Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/31/02. The contractual start date was in February 2012. The draft report began editorial review in October 2013 and was accepted for publication in April 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Professor Sniehotta is a co-applicant on a National Institute for Health Research (NIHR) Career Development Fellowship for Jean Adams, University of Newcastle (title: Financial incentives for health promoting behaviours). Professor Sniehotta is also a co-applicant on a related grant from the NIHR Health Technology Assessment (HTA) programme [title: Parental incentives and quasi-mandatory schemes for increasing uptake of immunisations in pre-school children (September 2012–July 2014). J Adams, B Bateman, B Gardner Sood, S Michie, J Shucksmith, FF Sniehotta, T Cresswell, L Ternant. Value: £275,419.00]. Professor Linda Bauld is chief investigator on a NIHR HTA grant [title: Facilitators and barriers to smoking cessation in pregnancy (May 2013–April 2015). Bauld L, Graham H, Sinclair L, Flemming K, Naughton F, Tappin D, Gorman D. Value: £250,753.00]. Professor Bauld is also coprincipal investigator on a study funded by the Chief Scientist Office, Scottish Government Health and Social Care Directorates, and the Glasgow Centre for Population Health and NHS Greater Glasgow and Clyde [title: Cessation in Pregnancy Incentives Trial (CPIT) (February 2011–December 2013). Tappin D, Bauld L, Briggs A and colleagues. Value: £850,000.00]. Professor David Tappin is co-applicant on a NIHR HTA grant [title: Facilitators and barriers to smoking cessation in pregnancy (May 2013–April 2015). Bauld L, Graham H, Sinclair L, Flemming K, Naughton F, Tappin D, Gorman D. Value: £250,753.00]. Professor Tappin is also coprincipal investigator on a study funded by the Chief Scientist Office, Scottish Government Health and Social Care Directorates, and the Glasgow Centre for Population Health and NHS Greater Glasgow and Clyde [title: Cessation in Pregnancy Incentives Trial: The CPIT (February 2011–December 2013). Tappin D, Bauld L, Briggs A and colleagues. Value: £850,000.00].
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Morgan et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
This monograph reports the findings of the Benefits of Incentives for Breastfeeding and Smoking cessation in pregnancy (BIBS) study. The BIBS study was funded to conduct evidence syntheses and primary qualitative and survey research, together with a discrete choice experiment (DCE), to develop an incentive taxonomy and to inform the design of an acceptable and feasible incentive intervention(s) with promise for improving smoking cessation in pregnancy and/or breastfeeding outcomes.
In this chapter we briefly describe what incentives are and how they are proposed to work to change behaviour. They are presented in the context of designing complex intervention trials to improve health outcomes. The health implications of smoking in pregnancy and breastfeeding are then considered with a brief history of incentives for these behaviours and the current policy context. The chapter concludes with the aims of the BIBS study and an overview of the chapters included in this report.
What are incentives and how do they work?
The Oxford English Dictionary1 defines an incentive as ‘a thing that motivates or encourages one to do something’. It is derived from the Latin word incentivum, ‘something that sets the tune or incites’, from incantare, ‘to chant or charm’. Incentives may be direct, as in a reward for attaining a goal, or indirect, for example reduced-price products or services. A financial incentive may also be a penalty imposed for not achieving a goal. Kane and colleagues2 argue that definitions of incentives often fail to distinguish their function and seldom consider the incentive within the larger environmental context. They propose that an incentive can function as a goal; as an external reinforcement of behaviour with the aim of increasing an individual’s internal motivation until it is sufficient; as reinforcement until learning is accomplished and a habit has formed; or as a way of focusing a person’s attention to a neglected area. For these reasons, we consider a broad definition of incentives delivered to and by a range of individuals and organisations in this study, as described later in this chapter.
Little is known about how incentives work in health care, how they might facilitate rather than erode informed choice and, crucially, how time and context modify effects. 3,4 In addition, new knowledge about behaviour, provided by neuroscience, is increasing rapidly and is beginning to inform theory. Incentive, motivation and behaviour theories are multitudinous, often overlap and are derived from a wide spectrum of disciplines besides health, including economics, psychology, sociology, education, law, business organisation and social policy. A review of these theories and of the neuroscience of incentives and rewards is beyond the scope of this study. Indeed, it has been asserted that there are too many theories of behaviour, with inherent problems around classification, labelling and the science underpinning the choice of theory. 5 In addition, Wise6 suggests that ‘Most writers have not come to grips with the problem of differentiating motivation from everything else’ (p. 161) with respect to human behaviour. This parallels the conclusion of Johnston and Dixon5 that there are gaps in the science of behaviour as applied to health. It is uncertain to what extent evidence and theories of incentives derived from non-health contexts are generalisable as this is a relatively new field of human behaviour research. Theories informing incentive interventions range from those targeting the individual only, which often stem from psychology and economics, to those that embrace the social network or apply a more ecological or systems approach to behaviour change. Economic theories focus on the values, costs and benefits of incentive interventions and can span all of these approaches. As many incentive interventions are multicomponent and complex,7 more than one theory of behaviour change from more than one discipline may explicitly or implicitly apply in an incentive programme.
The following paragraphs provide a brief overview of theories selected by the research team because they were considered particularly relevant to the BIBS study of incentives for health behaviour change in relation to smoking cessation in pregnancy and breastfeeding. By definition, incentives serve as motivators. One motivational theory,6,8 discussed in the context of neuroscience research and substance misuse, describes three main variables for motivation: drive, incentive and reinforcement. This builds on the work of Skinner,9 who described the reinforcement of behaviours through rewards. A similar distinction between incentive and reinforcement is made by Michie and colleagues10 in a recent taxonomy of behaviour change techniques (BCTs). Drive and incentive precede behaviour and energise it; reinforcement follows the first enactment of behaviour to establish memory and other processes that strengthen and sustain it, for example pleasure. Intrinsic drive exerts a push effect on action and extrinsic factors such as incentives exert a pull effect on action. Consistent incentives, and their reinforcement, result in conditioned learned responses, which establish behaviour.
In early incentive studies, for example that by Higgins and colleagues,11 individual learning theory and operant conditioning theory are applied whereby the behaviour is influenced or controlled by the consequences. Learning theories assume that incentives will increase the target behaviour and that withdrawing the incentives should result in the behaviour stopping. They are rooted in social cognitive theories, such as social learning theory12 and the theory of planned behaviour,13 and hypothesise that people deliberately consider the balance of anticipated positive and negative consequences of their behaviour. From this perspective, incentives might tip the balance towards a desirable behaviour. In addition, incentives can also be explained by expectancy-value models, in which an individual forms beliefs about the consequences of a behaviour, attributes a value to these beliefs and incentives can change the value and the likelihood of the behaviour occurring. Social cognition models, like the theory of planned behaviour, are developments of expectancy-value models. 13
It is important to understand the fit of incentives and rewards with more general theories of behaviour, such as dual process models, which have proposed that behaviour is underpinned by two separate, but interacting, systems: the cognitive, rational and reflective system as well as an impulsive, emotional, automatic system. 14,15 Although the relative importance of these two systems is often contested, incentives may exert their effects through either of these two behavioural systems. More specifically, Dixon and Johnston16 have recently proposed a cognitive route MAP (i.e. Motivation development, Action on motivation or Prompted/cued behaviour) to behaviour change. Whereas the motivation and action routes can be allocated to the reflective system of dual process models, the prompted route is akin to the automatic system. MAP has been used to inform interventions by organising 89 BCTs as affecting behaviour through either of these three proposed MAP routes. 16 Rewards delivered contingent on the behaviour are thereby considered to act via the prompted route, which bypasses the reflective system. Incentives, on the other hand, in which the actor is aware that a reward will be delivered contingent on a target behaviour or goal, were not considered in MAP, yet incentives have the potential to develop and to influence behaviour via the motivational route. The most recent taxonomy of BCTs17 lists 93 hierarchically clustered techniques agreed through expert consensus methods and includes both incentives (i.e. informing someone that future rewards or removal of future punishment will be contingent on performance of the behaviour) and rewards (i.e. arranging the delivery of a reward if there has been effort and/or progress towards performing the behaviour).
In a recent review of behaviour change intervention development frameworks, Michie and colleagues10 have systematically integrated psychological theory (in line with aspects of dual processing models and MAP) together with intervention functions (including BCTs) and policy categories. At the heart of the wheel is the COM-B system, which refers to three proposed essential conditions of a Behaviour system: Capability, Opportunity and Motivation. The outer layer of the wheel contains nine intervention functions, one of which is incentives. The intervention functions address deficits in the COM-B system and an outer ring of seven policy categories is outlined as a way to draw in specific intervention functions. Applying the behaviour change wheel to incentives highlights the complexity of incentive intervention development, both in terms of the mechanisms of action as well as from a policy perspective. Thus, to develop a successful incentive-based intervention, it is paramount to take into account which sources of behaviour (or route to behaviour within the MAP framework) are being targeted as well as how the intervention can be stimulated from a policy perspective to integrate evidence-based behaviour change practice into routine care.
It can be argued that wider systems and ecological theory are relevant to understanding incentive interventions as occurring within a complex sociocultural milieu. Such approaches view incentives as having multiple interactions at different levels: between personal attributes, situations and the local and distal features of place, including their structural attributes, cultures and meanings. 18,19 Individual and national economic milieu, media influences and local incentive cultures within schools, workplaces and shops are all likely to impact on incentive outcomes. For example, in a study by Allen and colleagues,20 it was found that a central reason for disengagement in a smoking cessation incentive programme was the change in the contractual relationship between the pharmacist and the client. Some participants associated attending the pharmacy with attending for methadone distribution, which pharmacists supervise, and were concerned about the potential stigma involved, which enhanced feelings of failure, guilt and shame.
In addition to the proposed mechanisms of action for the incentives described above, there are effect moderators operating through incentive delivery processes. These usually include a variety of associated activities and relationships, for example to establish behavioural targets, agree to a commitment contract, monitor performance or provide additional BCTs. 21 Incentives are unlikely to be similarly effective across all types of behaviour or in all contexts. Within health, differences would certainly be anticipated when comparing incentives for habitual behaviour change, in particular when overcoming a physiological addiction (such as smoking), and incentives for the establishment of a new skilled, performing behaviour (such as breastfeeding). Tversky and Kahneman22 propose that changes in behaviour may be more likely to be triggered to avoid losses than to realise rewards, and Deci and Ryan23 consider intrinsic motivation to be as important a factor in explaining human behaviour as extrinsic motivators such as economic incentives. Indeed, there are some surprising findings from studies that demonstrate changes of behaviour despite only a small proportion of the incentives offered actually being redeemed. 2 This suggests that mechanisms other than the actual incentive are responsible, for example triggering a socially desirable response, yet this has been relatively underexplored. Deci and colleagues24 argue that incentives can actually inhibit intrinsic motivation, with associated perceptions of ‘paternalism’ interfering with effectiveness. This complexity is compounded by the fact that these behaviours are socially, culturally and environmentally situated and have different meanings, which can change rapidly for the actors involved.
From the perspective of economic theory, financial incentives are used to change the behaviour of health-care consumers and providers to induce an optimal level of supply. The use of incentives increases the benefits associated with activities that require greater levels of effort, and so changes the costs and benefit balance associated with choices. By increasing benefits through the introduction of explicit incentives, policy-makers can increase the likelihood that behaviour changes in the desired direction, providing the benefits are perceived as exceeding the costs of the new behaviour. Behavioural economic theory acknowledges that people’s preferences may depend on timing, with more immediate desirable and certain outcomes (e.g. pleasure) valued more highly than distant and uncertain outcomes (e.g. potential health consequences). Such future uncertain benefits are valued less highly than those enjoyed immediately and with certainty, a process known as discounting. The costs of health-promoting behaviour (whether it is associated with additional financial costs or greater personal effort), on the other hand, are felt immediately. A measure of delayed discounting, which Yoon and colleagues25 equate to impulsivity, has shown that the greater discounting (impulsivity) of rewards predicts post partum smoking and substance misuse relapse. However, as Marteau and colleagues4 point out, many people do not act in the way that they retrospectively would have preferred to act, and much of our behaviour is triggered automatically by situations and environments. 26 Changes to the ‘choice architecture’ within an organisation or system can facilitate behaviour change and immediately associating incentives with a desired behaviour might therefore tip the balance. 27 There is strong evidence that financial penalty incentives, for example increased taxes, change behaviour. 28,29 Incentives as a means to redistribute resources to address health inequalities have recently gained popularity in low- and middle-income countries.
Evidence, complex intervention design and incentives
The BIBS study, reported in this monograph, provides an incentive evidence platform that takes theory, service-user, health professional and general public perspectives into account to inform the design of incentive intervention trials. It represents Phase I of the framework for the design of complex interventions as recommended by the Medical Research Council (MRC)30 and draws on the less linear development published in 2008. 31 A generic linear causal model for applying behaviour change theory to intervention design is described by Hardeman and colleagues32 and specifies techniques and measures for each step in a causal pathway. Another approach that we apply in this study is to develop a logic model, to understand the theory, intervention components, activities, processes and outcomes involved in a programme, as recommended by Armstrong and colleagues. 33
An important consideration was to define the target population for receipt of incentive interventions. In this study, the effectiveness of incentives delivered to consumers and to health-care providers is considered in Chapters 3 and 5. The focus is to change health behaviour outcomes. However, an alternative outcome of redistribution is apparent in some incentives literature, in which incentives are targeted to low-income populations. Health inequalities are increasing in the UK, with one in four children born into poverty. 34
Universalism, proportionate universalism35 or specific targeting is an important consideration for intervention design. Targeting can cause stigma and is unpopular with some, yet unhealthy lifestyle behaviours are socially patterned and health inequalities could be increased by universal incentive provision. Targeting has been used mostly in developing countries, for example conditional cash transfer or coresponsibility payments for pregnant women and families who attend antenatal, vaccination and child-care appointments have improved child health outcomes. 36 Such projects have been implemented in > 40 countries worldwide and aim to address poverty by providing cash to help families deal with their most urgent needs and use incentives to promote behaviours that aim to improve the well-being of children. However, their success and sustainability is questioned because of supply-side concerns in countries with weak health and education service infrastructure and shortages of service providers. 37,38
An example of targeting in the UK demonstrates the risks of incentivising a proxy outcome rather than the actual behaviour. The UK government’s Educational Maintenance Allowance programme was rolled out nationally in 2004 and was subsequently discontinued. This was a means-tested cash payment scheme conditional on attendance at post-compulsory education rather than achievements and had mixed outcomes. 39 The risk of implementing incentive schemes ahead of the evidence can be demonstrated by the example of the ‘smoke-free class’ competition for young people. This was widely implemented across some parts of Europe; however, a Cochrane review found no evidence that it had prevented young people from starting to smoke in the medium to long term. 40
Smoking in pregnancy: epidemiology, health impact and intervention effectiveness
Maternal smoking in pregnancy causes substantial harm, increasing the risk of miscarriage, stillbirth, prematurity, low birth weight, perinatal morbidity and mortality, neonatal or sudden infant death, asthma, attention deficit disorder, learning difficulties, obesity and diabetes. 41–50 Annual costs to the NHS of adverse events related to smoking in pregnancy have been estimated at between £8M and £64M for maternal outcomes and between £12M and £24M for infant outcomes. 51 Pregnancy is an opportunity to help women stop smoking before their own health is permanently compromised, which can significantly reduce rates of spontaneous preterm birth, small for gestational age babies and complicated pregnancies compared with those of non-smokers. 52
The 2010 UK Infant Feeding Survey found that at least 12% of mothers continued to smoke throughout their pregnancy, down from 17% in 2005. 53 Of survey respondents, 28% reported that they lived with at least one other person who smoked during their pregnancy and, in these situations, 30% continued to smoke compared with only 5% of mothers who did not live with any other smokers. 53 Of mothers who smoked in the year before or during their pregnancy, 54% gave up at some point before the birth.
The most recent Cochrane review of smoking cessation interventions for pregnant women found that those used in early pregnancy can reduce smoking in later pregnancy by around 6%. Cognitive–behavioural approaches were found to be effective and financial incentives were the single most effective intervention, but this latter finding was based on just four trials from the USA. 54 Nicotine replacement therapy (NRT) is licensed for use in pregnancy; however, adding a nicotine patch (15 mg per 16 hours) to behavioural support did not significantly increase the rate of abstinence from smoking until delivery or decrease the risk of adverse pregnancy or birth outcomes in one large UK trial. 55 National Institute for Health and Care Excellence (NICE) guidelines in the UK state that all pregnant women who smoke should be offered support to quit. 56 Self-help interventions have also been shown to be effective; however, the UK evidence for this is limited and may not be directly applicable. 57 In the UK, NHS stop smoking services are available for pregnant women, employing cognitive–behavioural approaches to cessation delivered by a trained adviser and including the offer of NRT when appropriate. 58 This follows the 2010 NICE guidance on smoking cessation interventions in pregnancy and following childbirth. 41 However, one challenge is that very few pregnant smokers access NHS support. In Scotland in 2006, for example, < 10% of pregnant smokers set a quit date with NHS services, suggesting that there is a need for new approaches that will encourage women to access support, potentially including the use of financial incentives. 59
Breastfeeding: epidemiology, health impact and intervention effectiveness
The World Health Organization (WHO) recommends exclusive breastfeeding (with no other liquids or solids) until the age of 6 months. 60 This policy is supported by the UK government; however, < 1% of UK women currently breastfeed until 6 months. 53 In 2010, only 46% of UK women exclusively provided breast milk to their infant at 1 week and 23% did so at 6 weeks. 53 In the UK, data from a 5-yearly Infant Feeding Survey show a steady increase in breastfeeding initiation, from 62% in 1990 to 76% in 2005 and 81% in 2010; however, increases in the duration of breastfeeding have been disappointing. For women who initiate breastfeeding, 19% stop breastfeeding before 2 weeks and 85% of these women report that they would have liked to have breastfed for longer, and 32% stop before 6 weeks. 53 When women were asked, one in five responded that more guidance and support from hospital staff, midwives and family would have helped them to continue breastfeeding for longer. Mothers express dissatisfaction with breastfeeding care61,62 and 30% report feeding problems in the early weeks. 53
There is good-quality evidence on the short- and long-term health benefits of breastfeeding for both mothers and infants. 63 Babies who are breastfed are at a lower risk of respiratory and gastrointestinal infections and hospitalisations for these conditions, allergies and leukaemia. Premature infants who are breastfed are at decreased risk of necrotising enterocolitis and suboptimal neurological development, with lower longer-term risks in adolescence of raised blood pressure and cholesterol levels. 63 Mothers who breastfeed are at reduced risk of breast cancer, ovarian cancer and type 2 diabetes. A recent health economic analysis estimated that, if 45% of women exclusively breastfed at 4 months and 75% of infants in neonatal units were breastfed while in hospital, over £17M of treatment costs could be saved in terms of gastrointestinal infections, lower respiratory infections, otitis media and necrotising enterocolitis, as well as there being gains in maternal breast cancer-related quality-adjusted life-years. 64
Systematic review evidence on interventions to increase the initiation and duration of breastfeeding suggests that increased professional and lay support are effective, particularly if this spans pregnancy and postnatal care, with multifaceted interventions reported as more likely to be effective. 65 However, the generalisability of these findings is uncertain as nine UK trials providing additional support since 2000 have not reported significant improvements in breastfeeding outcomes. 66 Qualitative studies suggest that current UK NHS support is not meeting women’s needs, particularly in the early weeks following birth,62 and qualitative evidence synthesis recommends a woman-centred approach. 67
Smoking and breastfeeding behaviour around childbirth
Smoking cessation and breastfeeding have a long history of being researched independently; however, recent studies suggest correlations and possibly causal relationships between the two. 68–70 Women who quit smoking in pregnancy and who breastfeed are more likely to abstain from smoking post partum for up to 12 months,69,70 and data from incentive intervention studies suggest a causal relationship between stopping smoking and increased breastfeeding duration. 68 UK data (2009/10) report that 41.5% of non-smoking mothers exclusively breastfed their babies at 10–14 days compared with 13.6% of mothers who smoked, with a similar pattern observed for partial breastfeeding rates and across maternal age groups and deprivation categories. 53 Considerable health inequalities are evidenced for both smoking in pregnancy and breastfeeding behaviours. Pregnant mothers aged ≤ 20 years are more than five times less likely to be breastfeeding at 4 months,71 are three times more likely to smoke before or during pregnancy and are less likely to quit smoking than mothers aged ≥ 35 years. 53 The breastfeeding initiation rate was 90% for mothers in managerial and professional occupations compared with 74% for mothers in routine and manual occupations, with a fourfold difference in smoking before or during pregnancy (14% and 40% respectively). 53 Mothers in routine and manual occupations are also less likely to attend parentcraft education classes or engage in health services that support behavioural change.
Incentives for smoking cessation and breastfeeding: the policy context
Given the health burden and considerable health inequalities observed for smoking in pregnancy and not breastfeeding, together with the limited reach and effectiveness of interventions to date, new innovative approaches to change these behaviours, such as incentives, seem attractive. UK governments are adopting a broader approach than the previous focus on individual behaviour change interventions, with reducing health inequalities as a priority. 35,72 In 2010, the UK government set up the Cabinet Office Behavioural Insight Unit, or ‘Nudge’ Unit, to consider how to encourage people to act in their own longer-term interests, as well as those of society, informed by behavioural economics theory. 27 Strategies that act at a population level, with a wider systems approach, and which can complement individually tailored brief interventions are suggested. Patient-centred care and self-care, which encourage individuals to take responsibility for their health, is a strong policy theme. 7,73
The 2010 public health White Paper in England73 compares providing incentives to local communities to forge partnerships to deliver better health outcomes and reduce health inequalities with incentivising individuals or health service providers, and considers the former more feasible. Local community development incentive schemes can have benefits beyond individual behaviour change, by increasing social capital in disadvantaged communities, fitting the current UK government’s vision of a ‘Big Society’. 74
Until recently, disincentives through increasing taxes on tobacco products or restricting where people can smoke (through smoke-free legislation) have been the dominant policy approach in the UK. However, such initiatives have commanded media attention. Some of these have included local financial incentive schemes for smoking cessation in pregnancy and grey literature on some of these schemes is included in our review. In addition, at least one incentive programme for the general adult population has been conducted in the UK. This is the Quit4U programme in Scotland that offers supermarket vouchers of £12.50 (2013 prices) per week to people living in disadvantaged areas of Tayside. 75 The Quit4U evaluation showed that the incentives helped to engage smokers and increased short-term quit rates; however, success rates at 12 months were only slightly higher than for other, existing smoking cessation services76 and some adverse impacts on client–professional relationships were reported, which impacted on engagement with services. 20 Concern has been expressed by others about the change in professional–patient relationships when financial incentives to either party are involved, particularly around mutual trust. 77
Incentives for feeding babies have a long history in the UK, dating back to when Winston Churchill introduced free national dried milk in 1942 to improve the health of children, famously saying ‘There is no finer investment for any community than putting milk into babies’. 78 The Welfare Food Scheme operated from 1942 until 2006 and removed a financial barrier to formula milk purchase. Indeed, it can be argued that this acted as a disincentive to breastfeed. On the other hand, breastfeeding has been perceived as cost free, but other costs for nursing bras, pumps and pads, and crucially the mother’s time, are incurred but rarely calculated in public policy. Until 2006, lower-income mothers were entitled to 900 g of formula milk a week for every child aged < 1 year and this was distributed through health centres. This scheme was replaced in 2006 by the current Healthy Start scheme,79 which provides vouchers to low-income families that include a pregnant woman or children aged < 4 years that can be exchanged for infant formula, liquid cow’s milk and fresh or frozen fruit and vegetables. The scheme does not offer any extra incentive to breastfeed. The NHS endorses the WHO International Code of Marketing of Breast Milk Substitutes;80 however, commercial formula milk samples, discount vouchers of < £90.00, cuddly toys and children’s clothes are available in the UK through mother-and-baby magazines, supermarket loyalty incentive schemes and websites.
Aims of the Benefits of Incentives for Breastfeeding and Smoking cessation in pregnancy study
The overarching aims of the BIBS study were to conduct evidence syntheses and primary qualitative and survey research, together with a DCE, to develop an incentive taxonomy and to inform the design of an acceptable and feasible incentive intervention(s) with promise for improving smoking cessation in pregnancy and/or breastfeeding outcomes. Aberdeenshire, Glasgow and Lancashire were purposively selected as the settings for primary data collection because of their diverse sociodemographic characteristics and their different incentive cultures for smoking cessation in pregnancy and breastfeeding (Box 1). A multiphase mixed-methods design87 with sequential stages was considered the most appropriate design to address the commissioning brief (see Appendix 1). The quantitative and qualitative methods converged (Figures 1 and 2) and integration of the findings occurred iteratively throughout the study to address both the a priori and emergent research questions. The Cessation in Pregnancy Incentives Trial (CPIT)88 was running concurrently with the BIBS study and the CPIT qualitative process evaluation data were incorporated into the BIBS analysis towards the end of the BIBS study. An overview of the CPIT is provided in Box 2.
Aberdeenshire has a mixed urban/town/rural population, with partners absent for long spells working offshore or in the fishing industry and oil industries, and there are pockets of affluence and deprivation. In Grampian in 2012, 14.5% of women were reported as current smokers at antenatal booking and 13.5% were reported as smoking at 10–14 days after birth. 81 In 2011/2, 58.4% of babies were being given some breast milk at 10–14 days, with 45.4% still receiving some breast milk at 6–8 weeks after birth. 81
Incentive culture: Aberdeenshire has the highest proportion in Scotland (71%) of smoking cessation services for pregnant women delivered through community pharmacists, who receive payments per person registering for smoking cessation support and for data collection. 53 In discussions between PH and providers in primary care and maternity services, many managers and practitioners are resistant to providing financial incentives to patients following adverse media publicity about a smoking cessation incentive scheme in neighbouring Tayside, which our collaborator Susan MacAskill evaluated. Our co-applicant mother-and-baby group is an example of a partnership community development project that has raised money from local businesses to provide non-financial incentives (a crèche and subsidised café).
GlasgowGlasgow has an urban multicultural population with a wide sociodemographic range from affluence to large areas of extreme disadvantage. A total of 50% of households are in areas of the highest material deprivation compared with 20% for Scotland as a whole. In 2012, 18.3% of women living in Greater Glasgow and Clyde were reported as current smokers at antenatal booking, with 15.3% smoking at 10–14 days after birth. 82 In 2011/2, 43.4% of babies were being given some breast milk at 10–14 days, with 33.9% still receiving some breast milk at 6–8 weeks after birth. 83
Incentive culture: The CPIT started in June 2011 and includes a qualitative element examining how incentives are perceived by recipients and providers. This Phase II randomised controlled trial is summarised in Box 2.
LancashireLancashire has a mixed urban, small town and rural population with a wide sociodemographic range. For 2007 Indices of Deprivation,84 six local districts (including Blackpool) are ranked within the top 50 in England and in some towns up to 35% of births are to women of South Asian origin. Blackpool has one of the highest rates of teenage pregnancy, one of the lowest breastfeeding initiation rates (56% compared with 74% for England) and one of the lowest rates for babies still breastfed at 6–8 weeks (24% compared with 47% for England). 85 Although smoking rates vary across the region, Blackpool has the highest overall rate with 30% of women smoking at the time of delivery in 2011/12, which is over twice the national average for England (13%). 86
Incentive culture: Lancashire is an innovative area for breastfeeding incentive schemes. The Be a Star campaign started in Lancashire in 2008 and promotes breastfeeding among 16- to 25-year-old mothers (see www.beastar.org.uk/archives/tag/be-a-star-adverts-lancashire). It originated as a partnership between one of the primary care trusts, the Little Angels breastfeeding peer support organisation and The Hub social marketing agency. Be a Star transforms local breastfeeding mums to look like models, celebrities, singers and actresses, making breastfeeding glamorous, sexy and appealing, and provides breastfeeding support. Be a Star has been rolled out across 15 primary care trusts in England with encouraging results. The strategic health authority provided funds to three areas in the North West (one of which is NHS Blackpool Primary Care Trust) to run incentive schemes with the aim of increasing breastfeeding duration at 6–8 weeks in 2011 by 5%. The community Star Buddies Breastfeeding Peer Supporters who are delivering the incentive scheme in Blackpool operate out of St Cuthbert’s and Palatine Children’s Centre (our co-applicant base).
FIGURE 1.
Flow diagram of the BIBS study.

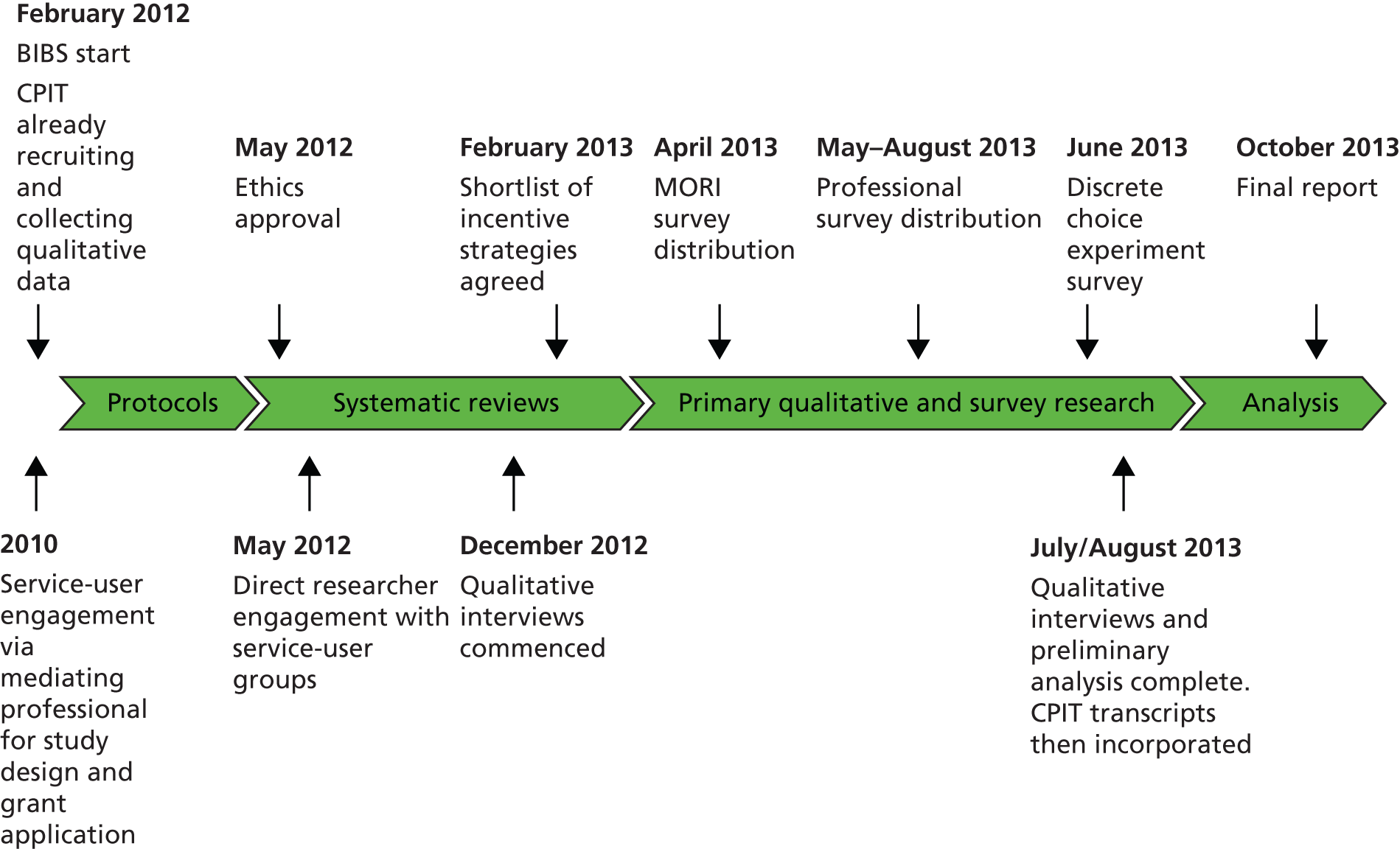
FIGURE 2.
Timeline of the multiphased mixed-methods approach. MORI, Market & Opinion Research International (Ipsos MORI).
Pregnancy is an opportunity for most young women to stop smoking before their own health is irreparably compromised. Quitting will protect their infants from miscarriage, stillbirth, low birth weight, sudden infant death syndrome, asthma, attention deficit disorder and adult cardiovascular disease. The NICE guideline Quitting Smoking in Pregnancy and Following Childbirth41 highlights the lack of evidence and recommends a research question: ‘Within a UK context, are incentives an acceptable, effective and cost-effective way to help pregnant women who smoke to quit?’. A Phase II exploratory trial was therefore run from June 2011 to December 2013 aimed at establishing a workable trial design based on individual randomisation; a generalisable regimen of intervention delivery; a primary biochemically verified outcome measure; methods to document cost-effectiveness; and a process evaluation to explore women’s’ and professionals’ views.
SettingMaternity services, Glasgow, UK.
PopulationPregnant women who self-reported as smokers at maternity booking, who had an expired carbon monoxide level of ≥ 7 parts per million (p.p.m.), who were at least 16 years of age, who could speak English and who were < 24 weeks’ gestation at maternity booking.
RecruitmentAll women in Glasgow are asked routinely at the maternity booking appointment if they are current smokers and all have a carbon monoxide breath test. Self-reported smokers and all who have a carbon monoxide level of ≥ 5 p.p.m. are automatically referred to the Stop Smoking Service, which attempts to contact all to discuss smoking and possible cessation.
The trial was discussed with women who satisfied the eligibility criteria outlined above when contact was successful, either by telephone or at the booking clinic (walk-ins). If interested, women were given an information sheet and asked for permission for their contact details to be passed to the research team. They were then contacted by the research team for formal telephone consent. Concealed randomisation followed.
InterventionThe control group received standard Stop Smoking Service care for pregnant women. This included the offer of a face-to-face appointment to discuss cessation and setting a quit date with telephone follow-up at 4 weeks after the quit date and the offer of telephone support for up to 12 weeks post quit date. Those who set a quit date were offered free NRT provided by pharmacy services for up to 16 weeks following their quit date.
Women in the intervention group were offered the routine service plus a £50.00 voucher if they attended their face-to-face appointment and set a quit date and a further £50.00 voucher if they had quit at the 4-week follow-up, which required a home visit to corroborate self-reported quitting by means of a carbon monoxide breath test (< 10 p.p.m.). Those who had quit at 4 weeks were contacted at 12 weeks and if they were still quit and this was corroborated by another carbon monoxide breath test carried out during a home visit they received a further £100.00 voucher. At a random time between 34 and 38 weeks’ gestation all participants were contacted to ascertain their current smoking status. Those who self-reported as having quit smoking were visited at home for corroboration of quit status using a carbon monoxide breath test and urine/saliva cotinine assays. Intervention participants who were confirmed to have quit by carbon monoxide breath test were sent a final voucher for £200.00. In total, £400.00 of voucher incentives were offered.
OutcomesThe primary outcome was self-reported quit at 34–38 weeks’ gestation, corroborated by either saliva (< 14.2 ng/ml) or urine (< 44.7 ng/ml) cotinine assay. A £25.00 voucher was given to all participants who provided primary outcome information.
Secondary outcomes were the proportion who set a quit date, birthweight and costs and benefits of the incentives intervention.
ResultsIn total, 612 women were enrolled over 15 months from December 2011 to March 2013, with two participants opting out of the control group. By May 2013, 480/610 (79%) participants had reached the primary outcome stage; 39 (16.2%) were lost to follow-up in the control group compared with 38 (15.9%) in the intervention group. In total, 49/241(20.3%) were cotinine-validated quit in the incentives group compared with 19/239 (7.9%) in the control group. Sensitivity analysis showed a significant improvement in the cotinine-validated quit rate whether those lost to follow-up were treated as all smokers or all non-smokers. This control group quit rate is similar to that seen in the Smoking, Nicotine and Pregnancy (SNAP) trial in Nottingham (7.6%),89 giving credibility to the intervention being generalisable to other geographical areas of the UK. The process evaluation found that the intervention was acceptable to pregnant women and professionals and that the trial was feasible to deliver, informing a future Phase III study.
ConclusionsThis Phase II trial has established a workable pragmatic trial design, for example the optimal sample size, which will reduce the risks associated with a future definitive Phase III multicentre randomised controlled trial. Stakeholder views about incentive payments for smoking cessation in pregnancy have been examined. A cost-effectiveness approach has been developed to inform data collection for a future definitive trial.
Trial registrationCurrent Controlled Trials ISRCTN87508788.
ReferenceTappin DM, Bauld L, Tannahill C, de Caestecker L, Radley A, McConnachie A, et al. The Cessation in Pregnancy Incentives Trial (CPIT): study protocol for a randomized controlled trial. Trials 2012;13:113.
Tappin D, Bauld L, Sinclair L, Boyd K, McKell J, Macaskill S, et al. Financial incentives for smoking cessation in pregnancy: randomised controlled trial. BMJ 2015;350. doi: http://dx.doi.org/10.1136/bmj.h134
Specific objectives were to:
-
Investigate the evidence for the effectiveness of incentive interventions delivered within or outside the NHS to (a) individuals and families or (b) organisations that aim to increase and sustain smoking cessation and breastfeeding.
-
Investigate the evidence for effective incentive delivery processes and how they work to increase and sustain smoking cessation and breastfeeding, including their acceptability and how they fit with existing barriers, facilitators and intrinsic and extrinsic motivators to behaviour change.
-
Systematically search for and identify incentive interventions in systematic reviews from other areas of health improvement, particularly for women of childbearing age to (a) assess fit with our evidence synthesis, (b) inform the primary research questions to investigate a shortlist of incentive strategies, and (c) identify research gaps where effective incentives for other behaviours have not been tested for smoking cessation and breastfeeding.
-
Investigate the acceptability and feasibility of a shortlist of promising incentive strategies and potential harms or adverse consequences from the perspectives of (a) women and partners; (b) health professionals, managers, policy-makers, research funders, ethics committee members, academics and other relevant stakeholders; and (c) the general public.
-
Develop an incentive taxonomy from the four objectives listed above.
-
Design a feasible trial: identify the target population, the active components and mechanisms of action of the intervention, the control group, the recruitment and delivery strategy, monitoring and outcome measurement and the effect size.
Definitions
The research team became aware early on that disciplines in applied health sciences use different terminologies that have multiple meanings and functions, which, as our service-user co-applicants pointed out, also have common usage or lay definitions. For terms that we identified as problematic in this respect, we referred to the Oxford English Dictionary1 for a common working definition; these are listed below:
-
Incentives include financial (positive or negative) and non-financial tangible incentives or rewards. By tangible, we mean free or reduced-cost items that have a monetary value or an exchange value, such as refreshments, baby products or services such as childcare or ironing (any setting). Our definition excludes intangible incentives such as supportive, motivational or persuasive relationships with professionals or peers. Incentives may be delivered directly or indirectly at a local, regional or national level by NHS or non-NHS organisations.
-
Incentive taxonomy – a classification of incentive characteristics in relation to behavioural change techniques.
-
Incentive typology – a classification of incentive types related to their meanings.
-
Incentive intervention logic model – a hypothetical description of a complex intervention and process whereby an incentive is delivered to a target population with the aim of sustaining or changing behaviour.
-
Incentive recipients may be women, families (collectively referred to in the report as consumers or service users) and/or NHS or non-NHS providers at the local, regional or national level. Incentive packages may benefit more than one group, for example local communities and parents.
-
Providers is an umbrella term referring to people, individually, in groups or in organisations, working in the NHS, government, voluntary sector or other organisations, who help women to stop smoking and/or to breastfeed. Providers also deliver incentive interventions and/or they may receive them.
When referring to human behaviour:
-
Intrinsic is a broad adjective referring to anything internal (states such as motivation, anxiety, pleasure; personal characteristics, for example age; thought processes such as planning; the senses; personal history) that is wholly within an individual, some of which can change over time. This definition of intrinsic is equivalent to the word internal and the two terms could be used interchangeably. The Oxford English Dictionary also describes another meaning in which intrinsic is the innate nature, or constitutional. Our interpretation is that this secondary definition implies a meaning of intrinsic as fixed rather than dynamic, which we do not wish to imply.
-
Extrinsic is used as an adjective in its broadest sense to refer to anything external to the individual (objects; other people; environments local to distal; cultures), some of which can change over time. This definition of extrinsic is equivalent to the word external and the two terms could be used interchangeably.
-
Influence is the capacity to have an effect on the character, development or behaviour of someone or something. We are using this term as an umbrella term rather than in a strict statistical sense and instead of using the more precise mediator or moderator terms that are sometimes used in relation to intervention design.
Our rationale for including incentives to providers is that a whole-systems approach is likely to be required. Changing recipients’ behaviour is likely to be less effective if there are barriers to the provision of support and thus changing provider behaviour may be more effective than individual incentives and the context in which incentives are delivered is likely to be important. The effect of incentivising both recipients and providers may be less than, the same as or greater than the sum of the two.
Report structure
This report is in two parts. Part 1 presents the evidence syntheses (stage 1 in Figure 1) and part 2 presents the primary qualitative, survey and DCE research (stages 2 and 3 in Figure 1). At the end of each part the findings are discussed together with the strengths and limitations. Each chapter concludes with the implications for the relevant outcomes described in Figure 1.
In part 1:
-
Chapter 2 describes how our co-applicant mother-and-baby groups contributed service-user perspectives throughout the study.
-
Chapter 3 presents a quantitative and qualitative evidence synthesis of the effectiveness and delivery processes of incentives to promote smoking cessation and breastfeeding in pregnancy. The review assesses incentive strategies at an individual recipient level as well as at provider and organisation levels. It develops an incentive taxonomy and the beginnings of an incentive typology, which are further developed and incorporated into an incentive logic model in Chapter 6. It concludes by considering the implications for a shortlist of incentive strategies, which is then refined by the findings presented in each of the subsequent chapters.
-
Chapter 4 presents a systematic narrative review of qualitative syntheses and a logic model to describe women’s perspectives on smoking in pregnancy and breastfeeding. This assists in understanding the barriers and facilitators, together with the overall balance of existing intrinsic and extrinsic motivators or demotivators for pregnant women and new mothers, for either initiating or sustaining smoking cessation or breastfeeding. The chapter concludes by considering how the findings inform the incentive taxonomy and the shortlist of incentive strategies.
-
Chapter 5 presents a scoping review of systematic reviews that have assessed the effectiveness of incentives for complex lifestyle behaviours relevant to women of childbearing age. The review identifies evidence of effective incentive strategies that support the findings presented in Chapter 2, as well as identifying knowledge gaps and incentive strategies that have not yet been evaluated for smoking cessation in pregnancy or breastfeeding. This review therefore triangulates findings from the earlier chapters and informs the shortlist of incentive strategies to take forward for further investigation in the primary qualitative and survey research.
In part 2:
-
Chapter 6 presents the findings of the qualitative research, in which interviews and focus groups were undertaken with pregnant and post partum women, partners, health professionals, experts, decision-makers and other key informants. The chapter begins with an incentive intervention logic model, which was developed during the course of the study according to our study data and applies the metaphor of a ‘ladder’ to understand how key components of incentive programmes (rungs) are perceived in relation to the life course and context facilitators of (rungs) and barriers to (missing or damaged rungs) smoking cessation in pregnancy and breastfeeding. A typology of incentive types and meanings expands the incentive taxonomy developed in Chapter 3 and is integrated into the logic model. Findings relevant to incentive intervention components and delivery processes for the shortlist of promising incentive strategies are described. Finally, we present participants’ views on potential unintended consequences of incentive interventions and issues relating to research studies.
-
Chapter 7 presents the findings of a survey to assess the acceptability of the shortlist of incentive strategies. We report findings from a Market & Opinion Research International (MORI) survey of the general public and a survey of health professionals who work with pregnant women, infants or new parents across Scotland and North West England.
-
Chapter 8 reports the findings from a DCE to inform the design of the most promising incentive strategy from the shortlist, which addresses incentives to improve smoking cessation outcomes in pregnancy.
-
Chapter 9 discusses the overall findings from the study. The most promising incentive trial design to emerge, together with other promising incentive strategies that require further development and research, are presented. The acceptability and feasibility of incentive interventions are considered, and the potential utility of the incentive logic model is discussed.
-
Chapter 10 summarises the main conclusions and implications for research.
Part 1
Chapter 2 Service-user engagement
For this project, a novel approach was adopted and we recruited two mother-and-baby groups, located in disadvantaged areas, as co-applicants. This chapter describes how we engaged service users through their groups, how they informed the research process and some reflections on collaborative approaches.
Background
Although there is a need to include patient and public involvement (PPI) in health research, which is a statutory requirement by virtue of the Health and Social Care Act 2001,90 and there is guidance and a growing pool of resources available on how this might be achieved,91 there is no ‘gold standard’ in terms of what constitutes best practice in PPI. The ways in which research teams approach PPI vary. 92 However, new strategies are being developed to actively and effectively involve the public to make PPI more meaningful. 93
The benefits of PPI include enhanced depth, credibility and applicability of findings, improved clarity of final reports and recommendations and an immediate link between practice-based evidence and evidence-based methodology. 94–96 Although it is recognised that PPI in research should begin at the earliest stage possible, in practice it is not easily achievable. Constraints on time, funding, ethics approvals and the availability of appropriate representatives often mean that PPI is not included until research is fairly well advanced. This is particularly so in studies such as BIBS, where the grant application was submitted in response to a commissioned National Institute for Health Research (NIHR) Health Technology Assessment (HTA) programme funding call. Involving patients or the public at a later stage, however, jeopardises and compromises one aim of PPI: to ensure that research is being carried out ‘with’ or ‘by’ members of the public rather than ‘to’, ‘about’ or ‘for’ them. It can marginalise them during the crucial project scoping and design phases, although the NIHR/HTA programme boards have PPI representation when deciding whether to commission research. Innovatively, we actively engaged in our project, from its inception, groups of service users who had not previously been involved in research, and who were untrained, by working with them as co-applicants.
Our aim was to foster a partnership approach97–99 to understand service-user perspectives on engaging in research, rather than imposing an unequal ‘researcher-dominant’ agenda. In the following sections we use a traditional structure to describe the process of service-user engagement, that is, using the headings of Methods and Findings, although it could be argued that this is inappropriate for such a categorisation, as the collaborative approach was dynamic and flexible, rather than fixed a priori. Service-user engagement was therefore conceived as an iterative, loosely structured process that was emergent and sensitive to the views and preferences of the service users involved. PH had the original idea based on her experience as a member of maternity service liaison committees; she had used action research methods successfully in a previous study100 and had conducted a longitudinal qualitative study with families recruited from disadvantaged areas to inform the design of interventions to improve breastfeeding outcomes. 62 NC, PH, HM and GT have extensive experience in conducting qualitative interviews with service users, including those living in disadvantaged areas.
Methods
Engaging service-user groups
At the grant application stage, service-user engagement was initiated by approaching maternity services, primary care and children’s centre managers to select two thriving, but diverse, groups operating in areas of high deprivation in Aberdeen and Lancashire. Managers identified group representatives to negotiate involvement with the study: a health visitor – Wendy Ratcliffe (Aberdeen) – and a community centre worker – Helen Cook (Blackpool) – both named in the grant application. Areas of high deprivation were considered most likely to be able to provide insights from service users who had experience of the target behaviours for change: smoking in pregnancy and formula feeding. 53 Moreover, the selected groups were considered to be particularly well suited to collaboration on the BIBS project: the Aberdeen group is user led, is successful in generating some funding and provides a café and crèche as incentives to attend; the Blackpool group is located in a local authority-run children’s centre. The settings for these groups were summarised in Chapter 1 (see Box 1) and the group profiles are summarised in Table 1.
| Group | Location | Distance for researcher to travel | Setting | Average attendance | Meetings | Purpose and structure | Funder |
|---|---|---|---|---|---|---|---|
| Mastrick Café Crèche, Aberdeen | One of the most disadvantaged areas of Aberdeen | Approximately 10-minute walk | One room within a larger family centre including a separate crèche room. Sofas, play area adjacent, toys, kitchen area, dining area, unobtrusive site manager in room nearby | Approximately seven mothers (two mothers left the group and two new mothers joined) and their babies, two (grandmothers fairly regular group membership) | Weekly, Wednesdays 1200–1400 (except during school holidays), plus some additional social/fundraising events | Set format: the café is facilitated by mothers who make and sell a cheap, healthy lunch costing £2.00 (subsidised through fundraising) to mothers and babies during the first hour, when, until early 2013, a health visitor was present to provide support. During the second hour the babies go into the crèche and the mothers enjoy a coffee and a catch-up or participate in training activities/listen to external speakers | Established through a partnership project between Aberdeen City Council, Homestart (see www.home-start.org.uk/) and NHS Grampian. The café crèche is now independent and self-funding |
| St Cuthbert’s and Palatine Children’s Centre, Blackpool | Covers a disadvantaged area of Blackpool | 18-mile drive | One room within a larger community centre with toys, books and soft-play facilities and seating for parents. A café is also located in this room for parents to purchase drinks and food | 16–20 families (discontinuous participation) | Weekly | Unstructured format. Mother-and-baby/toddler group in an informal setting where parents can drop in and out to interact with and receive support from children’s centre staff members and engage with peers. Children’s centre staff member in attendance throughout the session | Local government |
The group representatives were sent drafts of the grant proposal to discuss with the groups and they provided feedback during the planning stages. They negotiated reimbursement for their group’s refreshments and crèche costs as part of the grant application. Although the service-user collaborators were independent or local government representatives, rather than NHS groups, we considered it preferable to gain ethics committee approval before active engagement, particularly because the groups had not been involved in research before. When the study started (February 2012), service users assisted in developing the protocol and study information materials through feedback to the group representatives (HC and WR). Once ethics approval had been obtained (May 2012), the group representatives made the introductions between the service users and the fieldwork researchers. Researchers HM and GT attended 15 meetings between May 2012 and September 2013 to engage the service users by discussing study progress, gaining feedback and thus informing the research strategy. Reflecting on researcher perspectives is important and so reflexive diaries were kept and researcher observations were shared with the research team throughout.
Negotiating meeting frequency and types of visits
In Aberdeen, initial contact was made through the health visitor (WR); however, the researcher was able to exchange contact details with group members at her second visit and so the gatekeeper role shifted to the group’s treasurer, who shared it with one of the grandmothers who was responsible for organising meetings and events. This allowed for much less formal communication, good rapport building and thus intermittently ‘dropping in’ for informal lunch visits, as well as the more directed research sessions. The group met at a facility within walking distance of the university (approximately 10 minutes); this made visits easy to schedule and undertake. These circumstances were also fortunate in that, a few months into the project (September 2012), WR was relocated by her NHS employer and her replacement assumed a less prominent role within the group. In fact, after some further months (February/March 2013), health visitor support for the group was withdrawn altogether. However, this did not adversely affect engaging these service users and possibly strengthened a direct partnership between the researcher (HM) and the group. By contrast, in Blackpool, contact was initiated and continued through the community centre worker and therefore only formal research meetings were undertaken. The distance of travel for the researcher (18 miles by car) was a barrier to informal contacts. For both groups, long school holidays coincided with crucial research stages and were thus inopportune for our research schedule. For example, the final qualitative data analysis overlapped with the school summer holidays, although we were able to conduct an exercise involving our key qualitative findings with both groups (September 2013).
The Aberdeen group used Facebook (see www.facebook.com) as its primary communication mode; however, despite gaining ethical approval, the research team decided against engaging with the group using this social medium. Several concerns were discussed around maintaining appropriate boundaries, the best use of researcher time and a lack of published guidance. Using e-mail/telephone/text only did not appear to prevent or diminish engagement. Informal contacts and balancing communication traffic presented a challenge to the researcher (HM), who was confronted with some pressure for full group membership, such as being proactively invited to additional/extra activities: to assist at fundraising events and fêtes, to wear fancy dress to group seasonal parties and to attend mothers’ social evenings. The requirements of a researcher to remain objective and maintain a distance compared with a more anthropological approach to fieldwork were discussed with the research team. Of the six informal social events that took place during the course of this research, HM attended three to show goodwill. Researcher participation can therefore be considered as ‘active’101 as HM was able to gain insight into the cultural codes and rules for behaviour of the group.
Including service-user perspectives
Participatory approaches were employed and initially consisted of building good rapports with both groups by attending their meetings, informally socialising and observing in an unobtrusive manner to acknowledge the researchers’ roles as visitors to an established group. 101 This began with standing passively on the periphery, waiting to be invited in, eventually sitting comfortably on the sofas and even on the floor, and led to more active engagement, such as being asked to console crying babies. Once rapports were established, the researchers initiated requests for more directed sessions to gather feedback and data on important stages in the study.
