Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 06/45/02. The contractual start date was in June 2008. The draft report began editorial review in September 2013 and was accepted for publication in June 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Bruce Campbell, Paul Bachoo, Ian Chetter, Michael Gough, Jonothan Earnshaw, Tim Lees, Julian Scott and Sara A Baker declare that they receive direct payments in private practice for undertaking treatment of varicose veins using one or more of the treatments examined in the CLASS trial.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Brittenden et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Introduction
Health Technology Assessment-commissioned call
The original application for this study was submitted in 2006 in response to a Health Technology Assessment (HTA) programme-commissioned call (06/45) for studies involving foam sclerotherapy. The call specified a primary outcome of quality of life (QoL) at 6 months. As a result of this call, the Comparison of LAser, Surgery and foam Sclerotherapy (CLASS) trial was funded. This first chapter reflects the NHS practice and the evidence available at that time. The second chapter discusses changes in NHS practice and relevant literature published since 2006.
The burden of the problem
The treatment of patients with varicose veins results in a considerable workload and financial burden to the NHS. Visible varicose veins occur in up to 40% of men and 32% of women. 1 This resulted in approximately 37,500 operations being performed in the year April 2005 to March 2006 in England alone. 2 Approximately 20% of these operations are for recurrent varicose veins. 3 Varicose veins may result in pain, discomfort, itchiness and skin changes.
Throughout the UK prior to 2006, there were considerable variations in access to treatment for uncomplicated varicose veins. This may have been due to a belief on the part of those commissioning services, and some vascular surgeons, that it was a cosmetic procedure. 4 This was based on the results of a community-based study which showed no relationship between the presence of varicose veins, reflux in the main truncal veins and symptoms. 5 However, several studies had shown that many patients with varicose veins had reduced QoL, which was improved following treatment. 6–9 A randomised controlled trial (RCT) of surgery versus conservative management of patients with uncomplicated varicose veins estimated that the incremental cost per quality-adjusted life-year (QALY) gained was £4682, with a 70% probability that the cost per QALY would be lower than the National Institute for Health and Care Excellence (NICE) threshold of £20,000. 10
Treatment options
At that time, the established mainstay of treatment for incompetent varicose veins was surgery in the form of saphenofemoral junction (SFJ) ligation or small saphenous vein (SSV) ligation, stripping and multiple phlebectomies of non-trunk varicosities. Evidence at the time suggested a recurrence rate at 5 years for this kind of conventional surgery of 32% for great saphenous veins (GSVs) and 50% for small saphenous veins. 3
From the time of their introduction around 2000, foam sclerotherapy, endovenous laser ablation (EVLA) and radiofrequency ablation (RFA) had emerged as possible alternative treatment options that could be carried out under local anaesthetic. Foam sclerotherapy, EVLA and RFA aim to reduce the surgical trauma, bruising, scarring and time off work associated with conventional surgery. By 2006, NICE had published interventional procedures guidance on all these procedures, stating that there was adequate evidence on their safety and efficacy for use in the NHS. 11–13 These newer interventions had the potential to increase throughput of varicose vein patients without the need for expensive operating theatre facilities. However, this benefit may be offset by the need for the patient to return for further treatment.
In 2006, there were only two completed RCTs comparing the newer treatments against surgery, both with limited follow-up. One had been published,14 whereas the other, carried out by one of the CLASS co-applicants, had not. 15 These studies are discussed below (see Foam sclerotherapy and Endovenous laser ablation). Critics of the newer procedures pointed to the unknown effect of not treating tributaries at the saphenopopliteal junction and SFJ (an accepted principle of surgery aimed at minimising recurrence) and the need for several treatment sessions compared with ‘one-stop’ surgery.
Despite uncertainty about clinical effectiveness and cost-effectiveness, the use of these newer treatment options was increasing in the UK as alternatives to conventional surgery. A survey in 2006 of members of the Vascular Surgical Society of Great Britain and Ireland and the Venous Forum of the Royal Society of Medicine revealed that the vast majority of surgeons offered conventional surgery to NHS patients, with 27% of surgeons offering foam sclerotherapy, 19% offering EVLA and 3% offering RFA. 16 The following sections describe each of these treatments.
Foam sclerotherapy
Foam sclerotherapy is a development of conventional liquid sclerotherapy, aimed at more extensive and reliable ablation of veins through a process of chemical phlebitis. In 2006, use of foam sclerotherapy represented an ‘off-licence’ use of the licensed sclerosant. Several different liquid sclerosants of varying concentrations were being mixed with air to produce foam. The use of foam rather than the liquid sclerosant allows increased contact with the endothelium, and less mixing and dilution with venous blood. However, the foam could vary in consistency, which may affect efficacy. 17 Foam sclerotherapy induces irritation of the endothelium, leading to thrombosis.
A systematic review of the safety and efficacy of foam found that foam appeared efficacious in terms of obliterating the main trunk veins,18 but more than one treatment session may be required to achieve this. In a series of 500 patients, Cabrera19 achieved obliteration of the GSV in 81% of cases; 86% of patients achieved this after one injection, while 11% required two injections and 4% required a third. Other published data indicated that only 43% of patients undergoing foam sclerotherapy were adequately treated in a single treatment, with 48% requiring two sessions and 9% more sessions. 20 Across the studies included in the review, the median rate of recurrence or development of new varicose veins up to 10 years ranged from 3% to 28%, but the risk of recurrence or development of new veins was not significantly different to that of comparator treatments. 18 The authors concluded that there was insufficient evidence to compare the effectiveness of foam sclerotherapy reliably with other minimally invasive therapies or surgery. 18
In 2006, there was only one RCT of a commercial preparation for foam sclerotherapy [Varisolve® polidocanol microfoam (BTG International, London, UK)], which was a licensed product in which sclerosant was mixed with gas (oxygen and carbon dioxide). This was a three-arm study of 710 patients, which compared Varisolve® against either sclerotherapy (liquid or investigator-generated foam) or conventional surgery (ligation and stripping of the GSV). 14 No differences were detected in the primary outcome of technical success (ablation of the GSV) at 3 months. At 12 months, technical success was slightly higher in the surgery than in the Varisolve® group (86% vs. 79%, p = 0.11). Following Varisolve® foam sclerotherapy, patients required a median of 2 days to return to ‘normal activities’ compared with 13 days following surgery. QoL was not assessed. No RCTs were identified comparing foam sclerotherapy with EVLA.
The main safety concern regarding foam sclerotherapy was the potential for the foam to enter the deep venous system, with the risk of deep-vein thrombosis (DVT), and also to enter the systemic circulation, so reaching the heart and possibly the eye or brain via an atrial septal defect, which is present in 25% of the population. As a result, the NICE guidance for foam sclerotherapy recommended special arrangements for consent, audit and research,13 and limits were recommended on the amount of foam injected per session,21 necessitating additional treatment sessions to deal with non-truncal varicosities. In the systematic review, the incidence of DVT following treatment varied from 0.3% to 3%, while transient visual disturbance occurred in up to 2.8% of patients and transient ischaemic attack in 0% to 0.3% of patients. 18 The review reported one case of ischaemic stroke occurring immediately after injection, with partial recovery at 3 months; this occurred in a patient with a patent foramen ovale. 18 Other potential adverse events include thrombophlebitis (15–58%); early skin discolouration over the treated vein (11–50%); skin necrosis (0.01–0.9%); ulceration (0–7%); and allergy (0.3%). 18
Endovenous laser ablation
Endovenous laser ablation results in thermal ablation of the truncal veins. Most studies describing EVLA had used either 810- or 940-nm diode lasers based on a haemoglobin absorption peak to red/infrared light of 800–1000 nm. 22 The heat generated by the laser was believed to result in thermal damage to the endothelium and subendothelial layer, resulting in focal coagulative necrosis and shrinkage and leading to thrombotic occlusion of the vein. 23 However, histological studies at 3 and 6 months following EVLA indicate failure of endothelial regeneration and progressive damage to the muscle layers of the vein wall, resulting in further shrinkage and occlusion. 24 Studies had shown that between 30% and 99% of patients receiving EVLA require subsequent treatment for non-trunk varicosities. 22
It had been shown that successful occlusion was dependent on the energy used and could be achieved in all veins treated with ≥ 70 J/cm. 25 A RCT carried out by one of the CLASS co-investigators reported on 118 patients randomised to EVLA or surgery. 15 At 3-months follow-up, abolition of reflux was achieved in 98% of EVLA and 92% of surgical patients. EVLA patients had a quicker return to normal activities and work (p = 0.01). Improvements in the Aberdeen Varicose Vein Questionnaire (AVVQ) score, a disease-specific quality of life instrument, were similar in both groups. At 1 year follow-up, there was recanalisation in a minority of the EVLA-treated GSVs, but SFJ reflux remained abolished in 86% of patients who were available for follow-up.
A systematic review26 assessed the effectiveness and safety of EVLA in 13 case series involving 1289 patients (1631 limbs) with duplex-proven primary venous reflux; the mean length of follow-up ranged from 1 to 19 months. EVLA was effective in the short term, with occlusion of the GSV occurring in 88–100% of limbs. Mundy et al. 26 concluded that EVLA appeared to be safe, although there were two reported cases of incorrect positioning of the laser (within the deep venous system), which produced no long-term complications, and one reported DVT. Other reported complications of EVLA included superficial laser burns in 5% of patients in one study which used a very high laser energy; ecchymosis or skin discolouration (23–100% of limbs), which was generally self limiting; phlebitis in 1.6% of limbs; and saphenous paraesthesia in 1–36.5% of limbs. 26 The Australian Medical Services Advisory Committee, in its 2003 assessment report, concluded that EVLA and conventional surgery were similar in terms of safety. 27
The Comparison of LAser, Surgery and foam Sclerotherapy trial
It was against this background of continuing uncertainty about the relative clinical effectiveness and cost-effectiveness of foam sclerotherapy compared with surgery that the National Institute for Health Research (NIHR) HTA programme commissioned a call and the CLASS trial was funded. The CLASS trial was an 11-centre, three-arm comparison of foam sclerotherapy, EVLA and surgery, comparing the relative clinical effectiveness and cost-effectiveness of the three procedures. We chose to include EVLA in addition to foam sclerotherapy in our application because EVLA and foam were the most commonly used minimally invasive treatment options within the NHS at that time. We did not include RFA as, at the time of applying for funding, this technique was more costly and less suited to local anaesthesia on account of the contact time required between the probe and vein endothelium. However, developments since then have made RFA faster and suitable to be performed under a local anaesthetic. EVLA and RFA are now considered to be comparable techniques in terms of outcome. 28
Since the start of the CLASS trial, a further five RCTs comparing foam sclerotherapy against surgery and/or EVLA have been published,29–33 and also 10 RCTs which have compared EVLA against surgery. 15,29,31,34–40 These, and relevant changes in NHS practice regarding the treatment of varicose veins, are reviewed in Chapter 2. Despite these new studies, the 2013 NICE guidelines on the management of varicose veins found that the evidence comparing conventional surgery with foam sclerotherapy or with endovenous thermal ablation was of low quality. 28
The structure of the remainder of the monograph is as follows. Chapter 3 describes the methodology underpinning the CLASS trial. In Chapter 4 we describe the trial participants. Chapters 5 and 6 present the clinical effectiveness results up to 6 months. In Chapter 7, we discuss the clinical effectiveness results. Chapter 8 describes the development of an instrument to assess return to normal activity in terms of behavioural recovery and the trial results in terms of this outcome. Chapter 9 presents the within-trial cost-effectiveness analysis. In Chapter 10, we present economic modelling beyond the 6-month follow-up period. Finally, the overall results of the study are discussed in Chapter 11, together with implications for practice and recommendations for future research.
Chapter 2 Changes in practice and literature update
Recent changes in practice
Since the start of the CLASS trial, several surveys have shown an increased use of newer endovenous treatment options in the NHS. 16,41,42 Since 2006, specific codes for the minimally invasive treatment options have been introduced and Hospital Episode Statistics have shown that the most commonly used minimally invasive treatment is foam sclerotherapy, followed by EVLA. 43 However, overall, 70% of those having treatment of varicose veins in the NHS still undergo surgery. 43
Quality of life as an outcome measure has become increasingly important; the standard NHS contract for acute services in England requires that all licensed providers of NHS-funded varicose vein procedures ask patients to complete patient-reported outcome measure (PROM) questionnaires before and after surgery. This includes the disease-specific AVVQ, the generic European Quality of Life-5 Dimensions (EQ-5D) index and EQ-5D visual analogue scale (VAS). The PROMs data have shown that varicose vein treatment results in significant improvement in health for patients, with over 80% experiencing an improvement in the AVVQ and almost a 50% reduction in the AVVQ score from pre-operative values. 44 Despite this clear benefit, the number of varicose vein treatments being performed in the NHS has fallen (from approximately 36,650 in 2009–1028 to approximately 27,600 in 2011–122) owing to rationing of treatment, as a result of restrictions in referrals from primary to secondary care.
Literature update: randomised controlled trials of foam sclerotherapy alone versus surgery or endovenous treatments
EMBASE (1980 to week 37, 2012), Ovid MEDLINE (1946 to September week 2, 2012) and Ovid MEDLINE In-Process & Other Non-Indexed Citations were searched using terms designed to identify randomised comparisons of foam sclerotherapy, surgery and EVLA. In addition, the HTA database, Database of Abstracts of Reviews of Effects (DARE) and Cochrane Database of Systematic Reviews (CDSR) were searched using similar terms. Identified abstracts were screened for relevant papers. All searches were updated in 2013. In addition, the reference lists of identified papers were searched for any relevant papers.
At the time of submitting the proposal, there was only one RCT in which foam sclerotherapy was compared against surgery;14 by mid-2013 a further five RCTs had been published comparing foam sclerotherapy with surgery and/or thermal ablation (Table 1). 29,30–33 All these studies involved treatment to the GSV only. In two, foam sclerotherapy with concomitant phlebectomies was compared with EVLA. 29,32 The outcome of these studies in terms of QoL, technical success, return to normal activities, Venous Clinical Severity Scores (VCSSs), recurrence rates and costs are discussed below.
| Study | Number of patients, centres, vein involvement | Comparatorsa | Primary outcomes | Other outcomes |
|---|---|---|---|---|
| Biemans 201331 | 233, single centre, GSV | Foam sclerotherapy vs. EVLA vs. surgery | Anatomical success at 1, 3 and 12 months, post-operative neovascularisation | CEAP classification, complications, QoL (CIVIQ, EQ-5D) |
| Lattimer 201232 | 100, single centre, GSV | Foam sclerotherapy with phlebectomies vs. EVLA with phlebectomies | Technical success at 3 months | Cost, VCSS, QoL (AVVQ) up to 3 months, return to normal activities |
| Shadid 201233 | 460, three hospital sites, GSV | Foam sclerotherapy with delayed phlebectomies or further foam sclerotherapy vs. surgical stripping with high ligation | 2-year recurrence, defined as reflux combined with venous symptoms | Recurrent reflux, symptoms, QoL (EQ-5D), adverse events, direct hospital costs up to 2 years |
| Rasmussen 201129 | 500, two centres, GSV | Foam sclerotherapy with phlebectomies vs. EVLA, RFA or surgery | Technical success at 1 year (GSV closure) | Pain, absence from work and normal activity, QoL (AVVQ, SF-36), VCSS, recurrence rates up to 1 year |
| Figueiredo 200930 | 60, single centre, GSV and SSV | Foam sclerotherapy vs. surgery with phlebectomy | VCSS up to 6 months | Technical success at 6 months, treatment complications |
| Wright 200614 | 710, multicentre, GSV and SSV | Foam sclerotherapy (manufactured foam: Varisolve®) vs. surgery or sclerotherapy (liquid or investigator-generated foam) | Technical success at 3 months | Technical success at 12 months, return to normal activities |
In addition, there are four further RCTs which have compared foam sclerotherapy of the GSV with ligation of the SFJ against conventional surgery. 45–48 These are not discussed further because ligation of the SFJ is not considered minimally invasive treatment, and therefore its use with foam undermines the value of foam as a simple, minimally invasive treatment option. In addition, this type of treatment is not one which has been adopted in UK practice.
Quality of life
Quality of life was assessed in four of the above studies. In the study by Biemans et al. ,31 there was no difference in QoL [assessed by the disease-specific Chronic Venous Insufficiency Quality of Life Questionnaire (CIVIQ) or the EQ-5D] at 3 months or 1 year. At 3 months, there was no significant difference in AVVQ scores between treatment groups. 32 At 1 year, Rasmussen et al. 29 found significant improvements in the AVVQ and Short Form questionnaire-36 items (SF-36) scores in all treatment groups, but no difference between any of the treatment groups. Similarly, Shadid et al. 33 found no significant difference in the EQ-5D scores between treatment groups at 2 years.
Technical success
This was assessed in all six studies at various time points up to 2 years. At 1 year, the occlusion rate for foam sclerotherapy (73%) was significantly lower than for either surgery (88%, p < 0.02) or EVLA (89%, p < 0.02). 31 At 3 months, the technical success rate was found to be similar for foam sclerotherapy and EVLA (above-knee GSV occlusion rate 69% vs. 74%, p = 0.596). 32 At 6 months, Figueiredo et al. 30 found no statistically significant difference in technical success between patients randomised to foam sclerotherapy and those randomised to surgery (vein obliteration in 90% vs. 78%).
In the study by Wright et al. ,14 the technical success at 12 months was slightly higher in the surgery group (86%) than in the Varisolve® group (84%), but this did not reach statistical significance. Rasmussen et al. 29 found that the technical success rates at 12 months were significantly lower in patients receiving foam (84%) than in those receiving EVLA (94%), RFA (95%) and surgery (97%) (χ2 p < 0.001).
Duplex findings at medium-term follow-up
This has been assessed by Shadid et al. ,33 who found the presence of reflux to be greater in patients treated with foam sclerotherapy than in those receiving surgery at 2-year follow-up (35% vs. 21%, p = 0.003).
Return to normal activities
This was reported in three of the five studies. Wright et al. 14 found that the time to return to normal activities following treatment was shorter in the foam sclerotherapy group than in the surgical group (median 2 vs. 13 days, p < 0.001). In the study by Rasmussen et al. ,29 the median time to return to normal activities was shorter in the patients in the foam sclerotherapy and RFA groups (1 day in each) than in the EVLA (2 days) and surgery (4 days) groups (p < 0.001). The study by Lattimer et al. 32 found that the mean time to return to normal activities was shorter following foam (3 days) than EVLA (7.5 days) (p = 0.11).
Venous Clinical Severity Scores
The VCSSs were assessed in three studies, and improved significantly after the procedure in all groups, with no differences noted between groups. 29,30,32
Clinical, etiological, anatomical, pathological classification
One study considered the clinical, etiological, anatomical, pathological (CEAP) classification. 31 Although the CEAP classification improved after foam sclerotherapy, EVLA and surgery, there was no difference between groups at 3 or 12 months.
Clinical recurrence rates
In the study by Rasmussen et al. ,29 the 1-year clinical recurrence rates in those randomised to foam, surgery, EVLA and RFA were similar (14%, 15%, 12% and 7% respectively, p = 0.155).
A further study defined clinical recurrence in terms of a combined end point of reflux combined with venous symptoms at 2 years. 33 This end point was found to occur equally in patients randomised to foam sclerotherapy (11%) or surgery (9%) (p = 0.407).
Costs
These were reported in three of the studies, with all three reporting that foam was the least costly option. Lattimer et al. 32 calculated the cost of foam sclerotherapy to be approximately one-third of the cost of EVLA. In the study by Shadid et al. ,33 hospital costs over a 2-year period in patients receiving foam sclerotherapy were less than half of those in the surgery group. Rasmussen et al. 29 found that foam sclerotherapy was the cheapest option, and that EVLA and surgery were more expensive.
Literature update: randomised controlled trials comparing endovenous laser ablation with surgery
At the time of submitting the proposal, there was only one completed (but unpublished) RCT in which EVLA was compared against surgery. 15 By mid-2013, a further eight RCTs had been published which compared EVLA against surgery of the GSV,29,31,34–36,38–40,49–51 and one which compared EVLA against surgery to the SSV37 (Table 2). Two of these studies31,49 also included foam sclerotherapy; these are the only currently published studies which have compared foam sclerotherapy against EVLA. The outcomes of these studies in terms of QoL, technical success, return to normal activities, VCSS, recurrence rates and costs are discussed below.
| Study | Number of patients, centres, vein involvement | Comparatorsa | Primary outcomes | Other outcomes |
|---|---|---|---|---|
| Biemans 201331 | 233, single centre, GSV | Foam sclerotherapy vs. EVLA vs. surgery | Anatomical success at 1, 3 and 12 months, post-operative neovascularisation | CEAP, complications, QoL (CIVIQ, EQ-5D) |
| Samuel 201337 | 106, single centre, SSV | EVLA with phlebectomies vs. surgery | Technical success (abolition of reflux at 6 weeks) | Technical success, return to work and normal activities, complications, VCSS, QoL (AVVQ, EQ-5D, SF-36) up to 1 year |
| Rass 201238 | 400, two centres, GSV | EVLA vs. surgery | Clinically recurrent varicose veins at 2 years | Duplex-detected saphenofemoral recurrence, QoL (CIVIQ), adverse events, clinical and functional outcome (HVVSS) |
| Flessenkamper 201239 | 449, three centres, GSV | EVLA with phlebectomies ± high ligation vs. surgery | Venous reflux at proximal section of the GSV at 2 years (only 2-month data published) | Complications (including post-operative ecchymosis), CEAP |
| Carradice 201135,50 | 280, single centre, GSV | EVLA with phlebectomies vs. surgery | QoL (SF-36) | Clinical recurrent varicose veins, duplex-detected reflux, technical success, VCSS, QoL (AVVQ, EQ-5D), return to work and normal activities |
| Rasmussen 201129 | 500, two centres, GSV | EVLA with phlebectomies vs. foam, RFA or surgery | Technical success (GSV closure) at 1 year | Absence from work and normal activity, QoL (AVVQ, SF-36), VCSS, recurrence rates up to 1 year |
| Pronk 201040 | 122, single centre, GSV | EVLA with delayed sclerotherapy vs. surgery | Clinical recurrence and technical success up to 12 months | Recovery, complications, CEAP |
| Christenson 201034 | 200 limbs, single centre, GSV | EVLA vs. surgery | Duplex technical success at 2 years | VCSS, QoL (AVVQ, SF-36) |
| Darwood 200815 | 118, single centre, GSV | EVLA with delayed foam sclerotherapy vs. surgery | Duplex technical success, QoL (AVVQ) at 3 months | Return to normal activity and work, technical success, QoL (AVVQ) at 1 year, VCSS |
| Rasmussen 2007,36 2010,49 201351 | 121, two centres, GSV | EVLA with phlebectomies vs. surgery | Technical success, clinical recurrence at 6 months, 2 years and 5 years | VCSS, QoL (AVVQ, SF-36), costs |
Quality of life
Eight studies reported QoL, using instruments which included the AVVQ, SF-36, EQ-5D and the disease-specific CIVIQ. In all of these studies, no significant difference was noted in patients randomised to EVLA or surgery at various follow-up time points ranging from 3 months to 5 years. 15,29,31,34,35,37,38,50,51 Disease-specific QoL was found to be reduced in patients who developed a clinical recurrence compared with those who did not (p = 0.001). 35 In this study, the clinical recurrence rates were lower in patients who received EVLA than in those who underwent surgery at 1-year follow-up (p < 0.001). 35
Technical success
This was assessed in all 10 of the studies at various time points. In the study by Flessenkamper et al. , technical success (no inguinal venous reflux) was achieved after 2 months in 92% of the EVLA group, 98% of the EVLA/high-tie group and 100% of the standard surgery group. 37,39 Darwood et al. 15 found that, at 3-months follow-up, abolition of reflux was achieved in 94% of EVLA and 88% of surgical patients (p = 0.227), and that, by 1 year, technical success had reduced in both groups. At 6 months, Rasmussen et al. 36 reported no significant difference in technical success at 1 year (94% in the EVLA group and 98% in the surgery group, p > 0.05). In the later study by Rasmussen et al. ,29 technical success was 94% following EVLA compared with 96% following surgery (p = 0.543). Biemans et al. 31 found no difference in anatomical success following EVLA (89%) or surgery (88%).
In the study by Carradice et al. ,35 the technical success rate at 6 weeks was slightly lower in patients randomised to surgery (92%) than in those who underwent EVLA (99%) (p = 0.005). In the study by Pronk et al. ,40 the technical success was similar in both surgery (90%) and EVLA (91%) groups at 1 year.
In the study by Christenson,34 initial technical success (no detectable reflux at 12 days) was 99% in the EVLA group and 100% in the surgery group. The one study involving patients undergoing treatment to the SSV system found that the technical success (abolition of reflux) was greater in the EVLA group (96%) than in the surgery group (72%) at 6 weeks (p < 0.001). 37
Duplex findings at medium-term follow-up
Over a 2-year follow-up, recanalisation (partial or complete) occurred in 7% of the EVLA group and none of the surgery group (p = 0.051). 34 Rass et al. 38 found that patients in the EVLA group had a higher rate of duplex-detected saphenofemoral reflux than those undergoing surgery at 2 years (18% vs. 1%, p < 0.001).
At 5 years, there was no difference in the proportion of open refluxing GSVs between EVLA (18%) and surgery groups (10%) (p = 0.21). 51
Return to normal activities
In the study by Darwood,15 patients randomised to EVLA had a quicker return to normal activities and work than those randomised to surgery (p = 0.001 and p = 0.005 respectively). Similarly, Rass et al. 38 found that patients having EVLA returned to work more quickly than those having surgery; this was despite there being no difference in return to basic physical activities between the groups. Pronk et al. 40 found that recovery (mobility, self-care and daily activities) was better in patients randomised to surgery than in those randomised to EVLA at day 7 (p < 0.05); however, there was no difference in the mean number of days taken to restart daily activities, work and sport between the groups. In two studies by Rasmussen et al. ,29,36 there was no difference in return to normal activities and work between patients randomised to EVLA and surgery. In the one study to involve patients undergoing SSV treatment,37 patients who had EVLA returned to normal activities and work earlier than those undergoing surgery (p < 0.001).
Venous Clinical Severity Score
The VCSS was assessed in six studies, and scores improved significantly after treatment in all groups, with no differences noted between treatment groups. 15,29,34,37,49,51 In the study by Rass et al. ,38 an alternative assessment tool (the Homburg Varicose Vein Severity Score) was used; again there was no difference between treatment groups.
Clinical, etiological, anatomical, pathological classification
Three studies considered CEAP as an outcome measure. There was no difference in CEAP between intervention groups at 2 months39 and 1 year31,40 in patients undergoing EVLA versus surgery.
Clinical recurrence rates
These were reported in five studies. The clinical recurrence rates at 1 year, in the study by Pronk et al. ,40 were approximately 10% in both the EVLA and surgery groups. In contrast, Carradice et al. 35 found the clinical recurrence rate at 1 year to be lower after EVLA (4%) than after surgery (20%) (p < 0.001).
Rass et al. 38 reported recurrent varicose veins on clinical examination in 16.2% of the EVLA group versus 23.1% of the surgery group at 2 years (p = 0.15). Higher 2-year clinical recurrence rates were reported by Rasmussen et al. 49 (surgery 37%, EVLA 26%). In the 5-year results from this study, there was no difference in recurrence rates (surgery 55%, EVLA 47%, p = 0.72). 51 In the study by Rasmussen et al. 29 where four different treatment options were compared, clinical recurrence rates at 1 year were reported as 14% following foam, 15% following surgery, 12% following EVLA and 7% following RFA (p = 0.155).
Literature update: meta-analysis comparing foam with endovenous laser ablation and surgery (technical success, clinical recurrence rates and cost)
Treatment of recurrent varicose veins accounts for 20% of venous procedures in the NHS, and thus the long-term durability of any treatment is important both for the patient and for economic reasons. It is assumed that lower initial technical success rates will translate into higher clinical recurrence rates, reduced QoL, the need for further treatment and thus an increased cost to the NHS in the long term. The NICE meta-analysis of four studies comprising 966 randomised patients found that foam sclerotherapy was associated with a higher prevalence of reflux at 3–12 months (compared with conventional surgery), but there was not a large enough effect to show clear advantage for surgery. 28
A meta-analysis of 72 predominantly observational studies (average follow-up 32 months) found that foam sclerotherapy was less effective than surgery in terms of technical success rates, and EVLA was more effective than surgery, foam sclerotherapy or RFA. 52 A further meta-analysis found that foam sclerotherapy was associated with a higher clinical recurrence rate in patients with GSV incompetence than the other newer treatments. 53
A cost–utility analysis found that the incremental cost-effectiveness ratios (ICERs) at 5 years for foam sclerotherapy (vs. conservative care), EVLA (vs. foam sclerotherapy) and RFA (vs. EVLA) were £1366, £5799 and £17,350 per QALY respectively. 54 The ICER for conventional day-case surgery compared with RFA was £19,012. A further analysis undertaken by NICE found that endothermal treatment (i.e. EVLA or RFA) is the most cost-effective strategy, with an ICER of endothermal treatment compared with foam of £3161. 28 In both these analyses, the recurrence rate following the newer treatment options were based on estimates, owing to the lack of published data.
The systematic reviews and meta-analysis concluded that long-term data on clinical efficacy (particularly with regard to recurrence), QoL and costs are required from large high-quality prospective RCTs of foam sclerotherapy and other endovenous techniques, compared against each other and against surgery. 52,53,55,56
Chapter 3 Trial design
In this chapter, we describe the aims and objectives of the CLASS trial, and the trial design. In presenting this information, we have followed the Consolidated Standards of Reporting Trials – patient-reported outcomes (CONSORT PRO) guidance. 57 We also provide the sample size calculation and describe the statistical analysis for the clinical effectiveness data. The methods for the cost-effectiveness and economic modelling chapters are contained within those individual chapters.
Aims and objectives
The primary objective of the CLASS trial was to compare the clinical effectiveness and cost-effectiveness of two minimally invasive treatment modalities performed under local anaesthetic – foam sclerotherapy of the main great or small saphenous truncal and non-truncal varicosities, and EVLA including delayed foam sclerotherapy of non-truncal varicosities – against surgery, in respect of disease-specific QoL (as measured by the AVVQ) and generic QoL (as measured by the EQ-5D and SF-36) for each intervention at 6 months (and ultimately to 5 years) and cost-effectiveness as cost per QALY gained.
Following discussion with the HTA programme, the primary outcomes were extended to involve an analysis of EVLA versus foam sclerotherapy. Thus, the study is a three-way comparison of foam sclerotherapy, EVLA and surgery. The 5-year results will be presented at a later stage.
The secondary objectives were to compare the two novel interventions against conventional surgery in respect of:
-
clinical success, as determined by residual varicose veins, VCSS, complication rates and return to normal activities
-
technical success (duplex scan-verified partial or complete ablation of, or the presence of reflux in, the main great or small saphenous trunk veins) at 6 months and any development of deep venous incompetence and neovascularisation
-
the cost to the NHS and patients of each intervention and any subsequent care, including projected costs to 5 years, based on the 6-month costs via Markov modelling.
Overview of trial design
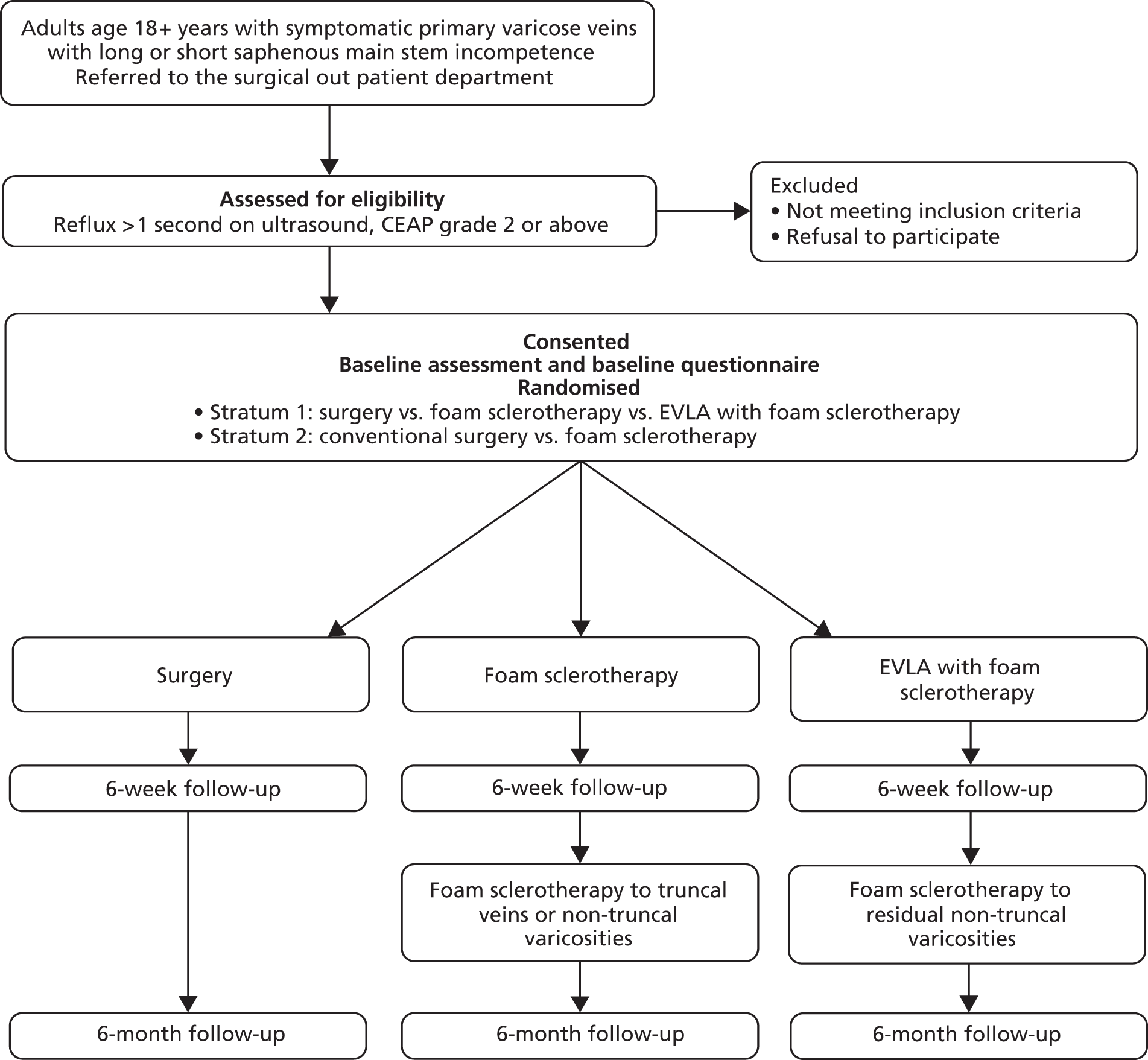
Comparison of LAser, Surgery and foam Sclerotherapy is a pragmatic, parallel-group trial designed to assess the clinical effectiveness and cost-effectiveness of (a) foam sclerotherapy of the main great or small saphenous trunk and non-trunk varicosities, and (b) EVLA of main truncal varicosities, including delayed foam sclerotherapy of non-trunk varicosities, when compared against surgery (the ‘control’ treatment). There were two strata; a recruitment site’s placement in one or the other stratum depended on the treatment options available at that site. Stratum A included eight hospitals which offered all three treatment options; thus, participants recruited in hospitals in this stratum were randomised to one of the three interventions. Stratum B included three hospitals which offered two treatment options (foam sclerotherapy and surgery), and patients recruited in hospitals in this stratum were randomised to one of these interventions.
The trial design is detailed in Figure 1.
FIGURE 1.
Overview of trial design.
Participants
Identification of patients
Patients referred from primary care to vascular surgery departments in 11 UK hospitals were identified by either a member of the clinical service or the local research nurse, and were recorded on the study log at each site.
Inclusion criteria
Adult patients (aged over 18 years) referred to the surgical outpatient department for treatment of primary varicose veins with symptomatic (CEAP grade 2 or above) great or small saphenous main truncal incompetence (reflux > 1 second on duplex scanning) were eligible for inclusion.
Exclusion criteria
The following exclusion criteria applied:
-
current DVT, acute superficial vein thrombosis
-
allergy to sclerosant
-
pregnancy or breast feeding
-
history of hypercoagulability
-
arterial disease (ankle–brachial pressure index < 0.8)
-
inability to mobilise post procedure
-
needle phobia
-
GSV or SSV < 3 mm in diameter or > 15 mm
-
tortuous veins considered to be unsuitable for EVLA owing to difficulties in passing the guide wire
-
inability to complete study questionnaires
-
history of migraines which are frequent, or migraines which are severe enough to require hospitalisation
-
other contraindications mentioned in the sodium tetradecyl sulphate (STS) (Fibrovein®, STD Pharmaceutical) prescribing information leaflet: varicosities caused by pelvic or abdominal tumours, cardiac failure, pulmonary oedema, local or systemic infection
-
patients who were not fit for a general anaesthetic because of significant systemic disease, morbid obesity or other causes.
As all varicose vein treatments should be used with care in patients taking oral contraceptives (OCs) or hormonal replacement therapy (HRT), the surgeon either asked the patient to discontinue the OC or HRT prior to treatment or prescribed heparin prophylaxis therapy.
Recruitment process
In trial centres where potentially eligible patients could be identified in advance of their attendance at an outpatient clinic, the postal summary information sheet (see Appendix 1, which contains all study paperwork, case report forms and questionnaires) was sent to them in advance of their appointment. Patients who were identified at an outpatient clinic were provided with the summary information sheet at the clinic (see Appendix 1).
The surgeon informed potentially eligible patients about the CLASS study, the different treatments available and the risks and benefits of the treatment options. The surgeon also provided patients with a study information leaflet, and an information leaflet providing detailed information about the alternative methods of treatment.
In some centres, the duplex scan was undertaken during this initial consultation; in such cases, only those patients who were eligible on the basis of the results of this scan were informed about the study.
All patients attending an outpatient clinic were logged on the study clinic log. If the patient was potentially interested in the study, his or her contact details were noted on the study clinic log. For patients who were not eligible for the study, or who were not interested in taking part, we recorded the reason for this on the study clinic log.
Around 1 week after the initial consultation, the research nurse telephoned patients who had indicated that they were potentially interested in taking part in the trial to ascertain whether or not this was still the case. If the patient was interested in taking part in the study, he or she was invited to a recruitment appointment at the clinic to provide informed consent. If the patient did not undergo duplex scanning at the initial consultation, this was undertaken at the recruitment appointment. Participants were asked to complete a baseline questionnaire. The baseline case report form was also completed at this appointment.
In participants who presented with bilateral varicose veins, the more severely affected leg (as determined by the participant) was nominated as the study leg. Where possible, the other leg was treated using the same treatment modality as the study leg, either at the same time as the study leg was treated, or sometime thereafter.
In some circumstances (for example, where the duplex scan was completed at the initial consultation and the patient lived a considerable distance from the recruitment clinic, or it was difficult for him or her to attend a recruitment clinic), the consent form and baseline questionnaire were sent to the participant, who was asked to complete these and return them by post. In these circumstances, the research nurse was available, by telephone, to answer any questions about the study.
If patients wanted to consent to the study at their initial outpatient appointment, this was also permitted.
Randomisation and allocation to intervention
Participants were randomised using a computer-generated randomisation system managed by the Centre for Healthcare Randomised Trials (CHaRT) at the University of Aberdeen. This was available to sites as a web-based or telephone system.
In the eight recruitment sites which offered all three interventions (stratum A), participants were randomly allocated 1 : 1 : 1 to EVLA, foam sclerotherapy or surgery using treatment allocation by minimisation. In the three sites which offered only two of the interventions (stratum B), participants were randomly allocated 1 : 1 to foam sclerotherapy or surgery. Each of these two strata (based on treatment options available at the trial centre) had its own separate treatment allocation application. For each application, the minimisation algorithm included centre, age (< 50 years, ≥ 50 years), sex, presence of GSV or SSV, and unilateral or bilateral veins.
After randomisation, participants were placed on the appropriate waiting list. The aim was to keep participants blinded to their treatment allocation until around 2 weeks prior to their treatment. Around 2 weeks prior to treatment, an appointment for treatment was issued by the hospital; at this time the trial office also informed the participant of his or her randomisation. However, at some sites, local processes meant that participants were informed of their randomisation by site staff in advance of this. The delay in informing participants about their treatment allocation was an attempt to minimise the possibility of unequal dropout between the arms.
A letter was sent to the participant’s general practitioner (GP) at trial entry to inform them that their patient had agreed to participate in the trial. Around the time that the participant was informed of his or her treatment allocation, a second letter was sent to the GP informing them of the allocation.
Trial interventions
Surgical treatment
The aim of surgical treatment is to perform saphenofemoral or saphenopopliteal ligation, ligate the groin or popliteal tributaries, remove the incompetent main varicosed truncal vein through inversion stripping and perform phlebectomies for non-truncal varicosities as a combined single procedure. Surgical treatment was performed under a general or regional anaesthetic.
Foam sclerotherapy
The aim of foam sclerotherapy is to fill the incompetent vein with sclerosant under ultrasound guidance by a process of chemical ablation. STS was used as the sclerosant; 3% was used for main truncal veins and 1% for non-truncal varicosities.
The patient was placed in the reverse Trendelenberg position. A needle was inserted into the incompetent GSV or SSV under ultrasound control. The leg was then raised and sclerosant foam [via 2-ml double-syringe Tessari technique, one part (0.5 ml) STS and three parts (1.5 ml) air, with at least 20 passages] injected. Immediately after injection, it was recommended that there was no movement of the patient or leg for 2–5 minutes, no Valsalva manoeuvre and no muscle activation. In line with the European consensus guidelines (published at the time of writing the protocol), a maximum of 12 ml of foam was recommended for use at one sitting. 21
At the 6-weeks appointment, the need for further foam sclerotherapy to truncal and/or non-truncal veins was assessed. The protocol allowed for a maximum of four treatment sessions of foam sclerotherapy to be offered if this was required to treat all the varicose veins and varicosities.
Sodium tetradecyl sulphate was purchased from routine NHS suppliers by each recruitment site. At the outset of the trial, the STS used was labelled as an investigational medicinal product (IMP) for use in the trial. Part way through the study, a substantial amendment was approved such that routine stocks of STS could be used in the trial and did not require to be labelled as an IMP.
Sodium tetradecyl sulphate was securely stored at room temperature or in a refrigerator in the ward, clinic or theatre. Minimum and maximum temperatures were recorded regularly by the study nurse. Temperature deviations were noted. STS would have been destroyed if the maximum storage temperature had exceeded 40 °C (stability data given in the prescribing information leaflet show that STS is stable for up to 6 months at 40 °C).
Endovenous laser ablation
The aim of EVLA is to destroy the incompetent vein by thermal ablation. EVLA involves cannulating the GSV at the lowest point of incompetence (mid-calf for SSV) under ultrasound guidance. The leg was treated flexed and externally rotated at the hip, with the knee slightly flexed. First a guide wire was inserted and then a 5-Fr catheter passed over this with the tip positioned 0.5–1 cm distal to the junction. The laser fibre was inserted as far as the tip of the catheter. The catheter was then withdrawn 2 cm so that the laser fibre protruded beyond the catheter. The table was then placed in the Trendelenberg position, and cold saline tumescent with lignocaine (Xylocaine®, AstraZeneca) infiltrated along the length of the trunk vein. This provided anaesthesia, compression of the vein around the catheter and absorption of heat. The laser fibre was fired continuously during stepwise or continuous withdrawal, aiming to achieve a target delivery of at least 70 J/cm. EVLA was carried out under local anaesthetic.
The treatment protocol allowed for the immediate treatment of a below-knee incompetent GSV with foam sclerotherapy if laser access was not possible at the site. If required, this was done at the same treatment session to the level of the mid-calf.
In one of the study sites (Hull), the protocol allowed phlebectomies for non-truncal varicosities to be performed at the same time as the EVLA.
At the 6-weeks appointment, the need for foam sclerotherapy to treat any non-truncal varicosities was assessed. The protocol allowed a maximum of four treatment sessions of foam sclerotherapy to be offered, if required, to treat all varicose veins.
Post-procedure compression
After all procedures, post-procedure compression was recommended for 10 days. For foam sclerotherapy, an attempt was made to standardise the type of bandaging and stockings used but this was not possible across all sites owing to local purchasing agreements.
Outcomes
The primary patient-reported outcome was disease-specific QoL (assessed at 6 months using the AVVQ58) and generic QoL (assessed at 6 months using the EQ-5D and SF-36 physical and mental component scores).
The AVVQ is an instrument designed to assess the perceived health of patients with varicose veins, and has been shown to be valid, reliable and responsive to change. 9,59,60 It is used as the disease-specific measure in the NHS PROMs. 44,61 The instrument comprises 12 questions and a set of manikin legs, on which participants are asked to draw their veins. Possible scores range from 0 to 100, though scores close to 100 can only be achieved if there are extensive veins covering the front and back of both legs.
The SF-36 has been validated and shown to be reliable. 61 It is widely used to assess generic QoL across different clinical conditions. The 36 questions in the SF-36 are scored as eight separate domains (vitality, physical functioning, bodily pain, general health, role – physical, role – emotional, social functioning, mental health) and as two summary scores (physical component summary, mental component summary). Though it may be presented as an overall score, we have not chosen to do this in CLASS on account of the lack of sensitivity. All scales are scored from 0 (worst QoL) to 100 (best QoL).
The EQ-5D was developed by the EuroQoL group as a single index valuation for health status. The version used in CLASS is the EQ-5D-3 levels (EQ-5D-3L), which has five questions (or dimensions: mobility, self-care, usual activities, pain/discomfort, anxiety/depression), each with three response options, and a VAS where respondents are asked to rate their current health-related quality of life (HRQoL). Responses to the five questions equate to 243 health states. Scores range from −0.594 to 1.
Secondary outcome measures included:
-
costs to the health service and patients and any subsequent care
-
clinical success of venous intervention at 6 weeks and 6 months
-
anatomical success of venous intervention at 6 weeks and 6 months
-
disease-specific and generic QoL (at 6 weeks: AVVQ, SF-36 physical and mental components and domains, EQ-5D and EQ-5D VAS; at 6 months: SF-36 domains and EQ-5D VAS)
-
behavioural recovery.
Measurement of secondary outcomes
Costs to the health service and participants and any subsequent care
This is fully described in Chapter 9. Projected 5-year costs are described in Chapter 10.
Clinical success
This was determined by the VCSS and a VAS at baseline, 6 weeks and 6 months. The VAS consisted of an unmarked line of 10 cm length, which had at the two extremes (1) no varicose veins on the left boundary, and (2) worst possible veins on the right boundary. This was completed by both the patient and the research nurse. It was used to assess the presence of varicose veins at baseline and residual varicose veins at 6 weeks and 6 months. Specific complications, which may affect clinical success, were recorded at the time of treatment and also at 6-weeks and 6-months follow-up.
Anatomical success
The duplex findings in the CLASS study were reported by an independent technician, using a standardised proforma, which recorded the presence of patency/obliteration and reflux (of one greater than 1 second at specific anatomical segments) (Box 1). The entire truncal vein was scanned, and if reflux and/or patency was identified at any site, this was recorded as occurring at the nearest site recorded on the proforma.
Groin – GSV (flush with common femoral vein, i.e. within 1 cm).
Groin – GSV (within 3 cm of common femoral vein).
Common femoral/superficial vein.
Mid-thigh – GSV.
Above knee – GSV.
Below knee – GSV.
SSV (flush with popliteal vein, i.e. within 1 cm).
SSV (within 3 cm of popliteal vein).
Popliteal vein.
Mid-calf – SSV.
The joint statement from the Venous Forum and Society of Interventional Radiology (2007)62 recommended reporting standards for endovenous ablation in the treatment of venous insufficiency. Anatomical success was defined as successful ablation of the entire treated segment of the target vein (absent flow or disappearance of the vein on duplex ultrasound). This guidance was used in the CLASS study. We defined complete anatomical success for the GSV as complete occlusion at the groin (within 3 cm of the common femoral vein), complete occlusion at mid-thigh and either an occluded or a patent but non-refluxing GSV above the knee. A partial success was defined as patency at one of the predefined segments of the treated GSV; this was further subclassified as refluxing or non-refluxing. Everything else was defined as a failure.
For the SSV, a complete success was defined as occlusion within 3 cm of the popliteal vein and complete occlusion at mid-calf. A partial success was defined as patency at one of the predefined segments of the treated GSV; this was further subclassified as refluxing or non-refluxing. Everything else was defined as a failure.
Where a participant had GSV and SSV involvement, a complete success for the whole study leg was achieved when there was a complete success for both GSV and SSV. A failure occurred when there was a failure for both GSV and SSV. If either GSV or SSV was a partial success, or one was a complete success and the other a failure, then it was considered to be a partial success for the whole leg. If there was a partial success for the whole leg and no reflux in either GSV or SSV, then it was classed as a partial success without reflux. Where the participant had GSV or SSV involvement only, then the outcome for the whole study leg was the same as the outcome for the vein.
Disease-specific behavioural recovery
The assessment of behavioural recovery required the development of a specific instrument, and this is discussed in Chapter 8. The timing and instruments used for data collection are summarised in Table 3; more detail is provided in Data collection.
| Time point | Completed by participant | Completed by research nurse/clinician/technician |
|---|---|---|
| Baseline (before randomisation) | Questionnaire completed at clinic (or by post) including: AVVQ EQ-5D SF-36 IPQ-R |
Personal details, GP, best contact, etc. Duplex scan Vein involvement VCSS CEAP classification Baseline demographic factors |
| After randomisation, before treatment | Questionnaire completed by post including: IPQ-R |
None; participant not at clinic |
| At treatment appointment(s) | VAS completed at clinic assessing pain of procedure | Procedural details Complications of procedure |
| 6 weeks after treatment | Questionnaire including: AVVQ EQ-5D SF-36 Time to return to work/normal activity Behavioural recovery questionnaire Recollection of pain during treatment and pain during follow-up |
Presence/absence DVT Presence/absence residual varicosity Anatomical success VCSS Complications For patients treated with EVLA or foam, details of further foam injections |
| 6 months after treatment | Questionnaire including: AVVQ EQ-5D SF-36 IPQ-R Economic questions |
Presence/absence DVT Presence/absence residual varicosity Anatomical success Complications VCSS |
Data collection
Recruitment appointment (baseline, before randomisation)
The disease-specific and generic QoL instruments (AVVQ, EQ-5D and SF-36) and the Illness Perception Questionnaire – Revised (IPQ-R)63 were combined into a single questionnaire for the participant to complete. Participants were asked to complete this questionnaire at baseline (at the recruitment visit). Participants could opt to complete this at home, and if they did not return this within 3 weeks, they were sent a reminder letter, a further copy of the questionnaire and a reply paid envelope. Early on in the recruitment phase, the randomisation system was amended such that participants could not be randomised until the questionnaire had been completed.
Participants were asked to rate their varicose veins on a VAS (from ‘no varicose veins’ to ‘the worst varicose veins I can imagine’). Independently, the research nurse also completed an identical VAS.
The baseline clinical form – incorporating CEAP and VCSS for both legs, duplex scan information and vein involvement in relation to the study leg, as well as some demographic information including height, weight, employment status, previous treatment and previous DVT – was also completed at the recruitment appointment.
Personal details, including GP details and a ‘best contact’, were also collected at recruitment. Participants were asked to nominate a best contact, ideally someone who did not live at the same address as them, who could be contacted if contact with the participant was lost.
After randomisation, before treatment
The pre-treatment questionnaire included the IPQ-R. Approximately 2 weeks before the treatment appointment, the questionnaire was sent to the participant, along with a reply paid envelope. Participants were asked to complete and return this before they attended for treatment. In view of the time frame for completion, reminder letters were not sent for this questionnaire. In some circumstances (e.g. when the treatment date was added retrospectively to the trial database, or immediately before the treatment appointment), it was not appropriate to send the questionnaire as it could not be completed before the treatment appointment.
Treatment appointment
A treatment-specific case report form (CRF) was completed by the treating surgeon (or delegate) after each treatment appointment. The information collected included information specific to the procedure, the grade of surgeon (and, if appropriate, the anaesthetist), how long the procedure took, details of the bandaging, any immediate complications associated with the treatment, whether or not the patient was hospitalised after the treatment and whether or not the contralateral leg was treated contemporaneously. If the participant was undergoing treatment other than the treatment to which he or she had been randomised, the CRF captured this information, together with information about the actual treatment received.
After bandaging, participants were asked to rate the pain experienced during treatment on a VAS ranging from no pain to the worst imaginable pain.
Six-weeks follow-up appointment
Participants were invited to attend for a 6-weeks follow-up appointment. At the appointment, the research nurse carried out a clinical examination of the study leg and completed a CRF incorporating the VCSS and CEAP. The technical success of the treatment was assessed by duplex scanning, performed by an independent, fully trained technician as described above.
Information about any complications or side effects was also recorded. The research nurse and participant assessed the presence of varicose veins using a VAS (ranging from ‘no varicose veins’ to ‘the worst varicose veins I can imagine’).
Participants treated with EVLA or foam sclerotherapy were assessed for further foam sclerotherapy treatment. In some cases this was carried out at the 6-weeks appointment; in other cases the participant returned to the clinic at a later date for this.
As at baseline, the disease-specific and generic QoL instruments (AVVQ, EQ-5D, SF-36) were combined into a single questionnaire for completion by the participant. The Behavioural Recovery After treatment for Varicose Veins (BRAVVO) instrument relating to behavioural recovery, including time to return to work/normal activities, was also included in this questionnaire (the development of this instrument is described in Chapter 8). Two questions on pain were also included. Participants were asked to rate, on a VAS ranging from no pain to the worst imaginable pain, the worst pain experienced while (1) having the treatment and (2) recovering after treatment.
Participants who opted to take the questionnaire home were provided with a reply paid envelope for its return. Participants who failed to return their questionnaire within 3 weeks were sent a reminder letter, a further copy of the questionnaire and a reply paid envelope. Participants who failed to attend for a follow-up appointment were offered a second appointment. If they failed to attend this, they were sent the questionnaire, covering letter and a reply paid envelope for its return. Again, those who failed to return their questionnaire within 3 weeks were sent a reminder letter, a further copy of the questionnaire and a reply paid envelope.
Six-months follow-up appointment
The 6-months follow-up took a similar form to the 6-weeks follow-up. Participants were invited to attend for a 6-months follow-up appointment. At the appointment, the research nurse carried out a clinical examination of the study leg and completed a CRF incorporating the VCSS and CEAP. The technical success of the treatment was assessed by duplex scanning (as described for the 6-weeks follow-up). Where possible, an individual patient was scanned by the same technician at each time point using the study designated duplex scanner. Information about complications and side effects of treatment was also recorded. The presence of varicose veins was assessed by both the participant and the research nurse using a VAS (as previously described). The research nurse also reviewed the hospital medical records to collect information on any hospital appointments or admissions.
The disease-specific and generic QoL instruments (AVVQ, EQ-5D, SF-36) and the IPQ-R were again combined into a single questionnaire for the participant to complete. Questions relating to resource use (primary and secondary care services), self-purchased health care, and participant time and travel costs were included in the 6-months questionnaire only (for more details see Chapter 9). Participants could opt to complete the questionnaire at home; the same reminder schedule was used as for the 6-weeks questionnaire. Participants who failed to attend for the 6-months appointment were offered a second appointment and, if they failed to attend this, a copy of the questionnaire was sent to them, along with a covering letter and a reply paid envelope, with a reminder 3 weeks later.
Data management
A secure, bespoke study database was developed which site staff could access over the internet. Password-protected access was provided such that sites could only view data from their own site. All data collected during the course of the research were kept strictly confidential and accessed only by members of the trial team. Patients’ details were stored under the guidelines of the 1988 Data Protection Act. 64 Patients were allocated an individual study number, and this number (rather than the participant’s name) was used to identify study paperwork.
Clinical data were entered into the database by the research nurse working in each hospital site, together with data from questionnaires completed at clinic. Data from questionnaires returned by post to the study office were entered by staff based there.
Staff in the study office worked closely with local research nurses to ensure that the data were as complete and accurate as possible. Extensive range and consistency checks further enhanced the quality of the data.
Pharmacovigilance and safety reporting
A serious adverse event (SAE) was defined as any medical occurrence that:
-
resulted in death
-
was life-threatening (i.e. the subject was at risk of death at the time of the event)
-
required inpatient hospitalisation or prolongation of existing hospitalisation
-
resulted in persistent or significant disability/incapacity
-
was a congenital anomaly/birth defect
-
was an important medical event, which may not have been immediately life-threatening or resulted in death or hospitalisation but may have jeopardised the patient or required intervention to prevent one of the other outcomes listed in the definition.
An adverse reaction was defined as an adverse event judged by either the reporting investigator or the sponsor as having a reasonable causal relationship to the medicinal product (i.e. STS).
An unexpected adverse reaction was defined as an adverse reaction, the nature and severity of which were not consistent with the applicable product information. We defined the following SAEs as potentially ‘expected’:
-
DVT (following foam sclerotherapy, EVLA, surgery)
-
pulmonary embolism (following foam sclerotherapy, EVLA, surgery)
-
anaphylactic shock (following foam sclerotherapy)
-
stroke (following foam sclerotherapy)
-
retinal arteriole occlusion (following foam sclerotherapy)
-
myocardial infarction (following foam sclerotherapy)
-
cutaneous necrosis and ulceration (following foam sclerotherapy)
-
epileptic fit (following foam sclerotherapy)
-
intra-arterial injection (following foam sclerotherapy)
-
injury to a major artery (common femoral or superficial femoral artery) (following surgery)
-
injury to a major vein (common femoral or popliteal vein) (following foam sclerotherapy, EVLA, surgery)
-
injury to a motor nerve (femoral, tibial or peroneal nerve) (following surgery)
-
transient ischaemic attack (following foam sclerotherapy)
-
migraine (following foam sclerotherapy).
All other SAEs were defined as unexpected.
Adverse events during, or immediately following, treatment were collected on the treatment CRF before discharge. In line with current clinical practice, participants were advised to contact their GP if they experienced an adverse event between the period following treatment and the 6-weeks follow-up appointment. At each follow-up visit, participants were asked if they had experienced any adverse events; these were collected on the appropriate follow-up CRF.
All SAEs were recorded as such using the SAE form, and reported to the trial office and to the sponsor within defined time lines. For all SAEs, the local principal investigator was asked to determine whether or not the event was likely to have been caused by study treatment.
Suspected unexpected serious adverse reactions (SUSARs) would have been reported to the Medicines and Healthcare Products Regulatory Agency (MHRA) and the Research Ethics Committee in accordance with prescribed time lines.
Trial oversight
The University of Aberdeen acted as sponsor for the study.
Independent trial steering and data monitoring committees were established. The Trial Steering Committee (TSC) comprised an independent chairperson (a vascular surgeon) and two further independent members (a vascular surgeon and a trials methodologist). The TSC met approximately annually over the course of the trial.
The Data Monitoring Committee (DMC) comprised an independent chairperson (a vascular surgeon) and two further independent members (a trials methodologist and a statistician). The DMC met approximately annually.
Ethics and regulatory approvals
The trial and subsequent amendments were reviewed and given a favourable opinion by Scotland A Research Ethics Committee (reference 08/MRE0024) and local research and development departments. The trial was classed as a clinical trial involving an investigational medicinal product (CTIMP) because of the use of STS in the foam sclerotherapy arm, and was therefore covered by the EU Clinical Trials Directive. Clinical trial authorisation (CTA) was provided from the MHRA (EudraCT 2008-001069-26, CTA 21583/0206/001). The trial was conducted according to the principles of good clinical practice and was registered and assigned an International Standard Randomised Controlled Trial Number (ISRCTN51995477).
Protocol amendments after trial initiation
A number of protocol revisions were made after trial initiation. These included:
-
clarification of the techniques for undertaking foam sclerotherapy and EVLA treatment
-
providing guidance on the labelling and storage of STS, and subsequently removing the requirement to label STS as an IMP
-
assessment of behavioural recovery at 6 weeks rather than 6 months
-
inclusion of the assessment of pain
-
revision of the ‘expected’ adverse events in light of new evidence
-
addition of an exclusion criterion relating to migraine.
Patient information leaflets were revised in light of new evidence. Adaptations of study administrative processes (for example the use of additional letters, revisions to letters, the use of the clinic log) were also implemented.
Sample size and power
At the outset of the study, we proposed a sample size of 1015 participants from six hospitals across the two strata. We anticipated that four hospital sites would offer three treatment options (surgery, foam sclerotherapy and EVLA; stratum A), and that two hospitals would offer two treatment options (surgery and foam sclerotherapy; stratum B). The proposed sample size is shown in Table 4. Based on previous studies,10,65 we suggested that it would be reasonable to expect differences between surgery and minimally invasive treatment (foam or EVLA) of approximately 0.25 of a standard deviation (SD) on the QoL instruments at 6-months follow-up (in particular, this would equate to a five-point shift in the EQ-5D score). This estimated difference of 0.25 SDs was observed in Short Form questionnaire-6 Dimensions (SF-6D) and EQ-5D scores in the small trial by Ratcliffe et al. 10 which compared conventional surgery with sclerotherapy.
| Stratum | EVLA | Foam | Surgery |
|---|---|---|---|
| Stratum A (four hospitals) | 245 | 245 | 245 |
| Stratum B (two hospitals) | – | 140 | 140 |
| Total | 245 | 385 | 385 |
Foam versus surgery
For this primary comparison, strata A and B can be combined without introducing any bias. A trial with 385 patients in each group (total 770 patients) will have at least 90% power at a 5% significance level to detect a change of 0.25 SDs in both AVVQ and EQ-5D. Adjusting for baseline score allows the sample size to be decreased by a factor of 1 − correlation squared, so including 385 participants allows for a 10% loss to follow-up at 6 months (assuming a correlation between baseline and 6-months scores of at least 0.31). A correlation of 0.31 is, in our experience, conservative for QoL studies, but should the loss to follow-up be 15%, the study would still have 90% power to detect a difference of 0.25 SDs. Cost savings will be sensitive to the number of participants with recurrent varicose veins requiring reintervention in each group. Allowing for additional loss to follow-up (up to 20%) by 5 years, the study will have 90% power to detect a 15% difference in recurrence from 32% in conventional surgery to 45% in the other groups (which would be funded separately).
Endovenous laser ablation versus surgery
For this primary comparison, only participants in stratum A provide a direct randomised comparison, giving 245 participants in each group (490 in total). This trial will have 80% power at 5% significance to detect a difference of 0.25 SDs. Given adjustment for baseline measures, this allows for a 10% loss to follow-up.
Recruitment to the trial was lower than anticipated for a number of reasons. These included a lower-than-anticipated proportion of varicose vein referrals who met the eligibility criteria, ‘rationing’ of varicose vein treatment at some sites leading to a sharp decline in the number of patients being referred for treatment, and a lower-than-anticipated proportion of eligible patients agreeing to take part. Additional recruitment sites were sought in an attempt to compensate for the recruitment shortfall, but few UK sites offered the appropriate treatment options to enable them to participate in the study. Despite some additional sites, and an extension to the recruitment period, the original recruitment targets were not met. Thus, the DMC and TSC were asked to consider a revised recruitment target of 779 (Table 5).
| Stratum | Total | EVLA | Foam sclerotherapy | Surgery |
|---|---|---|---|---|
| Stratum A | 635 | 211 | 211 | 211 |
| Stratum B | 144 | – | 72 | 72 |
| Total | 779 | 211 | 283 | 283 |
We provided the following justification for this request. The correlation, pooled across trial arms, between the AVVQ at baseline and 6 months post surgery was 0.39, and this has the effect of providing greater power than originally assumed. In the surgery versus foam comparison, the power achieved with the sample size of 283 in each arm would be equivalent to 334 in each arm if no correlation was observed. Similarly, the power achieved with the sample size of 211 in each arm of the surgery versus EVLA comparison would be equivalent to 249 in each arm if no correlation was observed.
However, at 6 months the response to follow-up was 89%, 1% of questionnaires did not include sufficient data to derive a valid AVVQ and the proportion of participants withdrawn (including those withdrawn prior to receiving an intervention) was 7%. Together, these factors indicated that a valid AVVQ could be expected in 82% of the planned sample.
Therefore, the sample size was effectively 275 (i.e. 82% of 334 in each arm of the surgery vs. foam comparison) for the purpose of determining power (Table 6).
| Target | Effective sample size in each arm | Detectable effect size with 90% power (SDs) | Power to detect 0.25 SDs (%) |
|---|---|---|---|
| Previous | 350 | 0.25 | 91 |
| Revised | 275 | 0.28 | 83 |
Similarly, the sample size was effectively 205 in each arm of the surgery versus EVLA comparison (Table 7).
| Target | Effective sample size in each arm | Detectable effect size with 80% power (SDs) | Power to detect 0.25 SDs (%) |
|---|---|---|---|
| Previous | 258 | 0.25 | 81 |
| Revised | 205 | 0.28 | 72 |
Note that although the proposed revised trial sample size was decreased, there was no change to the target differences assumed to be clinically important (0.25 SDs); the only amendment to the sample size calculation was a decrease in the power of the study to detect a 0.25-SD change. Both the DMC and TSC agreed this amendment.
