Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 13/12/01. The contractual start date was in July 2013. The draft report began editorial review in December 2013 and was accepted for publication in April 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Cooper et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Description of health problem
Premature ejaculation (PE) is a form of male sexual dysfunction. It is also referred to as early ejaculation, rapid ejaculation, rapid climax, premature climax and (historically) ejaculation praecox. Official definitions of PE have been set out in the Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition-Text Revision (DSM-IV-TR)1 and in the World Health Organization’s (WHO’s) International Classification of Diseases, Tenth Edition (ICD-10). 2 The DSM-IV-TR defines the condition as persistent or recurrent ejaculation with minimal sexual stimulation before, on or shortly after penetration and before the person wishes it. 1 Other definitions have also been proposed by the Second International Consultation on Sexual and Erectile Dysfunction3 and the International Society for Sexual Medicine (ISSM). 4 All four definitions consider time to ejaculation, inability to control or delay ejaculation and negative consequences of PE. However, there is no current consensus on quantification of the time to ejaculation, which is usually described by intravaginal ejaculatory latency time (IELT), i.e. the time taken by a man to ejaculate during vaginal penetration. 5
Aetiology, pathology and prognosis
According to the European Association of Urology (EAU), the aetiology of PE is unknown, with few data to support suggested biological and psychological hypotheses, including anxiety, penile hypersensitivity and 5-hydroxytryptamine (5-HT) receptor dysfunction, and the pathophysiology of PE is largely unknown. 6 PE can be either lifelong (primary) or acquired (secondary). 7 Lifelong PE is that which has been present since the person’s first sexual experiences, while acquired PE is that which begins later following normal ejaculation experiences. PE can occur secondary to another condition such as erectile dysfunction or prostatitis, in which case guidelines recommend treating the underlying condition first or concomitantly. 6,8 PE cannot be cured, but can be managed with behavioural and/or pharmacological treatment.
Epidemiology and prevalence
Epidemiological surveys in the USA and other countries suggest that PE as defined in the Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (DSM-IV)9 is the most common male sexual dysfunction, with prevalence rates of 20–30%. 3,10,11 The highest prevalence, 31% (among men aged 18–59 years), was found by the USA National Health and Social Life Survey study. 11 In a five-country European observational study, which included the UK, the prevalence of PE was 18%. 12
Impact of health problem
Men with PE are more likely to report lower levels of sexual functioning and satisfaction, and higher levels of personal distress and interpersonal difficulty, than men without PE. 5 They may also rate their overall quality of life lower than that of men without PE. 5 In addition, the partner’s satisfaction with the sexual relationship has been reported to decrease with increasing severity of the condition. 13
Measurement of disease
Diagnosis of PE is based on the patient’s medical and sexual history. 14,15 IELT can be either self-assessed or stopwatch measured. The EAU 2013 Guidelines on Male Sexual Dysfunction6 state that the use of IELT alone is not sufficient to define PE, and the need to assess PE objectively has led to the development of several questionnaires, including two questionnaires that can discriminate between patients who have PE and those who do not. These are the Premature Ejaculation Diagnostic Tool (PEDT)16,17 and the Arabic Index of Premature Ejaculation (AIPE). 18 Other questionnaires used to characterise PE and determine treatment effects include the Premature Ejaculation Profile (PEP),19 the Index of Premature Ejaculation (IPE)20 and the Male Sexual Health Questionnaire Ejaculatory Dysfunction. 21
Current service provision
Relevant national guidelines
Guidelines on PE include the EAU 2013 Guidelines on Male Sexual Dysfunction6 and the British Recommendations for the Management of Premature Ejaculation, 2006. 8
Management of the condition
The treatment of PE should attempt to alleviate concern about the condition as well as increase sexual satisfaction for the patient and the partner. 8 Descriptions of a recommended treatment pathway for the condition are varied. The British Association of Urological Surgeons suggest that counselling may help men with less troublesome PE but, for most men, the mainstay of long-term treatment is drugs. 22 The British Association for Sexual Health and HIV, Special Interest Group for Sexual Dysfunction, suggests that management of patients should be decided on a case-by-case basis that considers behavioural, local and systemic pharmacological treatments. 8 The EAU presents a definitive treatment pathway based on clinical diagnosis of the condition and treatment of PE based on whether or not the condition is either lifelong or acquired. There is currently no published literature that identifies a clinically significant threshold response to interventions for PE. 23
Description of technology under assessment
Summary of interventions
Treatments include behavioural techniques, anaesthetic creams and sprays, tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs), phosphodiesterase-5 (PDE5) inhibitors such as sildenafil, analgesics such as tramadol (Zydol SR®, Grünenthal) and other drug and non-drug interventions. 6,8 One antidepressant [dapoxetine (Priligy®, Menarini), a SSRI] has received approval for the treatment of PE in the UK. 24 To date, no other drug has been approved for PE in Europe or the USA and other medical treatments prescribed for PE are ‘off-label’ (the practice of prescribing treatments for an unapproved indication).
Chapter 2 Definition of the decision problem
Decision problem
Population and subgroups
The relevant population comprised all men aged ≥ 18 years with PE, both lifelong and acquired PE. Studies focusing specifically on men with PE secondary to another condition (such as erectile dysfunction or prostate conditions) were excluded if possible; however, this information was often not reported.
Interventions assessed
Treatment modalities included behavioural techniques, topical therapies, systemic therapies and other therapies.
Relevant comparators
Comparators included other interventions, waiting list control, placebo or no treatment.
Key outcomes
The key outcomes for this review were IELT, sexual satisfaction, control over ejaculation, relationship satisfaction, self-esteem and quality of life. As these outcomes in PE are assessed in the literature using different methods, and there is a lack of core validated outcome measures, any assessment methods were permitted for these outcomes.
Overall aim and objective of assessment
The aim and objective of this assessment were to systematically review the evidence for the clinical effectiveness of interventions for management of PE, in the form of a Health Technology Assessment (HTA) short report.
Chapter 3 Assessment of clinical effectiveness
A systematic review of the literature was undertaken to evaluate the clinical effectiveness of interventions for men with PE. A review of the evidence was undertaken in accordance with the general principles recommended in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. 25 The completed PRISMA checklist is presented in Appendix 1.
Methods for reviewing effectiveness
Identification of studies
The following electronic databases were searched from inception to 6 August 2013 for published and unpublished research evidence: MEDLINE; EMBASE; Cumulative Index to Nursing and Allied Health Literature (CINAHL); The Cochrane Library including the Cochrane Systematic Reviews Database; Cochrane Controlled Trials Register (CCRT); Database of Abstracts of Reviews of Effects and the HTA database; ISI Web of Science, including Science Citation Index, and the Conference Proceedings Citation Index-Science; The US Food and Drug Administration website and the European Medicines Agency website were also searched. All citations were imported into Reference Manager Software (version 12, Thomson ResearchSoft, Carlsbad, CA, USA) and any duplicates deleted.
Search terms were included a combination of medical subject headings (MeSHs) and free-text searches for terms around ‘premature ejaculation’. These included:
-
MeSHs: Ejaculation; Premature ejaculation.
-
Free-text search terms: premature$adj3 ejaculat$; early adj3 ejaculat$; rapid adj3 ejaculat$; rapid adj3 climax$; premature$adj3 climax$; ejaculat$adj3 pr?ecox.
Search filters (study design filters) were used to restrict the searches to randomised controlled trials (RCTs), reviews and guidelines. These were:
-
the RCT filter available from the Scottish Intercollegiate Guidelines Network26
-
the reviews filter available from the York Centre for Reviews and Dissemination27
-
the filter for guidelines available from the Health Evidence Bulletins Wales resource. 28
Details of the MEDLINE strategy are presented in Appendix 2. Existing reviews identified by the searches were obtained and examined for relevant RCT data. However, all bibliographic data sources were searched from inception; thus, existing reviews were not relied upon as the only source for identifying relevant RCTs.
Inclusion and exclusion criteria
Population
The relevant population included all men aged ≥ 18 years with PE, including both lifelong and acquired PE. Studies focusing specifically on men with PE secondary to another condition (such as erectile dysfunction or prostate conditions) were excluded; however, this information was often not reported.
As some formal definitions of PE have only recently been developed, studies were included whether or not they used a standard definition and all definitions used were recorded. Common definitions of PE include the following:
Included interventions
Behavioural interventions included psychological or psychosocial interventions to develop sexual management strategies that were either validated or described by investigators as being a treatment for PE treatment. Examples include:
-
‘Stop–start’ programme developed by Semans:16 the man or his partner stimulates the penis until he feels the urge to ejaculate, then stops until the sensation passes; this is repeated a few times before allowing ejaculation to occur. The aim is to learn to recognise the feelings of arousal in order to improve control over ejaculation.
-
‘Squeeze’ technique, proposed by Masters and Johnson:17 the man’s partner stimulates the penis until he feels the urge to ejaculate, then squeezes the glans of the penis until the sensation passes; this is repeated before allowing ejaculation to occur.
-
Sensate focus or sensate focusing:4 the man and his partner begin by focusing on touch which excludes breasts, genitals and intercourse, to encourage body awareness while reducing performance anxiety; this is followed by gradual reintroduction of genital touching and then full intercourse.
Topical treatments included:
-
Lidocaine–prilocaine, eutectic mixture of local anaesthetics (EMLA®, AstraZeneca), topical eutectic mixture for PE [(TEMPE), a combination of two medicines – lidocaine and prilocaine], dyclonine or lidocaine. These can be in the form of either a cream or an aerosol vehicle or a gel containing a local anaesthetic (Instillagel®, CliniMed).
Systemic treatments included:
-
SSRIs [e.g. fluoxetine, sertraline, citalopram (Cipramil®, Lundbeck), paroxetine, fluvoxamine and dapoxetine]. Dapoxetine is a short-acting SSRI that can be taken a few hours preintercourse rather than as a daily dose and is the only drug currently licensed for PE in the UK.
-
Serotonin–noradrenaline reuptake inhibitors (SNRIs) [e.g. duloxetine (Cymbalta®, Eli Lilly & Co Ltd), venlafaxine].
-
TCAs (e.g. clomipramine).
-
PDE5 inhibitors [e.g. sildenafil (Viagra), vardenafil (Levitra®, Bayer), tadalafil (Cialis®, Eli Lilly & Co Ltd)].
-
Alpha-blockers [e.g. terazosin (Hytrin®, AMCO), alfuzosin].
-
Opioid analgesics (e.g. tramadol).
Other therapies included:
-
acupuncture
-
Chinese medicine
-
delay device/desensitising band: a small device which the man can use together with stop–start and squeeze techniques to gradually improve control over ejaculation
-
yoga.
Combinations of therapies included drug plus behavioural therapies or combinations of drug therapies.
Excluded interventions
The following interventions not considered relevant to the UK setting were excluded:
-
Severance Secret cream (SS cream: a topical plant-based preparation comprising extracts of nine plants). Not currently available within the UK (Professor Kevan Wylie, Porterbrook Clinic, 2013, personal communication).
-
Antiepileptic drugs (e.g. gabapentin). Not currently included in the UK30 or European6 guidelines and not currently used in clinical practice in the UK (Professor Kevan Wylie, personal communication).
-
Antipsychotics [e.g. thioridazine (Melleril, Novartis, withdrawn worldwide in 2005), perphenazine (Trilafon, Merck Sharp & Dohme), levosulpiride]. Not currently included in the UK30 or European6 guidelines and not currently used in clinical practice in the UK (Professor Kevan Wylie, personal communication).
-
Antiemetics (e.g. metoclopramide). Not currently included in the UK30 or European6 guidelines and not currently used in clinical practice in the UK (Professor Kevan Wylie, personal communication).
-
Barbiturates (e.g. Atrium 300). Not currently included in the UK30 or European6 guidelines and not currently used in clinical practice in the UK (Professor Kevan Wylie, personal communication).
-
Beta-blockers (e.g. propranolol). Not currently included in the UK30 or European6 guidelines and not currently used in clinical practice in the UK (Professor Kevan Wylie, personal communication).
Comparators
Comparators included other interventions, waiting list control, placebo or no treatment.
Outcomes
The key outcomes for this review were:
-
IELT: studies that do not report this outcome objectively, but assess the outcome via another subjective measure such as a questionnaire, were included. Studies that assess ejaculation latency time in a laboratory setting, i.e. not intravaginally, were excluded.
-
Sexual satisfaction.
-
Control over ejaculation.
-
Relationship satisfaction.
-
Self-esteem.
Other outcomes included:
-
Quality of life.
-
Treatment acceptability.
-
Adverse events (AEs).
Included study types
Included study designs were restricted to RCTs, if available. If no RCT evidence was identified for a particular intervention, other study types (non-RCT) were considered. Owing to the time constraints of this short report, if RCTs were included in existing reviews, data were extracted from the review and not from the original RCT publication. RCTs not captured by existing reviews and those published subsequently to existing reviews were identified via the literature search and data were extracted directly from the RCT publication. RCTs reported in abstract form only were eligible for inclusion, provided adequate information was presented in the abstract. Studies using quasi-randomisation were excluded, providing other RCT evidence for the treatment of interest was available.
Non-English-language studies were excluded unless sufficient data could be extracted (from English-language abstracts and/or tables). Dissertations and theses were excluded.
Data abstraction strategy
Titles and abstracts of citations identified by the searches were screened for potentially relevant studies by one reviewer and a subset checked by a second reviewer (and a check for consistency undertaken). Full texts were screened by two reviewers. Details of studies identified for inclusion were extracted using a data extraction sheet. One reviewer performed data extraction of each included study. All numerical data were then checked against the original article by a second reviewer. Any disagreements were resolved by a third reviewer. When studies comprised duplicate reports (parallel publications), all associated reports were used to extract information.
Methods of data synthesis
When possible, data were pooled in a meta-analysis from RCTs reported in the existing reviews along with data extracted from additional RCTs not captured by the existing reviews. Meta-analysis of outcome data from all RCTs was then undertaken using Cochrane RevMan software (version 5.2, The Cochrane Collaboration, The Nordic Cochrane Centre, Copenhagen, Denmark). Outcomes reported as continuous data were estimated using a mean difference (MD) with 95% confidence interval (CI). Outcomes reported as dichotomous were estimated as relative risks (RRs) with associated 95% CI. When RCTs reported AEs in sufficient detail (e.g. the number of participants who experienced at least one AE), these were analysed as dichotomous data. Data from single-arm randomised crossover design studies were considered separately in the analysis to avoid a unit-of-analysis error. 31
Clinical heterogeneity across RCTs (the degree to which RCTs appear different in terms of participants, intervention type and duration and outcome type) and statistical heterogeneity were considered prior to data pooling. Statistical heterogeneity was assessed using the chi-squared test (p-value < 0.10 was considered to indicate statistically significant heterogeneity) in conjunction with the I-squared statistic. 32 For comparisons in which there was little apparent clinical heterogeneity and the I2-value was ≤ 40%, a fixed-effects model was applied. When there was little apparent clinical heterogeneity and the I2-value was > 40%, a random-effects model was applied. Effect estimates (estimated in RevMan as z-values) were considered significant at p < 0.05. Data were not pooled across RCTs for which heterogeneity was very high (I2-values of ≥ 75%).
Quality assessment of included studies
The methodological quality of systematic reviews used as a source of RCT data were assessed using the Assessing Methodological Quality of Systematic Reviews (AMSTAR) checklist. 33 This checklist consists of 11 items and has good face and content validity for measuring the methodological quality of systematic reviews. 33 Domain items with a ‘yes’ response are scored one point. ‘No’, ‘not applicable’ and ‘unclear’ responses score a zero. An overall score was estimated for each review by summing the total number of points. It was not possible to undertake quality assessment for RCTs for which data were extracted from existing reviews. Methodological quality of further RCTs identified from the literature search was assessed using the Cochrane Collaboration risk of bias assessment criteria. This tool addresses specific domains, namely sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data and selective outcome reporting. 34 We classified RCTs as being at overall ‘low risk’ of bias if they were rated as ‘low’ for each of three key domains – allocation concealment, blinding of outcome assessment and completeness of outcome data. RCTs judged as being at ‘high risk’ of bias for any of these domains were judged at overall ‘high risk’. Similarly, RCTs judged as being at ‘unclear risk’ of bias for any of these domains were judged at overall ‘unclear risk’.
Results
Quantity and quality of research available
The searches identified 2283 citations. Of these, 2181 citations were excluded, 2174 based on title and/or abstract information and seven that we were unable to obtain. One hundred and three (103) full-text articles were obtained as potentially relevant. Of these, 24 were excluded: eight were non-systematic reviews or treatment overviews, two were laboratory-based assessments, two were pharmacokinetic assessment studies and 12 were studies evaluating treatments not relevant to the UK setting. Details of the 24 excluded studies are presented in Appendix 3. In total, 78 articles from the searches were included in this assessment report comprising: 28 reviews, 47 primary study articles (relating to 38 studies) and three guideline articles (relating to two guidelines) (Figure 1).
FIGURE 1.
Study selection process: PRISMA flow diagram.
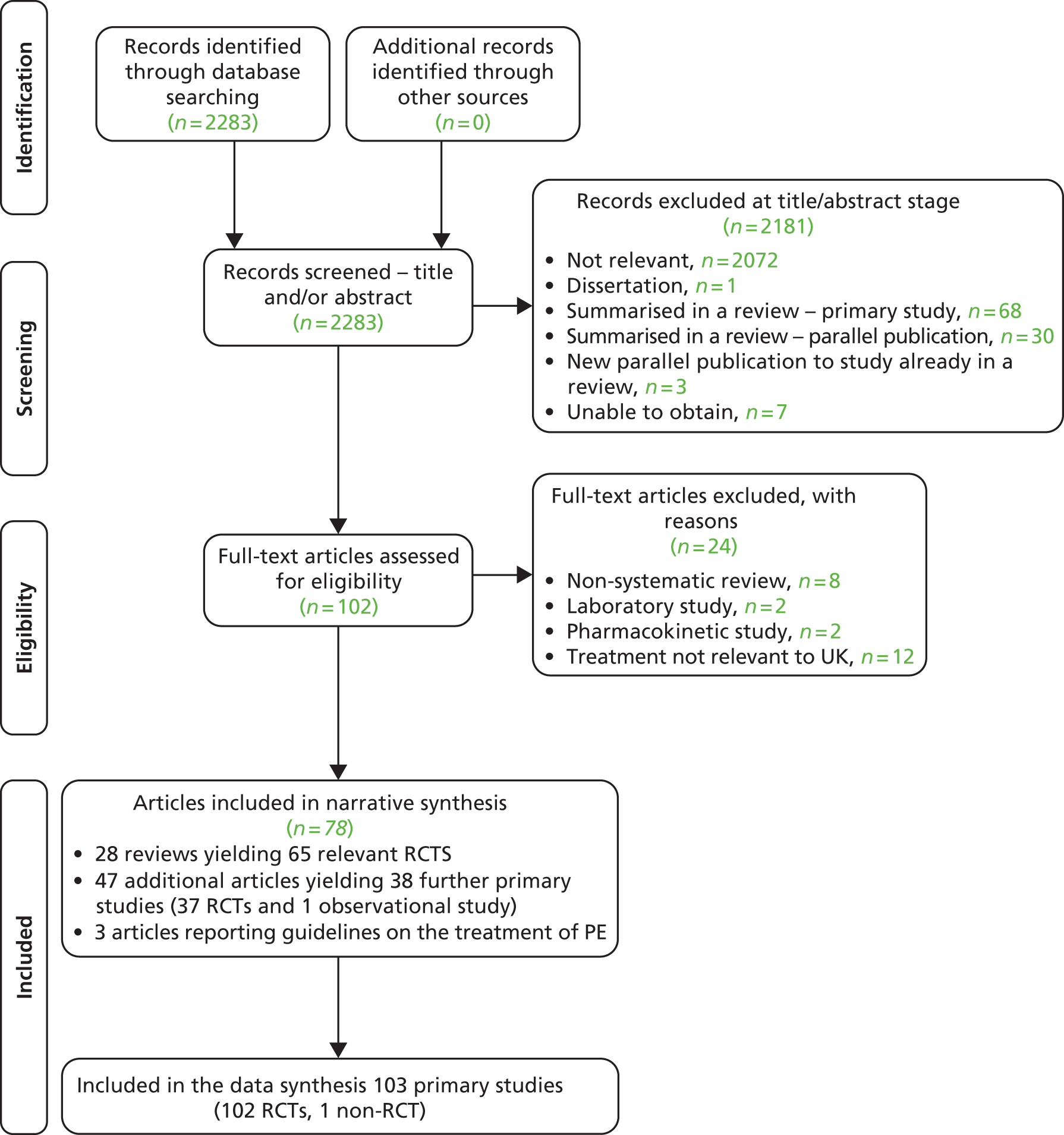
From these publications, a total of 103 primary studies (102 RCTs) are summarised in this review (Table 1). Sixty-five RCTs were extracted from existing reviews and 38 further studies from the literature search (see Table 1). All 65 RCTs reported in existing reviews were also captured by the searches for this assessment report. RCT evidence was available for all of the treatments of interest for this review, bar yoga. For yoga, one observational study was included (a non-RCT). Details of the AMSTAR33 quality assessment of included reviews and Cochrane risk of bias assessment34 for the RCTs not included by reviews are presented in Appendix 4.
| Intervention type | No. of reviews | No. of RCTs (extracted from reviews) | Further RCTs (not in reviews) | RCTs (total) |
|---|---|---|---|---|
| Behavioural therapies | 435–38 | 939–47 | 348–50 | 12 |
| Topical anaesthetics | 451–54 | 755–61 | 262,63 | 9 |
| SSRIs other than dapoxetine | 7: | 26: | 16: | 42: |
|
||||
|
|
|||
|
||||
|
|
|
||
|
||||
|
||||
| Dapoxetine | 8: | 885,113–119 (one non-licensed doses; data not included here115) | 1120 | 9 (8 for licensed doses) |
| SNRIs | 168 | 1 (duloxetine)121 | 2 (venlafaxine)122,123 | 3 |
| TCAs (clomipramine) | 352,68,69 | 1039,76,124–131 | 3107,132,133 | 13 |
| PDE5 | 537,134–137 | 1039,55,138–145 | 2101,120 | 12 |
| Alpha-blockers | 2 (various treatments)52,69 | 1146 | 1107 | 2 |
| Opioid analgesics (tramadol) | 3147–149 | 546,150–153 | 2154,155 | 7 |
| Acupuncture | 0 | 0 | 2156,157 | 2 |
| Chinese medicine | 0 | 0 | 5158–162 | 5 |
| Delay device | 0 | 0 | 1163 | 1 |
| Yoga | 0 | 0 | 1164 (non-RCT) | 1 (non-RCT) |
As titles and abstracts were screened for inclusion by one reviewer, a check for consistency was undertaken. A second reviewer screened approximately 10% of the references (n = 250) during the initial screening stage. At this stage, references tagged as potentially relevant by reviewer 1 included 5 out of 194 (3%) references excluded by reviewer 2, and references tagged as potentially relevant by reviewer 2 included 22 out of 211 (10%) references excluded by reviewer 1. This gave a kappa statistic of 0.65, generally classed as good agreement. The discrepancies appeared to be due to the very broad inclusion criteria (in terms of study type and intervention type) that were applied at the time of initial screening. The references for which there was a discrepancy related to article types such as comment articles, news articles and uncontrolled studies that were initially tagged as potentially relevant. However, later examination revealed that none of these articles were relevant for inclusion in the final review.
Assessment of effectiveness
Overall summary of results
An overall results summary from this assessment report for outcomes of IELT, sexual satisfaction, control over ejaculation and other secondary outcomes, plus AEs, following treatment with behavioural techniques anaesthetic creams and sprays, TCAs, SSRIs including dapoxetine, PDE5 inhibitors, analgesics (tramadol) and other interventions in the management of PE is provided in Table 2. A detailed assessment of the effectiveness for each treatment type then follows.
| Treatment | Better than | On outcomes of | Between-group difference significant | AEs with treatment |
|---|---|---|---|---|
| Behavioural therapies | ||||
| Behavioural therapy | Waiting list control | Duration of intercourse, sexual satisfaction, desire, self-confidence | Yes | No AE data available for behavioural therapies |
| Behavioural therapy plus pharmacotherapy | Pharmacotherapy alone | IELT, ejaculatory control, sexual satisfaction, sexual anxiety | Yes | |
| Behavioural therapy plus pharmacotherapy | Behavioural therapy alone | IELT, ejaculatory control, sexual satisfaction, sexual anxiety | Yes | |
| Pharmacotherapy alone | Behavioural therapy alone | IELT, sexual satisfaction | Yes | Various AEs associated with pharmacotherapy (nausea, vomiting, dry mouth, dizziness, flushing, diarrhoea) |
| Topical anaesthetics | ||||
| EMLA cream | Placebo | IELT | Yes | Loss of sensation, irritation and loss of erection (application ≥ 20 minutes) |
| TEMPE spray | Placebo | IELT ejaculatory control, sexual satisfaction and distress | Yes | |
| SSRIs currently not licensed for PE | ||||
| Citalopram | Placebo or no therapy | IELT, sexual satisfaction and measures of clinical improvement | Yes | |
| Escitalopram (Cipralex®, Lundbeck) | Placebo | IELT, sexual satisfaction | Yes | Nausea, headache, insomnia, dry mouth, diarrhoea, drowsiness, dizziness, somnolence, decreased libido and anejaculation |
| Fluoxetine | ||||
| Paroxetine | ||||
| Sertraline | Placebo | IELT, ejaculation control | Yes | |
| Clomipramine | Paroxetine | IELT | Yes | Similar to SSRIs |
| Fluvoxamine | Placebo | IELT | No | Not significant between fluvoxamine, fluoxetine, paroxetine and sertraline |
| SSRIs licensed for PE (dapoxetine) | ||||
| Dapoxetine 30 mg or 60 mg | Placebo | IELT, ejaculatory control, sexual satisfaction, patients reporting change | Yes | Similar to other SSRIs |
| Dapoxetine 60 mg | Dapoxetine 30 mg | Yes | ||
| SNRIs | ||||
| Duloxetine | Placebo | IELT | Yes | Dry mouth and nausea; more AEs with venlafaxine than placebo |
| Venlafaxine | Placebo | IELT | No | Significantly more treatment-related side effects than placebo |
| TCAs | ||||
| Clomipramine: oral | Placebo | IELT | Yes | More AEs with clomipramine than fluoxetine or sertraline |
| Clomipramine: nasal (4 mg) | Yes | Local irritation associated with nasal administration | ||
| PDE5 inhibitors | ||||
| PDE5 inhibitors | Placebo | IELT | Vardenafil or tadalafil, yes; sildenafil, no | Flushing, headache and palpitations |
| PDE5 inhibitors | SSRIs | Sertraline, yes; fluoxetine, no | ||
| PDE5 inhibitors plus SSRIs | SSRIs alone | Yes | ||
| PDE5 inhibitors | Behavioural therapy | Yes | ||
| Alpha-blockers | ||||
| Terazosin | Placebo | Ejaculation control | Yes | Headache, hypotension, drowsiness, ejaculation disorder |
| Opioid analgesics | ||||
| Tramadol | Placebo | IELT, various patient-reported outcomes, including sexual satisfaction | Yes | Erectile dysfunction, constipation, nausea, headache, somnolence, dry mouth, dizziness, pruritus, vomiting |
| Tramadol plus behavioural therapy | Behavioural therapy | Yes | ||
| Paroxetine | Tramadol | IELT | No | |
| Other therapies | ||||
| Acupuncture | Sham acupuncture | IELT | Yes | No AE, data available for acupuncture, Chinese medicine or yoga |
| Chinese medicine | Treatment as usual | Yes | ||
| Yoga (observational study) | Baseline | IELT | Yes | |
| Fluoxetine | Yoga | Yes | ||
| Desensitising band plus stop–start technique | Stop–start technique | IELT | Yes | Appear minimal when device used as directed |
Behavioural therapies
Characteristics of included studies: behavioural therapies
Behavioural therapies were evaluated by one Cochrane review35 and two further systematic reviews of behavioural therapies. 36,38 Nine RCTs evaluating behavioural therapies were identified from these and other reviews of pharmacological therapies. 39–47 A further three RCTs of behavioural therapy were identified by the literature search:48–50 one evaluated pelvic floor exercises compared with dapoxetine,48 one evaluated a multicomponent behavioural therapy intervention compared with paroxetine alone or in combination with the behavioural intervention49 and one evaluated an internet-based behavioural intervention compared with waiting list control. 50
The Cochrane review by Melnik et al. 35 and the systematic review by Melnik et al. 38 were conducted in Brazil. The review by Berner and Gunzler36 was undertaken in Germany. The Cochrane review by Melnik et al. 35 was awarded an overall AMSTAR quality score 7 out of 11. The systematic reviews by Berner and Gunzler36 and Melnik et al. 38 were awarded 6 and 3 out of 11, respectively. Details of the review type, the databases searched and dates, relevant included RCTs and the AMSTAR points awarded to these reviews is presented in Table 3. Full details of the AMSTAR assessment for these and all other include reviews are presented in Appendix 4.
| Author (country) review type | Databases searched and dates | Included RCTs relevant to this section | AMSTAR review quality assessment |
|---|---|---|---|
| Berner and Gunzler, 201236 (Germany) systematic review | CENTRAL, MEDLINE, CINAHL, Academic Search Premier, PsycINFO, PubMed and PSYNDEX between 1985 and 2009 | de Carufel and Trudel 2006,40 Oguzhanoglu et al. 2005,43 Trudel and Proulx 198745 | AMSTAR score, 6/11: |
|
|||
|
|||
|
|||
|
|||
|
|||
|
|||
| Melnik et al. 200938 (Brazil) systematic review | MEDLINE by PubMed (1966–2009), PsycINFO (1974–2009), EMBASE (1980–2009), Latin America and Caribbean Health Sciences Literature (1982–2009) and CENTRAL (The Cochrane Library, 2009, issue 1) | Abdel-Hamid et al. 2001,39 de Carufel and Trudel 2006,40 Li et al. 2006,42 Tang et al. 2004,44 Trudel and Proulx 1987,45 Yuan et al. 200847 | AMSTAR score, 3/11: |
|
|||
|
|||
|
|||
| Melnik et al. 201135 (Brazil) Cochrane review | MEDLINE, 1966–2010; PsycINFO, 1974–2010; EMBASE, 1980–2010; Latin America and Caribbean Health Sciences Literature, 1982–2010; and The Cochrane Library, 2010 | Abdel-Hamid et al. 2001,39 de Carufel and Trudel 2006,40 Li et al. 2006,42 Yuan et al. 200847 | AMSTAR score, 7/11: |
|
|||
|
|||
|
|||
|
|||
|
|||
|
|||
|
|||
|
All reviews varied in terms of which RCTs they included. In total, nine RCTs of behavioural therapies39–47 (total n = 505) were included in at least one systematic review. The method of IELT assessment (stopwatch) was reported for only five RCTs. 39,40,44,46,48 The duration of the RCTs included in the reviews ranged from 2 to 12 weeks. The behavioural therapies that were evaluated included the squeeze technique,39 functional–sexological treatment involving movement of the body, speed of sexual activity and education regarding sensuality,40 the stop–start technique plus squeeze technique,40 behavioural psychotherapy,42 stop–start technique alone,43 behavioural psychotherapy,44 ‘Bibliotherapy’ (consisting of introduction to PE, descriptions of squeeze technique, pause technique and sensate focusing), and sexual therapy for couples (sensate focus, stop–start technique and communication exercises). 45 The type of behavioural intervention was not specified for one RCT. 47
In addition to the RCTs captured in reviews of behavioural therapy, one RCT evaluating the stop–start technique compared with fluoxetine or placebo41 was captured in reviews of SSRIs (see Selective serotonin reuptake inhibitors currently not licensed for premature ejaculation) and one RCT evaluating a behavioural therapy that intervention was not specified compared with tramadol46 was captured in another review of pharmacological agents (see Opioid analgesics). Details of the RCTs extracted from reviews are presented in Table 4. All RCTs in reviews were captured by the search strategy for this assessment report.
| RCTs extracted from reviews | ||||||
|---|---|---|---|---|---|---|
| RCT (source) | Duration | Treatments | PE definition | Lifelong/acquired | IELT assessment | Other outcomes |
| Abdel-Hamid et al. 200139 (references to review in which the publication is included35,37,38,52,69,134,135,137,165) | Single-arm crossover. 5 × 4-week phases each with 2-week washout | Sildenafil 50 mg 1 hour precoitus | IELT ≤ 2 minutes | Lifelong | Stopwatch | Modified Erectile Dysfunction Inventory of Treatment Satisfaction, Arabic Anxiety Inventory (scale 0–30) |
| Clomipramine 25 mg 3–5 hours precoitus | ||||||
| Sertraline 50 mg 3–5 hours precoitus | ||||||
| Paroxetine 20 mg 3–5 hours precoitus | ||||||
| Squeeze technique | ||||||
| (Total n = 31) | ||||||
| de Carufel and Trudel 200640 (reviews35,36,38) | NR | Functional-sexological treatment (education on sensuality, body movements, speed of sexual activity) | IELT < 2 minutes | NR | Stopwatch | Perception of duration of intercourse, sexual satisfaction |
| Behavioural therapy (squeeze and stop–start techniques) | ||||||
| Waiting list control | ||||||
| (Total n = 36 couples) | ||||||
| Kolomaznik et al. 200241 (reviews52) | 8 weeks | Stop–start technique | NR | NR | IELT not assessed | Duration of coitus, subject report |
| Fluoxetine (dose NR) | ||||||
| Placebo | ||||||
| (Total n = 93) | ||||||
| Li et al. 200642 (reviews35,38) | 6 weeks | Behavioural therapy + chlorpromazine 50 mg/day (n = 45) | IELT < 1 minute | NR | Method NR | Self-Rating Anxiety Scale |
| Chlorpromazine 50 mg/day (n = 45) | Chinese Index Premature Ejaculation | |||||
| Oguzhanoglu et al. 200543 (review36) | 8 weeks | Stop–start technique (n = 16) | Ejaculation before or within several minutes | NR | Method NR | Anxiety |
| Fluoxetine 20 mg/day (n = 16) | Satisfaction with treatment | |||||
| Tang et al. 200444 (reviews35,37,38,134) | 6 weeks | Behavioural psychotherapy (n = 30) | NR | NR | Stopwatch | Patient/partner sexual satisfaction (0–5-point Likert scale) |
| Sildenafil 50 mg + behavioural psychotherapy (n = 30) | ||||||
| Trudel and Proulx 198749 (reviews36,38) | 12 weeks | Bibliotherapy (book on behavioural techniques) | IELT ≤ 5 minutes | NR | Method NR | NR |
| Bibliotherapy with therapist contact by phone | ||||||
| Sexual therapy for couples (sensate focus, stop–start, communication) | ||||||
| Waiting list control | ||||||
| (Total n = 25 couples) | ||||||
| Xiong et al. 201146 (reviews148,149) | 12 weeks | Behavioural modification alone (not reported which) (n = 36) | IELT ≤ 2 minutes | Lifelong | Stopwatch | IIEF |
| Tramadol 50 mg 2 hours preintercourse with behavioural modification (n = 36) | ||||||
| Yuan et al. 200847 (reviews35,38) | 2 weeks | Behavioural therapy (n = 32) | NR | NR | Method NR | Sexual satisfaction |
| Citalopram 20 mg/day (n = 32) | ||||||
| Behavioural therapy plus citalopram (n = 32) | ||||||
| Further RCTs identified by searches (not captured in reviews) | ||||||
| RCT (country), risk of bias | Duration | Treatments, numbers analysed/randomised (%) | PE definition | Lifelong/acquired | IELT assessment | Other outcomes |
| Pastore et al. 201248 (Italy), unclear | RCT 12 weeks | Pelvic floor muscle rehabilitation and electrical stimulation, three sessions/week (n = 19) | ISSM definition of PE | Lifelong | Stopwatch | None |
| Dapoxetine 30 mg or 60 mg on demand (n = 21) | ||||||
| Pelvic floor musclerehabilitation, 17/19 (89%); dapoxetine, 15/21 (71%) | ||||||
| Shao and Li 200849 (China), unclear | RCT 8 weeks | Paroxetine 10 mg/day (for first 4 weeks only) and behavioural therapy (n = 40) | NR | NR | CIPE5 | CIPE5 |
| Paroxetine 20 mg/day (n = 40) | ||||||
| Behavioural therapy (squeeze technique, sensate, focus, Qigong, acupoint tapping) (n = 40) | ||||||
| Paroxetine + behavioural therapy, 40/40 (100%); paroxetine, 40/40 (100%); behavioural therapy, 40/40 (100%) | ||||||
| van Lankveld et al. 200950 (the Netherlands), unclear | RCT 3 months | Internet-based sex therapy (sensate focus) (n = 22) | NR | NR | GRISS PE subscale | IIEF sexual desire, overall satisfaction, erectile dysfunction |
| Waiting list control (n = 18) | SEAR self-confidence | |||||
| Internet-based sex therapy, 21/22 (95%); waiting list control, 16/18 (89%) | Global Endpoint Question improvement/impairment of sexual functioning | |||||
The RCT by Pastore et al. 48 was conducted in Italy. Forty patients were randomised to pelvic floor muscle physiokinesitherapy (awareness of muscle contraction), comprising electrical stimulation of perineal floor, three 60-minute sessions per week, or to dapoxetine (30 mg or 60 mg on demand). IELT was assessed with a stopwatch. The duration was 12 weeks. The authors reported that 34 out of 40 (85%) patients completed the trial and IELT was stopwatch assessed. The RCT by Shao and Li49 was conducted in China. A total of 120 patients were randomised to paroxetine 10 mg per day (for the first 4 weeks) combined with behavioural therapy comprising the Masters and Johnson squeeze technique,17 sensate focus and Chinese traditional Qigong treatment (penis swinging and acupoint tapping), to paroxetine 20 mg per day, or to behavioural therapy only. The duration was 8 weeks. No objective assessment of IELT was reported. All patients (100%) were reported as completing the intervention. In the RCT by van Lankveld et al. ,50 an internet-based sex therapy based on the Masters and Johnson sensate focus technique was compared with waiting list control and 40 patients were randomised. The number and frequency of therapeutic contacts was left to the judgement of the therapist and the participant. No objective assessment of IELT was reported. The authors reported that 37 out of 40 (93%) patients completed the 3-month treatment programme. All three RCTs107,132,133 were considered to be at overall unclear risk of bias. 34
Details of the treatments evaluated, definition of PE, IELT assessment method, other outcomes assessed, study duration, along with the study country for the further RCTs not in reviews and the overall study quality assessment (AMSTAR33 for reviews and Cochrane risk of bias assessment34 for the RCTs not included by reviews) are presented in Table 4.
Assessment of effectiveness: behavioural therapies – intravaginal ejaculatory latency time outcomes
The reporting of IELT outcomes for RCTs included in the reviews was varied in terms of the treatment comparisons, the reporting of the assessment method, the outcome metric that was reported and the reporting of variance estimates and p-values. With the exception of the crossover study by Abdel-Hamid et al. 39 and the RCT by Xiong et al. ,46 no data were suitable to either estimate between-group differences for individual trial or pool data across studies in RevMan for this assessment report.
Intravaginal ejaculatory latency time: behavioural therapy compared with waiting list control
No variance estimates were reported for this outcome in the review by Berner and Gunzler. 36 Melnik et al. 35 reported that both functional sexological treatment and behavioural therapy significantly increased duration of intercourse compared with waiting list controls (functional sexological therapy: MD 6.87 minutes, 95% CI 5.10 to 8.64 minutes; behavioural therapy: MD 6.80 minutes, 95% CI 5.04 to 8.56 minutes) for one RCT. 40 p-values for the between-group differences were not reported. Summary results for these and across all other behavioural intervention trials are presented in Table 5.
| Comparison | Outcome | No. of RCTsa | No. of participants | Meta-analysis | Effect estimate (95% CI) | Favours | p-value | |
|---|---|---|---|---|---|---|---|---|
| IELT | ||||||||
| BT compared with waiting list control | IELT (minutes) | 240,45 | 36 | N/A | MD (two types BT vs. WL), 6.80, 6.87 (both significant)35,40 | BT | NR | |
| 25 | MD (three types BT vs. WL), 7.29, 8.84, 9.1136,45 | BT | NR | |||||
| BT with pharmacotherapy compared with pharmacotherapy alone | IELT | 142 | 90 | N/A | MD 1.11 (0.82 to 1.40)35 | BT + chlorpromazine | NR | |
| 147 | 96 | NR | BT + citalopram | NR | ||||
| CIPE5 ejaculatory latency score | 149 | 80 | N/A | MD 0.40 (0.18 to 0.62) | BT + paroxetine | 0.0003 | ||
| BT with pharmacotherapy compared with BT alone | IELT | 144 | 32 | N/A | MD 1.81 (NR) | BT + sildenafil | < 0.001 | |
| 146 | 72 | MD 1.65 (0.30 to 3.00) | BT + tramadol | 0.02 | ||||
| CIPE5 ejaculatory latency score | 149 | 80 | N/A | MD 0.60 (0.40 to 0.80) | BT + paroxetine | < 0.00001 | ||
| BT compared with pharmacotherapy | IELT | 148 | 32 | N/A | MD 1.22 (0.79 to 1.65) | Dapoxetine | < 0.00001 | |
| 147 | 96 | RR 0.52 (0.34 to 0.78)35 | Citalopram35 | NR35 | ||||
| 139 crossover | 31 | NS for clomipramine, sertraline and paroxetine | Sildenafil; NS for clomipramine, sertraline, paroxetine | < 0.00001; NS | ||||
| CIPE5 ejaculatory latency score | 149 | 80 | N/A | MD 0.20 (0.00 to 0.40) | Paroxetine | 0.05 | ||
| Other outcomes | ||||||||
| BT vs. waiting list control | Sexual satisfaction, desire, self-confidence | 240,50 | Varies | N/A | BT | < 0.0540,50 | ||
| BT with pharmacotherapy vs. pharmacotherapy alone | Ejaculatory control, sexual satisfaction and sexual anxiety | 442,44,47,49 | Varies | N/A | BT with pharmacotherapy (citalopram, chlorpromazine, sildenafil, paroxetine49) | < 0.0149; others NR | ||
| BT with pharmacotherapy vs. BT alone | Ejaculatory control, sexual satisfaction and sexual anxiety | 246,49 | Varies | N/A | BT with pharmacotherapy (paroxetine,49 tramadol46) | < 0.01;49 < 0.0546 | ||
| BT vs. pharmacotherapy | Ejaculatory control | 149 | 80 | N/A | Paroxetine49 | < 0.0149 | ||
| Sexual satisfaction | 149 | 80 | BT49 | < 0.0149 | ||||
| 147 | 96 | Citalopram | NR | |||||
Mean ejaculatory latency (minutes) post treatment in one trial45 was reported by Berner and Gunzler36 as follows: bibliotherapy without therapist contact, 11.05 minutes (change from baseline, p < 0.01); bibliotherapy with therapist contact by phone, 9.23 minutes (change from baseline, p < 0.01); sexual therapy for couples, 10.78 minutes (change from baseline, p < 0.01); and waiting list control, 1.94 minutes (improvement not significant, p-value not reported).
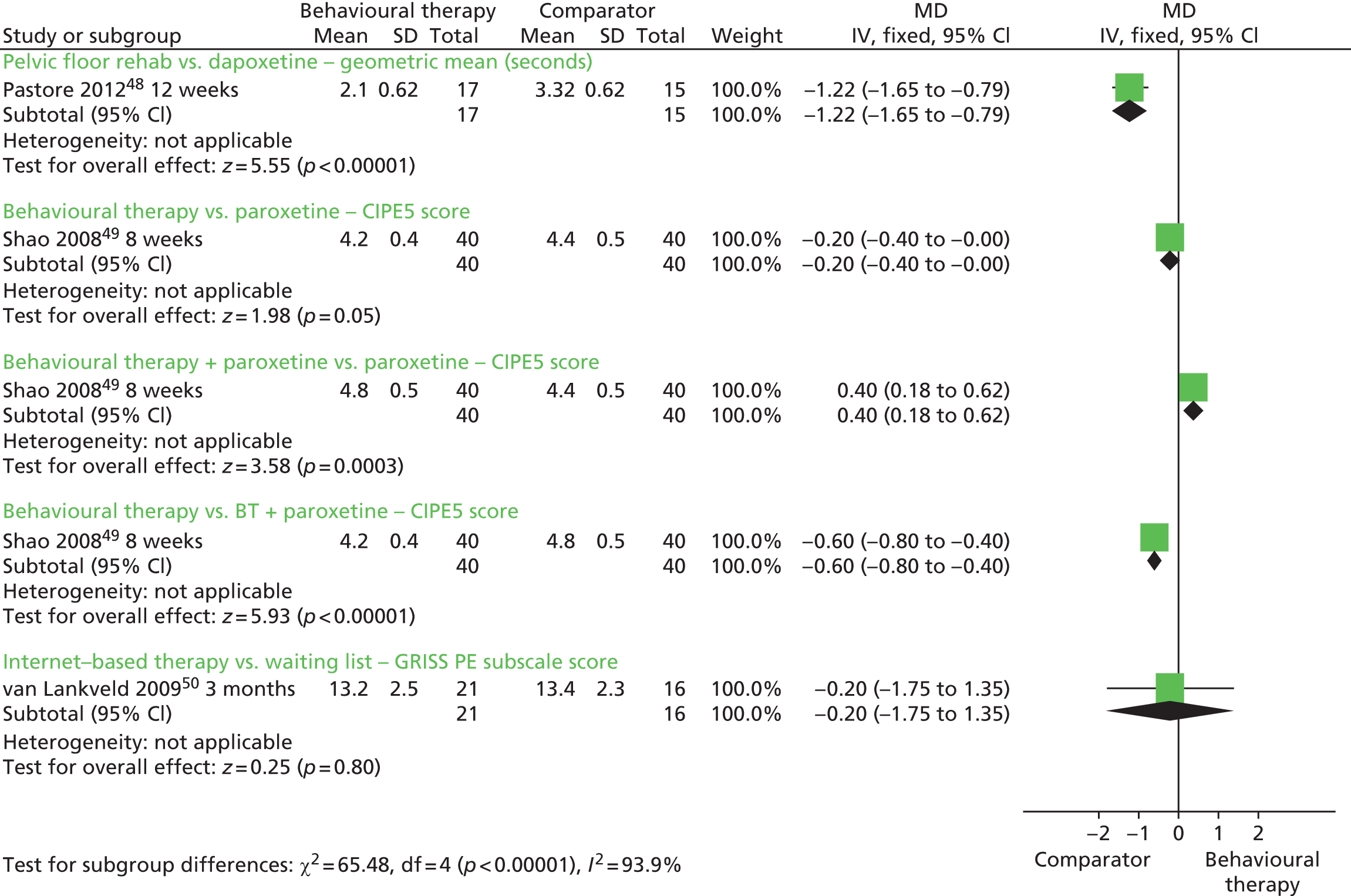
The between-group MD in the Golombok Rust Inventory of Sexual Satisfaction (GRISS) PE subscale score at 3 months based on one RCT50 (n = 37) was –0.20 minutes (fixed effect; 95% CI –1.75 to 1.35 minutes; p = 0.80) (Figure 2).
FIGURE 2.
Behavioural therapies compared with comparator: forest plot of IELT outcomes. CIPE5, Chinese Index of Premature Ejaculation 5 premature ejaculation-related items; df, degrees of freedom; IV, inverse variance; SD, standard deviation.
Intravaginal ejaculatory latency time: behavioural therapy with pharmacotherapy compared with pharmacotherapy alone
Melnik et al. 35 reported that behavioural therapy plus chlorpromazine was superior to chlorpromazine alone in increasing IELT (minutes) after treatment in one RCT42 (MD 1.11 minutes, 95% CI 0.82 to 1.40 minutes). A p-value for the between-group difference was not reported.
Melnik et al. 35 reported that, in one trial,47 citalopram combined with behavioural therapy compared with citalopram alone favoured the combined approach therapy (no data reported). p-values were not reported.
The between-group difference in the Chinese Index of Premature Ejaculation 5 PE-related items (CIPE5) ejaculatory latency score at 8 weeks based on one RCT49 (n = 80) was 0.40 minutes in favour of behavioural therapy combined with paroxetine 20 mg compared with paroxetine 20 mg alone [MD (fixed effect), 95% CI 0.18 to 0.62 minutes; p = 0.0003] (see Figure 2).
Intravaginal ejaculatory latency time: behavioural therapy with pharmacotherapy compared with behavioural therapy alone
Mean values (minutes) at week 6 for one trial44 were reported as 3.63 minutes for cognitive–behavioural therapy (CBT) plus sildenafil compared with 1.82 minutes for behavioural therapy alone. The p-value for between-group difference was reported as p < 0.001 in favour of behavioural therapy with sildenafil.
The between-group difference in mean IELT (minutes) at 12 weeks, based on one RCT46 (n = 72), was 1.65 minutes, significantly favouring tramadol with behavioural therapy compared with behavioural therapy alone (95% CI 0.30 to 3.00 minutes; p = 0.02). The forest plot for this analysis is presented as Figure 18 in the Opioid analgesics section of this assessment report.
The between-group difference in the CIPE5 ejaculatory latency score at 8 weeks based on one RCT49 (n = 80) was 0.60 minutes in favour of behavioural therapy combined with paroxetine 20 mg compared with behavioural therapy alone [MD (fixed effect), 95% CI 0.40 to 0.80 minutes; p < 0.00001] (see Figure 2).
Intravaginal ejaculatory latency time: behavioural therapy compared with pharmacotherapy
The between-group difference in geometric mean IELT (minutes) at 12 weeks based on one RCT48 (n = 32) was 1.22 minutes in favour of dapoxetine 30 mg or 60 mg compared with pelvic floor rehabilitation [MD (fixed effect) 95% CI 0.79 to 1.65 minutes; p < 0.0001] (see Figure 2).
Melnik et al. 35 reported that, in one trial,47 citalopram significantly improved IELT compared with behavioural therapy (RR 0.52, 95% CI 0.34 to 0.78). p-values were not reported.
The review by Berner and Gunzler36 reported that no outcome data were available for the one RCT evaluating this treatment comparison. 43
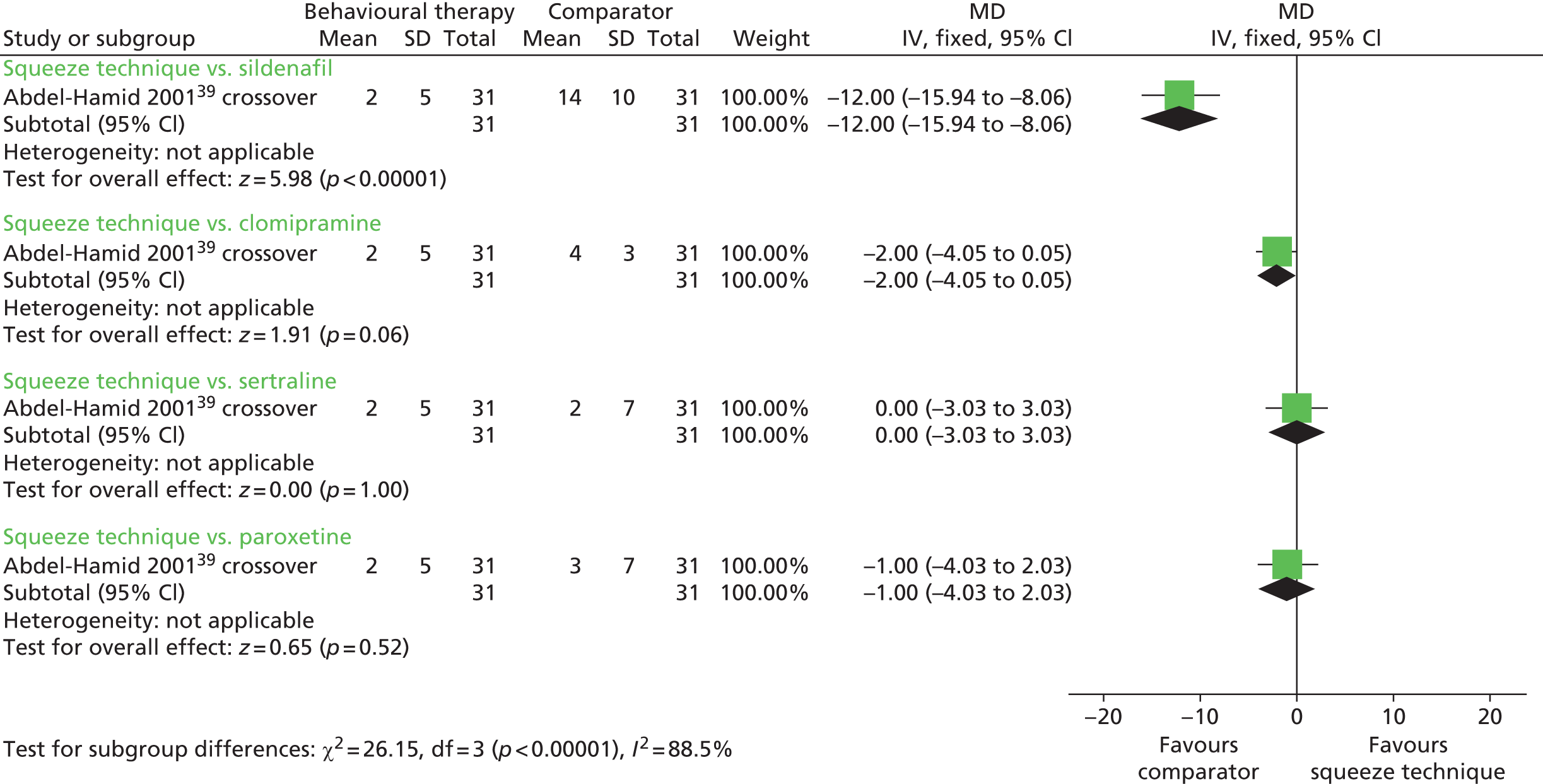
The between-group difference in mean IELT change (minutes) following a 4-week randomised crossover comparison39 was 12.00 minutes in favour of sildenafil compared with squeeze technique [MD (fixed effect) 95% CI 8.06 to 15.94 minutes; p < 0.00001]. Comparisons of squeeze technique with clomipramine, sertraline and paroxetine were not significant (Figure 3). A paired analysis could not be undertaken for approximation purposes for this study. Data from this trial were not pooled with other RCTs in any meta-analysis in this assessment report.
