to 6.21) | 3.48 (0.82 to 6.15) | 0.0108a | 0.20 (0.04 to 0.37) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 3.21 (0.53 to 5.89) | 0.0195a | – | ||||
No statistically significant difference was found between groups for the rest of the MHQ subscales including pain, although both groups’ pain improved over the follow-up time.
Health-related quality of life (Short Form Questionnaire-12 items and European Quality of Life-5 Dimensions)
There were no significant differences between the groups for overall health-related quality of life (HRQoL) scores as measured by the EQ-5D-3L and SF-12 (see Tables 33–36). There were significant differences in the SF-12 subscales roles physical and emotional, and the EQ-5D-3L health thermometer at 12 months. Effect sizes for these components were small.
| Questionnaire | Exercise programme, mean (SD) | Usual care, mean (SD) | ||||
|---|---|---|---|---|---|---|
| SF-12 aggregate physical scale | 33.8 (9.8) | 36.4 (10.5) | 35.6 (10.3) | 34.5 (9.5) | 35.4 (9.9) | 34.4 (10.2) |
| SF-12 aggregate mental scale | 48.1 (10.7) | 49.2 (10.4) | 51.1 (10.2) | 48.9 (11.0) | 49.7 (10.4) | 49.6 (9.9) |
| SF-12 physical functioning | 34.6 (30.2) | 42.3 (32.2) | 39.7 (32.0) | 37.2 (30.4) | 39.8 (31.0) | 37.9 (31.0) |
| SF-12 role-physical | 45.0 (27.0) | 51.6 (27.0) | 53.3 (26.5) | 47.9 (24.7) | 50.5 (25.0) | 48.5 (26.2) |
| SF-12 bodily pain | 49.5 (26.8) | 57.3 (26.6) | 57.7 (27.6) | 50.6 (25.4) | 54.3 (25.8) | 53.1 (26.4) |
| SF-12 general health | 45.0 (27.2) | 48.4 (27.1) | 48.8 (27.8) | 46.4 (25.7) | 48.8 (26.6) | 46.2 (25.5) |
| SF-12 vitality | 38.2 (24.7) | 40.2 (25.7) | 40.5 (25.3) | 37.8 (26.1) | 39.7 (25.3) | 37.3 (25.8) |
| SF-12 social functioning | 69.5 (28.6) | 72.8 (28.2) | 74.9 (27.0) | 72.1 (27.4) | 71.7 (26.9) | 71.8 (25.9) |
| SF-12 role-emotional | 67.4 (30.1) | 71.9 (29.0) | 77.5 (27.0) | 70.5 (28.5) | 73.5 (27.5) | 72.8 (27.1) |
| SF-12 mental health | 62.9 (20.9) | 66.3 (20.5) | 68.5 (21.0) | 64.2 (21.2) | 66.9 (20.4) | 66.4 (19.8) |
| SF-12 | Number of patients | Mean change (95% CI) | Mean treatment difference (95% CI) | p-value | Standardised mean difference (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Exercise programme | Usual care | Exercise programme | Usual care | ||||
| SF-12 aggregate physical scale (PCS) | |||||||
| 4 months | 218 | 225 | 2.04 (1.01 to 3.08) | 0.91 (0.03 to 1.80) | 1.08 (–0.22 to 2.37) | 0.1043 | 0.12 (–0.02 to 0.26) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 1.18 (–0.11 to 2.46) | 0.0743 | – | ||||
| 12 months | 207 | 216 | 1.19 (0.23 to 2.14) | 0.03 (–0.96 to 1.03) | 1.14 (–0.19 to 2.46) | 0.0931 | 0.12 (–0.02 to 0.26) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 0.93 (–0.35 to 2.22) | 0.1555 | – | ||||
| SF-12 aggregate mental scale (MCS) | |||||||
| 4 months | 218 | 225 | 0.46 (–0.66 to 1.59) | 0.58 (–0.56 to 1.73) | –0.26 (–1.70 to 1.18) | 0.7215 | –0.01 (–0.16 to 0.14) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | –0.16 (–1.58 to 1.27) | 0.8299 | – | ||||
| 12 months | 207 | 218 | 2.19 (0.75 to 3.63) | 0.41 (–0.89 to 1.71) | 1.63 (–0.01 to 3.26) | 0.0516a | 0.16 (–0.01 to 0.34) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 1.59 (–0.06 to 3.23) | 0.0593 | – | ||||
| SF-12 physical functioning | |||||||
| 4 months | 214 | 219 | 5.96 (2.50 to 9.41) | 2.28 (–0.33 to 4.90) | 3.22 (–0.86 to 7.30) | 0.1222 | 0.12 (–0.02 to 0.26) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 3.26 (–0.82 to 7.34) | 0.1181 | – | ||||
| 12 months | 204 | 211 | 3.31 (0.16 to 6.46) | 0.71 (–2.41 to 3.83) | 2.40 (–1.81 to 6.61) | 0.2643 | 0.09 (–0.06 to 0.23) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 1.58 (–2.61 to 5.76) | 0.4611 | – | ||||
| SF-12 role-physical | |||||||
| 4 months | 211 | 220 | 4.92 (2.07 to 7.76) | 2.73 (–0.05 to 5.50) | 1.80 (–1.86 to 5.45) | 0.3360 | 0.08 (–0.07 to 0.24) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 2.22 (–1.38 to 5.82) | 0.2268 | – | ||||
| 12 months | 202 | 215 | 6.68 (3.49 to 9.87) | 0.47 (–2.71 to 3.64) | 5.64 (1.55 to 9.73) | 0.0072a | 0.24 (0.07 to 0.41) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 5.43 (1.43 to 9.44) | 0.0082a | – | ||||
| SF-12 bodily pain | |||||||
| 4 months | 218 | 226 | 6.31 (3.16 to 9.45) | 3.65 (0.38 to 6.92) | 2.82 (–1.23 to 6.86) | 0.1732 | 0.10 (–0.07 to 0.28) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 3.80 (–0.21 to 7.81) | 0.0637 | – | ||||
| 12 months | 206 | 218 | 6.07 (2.83 to 9.30) | 2.64 (–0.42 to 5.70) | 3.84 (–0.24 to 7.91) | 0.0655 | 0.13 (–0.04 to 0.30) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 3.81 (–0.26 to 7.88) | 0.0670 | – | ||||
| SF-12 general health | |||||||
| 4 months | 218 | 226 | 1.65 (–1.17 to 4.48) | 1.66 (–1.16 to 4.47) | –0.14 (–3.83 to 3.54) | 0.9404 | 0.00 (–0.15 to 0.15) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | –0.37 (–3.98 to 3.25) | 0.8419 | – | ||||
| 12 months | 207 | 217 | 1.91 (–1.12 to 4.94) | –0.65 (–3.95 to 2.66) | 2.55 (–1.50 to 6.60) | 0.2179 | 0.10 (–0.07 to 0.27) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 1.96 (–2.06 to 5.98) | 0.3395 | – | ||||
| SF-12 vitality | |||||||
| 4 months | 216 | 224 | 0.93 (–2.07 to 3.92) | 1.90 (–0.92 to 4.72) | –0.45 (–4.17 to 3.27) | 0.8124 | –0.04 (–0.20 to 0.12) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 0.13 (–3.48 to 3.73) | 0.9454 | – | ||||
| 12 months | 207 | 217 | 1.45 (–1.71 to 4.61) | –0.58 (–3.78 to 2.63) | 2.50 (–1.51 to 6.51) | 0.2227 | 0.08 (–0.10 to 0.26) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 1.97 (–2.03 to 5.97) | 0.3347 | – | ||||
| SF-12 social functioning | |||||||
| 4 months | 218 | 226 | 1.38 (–1.67 to 4.43) | –0.88 (–4.02 to 2.25) | 1.85 (–2.10 to 5.80) | 0.3593 | 0.08 (–0.08 to 0.24) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 1.94 (–2.02 to 5.90) | 0.3369 | – | ||||
| 12 months | 207 | 218 | 2.42 (–1.37 to 6.20) | –1.03 (–4.63 to 2.56) | 3.26 (–1.17 to 7.68) | 0.1499 | 0.12 (–0.06 to 0.31) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 2.63 (–1.79 to 7.04) | 0.2445 | – | ||||
| SF-12 role-emotional | |||||||
| 4 months | 213 | 220 | 3.11 (–0.40 to 6.62) | 2.22 (–1.31 to 5.74) | –0.20 (–4.56 to 4.15) | 0.9267 | 0.03 (–0.14 to 0.20) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 0.07 (–4.23 to 4.37) | 0.9751 | – | ||||
| 12 months | 201 | 215 | 8.40 (4.45 to 12.34) | 2.21 (–1.70 to 6.12) | 5.25 (0.69 to 9.81) | 0.0246a | 0.21 (0.02 to 0.40) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 5.06 (0.50 to 9.61) | 0.0301a | – | ||||
| SF-12 mental health | |||||||
| 4 months | 214 | 219 | 1.99 (–0.61 to 4.58) | 2.45 (0.04 to 4.87) | –0.57 (–3.68 to 2.53) | 0.7176 | –0.02 (–0.19 to 0.15) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | –0.22 (–3.27 to 2.83) | 0.8877 | – | ||||
| 12 months | 206 | 214 | 4.13 (1.17 to 7.08) | 1.64 (–0.97 to 4.24) | 2.26 (–1.15 to 5.67) | 0.1943 | 0.12 (–0.07 to 0.31) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 2.29 (–1.16 to 5.74) | 0.1941 | – | ||||
| Questionnaire | Exercise programme, mean (SD) | Usual care, mean (SD) | ||||
|---|---|---|---|---|---|---|
| EQ-5D-3L health state | 0.57 (0.29) | 0.62 (0.25) | 0.62 (0.24) | 0.59 (0.26) | 0.60 (0.28) | 0.62 (0.25) |
| EQ-5D-3L VAS (your health today) | 66.1 (19.4) | 69.5 (19.4) | 69.5 (17.8) | 67.3 (18.1) | 67.7 (18.5) | 66.5 (19.8) |
| EQ-5D-3L | Number of patients | Mean change (95% CI) | Mean treatment difference (95% CI) | p-value | Standardised mean difference (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Exercise programme | Usual care | Exercise programme | Usual care | ||||
| EQ-5D-3L health statea | |||||||
| 4 months | 222 | 226 | 0.04 (0.01 to 0.07) | 0.01 (–0.03 to 0.04) | 0.02 (–0.02 to 0.06) | 0.3650 | 0.10 (–0.06 to 0.27) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 0.02 (–0.02 to 0.06) | 0.3813 | – | ||||
| 12 months | 214 | 220 | 0.03 (0.00 to 0.06) | 0.02 (–0.01 to 0.06) | 0.00 (–0.03 to 0.04) | 0.8547 | 0.02 (–0.14 to 0.18) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 0.00 (–0.03 to 0.04) | 0.8714 | – | ||||
| EQ-5D-3L VAS (your health today) | |||||||
| 4 months | 224 | 228 | 2.53 (0.11 to 4.96) | –0.01 (–2.33 to 2.32) | 2.19 (–0.77 to 5.15) | 0.1483 | 0.14 (–0.04 to 0.31) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 2.22 (–0.72 to 5.17) | 0.1402 | – | ||||
| 12 months | 214 | 220 | 2.14 (–0.09 to 4.37) | –1.10 (–3.68 to 1.48) | 3.13 (0.10 to 6.15) | 0.0432b | 0.17 (–0.01 to 0.35) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 2.51 (–0.47 to 5.50) | 0.0995 | – | ||||
Pain troublesomeness and self-efficacy
No statistically significant difference was reported between groups for pain troublesomeness at either follow-up time point (Tables 37 and 38).
| Questionnaire | Exercise programme, mean (SD) | Usual care, mean (SD) | ||||
|---|---|---|---|---|---|---|
| Pain troublesomeness overall score | 46.0 (22.2) | 39.8 (21.2) | 38.9 (23.3) | 48.5 (21.5) | 43.9 (21.3) | 43.5 (21.8) |
| Pain troublesomeness overall scorea | Number of patients | Mean change (95% CI) | Mean treatment difference (95% CI) | p-value | Standardised mean difference (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Exercise programme | Usual care | Exercise programme | Usual care | ||||
| 4 months | 215 | 224 | –5.44 (–7.91 to –2.97) | –4.64 (–7.23 to –2.05) | –2.16 (–5.36 to 1.04) | 0.1856 | –0.04 (–0.20 to 0.13) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | –2.70 (–5.91 to 0.50) | 0.0993 | – | ||||
| 12 months | 206 | 217 | –4.32 (–7.15 to –1.49) | –4.54 (–7.35 to –1.73) | –1.84 (–5.47 to 1.80) | 0.3226 | 0.01 (–0.17 to 0.19) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | –1.61 (–5.21 to 1.99) | 0.3810 | – | ||||
There was a small but statistically significant difference between groups for self-efficacy scores at both 4 and 12 months. The exercise programme group had a small but significant increase in self-efficacy compared with the usual care group (Tables 39 and 40).
| Questionnaire | Exercise programme, mean (SD) | Usual care, mean (SD) | ||||
|---|---|---|---|---|---|---|
| Self-efficacy (confidence to manage their condition) | 67.0 (20.3) | 74.2 (18.5) | 73.7 (18.6) | 68.7 (19.1) | 71.2 (20.2) | 70.3 (22.3) |
| Self-efficacy (confidence to manage their condition) | Number of patients | Mean change (95% CI) | Mean treatment difference (95% CI) | p-value | Standardised mean difference (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Exercise programme | Usual care | Exercise programme | Usual care | ||||
| 4 months | 216 | 226 | 5.78 (3.40 to 8.17) | 2.04 (–0.10 to 4.19) | 3.41 (0.53 to 6.29) | 0.0209a | 0.19 (0.03 to 0.35) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 3.38 (0.45 to 6.30) | 0.0244a | – | ||||
| 12 months | 205 | 217 | 5.19 (2.45 to 7.92) | 1.11 (–1.44 to 3.66) | 3.80 (0.41 to 7.19) | 0.0286a | 0.21 (0.02 to 0.40) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 3.21 (–0.19 to 6.62) | 0.0651 | – | ||||
Impairment measures
There were inconsistent findings for between-group comparisons of the impairment measures. Some found no difference between the groups whereas others found small improvements in favour of the exercise programme group.
Strength
Both groups had improvements in strength, with the exercise programme group showing greater improvements. Statistically significant between-group differences in favour of the exercise programme were apparent at 4 months for power grip and at 12 months for pinch grip (Tables 41 and 42).
| Measure | Exercise programme, mean (SD) | Usual care, mean (SD) | ||||
|---|---|---|---|---|---|---|
| Full hand grip force (N) | 134.2 (83.3) | 152.7 (87.1) | 156.6 (88.9) | 130.3 (73.1) | 139.2 (79.3) | 145.0 (81.8) |
| Pinch grip force (N) | 40.2 (21.1) | 45.1 (22.2) | 47.2 (25.0) | 39.1 (19.6) | 42.6 (21.1) | 42.7 (20.8) |
| Strength | Number of patients | Mean change (95% CI) | Mean treatment difference (95% CI) | p-value | Standardised mean difference (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Exercise programme | Usual care | Exercise programme | Usual care | ||||
| Full hand grip force (N, greater score = greater strength) | |||||||
| 4 months | 195 | 205 | 15.55 (10.17 to 20.93) | 7.35 (2.43 to 12.28) | 8.59 (1.39 to 15.80) | 0.0198a | 0.10 (0.01 to 0.20) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 9.29 (2.01 to 16.57) | 0.0129a | – | ||||
| 12 months | 171 | 184 | 15.77 (10.11 to 21.42) | 9.57 (3.66 to 15.48) | 6.55 (–1.60 to 14.69) | 0.1160 | 0.08 (–0.03 to 0.18) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 6.41 (–1.87 to 14.70) | 0.1303 | – | ||||
| Pinch grip force (N, greater score = greater strength) | |||||||
| 4 months | 192 | 204 | 4.29 (2.74 to 5.84) | 3.15 (1.60 to 4.70) | 1.29 (–0.87 to 3.46) | 0.2413 | 0.06 (–0.05 to 0.16) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 1.57 (–0.59 to 3.73) | 0.1547 | – | ||||
| 12 months | 169 | 182 | 5.33 (2.99 to 7.68) | 2.35 (0.63 to 4.06) | 3.16 (0.32 to 6.00) | 0.0301a | 0.15 (0.00 to 0.29) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 3.01 (0.13 to 5.88) | 0.0411a | – | ||||
Range of movement and dexterity
Apart from composite finger extension, there were no statistically significant between-group differences for ROM measures (Tables 43 and 44).
| Measure | Exercise programme, mean (SD) | Usual care, mean (SD) | ||||
|---|---|---|---|---|---|---|
| Active wrist flexion/extension (degrees) | 88.0 (29.6) | 94.6 (28.7) | 94.9 (29.8) | 90.1 (31.7) | 92.4 (32.2) | 95.5 (30.9) |
| Combined finger flexion (mm) | 13.0 (16.1) | 9.4 (14.6) | 9.1 (14.8) | 12.8 (16.1) | 9.7 (14.0) | 10.2 (14.1) |
| Composite finger extension (mm) | 21.3 (24.4) | 26.4 (21.0) | 27.6 (20.6) | 20.2 (25.2) | 21.8 (26.0) | 21.7 (26.4) |
| Thumb opposition score | 8.1 (1.9) | 8.4 (1.8) | 8.5 (1.8) | 8.0 (2.1) | 8.2 (2.0) | 8.1 (2.0) |
| ROM | Number of patients | Mean change (95% CI) | Mean treatment difference (95% CI) | p-value | Standardised mean difference (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Exercise programme | Usual care | Exercise programme | Usual care | ||||
| Active wrist flexion/extension score (degrees; greater score = greater movement) | |||||||
| 4 months | 196 | 205 | 4.84 (2.65 to 7.02) | 2.75 (0.63 to 4.87) | 2.08 (–0.87 to 5.02) | 0.1671 | 0.07 (–0.03 to 0.17) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 1.58 (–1.25 to 4.41) | 0.2750 | – | ||||
| 12 months | 173 | 183 | 4.56 (2.13 to 7.00) | 4.21 (1.73 to 6.68) | 0.21 (–3.13 to 3.55) | 0.9040 | 0.01 (–0.10 to 0.12) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 0.27 (–2.72 to 3.26) | 0.8587 | – | ||||
| Combined finger flexion (mm; lesser score = greater movement) | |||||||
| 4 months | 194 | 204 | –4.45 (–5.82 to –3.07) | –3.39 (–4.54 to –2.25) | –0.90 (–2.44 to 0.64) | 0.2543 | –0.07 (–0.18 to 0.05) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | –0.93 (–2.43 to 0.58) | 0.2281 | – | ||||
| 12 months | 172 | 183 | –3.92 (–5.48 to –2.36) | –3.20 (–4.51 to –1.89) | –0.89 (–2.67 to 0.89) | 0.3290 | –0.04 (–0.17 to 0.08) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | –0.64 (–2.40 to 1.13) | 0.4793 | – | ||||
| Composite finger extension (mm; greater score = greater movement) | |||||||
| 4 months | 194 | 196 | 4.04 (1.98 to 6.09) | 1.45 (–0.17 to 3.07) | 2.82 (0.34 to 5.30) | 0.0262a | 0.10 (0.00 to 0.21) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 2.55 (0.05 to 5.04) | 0.0068a | – | ||||
| 12 months | 171 | 175 | 4.81 (2.77 to 6.84) | 1.45 (–0.76 to 3.65) | 3.61 (0.73 to 6.50) | 0.0147a | 0.14 (0.01 to 0.26) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 4.05 (1.13 to 6.96) | 0.0068a | – | ||||
| Thumb opposition score (greater score = greater movement) | |||||||
| 4 months | 196 | 207 | 0.31 (0.13 to 0.50) | 0.18 (0.00 to 0.35) | –0.15 (–0.39 to 0.09) | 0.2133 | 0.07 (–0.05 to 0.20) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 0.13 (–0.10 to 0.37) | 0.2725 | – | ||||
| 12 months | 173 | 186 | 0.16 (–0.04 to 0.37) | 0.12 (–0.07 to 0.30) | –0.10 (–0.36 to 0.15) | 0.4233 | 0.02 (–0.11 to 0.16) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 0.10 (–0.16 to 0.36) | 0.4416 | – | ||||
Dexterity improved to a greater extent in the exercise programme group at 12 months and this difference was statistically significant compared with the usual care group. There was no significant difference between groups in the short term (Tables 45 and 46).
| Measure | Exercise programme, mean (SD) | Usual care, mean (SD) | ||||
|---|---|---|---|---|---|---|
| Dexterity: nine-hole peg test (seconds) | 27.2 (8.2) | 25.6 (7.3) | 25.1 (6.3) | 27.3 (9.4) | 26.5 (8.6) | 26.7 (10.6) |
| Dexterity: nine-hole peg test (seconds; lesser score = greater dexterity) | Number of patients | Mean change (95% CI) | Mean treatment difference (95% CI) | p-value | Standardised mean difference (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Exercise programme | Usual care | Exercise programme | Usual care | ||||
| 4 months | 196 | 207 | –1.39 (–1.97 to –0.81) | –0.74 (–1.50 to 0.03) | –0.72 (–1.61 to 0.17) | 0.1151 | –0.07 (–0.18 to 0.03) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | –0.64 (–1.53 to 0.26) | 0.1151 | – | ||||
| 12 months | 173 | 185 | –1.33 (–1.86 to –0.80) | –0.09 (–0.92 to 0.74) | –1.26 (–2.26 to –0.27) | 0.0134a | –0.14 (–0.25 to –0.03) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | –1.19 (–2.15 to –0.23) | 0.0156a | – | ||||
Disease activity
Comparisons of swollen and tender joint counts found a small to moderate effect of the exercise programme compared with the usual care groups in the short term only (Tables 47 and 48). On average, both groups achieve a one-point improvement, however the exercise programme participants achieve this in a shorter time frame (within 4 months) compared with the participants in the usual care group (within 12 months). No significant changes were reported for CRP and ESR levels either within or between groups at all time points indicating a relatively stable RA population that were then unchanged within and between groups.
| Measure | Exercise programme, mean (SD) | Usual care, mean (SD) | ||||
|---|---|---|---|---|---|---|
| MCP joint deformity (degrees) | 6.8 (8.4) | 5.4 (6.4) | 5.8 (7.7) | 7.4 (9.4) | 6.6 (8.4) | 6.9 (9.7) |
| Swollen joint count | 4.2 (4.8) | 3.2 (4.6) | 3.4 (4.9) | 4.1 (4.8) | 4.0 (5.3) | 3.3 (4.6) |
| Tender joint count | 5.0 (5.4) | 3.4 (4.5) | 3.9 (5.3) | 4.8 (5.1) | 4.4 (5.0) | 3.8 (5.1) |
| Disease activity | Number of patients | Mean change (95% CI) | Mean treatment difference (95% CI) | p-value | Standardised mean difference (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Exercise programme | Usual care | Exercise programme | Usual care | ||||
| MCP joint deformity (degrees; greater score = greater deformity) | |||||||
| 4 months | 196 | 202 | –0.92 (–1.57 to –0.27) | –0.59 (–1.32 to 0.15) | –0.59 (–1.48 to 0.30) | 0.1933 | –0.04 (–0.15 to 0.07) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | –0.66 (–1.53 to 0.21) | 0.1357 | – | ||||
| 12 months | 172 | 183 | –0.70 (–1.41 to 0.01) | –0.32 (–1.01 to 0.36) | –0.46 (–1.43 to 0.51) | 0.3539 | –0.04 (–0.15 to 0.07) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | –0.56 (–1.50 to 0.37) | 0.2369 | – | ||||
| Swollen joint count (both hands; greater score = greater number of joints affected) | |||||||
| 4 months | 196 | 209 | –1.05 (–1.58 to –0.53) | –0.12 (–0.73 to 0.48) | –0.90 (–1.65 to –0.16) | 0.0173a | –0.19 (–0.36 to –0.03) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | –0.87 (–1.50 to –0.23) | 0.0077a | – | ||||
| 12 months | 174 | 186 | –1.13 (–1.69 to –0.56) | –1.02 (–1.71 to –0.34) | –0.06 (–0.83 to 0.71) | 0.8854 | –0.02 (–0.21 to 0.16) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | –0.07 (–0.74 to 0.61) | 0.8844 | – | ||||
| Tender joint count (both hands; greater score = greater number of joints affected) | |||||||
| 4 months | 197 | 208 | –1.27 (–1.86 to –0.68) | –0.38 (–1.02 to 0.27) | –0.92 (–1.68 to –0.16) | 0.0177a | –0.17 (–0.34 to 0.00) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | –1.03 (–1.77 to –0.29) | 0.0069a | – | ||||
| 12 months | 174 | 186 | –0.96 (–1.69 to –0.23) | –1.15 (–1.86 to –0.43) | 0.14 (–0.76 to 1.03) | 0.7651 | 0.04 (–0.16 to 0.23) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 0.12 (–0.77 to 1.00) | 0.7955 | – | ||||
| ESR (log transformation) | |||||||
| 4 months | 138 | 138 | –0.04 (–0.15 to 0.07) | –0.09 (–0.20 to 0.02) | 0.01 (–0.14 to 0.16) | 0.9763 | 0.05 (–0.12 to 0.21) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 0.06 (–0.23 to 0.11) | 0.4864 | – | ||||
| 12 months | 119 | 133 | –0.04 (–0.18 to 0.10 | –0.10 (–0.23 to 0.03) | 0.02 (–0.16 to 0.20) | 0.8300 | 0.06 (–0.14 to 0.26) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | 0.02 (–0.18 to 0.15) | 0.8323 | – | ||||
| CRP (log transformation) | |||||||
| 4 months | 163 | 159 | –0.04 (–0.11 to 0.19) | –0.18 (–0.32 to 0.03) | 0.18 (–0.01 to 0.38) | 0.0645 | 0.21 (0.01 to 0.41) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | –0.32 (0.08 to 0.55) | 0.0093a | – | ||||
| 12 months | 142 | 149 | –0.14 (–0.29 to 0.02) | –0.12 (–0.29 to 0.05) | –0.08 (–0.28 to 0.12) | 0.4385 | –0.02 (–0.24 to 0.21) |
| Adjusted for centre, sex, age and drug groups (DMARD and oral steroids) | –0.03 (–0.24 to 0.19) | 0.8185 | – | ||||
Participant-rated improvement, benefit and satisfaction
The exercise programme group rated themselves as having greater global improvement in their hands and wrists than the usual care group at both 4 and 12 months (Wilcoxon test, p < 0.0001) (Table 49). They also reported significantly more benefit [p < 0.0001 (both categorical and ordinal) for both time points] and greater satisfaction from trial treatments (4 months: p < 0.0001 categorical, p = 0.0198 ordinal; 12 months: p < 0.0001 categorical, p = 0.347 ordinal) than the usual care group at both 4 and 12 months (Tables 50 and 51).
| Response | 4 months | 12 months | ||
|---|---|---|---|---|
| Exercise programme, n (%) | Usual care, n (%) | Exercise programme, n (%) | Usual care, n (%) | |
| Completely recovered | 1 (1) | 1 (< 1) | 3 (2) | 2 (1) |
| Much improved | 40 (19) | 20 (9) | 42 (20) | 16 (7) |
| Slightly improved | 72 (33) | 43 (19) | 47 (23) | 27 (13) |
| No change | 61 (28) | 89 (40) | 61 (30) | 89 (41) |
| Slightly worsened | 30 (14) | 60 (27) | 35 (17) | 61 (28) |
| Much worsened | 10 (5) | 10 (5) | 15 (7) | 17 (8) |
| Vastly worsened | 2 (1) | 1 (< 1) | 3 (2) | 4 (2) |
| Total | 216 | 224 | 206 | 216 |
| Response | 4 months | 12 months | ||
|---|---|---|---|---|
| Exercise programme, n (%) | Usual care, n (%) | Exercise programme, n (%) | Usual care, n (%) | |
| Substantial benefit | 62 (28.8) | 27 (12.6) | 64 (31.4) | 31 (14.6) |
| Moderate benefit | 111 (51.6) | 103 (47.9) | 101 (49.5) | 104 (48.8) |
| No benefit | 33 (15.4) | 83 (38.6) | 34 (16.7) | 76 (35.7) |
| Moderate harm | 9 (4.2) | 1 (0.5) | 3 (1.5) | 2 (0.9) |
| Substantial harm | – | 1 (0.5) | 2 (1.0) | – |
| Total | 215 | 215 | 204 | 213 |
| Response | 4 months | 12 months | ||
|---|---|---|---|---|
| Exercise programme, n (%) | Usual care, n (%) | Exercise programme, n (%) | Usual care, n (%) | |
| Extremely dissatisfied | 15 (6.9) | 6 (2.8) | 15 (7.4) | 8 (3.8) |
| Very dissatisfied | 10 (4.6) | 13 (6.0) | 17 (8.3) | 13 (6.1) |
| Somewhat dissatisfied | 1 (0.5) | 7 (3.2) | 2 (1.0) | 13 (6.1) |
| Neither satisfied nor dissatisfied | 14 (6.5) | 38 (17.6) | 22 (10.8) | 50 (23.5) |
| Somewhat satisfied | 23 (10.7) | 39 (18.1) | 21 (10.3) | 38 (17.8) |
| Very satisfied | 94 (43.5) | 83 (38.4) | 79 (38.7) | 70 (32.9) |
| Extremely satisfied | 59 (27.3) | 30 (13.9) | 48 (23.5) | 21 (9.9) |
| Total | 216 | 216 | 204 | 213 |
Hand exercise performance
Participants were encouraged to maintain their exercise routine once the exercise programme had finished. As would be expected, the proportion of participants who reported performing hand and wrist exercises was significantly greater in the exercise programme group than in the usual care group at both 4 and 12 months (p < 0.0001 at 4 months and p = 0.0185 at 12 months) (Table 52).