Directed data-gathering sessions with the service users occurred after information was provided in advance and on an agreed date. HM and GT sought informed consent to audio record discussions. Researchers involved the groups in the design, editing and presentation of intervention vignettes derived from a diverse sample of promising studies identified in the systematic reviews (see Appendix 2). The study vignettes were then used in interviews and focus groups to gain participants’ perspectives of incentive interventions and to assist with shortlisting promising incentive strategies. Pilot focus groups were undertaken to contribute to interview topic guide refinement. We were able to generate data that contributed to the qualitative analysis from five of those sessions [two individual interviews and three focus groups (see Chapter 6)]. In addition, group members piloted the DCE as the majority had a smoking history, constructed their own intervention ‘ladders’ using the logic model that we developed (see Chapter 6), commented on the lay summary and advised on future dissemination of study findings. Our co-applicant groups also assisted us in identifying and actively engaging ‘hard-to-reach’ women, who seldom access health services and participate in PPI initiatives even less often. Although they contributed to the development work leading to the shortlist of incentive strategies asked about in the MORI survey of the general public (see Chapter 7), a separate sample of independent general public participants was sought to pilot this to minimise the ‘group think’ that may occur through repeated discussion of a topic. 102
Findings
Collaborative approaches to incorporating service-user perspectives
The Aberdeen group environment, cohesiveness and set structure suited a directed format, that is, focused activities, to address research issues. Women in this group were used to undertaking training (e.g. food preparation, child first aid) and had regularly welcomed external speakers in the second hour of their weekly 2-hour meetings. During this time, women sat on comfortable sofas around a large coffee table or leaned in over the kitchen counter if they were making the tea/coffee. Meanwhile, their children were occupied in the crèche, which meant that the researcher (HM) could interact with women informally as well as undertaking researcher-led whole-group data collection sessions, with minimal issues around audio recording and using interactive materials, such as the intervention vignettes. Although computing/projector facilities were not available, whiteboard and printed copies were used and worked fairly well. The vignettes helped the group to become focused and positively engaged with the study in a more concrete and tangible way. On one occasion, however, internal politics caused tension because the session was led by the organiser, who was upset that day and prevented any research activities taking place because she was ‘telling off’ members of the group about rota duties and fulfilling their responsibilities (e.g. washing up and cleaning the carpets).
In Blackpool, the group’s drop-in format presented the researcher (GT) with the practical challenge of having to move between members to involve them in ‘chance encounters’. Although the service users were keen to participate and engage in discussions, group membership was discontinuous and so GT was unable to engage with the same parents at each visit. Despite these limitations, a number of women were involved in various activities on more than one occasion, and an advantage was that a wider number of service users were engaged in total (n = 12) compared with the Aberdeen group (n = 8). Further challenges were faced when trying to engage parents and carers in meaningful conversations when their infants/children were in attendance. However, during piloting of the DCE, additional support was provided by children’s centre staff to ‘supervise’ the children to enable close reading and discussion of the online format of this tool. Furthermore, although the noise levels (children playing, shouting, etc.) compromised the digital recordings of the discussions, GT also kept handwritten notes and transcribed the session recordings herself as soon as possible to facilitate accurate and comprehensive data documentation.
Relationship with the wider research team
Engaging the mother-and-baby groups with the wider research team was problematic. Inviting and encouraging group members to attend regular weekly or formal research team meetings at the university (e.g. grant holders’ meetings) proved impossible at both sites. The health visitor group facilitator from Aberdeen (WR) attended one meeting (September 2012) and suggested that the women would have been very nervous had any of them attended because of the formality. Her successor was invited to a subsequent meeting and expressed an interest in the associated paperwork, which was e-mailed to her; however, she did not attend. It was not possible for Blackpool group members to attend because of the distance to travel and they did not accept an invitation to attend by telephone.
Other members of the research team were invited to join HM and GT for visits to the mother-and-baby groups. Highly sceptical comments such as ‘You’ll be lucky!’ were made by academic colleagues and administrative staff when considering the potential take-up of such an invitation. PH accepted on two occasions and attended an Aberdeen mother-and-baby group Christmas ‘drop-in’ (December 2012) and observed and took notes while HM facilitated a ‘ladders’ session (September 2013) (see Substantive contributions, Ladders). NC similarly observed and took notes while GT facilitated a ‘ladders’ session in Blackpool (September 2013).
The importance for the groups of researchers going into their territory to gain PPI input was evident. They took pride in hosting researcher visits, for example enthusiastically offering refreshments and home baking (Aberdeen) or computer facilities for directed sessions (Blackpool). In addition, researchers witnessed conversations that are seldom encountered in formal health or research settings. For example, some group members were adversarial towards health professionals, one group described complex and disruptive domestic relationships and highly charged personal opinions were expressed about health behaviours (smoking during pregnancy) and on one occasion someone left the room. In the presence of health service staff, such accounts would have been unlikely.
Substantive contributions
Designing participant materials
Through the group representatives as mediators, service users helped us to rephrase several sections of the information sheet for both readability and acceptability before gaining ethics approval and undertaking the primary qualitative research (Figure 3).
FIGURE 3.
Comparison of the patient information leaflet before and following service-user input.

We revised the document to take account of the target participants’ style preferences, language and social and cultural contexts. In particular, they drew our attention to their unfamiliarity with the term ‘cessation’ and the predominance of formula feeding, either from their own personal experience or in their immediate family and social networks. Thus, they suggested that ‘help women to stop smoking’ and ‘try breastfeeding’ were more appropriate phrases, and they also pointed out that ‘encourage’ is persuasive, ‘smoking cessation’ is too technical and the word ‘breastfeeding’ alone implies a certainty. The last of these was a very important change as we noted that a mother in one of the groups disclosed that she was providing exclusive breast milk to her infant (on her information form) but pretended that she was formula feeding to other group members, using bottles of expressed breast milk that she allowed them to assume was formula by talking about preparing it. Breastfeeding was not a social norm and this woman anticipated that it could possibly be considered unacceptable by some.
Systematic review feedback and study vignettes
The mother-and-baby groups contributed to interpreting systematic review findings by providing feedback on a number of vignettes of studies included in the evidence syntheses, which were initially drafted by the researchers. Six vignettes were developed from studies that were selected either because they had statistically significant effects or because they involved an unusual or innovative approach. 103–108 Different vignette structures were tried out, for example presenting and discussing sections of the vignette sequentially compared with presenting the whole vignette. Presenting the vignette as a whole was the most popular format and this differed from the advice that we had received from our steering committee. Discussions around the vignettes assisted in talking interviewees through the interventions step by step and provided valuable insights into which incentives and programmes might be acceptable. This was particularly useful as very little detail around acceptability and processes is reported in the included studies (see Chapter 3), even when they are classified as using qualitative or mixed methods. 109–113
Piloting topic guides
We piloted draft interview topic guides in three focus groups with service-user mother-and-baby groups before recruiting participants. In Aberdeen, this involved trying a structured topic guide and the integration of study vignettes within the schedule and revisions. The final preferred version was unstructured with prompts for use if and when appropriate, for example opening with questions around what incentives were meant for women and using women’s conceptions of incentives to guide the interview.
The discrete choice experiment
The DCE was piloted with four mothers with a history of smoking from one group (Blackpool). When reading and answering each of the questions (using SurveyMonkey’s online format – see www.surveymonkey.com), the mothers were asked to use the ‘think aloud’ cognitive interviewing technique114 whereby they expressed their feelings and discussed any issues around the questions/process. This session was facilitated by GT, who audio recorded and transcribed the key points for team discussion. All participants in this session took the questionnaire seriously and engaged with the choices. Descriptions and explanations in the DCE were revised for better understanding and readability based on their comments.
‘Ladders’
In the final stages of the project, the ‘ladder’ logic model emerged through mixed-methods analysis of the BIBS study data and feedback from our mother-and-baby group co-applicants (see Chapter 6). This was taken to the groups in an interactive format so that women could engage and feed back on it before finalising it as a research output, but could also attempt to put together their own ‘ideal’ tailored interventions, using the components that either they or we identified. As researchers, we wanted to garner a sense of how the ladder might be communicated in lay terms and also to assess whether it could be used with potential trial participants as a tool to contribute to identifying important components and processes to optimise intervention codesign. This exercise proved popular and was successfully completed by participants in Aberdeen (n = 4; n = 3 reproduced with consent in Appendix 3) using a blank ladder and three envelopes labelled ‘life’, ‘incentive’ and ‘other’ rungs. Each envelope contained individual paper ‘rung’ cards with labels corresponding to barriers and facilitators, identified from study data, and some blank rungs for women’s own contributions (see Appendix 4). They then constructed their own smoking cessation or breastfeeding behaviour change programmes, applying star stickers to highlight those ‘rungs’ that they considered crucial. In retrospect, clarity could have been improved with separate ladders for barriers (damaged or missing rungs) and facilitators (rungs). However, women liked the simplicity of the ladder metaphor. They considered that it enabled them to set a clear goal and a direct means of reaching it while taking their personal circumstances and contexts into consideration and valuing their individual needs and preferences. Participants in this exercise commented that this model for intervention design would improve engagement as it felt personalised and suggested that it could work for other or multiple health behaviours. For example, some spontaneously talked about the implications of incentive rungs for other health behaviours, in particular healthy eating, which was considered too expensive to do. One woman commented that if she was ‘overhauling’ her health she might attempt to improve several aspects of her lifestyle, especially if she was being provided with incentives or rewards to help her do this. This suggests the relevance of incentives for addressing multiple health behaviours at this life stage. However, others expressed entrenched resistance to change; for example, when completing a ladder for stopping smoking, one woman responded to breastfeeding saying ‘no, no, no, no, no!’
In Blackpool, because of children being present in the room, a different format was adopted. For this exercise GT and NC constructed separate A4 sheets, which had a ladder diagram picture in the background, for the different ‘rungs’ (‘life’, ‘incentive’ and ‘other’). Mothers (n = 4) were requested to put a tick and/or a star by the rungs considered most important or relevant and a cross by those considered unimportant for behaviour change programmes. This method worked well, with the mothers agreeing with the ladder concept and engaging with the process. Three ladders, reproduced with consent, can be found in Appendix 5.
Lay summary
Mothers at the Aberdeen group read the first draft of the lay summary and commented. Two sentences were reworded according to their feedback and ‘promising’ was replaced with ‘potential’ as it was felt that the meaning of the former could not be easily understood without explanation and examples.
Dissemination
We took a poster presentation115 to discuss with one mother-and-baby group (Aberdeen) to show them how we are reporting and presenting results publicly. They reported that they liked the format, which was something none of the members had seen before, and were delighted that their group’s name had been included, expressing a sense of immense pride regarding being involved. At the final group meeting, mothers offered numerous suggestions, such as face-to-face briefings, making information available online on key web pages that parents may access, leaflets within community/health facilities and inclusion within school newsletters and local newspaper articles with a group and researcher photograph. We will also share the final report summary with the groups, several members having requested personal copies, and will explore involving the group members in our use of local press and social media (e.g. Facebook, see www.facebook.com; Mumsnet, see www.mumsnet.com/) to disseminate research findings.
We hope to retain connections with both groups so that potential future research collaborations can be realised.
Discussion
In summary, our flexible approach to service-user engagement was essential and positive interest from co-applicants was achieved. Participatory, qualitative and observational research methods were acceptable and facilitated substantive contributions to decision-making. It was important to start with social rapport building and move, at the pace of the groups, to more directive researcher-initiated agendas for gathering feedback and data at key research stages. Challenges experienced were the careful negotiation of boundaries; the use of social networking communication, which may allow consistency with group members but has potential drawbacks in terms of confidentiality and objectivity for researchers; having uninterrupted space for inclusion (e.g. difficult while supervising children) as this affects participation; and the presence of health professional/community staff, which may be inhibitive and thus accessing more private settings is important. However, it was possible to avoid the potential bias of relying on individual expert service users by involving two groups, both of which, to greater and lesser extents, had a turnover of attendees and a dynamic membership of women from more disadvantaged areas with experience of smoking in pregnancy and either short-term breastfeeding or formula feeding. We would have missed out on rich data from some of the harder-to-reach, more disadvantaged perspectives without the mother-and-baby groups’ involvement.
An important consideration is the grey area between a co-applicant PPI role and being a research participant in qualitative research. Aside from the impact that our approach had on research development through substantive contributions, and thus decision-making, the difference is subtle, especially when groups or individuals had had a dual role in that they were also involved in data generation (e.g. the interviews and focus groups included in Chapter 6). Qualitative research to inform trial design is recommended in the MRC framework for complex interventions. 30 This was undertaken in the BIBS study (see Chapter 6) and applies a more rigorous approach to sampling, data collection and analysis to ensure that a wide range of perspectives is incorporated. The single service-user perspective can be prone to selection bias. In addition, contrary to the norm116 with PPI involvement, we did not offer the mother-and-baby group participants any training in PPI. Our approach therefore falls somewhere in between conventional PPI and qualitative research.
We consider our engagement of harder-to-reach participants through collaborating with mother-and-baby groups to be a strength of our approach to PPI. Our ability to operate outside of the health service and develop trusting continuing relationships with independent groups facilitated substantive contributions, which had a direct impact on the development of our research. Another strength of our approach was the sense of pride experienced by the group members that their group was involved in the research and that they could host researcher visits, which challenged traditional power relations. Our observations were that some group members changed behaviours during the process, including stopping smoking and enrolling in college for further education. The extent to which the collaboration might have primed individual motivation to behave differently is uncertain, however, as others remained highly resistant to change in relation to either smoking and/or breastfeeding.
The limitations of this approach were that engagement with the wider research team was usually dependent on single researcher observations. Equally, the need to identify a legitimate manager or leader with a good rapport with the group to gain and maintain access until ethics approval is in place and the researcher has established a trusting relationship means that power differentials and representation are complex. This is not least manifested in naming co-applicants who are professionals and thus the service users themselves were involved only from the project’s inception and not its conception. Such issues of mediation include the translation of technical research language into lay terms, which is essential. The preparation of vignettes to substitute for publications relating to complex interventions and piloting long surveys such as the DCE mean that considerable work is needed to prepare and facilitate directed sessions. Best use of researcher time is therefore a consideration.
The implications of this approach are that the incorporation of service-users’ views throughout the research process has focused on women within the target population in the context of their real lives, with them informing the scientific elements of the study. We consider this to have been a novel, and crucial, approach to the development of an acceptable and feasible trial(s). It allowed for a deeper level of engagement, and developing ongoing ethically approved relationships with such groups needs further consideration as leaving the field is an issue once a partnership has been established but the funding stops. Gaps between funding and the need for input are not concepts familiar to service users. The use of social media to engage with groups is also an issue and comprehensive guidance is required.
Conclusions
There are advantages and disadvantages to engaging mother-and-baby groups in PPI as research co-applicants. Extensive involvement of the co-applicant groups as team members across a diverse range of research activities, from study onset to dissemination, resulted in novel contributions to the BIBS project’s development and decision-making. Regularly taking the research to the groups enabled harder-to-reach, less ‘professionalised’ and more confident PPI involvement than members of the research team have experienced when group representatives have been invited to the university setting.
However, there are methodological challenges. It is possible to overcome the issue of the ‘professional patient’ and to involve harder-to-reach communities, and multiple individuals, but not in conventional ways. Therefore, modified and flexible research methods, and off-site engagement, are required, but are not necessarily matched by participation in more traditional formal aspects of the research, such as university-based meetings. The question of PPI being an active partnership between researchers and the public remains an important one when power relations are still omnipresent.
Chapter 3 Review of the benefits of incentives for initiating and continuing smoking cessation in pregnancy or breastfeeding
This chapter describes evidence syntheses for the effectiveness of incentive interventions and their delivery processes for smoking cessation in pregnancy or breastfeeding. The section describing the methods for the review includes both smoking cessation and breastfeeding studies. The results are presented in three sections: results for incentives delivered to women for smoking cessation in pregnancy, results for incentives delivered to women for breastfeeding and finally results for incentives delivered to providers of care to improve smoking cessation in pregnancy or breastfeeding outcomes. The discussion and conclusions sections consider the review findings overall.
Methods
Research questions
-
What is the evidence for the effectiveness of incentive interventions delivered within or outside the NHS to (a) individuals and/or their families or (b) organisations that seek to increase and sustain (i) smoking cessation during pregnancy and/or within the first 6 months following birth and (ii) breastfeeding in the first 6 months following birth?
-
What is the evidence for effective incentive delivery processes (including acceptability, mechanisms of action, barriers/facilitators and intrinsic or extrinsic motivators to behavioural change) for recipients and/or providers to increase and sustain (i) smoking cessation during pregnancy and/or within the first 6 months following birth and (ii) breastfeeding in the first 6 months following birth?
Objectives
-
To systematically review incentive strategies for (i) smoking cessation in pregnancy and/or within the first 6 months following birth and (ii) breastfeeding within the first 6 months following birth to gather evidence to inform the design of an incentive intervention trial and to address the overall study objectives detailed in Chapter 1.
-
To integrate the quantitative and qualitative evidence on incentives for (i) smoking cessation in pregnancy and up to 6 months after birth and (ii) breastfeeding up to 6 months after birth.
For each of the two behaviours of interest – smoking cessation and breastfeeding – an integrated mixed-methods systematic review of effectiveness and delivery processes was undertaken. The effectiveness review was informed by the principles of Cochrane guidance on the conduct of systematic reviews,117 with particular reference to public health intervention guidance. 118 The process review was guided by the nature of the studies identified and the overall study research questions. The process review includes any data that meet the study inclusion criteria but are not included in the effectiveness review. The protocols were registered with PROSPERO (reference no. CRD42012001980) (see Appendix 6).
Identification of studies
Extensive electronic searches were conducted to identify reports of published, unpublished and ongoing studies that report data on the benefits of incentives for smoking cessation during pregnancy and/or within the first 6 months after birth and initiating and/or sustaining breastfeeding within the first 6 months after birth. The search strategy was designed to be highly sensitive and include appropriate subject headings and text-word terms. Full details of the search strategies used are reported in Appendix 7.
The databases searched were MEDLINE (1946 to February 2012), EMBASE (1974 to February 2012), Cumulative Index to Nursing and Allied Health Literature (CINAHL) (1981 to February 2012), Science Citation Index (1981 to February 2012), Social Science Citation Index (1981 to February 2012), Applied Social Sciences Index and Abstracts (1987 to February 2012), PsycINFO (1967 to February 2012) and Trials Register of Promoting Health Interventions (February 2012). In addition, the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, Issue 2, 2012) was searched for additional reports of randomised controlled trials (RCTs) and the Health Management Information Consortium database (1979 to February 2012) and the NHS Economic Evaluation Database (February 2012) were searched for economic literature. The Cochrane Database of Systematic Reviews (CDSR) (The Cochrane Library, Issue 2, 2012), Database of Abstracts of Reviews of Effects (DARE) (February 2012) and the HTA database (February 2012) were searched for relevant systematic reviews and reports. Relevant websites, including the WHO website (see www.who.int) and those of the United Nations Children’s Fund (UNICEF) (see www.unicef.org), the King’s Fund (see www.kingsfund.org.uk/), the Health Foundation (see www.health.org.uk), the Joanna Briggs Institute (see www.joannabriggs.edu.au), the Picker Institute (see www.pickereurope.org) and the Midwives Information and Resource Service (MIDIRS) (see www.midirs.org) were also searched. Co-applicants also used their networks to identify relevant studies.
Definition of an incentive
Incentives included financial (positive or negative) and non-financial but tangible incentives or rewards. Tangible incentives were defined as either free or reduced-cost items that have a monetary value (e.g. refreshments or baby products) or services with a monetary value (e.g. childcare or ironing). These incentives could be delivered directly or indirectly at local, regional or national level by health-care or other community and/or commercial providers. This excluded supportive, persuasive or motivational relationships with service providers or peers or educational materials such as information leaflets or non-commercial DVDs that reinforce verbal persuasive advice to stop smoking or to breastfeed.
Inclusion and exclusion criteria
The reviews included English-language studies of any study design (RCT, quasi-RCT, non-randomised interventions, mixed-method designs and qualitative studies) that met the definition of incentives and which were from a developed country as defined by the United Nations. 119 Grey literature was identified according to the Luxembourg definition of multiple document types produced by all levels of government, academia, business and organisations in electronic and print formats not controlled by commercial publishing, that is, in which publishing is not the primary activity of the producing body. 120
The purpose of the incentive could be (1) a behaviour change outcome or (2) participation in either an intervention or monitoring of the behaviour. We were interested in how incentives/rewards as BCTs were being used by researchers in intervention studies either alone or in combination with other BCTs.
The population of interest was women who were pregnant or those who had given birth within 6 months at the time of the intervention (either including or not including other family members) and/or health-care (e.g. NHS or other health-care companies), community (e.g. local authority or voluntary sector) or commercial (e.g. pharmacy) providers and/or stakeholders at local, regional or national level. Interventions could benefit one or more than one of these groups. The review concentrated on interventions specifically targeted at the population of interest and did not include studies analysing the population of interest as a subgroup within a wider population at whom the intervention was aimed.
Comparators under consideration included any alternative treatment arms to those of the interventions that represented either practices similar to those currently being provided in countries meeting the inclusion criteria for the review or possible alternative interventions that are realistic options that may be relevant to health-care and/or community providers in these countries, particularly (from a UK perspective) stakeholders within the NHS. Single-group before-and-after studies were also included.
Our primary outcomes were smoking cessation rates and relapse rates and any breast milk and exclusive breast milk feeding rates. All studies for the effectiveness review were required to provide data for at least one of these outcomes. In addition, when possible, we collected secondary outcomes related to the delivery of the intervention or outcomes related to the characteristics of the incentive mechanism. We were particularly interested in:
-
the context of the intervention:
-
the behaviour change theories informing the intervention
-
the setting within which the intervention was delivered
-
who the intervention was delivered by
-
how the intervention was delivered, for example in person, remote delivery, number of person contacts involved in delivery
-
-
for outcomes related to the characteristics of the incentive mechanism:
-
who is being incentivised (e.g. individuals, their families and/or provider organisations)
-
other incentive characteristics (e.g. size, duration, timing, delivery, ‘currency’, that is, financial or tangible non-financial)
-
the fidelity (compliance with the protocol) of the intervention and/or the incentive/reward mechanism
-
details of whether the incentive is contingent on something and if so what it is contingent on (e.g. abstinence from smoking, initiation or duration of breastfeeding, attendance at clinic, monitoring to validate primary outcomes either biochemically for smoking cessation or self-report, achieving other programme components)
-
differential effects, for example health inequalities evident.
-
The gold standard for biochemical validation of smoking outcomes is considered to be the level of cotinine in blood or saliva. Cotinine measurements are more reliable than exhaled carbon monoxide (CO) levels as levels of the latter can be undetectable within < 24 hours. 121 The half-life of cotinine in pregnant women between 16 and 40 weeks’ gestation is 8.8 hours compared with 16 hours for non-pregnant women and clearance to non-detectability takes place in about 44 hours. 122 Cotinine-level cut-offs vary, largely dependent on the levels of sensitivity and specificity chosen. The plasma level cut-off used is often 13.7 ng/ml but more recent studies in the pregnant population suggest that because of increased clearance of nicotine and cotinine during pregnancy a lower cut-off of 11.5 ng/ml may provide greater accuracy. 123 Saliva levels are thought to mirror plasma levels, with a cut-off of 13 ng/ml quoted for pregnant smokers in a recent study. 124 Urine cotinine levels are in theory more problematic in that the cotinine level should be corrected for dilution by measuring creatinine in the urine. In practice, the dilution effect may not be important and laboratories differ, with a cut-off of around 44.7 ng/ml. 125 Bedside assays for cotinine using urine and saliva are being developed. 126
Studies that reported secondary outcomes without also reporting at least one of the primary outcomes were excluded, with the exception of incentive studies that were not linked to a specific intervention but focused on experiences/perceptions of incentives for smoking around childbirth or breastfeeding (see Data extraction strategy). When interventions included both an incentive/reward behaviour change technique (IRBCT) component and general BCT components (e.g. support/motivational counselling), these studies were included regardless of the relative size of each component. The studies included in this evidence synthesis chapter are listed in Appendices 8 (smoking cessation) and 9 (breastfeeding).
Data extraction strategy
Abstracts identified by the search strategy were screened by two reviewers (JH and HM for smoking cessation and KR and HM or KR and PH for breastfeeding). Full-text papers of potentially relevant reports were obtained and independently assessed by two reviewers for inclusion in the effectiveness and process reviews (JH and KR for smoking cessation and KR and one of GT, NC or DT for breastfeeding). Qualitative and mixed-methods papers that met inclusion criteria only for the process review were independently assessed by two reviewers (HM and PH). Differences of opinion were resolved through review team discussion. Authors of individual papers were contacted for additional information when it was required to answer the research questions. The grey literature was assessed by two reviewers – for smoking cessation studies and KR and HM for breastfeeding studies – for inclusion in either the effectiveness or the process reviews; however, following team discussion it was considered more appropriate to use these data to inform triangulation of the findings for the entire study and to inform qualitative data collection. The grey literature is summarised in Appendix 10 and is referred to throughout the report.
As no purely qualitative studies were identified, the original analysis protocol was modified to maximise the information available on incentive delivery processes reported in the included studies. Studies for process data extraction were grouped into two categories:
-
Qualitative and mixed-method studies linked to identified RCTs and intervention studies, including any descriptive data reported as part of papers reporting quantitative outcomes. This is recommended in critical interpretive synthesis127 and in a review of smoking cessation interventions in pregnancy to ensure that findings are relevant for service users and practitioners. 128
-
Qualitative or descriptive studies not linked to any specific intervention. This included studies drawing on the experiences and perceptions of incentives for smoking cessation and breastfeeding around childbirth.
A single electronic mixed-methods data extraction form (see Appendix 11) for use in Microsoft Word (Microsoft Office Professional Plus 2010, Microsoft Corporation, Redmond, WA, USA) was developed and agreed through review team discussion, informed by the Cochrane public health guidance. 118 A single data extraction form was considered to be possible for the dual purposes of conducting a hypothesis-testing (effectiveness) review and a hypothesis-generating (process) review.
One reviewer extracted quantitative data from the included full-text papers first (JH for smoking cessation and KR, VHM or SJ for breastfeeding) and a second reviewer (KR for smoking cessation and KR, VHM, SJ, LJ or NC for breastfeeding) – depending on who originally extracted data from each study – checked the data extraction. The data extraction form was then passed to the process review team where one reviewer (HM) extracted hypothesis-generating data relating to the delivery of and perceptions about incentive interventions and a second reviewer (GT or PH) checked the data extraction. For the studies reporting qualitative data, identified themes were integrated with the mixed-methods review.
Behaviour change techniques
Intervention content was characterised by extracting BCTs for studies in which an intervention was delivered. Studies providing incentives to providers of care were excluded from BCT extraction. BCTs were extracted in two ways: (1) general BCTs (not involving incentives/rewards) were extracted to characterise the range of general BCTs employed and (2) IRBCTs were extracted for an in-depth examination of these components. General BCTs were extracted using behaviour-specific taxonomies17,129 to utilise the most appropriate frameworks to characterise intervention content. IRBCTs were excluded from the general BCT extraction as these were covered in the in-depth examination. In-depth examinations of IRBCTs were based on extractions using the recent BCT-v1,17 which provides the most comprehensive cover of incentive/reward-related techniques (i.e. taxonomy section ‘reward and punishment’ 10.1–10.11). The IRBCTs were coded in a modified taxonomy created for this study derived from the literature review (Table 2). This is based on the four dimensions inherent in the ‘reward and punishment’ BCTs from BCT-v1,17 namely contingency target (i.e. health behaviour such as self-reported smoking abstinence, preparatory behaviour such as intervention participation and engagement, or outcomes such as CO measurement), actor (i.e. participant, health-care professional, social other), content (i.e. material, social, self, non-specific) and type/awareness (i.e. aware of reward in advance and therefore acting as an incentive; not aware of reward; unclear). Social rewards were maintained for completeness, although they did not meet our inclusion criteria for the review. All BCTs were coded using a conservative approach, which required explicit written evidence for the use of a technique to avoid subjective inferences. Data were not extracted for the control arms because of poor-quality reporting, particularly of usual care.
| Category | Item | Description | Example |
|---|---|---|---|
| Type/awareness | Reward | Delivery of a reward | Participants receive money for confirmed SC or BF |
| Incentive | Informing someone of the delivery of a reward Also code as reward as we can assume that the incentive element will be delivered as promised |
Participants are told that they will receive money for being confirmed as a non-smoker | |
| Content | Material | Money, vouchers or other objects with a value | Participants receive money for verified SC or BF |
| Social | Accompanying verbal or non-verbal reward from the intervention facilitator or other social contacts | Intervention facilitator congratulates person for verified SC or BF | |
| Self-selected | Self-rewards selected by the target person for health behaviour change | The participant is asked to choose self-rewards for SC or BF | |
| Non-specific | Non-specific rewards or experiences that the individual values | Providing exercise classes to individuals for SC or BF | |
| Contingency target | Health behaviour | Performance of the health behaviour that constitutes the main target for change | Participants receive money for reporting SC or BF |
| Preparatory behaviour | Performance of any behaviours that might facilitate the performance of the target health behaviour Includes intervention attendance, intervention engagement, assessment attendance, agreeing to quit |
Participants receive money for attending intervention sessions | |
| Outcome | Achieving a verified outcome (i.e. physiological, biochemical or health outcome) related to the target behaviour | Participants receive money for verified SC on CO monitoring | |
| Other | Other contingency targets not performed by the target person for change | Health professional receives money for counselling participants | |
| Actor | Participant/patient | The target person for health behaviour change | People recruited to a SC or BF incentive intervention |
| Health-care professional | The health-care professional caring for people who intend to change their health behaviour | Health professional receives money for delivering SC or BF intervention | |
| Social other | A person with meaningful social relations with the target person for health behaviour change | Social support ‘buddies’ rewarded in a SC or BF intervention |
Smoking cessation studies
Behaviour change techniques were extracted using a smoking cessation-specific taxonomy129 that includes 41 smoking-relevant BCTs (the BCTs ‘provide rewards contingent on successfully stopping smoking’ and ‘provide rewards contingent on effort or progress’ were not rated as these were examined in the in-depth section based on the BCT-v1 taxonomy). BCTs were rated for the target behaviour of stopping smoking in the target population of pregnant women and/or those who had given birth within the last 6 months. The definition of the BCT ‘measure CO’ was extended to also include objective verification of smoking status through saliva samples. Two reviewers (HM and SUD) initially extracted BCTs for three studies that had been excluded from the review but which were very similar to included studies to pilot the extraction procedures and documents to obtain good inter-rater reliability. A consistent extraction and coding strategy was agreed following comparison and discussion. The reviewers then independently extracted BCTs from the included papers. Discrepancies in BCT codings were compared and resolved by a third senior reviewer (FFS).
Breastfeeding studies
A similar approach was used for the breastfeeding papers; however, in the absence of a behaviour-specific taxonomy for breastfeeding, the generic BCT-v1 taxonomy17 was applied. BCTs were rated for the target behaviour of breastfeeding in the target population of woman who were pregnant or who had recently given birth. The definition of the BCT ‘adding objects to environment’ was refined to explicitly exclude information-based materials such as leaflets and booklets as these were judged to be qualitatively different from the provision of objects such as breast pumps.
Quality assessment strategy
Two reviewers [JH and KR for smoking cessation and KR or VHM and SJ (acknowledgement) for breastfeeding] independently appraised the quality of studies included in the effectiveness review using the Cochrane Collaboration’s risk of bias tool130 for RCTs and/or (depending on the design of identified studies) an 18-question checklist for non-randomised studies and case series that was adapted from several sources including the Centre for Reviews and Dissemination’s guidance for those carrying out or commissioning reviews,131 Verhagen and colleagues,132 Downs and Black133 and the Graphic Appraisal Tool for Epidemiology (GATE). 134 This tool assesses bias and generalisability, sample definition and selection, description of the intervention, outcome assessment, adequacy of follow-up and performance of the analysis. The checklist was developed through the NICE Review Body for Interventional Procedures (ReBIP), a joint venture between the Health Services Research Unit at the University of Aberdeen and Health Services Research at the University of Sheffield for the NICE Interventional Procedures Programme. Any differences of opinion between independent reviewers were resolved through discussion with a third member of the team.
The quality of papers reporting qualitative data was independently assessed by two qualitative researchers HM or GT or NC; for one study authored by members of the research team, SM and VS (see Acknowledgements) assessed quality using a modified version of the Critical Appraisal Skills Programme (CASP)135 (see Appendix 12). Multimethod studies included in the effectiveness review were also assessed for risk of bias as described above. When consensus could not be reached, wider discussion with the research team took place to reach agreement. We also applied the Mixed Methods Assessment Tool (MMAT)136 to studies reporting quantitative and qualitative data, including survey studies; however, this was abandoned as it did not contribute additional useful information and it was insufficiently sensitive to detect quality issues with the surveys.
Data analysis
For the quantitative results we report means or changes in means or proportions between groups. For data that were reported only as figures (i.e. without information in the text of the article), data have been digitised using Engauge software (Digitizer version 4.1, see http://sourceforge.net/projects/digitizer/files/Engauge%20Digitizer/) to maximise the available information and report data as precisely as possible. For relevant outcomes, meta-analysis was carried out when appropriate to estimate a summary measure of effect, using Stata 13 (StataCorp LP, College Station, TX, USA), by calculating relative risk (RR) and risk difference (RD) statistics, using random-effects methods to account for heterogeneity. Inter-rater agreement was assessed using Cohen’s kappa137 if BCTs were identified in five or more instances. For studies not considered suitable for meta-analysis but in which primary outcomes were reported for intervention and control groups, RRs were calculated from the outcome data reported.
A narrative synthesis was conducted for the incentive process review. Process review analysis involved the qualitative research team (NC, PH, HM, GT) familiarising themselves with the studies and discussing the data extracted, developing initial descriptive themes and finally developing higher-order analytical and interpretive themes and concepts. Service-users’ perspectives were incorporated into this process through discussion with our co-applicant mother-and-baby groups (see Chapter 2). This cyclical and iterative process was used to identify the promising ‘ingredients’, processes and temporal aspects of incentive interventions most likely to contribute to effectiveness for smoking cessation in pregnancy and breastfeeding, including situation, context and environment themes.
We now present the results of the smoking cessation review. This is followed by the results of the breastfeeding review and the results of studies that incentivised providers.
Results of the smoking cessation review
Number of studies identified
We identified 1469 records from the primary searches for this review. After title and abstract screening, 1161 studies were considered not to be relevant and were excluded, leaving 215 studies for full-text assessment and a further 93 studies that were requested for background information only. An additional seven studies were identified by searching reference lists and from research team knowledge. In total, the full texts of 222 studies were therefore screened. In total, 23 studies (34 reports25,68,104,105,108–111,113,138–162) met our inclusion criteria. A flow diagram of the screening process is provided in Figure 4.
FIGURE 4.
Flow chart outlining the screening process for the review of incentives for smoking cessation in pregnancy and within 6 months of giving birth.
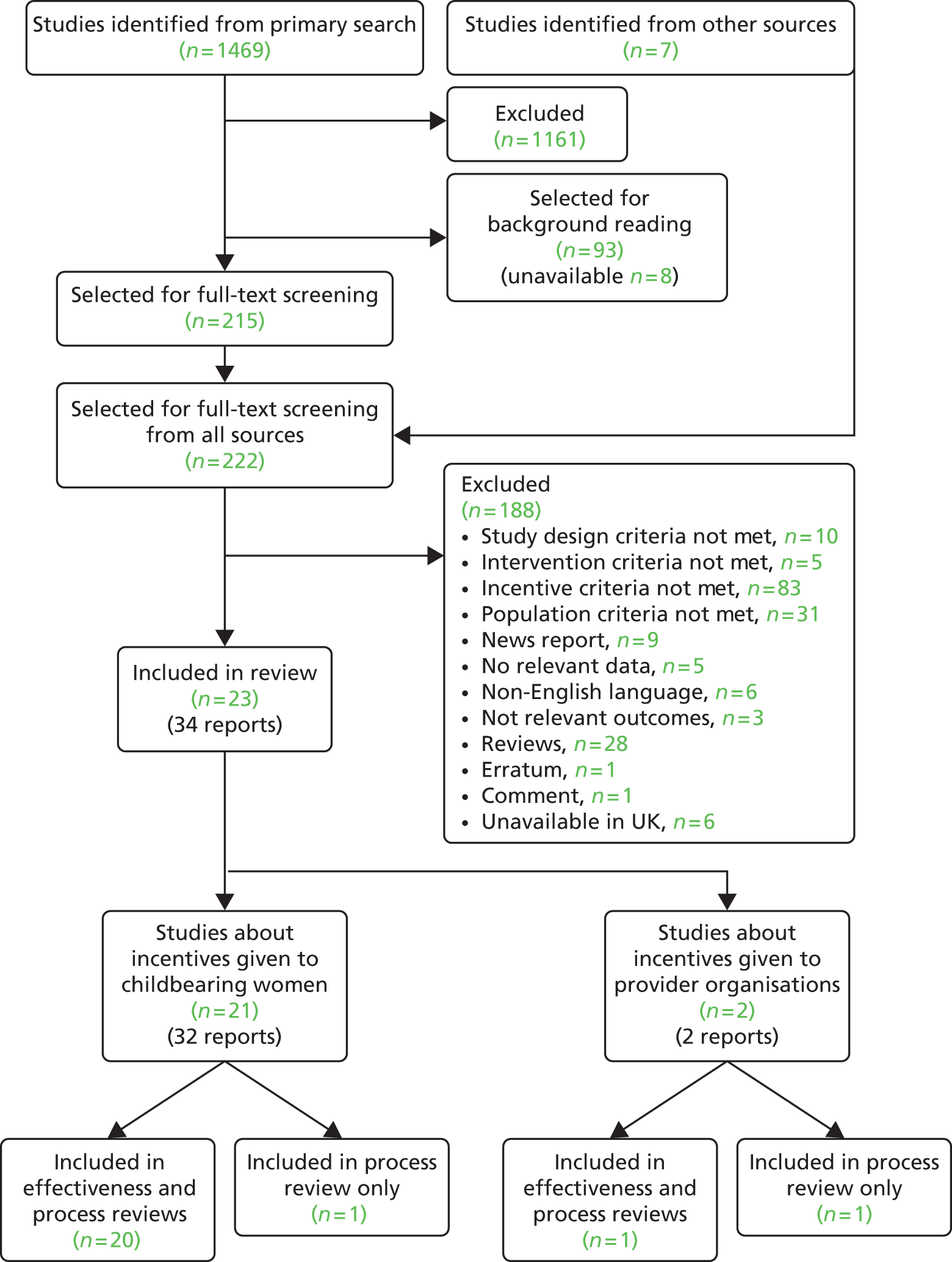
Included and excluded studies
Of the 23 studies included, 20 provided incentives to childbearing women. 104,105,108–111,113,138–150 One study was included in the process review only as it was a survey of acceptability and feasibility and did not provide incentives to women. 151 One study152 provided reimbursement to organisations providing care and one study included a question in a survey about reimbursement. 153 The surveys were included to inform the primary survey research to investigate the acceptability of incentives reported in Chapter 7. Studies providing incentives to organisations for either smoking cessation or breastfeeding are reported separately later in this chapter. The reasons for exclusion of assessed full-text papers are given in Figure 4.
There were reports in which it was unclear whether a study met our definition of an incentive. Decisions to include as an incentive study were made for the following:
-
A study by Ussher and colleagues150 in which free expert-led exercise sessions with free gym equipment and an exercise DVD were provided. As free expert tuition, a DVD and use of gym equipment has a cost, this met our criteria for an incentive intervention.
-
Multifaceted programmes providing incentives to women as part of usual care, for example the Women, Infants and Children (WIC) programme (see www.fns.usda.gov/wic/). The WIC programme provides federal grants to states for supplemental foods, health-care referrals and nutrition education for low-income pregnant, breastfeeding and non-breastfeeding post partum women and infants and children aged up to 5 years who are found to be at nutritional risk. It was decided to include only studies in which the aim was to investigate an incentive intervention according to our study definition rather than to include all WIC studies.
Decisions to exclude as an incentive study were made for the following:
-
Studies in which it was unclear whether an item provided was intended as an incentive or as a treatment or therapy that was being subsidised as part of the research were excluded. For example, NRT is provided free to NHS patients within the UK; however, in other countries this may not be the case. In such countries a pregnant smoker could be incentivised to stop smoking if they had free access to NRT.
Quality of the included studies
Details of the questions used to establish the quality of all included studies presenting quantitative outcome data are available in Appendix 13, where the individual assessment results for each question by study are reported.
Information is provided about each individual aspect of quality across all studies (Figure 5) and across all aspects of quality for each individual study (Figure 6). For both figures, answers of ‘yes’ represented the maximum possible level of quality. Therefore, in considering Figure 5, it can be seen that most studies (> 70%) had clearly described their inclusion and exclusion criteria, had undertaken data collection prospectively, had clearly defined their interventions and comparators, had followed up participants for long enough to detect important effects on outcomes and had considered and reported all of the important outcomes.
FIGURE 5.
Quality assessment results by quality appraisal question for all studies included in the review of incentives for smoking cessation in pregnancy.

FIGURE 6.
Quality assessment results by study for all aspects of quality for the review of incentives for smoking cessation in pregnancy.
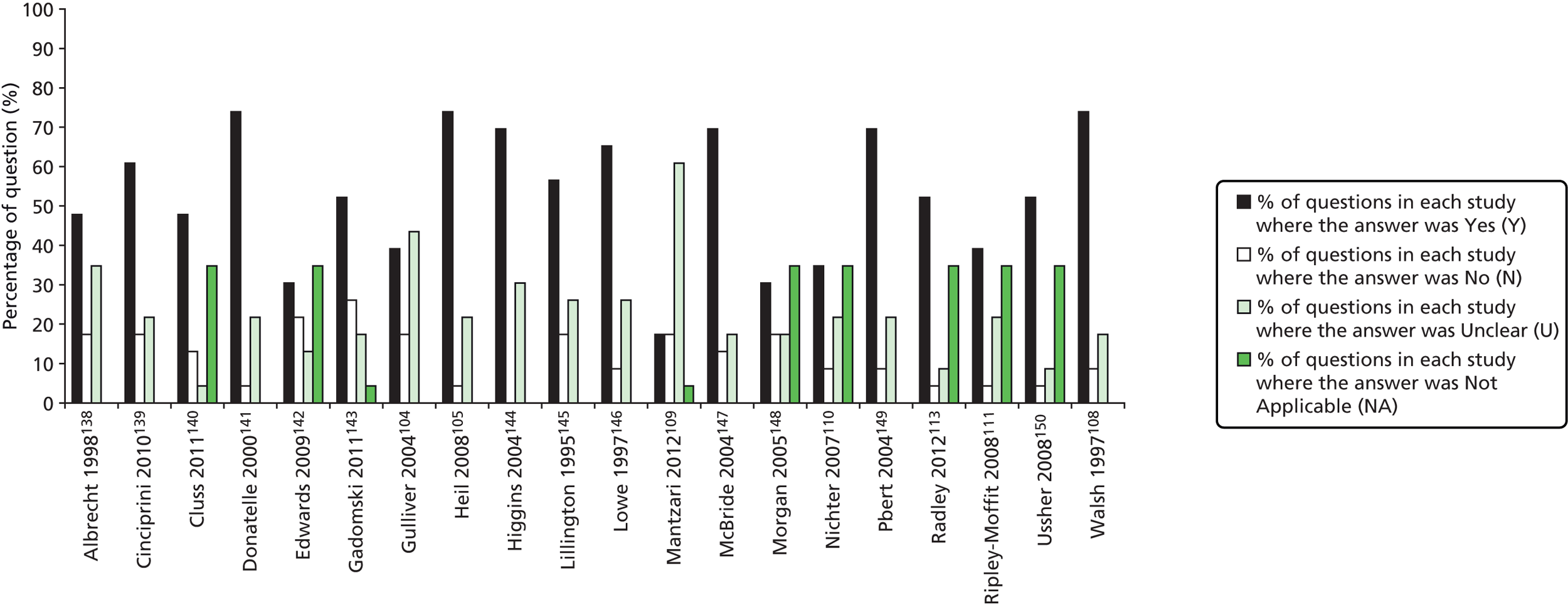
For some studies, details regarding the study population were absent and so it was less clear that participants were from a representative sample of a relevant patient population or that groups were comparable on demographic characteristics, although in some studies there was no comparator group and so it was not possible to answer this question or those regarding whether length of follow-up was similar between groups and whether groups were treated identically other than for the named intervention. For most studies (i.e. > 50% of studies) objective (valid and reliable) outcome measures had been used (e.g. biochemical validation of cessation).
More problematic aspects of study quality were the lack of clarity regarding the selection of patients and allocation concealment for studies reporting to be randomised. The effect of loss to follow-up on smoking cessation results was also unclear. Consistent with the Russell standard for outcome criteria in smoking cessation trials,163 we assumed for the purposes of our analysis that loss to follow-up would result in smoking relapse.
With regard to Figure 6, studies with the lowest proportion of ‘yes’ answers were those with an atypical study design. For example, the studies by Edwards and colleagues142 and Morgan and colleagues148 considered the effect of the interventions at a population level and the studies by Mantzari and colleagues,109 Nichter and colleagues110 and Ripley-Moffitt and colleagues111 were predominantly qualitative with additional quantitative results mentioned. In addition, several studies, including those by Cluss and colleagues140 and Ussher and colleagues,150 did not have comparator groups and therefore some of the questions considering the appropriateness of how groups were selected and treated did not apply.
The overall quality of the qualitative content of the four included qualitative and mixed-methods studies,109–111,113 assessed using CASP,135 was high and the detail is presented in Table 3.
| Study | CASP score | Main weaknesses |
|---|---|---|
| Mantzari 2012109 | 9/10 | CASP: 0 points given for reflexivity |
| Nichter 2007110 | 9/10 | CASP: 0.5 points given each for reflexivity and value of the research |
| Radley 2013113 | 7.5/10 | CASP: 0.5 points given each for data collection, data analysis and findings; 0 points given for reflexivity |
| Ripley-Moffitt 2008111 | 9/10 | CASP: 0 points given for reflexivity |
Characteristics of the included studies
Studies were classified from A to E according to incentive and study design. This classification is presented in Table 4.
| Category | Studies |
|---|---|
| Category A studies (n = 4) compare incentives contingent on validated smoking cessation outcomes with non-contingent incentives for participation | A: Donatelle 2000,141 Heil 2008,105 Higgins 2004,144 Mantzari 2012109 |
| Category B studies compare incentives contingent on validated smoking cessation outcomes (B1, n = 2 studies) or non-contingent incentives (B2, n = 5 studies) with no incentive | B1: Gadomski 2011,143 Walsh 1997108 |
| B2: Albrecht 1998,138 Edwards 2009,142 Lillington 1995,145 Lowe 1997,146 McBride 2004147 | |
| Category C studies evaluate incentives contingent on validated smoking cessation outcomes (C1, n = 2 studies) or non-contingent incentives (C2, n = 5 studies), but do not have a control group | C1: Morgan 2005,148 Radley 2013113 |
| C2: Cluss 2011,140 Gulliver 2004,104 Nichter 2007,110 Ripley-Moffitt 2008,111 Ussher 2008150 | |
| Category D studies are those in which both the intervention group and the control group receive the same contingent (D1, n = 0 studies) or non-contingent (D2, n = 2 studies) incentives and these studies are therefore comparing another intervention component | D2: Cinciripini 2010,139 Pbert 2004149 |
| Category E studies are those in which no incentive intervention is provided (n = 1) | E: Lynagh 2011151 |
The main characteristics of the 20 included studies that report incentives for smoking cessation in childbearing women are described in Table 5. Detailed characteristics of the incentive/reward are described in Table 6; the setting, provider and intensity of incentive delivery are summarised in Table 7 and the chronological patient journey through the intervention showing the timing of intervention components and BCTs offered to study participants is shown in Table 8.
| Study and category | Country | Study design as described | Total n participants | Incentive | Value of incentive | Contingency | Cessation biochemically validated | Smoking outcome | Main outcome reported towards the end of pregnancy (see Smoking outcome time point in bold) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Donatelle 2000141 A1 |
USA | RCT | 220 | Vouchers | Varied by arm | Varied by incentive | Yes | Cessation at end of pregnancy, 2 months post partum | I: 34/105 (32.4%) | ||
| C: 9/80 (11.3%) | |||||||||||
| RR (95% CI): 2.88 (1.47 to 5.65) | |||||||||||
| p-value: < 0.001 | |||||||||||
| Heil 2008105 A1 |
USA | RCT | 82 | Vouchers | High | Varied by arm | Yes | Cessation at end of pregnancy, 3 and 6 months post partum | I: 16/37 (43.2%) | ||
| C: 4/25 (16.0%) | |||||||||||
| RR (95% CI): 2.70 (1.02 to 7.14) | |||||||||||
| p-value: 0.024 | |||||||||||
| Higgins 2004144 A1 |
USA | RCT | 58 | Vouchers | High | Varied by arm | Yes | Cessation at end of pregnancy, 3 and 6 months post partum | I: 11/30 (36.7%) | ||
| C: 2/21 (9.5%) | |||||||||||
| RR (95% CI): 3.85 (0.95 to 15.61) | |||||||||||
| p-value: 0.029 | |||||||||||
| Mantzari 2012109 A1 |
UK | Non-randomised, comparative | 36 | Vouchers + £20.00 per interview | High | Yes | Yes | Cessation at unclear time point (7–9 months) | I: 8/19 (42.1%) | ||
| C: 4/15 (26.7%) | |||||||||||
| RR (95% CI): 1.58 (0.59 to 4.25) | |||||||||||
| p-value: 0.35 | |||||||||||
| Gadomski 2011143 B1 |
USA | Non-randomised, comparative | 858 | Vouchers | Moderate | Yes | Yes | Cessation at 3 and 6 months post partum | Not reported | ||
| Walsh 1997108 B1 |
Australia | RCTa | 293 | Lottery + gifts | Varied by incentive | Varied by incentive | Yes | Cessation at 4 weeks from baseline, end of pregnancy, early post partum (< 2 months) | I: 17/127 (13.4%) | ||
| C: 7/125 (5.6%) | |||||||||||
| RR (95% CI): 2.39 (1.03 to 5.56) | |||||||||||
| p-value: 0.035 | |||||||||||
| Albrecht 1998138 B2 |
USA | RCTb | 84 | Gift items | Low | No | Yes | Cessation at 4–6 weeks from baseline | Not reported | ||
| Edwards 2009142 B2 |
USA | Non-randomised, comparative | 8445 | Gift items | Low | No | No | Cessation at unclear time point | Not reported | ||
| Lillington 1995145 B2 |
USA | Cluster RCTb | 768 | Weekly lottery | Varied by incentive | No | No | Cessation at end of pregnancy and early (< 2 months) post partum | I: 106/208 (51.0%) | ||
| C: 263/560 (47.0%) | |||||||||||
| RR (95% CI): 1.09 (0.92 to 1.27) | |||||||||||
| p-value: 0.32 | |||||||||||
| Lowe 1997146 B2 |
USA | RCTa | 97 | Gift items | Low | No | Yes | Cessation at end of pregnancy | I: 32/45 (71.0%) | ||
| C: 29/52 (56.0%) | |||||||||||
| RR (95% CI): 1.28 (0.94 to 1.73) | |||||||||||
| p-value: 0.12 | |||||||||||
| McBride 2004147 B2 |
USA | RCTa | 625 | Gift items | Low | No | No | Cessation at end of pregnancy, early post partum (< 2 months) and 6 months post partum | I1: 118/193 (61.0%) | I2: 113/192 (59.0%) | |
| C: 119/198 (60.0%) | |||||||||||
| RR for I1 (95% CI): 1.02 (0.87 to 1.19) | RR for I2 (95% CI): 0.98 (0.83 to 1.15) | ||||||||||
| I1 p-value: 0.83 | I2 p-value: 0.80 | ||||||||||
| Morgan 2005148 C1 |
UK | Non-randomised, cohort | 86 | Gift items/rewards | Moderate | Yes | Yes | Cessation at 4 weeks from baseline | Not reported | ||
| Radley 2013113 C1 |
UK | Non-randomised, cohort | 383 | Weekly incentive voucher | High | Yes | Yes | Cessation at 4 weeks, 12 weeks and 3 months post partum | Not reported | ||
| Cluss 2011140 C2 |
USA | Non-randomised, cohort | 856 | Gift items | Low | No | Yes | Cessation at end of pregnancy | I: 119/856 (13.9%) | ||
| C: not applicable | |||||||||||
| RR (95% CI): not applicable | |||||||||||
| p-value: not applicable | |||||||||||
| Gulliver 2004104 C2 |
USA | RCTb | 20 | Gift items | High | Varied by incentive | Yes | Cessation at 4 weeks from baseline | not reported | ||
| Nichter 2007110 C2 |
USA | Non-randomised, cohort | 53 | Small gift + US$20.00 per interview | Moderate | No | Yes | Cessation at end of pregnancy | I: 16/53 (30.2%) | ||
| C: not applicable | |||||||||||
| RR (95% CI): not applicable | |||||||||||
| p-value: not applicable | |||||||||||
| Ripley-Moffitt 2008111 C2 |
USA | Non-randomised, cohort | 94 | Small cash incentive + gift card | Unclear | No | Yes | Cessation at 4 months post partum | Not reported | ||
| Ussher 2008150 C2 |
USA | Non-randomised, cohort | Study 1: 10; study 2: 22 | Not stated as incentives | Study 1: low/moderate; study 2: unclear | No | Yes | Study 1: cessation at 4 weeks from baseline and at end of pregnancy; Study 2: cessation at 1 week and 6 weeks post baseline, and at end of pregnancy | I1: 5/10 (50.0%) | I2: 3/22 (13.6%) | |
| C: not applicable | C: not applicable | ||||||||||
| RR (95% CI): not applicable | RR (95% CI): not applicable | ||||||||||
| p-value: not applicable | p-value: not applicable | ||||||||||
| Cinciripini 2010139 D2 |
USA | RCTb | 266 | US$ or gift card | Moderate | No | Yes | Cessation at 10, 12 and 26 weeks from baseline and 3 and 6 months post partum | I1: 40/129 (31.0%) | I2: 47/128 (36.7%) | |
| C: not applicable | |||||||||||
| RR (95% CI): not applicable | |||||||||||
| p-value: not applicable | |||||||||||
| Pbert 2004149 D2 |
USA | RCTb | 609 | Gift items | Unclear | No | Yes | Cessation at end of pregnancy, early post partum (< 2 months), 3 months post partum and 6 months post partum | I1: 82/278 (29.6%) | I2: 95/272 (35.0%) | |
| C: not applicable | |||||||||||
| RR (95% CI): not applicable | |||||||||||
| p-value: not applicable | |||||||||||
Most studies originated from the USA and they fell into two main categories: (1) contingent incentives for smoking cessation or (2) non-contingent incentives for participation in a smoking cessation programme and providing outcome data. This latter category included incentives for participation in another intervention component in the study or research to understand participants’ smoking behaviour. Within these two broad categories, a range of comparisons was present. Three studies were considered to have two incentive intervention arms. 138,139,147 The studies by Albrecht and colleagues138 and McBride and colleagues147 are B2 studies; both intervention arms involved incentives and these are compared with a comparator arm (usual care). The study by Cinciripini and colleagues139 is a category D study; both study arms received incentives and differed only in terms of another non-incentive component.
