Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 07/41/05. The contractual start date was in December 2010. The draft report began editorial review in December 2013 and was accepted for publication in April 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
none
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Peckham et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Smoking and severe mental illness
People with severe mental ill health (SMI), such as schizophrenia and bipolar disorder, are more likely to smoke1 and to smoke more heavily2 than the general population. The point prevalence of smoking among those with SMI has been estimated to be between 58% and 90%. 1,3 The presence of mental ill health is associated with an elevated risk of smoking by a factor of 2.7 [95% confidence interval (CI) 2.4 to 3.2]. 4 Smokers with SMI are more nicotine dependent, more likely to become medically ill and less likely to receive help in quitting, than the general population. 5 There are several reasons why people with SMI are more likely to smoke:
-
Compared with smokers without SMI, they smoke a greater number of cigarettes per day, and this is evident even before diagnosis. 6
-
They smoke each cigarette more intensely, extracting more nicotine per cigarette. 2,7
-
They are much less likely to receive advice to quit smoking from their general practitioner (GP)3 or mental health specialist. 8
People with SMI have a lot of time on their hands, and smoking is part of the ‘culture’ of mental health services (among both staff and patients). In addition, people with SMI often lack self-esteem and see the future as bleak; as a consequence, they may not be motivated to look after their physical health. 5 Many people with severe mental illness are also misinformed about the risks and benefits of smoking and those of nicotine dependence treatment. 9,10 They often fear and overestimate the medical risks of nicotine replacement therapy (NRT). 11 Many believe that smoking relieves depression and anxiety12 (although nicotine actually causes anxiety). Nicotine may also improve some aspects of cognitive dysfunction in schizophrenia, which could be a disincentive for patients to quit smoking. 13
Smoking contributes to the general poor physical health of those with SMI; in the UK the standardised mortality ratio (SMR) for all causes of death among people with schizophrenia has been reported to be 289 (95% CI 247 to 337), which means that people with schizophrenia have a mortality risk of just under three times that of the general population. 14 Although people with SMI are more likely than the general population to smoke, there is evidence that this is less likely to be recorded in primary care records or to be acted on for these patients than for the general population. 15 Burns and Cohen16 found that, although the annual general practice consultation rate is significantly higher among people with SMI (13–14 consultations a year) than in the general population (about three consultations per year), their health records are significantly less likely to include data relating to a variety of health promotion areas, including smoking advice. Recent studies show that people with mental health problems are just as likely to want to stop smoking as the general population – and are able to stop when offered evidence-based support. 17,18 However, research also shows that effective stop smoking treatment is not always offered to them. 19
It is within this context that a number of policy initiatives have emerged, which emphasise improving the physical care of those with SMI, including taking initiatives to facilitate smoking cessation and the promotion of smoke-free environments in secondary care services. 5,20 This has recently been the subject of National Institute for Health and Care Excellence (NICE) recommendations about the provision of smoking cessation services for people with SMI in order to help address this health inequality. 21
Existing knowledge
Smokers most commonly cite stress relief and enjoyment as their main reasons for smoking,22 although the major cause is nicotine dependence. Nicotine acts in the midbrain, creating impulses to smoke in the face of stimuli associated with smoking,23 and producing what may be thought of as a kind of ‘nicotine hunger’ (a feeling of need to smoke) when blood nicotine concentrations are depleted. Smokers also experience nicotine withdrawal symptoms: unpleasant mood swings and physical symptoms that occur on abstinence and are relieved by smoking. 24 Nicotine dependence is the main reason that most unassisted quit attempts fail within a week. 25 Cochrane systematic reviews24–34 and evidence-supported guidance from NICE,35,36 highlight that the following smoking cessation interventions (including medications used as smoking cessation aids) are helpful in helping smokers reduce their tobacco intake and quit smoking.
Nicotine replacement therapy
Six different forms of NRT are available for use as smoking cessation aids: nicotine patch, gum, lozenge, inhaler, nasal spray and sublingual tablet (microtab). These provide a ‘clean’ alternative source of nicotine without the other 4000 toxic chemicals found in cigarette smoke. All deliver a lower dose of nicotine than would be received through smoking, with the only difference being differing absorption rates as a result of different methods of delivery. A meta-analysis of more than 100 randomised control trials (RCTs) shows that all forms of NRT are roughly equally effective in aiding long-term cessation [odds ratio (OR) 1.77; 95% CI 1.66 to 1.88)]. 25 For those not ready to stop smoking, but who are interested in cutting down, NRT prescription has been shown to reduce smoking and to facilitate quit rates later on (reduce to stop, or cut down to quit). 37
Antidepressants and nicotine receptor agonists
Two non-nicotine pharmacotherapies have been licensed as smoking cessation aids. These are varenicline, a nicotinic acetylcholine receptor partial agonist [Chantix® (USA), Champix® (EU and other countries), Pfizer], and bupropion, a noradenaline and dopamine reuptake inhibitor which was first introduced as an atypical antidepressant (Zyban,® GlaxoSmithKline). Varenicline is almost certainly the most effective treatment to date (OR for 12 months’ continuous abstinence for varenicline vs. placebo 3.22; 95% CI 2.43 to 4.27). It is more efficacious than bupropion (OR for varenicline vs. bupropion 1.66, 95% CI 1.28 to 2.16). 32 However, its use in people with SMI may be limited by case reports of worsening of depression or mental health in populations with a previous history of mental health difficulties.
The US Food and Drug Administration (FDA) guidance on this matter states ‘some patients have reported changes in behaviour, agitation, depressed mood, suicidal thoughts or actions when attempting to quit smoking while taking varenicline or after stopping varenicline’. 38 It states that patients experiencing such changes should stop taking varenicline and contact their physician. A similar recommendation is made for bupropion. General recommendations are that these medications should be used in those whose mental state is stable. The association between varenicline use and exacerbation of mental illness, the frequency of which has yet to be ascertained, must be balanced against the very high risk of continued smoking. 39
Behavioural support
Advice, discussion and encouragement can be delivered via a range of means, from individual to group, open (rolling) or closed group, face to face, or over the telephone or internet. Meta-analyses of trials of multisession intensive behavioural support compared with brief advice found ORs of 1.56 (95% CI 1.32 to 1.84) for individual support and 2.04 (95% CI 1.60 to 2.60) for group support. 28,29 Regular support on the telephone is also effective. A meta-analysis of 10 trials of telephone support for people stopping smoking gave an OR of 1.64 (95 % CI 1.41 to 1.92). 34 There is some evidence to suggest that group support may be more effective in general than one-to-one support,40 and that it should involve multiple sessions. 40 There is also evidence that such sessions can be effective even if conducted over the telephone (OR 1.64, 95% CI 1.41 to 1.92). 34
The accumulated evidence for the use of current smoking cessation interventions has been distilled into clear recommendations for health-care professionals,41 and into a manual for those designing and delivering smoking cessation services. 42 In addition, guidance has been issued by the Royal Colleges of General Practitioners and Psychiatrists to guide the use of smoking cessation interventions for those with SMI. 43
Evidence on the effectiveness of smoking cessation strategies in SMI comes from a systematic review of randomised trials by Banham and Gilbody. 44 This review draws on the results of 10 RCTs of smoking cessation interventions among those with SMI and shows that combinations of behavioural support and pharmacotherapy (NRT and bupropion) are effective in facilitating smoking cessation. The evidence is strongest from bupropion, where the odds of quitting were improved fourfold [three trials; risk ratio (RR) 4.18, 95% CI 1.30 to 13.42]. The strongest evidence relates to NRT, where the addition of NRT tripled biochemically verified quit rates at 4 months (four trials; RR 2.77, 95% CI 1.48 to 5.16). There are, however, no trial-based data for varenicline.
Similar results were found following a recent Cochrane review assessing smoking cessation interventions in individuals with schizophrenia. 45 Smoking cessation rates were significantly higher among those taking bupropion than those taking placebo (seven trials; RR 2.78, 95% CI 1.02 to 7.58) with no report of serious adverse events (SAEs).
Rationale for the Smoking Cessation Intervention for Serious Mental Ill Health Trial
Despite the higher prevalence of smoking, a substantial proportion of people with SMI express a desire to quit. In a large population-based cohort, 30.5% of smokers with past-month mental illness had a self-reported ‘desire to quit’ (although this is lower than the rate of 42.5% among smokers without illness). 4 The introduction in 2004 of a new General Medical Services (GMS) contract46 created a policy impetus to improve the quality of primary care in priority areas. In terms of mental health, the new GMS contract specified that primary care is responsible for the provision of physical health care. Importantly, for smoking cessation initiatives, it ‘incentivises’ GPs to (1) produce a register of people with severe long-term mental health [Quality and Outcomes framework (QOF) indicator MH8 – the SMI register47] and (2) ensure that at least 90% of SMI patients have had a review that includes smoking status recorded within the previous 15 months (the QOF indicator MH9 – SMI health check48). This check includes patients seen in primary care, in secondary care and under shared care arrangements.
For those who are admitted to hospital, the introduction of smoke-free polices provides an opportunity to address smoking. This ban includes inpatient psychiatric units, although the complexities of the Mental Health Act49 have been interpreted by some hospitals as a requirement to provide smoking areas. The admission of an individual to hospital, while being stressful and occurring at a time of personal crisis, also provides a unique opportunity to provide general health advice and to engage individuals in interventions targeted at smoking reduction and cessation.
Recent guidance issued by NICE50 offers clear statements of purpose to make secondary care services (including mental health services) entirely smoke free and to promote a smoke-free culture among staff and users of services. Mental health services are highlighted as areas of priority and unmet need in relation to smoking cessation and there is clear guidance that services should be developed and implemented as a matter of some priority.
Smoking cessation services for people with SMI are not sufficiently evolved or embedded within the NHS. From the preceding discussion, we know ‘what works’ for smoking cessation in general; the purpose of the Smoking Cessation Intervention for Serious Mental Ill Health Trial (SCIMITAR) is to use enhancements of care to ensure that evidence-supported interventions are offered to (and taken up by) people with SMI and to see if smoking rates can be reduced. This technology represents a ‘complex health-care intervention’, and this study, therefore, uses the stepwise Medical Research Council complex interventions framework51 and the updated guidance52 to evaluate the clinical effectiveness, implementation and content of a bespoke smoking cessation (BSC) service for people with SMI.
Research objectives
-
To develop a BSC service based upon evidence-supported treatments for people with SMI.
-
To establish the acceptability and uptake of this BSC service by people with SMI in primary care and specialist mental health services.
-
To test the feasibility of recruitment and follow-up in a pilot trial of a BSC service among patients with SMI.
Chapter 2 Methods
Details of the methods used for the health economics can be found in Chapter 5 and details of the methods used in the qualitative substudy can be found in Chapter 6.
Study design
This study was a pragmatic, two-arm, parallel-group, pilot RCT. The setting was in primary care and specialist mental health services within three centres: York/Scarborough [principal investigator (PI) Professor Simon Gilbody), Manchester (Investigator Professor Helen Lester) and Hull (PI Professor Simon Gilbody). We recruited from both primary and secondary care settings. Given that this is a hard-to-reach population, several methods were used to try to identify and recruit eligible participants. A two-stage recruitment process was employed to check for eligibility, understanding of the study and to obtain consent. Participants were individually randomised to receive usual care or usual care plus a BSC service. Participants were followed up over the course 12 months, with data collected at 1 month, 6 months and 12 months post randomisation.
Approvals obtained
Ethical approval was sought and granted on 29 October 2010 by Leeds (East) Research Ethic Committee (10/H1306/72). Approval was also obtained from the relevant research and development departments (see Appendix 1).
Trial sites
The study was conducted in three sites in England. Sites recruited throughout the duration of the study.
Participant eligibility
Inclusion criteria
To be eligible for inclusion into this study participants needed to meet the following inclusion criteria:
-
aged 18 years and above
-
have SMI
-
are a smoker and express an interest in wanting to cut down smoking (though not necessarily quitting).
There is no agreed definition of SMI so we adopted a pragmatic definition of SMI,46,53 i.e. a documented diagnosis of schizophrenia or delusional/psychotic illness [International Classification of Diseases (ICD) 10 F20.X & F22.X or Diagnostic and Statistical Manual of Mental disorders (DSM) equivalent] or bipolar disorder (ICD F31.X or DSM equivalent). This SMI-inclusive diagnosis needed to have been made by specialist psychiatric services and have been documented in either the GP or psychiatric notes.
Exclusion criteria
People who:
-
were pregnant or breastfeeding
-
had comorbid drug or alcohol problems (as ascertained by the GP or mental health worker)
-
were non-English speakers
-
lacked capacity to participate in the trial (guided by the 2005 Mental Capacity Act54).
Serious mental illness patients who smoke while concurrently abusing substances may require additional medication or specialist advice, which was beyond the brief of the mental health smoking cessation practitioner (MHSCP) and this trial. Similarly, smoking cessation in pregnancy also requires specialist knowledge. It was planned that any participant who became pregnant during the course of the trial would be removed from the study and referred to local smoking cessation services specific to pregnancy.
Identifying participants
We used four methods to recruit participants: direct GP referral or following database screening, primary care referral following annual health check, secondary care recruitment – Care Programme Approach (CPA) and via Community Mental Health Teams (CMHTs) – and patient self-referral.
Direct general practitioner referral or following database screening
General practitioners are encouraged to offer opportunistic advice and information about smoking cessation services to all patients who smoke whenever they consult in primary care. GPs taking part in this study were provided with patient study information packs to give to patients with SMI who were receptive to participating in the trial. GPs then completed and faxed a referral form and patients’ consent to be contacted form to the SCIMITAR researchers, who approached the patient for recruitment.
General practitioner surgeries were also asked to consult their patient databases and SMI register, if available, to screen for potentially eligible participants. Patient information packs were sent from the GP practice inviting patients willing to take part in the study to return a completed consent to be contacted form to the SCIMITAR researchers, who then approached the patients to ascertain eligibility and recruitment. Following a database search, GPs were asked to provide details of the number of packs they had sent out to allow a return rate to be calculated.
Primary care referral following annual health check
At the time of the trial, annual primary care health checks for people with SMI55 (MH9) represented an opportunity to address smoking behaviour and to offer enhanced smoking cessation services within the context of a trial. Health checks are generally conducted by practice nurses, and we encouraged all primary care staff to make SMI smokers aware of the trial when they received their annual primary care health check. Patient information packs were given to interested and potentially eligible patients during their health check. Similar to GP referrals, practice nurses were instructed to complete referral forms and to fax the patients’ completed consent to be contacted form to the SCIMITAR researchers, who then approached the patients for eligibility and recruitment.
Secondary care recruitment – Care Programme Approach and via Community Mental Health Teams
A substantial proportion of people with SMI will be in receipt of the CPA, and will receive an annual review of their psychological, social and health-care needs. Study researchers worked with care co-ordinators and consultants to screen their entire caseloads for potentially eligible participants who matched the inclusion criteria. Participants identified as potentially suitable for the SCIMITAR trial were given a copy of the patient information pack by their care co-ordinator. The patient information pack contained a consent to be contacted form for potential participants to return to the research assistant giving permission for the researcher to contact them by telephone or letter, or in person to discuss the trial further.
Members of the CMHT were also invited to directly refer eligible patients to the research team, following a similar pathway as GP referrals.
Patient self-referral
Poster advertisements of the SCIMITAR trial and BSC service were displayed in venues where patients in secondary care often congregated (e.g. clozapine clinics, outpatient departments, day centres, etc.). The posters invited patients to contact study researchers if they were interested in participating in the study. The introduction of smoking bans in inpatient hospital services raised an ideal opportunity to offer smoking cessation services to patients who were interested in addressing their smoking behaviour. Therefore, we also advertised the BSC service in inpatient mental health settings. Interested participants contacted a SCIMITAR researcher, who sent out a patient information pack, including a consent to be contacted form.
Screening for eligibility
Potential participants identified by database screening or self-referral
Once a potential participant returned their consent to contact form the participant’s GP was contacted to check for exclusion criteria (pregnancy or known drug/alcohol problems) and their judgement on the appropriateness of the patient’s inclusion into the study. Once the GP had confirmed that it was appropriate for the participant to take part in the trial the participant was contacted by a trial researcher.
Potential participants referred by general practitioner or other health-care professional
If the patient was referred, the health-care professional giving them the information pack (GP, mental health specialist, practice nurse, CPA co-ordinator) explained the trial, assessed the patient for eligibility and screened for the given exclusion criteria. On receipt of a faxed referral form and signed consent to be contacted form, patients were contacted by a trial researcher.
The SCIMITAR researcher first approached the potential participant by telephone. After briefly explaining the trial, the researcher enquired about the patient’s smoking habits, specifically: (1) Do you smoke? (2) How much do you smoke? and (3) Would you seriously consider quitting or cutting down with a view to quitting within the next 6 months? These ensured that the patient currently smoked but was seriously contemplating quitting. The researcher also asked screening questions about pregnancy and breastfeeding, drug and alcohol use, which led to exclusion if present. The researcher then arranged a meeting at a mutually convenient time and venue.
Consenting participants
Potential participants who met with the SCIMITAR researcher were given the opportunity to clarify any points they did not understand and ask any questions. A full oral explanation of the trial was given by the SCIMITAR researcher. It was emphasised that the participants may withdraw their consent to participate at any time without loss of benefits to which they otherwise would be entitled. Participants were also informed that, by consenting, they agreed to their GP being informed of their participation in the trial and that their medical records may be inspected by regulatory authorities, but that their name would not be disclosed. Written informed consent was then obtained, with both the participant and the researcher signing and dating the consent forms prior to the patient being randomised.
Baseline assessment
Once participants had consented to take part in the trial, they completed the baseline questionnaires. In addition, height and weight measurements were taken in order to calculate the patient’s body mass index (BMI) and an exhaled breath carbon monoxide (CO) reading was taken. These made up the participants’ baseline data set. The participant was randomised on completion of this data set.
Randomisation
Simple randomisation was used following a computer-generated random number sequence. The SCIMITAR researcher contacted a secure randomisation line run by the York Trials Unit and, once given the details of the patient’s allocation, immediately informed the patient of his or her allocation and set up the first appointment with the MHSCP (if so allocated). A letter was sent to the GP and mental health specialist to be included in the patient’s records and to advise them on subsequent smoking cessation management. Owing to the nature of the intervention, it was not possible to blind participants, GPs, researchers or the MHSCPs to the treatment allocation.
Ineligible and non-consenting participants
All ineligible and non-consenting participants were referred back to their GP practice so that any general health-care advice on the importance of stopping smoking could be provided by the patients’ GP or community nurses.
Sample size
This pilot trial aimed to recruit 100 patients with SMI to obtain preliminary estimates of the effect size of the BSC service. Using the following assumptions (1) primary care QOF registers were assumed to give prevalence data for SMI of 0.5%; (2) an average of 2.5 whole-time equivalent GPs were assumed to work in each practice each with a list size of 1600 patients (4000 per practice); and (3) at least 80% of people with SMI smoke. If we were to recruit 25% of eligible patients in primary care, around 20 practices would enable us to recruit 100 patients over a 12-month period. This was a conservative assessment which did not allow for recruitment from secondary care, where recruitment is less easy to plan but was in addition to primary care.
Description of interventions
Trial intervention
This service intervention consisted of a mental health professional trained in smoking cessation interventions (MHSCP) who worked in conjunction with the patient and the patient’s GP or mental health specialist to provide a smoking cessation service individually tailored to each patient with SMI. The intervention was delivered in accordance the Smoking Cessation Manual: A Guide for Counsellors and Practitioners,42 which forms the basis of smoking cessation interventions in the NHS via the National Centre for Smoking Cessation Training (www.ncsct.co.uk).
This service was in line with current NICE guidelines for smoking cessation services56 and included support sessions specifically adapted for patients with SMI run by the MHSCP and GP-prescribed pharmacotherapies to aid smoking cessation (NRTs, bupropion or varenicline either separately or in combination, as decided by the GP), in addition to regular follow-up by the MHSCP. Examples of specific adaptations to the needs of those with SMI are (1) the need to make several assessments prior to setting a quit date; (2) recognising the purpose of smoking in the context of their mental illness, such as the use of smoking to relieve side effects from antipsychotic medication (and how this will be managed during a cessation attempt); (3) the need to involve other members of the multidisciplinary team in planning a successful quit attempt for those with complex care needs and multiagency programmes of care; (4) a greater need for home visits, rather than planned visits in GP surgeries; (5) providing additional face-to-face support following an unsuccessful quit attempt or relapse; and (6) informing the GP and psychiatrist of a successful quit attempt, such that they can review antipsychotic medication doses if metabolism changes. 57
Pharmacotherapies were provided as long as was deemed necessary, in line with NICE guidance, and were determined by the GP without the influence of the SCIMITAR trial team. In line with NICE recommendations, the MHSCP offered advice on the range of treatments options available to patients under the NHS (including medication, counselling and follow-up). It was not the remit of the trial to assess specific smoking cessation pharmacotherapies or treatments per se, although data on frequency of their usage were collected.
Participants were encouraged to (1) reduce smoking to quit,37 (2) set their own quit dates and (3) make several attempts to quit if their initial attempt failed. It is generally recommended that patients wait a few months after a failed quit attempt before trying again. This was not strictly enforced in this population and was left to the discretion of the MHSCP. All patients remained under the care of their GP and continued to receive their usual NHS treatment.
Bespoke smoking cessation interventions were in line with best practice guidance relevant to the provision of all NHS stop smoking interventions (including for those with mental illness). It sets out fundamental quality principles for the delivery of services and stop smoking support – stipulated in the Department of Health’s NHS Stop Smoking Services: Service and Monitoring Guide 2009/10. 3,58
In training our MHSCPs, we also paid attention to the content of the intervention to ensure that evidence-supported behaviour change techniques (BCTs) were incorporated. We followed contemporary best practice and incorporated evidence-supported BCTs59,60 in the following way:
-
During the design phase of SCIMITAR we reviewed existing trial data in this area (published in a systematic review by Banham and Gilbody). 44
-
We contacted the first authors of all existing SMI smoking cessation trials to obtain their smoking cessation manuals (10 manuals were obtained).
-
We classified the behavioural content of all existing mental health smoking cessation manuals using the taxonomy of BCTs developed by Abraham and Michie. 59
Finally, we identified those BCTs which were associated with a positive trial result (OR > 1.5) and incorporated these into our manualised SCIMITAR intervention.The specific evidence-supported mental health BCTs are summarised in Box 1.
-
Identify reasons for wanting and not wanting to stop smoking.
-
Measure CO.
-
Facilitate barrier identification and problem solving.
-
Facilitate relapse prevention and coping.