| Are you currently doing any hand/wrist exercises to help with your RA? | 4 months | 12 months | ||
|---|---|---|---|---|
| Exercise programme, n (%) | Usual care, n (%) | Exercise programme, n (%) | Usual care, n (%) | |
| No | 26 (12.0) | 75 (33.9) | 61 (29.8) | 88 (40.7) |
| Yes (total) | 190 (88.0) | 146 (66.1) | 144 (70.2) | 128 (59.3) |
| Daily | 95 (44.0) | 65 (29.4) | 32 (15.6) | 47 (21.8) |
| 3–4 times a week | 56 (25.9) | 40 (18.1) | 47 (22.9) | 37 (17.1) |
| 1–2 times a week | 23 (10.6) | 32 (14.5) | 49 (23.9) | 39 (18.1) |
| Other | 14 (6.5) | 9 (4.1) | 16 (7.8) | 5 (2.3) |
| Blank | 2 (0.9) | – | – | – |
| Total | 216 | 221 | 205 | 216 |
Medication usage
There were no significant differences in medication usage between the two groups at the two follow-up time points. There were small numbers of changes to medication over the 12 months (Tables 53–56).
| DMARD intensity | Baseline | 4 months | 12 months | |||
|---|---|---|---|---|---|---|
| Exercise programme, n (%) | Usual care, n (%) | Exercise programme, n (%) | Usual care, n (%) | Exercise programme, n (%) | Usual care, n (%) | |
| No DMARD medication | 19 (7.7) | 19 (7.9) | 16 (7.6) | 23 (10.3) | 23 (10.9) | 19 (8.8) |
| Single non-biologic DMARD | 103 (41.9) | 118 (48.8) | 83 (39.2) | 110 (49.3) | 74 (35.1) | 95 (44.0) |
| Comb non-biologic DMARDs | 72 (29.3) | 53 (21.9) | 57 (30.7) | 65 (21.5) | 65 (30.8) | 54 (25.0) |
| Biologic DMARDa | 52 (21.1) | 52 (21.5) | 48 (22.6) | 42 (18.8) | 49 (23.2) | 48 (22.2) |
| Oral steroids | 49 (19.9) | 52 (21.5) | 39 (18.4) | 45 (20.2) | 42 (19.9) | 40 (18.5) |
| NSAIDs | 106 (43.1) | 100 (41.3) | 85 (40.1) | 90 (40.4) | 92 (43.6) | 85 (39.4) |
| Analgesics | 89 (36.2) | 84 (34.7) | 69 (32.5) | 77 (30.0) | 72 (34.1) | 68 (31.5) |
| Medication | Baseline | 4 months | 12 months | |||
|---|---|---|---|---|---|---|
| Exercise programme, n (%) | Usual care, n (%) | Exercise programme, n (%) | Usual care, n (%) | Exercise programme, n (%) | Usual care, n (%) | |
| Extra NSAID | 9 (3.7) | 15 (6.2) | 10 (4.7) | 13 (5.8) | 12 (5.7) | 9 (4.2) |
| Steroid tablet | 2 (0.8) | 5 (2.1) | 1 (0.5) | 3 (1.3) | 3 (1.4) | 2 (0.9) |
| Steroid injection into joint | 1 (0.4) | – | 5 (2.4) | 1 (0.4) | 1 (0.5) | 2 (0.9) |
| Steroid injection into muscle | 2 (0.8) | 3 (1.2) | 2 (0.9) | 8 (3.6) | 4 (1.9) | 1 (0.5) |
| Change | Baseline to 4 months | Baseline to 12 months | ||
|---|---|---|---|---|
| Exercise programme, n (%) | Usual care, n (%) | Exercise programme, n (%) | Usual care, n (%) | |
| From no DMARD to DMARD | 1 (0.5) | 4 (1.8) | 2 (0.9) | 3 (1.4) |
| From DMARD to no DMARD | 2 (0.9) | 9 (4.0) | 11 (5.2) | 5 (2.3) |
| No change (on DMARD) | 195 (92.0) | 196 (87.9) | 186 (88.2) | 194 (89.8) |
| No change (not on DMARD) | 14 (6.6) | 14 (6.3) | 12 (5.7) | 14 (6.5) |
| Change | Baseline to 4 months | Baseline to 12 months | ||
|---|---|---|---|---|
| Exercise programme, n (%) | Usual care, n (%) | Exercise programme, n (%) | Usual care, n (%) | |
| From no biologic DMARD to biologic DMARD | 9 (4.2) | 4 (1.8) | 12 (5.7) | 6 (2.8) |
| From biologic DMARD to no biologic DMARD | 4 (1.9) | 6 (2.7) | 6 (2.8) | 4 (1.9) |
| No change (not on biologic DMARD) | 160 (75.5) | 175 (78.5) | 156 (73.9) | 164 (75.9) |
| No change (on biologic DMARD) | 39 (18.4) | 38 (17.0) | 37 (17.5) | 42 (19.4) |
Secondary analyses
Subgroup analyses
Analyses of various subgroups were conducted including age, sex, disease activity, medication and various baseline outcome measures. No statistically significant changes between subgroups were found (Table 57).
| Subgroup | 4 months | 12 months | ||||
|---|---|---|---|---|---|---|
| n (combined groups) | Treatment effect (95% CI) | p-value for difference between subgroups | n | Treatment effect (95% CI) | p-value for difference between subgroups | |
| Time since diagnosis (years) | ||||||
| < 5 | 116 | 6.57 (1.25 to 11.90) | 115 | 6.08 (0.22 to 11.94) | ||
| ≥ 5 | 284 | 3.08 (–0.15 to 6.31) | 0.2588 | 276 | 3.72 (0.21 to 7.22) | 0.4822 |
| < 10 | 200 | 4.17 (0.13 to 8.21) | 193 | 3.79 (–0.61 to 8.19) | ||
| ≥ 10 | 200 | 3.91 (0.09 to 7.73) | 0.9264 | 198 | 4.58 (0.38 to 8.78) | 0.7969 |
| Baseline MHQ overall function | ||||||
| < 52.5 | 220 | 5.08 (1.57 to 8.59) | 210 | 3.89 (–0.32 to 8.10) | ||
| ≥ 52.5 | 229 | 4.09 (0.55 to 7.62) | 0.6953 | 228 | 4.75 (0.97 to 8.53) | 0.7632 |
| Age (years) | ||||||
| < 60 | 147 | 3.86 (–1.23 to 8.95) | 142 | 4.69 (–0.73 to 10.12) | ||
| ≥ 60 | 302 | 5.25 (2.10 to 8.41) | 0.6323 | 296 | 4.07 (0.65 to 7.50) | 0.8443 |
| Type of referral | ||||||
| Clinic referrals | 363 | 5.51 (2.49 to 8.53) | 353 | 4.15 (0.94 to 7.35) | ||
| Review list mail-out | 86 | 1.19 (–4.63 to 7.00) | 0.2120 | 85 | 5.14 (–1.63 to 11.92) | 0.7884 |
| Gender | ||||||
| Male | 106 | 5.81 (–0.12 to 11.73) | 102 | 5.24 (–1.94 to 11.43) | ||
| Female | 343 | 4.36 (1.36 to 7.36) | 0.6528 | 336 | 4.10 (0.82 to 7.38) | 0.7428 |
| Baseline ESR (mm/hour) | ||||||
| < 16 | 166 | 4.30 (–0.30 to 8.90) | 166 | 6.04 (1.54 to 10.54) | ||
| ≥ 16 | 174 | 5.18 (0.80 to 9.56) | 0.7853 | 168 | 1.89 (–2.83 to 6.61) | 0.2102 |
| Baseline CRP (mg/l) | ||||||
| < 6 | 197 | 3.29 (–0.61 to 7.19) | 192 | 4.37 (–0.06 to 8.79) | ||
| ≥ 6 | 197 | 5.52 (1.30 to 9.74) | 0.4437 | 190 | 4.90 (0.40 to 9.40) | 0.8668 |
| SF-12 PCS (points) | ||||||
| < 34 | 225 | 6.36 (2.48 to 10.23) | 220 | 4.18 (0.13 to 8.24) | ||
| ≥ 34 | 223 | 2.95 (–0.73 to 6.64) | 0.2110 | 217 | 4.86 (0.74 to 8.98) | 0.8179 |
| SF-12 MCS (points) | ||||||
| < 50 | 225 | 5.51 (1.71 to 9.31) | 217 | 4.98 (0.75 to 9.21) | ||
| ≥ 50 | 224 | 3.79 (0.00 to 7.59) | 0.5290 | 221 | 3.78 (–0.19 to 7.75) | 0.6825 |
| Baseline DMARD therapy summary | ||||||
| Biological | 92 | 1.97 (–3.80 to 7.75) | 92 | 4.70 (–1.12 to 10.52) | ||
| Combination non-biological | 116 | 8.44 (2.47 to 14.41) | 114 | 6.20 (–0.09 to 12.49) | ||
| Single non-biological | 205 | 2.82 (–0.94 to 6.58) | 199 | 4.20 (0.10 to 8.50) | ||
| No DMARD | 35 | 8.51 (–0.96 to 17.98) | 0.2390 | 33 | –1.84 (–12.04 to 8.37) | 0.6261 |
Examination of the effect of subgroups on treatment compliance in the exercise programme group found that participants with increased age, disease activity (CRP) and SF-12 mental score were more likely to have attended all six sessions (Table 58). Note that no group has less than 60% adherence and this was not a pre-planned analysis.
| Subgroup | < 6 sessions | 6 sessions | p-value |
|---|---|---|---|
| n (%) | n (%) | ||
| Baseline MHQ | |||
| Baseline MHQ overall function < 52.5 (median) | 34 (27.4) | 90 (72.6) | |
| Baseline MHQ overall function ≥ 52.5 (median) | 28 (23.0) | 94 (77.0) | 0.42 |
| Time since diagnosis | |||
| < 5 years | 12 (19.7) | 49 (80.3) | |
| ≥ 5 years | 39 (24.8) | 118 (75.2) | 0.42 |
| < 10 years | 23 (21.1) | 86 (78.9) | |
| ≥ 10 years | 28 (25.7) | 81 (74.3) | 0.42 |
| Age | |||
| < 60 years | 31 (33.0) | 63 (67.0) | |
| ≥ 60 years | 31 (19.1) | 131 (80.9) | 0.03a |
| Gender | |||
| Female | 47 (25.0) | 141 (75.0) | |
| Male | 15 (25.9) | 43 (74.1) | 0.89 |
| Disease activity | |||
| ESR < 16 mm/hour (median) | 25 (26.6) | 70 (73.4) | |
| ESR ≥ 16 mm/hour (median) | 28 (30.1) | 65 (69.9) | 0.56 |
| CRP < 6 mg/l (median) | 21 (18.7) | 91 (81.3) | |
| CRP ≥ 6 mg/l (median) | 33 (30.8) | 74 (69.2) | 0.04a |
| SF-12 PCS | |||
| < 34 (median) | 35 (28.0) | 90 (72.0) | |
| ≥ 34 (median) | 27 (22.3) | 94 (77.7) | 0.30 |
| SF-12 MCS | |||
| < 50 (median) | 42 (32.6) | 87 (67.4) | |
| ≥ 50 (median) | 20 (17.1) | 97 (82.9) | 0.005a |
| Recruitment method | |||
| Clinic referral | 53 (26.8) | 145 (73.2) | |
| Rheumatology or therapy review list mail-out | 9 (18.7) | 39 (81.3) | 0.25 |
| Medication | |||
| Biologic DMARD | 15 (28.8) | 37 (71.2) | |
| Combination non-biologic DMARD | 16 (22.2) | 56 (77.8) | |
| Single non-biologic DMARD | 24 (23.3) | 79 (76.7) | |
| No DMARD | 7 (36.8) | 12 (63.2) | 0.52 |
Treatment preference
Analysis of participants’ baseline treatment preferences reveals that the primary treatment effect was higher (non-significantly) in the group of participants who expressed a preference to receive the exercise programme (Table 59).
| Preference | 4 months | 12 months | ||||||
|---|---|---|---|---|---|---|---|---|
| Exercise programme | Usual care | Mean treatment difference (95% CI) | p-value | Exercise programme | Usual care | Mean treatment difference (95% CI) | p-value | |
| Usual care or ‘do not mind’ | 130 | 137 | 2.79 (0.50 to 6.08) | 0.0974 | 125 | 136 | 2.39 (1.28 to 6.05) | 0.2024 |
| Exercise programme | 90 | 89 | 7.30 (4.01 to 10.60) | 0.0001 | 89 | 85 | 7.25 (3.10 to 11.40) | 0.0008 |
| Interaction p-value for difference in treatment effect between preference groups | 0.0913 | |||||||
Primary outcome by level of treatment compliance
The CACE estimates based on full attendance are slightly larger than the overall trial estimates at 4 and 12 months (Table 60). The CACE estimates based on the lower compliance threshold were close to the overall trial effect.
| Threshold attendance | Treatment effect, points on MHQ overall hand function (95% CI) | |
|---|---|---|
| 4 months | 12 months | |
| ≥ 3 sessions | 5.06 (2.97 to 7.18) | 4.59 (2.34 to 6.81) |
| ≥ 6 sessions | 5.84 (3.43 to 8.27) | 5.23 (2.62 to 8.01) |
Therapist effects
The main therapist was defined as the individual who was the therapist for the initial (assessment) session. A therapist was identified for 469 participants; for 10 participants no treatment log was available, five participants did not attend a session and for four participants no therapist was identified.
The primary outcome was modelled adjusting for baseline score, age, sex and centre. There was no significant therapist effect at 4 or 12 months (intraclass correlation coefficient = 0.00, p = 0.3106 and p = 0.4561 respectively).
Prospectively it was decided to try an alternative definition of the therapist, i.e. for the exercise programme arm, using the therapist who took the second session (first exercise session). Fifty-one participants who had a first exercise session had a different therapist from the one at assessment. The results of the analysis were very similar to that of the main therapist.
Multiple imputation analysis
A multiple imputation analysis was conducted to investigate the effect of missing data on the overall treatment effect for the primary outcome. The trial design allowed for a maximum of 25% missing primary outcome at 12 months. The actual rates achieved were considerably better at 12.2% in the exercise programme arm and 8.3% in the usual care arm. The 4% difference in these is small enough to make it unlikely to have a marked effect on the results.
In this multiple imputation analysis, the following baseline variables were used to estimate the primary outcome at 4 months and 12 months: age, SF-12 PCS, SF-12 MCS, troublesomeness of pain scale, confidence scale, ESR, CRP, finger joint deformity score, ROM summary score, combined finger flexion summary score, composite finger extension summary score, thumb opposition score, swollen joints score, tender joints score, dexterity score, full-grip strength score, pinch-grip strength score and DMARD intensity score.
As can be seen in Table 61, the analysis resulted in a slight reduction in the effect sizes at both 4 and 12 months as compared with the original analysis (treatment effect 4.6, 95% CI 2.22 to 6.97 and 4.35, 95% CI 1.60 to 7.10 respectively).
| 4 months | 12 months | ||
|---|---|---|---|
| Treatment effect (95% CI) | p-value | Treatment effect (95% CI) | p-value |
| 4.06 (1.36 to 6.76) | 0.0032 | 4.09 (1.11 to 7.07) | 0.0074 |
Repeated measures
To account for taking repeated measurements at various time points, a repeated measures analysis was conducted and adjusted for hospital, age and sex. Compared with the original analysis at 4 and 12 months, the results indicate virtually no difference between the two analyses (Table 62).
| 4 months | 12 months | ||
|---|---|---|---|
| Treatment effect (95% CI) | p-value | Treatment effect (95% CI) | p-value |
| 4.55 (1.90 to 7.19) | 0.0008 | 4.32 (1.46 to 7.17) | 0.0032 |
Adverse events
During the recruitment and follow-up period, 103 SAEs were reported to the trial team (Table 63). SAEs were classified through discussions with local principal investigators and the trial lead. As much information as possible was requested from the participant when a potential adverse event was noted, particularly with regard to likelihood of cause of trial treatment. No SAEs were deemed both unexpected and related to the trial involvement and therefore were not communicated to the MREC. The categories of SAE, likelihood of relatedness and reasons are summarised in Table 64.
| Relatedness of SAE | Exercise programme, n (% of patients in arm) | Usual care, n (% of patients in arm) |
|---|---|---|
| Unrelated | 27 (11) | 43 (17) |
| Unlikely | 18 (7) | 13 (5) |
| Possibly | 0 (0) | 2 (< 1) |
| Total | 45 (18) | 58 (24) |
| Category of SAE | Usual care | Exercise programme | ||
|---|---|---|---|---|
| Likelihood of relatednessa (n) | Reasons for SAE (n) | Likelihood of relatednessa (n) | Reasons for SAE (n) | |
| Death | Unrelated (2) | Cancer, Parkinson’s disease | Unrelated (0) | NA |
| Life-threatening condition | Unrelated (1) | Terminal illness | Unrelated (2) | Terminal illness (2) |
| Hospitalisation | Unrelated (3) | Stroke, fractured wrist, mastectomy | Unrelated (7) | Chest pain, foot surgery, fractured elbow, fractured wrist, septic knee replacement, stress fracture calcaneum, general body flare of RA requiring infusion |
| Medical intervention | Unrelated (1) | Steroid injection for flare | Unrelated (1) | Steroid injection for flare |
| Disability | Unrelated (20) | Flare of RA affecting whole body (18), flare of RA in hands with associated increased disease activity (2) | Unrelated (33) | Flare of RA affecting whole body (24), flare of RA in hands with associated increased disease activity (2), flare of RA due to stopping or changing medication (2), flare in RA in hands due to weather (1), flare in RA in hands due to stress (1), flare of RA in hands due to reaction to food (1), fractured leg (1), gout (1) |
| Unlikely (18) | Flare of RA affecting whole body (2), flare of RA in hands/wrists reason unknown (16) | Unlikely (13) | Flare of RA due to increase in activity outside trial (1), flare of RA in hands/wrists reason unknown (12) | |
| Possibly (0) | NA | Possibly (2) | Cervical spine and arm symptoms, flare of RA in hands | |
Chapter 5 Qualitative study
Introduction
This qualitative study was carried out to gain insight into participants’ experiences of the SARAH intervention with a group of individuals within the exercise programme arm of the main trial. We explored their experiences of the intervention at 4 and 12 months post randomisation and in particular examined how the trial intervention translated into daily life after they completed their therapy sessions. Our objectives were to:
-
explore the participants’ experience of taking part in the SARAH trial, including their satisfaction and its acceptability
-
explore how participants gauged the effectiveness of the SARAH intervention
-
explore the use of strategies used to improve adherence and facilitators and barriers to the SARAH exercise programme.
Methods
Sampling procedure
The interview study was carried out at four acute NHS trusts taking part in the SARAH trial; one trust yielded no eligible interview candidates. Trial participants were approached to take part in the interviews at their 4-month assessment. Willing participants were given an information sheet and verbal consent obtained for the interview researcher to telephone them if they were eligible for interview. As the assessors were blind to treatment allocation, all participants were informed about the interviews, but only those receiving the trial intervention were eligible for interview. Purposive sampling was done using the 4-month questionnaire, aiming to interview up to 10 participants who rated themselves as having benefited and up to 10 participants who had not benefited from the intervention in order to gain a range of experiences. Recruitment continued for approximately 1 year. Those eligible for interview were contacted by one of the researchers carrying out the interviews (EW and VN) and a convenient time and venue booked. At the beginning of the interview visit the researcher answered any questions arising from the information sheet and took written consent for the interview study (see Appendix 7).
Interviews
Five pilot interviews were carried out during the trial pilot study. These interviews informed the initial interview schedule and are not included in the analysis. Some changes were made to the interview schedule as the study progressed in response to the completed interviews, which is in line with an interpretative phenomenological analysis (IPA) approach (see Appendix 8 for interview schedules). Two researchers carried out the interviews, EW carried out the pilot and first six 4-month interviews and VN the remainder of the 4-month and all the 12-month interviews. Researcher notes were written immediately after the interviews to capture the researchers’ thoughts on the interviews and promote reflexivity across cases. Interviewees were given a pseudonym for anonymity.
Analysis
Analysis was carried out using IPA,104 which has been recommended as a suitable approach to investigate how an individual perceives a situation or to explore the meaning of particular events, experiences and states for the individual. 105 This approach has been used successfully to describe the experiences of patients with musculoskeletal problems such as chronic low back pain. 106 IPA does not require the testing of a hypothesis but allows the researcher to carry out exploratory work, thus reflecting the aims of this study. 105
All interviews were recorded using an Olympus DM10 digital voice recorder (Olympus Corporation, Shinjuku, Tokyo, Japan), transcribed verbatim, anonymised and checked by the interviewer. All transcripts were entered into NVivo, a computer software tool for managing qualitative data (NVivo V9, QSR, Portsmouth, UK).
The first six interviews by EW were used to explore passages of the data from which initial codes were developed. Codes were given definitions which were refined and ‘sharpened’ as the study progressed. EW and VN worked in collaboration to merge similar codes. Coding was also discussed with another experienced qualitative researcher (FT). After six interviews were completed, the 4-month interview codes were examined again by VN and FT and codes were grouped into four main headings. Subthemes and emergent themes across codes were explored with participant number counts. Analysis also involved cross-case comparisons to identify similarities and differences in the data.
Before each 12-month interview the 4-month transcripts and researcher notes were reread and additions made to the 12-month schedules which were specific to the individual. Interesting or ambiguous ideas and phrases were further explored in line with an IPA approach.
Results
Ten participants who had rated themselves as having benefited were identified (four males and six females) but only four participants who had rated themselves as having no benefit (three females and one male) were identified from the four NHS trusts (Figure 6).
FIGURE 6.
Strengthening And stretching for Rheumatoid Arthritis of the Hand interview study CONSORT chart. DNA, did not attend.

Fourteen trial participants were interviewed after their 4-month follow-up appointment. Twelve were interviewed at home and two at their workplace. Interviews followed a semistructured schedule and lasted 54 minutes on average. Thirteen interviews were carried out at 12 months (one trial participant declined for personal reasons) and lasted 46 minutes on average. Eleven of these interviews were carried out at home and two at their workplace.
Baseline characteristics of those interviewed were compared with those in the trial intervention arm (n = 246) of the total 490 trial participants (Table 65). The educational level groups showed a similar percentage of people with no qualifications. The interview study had a higher proportion of males to females and a higher number in employment. The mean of the MHQ scores of the interviewees showed slightly less disability although the standard deviation was similar.
| Characteristic | Trial participants | Interview study participants |
|---|---|---|
| Age | ||
| Mean (SD), range (years) | 61.3 (12.4), 27–94 | 61.4 (11.8), 44–82 |
| Mean years since diagnosis | ||
| Mean (SD), range (years) | 13.1 (10.7), 0–43 | 13.2 (11.9), 1–36 |
| Gender, n (%) | ||
| Male | 58 (23.6) | 5 (35.7) |
| Female | 188 (76.4) | 9 (64.3) |
| Ethnicity, n (%) | ||
| White | 238 (96.7) | 14 (100.0) |
| Other | 8 (3.3) | 0 (0) |
| Marital status, n (%) | ||
| Single | 24 (9.7) | 1 (7.1) |
| Married | 157 (63.8) | 9 (64.3) |
| Separated | 4 (1.6) | 0 (0) |
| Divorced | 19 (7.7) | 1 (7.1) |
| Widowed | 28 (11.4) | 3 (21.4) |
| Cohabiting | 14 (5.7) | 0 (0) |
| Employment status, n (%) | ||
| Full-time employed | 29 (11.8) | 2 (14.3) |
| Part-time employed | 26 (10.6) | 2 (14.3) |
| Self-employed | 11 (4.5) | 1 (7.1) |
| Unpaid work | 3 (1.2) | 0 (0) |
| Unemployed | 12 (4.9) | 1 (7.1) |
| Looking after home | 24 (9.8) | 1 (7.1) |
| Retired/inactive | 141 (57.3) | 7 (50.0) |
| Educational level, n (%) | ||
| Higher degree | 26 (10.9) | 1 (7.1) |
| NVQ4/5/degree or equivalent | 18 (7.5) | 3 (21.4) |
| Higher education below degree | 23 (9.6) | 1 (7.1) |
| NVQ3/GCE A-level equivalent | 18 (7.5) | 1 (7.1) |
| NVQ2/GCE O-level equivalent | 41 (17.2) | 2 (14.3) |
| NVQ1/CSE other grade equivalent | 24 (10.0) | 1 (7.1) |
| Foreign/other | 9 (3.8) | 0 (0) |
| No qualification | 80 (33.5) | 5 (35.7) |
| Missing | 7 (2.8) | 0 (0) |
| Medication intensity group, n (%) | ||
| Biologic DMARD therapy, n (%) | 52 (21.1) | 3 (21.4) |
| Combination non-biologic DMARD, n (%) | 72 (29.3) | 2 (14.3) |
| Single non-biologic DMARD, n (%) | 103 (41.9) | 8 (57.1) |
| No DMARD, n (%) | 19 (7.7) | 1 (7.1) |
| Michigan Hand Questionnaire baseline score 0 (worst)–100 (best) | ||
| Mean (SD), range (points) | 50.7 (16.3), 15–93 | 58.3 (16.8), 28–88 |
The findings are presented in seven sections:
-
acceptability and adherence to the SARAH intervention
-
establishing a routine: the key to success
-
the SARAH therapist: from teacher to facilitator
-
barriers to the SARAH exercises
-
gauging the personal effectiveness of the SARAH intervention
-
everyone is different; reasons the SARAH intervention might be effective for some people and not others
-
suggested changes to the SARAH intervention.
Acceptability and adherence to the Strengthening And stretching for Rheumatoid Arthritis of the Hand intervention
Acceptability of the Strengthening And stretching for Rheumatoid Arthritis of the Hand intervention
The SARAH intervention was well received, all 14 interviewees said they were satisfied with the programme and that they would recommend it to others. Although acceptability of the intervention as a whole was high, the interviewees did express preferences about the different elements of the programme and some elements worked better than others for different individuals.
The exercises
Half of the interviewees spoke of how easy the exercises were to understand and perform and eight spoke of the guidance and progression they had been given.
. . . so it was nice to start off at a level that you could cope with and then increase gradually as you went along.
Samantha; 4 months
There were more comments given about the exercises with the trial equipment which consisted of a selection of balls, putty and Theraband which provided different levels of resistance. The exercises involving the equipment often made interviewees split their exercises into those with and without equipment:
The easier ones from a personal point of view are the stretching ones. They’re quite good and you can do those when you’re . . . even in a meeting. They’re dead easy and after a bit you just . . . It becomes part of the routine. The ones that are less easy to fit in all of the time are the strengthening ones, which are the ones that I need to do more often I think because I want greater strength.
Kate; 4 months
The ball exercise was well received by twelve interviewees. Three of these spoke about changing the progression of the exercises:
. . . because as you got better, as you see, you could move from one up to a harder one and like at the weekend, I could go back onto the easier one you see, so it did help.
Harry; 4 months
One interviewee spoke about the balls being therapeutic:
In fact, that was therapeutic so that was quite nice. That’s when you get your stress balls don’t you? It’s a bit like that in a way.
Samantha; 4 months
Eleven interviewees commented on the putty exercises, which were well received by four individuals:
I think they [putty exercises] worked really well and they’re not too much. They’re not too complicated and once you get into the pattern of them.
Lucy; 4 months
Six interviewees spoke about difficulties regarding these exercises, three in general terms, and two had had to stop the putty exercises early on:
Well, I tried it for a couple of weeks but I couldn’t manage it, you know. It was just too difficult for me.
Sally; 4 months
One interviewee said that the difficulty was more to do with time and location:
I guess the greatest difficulty for me is actually making sure I could fit it into my day and particularly the ones where we were using the putty. Which actually do take longer to do and do require a slightly different environment.
Sadler; 4 months
One individual spoke of their difficulty and dislike for the exercise:
. . . the one I really don’t like, but I do is this one. You have to squeeze between the knuckles sideways like that and that is really quite . . . It’s not nice to do . . . It’s hard, it’s difficult.
Samantha; 4 months
One interviewee questioned the value of this exercise:
It was the only one that I found . . . You know, I couldn’t really see what it was actually doing for you, that one.
Joe; 4 months
Twelve interviewees commented on the Theraband exercise. Seven encountered some difficulties but had continued with them, sometimes at a lower repetition level. Some found the exercise harder to do than the others or that it could cause discomfort:
It was just more difficult.
Emily; 4 months
Two individuals actively disliked doing them:
Well, I hate the band. I still hate it now . . . I find that really uncomfortable . . . Yes, the actual exercise. I don’t like it at all and I still don’t. That’s the one . . . if I miss anything out, that’s the one I won’t do because I don’t like it.
Kate; 4 months
Two interviewees said it caused them pain:
It hurts and it doesn’t matter how we try it, it hurts . . . We just did very few. We just kept it really low and it just felt difficult.
Alice; 4 months
However, five interviewees had no problems with these exercises and one talked of liking a the progression of different bands:
It was a bit of a challenge, because I think I’ve now got a full range of everything because I’ve worked my way through and it was almost like an unsaid thing in my mind . . . I think I’ve got about three bands, four balls. I’ve got all of the stuff now because I gradually . . . and that was another positive in terms of because I was progressing.
Sadler; 4 months
The exercise booklet
The SARAH exercise booklet was mentioned by nine interviewees. Eight said it was useful and helped their technique:
I thought that was nicely presented. I thought it was very user friendly. It was done in such a way that I’ve always been able to read it and access it and with the visual cues and the fact that you’re shown . . . You can look at it as a picture and you can actually watch somebody in practice and I thought it was good.
Kate; 4 months
One person did not use the booklet:
To be quite honest, I didn’t really use it because I went through the exercises with [SARAH therapist] and then I went through them again the next week to make sure that I was doing them properly, so to be quite honest I didn’t really use the book at all.
Sally; 4 months
Completing the personal exercise guide
The personal exercise guide (PEG) promoted discussion around joint goal setting, the time and place of exercise and the participants’ confidence to do the exercises as set. When exercises were not completed, possible barriers to doing the exercises were explored. Both therapist and participant signed the PEG as a form of contract.
I think having that marker and actually trying to build it into your routine, which was a piece coming out in terms of right, okay create that commitment in terms of the contract and saying right, when are you going to do it, where are you going to do it, and actually building some of those key elements definitely for me works . . .
Sadler; 4 months
Thirteen interviewees spoke about goal setting. Six did not find goal setting useful:
Now I’m more and more aware that everybody keeps saying ‘You’ve got to set goals that you feel you’re achieving.’ No, I’m sorry. For me personally it’s not a big thing.