FIGURE 3.
Behavioural therapies, squeeze technique vs. SSRIs or TCAs – forest plot of IELT outcomes. df, degrees of freedom; IV, inverse variance; SD, standard deviation.
The between-group difference in the CIPE5 ejaculatory latency score at 8 weeks based on one RCT49 (n = 80) was 0.20 in favour of paroxetine 20 mg compared with behavioural therapy [MD (fixed effect), 95% CI 0.00 to 0.40 minutes; p = 0.05] (see Figure 2).
Assessment of effectiveness – behavioural therapies: other outcomes
With the exception of the RCTs by Pastore et al. 48 and Trudel and Proulx45 all of the included trials were reported as evaluating one or more other outcomes. However, these were diverse across the included trials and were often not reported in sufficient detail to permit any pooling across trials (Table 6).
| RCT, duration | Treatment | Outcome measure | Results | Between-group difference reported as significant | AEs |
|---|---|---|---|---|---|
| BT compared with waiting list control | |||||
| de Carufel and Trudel 2006,40 NR | Functional sexological treatment | Perception of duration of intercourse | Perception of duration of intercourse improved significantly in both treatments, but not in the waiting-list group (p < 0.05) | Yes | NR |
| BT | Couples’ sexual satisfaction | Both treatment groups had significant improvements over waiting list for couples’ sexual satisfaction | Yes | ||
| Waiting list control | |||||
| (Total n = 36 couples) | |||||
| Trudel and Proulx 1987,45 12 weeks | Bibliotherapy | NR | NR | NR | Different and high dropout rates across groups. No further data reported |
| Bibliotherapy with therapist contact | |||||
| Sexual therapy for couples | |||||
| Waiting list control | |||||
| (Total n = 25 couples) | |||||
| van Lankveld et al. 2009,50 treatment duration was 3 months, follow-up at 3 and 6 months post treatment | Internet-based sex therapy (n = 22) | IIEF sexual desire, overall satisfaction, erectile dysfunction | IIEF overall satisfaction and IIEF sexual desire: p-values for change from baseline for internet-based sex therapy of p < 0.05 | Yes (from baseline) | NR |
| Waiting list control (n = 18) | SEAR self-confidence | SEAR self-confidence score: p-value of 0.05 for change from 3-month to 6-month follow-up | Yes (at one time point) | ||
| GEQ improvement/impairment of sexual functioning | GEQ: no significant between-group difference (p > 0.05) | No | |||
| Combined therapies compared with monotherapy | |||||
| Li et al. 2006,42 6 weeks | BT + chlorpromazine (n = 45) | Self-Rating Anxiety Scale | Chlorpromazine + BT was superior to chlorpromazine alone for Self-Rating Anxiety Scale and for some CIPE questions (‘anxiety in sexual activity’, ‘partner sexual satisfaction’, ‘patient sexual satisfaction’, ‘control ejaculatory reflex’ and ‘ejaculatory latency’) | Yes | NR |
| Chlorpromazine (n = 45) | CIPE | ||||
| Shao and Li 2008,49 8 weeks | BT + paroxetine (n = 40) | CIPE5 | Ejaculation control: BT + paroxetine better than paroxetine, p < 0.01; or BT, p < 0.01; paroxetine better than BT, p < 0.01 | Yes | Four AEs (10%) in the paroxetine + BT group and 16 (40%) in the paroxetine group. No further details reported |
| Paroxetine 20 mg per day (n = 40) | Patient satisfaction: BT + paroxetine better than paroxetine, p < 0.01; or BT, p < 0.05; BT better than paroxetine, p < 0.01 | ||||
| BT (n = 40) | Partner satisfaction: BT + paroxetine better than paroxetine, p < 0.01; or BT, p < 0.05; BT better than paroxetine, p < 0.01 | ||||
| Sexual anxiety: BT + paroxetine better than paroxetine, p < 0.01; or BT, p < 0.01; BT vs. paroxetine, NS | |||||
| Tang et al. 2004,44 6 weeks | BT (n = 30) | Patient/partner sexual satisfaction (0–5-point Likert scale) | BT, 19/30 ‘satisfied’; BT + sildenafil, 26/30 ‘satisfied’. NR what point(s) of scale = ‘satisfied’. p-value for between-group difference, NR | Unclear | NR |
| Sildenafil + BT (n = 30) | |||||
| Combined therapies compared with monotherapy | |||||
| Xiong et al. 2011,46 12 weeks | Behaviour modification (n = 36) | IIEF | Tramadol + behaviour modification: mean change 4 | Yes | Any AE:
|
| Tramadol + behaviour modification (n = 36) | Behaviour modification alone: mean change 2 | ||||
| Between-groups p < 0.05 | |||||
| Yuan et al. 2008,47 2 weeks | Behavioural – therapy (n = 32) | Sexual satisfaction | Citalopram significantly improved the number of couples satisfied with their sex life vs. BT | Yes | NR |
| Citalopram (n = 32) | Citalopram + BT vs. citalopram favoured combined approach for satisfaction with sex life | ||||
| BT plus citalopram (n = 32) | |||||
| BT compared with pharmacotherapy | |||||
| Abdel-Hamid et al. 2001,39 5 × 4 week phases each separated by a 2-week washout | Squeeze technique | Erectile Dysfunction Inventory of Treatment Satisfaction scale 0–5: sexual satisfaction score | Clomipramine, 11; sertraline, 11; sildenafil, 30; paroxetine, 9; squeeze technique, 6 | NR | Headache, flushing, and nasal congestion: sildenafil, 18% |
| Sildenafil 50 mg – Clomipramine | Arabic Anxiety Inventory (scale 0–30) | Clomipramine, 11; sertraline, 10; sildenafil, 15; paroxetine, 12; squeeze technique, 3 | NR | The incidence of side effects was similar among groups (numbers NR) | |
| Sertraline | (Unclear if means or medians; no SD or p-values reported) | ||||
| Paroxetine | |||||
| (Total n = 31) | |||||
| Kolomaznik et al. 2002,41 8 weeks | Stop–start technique | Duration of coitus, subject report | NR | NR | NR |
| Fluoxetine | |||||
| Placebo | |||||
| Total n = 93 | |||||
| BT compared with pharmacotherapy | |||||
| Oguzhanoglu et al. 2005,43 8 weeks | Stop–start technique (n = 16) | Anxiety | State Anxiety change from baseline:
|
No | NR |
| Fluoxetine (n = 16) | No | ||||
Trait Anxiety change from baseline:
|
|||||
| Satisfaction with treatment | Satisfaction with treatment: p > 0.05 between groups | ||||
| Pastore et al. 2012,48 12 weeks | Pelvic floor muscle rehabilitation (n = 19) | None | – | – | Pelvic floor muscle rehabilitation, no side effects |
| Dapoxetine 30 or 60 mg (n = 21) | Dapoxetine: 30 mg nausea 1/8 (12.5%); 60 mg nausea, 2/7 (28.5%), diarrhoea 1/7 (14.0%) | ||||
| No severe AEs, no discontinuations due to AEs | |||||
Male perceptions of the duration of intercourse and couples’ sexual satisfaction were significantly improved with either functional sexological treatment (sensual education) or behavioural therapy (stop–start technique and squeeze technique) compared with waiting list control in one RCT. 40 One RCT50 reported a significant increase from baseline in International Index of Erectile Function (IIEF) measures of sexual satisfaction and desire, and on a measure of self-confidence associated with internet-based sex therapy based on a sensate focus technique compared with waiting list control. No difference was evident on an improvement/impairment of sexual functioning measure.
Behavioural psychotherapy combined with chlorpromazine was reported by one RCT as being more effective than chlorpromazine alone on a self-rated measure of anxiety and Chinese Index of Premature Ejaculation (CIPE) measures of sexual anxiety, sexual satisfaction and ejaculatory control. 42 Shao et al. 49 reported that CIPE measures of ejaculation control, patient/partner satisfaction and sexual anxiety were all significantly improved following treatment with behavioural therapy comprising squeeze technique, sensate focus and Chinese traditional treatment plus paroxetine compared with paroxetine alone. Yuan et al. 47 reported that behavioural therapy combined with citalopram was more effective at improving sexual satisfaction than citalopram alone.
Shao et al. 49 reported that CIPE measures of ejaculation control, patient/partner satisfaction and sexual anxiety were all significantly improved following treatment with behavioural therapy comprising squeeze technique, sensate focus and Chinese traditional treatment plus paroxetine compared with behavioural therapy alone. In one RCT,44 more patients receiving behavioural therapy plus sildenafil than patients receiving behavioural therapy alone reported ‘satisfied’ on a measure of sexual satisfaction. Xiong et al. 46 reported a between-group difference at 8 weeks of p < 0.05 on the IIEF favouring the tramadol plus behavioural therapy group compared with behavioural therapy alone.
Shao et al. 49 reported that paroxetine was significantly better than behavioural therapy on CIPE assessed ejaculation control. However, patient/partner satisfaction was significantly better following behavioural therapy than following paroxetine. No significant between-group difference was observed for sexual anxiety. Yuan et al. 47 reported that citalopram significantly increased the number of couples satisfied with their sex life compared with behavioural therapy alone. Oguzhanoglu et al. 43 reported no statistically significant between-group difference in satisfaction with treatment for stop–start technique compared with fluoxetine.
Assessment of safety: behavioural therapies – adverse events
Adverse event data were available for only 4 of the 12 included RCTs. Abdel-Hamid et al. 39 reported that the incidence of side effects was similar among groups and included headache, flushing and nasal congestion in 18% of the patients who received sildenafil. Pastore et al. 48 reported that dapoxetine was associated with nausea and diarrhoea whereas no AEs were reported for the pelvic floor rehabilitation group. In the RCT by Shao et al. ,49 the incidence of AEs was reported in the paroxetine group and the behavioural therapy combined with paroxetine group. However, the types of AEs were not reported. AEs for the behavioural therapy-only group were not reported. For one RCT,46 the between-group difference in relative risk (RR) at 12 weeks was 21.00 experiencing AEs [RR (random effects), 95% CI 1.28 to 345.41; p = 0.03] in favour of behavioural therapy alone compared with tramadol (lower risk). The forest plot for this analysis is presented as Figure 20 in the Opioid analgesics section of this assessment report.
Assessment of effectiveness: behavioural therapies – evidence summary
The current evidence base for behavioural therapy in the treatment of PE comprises 12 RCTs, nine captured in three low to good methodological quality systematic reviews and three further RCTs which are at overall unclear risk of bias. The quality of IELT outcome reporting across these trials is limited and does not facilitate any meaningful pooling across trials to be undertaken. However, individual trial results suggest that behavioural therapies are better than waiting list control in improving IELT, that behavioural therapies combined with pharmacological therapies are better than pharmacological agents alone (chlorpromazine, citalopram or paroxetine) and that behavioural therapies combined with pharmacological therapies (sildenafil, paroxetine or tramadol) are better than behavioural therapy alone in improving IELT in men with PE.
Various assessment methods in terms of ejaculation control, patients’/partners’ sexual satisfaction, anxiety and other patient-reported outcomes have been used across RCTs to measure the effectiveness of behavioural therapies. There is, however, some evidence to suggest that behavioural therapies combined with pharmacological therapies (paroxetine or tramadol) are better than behavioural therapy alone and that behavioural therapies combined with pharmacological therapies are better than pharmacotherapy alone (paroxetine, chlorpromazine, sildenafil or citalopram) in improving outcomes other than IELT. AE reporting across RCTs evaluating behavioural interventions is limited and AEs are often reported only for an adjuvant pharmacological agent or a pharmacological comparator. Adjuvant therapies to behavioural interventions that include SSRIs (dapoxetine, paroxetine) and PDE5 inhibitors (sildenafil) are reported to be associated with headache, flushing, nausea and diarrhoea.
Behavioural therapy alone appears to be more effective than no treatment in the treatment of PE. Behavioural therapy combined with pharmacological therapy appears more effective than behavioural therapy or pharmacological therapy alone. Comparisons between behavioural therapy and pharmacological therapies generally favour the pharmacological intervention for improvement in IELT, but are uncertain for other outcomes. AEs may be associated with adjuvant pharmacotherapy. The long-term efficacy of behavioural therapy in the treatment of PE is not evaluated in the current evidence base.
Topical anaesthetics
Characteristics of included studies: topical anaesthetics
Topical anaesthetics were evaluated by two systematic reviews51,53 and one ‘mini review’. 54 Two of these systematic reviews pooled data in a meta-analysis. 51,53 Trials of topical treatments were also included in one other review of pharmacological therapies. 52 A further two RCTs were identified, one of which evaluated EMLA (lidocaine and prilocaine) cream compared with electrical stimulation or placebo,62 while the other evaluated a lidocaine spray (Premjact, Boots Pharmaceuticals) compared with paroxetine. 63
One of the reviews of topical anaesthetics was conducted in the USA. 54 The two systematic reviews that pooled data in a meta-analysis were both undertaken in China. 51,53 The overall AMSTAR quality score of one of the reviews was 1 out of 11. 54 The two systematic reviews with a meta-analysis were scored as 4 out of 1151 and 5 out of 11. 53 Details of the review type, the databases searched and dates, relevant included RCTs and the AMSTAR points awarded to these reviews are presented in Table 7. Full details of the AMSTAR assessment for these and all other include reviews are presented in Appendix 4. The search methodology and inclusion criteria varied across these reviews. Pu et al. 51 pooled secondary outcome data from different domains of the same instrument in an overall summary effect estimate, in effect counting participants twice in the analysis. In the review by Xia et al. ,53 the authors pooled IELT effect estimates across studies using a standardised MD.
| Author (country), review type | Databases searched and dates | Included RCTs relevant to this section | AMSTAR review quality assessment |
|---|---|---|---|
| Morales et al. 200754 (USA), mini-review | MEDLINE 1966 to January 2004 | Atan et al. 2006,55 Atikeler et al. 2002,56 Busato and Galindo 2004,57 Dinsmore et al. 2007,59 Gittelman et al. 200661 | AMSTAR score, 1/11:
|
| Pu et al. 201351 (China), systematic and meta-analysis | Cochrane Central Register of Controlled Trials, PubMed (from 1980 to June 2012), and EMBASE (from 1980 to June 2012) | Atan et al. 2006,55 Atikeler et al. 2002,56 Busato and Galindo 2004,57 Carson et al. 2010,58 Dinsmore et al. 2007,59 Dinsmore and Wyllie 200960 | AMSTAR score, 4/11:
|
| Xia et al. 201353 (China), systematic and meta-analysis | The Cochrane Library, PubMed and EMBASE to October 2012 | Atikeler et al. 2002,56 Busato and Galindo 2004,57 Carson et al. 2010,58 Dinsmore et al. 2007,59 Dinsmore and Wyllie 200960 | AMSTAR score, 5/11:
|
The reviews above varied in terms of which RCTs they included. In total, seven RCTs (total n = 675) were included in at least one of these reviews. 55–61 IELT was reported as being assessed using a stopwatch in four RCTs57–60 and by patient self-report in one RCT. 56 The method of IELT assessment was not reported for two RCTs. 55,61 With the exception of the RCTs by Atikeler et al. 56 that evaluated the effects after more than five applications of treatment, and one trial reported as a crossover RCT,61 duration across trials ranged from 4 to 12 weeks. The topical anaesthetics evaluated included EMLA cream, TEMPE spray (containing lidocaine and prilocaine) and other topical anaesthetic creams (dyclonine cream and alprostadil cream). All of the RCTs compared topical anaesthetics with placebo. In addition, one RCT was identified that compared EMLA cream with sildenafil or EMLA cream combined with sildenafil. 55 This RCT is also evaluated in the section Phosphodiesterase-5 inhibitors of this assessment report. All RCTs in reviews were captured by the search strategy for this assessment report.
The RCT by Mallat et al. 62 was conducted in Tunisia. Patients were randomised, 30 per group, to EMLA, electrical stimulation or placebo. The trial was reported in abstract form only and the full details each treatment were not reported. The authors reported that 90 out of 90 (100%) patients completed the 12-week follow-up. The assessment method of IELT was not reported. The RCT by Steggall et al. 63 was conducted in the UK. Sixty patients were recruited to the trial and were randomised to either a lidocaine spray (Premjact) 10 minutes preintercourse or paroxetine 20 mg daily. Treatment duration was 2 months and the authors reported that 44 out of 60 (70%) patients completed the intervention. Both of these trials were considered to be at overall unclear risk of bias.
Details of the treatments evaluated, definition of PE, IELT assessment method, other outcomes assessed, study duration, along with the study country for the further RCTs not in reviews, and the overall study quality assessment (AMSTAR33 for reviews and Cochrane risk of bias assessment34 for the RCTs not included by reviews) are presented in Table 8.
| RCTs extracted from reviews | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RCT (source) | Duration | Treatments | PE definition | Lifelong/acquired | IELT assessment | Other outcomes | ||||||
| Atan et al. 200655 (reviews51,54) | 8 weeks | EMLA 15 minutes precoitus (n = 22) | DSM-IV | NR | IELT not assessed | NR | ||||||
| Sildenafil 50 mg 45 minutes precoitus (n = 20) | ||||||||||||
| EMLA + sildenafil (n = 22) | ||||||||||||
| Placebo (n = 20) | ||||||||||||
| All patients analysed | ||||||||||||
| Atikeler et al. 200256 (reviews152–154) | ≥ 5 applications | EMLA 2.5 g with condom: | IELT < 1 minute | Lifelong | Subject report | NR | ||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
| Placebo (n = 10) | ||||||||||||
| Busato and Galindo 200457 (reviews51,53,54) | 4–8 weeks | EMLA 2.5 g with condom 10–20 minutes precoitus (n = 24) | DSM-IV | Lifelong and acquired | Stopwatch | Sexual satisfaction (method NR) | ||||||
| Placebo (n = 18) | ||||||||||||
| (16 and 13 completed) | ||||||||||||
| Carson and Wyllie 201058 (reviews51,53) | 12 weeks | TEMPE spray 3 actuations (each 7.5 mg lidocaine, 2.5 mg prilocaine) 5 minutes precoitus (n = 167) | DSM-IV and ISSM | Lifelong and acquired | Stopwatch | IPE | ||||||
| Placebo (n = 82) | PEP | |||||||||||
| Dinsmore et al. 200759 (reviews51,53,54) | 4 weeks | TEMPE 3 actuations (each 7.5 mg lidocaine, 2.5 mg prilocaine) 15 minutes precoitus (n = 27) | DSM-IV | Lifelong | Stopwatch | IEC | ||||||
| Placebo (n = 28) | SQoL | |||||||||||
| (20 and 23 analysed) | ||||||||||||
| Dinsmore and Wyllie 200960 (reviews51,53) | 12 weeks | TEMPE 3 actuations (each 7.5 mg lidocaine, 2.5 mg prilocaine) 5 minutes precoitus (n = 200) | DSM-IV and ISSM | Lifelong and acquired | Stopwatch | IPE | ||||||
| Placebo (n = 100) | PEP | |||||||||||
| (191 and 99 analysed) | ||||||||||||
| Gittelman et al. 200661 (reviews54) | Single–arm crossover: duration NR | 5–20 minutes preintercourse: | NR | NR | Method NR | Sexual satisfaction – yes or no in ‘PSQ diary’a | ||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
|
||||||||||||
| (Total n = 30) | ||||||||||||
| Further RCTs identified by searches (not captured in reviews) | ||||||||||||
| RCT (country), risk of bias | Duration | Treatments, numbers analysed/randomised (%) | PE definition | Lifelong/acquired | IELT assessment | Other outcomes | ||||||
| Mallat et al. 201262 (Tunisia), unclear | 12 weeks | EMLA (n = 30) | NR | NR | Method NR | IIEF | ||||||
| Electric stimulation (n = 30) | ||||||||||||
| Placebo (n = 30) | ||||||||||||
| No details of treatments reported | ||||||||||||
| All groups (100%) | ||||||||||||
| Steggall et al. 200863 (UK), unclear | 2 months | Lidocaine 3–8 sprays 10 minutes precoitus | DSM-IV diagnosis plus IELT ≤ 3 minutes | Lifelong and acquired | Stopwatch | IIEF number of acts of coitus per week | ||||||
| Paroxetine 20 mg per day | IIEF intercourse satisfaction | |||||||||||
| (Total n = 60) | ||||||||||||
| Total n 44/60 (70%), n per group NR | ||||||||||||
Assessment of effectiveness: topical anaesthetics – intravaginal ejaculatory latency time outcomes
With the exception of the RCT by Atan et al. ,55 IELT outcomes were reported for all of the RCTs identified from existing reviews. The review by Morales et al. 54 reported that there was no statistical advantage in adding sildenafil to topical prilocaine-lidocaine treatment in the RCT by Atan et al. 55 No data or p-value were reported. The two further RCTs identified for inclusion in this assessment report both reported IELT outcomes, but without any variance estimates. Mallat et al. 62 reported a p-value for IELT of p < 0.001, but it was unclear if this was across or between groups, or whether this was for end of study values or change from baseline. Steggall et al. 63 reported a p-value for median IELT change from baseline of p = 0.038 for lidocaine spray and p < 0.0005 for paroxetine. These trials were therefore not included in any IELT meta-analysis in this assessment report.
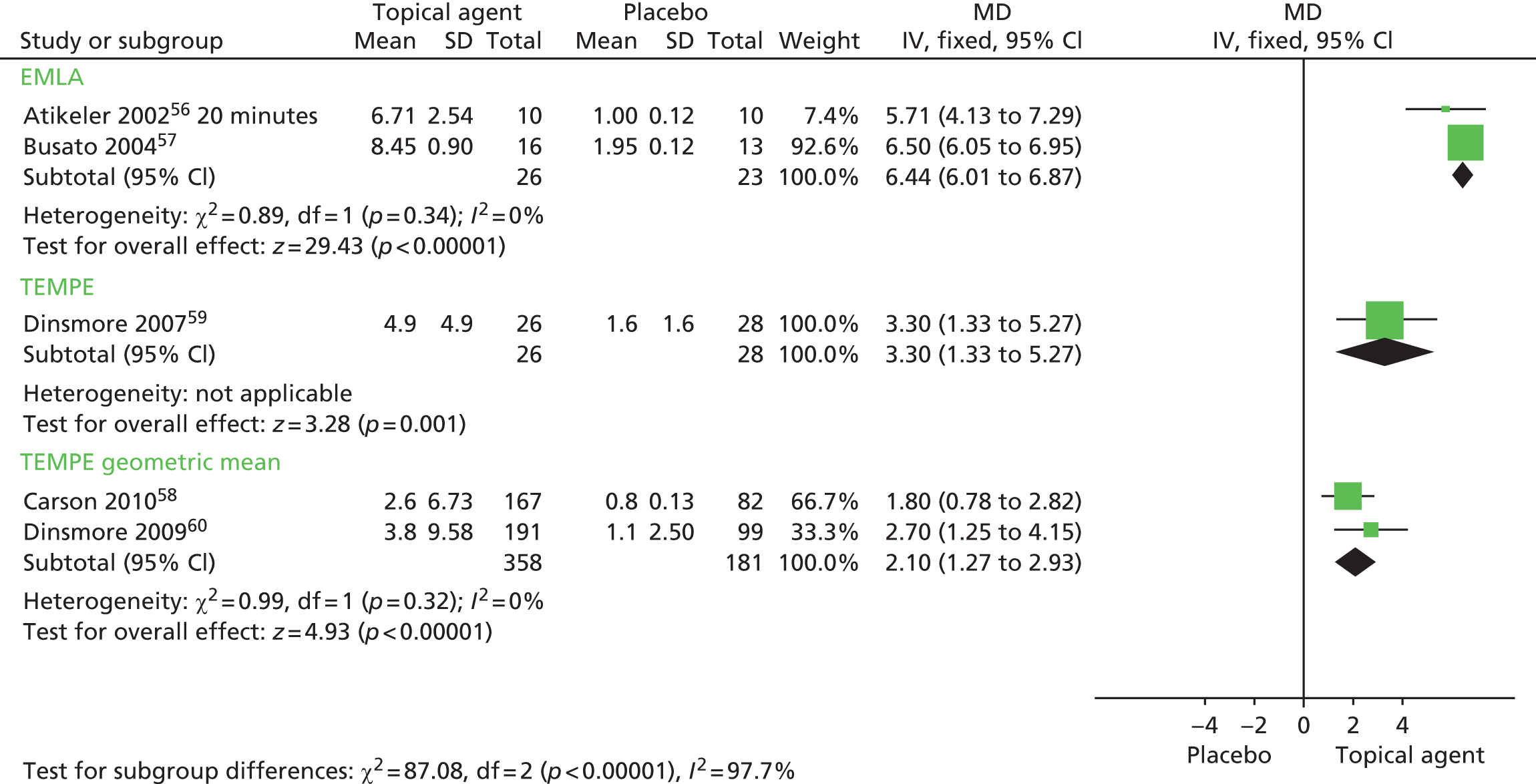
Meta-analysis of mean IELT (minutes) following an application of EMLA cream < 20 minutes preintercourse, based on two RCT study group comparisons (n = 49), displayed low heterogeneity (I2 = 0%). The pooled MD in IELT was 6.44 minutes, significantly favouring EMLA [MD (fixed effect); 95% CI 6.01 to 6.87 minutes; p < 0.00001]. The forest plot for this analysis is presented in Figure 4. Summary results for these and all other meta-analyses are presented in Table 9.
FIGURE 4.
Topical anaesthetics, EMLA cream or TEMPE spray compared with placebo: forest plot of IELT outcomes. df, degrees of freedom; IV, inverse variance; SD, standard deviation.
| Comparison | Outcome | No. of RCTsa | No. of participants | I 2 | Model | Effect estimate in minutes (95% CI) | Favours | p-value |
|---|---|---|---|---|---|---|---|---|
| IELT | ||||||||
| EMLA cream vs. placebo | IELT (minutes) | 256,57 | 49 | 0% | Fixed effect | MD 6.44 (6.01 to 6.87) | EMLA cream | < 0.00001 |
| TEMPE spray vs. placebo | IELT (minutes) | 159 | 54 | N/A | N/A | MD 3.30 (1.33 to 5.27) | TEMPE spray | 0.001 |
| TEMPE spray vs. placebo – geometric mean | IELT (minutes) | 258,60 | 539 | 0% | Fixed effect | MD 2.10 (1.27 to 2.93) | TEMPE spray | < 0.00001 |
| Dyclonine cream vs. placebo | IELT (minutes) | 155 crossover | 60 | N/A | N/A | MD 0.87 (0.71 to 1.03) | Dyclonine cream | < 0.00001 |
| Alprostadil cream vs. placebo | IELT (minutes) | 155 crossover | 60 | N/A | N/A | MD 1.41 (1.24 to 1.58) | Alprostadil cream | < 0.00001 |
| Dyclonine/alprostadil cream vs. placebo | IELT (minutes) | 155 crossover | 60 | N/A | N/A | MD 1.74 (1.58 to 1.90) | Alprostadil cream | < 0.00001 |
| Other outcomes | ||||||||
| EMLA cream vs. placebo | Other outcomes (various) | 257,62 | 119 | Two RCTs reported significant differences at 12 weeks in IPE ejaculatory control, sexual satisfaction and distress, and in PEP scores.58,60 | ||||
| TEMPE spray vs. placebo | Other outcomes (various) | 358–60 | 594 | Two RCTs reported significant differences at 12 weeks in IPE ejaculatory control, sexual satisfaction and distress and in PEP scores.58,59 One RCT reported no significant differences in Index of Ejaculatory Control and SQoL at 4 weeks60 | ||||
| AEs | ||||||||
| Topical anaesthetics (EMLA or TEMPE) vs. placebo | AEs | 655–60 | 704 | 0% | Fixed effect | RR 3.58 (1.71 to 7.48) | Placebo (fewer AEs) | 0.0007 |
| EMLA cream (applied ≤ 20 minutes) vs. placebo | AEs | 355–57 | 111 | N/A | Fixed effect | RR 9.06 (0.55 to 150.06) | NS | 0.12 |
| TEMPE spray vs. placebo | AEs | 358–60 | 593 | 0% | Fixed effect | RR 3.25 (1.50 to 7.02) | Placebo (fewer AEs) | 0.003 |
The between-group difference in mean IELT (minutes) based on one RCT (n = 54) was 3.30 minutes, significantly favouring TEMPE spray [MD (fixed effect); 95% CI 1.33 to 5.27 minutes; p = 0.001]. Meta-analysis of geometric mean IELT (minutes), based on two RCT study group comparisons (n = 49), displayed low heterogeneity (I2 = 0%). The pooled MD in IELT was 2.10 minutes, significantly favouring TEMPE spray [MD (fixed effect); 95% CI 1.27 to 2.93 minutes; p < 0.00001]. The forest plot for this analysis is presented in Figure 4.
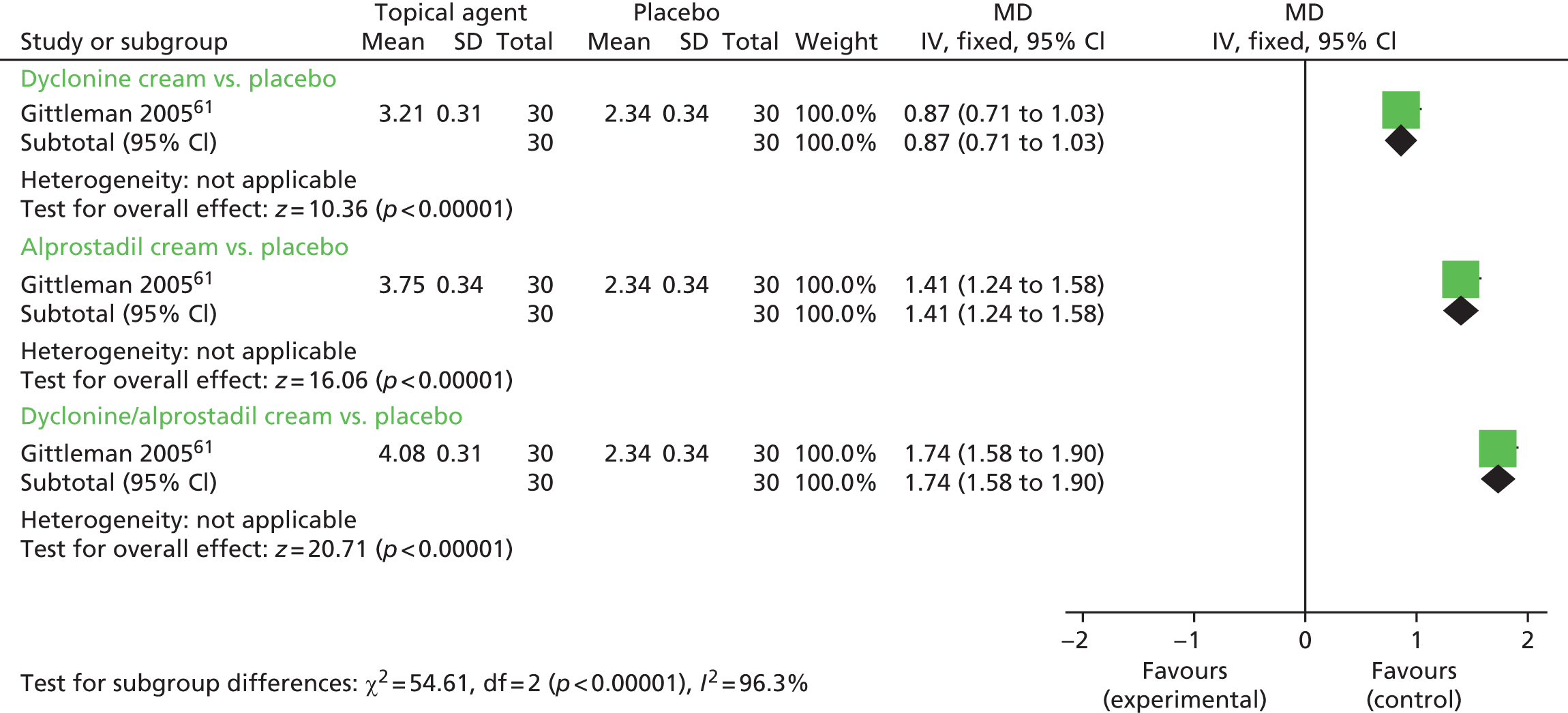
One single-arm randomised crossover trial (n = 30) evaluated three different topical anaesthetics. 61 The between-group differences in mean IELT (minutes) were 0.87 minutes in favour of dyclonine cream compared with placebo (95% CI 0.71 to 1.03 minutes; p < 0.00001); 1.41 minutes in favour of alprostadil cream compared with placebo (95% CI 1.24 to 1.58 minutes; p < 0.00001); and 1.74 minutes in favour of dyclonine/alprostadil cream compared with placebo (95% CI 1.58 to 1.90 minutes; p < 0.00001). The forest plot for this analysis is presented in Figure 5. A paired analysis could not be undertaken for approximation purposes for this study. Data from this trial were not pooled with other RCTs in any meta-analysis in this assessment report.
FIGURE 5.
Topical anaesthetics vs. placebo – forest plot of IELT outcomes. df, degrees of freedom; IV, inverse variance; SD, standard deviation.
Assessment of effectiveness: topical anaesthetics – other outcomes
Three RCTs did not report any effectiveness outcomes other than IELT. 55,56,63 Amongst the other RCTs, outcomes other than IELT were diverse across the included trials (Table 10).
| RCT, duration | Treatment | Outcome measure | Results | Between-group difference reported as significant | AEs |
|---|---|---|---|---|---|
| Atan et al. 2006,55 8 weeks | EMLA (n = 22) | NR | NR | NR | n/N (%) experiencing AEs: EMLA, 0/22 (0%); placebo, 0/20 (0%) (sildenafil and sildenafil + EMLA arms NR) |
| Sildenafil (n = 20) | |||||
| EMLA + Sildenafil (n = 15) | |||||
| Placebo (n = 20) | |||||
| Atikeler et al. 2002,56 ≥ 5 applications | EMLA:
|
NR | NR | NR | n/N (%) experiencing AEs: EMLA 20 minutes, 0/10 (0%); placebo, 0/10 (0%). Erection loss or numbness: 30-minute group, 6/10; 45-minute group, 10/10 |
| Placebo (n = 10) | |||||
| Busato and Galindo 2004,57 4–8 weeks | EMLA (n = 21) | Sexual satisfaction (method NR) | Sexual satisfaction: EMLA, 8.7; placebo, 4; p = 0.001 | Yes | n/N (%) experiencing AEs: EMLA, 5/16 (34%); placebo, 0/13 (0%) |
| Placebo (n = 21) | n/N reporting ‘great’ or ‘excellent’ satisfaction: EMLA, 6/16; 5/16; placebo, 3/13; 0/13 | Yes | EMLA-associated AEs: men, 2/29 retarded ejaculation, 2/29 loss of sensitivity, 2/29 penile irritation; women 1/29 decreased sensitivity | ||
| Carson and Wyllie 2010,58 12 weeks | TEMPE spray (n = 167) | IPE | Ejaculatory control (IPE): TEMPE, 11.6; placebo, 6.5 | Yes | n/N (%) experiencing AEs: TEMPE, 17/167 (10%); placebo, 1/82 (< 1%) |
| Placebo (n = 82) | Sexual satisfaction (IPE): TEMPE, 13.4; placebo, 8.6 | Yes | |||
| Distress (IPE): TEMPE, 6.1; placebo, 3.7 | Yes | ||||
| PEP | PEP ≥ 1 point improvement: p < 0.0001 (unclear if between groups or baseline) | Yes | |||
| Dinsmore et al. 2007,59 4 weeks | TEMPE spray (n = 27) | IEC | Ejaculatory control (IEC) change: TEMPE, 6.7; placebo, 3.0; p = 0.12 | No | n/N (%) experiencing AEs: TEMPE, 6/26 (23%); placebo, 4/28 (14%) |
| Placebo (n = 28) | SQoL | SQoL change: TEMPE, men 7.0, women 3.3. Placebo, men 5.5, women 1.8. p-value men, 0.48; women, 0.56 | No | Assume with TEMPE: hypoaesthesia, 3/26; erectile dysfunction, 1/26. Women: mild burning 1/26 | |
| Dinsmore and Wyllie 2009,60 12 weeks | TEMPE spray (n = 191) | IPE | Ejaculatory control (IPE): TEMPE, 14.3; placebo, 7.4 | Yes | n/N (%) experiencing AEs: TEMPE, 18/191 (9%); placebo 3/99 (3%) |
| Placebo (n = 99) | Sexual satisfaction (IPE): TEMPE, 14.8; placebo, 9.1 | Yes | |||
| Distress (IPE): TEMPE, 7.1; placebo, 4.5 | Yes | ||||
| PEP | PEP ≥ 1 point improvement: p < 0.001 (unclear if between groups or baseline) | Yes | |||
| Gittelman et al. 2006,61 NR (crossover) | Dyclonine cream | Sexual satisfaction – yes or no in ‘PSQ diary’a | % reporting ‘yes’: dyclonine cream, 73.3%; alprostadil cream, 83.3%; dyclonine/alprostadil cream, 86.7%; placebo cream, 66.7% | Unclear | Proportion experiencing AEs: dyclonine cream, 17.5%; alprostadil cream, 20%; dyclonine/alprostadil cream, 17.5%; placebo cream, 5%. Type not reported |
| Alprostadil cream | |||||
| Dyclon/alpro cream | |||||
| Placebo cream | |||||
| (Total n = 30) | |||||
| Mallat et al. 2012,62 12 weeks | EMLA (n = 30) | Number of coitus per week | Number of coitus: EMLA, 1.4; electric stimulation, 2.3; placebo, 1.3 | Unclear | No withdrawals caused by AEs across all treatments, but more AEs were associated with EMLA. No further details reported |
| Electric stimulation (n = 30) | IIEF intercourse satisfaction | IIEF satisfaction: EMLA, 10; electric stimulation, 14; placebo, 10 | Unclear | ||
| Placebo (n = 30) | |||||
| Steggall et al. 2008,63 8 weeks | Lidocaine spray | NR | NR | NR | NR |
| Paroxetine | |||||
| (Total n = 60) |
A statistically significant between-group difference in sexual satisfaction in favour of EMLA cream after 2 months was reported by Busato and Galindo. 57 There appeared to be no difference between EMLA cream and placebo on the IIEF. Number of coitus per week and sexual satisfaction values were reported by one RCT. 62
The between-group differences on the Index of Ejaculatory Control and Sexual Quality of Life for both men and women were reported as being not statistically significant at 4 weeks in one RCT. 59 However, two RCTs reported that TEMPE spray was significantly more effective than placebo at 12 weeks on the IPE measures including ejaculatory control, sexual satisfaction and distress and on the PEP. 58,60
In one crossover RCT, > 70% of patients allocated to receive a cream containing either dyclonine, alprostadil or both agents reported ‘yes’ for sexual satisfaction. 61 However, 66.7% in the placebo group also reported ‘yes’. A p-value for between-group difference was not reported.
Assessment of safety: topical anaesthetics – adverse events
Adverse events were not reported for one RCT. 63 When reported, AEs associated with topical anaesthetics included erectile dysfunction/loss of erection, loss of sensitivity/numbness (men and women) and irritation/burning (men and women).
Meta-analysis of patient numbers experiencing AEs following treatment with topical anaesthetics displayed low heterogeneity (I2 = 0%). The between-group difference in EMLA cream applied for ≥ 20 minutes compared with placebo was not statistically significant [RR 9.06 (fixed effect), 95% CI 0.55 to 150.06; p = 0.12]. However, Atikeler et al. 56 reported that EMLA cream caused 6 out of 10 men in the 30-minute application group and 10 out of 10 men in the 45-minute application group to report erection loss or numbness.
The pooled RR across three trials comparing TEMPE spray with placebo (593 participants) was 3.25 [RR (fixed effect); 95% CI 1.50 to 7.02; p = 0.003] in favour of placebo (lower risk). The forest plot for this analysis is presented in Figure 6. Results for these and all other meta-analyses are presented in Table 10.
FIGURE 6.
Topical anaesthetics compared with placebo: forest plot of AEs. df, degrees of freedom; IV, inverse variance.
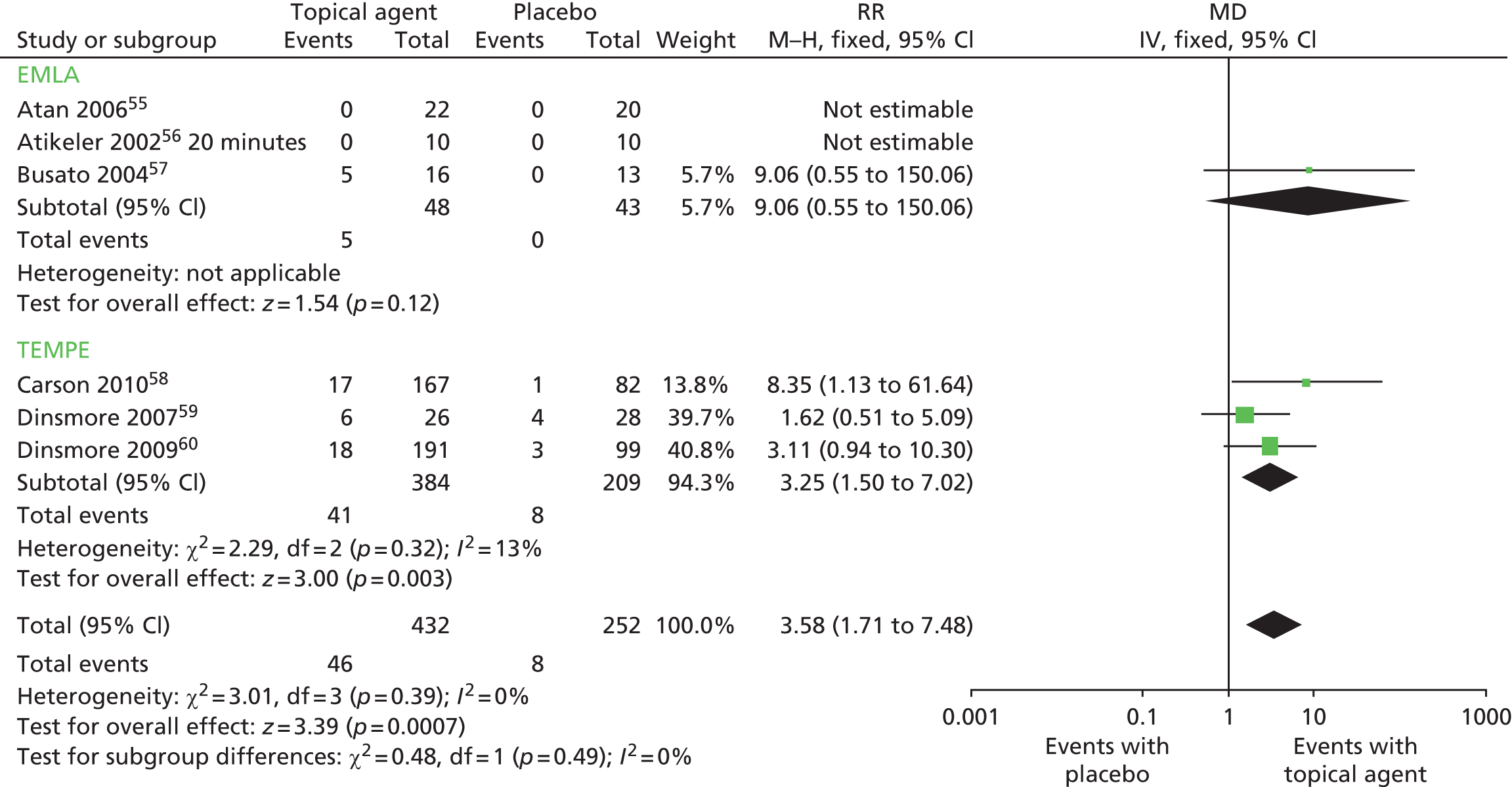
Assessment of effectiveness: topical anaesthetics – evidence summary
The current evidence base for topical anaesthetics in the treatment of PE comprises nine RCTs,54–63 seven55–61 captured in three low methodological quality systematic reviews51,53,54 and two further RCTs62,63 which are at overall unclear risk of bias. The pooled evidence across two RCTs56,57 comprising 49 participants suggests that EMLA cream is effective in significantly increasing IELT in men with PE compared with placebo (MD 6.44 minutes, 95% CI 6.01 to 6.87 minutes; p < 0.00001). Evidence from one RCT59 (54 participants) suggests that TEMPE spray is effective in significantly increasing IELT in men with PE compared with placebo (MD 3.30 minutes, 95% CI 1.33 to 5.27 minutes; p < 0.00001). Evidence from one crossover RCT61 suggests that creams containing dyclonine, alprostadil or both agents are more significantly more effective than placebo.
Various assessment methods in terms patient/partners sexual satisfaction and other outcomes have been used across RCTs to measure the effectiveness of topical anaesthetics. Evidence from three RCTs58–60 suggests significant improvements in sexual satisfaction with topical anaesthetics compared with placebo. However, two other RCTs that assessed the effects of topical anaesthetics or placebo suggests there is no difference in sexual satisfaction or intercourse frequency,57 or ejaculatory control and sexual quality of life. 58 Pooled evidence across trials suggests that topical anaesthetics are associated with significantly more AEs than placebo. AEs associated with topical anaesthetics include loss of sensitivity/numbness and irritation/burning for both men and women. Erectile dysfunction and loss of erection are also reported by men and appear to be related to treatment applications ≥ 20 minutes preintercourse.
Topical anaesthetics appear more effective than placebo in the treatment of PE. Loss of sensation and irritation are common AEs in both men and women, and there is more reporting of AEs associated with TEMPE spray than EMLA cream. Application of topical anaesthetics ≥ 20 minutes preintercourse is associated with erection loss. However, these findings should be interpreted with caution given the methodological quality of the available evidence. In addition, patient acceptability of this treatment modality (topical application) for PE has not been evaluated in the current evidence base.
Selective serotonin reuptake inhibitors currently not licensed for premature ejaculation
Characteristics of included studies: selective serotonin reuptake inhibitors
Selective serotonin reuptake inhibitors were evaluated by seven systematic reviews. 52,64–69 Four reviews focused specifically on SSRIs,64–67 while the others evaluated various treatments for PE including SSRIs. One review of SSRIs pooled data from RCTs comparing fluoxetine with placebo in a meta-analysis,64 and one pooled data from RCTs comparing citalopram, dapoxetine, fluoxetine, fluvoxamine and sertraline with placebo in a meta-analysis. 65 Details of the review type, the databases searched and dates, relevant included RCTs and the AMSTAR points awarded to these reviews is presented in Table 11.
| Author (country), review type | Databases searched and dates | Included RCTs relevant to this section | AMSTAR review quality assessment |
|---|---|---|---|
| Cong et al. 2012,64 (China), systematic review and meta-analysis | MEDLINE, EMBASE, PubMed, Ovid, CENTRAL, CBM and CNKI database July 1996 to May 2012 | Kara et al. 1996,75 Kim and Seo 1998,76 Mattos et al. 2008,141 Panshou and Xie 2004,80 Waldinger et al. 1998,81 Yilmaz et al. 199983 | AMSTAR score, 3/11:
|
| Huang et al. 200965 (China), systematic review and meta-analysis | MEDLINE, January 1950 to March 2008; EMBASE, January 1950 to March 2008; The Cochrane Library, Issue I 2008; and CNKI, January 1979 to March 2008 | Atmaca et al. 2002,70 Atmaca et al. 2003,71 Biri et al. 1998,89 Kara et al. 1996,75 Kim and Seo 1998,76 Mattos et al. 2008,141 McMahon and Touma 1999,84 Mendels et al. 1995,90 Novaretti et al. 2002,79 Panshou and Xie 2004,80 Safarinejad and Hosseini 2006,72 Safarinejad 2006,85 Waldinger et al. 1998,81 Yilmaz et al. 1999,83 Zhou 200791 | AMSTAR score, 1/11:
|
| McMahon and Porst 201168 (Australia), systematic review | PubMed 2004 | Atmaca et al. 2002,70 Kara et al. 1996,75 Mattos et al. 2008,141 Novaretti et al. 2002,79 Waldinger et al. 1998,81 Waldinger et al. 200182 | AMSTAR score, 2/11:
|
| Moreland and Makela 200566 (USA), described as a ‘mini review’ | NR | Atmaca et al. 2002,70 Biri et al. 1998,89 Kim and Seo 1998,76 Manasia et al. 2003,77 McMahon and Touma 1999,84 Mendels et al. 1995,90 Waldinger et al. 1997,87 Waldinger et al. 1998,81 Waldinger et al. 2001,73 Waldinger et al. 200182 | AMSTAR score, 0/11 |
| Richardson et al. 200569 (UK), systematic review | MEDLINE, 1966 to January 2003 and PsycINFO, 1872 to January 2003 | Abdel-Hamid et al. 2001,39 Kara et al. 1996,75 Kim and Seo 1998,76 McMahon and Touma 1999,84 Waldinger et al. 1997,87 Waldinger et al. 1998,81 Waldinger et al. 2001,82 Waldinger et al. 2001,73 Yilmaz et al. 199983 | AMSTAR score, 1/11:
|
| Waldinger et al. 200452 (the Netherlands), systematic review | MEDLINE (1966–2002), Web of Science, PICA, a and EMBASE (1980–2002) | Biri et al. 1998,89 Abdel-Hamid et al. 2001,39 Atmaca et al. 2002,70 Haensel et al. 1998,74 Kara et al. 1996,75 Kim and Seo 1998,76 Kolomaznik et al. 2002,41 McMahon and Touma 1999,84 Novaretti et al. 2002,79 Waldinger et al. 1994,86 Waldinger et al. 1997,87 Waldinger et al. 1998,81 Waldinger et al. 2001,73 Waldinger et al. 2001,82 Waldinger et al. 2003,88 Yilmaz et al. 199983 | AMSTAR score, 1/11:
|
| Wang et al. 200767 (China), systematic review | MEDLINE 1 January 1996 to 1 August 2006 | Atmaca et al. 2003,71 McMahon 1998,166 McMahon and Touma 1999,84 Murat Basar et al. 1999,78 Safarinejad and Hosseini 2006,72 Waldinger et al. 2001,82 Waldinger et al. 2001,73 Waldinger et al. 2003,88 Yilmaz et al. 199983 | AMSTAR score, 0/11 |
Three of the systematic reviews were conducted in China. 64,65,67 One review was conducted in Australia,68 one in the Netherlands,52 one in the USA66 and one in the UK. 69 The overall AMSTAR quality score was 1 out of 11 in three of the reviews,52,65,69 2 out of 11 in one review68 and 3 out of 11 in one review. 64 Two reviews scored 0 out of 11. 66,67 The review by Huang et al. 65 was the most comprehensive in terms of included RCTs evaluating SSRIs. However, the reviewers pooled data from single-arm crossover studies with separate treatment arm studies in a meta-analysis. Full details of the AMSTAR assessment for these and all other included reviews are presented in Appendix 4. The search methodology and inclusion criteria for studies were varied across these reviews. All RCTs in reviews were captured by the search strategy for this assessment report.
Twenty-six RCTs of SSRIs were evaluated across the seven reviews. 39,41,70–91,141,166 A further 16 RCTs additional to those already included reviews were identified for inclusion,92–107 resulting in a total of 42 RCTs that evaluated SSRIs. Fourteen of the 16 additional RCTs identified by the literature search were considered to be at overall unclear risk of bias. 92–94,96–102,104–107 Two were considered to be at overall high risk of bias. 95,103 The 16 additional RCTs were undertaken in China, Egypt, Georgia, Italy, the Islamic Republic of Iran, the Republic of Korea, the Netherlands, Saudi Arabia and Turkey. Across the 42 included RCTs:
-
Citalopram was assessed in nine RCTs. 70–73,92–96 Four RCTs were identified from reviews70–73 and five from the literature search. 92–96 Across these RCTS treatment doses ranged from 20 mg to 60 mg. Comparators included placebo, no therapy and other SSRIs. Duration ranged from 4 to 12 weeks.
-
Escitalopram (Cipralex®, Lundbeck) was evaluated in four RCTs, all identified from the literature search. 94,97–98 All prescribed daily dose of 10 mg. Comparators included placebo and other SSRIs. Duration ranged from 4 to 12 weeks.
-
Fluoxetine was assessed in 16 RCTs. 41,74–81,83,95,97,100–102,141 Eleven RCTs were identified from reviews41,74–83 and five from the literature search. 95,97,100–102 The doses evaluated were 10, 20 or 40 mg per day or 90 mg once weekly. Comparators included placebo, other SSRIs, clomipramine, fluoxetine plus tadalafil, and behavioural therapies (stop–start/squeeze technique). Duration ranged from 4 to 12 weeks.
-
Fluvoxamine was assessed in one RCT at a dose of 20 mg for 6 weeks, compared with placebo and other SSRIs (Waldinger et al. ,81 identified from a review).
-
Paroxetine was assessed in 13 RCTs. 39,73,81,82,84–88,97,103,104,105 Nine RCTs were identified from reviews39,73,81,82,84–88 and four from the literature search. 97,103,104,105 Doses were 20 mg or 40 mg (usually 20 mg as a daily dose). Comparators included placebo, other SSRIs, clomipramine, sildenafil, mirtazapine, nefazodone (Serzone, Bristol-Myers Squibb, discontinued 2005) and the squeeze technique. Duration ranged from 4 to 12 weeks.
-
Sertraline was assessed in 13 RCTs. 39,76,78,81,82,89–92,102,106,107,166 Nine RCTs were identified from reviews39,76,78,81,82,89–91,166 and four from the literature search. 92,102,106,107 Doses ranged from 50 mg to 200 mg (usually 50 mg as a daily dose). Comparators included placebo, other SSRIs, clomipramine, sildenafil, terazosin, mirtazapine, PDE5 inhibitors and behavioural therapies.
