Notes
Article history
The research reported in this issue of the journal was commissioned and funded by the HTA programme on behalf of NICE as project number 12/62/01. The protocol was agreed in June 2013. The assessment report began editorial review in January 2014 and was accepted for publication in May 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Michael Fisher has received consultancy fees from Daiichi Sankyo Company Ltd.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Greenhalgh et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Description of health problem
Acute coronary syndromes (ACSs) are life-threatening conditions associated with acute myocardial ischaemia with or without infarction. 1 These conditions usually result from a reduction in blood flow associated with a coronary artery becoming narrow or blocked through atherosclerosis (an accumulation of plaque containing fatty deposits or, less commonly, erosion of the endothelium) and atherothrombosis (a blood clot formed following the rupture of plaque). The classic symptom of ACS is chest pain or tightness, although many people (particularly women, the elderly and those with diabetes mellitus) may present with atypical pain or no pain at all. 2–4 Other symptoms may include breathlessness, sweating and nausea. 2–4
The underlying cause of ACS is build-up of atheroma within the wall of the coronary artery. This occurs over a number of years and is generally asymptomatic. 5 The risk factors for ACS are multifactorial and are the same as for cardiovascular (CV) disease. Among the non-modifiable risk factors are increasing age, sex (male) and a family history of premature coronary heart disease or premature menopause. Modifiable risk factors include smoking, diabetes mellitus (and impaired glucose tolerance), hypertension, dyslipidaemia, obesity and physical inactivity. 1,5 People with a history of myocardial infarction (MI) have an increased risk of recurrence or of other vascular events (e.g. stroke) when compared with the general population. 6
There are three main types of ACS diagnosed by clinical history, electrocardiography (ECG) and levels of cardiac enzymes: (1) ST segment elevation myocardial infarction (STEMI), (2) non-ST segment elevation myocardial infarction (NSTEMI) and (3) unstable angina (UA). A diagnosis of STEMI indicates that the affected artery is completely occluded, resulting in progressive necrosis of the area of heart muscle dependent on its blood supply. 5,7 The most common cause of a STEMI is complete and persistent occlusion of a coronary artery by a blood clot (thrombus). 8 A diagnosis of NSTEMI indicates partial or temporary blocking of an artery with limited tissue damage. 5,7 In the case of UA, clinical history suggests cardiac ischaemia, but without tissue death. 5,7
Over time, any damage sustained by the heart muscle results in scar tissue. The degree of the damage impacts on the overall ability of the heart to pump blood, which in turn impacts on the patient’s longer-term survival. 8 The timely treatment of ACS is imperative as almost half of potentially salvageable heart muscle is lost within 1 hour of the coronary artery being occluded, and two-thirds is lost within 3 hours. 8 One treatment for ACS is percutaneous coronary intervention (PCI), also known as coronary angioplasty. In PCI, the affected coronary artery is dilated using a balloon catheter and a stent is usually implanted to act as a scaffold and to hold open the artery wall. 9 All PCI procedures are accompanied by adjunctive treatment with antiplatelet drugs. These drugs are the focus of this review.
Treatment pathway
ST segment elevation myocardial infarction
The objective of treatment for patients with STEMI is rapid and sustained revascularisation. 10 The recommended treatment for people with confirmed STEMI is immediate (primary) PCI to the occluded artery. 9,11 Clinical guidelines produced by National Institute for Health and Care Excellence (NICE) (CG1678) recommend coronary angiography with follow-on PCI (if indicated) as the preferred treatment for acute STEMI if presentation is within 12 hours of the onset of symptoms and primary PCI can be delivered within 120 minutes. When PCI facilities are not immediately available, treatment with thrombolysis (pharmacological reperfusion achieved through the use of ‘clot-busting’ drugs) should be considered. 12 When STEMI persists despite thrombolytic treatment, PCI (rescue) in an appropriately equipped unit should be considered. 8
Unstable angina/non-ST segment elevation myocardial infarction
The objective of treatment for patients with UA/NSTEMI is to alleviate pain and anxiety, prevent recurrences of ischaemia and prevent, or limit, progression to further acute MI. 1 NICE Clinical Guideline CG9413 recommends that people presenting with UA/NSTEMI are initially treated with aspirin and antithrombin therapy. Their risk of further cardiac events should then be assessed using a risk score measurement tool that predicts 6-month mortality, such as the Global Registry of Acute Cardiac Events (GRACE). 14 In addition to a GRACE14 score, additional factors should be considered, including full clinical history [age, previous MI, previous coronary artery bypass grafting (CABG)], physical examination (including measurement of blood pressure and heart rate), and resting 12-lead ECG and blood tests (troponin I or T, creatinine, glucose and haemoglobin). Table 1 is adapted from NICE CG9413 and describes the risk categories of future CV events assigned to risk scores.
| Predicted 6-month mortality | Risk of future adverse CV events |
|---|---|
| ≤ 1.5% | Lowest |
| > 1.5–3.0% | Low |
| > 3.0–6.0% | Intermediate |
| > 6.0–9.0% | High |
| > 9.0% | Highest |
Patients considered to be at intermediate to high risk should be offered coronary angiography and follow-on PCI (if appropriate) within 96 hours of admission. 15 Patients with UA/NSTEMI who are clinically unstable or at high ischaemic risk should be offered angiography as soon as possible. 13 Patients at low risk should be treated medically; however, if ischaemia is subsequently experienced or is demonstrated on ischaemia testing, coronary angiography and delayed PCI (if appropriate) should be offered. 13
Epidemiology
The Myocardial Ischaemia National Audit Project5 (MINAP) is a national clinical audit of the management of heart attack. All hospitals in England, Wales and Belfast that admit patients with STEMI or NSTEMI contribute data (with the exception of Scarborough Hospital).
The most recent audit report5 presents analyses for admissions between April 2012 and March 2013. The audit recorded 80,974 patients with a final diagnosis of MI; 40% (32,665) of cases were diagnosed as STEMI and 60% (48,309) were diagnosed as NSTEMI. The average age of patients with STEMI and NSTEMI was 65 years and 72 years, respectively. 5
The authors of the report5 emphasise that the audit records the majority of admissions for STEMI but that NSTEMI admissions are under-represented.
Of the total number of patient admissions for STEMI, MINAP5 recorded that 68% (20,990) had primary PCI. The remaining patients received thrombolytic treatment (3%), no reperfusion treatment or treatment that was unclear (29%). 5
The Assessment Group (AG) notes that the MINAP5 data set does not include data for patients with UA as this condition does not fall under the audit’s MI remit. However, the AG is aware that, in England in 2012 to 2013, there were 54,000 finished consultant episodes and 32,000 patient admissions for UA. 16
British Cardiovascular Intervention Society Audit Data
The British Cardiovascular Intervention Society (BCIS) continuously audits interventional activity in the UK and the results are published annually. The most recent audit returns are for the year 2012. 17 The audit shows that there are currently 99 NHS PCI centres in the UK, almost double the number recorded in 2002. In 2012, 91,000 PCI procedures (for all indications) were carried out in the UK NHS, 27.4% in STEMI patients and 36.9% in UA/NSTEMI patients; the remainder were rescue or facilitated PCIs. A total of 24,631 PCIs for STEMI were conducted, the majority of which (23,842) were primary PCIs. The number of PCIs for STEMI has increased over time while the number of PCIs for UA/NSTEMI has remained stable.
Of patients referred for PCI in the UK in 2012, 74% were male and the average age was 64.9 years. 17 Approximately 20% had diabetes mellitus and 27% had had a previous MI. 17 One-quarter were current smokers and the majority (92%) were European. 17 It should be noted that these data are for an overall population of patients treated with PCI and, therefore, include patients other than those with ACS.
There are 85 NHS PCI centres in England and four in Wales. The total number of PCIs (all indications) performed in the NHS in England and Wales in 2012 was 75,217 and 3850, respectively. Almost 21,000 PCI procedures in England and 1000 in Wales were primary PCI procedures.
The BCIS audit data17 show that the number of PCIs performed in England and Wales has increased annually, although the rate of increase has slowed. In 2002, fewer than 30,000 procedures were carried out and, in contrast, almost 80,000 PCIs were conducted in 2012. The BCIS data describe the use of the radial artery (guidewire inserted through the wrist) as the access point for PCI. Radial access has risen to 65% of PCIs conducted in 2012 from 10% in 2004.
Antiplatelet treatment
Treatment with antiplatelet therapy is an established adjunct to PCI both before and for up to 12 months after the procedure (NICE CG1678 and NICE CG94). 13 The purpose of antiplatelet treatment is to inhibit the aggregation of platelets that can lead to thrombus formation and further vascular events including stent thrombosis. Dual antiplatelet therapy, aspirin plus prasugrel (Efient®, Daiichi Sankyo Company Ltd UK/Eli Lilly and Company Ltd), clopidogrel or ticagrelor (Brilique®, AstraZeneca), is the standard antiplatelet treatment in clinical practice in the UK.
Relevant national guidelines
A quality standard for ACS has been referred for consideration to NICE and, at the time of writing, was expected to be published in September 2014. 18,19 A treatment pathway for patients with ACS is also available on the NICE website. 20
A number of NICE guidance documents and NICE guidelines are relevant to this review. These are described in Table 2.
| NICE documentation | Recommendation |
|---|---|
| TA18221 (2009): prasugrel for the treatment of ACSs with PCI | Prasugrel in combination with aspirin is recommended as an option for preventing atherothrombotic events in people with ACS having PCI, only when:
|
| CG9413 (2010): UA and NSTEMI: the early management of UA and NSTEMI | Offer a 300-mg loading dose of clopidogrel to all patients with no contraindications who may undergo PCI within 24 hours of admission to hospital |
| In line with Prasugrel for the treatment of ACSs with PCI (TA182), prasugrel in combination with aspirin is an option for patients undergoing PCI who have diabetes or have had stent thrombosis with clopidogrel treatment | |
| It is recommended that treatment with clopidogrel in combination with low-dose aspirin should be continued for 12 months after the most recent acute episode of NSTEMI. Thereafter, standard care, including treatment with low-dose aspirin alone, is recommended | |
| TA23622 (2011): ticagrelor for the treatment of ACSs | Ticagrelor in combination with low-dose aspirin is recommended for up to 12 months as a treatment option in adults with ACS, that is, people:
|
| CG17223 (2013): secondary prevention in primary and secondary care for patients following a MI (CG172 is an update of CG48) | Aspirin should be offered to all people after a MI and continued indefinitely, unless individuals are aspirin intolerant or have an indication for anticoagulation |
| For patients with aspirin hypersensitivity, clopidogrel monotherapy should be considered as an alternative treatment | |
Clopidogrel is a treatment option for up to 12 months for:
|
|
| Ticagrelor is also recommended as per TA236 noted above | |
| Prasugrel for the treatment of ACS has not been incorporated in this guidance because this technology appraisal is currently scheduled for update | |
| There are special recommendations for antiplatelet therapy in people with an indication for anticoagulation | |
| CG1678 (2013): STEMI: the acute management of myocardial infarction with ST segment elevation | Following reperfusion therapy for STEMI, treatment with aspirin should be continued in line with CG48 MI secondary preventiona |
| The Guideline Development Group considered that treatment with clopidogrel is an established option in the pharmacological treatment of people with acute STEMI including people undergoing primary PCI. The Guideline Development Group were aware that a clopidogrel loading dose of 600 mg is not licensed in the UK, but is used widely in current practice, especially in people undergoing primary PCI | |
| Prasugrel was noted as a recommended treatment from TA182 and is the subject of this current appraisal | |
| Ticagrelor is recommended as in TA236 |
Description of technology under assessment
Intervention
The oral antiplatelet prasugrel, used within its licensed indication, is the focus of this review. The Summary of Product Characteristics (SPC) for prasugrel is available from the Electronic Medicines Compendium. 24
Prasugrel is a third-generation oral thienopyridine adenosine diphosphate receptor antagonist. It has a more rapid onset of action than clopidogrel as it requires only a single, relatively rapid metabolic step to produce the active agent (clopidogrel requires two steps). Prasugrel is prescribed as an adjunctive therapy to PCI to reduce platelet aggregation by irreversibly binding to P2Y12 receptors. It is available as 5-mg or 10-mg film-coated tablets. Prasugrel is given (with aspirin) as a single 60-mg loading dose and then continued at 10 mg daily for up to 12 months.
Prasugrel is licensed in Europe25 to be co-administered with aspirin, for the prevention of atherothrombotic events in patients with ACS (STEMI and UA/NSTEMI) undergoing primary or delayed PCI. As stated in the SPC, the use of prasugrel in patients with a history of stroke or transient ischaemic attack (TIA) is contraindicated, whereas in older (≥ 75 years) patients prasugrel is generally not recommended. For patients who weigh < 60 kg, the 60-mg loading dose of prasugrel should be used followed by a maintenance dose of 5 mg. 24 The SPC further states that, in patients with UA/NSTEMI in whom coronary angioplasty is performed within 48 hours after admission, the loading dose of prasugrel should be given only at the time of PCI.
NICE guidance (TA18221) limits the use of prasugrel (co-administered with aspirin) in the NHS to people with ACS having PCI only when:
-
immediate primary PCI for STEMI is necessary
-
stent thrombosis has occurred during clopidogrel treatment
-
the patient has diabetes mellitus.
In TA182,21 prasugrel was not recommended for patients with UA/NSTEMI who do not have diabetes mellitus or have not had a stent thrombosis following treatment with clopidogrel.
There is no patient access scheme in operation in the NHS for prasugrel.
The SPC for prasugrel highlights the increased bleeding risk for patients with ACS who are treated with prasugrel and aspirin. It is noted that the use of prasugrel in patients at increased risk of bleeding should be considered only when the benefits in terms of preventing ischaemic events are deemed to outweigh the risk of serious bleeding. 24
Current usage in the NHS
The decision paper26 presented to the Guidance Executive of NICE in June 2012 stated that the market share for prasugrel in terms of prescriptions had risen from 1% to 2% since 2011 and the monthly spend in the NHS had increased from approximately £400,000 to approximately £500,000. Data from the BCIS audit18 illustrate that prasugrel use has increased marginally between 2011 and 2012 (Table 3).
| Patient group | 2011 | 2012 |
|---|---|---|
| UA/NSTEMI | 1.5% | 2.6% |
| STEMI | 22% | 22.6% |
| UA/NSTEMI patients with diabetes mellitus | 1.7% | 2.8% |
The current British National Formulary (BNF)27 list price of prasugrel for both 5-mg and 10-mg tablets is £47.56 per pack of 28 tablets. The current Drug Tariff28 list price of aspirin 75 mg is 0.82 pence per pack of 28 tablets.
Comparators
The stated comparators to prasugrel in the final scope issued by NICE7 are clopidogrel (generic) and ticagrelor, both in combination with low-dose aspirin.
Clopidogrel
Clopidogrel is a thienopyridine and is available as a 300-mg and 75-mg film-coated tablet. The 300-mg tablet is intended as a loading dose for patients with ACS and treatment should be continued at 75 mg daily with aspirin (75–325 mg). Clopidogrel has a marketing authorisation for use in several patient groups relevant to this appraisal:
-
patients with MI (from a few days until < 35 days)
-
patients with STEMI in combination with aspirin who are eligible for thrombolytic therapy
-
patients with NSTEMI undergoing a stent placement following PCI, in combination with aspirin.
The AG notes that, according to its European Medicines Agency (EMA) licence, clopidogrel is not indicated for use in STEMI patients undergoing PCI. The patent for clopidogrel (Plavix, Sanofi) expired in 2010 and a number of generic versions are now licensed. This means that the cost of clopidogrel has substantially reduced since prasugrel was considered by NICE in 2009 (TA182). 21
In the SPC, increased bleeding risk with clopidogrel use is noted, as is a possible interaction with proton pump inhibitors. 29
The current Drug Tariff28 list price for clopidogrel is £1.71 per pack of 28 tablets.
Ticagrelor
Ticagrelor is a direct-acting P2Y12 receptor antagonist that has a different mechanism of action from the thienopyridines (prasugrel and clopidogrel). It has a rapid onset of action compared with clopidogrel and is a reversibly binding oral adenosine phosphate receptor antagonist. Ticagrelor is licensed in Europe30 (co-administered with aspirin) for the prevention of atherothrombotic events in adult patients with ACS (UA/NSTEMI or STEMI), including patients managed medically and those who are managed with PCI or CABG. Ticagrelor is administered as a 90-mg film-coated tablet. Treatment should be started with a single 180-mg loading dose (two 90-mg tablets) and then continued at 90 mg twice daily. The recommended use of ticagrelor is a single course of treatment up to 12 months with aspirin. 31
In the UK, NICE guidance (TA23622) recommends ticagrelor (with low-dose aspirin) for up to 12 months as a treatment option for adults with ACS:
-
with STEMI or
-
with NSTEMI or
-
patients admitted to hospital with UA.
The SPC31 for ticagrelor notes that patients treated with ticagrelor and aspirin are at increased risk of non-CABG major bleeding and are also more generally at risk of bleeds requiring medical attention but not fatal or life-threatening bleeds. Therefore, the SPC31 recommends that the use of ticagrelor in patients at known increased risk for bleeding should be balanced against the expected benefit in terms of prevention of atherothrombotic events. It is further noted that co-administration of ticagrelor with strong CYP3A4 inhibitors is contraindicated, as co-administration may lead to a substantial increase in exposure to ticagrelor. 31
Data from the 2012 BCIS audit report18 indicate that in 2012 ticagrelor was used in 3.74% of PCI procedures in patients with UA/NSTEMI and in 7.04% of PCI procedures in patients with STEMI. The current BNF price27 of ticagrelor is £54.60 per pack of 56 tablets.
In October 2013, AstraZeneca32 reported that it had received a demand from the US Department of Justice, Civil Division, seeking documents and information regarding the PLATO (PLATelet inhibition and patient Outcomes)33 trial, the pivotal trial that led to the regulatory authorisation of ticagrelor both in the US and in Europe. The AG is aware34 that the EMA has also contacted AstraZeneca requesting further information about the PLATO33 trial.
Chapter 2 Definition of the decision problem
Decision problem
The remit of this appraisal is to review and update (if necessary) the clinical effectiveness and cost-effectiveness evidence base described in TA182. 21 The key elements of the decision problem issued by NICE in the final scope7 for this appraisal are set out in Table 4.
| Interventions | Prasugrel in combination with aspirin |
| Population | Patients with ACS undergoing primary or delayed PCI |
| Comparators | Clopidogrel in combination with low-dose aspirin |
| Ticagrelor in combination with low-dose aspirin | |
| Outcomes | The outcome measures to be considered include:
|
| Economic analysis | The reference case stipulates that the cost-effectiveness of treatments should be expressed in terms of incremental cost per QALY gained |
| The reference case stipulates that the time horizon for estimating clinical effectiveness and cost-effectiveness should be sufficiently long to reflect any differences in costs or outcomes between the technologies being compared | |
| Costs should be considered from a NHS and Personal Social Services perspective | |
| Other considerations | If the evidence allows, the following subgroups will be considered: people with STEMI, UA/NSTEMI, people with diabetes mellitus |
| Guidance will be issued only in accordance with the marketing authorisation | |
| The availability of any patient access schemes for the interventions and comparators should be taken into account in the analysis |
Within this report, reference to the use of prasugrel, clopidogrel or ticagrelor indicates that these treatments are given concomitantly with low-dose aspirin as per their licensed indications.
Overall aims and objectives of assessment
The remit of this review is to appraise the clinical effectiveness and cost-effectiveness of prasugrel within its licensed indication for the treatment of ACS with PCI (review of NICE technology appraisal TA182). 21
Chapter 3 Assessment of clinical effectiveness
Methods for reviewing the clinical effectiveness evidence are described in this chapter. The methods for reviewing the cost-effectiveness evidence are described in Chapter 6.
Methods for reviewing effectiveness
In addition to searching the manufacturer’s submission for relevant references, the following databases were searched for studies of prasugrel:
-
EMBASE (Ovid) 1974 to 18 June 2013.
-
MEDLINE (Ovid) 1946 to Week 1 June 2013.
-
The Cochrane Library June 2013.
-
PubMed January 2010 to April 2013.
The results were entered into an EndNote X5 (Thomas Reuters, CA, USA) library and the references were deduplicated. Full details of the search strategies used are presented in Appendix 1.
The reference lists of included trials were searched for relevant trials. Information on trials in progress was sought from cardiology conference databases (European Society for Cardiology and the American College of Cardiology). The website clinicaltrials.gov was also searched for ongoing trials. In addition, advice was sought from the clinical advisor to the review.
Inclusion and exclusion criteria
Two reviewers (JG and NF) independently screened all titles and abstracts identified via searching and obtained full-paper manuscripts that were considered relevant by either reviewer (stage 1). The relevance of each study was assessed (JG/NF) according to the criteria set out below (stage 2), and studies that did not meet the criteria were excluded and their bibliographic details were listed alongside reasons for their exclusion. Any discrepancies were resolved by consensus and, when necessary, a third reviewer (AB) was consulted.
Study design
Only randomised controlled trials (RCTs) were included in the assessment of clinical effectiveness.
Interventions and comparators
The effectiveness of prasugrel within its licensed indication was assessed. Studies that compared prasugrel with clopidogrel or ticagrelor were considered for inclusion in the review.
Patient populations
Patients with ACSs who were to be treated with primary or delayed PCI constituted the relevant population.
Outcomes
Data on any of the following outcomes were included in the assessment of clinical effectiveness: non-fatal and fatal CV events, mortality from any cause, atherothrombotic events, incidence of revascularisation procedures, adverse effects of treatment (including bleeding events) and health-related quality of life (HRQoL).
Data extraction strategy
Data relating to both study design and quality were extracted by two reviewers (JG and KD) into an Excel spreadsheet (Microsoft Excel 2010; Microsoft Corporation, Redmond, WA, USA). The two reviewers cross-checked each other’s data extraction and, when multiple publications of the same study were identified, data were extracted and reported as a single study.
Quality assessment strategy
The quality of the clinical effectiveness studies was assessed independently by two reviewers (JG and KD) according to the Centre for Reviews and Dissemination at the University of York’s suggested criteria. 35 All relevant information is tabulated and summarised within the text of the report. Full details and results of the quality assessment strategy for clinical effectiveness studies are reported in Appendix 2.
Methods of data synthesis
The results of the clinical data extraction and clinical study quality assessment are summarised in structured tables and as a narrative description. An indirect treatment comparison of prasugrel with ticagrelor was planned.
Results
Quantity and quality of research available
A total of 1940 titles and abstracts were screened for inclusion in the review of clinical effectiveness evidence. The process of study selection is shown in Figure 1. Titles excluded at stage 2 (n = 111) are listed in Appendix 3 along with reasons for their exclusion. The AG identified the pivotal trial (TRITON-TIMI 3836) discussed in TA18221 but did not identify any new trials for inclusion in the review.
FIGURE 1.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart.

At stage 2, the AG excluded four clinical trials. 37–40 One of the trials37 compared prasugrel with clopidogrel in a population of Asian patients with ACS undergoing PCI. This was excluded as it was considered to be a dose-ranging trial with a clopidogrel control. The trial recruited 719 patients and randomised them to one of three dosing regimens of prasugrel or standard clopidogrel according to patient weight and age (< 60 kg and > 70 years or vice versa). The primary outcome was platelet aggregation at 4 hours after the loading dose. Secondary outcomes included major adverse cardiac events and CABG and non-CABG thrombolysis in myocardial infarction (TIMI) bleeding at 30 days and 90 days. The study was not powered to detect differences between treatments on the secondary outcomes. The JUMBO-TIMI (Joint Utilization of Medications to Block Platelets Optimally – Thrombolysis in Myocardial Infarction 26)38 trial was similarly excluded. In this trial, patients (n = 904) undergoing PCI were randomised to one of three prasugrel dosing regimens or to clopidogrel and followed up for 30 days.
Two further excluded trials39,40 included relevant comparators and patient populations but had pharmacodynamic (platelet aggregation) parameters. The AG considered that the trial populations were too small and the length of follow-up too short (5 days and 1 hour) to provide data relevant to this review.
Assessment of clinical effectiveness
The AG’s systematic search of clinical effectiveness evidence yielded one relevant RCT (TRITON-TIMI 3836) for inclusion in the review. This trial was the pivotal trial discussed in TA18221 and the key elements of this RCT are summarised in Table 5. The TRITON-TIMI 3836 trial included 13,608 patients and was conducted in 30 countries. Patients received a loading dose of either prasugrel or clopidogrel (60 mg or 300 mg, respectively) followed by daily maintenance doses of 10 mg or 75 mg, respectively.
| Design | Intervention | Inclusion criteria (main) | Exclusion criteria (main) | Outcomes |
|---|---|---|---|---|
| International (30 countries) multicentre, Phase III double-blind, double-dummy RCT comparing prasugrel with clopidogrel in patients undergoing PCI. Patients (n = 13,608) were randomised in a 1 : 1 ratio and stratified according to presentation [i.e. UA/NSTEMI (n = 10,074) or STEMI (n = 3534)]. Duration of study: 15 months (median). A total of 73 patients were recruited from the UK | Prasugrel (LD 60 mg/MD 10 mg). Clopidogrel (LD 300 mg/MD 75 mg). Loading dose administered before, during or after PCI. Maintenance dose was continued for a median period of 14.5 months | Moderate- to high-risk UA or NSTEMI patients: ischaemic symptoms of 10 minutes or longer within 72 hours of randomisation. TIMI risk score of ≥ 3 and either ST segment deviation of ≥ 1 mm or an elevated cardiac biomarker of necrosis. Patients with STEMI could be enrolled within 12 hours of symptom onset if primary PCI was planned or within 14 days if delayed PCI was planned following initial pharmacotherapy for STEMI | Patients at increased risk of bleeding: anaemia, thrombocytopenia, intracranial pathology including TIA or stroke (within the last 3 months), severe hepatic dysfunction, oral anticoagulants, chronic non-steroidal anti-inflammatory drug use, or use of any thienopyridine within 5 days | Primary: composite of CV death, non-fatal MI or non-fatal stroke during follow-up period. Secondary: composite of death from CV causes, non-fatal MI, non-fatal stroke, rehospitalisation owing to cardiac ischaemic event. Composite of all-cause death, non-fatal MI, non-fatal stroke, stent thrombosis. At 30 days and 90 days: primary composite end point, composite of CV death, non-fatal MI, UTVR. Safety: non-CABG-related bleeding, TIMI life-threatening bleeding, TIMI major or minor bleeding |
The results of the AG’s quality assessment of the TRITON-TIMI 3836 trial are presented in Appendix 2. Overall, the AG considers that the trial was robustly designed and of strong methodological quality.
As this report is an update of TA182,21 the AG has reproduced the original summary information for TRITON-TIMI 3836 in Appendix 4. The summary information presented includes:
-
patient baseline characteristics (overall trial population)
-
primary and secondary end point analyses (overall trial population)
-
prespecified subgroup analyses for diagnosis, sex, age, diabetic status, type of stent implanted, use of glycoprotein IIb/IIIa receptor agonist, renal function (overall trial population)
-
outcomes for STEMI patients (overall trial population)
-
primary outcome for UA/NSTEMI, STEMI, all ACSs, patients with diabetes mellitus, patients with stents (overall trial population)
-
outcomes for people with history of stroke/TIA
-
outcomes for people > 70 years or weighing < 60 kg
-
analyses of recurrent events following PCI (overall trial population).
A number of subgroup analyses relating to TRITON-TIMI 3836 have been published; the key publications are listed, along with a brief description, in Table 6. A more comprehensive list of associated publications is presented in Appendix 5 of this report. The paper by Wiviott (2011),42 which is directly relevant to this appraisal, focuses on a sub-population of patients from the TRITON-TIMI 3836 trial who are described as the ‘core clinical cohort’. This sub-population is discussed in TA18221 as the ‘target population’. The core clinical cohort comprises patients for whom prasugrel is licensed and who may be treated with the full recommended dose of prasugrel (60-mg loading dose followed by 10 mg daily). These patients have no history of stroke or TIA, are younger than 75 years and weigh more than 60 kg. The AG focuses on the clinical evidence relevant to this subgroup. The rationale for this focus is presented in Appendix 6.
| Reference | Title | Description |
|---|---|---|
| Wiviott et al. 200641 | Evaluation of prasugrel compared with clopidogrel in patients with ACSs: design and rationale for the TRITON-TIMI 38 | Paper describing the design of the TRITON-TIMI 38 trial |
| Wiviott et al. 200736 | Prasugrel compared with clopidogrel in patients with ACSs | Primary publication of TRITON-TIMI 38 trial |
| Wiviott et al. 201142 | Efficacy and safety of intensive antiplatelet therapy with prasugrel from TRITON-TIMI 38 in a core clinical cohort defined by worldwide regulatory agencies | Paper describing outcomes of core clinical cohort of patients from TRITON-TIMI 38 trial: patients have no known history of stroke or TIA, are aged below 75 years and weigh more than 60 kg. The core clinical cohort represents 10,804 of the 13,608 patients included in the overall trial cohort |
The core clinical cohort42 comprised 10,804 patients (79%) from the randomised population of the TRITON-TIMI 3836 trial. The characteristics of the patients in the core clinical cohort and the overall trial population are described in Table 7. The proportions of patients quoted in Table 7 (taken from Wiviott et al. 42) are not presented by trial arm. However, Wiviott et al. 42 states that patients in the core clinical cohort randomised to prasugrel and clopidogrel were well matched and that 50% of the core clinical cohort was randomised to prasugrel. 42 The AG notes that the patients in the overall trial population and the core clinical cohort appear to be similar in terms of baseline characteristics. In TA182,21 the overall trial population of TRITON-TIMI 3836 was considered to be younger and less likely to have experienced a prior MI than patients in clinical practice in England and Wales.
| Characteristic | Core clinical cohort, % (n = 10,804) | Overall trial population, % (n = 13,608) |
|---|---|---|
| Age (median) | NS | 61 years (median) |
| UA/NSTEMI | 73 | 74 |
| Male | 79 | 74 |
| White | 93 | 93 |
| Region | ||
| North America | 32 | 32 |
| South America | 4 | 4 |
| Western Europe | 25 | 26 |
| Eastern Europe | 25 | 25 |
| Africa/Asia/Middle East | 14 | 14 |
| Medical history | ||
| Hypercholesterolaemia | 56 | 56 |
| Hypertension | 62 | 64 |
| Diabetes mellitus | 22 | 23 |
| Previous MI | 17 | 18 |
| Previous CABG | 7 | 8 |
| Creatinine clearance < 60 ml/minute | 4 | 12 |
| Multivessel coronary intervention | 14 | 14 |
| Glycoprotein IIb/IIIa inhibitor | 56 | 55 |
| ACE/ARB | 75 | 76 |
| Beta-blocker | 89 | 88 |
| Statin | 93 | 92 |
| CCB | 16 | 18 |
| ASA | 100 | 99 |
Clinical efficacy in the core clinical cohort
The manufacturer submission (MS; Daiichi Sankyo Company Ltd/Eli Lilly and Company Ltd, 2013) and the Wiviott et al. 42 paper report the clinical outcomes for the core clinical cohort of patients from the TRITON-TIMI 3836 trial. It is emphasised by Wiviott et al. 42 that the core clinical cohort was identified in a post-hoc fashion defined by regulatory (EMA and the US Food and Drug Agency) criteria and should be considered as hypothesis generating.
The clinical efficacy outcomes for the core clinical cohort are presented in Table 8. For the primary composite end point of death from CV causes, non-fatal MI or non-fatal stroke, statistically significantly fewer events were recorded in the prasugrel arm (8.3%) than in the clopidogrel arm (11%) [hazard ratio (HR) = 0.74; 95% confidence interval (CI) 0.66 to 0.84; p < 0.0001]. Similarly, for the secondary composite end point (death from any cause, non-fatal MI, non-fatal stroke or non-CABG-related non-fatal TIMI major bleeding) statistically significantly fewer events were recorded in the prasugrel arm (10.2%) than in the clopidogrel arm (12.5%) (HR = 0.80; 95% CI 0.71 to 0.89; p < 0.001). The AG notes that the efficacy for both composite outcomes appears to be driven by the number of non-fatal MIs.
| End point | Clopidogrel, n/N (%) | Prasugrel, n/N (%) | HR (95% CI) | p-value |
|---|---|---|---|---|
| Primary | ||||
| Death from CV causes, non-fatal MI or non-fatal stroke | 569/5383 (11)a | 433/5421 (8.3)a | 0.74 (0.66 to 0.84) | < 0.001 |
| Secondary | ||||
| Death from any cause, non-fatal MI, non-fatal stroke, or non-CABG-related non-fatal TIMI major bleeding (net clinical benefit) | 641/5383 (12.5)a | 522/5421 (10.2)a | 0.80 (0.71 to 0.89) | < 0.001 |
| CV death or MI | 10.2% | 7.7% | 0.75 (0.66 to 0.85) | < 0.10 |
| CV death | 1.4% | 1.4% | 1.05 (0.75 to 1.46) | 0.78 |
| Death | 2.0% | 2.1% | 1.03 (0.78 to 1.37) | 0.82 |
| MI | 9.4% | 6.7% | 0.71 (0.62 to 0.81) | < 0.001 |
| Stroke | 1.0% | 0.8% | 0.75 (0.49 to1.15) | 0.19 |
| Stent thrombosis: definite | 2.0% | 0.8% | 0.41 (0.29 to 0.60) | < 0.001 |
| Stent thrombosis: definite/probable | 2.3% | 1.0% | 0.44 (0.31 to 0.62) | < 0.001 |
Statistically significant differences in favour of prasugrel were also reported for the outcomes of definite stent thrombosis (HR = 0.41, 95% CI 0.29 to 0.60; p < 0.001) and definite or probable stent thrombosis (HR = 0.44; 95% CI 0.31 to 0.62; p < 0.001). There were also statistically significantly fewer MIs in the prasugrel arm (6.7%) than in the clopidogrel arm (9.4%) (HR = 0.71, 95% CI 0.62 to 0.81; p < 0.001).
Efficacy across subgroups within the core clinical cohort
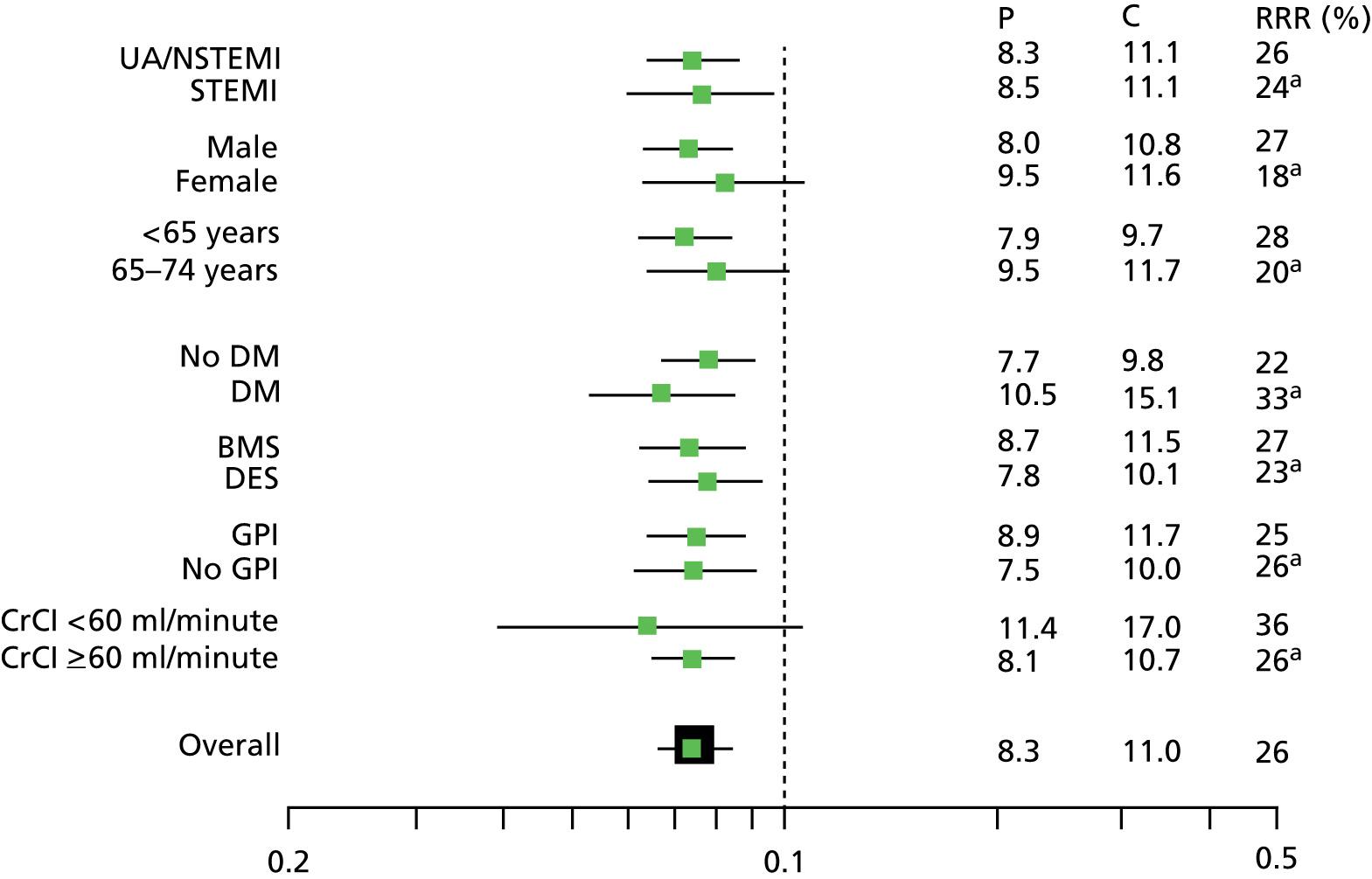
Wiviott et al. 42 present a forest plot that displays the relative effectiveness of prasugrel compared with clopidogrel across a range of subgroups within the core clinical cohort, including diagnostic group (UA/NSTEMI or STEMI), sex, age and diabetic status. The published forest plot is reproduced in Figure 2. The clinical effectiveness of prasugrel appears to be consistent across subgroups.
FIGURE 2.