Statistical analysis
The trial analysis was by intention to treat (all participants remained in their allocated group for analysis), giving the least biased estimate of effectiveness between interventions. Three comparisons were considered for the main trial analysis: (1) surgery versus foam sclerotherapy, (2) surgery versus EVLA and (3) EVLA versus foam sclerotherapy. Participants from all centres were included in the analysis of comparison (1), and participants from only those centres randomising to all three treatments were included in the analysis of comparisons (2) and (3). A single principal analysis of the randomised trial was planned when all participants had been followed up for 6 months after treatment. Study analyses were conducted according to a statistical analysis plan, using SAS version 9.3 (SAS Institute Inc., Cary, NC, USA).
The primary outcome measures (AVVQ, EQ-5D and SF-36 scores at 6 months) and all secondary outcome measures (AVVQ, EQ-5D and SF-36 scores at 6 weeks; VCSS, presence of residual varicosities, truncal vein ablation and complication rates at 6 weeks and 6 months; pain at time of treatment and at 6 weeks) were presented as summaries of descriptive statistics at each time point and comparisons between groups were analysed using generalised linear models (GLMs). All analyses were adjusted for minimisation covariates (sex, age group, saphenous involvement, disease laterality and centre) and, where appropriate, for baseline scores (for AVVQ, EQ-5D, SF-36 and VCSS). If there was a discrepancy between the minimisation covariate used in the randomisation process and the data recorded in the baseline clinical form, then the latter was used in the reporting of descriptive statistics and for adjustment in the analyses. No adjustment was made for multiple comparisons. However, for the secondary outcome measures, we considered differences to be significant only for p-values < 0.005. The models used to analyse the continuous outcomes were repeated measures mixed models with a compound symmetry covariance matrix and centre fitted as a random effect. Truncal vein ablation rates were analysed using ordinal logistic regression and complication rates were analysed using binary logistic regression. Estimates of treatment effect size were expressed as the fixed effect solutions in the mixed models and odds ratios (ORs) in the logistic regression models. For all estimates, 95% confidence intervals (CIs) were calculated and reported.
Sensitivity analyses were carried out on the primary outcome to investigate the impact of missing data under various assumptions following recently published recommendations. 66 Complete follow-up data were used in the sensitivity analyses, with values imputed for any missing AVVQ score at 6 months. The repeated measures models used in the primary analysis assumed data to be missing at random,67 whereas the sensitivity analyses used multiple imputation where data were assumed to be missing not at random, because we considered scenarios where there might be systematic differences between missing and observed values and also where this differed between groups. The first sensitivity analysis assumed no systematic difference and imputed values were obtained from the generation of 10 data sets and based purely on observed values (minimisation covariates and AVVQ scores at baseline and 6 weeks). The remaining sensitivity analyses adjusted the imputed values in the initial sensitivity analysis by either adding two points to the imputed AVVQ scores or subtracting two points. These adjustments were then repeated in one arm only, and repeated again by applying the adjustments in the other arm only. We considered two points on the AVVQ score to be more than the minimum clinically important difference, and hence a meaningful systematic difference to test in the sensitivity analyses.
The Aberdeen Varicose Vein Questionnaire
The AVVQ consists of 19 items (a diagram at the beginning on which respondents mark the location and extent of their varicose veins, followed by 12 ordinal response items, of which six require separate responses for each leg). The outcome measure is scored from 0 to 100 (higher values indicate worse QoL), and scores were calculated using weightings for each response in the questionnaire. Where a response was omitted by the respondent, the score for that item was removed from the denominator so that participants could still score between 0 and 100 even if the questionnaire was not fully completed. 59 If the participant had unilateral disease at the time of completing the questionnaire (determined from the veins drawn on the diagram), then, for unanswered questions which related to the healthy leg only, it was assumed that the response should be ‘no’ or ‘none at all’ and a value of 1 was imputed. Once this was executed, any questionnaires with more missing responses than non-missing responses (i.e. 10 missing items or more) were considered not to have valid scores.
The Venous Clinical Severity Score
The VCSS consists of 10 ordinal response items, each with four levels (coded from 0 to 3). The overall score was calculated as the sum of the values across each of the 10 responses, so that a VCSS score could potentially be in the range from 0 to 30 (higher scores indicate greater severity). If the response to question 7 was ‘no active ulcers’ and the responses to questions 8 and 9 (relating to active ulcers) were missing, then questions 8 and 9 were assigned a value of zero. For other missing items, if the equivalent response was available from the AVVQ then these missing responses were assigned the appropriate value. A valid VCSS was not obtainable for participants for whom there remained any missing responses.
Process evaluation
Background
As it was not possible for participants to be blinded to their treatment, several steps were taken to increase our confidence in the trial results based on the primary QoL outcomes. As much as possible, information provided to participants at recruitment, after randomisation and following treatment was standardised.
We also conducted a theory-based process evaluation to identify possible confounding variables, for example, expectations or concerns that might differ between treatment groups after participants were informed of the treatment they would receive and that might, in turn, influence recovery behaviours or QoL. The theoretical basis for this process evaluation was the common-sense self-regulation model (CS-SRM). 68
The CS-SRM proposes that people respond to a potential health threat in terms of their perceptions, beliefs and expectations (‘cognitive’ representations) and also in terms of anxieties and concerns (‘emotional’ representations). People use a range of coping behaviours (e.g. self-medicating, seeking social support or perhaps avoidance) to manage and regulate these representations. Systematic review evidence suggests that illness representations play a significant role in help-seeking behaviour and adherence to treatment recommendations. 69,70
Questionnaire measures have been developed and validated to assess the proposed domains of illness representations. The domains are illness identity; causes; timeline; consequences; control; coherence; and emotion. Table 8 presents the label, a brief explanation and an example questionnaire item for each of the domains of the illness perceptions framework.
| Domain label | Explanation | Example questionnaire item |
|---|---|---|
| Identity | What is the condition and its experienced symptoms? | Symptom checklist (see Identity domain) |
| Cause | What are the causes of the condition? | Cause checklist (see Identity domain) |
| Timeline (acute/chronic) | How long will the condition last? | My varicose veins will last a long time |
| Timeline (cyclical) | Is the condition experienced as episodes? | My symptoms come and go in cycles |
| Consequences | How serious are the consequences of the condition for the person’s everyday life? | My varicose veins have major consequences on my life |
| Personal control | To what extent does the person have control over managing or curing the condition? | Nothing I can do will affect my varicose veins (reverse code) |
| Treatment control | To what extent is treatment effective in managing or curing the condition? | My treatment will be effective in curing my varicose veins |
| Illness coherence | To what extent does the person understand the condition and the way the treatment is proposed to work? | My varicose veins are a mystery to me |
| Emotional representations | How worried is the person about having the condition? | When I think about my varicose veins I get upset |
Methods
The most frequently used, validated scale to assess illness representations is the IPQ-R. 63 The IPQ-R assesses each of the domains in the illness perceptions framework. This questionnaire was administered to trial participants at baseline, following randomisation and 6 months after treatment.
We were particularly interested in whether or not participants in any of the trial arms reported different illness perceptions after they were notified of the treatment to which they had been randomised (but before treatment). We were also interested in whether or not illness perceptions improved following treatment, particularly with regard to the domain of treatment control, as this would likely reflect participants’ perceptions of the effectiveness of their treatment, and timeline and consequences, as these would likely reflect the extent to which participants’ expectations were exceeded following treatment.
Analysis
Identity domain
In the IPQ-R, participants are presented with a number of symptoms, some that might be expected to occur as a result of varicose veins, and some unrelated to varicose veins. Participants are asked to identify symptoms that they have experienced since developing varicose veins, and whether or not they believe that these symptoms are related to their varicose veins. The IPQ-R was scored according to the method outlined on the Illness Perception Questionnaire (IPQ) website (www.uib.no/ipq). The ‘illness identity score’ is the number of symptoms that are both experienced by the participant and correctly identified as being related to their varicose veins. 63 The symptoms are pain, hardening of the skin, redness of the skin, swelling of the ankle, discolouration or brown staining on the leg, and breaks in the skin or ulcers on the leg. The questionnaire also lists unrelated symptoms (sleep difficulties, stiff joints, weight loss, dizziness, fatigue, sore eyes, breathlessness, loss of strength), but these questions do not count either positively or negatively towards the individual’s overall illness identity score. Possible identity scores range between 0 and 6.
The ‘percentage of symptoms correctly identified as being related to varicose veins’ is another way of representing illness identity,63 but differs from the identity score in that it only takes account of the subset of the six symptoms that the participants reported having experienced. These are calculated as a percentage for each participant. Where fewer than six symptoms are experienced, these percentages are higher than the corresponding identity score expressed as a proportion out of six.
Other domains
The other measures of illness perception are presented as mean scores out of 30 [for timeline (acute/chronic), consequences, personal control and emotional representations], 25 (for treatment control and illness coherence) or 20 [for timeline (cyclical)], with higher scores indicative of greater illness representation.
The results of the process evaluation for the comparison of foam sclerotherapy and surgery are presented in Chapter 5 and those for the comparison of EVLA, foam sclerotherapy and surgery in Chapter 6. Discussion of the process evaluation is given in Chapter 7.
Chapter 4 Baseline characteristics
This chapter provides a brief overview of all patients involved in the study and describes baseline characteristics of the whole cohort of participants pooled across treatment groups. Baseline data broken down by randomised groups are presented in Chapters 5 and 6.
Figure 2 shows the Consolidated Standards of Reporting Trials (CONSORT) diagram for the entire CLASS study. In total, 6592 patients with varicose veins were screened for eligibility over 48 months between November 2008 and October 2012. The mean age of those screened was 51 (SD 15) years, and 64% were female. Fifty-one per cent of those screened (3369 patients) met the eligibility criteria and 43% (2847 patients) were ineligible. The eligibility status was unknown (or not recorded in the study clinic log book) for the other 6%. Common reasons for exclusion were recurrent varicose veins, no reflux or reflux < 1 second, veins < 3 mm or > 15 mm in diameter and the presence of comorbidities. Of the 3369 eligible patients, 798 (24%) consented to participate in the trial and 2571 (76%) declined. The most frequent reason for declining to take part was a preference for one form of treatment, and therefore a wish not to undergo randomisation. The reasons for ineligibility and declining to take part are shown in Table 9.
FIGURE 2.
Consolidated Standards of Reporting Trials diagram.

| Non-randomised screened patients | n | % |
|---|---|---|
| Reason for ineligibility | 2847 | |
| Recurrence | 789 | 27.7 |
| No reflux or reflux < 1 second | 624 | 21.9 |
| Patient comorbidity | 384 | 13.5 |
| GSV or SSV < 3 mm in diameter or > 15 mm | 264 | 9.3 |
| Tortuous veins that are considered to be unsuitable for EVLA | 242 | 8.5 |
| Vein related – no further information | 179 | 6.3 |
| Asymptomatic CEAP grade 2 | 156 | 5.5 |
| Thrombosis (current deep-vein incompetence, acute superficial vein thrombosis) | 58 | 2.0 |
| Other | 151 | 5.3 |
| Reason for declining to take part | 2571 | |
| Patient preference for surgery | 838 | 32.6 |
| Patient preference for EVLA | 761 | 29.6 |
| Patient preference for foam sclerotherapy | 341 | 13.3 |
| Patient did not want foam sclerotherapy | 35 | 1.4 |
| Patient did not want surgery | 17 | 0.7 |
| Patient did not want EVLA | 1 | < 0.1 |
| Other reasona | 554 | 21.5 |
| Surgeon preference | 24 | 0.9 |
Thirteen patients (1.6%) were found to be ineligible after randomisation, including five patients with veins > 15 mm in diameter and three who were later discovered to have recurrent varicose veins. The remainder were ineligible because of patient comorbidities or lack of reflux. These patients were treated as post-randomisation exclusions, and therefore 785 participants were included in the formal trial population. 71
Eleven participating centres contributed to recruitment, each in one of two strata. The eight centres in stratum A randomised to all three treatment arms, and the three in stratum B randomised to foam sclerotherapy or surgery only, because they were unable to offer EVLA treatment. The numbers of randomised participants recruited, by centre, are shown in Table 10.
| Stratum | Centre | Foam | Surgery | EVLA | Total randomised | Percentage of total recruitment |
|---|---|---|---|---|---|---|
| A | Aberdeen | 72 | 74 | 74 | 220 | 28.0 |
| Hull | 55 | 56 | 54 | 165 | 21.0 | |
| Leeds | 35 | 36 | 35 | 106 | 13.5 | |
| Bournemouth | 22 | 24 | 24 | 70 | 8.9 | |
| Newcastle | 11 | 14 | 13 | 38 | 4.8 | |
| Sheffield | 5 | 4 | 6 | 15 | 1.9 | |
| Worcester | 4 | 3 | 2 | 9 | 1.1 | |
| Blackburn | 3 | 2 | 2 | 7 | 0.9 | |
| Stratum A total | 207 | 213 | 210 | 630 | 80.3 | |
| B | Gloucestershire | 37 | 35 | N/A | 72 | 9.2 |
| Exeter | 35 | 34 | N/A | 69 | 8.8 | |
| Sherwood Forest | 7 | 7 | N/A | 14 | 1.8 | |
| Stratum B total | 79 | 76 | N/A | 155 | 19.7 | |
| Total recruitment | 286 | 289 | 210 | 785 | ||
Recruitment and randomisation took place over a period of 48 months. Figure 3 shows the recruitment rate over time.
FIGURE 3.
Recruitment over time.

All 785 participants attended for baseline clinical assessment and 779 (99%) completed baseline questionnaires. At the time of primary treatment, 720 (92%) received their randomised allocation, 27 (3%) received a study treatment other than their randomised treatment and 38 (5%) did not receive any of the study treatments.
With regard to those patients who did not receive the treatment to which they were randomised, 10 were randomised to foam sclerotherapy but received EVLA (six patients) or surgery (four patients) as their primary treatment. Reasons given for this included unsuitability for foam (veins considered too wide, history of migraine, dizziness/double vision prior to treatment, needle phobia), patient preference and logistic reasons. Eleven patients were randomised to foam and did not have any treatment within the trial. The most common reason for this was patient preference; two patients failed to attend for treatment/pre-treatment review, and one participant suffered a major stroke prior to treatment and was excluded from treatment and follow-up at this point.
Two patients who were randomised to EVLA received surgery as their primary treatment. In one case, this was because the patient wished to have treatment under general anaesthetic; in the other case, the responsible consultant recommended surgery because of the extensive nature of the veins.
Five patients randomised to EVLA did not undergo treatment. Two patients failed to attend for treatment, two declined treatment and one patient had cardiac problems which led to cancellation of planned treatment. Seven participants were randomised to surgery but received foam sclerotherapy as their primary treatment. For two of these, the reason was medical (unfit for general anaesthetic and back problems/concerns regarding anaesthetic/positioning). For the other five, the reasons related to a preference for foam sclerotherapy. Eight participants randomised to surgery opted to have EVLA treatment. All but one of these patients expressed a preference for EVLA after randomisation. One patient attended for surgery but panicked and was then listed for EVLA. Twenty-two patients were randomised to surgery and did not receive any treatment. The majority of these patients indicated a preference not to undergo surgery, but one declined because she was undergoing tests for possible cancer and three moved out of the study area before surgery was carried out.
At 6 weeks after treatment, 709 (90%) attended clinic for follow-up examination and 670 (85%) completed questionnaires. At 6 months, 670 (85%) attended clinic for follow-up examination and 627 (80%) completed questionnaires.
Baseline characteristics of the study participants
The mean age of participants was 49.2 (SD 13.7) years and 57% were female, so the trial cohort was slightly younger and had fewer females than the overall group of patients with varicose veins who were initially screened (Table 11). The mean body mass index (BMI) was 27.3 kg/m2 (SD 4.6 kg/m2), 61% were in employment and 221 participants (28%) had varicose veins in both legs; these participants nominated their worst leg as their ‘study leg’. Eleven per cent of participants had undergone previous varicose vein treatment of their non-study leg and 1% had received previous sclerotherapy for varicose veins in their study leg.
| Characteristics | All participants | ||
|---|---|---|---|
| Randomised ( n ) | 785 | ||
| Age (years) ( n , mean, range) | 785 | 49.2 | 18–85 |
| Female ( N , n , %) | 785 | 445 | 56.7 |
| BMI (kg/m 2 ) ( n , mean, range) | 725 | 27.3 | 17–44 |
| Employment status | |||
| Self-employed (N, n, %) | 773 | 87 | 11.3 |
| Employed (N, n, %) | 773 | 468 | 60.5 |
| Other (N, n, %) | 773 | 218 | 28.2 |
| Laterality | |||
| Unilateral (N, n, %) | 785 | 564 | 71.8 |
| Bilateral (N, n, %) | 785 | 221 | 28.2 |
| Previous history of DVT ( N , n , %) | 776 | 19 | 2.4 |
| Previous treatment to contralateral leg ( N , n , %) | 779 | 85 | 10.9 |
| Foam sclerotherapy (N, n, %) | 779 | 16 | 2.1 |
| Surgery (N, n, %) | 779 | 61 | 7.8 |
| EVLA treatment (N, n, %) | 779 | 7 | 0.9 |
| Previous sclerotherapy to tributaries of study leg ( N , n , %) | 779 | 7 | 0.9 |
Quality of life
A valid AVVQ score (our primary outcome) was obtained for all but one of the participants who completed a baseline questionnaire, and the mean score was 17.9 (SD 9.5) (Table 12). The mean EQ-5D was 0.79 (SD 0.18).
| QoL measure | All participants | ||
|---|---|---|---|
| Randomised ( n ) | 785 | ||
| AVVQ score ( n , mean, SD) | 778 | 17.9 | 9.5 |
| EQ-5D score ( n , mean, SD) | 764 | 0.79 | 0.18 |
| VAS (n, mean, SD) | 771 | 80.5 | 15.8 |
| SF-36 summary scores | |||
| Physical component summary score (n, mean, SD) | 723 | 48.6 | 8.3 |
| Mental component summary score (n, mean, SD) | 723 | 51.8 | 9.2 |
| SF-36 domain scores | |||
| Physical functioning (n, mean, SD) | 738 | 50.1 | 8.5 |
| Role physical (n, mean, SD) | 772 | 50.0 | 9.1 |
| Bodily pain (n, mean, SD) | 772 | 47.3 | 8.9 |
| General health (n, mean, SD) | 772 | 49.5 | 8.4 |
| Vitality (n, mean, SD) | 776 | 51.3 | 9.4 |
| Social functioning (n, mean, SD) | 773 | 50.7 | 9.0 |
| Role emotional (n, mean, SD) | 770 | 51.0 | 8.8 |
| Mental health (n, mean, SD) | 774 | 51.6 | 9.4 |
For SF-36, the mean physical component score of 48.6 (SD 8.3) was lower than the mean mental component score of 51.8 (SD 9.2) and the population norm score for the physical component of 50 (SD 10). This indicates that, compared with the wider population, the study cohort had slightly poorer physical health at baseline and the low SDs suggest that the cohort was a relatively uniform group in terms of QoL. The main factor contributing to the lower physical component score was the bodily pain domain, with a subscale score of 47.3 (SD 8.9).
Physical activity
A summary of physical activity data collected at the baseline clinic assessment is presented in Table 13. Thirteen per cent of participants spent most of their time at work sitting (e.g. in an office) and 31% spent most of their time at work standing or walking without requiring much intense physical effort. Twenty-four per cent worked in jobs involving definite physical effort, including handling heavy objects and using tools, and 6% had employment involving vigorous physical activity including handling very heavy objects.
| Physical activity | All participants | ||
|---|---|---|---|
| Randomised ( n ) | 785 | ||
| Physical activity at work | |||
| Mostly sitting (N, n, %) | 761 | 101 | 13.3 |
| Mostly standing or walking (N, n, %) | 761 | 239 | 31.4 |
| Definite physical effort (N, n, %) | 761 | 180 | 23.7 |
| Vigorous physical effort (N, n, %) | 761 | 44 | 5.8 |
| Not in employment (N, n, %) | 761 | 197 | 25.9 |
| Physical activity in previous week | |||
| Physical activities (N, n, %) | 770 | 348 | 45.2 |
| Cycling (N, n, %) | 755 | 126 | 16.7 |
| Walking (N, n, %) | 769 | 756 | 98.3 |
| Housework/childcare (N, n, %) | 767 | 674 | 87.9 |
| Gardening (N, n, %) | 765 | 473 | 61.8 |
| Usual walking pace | |||
| Slow (N, n, %) | 771 | 48 | 6.2 |
| Steady/average (N, n, %) | 771 | 374 | 48.5 |
| Brisk (N, n, %) | 771 | 293 | 38.0 |
| Fast (N, n, %) | 771 | 56 | 7.3 |
In the week prior to recruitment, 45% of participants engaged in physical exercise such as swimming, jogging, aerobics, football, tennis and gym workouts (including 21% who had exercised for more than 3 hours).
Baseline characteristics of study leg
The majority of participants (653/785, 83%) had GSV reflux only, 56 (7%) had SSV reflux only and the remaining 76 (10%) had both GSV and SSV involvement (Table 14). The proportions of participants with left or right leg involvement were similar. The mean widest diameter below the SFJ was 8.7 mm for those with GSV involvement only, and for those with SSV involvement only, the mean widest diameter below the saphenopopliteal junction was 7.5 mm. Of those with any GSV involvement, 90% had reflux above the knee only. All participants had a CEAP classification of C2 or above (reflecting the inclusion criteria for the trial), and 55% had grade C2 veins. The mean VCSS at baseline was 5.0 (SD 2.5). The severity of varicose veins, assessed on a VAS from 0 (none) to 10 (worst possible), recorded higher scores (i.e. a perception of more varicose veins) when assessed by the participant (mean score 5.5, SD 2.2) than when assessed by the research nurse (mean score 3.8, SD 2.2).
| Study leg vein characteristics | All participants | ||
|---|---|---|---|
| Randomised ( n ) | 785 | ||
| Study leg | |||
| Right (N, n, %) | 785 | 382 | 48.7 |
| Left (N, n, %) | 785 | 403 | 51.3 |
| Saphenous involvement | |||
| GSV only (N, n, %) | 785 | 653 | 83.2 |
| Widest diameter (mm) (n, mean, range) | 587 | 8.7 | 3–15 |
| Reflux above knee only (N, n, %) | 520 | 500 | 96.2 |
| Reflux above and below knee (N, n, %) | 520 | 20 | 3.8 |
| SSV only (N, n, %) | 785 | 56 | 7.1 |
| Widest diameter (mm) (n, mean, range) | 50 | 7.5 | 3–15 |
| GSV and SSV (N, n, %) | 785 | 76 | 9.7 |
| Widest diameter GSV (mm) (n, mean, range) | 72 | 7.2 | 3–15 |
| Widest diameter SSV (mm) (n, mean, range) | 66 | 5.4 | 3–15 |
| Reflux above knee only (N, n, %) | 60 | 27 | 45.0 |
| Reflux above and below knee (N, n, %) | 60 | 33 | 55.0 |
| Deep-vein reflux ( N , n , %) | 767 | 100 | 13.0 |
| CEAP classification | |||
| C0 No visible or palpable signs of venous disease (N, n, %) | 782 | 0 | 0.0 |
| C1 Telangiectasis or reticular veins < 3 mm (N, n, %) | 782 | 0 | 0.0 |
| C2 Varicose veins > 3 mm (N, n, %) | 782 | 429 | 54.9 |
| C3 Oedema (N, n, %) | 782 | 102 | 13.0 |
| C4 Skin and subcutaneous changes (N, n, %) | 782 | 79 | 10.1 |
| C4a Pigmentation or eczema (N, n, %) | 782 | 130 | 16.6 |
| C4b Lipodermatosclerosis or atrophie blanche (N, n, %) | 782 | 11 | 1.4 |
| C5 Healed venous ulcer (N, n, %) | 782 | 20 | 2.6 |
| C6 Active venous ulcer (N, n, %) | 782 | 11 | 1.4 |
| VCSS ( n , mean, SD) | 778 | 5.0 | 2.5 |
| Presence of varicose veins | |||
| Assessed by participant (N, n, %) | 785 | 783 | 99.7 |
| VAS (n, mean, SD) | 785 | 5.5 | 2.2 |
| Assessed by research nurse (N, n, %) | 785 | 784 | 99.9 |
| VAS (n, mean, SD) | 785 | 3.8 | 2.2 |
Contralateral leg
The baseline CEAP classification and VCSSs are summarised in Table 15 for the 28% of participants with bilateral disease. The majority of these participants (63%) had a C2 CEAP classification for their contralateral leg; the distribution of CEAP classifications was similar for study legs and contralateral legs. The mean VCSS for contralateral legs was 3.8 (SD 2.3), which was lower than the mean score of 5.0 for the study legs.
| Non-study leg vein characteristics | All participants | ||
|---|---|---|---|
| Randomised ( n ) | 785 | ||
| Participants with bilateral disease ( N , n , %) | 785 | 221 | 28.2 |
| CEAP classification | |||
| C2 Varicose veins > 3 mm (N, n, %) | 213 | 135 | 63.4 |
| C3 Oedema (N, n, %) | 213 | 31 | 14.6 |
| C4 Skin and subcutaneous changes (N, n, %) | 213 | 16 | 7.5 |
| C4a Pigmentation or eczema (N, n, %) | 213 | 27 | 12.7 |
| C4b Lipodermatosclerosis or atrophie blanche (N, n, %) | 213 | 1 | 0.5 |
| C5 Healed venous ulcer (N, n, %) | 213 | 2 | 0.9 |
| C6 Active venous ulcer (N, n, %) | 213 | 1 | 0.5 |
| VCSS ( n , mean, SD) | 209 | 3.8 | 2.3 |
Results from the foam sclerotherapy versus surgery comparison, which included patients from all centres (n = 575), are described in Chapter 5. Results from the foam versus EVLA and EVLA versus surgery comparisons, which included patients in the centres which participated in the three arms (n = 630), are described in Chapter 6.
Chapter 5 Comparison of surgery and foam sclerotherapy
In this chapter we report the results for surgery compared with foam sclerotherapy, using data from all centres. A discussion of these results is included in Chapter 7.
Participants
Five hundred and eighty-six participants were randomised to either foam sclerotherapy or surgery; of these, 11 (2%) were post-randomisation exclusions (see Chapter 4), leaving a total of 575 participants included in the trial analysis (286 in the foam arm and 289 in the surgery arm). The CONSORT diagram (see Figure 2) describes the flow of participants in the trial.
The proportion receiving treatment as allocated appeared to be higher for foam sclerotherapy (93%) than for surgery (87%). Retention appeared to be slightly higher for foam, in terms of both follow-up clinic assessments and completion of participant questionnaires. The 6-weeks clinic was attended by 93% of participants randomised to foam sclerotherapy compared with 87% of those receiving surgery, and at 6 months the attendance rates were 88% and 82% respectively. The 6-weeks questionnaire was completed by 86% of participants randomised to foam sclerotherapy compared with 82% of those randomised to surgery, and at 6 months the response rates were 83% and 74% respectively. A slightly larger proportion of participants appeared to have withdrawn in the surgery arm at 6 months (11%) than in the foam arm (6%).
Baseline characteristics
Demographic details
The main baseline characteristics of study participants are shown in Table 16. There was a good balance between groups for most factors, particularly for age and sex, which were minimisation variables. There was a slight imbalance between groups in terms of bilateral disease. The data shown in Table 16 were used in the analysis when adjusting for minimisation factors.
| Participant characteristics | Randomised to foam sclerotherapy | Randomised to surgery | ||||
|---|---|---|---|---|---|---|
| Randomised ( n ) | 286 | 289 | ||||
| Age (years) ( n , mean, range) | 286 | 49.0 | 19–78 | 289 | 49.2 | 22–85 |
| Female ( N , n , %) | 286 | 162 | 56.6 | 289 | 163 | 56.4 |
| BMI (kg/m 2 ) ( n , mean, range) | 269 | 27.1 | 17–44 | 261 | 27.7 | 17–44 |
| Employment status | ||||||
| Self-employed (N, n, %) | 285 | 37 | 13.0 | 282 | 29 | 10.3 |
| Employed (N, n, %) | 285 | 169 | 59.3 | 282 | 179 | 63.5 |
| Other (N, n, %) | 285 | 79 | 27.7 | 282 | 74 | 26.2 |
| Laterality | ||||||
| Unilateral (N, n, %) | 286 | 215 | 75.2 | 289 | 196 | 67.8 |
| Bilateral (N, n, %) | 286 | 71 | 24.8 | 289 | 93 | 32.2 |
| Previous history of DVT ( N , n , %) | 284 | 4 | 1.4 | 286 | 9 | 3.1 |
| Previous treatment to contralateral leg ( N , n , %) | 284 | 30 | 10.6 | 287 | 28 | 9.8 |
| Foam sclerotherapy (N, n, %) | 284 | 10 | 3.5 | 287 | 2 | 0.7 |
| Surgery (N, n, %) | 284 | 20 | 7.0 | 287 | 22 | 7.7 |
| Laser treatment (N, n, %) | 284 | 0 | 0.0 | 287 | 4 | 1.4 |
| Previous sclerotherapy to tributaries of study leg ( N , n , %) | 284 | 0 | 0.0 | 287 | 4 | 1.4 |
Quality of life
Quality of life was assessed prior to the patient being randomised. QoL appeared to be slightly better in the foam group and this is reflected in the AVVQ, EQ-5D and each of the SF-36 components and subdomain scores (Table 17). For foam, the baseline AVVQ score was 17.6 (SD 10.0) and for surgery it was 18.2 (SD 9.2) (higher scores indicate worse QoL).
| QoL measure | Randomised to foam sclerotherapy | Randomised to surgery | ||||
|---|---|---|---|---|---|---|
| Randomised ( n ) | 286 | 289 | ||||
| AVVQ score ( n , mean, SD) | 284 | 17.6 | 10.0 | 284 | 18.2 | 9.2 |
| EQ-5D score ( n , mean, SD) | 279 | 0.80 | 0.18 | 279 | 0.78 | 0.18 |
| VAS (n, mean, SD) | 282 | 80.8 | 15.6 | 283 | 80.2 | 15.6 |
| SF-36 summary scores | ||||||
| Physical component summary score (n, mean, SD) | 275 | 48.9 | 8.2 | 275 | 48.2 | 8.8 |
| Mental component summary score (n, mean, SD) | 275 | 52.4 | 8.9 | 275 | 51.2 | 9.6 |
| SF-36 domain scores | ||||||
| Physical functioning (n, mean, SD) | 283 | 50.1 | 8.8 | 281 | 50.1 | 8.3 |
| Role physical (n, mean, SD) | 284 | 50.7 | 8.7 | 280 | 49.1 | 10.0 |
| Bodily pain (n, mean, SD) | 283 | 48.2 | 8.8 | 282 | 46.3 | 9.3 |
| General health (n, mean, SD) | 279 | 49.8 | 8.0 | 284 | 49.2 | 8.9 |
| Vitality (n, mean, SD) | 283 | 51.6 | 9.5 | 283 | 50.8 | 9.5 |
| Social functioning (n, mean, SD) | 283 | 51.5 | 8.1 | 283 | 49.8 | 9.9 |
| Role emotional (n, mean, SD) | 283 | 51.3 | 8.2 | 279 | 50.5 | 9.6 |
| Mental health (n, mean, SD) | 283 | 52.1 | 9.2 | 282 | 50.9 | 9.8 |
Physical activity
There was a good balance between the groups in terms of physical activity at baseline (Table 18).
| Physical activity | Randomised to foam sclerotherapy | Randomised to surgery | ||||
|---|---|---|---|---|---|---|
| Randomised ( n ) | 286 | 289 | ||||
| Physical activity at work | ||||||
| Mostly sitting (N, n, %) | 280 | 38 | 13.6 | 279 | 36 | 12.9 |
| Mostly standing or walking (N, n, %) | 280 | 102 | 36.4 | 279 | 90 | 32.3 |
| Definite physical effort (N, n, %) | 280 | 56 | 20.0 | 279 | 72 | 25.8 |
| Vigorous physical effort (N, n, %) | 280 | 15 | 5.4 | 279 | 13 | 4.7 |
| Not in employment (N, n, %) | 280 | 69 | 24.6 | 279 | 68 | 24.4 |
| Physical activity in previous week | ||||||
| Physical activities (N, n, %) | 284 | 129 | 45.4 | 282 | 124 | 44.0 |
| Cycling (N, n, %) | 280 | 39 | 13.9 | 278 | 55 | 19.8 |
| Walking (N, n, %) | 285 | 279 | 97.9 | 280 | 275 | 98.2 |
| Housework/childcare (N, n, %) | 284 | 254 | 89.4 | 280 | 243 | 86.8 |
| Gardening (N, n, %) | 283 | 187 | 66.1 | 279 | 172 | 61.6 |
| Usual walking pace | ||||||
| Slow (N, n, %) | 283 | 13 | 4.6 | 283 | 18 | 6.4 |
| Steady/average (N, n, %) | 283 | 129 | 45.6 | 283 | 144 | 50.9 |
| Brisk (N, n, %) | 283 | 121 | 42.8 | 283 | 100 | 35.3 |
| Fast (N, n, %) | 283 | 20 | 7.1 | 283 | 21 | 7.4 |
Varicose vein characteristics
Baseline characteristics describing the varicose veins in the participants’ study and contralateral legs are shown in Tables 19 and 20 respectively. The groups are well balanced across the majority of factors (except for deep-vein reflux, where 17% in the foam group were affected compared with 9% in the surgery group).
| Study leg vein characteristics | Randomised to foam sclerotherapy | Randomised to surgery | ||||
|---|---|---|---|---|---|---|
| Randomised ( n ) | 286 | 289 | ||||
| Study leg | ||||||
| Right (N, n, %) | 286 | 136 | 47.6 | 289 | 138 | 47.8 |
| Left (N, n, %) | 286 | 150 | 52.4 | 289 | 151 | 52.2 |
| Saphenous involvement | ||||||
| GSV only (N, n, %) | 286 | 232 | 81.1 | 289 | 239 | 82.7 |
| Widest diameter (mm) (n, mean, range) | 211 | 8.4 | 4–15 | 214 | 8.7 | 3–15 |
| Reflux above knee only (N, n, %) | 180 | 169 | 93.9 | 183 | 180 | 98.4 |
| Reflux above and below knee (N, n, %) | 180 | 11 | 6.1 | 183 | 3 | 1.6 |
| SSV only (N, n, %) | 286 | 21 | 7.3 | 289 | 21 | 7.3 |
| Widest diameter (mm) (n, mean, range) | 20 | 7.6 | 3–11 | 17 | 7.7 | 4–15 |
| GSV and SSV (N, n, %) | 286 | 33 | 11.5 | 289 | 29 | 10.0 |
| Widest diameter GSV (mm) (n, mean, range) | 31 | 7.3 | 3–14 | 28 | 7.6 | 3–15 |
| Widest diameter SSV (mm) (n, mean, range) | 30 | 5.0 | 3–10 | 26 | 5.4 | 3–8 |
| Reflux above knee only (N, n, %) | 26 | 11 | 42.3 | 22 | 10 | 45.5 |
| Reflux above and below knee (N, n, %) | 26 | 15 | 57.7 | 22 | 12 | 54.5 |
| Deep-vein reflux ( N , n , %) | 280 | 47 | 16.8 | 282 | 25 | 8.9 |
| CEAP classification | ||||||
| C0 No visible or palpable signs of venous disease (N, n, %) | 286 | 0 | 0.0 | 287 | 0 | 0.0 |
| C1 Telangiectasis or reticular veins < 3 mm (N, n, %) | 286 | 0 | 0.0 | 287 | 0 | 0.0 |
| C2 Varicose veins > 3 mm (N, n, %) | 286 | 169 | 59.1 | 287 | 147 | 51.2 |
| C3 Oedema (N, n, %) | 286 | 35 | 12.2 | 287 | 39 | 13.6 |
| C4 Skin and subcutaneous changes (N, n, %) | 286 | 30 | 10.5 | 287 | 32 | 11.1 |
| C4a Pigmentation or eczema (N, n, %) | 286 | 41 | 14.3 | 287 | 55 | 19.2 |
| C4b Lipodermatosclerosis or atrophie blanche (N, n, %) | 286 | 3 | 1.0 | 287 | 3 | 1.0 |
| C5 Healed venous ulcer (N, n, %) | 286 | 4 | 1.4 | 287 | 7 | 2.4 |
| C6 Active venous ulcer (N, n, %) | 286 | 4 | 1.4 | 287 | 4 | 1.4 |
| VCSS ( n , mean, SD) | 285 | 4.9 | 2.6 | 286 | 5.1 | 2.5 |
| Presence of varicose veins | ||||||
| Assessed by participant (N, n, %) | 286 | 286 | 100 | 289 | 288 | 99.7 |
| VAS (n, mean, SD) | 286 | 5.4 | 2.2 | 289 | 5.6 | 2.3 |
| Assessed by research nurse (N, n, %) | 286 | 286 | 100 | 289 | 288 | 99.7 |
| VAS (n, mean, SD) | 286 | 3.9 | 2.1 | 289 | 4.0 | 2.2 |
| Non-study leg vein characteristics | Randomised to foam sclerotherapy | Randomised to surgery | ||||
|---|---|---|---|---|---|---|
| Randomised ( n ) | 286 | 289 | ||||
| Participants with bilateral disease ( N , n , %) | 286 | 71 | 24.8 | 289 | 93 | 32.5 |
| CEAP classification | ||||||
| C2 Varicose veins > 3 mm (N, n, %) | 68 | 49 | 72.1 | 90 | 50 | 55.6 |
| C3 Oedema (N, n, %) | 68 | 8 | 11.8 | 90 | 15 | 16.7 |
| C4 Skin and subcutaneous changes (N, n, %) | 68 | 3 | 4.4 | 90 | 9 | 10.0 |
| C4a Pigmentation or eczema (N, n, %) | 68 | 6 | 8.8 | 90 | 15 | 16.7 |
| C4b Lipodermatosclerosis or atrophie blanche (N, n, %) | 68 | 0 | 0.0 | 90 | 0 | 0.0 |
| C5 Healed venous ulcer (N, n, %) | 68 | 2 | 2.9 | 90 | 0 | 0.0 |
| C6 Active venous ulcer (N, n, %) | 68 | 0 | 0.0 | 90 | 1 | 1.1 |
| VCSS ( N , mean, SD) | 67 | 3.8 | 2.4 | 88 | 3.9 | 2.2 |
Treatment received
Table 21 summarises the primary interventions received (i.e. excluding any delayed secondary foam treatments), summarised by randomised allocation. For participants randomised to foam, 96% of those who received treatment had their treatment as randomised (i.e. had foam sclerotherapy). The equivalent proportion in the surgery arm was 94%. The participants who did not undergo their randomised treatment are described in Chapter 4.
| Primary intervention | Randomised to foam sclerotherapy | Randomised to surgery | ||||
|---|---|---|---|---|---|---|
| Treated ( n ) | 275 | 267 | ||||
| Received foam (N, n, %) | 275 | 265 | 96.4 | 267 | 7 | 2.6 |
| Received surgery (N, n, %) | 275 | 4 | 1.5 | 267 | 252 | 94.4 |
| Received laser (N, n, %) | 275 | 6 | 2.2 | 267 | 8 | 3.0 |
| Treatment time (minutes) ( n , mean, SD) | 248 | 19.0 | 10.6 | 247 | 53.0 | 22.9 |
| Grade of surgeon | ||||||
| Consultant (N, n, %) | 271 | 208 | 76.8 | 261 | 153 | 58.6 |
| Consultant nurse (N, n, %) | 271 | 33 | 12.2 | 261 | 3 | 1.1 |
| Staff grade (supervised) (N, n, %) | 271 | 3 | 1.1 | 261 | 14 | 5.4 |
| Staff grade (unsupervised) (N, n, %) | 271 | 2 | 0.7 | 261 | 8 | 3.1 |
| Trainee (supervised) (N, n, %) | 271 | 14 | 5.2 | 261 | 42 | 16.1 |
| Trainee (unsupervised) (N, n, %) | 271 | 11 | 4.1 | 261 | 41 | 15.7 |
| Treatment to non-truncal varicosities ( N , n , %) | 270 | 84 | 31.1 | 260 | 233 | 89.6 |
| Concurrent contralateral treatment ( N , n , %) | 269 | 7 | 2.6 | 261 | 40 | 15.3 |
| Subcutaneous heparin (or derivative) ( N , n , %) | 262 | 13 | 5.0 | 238 | 115 | 48.3 |
| Overnight hospitalisation | ||||||
| Planned (N, n, %) | 268 | 0 | 0.0 | 203 | 8 | 3.9 |
| Unplanned (N, n, %) | 268 | 0 | 0.0 | 203 | 5 | 2.5 |
| Bandaging not according to protocol ( N , n , %) | 272 | 103a | 37.9 | 256 | 29 | 11.3 |
| Recommended duration of bandaging (if not for 10 days) (n, mean, SD) | 10 | 6.8 | 0.6 | 27 | 6.7 | 3.2 |
Procedure and treatment time
At the time of the primary intervention, more participants in the surgery arm had treatment to non-truncal varicosities (90%) than in the foam arm (31%) (see Table 21). More patients in the surgery arm (15%) had their contralateral leg treated at the same time than in the foam arm (3%).
The mean treatment duration (the time taken from preparation of the patient to completion of bandaging) was longer for surgery (53 minutes, SD 22.9 minutes) than for foam (19 minutes, SD 10.6 minutes). Foam sclerotherapy was performed by consultants much more often (77% of treatments) than surgery (59%). Only 8% of foam participants received treatment from a trainee, compared with 32% in the surgery group. The remainder of procedures were performed by staff grades or nurse consultants. Nearly all patients (98%) randomised to surgery had a general anaesthetic, with six receiving an epidural/spinal anaesthetic (Table 22). The anaesthetist was a consultant in 83% of cases.
| Anaesthetic details | Randomised to foam sclerotherapya | Randomised to surgery | ||||
|---|---|---|---|---|---|---|
| Received surgery ( n ) | 4 | 252 | ||||
| Type of anaesthetic | ||||||
| General (N, n, %) | 4 | 4 | 100.0 | 248 | 242 | 97.6 |
| Epidural/spinal (N, n, %) | 4 | 0 | 0.0 | 248 | 6 | 2.4 |
| Grade of anaesthetist | ||||||
| Consultant (N, n, %) | 4 | 3 | 75.0 | 245 | 204 | 83.3 |
| Associate specialist (N, n, %) | 4 | 0 | 0.0 | 245 | 5 | 2.0 |
| Registrar (N, n, %) | 4 | 1 | 25.0 | 245 | 27 | 11.0 |
| Staff grade (N, n, %) | 4 | 0 | 0.0 | 245 | 9 | 3.7 |
| Senior house officer (N, n, %) | 4 | 0 | 0.0 | 245 | 0 | 0.0 |
Primary treatment volume of foam
The total mean volume of foam administered was 9.0 ml (SD 2.9 ml). When the GSV alone was treated, the mean volume was 9.2 ml (SD 2.9 ml), with 8 ml (SD 3.0 ml) to the truncal vein and 1.2 ml (SD 2.2 ml) to the truncal varicosities (Table 23). There were six patients who received foam in excess of the 12-ml limit recommended in the protocol, without adverse consequences.
| Primary foam treatment | Randomised to foam sclerotherapy | Randomised to surgerya | ||||
|---|---|---|---|---|---|---|
| Received primary treatment of foam ( n ) | 265 | 7 | ||||
| Volume of foam (ml) ( n , mean, range) | 265 | 9.0 | 2–15 | 7 | 8.3 | 6–10 |
| GSV involvement only (total) (n, mean, range) | 215 | 9.2 | 2–15 | 5 | 7.6 | 6–10 |
| GSV (n, mean, range) | 215 | 8.0 | 0–15 | 5 | 6.8 | 6–8 |
| Non-truncal varicosities (n, mean, range) | 215 | 1.2 | 0–12 | 5 | 0.8 | 0–4 |
| SSV involvement only (total) (n, mean, range) | 20 | 6.8 | 2–12 | 1 | 10.0 | 10–10 |
| SSV (n, mean, range) | 20 | 5.4 | 0–12 | 1 | 10.0 | 10–10 |
| Non-truncal varicosities (n, mean, range) | 20 | 1.4 | 0–6 | 1 | 0.0 | 0–0 |
| GSV and SSV involvement (total) (n, mean, range) | 30 | 9.0 | 6–13 | 1 | 10.0 | 10–10 |
| GSV (n, mean, range) | 30 | 6.8 | 2–12 | 1 | 6.0 | 6–6 |
| SSV (n, mean, range) | 30 | 0.7 | 0–5 | 1 | 0.0 | 0–0 |
| Non-truncal varicosities (n, mean, range) | 30 | 1.4 | 0–6 | 1 | 4.0 | 4–4 |
Secondary or tertiary foam treatments
The numbers of participants who received secondary or tertiary treatments of foam sclerotherapy, along with a breakdown of the location of treatment, is shown in Table 24. Seventeen participants randomised to foam sclerotherapy (7%) received additional foam treatment to non-truncal varicosities, compared with one randomised to surgery (1%). There were two participants whose second foam treatment included foam sclerotherapy to the GSV and also to non-truncal varicosities. The one participant who received a tertiary treatment had treatment to both the SSV and GSV.
| Secondary foam treatment | Randomised to foam sclerotherapy | Randomised to surgerya | ||||
|---|---|---|---|---|---|---|
| No secondary foam treatment ( N , n , %) | 251 | 224 | 89.2 | 236 | 233 | 98.7 |
| One secondary foam treatment ( N , n , %) | 251 | 26 | 10.4 | 236 | 3 | 1.3 |
| to GSV (N, n, %) | 251 | 9 | 3.6 | 236 | 1 | 0.4 |
| to SSV (N, n, %) | 251 | 2 | 0.8 | 236 | 1 | 0.4 |
| to non-truncal varicosities (N, n, %) | 251 | 17 | 6.8 | 236 | 1 | 0.4 |
| Two secondary foam treatments ( N , n , %) | 251 | 1 | 0.4 | 236 | 0 | 0.0 |
| to GSV (N, n, %) | 251 | 1 | 0.4 | 236 | 0 | 0.0 |
| to SSV (N, n, %) | 251 | 1 | 0.4 | 236 | 0 | 0.0 |
| to non-truncal varicosities (N, n, %) | 251 | 0 | 0.0 | 236 | 0 | 0.0 |
Bandaging/compression
All participants had a bandage or stocking applied to their study leg, nearly all of which were full length. More foam participants (38%) than surgery participants (11%) received bandaging not according to protocol (see Table 21). The main reason for this difference was that the protocol specified the brand of bandaging/compression for patients undergoing foam sclerotherapy, whereas, for surgery, any type of bandaging for 10 days was sufficient. Of the 103 cases of foam participants whose bandaging was not according to protocol, only 10 were related to duration and the other 93 were related to the type of stockings.
Treatment outcome: quality of life
The QoL at 6 weeks and 6-months follow-up are shown in Tables 25 and 26, with the corresponding statistical analysis in Table 27.
| QoL measure | Randomised to foam sclerotherapy | Randomised to surgery | ||||
|---|---|---|---|---|---|---|
| Completed 6-weeks questionnaire ( N , n , %) | 286 | 247 | 86.4 | 289 | 237 | 82.0 |
| AVVQ score ( n , mean, SD) | 246 | 12.2 | 9.6 | 235 | 10.6 | 8.8 |
| EQ-5D score ( n , mean, SD) | 242 | 0.86 | 0.16 | 227 | 0.88 | 0.17 |
| VAS (n, mean, SD) | 244 | 80.6 | 17.3 | 232 | 83.1 | 15.5 |
| SF-36 summary scores | ||||||
| Physical component summary score (n, mean, SD) | 242 | 49.9 | 8.7 | 226 | 49.7 | 8.9 |
| Mental component summary score (n, mean, SD) | 242 | 52.3 | 9.0 | 226 | 51.7 | 8.9 |
| SF-36 domain scores | ||||||
| Physical functioning (n, mean, SD) | 245 | 50.8 | 8.9 | 235 | 51.5 | 8.1 |
| Role physical (n, mean, SD) | 246 | 50.3 | 9.6 | 235 | 48.1 | 10.3 |
| Bodily pain (n, mean, SD) | 244 | 50.2 | 9.2 | 229 | 49.1 | 10.1 |
| General health (n, mean, SD) | 245 | 50.8 | 9.0 | 232 | 52.2 | 8.9 |
| Vitality (n, mean, SD) | 245 | 52.3 | 9.7 | 232 | 51.7 | 9.3 |
| Social functioning (n, mean, SD) | 243 | 51.1 | 8.9 | 230 | 50.2 | 9.5 |
| Role emotional (n, mean, SD) | 246 | 51.2 | 8.6 | 234 | 50.0 | 10.4 |
| Mental health (n, mean, SD) | 245 | 52.5 | 8.8 | 232 | 52.3 | 9.0 |
| QoL measure | Randomised to foam sclerotherapy | Randomised to surgery | ||||
|---|---|---|---|---|---|---|
| Completed 6-months questionnaire (N, n , %) | 286 | 238 | 83.2 | 289 | 214 | 74.0 |
| AVVQ score ( n , mean, SD) | 236 | 9.1 | 7.9 | 213 | 7.8 | 7.5 |
| EQ-5D score ( n , mean, SD) | 235 | 0.90 | 0.17 | 206 | 0.88 | 0.20 |
| VAS (n, mean, SD) | 237 | 84.5 | 12.3 | 210 | 82.8 | 15.3 |
| SF-36 summary scores | ||||||
| Physical component summary score (n, mean, SD) | 232 | 52.3 | 8.5 | 204 | 52.4 | 8.9 |
| Mental component summary score (n, mean, SD) | 232 | 52.2 | 9.1 | 204 | 52.1 | 8.6 |
| SF-36 domain scores | ||||||
| Physical functioning (n, mean, SD) | 237 | 52.0 | 7.9 | 213 | 51.5 | 8.8 |
| Role physical (n, mean, SD) | 236 | 52.3 | 8.1 | 213 | 51.9 | 8.7 |
| Bodily pain (n, mean, SD) | 235 | 53.0 | 9.5 | 209 | 53.7 | 10.0 |
| General health (n, mean, SD) | 238 | 51.8 | 8.7 | 212 | 51.9 | 9.6 |
| Vitality (n, mean, SD) | 238 | 53.0 | 9.6 | 212 | 53.0 | 9.7 |
| Social functioning (n, mean, SD) | 235 | 52.6 | 8.2 | 211 | 51.9 | 9.0 |
| Role emotional (n, mean, SD) | 237 | 51.5 | 8.8 | 211 | 51.5 | 9.3 |
| Mental health (n, mean, SD) | 238 | 52.5 | 9.2 | 212 | 51.8 | 9.3 |
| QoL measure | Randomised to foam sclerotherapy | Randomised to surgery | Surgery vs. foam sclerotherapy | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | Effect sizea | 95% CI | p-value | |
| AVVQ score at baseline | 286 | 17.6 | 9.9 | 289 | 18.2 | 9.1 | N/A | N/A | N/A |
| AVVQ score at 6 weeks | 246 | 12.2 | 9.6 | 235 | 10.6 | 8.8 | –2.26 | –3.67 to –0.86 | 0.002 |
| AVVQ score at 6 months | 236 | 9.1 | 7.9 | 213 | 7.8 | 7.5 | –1.74 | –2.97 to –0.50 | 0.006 |
| EQ-5D at baseline | 286 | 0.80 | 0.18 | 289 | 0.78 | 0.17 | N/A | N/A | N/A |
| EQ-5D at 6 weeks | 242 | 0.86 | 0.16 | 227 | 0.88 | 0.17 | 0.02 | 0.00 to 0.05 | 0.071 |
| EQ-5D at 6 months | 235 | 0.90 | 0.17 | 206 | 0.88 | 0.20 | 0.01 | –0.02 to 0.04 | 0.732 |
| EQ-5D VAS at baseline | 286 | 80.8 | 15.5 | 289 | 80.2 | 15.5 | N/A | N/A | N/A |
| EQ-5D VAS at 6 weeks | 244 | 80.6 | 17.3 | 232 | 83.1 | 15.5 | 2.90 | 0.31 to 5.48 | 0.028 |
| EQ-5D VAS at 6 months | 237 | 84.5 | 12.3 | 210 | 82.8 | 15.3 | –1.23 | –3.42 to 0.96 | 0.270 |
| SF-36 physical component score at baseline | 286 | 48.9 | 8.0 | 289 | 48.2 | 8.6 | N/A | N/A | N/A |
| SF-36 physical component score at 6 weeks | 242 | 49.9 | 8.7 | 226 | 49.7 | 8.9 | 0.27 | –1.03 to 1.56 | 0.687 |
| SF-36 physical component score at 6 months | 232 | 52.3 | 8.5 | 204 | 52.4 | 8.9 | 1.03 | –0.25 to 2.30 | 0.114 |
| SF-36 mental component score at baseline | 286 | 52.4 | 8.7 | 289 | 51.2 | 9.4 | N/A | N/A | N/A |
| SF-36 mental component score at 6 weeks | 242 | 52.3 | 9.0 | 226 | 51.7 | 8.9 | –0.44 | –1.82 to 0.93 | 0.527 |
| SF-36 mental component score at 6 months | 232 | 52.2 | 9.1 | 204 | 52.1 | 8.6 | 0.23 | –1.10 to 1.56 | 0.738 |
| SF-36 Physical functioning at baseline | 286 | 50.1 | 8.7 | 289 | 50.1 | 8.2 | N/A | N/A | N/A |
| SF-36 Physical functioning at 6 weeks | 245 | 50.8 | 8.9 | 235 | 51.5 | 8.1 | 0.60 | –0.58 to 1.77 | 0.320 |
| SF-36 Physical functioning at 6 months | 237 | 52.0 | 7.9 | 213 | 51.5 | 8.8 | –0.28 | –1.39 to 0.84 | 0.625 |
| SF-36 Role physical at baseline | 286 | 50.6 | 8.6 | 289 | 49.1 | 9.9 | N/A | N/A | N/A |
| SF-36 Role physical at 6 weeks | 246 | 50.3 | 9.6 | 235 | 48.1 | 10.3 | –1.56 | –3.14 to 0.02 | 0.053 |
| SF-36 Role physical at 6 months | 236 | 52.3 | 8.1 | 213 | 51.9 | 8.7 | 0.65 | –0.53 to 1.83 | 0.278 |
| SF-36 Bodily pain at baseline | 286 | 48.1 | 8.8 | 289 | 46.3 | 9.2 | N/A | N/A | N/A |
| SF-36 Bodily pain at 6 weeks | 244 | 50.2 | 9.2 | 229 | 49.1 | 10.1 | –0.39 | –1.97 to 1.19 | 0.627 |
| SF-36 Bodily pain at 6 months | 235 | 53.0 | 9.5 | 209 | 53.7 | 10.0 | 1.95 | 0.42 to 3.47 | 0.012 |
| SF-36 General health at baseline | 286 | 49.8 | 7.9 | 289 | 49.2 | 8.8 | N/A | N/A | N/A |
| SF-36 General health at 6 weeks | 245 | 50.8 | 9.0 | 232 | 52.2 | 8.9 | 1.63 | 0.41 to 2.85 | 0.009 |
| SF-36 General health at 6 months | 238 | 51.8 | 8.7 | 212 | 51.9 | 9.6 | 0.46 | –0.82 to 1.75 | 0.480 |
| SF-36 Vitality at baseline | 286 | 51.6 | 9.5 | 289 | 50.8 | 9.4 | N/A | N/A | N/A |
| SF-36 Vitality at 6 weeks | 245 | 52.3 | 9.7 | 232 | 51.7 | 9.3 | –0.35 | –1.72 to 1.01 | 0.611 |
| SF-36 Vitality at 6 months | 238 | 53.0 | 9.6 | 212 | 53.0 | 9.7 | 0.37 | –0.96 to 1.69 | 0.589 |
| SF-36 Social functioning at baseline | 286 | 51.5 | 8.1 | 289 | 49.8 | 9.8 | N/A | N/A | N/A |
| SF-36 Social functioning at 6 weeks | 243 | 51.1 | 8.9 | 230 | 50.2 | 9.5 | –0.25 | –1.70 to 1.21 | 0.742 |
| SF-36 Social functioning at 6 months | 235 | 52.6 | 8.2 | 211 | 51.9 | 9.0 | 0.38 | –0.89 to 1.65 | 0.554 |
| SF-36 Role emotional at baseline | 286 | 51.3 | 8.2 | 289 | 50.5 | 9.4 | N/A | N/A | N/A |
| SF-36 Role emotional at 6 weeks | 246 | 51.2 | 8.6 | 234 | 50.0 | 10.4 | –1.06 | –2.57 to 0.44 | 0.164 |
| SF-36 Role emotional at 6 months | 237 | 51.5 | 8.8 | 211 | 51.5 | 9.3 | 0.49 | –0.95 to 1.92 | 0.505 |
| SF-36 Mental health at baseline | 286 | 52.1 | 9.2 | 289 | 51.0 | 9.7 | N/A | N/A | N/A |
| SF-36 Mental health at 6 weeks | 245 | 52.5 | 8.8 | 232 | 52.3 | 9.0 | 0.10 | –1.23 to 1.42 | 0.888 |
| SF-36 Mental health at 6 months | 238 | 52.5 | 9.2 | 212 | 51.8 | 9.3 | –0.04 | –1.40 to 1.32 | 0.956 |
Aberdeen Varicose Vein Questionnaire
At 6 weeks and 6 months, all QoL measures showed an apparent improvement compared with baseline. The treatment effect estimate for AVVQ (our primary outcome) at 6 weeks was −2.26 (95% CI −3.67 to −0.86, p = 0.002) in favour of surgery and at 6 months the estimate was −1.74 (95% CI −2.97 to −0.50, p = 0.006).
Sensitivity analyses
There were some missing AVVQ scores at 6 months (26% for the surgery arm and 17% for foam). Exploratory analysis shows that participants without a valid AVVQ score at 6 months had mean baseline AVVQ scores of 18.3 (SD 11.4) for foam and 18.2 (SD 10.3) for surgery. Those with an AVVQ score at 6 months had mean baseline scores of 17.4 (SD 9.7) for foam and 18.2 (SD 8.8) for surgery, indicating that there are differences in the missing data between groups. The mean AVVQ score at 6 weeks for participants with missing scores at 6 months is 11.8 for foam (slightly lower than the mean for all foam participants) and 7.7 for surgery (much lower than the mean for all surgery participants), which suggests that the treatment effect of −1.74 in favour of surgery may be underestimated in the primary analysis.
Table 28 demonstrates different estimates of the effect of treatment on the primary outcome when all missing AVVQ scores at 6 months have been imputed under varying assumptions. Under the ‘missing not at random’ assumption, the estimate of treatment effect is −2.00 (95% CI −3.30 to −0.70). Variously adding or subtracting two points to or from these imputed values, for either one arm at a time or both arms simultaneously, the resulting estimates range from −1.47 to −2.52, all significantly in favour of surgery. The missing AVVQ values for surgery would need to be at least 2.7 points higher (or the missing values for foam 4.0 points lower) than the imputed values for the difference between the groups to be non-significant.
| Sensitivity analysis | Effect sizea | 95% CI | p-value |
|---|---|---|---|
| Primary analysis (repeated measures, assuming missing at random) | –1.74 | –2.97 to –0.50 | 0.006 |
| Multiple imputation (assuming missing not at random) | –2.00 | –3.30 to –0.70 | 0.003 |
| All missing assumed to have AVVQ scores two points lower | –2.17 | –3.48 to –0.86 | 0.001 |
| All missing assumed to have AVVQ scores two points higher | –1.82 | –3.12 to –0.52 | 0.006 |
| Missing in foam group assumed to have AVVQ scores two points lower | –1.65 | –2.95 to –0.35 | 0.014 |
| Missing in foam group assumed to have AVVQ scores two points higher | –2.35 | –3.65 to –1.05 | 0.001 |
| Missing in surgery group assumed to have AVVQ scores two points lower | –2.52 | –3.83 to –1.22 | < 0.001 |
| Missing in surgery group assumed to have AVVQ scores two points higher | –1.47 | –2.77 to –0.17 | 0.027 |
Short Form questionnaire-36 items
There were no differences between foam and surgery for the overall physical and mental component scores or individual domains of the SF-36.
European Quality of Life-5 Dimensions
There were no differences in the EQ-5D or EQ-5D VAS between the foam and surgery groups.
Clinical outcomes
Venous Clinical Severity Score and presence of residual varicose veins
These outcomes at 6 weeks and 6 months are presented in Tables 29 and 30 respectively, with the estimates of treatment effect sizes when surgery is compared with foam shown in Table 31.
| Clinical outcome measure | Randomised to foam sclerotherapy | Randomised to surgery | ||||
|---|---|---|---|---|---|---|
| Clinic assessment at 6 weeks ( N , n , %) | 286 | 265 | 92.7 | 289 | 251 | 86.9 |
| VCSS ( n , mean, SD) | 251 | 2.2 | 2.0 | 230 | 1.8 | 2.0 |
| Presence of residual varicosities | ||||||
| Assessed by participant (N, n, %) | 261 | 221 | 84.7 | 242 | 173 | 71.5 |
| VAS (n, mean, SD) | 261 | 2.6 | 2.0 | 242 | 1.7 | 1.8 |
| Assessed by research nurse (N, n, %) | 261 | 197 | 75.5 | 242 | 125 | 51.7 |
| VAS (n, mean, SD) | 261 | 1.7 | 1.6 | 242 | 0.8 | 1.0 |
| CEAP classification | ||||||
| C0 No visible or palpable signs of venous disease (N, n, %) | 253 | 54 | 21.3 | 229 | 80 | 34.9 |
| C1 Telangiectasis or reticular veins < 3 mm (N, n, %) | 253 | 74 | 29.2 | 229 | 92 | 40.2 |
| C2 Varicose veins > 3 mm (N, n, %) | 253 | 92 | 36.4 | 229 | 33 | 14.4 |
| C3 Oedema (N, n, %) | 253 | 9 | 3.6 | 229 | 2 | 0.9 |
| C4 Skin and subcutaneous changes (N, n, %) | 253 | 6 | 2.4 | 229 | 6 | 2.6 |
| C4a Pigmentation or eczema (N, n, %) | 253 | 16 | 6.3 | 229 | 13 | 5.7 |
| C4b Lipodermatosclerosis or atrophie blanche (N, n, %) | 253 | 1 | 0.4 | 229 | 1 | 0.4 |
| C5 Healed venous ulcer (N, n, %) | 253 | 1 | 0.4 | 229 | 2 | 0.9 |
| C6 Active venous ulcer (N, n, %) | 253 | 0 | 0.0 | 229 | 0 | 0.0 |
| Clinical outcome measure | Randomised to foam sclerotherapy | Randomised to surgery | ||||
|---|---|---|---|---|---|---|
| Clinic assessment at 6 months ( N , n , %) | 286 | 251 | 87.8 | 289 | 236 | 81.7 |
| VCSS ( n , mean, SD) | 221 | 1.6 | 1.7 | 205 | 1.4 | 1.7 |
| Presence of residual varicosities | ||||||
| Assessed by participant (N, n, %) | 232 | 190 | 81.9 | 224 | 146 | 65.2 |
| VAS (n, mean, SD) | 232 | 2.3 | 1.9 | 224 | 1.4 | 1.6 |
| Assessed by research nurse (N, n, %) | 232 | 149 | 64.2 | 224 | 110 | 49.1 |
| VAS (n, mean, SD) | 232 | 1.2 | 1.3 | 224 | 0.7 | 1.0 |
| CEAP classification | ||||||
| C0 No visible or palpable signs of venous disease (N, n, %) | 222 | 60 | 27.0 | 212 | 74 | 34.9 |
| C1 Telangiectasis or reticular veins < 3 mm (N, n, %) | 222 | 81 | 36.5 | 212 | 85 | 40.1 |
| C2 Varicose veins > 3 mm (N, n, %) | 222 | 53 | 23.9 | 212 | 31 | 14.6 |
| C3 Oedema (N, n, %) | 222 | 11 | 5.0 | 212 | 3 | 1.4 |
| C4 Skin and subcutaneous changes (N, n, %) | 222 | 0 | 0.0 | 212 | 4 | 1.9 |
| C4a Pigmentation or eczema (N, n, %) | 222 | 15 | 6.8 | 212 | 12 | 5.7 |
| C4b Lipodermatosclerosis or atrophie blanche (N, n, %) | 222 | 1 | 0.5 | 212 | 1 | 0.5 |
| C5 Healed venous ulcer (N, n, %) | 222 | 1 | 0.5 | 212 | 2 | 0.9 |
| C6 Active venous ulcer (N, n, %) | 222 | 0 | 0.0 | 212 | 0 | 0.0 |
| Sensitivity analysis | Randomised to foam sclerotherapy | Randomised to surgery | Surgery vs. foama | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | Effect size | 95% CI | p-value | |
| VCSS at baseline | 286 | 4.9 | 2.6 | 289 | 5.1 | 2.5 | N/A | N/A | N/A |
| VCSS at 6 weeks | 251 | 2.2 | 2.0 | 230 | 1.8 | 2.0 | –0.52 | –0.85 to –0.19 | 0.002 |
| VCSS at 6 months | 221 | 1.6 | 1.7 | 205 | 1.4 | 1.7 | –0.23 | –0.53 to 0.07 | 0.130 |
| Patient VASb at baseline | 286 | 5.4 | 2.2 | 289 | 5.6 | 2.3 | N/A | N/A | N/A |
| Patient VASb at 6 weeks | 261 | 2.6 | 2.0 | 242 | 1.7 | 1.8 | –0.99 | –1.31 to –0.68 | < 0.001 |
| Patient VASb at 6 months | 232 | 2.3 | 1.9 | 224 | 1.4 | 1.6 | –0.95 | –1.27 to –0.63 | < 0.001 |
| Nurse VASc at baseline | 286 | 3.9 | 2.1 | 289 | 4.0 | 2.2 | N/A | N/A | N/A |
| Nurse VASc at 6 weeks | 261 | 1.7 | 1.6 | 242 | 0.8 | 1.0 | –0.87 | –1.09 to –0.65 | < 0.001 |
| Nurse VASc at 6 months | 232 | 1.2 | 1.3 | 224 | 0.7 | 1.0 | –0.50 | –0.71 to –0.30 | < 0.001 |
Each outcome showed an apparent improvement in both groups from baseline to 6 weeks and from 6 weeks to 6 months. The VCSS was significantly lower for surgery than for foam after 6 weeks, with an effect size of −0.52 (95% CI −0.85 to −0.19, p = 0.002). However, there was no difference between groups at 6 months, when the effect size reduced (−0.23, 95% CI −0.53 to 0.07; p = 0.130).