-
Facilitate action planning/know how to help identify relapse triggers.
-
Facilitate goal setting.
-
Advise on conserving mental resources.
-
Advise on stop-smoking medication.
-
Give options for additional and later support.
-
Assess current and past smoking behaviour.
-
Assess current readiness and ability to quit.
-
Assess nicotine dependence.
-
Assess physiological and mental functioning.
-
Elicit client views.
-
Monitor psychiatric medication levels and side effects throughout the quit attempt.
Control intervention
This was a usual-care control group in which participants were encouraged to consult with their GP or local NHS quit smoking services. GPs were given advice to follow current NICE guidelines for smoking cessation, without the additional support of a bespoke MHSCP. Usual care could include pharmacotherapies to aid smoking cessation (NRTs, bupropion or varenicline either separately or in combination), access to self-help materials and referral to local NHS stop smoking clinics (which would not be specifically tailored for the needs of those with SMI). Patients were encouraged to reduce smoking to quit and set their own quit dates, but were managed solely by their own GP or mental health specialist and, crucially, did not receive regular visits from a MHSCP. Details of NRT that control participants received were gathered by accessing patients’ GP notes and details of any smoking cessation management were requested from participants in the follow-up questionnaires.
Follow-up
Participants were followed up 1 month, 6 months and 12 months after randomisation.
Baseline assessments and 12-month follow-up were carried out face to face, while 1- and 6-month follow-ups were carried out by telephone interview, using paper questionnaires or via online questionnaires. If it was not possible to meet the participant for a face-to-face 12-month follow-up, a systematic approach was used to explore other avenues to collect data (self-report data only). Follow-up was carried out by researchers who were not blind to treatment allocation; however, the objective nature of the primary outcome eliminated any potential bias.
Outcomes
Primary outcomes
The primary outcome was whether patients had stopped or reduced smoking when assessed at 12 months post recruitment. This was determined by CO measurement, where abstinence is defined as CO < 10 p.p.m. In the absence of a CO measurement, self-reported smoking cessation was used.
Secondary outcomes
-
Self-reported number of cigarettes smoked per day.
-
Fagerstrom Test for Nicotine Dependence (FTND). 61
-
Motivation to quit (MTQ) questionnaire.
-
Self-reported number of attempts to quit and period of cessation.
-
Patient Health Questionnaire-9 items (PHQ-9). 62
-
Short Form questionnaire-12 items (SF-12). 63
-
Service utilisation.
-
Self-reported drug substitution (specifically cannabis use).
Table 1 gives details of the measures collected and the time points at which they were collected. Copies of the questionnaires can be found in Appendix 2.
| Assessment | Timeline (months post randomisation) | |||
|---|---|---|---|---|
| Baseline | 1 | 6 | 12 | |
| Eligibility and consent | ||||
| Eligibility | ✓ | |||
| Consent | ✓ | |||
| Background and follow-up | ||||
| Personal details | ✓ | |||
| Body mass index | ✓ | ✓ | ||
| Mental health details | ||||
| Mental health history | ✓ | |||
| Self-reported current mental health status | ✓ | ✓ | ✓ | ✓ |
| Current medications | ✓ | ✓ | ||
| Referrals to mental health services | ✓ | ✓ | ✓ | |
| Admissions to hospital related to mental health | ✓ | ✓ | ✓ | |
| Smoking details | ||||
| Smoking history | ✓ | |||
| Current smoking status | ✓ | ✓ | ✓ | ✓ |
| Use of smoking cessation services | ✓ | ✓ | ✓ | ✓ |
| CO measurement | ✓ | ✓ | ||
| Adverse event reporting | Ongoing collection | |||
| Questionnaires | ||||
| FTND questionnaire | ✓ | ✓ | ✓ | ✓ |
| MTQ questionnaire | ✓ | ✓ | ✓ | ✓ |
| PHQ-9 | ✓ | ✓ | ✓ | ✓ |
| Health-related quality of Life (SF-12) | ✓ | ✓ | ✓ | ✓ |
| Health state utility (EQ-5D) | ✓ | ✓ | ✓ | ✓ |
| Health economics/service utilisation questionnaire | ✓ | ✓ | ✓ | |
Participant engagement
Participant engagement was measured using the proportion of intervention participants who engaged with (1) contacts which were offered from a MHSCP; (2) medication when this was offered by their GP (as measured by the number of filled prescriptions issued by the GP); or (3) compliance with CO monitoring by MHSCPs. Smoking status at baseline and (where possible) follow-up were verified by exhaled CO. Readings < 10 p.p.m. confirmed that participants had not smoked recently (i.e. within 12 hours). Measurements above 10 p.p.m. indicated that the patient has not ceased smoking. At least two CO readings were taken; if participants claimed to have stopped but their CO readings were above 10 p.p.m., they were asked when they had last smoked and whether or not they had any minor relapses during their quit attempt.
Statistical analysis
All analyses were conducted on an intention-to-treat basis, including all randomised patients in the groups to which they were randomised. Analyses were conducted in STATA version 13 (StataCorp, College Station, TX, USA).
Baseline data
The baseline data were summarised by treatment group and described descriptively. No formal statistical comparisons were undertaken. Continuous measures were reported using means and standard deviations (SDs; median and range were also included where appropriate), whereas categorical data were reported using counts and percentages.
Primary analysis
The primary outcome was cessation of smoking at 12 months as measured by the breath CO test and self-reported smoking cessation in the absence of a breath measurement. The two treatment groups were compared using logistic regression with adjustment for prognostic variables: sex, age, number of cigarettes smoked at baseline and alcohol consumption. ORs and corresponding 95% CIs were obtained from this model.
Secondary analyses
The 1-, 6- and 12-month secondary outcomes analysed were self-reported smoking cessation, number of cigarettes smoked per day, dependence on smoking as assessed by the FTND questionnaire, level of motivation as assessed by the MTQ questionnaire, length of cessation of smoking, PHQ-9, BMI, SF-12 physical component score and SF-12 mental component score. Secondary outcomes were summarised descriptively, with no formal statistical comparisons undertaken. Continuous measures were reported using means and SDs (median and range was also included where appropriate), whereas categorical data were reported using counts and percentages.
Missing data
The numbers of patients analysed were reported for the primary and secondary outcomes for each treatment group. For the primary outcome, analysis was performed on complete cases only and cases without a CO measure or a self-reported smoking cessation result at 12 months were excluded from analysis. In a full trial, multiple imputation and mixed modelling would be considered in the presence of missing outcome data.
Qualitative substudy
We explored specific issues of acceptability and adherence of smoking cessation interventions among those with SMI and those who referred to and delivered the intervention. Full details of the qualitative substudy are given in Chapter 6.
Cost assessment
A cost assessment was carried out to estimate the cost of the alternative treatment strategies. A costing methodology was carried out in two steps. The first step was to measure resource use by trial patients in physical units. Health-care and community services resource use information was collected using an adapted health economic/service utilisation questionnaire included in the baseline and follow-up questionnaire (see Appendix 3). For medication use, the participating GP surgeries were asked to extract prescription information of participants during the trial period from their records at the end of the trial. Owing to the huge variety of medication that could be prescribed to participants, a list of antipsychotic medication was used to reduce the burden of data collection upon the GP surgeries (Table 2). Therefore, only the information on antipsychotic medication and prescriptions related to pharmacotherapy for stop smoking was collected from GP surgeries. The pharmacotherapy for stop smoking included NRT products, varenicline and bupropion. While the prescriptions of pharmacotherapy were collected from GP records, the usage of pharmacotherapy was also collected using the trial questionnaire, covering both prescription and over-the-counter purchases. The second step was to calculate the cost of resources used by applying market prices or national average unit costs. Costs were assessed from a NHS and Personal Social Service perspective. Intervention costs were based on delivery costs within the trial and included staff, equipment, supervision and appropriate annuitised capital costs. Missing data were estimated by using the average value among the same group at the same follow-up point.
| Chemical name | |
|---|---|
| Citalopram hydrobromide | |
| Clozapine | |
| Fluoxetine hydrochloride | |
| Flupentixol decanoate | |
| Fluphenazine decanoate | |
| Haloperidol decanoate | |
| Lithium carbonate | |
| Olanzapine | |
| Paroxetine hydrochloride | |
| Prochlorperazine maleate | |
| Procyclidine hydrochloride | |
| Quetiapine | |
| Risperidone | |
| Trihexyphenidyl hydrochloride | |
| Zuclopenthixol |
A cost-effectiveness analysis was undertaken at 12 months comparing resource use in the BSC group with resource use in the usual-care group using the incremental quit rate for the intervention over and above usual care. This cost-effectiveness analysis is undertaken to demonstrate the analysis that would be undertaken in a full trial. This is a pilot trial and results should be interpreted with extreme caution because of the small sample size. We do not conduct a full cost–utility analysis because of the very small sample size.
Adverse events
Clear guidance on the prescription of antismoking medications in the presence of SMI (including safety considerations) have been published and were made available to all GPs to help inform their prescribing decisions. A key feature of the SCIMITAR trial was to ensure that GPs manage antismoking medications within this framework and with their prior knowledge of the patient and their concomitant use of medication. This was with the aim of replicating real-life practice of the use of antismoking medications in primary care. The medication profile of the individual participants was reviewed by their GP or mental health specialist to assess any potential safety issues (in line with the latest practice guidance on the provision of smoking cessation interventions in the NHS). An important aspect of the design of this study was that the SCIMITAR team had no direct influence over prescribing decisions made by GPs since this was not a drug trial or an investigation of a medicinal product(s).
A standard operating procedure for detecting and reporting adverse events (AEs) was implemented. An AE was defined as any unexpected effect or untoward clinical event affecting the participant. It could be directly related, possibly related or completely unrelated to the intervention. It was also classed according to severity, either as a non-serious AE (including discomfort or slight worsening of symptoms) or as a SAE (which could be particularly harmful, dangerous or required hospitalisation).
The participant’s MHSCP, GP and mental health specialist were requested to inform the research team of any serious or non-serious AEs. In addition, participant responses to questions in the follow-up questionnaire relating to hospital admissions, attendance at accident and emergency, use of the emergency services or if the participant volunteered information which could potentially be classed as an AE or SAE, were followed up by the research team.
All AEs and SAEs were independently reviewed by a clinician and reported to the Data Monitoring and Ethics Committee and Trial Steering Committee. Any SAEs that were deemed to be related to the intervention and unexpected were reported to the Research Ethics Committee and sponsor within 7 days of notification.
Suicide protocol
A protocol for identifying and reporting suicide risk was implemented (see Appendix 6 for protocol). Question 9 on the PHQ-9, which asks if the patient ‘have you had thoughts that you would be better off dead or hurting yourself in some way?’, was used to identify any suicide risk.
If the participant indicated a response of 3 for this item, then the suicide protocol was implemented and the patient asked if he or she had talked to a GP, psychiatrist or care co-ordinator/community psychiatric nurse about these feelings. If the patient had not sought help, consent was sought to to inform the patient’s GP of the situation. If the patient refused, the relevant designated psychiatrist/health professional was contacted. If the patient agreed, the patient’s GP or psychiatrist was contacted immediately. A suicidal intent form was also completed and, where applicable, a ‘suicidal intent form: psychiatrist/health professional’ was completed. These forms were stored with the patient’s trial records.
Chapter 3 Changes to the protocol
Online questionnaires
In the original proposal participants would complete paper questionnaires and the answers would be manually entered into the database by the researcher. However, the possibility of participants completing the questionnaires online became available. As some participants may find this preferable to completing a paper questionnaire, they were given the additional option of completing questionnaires online via a secure website held on the university server.
Extension to end of study date
Owing to recruitment taking longer than anticipated an extension was requested and granted. It was originally planned that the study would end in May 2013. This was extended to November 2013. The extension was to allow sufficient time for the study team to collect all outstanding follow-up data from participants.
Twelve-month follow-up
In the original protocol, all 12-month follow-ups were to be carried out face to face. It became apparent because of the nature of the patient population being studied that this would not always be possible. To collect data from as many participants as possible, we decided that if a participant could not be met face to face, attempts would be made to collect 12-month data via a telephone interview or postal questionnaire. In these cases smoking abstinence or reduction would not be verified by CO measurements; however, self-reported quit rates would still be collected.
Gift vouchers
The SCIMITAR Trial management group offered participants taking part in the qualitative substudy a £10 gift voucher as a goodwill gesture and token of thanks for their time.
Chapter 4 Results
Recruitment
Recruitment started in May 2011 and ended in May 2012. Over the course of the trial, 45 GP surgeries in Manchester, York/Scarborough and Hull mailed out recruitment packs. Recruitment of at least one trial participant occurred in 25 of the 45 GP surgeries which mailed out packs. Twenty-nine CMHTs were enlisted to recruit participants, along with 21 other secondary care organisations and 14 tertiary care organisations. Four participants were recruited through direct referral, having seen a poster advertising the study.
In primary care, 1036 recruitment packs were mailed out by GP surgeries which resulted in 64 consent to contact forms being returned (a response rate of 6.2%). Of these, 51 people were recruited into the study. From secondary care 57 direct referrals were received, of which 42 were recruited and randomised (a rate of 74%); however, it was not possible to determine how many packs were given out by CMHTs and other secondary and tertiary care organisations.
A total of 97 patients were recruited to the trial. The rate of recruitment is shown in Figure 1. Recruitment occurred at three sites (York/Scarborough, Manchester and Hull) and was evenly distributed between primary and secondary care (Table 3). Table 4 shows how many people were recruited from each of the different secondary care organisations. Participant flow through the trial is shown in the Consolidated Standards of Reporting Trials (CONSORT) diagram (Figure 2). The conversion rates of each stage of the recruitment process in primary care are shown in Figure 3 and the conversion rate of each stage of the secondary care recruitment process is shown in Figure 4. It can be seen that a total GP list size of 466,734 led to 51 randomisations.
FIGURE 1.
Trial recruitment rate.

| Recruitment site | Recruitment method | Recruiting sites | |||
|---|---|---|---|---|---|
| York/Scarborough | Hull | Manchester | Total | ||
| Primary care | Database search | 25 | 9 | 15 | 49 |
| Self-referral | 1 | 1 | 0 | 2 | |
| Secondary care | Direct referral | 12 | 2 | 29 | 43 |
| Self-referral | 0 | 1 | 0 | 1 | |
| Unknown | 0 | 0 | 2 | 2 | |
| Total | 38 | 13 | 46 | 97 | |
| Centre | CMHT | Clozapine/depot clinic | Assertive outreach | Assisted housing | Other |
|---|---|---|---|---|---|
| York/Scarborough | 7 | 0 | 2 | 4 | 3 |
| Manchester | 14 | 4 | 3 | 5 | 10 |
| Hull | 8 | 1 | 0 | 3 | 0 |
| Total | 29 | 5 | 5 | 12 | 13 |
FIGURE 2.
A CONSORT diagram showing participant flow through the trial.

FIGURE 3.
Primary care randomisations from GP database searches.
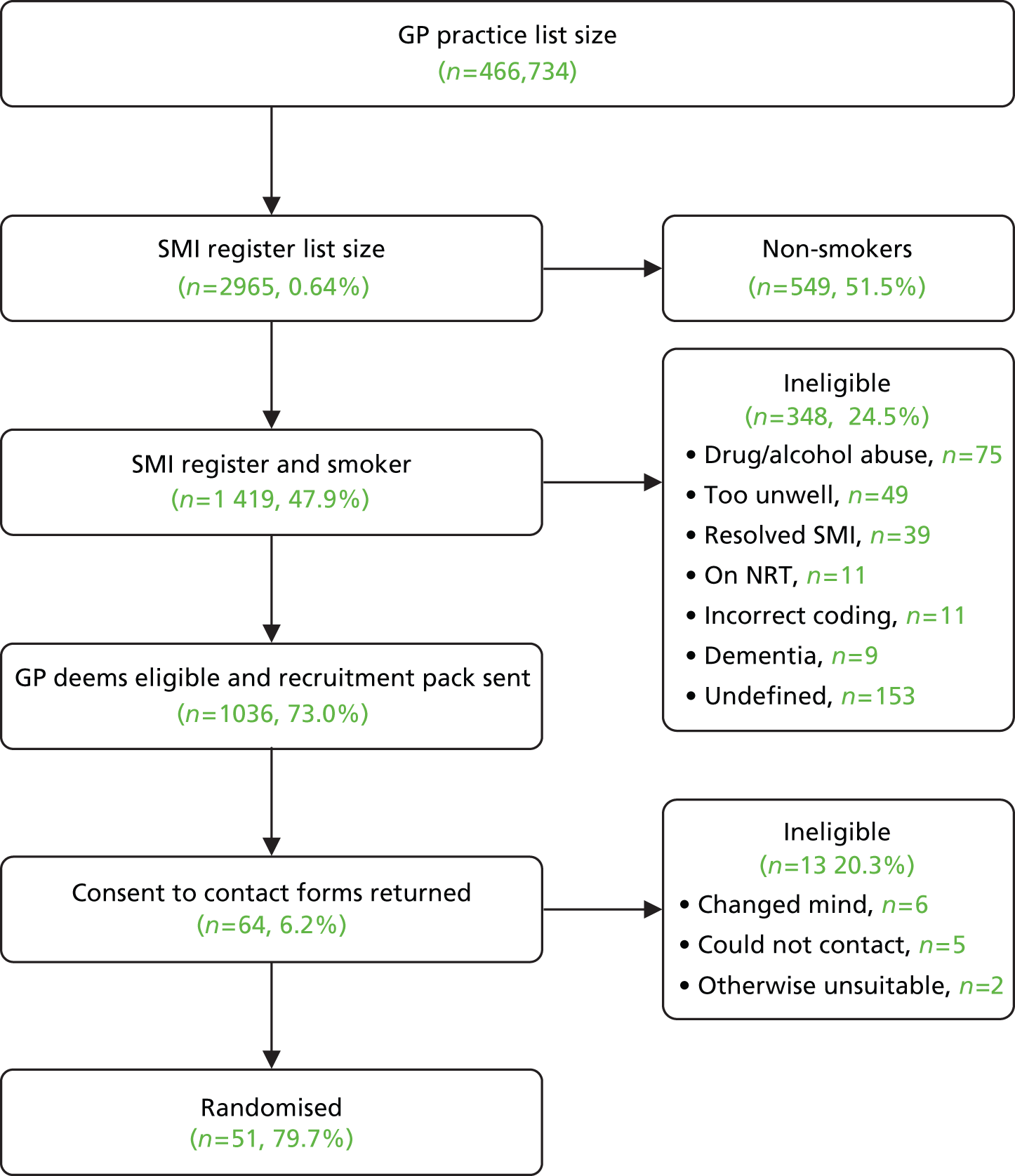
FIGURE 4.
Secondary care randomisations.
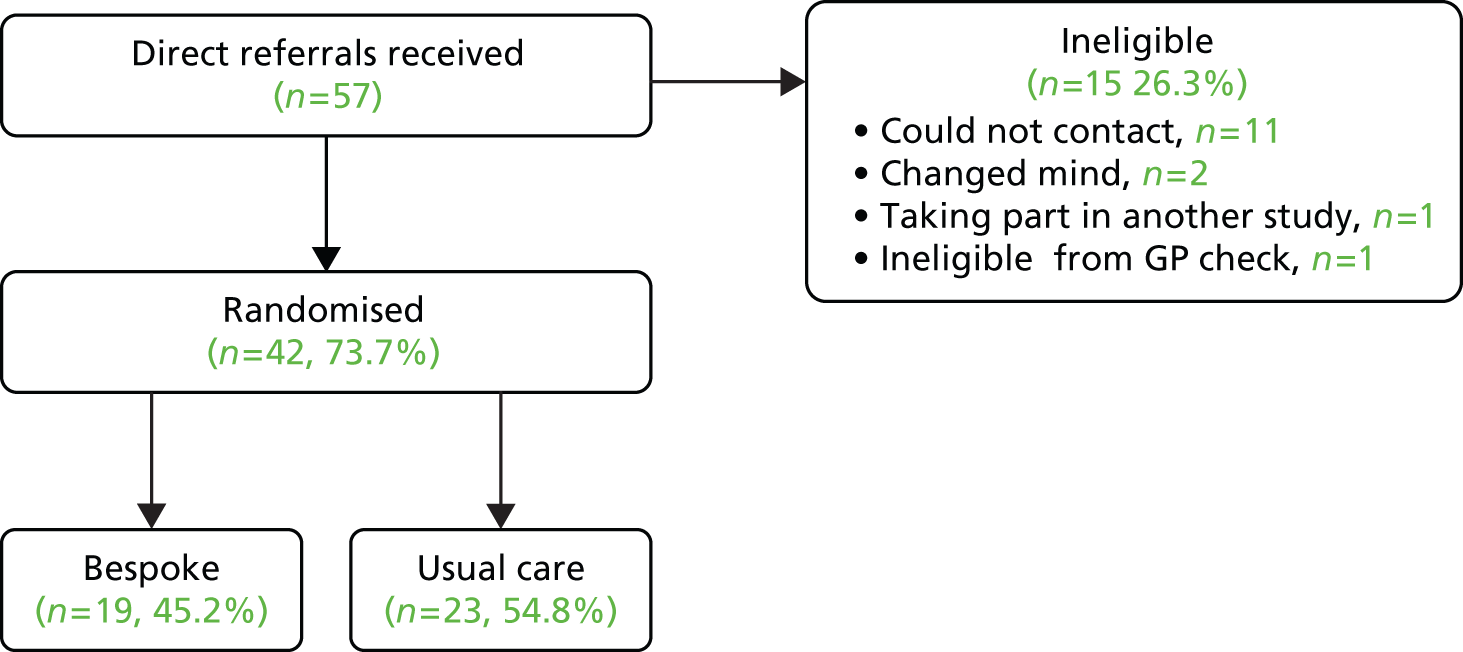
Of the 97 participants, 51 were randomised to usual GP care and 46 participants were randomised to BSC (Table 5).
| Group | Recruiting sites | |||
|---|---|---|---|---|
| York/Scarborough | Hull | Manchester | Total | |
| Usual GP care | 18 | 6 | 27 | 51 |
| BSC | 20 | 7 | 19 | 46 |
| Total | 38 | 13 | 46 | 97 |
Baseline data
The baseline characteristics of participants are summarised in Tables 6–10. Table 6 summarises participants by the prespecified prognostic factors. There were more male than female participants (59.8% vs. 40.2% respectively) and a greater proportion of men in the BSC group (69.6%) than in the usual GP care group (51.0%). There was some imbalance between the treatment groups with respect to alcohol consumption, with more alcohol consumption in the usual GP care group (62.7%) than in the BSC group (50.0%). The mean age of participants was 47 years, with a range from 19.1 to 73.3 years.