Key subgroups for primary efficacy end point (core clinical cohort). BMS, bare metal stent; CrCI, creatinine clearance; DES, drug-eluting stent; DM, diabetes mellitus; GPI, glycoprotein inhibitor; RRR, relative risk reduction. a, p-value was not significant. Reprinted from Am J Cardiol, vol. 108, Wiviott SD, Desai N, Murphy SA, Musumeci G, Ragosta M, Antman EM, et al. , Efficacy and safety of intensive antiplatelet therapy with prasugrel from TRITON-TIMI 38 in a core clinical cohort defined by worldwide regulatory agencies, pp. 905–11, 2011, with permission from Elsevier.
Efficacy across time in the core clinical cohort
It is noted in Wiviott et al. 42 that, in the core clinical cohort, prasugrel was more effective than clopidogrel for the primary end point at 30 days as well as at the 15-month follow-up (Table 9).
| End point | Clopidogrel (n = 5383) | Prasugrel (n = 5421) | HR (95% CI) | p-value |
|---|---|---|---|---|
| Primary: death from CV causes, non-fatal MI or non-fatal stroke | ||||
| 30 days | 7.0% | 5.0% | 0.70 (0.60 to 0.82) | < 0.0001 |
| 30 days to 15 months | 4.5% | 3.6% | 0.80 (0.65 to 0.97) | 0.027 |
Safety in the core clinical cohort
The key safety end point in the TRITON-TIMI 3836 trial was the rate of non-CABG-related TIMI major bleeding in the overall trial cohort at 15 months. The data for the safety end points at 15 months in the core clinical cohort are presented in Table 10. No statistically significant difference in non-CABG-related TIMI major bleeding was noted between patients in the prasugrel and clopidogrel arms; however, there was a significant difference in favour of clopidogrel when major and minor bleeding events were combined (3.0% vs. 3.9%) (HR = 1.26, 95% CI 1.02 to 1.57; p = 0.03).
| End point | Clopidogrel, n/N (%) | Prasugrel, n/N (%) | HR (95% CI) | p-value |
|---|---|---|---|---|
| Non-CABG-related TIMI major bleeding | 73/5337 (1.5) | 91/5390 (1.9) | 1.24 (0.91 to 1.69) | 0.17 |
| TIMI major or minor bleed | 3.0% | 3.9% | 1.26 (1.02 to 1.57) | 0.03 |
| Fatal TIMI major | 0.1% | 0.2% | 2.65 (0.70 to 9.97) | 0.14 |
| Intracranial haemorrhage | 0.3% | 0.2% | 0.69 (0.30 to 1.62) | 0.39 |
| TIMI major or minor bleeding | ||||
| 30 days | 1.6% | 1.9% | 1.21 (0.91 to 1.62) | 0.19 |
| 30 days to 15 months | 1.5% | 2.1% | 1.31 (0.95 to 1.79) | 0.97 |
Net clinical benefit
The analysis of the net clinical benefit outcome (death from any cause, non-fatal MI, non-fatal stroke or non-CABG-related non-fatal TIMI major bleeding) favoured the use of prasugrel in the core clinical cohort (12.5% in the clopidogrel group vs. 10.2% in the prasugrel group; HR 0.80, 95% CI 0.71 to 0.89; p < 0.001).
Health-related quality of life
Data relevant to HRQoL are available only for the TRITON-TIMI 3836 overall trial population and are not specific to the core clinical cohort. The HRQoL substudy was open to all TRITON-TIMI 3836 patients at participating sites in eight countries: the USA, Australia, Canada, Germany, Italy, Spain, the UK and France. HRQoL was evaluated using three instruments: (1) the Angina Frequency and Physical Limitations scales of the Seattle Angina Questionnaire; (2) the London School of Hygiene Dyspnoea Questionnaire; and (3) the European Quality of Life-5 Dimensions (EQ-5D) self-report questionnaire and the European Quality visual analogue scale. Assessments were taken at baseline and at days 30, 180, 360 and 450 (or last visit).
The HRQoL study recruited a much smaller sample than was initially planned (475 patients, compared with 3000 patients), and in TA18221 the representativeness of the substudy sample was considered to be unclear, as was the clinical utility of the results. Therefore, the AG was unable to draw any conclusions as to the HRQoL of patients treated with prasugrel or clopidogrel in the TRITON-TIMI 3836 trial. The results from the HRQoL study are presented in the MS.
Data relevant to key patient groups of the core clinical cohort
Specific clinical data relating to patients with STEMI, NSTEMI or diabetes mellitus in the core clinical cohort were not available from the MS. The AG notes from the forest plot in Figure 2 that the clinical effectiveness of prasugrel compared with clopidogrel was in evidence across the range of subgroups including STEMI, UA/NSTEMI and patients with and without diabetes. The manufacturer’s model enabled economic data pertaining to these patient groups to be extracted.
Overall summary of findings
All of the outcomes listed in the final scope issued by NICE were reported in the MS.
The clinical outcomes for the core clinical cohort of the TRITON-TIMI 3842 trial demonstrate statistically significant differences in favour of prasugrel compared with clopidogrel across a range of outcomes and clinical subgroups. In terms of safety (bleeding events), one statistically significant difference between prasugrel and clopidogrel was noted. The exception was for the combined outcome of TIMI major and minor bleeding, for which significantly more events occurred with prasugrel than with clopidogrel. No conclusions regarding HRQoL could be drawn owing to lack of data.
Clinical discussion points from TA182
It is noted in this report that the TRITON-TIMI 3836 trial was a well-designed trial. However, three key areas of uncertainty were raised at the time of TA18221 by the Appraisal Committee (AC) in respect of the TRITON-TIMI 3836 trial. The AC was concerned that the results of the TRITON-TIMI 3836 trial may not be generalisable to patients in England and Wales for the following reasons:
-
The loading dose of clopidogrel administered in the trial was 300 mg whereas a loading dose of 600 mg may be administered in clinical practice in England and Wales.
-
The majority of patients (74%) in the trial received the clopidogrel loading dose during the PCI procedure. In clinical practice in England and Wales, patients undergoing planned PCI receive the clopidogrel loading dose before the PCI procedure.
-
Clinical efficacy in the trial was largely driven by statistically significant differences in non-fatal MIs. Non-fatal MIs included both clinical MIs (symptoms) and non-clinical MIs (biomarkers and ECG readings). If only the incidence of clinical MIs were compared between treatment arms, there may be no differences in outcomes between the arms.
Clopidogrel loading dose: size
Manufacturer comments
The difference in size of the clopidogrel loading dose given to patients in the TRITON-TIMI 3836 trial (300 mg) and the dose (600 mg) most often used in clinical practice in England and Wales is addressed in the MS. The manufacturer acknowledges that there is variation in UK clinical practice as to whether 300 mg or 600 mg of clopidogrel is used in PCI treatment.
The manufacturer points out the inconsistency between clinical guidelines as to the recommended loading dose of clopidogrel (300 mg or 600 mg). For example, in NICE CG94,13 published in 2010, NICE recommends 300 mg while acknowledging that evidence exists to support the use of 600 mg. The Scottish Intercollegiate Guidelines Network (SIGN)43 guidelines recommend the use of a 300-mg loading dose, whereas the European Society for Cardiology (ESC) advocates both 300-mg and 600-mg loading doses. 10,11,44
The manufacturer states that the case for the additional benefit of 600 mg rather than 300 mg is not proven and cites the results of the CURRENT-OASIS (Clopidogrel and Aspirin Optimal Dose Usage to Reduce Recurrent Events–Seventh Organization to Assess Strategies in Ischemic Syndromes 7)45 trial, published in 2010. In this trial, patients with ACS (n = 25,806) who were scheduled for early angiography and PCI were randomised to receive a loading dose of 300 mg or 600 mg of clopidogrel and either high- or low-dose aspirin. The patients who received a 600-mg loading dose of clopidogrel and had a PCI continued with 150 mg of clopidogrel for the first 7 days and on day 8 received the standard 75-mg maintenance dose. Patients who received the 300-mg loading dose of clopidogrel and had a PCI continued on 75 mg of clopidogrel following the PCI procedure. The MS reports that in the overall trial population (which also includes the patients who did not undergo the scheduled PCI), the primary composite end point of death from CV causes, MI or stroke at 30 days was not statistically significantly different between the 600-mg arm (4.2%) and the 300-mg arm (4.4%) (HR = 0.94, 95% CI 0.83 to 1.06; p < 0.61); however, there was a statistically significant increase in bleeding events in the 600-mg arm (2.5%) compared with the 300-mg arm (2.0%) (HR = 1.24, 95% CI: 1.05 to 1.46; p < 0.01). This finding was consistent for subgroups of patients regardless of diagnosis (STEMI or NSTEMI).
The outcomes for the 69% of patients randomised to the CURRENT-OASIS 746 trial and who received PCI treatment after randomisation only are also reported in the MS. A statistically significant difference in the occurrence of the primary composite end point in favour of the 600-mg arm (3.9%) compared with the 300-mg arm (4.5%) is noted (HR 0.86, 95% CI 0.74 to 0.99; p = 0.039). However, the MS states that no statistical differences were noted for either the STEMI subgroup (HR 0.83, 95% CI 0.66 to 1.05; p < 0.117) or NSTEMI subgroup (HR 0.87, 95% CI 0.72 to 1.06; p < 0.167).
The manufacturer concludes that the results of the overall CURRENT-OASIS45 trial do not demonstrate any clear benefit associated with the use of a 600-mg loading dose of clopidogrel compared with a 300-mg dose and thus it is unlikely that the use of 600 mg of clopidogrel in the TRITON-TIMI 3836 trial would have changed the efficacy results, although it may have resulted in an increase in the number of bleeding events in the clopidogrel arm.
Assessment Group comments
The AG is aware that the licensed loading dose of clopidogrel is 300 mg and that this was the established loading dose in routine clinical practice in the USA when the TRITON-TIMI 3836 trial commenced. The AG notes that, in TA182,21 the manufacturer supported the case for the use of 300 mg of clopidogrel in the UK by reporting data from the Eli Lilly-sponsored AntiPlatelet Treatment Observational Registry47 and the IMS Health Acute Cardiovascular Analyser study. 48,49 These data indicated that, in 2007, 60–79% of ACS patients in the UK received the 300 mg licensed dose. Clinical advice to the AG is that clinical practice differs between PCI centres as to the loading dose of clopidogrel.
The AG agrees with the manufacturer that there are differences in the stated recommendations in the available clinical guidelines. The manufacturer correctly states that that the SIGN43 guidelines recommend a 300-mg loading dose of clopidogrel whereas the ESC10,11,44 guidelines recommend both 300 mg and 600 mg.
The most recent NICE guidelines for UA/NSTEMI (CG9413) state that most people admitted with UA/NSTEMI should be treated with a loading dose of 300 mg of clopidogrel. However, the guidelines further state that, if very early (< 24 hours) invasive intervention is planned, a higher loading dose should be considered, particularly in cases for which the procedure will be carried out within 6 hours. The guideline development group (GDG) responsible for CG9413 has stated in the guideline that as they were not able to formally review all the evidence for a 600-mg loading dose, they were not able to recommend this at the time of publication.
The recently published (July 2013) NICE guidelines CG1678 for patients with STEMI simply state that treatment with clopidogrel is an established option in the pharmacological treatment of people with acute STEMI, including people undergoing primary PCI. The GDG for CG1678 noted that a clopidogrel loading dose of 600 mg is not licensed in the UK but is used widely in current practice, especially in people undergoing PCI.
The AG agrees with the manufacturer’s conclusion that the results from the overall population of the CURRENT-OASIS 745 trial do not appear to support the use of a 600-mg loading dose of clopidogrel over a 300-mg dose. However, the AG considers that the results of the subgroup analysis45 of the 69% (17,263) of patients treated with PCI suggest that the trial protocol clopidogrel regimen of a 600-mg loading dose followed by 7 days at 150 mg and then 75 mg daily statistically significantly reduces CV events (including stent thrombosis) when compared with a loading dose of 300 mg followed by 75 mg daily. However, the AG also notes that the prevalence of bleeding events was statistically significantly greater in the 600-mg arm than in the 300-mg arm. In addition, the trial follow-up was for a period of 30 days and, therefore, longer-term outcomes are unknown. The AG notes that the findings of the PCI subgroup analysis of the CURRENT-OASIS 746 trial are based on subgroup analyses that are subject to statistical caveats; however, the findings are consistent with those of a meta-analysis comprising trials with PCI-treated patients. 50
In summary, the AG considers that the loading dose of clopidogrel given in the TRITON-TIMI 3836 trial may be inconsistent with the majority of clinical practice in England and Wales. Data to determine whether or not there is any difference in clinical efficacy between a 300-mg and 600-mg loading dose of clopidogrel are limited.
Timing of the clopidogrel loading dose
Manufacturer comments
In the MS, the manufacturer notes that the timing of the clopidogrel loading dose administered to patients in the TRITON-TIMI 3836 trial (79% of patients received treatment at the time of PCI) is different to the timing of the loading dose in clinical practice (clopidogrel is given prior to PCI whenever possible) in England and Wales. 21 However, the manufacturer also points out, citing data from the MINAP report,5 that door-to-treatment time in the UK is decreasing annually, thereby reducing the opportunity for preloading with clopidogrel.
The manufacturer restates the arguments put forward in their MS for TA18221 that changing the timing of the loading dose of clopidogrel in the trial would not have greatly impacted on the clinical efficacy outcomes of the trial. The manufacturer cites numerous sources of evidence derived from the analysis of the TRITON-TIMI 3836 trial to support their argument:
-
The effects of prasugrel were consistent over time. For the overall study period, the HR (0.81, 95% CI 0.73 to 0.90) is similar to the HR for the 0–3 days time period (HR 0.82, 95% CI 0.71 to 0.96) and the period from 3 days to the end of the study period (HR 0.80, 95% CI 0.70 to 0.93). An additional landmark analysis examining occurrence of MI, stent thrombosis and urgent target vessel revascularisation (UTVR) at 0–3 days and beyond 3 days confirmed sustained benefit over time.
-
In the case of patients treated with glycoprotein IIb/IIIa inhibitors, there was no evidence that the relative benefit of prasugrel compared with clopidogrel was reduced or that there was an excess need for bail-out glycoprotein IIb/IIIa inhibitor use during PCI in those patients randomised to clopidogrel in the study.
-
A group of patients received pretreatment up to 24 hours before PCI. The percentage of patients in this pretreated subgroup reaching the composite end point of CV death, non-fatal MI, or non-fatal stroke from randomisation through study end was 9.94% and 11.29% (unadjusted crude event rates) for patients pretreated with prasugrel and clopidogrel, respectively. Although the difference is not statistically significant for this subgroup, the difference supports the theory that, to a large extent, the timing of the loading dose did not influence overall efficacy.
Assessment Group comments
The AG considers that the evidence to support or refute the benefits of preloading with clopidogrel compared with clopidogrel at the time of PCI is equivocal; this means that whether or not patients in the trial would benefit more from clopidogrel compared with patients in the NHS in England and Wales remains unclear.
Clinical compared with non-clinical myocardial infarctions
Manufacturer’s comments
A point of discussion during the previous appraisal21 of prasugrel was that the definition of MI used in TRITON-TIMI 3836 included non-clinically detected MIs. The manufacturer states that the definition of MI in the TRITON-TIMI 3836 trial was based on the American College of Cardiology Task Force on Clinical Data Standards published in 2001. 51 This definition was prespecified and agreed with the regulatory agencies [United States Food and Drug Administration (FDA) and EMA] prior to the start of the trial. The AC and the Evidence Review Group (ERG) were concerned that, if the non-clinical MIs were excluded from the analyses, the resultant clinical difference in non-fatal MIs alone may not be statistically significant when comparing prasugrel with clopidogrel. In response, the manufacturer cited evidence from a reanalysis52 of the TRITON-TIMI 3836 trial MI (n = 1218 MIs). These MIs were reassessed according to the 2007 criteria of the Universal Definition of Myocardial Infarction (Table 11) developed by the European Society of Cardiology, the American College of Cardiology, the American Heart Association and the World Heart Federation Task Force. 53 Reviewers, who were blinded to treatment allocation, assessed the size and timing of all MIs and whether or not the MI was STEMI or NSTEMI. Of the 1218 MIs considered, 1163 had biomarker data to indicate the size. In the MS, the manufacturer reports that, when analysed according to non-clinical and clinical MIs, compared with clopidogrel, prasugrel demonstrated a significant reduction in MIs that was consistent across the spectrum of MIs of varying type, size and timing.
| Type | Description |
|---|---|
| Type 1 | Spontaneous MI caused by a primary coronary event, such as a plaque rupture in a coronary artery with less blood then flowing to the muscle |
| Type 2 | Secondary MI owing to either increased oxygen demand or decreased supply owing to other conditions such as spasm of the coronary artery or low blood oxygen from anaemia |
| Type 3 | Sudden cardiac death with evidence of MI but occurring before blood samples could be obtained or before the appearance of cardiac biomarkers in the blood |
| Type 4 | MI related to a PCI |
| Type 4a | MI associated with a PCI procedure |
| Type 4b | MI associated with stent thrombosis as documented by an angiography or at autopsy |
| Type 5 | MI associated with CABG |
The manufacturer also points to a further analysis54 of the TRITON-TIMI 3836 data in which the rate of CV death within 180 days was compared in people who had experienced a new MI and those who had not. Among patients who experienced a new MI of any type, the rate of CV death was significantly higher (6.5% vs. 1.3%; p < 0.001). This was the case even after adjustment for other risk factors (adjusted HR 5.2, 95% CI 3.8 to 7.1; p = 0.001). The manufacturer argues that these findings suggest that all MIs have prognostic implications.
In summary, the manufacturer claims that the results of the reanalysis52,54 of the MIs from the TRITON-TIMI 3836 trial demonstrate that treatment with prasugrel significantly reduces the risk of all MIs when compared with clopidogrel. The manufacturer also states that further evidence suggests that any type of MI is associated with a significantly increased risk of CV death, with a consistent relationship across all MI types as defined53 by the universal classification system.
Assessment Group comments
The AG considers that the manufacturer has provided a convincing case to support the hypothesis that prasugrel is effective across all types of MI when compared with clopidogrel. The AG also notes the finding that the reductions in MIs associated with small enzyme releases were not significantly different in the prasugrel-treated and clopidogrel-treated arms of the trial. This suggests that the clinical efficacy results were unlikely to have been driven by reductions in non-clinical MIs.
In summary, of the three key issues raised in TA18221 and discussed in this section, the AG considers that the size and timing of the loading dose of clopidogrel and the impact these factors have on the primary outcome of the TRITON-TIMI 3836 trial remain unclear. However, the reanalysis52,54 of the MIs by the manufacturer demonstrates that prasugrel was more effective than clopidogrel in preventing occurrence of MIs.
Stent thrombosis
In TA182,21 prasugrel is recommended for patients who have had a stent thrombosis during the course of treatment with clopidogrel. In the MS for the present review, the manufacturer describes the outcomes of related research conducted in collaboration with Professor Gershlick (Consultant Cardiologist, University Hospital of Leicester, Leicester, UK). The purpose of the research is to develop a method to identify patients at risk of stent thrombosis. The manufacturer reports that 20 risk factors for stent thrombosis have been identified, nine relating to patient factors, three relating to the lesion and eight relating to the PCI. These risk factors are presented in table 26 of the MS. The risk scores have subsequently been validated by the manufacturer using data from patients in the TRITON-TIMI 3836 trial. It is suggested in the MS that the risk scores could be used in clinical practice to identify patients at risk of stent thrombosis and thereby guide treatment decisions.
Comparison of prasugrel with ticagrelor
At the time of TA182,21 the standard comparator to prasugrel was clopidogrel. However, in 2010, NICE approved the use of ticagrelor as an antiplatelet treatment for patients with ACS (TA236). 22 The pivotal clinical trial assessing ticagrelor is the PLATO33 trial, in which ticagrelor is compared with clopidogrel in a population of ACS patients. Further information pertaining to the PLATO33 trial is presented in Appendix 7. In the MS (for ticagrelor), the manufacturer of ticagrelor (AstraZeneca) put forward a convincing case that a formal indirect treatment comparison between the TRITON-TIMI 3836 and PLATO33 trials would be inappropriate. The manufacturer’s case was accepted by both the ERG and the AC at the time of the ticagrelor appraisal (TA236). 22
Since the appraisal of ticagrelor, no new relevant RCTs have been conducted with either prasugrel or ticagrelor, nor is there any new direct evidence comparing prasugrel with ticagrelor. However, a number of authors have published indirect treatment comparisons using data from the TRITON-TIMI 3836 and PLATO33 trials. The AG considers that any comparison of the results of the TRITON-TIMI 3836 and PLATO33 trials is both problematic and inappropriate. Consequently, the AG has not conducted an indirect treatment comparison in this update of TA182. 21 The AG is of the opinion that the issues that mitigate against conducting such an indirect comparison remain unchanged from those presented and accepted during TA236 (ticagrelor). 22 Specifically, these refer to differences in the target populations, the usage of clopidogrel (loading dose and timing of administration) and differences in MI assessment. The AG notes that there is no indirect comparison presented in the MS and that the manufacturer agreed with the AC and the ERG in TA236 (ticagrelor)22 that such an indirect comparison would be inappropriate.
Problems with an indirect comparison of the TRITON-TIMI 38
The key features of the TRITON-TIMI 3836 and PLATO33 trials are described in Table 12 (reproduced from the MS for TA236). 22 Both trials were conducted in an ACS population, use clopidogrel as a comparator and report the same primary composite efficacy end point (death from CV causes, non-fatal MI, or non-fatal stroke during the follow-up period).
| Characteristic | TRITON-TIMI 38 | PLATO |
|---|---|---|
| Number of patients | 13,608 | 18,624 |
| Patient population | Patients with early invasively managed ACS scheduled for PCI (including STEMI and NSTEMI patients undergoing same admission PCI). Symptom onset within 72 hours | Broad ACS population (including STEMI). Symptom onset within 24 hours |
| Prior clopidogrel | Excluded | Allowed (including in-hospital prior to randomisation) |
| % STEMI | Capped at 26% (18% undergoing primary PCI) | 40.5% (all intended for primary PCI) |
| Clopidogrel load | Only 300 mg allowed | 300 mg or 600 mg |
| Timing of randomisation | Later: after angiography; after decision to perform PCI | Earlier: usually before angiography (if done) |
| Randomisation | Prasugrel 60-mg load and 10 mg once daily or clopidogrel 300-mg load and 75 mg once daily | Ticagrelor 180-mg load and 90 mg twice daily or clopidogrel 300- to 600-mg load and 75 mg once daily |
| Administration of study drug | Started in the time interval from randomisation up to 1 hour after PCI | Started immediately after randomisation |
| Primary efficacy end point | CV death/MI/stroke | CV death/MI/stroke |
| Primary safety end point | Non-CABG TIMI major bleeding | PLATO major bleeding |
| PCI | 99% (all at randomisation) | 61% (49% within 24 hours of randomisation) |
| CABG | 3.2% (0.35% on primary admission) | 10.2% (4.5% on primary admission) |
| Medical management only | 1.1% | 34% |
| Glycoprotein IIb/IIIa use | 54% | 27% |
| Follow-up | Up to 15 months | Up to 12 months |
Differences in the target population
The TRITON-TIMI 3836 trial recruited patients with ACS who were intended to be managed with PCI and were randomised just prior to the PCI. A more diverse range of patients was randomised to the PLATO33 trial; patients in PLATO33 were randomised at presentation and then investigators decided whether patients were to receive revascularisation treatment or medical therapy.
A TRITON-TIMI trial publication55 describes the results of a subgroup of patients with STEMI; however, this group included patients who were treated with primary or planned PCI. In the PLATO33 trial, all patients with STEMI were treated with primary PCI.
A subgroup analysis56 of the PLATO33 trial has also been published. This analysis describes the results of ACS patients who were intended for invasive treatment. However, as only 77% of this cohort actually underwent PCI it cannot be considered as a PCI-only cohort.
Differences in clopidogrel loading
The two trials33,36 differed as to the dosing and timing of administration of clopidogrel (the common comparator). The loading dose of clopidogrel administered in the TRITON-TIMI 38 trial36 was 300 mg, but, in the PLATO trial,33 loading doses of 300 mg or 600 mg were allowed. A total of 19.6% of clopidogrel-treated patients in the overall PLATO33 cohort, 26.8% in the cohort intended for invasive management and 38.6% in the STEMI cohort received 600 mg of clopidogrel.
In the TRITON-TIMI 3836 trial, most patients received their loading dose of clopidogrel in the time interval between the insertion of the guidewire for PCI up to 1 hour after the procedure, whereas, in the PLATO33 trial, most patients received their loading dose of clopidogrel before randomisation.
The issue of the size of loading dose and timing of administration of clopidogrel was discussed in detail earlier in this report (see Clinical discussion points from TA182). The AG is of the opinion that the differences in clopidogrel usage across the two trials must be considered problematic. The AG remains convinced that, for the reasons previously outlined, there are no reliable clinical data to permit a robust comparison of prasugrel with ticagrelor.
Differences in myocardial infarction assessment
The assessment of MIs across the two trials requires consideration. It was noted in TA23622 that determining whether or not a patient has a non-clinical MI during the angioplasty procedure is difficult, as any enzymatic changes observed may be wholly due to the original MI that triggered the procedure. A more definitive assessment can be made if multiple measurements of cardiac enzymes are taken between the initial event and the PCI procedure as it is then possible to differentiate a gradually falling pattern of enzymes and a subsequent rise after the PCI (consistent with a further MI having occurred at the time of the procedure). It was further noted in TA23622 that, in the TRITON-TIMI 3836 trial (with the exception of the STEMI primary PCI cohort), there was time for at least two preprocedure enzyme measurements to be taken, whereas, in the PLATO33 trial, only one preprocedure enzyme measurement was taken and any elevated enzymes could not be reliably attributed to either the index event or a new MI. The impact of the differences in MI assessment means that in the PLATO33 trial the majority of MIs included in the primary end point were clinical MIs, whereas almost half of those included in the TRITON-TIMI 3836 trial results were non-clinical only.
Differences in duration of trials
There was a difference in the length of follow-up of the two trials. The PLATO33 trial involved a median follow-up of 9 months, whereas the TRITON-TIMI 3836 trial followed patients for a median of 15 months. The AG is of the opinion that it is not appropriate to indirectly compare outcomes at 9 months with those at 15 months as the proportion of participants experiencing CV death, MI or stroke is likely to increase as the length of follow-up increases.
Differences in the primary analysis of the trials
The two trials33,36 also used different measures for the primary analysis. In anticipation of a lack of proportionality of hazards in the TRITON-TIMI 3836 trial, assessment of the primary outcome was made using the Gehan–Wilcoxon test for the primary analysis rather than the log-rank test. (The Gehan–Wilcoxon test assigns greater weight to earlier time points than the log-rank test.) The log-rank test was then used in a prespecified sensitivity analysis. In contrast, the Cox proportional hazards model was used for the primary analysis in the PLATO trial. 33 The AG is concerned about the impact that the different assumptions stated in these trials would have on the results of an indirect comparison.
Summary and critique of published indirect comparisons of prasugrel and ticagrelor
Four published indirect comparisons57–60 of prasugrel compared with ticagrelor were identified by the AG and the manufacturer during searching; the key features of these studies are described in Appendix 8. The quality of the four published indirect comparisons57–60 identified by the AG (and the manufacturer) was assessed using the assessment of multiple systematic reviews (AMSTAR)61 tool. The results are presented in Appendix 9.
The published indirect comparison of ticagrelor and prasugrel in patients with ACS conducted by Biondi-Zoccai et al. 57,62 was based on the results of the PLATO33 and TRITON-TIMI 3836 trials as well as on data from a 12-week dose-ranging trial that compared ticagrelor with clopidogrel in 990 patients with NSTEMI [Dose confIrmation Study assessing anti-Platelet Effects of AZD6140 vs. clopidogRel in non-ST segment Elevation myocardial infarction 2 (DISPERSE 2)]. 63 The total number of patients in the indirect comparison was 32,893. The results of the indirect comparison of prasugrel and ticagrelor demonstrated no statistically significant differences in overall death, non-fatal MI, non-fatal stroke, or their composite. 57 Prasugrel was associated with a significantly lower risk of stent thrombosis, and ticagrelor was associated with a significantly lower risk of any major bleeding and major bleeding associated with cardiac surgery. However, the risk of non-CABG-related major bleeding was similar for prasugrel and ticagrelor. The authors concluded that prasugrel and ticagrelor are superior to clopidogrel for ACS. The results of the indirect comparison suggest similar efficacy and safety of prasugrel compared with ticagrelor, whereas prasugrel appears more protective of stent thrombosis but causes more bleeding.
The AG’s main criticism of the indirect comparison in Biondi-Zoccai et al. 57 is that the findings are largely based on the outcomes of the TRITON-TIMI 3836 and PLATO33 trials. The substantial differences between the two trials (see Problems with an indirect comparison of TRITON-TIMI 38) render the results of the indirect comparison unreliable. The AG considers that results from the dose-ranging DISPERSE-263 trial make a negligible contribution to the results presented by Biondi-Zoccai et al. 57 as the length of follow-up was very short. The AG also notes that the published indirect comparison considered overall death (not CV death) as part of the primary composite end point.
The publication by Passaro et al. 59 presented a simplified network meta-analysis graph to improve the communicative value of the analysis undertaken by Biondi-Zoccai et al. 57 The analysis excluded the dose-ranging DISPERSE-263 trial and instead included the outcomes from the Clopidogrel in Unstable angina to prevent Recurrent Events (CURE)64 trial in which clopidogrel was compared with placebo in 12,562 patients with NSTEMI who were largely managed medically (only 21% of patients were treated with PCI). No rationale was given for the inclusion of the CURE64 trial. The AG assumes that the reason for inclusion was to enable the authors to expand the treatment network. The conclusions of this analysis concurred with those of Biondi-Zoccai et al. ,57 with the exception that no difference in major bleeding between prasugrel and ticagrelor was indicated. 59
As stated previously, the AG does not consider it appropriate to compare the results of the TRITON-TIMI 3836 and PLATO33 trials owing to their inherent differences.
The meta-analysis conducted by Chatterjee et al. 58 was intended to compare prasugrel and ticagrelor in patients with ACS or those undergoing coronary intervention for the same, or for significant coronary artery disease, by conducting a network meta-analysis. 58 Four studies, comprising a total of 34,126 patients, were included: PLATO,33 TRITON-TIMI 38,36 DISPERSE-263 and JUMBO-TIMI 26. 38 The JUMBO-TIMI 2638 trial was a dose-ranging Phase II trial comparing prasugrel with clopidogrel in 900 patients intended for PCI. The follow-up was limited to 30 days. Chatterjee et al. 58 found no difference in CV mortality or rates of MI among patients undergoing PCI but stated that CABG-related bleeding was lower with prasugrel than with ticagrelor. The authors concluded that prasugrel may be more effective than ticagrelor for preventing stent thrombosis and recurrent ischaemic events and warn that the credibility of any indirect comparison hinges on the similarity of the included trials and point to the differences in the patient populations included in the TRITON-TIMI 3836 and PLATO33 trials (randomised at presentation for PCI and randomised at presentation to the treatment centre, respectively). The authors acknowledge that this increases the likelihood of heterogeneity and recommend that a head-to-head trial of prasugrel and ticagrelor should be carried out.
The AG is of the opinion that the results of the TRITON-TIMI 3836 and PLATO33 trials have made a major contribution to the Chatterjee et al. 58 analysis and do not consider it appropriate to compare these two trials. The AG also considers that the length of follow-up of the DISPERSE-238 and JUMBO-TIMI 2638 trials was too short to provide data relevant to the current appraisal.
The work published by Steiner et al. 60 was intended to indirectly compare prasugrel, ticagrelor, high-dose clopidogrel and standard-dose clopidogrel in patients scheduled for PCI by undertaking a network meta-analysis from 14 eligible studies (48,982 patients). All studies are described in Appendix 7. The three largest studies are TRITON-TIMI 38,36 a substudy from the PLATO trial (PLATO-INVASIVE56) and CURRENT-OASIS 7 PCI. 45 These trials included patients with ACS and contributed almost 90% of patients in the analysis, whereas the other studies included stable or mixed study populations. A subgroup analysis was conducted on patients with ACS and treated with PCI using data from five studies: TRITON-TIMI 38,36 PLATO,33 CURRENT-OASIS 7,45 Han et al. 65 and DOSER. 66 This subgroup analysis corroborated the overall findings of the review which were that, for the majority of outcomes, there was no superiority of either prasugrel or ticagrelor and that prasugrel was associated with a significantly lower risk than ticagrelor for stent thrombosis but an increased risk of major or minor bleeding.
The AG is of the opinion that the overall network meta-analysis is not relevant to this review as the majority of included trials comprise stable or mixed study populations and are of short duration with primarily pharmacodynamics outcomes. The results of the ACS PCI subgroup are largely based on the comparison of the TRITON-TIMI 3836 and PLATO33 trials; the AG has previously stated this comparison to be inappropriate. The three other trials included in the subgroup analysis (CURRENT-OASIS 7,46 Han et al. 65 and DOSER66) compare high-dose clopidogrel with standard-dose clopidogrel and are of too short a duration to be of relevance to the current appraisal.
Discussion
One relevant RCT was identified for inclusion in this review, namely the TRITON-TIMI 3836 trial. This was an international, double-blind trial that recruited a large number of patients. The trial was robustly designed to demonstrate the clinical efficacy of prasugrel compared with clopidogrel in a population of patients with ACS who were treated with PCI. The outcomes for the core clinical cohort were considered relevant to this appraisal. Although the core clinical cohort comprised 79% of the overall trial population, this subgroup analysis was not prespecified in the original trial protocol42 and should, therefore, be considered as exploratory and hypothesis generating. Searching did not identify any trials of prasugrel compared with ticagrelor.
In the core clinical cohort, prasugrel was favoured over clopidogrel for the primary composite end point of death from CV causes, non-fatal MI, or non-fatal stroke. This effect appeared to be consistent across subgroups (including STEMI, UA/STEMI and patients with and without diabetes mellitus) and for the duration of the trial. Likewise, the benefit of prasugrel was statistically significantly greater for the secondary composite end point (death from any cause, non-fatal MI, non-fatal stroke, or non-CABG-related non-fatal TIMI major bleeding). The efficacy for both composite end points was driven by the reduced number of non-fatal MIs in the prasugrel arm. Other statistically significant differences in favour of prasugrel were reported for the outcomes of definite stent thrombosis and definite or probable stent thrombosis. There were no statistically significant differences noted between trial arms for the majority of the safety outcomes related to bleeding; however, there was a statistically significant difference in favour of clopidogrel when TIMI major and minor bleeds were combined. The calculated net clinical benefit also statistically significantly favoured prasugrel over clopidogrel. No reliable HRQoL outcome data for the patients in the TRITON-TIMI 38 trial were available.
No detailed clinical data were identified by the AG that related to key patient groups within the core clinical cohort, patients with STEMI or UA/NSTEMI or patients with diabetes mellitus.
The three areas of concern noted during TA18221 were reconsidered in this review. These centred around the generalisability of the TRITON-TIMI 3836 trial results to patients in clinical practice in England and Wales. The AG considers that the clinical evidence for the equivalence of a 300-mg loading dose of clopidogrel (administered in the trial) with the 600-mg loading dose often given in clinical practice remains uncertain. Similarly, the AG is of the opinion that the importance of timing of the administration of the clopidogrel loading dose on patient outcomes remains an issue. However, the AG considers that the case for the effectiveness of prasugrel compared with clopidogrel in preventing MIs of all types and sizes appears to be robust and indicates that prasugrel is more effective than clopidogrel at preventing MIs.
No indirect comparison of prasugrel with ticagrelor was conducted by the AG or the manufacturer. The AG did not conduct an indirect treatment comparison using data from the TRITON-TIMI 3836 and PLATO33 trials owing to irreconcilable differences between the trials. These differences were discussed in the appraisal of ticagrelor during TA236. 22 Four published indirect comparisons57–60 were considered to provide unreliable conclusions as they were based largely on data derived from the TRITON-TIMI 3836 and PLATO33 trials. The comparative effectiveness and safety of prasugrel compared with ticagrelor remains unknown.
Chapter 4 Assessment of cost-effectiveness
There are three distinct elements to this section on cost-effectiveness. First, the methods and results of a literature search for economic evidence describing prasugrel since the publication of the previous NICE guidance21 is presented. Second, a summary and critique of the economic model submitted by Daiichi Sankyo Company Ltd/Eli Lilly and Company Ltd is described (the AG notes that no other manufacturer submitted an economic model). Third, the AG’s independent economic model is described alongside comprehensive interpretation of the model’s results.
Systematic review of existing cost-effectiveness evidence
Search strategy
This review is an update of an existing review; however, searching was not date limited. In addition to searching the MS for relevant references, the following databases were searched for economic evaluations of prasugrel:
-
Ovid MEDLINE(R) (1946 to August Week 3 2013)
-
Ovid MEDLINE(R) In-Process & Other Non-Indexed Citations (searched 30 August 2013)
-
NHS EED (searched 30 August 2013)
-
EMBASE (1974 to 30 August 2013).
The results were entered into an EndNote X5 library (and the references were deduplicated electronically). Full details of the search strategy are presented in Appendix 1.
Inclusion and exclusion criteria
At stage 1, two reviewers (ABol and SB) independently screened all titles and abstracts. Full paper manuscripts of any titles and abstracts that were considered relevant by either reviewer were obtained when possible. At stage 2, the relevance of each study was assessed (ABol and SB) according to the criteria set out in Table 13. Studies that did not meet the criteria were excluded. Any discrepancies were resolved by consensus and, when necessary, a third reviewer was consulted.
| Variable | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Intervention or comparator | Prasugrel | Not prasugrel |
| Study design | Full economic evaluation | Methodological paper, letter,a abstractb |
| Perspective | UK or European perspective | Non-European perspective |
| Source of publication | Unrelated to previous appraisal | Related to previous appraisal (e.g. NICE/ERG/manufacturer) |
Data extraction and quality assessment strategy
In the AG’s review protocol,67 data relating to both study design and quality were planned to be extracted by two reviewers (ABol and SB) into an Excel spreadsheet (Microsoft Excel 2010; Microsoft Corporation, Redmond, WA, USA). It was also planned that all economic evaluations identified for inclusion in the review would be quality assessed according to the Drummond et al. 68 10-point checklist. However, no studies were identified for inclusion in the AG’s review.