Lizzy; 4 months
However, five found goal setting useful:
. . . think it was a good idea. You know, it’s something to strive for and you get a bit of feeling for life and if you don’t have a goal to go for then I think it’s half the battle really isn’t it?
Joe; 4 months
Two were unsure:
I’m not sure really. I suppose it gives you something to focus on and at the time when I did it, she asked me in the first session and then we wrote it down in the second session. So, whilst I was talking to her I thought, ‘I don’t know.’ You know, ‘I don’t really know what I want to achieve.’ But when you come home and you start doing things and then you realise what you can’t do and how you would like to do things slightly better.
Sally; 4 months
Daily diary sheets
Daily diary sheets were considered helpful by 10 interviewees, especially in the early stages of establishing the exercises. These were introduced in the PEG and were helpful in different ways, some interviewees spoke of more than one of these. Nine of these 10 interviewees said the sheets were useful for keeping track of their exercises and two said that it acted as a reminder:
[the diary sheets] made sure that you’d done them and you hadn’t forgotten, you know. I know now exactly what I have to do, so I needn’t fill it in every time now.
Harry; 4 months
Two spoke of the sheets making them accountable to the therapist:
What it did give me the opportunity to do was record where I’d modified the exercises. Report back to the therapist. Not for me.
Bert; 4 months
One interviewee said it helped to motivate them:
. . . it also gives you that motivation, because you can actually look at that and think hey, I actually done pretty well this week.
Sadler; 4 months
Adherence to the Strengthening And stretching for Rheumatoid Arthritis of the Hand intervention
Most interviewees managed to keep with their exercise programme, a majority at 4 months and just over half at 12 months. Eleven of the interviewees were still doing their exercises at 4 months, the majority because they had gained or were hoping to gain some benefit, and three interviewees said their exercises had become a habit. Doing exercise was deemed to be proactive by some, and three were doing them in part because they had committed themselves to the trial. Two main reasons for non-compliance emerged from the narrative: first, when hand function was felt to be too good to benefit from the exercise and, second, when an increase in symptoms prohibited exercise.
At 12 months, 7 of the 13 interviewees were still doing their exercises, although sometimes extensively reduced or modified, with only Harry and Joe keeping to their full regime:
. . . what I actually put it down to is not having to go back to the hospital, and have that follow-up and have the exercises reassessed, and tailored a little bit upwards or downwards, depending on how it goes. So I haven’t almost got to complete my homework and hand it in, and obviously the impact of work. Those are the real two key factors in terms of trying to fit things in. So where on average I would probably say I was doing my exercises four or five times a week, at around that . . . I would say I was probably now down to three times every fortnight.
Sadler; 12 months
Of the six who were no longer exercising, four gave reasons similar to the first interviews; their hand function being too good or their symptoms preventing them. Two had other medical comorbidities which impacted on their symptoms and their ability to exercise e.g. gout and a thyroid operation. The three interviewees who were not exercising at 4 months were still not exercising at 12 months for the same reasons.
Establishing a routine: the key to success
It was very clear from the narratives that establishing a routine was the key to successfully carrying out the SARAH exercises. Some interviewees found that establishing a routine was easier to do than others and they employed different strategies or ways of doing this.
‘Fitting in’ exercise into life
Some found it easy to fit exercise into their lives and established a routine:
I always believe in getting things done early of a morning. I’ve always done that, so it was the best way of doing it.
Joe; 4 months
Ten interviewees spoke of differing adaptations they had adopted in order to establish a routine. They described changes in the exercise programme itself (n = 6), the timing of when they chose to exercise (n = 7) or the location in which they did them (n = 2). Sometimes these modifications were in response to their symptoms:
Sometimes it will be the quantity I have changed. Sometimes I’m not able to do as much and other times I know I can do more and it may be for a week or 2 weeks that I can actually do more. [. . .] What else have I changed? I don’t always do them in the same routine. I get bored of the routine . . . I start off with the first two because the second one can be particularly painful, so I like to get that one over and done with.
Lizzy; 4 months
Others spoke about the difficulty this sometimes presented:
Introducing it into the routine and being able to manage time was probably the key difficulty.
Lizzy; 4 months
Three spoke of strategies they employed to facilitate this change in routine, some combining them with other activities such as back exercises and others of leaving reminders to prompt themselves:
It’s quiet. There’s nobody else there. It’s peaceful and I often whip through them and I do my spinal exercises then as well.
Kate; 4 months
Of the 11 still doing the exercises at 4 months, four had continued with their original programme of exercises at one sitting. Three had found it more convenient to split them into two daily sessions. The other four had modified them by doing them throughout the day:
I was terribly, terribly organised at first and I would come in in the evening before I started cooking supper and I would go through the whole lot of exercises, but then sometimes I was late getting in from work or I had to get the girls somewhere and actually, it started to become quite difficult and you’d think 20 minutes out of a day isn’t that much, so that’s when we started to . . . and also I was finding if I was stiff and sore and it was actually the occupational therapy [SARAH therapist] who said you can break it down. You don’t have to do them all at once, so I took that to heart and actually it did make it so much easier.
Alice; 4 months
At 12 months, only two had kept to their original set of exercises, but most had further modified their exercises to fit them into their life in a more flexible way:
Now I have done the routine so much that I know what the sets are, so I tick the day that I have done them and then I put a half tick if I have done some but haven’t had time to do all of them, because if it’s for example, weekend and the routine changes . . . So you might only have half a set done on a particular day, but then other days normally, say, especially in the week it is better for a routine, you know you can go through and do the whole daily exercise list . . .
Samantha; 12 months
Exercise becoming a habit
Three spoke about their routine becoming a habit. A habit seemed to be different from a routine – it was suggestive of behavioural change and the subconscious habituation of including exercises in their lives:
Introducing it into the routine and being able to manage time was probably the key difficulty, but building that piece in terms of right, this is an activity that is now inbred within your general activity of a day and it becoming a habit is then just something that now evolves through naturally, and I think that’s a key difference for me.
Sadler; 4 months
The Strengthening And stretching for Rheumatoid Arthritis of the Hand therapist: from teacher to facilitator
Interviewees were not directly asked about their relationship with the SARAH therapist. However, they offered insights into their interactions and the therapist’s role in helping them to exercise. Eight interviewees spoke about the therapist in the interviews. All their encounters were spoken about in a positive context and suggested that a good rapport was attained. The SARAH therapist was integral to the interviewees’ ability to establish their exercise routine. The therapist was portrayed as a collaborator who helped participants to exercise in the right way, at a time and routine which was negotiated with the individual:
I mean [the SARAH therapist] took a lot of time. I felt she spent a lot of time with me on each session making sure that I understood and could do the exercises.
Sally: 4 months
Interviewees provided examples of how the therapist facilitated their adherence with the exercise programme. Interviewees said that therapists were motivational and reassuring and provided feedback on how they were doing:
Having that weekly contact is certainly a huge motivator and it tends to make it much more of a focus in sort of the week and then when you’re sort of cut loose and you’re left to your own responsibilities you have to be really, really motivated.
Alice; 4 months
During the therapy sessions interviewees were encouraged to discuss their progress (or regression) and the therapist tailored the programme, progressing them when able and giving follow-up advice as needed. The therapist monitored exercises for accuracy and suitability:
The first time you’re shown them and the physiotherapist does them with you and shows you how to do them and then the next time you go back she’s sitting there and we were doing them together and then after that, it was down to see how well had I improved, or not improved and how perhaps there was a technique I could use that would be a little bit different.
Lizzy; 4 months
Four interviewees spoke of the therapist tailor-making their exercises rather than a ‘one size fits all’, which was appreciated:
She’s so lovely and she was so kind to me and the fact that they’re all sort of modified to suit. You know, it wasn’t that you had this goal that if you didn’t achieve X amount of exercises you couldn’t carry on. It was all how much I could do, so once I found out I was quite excited because I thought it could only help me. It couldn’t do any harm.
Alice; 4 months
The therapist provided input throughout the course of the programme but the role of the therapist varied at the different stages and in response to the needs of the patient. Initially exercises were taught but the PEG introduced behavioural elements which the therapist and patient signed up to. The therapist’s role changed from a teacher of exercise to a collaborator and facilitator through shared goal setting and reviewing how the exercises had or had not been carried out. Barriers were explored, problems solved and new goals were set if appropriate.
The type of exercise provision given was new to many interviewees as seeing a therapist for detailed exercise advice was not a common experience. Five people said that they had had varied or sporadic advice about exercising from different disciplines through the rheumatology clinic, however over half felt that they had had no specific advice about exercise:
No one has really given me advice about exercise . . . No one has actually said to me beforehand, ‘You need to do some exercise.’
Mark; 4 months
Barriers to the Strengthening And stretching for Rheumatoid Arthritis of the Hand exercises
Alongside the interviewees’ descriptions of factors which helped them to carry out the SARAH programme, barriers were also identified. The majority of interviewees described barriers to doing exercise with RA in general, and many of these applied to the doing the SARAH programme. There were some barriers that were specific to carrying out the SARAH programme. Barriers were classified as intrinsic and extrinsic barriers.
Intrinsic barriers
Intrinsic barrier came from within the individual. The most important was physical symptoms such as pain, stiffness, flare-ups, swelling or feeling unwell:
. . . but then they got more difficult to do to the stage where no, I can’t do anymore because my hands hurt so much.
Susan; 4 months
A negative mindset fostering poor motivation, procrastination and avoidance was also considered to be an internal barrier to exercise. Two spoke of a reluctance to exercise because the exercises reminded them of their RA:
Sometimes it’s attitude of mind, because there are definitely down days when things are not . . . You just don’t want to have it anymore and you want to give it to somebody else and that would certainly impact on whether or not . . . how well I did them. I know it did, because emotionally you have to be quite secure and stable don’t you?
Kate; 4 months
There were also challenges staying with the programme over time:
And I’d settled down to quite a pattern with the exercises. When I was off the hook with [the SARAH therapist] at occupational therapy, it became much more difficult to be disciplined about doing them every day. And after a period of time, I have to confess, that I ended up doing them probably three times a week, the whole programme, interspersed when I was sitting having a cup of coffee I would be doing odd ones just to keep it moving.
Lucy; 12 months
In relation to general exercises for RA, participants expressed concerns or fear around exercising with RA and the narratives were examined to see if this was a barrier to doing the SARAH programme. This did not appear to be the case, and in fact there was a noticeable absence of caution or fear of doing the SARAH exercises and five participants actually said that ‘no harm’ was associated with them:
Honestly, I can’t see that it can do any harm.
Lizzy; 12 months
Extrinsic barriers
Extrinsic factors were those surrounding the individual such as competing commitments on their time and changes in their routine which stopped them from exercising.
Competing priorities was given as the main barrier to doing the SARAH exercises, especially being busy with household and family commitments:
I was trying to catch up in the garden and do things in the garden and all of a sudden I was trying to do things in the house I hadn’t done for a while and the exercises sort of got put by the wayside and I thought well, at least I’m exercising doing things, so I didn’t worry too much.
Emily; 4 months
The time it took to fit the SARAH exercises into their day was an issue for some. Five interviewees spoke about the difficulties of exercising when they were out of their normal routine. Examples given were going on holiday, working away or visiting family. The trial equipment was also mentioned with this theme, as when away they were unlikely to take the equipment. Samantha also considered it antisocial to do the exercises when visiting family:
. . . we went down to my dad’s for a long weekend and so that was difficult because you’re doing something out of your routine, so anything out of routine, you’ve got to think; well where can I fit them in and that was tricky and sometimes it was antisocial as well, so where you should be joining in with something you couldn’t be doing these exercises.
Sam; 4 months
Gauging the personal effectiveness of the Strengthening And stretching for Rheumatoid Arthritis of the Hand intervention
We hoped to gain an understanding of the impact the SARAH intervention had on patient symptoms and how interviewees measured the personal effectiveness of the programme.
Ten interviewees felt that the SARAH exercise program had worked for them, although Emily and Lizzy were not sure if it was their medication or a combination of the intervention and medication. All 10 interviewees described how they felt the intervention had worked for them with physical improvements being the most prevalent. An improvement in function was most cited; functional gains were described by some in terms of being able to: do more in the garden (Samantha), do household tasks such as ironing and drying the dishes more easily (Harry), pick things up more easily (Lucy) and decrease their reliance on splints (Alice). Physical improvements were most often in strength or grip and flexibility or increased ROM:
Using the secateurs and also just to pull the stubborn weeds out. You can probably grip those better and pull them out easier and lifting certain things with my hands and so for example, the container off the lawnmower. I could probably do that a bit easier and maybe shopping bags a bit better. What else; maybe a couple of jars with the tin opener.
Samantha; 4 months
Some spoke of improvements in their symptoms with regard to their exercises. Joe stated that since doing the exercises he had had no flare-ups, and Alice said that her hands felt better after exercising.
Psychological constructs were difficult to characterise; some felt the benefit was ‘feeling better’ without further elucidation. Mark and Kate felt that the sessions had given them a focus, and Lizzy spoke of an increased awareness of her hands:
At 12 months, nine respondents still spoke of physical improvements:
I knew there was so much more flexibility in my hands. So the flexibility and, I suppose to a certain extent, the strength was quite significant.
Lucy; 12 months
However, they also spoke of a higher proportion of psychological improvements at 12 months when compared to 4 months. The most notable being in confidence and increased awareness, suggesting it took time for confidence in improved hand function to develop and translate into everyday function. Additional psychological benefits were a sense of empowerment, a hope of slowing their disease progress or maintaining their function.
Samantha, Alice and Lucy spoke of an increased confidence in their hand function:
It’s little things. I’m probably more confident with picking things up and I’m more confident with the manual dexterity. I mean my handwriting is still pretty appalling at times, but I was quite anxious about picking things up and dropping things and that’s less of an issue now.
Alice; 12 months
Alice and Sadler spoke of a confidence in the exercise programme itself, which had increased their confidence to continue with the exercises. Kate thought that it had empowered her to be in control of her RA, the SARAH intervention had given her hope that she could improve or control her symptoms:
Because emotionally and psychologically I feel more at ease with it and it’s about the power of thought for me, whereas before when I was first diagnosed with it I didn’t have any control over it whatsoever, whereas I’m actually doing something that’s practical that might help and ‘might’ is enough. It doesn’t have to be ‘will.’ It might and therefore, if that is in my hands to make that change I can do that. If it’s not in my hands I’m helpless and therefore that’s actually quite a difficult place to be, so the SARAH project for me I think has been really quite valuable because it’s empowered me.
Kate; 12 months
An increased awareness of their hands was spoken about by five interviewees. Participants had become more aware of addressing their limitations (Lucy), the importance of keeping moving and looking after their hands (Lizzy, Samantha) or that it gave them other ways of doing things (Alice):
Yes, they [the exercises] are valuable. Because they’ve made me realise how important it is to keep moving, it would be so easy to say, ‘Well I can’t do that’.
Lizzy; 12 months
Alice, Sadler and Samantha were hopeful that the exercises may possibly slow the rate of progress of their symptoms and talked about the exercises ‘maintaining what they had’:
And I don’t know if 5 years down the line I’m going to be so grateful I did this, and if this is going to help to maintain function and strength then I’m the only one who’s going to benefit from that, so if I don’t put it in I’m not gonna get it out. And it’s so little to ask to do.
Alice; 12 months
Four participants felt that the SARAH intervention had not worked for them: Jean because she hadn’t done the exercises, Susan because of a longstanding flare-up which hadn’t responded to treatment, Bert because he felt his hands were too good prior to randomisation (and he wasn’t expecting any improvement) and Sally because she hadn’t had any noticeable change yet (but was still hoping for change over the rest of the trial period).
Everyone is different: reasons the Strengthening And stretching for Rheumatoid Arthritis of the Hand intervention might be effective for some people and not others
There was recognition from interviewees that different things may work for different people and they were able to offer reasons as to why the SARAH intervention might be effective for some people and not for others. Almost all interviewees gave insights at 4 or 12 months giving rise to four main themes: (1) a positive mindset, (2) the level of disease, (3) reasons to exercise and (4) incorporating exercise into lives:
So I guess it’s all going to be about individuals in terms of how they’re . . . what their mental state is, what their total environment is, what else is going on, but ultimately, it’s that balance between spending the time doing it, either finding the time to do it in my case, putting yourself through the rigours of trying to do it and the potential discomfort that that may bring, versus the long-term benefit. It’s that balance which is ultimately going to be the equation that’s going to make it successful or not.
Sadler; 12 months
A positive mindset is important
Interviewees spoke of a positive mindset and that being motivated, committed and willing to put in the effort was desirable. Being self-disciplined and taking responsibility for your health were also considered to be useful traits. Conversely, not having the motivation or commitment, viewing the exercise as tricky, an effort or ‘easier not to do them’ could mean that it might not work. Being unrealistic about the amount of effort needed or wanting instant results also would not be useful:
Some people won’t do it for one thing. You’ve got to be motivated. You’ve got to want to do it. You’ve got to want to put the effort in, although this really is not a lot of effort. It’s actually quite minimal and I think you probably get a lot of benefit out of it, but I don’t know if everybody would see that
Kate; 4 months
The level of the disease was considered to be a contributing factor
Interviewees thought that giving exercise early in the disease process or when it was not severe might help the intervention to work. Many felt that it might not work if the disease was advanced, in a flare-up or causing pain and stiffness:
I think the exercises will work for people that have got arthritis as long as they’re not in a flare-up. I think if you were in a flare-up then no exercises are any good at all.
Susan; 4 months
People need reasons to exercise
Interviewees felt that people needed reasons to exercise, such as having a past experience of exercise being useful, the hope of some benefit or being accountable to the study and that these might make the intervention work. Conversely it might not work if they did not have a good enough reason to engage, preferred solely to take drugs rather than take exercise or weren’t accountable in some way.
Well, some might not keep to it you see. They might not . . . they might do it for a few days and then say, ‘Oh well that’s it’, or if they haven’t got a good enough reason to do it, you know?
Harry; 4 months
Beliefs such as ‘people may think they are irreparable’ may also stop them from engaging with an exercise programme. One person spoke of individuals having to balance many factors, alluding once more to competing priorities.
People need to incorporate exercise into their lives
Fitting the exercises into their lives and doing them regularly was thought to help make the intervention work. Conversely, not doing the exercise, not having the time or being out of routine might make it not work. One person said that people might need more encouragement, explanation, contact and some may prefer a different setting such as groups, reiterating how individual this may be.
Suggested changes to the Strengthening And stretching for Rheumatoid Arthritis of the Hand intervention
At 4 months, half the interviewees said that they would not change anything about the intervention. Three areas of potential change were identified from the remaining seven interviewees, and these seem to be mostly about practical administration of the intervention. Individual comments were given about the intervention content and suggestions included that daily diary sheets could be put in a ring folder with more space to write explanatory comments, the putty exercises could be further modified and the bag catch would have been better with a hook and loop fastening (rather than a clip fastening). Suggestions were given concerning the intervention format and setting. One interviewee suggested a group setting for interaction and accountability, but another felt a group setting may not work. Another interviewee suggested delivering the programme in a community setting for ease of parking.
At 12 months, however, suggestions given by six people all concerned ways in which people could be helped to ‘stay with’ the programme, three advocating groups. Three areas of change emerged. Firstly, more inventive ways were suggested to help to establish a routine and help incorporate the exercises into their lifestyle:
To change habits is quite difficult. [. . .] So I think it would be very good to carefully sit down with somebody and just honestly say where could you fit this in, in your routine, to help them maximise the chance of completing the exercises and saying ‘that exercise is really going to take you that long to do.’ This exercise, you’ve got to find that putty, where is it? . . . So if people haven’t got time to do that, they are not going to do it. So to really help them, with putting in place a plan that would fit in to their routine, and they’re are more likely to get it done.
Samantha; 12 months
Secondly, an increase of initial support by health-care professionals may help to establish a routine and then a ‘touching base’ after the end of the therapist input may be useful:
. . . but it needs to be fairly intense at the beginning just to guide the patient through it, what they are doing, what they are achieving or even keep an eye on what they are not achieving.
Bert; 12 months
Thirdly, interviewees gave ideas of other support systems which could be useful such as groups, peer/mentor support, text messages, email or ‘YouTube’ videos to help people stay engaged with the process:
It could have been done in a group but it was, because really you could all do the same exercises together in a group. But it is nicer when you’re one to one with a person because you can talk about other things as well.
Mark; 12 months
I don’t see why you couldn’t have half a dozen people at a table . . . and it might give . . . it might contribute towards the commitment element. That it would bring people together.
Lucy; 12 months
I think it would be useful to know what type of activity people enjoy, some people like groups, some people don’t. [. . .] I’d have hated it. But that’s just me. But other people would see it as a much more social event and would perhaps find support from being with other people. Sometimes it would have been nice to have another person who was on the trial to sort of say, ‘I’m really finding that one difficult’ . . . so that you had people that (you) could contact . . . I know that might not be feasible, but I do think actually that sort of mentor support is useful. Particularly if it’s such an ongoing long-term programme . . .
Alice; 12 months
One thing that’s just going through my mind is the use of the new technology and the internet and is there something, in terms of keeping in touch, that that could be factored in, because clearly it’s not easy to have that time, both from the patients perspective and from a healthcare professional perspective, people coming in, being on the road and coming in and meeting people. Could we use the internet or email or whatever, to actually do that. Is there any, almost like ‘YouTube’ video place that you could have access to, to remind you what you should be doing and things that . . .
Sadler; 12 months
Discussion
The SARAH programme is feasible and acceptable to patients with relatively stable RA symptoms. All interviewees said they were satisfied with the programme and would recommend it to others and they seemed confident in the delivery and continued use of the exercises. It has been reported that patients with RA are cautious about exercising owing to concern such as doing harm or damage. 107 However, interviewees in this study did not express these concerns regarding the SARAH exercise programme, providing further evidence that the programme was acceptable to patients.
The importance of establishing a routine was the major emergent theme throughout the interviews and the key to successfully carrying out the exercise intervention. Being out of routine was suggested as a barrier to doing the exercise programme. Establishing a routine was easy for some interviewees but others required support and encouragement. Interviewees found some elements of the programme challenging, for example the putty and Theraband exercises, which needed the most adaptation to the individual to ensure they were completed. This study has highlighted the importance of the therapist in enabling patients to establish a routine and incorporate the exercises into their lives and teaching patients to modify exercises if needed (e.g. if painful or during a flare-up). Competing priorities were highlighted as the biggest barrier to doing the exercises regularly. Physical symptoms experienced by individuals were another important barrier to exercising. The need for a reason to exercise was also emphasised. Individuals who thought that their hand function was good, and hence that they did not need to do the exercises, were also unlikely to carry out the programme.
Our study is in agreement with a previous interview study (n = 6) by Ronningen et al. ,69 who explored the effect of a hand exercise programme. This study found that those successful in maintaining a programme had incorporated it into their daily routine, and stated the importance of tailoring programmes to the individual allowing for changes in their symptoms or ability.
When asked about the effects of the intervention, the most striking difference between the two time points was the mention at 12 months of more psychological improvements alongside the physical. One possible mechanism for this may be that the physical improvements early on resulted in increased confidence (self-efficacy) in participants’ own abilities to carry out exercises or activity in general. Greater self-efficacy is often associated with better health outcomes and could potentially also mean that patients were less fearful of exercise or activity. Swardh et al. 108 (n = 18) explored patients with RA’s experiences of exercise maintenance. They concluded that individuals have differing needs in terms of context and support but advocated the importance of a self-efficacy approach to prepare someone for exercise maintenance, helping the patient to adapt and problem solve, a similar ethos to the SARAH intervention.
Implications for clinical practice
We have demonstrated that the behavioural elements of the SARAH intervention were successfully implemented and enhanced the more traditional exercise provision role of therapists. The use of behavioural strategies such as completing the PEG may not be familiar to all therapists. However, they were an important aspect of the SARAH exercise intervention so need to be delivered alongside the exercises. The role of the SARAH therapist changed from teacher to facilitator, enabling the patient to gain expertise and subsequently to exercise independently (Figure 7). Therapists who deliver the intervention need to be trained in all elements of the programme but training is straightforward and could be incorporated into current therapy departmental training programmes.
FIGURE 7.
Therapist and patient roles in the SARAH intervention.
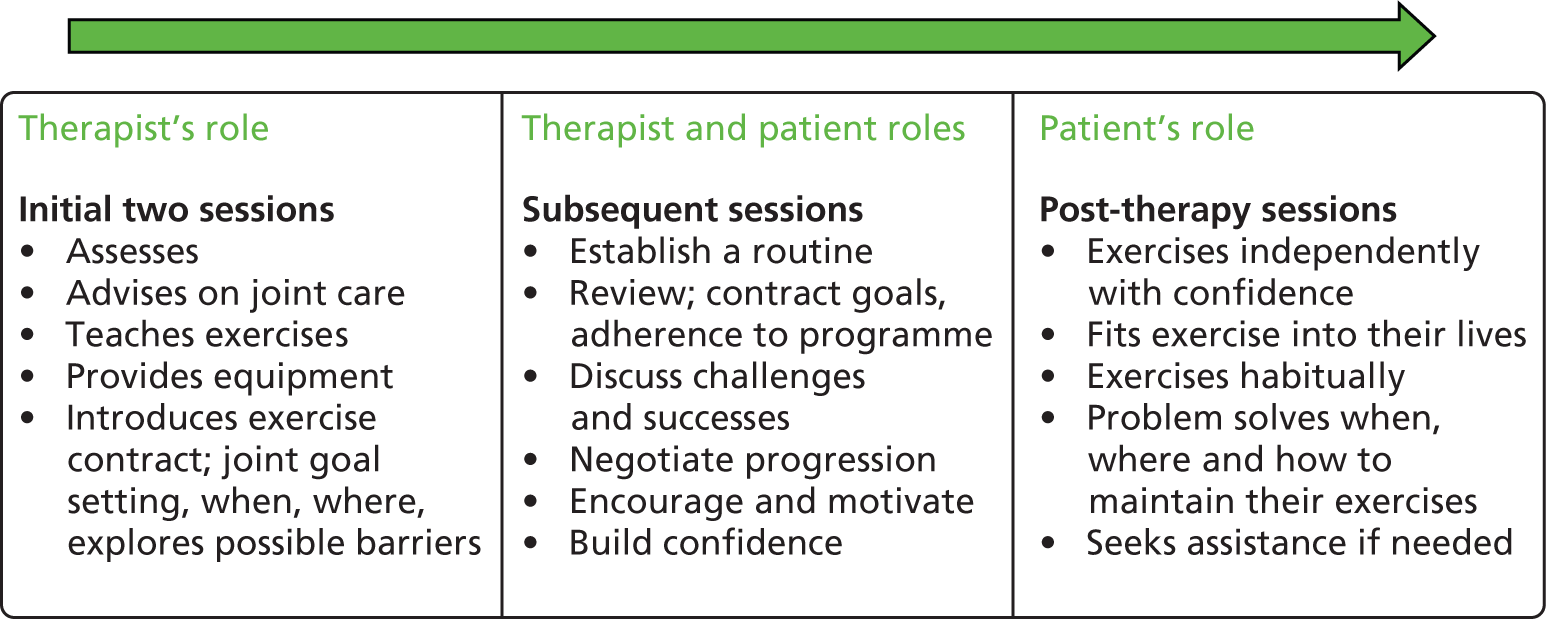
Further research
Several participants suggested the exercise programme could be delivered in a group setting, but this needs to be evaluated before it could be recommended.
Different methods of providing on-going support for patients as a way of ensuring long term adherence of the exercise programme were suggested. These ranged from ‘touching base’ visits to the therapist to the use of on-line resources such as ‘YouTube’. Evaluating ways of providing support is another area of further research.
Chapter 6 Health economics
Introduction
The cost-effectiveness analysis of the SARAH exercise intervention compared with usual care is presented in this chapter. Cost-effectiveness is estimated using a ‘within-trial’ analysis, based on health-care resource use and utility data collected alongside the clinical data over the 12-month trial period. We considered developing a model to predict longer-term impacts of the intervention, but concluded that in this case it would not help to quantify or resolve uncertainty for decision makers. The prospects for modelling are discussed further below.
The cost-effectiveness analysis was guided by an analysis plan prepared before data were available, and follows the NICE reference case109 and International Society for Pharmacoeconomics and Outcomes Research (ISPOR) recommendations on conducting economic evaluations alongside clinical trials. 110 The economic analysis uses an intention-to-treat approach. Effectiveness was measured using quality-adjusted life-years (QALYs), estimated by the area-under-the curve method from patient-reported health status at baseline, 4 and 12 months using the EQ-5D-3L questionnaire, with preference weights from a UK general population (the UK Social Tariff). 111 For comparison, we also estimated QALYs using the Short Form questionnaire-6 Dimensions (SF-6D) utility index. 112 NHS perspective was used for the costing. Costs were estimated for each participant over 12 months of follow-up, based on patient-reported use of a list of health services potentially influenced by hand function, RA status or side effects of treatment. Unit costs were obtained from national sources, and the cost of the intervention estimated from trial records. Given the 1-year time horizon, there was no need to discount costs or QALYs for time preference.
Unit cost data
Intervention costs
Participants in both treatment groups attended physiotherapy or occupational therapy appointments during the course of the trial. Assumptions used to cost the therapist time for these appointments are shown in Table 66. For the usual care group (control), the trial protocol specified a maximum of one and a half hours therapy spread over three sessions, although in practice some control patients attended more sessions than this (n = 6 and often for splinting issues). Usual care therapy included JP education and advice, simple mobility exercise for the hand and functional splinting.
| Section costed | Type of costing | Amount | Source |
|---|---|---|---|
| Training for exercise programme | Total therapist hours spent on training (hours) | 183 | Trial records |
| Number of therapists | 56 | Trial records | |
| Mean training time per therapist (hours) | 3.3 | ||
| Therapist cost per hour | £35 | PSSRU estimate (Curtis 2011)113 | |
| Total cost of training for trial | £6405 | ||
| Patients attending at least one session | 473 | Treatment logs | |
| Cost per patient attending at least one session | £13.54 | ||
| Treatment sessions | First session (minutes) | 45–60 | Estimate |
| Second session (minutes) | 45 | Estimate | |
| Next three sessions (minutes) | 30 | Estimate | |
| Final session (minutes) | 45 | Estimate |
Participants in the usual care and exercise group (experimental) received the usual care regimen supplemented with additional sessions of an optimised exercise programme delivered by specially trained physiotherapists and occupational therapists over a 12-week period. Participants were invited to attend six 30- to 45-minute sessions, given an individualised exercise programme to perform at home, and asked to keep a record of their activities. Training time for therapists to provide the optimised exercise programme ranged from 3 to 5 hours depending on site. In the base-case analysis we allocated the full cost of physiotherapist time spent on training (183 hours at £35 per hour) across the trial participants who attended at least one treatment session (n = 473) (see Table 66). This entails a conservative assumption that the training has no residual value. In practice, there would be a one-off cost for training physiotherapists new to the intervention, and then an on-going cost for training due to staff turnover and possibly also ‘top up’ training for existing staff.