Intervention content and format
Studies providing multicomponent incentive interventions to aid smoking cessation among pregnant women are summarised in Table 6. All incentive interventions involved interactions with incentive providers or other professionals, either directly or indirectly supporting smoking cessation, and we refer to these as the general BCT components of the intervention. However, communication at the time of delivering the incentive is under-reported and no study mentions observations or recordings of interactions during intervention delivery. The proportion of the intervention that can be attributed to the incentive and to the BCT component varied between studies and was sometimes difficult to assess. In category A studies105,109,141,144 the incentive was the dominant component. In some studies the primary study aim was to investigate separate BCT components, with the incentive of secondary importance, for example to improve recruitment, intervention uptake or reduce attrition. For category B–D studies, women were invited to participate in a range of activities to obtain the incentives, some of which were reported as usual care plus additional components110,111,138 or an enhanced programme of care108,145–149 or involved the implementation of a new protocol. 104,113,139,140,142,143,150 These programme components involved either one or a combination of advice, counselling, group sessions, written materials and motivational strategies and, in one study, a programme of supervised exercise. 150
| Study and category | Incentive/reward type | Awareness | Content | Contingency target | Actor |
|---|---|---|---|---|---|
| Donatelle 2000141 A1 |
Vouchers (US$50.00) | Unclear | Material | Outcome | Participant |
| Voucher (US$5.00) | Unclear | Material | Preparatory behaviour | Participant | |
| Vouchers (between US$25.00 and US$50.00) | Yes | Material | Outcomea | Social other | |
| Heil 2008105 A1 |
Vouchers (between US$6.25 and US$45.00) | Yes | Material | Outcome | Participant |
| Vouchers (between US$15.00 and US$25.00) | Yes | Material | Preparatory behaviour | Participant | |
| Higgins 2004144 A1 |
Vouchers (between US$6.25 and US$45.00) | Yes | Material | Outcome | Participant |
| Vouchers (between US$11.50 and US$20.00) | Yes | Material | Preparatory behaviour | Participant | |
| Mantzari 2012109 A1 |
Vouchers | Yes | Material | Health behaviour | Participant |
| Money (£20.00) | Yes | Material | Preparatory behaviour | Participant | |
| Vouchers (£10.00–40.00) | Yes | Material | Outcome | Participant | |
| Gadomski 2011143 B1 |
Vouchers (US$40.00) | Yes | Material | Outcome | Participant |
| Walsh 1997108 B1 |
Lottery (prize of A$75.00) | Yes | Material | Outcome | Participant |
| Albrecht 1998138 B2 |
‘Gifts and refreshments’ | No | Material | Preparatory behaviour | Participant |
| Edwards 2009142 B2 |
Gifts (e.g. bottles, baby bibs, sipper cups) | Unclear | Material | Preparatory behaviour | Participant |
| ‘Incentives’ | Unclear | Non-specific | Preparatory behaviour | Health professional | |
| Vouchers (US$20.00) | Yes | Material | Outcome | Participant | |
| Raffle ticket (to win a car seat) | Yes | Material | Preparatory behaviour | Participant | |
| Lillington 1995145 B2 |
Raffle prizes (e.g. baby items + US$100.00 grand prize) | Yes | Material | Preparatory behaviour | Participant |
| Cloth baby bib | No | Material | Preparatory behaviour | Participant | |
| Lowe 1997146 B2 |
Gifts (e.g. toothbrushes, chewing gum, nappy coupons) | No | Material | Preparatory behaviour | Participant |
| Praise | No | Social | Health behaviour | Participant | |
| McBride 2004147 B2 |
‘Gift items’ | No | Material | Preparatory behaviour | Participant |
| Morgan 2005148 C1 |
Rewards (e.g. aromatherapy massage, flowers, bubble bath) | No | Material | Health behaviour | Participant |
| Congratulatory postcard, certificate | No | Social | Health behaviour | Participant | |
| Radley 2013113 C1 |
Vouchers (£12.50) | Yes | Material | Outcome | Participant |
| Cluss 2011140 C2 |
Small baby gifts (< US$5.00 each) | Unclear | Material | Preparatory behaviour | Participant |
| Gulliver 2004104 C2 |
Raffle ticket (to win a car seat) | Yes | Material | Preparatory behaviour | Participant |
| Coupon for items on a list (brand new) | Yes | Material | Outcome | Participant | |
| Nichter 2007110 C2 |
Money (US$20.00) + gift (infant health kit) | Yes | Material | Preparatory behaviour | Participant |
| Ripley-Moffitt 2008111 C2 |
‘Cash incentive’ + ‘gift card’ | Unclear | Material | Preparatory behaviour | Participant |
| Ussher 2008150 C2 |
‘Supervised exercise sessions’ | Unclear | Non-specific | Preparatory behaviour | Participant |
| Cinciripini 2010139 D2 |
Money (US$40.00) | Unclear | Material | Preparatory behaviour | Participant |
| Voucher (US$15.00) | Unclear | Material | Preparatory behaviour | Participant | |
| Pbert 2004149 D2 |
Vouchers (for grocery store) | Yes | Material | Preparatory behaviour | Participant |
Incentive/reward-related elements
When extracting the IRBCT data, incentive and reward elements were identified reliably (kappa = 0.87). Out of the 20 studies, eight104,109–111,139,142,145,148 used multiple incentives (see Table 6) within their interventions, which varied in the four assessed incentive elements: material, social, self-selected and non-specific (see Table 2).
All studies included material incentives/rewards as this was a criterion for study inclusion. Two interventions specifically mentioned including social incentive/reward components such as congratulatory cards for being abstinent148 and health-care professional praise146 as well as the material incentive. In addition, one intervention offered supervised exercise classes. 150
Incentive categories emerged through analysis of the description of the incentives and the context in which they were delivered:
-
Earned rewards, for example vouchers contingent on biochemical validation of quit status as in the studies by Heil and colleagues,105 Higgins and colleagues144 and Donatelle and colleagues. 141
-
Prizes or awards – these could be either contingent on programme participation as in the study by Pbert and colleagues149 or contingent on stopping smoking. They were either predictable or unpredictable as in the raffles provided in the study by Gulliver and colleagues. 104
-
Gifts that were not contingent on either programme participation or quitting, with the aim of encouragement or increasing goodwill towards the programme deliverers or research team, as in the study by Edwards and colleagues. 142
-
Compensatory, for example voucher-based reinforcement therapy (VBRT). This was for participating in BCT programmes that might be demanding in time and commitment, as in the study by Cinciripini and colleagues. 139
Further analysis of the meanings of these emerging incentive categories is provided in Chapter 6, where qualitative data contribute to an incentive typology.
General behaviour change techniques
One study152 was excluded from BCT data extraction as the intervention was rolled out at the health system level rather than at the individual level and was thus not in line with the target behaviour.
When extracting the general BCT data, inter-rater agreement was acceptable, with a kappa of 0.78, ranging from 0.66 (‘offer/direct towards appropriate written materials’) to 0.94 (‘facilitate goal-setting’). Explicit reporting of BCTs underpinning interventions varied and this was often unclear or implicit. Altogether, 32 study arms were rated for the inclusion of 41 BCTs, including nine control/usual care arms and 23 intervention arms. Intervention arms were identified to use an average of 6.62 [standard deviation (SD) 3.1] BCTs, ranging from one109 to 12,145 emphasising that incentive interventions are complex and consist of various potentially active ingredients. Control/usual care arms used an average of 1.11 (SD 0.9) BCTs, ranging from zero109,143 to three. 138
Out of 41 BCTs rated, study arms were identified to have used 30 (Figure 7), with the BCTs used most often being ‘providing information on the consequences of smoking cessation’ (n = 24), ‘measuring CO’ (n = 18), which mostly took the form of objectively validating smoking status within intervention encounters, and ‘facilitating goal-setting’ (n = 15), which in most studies took the form of setting a formal quit date.
FIGURE 7.
General BCTs used in all intervention arms in the studies included in the review of incentives for smoking cessation in pregnancy.

In addition to the BCTs, six studies were also rated as being engaged in the WIC programme105,141–143,145,149 and one study was rated as being partially engaged in the WIC programme. 110 BCTs used in the WIC programme were recorded separately without inferring the presence of specific BCTs given the variation in WIC programme implementation.
The majority of individuals targeted to receive the incentives/rewards were judged to have been aware of these components (n = 12); of the remaining rewards, some were judged as not being known to participants (n = 5) and it was unclear whether participants were aware of the others (n = 5).
Setting and provider
Included studies were mostly from the USA, with three109,113,148 set in the UK (see Table 5). The studies varied in terms of their funding sources. One was funded by a research council108 and several were funded by private trusts. 104,109–111,142,151 One study was jointly funded by a private trust and a national health provider142 and the remainder were funded by national health or government institutes,113,138–140,143,144,146–150 with two receiving extra financial support from the WIC programme. 105,145 In addition, two explicitly relied on donations from local businesses to provide the incentives,104,108 which was framed as helping to reinforce the message of community support for smoking cessation through drawing women’s attention to it, as well as being cost-saving and making the programme and its continuation more attractive and sustainable to principal funders. The authors’ disciplinary backgrounds were also assorted and often involved multidisciplinary collaborations among research teams. A number of studies had a behavioural/psychological background108,109,139,147,149 and many involved public health/health researchers,113,141,142,146,150 with one overlapping both areas. 151 A number of studies were led by psychiatric specialists105,140,144 and two had other origins: education (paired with midwifery)148 and nursing. 138 Several had a cancer-specific derivation145–147 and two were approached by substance abuse/addiction experts. 104,111 One study was led by anthropologists who used ethnographic methods to understand participant perspectives. 110
The intervention settings and providers varied across the included studies (Table 7). A number of the studies were WIC based and were delivered in WIC clinics by WIC staff as an enhancement of usual care/smoking in pregnancy interventions. 105,141–143,145,149 One study recruited women through the WIC programme but women were interviewed in their own homes. 110 Similarly, other studies also involved improved or more intensive programmes designed for smoking cessation in pregnancy delivered alongside or through health services (e.g. the NHS) where contact with pregnant women was already taking place. 109,148 The others were mostly delivered in health-care settings but involved attending a non-standard clinic. 104,111,113,138–140,146,150 One intervention was delivered through an army hospital147 and others specifically recruited pregnant women to their studies and delivered the interventions in other settings, such as a university-based clinic,111,144 a teaching hospital108 and in schools. 138 Although most of the programmes were delivered in clinics by health professionals or research staff, some also involved trained workers carrying out home visits/offering telephone support,110,113,148 a helpline109,143 or telephone calls. 141,149 Details were often provided about the setting and staff providing the counselling-based components of the intervention, but little information was given regarding the process of providing incentives to participants. Some differentiation was made between the staff delivering the intervention, although in most cases this was carried out by usual-care health professionals: WIC staff,142,143 doctors and midwives,108 clinical psychologists,104 nurses138,146 and pharmacists. 113 The details reported were variable, but some noted when staff were specifically trained to deliver the intervention. 113,140,141,148 Study staff were also involved in delivering the intervention in many of the studies. 104,105,109–111,138,139,141,143–145,147,149 A number of the studies involved a community element whereby the smoking cessation message was explicitly reinforced either at routine clinic appointments in addition to intervention components108,140,146 or through community-based messages. 104
| Study and category | Setting | Who delivered the intervention | Mode of delivery | Recruitment (week of pregnancy) | Number of contacts when incentive provided | Number of contacts when no incentive was provided | Total number of contacts | Number of contacts with CO/saliva/urine monitoring | Intervention finish (week/month before or after birth) | Final follow-up contact (week/month before or after birth) | Total study contact period |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Donatelle 2000141 A1 |
WIC clinic | WIC programme or Singificant Other Supporter programme research staff | Face-to-face, telephone and social support | < 28 weeks | < 13 | Only if specimen positive | 13 | 13 | 2 months post partum | 2 months post partum | Maximum 10 months |
| Heil 2008105 A1 |
WIC clinic | Study staff at university-based research clinic | Face to face | ≤ 20 weeks | 36 | None | 36 | 36 | 12 weeks post partum | 24 weeks post partum | > 43 weeks |
| Higgins 2004144 A1 |
Three large group obstetric practices or one single-practitioner obstetric practice | Study staff at university-based research clinic | Face to face or telephone | ≥ 32 weeks | 36 | None | 36 | 36 | 12 weeks post partum | 24 week post partum | < 31 weeks |
| Mantzari 2012109 A1 |
Primary care trust/NHS Stop Smoking Services | Call centre staff, research staff | Telephone and face to face | Any – unclear | 9 | 1 | 10 | 8 | 2 months post partum | 2 months post partum | Unclear |
| Gadomski 2011143 B1 |
WIC clinic | WIC programme and research staff | Face to face and telephone | Unclear | 12 post-partum | ≤ 4 in pregnancy | 16 | 16 | 12 months post partum | < 12 months post partum | Unclear (> 12 months) |
| Walsh 1997108 B1 |
Urban teaching hospital | Doctors, midwives | Face to face or by mail | Any – unclear | 2 | 1 | 3 | 3 | 34–36 weeks | 6–12 weeks post partum | Unclear |
| Albrecht 1998138 B2 |
Prenatal clinics and local public schools | Project investigators, project nurse, clinic and school nurses | Face to face, group or face to face, group and a buddy | From 4 weeks to < 28 weeks | 8 | (Buddy arm – can have additional contact with buddy) | > 8 | 2 (baseline and at study finish) | 4–6 weeks after study entry | 4–6 weeks after study entry | 4–6 weeks |
| Edwards 2009142 B2 |
WIC clinic | Trained providers and various WIC staff | Face to face and telephone | Any – unclear | > 3 (unclear whether all incentives provided at baseline) | Unclear | > 3 | None | Post partum – unclear | Post partum – unclear | Unclear |
| Lillington 1995145 B2 |
WIC clinic | Study staff (bachelor’s degree/bilingual health educators) | Face to face or telephone and by mail | Any | 2 | 1 | 3 | 2 | 1 month after study entry | 6 weeks post partum | Unclear |
| Lowe 1997146 B2 |
Public health maternity clinics | Nurses, health educators, prenatal nurses | Face to face and buddy | Any – unclear | 1 | Clinic appointments (n = unclear) plus one process evaluation interview | > 1 | 1 | Unclear | Unclear | Unclear |
| McBride 2004147 B2 |
Womack Army Medical Centre (WAMC) | Study staff, health advisors | Letter, face to face, telephone and partner coaching | ≤ 20 weeks | 1 | Five study interviews, six counselling calls | 12 | Not applicable | 4 months post partum | 12 months post partum | 16 months |
| Morgan 2005148 C1 |
SureStart UK | SureStart midwife, other trained personnel | Face to face and telephone | Any | Unclear | Unclear | Minimum 6–8 | Each visit (minimum 6–8) | As long as women needed support | As long as women needed support | As long as women needed support |
| Radley 2013113 C1 |
Clinics and at home | Give it up for Baby development worker | Face to face | Any – unclear | Weekly during pregnancy | Weekly | Weekly | During pregnancy (although support available post partum) | Unclear – variable | Pregnancy and post partum – unclear | |
| Cluss 2011140 C2 |
Delivered in community health clinics. Referrals from a wide variety of sources including hospital obstetric providers, community clinics, drug and alcohol rehabilitation centres, school-based programmes for pregnant teens and self-referrals | Delivered by STOP counsellors. Close collaboration of programme staff with all referral sources including information and feedback | Face to face or telephone | Any | > 4 | None | > 4 | > 4 | Final session post partum (unclear) | Final session post partum (unclear) | Unclear as varied |
| Gulliver 2004104 C2 |
Private obstetric practices, self-referral through community flyers and newspaper ads | Research assistant, clinical psychologist | Telephone and face to face (individuals and couples) and groups | Any | Regular check-ins (unclear) | Only if positive specimen | Unclear | At every check-in | < 3 months post partum | < 3 months post partum | Unclear (pregnancy and < 3 months post partum) |
| Nichter 2007110 C2 |
WIC clinics and other local community sites | Advanced graduate anthropology students (female) | Face to face | < 28 weeks | 4 | 4 | 4 | 3 | Child’s birth | Child’s birth | From 28 weeks to birth |
| Ripley-Moffitt 2008111 C2 |
University and community clinics and local health departments | Clinic directors, prenatal co-ordinators, social workers, research staff | Face to face and telephone plus social support | < 30 weeks | 2 | 2 | 4 | 4 | 4 months post partum | 4 months post partum | > 6 months |
| Ussher 2008150 C2 |
Antenatal care at one London hospital | Antenatal clinic staff, scan clinic staff, hospital and community midwives | Telephone, letter, face to face | From 12 to < 20 weeks | Study 1: 6; study 2: 15 | Not applicable | Study 1: 6; study 2: 15 | Study 1: 6; study 2: 15 | < 8 months | < 8 months | Study 1: 5/6 weeks; study 2: 8/9 weeks |
| Cinciripini 2010139 D2 |
In clinic | Five doctoral-level clinical psychologists and two master’s-level counsellors | Telephone and face to face | ≤ 32 weeks | 16 | 2 | 18 | 17 | 6 months post partum | 6 months post partum | From 8 to < 14 months |
| Pbert 2004149 D2 |
WIC clinic | Research assistants | Face to face or telephone | Any | 5 | Only if positive specimen | 5 | 5 | 6 months post partum | 6 months post partum | > 6 months |
Intervention intensity and the participant journey
Studies provided multicomponent interventions to aid smoking cessation among pregnant women, with interventions (or even individual components within the intervention) provided at varying frequencies and time points. This is summarised in Tables 7 and 8. Intervention intensity refers to the number of contacts involved for patients from the start of the intervention until the final outcome data are collected; the study duration; and the length of individual encounters. The participant journey refers to a participant’s experience of the intervention components (time points and frequency) during the course of the intervention.
| Study and category | M2 | M3 | M4 | M5 | M6 | M7 | M8 | Birth | M1 | M2 | M3 | M4 | M5 | M6 | (M12)a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Donatelle 2000141 A1 |
WB£IG TWB£S |
TWB£S | TWB£S | TWB£S | TWB£S | TWB£S | WB£ TWB£S |
TWB£S | TWB£S | WB£ TWB£S |
|||||
| Heil 2008105 A1 |
WB£ Q B£B£B£B£B£ B£B£ B£B£ B£B£ |
B£B£ B£B£B£B£B£B£ |
B£ B£ B£ B£ |
B£ B£ |
WB£ B£ B£ |
WB£WB£ B£ B£ B£ B£ |
WB£ B£ B£ |
WB£ B£ B£ |
WB£ | ||||||
| Higgins 2004144 A1 |
WB£ Q B£B£B£B£B£ B£B£ B£B£ B£B£ |
B£B£ B£B£B£B£B£B£ |
B£ B£ B£ B£ |
B£ B£ |
WB£ B£ B£ |
WB£WB£ B£ B£ B£ B£ |
WB£ B£ B£ |
WB£ B£ B£ |
WB£ | ||||||
| Mantzari 2012109 A1 |
T G£ B£ B£ |
B£ B£ |
B£ | B£ G£ |
B£ | ||||||||||
| Gadomski 2011143 B1 |
Q GB |
GB | GB | GB | B£G(I) | B£G(I) | B£G(I) | B£G(I) | B£G(I) | B£G | B£G(I) B£G (I) B£G(I) B£G(I) B£G(I) B£G(I) |
||||
| Walsh 1997108 B1 |
GI£WSBR | WB£ | WB | WB | |||||||||||
| Albrecht 1998138 B2 |
GS£ | GS£ GS |
GS£ | GS£ GS£ |
GS£ | GS£ | |||||||||
| GSB£ | GSB£ GSB |
GSB£ | GSB£ GSB£ |
GSB£ | GSB£ | ||||||||||
| Edwards 2009142 B2 |
IGQ£W | G£W | G£W | ||||||||||||
| Lillington 1995145 B2 |
WGIQS | W£R W£ |
W£ W£ |
W£ W£ |
W£ W£ |
W£ W£ |
W£ W£ |
W | |||||||
| Lowe 1997146 B2 |
GSIR£ | R | R | R | R | R | R | ||||||||
| McBride 2004147 B2 |
WI£ | T | T | W | T | T | WT | T | W | W | |||||
| WI£ | T TF |
T TF |
W | T TF |
T TF |
WT TF |
T TF |
W | W | ||||||
| Morgan 2005148 C1 |
GWBI£ GWBQ GBT£ GBT£ |
GBT£ GBT£ (GB£)T (GB£)T |
T | T | T | T | T | ||||||||
| Radley 2013113 C1 |
GB£(T) | GB£(T) | GB£(T) | GB£(T) | GB£(T) | GB£(T) | GB£(T) | (GT) | (GT) | (GT) | (GT) | (GT) | (GT) | (GT) | |
| Cluss 2004140 C2 |
WB GB£QI | GB£QI | GB£QI | GB£QI | GB£QI | GB£QI | WB GB£QI |
GB£QI | |||||||
| Gulliver 2004104 C2 |
FGWIQB£ | GB£F | GB£F | GB£F | GB£F | GB£F | GB£F | GB£F | GB£F | GB£F | |||||
| Nichter 2007110 C2 |
£BG | £BG | £BG | £ | |||||||||||
| Ripley-Moffitt 2008111 C2 |
BW£ | TB | TB | GB£ | |||||||||||
| Ussher 2008150 C2 |
GI Study 1: £QB£B£B£B Study 2: £B£BQ£B£B£B£B£B£B |
Study 1: £B£BG Study 2: £B£B£B£B £B £B |
Study 2: £BG | W | |||||||||||
| Cinciripini 2010139 D2 |
GWB£ GWB£ Q |
GWB£ GWB£ GWB£ GWB£ |
GWB£ GWB£ GWB£ GWB£ |
WB£ | WB£ WB£ |
WB£ | WB£ | ||||||||
| Pbert 2004149 D2 |
WGB£R | R | R | WGB£R | WGB£R | R | WGB£R | R | R | WGB£R |
The time points at which women were recruited to the studies, when they first attended and when they received the first incentive varied and were often unclear. In particular, differentiation between contacts that were integrated with usual care and contacts that were separate from usual care was often unclear. In several studies it appears that recruitment could occur at any point during pregnancy. 104,109,113,140,142,144–146,148 When specific details were given, the earliest point at which women were reported to be recruited was at 4 weeks’ gestation138 and the latest was at 32 weeks’ gestation. 139 The incentive, or first incentive if more than one was given, was not necessarily provided at the point of recruitment; however, an incentive was given at initial intervention sessions in all studies with the exception of one. 143 In this study, the incentives (vouchers for nappy purchase) were not provided until the post partum period. In all of the other studies, incentives included items that were delivered during pregnancy. The overall number of contacts ranged from one to 36. In four of the studies, in addition to receiving the intervention, participants were also given usual care,108,138,144,147 whereas in three of the studies usual care was the control condition. 105,143,149 It might be assumed, however, that usual care was delivered in addition to the intervention in the remainder of the studies when this was not made clear, as well as for those in which it was the control condition. This, and the number of contacts that usual care might involve, was poorly reported throughout.
Some of the studies reported intensity in more detail with weekly attendance for the duration of the treatment and two involved daily monitoring for a period of 5 days. 105,144 Duration of treatment varied, with some studies delivering the programme components regularly over a short period of time, for example several, often consecutive, weeks,104,108,110,113,138–140,143,145,147–150 and some involving assessment at more spaced time points, for example at baseline, before delivery and postnatally. 111,141,142,146 Others were more varied, for example weekly to begin with and then after 3 months and 5 months. 109 End of treatment appears to have occurred before birth in some of the studies,138,140,150 although it is sometimes unclear. Six studies involved follow-up by way of an interview or assessment at a specified time point up to 6 months post partum. 105,139,143,144,147,149 In two studies contacts continued up to 1 year after birth,143,147 with one study providing monthly visits. 143 In another study an optional telephone helpline was available up to 1 year after birth. 113
Cinciripini and colleagues139 reported the mean duration of therapy sessions as 58 minutes (SD 10.1 minutes), with no significant differences in length of session between treatment groups, although in this study both treatment groups were incentivised to participate.
Ussher and colleagues150 reported the mean minutes of supervised exercise recorded for one of the studies they conducted that used exercise as the main component of the intervention, with supervised exercise increasing from 8.4 minutes (SD 3.5 minutes, n = 22) at the first visit to 19.9 minutes (SD 8.6 minutes, n = 16) on the first day of cessation, 23.3 minutes (SD 6.1 minutes, n = 6) 5 weeks after the first day of cessation and 18.5 minutes (SD 14.5 minutes, n = 6) 6 weeks after the first day of cessation.
Intervention fidelity/non-compliance
The fidelity of the intervention, in terms of whether the intervention was actually delivered or implemented in the way described in the protocol, was seldom reported. The intervention provided in the study by Gulliver and colleagues104 required participants to select a quit day; however, six of the 10 participants did not quit on their selected quit day.
Outcome measurement
In terms of incentive targets, 15 studies targeted preparatory behaviours (Figure 8). Preparatory behaviours targeted through incentives were intervention attendance and participation behaviours (n = 11), stating an intention to quit (n = 1), engaging in exercise (n = 1), counselling pregnant smokers (n = 1) or not specified (n = 3). Nine studies were identified as targeting only preparatory behaviours. 110,111,138–140,145,147,149,150 Six studies targeted preparatory behaviour in addition to outcomes (n = 4),105,141,142,144 health behaviour (n = 1)146 or outcomes and health behaviours simultaneously (n = 1)109 within the same intervention. Other studies targeted outcomes108,113,143 or health behaviours only. 148
FIGURE 8.
Combinations of contingency targets for incentive/rewards.

Outcome assessment varied depending on the study methodology used. Abstinence was point prevalence alone in 11 studies. 104,109,113,138,140–142,144,146,148,149 Seven studies attempted to quantify both point-prevalent and continuous abstinence. 105,108,139,143,145,147,150
Of the studies that had considered point-prevalent abstinence only, ten out of 11 (81.8%)104,109,113,138,140,141,144,146,148,149 had biochemically validated this. Seven studies104,109,113,138,140,144,148 had done so using CO levels, with the cut-offs ranging from ≤ 6 p.p.m. 144 to ≤ 10 p.p.m.. 104 The cut-off was unspecified in the studies by Morgan and colleagues148 and Radley and colleagues. 113 Two studies141,146 used thiocyanate to biochemically validate abstinence although only the study by Donatelle and colleagues141 specified the cut-off for this as 100 μg/ml. The studies by Donatelle and colleagues,141 Higgins and colleagues144 and Pbert and colleagues149 also used cotinine validation, with cut-offs ranging from 20 ng/ml to 80 ng/ml. The remaining study relied on self-reporting of smoking status. 142
Of the studies that used both point-prevalent and continuous abstinence, five out of seven (71.4%) had biochemically validated this. 105,108,139,143,150 Four studies105,139,143,150 had done so using CO levels, with the cut-offs ranging from < 4 p.p.m. 139 to < 8 p.p.m.,150 The studies by Cinciripini and colleagues139 and Heil and colleagues105 also used cotinine validation, with cut offs of < 15 ng/ml and ≤ 80 ng/ml respectively. The study by Walsh and colleagues108 used urinary cotinine validation, with a cut off of < 500 nmol/l. The remaining two studies did not use biochemical validation. 145,147
Of note, two studies had used biochemical validation methods for a different outcome, namely to quantify the deception rate, as it is possible to temporarily stop smoking to achieve a low CO level; however, this would be revealed by a raised cotinine level. 143,145 Gadomski and colleagues143 used salivary cotinine for this purpose (cut-off not specified) and Lillington and colleagues145 used a saliva cotinine cut-off of 20 ng/ml.
For the purposes of comparing data it was not always possible to utilise comparator data even if there had been a control group in the original study. For example, Gadomski and colleagues143 analysed data for an intervention with both gestational and post partum components. The provision of incentives applied to the post partum aspect of the intervention (vouchers for nappies); however, the authors did not have comparator data for this part of the intervention even though comparator data were available for the gestational part of the intervention (Anne Gadomski, Bassett Healthcare Research Institute, New York, May 2012, personal communication with JH).
In other cases it was not possible to derive outcome data for separate treatment groups. For example, the study by Albrecht and colleagues138 had three treatment arms comparing an intervention (Teen FreshStart), enhancement of the intervention with peer support (Teen FreshStart Plus Buddy) and a control group. However, results are reported comparing the enhanced intervention with combined data from the other two treatment arms; separate data for each of these arms are not available.
In two studies collecting population-level data had been attempted to consider the effectiveness of the interventions. 142,148 In the study by Edwards and colleagues142 data were gathered from forms that indicated whether or not a pregnant woman received a smoking cessation guide, which was provided only to women who expressed a desire to stop smoking (who were then offered counselling and access to a Smoking Quitline and provided with incentives). The study by Morgan and colleagues148 estimated the effect of a SureStart smoking cessation intervention by estimating the number of women who were reached through the incentive as a proportion of the estimated number of pregnant women smokers living within the SureStart area.
Actors: who the intervention targeted
The majority of studies targeted pregnant women who were smoking (Table 9). Engagement of social support in the intervention occurred in seven studies, although the format of this varied, with five involving ‘social supporters’ or ‘buddies’108,138,141,145,146 and two specifying that the father should perform this role. 104,147 In these studies a social supporter108,138,141,144,146 or partner104,147 was also registered to the programme and in one study141 also received incentives. Peer support was considered an essential element in two of the studies. 104,138 The involvement of the wider community was also a key factor in three of the studies104,108,140 and Cluss and colleagues140 concluded that community-reinforced interventions deliver results that are comparable to those of RCTs. In terms of equity pointers, most of the studies targeted low-income or disadvantaged women (or WIC programme participants); however, in some studies this was unclear. 110,111
| Study and category | Age (years) | Ethnicity | Relationship status | Weeks pregnant | Smoking behaviour | Education | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | White | Other | Married and/or living with a partner | Other | Mean | SD | Range | Average no. of cigarettes smoked per day pre pregnancy | Average no. of cigarettes smoked per day since pregnancy | Education < high school | Education – high school/GED | Education – vocational school, some college or more | Mean years of educational attainment | ||||||||||||
| n | % | n | % | n | % | n | % | Mean | SD | Mean | SD | n | % | n | % | n | % | Mean | SD | Range | |||||||
| Donatelle 2000141 A1 |
23.7 | 5.7 | 199 | 91.2 | 19 | 8.8 | 122 | 55.5 | 98 | 45.5 | 16.5 | 7.0 | ≤ 28 | 11.7 | 1.9 | ||||||||||||
| Heil 2008105 A1 |
24.3 | 5.2 | 72 | 93.7 | 5 | 6.3 | 14 | 18.0 | 63 | 82.0 | 9.2 | 3.2 | ≤ 20 | 18.5 | 7.7 | 8.7 | 5.8 | 11.9 | 2.3 | ||||||||
| Higgins 2004144 A1 |
22.7 | 4.9 | 50 | 94.3 | 3 | 5.7 | 12 | 22.6 | 41 | 77.4 | 14.0 | 7.2 | 23.0 | 11.3 | 10.3 | 8.7 | 11.7 | 2.0 | |||||||||
| Mantzari 2012109 A1 |
28.0 | NR | 17–43 | 34 | 94.4 | 2 | 5.6 | ||||||||||||||||||||
| Gadomski 2011143 B1 |
23.4 | NR | 702 | 90.9 | 71 | 9.1 | 14.1 | NR | 12.0 | NR | |||||||||||||||||
| Walsh 1997108 B1 |
≤ 26 | ||||||||||||||||||||||||||
| Albrecht 1998138 B2 |
31 | 37.0 | 53 | 63.0 | 0 | 0.0 | 84 | 100.0 | 4–28 | 6.4 | 6.2 | ||||||||||||||||
| Edwards 2009142 B2 |
|||||||||||||||||||||||||||
| Lillington 1995145 B2 |
26.8 | 5.3 | 18–43 | 20 | 3.6 | 536 | 96.4 | ||||||||||||||||||||
| Lowe 1997146 B2 |
|||||||||||||||||||||||||||
| McBride 2004147 B2 |
24.0 | 4.7 | 449 | 77.0 | 134 | 23.0 | 583 | 100.0 | 0 | 0.0 | 11.0 | 3.8 | ≤ 20 | 6.0 | 5.0 | 303 | 52.0 | ||||||||||
| Morgan 2005148 C1 |
|||||||||||||||||||||||||||
| Radley 2012113 C1 |
27.7 | 6.2 | |||||||||||||||||||||||||
| Cluss 2011140 C2 |
24.6 | 6.3 | 13–46 | 505 | 59.0 | 351 | 41.0 | 633 | 74.0 | 19.9 | 7.2 | ||||||||||||||||
| Gulliver 2004104 C2 |
26.7 | 4.3 | 19–35 | 15 | 75.0 | 5 | 25.0 | 12 | 62.0 | 8 | 38.0 | 9.7 | 5.6 | 12.9 | 1.5 | 10–16 | |||||||||||
| Nichter 2007110 C2 |
25.0 | 18–43 | 33 | 62.0 | 20 | 38.0 | 17 | 32.0 | 36 | 68.0 | 19.3 | 19 | 36.0 | 20 | 38.0 | 14 | 26.0 | ||||||||||
| Ripley-Moffit 2008111 C2 |
25.0 | NR | 55 | 58.5 | 39 | 41.5 | 54 | 57.4 | 10.5 | NR | 12.9 | NR | |||||||||||||||
| Ussher 2008150 C2 |
30.2 | 5.7 | 23 | 71.8 | 9 | 28.2 | 23 | 71.9 | 9 | 28.1 | 16.3 | 4.3 | 12–20 | 18.4 | 7.9 | 9.0 | 5.5 | 14.2 | 3.2 | ||||||||
| Cinciripini 2010139 D2 |
25.0 | 5.9 | 86 | 33.5 | 171 | 66.5 | 107 | 41.7 | 150 | 58.3 | 19.5 | 8.5 | ≤ 32 | 16.3 | 9.0 | 9.8 | 6.9 | 82 | 31.9 | 90 | 35.0 | 85 | 33.1 | ||||
| Pbert 2004149 D2 |
25.7 | 6.3 | 290 | 52.7 | 260 | 47.3 | 194 | 35.3 | 356 | 64.7 | 16.1 | 7.7 | 16.7 | 11.6 | 300 | 54.5 | 250 | 45.5 | |||||||||
Baseline population characteristics
Baseline characteristics data are summarised in Table 9. Several studies reported no baseline details of their study populations,108,142,146,148 although the studies by Lowe and colleagues146 and Walsh and colleagues108 indicated that there were no significant differences at baseline between the control and the experimental groups, without reporting further details.
Of those studies that reported baseline characteristics, with the exception of the study by Ussher and colleagues,150 the mean age of participants was ≤ 30 years. In terms of the ethnicity of participants, over half of the participants in each study population were white, apart from in the studies by Albrecht and colleagues,138 Cinciripini and colleagues139 and Lillington and colleagues,145 which was a study specifically designed to evaluate the effectiveness of a smoking cessation intervention among ethnic minority pregnant smokers.
Twelve studies reported the domestic status of participants,104,105,110,111,138–141,144,147,149,150 with more than half of study participants reported as single (or not married or living with a partner) in eight of the studies. 105,110,138–140,144,149,150 Gestational age was frequently prespecified in the inclusion criteria and therefore the maximum range for this characteristic can be derived from this information even when baseline characteristic data have not been reported, as was the case in the study by Walsh and colleagues,108 although range data were also provided separately too. 138,150 With the exception of the study by Heil and colleagues,105 in which the mean gestational age was 9.2 weeks, the mean gestational age for all studies reporting this information was between 10 and 20 weeks.
For smoking behaviour, the average number of cigarettes smoked per day before discovering they were pregnant exceeded 10 for all studies reporting this information. 105,111,139,143,144,149,150,152 Conversely, the reported average cigarette intake per day since discovering pregnancy was < 10 for all studies104,105,138,139,144,147,150 except for that by Higgins and colleagues,144 although it is worth noting that this study population had the highest mean pre-pregnancy cigarette intake of all those reporting this characteristic.
Apart from these characteristics there was less consistency in reported baseline data across studies; other characteristics reported included income, education, employment and mean Fagerström Test for Nicotine Dependence (FTND) scores. 164 Most studies targeted either disadvantaged areas or disadvantaged women. One study specifically aimed to recruit African American/Hispanic women145 and one was designed for pregnant adolescents who were still at school. 138
Effectiveness
The four category A studies, which compared incentives contingent on validated smoking cessation outcomes with non-contingent incentives for participation in a smoking cessation programme and providing outcome data, were considered sufficiently homogeneous for smoking cessation rates to be combined in a random-effects meta-analysis. 105,109,141,144 Figure 9 summarises the meta-analysis for biochemically validated cessation towards the end of pregnancy (from 7 months’ gestation onwards). The estimated RD was 0.23 [95% confidence interval (CI) 0.14 to 0.31], favouring the provision of contingent incentives. The RR of cessation was 2.58 (95% CI 1.63 to 4.07). All control groups in the studies included in this meta-analysis received some form of non-contingent incentive for participation in a smoking cessation programme and providing outcome data although the value provided varied.
FIGURE 9.
Meta-analysis for the effect of incentives contingent on biochemically validated smoking cessation compared with non-contingent incentives towards the end of pregnancy.
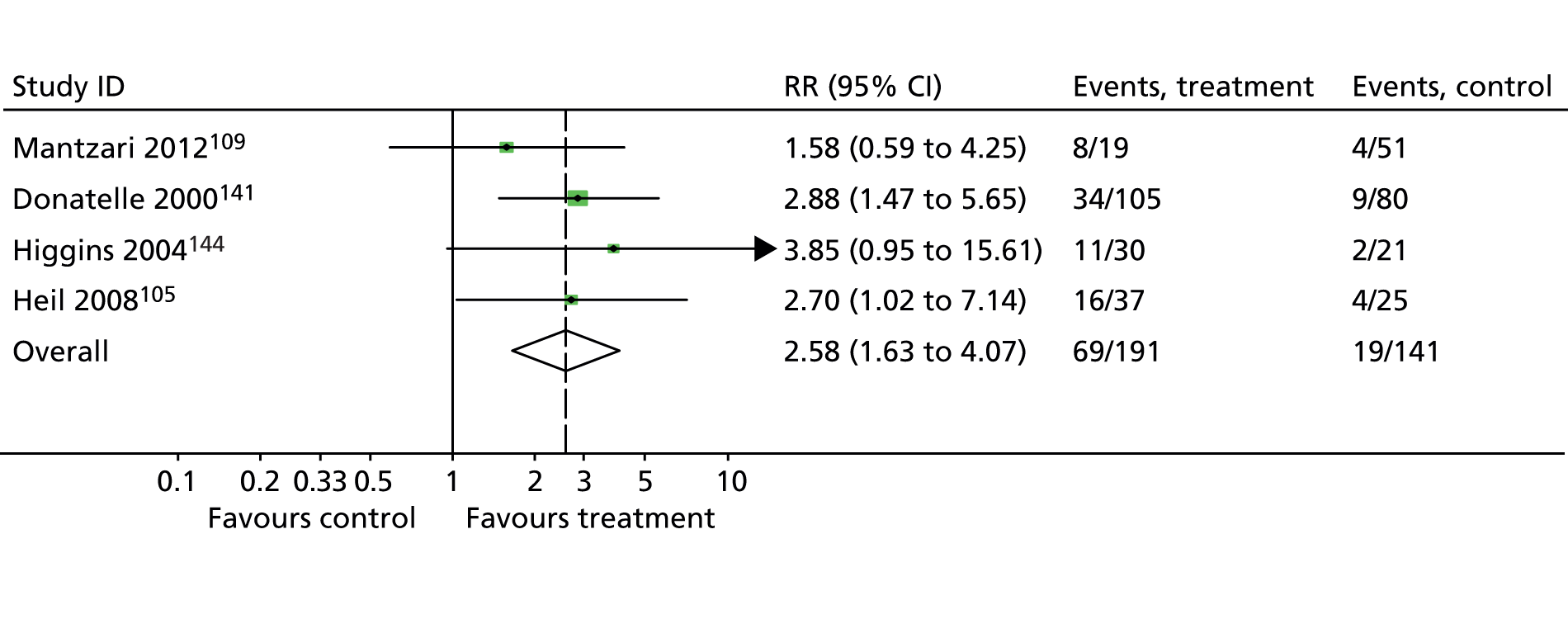
The study by Donatelle and colleagues141 also reported the proportion of those maintaining abstinence at 2 months post partum, with 21% (22/103) of participants receiving partner incentivisation as well as incentives for themselves maintaining abstinence at 2 months compared with 6% (6/102) of participants receiving incentives for themselves alone (RR 3.63, 95% CI 1.54 to 8.85; p = 0.001). At 3 months post partum, Heil and colleagues105 reported that 24% (9/37) of those receiving contingent vouchers were abstinent compared with 3% (1/40) of those receiving non-contingent vouchers (RR 9.72, 95% CI 1.29 to 73.12; p = 0.004). At the same time point, Higgins and colleagues144 reported that 33% (10/30) of participants receiving contingent vouchers were abstinent compared with no participants receiving non-contingent vouchers (p = 0.002).
At 6 months post partum, Heil and colleagues105 reported that 8% (3/37) of those receiving contingent vouchers were abstinent compared with 3% (1/40) of those receiving non-contingent vouchers (RR 3.32, 95% CI 0.36 to 30.58; p = 0.26). Higgins and colleagues144 reported that 27% (8/30) of participants receiving contingent vouchers were abstinent for the same period compared with no participants receiving non-contingent vouchers (p = 0.007).
All category B–D studies reported cessation rates at various time points and are summarised narratively. None of the category B studies comparing incentive interventions (intervention) with no incentive (control) were considered suitable by the research team to be included in the meta-analysis. For example, if other components of the intervention (e.g. counselling) had been provided multiple times it was not suitable to combine these data with data from studies in which the same component had been provided once as the effect of providing the incentive could be confounded with the effect of providing more intensive counselling. Category C studies had no control groups and in category D studies the incentive had been provided to all participants and these studies were therefore unsuitable for meta-analysis.
Smoking cessation outcomes
The effect of incentives on smoking cessation was our main outcome of interest. Apart from the meta-analysed data (see previous section), the reported numbers and percentages of those who quit smoking are provided. This outcome was reported for all studies as this was a prerequisite to meet the inclusion criteria for the effectiveness review. Details of smoking cessation rates at various time points during and beyond pregnancy are provided in Tables 10–15. Outcomes were sometimes reported at time points after the incentive programme had ceased (see Table 8). Intention-to-treat (ITT) and attrition data were often unclear, as detailed in Figure 9. RRs have been calculated from the reported smoking cessation rates and care was taken to use the most conservative estimate of the sample size (e.g. number randomised, number at the start of the study) to prevent any unintentional overinflation of the effectiveness of interventions. However, this was not possible in all cases because of the quality of reporting.
| Study | Category | Measured at | Sample size | Biochemically validated | Intervention | Control | RR (95% CI), p-value | ||
|---|---|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | ||||||
| Donatelle 2000141 | A1 | NR | |||||||
| Heil 2008105 | A1 | NR | |||||||
| Higgins 2004144 | A1 | NR | |||||||
| Mantzari 2012109 | A1 | NR | |||||||
| Gadomski 2011143 | B1 | NR | |||||||
| Walsh 1997108 | B1 | 4 weeks post baseline: approximately < 30 weeks’ gestation | 252 | ✓ (cotinine) | 20/127 | 15.7 | 2/125 | 1.6 | 9.84 (2.35 to 41.23), < 0.001 |
| Walsh 1997108 | B1 | 4 weeks post baseline: approximately < 30 weeks’ gestation | 252 | ✗ | 25/127 | 19.7 | 9/125 | 7.2 | 2.73 (1.33 to 5.62), 0.004 |
| Albrecht 1998138 | B2 | 4–6 weeks post baseline: approximately 8–34 weeks’ gestation | 33 | ✓ | 5/33 | 15.2 | NA | ||
| Albrecht 1998138 | B2 | 4–6 weeks post baseline: approximately 8–34 weeks’ gestation | 13 | ✓ | 3/13 | 23.1 | NA | ||
| Edwards 2009142 | B2 | Unclear | 11,210 | ✗ | 2044/8445 | 24.2 | 578/2765 | 20.9 | 1.16 (1.07 to 1.26), < 0.001 |
| Lillington 1995145 | B2 | NR | |||||||
| Lowe 1997146 | B2 | NR | |||||||
| McBride 2004147 | B2 | NR | |||||||
| Morgan 2005148 | C1 | NR | |||||||
| Radley 2013113 | C1 | NR | |||||||
| Cluss 2011140 | C2 | NR | |||||||
| Gulliver 2004104 | C2 | NR | |||||||
| Nichter 2007110 | C2 | NR | |||||||
| Ussher 2008,150 study 2 | C2 | 1 week post baseline: approximately 15 weeks’ gestation | 22 | ✓ | 4/22 | 18.2 | NA | ||
| Ussher 2008,150 study 1 | C2 | 4 weeks post baseline: approximately 18.4 weeks’ gestation | 10 | ✓ | 7/10 | 70.0 | NA | ||
| Ussher 2008,150 study 2 | C2 | 6 weeks post baseline: approximately 20 weeks’ gestation | 22 | ✓ | 4/22 | 18.2 | NA | ||
| Cinciripini 2010139 | D2 | NR | |||||||
| Pbert 2004149 | D2 | NR | |||||||
| Study | Category | Measured at | Sample size | Biochemically validated | Intervention | Control | RR (95% CI), p-value | ||
|---|---|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | ||||||
| Donatelle 2000141 | A1 | 8 months’ gestation | 185 | ✓ | 34/105 | 32.4 | 9/80 | 11.3 | 2.88 (1.47 to 5.65), < 0.001 |
| Heil 2008105 | A1 | ‘End of pregnancy’ | 62 | ✓ | 16/37 | 43.2 | 4/25 | 16 | 2.70 (1.02 to 7.14), 0.024 |
| Higgins 2004144 | A1 | ‘End of pregnancy’ | 51 | ✓ | 11/30 | 36.7 | 2/21 | 9.5 | 3.85 (0.95 to 15.61), 0.029 |
| Mantzari 2012109 | A1 | Approximately 32 weeks’ gestation (calculated from personal correspondence with author) | 34 | Unclear | 8/19 | 42.1 | 4/15 | 26.7 | 1.58 (0.59 to 4.25), 0.35 |
| Gadomski 2011143 | B1 | NR | |||||||
| Walsh 1997108 | B1 | Reported as ‘34th week of gestation’ | 252 | ✓ (cotinine) | 17/127 | 13.4 | 7/125 | 5.6 | 2.39 (1.03 to 5.56), 0.035 |
| Walsh 1997108 | B1 | Reported as ‘34th week of gestation’ | 252 | ✗ | 24/127 | 18.9 | 10/125 | 8.0 | 2.36 (1.18 to 4.73), 0.011 |
| Albrecht 1998138 | B2 | NR | |||||||
| Edwards 2009142 | B2 | NR | |||||||
| Lillington 1995145 | B2 | 9 months’ gestation | 768 | ✗ | 106/208 | 51.0 | 263/560 | 47.0 | 1.09 (0.92 to 1.27), 0.32 |
| Lowe 1997146 | B2 | 9 months’ gestation, stated as ‘at end of pregnancy’ | 97 | ✓ (cotinine) | 32/45 | 71.0 | 29/52 | 56.0 | 1.28 (0.94 to 1.73), 0.12 |
| McBride 2004147 | B2 | 28 weeks’ gestation | 391 | ✗ | 118/193 | 61.0 | 119/198 | 60.0 | 1.02 (0.87 to 1.19), 0.83 |
| McBride 2004147 | B2 | 28 weeks’ gestation | 390 | ✗ | 113/192 | 59.0 | 119/198 | 60.0 | 0.98 (0.83 to 1.15), 0.80 |
| Morgan 2005148 | C1 | NR | |||||||
| Radley 2013113 | C1 | NR | |||||||
| Cluss 2011140 | C2 | ‘Last session before delivery’ [calculated as being within the 7- to 9-month time frame as the mean (SD) length of time between this and delivery was reported in the paper as 44 (55) days, with a median of 22 days] | 856 | ✓ | 119/856 | 13.9 | NA | ||
| Gulliver 2004104 | C2 | NR | |||||||
| Nichter 2007110 | C2 | ‘End of term’, i.e. at the end of pregnancy | 53 | ✓ (cotinine) | 16/53 | 30.2 | NA | ||
| Ripley-Moffitt 2008111 | C2 | NR | |||||||
| Ussher 2008,150 study 2 | C2 | 8 months’ gestation | 10 | ✓ | 5/10 | 50.0 | NA | ||
| Ussher 2008,150 study 1 | C2 | 8 months’ gestation | 22 | ✓ | 3/22 | 13.6 | NA | ||
| Cinciripini 2010139 | D2 | 10 weeks post baseline: approximately 29.6 weeks’ gestation | 129 | ✓ (cotinine) | 51/129 | 39.5 | NA | ||
| Cinciripini 2010139 | D2 | 10 weeks post baseline: approximately 29.5 weeks’ gestation | 128 | ✓ (cotinine) | 58/128 | 45.3 | NA | ||
| Cinciripini 2010139 | D2 | 12 weeks post baseline: approximately 31.6 weeks’ gestation | 129 | ✓ (cotinine) | 40/129 | 31.0 | NA | ||
| Cinciripini 2010139 | D2 | 12 weeks post baseline: approximately 31.5 weeks’ gestation | 128 | ✓ (cotinine) | 47/128 | 36.7 | NA | ||
| Pbert 2004149 | D2 | 9 months’ gestation | 278 | ✓ (cotinine) | 82/278 | 29.5 | NA | ||
| Pbert 2004149 | D2 | 9 months’ gestation | 272 | ✓ (cotinine) | 95/272 | 35.0 | NA | ||
| Study | Category | Measured at | Sample size | Biochemically validated | Intervention | Control | RR (95% CI), p-value | ||
|---|---|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | ||||||
| Donatelle 2000141 | A1 | 2 months post partum | 205 | ✓ | 22/103 | 21 | 6/102 | 6 | 3.63 (1.54 to 8.85), 0.001 |
| Heil 2008105 | A1 | NR | |||||||
| Higgins 2004144 | A1 | NR | |||||||
| Mantzari 2012109 | A1 | NR | |||||||
| Gadomski 2011143 | B1 | NR | |||||||
| Walsh 1997108 | B1 | Approximately 9 weeks post partum (reported as range ‘6 to 12 weeks post partum’) | 252 | ✓ (cotinine) | 13/127 | 10.2 | 1/125 | 0.8 | 12.8 (1.7 to 96.3), 0.001 |
| Walsh 1997108 | B1 | Approximately 9 weeks post partum (reported as range ‘6 to 12 weeks post partum’) | 252 | ✗ | 17/127 | 13.4 | 8/125 | 6.4 | 2.1 (0.9 to 4.7), 0.064 |
| Albrecht 1998138 | B2 | NR | |||||||
| Edwards 2009142 | B2 | NR | |||||||
| Lillington 1995145 | B2 | 6 weeks post partum | 768 | ✗ | 80/208 | 38.5 | 174/560 | 31.1 | 1.24 (1.00 to 1.53), 0.053 |
| Lowe 1997146 | B2 | NR | |||||||
| McBride 2004147 | B2 | 2 months post partum | 391 | ✗ | 81/193 | 42.0 | 75/198 | 38.0 | 1.1 (0.9 to 1.4), 0.41 |
| McBride 2004147 | B2 | 2 months post partum | 192 | ✗ | 71/192 | 37.0 | NA | ||
| Morgan 2005148 | C1 | NR | |||||||
| Radley 2013113 | C1 | NR | |||||||
| Cluss 2011140 | C2 | NR | |||||||
| Gulliver 2004104 | C2 | NR | |||||||
| Nichter 2007110 | C2 | NR | |||||||
| Ripley-Moffitt 2008111 | C2 | NR | |||||||
| Ussher 2008,150 study 2 | C2 | NR | |||||||
| Ussher 2008,150 study 1 | C2 | NR | |||||||
| Cinciripini 2010139 | D2 | NR | |||||||
| Cinciripini 2010139 | D2 | 26 weeks post baseline: approximately 45.6 weeks’ gestation | 129 | ✓ (cotinine) | 21/129 | 16.3 | NA | ||
| Cinciripini 2010139 | D2 | 26 weeks’ gestation post baseline: approximately 45.5 weeks’ gestation | 128 | ✓ (cotinine) | 23/128 | 18.0 | NA | ||
| Pbert 2004149 | D2 | 1 month post partum | 269 | ✓ (cotinine) | 57/269 | 21.3 | NA | ||
| Pbert 2004149 | D2 | 1 month post partum | 233 | ✓ (cotinine) | 63/233 | 27.2 | NA | ||
| Study | Category | Measured at | Sample size | Biochemically validated | Intervention | Control | RR (95% CI), p-value | ||
|---|---|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | ||||||
| Donatelle 2000141 | A1 | NR | |||||||
| Heil 2008105 | A1 | 12 weeks post partum | 77 | ✓ | 9/37 | 24 | 1/40 | 3 | 9.72 (1.29 to 73.12), 0.004 |
| Higgins 2004144 | A1 | 12 weeks post partum | 51 | ✓ | 10/30 | 33 | 0/21 | 0 | p = 0.002 |
| Mantzari 2012109 | A1 | NR | |||||||
| Gadomski 2011143 | B1 | 3 months post partum | 264 | ✓ | 137/264 | 52.0 | NA | ||
| Gadomski 2011143 | B1 | 3 months post partum | 112 | ✓ | 86/112 | 77.0 | NA | ||
| Gadomski 2011143 | B1 | 3 months post partum | 17 | ✓ | 6/17 | 37.5 | NA | ||
| Walsh 1997108 | B1 | NR | |||||||
| Albrecht 1998138 | B2 | NR | |||||||
| Edwards 2009142 | B2 | NR | |||||||
| Lillington 1995145 | B2 | NR | |||||||
| Lowe 1997146 | B2 | NR | |||||||
| McBride 2004147 | B2 | NR | |||||||
| Morgan 2005148 | C1 | NR | |||||||
| Radley 2013113 | C1 | 3 months post partum | 393 | ✓ | 65/393 | 16.5 | NA | ||
| Cluss 2011140 | C2 | NR | |||||||
| Gulliver 2004104 | C2 | NR | |||||||
| Nichter 2007110 | C2 | NR | |||||||
| Ripley-Moffitt 2008111 | C2 | 4 months post partum | 94 | ✓ (cotinine) | 43/94 | 45.7 | NA | ||
| Ussher 2008,150 study 2 | C2 | NR | |||||||
| Ussher 2008,150 study 1 | C2 | NR | |||||||
| Cinciripini 2010139 | D2 | 3 months post partum | 129 | ✓ (cotinine) | 23/129 | 17.8 | NA | ||
| Cinciripini 2010139 | D2 | 3 months post partum | 128 | ✓ (cotinine) | 24/128 | 18.8 | NA | ||
| Pbert 2004149 | D2 | 3 months post partum | 269 | ✓ (cotinine) | 27/269 | 10.0 | NA | ||
| Pbert 2004149 | D2 | 3 months post partum | 233 | ✓ (cotinine) | 18/233 | 7.7 | NA | ||
| Study | Category | Measured at | Sample size | Biochemically validated | Intervention | Control | RR (95% CI), p-value | ||
|---|---|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | ||||||
| Donatelle 2000141 | A1 | NR | |||||||
| Heil 2008105 | A1 | 24 weeks post partum | 77 | ✓ | 3/37 | 8 | 1/40 | 3 | 3.32 (0.36 to 0.58), 0.26 |
| Higgins 2004144 | A1 | 24 weeks post partum | 51 | ✓ | 8/30 | 27 | 0/21 | 0 | p = 0.007 |
| Mantzari 2012109 | A1 | NR | |||||||
| Gadomski 2011143 | B1 | 6 months post partum | 264 | ✓ | 84/264 | 32.0 | NA | ||
| Gadomski 2011143 | B1 | 6 months post partum | 112 | ✓ | 72/112 | 64.0 | NA | ||
| Gadomski 2011143 | B1 | 6 months post partum | 17 | ✓ | 4/17 | 25.0 | NA | ||
| Walsh 1997108 | B1 | NR | |||||||
| Albrecht 1998138 | B2 | NR | |||||||
| Edwards 2009142 | B2 | NR | |||||||
| Lillington 1995145 | B2 | NR | |||||||
| Lowe 1997146 | B2 | NR | |||||||
| McBride 2004147 | B2 | 6 months post partum | 391 | ✗ | 71/193 | 37.0 | 65/198 | 33.0 | 1.12 (0.85 to 1.47), 0.41 |
| McBride 2004147 | B2 | 6 months post partum | 390 | ✗ | 69/192 | 36.0 | 65/198 | 33.0 | 1.09 (0.83 to 1.44), 0.52 |
| Morgan 2005148 | C1 | NR | |||||||
| Radley 2013113 | C1 | NR | |||||||
| Cluss 2011140 | C2 | NR | |||||||
| Gulliver 2004104 | C2 | NR | |||||||
| Nichter 2007110 | C2 | NR | |||||||
| Ripley-Moffitt 2008111 | C2 | NR | |||||||
| Ussher 2008,150 study 2 | C2 | NR | |||||||
| Ussher 2008,150 study 1 | C2 | NR | |||||||
| Cinciripini 2010139 | D2 | 6 months post partum | 129 | ✓ (cotinine) | 12/129 | 9.3 | NA | ||
| Cinciripini 2010139 | D2 | 6 months post partum | 128 | ✓ (cotinine) | 9/128 | 7.0 | NA | ||
| Pbert 2004149 | D2 | 6 months post partum | 269 | ✓ (cotinine) | 24/269 | 8.9 | NA | ||
| Pbert 2004149 | D2 | 6 months post partum | 233 | ✓ (cotinine) | 19/233 | 8.2 | NA | ||
| Study | Category | Measured at | Sample size | Biochemically validated | Intervention | Control | RR (95% CI), p-value | ||
|---|---|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | ||||||
| Donatelle 2000141 | A1 | NR | |||||||
| Heil 2008105 | A1 | NR | |||||||
| Higgins 2004144 | A1 | NR | |||||||
| Mantzari 2012109 | A1 | NR | |||||||
| Gadomski 2011143 | B1 | NR | |||||||
| Walsh 1997108 | B1 | NR | |||||||
| Albrecht 1998138 | B2 | NR | |||||||
| Edwards 2009142 | B2 | Unclear | 11,210 | ✗ | 2044/8445 | 24.2 | 578/2765 | 20.9 | 1.16 (1.07 to 1.26), p < 0.001 |
| Lillington 1995145 | B2 | NR | |||||||
| Lowe 1997146 | B2 | NR | |||||||
| McBride 2004147 | B2 | NR | |||||||
| Morgan 2005148 | C1 | 4 weeks post baseline: baseline gestation information not available | 86 | ✗ | 28/86 | 32.6 | NA | ||
| Radley 2013113 | C1 | 4 weeks post baseline: baseline gestation information not available | 393 | ✓ | 211/393 | 53.7 | NA | ||
| Radley 2013113 | C1 | 12 weeks post baseline: baseline gestation information not available | 393 | ✓ | 125/393 | 31.8 | NA | ||
| Cluss 2011140 | C2 | NR | |||||||
| Gulliver 2004104 | C2 | 4 weeks post baseline: baseline gestation information not available | 20 | ✓ | 11/20 | 54.0 | NA | ||
| Nichter 2007110 | C2 | NR | |||||||
| Ussher 2008,150 study 2 | C2 | NR | |||||||
| Ussher 2008,150 study 1 | C2 | NR | |||||||
| Cinciripini 2010139 | D2 | NR | |||||||
| Pbert 2004149 | D2 | NR | |||||||
In addition, Lowe and colleagues reported data for a historical control group for their study, published previously,165 which suggested a relapse rate of 35%. Lillington and colleagues145 provided an adjusted relapse rate to account for deception rates among participants, based on a method cited elsewhere. 145,166
McBride and colleagues147 provided data on abstinence among partners. Those whose pregnant partners had received care without partner involvement in the intervention and those who were involved in their partner’s intervention were incentivised groups. Of the 84 men in the partner-assisted group, 15 (17.9%) were abstinent when their partner was at 28 weeks’ gestation and 17 (20.2%) and 20 (23.8%) were abstinent when their partner had reached 2 months and 6 months post partum respectively.