| Characteristic | Usual GP care (N = 51) | BSC (N = 46) | Overall (N = 97) |
|---|---|---|---|
| Sex | |||
| Female, n (%) | 25 (49.0) | 14 (30.4) | 39 (40.2) |
| Male, n (%) | 26 (51.0) | 32 (69.6) | 58 (59.8) |
| Age (years) | |||
| Mean (SD) | 45.9 (12.8) | 47.8 (12.4) | 46.8 (12.6) |
| Median (range) | 46.4 (22.2–71.5) | 47.3 (19.1–73.3) | 47.2 (19.1–73.3) |
| Missing, n (%) | 2 (3.9) | 0 (0.0) | 2 (2.1) |
| Alcohol consumption at baseline | |||
| Yes, n (%) | 32 (62.7) | 23 (50.0) | 55 (56.7) |
| No, n (%) | 19 (37.3) | 23 (50.0) | 42 (43.3) |
| Number of cigarettes | |||
| Mean (SD) | 22.8 (13.2) | 25.8 (11.6) | 24.2 (12.5) |
| Median (range) | 20 (5–60) | 22.5 (5–60) | 20 (5–60) |
| Missing, n (%) | 0 (0.0) | 2 (4.3) | 2 (2.1) |
Table 7 summarises the general health of participants. Almost half of participants reported moderate health (48%) over the past year and a mean of 5.5 consultations with a GP in the last 12 months (range 0–40 consultations). The majority of participants (86%) felt that smoking had affected their health, and 63% of participants had been advised to stop smoking by their GP. The mean BMI of participants was 28.6 kg/m2 (range 17.9–43.1 kg/m2), which is categorised as overweight (normal weight BMI range is 18.5–25 kg/m2). Fifteen per cent of participants reported taking recreational drugs.
| Characteristic | Usual GP care (N = 51) | BSC (N = 46) | Overall (N = 97) |
|---|---|---|---|
| Health over the past year, n (%) | |||
| Excellent | 4 (8.0) | 2 (4.3) | 6 (6.3) |
| Good | 11 (22.0) | 12 (26.1) | 23 (24.0) |
| Moderate | 24 (48.0) | 22 (47.8) | 46 (47.9) |
| Poor | 9 (18.0) | 6 (13.0) | 15 (15.6) |
| Very poor | 2 (4.0) | 4 (8.7) | 6 (6.3) |
| Number of times consulted GP in the last 12 months | |||
| Mean (SD) | 5.4 (7.1) | 5.7 (6.2) | 5.5 (6.7) |
| Median (range) | 3 (0–40) | 3.5 (0–24) | 3 (0–40) |
| Missing, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Smoking has affected the state of your health, n (%) | |||
| Yes | 43 (84.3) | 40 (87.0) | 83 (85.6) |
| No | 8 (15.7) | 6 (13.0) | 14 (14.4) |
| GP or doctor advised you to quit smoking, n (%) | |||
| Yes | 30 (58.8) | 31 (67.4) | 61 (62.9) |
| No | 21 (41.2) | 15 (32.6) | 36 (37.1) |
| Recreational drugs at baseline, n (%) | |||
| Yes | 10 (19.6) | 5 (10.9) | 15 (15.5) |
| No | 41 (80.4) | 41 (89.1) | 82 (84.5) |
| BMI (kg/m2) | |||
| Mean (SD) | 29.1 (5.8) | 28.1 (5.7) | 28.6 (5.7) |
| Median (range) | 29.3 (18.5–43.1) | 27.3 (17.9–41.5) | 28.6 (17.9–43.1) |
| Missing, n (%) | 1 (2.0) | 0 (0.0) | 1 (1.0) |
Table 8 summarises the sociodemographic data of participants and Table 9 summarises their employment status. The majority of participants (87%) were white British and the next largest ethnic group was black or black British-Caribbean (5%). About 20% of the participants had General Certificates of Secondary Education (GCSEs)/O-levels as their highest educational qualification. Over half of participants (56%) were not employed but not seeking work because of ill health and 75% of those unemployed had not been employed for over 5 years. Over half of participants were single (57%), 20% were married or living with a partner and 19% were divorced or separated.
| Characteristic | Usual GP care (N = 51) | BSC (N = 46) | Overall (N = 97) |
|---|---|---|---|
| Ethnicity, n (%) | |||
| White – British | 41 (80.4) | 42 (93.3) | 83 (86.5) |
| White – Irish | 1 (2.0) | 0 (0.0) | 1 (1.0) |
| Any other white background | 2 (3.9) | 1 (2.2) | 3 (3.1) |
| Mixed – white and black Caribbean | 1 (2.0) | 0 (0.0) | 1 (1.0) |
| Any other mixed background | 1 (2.0) | 0 (0.0) | 1 (1.0) |
| Asian or Asian British – Pakistani | 1 (2.0) | 0 (0.0) | 1 (1.0) |
| Black or black British – Caribbean | 4 (7.8) | 1 (2.2) | 5 (5.2) |
| Black or black British – African | 0 (0.0) | 1 (2.2) | 1 (1.0) |
| Chinese | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Marital status, n (%) | |||
| Single | 32 (62.7) | 24 (52.2) | 56 (57.7) |
| Married | 4 (7.8) | 7 (15.2) | 11 (11.3) |
| Living with a partner/cohabiting | 5 (9.8) | 3 (6.5) | 8 (8.2) |
| Divorced/separated | 9 (17.6) | 9 (19.6) | 18 (18.6) |
| Widowed | 1 (2.0) | 1 (2.2) | 2 (2.1) |
| Never married | 0 (0.0) | 1 (2.2) | 1 (1.0) |
| Other | 0 (0.0) | 1 (2.2) | 1 (1.0) |
| Highest educational qualification | |||
| GCSE/O-level | 10 (19.6) | 9 (19.6) | 19 (19.6) |
| GCE A/AS-level or Scottish Higher | 1 (2.0) | 4 (8.7) | 5 (5.2) |
| NVQ/SVQ levels 1–3 | 6 (11.8) | 3 (6.5) | 9 (9.3) |
| BTEC certificate | 0 (0.0) | 1 (2.2) | 1 (1.0) |
| BTEC diploma | 2 (3.9) | 1 (2.2) | 3 (3.1) |
| Qualified teacher status | 1 (2.0) | 2 (4.3) | 3 (3.1) |
| Degree (first degree/ordinary degree) | 2 (3.9) | 1 (2.2) | 3 (3.1) |
| Postgraduate certificate | 7 (13.7) | 2 (4.3) | 9 (9.3) |
| Postgraduate diploma | 1 (2.0) | 0 (0.0) | 1 (1.0) |
| PhD | 0 (0.0) | 1 (2.2) | 1 (1.0) |
| Other | 15 (29.4) | 15 (32.6) | 30 (30.9) |
| Don’t know/no response | 6 (11.8) | 7 (15.2) | 13 (13.4) |
| Characteristic | Usual GP care (N = 51) | BSC (N = 46) | Overall (N = 97) |
|---|---|---|---|
| Employment, n (%) | |||
| Employed full-time | 4 (7.8) | 3 (6.5) | 7 (7.2) |
| Employed part-time | 2 (3.9) | 2 (4.3) | 4 (4.1) |
| Self-employed | 2 (3.9) | 0 (0.0) | 2 (2.1) |
| Retired | 5 (9.8) | 7 (15.2) | 12 (12.4) |
| Looking after family or home | 2 (3.9) | 0 (0.0) | 2 (2.1) |
| Student | 3 (5.9) | 0 (0.0) | 3 (3.1) |
| Voluntary worker | 6 (11.8) | 3 (6.5) | 9 (9.3) |
| Not employed but seeking work | 1 (2.0) | 1 (2.2) | 2 (2.1) |
| Not employed, but not seeking work because of ill health | 25 (49.0) | 29 (63.0) | 54 (55.7) |
| Not employed, but not seeking work for some other reason | 1 (2.0) | 1 (2.2) | 2 (2.1) |
| If unemployed, length of unemployment, n (%) | |||
| 4–12 months | 2 (7.4) | 0 (0.0) | 2 (3.6) |
| 1–2 years | 0 (0.0) | 1 (3.6) | 1 (1.8) |
| 2–5 years | 3 (11.1) | 5 (17.9) | 8 (14.5) |
| > 5 years | 20 (74.1) | 21 (75.0) | 41 (74.5) |
| Don’t know/no response | 2 (7.4) | 1 (3.6) | 3 (5.5) |
Table 10 summarises baseline mental health status. The most common severe mental health problems were schizophrenia or other psychotic illness (n = 57, 59%), schizoaffective disorder (n = 10, 10%) and bipolar disorder (n = 30, 31%). Over half of the participants (56%) had a CPA co-ordinator and 60% were under the care of a CMHT. On average, participants had twice (mean) in the last 10 years required psychiatric treatment in hospital, with a range of 1 to 15 periods of hospital treatment. Eighty per cent of participants described their condition as ‘stable’ and 8% described their condition as ‘unstable’ (though each participant had been judged to be stable from the point of view of their condition by either their GP or a responsible mental health professional). Almost all participants (99% of those who responded to the question) were taking a medication, the most common being olanzapine (23%) and clozapine (8%).
| Characteristic | Usual GP care (N = 51), n (%) | BSC (N = 46), n (%) | Overall (N = 97), n (%) |
|---|---|---|---|
| Do you have a CPA co-ordinator? | |||
| Yes | 25 (51.0) | 26 (61.9) | 51 (56.0) |
| No | 24 (49.0) | 16 (38.1) | 40 (44.0) |
| Do you have a CMHT? | |||
| Yes | 28 (58.3) | 27 (62.8) | 55 (60.4) |
| No | 20 (41.7) | 16 (37.2) | 36 (39.6) |
| Number of times needed psychiatric treatment in hospital in last 10 years | |||
| Mean (SD) | 1.7 (1.6) | 2.3 (3.1) | 2 (2.4) |
| Median (range) | 1 (0–6) | 1 (0–15) | 1 (0–15) |
| Missing | 1 (1.9) | 0 (0.0) | 1 (1.0) |
| Would you describe your condition as | |||
| Stable | 40 (80.0) | 37 (80.4) | 77 (80.2) |
| Unstable | 4 (8.0) | 4 (8.7) | 8 (8.3) |
| Unsure | 6 (12.0) | 5 (10.9) | 11 (11.5) |
| Do you take any medications? | |||
| Yes | 41 (100.0) | 34 (97.1) | 75 (98.7) |
| No | 0 (0.0) | 1 (2.9) | 1 (1.3) |
| Missing | 10 (19.6) | 11 (23.9) | 21 (21.6) |
| If yes, do you take the following medications | |||
| Haloperidol | 2 (3.9) | 1 (2.2) | 3 (3.1) |
| Fluphenazine | 0 (0.0) | 2 (4.3) | 2 (2.1) |
| Clozapine | 4 (7.8) | 4 (8.7) | 8 (8.2) |
| Olanzapine | 11 (21.6) | 11 (23.9) | 22 (22.7) |
| Fluvoxamine | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Duloxetine | 1 (2.0) | 0 (0.0) | 1 (1.0) |
| Propranolol | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Insulin | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Theophylline | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Cimetidine | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Flecainide | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Table 11 summarises smoking history of participants. The mean length of smoking was 27 years, with a range from 3 years to 60 years. Most participants smoked packet (66%) or hand-rolled cigarettes (53%) (or both). The median number of attempts to quit was three, with a range of 0 to 150 attempts. The mean duration of reported longest quit attempt was 43 days (median 8.5 days), with a range of 0 to 832 days. The most common self-reported previous strategies used to stop smoking were ‘cold turkey’ (70%), followed by nicotine skin patches (68%), nicotine chewing gum (52%) and nicotine inhalator (47%).
| Characteristic | Usual GP care (N = 51) | BSC (N = 46) | Overall (N = 97) |
|---|---|---|---|
| Length of time smoking (years) | |||
| Mean (SD) | 25.8 (12.2) | 28.5 (13.5) | 27.1 (12.9) |
| Median (range) | 25 (5–55) | 26.5 (3–60) | 25 (3–60) |
| Missing, (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Type of tobacco used, n (%) | |||
| Packet cigarettes | 38 (74.5) | 26 (56.5) | 64 (66.0) |
| Hand-rolled cigarettes | 26 (51.0) | 25 (54.3) | 51 (52.6) |
| Cigars | 0 (0.0) | 2 (4.3) | 2 (2.1) |
| Pipe | 0 (0.0) | 2 (4.3) | 2 (2.1) |
| Chewing tobacco | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Water pipe/Hookah/Sheesha pipe | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| If using roll-ups or pipe, amount of tobacco used per day (ounces) | |||
| Mean (SD) | 7.0 (6.7) | 9.1 (11.1) | 8.1 (9.2) |
| Median (range) | 7 (0–25) | 5.5 (0–50) | 7 (0–50) |
| n | 25 | 28 | 53 |
| Number of attempts to quit in the past | |||
| Mean (SD) | 3.2 (2.5) | 9.7 (25.7) | 6.3 (18.1) |
| Median (range) | 2 (0–12) | 3 (0–150) | 3 (0–150) |
| Missing, n (%) | 1 (1.9) | 0 (0.0) | 1 (1.0) |
| Longest quit attempt (days) | |||
| Mean (SD) | 47.5 (139.5) | 37.7 (64.2) | 42.8 (109.6) |
| Median (range) | 8 (0–832) | 9 (0–260) | 8.5 (0–832) |
| Missing, n (%) | 2 (3.9) | 1 (2.2) | 3 (3.1) |
| Previous methods used to stop smoking, n (%) | |||
| Nicotine chewing gum | 29 (56.9) | 21 (45.7) | 50 (51.5) |
| Nicotine skin patches | 32 (62.7) | 34 (73.9) | 66 (68.0) |
| Nicotine nasal spray | 7 (13.7) | 3 (6.5) | 10 (10.3) |
| Nicotine inhalator | 25 (49.0) | 21 (45.7) | 46 (47.4) |
| Nicotine microtab | 5 (9.8) | 5 (10.9) | 10 (10.3) |
| Nicotine lozenges | 5 (9.8) | 7 (15.2) | 12 (12.4) |
| Zyban | 2 (3.9) | 2 (4.3) | 4 (4.1) |
| Champix | 3 (5.9) | 3 (6.5) | 6 (6.2) |
| ‘Cold turkey’ | 37 (72.5) | 31 (67.4) | 68 (70.1) |
| Hypnosis | 6 (11.8) | 6 (13.0) | 12 (12.4) |
| Acupuncture | 2 (3.9) | 4 (8.7) | 6 (6.2) |
| Other | 2 (4.1) | 1 (2.2) | 3 (3.2) |
The reasons for smoking and their importance are summarised in Table 12. The most important reason given for smoking was helping to cope with stress (65%), followed by helping to relax (47%).
| Reasons for smoking | Usual GP care (N = 51), n (%) | BSC (N = 46), n (%) | Overall (N = 97), n (%) |
|---|---|---|---|
| It helps me relax | |||
| Very important | 22 (43.1) | 24 (52.2) | 46 (47.4) |
| Quite important | 26 (51.0) | 16 (34.8) | 42 (43.3) |
| Not important | 3 (5.9) | 6 (13.0) | 9 (9.3) |
| It helps break up my working time | |||
| Very important | 11 (21.6) | 12 (26.1) | 23 (23.7) |
| Quite important | 21 (41.2) | 13 (28.3) | 34 (35.1) |
| Not important | 19 (37.3) | 21 (45.7) | 40 (41.2) |
| It is something to do when I am bored | |||
| Very important | 14 (27.5) | 18 (39.1) | 32 (33.0) |
| Quite important | 28 (54.9) | 23 (50.0) | 51 (52.6) |
| Not important | 9 (17.6) | 5 (10.9) | 14 (14.4) |
| It helps me cope with stress | |||
| Very important | 34 (66.7) | 29 (63.0) | 63 (64.9) |
| Quite important | 14 (27.5) | 14 (30.4) | 28 (28.9) |
| Not important | 3 (5.9) | 3 (6.5) | 6 (6.2) |
| I enjoy it | |||
| Very important | 17 (33.3) | 18 (39.1) | 35 (36.1) |
| Quite important | 19 (37.3) | 16 (34.8) | 35 (36.1) |
| Not important | 15 (29.4) | 12 (26.1) | 27 (27.8) |
| It’s something I do with family and friends | |||
| Very important | 10 (19.6) | 7 (15.2) | 17 (17.5) |
| Quite important | 15 (29.4) | 17 (37.0) | 32 (33.0) |
| Not important | 26 (51.0) | 22 (47.8) | 48 (49.5) |
| It stops me putting on weight | |||
| Very important | 7 (13.7) | 8 (17.4) | 15 (15.5) |
| Quite important | 8 (15.7) | 6 (13.0) | 14 (14.4) |
| Not important | 36 (70.6) | 32 (69.6) | 68 (70.1) |
| It stops me getting withdrawal symptoms | |||
| Very important | 21 (41.2) | 17 (37.0) | 38 (39.2) |
| Quite important | 15 (29.4) | 19 (41.3) | 34 (35.1) |
| Not important | 15 (29.4) | 10 (21.7) | 25 (25.8) |
Table 13 summarises the reasons for giving up smoking. The most important reason cited for trying to give up smoking was that it is bad for health (86%).
| Reasons for giving up smoking | Usual GP care (N = 51), n (%) | BSC (N = 46), n (%) | Overall (N = 97), n (%) |
|---|---|---|---|
| It is expensive | |||
| Very important | 35 (68.6) | 29 (63.0) | 64 (66.0) |
| Quite important | 13 (25.5) | 9 (19.6) | 22 (22.7) |
| Not important | 3 (5.9) | 8 (17.4) | 11 (11.3) |
| It is bad for my health | |||
| Very important | 44 (86.3) | 39 (84.8) | 83 (85.6) |
| Quite important | 5 (9.8) | 6 (13.0) | 11 (11.3) |
| Not important | 2 (3.9) | 1 (2.2) | 3 (3.1) |
| I don’t like feeling dependent on cigarettes | |||
| Very important | 35 (68.6) | 31 (67.4) | 66 (68.0) |
| Quite important | 13 (25.5) | 10 (21.7) | 23 (23.7) |
| Not important | 3 (5.9) | 5 (10.9) | 8 (8.2) |
| It makes my clothes and breath smell | |||
| Very important | 22 (43.1) | 17 (37.0) | 39 (40.2) |
| Quite important | 18 (35.3) | 18 (39.1) | 36 (37.1) |
| Not important | 11 (21.6) | 11 (23.9) | 22 (22.7) |
| It is a bad example for children | |||
| Very important | 25 (49.0) | 26 (56.5) | 51 (52.6) |
| Quite important | 14 (27.5) | 12 (26.1) | 26 (26.8) |
| Not important | 12 (23.5) | 8 (17.4) | 20 (20.6) |
| It is unpleasant for people near me | |||
| Very important | 20 (39.2) | 22 (47.8) | 42 (43.3) |
| Quite important | 21 (41.2) | 14 (30.4) | 35 (36.1) |
| Not important | 10 (19.6) | 10 (21.7) | 20 (20.6) |
| It makes me less fit | |||
| Very important | 33 (64.7) | 34 (73.9) | 67 (69.1) |
| Quite important | 14 (27.5) | 11 (23.9) | 25 (25.8) |
| Not important | 4 (7.8) | 1 (2.2) | 5 (5.2) |
| People around me disapprove of my smoking | |||
| Very important | 17 (34.0) | 15 (32.6) | 32 (33.3) |
| Quite important | 16 (32.0) | 14 (30.4) | 30 (31.3) |
| Not important | 17 (34.0) | 17 (37.0) | 34 (35.4) |
| It is bad for the health of people near me | |||
| Very important | 26 (51.0) | 19 (41.3) | 45 (46.4) |
| Quite important | 17 (33.3) | 18 (39.1) | 35 (36.1) |
| Not important | 8 (15.7) | 9 (19.6) | 17 (17.5) |
Table 14 summarises the smoking behaviour of participants at baseline. Most of the participants smoked more than five cigarettes in the last week (96%). The mean number of cigarettes smoked per day is 25, with a range of 5 to 60. The mean CO reading is 24 p.p.m. with a range of 4 to 58 p.p.m. A CO reading > 20 p.p.m. indicates heavy smoking. The majority of participants (80%) said they smoke the same number of cigarettes every day. Table 15 summarises recent quit attempts. The median number of quit attempts in the last 6 months was three, with a range of 0 to 150 attempts. The mean length of the most recent quit attempt was 23 days, with a range of 0 to 180 days.
| Baseline smoking outcome | Usual GP care (N = 51) | BSC (N = 46) | Overall (N = 97) |
|---|---|---|---|
| Smoked in last week, n (%) | |||
| Not even a puff | 0 (0.0) | 1 (2.2) | 1 (1.0) |
| Yes, just a few puffs | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Yes, between one and five cigarettes | 2 (3.9) | 1 (2.2) | 3 (3.1) |
| Yes, more than five cigarettes | 49 (96.1) | 44 (95.7) | 93 (95.9) |
| Number of cigarettes per day | |||
| Mean (SD) | 23.3 (13.2) | 26.5 (12.0) | 24.8 (12.7) |
| Median (range) | 20 (5–60) | 25 (5–60) | 20 (5–60) |
| Missing, n (%) | 2 (4) | 3 (6.5) | 5 (5.2) |
| Breath CO reading (p.p.m.) | |||
| Mean (SD) | 24.7 (14.1) | 22.9 (13.2) | 23.8 (13.6) |
| Median (range) | 22 (4–57) | 21 (6–58) | 22 (4–58) |
| Missing, n (%) | 1 (2.0) | 1 (2.2) | 2 (2.1) |
| Following statement best describes you, n (%) | |||
| I smoke the same number of cigarettes every day | 37 (72.5) | 41 (89.1) | 78 (80.4) |
| I have cut down the number of cigarettes I smoke | 13 (25.5) | 4 (8.7) | 17 (17.5) |
| I smoke cigarettes but not every day | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| I have stopped smoking completely | 0 (0.0) | 1 (2.2) | 1 (1.0) |
| Baseline smoking outcome | Usual GP care (N = 51) | BSC (N = 46) | Overall (N = 97) |
|---|---|---|---|
| Number of quit attempts in last 6 months | |||
| Mean (SD) | 3.2 (2.5) | 9.8 (25.7) | 6.3 (18.1) |
| Median (range) | 2 (0–12) | 3 (0–150) | 3 (0–150) |
| Missing, n (%) | 1 (2.0) | 1 (2.2) | 2 (2.1) |
| Length of most recent quit attempt (days) | |||
| Mean (SD) | 38.1 (70.9) | 10.2 (29.9) | 23.4 (53.4) |
| Median (range) | 1 (0–180) | 0 (0–90) | 0 (0–180) |
| Denominator | 8 | 9 | 17 |
| FTND questionnaire score | |||
| Mean (SD) | 6.1 (2.2) | 6.0 (2.6) | 6.1 (2.4) |
| Median (range) | 6 (1–10) | 7 (0–10) | 6.5 (0–10) |
| Missing, n (%) | 0 (0.0) | 2 (4.3) | 2 (2.1) |
| MTQ questionnaire score | |||
| Mean (SD) | 13.4 (2.4) | 14.3 (2.3) | 13.8 (2.4) |
| Median (range) | 14 (6–18) | 14 (10–19) | 14 (6–19) |
| Missing, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Table 16 gives a breakdown of the answers to the FTND questionnaire score at baseline. The mean FTND score at baseline was 6.6, indicating moderate nicotine dependence, with a range of 0 to 10. The mean MTQ questionnaire score at baseline was 13.8, with a range of 6 to 19. Table 17 summarises the PHQ-9 and SF-12 scores. The mean PHQ-9 score at baseline was 9.2, indicating moderate levels of low mood but below the threshold for case-level depression (indicated by a score ≥ 10). The mean SF-12 physical component score at baseline was 45, with a range of 15 to 67. This is lower than the mean of the general UK population, indicating worse physical health than the general population. The mean SF-12 mental component score was 41, with a range of 13 to 64. This is about one SD lower than the mean of the general UK population, indicating worse mental health than the general population.