Results: quantity and quality of research available
After deduplication of 1449 references, a total of 1230 titles and abstracts were screened for inclusion at stage 1. Of these 1230 references, 1117 were immediately excluded because they did not include prasugrel as an intervention or a comparator. At stage 2, inclusion criteria were applied to 113 references. During stage 2, 98 references were excluded, leaving a possible 15 references available for potential inclusion and these are listed Table 14. Of the 15 potentially eligible references, none of the papers met the full inclusion criteria that were set by the AG.
| Study | Title | Comment |
|---|---|---|
| Mahoney et al.69 | Cost-effectiveness of prasugrel versus clopidogrel in patients with ACSs and planned percutaneous coronary intervention: results from the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-thrombolysis in MI TRITON-TIMI 38 | Non-European perspective |
| Serebruany70 | Letter by Serebruany regarding article “Cost-effectiveness of prasugrel versus clopidogrel in patients with ACSs and planned percutaneous coronary intervention: results from the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-thrombolysis in MI TRITON-TIMI 38” | Letter/linked to Mahoney69 |
| Mahoney et al.71 | Response to letter regarding article “Cost-effectiveness of prasugrel versus clopidogrel in patients with ACSs and planned percutaneous coronary intervention: results from the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-thrombolysis in MI TRITON-TIMI 38” | Letter/linked to Mahoney69 |
| Davies et al.72 | Prasugrel vs. clopidogrel in patients with ACSs undergoing percutaneous coronary intervention: a model-based cost-effectiveness analysis for Germany, Sweden, the Netherlands and Turkey | Related to previous appraisal (same economic model – TA182) |
| Mauskopf et al.73 | Cost-effectiveness of prasugrel in a US managed care population | Non-European perspective |
| Davies et al.74 | Is prasugrel cost-effective relative to clopidogrel in patients with ACSs undergoing percutaneous coronary intervention from the perspective of the UK national health service? A model-based analysis | Abstract |
| Davies et al.75 | Is prasugrel cost-effective relative to clopidogrel in patients with ACSs undergoing percutaneous coronary intervention from the perspective of the German health care system? A model-based analysis | Abstract |
| Davies et al.76 | Prasugrel vs. clopidogrel in patients with ACSs undergoing percutaneous coronary intervention: A Spanish model-based cost-effectiveness analysis | Abstract |
| Greenhalgh et al.15 | Prasugrel for the treatment of acute ACSs with percutaneous coronary intervention | NICE |
| Hill et al.77 | Prasugrel for the treatment of ACSs with percutaneous coronary intervention: NICE technology appraisal guidance | NICE/ERG |
| Keast et al.78 | Cost-effectiveness of prasugrel and clopidogrel for ACSs in a medicaid population | Abstract/non-European perspective |
| Mahoney et al.79 | Cost-effectiveness of prasugrel versus clopidogrel in patients with ACSs and planned PCI: Results from the TRITON-TIMI 38 trial from the German perspective | Abstract |
| Mondragon et al.80 | Cost-effectiveness of prasugrel versus clopidogrel in patients with ACSs undergoing percutaneous coronary intervention in the private sector in Mexico | Abstract/non-European perspective |
| Mondragon et al.81 | Cost-effectiveness of prasugrel versus clopidogrel in patients with ACSs undergoing percutaneous coronary intervention in the public health care system in Mexico | Abstract/non-European perspective |
| Rao et al.82 | A decision modelling approach to evaluate the cost-effectiveness of prasugrel versus clopidogrel in patients with planned percutaneous coronary intervention | Abstract |
The review carried out by the AG picked up the three studies69,72,73 that the manufacturer had identified for inclusion in the review of cost-effectiveness evidence presented in the MS. Two of these studies69,73 were carried out from a US perspective and the third study72 employed the model that was submitted to NICE for the evaluation of prasugrel in 2009 (TA18221); all three studies69,72,73 were therefore excluded from the review by the AG.
Studies by Davies et al.72,74–76
The AG notes that, of the 15 potentially eligible studies identified via electronic searching, four of the references were authored by Davies et al. ; one was a full paper72 and three were abstracts. 74–76 In the MS (p. 87), the manufacturer comments that the results of the analyses described in the full paper72 were generated by the same model as that submitted to NICE for the evaluation of prasugrel in 2009 (TA182). 21 This reference was therefore excluded from the review by the AG as the economic model described therein has been previously fully discussed and critiqued. However, as the full paper72 reports model results using costs and rehospitalisation rates specific to Germany, Sweden, the Netherlands and Turkey, the AG has reproduced the table of results from the main study72 and also the results of a sensitivity analysis where the price of clopidogrel has been set to zero (Table 15). The results of the Spanish model-based cost-effectiveness analysis presented in one of the abstracts have not been presented here as the abstract76 did not include sufficient population data to allow comparison with the other published model results. In summary, all of the individual country incremental cost-effectiveness ratio (ICER) estimates demonstrate the cost-effectiveness of prasugrel compared with clopidogrel in the overall licensed population and in four patient subgroups (UA/NSTEMI, STEMI, ACS diabetes and the core clinical cohort); when the price of clopidogrel is set to zero, prasugrel remains cost-effective compared with clopidogrel in the overall licensed population.
| Per patient costs, QALYs and ICER | Licensed population (n = 13,090) | UA/NSTEMI (n = 9669) | STEMI (n = 3421) | ACS diabetes (n = 2947) | Core cohort (n = 10,804) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| CLOP | PRA | CLOP | PRA | CLOP | PRA | CLOP | PRA | CLOP | PRA | |
| Germany | ||||||||||
| Total costs (€) | 19,942 | 20,725 | 19,990 | 20,751 | 19,804 | 20,652 | 18,995 | 19,817 | 21,428 | 22,220 |
| QALYs | 10.657 | 10.712 | 10.661 | 10.702 | 10.647 | 10.740 | 9.972 | 10.109 | 11.524 | 11.547 |
| ICER (€) | 14,350 | 18,530 | 9131 | 6025 | 14,487 | |||||
| Sweden | ||||||||||
| Total costs (€) | 27,003 | 27,345 | 27,020 | 27,330 | 26,954 | 27,388 | 25,633 | 26,021 | 29,128 | 29,481 |
| QALYs | 10.945 | 10.997 | 10.930 | 10.968 | 10.988 | 11.080 | 10.214 | 10.347 | 11.870 | 11.923 |
| ICER (€) | 6520 | 8016 | 4738 | 2910 | 6711 | |||||
| Netherlands | ||||||||||
| Total costs (€) | 13.646 | 14,147 | 13,667 | 14,152 | 13,587 | 14,132 | 13,049 | 13,566 | 14,626 | 15,132 |
| QALYs | 12.919 | 12.987 | 12.907 | 12.959 | 12.952 | 13.065 | 11.988 | 12.156 | 14.053 | 14.122 |
| ICER (€) | 7369 | 9378 | 4788 | 3080 | 7342 | |||||
| Turkey | ||||||||||
| Total costs (€) | 3789 | 4167 | 3796 | 4171 | 3769 | 4158 | 3591 | 3975 | 4074 | 4455 |
| QALYs | 9.521 | 9.573 | 9.518 | 9.558 | 9.531 | 9.616 | 8.810 | 8.937 | 10.366 | 10.419 |
| ICER | 7294 | 9371 | 4552 | 3036 | 7207 | |||||
| Licensed population: clopidogrel drug cost set at zero | ||||||||||
| ICER (€) | Germany (18,494) | Sweden (7058) | Netherlands (7634) | Turkey (14,251) | ||||||
Conclusions of the Assessment Group’s cost-effectiveness literature review
The AG did not identify any published papers that met the inclusion criteria for the review.
Review of the Eli Lilly and Company Ltd/Daiichi Sankyo Company Ltd economic model
Overview of manufacturer’s submitted model
Table 16 describes NICE’s reference case checklist and provides the manufacturer’s assessment of how the submitted economic model matches NICE’s checklist.
| NICE reference case requirements | Reference case | Does the de novo economic evaluation match the reference case? |
|---|---|---|
| Defining the decision problem | The scope developed by the Institute | Yes but timing and dose of comparator in UK does not match that used in the trial |
| Comparators | Therapies routinely used in the NHS, including technologies currently regarded as best practice | Economic evaluation was carried out from the perspective of the NHS – no PSS costs are described in the MS |
| Perspective on costs | NHS and PSS | Yes |
| Perspective on outcomes | All health effects on individuals | Time horizon chosen was a lifetime horizon so all relevant benefits are accounted for in the economic model; only in-trial drug and hospital costs are considered |
| Type of economic evaluation | Cost-effectiveness analysis | All outcome data up to 12 months are derived from a single Phase III RCT (TRITION-TIMI 38), which was appropriate. Four clinical studies were identified via ad hoc literature searches and used to estimate long-term risks up to 40 years |
| Synthesis of evidence on outcomes | Based on a systematic review | Although quality-of-life data were collected during the TRITON-TIMI 38 trial they were not used owing to small number of responses. Instead, published US EQ-5D scores were used |
| Measure of health benefits | QALYs | Valuations within the EQ-5D scores were calculated using time trade-off techniques |
| Source of data for measurement of HRQoL | Reported directly by patients and/or carers | Not stated in the MS |
| Source of preference data for valuation of changes in HRQoL | Representative sample of general public | Yes |
| Discount rate | An annual rate of 3.5% on both costs and QALYs | Yes |
| Equity weighting | An additional QALY has the same weight regardless of the other characteristics of the individuals receiving the health benefit | Yes, equal weighting regardless of characteristics |
In summary, the manufacturers have submitted the same economic model that they previously presented during the original appraisal of prasugrel for the treatment of ACS with PCI (TA182). 21 However, some aspects of the submitted model have been updated in the light of feedback generated during the original appraisal of prasugrel (TA182). 21 These revised aspects are:
-
use of sensitivity analysis encompassing the entire population as opposed to a ‘typical’ patient profile
-
removal of the functionality that allowed the user to choose to model 15 months of treatment (as the licence is only for 12 months)
-
conduct of scenario analysis using the ERG’s suggestions for utility values, amended long-term relative risk (RR) of mortality and reduced incidence of non-fatal MI
-
use of the generic (reduced) price of clopidogrel
-
updated costs.
The model was developed with the principle of simulating the TRITON-TIMI 3836 trial outcomes as closely as possible. There are two main phases to the model: the active treatment phase, which spans the duration of the clinical trial, and the post-treatment phase, which extrapolates outcomes and costs beyond events that took place during the treatment phase, up until death or lifetime horizon (base case 40 years). Within the trial period, there is an opening 3-day period, modelled using a decision tree, followed by 12 cycles, each of 1 month, up to 12 months. The transitions were time dependent. Long-term mortality was based on adjustment of population life tables to reflect prognostic implications of the events modelled over the short term. The model also permits some costs to accumulate after the end of the trial period.
Figure 3 shows the state transition diagram for the Markov model element of the TRITON-TIMI 3836 study. Patients enter the model at the point of the index ACS event, immediately prior to undergoing PCI. Exit occurs at death, or at completion of the model time horizon.
FIGURE 3.
Schema of manufacturer’s model. Note: the lightly dashed lines leading to ‘bleed end point event’ are intended to highlight that these do not represent transitions to health states that continue to impart prognostic effects in terms of long-term mortality, or permanent utility decrements. Patients remain in their origin states following bleed events, except when the event is fatal. Temporary utility decrements are applied at the time of major non-fatal bleeds. Rehospitalisation occurs in all states at rates determined by current and past clinical events. MI, myocardial infarction. Reproduced from MS.

Parameters and values
The parameters and values used in the economic model are displayed in Table 17.
| Parameter | Data | Source |
|---|---|---|
| General | ||
| Treatment duration | 12 months | SPC, treatment guidelines |
| Time horizon | 40 years | NICE reference case |
| Discounting | 3.5% | NICE reference case |
| Risk equations for transition probabilities | ||
| Primary events | Logistic regression for 3-day risk (OR) | Modelling working group based on TRITON baseline characteristics and end points results |
| Weibull regression for longer-term risk (HR) | ||
| Fatal bleeds, major bleeds, minor bleeds | Logistic regression for 3-day risk (OR) | Modelling working group based on TRITON baseline characteristics and end points results |
| Weibull regression for longer term risk (HR) | ||
| RRs for post-trial all-cause mortality [RR (95% CI)] | ||
| Angina | 1.21 (1.03 to 1.43) | Rosengren et al. (1998)83 |
| UA/NSTEMI | 1.55 (1.31 to 1.84) | Allen et al. (2006)84 |
| STEMI | 1.84 (1.52 to 2.20) | Allen et al. (2006)84 |
| Reinfarcted NSTEMI | 2.93 (2.34 to 3.66) | Mueller et al. (1995)85 |
| Reinfarcted STEMI | 3.48 (2.77 to 4.37) | Mueller et al. (1995)85 |
| Stroke | 2.39 (1.44 to 3.97) | Taneja et al. (2004)86 |
| Utility decrements compared with general population [EQ-5D time-trade-off utility scores (SE)] | ||
| ACS | 0.0409 (± 0.0002) | Sullivan et al. (2006)87 |
| Stroke | 0.0524 (± 0.0001) | Sullivan et al. (2006)87 |
| Major bleed | 25% decrement to population norm for 14 days | Assumption |
| Cost per hospitalisation (weighted) | ||
| Clopidogrel | £3070 | MS |
| Prasugrel | £3081 | MS |
| Drug acquisition costs | ||
| Clopidogrel | £0.24, loading dose | NHS Drug Tariff28 75 mg (28 tablets) £1.83 |
| £0.07/day, maintenance dose | ||
| Prasugrel | £10.20, loading dose | MIMS August 13 (based on £47.56 per pack of 28 tablets)88 |
| £1.70/day, maintenance dose | ||
Sources of evidence used to inform and develop the model
The TRITON-TIMI 3836 trial was the key source of clinical evidence described in the MS. Non-trial sources of clinical evidence were also identified via literature reviews to inform assumptions regarding additional clinical inputs, long-term extrapolation of mortality and HRQoL.
Baseline treatment strategy
The base case model uses a maximum treatment duration of 12 months, which matches the SPC24 and clinical practice in England and Wales. Aspirin use is continued up to 15 months for modelling purposes.
Baseline and relative risks of disease progression
There are two main phases to the model: the active treatment phase (duration of the trial) and the post-treatment phase, which extrapolates outcomes and costs beyond the duration of the trial up until death.
Separate risk equations for the primary end point events were modelled for UA/NSTEMI and STEMI populations. These analyses used logistic models for events occurring within 3 days, and Weibull models over the remainder of the trial period. Both primary efficacy and safety (bleed) end points predicted by these equations were disaggregated from their combinations into specific event types (e.g. CV death, non-fatal MI and stroke).
The primary end point risk equations played no part in predicting survival beyond the trial. RRs for all-cause mortality were applied to general population (life table-based) mortality rates adjusted to exclude deaths from CV causes. The RRs reflected the index ACS status and revascularisation of all patients in the trial, and the prognostic implications of a further MI or stroke within the trial period.
The estimation of transition probabilities and hospitalisation rates can be split into a number of sections. Table 18 provides an overview of these sections; further detail is provided beneath the table.
| Section | Period | Incident event | Type of event |
|---|---|---|---|
| Risk of primary end point event (CV death, MI, stroke) following PCI | 3 days | Logistic | Multinomial logistic for CV death, non-fatal MI and non-fatal stroke |
| 4 days to 12 months | Weibull | ||
| Risk of major and minor bleeds (including fatal) | 3 days | Logistic | Logistic for fatal bleeds, logistic for major vs. minor (no distinction between time periods) |
| 4 days to 12 months | Weibull | ||
| Risk of events and mortality following treatment phase | 12 months to 40 years | Cause elimination life tables adjusted for trial events RRs | Mortality and hospitalisations |
Risk of a primary end point
Probabilities of primary end point events were estimated from TRITON-TIMI 3836 trial data. Logistic regression was used to predict the occurrence of events during the initial (acute) 3-day period. Standard parametric time to event (survival) analysis (Weibull functions) was used to estimate the risk of events from day 4 to the end of treatment period (12 months).
The AG notes that, despite available clinical trial evidence, the model uses multinomial logistic regression analysis to derive risk equations to predict the probability that having experienced an event, the event is fatal MI, non-fatal MI, or a non-fatal stroke (MS, p. 98). The risk equations in the model focus on time to first event only, although, if a non-fatal event precedes a fatal event, primacy is given to the fatal event.
Risk of major and minor bleeds and mortality following a bleed
The risk of major and minor bleed was estimated using risk equations (MS, p. 98). The model definition of bleeds does not exclude CABG-related bleeds. Non-fatal bleeds are not treated as on-going health states within the model [such events incur only temporary reductions (14 days) in HRQoL and resource use consequences]; however, prognostic implications were captured by the events that occurred up to the end of the trial follow-up period.
Multiple events
Patients who experience a trial end point in some cases experienced multiple events. The risk equations focus on the time to first event only, although, if a non-fatal event preceded a fatal event, primacy was given to the fatal event. Long-term utility and life expectancy implications of clinical events were driven by the occurrence of a first event and were deemed to be unaffected by multiple occurrences. These events were recognised within the model in terms of associated rehospitalisations.
Extrapolation beyond the trial period
Based on treatment follow-up of 15 months in TRITON-TIMI 38,36 risk equations were developed in order to estimate the risk of primary efficacy and safety events for the cohorts of patients receiving prasugrel and clopidogrel. After the maximum treatment duration of 12 months, no additional treatment effect was accrued in either of the two treatment arms.
Patients who reached the end of the trial without suffering prognostic events could be expected to face a lower risk of mortality than patients who did suffer prognostic events. A literature review was conducted in order to identify potential sources for studies reporting long-term mortality rates in ACS PCI patients. As no studies that reported on long-term follow-up of revascularised ACS patients were identified, RRs from four studies83–85,89 of patients who had undergone revascularisation were used. Indirect comparisons were used to derive RRs of mortality compared with coronary heart disease-free patients for each health state included in the model.
The manufacturer adjusted actuarial life tables by RRs calculated by comparing life table mortality rates over the appropriate age ranges with cause elimination life tables for the UK. The MS states that ‘actuarial life tables were taken from the Government Actuarial Department and cause elimination life tables were calculated using Office for National Statistics data (excluding cause of death codes ICD-1-100-199)’ (MS, p. 101).
The RRs used to model the period beyond 12 months are shown in Table 19.
| Health state | Source | Details of study | Indirect RR (95% CI) vs. CHD-free mortality | |
|---|---|---|---|---|
| Non-revascularised | Revascularised | |||
| Angina | Rosengren et al. (1998)83 | Pooled RR for angina mortality 4–16 years after onset | 1.59 (1.16 to 2.20) | 1.21 (1.03 to 1.43) |
| NSTEMI | Allen et al. (2006)84 | Multivariate adjusted RR estimates for mortality in patients with NSTEMI (RR 1.28) or STEMI (RR 1.52) compared with patients with angina during 10-year follow-up | 2.04 (1.73 to 2.41) | 1.55 (1.31 to 1.84) |
| STEMI | 2.42 (2.03 to 2.88) | 1.84 (1.54 to 2.20) | ||
| Reinfarcted NSTEMI | Mueller et al. (1995)85 | RR for mortality in patients with reinfarction within 42 days (RR 1.89) | 3.85 (3.09 to 4.81) | 2.93 (2.34 to 3.66) |
| Reinfarcted STEMI | 4.58 (3.65 to 5.75) | 3.48 (2.77 to 4.37) | ||
| Stroke | Taneja et al. (2004)86 | RR for mortality in patients with a prior stroke at baseline during a 4-year follow-up of PRAIS-UK | – | 2.39 (1.44 to 3.97) |
Population
The populations described in the economic model reflect the patients enrolled in TRITON-TIMI 3836 (details presented in Table 20).
| Population | Description |
|---|---|
| All ACS | All patients other than those with prior stroke or TIA and including patients who are now recommended to be treated with a 5-mg maintenance dose |
| ACS core | Core clinical cohort, patients without prior TIA/stroke, aged < 75 years and weigh ≥ 60 kg |
| UA/NSTEMI | UA/NSTEMI licensed population (excluding prior TIA/stroke) |
| STEMI | STEMI licensed population (excluding prior TIA/stroke) |
| ACS diabetes | ACS licensed population with diabetes (excluding prior TIA/stroke) |
Interventions and comparators
The economic evaluation compares prasugrel in combination with aspirin and clopidogrel in combination with aspirin, at licensed doses. Consistent with both the TRITON-TIMI 3836 trial and the SPC,24 prasugrel is initiated with a single 60-mg loading dose and then continued at 10 mg once a day for up to 12 months in combination with aspirin (75–325 mg). Clopidogrel was initiated with a single 300-mg loading dose and then continued at 75 mg once a day in combination with aspirin for 12 months.
The manufacturer considered that a formal indirect comparison between prasugrel and ticagrelor was inappropriate and no economic analysis of this comparison has been presented in the MS.
Perspective, time horizon and discounting
The perspective for outcomes reflects all the direct health effects, whereas the perspective used for costs is that of the NHS. Outcomes are expressed in terms of life-years and quality-adjusted life-years (QALYs) gained. The time horizon is set at 40 years and, in line with the NICE Guide to the Methods of Technology Appraisal,90 both costs and benefits are discounted at 3.5%. A half-cycle adjustment was performed for both costs and outcomes (attributing events on the basis of average patient exposure over the course of each cycle).
Health-related quality of life
Although the TRITON-TIMI 3836 trial included a HRQoL substudy, the manufacturer reports that it was not possible to provide robust HRQoL estimates owing to the very small numbers of patients with events included within the analysis. The manufacturer, therefore, conducted a systematic review of the literature to identify HRQoL studies relevant to the modelled trial population. The MS (p. 102) includes details of the methods used in the systematic review. Mean utility decrements for ACS (0.049) and stroke/MI (0.052) were taken directly from a US study,87 which was designed to produce a specific list of preference weights for use in economic evaluations; the study used the US version of the EQ-5D.
To calculate utility weights for use in the economic evaluation, background UK population norms (free of disease) which vary by age and sex, as described by Kind et al. ,91 were applied to all patients in the trial. The utility decrements for ACS and stroke/MI were then used alongside these background utility estimates. Finally, the MS assumed that, for a major bleed, a decrement of 25% of the population (utility) norm was applicable for a 14-day period (25% decrement equates to a 0.007 utility toll).
Resources and costs
The key categories of cost estimates in the MS are related to (1) hospitalisations and (2) drug costs. Key cost parameter assumptions are presented in Table 21.
| Parameter | Assumption | Justification |
|---|---|---|
| Resource utilisation at index PCI | The costs of index ACS episodes and index hospitalisations were not included in the analyses | The costs of index hospitalisation were common to both arms |
| Costs of repeat hospitalisations | Only hospitalisations related to end points or to serious adverse events requiring rehospitalisation and potentially related to the ACS condition or the PCI intervention were included in the cost analysis | These represent all rehospitalisations clinically adjudicated as relevant to the trial population and intervention irrespective of adjudicated end points. Regression (Poisson) methods were used to predict rates of rehospitalisation conditional on clinical event histories |
| Rehospitalisations were valued at a weighted average unit cost per hospitalisation (using NHS reference costs) | DRGs were allocated to 2487 individual hospitalisations by clinical reviewer and then UK HRG4 codes matched by a UK clinical cardiologist | |
| Geographical variation in hospitalisation rates | Underlying differences in hospitalisation rates were applied by geographic location (based on economic substudy across eight countries) | Observed hospitalisation rates in the UK were lower than in the trial as a whole. The regression reflects this lesser propensity to hospitalise in the UK within the trial |
| Drug costs | Miscellaneous drug acquisition costs were included within the NHS reference costs applied to hospitalisations within the model. These may include antiplatelet costs (e.g. clopidogrel), but the acquisition cost continued to be applied during hospitalisations in the model, potential double counting | Double counting of antiplatelet drug acquisition costs would have no material effect on the ICER as these would constitute tiny proportions of hospital episode costs, apply to both arms, and leave average hospitalisation costs unaffected |
Drug acquisition costs
Patients were assumed to be treated with either aspirin and clopidogrel or aspirin and prasugrel for 12 months. The acquisition costs of prasugrel, clopidogrel and aspirin are shown in Table 22. No drug costs were applied beyond 12 months.
| Cost of loading dose (per day) | Cost of maintenance dose (per day) | Source | |
|---|---|---|---|
| Prasugrel | £10.20 | £1.70 | MS |
| Clopidogrel | £0.24 | £0.07 | MS |
| Aspirin | NA | £0.01 | MS |
Cost of hospitalisations in TRITON-TIMI 38
The TRITON-TIMI 3836 trial included a preplanned economic substudy which recorded the occurrence of rehospitalisations associated with serious adverse events over a 12-month period in eight countries: Australia, Canada, the USA, France, Germany, the UK, Spain and Italy. The hospitalisation substudy covered the trial period and focused on 2487 hospitalisations from 6705 patients. Individual US diagnostic-related groups (DRGs) were then assigned to each hospitalisation to facilitate a cost estimation for each episode. The assignments of DRGs were carried out by an expert who was blinded to the treatment arm of the study in which they occurred. Poisson regression was used to predict the rate of hospitalisations within the trial period according to clinical event history and geographical location to estimate the rates in the overall population. Patients who remained alive at the end of the trial continued to accrue life-years, QALYs and costs. No further incidence of clinical events was modelled during the extrapolation phase and the hospitalisation rates were estimated at the same constant rate per living patient in both arms.
For the UK economic evaluation, each DRG code was matched to a corresponding UK ‘NHS reference costs’ HRG4 code by a consultant cardiologist. The allocated unit costs were then used to calculate an average weighted unit cost per hospital episode for patients in the prasugrel and clopidogrel arms of TRITON-TIMI 38. 36 The manufacturer stated that a conservative approach was adopted as the average cost of hospitalisation in the clopidogrel arm was used for both treatment arms, despite evidence to suggest that the weighted average unit cost per hospitalisation episode may be more expensive in the prasugrel arm. Hospitalisation costs are presented in Table 23.
| Economic substudy sample | Clopidogrel (n = 3332) | Prasugrel (n = 3373) |
|---|---|---|
| Total hospitalisations (n) | 1259 | 1228 |
| Rate of rehospitalisation per month | 0.0256 | 0.0245 |
| Weighted average unit cost per hospitalisation episode (from trial) | £3070 | £3081 |
| Weighted average unit cost per hospitalisation (base case) | £3070 | £3081 |
Cost-effectiveness results
Five different subgroups are considered, namely (1) the whole ACS licensed population (excluding prior stroke/TIA), (2) the ACS core population (excluding those with prior stroke/TIA and patients weighing < 60 kg or aged ≥ 75 years), (3) the UA/NSTEMI licensed population (excluding those with prior stroke/TIA), (4) the STEMI licensed population (excluding those with prior stroke or TIA) and (5) the ACS–diabetes licensed population (excluding those with prior stroke or TIA). The base case ICERs generated by the manufacturer’s model for these five subgroups are presented in Table 24.
| Population | Whole ACS licensed population (excluding prior stroke/TIA) | ACS core (excluding prior stroke/TIA and patients weighing < 60 kg or aged ≥ 75 years) | UA/NSTEMI licensed population (excluding prior stroke/TIA) | STEMI licensed population (excluding prior stroke/TIA) | ACS diabetes, licensed population (excluding prior stroke/TIA) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | CLOP | PRA | Effect | CLOP | PRA | Effect | CLOP | PRA | Effect | CLOP | PRA | Effect | CLOP | PRA | Effect |
| Event probabilities | Ratio | Ratio | Ratio | Ratio | Ratio | ||||||||||
| CV death | 2.05% | 1.76% | 0.86 | 1.58% | 1.36% | 0.86 | 1.80% | 1.66% | 0.92 | 2.76% | 2.05% | 0.74 | 3.59% | 2.73% | 0.76 |
| MI | 8.49% | 6.43% | 0.76 | 8.15% | 6.20% | 0.76 | 8.60% | 6.61% | 0.77 | 8.17% | 5.91% | 0.72 | 10.64% | 6.72% | 0.63 |
| Stroke | 0.74% | 0.69% | 0.93 | 0.64% | 0.58% | 0.90 | 0.72% | 0.54% | 0.74 | 0.79% | 1.12% | 1.42 | 1.23% | 1.01% | 0.82 |
| Total combined end point | 11.28% | 8.87% | 0.79 | 10.37% | 8.14% | 0.79 | 11.13% | 8.80% | 0.79 | 11.71% | 9.08% | 0.78 | 15.46% | 10.46% | 0.68 |
| Fatal bleed | 0.00% | 0.11% | NA | 0.00% | 0.05% | NA | 0.00% | 0.11% | NA | 0.00% | 0.12% | NA | 0.00% | 0.15% | NA |
| Major bleed | 1.71% | 2.19% | 1.28 | 1.50% | 1.95% | 1.30 | 1.49% | 2.07% | 1.39 | 2.32% | 2.52% | 1.09 | 2.21% | 2.35% | 1.06 |
| Minor bleed | 1.93% | 2.51% | 1.30 | 1.49% | 1.98% | 1.33 | 1.69% | 2.40% | 1.42 | 2.61% | 2.82% | 1.08 | 2.70% | 2.93% | 1.08 |
| Total bleed | 3.64% | 4.81% | 1.32 | 2.99% | 3.97% | 1.33 | 3.18% | 4.58% | 1.44 | 4.93% | 5.46% | 1.11 | 4.91% | 5.42% | 1.11 |
| Results | Increment | Increment | Increment | Increment | Increment | ||||||||||
| Life-years | 13.14 | 13.21 | 0.07 | 14.14 | 14.20 | 0.07 | 13.16 | 13.21 | 0.05 | 13.09 | 13.20 | 0.11 | 12.35 | 12.52 | 0.17 |
| QALYs | 10.16 | 10.21 | 0.05 | 10.97 | 11.02 | 0.05 | 10.16 | 10.20 | 0.04 | 10.16 | 10.25 | 0.09 | 9.50 | 9.63 | 0.13 |
| Costs | £5469 | £6062 | £593 | £5867 | £6463 | £596 | £5480 | £6067 | £587 | £5437 | £6046 | £609 | £5209 | £5809 | £600 |
| Cost per life-year | £8847 | £8,979 | £11,661 | £5,337 | £3,550 | ||||||||||
| Cost per QALY | £11,660 | £11,796 | £15,452 | £6,987 | £4,675 | ||||||||||
Sensitivity analyses
A probabilistic sensitivity analysis was not undertaken. Univariate (one-way) sensitivity analysis was conducted by the manufacturer for selected model parameters, namely discounting, haemorrhage utility decrement, MI and stroke utility decrements, hospitalisation episodes, treatment duration, RR for all-cause mortality (post-trial phase) and time horizon. The results of the one-way sensitivity analysis are shown in Table 25.
| Model factor adjusted | Clopidogrel | Prasugrel | ICERs | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LYs | QALYs | Costs (£) | LYs | QALYs | Costs (£) | Δ LYs | Δ QALYs | Δ Costs (£) | £/LY | £/QALY | ||
| Base case | 14.14 | 10.97 | 5867 | 14.20 | 11.02 | 6463 | 0.07 | 0.05 | 596 | 8979 | 11,796 | |
| Discounting rates | 0.00% pa | 21.56 | 16.65 | 8917 | 21.68 | 16.74 | 9546 | 0.12 | 0.09 | 628 | 5147 | 6787 |
| 6.00% pa | 11.11 | 8.64 | 4622 | 11.16 | 8.68 | 5203 | 0.05 | 0.04 | 581 | 12,574 | 16,475 | |
| Haemorrhage disutility (120 days) | × 8 | 14.14 | 10.96 | 5864 | 14.20 | 11.01 | 6461 | 0.07 | 0.05 | 596 | 8979 | 11,851 |
| MI/stroke disutility | × 0.5 | 14.14 | 10.97 | 5864 | 14.20 | 11.02 | 6461 | 0.07 | 0.05 | 596 | 8979 | 11,966 |
| × 1.5 | 14.14 | 10.96 | 5864 | 14.20 | 11.01 | 6461 | 0.07 | 0.05 | 596 | 8979 | 11,630 | |
| Mortality RR | × 0.5 | 14.27 | 11.06 | 5916 | 14.30 | 11.09 | 6501 | 0.04 | 0.03 | 584 | 15,775 | 20,619 |
| × 1.5 | 14.05 | 10.90 | 5827 | 14.13 | 10.96 | 6431 | 0.09 | 0.07 | 605 | 6919 | 9096 | |
| Clopidogrel preloading adjustment | 70% | 14.15 | 10.97 | 5867 | 14.20 | 11.02 | 6461 | 0.06 | 0.04 | 593 | 10,631 | 13,959 |
| NHS reference costs (HRG) | × 0.5 | 14.14 | 10.97 | 2945 | 14.20 | 11.02 | 3535 | 0.07 | 0.05 | 590 | 8892 | 11,682 |
| × 0.8 | 14.14 | 10.97 | 4696 | 14.20 | 11.02 | 5290 | 0.07 | 0.05 | 594 | 8944 | 11,750 | |
| × 1.2 | 14.14 | 10.97 | 7032 | 14.20 | 11.02 | 7631 | 0.07 | 0.05 | 599 | 9014 | 11,841 | |
| × 1.5 | 14.14 | 10.97 | 8784 | 14.20 | 11.02 | 9386 | 0.07 | 0.05 | 602 | 9066 | 11,909 | |
Critique of submitted economic model
The AG’s critique of the manufacturer’s submitted economic model is the same as the original critique presented by the ERG during the original appraisal of prasugrel (TA182). 21 The AG and the ERG are the same academic research group.
As outlined in section 8.2.4.1 of the MS, at the time of the original appraisal, the ERG suggested amendments to the manufacturer’s economic model in the following six main areas:
-
life table calculations, which need to allow for competing risks
-
differences in discounting approaches
-
treatment costs, which should reflect usage and pack wastage
-
alternative utility values (i.e. those derived from the HODAR database)
-
reduced incidence of non-fatal MIs such that the underlying rate of MIs is 50% that recorded in the TRITON-TIMI36 trial
-
amended long-term RRs of mortality by ignoring the initial impact of ACS prior to TRITON-TIMI-related events (i.e. ignoring the sources from Rosengren et al. 83).
The AG agrees with the manufacturer that the first three points mentioned above lead to non-significant changes in the size of the ICER. The manufacturer carried out a scenario analysis to determine the effect of the remaining three amendments suggested by the ERG.
The impact of this scenario analysis on the results for the relevant subgroups is presented in Table 26.
| Population | UA/NSTEMI | STEMI | ACS–diabetes | ACS core | ||||
|---|---|---|---|---|---|---|---|---|
| Treatment | Clopidogrel | Prasugrel | Clopidogrel | Prasugrel | Clopidogrel | Prasugrel | Clopidogrel | Prasugrel |
| Life-years | 14.64 | 14.68 | 15.04 | 15.14 | 13.97 | 14.12 | 15.74 | 15.79 |
| QALYs | 9.74 | 9.76a | 10.04 | 10.11 | 9.26 | 9.36 | 10.51 | 10.54 |
| Costs (£) | 6047 | 6644 | 6203 | 6825 | 5809 | 6430 | 6487 | 7092 |
| Cost per life-year (£) | 16,713 | 5834 | 3952 | 11,509 | ||||
| Cost per QALY (£) | 25,504 | 8827 | 6002 | 17,439 | ||||
| Base case cost/QALY (£) | 15,542 | 6987 | 4675 | 11,796 | ||||
The results of the manufacturer’s scenario analysis show that, when comparing prasugrel with clopidogrel, all relevant ICERs remained within the £20,000 to £30,000 per QALY gained threshold.
However, the AG is of the opinion that the basic structure of the manufacturer’s economic model still requires further refinement. The main focus of the AG’s critique is the manufacturer’s projection of long-term survival. The AG’s specific concerns are outlined in detail in Independent economic assessment: results and Independent economic assessment: discussion.
In summary, the AG developed its own economic model for the following reasons:
-
The long-term model phase in the manufacturer’s submitted economic model was considered to be unsatisfactory and potentially not sufficiently reliable to generate a realistic representation of 39-years of follow-up.
-
The manufacturer’s decision model projects long-term (years 2–40) costs and outcomes solely in terms of mortality hazard rates fixed after 1 year, and takes no account of the effects of accumulating experience of CV events and disability.
-
The AG considered it appropriate to develop an economic model using the most reliable clinical evidence available and, therefore, preferred to use 3-year clinical data from the CAPRIE92 (Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events) trial instead of 15-month data from the TRITON-TIMI 3836 trial.
-
To fulfil the remit stated by NICE and to review fully the guidance for prasugrel issued in TA182,21 the AG was required to compare four patient subgroups (STEMI without diabetes mellitus, STEMI with diabetes mellitus, NSTEMI without diabetes mellitus and NSTEMI with diabetes mellitus). The structure of the decision model submitted by the manufacturer does not readily facilitate modelling these four subgroups in terms of cost-effectiveness.
Independent economic assessment: methods
Background and modelling rationale
The manufacturer of prasugrel has chosen to resubmit the same decision model previously employed for the NICE single technology appraisal (STA) of prasugrel in 2009. 21 This model comprised two distinct phases:
-
a short-term statistical model of the data from the TRITON-TIMI 3836 clinical trial (up to 15 months follow-up)
-
a long-term model projecting survival and hospitalisation of patients alive at the end of the first phase up to a maximum of 40 years.
In the ERG’s report prepared as part of the STA process, particular concern was expressed about the structure of this model. The ERG concluded that the initial phase of the model generated reliable outcome estimates:
Comparison of the mortality rate (all causes) obtained by Kaplan–Meier analysis of TRITON-TIMI 38 data (supplied by the manufacturer) with corresponding rates generated by the model at 30 days and 12 months indicate a good correspondence for treatment with clopidogrel and with prasugrel for all specified populations.
Greenhalgh et al. 2009, section 5.5.293
However, the long-term model phase was considered by the ERG to be less satisfactory and potentially not sufficiently reliable to generate a realistic representation of a further 39 years of follow-up:
In the long-term component of the submitted model there is an assumption that differences established between the prasugrel and clopidogrel arms of the TRITON-TIMI 38 trial will be preserved indefinitely at the level observed at the end of the trial. However, there is no reason to believe that further serious nonfatal events will not continue to occur to patients in both cohorts, and if events occurring during the trial are presumed to influence later survival, then it is also likely that any such events in subsequent periods will also have important effects. Since active treatment with clopidogrel or prasugrel will have ceased, it can be expected that event rates will be similar in both arms. As a result of this process it is likely that over time the disease history of patients will converge, and therefore any initial advantage for either treatment will be progressively attenuated. This effect would have become evident in the model results if the long-term model had been structured to reflect changes in health states over time.