Smoking reduction
Albrecht and colleagues138 reported that those randomised to one intervention group (intervention including social support) smoked on average four fewer cigarettes per day than those in the other intervention group (without social support) and the control group combined. Using digitised data reported in a figure, results indicate that the intervention group smoked an average of 2.8 cigarettes per day post intervention compared with an average of 5.8 in the other groups (i.e. three fewer cigarettes per day on average). Gulliver and colleagues104 reported that respondents who did not quit smoked three to eight cigarettes per day. Pbert and colleagues149 graphically showed the number of cigarettes smoked by those who did not quit. Digitised data for the reduction in cigarette intake are reported in Table 16.
| Measurement point | Average no. of cigarettes smoked per day (intervention group) | Average no. of cigarettes smoked per day (usual care group) | Between-group difference |
|---|---|---|---|
| Pre pregnancy | 18.1 | 21.1 | 3.0 |
| Baseline | 8.3 | 9.6 | 1.3 |
| 9 months’ gestation | 8.0 | 10.4 | 2.3 |
| 1 month post partum | 7.2 | 9.2 | 2.0 |
| 3 months post partum | 10.3 | 12.8 | 2.4 |
| 6 months post partum | 10.2 | 12.1 | 1.9 |
Cluss and colleagues140 reported the number of participants who reduced their cigarette intake even though they did not quit (217/797, 27.3%) but did not provide data on the average number of cigarettes smoked per day for these participants.
Alternative definitions of abstinence
Point-prevalent abstinence was the most commonly used method to define abstinence, likely because of the ability of biochemical measures to validate abstinence only across short time periods. However, several studies defined abstinence in alternative ways to account for consecutive periods of point-prevalent abstinence among participants.
The study by Cinciripini and colleagues139 distinguished between continuous abstinence, defined as no smoking on any day, and prolonged abstinence, defined as not having a relapse of either 7 consecutive days of smoking or smoking at least one cigarette over 2 consecutive weeks within the period of interest. In this study both treatment groups received incentives (as it compared different methods of providing behavioural support).
Continuous abstinence rates ranged from 21.0% (27/129) to 23.4% (30/128) at 3 months following treatment, which was on average 9.5 weeks (SD 4.6 weeks) post partum, from 10.9% (14/129) to 11.7% (15/128) at 3 months post partum, from 8.5% (11/129) to 11.1% (14/128) at 6 months post treatment, which was on average 17.5 weeks (SD 7.3 weeks) post partum and from 1.2% (3/129) to 3.1% (4/128) at 6 months post partum.
Prolonged abstinence rates ranged from 27.1% (35/129) to 31.3% (40/128) at 3 months following treatment (see above for definition), from 16.4% (21/128) to 18.6% (24/129) at 3 months post partum, from 14.1% (18/128) to 14.7% (19/129) at 6 months following treatment (see above for definition) and from 6.2% (8/129) to 7.8% (10/128) at 6 months post partum.
The study by Heil and colleagues105 reported that 24% of women receiving vouchers contingent on smoking abstinence sustained abstinence throughout the third trimester compared with 3% of those receiving non-contingent incentives. However, the time point at which this was established (and therefore the total numbers of women involved) is unclear.
McBride and colleagues147 reported sustained abstinence (‘not smoking at all four follow-ups’) among women but this was measured at 12 months’ follow-up and is therefore outwith the scope of this study. However, the authors also reported the proportion of women who were abstinent at 28 weeks’ gestation who remained abstinent at 2 and 6 months post partum. In total, 55% and 47% of women in the control group sustained abstinence at these time points, respectively, compared with 54% and 52% of women receiving the intervention without partner support and 60% and 52% of women who received both the intervention and social support. However, the total numbers of women in each group at each time point is unclear.
Pbert and colleagues149 reported subgroup data for those women who had spontaneously quit at baseline (i.e. who had been recent smokers until they discovered their pregnancy) who remained non-smokers at 9 months’ gestation and 1, 3 and 6 months post partum. In this study the control group received no intervention whereas the intervention group received vouchers as an incentive for completing interviews regarding their smoking status. Using ITT analysis based on the number of spontaneous quitters at baseline, in the intervention and control groups, respectively, 70.3% (57/81) and 78.2% (60/77) of women remained non-smokers at 9 months’ gestation compared with 40.1% (32/81) and 49.7% (38/77) at 1 month post partum, 12.8% (10/81) and 27.7% (21/77) at 3 months post partum and 11.7% (9/81) and 24.9% (19/77) at 6 months post partum.
Walsh and colleagues108 reported both self-reported and biochemically validated (urine cotinine of ≤ 500 nmol/l) continuous abstinence rates (abstinent on two or three consecutive follow-up visits). In this study the intervention group received incentives whereas the control group did not. Self-reported consecutive abstinence was 13% (17/127; 95% CI 8% to 21%) for participants in the experimental group compared with 3% (4/125; 95% CI 1% to 8%), for participants in the control group from first follow-up to the end of pregnancy, and 8% (10/127; 95% CI 4% to 14%) compared with 1% (1/125; 95% CI 0% to 4%) for the experimental and control groups, respectively, from first follow-up to the post partum follow-up. However, when biochemical validation was used, no control group participants were found to be abstinent on consecutive occasions. For the experimental group, consecutive abstinence from first follow-up to the end of pregnancy was 9% (12/127; 95% CI 5% to 16%) and from first follow-up to the post partum follow-up was 6% (8/127; 95% CI 3% to 12%).
The study by Gadomski and colleagues143 graphically reported time to first failed post partum test for those receiving incentives. Insufficient information was available to estimate data from this graph using digitising software. The study by Heil and colleagues105 reported that, for participants receiving contingent voucher incentives, the mean number of weeks of continuous abstinence as measured at the end of pregnancy was 9.7 weeks (SD 1.9 weeks) whereas, for those receiving non-contingent vouchers, the mean number of weeks of abstinence was 2.0 weeks (SD 0.8 weeks).
Changes in biochemically validated indicators of smoking cessation
Two studies reported changes in biochemical validation readings over the study time frame. The study by Albrecht and colleagues138 reported an average reduction in the CO level of 1.7 p.p.m. among those receiving the intervention (including incentives) plus social support at between 4 and 6 weeks post baseline compared with an increase of 0.9 p.p.m. among those receiving either the intervention (including incentives but without social support) or the control treatment (no incentive or intervention). Mean exhaled CO levels for the groups were also reported graphically and these data confirm the reported rates. The study by Heil and colleagues105 reported the mean decrease in urine cotinine levels from baseline for those receiving contingent voucher incentives (39 ng/ml) and those receiving non-contingent vouchers (33 ng/ml) as measured at the end of pregnancy.
Typology of smoking cessation in pregnancy quitters and non-quitters
Qualitative data from four studies109,110,111,113 illuminate how pregnant smokers are a heterogeneous group who attach different values to incentives and other BCT components. A typology of women engaging in an incentive intervention in Scotland describes six user groups. 113 The least successful at quitting were characterised as ‘breadline survivors’113 who felt pressured to quit109 and who were typically the most socially and financially disadvantaged,109,111 with an unsupportive or no partner. 110,111 They were ‘shifters’ whose changes in smoking behaviour included erratic reductions and increases. 110 Those who were most successful were ‘mothers to be’,113 ‘quitters’,110 with ‘a moral identity’110,111 for whom the child’s health was at the forefront of their decision-making, and they were women who had more stable lifestyles with more control over their environment. 110,113 Some women in a number of studies also reported concern for their baby, concern for self and concern for existing children as reasons for wanting to quit. 109,111 Also successful in the Scottish study were ‘enthusiastic amateurs’,113 who had a history of quit attempts and who benefited most from the support of those delivering the programme and the CO monitoring. 109 These women tended to have more stable lifestyles and to conceptualise the incentive as part of a wider rewards and social support structure,109 particularly valuing relationships established with their local community pharmacists. Women who cut down, ‘harm reducers’,110 or those who relapsed were more likely to have stressful, chaotic lives and perceive smoking as an essential strategy for coping. 110,111
Other groups identified by Radley and colleagues113 were ‘novice quitters’, ‘opportunists’ and ‘impulse shoppers’. ‘Novice quitters’ were typically younger, first-time mothers still living within the family home. They had limited motivation, were looking for a quick solution and were least likely to benefit from the incentive scheme. ‘Opportunists’ saw the scheme as a financial opportunity, had minimal engagement with other intervention components, were often light smokers and were relatively confident of their ability to give up. ‘Impulse shoppers’ were women with limited commitment and those who did not expect to succeed. They had tried to quit smoking numerous times but thought that they should try again as they were pregnant. They were not necessarily looking to participate in a scheme but were speculatively looking for advice. 113
Women seemed not to be aware of the effect of incentives on their motivation, except in one study109 in which some women reported a connection, for example ‘And then the vouchers give me incentive to, like, stop smoking’ (participant 14, incentivised group).
In most studies reporting qualitative data the incentives were described as an ‘added bonus’ in addition to the general BCTs received. 109 Where incentives seemed to play their greatest role, however, was in engaging more women in the study or programme and in achieving higher quit rates. 113 In one study, women in the intervention group who received incentives engaged more with stop smoking services and were more positive about their experiences than those in the control group. 109
Attrition
Attrition rates were reported (or calculable) for 14 studies. Details for different time points ranging from early follow-up in pregnancy through to around 6 months post partum are reported in Tables 17–20.
| Study | Category | Time of follow-up | Intervention | Control | RR (95% CI), p-value | ||
|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | ||||
| Donatelle 2000141 | A1 | NR | |||||
| Heil 2008105 | A1 | NR | |||||
| Higgins 2004144 | A1 | NR | |||||
| Mantzari 2012109 | A1 | NR | |||||
| Gadomski 2011143 | B1 | NR | |||||
| Walsh 1997108 | B1 | 4 weeks after first visit | 11/127 | 9.0 | 13/125 | 10.0 | 0.83 (0.39 to 1.79), 0.64 |
| Albrecht 1998138 | B2 | 4–6 weeks post baseline | 13/29 | 44.8 | 12/29 | 41.4 | 1.08 (0.60 to 1.96), 0.79 |
| Albrecht 1998138 | B2 | 4–6 weeks post baseline | 13/26 | 50.0 | 12/29 | 41.4 | 1.21 (0.68 to 2.16), 0.52 |
| Edwards 2009142 | B2 | NR | |||||
| Lillington 1995145 | B2 | NR | |||||
| Lowe 1997146 | B2 | NR | |||||
| McBride 2004147 | B2 | NR | |||||
| Morgan 2005148 | C1 | NR | |||||
| Radley 2013113 | C1 | NR | |||||
| Cluss 2011140 | C2 | NR | |||||
| Gulliver 2004104 | C2 | 1 month | 2/10 | 20.0 | NA | ||
| Nichter 2007110 | C2 | NR | |||||
| Ripley-Moffitt 2008111 | C2 | NR | |||||
| Ussher 2008,150 study 2 | C2 | 1 week post baseline | 6/22 | 27 | NA | ||
| Ussher 2008,150 study 1 | C2 | 1 week post baseline | 2/10 | 20 | NA | ||
| Ussher 2008,150 study 2 | C2 | 6 weeks post baseline | 14/22 | 64 | NA | ||
| Cinciripini 2010139 | D2 | NR | |||||
| Pbert 2004149 | D2 | NR | |||||
| Study | Category | Time of follow-up | Intervention | Control | RR (95% CI), p-value | ||
|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | ||||
| Donatelle 2000141 | A1 | 8 months’ gestation | 36/112 | 32.1 | 56/108 | 51.9 | 0.62 (0.45 to 0.86), 0.003 |
| Heil 2008105 | A1 | NR | |||||
| Higgins 2004144 | A1 | End of pregnancy | 3/30 | 10.0 | 0/23 | 0.0 | p = 0.12 |
| Mantzari 2012109 | A1 | NR | |||||
| Gadomski 2011143 | B1 | NR | |||||
| Walsh 1997108 | B1 | End of pregnancy | 13/127 | 10.0 | 11/125 | 9.0 | 1.16 (0.54 to 2.50), 0.70 |
| Albrecht 1998138 | B2 | NR | |||||
| Edwards 2009142 | B2 | NR | |||||
| Lillington 1995145 | B2 | End of pregnancy | 52/208 | 25.0 | 157/560 | 28.0 | 0.89 (0.68 to 1.17), 0.40 |
| Lowe 1997146 | B2 | End of pregnancy | 5/45 | 11.1 | 14/52 | 26.9 | 0.41 (0.16 to 1.06), 0.050 |
| McBride 2004147 | B2 | 28 weeks’ gestation | 40/193 | 20.7 | 42/198 | 21.2 | 0.98 (0.66 to 1.44), 0.91 |
| McBride 2004147 | B2 | 28 weeks’ gestation | 28/192 | 14.6 | 42/198 | 21.2 | 0.69 (0.44 to 1.06), 0.088 |
| Morgan 2005148 | C1 | NR | |||||
| Radley 2013113 | C1 | NR | |||||
| Cluss 2011140 | C2 | NR | |||||
| Gulliver 2004104 | C2 | NR | |||||
| Nichter 2007110 | C2 | NR | |||||
| Ripley-Moffitt 2008111 | C2 | NR | |||||
| Ussher 2008,150 study 2 | C2 | 8 months’ gestation | 18/22 | 81.8 | NA | ||
| Ussher 2008,150 study 1 | C2 | 8 months’ gestation | 5/10 | 50.0 | NA | ||
| Cinciripini 2010139 | D2 | NR | |||||
| Pbert 2004149 | D2 | End of pregnancy | 58/309 | 18.8 | 24/300 | 8.0 | 2.35 (1.50 to 3.67), < 0.001 |
| Study | Category | Time of follow-up | Intervention | Control | RR (95% CI), p-value | ||
|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | ||||
| Donatelle 2000141 | A1 | 2 months post partum | 58/112 | 52.0 | 56/108 | 52.0 | 1.00 (0.77 to 1.29), 0.99 |
| Heil 2008105 | A1 | NR | |||||
| Higgins 2004144 | A1 | 3 months post partum | 4/30 | 13.0 | 2/23 | 9.0 | 1.53 (0.31 to 7.66), 0.60 |
| Mantzari 2012109 | A1 | NR | |||||
| Gadomski 2011143 | B1 | NR | |||||
| Walsh 1997108 | B1 | 4–6 weeks post partum | 28/127 | 22.0 | 34/125 | 27.0 | 0.81 (0.52 to 1.25), 0.34 |
| Albrecht 1998138 | B2 | NR | |||||
| Edwards 2009142 | B2 | NR | |||||
| Lillington 1995145 | B2 | NR | |||||
| Lowe 1997146 | B2 | NR | |||||
| McBride 2004147 | B2 | 2 months post partum | 50/193 | 25.9 | 44/198 | 22.2 | 1.17 (0.82 to 1.66), 0.39 |
| McBride 2004147 | B2 | 2 months post partum | 42/192 | 21.9 | 44/198 | 22.2 | 0.98 (0.68 to 1.43), 0.93 |
| Morgan 2005148 | C1 | NR | |||||
| Radley 2013113 | C1 | NR | |||||
| Cluss 2011140 | C2 | NR | |||||
| Gulliver 2004104 | C2 | NR | |||||
| Nichter 2007110 | C2 | NR | |||||
| Ripley-Moffitt 2008111 | C2 | NR | |||||
| Ussher 2008,150 study 2 | C2 | NR | |||||
| Ussher 2008,150 study 1 | C2 | NR | |||||
| Cinciripini 2010139 | D2 | 3 months post partum | 22/128 | 17.2 | NA | ||
| Cinciripini 2010139 | D2 | 3 months post partum | 9/129 | 7.0 | NA | ||
| Pbert 2004149 | D2 | 1 month post partum | 59/309 | 19.1 | NA | ||
| Pbert 2004149 | D2 | 1 month post partum | 39/300 | 13.0 | NA | ||
| Pbert 2004149 | D2 | 3 months post partum | 116/309 | 37.5 | NA | ||
| Pbert 2004149 | D2 | 3 months post partum | 111/300 | 37.0 | NA | ||
| Study | Category | Time of follow up | Intervention | Control | RR (95% CI), p-value | ||
|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | ||||
| Donatelle 2000141 | A1 | NR | |||||
| Heil 2008105 | A1 | NR | |||||
| Higgins 2004144 | A1 | 6 months post partum | 4/30 | 13.0 | 3/23 | 13.0 | 1.02 (0.25 to 4.12), 0.98 |
| Mantzari 2012109 | A1 | NR | |||||
| Gadomski 2011143 | B1 | NRa | |||||
| Walsh 1997108 | B1 | NR | |||||
| Albrecht 1998138 | B2 | NR | |||||
| Edwards 2009142 | B2 | NR | |||||
| Lowe 1997146 | B2 | NR | |||||
| Lillington 1995145 | B2 | NR | |||||
| McBride 2004147 | B2 | 6 months post partum | 48/193 | 24.9 | 41/198 | 20.7 | 1.20 (0.83 to 1.73), 0.33 |
| McBride 2004147 | B2 | 6 months post partum | 35/192 | 18.2 | 41/198 | 20.7 | 0.88 (0.59 to 1.32), 0.54 |
| Morgan 2005148 | C1 | NR | |||||
| Radley 2013113 | C1 | NR | |||||
| Gulliver 2004104 | C2 | NR | |||||
| Nichter 2007110 | C2 | NR | |||||
| Ripley-Moffitt 2008111 | C2 | NR | |||||
| Ussher 2008,150 study 2 | C2 | NR | |||||
| Ussher 2008,150 study 1 | C2 | NR | |||||
| Cinciripini 2010139 | D2 | 6 months post partum | 54/128 | 42.2 | NA | ||
| Cinciripini 2010139 | D2 | 6 months post partum | 42/129 | 32.6 | NA | ||
| Pbert 2004149 | D2 | 6 months post partum | 113/221 | 51.1 | NA | ||
| Pbert 2004149 | D2 | 6 months post partum | 108/221 | 48.9 | NA | ||
Higgins and colleagues144 reported compliance as the proportion of monitoring sessions attended and found that 531 of 833 scheduled sessions (63.7%) were attended by women receiving vouchers contingent on cessation and 426 of 673 scheduled sessions (63.3%) were attended by women receiving non-contingent vouchers.
The study by Cinciripini and colleagues139 reported the proportions of respondents completing various numbers of therapy sessions as specified by the intervention, with 78% (208/266) completing seven sessions, 74% (197/266) completing eight sessions and 70% (186/266) completing nine sessions. The authors also reported that there were no between-group differences in treatment session attendance. In any case, in this study both groups received incentives.
In the study by Lillington and colleagues145 incentives were provided for the completion of behaviour change sheets. In total, 88% (136/155) of women completed one or more behaviour change sheet, 32% (50/155) completed between two and seven sheets, 19% (30/155) completed more than seven sheets and 8% (12/155) completed all 12 sheets.
Heil and colleagues105 reported compliance to be relatively high (83–95%) and not significantly different between the two treatment conditions at any assessment point, although no further details were provided.
Lowe and colleagues146 reported details of the proportions of participants who received individual components of the intervention. Of 45 participants, 93.9% (n = 42) received counselling, 97.9% (n = 44) received the calendar, 93.8% (n = 42) received a letter to give to their social supporter/’buddy’, all received a non-smoking contact, 66.7% (n = 30) received a physician’s letter to their home, 68.4% (n = 31) received reinforcement from a doctor, 71.1% (n = 32) received reinforcement from a registered nurse, 86.2% (n = 39) received ‘quit tips’ and 78.8% (n = 35) received deep-breathing instructions.
The study by Lowe and colleagues146 also reported the proportions of participants receiving various kinds of social support from their nominated social supporter/’buddy’. For the intervention group (who received incentives), 72.7% (n = 33) received support from a friend and the same proportion reported that they received help and praise from others and that their significant other understood stress. A total of 78.8% (n = 35) said that they received support from their significant other and 57.6% (n = 26) felt that they had the ability to deal with staying quit. Among the control (non-incentivised) group, 62.2% (n = 32) said that they received support from a friend, 72.4% (n = 38) received help and praise from others and 69.0% (n = 36) received support from their significant other. A total of 58.6% (n = 30) felt that their significant other understood their stress and 44.8% (n = 23) felt that they had the ability to deal with staying quit.
‘Deception’ rates
Some studies have tried to examine the issue of whether women ‘cheated’ (stated that they were abstinent when in fact they had smoked) to receive an incentive. ‘Deception’ was commonly measured by either comparing the difference between self-reported abstinence and biochemically validated abstinence or comparing different types of biochemical validation.
For example, the study by Gadomski and colleagues143 verified negative CO tests by comparing 10% of these samples with a saliva cotinine test to derive a deception rate (the number of positive saliva cotinine tests divided by the number of negative CO tests). For intervention groups there were large differences between samples (from 6% to 61%) but the time point at which these samples were verified was not reported, which is problematic as incentives (vouchers for nappies) were provided to intervention participants only post partum and not during pregnancy.
Walsh and colleagues108 reported inconsistencies between self-reported abstinence and biochemical validation (defined as a urine cotinine level of ≤ 500 nmol/l). Deception rates in self-reported abstinence were 12% for the experimental group (receiving the intervention) and 52% for the control group (not receiving the intervention), although there was some disparity in the proportion of respondents whose self-reported abstinence could be verified (86% in the experimental group and 78% in the control group). Pbert and colleagues149 reported the proportions of participants for whom saliva cotinine values were available, which was 58% at 9 months’ gestation, 49% at 1 month post partum, 43% at 3 months post partum and 46% at 6 months post partum, although it is not clear whether these percentages include those lost to follow-up at each time point. Problems with storage and transfer of samples were reported as the reason for this. Agreement between the self-reported and the cotinine-validated (with ≤ 20 ng/ml classified as abstinence) abstinence rates for the intervention and control groups, respectively, was high except at the final testing point: 89% and 92% at baseline, 84% and 94% at 9 months’ gestation, 81% and 88% at 1 month post partum, 71% and 90% at 3 months post partum and 40% and 80% at 6 months post partum. Both groups in this study received non-contingent incentives.
Lillington and colleagues145 also reported agreement between self-reported abstinence and saliva cotinine-verified abstinence (defined as < 20 ng/ml) at 6 months post partum. However, of the 111 saliva samples available for this time point, 23% (n calculated as 26 participants) exceeded the cut-off (of which 26% were from the intervention group and 21% were from the control group, although the denominators for both groups are unclear). The intervention group received incentives in this study although these were contingent on completing behaviour change worksheets and not on abstinence success.
This study also reported the reasons for not obtaining cotinine samples for validation, of which participant refusal was the reason in 48/143 (33.6%) cases. The study also reported that the intervention group (receiving incentives contingent on completing behaviour change sheets) had a lower refusal rate (23%) than the group not receiving incentives (33%), although the number of participants involved is not clear (numerators and denominators were not reported). 145
Costs
No studies reporting the cost-effectiveness of incentive interventions were identified; however, several other potentially relevant outcomes were reported by individual studies. Cost data were reported by five studies. 104,105,108,140,144 For the studies by Cluss and colleagues140 and Walsh and colleagues108 these data were estimates of the cost of providing the intervention.
For Cluss and colleagues,140 the cost per participant enrolled in the intervention was US$616.91 and the cost per abstainer was US$4433.87. The total cost of the intervention over the course of its 8-year implementation was US$527,631.00. A 2011 price year is assumed. This study had no control group.
For Walsh and colleagues,108 costs were reported as A$13.95 per participant for the intervention group (who received incentives) and A$1.83 for the control group, with the total cost per biochemically validated abstainer as measured at the end of pregnancy of A$121.41 for intervention participants and A$37.88 for control group participants (1997 price year assumed).
Two other studies105,144 reporting information on costs provided data on mean voucher earnings for those receiving vouchers contingent on cessation and for those receiving non-contingent vouchers. For the study by Heil and colleagues,105 voucher earnings as measured at the end of the intervention (12 weeks) in the contingent group ranged from US$0.00 to US$1180.00, with mean earnings of US$461.00 (SD US$456.00), and in the non-contingent group ranged from US$75.00 to US$670.00, with mean earnings of US$413.00 (SD US$163.00). A 2008 price year is assumed. For Higgins and colleagues,144 voucher earnings in the contingent group ranged from US$0.00 to US$1135.00, with mean earnings of US$397.00 (SD US$414.00), whereas voucher earnings in the non-contingent group ranged from US$35.00 to US$517.00, with mean earnings of US$313.00 (SD US$142.00). (A 2004 price year is assumed.)
The study by Gulliver and colleagues104 reported that funding did not exceed US$4000.00 (2004 price year) but noted that this figure did not include donated advertising costs and the costs of the incentives.
Implementation and sustainability
There were no data relevant to the sustainability of the incentive interventions in terms of the long-term benefits outweighing the costs, or to unintended effects once the research has finished. In two studies, incentives donated by local businesses were specifically sought to save costs and to make the programme more sustainable to principal funders. 104,108 Several authors commented that implementation in practice would require provider service changes;142 additional contacts over and above usual care to allow for monitoring/contingency;105,144 the enhancement or intensification of usual care contacts;104,108,143,146 a joined-up approach or case management;111 one-to-one specialist care in pregnancy;148 and additional care post partum to help to prevent relapse. 149 All of these suggestions are likely to have considerable resource implications. However, there was some disagreement in that the authors of two studies concluded that delivery could be effective even in brief (existing) usual care consultations in pregnancy. 145,149
Acceptability outcomes
For some studies, authors had conducted a pilot study to determine feasibility, for example the study by Higgins and colleagues144 was a pilot for the study by Heil and colleagues. 105 Other studies used a process evaluation, which included participants’ feedback,142,145,146,148 or planned to conduct an evaluation. 141 Four studies reported information on the acceptability of the interventions. Walsh and colleagues108 reported that, for midwives, the duration of the intervention was acceptable. This staff group spent significantly more time (t-test value of p < 0.0001 reported) discussing smoking with experimental (incentivised) patients (on average 7 minutes and 12 seconds) than with control (non-incentivised) patients (on average 1 minute 46 seconds).
The study by Cluss and colleagues140 reported that 90% of participants responding to a question about the quality of the service they received were ‘very satisfied’ and 98.5% said that the intervention had helped them deal more effectively with quitting smoking or cutting down their smoking intake. In addition, 95.5% said that they would refer a friend. In terms of the components that respondents found to be the single most helpful treatment, 40.8% cited the interventionist support, 30.5% indicated that CO monitoring helped, 14.8% stated that it was education about the effects of smoking, 5.1% said that it was the improvement to their health, 3.5% cited incentives, 2.1% said that it was problem-solving assistance and 3.3% said that it was another reason.
Ussher and colleagues150 reported that 30 out of 32 recruited participants (94%) had indicated that they would like to join a study involving randomisation to a physical activity (the main component of the intervention in this study) or the control condition.
Lynagh and colleagues151 surveyed acceptability and feasibility of incentives among outpatients at an antenatal clinic of a public hospital in Australia. The self-administered survey was distributed to women between 16 and 42 weeks’ gestation by clinic staff. There were quality limitations with the findings as numerators and denominators were not well reported. It was found that only 25% of 213 participants would endorse the idea of paying women to quit smoking (agreed or strongly agreed). In total, 60% disagreed or strongly disagreed that it was a good idea and 15% were undecided. In terms of feasibility, however, support for the idea that such a programme would be successful was greater and just over half thought that it might work (30%) or were undecided (22%). Among respondents, those who were smokers themselves were significantly more likely to think that incentives were both a good idea (p < 0.0001) and that they would be likely to work (p < 0.003).
Results of the breastfeeding review
We now consider the results of the evidence synthesis for studies that report incentives delivered to women to improve breastfeeding outcomes.
Number of studies identified
We identified 5408 records from the primary searches for this review. After title and abstract screening, 5092 studies were considered not to be relevant and were excluded, leaving 316 studies for full-text assessment; in addition, 10 studies were identified from other sources. In total, therefore, the full texts of 326 studies were screened, of which 19 studies (20 reports103,106,107,112,167–182) were included in the review. Two of the studies included in the effectiveness and process reviews were conference abstracts. 180,181 A flow chart of the screening process is provided in Figure 10.
FIGURE 10.
Flow chart outlining the screening process for the review of incentives for breastfeeding.
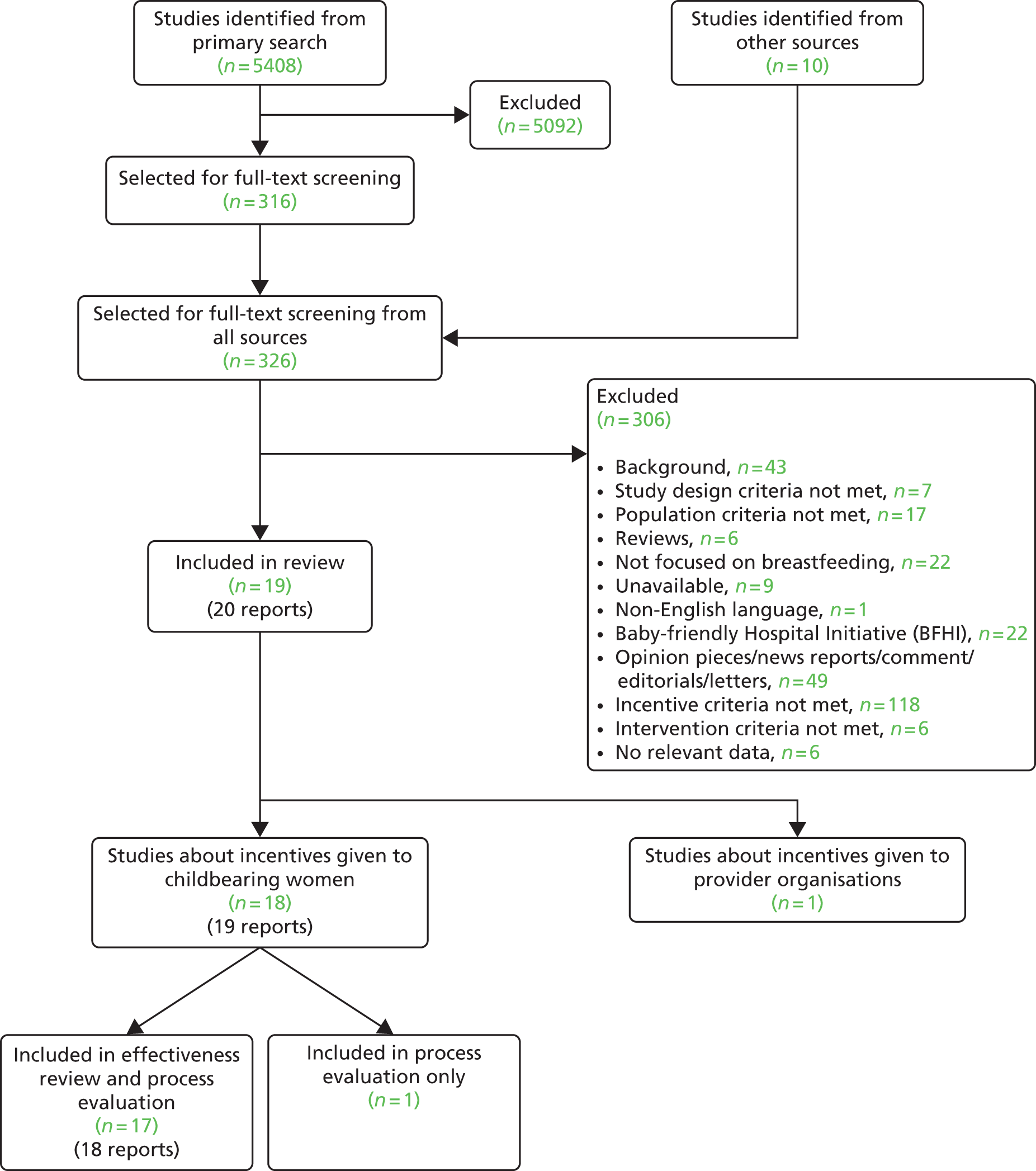
Included and excluded studies
Nineteen studies evaluating incentives for breastfeeding met our inclusion criteria because an incentive had been provided, even though evaluating the effect of incentivisation was not always the primary purpose of the study. Seventeen full study reports103,106,107,112,167–179 and two conference abstracts180,181 were included in the effectiveness and process review. One additional study was included in the process review only as it did not provide the required breastfeeding outcome data for inclusion. 182 Seventeen studies103,106,107,112,167,169–181 provided incentives to childbearing women and one study168 levied financial penalties against organisations for not meeting their own breastfeeding targets. The study providing incentives to organisations for breastfeeding168 is considered separately later in this chapter.
There were reports in which it was unclear whether the study met our definition of an incentive. Decisions to include as an incentive study were made for the following:
-
Organisational awards/quality standard achievements (e.g. ‘kite marks’) that have a tangible value such as a plaque, certificate or award ceremony, and usually require financial investment to attain, were included and can be considered commitment contracts. An example is the UNICEF Baby Friendly Initiative (BFI),183 which awards hospitals and communities with a plaque and can be considered as a commitment contract. The literature search identified 22 reports describing the BFI, which is a multifaceted and multilevel intervention. Hospitals and primary care organisations pay money to UNICEF UK to complete an accreditation process, commit to training staff and receive an esteemed award on achieving the quality standards. The reports identified by our search strategy represent a small proportion of a substantial body of literature that has recently been systematically reviewed by a team including co-applicant FD. 184 A full review of the BFI literature to identify data relating to the incentive component of interest is beyond the scope of our study because of the large number of publications. The Beake and colleagues184 review is summarised later in this chapter (see Results of incentives provided to organisations to improve smoking cessation in pregnancy and breastfeeding outcomes).
-
As with the review of studies providing incentives for smoking cessation in pregnancy, US WIC programme studies were included if they provided incentives over and above those routinely provided as part of the programme or reported qualitative findings about incentives.
-
Studies providing medical devices to assist the target behaviours that are not routinely provided by the UK health service, which women can keep and which potentially have a monetary exchange value. Studies providing breast pumps were therefore included.
Decisions to exclude as an incentive study were made for the following:
-
Studies providing medical devices to assist the target behaviours that are routinely provided free of charge by the UK health service as a treatment and which women can keep. Nipple shields were therefore excluded.
The reasons for exclusion of assessed full-text papers are given in Figure 10.
Quality of the included studies
Details of the questions used to establish the quality of 16 of the 18 included studies presenting quantitative outcome data are available in Appendix 14. 103,106,107,112,167–176,178,179 The quality of two conference abstracts180,181 was not assessed because of the limited data presented. The study by Wright and colleagues182 was not quality assessed as no appropriate tool was identified to assess the quality of case studies and it provided few data.
Information about quality across studies for each individual aspect of quality and across all aspects of quality for each individual study are provided in Figures 11 and 12 respectively. For both figures, answers of ‘yes’ represented the maximum possible level of quality. Therefore, in considering Figure 11, it can be seen that most studies recruited women at the same point in their pregnancies (88%103,106,107,167–172,174–179), had a follow-up period that was similar between groups (94%103,106,107,112,167,168,170–179) and long enough to detect important effects on outcomes of interest (100%103,106,107,112,167–179), used experienced staff (94%103,106,107,112,167,169–172,174–179), had appropriate facilities to perform the intervention (100%103,106,107,112,167–179) and reported all important outcomes (100%103,106,107,112,167–179).
FIGURE 11.
Quality assessment results by quality appraisal question for all studies included in the review of incentives provided to childbearing women for breastfeeding.
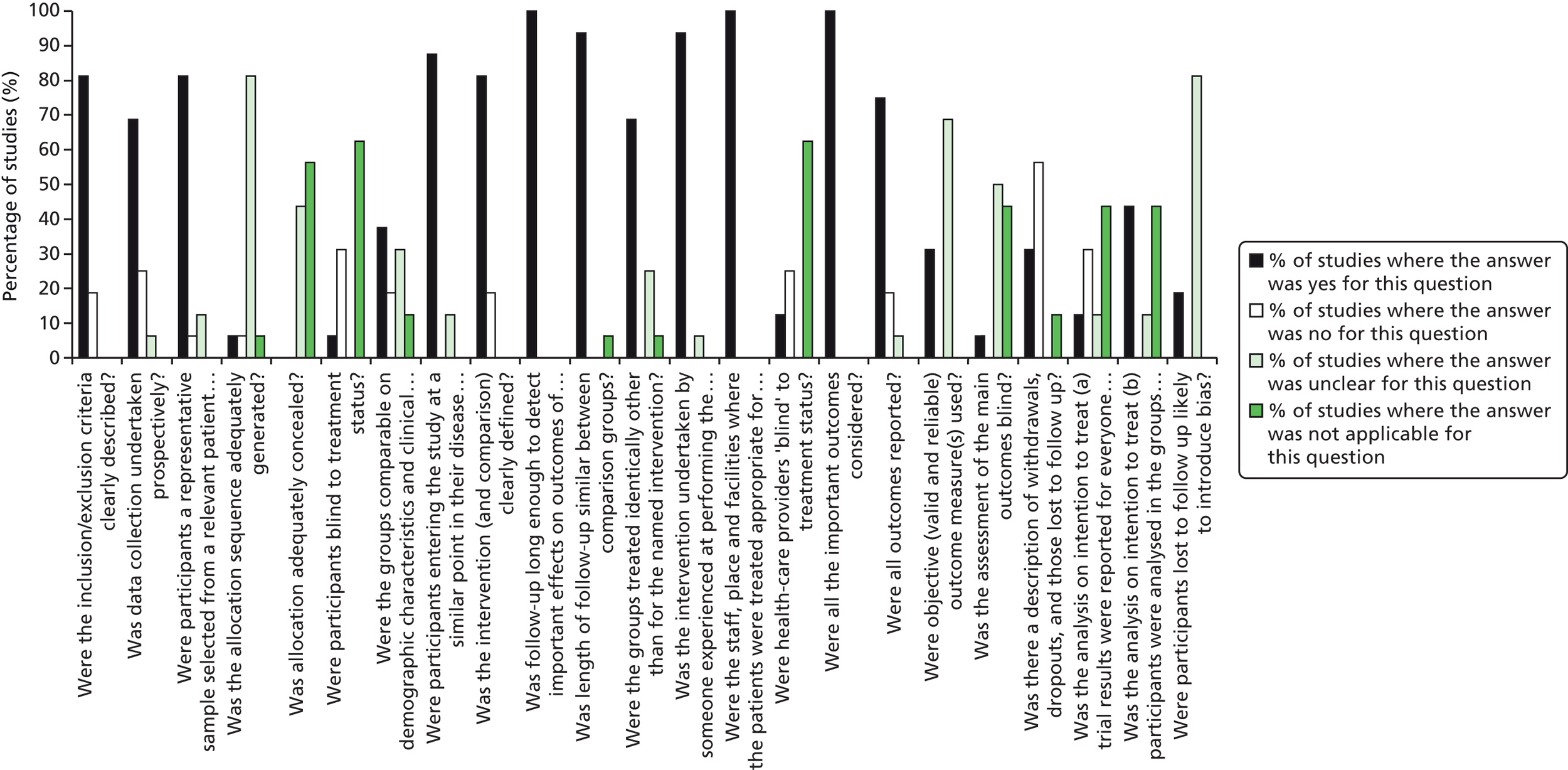
FIGURE 12.
Quality assessment results by study for all aspects of quality for all studies included in the review of incentives provided to childbearing women for breastfeeding.
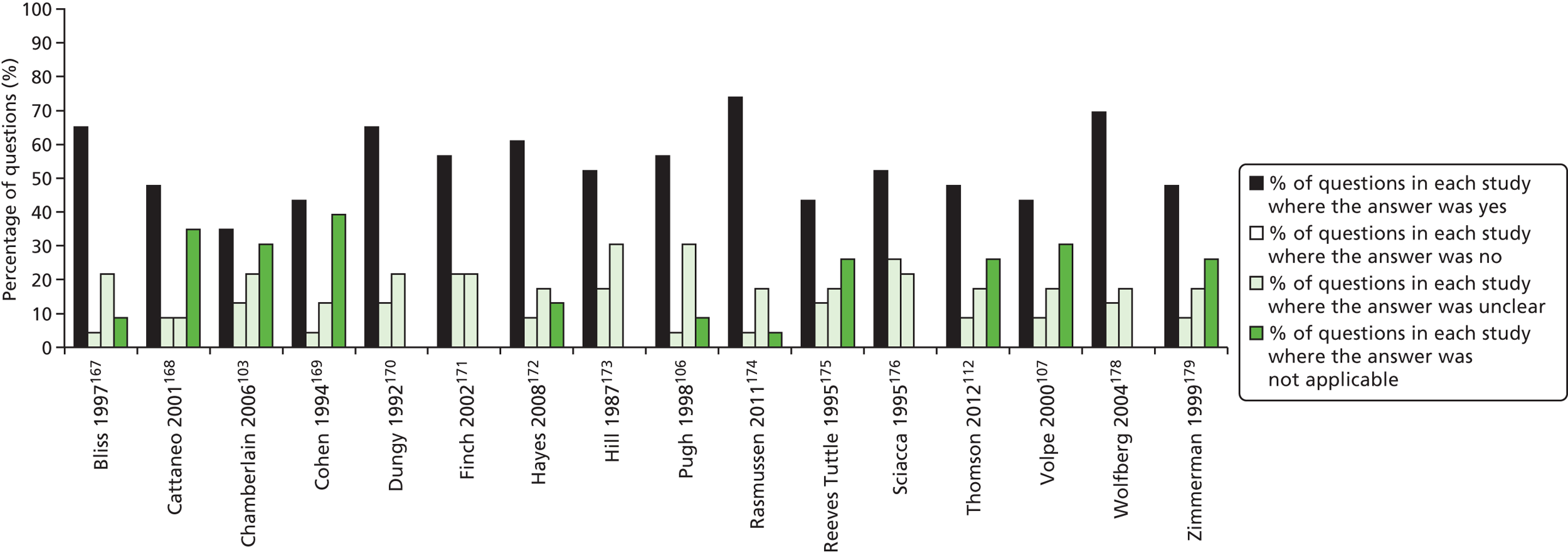
More problematic aspects of study quality within this group of studies were the lack of description of those lost to follow-up (31% of studies reported this adequately112,167,170,178,179) and an imbalance in demographic characteristics between groups (38% of studies reported this106,107,112,172,174,178); in addition, only two studies conducted analysis based on ITT. 173,174 Only five studies (25%) used a valid and reliable outcome measure. 112,167–169,174 For the purposes of this review, studies were considered to have a valid and reliable outcome measure if they reported a recognised description of how they defined breastfeeding. When studies were assessed for adequacy of reporting on randomisation and recruitment, that is, whether randomisation sequences had been adequately generated for RCTs or whether patients were recruited consecutively for non-randomised studies, only one study was considered adequate. 103 Because of the nature of the interventions, none of the participants was blind to treatment status and in only one study were caregivers blinded. 174
Two independent researchers (VS and SM) appraised the qualitative part of a mixed-methods study in one paper because it had been written by members of the research team involved in quality assessment. 112 The CASP score for this paper was high at 8 out of 10, with half a point given for ethical issues because of a lack of clarity around the consent procedure and only verbal information being provided and half a point given because of weak integration of mixed-methods analysis. No points were given for reflexivity.