Participants in the exercise programme arm who attended at least one session were given additional information and equipment for home exercises (Table 67). Each participant received an exercise booklet with a description of all the exercises they were to perform at home and in clinic, an exercise diary at each session (printed A4 sheet of paper), a PEG, a discharge advice information sheet and a spare exercise diary, a cardboard folder to hold all the paperwork, and a blue bag to keep this and the exercise materials in. Depending on whether or not there were any major changes to the participants’ goals or programme, they may have received between one and five personal PEGs. Exercise materials included putty, balls and Therabands. These were supplied according to each participant’s capabilities and may have changed as they progressed through the programme. The quantity and colour of the material (which reflects resistance level) that each participant received was individualised. In the cheapest case, a participant would have received 100 g of putty, one ball and a 1 m length of Theraband. In the most expensive case, materials would have been changed at every exercise session: 500 g putty, five balls and 4 m of Theraband.
| Resource item | Unit | Cost per unit (£) | Mean per participant | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Worst case | Best case | |||||||
| Quantity | Cost (£) | Quantity | Cost (£) | Quantity | Cost (£) | ||||
| Blue bag | Item | 1.7 | 1 | 1.7 | 1 | 1.7 | 1 | 1.7 | |
| Exercise booklet | Item | 1.43 | 1 | 1.43 | 1 | 1.43 | 1 | 1.43 | |
| Folder | Item | 1.85 | 1 | 1.85 | 1 | 1.85 | 1 | 1.85 | |
| Exercise diary | Item | 0.1 | 1 | 0.1 | 1 | 0.1 | 1 | 0.1 | |
| Discharge advice | Page | 0.02 | 1 | 0.02 | 1 | 0.02 | 1 | 0.02 | |
| Personal exercise guide | Item | 0.18 | 3 | 0.54 | 5 | 0.9 | 1 | 0.18 | |
| Putty | 100 g | 2.29 | 1.92 | 4.4 | 5 | 11.45 | 1 | 2.29 | |
| Ball | Yellow/red/green/blue | Item | 15.97 | 1.98 | 31.62 | 4 | 63.88 | 1 | 15.97 |
| Black | Item | 19.6 | 0 | 0 | 1 | 19.6 | 0 | 0 | |
| Subtotal | 31.62 | 83.48 | 15.97 | ||||||
| Theraband | Beige | 1 m | 1.21 | 1 | 1.21 | 1 | 1.21 | 1 | 1.21 |
| Yellow | 1 m | 1.3 | 0.42 | 0.55 | 1 | 1.3 | 0 | 0 | |
| Red | 1 m | 1.41 | 0 | 0 | 1 | 1.41 | 0 | 0 | |
| Green | 1 m | 1.49 | 0 | 0 | 1 | 1.49 | 0 | 0 | |
| Subtotal | 1.76 | 5.41 | 1.21 | ||||||
| Total cost of consumables | 43.42 | 0 | 106.34 | 0 | 24.75 | ||||
The estimated costs of hand therapy by the number of sessions attended are shown in Table 68. Following our base-case assumptions, the cost for a usual-care patient attending three sessions is £83, compared with £186 for a patient in the intervention group attending all six recommended sessions. Intervention costs were estimated at the individual patient level, based on the recorded number of session that they attended.
| Type of costing | Session type | Exercise programme (£) | Usual care (£) | ||||
|---|---|---|---|---|---|---|---|
| Base case | Worst case | Best case | Base case | Worst case | Best case | ||
| Therapist training | (If at least one session attended) | 6.77 | 13.54 | 0.00 | 0.00 | 0.00 | 0.00 |
| Consumables | 43.42 | 106.34 | 24.75 | 0.00 | 0.00 | 0.00 | |
| Therapist time | Session 1 | 30.63 | 35.00 | 26.25 | 30.63 | 26.25 | 35.00 |
| Session 2 | 26.25 | 26.25 | 26.25 | 26.25 | 26.25 | 26.25 | |
| Sessions 3–5 | 17.50 | 17.50 | 17.50 | – | – | – | |
| Session 6 | 26.25 | 26.25 | 26.25 | – | – | – | |
| Total cost by number of sessions attended | 0 sessions | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 1 session | 80.82 | 154.88 | 51.00 | 30.63 | 26.25 | 35.00 | |
| 2 sessions | 107.07 | 181.13 | 77.25 | 56.88 | 52.50 | 61.25 | |
| 3 sessions | 124.57 | 198.63 | 94.75 | 83.13 | 78.75 | 87.50 | |
| 4 sessions | 142.07 | 216.13 | 112.25 | 109.38 | – | 113.75 | |
| 5 sessions | 159.57 | 233.63 | 129.75 | 135.63 | – | 140.00 | |
| 6 sessions | 185.82 | 259.88 | 156.00 | 161.88 | – | 166.25 | |
| 7 sessions | – | – | – | 188.13 | – | 192.50 | |
| 8 sessions | – | – | – | 214.38 | – | 218.75 | |
The cost of training therapists is allocated across 473, 237 and 0 intervention participants for the worst-case, base-case and best-case scenarios respectively (473 patients attended at least one session in the trial). For simplicity, the cost of consumables is allocated to all intervention patients who attend at least one session. We conducted a simple one-way sensitivity analysis using the worst-case and best-case estimates (see Discussion).
Unit cost of medications
Information about prescribed medications was collected from participants in the baseline, 4-month and 12-month interviews. A list of all medications recorded was collated, and items related to RA were identified for inclusion in the economic analysis (Table 69). This list included NSAIDs, biological and non-biological disease-modifying drugs, analgesics and gastroprotective agents (possibly related to side effects of NSAID treatment). NHS net prices per mg for these medications were obtained from the British National Formulary (BNF). 115 Costs for individual patients were estimated based on their reported dose and frequency if this information was available, or otherwise on an assumed daily dose. This assumed dose was based on the World Health Organization defined daily dose (WHO DDD),116 or on BNF recommended doses if no WHO DDD was available. For the biologic disease-modifying drugs, doses were based on NICE recommended doses, and the cost of injections and infusions was also included based on NICE guidance117 (Table 70).
| Drug name | Units | Cost/mg (pence) | Dose/day (mg) | Cost/day (pence) | Source of dose estimate |
|---|---|---|---|---|---|
| NSAIDs | |||||
| Acemetacin | Capsules | 0.522 | 120 | 62.7 | WHO DDD |
| Aspirin | Tablet | 0.003 | 1200 | 3.9 | BNF |
| Celecoxib (Celebrex®, Pfizer) | Capsules | 0.359 | 200 | 71.8 | BNF |
| Diclofenac | Tablet | 0.461 | 100 | 46.1 | WHO DDD |
| Diclofenac/misoprostol (Arthrotec®, Pfizer) | Tablet | 0.399 | 100 | 39.9 | BNF |
| Etodolac | Capsules | 0.045 | 400 | 18.1 | WHO DDD |
| Etoricoxib | Tablet | 1.665 | 60 | 99.9 | WHO DDD |
| Ibuprofen | Tablet | 0.009 | 1200 | 10.3 | WHO DDD |
| Ibuprofen gel | Gel | 0.007 | 1500 | 10.5 | Underwood et al.114 |
| Indomethacin (indometacin) | Capsules | 0.321 | 100 | 32.1 | WHO DDD |
| Meloxicam | Tablet | 0.293 | 15 | 4.4 | WHO DDD |
| Mobic | Tablet | 4.133 | 15 | 62.0 | BNF |
| Nabumetone | Tablet | 0.017 | 1000 | 17.0 | WHO DDD |
| Naproxen | Tablet | 0.018 | 500 | 8.9 | WHO DDD |
| Piroxicam | Gel | 0.004 | 20 | 0.1 | WHO DDD |
| Biologic DMARDs | |||||
| Adalimumab (Humira®, Abbott Laboratories) | Injection | 880.350 | 2.9 | 2515.3 | BNF |
| Certolizumab | Injection | 178.75 | 14.3 | 2553.6 | BNF |
| Etanercept (Enbrel®, Amgen) | Injection | 357.52 | 7.1 | 2553.7 | BNF |
| Infliximab (Remicade®, Schering-Plough) | Infusion | 419.62 | 6.1 | 2547.7 | BNF |
| Rituximab | Infusion | 174.63 | 17.9 | 3118.4 | BNF |
| Tocilizumab | Infusion | 128.00 | 24 | 3108.6 | BNF |
| Non-biologic DMARDs | |||||
| Azathioprine | Tablet | 0.180 | 150 | 27.0 | WHO DDD |
| Ciclosporin (Capsorin®, Novartis Pharmaceuticals Ltd) | Tablet | 1.748 | 250 | 437.0 | WHO DDD |
| Gold (sodium aurothiomalate) | Injection | 22.460 | 7.1 | 160.4 | BNF |
| Hydroxychloroquine | Tablet | 0.043 | 516 | 22.1 | WHO DDD |
| Leflunomide | Tablet | 15.333 | 20 | 306.7 | WHO DDD |
| Methotrexate (oral) | Tablet | 4.671 | 2.5 | 11.7 | WHO DDD |
| Methotrexate (injection) | Injection | 197.067 | 1.1 | 211.1 | BNF |
| Prednisolone | Tablet | 4.671 | 10 | 46.7 | BNF |
| Sulfasalazine | Tablet | 0.026 | 2000 | 53 | BNF |
| Analgesics | |||||
| Buprenorphine | Tablet | 50.300 | 0.2 | 10.1 | BNF |
| Buprenophine (BuTrans Patches®, Purdue) | Patch | – | – | 112.1 | Assume ‘10’ patch |
| Capsaicin (Zacin®, Teva) | Cream | 0.039 | 1500 | 59.0 | Underwood et al.114 |
| Co-codamol (30 mg codeine, 500 mg paracetamol) | Tablet | 0.118 | 240 | 28.3 | BNF |
| Codeine | Tablet | 0.099 | 1000 | 99.4 | BNF |
| Co-dydramol | Tablet | 0.353 | 80 | 28.3 | BNF |
| Dihydrocodeine | Tablet | 0.165 | 150 | 24.8 | WHO DDD |
| Fentanyl patches | Patch | – | – | 91.3 | Assume ‘12’ patch |
| Gabapentin | Capsule | 0.073 | 1800 | 132.1 | WHO DDD |
| Morphine Sulphate [Minijet®, International medication systems (UK) Ltd] | Injection | 150.0 | 30 | 4500 | WHO DDD |
| Morphine Sulphate (Sevredol®, Napp Pharmaceuticals Ltd) | Tablet | 0.945 | 100 | 94.5 | WHO DDD |
| Oxycodone | Tablet | 12.543 | 75 | 940.7 | WHO DDD |
| Paracetamol | Tablet | 0.002 | 3000 | 6.0 | WHO DDD |
| Pregabalin | Tablet | 0.767 | 300 | 230.0 | WHO DDD |
| Codeine phosphate (Solpadol®, Sanofi-Synthelabo) | Caplet | 0.225 | 240 | 53.9 | BNF |
| Tramadol hydrocholoride and paracetamol (Tramacet®, Grüenthak Ltd, UK) | Tablet | 0.430 | 300 | 129.1 | BNF |
| Tramadol | Capsule | 0.076 | 300 | 22.8 | WHO DDD |
| Cocodamol (Tylex®, UBC Pharma Ltd) | Capsule | 0.257 | 240 | 61.6 | BNF |
| Gastrointestinal | |||||
| Lansoprazole | Capsule | 0.286 | 30 | 8.6 | WHO DDD |
| Nizatidine | Capsule | 0.164 | 300 | 49.3 | WHO DDD |
| Omeprazole | Capsule | 0.289 | 20 | 5.8 | WHO DDD |
| Pantoprazole | Tablet | 0.179 | 40 | 7.2 | WHO DDD |
| Ranitidine | Tablet | 0.016 | 300 | 4.9 | WHO DDD |
| Robeprazole Sodium | Tablet | 3.491 | 20 | 69.8 | BNF |
| Medication | Injections | Infusions | Administration cost (£) | |
|---|---|---|---|---|
| Per annum | Per annum | Per annum | Per day | |
| Adalimumab (Humira®, Abbott Laboratories) | 26 | 111 | 0.30 | |
| Certolizumab | 26 | 111 | 0.30 | |
| Etanercept (Enbrel®, Amgen) | 104 | 442 | 1.21 | |
| Infliximab (Remicade®, Schering-Plough) | 7 | 1078 | 2.95 | |
| Rituximab | 3.78 | 582 | 1.59 | |
| Tocilizumab | 13 | 2002 | 5.48 | |
Unit costs of other health services
The 4- and 12-month patient questionnaires included questions about their use of a range of other NHS services related to their hand arthritis (Table 71). Unit costs were collected for each of these items from national published sources: Department of Health reference costs118 or Personal Social Services Research Unit (PSSRU). 113
| Resource item | Unit | Mean cost (£ per unit) | Sources |
|---|---|---|---|
| Physiotherapist | Session | 38 | Ref cost: 650A |
| Occupational therapist | Session | 56 | Ref cost: 651A |
| General practitioner | Session | 31 | PSSRU |
| Rheumatologist | Session | 138 | Ref cost: 410 |
| Orthopaedic surgeon | Session | 99 | Ref cost: 110N |
| Other specialist | Session | 110 | Ref cost: weighted mean of orthopaedic and rheumatology outpatient |
| Nurse | Session | 49 | Ref cost: CN203AAF |
| Podiatrist/chiropodist | Session | 43 | Ref cost: 653 |
| Self-management group | Session | 5.8 | PSSRU: assume 1-hour group of six patients led by an occupational therapist |
| Occupational therapist | Hour | 35 | PSSRU |
| Inpatient stay | Episode | 2676 | Ref cost: elective and non-elective (long and short stay) HRG HB13Z, HB14B, HB14C, HB23B, HB23C, HB32A, HB33D, HB33E, HB53Z, HB54B, HB54C, HB62B, HB62C, HB72Z, HB91Z, HB99Z |
| Day case treatment | Episode | 1308 | Ref cost: day case HRG HB13Z, HB14B, HB14C, HB23B, HB23C, HB32A, HB33D, HB33E, HB53Z, HB54B, HB54C, HB62B, HB62C, HB72Z, HB91Z, HB99Z |
| X-rays | Test | 5 | Assumption |
| CT scan | Test | 93 | Ref cost: RA08Z–RA10Z |
| MRI scan | Test | 167 | Ref cost: RA01Z–RA03Z |
| Blood tests | Test | 3 | Ref cost |
Resource use and costs
Prescribed medication usage was acquired at baseline, 4 months and 12 months. Participants were asked to list the drugs that they were currently taking, and to report the dose and frequency of use. For the included range of medications (as listed in Table 69 above), the mean daily doses between time points were taken and assumed to be constant across the period, to give an estimate of daily dose (in mg) during the two time periods 0–4 months and 4–12 months. Where information about an individual patient’s dose or frequency of use was missing or difficult to interpret, we assumed an average daily dose as listed in Table 69. The resulting estimates of daily dose for each drug were then multiplied by unit costs and where relevant the costs of administering infusions and IV (see Table 70) were added. This yielded an estimate of the cost of prescribed medications for each individual over the two time intervals (Table 72). The mean cost per patient of prescribed medications over the 12 month trial period was similar for the treatment groups: £1641 for the exercise group compared with £1721 for the usual care group.
| Time point | Number of patients | Mean cost, £ (SE) | Mean treatment difference (95% CI)a | ||
|---|---|---|---|---|---|
| Exercise programme | Usual care | Exercise programme | Usual care | ||
| Mean cost per day | |||||
| Baseline | 246 | 242 | 5.00 (0.63) | 5.25 (0.65) | –0.25 (–2.03 to 1.52) |
| 4 months | 212 | 223 | 5.34 (0.73) | 5.07 (0.69) | 0.27 (–1.70 to 2.24) |
| 12 months | 211 | 216 | 5.42 (0.74) | 5.45 (0.74) | –0.04 (–2.09 to 2.01) |
| Total cost | |||||
| 0–4 months | 212 | 223 | 558.29 (74.53) | 548.01 (71.94) | 10.28 (–193.3 to 213.8) |
| 4–12 months | 198 | 207 | 1120.25 (155.67) | 1161.02 (157.88) | –40.77 (–477.0 to 395.5) |
| 0–12 months | 198 | 207 | 1641.16 (227.34) | 1721.27 (230.75) | –80.16 (–717.4 to 557.2) |
Information about the use of other relevant NHS services was obtained by patient self-report at 4 and 12 months. Reported estimates of health care use over 0–4 months and 4–12 months are given in Tables 73 and 74 respectively. These resource quantities were multiplied by the relevant unit costs (see Table 71) to provide estimates of the mean costs per patient from 0–4 months (Table 75) and 4–12 months (Table 76). Differences between the groups in the costs of health care use over these periods were modest, with wide CIs.
| Type of care | Number of patients | Mean quantity (SE) | Mean treatment difference (95% CI)a | ||
|---|---|---|---|---|---|
| Exercise programme | Usual care | Exercise programme | Usual care | ||
| Physiotherapist | 223 | 228 | 0.78 (0.17) | 0.36 (0.08) | 0.42 (0.05 to 0.79) |
| Occupational therapist | 223 | 227 | 0.52 (0.09) | 0.37 (0.07) | 0.14 (–0.09 to 0.37) |
| General practitioner | 223 | 228 | 0.88 (0.10) | 0.90 (0.12) | –0.02 (–0.33 to 0.29) |
| Rheumatologist | 223 | 228 | 0.86 (0.07) | 0.69 (0.06) | 0.16 (–0.02 to 0.34) |
| Orthopaedic surgeon | 223 | 228 | 0.11 (0.03) | 0.14 (0.03) | –0.04 (–0.12 to 0.05) |
| Other specialist | 222 | 227 | 0.15 (0.03) | 0.20 (0.04) | –0.05 (–0.15 to 0.05) |
| Nurse | 223 | 228 | 0.59 (0.07) | 0.64 (0.10) | –0.06 (–0.30 to 0.19) |
| Podiatrist/chiropodist | 223 | 228 | 0.26 (0.04) | 0.38 (0.06) | –0.12 (–0.27 to 0.03) |
| Self-management group | 222 | 228 | 0.16 (0.13) | 0.08 (0.07) | 0.08 (–0.21 to 0.36) |
| Inpatient stay | 223 | 227 | 0.02 (0.01) | 0.04 (0.01) | –0.02 (–0.05 to 0.01) |
| Day case treatment | 222 | 227 | 0.12 (0.02) | 0.10 (0.02) | 0.02 (–0.03 to 0.08) |
| X-ray | 223 | 228 | 0.29 (0.05) | 0.22 (0.03) | 0.07 (–0.05 to 0.18) |
| CT scan | 223 | 228 | 0.03 (0.01) | 0.04 (0.01) | –0.01 (–0.04 to 0.03) |
| MRI scan | 223 | 228 | 0.09 (0.03) | 0.04 (0.01) | 0.05 (–0.01 to 0.12) |
| Blood tests | 222 | 228 | 2.21 (0.14) | 2.05 (0.12) | 0.16 (–0.20 to 0.52) |
| Type of care | Number of patients | Mean quantity (SE) | Mean treatment difference (95% CI)a | ||
|---|---|---|---|---|---|
| Exercise programme | Usual care | Exercise programme | Usual care | ||
| Physiotherapist | 215 | 221 | 0.41 (0.12) | 0.18 (0.04) | 0.23 (–0.02 to 0.49) |
| Occupational therapist | 215 | 221 | 0.10 (0.04) | 0.09 (0.03) | 0.01 (–0.08 to 0.10) |
| General practitioner | 216 | 221 | 0.82 (0.10) | 0.98 (0.16) | –0.16 (–0.53 to 0.22) |
| Rheumatologist | 215 | 221 | 0.83 (0.06) | 0.89 (0.07) | –0.06 (–0.23 to 0.12) |
| Orthopaedic surgeon | 214 | 221 | 0.20 (0.05) | 0.12 (0.03) | 0.08 (–0.03 to 0.20) |
| Other specialist | 213 | 221 | 0.13 (0.03) | 0.25 (0.05) | –0.12 (–0.24 to 0.00) |
| Nurse | 215 | 221 | 0.63 (0.07) | 0.78 (0.09) | –0.15 (–0.37 to 0.07) |
| Podiatrist/chiropodist | 214 | 221 | 0.39 (0.07) | 0.46 (0.07) | –0.07 (–0.27 to 0.12) |
| Self-management group | 214 | 221 | 0.02 (0.02) | 0.01 (0.01) | 0.01 (–0.03 to 0.06) |
| Inpatient stay | 215 | 221 | 0.03 (0.01) | 0.02 (0.01) | 0.01 (–0.02 to 0.04) |
| Day case treatment | 213 | 219 | 0.14 (0.02) | 0.13 (0.02) | 0.02 (–0.05 to 0.08) |
| X-ray | 215 | 220 | 0.23 (0.03) | 0.26 (0.04) | –0.04 (–0.14 to 0.07) |
| CT scan | 215 | 220 | 0.03 (0.01) | 0.04 (0.01) | –0.01 (–0.04 to 0.02) |
| MRI scan | 214 | 220 | 0.04 (0.01) | 0.06 (0.02) | –0.03 (–0.07 to 0.02) |
| Blood tests | 214 | 218 | 2.19 (0.13) | 2.24 (0.15) | –0.05 (–0.43 to 0.34) |
| Type of care | Number of patients | Mean cost, £ (SE) | Mean treatment difference (95% CI)a | ||
|---|---|---|---|---|---|
| Exercise programme | Usual care | Exercise programme | Usual care | ||
| Physiotherapist | 223 | 228 | 29.82 (6.53) | 13.83 (3.01) | 15.99 (1.97 to 30.01) |
| Occupational therapist | 223 | 227 | 28.88 (5.14) | 20.97 (4.01) | 7.91 (–4.88 to 20.70) |
| General practitioner | 223 | 228 | 27.25 (3.18) | 27.87 (3.70) | –0.63 (–10.23 to 8.98) |
| Rheumatologist | 223 | 228 | 118.20 (10.22) | 95.63 (7.64) | 22.57 (–2.43 to 47.56) |
| Orthopaedic surgeon | 223 | 228 | 10.65 (2.86) | 14.33 (3.02) | –3.67 (–11.86 to 4.51) |
| Other specialist | 222 | 227 | 16.35 (3.37) | 21.81 (4.29) | –5.45 (–16.21 to 5.30) |
| Nurse | 223 | 228 | 28.78 (3.61) | 31.59 (4.88) | –2.81 (–14.78 to 9.16) |
| Podiatrist/chiropodist | 223 | 228 | 10.99 (1.88) | 16.22 (2.75) | –5.23 (–11.80 to 1.34) |
| Self-management group | 222 | 228 | 0.94 (0.74) | 0.48 (0.41) | 0.46 (–1.20 to 2.11) |
| Inpatient stay | 223 | 227 | 48.00 (23.84) | 94.31 (32.82) | –46.31 (–126.3 to 33.64) |
| Day case treatment | 222 | 227 | 159.08 (28.76) | 126.77 (25.74) | 32.31 (–43.45 to 108.1) |
| X-ray | 223 | 228 | 1.43 (0.24) | 1.10 (0.17) | 0.34 (–0.24 to 0.91) |
| CT scan | 223 | 228 | 2.50 (1.17) | 3.26 (1.27) | –0.76 (–4.16 to 2.64) |
| MRI scan | 223 | 228 | 14.98 (4.99) | 5.86 (2.04) | 9.12 (–4.16 to 2.64) |
| Blood tests | 222 | 228 | 6.64 (0.41) | 6.16 (0.37) | 0.48 (–0.61 to 1.56) |
| Total | 223 | 228 | 502.74 (44.46) | 478.55 (53.12) | 24.19 (–112.3 to 160.7) |
| Type of care | Number of patients | Mean cost, £ (SE) | Mean treatment difference (95% CI)a | ||
|---|---|---|---|---|---|
| Exercise programme | Usual care | Exercise programme | Usual care | ||
| Physiotherapist | 215 | 221 | 15.73 (4.65) | 6.88 (1.68) | 8.85 (–0.77 to 18.47) |
| Occupational therapist | 215 | 221 | 5.47 (2.04) | 4.81 (1.59) | 0.66 (–4.41 to 5.72) |
| General practitioner | 216 | 221 | 25.55 (3.08) | 30.44 (5.04) | –4.89 (–16.56 to 6.78) |
| Rheumatologist | 215 | 221 | 114.25 (8.15) | 122.39 (9.24) | –8.14 (–32.41 to 16.14) |
| Orthopaedic surgeon | 214 | 221 | 19.89 (5.04) | 11.65 (2.65) | 8.25 (–2.86 to 19.35) |
| Other specialist | 213 | 221 | 14.46 (3.60) | 27.87 (5.46) | –13.41 (–26.36 to –0.46) |
| Nurse | 215 | 221 | 31.00 (3.65) | 38.36 (4.17) | –7.36 (–18.28 to 3.55) |
| Podiatrist/chiropodist | 214 | 221 | 16.68 (3.20) | 19.85 (2.81) | –3.17 (–11.52 to 5.18) |
| Self-management group | 214 | 221 | 0.14 (0.14) | 0.05 (0.05) | 0.08 (–0.20 to 0.37) |
| Inpatient stay | 215 | 221 | 87.13 (32.47) | 48.43 (24.05) | 38.69 (–40.42 to 117.8) |
| Day case treatment | 213 | 219 | 184.23 (31.25) | 167.23 (29.58) | 16.99 (–67.54 to 101.5) |
| X-ray | 215 | 220 | 1.14 (0.16) | 1.32 (0.22) | –0.18 (–0.72 to 0.36) |
| CT scan | 215 | 220 | 2.60 (1.05) | 3.38 (1.18) | –0.79 (–3.89 to 2.31) |
| MRI scan | 214 | 220 | 6.24 (2.17) | 10.63 (3.33) | –4.38 (–12.24 to 3.47) |
| Blood tests | 214 | 218 | 6.57 (0.38) | 6.72 (0.45) | –0.14 (–1.30 to 1.02) |
| Total | 216 | 221 | 526.52 (52.56) | 498.28 (51.69) | 28.24 (–116.6 to 173.1) |
A summary of all included costs over the trial period is given in Table 77. This shows a significant difference between the groups in intervention costs – taking account of individually recorded attendance at the intervention sessions. The mean cost per participant for the exercise programme group was £171, compared with £44 for the usual care group: a difference of £127 (95% CI £122 to £131). Overall, taking account of costs for the intervention, for prescribed medications and for other NHS services, the estimated between-group difference in costs is £103 (95% CI –£622 to £828) per patient. This wide variation in health-care costs is common, owing to large outliers as some patients incur very high costs. This can be seen in the frequency distributions in Figure 8. The bimodal nature of these distributions is due to high costs incurred by a subgroup of patients on expensive biological disease-modifying drugs.
FIGURE 8.
Frequency distribution of total cost over 12 months by study arm. (a) Exercise programme; and (b) usual care.
| Types of cost | Number of patients | Mean cost, £ (SE) | Mean treatment difference (95% CI)a | ||
|---|---|---|---|---|---|
| Exercise programme | Usual care | Exercise programme | Usual care | ||
| Intervention | 238 | 235 | 171.19 (1.88) | 44.29 (1.19) | 126.89 (122.50 to 131.29) |
| NHS services | 208 | 216 | 1021.52 (706.88) | 975.42 (81.83) | 46.1 (–174.96 to 267.12) |
| Prescriptions | 198 | 207 | 1641.16 (227.34) | 1721.27 (230.75) | –80.12 (–717.44 to 557.21) |
| Total NHS cost | 197 | 203 | 2812.28 (254.34) | 2709.38 (266.62) | 102.90 (–622.18 to 827.98) |
Utility and quality-adjusted life-years
Utility scores were estimated using validated EQ-5D-3L and SF-12 questionnaires completed by patients at baseline, 4 months and 12 months.
The EQ-5D-3L questionnaire consists of five dimensions: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. For each dimension, the patient indicates the level of problems experienced on a 3-level scale: no problems, some problems or extreme problems. These responses were converted into utility scores using the UK social tariff. 111 EQ-5D-3L scores at baseline, 4 and 12 months for the exercise and usual care groups are shown in Table 78. Estimated by the simple area-under-the-curve method, mean QALYs attained over the trial period were slightly higher for the exercise group than for the usual care group: 0.01 (95% CI –0.03 to 0.05). However, this does not take account of the initial differences between the groups (Figure 9).
FIGURE 9.
European Quality of Life-5 Dimensions scores over 12 months’ follow-up.