Details of the treatments evaluated, definition of PE, IELT assessment method, other outcomes assessed, study duration, along with the study country for the further RCTs not in reviews, and the overall study quality assessment (AMSTAR for reviews and Cochrane risk of bias assessment34 for the RCTs not included by reviews) are presented in Table 12.
| Citalopram: RCTs extracted from reviews | ||||||
|---|---|---|---|---|---|---|
| RCT (source) | Duration | Treatments | PE definition | Lifelong/acquired | IELT assessment | Other outcomes |
| Atmaca et al. 200270 (reviews52,65,66,68) | 8 weeks | Citalopram 20–60 mg/day (n = 13) | Partially ISSM: DSM-III R167 | NR | Stopwatch | CGI-I, YSFI-II |
| Placebo (n = 13) | ||||||
| Atmaca et al. 200371 (reviews65,67) | 8 weeks | Citalopram 20–60 mg (n=15) | NR | NR | Method NR | NR |
| No therapy (n=15) | ||||||
| Safarinejad and Hosseini 200672 (reviews65,67) | 12 weeks | Citalopram 20 mg (n = 26) | NR | NR | Method NR | IIEF: intercourse satisfaction, coitus per week |
| Placebo (n = 25) | ||||||
| Citalopram: further RCTs identified by searches (not captured in reviews) | ||||||
| RCT (country) risk of bias | Duration | Treatments, number analysed/randomised (%) | PE definition | Lifelong/acquired | IELT assessment | Other outcomes |
| Farnia et al. 200893 (the Islamic Republic of Iran) unclear risk | 4 weeks | Citalopram 20 mg 4 h precoitus (n = 49) | DSM-IV-TR diagnosis | NR | Stopwatch | CIPE |
| Placebo (n = 43) | ||||||
| Completers: citalopram, 42/49 (86%); placebo, 38/43 (88%) | ||||||
| Shang et al. 201296 (China) unclear risk | Duration NR, 2-week follow-up and 4-week follow-up post treatment | Citalopram 20 mg/day (n = 40) | NR | NR | Treatment druation NR | Sexual satisfaction |
| Placebo (n = 40) | ||||||
| NR | ||||||
| Escitalopram: RCTs identified by searches (not captured in reviews) | ||||||
| Nada et al. 200998 (Egypt) unclear risk | 4 weeks (and further 2 months follow-up) | Escitalopram 10 mg/day (n = 15) | NR | NR | CIPE | NR |
| Placebo (n = 15) | ||||||
| NR | ||||||
| Safarinejad 200799 (the Islamic Republic of Iran) unclear risk | 12 weeks (then 3 and 6 months follow-up) | Escitalopram 10 mg/day (n = 138) | IELT < 2 minutes on 90% occasions | Lifelong, 82% | Stopwatch | IIEF intercourse satisfaction |
| Placebo (n = 138) | Acquired, 18% | Weekly coitus episodes | ||||
| Escitalopram, 128/138 (93%) | ||||||
| Placebo, 126/138 (91%) | ||||||
| Fluoxetine: RCTs extracted from reviews | ||||||
| RCT (source) | Duration | Treatments | PE definition | Lifelong/acquired | IELT assessment | Other outcomes |
| Haensel et al. 199874 (reviews52) | Crossover, 4 weeks per treatment | Fluoxetine 5 mg/day (2 weeks), then 10 mg/day (2 weeks) | DSM-IV | NR | Ejaculatory latency questionnaire | NR |
| Placebo (n = 15) | ||||||
| Kara et al. 199675 (reviews52,64,68,69) | 4 weeks | Fluoxetine 20 mg/day for 1 week then 40 mg/day (n = 9) | DSM-III168 | NR | Stopwatch | Hamilton Depression Scale |
| Placebo (n = 8) | ||||||
| Kolomaznik et al. 200241 (reviews52) | 8 weeks | Fluoxetine (dose NR) | NR | NR | IELT not assessed | Duration of coitus, subject report |
| Stop–start technique | ||||||
| Placebo (n = 93) | ||||||
| Manasia et al. 200377 (reviews66) | Duration NR | Fluoxetine 90 mg/week (n = 40) | NR | NR | Method NR | Sexual satisfaction ratings |
| Fluoxetine 20 mg/day (n = 40) | ||||||
| Mattos et al. 2008141 (reviews64,65,68) | 12 weeks | Fluoxetine 90 mg/week (n = 15) | DSM-IV + IELT ≤ 1.5 minutes | Lifelong | Stopwatch | NR |
| Tadalafil 20 mg 1–3 hours precoitus + fluoxetine 90 mg (n = 15) | ||||||
| Tadalafil (n = 15) | ||||||
| Placebo (n = 15) | ||||||
| Novaretti et al. 200279 (reviews52,65,68) | Crossover, 8 weeks | Fluoxetine 20 mg/d | NR | NR | Stopwatch | Hamilton Anxiety and Depression Scale; Beck Depression Inventory |
| Placebo (n = 50) | ||||||
| Panshou and Xie 200480 (reviews64,65) | 12 weeks | Fluoxetine 20 mg/day (n = 24) | DSM-IV | NR | Method NR | NR |
| Placebo (n = 20) | ||||||
| Yilmaz et al. 199983 (reviews52,64,65,68,69) | RCT, 4 weeks | Fluoxetine 20 mg/day (n = 20) | DSM-IV | NR | Self-report | Penile vibratory threshold and evoked potentials |
| Placebo (n = 20) | ||||||
| Fluoxetine: further RCTs identified by searches (not captured in reviews) | ||||||
| RCT (country) risk of bias | Duration | Treatments, number analysed/randomised (%) | PE definition | Lifelong/acquired | IELT assessment | Other outcomes |
| Ahn et al. 1996100 (the Republic of Korea) unclear risk | 6 weeks | Fluoxetine 20 mg/day (for 1 week) then 40 mg/day (n = 12) | NR | Lifelong | IELT assessed with questionnaire | Questionnaire assessing number of thrusts before ejaculation, frequency of coitus, libido and side effects of treatment |
| Placebo (n = 11) | ||||||
| Fluoxetine, 12/12 (100%) | ||||||
| Placebo, 11/11 (100%) | ||||||
| Culba et al. 2008101 (Turkey) unclear risk | 10 weeks | Fluoxetine 20 mg/day | NR | NR | IELT via visual scale, ELTQ, IIEF | IIEC |
| Fluoxetine 20 mg/day + tadalafil 20 mg twice weekly | PE question of CMASH questionnaire | |||||
| Placebo (n = 180) | ||||||
| Total 158/180 (88%) | ||||||
| Paroxetine: RCTs extracted from reviews | ||||||
| RCT (source) | Duration | Treatments | PE definition | Lifelong/acquired | IELT assessment | Other outcomes |
| McMahon and Touma 199984 (reviews52,65–67,69) | 2 × RCTs –crossover. Duration unclear | Study I:
|
NR | NR | Stopwatch | NR |
| NR | ||||||
| NR | ||||||
Study II:
|
NR | |||||
| NR | ||||||
| NR | ||||||
| Waldinger et al. 199486 (reviews52) | 6 weeks | Paroxetine (dose NR) | NR | NR | Questionnaire | NR |
| Placebo (n = 14) | ||||||
| Waldinger et al. 199787 (reviews52,66,69) | 8 weeks | Paroxetine 20 mg | IELT ≤ 1 minute > 50% of time | Lifelong | Clock with a second hand | NR |
| Paroxetine 40 mg (n = 34) | ||||||
| Waldinger et al. 200388 (reviews52,67) | 6 weeks | Paroxetine (n = 12) | IELT ≤ 1 minute | Lifelong | Stopwatch | NR |
| Mirtazapine (n = 12) | ||||||
| Paroxetine: RCTs identified by searches (not captured in reviews) | ||||||
| RCT (country) risk of bias | Duration | Treatments, numbers analysed/randomised (%) | PE definition | Lifelong/acquired | IELT assessment | Other outcomes |
| Giammusso et al. 1997103 (Italy) high risk | 6 months (and further 3 months follow-up) | Paroxetine 20 mg/day (n = 28) | NR | Lifelong | No objective assessment of IELT | Self-report control over ejaculation |
| Paroxetine 20 mg/day (for 2 weeks) then 10 mg/day (n = 34) | ||||||
| Paroxetine 20 mg, 27/28 (96%) | ||||||
| Paroxetine 10 mg, 16/34 (47%) | ||||||
| Khelaia et al. 2012104 (Georgia) unclear risk | 4 weeks | Paroxetine 20 mg/day (n = 26) | NR | NR | Method NR | IIEF: intercourse and overall satisfaction |
| Paroxetine 20 mg 2–3 hours precoitus (n = 28) | ||||||
| Placebo (n = 24) | ||||||
| NR | ||||||
| Sertraline: RCTs extracted from reviews | ||||||
| RCT (source) | Duration | Treatments | PE definition | Lifelong/acquired | IELT assessment | Other outcomes |
| Biri et al. 199889 (reviews52,65–67) | 4 weeks | Sertraline 50 mg (n = 22) | NR | NR | Ejaculatory latency questionnaire | NR |
| Placebo (n = 15) | ||||||
| McMahon et al. 1998166 (reviews52,65,67,69) | 4 weeks | Sertraline 50 mg (n = 19) | IELT < 1 minute | NR | Stopwatch | NR |
| Placebo (n = 18) | ||||||
| Mendels et al. 199590 (reviews52,65,66) | 8 weeks | Sertraline 50–200 mg (n = 22) | NR | NR | Self-report | Patient and partner satisfaction via scale |
| Placebo (n = 22) | ||||||
| Zhou et al. 200791 (reviews65) | 4 weeks | Sertraline (n = 24) | NR | NR | Method NR | NR |
| Placebo (n = 22) | ||||||
| Sertraline: RCTs identified by searches (not captured in reviews) | ||||||
| RCT (country) risk of bias | Duration | Treatments | PE definition | Lifelong/acquired | IELT assessment | Other outcomes |
| Arafa and Shamloul 2007106 (Egypt and Saudi Arabia) unclear risk | RCT (crossover), 4 weeks each (4-week washout) and 6-month follow-up | Sertraline 50 mg/day (n = 77) | IELT ≤ 2 minutes; < 31 on AIPE | Lifelong 11% | Stopwatch | AIPE frequency of intercourse (method NR) |
| Placebo (n = 70) | Acquired 89% | |||||
| AIPE scores reported for 147/147 (100%) | ||||||
| Tuncel et al. 2008107 (Turkey) unclear risk | 2 months, assessment ‘after eight sexual attempts’ | Sertraline 50 mg/day (n = 20) | WHO ICD-10 | NR | Not assessed | Clinical responses (assume control of ejaculation) self-assessed |
| Clomipramine 25 mg/day (n = 23) | ||||||
| Terazosin 5 mg/day (n = 25) | ||||||
| Placebo (n = 22) | ||||||
| All 100% | ||||||
| More than one SSRI: RCTs extracted from reviews | ||||||
| RCT (source) | Duration | Treatments | PE definition | Lifelong/acquired | IELT assessment | Other outcomes |
| Abdel-Hamid et al. 200139 (reviews37,134,135,137) | RCT crossover, 4 weeks each 2-week washout | Sildenafil 50 mg 1 hour precoitus | IELT ≤ 2 minutes | Lifelong | Stopwatch | Modified Erectile Dysfunction Inventory of Treatment Satisfaction, Arabic Anxiety Inventory (scale 0–30) |
| Clomipramine 25 mg 3–5 hours precoitus | ||||||
| Sertraline 50 mg 3–5 hours precoitus | ||||||
| Paroxetine 20 mg 3–5 hours precoitus | ||||||
| Squeeze technique (total n = 31) | ||||||
| Kim et al. 199876 (reviews52,64–66,69) | RCT crossover, 4 weeks each 1-week washout | Fluoxetine 40 mg | DSM-III | NR | Method NR | Patient self-reported questionnaire for patient and partner sexual satisfaction |
| Sertraline 100 mg | ||||||
| Clomipramine 50 mg | ||||||
| Placebo (total n = 36) | ||||||
| Murat Basar et al. 199978 (reviews67) | 4 and 8 weeks | Fluoxetine 20 mg for one week then 40 mg (n = 26) | NR | NR | Method NR | Results classified as unsuccessful, improvement and cure |
| Sertraline 50 mg (n = 31) | ||||||
| Safarinejad 200685 (reviews65) | 12 weeks | Paroxetine 20mg (n = 113) | NR | NR | IELT not assessed | Sexual satisfaction |
| Dapoxetine 60mg (n = 115) | ||||||
| Placebo (n = 112) | ||||||
| Waldinger et al. 199881 (reviews52,64–66,68,69) | 6 weeks | Fluoxetine 20 mg/day (n = 12) | IELT ≤ 1 minute | Lifelong | Stopwatch | Libido, erection hardness (questionnaire) |
| Fluvoxamine 100 mg/day (n = 12) | ||||||
| Paroxetine 20 mg/day (n = 12) | ||||||
| Sertraline 50 mg/day (n = 12) | ||||||
| Placebo (n = 12) | ||||||
| Waldinger et al. 200182 (reviews52,66–69) | 6 weeks | Paroxetine 20 mg/day (n = 12) | IELT ≤ 1 minute | Lifelong | Stopwatch | NR |
| Sertraline 50 mg/day (n = 12) | ||||||
| Nefazodone 400 mg/day (n = 12) | ||||||
| Placebo (n = 12) | ||||||
| Waldinger et al. 200173 (reviews52,66,67,69) | 6 weeks | Paroxetine 20 mg (n=15) | IELT ≤ 1 minute | Lifelong | Stopwatch | NR |
| Citalopram 20 mg (n=15) | ||||||
| More than one SSRI: RCTs identified by searches (not captured in reviews) | ||||||
| RCT (country) risk of bias | Duration | Treatments, number analysed/randomised (%) | PE definition | Lifelong/acquired | IELT assessment | Other outcomes |
| Akgül et al. 200892 (Turkey) unclear risk | 8 weeks | Citalopram 20 mg/day (n = 40) | IELT ≤ 2 minutes 75% of attempts | NR | IELT not assessed | IPE |
| Sertraline 50 mg/day (n = 40) | ||||||
| Citalopram, 40/40 (100%) | ||||||
| Sertraline, 40/40 (100%) | ||||||
| Arafa and Shamloul 200797 (Egypt and Saudi Arabia) unclear risk | 4 weeks | Fluoxetine 20 mg/day (n = 33) | IELT ≤ 2 minutes; < 31 on AIPE | All acquired | Stopwatch | AIPE frequency of intercourse (method NR) |
| Escitalopram 10 mg/day (n = 37) | ||||||
| Paroxetine 20 mg/day (n = 30) | ||||||
| All 100% | ||||||
| Nada et al. 201294 (Egypt) unclear risk | 6 weeks (and further 3 months follow-up) | Escitalopram 10 mg/day (n = 30) | NR | NR | IELT not assessed | CIPE overall |
| Citalopram 20 mg/day (n = 30) NR | ||||||
| Rezakhaniha and Sirosbakht 201095 (the Islamic Republic of Iran) high risk | 4 weeks | Fluoxetine 40 mg/day | NR | NR | Stopwatch | NR |
| Citalopram 40 mg/day (total n = 110) | ||||||
| Fluoxetine, 43; Citalopram, 34 | ||||||
| In total 7/110 (70%) | ||||||
| Waldinger et al. 2004105 (the Netherlands) unclear risk | 4 weeks | Paroxetine 20 mg/day (n = 15) | IELT ≤ 1 minute on > 90% occasions | Lifelong | Stopwatch | Questionnaire |
| Clomipramine 25 mg/day (n = 15) | Symptom Checklist-90 (SCL-90) | |||||
| Paroxetine, 15/15 (100%) | Dutch translation of UK side effect scale | |||||
| Clomipramine, 15/15 (100%) | ||||||
| Weixing et al. 2012102 (China) unclear risk | 6 and 12 weeks | Fluoxetine 20 mg | NR | NR | Self-report | Sexual satisfaction |
| Fluoxetine 30 mg | ||||||
| Sertraline 50 mg | ||||||
| Sertraline 100 mg | ||||||
| Squeeze technique (total n = 190) | ||||||
| 104/190 (55%) completed | ||||||
Assessment of effectiveness: selective serotonin reuptake inhibitors – intravaginal ejaculatory latency time outcomes
Previous reviews have pooled data from single-arm crossover studies with separate treatment arm studies in a meta-analysis. 64,65 Data from these trials39,74,76,79,84 have not been included in any meta-analysis of SSRIs in this assessment report.
Intravaginal ejaculatory latency time – selective serotonin reuptake inhibitors compared with placebo or no treatment
Mean IELT data with variance estimates were available for four RCTs. 70–72,96 A high level of heterogeneity was observed across these trials (I2 = 99%, meta-analysis not undertaken). Three of the four trials70,72,96 demonstrated a significant improvement in IELT for citalopram compared with placebo after 8–12 weeks (all p < 0.00001). The p-value for the between-group difference for one trial comparing citalopram with no therapy71 was p < 0.00001 (Figure 7). Summary results for these, and all other meta-analyses, are presented in Table 13.
FIGURE 7.
Selective serotonin reuptake inhibitors compared with placebo or no treatment: forest plot of IELT outcomes. df, degrees of freedom; IV, inverse variance; M–H, Mantel–Haenzel; SD, standard deviation.
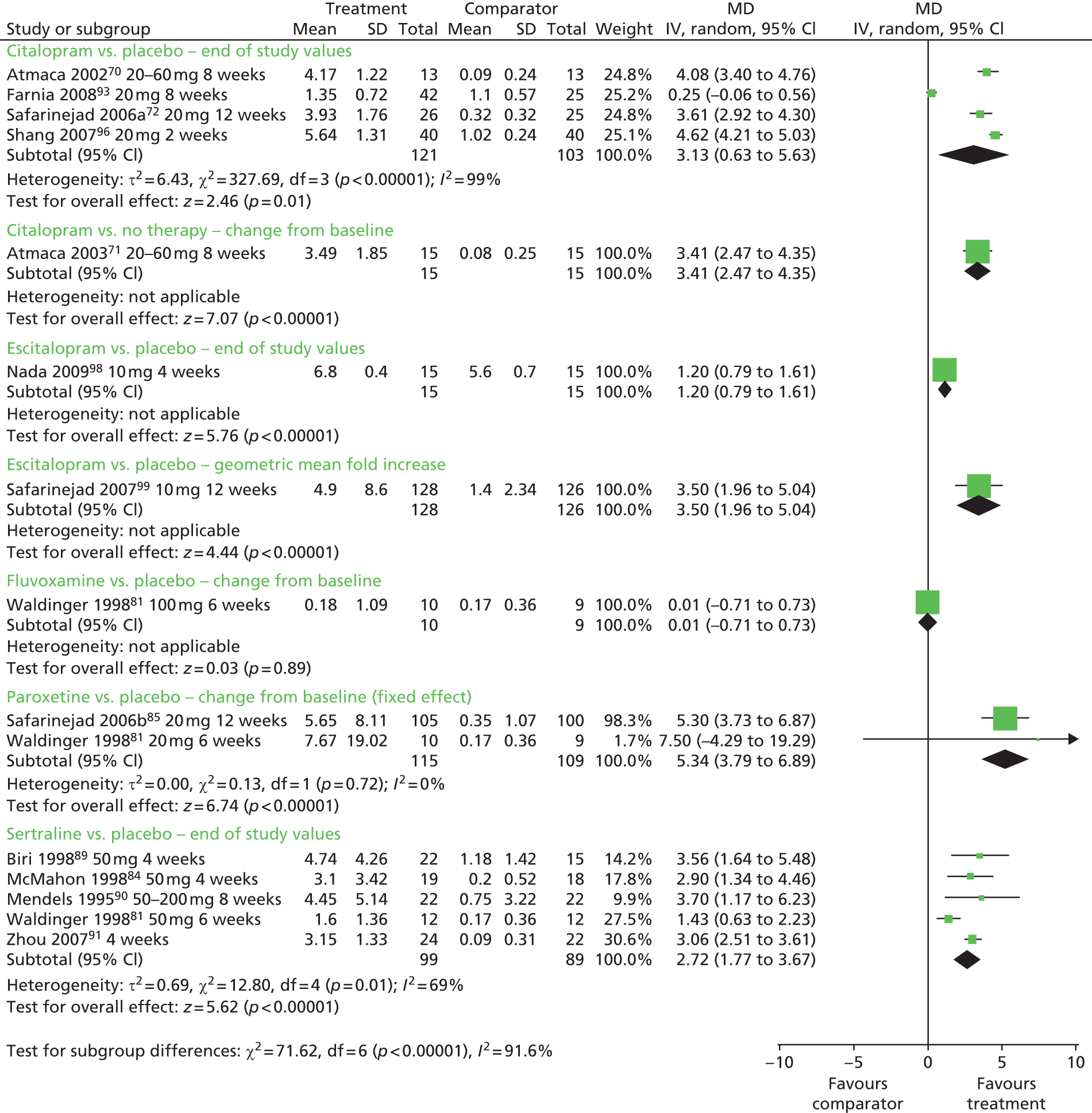
| Comparison | Outcome | Study duration | No. of RCTs | No. of participants | I 2 | Meta-analysis (model) | Effect estimate (MD) (95% CI) | Favours | p-value | |
|---|---|---|---|---|---|---|---|---|---|---|
| IELT | ||||||||||
| Citalopram vs. placebo | IELT (minutes) – end of study values | 2–12 weeks | 470,72,93,96 | 224 | 99% | Data not pooled | 4.08 (3.40 to 4.76) | Citalopram | < 0.00001 | |
| 0.25 (–0.06 to 0.56) | NS | 0.12 | ||||||||
| 3.76 (3.07 to 4.45) | Citalopram | < 0.00001 | ||||||||
| 4.62 (4.21 to 5.03) | Citalopram | < 0.00001 | ||||||||
| Citalopram vs. no therapy | IELT (minutes) – change from baseline | 8 weeks | 171 | 30 | N/A | N/A | 3.41 (2.47 to 4.35) | Citalopram | < 0.00001 | |
| Escitalopram vs. placebo | IELT (minutes) – end of study values | 4 weeks | 198 | 30 | N/A | N/A | 1.20 (0.79 to 1.61) | Escitalopram | < 0.00001 | |
| Escitalopram vs. placebo | IELT (minutes) – geometric mean fold increase | 12 weeks | 199 | 254 | N/A | N/A | 3.50 (1.96 to 5.04) | Escitalopram | < 0.00001 | |
| Fluoxetine vs. placebo | IELT (minutes) – end of study values | 4–12 weeks | 675,80,81,83,100,141 | 170 | N/A | Yes (fixed) | 2.41 (2.10 to 2.73) | Fluoxetine | < 0.00001 | |
| Fluvoxamine vs. placebo | IELT (minutes) – change from baseline | 6 weeks | 181 | 19 | N/A | N/A | 0.01 (–0.71 to 0.73) | NS | 0.98 | |
| Paroxetine vs. placebo | IELT (minutes) – change from baseline | 6–12 weeks | 281,85 | 70 | 0% | Yes (fixed) | 5.34 (3.79 to 6.89) | Paroxetine | < 0.00001 | |
| Paroxetine vs. clomipramine | IELT (minutes) – geometric mean fold increase | 4 weeks | 1105 | 30 | N/A | N/A | –2.29 (–2.97 to –1.61) | Clomipramine | < 0.00001 | |
| Sertraline vs. placebo | IELT (minutes) – end of study values | 4–8 weeks | 581,84,89–91 | 188 | 69% | Yes (random) | 2.72 (1.77 to 3.67) | Sertraline | < 0.00001 | |
| Comparison | Outcome | Study duration | No. of RCTs | Participants | Favours | |||||
| Other outcomes | ||||||||||
| Citalopram vs. placebo | Other effectiveness outcomes (various) | 4–12 weeks | 470,72,93,96 | Varies | Evidence from four RCTs suggests that sexual satisfaction and measures of clinical improvement are improved with citalopram | |||||
| Escitalopram vs. placebo | Other effectiveness outcomes (various) | 6–12 weeks | 294,99 | Varies | Evidence from one RCT99 suggests improved sexual satisfaction with escitalopram over placebo while another RCT94 suggests no difference from placebo on the Chinese Index of Sexual Function for PE scores | |||||
| Fluoxetine vs. placebo | Other effectiveness outcomes (various) | 6–8 weeks | 276,79,102 | Varies | Evidence from one crossover RCT79 suggests that sexual satisfaction is improved with fluoxetine over placebo, while evidence from another crossover and a RCT suggests improvements over sertraline and the squeeze technique76,102 | |||||
| Paroxetine vs. placebo | Other effectiveness outcomes (various) | 4–12 weeks | 285,104 | Varies | Two RCTs indicate that sexual satisfaction appears improved with paroxetine compared with placebo (significance levels unclear) | |||||
| Sertraline vs. placebo | Other effectiveness outcomes (various) | 4–8 weeks | 392,106,107 | Varies | Evidence from one RCT106 suggests significant improvement over placebo on AIPE; another reports improvements in ejaculation control.107 One RCT suggests no difference between sertraline and citalopram on IPE92 | |||||
The between-group difference in IELT in favour of escitalopram compared with placebo was significant for one RCT reporting end of study mean values98 and one reporting geometric mean fold increase99 (both p < 0.0001) (see Figure 7).
Meta-analysis of mean IELT (minutes) at 3–12 weeks’ follow-up, based on six RCT comparisons of fluoxetine at 20 mg or 40 mg daily, or 90 mg weekly (n = 170), displayed low heterogeneity (I2 = 0%). The pooled MD in IELT was 2.41 minutes, significantly favouring fluoxetine [MD (fixed effect); 95% CI 2.10 to 2.73 minutes; p < 0.00001] (Figure 8). Fluoxetine at 90 mg weekly was compared with 20 mg daily in one RCT. 77 IELT outcomes were reported without variance estimates or p-values. The between-group difference was reported as non-significant. For the comparison of fluoxetine alone compared with fluoxetine plus PDE inhibitor (tadalafil) reported in one RCT,141 refer to the section Phosphodiesterase-5 inhibitors.
FIGURE 8.
Selective serotonin reuptake inhibitors, fluoxetine compared with placebo: forest plot of IELT outcomes for end of study values. df, degrees of freedom; IV, inverse variance; SD, standard deviation.
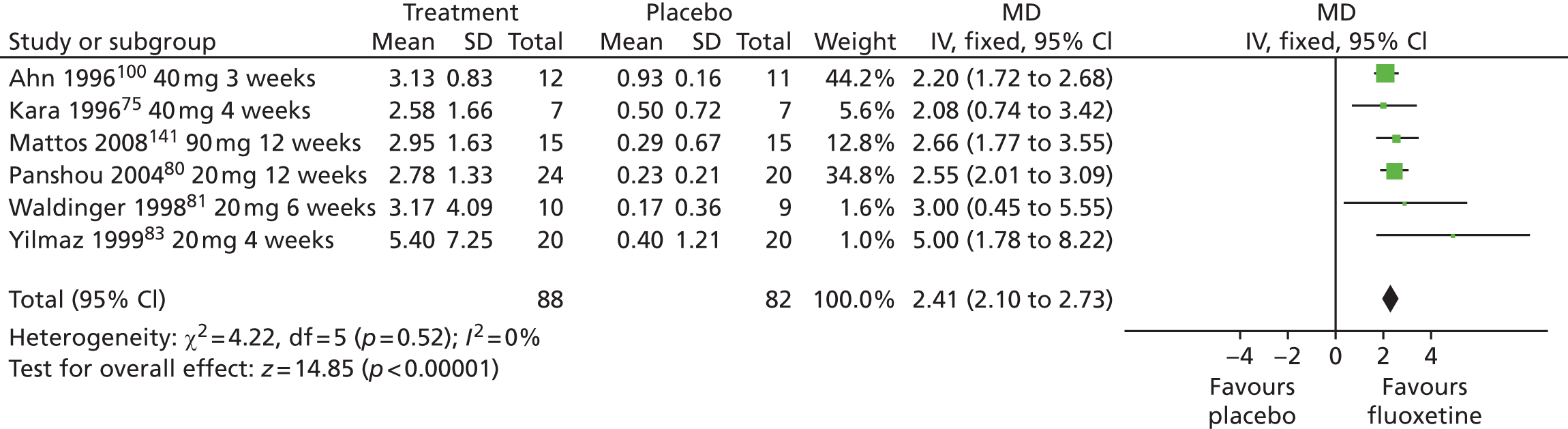
The between-group difference in change from baseline values after 6 weeks of treatment for one RCT comparing fluvoxamine with placebo81 was not significant (p = 0.98) (see Figure 7).
Meta-analysis of mean change from baseline IELT (minutes) at 6 or 12 weeks’ follow-up, based on two RCT comparisons of paroxetine at 20 mg (n = 70), displayed low heterogeneity (I2 = 0%). The pooled MD in IELT was 5.34 minutes, significantly favouring paroxetine [MD (fixed effect); 95% CI 3.79 to 6.89 minutes; p < 0.00001] (see Figure 7).
Meta-analysis of mean IELT (minutes) at 4, 6 or 8 weeks’ follow-up, based on five RCT comparisons of sertraline at 50 mg to 200 mg (n = 164), displayed moderate heterogeneity (I2 = 64%). The pooled MD in IELT was 2.72 minutes [MD (random effects); 95% CI 1.77 to 3.67 minutes; p < 0.00001] (see Figure 7).
Intravaginal ejaculatory latency time: selective serotonin reuptake inhibitors compared with other selective serotonin reuptake inhibitors or other treatments
Waldinger et al. 73 reported a fold increase in IELT for paroxetine 20 mg of 8.9-fold and for citalopram 20 mg of 1.8-fold. The fold was reported to be statistically significant increase for paroxetine (p < 0.001), but not for citalopram (p = 0.07). No variance estimates were reported.
The p-value for the between-group difference for one trial comparing a geometric mean fold increase between paroxetine and clomipramine105 was 2.29-fold [MD (random effects); 95% CI 1.61 to 2.97; p < 0.00001] in favour of clomipramine (figure not presented).
Assessment of effectiveness: selective serotonin reuptake inhibitors – other outcomes
Outcomes other than IELT were reported across the RCTs using a diversity of instruments (which were sometimes not reported) and outcome data. In a large proportion of the RCTs, a variance estimate for the outcome was not reported. Either p-values were not available or it was unclear if reported p-values were for between- or across-group comparisons (Table 14).
| RCT, duration | Treatment | Outcome measure | Results | Between-group difference significant |
|---|---|---|---|---|
| Citalopram vs. placebo or no therapy | ||||
| Atmaca et al. 2002,70 8 weeks | Citalopram 20–60 mg/day (n = 13) | CGI-I | Citalopram ‘much improved’, 4/13; (30.8%); ’very much improved’, 5/13 (38.5%). Placebo ‘much improved’, 1/13 (7.7%) | Unclear |
| Placebo (n = 13) | YSFI-II | Improved significantly with citalopram, compared with placebo (p-value NR) | Yes | |
| Sexual satisfaction | n/N ‘yes’: citalopram 9/13, placebo 1/13 | Unclear | ||
| Farnia et al. 2008,93 4 weeks | Citalopram 20 mg 4 hours precoitus (n = 49) | CIPE | CIPE between-group difference in IELT change from baseline at week 4, p = 0.002 | Yes |
| Placebo (n = 43) | ||||
| Safarinejad and Hosseini 2006,72 12 weeks | Citalopram 20 mg (n = 26) | IIEF: intercourse satisfaction domain | n/N ‘yes’: citalopram 23/26 (88.4%), placebo 10/25 (40.0%) | Yes |
| Placebo (n = 25) | Intercourse episodes per week | Significantly improved, citalopram (no p-value) | ||
| Shang et al. 2012,96 duration NR | Citalopram 20 mg/day (n = 40) | Sexual satisfaction | Mean (assume SD): citalopram – week 2, p < 0.01; week 4, p < 0.01 | Yes |
| Placebo (n = 40) | Placebo – week 2, p-value NR; week 4, p > 0.05 | |||
| Escitalopram vs. placebo | ||||
| Nada et al. 2012,94 2, 4 and 6 weeks’ treatment | Escitalopram 10 mg (n = 30) | CIPE overall score | Between-group difference: week 2, p = 0.51; week 4, p = 0.27; week 6, p = 0.32; 3-month post-treatment follow-up, p = 0.10 | No |
| Citalopram 20 mg (n = 30) | ||||
| Safarinejad 2007,99 12 weeks | Escitalopram 10 mg/day (n = 138) | Weekly coitus episodes | NR | |
| Placebo (n = 138) | IIEF intercourse satisfaction | Escitalopram: 12 weeks p = 0.01; 3 months p = 0.01; 6 months p = 0.01. Placebo: 12 weeks p = NS; 3 months p = NS; 6 months p = NS | Yes | |
| Sexual satisfaction | Between-groups: ‘satisfied’, p ≤ 0.001; ‘moderately satisfied’, p = NS; ‘dissatisfied’, p ≤ 0.001 | Yes | ||
| Fluoxetine vs. placebo | ||||
| Ahn et al. 1996,100 6 weeks | Fluoxetine 40 mg/day (n = 12) | Questionnaire assessing number of thrusts before ejaculation, frequency of coitus, libido and side effects of treatment | Number of patients with < 30/≥ 30 thrusts before ejaculation: fluoxetine, from baseline at 3 and at 6 weeks p < 0.05. Placebo change from baseline at 3 and at 6 weeks p > 0.05 | Yes |
| Placebo (n = 11) | ||||
| Novaretti et al. 2002,79 crossover 8 weeks | Fluoxetine 20 mg once daily | Sexual satisfaction | n/N ‘yes’: fluoxetine 34/50 (68%), placebo 5/50 (10%) | Unclear |
| Placebo once daily | Hamilton Anxiety and Depression Scale; Beck Depression Inventory | p-value between groups NR | ||
| Total n = 50 | NR | |||
| Fluoxetine vs. other treatments | ||||
| Arafa and Shamloul 2007,97 4 weeks | Fluoxetine 20 mg (n = 33) | AIPE | AIPE domains with change from baseline p < 0.05 all groups | Unclear |
| Escitalopram 10 mg (n = 37) | Frequency of intercourse | NR | ||
| Paroxetine 20 mg (n = 30) | ||||
| Culba et al. 2008,101 10 weeks | Fluoxetine 20 mg/day | IIEC | Patients who were treated with fluoxetine + tadalafil had better scores with both questionnaires | Unclear |
| Tadalafil + fluoxetine | PE question of CMASH questionnaire | Difference was NS compared with fluoxetine group. No data reported | ||
| Tadalafil 20 mg 2/weeks | ||||
| Placebo | ||||
| (Total n = 180) | ||||
| Kim and Seo 1998,76 each agent for 4 weeks, with 1-week washout | Fluoxetine 40 mg | Patient and partner sexual satisfaction: patient self-reported questionnaire | n/N ‘yes’: fluoxetine 23/36 (88.4%); sertraline 28/36 (77.7%); placebo 17/36 (47.2%); greater with clomipramine (NR) | Unclear |
| Sertraline 100 mg | p-value between groups NR | |||
| Clomipramine 50 mg | ||||
| Placebo | ||||
| Total n = 36 | ||||
| Murat Basar 1999,78 4 and 8 weeks | Fluoxetine 40 mg (n = 26) | The results were classified as unsuccessful, improvement and cure | Fluoxetine and sertraline, had the same efficacy. No data or p-value reported | Unclear |
| Sertraline 50 mg (n = 31) | ||||
| Weixing et al. 2012,102 6 and 12 weeks | Fluoxetine 20 or 30 mg | Sexual satisfaction | Sexual satisfaction was increased significantly in fluoxetine 30 mg. p-value NR | Unclear |
| Sertraline 50 or 100 mg | ||||
| Squeeze technique | ||||
| Total n = 104 | ||||
| Fluoxetine different doses | ||||
| Manasia et al. 2003,77 Duration NR | Fluoxetine 90 mg weekly (n = 40) | Sexual satisfaction ratings | Sexual satisfaction ratings did not significantly differ between the two groups. No data or p-value reported | Unclear |
| Fluoxetine 20 mg daily (n = 40) | ||||
| Paroxetine vs. placebo | ||||
| Khelaia et al. 2012,104 4 weeks | Paroxetine 20 mg/day (n = 26) | IIEF, intercourse satisfaction, overall satisfaction | Mean IIEF intercourse satisfaction scores | Unclear |
| Paroxetine 20 mg 2–3 hours precoitus (n = 28) | Mean IIEF overall satisfaction scores: p < 0.001, but unclear if change from baseline or for which group comparison | |||
| Placebo (n = 24) | ||||
| Safarinejad 2006,85 12 weeks | Paroxetine 20 mg (n = 113) | Sexual satisfaction | Sexual satisfaction assume n/N ‘yes’: paroxetine 97/105, placebo 30/100 | Unclear |
| Placebo (n = 112) | p-value for between-group difference NR | |||
| Dapoxetine 60 mg (n = 115) | ||||
| Sertraline vs. placebo | ||||
| Arafa and Shamloul 2007,106 crossover 4 weeks per treatment | Sertraline 50 mg/day | AIPE | Sertraline vs. baseline or placebo, p < 0.05 | Yes |
| Placebo | Frequency of intercourse – assessment method NR | Between-group (sertraline vs. placebo) difference in overall AIPE score, p < 0.001 | Yes | |
| Total n = 77 | Between-group (sertraline vs. placebo), change from other study phases, p > 0.05 | |||
| Mendels et al. 1995,90 8 weeks | Sertraline 50–200 mg (n = 22) | Patient and partner satisfaction measured using a numbered scale | Improved during the treatment period in the sertraline group. No data or p-value reported | Unclear |
| Placebo (n = 22) | ||||
| Sertraline vs. other treatments | ||||
| Abdel-Hamid et al. 2001,39 4 weeks | Sertraline 50 mg | EDITS (scale 0–5): sexual satisfaction score | Unclear if reported values are means or medians. No variance estimates or p-values reported | Unclear |
| Paroxetine 20 mg | Arabic Anxiety Inventory (scale 0–30) | Unclear if reported values are means or medians. No variance estimates or p-values reported | ||
| Clomipramine 25 mg | ||||
| Sildenafil 50 mg | ||||
| Squeeze technique | ||||
| Total n = 31 | ||||
| Akgül et al. 200892 | Sertraline 50 mg/day (n = 40) | IPE | Between-group difference at 8 weeks: p = 0.50 | No |
| Citalopram 20 mg/day (n = 40) | ||||
| Tuncel et al. 2008,107 treatment was for 2 months | Sertraline 50 mg/day (n = 23) | Clinical responses (assume control of ejaculation), self-assessed | Patients reporting ‘no change’, ‘improvement’, ‘under control’. All three treatments ‘superior to placebo’: p = 0.001 | Yes compared with placebo |
| Clomipramine 25 mg/day (n = 20) | No significant difference in efficacy between ‘medical treatments’: p = 0.537 | |||
| Terazosin 5 mg/day (n = 25) | ||||
| Placebo (n = 22) | ||||
| Studies with no data on other outcomes reported | ||||
| RCT | Treatments | |||
| Citalopram | ||||
| Atmaca et al. 200371 | Citalopram, no therapy | |||
| Escitalopram | ||||
| Nada et al. 200998 | Escitalopram, placebo | |||
| Fluoxetine | ||||
| Haensel et al. 199874 | Fluoxetine, placebo | |||
| Kara et al. 1996,75 Panshou and Xie 2004,80 Yilmaz et al. 199983 | Fluoxetine, stop–start, placebo | |||
| Kolomaznik et al. 200241 | Fluoxetine, tadalafil, fluoxetine + tadalafil, placebo | |||
| Mattos et al. 2008141 | Fluoxetine, citalopram | |||
| Fluvoxamine | ||||
| Rezakhaniha et al. 201095 | Fluvoxamine, fluoxetine, paroxetine, sertraline, placebo | |||
| Paroxetine | ||||
| Waldinger et al. 199787 | Paroxetine 20 mg, paroxetine 40 mg | |||
| Waldinger et al. 199881 | Paroxetine, placebo | |||
| McMahon and Touma 1999,84 Waldinger et al. 199486 | Paroxetine, clomipramine | |||
| Waldinger et al. 2004105 | Paroxetine, citalopram | |||
| Waldinger et al. 200173 | Paroxetine, sertraline, nefazodone, placebo | |||
| Waldinger et al. 200182 | Paroxetine, mirtazapine | |||
| Waldinger et al. 200386 | Paroxetine different doses | |||
| Sertraline | ||||
| Giammusso et al. 1997,103 Waldinger et al. 1997,87 Biri et al. 1998,89 McMahon 1998,166 Zhou 200791 | Sertraline, placebo | |||
Sexual satisfaction and intercourse satisfaction appeared improved in two RCTs compared with placebo. 92,96 The number of intercourse episodes per week also improved after treatment with citalopram in one RCT. 72 The proportion of patients reported as ‘much improved’ and ‘very much improved’ on a subjective measure of clinical improvement was greater with citalopram than placebo in one RCT. 70 One trial reported a significant between-group difference in favour of citalopram compared with placebo on the CIPE93 (see Table 14).
There was no between-group difference in escitalopram compared with placebo on the CIPE overall score at weeks 2, 4 or 6 in one RCT. 98 Intercourse satisfaction was reported as significantly improved at 3 and 6 months with escitalopram in one RCT99 (see Table 14).
The number of thrusts before ejaculation appeared greater with fluoxetine than placebo in one RCT. 100 Sexual satisfaction appeared improved with fluoxetine in two crossover RCTs compared with placebo. 78,79 There was no apparent between-group difference in sexual satisfaction between fluoxetine 20 mg daily or 90 mg weekly. 77 One RCT suggested an improvement in sexual satisfaction with fluoxetine 30 mg, compared with 20 mg, sertraline at 50 mg or 100 mg, or the squeeze technique. 102 One RCT suggested that there is no difference in change on the AIPE between fluoxetine and escitalopram97 (see Table 14).
No data were available.
Sexual satisfaction and IIEF satisfaction scores appeared improved with paroxetine when compared with placebo in two RCTs85,104 (see Table 14).
A significant between-group difference between sertraline and placebo on the AIPE and the frequency of intercourse was reported in one crossover study. 106 Patient and partner satisfaction improved during the treatment period in the sertraline group in one RCT. 90 A significant difference between sertraline and placebo on ejaculation control was reported by one RCT. 107 The same RCT reported that sertraline was comparable to both clomipramine and terazosin on this outcome. One RCT reported no significant between-group difference in sertraline or citalopram on the IPE92 (see Table 14).
Assessment of safety: selective serotonin reuptake inhibitors – adverse events summarised by existing reviews
The systematic review by Huang et al. 65 reported a summary table of the incidence of AEs for citalopram, fluoxetine, paroxetine and sertraline across the included studies. These data are adapted in Table 15. From these data, AEs affecting > 5% of patients appear to be:
-
citalopram: insomnia and nausea
-
fluoxetine: headache, insomnia, nausea, somnolence, erectile dysfunction, libido decrease
-
paroxetine: nausea and diarrhoea
-
sertraline: headache, dry mouth, dizziness, insomnia, nausea, somnolence, diarrhoea, anejaculation.
| Treatment | AE | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Headache, n/N (%) | Dry mouth, n/N (%) | Dizziness, n/N (%) | Insomnia, n/N (%) | Nausea n/N (%) | Somnolence, n/N (%) | Diarrhoea, n/N (%) | Erectile dysfunction, n/N (%) | Anejaculation, n/N (%) | Libido decrease, n/N (%) | |
| Citalopram | 6/167 (3.6) | 7/167 (4.2) | – | 3/44 (6.8) | 14/167 (8.4) | – | 5/138 (3.6) | 0/167 (0.0) | 0/29 (0.0) | – |
| Fluoxetine | 8/59 (13.6) | 3/86 (3.5) | 1/50 (2.0) | 4/59 (6.8) | 7/60 (11.7) | 24/101 (23.8) | – | 3/36 (8.3) | 2/44 (4.5) | 5/70 (7.1) |
| Paroxetine | 2/147 (1.4) | – | 2/105 (1.9) | 0/105 (0.0) | 8/105 (7.6) | – | 8/105 (7.6) | 1/147 (0.7) | 3/147 (2.0) | 5/147 (3.4) |
| Sertraline | 9/48 (18.8) | 10/84 (11.9) | 3/26 (11.5) | 3/26 (11.5) | 5/62 (8.1) | 15/121 (12.4) | 9/48 (18.8) | 4/99 (4.0) | 8/89 (9.0) | 0/37 (0.0) |
However, these data were reported by Huang et al. 65 as the overall number of incidents across included studies by AE as opposed to being reported for each included study. Therefore, it is unclear which of the included RCTs and single-arm randomised crossover trials contribute to the numbers in each AE. Thus, the differences in event rates may reflect the differences across the studies included by Huang et al. 65
Assessment of safety: selective serotonin reuptake inhibitors – adverse events for individual randomised controlled trials
Adverse event data were not available for 1441,71,72,74,84–88,90,91,94,96,98 out of the 4239,41,70–107,141,166 included RCTs evaluating SSRIs (Table 16). When AE data were reported, it was often unclear how many patients suffered AEs, what the AEs were or which group the AEs related to. Reporting of how many patients withdrew owing to AEs was limited across trials.
| RCT, duration | Treatment | AEs |
|---|---|---|
| Citalopram vs. placebo or no therapy | ||
| Atmaca et al. 2002,70 8 weeks | Citalopram 20–60 mg (n = 13) | Nausea and headache were reported in three subjects. Unclear which group |
| Placebo (n = 13) | ||
| Atmaca et al. 2003,71 8 weeks | Citalopram 20–60 mg (n = 15) | NR |
| No therapy (n = 15) | ||
| Farnia et al. 2008,93 4 weeks | Citalopram 20 mg (n = 49) | Twelve patients overall left the study (seven citalopram, five placebo). Five owing to headache and nausea (n by group NR). No other AE data reported |
| Placebo (n = 43) | ||
| Safarinejad and Hosseini 200672 | Citalopram vs. placebo | NR |
| Shang et al. 201296 | Citalopram vs. placebo | Treatment duration NR |
| Escitalopram vs. placebo | ||
| Nada et al. 2009,98 1 month | Escitalopram 10 mg (n = 15) | NR |
| Placebo (n = 15) | ||
| Nada et al. 2012,94 2, 4 and 6 weeks’ treatment | Escitalopram 10 mg (n = 30) | NR |
| Citalopram 20 mg (n = 30) | ||
| Safarinejad 2007,99 12 weeks | Escitalopram 10 mg (n = 138) | Escitalopram – 12/128 (9.4%) treatment-related AEs: nausea, 6/128 (4.7%); headache, 5/128 (3.9%); dry mouth, 4/128 (3.1%); diarrhoea, 4/128 (3.1%). Insomnia, drowsiness and dizziness were reported by < 1%. Four patients (3.1%) withdrew because of AEs (nausea, two; diarrhoea, one; headache, one) |
| Placebo (n = 138) | Placebo – 7/128 (5.5%) treatment-related AEs. Erectile dysfunction, 3/126 (2%). Two (1.6%) withdrew | |
| More AEs with escitalopram (p = 0.04) | ||
| Fluoxetine vs. placebo | ||
| Ahn et al. 1996,100 6 weeks | Fluoxetine 40 mg (n=12) | n/N (%) patients experiencing AEs: mild fatigue or yawning, 3/12 (25%); severe fatigue, 2/12 (16.7%); gastrointestinal discomfort, 0/12 (0%) |
| Placebo (n = 11) | n/N (%) patients experiencing AEs: mild fatigue or yawning, 0/11 (0%); severe fatigue, 1/11 (9.1%); gastrointestinal discomfort, 1/11 (9.1%) | |
| Haensel et al. 1998,74 4-week periods | Fluoxetine 10 mg | NR |
| Placebo (total n = 15) | ||
| Kara et al. 1996,75 4 weeks | Fluoxetine 40 mg (n = 9) | Two patients stopped because of side effects. Side effects were not described and it was unclear to which group these patients belonged |
| Placebo (n = 8) | ||
| Novaretti et al. 2002,79 crossover 8 weeks | Fluoxetine 20 mg once daily | Drowsiness (30%), headache (14%), insomnia (6%), decreased libido (4%), dry mouth (2%), dizziness (2%). Unclear if number of events or patients. Significant differences from placebo were noted |
| Placebo once daily (total n = 50) | ||
| Panshou and Xie 2004,80 12 weeks | Fluoxetine 20 mg (n = 24) | n/N experiencing AEs: fluoxetine, 7/24 (29%); placebo, 0/20 (0%) |
| Placebo (n = 20) | ||
| Yilmaz et al. 1999,83 | Fluoxetine 20 mg d (n = 20) | n/N experiencing AEs: fluoxetine, 10/20 (50%); placebo, 1/20 (5%) |
| Placebo (n = 20) | ||
| Fluoxetine vs. other treatments | ||
| Arafa and Shamloul 2007,97 4 weeks | Fluoxetine 20 mg (n = 33) | Drowsiness, anorexia and insomnia occurred in three patients on fluoxetine and three patients on escitalopram. Five patients on paroxetine complained of somnolence |
| Escitalopram 10 mg (n = 37) | ||
| Paroxetine 20 mg (n = 30) | ||
| Culba et al. 2008,101 10 weeks | Fluoxetine | Minor side effects due to tadalafil and fluoxetine were temporary. No data reported |
| Tadalafil + fluoxetine | ||
| Tadalafil | ||
| Placebo (total n = 180) | ||
| Kim and Seo 1998,76 each agent 4 weeks, 1-week washout | Fluoxetine 40 mg | Percentage experiencing AEs: fluoxetine 40 mg, 13%; sertraline 100 mg, 12%; clomipramine 50 mg, 23%; placebo, NR. p-value for clomipramine compared with sertraline and fluoxetine, p < 0.05. No other p-values reported |
| Sertraline 100 mg | ||
| Clomipramine 50 mg | ||
| Placebo (total n = 36) | ||
| Fluoxetine vs. other treatments | ||
| Kolomaznik et al. 2002,41 8 weeks | Fluoxetine | NR |
| Stop–start technique | ||
| Placebo (total n = 93) | ||
| Mattos et al. 2008,141 4 weeks | Fluoxetine 90 mg/week (n = 15) | Fluoxetine: yawning and somnolence (three patients), asthenia (three patients), nausea (one patient) |
| Tadalafil 20 mg daily + fluoxetine 90 mg (n = 15) | Fluoxetine + tadalafil: yawning and somnolence (three patients), nausea (two patients) palpitation (one patient), muscle soreness (one patient) | |
| Tadalafil (n = 15) | Tadalafil: headache (three patients), facial redness (two patients), palpitations (two patients) | |
| Placebo (n = 15) | ||
| Murat Basar et al. 1999,78 4 and 8 weeks | Fluoxetine 40 mg (n = 26) | Sertraline, fluoxetine had the same side effects. No data or p-value reported |
| Sertraline 50 mg (n = 31) | ||
| Rezakhaniha and Sirosbakht 2010,95 4 weeks | Fluoxetine 40 mg | Five patients withdrew owing to drug side effects such as headache, dizziness, insomnia and diarrhoea (NR which group) |
| Citalopram 40 mg d | ||
| Weixing et al. 2012,102 6 and 12 weeks | Fluoxetine 20 mg | AEs with fluoxetine and sertraline were drowsiness, headache, insomnia and diarrhoea. No data or p-values reported |
| Fluoxetine 30 mg | ||
| Sertraline 50 mg | ||
| Sertraline 100 mg | ||
| Squeeze technique (total n = 104) | ||
| Fluoxetine different doses | ||
| Manasia et al. 2003,77 duration NR | Fluoxetine 90 mg weekly (n = 40) | The occurrence of AEs did not significantly differ between the two groups. No data or p-value reported |
| Fluoxetine 20 mg daily (n = 40) | ||
| Mattos et al. 2008,141 4 weeks | Fluoxetine 90 mg/week (n = 15) | Fluoxetine: yawning and somnolence (three patients), asthenia (three patients), nausea (one patient) |
| Tadalafil 20 mg daily + fluoxetine 90 mg (n = 15) | Fluoxetine + tadalafil: yawning and somnolence (three patients), nausea (two patients) palpitation (one patient), muscle soreness (one patient) | |
| Tadalafil (n = 15) | Tadalafil: headache (three patients), facial redness (two patients), palpitations (two patients) | |
| Placebo (n = 15) | ||
| Fluvoxamine vs. other treatments | ||
| Waldinger et al. 199881 | Fluoxetine 20 mg (n = 12) | There were no statistically significant differences between the active treatment groups and the placebo group with respect to non-sexual side effects, including nausea and headache. No data or p-value reported |
| Fluvoxamine 100 mg (n = 12) | ||
| Paroxetine 20 mg (n = 12) | ||
| Sertraline 50 mg (n = 12) | ||
| Placebo (n = 12) (all once daily) | ||
| Paroxetine vs. placebo | ||
| Khelaia et al. 2012,104 4 weeks | Paroxetine 20 mg (n = 26) | ‘Drug related side effects’ were headache, drowsiness, nausea and dry mouth, but were mild an self-limited – n by group NR. Decreased libido was reported by four patients in the paroxetine daily group |
| Paroxetine on demand 20 mg (n = 28) | ||
| Placebo (n = 24) | ||
| McMahon and Touma 1999,84 crossover (single-arm), duration unclear | Study I: paroxetine 20 mg vs. placebo (total n = 26) | No AEs reported with paroxetine |
| Study II: paroxetine 20 mg vs. placebo (total n = 42) | ||
| Safarinejad 200685 | Paroxetine vs. dapoxetine vs. placebo | NR |
| Waldinger et al. 199486 | Paroxetine vs. placebo | NR |
| Paroxetine vs. other treatments | ||
| Waldinger et al. 200173 | Paroxetine 20 mg (n = 15) | AEs were not significantly different between the treatment groups. No data or p-value reported |
| Citalopram 20 mg (n = 15) | One patient discontinued on each treatment (two in total) | |
| Waldinger et al. 200182 | Paroxetine 20 mg (n = 12) | There were no statistically significant differences between the active treatment groups and the placebo group with respect to non-sexual side effects. No data or p-value reported |
| Sertraline 50 mg (n = 12) | Five did not complete because of side effects (paroxetine, three; sertraline, one; nefazodone, one) | |
| Nefazodone 400 mg (n = 12) | ||
| Placebo (n = 12) | ||
| Paroxetine vs. other treatments | ||
| Waldinger et al. 200388 | Paroxetine (n = 12) | NR |
| Mirtazapine (n = 12) | ||
| Waldinger et al. 2004,105 4 weeks | Paroxetine 20 mg (n = 15) | Difficulty concentrating, fatigue, sleepiness, restless, yawning, tremor, dry mouth, nausea, vomiting, loose stools, constipation, dizziness, perspiration, headache, decreased libido, difficulty attaining and maintaining erection. Six (20%) did not complete study: three owing to side effects (one on paroxetine, two on clomipramine) and three for non-medical/logistic reasons. Two drop-outs in first week, four in second week. Significant between-group differences in non-sexual side effects of treatment: day 1 sleepiness (more with paroxetine), p < 0.005; day one yawning (more with paroxetine), p < 0.05; day 2 nausea (more with clomipramine), p < 0.05 |
| Clomipramine 25 mg (n = 15) | ||
| Paroxetine different doses | ||
| Giammusso et al. 1997,103 3, 6 and 9 months | Paroxetine 20 mg (n = 28) | Paroxetine 20 mg – one patient withdrew from study owing to AEs (reported as ‘asentia’, unclear) |
| Paroxetine 20 mg 10 mg (n = 34) | Paroxetine 10 mg – non-serious AEs: nausea, sweating, reduced libido, drowsiness (n NR) | |
| Waldinger et al. 199787 | Paroxetine 20 mg vs. 40 mg | NR |
| Sertraline vs. placebo | ||
| Arafa and Shamloul 2007,106 crossover 4 weeks per treatment | Sertraline 50 mg | The authors report that sertraline was generally well tolerated. Most side effects were minor and none prompted withdrawal from the study. Drowsiness and anorexia occurred in one patient out of 47 (0.7%) patient. Two patients (1.4%) experienced minor gastrointestinal upset |
| Placebo (total n = 77) | ||
| Biri et al. 1998,89 8 weeks | Sertraline 50 mg (n = 22) | AEs not significantly different between groups. No data or p-value reported. After treatment with sertraline was discontinued, PE returned in 86.36% of patients |
| Placebo (n = 15) | ||
| McMahon and Touma 199984 | Sertraline vs. placebo | NR |
| Mendels et al. 199590 | Sertraline vs. placebo | NR |
| Zhou 200791 | Sertraline vs. placebo | NR |
| Sertraline vs. other treatments | ||
| Abdel-Hamid et al. 2001,39 4 weeks | Sertraline 50 mg | Headache, flushing and nasal congestion: 18% of participants in the sildenafil group (n NR). The incidence of side effects was similar among groups |
| Paroxetine 20 mg | ||
| Clomipramine 25 mg | ||
| Sildenafil 50 mg | ||
| Squeeze technique (total n = 31) | ||
| Akgül et al. 2008,92 8 weeks | Sertraline 50 mg (n = 40) | No serious AEs were detected in any of the patients. 3/40 patients (7.5%) in the citalopram group and 2/40 (5.0%) in the sertraline group had mild nausea at the beginning of the treatment |
| Citalopram 20 mg (n = 40) | ||
| Tuncel et al. 2008,107 2 months | Sertraline 50 mg (n=23) | n/N (%) reporting AEs were as follows. Clomipramine – headache, 8/23 (34.8%); hypotension, 1/23 (4%); drowsiness, 2/23 (8.6%); ejaculation disorder, 0/23 (0%). Sertraline – headache, 5/20 (25%); hypotension, 0/20 (0%); drowsiness, 3/20 (15%); ejaculation disorder, 0/20 (0%). Terazosin – headache, 5/25 (20%); hypotension, 3/25 (12%); drowsiness, 0/25 (0%); ejaculation disorder, 2/25 (8%). Placebo – headache, 2/22 (9.1%); hypotension, 0/22 (0%); drowsiness, 0/22 (0%); ejaculation disorder, 0/22 (0%). No significant differences between the ‘medical treatment groups’ in AEs – p = 0.204 |
| Clomipramine 25 mg (n=20) | ||
| Terazosin 5 mg (n = 25) | ||
| Placebo (n = 22) | ||
Nausea and headache were reported in two RCTs evaluating citalopram. 70,91 However, between-group differences with placebo groups were unclear.