Both the participant and nurse assessments (as assessed by the VAS) showed that there were fewer residual varicose veins for surgery than for foam at both follow-up time points [p < 0.001 (patient at 6 weeks –0.99, 95% CI –1.31 to –0.68, and at 6 months –0.95, 95% CI –1.27 to –0.63; nurse at 6 weeks –0.87, 95% CI –1.09 to –0.65, and at 6 months –0.50, 95% CI –0.71 to –0.30)].
The CEAP classification is presented for completeness, although it is generally accepted that this should not be used as a measure of treatment outcome. 72 By 6 months, 75% of those in the surgery arm were classed as CEAP C0 or C1, compared with 64% of those in the foam sclerotherapy arm.
Ablation rates
These are shown in Tables 32 and 33 for 6 weeks and 6 months respectively. The overall statistical analyses for the whole leg and the GSV only are shown in Table 34. The number of participants undergoing treatment to the SSV alone or in combination with the GSV was small, and therefore these subgroups were not subjected to statistical analysis.
| Anatomical success | Randomised to foam sclerotherapy | Randomised to surgery | ||||
|---|---|---|---|---|---|---|
| Duplex assessment at 6 weeks (N, n, %) | 286 | 265 | 92.7 | 289 | 251 | 86.9 |
| GSV involvement only | ||||||
| Complete success (N, n, %) | 205 | 112 | 54.6 | 192 | 162 | 84.4 |
| Partial success (N, n, %) | 205 | 56 | 27.3 | 192 | 21 | 10.9 |
| without reflux (N, n, %) | 205 | 47 | 22.9 | 192 | 12 | 6.3 |
| with reflux (N, n, %) | 205 | 9 | 4.4 | 192 | 9 | 4.7 |
| Failure (N, n, %) | 205 | 37 | 18.0 | 192 | 9 | 4.7 |
| SSV involvement only | ||||||
| Complete success (N, n, %) | 16 | 9 | 56.3 | 17 | 5 | 29.4 |
| Partial success (N, n, %) | 16 | 5 | 31.3 | 17 | 9 | 52.9 |
| without reflux (N, n, %) | 16 | 5 | 31.3 | 17 | 7 | 41.2 |
| with reflux (N, n, %) | 16 | 0 | 0.0 | 17 | 2 | 11.8 |
| Failure (N, n, %) | 16 | 2 | 12.5 | 17 | 3 | 17.6 |
| GSV and SSV involvement | ||||||
| GSV | ||||||
| Complete success (N, n, %) | 29 | 13 | 44.8 | 25 | 20 | 80.0 |
| Partial success (N, n, %) | 29 | 8 | 27.6 | 25 | 3 | 12.0 |
| without reflux (N, n, %) | 29 | 6 | 20.7 | 25 | 3 | 12.0 |
| with reflux (N, n, %) | 29 | 1 | 3.4 | 25 | 0 | 0.0 |
| Failure (N, n, %) | 29 | 8 | 27.6 | 25 | 2 | 8.0 |
| SSV | ||||||
| Complete success (N, n, %) | 27 | 5 | 18.5 | 23 | 2 | 8.7 |
| Partial success (N, n, %) | 27 | 4 | 14.8 | 23 | 5 | 21.7 |
| without reflux (N, n, %) | 27 | 3 | 11.1 | 23 | 2 | 8.7 |
| with reflux (N, n, %) | 27 | 0 | 0.0 | 23 | 3 | 13.0 |
| Failure (N, n, %) | 27 | 18 | 66.7 | 23 | 16 | 69.6 |
| Overall treatment of study leg | ||||||
| Complete success (N, n, %) | 246 | 125 | 50.8 | 230 | 167 | 72.6 |
| Partial success (N, n, %) | 246 | 77 | 31.3 | 230 | 51 | 22.2 |
| without reflux (N, n, %) | 246 | 64 | 26.0 | 230 | 34 | 14.8 |
| with reflux (N, n, %) | 246 | 13 | 5.3 | 230 | 17 | 7.4 |
| Failure (N, n, %) | 246 | 44 | 17.9 | 230 | 12 | 5.2 |
| Anatomical success | Randomised to foam sclerotherapy | Randomised to surgery | ||||
|---|---|---|---|---|---|---|
| Clinic assessment at 6 months (N, n, %) | 286 | 251 | 87.8 | 289 | 236 | 81.7 |
| GSV involvement only | ||||||
| Complete success (N, n, %) | 182 | 79 | 43.4 | 173 | 135 | 78.0 |
| Partial success (N, n, %) | 182 | 44 | 24.2 | 173 | 24 | 13.9 |
| without reflux (N, n, %) | 182 | 35 | 19.2 | 173 | 4 | 2.3 |
| with reflux (N, n, %) | 182 | 9 | 4.9 | 173 | 20 | 11.6 |
| Failure (N, n, %) | 182 | 59 | 32.4 | 173 | 14 | 8.1 |
| SSV involvement only | ||||||
| Complete success (N, n, %) | 17 | 7 | 41.2 | 14 | 4 | 28.6 |
| Partial success (N, n, %) | 17 | 3 | 17.6 | 14 | 4 | 28.6 |
| without reflux (N, n, %) | 17 | 2 | 11.8 | 14 | 4 | 28.6 |
| with reflux (N, n, %) | 17 | 1 | 5.9 | 14 | 0 | 0.0 |
| Failure (N, n, %) | 17 | 7 | 41.2 | 14 | 6 | 42.9 |
| GSV and SSV involvement | ||||||
| GSV | ||||||
| Complete success (N, n, %) | 26 | 6 | 23.1 | 21 | 16 | 76.2 |
| Partial success (N, n, %) | 26 | 7 | 26.9 | 21 | 2 | 9.5 |
| without reflux (N, n, %) | 26 | 6 | 23.1 | 21 | 1 | 4.8 |
| with reflux (N, n, %) | 26 | 1 | 3.8 | 21 | 1 | 4.8 |
| Failure (N, n, %) | 26 | 13 | 50.0 | 21 | 3 | 14.3 |
| SSV | ||||||
| Complete success (N, n, %) | 23 | 3 | 13.0 | 20 | 2 | 10.0 |
| Partial success (N, n, %) | 23 | 2 | 8.7 | 20 | 1 | 5.0 |
| without reflux (N, n, %) | 23 | 2 | 8.7 | 20 | 1 | 5.0 |
| with reflux (N, n, %) | 23 | 0 | 0.0 | 20 | 0 | 0.0 |
| Failure (N, n, %) | 23 | 18 | 78.3 | 20 | 17 | 85.0 |
| Overall treatment of study leg | ||||||
| Complete success (N, n, %) | 221 | 89 | 40.3 | 206 | 139 | 67.5 |
| Partial success (N, n, %) | 221 | 59 | 26.7 | 206 | 45 | 21.8 |
| without reflux (N, n, %) | 221 | 49 | 22.2 | 206 | 25 | 12.1 |
| with reflux (N, n, %) | 221 | 10 | 4.5 | 206 | 20 | 9.7 |
| Failure (N, n, %) | 221 | 73 | 33.0 | 206 | 22 | 10.7 |
| Sensitivity analysis | Randomised to foam sclerotherapy | Randomised to surgery | Surgery vs. foama | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | n | % | N | n | % | OR | 95% CI | p-value | |
| Truncal vein ablation | |||||||||
| Complete success at 6 weeks (whole leg) | 246 | 125 | 50.8 | 230 | 167 | 72.6 | |||
| Partial success without reflux at 6 weeks (whole leg) | 246 | 64 | 26.0 | 230 | 34 | 14.8 | |||
| Partial success with reflux at 6 weeks (whole leg) | 246 | 13 | 5.3 | 230 | 17 | 7.4 | |||
| Failure at 6 weeks (whole leg) | 246 | 44 | 17.9 | 230 | 12 | 5.2 | 3.07 | 2.05 to 4.59 | < 0.001 |
| Complete success at 6 months (whole leg) | 221 | 89 | 40.3 | 206 | 139 | 67.5 | |||
| Partial success without reflux at 6 months (whole leg) | 221 | 49 | 22.2 | 206 | 25 | 12.1 | |||
| Partial success with reflux at 6 months (whole leg) | 221 | 10 | 4.5 | 206 | 20 | 9.7 | |||
| Failure at 6 months (whole leg) | 221 | 73 | 33.0 | 206 | 22 | 10.7 | 3.37 | 2.26 to 5.02 | < 0.001 |
| Complete success at 6 weeks (GSV) | 205 | 112 | 54.6 | 192 | 162 | 84.4 | |||
| Partial success without reflux at 6 weeks (GSV) | 205 | 47 | 22.9 | 192 | 12 | 6.3 | |||
| Partial success with reflux at 6 weeks (GSV) | 205 | 9 | 4.4 | 192 | 9 | 4.7 | |||
| Failure at 6 weeks (GSV) | 205 | 37 | 18.0 | 192 | 9 | 4.7 | 5.12 | 3.09 to 8.48 | < 0.001 |
| Complete success at 6 months (GSV) | 182 | 79 | 43.4 | 173 | 135 | 78.0 | |||
| Partial success without reflux at 6 months (GSV) | 182 | 35 | 19.2 | 173 | 4 | 2.3 | |||
| Partial success with reflux at 6 months (GSV) | 182 | 9 | 4.9 | 173 | 20 | 11.6 | |||
| Failure at 6 months (GSV) | 182 | 59 | 32.4 | 173 | 14 | 8.1 | 4.94 | 3.07 to 7.93 | < 0.001 |
For the whole leg, the rate of successful ablation was significantly higher in the surgery group than in the foam sclerotherapy group at 6 weeks and 6 months (6 months OR 3.37, 95% CI 2.26 to 5.02; p < 0.001). Similar results were obtained for treatment to the GSV only at both time points (6 months OR 4.94, 95% CI 3.07 to 7.93; p < 0.001).
Pain
Immediately after treatment, the mean pain score for those randomised to surgery was 2.4 (SD 2.6) compared with 2.2 (SD 2.0) for those randomised to foam sclerotherapy (see Appendix 2, Table 106). At 6 weeks, the patients’ recollection of pain during treatment was higher than that recorded after treatment, and appeared to be higher for those randomised to surgery than for those randomised to foam sclerotherapy [4 (SD 3.0) vs. 3 (SD 2.4)]. The patients’ recollection of pain during recovery also appeared higher in the surgery group [4.3 (SD 2.8) vs. 3 (SD 2.4)].
Complications
Procedural complications
The event rate for any complication was similar in both groups (6% for foam and 7% for surgery) (Table 35). Six participants in the foam group (2%) and three in the surgery group (1%) each experienced two complications. In patients randomised to surgery, 2.5% had an unscheduled overnight admission following their treatment (see Table 21).
| Procedural complication | Randomised to foam sclerotherapy | Randomised to surgery | ||||
|---|---|---|---|---|---|---|
| Treated (n) | 275 | 267 | ||||
| Any procedural complicationa (N, n, %) | 275 | 17 | 6.2 | 267 | 19 | 7.1 |
| Wound haematoma (N, n, %) | 275 | 1 | 0.4 | 267 | 1 | 0.4 |
| Damage to major artery (N, n, %) | 275 | 0 | 0.0 | 267 | 0 | 0.0 |
| Damage to major vein (N, n, %) | 275 | 0 | 0.0 | 267 | 0 | 0.0 |
| Damage to major nerve (N, n, %) | 275 | 0 | 0.0 | 267 | 0 | 0.0 |
| Bleeding (N, n, %) | 275 | 0 | 0.0 | 267 | 2 | 0.7 |
| Visual disturbance/blurred vision (N, n, %) | 275 | 4 | 1.5 | 267 | 0 | 0.0 |
| Extravasation of foam sclerotherapy (N, n, %) | 275 | 0 | 0.0 | 267 | 1 | 0.4 |
| Allergic/anaphylactoid reaction (N, n, %) | 275 | 0 | 0.0 | 267 | 0 | 0.0 |
| Stroke (N, n, %) | 275 | 0 | 0.0 | 267 | 0 | 0.0 |
| Transient ischaemic attack (N, n, %) | 275 | 0 | 0.0 | 267 | 0 | 0.0 |
| Myocardial infarction (N, n, %) | 275 | 0 | 0.0 | 267 | 0 | 0.0 |
| Intra-arterial injection (N, n, %) | 275 | 0 | 0.0 | 267 | 0 | 0.0 |
| Epileptic fit (N, n, %) | 275 | 0 | 0.0 | 267 | 0 | 0.0 |
| Headache (N, n, %) | 275 | 2 | 0.7 | 267 | 1 | 0.4 |
| Transient confusion (N, n, %) | 275 | 0 | 0.0 | 267 | 0 | 0.0 |
| Panic attack (N, n, %) | 275 | 1 | 0.4 | 267 | 0 | 0.0 |
| Malaise (N, n, %) | 275 | 0 | 0.0 | 267 | 0 | 0.0 |
| Cough (N, n, %) | 275 | 1 | 0.4 | 267 | 0 | 0.0 |
| Chest tightness/heaviness (N, n, %) | 275 | 2 | 0.7 | 267 | 0 | 0.0 |
| Vasovagal (N, n, %) | 275 | 3 | 1.1 | 267 | 1 | 0.4 |
| Anaesthetic side effects (N, n, %) | 275 | 0 | 0.0 | 267 | 7 | 2.6 |
| Sickness (N, n, %) | 275 | 0 | 0.0 | 267 | 4 | 1.5 |
| Muscle pains (N, n, %) | 275 | 0 | 0.0 | 267 | 1 | 0.4 |
| Sore throat (N, n, %) | 275 | 0 | 0.0 | 267 | 3 | 1.1 |
| Damage to teeth, lip or tongue (N, n, %) | 275 | 0 | 0.0 | 267 | 0 | 0.0 |
| Other procedural complication (N, n, %) | 275 | 9 | 3.3 | 267 | 8 | 3.0 |
Later complications
Complications recorded at the time of the 6-week and 6-month assessments are shown in Tables 36 and 37. Estimates of the effect of treatment on complications are summarised across both follow-up time points in Table 38, with ORs comparing surgery with foam sclerotherapy. The overall complication rate was lower for surgery than for foam sclerotherapy at 6 weeks (OR 0.40, 95% CI 0.26 to 0.62; p < 0.001) and at 6 months (OR 0.64, 95% CI 0.44 to 0.92; p = 0.015).
| Complication | Randomised to foam sclerotherapy | Randomised to surgery | ||||
|---|---|---|---|---|---|---|
| Clinic assessment at 6 weeks (N, n, %) | 286 | 265 | 92.7 | 289 | 251 | 86.9 |
| Any complication at 6 weeks (N, n, %) | 265 | 219 | 82.6 | 251 | 168 | 66.9 |
| Numbness (N, n, %) | 265 | 15 | 5.7 | 251 | 45 | 17.9 |
| Persistent bruising (N, n, %) | 265 | 49 | 18.5 | 251 | 32 | 12.7 |
| Persist tenderness/discomfort (N, n, %) | 265 | 122 | 46.0 | 251 | 79 | 31.5 |
| Skin loss/ulceration (N, n, %) | 265 | 2 | 0.8 | 251 | 1 | 0.4 |
| Lumpiness (N, n, %) | 265 | 171 | 64.5 | 251 | 83 | 33.1 |
| Development of thread vein (N, n, %) | 265 | 27 | 10.2 | 251 | 21 | 8.4 |
| Skin staining (N, n, %) | 265 | 105 | 39.6 | 251 | 20 | 8.0 |
| Wound infection (N, n, %) | 265 | 2 | 0.8 | 251 | 23 | 9.2 |
| Backache (N, n, %) | 265 | 5 | 1.9 | 251 | 9 | 3.6 |
| Headache (N, n, %) | 265 | 13 | 4.9 | 251 | 4 | 1.6 |
| DVT (N, n, %) | 265 | 3 | 1.1 | 251 | 0 | 0.0 |
| Pulmonary embolus (N, n, %) | 265 | 0 | 0.0 | 251 | 0 | 0.0 |
| Stroke (N, n, %) | 265 | 0 | 0.0 | 251 | 0 | 0.0 |
| Myocardial infarction (N, n, %) | 265 | 0 | 0.0 | 251 | 0 | 0.0 |
| Loss of vision (N, n, %) | 265 | 4 | 1.5 | 251 | 0 | 0.0 |
| Damage to major artery (N, n, %) | 265 | 0 | 0.0 | 251 | 0 | 0.0 |
| Damage to major vein (N, n, %) | 265 | 1 | 0.4 | 251 | 0 | 0.0 |
| Damage to motor nerve (N, n, %) | 265 | 0 | 0.0 | 251 | 0 | 0.0 |
| Other complication (N, n, %) | 265 | 16 | 6.0 | 251 | 20 | 8.0 |
| Complication | Randomised to foam sclerotherapy | Randomised to surgery | ||||
|---|---|---|---|---|---|---|
| Clinic assessment at 6 months (N, n, %) | 286 | 251 | 87.8 | 289 | 236 | 81.6 |
| Any complication at 6 months (N, n, %) | 251 | 144 | 57.4 | 236 | 109 | 46.2 |
| Numbness (N, n, %) | 251 | 10 | 4.0 | 236 | 37 | 15.6 |
| Persistent bruising (N, n, %) | 251 | 38 | 15.2 | 236 | 40 | 17.0 |
| Skin loss/ulceration (N, n, %) | 251 | 2 | 0.8 | 236 | 0 | 0.0 |
| Lumpiness (N, n, %) | 251 | 67 | 26.6 | 236 | 17 | 7.2 |
| Development of thread vein (N, n, %) | 251 | 34 | 13.6 | 236 | 26 | 11.0 |
| Skin staining (N, n, %) | 251 | 92 | 36.6 | 236 | 24 | 10.2 |
| DVT (N, n, %) | 251 | 2 | 0.8 | 236 | 0 | 0.0 |
| Pulmonary embolus (N, n, %) | 251 | 0 | 0.0 | 236 | 0 | 0.0 |
| Other (N, n, %) | 251 | 8 | 3.2 | 236 | 12 | 5.0 |
| Complication type | Randomised to foam sclerotherapy | Randomised to surgery | Surgery vs. foama | ||||||
|---|---|---|---|---|---|---|---|---|---|
| N | n | % | N | n | % | OR | 95% CI | p-value | |
| Procedural complications at time of treatment | 275 | 17 | 6.2 | 267 | 19 | 7.1 | 1.07 | 0.55 to 2.09 | 0.848 |
| Any complication at 6 weeks | 265 | 219 | 82.6 | 251 | 168 | 66.9 | 0.40 | 0.26 to 0.62 | < 0.001 |
| Any complication at 6 months | 251 | 144 | 57.4 | 236 | 109 | 46.2 | 0.64 | 0.44 to 0.92 | 0.015 |
| Numbness at 6 weeks | 265 | 15 | 5.7 | 251 | 45 | 17.9 | 3.98 | 2.11 to 7.50 | < 0.001 |
| Numbness at 6 months | 251 | 10 | 4.0 | 236 | 37 | 15.6 | 5.39 | 2.50 to 11.62 | < 0.001 |
| Persistent bruising at 6 weeks | 265 | 49 | 18.5 | 251 | 32 | 12.7 | 0.63 | 0.37 to 1.06 | 0.080 |
| Persistent bruising at 6 months | 251 | 38 | 15.2 | 236 | 40 | 17.0 | 1.16 | 0.70 to 1.92 | 0.576 |
| Persist tenderness/discomfort at 6 weeks | 265 | 122 | 46.0 | 251 | 79 | 31.5 | 0.52 | 0.36 to 0.77 | 0.001 |
| Skin loss/ulceration at 6 weeks | 265 | 2 | 0.8 | 251 | 1 | 0.4 | 0.64 | 0.04 to 10.32 | 0.750 |
| Skin loss/ulceration at 6 months | 251 | 2 | 0.8 | 236 | 0 | 0.0 | N/C | N/C | N/C |
| Lumpiness at 6 weeks | 265 | 171 | 64.5 | 251 | 83 | 33.1 | 0.23 | 0.15 to 0.34 | < 0.001 |
| Lumpiness at 6 months | 251 | 67 | 26.6 | 236 | 17 | 7.2 | 0.18 | 0.10 to 0.33 | < 0.001 |
| Development of thread vein at 6 weeks | 265 | 27 | 10.2 | 251 | 21 | 8.4 | 0.75 | 0.40 to 1.40 | 0.364 |
| Development of thread vein at 6 months | 251 | 34 | 13.6 | 236 | 26 | 11.0 | 0.78 | 0.44 to 1.38 | 0.390 |
| Skin staining at 6 weeks | 265 | 105 | 39.6 | 251 | 20 | 8.0 | 0.12 | 0.07 to 0.21 | < 0.001 |
| Skin staining at 6 months | 251 | 92 | 36.6 | 236 | 24 | 10.2 | 0.16 | 0.10 to 0.28 | < 0.001 |
| Backache at 6 weeks | 265 | 5 | 1.9 | 251 | 9 | 3.6 | 2.16 | 0.67 to 6.95 | 0.198 |
| Headache at 6 weeks | 265 | 13 | 4.9 | 251 | 4 | 1.6 | 0.31 | 0.10 to 0.98 | 0.047 |
The event rates for cutaneous numbness (6 weeks OR 3.98, 95% CI 2.11 to 7.50; p < 0.001, and 6 months OR 5.39, 95% CI 2.50 to 11.62; p < 0.001) were significantly higher for surgery than for foam sclerotherapy. Wound infection occurred in 9.2% of the surgical patients at 6 weeks – a much higher proportion than the 0.8% of foam patients with wound infection – but clearly this complication only occurred in foam patients who had treatment other than that to which they had been randomised.
However, the rates for lumpiness (6 weeks OR 0.23, 95% CI 0.15 to 0.34; p < 0.001, and 6 months OR 0.18, 95% CI 0.10 to 0.33; p < 0.001), skin staining (6 weeks OR 0.12, 95% CI 0.07 to 0.21; p < 0.001, and 6 months OR 0.16, 95% CI 0.10 to 0.28; p < 0.001), persistent tenderness (6 weeks OR 0.52, 95% CI 0.36 to 0.77; p = 0.001) and headache (6 weeks OR 0.31, 95% CI 0.10 to 0.98; p = 0.047) were all significantly higher for foam sclerotherapy than for surgery. There were no differences for persistent bruising, skin loss/ulceration or development of thread vein at either time point. There was also no difference for backache at 6 weeks. At 6 months, data on wound infection, persistent tenderness, backache and headache were not collected.
Serious adverse events
Eleven SAEs were noted among those randomised to foam. Among these were three DVTs; all were assessed as related to treatment and expected. The other eight SAEs were not related to the foam treatment; full details are given in Table 39. Ten SAEs were noted among those randomised to surgery. Four of these (groin infection, post-operative infection, post-operative haematoma, injury to peroneal nerve) were assessed as being related to treatment. The other six SAEs were not related to treatment (see Table 39).
| Randomised | Treatment prior to SAE | Description of event | Related to treatment? | Expected? |
|---|---|---|---|---|
| Foam | Foam | DVT (non-occlusive tongue of thrombus extending from the SFJ to the common femoral vein); asymptomatic | Yes | Yes |
| Foam | Foam | Thrombophlebitis extending through the perforator mid-/proximal calf causing small DVT in the medial gastrocnemius vein | Yes | Yes |
| Foam | Foam | DVT (involving < 20% of lumen) in the right common femoral vein (asymptomatic) | Yes | Yes |
| Foam | Foam | Possible transient ischaemic attack. CT scan has shown ill-defined areas in subcortical white matter in both hemispheres; unclear if ischaemia or demyelinating | No | Yes |
| Foam | Foam | Headache associated with pre-syncope and vomiting (history of migraine with hemiplegia) | No | Yes |
| Foam | No treatment | Prior to treatment experienced right hemisphere cerebrovascular accident (stroke) | No | Yes |
| Foam | No treatment | Provisional diagnosis of myeloma | No | No |
| Foam | Foam | Chest pain – thought to be musculoskeletal | No | No |
| Foam | Foam | Urinary retention following elective laparoscopic left inguinal hernia repair | No | No |
| Foam | Foam | Breast cancer | No | No |
| Foam | Foam | Road traffic accident – no spinal or bony injury | No | No |
| Surgery | Surgery | Groin infection requiring further surgery for evacuation of haematoma | Yes | Yes |
| Surgery | Surgery | Injury to motor nerve (peroneal); presumably as a result of trauma during phlebectomies | Yes | Yes |
| Surgery | Surgery | Post-operative haematoma; deranged liver function tests | Yes | Yes |
| Surgery | Surgery | Post-operative infection – abscess to left calf; abscess of the pilonidal sinus | Yes | Yes |
| Surgery | Surgery | Surgery to wrong vein | No | No |
| Surgery | Surgery | Sectioned under the Mental Health Act | No | No |
| Surgery | Surgery | Road traffic accident – suffered right shoulder and neck pain | No | No |
| Surgery | Surgery | Endoscopic excision of an anal polyp | No | No |
| Surgery | Surgery | Acute upper-left quadrant pain – likely to have been caused by gall stone and cholecystitis | No | No |
| Surgery | Surgery | Breast enlargement | No | No |
Process evaluation: Illness Perception Questionnaire – Revised
Detailed descriptive results of the IPQ-R are given in Appendix 2 (see Table 108). There is little change in either randomised group in mean identity scores or the percentage of symptoms correctly identified as being related to varicose veins between baseline (recruitment) and after the participant is informed of his or her randomisation. The scores in each randomised group were similar at both time points. By 6 months, both measures of illness identity had fallen.
There is little difference between baseline and post-randomisation scores for all other domains. Scores were also similar between randomised groups at baseline and post treatment. Personal control and illness coherence scores increased marginally between baseline and 6 months; all other domain scores fell within this time scale.
Chapter 6 Comparison of surgery, endovenous laser ablation and foam sclerotherapy
This chapter reports the results of the three-arm comparison of surgery, EVLA and foam sclerotherapy, using data from the eight centres which randomised participants to all three treatment groups. This chapter will focus on comparing surgery with EVLA, and EVLA with foam sclerotherapy. Comparisons between foam sclerotherapy and surgery have been made using data from all centres and are reported in Chapter 5. A discussion of the results presented in this chapter is included in Chapter 7.
Participants
Six hundred and thirty-six participants were randomised in the three-arm centres, of which there were six post-randomisation exclusions, leaving a total of 630 participants included in this analysis (207 in the foam sclerotherapy arm, 213 in the surgery arm and 210 in the EVLA arm). The CONSORT diagram (see Figure 2) describes the flow of participants in the trial.
The proportion receiving treatment as allocated appeared to be higher for EVLA (97%) than for foam sclerotherapy (91%) and surgery (85%). Retention appeared to be lower for surgery, both for the follow-up clinic assessments and completion of participant questionnaires, than for foam sclerotherapy and EVLA. The 6-weeks clinic was attended by 85% of participants randomised to surgery compared with 91% for foam sclerotherapy and 92% for EVLA. At the 6-months follow-up, the attendance rates were 78%, 86% and 87% for surgery, foam sclerotherapy and EVLA respectively.
The 6-weeks questionnaire was completed by 81% of participants randomised to surgery compared with 88% for foam sclerotherapy and 89% for EVLA. At 6 months, the questionnaires were completed by 74%, 82% and 83% of participants for surgery, foam sclerotherapy and EVLA respectively. The largest proportion of withdrawals from the study at 6 months was in the surgery arm (13%), compared with foam sclerotherapy (7%) and EVLA (4%).
Baseline characteristics
Demographic details
The baseline characteristics of study participants are shown in Table 40. There was a good balance between groups for most factors, particularly for age and sex, which were minimisation variables. There was a slight imbalance between groups in terms of bilateral disease. The data shown in Table 11 were also used in the analysis when adjusting for minimisation factors.
| Participant characteristics | Randomised to EVLA | Randomised to foam sclerotherapy | Randomised to surgery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Randomised ( n ) | 210 | 207 | 213 | ||||||
| Age ( n , mean, range) | 210 | 49.7 | 18–80 | 207 | 48.3 | 19–78 | 213 | 48.4 | 22–85 |
| Female ( N , n , %) | 210 | 120 | 57.1 | 207 | 119 | 57.5 | 213 | 121 | 56.8 |
| BMI (kg/m 2 ) ( n , mean, range) | 195 | 27.0 | 17–42 | 192 | 27.1 | 17–44 | 188 | 27.8 | 20–44 |
| Employment status | |||||||||
| Self-employed (N, n, %) | 206 | 21 | 10.2 | 206 | 20 | 9.7 | 206 | 20 | 9.7 |
| Employed (N, n, %) | 206 | 120 | 58.3 | 206 | 123 | 59.7 | 206 | 138 | 67.0 |
| Other (N, n, %) | 206 | 65 | 31.6 | 206 | 63 | 30.6 | 206 | 48 | 23.3 |
| Laterality | |||||||||
| Unilateral (N, n, %) | 210 | 153 | 72.9 | 207 | 157 | 75.8 | 213 | 148 | 69.5 |
| Bilateral (N, n, %) | 210 | 57 | 27.1 | 207 | 50 | 24.2 | 213 | 65 | 30.5 |
| Previous history of DVT ( N , n , %) | 206 | 6 | 2.9 | 205 | 2 | 1.0 | 210 | 7 | 3.3 |
| Previous treatment to contralateral leg ( N , n , %) | 208 | 27 | 13.0 | 205 | 22 | 10.7 | 211 | 20 | 9.5 |
| Foam sclerotherapy (N, n, %) | 208 | 4 | 1.9 | 205 | 6 | 2.9 | 211 | 1 | 0.5 |
| Surgery (N, n, %) | 208 | 19 | 9.1 | 205 | 16 | 7.8 | 211 | 16 | 7.6 |
| EVLA (N, n, %) | 208 | 3 | 1.4 | 205 | 0 | 0.0 | 211 | 3 | 1.4 |
| Previous sclerotherapy to tributaries of study leg ( N , n , %) | 208 | 3 | 1.4 | 205 | 0 | 0.0 | 211 | 3 | 1.4 |
Quality of life
At baseline, the QoL for the surgery group appeared slightly worse than for the other two groups, and this was reflected in the AVVQ score, EQ-5D and each of the SF-36 components and domain scores (Table 41). The mean baseline AVVQ score was 18.1 (SD 9.1) for surgery, 17.8 (SD 9.1) for EVLA and 17.4 (SD 9.7) for foam (a higher AVVQ score indicates worse QoL).
| QoL measure | Randomised to EVLA | Randomised to foam sclerotherapy | Randomised to surgery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Randomised ( n ) | 210 | 207 | 213 | ||||||
| AVVQ score ( n , mean, SD) | 210 | 17.8 | 9.1 | 206 | 17.4 | 9.7 | 210 | 18.1 | 9.1 |
| EQ-5D score ( n , mean, SD) | 206 | 0.79 | 0.17 | 202 | 0.79 | 0.19 | 207 | 0.78 | 0.18 |
| VAS (n, mean, SD) | 206 | 80.6 | 16.3 | 205 | 80.6 | 16.3 | 210 | 80.0 | 15.5 |
| SF-36 summary scores | |||||||||
| Physical component summary score (n, mean, SD) | 204 | 48.6 | 7.9 | 199 | 48.4 | 8.7 | 202 | 48.2 | 9.0 |
| Mental component summary score (n, mean, SD) | 204 | 51.9 | 9.1 | 199 | 51.8 | 9.2 | 202 | 50.8 | 9.9 |
| SF-36 subscale scores | |||||||||
| Physical functioning (n, mean, SD) | 208 | 50.2 | 8.3 | 205 | 49.8 | 8.6 | 207 | 50.0 | 8.5 |
| Role physical (n, mean, SD) | 208 | 50.1 | 8.3 | 206 | 50.2 | 9.1 | 207 | 49.0 | 10.1 |
| Bodily pain (n, mean, SD) | 207 | 47.3 | 8.5 | 206 | 47.6 | 9.2 | 208 | 46.0 | 9.7 |
| General health (n, mean, SD) | 209 | 49.5 | 8.3 | 202 | 49.1 | 8.4 | 210 | 49.2 | 8.7 |
| Vitality (n, mean, SD) | 210 | 51.5 | 9.2 | 206 | 51.1 | 9.8 | 209 | 50.2 | 9.5 |
| Social functioning (n, mean, SD) | 207 | 50.8 | 8.9 | 205 | 50.9 | 8.4 | 209 | 49.8 | 9.9 |
| Role emotional (n, mean, SD) | 208 | 51.1 | 8.4 | 205 | 51.0 | 8.8 | 206 | 50.1 | 10.0 |
| Mental health (n, mean, SD) | 209 | 51.6 | 9.2 | 206 | 51.3 | 9.6 | 208 | 50.7 | 10.2 |
Physical activity
There was a good balance between the groups in terms of physical activity at baseline (Table 42).
| Physical activity | Randomised to EVLA | Randomised to foam sclerotherapy | Randomised to surgery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Randomised ( n ) | 210 | 207 | 213 | ||||||
| Physical activity at work | |||||||||
| Mostly sitting (N, n, %) | 202 | 27 | 13.4 | 201 | 30 | 14.9 | 205 | 30 | 14.6 |
| Mostly standing or walking (N, n, %) | 202 | 47 | 23.3 | 201 | 60 | 29.9 | 205 | 58 | 28.3 |
| Definite physical effort (N, n, %) | 202 | 52 | 25.7 | 201 | 43 | 21.4 | 205 | 62 | 30.2 |
| Vigorous physical effort (N, n, %) | 202 | 16 | 7.9 | 201 | 11 | 5.5 | 205 | 9 | 4.4 |
| Not in employment (N, n, %) | 202 | 60 | 29.7 | 201 | 57 | 28.4 | 205 | 46 | 22.4 |
| Physical activity in previous week | |||||||||
| Physical activities (N, n, %) | 204 | 95 | 46.6 | 205 | 93 | 45.4 | 206 | 94 | 45.6 |
| Cycling (N, n, %) | 197 | 32 | 16.2 | 201 | 23 | 11.4 | 202 | 38 | 18.8 |
| Walking (N, n, %) | 204 | 202 | 99.0 | 206 | 201 | 97.6 | 204 | 199 | 97.5 |
| Housework/childcare (N, n, %) | 203 | 177 | 87.2 | 205 | 186 | 90.7 | 204 | 178 | 87.3 |
| Gardening (N, n, %) | 203 | 114 | 56.2 | 204 | 130 | 63.7 | 203 | 118 | 58.1 |
| Usual walking pace | |||||||||
| Slow (N, n, %) | 205 | 17 | 8.3 | 204 | 12 | 5.9 | 207 | 14 | 6.8 |
| Steady/average (N, n, %) | 205 | 101 | 49.3 | 204 | 103 | 50.5 | 207 | 111 | 53.6 |
| Brisk (N, n, %) | 205 | 72 | 35.1 | 204 | 78 | 38.2 | 207 | 67 | 32.4 |
| Fast (N, n, %) | 205 | 15 | 7.3 | 204 | 11 | 5.4 | 207 | 15 | 7.2 |
Varicose vein characteristics
Tables 43 and 44 show the baseline characteristics of the varicose veins in the study leg and the contralateral leg. The groups are well balanced across the majority of factors (except for deep-vein reflux, where the rate was 18% for foam, 14% for EVLA and 9% for surgery).
| Study leg vein characteristics | Randomised to EVLA | Randomised to foam sclerotherapy | Randomised to surgery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Randomised ( n ) | 210 | 207 | 213 | ||||||
| Study leg | |||||||||
| Right (N, n, %) | 210 | 108 | 51.4 | 207 | 103 | 49.8 | 213 | 101 | 47.4 |
| Left (N, n, %) | 210 | 102 | 48.6 | 207 | 104 | 50.2 | 213 | 112 | 52.6 |
| Saphenous involvement | |||||||||
| GSV only (N, n, %) | 210 | 182 | 86.7 | 207 | 175 | 84.5 | 213 | 184 | 86.4 |
| Widest diameter (mm) (n, mean, range) | 162 | 9.1 | 3–15 | 154 | 8.2 | 4–15 | 159 | 8.6 | 4–15 |
| Reflux above knee only (N, n, %) | 157 | 151 | 96.2 | 146 | 138 | 94.5 | 152 | 150 | 98.7 |
| Reflux above and below knee (N, n, %) | 157 | 6 | 3.8 | 146 | 8 | 5.5 | 152 | 2 | 1.3 |
| SSV only (N, n, %) | 210 | 14 | 6.7 | 207 | 14 | 6.8 | 213 | 16 | 7.5 |
| Widest diameter (mm) ( n , mean, range) | 13 | 7.1 | 5–10 | 13 | 6.8 | 3–10 | 12 | 7.8 | 4–15 |
| GSV and SSV (N, n, %) | 210 | 14 | 6.7 | 207 | 18 | 8.7 | 213 | 13 | 6.1 |
| Widest diameter: GSV (mm) (n, mean, range) | 13 | 6.3 | 4–15 | 16 | 7.5 | 4–14 | 12 | 7.6 | 3–15 |
| Widest diameter: SSV (mm) (n, mean, range) | 10 | 6.8 | 3–15 | 15 | 4.9 | 3–10 | 11 | 5.1 | 3–7 |
| Reflux above knee only (N, n, %) | 12 | 6 | 50.0 | 13 | 7 | 53.8 | 9 | 5 | 55.6 |
| Reflux above and below knee (N, n, %) | 12 | 6 | 50.0 | 13 | 6 | 46.2 | 9 | 4 | 44.4 |
| Deep-vein reflux ( N , n , %) | 205 | 28 | 13.7 | 201 | 36 | 17.9 | 207 | 19 | 9.2 |
| CEAP classification | |||||||||
| C0 No visible or palpable signs of venous disease (N, n, %) | 209 | 0 | 0.0 | 207 | 0 | 0.0 | 211 | 0 | 0.0 |
| C1 Telangiectasis or reticular veins < 3 mm (N, n, %) | 209 | 0 | 0.0 | 207 | 0 | 0.0 | 211 | 0 | 0.0 |
| C2 Varicose veins > 3 mm (N, n, %) | 209 | 113 | 54.1 | 207 | 122 | 58.9 | 211 | 107 | 50.7 |
| C3 Oedema (N, n, %) | 209 | 28 | 13.4 | 207 | 28 | 13.5 | 211 | 29 | 13.7 |
| C4 Skin and subcutaneous changes (N, n, %) | 209 | 17 | 8.1 | 207 | 21 | 10.1 | 211 | 28 | 13.3 |
| C4a Pigmentation or eczema (N, n, %) | 209 | 34 | 16.3 | 207 | 29 | 14.0 | 211 | 38 | 18.0 |
| C4b Lipodermatosclerosis or atrophie blanche (N, n, %) | 209 | 5 | 2.4 | 207 | 2 | 1.0 | 211 | 0 | 0.0 |
| C5 Healed venous ulcer (N, n, %) | 209 | 9 | 4.3 | 207 | 3 | 1.4 | 211 | 5 | 2.4 |
| C6 Active venous ulcer (N, n, %) | 209 | 3 | 1.4 | 207 | 2 | 1.0 | 211 | 4 | 1.9 |
| VCSS ( n , mean, SD) | 207 | 5.0 | 2.5 | 206 | 4.8 | 2.5 | 210 | 5.0 | 2.4 |
| Presence of varicose veins | |||||||||
| Assessed by participant (N, n, %) | 210 | 209 | 99.5 | 207 | 207 | 100 | 213 | 213 | 100 |
| VAS (n, mean, SD) | 210 | 5.5 | 2.3 | 207 | 5.2 | 2.2 | 213 | 5.6 | 2.3 |
| Assessed by research nurse (N, n, %) | 210 | 210 | 100 | 207 | 207 | 100 | 213 | 212 | 99.5 |
| VAS (n, mean, SD) | 210 | 3.6 | 2.2 | 207 | 3.6 | 2.1 | 213 | 3.7 | 2.3 |
| Non-study leg vein characteristics | Randomised to EVLA | Randomised to foam sclerotherapy | Randomised to surgery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Randomised ( n ) | 210 | 207 | 213 | ||||||
| Participants with bilateral disease ( N , n , %) | 210 | 57 | 27.1 | 207 | 50 | 24.2 | 213 | 65 | 30.5 |
| CEAP classification | |||||||||
| C2 Varicose veins > 3 mm (N, n, %) | 55 | 36 | 65.5 | 47 | 30 | 63.8 | 62 | 31 | 50.0 |
| C3 Oedema (N, n, %) | 55 | 8 | 14.5 | 47 | 7 | 14.9 | 62 | 10 | 16.1 |
| C4 Skin and subcutaneous changes (N, n, %) | 55 | 4 | 7.3 | 47 | 3 | 6.4 | 62 | 7 | 11.3 |
| C4a Pigmentation or eczema (N, n, %) | 55 | 6 | 10.9 | 47 | 6 | 12.8 | 62 | 13 | 21.0 |
| C4b Lipodermatosclerosis or atrophie blanche (N, n, %) | 55 | 1 | 1.8 | 47 | 0 | 0.0 | 62 | 0 | 0.0 |
| C5 Healed venous ulcer (N, n, %) | 55 | 0 | 0.0 | 47 | 1 | 2.1 | 62 | 0 | 0.0 |
| C6 Active venous ulcer (N, n, %) | 55 | 0 | 0.0 | 47 | 0 | 0.0 | 62 | 1 | 1.6 |
| VCSS ( n , mean, SD) | 54 | 3.6 | 2.3 | 46 | 4.1 | 2.4 | 61 | 4.2 | 2.4 |
Treatment received
Tables 45–48 summarise the primary interventions received (i.e. excluding any delayed secondary foam treatments). For participants randomised to EVLA, 99% of those who received treatment had their treatment as randomised. The equivalent proportions were 95% and 93% in the foam sclerotherapy and surgery arms respectively. Details of those not receiving their randomised intervention are given in Chapter 4.
| Primary intervention | Randomised to EVLA | Randomised to foam sclerotherapy | Randomised to surgery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Treated ( n ) | 205 | 197 | 195 | ||||||
| Received foam (N, n, %) | 205 | 0 | 0.0 | 197 | 188 | 95.4 | 195 | 5 | 2.6 |
| Received surgery (N, n, %) | 205 | 2 | 1.0 | 197 | 3 | 1.5 | 195 | 182 | 93.3 |
| Received EVLA (N, n, %) | 205 | 203 | 99.0 | 197 | 6 | 3.0 | 195 | 8 | 4.1 |
| Treatment time (minutes) ( n , mean, SD) | 189 | 45.9 | 24.6 | 174 | 18.9 | 10.2 | 178 | 51.5 | 22.9 |
| Grade of surgeon | |||||||||
| Consultant (N, n, %) | 201 | 146 | 72.6 | 195 | 154 | 79.0 | 190 | 99 | 52.1 |
| Consultant nurse (N, n, %) | 201 | 0 | 0.0 | 195 | 16 | 8.2 | 190 | 3 | 1.6 |
| Staff grade (supervised) (N, n, %) | 201 | 1 | 0.5 | 195 | 3 | 1.5 | 190 | 10 | 5.3 |
| Staff grade (unsupervised) (N, n, %) | 201 | 7 | 3.5 | 195 | 2 | 1.0 | 190 | 6 | 3.2 |
| Trainee (supervised) (N, n, %) | 201 | 15 | 7.5 | 195 | 11 | 5.6 | 190 | 35 | 18.4 |
| Trainee (unsupervised) (N, n, %) | 201 | 32 | 15.9 | 195 | 9 | 4.6 | 190 | 37 | 19.5 |
| Treatment to non-truncal varicosities ( N , n , %) | 61a | 49 | 80.3 | 192 | 57 | 29.7 | 188 | 163 | 86.7 |
| Concurrent contralateral treatment ( N , n , %) | 203 | 20 | 9.9 | 193 | 6 | 3.1 | 192 | 23 | 12.0 |
| Subcutaneous heparin (or derivative) ( N , n , %) | 202 | 14 | 6.9 | 186 | 13 | 7.0 | 181 | 72 | 39.8 |
| Overnight hospitalisation | |||||||||
| Planned (N, n, %) | 197 | 0 | 0.0 | 190 | 0 | 0.0 | 161 | 6 | 3.7 |
| Unplanned (N, n, %) | 197 | 0 | 0.0 | 190 | 0 | 0.0 | 161 | 5 | 3.1 |
| Bandaging not according to protocol ( N , n , %) | 194 | 18 | 9.3 | 194 | 101b | 52.1 | 185 | 23 | 12.4 |
| Recommended duration of bandaging (if not for 10 days) (n, mean, SD) | 17 | 7.4 | 1.7 | 9 | 7.0 | 0.0 | 21 | 7.2 | 2.5 |
| Anaesthetic details | Randomised to EVLA | Randomised to foam sclerotherapy | Randomised to surgery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Received surgery ( N , n , %) | 2a | 3b | 182 | ||||||
| Type of anaesthetic | |||||||||
| General (N, n, %) | 2 | 2 | 100.0 | 3 | 3 | 100.0 | 179 | 174 | 97.2 |
| Epidural/spinal (N, n, %) | 2 | 0 | 0.0 | 3 | 0 | 0.0 | 179 | 5 | 2.8 |
| Grade of anaesthetist | |||||||||
| Consultant (N, n, %) | 2 | 1 | 50.0 | 3 | 2 | 66.7 | 176 | 145 | 82.4 |
| Associate specialist (N, n, %) | 2 | 1 | 50.0 | 3 | 0 | 0.0 | 176 | 2 | 1.1 |
| Registrar (N, n, %) | 2 | 0 | 0.0 | 3 | 1 | 33.3 | 176 | 25 | 14.2 |
| Staff grade (N, n, %) | 2 | 0 | 0.0 | 3 | 0 | 0.0 | 176 | 4 | 2.3 |
| Senior house officer (N, n, %) | 2 | 0 | 0.0 | 3 | 0 | 0.0 | 176 | 0 | 0.0 |
| EVLA details | Randomised to EVLA | Randomised to foam sclerotherapy | Randomised to surgery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Received EVLA ( n ) | 203 | 6 | 8 | ||||||
| GSV involvement only | |||||||||
| Watts (n, mean, range) | 168 | 13.8 | 7–30 | 2 | 13.0 | 12–14 | 5 | 13.6 | 12–14 |
| Length (cm) (n, mean, range) | 172 | 40.9 | 12–79 | 2 | 23.5 | 21–26 | 7 | 36.6 | 21–54 |
| J/cm (n, mean, range) | 170 | 82.5 | 40–188 | 2 | 71.9 | 67–77 | 6 | 71.6 | 59–91 |
| Wavelength | |||||||||
| 810 nm (N, n, %) | 134 | 90 | 67.2 | 1 | 1 | 100.0 | 5 | 4 | 80.0 |
| 1064 nm (N, n, %) | 134 | 10 | 7.5 | 1 | 0 | 0.0 | 5 | 0 | 0.0 |
| 1470 nm (N, n, %) | 134 | 34 | 25.4 | 1 | 0 | 0.0 | 5 | 1 | 20.0 |
| SSV involvement only | |||||||||
| Watts (n, mean, range) | 13 | 12.4 | 7–14 | 0 | N/A | N/A | 1 | 14.0 | N/A |
| Length (cm) (n, mean, range) | 13 | 19.4 | 7–45 | 0 | N/A | N/A | 1 | 12.0 | N/A |
| J/cm (n, mean, range) | 12 | 77.5 | 53–114 | 0 | N/A | N/A | 1 | 75.1 | N/A |
| Wavelength | |||||||||
| 810 nm (N, n, %) | 10 | 7 | 70.0 | 0 | N/A | N/A | 1 | 1 | 100.0 |
| 1064 nm (N, n, %) | 10 | 0 | 0.0 | 0 | N/A | N/A | 1 | 0 | 0.0 |
| 1470 nm (N, n, %) | 10 | 3 | 30.0 | 0 | N/A | N/A | 1 | 0 | 0.0 |
| GSV and SSV involvement | |||||||||
| Watts (GSV) (n, mean, range) | 9 | 17.6 | 10–30 | 0 | N/A | N/A | 0 | N/A | N/A |
| Length (cm) (GSV) (n, mean, range) | 9 | 39.8 | 27–56 | 0 | N/A | N/A | 0 | N/A | N/A |
| J/cm (GSV) (n, mean, range) | 9 | 100.3 | 61–165 | 0 | N/A | N/A | 0 | N/A | N/A |
| Wavelength (GSV) | |||||||||
| 810 nm (N, n, %) | 9 | 3 | 33.3 | 0 | N/A | N/A | 0 | N/A | N/A |
| 1064 nm (N, n, %) | 9 | 3 | 33.3 | 0 | N/A | N/A | 0 | N/A | N/A |
| 1470 nm (N, n, %) | 9 | 3 | 33.3 | 0 | N/A | N/A | 0 | N/A | N/A |
| Watts (SSV) (n, mean, range) | 5 | 9.6 | 7–14 | 0 | N/A | N/A | 0 | N/A | N/A |
| Length (cm) (SSV) (n, mean, range) | 5 | 15.6 | 5–26 | 0 | N/A | N/A | 0 | N/A | N/A |
| J/cm (SSV) (n, mean, range) | 5 | 63.7 | 36–82 | 0 | N/A | N/A | 0 | N/A | N/A |
| Wavelength (SSV) | |||||||||
| 810 nm (N, n, %) | 3 | 2 | 66.7 | 0 | N/A | N/A | 0 | N/A | N/A |
| 1064 nm (N, n, %) | 3 | 0 | 0.0 | 0 | N/A | N/A | 0 | N/A | N/A |
| 1470 nm (N, n, %) | 3 | 1 | 33.3 | 0 | N/A | N/A | 0 | N/A | N/A |
| Foam sclerotherapy to incompetent distal GSV at time of (or immediately following) EVLA treatment ( N , n , %) | 203 | 0 | 0.0 | 6 | 0 | 0.0 | 8 | 0 | 0.0 |
| Primary foam treatment | Randomised to EVLA | Randomised to foam sclerotherapy | Randomised to surgery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Received primary treatment of foam ( n ) | 0 | 188 | 5a | ||||||
| Volume of foam (ml) ( n , mean, range) | 0 | N/A | N/A | 188 | 9.1 | 2–15 | 5 | 7.6 | 6–10 |
| GSV involvement only (total) (n, mean, range) | 0 | N/A | N/A | 158 | 9.4 | 2–15 | 4 | 7.0 | 6–8 |
| GSV (n, mean, range) | 0 | N/A | N/A | 158 | 8.2 | 2–15 | 4 | 7.0 | 6–8 |
| Non-truncal varicosities (n, mean, range) | 0 | N/A | N/A | 158 | 1.2 | 0–8 | 4 | 0.0 | 0–0 |
| SSV involvement only (total) (n, mean, range) | 0 | N/A | N/A | 14 | 6.4 | 2–12 | 1 | 10.0 | 10–10 |
| SSV (n, mean, range) | 0 | N/A | N/A | 14 | 5.6 | 0–12 | 1 | 10.0 | 10–10 |
| Non-truncal varicosities (n, mean, range) | 0 | N/A | N/A | 14 | 0.8 | 0–5 | 1 | 0.0 | 0–0 |
| GSV and SSV involvement (total) (n, mean, range) | 0 | N/A | N/A | 16 | 9.2 | 6–13 | 0 | N/A | N/A |
| GSV (n, mean, range) | 0 | N/A | N/A | 16 | 6.8 | 3–12 | 0 | N/A | N/A |
| SSV (n, mean, range) | 0 | N/A | N/A | 16 | 0.7 | 0–5 | 0 | N/A | N/A |
| Non-truncal varicosities (n, mean, range) | 0 | N/A | N/A | 16 | 1.7 | 0–6 | 0 | N/A | N/A |
Procedure and treatment time
Treatment to non-truncal varicosities at the time of the primary intervention was performed in 87% of patients in the surgery arm, 30% in the foam sclerotherapy arm and 80% in the one study site in which concurrent EVLA and phlebectomies were performed (Hull). In all other centres, patients who were randomised to EVLA underwent delayed treatment as required, either at or after the 6-weeks follow-up, as stipulated in the protocol. Fewer patients in the foam arm (3%) appeared to have their contralateral leg treated at the same time than in the surgery (12%) and EVLA (10%) arms.
The mean treatment duration (the time taken from preparation of the patient to completion of bandaging) was shortest for those randomised to foam sclerotherapy (18.9, SD 10.2, minutes) (see Table 45). This compares to the other, far longer procedures for surgery (mean duration 51.5, SD 22.9, minutes) and EVLA (45.9, SD 24.6, minutes). Fewer consultants and more trainees performed surgery compared with the other treatments. Fifty-two per cent of surgery participants received treatment from a consultant surgeon, compared with 79% in the foam sclerotherapy group and 73% in the EVLA group. Trainees performed 38% of treatments in the surgical group, 23% in the EVLA group and 10% in the foam sclerotherapy group. The remainder of procedures were performed by the nurse consultants or staff grades.
Nearly all patients in the surgery group (97%) had a general anaesthetic, with five receiving an epidural/spinal anaesthetic (see Table 46). The anaesthetist was a consultant in 82% of cases.
Endovenous laser ablation treatment
Details specific to EVLA are shown in Table 47. Pooled across treatment arms, the mean length for the GSV was 40.5 cm (SD 14.0 cm) and the mean number of joules per centimetre was 82.9 (SD 29.1). The majority of treatments (73%) were given at 14 W with the other treatments being given at 7, 8, 10, 12, 25 or 30 W. The wavelengths used were 810 nm (66%), 1470 nm (26%) and 1064 nm in the remainder.
Primary treatment volume of foam
Among those randomised to foam sclerotherapy, the mean total volume of foam received was 9.1 ml (SD 3.0 ml) (see Table 48). In participants receiving treatment to the GSV only, the mean volume of foam used was 9.3 ml (SD 3.0 ml), with 8.2 ml (SD 3.0 ml) injected into the GSV and 1.1 ml (SD 2.1 ml) into non-truncal varicosities (see Table 48). There were five patients who received foam in excess of the 12 ml set out in the protocol without adverse complications.
Secondary or tertiary foam sclerotherapy treatment
Table 49 also shows the numbers of participants who received secondary or tertiary treatments of foam sclerotherapy, along with a breakdown of the location of that treatment. Twenty-five foam participants (14%), 42 EVLA participants (31%) and two surgery participants (1%) received secondary or tertiary foam treatments.
| Secondary foam treatment | Randomised to EVLA | Randomised to foam sclerotherapy | Randomised to surgery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| No secondary foam treatment ( N , n , %) | 136 | 94 | 69.1 | 178 | 153 | 86.0 | 167 | 165 | 98.8 |
| One secondary foam treatment ( N , n , %) | 136 | 39 | 28.7 | 178 | 24 | 13.5 | 167 | 2a | 1.2 |
| to GSV (N, n, %) | 136 | 6 | 4.4 | 178 | 8 | 4.5 | 167 | 0 | 0.0 |
| to SSV (N, n, %) | 136 | 1 | 0.7 | 178 | 2 | 1.1 | 167 | 1a | 0.6 |
| to non-truncal varicosities (N, n, %) | 136 | 34 | 25.0 | 178 | 16 | 9.0 | 167 | 1a | 0.6 |
| Two secondary foam treatments ( N , n , %) | 136 | 3 | 2.2 | 178 | 1 | 0.6 | 167 | 0 | 0.0 |
| to GSV (N, n, %) | 136 | 1 | 0.7 | 178 | 1 | 0.6 | 167 | 0 | 0.0 |
| to SSV (N, n, %) | 136 | 0 | 0.0 | 178 | 1 | 0.6 | 167 | 0 | 0.0 |
| to non-truncal varicosities (N, n, %) | 136 | 3 | 2.2 | 178 | 0 | 0.0 | 167 | 0 | 0.0 |
Bandaging/compression
All participants had a bandage or stocking applied to their study leg, and nearly all of these were full length. More foam participants (52%) received bandaging not according to protocol than surgery (12%) and EVLA (9%) participants (see Table 45). Of the 101 cases of foam participants whose bandaging was not according to protocol, only nine were related to duration and the other 92 were related to the unavailability of the type of bandaging and stocking specified in the protocol.