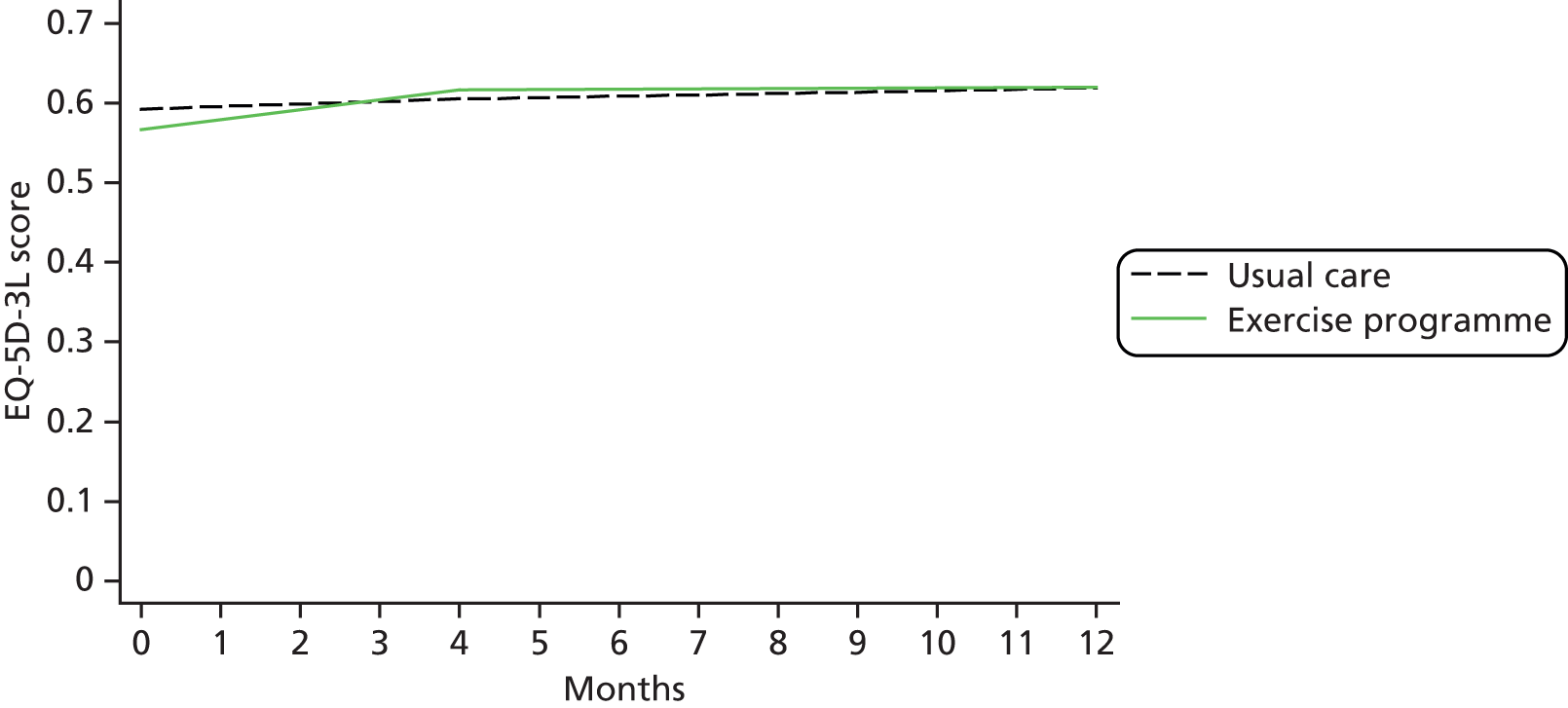
| Time point | Number of patients | Mean quantity (SE) | Mean treatment difference (95% CI) | ||
|---|---|---|---|---|---|
| Exercise programme | Usual care | Exercise programme | Usual care | ||
| Baseline | 244 | 240 | 0.57 (0.02) | 0.59 (0.02) | –0.03 (–0.07 to 0.02) |
| 4 months | 224 | 228 | 0.62 (0.02) | 0.60 (0.02) | 0.01 (–0.04 to 0.06) |
| 12 months | 215 | 221 | 0.62 (0.02) | 0.62 (0.02) | 0.00 (–0.05 to 0.05) |
| Change 0–12 months | 214 | 220 | 0.03 (0.01) | 0.02 (0.02) | 0.01 (–0.04 to 0.05) |
| QALYs | 207 | 215 | 0.62 (0.01) | 0.61 (0.02) | 0.01 (–0.03 to 0.05) |
The SF-12 consists of 12 items addressing eight domains of health: physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional and mental health. Patients indicate a level of problem on 3- or 5-level scales. The SF-12 was converted into a SF-6D utility score. 112 SF-6D results are shown in Table 79 and Figure 10. This yields a slightly higher and statistically different estimate of the QALY difference between the groups: 0.02 (95% CI 0.01 to 0.04).
FIGURE 10.
Short-Form questionnaire-6 Dimensions scores over 12 months’ follow-up.
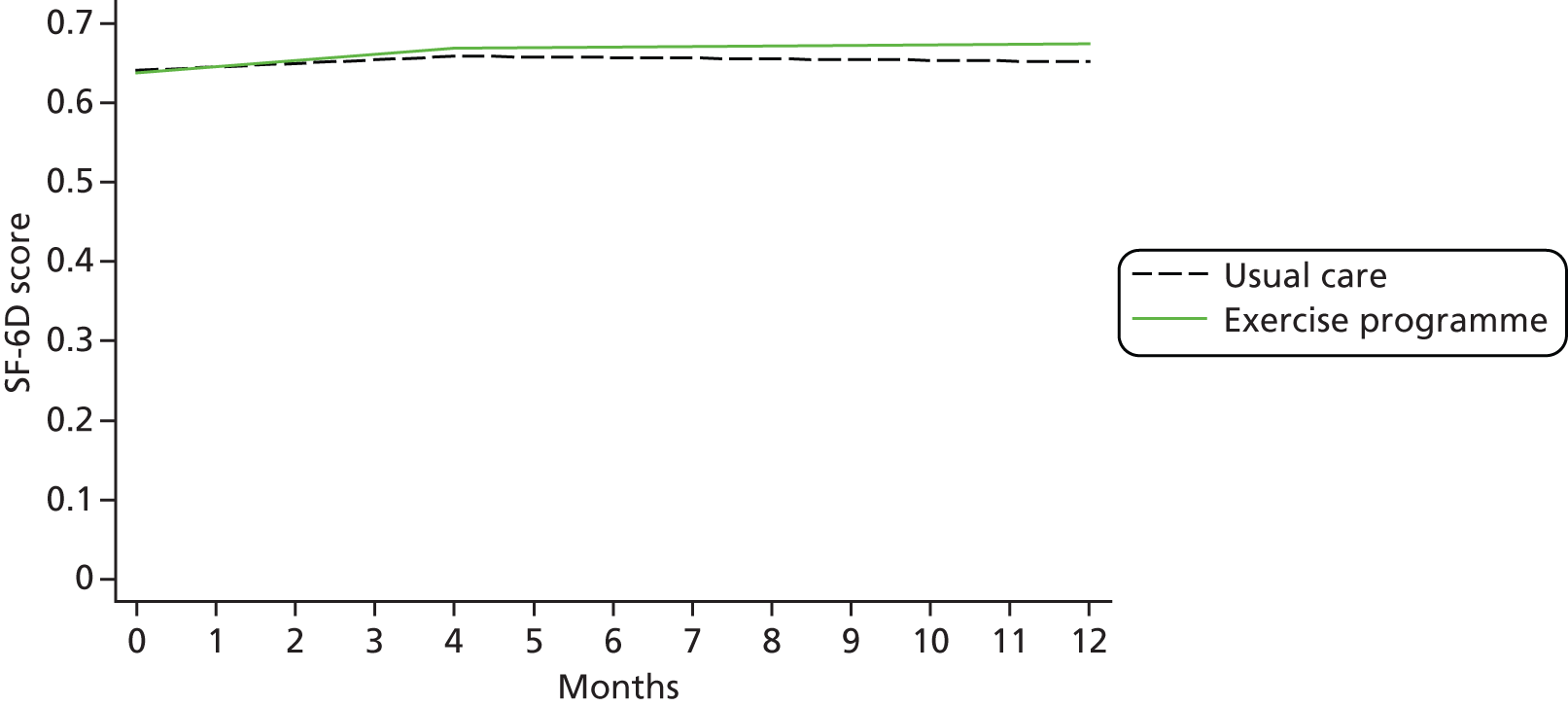
| Time point | Number of patients | Mean quantity (SE) | Mean treatment difference (95% CI) | ||
|---|---|---|---|---|---|
| Exercise programme | Usual care | Exercise programme | Usual care | ||
| Baseline | 245 | 238 | 0.64 (0.01) | 0.64 (0.01) | 0.00 (–0.02 to 0.02) |
| 4 months | 214 | 221 | 0.67 (0.01) | 0.66 (0.01) | 0.01 (–0.01 to 0.03) |
| 12 months | 203 | 216 | 0.67 (0.01) | 0.65 (0.01) | 0.02 (0.00 to 0.05) |
| Change 0–12 months | 203 | 212 | 0.02 (0.01) | 0.01 (0.01) | 0.02 (0.00 to 0.04) |
| QALYs | 193 | 201 | 0.67 (0.01) | 0.66 (0.01) | 0.02 (0.01 to 0.04) |
For comparison, we also show results based on the EQ-5D-3L VAS, which provides us with a value for the participant’s self-rated health at the time of survey completion on a scale of 0–100 (see Table 80 and Figure 11).
FIGURE 11.
Visual analogue scale scores over 12 months’ follow-up.

| Time point | Number of patients | Mean quantity (SE) | Mean treatment difference (95% CI) | ||
|---|---|---|---|---|---|
| Exercise programme | Usual care | Exercise programme | Usual care | ||
| Baseline | 246 | 242 | 66.11 (1.24) | 67.31 (1.16) | –1.20 (–4.54 to 2.14) |
| 4 months | 224 | 228 | 69.50 (1.30) | 67.72 (1.23) | 1.78 (–1.73 to 5.28) |
| 12 months | 214 | 220 | 69.54 (1.22) | 66.54 (1.33) | 2.99 (–0.56 to 6.55) |
| Change 0–12 months | 207 | 215 | 0.02 (0.01) | 0.00 (0.01) | 0.02 (0.00 to 0.05) |
| QALYs | 207 | 215 | 0.70 (0.01) | 0.68 (0.01) | 0.02 (–0.01 to 0.07) |
The EQ-5D-3L and SF-6D questionnaires give very different distributions of utility scores (Figures 12 and 13). The spread of scores is much wider with the EQ-5D-3L, with a small number of patients having negative scores (‘worse than death’) and others with a score just under 1 (‘perfect health’). In contrast, the SF-6D gives a much narrower range of scores.
FIGURE 12.
Frequency distribution of QALYs (EQ-5D-3L) by study arm. (a) Exercise programme; and (b) usual care.
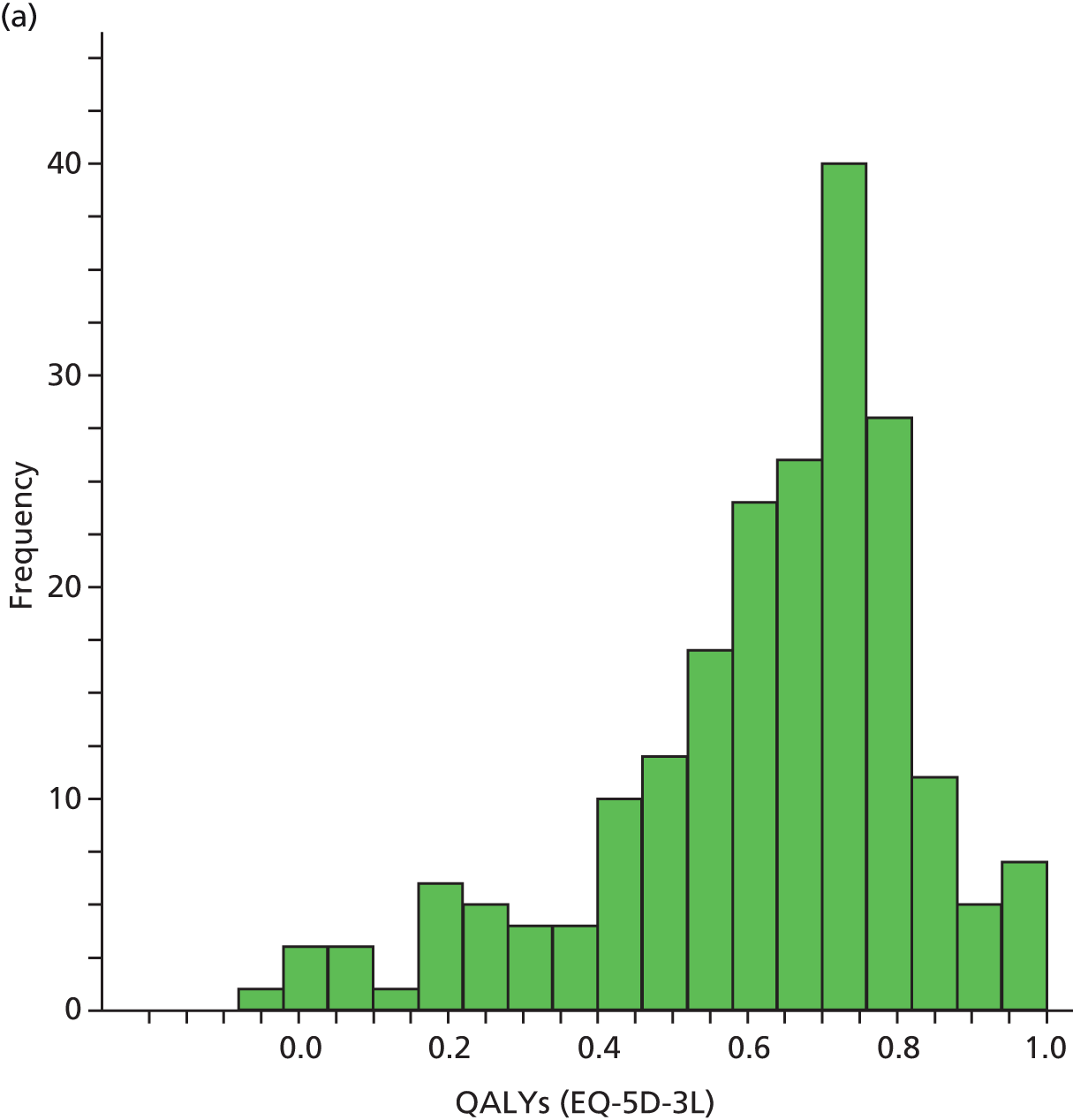
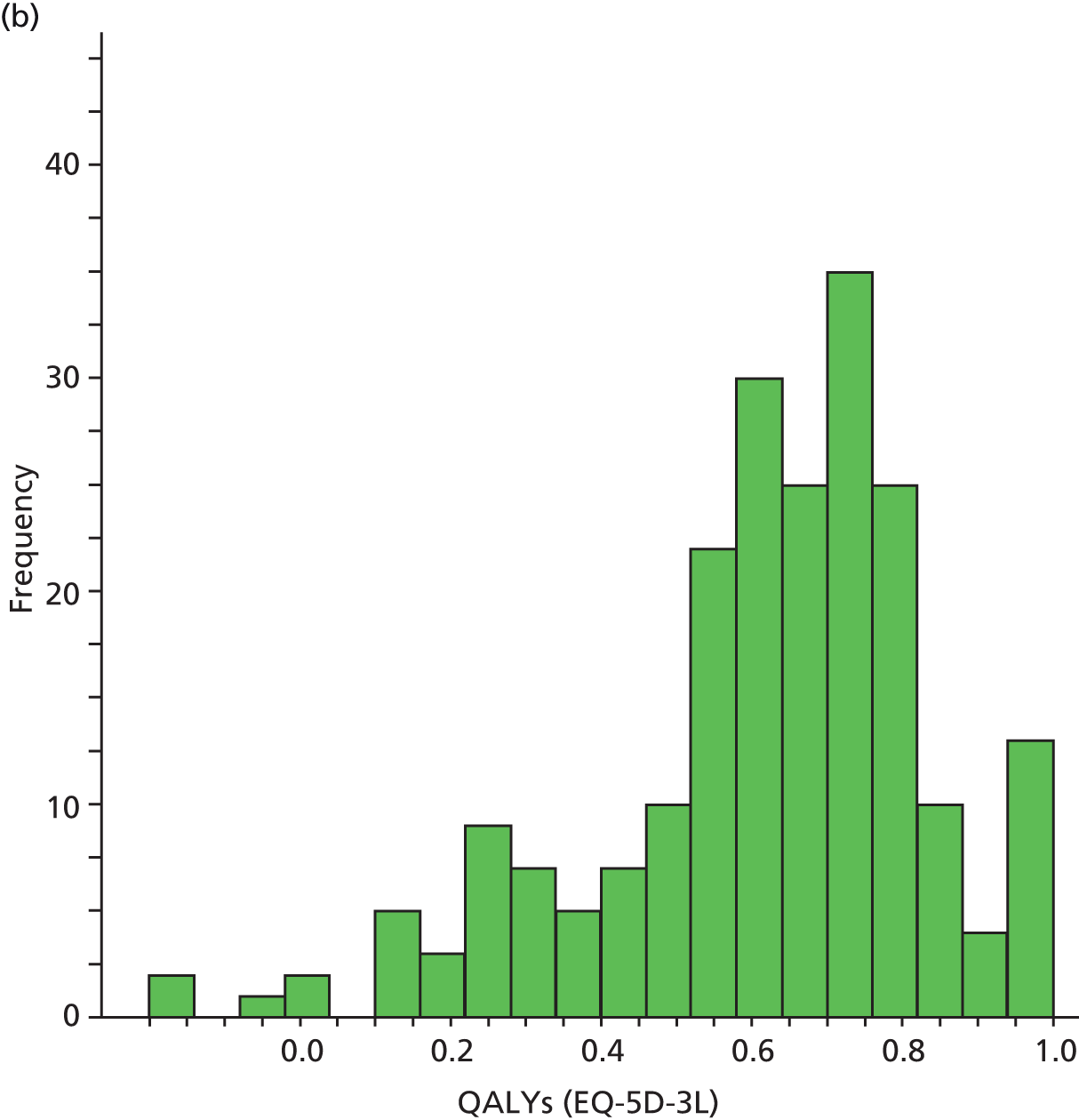
FIGURE 13.
Frequency distribution of QALYs (SF-6D) by study arm. Cost-effectiveness plane (EQ-5D-3L) 1000 bootstrap samples. (a) Exercise programme; and (b) usual care.
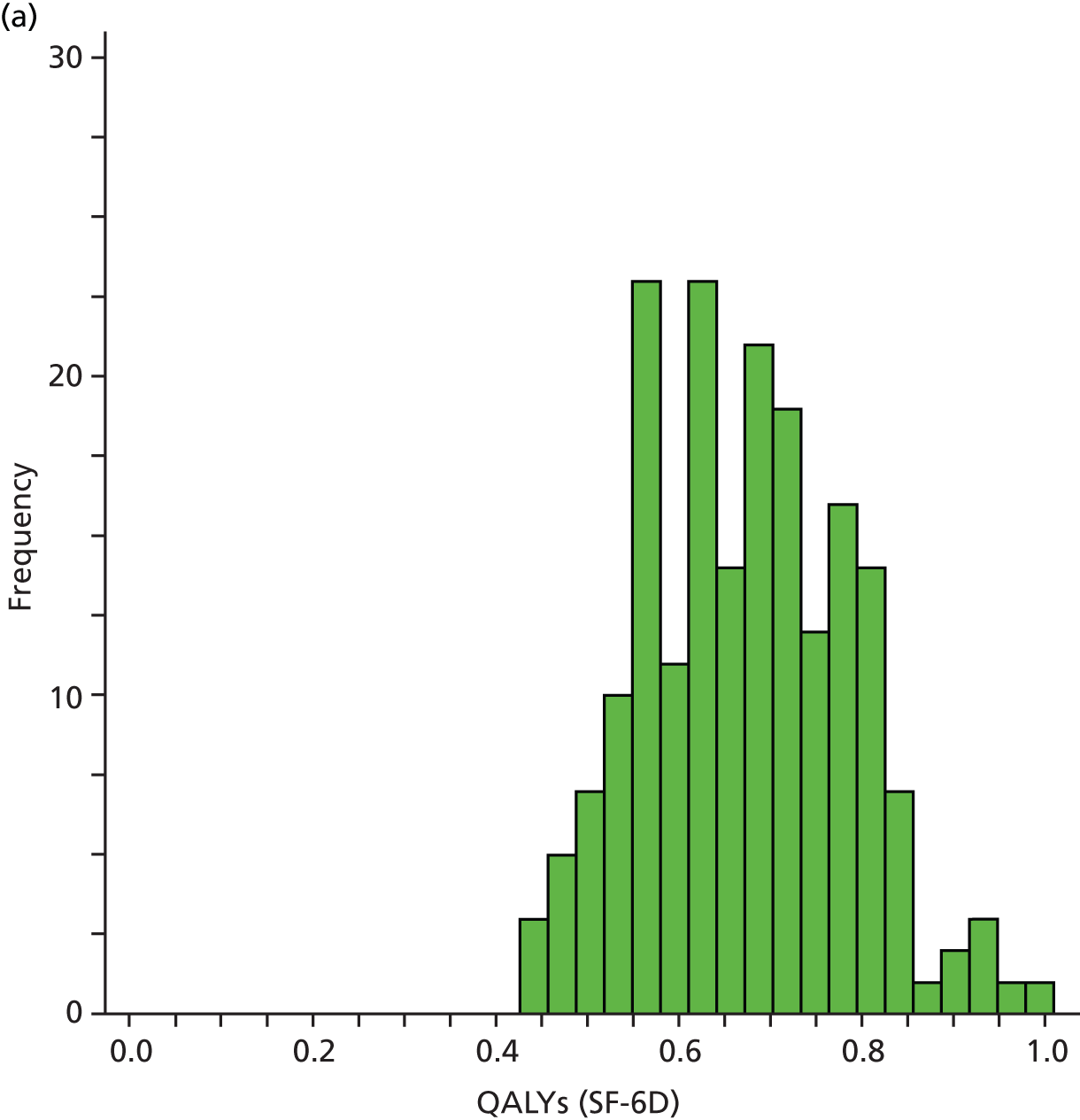
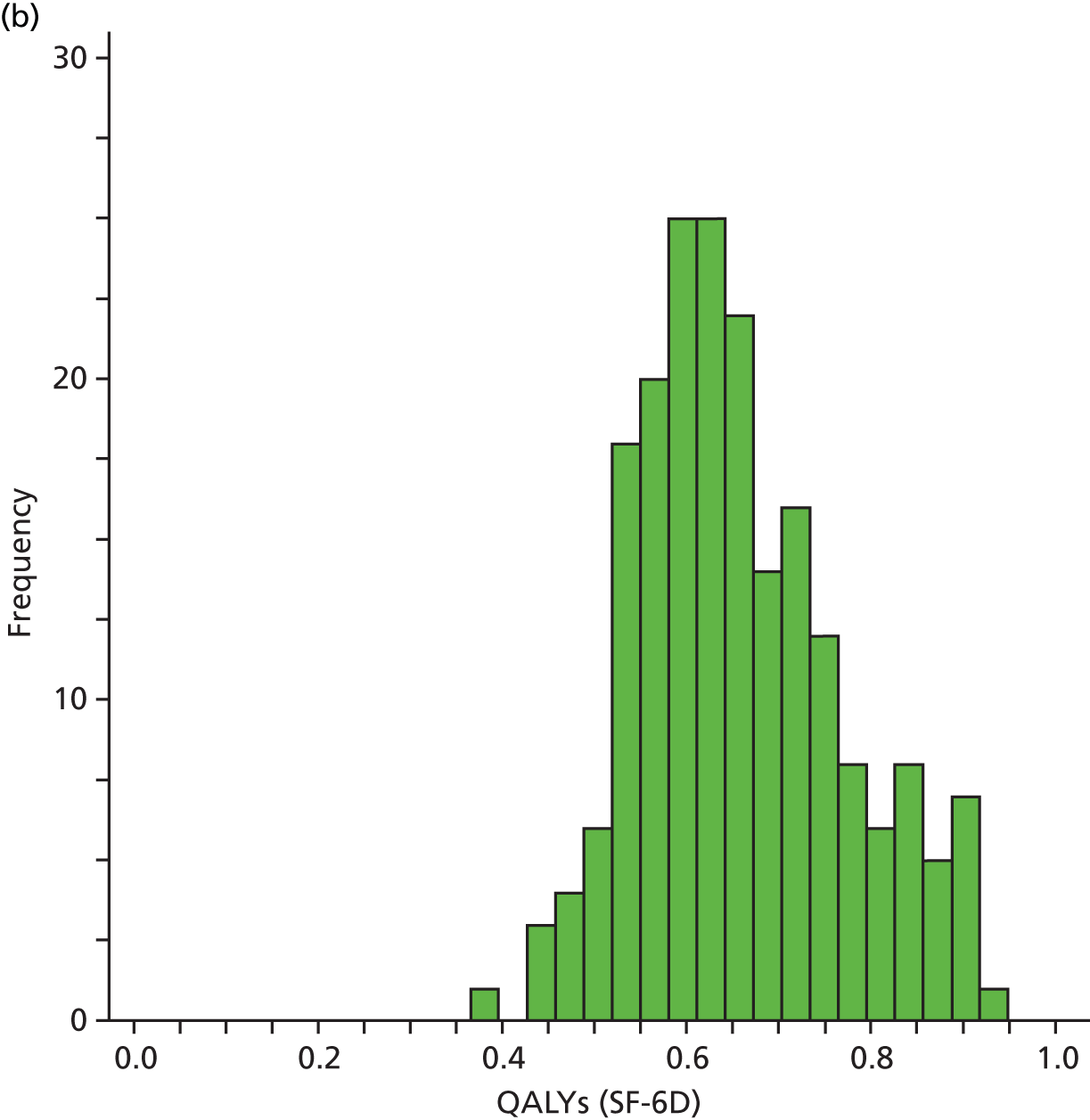
Cost-effectiveness analysis methods
The incremental cost-effectiveness of the exercise intervention compared with the usual care control was estimated by five methods (see Table 81). These analyses differed in assumptions about the distributions of costs and QALYs, whether or not correlations between costs and QALYs were accounted for, adjustment for baseline utility values and the handling of missing data. Analysis E is our preferred analysis, as it accounts for a range of potential biases and sources of uncertainty, as recommended in guidelines for economic analyses based on trials. 110 The other analyses provide supplementary information about the relative impact of these factors.
| Analysis | Treatment of missing data | Covariates | Assumed distribution for costs/effects | Correlation between costs and effects |
|---|---|---|---|---|
| A. t-test | Patients with full data | None | Normal/normal | No |
| B. GLM | Patients with full data | Baseline utility | Normal/gamma | No |
| C. SUR | Patients with full data | Baseline utility | Normal/normal | Yes |
| D. Bootstrap SUR | Patients with full data | Baseline utility | Non-parametric | Yes |
| E. Bootstrap SUR with imputation | Multiple imputation | Baseline utility | Non-parametric | Yes |
The five analyses were repeated using QALY estimates derived from EQ-5D-3L and SF-6D observations. Our primary analysis is that based on EQ-5D-3L data, as this is the utility measure currently recommended by NICE for the evaluation of cost-effectiveness of interventions in the NHS. 109
The first four analyses (A–D) included only patients for whom full data on costs and QALYs over the 12-month study period were available. For the analysis based on EQ-5D-3L data, complete economic data were available for 396 patients (195 in the exercise group and 201 in the usual care group). And for the SF-6D analysis, complete data were available for 374 patients (186 in the exercise group and 188 in the usual care group). The final analysis (E) used multiple imputation to include all 488 randomised patients. 119
Analysis A used simple, large sample methods to estimate differences in mean costs and in mean QALYs between the groups, with no adjustment for baseline utility. Analysis B used a regression approach to better reflect the nature of the data. 120 It is apparent that the distributions of costs are highly skewed (see Figure 8), such that ordinary least squares (OLS) assumptions of normality might not be appropriate; hence a generalised linear model was fitted for costs using a gamma distribution and identity link function. QALYs were estimated using an OLS regression with baseline utility (EQ-5D-3L or SF-6D scores) as a covariate, to adjust for any difference between groups. This method of adjusting for baseline utility differences is more efficient than estimation of QALYs using the ‘change from baseline’ method. 121 Because QALYs are not normally distributed (see Figure 12) we also attempted to apply a gamma and log-normal distribution for 1 QALY as the response, but this did not improve the fit.
The third analysis (C) also allowed for the likely correlation of costs and QALYs, which is potentially important for efficient estimation. 122 This used a seemingly unrelated regression (SUR) approach to estimate the costs and QALYs simultaneously. 123 Again, baseline utility was included as a covariate for QALYS. Analysis D repeated the SUR regression analysis, but using non-parametric bootstrapping of residuals to avoid the assumption that they were normally distributed. The results presented below are based on 10,000 bootstrap samples, which was sufficient to provide stable estimates of costs and effects.
The final analysis (E) combined the bootstrap SUR method with multiple imputation of missing cost and QALY data. 110 For each of 10,000 iterations: one set of missing values was imputed for utilities and costs; the SUR analysis was run on the imputed data set; non-parametric bootstrap samples were drawn from the SUR residuals for the two treatment groups; predicted values of costs and QALYs were calculated using the bootstrapped residuals; and mean costs and QALYs were estimated for the two treatment groups and saved. This procedure provided an empirical estimate of the sampling distribution for mean costs and effects from the two groups, which was used to estimate uncertainty around the cost-effectiveness statistics of interest. The multiple imputation procedure was conducted using the Stata version 9 ado file ‘ice’ (Stata Corp LP, College Station, TX, USA), and estimated missing values of EQ-5D-3L/SF-6D, drug costs and other health-care costs from the baseline, 4-month and 12-month assessments, adjusting for age and baseline values of MHQ overall scale, troublesomeness of pain scale and confidence scale.
Results for the various analyses are presented as incremental cost-effectiveness ratio (ICER) statistics – the ‘cost per QALY’. This is the estimated difference in mean costs between the exercise and usual care arms (the incremental cost), divided by the difference in mean QALYs between the arms (incremental effect). The ICERs can be compared against the benchmark thresholds for cost-effectiveness in the NHS context of £20,000 to £30,000 per QALY gained, as applied by NICE. 124 If the ICER is below £20,000 per QALY, this suggests that the intervention is a cost-effective alternative to usual care. Above £30,000 per QALY, the ICER suggests that the intervention is not cost-effective and, in between these figures, the result is indeterminate. We also present the results using incremental net benefit (INB) statistics, calculated by multiplying the incremental effects by an assumed monetary value of a QALY (the ‘cost-effectiveness threshold’) and subtracting the incremental cost. We calculate INB statistics based on the two cost per QALY thresholds of £20,000 and £30,000 per QALY. A positive INB suggests that the intervention is cost-effective compared with usual care at the defined threshold.
Uncertainty over the cost-effectiveness of the intervention is reflected in an estimated probability that the INB is positive. If this figure is greater than 0.5, it indicates that the intervention is more likely to be cost-effective than not. Finally, we present an expected value of perfect information (EVPI) statistic, which integrates the likelihood of making the wrong decision based on the mean INB statistic with the expected loss if the wrong decision is made. This provides an estimate of the maximum that it would be worth paying to obtain information to fully resolve uncertainty about the cost-effectiveness of the intervention.