Greenhalgh et al. 2009, section 5.5.393
As these serious concerns have not been addressed by the manufacturer in the model submitted for this reappraisal of prasugrel, the AG has developed a new decision model. The AG’s model accepts the manufacturer’s statistical model for the initial phase (up to 12 months), but replaces the long-term projection with a more detailed structure that provides an improved representation of subsequent CV events, accumulating patient histories, alteration in health states and associated care costs, as well as patient HRQoL.
Patient populations
The AG has structured its decision model to accommodate four mutually exclusive subgroups of the core clinical cohort population (i.e. all ACS patients excluding those with a history of TIA or stroke, those with body weight of < 60 kg or those aged > 75 years):
-
ACS patients treated with PCI for STEMI and with diagnosed diabetes
-
ACS patients treated with PCI for STEMI and without diagnosed diabetes
-
ACS patients treated with PCI for UA or NSTEMI and with diagnosed diabetes
-
ACS patients treated with PCI for UA or NSTEMI and without diagnosed diabetes.
These were the groups considered by the ERG to be important in the development of the final 2009 guidance related to prasugrel (TA18221) and they therefore form an appropriate basis for this review of the existing guidance.
Treatment options
No suitable clinical evidence has been identified that can provide the basis for a reliable comparison between prasugrel and ticagrelor. The AG model, therefore, has been developed as a simple comparison between dual antiplatelet therapy for 12 months from index PCI with either clopidogrel in combination with low-dose aspirin or prasugrel in combination with low-dose aspirin.
Model design and structure
The AG for this review also acted as AG for the reappraisal of clopidogrel and modified-release dipyridamole for the prevention of occlusive vascular events. That reappraisal was an update of NICE guidance TA9094 and resulted in the publication of TA210,95 which was issued in December 2010. In TA210,95 NICE made recommendations concerning the use of clopidogrel and modified-release dipyridamole for the prevention of occlusive vascular events. As part of the TA21095 guidance development process, the AG developed a detailed decision model to estimate the long-term health care and outcomes expected for patients receiving different strategies of long-term preventative treatment. The model took the form of an individual patient simulation. It was calibrated mainly using data provided by the manufacturer of clopidogrel from the CAPRIE92 clinical trial, supplemented with data provided by the manufacturer of dipyridamole from the PROFESS96 clinical trial and some additional published trial results. The additional data included follow-up results for 3 years from the start of preventative therapy. Supplementary details are provided in Appendix 6.
The AG has concluded that the MI subpopulation model used in the development of TA21095 (the TA21095 model), which was based largely on CAPRIE92 trial data, addresses very similar issues to those that are of concern to this review of TA182. 21 The AG’s clinical advisor has confirmed that CAPRIE92 data are an appropriate trial source for extrapolating long-term vascular events and that no better source has become available since 2010.
However, there is a significant practical drawback to using the individual patient simulation approach that was employed in the TA21095 model, namely the extended run times involved in generating model results, especially when carrying out probabilistic sensitivity analyses. The AG has therefore re-engineered the TA21095 model, and the current AG model for prasugrel employs a long-term Markov chain, which operates for up to 39 years of follow-up beyond the first 12 months of treatment with clopidogrel or prasugrel. This re-engineering has necessitated some compromises to the fully flexible logic of the TA21095 model, which allowed each patient to experience any number of occlusive vascular events at any time in any year. However, the frequency of these events is low, and restricting the Markov model to 12-month cycles and allowing only one event per cycle is unlikely to have a noticeable effect on the evaluation of treatments. In theory, the number of events per patient may be marginally understated, along with the related treatment costs and disutilities; however, as these apply in the same way to both arms of the evaluation, the impact on the assessment of comparative cost-effectiveness is believed to be negligible.
The annual transition matrix for the AG model is shown in Table 27. The matrix shows how the health state of a patient is altered depending on the type of vascular event suffered during the year and the most severe previous event experienced, including whether or not the patient had suffered a severely disabling stroke (modified Rankin Scale score 3–5).
| Health state at beginning of year | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Worst event | None | MI | Stroke | Stroke | MI | Stroke | Stroke | MI | Stroke | Stroke |
| Prior events | 0 | 1 | 1 | 1 | 2 | 2 | 2 | 3 + | 3 + | 3 + |
| Disabled | ND | ND | ND | D | ND | ND | D | ND | ND | D |
| Event in year | ||||||||||
| No event | None (0) ND | MI (1) ND | Stroke (1) ND | Stroke (1) D | MI (2) ND | Stroke (2) ND | Stroke (2) D | MI (3 +) ND | Stroke (3 +) ND | Stroke (3 +) D |
| Fatal MI | Dead | Dead | Dead | Dead | Dead | Dead | Dead | Dead | Dead | Dead |
| Non-fatal MI | MI (1) ND | MI (2) ND | Stroke (2) ND | Stroke (2) D | MI (2) ND | Stroke (3 +) ND | Stroke (3+) D | MI (3 +) ND | Stroke (3 +) ND | Stroke (3 +) D |
| Fatal HS | Dead | Dead | Dead | Dead | Dead | Dead | Dead | Dead | Dead | Dead |
| Non-fatal HS not disabling | Stroke (1) ND | Stroke (2) ND | Stroke (2) ND | Stroke (2) D | Stroke (3+) ND | Stroke (3 +) ND | Stroke (3 +) D | Stroke (3 +) ND | Stroke (3 +) ND | Stroke (3 +) D |
| Non-fatal HS disabling | Stroke (1) D | Stroke (2) D | Stroke (2) D | Stroke (2) D | Stroke (3 +) D | Stroke (3 +) D | Stroke (3 +) D | Stroke (3 +) D | Stroke (3 +) D | Stroke (3 +) D |
| Fatal IS/TIA | Dead | Dead | Dead | Dead | Dead | Dead | Dead | Dead | Dead | Dead |
| Non-fatal IS/TIA not disabling | Stroke (1) ND | Stroke (2) ND | Stroke (2) ND | Stroke (2) D | Stroke (3 +) ND | Stroke (3 +) ND | Stroke (3 +) D | Stroke (3 +) ND | Stroke (3 +) ND | Stroke (3 +) D |
| Non-fatal IS/TIA disabling | Stroke (1) D | Stroke (2) D | Stroke (2) D | Stroke (2) D | Stroke (3 +) D | Stroke (3 +) D | Stroke (3+) D | Stroke (3 +) D | Stroke (3 +) D | Stroke (3 +) D |
| OVD | Dead | Dead | Dead | Dead | Dead | Dead | Dead | Dead | Dead | Dead |
| NVD | Dead | Dead | Dead | Dead | Dead | Dead | Dead | Dead | Dead | Dead |
Patients enter the long-term model with the average number of vascular events experienced in the first 12 months following the index PCI event, estimated by the manufacturer’s short-term statistical model, apportioned between the first four states [None, MI(1)ND, Stroke(1)ND and Stroke(1)D] (see Table 27). The model then traces the long-term accumulating event history separately for males and females within each of the four subpopulations, using sex-specific parameter values (Table 28).
| Subgroup | Sex | Number of patients | Mean age (years) (at 1 year) | Clopidogrel | Prasugrel | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No event | Non-fatal MI only | Non-fatal stroke ± MI | Dead | No event | Non-fatal MI only | Non-fatal stroke ± MI | Dead | ||||
| STEMI diabetes | Females | 126 | 61.9 | 106.4 | 12.9 | 1.4 | 5.3 | 113.1 | 7.4 | 1.7 | 3.8 |
| Males | 387 | 59.0 | 327.6 | 42.7 | 2.7 | 14.0 | 348.2 | 23.9 | 4.8 | 10.1 | |
| STEMI no diabetes | Females | 358 | 60.1 | 323.5 | 23.8 | 2.6 | 8.2 | 329.3 | 19.0 | 2.7 | 7.1 |
| Males | 1876 | 56.5 | 1692.7 | 141.9 | 6.3 | 35.1 | 1724.0 | 109.7 | 12.0 | 30.4 | |
| UA/NSTEMI diabetes | Females | 559 | 62.5 | 484.8 | 53.3 | 5.9 | 15.2 | 507.1 | 34.6 | 3.8 | 13.4 |
| Males | 1229 | 60.3 | 1067.2 | 118.7 | 13.1 | 30.1 | 1117.2 | 77.2 | 8.4 | 26.3 | |
| UA/NSTEMI no diabetes | Females | 1138 | 61.1 | 1028.6 | 86.2 | 5.8 | 17.4 | 1044.5 | 71.0 | 4.6 | 18.0 |
| Males | 4641 | 58.1 | 4204.4 | 350.8 | 23.4 | 62.4 | 4269.4 | 288.9 | 18.6 | 64.1 | |
Assessment of uncertainty
A univariate sensitivity analysis has been performed on all model variables subject to uncertainty, and results are presented in the form of ‘torpedo’ diagrams ranking the 20 variables subject to greatest uncertainty in terms of influence on the deterministic estimated ICER per QALY gained for prasugrel compared with clopidogrel, as measured after 40 years’ follow-up.
A probabilistic sensitivity analysis has been carried out, using 1000 simulations and employing a standardised set of random variables selected to ensure full coverage of the uncertainty domain (sometimes referred to as orthogonal sampling), and incorporating correlated random variables as necessary.
Parameter sources and values
All the parameter values used in the Markov model for event incidence risk (Table 29), event fatality rates (Table 30) and RRs remain unchanged from those previously described in the AG report for the development of NICE guidance TA 210,95 with the exception of the RR applying to patients with/without diabetes (Table 31).
| Parameter | Sex | Meana | LCL | UCL |
|---|---|---|---|---|
| Risk of IS in year 1 | Male and female | 0.609% and 1.086% | 0.406% and 0.560% | 0.853% and 1.780% |
| Risk of HS in year 1 | Male and female | 0.096% | 0.033% | 0.191% |
| Proportion of stroke survivors disabled (modified Rankin Scale 3+) | Male and female | 35% | 33% | 37% |
| IS risk multiplier for stroke survivors not disabled (modified Rankin Scale 0–2) | Male and female | 0.945 | 0.851 | 1.039 |
| IS risk multiplier for stroke survivors disabled (modified Rankin Scale 3+) | Male and female | 1.201 | 1.031 | 1.370 |
| Annual risk of first MI in event-free ACS population treated with aspirin | Male and female | 2.052% and 2.393% | 2.010% and 2.255% | 2.095% and 2.530% |
| Annual risk of first IS in event-free ACS population treated with aspirin | Male and female | 0.300% and 0.774% | 0.251% and 0.694% | 0.349% and 0.854% |
| Annual risk of first HS in event-free ACS population treated with aspirin | Male and female | 0.096% | 0.033% | 0.191% |
| Annual risk of OVD in event-free ACS population treated with aspirin | Male and female | 0.646% and 0.863% | 0.609% and 0.594% | 0.683% and 1.132% |
| Short-term extra risk of MI after first MI event in ASC population treated with aspirin | Male and female | 3.287% | 3.272% | 3.303% |
| Long-term annual risk of MI after first MI event in ACS population treated with aspirin | Male and female | 5.787% | 5.766% | 5.809% |
| Short-term extra risk of IS after first MI event in ACS population treated with aspirin | Male and female | 1.608% | 1.598% | 1.618% |
| Long-term annual risk of IS after first MI event in ACS population treated with aspirin | Male and female | 1.837% | 1.827% | 1.847% |
| Long-term annual risk of HS after first MI event in ACS population treated with aspirin | Male and female | 0.190% | 0.189% | 0.191% |
| Parameter | Sex | Mean | LCL | UCL |
|---|---|---|---|---|
| MI fatality odds model: constant | Male | 0.00986 | 0.00553 | 0.01755 |
| MI fatality odds model: age coefficient | Male | 0.0455 | 0.0368 | 0.0541 |
| MI fatality odds model: constant | Female | 0.00801 | 0.00125 | 0.05124 |
| MI fatality odds model: age coefficient | Female | 0.0538 | –0.0192 | 0.1269 |
| MI subgroup odds multiplier for MI fatality | Male | 0.574 | 0.361 | 0.913 |
| Female | 0.584 | 0.269 | 1.267 | |
| IS fatality odds model: constant | Male | 0.00212 | 0.00040 | 0.011117 |
| IS fatality odds model: age coefficient | Female | 0.0520 | 0.0269 | 0.0770 |
| MI subgroup odds multiplier for IS fatality | Male and female | 1.673 | 0.772 | 3.626 |
| HS fatality | Male | 32.6% | 20.6% | 45.9% |
| Female | 59.9% | 37.7% | 80.1% | |
| Event (MI/stroke) order odds multiplier | ||||
| First event | Male and female | 0.791 | 0.693 | 0.904 |
| Second event | Male and female | 1.931 | 1.593 | 2.342 |
| Third event | Male and female | 4.398 | 2.936 | 6.587 |
| Event | RR | Standard error | LCL | UCL | Source |
|---|---|---|---|---|---|
| MI | 1.339 | 0.082 | 1.141 | 1.571 | Malmberg (2000);97 see table 3 |
| Stroke | 1.446 | 0.144 | 1.091 | 1.921 | Malmberg (2000);97 see table 3 |
| OVD | 2.121 | 0.262 | 1.269 | 3.544 | Kleinman (1988);98 see table 3, weighted average of males and females |
| NVD | 1.242 | 0.233 | 0.787 | 1.960 | Kleinman (1988);98 see table 3, weighted average of males and females |
Cost of medication
The cost of dual antiplatelet therapy in the first year, and the cost of continuing low-dose aspirin thereafter, is detailed in Table 32. Both clopidogrel and prasugrel usage has been adjusted to reflect actual usage in the clinical trial. The cost of a loading dose of 300-mg clopidogrel or 60-mg prasugrel is included.
| Detail | Clopidogrel | Prasugrel | Low-dose aspirin |
|---|---|---|---|
| Pack price (28 tablets) | £1.71 (Drug Tariff November 2013)28 | £47.56 (BNF October 2013)27 | £0.82 (Drug Tariff November 2013)28 |
| Cost of loading dose | £0.24 | £10.19 | – |
| Cost of 12 months’ supplya | £18.43a | £511.67a | £10.70 |
| Total dual antiplatelet therapy cost (year 1) | £29.37 | £532.56 | – |
| Annual maintenance cost | – | – | £10.70 |
Resource use estimation
Health-care costs and health-related utility values are applied for both time spent in each health state and as discrete single-event costs and disutilities.
Unit cost estimation
Unit costs used in the AG’s report for TA18221 have been uplifted using the Hospital and Community Health Services (HCHS) inflation index99 to 2012 prices. The revised costs are shown in Table 33.
| Cost component | Mean | Standard error | LCL | UCL |
|---|---|---|---|---|
| Event | ||||
| Fatal MI | £2373.68 | £121.11 | £2136.31 | £2611.05 |
| Non-fatal MI | £6165.21 | £314.55 | £5548.69 | £6781.73 |
| Fatal stroke | £9381.43 | £478.64 | £8443.29 | £10,319.57 |
| Non-fatal non-disabling stroke | £6858.64 | £349.93 | £6172.77 | £7544.50 |
| Non-fatal disabling stroke | £14,602.70 | £754.04 | £13,142.43 | £16,062.97 |
| OV death | £2407.50 | £122.83 | £2166.75 | £2648.25 |
| NV death | £2407.50 | £122.83 | £2166.75 | £2648.25 |
| Annual cost in health state | ||||
| Event free/MI only | £618.03 | £31.53 | £556.23 | £679.84 |
| Non-disabling stroke | £1804.06 | £92.04 | £1623.66 | £1984.47 |
| Disabling stroke | £5537.72 | £282.54 | £4983.95 | £6091.50 |
Health-related utility estimation
Utility parameter values are shown in Table 34.
| Utility component | Mean | Standard error | LCL | UCL |
|---|---|---|---|---|
| Event | ||||
| Fatal MI | –0.100 | – | 0.000 | –0.200 |
| Non-fatal MI | –0.037 | 0.056 | –0.147 | 0.073 |
| Fatal stroke | –0.100 | – | 0.000 | –0.200 |
| Non-fatal non-disabling stroke | 0.000 | – | 0.000 | –0.200 |
| Non-fatal disabling stroke | 0.000 | – | 0.000 | –0.200 |
| OV death | –0.100 | – | 0.000 | –0.200 |
| NV death | –0.100 | – | 0.000 | –0.200 |
| Utility in health state | ||||
| Event free/MI only | 0.874 | 0.003 | 0.869 | 0.880 |
| Non-disabling stroke (female) | 0.769 | 0.009 | 0.751 | 0.786 |
| Disabling stroke (female) | 0.418 | 0.013 | 0.392 | 0.443 |
| Non-disabling stroke (male) | 0.838 | 0.009 | 0.821 | 0.855 |
| Disabling stroke (male) | 0.487 | 0.013 | 0.463 | 0.512 |
| Annual age decrement | ||||
| All patients (male and female) | –0.0044 | 0.0004 | –0.0052 | –0.0035 |
Continuing utility on health states
The continuing health state EQ-5D utility value for patients who were event-free or suffered a non-fatal MI (but no strokes) and who were alive 12 months after the index PCI was derived from the economic substudy of the PLATO33 clinical trial and based on a weighted average of patients with no event or non-fatal MI after 12 months of follow-up. 100
Four separate utility parameters for patients suffering at least one stroke/TIA were sourced from a study of EQ-5D observations as part of the Oxford Vascular Study (OXVASC). 101 These reflect sex differences and mild compared with severe strokes (grades 0–2 vs. 3–5 in the modified Rankin Scale).
Age-related annual utility decrement and baseline adjustment
An annual loss of utility was estimated from the UK population EQ-5D norms by fitting a linear regression trendline to all participants aged > 35 years. 91 The decrement was used to adjust the initial health state utilities of each subgroup for the differences in mean age between the TRITON-TIMI 3842 cohort and the OXVASC101 patient sample. It was also applied annually to the results of the AG’s Markov model to reflect the average decline of utility score with advancing age.
Initial event disutility
Seven model events (four fatal and three non-fatal) can be expected to result in an additional utility decrement in the first year of follow-up during early recovery. For only one of these events (non-fatal MI) has it been possible to source a specific value, using an analysis of UK Prospective Diabetes Study trial results, which compares utility values for events occurring within 12 months with those occurring earlier. 102 Sources for non-fatal stroke parameters (mild and severe) gave contradictory figures, suggesting that there is no clear additional early disutility effect beyond the long-term continuing effect of a stroke. These parameters were therefore set to zero and made subject to a univariate sensitivity analysis. No sources could be found for disutility associated with the four types of fatal events (fatal MI, fatal stroke, other vascular death and non-vascular death). A notional value of –0.1 was assigned to each parameter, and a sensitivity analysis was conducted.
Discounting costs and outcomes
Both costs and outcomes were discounted annually at 3.5%. Univariate sensitivity analyses were carried out using discount rates of 0% and 6% for both costs and outcomes.
Time horizon
The model generates results annually at the end of each year from trial randomisation. However, deterministic results are reported at 1, 5, 10, 20 and 40 years, and probabilistic results at 5 and 40 years.
Key modelling assumptions
Long-term accumulating risks
The main objective of the AG’s model of prasugrel is to assess whether or not modelling the accumulation of risk-bearing disease events has the effect of causing the long-term experience of patients in both the comparator arms to converge. In this context, the AG considered that this objective could be mainly served through the explicit incorporation of strokes, and their associated elevated event risks and larger ongoing care costs, into the model. The AG also considered that some more marginal issues could be omitted so as to achieve modelling efficiency by generating rapid feedback of results to the user.
Main source of parameter values
The model employed in this appraisal is a simplified version of the individual patient simulation model developed for the NICE appraisal of clopidogrel and modified release dipyridamole which resulted in NICE guidance TA210. 95 The event risk and fatality risk parameters for that model have been preserved in the new formulation and were sourced primarily from analyses of results from the CAPRIE92 trial, which were kindly made available to the AG by the manufacturer of clopidogrel.
The AG sought clinical advice as to the suitability of using the CAPRIE92 data. This advice indicated that the CAPRIE92 trial results were the most appropriate basis for estimating long-term risk probabilities in the follow-up of ACS patients treated with PCI in the UK.
Annual cycles
The AG’s model involves annual cycles for 39 years beyond the index PCI event. This cycle length was adopted for convenience, recognising that it risks some inaccuracy in the number of events occurring each year. In the TA21095 model, individual patients may suffer multiple events in any year, and each contributes to modifying the future risk profile of the patient. By contrast, the AG’s model assumes that such events occur to separate individuals and the risk profile is only updated annually. The extent of any inaccuracy introduced as a result of this change is unclear, and could, in principle, either increase or decrease overall event rates. However, as the same risks apply to both prasugrel and clopidogrel patients, it is unlikely that incremental costs and outcomes will be affected.
Time horizon
The maximum time horizon (40 years) of the AG’s model could be considered to be excessively long, as the duration of the primary trial (TRITON-TIMI 3836) was no more than 15 months, and the CAPRIE trial,92 which was used for populating the risk parameters, had only 3 years of follow-up data. In particular, the stability of the risk equations used for advancing age might be called into question. With this in mind, model results are reported at various time points from 5 years, which represents a more cautious extrapolation.
Follow-up secondary prophylaxis is limited to low-dose aspirin in the model, partly for convenience but also to avoid the possibility of obscuring the primary comparison between prasugrel and clopidogrel use for the primary PCI. Similarly, no attempt has been made to incorporate various other aspects of guidance relating to post-stroke and post-MI care (including surgery and other medication options).
Secondary prophylaxis
No attempt has been made to incorporate the adverse effects of aspirin therapy, or the possibility of non-adherence to continuing aspirin treatment. In addition, the risk of bleeding events associated with long-term prophylaxis was not considered. For all these issues, patients in both arms will be similarly affected throughout follow-up, so that the net effect on incremental differences should be marginal.
Stroke-related disability
In line with the TA21095 model, the representation of stroke-related disability has been limited to two categories based on the modified Rankin Scale. The available data to calibrate the model with greater precision are not available and this approximation works well with a natural distinction between mild and severe dependency.
Validation and quality assurance
The AG’s long-term model has been cross-matched against the original individual patient model to ensure all formulae have been correctly implemented. In addition, check totals have been incorporated into each annual application to ensure that any discrepancies in patient totals, health state totals and event totals are readily identifiable. The starting values for the long-term model have been matched to the manufacturer’s model at 12 months for accuracy.
Independent economic assessment: results
Results from the AG’s model are presented separately for each of the four patient subgroups that were previously considered by the AC when formulating NICE guidance TA182. 21
For each subgroup, detailed deterministic cost-effectiveness estimates are presented across a range of time periods, namely 1, 5, 10, 20 and 40 years after the index PCI. A univariate sensitivity analysis is presented for the 40 years’ follow-up scenario. Probabilistic cost-effectiveness results are presented for 5 and 40 years’ follow-up, with a scatterplot of random replications and a cost-effectiveness acceptability curve (CEAC) for the 40 years’ follow-up scenario.
ST segment elevation myocardial infarction: diabetes subgroup
Deterministic results are detailed in Table 35 (life-years), Table 36 (QALYs), Table 37 (costs) and Table 38 (ICERs). The ICER at the end of the first year is high, owing to the inclusion of the full additional cost of treatment with prasugrel, while only modest health gains have accrued from the reduced incidence of MIs. Over time the estimated ICER decreases steadily, suggesting that incremental benefit continues to accrue over subsequent decades while incremental cost increases at a slower rate. The ICER for prasugrel compared with clopidogrel falls below £30,000 per QALY gained after 5 years.
| Follow-up | Mean time in health state | Life-years | ||||
|---|---|---|---|---|---|---|
| Treatment | Event free | MI(s) only | Mild stroke(s) +/– MI(s) | Severe stroke(s) +/– MI(s) | Total | Total discounted |
| 1 year | ||||||
| Clopidogrel | 0.923 | 0.054 | 0.003 | 0.001 | 0.981 | 0.981 |
| Prasugrel | 0.950 | 0.031 | 0.004 | 0.002 | 0.986 | 0.986 |
| Difference | +0.027 | –0.024 | +0.001 | +0.001 | +0.005 | +0.005 |
| 5 years | ||||||
| Clopidogrel | 3.953 | 0.557 | 0.066 | 0.037 | 4.612 | 4.320 |
| Prasugrel | 4.171 | 0.397 | 0.073 | 0.040 | 4.681 | 4.383 |
| Difference | +0.218 | –0.160 | +0.007 | +0.004 | +0.069 | +0.063 |
| 10 years | ||||||
| Clopidogrel | 6.865 | 1.250 | 0.234 | 0.134 | 8.483 | 7.375 |
| Prasugrel | 7.268 | 1.010 | 0.238 | 0.137 | 8.653 | 7.517 |
| Difference | +0.403 | –0.241 | +0.005 | +0.002 | +0.170 | +0.142 |
| 20 years | ||||||
| Clopidogrel | 10.429 | 2.339 | 0.640 | 0.373 | 13.780 | 10.664 |
| Prasugrel | 11.059 | 2.067 | 0.643 | 0.372 | 14.141 | 10.924 |
| Difference | +0.630 | –0.272 | +0.003 | –0.001 | +0.361 | +0.260 |
| 40 years | ||||||
| Clopidogrel | 12.151 | 2.894 | 0.925 | 0.529 | 16.499 | 11.823 |
| Prasugrel | 12.890 | 2.637 | 0.936 | 0.530 | 16.994 | 12.140 |
| Difference | +0.739 | –0.257 | +0.012 | +0.001 | +0.495 | +0.316 |
| Follow-up | Mean QALYs in health state | Event disutility (QALYs) | QALYs | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | Event free | MI(s) only | Mild stroke +/– MI(s) | Severe stroke +/– MI(s) | MI | Stroke | Death | Total | Total discounted |
| 1 year | |||||||||
| Clopidogrel | 0.837 | 0.049 | 0.002 | 0.001 | –0.005 | 0.000 | –0.003 | 0.882 | 0.882 |
| Prasugrel | 0.861 | 0.028 | 0.004 | 0.001 | –0.003 | 0.000 | –0.002 | 0.889 | 0.889 |
| Difference | +0.024 | –0.021 | +0.001 | 0.000 | +0.002 | 0.000 | +0.001 | +0.007 | +0.007 |
| 5 years | |||||||||
| Clopidogrel | 3.554 | 0.500 | 0.056 | 0.019 | –0.011 | –0.001 | –0.011 | 4.104 | 3.846 |
| Prasugrel | 3.750 | 0.356 | 0.062 | 0.021 | –0.009 | –0.001 | –0.011 | 4.168 | 3.904 |
| Difference | +0.196 | –0.144 | +0.006 | +0.002 | +0.003 | 0.000 | +0.001 | +0.064 | +0.059 |
| 10 years | |||||||||
| Clopidogrel | 6.108 | 1.108 | 0.197 | 0.066 | –0.020 | –0.003 | –0.022 | 7.434 | 6.475 |
| Prasugrel | 6.467 | 0.893 | 0.201 | 0.067 | –0.017 | –0.002 | –0.022 | 7.587 | 6.603 |
| Difference | +0.358 | –0.215 | +0.004 | +0.001 | +0.003 | 0.000 | 0.000 | +0.153 | +0.129 |
| 20 years | |||||||||
| Clopidogrel | 9.126 | 2.029 | 0.525 | 0.175 | –0.036 | –0.006 | –0.043 | 11.768 | 9.171 |
| Prasugrel | 9.676 | 1.787 | 0.528 | 0.175 | –0.033 | –0.006 | –0.044 | 12.083 | 9.400 |
| Difference | +0.550 | –0.241 | +0.003 | +0.000 | +0.003 | 0.000 | 0.000 | +0.314 | +0.228 |
| 40 years | |||||||||
| Clopidogrel | 10.499 | 2.473 | 0.742 | 0.240 | –0.046 | –0.009 | –0.070 | 13.828 | 10.054 |
| Prasugrel | 11.136 | 2.243 | 0.751 | 0.241 | –0.044 | –0.008 | –0.072 | 14.247 | 10.326 |
| Difference | +0.637 | –0.229 | +0.009 | +0.001 | +0.003 | 0.000 | –0.002 | +0.419 | +0.272 |
| Follow-up | Drug costs | Mean costs in health state | Event costs | Cost | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Event free | MI(s) only | Mild stroke +/– MI(s) | Severe stroke +/– MI(s) | MI | Stroke | Death | Total | Total discounted | |
| 1 year | ||||||||||
| Clopidogrel | 29 | 570 | 33 | 5 | 8 | 683 | 68 | 69 | 1465 | 1465 |
| Prasugrel | 533 | 587 | 19 | 7 | 12 | 386 | 101 | 51 | 1695 | 1695 |
| Difference | +503 | +16 | –15 | +3 | +4 | –297 | +33 | –18 | +230 | +230 |
| 5 years | ||||||||||
| Clopidogrel | 68 | 2443 | 344 | 119 | 204 | 1529 | 838 | 272 | 5817 | 5454 |
| Prasugrel | 572 | 2578 | 245 | 131 | 224 | 1169 | 915 | 257 | 6090 | 5723 |
| Difference | +504 | +135 | –99 | +13 | +20 | –361 | +77 | –16 | +273 | +269 |
| 10 years | ||||||||||
| Clopidogrel | 110 | 4243 | 773 | 422 | 744 | 2543 | 2589 | 528 | 11951 | 10277 |
| Prasugrel | 615 | 4492 | 624 | 430 | 756 | 2149 | 2646 | 519 | 12231 | 10552 |
| Difference | +505 | +249 | –149 | +9 | +12 | –394 | +56 | –9 | –280 | +275 |
| 20 years | ||||||||||
| Clopidogrel | 166 | 6446 | 1445 | 1154 | 2063 | 4040 | 6523 | 1041 | 22878 | 17013 |
| Prasugrel | 673 | 6835 | 1277 | 1160 | 2060 | 3651 | 6580 | 1050 | 23287 | 17363 |
| Difference | +507 | +389 | –168 | +6 | –3 | –390 | +58 | +9 | +409 | +351 |
| 40 years | ||||||||||
| Clopidogrel | 195 | 7510 | 1789 | 1668 | 2930 | 4801 | 9129 | 1681 | 29702 | 19904 |
| Prasugrel | 704 | 7966 | 1630 | 1689 | 2938 | 4437 | 9259 | 1723 | 30345 | 20351 |
| Difference | +508 | +457 | –159 | +21 | +8 | –364 | +130 | +42 | +643 | +447 |
| Follow-up | Total cost | Total QALYs | Incremental | ICER (£ per QALY) | |||
|---|---|---|---|---|---|---|---|
| Clopidogrel | Prasugrel | Clopidogrel | Prasugrel | Cost | QALYs | ||
| 1 year | £1465 | £1695 | 0.882 | 0.889 | +£230 | +0.007 | £31,915 |
| 5 years | £5454 | £5723 | 3.846 | 3.904 | +£269 | +0.059 | £4603 |
| 10 years | £10,277 | £10,552 | 6.475 | 6.603 | +£275 | +0.129 | £2139 |
| 20 years | £17,013 | £17,363 | 9.171 | 9.400 | +£350 | +0.228 | £1537 |
| 40 years | £19,904 | £20,351 | 10.054 | 10.326 | +£447 | +0.272 | £1640 |
Figure 4 displays the results of univariate sensitivity analysis, indicating that uncertainty from individual model parameters has a modest influence on the magnitude of the ICER in this subgroup: the discount rates for costs and outcomes cause the largest changes, but the ICER remains within the range £1000 to £2500 per QALY gained.
FIGURE 4.
Univariate sensitivity analysis: 20 most important parameters in determining the ICER for STEMI patients with diabetes.

Probabilistic analysis at the 40-year follow-up horizon for this subgroup yields a higher estimated ICER (£1732 per QALY gained) derived from very small incremental cost and QALY estimates (+£515 and +0.297, respectively). The scatterplot (Figure 5) and CEAC for this subgroup (Figure 6) indicate the relative cost-effectiveness of prasugrel despite the long-term erosion of incremental differences over time.
FIGURE 5.
Probabilistic sensitivity analysis scatterplot of prasugrel compared with clopidogrel for STEMI patients with diabetes.

FIGURE 6.
Cost-effectiveness acceptability curve of prasugrel compared with clopidogrel for STEMI patients with diabetes.

ST segment elevation myocardial infarction: no diabetes subgroup
Deterministic results are detailed in Table 39 (life-years), Table 40 (QALYs), Table 41 (costs) and Table 42 (ICERs). The ICER at the end of the first year is high, owing to the inclusion of the full additional cost of treatment with prasugrel, whereas only modest health gains have accrued from the reduced incidence of MIs. Over time the estimated ICER decreases steadily, suggesting that incremental benefit continues to accrue over subsequent decades while incremental cost increases at a slower rate. The ICER for prasugrel compared with clopidogrel falls below £30,000 per QALY gained at 10 years.
| Follow-up | Mean time in health state | Life-years | ||||
|---|---|---|---|---|---|---|
| Treatment | Event free | MI(s) only | Mild stroke(s) +/– MI(s) | Severe stroke(s) +/– MI(s) | Total | Total discounted |
| 1 year | ||||||
| Clopidogrel | 0.951 | 0.037 | 0.001 | 0.001 | 0.990 | 0.990 |
| Prasugrel | 0.960 | 0.029 | 0.002 | 0.001 | 0.992 | 0.992 |
| Difference | +0.008 | –0.008 | +0.001 | 0.000 | +0.001 | +0.001 |
| 5 years | ||||||
| Clopidogrel | 4.201 | 0.439 | 0.050 | 0.028 | 4.717 | 4.417 |
| Prasugrel | 4.269 | 0.382 | 0.055 | 0.031 | 4.736 | 4.434 |
| Difference | +0.068 | –0.057 | +0.005 | +0.003 | +0.019 | +0.017 |
| 10 years | ||||||
| Clopidogrel | 7.364 | 1.095 | 0.200 | 0.115 | 8.775 | 7.617 |
| Prasugrel | 7.491 | 1.008 | 0.205 | 0.118 | 8.823 | 7.657 |
| Difference | +0.127 | –0.087 | +0.005 | +0.003 | +0.048 | +0.040 |
| 20 years | ||||||
| Clopidogrel | 11.363 | 2.272 | 0.612 | 0.360 | 14.607 | 11.230 |
| Prasugrel | 11.564 | 2.171 | 0.617 | 0.363 | 14.714 | 11.307 |
| Difference | +0.201 | –0.101 | +0.005 | +0.002 | +0.107 | +0.076 |
| 40 years | ||||||
| Clopidogrel | 13.585 | 3.012 | 0.971 | 0.565 | 18.133 | 12.711 |
| Prasugrel | 13.827 | 2.916 | 0.979 | 0.568 | 18.291 | 12.808 |
| Difference | +0.242 | –0.096 | +0.008 | +0.003 | +0.158 | +0.097 |
| Follow-up | Mean QALYs in health state | Event disutility (QALYs) | QALYs | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | Event free | MI(s) only | Mild stroke +/– MI(s) | Severe stroke +/– MI(s) | MI | Stroke | Death | Total | Total discounted |
| 1 year | |||||||||
| Clopidogrel | 0.874 | 0.034 | 0.001 | 0.000 | –0.003 | 0.000 | –0.002 | 0.905 | 0.905 |
| Prasugrel | 0.882 | 0.026 | 0.002 | 0.001 | –0.002 | 0.000 | –0.001 | 0.907 | 0.907 |
| Difference | +0.008 | –0.008 | +0.001 | +0.000 | +0.001 | 0.000 | –0.000 | +0.002 | +0.002 |
| 5 years | |||||||||
| Clopidogrel | 3.825 | 0.398 | 0.044 | 0.015 | –0.009 | –0.001 | –0.009 | 4.262 | 3.992 |
| Prasugrel | 3.887 | 0.347 | 0.048 | 0.016 | –0.008 | –0.001 | –0.009 | 4.279 | 4.008 |
| Difference | +0.062 | –0.052 | +0.004 | +0.001 | +0.001 | 0.000 | 0.000 | +0.017 | +0.016 |
| 10 years | |||||||||
| Clopidogrel | 6.636 | 0.982 | 0.172 | 0.059 | –0.018 | –0.002 | –0.019 | 7.809 | 6.792 |
| Prasugrel | 6.751 | 0.903 | 0.177 | 0.060 | –0.017 | –0.002 | –0.019 | 7.852 | 6.828 |
| Difference | +0.114 | –0.079 | +0.005 | +0.002 | +0.001 | 0.000 | 0.000 | +0.043 | +0.036 |
| 20 years | |||||||||
| Clopidogrel | 10.067 | 1.990 | 0.512 | 0.175 | –0.034 | –0.005 | –0.040 | 12.664 | 9.805 |
| Prasugrel | 10.245 | 1.899 | 0.516 | 0.176 | –0.033 | –0.005 | –0.040 | 12.758 | 9.872 |
| Difference | +0.178 | –0.091 | +0.005 | +0.001 | +0.001 | 0.000 | 0.000 | +0.094 | +0.067 |
| 40 years | |||||||||
| Clopidogrel | 11.861 | 2.588 | 0.791 | 0.263 | –0.047 | –0.009 | –0.069 | 15.378 | 10.950 |
| Prasugrel | 12.072 | 2.502 | 0.798 | 0.265 | –0.046 | –0.009 | –0.070 | 15.512 | 11.033 |
| Difference | +0.211 | –0.087 | +0.007 | +0.002 | +0.001 | 0.000 | –0.001 | +0.133 | +0.084 |
| Follow-up | Drug costs | Mean costs in health state | Event costs | Cost | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Event free | MI(s) only | Mild stroke +/– MI(s) | Severe stroke +/– MI(s) | MI | Stroke | Death | Total | Total discounted | |
| 1 year | ||||||||||
| Clopidogrel | 29 | 588 | 23 | 2 | 4 | 463 | 36 | 37 | 1183 | 1183 |
| Prasugrel | 533 | 593 | 18 | 3 | 6 | 360 | 59 | 33 | 1605 | 1605 |
| Difference | +503 | +5 | –5 | +1 | +2 | –103 | +22 | –4 | +422 | +422 |
| 5 years | ||||||||||
| Clopidogrel | 69 | 2596 | 271 | 91 | 155 | 1263 | 725 | 228 | 5398 | 5045 |
| Prasugrel | 573 | 2638 | 236 | 99 | 170 | 1137 | 790 | 224 | 5867 | 5510 |
| Difference | +503 | +42 | –35 | +8 | +15 | –126 | +65 | –4 | +468 | +465 |
| 10 years | ||||||||||
| Clopidogrel | 113 | 4551 | 677 | 361 | 637 | 2293 | 2517 | 469 | 11617 | 9931 |
| Prasugrel | 616 | 4630 | 623 | 370 | 654 | 2153 | 2595 | 466 | 12108 | 10414 |
| Difference | +504 | +78 | –54 | +9 | +17 | –139 | +78 | –2 | +490 | +482 |
| 20 years | ||||||||||
| Clopidogrel | 175 | 7022 | 1404 | 1104 | 1994 | 3951 | 7095 | 957 | 23702 | 17354 |
| Prasugrel | 679 | 7147 | 1342 | 1113 | 2008 | 3810 | 7192 | 959 | 24249 | 17870 |
| Difference | +504 | +124 | –63 | +9 | +13 | –141 | +96 | +3 | +546 | +515 |
| 40 years | ||||||||||
| Clopidogrel | 213 | 8396 | 1861 | 1752 | 3129 | 4967 | 10868 | 1664 | 32850 | 21167 |
| Prasugrel | 718 | 8546 | 1802 | 1767 | 3146 | 4836 | 11002 | 1678 | 33493 | 21722 |
| Difference | +505 | +150 | –59 | +15 | +17 | –132 | +134 | +13 | +643 | +555 |
| Follow-up | Total cost | Total QALYs | Incremental | ICER (£ per QALY) | |||
|---|---|---|---|---|---|---|---|
| Clopidogrel | Prasugrel | Clopidogrel | Prasugrel | Cost | QALYs | ||
| 1 year | £1183 | £1605 | 0.905 | 0.907 | +£422 | +0.002 | £224,302 |
| 5 years | £5044 | £5510 | 3.992 | 4.008 | +£465 | +0.016 | £29,607 |
| 10 years | £9931 | £10,414 | 6.792 | 6.828 | +£482 | +0.036 | £13,370 |
| 20 years | £17,354 | £17,870 | 9.805 | 9.872 | +£516 | +0.067 | £7670 |
| 40 years | £21,167 | £21,722 | 10.950 | 11.033 | +£555 | +0.084 | £6626 |
Figure 7 displays the results of univariate sensitivity analyses, which indicate that uncertainty from the discounting rate for outcomes has the largest impact on the estimated ICER (ranging between £4000 and £9000 per QALY gained). Other individual model parameters have only a modest influence on the magnitude of the ICER in this subgroup.