Treatment outcome: quality of life
The QoL at 6-weeks and 6-months follow-up are shown in Tables 50 and 51, with the corresponding statistical analysis in Table 52.
| QoL measure | Randomised to EVLA | Randomised to foam sclerotherapy | Randomised to surgery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Completed 6-weeks questionnaire ( N , n , %) | 210 | 186 | 88.6 | 207 | 183 | 88.4 | 213 | 173 | 81.2 |
| AVVQ score ( n , mean, SD) | 184 | 10.6 | 8.5 | 183 | 11.8 | 8.6 | 171 | 11.0 | 9.2 |
| EQ-5D score ( n , mean, SD) | 184 | 0.89 | 0.15 | 181 | 0.85 | 0.17 | 164 | 0.86 | 0.18 |
| VAS (n, mean, SD) | 185 | 84.0 | 14.2 | 182 | 80.4 | 17.1 | 169 | 82.5 | 16.3 |
| SF-36 summary scores | |||||||||
| Physical component summary score (n, mean, SD) | 181 | 51.3 | 7.8 | 180 | 49.4 | 9.0 | 165 | 49.1 | 9.0 |
| Mental component summary score (n, mean, SD) | 181 | 53.6 | 6.9 | 180 | 52.3 | 9.3 | 165 | 51.3 | 9.6 |
| SF-36 subscale scores | |||||||||
| Physical functioning (n, mean, SD) | 185 | 52.5 | 6.8 | 182 | 50.3 | 9.3 | 171 | 51.3 | 8.2 |
| Role physical (n, mean, SD) | 185 | 51.9 | 7.6 | 183 | 49.7 | 10.0 | 172 | 47.8 | 10.5 |
| Bodily pain (n, mean, SD) | 183 | 51.4 | 9.3 | 181 | 50.0 | 9.3 | 168 | 48.0 | 10.2 |
| General health (n, mean, SD) | 181 | 51.8 | 8.2 | 181 | 50.3 | 9.6 | 169 | 51.9 | 9.1 |
| Vitality (n, mean, SD) | 183 | 54.3 | 8.2 | 181 | 52.3 | 9.8 | 169 | 50.8 | 9.5 |
| Social functioning (n, mean, SD) | 183 | 52.1 | 7.2 | 181 | 50.9 | 9.4 | 168 | 49.7 | 10.1 |
| Role emotional (n, mean, SD) | 185 | 52.7 | 7.2 | 183 | 51.0 | 8.8 | 171 | 49.9 | 10.4 |
| Mental health (n, mean, SD) | 183 | 54.0 | 7.3 | 181 | 52.3 | 8.8 | 169 | 51.8 | 9.7 |
| QoL measure | Randomised to EVLA | Randomised to foam sclerotherapy | Randomised to surgery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Completed 6-months questionnaire ( N , n , %) | 210 | 175 | 83.3 | 207 | 170 | 82.1 | 213 | 157 | 73.7 |
| AVVQ score ( n , mean, SD) | 173 | 7.9 | 8.4 | 169 | 8.9 | 8.1 | 156 | 7.6 | 7.6 |
| EQ-5D score ( n , mean, SD) | 172 | 0.90 | 0.17 | 167 | 0.88 | 0.19 | 151 | 0.87 | 0.21 |
| VAS (n, mean, SD) | 172 | 85.1 | 11.7 | 169 | 83.6 | 13.2 | 154 | 82.5 | 15.1 |
| SF-36 summary scores | |||||||||
| Physical component summary score (n, mean, SD) | 170 | 52.6 | 7.3 | 167 | 52.0 | 9.2 | 149 | 51.9 | 9.4 |
| Mental component summary score (n, mean, SD) | 170 | 53.5 | 7.7 | 167 | 51.8 | 9.7 | 149 | 51.7 | 8.9 |
| SF-36 domain | |||||||||
| Physical functioning (n, mean, SD) | 174 | 52.7 | 6.7 | 169 | 51.6 | 8.4 | 156 | 51.0 | 9.3 |
| Role physical (n, mean, SD) | 173 | 52.6 | 7.4 | 169 | 51.9 | 8.6 | 157 | 51.5 | 8.9 |
| Bodily pain (n, mean, SD) | 171 | 54.3 | 8.9 | 168 | 52.9 | 9.9 | 152 | 53.0 | 10.8 |
| General health (n, mean, SD) | 173 | 51.9 | 9.1 | 170 | 51.2 | 9.2 | 155 | 51.6 | 9.7 |
| Vitality (n, mean, SD) | 173 | 54.1 | 8.5 | 170 | 52.7 | 10.0 | 155 | 52.7 | 9.6 |
| Social functioning (n, mean, SD) | 173 | 52.7 | 7.9 | 168 | 52.2 | 9.0 | 154 | 51.5 | 9.5 |
| Role emotional (n, mean, SD) | 174 | 52.5 | 7.8 | 169 | 51.3 | 9.0 | 155 | 51.0 | 9.8 |
| Mental health (n, mean, SD) | 173 | 54.1 | 7.7 | 170 | 51.8 | 9.9 | 155 | 51.4 | 9.5 |
| QoL measure | Randomised to EVLA | Randomised to foam sclerotherapy | Randomised to surgery | Surgery vs. EVLA | EVLA vs. foam sclerotherapy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | Effect sizea | 95% CI | p-value | Effect sizeb | 95% CI | p-value | |
| AVVQ at baseline | 210 | 17.8 | 9.1 | 207 | 17.4 | 9.7 | 213 | 18.0 | 9.0 | N/A | N/A | N/A | N/A | N/A | N/A |
| AVVQ at 6 weeks | 184 | 10.6 | 8.5 | 183 | 11.8 | 8.6 | 171 | 11.0 | 9.2 | 0.21 | –1.38 to 1.79 | 0.797 | –1.71 | –3.27 to –0.15 | 0.032 |
| AVVQ at 6 months | 173 | 7.9 | 8.4 | 169 | 8.9 | 8.1 | 156 | 7.6 | 7.6 | –0.63 | –2.16 to 0.90 | 0.419 | –1.06 | –2.56 to 0.43 | 0.163 |
| EQ-5D at baseline | 210 | 0.79 | 0.17 | 207 | 0.79 | 0.19 | 213 | 0.78 | 0.18 | N/A | N/A | N/A | N/A | N/A | N/A |
| EQ-5D at 6 weeks | 184 | 0.89 | 0.15 | 181 | 0.85 | 0.17 | 164 | 0.86 | 0.18 | –0.02 | –0.05 to 0.01 | 0.126 | 0.04 | 0.01 to 0.07 | 0.004 |
| EQ-5D at 6 months | 172 | 0.90 | 0.17 | 167 | 0.88 | 0.19 | 151 | 0.87 | 0.21 | –0.02 | –0.05 to 0.02 | 0.405 | 0.02 | –0.01 to 0.06 | 0.164 |
| EQ-5D VAS at baseline | 210 | 80.6 | 16.1 | 207 | 80.6 | 16.2 | 213 | 80.0 | 15.4 | N/A | N/A | N/A | N/A | N/A | N/A |
| EQ-5D VAS at 6 weeks | 185 | 84.0 | 14.2 | 182 | 80.4 | 17.1 | 169 | 82.5 | 16.3 | –0.90 | –3.76 to 1.97 | 0.539 | 3.66 | 0.85 to 6.47 | 0.011 |
| EQ-5D VAS at 6 months | 172 | 85.1 | 11.7 | 169 | 83.6 | 13.2 | 154 | 82.5 | 15.1 | –2.09 | –4.57 to 0.39 | 0.099 | 1.70 | –0.72 to 4.12 | 0.167 |
| SF-36 physical component score at baseline | 210 | 48.6 | 7.8 | 207 | 48.4 | 8.5 | 213 | 48.2 | 8.7 | N/A | N/A | N/A | N/A | N/A | N/A |
| SF-36 physical component score at 6 weeks | 181 | 51.3 | 7.8 | 180 | 49.4 | 9.0 | 165 | 49.1 | 9.0 | –1.75 | –3.20 to –0.29 | 0.019 | 1.90 | 0.48 to 3.32 | 0.009 |
| SF-36 physical component score at 6 months | 170 | 52.6 | 7.3 | 167 | 52.0 | 9.2 | 149 | 51.9 | 9.4 | 0.07 | –1.41 to 1.55 | 0.923 | 0.66 | –0.78 to 2.09 | 0.368 |
| SF-36 mental component score at baseline | 210 | 51.9 | 9.0 | 207 | 51.8 | 9.0 | 213 | 50.9 | 9.7 | N/A | N/A | N/A | N/A | N/A | N/A |
| SF-36 mental component score at 6 weeks | 181 | 53.6 | 6.9 | 180 | 52.3 | 9.3 | 165 | 51.3 | 9.6 | –1.85 | –3.41 to –0.29 | 0.020 | 1.11 | –0.41 to 2.63 | 0.151 |
| SF-36 mental component score at 6 months | 170 | 53.5 | 7.7 | 167 | 51.8 | 9.7 | 149 | 51.7 | 8.9 | –1.33 | –2.91 to 0.24 | 0.096 | 1.54 | 0.01 to 3.06 | 0.048 |
| SF-36 Physical functioning at baseline | 210 | 50.2 | 8.2 | 207 | 49.8 | 8.5 | 213 | 50.0 | 8.4 | N/A | N/A | N/A | N/A | N/A | N/A |
| SF-36 Physical functioning at 6 weeks | 185 | 52.5 | 6.8 | 182 | 50.3 | 9.3 | 171 | 51.3 | 8.2 | –0.91 | –2.26 to 0.45 | 0.190 | 1.82 | 0.49 to 3.16 | 0.008 |
| SF-36 Physical functioning at 6 months | 174 | 52.7 | 6.7 | 169 | 51.6 | 8.4 | 156 | 51.0 | 9.3 | –1.03 | –2.33 to 0.26 | 0.117 | 0.77 | –0.50 to 2.03 | 0.234 |
| SF-36 Role physical at baseline | 210 | 50.1 | 8.3 | 207 | 50.2 | 9.1 | 213 | 49.0 | 10.0 | N/A | N/A | N/A | N/A | N/A | N/A |
| SF-36 Role physical at 6 weeks | 185 | 51.9 | 7.6 | 183 | 49.7 | 10.0 | 172 | 47.8 | 10.5 | –3.48 | –5.21 to –1.76 | 0.000 | 2.22 | 0.52 to 3.91 | 0.010 |
| SF-36 Role physical at 6 months | 173 | 52.6 | 7.4 | 169 | 51.9 | 8.6 | 157 | 51.5 | 8.9 | 0.16 | –1.22 to 1.54 | 0.821 | 0.41 | –0.94 to 1.76 | 0.550 |
| SF-36 Bodily pain at baseline | 210 | 47.3 | 8.5 | 207 | 47.6 | 9.2 | 213 | 46.0 | 9.5 | N/A | N/A | N/A | N/A | N/A | N/A |
| SF-36 Bodily pain at 6 weeks | 183 | 51.4 | 9.3 | 181 | 50.0 | 9.3 | 168 | 48.0 | 10.2 | –2.68 | –4.44 to –0.93 | 0.003 | 1.64 | –0.07 to 3.36 | 0.061 |
| SF-36 Bodily pain at 6 months | 171 | 54.3 | 8.9 | 168 | 52.9 | 9.9 | 152 | 53.0 | 10.8 | –0.11 | –1.92 to 1.71 | 0.909 | 1.30 | –0.46 to 3.06 | 0.146 |
| SF-36 General health at baseline | 210 | 49.5 | 8.3 | 207 | 49.1 | 8.3 | 213 | 49.2 | 8.6 | N/A | N/A | N/A | N/A | N/A | N/A |
| SF-36 General health at 6 weeks | 181 | 51.8 | 8.2 | 181 | 50.3 | 9.6 | 169 | 51.9 | 9.1 | 0.33 | –1.07 to 1.74 | 0.641 | 1.06 | –0.32 to 2.45 | 0.131 |
| SF-36 General health at 6 months | 173 | 51.9 | 9.1 | 170 | 51.2 | 9.2 | 155 | 51.6 | 9.7 | –0.08 | –1.60 to 1.44 | 0.917 | 0.35 | –1.14 to 1.83 | 0.646 |
| SF-36 Vitality at baseline | 210 | 51.5 | 9.2 | 207 | 51.1 | 9.7 | 213 | 50.2 | 9.4 | N/A | N/A | N/A | N/A | N/A | N/A |
| SF-36 Vitality at 6 weeks | 183 | 54.3 | 8.2 | 181 | 52.3 | 9.8 | 169 | 50.8 | 9.5 | –2.33 | –3.88 to –0.78 | 0.003 | 1.41 | –0.11 to 2.92 | 0.069 |
| SF-36 Vitality at 6 months | 173 | 54.1 | 8.5 | 170 | 52.7 | 10.0 | 155 | 52.7 | 9.6 | –0.49 | –2.07 to 1.10 | 0.546 | 0.90 | –0.64 to 2.44 | 0.253 |
| SF-36 Social functioning at baseline | 210 | 50.8 | 8.8 | 207 | 50.9 | 8.4 | 213 | 49.8 | 9.8 | N/A | N/A | N/A | N/A | N/A | N/A |
| SF-36 Social functioning at 6 weeks | 183 | 52.1 | 7.2 | 181 | 50.9 | 9.4 | 168 | 49.7 | 10.1 | –1.87 | –3.49 to –0.24 | 0.025 | 1.22 | –0.37 to 2.82 | 0.133 |
| SF-36 Social functioning at 6 months | 173 | 52.7 | 7.9 | 168 | 52.2 | 9.0 | 154 | 51.5 | 9.5 | –0.57 | –2.13 to 0.99 | 0.472 | 0.64 | –0.88 to 2.16 | 0.412 |
| SF-36 Role emotional at baseline | 210 | 51.1 | 8.3 | 207 | 51.0 | 8.7 | 213 | 50.1 | 9.8 | N/A | N/A | N/A | N/A | N/A | N/A |
| SF-36 Role emotional at 6 weeks | 185 | 52.7 | 7.2 | 183 | 51.0 | 8.8 | 171 | 49.9 | 10.4 | –2.42 | –4.05 to –0.80 | 0.004 | 1.60 | 0.00 to 3.19 | 0.050 |
| SF-36 Role emotional at 6 months | 174 | 52.5 | 7.8 | 169 | 51.3 | 9.0 | 155 | 51.0 | 9.8 | –0.77 | –2.39 to 0.85 | 0.351 | 0.96 | –0.62 to 2.54 | 0.231 |
| SF-36 Mental health at baseline | 210 | 51.6 | 9.2 | 207 | 51.3 | 9.6 | 213 | 50.7 | 10.1 | N/A | N/A | N/A | N/A | N/A | N/A |
| SF-36 Mental health at 6 weeks | 183 | 54.0 | 7.3 | 181 | 52.3 | 8.8 | 169 | 51.8 | 9.7 | –1.57 | –3.07 to –0.08 | 0.039 | 1.26 | –0.21 to 2.72 | 0.092 |
| SF-36 Mental health at 6 months | 173 | 54.1 | 7.7 | 170 | 51.8 | 9.9 | 155 | 51.4 | 9.5 | –2.11 | –3.71 to –0.52 | 0.010 | 2.06 | 0.51 to 3.61 | 0.009 |
Aberdeen Varicose Vein Questionnaire
At 6 weeks and 6 months, QoL measures in all treatment groups appeared better than at baseline. There was no significant treatment effect for AVVQ when surgery and EVLA were compared at 6 weeks (0.21, 95% CI −1.38 to 1.79; p = 0.797) or at 6 months (−0.63, 95% CI −2.16 to 0.90; p = 0.419). The AVVQ was similar for EVLA and foam (at 6 weeks −1.71, 95% CI −3.27 to −0.15; p = 0.032, and at 6 months −1.06, 95% CI −2.56 to 0.43; p = 0.163).
Sensitivity analyses
There were some missing AVVQ scores at 6 months (27% for surgery and 18% for both EVLA and foam). Exploratory analysis shows that participants without a valid AVVQ score at 6 months had mean baseline AVVQ scores of 18.8 (SD 11.7) for foam sclerotherapy, 18.6 (SD 9.3) for surgery and 20.7 (SD 10.5) for EVLA. Those with an AVVQ score at 6 months had mean baseline scores of 17.1 (SD 9.2) for foam, 17.9 (SD 9.0) for surgery and 17.32 (SD 8.7) for EVLA, indicating that non-respondents had higher mean scores at baseline and that the extent of this difference is not the same in each group (the biggest difference was observed in the EVLA arm). The mean AVVQ score at 6 weeks for participants with missing scores at 6 months is 13.1 (higher than the mean for all EVLA participants), 12.5 for foam sclerotherapy (slightly higher than the mean for all foam participants) and 8.8 for surgery (lower than the mean for all surgery participants), indicating that there are differences in the missing data between groups.
In Tables 53 and 54, different estimates of the effects of treatment on the primary outcome are shown when all missing AVVQ scores at 6 months were imputed under varying assumptions. When surgery is compared against EVLA, using the ‘missing not at random’ (MNAR) assumption, the estimate of treatment effect is −1.02 (95% CI −2.48 to 0.45, p = 0.174), an increase in the effect size estimated in the primary analysis (see Table 54). When variously adding or subtracting two points to the imputed values obtained from multiple imputation, either to one arm at a time or to both arms simultaneously, the resulting estimates range from −0.48 to −1.55 and represent an increase in the effect size in all but one instance (but always maintaining the same direction of effect). The missing AVVQ values for surgery would need to be at least 1.7 points lower (or the missing values for EVLA at least 2.6 points higher) than the values imputed under MNAR for there to be a significant difference in favour of surgery.
| Sensitivity analysis | Effect sizea | 95% CI | p-value |
|---|---|---|---|
| Primary analysis (repeated measures, assuming missing at random) | −0.63 | −2.16 to 0.90 | 0.419 |
| Multiple imputation (assuming MNAR) | −1.02 | −2.48 to 0.45 | 0.174 |
| All missing assumed to have AVVQ scores two points lower | −1.20 | −2.68 to 0.28 | 0.111 |
| All missing assumed to have AVVQ scores two points higher | −0.83 | −2.30 to 0.64 | 0.267 |
| Missing in surgery group assumed to have AVVQ scores two points lower | −1.55 | −3.03 to −0.08 | 0.039 |
| Missing in surgery group assumed to have AVVQ scores two points higher | −0.48 | −1.94 to 0.99 | 0.521 |
| Missing in EVLA group assumed to have AVVQ scores two points lower | −0.66 | −2.13 to 0.80 | 0.374 |
| Missing in EVLA group assumed to have AVVQ scores two points higher | −1.37 | −2.84 to 0.10 | 0.068 |
| Sensitivity analysis | Effect sizea | 95% CI | p-value |
|---|---|---|---|
| Primary analysis (repeated measures, assuming missing at random) | −1.06 | −2.56 to 0.43 | 0.163 |
| Multiple imputation (assuming MNAR) | −0.56 | −1.33 to 0.21 | 0.153 |
| All missing assumed to have AVVQ scores two points lower | −0.55 | −1.32 to 0.22 | 0.163 |
| All missing assumed to have AVVQ scores two points higher | −0.57 | −1.34 to 0.20 | 0.146 |
| Missing in foam group assumed to have AVVQ scores two points lower | −0.37 | −1.14 to 0.40 | 0.340 |
| Missing in foam group assumed to have AVVQ scores two points higher | −0.75 | −1.51 to 0.02 | 0.058 |
| Missing in EVLA group assumed to have AVVQ scores two points lower | −0.73 | −1.50 to 0.04 | 0.061 |
| Missing in EVLA group assumed to have AVVQ scores two points higher | −0.38 | −1.15 to 0.38 | 0.326 |
For the comparison between EVLA and foam, the MNAR estimate of treatment effect is smaller than the primary analysis at −0.56 (95% CI −1.33 to 0.21). Variations to this assumption result in estimates ranging from −0.38 to −0.75, and in all instances represent a decrease in the effect size (but always maintaining the same direction of effect). The missing AVVQ values for EVLA would need to be at least 2.5 points lower (or the missing values for foam at least 2.3 points higher) than the values imputed under MNAR for there to be a significant difference in favour of EVLA.
Short Form questionnaire-36 items
At 6 weeks and 6 months, QoL measures in all treatment groups appeared better than at baseline (see Tables 50 and 51).
Comparison of endovenous laser ablation with foam sclerotherapy
The overall SF-36 physical component score and physical domains were similar in patients who underwent EVLA (see Table 52).
The SF-36 mental component score was similar for the EVLA and foam groups at 6 weeks, but at 6 months a greater health gain was obtained with EVLA (1.54, 95% CI 0.01 to 3.06; p = 0.048). There were no statistical differences in the mental domain scores.
Comparison of surgery with endovenous laser ablation
There were no statistical differences in the SF-36 component score between surgery and EVLA. The individual domains of role physical, bodily pain, vitality, social functioning and role emotional showed a significant benefit in favour of EVLA at 6 weeks (p < 0.005) but not at 6 months.
European Quality of Life-5 Dimensions
At 6 weeks and 6 months, QoL measures in all treatment groups appeared better than at baseline.
Comparison of endovenous laser ablation with foam sclerotherapy
At 6 weeks there was a significantly greater improvement in the EQ-5D score (0.04, 95% CI 0.01 to 0.07; p = 0.004) in patients who underwent EVLA than in those who received foam sclerotherapy. This difference was not apparent at 6-months follow-up. There were no differences in the EQ-5D VAS at either time point.
Comparison of surgery with endovenous laser ablation
At 6 weeks and 6 months, no differences were noted in the EQ-5D and EQ-5D VAS scores between patients who received EVLA and those who underwent surgery.
Clinical outcomes
Venous Clinical Severity Score
These outcomes are presented in Table 55 (for 6 weeks) and Table 56 (for 6 months), with the estimates of treatment effect sizes for the comparisons of EVLA with surgery and EVLA with foam therapy in Table 57.
| Clinical outcome measure | Randomised to EVLA | Randomised to foam sclerotherapy | Randomised to surgery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinic assessment at 6 weeks ( N , n , %) | 210 | 193 | 91.9 | 207 | 189 | 91.3 | 213 | 180 | 84.5 |
| VCSS ( n , mean, SD) | 175 | 1.7 | 1.7 | 177 | 2.2 | 2.0 | 163 | 1.7 | 1.9 |
| Presence of residual varicosities | |||||||||
| Assessed by participant (N, n, %) | 187 | 152 | 81.3 | 185 | 154 | 83.2 | 173 | 125 | 72.3 |
| VAS (n, mean, SD) | 187 | 2.2 | 1.9 | 185 | 2.4 | 2.0 | 173 | 1.8 | 1.9 |
| Assessed by research nurse (N, n, %) | 186 | 137 | 73.7 | 185 | 129 | 69.7 | 173 | 83 | 48.0 |
| VAS (n, mean, SD) | 186 | 1.5 | 1.5 | 185 | 1.6 | 1.6 | 173 | 0.8 | 1.0 |
| CEAP classification | |||||||||
| C0 No visible or palpable signs of venous disease (N, n, %) | 176 | 29 | 16.5 | 179 | 30 | 16.8 | 161 | 49 | 30.4 |
| C1 Telangiectasis or reticular veins < 3 mm (N, n, %) | 176 | 50 | 28.4 | 179 | 55 | 30.7 | 161 | 73 | 45.3 |
| C2 Varicose veins > 3 mm (N, n, %) | 176 | 74 | 42.0 | 179 | 76 | 42.5 | 161 | 23 | 14.3 |
| C3 Oedema (N, n, %) | 176 | 4 | 2.3 | 179 | 3 | 1.7 | 161 | 1 | 0.6 |
| C4 Skin and subcutaneous changes (N, n, %) | 176 | 4 | 2.3 | 179 | 6 | 3.4 | 161 | 5 | 3.1 |
| C4a Pigmentation or eczema (N, n, %) | 176 | 11 | 6.3 | 179 | 9 | 5.0 | 161 | 8 | 5.0 |
| C4b Lipodermatosclerosis or atrophie blanche (N, n, %) | 176 | 1 | 0.6 | 179 | 0 | 0.0 | 161 | 1 | 0.6 |
| C5 Healed venous ulcer (N, n, %) | 176 | 3 | 1.7 | 179 | 0 | 0.0 | 161 | 1 | 0.6 |
| C6 Active venous ulcer (N, n, %) | 176 | 0 | 0.0 | 179 | 0 | 0.0 | 161 | 0 | 0.0 |
| Clinical outcome measure | Randomised to EVLA | Randomised to foam sclerotherapy | Randomised to surgery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinic assessment at 6 months ( N , n , %) | 210 | 183 | 87.1 | 207 | 178 | 86.0 | 213 | 167 | 78.4 |
| VCSS ( n , mean, SD) | 157 | 1.4 | 1.5 | 152 | 1.7 | 1.8 | 142 | 1.3 | 1.5 |
| Presence of residual varicosities | |||||||||
| Assessed by participant (N, n, %) | 168 | 122 | 72.6 | 162 | 132 | 81.5 | 155 | 101 | 65.2 |
| VAS (n, mean, SD) | 168 | 1.8 | 1.9 | 162 | 2.3 | 1.9 | 155 | 1.4 | 1.5 |
| Assessed by research nurse (N, n, %) | 167 | 90 | 53.9 | 162 | 101 | 62.3 | 155 | 73 | 47.1 |
| VAS (n, mean, SD) | 167 | 1.0 | 1.4 | 162 | 1.1 | 1.3 | 155 | 0.7 | 1.0 |
| CEAP classification | |||||||||
| C0 No visible or palpable signs of venous disease (N, n, %) | 159 | 31 | 19.5 | 153 | 26 | 17.0 | 149 | 42 | 28.2 |
| C1 Telangiectasis or reticular veins < 3 mm (N, n, %) | 159 | 71 | 44.7 | 153 | 65 | 42.5 | 149 | 69 | 46.3 |
| C2 Varicose veins > 3 mm (N, n, %) | 159 | 41 | 25.8 | 153 | 45 | 29.4 | 149 | 25 | 16.8 |
| C3 Oedema (N, n, %) | 159 | 3 | 1.9 | 153 | 8 | 5.2 | 149 | 1 | 0.7 |
| C4 Skin and subcutaneous changes (N, n, %) | 159 | 1 | 0.6 | 153 | 0 | 0.0 | 149 | 3 | 2.0 |
| C4a Pigmentation or eczema (N, n, %) | 159 | 7 | 4.4 | 153 | 8 | 5.2 | 149 | 7 | 4.7 |
| C4b Lipodermatosclerosis or atrophie blanche (N, n, %) | 159 | 2 | 1.3 | 153 | 0 | 0.0 | 149 | 0 | 0.0 |
| C5 Healed venous ulcer (N, n, %) | 159 | 3 | 1.9 | 153 | 1 | 0.7 | 149 | 2 | 1.3 |
| C6 Active venous ulcer (N, n, %) | 159 | 0 | 0.0 | 153 | 0 | 0.0 | 149 | 0 | 0.0 |
| Clinical outcome measure | Randomised to EVLA | Randomised to foam sclerotherapy | Randomised to surgery | Surgery vs. EVLA | EVLA vs. foam sclerotherapy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | Estimate of effect | 95% CI | p-value | Estimate | 95% CI | p-value | |
| VCSS at baseline | 210 | 5.0 | 2.5 | 207 | 4.8 | 2.5 | 213 | 5.0 | 2.4 | N/A | N/A | N/A | N/A | N/A | N/A |
| VCSS at 6 weeks | 175 | 1.7 | 1.7 | 177 | 2.2 | 2.0 | 163 | 1.7 | 1.9 | −0.02 | −0.39 to 0.36 | 0.929 | −0.52 | −0.89 to −0.15 | 0.006 |
| VCSS at 6 months | 157 | 1.4 | 1.5 | 152 | 1.7 | 1.8 | 142 | 1.3 | 1.5 | −0.11 | −0.46 to 0.25 | 0.560 | −0.26 | −0.61 to 0.09 | 0.148 |
| Patient VASa at baseline | 210 | 5.5 | 2.3 | 207 | 5.2 | 2.2 | 213 | 5.6 | 2.3 | N/A | N/A | N/A | N/A | N/A | N/A |
| Patient VASa at 6 weeks | 187 | 2.2 | 1.9 | 185 | 2.4 | 2.0 | 173 | 1.8 | 1.9 | −0.41 | −0.78 to −0.03 | 0.035 | −0.37 | −0.74 to 0.00 | 0.053 |
| Patient VASa at 6 months | 168 | 1.8 | 1.9 | 162 | 2.3 | 1.9 | 155 | 1.4 | 1.5 | −0.40 | −0.78 to −0.02 | 0.037 | −0.54 | −0.91 to −0.17 | 0.005 |
| Nurse VASa at baseline | 210 | 3.6 | 2.2 | 207 | 3.6 | 2.1 | 213 | 3.7 | 2.3 | N/A | N/A | N/A | N/A | N/A | N/A |
| Nurse VASa at 6 weeks | 186 | 1.5 | 1.5 | 185 | 1.6 | 1.6 | 173 | 0.8 | 1.0 | −0.76 | −1.04 to −0.48 | < 0.001 | −0.07 | −0.35 to 0.21 | 0.614 |
| Nurse VASa at 6 months | 167 | 1.0 | 1.4 | 162 | 1.1 | 1.3 | 155 | 0.7 | 1.0 | −0.28 | −0.54 to −0.02 | 0.037 | −0.12 | −0.38 to 0.14 | 0.354 |
The VCSS showed an apparent improvement (reduction in score) in all groups from baseline to 6 weeks, and from 6 weeks to 6 months. There were no differences between EVLA and foam or between surgery and EVLA in the VCSS at any time point.
Residual varicose veins
The presence of residual varicose veins as assessed by the participant and the research nurse show an apparent improvement (reduction in score) in all groups from baseline to 6 weeks and from 6 weeks to 6 months.
Comparison of endovenous laser ablation with foam sclerotherapy
There were no differences at 6 weeks between EVLA and foam sclerotherapy. Participants reported significantly fewer residual varicose veins in the EVLA group at 6-months follow-up (−0.54, 95% CI −0.91 to −0.17; p = 0.005). There were no differences in the nurse-reported scores.
Comparison of surgery with endovenous laser ablation
There were significantly fewer residual varicose veins following surgery than following EVLA as assessed by the nurse at 6 weeks (−0.76, 95% CI −1.04 to −0.48; p < 0.001), but not at 6 months. Results were similar for participant-reported residual varicose veins at both time points.
Clinical, etiological, anatomical, pathological classification
Although the CEAP is not generally considered an appropriate measure of outcome, it is included for completeness, but not statistically analysed. All participants had a CEAP classification of C2 or above at recruitment. By 6 weeks, the proportion of participants with a classification lower than C2 was highest in the surgery group (76%), compared with only 47% for foam and 45% for EVLA (see Table 55). At 6 months, the proportion of participants with a classification better than C2 remained highest in the surgery group (74%), compared with 64% for EVLA and 59% for foam (see Table 56).
Pain
Immediately after treatment, the mean pain scores for those randomised to foam sclerotherapy, surgery and EVLA were 2.2 (SD 2.0), 2.4 (SD 2.6) and 3.5 (SD 2.2) respectively (see Appendix 2, Table 107). Patient-reported pain is significantly higher for EVLA than for either surgery or foam sclerotherapy. At 6 weeks, patients’ recollection of pain during treatment was higher than that recorded after treatment for all three treatment modalities. Patients’ recollection of the pain they experienced during foam sclerotherapy (mean VAS 3.0, SD 2.5) was significantly lower than for either surgery (mean VAS 4.1, SD 3.0) or EVLA (mean VAS 4.4, SD 2.8). Patients’ recollection of pain during recovery was also significantly lower for foam sclerotherapy than for either surgery or EVLA. The pain experienced during recovery for surgery was significantly higher than that for EVLA.
Anatomical success
Success rates for truncal vein ablation at 6 weeks and 6 months are shown in Tables 58 and 59. The overall statistical analysis for the whole leg and the GSV only is shown in Table 60. The numbers undergoing treatment to the SSV alone or in combination with the GSV were small, and therefore these subgroups were not subjected to statistical analysis.
| Anatomical success | Randomised to EVLA | Randomised to foam sclerotherapy | Randomised to surgery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinic assessment at 6 weeks ( N , n , %) | 210 | 193 | 91.9 | 207 | 189 | 91.3 | 213 | 180 | 84.5 |
| GSV involvement only | |||||||||
| Complete success (N, n, %) | 153 | 127 | 83.0 | 152 | 96 | 63.2 | 143 | 122 | 85.3 |
| Partial success (N, n, %) | 153 | 23 | 15.0 | 152 | 36 | 23.7 | 143 | 15 | 10.5 |
| without reflux (N, n, %) | 153 | 13 | 8.5 | 152 | 29 | 19.1 | 143 | 9 | 6.3 |
| with reflux (N, n, %) | 153 | 10 | 6.5 | 152 | 7 | 4.6 | 143 | 6 | 4.2 |
| Failure (N, n, %) | 153 | 3 | 2.0 | 152 | 20 | 13.2 | 143 | 6 | 4.2 |
| SSV involvement only | |||||||||
| Complete success (N, n, %) | 12 | 6 | 50.0 | 11 | 5 | 45.5 | 12 | 3 | 25.0 |
| Partial success (N, n, %) | 12 | 4 | 33.3 | 11 | 4 | 36.4 | 12 | 7 | 58.3 |
| without reflux (N, n, %) | 12 | 4 | 33.3 | 11 | 4 | 36.4 | 12 | 5 | 41.7 |
| with reflux (N, n, %) | 12 | 0 | 0.0 | 11 | 0 | 0.0 | 12 | 2 | 16.7 |
| Failure (N, n, %) | 12 | 2 | 16.7 | 11 | 2 | 18.2 | 12 | 2 | 16.7 |
| GSV and SSV involvement | |||||||||
| GSV | |||||||||
| Complete success (N, n, %) | 9 | 4 | 44.4 | 15 | 9 | 60.0 | 11 | 9 | 81.8 |
| Partial success (N, n, %) | 9 | 2 | 22.2 | 15 | 3 | 20.0 | 11 | 0 | 0.0 |
| without reflux (N, n, %) | 9 | 1 | 11.1 | 15 | 1 | 6.7 | 11 | 0 | 0.0 |
| with reflux (N, n, %) | 9 | 1 | 11.1 | 15 | 1 | 6.7 | 11 | 0 | 0.0 |
| Failure (N, n, %) | 9 | 3 | 33.3 | 15 | 3 | 20.0 | 11 | 2 | 18.2 |
| SSV | |||||||||
| Complete success (N, n, %) | 6 | 1 | 16.7 | 16 | 2 | 12.5 | 11 | 1 | 9.1 |
| Partial success (N, n, %) | 6 | 1 | 16.7 | 16 | 3 | 18.8 | 11 | 3 | 27.3 |
| without reflux (N, n, %) | 6 | 1 | 16.7 | 16 | 2 | 12.5 | 11 | 1 | 9.1 |
| with reflux (N, n, %) | 6 | 0 | 0.0 | 16 | 0 | 0.0 | 11 | 2 | 18.2 |
| Failure (N, n, %) | 6 | 4 | 66.7 | 16 | 11 | 68.8 | 11 | 7 | 63.6 |
| Overall treatment of study leg | |||||||||
| Complete success (N, n, %) | 170 | 133 | 78.2 | 178 | 103 | 57.9 | 165 | 125 | 75.8 |
| Partial success (N, n, %) | 170 | 31 | 18.2 | 178 | 50 | 28.1 | 165 | 32 | 19.4 |
| without reflux (N, n, %) | 170 | 19 | 11.2 | 178 | 41 | 23.0 | 165 | 21 | 12.7 |
| with reflux (N, n, %) | 170 | 12 | 7.1 | 178 | 9 | 5.1 | 165 | 11 | 6.7 |
| Failure (N, n, %) | 170 | 6 | 3.5 | 178 | 25 | 14.0 | 165 | 8 | 4.8 |
| Anatomical success | Randomised to EVLA | Randomised to foam sclerotherapy | Randomised to surgery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinic assessment at 6 months ( N , n , %) | 210 | 183 | 87.1 | 207 | 178 | 86.0 | 213 | 167 | 78.4 |
| GSV involvement only | |||||||||
| Complete success (N, n, %) | 141 | 116 | 82.3 | 132 | 67 | 50.8 | 127 | 101 | 79.5 |
| Partial success (N, n, %) | 141 | 16 | 11.3 | 132 | 31 | 23.5 | 127 | 16 | 12.6 |
| without reflux (N, n, %) | 141 | 13 | 9.2 | 132 | 22 | 16.7 | 127 | 3 | 2.4 |
| with reflux (N, n, %) | 141 | 3 | 2.1 | 132 | 9 | 6.8 | 127 | 13 | 10.2 |
| Failure (N, n, %) | 141 | 9 | 6.4 | 132 | 34 | 25.8 | 127 | 10 | 7.9 |
| SSV involvement only | |||||||||
| Complete success (N, n, %) | 9 | 6 | 66.7 | 11 | 4 | 36.4 | 11 | 3 | 27.3 |
| Partial success (N, n, %) | 9 | 3 | 33.3 | 11 | 2 | 18.2 | 11 | 3 | 27.3 |
| without reflux (N, n, %) | 9 | 2 | 22.2 | 11 | 1 | 9.1 | 11 | 3 | 27.3 |
| with reflux (N, n, %) | 9 | 1 | 11.1 | 11 | 1 | 9.1 | 11 | 0 | 0.0 |
| Failure (N, n, %) | 9 | 0 | 0.0 | 11 | 5 | 45.5 | 11 | 5 | 45.5 |
| GSV and SSV involvement | |||||||||
| GSV | |||||||||
| Complete success (N, n, %) | 10 | 6 | 60.0 | 14 | 5 | 35.7 | 9 | 7 | 77.8 |
| Partial success (N, n, %) | 10 | 2 | 20.0 | 14 | 4 | 28.6 | 9 | 1 | 11.1 |
| without reflux (N, n, %) | 10 | 2 | 20.0 | 14 | 3 | 21.4 | 9 | 0 | 0.0 |
| with reflux (N, n, %) | 10 | 0 | 0.0 | 14 | 1 | 7.1 | 9 | 1 | 11.1 |
| Failure (N, n, %) | 10 | 2 | 20.0 | 14 | 5 | 35.7 | 9 | 1 | 11.1 |
| SSV | |||||||||
| Complete success (N, n, %) | 7 | 2 | 28.6 | 13 | 2 | 15.4 | 9 | 1 | 11.1 |
| Partial success (N, n, %) | 7 | 2 | 28.6 | 13 | 1 | 7.7 | 9 | 0 | 0.0 |
| without reflux (N, n, %) | 7 | 1 | 14.3 | 13 | 1 | 7.7 | 9 | 0 | 0.0 |
| with reflux (N, n, %) | 7 | 1 | 14.3 | 13 | 0 | 0.0 | 9 | 0 | 0.0 |
| Failure (N, n, %) | 7 | 3 | 42.9 | 13 | 10 | 76.9 | 9 | 8 | 88.9 |
| Overall treatment of study leg | |||||||||
| Complete success (N, n, %) | 156 | 123 | 78.8 | 156 | 73 | 46.8 | 147 | 104 | 70.7 |
| Partial success (N, n, %) | 156 | 24 | 15.4 | 156 | 41 | 26.3 | 147 | 28 | 19.0 |
| without reflux (N, n, %) | 156 | 20 | 12.8 | 156 | 31 | 19.9 | 147 | 15 | 10.2 |
| with reflux (N, n, %) | 156 | 4 | 2.6 | 156 | 10 | 6.4 | 147 | 13 | 8.8 |
| Failure (N, n, %) | 156 | 9 | 5.8 | 156 | 42 | 26.9 | 147 | 15 | 10.2 |
| Anatomical success | Randomised to EVLA | Randomised to foam sclerotherapy | Randomised to surgery | Surgery vs. EVLA | EVLA vs. foam sclerotherapy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | n | % | N | n | % | N | n | % | ORa | 95% CI | p-value | ORb | 95% CI | p-value | |
| Complete success at 6 weeks (whole leg) | 170 | 133 | 78.2 | 178 | 103 | 57.9 | 165 | 125 | 75.8 | ||||||
| Partial success without reflux at 6 weeks (whole leg) | 170 | 19 | 11.2 | 178 | 41 | 23.0 | 165 | 21 | 12.7 | ||||||
| Partial success with reflux at 6 weeks (whole leg) | 170 | 12 | 7.1 | 178 | 9 | 5.1 | 165 | 11 | 6.7 | ||||||
| Failure at 6 weeks (whole leg) | 170 | 6 | 3.5 | 178 | 25 | 14.0 | 165 | 8 | 4.8 | 1.11 | 0.63 to 1.95 | 0.723 | 2.42 | 1.48 to 3.95 | < 0.001 |
| Complete success at 6 months (whole leg) | 156 | 123 | 78.8 | 156 | 73 | 46.8 | 147 | 104 | 70.7 | ||||||
| Partial success without reflux at 6 months (whole leg) | 156 | 20 | 12.8 | 156 | 31 | 19.9 | 147 | 15 | 10.2 | ||||||
| Partial success with reflux at 6 months (whole leg) | 156 | 4 | 2.6 | 156 | 10 | 6.4 | 147 | 13 | 8.8 | ||||||
| Failure at 6 months (whole leg) | 156 | 9 | 5.8 | 156 | 42 | 26.9 | 147 | 15 | 10.2 | 0.62 | 0.36 to 1.07 | 0.087 | 4.67 | 2.81 to 7.77 | < 0.001 |
| Complete success at 6 weeks (GSV) | 153 | 127 | 83.0 | 152 | 96 | 63.2 | 143 | 122 | 85.3 | ||||||
| Partial success without reflux at 6 weeks (GSV) | 153 | 13 | 8.5 | 152 | 29 | 19.1 | 143 | 9 | 6.3 | ||||||
| Partial success with reflux at 6 weeks (GSV) | 153 | 10 | 6.5 | 152 | 7 | 4.6 | 143 | 6 | 4.2 | ||||||
| Failure at 6 weeks (GSV) | 153 | 3 | 2.0 | 152 | 20 | 13.2 | 143 | 6 | 4.2 | 1.17 | 0.61 to 2.26 | 0.636 | 3.10 | 1.78 to 5.42 | < 0.001 |
| Complete success at 6 months (GSV) | 141 | 116 | 82.3 | 132 | 67 | 50.8 | 127 | 101 | 79.5 | ||||||
| Partial success without reflux at 6 months (GSV) | 141 | 13 | 9.2 | 132 | 22 | 16.7 | 127 | 3 | 2.4 | ||||||
| Partial success with reflux at 6 months (GSV) | 141 | 3 | 2.1 | 132 | 9 | 6.8 | 127 | 13 | 10.2 | ||||||
| Failure at 6 months (GSV) | 141 | 9 | 6.4 | 132 | 34 | 25.8 | 127 | 10 | 7.9 | 0.82 | 0.44 to 1.53 | 0.531 | 4.83 | 2.76 to 8.48 | < 0.001 |
There were no differences in ablation success between surgery and EVLA. However, for all comparisons between EVLA and foam sclerotherapy, the OR was > 2, and the treatment effect on ablation rates is highly significant in favour of EVLA in each comparison (p < 0.001). At 6 months, the effect size in favour of EVLA over foam (GSV only) was 4.83 (95% CI 2.76 to 8.48, p < 0.001).
Complications
Procedural complications
The procedural complications noted at the time of the primary treatment are documented in Table 61. In the EVLA versus foam sclerotherapy comparison, the event rate for any procedural complication was significantly lower for EVLA (OR 0.17, 95% CI 0.05 to 0.60; p = 0.006). Similarly, in the surgery versus EVLA comparison, the event rate for any procedural complication was significantly higher for surgery (OR 5.41, 95% CI 1.73 to 16.89; p = 0.004). Five participants in the foam group (3%), five in the surgery group (3%) and none in the EVLA group experienced two procedural complications.
| Procedural complication | Randomised to EVLA | Randomised to foam sclerotherapy | Randomised to surgery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Treated (n) | 205 | 197 | 195 | ||||||
| Any procedural complicationa (N, n, %) | 205 | 2 | 1.0 | 197 | 13 | 6.6 | 195 | 16 | 8.2 |
| Wound haematoma (N, n, %) | 205 | 0 | 0.0 | 197 | 1 | 0.5 | 195 | 1 | 0.5 |
| Damage to major artery (N, n, %) | 205 | 0 | 0.0 | 197 | 0 | 0.0 | 195 | 0 | 0.0 |
| Damage to major vein (N, n, %) | 205 | 0 | 0.0 | 197 | 0 | 0.0 | 195 | 0 | 0.0 |
| Damage to major nerve (N, n, %) | 205 | 0 | 0.0 | 197 | 0 | 0.0 | 195 | 0 | 0.0 |
| Bleeding (N, n, %) | 205 | 0 | 0.0 | 197 | 0 | 0.0 | 195 | 2 | 1.0 |
| Visual disturbance/blurred vision (N, n, %) | 205 | 0 | 0.0 | 197 | 4 | 2.0 | 195 | 0 | 0.0 |
| Extravasation of foam sclerotherapy (N, n, %) | 205 | 0 | 0.0 | 197 | 0 | 0.0 | 195 | 1 | 0.5 |
| Allergic/anaphylactoid reaction (N, n, %) | 205 | 0 | 0.0 | 197 | 0 | 0.0 | 195 | 0 | 0.0 |
| Stroke (N, n, %) | 205 | 0 | 0.0 | 197 | 0 | 0.0 | 195 | 0 | 0.0 |
| Transient ischaemic attack (N, n, %) | 205 | 0 | 0.0 | 197 | 0 | 0.0 | 195 | 0 | 0.0 |
| Myocardial infarction (N, n, %) | 205 | 0 | 0.0 | 197 | 0 | 0.0 | 195 | 0 | 0.0 |
| Intra-arterial injection (N, n, %) | 205 | 0 | 0.0 | 197 | 0 | 0.0 | 195 | 0 | 0.0 |
| Epileptic fit (N, n, %) | 205 | 0 | 0.0 | 197 | 0 | 0.0 | 195 | 0 | 0.0 |
| Headache (N, n, %) | 205 | 0 | 0.0 | 197 | 2 | 1.0 | 195 | 1 | 0.5 |
| Transient confusion (N, n, %) | 205 | 0 | 0.0 | 197 | 0 | 0.0 | 195 | 0 | 0.0 |
| Panic attack (N, n, %) | 205 | 0 | 0.0 | 197 | 1 | 0.5 | 195 | 0 | 0.0 |
| Malaise (N, n, %) | 205 | 0 | 0.0 | 197 | 0 | 0.0 | 195 | 0 | 0.0 |
| Cough (N, n, %) | 205 | 0 | 0.0 | 197 | 0 | 0.0 | 195 | 0 | 0.0 |
| Chest tightness/heaviness (N, n, %) | 205 | 0 | 0.0 | 197 | 1 | 0.5 | 195 | 0 | 0.0 |
| Vasovagal (N, n, %) | 205 | 0 | 0.0 | 197 | 3 | 1.5 | 195 | 1 | 0.5 |
| Anaesthetic side effects (N, n, %) | 205 | 0 | 0.0 | 197 | 0 | 0.0 | 195 | 6 | 3.1 |
| Sickness (N, n, %) | 205 | 0 | 0.0 | 197 | 0 | 0.0 | 195 | 4 | 2.1 |
| Muscle pains (N, n, %) | 205 | 0 | 0.0 | 197 | 0 | 0.0 | 195 | 1 | 0.5 |
| Sore throat (N, n, %) | 205 | 0 | 0.0 | 197 | 0 | 0.0 | 195 | 2 | 1.0 |
| Damage to teeth, lip or tongue (N, n, %) | 205 | 0 | 0.0 | 197 | 0 | 0.0 | 195 | 0 | 0.0 |
| Other procedural complication (N, n, %) | 205 | 2 | 1.0 | 197 | 6 | 3.0 | 195 | 6 | 3.1 |
Later complications
Complications noted at the time of the 6-week and 6-month assessments are shown in Tables 62 and 63. Estimates of the effect of treatment on complications are summarised across both follow-up time points in Table 64, with ORs comparing surgery with EVLA, and EVLA with foam sclerotherapy.
| Complication | Randomised to EVLA | Randomised to foam sclerotherapy | Randomised to surgery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinic assessment at 6 weeks ( N , n , %) | 210 | 193 | 91.9 | 207 | 189 | 91.3 | 213 | 180 | 84.5 |
| Any complication at 6 weeks (N, n, %) | 193 | 103 | 53.4 | 189 | 149 | 78.8 | 180 | 118 | 65.6 |
| Numbness (N, n, %) | 193 | 22 | 11.4 | 189 | 10 | 5.3 | 180 | 30 | 16.7 |
| Persistent bruising (N, n, %) | 193 | 10 | 5.2 | 189 | 36 | 19.0 | 180 | 22 | 12.2 |
| Persistent tenderness/discomfort (N, n, %) | 193 | 41 | 21.2 | 189 | 76 | 40.2 | 180 | 57 | 31.7 |
| Skin loss/ulceration (N, n, %) | 193 | 0 | 0.0 | 189 | 2 | 1.1 | 180 | 1 | 0.6 |
| Lumpiness (N, n, %) | 193 | 36 | 18.7 | 189 | 104 | 55.0 | 180 | 51 | 28.3 |
| Development of thread vein (N, n, %) | 193 | 10 | 5.2 | 189 | 21 | 11.1 | 180 | 16 | 8.9 |
| Skin staining (N, n, %) | 193 | 18 | 9.3 | 189 | 66 | 34.9 | 180 | 11 | 6.1 |
| Wound infection (N, n, %) | 193 | 3 | 1.6 | 189 | 1 | 0.5 | 180 | 17 | 9.4 |
| Backache (N, n, %) | 193 | 4 | 2.1 | 189 | 5 | 2.6 | 180 | 7 | 3.9 |
| Headache (N, n, %) | 193 | 1 | 0.5 | 189 | 9 | 4.8 | 180 | 3 | 1.7 |
| DVT (N, n, %) | 193 | 0 | 0.0 | 189 | 2 | 1.1 | 180 | 0 | 0.0 |
| Pulmonary embolus (N, n, %) | 193 | 0 | 0.0 | 189 | 0 | 0.0 | 180 | 0 | 0.0 |
| Stroke (N, n, %) | 193 | 0 | 0.0 | 189 | 0 | 0.0 | 180 | 0 | 0.0 |
| Myocardial infarction (N, n, %) | 193 | 0 | 0.0 | 189 | 0 | 0.0 | 180 | 0 | 0.0 |
| Loss of vision (N, n, %) | 193 | 0 | 0.0 | 189 | 3 | 1.6 | 180 | 0 | 0.0 |
| Damage to major artery (N, n, %) | 193 | 0 | 0.0 | 189 | 0 | 0.0 | 180 | 0 | 0.0 |
| Damage to major vein (N, n, %) | 193 | 0 | 0.0 | 189 | 1 | 0.5 | 180 | 0 | 0.0 |
| Damage to motor nerve (N, n, %) | 193 | 0 | 0.0 | 189 | 0 | 0.0 | 180 | 0 | 0.0 |
| Other complication (N, n, %) | 193 | 10 | 5.2 | 189 | 14 | 7.4 | 180 | 17 | 9.4 |
| Complication | Randomised to EVLA | Randomised to foam sclerotherapy | Randomised to surgery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Clinic assessment at 6 months ( N , n , %) | 210 | 183 | 87.2 | 207 | 178 | 86.0 | 213 | 167 | 78.4 |
| Any complication at 6 months (N, n, %) | 183 | 89 | 48.6 | 178 | 94 | 52.8 | 167 | 77 | 46.1 |
| Numbness (N, n, %) | 183 | 17 | 9.2 | 178 | 5 | 2.8 | 167 | 28 | 16.8 |
| Persistent bruising (N, n, %) | 183 | 25 | 13.6 | 178 | 26 | 14.6 | 167 | 34 | 20.4 |
| Skin loss/ulceration (N, n, %) | 183 | 1 | 0.6 | 178 | 1 | 0.6 | 167 | 0 | 0.0 |
| Lumpiness (N, n, %) | 183 | 25 | 13.6 | 178 | 46 | 25.8 | 167 | 10 | 6.0 |
| Development of thread vein (N, n, %) | 183 | 24 | 13.2 | 178 | 23 | 13.0 | 167 | 19 | 11.4 |
| Skin staining (N, n, %) | 183 | 32 | 17.4 | 178 | 55 | 30.8 | 167 | 13 | 7.8 |
| DVT (N, n, %) | 183 | 0 | 0.0 | 178 | 1 | 0.6 | 167 | 0 | 0.0 |
| Pulmonary embolus (N, n, %) | 183 | 0 | 0.0 | 178 | 0 | 0.0 | 167 | 0 | 0.0 |
| Other (N, n, %) | 183 | 11 | 6.0 | 178 | 8 | 4.4 | 167 | 10 | 6.0 |
| Complication type | Randomised to EVLA | Randomised to foam sclerotherapy | Randomised to surgery | Surgery vs. EVLA | EVLA vs. foam sclerotherapy | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | n | % | N | n | % | N | n | % | ORa | 95% CI | p-value | ORb | 95% CI | p-value | |
| Procedural complications at treatment | 205 | 2 | 1.0 | 197 | 13 | 6.6 | 195 | 16 | 8.2 | 5.41 | 1.73 to 16.89 | 0.004 | 0.17 | 0.05 to 0.60 | 0.006 |
| Any complication at 6 weeks | 193 | 103 | 53.4 | 189 | 149 | 78.8 | 180 | 118 | 65.6 | 1.75 | 1.13 to 2.70 | 0.012 | 0.27 | 0.17 to 0.43 | < 0.001 |
| Any complication at 6 months | 183 | 89 | 48.6 | 178 | 94 | 52.8 | 167 | 77 | 46.1 | 0.90 | 0.58 to 1.40 | 0.640 | 0.82 | 0.54 to 1.25 | 0.357 |
| Numbness at 6 weeks | 193 | 22 | 11.4 | 189 | 10 | 5.3 | 180 | 30 | 16.7 | 1.70 | 0.91 to 3.17 | 0.096 | 2.50 | 1.12 to 5.60 | 0.025 |
| Numbness at 6 months | 183 | 17 | 9.2 | 178 | 5 | 2.8 | 167 | 28 | 16.8 | 2.11 | 1.05 to 4.24 | 0.037 | 3.85 | 1.35 to 10.99 | 0.012 |
| Persistent bruising at 6 weeks | 193 | 10 | 5.2 | 189 | 36 | 19.0 | 180 | 22 | 12.2 | 2.86 | 1.26 to 6.51 | 0.012 | 0.17 | 0.07 to 0.38 | < 0.001 |
| Persistent bruising at 6 months | 183 | 25 | 13.6 | 178 | 26 | 14.6 | 167 | 34 | 20.4 | 1.71 | 0.93 to 3.12 | 0.083 | 0.90 | 0.48 to 1.67 | 0.733 |
| Persistent tenderness/discomfort at 6 weeks | 193 | 41 | 21.2 | 189 | 76 | 40.2 | 180 | 57 | 31.7 | 1.90 | 1.16 to 3.11 | 0.011 | 0.36 | 0.22 to 0.58 | < 0.001 |
| Skin loss/ulceration at 6 weeks | 193 | 0 | 0.0 | 189 | 2 | 1.1 | 180 | 1 | 0.6 | N/C | N/C | N/C | N/C | N/C | N/C |
| Skin loss/ulceration at 6 months | 183 | 1 | 0.6 | 178 | 1 | 0.6 | 167 | 0 | 0.0 | N/C | N/C | N/C | 1.50 | 0.06 to 40.60 | 0.810 |
| Lumpiness at 6 weeks | 193 | 36 | 18.7 | 189 | 104 | 55.0 | 180 | 51 | 28.3 | 1.84 | 1.11 to 3.05 | 0.018 | 0.15 | 0.09 to 0.25 | < 0.001 |
| Lumpiness at 6 months | 183 | 25 | 13.6 | 178 | 46 | 25.8 | 167 | 10 | 6.0 | 0.43 | 0.19 to 0.97 | 0.041 | 0.38 | 0.21 to 0.68 | 0.001 |
| Development of thread vein at 6 weeks | 193 | 10 | 5.2 | 189 | 21 | 11.1 | 180 | 16 | 8.9 | 1.82 | 0.79 to 4.20 | 0.159 | 0.43 | 0.19 to 0.95 | 0.037 |
| Development of thread vein at 6 months | 183 | 24 | 13.2 | 178 | 23 | 13.0 | 167 | 19 | 11.4 | 0.80 | 0.41 to 1.57 | 0.520 | 1.03 | 0.55 to 1.96 | 0.917 |
| Skin staining at 6 weeks | 193 | 18 | 9.3 | 189 | 66 | 34.9 | 180 | 11 | 6.1 | 0.55 | 0.24 to 1.27 | 0.163 | 0.17 | 0.09 to 0.30 | < 0.001 |
| Skin staining at 6 months | 183 | 32 | 17.4 | 178 | 55 | 30.8 | 167 | 13 | 7.8 | 0.38 | 0.19 to 0.78 | 0.009 | 0.41 | 0.24 to 0.70 | 0.001 |
| Backache at 6 weeks | 193 | 3 | 1.6 | 189 | 1 | 0.5 | 180 | 17 | 9.4 | 7.56 | 2.00 to 28.58 | 0.003 | 3.26 | 0.33 to 32.68 | 0.314 |
| Headache at 6 weeks | 193 | 4 | 2.1 | 189 | 5 | 2.6 | 180 | 7 | 3.9 | 1.61 | 0.43 to 6.04 | 0.477 | 0.71 | 0.18 to 2.83 | 0.623 |
Comparison of endovenous laser ablation with foam
The event rates at 6 weeks for persistent bruising (OR 0.17, 95% CI 0.07 to 0.38; p < 0.001), persistent tenderness (OR 0.36, 95% CI 0.22 to 0.58; p < 0.001), lumpiness (OR 0.38, 95% CI 0.21 to 0.68; p < 0.001), skin staining (OR 0.41, 95% CI 0.24 to 0.70; p < 0.001), development of thread vein (OR 0.43, 95% CI 0.19 to 0.95; p = 0.037) and headache (OR 0.10, 95% CI 0.01 to 0.79; p = 0.029) were all significantly lower for EVLA than for foam. At 6 months, lumpiness (OR 0.38, 95% CI 0.21 to 0.68; p = 0.001) and skin staining (OR 0.41, 95% CI 0.24 to 0.70; p = 0.001) remained less frequent for EVLA than for foam. The event rate for cutaneous numbness (at both time points) is significantly higher for EVLA than for foam sclerotherapy (at 6 months, OR 3.85, 95% CI 1.35 to 10.99; p = 0.012).
Comparison of surgery with endovenous laser ablation
The rates for persistent bruising (OR 2.86, 95% CI 1.26 to 6.51; p = 0.012), persistent tenderness (OR 1.90, 95% CI 1.16 to 3.11; p = 0.011) and lumpiness (OR 1.84, 95% CI 1.11 to 3.05; p = 0.018) at 6 weeks were all significantly higher for surgery than for EVLA. At 6 months, cutaneous numbness (OR 2.11, 95% CI 1.05 to 4.24; p = 0.037), lumpiness (OR 0.43, 95% CI 0.19 to 0.97; p = 0.041) and skin staining (OR 0.38, 95% CI 0.19 to 0.78; p = 0.009) occurred more frequently for surgery than for EVLA.
Serious adverse events
Table 65 gives brief details of the seven SAEs reported in participants randomised to EVLA. One of these (pain in the contralateral leg treated at the same time as the study leg) was assessed as related to treatment. A further SAE (fall resulting in rib fracture) was assessed as possibly related to treatment because the participant was still wearing compression stockings at the time of the fall and was thought to be slightly incapacitated because of these. The other five SAEs were not related to treatment. Details of the SAEs in those randomised to surgery and foam sclerotherapy are given in Chapter 5 (see Table 39).
| Randomised | Treatment prior to SAE | Description of event | Related to treatment? | Expected? |
|---|---|---|---|---|
| EVLA | EVLA | Pain in contralateral leg (treated at same time as study leg); possibly related to osteoporosis | Yes | No |
| EVLA | EVLA | Fall; rib fracture | Possible | No |
| EVLA | EVLA | Surgery for fractured shoulder | No | No |
| EVLA | EVLA | Pain in thigh and groin; cellulitis | No | No |
| EVLA | EVLA | Injury at work – trauma to hand | No | No |
| EVLA | EVLA | Episode of palpitation | No | No |
| EVLA | No treatment | Migraine | No | No |
Process evaluation: Illness Perception Questionnaire – Revised
Detailed descriptive results of the IPQ-R are given in Appendix 2 (see Table 109). Within each randomised group, the mean identity scores and the percentage of symptoms correctly identified as being related to varicose veins at baseline (recruitment) and after the participant had been informed of his or her randomisation were similar. By 6 months, both measures of illness identity had fallen.
For all other measures of illness perception, there is very little difference between scores at baseline and post randomisation. For most of the domains, there were decreases in the mean scores between baseline and 6 months in all groups. The exceptions to this were for personal control and illness coherence, where the mean scores increased slightly in all groups. For all of these domains, the differences between surgery and EVLA, and EVLA and foam sclerotherapy were marginal.
Chapter 7 Clinical effectiveness
Primary outcome: Aberdeen Varicose Vein Questionnaire score
Main findings
This is the first RCT involving foam sclerotherapy to evaluate and report disease-specific QoL as a primary outcome measure. In all groups, disease-specific AVVQ scores improved over time (i.e. scores reduced). The health gain obtained in the AVVQ was lower in patients undergoing foam sclerotherapy than in those receiving surgery at 6 weeks (p = 0.006) and at 6 months (p = 0.0002). This equated to a difference in scores of 2.26 between the groups at 6 weeks and 1.74 at 6 months, which is likely to be of clinical significance. The health gains in the AVVQ in patients undergoing foam sclerotherapy and EVLA were similar, as were those in the EVLA and surgery comparison.
Comparison with published randomised controlled trials/patient-reported outcome measures
It is of note that the baseline AVVQ scores in the CLASS study (17.91) are similar to those observed in the PROMs for the NHS in England (18.53 in 2010). At 6 weeks there was a more than 5-point fall (improvement) in the AVVQ score compared with baseline in all treatment groups. In the surgery arm, by 6 months the AVVQ score more than halved compared with baseline (a fall of 10.4 points). Similarly, scores in the foam sclerotherapy and EVLA groups almost halved (falls of 8.5 and 9.9 points respectively). The reduction in AVVQ score (measured at least 3 months post treatment) observed in PROMs data for NHS England (7.9 points)44 sits within the values observed in CLASS at 6 weeks and 6 months. Thus, the CLASS results appear generalisable to that obtained in the NHS in England.
The 2007 study by Rasmussen et al. 36 reported similar baseline scores to those obtained in the CLASS study but had slightly greater health gains (a fall of 10.8 in the surgery group and 11.5 in the EVLA group at 6 months post treatment). Christenson et al. 34 reported higher baseline scores than both Rasmussen et al. (2007)36 and ourselves, and, by 12 months, a slightly greater improvement in patients undergoing EVLA or surgery.
We have shown, as have previous RCTs, that all three treatment modalities are associated with a health gain (reduction) in the AVVQ score. 15,29,32,34,36,37,50 Four of these studies assessed the AVVQ at 4–6 weeks. 29,36,37,50 In contrast to our findings, the previous study which compared foam with surgery and EVLA with foam assessed AVVQ at similar time points to CLASS and found no differences between AVVQ scores. 29 In common with CLASS, the studies that have assessed AVVQ at later time points have also found no difference between EVLA and surgery. 15,29,34,37,50 It is worth noting that the AVVQ was not the primary outcome measure in any of these previous studies; thus, they are unlikely to have been adequately powered to detect a difference.
With the exception of one site, we did not perform simultaneous phlebectomies in patients undergoing EVLA. This site (Hull) had previously performed a RCT which showed significant improvements in disease-specific QoL (assessed by AVVQ) in patients undergoing simultaneous compared with delayed phlebectomies following EVLA at 6 weeks and 3 months. 73
European Quality of Life-5 Dimensions
Main findings
In all treatment groups, a health gain (increase in EQ-5D score from baseline) was observed at the 6-weeks and 6-months time points. Most of the health gain was achieved between baseline and 6 weeks. There was a health gain at 6 weeks in patients randomised to EVLA compared with those randomised to foam for the EQ-5D (p = 0.004). There were no differences at 6 months. There were no differences in surgery versus EVLA or surgery versus foam sclerotherapy. At 6 months, there were no significant differences in the comparisons of foam sclerotherapy with surgery, EVLA with surgery or EVLA with foam sclerotherapy.
European Quality of Life-5 Dimensions visual analogue scale
At 6 weeks, the EQ-5D VAS score increased in patients randomised to surgery or EVLA. There was no change in those randomised to foam sclerotherapy. However, by 6 months there was a rise in all treatment groups (rise of 2.0–4.5). There were no statistical differences between groups at 6 weeks or 6 months.
Comparison with published randomised controlled trials/patient-reported outcome measures
The CLASS baseline EQ-5D scores (0.79) were similar to the baseline scores in the PROMs (0.77) for NHS England. 44 The health gain observed in CLASS (0.10–0.11) was similar to that seen in the PROMs (0.091). The EQ-5D VAS baseline score observed in CLASS (80.0–80.6) was similar to that observed in the PROMs (79.25). However, unlike the improvement seen in our study, in the PROMs the EQ-5D VAS showed no real change when assessed at least 3 months post treatment (fall of –0.098).
Similar to our findings, previous studies have shown an increase in EQ-5D scores at time points from 6 weeks to 2 years. In the study by Shadid,33 the mean change in the EQ-5D score from baseline to 2 years (a gain of 0.064 for foam sclerotherapy and 0.061 for surgery) was slightly lower than that obtained in the CLASS study at 6 months (a gain of approximately 0.1). In the study by Carradice et al. ,73 by 6 weeks, mean scores for both surgery and EVLA groups had increased to 1.0 (substantially higher than the mean scores observed in CLASS). The study by Samuel et al. ,37 which involved only patients with SSV involvement, also showed an increase in EQ-5D scores (of approximately 0.12 at 12 months for both surgery and EVLA groups), with the mean scores for surgery reaching 1.0 by 6 weeks. Unlike previous studies, CLASS showed an early improvement in the EQ-5D in patients receiving EVLA compared with foam and surgery compared with foam.
Short Form questionnaire-36 items
Main findings
The SF-36 physical component score was higher in all three treatment groups at 6 weeks than at baseline. Further increases in scores were observed at the 6-months time point.
There were no statistical differences between foam and surgery for the overall physical and mental component scores or domains of the SF-36. However, the general health domain showed a significant improvement for surgery compared with foam at 6 weeks (p < 0.005).
Patients randomised to EVLA had similar health gains to those who underwent foam sclerotherapy in the overall SF-36 physical component and individual physical domains at 6 weeks and 6 months. This difference was not apparent at 6-months follow-up. Similar improvements in the overall SF-36 mental component score and domains were shown for both EVLA and foam sclerotherapy.
At 6 weeks, patients randomised to EVLA had a greater health gain than those who underwent surgery in the SF-36 individual domains of vitality, social functioning and role emotional (p < 0.005). The overall physical scores were similar but the role physical and bodily pain domains were significantly improved for EVLA compared with surgery at 6 weeks (p < 0.005).
Comparison with published randomised controlled trials/patient-reported outcome measures
Short Form questionnaire-36 items scores were not collected in PROMs. One previous RCT involving a comparison of EVLA and surgery used the SF-36 as a primary outcome measure. 50 This study, by Carradice et al. , showed an initial reduction in some component scores at 1 week, but by 6 weeks significant improvements were detected in five of the eight domains (physical function, role physical, bodily pain, general health, vitality). Although the increases are in tune with those seen in CLASS, Carradice et al. 50 found no differences between groups. In contrast, in the CLASS trial there were significantly greater improvements for EVLA than for surgery in six of the domains (p < 0.05) at 6 weeks, with the improvement in the mental health domain remaining significant at 6 weeks. Four further RCTs which compared EVLA with surgery have also assessed generic QoL using the SF-36. 29,34,36,37 It is of note that all these studies assessed outcome early (1 month or 6 weeks), as was done in the CLASS study. 29,36,37,50 All reported improvements in some or all domains following treatment, but, unlike the CLASS trial, which showed a greater gain for EVLA than for surgery, no differences were detected. 29,34,36,37 One of these studies also included a comparison of EVLA and foam sclerotherapy, and found no difference between the treatment arms. 29 In contrast, in the CLASS trial, early benefits in favour of EVLA over foam sclerotherapy were found in two of the domains (p < 0.05), with a significant improvement in the mental health domain for EVLA at 6 months. The CLASS trial also showed significant benefits of surgery over foam sclerotherapy in two domains.
Venous Clinical Severity Score
Main findings
Up to 6 months, the VCSS improved in all three treatment groups. At 6 weeks, the improvement in the VCSS (reduction in score) was significantly greater in patients undergoing surgery than in those receiving foam sclerotherapy (0.52-point difference in scores, p = 0.002). A further, smaller reduction was observed in all groups at the 6-months follow-up, but at this stage there were no statistical differences between groups.
Comparison with published randomised controlled trials
The mean baseline score in CLASS (5.0) was within the range of baseline VCSS scores reported in previous RCTs (2.4–7). 15,29,32,34,36,37,50 All studies noted a reduction in VCSS at 3 months or longer.
In the CLASS study, the VCSS at 6 months was slightly higher (1.6) in the foam sclerotherapy group (i.e. that group had the most residual vein-related symptoms) than in the EVLA and surgery groups (1.4 and 1.3 respectively), but the differences were not statistically significant. The magnitude of the fall (3.1–3.7) was similar to those reported in some RCTs15,32 but greater than that observed by Rasmussen’s group,36,49 which reported lower baseline scores (2.4–2.8 and 5 respectively) than in CLASS.
The 6-months score was slightly higher than those of some studies (which had scores of 0–1), but many of these studies had lower baseline scores at the outset. 15,29,34,36,50 One previous single-site RCT73 found that the improvement in VCSS was significantly better at both 6 weeks and 3 months in patients who had EVLA and simultaneous phlebectomies than in those who underwent delayed phlebectomies. Although performing EVLA and simultaneous phlebectomies in the CLASS trial might therefore have further improved VCSS at 6 weeks, patients were given the option of foam sclerotherapy at 6 weeks, and thus it is unlikely that performing simultaneous phlebectomies would have influenced the 6-months VCSS score.
Residual varicose veins: visual analogue scale
Main findings
The VAS scores reported by the nurses were consistently lower (representing fewer varicose veins), at all time points and for all three treatment modalities, than the VAS scores reported by the patients. At 6 weeks, the patient-reported VAS scores were lower (fewer varicose veins) in the surgery group than in the foam sclerotherapy group (0.99-point difference, p < 0.001). Similarly, at 6 months the patient-reported VAS scores were lower (fewer varicose veins) in the surgery group than in the foam sclerotherapy group (0.95-point difference, p < 0.001). There were no differences between the EVLA and foam sclerotherapy groups at 6 weeks, but at 6 months the score was lower (fewer varicose veins) in the EVLA group (0.54-point difference, p = 0.005).
A similar pattern was seen for the nurse-reported VAS scores, with the exception that no differences were noted between the EVLA and foam sclerotherapy groups at 6-weeks and 6-months follow-up. The consistently lower scores recorded by the nurses compared with the patients may be due to the nurses’ prior exposure to patients with more complex and extensive varicose veins, which could have given them a higher ‘threshold’ in their judgements about the visual appearance of leg veins.
The presence of an increased number of residual varicosities in the patients undergoing foam at 6-weeks follow-up is explained by the fact that patients had not completed their treatment for calf varicosities by this stage (with the exception of patients at one centre which performed concomitant phlebectomies with EVLA).
Comparison with published randomised controlled trials
The presence of residual varicose veins was not reported in any of the previous RCTs. However, patient-reported cosmesis was recorded in the study by Darwood15 and showed no differences between patients undergoing EVLA and those receiving surgery. The findings in CLASS are an interesting and potentially important observation, because they suggest that surgeons involved in those trials did not see residual veins as an issue. By contrast, the expectation of other surgeons (and patients) is that treatment will get rid of all varicose veins in the treated leg. These contrasting aims and expectations are fundamental in judging the ‘success’ of any treatment for varicose veins and they are important to consider in interpreting the conclusions of any study.