| FTND question | Usual GP care (N = 51), n (%) | BSC (N = 46), n (%) | Overall (N = 97), n (%) |
|---|---|---|---|
| How soon after you wake up do you smoke your first cigarette? | |||
| ≤ 5 minutes | 26 (51.0) | 23 (50.0) | 49 (50.5) |
| 6–30 minutes | 23 (45.1) | 17 (37.0) | 40 (41.2) |
| > 30 minutes | 2 (3.9) | 6 (13.0) | 8 (8.2) |
| Do you find it difficult to stop smoking in no-smoking areas? | |||
| Yes | 23 (45.1) | 18 (39.1) | 41 (42.3) |
| No | 28 (54.9) | 28 (60.9) | 56 (57.7) |
| Which cigarette would you hate most to give up? | |||
| The first of the morning | 33 (64.7) | 35 (76.1) | 68 (70.1) |
| Other | 18 (35.3) | 11 (23.9) | 29 (29.9) |
| How many cigarettes per day do you usually smoke? | |||
| ≤ 10 | 10 (19.6) | 3 (6.7) | 13 (13.5) |
| 11–20 | 21 (41.2) | 17 (37.8) | 38 (39.6) |
| 21–30 | 7 (13.7) | 15 (33.3) | 22 (22.9) |
| ≥ 31 | 13 (25.5) | 10 (22.2) | 23 (24.0) |
| Do you smoke more frequently in the first hours after waking than during the rest of the day? | |||
| Yes | 33 (64.7) | 24 (52.2) | 57 (58.8) |
| No | 18 (35.3) | 22 (47.8) | 40 (41.2) |
| Do you smoke even if you are so ill that you are in bed most of the day? | |||
| Yes | 22 (43.1) | 20 (43.5) | 42 (43.3) |
| No | 29 (56.9) | 26 (56.5) | 55 (56.7) |
| Do you smoke hand-rolled cigarettes? | |||
| Yes | 25 (49.0) | 25 (55.6) | 50 (52.1) |
| No | 26 (51.0) | 20 (44.4) | 46 (47.9) |
| If yes, how many do you usually smoke per day? | |||
| Mean (SD) | 16.0 (10.9) | 29.1 (14.5) | 22.6 (14.3) |
| Median (range) | 15 (2–50) | 30 (6–60) | 20 (2–60) |
| Missing, n (%) | 2 (0.1) | 2 (0.1) | 4 (0.1) |
| How much tobacco do you usually use per day (g)? | |||
| Mean (SD) | 11.9 (12.5) | 14.5 (11.7) | 13.1 (12.0) |
| Median (range) | 7 (1–56) | 12.5 (1–50) | 8.2 (1–56) |
| Missing (n%) | 4 (0.2) | 6 (0.2) | 10 (0.2) |
| Secondary outcome | Usual GP care (N = 51) | BSC (N = 46) | Overall (N = 97) |
|---|---|---|---|
| PHQ-9 score | |||
| Mean (SD) | 8.7 (6.6) | 9.8 (7.1) | 9.2 (6.8) |
| Median (range) | 9 (0–22) | 8 (0–27) | 8 (0–27) |
| Missing, n (%) | 2 (3.9) | 1 (2.2) | 3 (3.1) |
| SF-12 physical component score | |||
| Mean (SD) | 45.3 (10.9) | 45.0 (10.9) | 45.2 (10.8) |
| Median (range) | 46.1 (15.4–67.0) | 43.0 (19.1–63.5) | 45.1 (15.4–67.0) |
| Missing, n (%) | 0 (0.0) | 1 (2.2) | 1 (1.0) |
| SF-12 mental component score | |||
| Mean (SD) | 40.8 (11.8) | 40.8 (13.1) | 40.8 (12.4) |
| Median (range) | 43.4 (16.2–62.7) | 42.9 (13.1–63.7) | 42.9 (13.1–63.7) |
| Missing, n (%) | 0 (0.0) | 1 (2.2) | 1 (1.0) |
Withdrawals
There were 15 participant withdrawals (15%) from the trial: five (10%) from the usual GP care group and 10 (22%) from the BSC group. A total of seven participants (7.2%) withdrew fully from the trial, while five participants (5.1%) withdrew from follow-up and three participants (3.1%) were too unwell to continue (Table 18). There are four categories of patient withdrawal:
-
Full withdrawal – participant withdrawn from the trial with regards completion of both postal questionnaires and collection of GP data.
-
Withdrawal from follow-up – participant has withdrawn from the completion of postal questionnaires, but agrees with the continuing collection of GP data.
-
Withdrawal from treatment – participant withdraws from trial intervention treatment, but agrees with continuing completion of postal questionnaires and collection of GP data.
-
Too unwell to continue – participant is deemed too unwell by medical staff to complete any questionnaires. This generally only occurs when a participant has been hospitalised.
| Withdrawal type | Usual GP care | BSC | Total |
|---|---|---|---|
| Full withdrawal | 2 | 5 | 7 |
| Withdrawal from follow-up | 2 | 3 | 5 |
| Withdrawal from treatment | 0 | 0 | 0 |
| Too unwell to continue | 1 | 2 | 3 |
| Total | 5 | 10 | 15 |
Follow-up
Participants were given the option of providing data face to face, via the telephone or by postal questionnaire. Of those who returned follow-up data at 12 months only one person declined a face-to-face visit and completed the follow-up by telephone; all the other participants who completed a 12 month follow-up did so face to face. Participants did not use the option of completing questionnaires online.
Primary outcome
Smoking cessation at 12 months was defined as a CO measure of < 10 p.p.m. or self-reported cessation if no CO measure was available. A CO measure of < 10 p.p.m. indicated no smoking in the last 8 hours and self-reported quit indicated no smoking within the last week. At 12 months, 64 participants had a CO measure and four participants had only a self-reported measure. Eight out of thirty-five participants (23%) had stopped smoking in the usual GP care arm and 12 out of 33 participants (36%) had stopped smoking in the BSC arm (Table 19).
| Primary outcome | Usual GP care (N = 51), n (%) | BSC (N = 46), n (%) | Overall (N = 97), n (%) | |||
|---|---|---|---|---|---|---|
| Number quit (CO verified) | 8 | 24.2a | 10 | 32.3a | 18 | 28.1a |
| Number with CO measure | 33 | 94.3a | 31 | 93.9a | 64 | 94.1a |
| Number quit (self-report only) | 0 | 0.0b | 2 | 100.0b | 2 | 50.0b |
| Number with self-report only | 2 | 5.7b | 2 | 6.1b | 4 | 5.9b |
| Total number quit | 8 | 22.9c | 12 | 36.4c | 20 | 29.4c |
| Total number with CO or self-reported measure | 35 | 100.0c | 33 | 100.0c | 68 | 100.0c |
A logistic regression of smoking cessation at 12 months on randomised group, adjusted for sex, age, number of cigarettes smoked at baseline and alcohol consumption at baseline, gave an OR of 2.9 (95% CI 0.8 to 10.5) for BSC compared with usual care (Table 20). This indicates that those randomised to BSC have greater odds of smoking cessation than those randomised to usual care, although this is not statistically significant. However, the analysis has been carried out on a small sample (complete cases, n = 65), so results should be interpreted with caution.
| Characteristic | OR | Standard error | 95% CI | p-value |
|---|---|---|---|---|
| BSC vs. usual care | 2.94 | 1.91 | 0.83 to 10.50 | 0.10 |
| Age | 0.97 | 0.02 | 0.93 to 1.02 | 0.30 |
| Male | 0.78 | 0.54 | 0.20 to 3.04 | 0.72 |
| Number of cigarettes smoked per day | 0.95 | 0.03 | 0.89 to 1.01 | 0.09 |
| Alcohol consumption | 1.23 | 0.80 | 0.35 to 4.38 | 0.75 |
Secondary outcomes
A summary of the smoking-related secondary outcome is given in Table 21 and a summary of the non-smoking-related secondary outcomes is given in Table 22.
| Outcome | Usual care | BSC | Overall | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Self-reported quit | n a | Frequencyb | % | n a | Frequencyb | % | n a | Frequencyb | % |
| 1 month | 40 | 2 | 5.0 | 42 | 4 | 9.5 | 82 | 6 | 7.3 |
| 6 months | 34 | 3 | 8.8 | 36 | 4 | 11.1 | 70 | 7 | 10.0 |
| 12 months | 35 | 4 | 11.4 | 33 | 5 | 15.2 | 68 | 9 | 13.2 |
| Number of cigarettes | n | Mean (SD) | Range | n | Mean (SD) | Range | n | Mean (SD) | Range |
| 1 month | 37 | 19.4 (12.3) | 0–50 | 38 | 18.4 (9.6) | 4–60 | 75 | 18.9 (11.0) | 0–60 |
| 6 months | 30 | 17.1 (11.6) | 1–50 | 31 | 16.8 (9.6) | 1–40 | 61 | 16.9 (10.5) | 1–50 |
| 12 months | 30 | 18.4 (11.6) | 5–50 | 26 | 20.1 (10.6) | 2–40 | 56 | 19.2 (11.1) | 2–50 |
| Number quit attempts | n | Mean (SD) | Range | n | Mean (SD) | Range | n | Mean (SD) | Range |
| 1 month | 40 | 1.1 (1.8) | 0–10 | 41 | 1.4 (2.8) | 0–15 | 81 | 1.2 (2.3) | 0–15 |
| 6 months | 33 | 0.9 (1.1) | 0–4 | 34 | 1.1 (1.1) | 0–4 | 67 | 1.0 (1.1) | 0–4 |
| 12 months | 35 | 0.7 (1.2) | 0–6 | 32 | 3.1 (7.5) | 0–32 | 67 | 1.9 (5.3) | 0–32 |
| Length of cessation | n | Mean (SD) | Range | n | Mean (SD) | Range | n | Mean (SD) | Range |
| 1 month | 12 | 0.3 (0.9) | 0–3 | 10 | 1.0 (1.8) | 0–5 | 22 | 0.59 (1.4) | 0–5 |
| 6 months | 11 | 1.3 (2.8) | 0–7 | 10 | 46.5 (72.9) | 0–180 | 21 | 22.8 (54.2) | 0–180 |
| 12 months | 8 | 1.8 (2.4) | 0–7 | 8 | 21.1 (42.5) | 0–120 | 16 | 11.4 (30.7) | 0–120 |
| FTND score | n | Mean (SD) | Range | n | Mean (SD) | Range | n | Mean (SD) | Range |
| 1 month | 40 | 5.5 (2.4) | 0–10 | 38 | 5.2 (2.1) | 0–9 | 78 | 5.3 (2.3) | 0–10 |
| 6 months | 32 | 4.8 (2.1) | 0–9 | 30 | 5.2 (2.3) | 1–9 | 62 | 5.0 (2.2) | 0–9 |
| 12 months | 29 | 4.9 (2.2) | 0–9 | 27 | 5.3 (2.0) | 1–9 | 56 | 5.1 (2.1) | 0–9 |
| MTQ score | n | Mean (SD) | Range | n | Mean (SD) | Range | n | Mean (SD) | Range |
| 1 month | 40 | 11.8 (2.4) | 7–17 | 41 | 14.2 (2.5) | 9–19 | 81 | 13.0 (2.7) | 7–19 |
| 6 months | 34 | 12.3 (3.1) | 4–19 | 33 | 12.6 (3.2) | 6–18 | 67 | 12.5 (3.1) | 4–19 |
| 12 months | 32 | 11.1 (3.1) | 4–18 | 33 | 12.1 (4.0) | 5–19 | 65 | 11.6 (3.6) | 4–19 |
| Secondary outcome | Usual care | BSC | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Range | n | Mean (SD) | Range | n | Mean (SD) | Range | |
| PHQ-9 score | |||||||||
| 1 month | 39 | 8.3 (6.5) | 0–27 | 41 | 9.2 (7.1) | 0–26 | 80 | 8.8 (6.8) | 0–27 |
| 6 months | 32 | 8.7 (7.0) | 0–23 | 33 | 9.6 (6.5) | 0–27 | 65 | 9.2 (6.7) | 0–27 |
| 12 months | 34 | 7.7 (7.3) | 0–23 | 33 | 11.2 (7.0) | 0–23 | 67 | 9.4 (7.3) | 0–23 |
| SF-12 physical component score | |||||||||
| 1 month | 40 | 45.4 (10.1) | 20.2–61.8 | 42 | 45.4 (11.2) | 18.8–64.4 | 82 | 45.4 (10.6) | 18.8–64.4 |
| 6 months | 34 | 46.9 (11.4) | 20.2–65.9 | 35 | 47.8 (11.1) | 23.1–72.4 | 69 | 47.43 (11.2) | 20.2–72.4 |
| 12 months | 33 | 45.8 (9.1) | 25.0–63.0 | 33 | 46.2 (11.1) | 22.0–61.6 | 66 | 46.0 (10.1) | 22.0–63.0 |
| SF-12 mental component score | |||||||||
| 1 month | 40 | 42.6 (10.2) | 21.8–61.7 | 42 | 39.9 (13.3) | 9.1–62.4 | 82 | 41.2 (11.9) | 9.1–62.4 |
| 6 months | 34 | 41.6 (10.7) | 22.2–59.5 | 35 | 37.1 (12.9) | 8.2–58.2 | 69 | 39.3 (12.0) | 8.2–59.5 |
| 12 months | 33 | 41.8 (11.0) | 16.2–61.3 | 33 | 39.1 (11.2) | 20.0–61.7 | 66 | 40.4 (11.1) | 16.2–61.7 |
| BMI (kg/m2) | |||||||||
| 12 months | 34 | 29.6 (6.5) | 17.4–46.7 | 33 | 27.8 (6.5) | 10.7–42.2 | 67 | 28.7 (6.5) | 10.7–46.7 |
The FTND questionnaire produces a score between 0 and 10, where a score of 1–2 indicates low dependence, 3–4 indicates low to moderate dependence, 5–7 indicates moderate dependence and 8–10 indicates high dependence. The FTND score slightly decreased over time for both randomised groups (Table 21). The usual-care group has a lower FTND score than the BSC group at 12 months, but the CIs overlap (Figure 5).
FIGURE 5.
Mean FTND questionnaire score and 95% CI by randomised group.
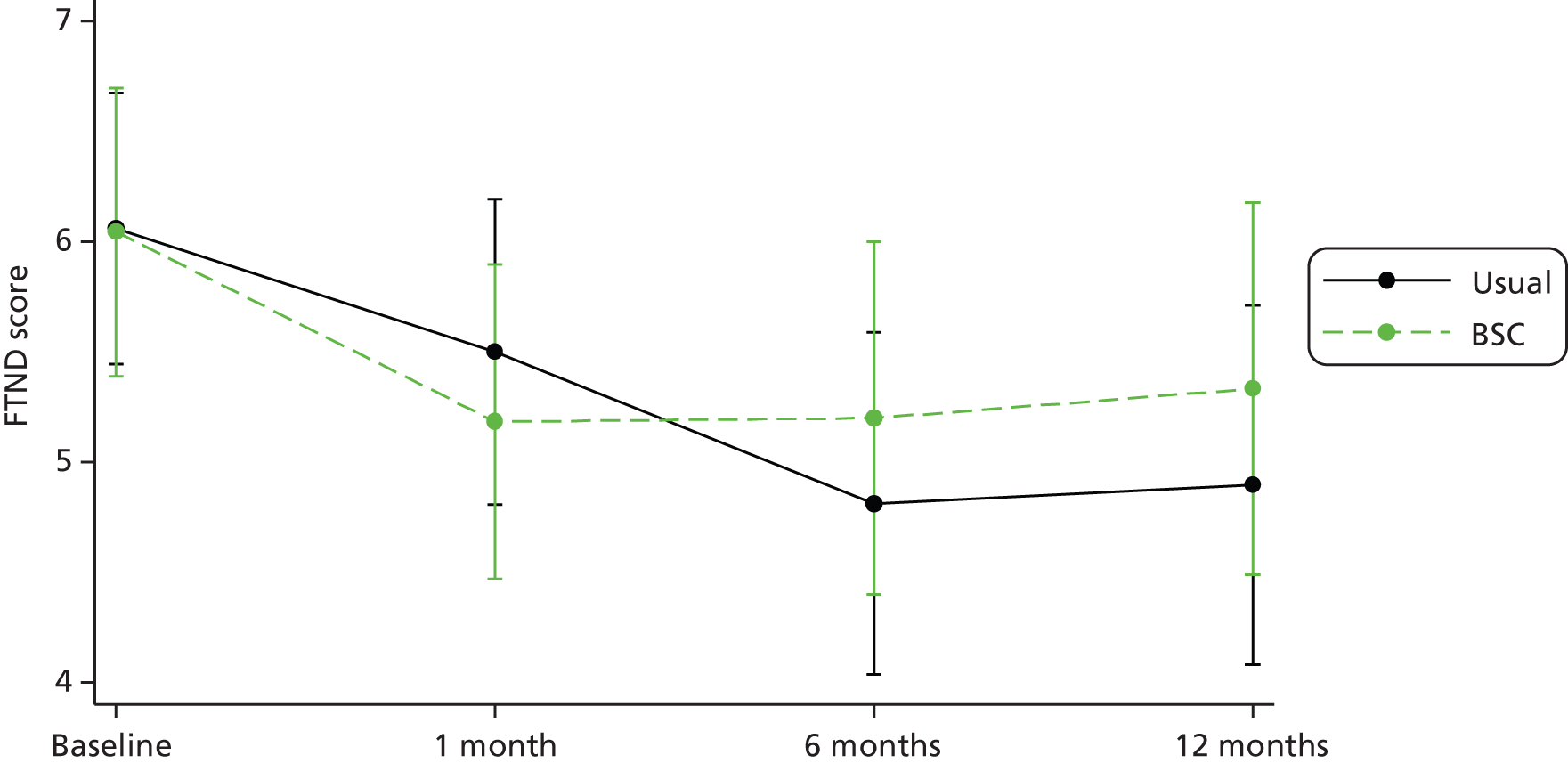
The MTQ questionnaire produces a score between 4 and 19, where a higher score indicates greater motivation to stop smoking. The MTQ score decreases over time in both randomised groups (Figure 6). The MTQ score is higher in the BSC group than in the usual-care group at all three time points and the CIs are not overlapping at 1 month.
FIGURE 6.
Mean MTQ questionnaire score and 95% CI by randomised group.

The PHQ-9 produces a score between 0 and 27, where scores of 5, 10, 15 and 20 are used as cut-off points for mild, moderate, moderately severe and severe depression respectively. The PHQ-9 score is fairly stable over the first 6 months and then increases at 12 months for the BSC group and decreases for the usual-care group (Figure 7). The PHQ-9 score is higher (indicating lowering of mood) in the BSC group than in the usual-care group, but the CIs are overlapping.
FIGURE 7.
Mean PHQ-9 score and 95% CI by randomised group.

The SF-12 physical component score ranges from 0 to 100, where 0 indicates the lowest level of health and 100 indicates the highest level of health measured by the scale. The physical component score appears to be fairly stable over time, increasing slightly at 6 months and then decreasing at 12 months (Figure 8). The physical component score is slightly higher in the BSC group than in the usual-care group at 6 and 12 months, indicating better physical health, but there is very little difference between the two groups.
FIGURE 8.
Mean SF-12 physical component score and 95% CI by randomised group. PCS, physical component score.

The SF-12 mental component score ranges from 0 to 100, where 0 indicates the lowest level of health and 100 indicates the highest level of health measured by the scale. The mental component score appears to be fairly stable over time for the usual-care group and decreases in the BSC group (Figure 9). The mental component score is lower in the BSC group than in the usual-care group, indicating lower composite mental health, but the CIs overlap at all time points.
FIGURE 9.
Mean SF-12 mental component score and 95% CI by randomised group. MCS, mental component score.