One RCT reported that escitalopram was associated with nausea, headache, dry mouth, diarrhoea, insomnia, drowsiness and dizziness and that significantly more AEs were experienced with escitalopram than with placebo. 99
A significant between-group difference compared with placebo in drowsiness, headache, insomnia, decreased libido, dry mouth and dizziness were reported by one crossover trial. 79 In one RCT,100 more patients treated with fluoxetine than with placebo experienced mild/severe fatigue and yawning. One crossover RCT reported that significantly more AEs were experienced with clomipramine than fluoxetine,76 and one RCT reported that both fluoxetine and sertraline caused the same type of AEs. 78
One trial reported that there were no statistically significant differences between fluvoxamine, fluoxetine, paroxetine and sertraline in non-sexual side effects, including nausea and headache. 81
One RCT reported paroxetine-associated AEs of headache, drowsiness, nausea and dry mouth,104 one reported that AEs were not significantly different between paroxetine and citalopram,73 one reported that AEs were not significantly different between paroxetine and sertraline,82 and one reported patients on paroxetine experiencing sleepiness and yawning early in treatment, whereas more patients on clomipramine experienced nausea. 105
Assessment of effectiveness: selective serotonin reuptake inhibitors – evidence summary
The current evidence base for SSRIs in the treatment of PE comprises 26 RCTs39,41,70–91,141,166 captured in seven52,64–69 low methodological quality systematic reviews and 16 further RCTs,92–107 two95,103 of which are at high risk of bias and 1492–94,96–102,104–107 care onsidered at unclear risk of bias.
Evidence from three70,72,96 out of four70,72,93,96 separate RCTs suggests that citalopram is significantly more effective than placebo in increasing IELT [MD 0.25 minutes (95% CI –0.06 to 0.56 minutes) to 4.62 minutes (95% CI 4.21 to 5.03 minutes); p < 0.00001]. However, a high level of heterogeneity is evident across these four trials. Citalopram is significantly more effective than no therapy (one RCT,71 30 participants). Evidence from four separate RCTs suggests that sexual satisfaction and measures of clinical improvement are improved with citalopram. 70,72,93,96 AEs with citalopram appear to be nausea, headache, insomnia and dry mouth although the magnitude and severity are unclear.
Evidence from one RCT98 reporting end of study mean values (30 participants) and one RCT99 reporting fold increase (i.e. by how many ‘fold’ the value in minutes at baseline had increased) (254 participants) indicates that escitalopram is significantly more effective than placebo in increasing IELT [MD 1.20 minutes (95% CI 0.79 to 1.61 minutes), p < 0.00001; geometric mean 3.50 minutes (95% CI 1.96 to 5.04 minutes), p < 0.00001]. Evidence from one RCT99 suggests that sexual satisfaction is improved with escitalopram. Evidence from one RCT suggests that there is no significant between-group difference for escitalopram compared with placebo on the Chinese Index of Sexual Function for PE scores. 94 Evidence from one RCT99 indicates that nausea, headache, dry mouth, diarrhoea, insomnia, drowsiness and dizziness are reported more with escitalopram than with placebo.
Pooled effects across six RCTs75,80,81,83,100,141 (170 participants) demonstrates that fluoxetine daily or weekly is significantly more effective than placebo at increasing IELT over 4–12 weeks [MD 2.41 minutes (95% CI 2.10 to 2.73 minutes); p < 0.00001]. Evidence from one RCT suggests that sexual satisfaction is improved with fluoxetine compared with placebo. 79 One RCT102 suggests that sexual satisfaction is improved with fluoxetine 30 mg compared with either fluoxetine 20 mg, sertraline at 50 mg or 100 mg, or the squeeze technique. There is evidence from one RCT77 that there is no apparent between-group difference in sexual satisfaction between fluoxetine 20 mg daily and 90 mg weekly. Evidence from one crossover79 indicates that fluoxetine is associated with more drowsiness, headache, insomnia, decreased libido, dry mouth and dizziness than placebo. Another crossover RCT78 indicates that both fluoxetine and sertraline cause the same AEs, and a further crossover RCT indicates that more AEs are experienced with clomipramine than with fluoxetine76 but that satisfaction ratings are greater with clomipramine. Evidence summarised by one systematic review65 suggests that > 5% patients treated with fluoxetine report headache, insomnia, nausea, somnolence, erectile dysfunction and libido decrease. However, the review is of overall low methodological quality.
Evidence from one RCT95 (19 participants) indicates that there is no significant difference between fluvoxamine and placebo in increase in IELT and that there is no significant differences between fluvoxamine, fluoxetine, paroxetine and sertraline in non-sexual side effects, including nausea and headache.
Pooled evidence across two RCTs81,85 (70 participants) demonstrates that paroxetine 20 mg is significantly more effective than placebo at increasing IELT over 6–12 weeks [MD 5.34 minutes (95% CI 3.79 to 6.89 minutes); p < 0.00001]. However, evidence from one RCT105 (30 participants) indicates that clomipramine is significantly more effective than paroxetine [2.29 minutes (95% CI 1.61 to 2.97 minutes); p < 0.00001]. Two RCTs85,104 indicate that sexual satisfaction and IIEF satisfaction scores appear improved with paroxetine compared with placebo. Paroxetine-associated AEs include headache, drowsiness, nausea and dry mouth. One RCT82 indicates that there is no significant difference in the occurrence of these events between paroxetine and sertraline. One RCT105 suggests that more patients on clomipramine than those on paroxetine experience nausea early in treatment.
Pooled effects across five RCTs79,81,84,89,90 (188 participants) suggest that sertraline 50 mg is significantly more effective than placebo at increasing IELT over 4–8 weeks [MD 2.72 minutes (95% CI 1.77 to 3.67 minutes); p < 0.00001]. However, a moderate level of heterogeneity is evident across these trials. Evidence from one RCT107 suggests a significant improvement in ejaculation control with sertraline compared with placebo. Evidence from one RCT106 also suggests a significant improvement over placebo on the AIPE. One RCT106 suggests that there is no significant difference between sertraline or citalopram on the IPE. One RCT90 suggests that both patient and partner satisfaction improved are improved with sertraline. One RCT107 indicates no significant differences between sertraline, clomipramine and terazosin in AEs including headache, hypotension, drowsiness and ejaculation disorder. Evidence summarised by one systematic review65 suggests that > 5% patients treated with sertraline report headache, dry mouth, insomnia, nausea, somnolence, diarrhoea and anejaculation. However, the review is of overall low methodological quality.
Selective serotonin reuptake inhibitors: evidence summary
There is evidence which suggests that, with the exception of fluvoxamine, SSRIs are more effective than placebo at increasing IELT in men with PE. Sexual satisfaction measures and other secondary outcomes also appear improved. However, the current evidence base comprises studies captured in low methodological quality reviews and further RCTs that are of unclear and high risk of bias. In addition, the evidence base is limited in terms of assessing the benefits of one SSRI compared with another SSRI in treating PE. AE data suggest that SSRIs are associated with a number of AEs. However, the choice of an appropriate SSRI for the treatment of PE in terms of a safety profile is unclear. Furthermore, long-term treatment effects and AE outcomes in the treatment of men with PE are not fully evaluated in the current literature. The RCTs evaluating SSRIs identified for inclusion in this assessment report evaluated treatments over 4 to 12 weeks and none reported a long-term follow-up or the effects when treatment with SSRIs is withdrawn. This, coupled with the limited treatment comparisons evaluated by RCTs assessing SSRIs (mainly placebo), prohibits any definitive conclusions regarding an appropriate choice of SSRI in terms of efficacy and safety for the treatment of men with PE.
Selective serotonin reuptake inhibitors licensed for premature ejaculation (dapoxetine)
Characteristics of included studies: dapoxetine
Dapoxetine as the primary treatment of investigation was evaluated by four systematic reviews of effectiveness,108–110,169 two of which pooled data in a meta-analysis. 108,110 One systematic review evaluated the risk–benefit assessment of dapoxetine including withdrawal data from Phase III trials,111 one review evaluated dapoxetine Phase II trials including pharmacokinetic and safety data112 and two further effectiveness reviews of SSRIs included studies of dapoxetine and other SSRIs. 65,67 One further RCT was identified that evaluated dapoxetine and dapoxetine plus a PDE5 inhibitor (mirodenafil). 120
Of four systematic reviews of effectiveness of dapoxetine, one was undertaken in Australia,169 one was undertaken in Ireland109 and two were undertaken in China. 108,110 The overall AMSTAR quality score was 1 out of 11 in one of the reviews,108 2 out of 11 in two of the reviews,109,110 and 4 out of 11 in one review. 167 Details of the review type, the databases searched and dates, included RCTs and the AMSTAR points awarded to these reviews of effectiveness are presented in Table 17. The two reviews of SRRIs that included some of the dapoxetine trials both scored 0 out of 11. 65,67 Details of these reviews are presented in Table 11 in the Characteristics of included studies: selective serotonin reuptake inhibitors section of this assessment report. Full details of the AMSTAR assessment for these and all other include reviews are presented in Appendix 4. The search methodology and inclusion criteria for studies varied across these. The two reviews including a meta-analysis108,110 both included different dosing arms from studies separately in the meta-analysis, but included the comparator arm (placebo) against each dosing arm, in effect counting participants twice in the analysis.
| Author (country), review type | Databases searched and dates | Included RCTs relevant to this section | AMSTAR review quality assessment |
|---|---|---|---|
| Luo et al. 2012108 (China), systematic and meta-analysis | PubMed, BIOSIS Previews (now part of the Web of Knowledge), The Cochrane Library, CNKI, Wangfang Database searched to 2011 | Buvat et al. 2009,113 Kaufman et al. 2009,116 McMahon et al. 2010,170 Pryor et al. 2006118 | AMSTAR score, 1/11:
|
| McCarty and Dinsmore 2012109 (Ireland), systematic review | PubMed, the Cochrane Database of Systematic Reviews, NHS Evidence and NICE to August 2011. Start date not reported | Buvat et al. 2009,113 Kaufman et al. 2009,116 McMahon et al. 2010,170 Safarinejad 2008119 | AMSTAR score, 2/11:
|
| McMahon 2012169 (Australia), systematic review | MEDLINE, Web of Science, PICA and EMBASE 1993 to April 2012 | Phase II studies: Hellstrom et al. 2004,114 Hellstrom et al. 2005115 Phase III studies: Buvat et al. 2009,113 Kaufman et al. 2009,116 McMahon et al. 2010,170 Pryor et al. 2006118 | AMSTAR score, 4/11:
|
| Wang et al. 2010110 (China) systematic and meta-analysis | The Cochrane Library, MEDLINE, EMBASE, CNKI, CBM, Chinese Science and Technology Periodical Database (VIP) from 1979 to 2009 | Buvat et al. 2009,113 Kaufman et al. 2008,116 Pryor et al. 2006,118 Safarinejad 2006,85 Safarinejad 2008119 | AMSTAR score, 2/11:
|
The reviews above varied in terms of which RCTs they included. In total, eight RCT85,113–116,118,119,170 reports (one118 integrating data from two RCTs) (total n = 6968) were included in at least one review of effectiveness. Seven RCTs were reported as being Phase III RCTs,85,113,116,118,119,170 (Pryor et al. 118 is an integrated analysis of two RCTs) and two RCTs as Phase II studies. 114,115 The IELT assessment method within the RCTs was not reported by any of the reviews. Duration of the RCTs included in these reviews was 2–4 weeks for the two Phase II trials and 9–24 weeks for the Phase III trials. The majority of the RCTs included within the reviews evaluated one or more dose level of dapoxetine compared with placebo. Only one RCT also evaluated paroxetine;85 however, no data for this comparison were reported in any review. This trial is also evaluated in the section Characteristics of included studies: selective serotonin reuptake inhibitors. Across the reviews, the dapoxetine doses evaluated were 20 mg, 30 mg, 40 mg, 60 mg and 100 mg on demand. As dapoxetine at 30 mg and 60 mg has received approval for the treatment of PE in the UK,24 these doses were used in the present review for analysis. One Phase II RCT evaluated doses of 20 mg and 40 mg and is not discussed further here. 115 Details of the RCTs extracted from these reviews are presented in Table 18. All RCTs in reviews were captured by the search strategy for this assessment report.
| RCTs extracted from reviews | ||||||
|---|---|---|---|---|---|---|
| RCT (source) | Duration | Treatments | PE definition | Lifelong/acquired | IELT assessment | Other outcomes |
| Buvat et al. 2009113 (reviews68,108–110) | Phase III, 24 weeks | Dapoxetine 30 mg (n = 388) on demand | NR | NR | Method NR | CGI of change, CCCB (unclear); sexual satisfaction, control over ejaculation, distress, interpersonal difficulty |
| Dapoxetine 60 mg (n = 389) on demand | ||||||
| Placebo (n = 385) | ||||||
| Hellstrom et al. 2004114 (reviews68,112) | Phase II, crossover, 72-hour washout, 2 weeks per treatment | Dapoxetine 60 mg on demand | NR | NR | Method NR | NR |
| Dapoxetine 100 mg on demand | ||||||
| Placebo | ||||||
| (Total n = 166) | ||||||
| Hellstrom et al. 2005115 (reviews67,68,112) | Phase II, crossover, no washout, 4 weeks per treatment | Dapoxetine 20 mg on demand | NR | NR | Method NR | NR |
| Dapoxetine 40 mg on demand | ||||||
| Placebo | ||||||
| (Total n = 154) | ||||||
| Kaufman et al. 2009116 (reviews68,108–110) | Phase II, 9 weeks | Dapoxetine 60 mg on demand | NR | NR | IELT not assessed | Global impression of change (PGI), perceived control over ejaculation, satisfaction with sexual intercourse, personal distress related to ejaculation |
| Dapoxetine 60 mg daily | ||||||
| Placebo | ||||||
| (Total n = 1238) | ||||||
| McMahon et al. 2010168 (reviews68,108,109) | Phase III, 12 weeks | Dapoxetine 30 mg (n = 354) on demand | NR | NR | Method NR | Sexual satisfaction, control over ejaculation, distress (CGI) |
| Dapoxetine 60 mg (n = 356) on demand | ||||||
| Placebo (n = 357) | ||||||
| Pryor et al. 2006118 (reviews65,67,68,108–110) | Two RCTs (integrated analysis), Phase III, 12 weeks | Dapoxetine 30 mg (n = 874) on demand | NR | NR | Method NR | Global impression of change (PGI), CCCB (unclear); sexual satisfaction, control over ejaculation |
| Dapoxetine 60 mg (n = 870) on demand | ||||||
| Placebo (n = 870) | ||||||
| Safarinejad 200685 (reviews65,110) | Phase III, 12 weeks | Dapoxetine 60 mg once daily (n = 115) | NR | NR | Method NR | Sexual satisfaction |
| Paroxetine 20 mg (n = 113) | ||||||
| Placebo (n = 112) | ||||||
| Safarinejad 2008119 (reviews109,110) | Phase III, 12 weeks | Dapoxetine 30 mg twice daily (n = 106) | NR | NR | Method NR | Sexual satisfaction |
| Placebo (n = 106) | ||||||
| Further RCTs identified by searches (not captured in reviews) | ||||||
| RCT (country), risk of bias | Duration | Treatments, numbers analysed/randomised (%) | PE definition | Lifelong/acquired | IELT assessment | Other outcomes |
| Lee et al. 2012120 (Republic of Korea), low risk | 12 weeks | Dapoxetine 30 mg + mirodenafil 50 mg, 1 to 3 hours precoitus (n = 63) | DSM-IV diagnosis | Lifelong | Stopwatch | Time from foreplay to beginning intercourse, OSAT, PEP |
| Dapoxetine 30 mg + placebo 1 to 3 hours precoitus (n = 57) | ||||||
| Dapoxetine + mirodenafil 62/63 (98%) | ||||||
| Dapoxetine + placebo, 56/57 (98%) | ||||||
The RCT by Lee et al. 120 was conducted in the Republic of Korea. Patients were randomised to dapoxetine 30 mg plus mirodenafil 50 mg per day (n = 63) or dapoxetine 30 mg plus placebo (n = 57). The trial was considered to be at overall low risk of bias. This trial is also evaluated in the section Characteristics of included studies: selective serotonin reuptake inhibitors.
Details of the treatments evaluated, definition of PE, IELT assessment method, other outcomes assessed, study duration, along with the study country for the further RCTs not in reviews, and the overall study quality assessment (AMSTAR33 for reviews and Cochrane risk of bias assessment34 for the RCTs not included by reviews) are presented in Table 18.
Assessment of effectiveness: dapoxetine – intravaginal ejaculatory latency time outcomes
Intravaginal ejaculatory latency time data were reported for six RCTs85,113–115,118,170 identified from existing reviews and the one further RCT120 identified for inclusion in this review. The report by Pryor et al. 118 comprised an integrated analysis of two RCTs. Data from this study have been evaluated as a single trial in this assessment report. Three trials were not included in the IELT analysis in this assessment report: one Phase II RCT that evaluated doses of 20 mg and 40 mg dapoxetine,115 one Phase II RCT for which no variance estimates or appropriate p-values were reported114 and one Phase III RCT for which no IELT data were available. 116
Meta-analysis of mean IELT (minutes) at 12 or 24 weeks’ follow-up, based on three RCT113,118,170 comparisons of dapoxetine 30 mg and placebo (n = 3036), displayed low heterogeneity (I2=28%). The pooled MD in IELT was 1.16 minutes, significantly favouring dapoxetine 30 mg [MD (fixed effect); 95% CI 0.94 to 1.39 minutes; p < 0.00001]. Meta-analysis of mean IELT (minutes) at 12 or 24 -weeks’ follow-up, based on five RCT85,113,118,119,170 comparisons of dapoxetine 60 mg compared with placebo (n = 3390), displayed low heterogeneity (I2 = 0%). The pooled MD in IELT was 1.66 minutes, significantly favouring dapoxetine 30 mg [MD (fixed effect); 95% CI 1.46 to 1.87 minutes; p < 0.00001]. The forest plot for this analysis is presented in Figure 9. Summary results for these and all other meta-analyses are presented in Table 19.
FIGURE 9.
Dapoxetine 30 mg or 60 mg compared with placebo: forest plot of IELT outcomes. df, degrees of freedom; IV, inverse variance; SD, standard deviation.
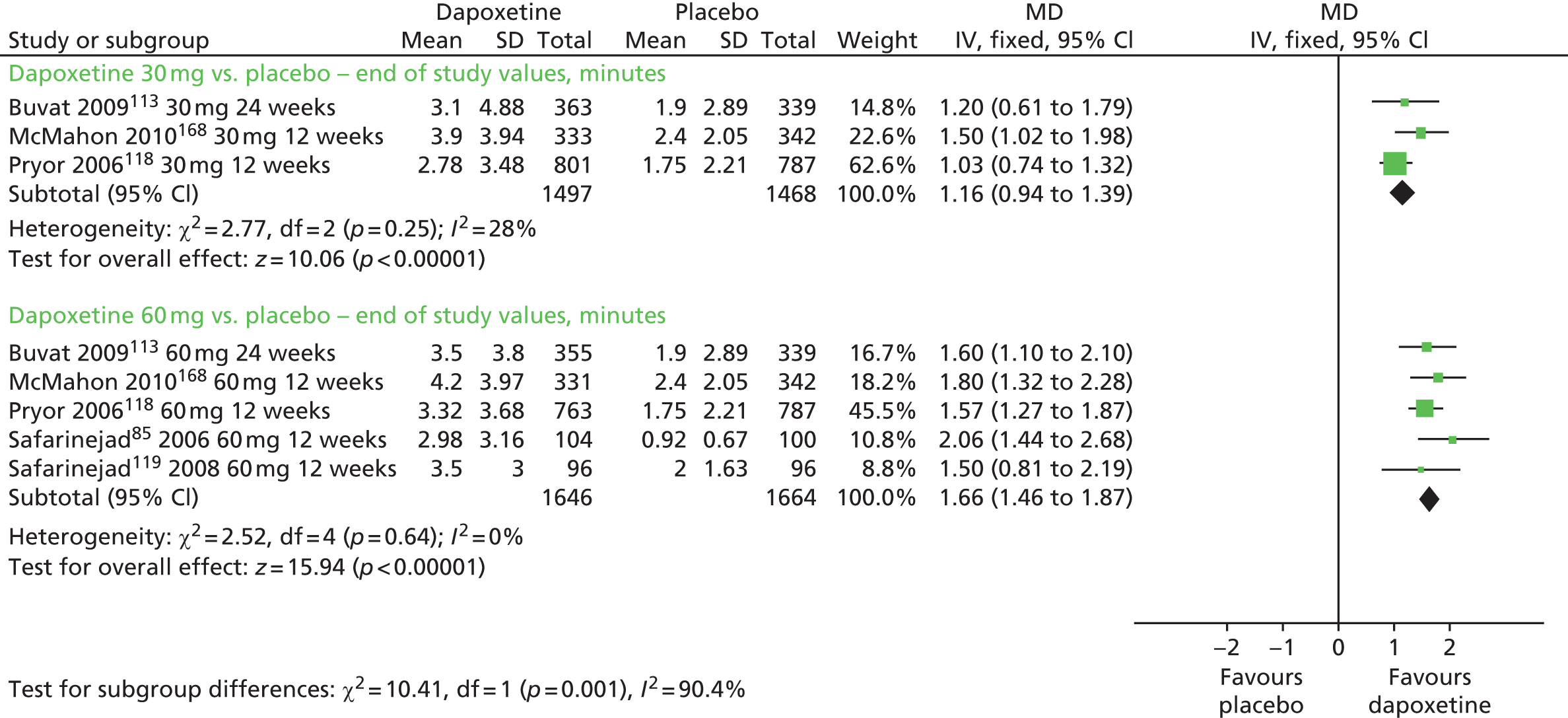
| Comparison | Outcome | Study duration | No. of RCTs | No. of participants | I 2 | Meta-analysis (model) | Effect estimate (95% CI) | Favours | p-value |
|---|---|---|---|---|---|---|---|---|---|
| IELT | |||||||||
| Dapoxetine 30 mg vs. placebo | IELT (minutes) – final values | 12–24 weeks | 3113,117,118 | 3036 | 28% | Yes (fixed) | MD 1.16 (0.94 to 1.39) | Dapoxetine 30 mg | < 0.00001 |
| Dapoxetine 60 mg vs. placebo | IELT (minutes) – final values | 12–24 weeks | 585,113,117–119 | 3390 | 0% | Yes (fixed) | MD 1.66 (1.46 to 1.87) | Dapoxetine 60 mg | < 0.00001 |
| Dapoxetine 60 mg vs. dapoxetine 30 mg | IELT (minutes) – final values | 12–24 weeks | 3113,117,118 | 3005 | 0% | Yes (fixed) | MD 0.46 (0.19 to 0.74) | Dapoxetine 60 mg | 0.0009 |
| 30 mg + mirodenafil vs. 30 mg + placebo | IELT (minutes) – final values | 12 weeks | 1120 | 120 | N/A | N/A | MD 2.20 (–0.89 to 5.29) | NS | 0.16 |
| Control over ejaculation – mean scores | |||||||||
| Dapoxetine 30 mg vs. placebo | Control over ejaculation: mean scores | 12 weeks | 1118 | 1588 | N/A | N/A | MD 0.60 (0.50 to 0.70) | Dapoxetine 30 mg | < 0.00001 |
| Dapoxetine 60 mg vs. placebo | Control over ejaculation: mean scores | 9–12 weeks | 2116,118 | 2202 | 86% | Data not pooled | MD 0.77 (0.67 to 0.87) | Dapoxetine 60 mg | < 0.00001 |
| MD 0.50 (0.33 to 0.67) | < 0.00001 | ||||||||
| Dapoxetine 30 mg vs. dapoxetine 60 mg | Control over ejaculation mean scores | 12 weeks | 1118 | 1564 | N/A | N/A | MD –0.17 (–0.28 to –0.06) | Dapoxetine 60 mg | 0.0002 |
| Control over ejaculation – patients reporting change | |||||||||
| Dapoxetine 30 mg vs. placebo | Control over ejaculation: patients reporting change | 24 weeks | 1113 | 723 | N/A | N/A | RR 2.05 (1.48 to 2.84) | Dapoxetine 30 mg | < 0.00001 |
| Dapoxetine 60 mg vs. placebo | Control over ejaculation: patients reporting change | 9–24 weeks | 2113,116 | 1369 | 76% | Data not pooled | RR 3.00 (2.21 to 4.07) | Dapoxetine 60 mg | < 0.00001 |
| RR 1.95 (1.46 to 2.59) | < 0.00001 | ||||||||
| Dapoxetine 30 mg vs. dapoxetine 60 mg | Control over ejaculation: patients reporting change | 24 weeks | 1113 | 712 | N/A | N/A | RR 0.68 (0.55 to 0.85) | Dapoxetine 60 mg | 0.0008 |
| Sexual satisfaction – mean scores | |||||||||
| Dapoxetine 30 mg vs. placebo | Sexual satisfaction: mean scores | 12 weeks | 1118 | 1588 | N/A | N/A | MD 0.51 (0.41 to 0.61) | Dapoxetine 30 mg | < 0.00001 |
| Dapoxetine 60 mg vs. placebo | Sexual satisfaction: mean scores | 9–12 weeks | 2116,118 | 2202 | 99% | Data not pooled | MD 0.61 (0.50 to 0.72) | Dapoxetine 60 mg | < 0.00001 |
| MD 0.50 (0.33 to 0.67) | < 0.00001 | ||||||||
| Dapoxetine 60 mg vs. dapoxetine 30 mg | Sexual satisfaction: mean scores | 12 weeks | 1118 | 1564 | N/A | N/A | MD 0.10 (0.00 to 0.20) | NS | 0.06 |
| Sexual satisfaction – patients reporting change | |||||||||
| Dapoxetine 30 mg vs. placebo | Sexual satisfaction: patients reporting change | 24 weeks | 1113 | 298 | N/A | N/A | RR 1.36 (1.14 to 1.62) | Dapoxetine 30 mg | 0.0007 |
| Dapoxetine 60 mg vs. placebo | Sexual satisfaction: patients reporting change | 9–24 weeks | 485,113,116,119 | 1745 | 89% | Data not pooled | RR 1.56 (1.32 to 1.80) | Dapoxetine 60 mg | < 0.00001 |
| RR 1.61 (1.32 to 1.98) | < 0.00001 | ||||||||
| RR 4.15 (2.59 to 6.63) | < 0.00001 | ||||||||
| RR 4.19 (2.68 to 6.55) | < 0.00001 | ||||||||
| Dapoxetine 30 mg vs. dapoxetine 60 mg | Sexual satisfaction: patients reporting change | 24 weeks | 1113 | 712 | N/A | N/A | RR 0.87 (0.75 to 1.00) | Dapoxetine 60 mg | 0.05 |
| Global impression of change – patients reporting change | |||||||||
| Dapoxetine 30 mg vs. placebo | Global impression of change: patients report change | 12–24 weeks | 3113,117,118 | 2950 | 48% | Yes (random) | RR 2.01 (1.69 to 2.38) | Dapoxetine 30 mg | < 0.00001 |
| Dapoxetine 60 mg vs. placebo | Global impression of change: patients report change | 9–24 weeks | 4113,116–118 | 3566 | 57% | Yes (random) | RR 2.26 (1.91 to 2.67) | Dapoxetine 60 mg | < 0.00001 |
| Dapoxetine 30 mg vs. dapoxetine 60 mg | Global impression of change: patients report change | 12–24 weeks | 3113,117,118 | 2950 | 0% | Yes (fixed) | RR 0.86 (0.80 to 0.90) | Dapoxetine 60 mg | < 0.0001 |
| Composite criteria for clinical benefit – patients reporting change | |||||||||
| Dapoxetine 30 mg vs. placebo | Composite criteria for clinical benefit: patients reporting change | 12–24 weeks | 2113,117 | 1376 | 0% | Yes (fixed) | RR 1.71 (1.40 to 2.08) | Dapoxetine 30 mg | < 0.00001 |
| Dapoxetine 60 mg vs. placebo | Composite criteria for clinical benefit: patients reporting change | 9–24 weeks | 3113,116,117 | 2029 | 66% | Yes (random) | RR 2.15 (1.64 to 2.82) | Dapoxetine 60 mg | < 0.00001 |
| Dapoxetine 30 mg vs. dapoxetine 60 mg | Composite criteria for clinical benefit: patients reporting change | 12–24 weeks | 2113,117 | 1375 | 76% | No | RR 0.68 (0.54 to 0.85) | Dapoxetine 60 mg | 0.0008 |
| RR 0.93 (0.76 to 1.14) | NS | 0.51 | |||||||
Meta-analysis of mean IELT (minutes) at 12 or 24 weeks’ follow-up, based on three RCT113,117,118 comparisons (n = 3005) displayed low heterogeneity (I2 = 0%). The pooled MD in IELT was 0.46 minutes, significantly favouring dapoxetine 60 mg [MD (fixed effect); 95% CI 0.19 to 0.74 minutes; p = 0.0009]. The forest plot for this analysis is presented in Figure 10.
FIGURE 10.
Dapoxetine 30 mg compared with 60 mg: forest plot of IELT outcomes. df, degrees of freedom; IV, inverse variance; SD, standard deviation.
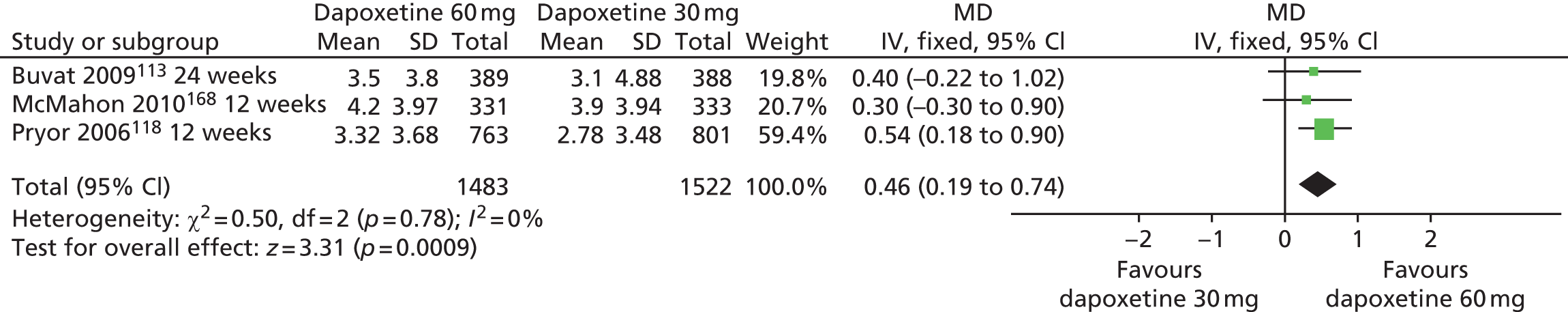
The between-group difference in mean IELT (minutes) at 4 weeks, based on one RCT120 (n = 118), was 1.50 minutes (95% CI –0.55 to 3.55 minutes; p=0.15). The between-group difference in mean IELT (minutes) at 12 weeks, based on one RCT120 (n = 118), was 2.20 minutes (95% CI –0.89 to 5.29 minutes; p=0.16). The forest plot for this analysis is presented in Figure 11.
FIGURE 11.
Dapoxetine + mirodenafil compared with dapoxetine + placebo: forest plot of IELT outcomes. df, degrees of freedom; IV, inverse variance; SD, standard deviation.

Assessment of effectiveness: dapoxetine – outcomes other than intravaginal ejaculatory latency time
With the exception of the RCTs by Safarinejad85 and Safarinejad,119 all Phase III RCTs and the RCT by Lee et al. 120 reported outcomes other than IELT. These outcomes included control over ejaculation, sexual satisfaction, global impression of change and a composite criterion for clinical benefit. However, the reporting of these outcomes varied across the included RCTs and differed in how the outcome was assessed (either as mean scores or as numbers of participants achieving a threshold). Results for between-group comparisons undertaken using RevMan for this assessment report for all secondary outcomes are presented in Table 19. All RCTs reporting these outcomes evaluated dapoxetine over 9–24 weeks.
Mean scores for this outcome were available for two Phase III RCTs. 116,118 High heterogeneity was observed for dapoxetine 60 mg compared with placebo (two RCTs,116,118 I2 = 86%, meta-analysis not undertaken). Numbers of patients reporting a change in this outcome were available for two Phase III RCTs. 113,116 High heterogeneity was observed for dapoxetine 60 mg compared with placebo (two RCTs,113,116 I2 = 76%, meta-analysis not undertaken). Between-group comparisons from individual RCTs estimated in RevMan for this assessment report (see Table 19) suggested that both dapoxetine 30 mg and dapoxetine 60 mg are significantly more effective then placebo on this outcome (MD, p < 0.0001 and p < 0.0001; RR, p < 0.0001 and p < 0.0001) and dapoxetine 60 mg is significantly more effective than dapoxetine 30 mg (MD, p = 0.0002; RR, p = 0.0008).
Mean scores for this outcome were available for two Phase III RCTs. 116,118 A high level of heterogeneity was observed for dapoxetine 60 mg compared with placebo (two RCTs,116,118 I2 = 99%, meta-analysis not undertaken). The number of patients reporting a change in this outcome was available for four Phase III RCTs. 85,113,116,119 High heterogeneity was observed for dapoxetine 60 mg compared with placebo (four RCTs,85,113,116,119 I2 = 89%, meta-analysis not undertaken). Between-group comparisons from individual RCTs in RevMan for this assessment report (see Table 19) suggested that both dapoxetine 30 mg and dapoxetine 60 mg are significantly more effective than placebo on this outcome (MD, p < 0.0001 and p < 0.0001; RR, p = 0.0007 and p < 0.0001) and that dapoxetine 60 mg is significantly more effective than dapoxetine 30 mg on the number of patients reporting a change in this outcome (MD, p = 0.06; RR, p = 0.05) (see Table 19).
The numbers of patients reporting a change in this outcome were available for four Phase III RCTs. 113,116–118 Pooled effects across RCTs suggested that both dapoxetine 30 mg and dapoxetine 60 mg were significantly more effective than placebo (RR, p < 0.0001 and p < 0.0001) and that dapoxetine 60 mg is significantly more effective than dapoxetine 30 mg (RR, p < 0.0001) (see Table 19).
The numbers of patients reporting a change in this outcome were available for three Phase III RCTs (Buvat et al. , 2009,113 Kaufman et al. , 2009,116 McMahon et al. , 2010117). Pooled effects across RCTs suggested that both dapoxetine 30 mg and dapoxetine 60 mg were significantly more effective than placebo (RR, p < 0.0001 and p < 0.0001) (see Table 19). High heterogeneity was observed for dapoxetine 30 mg compared with dapoxetine 60 mg (two RCTs, I2 = 76%, meta-analysis not undertaken). Between-group comparisons from individual RCTs estimated in RevMan for this assessment report for one RCT suggested that dapoxetine 30 mg was significantly more effective than dapoxetine 60 mg on this outcome (RR, p = 0.0008) (see Table 19).
The RCT by Lee et al. 120 reported no statistically significant between-group difference in time from foreplay to beginning intercourse between dapoxetine plus mirodenafil and mirodenafil alone. Nor was any statistically significant between-group difference evident in overall sexual act time (OSAT) at week 4 or 12. The authors reported statistically significant between-group differences in favour of dapoxetine plus mirodenafil on the PEP domains of perceived control over ejaculation (p = 0.019), interpersonal difficulty related to ejaculation (p = 0.013) and the overall index score (p = 0.046).
Assessment of safety: dapoxetine – adverse events
Adverse event and withdrawal data for RCTs from reviews are summarised from the reports by McCarty and Dinsmore,109 McMahon and Porst,68 Hutchinson et al. 111 and Kendirci et al. 112 in Table 20.
| AEs (%) | Dapoxetine 30 mg | Dapoxetine 60 mg | Dapoxetine + mirodenafil | Placebo | References |
|---|---|---|---|---|---|
| Nausea | 16.5 | 30.6 | 2.9 | Buvat et al. 2009113 (24 weeks) | |
| 5.8 | 0.7 | Hellstrom et al. 2004114 (2 weeks) | |||
| 15.3 | 1.6 | Kaufman et al. 2009116 (9 weeks) | |||
| 10.5 | 26.4 | 2.0 | McMahon 2010117 (12 weeks) | ||
| 8.7 | 20.1 | 1.9 | Pryor et al. 2006118 (12 weeks) | ||
| 5.4 | 1.0 | Safarinejad 2008119 (12 weeks) | |||
| 10.7 | 8.1 | Lee et al. 2012120 (12 weeks) | |||
| Diarrhoea | 3.9 | 11.3 | 1.6 | Buvat et al. 2009113 (24 weeks) | |
| 5.0 | 0.7 | Hellstrom et al. 2004114 (2 weeks) | |||
| 6.1 | 2.0 | Kaufman et al. 2009116 (9 weeks) | |||
| 2.0 | 1.7 | 0.8 | McMahon et al. 2010168 (12 weeks) | ||
| 3.9 | 6.8 | 1.4 | Pryor et al. 2006118 (12 weeks) | ||
| 5.4 | 0.0 | Safarinejad 2008119 (12 weeks) | |||
| 3.6 | 4.8 | Lee et al. 2012120 (12 weeks) | |||
| Headache | 6.4 | 13.6 | 8.3 | Buvat et al. 2009113 (24 weeks) | |
| 4.3 | 0.0 | Hellstrom et al. 2004114 (2 weeks) | |||
| 8.1 | 6.1 | Kaufman et al. 2009116 (9 weeks) | |||
| 3.4 | 4.8 | 2.0 | McMahon 2010117 (12 weeks) | ||
| 5.9 | 6.8 | 4.0 | Pryor et al. 2006118 (12 weeks) | ||
| 4.3 | 1.0 | Safarinejad 2008119 (12 weeks) | |||
| 5.4 | 12.9 | Lee et al. 2012120 (12 weeks) | |||
| Dizziness | 7.7 | 13.4 | 2.6 | Buvat et al. 2009113 (24 weeks) | |
| 2.2 | 0.0 | Hellstrom et al. 2004114 (2 weeks) | |||
| 10.2 | 2.9 | Kaufman et al. 2009116 (9 weeks) | |||
| 10.5 | 18.8 | 3.9 | McMahon et al. 2010168 (12 weeks) | ||
| 3.0 | 6.2 | 0.8 | Pryor et al. 2006118 (12 weeks) | ||
| 3.2 | 0.0 | Safarinejad 2008119 (12 weeks) | |||
| 8.9 | 9.7 | Lee et al. 2012120 (12 weeks) | |||
| Somnolence | 3.9 | 7.2 | 1.0 | Buvat et al. 2009113 (24 weeks) | |
| 2.9 | 0.7 | Hellstrom et al. 2004114 (2 weeks) | |||
| 3.7 | 0.8 | Kaufman et al. 2009116 (9 weeks) | |||
| 3.4 | 6.2 | 0.6 | McMahon et al. 2010168 (12 weeks) | ||
| 3.2 | 3.7 | 0.2 | Pryor et al. 2006118 (12 weeks) | ||
| Vomiting | 1.3 | 3.1 | 0.5 | Buvat et al. 2009113 (24 weeks) | |
| 0.3 | 2.5 | 0.0 | McMahon et al. 2010168 (12 weeks) | ||
| Palpitation | 1.8 | 6.5 | Lee et al. 2012120 (12 weeks) | ||
| Facial flushing | 1.8 | 3.2 | Lee et al. 2012120 (12 weeks) | ||
| Any AE | 32.1 | 45.2 | Lee et al. 2012120 (12 weeks) | ||
| Withdrawals (owing to AE) | 3.9 | 8.2 | 1.3 | Buvat et al. 2009113 (24 weeks) | |
| 1.7 | 5.1 | 0.3 | McMahon et al. 2010168 (12 weeks) | ||
| 4.0 | 10.0 | 0.9 | Pryor et al. 2006118 (12 weeks) | ||
| 3.5 | 0.0 | Safarinejad 200685 (12 weeks) | |||
| 5.7 | 0.0 | Safarinejad 2008119 (12 weeks) | |||
| Withdrawals (overall) | 42.8 | 46.8 | 50.9 | Buvat et al. 2009113 (24 weeks) | |
| 28.5 | 31.2 | 17.4 | McMahon et al. 2010168 (12 weeks) | ||
| 22.7 | 29.7 | 22.8 | Pryor et al. 2006118 (12 weeks) | ||
| 8.7 | 8.9 | Safarinejad 200685 (12 weeks) | |||
| 12.3 | 9.4 | Safarinejad 2008119 (12 weeks) | |||
| 0.0 | 0.7 | Hellstrom et al. 2004114 (2 weeks) |
These reviewers concluded that, among the Phase II studies, the most commonly reported AEs were nausea, diarrhoea, headache and dizziness, and that the incidence of most AEs appeared to be dose dependent. Amongst the Phase III studies, the most common treatment-related AEs included nausea, dizziness and headache.
Across the included RCTs, insufficient data for numbers of patients experiencing AEs were available for any meaningful pooling in a meta-analysis.
Assessment of effectiveness: dapoxetine – evidence summary
The current evidence base for dapoxetine at 30 mg and 60 mg on demand (approved doses for the treatment of PE in the UK24) in the treatment of PE comprises one Phase II RCT114 and six Phase III RCT reports. 85,113,116,118,119,170 These RCTs are captured in six systematic reviews of effectiveness which are of low to moderate methodological quality. 65,67,108–110,169 One further RCT120 evaluating the effects of dapoxetine combined with a PDE5 inhibitor (mirodenafil) is at overall low risk of bias. The pooled evidence across three RCTs113,117,118 including 3036 participants and across five RCTs85,113,117–119 comprising 3390 participants suggests that both dapoxetine 30 mg and dapoxetine 60 mg increase IELT in men with PE to a significantly greater extent than placebo (30 mg: MD 1.16 minutes, 95% CI 0.94 to 1.39 minutes; p < 0.00001; 60 mg: MD 1.66 minutes, 95% CI 1.46 to 1.87 minutes; p < 0.00001). The pooled evidence across three RCTs113,117,118 including 3005 participants suggests that dapoxetine 60 mg is significantly more effective in increasing IELT in men with PE when compared with dapoxetine 30 mg (MD 0.46 minutes, 95% CI 0.19 to 0.74 minutes; p = 0.0009). Evidence from one RCT120 (120 participants) showed no statistically significant difference in IELT between dapoxetine 30 mg combined with mirodenafil and dapoxetine 30 mg alone. Among the Phase III trials, treatment duration ranged from 9 to 24 weeks. The effects of longer-term treatment with dapoxetine for PE or the effects once treatment is withdrawn have not been evaluated in the current evidence base.
Evidence from individual Phase III RCTs suggests that both dapoxetine 30 mg and dapoxetine 60 mg are significantly more effective than placebo and that dapoxetine 60 mg is significantly more effective than dapoxetine 30 mg, on outcomes of ejaculatory control, sexual satisfaction, global impression of change and clinical benefit. However, the assessment and reporting of these outcomes is variable across trials. High levels of heterogeneity were observed when trials were pooled. These findings should be interpreted with caution given the observed levels of between-study heterogeneity.
The most commonly reported AEs with dapoxetine are nausea, diarrhoea, headache, dizziness and appear to be dose dependent. From the current evidence base there are no data regarding possible long-term AEs of dapoxetine in the treatment of PE.
The findings for dapoxetine are based on meta-analyses of RCT data extracted from existing reviews and meta-analyses. From a review presenting withdrawal data from Phase III trials, it is apparent that previous reviews have meta-analysed RCT data across per-protocol (patients completing) and intention-to-treat populations. 111 Thus, an attrition bias may be present. The results for dapoxetine in this assessment report should therefore be interpreted with caution.
Serotonin–noradrenaline reuptake inhibitors
Characteristics of included studies: serotonin–noradrenaline reuptake inhibitors
One RCT evaluating duloxetine was identified from one review. 68 The review was undertaken in Australia and was awarded an AMSTAR score of 2 out of 11 (see Table 11 in the Characteristics of included studies: selective serotonin reuptake inhibitors section and Appendix 4). A further two RCTs were identified, both of which evaluated venlafaxine compared with placebo. 122,123
Duloxetine 80 mg was compared with placebo in one trial. 121 The duration was 12 weeks and IELT was assessed using a stopwatch. This RCT was captured by the search strategy for this assessment report.
The trial by Kilic et al. 122 was undertaken in Turkey and was a randomised crossover design trial recruiting 31 patients. Patients were randomised to venlafaxine extended-release 75 mg per day or placebo: 2 weeks treatment, 1 week washout, 2 weeks treatment. IELT was assessed using a stopwatch. The authors reported that 21 out of 31 (67.7%) patients completed the trial. This trial was considered at overall high risk of bias. The RCT by Safarinejad123 was conducted in the Islamic Republic of Iran. Two hundred and twenty patients were randomised to either venlafaxine extended-release 75 mg per day or placebo. IELT was assessed using a stopwatch. Treatment duration was 12 weeks and the authors reported that 192 out of 222 (86%) patients completed the intervention. This trial was considered to be at overall unclear risk of bias.
Details of these trials are presented in Table 21.
| RCTs extracted from reviews | ||||||
|---|---|---|---|---|---|---|
| RCT (source) | Duration | Treatments | PE definition | Lifelong/acquired | IELT assessment | Other outcomes |
| Athanasios et al. 2007121 (review68) | 12 weeks | Duloxetine 40 mg twice daily (n = 10) | Partially ISSM: IELT ≤ 4 minutes | NR | Stopwatch | CGI-I |
| Placebo twice daily (n = 10) | ||||||
| (Following 1 week titration with duloxetine 20 mg or placebo twice daily) | ||||||
| Further RCTs identified by searches (not captured in reviews) | ||||||
| RCT (country), risk of bias | Duration | Treatments, numbers analysed/randomised (%) | PE definition | Lifelong/acquired | IELT assessment | Other outcomes |
| Kilic et al. 2005122 (Turkey), high | Crossover: 2 weeks treatment, 1 week washout, 2 weeks treatment | Venlafaxine extended-release 75 mg per day | IELT < 2 minutes on 50% occasions | All lifelong | Stopwatch | Sexual satisfaction of patient and partner – assessment method not reported |
| Placebo | ||||||
| 21/31 (67.7%) | ||||||
| Safarinejad 2008123 (the Islamic Republic of Iran), unclear | 12 weeks | Venlafaxine extended-release 75 mg per day | IELT < 2 minutes on 90% occasions | Lifelong, 83 | Stopwatch | IIEF intercourse satisfaction |
| Placebo | Acquired, 119 | Weekly coitus episodes | ||||
| Venlafaxine, 94/112 (84%) | ||||||
| Placebo, 98/110 (89%) | ||||||
Assessment of effectiveness: serotonin–noradrenaline reuptake inhibitors – intravaginal ejaculatory latency time outcomes
The crossover trial by Kilic et al. 122 reported that there was no statistically significant between-group difference in IELT post treatment (p = 0.144) while no variance estimates were reported for the RCT by Safarinejad. 123 The author reported that, during the study (fortnightly assessment points), there was no significant differences between venlafaxine and placebo (p = 0.10). After 12 weeks, IELT did not differ significantly between the two groups (p = 0.10 for geometric mean fold increase).
The between-group difference in mean IELT for one RCT evaluating this comparison121 was 1.52 minutes [MD (fixed effect), 95% CI 0.08 to 2.24 minutes; p < 0.00001] in favour of duloxetine at 12 weeks (estimated for this assessment report using RevMan; figure not presented and, therefore, there is no figure for this comparison in the report).
Assessment of effectiveness: serotonin–noradrenaline reuptake inhibitors – other outcomes
The RCT by Athanasios et al. 121 assessed score on the Clinical Global Impression – Improvement (CGI-I) scale. The trial by Kilic et al. 122 assessed sexual satisfaction, but did not report the instrument used. Safarinejad123 assessed IIEF intercourse satisfaction and number of coitus episodes weekly.
The proportion of patients reported as ‘much improved’ and ‘very much improved’ on a subjective measure of clinical improvement was greater with duloxetine than with placebo in one RCT. 121
The trial by Kilic et al. 122 reported no statistically significant between-group difference in patient or partner sexual satisfaction between venlafaxine compared with placebo. Safarinejad123 also reported no significant between-group difference in IIEF sexual satisfaction or number of episodes of coitus per week.