FIGURE 7.
Univariate sensitivity analysis: 20 most important parameters in determining the ICER for STEMI patients without diabetes.
Probabilistic analysis at the 40-year follow-up horizon for this subgroup yields a higher estimated ICER (£7073 per QALY gained) derived from small incremental cost and QALY estimates (+£609 and +0.086, respectively). The scatterplot (Figure 8) and CEAC for this subgroup (Figure 9) indicate the relative cost-effectiveness of prasugrel despite the long-term erosion of incremental differences over time.
FIGURE 8.
Probabilistic sensitivity analysis scatterplot of prasugrel compared with clopidogrel for STEMI patients without diabetes.

FIGURE 9.
Cost-effectiveness acceptability curve of prasugrel compared with clopidogrel for STEMI patients without diabetes.

Unstable angina/non-ST segment elevation myocardial infarction: diabetes subgroup
Deterministic results are detailed in Table 43 (life-years), Table 44 (QALYs), Table 45 (costs) and Table 46 (ICERs). The ICER at the end of the first year is high, owing to the inclusion of the full additional cost of treatment with prasugrel, whereas only modest health gains have accrued from the reduced incidence of MIs. Over time, the estimated ICER decreases steadily, suggesting that incremental benefit continues to accrue over subsequent decades, while incremental cost increases at a slower rate. The ICER for prasugrel compared with clopidogrel falls below £30,000 per QALY gained after 5 years.
| Follow-up | Mean time in health state | Life-years | ||||
|---|---|---|---|---|---|---|
| Treatment | Event free | MI(s) only | Mild stroke(s) +/– MI(s) | Severe stroke(s) +/– MI(s) | Total | Total discounted |
| 1 year | ||||||
| Clopidogrel | 0.934 | 0.048 | 0.003 | 0.002 | 0.987 | 0.987 |
| Prasugrel | 0.954 | 0.031 | 0.002 | 0.001 | 0.989 | 0.989 |
| Difference | +0.020 | –0.017 | –0.001 | –0.000 | +0.002 | +0.002 |
| 5 years | ||||||
| Clopidogrel | 4.032 | 0.513 | 0.071 | 0.040 | 4.656 | 4.361 |
| Prasugrel | 4.198 | 0.400 | 0.060 | 0.035 | 4.692 | 4.394 |
| Difference | +0.166 | –0.113 | –0.012 | –0.005 | +0.036 | +0.033 |
| 10 years | ||||||
| Clopidogrel | 6.986 | 1.172 | 0.242 | 0.139 | 8.540 | 7.426 |
| Prasugrel | 7.291 | 1.004 | 0.060 | 0.126 | 8.639 | 7.508 |
| Difference | +0.305 | –0.168 | –0.012 | –0.013 | +0.099 | +0.083 |
| 20 years | ||||||
| Clopidogrel | 10.536 | 2.202 | 0.645 | 0.371 | 13.754 | 10.667 |
| Prasugrel | 11.009 | 2.015 | 0.606 | 0.349 | 13.980 | 10.827 |
| Difference | +0.473 | –0.186 | –0.038 | –0.022 | +0.226 | +0.161 |
| 40 years | ||||||
| Clopidogrel | 12.127 | 2.690 | 0.907 | 0.510 | 16.233 | 11.733 |
| Prasugrel | 12.675 | 2.515 | 0.870 | 0.487 | 16.547 | 11.930 |
| Difference | +0.548 | –0.176 | –0.037 | –0.023 | +0.313 | +0.197 |
| Follow-up | Mean QALYs in health state | Event disutility (QALYs) | QALYs | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | Event free | MI(s) only | Mild stroke +/– MI(s) | Severe stroke +/– MI(s) | MI | Stroke | Death | Total | Total discounted |
| 1 year | |||||||||
| Clopidogrel | 0.842 | 0.043 | 0.003 | 0.001 | –0.004 | 0.000 | –0.002 | 0.883 | 0.883 |
| Prasugrel | 0.860 | 0.028 | 0.002 | 0.001 | –0.003 | 0.000 | –0.002 | 0.887 | 0.887 |
| Difference | +0.018 | –0.015 | –0.001 | 0.000 | +0.001 | 0.000 | 0.000 | +0.003 | +0.003 |
| 5 years | |||||||||
| Clopidogrel | 3.602 | 0.457 | 0.061 | 0.020 | –0.011 | –0.001 | –0.011 | 4.118 | 3.858 |
| Prasugrel | 3.750 | 0.356 | 0.050 | 0.017 | –0.009 | –0.001 | –0.011 | 4.154 | 3.892 |
| Difference | +0.148 | –0.101 | –0.010 | –0.003 | +0.002 | 0.000 | 0.000 | +0.037 | +0.034 |
| 10 years | |||||||||
| Clopidogrel | 6.178 | 1.032 | 0.202 | 0.067 | –0.020 | –0.002 | –0.022 | 7.434 | 6.477 |
| Prasugrel | 6.447 | 0.883 | 0.181 | 0.061 | –0.017 | –0.002 | –0.022 | 7.530 | 6.557 |
| Difference | +0.270 | –0.149 | –0.021 | –0.006 | +0.002 | 0.000 | 0.000 | +0.095 | +0.080 |
| 20 years | |||||||||
| Clopidogrel | 9.164 | 1.897 | 0.522 | 0.171 | –0.035 | –0.006 | –0.045 | 11.668 | 9.114 |
| Prasugrel | 9.575 | 1.733 | 0.490 | 0.160 | –0.033 | –0.006 | –0.045 | 11.874 | 9.261 |
| Difference | +0.411 | –0.165 | –0.032 | –0.011 | +0.002 | 0.000 | 0.000 | +0.205 | +0.147 |
| 40 years | |||||||||
| Clopidogrel | 10.426 | 2.285 | 0.719 | 0.227 | –0.044 | –0.009 | –0.071 | 13.533 | 9.919 |
| Prasugrel | 10.896 | 2.129 | 0.688 | 0.216 | –0.042 | –0.008 | –0.072 | 13.806 | 10.095 |
| Difference | +0.470 | –0.156 | –0.031 | –0.011 | +0.002 | 0.000 | –0.002 | +0.273 | +0.176 |
| Follow-up | Drug costs | Mean costs in health state | Event costs | Cost | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Event free | MI(s) only | Mild stroke +/– MI(s) | Severe stroke +/– MI(s) | MI | Stroke | Death | Total | Total discounted | |
| 1 year | ||||||||||
| Clopidogrel | 29 | 577 | 30 | 6 | 10 | 603 | 80 | 47 | 1383 | 1383 |
| Prasugrel | 533 | 590 | 19 | 4 | 8 | 393 | 52 | 43 | 1642 | 1642 |
| Difference | +503 | +13 | –10 | –2 | –3 | –210 | –27 | –4 | +259 | +259 |
| 5 years | ||||||||||
| Clopidogrel | 69 | 2492 | 317 | 129 | 222 | 1436 | 829 | 262 | 5755 | 5391 |
| Prasugrel | 572 | 2594 | 247 | 107 | 195 | 1171 | 691 | 259 | 5837 | 5487 |
| Difference | +504 | +103 | –70 | –21 | –27 | –265 | –138 | –3 | +82 | +96 |
| 10 years | ||||||||||
| Clopidogrel | 110 | 4318 | 724 | 437 | 770 | 2421 | 2430 | 533 | 11743 | 10102 |
| Prasugrel | 614 | 4506 | 621 | 392 | 698 | 2129 | 2149 | 535 | 11644 | 10054 |
| Difference | +504 | +189 | –104 | –46 | –72 | –292 | –281 | +1 | –99 | –47 |
| 20 years | ||||||||||
| Clopidogrel | 166 | 6512 | 1361 | 1163 | 2055 | 3848 | 5891 | 1075 | 22071 | 16476 |
| Prasugrel | 672 | 6804 | 1246 | 1094 | 1933 | 3557 | 5478 | 1090 | 21872 | 16362 |
| Difference | +506 | +292 | –115 | –69 | –122 | –292 | –413 | +15 | –199 | –114 |
| 40 years | ||||||||||
| Clopidogrel | 192 | 7495 | 1663 | 1636 | 2822 | 4519 | 7987 | 1706 | 28019 | 19015 |
| Prasugrel | 699 | 7834 | 1554 | 1569 | 2697 | 4243 | 7575 | 1743 | 27915 | 18939 |
| Difference | +507 | +339 | –108 | –67 | –125 | –275 | –412 | +37 | –105 | –77 |
| Follow-up | Total cost | Total QALYs | Incremental | ICER (£ per QALY) | |||
|---|---|---|---|---|---|---|---|
| Clopidogrel | Prasugrel | Clopidogrel | Prasugrel | Cost | QALYs | ||
| 1 year | £1383 | £1642 | 0.883 | 0.887 | +£259 | +0.003 | £76,856 |
| 5 years | £5391 | £5487 | 3.858 | 3.892 | +£96 | +0.034 | £2846 |
| 10 years | £10,102 | £10,054 | 6.477 | 6.557 | –£47 | +0.080 | Dominant |
| 20 years | £16,476 | £16,362 | 9.114 | 9.261 | –£114 | +0.147 | Dominant |
| 40 years | £19,015 | £18,939 | 9.919 | 10.095 | –£77 | +0.176 | Dominant |
Figure 10 displays the results of univariate sensitivity analyses, which indicate that uncertainty from event incidence and fatality rates have the largest effect on the estimated ICER (ranging between –£1000 and £400 per QALY gained). Other individual model parameters have only a modest influence on the magnitude of the ICER in this subgroup.
FIGURE 10.
Univariate sensitivity analysis: 20 most important parameters in determining the ICER for UA/NSTEMI patients with diabetes.

Probabilistic analysis at the 40-year follow-up horizon for this subgroup confirms that prasugrel dominates clopidogrel with a small net cost saving and positive incremental benefit (–£120 and +0.191, respectively). The scatterplot (Figure 11) and CEAC for this subgroup (Figure 12) indicate the cost-effectiveness of prasugrel despite the long-term erosion of incremental differences over time.
FIGURE 11.
Probabilistic sensitivity analysis scatterplot of prasugrel compared with clopidogrel for UA/NSTEMI patients with diabetes.

FIGURE 12.
Cost-effectiveness acceptability curve of prasugrel compared with clopidogrel for UA/NSTEMI patients with diabetes.

Unstable angina/non-ST segment elevation myocardial infarction: no diabetes subgroup
Deterministic results are detailed in Table 47 (life-years), Table 48 (QALYs), Table 49 (costs) and Table 50 (ICERs). The ICER at the end of the first year is high, owing to the inclusion of the full additional cost of treatment with prasugrel, whereas only modest health gains have accrued from the reduced incidence of MIs. Over time the estimated ICER decreases steadily, suggesting that incremental benefit continues to accrue over subsequent decades while incremental cost increases at a slower rate. The ICER for prasugrel compared with clopidogrel falls below £30,000 per QALY gained after 10 years.
| Follow-up | Mean time in health state | Life-years | ||||
|---|---|---|---|---|---|---|
| Treatment | Event free | MI(s) only | Mild stroke(s) +/– MI(s) | Severe stroke(s) +/– MI(s) | Total | Total discounted |
| 1 year | ||||||
| Clopidogrel | 0.953 | 0.038 | 0.002 | 0.001 | 0.993 | 0.993 |
| Prasugrel | 0.960 | 0.031 | 0.001 | 0.001 | 0.993 | 0.993 |
| Difference | +0.007 | –0.007 | –0.000 | 0.000 | 0.000 | 0.000 |
| 5 years | ||||||
| Clopidogrel | 4.204 | 0.443 | 0.053 | 0.030 | 4.730 | 4.429 |
| Prasugrel | 4.262 | 0.398 | 0.051 | 0.028 | 4.737 | 4.435 |
| Difference | +0.058 | –0.046 | –0.004 | –0.002 | +0.007 | +0.006 |
| 10 years | ||||||
| Clopidogrel | 7.348 | 1.092 | 0.206 | 0.118 | 8.764 | 7.611 |
| Prasugrel | 7.454 | 1.023 | 0.197 | 0.113 | 8.787 | 7.630 |
| Difference | +0.106 | –0.069 | –0.009 | –0.005 | +0.024 | +0.019 |
| 20 years | ||||||
| Clopidogrel | 11.249 | 2.219 | 0.607 | 0.354 | 14.429 | 11.125 |
| Prasugrel | 11.417 | 2.139 | 0.593 | 0.345 | 14.494 | 11.169 |
| Difference | +0.167 | –0.079 | –0.015 | –0.009 | +0.064 | +0.044 |
| 40 years | ||||||
| Clopidogrel | 13.248 | 2.863 | 0.924 | 0.530 | 17.565 | 12.454 |
| Prasugrel | 13.446 | 2.788 | 0.909 | 0.520 | 17.663 | 12.512 |
| Difference | +0.198 | –0.075 | –0.015 | –0.010 | +0.099 | +0.058 |
| Follow-up | Mean QALYs in health state | Event disutility (QALYs) | QALYs | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | Event free | MI(s) only | Mild stroke +/– MI(s) | Severe stroke +/– MI(s) | MI | Stroke | Death | Total | Total discounted |
| 1 year | |||||||||
| Clopidogrel | 0.869 | 0.034 | 0.001 | 0.000 | –0.003 | 0.000 | –0.001 | 0.901 | 0.901 |
| Prasugrel | 0.875 | 0.028 | 0.001 | 0.000 | –0.002 | 0.000 | –0.001 | 0.901 | 0.901 |
| Difference | +0.006 | –0.006 | 0.000 | 0.000 | +0.001 | 0.000 | 0.000 | 0.000 | 0.000 |
| 5 years | |||||||||
| Clopidogrel | 3.799 | 0.399 | 0.046 | 0.015 | –0.009 | –0.001 | –0.009 | 4.241 | 3.972 |
| Prasugrel | 3.851 | 0.358 | 0.043 | 0.014 | –0.008 | –0.001 | –0.010 | 4.248 | 3.979 |
| Difference | +0.052 | –0.041 | –0.003 | –0.001 | +0.001 | 0.000 | –0.000 | +0.007 | +0.007 |
| 10 years | |||||||||
| Clopidogrel | 6.571 | 0.971 | 0.175 | 0.059 | –0.018 | –0.002 | –0.020 | 7.736 | 6.732 |
| Prasugrel | 6.666 | 0.909 | 0.167 | 0.057 | –0.017 | –0.002 | –0.020 | 7.760 | 6.751 |
| Difference | +0.095 | –0.062 | –0.008 | –0.002 | +0.001 | 0.000 | 0.000 | +0.024 | +0.020 |
| 20 years | |||||||||
| Clopidogrel | 9.892 | 1.929 | 0.502 | 0.169 | –0.034 | –0.005 | –0.042 | 12.411 | 9.637 |
| Prasugrel | 10.039 | 1.859 | 0.490 | 0.164 | –0.033 | –0.005 | –0.042 | 12.471 | 9.678 |
| Difference | +0.147 | –0.071 | –0.013 | –0.005 | +0.001 | 0.000 | 0.000 | +0.060 | +0.042 |
| 40 years | |||||||||
| Clopidogrel | 11.494 | 2.446 | 0.746 | 0.243 | –0.046 | –0.008 | –0.070 | 14.804 | 10.655 |
| Prasugrel | 11.666 | 2.379 | 0.733 | 0.238 | –0.045 | –0.008 | –0.071 | 14.892 | 10.708 |
| Difference | +0.172 | –0.067 | –0.013 | –0.005 | +0.001 | 0.000 | –0.001 | +0.087 | +0.053 |
| Follow-up | Drug costs | Mean costs in health state | Event costs | Cost | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | Event free | MI(s) only | Mild stroke +/– MI(s) | Severe stroke +/– MI(s) | MI | Stroke | Death | Total | Total discounted | |
| 1 year | ||||||||||
| Clopidogrel | 29 | 589 | 23 | 3 | 5 | 471 | 45 | 26 | 1192 | 1192 |
| Prasugrel | 533 | 593 | 19 | 2 | 4 | 388 | 37 | 28 | 1604 | 1604 |
| Difference | +503 | +4 | –4 | –1 | –1 | –83 | –8 | +1 | +413 | +413 |
| 5 years | ||||||||||
| Clopidogrel | 69 | 2598 | 274 | 96 | 165 | 1274 | 743 | 228 | 5447 | 5091 |
| Prasugrel | 573 | 2634 | 246 | 90 | 156 | 1168 | 693 | 229 | 5787 | 5437 |
| Difference | +503 | +36 | –28 | –7 | –10 | –106 | –50 | +1 | +340 | +346 |
| 10 years | ||||||||||
| Clopidogrel | 112 | 4541 | 675 | 371 | 654 | 2287 | 2467 | 482 | 11590 | 9920 |
| Prasugrel | 616 | 4607 | 632 | 355 | 627 | 2169 | 2357 | 485 | 11848 | 10200 |
| Difference | +503 | +66 | –43 | –16 | –27 | –119 | –111 | +2 | +257 | +280 |
| 20 years | ||||||||||
| Clopidogrel | 173 | 6952 | 1371 | 1096 | 1961 | 3870 | 6680 | 1000 | 23103 | 17002 |
| Prasugrel | 677 | 7056 | 1322 | 1069 | 1911 | 3748 | 6505 | 1006 | 23293 | 17239 |
| Difference | +504 | +103 | –49 | –27 | –50 | –122 | –175 | +6 | +190 | +237 |
| 40 years | ||||||||||
| Clopidogrel | 207 | 8188 | 1769 | 1667 | 2934 | 4753 | 9799 | 1693 | 31010 | 20328 |
| Prasugrel | 711 | 8310 | 1723 | 1640 | 2880 | 4637 | 9622 | 1707 | 31230 | 20576 |
| Difference | +504 | +123 | –46 | –27 | 54 | –116 | –178 | +14 | +220 | +248 |
| Follow-up | Total cost | Total QALYs | Incremental | ICER (£ per QALY) | |||
|---|---|---|---|---|---|---|---|
| Clopidogrel | Prasugrel | Clopidogrel | Prasugrel | Cost | QALYs | ||
| 1 year | £1192 | £1604 | 0.90097 | 0.90134 | +£413 | +0.00037 | £1,101,662 |
| 5 years | £5091 | £5437 | 3.972 | 3.979 | +£346 | +0.007 | £52,288 |
| 10 years | £9920 | £10,200 | 6.732 | 6.751 | +£280 | +0.020 | £14,276 |
| 20 years | £17,002 | £17,239 | 9.637 | 9.678 | +£237 | +0.042 | £5688 |
| 40 years | £20,328 | £20,576 | 10.655 | 10.708 | +£248 | +0.053 | £4667 |
Figure 13 displays the results of univariate sensitivity analyses, which indicate that uncertainty from discounting rates, and event incidence and fatality rates have the largest effect on the estimated ICER (ranging between £2500 and £6500 per QALY gained). Other individual model parameters have only a modest influence on the magnitude of the ICER in this subgroup.
FIGURE 13.
Univariate sensitivity analysis: 20 most important parameters in determining the ICER for UA/NSTEMI patients without diabetes.

Probabilistic analysis at the 40-year follow-up horizon for this subgroup yields a lower estimated ICER of £4154 per QALY gained, derived from small incremental cost and QALY estimates (+£212 and +0.051, respectively). The scatterplot (Figure 14) and CEAC for this subgroup (Figure 15) indicate the relative cost-effectiveness of prasugrel despite the long-term erosion of incremental differences over time.
FIGURE 14.
Probabilistic sensitivity analysis scatterplot of prasugrel compared with clopidogrel for UA/NSTEMI patients without diabetes.
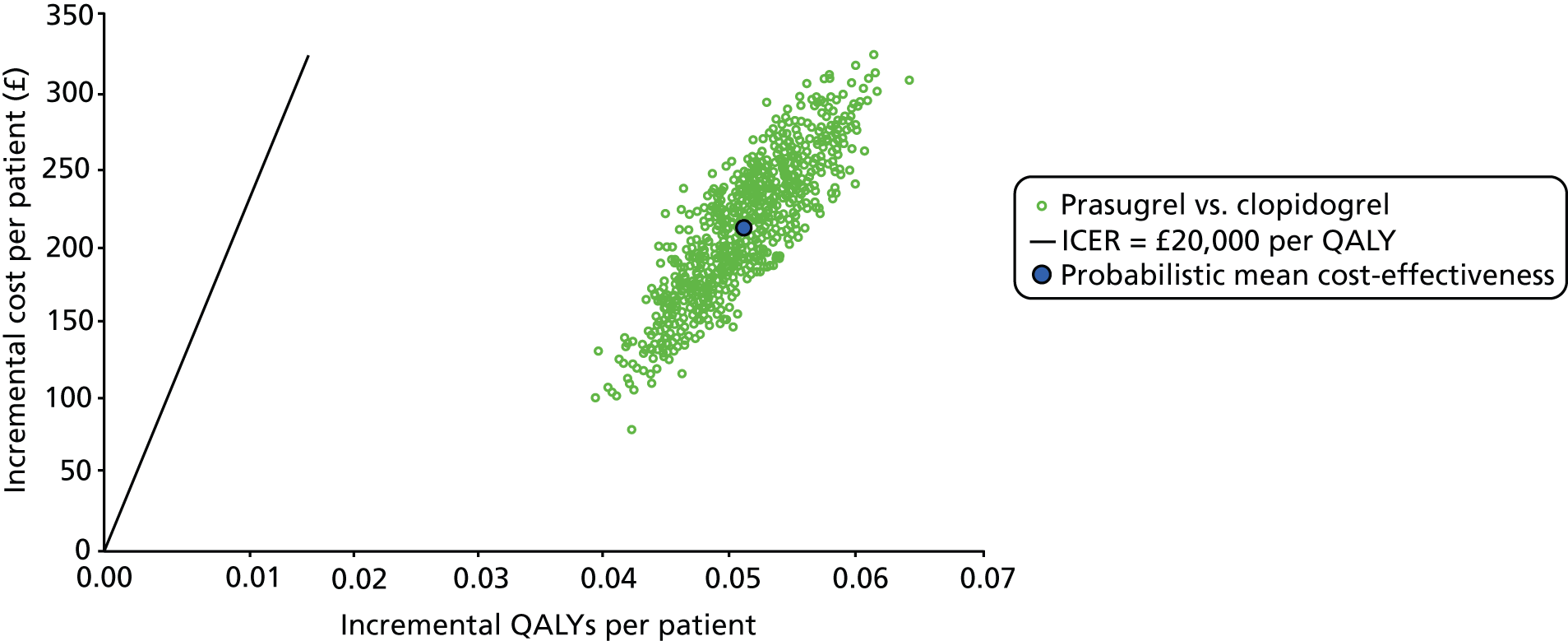
FIGURE 15.
Cost-effectiveness acceptability curve of prasugrel compared with clopidogrel for UA/NSTEMI patients without diabetes.
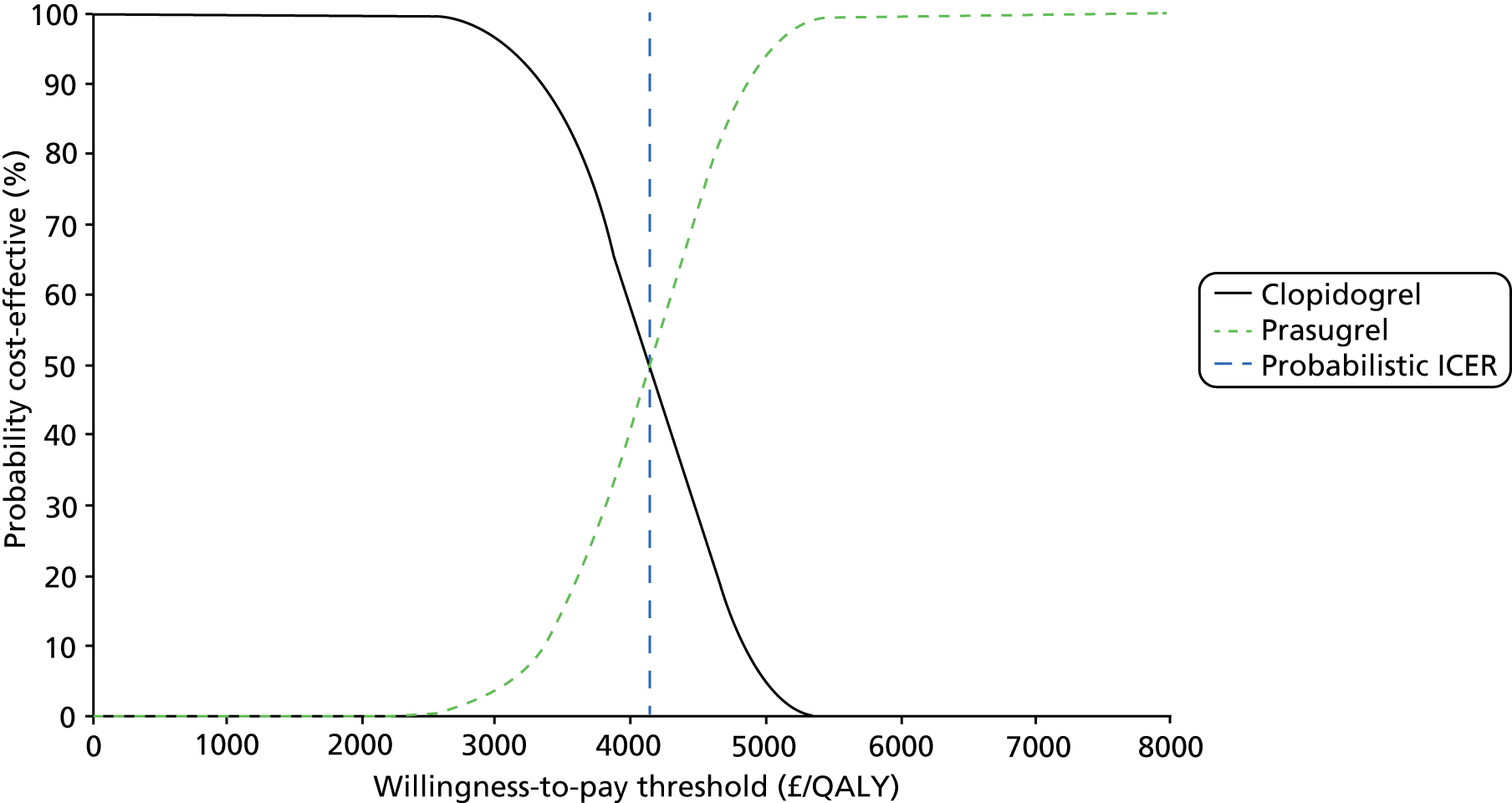
Independent economic assessment: discussion of cost-effectiveness evidence
The main concern expressed by the ERG in its critique of the manufacturer’s original submission in 2009 was that the very basic nature of projecting patient survival beyond the short follow-up period of the TRITON-TIMI 3836 trial perpetuated a small effectiveness advantage over a period of 40 years. This projection method failed to allow the possibility of initial health gain being progressively attenuated and thus worsened the apparent economic comparison of prasugrel compared with clopidogrel. The application of the findings of the CAPRIE92 trial in a similar patient population over a longer follow-up period to populate a long-term model has allowed the issue of clinical and economic benefit to be reassessed in a structured manner. The results from the AG’s model suggest that attenuation of the initial benefits is indeed likely to occur, but that it is closely matched by narrowing of the initial cost difference so that estimated ICERs tend to reduce progressively rather than increase.
Simulation of the TRITON-TIMI 3836 trial population within the AG’s decision model as four mutually exclusive subgroups has facilitated a reconsideration of the strength of evidence underlying the previous NICE guidance21 which excluded patients from treatment with prasugrel if they had not suffered from a STEMI event, or been diagnosed with diabetes. Both the deterministic and probabilistic analyses have confirmed that, within 5–10 years, and in all four subgroups, it appears likely that prasugrel is a cost-effective treatment option when compared with clopidogrel at a willingness-to-pay threshold of £20,000 to £30,000 per QALY gained. At the full 40-year time horizon, all estimated ICERs are less than £10,000 per QALY gained, indicating confidence in this interpretation of the available evidence.
This economic analysis has developed beyond the previous assessment, using results from a large study (CAPRIE92 data) over a longer period (3 years) and, therefore, serves to strengthen the case that was previously presented for consideration. However, any long-term modelling exercise is vulnerable to major assumptions about the continuation of early outcome gains, far beyond any possibility of experimental validation through an extended clinical trial. It is likely that the only viable approach to obtaining corroborative evidence would be from an extended patient register, tracing patients’ subsequent health and health-care careers over decades.
Assessment of factors relevant to the NHS and other parties
The AG considers that any changes to the patient population eligible for prasugrel made as a result of this appraisal would not substantially affect resource use in the NHS in England and Wales.
Chapter 5 Discussion
The remit of this review was to update the evidence underpinning TA18221 NICE guidance for the use of prasugrel in the NHS. In TA182,21 only one RCT (TRITON-TIMI 3836) compared prasugrel with clopidogrel in patients presenting with ACS who were intended for treatment with PCI. No new trials were identified for inclusion in this update, which means that the present review is largely based on the clinical evidence available for TA182. 21
Statement of principal findings
Clinical effectiveness
This review focused on the health outcomes of the subgroup of patients discussed in TA18221 and for whom the full dose of prasugrel is licensed, namely the core clinical cohort (i.e. patients without a history of TIA or stroke, those with body weight of < 60 kg or those aged > 75 years). This group of patients constituted 79% of the overall population of TRITON-TIMI 38. 36 In the core clinical cohort, all non-bleeding clinical outcomes of the TRITON-TIMI 3836 trial favoured the use of prasugrel compared with clopidogrel. These findings held over time and across subgroups of patients, including those with STEMI and UA/NSTEMI. There was a statistically significant difference in event rates in favour of clopidogrel when major and minor bleeding rates were combined.
A clinical comparison of prasugrel with ticagrelor was not carried out by the AG (or the manufacturer of prasugrel). There were two reasons for this. First, there was no direct RCT evidence comparing prasugrel with ticagrelor and, second, it was not possible to conduct an indirect comparison as there were irreconcilable differences between the two pivotal trials33,36 (including timing and dosing of clopidogrel and assessment of MI). Thus, the comparative effectiveness and safety of prasugrel compared with ticagrelor still remain unknown.
Cost-effectiveness
In the AG’s independent economic model, the outcomes of the TRITON-TIMI 3836 trial population were simulated as four mutually exclusive subgroups: STEMI without diabetes mellitus, STEMI with diabetes mellitus, NSTEMI without diabetes mellitus and NSTEMI with diabetes mellitus. This approach has allowed the AG to reconsider the strength of evidence underlying the previous NICE guidance21 that excluded patients from treatment with prasugrel if they had not suffered a STEMI event, or had not been diagnosed with diabetes. The new model confirmed that, using a £20,000 to £30,000 per QALY gained threshold, within 5–10 years, it appears likely that prasugrel is a cost-effective treatment option when compared with clopidogrel for all four subgroups.
Strengths and limitations of the assessment
The main strength of this review is that, despite some remaining areas of uncertainty, the case for prasugrel compared with clopidogrel appears to have been strengthened. The results of the AG’s independent economic model confirm the cost-effectiveness of prasugrel compared with clopidogrel, at a threshold of £20,000 to £30,000 per QALY gained, for key groups of patients with ACS who are to be treated with PCI. The structure of the AG’s model differs from the model developed by the manufacturer in that it uses the most up-to-date clinical evidence available (from the CAPRIE92 trial) and compares four patient subgroups (STEMI without diabetes mellitus, STEMI with diabetes mellitus, NSTEMI without diabetes mellitus and NSTEMI with diabetes mellitus). A particular strength of the AG’s economic model is that is provides assessments at specific time periods within the modelled time horizon of 40 years.
Both the AG and the manufacturer demonstrate the cost-effectiveness of prasugrel compared with clopidogrel at a threshold of £20,000 to £30,000 per QALY gained. However, the AG acknowledges that any long-term modelling exercise is vulnerable to major assumptions about the continuation of early health outcome gains, and it is noted that both the manufacturer’s and the AG’s models rely on extrapolating relatively short-term results from beyond the end of the trial to a further 40 years.
Since TA182,21 the patent for clopidogrel has expired. In TA182,21 the assessment of the cost-effectiveness of prasugrel was based on the non-generic price of clopidogrel using the economic model submitted by the manufacturer of prasugrel. A key strength of this update is that the AG has been able to reassess the cost-effectiveness of prasugrel compared with clopidogrel using the generic price of clopidogrel in an independent economic model.
The clinical effectiveness and cost-effectiveness findings of the report are limited by the nature of the available clinical evidence. Since TA182,21 no new clinical evidence has become available to support the use of prasugrel compared with clopidogrel. In the short-term, all clinical effectiveness data used in the model were derived from a single RCT (TRITON-TIMI 38). 36 In the longer term, all clinical effectiveness data used in the model were primarily derived from a single RCT (CAPRIE). 92 The AG notes that both RCTs recruited large numbers of patients and were well conducted and well reported.
The AG notes that, although the TRITON-TIMI-3836 trial was considered to be of a robust design, the majority (93%) of the trial population was white Caucasian. This does not negate the findings of this report, but it must be considered as a limitation to the applicability of the recommendations.
Uncertainties
The three areas of uncertainty noted by the AC for TA18221 were reconsidered in this review. These centred on the generalisability of the TRITON-TIMI 3836 trial results to patients in clinical practice in the UK. The AG is of the opinion that the clinical evidence for the equivalence of a 300-mg loading dose of clopidogrel (administered in TRITON-TIMI 3836) with a 600-mg loading dose (often given in clinical practice in the UK) remains uncertain. Similarly, the importance of timing of the administration of the loading dose of clopidogrel on patient outcomes remains unresolved and differs between the TRITON-TIMI 3836 trial and clinical practice in the NHS in England and Wales. The AG considers that the case for the clinical effectiveness of prasugrel compared with clopidogrel in preventing MIs of all types and sizes appears to be robust.
Part of the remit for this review was to consider the efficacy of prasugrel compared with ticagrelor for patients with ACS who are to be treated with PCI. As no head-to-head trial has been conducted comparing these two treatments, the AG considered the possibility of an indirect treatment comparison using data from the TRITON-TIMI 3836 and PLATO33 trials; however, the AG concluded that the key differences between the two trials made any comparison unreliable. Thus, the comparative clinical effectiveness and safety of prasugrel compared with ticagrelor remains unknown. However, the AG is aware of a RCT that commenced recruiting patients in September 2013. 103,104 The Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment (ISAR-REACT) 5103,104 trial is designed to assess whether or not ticagrelor is superior to prasugrel in patients with ACS and planned invasive strategy. The primary outcome is the composite of death, MI or stroke at 12 months in a planned patient population of 4000. The results of the ISAR-REACT 5103,104 trial will allow a formal comparison of the efficacy of prasugrel compared with ticagrelor.
Chapter 6 Conclusions
Suggested research priorities
It would be most valuable to have well-audited data on defined ACS patient groups from a long-term clinical registry of all UK patients receiving prasugrel, ticagrelor and clopidogrel and who are treated with a PCI. Such a data source could provide a basis for research and audit to inform future assessments of these antiplatelet treatments.
A database that allows comparison of populations or regions within the UK NHS in which all patients are uniformly treated with one or other of the antiplatelet agents would be a useful and informative resource.
It is suggested that any future trials in this area should focus on the comparison of prasugrel with ticagrelor and recruit patients with ACS who are to be treated with a PCI. It is anticipated that the results of the ISAR-REACT 5103,104 trial, if conducted well, could fill the current gap in evidence related to the comparative efficacy and safety of prasugrel compared with ticagrelor.
Acknowledgements
We thank Dr Alex Hobson, Consultant Cardiology Interventionalist at Portsmouth Hospitals NHS Trust, Portsmouth, UK, and Dr Kathleen Boyd, Health Economist at Glasgow University, Glasgow, UK, for their comments on the final version of this report. Dr Hobson has received reimbursement from Eli Lilly for attending a conference.
Contributions of authors
Janette Greenhalgh was the project lead and conducted the review of clinical evidence.
Adrian Bagust conducted the critical appraisal of manufacturers’ economic model and development of de novo economic model.