Visual analogue scale score and further treatment of residual varicosities with foam sclerotherapy
The patient-reported VAS scores at 6 weeks for the foam sclerotherapy, EVLA and surgery groups were 2.6, 2.2 and 1.7 respectively. The decision to proceed with further treatment of residual varicosities at the 6-weeks stage in the foam sclerotherapy and EVLA groups was patient led. In the CLASS study, excluding the patients who underwent concurrent phlebectomies in Hull, 31% of the EVLA patients underwent foam sclerotherapy to their residual varicosities, including 2% who underwent a second foam sclerotherapy treatment. In the foam sclerotherapy group, 31% had treatment to their calf varicosities at their primary treatment session and a further 7% received further foam sclerotherapy to their residual varicosities. The presence of complications relating to treatment at the 6-weeks time point (see Complications) may have influenced the patients’ decision regarding whether or not they should proceed with further foam sclerotherapy treatment to residual varicosities.
Comparison with published randomised controlled trials
In the six studies14,29–33 which involved foam sclerotherapy, only three14,30,33 administered delayed foam sclerotherapy for residual tributaries (see Chapter 2). Similar to our study, in the Varisolve® study14 8% of patients had a further treatment session. In contrast, in the study by Figueiredo,30 all but 3 of the 27 patients underwent one or more further treatment session. In the remaining study33 which offered delayed phlebectomies or foam sclerotherapy, the number of patients who underwent further treatment is not stated.
In studies involving EVLA, only two used foam sclerotherapy to treat residual tributaries at a later stage. 15,40 In the Darwood study,15 this was performed in 36% of patients, which is a similar rate to the CLASS trial. Details regarding the number of patients who had delayed foam sclerotherapy in the other study are not published. 40 The remainder of the studies treated the calf varicosities at the same time as the main truncal veins. Given that few patients randomised to EVLA underwent delayed treatment of residual varicosities in the CLASS trial, a policy of concomitant treatment of calf varicosities and the main truncal vein may result in a considerable number of patients receiving unnecessary treatment to calf varicosities.
Duplex-detected ablation of the main truncal vein (great saphenous vein/small saphenous vein)
The joint statement from the Venous Forum and the Society of Interventional Radiology62 recommended reporting standards for endovenous ablation in the treatment of venous insufficiency. Anatomical success was defined as successful ablation of the entire treated segment of the target vein (absent flow or disappearance of the vein on duplex ultrasound). This guidance was used in the CLASS study; the duplex findings were reported by independent technicians at set anatomical locations.
In accordance with the above statement, we defined complete anatomical success for the GSV as complete occlusion at the groin (within 3 cm of the common femoral vein), complete occlusion at mid-thigh and either an occluded or a patent but non-refluxing GSV above the knee. It is of note that 90% of patients in CLASS who underwent GSV treatment had reflux above the knee only at baseline. The justification for not including the recorded ‘within 1 cm of the common femoral vein’ site in our definition was that, with the exception of one centre, this section was not treated. For foam sclerotherapy, our practice was to apply manual compression at the junction to reduce passage of foam into the common femoral vein.
Main findings
We have presented the anatomical success rates achieved for the truncal veins of the patient’s study leg and for those patients undergoing treatment to the GSV alone. Higher rates of ablation success were obtained for patients undergoing treatment of the GSV alone than for those undergoing treatment of the SSV alone, or the GSV and SSV combined. There were no statistically significant differences in anatomical success between surgery and laser at either the 6-weeks or the 6-months time point, but both were superior to foam at these time points (p < 0.001).
The anatomical success achieved with foam sclerotherapy did not improve between the 6-weeks and 6-months follow-ups. The protocol allowed for further foam sclerotherapy to be given at the surgeon’s discretion to any patent truncal vein at or after the 6-weeks follow-up, but in practice this was only performed in 12 participants randomised to foam sclerotherapy. There may be a number of reasons why further foam sclerotherapy to the truncal vein was not administered. For instance, it is often difficult to treat segmental isolated sections of the GSV, and the need to treat non-refluxing patent sections of the GSV is also questionable. It should be noted that of those patients who were considered to have had a partial anatomical success following foam sclerotherapy, only 4% had GSV reflux present at 6 weeks.
The definition of anatomical success for the SSV disadvantaged surgery, as the vein was not stripped in the majority of centres in the CLASS study (only 15 patients had stripping of the SSV performed). The number of patients undergoing SSV treatment was low (n = 56), and therefore we have not performed a statistical analysis of anatomical success within this group. The proportion of patients who underwent combined GSV and SSV treatment was also very low and, similarly, was not subjected to formal statistical analysis. Overall, the results for the SSV appear inferior to those obtained for the GSV, but the ablation success rates at 6 months appeared to be higher for EVLA than for either surgery or foam sclerotherapy.
In CLASS, the impact of anatomical success on symptoms, residual varicose veins and QoL following treatment is unclear. Despite the significant reduction in anatomical success following foam sclerotherapy, the VCSS, presence of residual varicose veins (nurse assessed) and generic QoL were similar to those achieved following EVLA and surgery. Thus, although anatomical success rates may be important in terms of future clinical recurrences, they have little effect (if any) on symptoms or QoL.
Comparison with published randomised controlled trials
The anatomical success rates for the GSV observed at 6 months in CLASS were lower than those reported in most previous RCTs for all three treatment modalities. This difference is most notable for the complete anatomical success achieved in CLASS for foam sclerotherapy (51% in the three-arm centres included in stratum A; 43% in the two-arm centres in stratum B), when compared against success rates of between 72% and 94% in previous studies where success was assessed at between 3 and 12 months. 14,29,30,33 In the study by Lattimer et al. ,32 success at 3 months was reported for above and below the knee separately (69% and 44% respectively). The lower anatomical success rates for foam sclerotherapy occurred despite the majority of procedures being performed by consultant surgeons in CLASS, so inexperience is unlikely to be a factor. The reasons for the lower anatomical success rate achieved in stratum B compared with stratum A are likely to be multifactorial and reflect NHS practice across the UK. For surgery and EVLA, the difference between CLASS and previous studies is less marked.
At 6 months, 82% of those undergoing EVLA in CLASS had complete anatomical success; this is slightly lower than the range reported in most previous RCTs (84–99%). 15,29,34–36 Again, in the study by Lattimer,32 lower success rates were observed (74% above knee and 15% below knee).
Although lower than for many previous RCTs, the anatomical success rate of 78% at 6 months following surgery does lie within the range of success rates reported in previous RCTs (72–100%). 14,15,29,30,33–37
There are a number of possible reasons for the lower anatomical success rates observed in CLASS. Firstly, in the CLASS study anatomical success in all but one centre was reported by independent, accredited vascular technicians. This was not the case in previous studies, which could have resulted in a bias towards reporting favourable outcomes in those studies. Secondly, the definitions of anatomical success (and/or failure) vary considerably between studies and many were less stringent in terms of complying with the joint statement of the Venous Forum, particularly for studies involving foam. 62 With the exception of one previous RCT,32 outcomes were reported as success or failure, yet the definitions of failure varied considerably (Table 66).
| Study | Anatomical outcome |
|---|---|
| Rasmussen 201129 | Success: closed or absent GSV with lack of flow Failure: open part of the treated GSV above the knee of > 10 cm in length |
| Rasmussen 200736 | Success: closed or absent GSV or closed GSV with lack of flow Failure: open part of the treated vein of > 5 cm in length |
| Carradice 200935 | Success: absent GSV in thigh; closed or absent GSV with absent flow in treated segment of thigh |
| Darwood 200815 | Success: abolition of reflux in the treated GSV segment |
| Christenson 201034 | Success: absent GSV, closed or absent GSV with no flow including absent junctional reflux |
| Wright 200714 | Success: closed or absent GSV including absent junctional reflux |
| Samuel 201337 | Success: abolition of SSV reflux |
| Lattimer 201332 | Different criteria for success, including:
|
| Rass 201138 | Success: closed or absent GSV |
| Figueiredo 200930 | Foam sclerotherapy: (1) total occlusion, (2) partial recanalisation without reflux, (3) partial recanalisation with reflux, (4) total recanalisation [success is defined as (1) and (2)] Surgery: failure defined as presence of reflux or residual varicose veins |
| Biemans 201331 | EVLA and foam sclerotherapy: success defined as complete obliteration, without flow or reflux, of the GSV at the level of the mid-thigh Surgery: success defined as absence of GSV in saphenous compartment at mid-thigh level |
This variation means that the duplex findings said to represent success in one study (for example the Rasmussen study29) might be considered only a partial success (or even a failure) in the CLASS study. Indeed, if the complete and partial non-refluxing success rates in the CLASS trial were combined, these would give ablation rates comparable with those of the Rasmussen paper for EVLA (91.4%). However, rates still remain lower for surgery (82%) and foam sclerotherapy (67%). Nevertheless, the results achieved with foam are comparable with the 69% rate obtained in the study by Latimer et al. 32 and the 68% rate obtained by surgeons in the study by Wright et al. 14 Thirdly, it is of note that most previous RCTs were conducted in single centres by enthusiasts in the field. This raises the possibility that these surgeons achieved better outcomes than the generality of vascular surgeons and their trainees and/or that a bias resulted towards reporting favourable outcomes. In CLASS, consultants performed 59%, 73% and 77% of surgical procedures, EVLA and foam sclerotherapy respectively.
The findings of CLASS were in accord with those in the Rasmussen29 and Biemans31 studies which showed significantly better technical success rates for surgery than for foam sclerotherapy. Only one study found an advantage of foam sclerotherapy over surgery. 30
In two29,31 of the three RCTs29,31,32 which compared foam sclerotherapy with EVLA, technical success was significantly higher in patients randomised to EVLA, which is similar to our findings in CLASS.
Of the 10 studies that compared EVLA and surgery, the majority (six) reported no significant difference in technical success rates and were thus similar to the CLASS study. 29,31,34,36,39,40 In contrast, three studies reported a significantly higher technical success rate for EVLA15,35,37 and one for surgery. 38
Complications
Serious adverse events
Ten SAEs were reported in patients randomised to foam sclerotherapy, but only three of these were related to the treatment. All three were non-occlusive DVT. There were seven SAEs in patients randomised to EVLA, of which one was related to the procedure (prolonged discomfort in non-study leg treated simultaneously). In patients randomised to surgery, 4 of 10 reported SAEs were secondary to the operation (two infections, one haematoma and one peroneal nerve injury).
Procedural complications
These were more common in patients randomised to surgery and foam sclerotherapy than in those undergoing EVLA (surgery 7.1–8.2%, foam 6.2–6.6%, EVLA 1%; p < 0.001). Patients appeared to report less pain immediately following the treatment with foam sclerotherapy than after EVLA or surgery. Patients’ later recollection of pain experienced at the time of treatment and during recovery was also lower for foam sclerotherapy.
The majority of the complications in the surgery group were anaesthetic related. Following foam sclerotherapy, visual disturbance was experienced by 1.5% of patients, but there were no reports of more serious complications such as stroke, despite historical concerns about the possible effect of foam entering the systemic circulation.
Later complications
At 6 weeks, complications were increased in patients randomised to foam compared with EVLA or surgery (p < 0.05) and in patients undergoing surgery compared with EVLA. At 6 weeks, skin staining, bruising, persistent tenderness/discomfort, development of thread veins and lumpiness were statistically more common in the patients randomised to foam sclerotherapy than in those who had surgery or EVLA. Cutaneous numbness was statistically more common in patients randomised to surgery (17.6%) or EVLA (12%) than in those undergoing foam sclerotherapy (4.4%).
At 6 months, the total complication rate was higher for foam than for surgery (p < 0.05). There were no differences at this time point between EVLA and foam sclerotherapy. At 6 months, the proportion of patients who had skin staining had doubled in the EVLA group, presumably because of subsequent foam sclerotherapy for residual varicosities. Lumpiness was more common in the EVLA and foam sclerotherapy groups than in those undergoing surgery. Persistent bruising and cutaneous numbness were more common in those patients randomised to surgery.
Persistent lumpiness may have accounted for the increase in patient- and nurse-reported residual veins (VAS) at 6 months, because any lumps on the legs can mimic varicose veins. The presence of lumpiness and skin staining after foam sclerotherapy may have made some patients less willing to undergo further treatment sessions. Lumpiness, skin staining and tenderness are so common after foam sclerotherapy that it would be reasonable to consider them as expected sequelae rather than ‘complications’. It is certainly important that patients be warned that they will occur and that they may take a long time to resolve.
Comparison with published randomised controlled trials
The type and frequency of complications observed in CLASS were similar to those documented in previous RCTs. It is worthy of note that no DVTs were identified in the patients undergoing EVLA or surgery, but three occurred in patients undergoing foam sclerotherapy. The role of DVT prophylaxis with low-molecular-weight heparin in conjunction with foam sclerotherapy has not been established and only 5% of patients received this in CLASS. This is similar to the proportion who received it in the EVLA group (7%) but much lower than that in the surgery group (48%).
Illness perceptions
No previous RCTs have assessed illness perception. We assessed illness perceptions at two time points before treatment (at recruitment, and after the participant was informed of his or her randomisation) and at 6 months. The reason for assessing illness perceptions at two time points before treatment was to assess whether or not the illness perceptions of participants in any of the trial arms changed after they were notified of the treatment to which they had been randomised. If there had been differences in illness perceptions between either the trial arms or the two time points, this may have influenced recovery behaviours or participants’ self-reporting of QoL post treatment. In particular, if there had been differences between randomised groups, this may have introduced bias into the study. However, illness perceptions between baseline and after the participants were informed of their randomisation were very similar, as were illness perceptions between randomised groups at both time points. This suggests that revealing the randomised treatment allocation to participants (as was necessary in a trial where blinding of participants was not possible) did not introduce bias in terms of their reported illness perceptions. This increases our confidence in the QoL outcomes at 6 weeks and 6 months.
Illness identity was reduced by 6 months in all groups, and this probably reflects a reduced number of symptoms experienced by the participants after treatment. In those patients randomised to EVLA or foam, the percentage of symptoms correctly identified as being related to varicose veins increased; in part this is again likely to reflect a reduced number of symptoms, but may also reflect increased understanding of those symptoms likely to be related to varicose veins.
For all three treatments, the timeline domain relating to acute or chronic condition decreased. This means that participants’ views that varicose veins last a long time had changed, and that they believed the timeline was shorter. Scores for the treatment control domain also reduced by 6 months. Both of these observations suggest that participants recognised some effectiveness of their treatment.
The consequences domain had reduced by 6 months in all groups, indicating that participants felt that the consequences of having varicose veins were less severe than they had previously reported. Emotional representations also reduced by 6 months in all groups. The findings across these domains are likely to reflect the effect of treatment on the varicose veins. As previously shown, treatment improves QoL and clinical outcomes; this analysis shows that there is also a benefit of treatment to patients at an emotional level.
Chapter 8 Behavioural recovery after treatment for varicose veins
As described in Chapter 3, one of the secondary outcomes in CLASS was ‘behavioural recovery’, or return to normal activities, which is regarded as an aspect of clinical success. One of the studies published prior to CLASS being funded showed that return to normal activities was considerably shorter following foam sclerotherapy than after surgery. 14 However, in that study it is not clear if ‘normal activities’ were defined or described for participants. We hypothesised that after any particular treatment there may be earlier return to some activities but later return to others, when compared against other treatments. With this in mind, we searched for suitable instruments to assess different types of normal activity, but found none. We therefore developed an instrument, BRAVVO, to assess distinct aspects of normal activities. In this chapter, we describe the theoretical underpinning of the development of the instrument and the development process. We also present the trial results generated by the BRAVVO instrument.
Theoretical background
The development and content validation of the BRAVVO questionnaire was informed by the World Health Organization (WHO) International Classification of Disability and Function (ICF) model. 74 The ICF model proposes that ‘impairment’ (defined as problems in body function or structure) is only one component of health outcome, the aspect that is based on a medical model of health or disease. The other two components of the ICF model are ‘activity’ (tasks or actions that an individual is capable of doing in an idealised situation) and ‘participation’ (what the individual actually does in an everyday, ‘real world’ situation). Variation in activity and participation is not fully explained by impairment, and so these constructs are important additional indicators of health outcome. It has been proposed that activity and participation can be defined in behavioural terms;75 thus, the assessment of activity and participation is potentially a useful indicator of health outcome following treatment for varicose veins, over and above the AVVQ (which is primarily a measure of impairment). The BRAVVO questionnaire was therefore developed as an instrument to assess the activity and participation components of the ICF model following treatment for varicose veins. Being able to return to these activity or participation behaviours following treatment suggests recovery in terms of these behaviours, and we have termed this ‘behavioural recovery’.
Development of an instrument to assess return to normal activities (behavioural recovery)
Methods
We developed a questionnaire to assess behavioural recovery. An interview study was carried out to identify normal activities and ‘milestone’ behaviours to incorporate into the questionnaire.
Eligibility criteria
Patients who had recently undergone treatment for their varicose veins at Aberdeen Royal Infirmary (ARI) were eligible to participate in the interview study if they
-
had undergone recent treatment for their varicose veins (surgery, EVLA or foam sclerotherapy within the previous 6–12 weeks)
-
were 18 years of age or older
-
could speak English and were able to participate in the interview
-
consented to participate.
Recruitment
Potential participants were identified from treatment lists at ARI and purposively sampled to provide a balance of those who received each of the three forms of treatment (surgery, EVLA or foam sclerotherapy). In addition to sampling from the three treatment options, diversity sampling was used in an attempt to gain a mix with regard to sex, age and rural–urban location.
Potential participants were invited, by letter sent from a vascular surgeon based at ARI, to take part in the interview study. They were each provided with a study information leaflet, invited to make contact with the research team for clarification of queries or further information, and asked to return a reply-paid slip or make contact by telephone if they wished to participate in the study. Study paperwork is included in Appendix 1.
Patients who wished to participate in the study were then contacted by telephone to arrange an interview. Those who wished to participate but were unable to travel to the hospital campus were asked if they would be prepared to participate in an interview in their home. Participants were compensated for costs incurred in taking part (e.g. travel, parking, child care).
Interview schedule
A topic guide (see Appendix 3) was prepared to assist the interviewer in eliciting behavioural milestones that patients regarded as significant. For the first 11 interviews, milestones were explained as ‘Things that you looked forward to doing for the first time, were worried about doing for the first time, or felt pleased that you had achieved when you did them for the first time, after your treatment for varicose veins.’ Based on the reports of several participants that there was nothing in particular that they looked forward to, this was altered to ‘What couldn’t you do straight after your treatment?’
Interviews
At the interview, participants were asked to sign a consent form indicating their agreement to participate in the study and to be audio-taped. An experienced interviewer (DB) used the topic guide to ask open questions (followed by appropriate prompts as required) to identify the actions that patients regarded as ‘milestones’ during their recovery. Interviews were audio-taped and transcribed verbatim. The interviewer then anonymised the transcripts.
Analysis of interview data
Interview transcripts (n = 17) were content analysed in four stages in order to identify appropriate items to include in a questionnaire.
First, one researcher (DB) identified, by highlighting, each utterance (or unit of text) that referred to a behaviour (‘behavioural description’). To validate this first step, a second researcher (JF) read two transcripts to identify omissions in highlighting. No omissions were identified.
Second, each unit of highlighted text was pasted into a coding table and a ‘label’ (unique descriptor) was generated to describe each of the behaviours. Two researchers (DB, JF) independently coded five transcripts, discussing disagreements until consensus about these labels was reached. The 12 other transcripts were coded by a single researcher (DB) using this set of labels as a guide, and additional labels proposed when required.
In the third step of the analysis, using data from all the interviews, two researchers (DB, SC) independently allocated the units of text identified in the first step to one of the labels identified in the second step. A frequency table (representing the number of times a particular behaviour had been mentioned across all the interviews) was generated. Intercoder agreement was assessed and any additional labels identified by either researcher were included in the frequency table. The relevance or importance of each behaviour was assumed to be reflected by the frequency data. 76
Finally, in the fourth stage of the content analysis, three researchers (DB, JF, SC) discussed the frequency table and identified (i) the most frequently mentioned behaviours (that merited a questionnaire item) and (ii) less frequently mentioned behaviours that could be grouped together to generate a questionnaire item which would encapsulate these behaviours. The behaviours identified using these methods were used to generate items for the BRAVVO questionnaire.
International Classification of Disability and Function classification of behaviours included in the Behavioural Recovery After treatment for Varicose Veins questionnaire
Distinguishing between impairment and the other constructs (activity and participation) within the ICF model is relatively straightforward. However, the distinction between activity and participation is contextual and somewhat subjective. For example, the behaviour of ‘walking for 5 minutes’ should be coded as activity, as it is the execution of a task or action by an individual that could be performed in an idealised situation (such as on a treadmill during a health-care assessment). However, the behaviour of ‘walking to work for 5 minutes’ could be coded as participation as it relates to involvement in a ‘real world’ situation.
In order to identify the ICF constructs measured by the BRAVVO questionnaire, two health psychologists (who had not been involved with the questionnaire development, and had experience of the ICF model) independently coded each of the behaviours contained within the questionnaire (e.g. driving a car) as measuring none, one or more than one of the ICF constructs (impairment, activity, participation). The research team compared the health psychologists’ coding and discussed any disagreements, with reference to the context within which the item was framed, until consensus was achieved.
Results
Participants
Seventeen participants (12 female and five male) who had received treatment for varicose veins (eight had been treated with EVLA, five with foam sclerotherapy and four with surgery) were interviewed. As some interviews were delayed owing to the personal circumstances of participants, time from treatment to interview was more variable than planned (range 8–19 weeks). At interview, the participants’ age range was 30–67 years (mean 48.6 years). Six participants were resident in Aberdeen city and the remainder in the surrounding area (including commuter towns and more rural areas). Hence, a reasonable level of diversity was achieved in this sample. Twelve interviews were conducted in an office at the hospital campus; five were conducted in the participants’ homes.
Generation of labels (unique descriptors)
For five transcripts selected at random (two from participants who had undergone EVLA, two from foam and one from surgery), two researchers independently proposed labels to describe each identified behaviour. One researcher proposed 22 labels and the other proposed 30 labels. There was considerable overlap between the labels proposed by each researcher. However, some of the labels proposed by one researcher were more specific than those proposed by the other. For example, one researcher labelled going out socially, going to the cinema and going to a restaurant as three separate behaviours whereas the other researcher labelled them as a single behaviour. Following discussion of the proposed labels, 29 labels were agreed (see Table 67). Analysis of the text from the remaining transcripts yielded 12 further labels, making a total of 41 labels (see Table 67).
Frequencies for each behaviour
For all 17 interview transcripts, two researchers independently allocated each behaviour identified in the transcripts to one of the 41 labels. The level of agreement between researchers was 96%. Four additional labels were generated at this step, resulting in a final total of 45 labels. The number of participants who mentioned each behaviour was summarised in a frequency table. The frequency data (Table 67) thus generated were assumed to be an indicator of the relevance or importance of each milestone across the patient sample. 76
| Behaviour label | Frequency |
|---|---|
| Having a bath/showera | 15 |
| Full return to normal work/employmenta | 12 |
| Bending leg(s)a | 11 |
| Driving – generalb | 11 |
| Wearing clothing that exposes the legsa | 11 |
| Walking long distances (> 20 minutes)a | 9 |
| Walking short distances (< 20 minutes)a | 8 |
| Standing still for a long time (e.g. > 15 minutes)a | 7 |
| Air travela | 5 |
| Caring for childrena | 5 |
| Doing houseworka | 5 |
| Going out sociallya | 5 |
| Lifting heavy objectsa | 5 |
| Partial return to normal work/employmenta | 5 |
| Sitting in cara | 5 |
| Walking – generalb | 5 |
| Driving – long distancea | 4 |
| Physical activity with childrena | 4 |
| Shoppinga | 4 |
| Swimminga | 4 |
| Dancingc | 3 |
| Driving – short distancea | 3 |
| Golfc | 3 |
| Hill-walkingc | 3 |
| Kneelingc | 3 |
| Sitting down/getting up from chaira | 3 |
| Using gym equipment, e.g. exercise bikec | 3 |
| Circuit training/going to gyma | 2 |
| Climbing steps or stairsc | 2 |
| Getting in and out of cara | 2 |
| Going to the cinema/theatrea | 2 |
| Horse ridinga | 2 |
| Running/jogginga | 2 |
| Sleeping properly/getting to sleepc | 2 |
| Curlingb | 1 |
| Cutting grassc | 1 |
| Cyclinga | 1 |
| Divingc | 1 |
| Fully participating in club/organisation activityc | 1 |
| Going to a restauranta | 1 |
| Having friends overa | 1 |
| Pole dancingc | 1 |
| Running the homea | 1 |
| Stand and tanb | 1 |
| Stretching leg outc | 1 |
Generation of questionnaire items
Based on these frequencies, the researchers agreed on the items to be included in the final questionnaire, sometimes by collapsing labels for infrequently mentioned activities (e.g. the labels for golfing, horse riding and swimming were collapsed into a single label, ‘sporting activity or exercise’). Although ‘air travel’ was mentioned in five interviews, it was not included as a questionnaire item because it was unlikely to be relevant to all potential trial participants. However, if an individual trial participant felt this behaviour was important to them, they could include it in the open behavioural item (‘Anything else that you do that is important to you . . .’). The words ‘without discomfort’ were added to behaviours that patients reported doing during early stages of recovery, when they had noted doing them without discomfort as a milestone.
During the interviews, participants discussed the bandages and compression stockings worn after treatment. Although wearing them was not considered to be a ‘milestone’ behaviour, interview participants reported being pleased and relieved to be able to stop wearing their stocking. Therefore, it was agreed that a question would be included in the questionnaire about the length of time for which compression stockings were worn.
Designing the Behavioural Recovery After treatment for Varicose Veins questionnaire
The final version of the questionnaire contained 15 behavioural items (two of which gave participants the opportunity to describe a social activity and a physical activity), one open behavioural item, one item about wearing the support stocking and one sentence completion item (‘To help my recovery, I . . .’).
We developed a standard response format for the 15 behavioural items and the open behavioural item. Box 2 illustrates the format of the behavioural recovery items and response options; the full BRAVVO instrument is included in the 6-weeks questionnaire contained in Appendix 1. For the three items that asked participants about an important social or physical activity, or anything else important to them (the open behavioural item), the response option ‘I don’t normally do this’ was omitted.
□ I don’t normally do this.
□ I normally do this, but haven’t done so since my treatment.
□ I have done this since my treatment. I did it for the first time:
□ on the day of my treatment OR □ days after my treatment OR □ weeks after my treatment.
The response options for the item about wearing the support stocking were ‘not at all’, ‘day and night for ____ days, then during the day only for ____ days’ and a free-text option to record any other pattern of use.
Preliminary versions of the questionnaire were pilot tested with three patients (who had agreed to take part in the interview study). This pilot testing suggested that the BRAVVO questionnaire was acceptable to participants, and was comprehensible and thus appropriate for self-completion. We did not change the items included in the questionnaire, but minor refinements to the wording and formatting of the questionnaire were made in response to pilot testing.
International Classification of Disability and Function classification of behaviours included in the Behavioural Recovery After treatment for Varicose Veins questionnaire
Box 3 presents the ICF classification of the 15 behavioural items. There was full agreement between the health psychologists on the ICF construct measured by 11 of the 15 behaviours coded. There was partial agreement for two behaviours (‘partial return to normal work/employment’, ‘full return to normal work/employment’), with one health psychologist coding these behaviours as participation and the other coding them as measuring both activity and participation. There was also partial agreement on a further two behaviours (‘doing housework’ and ‘sporting activity or exercise’), with one health psychologist coding these as activity and the other as both activity and participation. The research team discussed the four behaviours for which there was partial agreement and consensus was achieved that all four measured participation. The rationale for this was that all relate to involvement in an everyday, ‘real world’ situation. In addition, in the case of ‘doing housework’ and ‘sporting activity or exercise’, there is a lack of behavioural specificity of the tasks or actions involved (i.e. housework could involve light dusting or scrubbing floors). This may make responses to them more difficult to interpret in terms of behavioural recovery.
-
Bending the leg(s) (without discomfort).
-
Lifting heavy objects (without discomfort).
-
Moving from a standing to a sitting position (without discomfort).
-
Standing still for a long time, i.e. more than 15 minutes (without discomfort).
-
Walking short distances, i.e. less than 20 minutes (without discomfort).
-
Walking long distances, i.e. more than 20 minutes.
-
Having a bath/shower.
-
Driving a car.
-
Doing housework.
-
Looking after children.
-
Wearing clothes that show the legs.
-
Partial return to normal work/employment.
-
Full return to normal work/employment.
-
Going out socially (such as going to the cinema, theatre, restaurant, etc.).
-
Sporting activity or exercise (such as swimming, going to the gym, cycling, running, jogging, horse riding, hill walking, golf, etc.).
Use of the Behavioural Recovery After treatment for Varicose Veins questionnaire in Comparison of LAser, Surgery and foam Sclerotherapy
Methods
Data collection
As described in Chapter 3, we incorporated the BRAVVO questionnaire into the larger CLASS questionnaire administered 6 weeks following treatment. Participants in the CLASS trial were invited to complete this at their 6-weeks follow-up appointment. Participants who failed to attend for their 6-weeks appointment were sent a questionnaire to complete at home.
Statistical analysis
Data from the BRAVVO questionnaire were analysed within an interval-censored time-to-event framework using flexible parametric survival models. 77 For each behaviour item, each participant’s response was converted into the number of days to return to the behaviour. If a participant indicated that return to the behaviour was on the day of the procedure, this was assumed to be interval censored between day 0 and day 1. If a participant indicated that return to the behaviour was after a number of weeks, not days, this was assumed to be interval censored between the previous week and the week indicated. For example, if a participant reported 5 weeks, it was assumed that the return to the behaviour took place between 28 and 35 days. If a participant indicated that they had not returned to a behaviour they usually did, they were right censored at 42 days. Participants who indicated that they did not normally do the specific behaviour were not included in analysis of that behaviour. No missing data were imputed.
We reported the number of days for 50% and 90% of participants to return to each behaviour, estimated from the parametric survival models (the 50% value represents the median time to return to that behaviour). This allows extrapolation beyond the 42-day cut-off for behaviours where 90% of participants had not returned to the activity by 42 days.
The models were fitted on the log cumulative hazard scale, using a restricted cubic spline with one knot to model the baseline hazard function. Treatment effects are presented as hazard ratios (HRs) and 95% CIs. All analyses were carried out in Stata 12 (StataCorp LP, College Station, TX, USA). 78 Flexible parametric survival models were fitted using the stpm command. 79
The item relating to ‘anything else that you do that is important to you, not already mentioned’ was only completed by 99 participants (12%). These participants described a wide range of behaviours (including cooking, cycling, gardening and yoga) that might be expected to have a different recovery trajectory. For these reasons, we considered it inappropriate to summarise data or calculate HRs for this item.
Comparison of Behavioural Recovery After treatment for Varicose Veins results by randomised group
Foam sclerotherapy versus surgery
Participants randomised to foam sclerotherapy were able to carry out most of the behaviours (both activity and participation behaviours) more quickly than those randomised to surgery (Table 68). In general, the median time to return to the activity behaviours was 5 days or fewer for those randomised to foam and up to 9 days for those randomised to surgery. In both groups, there was greater variation in the median time to return to the participation behaviours than the activity behaviours. For all but two of the behaviours, the HRs comparing foam with surgery indicated that return to the behaviour took longer in the surgery arm. The two behaviours for which there was no evidence of a difference in the time to recover between the trial arms were ‘having a bath or shower’ and ‘wearing clothes that show the legs’.
| Questionnaire item | Proportion carrying out activity (%) | Number of days until 50% (and 90%) of participants can carry out activitya | HRb (95% CI) | |
|---|---|---|---|---|
| Foam | Surgery | |||
| Activity items | ||||
| Bending the legs without discomfort | 50 | 3.0 | 4.6 | 1.38 (1.14 to 1.67) |
| 90 | 14.1 | 21.3 | ||
| Lifting heavy objects without discomfort | 50 | 4.8 | 9.8 | 1.97 (1.59 to 2.44) |
| 90 | 16.9 | 34.5 | ||
| Moving from standing to sitting without discomfort | 50 | 1.9 | 3.7 | 1.63 (1.35 to 1.97) |
| 90 | 9.3 | 17.5 | ||
| Standing still for a long time (> 15 minutes) without discomfort | 50 | 3.9 | 7.1 | 1.67 (1.36 to 2.05) |
| 90 | 15.8 | 28.7 | ||
| Walking short distances (< 20 minutes) without discomfort | 50 | 1.9 | 4.4 | 2.00 (1.65 to 2.42) |
| 90 | 8.2 | 19.1 | ||
| Walking long distances (> 20 minutes) | 50 | 4.5 | 8.0 | 1.76 (1.45 to 2.14) |
| 90 | 15.2 | 27.1 | ||
| Having a bath or shower | 50 | 5.4 | 4.9 | 0.85 (0.70 to 1.03) |
| 90 | 11.4 | 10.3 | ||
| Driving a car | 50 | 4.1 | 7.0 | 1.78 (1.45 to 2.19) |
| 90 | 12.4 | 21.1 | ||
| Participation items | ||||
| Doing housework | 50 | 2.1 | 4.5 | 2.10 (1.72 to 2.56) |
| 90 | 7.3 | 15.7 | ||
| Looking after children | 50 | 1.2 | 3.5 | 2.20 (1.61 to 3.00) |
| 90 | 6.2 | 17.9 | ||
| Wearing clothes that show the legs | 50 | 12.4 | 12.8 | 1.03 (0.78 to 1.35) |
| 90 | 56.6 | 58.7 | ||
| Partial return to normal work/employment | 50 | 4.4 | 9.9 | 2.16 (1.72 to 2.72) |
| 90 | 15.4 | 34.2 | ||
| Full return to normal work/employment | 50 | 4.8 | 11.7 | 2.56 (2.05 to 3.21) |
| 90 | 14.9 | 36.2 | ||
| Going out socially | 50 | 7.1 | 9.3 | 1.29 (1.06 to 1.57) |
| 90 | 25.8 | 34.0 | ||
| Sporting activity or exercise | 50 | 15.7 | 21.8 | 1.33 (1.05 to 1.68) |
| 90 | 62.6 | 86.7 | ||
Endovenous laser ablation versus surgery
For seven of the eight activity behaviours, return to the behaviour took longer for those randomised to surgery than for those randomised to EVLA (Table 69). Return to the other activity behaviour (having a bath or shower) was quicker after surgery than after EVLA. For six of the seven participation behaviours, return to the behaviour took longer for those randomised to surgery than for those randomised to EVLA. There was no evidence of a difference in time to return to the other participation behaviour (wearing clothes that show the legs).
| Questionnaire item | Proportion carrying out activity (%) | Number of days until 50% (and 90%) of participants can carry out activitya | HRb (95% CI) | |
|---|---|---|---|---|
| EVLA | Surgery | |||
| Activity items | ||||
| Bending the legs without discomfort | 50 | 2.7 | 4.6 | 1.49 (1.1 to 1.75) |
| 90 | 12.6 | 21.3 | ||
| Lifting heavy objects without discomfort | 50 | 5.9 | 9.8 | 1.79 (1.39 to 2.27) |
| 90 | 20.5 | 34.5 | ||
| Moving from standing to sitting without discomfort | 50 | 2.2 | 3.7 | 1.56 (1.27 to 1.96) |
| 90 | 10.4 | 17.5 | ||
| Standing still for a long time (> 15 minutes) without discomfort | 50 | 4.8 | 7.1 | 1.41 (1.11 to 1.79) |
| 90 | 20.0 | 28.7 | ||
| Walking short distances (< 20 minutes) without discomfort | 50 | 3.0 | 4.4 | 1.30 (1.04 to 1.61) |
| 90 | 13.2 | 19.1 | ||
| Walking long distances (> 20 minutes) | 50 | 5.6 | 8.0 | 1.53 (1.06 to 1.67) |
| 90 | 19.8 | 27.1 | ||
| Having a bath or shower | 50 | 5.5 | 4.9 | 0.74 (0.59 to 0.93) |
| 90 | 12.8 | 10.3 | ||
| Driving a car | 50 | 4.4 | 7.0 | 1.82 (1.43 to 2.33) |
| 90 | 12.7 | 21.1 | ||
| Participation items | ||||
| Doing housework | 50 | 2.5 | 4.5 | 1.89 (1.49 to 2.38) |
| 90 | 8.4 | 15.7 | ||
| Looking after children | 50 | 1.9 | 3.5 | 1.61 (1.15 to 2.27) |
| 90 | 8.8 | 17.9 | ||
| Wearing clothes that show the legs | 50 | 14.6 | 12.8 | 0.97 (0.69 to 1.35) |
| 90 | 75.1 | 58.7 | ||
| Partial return to normal work/employment | 50 | 6.3 | 9.9 | 1.75 (1.33 to 2.27) |
| 90 | 21.1 | 34.2 | ||
| Full return to normal work/employment | 50 | 7.7 | 11.7 | 1.79 (1.37 to 2.27) |
| 90 | 23.5 | 36.2 | ||
| Going out socially | 50 | 6.9 | 9.3 | 1.41 (1.12 to 1.75) |
| 90 | 23.9 | 34.0 | ||
| Sporting activity or exercise | 50 | 14.2 | 21.8 | 1.47 (1.12 to 1.92) |
| 90 | 55.5 | 86.7 | ||
Endovenous laser ablation versus foam sclerotherapy
There was little difference in the time taken to return to the majority of activity and participation behaviours between those randomised to EVLA and those randomised to foam (Table 70). Exceptions to this were walking short and long distances, looking after children and full return to normal work/employment, where return to the activity was longer for the EVLA group than for the foam group.
| Questionnaire item | Proportion carrying out activity (%) | Number of days until 50% (and 90%) of participants can carry out activitya | HRb (95% CI) | |
|---|---|---|---|---|
| EVLA | Foam | |||
| Activity items | ||||
| Bending the legs without discomfort | 50 | 2.7 | 3.0 | 0.94 (0.75 to 1.17) |
| 90 | 12.6 | 14.1 | ||
| Lifting heavy objects without discomfort | 50 | 5.9 | 4.8 | 1.11 (0.87 to 1.42) |
| 90 | 20.5 | 16.9 | ||
| Moving from standing to sitting without discomfort | 50 | 2.2 | 1.9 | 1.12 (0.90 to 1.40) |
| 90 | 10.4 | 9.3 | ||
| Standing still for a long time (> 15 minutes) without discomfort | 50 | 4.8 | 3.9 | 1.14 (0.90 to 1.44) |
| 90 | 20.0 | 15.8 | ||
| Walking short distances (< 20 minutes) without discomfort | 50 | 3.0 | 1.9 | 1.48 (1.19 to 1.84) |
| 90 | 13.2 | 8.2 | ||
| Walking long distances (> 20 minutes) | 50 | 5.6 | 4.5 | 1.32 (1.05 to 1.66) |
| 90 | 19.8 | 15.2 | ||
| Having a bath or shower | 50 | 5.5 | 5.4 | 1.19 (0.96 to 1.48) |
| 90 | 12.8 | 11.4 | ||
| Driving a car | 50 | 4.4 | 4.1 | 0.95 (0.74 to 1.21) |
| 90 | 12.7 | 12.4 | ||
| Participation items | ||||
| Doing housework | 50 | 2.5 | 2.1 | 1.03 (0.82 to 1.29) |
| 90 | 8.4 | 7.3 | ||
| Looking after children | 50 | 1.9 | 1.2 | 1.45 (1.04 to 2.02) |
| 90 | 8.8 | 6.2 | ||
| Wearing clothes that show the legs | 50 | 14.6 | 12.4 | 1.17 (0.83 to 1.64) |
| 90 | 75.1 | 56.6 | ||
| Partial return to normal work/employment | 50 | 6.3 | 4.4 | 1.17 (0.89 to 1.52) |
| 90 | 21.1 | 15.4 | ||
| Full return to normal work/employment | 50 | 7.7 | 4.8 | 1.43 (1.11 to 1.85) |
| 90 | 23.5 | 14.9 | ||
| Going out socially | 50 | 6.9 | 7.1 | 0.88 (0.70 to 1.10) |
| 90 | 23.9 | 25.8 | ||
| Sporting activity or exercise | 50 | 14.2 | 15.7 | 0.80 (0.61 to 1.04) |
| 90 | 55.5 | 62.6 | ||
Compression stockings
We asked how long participants wore their stocking constantly (i.e. day and night); those in the foam arm reported wearing their stocking longer (median 10 days) than those in both the surgery and EVLA arms (median 7 days for each). We also asked about the number of days (or nights) they wore the stocking after they stopped wearing it constantly. We calculated the total number of days that participants wore their stocking (wearing constantly, and then wearing for part of the day or night only); the median time was shortest for foam (12 days) compared with 14 days for EVLA and 17 days for surgery.
Discussion
In this chapter, we have described the development of the BRAVVO questionnaire as an instrument to assess behavioural recovery, and reported findings from the CLASS trial in which the instrument was used. This is a new approach to investigating and describing return to normal activities after treatment of varicose veins which may be useful for more widespread application.
Strengths and limitations of the Behavioural Recovery After treatment for Varicose Veins instrument
The instrument has a number of limitations. First, although care was taken to recruit a diverse sample of participants, this was essentially a self-selected sample of people in one geographical region of the UK, who might not be typical of patients in general. Second, there were constraints associated with developing a questionnaire that would be suitable and relevant for use by a wide range of trial participants but sufficiently short to minimise participant fatigue. Finally, despite pilot testing the questionnaire, the level of missing data was slightly higher for BRAVVO than for other instruments used within CLASS. It may be beneficial to consider if rephrasing the questions or response options, or reformatting the questions, may reduce the level of missing data.
Despite these limitations, the BRAVVO questionnaire represents a systematic first step in identifying the behaviours that may be used to monitor recovery, as well as the actions that patients may take to influence their own recovery following treatment for varicose veins. For the first time, it allows researchers to provide meaningful information to patients about their early recovery and what they may expect to achieve after their treatment.
Comparison of LAser, Surgery and foam Sclerotherapy results in the context of previous research
Return to normal activities
In contrast to previous studies,14,15,29,32,36–38,40 where it appears that participants were asked about return to ‘normal activities’, ‘full activity’, ‘daily activity’ or ‘basic physical activities’, and/or return to work and sporting activities, the BRAVVO questionnaire asks about specific behaviours, all of which are likely to contribute to ‘normal activities’, and all of which were identified by patients as important milestones following their treatment. It provides much more specific and meaningful insights into the wide range of aspects of recovery which are important to patients.
Across the specific behaviours included in BRAVVO, there was variation in the median time to return to the behaviour (i.e. the time that it took for 50% of participants to return to the behaviour). For example, the median time for different behaviours varied from 1 day to 15 days in the foam arm, from 2 days to 14 days in the EVLA arm and from 3 days to 21 days in the surgery arm. This suggests that simply asking about generic ‘normal activities’ (as in previous studies) may miss important differences between different behaviours. This strengthens the rationale for asking about specific behaviours rather than asking a generic question about ‘normal activities’. Information about recovery in terms of specific behaviours could be incorporated into guidance for patients regarding what to expect in the post-treatment period.
Previous studies reporting return to ‘normal activity’ (i.e. using the terms outlined above) have reported median values of up to 3 days following foam,14,29,32 up to 7 days following EVLA15,29,32,37,38 and up to 21 days following surgery. 15,29,37,38 For foam, the median time for some of the behaviours asked about in CLASS was 3 days or fewer; for other behaviours, the median time was considerably longer (up to 15 days). For EVLA, the median time for the majority of behaviours we asked about was 7 days or fewer; only two behaviours had a median time greater than this. For surgery, none of the behaviours we asked about had a median recovery time longer than 21 days. Although this might suggest that the BRAVVO questionnaire has captured similar data to the single question used in previous studies, the data from the BRAVVO questionnaire are more informative. This is because it provides data across a range of specific behaviours which have different recovery trajectories.
The distribution of time to return to each of the behaviours indicates that there is a proportion of people who take much longer to return to the behaviour than would be expected. The extent of this delay in recovery is hard to justify, particularly in light of the information and advice that was given in the study patient information leaflet (PIL), where we suggested that participants should aim to get back to all their normal activities as soon as they were able, but that strenuous activity/contact sport should be avoided for 1–2 weeks. There may have been a number of external influences that had an impact on participants’ recovery, including misinformation and fear. Although attitudes to recovery and returning to normal activities have changed in secondary care, this may not have filtered into primary care or the ‘public knowledge’. Fear of activity or fear of pain caused by activity has been documented following surgery for other conditions. 80,81 It is possible that some people undergoing treatment for varicose veins will experience similar fears and this may limit or restrict activity following their treatment. In addition, anecdotal evidence suggests that many patients fear ‘doing damage’ to their leg following treatment, and particularly following surgery. These fears may contribute to delays in recovery.
Two previous studies have observed that patients return to normal activities more quickly after foam than after surgery. 14,29 For all but two of the behaviours we asked about in CLASS, we showed a similar pattern of results. The exceptions were wearing clothes that showed the legs and showering/bathing. Our observation about showering and bathing is likely to have occurred as a result of the information contained in the study PIL. Participants undergoing foam sclerotherapy or EVLA were advised to wear their stocking for 10 days constantly (i.e. day and night). Those in the surgery group were advised that their bandages would be removed the day after the operation, after which they should wear a stocking for 10 days, but that it was reasonable to remove the stocking after 4 or 5 days, providing they were active. Following surgery, they were also told that a shallow bath might be possible if they could raise their leg to keep the stocking dry.
In three previous studies,15,29,37 the time to return to normal activities was shorter following EVLA than following surgery. For all but two of the behaviours we asked about in CLASS, the findings were similar in that those randomised to EVLA could carry out the behaviour more quickly than those randomised to surgery. One of these behaviours was having a bath or shower, and those randomised to surgery were able to do this earlier after treatment than those randomised to EVLA. The likely reason for this, as discussed above, is the information provided in the PIL. Two previous RCTs comparing surgery and EVLA did not find a difference in mean time to return to daily activities,36,40 and in one further study there was no difference in median time to return to ‘basic activity’ between surgery and EVLA. 38
There have been two previous RCTs which showed a quicker return to normal activities in patients undergoing foam sclerotherapy than in patients undergoing EVLA. 29,32 For four of the behaviours we asked about in CLASS (walking short and long distances, looking after children, full return to normal work/employment), participants in the foam arm returned to the behaviour more quickly than participants in the EVLA arm. For all other behaviours, there was no difference between the arms.
Return to work
Two of the behaviours identified in the interview study, and included in the BRAVVO questionnaire, related to return to work/employment (partial return and full return). As might be expected, across all three arms the median time to full return to work was longer than that for partial return to work. In previous studies, return to work is reported as a single item, without any distinction between partial and full return, which may be of substantial importance to patients, their employers and the economy as a whole. 15,29,36–38,40
The median time to return to either partial or full employment was significantly longer following surgery than either foam or EVLA. Although there was no significant difference in the median time to partial return to employment following foam or EVLA, the median time to return to full employment was significantly longer following EVLA than following foam. This latter finding is perhaps surprising because, in the PIL, the advice about return to work was identical for foam and EVLA. The advice stated that ‘most people are able to return to work within two to three days of treatment, but some people go back the following day or even the same day’. That the time to return to work following surgery was longer than for either foam or EVLA is less surprising. The advice in our PIL for surgery stated that people can return to office or sedentary work after 2–3 days, and that most people will be back at work within 1 week after surgery to one leg and 2 weeks after surgery to both legs. It also stated that there is no reason to remain off work for this long, if it can be managed with reasonable comfort.
In addition to the information given in the PIL and by the vascular surgeon about return to work, a number of factors are likely to mediate return to work after treatment for varicose veins. These may include a person’s employment status (employed or self-employed), the sickness benefits they are entitled to, the type of work they are employed to do, how long they are ‘signed off’ for by their doctor and the views of their employer on return to work after an operation.
In CLASS, the median time to full return to normal work/employment following surgery was 11 days. This is within the range previously reported. Studies have reported median times of 4.3,29 11.8,38 1715 and 21 days. 37
For EVLA, the median time to full return to normal work/employment was 7 days; this is within the range previously reported (4–10 days). 15,29,36–38,40
For foam sclerotherapy, the median time to full return to work/employment was 4 days. In the one previous study that reported the median number of days for return to work, the median time was shorter (2.9 days) than that found in CLASS (partial return 4.4 days, full return 4.8 days). 29
In our comparison of surgery versus foam sclerotherapy, we showed that there were significant differences in both partial and full return to work in favour of foam. The one previous study reporting this outcome has shown that return to work was quicker following foam than following surgery. 29
The CLASS trial showed that return to work following EVLA was quicker than following surgery. This is in line with the findings of three previous RCTs. 15,37,38 In the study by Rasmussen et al. ,29 there was no difference in median time to return to work between those undergoing EVLA and those undergoing surgery. Two further RCTs did not find a difference in time to return to work;36,40 however, in these studies mean times were compared rather than median times, and this may not be the most appropriate measure if the distribution is skewed.
In CLASS, there was no difference in time to partial return to work following foam or EVLA, but full return to work was quicker following foam than following EVLA. The one previous study to report a comparison of foam and EVLA in relation to return to work found that it was quicker following foam than following EVLA. 29
Further development of the Behavioural Recovery After treatment for Varicose Veins questionnaire
The 14 core behavioural items were designed to be scored along a time scale (number of days after treatment to when each behaviour was first performed), and this is how the findings from the CLASS trial are presented. It may be possible to explore the psychometric properties of the instrument and develop a method of producing an overall score for the behavioural items of the questionnaire. Through a systematic process of independent coding of the behaviours contained within the questionnaire by two health psychologists with experience of the ICF model, we have produced data to show that eight of the items measure activity behaviours and seven measure participation behaviours. It may be possible to score these two classes of behaviours as separate subscales.
The results from the application of BRAVVO in the CLASS trial provide useful data for clinicians as they guide patients through their recovery phase. Similar data following treatment for other conditions may also be helpful for both clinicians and patients. Although some of the items in BRAVVO may be transferable to other conditions, we would recommend using similar methods to those described here to identify relevant items to populate other treatment-specific behavioural recovery questionnaires, and the appropriate time frame at which to assess these. During the funding process, there was discussion regarding the most appropriate time point at which to assess behavioural recovery. Although initially planned for the 6-months follow-up, clinical expertise and interview data indicated that, following treatment for varicose veins, it would be more appropriate to collect this type of data at the 6-weeks follow-up. There were two reasons for this. First, the validity of patient recall data was likely to be higher if patients were recalling events over the previous 6 weeks than over the previous 6 months. Second, variation in recovery was likely to be greater over a period of 6 weeks, as most patients would consider themselves to be fully recovered well before the 6-months follow-up. Hence, BRAVVO data at 6 weeks were likely to have more explanatory value.
Conclusions
Development of the BRAVVO instrument represents a systematic first step in identifying the actions that patients may take to influence their own recovery following treatment for varicose veins, as well as the behaviours that may be used to monitor recovery. Using this questionnaire, we have shown that patients are able to return to a wide range of behaviours more quickly following foam or EVLA than following surgery.
Chapter 9 Trial-based cost-effectiveness analysis
Introduction
The purpose of this chapter is to report on the economic analysis that was conducted using individual participant cost and effect data collected alongside the RCT. Two comparisons were considered for the analysis: (1) foam sclerotherapy versus surgery; and (2) EVLA versus foam sclerotherapy versus surgery. As with the clinical effectiveness analyses, the first comparison was carried out using data from all recruiting centres, whereas the three-arm comparison was based only on data from the eight centres that randomised participants to all three treatment options. The methods utilised are described below and, following this, the results are presented.
Methods
This economic evaluation was constructed as a cost-effectiveness analysis, the measure of effect being QALYs over the 6-month trial follow-up period. The primary economic outcome was expressed as the incremental cost per QALY for each treatment option. QALY (utility) weights were derived from participant responses to the EQ-5D82 at baseline, 6 weeks and 6 months. However, to test the robustness of the results to the choice of health state utility instrument, and to enable a comparison with the only other published, UK-based economic evaluation in this area,65 QALYs were also estimated using responses to the SF-36 via the SF-6D scoring algorithm. 83 For each participant, the area under the curve was calculated to determine the QALYs gained. Total NHS, individual participant and indirect costs were estimated for each participant based on resource use data collected on case report forms and participant questionnaires (see Appendix 1). Incremental costs and QALYs associated with the alternative treatment options were estimated using GLMs, adjusted for baseline EQ-5D score and the minimisation covariates. A ceiling ratio of £20,000 per QALY (i.e. a treatment was only deemed cost-effective if it cost less than £20,000 per extra QALY gained) was used to determine cost-effectiveness (i.e. the ICER). 28
Non-parametric bootstrapping84 was used to generate CIs for the estimated mean incremental costs and effects, and to summarise the uncertainty surrounding the ICERs. To illustrate the uncertainty surrounding the estimates of cost-effectiveness, cost-effectiveness acceptability curves (CEACs) were derived using the bootstrapped estimates of incremental costs and effects. CEACs demonstrate the probability of an intervention being cost-effective at different ceiling ratios of decision-makers’ willingness to pay (WTP) per QALY. To test the robustness of results derived from the base-case analysis, key parameters were subjected to deterministic sensitivity analyses. Such analyses examine the effect of estimated or uncertain parameters on the decision.
Unit cost estimation
Unit costs for all resources were obtained for the financial year 2010–11 and were acquired, where possible, from national sources including the British National Formulary,85 NHS Reference Costs (2011)86 and the Unit Costs of Health and Social Care [Personal Social Services Research Unit (PSSRU) 2011]. 87 Where national sources were not available, other sources were utilised, for example finance departments of participating centres.
NHS costs
Total NHS costs included health service resource use associated with initial treatment, all subsequent contact with primary care (e.g. GP contacts) and secondary care contact potentially related to the participants’ varicose veins (e.g. hospital admissions, outpatient appointments).
Cost of treatment procedures
All treatment strategies were performed as day-case procedures; however, the locations and settings differed. The unit costs of performing each of the three treatment strategies were estimated using a ‘bottom-up’ approach. These costs were collected via a centre-specific resource use and costing questionnaire, allowing us to capture resource use not recorded on the trial CRFs, and also to cost the treatment strategies in a centre-specific manner. The additional questionnaire is shown in Appendix 4. Information collected included average participant waiting time (before and after treatment), location of treatment and recovery, nursing staff input, equipment use and consumables used for procedures.
The grade of the surgeon or nurse performing each procedure (nurses performed some foam sclerotherapy treatments), and whether or not they were supervised by a consultant, was recorded on the CRF of each participant receiving treatment. For surgery, the grade of the anaesthetist present was recorded. The times of entering and leaving the operating theatre or treatment room were used as a measure of the total time requirement for all staff (including nursing and support staff) present for the procedure. Further information, relating to the numbers of nursing and support staff who were typically present for the different types of procedure and their salary bands, was collected using the centre-specific resource use and costing questionnaire. This staffing information, together with treatment duration times (obtained for individual participants), was combined with national unit cost data (PSSRU 2011)87 to estimate the total cost of staff time for each procedure. The unit costs applied for each grade of staff are presented in Table 71.
| Staff | Unit cost per hour (£) | Cost per minute (£) | Source |
|---|---|---|---|
| Nursing staff | |||
| Band 2 | 20 | 0.34 | PSSRU (2011)87 |
| Band 3 | 24 | 0.40 | PSSRU (2011)87 |
| Band 4 | 30 | 0.50 | PSSRU (2011)87 |
| Band 5 | 82 | 1.37 | PSSRU (2011)87 |
| Band 6 | 107 | 1.78 | PSSRU (2011)87 |
| Band 7 | 129 | 2.15 | PSSRU (2011)87 |
| Band 8b (consultant nurse) | 147 | 2.45 | PSSRU (2011)87 |
| Medical staff | |||
| Foundation Year 1 | 33 | 0.55 | PSSRU (2011)87 |
| Foundation Year 2 core trainee, CCT | 42 | 0.69 | PSSRU (2011)87 |
| Specialty trainee | 59 | 0.99 | PSSRU (2011)87 |
| Staff grade | 95 | 1.59 | PSSRU (2011)87 |
| Associate specialist | 131 | 1.88 | PSSRU (2011)87 |
| Consultant medical | 136 | 2.27 | PSSRU (2011)87 |
An extensive list of minor consumables was generated from the trial centre which had recruited most participants and this cost was applied directly to all procedures. However, major consumables and items of capital equipment (which could have a significant impact on overall costs) were collected from a number of participating centres. An overview of the costing approach is described below.
Capital equipment was costed by asking participating centres to report the current market price for major equipment items used. Two major pieces of capital equipment – duplex ultrasound machines and laser generators – were required for foam sclerotherapy and EVLA treatments. Centres reported using Sonosite Micromaxx® or Sonosite M-turbo® ultrasound machines (Sonosite, Inc., Bothell, WA, USA); laser machines reported were Fotona Lasers XP-2® (Fotona, San Clemente, CA, USA) and Biolitec Lasers® (Biolitec, East Longmeadow, MA, USA). However, centres offering EVLA reported receiving laser machines on loan from suppliers. Because the NHS did not incur a cost for any laser generator, no cost was applied for the usage of this capital item in the base-case analysis (a sensitivity analysis was conducted to test this assumption). The initial outlay costs of duplex ultrasound machines were annuitised over the expected serviceable working life of these devices, using an annual depreciation rate of 3.5% to account for the opportunity cost of the investment over time. The estimated equivalent annual cost was then divided by an estimate of annual patient throughput to give an estimated cost per use of £8.78.
The unit costs of staff time incorporated an allocation of overhead and capital (building space) costs attributable to individual grades of staff, but there was nevertheless some concern that these unit costs would not fully capture the opportunity cost associated with procedures which utilised operating theatres. All surgical procedures for the GSV and SSV were performed in an operating theatre. Therefore, a secondary analysis was undertaken, whereby an extra allocated cost per minute for use of an operating theatre was applied to procedures for which a theatre was stated as the main location for treatment. An additional cost of £3.64 per minute, derived from Information Services Division (ISD) data,88 was calculated from the total allocated costs attributable to theatre use divided by total theatre operating time at three large teaching hospitals in Scotland. These allocated costs include the cost of theatre equipment and cleaning of consumables. Therefore, for the secondary analysis, the bottom-up estimated costs of equipment use and cleaning were dropped from total cost estimates for procedures carried out in theatre (to avoid double counting).
Foam sclerotherapy
Foam sclerotherapy was, for the most part, performed in a clinic setting. The nursing staff requirements reported for this procedure varied from one to two nurses (band 5 or 6), with some centres also reporting the presence of a health-care assistant or nurse runner.
Foam sclerotherapy treatment involves the injection of a foam sclerosant into the affected vein(s), guided by ultrasound. The unit cost of the ultrasound machine usage was also incorporated for each treatment session. One centre reported using a micropuncture kit for venous access, whereas others reported using an intravenous cannula (a small, flexible plastic tube that is inserted into the vein). The cost of the micropuncture kit (which incurs a higher cost than the intravenous cannula) was only applied to the centre that reported using it. Table 72 shows the unit costs, over and above staff time, of items of resource use included in the cost calculation for foam sclerotherapy treatment.
| Resource use item | Mean unit cost (£) | Assumptions | Source |
|---|---|---|---|
| Foam sclerotherapy | STS 3%: 5.52 per amp STS 1%: 4.54 per amp |
3% used for GSV and SSV 1% used for non-truncal varicosities |
Participating centre (lead centre) (2013) |
| Consumables | 50.20 | Centre using micropuncture kit | Participating centres, resource use and costing questionnaire (2013) |
| Consumables | 26.23 | Applied to all centres other than that using micropuncture kit | Participating centres, resource use and costing questionnaire (2013) |
| Capital equipment (ultrasound machine) | 8.78 | Per usage | Participating centres, resource use and costing questionnaire (2013) |
| Preparation cost of clinic/theatre | 24.86 | Applied to all centres | Clinical opinion (2013), PSSRU (2011)87 |
| Recovery cost | 1.70 | 10 minutes in recovery area | Clinical opinion (2013), PSSRU (2011)87 |
Endovenous laser ablation
Centres reported varied locations for performing EVLA. These included outpatient clinic room, treatment room, short-stay ward and day-case theatre (a minority of centres reported performing procedures here). All procedures were performed under local anaesthetic, regardless of location. The unit cost of staff time was estimated as outlined in Table 71.
Disparities were evident among centres performing EVLA in terms of staff (additional to the surgeon) present at the procedure. On average, centres reported the presence of two additional staff for procedures performed in a clinic or treatment room setting, whereas those that used a theatre or short-stay ward to perform EVLA reported an average of five additional staff. Additional staff types included theatre nurses, vascular nurse specialists, health-care assistants/support workers, trainee operating department practitioners and nurse runners. Although the base-case analysis incorporated staff present in a centre-specific manner, a sensitivity analysis was conducted to assess the impact if EVLA was performed in a clinic or treatment room setting only and adopted a similar staff profile to foam sclerotherapy.
Endovenous laser ablation requires the use of both an ultrasound machine to identify the veins that need treatment, and a laser generator to deliver the energy to the vein via the laser fibre. Although it was assumed that the laser generator would generally be loaned free of charge to the NHS, this still requires the use of a laser fibre. The price of this non-reusable laser fibre (£256) is a key driver in the overall cost of this treatment. Table 73 shows the unit costs included in the cost calculation for the EVLA procedure. In addition, the section of the resource use and costing questionnaire concerned with EVLA captured the average length of time spent on the ward both before and after the procedure.
| Resource use item | Unit cost (£) | Assumptions | Source |
|---|---|---|---|
| Consumables | 65.06 | Applied to all centres | Participating centre (lead centre) (2013) |
| Laser fibre | 256 | Includes catheter and guide wire | Participating centre (lead centre) (2013) |
| Capital equipment (ultrasound machine) | 8.78 | Per usage | Participating centres, resource use and costing questionnaire (2013) |
| Preparation cost of clinic/theatre | 23.78 | Applied to all centres | Clinical opinion (2013), PSSRU (2011)87 |
| Recovery cost | 31.85 | Estimated from resource use and costing questionnaire | Clinical opinion (2013), PSSRU (2011)87 |
Surgery
The staffing requirements for surgery were again costed in a participant-/centre-specific way. All procedures are carried out in an operating theatre under general or epidural/spinal anaesthetic. Surgery also requires additional staff time to look after the patient in the recovery room and recovery ward after the procedure, during recovery from anaesthesia.
Centres specified a range of different nursing and support staff present for this procedure, with an average of five staff in addition to the surgeon and anaesthetist. These staff included anaesthetic nurses, scrub nurses, assistant scrub nurses, health-care assistants, nurse runners, operating department practitioners and recovery nurses.
The main capital equipment items required for the surgical procedure are the electrocardiograph (ECG), pulse oximeter and non-invasive blood pressure monitor. A cost per patient was calculated in a similar way to that for use of the ultrasound machine.
Time spent by participants in the recovery room after the procedure was also recorded on the CRFs. It was assumed that the cost of the recovery room time would include half the time of a band 5 nurse, while further time spent on a recovery ward after leaving the recovery room would incur one-quarter of the time of a band 5 nurse. Table 74 outlines the cost items, over and above the staffing costs for the procedure and recovery room time, required for the estimation of the total costs of surgery. Information was collected from the additional resource use and costing questionnaire on the average time spent by participants on the day-case ward before and after treatment.
| Resource use item | Unit cost (£) | Assumptions | Source |
|---|---|---|---|
| Consumables | 159.56 | Applied to all centres | Participating centre (lead centre) (2013) |
| Capital equipment (ECG, pulse oximeter and blood pressure monitor) | 4.15 | Per usage | Participating centres, resource use and costing questionnaire (2013) |
| Preparation cost of theatre | 31.36 | Applied to all centres | Clinical opinion (lead centre) (2013), PSSRU (2011)87 |
| Time on ward after leaving the recovery room | 47.60 | One-quarter of the time of a band 5 nurse for 140 minutes | Participating centres, resource use and costing questionnaire (2013), PSSRU (2011)87 |
Follow-up health service use costs
Data on the use of secondary health-care services (hospital admissions and outpatient attendances) were collected using a combination of clinical assessment forms (completed at 6 weeks and 6 months) and a data abstraction form completed by the research nurse at each centre based on each participant’s medical records. Data on the use of primary care services over the follow-up period were collected from the 6-months, patient-completed questionnaire. The clinical assessment and data abstraction forms captured information regarding any hospital admissions and any additional treatment to the study leg (outside the CLASS trial protocol). Reported hospital admissions were reviewed by clinicians on the trial team, and those identified as possibly related to treatment were costed using the appropriate NHS reference cost (Department of Health 2011). 86 The average costs of the treatment strategies, derived from the bottom-up micro-costing of treatment strategies, were applied for additional reported varicose vein treatments to the study leg that were not recorded in the CLASS treatment CRFs.