Details of these outcomes and AEs are presented in Table 22.
| RCT, duration | Treatment | Outcome measure | Results | Between-group difference reported as significant | AEs: venlafaxine vs. placebo |
|---|---|---|---|---|---|
| Athanasios et al. 2007,121 12 weeks | Duloxetine 80 mg (n = 10) | CGI-I | Duloxetine ‘much improved’, 40%; (4/10); ’very much improved’, 40% (4/10) | Unclear | Nausea and dry mouth were reported in three subjects. Unclear which group |
| Placebo (n = 10) | Placebo ‘much improved’, 10% (1/10) | ||||
| p-value NR | |||||
| Kilic et al. 2005,122 2 weeks treatment, 1 week washout, 2 weeks treatment | Venlafaxine 75 mg | Sexual satisfaction of patient and partner – assessment method NR | No statistical difference was found in increases of sexual satisfaction scores of both patient and partner groups between venlafaxine and placebo (p = 0.080 for patients and p = 0.067 for partners) | No | Venlafaxine vs. placebo (n = 21 with data) |
| Placebo | Any AE, 48% vs. 29% | ||||
| (Total n = 31) | Exhaustion, 10% vs. 5% | ||||
| Drowsiness, 24% vs. 10% | |||||
| Stagnation, 10% vs. 5% | |||||
| Sweating, 5% vs. 0% | |||||
| Gnashing of teeth, 5% vs. 5% | |||||
| Tension, 10% vs. 5% | |||||
| Dry mouth, 19% vs. 14% | |||||
| Reduced potency, 0% vs. 5% | |||||
| Increased potency, 5% vs. 0% | |||||
| Reduced libido, 5% vs. 5% | |||||
| Nausea, 19% vs. 0% | |||||
| Palpitation, 5% vs. 0% | |||||
| Sleeplessness, 5% vs. 5% | |||||
| Reduced attention, 5% vs. 0% | |||||
| Headache, 5% vs. 0% | |||||
| Only nausea was significantly higher in patients who took venlafaxine. No withdrawals due to side effects | |||||
| Safarinejad 2008,123 12 weeks | Venlafaxine 75 mg (n = 112) | IIEF intercourse satisfaction | Mean IIEF intercourse satisfaction post treatment: | No | Venlafaxine (n = 112) vs. placebo (n = 110): |
| Placebo (n = 110) | Weekly coitus episodes |
|
Any treatment-related AE, 29% vs. 7% | ||
| Mean number of acts of coitus per week post treatment: | No | Nausea, 27% vs. 1% | |||
|
Dry mouth, 20% vs. 0% | ||||
| No variance estimates or p-value reported | Agitation, 11% vs. 1% | ||||
| Constipation, 10% vs. 0% | |||||
| Headache, 8% vs. 2% | |||||
| Dizziness, 3% vs. 2% | |||||
| Erectile dysfunction, 2% vs. 2% | |||||
| Loss of libido, 3% vs. 2% | |||||
| More AEs were associated with venlafaxine (p = 0.02) |
Assessment of safety: serotonin–noradrenaline reuptake inhibitors – adverse events
Dry mouth and nausea were reported in one RCT evaluating duloxetine;121 however, it was unclear whether this was in the duloxetine or placebo group. The two trials that evaluated venlafaxine both reported proportions of patient experiencing specific AEs of treatment. 122,123 The trial by Kilic et al. 122 reported that only nausea was significantly higher with venlafaxine than with placebo. Safarinejad123 reported that significantly more AEs were associated with venlafaxine.
Assessment of effectiveness: serotonin–noradrenaline reuptake inhibitors – evidence summary
The current evidence base for SNRIs in the treatment of PE comprises three RCTs,121–123 one captured in a low methodological quality systematic review121 and two further RCTs,122,123 one122 of which is at overall high risk of bias and the other at overall unclear risk of bias. 123
There is evidence from one RCT121 (20 participants) that duloxetine is significantly more effective than placebo in increasing IELT (MD 1.52 minutes, 95% CI 0.08 to 2.24 minutes; p < 0.00001). Measures of clinical improvement appear improved with duloxetine. Duloxetine-associated side effects are reported to be dry mouth and nausea. Evidence from two RCTs122,123 suggests that venlafaxine is not effective at increasing IELT in men with PE when compared with placebo. Venlafaxine is associated with significantly more treatment-related side effects than placebo.
The long-term efficacy and side effects of these treatments along with patient acceptability are not assessed in the current evidence base.
Tricyclic antidepressants
Characteristics of included studies: tricyclic antidepressants
Two single-arm randomised crossover RCTs39,76 were captured in several reviews (see Characteristics of included studies: selective serotonin reuptake inhibitors, Table 11). Both evaluated oral clomipramine. Eight further RCTs124–131 that also evaluated oral clomipramine were identified from three reviews of low methodological quality. 52,68,69 Full details of the AMSTAR assessment for these and all other included reviews are presented in Appendix 4. A further three RCTs were identified from the literature search,107,132,133 and the RCT by Tuncel et al. 107 evaluated clomipramine, sertraline, terazosin and placebo. The trials by Akilov et al. 132 and Leaker et al. 133 both evaluated nasally inhaled clomipramine. The trial by Tuncel et al. 107 is also evaluated in sections Phosphodiesterase-5 inhibitors and Alpha-blockers.
In total, 10 trials were identified from reviews. 39,76,124–131 Of the trials identified as having as crossover design, by Abdel-Hamid et al. ,39 evaluated clomipramine 25 mg, sildenafil 50 mg, paroxetine 20 mg, sertraline 50 mg and the squeeze technique over five separate 4-week treatment phases. IELT was assessed using a stopwatch. Kim and Seo76 evaluated clomipramine 50 mg, fluoxetine 40 mg, sertraline 100 mg and placebo over 4-week treatment phases. The method of IELT assessment was not reported. Of the other trials, Althof et al. 124 evaluated clomipramine 25 mg, clomipramine 50 mg or placebo in 15 couples (unclear from existing reviews if crossover or pairwise comparison) over 2–7 weeks. Girgis et al. ,125 Goodman,126 Haensel et al. ,127 Montorsi et al. ,128 Porto,129 Segraves et al. 130 and Strassberg et al. 131 all evaluated clomipramine compared with placebo. The total number of participants per trial ranged from 16 to 33; however, numbers by treatment group were not reported and it was unclear from the reviews from which these trials were extracted which, if any, were crossover design trials. Duration across these trials ranged from 2 to 6 weeks. IELT was reported as being assessed using subject report or questionnaire. All RCTs in reviews were captured by the search strategy for this assessment report.
Details of these RCTs extracted from reviews are presented in Table 23.
| RCTs extracted from reviews | ||||||
|---|---|---|---|---|---|---|
| RCT | Duration | Treatments | PE definition | Lifelong/acquired | IELT assessment | Other outcomes |
| Abdel-Hamid et al. 200139 (reviews35,37,38,52,69,134,135,137,165) | Crossover (single-arm) 5 × 4 week phases each separated by a 2-week washout | Clomipramine 25 mg 3–5 hours precoitus | IELT ≤ 2 minutes | Lifelong | Stopwatch | Modified Erectile Dysfunction Inventory of Treatment Satisfaction, Arabic Anxiety Inventory (scale 0–30) |
| Sildenafil 50 mg 1 hour precoitus | ||||||
| Sertraline 50 mg 3–5 hours precoitus | ||||||
| Paroxetine 20 mg 3–5 hours precoitus | ||||||
| Squeeze technique | ||||||
| Total n = 31 | ||||||
| Althof et al. 1995124 (reviews68,69) | 2–7 weeks | Clomipramine 25 mg/day | IELT < 2 minutes | NR | Stopwatch | Symptom Checklist-90-Revised, Dyadic Adjustment Scale, State-Trait Anxiety Inventory, Harder Self- Esteem Inventory |
| Clomipramine 50 mg/day | ||||||
| Placebo | ||||||
| Total n = 15 couples | ||||||
| Kim and Seo 199876 (reviews52,64–66,69) | Crossover (single-arm). Each agent for 4 weeks, with 1-week washout | Clomipramine 50 mg | DSM-III | NR | Method NR | A patient self-reported questionnaire was used to obtain information about patient and partner sexual satisfaction |
| Fluoxetine 40 mg | ||||||
| Sertraline 100 mg | ||||||
| Placebo | ||||||
| Total n = 136 | ||||||
| Girgis et al. 1982125 (reviews52) | 6 weeks | Clomipramine | NR | NR | Questionnaire | NR |
| Placebo | ||||||
| Total n = 139 | ||||||
| Goodman 1980126 (reviews52) | 4–16 weeks | Clomipramine | NR | NR | Questionnaire | NR |
| Placebo | ||||||
| Total n = 116 | ||||||
| Haensel et al. 1996127 (reviews52,69) | 6 weeks | Clomipramine 25 mg as needed (12–24 hours precoitus) | DSM-IV | 8 men with primary PE | Subject report | Pelvic thrusts and time ejaculation, orgasm sooner than desired, within 1 to 2 minutes and after fewer than 10 pelvic thrusts |
| Placebo | ||||||
| Total n = 14 | ||||||
| RCTs extracted from reviews | ||||||
| RCT | Duration | Treatments | PE definition | Lifelong/acquired | IELT assessment | Other outcomes |
| Montorsi et al. 1995,128 (reviews52) | 8 weeks | Clomipramine | NR | NR | Questionnaire | NR |
| Placebo | ||||||
| Total n = 33 | ||||||
| Porto 1981,129 (reviews52) | 5 weeks | Clomipramine | NR | NR | Subject report | NR |
| Placebo | ||||||
| Total n = 20 | ||||||
| Segraves et al. 1993,130 (reviews52) | 3–5 weeks | Clomipramine 25–50 mg as needed (6 hours precoitus) | NR | NR | Subject report | NR |
| Placebo | ||||||
| Total n = 20 | ||||||
| Strassberg et al. 1999,131 (reviews52) | 2 weeks per treatment | Clomipramine 25 mg as needed (4–6 hours precoitus) | NR | NR | Subject report | NR |
| Placebo | ||||||
| Total n = 23 | ||||||
| Further RCTs identified by searches (not captured in reviews) | ||||||
| RCT (country), risk of bias | Duration | Treatments Numbers analysed/randomised (%) | PE definition | Lifelong/acquired | IELT assessment | Other outcomes |
| Akilov et al. 2011132 (Uzbekistan), unclear | 8 weeks | Clomipramine 4 mg nasal spray (n = 19) | IELT < 2 minutes during least 6 last months | NR | Self-reported | CIPE |
| Placebo nasal spray (n = 15) | IIEF-5 | |||||
| Total n 33/34 (97%), n by group NR | ||||||
| Leaker et al. 2008133 (UK), unclear | Crossover: each treatment for five occasions | Inhaled clomipramine 1 mg or placebo (not described) | IELT of 2 minutes during run-in | NR | Stopwatch | NR |
| Inhaled clomipramine 2 mg or placebo (not described) | ||||||
| Before intercourse for a maximum of five occasions | ||||||
| (n NR) | ||||||
| 39 analysed in intention to treat | ||||||
| Tuncel et al. 2008107 (Turkey), unclear | Treatment 2 months, assessment ‘after eight sexual attempts’ | Clomipramine 25 mg/day (n = 23) | WHO ICD-10 | NR | IELT not assessed | Clinical responses (assume control of ejaculation) self-assessed |
| Sertraline 50 mg/day (n = 20) | ||||||
| Terazosin 5 mg/day (n = 25) | ||||||
| Placebo (n = 22) | ||||||
| Clomipramine, 23/23 (100%) | ||||||
| Sertraline, 20/20 (100%) | ||||||
| Terazosin, 25/25 (100%) | ||||||
| Placebo, 22/22 (100%) | ||||||
The RCT by Akilov et al. 132 was conducted in Uzbekistan and patients were randomised, 19 to a clomipramine 4 mg nasal spray and 15 to a placebo nasal spray. The authors reported that 33 out of 34 (97%) completed the 8-week follow-up. IELT was via patient self-report. The RCT by Leaker et al. 133 was conducted in the UK and inhaled clomipramine 1 mg or placebo (not described) before intercourse for a maximum of five occasions was compared with inhaled clomipramine 2 mg or placebo before intercourse for a maximum of five occasions in a randomised crossover design study. Thirty-nine patients were reported as included in an intention-to-treat analysis. IELT was assessed using a stopwatch and both RCTs were reported in abstract form only. The RCT by Tuncel et al. 107 was undertaken in Turkey and 90 patients were randomised to receive clomipramine 25 mg per day, sertraline 50 mg, terazosin 5 mg or placebo. Treatment was for 2 months and IELT was not assessed. The authors reported that 90 out of 90 (100%) patients completed the trial. Treatment was for 2 months and IELT was not assessed. All three RCTs were considered to be at overall unclear risk of bias. 107,132,133
Details of the treatments evaluated, definition of PE, IELT assessment method, other outcomes assessed, study duration, along with the study country for the further RCTs not in reviews, and the overall study quality assessment (AMSTAR33 for reviews and Cochrane risk of bias assessment34 for the RCTs not included by reviews) are presented in Table 23.
Assessment of effectiveness: tricyclic antidepressants – intravaginal ejaculatory latency time outcomes
Intravaginal ejaculatory latency time outcomes were reported by the two crossover RCTs39,76 identified from existing reviews and the two further RCTs132,133 evaluating nasal administration identified for inclusion in this review. IELT data with variance estimates or p-values were not available for the remaining RCTs identified from reviews; however, the review summaries of data for TCAs are reported in the next section, Intravaginal ejaculatory latency time: clomipramine compared with placebo – summary data from existing reviews.
When IELT data post treatment were reported for the RCT by Althof et al. ,124 a latency increase of 3.37 minutes with clomipramine 25 mg and of 6.98 minutes with clomipramine 50 mg was reported. p-values or variance estimates were not reported. Placebo was reported as not significantly different from baseline; however, no data were reported. For the RCT by Haensel et al. ,127 an increase in latency from 2 to 8 minutes was reported (p-value not reported). For the RCT by Strassberg et al. ,131 post-treatment IELT was 3.82 minutes with clomipramine, compared with 0.87 minutes with placebo (p-value not reported).
When IELT was summarised across trials by reviews, Waldinger et al. 52 reported that, across RCTs, non-RCTs and single-arm studies, the mean percentage increase in delaying ejaculation was 512% (95% CI 234% to 1122%) with clomipramine. The reviewers reported a p-value compared with placebo of p < 0.001. Richardson et al. 69 estimated the mean increase in latency over baseline or placebo, combining data from different trials weighted by sample size. The latency increase was 3.66 minutes with clomipramine 25 mg and 5.31 minutes with clomipramine 50 mg. The reviewers reported a significant increase in latency for active treatment compared with baseline or placebo (p-values not reported).
The between-group difference in mean IELT change (minutes) following a 4-week randomised crossover comparison39 was 10.00 minutes in favour of sildenafil compared with clomipramine [MD (fixed effect); 95% CI 6.32 to 13.68 minutes; p < 0.00001]. Comparisons of clomipramine 25 mg with sertraline, paroxetine or the squeeze technique were not statistically significant (Figure 12). A paired analysis could not be undertaken for approximation purposes for this study. Data from this trial were not pooled with other RCTs in any meta-analysis in this assessment report. Summary results for these and all other meta-analyses are presented in Table 24.
FIGURE 12.
Tricyclic antidepressants, clomipramine compared with PDE5 inhibitors: forest plot of IELT outcomes. df, degrees of freedom; IV, inverse variance.

| Comparison | |||||||
|---|---|---|---|---|---|---|---|
| IELT | Outcome | Study duration | No. of RCTs | No. of participants | Effect estimate (MD) (95% CI) | Favours | p-value |
| Clomipramine vs. sildenafil | IELT (minutes) – end of study values | Crossover 4-week phases | 139 | 31 per treatment | –10.00 (–13.68 to – 6.32) | Sildenafil | < 0.00001 |
| Clomipramine vs. sertraline | IELT (minutes) – end of study values | Crossover 4-week phases | 139 | 31 per treatment | 2.00 (–0.68 to 4.68) | NS | 0.14 |
| Clomipramine vs. paroxetine | IELT (minutes) – end of study values | Crossover 4-week phases | 139 | 31 per treatment | 1.00 (–1.68 to 3.68) | NS | 0.46 |
| Clomipramine vs. squeeze technique | IELT (minutes) – end of study values | Crossover 4-week phases | 139 | 31 per treatment | 2.00 (–0.05 to 4.05) | NS | 0.06 |
| Clomipramine vs. fluoxetine | IELT (minutes) – end of study values | Crossover 4-week phases | 176 | 36 per treatment | 3.45 (1.16 to 5.74) | Clomipramine | 0.003 |
| Clomipramine vs. sertraline | IELT (minutes) – end of study values | Crossover 4-week phases | 176 | 36 per treatment | 1.48 (–1.38 to 4.34) | NS | 0.31 |
| Clomipramine vs. placebo | IELT (minutes) – end of study values | Crossover 4-week phases | 176 | 36 per treatment | 3.48 (0.97 to 5.99) | Clomipramine | 0.007 |
| Clomipramine vs. placebo | IELT various subjective and objective assessment measures | Varies | Varies – review summaries | Varies | Review summaries (including non-RCTs): % increase in delay in ejaculation: 512% (234–1122%)52 | Clomipramine | NR |
| Latency increase over baseline or placebo: clomipramine 25 mg, 3.66 minutes; 50 mg, 5.31 minutes | |||||||
| Inhaled 4mg clomipramine vs. placebo | IELT (minutes) – end of study values | 8 weeks | 1132 | 34 | 1.68 (1.06 to 2.29) | Clomipramine 4 mg | < 0.00001 |
| Inhaled clomipramine 1 or 2 mg vs. placebo | IELT (minutes) – end of study values | Crossover each five occasions | 1133 | 39 | Not assessed | NS (1 mg), clomipramine 2 mg | NR, 0.0108 |
| Other outcomes | |||||||
| Clomipramine vs. PDE5 inhibitors, SSRIs or placebo | Other effectiveness outcomes (various) | Crossover 4-week phases | 239,76 | 31 and 36 per treatment | Evidence from one crossover trial suggests treatment satisfaction scores was lower with clomipramine than sildenafil or paroxetine.39 Evidence from one crossover trial suggests that sexual satisfaction rating was greater with clomipramine than other therapies76 | ||
| 2 months | 1107 | 90 | Evidence from one RCT suggests no difference between clomipramine, sertraline and terazosin in ejaculatory control | ||||
| Inhaled clomipramine vs. placebo | Other effectiveness outcomes (various) | 8 weeks or five occasions | 2132,133 | 34 and 39 | Evidence from one RCT suggested nasal clomipramine improved CIPE scores but not IIEF-5 scores.132 Evidence from one RCT suggested ejaculatory control was statistically improved for clomipramine 2 mg spray, but not 1 mg spray, over placebo133 | ||
| Clomipramine (oral) vs. placebo | AEs | 2–7 weeks | 276,124 | 15 couples | Evidence from one RCT suggests a greater proportion of reporting of dry mouth, feeling ‘different’, and constipation with 50 mg compared with 25 mg.76 Evidence from one crossover trial suggests a greater proportion of patients receiving clomipramine experienced AEs than when receiving fluoxetine, sertraline or placebo124 | ||
| Inhaled clomipramine vs. placebo | AEs | RCT 8 weeks | 1132 | 34 | Clomipramine 4 mg, nasal irritation, dry mouth and headache | ||
| Crossover five occasions | 1133 | 39 | Clomipramine 1 and 2 mg – dose-related: local irritation (cough, throat irritation, respiratory tract irritation) | ||||
| 2 months | 1107 | 90 | Evidence from one RCT suggests no difference between clomipramine, sertraline and terazosin in AEs | ||||
The crossover trial by Kim and Seo76 reported that mean [standard deviation (SD)] post-treatment IELT (minutes) was 2.30 minutes (SD 2.08 minutes) with fluoxetine 40 mg, 4.27 minutes (SD 5.68 minutes) with sertraline 100 mg, 5.75 minutes (SD 6.68 minutes) with clomipramine 50 mg and 2.27 minutes (SD 3.78 minutes) with placebo, and that IELT was significantly increased in all treatment phases (p < 0.001). The between-group comparisons from this study estimated in RevMan for this assessment report are presented in Figure 13. The between-group difference in mean IELT (minutes) was 3.45 minutes in favour of clomipramine 100 mg compared with fluoxetine [MD (fixed effect); 95% CI 1.65 to 5.75 minutes; p = 0.003] and 3.48 minutes in favour of clomipramine 100 mg compared with placebo [MD (fixed effect); 95% CI 0.97 to 5.99 minutes; p = 0.007]. The comparison of clomipramine with sertraline was not statistically significant (Figure 13). A paired analysis could not be undertaken for approximation purposes for this study. Data from this trial were not pooled with other RCTs in any meta-analysis in this assessment report.
FIGURE 13.
Tricyclic antidepressants, clomipramine compared with SSRIs or placebo: forest plot of IELT outcomes. df, degrees of freedom; IV, inverse variance.
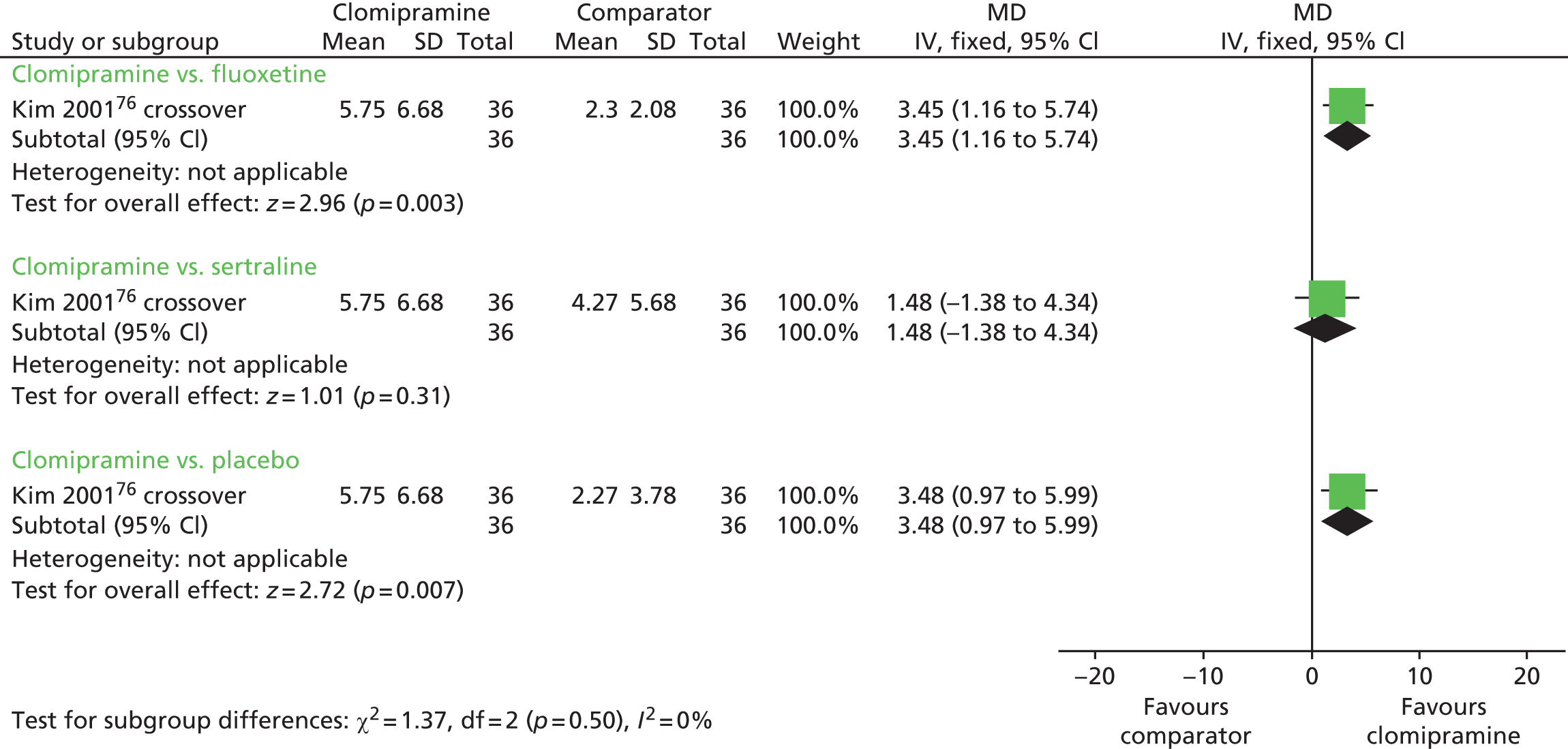
The between-group difference in mean IELT (minutes) post treatment, based on one RCT132 (n = 34), was 1.68 minutes [MD (fixed effect) 95% CI 1.06 to 2.29 minutes; p < 0.00001] in favour of the clomipramine spray (figure not presented). The RCT by Leaker et al. 133 reported end of study IELT values without variance estimates. The authors reported a p-value of p = 0.0108 for the comparison of inhaled clomipramine 2 mg compared with placebo and that the comparison of inhaled clomipramine 1 mg with placebo was not statistically significant (p-value not reported).
Assessment of effectiveness: tricyclic antidepressants – other outcomes
With the exception of the RCTs that were reported only in the review by Waldinger et al. ,52 all of the included trials reported one or more outcomes in addition to IELT. However, these outcomes were diverse across the include trials and were often not reported in sufficient detail to permit any pooling across trials (Table 25).
| RCT, duration | Treatment | Outcome measure | Results of sexual satisfaction score | Between-group difference reported as significant | AEs |
|---|---|---|---|---|---|
| Abdel-Hamid et al. 2001,39 4 weeks | Clomipramine 25 mg | Sexual satisfaction score | Sildenafil, 3; clomipramine, 1.1; sertraline, 1; paroxetine, 1.2; squeeze technique, 0.6 | Unclear | Headache, flushing, and nasal congestion: sildenafil, 18% |
| Sertraline 50 mg | EDITS (scale 0–5) | Clomipramine, 11; sertraline, 11; sildenafil, 30; paroxetine, 9; squeeze technique, 6 | Unclear | The incidence of side effects was similar among groups. Numbers NR | |
| Sildenafil 50 mg | Arabic Anxiety Inventory (scale 0–30) | Unclear if reported values are means or medians. No variance estimates or p-values reported | |||
| Paroxetine 20 mg | |||||
| Squeeze technique | |||||
| Total n = 31 | |||||
| Akilov et al. 2011,132 8 weeks | Clomipramine 4 mg nasal spray (n = 19) | CIPE | CIPE change from baseline: nasal clomipramine – p < 0.05; placebo – NR | Unclear | Nasal irritation: Cl spray, n = 3; placebo, n = 1 |
| Placebo nasal spray (n = 15) | IIEF-5 | IIEF-5 change from baseline: nasal clomipramine – p > 0.01; placebo – p > 0.01 | Unclear | Dry mouth and headache: Cl spray: n = 2 (caused discontinuation in n = 1) | |
| Althof et al. 1995,124 2 to 7 weeks | Clomipramine 25 mg | Symptom Checklist- 90-Revised, Dyadic Adjustment Scale, State-Trait Anxiety Inventory, Harder Self-Esteem Inventory | NR | NR | Clomipramine 25 mg/day: dry mouth (7%), feeling ‘different’ (8%), constipation (1%) |
| Clomipramine 50 mg | Clomipramine 50 mg/day: dry mouth (33%), feeling ‘different’ (21%), constipation (18%) | ||||
| Placebo | |||||
| Total n = 15 couples | |||||
| Haensel et al. 1966,127 6 weeks | Clomipramine 25 mg | Pelvic thrusts and time ejaculation, orgasm sooner than desired, within 1–2 minutes and after fewer than 10 pelvic thrusts | NR | NR | NR |
| Placebo | |||||
| Total n = 24 | |||||
| Montorsi et al. 1995,128 | Clomipramine | NR | NR | NR | NR |
| Placebo | |||||
| Porto 1981129 | Clomipramine | NR | NR | NR | NR |
| Placebo | |||||
| Segraves et al. 1993130 | Clomipramine | NR | NR | NR | NR |
| Placebo | |||||
| Strassberg et al. 1999131 | Clomipramine | NR | NR | NR | NR |
| Placebo | |||||
| Kim and Seo 1998,76 each agent for 4 weeks, with 1-week washout | Clomipramine 50 mg | Patient self-reported questionnaire on patient and partner sexual satisfaction | Sexual satisfaction, n/N ‘yes’: satisfaction rating greater with clomipramine (n NR). p-value for between-group difference NR | Results of sexual satisfaction ratings were reported as statistically significant | Percentage experiencing AEs: clomipramine 50 mg, 23%; fluoxetine 40 mg, 13%; sertraline 100 mg, 12%. Placebo, NR. p-value for clomipramine vs. sertraline and fluoxetine p < 0.05 |
| Fluoxetine 40 mg | |||||
| Sertraline 100 mg | |||||
| Placebo | |||||
| Total n = 36 | |||||
| Leaker et al. 2008,133 each treatment for five occasions | Inhaled clomipramine 1 mg or placebo | IVELT sexual satisfaction score | IVELT – no data or p-value | Unclear | Dose-related incidence of AEs characterised by local irritation for the 2 mg group – cough, 70%; throat irritation, 70%; and respiratory tract irritation 35% |
| Inhaled clomipramine 2 mg or placebo | Ejaculatory control (no details of instrument) | Ejaculatory control: inhaled clomipramine 1 mg vs. placebo – p-value NR; inhaled clomipramine 2 mg vs. placebo – p = 0.0082 | Yes for clomipramine 2 mg spray | ||
| 39 analysed | |||||
| Tuncel et al. 2008,107 treatment was for 2 months | Clomipramine 25 mg/day (n = 20) | Clinical responses (assume control of ejaculation), self-assessed | Ejaculation control: n/N (%) reporting ‘no change’, ‘improvement’, ‘under control’ | Yes compared with placebo | % AEs: Clomipramine – headache, 34.8%; hypotension, 4%; drowsiness, 8.6%; ejaculation disorder, 0% |
| Sertraline 50 mg/day (n = 23) | All three treatments were ‘superior to placebo’ – p = 0.0017 | Sertraline – headache, 25%; hypotension, 0%; drowsiness, 15%; ejaculation disorder, 0% | |||
| Terazosin 5 mg/day (n = 25) | No significant difference in efficacy between ‘medical treatments’ – p = 0.53 | Terazosin – headache, 20%; hypotension, 12%; drowsiness, 0%; ejaculation disorder, 8% | |||
| Placebo (n = 22) | Placebo – headache, 9.1%; hypotension, 0%; drowsiness, 0%; ejaculation disorder, 0% | ||||
| No significant differences between ‘medical treatment groups’ – p = 0.204 |
In the crossover study by Abdel-Hamid et al. ,39 Erectile Dysfunction Inventory of Treatment Satisfaction (EDIT) scores appeared lower with clomipramine than with sildenafil or paroxetine. Kim and Seo76 reported that a sexual satisfaction rating was greater with clomipramine than other therapies; however, no data for clomipramine or p-value were reported. Tuncel et al. 107 reported that clomipramine, sertraline and terazosin were all significantly better than placebo on ejaculation control, but that there was no significant difference between the active treatments on this outcome.
Akilov et al. 132 reported that CIPE scores improved significantly with nasal clomipramine; however, there was no significant change in the five-item version of the IIEF scores. Leaker et al. 133 assessed IELT sexual satisfaction but did not report any outcome data. The between-group difference in ejaculatory control between inhaled clomipramine and placebo was statistically significant in favour of clomipramine 2 mg spray, but not 1 mg spray.
Assessment of safety: tricyclic antidepressants – adverse events
Althof et al. 124 reported the proportion of patients receiving clomipramine 25 mg or 50 mg who experienced dry mouth, feeling ‘different’ and constipation (number not reported). The proportions were noticeably higher in the 50 mg group than in the 25 mg group (see Table 25). Proportions for the placebo group were not reported. Tuncel et al. 107 reported that there were no significant differences between clomipramine, sertraline and terazosin in the number of patients reporting AEs of headache, hypotension, drowsiness and ejaculation disorder.
Abdel-Hamid et al. 39 reported that the incidence of side effects was similar among groups, but the types of side effects associated with clomipramine were not reported. A greater proportion of patients receiving clomipramine experienced AEs than when receiving fluoxetine, sertraline or placebo in the crossover trial by Kim and Seo. 76 The authors reported that the between-group difference compared with placebo was significant. No other statistical comparison between groups was reported.
Akilov et al. 132 reported that the most common side effect with nasal clomipramine was nasal irritation. Leaker et al. 133 reported that the incidence of AEs of local irritation cough, sore throat and respiratory tract infection was dose related (1 mg or 2 mg).
Assessment of effectiveness: tricyclic antidepressants – evidence summary
The current evidence base for clomipramine in the treatment of PE comprises 13 RCTs, 10 captured in low to moderate methodological quality systematic reviews39,76,107,124–133 and three further RCTs which are at overall unclear risk of bias. 39,76,124–131 Both oral and nasal administration of clomipramine is evaluated in the evidence base. The quality of reporting in some reviews does not facilitate data extrapolation of IELT and other data from RCTs therein.
Evidence from one crossover trial suggests that oral sildenafil is more effective than oral clomipramine in increasing IELT in men with PE. 39 Evidence from another crossover trial suggests that oral clomipramine is more effective than fluoxetine at increasing IELT. 76 There is evidence from one RCT (39 participants)132 that clomipramine administered nasally (spray) at 4 mg is significantly effective when compared with placebo at increasing IELT [1.68 minutes (95% CI 1.06 to 2.29 minutes); p < 0.00001)]. Evidence from a further crossover trial (39 participants) suggests that inhaled clomipramine at 2 mg is also significantly effective compared with placebo. 133 No significant effects are evident at 1 mg. Summary evidence from one review52 that estimated a weighted mean increase in IELT across included studies and one review that estimated a mean percentage increase in IELT across RCTs, non-RCTs, and from single-arm studies, suggests that oral clomipramine may be more effective than placebo on this outcome. 69
Various assessment methods in terms of treatment satisfaction, sexual satisfaction and ejaculation control have been used across RCTs to measure the effectiveness of clomipramine. Evidence from one crossover trial suggests that treatment satisfaction is greater with oral sildenafil and paroxetine than with oral clomipramine. 39 Evidence from one crossover trial suggests that sexual satisfaction is greater with oral clomipramine than with SSRIs (fluoxetine and sertraline). 39 Evidence from one RCT suggests that there is no difference between oral clomipramine, sertraline and terazosin in effect on ejaculatory control. 107 Evidence from one RCT suggests that ejaculatory control is better with inhaled clomipramine at 2 mg than 1 mg. 133 Evidence from one crossover trial suggests that clomipramine is associated with a greater incidence of AEs than fluoxetine or sertraline; however, the nature of the AEs is unknown. 76 Evidence from one RCT suggests that there is no significant difference between oral clomipramine, sertraline and terazosin in the number of patients reporting AEs of headache, hypotension, drowsiness and ejaculation disorder. 107
Nasal clomipramine is associated with nasal, throat and respiratory tract irritation, with greater incidence at 2 mg than 1 mg application. 133
Clomipramine appears to be more effective than fluoxetine or paroxetine but not as effective as sildenafil in the treatment of PE. However, these findings should be interpreted with caution given that they are extrapolated from poorly reported crossover observations with low patient numbers. Inhaled clomipramine appears effective at increasing IELT but efficacy appears to be dose dependent, as do treatment-related side effects of application-associated irritation. The current evidence base for oral administration in the treatment of PE in terms of both efficacy and safety of clomipramine along with patient acceptability is limited.
Phosphodiesterase-5 inhibitors
Characteristics of included studies: phosphodiesterase-5 inhibitors
Phosphodiesterase-5 inhibitors were evaluated by five systematic reviews,37,134–137 one of which pooled data in a meta-analysis. 134 Two further RCTs evaluating PDE5 inhibitors were identified. 101,120
Two of the systematic reviews were conducted in Italy,134,135 one review was conducted in Australia,136 one in Israel137 and one in the USA. 37 Details of the review type, the databases searched and dates, included RCTs and the AMSTAR quality assessment for these reviews of effectiveness are presented in Table 26. The overall AMSTAR quality score was 2 out of 11 in three of the reviews,134,136,137 3 out of 11 in one review135 and 4 out of 11 in one review. 37 However, the review by Asimakopoulos et al. 134 was the most comprehensive in terms of included studies. Full details of the AMSTAR assessment for these and all other included reviews are presented in Appendix 4. The search methodology and inclusion criteria for studies were varied across these reviews. In the review by Asimakopoulos et al. ,134 which included a meta-analysis, the authors pooled IELT effect estimates across studies using a standardised MD. These authors also pooled data across different study types (observation studies and RCTs) in the same meta-analysis.
| Author (country), review type | Databases searched and dates | Included RCTs relevant to this section | AMSTAR review quality assessment |
|---|---|---|---|
| Asimakopoulos et al. 2012134 (Italy), systematic review and meta-analysis | PubMed January 1990 and June 2011 | Abdel-Hamid et al. 2001,39 Atan et al. 2006,55 Aversa et al. 2009,138 Hosseini and Yarmohammadi 2007,139 Mathers et al. 2009,140 Mattos et al. 2008,141 McMahon et al. 2005142 Tang et al. 2004,143 Wang et al. 2007,144 Zhang et al. 2005145 | AMSTAR score, 2/11:
|
| Aversa et al. 2011135 (Italy), systematic review | MEDLINE up to a May 2010. No start date | Abdel-Hamid et al. 2001,39 Aversa et al. 2009,138 Hosseini et al. 2007,139 Mathers et al. 2009,140 Mattos et al. 2008,141 McMahon et al. 2005,142 Wang et al. 2007144 | AMSTAR score, 3/11:
|
| Burton and Liday 2011136 (Australia), systematic review | MEDLINE (January 1980–April 2011) and International Pharmaceutical Abstracts (January 1970–April 2011) | Hosseini and Yarmohammadi 2007,139 Mattos et al. 2008,141 | AMSTAR score, 2/11:
|
| Chen et al. 2007137 (Israel), systematic review | MEDLINE 1 January 1990 to 28 February 2007 | Abdel-Hamid et al. 2001,39 Atan et al. 2006,55 McMahon et al. 2005142 | AMSTAR score, 2/11:
|
| McMahon et al. 200637 (USA), systematic review | MEDLINE, Web of Science, PICAa and EMBASE between 1998 and 2005 | Abdel-Hamid et al. 2001,39 Atan et al. 2006,55 McMahon et al. 2005,142 Tang et al. 2004,143 Zhang et al. 2005145 | AMSTAR score, 4/11:
|
The reviews above varied in terms of which RCTs they included. In total, 10 RCTs39,55,138–145 (total 795 participants) were included in the review by Asimakopoulos et al. 134 The other reviews included different subsets of these RCTS. Seven RCTs assessed sildenafil. 39,55,139,142–145 Among these trials the dose was 50 mg or greater, administered a few hours preintercourse. Sildenafil was combined with fluoxetine in one trial139 and with behavioural therapy in another,143 i.e. there was no sildenafil-only arm in these two trials. One RCT assessed tadalafil 20mg one to 36 hours preintercourse141 and two RCTs assessed vardenafil. 138,140 The vardenafil doses for these RCTs were not available from any reviews.
Intravaginal ejaculatory latency time was reported as being measured using a stopwatch in all but one RCT. 55 When reported, duration of the RCTs included in the reviews ranged from 4 weeks to 4 months. Comparators to PDE5 inhibitors within these RCTs were SSRIs (various), clomipramine, behavioural therapy (squeeze technique), CBT, topical anaesthetics (EMLA cream) and placebo. Details of the RCTs extracted from these reviews are presented in Table 27. All RCTs in reviews were captured by the search strategy for this assessment report.
| RCTs extracted from reviews | ||||||
|---|---|---|---|---|---|---|
| RCT (source) | Duration | Treatments | PE definition | Lifelong/acquired | IELT assessment | Other outcomes |
| Abdel-Hamid et al. 200139 (reviews37,134,135,137) | RCT crossover | Sildenafil 50 mg 1 hour precoitus | IELT ≤ 2 minutes | Lifelong | Stopwatch | Modified Erectile Dysfunction Inventory of Treatment Satisfaction, Arabic Anxiety Inventory (scale 0–30) |
| 4 weeks each 2-week washout | Clomipramine 25 mg 3–5 hours precoitus | |||||
| Sertraline 50 mg 3–5 hours precoitus | ||||||
| Paroxetine 20 mg 3–5 hours precoitus | ||||||
| Squeeze technique (total n = 31) | ||||||
| Atan et al. 200655 (reviews37,134,137) | 8 weeks | Sildenafil 50 mg 45 minutes precoitus (n = 20) | DSM-IV | Lifelong and acquired | IELT not assessed | Self-reported improvement: ‘no change’, ‘improvement’, ‘cure’ |
| Sildenafil 50 mg 45 minutes precoitus + topical EMLA 15 minutes precoitus (n = 15) | ||||||
| Topical EMLA 15 minutes precoitus (n = 22) | ||||||
| Placebo (n = 20) | ||||||
| Aversa et al. 2009138 (reviews134,135) | NR | Vardenafil (n = 31) | NR | Lifelong | Stopwatch | IPE |
| Placebo (n = 11) | ||||||
| Hosseini et al. 2007139 (reviews134–136) | 4 months | Sildenafil 50 mg 1 hour precoitus + fluoxetine 20 mg 2–3 hours precoitus (n = 43) | NR | Lifelong | Stopwatch | Intercourse satisfaction (instrument not reported) |
| Fluoxetine 10 mg twice daily for 4 weeks then 20 mg 3 hours precoitus (n = 48) | ||||||
| Mathers et al. 2009140 (reviews134,135) | NR | Behavioural therapy (not described) followed by vardenafil (n = 36) | NR | Lifelong | Stopwatch | PE grade |
| Behavioural therapy followed by sertraline (n = 36) | ||||||
| Mattos et al. 2008141 (reviews134–136) | 12 weeks | Tadalafil 20 mg 1–36 hours precoitus (n = 15) | NR | Lifelong | Stopwatch | NR |
| Fluoxetine 90 mg weekly (n = 15) | ||||||
| Tadalafil + fluoxetine (n = 15) | ||||||
| Placebo (n = 15) | ||||||
| McMahon et al. 2005142 (reviews37,134,135,137) | 8 weeks | Sildenafil 50–100 mg 1 hour precoitus (n = 78) | DSM-IV, IELT ≤ 2 minutes | Lifelong | Stopwatch | IPE |
| Placebo (n = 79) | ||||||
| Tang et al. 2004143 (reviews37,134) | 6 weeks | Sildenafil 50mg + behavioural therapy (n = 30) | NR | NR | Stopwatch | Patient/partner sexual satisfaction (0–5-point Likert scale) |
| Behavioural therapy (n = 30) | ||||||
| Wang et al. 2007144 (reviews134,135) | NR | Sildenafil as needed (n = 60) | NR | Lifelong | Stopwatch | PE grade, intercourse satisfactory score, frequency of intercourse |
| Paroxetine (n = 60) | ||||||
| Squeeze technique (n = 60) | ||||||
| Zhang et al. 2005141 (reviews37,134) | 12 weeks | Sildenafil 50 mg + sertraline 50 mg 4–6 hours precoitus | NR | Lifelong and acquired | Stopwatch | IIEF |
| Sertraline 50 mg 4–6 hours precoitus | ||||||
| (Total n = 72) | ||||||
| Further RCTs identified by searches (not captured in reviews) | ||||||
| RCT (country) risk of bias | Duration | Treatments, numbers analysed/randomised (%) | PE definition | Lifelong/acquired | IELT assessment | Other outcomes |
| Culba et al. 2008101 (Turkey), unclear | 10 weeks | Tadalafil 20 mg twice weekly + fluoxetine 20 mg per day | NR | NR | Visual scale of ELTQ | IIEF |
| Fluoxetine 20 mg per day | IIEC | |||||
| Placebo | PE question of CMASH questionnaire | |||||
| (Total n = 180) | ||||||
| Total 158/180 (88%) | ||||||
| Lee et al. 2012120 (Republic of Korea), low | 12 weeks | Mirodenafil 50 mg + dapoxetine 30 mg, 1–3 hours precoitus (n = 63) | DSM-IV | Lifelong | Stopwatch | Time from foreplay to beginning intercourse |
| Dapoxetine 30 mg + placebo, 1–3 hours precoitus (n = 57) | OSAT | |||||
| Mirodenafil + dapox, 62/63 (98%) | PEP | |||||
| Dapoxetine + placebo, 56/57 (98%) | ||||||
The RCT by Culba et al. 101 was undertaken in Turkey and patients were randomised to fluoxetine 20 mg per day plus tadalafil 20 mg twice weekly, fluoxetine 20 mg per day alone, or placebo. The authors reported that 158 out of 180 (88%) completed the 10-week follow-up. This study was reported in abstract form only and outcome data were not presented by the treatments evaluated. This trial was considered to be at overall unclear risk of bias. The RCT by Lee et al. 120 was undertaken in the Republic of Korea and patients were randomised to dapoxetine 30 mg plus mirodenafil 50 mg or dapoxetine 30 mg plus placebo. All agents were taken 1–3 hours preintercourse. IELT was assessed using a stopwatch. In each group, 98% of patients were analysed. This trial was considered to be at overall low risk of bias and is also evaluated in the section Selective serotonin reuptake inhibitors licensed for premature ejaculation (dapoxetine).
Details of the treatments evaluated, definition of PE, IELT assessment method, other outcomes assessed, study duration, along with the study country for the further RCTs not in reviews, and the overall study quality assessment (AMSTAR33 for reviews and Cochrane risk of bias assessment34 for the RCTs not included by reviews) are presented in Table 27.
Assessment of effectiveness: phosphodiesterase-5 inhibitors – intravaginal ejaculatory latency time outcomes
For three RCTs, IELT data suitable for meta-analysis were not available. One RCT55 that evaluated sildenafil and EMLA cream did not assess IELT. Post-treatment IELT data were available for one RCT assessing sildenafil and fluoxetine;139 however, no variance estimates or p-values were reported. In one RCT assessing tadalafil and fluoxetine,101 no IELT data were reported. These trials were therefore not included in any IELT meta-analysis in this assessment report.
Evidence synthesis intravaginal ejaculatory latency time
The between-group difference in mean increase in IELT (minutes) was 2.59 minutes in favour of tadalafil compared with placebo at 8 weeks [MD (fixed effect); 95% CI 1.28 to 3.90 minutes; p = 0.0001]. However, the between-group difference at 12 weeks between sildenafil and placebo was not significant [MD (fixed effect) 1.03 minutes; 95% CI –0.39 to 2.45 minutes; p = 0.16]. The pooled effect estimate across these RCTs (I2 = 59.9%, random effects) was 1.84 minutes (95% CI 0.31 to 3.36 minutes; p = 0.02). The between-group difference in geometric mean increase in IELT from one RCT138 was 3.80 minutes in favour of vardenafil compared with placebo [MD (fixed effect); 95% CI 3.30 to 4.30 minutes; p < 0.00001]. The forest plot for this analysis is presented in Figure 14. Results for this and all other meta-analyses are presented in Table 28.
FIGURE 14.
Phosphodiesterase-5 inhibitors compared with placebo: forest plot of IELT outcomes. df, degrees of freedom; IV, inverse variance.

| Comparison | Outcome | n RCTsa | Participants | Model | MD effect estimate (95% CI) | Favours | p-value |
|---|---|---|---|---|---|---|---|
| Vardenafil vs. placebo | IELT (minutes) – geometric mean change from baseline | 1138 | 42 | N/A | 3.80 (3.30 to 4.30) | Vardenafil | < 0.00001 |
| Sildenafil vs. placebo | IELT (minutes) – end of study values | 1142 | 157 | N/A | 1.03 (–0.39 to 2.45) | NS | 0.16 |
| Tadalafil vs. placebo | IELT (minutes) – change from baseline | 1141 | 30 | N/A | 2.59 (1.28 to 3.90) | Tadalafil | 0.0001 |
| Sildenafil vs. sertraline | IELT (minutes) – single-arm, randomised crossover | 139 crossover | 31 | N/A | 12.00 (7.70 to 16.30) | Sildenafil | < 0.00001 |
| Sildenafil vs. paroxetine | IELT (minutes) – single-arm, randomised crossover | 139 crossover | 31 | N/A | 11.00 (6.70 to 15.30) | Sildenafil | < 0.00001 |
| Sildenafil vs. paroxetine | IELT (minutes) – change from baseline | 1144 | 120 | N/A | 1.26 (0.81 to 1.71) | Sildenafil | < 0.00001 |
| Tadalafil vs. fluoxetine | IELT (minutes) – change from baseline | 1145 | 30 | N/A | –0.06 (–1.56 to 1.44) | NS | 0.94 |
| BT then vardenafil vs. BT then sertraline | IELT (minutes) – change from baseline | 1140 | 72 | N/A | 1.89 (0.54 to 3.24) | Vardenafil | 0.006 |
| Sildenafil vs. clomipramine | IELT (minutes) – single-arm, randomised crossover | 139 crossover | 31 | N/A | 10.00 (6.32 to 13.68) | Sildenafil | < 0.00001 |
| PDE5 inhibitors + SSRIs vs. SSRIs | IELT (minutes) | 3120,141,145 | 222 | Fixed effect I2 = 0% | 1.70 (1.64 to 1.76) | PDE5 + SSRI | < 0.00001 |
| Sildenafil vs. squeeze technique | IELT (minutes) – single-arm, randomised crossover | 139 crossover | 31 | N/A | 12.00 (8.06 to 15.94) | Sildenafil | < 0.00001 |
| Sildenafil vs. squeeze technique | IELT (minutes) – change from baseline | 1144 | 120 | N/A | 3.56 (3.16 to 3.96) | Sildenafil | < 0.00001 |
| Sildenafil + BT vs. BT | IELT (minutes) – end of study values | 1143 | 60 | N/A | 1.81 (1.53 to 2.09) | Sildenafil | < 0.00001 |
The between-group difference in mean IELT change (minutes) following a 4-week randomised crossover comparison39 was 12.00 minutes in favour of sildenafil compared with sertraline [MD (fixed effect); 95% CI 7.70 to 16.30 minutes; p < 0.00001], 11.00 minutes in favour of sildenafil compared with paroxetine [MD (fixed effect); 95% CI 6.70 to 15.30 minutes; p < 0.00001] and 10.00 minutes in favour of sildenafil compared with clomipramine [MD (fixed effect) 95% CI 6.32 to 13.68 minutes; p < 0.00001]. A paired analysis could not be undertaken for approximation purposes for this study. Data from this trial were not pooled with other RCTs in any meta-analysis in this assessment report. This trial is also evaluated in the Behavioural interventions, Selective serotonin reuptake inhibitors not currently licensed for premature ejaculation, Selective serotonin reuptake inhibitors licensed for premature ejaculation and Tricyclic antidepressants sections.
The between-group difference in mean increase in IELT was 1.26 minutes in favour of sildenafil compared with paroxetine (duration unclear) [MD (fixed effect); 95% CI 0.81 to 1.71 minutes; p < 0.00001]. The between-group difference in mean increase in IELT (minutes) between tadalafil and fluoxetine at 12 weeks was –0.06 minutes [MD (fixed effect); 95% CI –1.56 to 1.44 minutes; p = 0.94].
The between-group difference in mean increase in IELT was 1.89 minutes in favour of behavioural therapy followed by vardenafil compared with behavioural therapy followed by sertraline (duration unclear) [MD (fixed effect); 95% CI, 0.54 to 3.24 minutes; p = 0.006]. A moderate level of heterogeneity was observed across the non-crossover RCTs comparing PDE5 inhibitors with SSRIs (I2 = 47%). The between-group difference in mean increase in IELT across these RCTs (random effects) was 1.14 minutes [95% CI 0.31 to 1.96 minutes; p = 0.007].
The forest plot for these comparisons is presented in Figure 15.
FIGURE 15.
Phosphodiesterase-5 inhibitors compared with SSRIs or TCAs: forest plot of IELT outcomes. df, degrees of freedom; IV, inverse variance.

Meta-analysis of mean IELT (minutes) at 12 weeks, based on three RCT comparisons120,141,145 (222 participants), displayed low heterogeneity (I2 = 0%). The pooled MD in IELT in favour of PDE5 inhibitors plus SSRIs compared with PDE5 inhibitors alone was 1.70 minutes [MD (fixed effect); 95% CI 1.64 to 1.76 minutes; p < 0.00001]. Of note, the trial evaluating sildenafil plus sertraline by Zhang et al. ,145 which was highly significant, was awarded 99.9% of the weight in the analysis. The forest plot for this analysis is presented in Figure 16.
FIGURE 16.
Phosphodiesterase-5 inhibitors plus SSRIs compared with SSRIs: forest plot of IELT outcomes. df, degrees of freedom; IV, inverse variance.
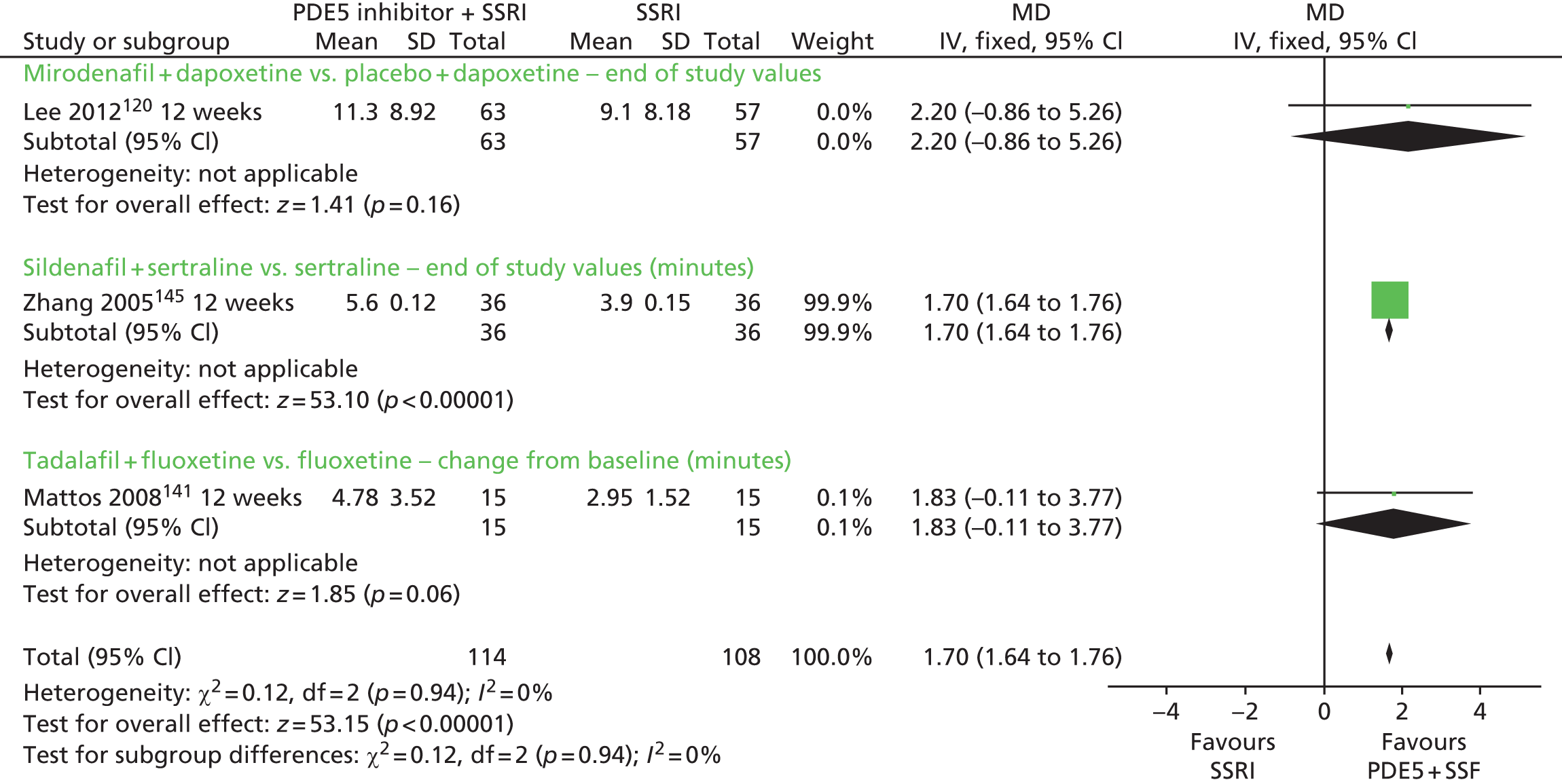
The between-group difference in mean IELT change (minutes) following a 4-week randomised crossover comparison39 was 12.00 minutes in favour of sertraline compared with the squeeze technique [MD (fixed effect); 95% CI 8.06 to 15.94 minutes; p < 0.00001]. A paired analysis could not be undertaken for approximation purposes. Data from this trial were not pooled with other RCTs.