Cost-effectiveness results
The results of an incremental cost-effectiveness analysis of the SARAH exercise programme compared with usual care are presented in Tables 82 and 83, based on the EQ-5D-3L and SF-6D outcomes respectively.
| Outcome | Mean treatment difference (95% CI) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A. t-tests | B. GLM regression | C. SUR regression | D. Bootstrap SUR | E. Bootstrap SUR with imputation | |||||||
| Total NHS cost (£) | 115.69 | (–615.6 to 847.0) | 115.68a | (–615.9 to 847.2) | 115.69b | (–611.6 to 842.9) | 113.31 | (–611.7 to 843.2) | 206.40 | (–495.12 to 907.53) | |
| QALYs (EQ-5D-3L) | 0.014 | (–0.029 to 0.056) | 0.012c | (–0.016 to 0.040) | 0.012b | (–0.016 to 0.040) | 0.012 | (–0.016 to 0.040) | 0.012 | (–0.017 to 0.040) | |
| ICER (£ per QALY) | 8564 | 9572 | 9549 | 9364 | 17,941 | ||||||
| INB (£) | £20,000 | 154 | 126 | 127 | 129 | 24 | |||||
| £30,000 | 290 | 247 | 248 | 250 | 139 | ||||||
| P (INB > 0) | £20,000 | 0.66 | 0.63 | 0.63 | 0.60 | 0.52 | |||||
| £30,000 | 0.78 | 0.75 | 0.75 | 0.66 | 0.59 | ||||||
| EVPI | £20,000 | 143 | 186 | ||||||||
| £30,000 | 138 | 178 | |||||||||
| Outcome | Mean treatment difference (95% CI) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A. t-tests | B. GLM regression | C. SUR regression | D. Bootstrap SUR | E. Bootstrap SUR with imputation | |||||||
| Total NHS cost (£) | 107.94 | (–641.0 to 856.9) | 107.93a | (–640.8 to 856.7) | 107.94b | (–636.6 to 852.5) | 100.34 | (–644.1 to 856.2) | 206.89 | (–494.5 to 920.7) | |
| QALYs (SF-6D) | 0.018 | (–0.005 to 0.041) | 0.014c | (0.001 to 0.028) | 0.015b | (0.001 to 0.028) | 0.015 | (0.001 to 0.028) | 0.009 | (–0.004 to 0.021) | |
| ICER (£ per QALY) | 5986 | 7455 | 7440 | 6823 | 23,288 | ||||||
| INB (£) | £20,000 | 253 | 182 | 182 | 194 | –29 | |||||
| £30,000 | 433 | 326 | 327 | 341 | 60 | ||||||
| P (INB > 0) | £20,000 | 0.75 | 0.68 | 0.68 | 0.68 | 0.47 | |||||
| £30,000 | 0.87 | 0.80 | 0.81 | 0.78 | 0.56 | ||||||
| EVPI | £20,000 | – | – | – | 88 | 176 | |||||
| £30,000 | – | – | – | 59 | 147 | ||||||
The estimated mean health-care cost with the intervention was approximately £200 higher than the mean cost under usual care based on our preferred analysis (method E using EQ-5D-3L data), but there was a wide CI around this estimate (95% CI –£495 to £908). The results were more favourable when estimated by the other methods of analyses (A to D) and for the EQ-5D-3L and SF-6D complete case data sets.
The estimated difference in mean QALYs accrued over the 12-month period was approximately 0.01 greater in the intervention group than in the usual care group (analysis E using EQ-5D-3L data), with a CI of –0.02 to 0.04. The magnitude of QALY gain was similar across the different methods of estimation, and with the EQ-5D-3L and SF-6D.
These results suggest that the hand exercise programme costs around £17,941 more per additional QALY gained than the usual care control. This figure was rather more favourable under the other methods of analysis based on complete case data set.
Patients excluded from the economic complete case analyses were different from those included. Overall, 92 patients (41 control and 51 intervention) could not be included in the EQ-5D-3L based analysis because one or more cost or utility observations were missing. At baseline, the excluded patients tended to be younger (mean age 59 vs. 63, p < 0.001), with worse hand function (mean MHQ score 47 vs. 52 points, p < 0.01), more troublesome pain (52 vs. 46, p < 0.01), poorer confidence in self-efficacy (64 vs. 69, p < 0.05), lower EQ-5D-3L scores (0.48 vs. 0.60, p < 0.001) and higher daily drug costs (£4.64 vs. £7.21, p < 0.05). Similar differences were found between the patients included/excluded from the SF-6D complete case economic analysis.
Given the wide CIs for the incremental cost and QALY estimates, it is not surprising that a high level of uncertainty over cost-effectiveness was observed. Figure 14 shows the results for 10,000 bootstrap samples based on EQ-5D-3L analysis with imputation, where there is a 52% chance that the intervention is cost-effective at a threshold of £20,000 per QALY and 59% chance at a threshold of £30,000 per QALY. This uncertainty is also shown in the cost-effectiveness acceptability curve (CEAC) in Figure 15. Analysis of the SF-6D data yields a rather lower estimated probability that the intervention is cost-effective: 47% at £20,000 per QALY and 56% at £30,000 per QALY. Finally, the EVPI estimates indicate a maximum value of further research in the region of £200 per patient.
FIGURE 14.
Bootstrap samples using SUR and imputation of missing data (analysis E): CEAC (EQ-5D-3L). Controlling for baseline utility and missing data.
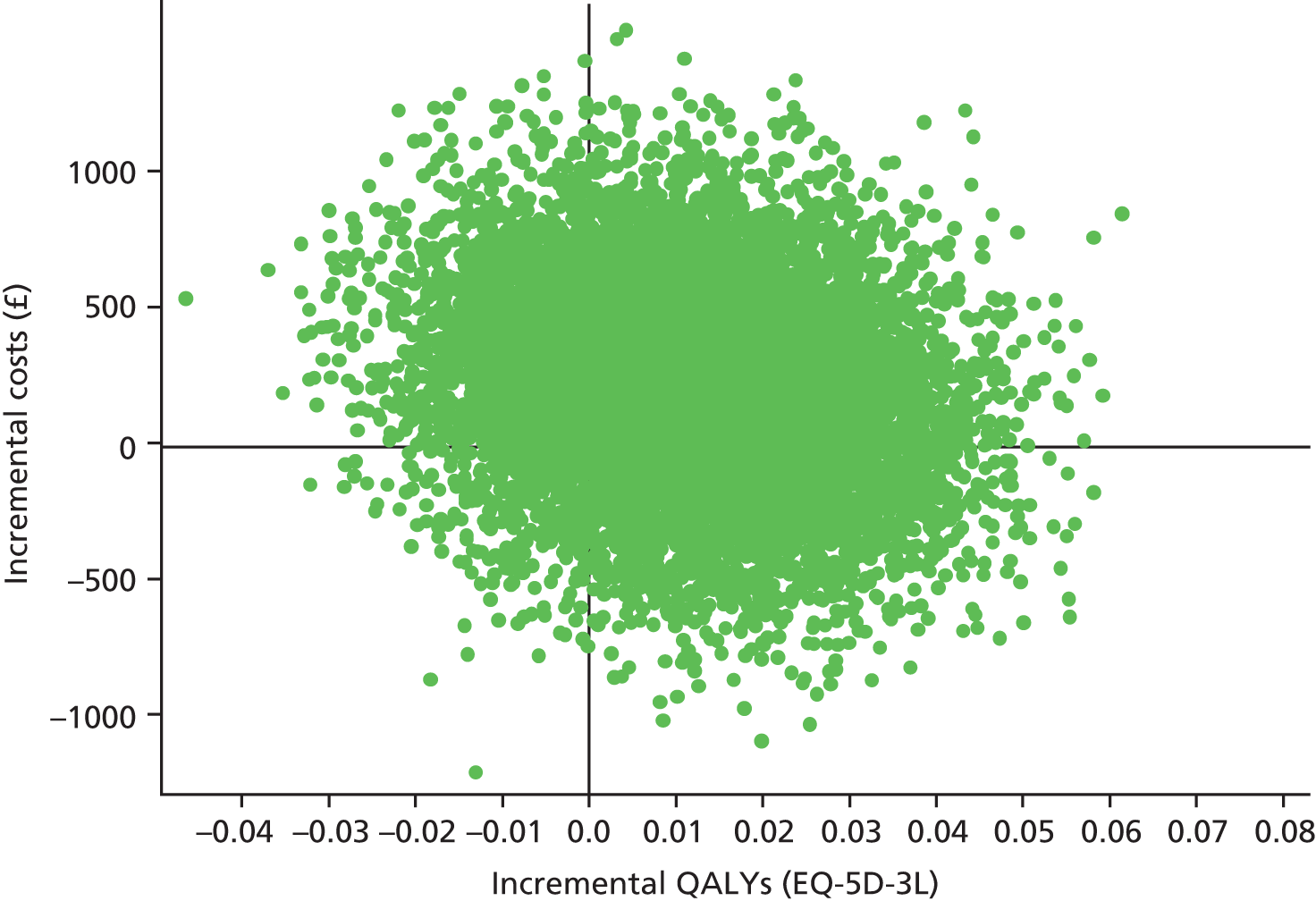
FIGURE 15.
Cost-effectiveness acceptability curve based on analysis E (bootstrap SUR with imputation).

Discussion
Analysis of the costs and effects of the hand exercise programme over the 12-month trial period indicates, on balance, this is likely to be a cost-effective use of NHS resources. At £17,941 per QALY gained based on EQ-5D-3L scores at 0, 4 and 12 months, the estimated ICER is within NICE’s lower cost-effective thresholds of £20,000. Using the SF-6D method for quantifying HRQoL, the estimated ICER was slightly less attractive, but still within the upper £30,000 per QALY threshold. There is, however, a high degree of uncertainty over these estimates, owing to the small magnitude of QALY gain observed and the very wide variation in the costs of rheumatoid-related health-care for individual patients.
Missing data might also have introduced a bias into the health economic results. Although overall rates of data completion in the trial were very good, the complete resource use and utility data across all time points that are needed to estimate QALYs and total costs for the year of follow-up were available for fewer patients: 92 (19%) patients could not be included in the EQ-5D-3L based economic analysis, and 114 (23%) could not be included in the SF-6D analysis. Furthermore, the patients with missing data did appear to be different from those with complete data: at baseline the former tended to be younger, with worse hand function, pain, self-efficacy and quality of life, and higher drug costs. These differences may explain the rather less attractive estimates of cost-effectiveness obtained when multiple imputation was introduced to investigate the effect of missing data (analysis E, bootstrap SUR with imputation), compared with our complete case analyses (analyses A–D). The probabilistic results for analysis E incorporate an estimate of uncertainty related to the imputation procedure. However, there is a further element of structural uncertainty related to the choice of the imputation model that is not quantified in the above estimates.
The analysis was limited to a 12-month time horizon. There was an observed difference in reported quality of life at this end point – not statistically significant for the EQ-5D-3L, but it was significant for the SF-6D, and for the primary trial outcome of MHQ score. It is likely that this difference would have persisted for some time after 12 months. This, coupled with the lack of evidence for any difference in health-care costs other than the initial cost of delivering the intervention, suggests that the cost-effectiveness would be better in the longer-term than that estimated. We therefore considered using modelling to extrapolate the trial results, and to obtain a better long-run estimate of cost-effectiveness and of the uncertainty over this figure. However, there is no reliable means for estimating the likely persistence of the quality-of-life gap beyond 12 months. We therefore did not believe that modelling would help to resolve or further quantify uncertainty in this case. Furthermore, any extrapolation would only be likely to reduce the estimated ICERs, and would therefore not change the conclusion that the exercise programme is likely to be cost-effective.
Another potential bias that might have led to an underestimation of cost-effectiveness is double-counting of the costs of the therapy sessions. Patients were asked about their use of a range of NHS services, including physiotherapy and occupational therapy, at the 4-month and 12-month assessments. They were asked not to include services that were part of the exercise programme intervention, as these were to be costed separately based on attendance records at the exercise sessions. However, it is possible that some patients might not have understood the difference between intervention and other routine therapy sessions. The intervention group did report more frequent use of physiotherapy and occupational therapy than the control group (see Tables 73 and 74), which translated to a mean additional cost of £33 per patient. If we assumed that all of the difference in therapy costs was down to double counting, the correct ICERs would be somewhat lower than the above estimates (£15,825 per QALY based on EQ-5D-3L estimates, analysis E).
Our conservative assumption regarding the training costs of physiotherapists and occupational therapists may have also led to underestimation of cost-effectiveness. A simple sensitivity analysis shows that if the ongoing training costs were half those incurred within the trial (£6.77 per patient), the ICER would fall to £17,395. Using the best-case scenario for the cost of consumables (£24.75) reduced the ICER to £16,361 and using the worst-case scenario (£106.34) increased the ICER to £23,453. The high degree of uncertainty around the cost-effectiveness estimates suggests that further research that would reduce this uncertainty might be worthwhile.
Chapter 7 Extended follow-up study
Introduction
Having confirmed that the SARAH exercise programme improved hand function at 4 and 12 months compared with usual care alone, a decision was made to carry out an extended follow-up. The aim of the extended follow-up was to study the effects of the SARAH exercise programme beyond 12 months. We had successfully completed a similar extended follow-up for another Health Technology Assessment (HTA) funded study, the Back Skills Training Trial125 and we used similar follow-up procedures.
Methods
Data collection
Data collection was by postal questionnaire. The questionnaires were sent to participants at the same time (between September 2012 and January 2013) regardless of when they were randomised, so that the time to extended follow-up varied. Participants were sent a questionnaire and a response form so that they could indicate whether or not they wish to participate in the extended follow-up. On the response form, there was also an option for participants to request to complete the questionnaire over the telephone. We designed a shortened version of the questionnaire containing only ‘core’ outcome measures, to be asked and answered verbally, via telephone, by participants who had indicated they wish to do so. A reminder letter encouraging participants to return the questionnaire was sent after 2 weeks to those who have not yet returned their questionnaire.
To reduce the burden on participants, the number of outcome measures collected was reduced compared with the earlier time points, and included the outcomes where significant differences between the two groups were observed at earlier time points. The following outcomes were collected:
-
MHQ – overall hand function subscale (primary outcome)
-
MHQ – ADLs subscale
-
MHQ – work subscale
-
rating of troublesomeness
-
participant-rated improvement
-
Arthritis Self-efficacy Scale
-
current hand exercise performance
-
HRQoL (SF-12 and EQ-5D-3L)
-
health-related resource use
-
time off work.
Analysis of extended follow-up
We used the same data analysis methods for the extended follow-up as approved by DMEC for the main trial. The analysis was intention to treat. We investigated differences in baseline characteristics of participants completing and not completing the extended follow-up to assess selection bias.
The outcome measures based on scores from extended follow-up were analysed as a single time point as for the earlier follow-up. The estimates were added to a reduced version of the previous outcome table. For the MHQ overall hand function score, the inclusion of a time covariate was investigated. The repeated measures and multiple imputation estimates for MHQ overall hand function were also calculated for the extended follow-up time point.
Ethical approval and monitoring
The Oxford C Multicentre Research Ethics Committee approved the extended follow-up study as a substantial amendment in September 2012. The research and development departments of each participating centre gave approval to implement the amendment.
The extended follow-up was supported by the DMEC and the TSC.
Results
Follow-up
There were 25 withdrawals and two unrelated deaths following the main study (one death occurred between the 12-month follow-up and extended follow-up) so 461 participants were eligible to be approached to take part in the extended follow-up. In total, 328 (67% of the original cohort) participants provided data for the extended follow-up analysis (Table 84).
| Response category | Exercise programme, n (%) | Usual care, n (%) |
|---|---|---|
| Allocation | 246 (100) | 244 (100) |
| Withdrew | 17 (7) | 10 (2) |
| Died | 0 (0) | 2 (1) |
| Eligible for extended follow-up | 229 (93) | 232 (95) |
| Unable to contact (no current contact details available) | 3 (1) | 2 (< 1) |
| Sent extended follow-up questionnaire | 226 (92) | 231 (95) |
| Provided extended follow-up data | 155 (63) | 173 (71) |
| Full questionnaire | 153 (62) | 172 (70) |
| Core questionnaire (phone) | 2 (< 1) | 1 (< 1) |
| Declined to participate | 18 (7) | 11 (5) |
| Unable to contact | 6 (3) | 3 (1) |
| Died | 2 (< 1) | 0 (0) |
| Did not respond | 48 (20) | 45 (19) |
The time from randomisation to when the participant completed the extended follow-up is reported in Table 85.
| Time | Exercise programme, median (IQR) | Usual care, median (IQR) |
|---|---|---|
| Time in months to extended follow-up | 25.8 (22.0–30.8) | 26.0 (22.2–29.9) |
Comparisons of those retained versus lost to follow-up
Baseline characteristics of participants who did and did not complete the extended follow-up questionnaire are provided in Table 86. The groups were broadly similar based on age, gender, duration of disease and EQ-5D-3L scores. Those who were lost to follow-up had worse hand function at baseline (approximately 6 points lower on the MHQ) than those retained in the study. They also had slightly lower SF-12 scores.
| Characteristic by arm | Participants completing the extended follow-up | Participants not completing the extended follow-up |
|---|---|---|
| Exercise programme | ||
| Age (years) at randomisation, mean (SD) | 62.9 (11.0) | 58.6 (14.0) |
| Sex, percentage female | 77.4 | 74.7 |
| Disease duration (years), mean (SD) | 12.4 (10.8) | 14.4 (10.4) |
| Baseline MHQ hand function, mean (SD) | 53.9 (15.1) | 48.9 (14.8) |
| SF-12 PCS, mean (SD) | 35.4 (9.7) | 31.1 (9.4) |
| SF-12 MCS, mean (SD) | 49.7 (10.5) | 45.5 (10.7) |
| EQ-5D-3L health state, mean (SD) | 0.6 (0.3) | 0.5 (0.3) |
| Change in MHQ hand function baseline to 12 months, mean (SD) | 7.2 (13.6) | 9.8 (16.8) |
| Change in SF-12 PCS baseline to 12 months, mean (SD) | 0.8 (7.2) | 2.3 (6.3) |
| Change in SF-12 MCS baseline to 12 months, mean (SD) | 2.1 (9.9) | 2.4 (12.4) |
| Change in EQ-5D-3L health state baseline to 12 months, mean (SD) | 0.0 (0.2) | 0.0 (0.2) |
| Usual care | ||
| Age (years), mean (SD) | 64.3 (10.8) | 61.5 (12.1) |
| Sex, percentage female | 74.0 | 81.2 |
| Disease duration (years), mean (SD) | 14.7 (12.5) | 12.3 (10.7) |
| Baseline MHQ hand function, mean (SD) | 54.1 (15.6) | 47.0 (17.4) |
| SF-12 PCS, mean (SD) | 35.4 (9.7) | 32.1 (8.5) |
| SF-12 MCS, mean (SD) | 50.4 (10.4) | 45.1 (11.7) |
| EQ-5D-3L health state, mean (SD) | 0.6 (0.2) | 0.5 (0.3) |
| Change in MHQ hand function baseline to 12 months, mean (SD) | 3.2 (16.0) | 4.8 (16.3) |
| Change in SF-12 PCS baseline to 12 months, mean (SD) | –0.1 (7.6) | 0.5 (6.7) |
| Change in SF-12 MCS baseline to 12 months, mean (SD) | 0.2 (9.5) | 1.1 (10.8) |
| Change in EQ-5D-3L health state baseline to 12 months, mean (SD) | 0.0 (0.2) | 0.0 (0.3) |
| Combined | ||
| Age (years), mean (SD) | 63.6 (10.9) | 59.8 (13.2) |
| Sex, percentage female | 75.5 | 77.8 |
| Disease duration (years), mean (SD) | 13.7 (11.8) | 13.5 (10.6) |
| Baseline MHQ hand function, mean (SD) | 54.0 (15.4) | 48.1 (15.9) |
| SF-12 PCS, mean (SD) | 35.4 (9.7) | 31.5 (9.0) |
| SF-12 MCS, mean (SD) | 50.1 (10.4) | 45.3 (11.1) |
| EQ-5D-3L health state, mean (SD) | 0.6 (0.3) | 0.5 (0.3) |
| Change in MHQ hand function baseline to 12 months, mean (SD) | 5.1 (15.0) | 7.5 (16.7) |
| Change in SF-12 PCS baseline to 12 months, mean (SD) | 0.3 (7.5) | 1.5 (6.5) |
| Change in SF-12 MCS baseline to 12 months, mean (SD) | 1.1 (9.7) | 1.8 (11.7) |
| Change in EQ-5D-3L health state baseline to 12 months, mean (SD) | 0.0 (0.2) | 0.0 (0.3) |
Missing data
The number of missing data on the returned questionnaires was minimal (Table 87).
| Missing items/scales questionnaires | Extended follow-up | |
|---|---|---|
| Exercise programme (n = 155) | Usual care (n = 173) | |
| MHQ | ||
| MHQ: overall hand function both | 1 | – |
| MHQ: ADLs | 2 | 1 |
| MHQ: work | 8 | 4 |
| Secondary outcomes | ||
| Pain troublesomeness: any missing | 2 | – |
| Arthritis self-efficacy scale: any missing | 4 | 1 |
| Participant-rated benefit | 2 | 1 |
| EQ-5D-3L any items | 2 | – |
| SF-12 any missing items | – | – |
| Health resource use sections | ||
| GP visits because of RA | 5 | 2 |
| Nights in NHS hospital (RA) | 3 | – |
| Work | ||
| Days off work | 3 | 1 |
| Current exercises | 2 | – |
Outcomes and estimation
Primary outcome: hand function
Both groups continued to report improved hand function compared with baseline at the extended follow-up with greater benefit observed in the exercise group. The difference between the two groups was no longer statistically significant (Figure 16 and Table 88).
FIGURE 16.
Michigan Hand Outcome Questionnaire overall hand function subscale scores over time.

| MHQ overall hand function (both hands) | Number of patients analysed | Mean change score (95% CI) | Mean treatment difference (95% CI) | p-value | Standardised mean difference (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Exercise programme | Usual care | Exercise programme | Usual care | ||||
| Extended follow-up | 154 | 173 | 3.76 (1.50 to 6.02) | 1.97 (–0.45 to 4.39) | 1.74 (–1.46 to 4.95) | 0.2875 | 0.11 (–10.00 to 0.32) |
| Adjusted for centre, sex and age | 1.72 (–1.53 to 4.96) | 0.3007 | |||||
| Adjusted for centre, sex, age and time of follow-up | 1.93 (–1.33 to 5.19) | 0.2469 | |||||
In the adjusted analysis of the primary outcome, the time from randomisation to receipt of follow-up in months was included as a covariate. As shown in Table 88 this resulted in a small change to the estimated treatment effect from 1.74 to 1.93. A test to determine whether there was a difference in the follow-up effect between treatments was not significant (p = 0.2607).
Repeated measures analysis of overall hand function
As with the main trial analysis, the repeated measures estimate uses the overall hand function scores at all time points and is adjusted for hospital, age and sex. The treatment difference estimated from baseline to the extended follow-up is 1.49 (95% CI –1.68 to 4.65; p = 0.3585).
Multiple imputation estimate for overall hand function
To take some account of the effects of the data being incomplete, the same method of multiple imputation analysis was used here as in the main trial analysis with the addition of the extra time point. The estimate of treatment difference from baseline to extended follow-up is 1.31 (95% CI –2.08 to 4.71; p = 0.4404).
Complier average causal effect
The CACE estimates based on full attendance and for the lower compliance threshold are slightly larger than the overall trial estimates for the extended follow-up but they remain non-significant.
Complier average causal effect estimates for 0–24 months with bootstrap 95% CI:
Other outcomes
Michigan Hand Outcome Questionnaire activities of daily living and work subscales
Both groups had higher MHQ ADLs scores and MHQ work at the extended follow-up than at baseline. Greater improvement from baseline was observable in the exercise arm but the difference between the two groups was no longer significant (Table 89).
| MHQ | Number of patients | Mean change score (95% CI) | Mean treatment difference (95% CI) | p-value | Standardised mean difference (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Exercise programme | Usual care | Exercise programme | Usual care | ||||
| MHQ ADLs (both hands) | |||||||
| Extended follow-up | 152 | 172 | 3.15 (0.30 to 6.01) | 2.34 (0.03 to 4.66) | 0.85 (–2.71 to 4.42) | 0.6401 | 0.03 (–0.12 to 0.18) |
| MHQ work | |||||||
| Extended Follow-up | 147 | 168 | 7.76 (4.67 to 10.84) | 5.81 (2.97 to 8.65) | 1.84 (–2.22 to 5.90) | 0.3745 | 0.09 (–0.10 to 0.28) |
Health-related quality of Life (Short Form questionnaire-12 items and European Quality of Life-5 Dimensions)
There was no difference between the two treatments as measured by the SF-12. The majority of SF-12 subscales were similar to the baseline scores at the extended follow-up in both groups. The exceptions were the social functioning subscale, with both groups demonstrating a reduction in social functioning compared with baseline, although there was no difference between the groups. Both groups also reported a reduction in their general health compared with baseline, but there was no difference between the two groups.
There was no difference between the two treatments as measured by the EQ-5D-3L (Tables 90 and 91).
| SF-12 | Number of patients | Mean change score (95% CI) | Mean treatment difference (95% CI) | p-value | Standardised mean difference (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Exercise programme | Usual care | Exercise programme | Usual care | ||||
| SF-12 aggregate physical scale (PCS) | |||||||
| Extended follow-up | 153 | 173 | 0.19 (–1.16 to 1.54) | 0.51 (–1.66 to 0.64) | 0.69 (–1.01 to 2.40) | 0.4271 | 0.07 (–0.11 to 0.26) |
| SF-12 aggregate mental scale (MCS) | |||||||
| Extended follow-up | 153 | 173 | 0.27 (–1.25 to 1.78) | 0.21 (–1.23 to 1.66) | –0.22 (–2.11 to 1.67) | 0.8215 | 0.00 (–0.19 to 0.20) |
| SF-12 physical functioning | |||||||
| Extended follow-up | 150 | 167 | 1.33 (–2.78 to 5.45) | –2.25 (–6.03 to 1.54) | 3.21 (–2.12 to 8.54) | 0.2388 | 0.12 (–0.07 to 0.30) |
| SF-12 role-physical | |||||||
| Extended follow-up | 149 | 169 | 2.18 (–1.38 to 5.74) | 1.11 (–2.23 to 4.45) | 1.11 (–3.47 to 5.69) | 0.6362 | 0.04 (–0.15 to 0.23) |
| SF-12 bodily pain | |||||||
| Extended follow-up | 153 | 172 | 2.12 (–1.99 to 6.24) | 2.76 (–0.84 to 6.36) | –0.53 (–5.40 to 4.35) | 0.8322 | –0.02 (–0.23 to 0.19) |
| SF-12 general health | |||||||
| Extended follow-up | 152 | 173 | –3.09 (–6.48 to 0.30) | –4.13 (–7.41 to –0.86) | 0.65 (–3.58 to 4.87) | 0.7648 | 0.04 (–0.14 to 0.22) |
| SF-12 vitality | |||||||
| Extended follow-up | 150 | 173 | 2.00 (–1.67 to 5.67) | 0.14 (–3.08 to 3.37) | 1.81 (–2.60 to 6.22) | 0.4227 | 0.07 (–0.12 to 0.27) |
| SF-12 social functioning | |||||||
| Extended follow-up | 153 | 173 | –4.41 (–8.86 to –0.15) | –3.18 (–6.90 to 0.54) | –1.38 (–6.39 to 3.62) | 0.5887 | –0.04 (–0.25 to 0.16) |
| SF-12 role-emotional | |||||||
| Extended follow-up | 150 | 171 | 4.08 (–0.15 to 8.32) | 1.46 (–2.35 to 5.27) | 1.15 (–3.80 to 6.10) | 0.6500 | 0.09 (–0.10 to 0.28) |
| SF-12 mental health | |||||||
| Extended follow-up | 150 | 168 | –0.33 (–3.67 to 3.00) | 0.97 (–2.03 to 3.96) | –1.44 (–5.45 to 2.58) | 0.4842 | –0.06 (–0.27 to 0.15) |
| EQ-5D-3L | Number of patients | Mean change score (95% CI) | Mean treatment difference (95% CI) | p-value | Standardised mean difference (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Exercise programme | Usual care | Exercise programme | Usual care | ||||
| EQ-5D-3L health statea | |||||||
| Extended follow-up | 152 | 172 | –0.01 (–0.05 to 0.03) | –0.01 (–0.05 to 0.02) | –0.01 (–0.06 to 0.04) | 0.6511 | 0.01 (–0.18 to 0.20) |
| EQ-5D-3L VAS (your health today) | |||||||
| Extended follow-up | 151 | 171 | –1.42 (–4.23 to 1.38) | –1.91 (–4.49 to 0.68) | –0.03 (–3.20 to 3.80) | 0.8667 | 0.03 (–0.18 to 0.23) |
Pain troublesomeness and self-efficacy
At the previous follow-up point, both groups had reported less pain than at baseline, and this continued in the control group at extended follow-up. The pain scores reported in the exercise arm were similar to baseline scores. The difference between the two groups was not significant (Table 92). Self-efficacy scores remained slightly higher in the exercise arm than in the control arm, but the difference was not significant (Table 93).
| Pain troublesomeness overall scorea | Number of patients | Mean change score (95% CI) | Mean treatment difference (95% CI) | p-value | Standardised mean difference (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Exercise programme | Usual care | Exercise programme | Usual care | ||||
| Extended follow-up | 153 | 173 | 0.20 (–2.98 to 3.38) | –3.79 (–6.93 to –0.64) | 2.68 (–1.41 to 6.77) | 0.2002 | 0.18 (–0.02 to 0.39) |
| Confidence in performing tasks overall score | Number of patients | Mean change score (95% CI) | Mean treatment difference (95% CI) | p-value | Standardised mean difference (95% CI) | ||
|---|---|---|---|---|---|---|---|
| Exercise programme | Usual care | Exercise programme | Usual care | ||||
| Extended follow-up | 151 | 172 | 2.96 (0.03 to 5.90) | 0.22 (–2.34 to 2.78) | 2.11 (–1.37 to 5.59) | 0.2347 | 0.14 (–0.06 to 0.34) |
Participant rated improvement
There was no difference between the two groups as measured by the participant-rated improvement (Table 94).
| Response | Extended follow-up | |
|---|---|---|
| Exercise programme, n (%) | Usual care, n (%) | |
| Completely recovered | – | 1 (0.6) |
| Much improved | 25 (16.3) | 22 (12.8) |
| Slightly improved | 24 (15.7) | 17 (9.9) |
| No change | 58 (37.9) | 75 (43.6) |
| Slightly worsened | 35 (22.9) | 41 (23.8) |
| Much worsened | 7 (4.6) | 15 (8.7) |
| Vastly worsened | 4 (2.6) | 1 (0.6) |
| Total | 153 | 172 |
Current hand exercise performance
There was no difference in current exercise performance between the two groups (Table 95).
| Are you currently doing any hand/wrist exercises to help with your RA? | Extended follow-up | |
|---|---|---|
| Exercise programme, n (%) | Usual care, n (%) | |
| No | 57 (37.3) | 74 (42.8) |
| Yes, total | 96 (62.7) | 99 (57.2) |
| Yes, daily | 20 (13.2) | 38 (22.1) |
| Yes, 3–4 times a week | 28 (18.5) | 22 (12.8) |
| Yes, 1–2 times a week | 42 (27.8) | 35 (20.3) |
| Yes, other | 6 (4.0) | 3 (1.7) |
| Yes, blank | – | 1 (0.6) |
| Total | 151 | 172 |
Health resource use
Four participants in the exercise intervention arm and six participants in the usual care arm reported spending at least one night in a NHS hospital because of their RA within the previous 4 months. The mean number of nights is not reported since four of the six participants in the usual care arm did not report how many nights and one participant in the intervention arm reported spending 28 nights in hospital. There was no difference in visits to their general practitioner or NHS therapists because of RA between the two groups (Table 96).
| Type of care | Number of patients | Mean quantity (SE) | Mean treatment difference (95% CI) | ||
|---|---|---|---|---|---|
| Exercise programme | Usual care | Exercise programme | Usual care | ||
| General practitioner visits due to RA | 51/150 | 61/171 | 0.678 (0.110) | 0.564 (0.074) | 0.117 (–0.143 to 0.377) |
| NHS therapist visits due to RA | 29/152 | 27/172 | 0.467 (0.131) | 0.394 (0.109) | 0.073 (–0.261 to 0.407) |
Time off work
Of those participants working, 3/41 in the exercise intervention arm and 7/47 in the usual care arm reported taking time off work during the previous 4 months because of their RA. The number of days off work is not presented as this was dominated by two participants in the usual care arm losing 16 weeks and 8 weeks. Changes to work hours owing to RA are reported in Table 97. There was no difference between the two groups.
| Changes to hours of employment owing to RA | Extended follow-up | |
|---|---|---|
| Exercise programme | Usual care | |
| No, stayed the same | 33 | 34 |
| Yes, increased | – | 1 |
| Yes, decreased | 2 | 3 |
| Not applicable | 117 | 134 |
Discussion
Improvements in the primary outcome compared with baseline were still observable in both groups more than 2 years after randomisation. MHQ overall hand function scores at the extended follow-up remained higher than baseline scores despite a reduction over time compared with earlier follow-up points. Greater benefit persisted in the exercise group although this was no longer significantly different between groups. A similar pattern was observable in the MHQ ADLs and work subscale scores. We demonstrated at earlier time points that the exercise programme did not result in increased pain and this was still true at the extended follow-up.