Characteristics of the included studies
Initially, studies included in the review of incentives provided to childbearing women for breastfeeding were classified according to study design, as described in the smoking cessation review. This classification is presented in Table 21.
| Category | Studies |
|---|---|
| Category A studies (n = 0) compare contingent incentives for validated breastfeeding outcomes with non-contingent incentives | No studies |
| Category B studies (n = 13) compare incentives with no incentive, a very small incentive or a disincentive (gift packs including infant formula) | Bai 2010,180 Bliss 1997,167 Chamberlain 2006,103 Chiasson 2011,181 Dungy 1992,170 Finch 2002,171 Pugh 1998,106 Rasmussen 2011,174 Reeves Tuttle 1995,175 Sciacca 1995176 (control group given very small incentive by comparison), Thomson 2012,112 Volpe 2000,107 Zimmerman 1999179 |
| Category C studies (n = 3) evaluate incentives but do not have a control group | Cohen 1994,169 Hayes 2008,172 Wright 2012182 |
| Category D studies (n = 2) are those in which both the intervention group and the control group received the same incentives and these studies are therefore comparing another intervention component | Hill 1987,173 Wolfberg 2004178 |
| Category E studies (n = 0) are those in which no incentive intervention was provided | No studies |
Unlike the incentives in the smoking cessation review, there were no category A studies or other categories of study suitable for meta-analysis. Thus, to maximise the potential of the narrative evidence synthesis, studies were categorised according to the type of incentive, with the order within each category determined, first, by study design, with randomised studies placed before other intervention designs, and, second, by sample size (Table 22). This order is maintained for all tables in this chapter.
| Incentive | Studies |
|---|---|
| Includes a breast pump (and possibly other secondary incentive items, e.g. feeding-related items) | RCTs (n = 4): Bliss 1997,167 Dungy 1992,170 Hayes 2008,172 Rasmussen 2011174 |
| Historically controlled study (n = 1): Chamberlain 2006103 | |
| Non-RCT (n = 1): Bai 2010180 | |
| Case series (n = 1): Cohen 1994169 | |
| Breast pump, vouchers, gifts and raffle for both parents | RCT (n = 1): Sciacca 1995176 |
| Includes food packages | RCT (n = 1): Finch 2002171 |
| Historically controlled study (n = 1): Chiasson 2011181 | |
| Gifts and vouchers | Historically controlled studies (n = 4): Reeves Tuttle 1995,175 Thomson 2012,112 Volpe 2000,107 Zimmerman 1999179 |
| Case study (n = 1): Wright 2012182 | |
| Household tasks | RCT (n = 1): Pugh 1998106 |
| Cash for participation in both study arms | RCT (n = 2): Hill 1987,173 Wolfberg 2004178 |
The main characteristics of the studies, most of which originated in the USA (17/18), are described in Table 23.
| Study | Country | Study design | No. randomised/recruited | Incentive | Contingency | Primary outcome | Follow-up | Main outcomes reported |
|---|---|---|---|---|---|---|---|---|
| Bliss 1997167 | USA | RCT | 1625 | Manual breast pump and formula discharge packs (three intervention arms) | Initiation | Breastfeeding duration | 6 weeks and 4 and 6 months | Mean duration (weeks) of any breastfeeding: intervention (pump only) 16.0; intervention (pump + formula) 15.7; control 15.7 |
| Dungy 1992170 | USA | RCT | 146 | Manual breast pump + gift items | Initiation | Breastfeeding continuation rates | 2, 4, 6 and 8 weeks | Prevalence of exclusive breastfeeding (weeks): intervention: 2 weeks 72.7% (32/44), 4 weeks 54.5% (24/44), 6 weeks 45.5% (20/44), 8 weeks 36.4% (16/44); control: 2 weeks 53.5% (23/43), 4 weeks 34.9% (15/43), 6 weeks 30.2% (13/43), 8 weeks 23.3% (10/43) |
| Hayes 2008172 | USA | RCT | 280 | Electric or manual breast pump | Participation | Breastfeeding duration | 6 and 12 months | Breastfeeding for ≥ 6 months: electric pump 72.3% (94/130); manual pump: 76.8% (76/99) Median duration (months) of any breastfeeding: electric pump 12 (95% CI 8 to 12); manual pump 11 (95% CI 9 to 14) |
| Rasmussen 2011174 | USA | RCT | 34 | Electric or manual breast pump (two intervention arms) | Participation | Breastfeeding continuation rates | 7, 30 and 90 days | Median duration (weeks) of exclusive breastfeeding (IQR): electric pump 0.7 (0.1–2.7); manual pump 2.3 (0.4–4.4); control 4.4 (1.1–9.4) Median duration (weeks) of any breastfeeding (IQR): electric pump 4.0 (2.4–8.4); manual pump 13.4 (2.1–36.0); control 26.6 (9.4–44.6) |
| Chamberlain 2006103 | USA | Historically controlled study | Unclear | Breast pump (type unspecified) | Participation | Breastfeeding initiation rates | Initiation | Breastfeeding initiation: intervention: general 74%, NICU 64%; control: general 16%, NICU 34.5% |
| Bai 2010180 | USA | Quasi-experimental | 450 | Manual breast pump | Participation | Exclusive breastfeeding continuation | 2, 4 and 12 weeks | Mean (±SD) exclusive breastfeeding rate (weeks): intervention 8.1 (±5.0); control 6.0 (±4.8) Exclusive breastfeeding at 12 weeks: intervention 60.4%; control 37.8% |
| Cohen 1994169 | USA | Case series | 187 | Electric breast pump + gift items | Initiation | Breastfeeding duration and behaviour | Unclear | Average duration (months) of any breastfeeding: 8.1. Since programme inception (4-year period), 74% (n = 139) of mothers who returned to work breastfeeding continued to breastfeed for a least 6 months compared with a national average of 10% |
| Sciacca 1995176,177 | USA | RCT | 68 | Breast pump, vouchers, gifts and raffle for both parents | Participation and continuation of breastfeeding | Exclusive and any breastfeeding rates at hospital discharge and continuation rates | 2 and 6 weeks and 3 months | Exclusive breastfeeding: at discharge: intervention 23/26 (88.5%), control 16/29 (55.2%); at 2 weeks: intervention 21/26 (80.8%), control 10/29 (34.5%); at 6 weeks: intervention 13/26 (50.0%), control 7/29 (24.1%); at 3 months: intervention 11/26 (42.3%), control 5/29 (17.2%) Any breastfeeding: at discharge: intervention 3/26 (11.5%), control 7/29 (24.1%); at 2 weeks: intervention 4/26 (15.4%), control 6/29 (20.7%); at 6 weeks: intervention 8/26 (30.8%), control 2/29 (6.9%); at 3 months: intervention 5/26 (19.2%), control 2/29 (6.9%) |
| Finch 2002171 | USA | RCT | 60 | Food packages + voucher | Initiation, continuation, exclusivity | Exclusive and partial breastfeeding initiation and continuation rates | Initiation and 8 weeks | Breastfeeding initiation: no significant difference between the two groups Median duration (weeks) of exclusive breastfeeding (min./max.): intervention 12 (7/12+) (n = 9/19); control 12 (5/12+) (n = 5/29) Median duration (weeks) of partial breastfeeding (min./max.): intervention 5 (1/12+) (n = 6/19); control 12 (1/12+) (n = 15/29) |
| Chiasson 2011181 | USA | Historically controlled study | Unclear | Food packages | Initiation and continuation | Breastfeeding initiation rates | Unclear | Breastfeeding initiation rates increased from 72.2% (pre implementation) to 75.5% (post implementation) |
| Reeves Tuttle 1995175 | USA | Historically controlled study | 412 | Vouchers + gift items | Participation, initiation and continuation | Breastfeeding initiation rates and continuation rates | By day 3 and at 3–6 weeks | Breastfeeding at initiation: intervention 24/63 (38.1%), control NR; at 3–6 weeks: intervention 11/63 (17.5%), control 19/349 (5.4%) |
| Thomson 2012112 | UK | Qualitative and historically controlled study | 266 in historically controlled arm | Vouchers + gift items | Initiation and continuation | Breastfeeding at 6–8 weeks in historically controlled arm | 6–8 weeks | Any breastfeeding at 6–8 weeks: intervention 57/94 (60.6%); control 119/172 (69.2%) |
| Volpe 2000107 | USA | Historically controlled study | 91 | Gift items | Participation | Breastfeeding initiation rates defined as at least once a day for at least 3 days | Initiation | Breastfeeding at initiation: intervention 28/43 (65.1%); control 7/48 (14.6%) |
| Zimmerman 1999179 | USA | Historically controlled study | 737 | Gift items | Participation | Exclusive and partial breastfeeding rates | Hospital discharge and 2 weeks | Exclusive breastfeeding at discharge: baseline 67 (36%), year 1 74 (51%), year 2 222 (55%); at 2 weeks: baseline 37 (20%), year 1 44 (24%), year 2 115 (30%) Partial breastfeeding at discharge: baseline 26 (14%), year 1 14 (10%), year 2 47(12%); at 2 weeks: baseline 28 (15%), year 1 35 (24%), year 2 105 (27%) |
| Wright 2012182 | USA | Case study | Approximatley 100 | Gift items | Participation | NR | NA | NA |
| Pugh 1998106 | USA | RCT | 60 | Non-nursing tasks | Initiation and continuation | Breastfeeding duration and breastfeeding continuation rate | Initiation and 6 months | Mean breastfeeding duration (days): intervention 136.3; control 88.3 Breastfeeding at 6 months: intervention 50%; control 27% |
| Hill 1987173 | USA | RCT | 64 | Cash | Participation | Breastfeeding continuation rates | ≥ 6 weeks | Breastfeeding at ≥ 6 weeks: intervention 12/31 (39%); control 10/33 (30%) |
| Wolfberg 2004178 | USA | RCT | 59 couples | Cash | Participation | Breastfeeding initiation rates and continuation rates | Initiation and 4, 6 and 8 weeks | Breastfeeding at initiation: intervention 20/27 (74%), control 13/32 (41%); at week 4: intervention 10/26 (38%), control 11/31 (35%); at weeks 6 and 8: intervention 9/26 (35%), control 6/31 (19%) |
Incentive/reward-related elements
The detail of the incentive interventions provided are summarised in Table 24. Studies provided multicomponent interventions to encourage new mothers to breastfeed, with interventions (or even individual components within the intervention) being provided with varying frequency, intensity and duration (Table 25). Three studies were considered to have more than one incentive intervention arm for the outcome of interest (i.e. breastfeeding). The four-arm RCT by Bliss and colleagues167 compared discharge packs containing manual breast pumps alone, manual breast pumps with infant formula or formula alone with a control group receiving neither formula nor a pump. The RCT by Rasmussen and colleagues174 compared two incentive intervention arms, a manual breast pump or the loan of an electric breast pump for 14 days, with a control group receiving no pump. The non-RCT by Bai and colleagues180 compared discharge packs containing a manual breast pump and breastfeeding information, discharge packs with information only and ‘commercial’ discharge packs that contained infant formula. The incentive components varied across the studies and ranged from a breast pump103,172,174,180 to a combination of a breast pump and feeding-related items,167,169,170 a breast pump, vouchers, gifts and a raffle for both parents,176 enhanced food packages in the context of the WIC programme,171,181 gift items or vouchers107,112,175,179,182 or cash. 173,178 Only one study provided non-material incentives as a help with non-nursing tasks. 106 In all studies incentives were provided at consistent and predictable time points. In addition, in the study by Sciacca and colleagues,176 a raffle was provided at 3 months post partum with a wide range of gifts and experiences on offer.
| Study | Incentive/reward type | Awareness | Content | Contingency target | Actor |
|---|---|---|---|---|---|
| Bliss 1997167 | Manual breast pump | Unclear | Material | Preparatory behaviour | Participant |
| Hayes 2008172 | Electric breast pump (loan) | Unclear | Material | Preparatory behaviour | Participant |
| Manual breast pump (loan) | Unclear | Material | Preparatory behaviour | Participant | |
| Dungy 1992170 | Manual breast pump + gifts (e.g. breast pads, breast cream). Total value US$15.00 | Unclear | Material | Preparatory behaviour | Participant |
| Rasmussen 2011174 | Manual breast pump | Unclear | Material | Preparatory behaviour | Participant |
| Electric breast pump (loan for 10–14 days) | Unclear | Material | Preparatory behaviour | Participant | |
| Chamberlain 2006103 | Breast pump (type unspecified) | Unclear | Material | Preparatory behaviour | Participant |
| Cohen 1994169 | Electric breast pump + gifts (expressing kit) | Unclear | Material | Preparatory behaviour | Participant |
| Bai 2010180 | Manual breast pump | Unclear | Material | Preparatory behaviour | Participant |
| Sciacca 1995176 | Breast pump (type unspecified) + gifts (e.g. baby powder, nappies) | Yes | Material | Preparatory behaviour | Participant |
| Gifts (e.g. coupon for haircut, lunch or breakfast for two, clothing voucher, infant carrier) | Yes | Material | Preparatory behaviour | Participant + social other | |
| Gifts (e.g. ’pair of tickets to a Northern Arizona football game’) | Yes | Material | Preparatory behaviour | Social other | |
| Gifts (e.g. bag of nappies, baby wipes) + raffle (e.g. trip to Grand Canyon, US$100.00 grocery voucher, tool kit) | Yes | Material | Health behaviour | Participant | |
| Finch 2002171 | Food package (US$50.00) + gift certificate (US$25.00) | Unclear | Material | Health behaviour | Participant |
| Chiasson 2011181 | Food package | Unclear | Material | Health behaviour | Participant |
| Zimmerman 1999179 | Gifts (nursing pads, t-shirt, fridge magnet, safety plug guard). Total value US$8.00 | Unclear | Material | Preparatory behaviour | Participant |
| Reeves Tuttle 1995175 | Vouchers + gifts (baby-care products, certificate with infant photograph) | Yes | Material | Preparatory behaviour | Participant |
| Volpe 2000107 | Gifts (chocolate, safety plug guard, perfume) | Yes | Material | Preparatory behaviour | Participant |
| Thomson 2012112 | Gifts (picture frame, healthy treats, swimming vouchers, magazine, cakes/hot drinks). Total value £71.99 | Yes | Material | Preparatory behaviour | Participant |
| Wright 2012182 | Gifts (water bottle, e.g. baby blanket, reminder bracelet) | Unclear | Material | Preparatory behaviour | Participant |
| Pugh 1998106 | Non-nursing tasks including housework or childcare | Yes | Social | Preparatory behaviour | Participant |
| Wolfberg 2004178 | Money (US$25.00) | Unclear | Material | Preparatory behaviour | Social other |
| Money (US$25.00) | Unclear | Material | Preparatory behaviour | Participant | |
| Hill 1987173 | Money (US$5.00) | Unclear | Material | Preparatory behaviour | Participant |
| Study | Setting | Who delivered the intervention | Mode of delivery | Recruitment timing | No. of contacts when incentive provided | No. of contacts when no incentive provided | Total no. of contacts | Intervention finish (week/month before or after birth) | Final follow-up contact (week/month before or after birth) | Total study contact period |
|---|---|---|---|---|---|---|---|---|---|---|
| Bliss 1997167 | Regional tertiary hospital with a Certificate of Intent to become a WHO/UNICEF BFI hospital | Staff nurses | Face to face | Postnatal before discharge | 1 | 3 | 4 | Unclear | 6 months post partum | 6 months |
| Hayes 2008172 | WIC clinic | Unclear | Face to face | Last prenatal or first post-partum appointment | 1 | 2 | 3 | Unclear | 12 months post partum | 12 months |
| Dungy 1992170 | Private hospital | Trained female interviewee | Face to face | < 48 hours after delivery | 1 | 5 | 6 | Unclear | 8 weeks post partum | 8 weeks |
| Rasmussen 2011174 | Rural hospital | Research assistants | Face to face | Prenatal | 1 | 11 | 12 | 2 weeks post partum | 90 days post partum | 90 days |
| Chamberlain 2006103 | Inner city hospital/disadvantaged area – general ward and a neonatal intensive care unit | Hospital staff | Unclear | Birth | 1 | NA | 1 | Unclear | None (routine data) | NA |
| Cohen 1994169 | At work (aeronautics company, water and power utilities company) | Lactation professional | Face to face (individual and family) | Prenatal | 1 | Unclear | Unclear | Unclear | Unclear | Unclear |
| Bai 2010180 | Two hospitals | Unclear | Face to face | Post partum | 1 | 3 | 4 | Unclear | 12 weeks post partum | 12 weeks |
| Sciacca 1995176 | Two WIC clinics | Unclear | Groups, couples + peer counselling | Prenatal | > 10 | Additional peer counselling | > 10 | 3 months post partum | 3 months post partum | 4–11 months |
| Finch 2002171 | WIC clinic | Trained counsellor | Group | Prenatal | Unclear – regular packages | 1 – education plus counselling | Unclear | 2 months post partum | Unclear | Unclear |
| Chiasson 2011181 | WIC clinic | WIC staff | Unclear | Unclear | Unclear – regular packages | Unclear | Unclear | Unclear | Unclear | Unclear |
| Zimmerman 1999179 | Inner-city clinic (university affiliated) | Clinic nutritionist | Group + peer support | Prenatal (third trimester) | 1 | Monthly breastfeeding support group | Unclear | 1 week post partum | 2 weeks post partum | Unclear |
| Reeves Tuttle 1995175 | WIC clinic | Research staff, Hmong peer counsellors | Group and individual face to face | Prenatal (third trimester) | 4 | Additional telephone calls to hospital/mother from 38 weeks to check whether infant born | Unclear | 3–6 weeks post partum | 3–6 weeks post partum | 4–5 months |
| Volpe 2000107 | High school | Nurse clinician/lactation consultant | Group | 3 weekly classes | > 3 | Weekly visits for those who continued breastfeeding | Unclear | Unclear | Unclear | Unclear |
| Thomson 2012112 | Participants recruited on the postnatal ward of a hospital in North West England disadvantaged area. Intervention delivered in participants’ homes, community settings or children’s centres | Peer supporters | Face to face peer support | Postnatal | > 8 | Unclear | > 8 | 8 weeks post partum | 8 weeks post partum | 8 weeks |
| Wright 2012182 | WIC clinic (room remodelled to include art, television and DVD player) | Project staff | Groups and telephone calls | NA | 1 | Unclear | Unclear | Unclear | Unclear | Unclear |
| Pugh 1998106 | Participants recruited at community hospital. Intervention delivered in participants’ homes | Community health nurse | Home help | < 24 hours of delivery | 3 | 5 | 8 | 6 weeks post partum | 6 months post partum | 6 months |
| Wolfberg 2004178 | Local hospital | Peer educator (black, also a father) | Groups for fathers, telephone calls for mothers | Prenatal (third trimester) | 1 (father) + 0 (mother) | 1 (father) + 4 (mother) | 2 (father) + 4 (mother) | Unclear (third trimester) | 8 weeks post partum | 8 weeks |
| Hill 1987173 | Midwestern tertiary university hospital | Antenatal hospital staff | Groups | Prenatal | 1 | 1 | 2 | 6 weeks post partum | 6 weeks post partum | Unclear |
All incentive interventions involved interactions with incentive providers or other professionals either explicitly or implicitly supporting breastfeeding and we refer to this as the general BCT component of the intervention. These were extracted for each study when reported. However, general BCT components are frequently under-reported and no study mentions observations or recordings of interactions during intervention delivery to assess fidelity or content. We could not extract data for the control arms because of poor-quality reporting, particularly of usual care.
The proportions of the intervention that can be attributed to the incentive and to the general BCT component varied between studies and were sometimes difficult to assess. Across studies, women were invited to participate in a range of activities to obtain the incentives, some of which were reported as usual care plus additional components,103,170,171,179,181 an enhanced programme of care106,112,175,176,178,182 or involved the implementation of a new protocol. 107,167,169,172–174,180
General behaviour change techniques
One study was excluded from BCT extraction as the intervention was rolled out to providers at the health system level rather than at the individual level168 and was thus not in line with the target behaviour. Two studies were excluded from BCT extraction as they were reported only in abstract form, which prevents accurate judging of intervention content. 180,181 Moreover, one study178 targeted partners of pregnant women and BCTs were rated only in relation to increasing breastfeeding in women (i.e. support-related BCTs) rather than rating the BCTs used to target partners’ support behaviours (e.g. information provision and instruction techniques).
Applying the 93-point BCT taxonomy described by Michie and colleagues17 to the included studies resulted in an acceptable inter-rater agreement, with a kappa of 0.77, ranging from 0.69 (‘instruction on how to perform the behaviour’) to 0.88 (‘adding objects to the environment’). Explicit reporting of BCTs underpinning interventions varied and reporting was often unclear or implicit. Altogether, 27 study arms were rated for inclusion of 82 BCTs, including seven control/usual care arms and 20 intervention arms. Intervention arms used an average of 4.34 (SD 2.8) BCTs, ranging from one170,173 to 12,175 whereas control/usual care arms used an average of 1.7 (SD 2.5) BCTs, ranging from zero106,107,175 to five. 176
Out of 82 BCTs rated, study arms were identified to have used 22 (Figure 13), with the BCTs used most often used being ‘Adding objects to the environment’ (n = 14), which, in most studies, took the form of providing breast pumps, ‘Social support (unspecified)’ (n = 12), ‘Instruction on how to perform the behaviour’ (n = 11) and ‘Information about health consequences’ (n = 10). As in the smoking cessation review, WIC studies were recorded separately without inferring the presence of specific BCTs given the variation in WIC programme implementation. Six studies were rated as being engaged in the WIC programme171,172,175,176,181,182 in addition to the identified BCTs.
FIGURE 13.
Behaviour change techniques used in studies included in the review of incentives for breastfeeding.

Incentive and reward elements were identified reliably (kappa = 0.73). Out of the 18 studies, two176,178 used multiple incentives within an intervention, which varied across the four assessed incentive elements: contingency target, actor, type and content (see Table 24). Two studies used different rewards/incentives for different experimental groups within the same study. 172,174 The majority of studies were rated as unclear in relation to whether participants were aware of the incentives/rewards (n = 13), with the remaining rewards/incentives rated as being known to participants (n = 5).
General BCT components included peer support or counselling in five studies, although the formality of this varied from its being a key component, that is, having a nominated peer supporter,107,112,176 to the intervention involving membership of a peer support group107,179 or a trained Hmong peer counsellor. 175 Professional support was offered in one study182 and professional counselling was offered in a number of studies. 106,107,169,175 Proactive telephone calls were made in five studies, with the purpose being predominantly to collect outcome data167,170,173,180 although in one study new breastfeeding mothers were offered reactive breastfeeding support by telephone. 182
Setting and provider
The studies included in the review were typically conducted in the USA (17/18), with only one carried out in the UK112 (see Table 23). Several studies were funded by private sources106,107,167,170,172,178,179,182 whereas others were funded by private and university funds180 or national health or government institutes,175,176,185 one of which explicitly reported the receipt of extra financial support from the WIC programme. 175 One study was jointly funded by a charity, breast pump distributors and local insurance companies, relying on donations from local businesses to provide the incentives. 103 Authors in one study declared research consultancy for a breast pump manufacturer169 and another involved the breast pump manufacturer for distribution. 174 In four studies the funding source was unclear. 169,171,173,181 For the study conducted in the UK, funding was received from the NHS and a breastfeeding charity. 112
The lead authors’ disciplinary backgrounds were also assorted, including lactation specialists,107,179 nutritionists,175 paediatricians/maternal health clinicians,169,170,172,178 public health/health research researchers112,182 and psychologists,170 and those with a health education background. 106,171 Five study teams involved members from across these disciplines. 103,167,174,176,180 Details were not reported for two studies. 172,181
The intervention settings and providers delivering the interventions varied across the included studies (see Table 25). Six of the studies recruited women in WIC clinics and provided the intervention as a supplement to usual care. 171,172,175,176,181,182 Similarly, other studies recruited in centres where concurrent contact with pregnant women was already taking place. 103,106,112,167,170,173,179,180 One study was conducted with employees in a work setting169 and one was conducted at a school with adolescent school pupils who were pregnant. 107
Little information was given regarding the process of providing incentives to participants (see Table 25). Some differentiation was made between the staff delivering the incentive and the staff delivering the general BCT components of the intervention, although in most cases this was carried out by usual care health professionals: WIC staff,172,176,181 doctors and midwives,103,106,173 lactation consultants or nutritionists,107,169,179 hospital staff,167,180 trained interviewers or counsellors,112,170,171,178 peer supporters112 and research staff. 174,182 The details reported were often unclear but some noted when staff were specifically trained to deliver the intervention. 170,175
Intervention intensity and the participant journey
Intensity refers to the number of contacts involved for patients from the start of the intervention until the final outcome data are collected. Studies provided multicomponent interventions to encourage women to breastfeed, with interventions (or even individual components within the interventions) being provided at varying frequencies and time points, and this is summarised in Tables 25 and 26.
| Study | M6 | M7 | M8 | Delivery | M1 | M2 | M3 | M4 | M5 | M6 | (M12) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bliss 1997167 | G£ | T | T | T | |||||||
| Hayes 2008172 | I | £ | W | W | |||||||
| Dungy 1992170 | G £ |
T T |
T T |
||||||||
| Rasmussen 2011174 | W T |
£G | G TTTTTTT T |
T | |||||||
| Chamberlain 2006103 | £ | ||||||||||
| Cohen 1994169 | G(F) | (G or T) | £I | G | G | (G or T) | (G or T) | (G or T) | (G or T) | (G or T) | |
| Bai 2010180 | £ | T T |
T | ||||||||
| Sciacca 1995176 | G££££ | GGG(GG) £ |
S£ | G£ | G£ | ||||||
| Finch 2002171 | |||||||||||
| Chiasson 2011181 | |||||||||||
| Zimmerman 1999179 | (S) | G£(S) | (S) | S£(S) | (S) | (S) | (S) | (S) | (S) | (S) | |
| Reeves Tuttle 1995175 | GI£ | G£ | (T) | G£ | G£ | ||||||
| Volpe 2000107 | G£G£ G£ |
(ST) | (ST) | (ST) | (ST) | (ST) | (ST) | (ST) | |||
| Thomson 2012112 | S£S£ S£S£ |
S£S£S£S£ | |||||||||
| Wright 2012182 | G£? (ST) |
||||||||||
| Pugh 1998106 | G | GT£T G£ |
£T | (T) | (T) | (T) | G/T | ||||
| Wolfberg 2004178 | G£Fa | TT | T£ | ||||||||
| Hill 1987173 | GW | T£ |
The time points at which women were recruited to the studies, first received any intervention components or received the first incentive varied and were often unclear, in particular how the intervention integrated with usual care. For most studies, recruitment occurred in late pregnancy at some point during the third trimester. 107,169,172–176,178,179,182 In six studies, recruitment occurred around or just after delivery103,106,167,170,180 or early post partum. 112 In two studies it was unclear when recruitment occurred and our interpretation is that recruitment was post partum. 171,181
Incentives were given between the third trimester of pregnancy and 3 months post partum. The incentive, or first incentive if more than one was given, was not necessarily provided at the point of recruitment. In four studies, participants were provided with general BCT interventions prior to birth but did not receive an incentive until early post partum. 106,169,171,173
The overall number of formal contacts ranged from one103,182 to 12,112 with one study explicitly reporting that additional support was available in person (peer/professional counselling) or by telephone but that this was not scheduled. 169 Sometimes the availability of extra contact was implied but was not sufficiently reported to determine how often or how much a participant might encounter additional BCT components. Contacts at which the delivery of incentives took place were more clearly reported and these ranged from once103,169,170,173,182 to eight times,112 with one study176 reporting raffles of additional incentives. For the studies providing breast pumps103,167,169,170,172,174,180 the incentive was provided once but was taken home and could be used continuously. Although the point of recruitment was mostly unclear or flexible (i.e. not a named week in pregnancy), it seems that the longest period of contact could have been approximately 12 months. 172 It was not always possible to define when the intervention ended as support appeared to be available for those who sought it up to a later stage in many of the studies,106,107,169,179 for example through the availability of additional support if required. 169 When a breast pump was provided, data on reported frequency of use were not given. The mean duration of sessions for the delivery of the intervention was not reported clearly enough to draw any meaningful conclusions.
Intervention fidelity/non-compliance
There was minimal reporting of issues around intervention fidelity or compliance with the intervention, except in the study by Rasmussen and colleagues174 in which it was noted that most participants violated the protocol by pumping (control group) or using a different pump from that given (intervention group).
Outcome measurement
Feeding outcomes were self-reported and not validated as, unlike in smoking cessation, no biochemical test is available to confirm outcome status. In terms of incentive targets, all except three studies171,176,181 targeted preparatory behaviours (Figure 14). Targeted preparatory behaviours were pumping of breast milk (n = 7103,167,169,170,172,174,176), intervention attendance and participation (n = 8107,112,173,175,176,178,179,182) or others that were not specified (n = 3106,171,176). One study176 targeted preparatory behaviours as well as health behaviour and two studies171,181 targeted health behaviours only.
FIGURE 14.
Combinations of contingency targets for incentive/rewards.
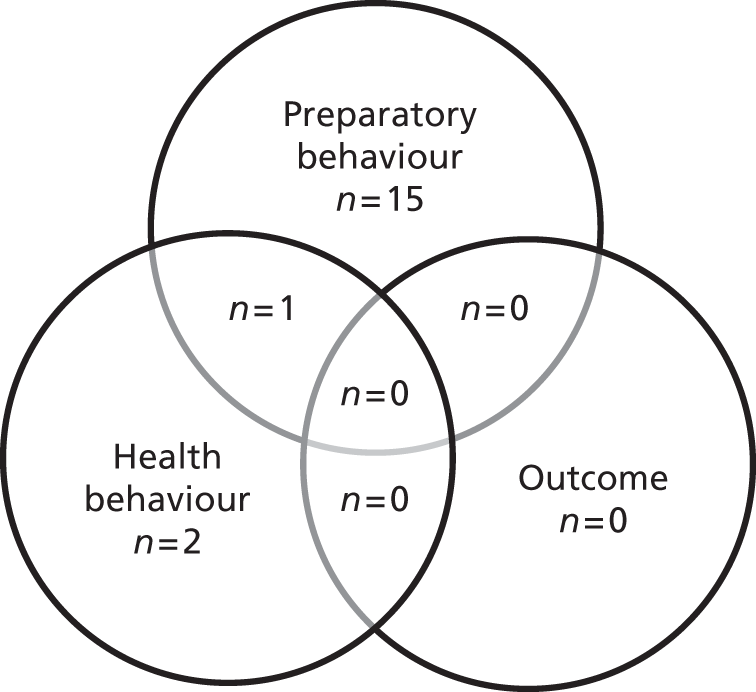
Studies varied in their selection and definition of feeding outcomes and there was poor consistency in outcome reporting, as noted by Hector. 186 Some studies differentiated between exclusive breastfeeding (no other liquid supplementation), any or partial breastfeeding (some breast milk and some supplementation) and formula only,112,170,171,174,176,179 but in others the extent of breastfeeding was not specified or it was unclear. 106,172,173,175,178,181 Bliss and colleagues167 categorised ‘full breastfeeding’ as daily breastfeeding with no regular formula feeding. In two studies exclusive and partial breastfeeding were defined but there was no differentiation between the two in the results. 169,182 Breastfeeding initiation was also defined differently among studies, ranging from any breastfeeding one or more times daily for at least 3 days post partum107 to still breastfeeding on day 4 following delivery174 and breastfeeding at discharge from hospital. 179 Two studies reported only exclusive breastfeeding rates but did not provide a definition. 103,180 None of the studies reported recall time (e.g. reporting breastfeeding within the last 24 hours) as recommended by Hector. 186 Frequency of breast pumping, volume of milk expression and feeding ratio (at breast or using expressed breast milk) were also not reported. One study did ask women in the intervention group to pump after five nursing sessions of their choice every day for 10 minutes on each breast until their milk came in or until their infants were 5 days old. 174
Studies varied in their data collection points and follow-up times, making comparisons between studies difficult. Rates of breastfeeding were assessed at initiation,103,106,107,171,178,181 1 week,174 2 weeks,170,176,179,180 4 weeks,170,174,178,180 6 weeks,167,170,173,176,178 8 weeks,112,171,178 12 weeks,174,176,180 4 months,167 6 months106,167,172 or 12 months172 or at hospital discharge. 175,176,179 Reeves Tuttle and Dewey175 assessed breastfeeding continuation rates at 3–6 months. In three studies breastfeeding initiation only was measured and no follow-up data were collected103,107,181 and in another study it was unclear when follow-up contacts were made. 169
All studies except that by Wright and colleagues182 utilised a comparator group to compare breastfeeding outcomes, although these varied and were not always control groups who received no treatment or a standard treatment. Six studies used a historical control group within the setting in which the subsequent intervention took place. These ranged from control data collected in the 6–12 months before the start of the intervention107,112,175,179,181 to data collected up to 4 years before the intervention in one pre-post BFI implementation study. 103 Cohen and colleagues169 compared 6-month breastfeeding rates over a 5-year work-based lactation programme with population-level breastfeeding data.
Actors: who the intervention targeted
The majority of studies appeared to opportunistically target all women without complications receiving maternity-related care from the provider settting. 103,106,112,167,170,173,179,180 One targeted the partners of women seeking prenatal care178 and another encouraged fathers to participate. 176 The remaining studies targeted specific groups, for example the study by Cohen and colleagues169 recruited employees of two companies who were returning to work for at least 16 hours per week following maternity leave and the study by Volpe and colleagues107 targeted pregnant adolescents. One study targeted Hmong women in California175 and another was conducted in an area with a predominantly non-white population, with predominantly black Caribbean and African American women participating. 179 Although one study did not specify targeting black women, only data relating to black women (both US born and non-US born) were presented. 103 One study targeted only obese women. 174
In terms of equity pointers, nine studies targeted low-income or disadvantaged women (or WIC programme participants)103,112,171,172,175,176,179,181,182 whereas other studies involved predominantly higher socioeconomic status populations170,180 or were concerned with factors such as employment,169 age107 or weight. 174 The remaining studies did not target any specific socioeconomic group and their samples appeared to contain those of reasonably diverse socioeconomic status. 106,167,173,178
Baseline and demographic characteristics
Demographic characteristics data are summarised in Table 27. Most studies provided some demographic data to describe their participants, but this varied in quantity and in its categorisation making comparisons across studies difficult. Two studies did not provide any demographic information other than stating that the interventions targeted WIC attendees. 181,182 Thomson and colleagues112 provided information about the age of the study participants only and Reeves Tuttle and Dewey175 provided demographic data on the intervention group only.
| Study | Age (years) | Ethnicity | Relationship status | Type of pregnancy/mode of birth | Type of feeding method at baseline | Education level | Socioeconomic status: income (monthly or yearly) | |||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White | Black | Other | Married and/or living with partner | Other | Primiparous | Multiparous | Vaginal | Caesarean section | Breast milk | Formula | Both | Completed high school | Less than high school | College or higher | ||||||||||||||||||||
| Mean | (SD) | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | Mean | % | |
| Bliss 1997167 | 1227 | 77 | 101 | 6 | 270 | 17 | ||||||||||||||||||||||||||||
| Hayes 2008172 | 106 | 100 | 95 | 39 | 149 | 61 | 126 | 51 | 116 | 47 | 96 | 39 | ||||||||||||||||||||||
| Dungy 1992170 | 29 | 135 | 91.8 | 2 | 1.4 | 9 | 6 | 139 | 94.6 | 8 | 5.4 | 140 | 95.9 | 6 | 4.1 | 46 | 31.3 | 5 | 3.4 | 96 | 65.8 | US$30,000.00/yeara | ||||||||||||
| Rasmussen 2011174 | 29.1 | 24 | 71 | 10 | 29 | 15 | 44.1 | 19 | 55.9 | 24 | 70.6 | 10 | 29.4 | 24 | 70.6 | |||||||||||||||||||
| Chamberlain 2006103 | 100 | |||||||||||||||||||||||||||||||||
| Cohen 1994169 | 31.4 | 94 | 50.3 | 31 | 16.6 | 62 | 33.2 | |||||||||||||||||||||||||||
| Bai 2010180 | 31.6 | 223 | 82.6 | 238 | 88 | 191 | 71 | |||||||||||||||||||||||||||
| Sciacca 1995176 | 36 | 65.5 | 68 | 100 | ||||||||||||||||||||||||||||||
| Finch 2002171 | 2 | 4.2 | 32 | 66.7 | 14 | 29.1 | ||||||||||||||||||||||||||||
| Chiasson 2011181 | ||||||||||||||||||||||||||||||||||
| Zimmerman 1999179 | 28 | 15 | 88 | 47 | 72 | 38 | 65 | 35 | 146 | 78 | 67 | 36 | 95 | 51 | 26 | 14 | ||||||||||||||||||
| Reeves Tuttle 1995175 | 25.9 (6.8) | 412 | 100 | |||||||||||||||||||||||||||||||
| Volpe 2000107 | 16.2 | 57 | 62.6 | 24 | 26.4 | 10 | 11.0 | |||||||||||||||||||||||||||
| Thomson 2012112 | 29.1 | |||||||||||||||||||||||||||||||||
| Wright 2012182 | ||||||||||||||||||||||||||||||||||
| Pugh 1998106 | 24.4 | 55 | 93 | 47 | 78 | 13 | 22 | 60 | 100 | 60 | 100 | 58 | 97 | |||||||||||||||||||||
| Wolfberg 2004178 | 50 | 84.7 | 35 | 59.3 | 16 | 27.1 | ||||||||||||||||||||||||||||
| Hill 1987173 | 61 | 95 | 44 | 69 | 27 | 42 | 37 | 58 | 0 | |||||||||||||||||||||||||
Only two studies provided data on baseline infant feeding method. In a historically controlled study, Zimmerman and colleagues179 compared the proportions of women exclusively and partially breastfeeding and formula feeding prior to the intervention with the proportions of women exclusively and partially breastfeeding and formula feeding in years 1 and 2 of the intervention period. An evaluation of the effect of discharge packs on continued breastfeeding rates by Dungy and colleagues170 excluded women who did not breastfeed and at baseline the study sample consisted of 140 women (96%) who exclusively breastfed and six women (4%) who mixed fed while in hospital.
When reported, the mean age of participants across studies varied between 16.2 years in a study that specifically focused on adolescents107 to 31.6 years. 180 Some studies did not provide an overall description of participants’ ages but rather presented findings according to specific age groups, for example < 21 years, 21–30 years, > 30 years. 167,171,176 Two studies gave the age of intervention participants only. 175,179
The ethnicity of participants was described in all but four studies. 112,174,181,182 Most studies that described ethnicity had a reasonably diverse sample of Caucasian, black and other ethnicities. Exceptions to this were the studies by Hayes and colleagues,172 who classified the ethnicity of their sample as Hawaiian and non-Hawaiian, Reeves Tuttle and Dewey,175 whose sample consisted of Hmong immigrants to the USA, and Chamberlain and colleagues,103 who provided demographic and outcome data for black women only. In some studies the majority of the sample were white Caucasian women (95%,173 93%,106 92%,170 83%,180 66%176 and 63%107) whereas in others the majority were black (85%178 and 67%171).
Relationship status was reported in seven studies, six of which reported that the majority of their participants were married or living with their partner (95%,170 88%,180 78%,106 71%,174 69%173 and 59%178). In the study by Hayes and colleagues172 it was reported that the majority (61%) of the sample were not married but it was not clear whether this included women living with their partner.
Few studies clarified whether women were primiparous or multiparous when entering the study. Two studies consisted of entirely primiparous women,106,176 but only Sciacca and colleagues176 stated that primiparity was an eligibility criterion as an attempt to control for the influence of previous feeding experiences on current infant feeding decisions. Four other studies included both primiparous and multiparous women. 172–174,179
Only three studies described their participants’ mode of birth, including women who had caesarean and vaginal births. 174,179 Pugh and Milligan106 described their participants as having vaginal births only but were not explicit whether this was a criterion for inclusion to the study.
Level of education was described in eight studies106,170,172–176,178 but this was often categorised differently, making comparisons across studies difficult. Socioeconomic status was also described differently across studies, being assessed either in relation to whether participants received Medicaid (a US health programme for families and individuals with low income and resources) or WIC services167,171,174–176,178 and/or by salary. 106,169,170,173,175
Some studies indicated that there were no significant differences in demographic characteristics between control and experimental groups without reporting further details106,112,173–175,178 whereas other studies did not compare control and intervention group demographics. 107,167,169,170 Hayes and colleagues,172 Bai and colleagues,180 Sciacca and colleagues176 and Finch and Daniel171 reported no significant differences between treatment groups at a significance level of p > 0.05. In a historically controlled study, Zimmerman and colleagues179 found a significant difference between year 2 data and baseline data with regard to ethnicity (p < 0.005).
Effectiveness
Breastfeeding rates
The effect of incentives on the proportion of women exclusively or partially breastfeeding was the main outcome of interest. Breastfeeding rates at various time points are provided in Tables 28–30 and they are summarised narratively because none of the studies was considered suitable to be included in a meta-analysis. There was too much variation among intervention components and, for example, if other components of the intervention (e.g. counselling) had been provided multiple times it was not suitable to combine these data with studies in which the same component had been provided once. The effect of providing the incentive could be confounded with the effect of providing more intensive counselling. Likewise, studies with no control group and studies in which the incentive had been provided to all participants were equally unsuitable for meta-analysis.
| Study | Category | Type of breastfeeding | Time point | Intervention | Control | RR (95% CI), p-value | ||
|---|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | |||||
| Rasmussen 2011174 | Breast pump – electric | Unclear | 4 days postpartum | 12/13 | 92.3 | 12/12 | 100.0 | 0.92 (0.79 to 1.08), 0.33 |
| Rasmussen 2011174 | Breast pump – manual | Unclear | 4 days postpartum | 7/9 | 77.8 | 12/12 | 100.0 | 0.78 (0.55 to 1.10), 0.086 |
| Chamberlain 2006,103 US-born black participants | Breast pumps and implementing BFI | Unclear | Initiation | NR | 74 | NR | 16 | |
| Chamberlain 2006,103 non-US-born black participants | Breast pumps and implementing BFI | Unclear | Initiation | NR | 96 | NR | 43 | |
| Sciacca 1995176 | Breast pumps, gifts, vouchers, football tickets and raffle gifts | Exclusive | Discharge | 23/26 | 88.5 | 16/29 | 55.2 | 1.60 (1.12 to 2.29), 0.007 |
| Sciacca 1995176 | Breast pumps, gifts, vouchers, football tickets and raffle gifts | Any | Discharge | 26/26 | 100.0 | 23/29 | 79.3 | 1.26 (1.05 to 1.52), 0.014 |
| Chiasson 2011181 | Food packages | Unclear | Initiation | NR | 75.5 | NR | 72.2 | |
| Zimmerman 1999,179 year 1 | Gifts and vouchers | Exclusive | Discharge | 74/144 | 51.4 | 67/188 | 35.6 | 1.44 (1.12 to 1.85), 0.004 |
| Zimmerman 1999,179 year 1 | Gifts and vouchers | Any | Discharge | 88/144 | 61.1 | 93/188 | 49.5 | 1.24 (1.02 to 1.50), 0.035 |
| Zimmerman 1999,179 year 2 | Gifts and vouchers | Exclusive | Discharge | 222/405 | 54.8 | 67/188 | 35.6 | 1.54 (1.24 to 1.90), < 0.001 |
| Zimmerman 1999,179 year 2 | Gifts and vouchers | Any | Discharge | 269/405 | 66.4 | 93/188 | 49.5 | 1.34 (1.14 to 1.58), < 0.001 |
| Reeves Tuttle 1995175 | Gifts and vouchers | Unclear | Initiation | 24/63 | 38.1 | NR | NR | |
| Volpe 2000107 | Gifts and vouchers | Unclear | Initiation | 28/43 | 65.1 | 7/48 | 14.6 | 4.47 (2.18 to 9.16), < 0.001 |
| Study | Category | Type of breastfeeding | Time point | Intervention | Control | RR (95% CI), p-value | ||
|---|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | |||||
| Dungy 1992170 | Breast pumps | Unclear | 2 weeks | 32/44 | 72.7 | 23/43 | 53.5 | 1.36 (0.98 to 1.90), 0.063 |
| Sciacca 1995176 | Breast pumps, gifts, vouchers, football tickets and raffle gifts | Exclusive | 2 weeks | 21/26 | 80.8 | 10/29 | 34.5 | 2.34 (1.37 to 4.00), < 0.001 |
| Sciacca 1995176 | Breast pumps, gifts, vouchers, football tickets and raffle gifts | Any | 2 weeks | 25/26 | 96.2 | 23/29 | 79.3 | 1.21 (0.99 to 1.48), 0.061 |
| Zimmerman 1999,179 year 1 | Gift items and vouchers | Exclusive | 2 weeks | 44/154 | 28.6 | 37/186 | 19.9 | 1.44 (0.98 to 2.10), 0.061 |
| Zimmerman 1999,179 year 1 | Gift items and vouchers | Any | 2 weeks | 79/154 | 51.3 | 65/186 | 34.9 | 1.47 (1.14 to 1.88), 0.002 |
| Zimmerman 1999,179 year 2 | Gift items and vouchers | Exclusive | 2 weeks | 115/389 | 29.6 | 37/186 | 19.9 | 1.49 (1.07 to 2.06), 0.014 |
| Zimmerman 1999,179 year 2 | Gift items and vouchers | Any | 2 weeks | 220/389 | 56.6 | 65/186 | 34.9 | 1.62 (1.31 to 2.01), < 0.001 |
| Study | Category | Type of breastfeeding | Time point | Intervention | Control | RR (95% CI), p-value | ||
|---|---|---|---|---|---|---|---|---|
| n/N | % | n/N | % | |||||
| Bliss 1997167 | Breast pumps | Exclusive | 6 weeks | 240/419 | 57.3 | 196/360 | 54.4 | 1.05 (0.93 to 1.19), 0.43 |
| Bliss 1997167 | Breast pumps | Partial | 6 weeks | 87/419 | 20.8 | 88/360 | 24.4 | 0.85 (0.65 to 1.10), 0.22 |
| Bliss 1997167 | Breast pumps | Exclusive | 4 months | 141/403 | 35.0 | 123/346 | 35.5 | 0.98 (0.81 to 1.20), 0.87 |
| Bliss 1997167 | Breast pumps | Partial | 4 months | 79/403 | 19.6 | 53/346 | 15.3 | 1.28 (0.93 to 1.76), 0.12 |
| Bliss 1997167 | Breast pumps | Exclusive | 6 months | 90/388 | 23.2 | 79/331 | 23.9 | 0.97 (0.75 to 1.27), 0.83 |
| Bliss 1997167 | Breast pumps | Partial | 6 months | 59/388 | 15.2 | 42/331 | 12.7 | 1.20 (0.83 to 1.73), 0.33 |
| Dungy 1992170 | Breast pumps | Unclear | 4 weeks | 24/44 | 54.5 | 15/43 | 34.9 | 1.56 (0.96 to 2.55), 0.065 |
| Dungy 1992170 | Breast pumps | Unclear | 6 weeks | 20/44 | 45.5 | 13/43 | 30.2 | 1.50 (0.86 to 2.63), 0.14 |
| Dungy 1992170 | Breast pumps | Unclear | 8 weeks | 16/44 | 36.4 | 10/43 | 23.3 | 1.56 (0.80 to 3.05), 0.18 |
| Rasmussen 2011174 | Breast pumps – electric | Exclusive | 30 days | 2/13 | 15 | 5/12 | 42 | 0.37 (0.09 to 1.56), 0.14 |
| Rasmussen 2011174 | Breast pumps – manual | Exclusive | 30 days | 3/9 | 33 | 5/12 | 42 | 0.80 (0.26 to 2.50), 0.70 |
| Rasmussen 2011174 | Breast pumps – electric | Partial | 30 days | 3/13 | 23 | 8/12 | 67 | 0.35 (0.12 to 1.01), 0.028 |
| Sciacca 1995176 | Breast pumps, gifts, vouchers, football tickets and raffle gifts | Exclusive | 6 weeks | 13/26 | 50.0 | 7/29 | 24.1 | 2.07 (0.98 to 4.39), 0.047 |
| Sciacca 1995176 | Breast pumps, gifts, vouchers, football tickets and raffle gifts | Any | 6 weeks | 21/26 | 80.8 | 9/29 | 31.0 | 2.60 (1.47 to 4.62), < 0.001 |
| Reeves Tuttle 1995175 | Gift items and vouchers | Unclear | 3–6 weeks | 11/63 | 17.5 | 19/349 | 5.4 | 3.21 (1.60 to 6.41), < 0.001 |
| Thomson 2012112 | Gift items and vouchers | Any | 6–8 weeks | 57/94 | 60.6 | 119/172 | 69.2 | 0.88 (0.72 to 1.06), 0.16 |
| Pugh 1998106 | Household tasks | Unclear | 6 months | 15/30 | 50.0 | 8/30 | 26.7 | 1.88 (0.94 to 3.75), 0.063 |
Effect sizes were calculated when data were available (see Tables 28–30). We found a significant effect of breast pumps when they were given with vouchers, gifts and a raffle for both parents on both exclusive breastfeeding at discharge, 2 weeks post partum and 6 weeks post partum and any breastfeeding at discharge and 6 weeks post partum. 176 A significant effect of gifts and vouchers was found on initiation of breastfeeding,107 breastfeeding at 3–6 weeks post partum175 and exclusive and any breastfeeding at discharge and 2 weeks post partum. 179 A small effect size was observed for the provision of electric breast pumps for partial breastfeeding at 30 days post partum. 174
Reporting of ITT and attrition data was poor, as detailed in Figure 11. In addition, some studies referred to between-group comparisons at a certain time point and other studies referred to within-group comparisons from baseline to different time points. We would have preferred to use ITT analysis because it provides a more conservative estimate of effect. When the total number in the sample for reported percentages was not obvious, care was taken to use the most conservative estimate of the sample size (e.g. number randomised, number at the start of the study) to prevent any unintentional overinflation of the effectiveness of interventions. However, this was not possible in all cases because of the quality of reporting.
Breast pumps
Seven US studies evaluated the effect of breast pump provision on breastfeeding outcomes. 103,167,169,170,172,174,180 The four RCTs167,170,172,174 appeared to show conflicting results for breastfeeding continuation and few patterns could be observed because of the heterogeneity of the included studies.
A large RCT by Bliss and colleagues167 of 1625 women provided the women with one of four free discharge packs that were identical in every respect except that one contained powdered infant formula, one a manual breast pump, one both formula and a pump and one neither. The contents of the discharge packs had a limited overall effect on feeding method and breastfeeding duration. Subgroup analyses suggested that there may have been a significant positive effect of breast pump provision (or a negative effect of giving mothers infant formula) on exclusive breastfeeding rates among women who intended to breastfeed for at least 6 months (mothers receiving formula were less likely to exclusively breastfeed at 6 weeks than women receiving breast pumps; p = 0.003) or in women who had not returned to work by 6 weeks post partum (mothers receiving formula were more likely to breastfeed partially at 6 weeks than women receiving breast pumps; p = 0.002).
A smaller RCT by Dungy and colleagues170 of 146 women, which also compared hospital discharge packs containing either a manual breast pump or infant formula, reported a higher prevalence of exclusive breastfeeding among women who received breast pump discharge packs than among those who received formula discharge packs up to 8 weeks post partum. Breast pump provision was associated with longer exclusive breastfeeding than infant formula (mean 4.18 and 2.78 weeks respectively; p < 0.05). It is not clear from either study, however, whether the observed effect was due to the provision of breast pumps (higher breastfeeding rates) or the inclusion of infant formula in discharge packs (lower breastfeeding rates).
A RCT by Hayes and colleagues172 comparing the provision of manual or electric breast pumps to 280 WIC women returning to work or school in Hawaii found no significant difference between the two groups in the proportion of women breastfeeding for at least 6 months. Both groups continued to breastfeed on average for at least 11 months, suggesting that manual pumps have a similar effect on breastfeeding rates as electric pumps. As the study did not include a control group (no breast pump), no conclusions can be made about the effectiveness of the availability of breast pumps on breastfeeding duration.
A small RCT by Rasmussen and colleagues174 of 34 obese women who received a manual pump up until 14 days post partum, an electric pump up until 14 days post partum or no pump found no significant differences in adjusted analyses among treatment groups in the proportion of women still breastfeeding up to 90 days post partum. During data collection it was discovered that most participants violated the protocol either by pumping (all control participants) or by using a different pump from the one assigned and this, together with the limited sample size, may have contributed to the lack of difference detected between these groups of obese women.
A non-randomised controlled intervention study by Bai and colleagues,180 which consecutively enrolled 270 mothers with newborn infants to a control group (received commercial discharge pack including infant formula) and an intervention group (received discharge pack with either breastfeeding information or information plus a manual breast pump) found that the duration of exclusive breastfeeding was significantly longer in women who received a pump (8.1 ± 5.0 weeks) than in women who received infant formula (6.0 ± 4.8 weeks) (p = 0.015) (see Table 23).
Two observational studies evaluated the effect of breast pump provision on breastfeeding outcomes. A case series study by Cohen and colleagues,169 which provided women returning to work at two corporations with breast pumps and facilities to express and store milk, reported that, since programme inception (a 4-year period), a larger proportion of women continued to breastfeed for at least 6 months than in national average data187 (74.3% vs. 10%) (see Table 23). The average duration of breastfeeding was 8.1 months. Chamberlain and colleagues103 provided free electric breast pumps at the time of implementing the BFI standards and compared breastfeeding rates before and after BFI accreditation. In the general hospital setting, breastfeeding rates increased overall by 288% (see Table 23).
Breast pump and vouchers, gifts and raffle for both parents
Sciacca and colleagues176 randomised 68 women to receive either an intervention consisting of a variety of incentives for women and their partners (see Table 24) to participate in a breastfeeding class for expectant couples and an educational series on childbirth, or usual breastfeeding education with infant shirt and free nappies if breastfeeding at 3 months (control group). Incentives included a breast pump, baby gifts (baby products, nappies, infant carrier, stuffed animals), vouchers (e.g. for clothing, breakfast/lunch), football tickets for the partners and a raffle of more expensive items (e.g. US$100.00 of groceries, trip to the Grand Canyon, tool kit). Intervention group mothers received nappies if they were still breastfeeding at 3 months post partum and if women were breastfeeding for at least 50% of the time at hospital discharge, 6 weeks and 3 months post partum they were also eligible for entry into a prize raffle. A significantly higher percentage of women in the intervention group reported exclusively breastfeeding at each measurement point (hospital discharge and 2 weeks, 6 weeks and 3 months post partum) (p < 0.05) (see Table 23).
Food packages
A RCT by Finch and Daniel171 was undertaken in 60 women who were randomised prenatally to receive either a breastfeeding education intervention or usual prenatal education. Enhanced WIC food packages worth US$50.00 per month were offered to mothers who exclusively breastfed for at least 2 months and they also received a US$25.00 shopping mall certificate. Although it was stated that access to breast pumps was also included as an incentive in this study, the details of this provision were unclear. No significant difference in the number of women initiating breastfeeding between the two groups was observed, and subgroup analyses were based on too few participants to be meaningful (i.e. five vs. nine participants) (see Table 23).
Chiasson and colleagues181 conducted a historically controlled study of WIC participants, which evaluated a change in WIC policy whereby women who reported breastfeeding received enhanced food packages. Comparing two 6-month periods, 75.5% of women initiated breastfeeding following the policy change compared with 72.2% before implementation of the policy change.