Angela Boland supported the review process (clinical and economics).
Kerry Dwan conducted the clinical quality assessment and data extraction and was the statistical advisor.
Sophie Beale supported the review process (economics).
Nigel Fleeman conducted the literature selection and data management.
Joanne McEntee was the pharmacy advisor.
Yenal Dundar conducted the literature searching.
Marty Richardson conducted the data extraction and was the statistical advisor.
Michael Fisher was the clinical advisor.
All authors read and commented on draft versions of the report.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Egton Medical Information Systems Ltd . Acute Coronary Syndromes 2013. www.patient.co.uk/doctor/acute-coronary-syndrome (accessed April 2013).
- Arslanian-Engoren C, Engoren M. Physiological and anatomical bases for sex differences in pain and nausea as presenting symptoms of acute coronary syndromes. Heart Lung 2010;39:386-93. http://dx.doi.org/10.1016/j.hrtlng.2009.10.013.
- Dey S, Flather MD, Devlin G, Brieger D, Gurfinkel EP, Steg PG, et al. Sex-related differences in the presentation, treatment and outcomes among patients with acute coronary syndromes: the Global Registry of Acute Coronary Events. Heart 2009;95:20-6. http://dx.doi.org/10.1136/hrt.2007.138537.
- Hasin T, Hochadel M, Gitt AK, Behar S, Bueno H, Hasin Y. Comparison of treatment and outcome of acute coronary syndrome in patients with versus patients without diabetes mellitus. Am J Cardiol 2009;103:772-8. http://dx.doi.org/10.1016/j.amjcard.2008.11.034.
- MINAP . How the NHS Cares for Patients With Heart Attack. Annual Public Report April 2012–March 2013 2013. www.hqip.org.uk/assets/NCAPOP-Library/NCAPOP-2013-14/MINAP-Audit-Report-2013-LOW.pdf (accessed November 2013).
- Greenhalgh J, Bagust A, Boland A, Martin Saborido C, Oyee J, Blundell M, et al. Clopidogrel and modified-release dipyridamole for the prevention of occlusive vascular events (review of Technology Appraisal No. 90): a systematic review and economic analysis. Health Technol Assess 2011;15. http://dx.doi.org/10.3310/hta15310.
- NICE . Prasugrel With Percutaneous Coronary Intervention for Treating Acute Coronary Syndrome (Review of TA182): Final Scope 2013. http://guidance.nice.org.uk/TA/WaveR/141 (accessed 2013 October).
- NICE . Myocardial Infarction With ST-Segment-Elevation (STEMI): The Acute Management of Myocardial Infarction With ST-Segment-Elevation (STEMI): NICE Clinical Guideline CG167 2013. http://publications.nice.org.uk/myocardial-infarction-with-st-segment-elevation-cg167 (accessed November 2013).
- National Institute for Cardiovascular Outcomes Research . National Audit of Percutaneous Coronary Interventional Procedures Public Report 2011 2011. www.ucl.ac.uk/nicor/audits/adultcardiacintervention/publicreports/documents/pcireport2012 (accessed April 2013).
- Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2011;32:2999-3054. http://dx.doi.org/10.1093/eurheartj/ehr236.
- Steg PG, James SK, Atar D, Badano LP, Blomstrom-Lundqvist C, Borger MA, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012;33:2569-619. http://dx.doi.org/10.1093/eurheartj/ehs215.
- Egton Medical Information Systems Ltd . Percutaneous Coronary Intervention 2013. www.patient.co.uk/doctor/percutaneous-coronary-intervention (accessed April 2013).
- NICE . The Early Management of Unstable Angina and Non-ST-Segment-Elevation Myocardial Infarction: NICE Clinical Guideline 94 2010. www.nice.org.uk/guidance/cg94/evidence/cg94-unstable-angina-and-nstemi-full-guidance-and-appendices2 (accessed November 2013).
- Global Registry of Acute Coronary Events . GRACE ACS Risk Model 2013. www.outcomes-umassmed.org/grace/acs_risk/acs_risk_content.html (accessed May 2013).
- Greenhalgh J, Bagust A, Boland A, Saborido CM, Fleeman N, McLeod C, et al. Prasugrel for the treatment of acute coronary artery syndromes with percutaneous coronary intervention. Health Technol Assess 2010;14. http://dx.doi.org/10.3310/hta14Suppl1/05.
- Health and Social Care Information Centre . Hospital Episode Statistics: Admitted Patient Care 2012–13. Diagnosis n.d. www.hscic.gov.uk/catalogue/PUB12566/hosp-epis-stat-admi-summ-rep-2012-13-rep.pdf (accessed November 2013).
- British Cardiovascular Intervention Society . BCIS Audit Returns – Adult Interventional Procedures 2012. www.bcis.org.uk/pages/page_box_contents.asp?pageid=780&navcatid=11 (accessed November 2013).
- NICE . NICE Quality Standards: QS Forward Planner 2013. www.nice.org.uk/guidance/qualitystandards/qualitystandards.jsp?domedia=1&mid=6D55F322-CBF6-F672–00B5C83632967E35 (accessed December 2013).
- NICE quality standards [QS68] . Acute Coronary Syndromes (Including Myocardial Infarction) n.d. www.nice.org.uk/guidance/qs68 (accessed November 2013).
- NICE . NICE Pathways: Acute Coronary Syndrome Overview 2013. http://pathways.nice.org.uk/pathways/acute-coronary-syndrome (accessed December 2013).
- NICE . Prasugrel for the Treatment of Acute Coronary Syndromes With Percutaneous Coronary Intervention: TA182 2009. http://publications.nice.org.uk/prasugrel-for-the-treatment-of-acute-coronary-syndromes-with-percutaneous-coronary-intervention-ta182 (accessed October 2013).
- NICE . Ticagrelor for the Treatment of Acute Coronary Syndromes (ACS): TA236 2011. http://publications.nice.org.uk/ticagrelor-for-the-treatment-of-acute-coronary-syndromes-ta236 (accessed November 2013).
- NICE . MI – Secondary Prevention. Secondary Prevention in Primary and Secondary Care for Patients Following a Myocardial Infarction: NICE Clinical Guideline CG172 2013. http://publications.nice.org.uk/mi-secondary-prevention-cg172 (accessed November 2013).
- Electronic Medicines Compendium . Efient – SPC 2014. www.medicines.org.uk/emc/medicine/21504 (accessed February 2014).
- EMEA . European Public Assessment Report for Efient 2009. www.emea.europa.eu/humandocs/PDFs/EPAR/Efient/H-984-en6.pdf (accessed November 2013).
- NICE . Acute Coronary Syndrome – Prasugrel. Appendix A: Decision Paper Presented to the Institute’s Guidance Executive 2012. http://guidance.nice.org.uk/TA182/ReviewDecisionJune12/ReviewDecisionAppendix/pdf/English (accessed 2013 October).
- British National Formulary. London: BMJ Group and Pharmaceutical Press; n.d.
- NHS Prescription Services . Electronic Drug Tariff 2013 n.d. www.ppa.org.uk/edt/November_2013/mindex.htm (accessed November 2013).
- Electronic Medicines Compendium . Clopidogrel – SPC 2013. www.medicines.org.uk/emc/medicine/24206/SPC/Plavix+300mg+tablets/#CONTRAINDICATIONS (accessed October 2013).
- European Medicines Agency . Brilique 2013. www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/001241/human_med_001398.jsp&mid=WC0b01ac058001d124 (accessed December 2013).
- Electronic Medicines Compendium . Brilique – SPC 2013. www.medicines.org.uk/emc/medicine/23935/SPC/Brilique+90+mg+film+coated+tablets/ (accessed October 2013).
- AstraZeneca PLC. Third Quarter and Nine Months Results (2013) 2013. www.astrazeneca.com/cs/Satellite?blobcol=urldata&blobheader=application%2Fpdf&blobheadername1=Content-Disposition&blobheadername2=MDT-Type&blobheadervalue1=inline%3B+filename%3DPress-release.pdf&blobheadervalue2=abinary%3B+charset%3DUTF-8&blobkey=id&blobtable=MungoBlobs&blobwhere=1285660598284&ssbinary=true (accessed November 2013).
- Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045-57. http://dx.doi.org/10.1056/NEJMoa0904327.
- FiercePharma . EU Watchdogs Demand Info on AstraZeneca’s Disputed Brilinta Trial 2013. www.fiercepharma.com/story/eu-watchdogs-demand-info-astrazenecas-disputed-brilinta-trial/2013–11–08?utm_medium=nl&utm_source=internal (accessed November 2013).
- CRD’s Guidance for Undertaking Reviews in Healthcare: Systematic Reviews. University of York, York: Centre for Reviews and Dissemination; 2009.
- Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2007;357:2001-15. http://dx.doi.org/10.1056/NEJMoa0706482.
- Ge J, Zhu J, Hong BK, Boonbaichaiyapruck S, Goh YS, Hou CJ, et al. Prasugrel versus clopidogrel in Asian patients with acute coronary syndromes: design and rationale of a multi-dose, pharmacodynamic, phase 3 clinical trial. Curr Med Res Opin 2010;26:2077-85. http://dx.doi.org/10.1185/03007995.2010.502048.
- Wiviott SD, Antman EM, Winters KJ, Weerakkody G, Murphy SA, Behounek BD, et al. Investigators J-T. Randomized comparison of prasugrel (CS-747, LY640315), a novel thienopyridine P2Y12 antagonist, with clopidogrel in percutaneous coronary intervention: results of the Joint Utilization of Medications to Block Platelets Optimally (JUMBO)-TIMI 26 trial. Circulation 2005;111:3366-73. http://dx.doi.org/10.1161/CIRCULATIONAHA.104.502815.
- Alexopoulos D, Xanthopoulou I, Gkizas V, Kassimis G, Theodoropoulos KC, Makris G, et al. Randomized assessment of ticagrelor versus prasugrel antiplatelet effects in patients with ST-segment-elevation myocardial infarction. Circulation Cardiovasc Interv 2012;5:797-804. http://dx.doi.org/10.1161/CIRCINTERVENTIONS.112.972323.
- Xanthopoulou I, Theodoropoulos KF, Kassimis G, Gizas V, Tsigkas G, Koutsogiannis N, et al. Ticagrelor vs prasugrel in patients with ST elevation myocardial infarction undergoing primary percutaneous coronary intervention. Eur Heart J 2012;33.
- Wiviott SD, Antman EM, Gibson CM, Montalescot G, Riesmeyer J, Weerakkody G, et al. Investigators T-T. Evaluation of prasugrel compared with clopidogrel in patients with acute coronary syndromes: design and rationale for the TRial to assess Improvement in Therapeutic Outcomes by optimizing platelet InhibitioN with prasugrel Thrombolysis In Myocardial Infarction 38 (TRITON-TIMI 38). Am Heart J 2006;152:627-35. http://dx.doi.org/10.1016/j.ahj.2006.04.012.
- Wiviott SD, Desai N, Murphy SA, Musumeci G, Ragosta M, Antman EM, et al. Efficacy and safety of intensive antiplatelet therapy with prasugrel from TRITON-TIMI 38 in a core clinical cohort defined by worldwide regulatory agencies. Am J Cardiol 2011;108:905-11. http://dx.doi.org/10.1016/j.amjcard.2011.05.020.
- SIGN . Acute Coronary Syndromes 2007. www.sign.ac.uk/pdf/sign93.pdf (accessed October 2013).
- Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, et al. Guidelines on myocardial revascularization. Eur Heart J 2010;31:2501-55. http://dx.doi.org/10.1093/eurheartj/ehq277.
- Mehta SR, Bassand JP, Chrolavicious S, Diaz R, Eikelboom J, Fox KAA, et al. Dose comparisons of clopidogrel and aspirin in acute coronary syndromes. N Engl J Med 2010;363:930-42. http://dx.doi.org/10.1056/NEJMoa0909475.
- Mehta SR, Tanguay J-F, Eikelboom JW, Jolly SS, Joyner CD, Granger CB, et al. Double-dose versus standard-dose clopidogrel and high-dose versus low-dose aspirin in individuals undergoing percutaneous coronary intervention for acute coronary syndromes (CURRENT-OASIS 7): a randomised factorial trial. Lancet 2010;376:1233-43. http://dx.doi.org/10.1016/S0140-6736(10)61088-4.
- Bakhai A, Iñiguez A, Ferrieres J, Needs N, Schmitt C, Sartral M, et al. International comparison of health care resources and quality of life in acute coronary syndrome patients in 2007: results from the AntiPlatelet Treatment Observational Registry (APTOR). Value Health 2008;11:A209-10.
- McCollam P, Bae J, Nasuti P, Anger C. Clopidogrel Patterns of Use in Acute Coronary Syndrome Patients Undergoing Percutaneous Coronary Intervention in 5 European Countries n.d.
- McCollam P, Bae J, Nasuti P, Yeung E, Hamad B. Clopidogrel Patterns of Use in Acute Coronary Syndrome Patients Undergoing Percutaneous Coronary Intervention in 5 European Countries n.d.
- Lotrionte MMD, Biondi-Zoccai GGLMD, Agostoni PMD, Abbate AMD, Angiolillo DJMDP, Valgimigli MMDP, et al. Meta-Analysis Appraising High Clopidogrel Loading in Patients Underqoinq Percutaneous Coronary Intervention(dagger). Am J Cardiol 2007;100:1199-206. http://dx.doi.org/10.1016/j.amjcard.2007.05.048.
- Cannon CP, Battler A, Grindis RG, Cox JL, Ellis SG, Every NR, et al. American College of Cardiology key data elements and definitions for measuring the clinical management and outcomes of patients with acute coronary syndromes. J Am Coll Cardiol 2001;38:2114-30. http://dx.doi.org/10.1016/S0735-1097(01)01702-8.
- Morrow DA, Wiviott SD, White HD, Nicolau JC, Bramucci E, Murphy SA, et al. Effect of the novel thienopyridine prasugrel compared with clopidogrel on spontaneous and procedural myocardial infarction in the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction 38: an application of the classification system from the universal definition of myocardial infarction. Circulation 2009;119:2758-64. http://dx.doi.org/10.1161/CIRCULATIONAHA.108.833665.
- Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M, et al. Universal definition of myocardial infarction. Eur Heart J 2007;28:2525-38. http://dx.doi.org/10.1093/eurheartj/ehm355.
- Bonaca MP, Wiviott SD, Braunwald E, Murphy SA, Ruff CT, Antman EM, et al. American College of Cardiology/American Heart Association/European Society of Cardiology/World Heart Federation universal definition of myocardial infarction classification system and the risk of cardiovascular death: observations from the TRITON-TIMI 38 trial (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel-Thrombolysis in Myocardial Infarction 38). Circulation 2012;125:577-83. http://dx.doi.org/10.1161/CIRCULATIONAHA.111.041160.
- Montalescot G, Wiviott SD, Braunwald E, Murphy SA, Gibson CM, McCabe CH, et al. Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): double-blind, randomised controlled trial. Lancet 2009;373:723-31. http://dx.doi.org/10.1016/S0140-6736(09)60441-4.
- Cannon CP, Harrington RA, James S, Ardissino D, Becker RC, Emanuelsson H, et al. Comparison of ticagrelor with clopidogrel in patients with a planned invasive strategy for acute coronary syndromes (PLATO): a randomised double-blind study. Lancet 2010;375:283-93. http://dx.doi.org/10.1016/S0140-6736(09)62191-7.
- Biondi-Zoccai G, Lotrionte M, Agostoni P, Abbate A, Romagnoli E, Sangiorgi G, et al. Adjusted indirect comparison meta-analysis of prasugrel versus ticagrelor for patients with acute coronary syndromes. Int J Cardiol 2011;150:325-31. http://dx.doi.org/10.1016/j.ijcard.2010.08.035.
- Chatterjee S, Ghose A, Sharma A, Guha G, Mukherjee D, Frankel R. Comparing newer oral anti-platelets prasugrel and ticagrelor in reduction of ischemic events-evidence from a network meta-analysis. J Thromb Thrombolysis 2013;36:223-32. http://dx.doi.org/10.1007/s11239-012-0838-z.
- Passaro D, Fadda V, Maratea D, Messori A. Anti-platelet treatments in acute coronary syndrome: simplified network meta-analysis. Int J Cardiol 2011;150:364-7. http://dx.doi.org/10.1016/j.ijcard.2011.05.083.
- Steiner S, Moertl D, Chen L, Coyle D, Wells GA. Network meta-analysis of prasugrel, ticagrelor, high- and standard-dose clopidogrel in patients scheduled for percutaneous coronary interventions. Thromb Haemost 2012;108:318-27. http://dx.doi.org/10.1160/TH11-08-0586.
- Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol 2007;7. http://dx.doi.org/10.1186/1471-2288-7-10.
- Biondi-Zoccai G, D’Ascenzo F, Abbate A, Agostoni P, Modena MG. Agreement between adjusted indirect comparison and simplified network meta-analyses on prasugrel and ticagrelor. Int J Cardiol 2011;151:228-9. http://dx.doi.org/10.1016/j.ijcard.2011.06.036.
- Cannon CP, Husted S, Harrington RA, Scirica BM, Emanuelsson H, Peters G, et al. Investigators D-. Safety, tolerability, and initial efficacy of AZD6140, the first reversible oral adenosine diphosphate receptor antagonist, compared with clopidogrel, in patients with non-ST-segment elevation acute coronary syndrome: primary results of the DISPERSE-2 trial. J Am Coll Cardiol 2007;50:1844-51. http://dx.doi.org/10.1016/j.jacc.2007.07.053.
- Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK. Clopidogrel in unstable angina to prevent recurrent events trial I. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001;345:494-502. http://dx.doi.org/10.1056/NEJMoa010746.
- Han YL, Wang B, Li Y, Xu K, Wang SL, Jing QM, et al. A high maintenance dose of clopidogrel improves short-term clinical outcomes in patients with acute coronary syndrome undergoing drug-eluting stent implantation. Chin Med J (Engl) 2009;122:793-7.
- Aradi D, Vorobecsuk A, Pinter T. Doubling the maintenance dose of clopidogrel in patients with high post-clopidogrel platelet reactivity after percutaneous coronary intervention: the DOSER randomized placebo-controlled trial. Eur Heart J 2010;31.
- Greenhalgh J, Bagust A, Boland A, Dwan K, Beale S, Fleeman N, et al. Prasugrel With Percutaneous Coronary Intervention for Treating Acute Coronary Syndromes (Review of TA182) 2013. www.nice.org.uk/nicemedia/live/13908/64290/64290.pdf (accessed 2013 December).
- Drummond M, Stoddart G, Torrance G. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 1997.
- Mahoney EM, Wang K, Arnold SV, Proskorovsky I, Wiviott S, Antman E, et al. Cost-effectiveness of prasugrel versus clopidogrel in patients with acute coronary syndromes and planned percutaneous coronary intervention: results from the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-thrombolysis in myocadial infarction TRITON-TIMI 38. Circulation 2010;121:71-9. http://dx.doi.org/10.1161/CIRCULATIONAHA.109.900704.
- Serebruany VL. Letter by Serebruany regarding article ‘Cost-effectiveness of prasugrel versus clopidogrel in patients with acute coronary syndromes and planned percutaneous coronary intervention: results from the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-thrombolysis in myocardial infarction TRITON-TIMI 38’. Circulation 2010;122. http://dx.doi.org/10.1161/CIRCULATIONAHA.110.936757.
- Mahoney EM, Wang K, Arnold SV, Cohen DJ, Proskorovsky I, Wiviott S, et al. Response to letter regarding article ‘Cost-effectiveness of prasugrel versus clopidogrel in patients with acute coronary syndromes and planned percutaneous coronary intervention: results from the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-thrombolysis in myocardial infarction TRITON-TIMI 38’. Circulation 2010;122. http://dx.doi.org/10.1161/CIRCULATIONAHA.110.951889.
- Davies A, Bakhai A, Schmitt C, Barrett A, Graham-Clarke P, Sculpher M. Prasugrel vs clopidogrel in patients with acute coronary syndrome undergoing percutaneous coronary intervention: a model-based cost-effectiveness analysis for Germany, Sweden, the Netherlands and Turkey. J Med Econ 2013;16:510-21. http://dx.doi.org/10.3111/13696998.2013.768998.
- Mauskopft JA, Graham JB, Ramaswamy K, Zagar AJ, Magnuson EA, Cohen DJ, et al. Cost-effectiveness of prasugrel in a US managed care population. J Med Econ 2011;15:166-74. http://dx.doi.org/10.3111/13696998.2011.637590.
- Davies A, Sculpher M, Schmitt C, Barrett A, Baird J, Zanotti G, et al. Prasugrel cost-effective relative to clopidogrel in patients with acute coronary syndrome undergoing percutaneous coronary intervention from the perspective of the UK national health service? A model-based analysis. Value Health 2009;12. http://dx.doi.org/10.1016/S1098-3015(10)74616-7.
- Davies A, Sculpher M, Schmitt C, Barrett A, Clouth J, McCollam PL, et al. Is prasugrel cost-effective relative to clopidogrel in patients with acute coronary syndrome undergoing percutaneous coronary intervention from the perspective of the German health care system? A model-based analysis. Value Health 2009;12. http://dx.doi.org/10.1016/S1098-3015(10)74628-3.
- Davies A, Sculpher MJ, Barrett A, Valladares A, Huete T, Dilla T. Prasugrel vs. clopidogrel in patients with acute coronary syndrome undergoing percutaneous coronary intervention: a Spanish model-based cost-effectiveness analysis. Value Health 2010;13. http://dx.doi.org/10.1016/S1098-3015(11)72428-7.
- Hill RA, Chung H, George E, Longson C, Stevens A. Prasugrel for the treatment of acute coronary syndromes with percutaneous coronary intervention: NICE technology appraisal guidance. Heart 2010;96:1407-8. http://dx.doi.org/10.1136/hrt.2010.202853.
- Keast S, Burns CF, Harrison D, Nesser N, Lambert T. Cost-effectiveness of prasugrel and clopidogrel for acute coronary syndrome in a medicaid population. J Manag Care Pharm 2010;16.
- Mahoney EM, Wang K, McCollam PL, Schmitt C, Cohen DJ. Cost-effectiveness of prasugrel versus clopidogrel in patients with acute coronary syndromes and planned PCI: Results from the triton-timi 38 trial from the German perspective. Value Health 2009;12:A328-9. http://dx.doi.org/10.1016/S1098-3015(10)74614-3.
- Mondragon R, Arrieta-Maturino E, Vargas-Valencia JJ, Ramirez-Gamez J, Martinez-Fonseca J, Guzman-Sotelo M. Cost-effectiveness of prasugrel versus clopidogrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention in the private sector in Mexico. Value Health 2011;14. http://dx.doi.org/10.1016/j.jval.2011.08.1515.
- Mondragon R, Arrieta-Maturino E, Vargas-Valencia JJ, Martinez-Fonseca J, Guzman-Sotelo M, Galindo-Suarez RM, et al. Cost-effectiveness of prasugrel versus clopidogrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention in the public health care system in Mexico. Value Health 2011;14. http://dx.doi.org/10.1016/j.jval.2011.08.1579.
- Rao S, Lin FJ, Ojo O, Patel V, Yu S, Zhan L, et al. A decision modeling approach to evaluate the cost-effectiveness of prasugrel versus clopidogrel in patients with planned percutaneous coronary intervention. Value Health 2011;14:A39-40. http://dx.doi.org/10.1016/j.jval.2011.02.229.
- Rosengren A, Wilhelmsen L, Hagman M, Wedel H. Natural history of myocardial infarction and angina pectoris in a general population sample of middle-aged men: a 16 year follow up of the Primary Prevention Study, Goteberg, Sweden. J Intern Med 1998;244:495-50. http://dx.doi.org/10.1111/j.1365-2796.1998.00394.x.
- Allen L, O’ Donnell C, Camargo CJ, Giugliano R, Lloyd-Jones D. Comparison of long-term mortality across the spectrum of acute coronary syndromes. Am Heart J 2006;151:1065-71. http://dx.doi.org/10.1016/j.ahj.2005.05.019.
- Mueller H, Forman S, Menegus M, Cohen L, Knatterud G, Braunwald E. Prognostic significance of nonfatal reinfarction during 3-year follow up: results of the Thrombolysis in Myocardial Infarction (TIMI) phase II clinical trial. The TIMI Investigators. J Am Coll Cardiol 1995;26:900-7. http://dx.doi.org/10.1016/0735-1097(95)00270-1.
- Taneja A, Collinson J, Flather M, Bakhai A, de Arenaza D, Wang D, et al. Mortality following non-ST elevation acute coronary syndrome: 4 years follow up of the PRAIS UK Registry (Prospective Registry of Acute Ischaemic Syndromes in the UK). Eur Heart J 2004;25:2013-18. http://dx.doi.org/10.1016/j.ehj.2004.08.009.
- Sullivan P, Ghushchyan V. Preference based EQ-5D index scores for chronic conditions in the United States. Med Decis Making 2006;26:410-20. http://dx.doi.org/10.1177/0272989X06290495.
- MIMS . Prescription Drug Database: MIMS 2013. www.mims.co.uk/ (accessed November 2014).
- Mehta S, Cannon C, Fox K. Routine vs selective invasive strategies in patients with acute coronary syndromes: a collaborative meta-analysis of randomized trials. J Am Med Assoc 2005;293:2908-17. http://dx.doi.org/10.1001/jama.293.23.2908.
- Guide to the Methods of Technology Appraisal. London: NICE; 2013.
- Kind P, Hardman G, Macran S. UK Population Norms for EQ-5D. Centre for Health Economics Discussion Paper (172). York: University of York; 1999.
- CAPRIE Steering Committee . A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996;348:1329-39. http://dx.doi.org/10.1016/S0140-6736(96)09457-3.
- Greenhalgh J, Bagust A, Boland A, Martin Saborido C, Fleeman N, McLeod C, et al. Prasugrel for the Treatment of Acute Coronary Syndromes with Percutaneous Coronary Intervention: A Single Technology Appraisal. Liverpool: Liverpool Reviews and Implementation Group, University of Liverpool; 2009.
- NICE . Clopidogrel and Modified-Release Dypiridamole in the Prevention of Occlusive Vascular Events: TA90 (replaced by TA210) 2005. www.nice.org.uk/TA090 (accessed December 2013).
- NICE . Vascular Disease – Clopidogrel and Dipyridamole (TA210) 2010. http://guidance.nice.org.uk/TA210 (accessed December 2013).
- Diener H-C, Sacco RL, Yusuf S, Cotton D, Ounpuu S, Lawton WA, et al. Effects of aspirin plus extended-release dipyridamole versus clopidogrel and telmisartan on disability and cognitive function after recurrent stroke in patients with ischaemic stroke in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial: a double-blind, active and placebo-controlled study. Lancet Neurol 2008;7:875-84. http://dx.doi.org/10.1016/S1474-4422(08)70198-4.
- Malmberg K, Yusuf S, Gerstein H, Brown J, Zhao F, Humt D, et al. Impact of diabetes on long-term prognosis in patients with unstable angina and non-Q-wave myocardial infarction. Circulation 2000;102:1014-19. http://dx.doi.org/10.1161/01.CIR.102.9.1014.
- Kleinman J, Donahue R, Harris M, Finucane F, Madans J, Brock D. Mortality among diabetics in a national sample. Am J Epidemiol 1988;128:389-401.
- Curtis L. Unit Costs of Health and Social Care 2012 (PSSRU) 2012. www.pssru.ac.uk/project-pages/unit-costs/2012/ (accessed November 2014).
- AstraZeneca . Ticagrelor for the Treatment of Acute Coronary Syndromes: Manufacturer Submission 2010. www.nice.org.uk/nicemedia/live/12169/55171/55171.pdf (accessed November 2014).
- Arias O, Ouellet M, Gray A, Wolstenholme J, Rothwell PM, Luengo-Fernandez R. Mapping the modified Rankin Scale (mRS) measurement into the generic EuroQol (EQ-5D) health outcome. Med Decis Making 2010;30:341-54. http://dx.doi.org/10.1177/0272989X09349961.
- Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62). Med Decis Making 2002;22:340-9. http://dx.doi.org/10.1177/027298902400448902.
- ClinicalTrials.gov . Prospective, Randomised Trial of Ticagrelor Versus Prasugrel in Patients With Acute Coronary Syndrome (ISAR-REACT 5) 2013. http://clinicaltrials.gov/ct2/show/NCT01944800?term=ticagrelor&rank=41 (accessed November 2014).
- Schulz S, Angiolillo D, Antoniucci D, Bernlochner I, Hamm C, Jaitner J, et al. Randomized Comparison of Ticagrelor versus Prasugrel in Patients with Acute Coronary Syndrome and Planned Invasive Strategy—Design and Rationale of the Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment (ISAR-REACT) 5 Trial. J Cardiovasc Transl Res 2014;7:91-100. http://dx.doi.org/10.1007/s12265-013-9527-3.
- Antman EM, Wiviott SD, Murphy SA, Voitk J, Hasin Y, Widimsky P, et al. Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a TRITON-TIMI 38 (TRial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet InhibitioN with Prasugrel-Thrombolysis In Myocardial Infarction) analysis. J Am Coll Cardiol 2008;51:2028-33. http://dx.doi.org/10.1016/j.jacc.2008.04.002.
- Hochholzer W, Wiviott SD, Antman EM, Contant CF, Guo J, Giugliano RP, et al. Predictors of bleeding and time dependence of association of bleeding with mortality: Insights from the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-thrombolysis in myocardial infarction 38 (TRITON-TIMI 38). Circulation 2011;123:2681-9. http://dx.doi.org/10.1161/CIRCULATIONAHA.110.002683.
- Laynez A, Sardi G, Torguson R, Xue Z, Suddath WO, Satler LF, et al. Safety and efficacy of prasugrel use in patients undergoing percutaneous coronary intervention and anticoagulated with bivalirudin. Am J Cardiol 2013;111:516-20. http://dx.doi.org/10.1016/j.amjcard.2012.10.035.
- Mega JL, Close SL, Wiviott SD, Shen L, Hockett RD, Brandt JT, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. New Engl J Med 2009;360:354-62. http://dx.doi.org/10.1056/NEJMoa0809171.
- Mega JL, Close SL, Wiviott SD, Shen L, Walker JR, Simon T, et al. Genetic variants in ABCB1 and CYP2C19 and cardiovascular outcomes after treatment with clopidogrel and prasugrel in the TRITON-TIMI 38 trial: a pharmacogenetic analysis. Lancet 2010;376:1312-19. http://dx.doi.org/10.1016/S0140-6736(10)61273-1.
- Michelson AD, Frelinger AL, Braunwald E, Downey WE, Angiolillo DJ, Xenopoulos NP, et al. Pharmacodynamic assessment of platelet inhibition by prasugrel vs. clopidogrel in the TRITON-TIMI 38 trial. Eur Heart J 2009;30:1753-63. http://dx.doi.org/10.1093/eurheartj/ehp159.
- Murphy SA, Antman EM, Wiviott SD, Weerakkody G, Morocutti G, Huber K, et al. Reduction in recurrent cardiovascular events with prasugrel compared with clopidogrel in patients with acute coronary syndromes from the TRITON-TIMI 38 trial. Eur Heart J 2008;29:2473-9. http://dx.doi.org/10.1093/eurheartj/ehn362.
- O’Donoghue M, Antman EM, Braunwald E, Murphy SA, Steg PG, Finkelstein A, et al. The efficacy and safety of prasugrel with and without a glycoprotein IIb/IIIa inhibitor in patients with acute coronary syndromes undergoing percutaneous intervention: a TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel-Thrombolysis In Myocardial Infarction 38) analysis. J Am Coll Cardiol 2009;54:678-85. http://dx.doi.org/10.1016/j.jacc.2009.05.025.
- O’Donoghue ML, Braunwald E, Antman EM, Murphy SA, Bates ER, Rozenman Y, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet 2009;374:989-97. http://dx.doi.org/10.1016/S0140-6736(09)61525-7.
- Pride YB, Wiviott SD, Buros JL, Zorkun C, Tariq MU, Antman EM, et al. Effect of prasugrel versus clopidogrel on outcomes among patients with acute coronary syndrome undergoing percutaneous coronary intervention without stent implantation: a TRial to assess Improvement in Therapeutic Outcomes by optimizing platelet inhibitioN with prasugrel (TRITON)-Thrombolysis in Myocardial Infarction (TIMI) 38 substudy. Am Heart J 2009;158. http://dx.doi.org/10.1016/j.ahj.2009.06.021.
- Pride YB, Tung P, Mohanavelu S, Zorkun C, Wiviott SD, Antman EM, et al. Angiographic and clinical outcomes among patients with acute coronary syndromes presenting with isolated anterior ST-segment depression: a TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel-Thrombolysis In Myocardial Infarction 38) substudy. JACC Cardiovasc Interv 2010;3:806-11. http://dx.doi.org/10.1016/j.jcin.2010.05.012.
- Riesmeyer JS, Salazar DE, Weerakkody GJ, Ni L, Wrishko RE, Ernest CS, et al. Relationship between exposure to prasugrel active metabolite and clinical outcomes in the TRITON-TIMI 38 substudy. J Clin Pharmacol 2012;52:789-97. http://dx.doi.org/10.1177/0091270011406280.
- Ruff CT, Giugliano RP, Antman EM, Murphy SA, Lotan C, Heuer H, et al. Safety and efficacy of prasugrel compared with clopidogrel in different regions of the world. Int J Cardiol 2012;155:424-9. http://dx.doi.org/10.1016/j.ijcard.2010.10.040.
- Scirica B, Morrow D, Antman E, Bonaca M, Murphy S, Braunwald E, et al. Timing and clinical setting of cardiovascular death or myocardial infarction following PCI for ACS-observations from the TRITON-TIMI 38 trial. J Am Coll Cardiol 2012;1. http://dx.doi.org/10.1016/S0735-1097(12)60341-6.
- Smith PK, Goodnough LT, Levy JH, Poston RS, Short MA, Weerakkody GJ, et al. Mortality benefit with prasugrel in the TRITON-TIMI 38 coronary artery bypass grafting cohort: risk-adjusted retrospective data analysis. J Am Coll Cardiol 2012;60:388-96. http://dx.doi.org/10.1016/j.jacc.2012.03.030.
- Udell JA, Braunwald E, Antman EM, Murphy SA, Montalescot G, Wiviott SD. Benefit of prasugrel in ST-elevation myocardial infarction according to timing of percutaneous coronary intervention: Insight from the triton-TIMI 38 study. Circulation 2011;124. http://dx.doi.org/10.1016/j.jcin.2014.01.160.
- Wiviott SD, Braunwald E, Angiolillo DJ, Meisel S, Dalby AJ, Verheugt FW, et al. Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-Thrombolysis in Myocardial Infarction 38. Circulation 2008;118:1626-36. http://dx.doi.org/10.1161/CIRCULATIONAHA.108.791061.
- Wiviott SD, Braunwald E, McCabe CH, Horvath I, Keltai M, Herrman JP, et al. Intensive oral antiplatelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary syndromes treated with percutaneous coronary intervention and stenting in the TRITON-TIMI 38 trial: a subanalysis of a randomised trial. Lancet 2008;371:1353-63. http://dx.doi.org/10.1016/S0140-6736(08)60422-5.
- Wrishko RE, Ernest CS, Small DS, Li YG, Weerakkody GJ, Riesmeyer JR, et al. Population pharmacokinetic analyses to evaluate the influence of intrinsic and extrinsic factors on exposure of prasugrel active metabolite in TRITON-TIMI 38. J Clin Phamacol 2009;49:984-98. http://dx.doi.org/10.1177/0091270009337942.
- Greenhalgh J, Bagust A, Boland A, Dwan K, Beale S, Fleeman N, et al. The Clinical and Cost-Effectiveness of Prasugrel with Percutaneous Coronary Intervention for Treating Acute Coronary Syndromes (Review of TA182). Liverpool: Liverpool Reviews and Implementation Group, University of Liverpool; 2013.
- Abuzahra M, Pillai M, Caldera A, Hartley WB, Gonzalez R, Bobek J, et al. Comparison of higher clopidogrel loading and maintenance dose to standard dose on platelet function and outcomes after percutaneous coronary intervention using drug-eluting stents. Am J Cardiol 2008;102:401-3. http://dx.doi.org/10.1016/j.amjcard.2008.03.073.
- Angiolillo DJ, Bernardo E, Palazuelos J, Desai B, Weisberg I, Alfonso F, et al. Functional impact of high clopidogrel maintenance dosing in patients undergoing elective percutaneous coronary interventions. Results of a randomized study. Thromb Haemost 2008;99:161-8. http://dx.doi.org/10.1160/TH07-09-0562.
- Palmerini T, Barozzi C, Tomasi L, Sangiorgi D, Marzocchi A, De Servi S, et al. A randomised study comparing the antiplatelet and antiinflammatory effect of clopidogrel 150 mg/day versus 75 mg/day in patients with ST-segment elevation acute myocardial infarction and poor responsiveness to clopidogrel: results from the DOUBLE study. Thromb Res 2010;125:309-14. http://dx.doi.org/10.1016/j.thromres.2009.06.016.
- Price MJ, Berger PB, Teirstein PS, Tanguay J-F, Angiolillo DJ, Spriggs D, et al. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA 2011;305:1097-105. http://dx.doi.org/10.1001/jama.2011.290.
- Aleil B, Jacquemin L, De Poli F, Zaehringer M, Collet J-P, Montalescot G, et al. Clopidogrel 150 mg/day to overcome low responsiveness in patients undergoing elective percutaneous coronary intervention: results from the VASP-02 (Vasodilator-Stimulated Phosphoprotein-02) randomized study. JACC Cardiovasc Interv 2008;1:631-8. http://dx.doi.org/10.1016/j.jcin.2008.09.004.
- von Beckerath N, Kastrati A, Wieczorek A, Pogatsa-Murray G, Sibbing D, Graf I, et al. A double-blind, randomized study on platelet aggregation in patients treated with a daily dose of 150 or 75 mg of clopidogrel for 30 days. Eur Heart J 2007;28:1814-19. http://dx.doi.org/10.1093/eurheartj/ehl489.
- Alexopoulos D, Xanthopoulou I, Davlouros P, Plakomyti T-E, Panagiotou A, Mavronasiou E, et al. Prasugrel overcomes high on-clopidogrel platelet reactivity in chronic coronary artery disease patients more effectively than high dose (150 mg) clopidogrel. Am Heart J 2011;162:733-9. http://dx.doi.org/10.1016/j.ahj.2011.07.026.