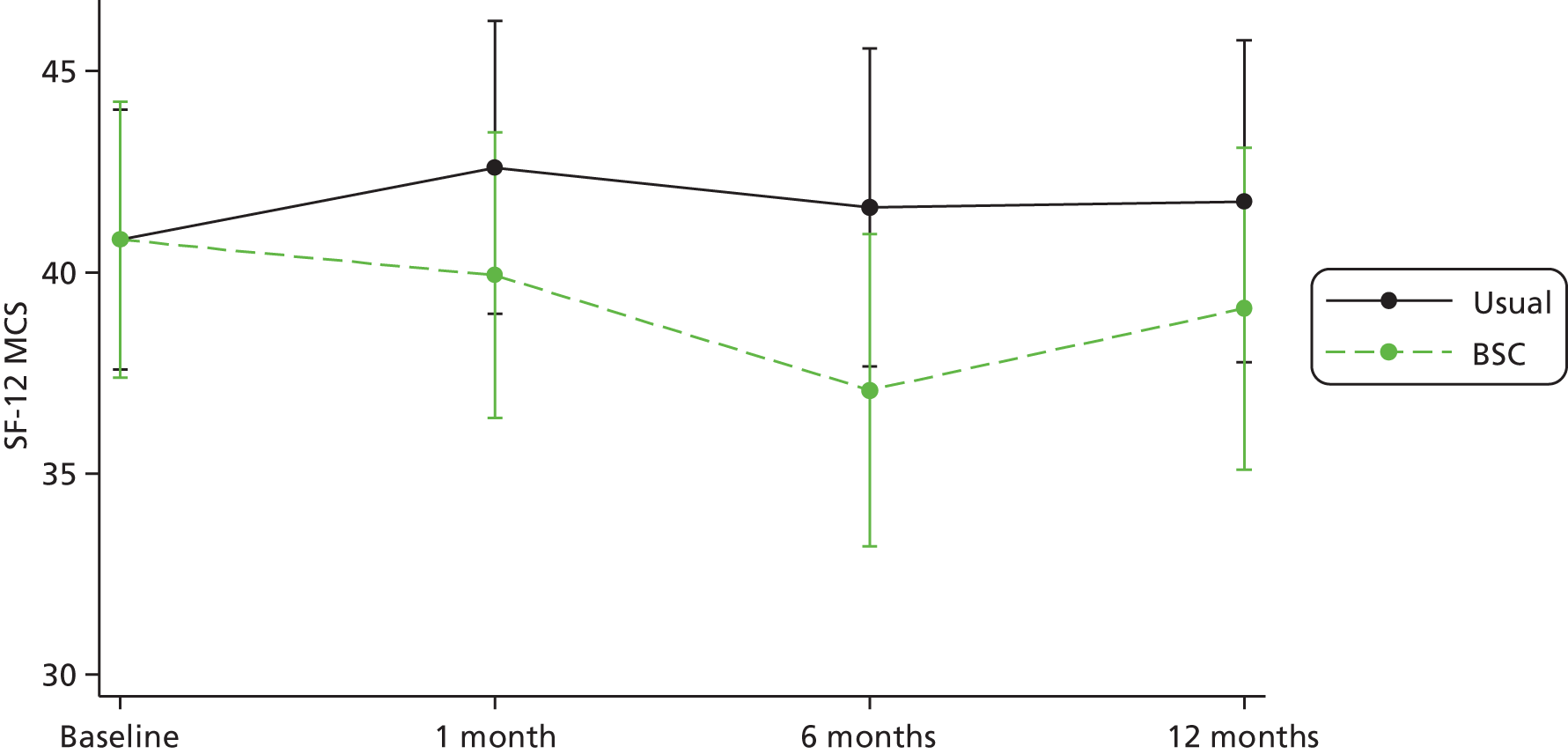
The number of cigarettes smoked per day appears to be fairly stable over time (Figure 10). At 12 months, fewer cigarettes were smoked in the BSC arm than in the usual-care group.
FIGURE 10.
Histograms of number of cigarettes smoked per day by randomised group at 12 months. (a) BSC; and (b) usual GP care.


The number of attempts to quit in the last 6 months appears to decrease at 6 months and increase at 12 months. The number of attempts to quit is greater in the BSC arm than in the usual-care arm at all time-points, including at 12 months (Figure 11).
FIGURE 11.
Histograms of number of attempts to quit by randomised group at 12 months. (a) BSC; and (b) usual GP care.
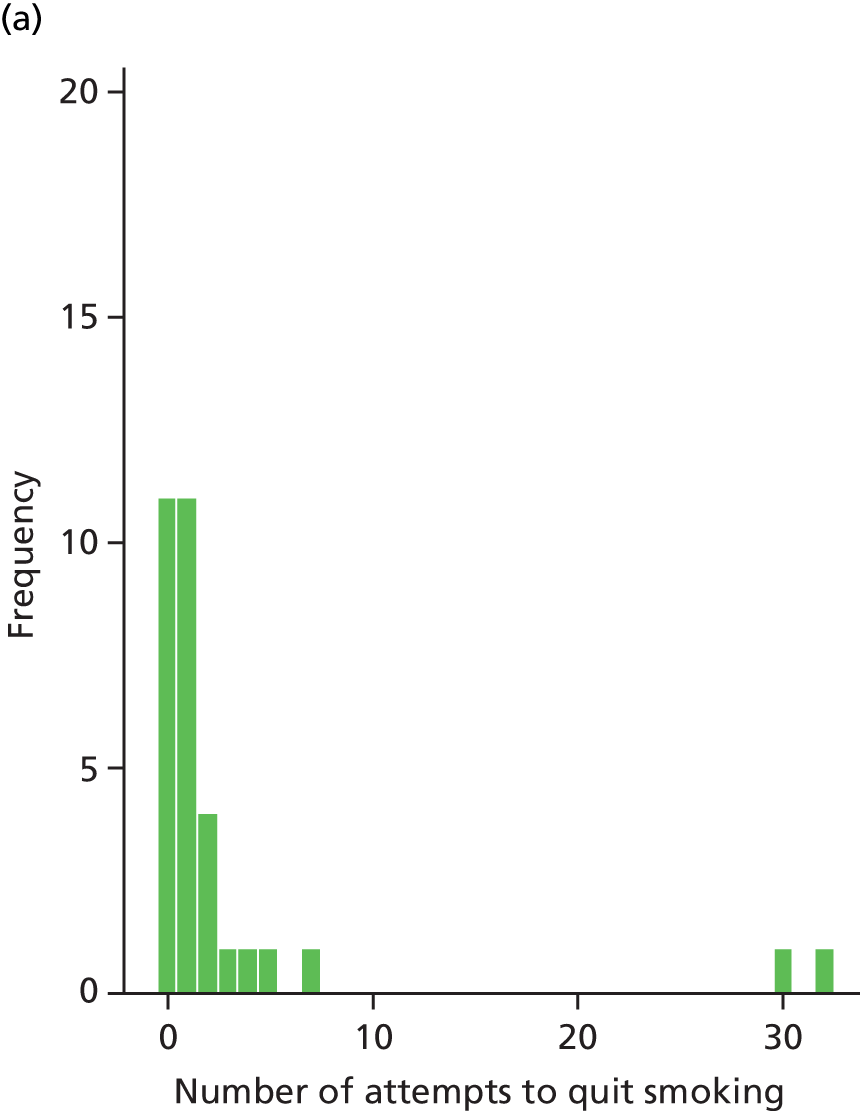
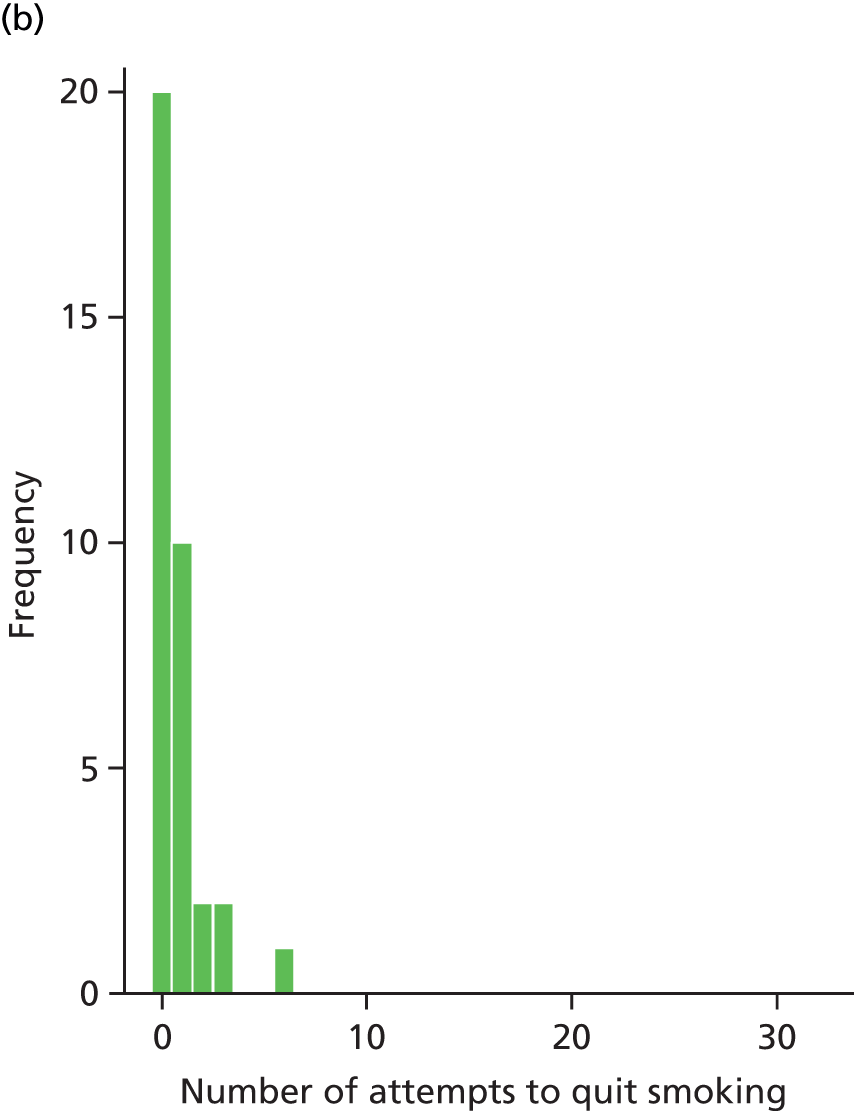
The length of the most recent quit attempt has a large range in the BSC group at 6 and 12 months (Table 22). The length is shorter at 1 month in the BSC group than in the usual-care group, but longer at 6 and 12 months. Figure 12 give details of the percentage of self-reported smoking cessation by randomised group.
FIGURE 12.
Percentage of self-reported smoking cessation and 95% CI by randomised group.
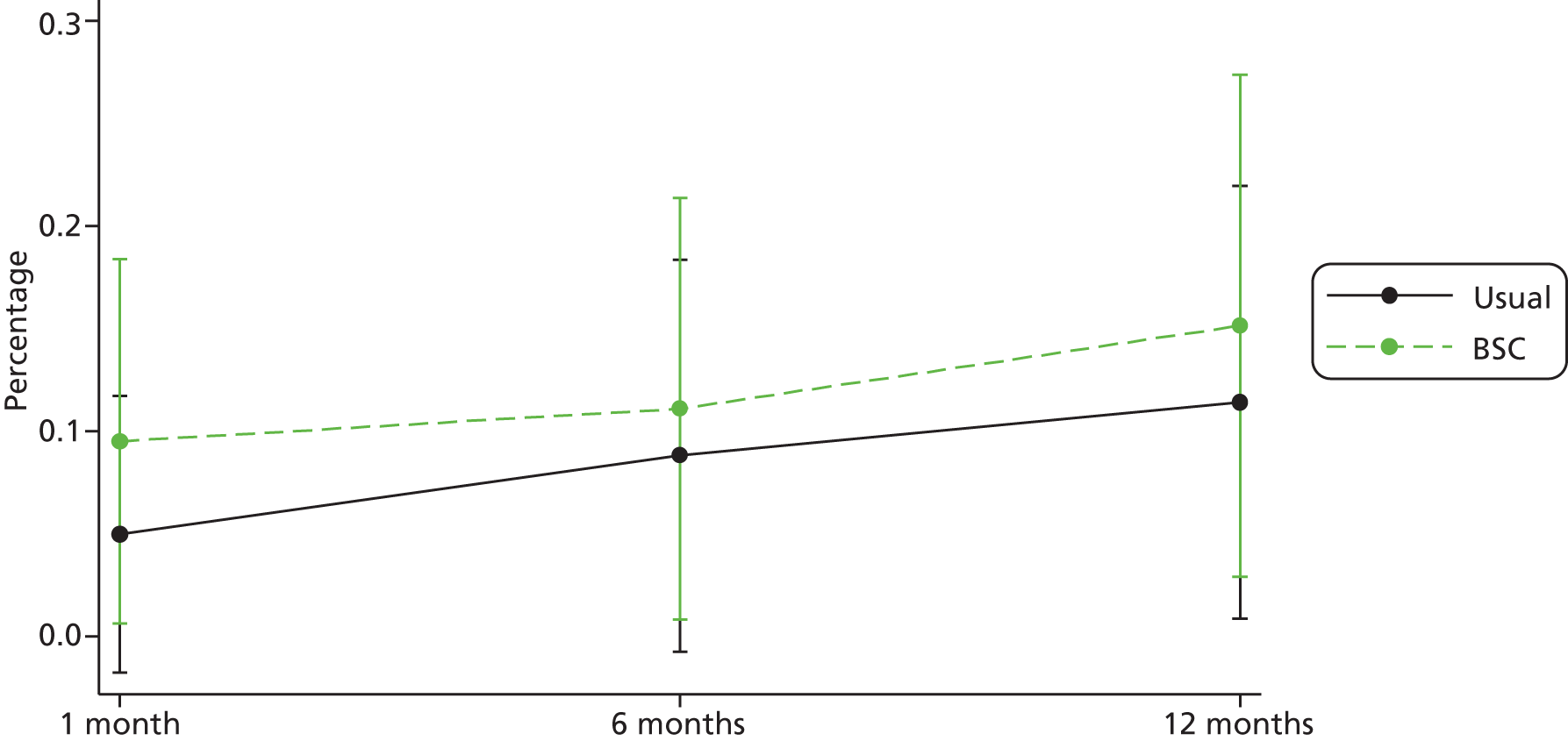
The number of participants self-reporting cessation increased slightly over time (Table 22). The self-reported quit rate was higher in the BSC group than in the usual-care group at all time-points, but the CIs overlap. A cross-tabulation of self-reported quit with CO-measured quit showed moderate agreement between the two measures (κ = 0.48). There were no participants who had quit according to the self-report measure and not quit according to the CO measure, but there were 11 participants who had quit according to the CO measure but did not self-report quitting (Table 23). However, note that a CO measure of < 10 p.p.m. indicates no smoking in the last 8 hours and self-reported quit indicates no smoking within the last week.
| Outcome | CO-measured smoking | CO-measured cessation | Total |
|---|---|---|---|
| Self-reported smoking | 46 | 11 | 57 |
| Self-reported cessation | 0 | 7 | 7 |
| Total | 46 | 18 | 64 |
At 12 months, there were two participants who reported drug use in the BSC arm (6%) and four in the usual-care arm (12%).
Adverse events
There were 21 AEs among 17 participants during the course of the trial. Of these, 11 were classed as serious. More participants in the BSC group (23.9%) than in the usual GP care group (11.8%) experienced one or more AEs. The six AEs that were definitely or probably related to the intervention were all non-serious AEs (Table 24).
| Event type | Relationship to study | Usual GP care | BSC | Total |
|---|---|---|---|---|
| SAE | Definitely related | 0 | 0 | 0 |
| Probably related | 0 | 0 | 0 | |
| Unlikely to be related | 1 | 4 | 5 | |
| Unrelated | 0 | 6 | 6 | |
| Non-serious AE | Definitely related | 1 | 0 | 1 |
| Probably related | 2 | 3 | 5 | |
| Unlikely to be related | 0 | 0 | 0 | |
| Unrelated | 1 | 2 | 3 | |
| Total | 5a | 15 | 20 |
Of the participants who experienced SAEs, eight were hospitalised as a result of deterioration in their mental health; one participant had surgery for an existing problem, two participants were hospitalised for illnesses unlikely to be related to the study and one participant died as a result of lung cancer.
Of the participants reporting AEs, one experienced a deterioration in their mental health that did not require hospitalisation, four participants experienced side effects of NRT products (burning mouth, feeling sleepy, headaches), two participants experienced side effects of smoking cessation medication (headaches, nightmares) and one participant had an unrelated complaint (ear infection).
Summary of findings
At 12 months, 36% of participants had stopped smoking in the BSC group, compared with 23% in the usual-care group. The adjusted OR was 2.9 (95% CI 0.8 to 10.5), indicating a greater likelihood of smoking cessation in the BSC group than the usual-care group, but the difference is not statistically significant.
In terms of smoking-related outcomes at 12 months, the BSC group generally performed better than the usual-care group. At 12 months the FTND questionnaire score, MTQ score, number of cigarettes smoked per day and number of cessation attempts were higher in the BSC group than in the usual-care group. In addition, the duration of cessation was longer in the BSC group.
In terms of mental health outcomes at 12 months, the BSC group had a slightly lower mean mental component score (a measure of psychological well-being incorporating anxiety and depression) and a slightly higher PHQ-9 score (a measure of depression). Mental health outcome – as measured by these metrics – was not different between groups at either 1 or 6 months. In terms of physical health outcomes at 12 months, the BSC group fared better than the usual-care group overall, with slightly higher mean physical component scores and slightly lower mean BMI.
Chapter 5 Health economic analysis
The health economic analysis for the SCIMITAR pilot trial examined the feasibility of a cost-effectiveness analysis in a full trial of supplementing usual care with BSC compared with usual care alone. The costs of both BSC and usual care were identified, measured and valued. The intervention stage consisted of recruitment, follow-up and assessment. Costs were recorded for both intervention and control groups at baseline, 6-month follow-up and 12-month follow-up. European Quality of Life-5 Dimensions (EQ-5D) data were collected at baseline, and at 6- and 12-month follow-up.
Costs
Intervention costs were calculated based on training costs and the costs of delivering the intervention.
Training costs
At the pre-intervention stage, the cost of training MHSCPs was recorded. Training was provided by a NHS Stop Smoking Services trainer and a pharmacist and lasted for 1 day at a cost of £650. Five MHSCPs participating in the trial received training. Based on their wage bands, the cost of 1 day’s training was calculated as £745, in total. The total training cost during this period was estimated to be £1395.
Intervention costs
For the intervention group, the costs of BSC included staff cost of MHSCPs and other relevant expenses (e.g. telephone, travel, CO monitor, etc.).
The individual cost for contacts with MHSCPs was calculated according to the contact and non-contact time recorded on the treatment log. The working time of all MHSCPs was 290 hours in total, including contact and non-contact time. The wage rates for the five MHSCPs were also recorded. The total staff cost was, therefore, estimated to be £5810.
The cost of CO monitors was calculated for 1 year of its 3 life-years. The price of a CO monitor was £196. In the trial, five CO monitors were used by MHSCPs for 12 months. The allocated 1-year cost for five CO monitors was, therefore, £327. The mean recorded expenses including travel and telephone were £55.86 per participant.
The total cost of providing the incremental cost of BSC over and above usual care was £221 (SD £160).
Medication costs (prescriptions)
The details of antipsychosis medication and pharmacotherapy for prescriptions for smoking cessation products during the trial period were extracted from GP records through participating GP surgeries. The corresponding prices were taken from the British National Formulary and prescription cost analysis 2012, where applicable. 64,65 The proportion of participants who had taken antipsychotic medication was similar in each group (50% in the BSC group; 47% in the usual-care group). The mean cost of antipsychotic medication prescription in BSC group was £474, compared with £428 in the usual-care group. During the trial period, GP records showed that 22 participants (48%) in the BSC group had used pharmacotherapy for smoking cessation, whereas only 10 participants (19%) in the usual-care group did so. The mean cost of pharmacotherapy per participant was £62 in the BSC group and £17 in the usual-care group.
Health-care and community services costs
Items on the health-care service use questionnaire were analysed for completeness in order to assess the feasibility of collecting wider service use data from patients in a full trial. Other than data missing because of loss of follow-up, the number of missing data with regard to health-care resources and social service section was relatively low in the pilot trial. Table 25 presents the number of missing data items by question at baseline and follow-ups for each group. Most of the missing data were a consequence of participants not responding to the whole section of the questionnaire.
| Health-care and social service item | Baseline | 6 months | 12 months | ||
|---|---|---|---|---|---|
| BSC | Usual care | BSC | Usual care | ||
| A&E | 0 | 2 | 0 | 0 | 1 |
| Hospital admission | 0 | 2 | 1 | 0 | 1 |
| Outpatient appointment | 0 | 3 | 2 | 0 | 1 |
| Day case/procedure | 0 | 2 | 1 | 0 | 3 |
| 999 emergency ambulance | 0 | 2 | 0 | 0 | 1 |
| Patient transport service | 0 | 1 | 0 | 0 | 1 |
| GP – home | 0 | 3 | 1 | 0 | 1 |
| GP – surgery | 0 | 3 | 1 | 0 | 1 |
| GP – telephone | 0 | 4 | 1 | 0 | 1 |
| Practice nurse | 0 | 3 | 1 | 0 | 1 |
| District nurse, health visitor | 0 | 4 | 1 | 0 | 1 |
| Care co-ordinator, case manager, key worker | 0 | 4 | 1 | 0 | 1 |
| Psychiatrist | 0 | 3 | 1 | 0 | 1 |
| Clinical psychologist | 0 | 4 | 1 | 0 | 1 |
| CPN | 0 | 2 | 1 | 0 | 1 |
| CAMHS worker | 0 | 4 | 1 | 0 | 1 |
| Counsellor | 0 | 4 | 1 | 0 | 2 |
| Family therapist | 0 | 4 | 1 | 0 | 1 |
| Art/drama/music/occupational therapist | 0 | 4 | 1 | 1 | 1 |
| Social worker | 0 | 3 | 1 | 1 | 1 |
| Family support worker | 0 | 4 | 1 | 1 | 1 |
| Accommodation key worker | 0 | 4 | 1 | 1 | 1 |
| Drug/alcohol support worker | 0 | 4 | 1 | 1 | 1 |
| NHS Direct telephone helpline | 0 | 4 | 1 | 1 | 1 |
| Day centre/drop-in centre | 0 | 5 | 1 | 1 | 1 |
Health-care resources and community services used by patients were self-reported using a health economic/service utilisation questionnaire (see Appendix 3). The total volume of usage was calculated by summing the total number of episodes that occurred or the total number of contacts in each group. National average unit costs of corresponding services were applied to quantities recorded to derive the total cost of health-care resources and community services for each trial participant. National average unit costs were extracted from published sources where applicable (Table 26). The quantities of resources used during the trial period are reported in Table 27.