Gifts
Zimmerman and colleagues179 conducted a historically controlled study that compared breastfeeding outcomes pre and post intervention, which consisted of prenatal breastfeeding education, postnatal gift packs worth approximately US$8.00 and breastfeeding support groups. The rate of exclusive breastfeeding at discharge increased significantly from 36% (n = 67) at baseline to 51% (n = 74) in year 1 (p < 0.05) and 55% (n = 222) in year 2 (p < 0.05). At 2 weeks the proportion of women breastfeeding increased from 35% (n = 65) at baseline to 48% (n = 79) in year 1 (p < 0.05) and 57% (n = 220) in year 2 (p < 0.05).
Reeves Tuttle and Dewey175 conducted a historically controlled study that compared an intervention group of women (n = 63) who received pre- and postnatal breastfeeding support with a control group of women (n = 349) who had delivered infants within the previous 8 months. The intervention group received incentives at each phase of the intervention, which included childbirth and baby-care magazines, baby-care and post partum product samples and coupons, night lights and, for those who were still breastfeeding at 3–6 weeks post partum, a certificate of accomplishment with their infant’s picture. At 3–6 weeks, a significantly higher proportion of women in the intervention group (17.5%, 11/63) than in the control group (5.4%, 19/349) continued to breastfeed (p = 0.002).
The study by Volpe and Bear107 was a historically controlled study that compared pregnant teenagers who were still at school who were provided with an education programme and education-linked gifts (chocolate cigars, vials of perfume and electric socket covers) with the previous year’s cohort of pregnant teenagers who received no specific breastfeeding intervention. In the intervention group, 65.1% (28/43) initiated breastfeeding whereas in the control group 14.6% (7/48) initiated breastfeeding (p < 0.001).
A mixed-methods study by Thomson and colleagues112 investigated the impact of an incentive intervention delivered within an existing 8-week breastfeeding support programme. The incentive consisted of weekly home visits by peer supporters for 8 weeks to deliver eight different incentives (a picture frame, healthy treats, a swimming voucher, a pamper gift set, a glossy magazine, a pamper session, a family ready meal and a hot drink/cake voucher) with a monetary value of £71.99 per woman. For those who completed the full 8-week peer support programme, 40 women (75.5%) in the intervention group exclusively breastfed compared with 74 women (68.5%) who took part before the incentive intervention was introduced, which was not statistically significant (see Table 23).
Household tasks
Pugh and Milligan106 randomised 60 women to either an intervention (n = 30) that involved structured teaching and support, including home visits from a nurse and telephone calls from a lactation consultant, or usual care (n = 30). The home visits included help with domestic tasks of the woman’s choice. Women in the intervention group breastfed for longer than women in the control group (136.3 days and 88.3 days respectively) and, at 6 months, 50% of the women in the intervention group were still breastfeeding compared with 27% of women in the control group (n not given). However, because of the skewness of the data no statistically significant differences were detected. Fatigue scores were significantly higher in the control group than in the intervention group at 14 days post partum [9.5 ± 7.6 vs. 6.9 ± 5.7 (maximum score 30, indicating a high level of fatigue); p < 0.05].
Financial incentives primarily for engaging in the study
Wolfberg and colleagues178 randomised 59 fathers to either a 2-hour intervention class on infant care and breastfeeding promotion or a class on infant care only (control group). Both intervention and control group participants received US$25.00 for attendance. The pregnant women received US$25.00 if they completed a series of interviews about the intervention/control programmes. Women whose partners attended the breastfeeding class were more likely to initiate breastfeeding (74%, 20/27) than those whose partners attended the control class (41%, 13/32) (p = 0.02). There was no significant difference between groups for breastfeeding duration.
Hill173 randomised 64 women to either a prenatal breastfeeding education programme or a control group with no prenatal breastfeeding education programme. Each individual received US$5.00 remuneration for participation in the study. In the intervention group 61% (19/31) of women initiated breastfeeding whereas in the control group 45% (15/33) of women initiated breastfeeding. At 6 weeks 39% (12/31) of women in the intervention group breastfed for ≥ 6 weeks compared with 23% (7/31) in the control group. Although breastfeeding knowledge was significantly greater in women in the intervention group than in women in the control group (p < 0.001), the differences in breastfeeding outcomes were not statistically significant.
Attrition
Attrition rates were reported (or calculable) for eight of 18 studies included in the effectiveness review. 112,170,171,174,176,178–180 Details for different time points are reported in Table 31. In two studies171,176 the attrition rate was higher in the intervention arm than in the control arm (by 9.4%176 and 33.4%171). Reasons provided for dropouts in the study by Finch and Daniel were miscarriage or infant death, relocation and participants missing appointments. 171 Sciacca and colleagues176 did not provide reasons for attrition. In one study112 an attrition rate of around 60% was reported in both the intervention arm and the control arm, although reasons for dropping out were not provided. In the study by Rasmussen and colleagues174 there was no attrition among the 13 women receiving the electric breast pump but three of 12 women receiving a manual pump (25%) and two of 14 women in the control group (14.3%) did not complete the study. Reasons provided were because of a change of care, infant birth injury or going into foster care, and deciding not to breastfeed. Bai and colleagues180 reported attrition rates at 12 weeks post partum in the three groups studied: provision of a breast pump (35%), provision of breastfeeding information (45%) or no provision of a pump/information (40%); however, no reasons for dropping out were given. Two further studies reported attrition rates: Zimmerman and colleagues179 reported attrition rates of 0.5% at 2 weeks post partum, 0% in year 1 of the intervention and 3.7% in year 2 of the intervention; Wolfberg and colleagues178 reported attrition rates at 8 weeks of 3.7% and 3.1% in the intervention and control groups respectively. Neither study provided reasons for dropping out. None of the studies described above attempted to compare the demographic characteristics of those who completed the study and those who did not.
| Study | Time of follow-up | Intervention | Control | ||
|---|---|---|---|---|---|
| n/N | % | n/N | % | ||
| Bliss 1997167 | NR | ||||
| Hayes 2008172 | NR | ||||
| Dungy 1992170 | Attrition rate at 8 weeks post partum for both treatment groups combined was 60% (87/146) | ||||
| Rasmussen 2011174 | Before or immediately after delivery | 3/12a | 25.0 | 2/14 | 14.3 |
| 0/13b | 0.0 | ||||
| Chamberlain 2006103 | NA | ||||
| Cohen 1994169 | NR | ||||
| Bai 2010180 | 12 weeks post partum | 53/150 | 35 | 60/150 | 40 |
| Sciacca 1995176 | 2 weeks post partum | 8/32 | 25.0 | 5/32 | 15.6 |
| Finch 2002171 | 2 months post partum | 11/30 | 36.7 | 1/30 | 3.3 |
| Chiasson 2011181 | NA | ||||
| Zimmerman 1999179 | 2 weeks post partum | 0/144c | 0.0 | 1/188 | 0.5 |
| 15/405d | 3.7 | ||||
| Reeves Tuttle 1995175 | NR | ||||
| Volpe 2000107 | NR | ||||
| Thomson 2012112 | 8 weeks | 83/136 | 61.0 | 164/272 | 60.3 |
| Wright 2012182 | NR | ||||
| Pugh 1998106 | NR | ||||
| Wolfberg 2004178 | 8 weeks post partum | 1/27 | 3.7 | 1/32 | 3.1 |
| Hill 1987173 | NR | ||||
Dungy and colleagues170 reported a combined attrition rate for both treatment groups of 60% (87/146). No reasons were provided for dropping out. They calculated that the sociodemographic and breastfeeding attitude measures obtained during interviews conducted in hospital were not significantly different between the women from whom follow-up data were obtained and those who could not be contacted for postdischarge interviews.
Costs
No studies reporting the cost-effectiveness of incentive interventions were identified. Data related to the costs of implementing the incentive were reported by eight studies. 103,112,169–171,173,178,179 The ways in which costs were reported varied and so it is difficult to draw direct comparisons between them. However, in the eight studies the cost per participant can be approximated. The values range from a cost of approximately US$5.00 per participant173 to US$500.00 per employee,169 which did not include the overall outlay for the employers concerned to furnish on-site breastfeeding accommodation.
Between these two extremes of incentive costs was a range of other incentive values. In one study a cost of between US$175.00 and US$320.00 per woman was given for the provision of a breast pump. 103 Other studies that provided breast pumps as incentives for breastfeeding, either as gifts or on loan, did not report the costs associated with this provision. 167,172,174,180
In another study a cost of £71.99 per woman was cited to finance a series of eight gift items,112 whereas in a further two studies costs of US$25.00 for fathers and US$25.00 for mothers (total per couple US$50.00)178 and US$15.00 per discharge packet (US$11.75 of which was for a manual breast pump)170 were quoted.
In two studies cost data were reported but it was difficult to determine a precise cost per participant. For example, in one study, approximately US$1000.00 per annum was budgeted for the provision of refreshments at breastfeeding education sessions; therefore, the cost per participant cannot be easily determined. 179 In the other study, although the cost of the WIC food package provided as an incentive to breastfeed was reported as having a value of > US$50.00 per month and the additional cost of a one-off US$25.00 gift card was fixed, the cost per woman was difficult to estimate given that individuals were eligible for the incentives for different time periods. 171
In the remaining studies, a range of incentives was provided and these appeared to differ in value quite considerably, but data were not available to calculate or estimate total costs. Sciacca and colleagues176 provided a range of incentives, combining small gift items with a low value, for example baby wipes, haircuts and breakfast vouchers, with raffle gifts that had a much higher value, for example sports tickets, a holiday voucher and US$100.00 for groceries. This study raffled some of the incentives and so the total provision is difficult to ascertain.
Implementation and sustainability
There were no data relevant to the sustainability of the incentive interventions in terms of the long-term benefits outweighing the costs or to unintended effects once the research had finished. It is clear that provider changes would need to be implemented to improve the success of any incentives programme but it was concluded among several studies that programmes could be most effectively implemented and delivered through enhancing or intensifying usual care contacts and engaging staff through raising awareness or increased training. 175,179,182 In some studies, one-to-one specialist care169 and extended care post partum to help sustain breastfeeding106,112 were also considered necessary. However, two studies concluded that delivery could be effective even in brief or short-term incentive interventions. 103,175
Acceptability outcomes
One study reported information on the acceptability of the intervention. Thomson and colleagues112 reported that almost all of the women perceived that ongoing support from the programme had enabled them to breastfeed for longer. The weekly receipt of gifts reinforced and recognised their breastfeeding achievements in the context of breastfeeding being perceived as arduous and difficult.
In addition, Dungy and colleagues170 reported that provision of a breast pump helped to change attitudes around the ease of breastfeeding and Finch and Daniel171 reported that 89% (16/18) of women valued incentives. Chamberlain and colleagues103 reported that insurance companies favoured including breast pumps on their list of items that can be claimed because it allowed them to gain more clients as breast pump provision is popular.
Results of the review of incentives provided to organisations to improve smoking cessation in pregnancy and breastfeeding outcomes
This section reports the results of the evidence synthesis for incentives provided to organisations to improve smoking cessation in pregnancy and breastfeeding outcomes. Three studies have been included in this section of the review. Two provided incentives to organisations – one reimbursed an organisation for the provision of smoking cessation counselling152 and one provided a financial incentive for breastfeeding promotion168 – and one collected survey data, which included attitudes towards reimbursement of health-care providers in relation to providing a smoking cessation intervention. 153 The studies that gave incentives to care-providing organisations are summarised in Table 32.
| Study | Country | Study design as described | Total n participants | Incentive | Primary outcome |
|---|---|---|---|---|---|
| Smoking cessation | |||||
| Latts 2002152 | USA | Pilot project to implement a new smoking in pregnancy counselling system in a health maintenance organisation | 18 physician practices representing 27 office sites and 80 physicians. Training was provided to 66 staff members | US$150.00 for each pregnant smoker counselled | Reimbursement received for providing counselling to four of 21 smokers identified |
| Hartmann 2007153 | USA | Survey of prenatal care providers about smoking cessation interventions. One of 18 questions asked about the influence of reimbursement on providers’ willingness to provide a smoking cessation intervention | 844 health professionals consisting of obstetricians (50%), midwives (18%), family medicine physicians (15%), nurse practitioners (13%) and physician assistants (4%) | No incentive provided | Over half of providers reported that reimbursement was at least ‘somewhat’ influential in relation to their willingness to provide smoking cessation services, with no difference between provider types |
| Breastfeeding | |||||
| Cattaneo 2001168 | Italy | Breastfeeding promotion programme | Six local health authorities, 18 public hospitals | Financial penalty for not reaching a self-set breastfeeding prevalence target | Breastfeeding |
Smoking cessation in pregnancy
The smoking cessation review identified one study that had considered the effect of incentivising a care-providing organisation. 152 The intervention involved training 66 staff in smoking cessation counselling in obstetric practices delivering health maintenance organisation (HMO) care. The HMO was billed US$150.00 per woman, in increments of US$25.00 per 10 minutes, for providing counselling to women. Only 18 of the 33 obstetric practices agreed to participate. Before and following the intervention, reviews of medical charts of patients were undertaken to collect data on the identification of smoking status and the provision of advice to stop smoking. Smoking status was identified in 96% (175/182) of patients post intervention compared with 90% (198/220) of patients before the intervention. Smoking status identification was carried out at the first obstetric visit for 99% (196/198) at baseline and in 100% of cases (171/171) after the intervention. For the 12% (21/170) of women currently smoking in pregnancy at the time of the survey, documentation of advice to stop smoking actually worsened, from a baseline of eight out of 13 (62%) to four out of 17 (24%) after the intervention. The HMO received claims for reimbursement for only four out of 21 (19.1%) identified smokers who received counselling over the study period.
Patients were asked to recall components of the care provided to them in a follow-up survey, which had a response rate of 59% (505/849) at baseline and 33% (250/753) after the intervention. For the respondents who reported being current or former smokers, 96.8% (120/124) recalled being asked whether they were a smoker after the intervention compared with 90.2% (74/82) at baseline. However, only 64.5% (20/31) recalled being advised to quit by their obstetric provider after the intervention compared with 85.9% (61/71) before the intervention. After the intervention 3.2% (1/31) recalled receiving smoking cessation counselling compared with 11.3% (8/71) before the intervention. At both time points no participant could recall being referred to a smoking cessation programme.
Staff acceptability for eligible staff still in employment was reported for 56.9% (29/51) who responded to a post-intervention survey. Of the 29 respondents, 23 indicated that they had used their training to provide smoking cessation counselling, three reported that they did not see any smokers to counsel, five did not see any smokers from the participating HMO, one reported having no interest in counselling smokers and one felt unqualified to counsel smokers despite training. The authors identified several barriers to participation and engagement in the intervention, including the presence of office managers who themselves smoked. They suggest that only the more enthusiastic physicians participated, yet despite this the intervention resulted in a decrease in the provision of counselling.
Prenatal care provider organisation attitudes were captured in a survey about best practice in providing a smoking cessation intervention. 153 Within a mailed survey of 18 items, one question asked about ‘the influence of reimbursement on providers’ willingness to provide smoking cessation intervention’, with respondents rating their willingness on a Likert scale from ‘none’ to ‘very substantial’. Numerators and denominators were infrequently reported in this study. More than half of the 844 respondents, who comprised obstetricians (50%), midwives (18%), family medicine physicians (15%), nurse practitioners (13%) and physician assistants (4%) from an area in the USA, reported that reimbursement was ‘somewhat’ influential on their willingness to provide smoking cessation counselling, with no difference between provider types. Of those who thought that reimbursement would be influential, 40% were less likely to provide best practice. The most commonly reported barriers to providing smoking cessation services were time and a perceived lack of patient interest. Appropriate reimbursement was recommended for further investigation.
Breastfeeding
The breastfeeding review identified one study that considered the effect of incentivising a care-providing organisation. 168 In addition, there were 22 studies188–209 relating to the WHO/UNICEF BFI. 183 The BFI is an accreditation award that is achieved by meeting evidence-based and quality standards and can be considered a commitment contract. The BFI is a significant health-care intervention with multiple components that aim to increase breastfeeding rates. Only a small proportion of BFI studies were identified by our search strategy and we did not identify any studies specifically investigating the incentive component of BFI among the 22 studies identified and hence only a brief overview is provided below.
Beake and colleagues184 conducted a systematic review to assess whether a structured programme such as the WHO/UNICEF BFI implemented in maternity acute care settings is more likely to be associated with higher rates of initiation and duration of exclusive breastfeeding than no structured programme. The ‘structured programme’ included a multifaceted approach to support breastfeeding that targeted change at organisational, service delivery and individual behaviour levels. Studies that considered only community-based interventions were excluded. An extensive search of literature published in 1992–2010 was undertaken and methodological quality was assessed using checklists developed by the Joanna Briggs Institute. Twenty-six studies were included: one cluster RCT, two controlled trials, one cross-sectional study, two descriptive studies, 15 cohort studies and five systematic reviews.
Because of clinical and methodological heterogeneity of study designs it was not possible to combine studies or individual outcomes in meta-analyses. Most studies found a statistically significant improvement in breastfeeding initiation following introduction of a structured breastfeeding programme, although effect sizes varied. An impact on the duration of exclusive breastfeeding and duration of any breastfeeding to 6 months was also evident, although not all studies found statistically significant differences. Few studies controlled for any potential confounding factors, and the impact of bias has to be considered. It was concluded that structured programmes compared with standard care positively influence the initiation and duration of exclusive breastfeeding and any breastfeeding. In health-care settings with low breastfeeding initiation and duration rates, structured programmes may have a greater benefit. The generalisability of this evidence to the UK context is still debated as, to date, increased breastfeeding duration beyond the first week after birth is uncertain. 210 This is perhaps not surprising given that the effects of the more recently implemented BFI in the community, which aims to sustain breastfeeding after hospital discharge, have not been reported.
Cattaneo and colleagues168 conducted an observational study in Italy of a breastfeeding promotion programme that attributed financial incentives to local health authorities (LHAs) for complying with work plans and achieving targets set by the LHAs themselves. Financial penalties were levied against LHAs that did not comply with or did not achieve the targets. None of the hospitals in the LHAs had been designated ‘baby friendly’. The breastfeeding work plans and targets varied across the LHAs and included process or activity objectives, such as policy development, training of health professionals, education of mothers and improving co-ordination and integration of teams and activities, and outcomes measures, such as increasing breastfeeding rates. The penalty for not achieving their own work plans and targets or not participating in the collection of data was a fixed percentage (0.5 per thousand of the amount paid by the region to the LHA every year) (Adriano Cattaneo, Istituto per I’lnfanzia, Trieste, Italy, May 2013, personal communication).
Ten hospitals across six LHAs set up a breastfeeding reporting system in 1998 and defined breastfeeding promotion activities for 1999. High variability in breastfeeding outcomes at hospital discharge was recorded among LHAs. Overall, between 1998 and 1999, there was a statistically significant trend towards an increased exclusive breastfeeding rate at discharge (from 37% to 49%; p < 0.001), although two LHAs showed either no difference or a reduction in the exclusive breastfeeding rate. The overall rate of predominant breastfeeding at discharge decreased (from 46% to 39%; p < 0.001) but full breastfeeding rates did not change in four of the six LHAs. The rate of complementary breastfeeding at discharge also declined from 15% in 1998 to 10% in 1999 (p < 0.001).
The variability in breastfeeding outcomes among LHAs was much smaller at 16–19 weeks than at hospital discharge. Overall, there was a small but significant increase in exclusive breastfeeding rates between 1998 and 1999 (from 26% to 30%; p < 0.001) with an associated increase in the rate of full breastfeeding (from 38% to 41%; p < 0.001) and a decrease in the rate of complementary breastfeeding (from 25% to 23%; p < 0.05).
Published data are not available for subsequent years but as all six LHAs achieved their targets for every year that the programme was active (1998–2004) no penalties were applied (Adriano Cattaneo, personal communication). The data suggest an association of a financial incentive mechanism with improved breastfeeding rates. As the financial mechanism contributed to the establishment of a reporting system for breastfeeding and to the development of work plans and targets, the effect may result from the introduction of a surveillance system with regular feedback or the modified practices carried out in each LHA to achieve the objectives, many of which are known to be effective in improving breastfeeding outcomes. Although this intervention shows promise, there was a sharp fall in breastfeeding rates at hospital discharge compared with around 4 months (16–19 weeks), suggesting that changing hospital practices is not sufficient to prolong breastfeeding, even when initiation is high.
Discussion
Incentives for smoking cessation in pregnancy
In relation to smoking cessation, there is good evidence that pregnant women provided with contingent voucher incentives with biochemical validation of outcomes are more likely to stop smoking by the end of pregnancy than those who receive non-contingent incentives (for participation in a smoking cessation programme and providing outcome data) alone. This is shown by the four studies meta-analysed for end-of-pregnancy data in our results. 105,109,141,144 From the post-partum data available from three of the four studies meta-analysed the difference in abstinence rates between the groups is carried into the early months of the postpartum period (≤ 3 months) and is statistically significant. However, by 6 months post partum, data available from two studies indicate that the difference is no longer statistically significant.
The meta-analysis provides some information on the effect of varying the level and type of incentives. However, the evidence on incentives more generally is varied. At the end of pregnancy, cessation rates ranged from 13.4% to 71.0% for incentivised groups compared with 6.0–60.0% for control groups provided with smaller non-contingent incentives for participation. Intervention groups achieved abstinence rates of 10.2–42.0% for the early post-partum period (from 0 to 2 months) whereas control groups achieved abstinence rates of 0.8–38.0% for the same period. At 6 months post partum, intervention groups and control groups achieved cessation rates of 36.0–37.0% and 0–33.0% respectively. The findings of our meta-analysis are consistent with the findings of others. 54,211 A study by Sexton and Hebel212 was included in an earlier meta-analysis of incentives for smoking cessation in pregnancy54 but was excluded from our review. The abstract was not picked up through our search and the full-text paper reporting the trial outcomes does not mention incentives or rewards at all. It does highlight that intervention strategies were reviewed throughout the study, and new ideas and approaches were incorporated. The paper was similarly excluded from a recent NICE review of the incentives evidence. 41 Reviews by Higgins and colleagues211 and Sigmon and Patrick213 did not include any new eligible published studies.
Aside from meta-analysed data described above, reported rates of abstinence included maximum values for incentivised groups that exceeded the maximum values for control groups, but there is overlap in the ranges and it is very difficult to draw any firm conclusions about the effectiveness of incentives from these data, particularly as the other components of the interventions being provided also varied widely.
It was also difficult to consider the effectiveness of interventions when studies did not have a comparator (non-incentivised) group. Cessation rates for these studies ranged from 13.6% to 50% at the end of pregnancy, from 21.3% to 37.0% during the early post partum period, from 7.9% to 77.0% at 3 months post partum and from 7.0% to 64.0% at 6 months post partum.
An added complication in comparing cessation between studies is how smoking status was measured. Studies used widely varying primary outcomes, including point prevalence, prolonged and continuous abstinence, measured at a number of different points during pregnancy and post partum. This reflects the lack of consistency in the field as to how smoking cessation in pregnancy should be measured. Although for general population smoking studies the Russell standard163 is often used, providing some consistency, a similar standard is not currently available for studies involving pregnant women.
Biochemical verification of smoking cessation was common, although the methods for determining abstinence and the cut-offs used varied and were problematic. It could be argued that standards for biochemically validating smoking cessation may not be adequate when incentive payments to individuals are made. CO and cotinine levels are both likely to be lower among pregnant smokers than among non-pregnant smokers because of more rapid clearing of these metabolites of smoking. 121 Before the results of the studies described above are accepted as true, there needs to be further study of pregnant smokers’ ability to ‘game’ the system to receive incentive payments while still smoking. One way to look at CO biochemical verification would be to make it clear that incentives are paid on self-report corroborated by CO level, but to take a cotinine level at the same time to assess gaming. A second method would be to use blood samples that are taken routinely at an appropriate time in pregnancy for other reasons (i.e. at 36 weeks’ gestation). These routine blood samples could be used to assess the extent of gaming at the later pregnancy assessment for smoking cessation (corroborated by cotinine or CO level). It would be prudent to accept the findings with reservations until it is clear that gaming is minimal among pregnant smokers who are offered financial incentives for smoking cessation.
Some studies reported outcomes other than smoking cessation. Although some reported reductions in cigarette consumption,104,138,140,149 study design makes it difficult to determine whether or not the incentive component of the interventions prompted the reductions.
Incentives for breastfeeding
With regard to breastfeeding, there is currently insufficient evidence to formulate conclusions on the effectiveness of incentives to improve breastfeeding outcomes. The lack of good-quality RCTs prohibited meta-analysis and the heterogeneity of the studies limits comparisons. The studies included in this review were different in a number of aspects, such as the type and complexity of the intervention, the comparison groups, study design, sample size, outcome definitions, follow-up times and the population studied.
In total, 13 category B studies (see Table 21) compared an incentive of any type with no incentive or a much smaller incentive. Breastfeeding rates at initiation or at hospital discharge ranged from 38.1% to 88.5% in incentivised groups and from 14.6% to 72.2% in non-incentivised groups. 103,107,175,176,179,181 The average duration of exclusive breastfeeding ranged from 0.7 to 12 weeks for incentivised groups and from 2.78 to 12 weeks for non-incentivised groups. 170,171,174,180 The average duration of any breastfeeding ranged from 4.0 to 136.3 weeks for incentivised groups and from 15.7 to 88.3 weeks for non-incentivised groups. 106,167,171,174 Reported breastfeeding initiation and duration rates in intervention and control groups therefore showed considerable overlap, limiting the ability to formulate conclusions about the effectiveness of incentives from these data.
The most commonly used incentive involved providing access to a breast pump. Seven studies evaluated the effect of provision of only a breast pump on breastfeeding outcomes and one evaluated the effect of the provision of a breast pump plus other gifts and vouchers. Of the studies that investigated breast pumps only, four RCTs,167,170,172,180 ranging in size from 34 to 1625 women, provided little consensus. As statistically significant findings were identified only when women given breast pumps were compared with those given infant formula,167,170 it is not possible to ascertain whether it was the incentive of a breast pump to breastfeed or the incentive of infant formula to formula feed that produced the observed effect. When breast pumps were provided with a number of other incentives such as gift items, vouchers and raffle prizes in a small RCT of 55 women,176 the proportion of women who exclusively breastfed was significantly higher in the intervention group than in the control group (who received usual care and small incentives). The remaining studies evaluating breast pumps included a conference abstract180 and two observational studies103,169 and so the results should be treated with caution. In summary, studies investigating the effectiveness of breast pumps as incentives to breastfeed are limited by sample size, restrictions on breast pump availability, contamination of intervention and control groups, short follow-up times and lack of adequate control groups. A Cochrane review214 (currently withdrawn) investigated the influence of the provision of commercial discharge packs that include infant formula samples and/or infant formula promotional materials on the duration and exclusivity of breastfeeding. Commercial discharge packs reduced the number of women exclusively breastfeeding at all times from 0 to 6 months but had no significant effect on non-exclusive breastfeeding. There are also ethical issues concerning the provision of infant formula incentives, which would contravene the WHO International Code of Marketing of Breast Milk Substitutes. 80 Given the reported problems with intervention fidelity,174 there is a need for an adequately powered cluster RCT that compares breast pump provision with usual care to clarify the impact of breast pumps on breastfeeding rates.
Overall, it is difficult to draw any conclusions about the effectiveness of incentives from these data, particularly as the incentives differed and most interventions consisted of a number of BCT components. For example, most studies incorporated an education and/or a support element in their intervention, with the incentive provided either to encourage continuation in the programme or as a reward for continuing to breastfeed. 106,107,171,173,175,176,178,179
It was also difficult to consider the effectiveness of interventions when studies did not have a adequate comparator (non-incentivised) group. A number of studies either did not include a non-incentivised control group169,172,182 or used historical control subject, which may be subject to strong selection bias. 103,107,112,175,179,181
Incentive intervention delivery for smoking cessation and breastfeeding
The frequency and intensity of the general BCTs provided to women in the studies is a potential confounder. In lifestyle behaviour change interventions for diet and exercise, frequent contacts have been shown to be associated with effectiveness, together with self-regulatory BCTs such as goal-setting. 215 Greaves and colleagues215 also undertook causal analysis which showed that including social support in interventions increased effectiveness. However, in both the smoking cessation and breastfeeding incentive reviews it was often difficult to determine the frequency and duration of contacts and how these related to contacts taking place as part of usual care in either the intervention or the control arms. Therefore, the data that we report are based on our interpretations of the authors’ accounts. The nature of communication at the time of delivering the incentives is under-reported but could be crucial in terms of empowering, motivating or encouraging women; conversely, providers could also be seen as functional, brusque and disempowering. No studies mention observations or recordings of interactions to investigate their nature, and such process evaluation would be important in future trials.
Data on the acceptability of incentive interventions to participants or providers are an important aspect of the BIBS study; however, they were very limited and methods were not comparable between studies. The acceptability of interventions to research participants was high except in the one study that sampled a group of pregnant women not currently engaged with any incentive scheme, in which it was more mixed. 151 In terms of satisfaction among those receiving support, feedback was generally positive. However, it was not always the incentives themselves that could be directly linked to satisfaction levels. It is worth noting that the participants in the study by Cluss and colleagues140 reported that they were satisfied with the quality of service that they received and that the intervention had helped them but that a low proportion cited incentives as being the single most important component of the intervention. This may be an effect of participants comparing the incentive components with the other intervention components, or a bias whereby participants did not want to admit that they were most motivated by the incentives (depending on the data collection method used to elicit satisfaction).
Dropout or loss to follow-up in studies was an issue for both smoking cessation and breastfeeding studies. A wide range of attrition rates was reported and again the results are likely to be confounded by both the study population being investigated and the interventions being provided by the different studies. For smoking cessation studies that reported multiple attrition rates over time, these increased, although only six studies reported multiple end points for attrition data. Attrition rates in uncontrolled studies ranged from 20% to 64% within 2 months of baseline, from 4.9% to 50% at the end of pregnancy, from 7.0% to 37.5% in the early post-partum period and from 32.6% to 51.1% at 6 months post partum. For controlled studies, in the immediate post-baseline period attrition rates ranged from 9.0% to 50.0% for intervention participants and from 10.0% to 41.4% for control participants. These ranges were 10.0–32.0% and 0–51.5% at the end of pregnancy, 13.0–52.0% and 9.0–52.0% for the early postpartum period and 13.0–24.9% and 13.0–20.7% at 6 months post partum respectively. Eight of 18 breastfeeding studies reported information regarding attrition rates. Attrition rates ranged from 0% to 25% (intervention) and from 0.5% to 15.6% (control) in the period before delivery to 2 weeks post partum and from 3.7% to 61% (intervention) and from 3.1% to 60.3% (control) at 8 weeks post partum and were 35% (intervention) and 40% (control) at 12 weeks post partum. These findings are consistent with a recent Cochrane review which found that incentives have a small effect on attrition in research studies. 216
Costs varied in terms of what was reported (e.g. cost per participant or cost of implementing the entire intervention), what was provided (e.g. the value of incentives provided to participants), the currencies used (depending on the origin of each study reporting costs) and the price year (depending on when each study reporting costs was published), and it is therefore difficult to compare the results.
Incentives for providers of services for smoking cessation in pregnancy and breastfeeding
One intervention study of incentives for providers of services for smoking cessation in pregnancy was identified, which appeared to show some adverse effects on outcomes. 152 However, the methods used to establish effectiveness of the intervention in this study were not robust.
Only one study was identified that investigated breastfeeding incentives for providers. 168 Although associations between financial incentives and improved breastfeeding outcomes were made, the study is limited by its observational design and its findings should be treated with caution. Both the study by Cattaneo and colleagues168 and the BFI183 data suggest that commitment contracts show promise, but the role of incentives in these multicomponent interventions is uncertain. In 2012 the US Joint Commission introduced targets for exclusive breastfeeding at the time of hospital discharge as one of several mandatory requirements for maternity unit accreditation. 217
It is likely that pilot schemes and other interventions may not be widely published and therefore were not identified by our literature search, even though they may have been implemented in practice. Very limited acceptability data on incentives for providers were reported from one question in a survey153 and conclusions cannot be drawn.
Strengths and limitations
This review was comprehensive and the search strategy was particularly sensitive, covering a wide range of materials and types of study. However, it is possible that some multicomponent interventions that included incentive components may have been missed because of how they were reported. Nevertheless, no new studies were included in a systematic review of incentives for smoking cessation in pregnancy identified after our literature search had been completed, and the conclusions drawn are similar. 211 The inclusion criteria were broad enough to capture all possible kinds of incentives being provided and all types of study design. The breadth of the inclusion criteria and the multidisciplinary, mixed-methods evidence synthesis approach are important aspects of the BIBS study design. This approach assists in understanding the mechanisms of action for incentives for complex behaviours, informs the primary qualitative research (see Chapter 6) and informs the design and delivery of incentive intervention trials. This resulted in novel systematic review approaches, for example detailing the variation in incentive intervention journeys. The poor reporting of intervention details in studies included in the systematic reviews and the difficulties that this causes for replication and implementation has been highlighted. 218 BCT taxonomies can be helpful, as we have demonstrated; however, they capture only what is reported rather than what actually happened. Our approach develops the graphical methods that have been suggested for individual trial reporting by Perera and colleagues,219 which aim to capture content, delivery and chronology. Our approach demonstrates how this can be applied in a systematic review. It provides important detail about variation in content and intensity over time, which has the potential to confound an intervention and has implications for the costs of implementation and sustainability.
This study was hampered by the quality of data available and the multicomponent nature of the interventions, whereby the provision of incentives was not usually all that was being compared between groups. It was not always possible to identify the active component(s) of the intervention or whether synergy or opposition was occurring. In some cases, studies met our inclusion criteria by virtue of having provided token participation incentives to both groups and were therefore comparing another facet of the intervention than the incentive. As a result, both the intervention and ‘control’ groups in studies had to be treated as multiple intervention groups, as both received incentives. This also affected the analysis and quality assessment as studies that reported to be randomised had to be treated as containing non-randomised data, as this was the case for the data that we were interested in. Particularly important is the absence of a biochemical or other suitable method for validating breastfeeding outcomes. Self-report is relied on with the inherent and under-reported risks of gaming.
There is likely to be a great deal of confounding in studies reporting the effectiveness of incentives as, with all complex intervention studies, there was much variation in the other intervention components being provided. The under-reporting of intervention delivery processes, the absence of reported observations or recordings of interactions and the sparse qualitative data on patient experiences are potentially important. For example, relationships and communication with incentive intervention providers could be crucial in terms of empowering, motivating and encouraging women or, conversely, providers could be perceived as functional, brusque or disempowering. Most of the evidence is from US studies and the generalisability of the findings is unknown, particularly as many US women of childbearing age are not insured for health care and this is a barrier to health-care treatment. They may therefore behave differently from their UK counterparts.
Conclusions
The results indicate that providing high-value voucher incentives contingent on biochemically validated smoking cessation or providing voucher incentives to women and their social supporters, compared with providing non-contingent incentives for participation in a smoking cessation programme and providing outcome data, shows effectiveness observed up to 3 months post partum.
However, for both smoking cessation in pregnancy and breastfeeding the overall effect of providing incentives compared with no incentives is less clear, and the minimum size and the optimal frequency of incentives is uncertain. Furthermore, it is not clear to what extent other BCT components are synergistic or oppose the incentive effects or how incentive interventions interact with other aspects of usual care. Biochemical validation is important to detect deception.
For both smoking cessation and breastfeeding, further evidence on incentives is required to understand the possible effects and variation in effects of providing different types of incentives. We are aware of two UK incentive intervention studies on smoking cessation in pregnancy in progress, the CPIT88 and a single-arm intervention study. 220 In addition, a feasibility study for a financial incentive trial for breastfeeding is under way. 221 At present, most evidence is of varied quality and the incentivisation components of interventions are not sufficiently detailed to be certain of their effect compared with the effects of the other BCT components. In addition, the frequency and duration of contacts is a likely confounder. This is of crucial importance to multicentre trial design as usual care is variable and it is unrealistic to expect usual care to change substantially to accommodate an incentive intervention.
There is a paucity of qualitative research to understand the perspectives of those receiving and delivering incentive interventions. Further research is also required to establish the effectiveness of incentivising organisations to provide smoking cessation and breastfeeding interventions.
Pointers for the shortlist of promising incentive strategies
The incentive strategy with the most promising evidence of effectiveness is vouchers contingent on biochemically validated smoking cessation, provided either just to women or to women and their social supporters, compared with non-contingent incentives (which may be small payments for taking part and providing outcome data) extending from early pregnancy to late pregnancy.
The following incentive strategies show some promise but caution is required because of the quality of the data:
-
Vouchers contingent on biochemically validated smoking cessation, provided either just to women or to women and their social supporters, compared with non-contingent incentives (which may range from small payments for taking part to potentially larger payments for providing regular outcome data) extending from pregnancy until 3 months after birth.
-
Commitment contracts with either financial penalties for not meeting point prevalence breastfeeding targets or esteemed non-financial accreditation awards for investment in and meeting quality criteria.
-
Vouchers and gifts, which may include a breast pump, contingent on self-reported breastfeeding compared with no incentive or a much smaller incentive.
-
Incentives to reduce attrition in research studies.
There is insufficient evidence for the following incentive strategies:
-
Breast pump provision as an incentive to either increase the exclusivity of breast milk provision or prolong the duration of breastfeeding.
-
Multicomponent interventions in which one or several BCTs are combined with an incentive(s). It is unknown whether combinations of different components have synergistic or opposition effects for outcomes. This includes incentives for preparatory behaviours in addition to incentives for verified outcomes.
-
Incentives to providers of smoking cessation services.
The following are pointers for the acceptability of incentives and any unintended consequences:
-
Very few data are available and the data quality is variable.
-
Incentive interventions appear to be acceptable to those who receive them although differentiation between the incentive and other BCT components is difficult.
-
The acceptability of incentives to those who are not eligible to receive them is uncertain.
-
The acceptability of incentives delivered to providers of care to pregnant women is uncertain, particularly for smoking cessation. No data are available for breastfeeding.
-
Data on unintended consequences were generally not reported. Protocol violation was reported in one breast pump trial, which suggests that individual randomisation may be inappropriate.
The following are pointers for the development of an incentive taxonomy:
-
The IRBCT taxonomy was developed from existing BCT taxonomies and informed qualitative data collection (see Chapter 6).
-
A wide variety of incentives was delivered in the included studies and a typology began to emerge through discussion with service users. This typology is developed further in the primary qualitative research (see Chapter 6).
The wide variety of BCTs delivered alongside the incentives, and the variation in delivery mode, duration and intensity, need to be taken into account.
Chapter 4 Review of reviews of the barriers and facilitators experienced by women for smoking cessation in pregnancy and breastfeeding
In this chapter, reviews of qualitative research on women’s perspectives on smoking cessation in pregnancy and breastfeeding are thematically compared and contrasted to identify the barriers and facilitators for these behaviours. The review methods are described followed by a logic model for presenting the barrier and facilitator themes identified from the smoking cessation in pregnancy and the breastfeeding reviews. The chapter concludes by summarising the common themes for both behaviours and the behaviour-specific themes and considers how these inform the development of an incentive taxonomy for the design of incentive trials.
Methods
Aim
To understand how women’s experiences of the barriers to and facilitators of smoking cessation in pregnancy and breastfeeding fit with the evidence synthesis reviews on the effectiveness and delivery of incentive interventions.
Objectives
-
To identify systematic and narrative reviews of service-user perspectives on smoking cessation in pregnancy and breastfeeding.
-
To undertake a narrative evidence synthesis of qualitative reviews and thematically describe the barriers to, facilitators of and intrinsic and extrinsic motivators and demotivators of smoking cessation in pregnancy and breastfeeding.
-
To understand how the evidence on the barriers to and facilitators of smoking cessation in pregnancy and breastfeeding fits with the evidence synthesis for incentive interventions. In turn, this will inform the primary qualitative and survey data collection and contribute to both the shortlist of promising incentive strategies and the development of an incentive taxonomy.
Inclusion criteria
Types of studies
English-language reviews reporting qualitative evidence syntheses from developed countries only were included.
Population
The population of interest is pregnant or post-partum women up to 6 months after birth. The reviews may, in addition, include the perspectives of family members or professionals.
Subject/topic
Qualitative data describing women’s experiences of smoking in pregnancy or infant feeding and the barriers to and facilitators of sustained behaviour change were included.
Exclusion criteria
Surveys and predominantly quantitative reviews in which only one or two qualitative studies were included and studies reporting the perspectives of health professionals only were excluded. Reviews in which health professionals’ and women’s perspectives were given equivalent weight and the findings could be distinguished, for example the study by Baxter and colleagues222 on smoking cessation, were included. Similarly, in a review that included quantitative and qualitative studies relating to the perspectives of adolescents on breastfeeding, only the findings from the qualitative studies were included. 223
Search strategy
The literature was searched to identify reviews or syntheses of qualitative studies describing women’s perspectives on breastfeeding and on smoking in pregnancy. The databases searched were MEDLINE (1946 to August 2012), MEDLINE-In-Process & Other Non-Indexed Citations (7 August 2012), EMBASE (1974 to August 2012), CINAHL (1981 to August 2012) and PsycINFO (1806 to August 2012). Searches were limited to English-language publications but no date restrictions were imposed. Our existing files of studies relevant to breastfeeding, compiled for other research projects, were cross-checked as a supplement to the literature searches.
Data extraction strategy
Two qualitative researchers (HM and PH) screened the abstracts, independently screened the full-text papers and reached agreement on those to be included. All of the papers were read initially by HM and PH to identify text relating to the a priori broad themes of the barriers to and facilitators of smoking cessation in pregnancy and breastfeeding. The intention was to identify themes that could be applied across both behaviours to facilitate the constant comparative method of data analysis. 224 The identification of themes was undertaken within the broader context of understanding the mechanisms of action of how incentive interventions might help to change behaviour, as a platform to design future trials. Data were extracted by two researchers (HM and PH) and summarised into Microsoft Excel tables (Microsoft Office Professional Plus 2010, Microsoft Corporation, Redmond, WA, USA). With wider team involvement (SUD, GT, NC and FD), themes were compared and contrasted, first across the studies within each behaviour (smoking cessation in pregnancy and breastfeeding) and then across the reviews for both behaviours, to produce an integrated thematic anaysis. A logic model emerged (see Figure 16) through considering the fit between barrier and facilitator themes for smoking cessation and breastfeeding and more general theories of motivation and behaviour.
We drew in particular on the theories that are summarised in Chapter 1. 6,10,16 Interventions and BCTs are at the bottom of the model as they are extrinsic influences. This allows the model to incorporate how the evidence synthesis and the shortlist of incentive strategies described in Chapter 3 fit. To present the barrier and facilitator themes we apply a socioecological theory of behaviour,19 which considers the micro, meso and macro levels of context in which behaviours are situated.
Findings
Number of studies identified
In total, 386 studies were identified, 384 from the primary literature searches and two from additional sources. Of these, 36 were selected for full-text screening and 13 were subsequently included in the analysis. The screening process is detailed in Figure 15.
FIGURE 15.
Flow chart of the number of potentially relevant reports identified and the number subsequently included and excluded from the review.
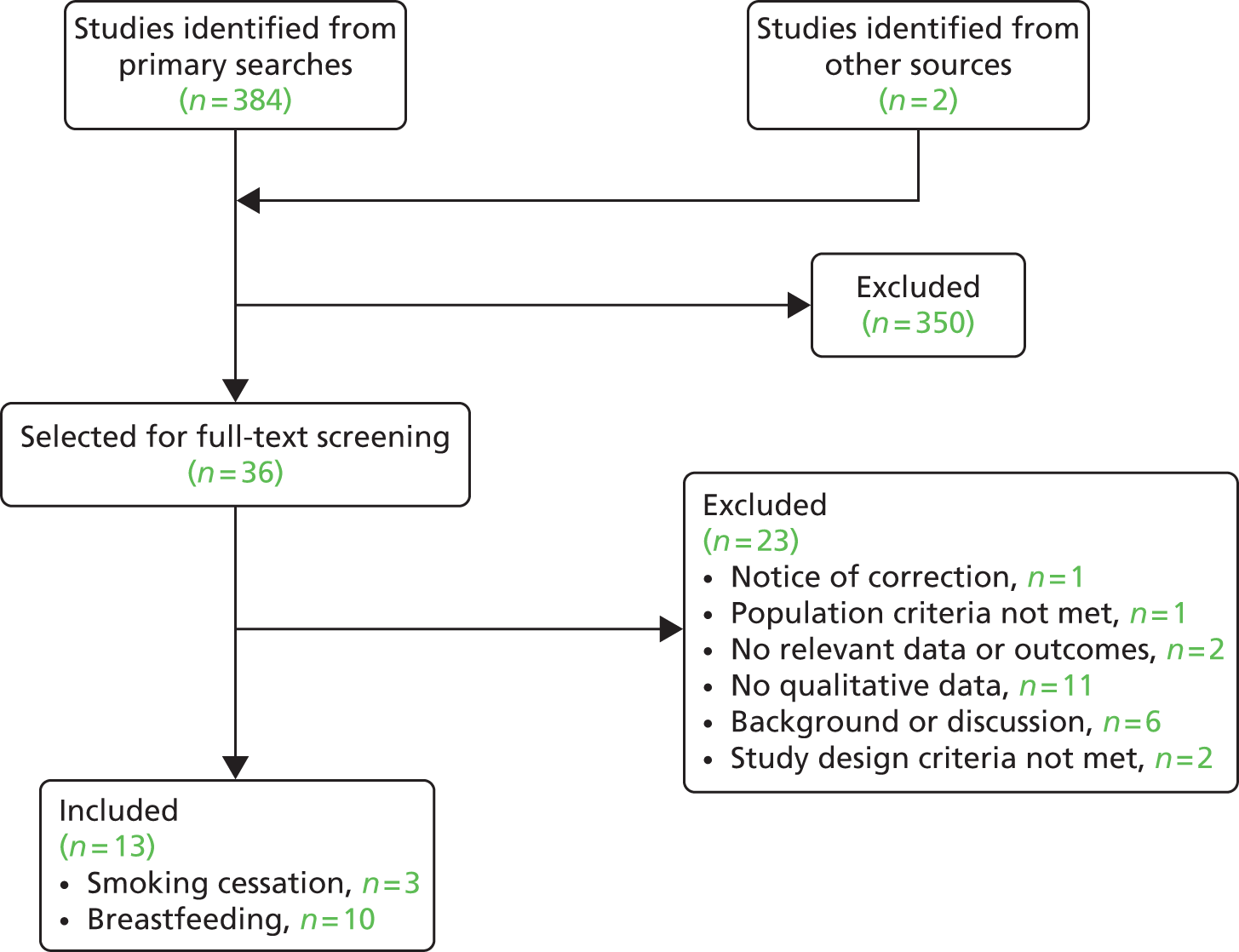
The logic model
The logic model that emerged from our analysis (Figure 16) reflects the complexity of dynamic social lives, relationships and situations.
FIGURE 16.
Barriers and facilitators logic model. M-DIR, motivation-drive: incentive and reinforcement.
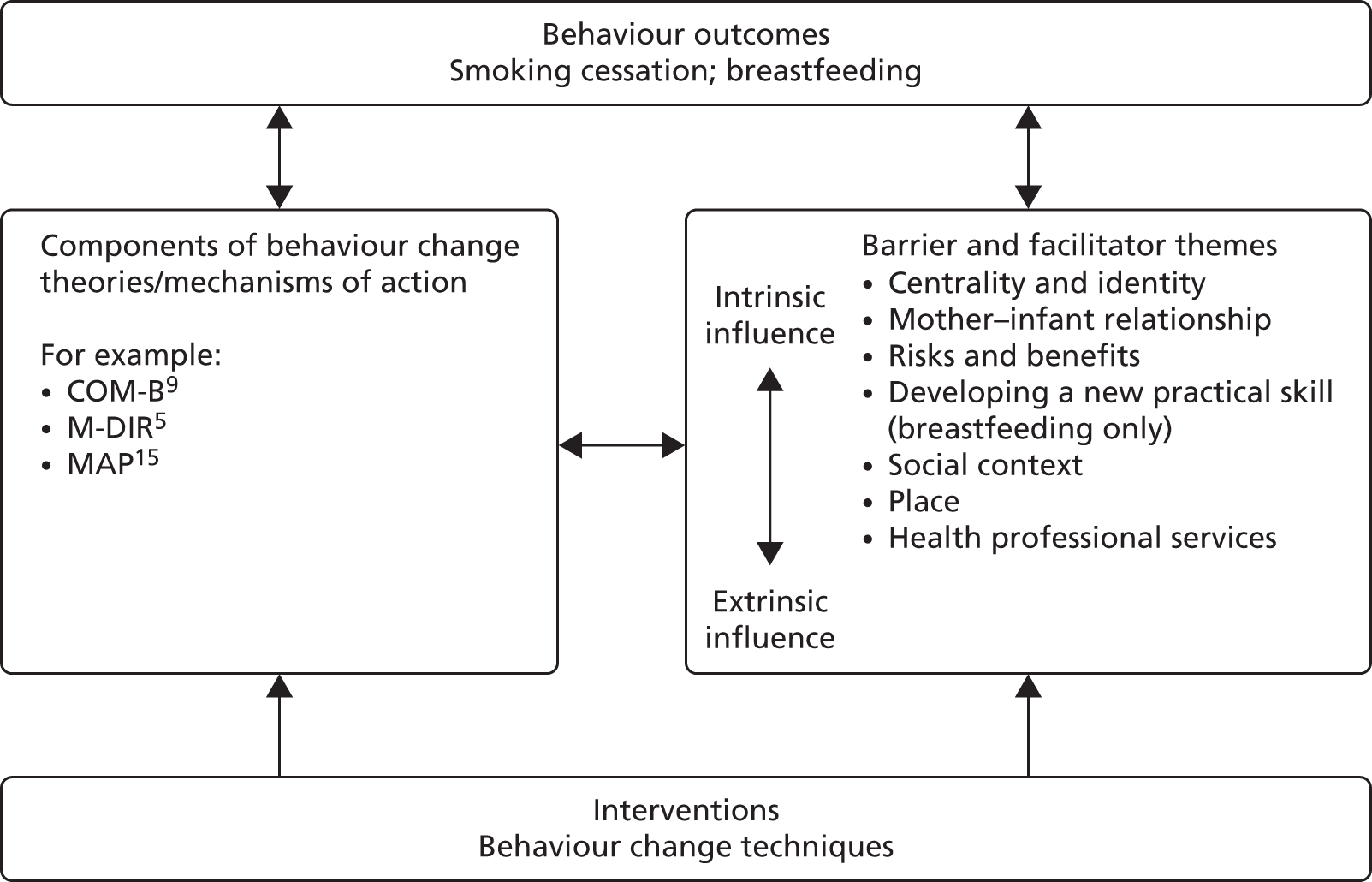
Rather than a neat dichotomy of categories for barriers or facilitators, the presented themes should be considered as a dynamic continuum of influences that are either intrinsic (wholly internal to the woman) or extrinsic (wholly external to the woman) or a combination at any one point in time. A woman has control over some intrinsic and some extrinsic influences and little or no control over others. Similarly, these intrinsic and extrinsic influences interact with the variety of motivation and other components of incentive and behaviour change theories that were highlighted in Chapter 1. Although an objective was to review the intrinsic and extrinsic motivators for behaviour change, this conceptualisation was found to be too narrow to apply to the data extracted from the reviews. The themes for the barriers and facilitators are ordered from predominantly intrinsic influences to those in which extrinsic influences are more dominant, with health professional involvement considered as the most extrinsic influence as health professionals are often the least integral to women’s social lives in which the behaviours are situated and performed. Increasing specialisation in maternal and infant care is evident in the data, particularly for smoking cessation services in pregnancy and formal peer support for breastfeeding. However, for smoking cessation and breastfeeding, it is optional for women to access health professionals for additional support and for health professionals to engage in changing or sustaining the target behaviours.