Unit costs from nationally available data were used to cost primary care contacts (Table 75). Participants were asked whether or not they had been in contact with a GP as a result of their varicose veins (visit to the GP, telephone call and/or GP home visit), and if so, how many contacts they had over the previous 6 months. Questions relating to appointments with other health-care workers (e.g. community or practice nurse, NHS physiotherapist, NHS occupational therapist) as a result of varicose veins were also recorded and costed in a similar manner.
| Practitioner | Unit cost (£) | Assumption | Source |
|---|---|---|---|
| GP clinic visit | 36 | 11.7-minute surgery visit | PSSRU (2011)87 |
| GP home visit | 120 | Home visit lasting 23.4 minutes (including travel time) | PSSRU (2011)87 |
| GP telephone conversation | 22 | 7.1-minute telephone conversation | PSSRU (2011)87 |
| Community nurse | 18.67 | 20-minute visit | PSSRU (2011)87 |
| Practice nurse | 12 | GP nurse | PSSRU (2011)87 |
| NHS physiotherapist | 17 | 30-minute clinic visit | PSSRU (2011)87 |
| NHS occupational therapist | 17 | 30-minute clinic visit | PSSRU (2011)87 |
Follow-up assessment and outpatient costs
An additional outpatient follow-up assessment cost was applied to all participants who received just one treatment of EVLA or foam sclerotherapy. This was done because the CLASS trial was designed to assess the clinical effectiveness and cost-effectiveness of EVLA and foam sclerotherapy with additional further foam sclerotherapy as required. Participating centres reported that it is normal practice for all participants, having received initial treatment with EVLA or foam sclerotherapy, to return for a follow-up appointment to assess the need for further treatment. We applied the cost of an outpatient follow-up attendance (£123) with duplex scan (£53) for all participants in the data set who attended their 6-weeks clinical assessment following one treatment session with EVLA or foam sclerotherapy. For participants who had further treatment following initial treatment with EVLA or foam sclerotherapy, we assumed that this was done at their planned follow-up appointment when the decision was made that it was needed, and so no additional clinical assessment cost was applied to these participants. However, because the majority of participants receiving EVLA or foam sclerotherapy as their initial treatment did not receive any subsequent treatment in the trial, we also carried out a sensitivity analysis in which routine follow-up costs were excluded for all participants. As surgery patients would not routinely be seen at 6 weeks after surgery in normal NHS practice, no costs were attributed for this.
In addition, some participants incurred unplanned outpatient appointments during the 6-month follow-up period, which could potentially have been related to their varicose veins treatment (e.g. surgical and medical outpatient appointments). These were costed using the appropriate NHS reference cost (Department of Health 2011)86 and included in the analysis.
Total NHS cost
The total cost to the health service was computed by summing the estimated treatment and follow-up costs for each participant in the data set. If one of the component costs was missing, then that participant was dropped from the analysis of complete case data. Two total NHS cost variables were computed, one excluding the additional cost applied to procedures carried out in theatre, and one which included this extra cost. This was to ensure that the opportunity cost associated with procedures which utilised operating theatres was accurately captured.
Costs directly incurred by the participant and indirect costs
Individual participant costs were estimated based on responses to the health-care utilisation questions included in the 6-months questionnaire. Individual participant costs comprised three main elements: self-purchased health care; travel costs of making return visit(s) to NHS health-care provider(s); and the time costs incurred while travelling to and attending NHS care. The estimation of travel costs required information from participants regarding the number of visits to, for example, their GP, and the unit cost of making a return journey to each type of health-care provider. The cost of participant time was estimated in a similar manner. Participants were asked how long they spent travelling to and attending their last visit to each type of health-care provider. They were asked what activity they would otherwise have been doing (e.g. paid work, housework, leisure activities) had they not attended the health-care provider, enabling a calculation of the opportunity cost associated with their health-care contact. These data were presented in natural units and costed using standard economic conventions, such as the Department of Transport estimates for the value of leisure time. 89 Table 76 presents the unit costs used to value participant time in the analysis. These unit time costs were combined with estimates of the number of health-care contacts derived from the 6-months questionnaire. Costs were also acquired for any additional private health care that participants had for their varicose veins which was recorded in the 6-months questionnaire.
| Activity | Cost per hour (day), £ | Assumption | Source |
|---|---|---|---|
| Paid work | 12.62 (100.96) | Median hourly earnings, excluding overtime | ONS (2011)90 |
| Housework | 10.10 (80.77) | Annual salary of £21,000 | NHS Pay Review Body 2012 Report91 |
| Child care | 12.62 (100.96) | Median hourly earnings, excluding overtime | ONS (2011)90 |
| Caring for relative/friend | 12.62 (100.96) | Median hourly earnings, excluding overtime | ONS (2011)90 |
| Unemployed | 4.46 (35.68) | Values of non-working time | TAG (2011)89 |
| Voluntary work | 12.62 (100.96) | Median hourly earnings, excluding overtime | ONS (2011)90 |
| Leisure activities | 4.46 (35.68) | Values of non-working time | TAG (2011)89 |
Indirect costs were defined as the production losses resulting from treatment when the participant was unable to return to normal activity. Information regarding participants’ recovery was collected in the 6-weeks questionnaire. Included in this section was the length of time to return to normal activities, comprising doing housework, looking after children and partial or full return to normal work/employment. As with the calculation of participant time cost, these data were recorded in natural units and costed using standard economic conventions (see Table 76). This estimate gave the cost of days lost associated with the participants’ health care. Individual participant, indirect and NHS costs were combined and a further analysis was conducted.
Health outcome measures
The EQ-5D and SF-36 were completed by participants at baseline, 6 weeks and 6 months using a self-completion questionnaire, ensuring that all movements in HRQoL which occurred during the follow-up period were accurately captured. A preference-based index score was derived for each participant’s response to the EQ-5D using the UK population time trade-off tariff,82 and responses to the SF-36 were converted into a preference-based index score using the SF-6D scoring algorithm. 83 Although both these index scores are anchored on full health (1) and death (0), making them suitable for estimating QALYs for individual participants, the EQ-5D is the instrument favoured by NICE for this purpose. Therefore, the EQ-5D was used to estimate participant QALYs in the base-case economic analysis.
Quality-adjusted life-years for each participant were computed by assuming that changes in utility between measures at adjacent time points follow a straight line between the points. The average utility over each time period (baseline to 6 weeks, and 6 weeks to 6 months) was calculated and multiplied by the duration of that time to compute the corresponding QALYs. If an EQ-5D value was missing for a participant at any time point, that patient was dropped from the analysis of complete case data.
Statistical analysis of economic data
The economic analysis was conducted by intention to treat. Cost, QALY and resource use data were summarised and analysed using Stata version 12.1. 78 The mean incremental costs associated with EVLA and foam sclerotherapy versus surgery were estimated using a GLM, with adjustment for minimisation variables (age < 50 years, sex, SSV or GSV, unilateral or bilateral) and participant baseline values as appropriate (e.g. baseline utility scores). A GLM model allows for heteroscedasticity by specifying a distributional family, which reflects the relationship between the mean and variance. 84 To test the appropriate family, a modified Parks test was conducted. The test identified the Gamma family, which allows for right skewness in the cost data and assumes that the variance is proportional to the square of the mean, as appropriate. The specification of a link function is also required for a GLM model; this specifies the relationship between the set of regressors and the conditional mean. 84 The link test recognised the identity link as the appropriate link function. The identity link leaves the interpretation of the coefficients unchanged from that of the ordinary least squares (OLS) regression, in that the covariates act additively on the mean. To further ensure that the correct model was specified, the Akaike information criterion (AIC) was used to assess the model fit. An OLS regression was implemented to estimate mean incremental differences in QALYs associated with EVLA and foam sclerotherapy, adjusting for minimisation variables and participant baseline values in a similar manner. To account for the potential lack of independence in costs and outcomes for participants within centres, cluster robust standard errors and CIs are reported for the estimated mean incremental costs and effects.
To characterise the uncertainty surrounding the estimates of incremental cost-effectiveness, clustered bootstrapping was used to generate 1000 estimates of the mean costs and effects by treatment allocation group. CEACs were generated using these 1000 estimates, using the net monetary benefit (NMB) approach. The NMB associated with a given treatment option is given by the formula:
where effects are measured in QALYs and Rc is the ceiling ratio of WTP per QALY. Using this formula, the strategy with greatest NMB was identified for each of the 1000 bootstrapped replicates of the analysis, for different ceiling ratios of WTP per QALY. Plotting the proportion of bootstrap iterations favouring each treatment option (in terms of the NMB) against increasing WTP per QALY produces the CEAC for each treatment option. These curves present graphically the probability of each treatment strategy being considered optimal at different levels of WTP per QALY gained.
Deterministic sensitivity analysis
Deterministic sensitivity analyses were conducted, focusing on the impact of altering key costing assumptions and restricting the comparisons to subgroups of the population. All the sensitivity analyses were conducted with the exclusion of additional operating theatre costs. We assessed the impact of excluding the routine follow-up costs (the cost of an outpatient follow-up appointment and duplex ultrasound scan) following EVLA and foam sclerotherapy. This was to reflect that the majority of participants receiving these treatment strategies as their initial treatment did not receive any further foam sclerotherapy treatment in the trial. We tested the impact of restricting the comparisons to subgroups of the population (e.g. those with unilateral disease only and also those with unilateral disease and involvement of the GSV only). The impact of using the SF-6D to calculate QALYs was assessed. Two further sensitivity analyses were conducted for the three-arm comparison, assessing the impact on the results for EVLA of (i) incorporating a staff profile if the procedure was performed in a clinic/treatment room setting, and (ii) including the cost per usage of the laser generator.
As the base-case cost-effectiveness analyses were conducted for participants with complete cost and QALY data, multiple imputation was carried out using Stata’s chained equations procedure to replace each missing resource use, cost and EQ-5D variable with a different plausible value in 10 imputed data sets. 78 Ten imputations was found to produce stable estimates of incremental costs and effects. The cost and QALY analysis models were then run across the 10 imputed data sets and combined using Rubin’s rules92 to produce a single set of results. Multiple imputation was conducted on both the full data set and the data that remained when those participants receiving no treatment and follow-up (and thus incurring no costs) were dropped.
Results
The results from the two-arm comparison (foam sclerotherapy vs. surgery) are described below, and following this, the results from the three-arm comparison (EVLA vs. foam sclerotherapy vs. surgery) are presented.
Foam sclerotherapy versus surgery
Resource use and costs
Table 77 documents the main components included in the treatment cost estimation for participants (randomised to foam sclerotherapy or surgery). Of the 286 participants randomised to foam sclerotherapy, 268 presented for their allocated treatment, although two of these participants were found to be unsuitable for foam sclerotherapy treatment. Ten participants randomised to foam sclerotherapy received either surgery (n = 4) or EVLA (n = 6). Eleven participants randomised to foam sclerotherapy received no treatment within CLASS. The mean number of treatments for those participants receiving foam sclerotherapy was 1.12. The surgical arm included 289 participants. Of these, 253 received their allocated treatment, but 19 received treatment in the form of foam sclerotherapy (n = 11) or EVLA (n = 8). The mean number of foam sclerotherapy treatments among those receiving this treatment modality was similar to that in the foam group (1.09 treatments). Twenty-one participants randomised to surgery received no treatment within CLASS.
| Treatment details | Randomised to foam sclerotherapy | Randomised to surgery | ||||
|---|---|---|---|---|---|---|
| Number randomised | 286 | 289 | ||||
| Treatment received | ||||||
| Presented for foam (n, %) | 268 | 93.7 | 11 | 3.8 | ||
| Number of foam treatments (n, mean, SD) | 268 | 1.12 | 0.35 | 11 | 1.09 | 0.30 |
| Presented for surgery (n, %) | 4 | 1.4 | 253 | 87.5 | ||
| Presented for EVLA (n, %) | 6 | 2.1 | 8 | 2.8 | ||
| No recorded treatment (n, %) | 11 | 3.9 | 21 | 7.3 | ||
| Treatment durationsa | ||||||
| Cumulative procedure time (minutes) | ||||||
| Foam sclerotherapy (n, mean, SD) | 261 | 29.70 | 16.53 | 288 | 1.10 | 7.08 |
| Surgery (n, mean, SD) | 285 | 0.78 | 7.63 | 270 | 64.56 | 33.67 |
| EVLA (n, mean, SD) | 283 | 0.62 | 6.05 | 288 | 1.20 | 7.89 |
| Treatment costs | ||||||
| Staff procedure costs | ||||||
| Foam sclerotherapy (n, mean, SD) | 261 | £144 | £87 | 288 | £5 | £36 |
| Surgery (n, mean, SD) | 285 | £8 | £75 | 270 | £599 | £340 |
| EVLA (n, mean, SD) | 283 | £4 | £37 | 288 | £8 | £50 |
| Recovery time costs (n, mean, SD) | 285 | £3 | £9 | 271 | £72 | £34 |
| Consumable costs (n, mean, SD) | 286 | £44 | £52 | 289 | £150 | £54 |
| Theatre use (n, mean, SD) | 260 | £23 | £50 | 269 | £238 | £121 |
| Equipment costs (n, mean, SD) | 286 | £9 | £4 | 289 | £4 | £2 |
| Preparation costs (n, mean, SD) | 286 | £27 | £10 | 289 | £29 | £9 |
| Total treatment costs | ||||||
| Foam sclerotherapy (n, mean, SD) | 261 | £218 | £104 | 288 | £8 | £49 |
| Surgery (n, mean, SD) | 285 | £10 | £103 | 270 | £859 | £429 |
| EVLA (n, mean, SD) | 283 | £8 | £76 | 288 | £17 | £108 |
| Total staff procedure costs (n, mean, SD) | 257 | £159 | £107 | 268 | £617 | £321 |
| Total treatment costsb (n, mean, SD) | 257 | £241 | £142 | 268 | £891 | £391 |
For participants randomised to foam sclerotherapy, the mean cumulative time spent receiving this treatment modality was 29.7 minutes. This was considerably shorter than the time spent by participants receiving surgery in the surgery group (64.56 minutes). Staff costs for the procedure were derived from the duration times and the grades and numbers of staff present for each procedure; therefore, the overall staff costs were considerably higher in the surgery arm (£599 compared with £144 for foam sclerotherapy).
A considerable difference was also evident between the foam sclerotherapy and surgery arms in terms of recovery, consumable and theatre use costs. The estimated total mean treatment costs, inclusive of staff costs, consumables, equipment use, clinic/theatre preparation and recovery time costs, amounted to £241 for foam sclerotherapy and £891 for surgery.
Table 78 shows follow-up resource use and costs over the 6-month follow-up period, by treatment allocation group. Resource use and costs were generally similar for the foam sclerotherapy and surgery arms, although unplanned outpatient appointments were slightly more frequent in the foam sclerotherapy arm and primary care use was slightly higher in the surgery arm. Attendance at the 6-week and 6-month assessments was > 90% in both arms; however, only the cost of the 6-week assessment was included in the economic analysis for participants receiving foam sclerotherapy. There were no reports of hospital admissions related to treatment in the foam sclerotherapy group, and the number reporting this outcome in the surgical group was low (0.02 of those randomised to surgery).
| Post-treatment care costs | Randomised to foam sclerotherapy | Randomised to surgery | ||||
|---|---|---|---|---|---|---|
| Number randomised | 286 | 289 | ||||
| Primary care | ||||||
| GP consultations (n, mean, SD) | 234 | 0.36 | 0.74 | 213 | 0.56 | 1.19 |
| Other health professional consultations (n, mean, SD) | 234 | 0.28 | 1.96 | 213 | 0.44 | 1.05 |
| Total cost of GP consultations (n, mean, SD) | 234 | £13 | £27 | 213 | £20 | £42 |
| Total cost of consultations (other health professionals) (n, mean, SD) | 234 | £4 | £30 | 213 | £6 | £15 |
| Total primary care costs (n, mean, SD) | 234 | £17 | £40 | 213 | £26 | £44 |
| Secondary care (planned) | ||||||
| Attendance at 6-week assessmenta (n, mean, SD) | 265 | 260 | 98.1 | 251 | 241 | 96.0 |
| Attendance at 6-month assessmentb (n, mean, SD) | 250 | 230 | 92.0 | 235 | 217 | 92.3 |
| 6-week assessment costa (n, mean, SD) | 265 | £173 | £24 | 251 | £169 | £35 |
| 6-month assessment costb (n, mean, SD) | 250 | £162 | £48 | 235 | £163 | £47 |
| Secondary care (unplanned) | ||||||
| Unplanned medical/surgical outpatient appointments (n, mean, SD) | 286 | 0.25 | 1.15 | 289 | 0.20 | 0.77 |
| Cost of unplanned outpatient appointments (n, mean, SD) | 286 | £35 | £138 | 289 | £29 | £93 |
| Admissions (n, mean, SD) | 272 | 0 | 0 | 254 | 0.02 | 0.12 |
| Admission costs (n, mean, SD) | 272 | £0 | £0 | 254 | £5 | £42 |
Table 79 documents the total mean NHS costs associated with both treatment strategies to 6-months follow-up. These are reported for both the primary (excluding the additional cost for operating theatre use) and secondary (including the extra cost for operating theatre use) analysis of NHS costs. For the primary analysis, the mean total cost for those randomised to foam sclerotherapy was £458, compared with £1057 for those randomised to surgery. When the additional cost of theatre (£3.64 per minute in the operating theatre) is included, the mean total cost of foam sclerotherapy increases slightly to £479, and the mean total cost of surgery increases to £1314.
| NHS costs | Randomised to foam sclerotherapy | Randomised to surgery | ||||
|---|---|---|---|---|---|---|
| Number randomised | 286 | 289 | ||||
| Total NHS costs (primary analysis) (n, mean, SD) | 206 | £458 | £201 | 196 | £1057 | £327 |
| Total NHS costs (secondary analysis) (n, mean, SD) | 206 | £479 | £215 | 196 | £1314 | £399 |
Health outcomes: European Quality of Life-5 Dimensions and Short Form questionnaire-6 Dimensions
The EQ-5D and SF-6D results are summarised by treatment allocation group in Table 80. These data showed a general improvement in generic HRQoL in both foam sclerotherapy and surgery treatment allocation groups between baseline and 6 weeks, and between 6 weeks and 6 months. The increase in the EQ-5D score between baseline and 6 weeks was more marked in the surgery arm, whereas the improvement was greater in the foam sclerotherapy arm between 6 weeks and 6 months. Overall, the QALYs accrued over 6 months were slightly lower in the surgery arm, although this was likely to have been due to a slight imbalance in EQ-5D scores at baseline.
| HRQoL | Randomised to foam sclerotherapy | Randomised to surgery | ||||
|---|---|---|---|---|---|---|
| Number randomised | 286 | 289 | ||||
| EQ-5D | ||||||
| Baseline (n, mean, SD) | 279 | 0.803 | 0.179 | 279 | 0.783 | 0.178 |
| 6 weeks (n, mean, SD) | 242 | 0.860 | 0.161 | 227 | 0.876 | 0.169 |
| 6 months (n, mean, SD) | 235 | 0.895 | 0.174 | 206 | 0.881 | 0.202 |
| Total QALYs (n, mean, SD) | 217 | 0.435 | 0.072 | 188 | 0.432 | 0.082 |
| SF-6D | ||||||
| Baseline (n, mean, SD) | 272 | 0.767 | 0.122 | 269 | 0.754 | 0.128 |
| 6 weeks (n, mean, SD) | 240 | 0.774 | 0.121 | 218 | 0.765 | 0.116 |
| 6 months (n, mean, SD) | 227 | 0.805 | 0.122 | 199 | 0.802 | 0.127 |
| Total QALYs (n, mean, SD) | 202 | 0.392 | 0.054 | 166 | 0.390 | 0.052 |
Incremental cost-effectiveness: primary analysis
It was found that surgery was associated with a slight gain in QALYs following adjustment for baseline EQ-5D and minimisation variables, but it was also associated with a significantly higher health service cost than foam sclerotherapy (Table 81). Therefore, foam sclerotherapy was less costly than surgery (cost saving), but at the expense of HRQoL, which was lower. 84 The ICER was £102,106, which represents the cost saving per QALY lost with foam sclerotherapy versus surgery. NICE generally judges an intervention as being cost-effective if the additional cost required to fund it is < £20,000 per QALY gained (i.e. the ICER is < £20,000). Conversely, assuming equal value placed on a QALY gained and a QALY lost, one would require an ICER > £20,000 of cost savings before the decision-maker would be willing to sacrifice one QALY. As the ICER is above the threshold value, based on this decision rule, foam sclerotherapy would have a favourable ICER.
| Intervention | Incremental costs (95% CI), £ | Incremental QALYs (95% CI) | ICER (£) |
|---|---|---|---|
| Surgery | – | – | – |
| Foam sclerotherapy | −602 (−740 to −464) | −0.006 (−0.021 to 0.009) | 102,106 |
To explore the uncertainty surrounding the estimate of cost-effectiveness, a CEAC was derived using the results of 1000 bootstrapped replicates of the estimated mean incremental costs and effects. Figure 4 shows the empirical estimate of the joint distribution of mean incremental costs and effects (for foam sclerotherapy vs. surgery) obtained using the results of the bootstrap replicates. The estimates indicate that foam sclerotherapy is significantly less costly, with a non-significant tendency to produce fewer QALYs than surgery. Applying a ceiling WTP ratio of £20,000 per QALY, Figure 5 illustrates that, when using the EQ-5D as the measure of outcome, foam sclerotherapy has a 99.8% probability of being considered cost-effective at 6 months. The sensitivity of these findings to missing data is assessed using multiple imputation (see Deterministic sensitivity analysis).
FIGURE 4.
Incremental cost-effectiveness scatterplot for foam sclerotherapy vs. surgery at 6 months.
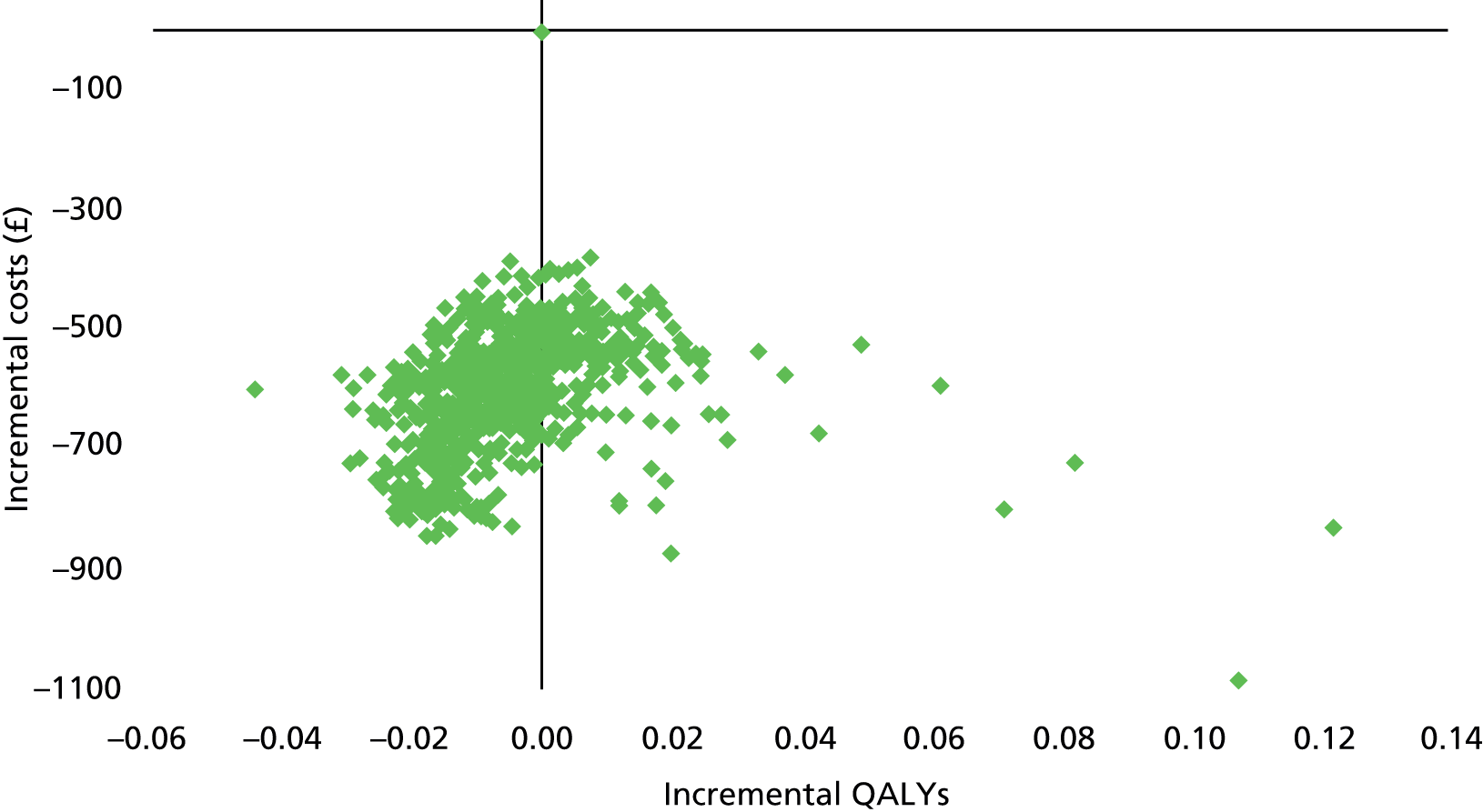
FIGURE 5.
Cost-effectiveness acceptability curves for foam sclerotherapy vs. surgery at 6 months.

Incremental cost-effectiveness: secondary analysis
The secondary analysis (incorporating an additional cost for the use of an operating theatre) reinforces the findings of the primary analysis, increasing the cost saving associated with foam sclerotherapy. Under these alternative costing assumptions, the ICER increases to £142,973, representing the cost saving per QALY lost for foam sclerotherapy versus surgery (Table 82).
| Intervention | Incremental costs (95% CI), £ | Incremental QALYs (95% CI) | ICER (£) |
|---|---|---|---|
| Surgery | – | – | – |
| Foam sclerotherapy | −843 (−1004 to −682) | −0.006 (−0.021 to 0.009) | 142,973 |
Similar to the primary analysis, although the cluster falls further below zero on the cost axis (i.e. cost saving), Figure 6 illustrates that foam sclerotherapy has a significantly lower cost with a non-significant tendency to produce fewer QALYs. Figure 7 shows that when the additional cost of operating theatre use is included, foam sclerotherapy retains the higher probability of being considered cost-effective at 6 months up to the ceiling WTP of > £130,000 per QALY.
FIGURE 6.
Incremental cost-effectiveness scatterplot for foam sclerotherapy vs. surgery, including additional theatre use costs at 6 months.

FIGURE 7.
Cost-effectiveness acceptability curves for foam sclerotherapy vs. surgery, including additional theatre use costs at 6 months.

Deterministic sensitivity analysis
The overall conclusions drawn from the base-case analysis remain robust to changes in the key costing assumptions and restriction to subgroups (Table 83). Under the first adjustment (excluding routine follow-up costs for foam sclerotherapy), the ICER increases, representing a greater cost saving per QALY lost. Scenario 2 (restricting the analysis to participants with unilateral disease only) has a broadly similar ICER value to that obtained in the base-case analysis. However, by restricting the analysis to participants with unilateral disease and GSV involvement only, the ICER, and hence the case for cost-effectiveness, reduces considerably, with a lower cost saving per QALY lost. A greater cost saving per QALY lost is evident when the SF-6D is used to calculate QALYs. The results appear robust to the missing data, with similar results obtained using multiple imputation to replace missing cost and utility values. For all analyses, the ICER remains above a threshold value of £20,000–30,000 per QALY. These analyses demonstrate that foam sclerotherapy remains the preferred option from a cost-effectiveness perspective, a conclusion that remains robust under a range of plausible assumptions.
| Intervention | Incremental cost (95% CI), £ | Incremental QALYs (95% CI) | ICER (vs. surgery) (£) |
|---|---|---|---|
| Scenario 1: routine follow-up costs excluded following foam sclerotherapy and EVLA | |||
| Surgery | – | – | – |
| Foam sclerotherapy | −753 (−903 to −604) | −0.006 (−0.021 to 0.009) | 127,682 |
| Scenario 2: analysis based on participants with unilateral disease only | |||
| Surgery | – | – | – |
| Foam sclerotherapy | −553 (−694 to −412) | −0.006 (−0.031 to 0.020) | 99,867 |
| Scenario 3: analysis based on participants with unilateral disease and only GSV involvement | |||
| Surgery | – | – | – |
| Foam sclerotherapy | −561 (−722 to −400) | −0.018 (−0.044 to 0.009) | 31,771 |
| Scenario 4: analysis based on QALYs derived from the SF-6D | |||
| Surgery | – | – | – |
| Foam sclerotherapy | −602 (−740 to −464) | −0.001 (−0.013 to 0.011) | 478,055 |
| Scenario 5: analysis based on full multiple imputation data sets | |||
| Surgery | – | – | – |
| Foam sclerotherapy | −553 (−676 to −430) | −0.009 (−0.024 to 0.007) | 64,600 |
| Scenario 6: analysis based on multiple imputation data sets with participants receiving no treatment and follow-up dropped | |||
| Surgery | – | – | – |
| Foam sclerotherapy | −581 (−721 to −441) | −0.008 (−0.023 to 0.006) | 68,901 |
Endovenous laser ablation versus foam sclerotherapy versus surgery
Resource use and costs
This analysis includes all participants recruited in the eight centres who were randomised to all three treatment options. Table 84 illustrates the resources used and corresponding costs of treatment by treatment allocation group (EVLA, foam sclerotherapy and surgery). Forty-nine of the participants randomised to EVLA (n = 210) underwent either foam sclerotherapy (n = 46) or surgery (n = 3). Of the 207 participants randomised to receive foam sclerotherapy, nine received either EVLA (n = 6) or surgery (n = 3). Of the participants randomised to surgery (n = 213), 17 participants underwent either EVLA (n = 8) or foam sclerotherapy (n = 9). More participants in the surgery arm received no treatment (n = 17) than in the foam sclerotherapy (n = 10) or EVLA (n = 5) arms.
| Treatment details | Randomised to EVLA | Randomised to foam sclerotherapy | Randomised to surgery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number randomised | 210 | 207 | 213 | ||||||
| Treatment received | |||||||||
| Presented for EVLA (n, %) | 203 | 96.7 | 6 | 2.9 | 8 | 3.8 | |||
| Presented for foam (n, %) | 46 | 21.9 | 191 | 92.3 | 9 | 4.23 | |||
| Number of foam treatments (n, mean, SD) | 46 | 1.07 | 0.25 | 191 | 1.15 | 0.39 | 9 | 1.00 | 0.00 |
| Presented for surgery (n, %) | 3 | 1.4 | 3 | 1.5 | 183 | 85.9 | |||
| No recorded treatment (n, %) | 5 | 2.4 | 10 | 4.8 | 17 | 8.0 | |||
| Treatment durationsa | |||||||||
| Cumulative procedure time (minutes) | |||||||||
| EVLA (n, mean, SD) | 194 | 59.41 | 28.48 | 204 | 0.86 | 7.11 | 212 | 1.63 | 9.17 |
| Foam sclerotherapy (n, mean, SD) | 197 | 4.41 | 11.50 | 185 | 29.34 | 18.08 | 212 | 0.89 | 4.72 |
| Surgery (n, mean, SD) | 209 | 0.69 | 7.08 | 207 | 1.07 | 8.95 | 197 | 62.60 | 34.06 |
| Treatment costs | |||||||||
| Staff procedure costs | |||||||||
| EVLA (n, mean, SD) | 194 | £326 | £154 | 204 | £5 | £43 | 212 | £10 | £58 |
| Foam sclerotherapy (n, mean, SD) | 197 | £19 | £52 | 185 | £137 | £91 | 212 | £4 | £20 |
| Surgery (n, mean, SD) | 209 | £6 | £64 | 207 | £10 | £88 | 197 | £615 | £362 |
| Recovery time costs (n, mean, SD) | 209 | £32 | £10 | 207 | £4 | £11 | 198 | £74 | £37 |
| Consumable costs (n, mean, SD) | 210 | £322 | £58 | 207 | £50 | £60 | 213 | £150 | £58 |
| Theatre use (n, mean, SD) | 193 | £31 | £96 | 185 | £14 | £46 | 196 | £231 | £122 |
| Equipment costs (n, mean, SD) | 210 | £11 | £4 | 207 | £10 | £4 | 213 | £4 | £2 |
| Preparation costs (n, mean, SD) | 210 | £29 | £12 | 207 | £28 | £12 | 213 | £29 | £9 |
| Total treatment costs | |||||||||
| EVLA (n, mean, SD) | 194 | £699 | £195 | 204 | £11 | £90 | 212 | £23 | £126 |
| Foam sclerotherapy (n, mean, SD) | 197 | £33 | £79 | 185 | £214 | £111 | 212 | £6 | £33 |
| Surgery (n, mean, SD) | 209 | £9 | £94 | 207 | £14 | £121 | 197 | £876 | £457 |
| Total staff procedure costs (n, mean, SD) | 183 | £349 | £163 | 182 | £157 | £118 | 195 | £637 | £340 |
| Total treatment costsb (n, mean, SD) | 183 | £737 | £204 | 182 | £245 | £161 | 195 | £916 | £412 |
The cumulative mean time spent in treatment (see Table 84) was again considerably lower for those participants randomised to foam sclerotherapy than it was for those randomised to surgery. Participants randomised to EVLA spent slightly less time in treatment than those randomised to surgery. It should be noted that the small difference in time spent in treatment between EVLA and surgery (based on intention to treat) was influenced by the fact that a higher proportion of participants in the surgery arm received treatment other than that to which they had been allocated, or no treatment. The mean time from entering to leaving the treatment room was similar for EVLA (59.41 minutes) and surgery (62.60 minutes). The cumulative duration of time spent receiving foam sclerotherapy was considerably lower (29.34 minutes). Based on the treatment duration estimates, coupled with information on the numbers and grades of staff present at each procedure, the estimated staff procedure costs were £326, £137 and £615 for participants randomised to EVLA, foam sclerotherapy and surgery respectively.
Recovery time costs were highest, as expected, for participants randomised to surgery. Consumables were considerably higher in the EVLA group, on account of the high cost of the laser fibre (unit cost £256). Additional costs for the use of theatre were higher in the surgery arm, whereas equipment and preparation costs were generally similar across the EVLA, foam sclerotherapy and surgery groups.
As in the comparison of foam sclerotherapy versus surgery, in this analysis the treatment cost data showed foam sclerotherapy to be the least costly treatment option. Total treatment costs, inclusive of staff costs, consumables, equipment use, clinic/theatre preparation and recovery time costs, were £737, £245 and £916 for participants randomised to EVLA, foam sclerotherapy and surgery respectively.
Table 85 shows the follow-up resource use and cost data up to 6 months post treatment. Numbers of reported GP and other health professional consultations were slightly higher in the surgery group than in the EVLA and foam sclerotherapy groups; they were similar in each of these two groups. Attendance for the 6-week and 6-month assessments were > 80% in all treatment allocation groups. Numbers of unplanned outpatient appointments were similar among the three groups. No hospital admissions were reported in either the EVLA or the foam sclerotherapy group, and only a low number were observed in the surgery arm.
| Post-treatment care costs | Randomised to EVLA | Randomised to foam sclerotherapy | Randomised to surgery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number randomised | 210 | 207 | 213 | ||||||
| Primary care | |||||||||
| GP consultations (n, mean, SD) | 170 | 0.36 | 0.94 | 168 | 0.33 | 0.69 | 156 | 0.57 | 1.28 |
| Other health professional consultations (n, mean, SD) | 170 | 0.18 | 0.58 | 168 | 0.35 | 2.30 | 156 | 0.42 | 1.10 |
| Total cost of GP consultations (n, mean, SD) | 170 | £14 | £40 | 168 | £11 | £24 | 156 | £20 | £45 |
| Total cost of consultations with other health professionals (n, mean, SD) | 170 | £2 | £8 | 168 | £5 | £35 | 156 | £6 | £16 |
| Total primary care costs (n, mean, SD) | 170 | £16 | £43 | 168 | £16 | £42 | 156 | £26 | £47 |
| Secondary care (planned) | |||||||||
| Attendance at 6-week assessmenta (n, mean, SD) | 192 | 184 | 95.8 | 189 | 184 | 97.4 | 180 | 172 | 95.6 |
| Attendance at 6-month assessmentb (n, mean, SD) | 182 | 164 | 90 | 177 | 160 | 90.4 | 166 | 154 | 92.8 |
| 6-week assessment costa (n, mean, SD) | 192 | £169 | £35 | 189 | £172 | £28 | 180 | £168 | £36 |
| 6-month assessment costb (n, mean, SD) | 182 | £159 | £53 | 177 | £159 | £52 | 165 | £163 | £46 |
| Secondary care (unplanned) | |||||||||
| Unplanned medical/surgical outpatient appointments (n, mean, SD) | 210 | 0.30 | 0.77 | 207 | 0.24 | 1.29 | 213 | 0.24 | 0.86 |
| Cost of unplanned outpatient appointments (n, mean, SD) | 210 | £39 | £97 | 207 | £31 | £149 | 213 | £34 | £103 |
| Admissions (n, mean, SD) | 199 | 0 | 0 | 195 | 0 | 0 | 183 | 0.02 | 0.15 |
| Admission costs (n, mean, SD) | 199 | £0 | £0 | 195 | £0 | £0 | 183 | £7 | £49 |
Table 86 shows the mean total NHS costs by treatment allocation group to 6-months’ follow-up. As expected, total mean NHS costs (excluding additional operating theatre costs) were considerably higher in the surgery arm (£1113), with foam sclerotherapy being the least costly (£453), followed by EVLA (£951). A similar ranking was evident when additional theatre costs were included, with the difference in cost between surgery and EVLA and foam sclerotherapy increasing substantially.
| NHS costs | Randomised to EVLA | Randomised to foam sclerotherapy | Randomised to surgery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number randomised | 210 | 207 | 213 | ||||||
| Total NHS costs (excluding all theatre costs) (n, mean, SD) | 142 | £951 | £179 | 144 | £453 | £225 | 141 | £1113 | £332 |
| Total NHS costs (including theatre costs) (n, mean, SD) | 142 | £975 | £205 | 144 | £465 | £239 | 141 | £1367 | £404 |
Health outcomes: European Quality of Life-5 Dimensions and Short Form questionnaire-6 Dimensions
The EQ-5D and SF-6D scores are presented by treatment allocation group and time point in Table 87. An improvement in HRQoL was observed between baseline and 6 weeks in all treatment allocation groups (the increase being more pronounced in the EVLA-treated group in particular). A further increase in HRQoL was evident in all three treatment arms between 6 weeks and 6 months. However, the largest increase during this time interval occurred in participants allocated to foam sclerotherapy. Overall, the mean unadjusted QALYs accrued over 6 months were highest in the EVLA group.
| QoL measure | Randomised to EVLA | Randomised to foam sclerotherapy | Randomised to surgery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number randomised | 210 | 207 | 213 | ||||||
| EQ-5D | |||||||||
| Baseline (n, mean, SD) | 206 | 0.792 | 0.169 | 202 | 0.793 | 0.187 | 207 | 0.777 | 0.184 |
| 6 weeks (n, mean, SD) | 184 | 0.894 | 0.145 | 181 | 0.853 | 0.172 | 164 | 0.864 | 0.180 |
| 6 months (n, mean, SD) | 172 | 0.903 | 0.171 | 167 | 0.884 | 0.192 | 151 | 0.872 | 0.212 |
| Total QALYs (n, mean, SD) | 155 | 0.443 | 0.071 | 159 | 0.431 | 0.078 | 139 | 0.426 | 0.086 |
| SF-6D | |||||||||
| Baseline (n, mean, SD) | 201 | 0.759 | 0.121 | 197 | 0.760 | 0.125 | 198 | 0.752 | 0.131 |
| 6 weeks (n, mean, SD) | 176 | 0.793 | 0.114 | 179 | 0.771 | 0.123 | 159 | 0.758 | 0.120 |
| 6 months (n, mean, SD) | 165 | 0.821 | 0.112 | 164 | 0.802 | 0.127 | 146 | 0.794 | 0.133 |
| Total QALYs (n, mean, SD) | 139 | 0.402 | 0.048 | 151 | 0.391 | 0.056 | 122 | 0.387 | 0.054 |
Incremental cost-effectiveness: primary analysis
Based on an adjusted regression analysis of participants with complete cost and QALY data (n = 389), EVLA was found to produce a small (non-significant) increase in QALYs over both surgery and foam sclerotherapy at 6 months. In comparison with surgery, foam sclerotherapy had an ICER of £103,633 per QALY lost at 6 months, whereas EVLA was found to be ‘dominant’, that is, less costly and marginally more effective (Table 88). The ICER for EVLA versus foam sclerotherapy was £26,107 per QALY gained (EVLA was more costly and more effective than foam sclerotherapy). Therefore, applying a ceiling WTP threshold of £20,000, foam sclerotherapy was the favourable option from a cost-effectiveness perspective, accruing more NMB at the 6-month time frame.
| Intervention | Incremental cost (95% CI), £ | Incremental QALYs (95% CI) | ICER (vs. surgery) (£) | ICER (EVLA vs. foam sclerotherapy) (£) | NMB ranking at Rc £20,000 |
|---|---|---|---|---|---|
| Surgery (n = 122) | – | – | – | – | 3 |
| Foam sclerotherapy (n = 129) | −662 (−826 to −498) | −0.006 (−0.030 to 0.017) | 103,633 | – | 1 |
| EVLA (n = 134) | −156 (–316 to 4) | 0.013 (−0.003 to 0.029) | Dominant | 26,107 | 2 |
The joint distribution of the incremental costs and effects for EVLA and foam sclerotherapy versus surgery, obtained from the bootstrap replicates of the analysis models, are plotted in Figure 8. Based on the analysis of complete case data, the majority of the points for EVLA fall below zero on the cost axis (i.e. cost saving) and above zero on the QALYs axis (increased QALYs), indicating a high probability of EVLA being both less costly and more effective than surgery.
FIGURE 8.
Incremental cost-effectiveness scatterplot for EVLA and foam sclerotherapy vs. surgery at 6 months.
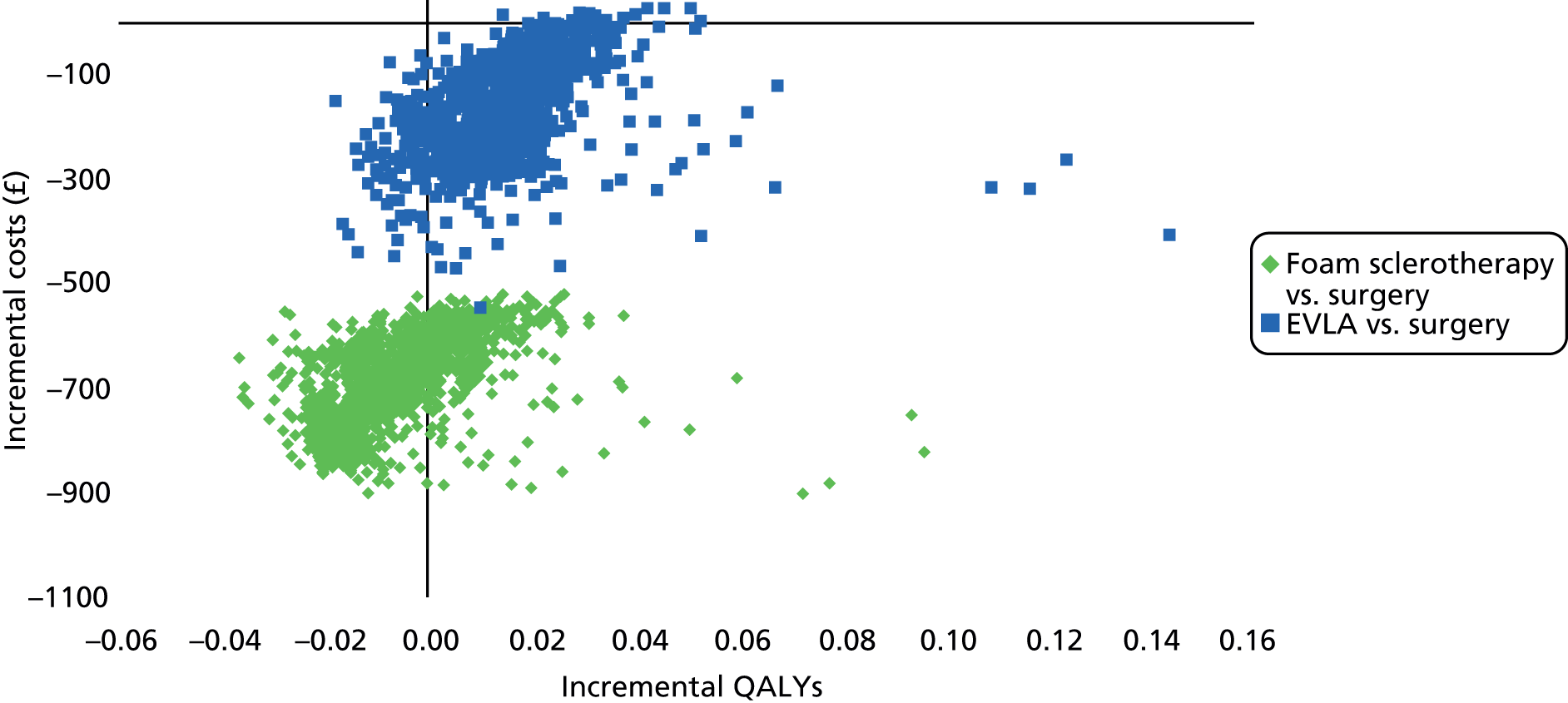
To characterise the uncertainty surrounding the cost-effectiveness estimates, a CEAC (Figure 9) was derived from the bootstrapped estimates of mean incremental costs and effects. At a ceiling WTP threshold of £20,000 per QALY, foam sclerotherapy has a 78.9% chance of being cost-effective, followed by EVLA (20.9%) and surgery (0.2%). However, at a WTP threshold of ≈ £25,000, the CEACs for EVLA and foam sclerotherapy cross, and EVLA has the highest probability of being the optimal strategy on grounds of cost-effectiveness. Applying a ceiling WTP threshold of £30,000 per QALY, EVLA has a 59.4% probability of being cost-effective. To assess the impact on these findings of missing data, multiple imputations were conducted.
FIGURE 9.
Cost-effectiveness acceptability curves for EVLA, foam sclerotherapy and surgery at 6 months.

Incremental cost-effectiveness: secondary analysis including additional costs for use of theatre
Applying the additional cost for the use of an operating theatre results in an increase to the reported incremental cost savings associated with EVLA and foam sclerotherapy (Table 89). A greater cost saved per QALY lost is evident for foam sclerotherapy versus surgery. The addition of extra theatre costs has very little impact on the ICER for EVLA versus foam sclerotherapy, and the NMB rankings remain the same.
| Intervention | Incremental cost (95% CI), £ | Incremental QALYs (95% CI) | ICER (vs. surgery) (£) | ICER (EVLA vs. foam sclerotherapy) (£) | NMB ranking at Rc £20,000 |
|---|---|---|---|---|---|
| Surgery (n = 122) | – | – | – | – | 3 |
| Foam sclerotherapy (n = 129) | −911 (−1104 to −718) | −0.006 (−0.030 to 0.017) | 142,649 | – | 1 |
| EVLA (n = 134) | −395 (−591 to −200) | 0.013 (−0.003 to 0.029) | Dominant | 26,625 | 2 |
The joint distribution of the incremental costs and effects are shown for EVLA and foam sclerotherapy versus surgery in Figure 10. These follow a similar pattern to those reported for the primary analysis, although the clusters fall further below zero on the cost axis (i.e. greater incremental cost saving). The surgical group retains a very low probability of being cost-effective (Figure 11) across all feasible ceiling WTP ratios, and at £20,000 foam sclerotherapy has an 81.4% probability of being cost-effective. The CEACs for EVLA and foam sclerotherapy begin to converge at ≈ £15,000 and again cross at a WTP threshold of ≈ £25,000 per QALY gained.
FIGURE 10.
Incremental cost-effectiveness scatterplot for EVLA and foam sclerotherapy vs. surgery, including additional theatre use costs at 6 months.

FIGURE 11.
Cost-effectiveness acceptability curves for EVLA, foam sclerotherapy and surgery, including additional theatre use costs at 6 months.
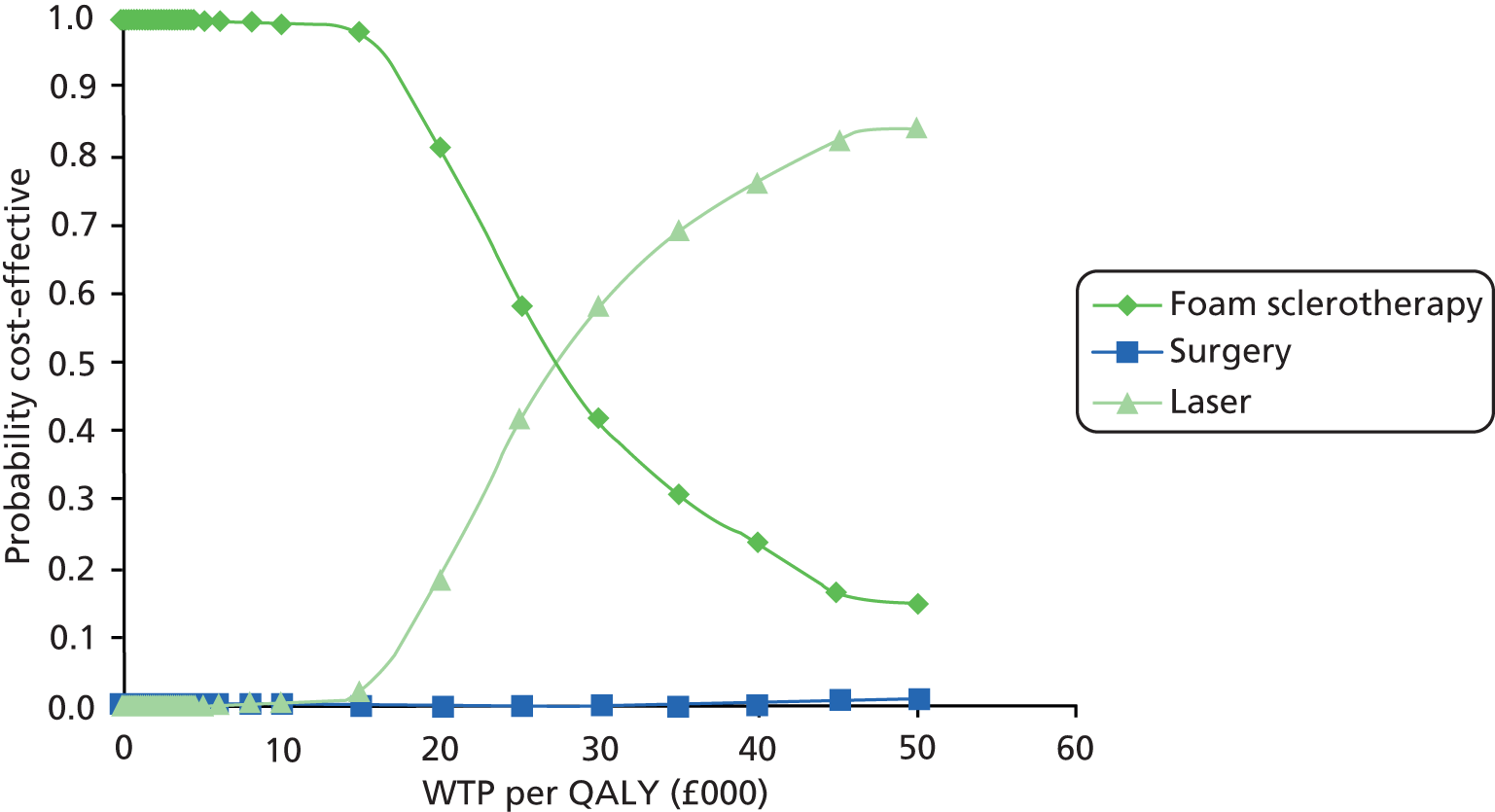
Deterministic sensitivity analysis
The sensitivity analyses conducted (Table 90) demonstrate that foam sclerotherapy appears more cost-effective under several scenarios: specifically, when the routine follow-up cost is excluded (scenario 1), the cost per laser generator usage is incorporated (scenario 3) or QALYs are calculated from the SF-6D (scenario 6). The base-case results also appear robust to the missing data with similar results, favouring foam sclerotherapy, obtained using multiple imputation to replace missing cost and EQ-5D values (scenarios 7 and 8). However, the cost-effectiveness of EVLA versus foam sclerotherapy did improve substantially under certain scenarios. By applying the same staff profile for EVLA as for foam sclerotherapy, EVLA appears the favourable option, with an ICER of £17,146 per QALY gained. Under this scenario (scenario 2), there is a greater cost saving estimate for EVLA versus surgery. The ICER for EVLA versus foam sclerotherapy also falls below the £20,000 threshold when the analysis is restricted to subgroups (i.e. those with unilateral disease only and participants with unilateral and GSV involvement only).
| Intervention | Incremental cost (95% CI), £ | Incremental QALYs (95% CI) | ICER (vs. surgery) (£) | ICER (EVLA vs. foam sclerotherapy) (£) | NMB ranking at Rc £20,000 |
|---|---|---|---|---|---|
| Scenario 1: routine follow-up costs excluded following foam sclerotherapy and EVLA | |||||
| Surgery | – | – | – | – | 3 |
| Foam sclerotherapy | −805 (−998 to −612) | −0.006 (−0.030 to 0.017) | 126,056 | – | 1 |
| EVLA | −292 (−477 to −107) | 0.013 (−0.003 to 0.029) | Dominant | 26,494 | 2 |
| Scenario 2: EVLA performed with same staff profile as foam sclerotherapy | |||||
| Surgery | – | – | – | – | 3 |
| Foam sclerotherapy | −658 (−828 to −488) | −0.006 (−0.030 to 0.017) | 103,058 | – | 2 |
| EVLA | −326 (−424 to −228) | 0.013 (−0.003 to 0.029) | Dominant | 17,146 | 1 |
| Scenario 3: cost of laser machine usage incorporated | |||||
| Surgery | – | – | – | – | 3 |
| Foam sclerotherapy | −662 (−826 to −499) | −0.006 (−0.030 to 0.017) | 103,713 | – | 1 |
| EVLA | −123 (−282 to 37) | 0.013 (−0.003 to 0.029) | Dominant | 27,865 | 2 |
| Scenario 4: analysis based on participants with unilateral disease only | |||||
| Surgery | – | – | – | – | 3 |
| Foam sclerotherapy | −614 (−776 to −451) | −0.009 (−0.048 to 0.030) | 67,780 | – | 2 |
| EVLA | −105 (−271 to 61) | 0.019 (0.001 to 0.036) | Dominant | 18,402 | 1 |
| Scenario 5: analysis based on participants with unilateral disease and only GSV involvement | |||||
| Surgery | – | – | – | – | 3 |
| Foam sclerotherapy | −623 (−797 to −448) | −0.020 (−0.058 to 0.018) | 31,300 | – | 2 |
| EVLA | −114 (−281 to 53) | 0.011 (−0.005 to 0.027) | Dominant | 16,422 | 1 |
| Scenario 6: analysis based on QALYs derived from the SF-6D | |||||
| Surgery | – | – | – | – | 3 |
| Foam sclerotherapy | −662 (−826 to −498) | −0.002 (−0.020 to 0.016) | 340,775 | – | 1 |
| EVLA | −156 (−316 to 4) | 0.012 (−0.001 to 0.026) | Dominant | 35,920 | 2 |
| Scenario 7: analysis based on full multiple imputation data sets | |||||
| Surgery | – | – | – | – | 3 |
| Foam sclerotherapy | −585 (−730 to −440) | −0.010 (−0.031 to 0.010) | 56,217 | – | 1 |
| EVLA | −103 (−255 to 47) | 0.007 (−0.008 to 0.021) | Dominant | 31,589 | 2 |
| Scenario 8: analysis based on multiple imputation data sets with participants receiving no treatment and follow-up dropped | |||||
| Surgery | – | – | – | – | 3 |
| Foam sclerotherapy | −621 (−782 to −459) | −0.009 (−0.030 to 0.011) | 65,523 | – | 1 |
| EVLA | −126 (−281 to 28) | 0.007 (−0.007 to 0.021) | Dominant | 30,723 | 2 |
The overall conclusions drawn from the base-case analysis again remain robust to several of the sensitivity analyses presented. EVLA could offer a cost-effective alternative if the treatment is performed with a similar staff profile to foam sclerotherapy. The subgroup analyses results also suggest that EVLA continues to dominate surgery, and has a favourable ICER when compared with foam sclerotherapy.
Costs directly incurred by participants and indirect costs
A further analysis was conducted incorporating both participant and indirect costs into the analysis. Participant costs comprised three main elements: self-purchased health care; travel costs for making a return visit(s) to NHS health care; and time costs of travelling and attending NHS health care. Indirect costs were defined as the production losses resulting from treatment when the participant was unable to return to normal activity. The cost of days lost was estimated using the same unit costs applied to participant time. These were calculated as outlined above (see Methods). Where a participant’s own reported costs associated with a specific type of health service visit were missing, the mean cost by centre for that type of visit was applied.
Foam sclerotherapy versus surgery
Table 91 illustrates the mean indirect and participant costs of attending GP and outpatient appointments and also hospital admissions over the follow-up period. Mean indirect costs for foam sclerotherapy and surgery were £420 and £989 respectively. The costs associated with attending GP appointments were similar between groups, whereas the cost of attending outpatient appointments was somewhat higher in the foam sclerotherapy group. This was due to the assumption that participants would routinely attend a follow-up outpatient assessment appointment after foam sclerotherapy, but would not do so after surgery. The surgery participants incurred a higher mean cost in terms of hospital admissions, but this cost was very small owing to the low number of additional admissions (related to varicose veins treatment) which occurred during the follow-up period. Combining the indirect and participant costs with the total NHS costs has very little impact on the overall findings (Table 92).
| Costs | Foam sclerotherapy | Surgery | ||||
|---|---|---|---|---|---|---|
| Number of participants | 286 | 289 | ||||
| Indirect costs (n, mean, SD) | 223 | £420 | £554 | 220 | £989 | £788 |
| Participant GP cost (n, mean, SD) | 234 | £4 | £18 | 213 | £4 | £13 |
| Participant outpatient cost (n, mean, SD) | 286 | £17 | £53 | 289 | £4 | £13 |
| Participant hospital cost (n, mean, SD) | 272 | £0 | £0 | 254 | £1 | £5 |
| Total participant cost (n, mean, SD) | 232 | £22 | £63 | 210 | £9 | £21 |
| Total costs primary analysis | ||||||
| Total participant, NHS and indirect costs (n, mean, SD) | 180 | £895 | £611 | 175 | £2053 | £889 |
| Intervention | Incremental costs (95% CI), £ | Incremental QALYs (95% CI) | ICER (£) |
|---|---|---|---|
| Surgery | – | – | – |
| Foam sclerotherapy | −1173 (−1371 to −974) | −0.006 (−0.021 to 0.009) | 198,802 |
To explore the uncertainty surrounding the cost-effectiveness estimate, a CEAC was derived from the results of 1000 bootstrapped replicates of mean incremental costs and effects. Figure 12 illustrates that foam sclerotherapy has a 100% probability of being the preferred option from a cost-effectiveness perspective at 6 months.
FIGURE 12.
Cost-effectiveness acceptability curves, including NHS, indirect and participant costs: foam sclerotherapy vs. surgery.

Endovenous laser ablation versus foam sclerotherapy versus surgery
Table 93 documents the mean indirect and participant costs associated with use of health services over the follow-up period. The surgery treatment strategy incurred the highest mean indirect costs (£1075), followed by EVLA (£628) and foam sclerotherapy (£466). Mean participant costs of attending GP appointments were slightly higher in the surgery group, whereas higher costs of attending outpatient appointments were observed in the EVLA and foam sclerotherapy groups; again, this was due to the assumed need to attend a routine assessment appointment following these treatment modalities. As for the analysis of foam sclerotherapy versus surgery, combining the mean indirect and mean participant costs with NHS costs had very little impact on the incremental cost-effectiveness findings in this analysis (Table 94).
| Costs | EVLA | Foam sclerotherapy | Surgery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of participants | 210 | 207 | 213 | ||||||
| Indirect costs (n, mean, SD) | 174 | £628 | £653 | 164 | £466 | £597 | 164 | £1075 | £852 |
| Participant GP cost (n, mean, SD) | 170 | £2 | £7 | 168 | £4 | £21 | 156 | £5 | £15 |
| Participant outpatient cost (n, mean, SD) | 210 | £14 | £22 | 207 | £14 | £38 | 213 | £4 | £14 |
| Participant hospital cost (n, mean, SD) | 199 | £0 | £0 | 195 | £0 | £0 | 183 | £1 | £6 |
| Total participant cost (n, mean, SD) | 167 | £17 | £24 | 167 | £19 | £48 | 153 | £11 | £23 |
| Total participant, NHS and indirect costs (n, mean, SD) | 124 | £1541 | £632 | 127 | £945 | £661 | 129 | £2196 | £930 |
| Intervention | Incremental cost (95% CI), £ | Incremental QALYs (95% CI) | ICER (vs. surgery) (£) | ICER (EVLA vs. foam sclerotherapy) (£) | NMB ranking at Rc £20,000 |
|---|---|---|---|---|---|
| Surgery | – | – | – | – | 3 |
| Foam sclerotherapy | −1271 (−1459 to −1083) | −0.006 (−0.030 to 0.017) | 198,977 | – | 1 |
| EVLA | −660 (−849 to −471) | 0.013 (−0.003 to 0.029) | Dominant | 31,546 | 2 |
To characterise the uncertainty surrounding the cost-effectiveness estimates, a CEAC was derived (Figure 13). At a ceiling WTP threshold of £20,000 per QALY, foam sclerotherapy had an 85.4% probability of being cost-effective, whereas EVLA had a 14.6% chance and surgery had a 0% probability of being the preferred option. At a WTP threshold of £30,000 per QALY, foam sclerotherapy retains the highest probability (54.5%) of being the cost-effective option, followed by EVLA (probability of 45.5%), and the probability of surgery being the preferred option does not increase. The CEACs for foam sclerotherapy and EVLA do not cross until the WTP threshold is > £30,000.
FIGURE 13.
Cost-effectiveness acceptability curves, including NHS, indirect and participant costs: EVLA vs. foam sclerotherapy vs. surgery.

Discussion
Summary of key results
Based on the primary analysis of foam sclerotherapy versus surgery data, foam was found to result in a cost saving of £102,106 per QALY lost in comparison with surgery. Considering that society might be required to receive compensation of at least £20,000 for a QALY loss, this result is highly favourable for foam, suggesting good value for money for the NHS in comparison with surgery. The high value of the ICER is driven by the comparatively modest cost of foam sclerotherapy treatment. The results were robust to a range of alternative assumptions relating to unit cost estimation, restriction of the analysis to specific subgroups and multiple imputation of missing data.
For the analysis of EVLA versus foam sclerotherapy versus surgery, very similar results were obtained for the comparison between foam sclerotherapy and surgery. From the three-way comparison of data at 6-months follow-up, EVLA was found to be less costly and marginally more effective than surgery, and therefore ‘dominant’ (i.e. better than surgery in terms of both costs and QALYs). In the base-case analysis it was estimated that the ICER for EVLA versus foam sclerotherapy was ≈ £26,000. Therefore, adopting the same ceiling WTP ratio of £20,000 per QALY, foam sclerotherapy produced the greatest NMB at 6 months, followed by EVLA, followed by surgery. Considering the results of the probabilistic analysis, foam sclerotherapy had the greatest probability of being cost-effective (≈ 78%) at a threshold value of £20,000 per QALY, whereas EVLA had a ≈ 21% chance and surgery a < 1% chance. However, increasing the WTP threshold to £30,000, EVLA was found to have the higher probability (≈ 60%) of being the most cost-effective option.
Further, EVLA was found to generate greater NMB under certain scenarios presented in the sensitivity analysis, namely, when EVLA incorporated a similar staff profile to foam sclerotherapy (performing the procedure with the presence of a surgeon and one or two additional nurses); when the analysis was restricted to include participants with unilateral disease only; and when the analysis was based on participants with unilateral and GSV involvement only. The base-case results demonstrated that EVLA generated a cost saving of £156 compared with surgery. This cost saving increased when EVLA was assumed to adopt a similar staff profile to foam sclerotherapy (£326). However, when the cost per usage of the laser generator (assumed as loaned free of charge) was included in the EVLA treatment, the cost saving versus surgery reduced (£123).
Explanation of results
The ICER for foam sclerotherapy versus surgery in both analyses (foam sclerotherapy vs. surgery and EVLA vs. foam sclerotherapy vs. surgery) showed that foam sclerotherapy had reduced effectiveness but at a lower cost. The ICER remained robust to all scenarios presented in the sensitivity analyses. On the basis of the data presented, it would be accepted NHS practice to conclude that foam sclerotherapy would be considered the favourable option from a cost-effectiveness perspective at the 6-month time frame.