| Resource | Unit cost | Sources |
|---|---|---|
| A&E (admitted) | £147/episode | Department of Health 201266 |
| A&E (not admitted) | £95/episode | Department of Health 201266 |
| Hospital admission | £1236/episode | Department of Health 201266 |
| Outpatient appointment | £131/episode | Department of Health 201266 |
| Day case/procedure | £681/episode | Department of Health 201266 |
| Emergency ambulance | £98/episode | Department of Health 201266 |
| Patient transport service | £34/episode | Department of Health 2011,67 inflated with HCHS Index to 2011–12 prices66 |
| GP – home visit | £4.7/minute × 11.4 minutes/visit = £54/visit | Curtis 201268 |
| GP – surgery | £3.7/minute × 11.7 minutes/visit = £43/visit | Curtis 201268 |
| GP – telephone | £3.7/minute × 7.1 minutes/telephone call = £26/telephone call | Curtis 201268 |
| GP – practice nurse | £45/hour × 15.5 minutes/contact = £12/contact | Curtis 201268 |
| District nurse/health visitor | £39/contact | Department of Health 2012.66 (There was a different unit cost for health visitor: £44/visit. Since we were unable to distinguish the utilisation between the two, we opted for the unit cost for the more specific role) |
| Care co-ordinator/case manager/key worker | £67/hour of face-to-face contact × 1 hour/contact = £67/contact | Curtis 2012.68 (Data were not available on these specific roles. As we learned during the trial, these roles could be held by CPN or other personnel. It was not possible to determine the individuals held these roles. We used unit cost for CPN to estimate the costs. No data available on the average duration per contact, we assumed a 1-hour contact) |
| Community psychiatrist | £319/face-to-face contact | Curtis 201268 |
| Clinical psychologist | £141/contact | Department of Health 201266 |
| CPN | £67/hour of face-to-face contact × 1 hour/contact = £67/contact | Curtis 2012.68 (No data available on the average duration per contact, we assumed a 1-hour contact) |
| CAMHS worker/STAR worker or advocate | £244/contact | Department of Health 2012.66 (Data were only available for CAMHS worker. As we were unable to distinguish the utilisation between the two, we used the unit cost for CAMHS worker to estimate both) |
| Counsellor (NHS, school/college or private) | £59/consultation | Curtis 2012.68 (We were unable to distinguish the utilisation of counsellor from public or private sectors. We estimated the costs using the unit cost in public sector) |
| Family therapist | £66/contact | Department of Health 2012.66 (No data available specifically on family therapist. The unit cost here was estimated based on all community therapy provided by NHS) |
| Art/drama/music/occupational therapist | £71/contact | Department of Health 2012.66 (No data available on art/drama/music therapy but occupational therapy. We used the unit cost for occupational therapy to estimate the costs in the other area) |
| Social worker | £156/hour of face-to-face contact × 1 hour/face-to-face contact = £156/face-to-face contact | Curtis 2012.68 (No data available on the average duration per face-to-face contact. We assumed a 1-hour contact) |
| Family support worker | £49/hour of client-related work × 1 hour of client-related work/contact = £49/contact | Curtis 2012.68 (No data available on the average duration of client-related work for one contact. We assumed a 1-hour workload) |
| Accommodation key worker | £156/hour of face-to-face contact × 1 hour/face-to-face contact = £156/face-to-face contact | Curtis 2012.68 (No data available on accommodation key worker. We used the unit cost for social worker to estimate the cost. No data available on the average duration per face-to-face contact. We assumed a 1-hour contact) |
| Drug and alcohol support worker | £113/contact | Department of Health 201266 |
| Day centre/drop-in centre | £30/session | Curtis 201268 |
| Community pharmacist | £125/hour of direct clinical activities × 5 minutes = £10/contact | Curtis 2012,68 Wu et al. 200969 |
| NHS Stop Smoking Services helpline | £6/telephone call | Wu et al. 2009,69 £5.93/telephone call in 2009, inflated with HCHS Index to 2011–12 prices66 |
| Podiatrist | £40/appointment | Department of Health 201266 |
| Crisis team | £184/contact | Curtis 201268 |
| Dentist | £96/appointment | Department of Health 201266 |
| Therapy centre | £66/contact | Department of Health 2012.66 (Based on the responses, we were not able to determine what therapy this participant went to. The unit cost here was estimated based on all community therapy provided by NHS) |
| Social care support for mental health | £169/week for 10 people | Curtis 201268 |
| Physiotherapist | £46/contact | Department of Health 201266 |
| Daily care | £67/week for 20 people | Curtis 201268 |
| Resources | Number of patients (%) | Total use (number of contacts) | ||
|---|---|---|---|---|
| BSC | Usual care | BSC | Usual care | |
| A&E (admitted) | 6 (13) | 1 (2) | 6 | 1 |
| A&E (not admitted) | 9 (20) | 7 (14) | 12 | 8 |
| Hospital admission | 4 (9) | 2 (4) | 7 | 2 |
| Outpatient appointment | 23 (50) | 20 (39) | 116 | 53 |
| Day case/procedure | 6 (13) | 6 (12) | 8 | 12 |
| Emergency ambulance | 7 (15) | 4 (8) | 9 | 6 |
| Patient Transport Service | 4 (9) | 2 (4) | 111 | 22 |
| GP home visit | 5 (11) | 3 (6) | 9 | 6 |
| GP surgery | 35 (76) | 33 (65) | 233 | 182 |
| GP telephone | 11 (24) | 13 (25) | 31 | 32 |
| GP practice nurse | 28 (61) | 29 (57) | 122 | 131 |
| District nurse/health visitor | 3 (7) | 4 (8) | 10 | 19 |
| Care co-ordinator/case manager/key worker | 19 (41) | 21 (41) | 961 | 243 |
| Community psychiatrist | 26 (57) | 25 (49) | 73 | 87 |
| Clinical psychologist | 7 (15) | 7 (14) | 79 | 205 |
| CPN | 18 (39) | 13 (25) | 278 | 140 |
| CAMHS worker/STAR worker or advocate | 10 (22) | 8 (16) | 745 | 228 |
| Counsellor (NHS, school/college or private) | 3 (7) | 4 (8) | 30 | 78 |
| Family therapist | 2 (4) | 0 (0) | 10 | 0 |
| Art/drama/music/occupational therapist | 3 (7) | 8 (16) | 244 | 222 |
| Social worker | 7 (15) | 4 (8) | 134 | 32 |
| Family support worker | 1 (2) | 0 (0) | 2 | 0 |
| Accommodation key worker | 3 (7) | 1 (2) | 19 | 24 |
| Drug and alcohol support worker | 3 (7) | 4 (8) | 17 | 37 |
| Day centre/drop-in centre | 8 (17) | 8 (16) | 223 | 221 |
| Community pharmacist | 7 (15) | 9 (18) | 17 | 23 |
| NHS Stop Smoking Services helpline | 1 (2) | 3 (6) | 1 | 8 |
| Podiatrist | 1 (2) | 0 (0) | 1 | 0 |
| Crisis team | 1 (2) | 0 (0) | 14 | 0 |
| Dentist | 19 (41) | 19 (37) | 54 | 46 |
| Therapy centre | 0 (0) | 1 (2) | 0 | 108 |
| Social care support for mental health | 1 (2) | 0 (0) | 12 | 0 |
| Physiotherapist | 1 (2) | 1 (2) | 10 | 12 |
| Daily care | 0 (0) | 1 (2) | 0 | 182 |
Total costs
Mean total health-care costs are presented in Table 28 for BSC and usual care. Mean total cost per participant in the BSC group was £12,674, compared with £6867 in the usual-care group. The health-care resources/community services were the main cost driver in both groups, representing the majority (94%) of the costs incurred.
| Cost item | BSC (n = 46) (SD) | Usual care (n = 51) (SD) |
|---|---|---|
| BSC intervention | £221 (£160) | £0 (£0) |
| Antipsychosis medicine prescription | £474 (£913) | £428 (£782) |
| Pharmacotherapy for stop smoking prescription | £62 (£132) | £17 (£60) |
| Health-care resources/community services | £11,917 (£16,601) | £6421 (£6089) |
| Total | £12,674 (£16,595) | £6867 (£6026) |
Mean costs and their SDs were calculated. The SDs demonstrated a high variance among costs, especially the cost of health-care resources/community services. The exact range of each cost component is shown in Table 29. Because of the small sample size in both groups, low-frequency, high-tariff costs can have a significant impact upon the mean cost. One participant in the BSC group stayed in rehabilitation for 6 months immediately prior to the trial and subsequently received multiple services on a daily basis for at least another 6 months. No patients in the usual-care group were observed to have similar episodes; hence, in the small sample, the average in the BSC group may be inflated by this case. This is a common problem in the analysis of pilot trials with small populations, where small sample size, even under random allocation, can result in some baseline imbalances.
| Cost item | BSC (n = 46) | Usual care (n = 51) |
|---|---|---|
| BSC intervention | £37–£824 | – |
| Antipsychosis medicine prescription | £0–£3712 | £0–£3247 |
| Pharmacotherapy for stop smoking prescription | £0–£706 | £0–£300 |
| Health-care resources/community services | £352–£96,896 | £86–£33,217 |
| Total cost | £716–£97,232 | £343–£33,217 |
It should also be noted that the greatest proportion of cost was accounted for by the utilisation of wider health care and social services outside the trial, which was considerably higher in the intervention group (Figure 13). The highest utilisation of health care and social services occurred in the intervention group, and the utilisation level remained higher in the intervention group when the outliers were excluded (as shown by the BSC* line in Figure 13). Although there was a consistent difference in health-care and social services usage between two groups, the limited sample size prevented us from concluding a statistically significant difference.
FIGURE 13.
Mean cost of health care and social services in the last 6 months at baseline, 6 months and 12 months. UC, usual care.
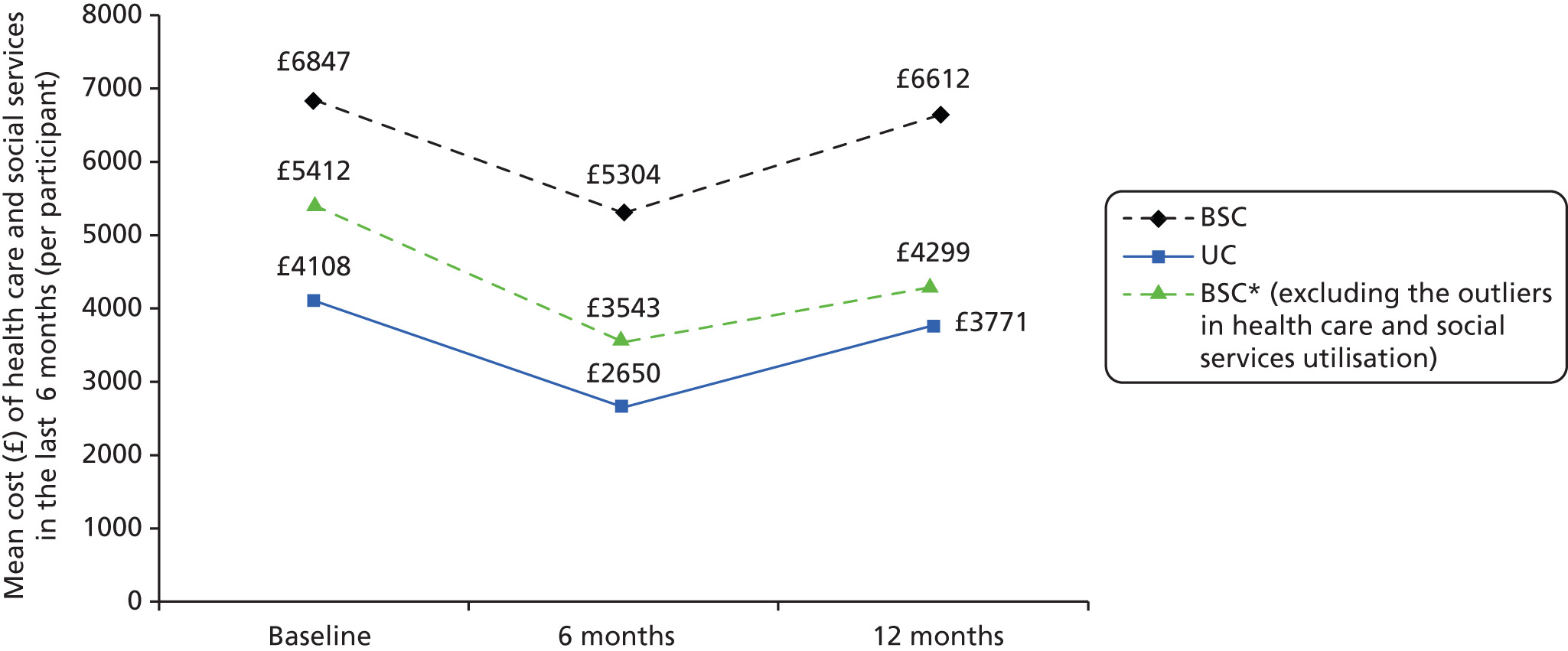
European Quality of Life-5 Dimensions
Quality-adjusted life-years (QALYs) were derived from EQ-5D data by calculating the area under the curve derived from EQ-5D at each time point. 70 EQ-5D data were collected at baseline and at the 1 month, 6 months and 12 months time points. Missing data at each point were replaced with the mean value in the relevant group. Figures 14–16 show the proportion of participants who reported a problem in each individual domain of EQ-5D (i.e. domain score of 2 or 3) over the trial period in each group. Similar patterns in mobility and usual activities were evident in both groups. However, the patterns in other domains were almost opposite between the two groups. During the 12-month trial period, participants in the BSC group gained a mean of 0.65 QALYs (95% CI 0.58 to 0.72 QALYs), while participants in the usual-care group gained, on average, 0.69 QALYs (95% CI 0.63 to 0.75 QALYs).
FIGURE 14.
Mean EQ-5D score at baseline and at 1 month, 6 months and 12 months. UC, usual care.

FIGURE 15.
Proportion of the participants who reported a problem (minor or severe) in each individual domain of EQ-5D: BSC (intervention group).

FIGURE 16.
Proportion of the participants who reported a problem (minor or severe) in each individual domain of EQ-5D: usual care (control group).

We have not undertaken a full incremental cost-effectiveness analysis, as this is a pilot trial and was not powered to detect significant differences in cost-effectiveness. The aim of this pilot is to assess the feasibility of conducting a full economic evaluation and to investigate the completeness of data. We should also note that a 12-month trial may not demonstrate the potential long-term impact on health as well as health-care and social services utilisation. It is likely that cost savings and quality-of-life gains as a result of smoking cessation would extend beyond the 12-month follow-up in this study. 71 Hence, a full-trial analysis would use longer-term modelling to project costs and outcomes beyond 12 months.
Cost-effectiveness
Total health-care and social care costs were combined with the primary outcome of the trial to estimate the cost per quitter at 12 months. As this is a pilot trial, which is not powered to estimate cost-effectiveness, we report a simple incremental cost-effectiveness ratio (ICER) by combining the costs with the number of successful quitters at 12 months; the incremental cost was £58,197 per quitter. This ICER should be treated with caution because of the small sample size and large variance of total cost. In a pilot trial, high-tariff, low-frequency costs can have a large impact on the overall ICER if these high-cost cases fall into a treatment arm by chance. Therefore, the main aim of the economic analysis of this trial has been to pilot questionnaires and assess the feasibility of collecting such data in a larger trial.
Smoking cessation help beyond the trial
Beyond our analysis perspective, 19 participants in the BSC group and 14 participants in the usual-care group used resources other than NHS-funded resources regarding smoking cessation, including other helplines, the internet and self-help booklets. Among these participants, eight in the BSC group used other helplines, ranging from one to nine times during the trial period, while six participants in the usual-care group used other helplines between 1 and 24 times. Similar numbers of participants used the internet in both groups (seven in the BSC group vs. eight in the usual-care group), but the participants in the usual group appeared to use the internet more frequently (one or two times in 12 months in BSC group vs. 2–10 times in 12 months in usual-care group). Nine participants in each group used self-help booklet for advice to stop smoking. Except for one participant in the BSC group who reported using the booklet 40 times during the trial period, the remainder of participants used the self-help booklet fewer than ten times in the same period. These results could indicate an unmet need for smoking cessation support in this population.
The results from self-report in the follow-up questionnaire indicated that in both groups more participants were using smoking cessation products at 12 months than at baseline (36 in the BSC group vs. 21 in the usual-care group), but the proportion in the BSC group remained higher than in the usual-care group (78% vs. 41%). Comparing data extracted from GP records and participants’ self-report demonstrated that, while participants in the BSC group remained more likely to use pharmacotherapy than those in the usual-care group, participants in both groups tended to use pharmacotherapy obtained from other sources in addition to, or instead of, that obtained through GP prescription (Table 30). Considering that the participants in this sample rarely seek help through NHS Stop Smoking Services, it was reasonable to assume that products not covered by GP prescription were purchased over the counter. This also suggests that participants in the usual-care group tended to obtain their products over the counter. Overall, 42% of the pharmacotherapy cost was spent over the counter in the BSC group, whereas 66% was spent over the counter in the usual-care group. Furthermore, the results also indicated that participants in the BSC group were more likely to receive other NRT products (Figure 17). However, this observation was not evident when using self-report information. Although participants in the BSC group appeared to be more likely to receive multiple pharmacotherapies, the range of products used was similar in both groups (Figure 18).
| Trial arm | Pharmacotherapy cost per participant | |
|---|---|---|
| By prescription (SD), recorded by GP surgeries | By prescription and over the counter (SD), reported by participants | |
| BSC | £62 (£132) | £106 (£138) |
| Usual care | £17 (£60) | £50 (£56) |
FIGURE 17.
Number of participants using pharmacotherapy during trial period by GP records, by group and pharmacotherapy. UC, usual care.

FIGURE 18.
Number of participants using pharmacotherapy during trial period by self-report, by group and pharmacotherapy. UC, usual care.

Piloting the health-care and social care service use questionnaire
In order to explore the feasibility and scope of analysis full of a economic evaluation, a questionnaire was used to collect data on health-care resources and community services utilisation. While a comprehensive list of services available was essential to the analysis, the pilot identified several issues when using this questionnaire with this population.
Firstly, by compiling a complete service list, some of the community services were potentially overlapping. For example, the duties of a care co-ordinator, case manager and key worker could be carried out by a community psychiatric nurse (CPN) or a district nurse, since these positions are in some cases interchangeable between different staff members. Listing roles separately appeared to result in confusion and possible double counting. However, a lack of conclusive evidence means that we cannot determine if double counting occurred. Consequently, although we are aware of it, the results reported here were not adjusted for this possibility. It is something which would be addressed in a full trial.
Secondly, the groupings of services highlighted potential issues regarding costs. For instance, a health visitor could be a district nurse or other personnel, depending on the situation. The average cost per health visitor visit is higher than for a district nurse, so further distinctions need to be made. Although it might not cause problems on the participants’ side, the cost cannot be valued properly when the two are listed as one service.
In addition, some of the services are available from both public and private sectors. Without supervision, the nature of the self-report questionnaire might lead to a result that included the private sector, regardless of the intention. As the final two open questions regarding usage of other services indicated, although the questions were clearly listed under community service, some participants’ responses indicated that they might not have a clear idea of the nature of service providers. Some of the services mentioned were apparently not provided by the NHS or social care service, while others were provided by both private and public sector. Without further information, we were unable to determine what proportion of the services, if any, which were provided by public sector.
It should also be noted that, while we attempted to determine pharmacotherapy use from GP records in order to derive more accurate estimates, there were discrepancies between GP records and self-report which could not be fully explained by out-of-pocket purchase. This could result from recall error or participants failing to follow instructions. However, although prescription information collected from GP surgeries is believed to be more reliable, it is unlikely to be replicated in a full trial with a larger sample size. The workload could be too high for GP surgeries to respond.
Because of the nature of this population, any information collected through personal recall could be less reliable than under normal circumstances. Therefore, cost assessment regarding this population should be interpreted with caution.
Summary of findings
The incremental cost of providing the BSC intervention over and above usual care was estimated at £221 (SD £160) per participant. When the wider use of health-care and social care and prescriptions is included, the total cost in the BSC group was £12,674 (SD £16,596) per participant, compared with £6867 (SD £6026) per participant in the usual-care group. However, because of the small sample size, we recommend that these results should be treated with caution as the means are influenced by extreme values. Combining costs with the number of successful quitters at 12 months, the incremental cost was £58,197 per quitter. However, these results are from a pilot trial which is not powered to detect a significant difference from an economic perspective. It is also likely that in the longer term, beyond the 12-month follow-up, cost savings may accrue as a result of successful quits. Furthermore, improvements in health-related quality of life would be expected beyond the trial follow-up, which would be modelled in a full trial.
The pilot trial demonstrates the feasibility of conducting a full economic evaluation in a sufficiently powered trial of BSC over and above usual care. Several issues with regard to questionnaire design have been identified which would improve the accuracy and completeness in the collection of service use data.
Chapter 6 Engagement with the bespoke smoking cessation intervention to patients and professionals
Background/introduction
We explored specific issues of acceptability and engagement with the BSC interventions among patients with SMI and with professionals who delivered the intervention. An understanding of these issues is essential for improving the implementation of a BSC service and informing the design of the intervention in subsequent definitive trials. The aim of the substudy was to qualitatively explore, from both patient and therapist perspectives, perceptions of the need for smoking cessation services for this population and their experience of delivering or receiving the bespoke intervention. In particular, we aimed to explore how the bespoke intervention differed to any previous experiences of smoking cessation in usual care, and to identify barriers and facilitators of implementing or engaging with the intervention in practice.
Methods
In-depth semistructured interviews were conducted with a purposive sample including participants who completed the intervention and those who struggled to engage, and to compare those who sustained engagement with those who struggled or withdrew from treatment, and to identify barriers and facilitators to patient engagement. All participants in the intervention arm who had completed their treatment with the MHSCP or withdrawn from treatment were invited to take part. We did not preselect participants based on any other specific criteria, such as sex or smoking history. Participants who responded by post with an expression of interest or who verbally informed either their MHSCP or the research team that they were interested were contacted by telephone to discuss participation. We performed a comparison of the interviewed sample to the full trial sample on predetermined variables (including age, sex, ethnicity, number of previous quit attempts, smoking history and SMI diagnosis) to determine the representativeness of the interview sample compared with the patient sample as a whole.
We also conducted semistructured interviews with the MHSCPs to gain their perspectives on acceptability and delivery of smoking cessation services for SMI.
In-depth interview topic guides addressed the following issues:
-
Characteristics of the recipients: what are the specific features of SMI that need to be anticipated and accommodated in delivering BSC?
-
Mode and setting of delivery: is BSC best delivered in patients’ homes, GP surgeries or day-hospital settings? Is BSC best delivered face to face, in groups or over the telephone? What is an ideal contact time and number of sessions?
-
Prior experience of smoking cessation, including support received from other primary care or mental health professionals.
-
Acceptability of the intervention to patients, and satisfaction with the BSC, particularly in comparison with previous smoking cessation interventions received.
-
Patients’ engagement with the intervention, with specific reference to barriers and facilitators to working with the MHSCPs.
-
Implementation in routine care, including perceptions of who is best to deliver the BSC and any anticipated barriers to implementation.