The between-group difference in mean IELT (minutes) post treatment (duration unclear) was 3.56 minutes in favour of sildenafil compared with the squeeze technique [MD (fixed effect); 95% CI 3.16 to 3.96 minutes; p < 0.00001]. The between-group difference in mean IELT change (minutes) post treatment was 1.81 minutes in favour of sildenafil plus behavioural therapy compared with behavioural therapy (duration 4 weeks) [MD (fixed effect); 95% CI 1.53 to 2.09 minutes; p < 0.00001]. A high level of heterogeneity was observed across the non-crossover RCTs comparing PDE5 inhibitors with behavioural interventions (I2 = 97%, meta-analysis not undertaken). The forest plot for this analysis is presented in Figure 17.
FIGURE 17.
Phosphodiesterase-5 inhibitors compared with behavioural interventions: forest plot of IELT outcomes. df, degrees of freedom; IV, inverse variance.
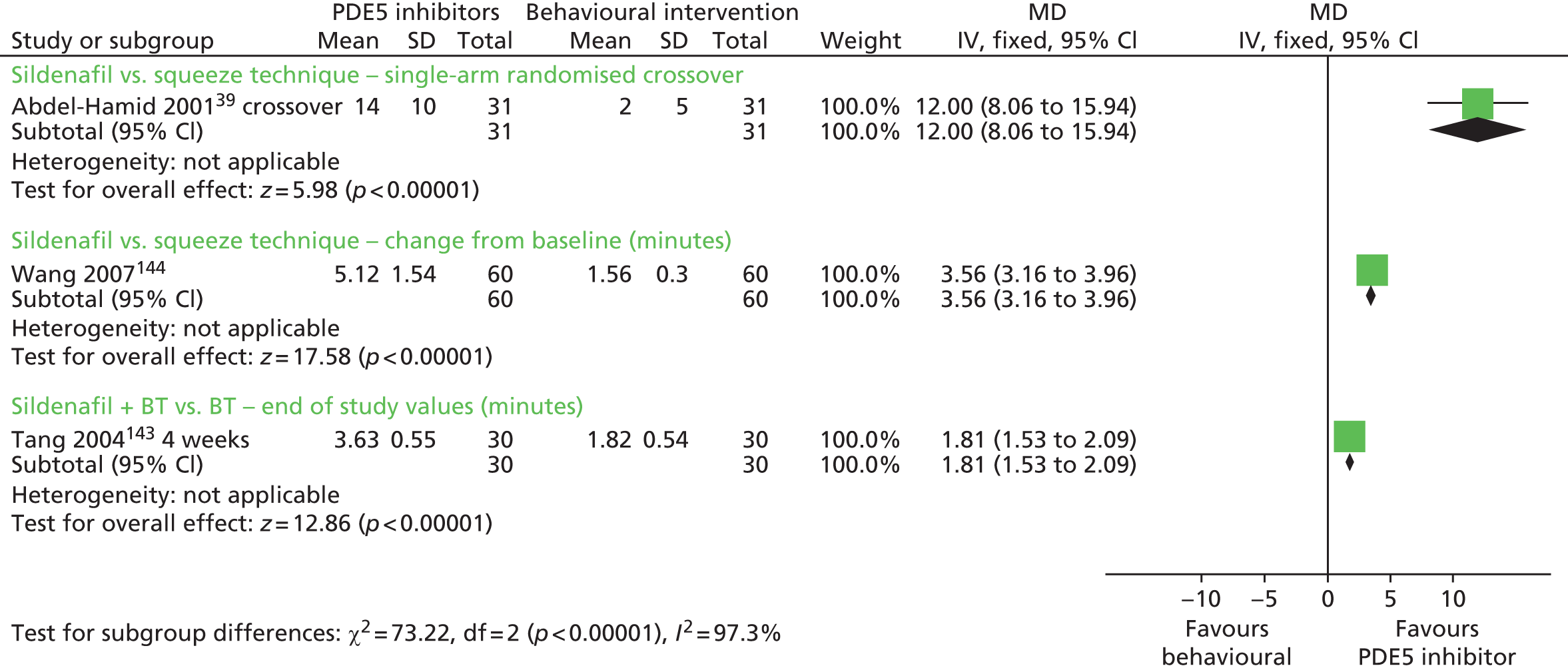
Assessment of effectiveness: phosphodiesterase-5 inhibitors – other outcomes
Outcomes other than IELT were reported across the RCTs that were captured in reviews using a diversity of instruments (sometimes not reported which) and outcome data. In some instances it was unclear if the metric was an end of study or change from baseline value, or if the value was a mean or median. In a large proportion of the RCTs, a variance estimate for the outcome was not reported. Either p-values were not available for the majority of the RCTs or, when they had been reported, it was unclear if this was for a between- or across-group comparison (Table 29).
| RCT, duration | Treatment | Outcome measure | Results | Between-group difference reported as significant | AEs |
|---|---|---|---|---|---|
| Abdel-Hamid et al. 2001,39 4 weeks | Sildenafil 50 mg | EDITS (scale 0–5): sexual satisfaction score | Clomipramine, 11; sertraline, 11; sildenafil, 30; paroxetine, 9; squeeze technique, 6 | NR | Headache, flushing, and nasal congestion: sildenafil, 18% |
| Clomipramine 25 mg | Arabic Anxiety Inventory (scale 0–30) | Clomipramine, 11; sertraline, 10; sildenafil, 15; paroxetine, 12; squeeze technique, 3 | NR | The incidence of side effects was similar among groups (numbers NR) | |
| Sertraline 50 mg | (Unclear if means or medians; no SD or p-values reported) | ||||
| Paroxetine 20 mg | |||||
| Squeeze technique | |||||
| (Total n = 31) | |||||
| Atan et al. 2006,55 8 weeks | Sildenafil 50 mg (n = 20) | Self-reported improvement: ‘no change’, ‘improvement’, ‘cure’ | ‘Improvement’ or ‘cure’: Sildenafil, 55% (p > 0.05) | Unclear | Headache: sildenafil, 26%; flushing: sildenafil, 26%. Only patients receiving sildenafil experienced side effects |
| Sildenafil 50 mg + EMLA (n = 15) | Sildenafil + EMLA, 86% | ||||
| EMLA (n = 22) | EMLA, 77% | ||||
| Placebo (n = 20) | Placebo, 40% (NR if p-value across or between-groups) | ||||
| Aversa et al. 2009,138 duration NR | Vardenafil (n = 31) | IPE: sexual satisfaction | % increase (fold increase): vardenafil, 114% (twofold) | Unclear | NR |
| Placebo (n = 11) | Placebo, % NR, (0-fold) (p-value NR) | ||||
| Culba et al. 2008,101 10 weeks | Tadalafil 20 mg + fluoxetine 20 mg | IELT via visual scale ELTQ and IIEF | Patients who were treated with fluoxetine + tadalafil had better scores than placebo with all questionnaires | Yes | Minor side effects owing to tadalafil and fluoxetine were temporary. No data reported |
| Fluoxetine 20 mg | IIEC | Difference was not significant compared with fluoxetine group. No data reported | |||
| Placebo | PE question of CMASH questionnaire | ||||
| (Total n = 180) | |||||
| Hosseini et al. 2007,139 4 months | Sildenafil 50 mg + fluoxetine 20 mg (n = 43) | Sexual satisfaction, instrument NR | % increase (fold increase): sildenafil + fluoxetine, 55% (3.3-fold) | Unclear | NR |
| Fluoxetine 20 mg | Fluoxetine, 20% (1.2-fold) (p-value NR) | ||||
| Lee et al. 2012,120 12 weeks | Mirodenafil 50 mg + dapoxetine 30 mg (n = 63) | Time from foreplay to beginning intercourse | Significant between-group difference in: | Yes, but only some OSAT and PEP outcomes | See dapoxetine section in this assessment report |
| Dapoxetine 30 mg + placebo (n = 57) | OSAT | OSAT week 4, p = 0.049; week 8, p = 0.026; week 4 to 8, p = 0.040 | |||
| PEP | PEP week 12: perceived control over ejaculation, p = 0.019; interpersonal difficulty related to ejaculation, p = 0.013; index score, p = 0.046 | ||||
| Mathers et al. 2009,140 duration NR | BT then vardenafil (n = 36) | NR | NR | N/A | Tadalafil: headache (3 patients), facial redness (2 patients), palpitation (2 patients) |
| BT then sertraline (n =36) | Fluoxetine: yawning and somnolence (3 patients), asthenia (3 patients), nausea (1 patient) | ||||
| Fluoxetine + tadalafil: yawning and somnolence (3 patients), nausea (2 patients), palpitation (1 patient), muscle soreness (1 patient) | |||||
| Mattos et al. 2008,141 12 weeks | Tadalafil 20 mg (n = 15) | NR | NR | N/A | NR |
| Tadalafil 20 mg + fluoxetine 90 mg (n = 15) | |||||
| Fluoxetine 90 mg (n = 15) | |||||
| Placebo (n = 15) | |||||
| McMahon et al. 2005,142 8 weeks | Sildenafil 50–100 mg (n = 78) | IPE: sexual satisfaction | Sildenafil, 3.1; placebo, 2.2 | Unclear | NR |
| Placebo (n = 79) | IPE: ejaculatory control | Sildenafil, 1.8; placebo, 1.2 | Unclear | NR | |
| IPE: ejaculatory confidence | Sildenafil, 2.2; placebo, 1.3 | Unclear | NR | ||
| Global efficacy | Sildenafil, 48%; placebo, 16% | Unclear | NR | ||
| Tang et al. 2004,143 6 weeks | Sildenafil 50mg + BT (n = 30) | Patient/partner composite sexual satisfaction (0–5-point Likert scale) | Sildenafil + BT, 26/30 ‘satisfied’ | Yes | NR |
| BT (n = 30) | BT, 19/30 ‘satisfied’ | ||||
| Estimated p-value 0.04 | |||||
| Wang et al. 2007,144 duration NR | Sildenafil (n = 60) | Change in intercourse satisfactory score (instrument NR) | % increase (fold increase): sildenafil, 164% (threefold) | Unclear | NR |
| Paroxetine (n = 60) | Paroxetine, 115% (2.2-fold) | ||||
| Squeeze technique (n = 60) | Squeeze technique, 53% (1.8-fold) | ||||
| p-values NR | |||||
| Zhang et al. 2005,145 12 weeks | Sildenafil 50 mg + sertraline 50 mg | IIEF: sexual satisfaction | Sildenafil + sertraline, 13.8 | Yes | Sildenafil + sertraline had more AEs (headache, flushing). Numbers NR |
| Sertraline 50 mg | Sertraline, 10.8 (p < 0.001 between groups) | Yes | |||
| (Total n = 72) | Intercourse frequency (per week) | Sertraline + sildenafil, 2.7 | |||
| Sertraline, 1.9 (p < 0.005 between groups) |
Where between-group differences were estimatable, sildenafil plus behavioural therapy appeared to be more effective than behavioural therapy alone in the number of patients answering ‘satisfied’ on a patient/partner sexual satisfaction Likert scale (p = 0.04). Sildenafil plus sertraline also appeared to be more effective than sertraline alone on the IIEF sexual satisfaction and intercourse frequency domains (p < 0.001).
Across the RCTs, p-values for outcomes other than IELT either were not reported or, if they were, it was unclear whether the comparison was between groups or from baseline. The available data suggest that, in terms of secondary outcomes to IELT, PDE5 inhibitors are better than placebo and that PDE5 inhibitors combined with another therapy (SSRI or behavioural therapy) are better than the other therapy alone.
Assessment of safety: phosphodiesterase-5 inhibitors – adverse events
Of all the included RCTs, AE data were available for only a subset of trials evaluating sildenafil,39,55,145 for which it was reported that sildenafil was associated with a greater incidence of flushing and headache. However, data from these trials were insufficient for any meaningful pooling to be undertaken.
Assessment of effectiveness: phosphodiesterase-5 inhibitors – evidence summary
The current evidence base for PDE5 inhibitors in the treatment of PE comprises 10 RCTs39,55,138–145 captured in five systematic reviews37,134–137 of low to moderate methodological quality reviews and two further RCTs,101,120 one of which is at overall low risk of bias and the other at overall unclear risk.
Evidence from two RCTs138,141 suggests that vardenafil (42 participants) and tadalafil (30 participants) are both significantly effective in increasing IELT in men with PE [MD 3.80 minutes (95% CI 3.30 to 4.30 minutes; p = 0.0001) and 2.59 minutes (95% CI 1.28 to 3.90 minutes; p < 0.00001), respectively] when compared with placebo. Evidence from one RCT (157 participants) suggests that there is no statistically significant difference between sildenafil and placebo. 142
In comparison with SSRIs, sildenafil appears significantly more effective than paroxetine (one RCT,144 120 participants) [MD 1.26 minutes (95% CI 0.81 to 1.71 minutes)] and vardenafil (preceded by behavioural therapy) appears significantly more effective than sertraline preceded by behavioural therapy (one RCT,140 72 participants) [MD 1.89 minutes (95% CI 0.54 to 3.24 minutes); p < 0.00001 and p = 0.006, respectively]. No significant difference was evident between tadalafil and fluoxetine. A crossover RCT of 31 participants also suggests that sildenafil is more effective than paroxetine, sertraline or clomipramine. 39 No significant difference was evident between tadalafil and fluoxetine from one RCT. 145 Pooled effects across three RCTs120,141,145 (222 participants) suggests that PDE5 inhibitors in combination with a SSRI are significantly more effective than a SSRI alone with sildenafil plus sertraline demonstrating the greatest significant effect [MD 1.70 minutes (95% CI 1.64 to 1.76 minutes); p < 0.0001].
In comparison with behavioural interventions, sildenafil appears to be significantly more effective than the squeeze technique (one RCT144 with 120 participants and one crossover RCT39 with 31 participants) [(data not pooled) and one RCT144 with 120 participants (MD 3.56 minutes, 95% CI 3.16 to 3.96 minutes)], and sildenafil combined with behavioural therapy is significantly more effective than behavioural therapy alone (one RCT,143 60 participants) [MD 1.81 minutes (95% CI 1.53 to 2.09 minutes)].
Various assessment methods have been used across RCTs to measure effectiveness in terms of patient/partner sexual satisfaction, and other outcomes, although the between-group significance is often unclear or not reported. Outcomes appear to favour PDE5 inhibitors in comparison with placebo and PDE5 inhibitors combined with another therapy (SSRI or behavioural therapy) compared with another therapy (SSRI or behavioural therapy) alone. However, in the current evidence base, data are poorly reported and do not permit any meaningful interpretation of the efficacy of PDE5 inhibiters on efficacy outcomes other than IELT.
There is some evidence suggesting that both sildenafil and tadalafil are associated with a greater incidence of flushing and headache, and that tadalafil is also associated with palpitations. However, these data are difficult to extrapolate in order to estimate any between-group comparisons with other treatments. In addition, AE data are limited across the current evidence base for other PDE5 inhibitors.
Certain PDE5 inhibitors have been evaluated against placebo, while others are evaluated against SSRIs or behavioural therapy, or, in combination with a SSRI or behavioural therapy, have been evaluated against SSRI monotherapy or behavioural monotherapy. This variability of treatment comparisons in RCTs assessing PDE5 inhibitors limits definitive conclusions regarding an appropriate choice in terms of efficacy and safety for the treatment of men with PE. In addition, the long-term effects of PDE5 inhibitors in the treatment of PE are not evaluated in the current evidence base.
Alpha-blockers
Characteristics of included studies: alpha-blockers
Two RCTs were identified that evaluated alpha-blockers107,146 and both were captured by the search strategy for this assessment report. The RCT by Cavallini146 was evaluated by two systematic reviews evaluating pharmacotherapies. 52,69 The overall AMSTAR quality score was 1 out of 11 for both of these reviews (see Table 11). Full details of the AMSTAR assessment for these and all other included reviews are presented in Appendix 4. The RCT by Tuncel et al. 107 was identified by the literature search.
Cavallini146 included men with primary PE with an IELT ≤ 1 minute on more than 50% of occasions. Ninety-one patients were allocated to alfuzosin 6 mg, terazosin 5 mg or vitamin C 1 mg in a crossover design trial, 2 months per treatment phase. Ejaculatory control was assessed by patient self-report. The RCT by Tuncel et al. 107 was undertaken in Turkey and 90 patients were randomised to receive clomipramine 25 mg per day, sertraline 50 mg, terazosin 5 mg or placebo. Treatment duration was 2 months, but IELT was not assessed. The authors reported that 90 out of 90 (100%) patients completed the trial. This trial considered to be at overall unclear risk of bias. This trial is also evaluated in the SSRIs inhibitors and PDE5 inhibitors sections of this report.
Details of these trials are presented in Table 30.
| RCTs extracted from reviews | ||||||
|---|---|---|---|---|---|---|
| RCT (source) | Duration | Treatments | PE definition | Lifelong/acquired | IELT assessment | Other outcomes |
| aCavallini 1995146 (reviews52,69) | 2 months per treatment | Alfuzosin 6 mg | IELT ≤1 minute | Lifelong | Method NR | Ejaculatory control |
| Terazosin 5 mg | ||||||
| Vitamin C 1 mg | ||||||
| Total n = 91 | ||||||
| Further RCTs identified by searches (not captured in reviews) | ||||||
| RCT (country), risk of bias | Duration | Treatments, numbers analysed/randomised (%) | PE definition | Lifelong/acquired | IELT assessment | Other outcomes |
| Tuncel et al. 2008107 (Turkey), unclear | 2 months, assessment ‘after eight sexual attempts’ | Terazosin 5 mg/day (n = 25) | WHO ICD-10 | NR | IELT not assessed | Clinical responses (assume control of ejaculation) self-assessed |
| Clomipramine 25 mg/day (n = 23) | ||||||
| Sertraline 50 mg/day (n = 20) | ||||||
| Placebo (n = 22) | ||||||
| Terazosin, 25/25 (100%) | ||||||
| Clomipramine, 23/23 (100%) | ||||||
| Sertraline, 20/20 (100%) | ||||||
| Placebo, 22/22 (100%) | ||||||
Assessment of effectiveness: alpha-blockers – intravaginal ejaculatory latency time outcomes
An objective assessment of IELT was not reported by either of the two RCTs evaluating alpha-blockers identified for inclusion in this assessment report.
Assessment of effectiveness – alpha-blockers: other outcomes
Details of outcome results other than IELT and AEs are presented in Table 31.
| RCT duration | Treatment | Outcome measure | Results | Between-group difference reported as significant | AEs |
|---|---|---|---|---|---|
| aCavallini 1995,146 2 months per treatment | Alfuzosin 6 mg | Ejaculatory control | Percentage of ‘positive’ results: alfuzosin, 46.2%; terazosin, 53.7%; vitamin C, 24.2% | Unclear | NR |
| Terazosin 5 mg | |||||
| Vitamin C 1 mg | |||||
| Total n = 91 | |||||
| Tuncel et al. 2008,107 treatment was for 2 months | Sertraline 50 mg/day (n = 23) | Ejaculation control, self-assessed | Ejaculation control: n/N (%) reporting ‘no change’, ‘improvement’, ‘under control’ | Yes compared with placebo | % AEs: |
| Clomipramine 25 mg/day (n = 20) | All three treatments were ‘superior to placebo’ – p = 0.001 | Clomipramine – headache, 34.8%; hypotension, 4%; drowsiness, 8.6%; ejaculation disorder, 0% | |||
| No significant difference in efficacy between ‘medical treatments’ – p = 0.537 | Sertraline – headache, 25%; hypotension, 0%; drowsiness, 15%; ejaculation disorder, 0% | ||||
| Terazosin 5 mg/day (n = 25) | Terazosin – headache, 20%; hypotension, 12%; drowsiness, 0%; ejaculation disorder, 8% | ||||
| Placebo (n = 22) | Placebo – headache, 9.1%; hypotension, 0%; drowsiness, 0%; ejaculation disorder, 0% | ||||
| No significant differences between ‘medical treatment groups’ – p = 0.204 |
Tuncel et al. 107 reported that terazosin, clomipramine and sertraline were all significantly better than placebo on ejaculation control, but that there was no significant difference between the active treatments on this outcome.
A significant ejaculatory latency increase was reported for the RCT by Cavallini. 146 The proportion of patients by treatment group with a ‘positive’ result for this outcome was reported as 46.2% with alfuzosin, 53.7% with terazosin and 24.2% with vitamin C. However, no p-values were reported and it was unclear whether the reported ‘significant increase’ was across or between groups.
Assessment of safety: alpha-blockers – adverse events
Tuncel et al. 107 reported that there were no significant differences between clomipramine, sertraline and terazosin in the number of patients reporting AEs of headache, hypotension, drowsiness and ejaculation disorder.
Adverse events were not reported for the RCT by Cavallini. 146
Assessment of effectiveness: alpha-blockers – evidence summary
The current evidence base for alpha-blockers in the treatment of PE comprises two RCTs,39,107 one captured in low methodological quality systematic reviews39 and one further RCT which is at overall unclear risk of bias. 107 An assessment of IELT is not reported for either these trials.
Ejaculation control is reported by both RCTs assessing alpha-blockers. Evidence from one of these trials suggests that terazosin, clomipramine and sertraline were all significantly better than placebo on the outcome of ejaculation control,107 but that there is no significant difference between the active treatments on this outcome. Other RCT evidence for this outcome is unclear. 39
One RCT suggests that there is no significant difference between terazosin, clomipramine and sertraline and in the number of patients reporting AEs of headache,107 hypotension, drowsiness and ejaculation disorder. However, this observation should be interpreted with caution given the unclear methodological quality of the trial. The current evidence base for alpha-blockers in the treatment of PE in terms IELT and other secondary outcomes is limited.
Opioid analgesics
Characteristics of included studies: opioid analgesics
Tramadol was evaluated by three systematic reviews,147–149 two of which pooled data in a meta-analysis. 148,149 A further two RCTs were identified, one of which evaluated tramadol at 25 mg, 50 mg and 100 mg per day doses (no other comparator or placebo arm),154 while the other evaluated 25 mg per day against placebo. 155
The three systematic reviews were all conducted in China. 147–149 The overall AMSTAR quality score was 1 out of 11 in one of the reviews,147 2 out of 11 in another148 and 6 out of 11 in the last. 149 Details of the review type, the databases searched and dates, relevant included RCTs and the AMSTAR points awarded to these reviews, are presented in Table 32. Full details of the AMSTAR assessment for these and all other include reviews are presented in Appendix 4. The search methodology and inclusion criteria varied across these reviews. Of the two reviews including a meta-analysis, the review by Wu et al. 148 pooled data across different study types (observational studies and RCTs) using a MD. The authors also included different dosing arms from studies separately in the meta-analysis, but included the comparator arm (placebo) against each dosing arm in effect counting participants twice in the analysis. Likewise, the authors also pooled together data from the same arm at different time points (i.e. the same study group was counted twice in the analysis). In the review by Yang et al. ,149 the authors pooled IELT effect estimates across studies using a standardised MD.
| Author (country) review type | Databases searched and dates | Included RCTs relevant to this section | AMSTAR review quality assessment |
|---|---|---|---|
| Wong and Malde 2013147 (China) systematic review | PubMed 2006 to March 2012 | Alghobary et al. 2010,150 Bar-Or et al. 2012,151 Kaynar et al. 2012,152 Safarinejad and Hosseini 2006153 | AMSTAR score, 1/11:
|
| Wu et al. 2012148 (China) systematic review and meta-analysis | The Cochrane Library, MEDLINE, EMBASE, and Science Citation Index Expanded Until the end of February 2012, with no lower date limit | Alghobary et al. 2010,150 Bar-Or et al. 2012,151 Kaynar et al. 2012,152 Safarinejad and Hosseini 2006,153 Xiong et al. 201146 | AMSTAR score, 2/11:
|
| Yang et al. 2013149 (China) systematic review and meta-analysis | PubMed, EMBASE, CCRT and the Cochrane Database of Systematic Reviews 1980 to April 2012 all databases | Bar-Or et al. 2012,151 Kaynar et al. 2012,152 Safarinejad and Hosseini 2006,153 Xiong et al. 201146 | AMSTAR score, 6/11:
|
The reviews above varied in terms of which RCTs they included. In total, five RCTs46,150–153 (total n = 863) were included in at least one review. IELT was reported as being assessed using a stopwatch in all five RCTs. Duration of the RCTs included in these reviews ranged from 6 to 12 weeks and comparators to tramadol within the RCTs included in these review were behavioural therapy, paroxetine, or placebo. Tramadol doses varied from 25 mg to 89 mg, taken as needed, usually 2–3 hours preintercourse. Details of the RCTs extracted from these reviews are presented in Table 33. All RCTs in reviews were captured by the search strategy for this assessment report.
| RCTs extracted from reviews | ||||||
|---|---|---|---|---|---|---|
| RCT (source) | Duration | Treatments | PE definition | Lifelong/acquired | IELT assessment | Other outcomes |
| Alghobary et al. 2010150 (reviews147,148) | Crossover weeks per treatment (2-week washout) | Tramadol 50 mg (n = 35) | NR | Lifelong | Stopwatch | AIPE |
| Paroxetine 20 mg (n = 35) | ||||||
| 2–3 hours preintercourse | ||||||
| Bar-Or et al. 2012151 (reviews147–149) | 12 weeks | Tramadol 62 mg (n = 206) | Ejaculation ≤ 1 minute | Lifelong | Stopwatch | PEP |
| Tramadol 89 mg (n = 198) | ||||||
| Placebo (n = 200) | ||||||
| 2–8 hours preintercourse | ||||||
| Kaynar et al. 2012152 (reviews147–149) | 8 weeks | Tramadol 25 mg (n = 30) | IELT ≤ 2 minutes during 90% intercourse episodes | Lifelong | Stopwatch | AEC |
| Placebo (n = 30) | Sexual satisfaction scores | |||||
| 2 hours preintercourse | ||||||
| Safarinejad 2006153 (reviews147–149) | 8 weeks | Tramadol 50 mg (n = 29) | IELT ≤ 2 minutes during 90% coitus | Lifelong | Stopwatch | IIEF |
| Placebo (n = 28) | ||||||
| 2 hours preintercourse | ||||||
| Xiong et al. 201146 (reviews148,149) | 12 weeks | Tramadol 50 mg 2 hours preintercourse with behavioural therapy (NR which) (n = 36) | IELT ≤ 2 minutes | Lifelong | Stopwatch | IIEF |
| Behavioural therapy alone (n = 36) | ||||||
| Further RCTs identified by searches (not captured in reviews) | ||||||
| RCT (country risk of bias) | Duration | Treatments, numbers analysed/randomised (%) | PE definition | Lifelong/acquired | IELT assessment | Other outcomes |
| Eassa and El-Shazly 2013,154 (Egypt) unclear | 24 weeks | Tramadol 25 mg (n = 100) | NR | Lifelong | Stopwatch | None |
| Tramadol 50 mg (n = 100) | ||||||
| Tramadol 100 mg (n = 100) | ||||||
| 2–3 hours preintercourse | ||||||
| Tramadol 25 mg, 100/100 (100%) | ||||||
| Tramadol 50 mg, 100/100 (100%) | ||||||
| Tramadol 100 mg, 100/100 (100%) | ||||||
| Generali and Cada 2006155 (USA) unclear | 8 weeks | Tramadol 50 mg | NR | NR | Method NR | IIEF number of acts of coitus per week |
| Placebo | IIEF intercourse satisfaction | |||||
| 2 hours preintercourse | ||||||
| (Total n = 64) | ||||||
| Total n 57/64 (89%), n per group NR | ||||||
The RCT by Eassa and El-Shazly154 was conducted in Egypt and patients were randomised 100 per group to tramadol at 25 mg, 50 mg and 100 mg 2 to 3 hours preintercourse. The authors reported that all patients completed the 24 week follow-up and IELT was stopwatch assessed. Of note, the authors reported a mean baseline IELT of 2.82, 2.79 and 2.99 minutes for each of the treatment groups, respectively. This was noticeably higher than any other RCT, for any treatment, identified for inclusion in this assessment report. The RCT by Generali and Cada155 was conducted in the USA. Patients were randomised to tramadol 50 mg 2 hours before intercourse or placebo. Fifty-seven patients completed the 8-week study and the IELT assessment method was not reported. Variance estimates for the outcome data were not reported by the authors and were imputed for this assessment report using the reported p-values employing methods detailed in the Cochrane Reviewer’s Handbook. 31 Both of these trials were considered to be at overall unclear risk of bias.
Details of the treatments evaluated, definition of PE, IELT assessment method, other outcomes assessed, study duration, along with the study country for the further RCTs not in reviews, and the overall study quality assessment (AMSTAR33 for reviews and Cochrane risk of bias assessment34 for the RCTs not included by reviews) are presented in Table 33.
Assessment of effectiveness: opioid analgesics – intravaginal ejaculatory latency time outcomes
Intravaginal ejaculatory latency time outcomes were reported for all of the RCTs identified from existing reviews and the two further RCTs identified for inclusion in this review.
Meta-analysis of mean IELT change (minutes) at 8- or 12-week follow-up, based on five RCT study group comparisons from four RCTs (n = 776),151–153,155 displayed moderate heterogeneity (I2 = 70%). The pooled MD in IELT was 1.35 minutes, significantly favouring tramadol [MD (random effects) 95% CI 0.63 to 2.07 minutes; p = 0.0002]. The forest plot for this analysis is presented in Figure 18. Summary results for these, and all other meta-analyses, are presented in Table 33.
FIGURE 18.
Opioid analgesics, tramadol compared with comparator: forest plot of IELT outcomes. df, degrees of freedom; IV, inverse variance.
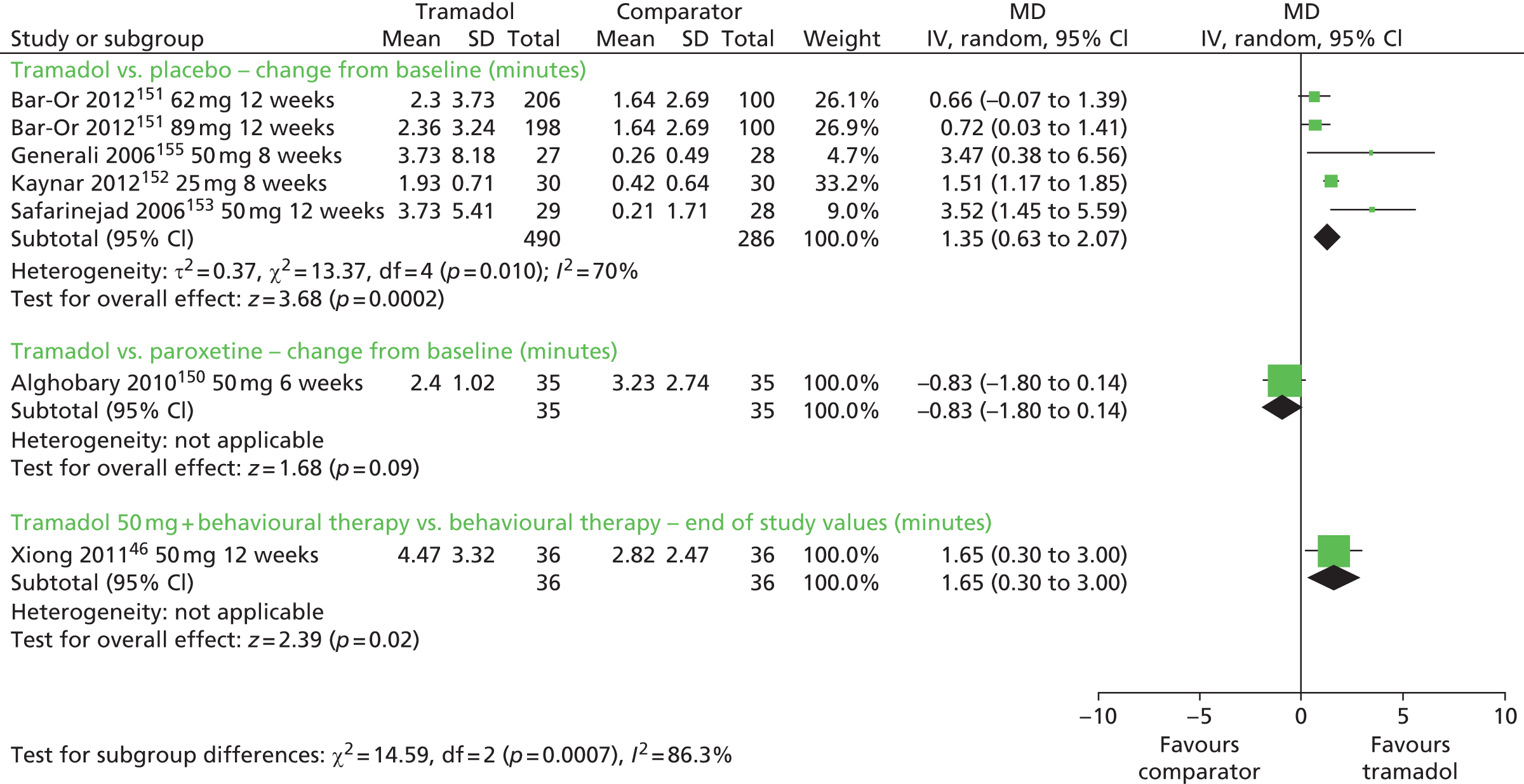
The between-group difference in mean IELT change (minutes) at 6 weeks, based on one RCT150 (n = 70) was –0.83 minutes (95% CI –1.80 to 0.14 minutes; p = 0.09). The forest plot for this analysis is presented in Figure 18.
The between-group difference in mean IELT (minutes) at 12 weeks, based on one RCT46 (n = 72) was 1.65 minutes, significantly favouring tramadol with behavioural therapy (95% CI 0.30 to 3.00 minutes; p = 0.02). The forest plot for this analysis is presented in Figure 18.
One RCT154 (n = 300) evaluated three different doses of tramadol. The between-group differences in mean IELT (minutes) at 24 weeks were 10.65 minutes in favour of tramadol 50 mg compared with 25 mg (95% CI 9.76 to 10.76 minutes; p < 0.00001); 23.32 minutes in favour of tramadol 100 mg compared with 25 mg (95% CI 22.59 to 24.05 minutes; p < 0.00001); and 13.06 minutes in favour of tramadol 100 mg compared with 50 mg (95% CI 12.33 to 13.79 minutes; p < 0.00001). The forest plot for this analysis is presented in Figure 19.
FIGURE 19.
Opioid analgesics, tramadol different doses: forest plot of IELT outcomes. df, degrees of freedom; IV, inverse variance.

Assessment of effectiveness: opioid analgesics – other outcomes
With the exception of the RCT by Eassa and El-Shazly154 that did not report any outcomes other than IELT, all of the included trials reported one or more other outcomes. However, these were diverse across the include trials and were often not reported in sufficient detail to permit any pooling across trials (Table 34).
| RCT duration | Treatment | Outcome measure | Results | Between-group difference reported as significant | AEs |
|---|---|---|---|---|---|
| Bar-Or et al. 2012,151 12 weeks | Tramadol 62 mg (n = 206) | PEP: 4 measures (intercourse satisfaction, control over ejaculation, ejaculation-related distress, ejaculation-related interpersonal difficulty) | Mean change for all 4 measures significantly higher in both tramadol groups than placebo (p < 0.05 for all) | Yes | Any AE, tramadol 62 mg: 12% |
| Tramadol 89 mg (n = 198) | Female partner PEP scores | Significantly more had improvement (≥ 1 category) for tramadol than placebo on all four measures | Yes | Tramadol 89 mg: 16% | |
| Placebo (n = 200) | Placebo: 7% | ||||
| Generali et al. 2006,155 8 weeks | Tramadol 50 mg | IIEF: number of acts of coitus per week | Tramadol: mean change 1.23; placebo: mean change 0.2 | Unclear | Any AE: tramadol: 28%; placebo: 16%, p < 0.05 |
| Placebo | IIEF: intercourse satisfaction | Tramadol: mean change 4; placebo: mean change –1 | Unclear | Nausea: tramadol: 16%; placebo: 3%, p < 0.05. No differences between groups for vomiting (6.2% each), dizziness (3.1% vs. 6.2%), or constipation (2.6% vs. 0.0%) | |
| Kaynar et al. 2012,152 8 weeks | Tramadol 25 mg (n = 30) | AEC score | Tramadol: mean increase 2.00; placebo: mean increase 0.57. Tramadol better than placebo (p < 0.001) | Yes | Any AE: tramadol: 27%; placebo: 0% |
| Placebo (n = 30) | Sexual satisfaction scores | Tramadol: mean increase 1.80 (SD 0.98); placebo: mean increase 0.53 (SD 0.92). Tramadol better than placebo (p < 0.001) | Yes | Mild nausea/headache: tramadol: 20% | |
| Mild somnolence: tramadol: (6.5%) | |||||
| Safarinejad 2006,153 8 weeks | Tramadol 50 mg (n = 29) | IIEF: intercourse satisfaction | Tramadol: mean change 4 | Yes | Any AE: tramadol: 28% |
| Placebo (n = 28) | Placebo: mean change –1 | Placebo: 16% (mainly nausea) | |||
| Between-groups p < 0.05 | |||||
| Alghobary et al. 2010,150 6 weeks per treatment | Tramadol 50 mg (n = 35) | AIPE | Tramadol: improved at 6 weeks but not at 12 weeks | Unclear | No AEs reported |
| Paroxetine 20 mg (n = 35) | Libido | Paroxetine: improved at 6 weeks (p < 0.05) and 12 weeks (p < 0.05) | No | ||
| Difference between groups not significant. Tramadol group had less rigid erections than paroxetine group (p < 0.05) | |||||
| Xiong et al. 2011,46 12 weeks | Tramadol 50 mg + BT (behaviour modification) (n = 36) | IIEF | Tramadol + BT: mean change 4 | Yes | Any AE: tramadol: 28%; placebo: 0% |
| Behaviour modification (n = 36) | BT alone: mean change 2 | Tramadol: nausea (11.1%), vomiting (2.8%), dry mouth (5.6%), dizziness (8.3%) | |||
| Between-groups p < 0.05 | |||||
| Eassa and El-Shazly 2013,154 24 weeks | Tramadol 25, 50 and 100 mg (n = 100 per group) | No other outcomes reported | N/A | N/A | Tramadol 25 mg – somnolence (100%); pruritus (100%) |
| Tramadol 50 mg – somnolence (100%); pruritus (100%); dizziness (18%); headache (16%); dry mouth (13%) | |||||
| Tramadol 100 mg – somnolence (100%); pruritus (100%); dizziness (38%); headache (30%); dry mouth (20%); nausea (20%); vomiting (17%) |
Bar-Or et al. 151 reported an improvement in 62-mg and 89-mg tramadol dose groups compared with placebo on measures of the PEP (p < 0.05 for all). Generali and Cada155 reported a change from baseline in the IIEF mean number of acts of coitus per week and mean intercourse satisfaction associated with tramadol (p < 0.05). However, p-values were not reported for the placebo group (data by group not reported). Kaynar et al. 152 reported improvements on ability of ejaculation control (AEC) (AEC score: placebo increased from 0.93 to 1.50; tramadol increased from 0.83 to 2.83) and sexual satisfaction scores (placebo increased from 0.80 to 1.33) for tramadol over placebo (p < 0.001 for both), although the instrument was not described. Safarinejad and Hosseini153 reported a between-group difference of p < 0.05 on the IIEF intercourse satisfaction score (tramadol mean change 4, placebo –1).
Xiong et al. 46 reported a significant between-group difference in IIEF intercourse satisfaction (p < 0.05) in favour of tramadol plus behavioural therapy.
Alghobary et al. 150 was the only RCT to employ the AIPE. The reviewers reported that paroxetine improved AIPE at 6 weeks (p < 0.05) and 12 weeks (p < 0.05), whereas tramadol improved AIPE at 6 weeks but not at 12 weeks.
Xiong et al. 46 reported a between-group difference at 8 weeks of p < 0.05 on the IIEF favouring the tramadol group. This trial is also evaluated in the Behavioural therapies section.
Assessment of safety: opioid analgesics – adverse events
No AEs were reported for the RCT by Alghobary et al. 150 When reported, AEs associated with tramadol included erectile dysfunction, constipation, nausea, headache, somnolence, dry mouth, dizziness, pruritus (itching) and vomiting. Numbers of patients by treatment groups experiencing AEs were reported by all RCTs. The trial by Eassa and El-Shazly,154 which compared tramadol at different doses, reported that all patients in the trial experienced one or more AEs (all experienced somnolence and pruritus).
Meta-analysis of numbers experiencing AEs at 8- or 12-week follow-up displayed low heterogeneity (I2 = 0%). The pooled RR across trials was 2.14 experiencing AEs [RR (fixed effect) 95% CI 1.36 to 3.38; p = 0.001] in favour of placebo (lower risk). The forest plot for this analysis is presented in Figure 20. Results for these, and all other meta-analyses, are presented in Table 35.
FIGURE 20.
Opioid analgesics, tramadol compared with comparator: forest plot for AEs. df, degrees of freedom; IV, inverse variance; M–H, Mantel–Haenzel.

| Comparison | Outcome | n RCTs | Participants | I 2 | Model | Effect estimate (95% CI) | Favours | p-value |
|---|---|---|---|---|---|---|---|---|
| IELT | ||||||||
| Tramadol vs. placebo | IELT (minutes) – change from baseline | 5151–153,155 | 776 | 70% | Random-effects | MD 1.35 (0.63 to 2.07) | Tramadol | 0.0002 |
| Tramadol vs. paroxetine | IELT (minutes) – change from baseline | 1150 | 70 | N/A | N/A | MD –0.83 (–1.80 to 0.14) | Between-group difference NS | 0.09 |
| Tramadol 50 mg + BT vs. BT | IELT (minutes) – end of study values | 146 | 72 | N/A | N/A | MD 1.65 (0.30 to 3.00) | Tramadol | 0.02 |
| Tramadol 50 mg vs. 25 mg | IELT (minutes) – final values, minutes | 1154 | 200 | N/A | N/A | MD 10.26 (9.76 to 10.76) | Tramadol | < 0.0001 |
| Tramadol 100 mg vs. 25 mg | IELT (minutes) – final values, minutes | 1154 | 200 | N/A | N/A | MD 23.32 (22.59 to 24.05) | Tramadol | < 0.0001 |
| Tramadol 100 mg vs. 50 mg | IELT (minutes) – final values, minutes | 1154 | 200 | N/A | N/A | MD 13.06 (12.33 to 13.79) | Tramadol | < 0.0001 |
| Other outcomes | ||||||||
| Tramadol vs. placebo | AEs | 4151–153,155 | 587 | 0% | Fixed effect | RR 2.14 (1.36 to 3.38) | Placebo (fewer AEs) | 0.001 |
| Tramadol 50 mg + BT vs. BT | AEs | 146 | 72 | N/A | N/A | RR 21.00 (1.28 to 345.41) | Placebo (fewer AEs) | 0.03 |
| Comparison | Outcome | n RCTs | Participants | I 2 | Model | Favours | ||
| Other outcomes | ||||||||
| Tramadol vs. placebo | Other effectiveness outcomes (various) | 546,151–153,155 | Varies | N/A | N/A | Tramadol significantly more effective than placebo on: PEP,151 AEC score152 IIEF-IS,153 IIEF,46; unclear on IIEF-NC and IS155 | ||
| Tramadol vs. paroxetine | Other effectiveness outcomes (various) | 1150 | 70 | N/A | N/A | Between-group difference not significant/unclear in one study AIPE150 | ||
| Tramadol 50 mg + BT vs. BT | Other effectiveness outcomes (various) | 146 | 72 | N/A | N/A | Tramadol + BT significantly more effective than BT alone in one study46 IIEF | ||
The between-group difference in RR at 12 weeks was 21.00 [RR (random effects) 95% CI 1.28 to 345.410; p = 0.03] in favour of behavioural therapy alone (lower risk). The forest plot for this analysis is presented in Figure 20. An assessment of between study heterogeneity could not be undertaken for this comparison as only one trial was included.
Assessment of effectiveness: opioid analgesics – evidence summary
The current evidence base for tramadol in the treatment of PE comprises seven RCTs,46,150–155 five46,150–153 captured in three low to moderate methodological quality systematic reviews and two further RCTs154,155 which are at overall unclear risk of bias. The pooled evidence across five RCT study groups151–153,155 (776 participants) suggests that tramadol is effective in increasing IELT in men with PE when compared with placebo [MD 1.35 minutes (95% CI 0.63 to 2.07 minutes); p = 0.0002]. Evidence from one RCT46 (72 participants) suggests that tramadol combined with behavioural therapy is significantly more effective than behavioural therapy alone in increasing IELT [MD 1.65 minutes (95% CI 0.30 to 3.00 minutes); p = 0.02]. The evidence from one RCT150 (70 participants) suggests that there is no statistically significant difference in IELT between tramadol and paroxetine.
Various assessment methods in terms of ejaculation control, patient/partners sexual satisfaction, anxiety and other patient-reported outcomes have been used across RCTs to measure the effectiveness of tramadol. Four46,151–153 out of five RCTs46,151–153,155 reported that tramadol was significantly more effective than placebo for various patient-reported outcomes, while one RCT155 did not report any significant between-group differences. Pooled evidence across trials151–153,155 (587 participants) suggests that tramadol is associated with significantly more AEs including erectile dysfunction, constipation, nausea, headache, somnolence, dry mouth, dizziness, pruritus (itching) and vomiting, than placebo or behavioural therapy over 8–12 weeks of treatment. Addiction to tramadol by patients treated with this agent for PE is not assessed in the current evidence base. Likewise, patient acceptability of treatment is not reported.
Tramadol appears more effective than placebo or behavioural therapy in the treatment of PE. However, these findings should be interpreted with caution given the observed levels of between-study heterogeneity and the methodological quality of the available evidence. In addition, the variability across placebo-controlled trials in terms of the tramadol dose evaluated and the treatment duration does not permit any assessment of a safe and effective minimum daily dose. Furthermore, the long-term effects and side effects of the treatment for men with PE have not been evaluated in the current evidence base.
Other therapies: acupuncture
Characteristics of included studies: acupuncture
No RCTs evaluating acupuncture were included in any of the systematic reviews identified for inclusion in this assessment report. Two RCTs were identified through the literature searches, one of which evaluated acupuncture compared with citalopram,156 while the other evaluated acupuncture compared with sham acupuncture or paroxetine. 157
The RCT by Chen156 was conducted in China. A total of 111 patients were randomised to daily acupuncture or citalopram (described as Sailete tablets) 20 mg per day. The trial was reported in Chinese with an English-language abstract. Treatment duration was 4 weeks and the authors reported that 111 out of 111 (100%) patients completed the trial, but the assessment method of IELT was not reported. The RCT by Sunay et al. 157 was conducted in Turkey and 90 patients were recruited to the trial and were randomised to either acupuncture twice a week, sham acupuncture twice a week or paroxetine 20 mg per day. The authors reported that 90 out of 90 (100%) patients completed the intervention. Both of these trials were considered to be at overall unclear risk of bias.
Details of the treatments evaluated, definition of PE, IELT assessment method, other outcomes assessed, study duration, along with the study country for the further RCTs not in reviews and the overall study quality assessment (Cochrane risk of bias assessment34) are presented in Table 36.
| RCTs identified by searches (not captured in reviews) | ||||||
|---|---|---|---|---|---|---|
| RCT (country) risk of bias | Duration | Treatments, numbers analysed/randomised (%) | PE definition | Lifelong/acquired | IELT assessment | Other outcomes |
| Chen 2009156 (China) unclear risk | 4 weeks | Acupuncture daily (n = 56) | NR | NR | No objective assessment | Chinese index of sexual function for PE (CIPE) |
| Citalopram 20 mg per day (n = 55) | ||||||
| Acupuncture daily 56/56 (100%) | ||||||
| Citalopram 55/55 (100%) | ||||||
| Sunay et al. 2011,157 (Turkey) unclear risk | 4 weeks | Acupuncture two times weekly (n = 30) | IELTs of 2 minutes in > 70% of attempts | Lifelong 66%, acquired 34% | Stopwatch | PEDT |
| Sham acupuncture two times weekly (n = 30) | ||||||
| Paroxetine 20 mg per day (n = 30) | ||||||
| Acupuncture 30/30 (100%) | ||||||
| Sham acupuncture 30/30 (100%) | ||||||
| Paroxetine 30/30 (100%) | ||||||
Assessment of effectiveness: acupuncture – intravaginal ejaculatory latency time outcomes
The RCT by Chen156 employed the CIPE. The CIPE has 10 questions focusing on libido, erectile function, ejaculatory latency, sexual satisfaction and difficulty in delaying ejaculation, self-confidence and depression. However, the authors only reported an overall score (see Assessment of effectiveness: acupuncture – other outcomes). Sunay et al. 157 reported IELT outcomes as median and mean rank values post treatment and change from baseline. The mean rank increase with paroxetine, acupuncture and sham acupuncture were 1.38 minutes, 1.10 minutes and 0.55 minutes, respectively. The authors reported that statistically significant between-group differences were determined for mean rank IELTs for paroxetine compared with sham acupuncture in favour of paroxetine (p = 0.001), acupuncture compared with sham acupuncture in favour of acupuncture (p = 0.001) and paroxetine compared with acupuncture in favour of paroxetine (p = 0.001) after treatment.
Assessment of effectiveness: acupuncture – other outcomes
Chen156 reported that the change from baseline in cumulative CIPE scores were statistically significant with both acupuncture and with citalopram and that the between-group difference post treatment was statistically significant in favour of acupuncture (Table 37). The RCT by Sunay et al. 157 reported that median PEDT scores were significantly improved from baseline in both the acupuncture and paroxetine groups, but not in the sham acupuncture group. The authors also reported that both acupuncture and paroxetine were significantly better than sham acupuncture on this outcome; however, that there was no statistically significant between-group difference between acupuncture and paroxetine. Similarly, that no significant differences were found between PEDT subscores (ejaculation control, frequency, minimal stimulation, distress, interpersonal difficulty) for the paroxetine and acupuncture groups before and after treatment, but significant differences were determined between the paroxetine and placebo groups and between the acupuncture and placebo groups after treatment.
| RCT, duration | Treatment | Outcome measure | Results | Between-group difference reported as significant | AEs |
|---|---|---|---|---|---|
| Chen 2009,156 4 weeks | Acupuncture daily (n = 56) | CIPE | Change from baseline: acupuncture, p < 0.01; citalopram, p < 0.05 | Yes | NR |
| Citalopram 20 mg per day (n = 55) | Between group difference in post-treatment scores, p < 0.05 (favouring acupuncture) | ||||
| Sunay et al. 2011,157 4 weeks | Acupuncture 2 × weekly (n = 30) | PEDT | Change from baseline: paroxetine, p = 0.001; acupuncture, p = 0.001 | Yes for acupuncture and paroxetine from baseline and vs. sham acupuncture | No AEs were reported by patients |
| Sham acupuncture 2 × week (n = 30) | Sham acupuncture, p = 0.314 | ||||
| Paroxetine 20 mg per day (n = 30) | Between-group differences: paroxetine vs. acupuncture, p = NS; paroxetine vs. sham p = 0.001; acupuncture vs. sham, p = 0.001 | No between acupuncture and paroxetine |
Assessment of safety: acupuncture – adverse events
Adverse event data were not reported for the RCT by Chen. 156 Sunay et al. 157 reported that no questionnaire was used to evaluate the side effects; however, no side effects were observed in any of the patients.
Assessment of effectiveness: acupuncture – evidence summary
The current evidence base for acupuncture in the treatment of PE comprises two RCTs156,157 that compare acupuncture with SSRIs (citalopram and paroxetine) that are at overall unclear risk of bias. Evidence from one of these RCTs157 suggests that both acupuncture and paroxetine are both effective in increasing IELT in men with PE when compared with sham acupuncture. However, that paroxetine is more effective than acupuncture in increasing IELT.
Evidence from one RCT156 suggests that subjective measures of libido, erectile function, ejaculatory latency, sexual satisfaction and difficulty in delaying ejaculation, self-confidence and depression are significantly improved with both acupuncture and citalopram and that the difference is greater with acupuncture. Conversely, evidence from one RCT157 suggests that there is no statistically significant difference in subjective measures of ejaculation control, frequency, minimal stimulation, distress and interpersonal difficulty, between acupuncture and paroxetine. Treatment-related AEs for acupuncture in the treatment of PE are not well reported in the current literature.