Findings for smoking cessation in pregnancy
Study characteristics
Three reviews,222,225,226 which included women’s accounts of the barriers to and facilitators of smoking behaviour change in pregnancy or engagement with smoking cessation services, were identified (Table 33). Graham and colleagues225 conducted a systematic review of qualitative studies to address the importance of everyday life to smoking status during pregnancy, a context that is often overlooked by research on public health issues but which is crucial for understanding how barriers and facilitators work and is therefore central to intervention design. Baxter and colleagues222 conducted a systematic review to investigate factors enabling or discouraging the uptake of smoking cessation services by pregnant smokers and they suggest 10 aspects of service delivery that may have an influence on the uptake of interventions. They identified that variation in practice can lead to conflicting advice being given to women and recommended that greater training and use of protocols is needed in this area. Ingall and Cropley226 reviewed qualitative studies that investigated the psychological and social factors around women attempting to quit smoking during pregnancy.
| Study | Country/countries | Papers included | Aim | Population | Type | Key results |
|---|---|---|---|---|---|---|
| Baxter 2010222 | UK, Sweden, France, USA, Australia, New Zealand, South Africa and one international study | 23 | To investigate the uptake of smoking cessation interventions (delivery of services to women who smoke) | Pregnant or recently pregnant | Systematic review and qualitative synthesis | 10 aspects of service delivery may have an influence on the uptake of interventions: if smoking broached by health professional, content of advice and information provided, manner of communication, having service protocols, follow-up discussion, staff confidence in skills, time and resource constraints, staff perceptions of ineffectiveness, differences between professionals, obstacles to accessing interventions |
| Graham 2012225 | High-income countries, UK | 27 (24 studies) | To enrich the evidence base informing policies and interventions to reduce smoking in pregnancy by including evidence from qualitative studies | Pregnant smokers | Systematic review of qualitative studies/meta-ethnography | Four dimensions with implications for design and delivery of interventions: role of partners, motivation to quit for pregnancy rather than for good, prominence of cutting down as method of quitting and alternative to quitting, different concepts of risk that underpin scientific evidence and professional advice on the one hand and guide everyday life on the other |
| Ingall 2010226 | UK, USA, Sweden, Canada, Australia | 7 | To examine and evaluate qualitative studies that have investigated the psychological and social factors around women attempting to quit smoking during pregnancy | Pregnant smokers and when changes made to smoking behaviour in pregnancy discussed post partum | Systematic review of qualitative studies | Women are aware of health risks to foetus; however, knowledge of potential health risks not sufficient to motivate them to quit. Several barriers identified including willpower, role, meaning of smoking, issues with cessation provision, changes in relationship interactions, understanding of facts, changes in smell and taste and the influence of family and friends |
The centrality of smoking to identity and the body
The dominant theme was the centrality of smoking in the everyday life of some pregnant women, her identity, her strategies to cope with stress and adversity and the experience of withdrawal symptoms relating to addiction. Graham and colleagues225 identified that one of the key barriers to pregnant women’s smoking cessation includes the centrality of smoking to their lives, a finding supported by Ingall and Cropley. 226 For many women who smoke during their pregnancy, smoking is already enmeshed in the everyday, in terms of their own identities as smokers, both before conception and as the pregnancy develops. The meaning that smoking has for them is fundamentally important. In what are often constraining circumstances of financial pressure, in which coping mechanisms are developed to balance stress, smoking appears to bring about psychosocial benefits that help women to alleviate stress, as well as boredom, and can bring pleasure, comfort and reward. Women believe that their reliance on smoking for these reasons is less problematic and less dangerous than the use of other substances. 225
Addiction is similarly fundamental to the centrality of smoking in women’s lives, with a sense of smoking controlling them and quitting being something beyond their control. 225 This relates to the physiological addiction to or dependence on nicotine. Smoking is therefore necessary to avoid withdrawal effects. Smoking is also a habit that produces perceived desirable effects and consequences. Dependence is based on physical continuity, coupled with a lack of willpower and self-doubt, as well as a lack of confidence around women’s ability to maintain abstinence. 225,226 These daily struggles occur in the context of everyday life circumstances that are often already difficult, as smoking in pregnancy is more prevalent among women who are more economically and socially disadvantaged. 53 Reconciling daily struggles with smoking cessation and other life adversities could determine whether or not a woman can successfully change her behaviour, even when she would like to. It may be that cutting down might be more possible and sustainable in some cases and that promotion of this approach might facilitate a positive behaviour change in the direction desired to improve health, and this is often condoned by health professionals. 225 Ingall and Cropley226 highlight, however, that this is a barrier to complete smoking cessation and it might lead to compensatory smoking whereby women inhale more deeply or smoke more of each cigarette.
The pregnancy
Associations of smoking with stress relief, pleasure, comfort and reward are deeply embedded within smokers’ everyday lives and, for women, this does not automatically alter as a result of becoming or being pregnant. These associations therefore constitute a significant barrier to behaviour change, even when compounded with feelings of shame and guilt about smoking continuation. 226 Graham and colleagues225 discuss how smoker identity is particularly problematic for pregnant women, especially as their pregnancies develop and are increasingly visible, with societal attitudes and overt or perceived social disapproval encouraging feelings of guilt. The pregnant smoker identity is not one that many women are comfortable with and so this consideration may serve as a facilitator to behaviour change during the course of pregnancy. Ingall and colleagues226 and Graham and colleagues225 found that this often consists of cutting down or multiple attempts to stop smoking, with relapse after varying periods of being abstinent. Most women will not have planned to give up smoking before the pregnancy. Moreover, the additional willpower that women consider necessary to quit completely may not be considered achievable. 226 As such, women are not ready to sacrifice the imperative smoker identity in favour of the pregnant woman one, especially when they see pregnancy as a short-term condition and the expectation of smoking cessation as temporary, with many returning to their previous levels of smoking post partum. 225,226 Furthermore, it seems that, for many women, life is even more complex in the context of their pregnancy and so, because smoking often serves as a crutch in times of stress, smoking may continue or even increase despite the pregnancy. 225
Risks and benefits
In addition to well-established issues of identity and everyday life that serve as barriers to smoking cessation in pregnancy, all three reviews222,225,226 found that many women also have deep-seated beliefs that the risks of smoking in pregnancy are exaggerated. Graham and colleagues225 found that women give more weight to the experiences of friends and family than to scientific evidence or health professional advice. Baxter and colleagues222 identified that women perceive a lack of ‘proof’ that smoking is harmful to the baby. Ingall and Cropley226 found that health professionals’ advice is followed less than personal experience or the experiences of family and friends, particularly when accounts of smoking during pregnancy appear to result in a healthy baby. This knowledge intensifies scepticism about public health messages around the dangers of smoking in pregnancy and promotes ideas about it not being dangerous enough to warrant giving up. Moreover, the stress of receiving such public health messages, which try to instil a notion of harm, can increase smoking, again because women turn to it as a source of stress relief. Even when there is an awareness or acknowledgement of the harmful effects of smoking, Ingall and Cropley226 distinguish between knowledge and action, and conclude that understanding risk does not necessarily lead to quitting.
Graham and colleagues225 found that for some women, however, an increased awareness of the risks of smoking for their unborn child, especially low birth weight but also miscarriage and damage to the baby, serves as a facilitator for smoking cessation, particularly when a woman’s primary motivation is the protection of her unborn child. They found that knowing about the risks can operate as a temporary quitting motivator and that receiving personalised feedback following maternity care procedures, such as ultrasound and heart beat measures, and imagining the baby in the womb can facilitate change. However, as Graham and colleagues225 note, even when the health risks extend into childhood and the teenage years, many women still believe that the risks are not hazardous enough to warrant quitting. Compounding this issue is an additional concern about the weight gain that might occur with stopping smoking, in addition to the weight gained from being pregnant. The desire to lose weight after birth is also a consideration around relapse or continued smoking. However, countering this, the physical condition of being pregnant may trigger change,225 in particular changes in maternal body shape as the pregnancy progresses, which make smoking when pregnant more difficult to conceal. 225 Changes in smell and taste226 or morning sickness225 causing aversion to smoking are also intrinsic motivators, but they are not straightforward because the influence of family and friends remains unaccounted for. Women do not necessarily reconcile beliefs and knowledge about the dangers of smoking during pregnancy with public health messages because many share and enjoy smoking with friends and family members225 and quitting can adversely affect their relationships with these people. 226
Social context
Graham and colleagues225 and Ingall and colleagues226 both found that a major barrier to smoking cessation for pregnant women is that they are often around others who smoke. What is more, smoking might function as an axis for a significant relationship, particularly with a partner, and their smoking status can play a central role. Graham and colleagues225 note that many partners who smoke do not quit during a woman’s pregnancy. The contrast between a relationship in which a partner smokes, which may be perceived as taunting or controlling to some degree, and a relationship in which ongoing encouragement and support are provided illustrates how a close, interpersonal relationship can serve as a barrier to or a facilitator of behaviour change. The latter might involve verbal encouragement, actually quitting together with a partner or ‘buddy’,225 a more peaceful home environment where a partner is a non-smoker226 or the presence of older children/other members of the close network who find smoking unacceptable. 225 The former might be more influential when a partner smokes at home and the woman perceives increased pressure to police the home225,226 or when women themselves feel that their smoking behaviour is being policed,225 both creating conflict, which can increase stress levels and thus perpetuate one of the triggers for continued smoking.
Place
Ingall and Cropley226 note that the immediate environment can be influential on women’s success in quitting smoking and that, when giving up or cutting down, a smoke-free home is important. Graham and colleagues225 concluded that pregnancy gives women the moral authority to insist on establishing smoke-free areas at home and to change the behaviour of others. However, as noted in the previous section, the ‘policing’ that is necessary if a partner smokes can become a drain on women. Therefore, women are often marginalised and exposed to stigma and pressure at home. 226 Women can perceive that there is no refuge, nowhere to escape from the daily stress of smoking. Women also experience the same pressures outside of the home when they are pregnant and smoking, as it is considered socially unacceptable and is often met with overt disapproval. As Graham and colleagues225 note, women might then be tempted to conceal their pregnancy or conceal their smoking to avoid stigma as they continue to smoke while pregnant. As the pregnancy becomes more visible women can be encouraged to quit smoking, to do the ‘right thing’ and to avoid places associated with smoking so that they avoid temptation. 225 Ingall and Cropley226 suggest that quitting can reduce conflict and therefore the desire for a peaceful environment acts as a motivating factor. However, smoking is a physiological addiction and these situations and environments as motivators conflict with the sense of the cigarettes being in control and the centrality of smoking to women’s lived experiences.
Health professional services
There are many obstacles to accessing services and women perceive issues such as long sessions, lack of time and mobility restrictions as barriers. 222 Therefore, women’s commitment to smoking cessation services is often compromised. This can lead to their disengagement with services and/or personnel. When the public health message recommends smoking cessation in pregnancy, barriers for pregnant women also include negative perceptions of care-provider counselling and unsatisfactory or inconsistent information.
Using smoking cessation advisors to deliver specialised services can increase the quality and consistency of the services. However, Baxter and colleagues222 note that women reported fearing that they would disappoint a smoking advisor and so do not attend appointments. In addition, they feared failure in general and feeling disappointed in themselves. Cutting down might be promoted by health professionals in certain instances225 and there is certainly a perception among women that some health professionals condone this in pregnancy because a lower level of smoking is assumed to be less hazardous. However, what Graham and colleagues225 found across studies was that this is just another example of conflicting advice given to women. Ingall and Cropley226 indicate that the relationship with a midwife in particular is crucial; however, Baxter and colleagues222 note the potential conflict perceived by midwives who do not wish to discuss smoking in pregnancy because it can increase a woman’s sense of guilt and compromise their relationship with women. Moreover, Baxter and colleagues222 found that women’s smoking status is not always recorded and, even when it is, it is not always questioned and therefore women are not actively engaged in discussions about issues pertaining to smoking or their readiness to change. However, some women reported such discussions as positive and important. 222 When women are confronted, they note that this quite often consists of being given information and advice as opposed to being persuaded to quit. They report feeling insulted and leaving consultations feeling resentful about the health professional’s manner of communication and as though they have been judged. 222 This is a barrier, especially because Baxter and colleagues222 also note that health professionals acknowledge that their approach is inadequate. Lack of protocol and follow-up, low staff confidence level in their skills and the impact of time and resource constraints make it difficult for health professionals to facilitate smoking cessation effectively. Furthermore, women seem to feel that there is a lack of enthusiasm or empathy from health-care professionals and that any support they do receive is short term. These considerations all serve as barriers to smoking cessation in what is already a complex context. What Graham and colleagues225 considered as factors that might minimise these barriers, and perhaps serve as facilitators to smoking cessation, were ongoing encouragement and personalised support solutions, which Ingall and Cropley226 call a ‘tailored approach’ and conclude is necessary within a smoking cessation programme. Ingall and Cropley226 suggest better multidisciplinary teamwork between doctors, midwives and health visitors. In addition, it is recommended that smoking cessation programmes include the provision of NRT in the form of patches, which women generally view favourably and regard as important.
Findings for breastfeeding
Study characteristics
The included reviews report women’s accounts of breastfeeding behaviour and patterns and consider breastfeeding support,67,223,227 women’s experiences of breastfeeding,228–230 formula milk feeding,231 breastfeeding among adolescent mothers,223 decision-making about feeding232,233 and current research in the subfields of anthropology and related disciplines234 (Table 34). Throughout the included evidence reviews, themes that emerged can be applied to understand the barriers to and facilitators of initiating and sustaining breastfeeding. Nelson230 notes that, in industrialised societies, breastfeeding is a ‘voluntary decision to act’ and not a necessity for infant survival and therefore the reality is that breastfeeding is a choice that is influenced by many factors. Intrinsic influences were less dominant in the breastfeeding literature than they were in the smoking literature. However, attachment to the infant as both a barrier and a facilitator was more apparent than in the smoking literature where, apart from ultrasound images and heartbeat recordings, the infant was a more theoretical and less tangible influence. For breastfeeding, the extrinsic relational concept of ‘support’ was dominant throughout the reviews, with some considering who provides the support and others focusing on the nature of the support. So, once again, clear divisions between support from social networks and support from health professionals are to some extent artificial.
| Study | Country/countries | Papers included | Aim | Population | Type | Key results |
|---|---|---|---|---|---|---|
| Atchan 2011232 | Unclear (although mainly Australian context) | 86 | To review the literature to explore the factors that influence women’s decisions about breastfeeding and their reasons for not initiating breastfeeding | Women, although unclear | Narrative review | Relatively few studies explore experiences of women who decide not to initiate breastfeeding |
| Burns 2010228 | Canada, UK, Australia, USA, New Zealand | 17 | To better understand the social phenomenon of breastfeeding by making the hidden obvious | Varied – women with experience of breastfeeding | Meta-ethnography | Two overarching themes emerged: breastfeeding was described in terms of expectation and reality, whereas the emotional aspects of breastfeeding were expressed in connected or disconnected terms |
| Hall Moran 2007223 | USA, UK, Australia | 7 | To review the evidence on the nature of support for breastfeeding adolescent mothers | Adolescent breastfeeding mothers | Systematic review – narrative and thematic | Five types of support identified: emotional, esteem, instrumental, informational and network. Emotional, esteem and network most helpful |
| Lakshman 2009231 | UK, USA, New Zealand, Australia | 6 qualitative and 17 quantitative studies | To understand how formula feeding decisions are made | Parents of either sex | Systematic review | Mothers who bottle fed experienced negative emotions. Mothers reported receiving little information on bottle-feeding and did not feel empowered to make decisions. Mistakes in preparation of bottle-feeds were common |
| Larsen 2008229 | UK, Ireland, Sweden, Australia | 7 | To examine what has affected a mother’s confidence in breastfeeding when she gives up breastfeeding | Mothers, midwives, fathers, child health nurses, people in network | Meta-synthesis | Confidence in breastfeeding is shaped by shattered expectations and is affected on an immediate level by mothers’ expectations, the network and the breastfeeding experts and on a discourse level by the discourses: breastfeeding as nature, the female body as a machine and the note of caution |
| McInnes 2008227 | UK, USA, Australia, New Zealand, Sweden, Canada | 47 | To synthesise mothers’ and health-care professionals’ experiences and perceptions of breastfeeding support | Women, fathers and health-care professionals | Narrative synthesis and secondary thematic analysis | Five themes emerged in health service support of breastfeeding: the mother–health professional relationship, skilled help, pressures of time, medicalisation of breastfeeding and the ward as a public place |
| Nelson 2006230 | USA, Australia, Canada, UK | 15 | To synthesise qualitative breastfeeding studies related to maternal breastfeeding experience | Women with breastfeeding experience | Meta-ethnographic, comparative | Breastfeeding is an engrossed journey that is very physical and requires maternal commitment, adaptation and support from multiple sources. Breastfeeding is also shown to have a significant personal impact on mothers and to require time for resolution on discontinuing |
| Nelson 2012233 | USA, UK, Australia | 14 | To synthesise the findings of qualitative research studies related to infant feeding decision-making | Pregnant or post-partum women, primiparas, adolescents, fathers | Meta-ethnographic, comparative | Two processes emerged as central: making a personal choice and defending the choice. Six themes are indicative of issues that affected these processes: knowing breast is best, disapproval of public breastfeeding, sense of personal comfort, level of confidence/commitment, need for support, perceived lifestyle compatibility |
| Schmied 201167 | UK, USA, Australia, Canada, New Zealand, Tanzania | 31 | To examine women’s perceptions and experiences of breastfeeding support, either professional or peer | Primiparas and multiparas who initiated breastfeeding | Meta-ethnography | Support for breastfeeding occurred along a continuum from authentic presence at one end, perceived as effective support, to disconnected encounters at the other, perceived as ineffective or even discouraging or counterproductive. Facilitative vs. reductionist approaches as contrasting styles |
| van Esterik 2002234 | Unclear | NA | To examine current research in the subfields of anthropology and related disciplines on the biocultural process of breastfeeding and broader questions of infant and young child feeding | NA | Narrative review | The narrow speciality of infant feeding has broad implications for the discipline. Themes: sexuality, reproduction, embodiment, subjective experience, problems breastfeeders face in bottle-feeding culture |
The centrality of feeding to identity and the body
There are intrinsic facilitators for breastfeeding, which are discussed in the context of the pleasure and intimacy that women can experience through breastfeeding,228,234 as well as feelings of connection and confidence. 228 Breastfeeding is tied in with the concept of being a good mother, which provides a sense of pride and self-worth. 228,233 Belief that breastfeeding influences bonding and attachment with their infants is closely linked with what Burns and colleagues228 put forward as another facilitator: faith in the body. However, women have also described barriers that are related to increased negative awareness of their bodies, such as pain and discomfort. Nelson’s 2006 meta-synthesis230 found that many women perceived breastfeeding as messy and felt disgusted or that their breasts were repulsive, whereas the same author’s 2012 meta-synthesis233 discusses a sense of personal comfort with the physical act of breastfeeding and perceptions of control. Both of Nelson’s reviews230,233 found that discomfort encouraged feeling out of control. In addition, van Esterik234 talks about a loss of self and agency. Breastfeeding is also portrayed as emotionally and physically demanding and time-consuming and a lack of confidence can increase anxiety, thus creating a barrier to continuing. In cultures that sexualise women’s breasts, difficulties around the body and image, sexual identity and relations can inhibit breastfeeding,223,232,234 especially if the body is seen as a ‘machine’. 229 Breastfeeding is the sole responsibility of the mother223,229 and this can act as a barrier, especially if women are less likely to try new things. 232 Overall body image and sensations were influential, as they were in the reviews of smoking when pregnant. The public–private interface, with concern around the judgements of others, was also a common theme.
The mother–infant relationship
The extent to which mothers consider their baby separate or attached, both physically and emotionally, while breastfeeding can cause conflicts. Nelson230 reported that some women feel as though their breasts now belong to their infant, which creates an emotional burden and results in cravings for separation. Conversely, some women interpret the physical bond as promoting closeness and a better understanding of their infant’s needs. The physical bond can affect women’s perceptions of autonomy and control, especially as the infant must also ‘know’ or learn what to do. 228 Mothers’ observations of happy and contented infants are key facilitators for breastfeeding continuation,228 with discontented babies cited as a justification for feeding behaviour change. Nevertheless, there are beliefs about not producing enough milk, or milk of sufficient quality, that lead women to formula feed because they feel that they are unable to satisfy their infants. 228 There are also issues around what constitutes effective mothering and many women believe that the pain and discomfort that they feel when breastfeeding inhibits their ability to be effective mothers. Women may therefore give up breastfeeding because of a perception of having problems feeding and then feel guilty.
Risks and benefits
The dominant Western sociocultural and health professional discourse, ‘breast is best’, is the primary reason why many women choose to breastfeed. 228,230 However, ‘breast is best’ can contribute to a sense of disillusionment, guilt and failure, which some breastfeeding women describe as a result of receiving what they consider to be technical (and often unrealistic) advice from health professionals. In particular, there is confusion caused by the idea that some health professionals view bottle-feeding as ‘safer’, an idea that many women feel has been communicated to them. Lakshman and colleagues231 note that this is a barrier to breastfeeding for women, especially when they can be more reassured that their baby is getting enough milk when they bottle-feed. When there is any breast pain or discomfort, or indeed the mother is anxious, this might impact negatively on the mother–infant relationship and, because of scientific advances, formula milk might be an acceptable alternative. ‘Breast is best’ may therefore be contested when formula is seen as not harmful233 or when breastfeeding can be perceived as ‘risky’. 234
Breastfeeding: developing a practical skill
Support for breastfeeding was the dominant theme – both as a barrier and a facilitator for breastfeeding, particularly the availability, characteristics, amount and quality of the support and the emotional impact on maternal well-being. Breastfeeding can be perceived as being natural and therefore easy or automatic,229 but, in contrast to smoking, it is a learned skill that requires practice and perseverance to develop. Nelson230 calls it an engrossing, personal journey, which takes account of the maternal self-sacrifice, time and life adaptations that breastfeeding requires. However, the dominant theme is that this personal journey requires support from others, with other intrinsic and extrinsic influences less evident in the reviews.
In the review by Hall Moran and colleagues,223 which looked at breastfeeding support in the context of adolescent mothers, a useful framework of support types was applied, derived from Sarafino. 235 Network support, emotional support, esteem support, informational support and instrumental support are each relevant to feeding choices from initiation through to sustaining breastfeeding. In addition to the everyday life context, and social network, in which women are situated, Hall Moran and colleagues223 found that having a nominated supporter, whether that person was new, that is, a health professional or peer counsellor, or familiar, that is, a partner or family member, was effective as a facilitator for breastfeeding. This was also found by Schmied and colleagues67 whose categories of support, such as ‘authentic presence’, ‘being there’, ‘empathy’ and ‘sharing the experience’, can refer to any supporter (professional or peer). Whatever the source, one-to-one care, and its continuity, can make women feel emotionally supported, which is a priority for women. Schmied and colleagues67 described the ideal supporter as someone who is responsive, who shares the experience and who provides affirmation. For Nelson,230 support is not reducible to individuals, however, but rather ‘it takes a village’ (p. e17). This includes partners, mothers, friends, groups, counsellors and professionals all providing support.
Emotional and esteem support are described by McInnes and Chambers227 as being about praise and building self-confidence. However, women have described feelings of being watched and judged as opposed to the more facilitative perception of support, regardless of whether or not they are successful. Therefore, support from others is important for women not only in underlying emotional terms but also because it can help to improve women’s feelings of well-being when breastfeeding. Schmied and colleagues67 describe the ideal as a facilitative approach, in which there is encouragement for breastfeeding and encouraging dialogue as opposed to ‘disconnected encounters’, which appear to involve undermining, blaming, pressuring and communicating a lack of time and which therefore serve as barriers.
Schmied and colleagues67 also suggest that the ideal facilitative approach should involve realistic, accurate and sufficiently detailed information. This is in contrast to the reductionist approach, which creates barriers through the delivery of conflicting information and advice, or standardised rather than individually tailored information. Hall Moran and colleagues223 suggest that the facilitative-type approach is particularly important for adolescent mothers who can be made to feel important through breastfeeding, as well as being better accepted as mothers, rather than problematised as ‘teenage mothers’. The encouragement that they are given acts as a facilitator compared with a lack of encouragement, which can lead to rapid disillusionment and early cessation.
Social context
The social context in which the woman is situated is crucial to her ability to deal with the barriers and facilitators that initiating and sustaining breastfeeding present. Having a mother who breastfed or who actively supports breastfeeding, as well as a supportive partner who is encouraging and shifts his perception of the breast from sexual to functional,232 have been identified as key facilitators in reviews. 223,227,228,230,232,233 Conflict can occur if these people encourage supplementing or weaning. 230 Conversely, if bottle-feeding with formula is a cultural norm in the immediate family then this will act as a barrier to breastfeeding. 223 Nelson230,233 also noted that ‘perceived lifestyle compatibility’ can act as a barrier when work or school commitments for the mother or other children may be construed as barriers. In contrast, membership in groups with shared interests or activities and peer support encourages breastfeeding when women can talk about their situation. 223 This can involve the generation of new friendships, especially when, as Burns and colleagues228 and Hall Moran and colleagues223 highlight, connections with other mothers experiencing the same emotions and challenges are forged. This can be formalised and facilitated through health or social care agencies connecting a woman with a dedicated peer supporter, which can be considered as more similar to professional support.
Place
The issue of public breastfeeding is cited as a key barrier within a number of reviews. 223,227,230,232–234 McInnes and Chambers227 suggest that hospital set-ups do not necessarily facilitate good relationships between mother and infant, when women might feel isolated and distressed. This is further complicated by the adverse effects of birth and inadequate pain relief, or when the mother and infant might be separated or accommodated within semi-public postnatal wards. Breastfeeding in public, whether in hospital, at home or outside the home, is potentially embarrassing and a tricky public performance, even in the presence of family or friends. 233,234 As Larsen and colleagues229 also reported, there is considerable pressure on many women to return to work after giving birth and therefore breastfeeding might be considered an unsuitable choice. Other family or social commitments contribute to lifestyle compatibility, besides the return to work,233,234 and to a wider need to manage feeding in everyday life and especially in unsupportive environments. 230,232,234 Negative attitudes towards breastfeeding affect women’s ability, willingness or commitment to breastfeed at home and elsewhere, and many women refer to breastfeeding as an ‘inconvenience’ or embarrassment. 228
Health professional services
Burns and colleagues228 suggest that opportunities for women to explore and articulate their experiences of breastfeeding are important and, more crucially, can influence the ways in which health professionals might represent breastfeeding during pregnancy and into the postnatal period. There were varied perceptions of postnatal support and individual experiences were wide-ranging, from encouragement through to discouragement. Overall, the reviews presented professional support as essential and the perceived insufficiencies recounted by women as major barriers to breastfeeding. Hall Moran and colleagues223 suggest that direct, tangible assistance, including practical help, is important, but it can be perceived as a barrier if it is given in a rushed or uncaring, or indeed fragmented, way. This is supported by Schmied and colleagues,67 who suggest that an authentic presence, characterised by being there, empathy, taking time and touching base, can facilitate breastfeeding. In addition, women can be reluctant to seek professional support that might facilitate breastfeeding. 223,233
McInnes and Chambers227 discuss how lack of knowledge can act as a barrier to receiving and accepting support for breastfeeding and likewise this is the case for women who feel that they have been denied information about bottle-feeding/formula. 232 McInnes and Chambers227 note that lack of resources can often mean that staff do not engage in the relevant training and this can result in inaccurate or inconsistent advice being given. Lack of emotional support is a strong theme, for example encouragement and reassurance,67,223,229,230 which also impacts on and compounds women’s self-esteem. Mixed messages, such as those surrounding the risks and benefits of breast or formula milk, or conflicting information on the practicalities of and instrumental support for breastfeeding, are described. 67,227–229 Women have described feeling pressured to breastfeed67,227,229 as well as to formula feed. 233 Health professionals are perceived by some mothers to be taking over, delivering standardised care, attributing problems to the mother’s or the infant’s personality and even projecting a lack of belief in the mother’s ability. 227 Lack of health professional time and fragmented support for women is emphasised across many of the reviews. 223,227,228,230,233 Instrumental support with positioning and attachment is required, but unhelpful and inappropriate practices such as insensitive and physically intrusive touching (doing not showing) cause distress or embarrassment. 227,229,233 This is especially important when, as Burns and colleagues228 state, there is confusion around exposing breasts and being a ‘good’ woman. McInnes and Chambers227 suggest that technical advice or ‘medicalisation’ can be a barrier for women and that advice should be based on real experiences and delivered in lay language, both of which could bridge the gap between theory and practice. In the review by Burns and colleagues,228 biomedical descriptions of female bodily experiences are hypothesised to suppress women’s own embodied descriptions of breastfeeding and have the potential to impact on the mother–infant relationship.
Discussion
For smoking cessation in pregnancy, intrinsic barriers were the dominant theme around the centrality of smoking to the everyday life of a pregnant woman, her identity, her strategies to cope with stress and adversity and the experience of withdrawal symptoms relating to addiction. In contrast, for breastfeeding, the extrinsic facilitator and barrier ‘support’ was the dominant theme, particularly the availability, characteristics, amount and quality of the support and the emotional impact on maternal well-being. Intrinsic influences for breastfeeding around identity as a ‘good mother’, confidence and attachment were described. For both behaviours, the well-being and health of the baby is an important intrinsic and, once born, extrinsic motivator, with accompanying feelings of guilt if smoking is continued or formula milk given. However, for both behaviours there is some disbelief about the associated adverse health outcomes, reinforced by witnessed personal, family or network experiences. The social context and the places where smoking and breastfeeding are situated were relevant to both behaviours, with partners, relatives, friends and network relationships acting as either barriers or facilitators, or demotivators or motivators, to both the initiation of the desired behaviour and sustaining the behaviour. Particularly for breastfeeding, professional support was reported as a facilitator when it was confidence boosting, skilled, accessible, consistent and timely; alternatively, it could be seen as a barrier if it was perceived as judgemental, pressurising, rushed or conflicting. Communication and support that are sensitive to each woman’s unique situation and the intrinsic and extrinsic influences that she is experiencing would appear to impact on both smoking and breastfeeding outcomes.
Strengths and limitations
As far as we are aware, this is the first qualitative synthesis to compare and contrast the barriers to and facilitators of smoking cessation in pregnancy and breastfeeding from the perspectives of women and it provides valuable insights into the data collected and reported by the respective academic researchers. Qualitative researchers and academics have considered the behaviours separately, with limited cross-reference or collaborations to share understanding. Indeed the socioeconomic patterning of the behaviours received little attention, particularly in the breastfeeding reviews. This could reflect the data collected from women in the original studies, the focus of the analysis or the second- and third-order interpretations by the review researchers. Health inequalities and social patterning of the two behaviours are more evident in surveys. Lifestyle behaviours that are undesirable from a health perspective, such as smoking and formula milk feeding, are known to be clustered in younger mothers from more disadvantaged backgrounds, particularly in the northern regions of the UK. 53 By not including observational studies in this review, some barrier and facilitator themes might be under-represented. For example, in the 2010 UK Infant Feeding Survey the most common reasons for stopping breastfeeding are the baby not sucking or rejecting the breast (33%), painful breasts or nipples (22%) and the mother feeling that she had insufficient milk (17%). 53 This review did not include important qualitative studies on the perspectives of partners on women smoking in pregnancy or post partum smoking as they were excluded from the included studies.
There are considerable limitations in applying an a priori framework of barriers and facilitators to papers whose aims were different. The amount of data relevant to our analysis that was omitted from the published accounts, perhaps because of strict word limits, is unknown. The review does not assess the quality of the reviews or the included papers, and this was inconsistently reported in the included studies. Groupthink102 is a consideration as the included papers in these reviews cite each other, with considerable overlap, and therefore can over- or underemphasise some themes. Additional reasons given by women for stopping breastfeeding after the first few weeks include the baby feeding too often/being constantly hungry (10%) or breastfeeding taking too long/being too tiring (8%). 53 This would suggest that intrinsic influences and the relationship with the infant may be more important than the dominant theme of support emerging from the reviews. Applying the constant comparative approach to the raw data in the transcripts in the original papers in each review would be a methodologically more rigorous approach, albeit very resource intensive and beyond the scope of this review.
Conclusions
This synthesis illustrates the similarities in and differences between the barriers to and facilitators of stopping smoking in pregnancy, and not relapsing, and initiating and sustaining breastfeeding. It provided an important evidence platform for informing the qualitative interview data collection and analysis and the development of a taxonomy of incentive strategies (see Chapter 6).
Key themes for barriers to and facilitators of smoking cessation and breastfeeding
In conclusion, the common dynamic themes for barriers to and facilitators of both smoking cessation and breastfeeding are:
-
centrality to the concept of self
-
the resistance to adopting a new identity and not trying a new behaviour, for example being a non-smoker for good or being a breastfeeding mother
-
time: maternal ‘me time’ and family/social time conflicting with the future optimal health of the baby
-
stress, anxiety, adversity and pressure
-
feelings of failure and guilt around being a ‘good mother’
-
beliefs and disbeliefs about health messages
-
body image and the private–public interface
-
social and cultural norms, particularly around lifestyles and the places where smoking and breastfeeding are situated, both within and outside the home
-
pleasure and control crossover, for example the pleasure that cigarettes and breastfeeding can both provide and the sense of control over situations that vary in their adversity
-
relationships with family and friends
-
experiences of information and support provided by health professionals, their timeliness and how they are tailored in relation to a woman’s needs.
Behaviour-specific themes for barriers to and facilitators of smoking cessation and breastfeeding are:
-
for smoking cessation: the addiction to nicotine and strategies that aim to address this such as NRT
-
for breastfeeding: learning and developing a new practical and performing skill and the additional skilled support that is required to do this.
The following are pointers for the development of an incentive taxonomy:
-
Applying dichotomies: barriers/facilitators and intrinsic/extrinsic motivators and demotivators for smoking cessation in pregnancy and breastfeeding do not capture the complexity and dynamic interactions evident in the reviews.
-
A logic model emerged that appears to fit the data. This incorporates influences on a continuum between intrinsic (wholly within self) and extrinsic (wholly outside of self) and which are in a dynamic relationship with the components identified in behaviour change theories, their mechanisms of action and the themes relating to the barriers to and facilitators of achieving the behaviour outcomes.
-
The level of perceived control that individuals have over intrinsic or extrinsic influences is an important consideration.
-
An incentive taxonomy will need to incorporate the common and behaviour-specific themes identified.
The following are pointers for the shortlist of promising incentive strategies:
-
Any incentive strategy will need to address the complexity of women’s real-life situations, particularly as those who continue to smoke during pregnancy and who choose to formula feed or to stop breastfeeding early are more likely to experience disadvantage in other aspects of their lives.
-
Relationships with health professionals during routinely provided care can be influential in both positive and negative directions. What happens during routine maternity care is likely to interact with any incentive intervention.
Chapter 5 How the evidence on incentives for other lifestyle behaviours contributes to the Benefits of Incentives for Breastfeeding and Smoking cessation in pregnancy study
This chapter presents a scoping review of evidence syntheses of incentives for other complex lifestyle behaviours, particularly for populations relevant to women of childbearing age. Following a description of the methods, we present the findings, first, for incentives offered to consumers and, second, for incentives offered to providers. The chapter concludes with a summary and the implications for the shortlist of incentive strategies and future research.
Methods
This scoping review of evidence syntheses of incentives for other complex lifestyle behaviours was undertaken once the smoking cessation and breastfeeding incentive evidence syntheses (see Chapter 3) were complete. The methodological rationale for doing this was threefold. First, incentive interventions that aim to change complex lifestyle behaviours from an increasingly popular research field and the evidence base is changing rapidly. Second, as incentive interventions are a relatively recent development, publications are dominated by a few groups of authors, mostly in North America, as illustrated by the meta-analysis for smoking cessation in pregnancy (see Figure 9). There is a risk of pursuing potentially less effective incentives and generating citation bias whereas new or potentially more effective strategies, which are less publicised, may be overlooked. Third, there is a danger that interventions leap ahead of behaviour change theory about how incentives work, and this may be evident from the wider literature. As our evidence syntheses for breastfeeding and smoking cessation in pregnancy identified few intervention studies delivering incentives for health-care providers or organisations, this was a particular area of relevance.
The definitions in Chapter 1 apply. ‘Consumers’ and ‘providers’ are used as umbrella terms, as not all people in receipt of incentives were patients. Similarly, providers included a wide range of people and professionals in differing health service systems who deliver services for people with the target behaviour.
Research question
How do evidence syntheses of incentive interventions from other areas of health improvement, particularly for women of childbearing age, (1) fit with our evidence syntheses for smoking cessation in pregnancy and breastfeeding, (2) inform the primary research questions relating to the development of a shortlist of incentive strategies and (3) help to identify research gaps where effective incentives for other behaviours have not been tested for smoking cessation in pregnancy and breastfeeding?
Objectives
-
To systematically search for and identify evidence syntheses of incentive interventions for other complex lifestyle behaviours that are relevant to women of childbearing age and health-care providers.
-
To assess the fit of the scoping review findings with our evidence syntheses of incentives for smoking cessation and breastfeeding and triangulate the findings.
-
To identify knowledge gaps where incentives for other behaviours show promise but (1) have not been tested for smoking cessation in pregnancy or breastfeeding and (2) have received little research interest to date for any complex lifestyle behaviour.
-
To provide any new information relevant to the development of a shortlist of incentive strategies for smoking cessation in pregnancy and breastfeeding and to inform primary qualitative and survey data collection in the BIBS study.
Identification of studies
Literature searching was carried out in two stages. First, to inform the grant application for the project, a scoping search was undertaken to identify studies of incentives for any lifestyle behaviour in any population. Second, a search was undertaken to inform the review of incentive reviews, which was specifically designed to identify systematic reviews of incentives for lifestyle behaviours, other than smoking and breastfeeding, in women of childbearing age. A decision was made to limit the number of databases searched to adequately address the research question but to allow the searches to remain feasible for the project team and resources. No language or date restrictions were imposed on the searches. Databases searched were MEDLINE (1946 to November Week 3 2012), MEDLINE-In-Process & Other Non-Indexed Citations (19 November 2012), EMBASE (1974 to 2012 Week 47), CINAHL (1981 to December 2012), CDSR (Issue 12, 2012) and The Cochrane Library Technology Assessments (Issue 4, 2012). Other websites consulted were the King’s Fund (see www.kingsfund.org.uk/), EPPI-Centre (see http://eppi.ioe.ac.uk/cms/) and the Health Foundation (see www.health.org.uk/).
Inclusion and exclusion criteria
The reviews included English-language studies reporting evidence syntheses that met the definition of an incentive (see Chapter 1) and included studies conducted in a developed country and reported between 2003 and October 2012. These dates were based on the findings from our scoping review for the grant application, in which no new primary studies or findings were reported in systematic reviews published before 2003.
The population of interest was adult women of childbearing age, with or without family members and health-care providers. For health-care providers, the evidence synthesis was included only if it reported on studies relevant to a UK NHS context.
Primary outcomes relating to the following complex lifestyle behaviours were included, in which the incentives are delivered either to the women or to health-care providers: weight management, drug and alcohol addiction, exercise and smoking (not in the context of pregnancy). Evidence syntheses reporting outcomes for only simple behaviour change, defined as a one-off behaviour (e.g. a clinic or screening appointment attendance, an immunisation), were excluded, although some of the reviews examined these alongside incentives for complex lifestyle behaviours. 236 Simple behaviour change interventions (e.g. turning up for a mammogram) are (usually) performed less frequently than healthy eating. They were excluded as it is widely recognised that breaking or forming a behaviour habit such as smoking or breastfeeding is unlikely to be achieved by a once-only intervention. 3
Data were extracted from the most recently published reviews first, before working chronologically backwards. For lifestyle behaviours under consideration, a date was reached when a review included only studies that were included in later or more comprehensive reviews. When this occurred, earlier studies addressing similar research questions were excluded. For reviews of incentives provided to health service organisations, no new studies or findings were identified in systematic reviews published before 2009 and this was the cut-off date for inclusion in this review.
Data extraction strategy
Abstracts identified by the search strategy were screened by two of three reviewers (PH, HM and FS) and agreement was reached on which to exclude at this stage. Full-text papers of potentially relevant reviews were obtained and assessed by two reviewers for inclusion (PH and either DT, GT, FD, LB, NC or SF). Differences of opinion were resolved through review team discussion. Data were extracted by one reviewer (PH) and cross-checked by a second (DT, GT, FD, LB, NC or SF). The following data were extracted:
-
authors
-
title of the review
-
the behaviour targeted for change
-
the population receiving the incentive intervention
-
summary of effectiveness findings reported by the authors that are of relevance to the BIBS study
-
summary of incentive delivery process findings reported by the authors that are of relevance to the BIBS study
-
any findings that refute the BIBS study review findings for smoking cessation in pregnancy or breastfeeding
-
any findings for incentive strategies that were not identified in the BIBS study evidence syntheses for smoking cessation in pregnancy or breastfeeding
-
any hypotheses arising from the review that inform primary data collection for the BIBS study.
An electronic form was used to record data. The quality of the reviews was not assessed as the aim of this scoping review was not formally to synthesise the evidence presented but to inform the subsequent stages of the BIBS study. A narrative thematic analysis of the data was undertaken, with themes discussed by the research team and assessed for their relevance and contribution to the BIBS study in an iterative process.
Results
Number of studies identified
We identified 255 records from the primary searches for this review. An additional three studies were identified from other sources. After title and abstract screening, 223 studies were considered not to be relevant and were excluded, leaving 35 studies for full-text assessment, of which 17 studies met our inclusion criteria. 2,3,236–250 A flow diagram of the screening process is provided in Figure 17.
FIGURE 17.
Flow chart of the number of potentially relevant reports identified and the number subsequently included and excluded from the review.

Included studies
Of the 17 evidence syntheses included, 10 provided incentives to consumers and were relevant to women of childbearing age,3,237–245 one focused on young people aged 11–19 years,236 one provided incentives to both consumers and health service providers2 and five delivered incentives to health service providers relevant to the UK NHS and included some studies in which the incentive targeted a complex lifestyle behaviour. 246–250
Excluded studies
The reasons for exclusion of assessed full-text reviews are given in Table 35.
Characteristics of included studies
All included reviews reported outcomes for some complex lifestyle behaviours, with eight reviews including studies reporting outcomes for adult smoking cessation, either alone237,238 or combined with other lifestyle behaviours. 3,236,239 Six reviews included studies reporting outcomes for weight management or diet alone241,244 or in addition to other lifestyle behaviours. 2,3,236,239,240,242 Six reviews included addiction to drugs, including cocaine, alcohol or opiates alone243 or in combination with tobacco240,242 or other lifestyle behaviours. 3,236,245 One review included incentives for physical activity in addition to other lifestyle behaviours. 239 The study by Kavanagh and colleagues239 was a scoping review of study titles and abstracts only; all other studies reviewed full-text publications and reports of intervention studies. In the following sections we present key points relevant to the aims and objectives of the BIBS study; Table 36 summarises the data on consumer incentives.
| Study and title | Behaviour | Population | Summary of effectiveness findings | Summary of incentive delivery process findings | Findings that were not identified in the BIBS smoking in pregnancy and/or breastfeeding reviews |
|---|---|---|---|---|---|
| Cahill 2008237 Quit and win contests for smoking cessation |
Smoking cessation | Adult smokers within the targeted community | Quit rates were significantly higher (8–20%) for quit and win competitions than in the control groups at the 12-month assessment. None of the included studies was a true RCT as the control populations were different and more generic than the intervention populations. The population impact was small, ranging from one in 1400 to one in 160 smokers quitting. Two factors were consistent predictors of success: the assistance of supportive others (partner, cohabiting non-smokers, family and friends, workmates) and abrupt cold turkey withdrawal rather than reducing smoking. The average cessation rates stayed relatively stable and did not appear to be diluted by increased participation; therefore, enhancing participation is important to increase absolute numbers of quitters. Reported quit rates are higher in developed countries. Cost-effectiveness could not be determined. No dose–response effect was observed | Predominantly female, younger, better-educated, heavier smokers with previous quit attempts who are in the contemplation or preparation stage for change register with quit and win contests. In one of the five included studies, women and low-income groups were as likely to quit as men and high-income groups and show particular promise. Quit and win contests might be attractive to women. Deception rates, when they could be measured, were high and may compromise the validity of the intervention. Recruitment was through self-selection via mass media advertising campaigns, which included television, schools and workplaces. Raffle prizes included a holiday to Disneyworld. Volunteers delivered the intervention and prizes were donated. Biochemical outcome validation was usually for prize winners only and nearly always this included CO testing only, which can be falsified by not smoking for a few hours | Settings were relatively large geographical areas with no targeting of specific smoking populations. Population impact calculation = (quit rate × % of smokers who participated in the intervention) facilitates comparison between intervention settings and calculation of number needed to participate for long-term abstinence. Relationship between community involvement in competitions, financial motivation and the value of the prize |
| Cahill 2011238 Competitions and incentives for smoking cessation |
Smoking cessation | Adult smokers of either sex in any setting. Excludes pregnant smokers | Of 19 heterogeneous studies, only one demonstrated a significantly higher quit rate for the incentives group beyond 6 months. No evidence that participants who committed their own money did better than those who did not (four studies) or that contingent rewards enhanced success rates over fixed payment schedules (four studies). Recruitment rates can be improved by rewarding participation but this does not lead to higher quit rates | Incentives of < US$750.00 together with referral to independently funded local smoking cessation services were components of interventions reporting prolonged abstinence. Early success dissipates when the rewards disappear. Most studies were set in the workplace and were based in the USA. Most studies were multicomponent. Little evidence that levels of deception differed between intervention and control groups and rates were low. People responding indirectly (not face to face) to a question about their smoking and not expecting to have their answer checked biochemically showed a higher level of discrepancy | Cost-effectiveness could not be assessed as the efficacy of most interventions was not demonstrated. Two studies provided lottery tickets for a prize draw for smoking buddies. Generalisability of the findings to populations with diverse regional, socioeconomic and ethnic mixes. Random biochemical testing and cross-checking with family and friends. Merits of cash vs. payments in kind (e.g. grocery vouchers) are unknown |
| Jochelson 20073 Paying the patient. Improving health using financial incentives |
Any | Patients | Financial incentives are effective for simple, time-limited behaviours but not for complex behaviours. They encourage participation and reduce attrition in lifestyle programmes, but healthier behaviour is not maintained once the reward is withdrawn. They may be useful as one part of a multifaceted programme. There is limited evidence on behaviour deposit contracts with payments made (or forfeited) from the deposit if goals met (not met) | Higher-value incentives increased participation. Better outcomes were reported for incentives delivered immediately (vs. delayed) after testing a specimen and interventions that provided feedback and increased self-efficacy. Consistent and periodic rewards may support behaviour change compared with one-off rewards. A controlling or humiliating communication style can undermine behaviour change compared with praise and encouragement | Penalising poor performance may reinforce the sense of personal failure and attrition rates may be higher. Group weight loss contracts may be more effective than individual, with group participants less likely to regain weight |
| Kane 20042 Economic incentives for preventive care |
Health promotion and preventative care pre-diagnosis of a disease | Providers and consumers | Little evidence available to support explicit provider financial incentives. Consumer economic incentives are effective for simple preventative care and distinct short-term behavioural goals that are well defined. There were no long-term benefits. There is minimal evidence for a dose–response effect for provider incentives. For consumer incentives the threshold dose appears low. Facilitative incentives to increase engagement in a new behaviour and programme participation are effective. Studies using disincentives showed significant effects. Coupons were preferred to gifts. The cost-effectiveness and effectiveness of provider incentives for complex behaviours are relatively unexplored | Low reporting of process data, e.g. timing, time lag between behaviour and incentive, frequency, performance feedback (private or public) or who received the payment (individual or organisation) for provider incentives. ‘Improvements in chart documentation procedures may account for the positive effects’. For consumers, coupons that are more convenient and flexible may be preferred to gifts. Some were never redeemed. The effects of gift incentives were sometimes confounded by lottery and competition processes | Process factors: how the readiness stage of a provider to change behaviour may affect the salience of the incentive programme; barriers such as attitudes regarding the perceived accuracy of the data; provider work-flow decisions when only a portion of the patient base may be affected by an incentive programme; the providers’ level of knowledge of guidelines; the effects of peer pressure and performance norms; continuity of care; fear of retribution; and professional pride. The potential synergy between individual-level and system-level incentives is untested |
| Kavanagh 2006236 A systematic review of the evidence for incentive schemes to encourage positive health and other social behaviours in young people |
Any health promotion, education or social behaviour | Young people aged 11–19 years | ‘Useful’ in some settings for some groups, particularly for simple or single-event preventative health behaviours. School-based competitions reduced smoking behaviours in a small number of studies. No evidence for sustained behaviour change. Unintended consequences can occur if a proxy outcome such as attendance is rewarded rather than the behaviour outcome as in the UK government’s Educational Maintenance Allowance (EMA) programme. The EMA targets means-tested household income and provides cash incentives for post-compulsory education attendance. Some incentive programmes do not achieve their intended demographic reach, with more advantaged groups benefiting from the programmes. The cost-effectiveness and the size and type of incentive that is sufficient to motivate young people are unknown | Attributes of the study setting, resources and interactions had an impact on intervention outcomes. Incentives are perceived favourably by young people. If targets are not met, self-esteem can be reduced; incentives perceived negatively can cause some harm, such as undesirable peer pressure. Incentives targeted at some groups and not others are perceived as unethical and inequitable by some. Consistent monitoring and rewards are important. Robust systems with skilled staff to provide intensive support are required. Commitment, organisation of services and characteristics of the populations served possibly contributed to differential effects. A team-orientated atmosphere can reinforce compliance. Cash to reward staff collectively to fund lunches and ‘treats’ was valued. Competitions may be more suitable for younger teenagers before commencing smoking. Views of young people were not taken into account in the intervention designs | Experiential incentives including holidays and social outings; edible treats – ice cream, pizza; achievement recognition by peers/others – public presentation of certificates, applause. The EMA can be considered as a redistribution programme and was not perceived as unethical or inequitable – unlike some other targeted incentive interventions. How staff interpret reasons for non-compliance varies. Tracking and reminder systems for providers are important. Personal provider experience of the behaviour, praise and positive feedback from clients and from staff influenced compliance |
| Kavanagh 2009239 Incentives to improve smoking, physical activity dietary and weight management behaviours: a scoping review of the research evidence |
Smoking, diet, physical activity, healthy weight maintenance | Any consumer | Scoping review of study abstracts that recommends full in-depth systematic reviews for smoking cessation and weight loss in overweight or obese people | Uncertainty about the effects of incentives for low socioeconomic status groups | |
| Lussier 2006240 A meta-analysis of VBRT for substance use disorders |
Illicit drug, alcohol or tobacco dependence | Any | Meta-analysis of 30 studies showed that VBRT increased short-term abstinence with an average effect size of r = 0.32 (95% CI 0.26 to 0.38) compared with the control. More immediate voucher delivery at the clinic visit resulted in almost twice the effect size of delayed vouchers. Incentives with an average daily voucher earning of US$5.00 were associated with smaller effect sizes than those with an average daily voucher earning of between US$5.00 and < US$16.00, with a dose–response relationship observed. VBRT increased clinic attendance with an average effect size of 0.15 (95% CI 0.02 to 0.28) | Duration of the intervention was not a moderator of effect size unlike in the review by Prendergast et al.,242 which included these studies and a wider range of contingency management interventions. Abstinence was for around 12 weeks in most studies. There were larger effect sizes for targeting a single drug than for targeting multiple drugs. Unlike in the study by Prendergast et al.,242 there were also reported effects of VBRT for tobacco use | Delays between monitoring, feedback and receipt of the voucher might weaken the reinforcement effect. Monetary value of incentive may moderate the effect – dose–response curve observed. Effects beyond the intervention period – mixed results |
| Paul-Ebhohimhen 2008241 Systematic review of the use of financial incentives in treatments for obesity and overweight |
Weight loss as a proxy measure of diet or physical activity | Obese and overweight mostly female patients | No significant effect of financial incentives on weight loss or maintenance at 12 and 18 months. Weak trends were observed for (1) more positive effects for incentives valued at ≥ 1.2% of personal disposable income and (2) reversal of effects for studies with 30-month follow-up data | Additional motivators such as providing food or a personal exercise trainer at groups. The contracting processes could be time-consuming. Attrition rates were high in some studies. Weak trends were observed (1) in favour of rewarding behaviour change rather than weight change; (2) for rewards for group rather than individual performance; (3) for incentives delivered by non-psychologists rather than psychologists. There were few data on lower socioeconomic and ethnic groups | Financial deposits with refunds made for either weight loss, compliance with behaviour change or attendance at sessions. Behaviour change rewards (self-report and not validated) compared with primary outcome (validated weight) rewards |
| Prendergast 2006242 Contingency management for treatment of substance use disorders: a meta-analysis |
Illicit drug, alcohol or tobacco dependence | Juveniles or adults | Meta-analysis showed that contingency management increased abstinence with a median effect size of d = 0.42 (95% CI 0.35 to 0.50). The magnitude of the effect size declined over time and was greater for a single illicit drug than for tobacco or multiple drug use. Larger effect sizes were associated with studies conducted in the 1970s and 1980s, higher researcher involvement and shorter (< 25 weeks) treatment duration, with higher effect sizes reported at < 11 weeks | VBRT (the voucher value escalates with each successive negative urine sample and is reset to a lower value for a positive sample). Support offered in addition to the incentive was often unclear | Shorter interventions may be more effective than longer interventions – possibly related to the chronic relapsing nature of substance misuse. ‘Fishbowl’ variable reinforcement interventions using either prize draws for financial incentives or documented praise. The aim of the fishbowl approach was to reduce costs |
| Roozen 2004243 A systematic review of the effectiveness of the community reinforcement approach in alcohol, cocaine and opioid addiction |
Alcohol, cocaine and opioid addiction | Adults aged 18–65 years with substance dependence excluding those for whom substance dependence was not the main diagnosis (i.e. adults with mental health problems who also had a substance dependency) | Evidence that the community reinforcement approach (CRA) with incentives is effective for cocaine addiction compared with usual care (two studies) but limited evidence for opioid addiction (one study). Evidence that CRA with abstinence-contingent incentives is more effective than CRA with non-contingent incentives for cocaine abstinence (two studies). CRAFT (Community Reinforcement Approach including Family Training) to achieve a lifestyle that is more rewarding than substance misuse. Included trials have high internal validity but new trials should focus on high external validity. Little follow-up data beyond 6 months | All four cocaine CRA with incentives studies were conducted by teams including the same author (Higgins). It is unknown which components of CRA are most effective and which are necessary or superfluous. Authors suggest that ‘The favourable results appear attributable to the inclusion of a significant other in the therapy and the use of reinforcement contingent incentives’. The social, vocational and recreational activities are a crucial component of CRA: peer group social reinforcement could improve outcomes. Feasibility of implementation in practice is questioned as this approach is labour intensive and has a high cost. CRA can be provided in five sessions over 4–6 weeks (< 50 hours per person) and can be tailored to individual goals | CRA is a multifaceted biopsychosocial approach that acknowledges the role of the environment and influences on habits and develops alternative rewarding social activities that are incompatible with substance misuse. CRA integrates cognitive–behavioural interventions including a treatment manual with incentive and pharmaceutical interventions to bring about change in environmental contingencies such as work, recreation and family involvement |
| Sutherland 2008245 Paying the patient: does it work? |
Any | Patients and employees/community members | Even relatively small incentives can influence individuals’ health-related behaviours, but effects diminish over time. Worksite incentive schemes have high adherence and seem promising for diet and exercise interventions. Few studies report long-term follow-up | Some but not all incentives studies increased attendance at pre- and postnatal care appointments. Selection effects occur when only the most motivated engage in the programme. Limited data on sample characteristics to know whether tailoring the incentive to certain populations would increase effectiveness. Information on overall programme costs was very limited | |
| Wall 2006244 Effectiveness of monetary incentives in modifying dietary behaviour: a review of randomized, controlled trials |
Dietary behaviour | Community-based populations | All four included trials found a positive effect of incentives on healthy eating or weight loss compared with the control group. Growing observational evidence for price reduction strategies increasing the purchase of healthy food and the increased sensitivity to price of those on lower incomes | Uncertainty about sustained positive effects. The small number of studies and small sample sizes precluded the drawing of any conclusions about the form or level or incentive. No studies assessed effects according to socioeconomic or ethnic group. No studies assessed cost-effectiveness |
Consumer incentives: findings that support the Benefits of Incentives for Breastfeeding and Smoking cessation in pregnancy study evidence syntheses of incentives to increase smoking cessation in pregnancy and/or breastfeeding (see Chapter 3)
Effectiveness of incentives for lifestyle behaviour change
-
Effectiveness is short term and dissipates when the incentives are withdrawn. 2,3,238,241,242,245 Short-term programmes may have more effect than long-term programmes. 242
-
Uncertain whether there is a dose–response curve. 2,237,240 Even small incentives can influence health-related behaviours. 2,245
-
Consistent periodic incentives (compared with one-off incentives)3 delivered as rewards immediately after (vs. delayed) and contingent on a validated test to confirm change are effective. 3,236,240,242,243
-
Abrupt withdrawal of cigarettes rather than reducing the level of smoking consistently predicted successful smoking cessation. 237
-
Larger effect sizes are seen with higher researcher involvement. 242
Other components of incentive interventions
Incentive intervention design and delivery
-
Incentives can increase recruitment and participation rates and this may increase the absolute numbers of quitters. 3,236–238
-
Predominantly female, younger, better-educated, heavier smokers with previous quit attempts who are in the contemplation or preparation stage for change register with quit and win contests. 237
-
Attrition rates can be high241 and selection effects occur when only the most motivated engage in the programme; therefore, comparison between participants and non-participants is recommended. 245
-
Studies of incentive-based interventions should take into account the views of participants in designing and delivering the interventions. 236
Consumer incentives: gaps in the evidence and findings that show promise and need further investigation for smoking cessation in pregnancy and breastfeeding (see Chapter 3)
Effectiveness of incentives for lifestyle behaviour change
-
Voucher-based reinforcement therapy (the voucher value escalates with each successive negative urine sample and is reset to a lower value for a positive sample) has comparable efficacy to variable ‘fishbowl’ reinforcement using either prize draws (lower cost) for financial incentives or documented praise. 242
-
There are limited data on the costs of schemes or cost-effectiveness. 237,243–245 The contracting process can be time-consuming241 and robust systems with committed, skilled staff to provide intensive support are required. Sustainability is a concern. 236,243
-
Quit and win competitions with easy to enter raffles, enticing prizes such as holidays or social activities, refreshed each year. 237
-
Coupons that are convenient and flexible may be preferred to gifts. 2
-
Workplace incentives have high adherence and show promise. 237,245
Other components of incentive interventions
-
Achievement rewarded through recognition by peers/others, for example public presentation of certificates, applause for young people. 236
-
Community reinforcement through social or recreational activities243 and donation of prizes, together with health professional support and social marketing approaches. 237
-
The interagency communication and human resources necessary to sustain complex interventions and sustain behaviour change are unknown. 236
-
Incentives delivered to a group of participants, including social support networks, peers and families, with the aim of setting new peer norms. 3,241
Incentive intervention design and delivery
-
Unintended consequences can occur if a proxy outcome such as attendance is rewarded rather than the behaviour outcome. If targets are not met, self-esteem can be reduced, and incentives perceived negatively can cause harm, such as undesirable peer pressure. 236
-
The effect of media publicity to assist in recruitment is uncertain. 237
-
There is uncertainty about incentives delivered by non-professionals or volunteers,237,241 including those with personal experience of the behaviour,236 compared with those delivered by health professionals.