Qualitative interviews were held at the end of treatment and ran throughout the duration of the data collection period. An experienced qualitative researcher facilitated all interviews and ethical approval was obtained by the relevant local NHS research ethics committee. Written consent was collected from all of the participants. Participants were asked to consent to the discussions or interviews being recorded and were informed that all identifiable data would be removed once transcribed. Participants were informed that they could remove themselves from the group or stop the interview at any time and did not have to answer any questions they were uncomfortable with.
After completion of the interview, as a token of thanks for their time, the participant was offered a £10 gift voucher as a good-will gesture.
Changes to the original protocol
In the original protocol we intended to interview practice staff who had been involved in the delivery of the intervention (GPs, practice nurses). However, during the study it became apparent that such staff had minimal involvement in the intervention itself and, therefore, we did not interview GPs directly. We did, however, modify the topic guides for both patients and MHSCPs to ask specifically about their interactions with GPs to ensure any relevant issues were captured (and to reconsider the need for interviewing GPs if it became apparent that their involvement was greater than expected, although the interviews confirmed our perceptions that GPs had minimal involvement in delivering or referring to the intervention). The following results are, therefore, from the patient and MHSCP interviews.
Participant characteristics
Interviews took place between August 2012 and January 2013. Thirteen patients were recruited from across the three recruitment sites (five from Manchester, six from York and two from Hull) and three MHSCPs, one from each site, were interviewed.
Comparison of the qualitative subsample to the trial population
Of the 13 patients, two were female (although the trial sample as a whole was 60% male, which suggests that women were under-represented in the qualitative sample.) The average age was 50 years (range 32–68 years). The trial population had smoked for a mean of 27 years and the median number of quit attempts was three; in the qualitative sample, the participants had smoked an average of 32 years and had tried to quit five times, indicating that the smoking history of the qualitative sample is fairly representative of that of the trial population as a whole. All of the participants in the study were white British. Although this group made up the majority of the trial population (85%) this does suggest that further qualitative work may be needed with black and minority ethnic participants to determine if the results are representative. Consistent with the trial population as a whole, the majority of the qualitative sample were unemployed and not seeking work due to ill health. Regarding diagnosis, five of the patients had bipolar disorder, six had schizophrenia (three reported paranoid schizophrenia) and two had experienced depression with psychotic symptoms.
All three MHSCPs were female and white British.
Analysis
Each in-depth interview was digitally recorded and transcribed verbatim. Transcripts were checked and anonymised to remove identifying details.
Transcripts were read independently by two researchers and analysed using the constant comparison (CC) method. The CC method aims to inductively develop themes through categorising and coding data and exploring connections between them, repeating the cycle across the data set until theoretical saturation is achieved. Emergent themes were discussed and verified with a third researcher. Analysis was completed prior to the quantitative analysis being complete, and was therefore blind to study outcome.
The MHSCP transcripts were initially analysed independently from the patient transcripts, but the analysis was combined when preliminary readings suggested consensus in core themes across both the patient and professional data sets and also indicated that novel insights could be synthesised across the two samples to provide a holistic picture of the intervention. Similarly, we did not analyse the data of engaged and disengaged patients separately, partly because only two of the participants were formally considered to have ‘disengaged’ (having been discharged because of a lack of contact with the MHSCP), but also because analysis suggested this separation did not reflect differences in experienced barriers and facilitators as we has assumed it would. Both the disengaged patients reported positive experiences with the intervention itself, suggesting other circumstances may have contributed to their disengagement, and even patients considered to have engaged with treatment reported difficulties maintaining motivation and planning future sessions. This suggests that engagement was less reflective of the acceptability of the intervention and more indicative of the chaotic nature of this population, which was a recurrent theme in the data (theme 3 below).
Main findings
We identified four primary themes. Themes 1 and 2 reflected the lack of support for smoking cessation in current services and, consequently, the perceived benefits of the BSC intervention which was more tailored to this population. Themes 3 and 4 reflect challenges and barriers reported by patients and professionals, including difficulties sustaining engagement and difficulties liaising with primary care.
Theme 1: NHS smoking cessation services were not responsive to the needs of people with severe mental ill health
Interviews revealed the perceived unsuitability of generic stop smoking services for patients with SMI, emphasising the need for sensitised intervention, which was reported by both patients and professionals. This included issues around the lack of support for smoking cessation (both implicit and explicit) from other health professionals in primary care and mental health services, and concerns about stigma when accessing generic services
I’ve actually had a doctor turn round and say, after quite an episode, which was quite a lengthy episode, and I talked about giving up, he said, oh no, you don’t want to be giving up at the moment. So it was kind of like a medical permission to carry on smoking . . . The doctor might say, as he said, terrible thing smoking. But never actually say, you should give up, and I’ll refer you. I’ve had to ask for that. The last thing you want to think about is giving up, that sort of comment comes across.
Y1085
I did have one chap that came, which was … and he’d been to normal standard NHS services, and he’d been to a group, and he had a diagnosis of bipolar, and . . . she’d given them all a prescription request sheet for Champix. And he went to see his GP and his GP said, ‘I’m not giving you Champix, you’ve got bipolar.’ So he came back next week, and he was the only one in the room that hadn’t been given the Champix. And he said he felt really awkward. ‘How do I explain why I couldn’t have the Champix?’ He said, ‘I didn’t want to tell them it’s because I had a mental health problem.’
MHSCP1
Theme 2: participants valued the mental health background of smoking cessation practitioners and the flexibility of the bespoke intervention
Perceived benefits included the mental health background of the MHSCP and the greater flexibility of the intervention. The mental health background of the MHSCP was considered essential, especially when contrasted with the generic smoking cessation services available. Patients reported that MHSCPs had a of better understanding of their condition and also adopted a more supportive, collaborative relationship with them.
It wasn’t just a stop smoking clinic for Tom, Dick and Harry, she understood the mental health side, which is obviously a big concern . . . Because I wouldn’t go to a normal – because I’m frightened . . . Well [the MHSCP] knows what I’ve got. Whereas if you go to a normal stop smoking thing and they know you’ve got mental health problems then it’s stigma isn’t it? . . . you’ve got to trust the person who you’re talking to and be comfortable with them, especially on mental health issues, because if you’re talking to somebody who doesn’t understand then you think well, you’re not on the same wavelength as me, you don’t understand me.
H1098
Second, the flexibility and personalisation of the intervention were valued, in terms of where and when sessions were held, allowing for both cutting down and quit targets, and tailoring the intervention depending on the patients’ condition and circumstances.
It was individual to the person really, flexible to their needs, like seeing them when they wanted within reason and then not putting too much pressure on them . . . just tailored to the person see what works for each person . . . It was interesting how each person was completely different what they wanted to do and what they wanted from me and how motivated they were and everything . . . you can’t just say ‘I’ve got to read this script.’
MHSCP2
Theme 3: there were additional challenges for people with severe mental ill health with regard to smoking cessation
Both patients and professionals acknowledged the challenges of smoking cessation in this population; patients reported that motivation could waver and that having help available at the right time was important. MHSCPs noted that this patient group struggles with planning and organisation. Proactive follow-up was necessary to try to sustain patient engagement, although this could be problematic, particularly if patients suffered an acute episode.
It [starting the intervention] was over Christmas, and before Christmas I really, really wanted to quit, and I was ready to quit. But when I saw [the MHSCP], I don’t think I was ready to quit . . . When things get a bit rough, I start smoking. And that really [happened] actually about a couple of months before I started seeing [the MHSCP]. If I’d have started seeing her in the first place, it would have been a different tale. I would have quit, and I know I would. Timing, timing. Getting the timing right.
Y1084
She disengaged and was texting me saying, ‘Oh I’ve not done too well this week so can you come next week?’ And I’d go and she wouldn’t be there . . even if I could say only one of my clients attended every appointment [but] none of them did . . . I think it’s reflective of the patient group really . . . they’re just so chaotic.
MHSCP2
Theme 4: the need for integration of smoking cessation services between primary and secondary care
Potential barriers to implementation were also evident. MHSCPs reported that it would be better if the smoking cessation could be integrated with existing mental health support but questioned whether or not resources in terms of time and cost would be available to support this and also if the workers would prioritise smoking cessation given other demands.
You could put this work into main stream, you know, into CPNs work, but I don’t know that everybody would do it, that’s the thing, and how much time and attention they would give, because you need to be quite focused.
MHSCP3
Whether if they said to people in CMHTs just get somebody who does a specific smoking cessation speciality I don’t know if it would work because say at [Community Residential Unit] they had a smoking cessation worker there who I met and I’m like ‘Well why am I here like?’ And it’s because her role just was eclipsed and she was just doing the general support work. So you’d have to have a specific . . . you’d have to be quite regimented in doing your work.
MHSCP2
Both patients and professionals referred to difficulties encountered by MHSCPs when liaising with primary care services, specifically when trying to organise NRT for patients.
If the GP wouldn’t prescribe . . . then you’re chasing it up and then when the client goes it’s not there and they get annoyed that they’ve wasted a visit to the doctors. Some GP surgeries refused to do it on my recommendation and had to see the client. So then the client had to make an appointment with the GP which just didn’t happen. So then I’d say well I’ll give you a letter to take with the doc . . . and then they lose the letter.
MHSCP2
I would have said, if anything, my own doctor’s let [the MHSCP] down because she would put things in to request for things that I needed, but they weren’t coming through quick enough . . . I think we used to sometimes do texts, can I just check, have you spoken to my doctor? And she’d say, I’ve written the letter. And I’d go across and try and pick up my prescription, and it just wouldn’t be ready.
H1066
Summary of themes
Overall, the findings of the qualitative substudy support the need for a sensitised and BSC intervention for this population. Providing this through mental health trained workers was perceived to be most appropriate by both patients and professionals. Challenges to be addressed include difficulties in helping patients to manage their cessation plans, and better communication or integration with primary care to organise prescribing. Implementation in routine care settings, particularly considering who would take on the MHSCP role and cost implications of this, should also be explored.
Limitations
Only three MHSCPs could be interviewed given that this was a pilot study. Future work should explore whether or not larger cohorts of MHSCPs report similar experiences. Although the patient sample is fairly small, the participants interviewed included patients with both bipolar disorder and schizophrenia and were largely representative of the overall trial population. The consistency in emergent themes across the patients also supports the representativeness of the results. However, women and black and minority ethnic participants were under-represented and further research is necessary to explore the acceptability of the BSC intervention to this population.
Chapter 7 Discussion
This report presents the results of the first UK trial of a BSC intervention designed specifically for people with severe mental ill health. The SCIMITAR trial was commissioned by the National Institute for Health Research Health Technology Assessment programme in view of the clinical need of this population and the widening health inequalities which exist in relation to smoking and smoking-related illness. The SCIMITAR trial is a pilot study, which now paves the way for a fully powered trial to assess clinical effectiveness and cost-effectiveness. The SCIMITAR programme has followed a developmental pathway to produce a feasible intervention to the point at which this can now be evaluated within the context of a definitive trial. We have first drawn upon existing evidence (taken from high-quality systematic reviews) of ‘what works’ in helping people to cut down or quit smoking. 25 We have also conducted a systematic review of ‘what works’ in relation to people with SMI, and have shown that the same pharmacological and behavioural approaches to smoking cessation are effective amongst people with SMI as with the rest of the population. 44 Despite this evidence, it is clear that people with SMI do not access conventional NHS quit smoking services, and a coherent response is to design a service and intervention that ensures that evidence-supported pharmacotherapies and BCTs are applied with specific reference to the needs of people with SMI.
The BSC intervention at the centre of the SCIMITAR pilot trial was designed to address the unmet needs and barriers to accessing smoking cessation interventions for this population. We will now review the main findings and address the main objectives of the SCIMITAR pilot trial in turn before considering whether or not the SCIMITAR trial can now be scaled up as a fully powered RCT.
Main findings
The main finding of the SCIMITAR trial is that smoking cessation can be achieved among people with SMI and that the use of a BSC intervention increased the chances of sustained quitting, as estimated by a biochemically verified outcome measure (exhaled CO). 72 The observed odds of successful quitting at 12 months were almost three times higher among those who received BSC (OR 2.9, 95% CI 0.8 to 10.5), although this value was calculated using a small sample and, therefore, requires cautious interpretation.
A range of secondary outcomes were also measured and there was a general direction of effect in favour of BSC in relation to the (1) number of cigarettes smoked, (2) number of quit attempts, (3) reported nicotine dependence and (4) reported MTQ. Taken together, the positive overall primary outcome and consistency of direction of effect among primary and secondary outcomes (reduced number of cigarettes smoked, increased number of quit attempts, increased MTQ) add weight to the hypothesis that BSC is effective for this group. The consistency of findings from the pilot trial, alongside systematic review evidence44 represents an accumulation of evidence. Ultimately the clinical effectiveness of a BSC intervention can really be tested only within a fully powered RCT. The pilot trial also found some evidence of deterioration in mental health in the intervention group compared with the usual-care group. This finding is not consistent with other evidence, where smoking cessation tends to improve mental health. 73 However, this does indicate that mood should be monitored in clinical practice and the safety of smoking cessation should be tested in a fully powered trial. The various findings and experience from conducting a pilot trial will now be considered in turn in order to inform the design of a fully powered definitive RCT.
Is it possible to recruit people with severe mental ill health to a trial of a smoking cessation intervention?
At the outset of the SCIMITAR pilot trial there was genuine uncertainty as to whether or not sufficient people with SMI would express an interest in a smoking cessation intervention and agree to undergo randomisation. An important finding from the SCIMITAR pilot trial is that it was possible to recruit a mixed population of people with SMI and successfully randomise almost 100 participants to the trial. This finding is in line with research evidence that shows that the proportion of smokers with SMI who express a desire to cut down or quit smoking is now broadly in line with expressions of desire to cut down/quit within the general population.
The participants recruited to the SCIMITAR pilot trial were largely middle-aged people of both sexes with heavy tobacco addiction and long smoking histories (mean duration of smoking 27 years). The participants were recruited from both primary and secondary care settings. A successful fully powered trial would, therefore, be able to recruit participants from both of these settings. The availability of primary care computer records allowed GPs to write to their patients directly and offer them the opportunity to participate in a trial. The experience of recruiting in secondary care was more mixed and the geographical areas which were the most successful in recruiting participants were those where there were well-integrated teams of research workers and good engagement between the Mental Health Research Network (MHRN) and local NHS services. The resources required to populate a fully powered trial can be estimated from the current study. We anticipate that more than 100 general practices would need to be enlisted, with direct GP approaches to potentially eligible patients. With respect to secondary care, we judge that four mental health trusts would need to be enlisted, with preference given to those trusts where there is an embedded model of research support offered by a research network such as that currently offered by the MHRN.
Is the treatment acceptable to participants and health professionals from primary and secondary care?
The SCIMITAR trial found that participants who underwent randomisation generally engaged with BSC services. In the qualitative evaluation of the bespoke intervention it was found that participants valued the fact that smoking cessation therapists were drawn from staff working within mental health services. Smoking cessation practitioners had a familiarity with SMI and the specific needs of that group, and this was seen as a positive aspect of the intervention by participants. There was a coherent theme within the qualitative interviews that people with SMI felt excluded from conventional smoking cessation services and that the less-flexible and time-limited nature of NHS Stop Smoking Services were seen as barriers to successful treatment. By addressing these factors, the SMI participants felt that their smoking was more readily addressed and they felt less stigmatised than might have been the case in conventional services. Participants were attracted to a service which offered the prospect of cutting down prior to quitting, and they appreciated the opportunity to receive NRT prior to setting a quit date. In the control group, there was a lack of engagement with conventional NHS quit smoking services despite control participants being given smoking cessation literature and encouraged to visit their GP or NHS quit smoking service.
Engagement with the bespoke intervention was good: 41 out of 46 participants attended at least one session and the mean number of sessions was 10. The intervention was clearly more intensive than that which would be offered in conventional NHS services and the overall cost of BSC was £283 (£221 practitioner costs and £62 medication costs).
Within the SCIMITAR pilot trial participants were encouraged to choose an appropriate form of smoking cessation medication in collaboration with their GP. The mainstay of treatment was NRT and only two participants were prescribed varenicline. None was prescribed bupropion. Qualitative interview data showed that GPs were very reluctant to prescribe smoking cessation products other than NRT. It was also noted that participants experienced difficulties in obtaining supplies of NRT from their GPs, and in a future trial it might be more acceptable for participants to be prescribed NRT medication by their secondary care provider. This recommendation is in line with 2013 NICE guidance on smoking cessation provisions for people with mental ill health, which recommends that mental health services make this provision for smokers who use their services. 50
In a definitive trial we would propose that the mainstay of treatment should be NRT and that this be provided within mental health services rather than from the GP (when participants are in receipt of secondary mental health care).
Is it possible to achieve follow-up of people with severe mental ill health within a trial?
The SCIMITAR pilot trial sought to establish the feasibility of follow-up in both the short and longer term (12 months). Smoking cessation trials conventionally focus on short-term quit rates and it is important to also judge the longer-term impact of programmes. The SCIMITAR pilot trial showed that biologically verified long-term outcomes could be achieved and it was shown that 70% of participants then agreed to giving a CO measurement. The importance of using a biologically verified smoking cessation outcome was also underlined when biologically verified and self-report data were compared. There was moderate concordance between gold standard CO smoking status and self-report with a kappa value of 0.48.
In addition, several participants who reported being smokers were found not to have smoked when their CO was tested. This point prevalence non-smoking status is a potentially less rigorous measure of abstinence, and a future trial should consider a higher level of evidence such that non-smoking participants must be self-reported non-smokers and must be abstinent on CO testing. This is in line with the Russell standards of reporting.
In moving forward to a definitive trial it will be important to record a Russell-standard outcome at all follow-up points, in line with evidence-supported recommendation on the standards of smoking cessation trials. 72 An additional recommendation might be that a small financial payment may improve follow-up rates at all time points and that 90% follow-up could be achieved by this means.
How large would a definitive trial need to be?
The SCMITAR pilot trial has established the important parameters to allow the sample size to be calculated for a definitive trial. A fully definitive trial with sufficient power to detect a 15% reduction in smoking would require a sample size of 296 participants (baseline quit rate 23%, two-sided, α = 0.05, β = 80%). Firstly, we have established the baseline 12-month quit rate for smokers with SMI. This quit rate lies within the range of quit rates expected in non-SMI populations, and allows a reasonable control quit rate to be set for a power calculation. Secondly, the SCIMITAR trial provides a range of plausible effect sizes which are broadly in line with the quit rates seen in a review of smoking cessation interventions in SMI44 (pooled relative risk estimates 2.74, 95% CI 1.10 to 6.81), and are also in line with effect sizes observed in non-SMI populations for a NRT-based intervention. 41
A full trial with sufficient power to detect a relative increase in quitting of 1.7-fold would require a sample size of 260 participants (baseline quit rate 23%, two-sided, α = 0.05, β = 80%). However, a control group quit rate of 23% may be considered high, so we instead consider a more plausible value of 20%. In this case, we would require a sample size of 314 participants (again with RR = 1.7, α = 0.05, β = 0.8). All sample sizes would need to be inflated to allow for 15–30% loss to follow-up.
Limitations of the Smoking Cessation Intervention for Serious Mental Ill Health Trial pilot study
The SCIMITAR pilot study had insufficient power to detect a plausible effect size, but as a pilot trial was not designed to detect a difference.
We found that there was a withdrawal rate of 15% from the trial, making the trial potentially open to biases of unrepresentative participants in the follow-up and differential attrition between arms. The dropout rate was higher in the intervention than in the control arm, and a future trial will have to ensure that follow-up and retention are maximised. Nevertheless, the withdrawal rate was lower than that seen in comparable trials in SMI populations44 and is, in part, a feature of the nature of the population within the trial, who are prone to periods of illness that in turn might impact on motivation and ability to remain in longer-term follow-up studies. A further 15% of participants did not complete a 12-month follow-up, meaning that 70% of participants completed their 12-month follow-up. Initial follow-up at 12 months was lower than we had hoped for; therefore, we initiated a more robust method of following people up at 12 months. This involved telephoning participants at different times of the day and in some cases working with the participant’s care co-ordinator to arrange times for follow-up visits to be completed. The implementation of this more robust method led to an increase in our 12-follow-up rate; hence, we would use these strategies in a future trial to ensure a higher level of follow-up.
A third limitation is the absence of a biologically verified quit outcome at 1 and 6 months, and a future trial should seek to capture short- and medium-term quit with a CO-verified measure. The methods to collect this outcome and to maximise follow-up could replicate those used at 12 months.
Finally, this was a pragmatic evaluation of a complex intervention: combining case management, pharmacological treatment, behavioural support and evidence-supported behaviour change techniques. We have described the developmental phase of this complex intervention. However, it is not possible within the context of a pragmatic health technology assessment trial (either pilot or fully powered) to disaggregate the relative contributions of these elements. This remains a topic for future research if the clinical effectiveness of bespoke cessation is ultimately demonstrated in a fully powered trial.
Conclusions
The SCIMITAR pilot trial has shown that it is possible to recruit to a trial of a BSC intervention for people with SMI. Follow-up in 70% of participants has been achieved using a biologically verified measure smoking status at 12 months. The preliminary estimates of clinical effectiveness are supportive of BSC across a range of primary and secondary outcomes. The clinical effectiveness and cost-effectiveness of bespoke smoking can now be established in a fully powered trial. A recruitment strategy for a fully powered trial should enlist participants from primary and secondary care and the SCIMITAR pilot trial has delineated the relative strengths and practical limitations of approaches in both of these settings. Both approaches should be used in a definitive trial.
Implications for health care
Although it is important to ensure that there is equitable provision of smoking cessation services for all populations, it would be premature to invest in BSC services without the results of a definitive clinical trial.
Recommendations for future research
A definitive trial is now needed to establish the clinical effectiveness and cost-effectiveness of BSC services for people with SMI. The SCMITAR trial forms a template for this trial, with some modification which follow from the experience of conducting this pilot trial.
Acknowledgements
We would like to thank the participants for taking part in the trial, the GPs and secondary and tertiary care staff for recruiting participants to the study and completing trial documentation, the Trial Steering Committee and Data Monitoring and Ethics Committee members for the overseeing the study, and Helen Hartley of the Leeds Stop Smoking Services for proving training for the MHSCP.
We are also grateful to Dr Fabiana Lorencatto and Dr Andy McEwan, Director of the NHS Centre for Smoking Cessation and Training, for their advice on the use of evidence-supported smoking cessation interventions and their adaptation to people with SMI.
The SCIMITAR trial benefited from the support of the north-east and north-west hubs of the MHRN, and the North East Yorks and North Lincs Comprehensive Local Research Network (NEYNL CLRN).