- Wiviott SD, Trenk D, Frelinger AL, O’Donoghue M, Neumann F-J, Michelson AD, et al. Prasugrel compared with high loading- and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation 2007;116:2923-32. http://dx.doi.org/10.1161/CIRCULATIONAHA.107.740324.
Appendix 1 Literature search strategies
OvidSP MEDLINE(R)
1946 to June Week 1 2013
| 1 | exp Acute Coronary Syndrome/ |
| 2 | (coronary adj syndrome$).ti,ab. |
| 3 | exp Angina, Unstable/ |
| 4 | (unstable adj2 angina).ti,ab. |
| 5 | exp Myocardial Infarction/ |
| 6 | (myocard$ adj infarct$).ti,ab. |
| 7 | heart infarct$.ti,ab. |
| 8 | exp Myocardial Ischemia/ |
| 9 | (myocard$ adj isch?emi$).ti,ab. |
| 10 | (isch?emic adj3 heart).ti,ab. |
| 11 | or/1-10 |
| 12 | (Prasugrel or Effient or Efient).af |
| 13 | 11 and 12 |
| 14 | animal/ not (animal/ and human/) |
| 15 | 13 not 14 |
| 16 | Limit 15 to (English language) |
OvidSP EMBASE
1974 to 2013 June 18
| 1 | exp unstable angina pectoris/ or exp acute coronary syndrome/ or heart infarction/ or heart muscle ischemia/ or ischemic heart disease/ |
| 2 | (coronary adj syndrome$).ti,ab. |
| 3 | (unstable adj2 angina).ti,ab. |
| 4 | (myocard$ adj infarct$).ti,ab. |
| 5 | heart infarct$.ti,ab. |
| 6 | (myocard$ adj isch?emi$).ti,ab. |
| 7 | (isch?emic adj3 heart).ti,ab. |
| 8 | or/1-7 |
| 9 | (Prasugrel or Effient or Efient).af |
| 10 | 8 and 9 |
| 11 | limit 10 to (human and english language) |
The Cochrane Library Searches
Prasugrel or Effient or Efient:ti,ab,kw (word variations have been searched).
Appendix 2 Quality assessment of included trial
| Trial | Randomisation | Baseline comparability | Eligibility criteria specified | Co-interventions identified | Blinding | Withdrawals | ITT | Other outcomes | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Truly random | Allocation concealment | Number stated | Presented | Achieved | Assessors | Administration | Participants | Procedure assessed | > 80% in final analysis | Reasons stated | |||||
| Wiviott et al. 200736 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓a | ✓ | ✓ | ✓ | NS | ✓ | ✓ | ✓ | ✗ |
Appendix 3 Table of excluded studies with rationale
| Paper | Reason for exclusion |
|---|---|
| National Horizon Scanning Centre. Prasugrel for Acute Coronary Artery Syndrome with Percutaneous Coronary Intervention: Horizon Scanning Technology Briefing. Birmingham: National Horizon Scanning Centre (NHSC); 2007, issue 2, page 6. URL: www.crd.york.ac.uk/CRDWeb/ShowRecord.asp?AccessionNumber=32007000470&UserID=0#.VKwJkdKDl8E (accessed July 2013) | Abstract of review |
| NICE. Prasugrel for the Treatment of Acute Coronary Syndromes with Percutaneous Coronary Intervention. Health Technology Assessment Database. URL: www.crd.york.ac.uk/CRDWeb/ShowRecord.asp?AccessionNumber=32011000084&UserID=0 (accessed November 2013) | Abstract of TA182 |
| Canadian Agency for Drugs and Technologies in Health. Clopidogrel, Prasugrel and Ticagrelor in Adults with Acute Coronary Syndrome: A Review of the Clinical Effectiveness. Health Technology Assessment Database, 2011. URL: www.crd.york.ac.uk/CRDWeb/ShowRecord.asp?AccessionNumber=32011001503&UserID=0 (accessed November 2013) | Abstract of systematic reviews |
| National Horizon Scanning Centre. Prasugrel (Efient) for the Prevention of Atherothrombotic Events in Patients with Acute Coronary Syndromes who will be Managed Without Acute Coronary Revascularisation in Combination with Aspirin. Birmingham: National Horizon Scanning Centre (NHSC); 2011 | Horizon scanning document |
| British Journal of Cardiology. News from the ESC Congress 2012. Br J Cardiol 2012;19:152–5 | Meeting report |
| American Journal for Cardiology, 18th Annual Interventional Vascular Therapeutics Angioplasty Summit-Transcatheter Cardiovascular Therapeutics Asia Pacific Symposium, TCTAP Hong Kong, 23–6 April 2013 | Meeting report |
| Journal of American College of Cardiology. JACC Official Highlights from the ACC. 13 62nd Annual Scientific Session and Expo: MD Conference Express. March 9–12 2013. URL: www.nxtbook.com/nxtbooks/md_conference_express/acc2013/#/0 (accessed November 2013) | Meeting report |
| Society for Cardiovascular Angiography and Interventions’ 36th Annual Scientific Sessions. Catheterization and Cardiovascular Interventions 2013;81:S1. Orlando, Florida, 8–11 May 2013 | Meeting report |
| Aalbers J. Prasugrel study addresses timing of thienopyridine loading dose in NSTEMI patients pre-PCI (the ACCOAST study). Cardiovasc J Afr 2011;22:168 | Letter |
| Abdel-Latif A, Moliterno DJ. Prasugrel versus clopidogrel in primary PCI: Considerations of the TRITON-TIMI 38 substudy. Curr Cardiol Rep 2009;11:323–4 | Report of a TRITON-TIMI 38 substudy |
| Alexander W. TRITON-TIMI 38: Clopidogrel and prasugrel. Pharm Ther 2008;33:51 | Report of a TRITON-TIMI 38 |
| Alexander W. FDA advisory committee meeting on prasugrel for acute coronary syndromes. Pharm Ther 2009;34:155–6 | FDA discussion of prasugrel |
| Alexander W. Cardiovascular research technologies 2012. Pharm Ther 2012;37:186–9 | Discussion document |
| Alexander W. Transcatheter cardiovascular therapeutics 2012. Pharm Ther 2012;37:709–10 | Meeting review |
| Alexopoulos D, Theodoropoulos KC, Stavrou EF, Xanthopoulou I, Kassimis G. Tsigkas G, et al. Prasugrel versus high dose clopidogrel to overcome early high on clopidogrel platelet reactivity in patients with ST elevation myocardial infarction. Cardiovasc Drugs Ther 2012;26:393–400 | Platelet reactivity trial. 30-day outcomes |
| Alexopoulos D, Xanthopoulou I, Gkizas V, Kassimis G, Theodoropoulos KC, Makris G, et al. Randomized assessment of ticagrelor versus prasugrel antiplatelet effects in patients with ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv 2012;5:797–804 | Platelet reactivity trial. 30-day outcomes |
| Aradi D, Komocsi A, Price M, Cuisset T, Ari H, Hazarbasanov D, et al. Efficacy and safety of intensified antiplatelet therapy on the basis of platelet reactivity testing in patients after PCI: systematic review and meta-analysis. EuroIntervention 2012;8:N109 | Platelet function studies |
| Aradi D, Komocsi A, Price M, Cuisset T, Ari H, Hazarbasanov D, et al. Efficacy and safety of intensified antiplatelet therapy on the basis of platelet reactivity testing in patients after percutaneous coronary intervention: Systematic review and meta-analysis. J Am Coll Cardiol 2012;60:B218 | Abstract of systematic review |
| Aradi D, Komocsi A, Vorobcsuk A, Serebruany VL. Impact of clopidogrel and potent P2Y12-inhibitors on mortality and stroke in patients with acute coronary syndrome or undergoing percutaneous coronary intervention: A systematic review and meta-analysis. Thromb Haemost 2013;109:93–101 | Systematic review |
| Aradi D, Pinter T, Magyari B, Konyi A, Vorobcsuk A, Horvath IG, et al. Optimizing P2Y12-receptor inhibition in acute coronary syndrome patients after PCI using platelet function testing: Impact of prasugrel versus high-dose clopidogrel. J Am Coll Cardiol 2013;1:E1922 | Registry study |
| Aradi D, Serebruany VL. No benefit of new-generation antiplatelet agents on stroke compared to clopidogrel. Eur Heart J 2011;32:555 | Abstract of systematic review |
| Armero S, Bonello L, Berbis J, Camoin-Jau L, Lemesle G, Jacquin L, et al. Rate of nuisance bleedings and impact on compliance to prasugrel in acute coronary syndromes. Am J Cardiol 2011;108:1710–13 | Not RCT |
| Arnesen H. Thrombocardiology: an update. Expert Rev Cardiovasc Ther 2010;8:331–3 | Meeting review |
| Baron TH, Kamath PS, McBane RD. Management of antithrombotic therapy in patients undergoing invasive procedures. N Engl J Med 2013;368:2113–24 | Review |
| Beigel R, Fefer P, Fink N, Grupper A, Varon D, Hod H, et al. The immediate antiplatelet effect of prasugrel versus clopidogrel in patients undergoing primary angioplasty for ST-elevation myocardial infarction-implications for reperfusion. J Am Coll Cardiol 2012;1:E503 | Platelet function study |
| Bellemain-Appaix A, Brieger D, Beygui F, Silvain J, Pena A, Cayla G, et al. New P2Y12 inhibitors versus clopidogrel in percutaneous coronary intervention: A meta-analysis. J Am Coll Cardiol 2010;56:1542–51 | Systematic review discussed in main report |
| Biondi-Zoccai G, D’Ascenzo F, Abbate A, Agostoni P, Modena MG. Agreement between adjusted indirect comparison and simplified network meta-analyses on prasugrel and ticagrelor. Int J Cardiol 2011;151:228–9. [Reply to Passaro et al. Int J Cardiol 2011;150:364–7] | Letter |
| Biondi-Zoccai G, Lotrionte M, Agostoni P, Abbate A, Romagnoli E, Sangiorgi G, et al. Adjusted indirect comparison meta-analysis of prasugrel versus ticagrelor for patients with acute coronary syndromes. Int J Cardiol 2011;150:325–31 | Abstract of indirect treatment comparison discussed in main report |
| Biondi-Zoccai G, Lotrionte M, Moretti C, Sciuto F, Omede P, Abbate A, et al. Comparing ticagrelor versus prasugrel for the treatment of patients with acute coronary syndromes: Evidence from a 32,983-patient adjusted indirect comparison meta-analysis. EuroIntervention 2010;6(Suppl. H) | Indirect treatment comparison discussed in main report |
| Canadian Agency for Drugs and Technologies in Health. Clopidogrel, Prasugrel and Ticagrelor in Adults with Acute Coronary Syndrome: a Review of the Clinical Effectiveness. Ottawa: Canadian Agency for Drugs and Technologies in Health (CADTH); 2011 | Various systematic reviews |
| Canadian Agency for Drugs and Technologies in Health. Clopidogrel, Prasugrel and Ticagrelor in Adults with Acute Coronary Syndrome: A Review of the Clinical Effectiveness, Cost Effectiveness and Guidelines. Ottawa: Canadian Agency for Drugs and Technologies in Health (CADTH); 2012 | Systematic review but not relevant to review |
| Capodanno D, Tamburino C. Cyphering the statistical and clinical significance of prasugrel in the TRITON-TIMI 38 trial. Int J Cardiol 2011;146:242–3 | Theoretical paper |
| Cattaneo M. New P2Y12 inhibitors. Circulation 2010;121:171–9 | Discussion |
| Collet JP, Cuisset T, Range G, Cayla G, Elhadad S, Pouillot C, et al. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N Engl J Med 2012;367:2100–9 | Platelet function and tailored treatment trial |
| De Servi S, Savonitto S. How to explain the reduced cardiovascular mortality in the ticagrelor arm of the PLATO trial? Int J Cardiol 2011;149:265–7 | Discussion |
| Dowdall M. Clopidogrel treatment prior to percutaneous coronary intervention questioned by results of recent analysis. Intervent Cardiol 2013;5:13–14 | Discussion |
| Dridi NP, Johansson PI, Clemmensen P, Engstrom T, Radu M, Pedersen F, et al. Thrombocytes and individualization of oral antiplatelet treatment after percutaneous coronary intervention (tailor). J Am Coll Cardiol 2012;60:B215 | Platelet function study |
| Erlinge D, Ten Berg J, Foley D, Angiolillo DJ, Wagner H, Brown PB, et al. Reduction in platelet reactivity with prasugrel 5 mg in low-body-weight patients is noninferior to prasugrel 10 mg in higher-body-weight patients: results from the FEATHER trial. J Am Coll Cardiol 2012;60:2032–40 | Crossover study |
| Floyd JS, Serebruany VL. Prasugrel as a potential cancer promoter: review of the unpublished data. Arch Int Med 2010;170:1078–80 | Review |
| Freeman MK. Thienopyridine antiplatelet agents: focus on prasugrel. Consult Pharm 2010;25:241–257 | Review |
| Garrett AD. Ticagrelor tops prasugrel in pharmacodynamic study. Drug Top 2012;156:P43. | News article |
| Ge J, Zhu J, Hong BK, Boonbaichaiyapruck S, Goh YS, Hou CJ, et al. Prasugrel versus clopidogrel in Asian patients with acute coronary syndromes: design and rationale of a multi-dose, pharmacodynamic, phase 3 clinical trial. Curr Med Res Opin 2010;26:2077–85 | Dose-ranging trial |
| Giugliano RP, Braunwald E. The year in non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol 2010;56:2126–38 | Review of guidelines |
| Giugliano RP, Braunwald E. The year in non ST-segment elevation acute coronary syndrome. JAm Coll Cardiol 2011;58:2342–54 | Review of guidelines |
| Giugliano RP, Braunwald E. The year in non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol 2012;60:2127–39 | Review of guidelines |
| Goodwin MM, Desilets AR, Willett KC. Thienopyridines in acute coronary syndrome. Ann Pharmacother 2011;45:207–17 | Review |
| Greenhalgh J, Bagust A, Boland A, Saborido CM, Fleeman N, McLeod C, et al. Prasugrel for the treatment of acute coronary artery syndromes with percutaneous coronary intervention. Health Technol Assess 2010;14(Suppl. 1) | Short version of TA182 ERG report |
| Hamilos M, Kochiadakis G, Skalidis E, Igoumenidis N, Saloustros I, Psathakis E, et al. Prasugrel is associated with higher levels of P2Y12 blockade and less periprocedural myonecrosis than clopidogrel in patients undergoing coronary angioplasty for stable coronary artery disease. Eur Heart J 2012;33:41 | Not patient group |
| Hill RA, Chung H, George E, Longson C, Stevens A. Prasugrel for the treatment of acute coronary syndromes with percutaneous coronary intervention: NICE technology appraisal guidance. Heart 2010;96:1407–8 | Discussion of NICE decision |
| IQWiG. Prasugrel bei akutem Koronarsyndrom. [Prasugrel in the treatment of acute coronary syndrome] Cologne: Institut fuer Qualitaet und Wirtschaftlichkeit im Gesundheitswesen (IQWiG). IQWiG-Berichte 89; 2011 | German HTA |
| Jakubowski JA, Riesmeyer JS, Close SL, Leishman AG, Erlinge D. TRITON and beyond: new insights into the profile of prasugrel. Cardiovasc Ther 2012;30:e174–82 | Review of prasugrel studies to 2007 |
| Jakubowski JA, Winters KJ, Naganuma H, Wallentin L. Prasugrel: a novel thienopyridine antiplatelet agent. A review of preclinical and clinical studies and the mechanistic basis for its distinct antiplatelet profile. Cardiovasc Drug Rev 2007;25:357–74 | Review of prasugrel studies up to 2012 |
| Jeong YH, Tantry US, Gurbel PA. Importance of potent P2Y(12) receptor blockade in acute myocardial infarction: focus on prasugrel. Expert Opin Pharmacother 2012;13:1771–96 | Review |
| Lange CG. Is prasugrel more effective than clopidogrel at preventing future cardiac events? JAAPA 2011;24:52, 55 | Review |
| Lee DH, Kim MH, Park TH, Park JS, Park K, Zhang HZ, et al. Comparison of prasugrel and clopidogrel reloading on high platelet reactivity in clopidogrel-loaded patients undergoing percutaneous coronary intervention (PRAISE-HPR): a study protocol for a prospective randomized controlled clinical trial. Trials 2013;14:62 | Platelet function study |
| Lopes RD, Becker RC, Alexander JH, Armstrong PW, Califf RM, Chan MY, et al. Highlights from the III International Symposium of Thrombosis and Anticoagulation (ISTA), October 14–16, 2010, Sao Paulo, Brazil. J Thromb Thrombolysis 2011;32:242–66 | Meeting review |
| Lopes RD, Becker RC, Newby LK, Peterson ED, Hylek EM, Granger CB, et al. Highlights from the IV International Symposium of Thrombosis and Anticoagulation (ISTA), October 20–21, 2011, Salvador, Bahia, Brazil. J Thromb Thrombolysis 2012;34:143–63 | Meeting review |
| Lopes RD, Granger CB. Interpreting the TRITON results in light of the event adjudication process. Cardiology 2010;115:89–90 | Commentary |
| Lynch DR Jr, Dantzler DM Jr, Zhao D. Prasugrel versus clopidogrel for acute coronary syndromes. N Engl J Med 2013;368:188 | Letter |
| Manolis AS, Manolis TA, Papadimitriou P, Koulouris S, Melita H. Combined antiplatelet therapy: still a sweeping combination in cardiology. Cardiovasc Hematol Agents Med Chem 2013;1:136–67 | Not RCT |
| Mariani M, Mariani G, De Servi S. Efficacy and safety of prasugrel compared with clopidogrel in patients with acute coronary syndromes: results of TRITON-TIMI 38 trials. Expert Rev Cardiovasc Ther 2009;7:17–23 | Expert review |
| Martin MT, Spinler SA, Nutescu EA. Emerging antiplatelet therapies in percutaneous coronary intervention: a focus on prasugrel. Clin Ther 2011;33:425–42 | Review |
| Mauri L, Kereiakes DJ, Normand SL, Wiviott SD, Cohen DJ, Holmes DR, et al. Rationale and design of the dual antiplatelet therapy study, a prospective, multicenter, randomized, double-blind trial to assess the effectiveness and safety of 12 versus 30 months of dual antiplatelet therapy in subjects undergoing percutaneous coronary intervention with either drug-eluting stent or bare metal stent placement for the treatment of coronary artery lesions. Am Heart J 2010;160:1035–41 | Not comparators of interest |
| Mohammad RA, Goldberg T, Dorsch MP, Cheng JW. Antiplatelet therapy after placement of a drug-eluting stent: a review of efficacy and safety studies. Clin Ther 2010;32:2265–81 | Review |
| Montalescot, G. Benefits for specific subpopulations in TRITON-TIMI 38. Eur Heart J 2009[Suppl. 11(G)]:G18–24 | Discussion |
| Montalescot G, Collet JP, Vicaut E, Cayla G, Cuisset T, Elhadad S, et al. A randomized trial of bedside platelet function monitoring to adjust antiplatelet therapy versus standard of care in patients undergoing drug eluting stent implantation: the ARCTIC study. Circulation 2012;126:2777 | ARCTIC platelet function study |
| Montalescot G, Sideris G, Cohen R, Meuleman C, Bal dit Sollier C, Barthelemy O, et al. Prasugrel compared with high-dose clopidogrel in acute coronary syndrome. The randomised, double-blind ACAPULCO study. Thromb Haemost 2010;103:213–23 | Not patient population |
| Motovska Z, Kala P. Benefits and risks of clopidogrel use in patients with coronary artery disease: evidence from randomized studies and registries. Clin Ther 2008;30(part 2):2191–202 | Review |
| Navarese EP, Verdoia M, Schaffer A, Suriano P, Kozinski M, Castriota F, et al. Ischaemic and bleeding complications with new, compared to standard, ADP-antagonist regimens in acute coronary syndromes: a meta-analysis of randomized trials. QJM 2011;104:561–9 | Meta-analysis |
| Neumann FJ. Balancing efficacy and safety in the TRITON-TIMI 38 trial. Eur Heart J 2009(Suppl. 11)(G): G14–17 | Review |
| Oberhansli M, Lehner C, Puricel S, Lehmann S, Togni M, Stauffer JC, et al. A randomized comparison of platelet reactivity in patients after treatment with various commercial clopidogrel preparations: the CLO-CLO trial. Arch Cardiovasc Dis 2012;105:587–92 | Clopidogrel dosing study |
| Oh EY, Abraham T, Saad N, Rapp JH, Vastey FL, Balmir E. A comprehensive comparative review of adenosine diphosphate receptor antagonists. Expert Opin Pharmacother 2012;13:175–91 | Systematic review |
| Parodi G, Valenti R, Bellandi B, Migliorini A, Marcucci R, Comito V, et al. Comparison of prasugrel and ticagrelor loading doses in ST-segment elevation myocardial infarction patients: RAPID (Rapid Activity of Platelet Inhibitor Drugs) primary PCI study. J Am Coll Cardiol 2013;61:1601–6 | Platelet function study |
| Passaro D, Fadda V, Maratea D, Messori A. Anti-platelet treatments in acute coronary syndrome: simplified network meta-analysis. Int J Cardiol 2011;150:364–7 | Discussed in main body of this AG report |
| Rabasseda X. A report from the 60th Annual Scientific Session & Expo and I2 (Innovation and Intervention) Summit of the American College of Cardiology April 2–5, 2011 – New Orleans, Louisiana USA). Drugs Today 2011;47:381–400 | Meeting review |
| Rabasseda X. Highlights from the American College of Cardiology 2012 Annual Meeting: March 24–27, 2012 – Chicago, Illinois, USA. Drugs Future 2012;37:379–87 | Meeting review |
| Ramanakumar A, Bajaj R, Singh A, Dani S, Basheer Z, Hannan J. Comparison of prasugrel 60 Mg vs. clopidogrel 600 Mg loading doses in patients undergoing primary PCI for acute STEMI. Cardiovasc Interv 2013;1:S7 | Not randomised |
| Scott DM, Norwood RM, Parra D. P2Y12 inhibitors in cardiovascular disease: focus on prasugrel. Ann Pharmacother 2009;43:64–76 | Review |
| Serebruany VL. Excess rates of nonfatal myocardial infarction in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel (preventing clinical events or chasing enzymatic ghosts?). Am J Cardiol 2008;101:1364–6 | Comment |
| Serebruany VL. Delays of event adjudication in the TRITON trial. Cardiology 2010;115:217–20 | Comment |
| Serebruany VL. Mortality in the TRITON trial: update from the FDA prasugrel action package. Am J Cardiol 2010;105:1356–7. | Comment |
| Serebruany VL. The TRITON versus PLATO trials: differences beyond platelet inhibition. Thromb Haemost 2010;103:259–61 | Comment |
| Serebruany VL. Timing of thienopyridine loading and outcomes in the TRITON trial: the FDA Prasugrel Action Package outlook. Cardiovasc Revasc Med 2011;12:94–8 | Comment |
| Serebruany VL, Midei MG, Meilman H, Malinin AI, Lowry DR. Platelet inhibition with prasugrel (CS-747) compared with clopidogrel in patients undergoing coronary stenting: the subset from the JUMBO study. Postgrad Med J 2006;82:404–10 | Comment |
| Siller-Matula JM, Francesconi M, Dechant C, Jilma B, Maurer G, Delle-Karth G, et al. Personalized antiplatelet treatment after percutaneous coronary intervention: The MADONNA study. Eur Heart J 2012;33:41 | Not RCT |
| Silvain J, Bellemain-Appaix A, Barthelemy O, Beygui F, Collet JP, Montalescot G. Optimal use of thienopyridines in Non-ST-elevation acute coronary syndrome following CURRENT-OASIS 7. Circulation Cardiovasc Interv 2011;4:95–103 | Review |
| Singh T, Cuomo L, Cohen M, Ahmad HA, Aronow WS. Use of antiplatelet therapy after percutaneous coronary intervention with bare-metal stents and different types of drug-eluting stents. Curr Clin Pharmacol 2013;8:59–66 | Not relevant comparators |
| Skalli S, Garcia Palop B, Faudel A, Nouvel M, Parat S, Jacob X, et al. Are prasugrel and clopidogrel equally effective and safe? Int J Clin Pharm 2012;34:258 | Review |
| Smith PK, Goodnough LT, Levy JH, Poston RS, Short MA, Weerakkody GJ, et al. Mortality benefit with prasugrel in the TRITON-TIMI 38 coronary artery bypass grafting cohort: risk-adjusted retrospective data analysis. J Am Coll Cardiol 2012;60:388–96 | Subgroup analysis from TRITON-TIMI 38 |
| Sorich MJ, Vitry A, Ward MB, Horowitz JD, McKinnon RA. Prasugrel vs. clopidogrel for cytochrome P450 2C19-genotyped subgroups: integration of the TRITON-TIMI38 trial data. J Thrombos Haemost 2010;8:1678–84 | Genotype study |
| Spinler SA, Rees C. Review of prasugrel for the secondary prevention of atherothrombosis. J Manag Care Pharm 2009;15:383–95 | Review |
| Steiner S, Chen L, Coyle D, Wells GA. Indirect treatment comparison of novel antiplatelet drugs directed against the ADP receptor compared to placebo-evaluation by three different statistical approaches. J Cardio Rehab Prev 2011;31:E8 | Abstract of network meta-analysis discussed in present report |
| Steiner, S, Chen L, Coyle D and Wells GW. Effects of prasugrel, ticagrelor and high dose clopidogrel compared to placebo evaluated by three different statistical approaches for indirect treatment comparisons. Eur Heart J 2011;32:252 | Abstract of network meta-analysis discussed in present report |
| Steiner S, Chen L, Coyle D, Wells GW. Effects of prasugrel, ticagrelor and high dose clopidogrel compared to placebo evaluated by three different statistical approaches for indirect treatment comparisons. Eur Heart J 2011;32:252 | Network meta-analysis discussed in present report |
| Steiner S, Moertl D, Chen L, Coyle D, Wells GA. Network meta-analysis of prasugrel, ticagrelor, high- and standard-dose clopidogrel in patients scheduled for percutaneous coronary interventions (provisional abstract). 2012;108;318–27 | Abstract of network meta-analysis discussed in present report |
| Storey RF. Pharmacology and clinical trials of reversibly-binding P2Y12 inhibitors. Thromb Haemost 2011;105(Suppl. 1):75–81 | Not RCT |
| Storey RF, Bliden KP, Patil SB, Karunakaran A, Ecob R, Butler K, et al. Incidence of dyspnea and assessment of cardiac and pulmonary function in patients with stable coronary artery disease receiving ticagrelor, clopidogrel, or placebo in the ONSET/OFFSET study. J Am Coll Cardiol 2010;56:185–93 | Not intervention |
| Testa L, Bhindi R, Van Gaal WJ, Latini RA, Pizzocri S, Lanotte S, et al. What is the risk of intensifying platelet inhibition beyond clopidogrel? A systematic review and a critical appraisal of the role of prasugrel. QJM 2010;103:367–77 | Systematic review |
| Ukena C, Bohm M, Schirmer SH. Hot topics in cardiology: Data from IABP-SHOCK II, TRILOGY-ACS, WOEST, ALTIDUDE, FAME II and more. Clin Res Cardiol 2012;101:861–74 | Meeting review |
| Unger EF. Weighing benefits and risks – the FDA’s review of prasugrel. N Engl J Med 2009;361:942–5 | Summary of FDA review |
| Veverka A, Hammer JM. Prasugrel: a new thienopyridine inhibitor. J Pharm Pract 2009;22:158–65 | Review |
| Wiviott SD. Intensity of antiplatelet therapy in patients with acute coronary syndromes and percutaneous coronary intervention: the promise of prasugrel? Cardiol Clin 2008;26:629–37 | Discussion |
| Wiviott SD. Prasugrel: TRITON-TIMI 38 stent trial. Clin Res Cardiol 2008;97:410 | Abstract of TRITON-TIMI 38 substudy |
| Wiviott SD, Antman EM, Braunwald E. Mortality in the TRITON trial: update from the FDA prasugrel action package. Am J Cardiol 2010;106:293–4 | Response to letter |
| Wiviott SD, Antman EM, Braunwald E. Prasugrel. Circulation 2010;122:394–403 | Review |
| Wiviott SD, Antman EM, Winters KJ, Weerakkody G, Murphy SA, Behounek BD, et al. Randomized comparison of prasugrel (CS-747, LY640315), a novel thienopyridine P2Y12 antagonist, with clopidogrel in percutaneous coronary intervention: results of the Joint Utilization of Medications to Block Platelets Optimally (JUMBO)-TIMI 26 trial. Circulation 2005;111:3366–73 | Dose-ranging trial |
| Wiviott SD, Braunwald E, Murphy SA, Antman EM, Investigators T-T. A perspective on the efficacy and safety of intensive antiplatelet therapy in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-thrombolysis in myocardial infarction 38. Am J Cardiol 2008;101:1367–70 | Response to letter |
| Wiviott SD, Trenk D, Frelinger AL, O’Donoghue M, Neumann FJ, Michelson AD, et al. Prasugrel compared with high loading- and maintenance-dose clopidogrel in patients with planned percutaneous coronary intervention: the Prasugrel in Comparison to Clopidogrel for Inhibition of Platelet Activation and Aggregation-Thrombolysis in Myocardial Infarction 44 trial. Circulation 2007;116:2923–32 | Crossover trial |
| Wouter Jukema J, Collet JP, De Luca L. Antiplatelet therapy in patients with ST-elevation myocardial infarction undergoing myocardial revascularisation: beyond clopidogrel. Curr Med Res Opin 2012;28:203–11 | Review |
| Xanthopoulou I, Theodoropoulos KF, Kassimis G, Gizas V, Tsigkas G, Koutsogiannis N, et al. Ticagrelor vs prasugrel in patients with ST elevation myocardial infarction undergoing primary percutaneous coronary intervention. Eur Heart J 2012;33:41 | Platelet function study |
| Yokoi H, Kimura T, Isshiki T, Ogawa H, Ikeda Y. Pharmacodynamic assessment of a novel P2Y12 receptor antagonist in Japanese patients with coronary artery disease undergoing elective percutaneous coronary intervention. Thrombos Res 2012;129:623–8 | Not patient group |
Appendix 4 Selected data taken from Evidence Review Group report for TA182 appraisal
All data are for the overall population unless otherwise stated.
Summary of baseline characteristics of patients in TRITON-TIMI 38
| Characteristic | Prasugrel (n = 6813) | Clopidogrel (n = 6795) |
|---|---|---|
| UA or NSTEMI (%) | 74 | 74 |
| STEMI (%) | 26 | 26 |
| Age (median) (years) | 61 | 61 |
| ≥ 75 years (%) | 13 | 13 |
| Female (%) | 25 | 27 |
| White race (%) | 92 | 93 |
| Region of enrolment (%) | ||
| North America | 32 | 32 |
| Western Europe | 26 | 26 |
| Eastern Europe | 24 | 25 |
| Middle East, Africa, Asia-Pacific | 14 | 14 |
| South America | 4 | 4 |
| Medical history (%) | ||
| Hypertension | 64 | 64 |
| Hypercholesterolaemia | 56 | 56 |
| Diabetes mellitus | 23 | 23 |
| Tobacco use | 38 | 38 |
| Previous MI | 18 | 18 |
| Previous CABG | 8 | 7 |
| Creatinine clearance < 60 ml/minute | 11 | 12 |
| Index procedure (%) | ||
| PCI | 99 | 99 |
| CABG | 1 | 1 |
| Stent | 94 | 95 |
| Bare-metal stent only | 48 | 47 |
| ≥ 1 drug-eluting stent | 47 | 47 |
| Multivessel PCI | 14 | 14 |
| Timing of study drug administration (%)a | ||
| Before PCI | 26 | 25 |
| During PCI | 73 | 74 |
| After PCI | 1 | 1 |
Primary end point analysis
These results are for the overall trial population (n = 13,608), which includes patients with a history of stroke or TIA. At the end of the trial period, there was a statistically significant reduction in the primary end point in the prasugrel arm compared with the clopidogrel arm. This result was largely attributable to differences in the occurrence of non-fatal MI. The ERG notes that there are no statistically significant differences in mortality (CV death or death from all causes) or non-fatal stroke between the groups.
TRITON-TIMI 38: efficacy results at 15 months (overall cohort)
| End point | Clopidogrel (N = 6795) | Prasugrel (N = 6813) | HR (95% CI) | p-valuea |
|---|---|---|---|---|
| n (%) | n (%) | |||
| Primary | ||||
| Death from CV causes, non-fatal MI or non-fatal stroke | 781 (12.1) | 643 (9.9) | 0.81 (0.73 to 0.90) | < 0.001 |
| Death from CV causes | 150 (2.4) | 133 (2.1) | 0.89 (0.70 to 1.12) | 0.31 |
| Non-fatal MI | 620 (9.5) | 475 (7.3) | 0.76 (0.67 to 0.85) | < 0.001 |
| Non-fatal stroke | 60 (1.0) | 61 (1.0) | 1.02 (0.71 to 1.45) | 0.93 |
| Secondary | ||||
| Death from any cause | 197 (3.2) | 188 (3.0) | 0.95 (0.78 to 1.16) | 0.64 |
| Death from CV causes, non-fatal MI or UTVR | 798 (12.3) | 652 (10.0) | 0.81 (0.73 to 0.89) | < 0.001 |
| Death from CV causes | 150 (2.4) | 133 (2.1) | 0.89 (0.70 to 1.12) | 0.31 |
| Non-fatal MI | 620 (9.5) | 475 (7.3) | 0.76 (0.67 to 0.85) | < 0.001 |
| UTVR | 233 (3.7) | 156 (2.5) | 0.66 (0.54 to 0.81) | < 0.001 |
| Stent thrombosisb | 142 (2.4) | 68 (1.1) | 0.48 (0.36 to 0.64) | < 0.001 |
| Death from CV causes, non-fatal MI, non-fatal stroke or rehospitalisation for ischaemia | 938 (14.6) | 797 (12.3) | 0.84 (0.76 to 0.92) | < 0.001 |
Secondary end points
Statistically significant reductions in favour of prasugrel were found for three secondary clinical end points: (1) composite end point of CV death, non-fatal MI or UTVR; (2) composite end point of death from CV causes, non-fatal MI, non-fatal stroke or rehospitalisation for ischaemia; and (3) stent thrombosis.
Results of the secondary analyses in respect of the primary composite end point were presented at 3 days, 30 days, 90 days and day 4 to day 90. The CEs all show a statistically significant benefit of prasugrel over time.
TRITON-TIMI: primary efficacy outcomes at 3 days, 30 days, 90 days and day 4 to day 90 (overall cohort)
| End point | Time | Clopidogrel (N = 6795) (%) | Prasugrel (N = 6813) (%) | HR for prasugrel (95% CI) | p-value |
|---|---|---|---|---|---|
| Death from CV causes, non-fatal MI, non-fatal stroke | 3 days | 5.6 | 4.7 | 0.82 (0.71 to 0.96) | < 0.01 |
| 30 days | 7.4 | 5.7 | 0.77 (0.67 to 0.88) | < 0.01 | |
| 90 days | 8.4 | 6.8 | 0.80 (0.71 to 0.90) | < 0.001 | |
| Day 4 to 90 | 6.9 | 5.6 | 0.80 (0.70 to 0.93) | < 0.003 | |
| Death from CV causes, non-fatal MI, UTVR | 30 days | 7.4 | 5.9 | 0.78 (0.69 to 0.89) | < 0.01 |
| 90 days | 8.7 | 6.9 | 0.79 (0.70 to 0.90) | < 0.01 |
Prespecified subgroup analyses
The subgroups included in the MS are as follows: UA/NSTEMI, STEMI, males, females, < 65 years, 65–74 years, ≥ 75 years, diabetes mellitus, type of stent, use of glycoprotein IIb/IIIa receptor antagonist, and renal function. The MS presents a forest plot showing the primary efficacy end point results within selected subgroups for the overall trial cohort. The forest plot shows a statistically significant benefit of prasugrel for all subgroups with the exception of females, patients aged ≥ 65 years and patients with creatinine clearance of < 60 ml/minute.
ST segment elevation myocardial infarction patient subgroup
The MS presents data relevant to the STEMI cohort. The relevant text can be found on page 53 of the MS. It is emphasised in the MS that the trial was not powered to compare the effects of prasugrel with clopidogrel in the STEMI population. A total of 3534 STEMI patients were randomised. The primary end point (CV death, non-fatal MI or non-fatal stroke) was statistically significantly reduced with prasugrel at 30 days (HR 0.68, p = 0.002) and 15 months (HR 0.79, 95% CI 0.65 to 0.97; p = 0.02). The secondary end point (CV death, MI or UTVR) was also statistically significantly reduced with prasugrel at 30 days (p = 0.02) and 15 months (p = 0.03). Stent thrombosis and the composite of CV death or non-fatal MI were reported to be statistically significantly reduced with prasugrel at 30 days and 15 months.
At 15 months, no statistically significant difference was reported between the prasugrel arm and the clopidogrel arm of the trial for non-CABG-related TIMI major bleeding (HR 1.11, 95% CI 0.70 to 1.77; p = 0.65). The MS concludes that for STEMI patients who are treated with PCI, prasugrel offers a greater reduction in ischaemic events without an excess risk in major bleeding.