Similar results were found for foam sclerotherapy versus surgery in the three-way comparison. The ICER remained robust to all scenarios presented in the sensitivity analysis. EVLA was found to give a small increase in QALYs compared with both foam sclerotherapy and surgery. EVLA was, on average, less costly and more effective than surgery, and was thus dominant. However, the ICER for EVLA versus foam sclerotherapy showed that although it produced higher QALYs, it did so at an increased cost. The ICER for EVLA versus foam sclerotherapy in the base-case analysis was higher than the accepted threshold, and for this reason foam sclerotherapy remains the preferred option from a cost-effectiveness perspective at 6 months.
Strengths and limitations
One of the key strengths of this study was the multicentre RCT design, which comprised 11 centres around the UK, adding to the applicability of results more widely. The inclusion of a comprehensive cost-effectiveness analysis, based on best practice guidelines, within a RCT is another key strength. A detailed micro-costing method was implemented, ensuring that variations in treatment strategy costs across participating centres were accurately reflected in the analysis. To ensure that results were robust to the primary outcome measure (EQ-5D), QALYs were also estimated using responses from the SF-36 (converted into the SF-6D).
Some data were missing for cost and QALY outcomes. However, multiple imputation of missing resource use, cost and EQ-5D data did not alter the conclusions of the analysis. It should be noted that the results of the trial-based cost-effectiveness analysis pertain only to a short period of follow-up (6 months). Therefore, any differences across treatment arms in terms of recurrence over the longer term, and the associated cost and QALY implications, were not addressed in this phase of the study. For this reason, we developed an economic model (see Chapter 10) to extrapolate results from the 6-month trial-based analysis over a longer time horizon. In addition, further data will be collected from participants after 5 years.
Summary/conclusions
To summarise, within the trial period it was found that foam sclerotherapy had the highest probability of being the most cost-effective option under base-case assumptions. These results remained robust under all scenarios presented in the sensitivity analysis for foam sclerotherapy versus surgery. However, our analysis shows that under certain circumstances in the three-arm analysis (EVLA vs. foam sclerotherapy vs. surgery), EVLA may generate the greatest NMB in comparison with foam sclerotherapy and surgery. Data from the subgroup analyses would suggest that EVLA is more likely to be cost-effective in the subgroup of participants treated for unilateral GSV involvement only. Further, if EVLA could be performed using a similar staff profile to foam sclerotherapy, it may offer a cost-effective approach to treatment.
Although these results provide useful information on the short-term costs and effects of the different procedures, they are not sufficient for determining the optimal approach to treatment over a longer time horizon. This is because any differential effects of the treatments on costs and outcomes may persist far into the future. In addition, the risk of clinical recurrence beyond 6 months may be found to differ significantly between the alternative treatment strategies, altering the cost-effectiveness rankings of treatments in the long term. Therefore, Chapter 10 reports on the findings of a decision modelling exercise, designed to extrapolate these cost-effectiveness findings over extended time horizons. Extended follow-up of the CLASS cohort to 5 years will provide a means of validating this modelling exercise in the future.
Chapter 10 Cost-effectiveness modelling
Introduction
The aim of this chapter is to assess the cost-effectiveness of the alternative treatment modalities for varicose veins over the longer term. The within-trial analysis provides useful information on the short-term costs and effects of the alternative interventions, but it is important to discover whether or not the effects of treatment on outcomes persist into the future. This is because recurrence of varicose veins in the long term after any particular treatment will lead to reductions in QoL and further costs. In order to address this issue, additional information was gained through modelling expected future costs and outcomes over longer time horizons (through to 5 and 10 years). The specific objectives of this chapter are to:
-
describe the development of a model to extrapolate the costs and consequences of surgery, foam sclerotherapy and EVLA treatments over extended time horizons of 5 and 10 years
-
compare the treatment modalities incrementally in terms of costs and QALYs, using the NMB framework to identify the optimal strategy.
Methods
General structure of the model
A cost-effectiveness Markov model was developed using TreeAge Pro 2012, R2.1 (TreeAge Software, Inc., Williamstown, MA, USA). The model was based on care pathways developed in consultation with clinical members of the team, describing the possible temporal sequences of events that patients may experience following their initial treatment. The model structure describes the logical and temporal sequence of events from the initial treatment until the patient’s death.
The model was constructed to simulate transitions between discrete health states on a 6-month time interval (Markov cycle). For the first 6-month cycle, model data were taken directly from CLASS trial data. Beyond 6 months, the best available evidence on the risk of recurrence following initial treatment with the alternative treatment modalities was used to model subsequent costs and consequences over an extended time horizon. The model was designed to inform the optimal approach to treatment in patients considered clinically suitable for all three surgical procedures (as was the case for all patients randomised in the CLASS trial). It simultaneously compared the three treatment modalities, but did not include a no treatment (or conservative management) arm. As the model focused on the three-way treatment comparison, the model input parameters were based only on data from centres that randomised patients to all three procedures.
Modelled cohort
The model was analysed for a cohort of patients with mean age and sex matching those of participants recruited at CLASS trial sites which offered randomisation between all three treatment modalities. Initially, the model was populated based on cost and utility data obtained from all randomised patients, but the impact of basing model inputs on patients with unilateral disease only (and those with unilateral disease and GSV involvement only) was also assessed.
Definition of health states
For the base-case analysis, a simple model was specified using five main states: ‘pre-treatment primary varicose veins’, ‘post-primary treatment of varicose veins’ (following surgery, foam sclerotherapy or EVLA), ‘clinical recurrence’, ‘post treatment of recurrent varicose veins’ and ‘death’ from all causes (Figure 14). All patients commenced in the ‘pre-treatment primary varicose veins’ state, and then moved to the corresponding post-treatment state for the start of the second model cycle. Over subsequent model cycles, a constant risk of clinical recurrence, specific to the initial treatment modality received, was applied to patients in the post-treatment states. Following clinical recurrence, patients could either present for further treatment (surgery, foam sclerotherapy or EVLA), and then transit to the ‘post treatment of recurrent varicose veins’ state, or they could remain in the ‘clinical recurrence’ state. For patients experiencing a second recurrence following secondary treatment, a simplifying assumption was made that these patients would not proceed to further treatment. The impact of allowing any number of further repeat treatments for clinical recurrence was also assessed through sensitivity analysis.
FIGURE 14.
Diagram showing the model structure. pACM, probability of death from all causes; Tx1, ultrasound-guided foam sclerotherapy; Tx2, surgery; Tx3, EVLA.
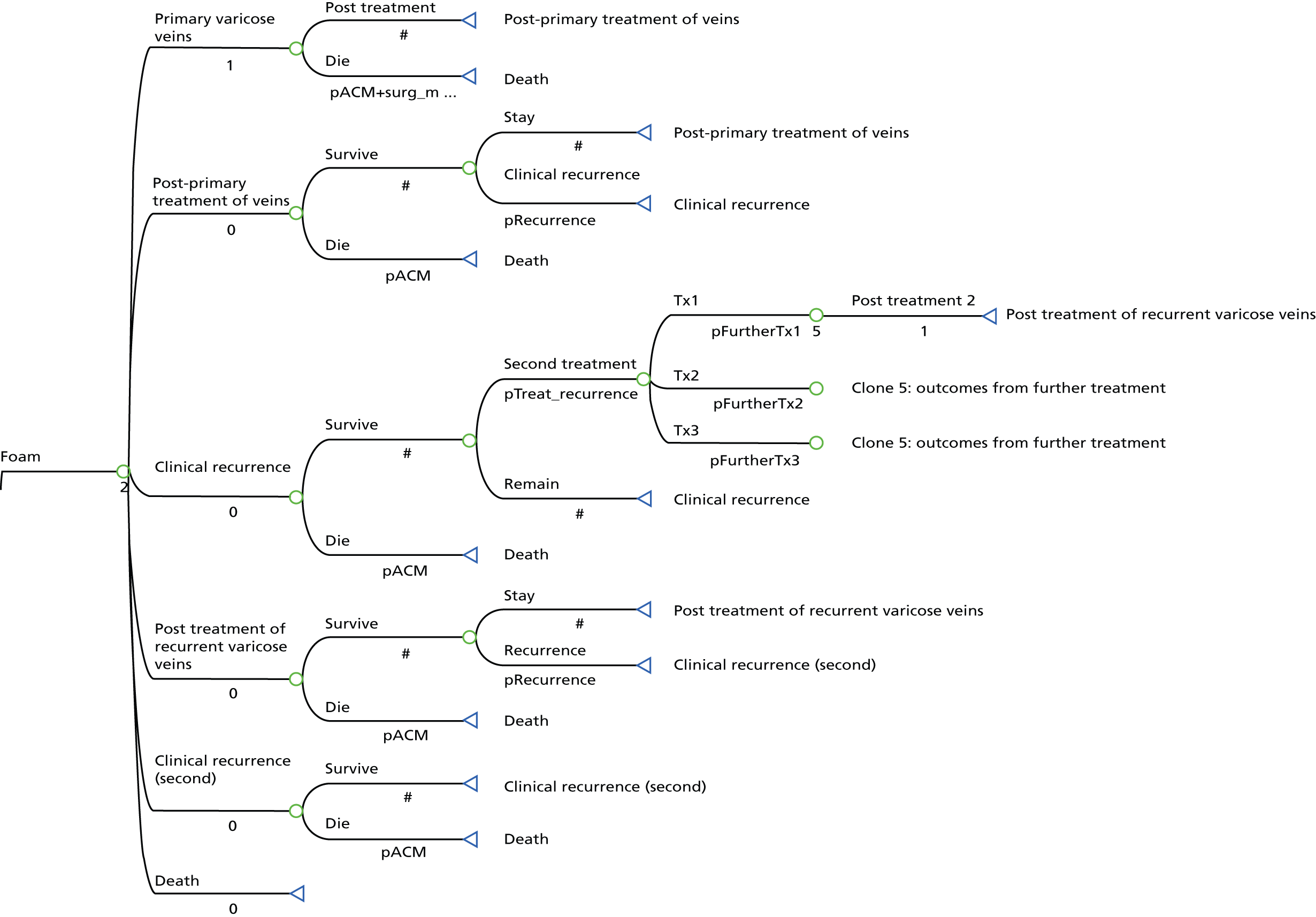
Clinical recurrence
To inform the risk of recurrence in the model, existing systematic reviews assessing the longer-term clinical effectiveness of the alternative treatment modalities for varicose veins were consulted. 28,52,56,93 The most recent of these was carried out for NICE Clinical Guideline 168 on varicose veins in the legs. 28 Within the review prepared for the NICE evaluation, the authors presented the findings of a random-effects network meta-analysis of treatment-specific probabilities of clinical recurrence over time. This analysis was based on summary data from eight RCTs that included clinical recurrence as an outcome, seven comparing surgery against endothermal treatment (including EVLA), two comparing surgery against foam sclerotherapy and one comparing foam sclerotherapy against endothermal treatment. The duration of follow-up in the majority of included studies was 12 or 24 months, although one small trial comparing surgery and endothermal treatment reported follow-up to 36 months. 94 It should be noted that the endothermal treatments (EVLA and endovenous radiofrequency ablation) were pooled for the purpose of this meta-analysis. The monthly probabilities of recurrence presented in the updated NICE guideline were applied in the model, transformed into constant 6-month probabilities (Table 95). Application of these probabilities yields predicted recurrence rates at 5 years of ≈ 40% for foam sclerotherapy, ≈ 37% for surgery and ≈ 29% for EVLA. It should be noted that plans are in place to assess clinical recurrence at 5 years after treatment through extended follow-up of the CLASS trial participants, but these data are not yet available. Sensitivity analysis was used to assess the impact of varying the recurrence rates on the cost-effectiveness of the alternative treatment modalities.
| Treatment | Mean | Standard error | Assumed distributional form |
|---|---|---|---|
| Surgery | 0.008818 | 0.00306 | Beta |
| Foam sclerotherapy | 0.0115 | 0.009929 | Beta |
| EVLA | 0.006532 | 0.003448 | Beta |
Utilities estimates
The utility estimate for pre-treatment primary varicose veins was taken as the mean baseline EQ-5D score across all treatment arms of the CLASS trial. For the base-case model, the utility values applied to the post-treatment states were derived by regressing the 6-month EQ-5D values on treatment group, baseline EQ-5D score and the minimisation variables (Table 96). The method of recycled predictions84 was used to recover the mean estimated 6-month utility value following surgery, and the estimated mean utility increments associated with foam sclerotherapy and EVLA were applied to patients receiving these treatment modalities. Cluster robust standard errors, obtained from the regression analysis of utility data, were used to fit distributions to the incremental utility parameters for probabilistic sensitivity analysis (PSA). Further, the utility pay-off in the first model cycle was adjusted so that the QALYs generated in the first 6 months were consistent with those derived from the within-trial analysis. It was assumed that following clinical recurrence, patients would revert back to the pre-treatment EQ-5D utility value. This assumption is conservative in favour of treatments with higher clinical recurrence rates, because some previous evidence suggests that the utility decrement associated with recurrence might be greater than the utility increment associated with initial treatment success. 28
| State | EQ-5D utility value | Standard error | Assumed distributional form |
|---|---|---|---|
| Pre-treatment primary varicose veins | 0.790 | 0.009 | Beta |
| Post-treatment for primary veins (surgery) | 0.884 | 0.0104a | Beta |
| Post-treatment utility increment (foam sclerotherapy) | −0.009 | 0.018b | Normal |
| Post-treatment utility increment (EVLA) | 0.016 | 0.0121b | Normal |
| Clinical recurrence | 0.790 | 0.009 | Beta |
Resource use and costs
The costs included in the model were derived from the analysis of patient-level cost data collected in the CLASS trial (reported in Chapter 9), supplemented by assumptions about further resource use associated with clinical recurrence. The model-based analysis adopted a health and social care perspective, although no social care costs were identified as being relevant to the comparison of the alternative treatment modalities.
Initial treatment costs
For the initial 6-month cycle, we employed the same general linear regression model used to analyse the CLASS patient-level cost data. However, for the purpose of populating the economic model, the analysis of cost data was not restricted to patients with complete QALY data. The approach of recycled predictions was used to recover the estimated mean cost in the surgery arm (at 6 months), adjusted for any potential imbalance in the covariates at baseline. This cost accounts for the initial treatment, any perioperative complications experienced and any subsequent resource use occurring within the first 6 months of follow-up (Table 97). The incremental costs associated with foam sclerotherapy and EVLA, compared with surgery (derived from the regression model), were applied to recover the expected costs for patients receiving these treatment modalities in the model.
Costs of recurrence
Assumptions were required regarding the approach to management of clinical recurrence. In the base case it was assumed that the approach to initial treatment would not determine the approach to treating clinical recurrence. Initially, the assumptions regarding the treatment of clinical recurrence were taken from the modelling exercise undertaken to inform the NICE guideline. 28 Based on consultation with the NICE Guideline Development Group (GDG), the authors of the NICE report assumed that 75% of patients experiencing a recurrence would receive further interventional treatment, and that regardless of initial treatment modality, 12% would receive surgery, 42% would receive foam and 46% would receive EVLA. For the costs of these procedures for recurrent varicose veins, we applied the same estimated 6-month mean costs as applied for the initial treatment, derived from our analysis of the directly collected trial data (see Table 97). However, we also assessed the impact of applying the NHS reference cost for the treatment of recurrent varicose veins86 in a sensitivity analysis.
It was further assumed that patients receiving treatment for recurrence would spend 1 year on average in the ‘clinical recurrence’ state prior to receiving treatment. The impact of this assumption was assessed through sensitivity analysis. In the economic model developed for the NICE guideline, it was also assumed that patients experiencing a clinical recurrence would, on average, incur the cost of a further 2.5 GP visits (£75)87 and subsequent assessment in an outpatient setting (£165) – including a duplex scan (£52.84)86 – prior to treatment. To retain consistency with this previous analysis, these additional costs were also incorporated in the current model.
Complications
Although complications have the potential for significant cost and outcome implications, it was assumed that serious long-term complications would be rare and that these would not differentially affect resource use across the treatment groups. In addition, the differential impact of any short-term complications on resource use is implicitly captured in the model via application of the 6-month cost estimates obtained from the trial-based cost-effectiveness analysis (see Chapter 9), while the impact of any complications on health status is captured through application of the 6-month QALY and EQ-5D estimates. Therefore, resource use associated with any long-term complications, other than clinical recurrence, was not explicitly included in the model.
Analysis
The model was initially run over a 5-year time horizon, which is the duration to which the CLASS study follow-up will ultimately be extended in order to provide a validity check on initial model-based estimates of cost-effectiveness. Following this, we explored the impact of extending the model time horizon to 10 years, assuming constant risks of clinical recurrence. Future costs and QALYs were discounted at a rate of 3.5% per annum in line with the NICE reference case. 96 The model-based analysis was restricted to the health and social care perspective, comparing the expected health service costs and QALYs generated by each treatment modality incrementally from the least costly to the most costly. In order to help identify the optimal approach to treatment, the NMB framework was used, where the NMB for any given strategy is equal to the accrued QALYs multiplied by the ceiling ratio (Rc) of WTP per QALY gained, minus the strategy costs.
To help interpret the results for decision-making, the accepted value of £20,000 was placed on the Rc of WTP per QALY,96 and the strategy generating the greatest NMB at this value of Rc identified. In order to characterise the uncertainty surrounding selection of the optimal strategy, the model was analysed probabilistically and the mean costs and effects reported. Each input parameter in the model was assigned an appropriate distribution reflecting the uncertainty surrounding it. The probabilistic analysis proceeded by randomly selecting a value from the assigned distribution for each model parameter, and recomputing the model results. This process was repeated 10,000 times to produce an empirical estimate of the uncertainty surrounding the model-based estimates of NMB. Distributions for the incremental cost and utility parameters (for foam and EVLA vs. surgery) applied in the model were defined using the mean regression-based estimates with cluster robust standard errors (see Tables 96 and 97).
Deterministic sensitivity analysis
Further deterministic sensitivity analysis was undertaken to assess the robustness of the findings to various parameter and structural assumptions applied in the base-case analysis. Deterministic sensitivity analysis assessed the impact on findings of:
-
applying additional overhead costs to reflect the opportunity cost of operating theatre space for procedures using this resource
-
basing the model cost and utility input parameters on the estimates obtained from the trial-based multiple imputation analysis
-
basing the model cost and utility input parameters on the trial-based estimates obtained for patients with unilateral disease only (to eliminate any potential bias in estimated cost-effectiveness associated with patients receiving simultaneous treatment for their contralateral leg)
-
altering assumptions about the proportional distribution of patients receiving different treatment modalities (surgery, foam sclerotherapy and EVLA) for clinical recurrence
-
altering assumptions about the proportion of patients with clinical recurrence who proceed to further surgical treatment
-
increasing/decreasing the duration of time between clinical recurrence and subsequent treatment
-
allowing patients to come back for further surgical treatment for any number of clinical recurrences, over an extended time horizon
-
applying the NHS reference cost for treatment of recurrent unilateral varicose veins, rather than the bottom-up trial-based estimates of mean procedure costs
-
assuming equal utility between treatment arms at 6 months
-
basing utility inputs on responses to the SF-36, scored via the SF-6D.
Sensitivity to alternative structural assumptions
Given the uncertainty surrounding the estimated differences in generic HRQoL between treatment arms at 6 months, an alternative model specification was also assessed using pre- and post-treatment health states defined by clinical severity. Within this model it was assumed that changes in QoL are determined solely by transitions across the clinical severity states, and are not otherwise influenced by the treatment modality received.
Four mutually exclusive clinical states were defined based on the VCSS. 97 This instrument was chosen as the basis for defining the health states as it reports the presence of varicose veins and the severity of symptoms based on an objective clinical assessment, rather than the patients’ subjective perception. It was felt desirable that the severity states in the model should reflect underlying clinical status rather than perceived status. The defined states were also found to correlate reasonably well with participants’ EQ-5D scores, which reflect participants’ self-reported general health status and are used for estimating QALYs within the model. The states chosen for this model are summarised in Table 98.
| State | Description |
|---|---|
| State 2: pre treatment | VCSS score 1–3 prior to treatment |
| State 3: pre treatment | VCSS score 4–6 prior to treatment |
| State 4: pre treatment | VCSS score > 6 prior to treatment |
| State 1: post treatment | VCSS score 0 post treatment |
| State 2: post treatment | VCSS score 1–3 post treatment |
| State 3: post treatment | VCSS score 4–6 post treatment |
| State 4: post treatment | VCSS score > 6 post treatment |
| State 2: recurrence | VCSS score 1–3 post recurrence |
| State 3: recurrence | VCSS score 4–6 post recurrence |
| State 4: recurrence | VCSS score > 6 post recurrence |
| Dead | Death from all causes |
Figure 15 provides a diagram of this alternative model structure. The modelled cohort was initially spread across the pre-treatment states 2–4, reflecting the fact that all participants in the CLASS trial had varicose veins at baseline. Probabilities of transition to the alternative post-treatment states were derived using a multivariate ordinal logistic regression model. The ordinal post-treatment VCSS state at 6 months was regressed on treatment allocation group, pre-treatment VCSS state and the minimisation factors [age group, sex, vein involvement (GSV/SSV/both) and laterality (unilateral/bilateral)]. This model predicts the probability of transition to each of the clinical severity states at 6 months, based on treatment allocation group adjusted for baseline disease status and the minimisation covariates. These treatment-specific predicted probabilities of moving to the alternative post-treatment severity states were applied in the decision model.
FIGURE 15.
Diagram showing the alternative model structure. Drop 1, drop 1 severity state; Drop 2, drop 2 severity states; pACM, probability of death from all causes; pGrp1, probability of moving to clinical severity state 1 following treatment; pGrp2, probability of moving to clinical severity state 2 following treatment; pGrp3, probability of moving to clinical severity state 3 following treatment; pGrp4, probability of moving to clinical severity state 4 following treatment; propGrp2, proportion starting model in clinical severity state 2; propGrp3, proportion starting model in clinical severity state 3; propGrp4, proportion starting model in clinical severity state 4; Tx1, ultrasound-guided foam sclerotherapy; Tx2, surgery; Tx3, EVLA.
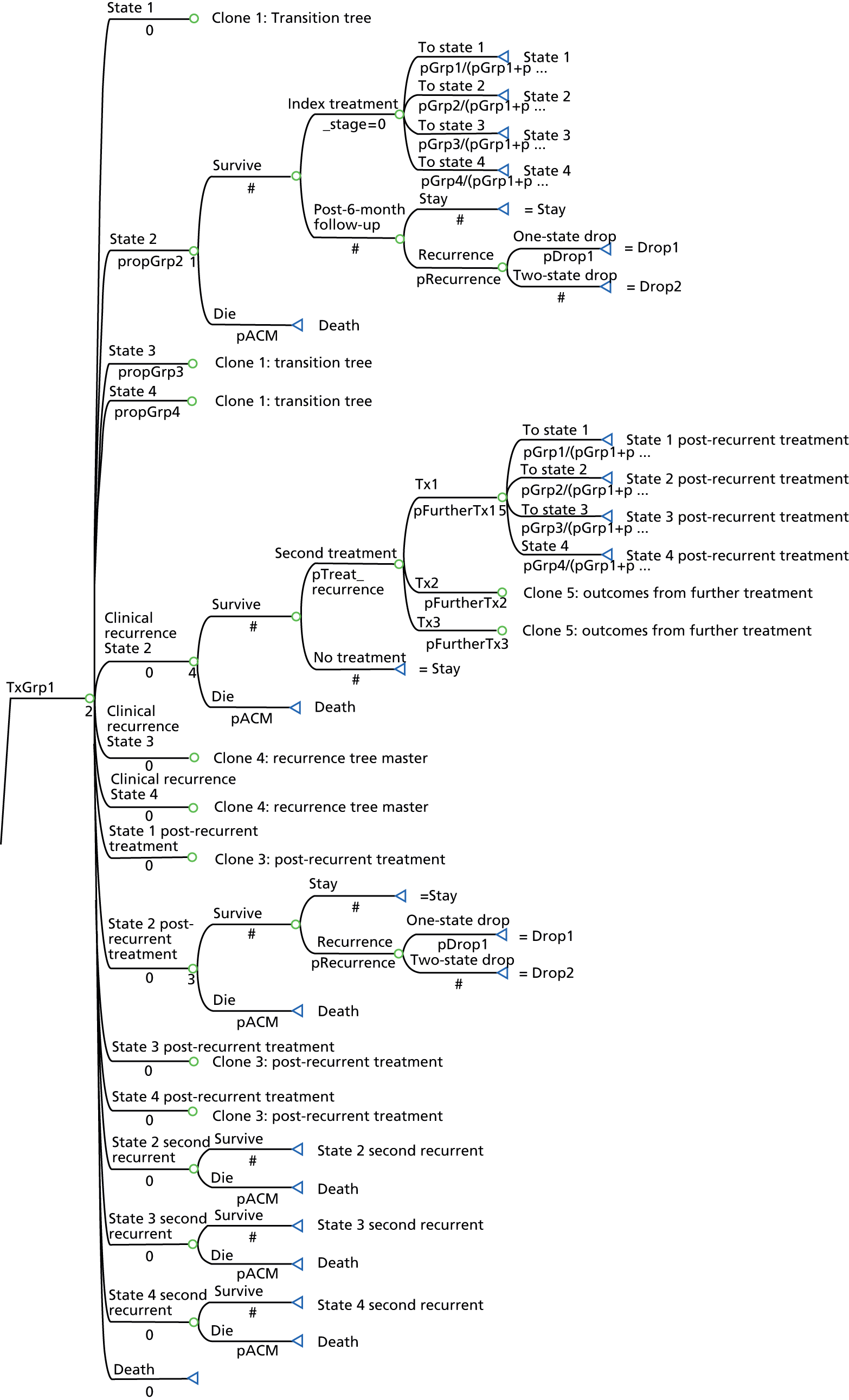
The utility value for each model state was taken as the estimated mean EQ-5D index score for patients in that state (Table 99). In this structural specification, no adjustments were made to the mean state utility values by treatment allocation group. This modelling assumption constrains the modelled QALYs to be influenced only by the impact of the alternative treatments on the probabilities of transition to the different clinical health states following treatment, as well as the risk of recurrence over time. A further assumption applied in this model was that clinical recurrence results in the patient moving down one clinical severity state (apart from those patients already in state 4 following initial treatment), taking the EQ-5D utility value back to the pre-treatment level for that state. This alternative model was analysed in the same way as described above, using 10,000 probabilistic iterations.
| State | EQ-5D utility value | Standard error | Distribution for PSA |
|---|---|---|---|
| State 2: pre treatment | 0.809 | 0.0117 | Beta |
| State 3: pre treatment | 0.802 | 0.0083 | Beta |
| State 4: pre treatment | 0.745 | 0.0179 | Beta |
| State 1: post treatment | 0.940 | 0.0104 | Beta |
| State 2: post treatment | 0.894 | 0.0099 | Beta |
| State 3: post treatment | 0.799 | 0.0338 | Beta |
| State 4: post treatment | 0.754 | 0.0203 | Beta |
| State 2: recurrence | 0.809 | 0.0117 | Beta |
| State 3: recurrence | 0.802 | 0.0083 | Beta |
| State 4: recurrence | 0.745 | 0.0179 | Beta |
Results
Base-case analysis
Table 100 presents the findings of the base-case modelling exercise over a 5-year time horizon. Mean costs and effects are reported based on 10,000 probabilistic iterations of the model. The findings indicate increased costs and QALYs associated with EVLA in comparison with foam sclerotherapy. The incremental cost per QALY gained (EVLA vs. foam) is below the accepted Rc of £20,000. Surgery is associated with increased costs compared with EVLA, but on average produces slightly fewer QALYs over 5 years. This is driven by the slightly lower number of QALYs observed for surgery at 6-months follow-up, a slightly lower EQ-5D score applied at 6 months and beyond, and a slightly higher clinical recurrence rate applied to surgery compared with EVLA. This leads to surgery being dominated by EVLA, that is, EVLA is less costly and more effective. Applying a ceiling WTP ratio of £20,000 per QALY to help interpret the probabilistic results, EVLA had the highest probability (78.7%) of being cost-effective, with foam sclerotherapy second (16.8%) and surgery third (4.5%).
| Strategy | Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | Incremental cost per QALY (£) | Probability cost-effective at Rc £20,000 |
|---|---|---|---|---|---|---|
| Foam sclerotherapy | 664 | 0 | 4.000 | 0 | 0 | 0.168 |
| EVLA | 1095 | 431 | 4.119 | 0.118 | 3640 | 0.787 |
| Surgery | 1300 | 206 | 4.040 | −0.078 | Dominated | 0.045 |
Figure 16 plots the proportion of probabilistic iterations favouring each of the alternative strategies (in terms of NMB) at increasing levels of WTP per QALY. This figure shows that as WTP increases beyond £30,000, EVLA has ≈ 80% chance of being considered the optimal strategy from a cost-effectiveness perspective.
FIGURE 16.
Cost-effectiveness acceptability curve based on 5-year time horizon.
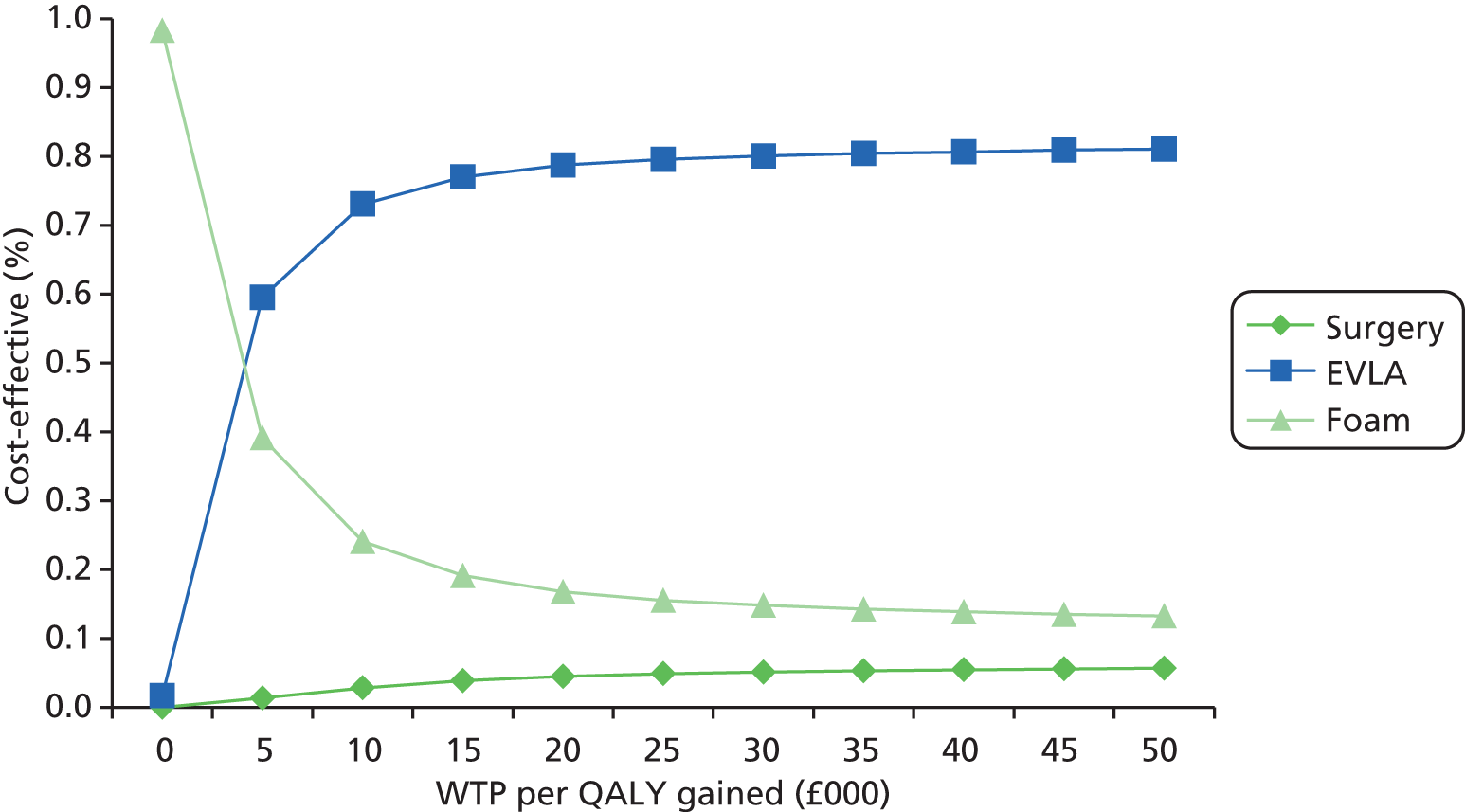
Table 101 and Figure 17 summarise the results when adopting a 10-year time horizon (assuming a constant risk of recurrence over time). A similar pattern of results is obtained, although the incremental cost per QALY gained for EVLA versus foam decreases to £2065. There is also slightly more uncertainty over the optimal treatment modality when adopting the 10-year time horizon (see Figure 17), but EVLA retains the highest chance (76%) of being cost-effective at a ceiling ratio of £20,000 per QALY.
| Strategy | Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | Incremental cost per QALY (£) | Probability cost-effective at Rc £20,000 |
|---|---|---|---|---|---|---|
| Foam sclerotherapy | 815 | 0 | 7.265 | 0 | 0 | 0.165 |
| EVLA | 1238 | 424 | 7.470 | 0.205 | 2065 | 0.76 |
| Surgery | 1475 | 237 | 7.332 | −0.138 | −1716 | 0.075 |
FIGURE 17.
Cost-effectiveness acceptability curve based on 10-year time horizon.
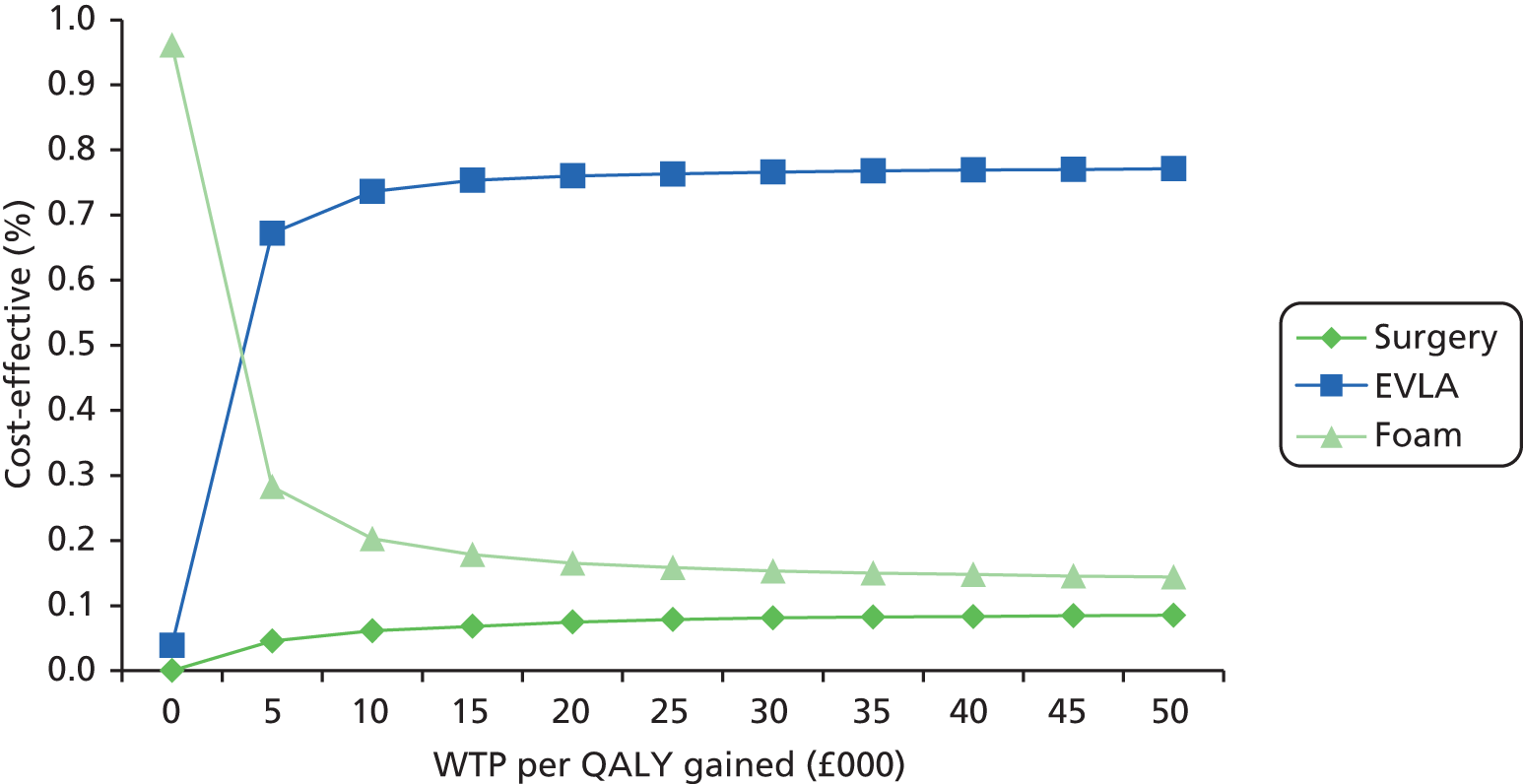
Deterministic sensitivity analysis
Table 102 demonstrates the sensitivity of the base-case modelling results to uncertainty surrounding several key input parameters and modelling assumptions. In general, the findings are robust to most changes. However, the ordering of strategies does partially switch when using the full multiple imputation data set to inform the cost and utility parameters of the model (see scenario 2 in Table 102). Under this scenario, the mean increased cost of surgery in relation to foam and EVLA drops somewhat, owing to a cluster of patients in the surgery arm, receiving no treatment within CLASS, being assigned costs in line with the mean treatment cost (across all arms) in the imputation analysis. These patients were dropped out of the complete case analysis owing to withdrawal from follow-up. Using multiple imputation to put these patients back into the analysis reduces the mean cost of surgery and, consequently, increases its chances of being cost-effective. However, EVLA still retains the highest chance of being cost-effective. Further, when the patients receiving no treatment and no follow-up are dropped from the multiple imputation analysis, the relative order of the strategies is restored to that observed for the base-case analysis (see scenario 3 in Table 102). The base-case findings were also found to hold when restricting the analysis to patients with unilateral disease only and to patients with unilateral disease and GSV involvement only.
| Strategy | Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | Incremental cost per QALY (£) | Probability cost-effective at Rc £20,000 |
|---|---|---|---|---|---|---|
| Scenario 1: including additional overhead costs for procedures carried out in theatre | ||||||
| Foam sclerotherapy | 684 | – | 4.000 | – | – | 0.174 |
| EVLA | 1122 | 438 | 4.119 | 0.118 | 3703 | 0.8 |
| Surgery | 1561 | 439 | 4.040 | −0.078 | Dominated | 0.027 |
| Scenario 2: cost and utility inputs based on full multiple imputation analysis | ||||||
| Foam sclerotherapy | 677 | – | 3.975 | – | – | 0.114 |
| EVLA | 1094 | 417 | 4.116 | 0.142 | 2943 | 0.769 |
| Surgery | 1237 | 144 | 4.057 | −0.060 | Dominated | 0.116 |
| Scenario 3: cost and utility inputs based on multiple imputation analysis, with participants receiving no treatment and follow-up dropped | ||||||
| Foam sclerotherapy | 665 | – | 3.996 | – | – | 0.174 |
| EVLA | 1097 | 432 | 4.115 | 0.119 | 3626 | 0.738 |
| Surgery | 1272 | 175 | 4.044 | −0.071 | Dominated | 0.088 |
| Scenario 4: applying cost and utility input parameter values based only on patients with unilateral disease and no simultaneous treatment to contralateral leg | ||||||
| Foam sclerotherapy | 646 | – | 3.990 | – | – | 0.172 |
| EVLA | 1081 | 436 | 4.138 | 0.148 | 2947 | 0.778 |
| Surgery | 1233 | 152 | 4.037 | −0.101 | Dominated | 0.051 |
| Scenario 5: applying cost and utility input parameter values based only on patients with unilateral disease and GSV involvement | ||||||
| Foam sclerotherapy | 646 | – | 3.929 | – | – | 0.115 |
| EVLA | 1085 | 439 | 4.107 | 0.179 | 2456 | 0.784 |
| Surgery | 1251 | 166 | 4.039 | −0.069 | Dominated | 0.102 |
| Scenario 6: 60% of patients with clinical recurrence proceed to further treatment | ||||||
| Foam sclerotherapy | 622 | – | 3.995 | – | – | 0.168 |
| EVLA | 1066 | 444 | 4.115 | 0.120 | 3702 | 0.786 |
| Surgery | 1262 | 196 | 4.035 | −0.080 | Dominated | 0.046 |
| Scenario 7: 90% of patients with clinical recurrence proceed to further treatment | ||||||
| Foam sclerotherapy | 706 | – | 4.006 | – | – | 0.168 |
| EVLA | 1124 | 418 | 4.123 | 0.117 | 3576 | 0.788 |
| Surgery | 1339 | 215 | 4.045 | −0.077 | Dominated | 0.044 |
| Scenario 8: all patients with clinical recurrence receive conventional surgery | ||||||
| Foam sclerotherapy | 734 | – | 3.999 | – | – | 0.169 |
| EVLA | 1143 | 409 | 4.118 | 0.119 | 3451 | 0.785 |
| Surgery | 1363 | 221 | 4.039 | −0.079 | Dominated | 0.046 |
| Scenario 9: all patients with clinical recurrence receive foam | ||||||
| Foam sclerotherapy | 603 | – | 3.997 | – | – | 0.17 |
| EVLA | 1053 | 450 | 4.116 | 0.119 | 3768 | 0.786 |
| Surgery | 1245 | 192 | 4.037 | −0.079 | Dominated | 0.045 |
| Scenario 10: application of NHS reference cost (£1216) to all treatments for clinical recurrence | ||||||
| Foam sclerotherapy | 755 | – | 4.000 | – | – | 0.169 |
| EVLA | 1157 | 402 | 4.119 | 0.118 | 3401 | 0.786 |
| Surgery | 1383 | 225 | 4.040 | −0.078 | Dominated | 0.046 |
| Scenario 11: 2-year delay between clinical recurrence and receiving further treatment | ||||||
| Foam sclerotherapy | 605 | – | 3.987 | – | – | 0.172 |
| EVLA | 1049 | 444 | 4.108 | 0.122 | 3656 | 0.782 |
| Surgery | 1242 | 192 | 4.027 | −0.081 | Dominated | 0.047 |
| Scenario 12: allowing any number of repeat treatments for subsequent clinical recurrences, with a 10-year time horizon | ||||||
| Foam sclerotherapy | 927 | – | 7.283 | – | – | 0.164 |
| EVLA | 1285 | 358 | 7.478 | 0.196 | 1831 | 0.765 |
| Surgery | 1547 | 262 | 7.345 | −0.134 | Dominated | 0.071 |
| Scenario 13: assuming no difference in post-treatment utility scores between alternatives | ||||||
| Foam sclerotherapy | 664 | – | 4.033 | – | – | 0.549 |
| EVLA | 1095 | 431 | 4.052 | 0.019 | 22,268 | 0.378 |
| Surgery | 1300 | 206 | 4.039 | −0.013 | Dominated | 0.073 |
| Scenario 14: assuming no difference in post-treatment utility scores between alternatives, with a 10-year time horizon | ||||||
| Foam sclerotherapy | 815 | – | 7.311 | – | – | 0.42 |
| EVLA | 1238 | 424 | 7.365 | 0.054 | 7881 | 0.454 |
| Surgery | 1475 | 237 | 7.328 | −0.036 | Dominated | 0.127 |
| Scenario 15: utility inputs based on participant responses to the SF-36 (scored using the SF-6D) | ||||||
| Foam sclerotherapy | 665 | – | 3.706 | – | – | 0.216 |
| EVLA | 1095 | 431 | 3.772 | 0.066 | 6503 | 0.782 |
| Surgery | 1301 | 205 | 3.706 | −0.066 | Dominated | 0.003 |
| Scenario 16: equal recurrence rates applied following EVLA and surgery | ||||||
| Foam sclerotherapy | 664 | – | 4.002 | – | – | 0.169 |
| EVLA | 1095 | 431 | 4.119 | 0.117 | 3691 | 0.78 |
| Surgery | 1255 | 160 | 4.053 | −0.066 | Dominated | 0.052 |
When applying the assumption of no difference in generic QALYs between the strategies up to 6 months, and no difference in the mean EQ-5D score at 6 months, foam sclerotherapy has the highest probability of being cost-effective at 5 years (see scenario 13 in Table 102), with EVLA second and surgery third. However, the base-case ordering is restored when the time horizon for this analysis is extended to 10 years (see scenario 14 in Table 102).
Secondary analysis for bilateral varicose veins
As well as assessing the sensitivity of the base-case cost-effectiveness findings to alternative parameter values and assumptions, a further model-based analysis was undertaken to ascertain the likely cost-effectiveness of using the alternative treatment modalities in patients with bilateral disease. Several assumptions were required for this analysis, as participants with bilateral disease in the CLASS trial were assigned only one study leg to be treated, in accordance with the CLASS protocol. Therefore, the CLASS data are not ideally suited to assessing the cost-effectiveness of using the individual treatment modalities (where clinically appropriate) to treat bilateral varicose veins.
To inform this analysis, it was assumed that QALY and utility gains following bilateral treatment would follow the same pattern as observed for the treatment of unilateral veins. This assumes that using the alternative treatment modalities in both legs would result in similar outcomes for both legs, and that the worst leg determines patients’ QoL. It was further assumed that for surgery, treatment of both legs would be carried out in a single session, whereas for foam and EVLA the second leg would be treated in a separate session. Therefore, in this model the cost of surgery (for unilateral disease) was inflated by 1.23, the ratio of the mean surgery cost for unilateral disease over the mean surgery cost for patients in the CLASS trial having surgery for their contralateral leg at the same time. For foam and EVLA, we added the full cost of an additional treatment session, based on the mean cost estimates for unilateral treatment derived from the trial data. These assumptions are conservative in favour of surgery, as EVLA may also be carried out for the second leg within a single treatment session.
Finally, to reflect the fact that costs associated with clinical recurrence are likely be higher in patients with bilateral disease, we applied the NHS reference cost (£1611) for the treatment of recurrent bilateral varicose veins. 86
The results of this analysis are presented in Table 103. They show that under this scenario EVLA becomes the most costly option. However, as a result of application of the estimated QALY and utility gains for EVLA versus surgery in the model, and application of a slightly lower estimated recurrence risk for EVLA, EVLA retains an estimated QALY gain of 0.1 over surgery at 5 years. The consequence of this is that EVLA has an ICER below £20,000 in comparison with surgery, and the highest chance of being cost-effective at this threshold.
| Strategy | Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | Incremental cost per QALY (£) | Probability cost-effective at Rc £20,000 |
|---|---|---|---|---|---|---|
| Scenario 1: base-case bilateral treatment scenario | ||||||
| Foam sclerotherapy | 1137 | – | 3.990 | – | – | 0.206 |
| Surgery | 1631 | 493 | 4.037 | 0.047 | 10,440 | 0.09 |
| EVLA | 1953 | 323 | 4.138 | 0.101 | 3207 | 0.704 |
| Scenario 2: applying the same EQ-5D utility weight for EVLA and surgery at 6 months | ||||||
| Foam sclerotherapy | 1137 | – | 3.990 | – | – | 0.204 |
| Surgery | 1631 | 493 | 4.105 | 0.115 | 4290 | 0.249 |
| EVLA | 1953 | 323 | 4.138 | 0.033 | 9799 | 0.574 |
| Scenario 3: applying the same recurrence risk for EVLA and surgery | ||||||
| Foam sclerotherapy | 1137 | – | 3.990 | – | – | 0.204 |
| Surgery | 1548 | 411 | 4.050 | 0.060 | 6891 | 0.111 |
| EVLA | 1953 | 406 | 4.138 | 0.088 | 4591 | 0.685 |
| Scenario 4: applying scenarios 2 and 3 simultaneously | ||||||
| Foam sclerotherapy | 1137 | – | 3.990 | – | – | 0.21 |
| Surgery | 1548 | 411 | 4.121 | 0.131 | 3128 | 0.505 |
| EVLA | 1953 | 406 | 4.138 | 0.017 | 24,341 | 0.284 |
The impact on the results of applying the same EQ-5D utility weight for surgery and EVLA at 6 months and beyond (see scenario 2 in Table 103), and of applying the same clinical recurrence risk (see scenario 3 in Table 103), was also assessed. The findings are robust to these changes individually, but when applied simultaneously, the ICER for EVLA rises above £20,000 per QALY gained, and surgery has the higher chance of being cost-effective (see scenario 4 in Table 103).
Sensitivity to alternative structural assumptions
Table 104 and Figure 18 summarise the results obtained when using alternative structural assumptions to construct the model. With this model, it was assumed that the alternative treatments have no differential impact on the generic HRQoL of patients, other than that driven by their effects on the clinical severity of patients’ venous disease (as assessed by the VCSS).
| Strategy | Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | Incremental cost per QALY (£) | Probability cost-effective at Rc £20,000 |
|---|---|---|---|---|---|---|
| Foam sclerotherapy | 667 | – | 4.022 | – | – | 0.302 |
| EVLA | 1096 | 429 | 4.063 | 0.042 | 10,329 | 0.397 |
| Conventional surgery | 1301 | 205 | 4.070 | 0.006 | 31,977 | 0.301 |
FIGURE 18.
Cost-effectiveness acceptability curve based on the alternative model structure with a 5-year time horizon.

In line with the clinical analysis of the VCSS score, this model predicts higher QALYs with EVLA than with foam, and slightly higher QALYs with surgery than with EVLA. However, the incremental cost per QALY gained with surgery versus EVLA remains slightly above £30,000 at 5 years, and, consequently, the probability of surgery being cost-effective remains lower than that for EVLA at the ceiling ratio of £20,000 per QALY. Furthermore, increasing the time horizon for this analysis to 10 years (Table 105, Figure 19) increases the chance of EVLA being the preferred option owing to its slightly lower estimated clinical recurrence rate.
| Strategy | Cost (£) | Incremental cost (£) | QALYs | Incremental QALYs | Incremental cost per QALY (£) | Probability cost-effective at Rc £20,000 |
|---|---|---|---|---|---|---|
| Foam sclerotherapy | 817 | – | 7.314 | – | – | 0.251 |
| EVLA | 1240 | 422 | 7.409 | 0.094 | 4474 | 0.440 |
| Conventional surgery | 1476 | 237 | 7.406 | −0.003 | Dominated | 0.309 |
FIGURE 19.
Cost-effectiveness acceptability curve based on the alternative model structure with a 10-year time horizon.
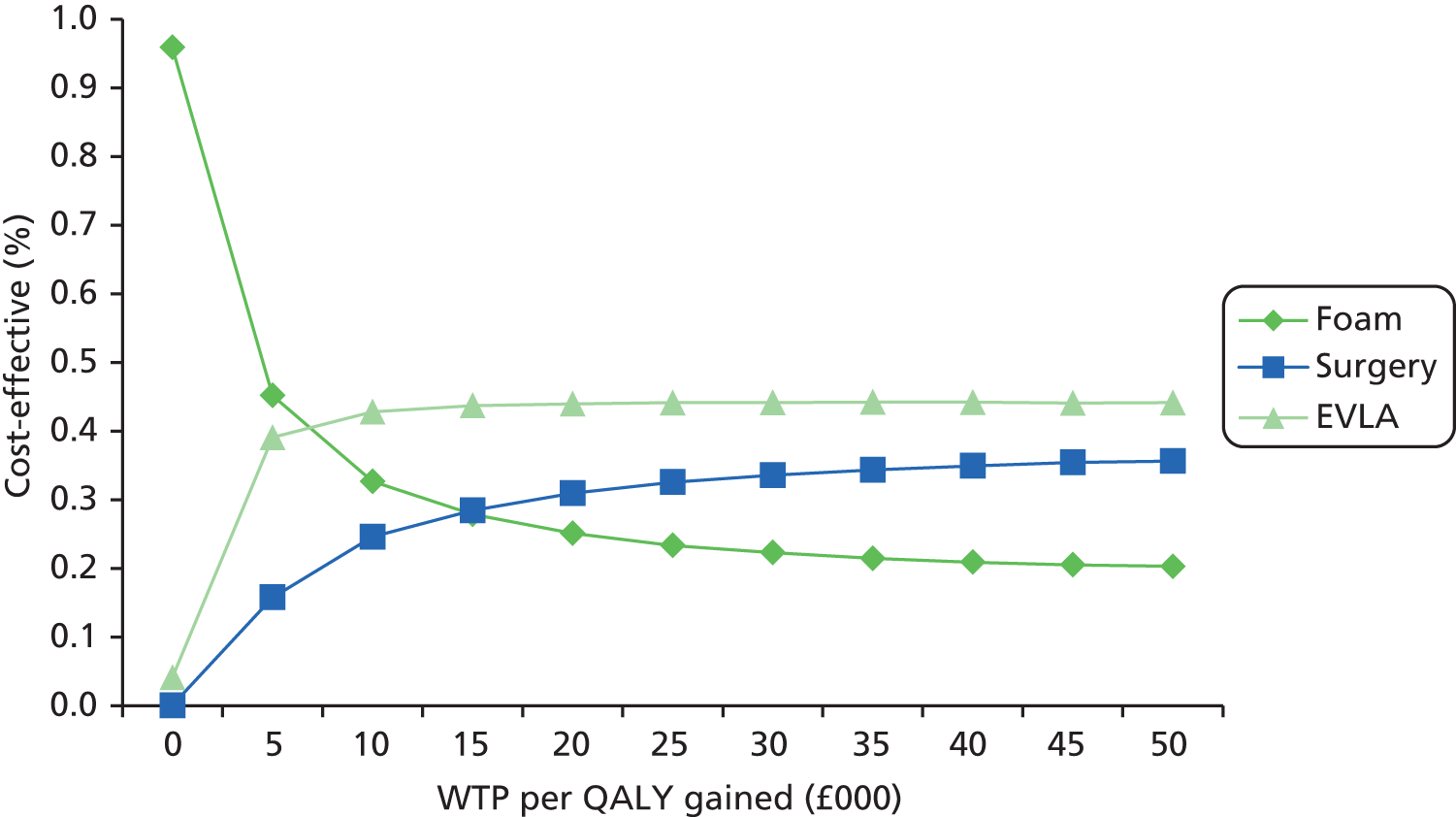
Given the slightly counterintuitive impact of applying the observed mean EQ-5D utility scores for patients in clinical severity states 3 and 4 following primary treatment (the mean values for these states were lower post treatment than they were pre treatment, resulting in modelled recurrence leading to a utility increase for some patients), we also examined the impact of applying the mean EQ-5D utility values obtained across the baseline and 6-months time points for these states in the model. This had very little impact on the results (Figure 20).
FIGURE 20.
Cost-effectiveness acceptability curve based on the alternative model structure with a 5-year time horizon (assuming equal utility for states 3 and 4 both pre and post treatment).

Discussion
Summary of key results
The modelling in this chapter suggests that, over a 5- and 10-year time horizon, EVLA is likely to be the preferred option on grounds of cost-effectiveness, costing only £3640 per QALY gained in comparison with foam sclerotherapy, and costing less and producing slightly more QALYs than surgery. Based on probabilistic analysis, EVLA had a ≈ 79% chance of being cost-effective at a ceiling WTP ratio of £20,000 per QALY.
These findings, based on extrapolation of the incremental costs and outcomes obtained from the analysis of complete trial data, were generally found to be robust to uncertainty surrounding various model parameter inputs and assumptions, including multiple imputation of missing data and the basing of model inputs on patients with unilateral disease. Although the CLASS data are not ideally suited to assessing the cost-effectiveness of the alternative treatment modalities for patients with bilateral disease (where clinically appropriate), a further sensitivity analysis was carried out to address this question, applying a number of different assumptions (see Table 103). Under most of the scenarios, EVLA retained the highest probability of being cost-effective. However, when it was assumed that EVLA and surgery have exactly the same utility outcome at 6 months, and also the same clinical recurrence rate, surgery had the higher chance of being cost-effective. This last result was still based on the conservative assumption (favouring surgery) that when using EVLA to treat bilateral disease, treatment of the second leg would generally be carried out in a separate treatment session. Therefore, it is likely that EVLA should also be preferred on grounds of cost-effectiveness for the treatment of bilateral disease in situations where all three treatment modalities are clinically viable options for the treatment of both legs.
The model-based probability of EVLA being the preferred option at the ceiling WTP ratio of £20,000 per QALY was found to be somewhat sensitive to the application of alternative model structuring assumptions. The base-case model extrapolated estimates of the direct effects of the alternative treatments on generic HRQoL (as measured by the EQ-5D). This translated into a small gain in QALYs for EVLA over surgery which, coupled with the lower cost of EVLA, resulted in it having a high probability of being cost-effective compared with surgery. However, the estimated QALY and utility gains associated with EVLA were not significant at 6 months. Therefore, we assessed the impact of (1) setting equal utility gains following the alternative treatment options; and (2) mapping changes in utility to transitions across health states defined by the VCSS. Both these approaches resulted in greater uncertainty surrounding selection of optimal treatment modality on grounds of cost-effectiveness. However, EVLA did retain the higher probability of being cost-effective at the WTP threshold of £20,000 per QALY.
Explanation of findings
The finding that EVLA has the highest chance of being the most cost-effective option is driven by a number of factors: (1) its greater estimated QALY and utility gains at 6 months versus foam sclerotherapy and, to a lesser extent, surgery; (2) its lower cost at 6 months compared with surgery; and (3) its slightly lower estimated clinical recurrence rate compared with foam sclerotherapy and surgery. Although none of the estimated differences between EVLA and surgery (with respect to post-treatment EQ-5D values and the probability of recurrence) were significant at the traditional 5% level, it was the mean estimates or estimated mean differences (between the alternative treatment modalities) that were used as model inputs (with the uncertainty surrounding each input appropriately characterised as a probabilistic distribution). The uncertainty surrounding each model parameter was then simultaneously propagated through the model (using 10,000 probabilistic iterations) to characterise the uncertainty surrounding the model outputs, that is, the estimated mean costs and effects of the alternative treatment modalities. This uncertainty surrounding the model outputs was then expressed as a probability of each strategy being cost-effective when using a ceiling ratio of £20,000 to value the model outcome (QALYs).
Strengths and limitations
The model was populated, where possible, using estimates of the mean difference in costs and effects derived from the analysis of individual patient data on resource use and outcomes collected prospectively alongside the CLASS study. Therefore, the results should be internally valid and generalisable across settings in the UK.
In the absence of data on long-term clinical recurrence for the CLASS cohort, the risk of clinical recurrence was modelled using data derived from a network meta-analysis of existing trials which reported clinical recurrence as an outcome. 28 Although this provides the best current source of evidence on recurrence, the quality of trials included in this network meta-analysis varied, and EVLA and endothermal radiofrequency ablation were pooled for the analysis. In addition, some RCTs included subjects undergoing only high tie without stripping, which would result in a higher clinical recurrence rate. In CLASS, all patients had preoperative scans to determine the involvement of single or multiple truncal veins, and all affected truncal veins were stripped. Furthermore, follow-up generally extended to only 12 or 24 months in the included trials (only one small trial94 reported outcomes at 36 months) and definitions of clinical recurrence were not always well defined and varied from study to study. Therefore, uncertainty remains regarding the applicability of these recurrence rates to patients in the CLASS trial (and more generally), and also the risks of recurrence beyond 24 months and the utility impact of clinical recurrence according to varying definitions. This underlines the importance of collecting further data on clinical recurrence and its impact on generic HRQoL via the extended follow-up of CLASS participants. Plans are in place to do this at 5 years, which will provide a means of validating and updating the modelling undertaken in this chapter.
Comparison with other cost-effectiveness studies
In general, the modelling approach used in this chapter is consistent with that used in previous economic modelling studies of treatments for varicose veins. Michaels et al. 65 previously developed a Markov model to assess the cost-effectiveness of conventional liquid sclerotherapy in comparison with surgery for patients with moderate and severe uncomplicated varicose veins. Four severity states were defined using an anatomical classification combined with evidence for the presence/absence of reflux, which was used to estimate the risk of subsequent clinical recurrence. The clinical severity states were defined as pre- and post-treatment states, in a similar manner to the way in which the clinical states were defined in the secondary analysis model described in this chapter. However, the states in our model were based on the VCSS rather than the anatomical distribution of varicose veins. In addition, Michaels et al. 65 assigned the same utility weights (obtained from post-treated patients) to their severity states both pre and post treatment (owing to a lack of correlation between baseline utility values and the anatomical health states). This may have resulted in a lack of sensitivity for capturing changes in utility associated with remaining in the same severity state following treatment.
A further difference between the CLASS and Michaels models relates to the fact that Michaels et al. 65 explicitly included some complications of surgery and sclerotherapy in their model, whereas the impact of any complications was implicitly captured in the models described in this chapter by applying the mean costs and QALYs observed for patients enrolled in the CLASS trial at 6-months follow-up. Further, observed utility at 6 months was extrapolated beyond this time point, and was assumed to capture the utility impact of any differences in complication rates between the treatment options at 6 months and beyond.
Based on their model, Michaels et al. 65 reported an ICER for surgery versus conventional liquid sclerotherapy of £1728 per QALY based on a 10-year time horizon. However, the relevance of this prior analysis is now questionable as foam sclerotherapy has superseded the use of conventional liquid sclerotherapy. Furthermore, for purposes of decision-making, it is not appropriate to consider the relative cost-effectiveness of sclerotherapy and surgery in isolation of the other appropriate comparator, EVLA.
Gohel et al. 54 similarly developed a Markov model to assess the cost-effectiveness of conservative management, foam sclerotherapy, EVLA, RFA and surgery. This was a simpler model than that developed by Michaels et al. ,65 simulating the continued success or recurrence of reflux in patients following either a completely successful or a partially successful (residual varicosities or incomplete occlusion) primary treatment. The effects of the alternative treatments on initial outcome at 3 months and subsequent recurrence of reflux were based on reviews of existing RCTs, and the costs of treatments were adapted from NHS reference costs. 86 Finally, health state utilities associated with primary varicose veins and successfully treated varicose veins were taken from a previous trial of surgery. It was assumed that patients not experiencing a successful primary treatment would remain at the pre-treatment utility level, but that those patients with residual varicosities would be offered additional foam sclerotherapy (assumed successful) and those with incomplete occlusion at 3 months would receive another treatment with the same probability of success as the primary treatment.
Based on this model, Gohel et al. 54 estimated that EVLA carried out under local anaesthetic would have the highest chance of being cost-effective at a ceiling ratio of £20,000 per QALY, with day-case surgery having the next highest probability, followed by RFA and foam sclerotherapy. Although the finding that EVLA has the highest probability of being cost-effective is consistent with our analysis, the probabilistic ordering of strategies from the model by Gohel et al. 54 was somewhat different to ours. Although it is difficult to unpick all the reasons for this, it may be partly explained by the fact that Gohel et al. 54 used estimates for the recurrence of reflux as the QoL driver (rather than clinical recurrence) and also applied less precise costs to their alternative treatment modalities (basing these on NHS reference costs). Furthermore, differences in model structure may also explain the differences in findings.
The structure and assumptions applied in our primary analysis are more in line with those used in the model developed to inform the recent NICE Clinical Guideline 168. 28 However, some differences do exist in terms of the cost and utility estimates applied in the models. Based on the direct collection of individual patient-level resource use data through CLASS CRFs and questionnaires, combined with a survey of participating centres to collect information on additional resource use, we estimated a somewhat narrower difference in cost between EVLA and surgery compared with that inferred from the estimates used in the NICE guideline model. This was due to differences in our estimated mean treatment durations (based on prospectively collected patient-level data) as well as differences in the reported staffing profile for the different procedures. Our estimates are more likely to be generalisable to UK practice because they were derived using time and staffing data collected prospectively alongside the CLASS trial rather than clinical opinion. Furthermore, rather than assuming equal utilities following different treatment modalities at 6 months (as was done in the NICE guideline model), we applied the estimated mean incremental differences between foam and surgery and between EVLA and surgery. For the first 6-month cycle, the model was also specified to return the mean QALYs observed for patients in alternative treatment allocation arms of the CLASS trial.
Despite the differences between our analysis and that used to inform development of NICE Clinical Guideline 168, the conclusions of our analysis are generally similar. EVLA retained the highest probability of being cost-effective under most scenarios tested. However, the NICE guideline also concluded that foam sclerotherapy would be the most cost-effective option in situations where EVLA is not considered a viable option, based on an ICER for surgery versus foam > £20,000 per QALY gained. Although our modelling was based on data that do not allow us to directly address this question (suitability for all three treatment modalities was an inclusion criteria in the CLASS trial), it does indicate that, for the CLASS cohort, the incremental cost per QALY gained for surgery versus foam is generally below £20,000.