This trial is dedicated to the memory of Professor Helen Lester (1961–2013), and is a celebration of her work and contribution to the care and well-being of people with SMI. This was her abiding passion and will be her lasting contribution.
Contribution of authors
Simon Gilbody and Mei-See Man wrote the original protocol.
Simon Gilbody, Jinshou Li, Susan Michie and Tim Bradshaw were co-applicants on the Health Technology Assessment application and refined the protocol.
Simon Gilbody was the chief investigator and oversaw the study.
Mei-See Man, Natasha Mitchell and Emily Peckham were trial mangers.
Taeko Becque designed and conducted the clinical analysis.
Jinshou Li and Steve Parrott designed and undertook the economic analysis.
The writing team consisted of Taeko Becque, Simon Gilbody, Sarah Knowles, Mei-See Man, Emily Peckham, Claire Planner and Charles Shepherd who drafted the report.
Collaborations
The SCIMITAR collaborators (current and past) are:
Katie Atherton, Taeko Becque, Tim J Bradshaw, Helen Cox, Ben Cross, Jane Dallender, Emma Davies, Rhian Gabe, Linda Gask, Simon Gilbody, Edward Greenward, Kerin Hannon, Laura Hermann, Catherine Hewitt, Hayley Jackson, Sarah Knowles, Helen Lester, Jinshou Li, Andy McEwen, Mei-See Man, Sarah Mercer, Susan Michie, Natasha Mitchell, Ann Mortimer, Emily Peckham, Claire Planner, David Richards, Kath Richardson, Charles Shepherd, Maggie Stronach, David Torgerson, Muhammad Usman, Ian Watt and Robert West.
Contributions of collaborators
Catherine Hewitt, David Richards, David Torgerson and Ian Watt were co-applicants on the Health Technology Assessment application and refined the protocol.
Rhian Gabe and Catherine Hewitt designed the clinical analysis.
Catherine Hewitt oversaw the conduct of the analysis.
Trial Steering Committee members
Professor Ann McNeil (independent chairperson), Professor of Tobacco Addiction, King’s College London, London, UK.
Dr David Shiers, retired GP, North Staffordshire.
Dr Tom Hughes, Consultant psychiatrist, Leeds and York Partnership NHS Foundation Trust, Leeds, UK.
Data Monitoring and Ethics Committee members
Dr David Osborn (independent chairperson), Reader in Community Psychiatric Epidemiology, Mental Health Science Unit, University College London, London, UK.
Dr Elena Ratschen, Lecturer in Epidemiology/Tobacco Control, Faculty of Medicine and Health Sciences, University of Nottingham, Nottingham, UK.
Dr Richard Emsley, Lecturer in Biostatistics, Centre for Biostatistics, University of Manchester, Manchester, UK.
Patient and public involvement in research
The SCIMITAR trial benefited from involvement of users of mental health services and carers of people with SMI throughout the research period. Our Trial Steering Committee included representation from a carer. Our protocol and study materials were scrutinised and supported by users and carers in the north-west of England and by the user and carer groups of our local MHRN.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
Publications
Bradshaw T, Davies E, Stronach M, Hermann L. Support for people with serious mental illness to cut down or stop smoking. Mental Health Pract 2014;17:14–20. URL: http://dx.doi.org/10.7748/mhp2014.03.17.6.14.e890
Gilbody S, Peckham E, Man M-S, Mitchell N, Li J, Becque T, et al. Bespoke smoking cessation for people with severe mental ill health (SCIMITAR): a pilot randomised controlled trial. Lancet Psychiatry 2015; in press. http://dx.doi.org/10.1016/S2215-0366(15)00091-7
References
- McDonald C. Cigarette smoking in patients with schizophrenia. Br J Psychiatry 2000;176:596-7. http://dx.doi.org/10.1192/bjp.176.6.596-b.
- Olincy A, Young DA, Freedman R. Increased levels of the nicotine metabolite cotinine in schizophrenic smokers compared to other smokers. Biol Psychiatry 1997;42:1-5. http://dx.doi.org/10.1016/S0006-3223(96)00302-2.
- McCreadie R, Kelly C. Patients with schizophrenia who smoke. Br J Psychiatry 2000;176. http://dx.doi.org/10.1192/bjp.176.2.109.
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness a population-based prevalence study. J Am Med Assoc 2000;284:2606-10. http://dx.doi.org/10.1001/jama.284.20.2606.
- Phelan M, Stradins L, Morrison S. Physical health of people with severe mental illness: can be improved if primary care and mental health professionals pay attention to it. BMJ 2001;322:443-4. http://dx.doi.org/10.1136/bmj.322.7284.443.
- Weiser M, Reichenberg A, Grotto I. Higher rates of cigarette smoking in male adolescents before the onset of schizophrenia: a historical-prospective cohort study. Am J Psychiatry 2004;161:1219-23. http://dx.doi.org/10.1176/appi.ajp.161.7.1219.
- Williams JM, Ziedonis DM, Abanyie F. Increased nicotine and cotinine levels in smokers with schizophrenia and schizoaffective disorder is not a metabolic effect. Schizophr Res 2005;79:323-35. http://dx.doi.org/10.1016/j.schres.2005.04.016.
- Himelhoch S, Daumit G. To whom do psychiatrists offer smoking-cessation counseling?. Am J Psychiatry 2003;160:2228-30. http://dx.doi.org/10.1176/appi.ajp.160.12.2228.
- Baker A, Richmond R, Haile M, Lewin T, Carr R. Characteristics of smokers with a psychotic disorder and implications for smoking interventions. Psychiatry Res 2006;150:141-52. http://dx.doi.org/10.1016/j.psychres.2006.05.021.
- Carosella AM, Ossip-Klein DJ, Owens CA. Smoking attitudes, beliefs, and readiness to change among acute and long term care inpatients with psychiatric diagnoses. Addict Behav 1999;24:331-4. http://dx.doi.org/10.1016/S0306-4603(98)00096-3.
- Esterberg ML, Compton ML. Smoking behaviour in persons with a schizophrenia-spectrum disorder: a qualitative investigation of the transtheoretical model. Soc Sci Med 2005;61:293-30. http://dx.doi.org/10.1016/j.socscimed.2004.11.057.
- Addington J, el-Guebaly N, Addington D, Hodgins D. Readiness to stop smoking in schizophrenia. Can J Psychiatry 1997;42:49-52.
- Sacco KA, Termine A, Seyal A. Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia: involvement of nicotinic receptor mechanisms. Arch Gen Psychiatry 2005;62:649-59. http://dx.doi.org/10.1001/archpsyc.62.6.649.
- Brown S, Kim M, Mitchell C, Inskip H. Twenty-five year mortality of a community cohort with schizophrenia. Br J Psychiatry 2010;196:116-21. http://dx.doi.org/10.1192/bjp.bp.109.067512.
- Kendrick T, Burns T, Freeling P. Provision of care to general practice patients with disabling long-term mental illness: a survey in 16 practices. Br J Gen Pract 1994;44:301-5.
- Burns T, Cohen A. Items of service payments for general practitioner care of severely mentally ill patients: does the money matter?. Br J Gen Pract 1998;48:1415-16.
- Jochelson K, Majrowski B. Clearing the Air: Debating Smoke-free Policies in Psychiatric Units. London: The King’s Fund; 2006.
- Siru R, Hulse GK, Tait RJ. Assessing motivation to quit smoking in people with mental illness: a review. Addiction 2009;104:719-33. http://dx.doi.org/10.1111/j.1360-0443.2009.02545.x.
- Ratschen E, Britton J, Doody GA, Leonardi-Bee J, McNeill A. Tobacco dependence, treatment and smoke-free policies: a survey of mental health professionals’ knowledge and attitudes. Gen Hosp Psych 2009;31:576-82. http://dx.doi.org/10.1016/j.genhosppsych.2009.08.003.
- Lester H. Shared care for people with mental illness: a GP’s perspective. Adv Psychiatr Treat 2005;11:133-9. http://dx.doi.org/10.1192/apt.11.2.133.
- NICE Public Health Guidance 48, Smoking Cessation in Secondary Care in Acute, Maternity and Mental Health Services. London: NICE; 2013.
- West R, Corrigall WA. Understanding Nicotine and Tobacco Addiction. Chichester: John Wiley & Sons; 2006.
- Balfour DJK. The neurobiology of tobacco dependence: A preclinical perspective on the role of the dopamine projections to the nucleus. Nicotine Tob Res 2004;6:899-912. http://dx.doi.org/10.1080/14622200412331324965.
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res 2007;9:315-27. http://dx.doi.org/10.1080/14622200701188919.
- Silagy C, Lancaster T, Stead L, Mant D, Fowler G. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 2004;3.
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smoke. Addiction 2004;99:29-38. http://dx.doi.org/10.1111/j.1360-0443.2004.00540.x.
- Lancaster T, Stead L. Physician advice for smoking cessation. Cochrane Database Syst Rev 2004;4.
- Lancaster T, Stead LF. Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev 2005;2. http://dx.doi.org/10.1002/14651858.CD001292.pub2.
- Lancaster T, Stead LF. Group behaviour therapy programmes for smoking cessation. Cochrane Database Syst Rev 2005;2. http://dx.doi.org/10.1002/14651858.CD001007.pub2.
- Lancaster T, Stead LF. Self-help interventions for smoking cessation. Cochrane Database Syst Rev 2005;3. http://dx.doi.org/10.1002/14651858.CD001118.pub2.
- Lancaster T, Perera R, Stead LF. Telephone counselling for smoking cessation. Cochrane Database Syst Rev 2006;3. http://dx.doi.org/10.1002/14651858.CD002850.pub2.
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev 2007;3. http://dx.doi.org/10.1002/14651858.CD006103.pub3.
- Hughes JR, Stead LF, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev 2007;1. http://dx.doi.org/10.1002/14651858.CD000031.pub3.
- Stead LF, Perera R, Lancaster T. Telephone counselling for smoking cessation (Review). Cochrane Database Syst Rev 2007;3.
- Brief Interventions and Referral for Smoking Cessation in Primary Care and Other Settings: NICE Public Health Intervention Guidance. London: NICE; 2006.
- Smoking Cessation Services, Including the Use of Pharmacotherapies, in Primary Care, Pharmacies, Local Authorities and Workplaces. London: NICE; 2007.
- Wang D, Connock M, Barton P, Fry-Smith A, Aveyard P, Moore D. ‘Cut down to quit’ with nicotine replacement therapies in smoking cessation: a systematic review of effectiveness and economic analysis. Health Technol Assess 2008;12.
- Pfizer Labs . Medication Guide Chantix® 2012. www.fda.gov/downloads/Drugs/DrugSafety/ucm088569.pdf (accessed 19 May 2014).
- Schroeder SA. A 51-year-old woman with bipolar disorder who wants to quit smoking. JAMA 2009;301:522-31. http://dx.doi.org/10.1001/jama.2008.982.
- McEwen A, West R, McRobbie H. Effectiveness of specialist group treatment for smoking cessation vs. one-to-one treatment in primary care. Addict Behav 2006;31:1650-60. http://dx.doi.org/10.1016/j.addbeh.2005.12.014.
- Aveyard P, West R. Managing smoking cessation. BMJ 2007;335:37-41. http://dx.doi.org/10.1136/bmj.39252.591806.47.
- McEwen A, Hajek P, McRobbie H, West R. Smoking Cessation Manual: A Guide for Councellors and Practitioners. London: Blackwell Publishing Ltd; 2006.
- Primary Care Guidance on Smoking and Mental Health. London: The forum for mental health in primary care; 2008.
- Banham L, Gilbody S. Smoking cessation in severe mental illness: what works?. Addiction 2010;105:1176-89.
- Tsoi DT, Porwal M, Webster AC. Interventions for smoking cessation and reduction in individuals with schizophrenia. Cochrane Database Syst Rev 2010;6. http://dx.doi.org/10.1002/14651858.CD007253.pub2.
- Revisions to the GMS Contract, 2006/7. Delivering Investment in General Practice. London: British Medical Association; 2006.
- The Practice can Produce a Register of People with Schizophrenia, Bipolar Disorder and Other Psychoses. Leeds: NHS Information Centre for Health and Social Care; 2012.
- The Percentage of Patients with Schizophrenia, Bipolar Affective Disorder and Other Psychoses with a Review Recorded in the Preceding 15 Months. In the Review there Should be Evidence that the Patient has been Offered Routine Health Promotion and Prevention Advice Appropriate to their Age, Gender and Health Status. Leeds: NHS Information Centre for Health and Social Care; 2011.
- Mental Health Act. London: Her Majesty’s Stationery Office; 1983.
- Smoking Cessation in Secondary Care: Acute, Maternity and Mental Health Services. London: NICE; 2013.
- Campbell M, Fitzpatrick R, Haines A, Kinmonth AL, Sandercock P, Spiegelhalter D, et al. Framework for design and evaluation of complex interventions to improve health. BMJ 2000;321:694-6. http://dx.doi.org/10.1136/bmj.321.7262.694.
- Craig P, Dieppe P, Macintyre S, Mitchie S, Nazareth I, Petticrew M. Developing and evaluating complex interventions: the new Medical Research Council guidance. BMJ 2008;337:979-83. http://dx.doi.org/10.1136/bmj.a1655.
- Cohen A, Hove M. Physical Health of the Severe and Enduring Mentally Ill. London: Sainsbury Centre for Mental Health; 2001.
- Mental Capacity Act. London: The Stationery Office; 2005.
- Revisions to the GMS Contract, 2008/9. Delivering Investment in General Practice. London: British Medical Association; 2008.
- Smoking Cessation Services in Primary Care, Pharmacies, Local Authories and Workplaces, Particularly for Manual Working Groups, Pregnant Women and Hard to Reach Communities. London: NICE; 2008.
- Hartley H. ‘The Tip of the Iceberg’ – A Review of Smoking Cessation Work: Mental Health and Learning Disabilities. Leeds: Leeds Community Healthcare, Leeds NHS; 2009.
- NHS Stop Smoking Services: Service and Monitoring Guide 2009/10. London: DH; 2009.
- Abraham C, Michie S. A taxonomy of behaviour change techniques used in interventions. Health Psychol 2008;27:379-87. http://dx.doi.org/10.1037/0278-6133.27.3.379.
- Michie S, Hyder N, Walia A, West R. Development of a taxonomy of behaviour change techniques used in individual behavioural support for smoking cessation. Addict Behav 2011;36:315-19. http://dx.doi.org/10.1016/j.addbeh.2010.11.016.
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. Addiction 1991;86:1119-27. http://dx.doi.org/10.1111/j.1360-0443.1991.tb01879.x.
- Gilbody S, Richards D, Barkham M. Diagnosing depression in primary care using self-completed instruments: a UK validation of the PHQ9 and CORE-OM. Br J Gen Pract 2007;57:650-2.
- Ware JE, Kosinski M, Dewey JE. How to Score Version Two of the SF-36 Health Survey. Lincoln, RI: Quality Metric Inc.; 2000.
- British National Formulary. No. 63. London: BMA and RPS; 2012.
- Prescription Cost Analysis England 2012. Leeds: Health and Social Care Information Centre; 2013.
- Reference Costs 2011–12. London: DH; 2012.
- Reference Costs 2009–10. London: DH; 2011.
- Curtis L. Unit Costs of Health and Social Care 2012. University of Kent: Personal Social Services Research Unit; 2012.
- Wu Q, Parrott S, Godfrey C, Gilbert H, Nazareth I, Leurent B, et al. Cost-Effectiveness of computer-tailored Smoking Cessation Advice in Primary carE: a randomised trial (ESCAPE). Nicotine Tob Res 2014;16:270-8. http://dx.doi.org/10.1093/ntr/ntt136.
- Richardson G, Manca A. Calculation of quality adjusted life years in the published literature: a review of methodology and transparency. Health Econ 2004;13:1203-10. http://dx.doi.org/10.1002/hec.901.
- Smoking and Mental Health: A Report by the Tobacco Advisory Group. London: Royal College of Physicians; 2013.
- West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction 2005;100:299-303. http://dx.doi.org/10.1111/j.1360-0443.2004.00995.x.
- Taylor G, McNeill A, Girling A, Farley A, Lindson-Hawley N, Aveyard P. Change in mental health after smoking cessation: systematic review and meta-analysis. BMJ 2014;348. http://dx.doi.org/10.1136/bmj.g1151.
Appendix 1 Regulatory approvals
| Trust | Research and development approval granted |
|---|---|
| NHS East Riding of Yorkshire | 31 January 2011 |
| NHS Hull | 10 February 2011 |
| Humber NHS Foundation Trust | 12 January 2011 |
| NHS North Yorkshire and York | 20 January 2011 |
| Greater Manchester West Mental Health NHS Foundation Trust | 7 January 2011 |
| Manchester Primary Care Trust | 6 December 2010 |
| Manchester Mental Health and Social Care Trust | 17 January 2011 |
| Salford Primary Care Trust | 19 January 2011 |
| Stockport Primary Care Trust | 8 December 2010 |
| NHS Lincolnshire and North East Lincolnshire Care Trust Plus | 21 March 2012 |
| Tees Esk and Wear Valleys NHS Foundation Trust | 23 December 2011 |
| NAViGO Health and Social Care Community Interest Company | 10 January 2012 |
Appendix 2 Patient information sheets and consent form
Appendix 3 Data collection forms
Appendix 4 Advertising materials

Appendix 5 Flow chart for Smoking Cessation Intervention for Serious Mental Ill Health Trial
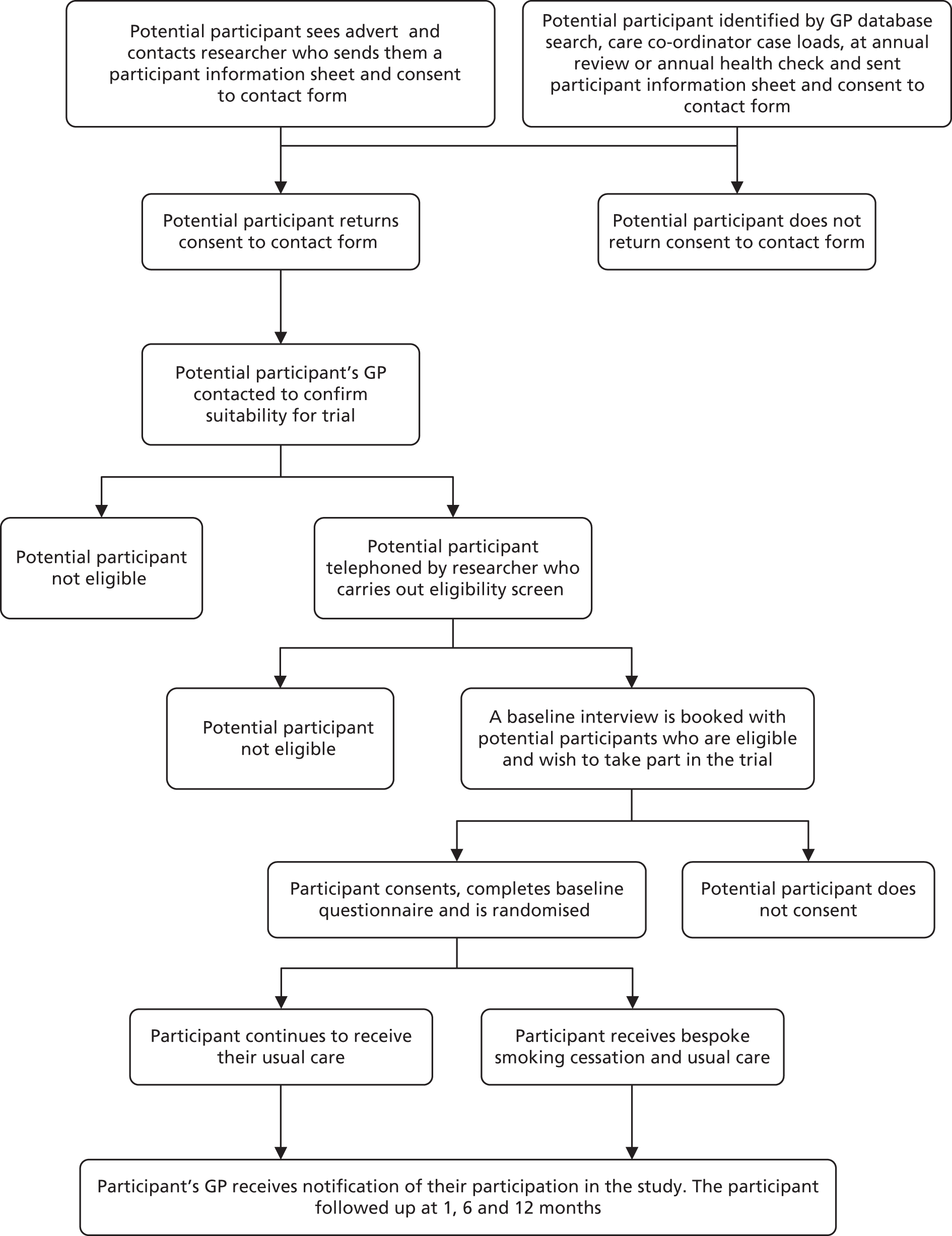
Appendix 7 Qualitative interviews: participant information sheets
List of abbreviations
- AE
- adverse event
- BCT
- behaviour change technique
- BMI
- body mass index
- BSC
- bespoke smoking cessation
- CI
- confidence interval
- CMHT
- Community Mental Health Team
- CPA
- Care Programme Approach
- CPN
- community psychiatric nurse
- CO
- carbon monoxide
- CONSORT
- Consolidated Standards of Reporting Trials
- DSM
- Diagnostic and Statistical Manual of Mental Disorders
- EQ-5D
- European Quality of Life-5 Dimensions
- FTND
- Fagerstrom Test for Nicotine Dependence
- GCSE
- General Certificate of Secondary Education
- GMS
- General Medical Services
- GP
- general practitioner
- ICD
- International Classification of Diseases
- ICER
- incremental cost-effectiveness ratio
- MHRN
- Mental Health Research Network
- MHSCP
- mental health smoking cessation practitioner
- MTQ
- motivation to quit
- NICE
- National Institute for Health and Care Excellence
- NRT
- nicotine replacement therapy
- OR
- odds ratio
- PHQ-9
- Patient Health Questionnaire-9 items
- PI
- principal investigator
- QALY
- quality-adjusted life-year
- QOF
- Quality and Outcomes Framework
- RCT
- randomised controlled trial
- SAE
- serious adverse event
- SCIMITAR
- Smoking Cessation Intervention for Serious Mental Ill Health Trial
- SD
- standard deviation
- SF-12
- Short Form questionnaire-12 items
- SMI
- severe mental ill health