Acupuncture appears more effective than citalopram but not paroxetine in the treatment of PE. The AEs associated with acupuncture in the treatment of PE are unclear. However, these finding should be interpreted with caution given the limited available evidence for this treatment.
Other therapies: Chinese medicine
Characteristics of included studies: Chinese medicine
No RCTs evaluating Chinese medicine were included in any of the systematic reviews identified for inclusion in this assessment report, but five RCTs158–162 were identified through the literature searches. One compared Chinese medicine combined with sertraline and counselling with sertraline alone,158 one compared Chinese medicine with treatment as usual,159 one compared Chinese medicine with fluoxetine,160 one compared Chinese medicine alone with Chinese medicine combined with trazodone [a serotonin antagonist and reuptake inhibitor (SARI) antidepressant]161 and one compared Chinese medicine adjuvant to behavioural therapy with behavioural therapy alone. 162
All five RCTs158–162 were undertaken in China and three were reported in Chinese with an English-language abstract. 158,160,161 Pei and Shi158 randomised 110 patients to Wu Bei Zi (Galla Chinensis) and Xi Xin (Asari Herba) combined with sertraline and counselling or sertraline alone; no further treatment details were reported. The assessment method of IELT was not reported. Treatment duration was 4 weeks and the authors reported that 110 out of 110 (100%) patients completed the trial. In the trial by Song et al. ,159 68 patients were randomised to Uighur medicine (ingredients: Radix anacycli pyrethri, Mastiche, Fructus Cardamomi, Rhizoma Cyperi, Stigma Croci, Semen Myristicae, Radix Curcumae, Folium Syringae oblatae, Radix et Rhizoma Nardostachyos, Fructus Tsaoko and Flos Rosae rugosae), four tablets twice a day or treatment as usual (no tablets). IELT was assessed by a questionnaire designed for the study and all patients were reported as completing the 15-day trial. Sun et al. 160 evaluated Yimusake (Arabian Olibanum, Moschus, Stigma Croci, Testis Et penis Bovis seu Bubali, Ambra Grisea, Semen Myristicae, Rhizoma Alpiniae Officinarum, Flos Caryophylli, Salep, Semen Strychni, Pericarpium Papaveris) 1.5 g per day, fluoxetine 20 mg per day, and Yimusake 1.5 g combined with fluoxetine 20 mg per day. Thirty-eight patients were randomised to each of the three treatment groups and all were reported as completing. The IELT assessment method was not reported, but duration was 4 weeks. The RCT by Xu et al. 161 compared Yimusake 50 mg per day with Yimusake 50 mg per day combined with trazodone 50 mg per day. The IELT assessment method was not reported, but duration was 4 weeks. The authors reported that 68 out of 68 (100%) patients completed the trial. The RCT by Zhang et al. 162 randomised 28 patients to Xuanju compound (Formica fusca, Herba epimedii, Fructus cnidii and Fructus lycii) with sensate focus and 24 patients to sensate focus alone. The IELT assessment method was not reported, but treatment was for 4 weeks and all patients (100%) were reported as completing. All five trials were at overall unclear risk of bias.
Details of treatments evaluated, definition of PE, IELT assessment method, other outcomes assessed, study duration and country for the further RCTs not in reviews, and the overall study quality assessment (Cochrane risk of bias assessment34) are presented in Table 38.
| RCTs identified by searches (not captured in reviews) | ||||||
|---|---|---|---|---|---|---|
| RCT (country) risk of bias | Duration | Treatments, numbers analysed/randomised (%) | PE definition | Lifelong/acquired | IELT assessment | Other outcomes |
| Pei and Shi 2008158 (China) unclear | 4 weeks | Chinese medicine (Wu Bei Zi and Xi Xin) + sertraline + sexual counselling (n = 60) | NR | NR | IELT not assessed | Total effectiveness rates |
| Sertraline (n = 50) | ||||||
| Chinese medicine + sertraline + sexual counselling, 60/60 (100%) | ||||||
| Sertraline, 50/50 (100%) | ||||||
| Song et al. 2007159 (China) unclear | 15 days | Chinese medicine (Uighur) (n = 35) | American Urological Association and DSM-IV diagnosed; IELT ≤ 2 minutes | Acquired and lifelong (n NR) | Questionnaire designed for the study | CIPE5 and CIPE10 Sexual partner’s satisfaction rate and ‘wish fulfilment’ (assume ejaculation control) |
| Treatment as usual (n = 33) | ||||||
| Chinese medicine, 35/35 (100%) | ||||||
| Treatment as usual, 33/33 (100%) | ||||||
| Sun et al. 2010160 (China) unclear | 4 weeks | Chinese medicine (Yimusake 1.5 g/day) (n = 38) | NR | NR | Method NR | Patient and partners intercourse satisfaction |
| Fluoxetine 20 mg per day (n = 38) | ||||||
| Chinese medicine + fluoxetine (n = 38) | ||||||
| Chinese medicine, 38/38 (100%) | ||||||
| Fluoxetine, 38/38 (100%) | ||||||
| Chinese medicine + fluoxetine, 38/38 (100%) | ||||||
| Xu et al. 2012161 (China) unclear | 4 weeks | Chinese medicine (Yimusake 50 mg/day) (n = 32) | NR | Lifelong | Method NR | Patients ‘cured’, ‘improved’, ‘unimproved’, total ‘efficacious’ |
| Chinese medicine + trazodone 50 mg/day (n = 36) | ||||||
| Chinese medicine, 32/32 (100%) | ||||||
| Chinese medicine + trazodone, 36/36 (100%) | ||||||
| Zhang et al. 2006162 (China) unclear | 4 weeks | Chinese medicine (Xuanju) + sensate focus (n = 28) | NR | NR | IELT not assessed | Sexual satisfaction |
| Placebo + sensate focus (n = 24) | ||||||
| Chinese medicine + sensate focus, 28/28 (100%) | ||||||
| Sensate focus, 24/24 (100%) | ||||||
Assessment of effectiveness: Chinese medicine – intravaginal ejaculatory latency time outcomes
With the exception of the RCTs by Pei and Shi158 and Zhang et al. ,162 IELT outcomes were reported for all of the included trials.
The between-group difference in mean IELT change (minutes) at 4 weeks, based on one RCT (n = 68) was 1.57 minutes (95% CI 1.11 to 2.03 minutes; p < 0.00001) in favour of Chinese medicine. 159
The between-group difference in mean IELT change (minutes) after 15 days, based on one RCT (n = 76) was 0.60 minutes (95% CI 0.19 to 1.01 minutes; p = 0.004) in favour of fluoxetine. 160
The between-group difference in mean IELT change (minutes) after 15 days, based on one RCT (n = 76) was 2.50 minutes (95% CI 2.08 to 2.92 minutes; p < 0.00001) in favour of Chinese medicine combined with fluoxetine. 160
The between-group difference in mean IELT change (minutes) after 15 days, based on one RCT (n = 76) was 1.90 minutes (95% CI 1.47 to 2.33 minutes; p < 0.00001) in favour of Chinese medicine combined with fluoxetine. 160
The between-group difference in mean IELT change (minutes) at 4 weeks, based on one RCT (n = 68) was not significant (MD 0.08 minutes 95% CI –0.19 to 0.35 minutes; p = 0.56). 161
The forest plot for these analyses is presented in Figure 21.
FIGURE 21.
Chinese medicine compared with comparator: forest plot of IELT outcomes. IV, inverse variance.
Assessment of effectiveness: Chinese medicine – other outcomes
A greater proportion of patients receiving Chinese medicine combined with sertraline and sexual counselling than those receiving sertraline alone reported an effectiveness rating of ‘effective’ or ‘improved’ in the RCT by Pei and Shi. 158 The between-group difference in the number of patients reporting ‘effective’ or ‘improved’ estimated using RevMan for this assessment reported was 1.21 in favour of Chinese medicine combined with sertraline and sexual counselling compared with sertraline alone [RR (fixed effect), 95% CI 1.01 to 1.43; p = 0.03] (figure not presented).
Song et al. 159 reported a statistically significant between-group difference in Chinese medicine compared with care as usual in favour of Chinese medicine on sexual satisfaction and ejaculation control measures of the Chinese index of sexual function for PE scale for PE-related items. Sun et al. 160 reported that Chinese medicine combined with fluoxetine was significantly better than fluoxetine alone or Chinese medicine alone, on a measure of patient and partner intercourse satisfaction. Xu et al. 161 reported the number of patients as ‘total efficacious’ (assume ‘improved’ or ‘cured’). The between-group difference was not significant (p = 0.27). In the RCT by Zhang et al.,162 a greater proportion of patients in the Chinese medicine combined with behavioural therapy than those in the behavioural therapy alone group reported a ‘cure rate’ of ‘cured’ or ‘improved’ on an overall ‘Cure rate and rate of sexual satisfaction improvement’ rating. The between-group difference in the number of patients reporting ‘cured’ or ‘improved’ estimated using RevMan for this assessment reported was 1.92 in favour of Chinese medicine combined with behavioural therapy [RR (fixed effect), 95% CI 1.27 to 2.92; p < 0.00001] (figure not presented).
Details of outcomes other than IELT and AEs are presented in Table 39.
| RCT Duration | Treatment | Outcome measure | Results | Between-group difference reported as significant | AEs |
|---|---|---|---|---|---|
| Pei and Shi 2008,158 4 weeks | Chinese medicine (Wu Bei Zi and Xi Xin) + sertraline + sexual counselling (n = 60) | Total effectiveness rates | Chinese medicine + sertraline + counselling: ‘effective’, 53.3%; ‘improved’, 38.3%; ‘ineffective’, 8.4%; ‘total’, 91.6% | Unclear | NR |
| Sertraline (n = 50) | Sertraline: ‘effective’, 40.0%; ‘improved’, 36.0%; ‘ineffective’, 24.0%; ‘total’, 76.0% | ||||
| Song et al. 2007,159 15 days | Chinese medicine (Uighur) (n = 35) | Chinese index of sexual function for PE | CIPE5, CIPE10, satisfaction and control were improved following treatment at p < 0.01 and were different from the control group at p < 0.01 | Yes | NR |
| Treatment as usual (n = 33) | CIPE10 and the scale for five PE-related items | ||||
| CIPE5 | |||||
| Sun et al. 2010,160 4 weeks | Chinese medicine (Yimusake) (n = 38) | Patient and partners intercourse satisfaction | Patients intercourse satisfaction change from baseline (same for partner satisfaction): Chinese medicine, p < 0.05; fluoxetine, p < 0.05; Chinese medicine + fluoxetine, p < 0.01 | Yes | AEs with Chinese medicine + fluoxetine were not significantly different to Chinese medicine or fluoxetine alone. No data or p-value |
| Fluoxetine (n = 38) | Between-group (same for partner satisfaction): Chinese medicine + fluoxetine vs. Chinese medicine, p < 0.05 | ||||
| Chinese medicine + fluoxetine (n = 38) | Chinese medicine + fluoxetine vs. fluoxetine, p < 0.05 | ||||
| Xu et al. 2012,161 4 weeks | Chinese medicine (Yimusake) (n = 32) | Patients ‘cured’, ‘improved’, ‘unimproved’, total ‘efficacious’ | Total ‘efficacious’: Chinese medicine: 18/32 (56.25%) | No | NR |
| Chinese medicine + trazodone (n = 36) | Chinese medicine + trazodone: 25/36 (69.44%) | ||||
| Between-group difference, p = 0.27 | |||||
| Zhang et al. 2006,162 4 weeks | Chinese medicine (Xuanju) + sensate focus (n = 28) | ‘Cure rate’ and rate of sexual satisfaction improvement | Between-group difference in number improved p < 0.05 | Yes | NR |
| Sensate focus (n = 24) | Between-group difference in n cured p < 0.01 (both favouring Chinese medicine + sensate) |
Assessment of safety: Chinese medicine – adverse events
Reporting of AEs was only available for one of the included RCTs160 for which it was reported that the AEs observed with Chinese medicine combined with fluoxetine were not significantly different to those observed with Chinese medicine alone or fluoxetine alone. However, no details of the AEs assessed or a p-value for between-group differences were reported.
Assessment of effectiveness: Chinese medicine – evidence summary
The current evidence base for Chinese medicine in the treatment of PE comprises five RCTs all at unclear risk of bias. One comparing Wu Bei Zi and Xi Xin combined with sertraline and counselling with sertraline alone, one comparing Uighur medicine with treatment as usual, one comparing Yimusake with fluoxetine or Yimusake combined with fluoxetine, one comparing Yimusake with Yimusake combined with trazodone, and one comparing Xuanju compound plus sensate focus with sensate focus alone. No placebo-controlled trials of any Chinese medicine have been identified from the current literature. Evidence from one RCT159 suggests Chinese medicine is significantly more effective than treatment as usual (no tablet) in increasing IELT in men with PE (1.57 minutes, 95% CI 1.11 to 2.03; p < 0.00001). One RCT160 suggests that fluoxetine is better than Chinese medicine and that Chinese medicine combined with fluoxetine is significantly better than Chinese medicine alone or fluoxetine alone in increasing IELT [(0.60 minutes, 95% CI 0.19 to 1.01; 2.50 minutes, 95% CI 2.08 to 2.92; and 1.90 minutes, 95% CI 1.47 to 2.33 minutes), p = 0.004, p < 0.00001 and p < 0.00001, respectively]. One RCT159 suggests no significant difference in IELT between Chinese medicine combined with trazodone and Chinese medicine alone.
Evidence from one RCT each suggests that CIPE-assessed sexual satisfaction and ejaculation control are better with Chinese medicine than treatment as usual and that a subjective measure of intercourse satisfaction is better with Chinese medicine combined with a SSRI than Chinese medicine or SSRI alone. Treatment-related AEs for Chinese medicine in the treatment of PE are not well reported in the current literature.
Limited evidence suggests that Chinese medicine may be effective in the treatment of PE and that greater efficacy is evident when Chinese medicine is combined with a SSRI. However, AEs associated with Chinese medicine, with or without these secondary agents, in the treatment of PE are unclear. The long-term effects of Chinese medicine in the treatment of PE and patient acceptability of the treatment are not evaluated in the current evidence base.
Other therapies: delay devices
Characteristics of included studies: delay devices
No studies evaluating delay devices were included in any of the systematic reviews identified for inclusion in this assessment report. One RCT was identified through the literature searches which evaluated a novel desensitising band. 163
The study was undertaken in the UK and PE was defined by DSM-IV diagnosis. 163 The numbers of lifelong/acquired PE was not reported. The device evaluated was a desensitising ring comprising a stretchable latex ring with stimulating ridged plate which was used three times per week combined with the stop–start technique which was compared with CBT (six sessions with a trained therapist) combined with the stop–start technique. Twenty-six patients were randomised to each treatment group. The trial was reported in conference poster format and treatment duration was unclear (possibly eight weeks). Assessment was at the end of therapy and three months post treatment. The authors assessed PE and other subscales of the GRISS questionnaire. The authors reported that 52 out of 52 (100%) patients completed the study. This trial was considered at overall unclear risk of bias.
Assessment of effectiveness: delay devices – intravaginal ejaculatory latency time outcomes
Wise et al. 163 reported that the mean latency for coitus at completion was 8.8 minutes in the desensitising band group and 2.6 minutes in the CBT group and that the between-group difference favouring the desensitising band was significant (p < 0.002). However, it was unclear how this outcome was assessed as the authors reported that stopwatches were not used.
Wise et al. 163 reported that 16 out of 26 (62%) patients in the desensitising band group reported an improvement in latency, compared with 11 out of 26 (42%) in the CBT group. The between-group difference estimated using RevMan for this assessment report was 1.60 [RR (fixed effect), 95% CI 0.90 to 2.84; p = 0.11] (figure not presented).
Assessment of effectiveness: delay devices – other outcomes
Wise et al. 163 reported that the GRISS subscales showed no statistically significant differences between groups except in the PE subscale. The GRISS mean rank score was reported as being significantly lower (better) in the desensitising band group compared with the CBT group at 8 weeks (p ≤ 0.05) and 3 months (p < 0.05).
Assessment of safety: delay devices – adverse events
Adverse events were not reported in the RCT by Wise et al. 163 A case study (six patients) report from the same research group171 reported that the only side effect associated with the desensitising band was slight soreness with over-use which was resolved when used as instructed.
Assessment of effectiveness: delay devices – evidence summary
The current RCT evidence base for delay devices in the treatment of PE comprises one study that compares a desensitising band combined with the stop–start technique compared with behavioural therapy combined with the stop–start technique. 163 The RCT is considered to be at overall unclear risk of bias. Evidence from this study suggests that a desensitising band combined with the stop–start technique is more effective than behavioural therapy combined with the stop–start technique in increasing IELT in men with PE.
Evidence from the same RCT suggests that GRISS questionnaire assessed IELT appears improved with the desensitising band and is continued with use over 3 months. Evidence from one case series study suggests that soreness is reported with over-use but appears resolved when the device is used as instructed. 171
Evidence from one RCT,163 that is considered to be at unclear risk of bias, suggests that desensitising bands combined with the stop–start technique appear effective in increasing IELT in men with PE. The effects of desensitising bands alone on PE are not evaluated in the current evidence base. AEs appear minimal when these devices are used as directed.
Other therapies: yoga
Characteristics of included studies: yoga
No RCTs evaluating yoga were included in any of the systematic reviews identified for inclusion in this assessment report. One observational study (non-RCT) was identified through the literature searches which evaluated yoga compared with fluoxetine. 164 In the absence of any RCT evidence for the effects of yoga in the treatment of PE, this study was included in this assessment report.
The study was undertaken in India and PE was defined by DSM-IV diagnosis. 164 The number of patients with lifelong/acquired PE was not reported. Yoga (14 active and passive postures for 1 hour each day) was compared with fluoxetine, 20–60 mg per day (single dose). Patients self-selected to treatment groups and study duration was 12 weeks. IELT was assessed using a stopwatch and partner satisfaction (‘good’, ‘fair’, ‘poor’ responses) was also assessed. The authors reported that 68 out of 68 (100%) patients completed the study. This trial was considered at overall high-risk of bias.
Assessment of effectiveness: yoga – intravaginal ejaculatory latency time outcomes
The observational study by Dhikav et al. 164 reported that the mean post-treatment IELT at the 8-week follow-up was 1.07 minutes (SD 0.49 minutes) in the yoga group compared with 1.88 minutes (SD 0.59 minutes) in the fluoxetine groups. The authors reported that the change from baseline was significant in both groups (p < 0.0001). The between-group difference estimated using RevMan for this assessment report was 0.81 minutes in favour of fluoxetine [MD (fixed effect), 95% CI 0.55 to 1.08 minutes; p < 0.0001] (figure not presented).
Assessment of effectiveness: yoga – other outcomes
Dhikav et al. 164 reported that in the yoga group, partner satisfaction was rated as ‘good’ by 25 out of 38 (65.6%) patients, ‘fair’ by 13 out of 38 (34.2%) patients and ‘poor’ by 0 out of 38 (0.0%) patients. No data were reported for the fluoxetine group.
Assessment of safety: yoga – adverse events
Dhikav et al. 164 reported that there were no significant side effects or dropouts during course of treatment with yoga; however, no data were reported. The authors reported numbers of patients experiencing AEs in the fluoxetine group of: nausea 14 out of 30 (46.7%); vomiting, 4 out of 30 (13.3%); anxiety, 4 out of 30 (13.3%); and insomnia, 8/30 (26.7%).
Assessment of effectiveness: yoga – evidence summary
The current evidence base for yoga in the treatment of PE comprises one observational study that compares yoga with fluoxetine. The study is considered to be at overall high risk of bias base on participants self-selecting to treatment groups (selection bias). 164 In this study, both yoga and fluoxetine were reported as significantly effective at increasing IELT following treatment. However, the between-group estimate post treatment for this study suggests that fluoxetine is more effective than yoga in increasing IELT in men with PE. However, these results should be interpreted with caution given the possibility of selection bias in this study.
Evidence from the same study suggests that a high proportion of partners report a satisfaction rating of yoga of ‘good’. No data for fluoxetine are reported for this outcome. AEs associated with fluoxetine include nausea, vomiting, anxiety and insomnia, and AEs associated with yoga are not reported.
Based on one observational study that is considered to be at high risk of selection bias, fluoxetine appears more effective than yoga in the treatment of PE, but is associated with AEs. The long-term effects of yoga in treating men with PE and patient acceptability compared with fluoxetine are not adequately assessed in the current evidence base.
Chapter 4 Discussion
The purpose of this report was to systematically review the evidence for interventions in the treatment of PE in men and to summarise this in the form of a short report. The treatments evaluated were those relevant to the UK setting. RCTs in adult men with PE that evaluated a treatment of interest compared with other interventions, waiting list control, placebo or no treatment were eligible for inclusion. When RCT evidence was not available, other study types were considered. RCTs were identified from existing systematic reviews and through literature searching. Data for RCT publications reported in existing systematic reviews were extrapolated from the review article (not from the original RCT publications). Methodological quality of included reviews and additional RCTs was assessed. The primary outcome was IELT; other outcomes included sexual satisfaction, control over ejaculation, relationship satisfaction, self-esteem, quality of life, treatment acceptability and AEs. When possible, data were pooled across trials in a meta-analysis.
Statement of principal findings
Behavioural interventions
The evidence for behavioural therapy was reported in 12 RCTs:39–50 nine39–47 captured in two low-quality reviews and one moderate quality Cochrane review, plus three further RCTs48–50 of unclear methodological quality. The quality of reporting and diversity of outcome data did not permit pooling of effect estimates. Individual trial results suggest that behavioural therapies improved both IELT and sexual satisfaction compared with waiting list control. Behavioural therapies combined with pharmacological therapies (PDE5 inhibitors, SSRIs, chlorpromazine, tramadol) were better than behavioural therapy alone or pharmacological agents alone in improving IELT, sexual satisfaction, sexual anxiety and ejaculation control. No AEs specific to behavioural therapies were reported.
Topical anaesthetics
The evidence for topical anaesthetics was reported in nine RCTs,55–63 seven55–61 captured in three low methodological quality systematic reviews and two further RCTs62,63 of unclear methodological quality. Pooled evidence across RCTs suggests that both EMLA cream and TEMPE spray are more effective than placebo in increasing IELT [MD 6.44 minutes, 95% CI 6.01 to 6.87 minutes (p < 0.00001); and 3.30 minutes, 95% CI 1.33 to 5.27 minutes (p = 0.001), respectively]. AEs include loss of sensation and irritation for both men and women. Application of topical anaesthetics for ≥ 20 minutes preintercourse appears to be associated with erection loss.
Selective serotonin reuptake inhibitors currently not licensed for premature ejaculation
The evidence for SSRIs other than dapoxetine was reported in 42 RCTs,39,41,70–107,141,166 2639,41,70–91,141,166 captured in seven52,64–69 low methodological quality systematic reviews and 16 further RCTs,92–107 1492–94,96–100,104–107 of unclear methodological quality and two95,103 at high risk of bias. Treatment duration was 4–12 weeks. Evidence suggests that citalopram is significantly more effective in increasing IELT than placebo (MD 4.08 minutes, 95% CI –3.40 to 4.76 minutes; MD 4.62 minutes, 95% CI 4.21 minutes to 5.03 minutes; both p < 0.00001). Citalopram is also significantly more effective than no treatment (MD 3.14 minutes, 95% CI 2.47 minutes to 4.35 minutes; p < 0.00001). Escitalopram significantly increased IELT compared with placebo (MD 1.2 minutes, 95% CI 0.79 to 1.61 minutes; geometric mean 3.5 minutes, 95% CI 1.96 to 5.04 minutes; both p < 0.00001). Fluoxetine significantly increased IELT compared with placebo (MD 2.41 minutes, 95% CI 2.10 to 2.73 minutes; p < 0.00001). There was no significant difference in IELT between fluvoxamine and placebo. Paroxetine significantly increased IELT compared with placebo (MD 5.34 minutes, 95% CI 3.79 to 6.89 minutes; p < 0.00001) and improved sexual satisfaction. Sertraline significantly increased IELT compared with placebo (MD 2.72 minutes, 95% CI 1.77 to 3.67 minutes; p < 0.00001) and improved ejaculation control. AEs included nausea, headache, insomnia, dry mouth, diarrhoea, drowsiness, dizziness, somnolence, decreased libido and anejaculation.
Selective serotonin reuptake inhibitors licensed for premature ejaculation (dapoxetine)
The evidence for dapoxetine at 30 mg or 60 mg on demand (approved doses in the UK) came from eight RCTs113–116,118–120,170 including one Phase II RCT114 and six Phase III RCT85,113,116,118,119,170 reports captured in six systematic reviews65,67,68,108–110 of low to moderate quality, plus one further RCT of low quality. The pooled evidence across RCTs suggests that dapoxetine 30 mg (three RCTs113,118,170) and 60 mg (five RCTs85,113,118,119,170) both significantly increased IELT compared with placebo (MD 1.16 minutes, 95% CI 0.94 to 1.39 minutes; and 1.66 minutes, 95% CI 1.46 to 1.87 minutes; both p < 0.00001). Dapoxetine 60 mg was significantly more effective than 30 mg (MD 0.46 minutes, 95% CI 0.19 to 0.74 minutes; p = 0.0009). Similar effects were evident for ejaculatory control, sexual satisfaction, global impression of change and clinical benefit. There was no significant difference in IELT between dapoxetine 30 mg combined with mirodenafil (PDE5 inhibitor) and dapoxetine 30 mg alone. AEs included nausea, diarrhoea, headache and dizziness and appear to be dose dependent.
Serotonin–noradrenaline reuptake inhibitors
The evidence for SNRIs was reported in three RCTs,121–123 one121 captured in a low-quality systematic review, plus two further RCTs,122,123 one123 of unclear quality and one122 at high risk of methodological bias. Evidence from one RCT121 indicated that duloxetine was significantly better than placebo in increasing IELT (MD 1.52 minutes, 95% CI 0.08 to 2.24 minutes; p < 0.00001). Evidence from two RCTs122,123 suggests that venlafaxine is not effective at increasing IELT compared with placebo. Duloxetine-associated side effects are reported as dry mouth and nausea, and venlafaxine caused more side effects than placebo.
Tricyclic antidepressants
The evidence for clomipramine was reported in 13 RCTs,39,76,107,124–133 1039,76,124–131 captured in low-to-moderate methodological quality systematic reviews, plus three further RCTs107,132,133 of unclear quality. Both oral and nasal administration of clomipramine is evaluated in these trials. Existing study evidence summarised by reviews suggests that oral clomipramine might be better than placebo at increasing IELT,52,69 but the reviews are of low methodological quality and report pooled estimates based on RCT and observational data. Inhaled clomipramine 4 mg appears effective at increasing IELT compared with placebo (1.68 minutes, 95% CI 1.06 to 2.29 minutes; p < 0.00001). Crossover trial evidence suggests efficacy with 1 mg or 2 mg appears to be dose dependent, as do treatment-related side effects of local irritation associated with nasal administration.
Phosphodiesterase-5 inhibitors
The evidence for PDE5 inhibitors was reported in 12 RCTs,39,55,101,120,138–145 1039,55,138–145 captured in five37,134–137 systematic reviews of low to moderate methodological quality and two further RCTs97,116 of low and unclear quality. Based on one RCT each, vardenafil138 and tadalafil141 both significantly increased IELT compared with placebo, (MD 3.80 minutes, 95% CI 3.30 to 4.30 minutes; and 2.59 minutes, 95% CI 1.28 to 3.90 minutes; p < 0.00001 and p = 0.0001, respectively). There was no significant difference in IELT between sildenafil and placebo in one RCT. 142 Sexual satisfaction favoured PDE5 inhibitors compared with placebo. Combined therapy (sildenafil plus sertraline or behavioural therapy) was better than sildenafil alone. Some RCTs provided evidence that PDE5 inhibitors increased IELT more than SSRIs; however, no significant difference was evident for some RCTs. AEs included flushing, headache and palpitations.
Alpha-blockers
The evidence for alpha-blockers was reported in two RCTs,39,107 one39 captured in low methodological quality systematic reviews and one further RCT107 of unclear quality. IELT was not reported for either trial. Evidence from one RCT107 suggested that terazosin, clomipramine and sertraline are all significantly better than placebo on ejaculation control, with no significant difference between active treatments. The same RCT reported no significant difference between terazosin, clomipramine and sertraline in the number of patients reporting AEs of headache, hypotension, drowsiness and ejaculation disorder.
Opioid analgesics: tramadol
The evidence for tramadol was reported in seven RCTs,46,150–155 five46,150–153 captured in three147–149 low to moderate methodological quality systematic reviews and two further RCTs154,155 of unclear methodological quality. Pooled evidence suggested that tramadol significantly increased IELT compared with placebo (MD 1.35 minutes, 95% CI 0.63 to 2.07 minutes; p = 0.0002) and improved sexual satisfaction. One RCT46 suggested that tramadol combined with behavioural therapy was significantly more effective than behavioural therapy alone (MD 1.65 minutes, 95% CI 0.30 to 3.00 minutes; p = 0.02). One RCT150 found no statistically significant difference in IELT between tramadol and paroxetine. Tramadol was associated with significantly more AEs than placebo, including erectile dysfunction, constipation, nausea, headache, somnolence, dry mouth, dizziness, pruritus (itching) and vomiting. Addiction to tramadol was not assessed.
Other therapies: acupuncture
The current evidence base for acupuncture comprises two RCTs156,157 of unclear methodological quality comparing acupuncture with SSRIs (citalopram and paroxetine). Acupuncture appeared to be more effective than sham acupuncture or citalopram but paroxetine appeared to be more effective than acupuncture. The AEs associated with acupuncture are unclear and the evidence base for this treatment is limited.
Other therapies: Chinese medicine
The current evidence base for Chinese medicine comprises five RCTs158–162 of unclear methodological quality. None was placebo controlled. These trials suggest that Chinese medicine is more effective than treatment as usual (1.57 minutes, 95% CI 1.11 to 2.03 minutes; p < 0.00001) but that fluoxetine is better than Chinese medicine (0.60 minutes, 95% CI 0.19 to 1.01 minutes; p = 0.004) in increasing IELT. AEs were not well reported. The lack of any placebo comparisons in PE trials coupled with limited evidence-based information regarding the efficacy and safety of Chinese medicine compounds limits the interpretation of results.
Other therapies: delay devices
The current evidence base for delay devices comprises one RCT163 of unclear methodological quality. This trial indicated that a desensitising band combined with the stop–start technique increased IELT more than behavioural therapy combined with the stop–start technique. Soreness is reported with overuse but appears resolved when the device is used as instructed.
Other therapies: yoga
The current evidence base for yoga comprises one observational study (non-RCT)164 comparing yoga with fluoxetine. This study reported that a high proportion of partners reported a satisfaction rating of yoga of ‘good’. However, the IELT data suggested that fluoxetine is more effective than yoga. AEs associated with yoga were not reported. These findings are limited by non-randomised trial design and no RCTs assessing yoga for the treatment of PE were identified.
Strengths and limitations of the assessment
Strengths
Methodological considerations
This report has systematically reviewed the evidence for a range of treatments for PE. RCT evidence reported in existing reviews along with further identified RCTs was included. Our literature search covered all dates (from database inception to August 2013) in order to capture any studies missed by existing reviews in addition to those published more recently. The current evidence base includes several systematic reviews of PE treatments, many of which do not report a meta-analysis. Where meta-analyses are undertaken, methodological errors are evident. These include combining RCTs with observational studies (and not reporting which are which), double-counting participants within the meta-analyses (including the control group from a RCT twice when different treatments are assessed), pooling data from crossover and pairwise RCTs (double counting for crossover trials), pooling between-group comparisons on questionnaire domains (subgroups) as an overall effect for the same trial (double counting), and applying a standardised MD to pool IELT effects where a MD is statistically more appropriate. This assessment report has pooled data across RCTs, when appropriate, in a meta-analysis using a MD to summarise IELT outcomes, has avoided double-counting of participants in the analysis and has considered pairwise and crossover RCT data separately. Furthermore, a formal assessment of methodological quality was undertaken. This was undertaken for both reviews from which RCT data were extrapolated and for any further RCTs identified by the searches not included in reviews.
Range of interventions assessed
The treatments evaluated in this assessment report were those relevant to the UK setting. In addition to treatments currently recommended in clinical practice, other treatments, including Chinese medicine, acupuncture, yoga and delay devices, were also evaluated, as patients might access these outside clinical practice. These treatments have not previously been reviewed in the management of PE.
Limitations
Methodological considerations
This assessment report summarises a wide range of interventions from a large volume of trial evidence and was undertaken within a limited timeframe. While RCT publications not already included in a review were obtained in full and data extracted (and checked by a second reviewer), data for RCTs reported in reviews were extracted (and checked) from the review article and not the original RCT publication. While data extraction from reviews was optimised when more than one review reported data for the same RCT, the reliability of the data extraction within the reviews cannot be guaranteed by this assessment report.
The methodological quality of the majority of existing reviews was low. Only four reviews reported independent double data extraction36,53,135,149 (see Appendix 4). Reported search strategies varied in terms of the search dates and resources searched. The search strategy for this assessment report covered all dates (from database inception to August 2013) in order to capture any studies missed by existing reviews. Within this assessment report, although quality assessment was undertaken for RCTs not included in reviews, the methodological quality of individual RCTs reported in existing reviews was not assessed. Of the nine existing reviews that reported undertaking quality assessment,35,36,38,51,53,64,65,108,110 quality scores were reported by only four,35,51,53,64 across which the assessment method was diverse, including use of an assessment instrument not appropriate for RCTs53 (the Newcastle–Ottawa Scale for assessing the quality of non-randomised studies in meta-analyses).
Although the search strategy for this assessment report was comprehensive, the possibility of a publication bias cannot be discounted. Nonetheless, given the unclear methodological quality of the majority of included RCTs, coupled with the variability of treatment effects on IELT, it could be considered unlikely that any additional unpublished data would contribute significantly to the overall findings.
Nature of the available evidence
Most trials comprised men with primary PE without a concomitant condition and excluded those with erectile dysfunction. When reported, men were mainly recruited from specialist sexual health settings. For this reason, effectiveness of in men with secondary PE, PE concomitant to another condition, or not attending specialised clinics, is less certain. Trials were undertaken in a variety of European Union (EU) and non-EU countries. Variability in cultural attitudes towards PE and acceptability of the various treatments in trial populations, coupled with variability in PE definitions and IELT entry criteria, also limits the generalisability of the findings.
Within the current evidence base, there are very few RCTs of robust methodological quality that compare one treatment with another in pairwise comparisons. A network meta-analysis has not been undertaken to date. It is therefore difficult to make comparisons of efficacy between treatments. The only treatment licensed for PE in the UK is dapoxetine, which has demonstrated modest but statistically significant improvements in IELT and other outcomes, but is associated with AEs similar to those of other SSRIs. Although some other treatments (e.g. topical) have shown greater IELT improvements than dapoxetine, other treatments have not been so extensively investigated.
Treatment duration within RCTs ranged from 2 to 24 weeks. No studies reported long-term follow-up (> 6 months) of patients either continuing on or withdrawing from treatment; thus, there was no assessment of long-term safety and efficacy, or effects of treatment withdrawal.
The majority of RCTs assessed IELT and, when reported, the assessment method was mainly by stopwatch. The duration of treatment effects on IELT ranged from < 0.50 minutes to > 6.00 minutes. Many interventions also demonstrated improvements in ejaculation control, sexual satisfaction and other outcomes. However, these outcomes were often measured using different assessment scales and the reporting of outcome data was often limited. IELT is reported to have a significant direct effect on perceived control over ejaculation, but not a significant direct effect on ejaculation-related personal distress or satisfaction with sexual intercourse. 172 There is currently no published literature which identifies a clinically significant threshold response to intervention. 23 Although the observed increases in IELT were statistically significant in favour of active treatments, it is difficult to quantify how acceptable and meaningful these changes are for men with PE, without being able to evaluate the relationship between IELT, ejaculation control and sexual satisfaction within the current RCT evidence base.
Adverse event reporting, both in reviews and in further RCTs, was limited. Although the nature of AEs associated with specific treatments could be identified, evidence surrounding proportions of patients withdrawing from treatment owing to AEs was either unclear or not reported. Furthermore, patient adherence to and acceptability of PE treatments has not yet been fully evaluated in the current evidence base.
Assessment of factors relevant to the NHS and other parties
Key considerations include the following:
-
The treatment duration among RCTs ranged from 2 to 24 weeks (maximum of 12 weeks for many treatments). Thus, there is limited evidence regarding long-term safety and effectiveness of treatments.
-
The effects of many treatments may be expected to end when treatment is stopped. This may be of particular concern following cessation of pharmacological agents. Behavioural modifications that are acquired through counselling might also not endure long term without continued support.
-
Some AE data were available from the included RCTs, but some key safety concerns were not assessed. These include possible long-term effects of SSRIs8 and the addiction potential of tramadol.
-
Different interventions have different modes of action and patients may have a preference, for example a preference for non-pharmacological interventions, or for pharmacological agent that can be taken as needed rather than every day. Having available a range of treatment options (to be used individually or in combination) would be a useful approach to individual patient management.
-
It is important to consider the balance between IELT and other effectiveness outcomes compared with AEs and inconvenience. Some patients may consider small increases of a few minutes in IELT to outweigh any treatment-related AEs, while others may not.
-
In the UK, there are currently only a few specialised treatment centres for PE, and a general practitioner (GP) referral to one of these may have long waiting times. A range of treatment options should be available to GPs as a first-line approach for patients presenting with PE.
Chapter 5 Conclusions
Implications for service provision
Several interventions provided statistically significant improvements of between 1 and 6 minutes in time to ejaculation (IELT). These include pharmacological interventions (SSRIs and other antidepressants, PDE5 inhibitors, tramadol), topical anaesthetics and behavioural therapies. Many interventions also demonstrated improvements in sexual satisfaction and other outcomes. Behavioural therapy combined with pharmacotherapy was better than behavioural therapy or pharmacotherapy alone. Pharmacological and topical therapies are associated with some AEs. Trial duration was a maximum of 12 weeks for most interventions (24 weeks for dapoxetine and tramadol). Different interventions have different modes of action and individual patients may have a preference for pharmacological or behavioural interventions, so maintaining a range of options (to be used individually or in combination) may remain a useful approach in the treatment of PE.
Suggested research priorities
The suggested research priorities when evidence is most unclear are as follows.
Long-term safety and effectiveness
Assessment of long-term safety and effectiveness (> 6 months) is required to evaluate whether or not initial treatment effects are maintained long term, whether or not dose escalation is required, how soon treatment effects end following treatment cessation, and whether or not treatments can be stopped and resumed at a later time. In addition, it is important to assess the AEs associated with long-term treatment (e.g. long-term effects of SSRIs and the addiction potential of tramadol) and whether or not different doses have differing AE profiles. These research questions might be addressed by reviewing the literature surrounding the use of these treatments in other conditions (e.g. SSRIs in the management of depression). Any evidence gaps could be addressed through longer-term studies in PE; this may include observational studies or longer-term follow-up of RCT participants.
Comparison between treatments
The majority of treatments evaluated by this report provide improvements in IELT and other outcomes compared with placebo or no treatment, but are associated with AEs. The current evidence base does not include sufficient direct comparisons between treatments to inform any judgement regarding the ‘best treatment’. Future research could consider a mixed treatment comparison/network meta-analysis approach and/or further head-to-head trials, as well as assessment of cost-effectiveness of the different interventions. Given that dapoxetine has been specifically developed for PE and has been extensively evaluated for this indication, head-to-head comparisons between this and other treatments might be informative. The effect of treatments used sequentially or in combination should also be further assessed. However, as patients are likely to have preferences for different types of treatment (e.g. pharmacological or behavioural), maintaining a range of options may remain a useful approach.
In terms of behavioural therapies, given the diversity of interventions in terms of technique, duration and delivery, further research is required to establish the components and intensity of intervention that are most effective. This could be addressed via further RCTs comparing different behavioural interventions in a head-to-head manner.
Clinical significance of outcomes and risk–benefit assessment
Future research should also consider an evaluation of the clinical significance of IELT increases, which may include assessment of the relationship between increases in IELT, ejaculatory control and sexual satisfaction, and whether or not increases of a few minutes in IELT are more meaningful to some patients than others. The trade-off between improvements in IELT and other clinical effectiveness outcomes compared with AEs and inconvenience should also be further assessed. Patient and partner acceptability of the different types of treatment (systemic, topical, behavioural) should also be further evaluated.
Acknowledgements
Many thanks to our clinical advisors for providing support and advice for this review: Professor Kevan Wylie, Porterbrook Clinic, Sexual Medicine, Sheffield; Dr Leila Frodsham, Chair, Institute of Psychosexual Medicine, London.
Thanks to Dr Catherine Hood, Specialist in Psychosexual Medicine, St George’s Hospital London, for reviewing the draft report.
Thanks to Gill Rooney at School of Health and Related Research (ScHARR) for providing administrative support and preparing and formatting the report.
Thanks to Kate Ren, Yelan Guo and Ji Hee Youn, at ScHARR for their help with translation.
This report was commissioned by the National Institute for Health Research (NIHR) HTA. The views expressed in this report are those of the authors and not necessarily those of the NIHR HTA programme. The final report and any errors remain the responsibility of the University of Sheffield. Eva Kaltenthaler and Matt Stevenson are guarantors.
About School of Health and Related Research
The ScHARR is one of the nine departments that constitute the Faculty of Medicine, Dentistry and Health at the University of Sheffield, Sheffield, UK. ScHARR specialises in health services and public health research, and the application of health economics and decision science to the development of health services and the improvement of public health.
The ScHARR Technology Assessment Group (ScHARR-TAG) synthesises research on the clinical effectiveness and cost-effectiveness of health-care interventions for the NIHR HTA programme on behalf of a range of policy makers, including the National Institute for Health and Care Excellence. ScHARR-TAG is part of a wider collaboration of a number of units from other regions including Southampton Health Technology Assessment Centre (SHTAC), University of Southampton, Southampton, UK; Aberdeen HTA Group, University of Aberdeen, Aberdeen, UK; Liverpool Reviews & Implementation Group, University of Liverpool, Liverpool, UK; Peninsular Technology Assessment Group (PenTAG), University of Exeter, Exeter, UK; the NHS Centre for Reviews and Dissemination, University of York, York, UK; Warwick Evidence, University of Warwick, Warwick, UK; the BMJ Group, London, UK, and Kleijnen Systematic Reviews, York, UK.
Contributions of authors
Katy Cooper and Marrissa Martyn-St James carried out the systematic review and quality assessment of the studies and wrote the report.
Eva Kaltenthaler contributed to and checked the review.
Kath Dickinson and Anna Cantrell carried out the literature searches.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 2000.
- International Classification of Diseases and Related Health Problems. Geneva: WHO; 1994.
- Porst H, Montorsi F, Rosen RC, Gaynor L, Grupe S, Alexander J. The Premature Ejaculation Prevalence and Attitudes (PEPA) survey: prevalence, comorbidities, and professional help-seeking. Eur Urol 2013;51:816-23. http://dx.doi.org/10.1016/j.eururo.2006.07.004.
- McMahon C, Abdo C, Incrocci L, Perelman M, Rowland D, Waldinger M, et al. Disorders of orgasm and ejaculation in men. J Sex Med 2004 n.d.;1:58-65. http://dx.doi.org/10.1111/j.1743-6109.2004.10109.x.
- Rowland DL, Patrick DL, Rothman M, Gagnon DD. The psychological burden of premature ejaculation. J Urol 2007;177:1065-70. http://dx.doi.org/10.1016/j.juro.2006.10.025.
- Wespes EC, Eardley I, Giuliano F, Hatzichristou D, Hatzimouratidis KK, Moncada I, et al. Guidelines on Male Sexual Dysfunction: Erectile Dysfunction and Premature Ejaculation. Arnhem: European Association of Urology; 2013.
- Godpodinoff ML. Premature ejaculation: clinical subgroups and etiology. J Sex Marital Ther 1989;15:130-4. http://dx.doi.org/10.1080/00926238908403817.
- Richardson D, Goldmeier D, Green J, Lamba H, Harris JRW. Recommendations for the management of premature ejaculation: BASHH Special Interest Group for Sexual Dysfunction. Int J STD AIDS 2006;17:1-6. http://dx.doi.org/10.1258/095646206775220540.
- Diagnostic and Statistical Manual of Mental Disorders: DSM-IV. Washington, DC: American Psychiatric Association; 1994.
- Laumann EO, Nicolosi A, Glasser DB, Paik A, Gingell C, Moreira ED, et al. Sexual problems among women and men aged 40–80 y: prevalence and correlates identified in the global study of sexual attitudes and behaviors. J Impot Res 2005;17:39-57. http://dx.doi.org/10.1038/sj.ijir.3901250.
- Laumann EO, Paik A, Rosen RC. Sexual dysfunction in the United States: prevalence and predictors. JAMA 1999;281:537-44. http://dx.doi.org/10.1001/jama.281.6.537.
- Giuliano F, Patrick DL, Porst H, La Pera G, Kokoszk A, Merchant S, et al. Premature ejaculation: results from a five-country European observational study. Eur Urol 2008;53:1048-57. http://dx.doi.org/10.1016/j.eururo.2007.10.015.
- Byers ES, Grenier G. Premature or rapid ejaculation: heterosexual couples’ perceptions of men’s ejaculatory behavior. Arch Sexl Behav 2003;32:261-70. http://dx.doi.org/10.1023/A:1023417718557.
- Shabsigh R. Diagnosing premature ejaculation: a review. J Sex Med 2006;3:318-23. http://dx.doi.org/10.1111/j.1743-6109.2006.00307.x.
- Sharlip I. Diagnosis and treatment of premature ejaculation: the physician’s perspective. J Sex Med 2005;2:103-9. http://dx.doi.org/10.1111/j.1743-6109.2005.20370.x.
- Semans JH. Premature ejaculation: a new approach. South Med J 1953;49:353-8. http://dx.doi.org/10.1097/00007611-195604000-00008.
- Masters W, Johnson V. Human Sexual Inadequacy. Boston, MA: Little, Brown and Company; 1970.
- Arafa M, Shamloul R. Development and evaluation of the Arabic Index of Premature Ejaculation (AIPE). J Sex Med 2007;4:1750-6. http://dx.doi.org/10.1111/j.1743-6109.2006.00213.x.
- Patrick DL, Giuliano F, Ho KF, Gagnon DD, McNulty P, Rothman M. The premature ejaculation profile: validation of self-reported outcome measures for research and practice. BJU Int 2009;103:358-64. http://dx.doi.org/10.1111/j.1464-410X.2008.08041.x.
- Althof S, Rosen R, Symonds T, Mundayat R, May K, Abraham L. Development and validation of a new questionnaire to assess sexual satisfaction, control, and distress associated with premature ejaculation. J Sex Med 2006;3:465-75. http://dx.doi.org/10.1111/j.1743-6109.2006.00239.x.
- Rosen RC, Catania JA, Althof SE, Pollack LM, O’Leary M, Seftel AD, et al. Development and validation of four-item version of male sexual health questionnaire to assess ejaculatory dysfunction. Urology 2007;69:805-9. http://dx.doi.org/10.1016/j.urology.2007.02.036.
- The British Association of Urological Surgeons . Premature Ejaculation 2013. www.baus.org.uk/patients/symptoms/Premature+ejaculation (accessed October 2013).
- McMahon CG. Clinical trial methodology in premature ejaculation observational, interventional, and treatment preference studies – part II – study design, outcome measures, data analysis, and reporting. J Sex Med 2008;5:1817-33. http://dx.doi.org/10.1111/j.1743-6109.2008.00837.x.
- European Medicines Agency . Questions and Answers on Priligy (Dapoxetine, 30 mg and 60 mg Tablets). Outcome of a Procedure Under Article 29 of Directive 2001 83 EC As Amended 2012. www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Priligy_29/WC500116880.pdf (accessed July 2013).
- Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group: Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLOS Med 2009;6. http://dx.doi.org/10.1371/journal.pmed.1000097.
- Scottish Intercollegiate Guidelines Network . SIGN Search Filters n.d. www.sign.ac.uk/methodology/filters.html#random (accessed 6 August 2013).
- Lee E, Dobbins M, DeCorby K, McRae L, Tirilis D, Husson H. An optimal search filter for retrieving systematic reviews and meta-analyses. BMC Med Res Methodol 2012;12. http://dx.doi.org/10.1186/1471-2288-12-51.
- Health Evidence Bulletins Wales . Health Evidence Bulletins Wales, Filter for Guidelines n.d. http://hebw.cf.ac.uk/projectmethod/appendix2.htm (accessed 6 August 2013).
- McMahon CG, Althof S, Waldinger MD, Porst H, Dean J, Sharlip I, et al. An evidence-based definition of lifelong premature ejaculation: report of the International Society for Sexual Medicine Ad Hoc Committee for the Definition of Premature Ejaculation. BJU Int 2008;102:338-50. http://dx.doi.org/10.1111/j.1464-410X.2008.07755.x.
- Richardson D, Goldmeier D. BASHH Special Interest Group for Sexual Dysfunction. Recommendations for the management of retarded ejaculation: BASHH Special Interest Group for Sexual Dysfunction. Int J STD AIDS 2006;17:7-13. http://dx.doi.org/10.1258/095646206775220450.
- Higgins JPT, Deeks JJ, Altman DA, Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Updated March 2011). n.d.
- Higgins JPTH, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. http://dx.doi.org/10.1136/bmj.327.7414.557.
- Shea B, Grimshaw J, Wells G, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 2007;7. http://dx.doi.org/10.1186/1471-2288-7-10.
- Higgins JPT, Altman DG, Sterne JAC, Higgins JPT, Green S. behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group . Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (Updated March 2011) n.d. http://handbook.cochrane.org/ (accessed 23 January 2015).
- Melnik T, Althof S, Atallah AN, Puga MEDS, Glina S, Riera R. Psychosocial interventions for premature ejaculation. Cochrane Database Syst Rev 2011;8.
- Berner M, Gunzler C. Efficacy of psychosocial interventions in men and women with sexual dysfunctions–a systematic review of controlled clinical trials: part 1 – the efficacy of psychosocial interventions for male sexual dysfunction. J Sex Med 2012;9:3089-107. http://dx.doi.org/10.1111/j.1743-6109.2012.02970.x.
- McMahon CG, McMahon CN, Leow LJ, Winestock CG. Efficacy of type-5 phosphodiesterase inhibitors in the drug treatment of premature ejaculation: a systematic review. BJU Int 2006;98:259-72. http://dx.doi.org/10.1111/j.1464-410X.2006.06290.x.
- Melnik T, Glina S, Rodrigues OMJ. Psychological intervention for premature ejaculation. Nat Rev Urol 2009;6:501-8. http://dx.doi.org/10.1038/nrurol.2009.147.
- Abdel-Hamid IA, El Naggar EA, El Gilany AH. Assessment of as needed use of pharmacotherapy and the pause-squeeze technique in premature ejaculation. Int J Impot Res 2001;13:41-5. http://dx.doi.org/10.1038/sj.ijir.3900630.
- de Carufel F, Trudel G. Effects of a new functional-sexological treatment for premature ejaculation. J Sex Marital Ther 2006;32:97-114. http://dx.doi.org/10.1080/00926230500442292.
- Kolomaznik M, Cervinka L, Kutilek S, Pohanka M, Skibova J, Taus L, et al. Fluoxetine in the treatment of ejaculatio praecox. Psychiatrie 2002;6:6-9.
- Li P, Zhu GS, Xu P, Sun LH, Wang P. Interventional effect of behaviour psychotherapy on patients with premature ejaculation. Zhonghua Nan Ke Xue 2006;12:717-19.
- Oguzhanoglu NK, Ozdel O, Aybek Z. The efficacy of fluoxetine and a stop-start technique in the treatment of premature ejaculation and anxiety. J Clin Psychopharmacol 2005;25:192-4. http://dx.doi.org/10.1097/01.jcp.0000161449.50969.28.
- Tang W, Ma L, Zhao L, Liu Y, Chen Z. Clinical efficacy of Viagra with behavior therapy against premature ejaculation. Zhonghua Nan Ke Xue 2004;10:366-7.
- Trudel G, Proulx S. Treatment of premature ejaculation by bibliotherapy: an experimental study. J Sex Marital Ther 1987;2:163-7. http://dx.doi.org/10.1080/02674658708407860.
- Xiong GG, Wu FH, Chen SH, Yao WL. Safety and efficacy of tramadol hydrochloride with behavioral modification in the treatment of premature ejaculation. Zhonghua Nan Ke Xue 2011;17:538-41.
- Yuan P, Dai J, Yang Y, Guo J, Liang R. A comparative study on treatment for premature ejaculation: Citalopram used in combination with behavioral therapy versus either citalopram or behavioral therapy alone. Chin J Androl 2008;22:35-8.
- Pastore AL, Palleschi G, Leto A, Pacini L, Iori F, Leonardo C, et al. A prospective randomized study to compare pelvic floor rehabilitation and dapoxetine for treatment of lifelong premature ejaculation. Int J Androl 2012;35:528-33. http://dx.doi.org/10.1111/j.1365-2605.2011.01243.x.
- Shao X, Li J. Clinical study on treatment of premature ejaculation with Paroxetine and behavior-therapy. Chin J Androl 2008;22:18-20.
- van Lankveld JJDM, Leusink P, van Diest S, Gijs L, Slob AK. Internet-based brief sex therapy for heterosexual men with sexual dysfunctions: a randomized controlled pilot trial. J Sex Med 2009;6:2224-36. http://dx.doi.org/10.1111/j.1743-6109.2009.01321.x.
- Pu C, Yang L, Liu L, Yuan H, Wei Q, Han P. Topical anesthetic agents for premature ejaculation: a systematic review and meta-analysis. Urology 2013;81:799-804. http://dx.doi.org/10.1016/j.urology.2012.12.028.
- Waldinger MD, Zwinderman AH, Schweitzer DH, Olivier B. Relevance of methodological design for the interpretation of efficacy of drug treatment of premature ejaculation: a systematic review and meta-analysis. Int J Impot Res 2004;16:369-81. http://dx.doi.org/10.1038/sj.ijir.3901172.