Primary efficacy results for the unstable angina/non-ST segment elevation myocardial infarction, ST segment elevation myocardial infarction and all acute coronary syndrome groups in the TRITON-TIMI 38 trial
TRITON-TIMI 38: primary efficacy for unstable angina/non-ST segment elevation myocardial infarction, ST segment elevation myocardial infarction and all acute coronary syndrome groups (European Public Assessment Report)
Primary efficacy end point and components at study end
| Event | Prasugrel, n (%)a | Clopidogrel, n (%)a | HR (95% CI)b | p-valuec |
|---|---|---|---|---|
| UA/NSTEMI | N = 5044 | N = 5030 | ||
| CV death, non-fatal MI or non-fatal stroke | 469 (9.30) | 565 (11.23) | 0.820 (0.726 to 0.927) | 0.002 |
| CV death | 90 (1.78) | 92 (1.83) | 0.979 (0.732 to 1.309) | 0.885 |
| Non-fatal MI | 357 (7.08) | 464 (9.22) | 0.761 (0.663 to 0.873) | < 0.001 |
| Non-fatal stroke | 40 (0.79) | 41 (0.82) | 0.979 (0.633 to 1.513) | 0.922 |
| All cause death | 130 (2.58) | 121 (2.41) | 1.076 (0.840 to 1.378) | 0.563 |
| All MI | 366 (7.26) | 476 (9.46) | 0.760 (0.663 to 0.871) | < 0.001 |
| All stroke | 49 (0.97) | 46 (0.91) | 1.068 (0.714 to 1.597) | 0.748 |
| STEMI | N = 1769 | N = 1765 | ||
| CV death, non-fatal MI or non-fatal stroke | 174 (9.84) | 216 (12.24) | 0.793 (0.649 to 0.968) | 0.019 |
| CV death | 43 (2.43) | 58 (3.29) | 0.738 (0.497 to 1.094) | 0.129 |
| Non-fatal MI | 118 (6.67) | 156 (8.84) | 0.746 (0.588 to 0.948) | 0.016 |
| Non-fatal stroke | 21 (1.19) | 19 (1.08) | 1.097 (0.590 to 2.040) | 0.770 |
| All cause death | 58 (3.28) | 76 (4.31) | 0.759 (0.539 to 1.068) | 0.113 |
| All MI | 119 (6.73) | 157 (8.90) | 0.748 (0.589 to 0.949) | 0.016 |
| All stroke | 26 (1.47) | 25 (1.42) | 1.032 (0.596 to 1.787) | 0.911 |
| All ACS | N = 6813 | N = 6795 | ||
| CV death, non-fatal MI or non-fatal stroke | 643 (9.44) | 781 (11.49) | 0.812 (0.732 to 0.902) | < 0.001 |
| CV death | 133 (1.95) | 150 (2.21) | 0.886 (0.701 to 1.118) | 0.307 |
| Non-fatal MI | 475 (6.97) | 620 (9.12) | 0.757 (0.672 to 0.853) | < 0.001 |
| Non-fatal stroke | 61 (0.90) | 60 (0.88) | 1.016 (0.712 to 1.451) | 0.930 |
| All cause death | 188 (2.76) | 197 (2.90) | 0.953 (0.781 to 1.164) | 0.639 |
| All MI | 485 (7.12) | 633 (9.32) | 0.757 (0.673 to 0.852) | < 0.001 |
| All stroke | 75 (1.10) | 71 (1.04) | 1.055 (0.763 to 1.460) | 0.745 |
Patients with diabetes mellitus
TRITON-TIMI 38: clinical events by diabetic status
| End point | Clopidogrel (%) | Prasugrel (%) | HR (95% CI) | p-value | p-value for the subgroup analyses that compare diabetes with no diabetes |
|---|---|---|---|---|---|
| Patients without diabetes mellitus | N = 5225 | N = 5237 | |||
| Primary efficacy end point of death from CV causes, non-fatal MI or non-fatal stroke | 10.6 | 9.2 | 0.86 (0.76 to 0.98) | 0.02 | |
| Death from CV causes or MI | 10.0 | 8.5 | 0.85 (0.75 to 0.97) | 0.01 | |
| Fatal or non-fatal MI | 8.7 | 7.2 | 0.82 (0.72 to 0.95) | 0.006 | |
| Death from CV causes | 1.9 | 1.7 | 0.91 (0.68 to 1.23) | 0.53 | |
| Stent thrombosis | 2.0 | 0.9 | 0.45 (0.31 to 0.65) | < 0.001 | |
| Death from CV causes, non-fatal MI, non-fatal stroke or major bleeding event | 12.3 | 11.5 | 0.92 (0.82 to 1.03) | 0.16 | |
| Patients with diabetes mellitus | N = 1570 | N = 1576 | |||
| Primary efficacy end point of death from CV causes, non-fatal MI or non-fatal stroke | 17.0 | 12.2 | 0.70 (0.58 to 0.85) | < 0.001 | 0.09 |
| Death from CV causes or MI | 15.4 | 10.8 | 0.68 (0.56 to 0.84) | < 0.001 | 0.08 |
| Fatal or non-fatal MI | 13.2 | 8.2 | 0.60 (0.48 to 0.76) | < 0.001 | 0.02 |
| Death from CV causes | 4.2 | 3.4 | 0.85 (0.58 to 1.24) | 0.40 | 0.78 |
| Stent thrombosis | 3.6 | 2.0 | 0.52 (0.33 to 0.84) | 0.007 | 0.63 |
| Death from CV causes, non-fatal MI, non-fatal stroke or major bleeding event | 19.2 | 14.6 | 0.74 (0.62 to 0.89) | 0.001 | 0.05 |
TRITON-TIMI 38: bleeding rates by diabetes mellitus status
| End point | Patients with diabetes mellitus (n = 3146) % | Patients without diabetes mellitus (n = 10,462) % | HR (95% CI) | p-value |
|---|---|---|---|---|
| Major non-CABG-related bleeding event | 2.6 | 2.0 | 1.28 (0.97 to 1.68) | 0.08 |
| Major non-CABG-related or minor bleeding event | 4.8 | 4.2 | 1.15 (0.95 to 1.41) | 0.15 |
TRITON-TIMI 38: bleeding rates for prasugrel compared with clopidogrel by diabetes mellitus status
| End point | Clopidogrel % | Prasugrel % | HR (95% CI) | p-value | p-value for the subgroup analyses that compare diabetes with no diabetes |
|---|---|---|---|---|---|
| Patients without diabetes mellitus | N = 5225 | N = 5237 | |||
| Major non-CABG-related bleeding event | 1.6 | 2.4 | 1.43 (1.07 to 1.91) | 0.02 | |
| Major non-CABG-related or minor bleeding event | 3.6 | 4.9 | 1.32 (1.08 to 1.61) | 0.006 | |
| Patients with diabetes mellitus | N = 1570 | N = 1576 | |||
| Major non-CABG-related bleeding event | 2.6 | 2.5 | 1.06 (0.66 to 1.69) | 0.81 | 0.29 |
| Major non-CABG-related or minor bleeding event | 4.3 | 5.3 | 1.30 (0.92 to 1.82) | 0.13 | 0.93 |
Patients with stents
In this group, 6461 patients received bare-metal stents, 5743 patients received drug-eluting stents and 640 patients received both types of stent. In the ‘stented’ group as a whole, the occurrence of the primary end point was reduced in the prasugrel arm compared with the clopidogrel arm (9.7% compared with 11.9%, HR 0.81; p = 0.0001). Similar results were reported for drug-eluting stents and bare-metal stents.
Efficacy and bleeding and net clinical benefit in selected subpopulations
TRITON-TIMI 38: efficacy, bleeding and net clinical benefit in selected populations
| End point | Clopidogrel n/N (%) | Prasugrel n/N (%) | HR for prasugrel (95% CI) | p-value |
|---|---|---|---|---|
| History of stroke or TIA | ||||
| Death from CV causes, non-fatal MI, non-fatal stroke (primary efficacy end point) | 35/256 (14.4) | 47/262 (19.1) | 1.37 (0.89 to 2.13) | 0.15 |
| Non-CABG-related TIMI major bleeding | 6/252 (2.9) | 14/257 (5.0) | 2.46 (0.94 to 6.42) | 0.06 |
| Death from any cause, non-fatal MI, non-fatal stroke, or non-CABG-related non-fatal TIMI major bleeding | 39/256 (16.0) | 57/262 (23.0) | 1.54 (1.02 to 2.32) | 0.04 |
| Aged ≥ 75 years, body weight < 60 kg, or history of stroke or TIA | ||||
| Death from CV causes, non-fatal MI, non-fatal stroke (primary efficacy end point) | 199/1347 (16.0) | 198/1320 (16.1) | 1.02 (0.84 to 1.24) | 0.83 |
| Non-CABG-related TIMI major bleeding | 38/1328 (3.3) | 52/1305 (4.3) | 1.42 (0.93 to 2.15) | 0.10 |
| Death from any cause, non-fatal MI, non-fatal stroke, non-CABG-related non-fatal TIMI major bleeding | 239/1347 (19.0) | 249/1320 (20.2) | 1.07 (0.90 to 1.28) | 0.43 |
TRITON-TIMI 38 recurrent events analysis
This analysis compared the number of subsequent events (after the first event within the primary end point) that occurred within each arm of the trial. More subsequent events were recorded in the clopidogrel arm than in the prasugrel arm (115 compared with 58; p < 0.001).
Appendix 5 Publications related to the TRITON-TIMI 38 trial
| Author/year | Title | Description |
|---|---|---|
| Wiviott et al. 200641 | Evaluation of prasugrel compared with clopidogrel in patients with acute coronary syndromes: design and rationale for the TRial to assess Improvement in Therapeutic Outcomes by optimising platelet InhibitioN with prasugrel Thrombolysis In Myocardial Infarction 38 (TRITON-TIMI 38) | Paper describing the design of the TRITON-TIMI 38 trial |
| Wiviott et al. 2007 36 | Prasugrel versus clopidogrel in patients with acute coronary syndromes | Primary publication of TRITON-TIMI 38 trial |
| Wiviott et al. 201142 | Efficacy and safety of intensive antiplatelet therapy with prasugrel from TRITON-TIMI 38 in a core clinical cohort defined by worldwide regulatory agencies | Paper describing outcomes of ‘core clinical cohort’ of patients from TRITON-TIMI 38 trial: patients no known history of stroke or TIA, aged below 75 years and weighing more than 60 kg. The core clinical cohort represent 10,804 of the 13,608 patients included in the overall trial cohort |
| Antman et al. 2008105 | Early and late benefits of prasugrel in patients with acute coronary syndromes undergoing percutaneous coronary intervention: a TRITON-TIMI 38 (TRial to Assess Improvement in Therapeutic Outcomes by Optimising Platelet InhibitioN with Prasugrel-Thrombolysis In Myocardial Infarction) analysis | Paper reporting on the effects of both the loading dose and the maintenance dose of prasugrel in the TRITON-TIMI 38 trial (n = 13,608) |
| Bonaca et al. 201254 | American College of Cardiology/American Heart Association/European Society of Cardiology/World Heart Federation universal definition of myocardial infarction classification system and the risk of cardiovascular death: observations from the TRITON-TIMI 38 trial (Trial to Assess Improvement in Therapeutic Outcomes by Optimising Platelet Inhibition With Prasugrel-Thrombolysis in Myocardial Infarction 38) | Paper reporting the risk of CV death for patients in the TRITON-TIMI 38 trial according to the individual MI subtypes defined in the universal definition of MI classification system |
| Hochholzer et al. 2011106 | Predictors of bleeding and time dependence of association of bleeding with mortality: insights from the Trial to Assess Improvement in Therapeutic Outcomes by Optimising Platelet Inhibition With Prasugrel-Thrombolysis in Myocardial Infarction 38 (TRITON-TIMI-38) | Paper reporting the major predictors of serious bleeding in patients in the TRITON-TIMI 38 trial |
| Laynez et al. 2011107 | Safety and efficacy for the use of prasugrel in patients undergoing percutaneous coronary intervention and anticoagulated with bivalirudin | Paper presenting the results of a study that compared prasugrel and clopidogrel antiplatelet therapy in patients with ACS undergoing PCI with bivalirudin, rather than heparin, anticoagulation |
| aMega et al. 2009108 | Cytochrome p-450 polymorphisms and response to clopidogrel | Paper reporting an analysis of clinical outcomes for clopidogrel-treated patients who could be classified as carriers or non-carriers of the reduced function CYP2C19 allele (n = 1459) |
| Mega et al. 2010109 | Genetic variants in ABCB1 and CYP2C19 and cardiovascular outcomes after treatment with clopidogrel and prasugrel in the TRITON-TIMI 38 trial: a pharmacogenetic analysis | Paper reporting an analysis of the association between ABCB1 3435C->T and reduced function alleles of CYP2C19 (n = 2932 patients) and clinical outcomes in the TRITON-TIMI 38 trial |
| Michelson et al. 2009110 | Pharmacodynamic assessment of platelet inhibition by prasugrel vs. clopidogrel in the TRITON-TIMI 38 trial | Paper reporting the outcome of analyses of platelet function between prasugrel- and clopidogrel-treated patients (n = 125) in the TRITON-TIMI 38 trial |
| Montalescot et al. 200955 | Prasugrel compared with clopidogrel in patients undergoing percutaneous coronary intervention for ST-elevation myocardial infarction (TRITON-TIMI 38): double-blind, randomised controlled trial | Paper reporting the clinical outcomes for the STEMI subgroup of patients (n = 3534) from the TRITON-TIMI 38 trial |
| Morrow et al. 200952 | Effect of the novel thienopyridine prasugrel compared with clopidogrel on spontaneous and procedural myocardial infarction in the Trial to Assess Improvement in Therapeutic Outcomes by Optimising Platelet Inhibition with Prasugrel-Thrombolysis in Myocardial Infarction 38: an application of the classification system from the universal definition of myocardial infarction | Paper reporting the reassessment of the MIs recorded in the TRITON-TIMI 38 trial using a new universal definition of MI developed by the Joint task force of the ESC, American College of Cardiology Foundation, American Heart Association and World Heart Federation |
| Murphy et al. 2008111 | Reduction in recurrent cardiovascular events with prasugrel compared with clopidogrel in patients with acute coronary syndromes from the TRITON-TIMI 38 trial | Paper reporting on the efficacy of prasugrel compared with clopidogrel in reducing the occurrence of subsequent ischaemic events (following a non-fatal trial event) in the Reduction in recurrent CV events with prasugrel compared with clopidogrel in patients with acute coronary syndromes from the TRITON-TIMI 38 trial |
| O’Donoghue et al. 2009112 | The efficacy and safety of prasugrel with and without a glycoprotein IIb/IIIa inhibitor in patients with acute coronary syndromes undergoing percutaneous intervention: a TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimising Platelet Inhibition With Prasugrel-Thrombolysis In Myocardial Infarction 38) analysis | Paper reporting clinical outcomes for patients who did and did not receive treatment with glycoprotein IIb/IIIa inhibitors during the PCI procedure in the TRITON-TIMI 38 trial |
| aO’Donoghue et al. 2009113 | Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials | Paper reporting clinical outcomes for patients who were treated with proton-pump inhibitors in the PRINCIPLE-TIMI 44 trial (n = 201) and the TRITON-TIMI 38 trial (n = 4529) |
| Pride et al. 2009114 | Effect of prasugrel versus clopidogrel on outcomes among patients with acute coronary syndrome undergoing percutaneous coronary intervention without stent implantation: a TRial to assess Improvement in Therapeutic Outcomes by optimising platelet inhibitioN with prasugrel (TRITON)-Thrombolysis in Myocardial Infarction (TIMI) 38 substudy | Paper reporting the clinical outcomes of patients (n = 569) who did not receive stents as part of the PCI procedure in the TRITON-TIMI 38 trial |
| Pride et al. 2010115 | Angiographic and clinical outcomes among patients with acute coronary syndromes presenting with isolated anterior ST-segment depression: a TRITON-TIMI 38 (Trial to Assess Improvement in Therapeutic Outcomes by Optimising Platelet Inhibition With Prasugrel-Thrombolysis In Myocardial Infarction 38) substudy | Paper reporting clinical outcomes for a subgroup of patients (n = 1198) with isolated anterior ST-segment depression on 12-lead electrocardiogram in the TRITON-TIMI 38 trial |
| Riesmeyer et al. 2012116 | Relationship between exposure to prasugrel active metabolite and clinical outcomes in the TRITON-TIMI 38 substudy | Paper reporting the outcomes of a study designed to identify the effect of increased exposure to the prasugrel active on bleeding risk |
| Ruff et al. 2012117 | Safety and efficacy of prasugrel compared with clopidogrel in different regions of the world | To determine whether or not there were differential effects of prasugrel compared with clopidogrel in the TRITON-TIMI 38 study according to geographical region |
| Scirica et al. 2012118 | Timing and clinical setting of cardiovascular death or myocardial infarction following PCI for ACS-observations from the TRITON-TIMI 38 trial | Paper reporting the outcomes of an analysis from the TRITON-TIMI 38 study of the time of occurrence of new cardiac events (MI/stent thrombosis) and the setting of those events (peri procedural/procedural/spontaneous) |
| Smith et al. 2012119 | Mortality benefit with prasugrel in the TRITON-TIMI 38 coronary artery bypass grafting cohort: risk-adjusted retrospective data analysis | The objective of this study was to characterise the bleeding, transfusion and other outcomes of patients related to the timing of prasugrel or clopidogrel withdrawal before CABG |
| Udell et al. 2011120 | Benefit of prasugrel in ST-elevation myocardial infarction according to timing of percutaneous coronary intervention: Insight from the TRITON-TIMI 38 study | Conference abstract reporting the clinical outcomes of the STEMI subgroup of patients (n = 3534) from the TRITON-TIMI 38 trial. A sensitivity analysis that after the exclusion of procedural MIs |
| Wiviott et al. 2008121 | Greater clinical benefit of more intensive oral antiplatelet therapy with prasugrel in patients with diabetes mellitus in the trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel-Thrombolysis in Myocardial Infarction 38 | Paper reporting the clinical outcomes for the subgroup of patients with diabetes mellitus (n = 3146) from the TRITON-TIMI 38 trial |
| Wiviott et al. 2008122 | Intensive oral antiplatelet therapy for reduction of ischaemic events including stent thrombosis in patients with acute coronary syndromes treated with percutaneous coronary intervention and stenting in the TRITON-TIMI 38 trial: a sub-analysis of a randomised trial | Paper reporting the outcomes for the subgroup of patients from the TRITON-TIMI 38 trial who were treated with stents (n = 12,844) |
| Wrishko et al. 2009123 | Population pharmacokinetic analyses to evaluate the influence of intrinsic and extrinsic factors on exposure of prasugrel active metabolite in TRITON-TIMI 38 | Pharmacodynamic substudy of TRITON-TIMI 38 |
Appendix 6 Definition of the decision problem and patient populations and details of the independent economic model
Definition of the decision problem and patient populations
This section is relevant to Sections 4.1 and 6.3.2 of the Assessment Report124 and is intended to provide the rationale for the specific patient populations considered by the AG in the Assessment Report.
The final scope7 issued by NICE (described in table 4 of the Assessment Report) for this appraisal identifies the relevant population as patients with ACS undergoing primary or delayed PCI. It further states that if the evidence allows, subgroups of patients will be considered, including people with UA/NSTEMI, STEMI and people with diabetes mellitus. Finally, the scope specifies that guidance will only be issued in accordance with the marketing authorisation.
The remit of the AG was to appraise the clinical effectiveness and cost-effectiveness of prasugrel within its licensed indication for the treatment of ACS with PCI and was a review of an existing technology appraisal, TA182. 21
No new RCT evidence for prasugrel has been published and the clinical evidence base for the effectiveness of prasugrel remains unchanged from that considered for TA182. 21 The AG has therefore taken its starting position for this multiple technology appraisal as a reassessment of the evidence from TA182. 21
Core clinical cohort
The evidence for TA18221 was based on a single RCT, the TRITON-TIMI-3836 trial. The TRITON-TIMI-3836 trial included 13,608 patients with ACS who were to be treated with PCI. The relevance of the evidence from the overall TRITON-TIMI 3836 trial population was constrained by the marketing authorisation, which excludes patients with prior stroke or TIA and patients with active peptic ulcer disease, and restricts use in patients over the age of 75 years and in those weighing < 60 kg to a lower 5-mg dose to limit the risk of severe bleeding.
In their evidence submission for TA182,21 the manufacturer identified a reduced population from the TRITON-TIMI-3836 trial that they referred to as the ‘target population’, and the manufacturer considered the ‘target population’ to be the most relevant for providing data for the development of the economic model. The ERG and the AC agreed with this selection, as the excluded patients were either explicitly excluded from the marketing authorisation or were not supported by trial evidence (as the trial was based on the full 10-mg dose). It is therefore this ‘target population’ that is the focus of the Assessment Report and is described as the ‘core clinical cohort’ in the manufacturer’s latest evidence submission (review of TA1822). This cohort comprised 10,804 (79%) patients from the overall trial population.
Specific subpopulations identified in the Assessment Report
During the process of TA182,2 an appraisal consultation document was issued (June 2009) that restricted the use of prasugrel to patients undergoing PCI as primary treatment for patients with a STEMI event, as well as those suffering stent thrombosis while under treatment with clopidogrel. In a response to the appraisal consultation document, the manufacturer of prasugrel suggested that several other high-risk patient groups should also be considered, in particular those diagnosed with diabetes.
In preparation for the second meeting of the AC, the Chairperson requested that the ERG should provide cost-effectiveness estimates relating to four mutually exclusive subgroups defined by the type of index event (STEMI compared with UA/NSTEMI) and whether or not patients were diagnosed with diabetes mellitus. These results were provided by the ERG and then considered by the AC. The AC concluded that prasugrel could be recommended for three of the four subgroups, but that the results for UA/NSTEMI non-diabetic patients did not support a positive recommendation.
As the evidence base has not changed since the publication of TA182,2 the AG has taken the view that the review of the existing guidance should involve a reassessment of the same subgroups of the same trial cohort and has developed its economic model on this basis.
Independent economic model
The purpose of this section is to provide further information relevant to the independent economic model in respect of the model design and structure (section 6.3.4 of the Assessment Report), the data source for the key patient groups of the core clinical cohort (page 36 of the Assessment Report) and parameter sources and values (section 6.3.6 of the Assessment Report).
The manufacturer’s decision model comprises two parts:
-
a statistical model to represent the main clinical outcomes of the trial during the first 12 months of follow-up until the trial treatments clopidogrel or prasugrel have finished
-
a long-term model based on modified life table data to represent survival for up to an additional 39 years.
The AG found the short-term statistical model to be an accurate representation of the reported trial outcomes and was content to employ the results of this part of the manufacturer’s model unaltered. The specifications of the statistical outcome functions are shown in table 17 of the AG report. However, the AG considers that the long-term life table extrapolation is unrealistically simple and does not adequately represent the likelihood of patients suffering multiple additional CV events in their lifetime and the associated disutility and costs associated with such events.
The AG has therefore extracted the outcomes from the manufacturer’s short-term model for the four mutually exclusive subgroups of the ‘core clinical cohort’ and employed these as the initial conditions for surviving patients entering the AG’s long-term state-transition model.
The details of these outcome data from the manufacturer’s short-term model are fully described in table 28 of the AG report, covering 10,314 patients in the original ‘core clinical cohort’ (but excluding additionally those with peptic ulcer disease who had been previously included in the manufacturer’s analysis despite the explicit contraindication shown in the SPC). Costs, survival time and utility/disutility values during the first year (short term) are estimated on the same basis as in the AG’s long-term model. Specific clinical data relating to patients with STEMI, UA/NSTEMI or diabetes mellitus in the core clinical cohort were not available from the MS or the most recent publication.
The possible interstate transitions from year to year in the AG’s long-term model are represented in detail in table 27 of the AG report. Figure 16 provides a graphic representation.
FIGURE 16.
If no event occurs in year, the patient remains in the same health state. D, disabled; ND, not disabled. Boxes with green edges represent health states; boxes with black edges represent events. Green shading represents a ‘sink state’ and boxes without shading represent temporal states. The dashed lines linking ‘prior stroke ND’ states at the beginning and end of a year indicate that a new non-disabling stroke event can occur. The blue dashed lines represent the transition to death. The solid lines linking ‘prior stroke D’ states at the beginning and end of a year indicate that this pathway is mandatory for any patient who suffered a prior disabling stroke, regardless of the severity of stroke suffered during the year.

The main source of data used to populate the AG’s long-term model is the CAPRIE92 clinical trial. This was a double-blind placebo comparison of clopidogrel with aspirin involving 19,185 patients with atherosclerotic vascular diseases manifested as either ischaemic stroke (IS), MI or symptomatic peripheral arterial disease. Only CAPRIE92 data from 5741 MI patients without prior history of other vascular events were used to populate the AG’s long-term model. Follow-up of patients continued for up to 3 years (mean 1.9 years). The primary outcome was the first occurrence of IS, MI or vascular death. Secondary outcomes included: the first occurrence of IS, MI, amputation or vascular death; vascular death; overall net benefit; any stroke (including primary intracranial haemorrhage), MI or death from any cause; and death from any cause.
The manufacturer of clopidogrel kindly carried out extensive reanalyses of the CAPRIE92 trial data as specified by the AG, in order to estimate independent event hazards adjusted to age, sex and event history. Full details of the estimated event rates (appendix 10) and event fatality rates (appendix 11) are provided in the full AG report for TA210.
Appendix 7 Details of the PLATelet inhibition and patient Outcomes trial
Key trial characteristics
The recommendations made in the NICE guidance TA23622 were based on a single RCT known as the PLATO33 trial. The PLATO33 trial was an international, multicentre, double-blind, double-dummy Phase III trial comparing ticagrelor plus aspirin with clopidogrel plus aspirin in 18,624 patients admitted to hospital with ACS with or without STEMI. It is important to note that patients were randomised to the trial irrespective of planned intervention and, therefore, the patient population included ACS patients who were to be medically managed as well as those who were to undergo PCI. The trial follow-up was for 12 months, however, the AG notes that the trial protocol stipulated that once the requisite number of events (1780) had accrued, patients were required to leave the trial after their 6-month or 9-month visit. The key trial characteristics are described in the table below.
PLATO trial key characteristics
| PLATO trial design and patients | Intervention/comparator | Key inclusion criteria | Key exclusion criteria | Outcomes |
|---|---|---|---|---|
|
|
|
|
|
PLATelet inhibition and patient Outcomes trial outcomes
The results of the PLATO33 trial for the overall trial population at 12 months showed a statistically significant benefit of ticagrelor was found for the primary composite end point [9.8% compared with 11.67% (HR 0.84, 95% CI 0.77 to 0.92; p < 0.001)]. When the individual components of the composite end point are disaggregated, the reduction in the primary end point is driven by statistically significant reductions in death from vascular causes (HR 0.79, 95% CI 0.69 to 0.91; p = 0.001) and MI (HR 0.84, 95% CI 0.75 to 0.95; p = 0.005).
A novel system for categorising bleeding events was utilised in the PLATO33 trial. There were no statistically significant differences between the two arms of the trial for the end points of PLATO major bleed (primary safety end point) and PLATO major fatal/life-threatening bleed; however, statistically significant differences in favour of clopidogrel are in evidence for the end points of PLATO total major + minor bleed (HR 1.11, 95% CI 1.03 to 1.20; p=0.008) and PLATO non-CABG major bleed (HR 1.19, 95% CI 1.02 to 1.38; p = 0.03).
PLATelet inhibition and patient Outcomes trial subgroup analyses
The results of analyses that assess the clinical effectiveness of ticagrelor compared with clopidogrel in the range of patient populations included in the PLATO33 trial are summarised in the table below. The patient populations include people intended for early angiography, people managed medically, people with STEMI, people who were treated with CABG and people with diabetes. With the exception of the subgroup of patients treated with CABG and people with diabetes, a statistically significant benefit for ticagrelor compared with clopidogrel is recorded.
| Trial name | Patient group (n) | Ticagrelor (KM%/12 months) | Clopidogrel (KM%/12 months) | HR (95% CI) | p-value |
|---|---|---|---|---|---|
| PLATO | All ACS (18,624) | 9.8 | 11.7 | 0.84 (0.77 to 0.92) | < 0.001 |
| PLATO-INVASIVE | Intended for early angiography (13,408) | 9.0 | 10.7 | 0.84 (0.75 to 0.94) | 0.0025 |
| PLATO-MEDICAL | Conservative management (5216) | 12.0 | 14.3 | 0.85 (0.73 to 1.00) | 0.04 |
| PLATO-STEMI | STEMI with PCI | ||||
| STEMI or LBBB at presentation (7544) | 9.4 | 10.8 | 0.87 (0.75 to 1.01) | 0.07 | |
| LBBB/STEMI at presentation or STEMI at discharge (8430) | 9.3 | 11.0 | 0.85 (0.74 to 0.97) | 0.02 | |
| PLATO-CABG | CABG (1261) | 10.6 | 13.1 | 0.84 (0.60 to 1.16) | 0.29 |
| PLATO-DIABETES | With diabetes mellitus (4622) | 14.1 | 16.2 | 0.88 (0.76 to 1.03) | NR |
| Without diabetes mellitus (13,951) | 8.4 | 10.2 | 0.83 (0.74 to 0.93) | NR |
PLATO health-related quality of life
The PLATO33 trial included a Health Economics and Quality of Life substudy. This substudy employed the paper version of the EQ-5D questionnaire and the manufacturer converted the EQ-5D scores to utility values to inform the cost-effectiveness analyses presented in the manufacturer’s submission for TA236 using the UK tariff weightings.
Of the total number of 18,624 patients, 15,212 (82%) had a utility score calculated at discharge from the index hospitalisation (visit 1). At visit 4 (6 months) and visit 6 (12 months) the percentage of patients in the full cohort with a utility score was 80% and 79%, respectively. Of the 10,686 patients who were eligible for a 12-month follow-up (referred to as the 12-month cohort), 8840 (83%) had a utility score calculated at visit 1. The corresponding percentage of patients in the 12-month cohort with utility score at visit 4 and visit 6 was 81% and 80%, respectively.
No differences were found between ticagrelor and clopidogrel for any of the items on the EQ-5D.
Appendix 8 Key characteristics of identified indirect comparisons of prasugrel and ticagrelor
| Publication | Objective | Trials included; length of follow-up | Comparator 1 | Comparator 2 | Patient group (n) | Primary outcomes of the meta-analysis |
|---|---|---|---|---|---|---|
| Biondi-Zoccai et al. 201157 | To perform an indirect comparison meta-analysis of prasugrel vs. ticagrelor in patients with ACS | TRITON-TIMI 38 2007;36 15 months | Prasugrel; 60-mg LD/10 mg daily | Clopidogrel 300-mg LD/75 mg daily | All ACS (13,608) |
|
| PLATO 2009;33 9 months | Ticagrelor; 180-mg LD/90 mg twice daily | Clopidogrel 300- to 600-mg LD/75 mg daily | All ACS (18,624) | |||
| DISPERSE-2 2007;63 3 months | Ticagrelor; 90 mg twice dailya | Clopidogrel 300-mg LD/75 mg daily | NSTEMI (661) | |||
| Passaro et al. 201159 | Presentation of a simplified network meta-analysis graph to improve the communicative value of the analysis by Biondi-Zoccai | TRITON-TIMI 38 2007;36 15 months | Prasugrel | Clopidogrel 300-mg LD/75 mg daily | All ACS (13,608) |
|
| PLATO 2009;33 9 months | Ticagrelor | Clopidogrel 300- to 600-mg LD/75 mg daily | All ACS (18,624) | |||
| CURE 2001;64 3–12 months | Clopidogrel 300-mg LD/75mg daily | Placebo | NSTEMI (12,562) | |||
| Chatterjee et al. 201358 | To compare the relative efficacies of prasugrel and ticagrelor in the reduction of meaningful clinical end points in patients with ACS or CAD intended for PCI treatment using a network meta-analysis of published data | TRITON-TIMI 38 2007;36 15 months | Prasugrel | Clopidogrel 300-mg LD/75 mg daily | All ACS (13,608) |
|
| PLATO 2009;33 9 months | Ticagrelor | Clopidogrel 300- to 600-mg LD/75 mg daily | All ACS (18,624) | |||
| DISPERSE-2 2007;63 3 months | Ticagrelor; 90 mg twice dailya | Clopidogrel 300-mg LD/75 mg daily | NSTEMI (661) | |||
| JUMBO-TIMI 26 2005;38 30 days | Prasugrel; three different dosing regimens | Clopidogrel 300-mg LD/75 mg daily | ACS intended for PCI (904) | |||
| Steiner et al. 201260 | To compare the efficacy and safety of prasugrel, ticagrelor and high-dose clopidogrel in patients undergoing PCI | Abuzahra 2008;125 30 days | Clopidogrel 600-mg LD/150 mg daily | Clopidogrel 300-mg LD/75 mg daily | ACS: 44%, SCAD: 56% (119) |
|
| Angiolillo 2008;126 30 days | Clopidogrel 600-mg LD/150 mg daily | Clopidogrel 300-mg LD/75 mg daily | SCAD (40) | |||
| DOSER 2010;66 30 days | Clopidogrel 600-mg LD/150 mg daily | Clopidogrel 300-mg LD/75 mg daily | SCAD, HTPR (74) | |||
| DOUBLE 2010;127 1 month | Clopidogrel 300mg LD/150 mg daily | Clopidogrel 300mg LD/75 mg daily | STEMI (54) | |||
| GRAVITAS 2011;128 6 months | Clopidogrel 300- to 600-mg LD/150 mg daily | Clopidogrel 300- to 600-mg LD/75 mg daily | ACS: 40%, SCAD: 60%, HTPR: 100% (2214) | |||
| Han et al. 2009;65 30 days | Clopidogrel 600-mg LD/150 mg daily | Clopidogrel 600-mg LD/75 mg daily | ACS (813) | |||
| OASIS 7 PCI 2010;46 30 days | Clopidogrel 600-mg LD/150 mg daily | Clopidogrel 300-mg LD/75 mg daily | ACS (17,263) | |||
| VASP-02 2008;129 14 days | Clopidogrel 300- to 600-mg LD/150 mg daily | Clopidogrel 300- to 600-mg LD/75 mg daily | Stable CAD (153) | |||
| Von Beckerath et al. 2007;130 30 days | Clopidogrel 600-mg LD/150 mg daily | Clopidogrel 300- to 600 mg LD/75 mg daily | Stable CAD (60) | |||
| JUMBO-TIMI 26 2005;38 30 days | Prasugrel; three different dosing regimens | Clopidogrel 300-mg LD/75 mg daily | ACS intended for PCI (904) | |||
| TRITON-TIMI 38 2007;36 15 months | Prasugrel | Clopidogrel 300-mg LD/75 mg daily | All ACS (13,608) | |||
| Alexopolous 2011;131 30 days | Clopidogrel 600-mg LD/10 mg prasugrel daily | Clopidogrel 300- to 600-mg LD/150 mg daily | ACS: 70%, Stable CAD: 30%, HTPR: 100% (71) | |||
| PRINCIPLE-TIMI 44 2007;132 15 days | Prasugrel 60-mg LD/10 mg daily | Clopidogrel 600-mg LD/150 mg daily | Stable CAD (201), 55% PCI | |||
| PLATO INVASIVE 2009;56 9 months | Ticagrelor 180-mg LD/180 mg daily | Clopidogrel 300- to 600-mg LD/75 mg daily | ACS (13,408), 77% PCI |
Appendix 9 Quality assessment of identified indirect comparisons of prasugrel and ticagrelor
None of the indirect comparisons stated whether or not the design was a priori. Biondi-Zoccai et al. 57 did not perform a comprehensive search strategy, assess the quality of included studies or assess publication bias. Chatterjee et al. 58 did not state whether or not there was duplicate selection or data extraction and did not provide a list of excluded studies or study characteristics. They also did not provide a breakdown of results of the quality assessment or use it in formulating conclusions, although they did state that all included studies were judged to be at a low risk of bias. The assessment was not applicable to the article by Passaro et al. 59 as the primary aim of this was to present a simplified network meta-analysis graph based on the review by Biondi-Zoccai et al. 57 The review by Steiner et al. 60 did not provide a list of excluded studies, assess publication bias or use the quality assessment in formulating conclusions.
Quality of the identified indirect comparisons
| Review | A priori design provided? | Duplicate selection/data extraction? | Comprehensive literature search? | Publication status used as an inclusion criterion? | List of studies provided? | Study characteristics provided? | Scientific quality of included studies assessed? | Scientific quality of included studies used appropriately? | Appropriate methods used to combine findings? | Publication bias assessed? | COIs stated? |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Biondi-Zoccai et al. 201157 | NS | NS | No | Yes | Yes | Yes | No | No | Yes | No | Yes |
| Chatterjee et al. 201358 | NS | NS | Yes | Yes | No, excluded studies not given | No | Yesa | No | Yes | Yes | Yes |
| Passaro et al. 201159 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Steiner et al. 201260 | NS | Yes | Yes | Yes | No, excluded studies not given | Yes | Yes | No | Yes | No | Yes |
List of abbreviations
- AC
- Appraisal Committee
- ACS
- acute coronary syndrome
- AG
- Assessment Group
- BCIS
- British Cardiovascular Intervention Society
- BNF
- British National Formulary
- CABG
- coronary artery bypass grafting
- CAPRIE
- Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events
- CEAC
- cost-effectiveness acceptability curve
- CI
- confidence interval
- CURE
- Clopidogrel in Unstable angina to prevent Recurrent Events
- CURRENT-OASIS
- Clopidogrel and Aspirin Optimal Dose Usage to Reduce Recurrent Events−Seventh Organization to Assess Strategies in Ischemic Syndromes
- CV
- cardiovascular
- DISPERSE-2
- Dose confIrmation Study assessing anti-Platelet Effects of AZD6140 vs. clopidogRel in non–ST segment Elevation myocardial infarction 2
- DRG
- diagnostic-related group
- ECG
- electrocardiograph
- EMA
- European Medicines Agency
- EQ-5D
- European Quality of Life-5 Dimensions
- ERG
- Evidence Review Group
- FDA
- United States Food and Drug Administration
- GDG
- guideline development group
- HR
- hazard ratio
- HRQoL
- health-related quality of life
- ICER
- incremental cost-effectiveness ratio
- IS
- ischaemic stroke
- ISAR-REACT
- Intracoronary Stenting and Antithrombotic Regimen: Rapid Early Action for Coronary Treatment
- JUMBO-TIMI
- Joint Utilization of Medications to Block Platelets Optimally – Thrombolysis in Myocardial Infarction
- MI
- myocardial infarction
- MINAP
- Myocardial Ischaemia National Audit Project
- MS
- manufacturer submission
- NICE
- National Institute for Health and Care Excellence
- NSTEMI
- non-ST segment elevation myocardial infarction
- OXVASC
- Oxford Vascular Study
- PCI
- percutaneous coronary intervention
- PLATO
- PLATelet inhibition and patient Outcomes trial
- QALY
- quality-adjusted life-year
- RCT
- randomised controlled trial
- RR
- relative risk
- SIGN
- Scottish Intercollegiate Guidelines Network
- SPC
- Summary of Product Characteristics
- STA
- single technology appraisal
- STEMI
- ST segment elevation myocardial infarction
- TIA
- transient ischaemic attack
- TIMI
- thrombolysis in myocardial infarction
- TRITON-TIMI
- Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition with Prasugrel Thrombolysis in Myocardial Infarction
- UA
- unstable angina
- UTVR
- urgent target vessel revascularisation