The majority of subscales in the HRQoL measures were similar to baseline scores at the extended follow-up and there were no differences between the two groups. Some improvement was observed in aspects of HRQoL at earlier time points, but it is not surprising that this effect diminished over time as the intervention was specifically focused to the hand. An interesting finding was that both groups reported reduced general health ratings and social interaction scores measured by the SF-12 compared with baseline. This may reflect the impact of RA as a systemic disease on general health and subsequently social functioning as individuals with RA often have comorbidities such as cardiovascular problems. 126
The mean follow-up time point was approximately 26 months post randomisation. Participants who were allocated to the exercise intervention had completed treatment with the therapist approximately 3 months post randomisation and so for some participants it had been 2 years since they had attended an appointment with a therapist. Therefore, it is unsurprising that the added benefit from attending the therapy sessions had reduced over time. The qualitative study demonstrated that the encouragement and support provided by the therapists was integral to them successfully carrying out their exercise programme regularly. The data suggest that by the extended follow-up many of the participants who were allocated to the exercise arm were no longer doing the exercises they were given as part of the study. At 4 months, 88% of participants reported doing hand/wrist exercises for their RA, and this fell to 70% at 12 months’ follow-up. There was a further reduction to 63% at the extended follow-up. Specifically, the number of participants who reported doing daily hand/wrist exercises (as per the exercise intervention protocol) had fallen from 44% at 4 months follow-up to only 13% at the extended follow-up. Reported exercise behaviour was very similar in the two arms of the study at the extended follow-up time point. RA is a progressive disease so regular exercise of sufficient intensity is needed to maintain muscle strength and flexibility. It is likely that participants in the exercise intervention arm were no longer achieving a sufficient level of exercise intensity to maintain the functional improvements they achieved earlier on in the study compared with the control group.
It was proposed that another mechanism by which the intervention improved function was bolstering self-efficacy and the participants’ ability to self-manage their condition. This effect had also diminished over time and the difference between the groups was no longer evident. This may be because it had been such a long time since receiving encouragement and support from the therapist.
The response rate for extended follow-up was lower than for the main study. This was not unexpected as it was a postal questionnaire rather than the face-to-face appointment used for the earlier follow-up assessments. Also, as this was not planned at the outset of the study and participants had not been made aware of the extended follow-up, we limited the contact with participants to reminders by post so as not to place undue pressure on participants to respond. We only phoned those who requested to complete the questionnaire by phone. In total, 67% of participants provided follow-up data, which means the analysis is slightly underpowered to detect a difference in the primary outcome. Loss to follow-up could introduce bias and there were some differences in baseline characteristics of those who responded and those who did not. Most notably, those who did not return the questionnaire had poorer hand function at baseline compared with those who did return the questionnaire. The difference was approximately 6 points on the MHQ. Multiple imputation techniques were used to estimate the effect of missing data and the results were largely similar, indicating that the missing data did not overly influence the findings. Overall, we are confident that the participants providing data were a good representation of the total cohort.
These results show that an exercise programme delivered over 12 weeks benefited participants for 12 months, but after this time point a reduction in benefit was observed. These findings raise important questions regarding how patients might be encouraged to carry out exercise programmes long term in the hope that improvements will be maintained. In the qualitative study, interviewees did suggest that ongoing contact with the therapist would help them to continue with their exercises. Further research needs to evaluate methods of providing support for patients to assist them to continue with long-term exercise programmes.
Chapter 8 Discussion
This discussion provides an overview of findings, issues surrounding internal and external validity of the study and its analyses and provides interpretation for clinical and policy arenas. Recommendations for further research are also provided.
Aims and overview of the trial findings
Hand and wrist dysfunction are a significant and common concern for people with RA. Clinical guidelines recommend treatments targeted specifically at hands to improve impairments and resulting disabilities. Prior to the SARAH trial, no high-quality evidence existed regarding the clinical effectiveness of exercise specifically for RA hands, although findings from small-scale studies were promising. Also, no evidence existed of cost related to exercise interventions of hand therapy for RA and subsequent cost-effectiveness analyses. Therefore, the aim of the study was to estimate the clinical effectiveness and cost-effectiveness of adding an optimised exercise programme to best practice usual care. The addition of an embedded qualitative study provided the participants’ perspective and assists with explaining and interpreting the findings.
For the primary outcome of overall hand function, an optimised programme of stretching and strengthening exercises provided significant improvements in both the short and long term (4 and 12 months) when added to best practice usual care. Maintenance of an effect at 12 months is impressive considering the intervention was delivered in the first 4 months and then relied on patients to self-manage. The finding from the primary outcome is supported by findings from some, but not all, secondary outcome measures: participants reported greater ratings of global improvement, greater benefit from treatment and greater treatment satisfaction at both time points. As well as improving hand function, the exercise programme resulted in significant improvement in self-efficacy. Physical and psychological benefits from the exercise programme were reported by participants of the qualitative study. The pattern of changes are consistent with an intervention that effects hand function but not the broader effects of RA such as impaired mobility (although swollen and tender joints in the hands and wrists were significantly improved in the short term). The findings from the extended follow-up study that there was no benefit of the exercise programme over usual care alone at approximately 2 years after randomisation and treatment is at odds with the original study time points but not unexpected considering the lack of therapist support and reduction in proportion of patients continuing to perform specific exercises.
With regard to impairment measures, there were improvements in strength, ROM and dexterity in both groups. The exercise programme group had greater gains in power grip strength at 4 months and pinch grip strength at 12 months. Improvements in dexterity, measured by the nine-hole peg test, were sustained in the exercise programme. Whether or not further sessions or longer treatment duration would have resulted in greater changes in impairment measures is a matter of conjecture, although previous studies have not reported consistent links between improved function and changes in impairment measures.
The intervention is likely to be cost-effective, although there is considerable uncertainty owing to difficulties in estimating costs and the small magnitude of the observed between-group difference in EQ-5D-3L scores (there is a 59–66% likelihood that it is cost-effective at an upper NICE threshold of £30,000 per additional QALY gained for the base-case analysis). Results suggest that the hand exercise programme costs between £6000 and £23,000 more per additional QALY gained over a 12-month time horizon, compared with usual care and depending on the measure and methods of analysis used. These figures are within current willingness-to-pay levels applied by NICE. The exercise programme is also a cheap intervention, costing around one-tenth of the cost of average medications for the patients in this study over 12 months.
This trial also proved that the addition of an optimised exercise programme was safe. There was, on average, no increase in pain or disease activity, no adverse events attributable to treatments and a short-term reduction in joint tenderness and swelling.
Internal validity and methodological limitations
According to the sample size calculation, data from a minimum of 352 participants were required to show a moderate mean effect size difference of 0.3 at 12 months. We recruited 490 participants and had a lower than estimated loss to follow-up rate of 10%, producing 438 participants with primary outcome data at 12 months, which provided ample statistical power to detect the difference originally specified.
Randomisation resulted in two well-matched groups. Demographic data, self-report functional measures, clinical impairment measures and disease stability and duration were all very similar. The small difference between arms in proportions of participants taking single or combination DMARDs is very unlikely to be a significant imbalance. Randomisation was stratified by site and used variable block length, minimising the chances of research staff anticipating and allocating treatments for certain participants.
It was intended that outcome assessors (research clinicians) would remain blind to allocation throughout the trial. It was not possible to blind participants and therapists because of the nature of the interventions tested. We asked the participants not to disclose the treatments they received within the trial; however, with face-to-face assessments (as opposed to postal questionnaires) there is a risk that unblinding can occur, and it appears that outcome assessors were unblinded in approximately one-quarter of cases. The rate indicates the difficulty in maintaining blinding in pragmatic rehabilitation trials of this nature. Post-hoc sensitivity analysis indicated that this does not have a significant impact on findings. It is also to be noted that the primary outcome measure was participant self-report and, therefore, unlikely to have been influenced by unblinding of the outcome assessor anyway.
The interventions in both arms were well received by participants according to the results of the satisfaction with treatment question and qualitative study, and well delivered by the therapists as determined by quality assurance checks. Attendance rates were above those normally expected for NHS out-patient therapy (10% non-attendance according to the Audit Commission)127 and adherence to the treatment protocols was good, with 75% of participants in the exercise programme arm completing all six sessions of treatment. Progression of exercises was regarded as key in order to achieve the physiological changes required for strengthening and stretching of hands and wrists. The treatment logs provided evidence that approximately 80% of participants in the experimental arm progressed between their first and last sessions as intended. We found no significant therapist effects at either time point, implying a well-standardised intervention.
A small proportion of participants (5%) withdrew, and these were generally distributed evenly across the two trial arms. There was a trend for a higher proportion of loss to follow-up among participants in the exercise programme and mostly at their time of treatment, potentially because of the greater number of sessions and demands on their time, but this was within the realms of chance. There were no significant baseline differences between those retained and those lost to follow-up although there was a slight trend for lost participants to be younger and male – a common finding in pragmatic clinical trials. Where participants were unable to attend face-to-face clinic assessments, collecting core data by postal questionnaire or telephone proved successful in retaining an extra 10–15% of original participants. Overall, our estimates of treatment effectiveness appear insensitive to missing data.
Medication can have a far greater influence on symptoms and disability than an exercise programme for RA so we investigated this as part of the trial. There were no significant differences in medication type, usage and changes at the follow-up time points, suggesting that the changes found in hand function were due to the addition of the exercise programme.
External validity and generalisability of the findings
Overall, we believe the generalisability of the findings of the trial is good. Originally we had planned to recruit from 10 NHS trusts; however, a suboptimal recruitment rate meant that we involved a further seven NHS trusts. The advantage of this was a greater range of centres in terms of geography and size. We recruited from large university teaching hospitals as well as small district general hospitals. The training provided to the staff in these centres was brief, with most NHS therapy staff already having sufficient expertise to deliver the exercise programme intervention. As a result we believe the implementation of this intervention into the NHS would be straightforward and involve relatively small cost.
We consider the population recruited into this trial to be representative of patients who would be referred from NHS rheumatology clinics to hand therapy for treatment of hand and wrist problems. The majority of study participants were female (76%), which reflects the estimates that prevalence in women is three times that of men. 1 The average age of participants was 63 years, which is consistent with the estimate of 70+ years as the peak age of prevalent RA. 128 A previous RCT reported a cohort of very similar characteristics. 23 There exists the possibility that those recruited into the study form only a proportion of RA patients who typically present to rheumatology clinics, with some suggestions that newly diagnosed patients (i.e. younger with shorter disease duration) and those suffering from greater instability or ‘flare-ups’ form a significant percentage of the caseload.
There were few recently diagnosed patients in this study. The inclusion criteria for the study stipulated that patients needed to be on stable medications for at least 3 months. This may have resulted in a higher proportion of patients with longer disease duration being included in the study as patients are less likely to be on stable medication regimes when newly diagnosed. Our qualitative study found that one of the main barriers to regular exercise was flare-ups or increased symptoms. This suggests that stable symptoms are an important aspect of selecting patients for this intervention as uncontrolled symptoms make exercising difficult.
The participants in this study had relatively low inflammatory disease activity, as shown by the low ESR and CRP in both treatment groups at baseline. This would be in keeping with the idea that they represent a group who have been established and stabilised on a successful treatment regime, and thus very few patients required changes in DMARD treatment during the course of the study. It is important to note, however, that this does not mean that these were patients whose RA had burnt out. On the contrary, approximately half the participants were being treated with either biologic DMARDs or combination non-biologic DMARDs, which are the two most intensive forms of drug therapy for RA. About one-fifth of participants were being treated with biologic DMARDs, which signifies that in the relatively recent past their arthritis was so active that they fulfilled the NICE guidelines for starting these new and expensive agents. Thus, the results of the study suggest that, when active disease has been brought under control using an appropriate DMARD regime, the addition of a relatively inexpensive exercise programme can maximise the beneficial effect to the patient. The qualitative study also suggests that it would be better to introduce exercise once active joint inflammation has been controlled, since pain or flaring in joints was identified as a disincentive to carrying out exercises.
Our cohort had a slightly higher proportion of participants of white ethnicity compared with the British population as a whole (97% vs. 91% respectively),129 but the prevalence of RA in ethnic minorities is not well known or understood so it is difficult to infer what influence this may have on the generalisability of our results. It is not unusual to have difficulty recruiting ethnic minority patients with RA into trials and we would echo calls for further research in this area.
The source of recruitment for the study (i.e. approached while attending rheumatology clinic or from mail-out to patients on rheumatology or therapy review lists) was equal in the two groups and did not appear to have any effect on treatment outcomes. Other subgroup analyses examining baseline disease duration, age, sex, disease activity, medication, hand function (MHQ) and HRQoL (SF-12) indicate that these factors also did not affect the primary outcome measure.
Interpretation and implications for clinical practice and policy
Although a statistically significant difference in hand function at 12 months was found between the two groups, it is difficult to state the degree of clinical benefit provided by the exercise programme over and above the usual care intervention. The difference in scores between the groups was between 4–5 points on the MHQ overall hand function scale in favour of the exercise group. Although the responsiveness to change of the MHQ has been reported elsewhere,41 the minimum clinically important difference for the MHQ has not been established in a conservatively treated RA population. Previous work has investigated this property of the MHQ following MCP joint arthroplasty with the minimum clinically important difference of the hand function subscale reported at 13 points. 130 These findings suggest that the change in hand function in this study did not reach clinical relevance as it was less than half of the change that would be required for the participant to describe an improvement in satisfaction with hand function.
An alternative explanation is that a conservative intervention, such as the exercise programme used in this study, resulted in a clinical change almost half of that previously described for joint surgery, a procedure vastly more invasive and costly that would be expected to result in a far greater difference between pre- and post-intervention assessments. Viewed from this perspective, the change score recorded as part of this study is good. The extended follow-up demonstrated that the benefit of the exercise programme was reduced but not completely diminished at a mean follow-up time of 26 months post randomisation. This is an encouraging finding as the participants received the intervention in the 4 first months post randomisation but the benefit was observed for a considerable time beyond this period. As RA is a chronic disease it is most likely that patients need to continue with regular exercise at an adequate level to maintain improvements for longer time periods. The participants in this study did not receive any further support to continue their exercises beyond the duration of their appointments with the therapists when they were left to exercise independently. Finding cost-effective ways to support patients to continue with a regular independent exercise programme at a sufficient dose could potentially extend the benefits already demonstrated by this study.
For the purposes of the trial, the control intervention was standardised across all sites in accordance with what would be considered ‘best practice’ usual care, with the quality of this care being high. Prior to the trial, considerable variation in normal practice existed across the centres, with some offering little or no treatment. Therefore, involvement of these ‘no treatment’ centres in the trial resulted in additional care for their patients irrespective of which arm they were allocated to. The subsequent improvement described for those in the control intervention may include the effect of the greater than normal treatment at some sites, suggesting that the mean treatment difference between the groups may be underestimated for some NHS trusts currently.
From the 12-month interviews it was apparent that, in addition to functional improvement, interviewees reported psychological benefits, primarily in their confidence levels. This reported benefit was also mirrored in the main trial findings, with those receiving the exercise intervention reporting higher levels of self-efficacy to manage their arthritis. Improvement in confidence may go some way to explain why the exercise intervention improved function despite no impact on participants’ pain or symptoms.
One of the pleasing aspects of the study was the high number of participants who completed the exercise programme in full with its associated procedures (74%). This in part can be explained by the motivation of the participants, but is also due to the design of the intervention, which made every effort to include measures to increase adherence. The importance of maximising adherence is highlighted by the results of the CACE analysis, which, despite being limited to some extent by the small number of participants that did not attend all six sessions, revealed a greater treatment effect for those who completed treatment in full. This may be an important factor for therapists to consider when providing information to patients regarding how to gain maximum benefit from an exercise programme. It also suggests that a minimum number of sessions or duration of treatment is required in order to effect the necessary changes, physiological or otherwise, that result in improved hand function.
Participants in the interview study were advocates of exercise, which is perhaps unsurprising as they had agreed to participate in a research project involving exercise. Patients seen in normal clinical practice may not hold such beliefs about the benefit of exercise, which could potentially impact on their adherence. The interview study identified several factors that are important for future implementation of the SARAH intervention into clinical practice. The key to adherence was the ability to ‘establish a routine’. Interviewees reported the importance of being able to integrate the programme into their daily routine as being essential to regular completion of the exercise programme. The behavioural strategies (daily exercise diary and PEG) used in the study were reported to be helpful tools to assist in establishing a routine; thus, this would be an essential component to delivering the exercise programme in clinical practice. The therapists also had a pivotal role in teaching, supporting and motivating patients to carry out the exercises at home. It was also suggested that interviewees need to be motivated to do this and, if motivational factors are lacking, then adherence to the exercise programme is unlikely. Demotivating factors were often linked to the symptoms experienced by patients. For example, if they perceived their symptoms to be too mild then they were unlikely to be motivated to exercise because of a lack of potential benefit. However, severe symptoms such as those experienced during a flare-up were also potential barriers to carrying out the programme. Other potential barriers spoken about were competing priorities, lack of time and having a negative mindset. These are important factors for therapists to consider when trying delivering the SARAH intervention in clinical practice.
Other literature
Only one relevant RCT has been published since the SARAH trial began. Cima et al. 131 randomised 20 women with deformed RA hands to receive an intensive strengthening and co-ordination exercise programme or no treatment. The results showed that function and grip strength were significantly improved in patients in the exercise arm but were only for short-term measurement (follow-up 10 weeks). This reinforces the importance of the SARAH trial findings in terms of their definitive nature and long-term follow-up.
We also await the imminent publication of another UK-based RCT evaluating exercise for the upper limb in early RA patients [the Education, Self-management and Upper limb Exercise training in People with Rheumatoid arthritis (EXTRA) study, n = 108]. 132 This study found short-term improvements in function and self-efficacy and reduction in impairment when compared to usual care, but these were not maintained in the longer term (9 months). An important message from this study is that a similar exercise programme to that used in SARAH was found to be effective and safe in RA patients with average disease duration of 20 months.
Further research
With the findings of the extended follow-up indicating that participants found it hard to maintain the exercise programme beyond 1 year, it would be beneficial to explore the effects of different motivational techniques, such as top-up contacts, on adherence to the programme. Investigation of effectiveness of the SARAH exercise programme in a population with shorter disease duration also appears to be warranted.
The individualised exercise intervention has been shown to be likely to be cost-effective over a 12-month period, but some participants of the interview study felt that it could be implemented in a group setting. This could potentially make it even more cost-effective and time-efficient. Interviewees suggested that benefits such as peer support and improved commitment could be gained from undertaking the exercise programme along with others with a similar condition. Delivering the exercise programme in a group setting would provide different challenges in design and would obviously need further evaluation before it could be recommended. Further study of implementation could include evaluation of how therapists are able to prioritise the SARAH exercise programme within time and resource constraints of current clinical practice and existing modes of hand exercise prescription.
Current NICE guidance recommends an annual review for all patients with RA. 13 Evaluation of whether or not this should include a therapist review to reinforce the importance of hand exercise and JP is warranted. Core elements of the exercise programme (e.g. the behavioural activation approach) could be tested in other areas of the body for RA or for other musculoskeletal conditions.
Acknowledgements
Contributions of authors
Mark A Williams (research fellow, clinical trials): study lead, study design, development of interventions, supervision, writing and reviewing the report.
Esther M Williamson (research fellow, clinical trials): qualitative study design and conduct, development of interventions, writing and reviewing the report.
Peter J Heine (clinical research fellow, physiotherapy): development of interventions, quality assurance of interventions, study management, writing and reviewing the report.
Vivien Nichols (research associate, qualitative): qualitative study design and conduct, writing and reviewing the report.
Matthew J Glover (research assistant, health economics): designing and conducting economic analysis, writing and reviewing the report.
Melina Dritsaki (research fellow, health economics): designing and conducting economic analysis, writing and reviewing the report.
Jo Adams (senior lecturer, occupational therapy): southern hub lead, development of interventions, quality assurance of interventions, writing and reviewing the report.
Sukhdeep Dosanjh (trial co-ordinator, emergency care and rehabilitation): study management, writing and reviewing the report.
Martin Underwood (Professor, primary care): study conception and design, study supervision, writing and reviewing the report.
Anisur Rahman (Professor, rheumatology): study supervision, study design, reviewing the report.
Christopher McConkey (Statistician): designing and conducting statistical analysis, writing and reviewing the report.
Joanne Lord (Reader, Health Economics): designing and conducting economic analysis, supervision, writing and reviewing the report.
Sarah E Lamb (Professor, rehabilitation; Director of Clinical Trials Unit): chief investigator, led funding application, study conception and design, development of interventions, supervision, writing and reviewing the report.
Strengthening And stretching for Rheumatoid Arthritis of the Hand hospitals
Basingstoke and North Hampshire Hospitals NHS Foundation Trust (North Hampshire Hospital), Derby Hospitals NHS Foundation Trust (Royal Derby Hospital), Dorset Primary Care Trust (Victoria Hospital), George Eliot Hospital NHS Trust (George Eliot Hospital), Heart of England NHS Foundation Trust (Solihull Hospital), Nuffield Orthopaedic Centre NHS Trust (Nuffield Orthopaedic Centre), Poole Hospital NHS Foundation Trust (Poole General Hospital), Portsmouth Hospitals NHS Trust (Queen Alexandra Hospital), Royal Bournemouth NHS Foundation Trust (Christchurch Hospital), Royal National Hospital for Rheumatic Diseases NHS Foundation Trust (Bath Royal National Hospital for Rheumatic Diseases), South Warwickshire General Hospitals NHS Trust (Warwick Hospital and Stratford-Upon-Avon Hospital), Sussex Community NHS Trust (Bognor Regis War Memorial Hospital), Winchester and Eastleigh Health Care Trust (Royal Hampshire County Hospital), Worcestershire Acute Hospitals NHS Trust (Worcestershire Royal Hospital; Alexandra Hospital, Redditch; Kidderminster Hospital and Treatment Centre), Wrightington, Wigan and Leigh NHS Foundation Trust (Wrightington Hospital), University Hospitals Coventry & Warwickshire [University Hospital (Walsgrave site) and Rugby St Cross Hospital], University Hospitals of Leicester NHS Trust (Leicester Royal Infirmary and Leicester General Hospital).
Strengthening And stretching for Rheumatoid Arthritis of the Hand trial team
Chief investigator: Professor Sarah E Lamb.
Co-investigators: Dr Jo Adams, Professor Martin Underwood, Dr Chris Bridle, Christopher McConkey, Dr Joanne Lord and Professor Anisur Rahman.
Trial lead: Dr Mark A Williams.
Trial co-ordination/administration: Dr Sukhdeep Dosanjh, Sarah Lowe, Amy Campbell and Laura Rattigan.
Research fellows/associates: Peter J Heine, Dr Ester M Williamson and Vivien Nichols.
Trial statistician: Christopher McConkey.
Health economists: Dr Joanne Lord, Ms Catriona Crossan, Dr Melina Dritsaki, Mr Matthew J Glover.
Research clinicians (recruitment and data collection)
Olivia Neely, Catherine Gibson, Karen Hotchkiss, Frances Chilton, Jessica Thrush, Catherine Minns-Lowe, Ann Birch, Linda Webber, Nicola Clague, Sue Kennedy, Kevin Spear, Sandi Derham, Dr Jenny Lewis, Sarah Bradley, Julie Cottrell, Paula White, Carole Frosdick, Jennifer Wilson, Nicola Bassett-Burr and Maggie Walsh.
Intervention therapists (delivery of treatments)
Lynda Myshrall, Jane Tooby, Cherry Steinberg, Mary Grant, Roslyn Handley, Fiona Jones, Clare Pheasant, Kate Hynes and Sue Kelly (University Hospitals Coventry and Warwickshire NHS Trust); Joanne Newbold, Sally Thurgarland (George Eliot Hospital NHS Trust); Jane Dickenson and Lucy Mann (South Warwickshire Hospital NHS Trust); Alison Hinton, Rachel Chapman, Sunita Farmah, Collette James, Janice Wiltshire and Jane Simons (Worcester Acute Hospitals NHS Trust); Jane Martindale, Susan Hesketh and Alison Gerrard (Wrightington, Wigan & Leigh NHS Trust); Kirsty Bancroft and Corinna Cheng (Poole Hospital NHS Trust); Caroline Wood (Royal Bournemouth NHS Trust); Lisa Small, Karen Coales, Helen Ibbunson and Anne Bonsall (Bath Royal National Hospital for Rheumatic Diseases); Caroline Mountain, Jonathan Gibbons, Esther Mavurah and Hannah Susans (Portsmouth Hospitals NHS Trust); Nicola Spear, Becky Shaylor and Leon Ghulam (Basingstoke & North Hampshire Hospital NHS Trust); Sarah Wastell (Dorset NHS Trust); Christina MacLeod, Sapphire Patterson and Jane Vadher (Winchester & Eastleigh Healthcare NHS Trust); Karen Barker, Sue Gosling, Lizelle Sander-Danby, Jon Room, Aimee Fenn, Anne Richards, Pam Clarke, Gill Rowbotham and Nicky Nolan (Nuffield Orthopaedic Centre NHS Trust); Lorraine Kendall (Bognor Regis War Memorial Hospital); Claire Charnley (Solihull Hospital); Laura Richardson and Kate Wakefield (Leicester Royal Infirmary); and, Victoria Jansen, Liz Radbourne and Julie Tougher (Royal Derby Hospital).
Trial Steering Committee
Professor Alison Hammond (chairperson), Dr Chris Deighton, Dr Chris McCarthy, Professor Sarah E Lamb, Dr Mark Williams and Mr John Wright (user representative).
Data Monitoring Committee
Mr Ed Juszczak, Professor Paul Dieppe and Dr Helen Frost.
Other acknowledgements
Dr Francine Toye for expert advising on design and conduct of the qualitative study.
The SARAH trial is funded by the National Institute for Health Research Health Technology Assessment programme (NIHR HTA), project number 07/32/05.
This report has been developed in association with the NIHR Collaboration for Leadership in Applied Health Research and Care Oxford and the NIHR Biomedical Research Unit Funding Scheme (Lamb, Williams and Williamson).
This project benefited from facilities funded through Birmingham Science City Translational Medicine Clinical Research and Infrastructure Trials Platform, with support from Advantage West Midlands.
Publications
SARAH Trial Team, Adams J, Bridle C, Dosanjh S, Heine P, Lamb S, et al. Strengthening And stretching for Rheumatoid Arthritis of the Hand (SARAH): design of a randomised controlled trial of a hand and upper limb exercise intervention – ISRCTN89936343. BMC Musculoskelet Disord 2012;13:230.
Heine PJ, Williams MA, Williamson E, Bridle C, Adams J, O’Brien A, et al. Development and delivery of an exercise intervention for rheumatoid arthritis: Strengthening And stretching for Rheumatoid Arthritis of the Hand (SARAH) trial. Physiotherapy 2012;98:121–30.