Conclusion of model-based cost-effectiveness analysis
Overall, our modelling suggests that for patients in whom all three treatment modalities are a clinically viable option, EVLA has the highest probability of being cost-effective at accepted thresholds of WTP per QALY. This finding is consistent with the results of recent modelling undertaken to inform the NICE clinical guideline on the management of varicose veins. However, we cannot rule out the possibility that surgery may be the preferred option in patients in whom EVLA is not viable. We cannot directly address this question, as our modelling was based on cost and utility inputs derived from patients eligible for all three treatment options.
Chapter 11 Final discussion
The CLASS trial has shown that at 6-months follow-up all three treatment modalities – foam, EVLA and surgery – improved disease-specific and generic QoL and achieved similar improvements in the VCSS. EVLA and surgery were broadly equivalent in terms of the improvements in disease-specific QoL, VCSS and anatomical success (ablation of GSV or SSV trunks). However, EVLA showed greater early improvements in four of the eight SF-36 domains than surgery, although these were not present at 6 months.
In this first RCT involving foam sclerotherapy to evaluate and report disease-specific QoL as a primary outcome measure, the health gain achieved with foam sclerotherapy was significantly lower than that for surgery at 6-months follow-up. At 6 weeks the health gain in the AVVQ was also significantly lower for foam sclerotherapy than for surgery. At 6 months, the health gain in the SF-36 mental component was also significantly lower for foam sclerotherapy than for EVLA. The EQ-5D health gain with foam sclerotherapy at 6 weeks was also significantly lower than with EVLA, but there were no differences at 6 months.
As well as having lower QoL health gains, patients who had foam sclerotherapy had more residual varicose veins than those undergoing EVLA or surgery at 6-months follow-up. Foam sclerotherapy was also associated with a significantly greater rate of procedural complications than EVLA, and of complications at 6 weeks than surgery and EVLA. At 6 months, the overall complication rate remained higher for foam sclerotherapy than for surgery. However, the recovery and return to normal activities was quicker for foam and EVLA than for surgery. Furthermore, at 6 weeks patients’ recollection of the pain experienced at the time of the procedure and in the recovery was less following foam sclerotherapy than either EVLA or surgery.
The anatomical success rate was significantly lower for foam sclerotherapy than for surgery or EVLA. This is likely to result in a greater risk of developing recurrent varicose veins and need for further treatment in patients who underwent foam sclerotherapy. In contrast, the trial-based cost-effectiveness analysis showed that at 6 months foam sclerotherapy had the lowest costs, followed by EVLA and then surgery. Based on consideration of costs and QALYs at 6 months, foam sclerotherapy had the highest probability of being considered cost-effective at the accepted Rc of £20,000 per QALY. However, foam sclerotherapy was not associated with the greatest clinical benefit.
Some differences were evident between the cost estimates incorporated in our analysis and those used in the recent NICE guideline. 28 We estimated a somewhat larger cost difference between foam sclerotherapy and EVLA, whereas a somewhat smaller cost difference was estimated between EVLA and surgery. The main reason for this was that in some centres within the CLASS trial EVLA was performed in a theatre setting, which required a larger number of staff to be present, whereas in others it was performed in a clinic setting, which used the same staff profile as foam. A sensitivity analysis was conducted to test the impact of EVLA incorporating a similar staff profile to foam sclerotherapy. Under this scenario, the ICER of EVLA versus foam sclerotherapy fell below the accepted threshold of £20,000 per QALY gained. Further, the cost saving from EVLA versus surgery significantly increased. Thus, when EVLA was performed in a clinic setting with a similar staff profile to that used for foam, it produced the greatest NMB at 6 months compared with both foam sclerotherapy and surgery.
However, these early results cannot be used to determine definitive recommendations for the treatment of varicose veins because late recurrence rates and the need for further treatment also need to be considered. This is a very important determinant of cost-effectiveness in the longer term. This underlines the importance of the 5-year follow-up of patients in the CLASS study.
Markov modelling based on the trial data and the limited data currently available on longer-term recurrence rates suggests that, at 5 years, EVLA has the highest probability of being cost-effective (≈ 79%), followed by foam sclerotherapy (≈ 17%) and surgery (≈ 5%), for patients considered clinically suitable for all three treatment options. It should be noted, however, that the outcome of this model is quite sensitive to the projected recurrence rates. Data from clinical recurrence rates at 5 years are not available from controlled trials at present, with the exception of the recent study by Rasmussen51 comparing EVLA with surgery. The recurrence rates used for the CLASS modelling were based on figures used in the recent NICE economic analysis. 28 Using these assumptions, our analysis suggests that EVLA is likely to be the treatment of choice for suitable patients, based on considerations of both clinical effectiveness and cost-effectiveness. Although the CLASS findings are not directly applicable to patients considered clinically unsuitable for EVLA (suitability for all three treatment options was an inclusion criterion in the CLASS trial), our modelling does suggest that the incremental cost per QALY gained with surgery versus foam sclerotherapy will fall below £20,000 by 5 years in the CLASS cohort. In a two-way comparison between foam sclerotherapy and surgery, we found surgery to have the higher probability of being cost-effective at 5 years, although a great deal of uncertainty surrounds this finding owing to the significantly higher cost of surgery and uncertainty relating to its generic QoL and longer-term benefits over foam.
If the above model-based findings are confirmed by long-term follow-up of the CLASS cohort, and considered generalisable to patients not suitable for EVLA, then conventional surgery may be preferred over foam sclerotherapy on grounds of cost-effectiveness in these patients. Furthermore, other clinical benefits, such as the significantly greater improvement in disease-specific QoL, reductions in some complications at 6 months and the higher anatomical success rate associated with surgery, may also be considered when determining the choice of treatment for patients not suitable for EVLA. The recent NICE clinical guideline,28 which recommends foam sclerotherapy as the preferred treatment in patients who are not suitable for EVLA, therefore presents a dilemma for clinicians, patients and commissioners in terms of balancing clinical effectiveness, patient choice and cost when choosing which treatments to offer if the patient is not suitable for EVLA.
Strengths and limitations
Proportion of ineligible patients and overall recruitment rates
In this study, 43% of screened patients were found to be ineligible for inclusion in the trial. The main reason for this was the presence of recurrent varicose veins (28%) and the lack of truncal GSV or SSV reflux (22%). The proportion of ineligible patients is higher than in previous studies, where review of case notes or other methods of screening in advance of the patient’s appointment may have been used. The lack of advance screening in CLASS is evident from the number of patients who were excluded because they either had recurrent veins or did not have symptomatic varicose veins.
Of the eligible patients, only 24% were recruited to CLASS. This is lower than most of the previously published RCTs involving varicose veins,38,40,50 although the 2007 Rasmussen study36 only achieved an 11% recruitment rate and the study by Darwood et al. 15 failed to reach its recruitment target. A previous HTA-funded study involving vascular surgeons recruiting patients with severe limb ischaemia also experienced similar recruitment rates to the CLASS study (29%), and this was attributed to a lack of clinical equipoise. 98
Among the patients who were eligible but declined to take part, 78% either had a preference for one of the treatment options or did not wish to undergo one of the treatment options within the study. More patients declined to take part in the study because they wanted surgery (33%) than declined because they did not want surgery (< 1%). For EVLA, a similar proportion of patients expressed a preference (30%) and 1% declined because they did not want this treatment. In contrast, the proportion of patients who preferred foam sclerotherapy was much lower (preference for foam 13%). Clearly, many factors may have affected a patient’s decision to take part in the study and preference for one treatment over another. Although surgeon preference was cited infrequently as a reason (1%), the treating vascular surgeon is likely to have had a significant influence on the patient’s decision-making.
In order to explore this further, a study was performed at one of our sites. This found that the surgeons presented balanced descriptions of the treatments but that they made the assumption that the patients might have a preference, and patients, in turn, felt that they were expected to have a preference.
Revised target sample size
The original trial sample size of 1015 (surgery vs. foam, 90% power, 5% significance, EVLA, EVLA versus foam or surgery 80% power, 5% significance) was revised to 779 based on data which showed that the correlation between AVVQ at baseline and at 6 months was better than originally assumed. This analysis was prompted by the lower than expected recruitment rate. The reduction in sample size did not lead to any reduction in the predefined clinically important difference in QoL, but may have disadvantaged the EVLA arm in which the power was lower. This revision was approved by both the TSC and DMC.
Number of statistical comparisons
There were a large number of comparisons involving primary and secondary outcomes, and therefore it may be inferred that some differences may have occurred by chance. Thus, for the secondary outcome measures, we consider differences to be significant only for p-values < 0.005.
Generalisability
Despite the fact that many eligible patients chose not to take part, those who did participate appear broadly similar to those in other RCTs (see Chapter 7), with the exception of a lower than expected proportion of females (56% of participants were female, but 75% of those invited to participate were female). Although most (or all) of the previous RCTs involving varicose veins have had higher proportions of female participants, our experience is consistent with a number of other studies which have shown that females are less likely than males to participate and are more difficult to recruit to RCTs. 99–104 It has been suggested that this may reflect less favourable attitudes towards medical research among women. It is of note that the mean baseline AVVQ score for women was slightly higher than for men (18.2 vs. 17.4), although this difference was not statistically significant and was unlikely to have had a bearing on their recruitment to the study.
The CEAP classification grade, VCSS (pre/post treatment) and QoL (pre/post treatment) were similar to those in other RCTs. 15,29–34,37,38,49–51 The QoL values were also similar to those published in NHS England PROMs. Given this, and the fact that less than 20% of patients were excluded because the vein diameter was too small, large or tortuous, the results of this study appear generalisable to the majority of patients seeking treatment for primary varicose veins.
Truncal ablation rates
The reasons for the lower ablation rates observed in the CLASS study compared with previous reports have been fully discussed in Chapter 7. Although the complete success rates for the GSV are at the lower end of those published in other RCTs, many of these defined ‘technical success’ as the combination of complete ablation and partial success with no reflux. The overall ‘technical success’ rate for CLASS is comparable (91% for EVLA and 82% for surgery). The results for foam (67% complete and partial with no reflux) remain lower than in some studies, but are comparable with those in the RCT by Latimer et al. 32 and those achieved by surgeons in the study by Wright et al. 14 It is important to note that, despite the apparently lower ablation rates, the improvements in QoL and VCSS were comparable with those published in previous RCTs15,29–34,37,38,49–51 and in the PROMs for NHS England. In addition, this is the largest multicentre RCT to date involving EVLA, foam and surgery, and its results reflect those of the generality of vascular surgeons and their trainees, rather than those of enthusiasts in single centres.
Unlike those in previous RCTs, the duplex scans by which truncal ablation was assessed were performed by independent, accredited vascular technologists, with the exception of those performed at one centre. This was done in order to minimise the bias which can occur when surgeons who have treated patients do the follow-up scans. An attempt was made to quality assure the scans, but as none of the sites were able to video-record a whole examination, this proved impossible. An audit of still images taken at set anatomical locations was of no value owing to the lack of anatomical landmarks on the images, which meant that the duplex scanning site could not be verified. There was also no means of standardising the angle of the probe, or probe or calf compression. These difficulties reflect the general limitations of duplex scanning and are not specific to our study. The technique for performing the scan was standardised prior to commencing the trial following discussion between the vascular technologists at the 10 centres, and results were recorded on a set proforma. These dedicated vascular technologists are likely to be more skilled and reliable than the varied clinicians who scanned in other studies.
Concomitant phlebectomies
The issue of whether or not varicosities should be treated at the same time as the main truncal veins remains controversial. This is highlighted as an area of future research in the recent NICE clinical guideline. 28 One previous single-centre RCT has shown significant improvements in disease-specific QoL and VCSS at 6 weeks and 3 months following concomitant, as opposed to delayed, phlebecotomies. 73
In CLASS, patients in the EVLA group only received treatment to the main truncal vein, without concomitant phlebectomies (with the exception of patients at one site), unlike those in the surgery group. Nevertheless, we observed significant improvements in the AVVQ and VCSS in patients undergoing either surgery or EVLA compared with foam sclerotherapy at 6 weeks. Importantly, there was no difference in these outcomes in patients undergoing EVLA or surgery at the 6-weeks follow-up. These findings suggest that the strategy of performing concurrent phlebectomies in patients undergoing EVLA is unnecessary, with respect to improving QoL or VCSS in the short term. Furthermore, approximately one-third of patients who underwent EVLA in CLASS elected to have treatment for residual varicosities at or after 6-weeks follow-up, so it could be inferred that the use of concomitant phlebectomies would have been unnecessary treatment for the remaining two trials of participants. It is unclear if this will affect future recurrence rates.
The CLASS trial also raises concerns regarding whether or not foam sclerotherapy is the most appropriate means to treat any residual varicose veins. EVLA showed benefits over foam sclerotherapy in terms of disease-specific and generic QoL as well as reduced complications at 6 weeks. These benefits did not persist to 6 months, by which stage one-third of the patients in the EVLA group had undergone foam sclerotherapy.
Clinical, etiological, anatomical, pathological grade
The majority of patients in the CLASS study had CEAP grade 2 varicose veins, which is consistent with other studies and with the known distribution of venous disease in the population. NICE referral guidelines for varicose veins published in 2001 recommended referral of symptomatic primary varicose veins, which are associated with impaired QoL. 105 It is apparent that rationing is being imposed in most areas, which means that only patients with more severe CEAP grades are offered referral for treatment. Thus, the results from CLASS may not be as generalisable to current NHS practice as they should be if referral guidelines were adhered to. The study was designed before this rationing became widespread. Within CLASS, there was evidence of a clear inequity in the provision of treatment for varicose veins in centres across the country, which has arisen primarily as a result of cost pressures and, indeed, this influenced differences in recruitment rates between centres. It is not clear what the long-term health and QoL effects of not treating many people with varicose veins will be. If it does lead to an increased incidence of venous ulceration, this will have substantial cost consequences for the NHS, in addition to increased morbidity and decreased QoL for affected patients.
Sensitivity of the European Quality of Life-5 Dimensions to detect differences between treatment modalities
As would be expected in patients undergoing treatment for varicose veins, the AVVQ, designed specifically to assess QoL in these patients, was more sensitive to change than either of the generic QoL instruments (SF-36 and EQ-5D). This sensitivity was demonstrated in terms of the magnitude of change from baseline at both 6 weeks and 6 months post treatment and was crucial in confirming improvements both across and between the treatment groups. The AVVQ showed differences between groups at 6 weeks which favoured surgery and EVLA over foam sclerotherapy, and differences at 6 months which favoured surgery over foam sclerotherapy. By contrast, the EQ-5D showed only a benefit for EVLA over foam sclerotherapy at 6 weeks but no differences at 6 months. However, the EQ-5D was principally used in the evaluation of cost-effectiveness as it is the instrument routinely used for this type of QoL analysis. 28
Lack of inclusion of radiofrequency ablation as a treatment option
We chose to include EVLA and foam sclerotherapy in the CLASS trial because these were the two most widely used of the newer, local anaesthetic treatment options within the NHS at that time. We considered RFA as an alternative to EVLA, but it was more costly and less suited for local anaesthesia owing to the longer contact time required between the probe and the vein endothelium. In addition, EVLA seemed to be in greater demand from patients and to have found favour with more vascular specialists at the time when the trial was being designed. However, subsequent developments have made RFA faster, such that it is now routinely performed under a local anaesthetic. Nevertheless, it remains more expensive than EVLA.
Endovenous laser ablation and RFA are now considered to be comparable techniques in terms of outcome. Furthermore, in the recent NICE Clinical Guideline 16828 RFA was considered equivalent to EVLA; the two treatments were grouped together throughout the recommendations as ‘endothermal ablation’.
Behavioural recovery
The 14-item BRAVVO questionnaire was made up of items and behaviours which patients identified as being important to their recovery, many of which have not been considered in previous studies. Development of the BRAVVO instrument represents an important first step in identifying the behaviours that patients perceive to be important and will allow for a more detailed explanation to patients of the anticipated recovery following treatment, as well as a more sensitive comparison of the effect of different treatments on recovery.
Comparison of LAser, Surgery and foam Sclerotherapy 5-year follow-up
Treatment of recurrent varicose veins accounts for 20% of venous procedures performed in the NHS, and was responsible for ineligibility in 28% of patients considered for this trial; thus, the durability of primary treatment is important both for patients and for economic reasons. Few RCTs involving foam sclerotherapy or EVLA have assessed clinical recurrence rates, and they provide only limited short- to medium-term (i.e. 2-year) clinical results. The 5-year recurrence rate for EVLA has recently been reported in one small RCT51 but has not been reported for any RCT of foam sclerotherapy. 14,29–33,51 Patients with recurrent varicose veins have significantly worse QoL than patients with primary varicose veins106 and show less improvement in QoL after treatment,7,107 so using a primary treatment which minimises the risk of recurrence is an important consideration.
Implications for practice
The CLASS trial has shown that EVLA (performed under a local anaesthetic, in a predominantly clinic-based setting) has the highest probability of being cost-effective at accepted thresholds of WTP per QALY. This finding is consistent with the results of recent modelling undertaken to inform the NICE clinical guideline28 on the management of varicose veins. The CLASS trial cannot directly inform the choice between surgery and foam in patients in whom EVLA is not a treatment option, as eligibility for all three treatment options was an inclusion criterion of the CLASS trial. However, less than 20% of patients were ineligible for the CLASS trial because the vein was too tortuous (9%), or either too small or too large in diameter (9%). Thus, the majority of patients with primary veins referred for treatment in the NHS appear to be suitable for thermal ablation. Furthermore, in CLASS only one eligible potential participant declined to be randomised because he/she did not wish to undergo EVLA.
For patients in whom thermal ablation may be unsuitable or declined, the results from the CLASS trial suggest that surgery rather than foam sclerotherapy should be considered. In a two-way comparison between foam and surgery, surgery was found to have the greatest probability of being cost-effective at 5 years, although a great deal of uncertainty surrounds this finding owing to the significantly higher cost of surgery and lack of long-term recurrence rate data for both interventions. However, surgery was associated with greater gains in the AVVQ at 6 months, a higher truncal ablation rate and reduced residual varicose veins compared with foam sclerotherapy. There were no differences in terms of the VCSS or complication rates, but return to normal activities was quicker following foam sclerotherapy than following surgery.
The CLASS trial also raises concerns regarding whether or not foam sclerotherapy is the most appropriate means to treat non-truncal varicosities in patients undergoing EVLA.
Recommendations for research
The CLASS trial has highlighted the need for long-term outcome data from RCTs on QoL, recurrence rates and costs for foam sclerotherapy and other endovenous techniques, compared against each other and against surgery. With one recent exception,51 follow-up from RCTs involving foam sclerotherapy or EVLA is currently limited to 2 years. In the current absence of data on long-term clinical recurrence, the risk of clinical recurrence in CLASS was modelled using the network meta-analysis performed by NICE28 which included recurrence rates up to 2 years. Although this provides the best current source of evidence on recurrence, uncertainty remains regarding the applicability of these recurrence rates to patients in the CLASS trial and the risks of recurrence beyond 24 months. This underlines the importance of collecting further data on clinical recurrence and its impact on generic QoL via the extended follow-up of CLASS participants.
We have previously discussed the controversial issue of whether or not varicosities should be treated at the same time as the main truncal veins. The CLASS trial provides further impetus for future research in this area as highlighted in the recent NICE clinical guideline. 28
Conclusion
The CLASS trial is the largest multicentre trial to have compared surgery with the two most commonly performed newer treatment options, namely foam sclerotherapy and thermal ablation by EVLA. It has comprehensively assessed both the clinical effectiveness and cost-effectiveness of these treatment options within the NHS. The 6-month outcomes and 5-year economic model clearly suggest that EVLA should be considered as the first-line treatment in patients with varicose veins. In patients not suitable for EVLA, surgery rather than foam sclerotherapy should be considered on grounds of clinical effectiveness and cost-effectiveness.
We await the 5-year results of CLASS, which are essential to determine recurrence rates and the true cost-effectiveness of EVLA, foam sclerotherapy and surgery.
Acknowledgements
The authors wish to thank Janice Cruden for her secretarial support and data management; Gladys McPherson and the programming team at CHaRT; Tracey Davidson, Lynda Constable, Jackie Ellington, Laura Elliott and Yvonne Fernie for help with scoring the AVVQ; our original economists in the group, Luke Vale and Laura Ternent; members of the Project Management Group for their ongoing advice and support of the trial; the independent members of the TSC and DMC; and the staff at recruitment sites who facilitated recruitment, treatment and follow-up of trial participants.
Project Management Group
Julie Brittenden, Jennifer Burr, Marion Campbell, Seonaidh Cotton, Janice Cruden, Tracey Davidson, Jill Francis, Alison McDonald, Gladys McPherson, John Norrie, Craig Ramsay and Samantha Wileman.
Independent members of the Trial Steering Committee
Alun Davies (chairperson), Ian Loftus and Jane Nixon.
Independent members of the Data Monitoring Committee
Gerry Stansby (chairperson), Winston Banya and Marcus Flather.
Recruitment sites
Aberdeen
J Brittenden (lead clinician), P Bachoo, M Balment, I Cadle, M Christie, A Colledge, P Cooper, S Flockhart, G Kuhan, K McMullan, E Munro, S Rajagopalan, V Revanur, M Sharp, L Sleigh, L Stuart, L Swan, A Tambyraja, A Wilson, E Wilson and V Vaughn.
Blackburn
S Hardy (lead clinician), W Goddard and K Hargreaves.
Bournemouth
D Rittoo (lead clinician), I Ali, SJA Baker, R Bower, A Ferguson, D Foy, M Hiscock, C Lee, M Lunt, S Parvin, S Smith, LJ Stephenson and L Wijesinghe.
Exeter
B Campbell (lead clinician), A Cowan, D Birchley, F Hall, B Kemp, L Park, A Peters, P Niblet, F Summers, J Thompson and R Wilkinson.
Gloucester
J Earnshaw (lead clinician), G Bishop, V Cannon, D Cooper, K Harvey, A Kindon, D Parkin, C Rodd and J Symonds.
Hull
I Chetter (lead clinician), S Brown, D Carradice, S Deacon, J Hatfield, V Lowthorpe, B McCloy, P McCollum, S Moffat, N Samuel, T Wallace and A Welburn.
Leeds
J Scott, M Gough (lead clinicians), C Bedford, D Berridge, N Dewhirst, M Gough, S Homer-Vanniasinkam, P Kent, A Mavor, D Russell, P Smalley, M Troxler, D Watson and J Woods.
Newcastle
T Lees (lead clinician), M Allen, T Barakat, L Cassidy, R Challis, MJ Clarke, V Cooke, S Kappadath, D Lambert, J McCaslin, A Nath, C Oates, N Parr, A Tenna, LE Wales, A Watson, V Wealleans, V White, L Wilson and MG Wyatt.
Sheffield
D Dodd (lead clinician), K Armitage, J Beard, E Calton, P Chan, N Fenwick, D Ford, K Haran, A Lambe, R Lonsdale, N Mills, R Mottram, E Mulkern, R Nair, A Nassef, S Nawaz, K Ryalls, J Sorrell and H Trender.
Sherwood Forest
KR Makhdoomi (lead clinician), B Rorison, S Haigh, K Hollis, L Munson, D Nix, J Taylor and C Wearn.
Worcester
I Nyamekye (lead clinician), W Hayes, P Matheson, H Morrow, H Perry, A Rosoman, A White and M Williams.
The Health Services Research Unit is funded by the Chief Scientist Office of the Scottish Government Health Directorate.
Contributions of authors
Julie Brittenden (chief investigator) contributed to the conception and design of the trial, the conduct of the trial, the interpretation of the results and the writing/editing of the report.
Seonaidh C Cotton (trials manager, triallist) was responsible for the day-to-day management of the trial, contributed to the interpretation of data and made significant contributions to drafting the monograph.
Andrew Elders (statistician) conducted the statistical analyses, contributed to drafting the statistical methods section in Chapter 3, drafted the clinical effectiveness results in Chapters 4–6 and reviewed the final manuscript.
Emma Tassie (research assistant) undertook the trial-based cost-effectiveness analysis and drafted the chapter on cost-effectiveness analysis, under the supervision of Graham Scotland (senior research fellow).
Graham Scotland (senior research fellow) supervised the trial-based cost-effectiveness analysis and carried out the economic modelling with support from Emma Tassie.
Craig R Ramsay (professor and triallist) contributed to the design of the trial, the conduct of the trial, the statistical analysis and interpretation of the results and the preparation of the report.
John Norrie (CHaRT Director) contributed to the conception and the design of the trial, the conduct of the trial, the interpretation of the results and the preparation of the report.
Jennifer Burr (triallist) contributed to the design of the study, oversight of the study conduct and writing of the final report.
Jill Francis (health psychologist) led on the conception and design of the study of behavioural recovery and development of the BRAVVO questionnaire; contributed to analysis and interpretation of behavioural data and illness representations data; made significant contributions to drafting the BRAVVO chapter of the monograph and contributed to drafting the other chapters; and gave approval of the version to be published.
Samantha Wileman (quality assurance manager) contributed to oversight and conduct of the study, and preparation of the monograph.
Bruce Campbell (principal investigator) contributed to the design of the study, design of the patient information leaflet, recruitment of participants and interpretation of data, and contributed extensively to the writing of the final report.
Paul Bachoo (co-investigator) contributed to recruitment of participants, interpretation of data and editing the final report.
Ian Chetter (principal investigator) contributed to the conception and design of the study, acquisition and interpretation of data, drafting and revising of the report, and final approval of the publication.
Michael Gough (joint principal investigator) contributed to the conception and design of the study, recruitment of participants, revising of the report and final approval of the publication.
Jonothan Earnshaw (principal investigator) contributed to the conception and design of the study, recruitment of participants, revising of the report and final approval of the publication.
Tim Lees (principal investigator) contributed to the follow-up design, recruitment of participants, revising of the report and final approval of the publication.
Julian Scott (joint principal investigator) contributed to the follow-up design, recruitment of participants, revising of the report and final approval of the publication.
Sara A Baker (consultant vascular nurse) contributed to the design of the study, recruitment of participants, interpretation of data and writing of the final report.
Graeme MacLennan (senior statistician) undertook the statistical analysis of the BRAVVO questionnaire and contributed to the writing of the corresponding chapter.
Maria Prior (research fellow) contributed to the analysis of the BRAVVO questionnaire data, assisted in writing this chapter of the report, commented on other aspects of the study and contributed to the preparation of the report.
Denise Bolsover (research fellow) was involved in the delivery of the study of behavioural recovery and development of the BRAVVO questionnaire; contributed to analysis and interpretation of behavioural data; and made contributions to drafting the BRAVVO chapter of the monograph.
Marion K Campbell (professor and director, triallist) contributed to the design and conduct of the trial, the interpretation of the results and the preparation of the report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Ladropoulos N, Leaon M, Nicolaides AN, Giannoukas AD, Volteas N, Chan P. Superficial venous insufficiency: correlation with anatomical extent of reflux with clinical symptoms and signs. J Vasc Surg 1994;20:953-8. http://dx.doi.org/10.1016/0741-5214(94)90233-X.
- Health and Social Care Information Centre . Hospital Episode Statistics n.d. www.hesonline.nhs.uk/Ease/servlet/ContentServer?site=1937&categoryID=192 (accessed 23 August 2013).
- van Rij AM, Jiang P, Solomon C, Christie RA, Hill GB. Recurrence after varicose vein surgery: A prospective long-term clinical study with duplex ultrasound scanning. J Vasc Surg 2003;38:935-43. http://dx.doi.org/10.1016/S0741-5214(03)00601-3.
- Beale RJ, Gough MJ. Treatment options for primary varicose veins – a review. Eur J Vasc Endovasc Surg 2005;30:83-95. http://dx.doi.org/10.1016/j.ejvs.2005.02.023.
- Bradbury A, Evans CJ, Allan P, Lee AJ, Ruckley CV, Fowkes FG. The relationship between lower limb symptoms and superficial and deep venous reflux on duplex ultrasonography: The Edinburgh Vein Study. J Vasc Surg 2000;32:921-31. http://dx.doi.org/10.1067/mva.2000.110509.
- Subramonia S, Lees T. Sensory abnormalities and bruising after long saphenous vein stripping: impact on short-term quality of life. J Vasc Surg 2005;42:510-14. http://dx.doi.org/10.1016/j.jvs.2005.05.021.
- Mackenzie RK, Lee AJ, Paisley A, Burns P, Allan PL, Ruckley CV, et al. Patient, operative and surgeon factors that influence the effect of superficial venous surgery on disease-specific quality of life. J Vasc Surg 2002;36:896-902. http://dx.doi.org/10.1067/mva.2002.128638.
- Mackenzie RK, Paisley A, Allan PL, Lee AJ, Ruckley CV, Bradbury AW. The effect of long saphenous stripping on quality of life. J Vasc Surg 2002;35:1197-203. http://dx.doi.org/10.1067/mva.2002.121985.
- Smith JJ, Garratt AM, Guest M, Greenhalgh RM, Davies AH. Evaluating and improving health-related quality of life in patients with varicose veins. J Vasc Surg 1999;30:710-19. http://dx.doi.org/10.1016/S0741-5214(99)70110-2.
- Ratcliffe J, Brazier JE, Campbell WB, Palfreyman S, MacIntyre JB, Michaels JA. Cost-effectiveness analysis of surgery versus conservative treatment for uncomplicated varicose veins in a randomised clinical trial. Br J Surg 2006;93:182-6. http://dx.doi.org/10.1002/bjs.5263.
- Endovenous Laser Treatment of the Long Saphenous Vein. London: NICE; 2004.
- Radiofrequency Ablation of Varicose Veins. London: NICE; 2003.
- Ultrasound-guided Foam Sclerotherapy for Varicose Veins. London: NICE; 2006.
- Wright D, Gobin JP, Bradbury A, Coleridge-Smith P, Spoelstra H, Berridge D, et al. Varisolve polidocanol microfoam compared with surgery or sclerotherapy in the management of varicose veins in the presence of trunk vein incompetence: European randomised controlled trial. Phlebology 2006;21:180-90. http://dx.doi.org/10.1258/026835506779115807.
- Darwood RJ, Theivacumar N, Dellagrammaticas D, Mavor AI, Gough MJ. Randomized clinical trial comparing endovenous laser ablation with surgery for the treatment of primary great saphenous varicose veins. Br J Surg 2008;95:294-301. http://dx.doi.org/10.1002/bjs.6101.
- Winterborn RJ, Corbett CR. Treatment of varicose veins: the present and the future – a questionnaire survey. Ann R Coll Surg Engl 2008;90:561-4. http://dx.doi.org/10.1308/003588408X318228.
- Guex JJ. Foam sclerotherapy: an overview of use for primary venous insufficiency. Sem Vasc Surg 2005;18:25-9. http://dx.doi.org/10.1053/j.semvascsurg.2004.12.008.
- Jia X, Mowatt G, Burr JM, Cook JA, Fraser C. Systematic review of the safety and efficacy of foam sclerotherapy for venous disease of the lower limbs. Br J Surg 2007;94:925-36. http://dx.doi.org/10.1002/bjs.5891.
- Cabrera J, Cabrera JJ, Garcia-Olmedo MA. Treatment of varicose long saphenous veins with sclerosant in microfoam form: long-term outcomes. Phlebology 2000;15:19-23. http://dx.doi.org/10.1007/s005230070032.
- Smith PC. Chronic venous disease treated by ultrasound guided foam sclerotherapy. Eur J Vasc Endovasc Surg 2006;32:577-83. http://dx.doi.org/10.1016/j.ejvs.2006.04.033.
- Breu FX, Guggenbichler S. European consensus meeting on foam sclerotherapy, April 4–6, 2003, Tegernsee, Germany. Dermatol Surg 2004;30:709-17. http://dx.doi.org/10.1111/j.1524-4725.2004.30209.x.
- Beale RJ, Mavor AID, Gough MJ. Minimally invasive treatment for varicose veins: a review of endovenous laser treatment and radiofrequency ablation. Int J Low Extrem Wounds 2004;3:188-97. http://dx.doi.org/10.1177/1534734604272245.
- Proebstle TM, Sandhofer M, Kargl A, Gul D, Rother W, Knop J, et al. Thermal damage of the inner vein wall during endovenous laser treatment; key role of energy absorption by intravascular blood. Dermatol Surg 2002;28:596-600.
- Bush RG, Shamma HN, Hammond KA. 940-nm laser for treatment of saphenous insufficiency: histological analysis and long-term follow-up. Photomed Laser Surg 2005;23:15-9. http://dx.doi.org/10.1089/pho.2005.23.15.
- Timpermann PE, Sichlau M, Ryu R. Greater energy delivery improves treatment success of endovenous laser treatment of incompetent saphenous veins. J Vasc Interv Radiol 2004;15:1061-3. http://dx.doi.org/10.1097/01.RVI.0000130382.62141.AE.
- Mundy L, Merlin TL, Fitridge RA, Hiller JE. Systematic review of endovenous laser treatment for varicose veins. Br J Surg 2005;92:1189-94. http://dx.doi.org/10.1002/bjs.5142.
- Medical Services Advisory Committee . Endovenous Laser Treatment for Varicose Veins 2003. www.msac.gov.au/internet/msac/publishing.nsf/Content/CA1C4AC50025A411CA2575AD0082FDCA/$File/1059-Assessment-Report.pdf (accessed 1 August 2006).
- Varicose Veins in the Legs. NICE; 2013.
- Rasmussen LH, Lawaetz M, Bjoern L, Vennits B, Blemings A, Eklof B. Randomised clinical trial comparing endovenous laser ablation, radiofrequency ablation, foam sclerotherapy and surgical stripping for great saphenous varicose veins. Br J Surg 2011;98:1079-87. http://dx.doi.org/10.1002/bjs.7555.
- Figueiredo M, Araujo S, Barros NJ, Miranda FJ. Results of surgical treatment compared with ultrasound-guided foam sclerotherapy in patients with varicose veins: a prospective randomised study. Eur J Vasc Endovasc Surg 2009;38:758-63. http://dx.doi.org/10.1016/j.ejvs.2009.07.015.
- Biemans AAM, Kockaert M, Akkersdijk GP, van den Bos RR, De Maeseneer MGR, Cuypers P, et al. Comparing endovenous laser ablation, foam sclerotherapy, and conventional surgery for great saphenous varicose veins. J Vasc Surg 2013;58:727-34. http://dx.doi.org/10.1016/j.jvs.2012.12.074.
- Lattimer CR, Azzam M, Kalodiki E, Shawish E, Trueman P, Geroulakos G. Cost and effectiveness of laser with phlebectomies compared with foam sclerotherapy in superficial venous insufficiency. Early results of a randomised controlled trial. Eur J Vasc Endovasc Surg 2012;43:594-600. http://dx.doi.org/10.1016/j.ejvs.2012.01.032.
- Shadid N, Ceulen R, Nelemans P, Dirksen C, Veraart J, Schurink GW, et al. Randomized clinical trial of ultrasound-guided foam sclerotherapy versus surgery for the incompetent great saphenous vein. Br J Surg 2012;99:1062-70. http://dx.doi.org/10.1002/bjs.8781.
- Christenson JT, Gueddi S, Gemayel G, Bounameaux H. Prospective randomised trial comparing endovenous laser ablation and surgery for treatment of primary great saphenous varicose veins with a 2-year follow-up. J Vasc Surg 2010;52:1234-41. http://dx.doi.org/10.1016/j.jvs.2010.06.104.
- Carradice D, Mekako AI, Mazari FA, Samuel N, Hatfield J, Chetter IC. Clinical and technical outcomes from a randomised clinical trial of endovenous laser ablation compared with conventional surgery for great saphenous varicose veins. Br J Surg 2011;98:1117-23. http://dx.doi.org/10.1002/bjs.7615.
- Rasmussen LH, Bjoern L, Lawaetz M, Blemings A, Lawaetz B, Eklof B. Randomised trial comparing endovenous laser ablation of the great saphenous vein with high ligation and stripping in patients with varicose veins: short-term results. J Vasc Surg 2007;46:308-15. http://dx.doi.org/10.1016/j.jvs.2007.03.053.
- Samuel N, Carradice D, Wallace T, Mekako A, Hatfield J, Chetter I. Randomised clinical trial of endovenous laser therapy versus conventional surgery for short saphenous varicose veins. Ann Surg 2013;257:419-26. http://dx.doi.org/10.1097/SLA.0b013e318275f4e4.
- Rass K, Frings N, Glowacki P, Hamsch C, Graber S, Vogt T, et al. Comparable effectiveness of endovenous laser ablation and high ligation with stripping of the great saphenous vein: two-year results of a randomized clinical trial (RELACS study). Arch Dermatol 2012;148:49-58. http://dx.doi.org/10.1001/archdermatol.2011.272.
- Flessenkamper I, Hartmann M, Stenger D, Roll S. Endovenous laser ablation with and without high ligation compared with high ligation and stripping in the treatment of great saphenous varicose veins: initial results of a multicentre randomised controlled trial. Phlebology 2013;28:16-23.
- Pronk P, Gauw SA, Mooij MC, Gaastra MT, Lawson JA, van Goethem AR, et al. Randomised controlled trial comparing sapheno-femoral ligation and stripping of the great saphenous vein with endovenous laser ablation (980 nm) using local tumescent anaesthesia: one year results. Eur J Vasc Endovasc Surg 2010;40:649-56. http://dx.doi.org/10.1016/j.ejvs.2010.08.007.
- Edwards AG, Baynham S, Lees T, Mitchell DC. Management of varicose veins: a survey of current practice by members of the Vascular Society of Great Britain and Ireland. Ann R Coll Surg Engl 2009;91:77-80. http://dx.doi.org/10.1308/003588409X358953.
- Shepherd AC, Gohel MS, Hamish M, Lim CS, Davies AH. Endovenous treatments for varicose veins – over-taking or over-rated?. Phlebology 2010;25:38-43. http://dx.doi.org/10.1258/phleb.2009.008091.
- Kanwar A, Hansrani M, Lees T, Stansby G. Trends in varicose vein therapy in England: radical changes in the last decade. Ann R Coll Surg Engl 2010;92:341-6. http://dx.doi.org/10.1308/003588410X12518836440649.
- Health and Social Care Information Centre (HSCIC) . PROMs Outcomes Summary by Provider, April 2010–March 2011 2012. www.hscic.gov.uk/catalogue/PUB07049/fina-prom-eng-apr-10-mar-11-pre-post-tab3.xls (accessed 23 August 2013).
- Liu X, Jia X, Guo W, Xiong J, Zhang H, Liu M, et al. Ultrasound-guided foam sclerotherapy of the great saphenous vein with sapheno-femoral ligation compared to standard stripping: a prospective clinical study. Int Angiol 2011;30:321-6.
- Abela R, Liamis A, Prionidis I, Mathai J, Gorton L, Browne T, et al. Reverse foam sclerotherapy of the great saphenous vein with sapheno-femoral ligation compared to standard and invagination stripping: a prospective clinical series. Eur J Vasc Endovasc Surg 2008;36:485-90. http://dx.doi.org/10.1016/j.ejvs.2008.06.029.
- Bountouroglou DG, Azzam M, Kakkos SK, Pathmarajah M, Young P, Geroulakos G. Ultrasound-guided foam sclerotherapy combined with sapheno-femoral ligation compared to surgical treatment of varicose veins: early results of a randomised controlled trial. Eur J Vasc Endovasc Surg 2006;31:93-100. http://dx.doi.org/10.1016/j.ejvs.2005.08.024.
- Kalodiki E, Azzam M, Lattimer CR, Shawish E, Zambas N, Geroulakos G. Randomised controlled trial of ultrasound guided foam sclerotherapy combined with sapheno-femoral ligation compared to surgical treatment of varicose veins: five-year results. J Vasc Surg 2011;53:259-60. http://dx.doi.org/10.1016/j.jvs.2010.11.025.
- Rasmussen LH, Bjoern L, Lawaetz M, Blemings A, Eklof B. Randomised clinical trial comparing endovenous laser ablation with stripping of the great saphenous vein: clinical outcome and recurrence after 2 years. Eur J Vasc Endovasc Surg 2010;39:630-5. http://dx.doi.org/10.1016/j.ejvs.2009.11.040.
- Carradice D, Mekako AI, Mazari FAK, Samuel N, Hatfield J, Chetter IC. Randomised clinical trial of endovenous laser ablation compared with conventional surgery for great saphenous varicose veins. Br J Surg 2011;98:501-10. http://dx.doi.org/10.1002/bjs.7394.
- Rasmussen LH, Lawaetz M, Bjoern L, Blemings A, Eklof B. Randomized clinical trial comparing endovenous laser ablation and stripping of the great saphenous vein with clinical and duplex outcome after 5 years. J Vasc Surg 2013;58:421-6. http://dx.doi.org/10.1016/j.jvs.2012.12.048.
- van den Bos R, Arends L, Kockaert M, Neumann M, Nijsten T. Endovenous therapies of lower extremity varicosities: a meta-analysis. J Vasc Surg 2009;49:230-9. http://dx.doi.org/10.1016/j.jvs.2008.06.030.
- Luebke T, Brunkwall J. Systematic review and meta-analysis of endovenous radiofrequency obliteration, endovenous laser therapy, and foam sclerotherapy for primary varicosis. J Cardiovasc Surg (Torino) 2008;49:213-33.
- Gohel MS, Epstein DM, Davies AH. Cost-effectiveness of traditional and endovenous treatments for varicose veins. Br J Surg 2010;97:1815-23. http://dx.doi.org/10.1002/bjs.7256.
- Berridge D, Lees T, Earnshaw JJ. The VEnous INtervention (VEIN) Project. Phlebology 2009;24:1-2. http://dx.doi.org/10.1258/phleb.2009.09s001.
- Nesbitt C, Eifell RK, Coyne P, Badri H, Bhattacharya V, Stansby G. Endovenous ablation (radiofrequency and laser) and foam sclerotherapy versus conventional surgery for great saphenous vein varices (review). Cochrane Database Syst Rev 2011;10.
- Calvert M, Blazeby J, Altman DG, Revicki DA, Moher D, Brundage MD, et al. Reporting of patient-reported outcomes in randomized trials: the CONSORT PRO extension. JAMA 2013;309:814-22. http://dx.doi.org/10.1001/jama.2013.879.
- Michaels JA, Brazier JE, Campbell WB, Macintyre JB, Palfreyman SJ, Ratcliffe J. Randomized clinical trial comparing surgery with conservative treatment for uncomplicated varicose veins. Br J Surg 2006;93:175-81. http://dx.doi.org/10.1002/bjs.5264.
- Garratt AM, Macdonald LM, Ruta DA, Russell IT, Buckingham JK, Krukowski ZH. Towards measurement of outcome for patients with varicose veins. Qual Health Care 1993;2:5-10. http://dx.doi.org/10.1136/qshc.2.1.5.
- Garratt AM, Ruta DA, Abdalla MI, Russell IT. Responsiveness of the SF-36 and a condition-specific measure of health for patients with varicose veins. Qual Life Res 1996;5:223-34. http://dx.doi.org/10.1007/BF00434744.
- Ware JE. SF-36® Health Survey Update n.d. www.sf-36.org/tools/sf36.shtml (accessed 23 August 2013).
- Kundu S, Lurie F, Millward SF, Padberg F, Vedantham S, Elias S, et al. Recommended reporting standards for endovenous ablation for the treatment of venous insufficiency: joint statement of the American Venous Forum and the Society of Interventional Radiology. J Vasc Surg 2007;46:582-9. http://dx.doi.org/10.1016/j.jvs.2007.05.025.
- Moss-Morris R, Weinman J, Petrie KJ, Horne R, Cameron LD, Buick D. The Revised Illness Perception Questionnaire (IPQ-R). Psychol Health 2002;17:1-16. http://dx.doi.org/10.1080/08870440290001494.
- Data Protection Act 1988. London: The Stationery Office; 1988.
- Michaels JA, Campbell WB, Brazier JE, MacIntyre JB, Palfreyman SJ, Ratcliffe J, et al. Randomised clinical trial, observational study and assessment of cost-effectiveness of the treatment of varicose veins (REACTIV trial). Health Technol Assess 2006;10. http://dx.doi.org/10.3310/hta10130.
- White IR, Horton NJ, Carpenter J, Pocock SJ. Strategy for intention to treat analysis in randomised trials with missing outcome data. BMJ 2011;342. http://dx.doi.org/10.1136/bmj.d40.
- Fairclough DL. Design and Analysis of Quality of Life Studies in Clinical Trials. Boca Raton, FL: CRC Press; 2002.
- Leventhal H, Leventhal EA, Cameron L, Baum A, Taylor SE, Singer JE. Handbook of Health Psychology. Hillsdale, NJ: Lawrence Erlbaum; 2001.
- Liu R, Skelly M, Weinman J. Effects of background stress and anxiety on postoperative recovery. Anaesthesia 1994;49:382-6. http://dx.doi.org/10.1111/j.1365-2044.1994.tb03467.x.
- Philips HC. Avoidance behaviour and its role in sustaining chronic pain. Behav Res Ther 1987;25:273-9. http://dx.doi.org/10.1016/0005-7967(87)90005-2.
- Fergusson D, Aaron SD, Guyatt G, Hebert P. Post-randomisation exclusions: the intention to treat principle and excluding patients from analysis. BMJ 2002;325:652-4. http://dx.doi.org/10.1136/bmj.325.7365.652.
- Kundu S, Lurie F, Millward SF, Padberg FTJ, Vedantham S, Elias SM, et al. Recommended reporting standards for endovenous ablation for the treatment of venous insufficiency: joint statement of the American Venous Forum and the Society of Interventional Radiology. J Vasc Interv Radiol 2009;20:417-24. http://dx.doi.org/10.1016/j.jvir.2009.04.019.
- Carradice D, Mekako AI, Hatfield J, Chetter IC. Randomised clinical trial of concomitant or sequential phlebectomy after endovenous laser therapy for varicose veins. Br J Surg 2009;96:369-75. http://dx.doi.org/10.1002/bjs.6556.
- World Health Organization (WHO) . International Classification of Functioning, Disability and Health 2001. www.who.int/classifications/icf/en/ (accessed 23 August 2013).
- Dixon D, Johnston M, French DP, Kaptein AA, Vedhara K, Weinmann J. Health Psychology. Oxford: Wiley-Blackwell; 2010.
- Francis JJ, Duncan EM, Prior ME, MacLennan G, Marshall AP, Wells EC, et al. Comparison of four methods for assessing the importance of attitudinal beliefs: an international Delphi study in intensive care settings. Br J Health Psychol 2014;19:274-91. http://dx.doi.org/10.1111/bjhp.12066.
- Royston P, Parmar MKB. Flexible proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med 2002;21:2175-97. http://dx.doi.org/10.1002/sim.1203.
- Stata Multiple-Imputation Reference Manual Release 12. College Station, TX: StataCorp LP; 2012.
- Royston P. Flexible alternatives to the Cox model, and more. Stata J 2001;1:1-28.
- Ross MD. The relationship between functional levels and fear-avoidance beliefs following anterior cruciate ligament reconstruction. J Orthop Traumatol 2010;11:237-43. http://dx.doi.org/10.1007/s10195-010-0118-7.
- Lethem J, Slade PD, Troup JD, Bentley G. Outline of a Fear-Avoidance Model of exaggerated pain perception – I. Behav Res Ther 1983;21:401-8. http://dx.doi.org/10.1016/0005-7967(83)90009-8.
- Dolan P, Gudex C, Kind P, Williams A. The time trade-off method: results from a general population study. Health Econ 1996;5:141-54. http://dx.doi.org/10.1002/(SICI)1099-1050(199603)5:2<141::AID-HEC189>3.0.CO;2-N.
- Brazier J, Roberts J, Deverill M. The estimation of a preference-based measure of health from the SF-36. J Health Econ 2002;21:271-92. http://dx.doi.org/10.1016/S0167-6296(01)00130-8.
- Glick H, Doshi J, Sonnad S, Polsky D. Economic Evaluation in Clinical Trials. New York, NY: Oxford University Press; 2007.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2012.
- Department of Health . NHS Reference Costs 2010–11 2011. www.gov.uk/government/publications/2010-11-reference-costs-publication (accessed October 2012).
- Curtis L. Unit Costs of Health and Social Care 2011. Canterbury: PSSRU, University of Kent; 2011.
- ISD Scotland . ISD Scotland 63 n.d. www.isdscotland.org/Health-Topics/Finance/Costbook/Speciality-Costs/Overhead.asp (accessed October 2012).
- Department for Transport . Value of Time and Operating Costs: Transport Analysis Guidance (TAG) 2013. www.dft.gov.uk/webtag/documents/archive/1208/unit3.5.6.pdf (accessed August 2013).
- Office for National Statistics (ONS) . 2011 Annual Survey of Hours and Earnings (SOC 2000) 2011. www.ons.gov.uk/ons/dcp171778_241497.pdf (accessed December 2012).
- Twenty-sixth Report 2012. London: The Stationery Office; 2012.
- Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, NY: J. Wiley & Sons; 1987.
- Siribumrungwong B, Noorit P, Wilasrusmee C, Attia J, Thakkinstian A. A systematic review and meta-analysis of randomised controlled trials comparing endovenous ablation and surgical intervention in patients with varicose veins. Eur J Vasc Endovasc Surg 2012;44:214-23. http://dx.doi.org/10.1016/j.ejvs.2012.05.017.
- Perala J, Rautio T, Biancari F, Ohtonen P, Wiik H, Heikkinen T, et al. Radiofrequency endovenous obliteration versus stripping of the long saphenous vein in the management of primary varicose veins: 3-year outcome of a randomized study. Ann Vasc Surg 2005;19:669-72. http://dx.doi.org/10.1007/s10016-005-6613-2.
- Office for National Statistics (ONS) . Interim Life Tables 2007–2009 n.d. www.ons.gov.uk/ons/publications/re-reference-tables.html?edition=tcm%3A77-61850 (accessed 27 August 2013).
- National Institute for Health and Care Excellence (NICE) . Guide to the Methods of Technology Appraisal 2013 n.d. www.nice.org.uk/article/chapter/1-introduction (accessed 15 June 2013).
- Vasquez MA, Rabe E, McLafferty RB, Shortell CK, Marston WA, Gillespie D, et al. Revision of the venous clinical severity score: venous outcomes consensus statement: special communication of the American Venous Forum Ad Hoc Outcomes Working Group. J Vasc Surg 2010;52:1387-96. http://dx.doi.org/10.1016/j.jvs.2010.06.161.
- Bradbury AW, Adam DJ, Bell J, Forbes JF, Fowkes FG, Gillespie I, et al. Multicentre randomised controlled trial of the clinical and cost-effectiveness of a bypass-surgery-first versus a balloon-angioplasty-first revascularisation strategy for severe limb ischaemia due to infrainguinal disease. The Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) trial. Health Technol Assess 2010;14. http://dx.doi.org/10.3310/hta14140.
- Anon . Recruitment of women to clinical trials. Lancet 2001;358. http://dx.doi.org/10.1016/S0140-6736(01)06078-0.
- Benson JR. Recruitment of women into trials. Lancet 2002;359. http://dx.doi.org/10.1016/S0140-6736(02)07341-5.
- Thornton H, Dixon-Woods M. Recruitment of women into trials. Lancet 2002;359. http://dx.doi.org/10.1016/S0140-6736(02)07342-7.
- Smyth A, Hyman-Taylor P, Lewis S, Knox A, Stephenson T. Recruitment of women into trials. Lancet 2002;359:165-6. http://dx.doi.org/10.1016/S0140-6736(02)07344-0.
- Creel AH, Losinia E, Mandi LA, Marx RJ, Mahomed NN, Martin SD, et al. An assessment of willingness to participate in a randomised trial of arthroscopic knee surgery in patients with osteoarthritis. Contemp Clin Trials 2005;26:169-78. http://dx.doi.org/10.1016/j.cct.2004.12.010.
- Myles PS, Fletcher HE, Cairo S, Madder H, McRae R, Cooper J, et al. Randomised trial of informed consent and recruitment for clinical trials in the immediate preoperative period. Anaesthesiology 1999;91:969-78. http://dx.doi.org/10.1097/00000542-199910000-00016.
- Referral Advice: A Guide to Appropriate Referral From General to Specialist Services: Varicose Veins. London: NICE; 2001.
- Beresford T, Smith JJ, Brown L, Greenhalgh RM, Davies AH. A comparison of health-related quality of life of patients with primary and recurrent varicose veins. Phlebology 2003;18:35-7. http://dx.doi.org/10.1258/026835503321236885.
- Darvall KAL, Bate GR, Adam DJ, Bradbury AW. Recovery after ultrasound-guided foam sclerotherapy compared with conventional surgery for varicose veins. Br J Surg 2009;96:1262-7. http://dx.doi.org/10.1002/bjs.6754.
Appendix 1 Trial paperwork
Appendix 2 Additional trial results tables
| Pain VAS scores | Randomised to foam sclerotherapy | Randomised to surgery | Surgery vs. foam sclerotherapya | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | Effect size | 95% CI | p-value | |
| Pain VAS scores completed immediately after treatment | 226 | 2.2 | 1.9 | 163 | 2.3 | 2.6 | 0.06 | −0.38 to 0.50 | 0.796 |
| Pain VAS scores completed at 6 weeks | |||||||||
| During treatment | 240 | 3.0 | 2.4 | 227 | 4.0 | 3.0 | 1.04 | 0.57 to 1.52 | < 0.001 |
| During recovery | 239 | 3.0 | 2.4 | 229 | 4.3 | 2.8 | 1.21 | 0.76 to 1.66 | < 0.001 |
| Pain VAS scores | Randomised to EVLA | Randomised to foam sclerotherapy | Randomised to surgery | Surgery vs. EVLA | EVLA vs. foam | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | Effect size | 95% CI | p-value | Effect size | 95% CI | p-value | |
| Pain VAS scores completed immediately after treatment | 171 | 3.5 | 2.2 | 170 | 2.2 | 2.0 | 137 | 2.4 | 2.6 | −1.12 | −1.61 to −0.62 | < 0.001 | 1.25 | 0.79 to 1.72 | < 0.001 |
| Pain VAS scores completed at 6 weeks | |||||||||||||||
| Pain during treatment | 178 | 4.4 | 2.8 | 176 | 3.0 | 2.5 | 165 | 4.1 | 3.0 | −0.23 | −0.78 to 0.33 | 0.418 | 1.40 | 0.86 to 1.95 | < 0.001 |
| Pain during recovery | 178 | 3.4 | 2.6 | 176 | 2.8 | 2.3 | 166 | 4.2 | 2.7 | 0.79 | 0.28 to 1.30 | 0.002 | 0.60 | 0.10 to 1.11 | 0.019 |
| Illness perception component | Randomised to foam sclerotherapy | Randomised to surgery | ||||
|---|---|---|---|---|---|---|
| Identity score | ||||||
| Baseline (n, mean, SD) | 265 | 2.6 | 1.5 | 274 | 2.9 | 1.6 |
| Post randomisation (n, mean, SD) | 137 | 2.7 | 1.5 | 117 | 3.1 | 1.6 |
| 6 months (n, mean, SD) | 212 | 2.4 | 1.6 | 179 | 2.1 | 1.7 |
| Percentage of symptoms correctly identified as being related to varicose veins | ||||||
| Baseline (n, mean, SD) | 263 | 74.7% | 29.8% | 274 | 75.2% | 27.4% |
| Post randomisation (n, mean, SD) | 136 | 79.3% | 27.1% | 121 | 78.3% | 25.8% |
| 6 months (n, mean, SD) | 205 | 82.1% | 27.2% | 154 | 77.1% | 31.4% |
| Timeline acute/chronic | ||||||
| Baseline (n, mean, SD) | 280 | 23.7 | 4.8 | 280 | 23.6 | 4.5 |
| Post randomisation (n, mean, SD) | 142 | 23.5 | 4.6 | 125 | 23.8 | 4.8 |
| 6 months (n, mean, SD) | 228 | 21.0 | 5.2 | 196 | 21.2 | 5.1 |
| Timeline cyclical | ||||||
| Baseline (n, mean, SD) | 279 | 10.7 | 3.4 | 280 | 10.9 | 3.2 |
| Post randomisation (n, mean, SD) | 142 | 10.5 | 3.1 | 126 | 11.0 | 3.3 |
| 6 months (n, mean, SD) | 228 | 9.6 | 3.2 | 199 | 9.9 | 3.2 |
| Consequences | ||||||
| Baseline (n, mean, SD) | 281 | 15.9 | 4.2 | 281 | 16.1 | 4.4 |
| Post randomisation (n, mean, SD) | 142 | 16.1 | 4.0 | 126 | 15.6 | 4.3 |
| 6 months (n, mean, SD) | 227 | 13.6 | 4.0 | 196 | 13.9 | 4.1 |
| Personal control | ||||||
| Baseline (n, mean, SD) | 279 | 18.6 | 4.2 | 279 | 18.3 | 3.8 |
| Post randomisation (n, mean, SD) | 142 | 18.7 | 3.8 | 126 | 18.4 | 4.2 |
| 6 months (n, mean, SD) | 227 | 19.0 | 4.2 | 197 | 18.9 | 4.1 |
| Treatment control | ||||||
| Baseline (n, mean, SD) | 279 | 19.5 | 2.5 | 278 | 19.3 | 2.3 |
| Post randomisation (n, mean, SD) | 141 | 19.1 | 2.5 | 126 | 19.0 | 2.3 |
| 6 months (n, mean, SD) | 227 | 18.8 | 2.8 | 197 | 18.4 | 2.9 |
| Illness coherence | ||||||
| Baseline (n, mean, SD) | 276 | 17.7 | 4.1 | 277 | 17.6 | 3.7 |
| Post randomisation (n, mean, SD) | 142 | 17.9 | 4.1 | 125 | 18.2 | 4.2 |
| 6 months (n, mean, SD) | 225 | 18.2 | 3.8 | 196 | 18.6 | 3.9 |
| Emotional representations | ||||||
| Baseline (n, mean, SD) | 281 | 15.4 | 4.5 | 280 | 14.9 | 4.7 |
| Post randomisation (n, mean, SD) | 142 | 15.6 | 4.5 | 126 | 15.1 | 5.0 |
| 6 months (n, mean, SD) | 228 | 13.4 | 4.6 | 200 | 13.5 | 4.3 |
| Illness perception | Randomised to EVLA | Randomised to foam sclerotherapy | Randomised to surgery | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Identity score | |||||||||
| Baseline (n, mean, SD) | 195 | 2.7 | 1.5 | 193 | 2.6 | 1.6 | 202 | 2.9 | 1.5 |
| Post randomisation (n, mean, SD) | 84 | 2.9 | 1.7 | 88 | 2.6 | 1.5 | 73 | 3.1 | 1.7 |
| 6 months (n, mean, SD) | 141 | 2.2 | 1.7 | 149 | 2.3 | 1.6 | 125 | 2.1 | 1.7 |
| Percentage of symptoms correctly identified as being related to varicose veins | |||||||||
| Baseline (n, mean, SD) | 200 | 72.4% | 31.1% | 193 | 73.8% | 30.1% | 201 | 75.1% | 28.4% |
| Post randomisation (n, mean, SD) | 83 | 75.8% | 27.8% | 87 | 78.7% | 27.6% | 77 | 77.1% | 27.1% |
| 6 months (n, mean, SD) | 133 | 79.0% | 32.5% | 147 | 79.6% | 29.2% | 108 | 74.2% | 33.1% |
| Timeline acute/chronic | |||||||||
| Baseline (n, mean, SD) | 209 | 23.6 | 4.7 | 206 | 23.4 | 4.8 | 206 | 23.1 | 4.5 |
| Post randomisation (n, mean, SD) | 90 | 23.1 | 4.7 | 92 | 23.4 | 4.7 | 80 | 23.4 | 4.5 |
| 6 months (n, mean, SD) | 161 | 20.6 | 4.8 | 166 | 20.8 | 5.1 | 141 | 21.0 | 5.1 |
| Timeline cyclical | |||||||||
| Baseline (n, mean, SD) | 208 | 10.5 | 3.3 | 203 | 11.1 | 3.3 | 209 | 11.0 | 3.3 |
| Post randomisation (n, mean, SD) | 90 | 10.4 | 3.0 | 92 | 10.7 | 3.1 | 81 | 11.1 | 3.5 |
| 6 months (n, mean, SD) | 159 | 9.5 | 3.1 | 164 | 9.7 | 3.1 | 144 | 10.1 | 3.2 |
| Consequences | |||||||||
| Baseline (n, mean, SD) | 209 | 16.4 | 4.3 | 206 | 16.2 | 4.3 | 207 | 16.0 | 4.2 |
| Post randomisation (n, mean, SD) | 90 | 16.0 | 3.6 | 92 | 16.0 | 3.8 | 81 | 15.7 | 4.7 |
| 6 months (n, mean, SD) | 160 | 13.8 | 3.6 | 165 | 13.6 | 3.9 | 141 | 14.0 | 4.0 |
| Personal control | |||||||||
| Baseline (n, mean, SD) | 209 | 18.5 | 4.1 | 205 | 18.7 | 4.1 | 206 | 18.6 | 3.7 |
| Post randomisation (n, mean, SD) | 89 | 18.7 | 4.1 | 92 | 18.6 | 3.9 | 81 | 18.7 | 4.3 |
| 6 months (n, mean, SD) | 158 | 18.9 | 3.9 | 165 | 19.3 | 4.1 | 142 | 19.3 | 4.1 |
| Treatment control | |||||||||
| Baseline (n, mean, SD) | 209 | 19.6 | 2.3 | 205 | 19.3 | 2.5 | 205 | 19.3 | 2.3 |
| Post randomisation (n, mean, SD) | 90 | 19.2 | 2.0 | 91 | 18.9 | 2.3 | 81 | 18.9 | 2.2 |
| 6 months (n, mean, SD) | 157 | 18.8 | 2.5 | 165 | 18.8 | 2.5 | 142 | 18.3 | 3.0 |
| Illness coherence | |||||||||
| Baseline (n, mean, SD) | 208 | 17.4 | 3.8 | 203 | 17.4 | 4.1 | 206 | 17.5 | 3.7 |
| Post randomisation (n, mean, SD) | 90 | 17.8 | 3.8 | 92 | 17.3 | 4.2 | 81 | 17.6 | 4.3 |
| 6 months (n, mean, SD) | 156 | 17.9 | 3.3 | 163 | 17.7 | 3.9 | 141 | 18.4 | 3.8 |
| Emotional representations | |||||||||
| Baseline (n, mean, SD) | 208 | 16.1 | 4.7 | 205 | 15.7 | 4.5 | 209 | 14.9 | 4.6 |
| Post randomisation (n, mean, SD) | 90 | 16.2 | 4.8 | 92 | 15.6 | 4.4 | 81 | 15.4 | 5.4 |
| 6 months (n, mean, SD) | 159 | 13.6 | 4.2 | 164 | 13.5 | 4.5 | 145 | 13.5 | 4.6 |
Appendix 3 Behavioural Recovery After treatment for Varicose Veins study paperwork
Appendix 4 Resource use and costing questionnaire
List of abbreviations
- ARI
- Aberdeen Royal Infirmary
- AVVQ
- Aberdeen Varicose Vein Questionnaire
- BMI
- body mass index
- BRAVVO
- Behavioural Recovery After treatment for Varicose Veins
- CEAC
- cost-effectiveness acceptability curve
- CEAP
- clinical, etiological, anatomical, pathological
- CHaRT
- Centre for Healthcare Randomised Trials
- CI
- confidence interval
- CIVIQ
- Chronic Venous Insufficiency Quality of Life Questionnaire
- CLASS
- Comparison of LAser, Surgery and foam Sclerotherapy
- CONSORT
- Consolidated Standards of Reporting Trials
- CRF
- case report form
- CS-SRM
- common-sense self-regulation model
- CTA
- clinical trial authorisation
- DMC
- Data Monitoring Committee
- DVT
- deep-vein thrombosis
- ECG
- electrocardiograph
- EQ-5D
- European Quality of Life-5 Dimensions
- EVLA
- endovenous laser ablation
- GLM
- generalised linear model
- GP
- general practitioner
- GSV
- great saphenous vein
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- HRT
- hormonal replacement therapy
- HTA
- Health Technology Assessment
- ICER
- incremental cost-effectiveness ratio
- ICF
- International Classification of Disability and Function
- IMP
- investigational medicinal product
- IPQ-R
- Illness Perception Questionnaire – Revised
- MHRA
- Medicines and Healthcare Products Regulatory Agency
- MNAR
- missing not at random
- NICE
- National Institute for Health and Care Excellence
- NMB
- net monetary benefit
- OC
- oral contraceptive
- OLS
- ordinary least squares
- OR
- odds ratio
- PIL
- patient information leaflet
- PROM
- patient-reported outcome measure
- PSA
- probabilistic sensitivity analysis
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QoL
- quality of life
- Rc
- ceiling ratio
- RCT
- randomised controlled trial
- RFA
- radiofrequency ablation
- SAE
- serious adverse event
- SD
- standard deviation
- SF-6D
- Short Form questionnaire-6 Dimensions
- SF-36
- Short Form questionnaire-36 items
- SFJ
- saphenofemoral junction
- SSV
- small saphenous vein
- STS
- sodium tetradecyl sulphate
- TSC
- Trial Steering Committee
- VAS
- visual analogue scale
- VCSS
- Venous Clinical Severity Score
- WTP
- willingness to pay