- Xia JD, Han YF, Zhou LH, Chen Y, Dai YT. Efficacy and safety of local anaesthetics for premature ejaculation: a systematic review and meta-analysis. Asian J Androl 2013;15:497-502. http://dx.doi.org/10.1038/aja.2012.174.
- Morales A, Barada J, Wyllie MG. A review of the current status of topical treatments for premature ejaculation. BJU Int 2007;100:493-501. http://dx.doi.org/10.1111/j.1464-410X.2007.07051.x.
- Atan A, Basar MM, Tuncel A, Ferhat M, Agras K, Tekdogan U. Comparison of efficacy of sildenafil-only, sildenafil plus topical EMLA cream, and topical EMLA-cream-only in treatment of premature ejaculation. Urology 2006;67:388-91. http://dx.doi.org/10.1016/j.urology.2005.09.002.
- Atikeler MK, Gecit I, Senol FA. Optimum usage of prilocaine-lidocaine cream in premature ejaculation. Andrologia 2002;34:356-9. http://dx.doi.org/10.1046/j.1439-0272.2002.00511.x.
- Busato W, Galindo CC. Topical anaesthetic use for treating premature ejaculation: a double-blind, randomized, placebo-controlled study. BJU Int 2004;93:1018-21. http://dx.doi.org/10.1111/j.1464-410X.2003.04773.x.
- Carson C, Wyllie M. Improved ejaculatory latency, control and sexual satisfaction when PSD502 is applied topically in men with premature ejaculation: results of a phase III, double-blind, placebo-controlled study. J Sex Med 2010;7:3179-89. http://dx.doi.org/10.1111/j.1743-6109.2010.01913.x.
- Dinsmore WW, Hackett G, Goldmeier D, Waldinger M, Dean J, Wright P, et al. Topical eutectic mixture for premature ejaculation (TEMPE): a novel aerosol-delivery form of lidocaine-prilocaine for treating premature ejaculation. BJU Int 2007;99:369-75. http://dx.doi.org/10.1111/j.1464-410X.2006.06583.x.
- Dinsmore WW, Wyllie M. Results of a phase III, randomized, placebo-controlled study show that PSD502 improves ejaculatory latency and patient reported outcomes, with benefit maintained over 3 months. J Sex Med 2009;6:418-19.
- Gittelman M, Mo J, Lu M. Synergistic effect of meatal application of dyclonine/alprostadil cream for the treatment of early ejaculation (EE) in a double-blind and crossover study. J Sex Med 2006;3.
- Mallat F, Hmida W, Hidoussi A, Slama A, Jaidane M, Ben SN, et al. Comparison of efficacy of electric stimulation and topical EMLA cream in treatment of premature ejaculation: prospective and comparative study. Eur Urol Suppl 2012;11.
- Steggall MJ, Fowler CG, Pryce A. Sex Relationship Ther 2008;23:365-76. http://dx.doi.org/10.1080/14681990802545532.
- Cong W, Zhao W-D, Zhong Z-H. Effectiveness and safety of fluoxetine for premature ejaculation: a meta-analysis. Chin J Evidence-Based Med 2012;12:1510-15.
- Huang XK, Lu YP, Luo SW, Wang F, Xie ZY, Wang XD. Efficacy and safety of selective serotonin re-uptake inhibitors in the treatment of premature ejaculation: a systematic evaluation. Zhonghua Nan Ke Xue 2009;15:248-55.
- Moreland AJ, Makela EH. Selective serotonin-reuptake inhibitors in the treatment of premature ejaculation. Ann Pharmacother 2005;39:1296-301. http://dx.doi.org/10.1345/aph.1E069.
- Wang WF, Chang L, Minhas S, Ralph DJ. Selective serotonin reuptake inhibitors in the treatment of premature ejaculation. Chin Med J 2007;120:1000-6.
- McMahon CG, Porst H. Oral agents for the treatment of premature ejaculation: review of efficacy and safety in the context of the recent International Society for Sexual Medicine criteria for lifelong premature ejaculation. J Sex Med 2011;8:2707-25. http://dx.doi.org/10.1111/j.1743-6109.2011.02386.x.
- Richardson D, Green J, Ritcheson A, Goldmeier D, Harris JRW. A review of controlled trials in the pharmacological treatment of premature ejaculation. Int J STD AIDS 2005;16:651-8. http://dx.doi.org/10.1258/095646205774357299.
- Atmaca M, Kuloglu M, Tezcan E, Semercioz A. The efficacy of citalopram in the treatment of premature ejaculation: a placebo-controlled study. Int J Impot Res 2002;14:502-5. http://dx.doi.org/10.1038/sj.ijir.3900918.
- Atmaca M, Kuloglu M, Tezcan E, Ustundag B, Semercioz A. Serum leptin levels in patients with premature ejaculation before and after citalopram treatment. BJU Int 2003;91:252-4. http://dx.doi.org/10.1046/j.1464-410X.2003.04052.x.
- Safarinejad MR, Hosseini SY. Safety and efficacy of citalopram in the treatment of premature ejaculation: a double-blind placebo-controlled, fixed dose, randomized study. Int J Impot Res 2006;18:164-9. http://dx.doi.org/10.1038/sj.ijir.3901384.
- Waldinger MD, Zwinderman AH, Olivier B. SSRIs and ejaculation: a double-blind, randomized, fixed-dose study with paroxetine and citalopram. J Clin Psychopharmacol 2001;21:556-60. http://dx.doi.org/10.1097/00004714-200112000-00003.
- Haensel SM, Klem TM, Hop WC, Slob AK. Fluoxetine and premature ejaculation: a double-blind, crossover, placebo-controlled study. J Clin Psychopharmacol 1998;18:72-7. http://dx.doi.org/10.1097/00004714-199802000-00012.
- Kara H, Aydin S, Yucel M, Agargun MY, Odabas O, Yilmaz Y. The efficacy of fluoxetine in the treatment of premature ejaculation: a double-blind placebo controlled study. J Urol 1996;156:1631-2. http://dx.doi.org/10.1016/S0022-5347(01)65467-3.
- Kim CS, Seo KK. Efficacy and safety of fluoxetine, sertraline and clomipramine in patients with premature ejaculation: a double-bind, placebo controlled study. J Urol 1998;159:425-7. http://dx.doi.org/10.1016/S0022-5347(01)63940-5.
- Manasia P, Pomerol J, Ribe N, Gutierrez del Pozo R, Alcover Garcia J. Comparison of the efficacy and safety of 90 mg versus 20 mg fluoxetine in the treatment of premature ejaculation. J Urol 2003;170:164-5. http://dx.doi.org/10.1097/01.ju.0000071040.12407.32.
- Murat Basar M, Atan A, Yildiz M, Baykam M, Aydoganli L. Comparison of sertraline to fluoxetine with regard to their efficacy and side effects in the treatment of premature ejaculation. Arch Esp Urol 1999;52:1008-11.
- Novaretti JPT, Pompeo ACL, Arap S. Selective serotonin re-uptake inhibitor in the treatment of premature ejaculation. International Braz J Urol 2002;28:116-22.
- Panshou H, Xie L. Efficacy of fluoxetine in the treatment of premature ejaculation. Urology 2004;19:400-1.
- Waldinger MD, Hengeveld MW, Zwinderman AH, Olivier B. Effect of SSRI antidepressants on ejaculation: a double-blind, randomized, placebo-controlled study with fluoxetine, fluvoxamine, paroxetine, and sertraline. J Clin Psychopharmacol 1998;18:274-81. http://dx.doi.org/10.1097/00004714-199808000-00004.
- Waldinger MD, Zwinderman AH, Olivier B. Antidepressants and ejaculation: a double-blind, randomized, placebo-controlled, fixed-dose study with paroxetine, sertraline, and nefazodone. J Clin Psychopharmacol 2001;21:293-7. http://dx.doi.org/10.1097/00004714-200106000-00007.
- Yilmaz U, Tatlisen A, Turan H, Arman F, Ekmekcioglu O. The effects of fluoxetine on several neurophysiological variables in patients with premature ejaculation. J Urol 1999;161:107-11. http://dx.doi.org/10.1016/S0022-5347(01)62078-0.
- McMahon CG, Touma K. Treatment of premature ejaculation with paroxetine hydrochloride as needed: 2 single-blind placebo controlled crossover studies. J Urol 1999;161:1826-30. http://dx.doi.org/10.1016/S0022-5347(05)68816-7.
- Safarinejad MR. Comparison of dapoxetine versus paroxetine in patients with premature ejaculation: a double-blind, placebo-controlled, fixed-dose, randomized study. Clin Neuropharmacol 2006;29:243-52. http://dx.doi.org/10.1097/01.WNF.0000228210.12194.46.
- Waldinger MD, Hengeveld MW, Zwinderman AH. Paroxetine treatment of premature ejaculation: a double-blind, randomized, placebo-controlled study. Am J Psychiatry 1994;151:1377-9. http://dx.doi.org/10.1176/ajp.151.9.1377.
- Waldinger MD, Hengeveld MW, Zwinderman AH. Ejaculation-retarding properties of paroxetine in patients with primary premature ejaculation: a double-blind, randomized, dose–response study. BJU Int 1997;79:592-5. http://dx.doi.org/10.1046/j.1464-410X.1997.00102.x.
- Waldinger MD, Zwinderman AH, Olivier B. Antidepressants and ejaculation: a double-blind, randomized, fixed-dose study with mirtazapine and paroxetine. J Clin Psychopharmacol 2003;23:467-70. http://dx.doi.org/10.1097/01.jcp.0000088904.24613.e4.
- Biri H, Isen K, Sinik Z, Onaran M, Kupeli B, Bozkirli I. Sertraline in the treatment of premature ejaculation: a double-blind placebo controlled study. Int Urol Nephrol 1998;30:611-15. http://dx.doi.org/10.1007/BF02550555.
- Mendels J, Camera A, Sikes C. Sertraline treatment for premature ejaculation. J Clin Psychopharmacol 1995;15:341-6. http://dx.doi.org/10.1097/00004714-199510000-00006.
- Zhou X. Efficacy of Zoloft in the Treatment of Premature Ejaculation 2007.
- Akgül T, Karakan T, Ayyildiz A, Germiyanoglu C. Comparison of sertraline and citalopram for treatment of premature ejaculation. Urol J 2008;5:41-5.
- Farnia V, Raisi F, Mohseni MG, Atharikia D, Ghafuri Z. On-demand treatment of premature ejaculation with citalopram: a randomized double-blind study. Acta Med Iranica 2009;47:353-5.
- Nada EA, Saleh RSA, Ali MA, Azab HM. A comparison between citalopram and escitalopram in treatment of patients with premature ejaculation: a double-blind controlled clinical study. J Sex Med 2012;9:261-2.
- Rezakhaniha B, Sirosbakht S. Efficacy of selective serotonin reuptake inhibitor (SSRI) in patient with premature ejaculation. Iran J Reprod Med 2010;8:55-9.
- Shang XJ, Geng Q, Zhang K, Xia XY, Shao Y, Huang YF. Efficacy of citalopram on premature ejaculation: a clinical observation. Zhonghua Nan Ke Xue 2012;18:1097-100.
- Arafa M, Shamloul R. Efficacy of sertraline hydrochloride in treatment of premature ejaculation: a placebo-controlled study using a validated questionnaire. Int J Impot Res 2006;18:534-8. http://dx.doi.org/10.1038/sj.ijir.3901469.
- Nada EA, Saleh R, Abu AM. The use of escitalopram in treatment of patients with premature ejaculation: A randomized, double-blind, placebo-controlled study. J Sex Med 2009;6.
- Safarinejad MR. Safety and efficacy of escitalopram in the treatment of premature ejaculation: a double-blind, placebo-controlled, fixed-dose, randomized study. J Clin Psychopharmacol 2007;27:444-50. http://dx.doi.org/10.1097/jcp.0b013e31814b98d4.
- Ahn TY, Park H, Choi EH, Choo MS, Park T. Fluoxetine as a treatment for premature ejaculation: a double-blind, randomized, placebo-controlled study. Korean J Urol 1996;37:926-31.
- Culba M, Ozkan L, Ozkurkcugil C, Gokalp A. Comparison of fluoxetine vs. fluoxetine plus tadalafil in the treatment of premature ejaculation – a double blind, placebo control study. J Sex Med 2008;5:60-1.
- Weixing Z, Junchang Q, Rui W, Lei W, Jie Z. Effect evaluation of paroxetine and sertraline in the treatment of premature ejaculation. Chin J Androl 2012;26:41-3.
- Giammusso B, Morgia G, Spampinato A, Motta M. Paroxetine as a treatment for premature ejaculation. Arch Ital Urol Androl 1997;69:11-3.
- Khelaia AV, Bochorishvili GG, Managadze LG. Assessment of paroxetine in the treatment of premature ejaculation and dosage optimization. Eur Urol Suppl 2012;11.
- Waldinger MD, Zwinderman AH, Olivier B. On-demand treatment of premature ejaculation with clomipramine and paroxetine: a randomized, double-blind fixed-dose study with stopwatch assessment. Eur Urol 2004;46:510-16. http://dx.doi.org/10.1016/j.eururo.2004.05.005.
- Arafa M, Shamloul R. A randomized study examining the effect of 3 SSRI on premature ejaculation using a validated questionnaire. Ther Clin Risk Manag 2007;3:527-31.
- Tuncel A, Aslan Y, Basar MM, Atan A. Efficacy of clomipramine, sertraline and terazosin treatments in premature ejaculation. Turkish J Med Sci 2008;38:59-64.
- Luo YH, Hou Q, Zheng SB. Meta-analysis of dapoxetine on demand in the treatment of premature ejaculation. Zhonghua Nan Ke Xue 2012;18:930-5.
- McCarty E, Dinsmore W. Dapoxetine: an evidence-based review of its effectiveness in treatment of premature ejaculation. Core Evid 2012;7:1-14. http://dx.doi.org/10.2147/CE.S13841.
- Wang YB, Mao Y, Wei Q, Wu TX, Dong Q. Dapoxetine in treatment of premature ejaculation: a systematic review. Beijing Da Xue Xue Bao 2010;42:425-32.
- Hutchinson K, Cruickshank K, Wylie K. A benefit-risk assessment of dapoxetine in the treatment of premature ejaculation. Drug Saf 2012;35:359-72. http://dx.doi.org/10.2165/11598150-000000000-00000.
- Kendirci M, Salem E, Hellstrom W. Dapoxetine, a novel selective serotonin transport inhibitor for the treatment of premature ejaculation. Ther Clin Risk Manag 2007;3:277-89. http://dx.doi.org/10.2147/tcrm.2007.3.2.277.
- Buvat J, Tesfaye F, Rothman M, Rivas DA, Giuliano F. Dapoxetine for the treatment of premature ejaculation: results from a randomized, double-blind, placebo-controlled phase 3 trial in 22 countries. Eur Urol 2009;55:957-67. http://dx.doi.org/10.1016/j.eururo.2009.01.025.
- Hellstrom W, Gittelman M, Althof S, Ho KF, Kell S. Dapoxetine HCl for the treatment of premature ejaculation: a Phase II, randomized, double-blind, placebo-controlled study. J Sex Med 2004;1.
- Hellstrom WJ, Althof S, Gittelman M, Ho KF, Kell S. Dapoxetine for the treatment of men with premature ejaculation (PE): dose-finding analysis. J Urol 2005;173.
- Kaufman JM, Rosen RC, Mudumbi RV, Tesfaye F, Hashmonay R, Rivas D. Treatment benefit of dapoxetine for premature ejaculation: results from a placebo-controlled phase III trial. BJU Int 2009;103:651-8. http://dx.doi.org/10.1111/j.1464-410X.2008.08165.x.
- McMahon CG. Dapoxetine as treatment for premature ejaculation. J Sex Med 2013;10.
- Pryor JL, Althof SE, Steidle C, Rosen RC, Hellstrom WJG, Shabsigh R, et al. Efficacy and tolerability of dapoxetine in treatment of premature ejaculation: an integrated analysis of two double-blind, randomised controlled trials. Lancet 2006;368:929-37. http://dx.doi.org/10.1016/S0140-6736(06)69373-2.
- Safarinejad MR. Safety and efficacy of dapoxetine in the treatment of premature ejaculation: a double-blind, placebo-controlled, fixed-dose, randomized study. Neuropsychopharmacology 2008;33:1259-65. http://dx.doi.org/10.1038/sj.npp.1301500.
- Lee WK, Cho ST, Lee YS, Oh CY, Yu CH, Cho JS, et al. Comparison between on-demand dosing of dapoxetine alone and dapoxetine plus mirodenafil in patients with premature ejaculation; prospective, randomized, doubleblind, and placebo-controlled. J Sex Med 2012;9:203-4.
- Athanasios Z, Polyanthi P, George K. The efficacy of duloxetine in the treatment of premature ejaculation. Int Urol Nephrol 2013;39:115-18. http://dx.doi.org/10.1007/s11255-006-6659-2.
- Kilic S, Ergin H, Baydinc YC. Venlafaxine extended release for the treatment of patients with premature ejaculation: a pilot, single-blind, placebo-controlled, fixed–dose crossover study on short-term administration of an antidepressant drug. Int J Androl 2005;28:47-52. http://dx.doi.org/10.1111/j.1365-2605.2005.00507.x.
- Safarinejad MR. Safety and efficacy of venlafaxine in the treatment of premature ejaculation: a double-blind, placebo-controlled, fixed-dose, randomised study. Andrologia 2008;40:49-55. http://dx.doi.org/10.1111/j.1439-0272.2008.00813.x.
- Althof SE, Levine SB, Corty EW, Risen CB, Stern EB, Kurit DM. A double–blind crossover trial of clomipramine for rapid ejaculation in 15 couples. J Clin Psychiatry 1995;56:402-7.
- Girgis SM, El-Haggar S, El-Hermouzy S. A double-blind trial of clomipramine in premature ejaculation. Andrologia 1982;14:364-8. http://dx.doi.org/10.1111/j.1439-0272.1982.tb02278.x.
- Goodman RE. An assessment of clomipramine (Anafranil) in the treatment of premature ejaculation. J Int Med Res 1980;8:53-9.
- Haensel SM, Rowland DL, Kallan KT. Clomipramine and sexual function in men with premature ejaculation and controls. J Urol 1996;156:1310-15. http://dx.doi.org/10.1016/S0022-5347(01)65576-9.
- Montorsi F, Guazzoni G, Trimboli F, Rigatti P, Pizzini G, Miani A. Clomipramine for premature ejaculation: A randomized, double-blind, placebo controlled study. Acta Urol Ital 1995;9:5-6.
- Porto R. Double-blind trial of clomipramine in premature ejaculation. Medecine Et Hygiene 1981;39:1249-53.
- Segraves RT, Saran A, Segraves K, Maguire E. Clomipramine versus placebo in the treatment of premature ejaculation: a pilot study. J Sex Marital Ther 1993;19:198-200. http://dx.doi.org/10.1080/00926239308404904.
- Strassberg DS, de Gouveia Brazao CA, Rowland DL, Tan P, Slob AK. Clomipramine in the treatment of rapid (premature) ejaculation. J Sex Marital Ther 1999;25:89-101. http://dx.doi.org/10.1080/00926239908403982.
- Akilov A, Shavakhabov SH, Fozilov A, Rustamov R. Efficacy and safety of clomipramine hydrochloride containing nasal spray for the pharmacotherapy of premature ejaculation. Eur Urol Suppl 2011;10:158-9. http://dx.doi.org/10.1016/S1569-9056(11)60457-0.
- Leaker B, Kell P, Main M, Ralph D. A double blind, placebo controlled, randomised crossover study to investigate the effect of inhaled doses of VR776 on intravaginal ejaculatory latency in patients with premature ejaculation. J Sex Med 2008;5.
- Asimakopoulos AD, Miano R, Finazzi Agro E, Vespasiani G, Spera E. Does current scientific and clinical evidence support the use of phosphodiesterase type 5 inhibitors for the treatment of premature ejaculation? a systematic review and meta-analysis. J Sex Med 2012;9:2404-16. http://dx.doi.org/10.1111/j.1743-6109.2011.02628.x.
- Aversa A, Francomano D, Bruzziches R, Natali M, Spera G, Lenzi A. Is there a role for phosphodiesterase type-5 inhibitors in the treatment of premature ejaculation?. Int J Impot Res 2011;23:17-23. http://dx.doi.org/10.1038/ijir.2010.34.
- Burton TD, Liday C. The comparison of combination SSRI and PDE-5 inhibitor therapy to SSRI monotherapy in men with premature ejaculation. Ann Pharmacother 2011;45:1000-4. http://dx.doi.org/10.1345/aph.1Q008.
- Chen J, Keren-Paz G, Bar-Yosef Y, Matzkin H. The role of phosphodiesterase type 5 inhibitors in the management of premature ejaculation: a critical analysis of basic science and clinical data. Eur Urol 2007;52:1331-9. http://dx.doi.org/10.1016/j.eururo.2007.08.005.
- Aversa A, Pili M, Francomano D, Bruzziches R, Spera E, La Pera G, et al. Effects of vardenafil administration on intravaginal ejaculatory latency time in men with lifelong premature ejaculation. Int J Impot Res 2009;21:221-7. http://dx.doi.org/10.1038/ijir.2009.21.
- Hosseini MM, Yarmohammadi H. Effect of fluoxetine alone and in combination with sildenafil in patients with premature ejaculation. Urol Int 2007;79:28-32. http://dx.doi.org/10.1159/000102909.
- Mathers MJ, Klot T, Roth S, Lummen G, Sommer F. Safety and efficacy of vardenafil versus sertraline in the treatment of premature ejaculation: a randomised, prospective and crossover study. Andrologia 2009;41:169-75. http://dx.doi.org/10.1111/j.1439-0272.2008.00910.x.
- Mattos RM, Marmo Lucon A, Srougi M. Tadalafil and fluoxetine in premature ejaculation: prospective, randomized, double-blind, placebo-controlled study. Urol Int 2008;80:162-5. http://dx.doi.org/10.1159/000112607.
- McMahon CG, Stuckey BGA, Andersen M, Purvis K, Koppiker N, Haughie S, et al. Efficacy of sildenafil citrate (Viagra) in men with premature ejaculation. J Sex Med 2005;2:368-75. http://dx.doi.org/10.1111/j.1743-6109.2005.20351.x.
- Tang W, Ma L, Zhao L, Liu Y. Efficacy and safety of sertraline, viagra and cardura in patients with premature ejaculation. Chin J Androl 2004;18:20-2.
- Wang WF, Wang Y, Minhas S, Ralph DJ. Can sildenafil treat primary premature ejaculation? A prospective clinical study. Int J Urol 2007;14:331-5. http://dx.doi.org/10.1111/j.1442-2042.2007.01606.x.
- Zhang XS, Wang YX, Huang XY, Leng J, Li Z, Han YF. Comparison between sildenafil plus sertraline and sertraline alone in the treatment of premature ejaculation. Zhonghua Nan Ke Xue 2005;11:520-5.
- Cavallini G. Alpha-1 blockade pharmacotherapy in primitive psychogenic premature ejaculation resistant to psychotherapy. Eur Urol 1995;28:126-30.
- Wong BLK, Malde S. The use of tramadol ‘on-demand’ for premature ejaculation: a systematic review. Urology 2013;81:98-103. http://dx.doi.org/10.1016/j.urology.2012.08.037.
- Wu T, Yue X, Duan X, Luo D, Cheng Y, Tian Y, et al. Efficacy and safety of tramadol for premature ejaculation: a systematic review and meta-analysis. Urology 2012;80:618-24. http://dx.doi.org/10.1016/j.urology.2012.05.035.
- Yang L, Qian S, Liu H, Liu L, Pu C, Han P, et al. Role of tramadol in premature ejaculation: a systematic review and meta-analysis. Urol Int 2013;91:197-205. http://dx.doi.org/10.1159/000348826.
- Alghobary M, El-Bayoumy Y, Mostafa Y, Mahmoud EHM, Amr M. Evaluation of tramadol on demand vs. daily paroxetine as a long-term treatment of lifelong premature ejaculation. J Sex Med 2010;7:2860-7. http://dx.doi.org/10.1111/j.1743-6109.2010.01789.x.
- Bar-Or D, Salottolo KM, Orlando A, Winkler JV, Tramadol ODT. Study Group . A randomized double-blind, placebo-controlled multicenter study to evaluate the efficacy and safety of two doses of the tramadol orally disintegrating tablet for the treatment of premature ejaculation within less than 2 minutes. Eur Urol 2012;61:736-43. http://dx.doi.org/10.1016/j.eururo.2011.08.039.
- Kaynar M, Kilic O, Yurdakul T. On-demand tramadol hydrochloride use in premature ejaculation treatment. Urology 2012;79:145-9. http://dx.doi.org/10.1016/j.urology.2011.09.031.
- Safarinejad MR, Hosseini SY. Safety and efficacy of tramadol in the treatment of premature ejaculation: a double-blind, placebo-controlled, fixed-dose, randomized study. J Clin Psychopharmacol 2006;26:27-31. http://dx.doi.org/10.1097/01.jcp.0000195110.79027.3f.
- Eassa BI, El-Shazly MA. Safety and efficacy of tramadol hydrochloride on treatment of premature ejaculation. Asian J Androl 2013;15:138-42. http://dx.doi.org/10.1038/aja.2012.96.
- Generali J, Cada DJ. Tramadol: premature ejaculation. Hosp Pharm 2006;41:1048-50. http://dx.doi.org/10.1310/hpj4111-1048.
- Chen ZX. Control study on acupuncture and medication for treatment of primary simple premature ejaculation. Zhongguo Zhen Jiu 2009;29:13-5.
- Sunay D, Sunay M, Aydogmus Y, Bagbanci S, Arslan H, Karabulut A, et al. Acupuncture versus paroxetine for the treatment of premature ejaculation: a randomized, placebo-controlled clinical trial. Eur Urol 2011;59:765-71. http://dx.doi.org/10.1016/j.eururo.2011.01.019.
- Pei JT, Shi ZH. An effective combined therapy for simple premature ejaculation. Zhonghua Nan Ke Xue 2008;14:731-3.
- Song GH, Halmurat U, Geng JC, Feng LC, Yilihamujiang S, Ma C, et al. Clinical study on the treatment of premature ejaculation by Uighur medicine gu-jing-mai-si-ha tablet. Chin J Integr Med 2007;13:185-9. http://dx.doi.org/10.1007/s11655-007-0185-7.
- Sun Z, Li Y, Zhang R, Chen L, Wang Y, Wang X, et al. Clinical study on treatment of premature ejaculation with Yimusake Pian. Chin J Androl 2010;24:46-52.
- Xu JX, Gao G, Xu N, Yang YY. Yimusake alone or combined with trazodone hydrochloride for primary premature ejaculation. Zhonghua Nan Ke Xue 2012;18:376-8.
- Zhang FB, Tian Y, Du LD. Xuanju compound capsule combined with erogenous focus exercise is effective for premature ejaculation. Zhonghua Nan Ke Xue 2006;12:1139-40.
- Wise MJ, Pujol M, Baggaley MR, Crowe M. A Novel Treatment for Premature Ejaculation: A Randomized, Controlled Trial n.d.
- Dhikav V, Karmarkar G, Gupta M, Anand KS. Yoga in premature ejaculation: a comparative trial with fluoxetine. J Sex Med 2007;4:1726-32. http://dx.doi.org/10.1111/j.1743-6109.2007.00603.x.
- Hatzimouratidis K, Amar E, Eardley I, Giuliano F, Hatzichristou D, Montorsi F, et al. Guidelines on male sexual dysfunction: erectile dysfunction and premature ejaculation. Eur Urol 2010;57:804-14. http://dx.doi.org/10.1016/j.eururo.2010.02.020.
- McMahon CG. Treatment of premature ejaculation with sertraline hydrochloride: a single-blind placebo controlled crossover study. J Urol 1998;159:1935-8. http://dx.doi.org/10.1016/S0022-5347(01)63201-4.
- Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 1987.
- Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 1980.
- McMahon CG. Dapoxetine: a new option in the medical management of premature ejaculation. Ther Adv Urol 2012;4:233-51. http://dx.doi.org/10.1177/1756287212453866.
- McMahon C, Kim SW, Park NC, Chang CP, Rivas D, Tesfaye F, et al. Treatment of premature ejaculation in the Asia-Pacific region: results from a phase III double-blind, parallel-group study of dapoxetine. J Sex Med 2010;7:256-68. http://dx.doi.org/10.1111/j.1743-6109.2009.01560.x.
- Zamar A. Prolong (TM): review of a newly approved treatment for premature ejaculation in Europe. J Sex Med 2012;9.
- Patrick DL, Rowland D, Rothman M. Interrelationships among measures of premature ejaculation: the central role of perceived control. J Sex Med 2007;4:780-8. http://dx.doi.org/10.1111/j.1743-6109.2007.00464.x.
- PRISMA . The PRISMA Statement n.d. www.prisma-statement.org (accessed 23 January 2015).
- Althof SE. Psychological approaches to the treatment of rapid ejaculation. JMHG 2006;3:180-6.
- Althof SE. Psychological interventions for delayed ejaculation/orgasm. Int J Impot Res 2012;24:131-6. http://dx.doi.org/10.1038/ijir.2012.2.
Appendix 1 Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist
| TITLE | |||
|---|---|---|---|
| Title | 1 | Identify the report as a systematic review, meta-analysis, or both | i (title page) |
| ABSTRACT | |||
| Structured summary | 2 | Provide a structured summary including, as applicable: background; objectives; data sources; study eligibility criteria, participants, and interventions; study appraisal and synthesis methods; results; limitations; conclusions and implications of key findings; systematic review registration number | v–vi |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of what is already known | 3 |
| Objectives | 4 | Provide an explicit statement of questions being addressed with reference to participants, interventions, comparisons, outcomes, and study design (PICOS) | 3 |
| METHODS | |||
| Protocol and registration | 5 | Indicate if a review protocol exists, if and where it can be accessed (e.g. web address), and, if available, provide registration information including registration number | vi |
| Eligibility criteria | 6 | Specify study characteristics (e.g. PICOS, length of follow-up) and report characteristics (e.g. years considered, language, publication status) used as criteria for eligibility, giving rationale | 5–7 |
| Information sources | 7 | Describe all information sources (e.g. databases with dates of coverage, contact with study authors to identify additional studies) in the search and date last searched | 5 |
| Search | 8 | Present full electronic search strategy for at least one database, including any limits used, such that it could be repeated | 169 |
| Study selection | 9 | State the process for selecting studies (i.e. screening, eligibility, included in systematic review, and, if applicable, included in the meta-analysis) | 8 |
| Data collection process | 10 | Describe method of data extraction from reports (e.g. piloted forms, independently, in duplicate) and any processes for obtaining and confirming data from investigators | 8 |
| Data items | 11 | List and define all variables for which data were sought (e.g. PICOS, funding sources) and any assumptions and simplifications made | 7 |
| Risk of bias in individual studies | 12 | Describe methods used for assessing risk of bias of individual studies (including specification of whether this was done at the study or outcome level), and how this information is to be used in any data synthesis | 8 |
| Summary measures | 13 | State the principal summary measures (e.g. RR, difference in means) | 8 |
| Synthesis of results | 14 | Describe the methods of handling data and combining results of studies, if done, including measures of consistency (e.g. I2) for each meta-analysis | 8 |
| Risk of bias across studies | 15 | Specify any assessment of risk of bias that may affect the cumulative evidence (e.g. publication bias, selective reporting within studies) | N/A |
| Additional analyses | 16 | Describe methods of additional analyses (e.g. sensitivity or subgroup analyses, meta-regression), if done, indicating which were pre-specified | N/A |
| RESULTS | |||
| Study selection | 17 | Give numbers of studies screened, assessed for eligibility, and included in the review, with reasons for exclusions at each stage, ideally with a flow diagram | 9 |
| Study characteristics | 18 | For each study, present characteristics for which data were extracted (e.g. study size, PICOS, follow-up period) and provide the citations | 13, 29, 42, 71, 84, 86, 102, 118, 121, 133, 136, 142 and 143 |
| Risk of bias within studies | 19 | Present data on risk of bias of each study and, if available, any outcome level assessment (see item 12) | 174 |
| Results of individual studies | 20 | For all outcomes considered (benefits or harms), present, for each study: (a) simple summary data for each intervention group (b) effect estimates and CIs, ideally with a forest plot | 18, 31, 44, 75, 84, 89, 107, 119, 125, 134, 138, 143 and 144 |
| Synthesis of results | 21 | Present results of each meta-analysis done, including CIs and measures of consistency | 23, 35, 54, 76, 97, 108, 126 and 139 |
| Risk of bias across studies | 22 | Present results of any assessment of risk of bias across studies (see Item 15) | N/A |
| Additional analysis | 23 | Give results of additional analyses, if done (e.g. sensitivity or subgroup analyses, meta-regression [see Item 16]) | N/A |
| DISCUSSION | |||
| Summary of evidence | 24 | Summarise the main findings including the strength of evidence for each main outcome; consider their relevance to key groups (e.g. healthcare providers, users, and policy makers) | 145 |
| Limitations | 25 | Discuss limitations at study and outcome level (e.g. risk of bias), and at review-level (e.g. incomplete retrieval of identified research, reporting bias) | 147 |
| Conclusions | 26 | Provide a general interpretation of the results in the context of other evidence, and implications for future research | 149 |
| FUNDING | |||
| Funding | 27 | Describe sources of funding for the systematic review and other support (e.g. supply of data); role of funders for the systematic review | vi and xxiii |
Appendix 2 Literature search strategies
MEDLINE
The following strategy was developed for use in MEDLINE. This strategy was subsequently translated in accordance with the other databases searched.
MEDLINE search strategy
-
exp Ejaculation/
-
exp Premature Ejaculation/
-
(premature$ adj3 ejaculat$).ti,ab.
-
(early adj3 ejaculat$).ti,ab.
-
(rapid adj3 ejaculat$).ti,ab.
-
(rapid adj3 climax$).ti,ab.
-
(premature$ adj3 climax$).ti,ab.
-
(ejaculat$ adj3 pr?ecox).ti,ab.
-
or/1-8
Filter 1: randomised controlled trials
-
Randomized Controlled Trials as Topic/
-
randomized controlled trial/
-
Random Allocation/
-
Double Blind Method/
-
Single Blind Method/
-
clinical trial/
-
clinical trial, phase i.pt.
-
clinical trial, phase ii.pt.
-
clinical trial, phase iii.pt.
-
clinical trial, phase iv.pt.
-
controlled clinical trial.pt.
-
randomized controlled trial.pt.
-
multicenter study.pt.
-
clinical trial.pt.
-
exp Clinical Trials as topic/
-
or/10-24
-
(clinical adj trial$).tw.
-
((singl$ or doubl$ or treb$ or tripl$) adj (blind$3 or mask$3)).tw.
-
PLACEBOS/
-
placebo$.tw.
-
randomly allocated.tw.
-
(allocated adj2 random$).tw.
-
26 or 27 or 28 or 29 or 30 or 31
-
25 or 32
-
case report.tw.
-
letter/
-
historical article/
-
34 or 35 or 36
-
33 not 37
Filter 2: reviews
-
review.ab.
-
review.pt.
-
meta-analysis.ab.
-
meta-analysis.pt.
-
meta-analysis.ti.
-
or/10-14
-
letter.pt.
-
comment.pt.
-
editorial.pt.
-
or/16-18
-
15 not 19
Filter 3: guidelines
-
guideline.pt.
-
practice guideline.pt.
-
exp Guideline/
-
health planning guidelines/
-
10 or 11 or 12 or 13
Appendix 3 Table of excluded studies with rationale
| Author and Year | Reason for exclusion |
|---|---|
| Abdallah H, Abdelnasser T, Hosny H, Selim O, Al-Ahwany A, Shamloul R. Treatment of premature ejaculation by glans penis augmentation using hyaluronic acid gel: a pilot study. Andrologia 2012;44(Suppl. 1):650–3 | Treatment not relevant to UK setting |
| Abdel-Hamid IA. Pharmacologic treatment of rapid ejaculation: levels of evidence-based review. Curr Clin Pharmacol 2006;1:243–54 | Non-systematic review/treatment overview |
| Bandolier. Premature ejaculation treatments reviewed. Bandolier 2004;11:3 | Non-systematic review/treatment overview |
| Basar MM, Yilmaz E, Ferhat M, Basar H, Batislam E. Terazosin in the treatment of premature ejaculation: a short-term follow-up. Int Urol Nephrol 2005;37:773–7 | Treatment not relevant to UK setting |
| Burner M, Tahrat A. Double blind trial of atrium 300 in the treatment of sexual disorders. Psychol Med 1978;10:1165–71 | Treatment not relevant to UK setting |
| Demirta A, Hali F, Ekmekciogl O. The effects of sildenafil, vardenafil and tadalafil on ejaculation latency time in premature ejaculators: a double blind, randomized, placebo controlled laboratory setting study. J Sex Med 2009;6:93–4 | Laboratory study |
| Dresser MJ, Desai D, Gidwani S, Seftel AD, Modi NB. Dapoxetine, a novel treatment for premature ejaculation, does not have pharmacokinetic interactions with phosphodiesterase-5 inhibitors. Int J Impot Res 2006;18:104–10 | Pharmacokinetic study |
| Dogan S, Dogan M. Premature ejaculation, treatment of the premature ejaculation and efficacy of selective serotonin reuptake inhibitors in the treatment of premature ejaculation. Klinik Psikofarmakoloji Bulteni 2007;17:87–99 | Non-systematic review/treatment overview |
| El-Seweifi A. Partial penile neurectomy for management of ejaculatio praecox. J Mens Health 2010;7:282–3 | Treatment not relevant to UK setting |
| Feige AM, Pinsky MR, Hellstrom WJG. Dapoxetine for premature ejaculation. Clin Pharmacol Ther 2011;89:125–8 | Non-systematic review/treatment overview |
| Ginsberg DL. Gabapentin treatment of premature ejaculation. Prim Psychiatry 2004;11:20–1 | Treatment not relevant to UK setting |
| Giuliano F, Patrick DL, Porst H, La Pera G, Kokoszka A, Merchant S, et al. Premature ejaculation: results from a five-country European observational study. Eur Urol 2008;53:1048–57 | Non-systematic review/treatment overview |
| Gokce A, Halis F, Demirtas A, Ekmekcioglu O. The effects of three phosphodiesterase type 5 inhibitors on ejaculation latency time in lifelong premature ejaculators: a double-blind laboratory setting study. BJU Int 2011;107:1274–7 | Laboratory study |
| Greco E, Polonio-Balbi P, Speranza JC. Levosulpiride: a new solution for premature ejaculation? Int J Impot Res 2002;14:308–9 | Treatment not relevant to UK setting |
| Guan ZC, Shi BT, Wang R. Resiniferatoxin for treatment of premature ejaculation: a new medical therapy. J Sex Med 2010;7:177 | Treatment not relevant to UK setting |
| Gurkan L, Oommen M, Hellstrom WJG. Premature ejaculation: current and future treatments. Asian J Androl 2008;10:102–9 | Non-systematic review/treatment overview |
| Hakobyan AE, Nersisyan NR, Azatyan RE, Azizian A, Grigoryan AD. New approach to premature ejaculation treatment. J Sex Med 2011;8:175–6 | Treatment not relevant to UK setting |
| Hoy SM, Scott LJ. Dapoxetine: in premature ejaculation. Drugs 2010;70:1433–43 | Non-systematic review/treatment overview |
| Modi NB, Dresser MJ, Simon M, Lin D, Desai D, Gupta S. Single- and multiple-dose pharmacokinetics of dapoxetine hydrochloride, a novel agent for the treatment of premature ejaculation. J Clin Pharmacol 2006;46:301–9 | Pharmacokinetic study |
| Morales A, Black A, Clark-Pereira J, Emerson L. A novel approach to premature ejaculation: extracorporeal functional magnetic stimulation. Can J Urol 2009;16:4458–62 | Treatment not relevant to UK setting |
| Porst H. An overview of pharmacotherapy in premature ejaculation. J Sex Med 2011;8(Suppl. 4):335–41 | Non-systematic review/treatment overview |
| Riley AJ, Riley EJ. Amitriptyline-perphenazine and the squeeze technique in premature ejaculation. J Pharmacother 1979;2:136–40 | Treatment not relevant to UK setting |
| Safarinejad MR. Safety and efficacy of venlafaxine in the treatment of premature ejaculation: a double-blind, placebo-controlled, fixed-dose, randomised study. Andrologia 2008;40:49–55 | Treatment not relevant to UK setting |
| Zhang GX, Yu LP, Bai WJ, Wang XF. Selective resection of dorsal nerves of penis for premature ejaculation. Int J Androl 2012;35:873–9 | Treatment not relevant to UK setting |
Appendix 4 Quality assessment
The Assessing Methodological Quality of Systematic Reviews33 assessment of included reviews
| Review author, review type, interventions | 1. Was an a priori design provided? | 2. Was there duplicate study selection and data extraction? | 3. Was a comprehensive literature search performed? | 4. Was the status of publication (i.e. grey literature) used as an inclusion criterion? | 5. Was a list of studies (included and excluded) provided? | 6. Were the characteristics of the included studies provided? | 7. Was the scientific quality of the included studies assessed and documented? | 8. Was the scientific quality of the included studies used appropriately in formulating conclusions? | 9. Were the methods used to combine the findings of studies appropriate? | 10. Was the likelihood of publication bias assessed? | 11. Was the conflict of interest included? | Total AMSTAR score (total number of ‘Yes’) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Althof 2006;174 Althof 2012,175 narrative, behavioural | No | No | No | No | No | No | No | No | No | No | No | 0 |
| Asimakopoulos et al. 2012,134 systematic with MA, PDE5 | Unclear | Unclear | No | No | No | Yes | Unclear | Unclear | No | No | Yes | 2 |
| Aversa et al. 2011,135 systematic, PDE5 | Unclear | Yes | No | No | No | Yes | No | No | N/A | No | Yes | 3 |
| Berner and Gunzler 2012,36 systematic, behavioural | Yes | Yes | Yes | No | No | Yes | Yes | Yes | N/A | No | Yes | 6 |
| Burton and Liday 2011,136 narrative, SSRIs vs. PDE5 | Unclear | Unclear | No | No | No | Yes | No | No | N/A | No | Yes | 2 |
| Chen et al. 2007,137 systematic, PDE5 | Unclear | Unclear | No | No | No | Yes | No | No | N/A | No | Yes | 2 |
| Cong et al. 2012,64 systematic with MA Fluoxetine | Unclear | Unclear | Yes | No | No | No | Yes | No | No | Yes | No | 3 |
| Hatzimouratidis et al. 2010,165 guidelines, systemic, topical and behavioural | Unclear | Unclear | Unclear | Unclear | No | No | No | Unclear | N/A | No | Yes | 1 |
| Huang et al. 2009,65 systematic with MA, SSRIs – mixed | Unclear | Unclear | Unclear | Unclear | No | No | Yes | Unclear | No | Unclear | Unclear | 1 |
| Hutchinson et al. 2012,111 systematic, review of AEs and withdrawals associated with dapoxetine | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Kendirci et al. 2007,112 communication on preclinical and clinical data, dapoxetine, communication on preclinical data | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| Luo et al. 2012,108 systematic with MA, dapoxetine | Unclear | Unclear | Unclear | Unclear | No | No | Yes | Unclear | No | Unclear | Unclear | 1 |
| McCarty and Dinsmore 2012,109 systematic, dapoxetine | Unclear | Unclear | No | No | No | Yes | No | No | N/A | No | Yes | 2 |
| McMahon et al. 2006,37 systematic, PDE5 | Unclear | Unclear | Yes | Yes | No | Yes | No | No | No | No | Yes | 4 |
| McMahon and Porst 2011,68 systematic, mixed – systemic treatments | Unclear | Unclear | No | No | No | Yes | No | No | N/A | No | Yes | 2 |
| McMahon et al. 2012,169 systematic, dapoxetine | Unclear | Unclear | Yes | Yes | No | Yes | Yes | No | N/A | No | Yes | 5 |
| Melnik et al. 2009,38 systematic, behavioural | Unclear | Unclear | Yes | Yes | No | No | Yes | No | N/A | No | No | 3 |
| Melnik et al. 2011,35 Cochrane review, behavioural | Yes | Unclear | Yes | Yes | No | Yes | Yes | Yes | Yes | Unclear | Yes | 7 |
| Morales et al. 2007,54 mini-review, topical anaesthetics | Unclear | Unclear | Unclear | Unclear | No | Unclear | No | No | N/A | No | Yes | 1 |
| Moreland and Makela 2005,66 systematic, SSRIs – mixed | Unclear | Unclear | No | No | No | No | No | No | N/A | No | No | 0 |
| Pu et al. 2013,51 systematic with MA, topical anaesthetics | Unclear | Unclear | Yes | Yes | No | Yes | Yes | No | No | No | No | 4 |
| Richardson et al. 2005;69 Richardson et al. 2006,8 systematic, mixed – systemic treatments | Unclear | Unclear | No | No | No | Yes | No | No | No | No | No | 1 |
| Waldinger et al. 2004,52 systematic with MA, mixed – systemic and topical treatments | Unclear | Unclear | No | No | No | Yes | No | No | No | No | No | 1 |
| Wang et al. 2007,67 systematic, SSRIs – mixed | Unclear | Unclear | No | Unclear | No | No | No | No | N/A | No | No | 0 |
| Wang et al. 2010,110 systematic with MA, dapoxetine | Unclear | Unclear | Unclear | Unclear | No | Yes | Yes | Unclear | No | Unclear | Unclear | 2 |
| Wong and Malde 2013,147 systematic, tramadol | Unclear | Unclear | No | No | No | Yes | No | No | N/A | No | No | 1 |
| Wu et al. 2012,148 systematic with MA, tramadol | Unclear | Unclear | No | Unclear | No | Yes | Yes | No | No | No | No | 2 |
| Xia et al. 2013,53 systematic with MA, topical anaesthetics | Unclear | Yes | No | No | No | Yes | Yes | No | Yes | No | Yes | 5 |
| Yang et al. 2013,149 systematic with MA, tramadol | Unclear | Yes | Yes | No | No | Yes | Yes | No | Yes | No | Yes | 6 |
Risk of bias assessment34 for the randomised controlled trials not included by reviews
| Study, broad area | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Overall riska |
|---|---|---|---|---|---|---|---|
| Ahn et al. 1996,100 SSRIs – fluoxetine vs. placebo | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Akgül et al. 2008,92 SRRIs – citalopram vs. sertraline | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk | Unclear risk |
| Akilov et al. 2011,132 TCAs – nasal clomipramine vs. placebo | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Low risk | Unclear risk |
| Arafa and Shamloul 2006,97 SSRIs – sertraline vs. placebo | Unclear risk | Unclear risk | High risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Arafa and Shamloul 2007,106 SSRIs – fluoxetine vs. escitalopram vs. paroxetine | Unclear risk | Unclear risk | High risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Athanasios et al. 2007,121 SSRIs – duloxetine vs. placebo | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Chen 2009,156 acupuncture – acupuncture vs. citalopram | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Unclear risk | Unclear risk |
| Culba et al. 2008,101 SSRIs – fluoxetine vs. fluoxetine + PDE5 vs. placebo | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | High risk | Unclear risk |
| Dhikav et al. 2007,164 yoga – yoga vs. fluoxetine | High risk | High risk | High risk | Unclear risk | Low risk | Low risk | High risk |
| Eassa and El-Shazly 2013,154 tramadol – tramadol different doses | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Farnia et al. 2009,93 SSRIs – citalopram vs. placebo | Unclear risk | Unclear risk | Low risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Generali and Cada 2006,155 tramadol – tramadol vs. placebo | Unclear risk | Unclear risk | Low risk | Unclear risk | Low risk | High risk | Unclear risk |
| Giammusso et al. 1997,103 SSRIs – paroxetine 20 mg vs. paroxetine 10 mg | Unclear risk | Unclear risk | Unclear risk | Unclear risk | High risk | Unclear risk | High risk |
| Khelaia et al. 2012,104 SSRIs – paroxetine daily vs. paroxetine on-demand vs. placebo | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Unclear risk |
| Kilic et al. 2005,122 SNRIs – venlafaxine vs. placebo | Unclear risk | Unclear risk | High risk | Unclear risk | High risk | Low risk | High risk |
| Leaker et al. 2008,133 TCAs – inhaled clomipramine: 1 mg vs. 2 mg vs. placebo | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Unclear risk | High risk | Unclear risk |
| Lee et al. 2012,120 dapoxetine – dapoxetine adjuvant to PDE5 (mirodenafil) or placebo | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Mallat et al. 2012,62 topical anaesthetics – electric stimulation vs. EMLA | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Unclear risk | Unclear risk |
| Nada et al. 2009,98 SSRIs – escitalopram vs. placebo | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Unclear risk |
| Nada et al. 2012,94 SSRIs – escitalopram vs. citalopram | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Unclear risk |
| Pastore et al. 2012,48 behavioural – pelvic floor vs. dapoxetine | Low risk | Unclear risk | High risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Pei and Shi 2008,158 Chinese medicine – Chinese medicine (Wu Bei Zi and Xi Xin) + sertraline + sexual counselling vs. sertraline | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Unclear risk | Unclear risk |
| Rezakhaniha and Sirosbakht 2010,95 SSRIs – fluoxetine vs. citalopram | Unclear risk | Unclear risk | Unclear risk | Unclear risk | High risk | High risk | High risk |
| Safarinejad 2007,99 SSRIs – escitalopram vs. placebo | Low risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Safarinejad 2008,123 SNRIs – venlafaxine vs. placebo | Low risk | Low risk | Low risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Shang et al. 2012,96 SSRIs – citalopram vs. placebo | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Shao and Li 2008,49 behavioural – behavioural therapy vs. paroxetine | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Song et al. 2007,159 Chinese medicine – Uighur medicine vs. treatment as usual | Low risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Steggall et al. 2008,63 topical anaesthetics – lidocaine spray vs. paroxetine | Unclear risk | Unclear risk | High risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Sun et al. 2010,160 Chinese medicine – Yimusake tablet vs. fluoxetine | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Sunay et al. 2011,157 acupuncture – acupuncture vs. paroxetine | Low risk | Unclear risk | Low risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Tuncel et al. 2008,107 SSRIs – clomipramine (TCA) vs. sertraline vs. terazosin (alpha-blocker) vs. placebo | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | High risk | Unclear risk |
| van Lankveld et al. 2009,50 behavioural – internet-based sensate focus vs. waiting list control | Low risk | Unclear risk | High risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Waldinger et al. 2004,105 SSRIs – paroxetine vs. clomipramine | Low risk | Low risk | Low risk | Unclear risk | Low risk | High risk | Unclear risk |
| Weixing et al. 2012,102 SSRIs – fluoxetine or sertraline vs. squeeze technique | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Unclear risk |
| Wise et al. 2004,163 delay device – desensitising band + stop–start technique vs. behavioural therapy + stop–start technique | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | High risk | Unclear risk |
| Xu et al. 2012,161 Chinese medicine – Yimusake tablet vs. Yimusake tablet + trazodone (SARI antidepressant) | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Low risk | Unclear risk |
| Zhang et al. 2006,162 Chinese medicine – Xuanju compound vs. no treatment (both adjuvant to sensate focus) | Unclear risk | Unclear risk | Unclear risk | Unclear risk | Low risk | Unclear risk | Unclear risk |
Glossary
- Anejaculation
- Inability to ejaculate.
- Bibliotherapy
- Expressive therapy that uses an individual’s relationship to the content of books.
- Hypoaesthesia
- Diminished sensitivity to pain.
- Intravaginal ejaculatory latency time
- Time taken by a man to ejaculate during vaginal penetration.
- Libido
- Sexual drive or desire for sexual activity.
- Sensate focus
- A focus on the patient’s own varied sense experience, rather than viewing orgasm as the sole goal of sex.
- Somnolence
- Strong desire for sleep.
- Squeeze technique
- Application of firm pressure with thumb and forefinger below head of penis.
- Stop–start/pause technique
- Pausing action when approaching ‘point of no return’.
List of abbreviations
- AE
- adverse event
- AEC
- Ability of Ejaculation Control
- AIPE
- Arabic Index of Premature Ejaculation
- AMSTAR
- Assessing Methodological Quality of Systematic Reviews
- CBT
- cognitive–behavioural therapy
- CCRT
- Cochrane Controlled Trials Register
- CGI-I
- Clinical Global Impression – Improvement
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CIPE
- Chinese Index of Premature Ejaculation
- CIPE5
- Chinese Index of Premature Ejaculation 5 premature ejaculation-related items
- DSM-IV
- Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition
- DSM-IV-TR
- Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition-Text Revision
- EAU
- European Association of Urology
- EMLA®
- eutectic mixture of local anaesthetics
- EU
- European Union
- GP
- general practitioner
- GRISS
- Golombok Rust Inventory of Sexual Satisfaction
- HTA
- Health Technology Assessment
- ICD-10
- International Classification of Diseases, Tenth Edition
- IELT
- intravaginal ejaculatory latency time
- IIEF
- International Index of Erectile Function
- IPE
- Index of Premature Ejaculation
- ISSM
- International Society for Sexual Medicine
- MD
- mean difference
- MeSH
- medical subject heading
- NIHR
- National Institute for Health Research
- OSAT
- overall sexual act time
- PDE5
- phosphodiesterase-5
- PE
- premature ejaculation
- PEDT
- Premature Ejaculation Diagnostic Tool
- PEP
- Premature Ejaculation Profile
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- RCT
- randomised controlled trial
- RR
- relative risk
- SARI
- serotonin antagonist and reuptake inhibitor
- ScHARR
- School of Health and Related Research
- ScHARR-TAG
- School of Health and Related Research Technology Assessment Group
- SD
- standard deviation
- SNRI
- serotonin–noradrenaline reuptake inhibitor
- SSRI
- selective serotonin reuptake inhibitor
- TCA
- tricyclic antidepressant
- TEMPE
- topical eutectic mixture for premature ejaculation
- WHO
- World Health Organization