Lamb SE, Williamson EM, Heine PJ, Adams J, Dosanjh S, Dritsaki M, et al. Exercises to improve function of the rheumatoid hand: a randomised controlled trial. Lancet 2015;385:421–9.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Symmons D, Turner G, Webb R, Asten P, Barrett E, Lunt M, et al. The prevalence of rheumatoid arthritis in the United Kingdom: new estimates for a new century. Rheumatology 2002;41:793-800. http://dx.doi.org/10.1093/rheumatology/41.7.793.
- Young A, Dixey J, Cox N, Davies P, Devlin J, Emery P, et al. How does functional disability in early rheumatoid arthritis (RA) affect patients and their lives? Results of 5 years of follow-up in 732 patients from the Early RA Study (ERAS). Rheumatology 2000;39:603-11. http://dx.doi.org/10.1093/rheumatology/39.6.603.
- Taleisnik J, Ruby L, Cooney WP, Linscheid RL, Dobyns JH. The Wrist. 2. St Louis, MO: Mosby; 1998.
- Adams J, Burridge J, Mullee M, Hammond A, Cooper C. Correlation between upper limb functional ability and structural hand impairment in an early rheumatoid population. Clin Rehabil 2004;18:405-13. http://dx.doi.org/10.1191/0269215504cr732oa.
- Jones E, Hanly JG, Mooney R, Rand LL, Spurway PM, Eastwood BJ, et al. Strength and function in the normal and rheumatoid hand. J Rheumatol 1991;18:1313-18.
- Horsten NC, Ursum J, Roorda LD, van Schaardenburg D, Dekker J, Hoeksma AF. Prevalence of hand symptoms, impairments and activity limitations in rheumatoid arthritis in relation to disease duration. J Rehabil Med 2010;42:916-21. http://dx.doi.org/10.2340/16501977-0619.
- de la Mata Llord J, Palacios Carvajal J. Rheumatoid arthritis: are outcomes better with medical or surgical management?. Orthopedics 1998;21:1085-6.
- Singh JA, Christensen R, Wells GA, Suarez-Almazor ME, Buchbinder R, Lopez-Olivo MA, et al. Biologics for rheumatoid arthritis: an overview of Cochrane reviews. Cochrane Database Syst Rev 2009;7.
- Suarez-Almazor ME, Belseck E, Shea B, Wells G, Tugwell P. Methotrexate for rheumatoid arthritis. Cochrane Database Syst Rev 2000;2.
- American College of Rheumatology . Guidelines for the management of rheumatoid arthritis: 2002 Update. Arthritis Rheum 2002;46:328-46. http://dx.doi.org/10.1002/art.10148.
- Lard LR, Visser H, Speyer I, vander Horst-Bruinsma IE, Zwinderman AH, Breedveld FC, et al. Early versus delayed treatment in patients with recent-onset rheumatoid arthritis: comparison of two cohorts who received different treatment strategies. Am J Med 2001;111:446-51. http://dx.doi.org/10.1016/S0002-9343(01)00872-5.
- Nurmohamed MT, Dijkmans BA. Are biologics more effective than classical disease-modifying antirheumatic drugs?. Arthritis Res Ther 2008;10. http://dx.doi.org/10.1186/ar2491.
- Deighton C, O’Mahony R, Tosh J, Turner C, Rudolf M, Group GD. Management of rheumatoid arthritis: summary of NICE guidance. BMJ 2009;338. http://dx.doi.org/10.1136/bmj.b702.
- Luqmani R, Hennell S, Estrach C, Basher D, Birrell F, Bosworth A, et al. British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of rheumatoid arthritis (after the first 2 years). Rheumatology 2009;48:436-9. http://dx.doi.org/10.1093/rheumatology/ken450a.
- Hammond A. Rehabilitation in rheumatoid arthritis: a critical review. Musculoskelet Care 2004;2:135-51. http://dx.doi.org/10.1002/msc.66.
- Rajbhandary R, Khezri A, Panush RS. Rheumatoid cachexia: what is it and why is it important?. J Rheumatol 2011;38:406-8. http://dx.doi.org/10.3899/jrheum.101036.
- Dellhag B, Hosseini N, Bremell T, Ingvarsson PE. Disturbed grip function in women with rheumatoid arthritis. J Rheumatol 2001;28:2624-33.
- Van den Ende C, Vliet Vlieland T, Munneke M, Hazes J. Dynamic exercise therapy in rheumatoid arthritis: a systematic review. Br J Rheumatol 1998;37:677-87. http://dx.doi.org/10.1093/rheumatology/37.6.677.
- Brighton SW, Lubbe JE, van der Merwe CA. The effect of a long-term exercise programme on the rheumatoid hand. Br J Rheumatol 1993;32:392-5. http://dx.doi.org/10.1093/rheumatology/32.5.392.
- Buljina AI, Taljanovic MS, Avdic DM, Hunter TB. Physical and exercise therapy for treatment of the rheumatoid hand. Arthritis Rheum 2001;45:392-7. http://dx.doi.org/10.1002/1529-0131(200108)45:4<392::AID-ART353>3.0.CO;2-2.
- Dellhag B, Wollersjo I, Bjelle A. Effect of active hand exercise and wax bath treatment in rheumatoid arthritis patients. Arthritis Care Res 1992;5:87-92. http://dx.doi.org/10.1002/art.1790050207.
- Hoenig H, Groff G, Pratt K, Goldberg E, Franck W. A randomized controlled trial of home exercise on the rheumatoid hand. J Rheumatol 1993;20:785-9.
- O’Brien AV, Jones P, Mullis R, Mulherin D, Dziedzic K. Conservative hand therapy treatments in rheumatoid arthritis--a randomized controlled trial. Rheumatology 2006;45:577-83. http://dx.doi.org/10.1093/rheumatology/kei215.
- Lemmey A, Marcora S, Chester K, Wilson S, Casanova F, Maddison P. Effects of high-intensity resistance training in patients with rheumatoid arthritis: a randomized controlled trial. Arthritis Rheum 2009;61:1726-34. http://dx.doi.org/10.1002/art.24891.
- Westby MD. A health professional’s guide to exercise prescription for people with arthritis: a review of aerobic fitness activities. Arthritis Rheum 2001;45:501-11. http://dx.doi.org/10.1002/1529-0131(200112)45:6<501::AID-ART375>3.0.CO;2-Y.
- Meichenbaum D, Turk D. Facilitating Treatment Adherence: A Practitioner’s Guidebook. New York, NY: Plenum Press; 1987.
- Rejeski W, Craven T, Ettinger WJ, McFarlane M, Shumaker S. Self-efficacy and pain in disability with osteoarthritis of the knee. J Gerontol B Psychol Sci Soc Sci 1996;51:P24-9. http://dx.doi.org/10.1093/geronb/51B.1.P24.
- Hammond A, Freeman K. The long-term outcomes from a randomized controlled trial of an educational-behavioural joint protection programme for people with rheumatoid arthritis. Clin Rehabil 2004;18:520-8. http://dx.doi.org/10.1191/0269215504cr766oa.
- Looking After Your Joints When You Have Arthritis. Chesterfield: Arthritis Research Campaign; 2007.
- Hammond A, Freeman K. One-year outcomes of a randomized controlled trial of an educational-behavioural joint protection programme for people with rheumatoid arthritis. Rheumatology 2001;40:1044-51. http://dx.doi.org/10.1093/rheumatology/40.9.1044.
- Hammond A. What is the role of the occupational therapist?. Best Pract Res Clin Rheumatol 2004;18:491-505. http://dx.doi.org/10.1016/j.berh.2004.04.001.
- Egan M, Brosseau L, Farmer M, Ouimet MA, Rees S, Wells G, et al. Splints/orthoses in the treatment of rheumatoid arthritis. Cochrane Database Syst Rev 2003;1.
- Cooper NJ. Economic burden of rheumatoid arthritis: a systematic review. Rheumatology 2000;39:28-33. http://dx.doi.org/10.1093/rheumatology/39.1.28.
- Yelin E, Wanke LA. An assessment of the annual and long-term direct costs of rheumatoid arthritis: the impact of poor function and functional decline. Arthritis Rheum 1999;42:1209-18. http://dx.doi.org/10.1002/1529-0131(199906)42:6<1209::AID-ANR18>3.0.CO;2-M.
- National Rheumatoid Arthritis Society . The Economic Burden of Rheumatoid Arthritis 2010.
- World Medical Association . World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Nurs Ethics 2002;9:105-9. http://dx.doi.org/10.1191/0969733002ne486xx.
- Keep Moving – How a Few Simple Exercises Can Make You Feel Better About Yourself and Your Arthritis. Chesterfield: Arthritis Research Campaign; 2005.
- Rheumatoid Arthritis. Chesterfield: Arthritis Research Campaign; 2005.
- Massy-Westropp N, Krishnan J, Ahern M. Comparing the AUSCAN Osteoarthritis Hand Index, Michigan Hand Outcomes Questionnaire, and Sequential Occupational Dexterity Assessment for patients with rheumatoid arthritis. J Rheumatol 2004;31:1996-2001.
- van der Giesen F, Nelissen R, Arendzen J, de Jong Z, Wolterbeek R, Vliet Vlieland T. Responsiveness of the Michigan Hand Outcomes Questionnaire–Dutch language version in patients with rheumatoid arthritis. Arch Phys Med Rehabil 2008;89:1121-6. http://dx.doi.org/10.1016/j.apmr.2007.10.033.
- Adams J, Mullee M, Burridge J, Hammond A, Cooper C. Responsiveness of self-report and therapist-rated upper extremity structural impairment and functional outcome measures in early rheumatoid arthritis. Arthritis Care Res 2010;62:274-8. http://dx.doi.org/10.1002/acr.20078.
- SARAH Trial Team, Adams J, Bridle C, Dosanjh S, Heine P, Lamb SE, . Strengthening and stretching for rheumatoid arthritis of the hand (SARAH): design of a randomised controlled trial of a hand and upper limb exercise intervention – ISRCTN89936343. BMC Musculoskelet Disord 2012;13. http://dx.doi.org/10.1186/1471-2474-13-230.
- Parsons S, Carnes D, Pincus T, Foster N, Breen A, Vogel S, et al. Measuring troublesomeness of chronic pain by location. BMC Musculoskelet Disord 2006;7. http://dx.doi.org/10.1186/1471-2474-7-34.
- Lorig K. Outcome Measures for Health Education and Other Health Care Interventions. Thousand Oaks, CA: Sage Publications; 1996.
- Brooks R. EuroQol: the current state of play. Health Policy 1996;37:53-72. http://dx.doi.org/10.1016/0168-8510(96)00822-6.
- Jenkinson C, Layte R. Development and testing of the UK SF-12 (short form health survey). J Health Serv Res Policy 1997;2:14-8.
- Norkin CC, White DJ. Measurement of Joint Motion: A Guide to Goniometry. Philadelphia, PA: F.A. Davis; 2009.
- Ellis B, Bruton A. A study to compare the reliability of composite finger flexion with goniometry for measurement of range of motion in the hand. Clin Rehabil 2002;16:562-70. http://dx.doi.org/10.1191/0269215502cr513oa.
- Kapandji A. Clinical test of apposition and counter-apposition of the thumb. Ann Chir Main 1986;5:67-73. http://dx.doi.org/10.1016/S0753-9053(86)80053-9.
- Fuchs HA, Brooks RH, Callahan LF, Pincus T. A simplified twenty-eight-joint quantitative articular index in rheumatoid arthritis. Arthritis Rheum 1989;32:531-7. http://dx.doi.org/10.1002/anr.1780320504.
- Mathiowetz V, Weber G, Kashman N, Volland G. Adult’s norms for 9-hole peg test of finger dexterity. Occup Ther J Res 1985;5:24-38.
- Helliwell P, Howe A, Wright V. Functional assessment of the hand: reproducibility, acceptability, and utility of a new system for measuring strength. Ann Rheum Dis 1987;46:203-8. http://dx.doi.org/10.1136/ard.46.3.203.
- Fess E, Moran C. Clinical Assessment Recommendations. Philadelphia, PA: American Society of Hand Therapists; 1981.
- Lineker S, Badley E, Hawker G, Wilkins A. Determining sensitivity to change in outcome measures used to evaluate hydrotherapy exercise programs for people with rheumatic diseases. Arthritis Care Res 2000;13:62-5. http://dx.doi.org/10.1002/1529-0131(200002)13:1<62::AID-ART9>3.0.CO;2-J.
- Lachin JM. Statistical considerations in the intent-to-treat principle. Control Clin Trials 2000;21:167-89. http://dx.doi.org/10.1016/S0197-2456(00)00046-5.
- Moher D, Schulz K, Altman D. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet 2001;357:1191-4. http://dx.doi.org/10.1016/S0140-6736(00)04337-3.
- Dunn G, Maracy M, Tomenson B. Estimating treatment effects from randomized clinical trials with noncompliance and loss to follow-up: the role of instrumental variable methods. Stat Methods Med Res 2005;14:369-95. http://dx.doi.org/10.1191/0962280205sm403oa.
- Chung K, Pillsbury M, Walters M, Hayward R. Reliability and validity testing of the Michigan Hand Outcomes Questionnaire. J Hand Surg Am 1998;23:575-87. http://dx.doi.org/10.1016/S0363-5023(98)80042-7.
- Ware JE, Kosinski M, Turner-Bowker DM, Gandek B. SF-12v2: How to Score Version 2 of the SF-12 Health Survey. Lincoln, RI: Quality Metric Incorporated; 2002.
- Perneger TV, Burnand B. A simple imputation algorithm reduced missing data in SF-12 health surveys. J Clin Epidemiol 2005;58:142-9. http://dx.doi.org/10.1016/j.jclinepi.2004.06.005.
- O’Brien A, Jones P, Mullis R, Mulherin D, Dziedzic K. Conservative hand therapy treatments in rheumatoid arthritis--a randomized controlled trial. Rheumatology 2006;45:577-83. http://dx.doi.org/10.1093/rheumatology/kei215.
- Kennedy T, McCabe C, Struthers G, Sinclair H, Chakravaty K, Bax D, et al. BSR guidelines on standards of care for persons with rheumatoid arthritis. Rheumatology 2005;44:553-6. http://dx.doi.org/10.1093/rheumatology/keh554.
- Scottish Intercollegiate Guidelines Network (SIGN) . Management of Early Rheumatoid Arthritis. A National Clinical Guideline 2000. www.sign.ac.uk/guidelines/fulltext/48/index.html (accessed 31 March 2009).
- Rheumatoid Arthritis: The Management of Rheumatoid Arthritis in Adults. London: NICE; 2009.
- Kennedy N, Sayers K, Cassidy C, Ward S, Bresnihan B, Fitzgerald O, et al. The effect of a period of rehabilitation in a rheumatic diseases unit. Br J Ther Rehabil 2001;8:29-36.
- Henderson S, McMillan I. Pain and function: occupational therapists’ use of orthotics in rheumatoid arthritis. Br J Occup Ther 2002;65:165-71.
- Hammond A. Joint protection education: what are we doing?. Br J Occup Ther 1997;60:401-6.
- Hurkmans E, van der Giesen F, Vliet Vlieland T, Schoones J, Van den Ende E. Dynamic exercise programs (aerobic capacity and/or muscle strength training) in patients with rheumatoid arthritis. Cochrane Database Syst Rev 2009;4.
- Rønningen A, Kjeken I. Effect of an intensive hand exercise programme in patients with rheumatoid arthritis. Scand J Occup Ther 2008;15:173-83. http://dx.doi.org/10.1080/11038120802031129.
- Brorsson S, Hilliges M, Sollerman C, Nilsdotter A. A 6-week hand exercise programme improves strength and hand function in patients with rheumatoid arthritis. J Rehabil Med 2009;41:338-42. http://dx.doi.org/10.2340/16501977-0334.
- de Jong Z, Munneke M, Zwinderman A, Kroon H, Ronday K, Lems W, et al. Long term high intensity exercise and damage of small joints in rheumatoid arthritis. Ann Rheum Dis 2004;63:1399-405. http://dx.doi.org/10.1136/ard.2003.015826.
- Wessel J. The effectiveness of hand exercises for persons with rheumatoid arthritis: a systematic review. J Hand Ther 2004;17:174-80. http://dx.doi.org/10.1197/j.jht.2004.02.006.
- Ottawa Panel . Ottawa Panel evidence-based clinical practice guidelines for therapeutic exercises in the management of rheumatoid arthritis in adults. Phys Ther 2004;84:934-72.
- Williams MA, Heine PJ, Bruce J, Brosseau L, Lamb S. Exercise Therapy for the Rheumatoid Hand. Cochrane Database Syst Rev 2012;4.
- McLaughlin JE, Reynolds WJ. An evaluation of therapeutic exercise on hand function in rheumatoid arthritis. Physiother Can 1973;25:71-6.
- Flatt A. The Care of the Rheumatoid Hand. St. Louis, MO: Mosby; 1974.
- Flatt A. The Care of the Rheumatoid Hand. St. Louis, MO: Mosby; 1963.
- Hammond A, Kidao R, Young A. Hand impairment and function in early rheumatoid arthritis. Arthritis Rheum 2000;43.
- Marcora S, Lemmey A, Maddison P. Can progressive resistance training reverse cachexia in patients with rheumatoid arthritis? Results of a pilot study. J Rheumatol 2005;32:1031-9.
- Kraemer W, Adams K, Cafarelli E, Dudley G, Dooly C, Feigenbaum M, et al. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc 2002;34:364-80. http://dx.doi.org/10.1097/00005768-200202000-00027.
- Adams J, Cavolina J, Read H, Larkin E. Differences in hand functional ability in men and women with and without rheumatoid arthritis. Ann Rheum Dis 2007;66.
- Dellhag B, Bjelle A. A five-year followup of hand function and activities of daily living in rheumatoid arthritis patients. Arthritis Care Res 1999;12:33-41. http://dx.doi.org/10.1002/1529-0131(199902)12:1<33::AID-ART6>3.0.CO;2-4.
- Dellhag B, Burckhardt C. Predictors of hand function in patients with rheumatoid arthritis. Arthritis Care Res 1995;8:16-20. http://dx.doi.org/10.1002/art.1790080106.
- Wehbé M. Tendon gliding exercises. Am J Occup Ther 1987;41:164-7. http://dx.doi.org/10.5014/ajot.41.3.164.
- Mugii N, Hasegawa M, Matsushita T, Kondo M, Orito H, Yanaba K, et al. The efficacy of self-administered stretching for finger joint motion in Japanese patients with systemic sclerosis. J Rheumatol 2006;33:1586-92.
- Borg G. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982;14:377-81. http://dx.doi.org/10.1249/00005768-198205000-00012.
- Steultjens EM, Dekker J, Bouter LM, van Schaardenburg D, van Kuyk MA, van den Ende CH. Occupational therapy for rheumatoid arthritis. Cochrane Database Syst Rev 2004;1.
- Malcus-Johnson P, Carlqvist C, Sturesson AL, Eberhardt K. Occupational therapy during the first 10 years of rheumatoid arthritis. Scand J Occup Ther 2005;12:128-35. http://dx.doi.org/10.1080/11038120510031716.
- Vliet Vlieland TP. Non-drug care for RA – is the era of evidence-based practice approaching?. Rheumatology 2007;46:1397-404. http://dx.doi.org/10.1093/rheumatology/kem149.
- Hammond A, Klompenhouwer P. Getting evidence into practice: implementing a behavioural joint protection education programme for people with rheumatoid arthritis. Br J Occup Ther 2005;68:25-33.
- Vliet Vlieland T. Rehabilitation of people with rheumatoid arthritis. Best Pract Res Clin Rheumatol 2003;17:847-61. http://dx.doi.org/10.1016/S1521-6942(03)00043-3.
- Stern E. Grip strength and finger dexterity across five styles of commercial wrist orthoses. Am J Occup Ther 1996;50:32-8. http://dx.doi.org/10.5014/ajot.50.1.32.
- Adams J, Burridge J, Mullee M, Hammond A, Cooper C. The clinical effectiveness of static resting splints in early rheumatoid arthritis: a randomized controlled trial. Rheumatology 2008;47:1548-53. http://dx.doi.org/10.1093/rheumatology/ken292.
- McGuigan M, Egan A, Foster C. Salivary cortisol responses and perceived exertion during high intensity and low intensity bouts of resistance exercise. J Sports Sci Med 2004;3:8-15.
- Michie S, Rumsey N, Fussell A, Hardeman W, Johnston M, Newman S, et al. NHS Health Trainer Handbook. London: Department of Health; 2008.
- Bandura A. Self-Efficacy: The Exercise of Control. New York, NY: WH Freeman; 1997.
- Sheeran P, Stroebe W, Hewstone M. European Review of Social Psychology. Chichester: Wiley; 2002.
- Gollwitzer PM. Implementation intentions: strong effects of simple plans. Am Psychol 1999;54:493-50. http://dx.doi.org/10.1037/0003-066X.54.7.493.
- Gollwitzer P, Sheeran P, Zanna M. Advances in Experimental Social Psychology. Volume 38. San Diego, CA: Elsevier Academic Press; 2006.
- Abraham C, Michie S. A taxonomy of behavior change techniques used in interventions. Health Psychol 2008;27:379-87. http://dx.doi.org/10.1037/0278-6133.27.3.379.
- Moseley G. Do training diaries affect and reflect adherence to home programs?. Arthritis Rheum 2006;55:662-4. http://dx.doi.org/10.1002/art.22086.
- A Framework for Development and Evaluation of RCTs for Complex Interventions to Improve Health. London: Medical Research Council; 2000.
- NHS Careers . Agenda For Change – Pay Rates n.d. www.nhscareers.nhs.uk/working-in-the-nhs/pay-and-benefits/agenda-for-change-pay-rates/ (accessed 5 December 2014).
- Smith J, Flowers P, Larkin M. Interpretative Phoneomological Analysis: Theory, Method and Research. London: Sage Publications; 2009.
- Smith J, Osborn M, Smith J. Interpretative Phenomenological Analysis. London: Sage Publications; 2003.
- Osborn MS, Smith JA. The personal experience of chronic benign low back pain: an interpretive phenomenological analysis. Br J Health Psychol 1998;3:65-83. http://dx.doi.org/10.1111/j.2044-8287.1998.tb00556.x.
- Law RJ, Breslin A, Oliver EJ, Mawn L, Markland DA, Maddison P, et al. Perceptions of the effects of exercise on joint health in rheumatoid arthritis patients. Rheumatology 2010;49:2444-51. http://dx.doi.org/10.1093/rheumatology/keq299.
- Swardh E, Biguet G, Opava CH, Swardh E, Biguet G, Opava CH. Views on exercise maintenance: variations among patients with rheumatoid arthritis. Phys Ther 2008;88:1049-60. http://dx.doi.org/10.2522/ptj.20070178.
- Guide to the Methods of Technology Appraisal. London: Department of Health; 2008.
- Ramsey S, Willke R, Briggs A, Brown R, Buxton M, Chawla A, et al. Good research practices for cost-effectiveness analysis alongside clinical trials: the ISPOR RCT-CEA Task Force report. Value Health 2005;8:521-33. http://dx.doi.org/10.1111/j.1524-4733.2005.00045.x.
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D: Centre for Health Economics. York: University of York; 1999.
- Brazier JE, Roberts J. The estimation of a preference-based measure of health from the SF-12. Med Care 2004;42:851-9. http://dx.doi.org/10.1097/01.mlr.0000135827.18610.0d.
- Curtis L. Unit Costs of Health and Social Care 2011. Kent: Personal Social Services Research Unit, University of Kent; 2011.
- Underwood M, Ashby D, Carnes D, Castelnuovo E, Cross P, Harding G, et al. Topical or oral ibuprofen for chronic knee pain in older people. The TOIB study. Health Technol Assess 2008;12. http://dx.doi.org/10.3310/hta12220.
- British National Formulary 63. London: Pharmaceutical Press; 2012.
- ATC/DDD Index 2012. Copenhagen: World Health Organization; 2012.
- Tocilizumab for the Treatment of Rheumatoid Arthritis. London: Department of Health; 2012.
- Reference Costs 2010–2011 Publication. London: Department of Health; 2011.
- Briggs A, Clark T, Wolstenholme J, Clarke P. Missing . . . presumed at random: cost-analysis of incomplete data. Health Econ 2003;12:377-92. http://dx.doi.org/10.1002/hec.766.
- Hoch JS, Briggs AH, Willan AR. Something old, something new, something borrowed, something blue: a framework for the marriage of health econometrics and cost-effectiveness analysis. Health Econ 2002;11:415-30. http://dx.doi.org/10.1002/hec.678.
- Manca A, Hawkins N, Sculpher MJ. Estimating mean QALYs in trial-based cost-effectiveness analysis: the importance of controlling for baseline utility. Health Econ 2005;14:487-96. http://dx.doi.org/10.1002/hec.944.
- Willan AR, Briggs AH, Hoch JS. Regression methods for covariate adjustment and subgroup analysis for non-censored cost-effectiveness data. Health Econ 2004;13:461-75. http://dx.doi.org/10.1002/hec.843.
- Fiebig DG, Baltargi BH. Seemingly Unrelated Regression. Malden, MA: Blackwell Publishing; 2001.
- Earnshaw J, Lewis G. NICE Guide to the Methods of Technology Appraisal: pharmaceutical industry perspective. Pharmacoeconomics 2008;26:725-7. http://dx.doi.org/10.2165/00019053-200826090-00002.
- Lamb SE, Mistry D, Lall R, Hansen Z, Evans D, Withers EJ, et al. Group cognitive behavioural interventions for low back pain in primary care: extended follow-up of the Back Skills Training Trial (ISRCTN54717854). Pain 2012;153:494-501. http://dx.doi.org/10.1016/j.pain.2011.11.016.
- Gullick NJ, Scott DL. Co-morbidities in established rheumatoid arthritis. Best Pract Res Clin Rheumatol 2011;25:469-83. http://dx.doi.org/10.1016/j.berh.2011.10.009.
- Outpatients – Review of National Findings. London: Audit Commission; 2003.
- CG79 Rheumatoid Arthritis 2009. London: Department of Health; n.d.
- Ethnicity and Religion. Newport: Palgrave Macmilian; 2006.
- Shauver M, Chung K. The minimal clinically important difference of the Michigan hand outcomes questionnaire. J Hand Surg Am 2009;34:509-14. http://dx.doi.org/10.1016/j.jhsa.2008.11.001.
- Cima SR, Barone A, Porto JM, de Abreu DC. Strengthening exercises to improve hand strength and functionality in rheumatoid arthritis with hand deformities: a randomized, controlled trial. Rheumatol Int 2012;33:725-32.
- Bearne L, Manning V, Scott D, Choy E, Hurley M. A Brief Exercise and Self Management Programme Improves Upper Limb Disability in People With Early Rheumatoid Arthritis n.d.
Appendix 1 Patient information sheet
Appendix 2 Consent form
Appendix 3 Case report forms
Appendix 4 Impairment measures scoring
Metacarpophalangeal joint deformity score (degrees, four finger measurements for each hand). Summarised as a single score for both hands representing the average of left and right. This will be calculated if all finger scores are available as the average of the individual finger scores. For each patient the number of missing finger scores will also be counted.
Active wrist flexion (degrees, two measurements) and active wrist extension (degrees, two measurements). Summarised as a single score for both hands representing the average of left and right. This will be calculated if both scores are present as the sum of flexion and extension.
Combined finger flexion (mm, four measurements for each hand). Summarised as a single score for both hands representing the average of left and right. This will be calculated if all finger flexion scores are present as the average of the individual finger scores. For each patient the number of missing finger scores will also be counted.
Composite finger extension method A or method B (mm, two measurements for each hand). Summarised as a single score for both hands representing the average of left and right. This will be calculated if all the finger extension scores are available as the average of the individual finger scores, method A being counted as positive, method B as negative. For each patient the number of missing finger scores is counted.
Kapandji thumb opposition score (a count for each hand).
Swollen joint count (overall count for both hands combined).
Tender joint count (overall count for both hands combined).
Dexterity: observation of timed nine-hole peg test (seconds, one measurement for each hand).
Full-hand grip (newtons, three measurements for each hand). The maximum will be used for each hand.
Tripod pinch strength (newtons, three measurements for each hand). The maximum will be used for each hand.
Appendix 5 Example of exercise diary
Appendix 6 Personal exercise guide sheet
Appendix 7 Qualitative study consent form
Appendix 8 Qualitative study interview schedules
List of abbreviations
- ACR
- American College of Rheumatology
- ADL
- activity of daily living
- AIMS2
- Arthritis Impact Measurement Scale 2
- ARC
- Arthritis Research Campaign
- BNF
- British National Formulary
- CACE
- complier average causal effect
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CONSORT
- Consolidated Standards of Reporting Trials
- CRP
- C-reactive protein
- DMARD
- disease-modifying anti-rheumatic drug
- DMEC
- Data Monitoring and Ethics Committee
- EQ-5D-3L
- European Quality of Life-5 Dimensions-3 Levels
- ESR
- erythrocyte sedimentation rate
- EVPI
- expected value of perfect information
- HRQoL
- health-related quality of life
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- INB
- incremental net benefit
- IP
- interphalangeal
- IPA
- interpretative phenomenological analysis
- IV
- intravenous
- JP
- joint protection
- MCP
- metacarpophalangeal
- MCS
- Mental Component Score of the SF-12 Questionnaire
- MHQ
- Michigan Hand Outcome Questionnaire
- MREC
- Multicentre Research Ethics Committee
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NSAID
- non-steroidal anti-inflammatory drug
- OLS
- ordinary least squares
- PCS
- Physical Component Score of the SF-12 questionnaire
- PEG
- personal exercise guide
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- RA
- rheumatoid arthritis
- RCT
- randomised controlled trial
- ROM
- range of movement
- SAE
- serious adverse event
- SARAH
- Strengthening And stretching for Rheumatoid Arthritis of the Hand
- SF-12
- Short Form questionnaire-12 items
- SF-6D
- Short Form questionnaire-6 Dimensions
- SUR
- seemingly unrelated regression
- TMG
- Trial Management Group
- TNF
- tumour necrosis factor
- TSC
- Trial Steering Committee
- VAS
- visual analogue scale
- WHO DDD
- World Health Organization defined daily dose