-
Tracking and reminder systems for providers, with a team-orientated atmosphere, can reinforce compliance. Cash to reward staff collectively to fund lunches and ‘treats’ was valued. 236
-
A controlling or humiliating communication style can undermine behaviour change whereas praise, positive feedback and encouragement help. 3,236
-
There are limited data reported on effectiveness for lower socioeconomic and ethnic groups and therefore it is not known whether tailoring the incentive to certain populations would increase effectiveness. 239,241,244,245
-
Some incentive programmes do not achieve their intended demographic reach, with more advantaged groups benefiting from the programmes. 236
-
Incentives targeted at some groups and not others are perceived as unethical and inequitable by some. When eligibility for incentives is means tested for household income, such redistribution incentives are more acceptable. 236
-
Levels of deception determined through random checks and cross-checks with family and friends varied but were higher in high-risk populations (e.g. drug addicts) and when checks were random and unexpected. 237,238
Incentives delivered to health-care providers
The studies included in the reviews of incentive interventions delivered to health-care providers addressed only one of our identified complex lifestyle behaviours: smoking cessation. In addition, a wide range of simple behaviours related to primary and secondary disease prevention and quality of care or treatment were addressed. In the majority of reviews, process indicators for documenting smoking status, providing advice or referral to specialist services rather than quit outcomes were incentivised. The eligibility criteria for each review varied from narrow to broad, which resulted in considerable variation in the number of studies included, the number of different outcomes or process indicators reported and the range of study designs. There was considerable overlap both in the included studies and in the reported findings. It could therefore be difficult to attribute the reported findings to the smoking cessation studies. However, all studies reported on changes in provider behaviour as a result of an incentive intervention. The providers incentivised were predominantly doctors, either individually or as groups.
The reviews highlight that the popularity of incentives to change provider behaviour is partly because educational strategies involving protocols and guidelines alone have had limited success at improving the quality of care, are expensive and are difficult to sustain. 268 The underpinning theory is that financial incentives will change health professionals’ behaviour to improve the quality and/or quantity of care or reach targets, with the assumption that this will translate into improved patient outcomes, resulting in reduced costs and improved access to care. However, health professionals often have high levels of intrinsic motivation to improve patients’ health and well-being and extrinsic incentives can crowd this out with potentially detrimental consequences for health care. 269 A small extrinsic incentive may be effective for a behaviour that the provider values highly. Conversely, a large incentive may be required to change a behaviour that is not considered important to the provider. The individual behavioural response to financial incentives may be more complex than generally considered by most empirical economic analyses, as health professionals are motivated by more than financial incentives269 and gaming the system is a concern. 2
Kane and colleagues2 identify several forms that incentives for providers can take: pay per service provided (often called fee-for-service); pay per service with a bonus or penalty paid based on assessed performance [often called fee-for-service with withhold or pay for performance (P4P)]; on the basis of the costs of providing a service (often called cost-based or retrospective payment); a fixed payment (often called capitation or prospective payment); or a mixture of payment types. P4P became part of the UK general practitioner (GP) primary care contract in 2004. This is known as the Quality and Outcomes Framework (QOF) and financially rewards general practices for recording smoking behaviour and offering smoking cessation services, particularly for long-term conditions such as diabetes and heart and lung disease. 270 This is supported by prompts from electronic medical records available during the consultation. It does not incentivise smoking quit outcomes and the recording of these is inconsistent. A maximum of 1000 points per annum can be earned by GPs for meeting quality process and outcome targets and 79 of these are for smoking indicators. Practices are paid on average £130.50 per point (2011/12 prices). 270 Currently, QOF does not include any complex lifestyle behaviours relating to pregnancy, unless the woman has one of the target diseases.
Overall, there is little evidence to date that would support provider incentives to improve complex lifestyle behaviour change outcomes for consumers and this area is relatively unexplored. However, undoubtedly incentives do change doctors’ behaviour in supporting and documenting behaviour change. In the following sections key bullet points relevant to the aims and objectives are presented; Table 37 summarises the data on provider incentives.
| Study and title | Behaviour | Population | Summary of effectiveness findings | Summary of incentive delivery process findings | Relevant evidence gaps that apply to smoking in pregnancy and/or breastfeeding | Incentive strategies/themes to explore in primary research |
|---|---|---|---|---|---|---|
| Flodgren 2011246 An overview of reviews evaluating the effectiveness of financial incentives in changing health-care professional behaviours and patient outcomes |
Aspects of the quality, quantity or targeting of care | Health professionals | Incentives were categorised by the unit of reimbursement rather than considering the unit of effect. Studies looked at the impact of paying per unit of time; paying per unit of service; paying for providing to specific types of patients; and paying for providing a prespecified level of care or providing a change in activity or quality of care. Payment was generally effective for providing (1) a service, episode or visit, (2) care for a patient or specific population and (3) a prespecified level of care or change of activity or quality of care. Financial incentives improved processes of care, referral and admissions and prescribing cost outcomes. There was no evidence for an effect of financial incentives on patient outcomes. There was mixed effectiveness of incentives for consultations or visits and incentives were ineffective for improving compliance with guidelines or for working for a specified time | No studies evaluated adverse or unexpected effects. No studies investigated variable ‘doses’ of incentive. The studies had methodological weaknesses and the generalisability of the findings is uncertain. Doses of incentives were poorly described | Adverse or unexpected effects; dose–response effects for incentives; how incentives interact with intrinsic motivation | The relationship between the dose of the incentive and the value or intrinsic motivation of the behaviour that it seeks to change; the effectiveness of different payment strategies, i.e. meeting a target level, targeting specific groups (good way of avoiding patient selection), paying per service provided |
| Gillam 2012247 Pay-for-performance in the United Kingdom: impact of the Quality and Outcomes Framework: a systematic review |
Process and intermediate outcome measures in a wide range of domains | General practices | Quality of care for incentivised conditions during the first year improved at a faster rate than the pre-intervention trend and subsequently returned to previous rates of improvement. Achievement for non-incentivised conditions worsened in relative terms. The impact remains uncertain. Conflicting findings because of the diverse indicators and study heterogeneity | Inequalities in the process of care narrowed between the most and the least deprived population quintiles. Ethnic and age-related variation were both reduced. Greater consistency and improved organisation of care. Some doctors reported improved recording and teamwork and some nurses reported enhanced specialist skills. Protocol-driven care increased, with person centeredness and continuity perceived as being negatively affected and patients’ satisfaction with continuity of care decreasing. Care should be taken over the weight given to ‘perceived’ changes although some of these were supported by studies of patient experience | Few studies evaluated cost-effectiveness and this varied according to the indicator. The costs of the scheme are substantial and the opportunity costs are unknown. The effects of incentives and the availability of comparative outcome data on teamwork, hierarchies, morale and the primary care provider–patient relationship are also unknown. There was little evidence of gaming. Linking the size of the financial reward to the public health impact of individual indicators was recommended | Impact on non-incentivised care, teamwork, staff–staff and staff–patient relationships, organisational hierarchies and workload; ethical issues; the extent to which incentives should be linked to protocols; scientific–bureaucratic medicine as a threat to professionalism; comparison of data between colleagues and teams |
| Hamilton 2013250 Effectiveness of providing financial incentives to healthcare professionals for smoking cessation activities: systematic review |
Smoking cessation | Individual or groups of health-care providers (doctors, nurses or other members of health-care teams) | Financial incentives appear to improve recording of smoking behaviour, the provision of smoking cessation advice and referral to stop smoking services. Three studies reported smoking quit rates and the results were mixed. Multicomponent interventions are more effective than single-component interventions. Cost-effectiveness was examined in one study only and depended on the training and medication components of the intervention | Four studies examined effects on individual doctors, with the remainder examining incentives provided to groups of health-care providers. Financial incentives may have more effect when combined with other interventions, e.g. education, subsidised smoking cessation prescriptions. There is evidence of GPs’ perceived lack of expertise in changing smoking behaviours and overcoming this could increase the effectiveness of incentives | Interactions between health-care provider training and incentive provision. Effectiveness of incentives for nurses and non-doctor staff is unknown. Price elasticity and the optimum incentive level are unknown. Evidence on the response to incentives appears to be clearest for two types of interventions: measuring smoking status and prescribing, which are both relatively easy to implement | Individual or group provider incentives; the other components that might increase the effectiveness of financial incentives delivered to providers |
| Scott 2011248 The effect of financial incentives on the quality of health care provided by primary care physicians |
Patient well-being, which included smoking cessation | Primary care physicians or teams | The effects of financial incentives on the quality of primary care were ‘modest and variable’. For the three included smoking cessation trials, there were statistically significant effects on referral rates and recording of smoking status, but not on measures of smoking cessation | Studies used a variety of payment mechanisms including single-threshold target payments, a fixed fee per patient achieving an outcome (smoking quit) and payments based on the relative ranking of medical groups’ performance (tournament-based pay). The size of the incentives as a percentage of total revenue is unknown | A stronger theoretical basis for incentive schemes and more rigorous study design; degree of attachment between patients and physicians; how the incentive payments were distributed and used within teams | Different payment mechanisms; how the level of attachment between provider and patient might interact with incentives to providers |
| Van Herck 2010249 Systematic review: effects, design choices, and context of pay-for-performance in health care |
Any medical condition including prevention | Primary care or acute hospital care providers | Effects were variable from absent to strongly beneficial, relating to the design choices and context. In general, there was about a 5% improvement because of P4P. It seems effective to support uniform minimum standards. Positive results were reported for smoking cessation. Ceiling effects occur. Financial rewards seemed to generate more positive results than competitive approaches in which there were winners and losers. Equity is improving in the UK. Unintended effects on non-incentivised interventions or conditions were identified. Positive and negative spillover effects were reported. There is limited evidence that gaming occurs with P4P | Recommended P4P programme best practice hypotheses and recommendations: targets should be selected and defined on the basis of room for improvement; use process and intermediary outcome measures as targets; implement a uniform design; involve stakeholders and communicate information thoroughly and directly; focus on both quality improvement and achievement; distribute incentives to the individual and/or the team rather than at the organisation level. The dose–response relationship is not known. Incentives worked better when they were introduced to a uniform health-care system as opposed to a fragmented payment-based system | There is little evidence on the co-ordination and continuity of care or patient centeredness. Few studies measured gaming or unintended consequences; however, these seem minimal | Importance of communication and awareness of provider incentive programmes; organisational aspects; incentivising individuals or teams |
Provider incentives: gaps in the evidence and findings that show promise and need further investigation for smoking cessation in pregnancy and breastfeeding
Effectiveness of incentives for lifestyle behaviour change
-
The effectiveness of provider incentives for complex lifestyle behaviour change is relatively unexplored and both studies and indicators are diverse. 246–250 Recording of smoking behaviour, provision of smoking cessation advice and referral rates to stop smoking services increase. 248,250
-
Improvements occur at the fastest rate in the first year of a programme and subsequently return to the pre-intervention rates of improvement. 247
-
Achievements for non-incentivised conditions worsen. 247
-
Provider incentives can narrow health inequalities between the most and the least deprived populations. 247,249
-
P4P seems effective to support uniform minimum standards. Ceiling effects are observed. 249
-
Guaranteed incentives seem more effective than competitions in which there are winners and losers. 249
-
Incentives result in greater consistency and improved organisation of care. Some doctors report improved teamwork and some nurses report enhanced specialist skills. 247
-
Protocol-driven care increases whereas person centeredness, patient satisfaction and continuity of care can decline. 247
-
Little is known about cost-effectiveness, price elasticity, the optimum size of incentives or opportunity costs. Costs can be considerable and depend on the other intervention components delivered. 246–250
Other components of incentive interventions
-
Incentives as one component in multicomponent interventions are more effective than incentives alone, for example incentives combined with education and subsidised medication for smoking cessation. 250
Incentive intervention design and delivery
-
A stronger theoretical basis for incentive schemes and more rigorous study design, with attention to tensions between scientific–bureaucratic and professional models of health care, are needed. 248
-
Most of the evidence relates to doctors or general practices rather than nurses or other maternity care providers. The differences in effect between incentivising individuals and incentivising groups are uncertain. 247,250
-
Little is known about the prevalence of gaming or unintended consequences as they are seldom evaluated. 246,247,249
-
Little is known about the impact of provider incentives on provider–patient relationships, provider–provider relationships, teamwork or morale. 247,248
-
Recommendations: select and define targets on the basis of room for improvement, involve stakeholders and communicate information thoroughly and directly and focus on both quality improvement and achievement. 249
Discussion
The evidence supporting incentives delivered to consumers to change lifestyle behaviours is strongest for contingency management programmes with varying levels of additional support addressing illicit drug, alcohol and tobacco use. Meta-analysis of included studies was possible for these behaviours and showed short-term effectiveness of around 12 weeks. 240,242 Other studies in reviews of incentive strategies for smoking cessation, including competitions,237,238 were so heterogeneous that it was not considered appropriate to calculate an overall combined effect. The methodological quality was variable, particularly for verification of smoking outcomes using CO monitoring. Presently, evidence does not support incentive interventions for weight loss through diet or exercise. 236,241 There is an absence of sustained longer-term behaviour change. Unknowns include the most effective dose including ceiling effects; the nature of the dose–response relationship; and the optimal proportions of incentive to psychosocial support for effectiveness, as well as the characteristics of the support. The reach of incentive interventions is poorly reported; many studies have small sample sizes and the question of selection bias arises. Indeed, typologies of the impact of incentives on five groups of individuals – the ‘lucky ones’, the ‘yes I can’ group, the ‘I’ll do it tomorrow’ group, the ‘unlucky ones’ and the ‘leave me alone’ group – have implications for whether incentives should be universal, targeted or abandoned. 271 Unintended consequences are reported in some studies, with gaming to receive the incentive seeming more likely if the incentive rewards attendance rather than the actual desired behaviour. Incentive studies for complex lifestyle behaviours are therefore predominantly hypothesis generating and have largely targeted individuals and further research into targeting other populations is indicated, for example families, communities and workplaces.
The evidence supporting incentives to change provider behaviour is predominantly for provider process outcomes not relevant to the complex lifestyle behaviours of interest. In the included studies, only interim process indicators for smoking cessation were relevant to our review. These included relatively simple health professional behaviours to change, such as documenting smoking status, the provision of advice or referral to stop smoking services. There was no reported evidence relating to incentives delivered to providers to change consumer complex lifestyle behaviours. A recent analysis of incentives for smoking counselling implemented as part of the UK QOF payments questions their utility. 272 Coleman272 argues that incentives may not be having the desired effect and that they could adversely affect patient-centred care and that indicators of meeting targets require rigorous evaluation.
Research has focused on incentives delivered to doctors rather than to nurses, midwives or maternity staff, thus limiting their generalisability to smoking in pregnancy and breastfeeding. The UK evidence is dominated by the QOF financial incentives provided to general practices, whereas US incentives have largely been paid to individual doctors. There is an absence of evidence on how the financial incentives are distributed within practices. UK GPs have independent contractor status and employ practice nurses and other staff. It is unclear to what extent each team member perceives the QOF as a financial incentive or as a managerial tool, as electronic reminder systems are commonly used that can trigger intrinsic motivation to provide quality care. Findings suggest that, for complex behaviours, multifaceted programmes might be more promising than single-component interventions. Components might include electronic prompts, education of health-care providers or subsidised smoking cessation medication, as well as a financial payment for meeting a target level. However, the evidence is dominated by a few well-researched programmes, in particular the QOF.
Commitment contracts for lifestyle behaviours have been considered for consumers to bring the risk of loss into the present, reduce temptation and increase motivation,273 but these have received less attention for providers. In an overview of P4P for health service providers by the King’s Fund, which did not meet our inclusion criteria, Appleby and colleagues255 conclude that giving priority to the prevention of illness will require a radical rethink of the incentives needed. A recently published checklist helps decision-makers to assess when incentives might do more good than harm, to help prevent premature or inappropriate implementation. 274 Importantly, the checklist highlights the need for systems and structures to be in place to facilitate a provider incentive intervention.
Strengths and limitations
This was a scoping review of evidence syntheses of incentives for lifestyle behaviour change and we cannot guarantee that every relevant review was identified by our search strategy. The aims of the review were very specific to the BIBS study and the data extracted are therefore selective and purposive. A recently published review found no convincing evidence that effects were different between incentives for different groups of behaviours: long-term (> 6 months) smoking cessation, attendance for vaccination or screening, or all behaviours. 275 This study reports some evidence that effect size increased with increasing incentive value in smoking cessation studies with follow-up beyond 6 months. One further narrative review of contingent incentives for smoking cessation was identified after completion of this review. The study by Sigmon and Patrick213 confirms findings on the effectiveness of incentives and discusses some new aspects of programme delivery. For example, technological innovations such as internet technology and smart phones to increase uptake of incentive interventions, with video recordings using a webcam to verify CO monitoring, are being developed and show promise. 276 We made every attempt to identify reviews between our cut-off dates by searching reference lists of the reviews that we included. We did not include general reviews for the included complex behaviours, some of which include incentive studies, for example a review of smoking cessation in pregnancy. 54 Incentives for increasing exercise are therefore likely to have been missed as no stand-alone review was identified. As exercise involves the establishment of a new behaviour, this could have relevance for breastfeeding, for example one recent study investigated a loyalty card scheme to increase the uptake of exercise in the workplace. 277
Conclusions
In the following section we consider how the evidence presented in this chapter supports, refutes or does not contribute to the development, acceptability and any unintended consequences of the shortlist of incentive strategies for smoking cessation and breastfeeding commenced in Chapter 3.
The following pointers were incorporated into discussions with service users (see Chapter 2) and the qualitative interviews (see Chapter 6), and the shortlist was finalised at a grant holders’ meeting in February 2013. The final shortlist taken forward for further qualitative investigations and the survey research is presented in Chapter 6.
Pointers for the incentive strategy shortlist
-
Supports contingent shopping vouchers for proven smoking cessation in pregnancy and for breastfeeding, with regular monitoring, feedback and support provided by someone with expertise. The effects appear to be short term; however, as the maximum benefit period is the 9 months of pregnancy plus 6 months for breastfeeding,60 this may be less important than for other lifestyle behaviours such as obesity.
-
Supports incentives beyond the individual as involvement of others in an incentive intervention (partner/cohabitant, family, friends, work colleagues) consistently predicted successful smoking cessation. However, there is a gap in the evidence with regard to incentives delivered to groups rather than individuals, for example social support networks, peers and families.
-
Supports the strategy of financial incentives delivered to providers for improving outcomes relevant to the quality of care and for changing service provider and professional behaviour. However, their impact on actual lifestyle behaviour outcomes is unknown. Given the low number of studies identified that tested provider incentives for smoking cessation in pregnancy and breastfeeding (see Chapter 3), these warrant further investigation. The findings strengthen the case for more research to evaluate provider incentives and commitment contracts for breastfeeding, particularly as support was identified as the dominant barrier/facilitator in Chapter 4 and there is some evidence of improved breastfeeding outcomes from multicomponent accreditation interventions that include an award (see Chapter 3).
-
This review does not contribute any relevant evidence on the effect of the provision of free or subsidised breast pumps. However, returning to work is a trigger for breastfeeding behaviour change and workplace interventions show some promise.
Pointers for the acceptability of incentives and any unintended consequences
-
There is uncertainty and about the acceptability and effectiveness of targeting incentives to some populations and the impact of incentives on health inequalities.
-
Gaming is an unintended consequence, particularly if attendance or a preparatory behaviour is incentivised rather than the target behaviour.
-
Neglect of non-incentivised areas of care can occur when incentivising providers.
Implications for research
Many hypotheses are generated by the reviews and warrant further research; in particular, multicomponent interventions, the involvement of social support networks, different settings for incentive programmes such as the workplace, the use of raffles and community engagement all show promise and warrant more robust evaluation. The review of incentive reviews has identified both gaps in the evidence and emergent themes, which have informed the qualitative study and survey data collection described in Chapters 6 and 7 respectively.
Part 2
Chapter 6 Primary qualitative study investigating perspectives on incentives
In this chapter we present qualitative research that explores the mechanisms of action and interactions of incentives, a shortlist of incentive strategies and the unintended consequences of incentives. An incentive ‘ladder’ logic model for behaviour change is used. This was developed during the course of the study from the analysis and synthesis of the mixed-methods data and in collaboration with our service-user co-applicants (see Chapter 2). We consider the ladder model to be a useful metaphorical aid to explore how the components and delivery of incentive programmes fit with the behaviours, which are enmeshed in women’s life courses and contexts.
Methods
Design
This primary qualitative research was part of a mixed-methods approach that included the three evidence syntheses (see Chapters 3–5), general public and health professional surveys (see Chapter 7) and a DCE (see Chapter 8). In addition, co-applicant service users contributed to the design and the data (see Chapter 2), and independently collected qualitative interview and focus group data from the CPIT88 (see Box 2) were included in the final analysis. A timeline for the study is given in Figure 2. The mixed-methods approach was informed by different theoretical approaches and used both deductive and inductive reasoning.
The shortlist of promising incentive strategies (Table 38) was agreed by consensus at a research team meeting in February 2013 by considering the evidence syntheses (see Chapters 3–5), service-user input (see Chapter 2) and early qualitative interview data. It was unclear from the evidence syntheses in Chapter 3 what other components, for example BCTs, might be needed as well as the incentive as no trial tested an incentive alone compared with no incentive. A framework approach278 was therefore applied early in the data collection and analysis to inform incentive trial design for the shortlist of incentive strategies. Trial design has fixed components and processes that tie in with Consolidated Standards of Reporting Trials (CONSORT) guidelines for reporting trials. 279 The shortlist of incentive strategies in turn informed the design of a survey (see Chapter 7) to measure acceptability and the results then fed back into the qualitative data collection and analysis. In particular, open questions were included in the survey about the consequences of incentive programmes (see Chapter 7).
| No. | Details |
|---|---|
| 1 | Incentives for women who prove that they have stopped smoking during pregnancy |
| 2 | Incentives for women for 2 months after the birth of their baby if they prove that they are still not smoking |
| 3 | Incentives beyond the individual, for example incentives for a partner or a buddy or for a smoke-free home |
| 4 | Incentives for women who prove that they are breastfeeding |
| 5 | A breast pump provided for free on the NHS |
| 6 | Additional funding for local health services if they reach targets for the number of women who prove that they have stopped smoking during pregnancy, or penalties if targets are not met |
| 7 | Additional funding for local health services if they reach targets for the number of women who prove that they are breastfeeding, or penalties if targets are not met |
A grounded theory approach280 was applied concurrently to develop an incentive taxonomy and to understand the mechanisms of action of incentives. This is appropriate as it is a relatively unexplored area. We used an iterative approach to collecting data, analysis, refining our research questions, theoretical sampling, revising the topic guides and refining the analysis. A range of qualitative methods was also integrated, including unstructured interviews, structured interviews with vignettes, focus groups, interactive discussions, observations with mother-and-baby groups and open questions on surveys.
Key research questions
Our research questions were:
-
How do incentives alter the balance of existing intrinsic and extrinsic motivators/demotivators?
-
How do incentives interact with the environmental, organisational, social and cultural facilitators of and barriers to behaviour change?
-
Is the shortlist of incentive strategies acceptable and feasible?
-
What are likely to be the unintended consequences for the incentivised and the non-incentivised?
Study settings
The settings for conducting this research included primary and secondary health services and local authority community and voluntary sector services (e.g. antenatal clinics, children and family centres, mother-and-baby groups). Aberdeenshire, Lancashire and Glasgow (the CPIT88) were purposively selected for their diverse sociodemographic characteristics and their different incentive cultures for smoking cessation in pregnancy and breastfeeding (see Box 1).
Sampling and recruitment
The purposive, theoretical and snowball sampling strategy is summarised in Table 39. 281 This was flexibly implemented over time, with snowball sampling included to identify harder-to-reach, more disadvantaged participants and to search for disconfirming perspectives. For example, through a midwifery contact we were able to gain access to a Barnardo’s group for teenage mothers.
| Sample | Recruitment strategy |
|---|---|
| Pregnant women and mothers/partners/significant others from the first trimester until 6 months after birth |
|
| Providers of care/stakeholders: midwives, health visitors, obstetricians, paediatricians, GPs, public health specialists, pharmacists, voluntary sector, children and family centre staff |
|
| UK experts/decision-makers: UK government policy-makers for maternal and child health and public health, research ethics and research governance personnel, expert advisers, voluntary sector |
|
Benefits of Incentives for Breastfeeding and Smoking cessation in pregnancy study data collection
Qualitative data collection started in June 2012 and finished in early August 2013. Three postdoctoral researchers (NC, HM and GT) conducted qualitative interviews and focus groups in Aberdeenshire and Lancashire with the participants and conference attendees detailed in Table 40. They worked closely with the two mother-and-baby group co-applicants, informed by participatory research methods, which are described in detail in Chapter 2. Qualitative sampling strategies, topic guide refinement, data collection and analysis were iterative to address specific research questions as they arose and were refined throughout the study. Once interview participants’ personal or professional trajectory vis-à-vis smoking in pregnancy and feeding intention/experience had been established, members of the research team explored interviewees’ understandings of ‘incentives’, specifically as defined within the project: as financial or non-financial tangible incentives or rewards. Participants were asked how they conceptualised incentives, what various types of incentive might mean and whether or not they were acceptable. Early interviews explored the processes through which existing barriers to and facilitators of smoking cessation and breastfeeding could potentially interact with both incentives and other intervention components, including the BCTs identified in the evidence synthesis findings (see Chapter 3). Interviews were open-ended, audio recorded and transcribed verbatim. The lengths of the interviews ranged from approximately 15 minutes to 100 minutes. Topic guides changed over time as the analysis progressed, to ensure that key issues were covered (see Appendix 15). Although initial topic guides were informed by and explored issues relating to Chapters 3–5 and the shortlist of promising incentive strategies, as the analysis progressed the sampling strategy and topic guides were modified to develop theory about incentive interventions and explore the acceptability and feasibility of the shortlist of incentives and the unintended consequences of incentives.
Intervention vignettes, used by PH in previous qualitative research to inform the design of an intervention,62 were employed to facilitate more directed discussions when appropriate/feasible (see Appendices 2 and 16). Vignettes were developed from studies that either were effective or involved an unusual/notable approach. Three studies for each of the target behaviours were selected. 103–108 As reported in Chapter 2, the mother-and-baby groups contributed to interpreting systematic review findings by providing feedback on a number of vignettes of studies included in the evidence syntheses. Initially, vignettes were referred to assist participants who struggled to conceptualise incentives. Therefore, they were not used in all interviews because some participants spoke more confidently than others. Later, more focused discussions around the vignettes helped the research team to gain valuable participant insights into aspects of content and delivery as well as to generate data around what might be acceptable incentives for the target population. Vignettes were also used in interviews with health professionals and the studies represented were based on the shortlist. 82,103,105,168 These vignette data therefore enabled us to refine the shortlist.
Free-text responses by health professionals about the consequences of incentive schemes in response to open questions in the survey investigating the acceptability of the shortlist of incentive strategies (see Chapter 7) were entered onto a Microsoft Excel spreadsheet. The questions were:
-
We would like you to imagine that your local health service is going to run a scheme that provides incentives for stopping smoking in pregnancy. What do you think the consequence might be for participants and/or staff? (1) Positive consequences? (free text); (2) negative consequences? (free text).
-
We would like you to imagine that your local health service is going to run a scheme that provides incentives for breastfeeding. What do you think the consequence might be for participants and/or staff? (1) Positive consequences? (free text); (2) negative consequences? (free text).
Data collection for the Cessation in Pregnancy Incentives Trial
Data collection for the qualitative research element of the CPIT Phase II RCT began in April 2012 and ended in October 2012. Two experienced qualitative researchers (SM, JM) conducted semi-structured qualitative interviews with women participating in the trial and professional stakeholders. Interviews explored various topics to address the following research question: ‘Can incentives be introduced in a way that is feasible and acceptable to women and service providers and are there any unintended consequences?’
One-to-one interviews with trial participants were conducted on a face-to-face basis in participants’ homes located within the boundaries of the CPIT study site: NHS Greater Glasgow and Clyde. Qualitative interview participants were recruited as part of the process of recruitment for the wider trial. In total, 100 trial participants were approached to achieve a target qualitative sample of 20 women, which incorporated an equal balance across intervention and control groups and age groups (< 25 years and 25+ years). Sampling also involved an approximate 70/30 split with regard to participant location in the Glasgow or Clyde area, reflecting the population ratio within the health board area. Additionally, interviews were planned to incorporate different stages post setting a quit date (SQD), that is, SQD + 4 weeks, SQD + 12 weeks and SQD + 20 weeks.
Interviews began with discussion of individuals’ experiences of smoking cessation services and their recent smoking cessation attempt and progressed to cover views on the use of incentives to encourage smoking cessation in pregnancy; their experience of, or opinions on, trial processes, including monitoring at key stages; and the likelihood of becoming involved in gaming. The initial topic guide used within interviews (see Appendix 17) evolved during the period of data collection to take account of interview experiences, specific requests from the trial team and participants’ different stages post SQD. Every woman who took part in a qualitative interview for the trial was given £20.00 in cash as a thank you for participation.
Professional views were gathered in 10 one-to-one interviews and two focus groups involving 23 individuals overall. Interviews with professionals were mainly conducted face to face in the workplace although three interviews were conducted by telephone. Professionals were invited to participate in a study interview based on their role within the delivery of the trial or their professional interest in routine service delivery among pregnant women within NHS Greater Glasgow and Clyde. The content of the interview varied depending on the occupation of the participant but it followed a topic guide that covered personal experiences of trial implementation, including the impact on routine service delivery, and elicited views on key aspects of the intervention and trial, as in interviews with trial participants (see Appendix 18). Interviews lasted for between 25 and 80 minutes and all were audio recorded and transcribed verbatim.
Ethical approval for incorporating the qualitative transcripts from the CPIT into the BIBS study was granted by the West of Scotland REC2 on 25 May 2011.
Data analysis
All qualitative data were entered into NVivo10 software (QSR International, Warrington, UK) to facilitate data organisation, coding and retrieval. In addition, free-text responses to open questions in the health professional survey on the perceived consequences of incentive programmes were entered onto a Microsoft Excel spreadsheet and content analysis was used to triangulate the analysis of the interview data. Analysis was informed by the framework method,278 which is well established as a transparent, systematic and rigorous data management tool in applied policy research. One of the strengths of the framework method is its potential to summarise data into thematic matrices, to look for patterns or explanations. Initially, three researchers (NC, HM and GT) identified key themes and categories independently by reading transcripts of and listening to the first four participant and four provider interviews. Through wider research team transcript reading and discussion, a single tree structure coding index (see Appendix 19) was agreed and applied in NVivo10 to the data sets from the separate sites, with merges of data sets every 2–4 weeks. The researchers undertook a detailed analysis of the data with regular discussion several times a week between sites to ensure consistency and to search for disconfirming perspectives. Drafts of the findings and analysis were circulated before weekly meetings with ongoing feedback provided by the project lead (PH). The emerging mixed-methods analysis, drawing on data from other stages of the BIBS study, and the framework matrices, informed and helped to develop an incentive logic model. CPIT transcripts were then added into NVivo10 software in July 2013 and incorporated into the analysis. This was to minimise bias of interpretation, as those participating in an incentive trial for smoking cessation in pregnancy are likely to bring very different perspectives to the data. The research team did not share the analysis of the data with the CPIT researchers until a final draft of this chapter was available, when they were asked to check it for accuracy.
When presenting the findings we refer to an intervention that aims to change smoking or breastfeeding behaviour as ‘a programme’, regardless of whether the reference point in the data is a research study (data collected using intervention vignettes), an actual intervention experienced, for example as part of usual maternity care or delivered in the community by the voluntary sector, or a potential future service delivered by health-care or other agencies. The collective term ‘participant’ is used within the text to indicate that all participant groups (women/partners, providers and experts) provided similar comments. When the points raised specifically refer to certain groups, this has been made explicit within the text. We refer to ‘providers’ as those who deliver a behaviour-related programme, with specific reference made to professional groups only when appropriate. The findings are supported by quotations from participants followed by a reference, for example ‘FG5, I, mother’. The first code is the participant identification number, which can be cross-referenced to the detailed participant characteristics provided in Appendices 20–23, preceded by letters that relate to whether the participant took part in a focus group (FG), an interactive discussion (IA) or a telephone interview (T). If there is no preceding letter the participant took part in a face-to-face interview. The second code (the presence of an ‘I’) relates to whether the participant was/had been involved in an incentive programme. If there is no ‘I’ then the participant has no direct experience of an incentive programme. The last code provides a narrative description of who the participant is. To protect confidentiality, study sites and all quotations have been anonymised.
Findings
Sample characteristics
There were three main sample populations: (1) pregnant women and new mothers and their partners or significant others until 6 months after birth, (2) providers, who could either deliver or receive incentives to support women to initiate or maintain smoking cessation or breastfeeding and (3) experts and decision-makers in a management/co-ordinator position potentially responsible for implementing incentive programmes, for example a policy-maker at local, regional or national level or a member of a research ethics committee (Table 40). Some from each sample had had experience of incentive programmes for either smoking cessation in pregnancy or breastfeeding. Overall, a total of 177 participants took part in 16 focus groups, 55 face-to-face interviews and 19 telephone interviews. In addition, approximately 63 conference attendees at three conferences took part in audio-recorded interactive discussion sessions. Detailed characteristics of the sample are provided in Appendices 20–23 and are summarised in Table 41. There were 432/497 (86.9%) responses to the open survey questions (see Chapter 7 for the survey questions).
| Participants | No. interviewed | Totals and format |
|---|---|---|
| Mother-and-baby groups: co-applicants: | Participants n = 12, focus groupsa n = 3, face-to-face interviews n = 2 | |
| Aberdeenshire | n = 6 | |
| Blackpool | n = 6 | |
| Pregnant women and recent parents:b | Participants n = 88, focus groupsa n = 8, face-to-face interviews n = 39, telephone interviews n = 6 | |
| Pregnant women | n = 38c | |
| Postnatal women | n = 45 | |
| Partners | n = 5 | |
| Providers:d | Participants n = 53 Focus groupsa n = 10 Face-to-face interviews n = 13 Telephone interviews n = 6 |
|
| Midwives | n = 11 | |
| Nurse | n = 1 | |
| Health visitors | n = 12 | |
| Doctors: paediatricians, obstetricians, GPs | n = 5 | |
| Public health staff | n = 3 | |
| Smoking cessation specialists/staff | n = 11 | |
| Voluntary sector/children’s centre staff | n = 2 | |
| Pharmacists | n = 7 | |
| Incentive scheme administrator | n = 1 | |
| Experts and decision-makers | n = 24 | Participants n = 24, focus groupsa n = 4, face-to-face interviews n = 3, telephone interviews n = 7 |
| Public health, maternal and infant health conferences | Range of participants per session involving policy-makers, decision-makers, experts and some practitioners | Participants n = ≈63, interactive recorded group discussions at conferences n = 3 |
| Characteristic | Women/partners | Not recorded |
|---|---|---|
| Ethnicity | 78 (88.6%) white, 9 (10.2%) black and minority ethnic | 1 (1.1%) |
| Marital status | 68 (77.3%) married, 18 (20.5%) divorced/single | 2 (2.3%) |
| Currently employed | 43 (48.9%) employed, 40 (45.5%) unemployed | 5 (5.7%) |
| Smoking status | 26 (29.5%) never smoked, 24 (27.3%) currently smoking, 37 (42.0%) previously quit | 1 (1.1%) |
| Previous infant feeding behaviours (n = 58)a | 51 (87.9%) previous experience of breastfeeding, 4 (6.9%) used formula only | 3 (5.2%) |
| Current infant feeding intentions (n = 18)a | 11 (61.1%) planned to breastfeed, 4 (22.2%) planned to mixed feed, 3 (16.7%) planned to formula feed | – |
Initially, problems were encountered in the Aberdeenshire area in relation to recruitment. There appeared to be some resistance to the study by gatekeepers and thus recruiting both service users and health professionals proved difficult. Higher-level managers were supportive and this was cascaded down, for instance by facilitating meetings with middle management; however; it was largely unsuccessful. For example, on one occasion, a focus group was organised for local health visitors, during work time, on site at their office base. Despite being told to expect between six and eight participants, nobody attended. Nevertheless, by engaging a wider network of champions and through chance encounters (especially two made through the mother-and-baby group co-applicant in Aberdeen), both women and health professional participants in the Aberdeenshire area were able to be recruited to quota. In Lancashire, where there has been an incentive culture, no problems were encountered.
Overview of the findings
First we present a logic model that emerged from the data analysis and which addresses the first two research questions concerning the mechanisms of action of incentives and how they interact with the environmental, organisational, social and cultural facilitators of and barriers to behaviour change. Data are then presented to support the logic model. How incentive programmes might fit with everyday lives is therefore considered first, as this was of paramount importance to women. Although the interview topic guides focused on incentives, women’s accounts constantly returned to smoking and breastfeeding within their everyday lives, with the interviewer needing to bring the topic back to incentives. Views about incentive programmes are then presented. Incentive strategies relating to the shortlist (see Table 38) are then considered in depth. Data on incentives contingent on verified outcomes, incentivising preparatory behaviours and for multiple health behaviours are presented. A typology of potential incentive components and their meanings to women is then detailed as well as the psychosocial and BCT intervention components that women felt should accompany incentive components. Other programme components in relation to goal-setting, monitoring and proof, help and support, and information and education are presented. This is followed by the context and delivery processes that are important for informing incentive programmes and trial design, for example the setting, who provides the programme and whether incentives should be targeted or universal. Providing incentives beyond the individual to partners, buddies, communities and providers of care is discussed, following which the mode, timing and intensity of delivery are presented. The final sections consider who pays for incentives, the unintended consequences and views on the conduct of research into incentives.
An incentive ‘ladder’ logic model for behaviour change
The research team considered the interactions and fit between women’s lives, behaviour change, incentives and other intervention components in a programme and how to represent the complex interactions that emerged through data analysis. After careful consideration of other symbolic challenges, such as mountains to climb and pathways to navigate, and through informal conversations with our mother-and-baby group co-applicant collaborators, the team identified resonance between a ‘ladder’ as a metaphorical concept and the ‘rungs’ as what an individual woman might need to achieve the behaviour outcome(s). Ladders are universally comprehensible and accessible, whereas climbing a mountain, for example, would be unfamiliar to city residents without resources for transport. The ladder concept is represented diagrammatically in an incentive logic model (Figure 18) and summarised in Table 42. The logic model incorporates and builds on the four categories detailed in the taxonomy of IRBCTs (see Table 2): type/awareness, content, contingency target and actor. The taxonomy of IRBCTs is derived from a general taxonomy of BCTs,17 which are included as other intervention components.
FIGURE 18.
Incentive ladder logic model: two interacting metaphorical ‘ladders’ with complete, missing or broken rungs are joined by a platform representing sustained behaviour change.

| Metaphor | Represents | Examples |
|---|---|---|
| Ladder | A behaviour change process, which includes life course and context rungs and incentive programme rungs | Even though a woman may be engaging in a behaviour change incentive programme, smoking and infant feeding are socially located and influenced |
| Life course and context rungs | Something/someone/a situation in the woman’s life that helps/facilitates or motivates her to climb the ladder and supports her at each step | Rungs may be a woman’s desire and willpower to change, positive health decisions influenced by pregnancy/birth events, routine maternity service care, social network/partner/family friends, the environment/place/community/media or a combination of these |
| Damaged rungs | Intrinsic or extrinsic barriers or demotivators that a woman may encounter | A rung might break when a woman and her partner both quit smoking but the partner relapses, thus weakening social support |
| Missing rungs | Lack of contemplation of the behaviour, independence from or rejection of rungs | A rung might be missing, for example when no one in the immediate social network has breastfed or a woman does not believe the health evidence |
| Incentive/reward programme rungs | Incentive/reward component(s), contingency and verification of the behaviour, other intervention components, programme processes and modes of delivery | An intervention that includes an incentive and other components, for example CO monitoring, BCTs such as goal-setting, support, feedback |
The configuration of rungs in each woman’s ladder can differ considerably. The rungs represent intrinsic and/or extrinsic influences as well as programme components in the ladder to achieve the behaviour outcome(s). However, the ways in which these motivators/facilitators/components fit together might be flawed from the woman’s perspective, or some rungs might compensate for others or need to be replaced or (re)introduced. Broken or missing rungs represent something/someone/a situation that impedes, reverses or destabilises, or is a barrier to taking another step towards behaviour change.
An incentive might be one or more rung(s) among several on a ladder. These rungs might appear in conjunction with other rungs, such as an additional psychosocial intervention or BCT components to bolster the rungs. Although an incentive programme may offer a standard package of rungs, each woman’s ladder will vary in how complete, broken or damaged it is, and the condition of the ladder can change very quickly and unpredictably, particularly for those with more chaotic or disadvantaged lives.
The length of the ladder will vary for each programme and for the behaviour change process that each woman will go through. From the literature reviews, a woman’s journey through an intervention programme (see Chapter 3) may involve relatively short ladders with few rungs (intervention components or processes) provided over a short time period, for example the trials by Chamberlain and colleagues103 or Hayes and colleagues,172 or longer ladders with many closely spaced rungs to start with and then longer spaces between rungs, for example the trials by Heil and colleagues105 or Higgins and colleagues. 144 Similarly, ladder length can be determined by when the final outcome or goal is set. Goals may be relatively short term or longer term, requiring more effort, for example maintaining smoking cessation until the end of pregnancy or until 4 months after birth or breastfeeding for 6 months compared with 6 weeks. In contrast to a drug trial, in which the drug is all that is needed for the change in outcome, our data suggest that an incentive in isolation will seldom be sufficient to maintain behaviour change, a finding supported by the lifestyle behaviour change literature reviewed in Chapter 5. Instead, a combination of rungs is required to support a woman’s journey. Some women require very few or no intervention component rungs to change their behaviour as they have sufficient rungs within their own intrinsic (e.g. motivation, confidence, resilience) or extrinsic (e.g. support from partner, family and friends) resources. For example, one woman in a co-applicant mother-and-baby group meeting to discuss ‘ladders’ reported that she stopped smoking immediately when she developed chest pains and no additional help was required, whereas another woman said that ‘the ladder keeps getting longer and longer’. Pregnancy is a sufficient trigger and motivator for maintaining behaviour change for some, who as a result may never need to engage in an intervention programme. Others require many and frequent intervention programme rungs to succeed. Some women choose to restart smoking or stop breastfeeding and leave the ladder; however, some programmes allow for behaviour relapse by resetting the value of the incentive to a lower level and by setting a new quit date (e.g. studies by Heil and colleagues105 and Higgins and colleagues144) and women may therefore be afforded a ‘second chance’ or repaired rung(s).
In interviews, perspectives on which outcomes should be incentivised were considered and are represented at the top of the ladder model (see Figure 18). When discussing the shortlist of incentive strategies, the outcomes chosen were smoking cessation for the duration of pregnancy and breastfeeding for 6 months after birth, which are used in the surveys (see Chapter 7). Preparatory behaviours were included in the systematic reviews (see Chapter 3). Preparatory behaviours discussed included attending educational or support sessions or groups; cutting down on the number of cigarettes smoked; and changing the performance of the behaviour such as ensuring a smoke-free home and using a breast pump.
In the logic model, maintenance is when a level of ideal, desired or suboptimal behaviour occurs and is sustained. Suboptimal behaviour change is important to some women, for example only social intermittent smoking or non-exclusive breastfeeding to allow expression of pre-pregnancy social identity. This allows a woman to engage in behaviour change on terms that seem appropriate to her but which may fall short of achieving the ideal behaviour (no smoking and exclusive breastfeeding) and ultimately optimal health and well-being.
The data to support the incentive ladder logic model are now presented, starting with the everyday life rungs on the left side of the ladder. The following themes were identified: intrinsic and extrinsic influences within everyday life; pregnancy and birth: routine care and opportunities; and the private–public interface. These fit closely with the themes described in the literature review of the barriers to and facilitators of smoking cessation in pregnancy and breastfeeding (see Chapter 4).
Everyday life: smoking cessation in pregnancy, breastfeeding and incentive intervention programmes
Intrinsic influences
A broad typology emerged for the intrinsic influences on engagement with an incentive programme: ‘give it a try’, ‘non-contemplators’ and ‘do-it-aloners’. For some women who smoke, no intervention programme is considered necessary. These women can be typified as ‘non-contemplators’ or ‘do-it-alone quitters’. Pregnancy could act as a strong influence – ‘I just stopped straight away, willpower’ – and the do-it-alone quitters did not express the need for any extrinsic influence to make the effort and persevere. However, many of these women acknowledged that they were likely to relapse and that an incentive programme might help them to maintain their quit smoking status.
‘Non-contemplators’ have no intention of giving up smoking during their pregnancy because they are not intrinsically motivated to quit and smoking is central to their identity, often in spite of feeling embarrassed or conscious of the potential risks:
But I didn’t quit smoking through mine, because I just didn’t feel that my baby . . . because everything was perfect in all the scans and that, all the antenatals and stuff like that. I just didn’t feel that it was having an effect on him. If they’d have told me that he was too small or something, then that would have gave me more reason to quit, but nothing ever came up like that, so that just didn’t give me a reason to quit. Obviously having a baby is a good enough reason to quit but I don’t know.
FG3, mothers
Women could find it difficult to articulate the reason for not considering stopping smoking, as illustrated by the ‘I don’t know’ at the end of the previous quote. Our interpretation is that the centrality of smoking to some women’s everyday lives was a significant barrier to either their thinking about engaging in a programme or the perceived potential for a successful outcome.
Smoking might be couched in different ways: some women described their smoking passively, as a habit or addiction, whereas others looked at it as enjoyable or as a treat that they actively earned:
I roll my own fags and all the rest of it so by the time pay day comes on a Tuesday, well it used to come on a Tuesday, my reward instead of going out and buying an item of clothing, I’d go and buy my fags. I’ve paid all my bills, everything is done, cleaning and I’m buying a packet of fags.
FG8, I, mothers
For these women, engaging in a programme is not on their agenda and thus, regardless of content, it would not be helpful, particularly if it is likely to increase feelings of fear and anxiety. There were accounts of how smoking provides relief and how drawing too much attention to it might exacerbate the behaviour. Being made aware of the health risks of smoking in some cases actually resulted in an increase in women’s smoking, as discussed in Information and education.
Being confronted with personal experience of the effects of smoking on the baby could influence a woman’s reflections on her behaviour and result in her deciding to ‘give it a try’. For one, at the worst extreme, a personal experience of losing a baby motivated contemplation of change:
’Cos I had a wee baby, so I did, and he was born asleep and I was smoking through that pregnancy, so I just didn’t want to take any risks in this pregnancy. So I just wanted to stop.
30, I, pregnant woman
As with smoking, women’s deep-seated feelings about and experiences of breastfeeding affect their infant feeding intention and actual behaviour and, for some, a programme was not considered desirable or necessary. Often the infant feeding choice had already been made during pregnancy: ‘you decide when you are pregnant if you want to do that kind of thing or not’. Non-contemplation could be because of lifestyle implications – ‘I sat there and I thought, why would I want to breastfeed when I can’t go out?’ – or because the idea of breastfeeding is abhorrent or has other connotations:
I think it is a personal choice, some people don’t feel comfortable with that sort of closeness, it is a sexual area in some people’s views and they don’t want their child on it.
FG8, I, mothers and liaison worker for young mums
However, as with quitting smoking, some women have a ‘do-it-alone’ attitude and they already want to try because they are intrinsically motivated towards breastfeeding and they assume that they will do it. Even though some women talked about breastfeeding coming ‘naturally’, this was not always their expectation and many were aware of potential difficulties: ‘you never know until you start’ and ‘you think “boob”, but it’s not always the case’. They questioned how they could be supported by an incentive programme should they go on to encounter difficulties when they actually start breastfeeding because they believe that what is important is their own intrinsic motivation and resilience and that, ultimately, it is down to them: ‘If it goes wrong, well you’re on your own. You are’.
Many acknowledged, however, that their own intrinsic motivation might be affected by their infant’s behaviour and how that is interpreted, particularly the effort/learning required on both sides and the extent to which infant feeding decisions appear reversible or irreversible.
Personal history of infant feeding experiences was very important for those with children. There is less opportunity for women to even consider breastfeeding when they have previously experienced adverse events that may be too strong a barrier for any programme to overcome, such as intense pain or perceived failure because of insufficient milk or latching problems.
Extrinsic influences
Many women expressed concern that other influences in their lives, beyond their own internal smoking and breastfeeding identities, would affect their ability to give up smoking or try breastfeeding. When they did not fit the ‘non-contemplator’ or ‘do-it-aloner’ categories above, and sometimes when they did, many women talked about the magnitude of extrinsic influences. In particular, concerns were expressed around interpersonal partner and family relationships, home life situations and wider norms and support available within their immediate network. When considered as rungs on the metaphorical ladder, women’s accounts of these extrinsic influences could be interpreted as fixed, damaged or missing for either positive or negative influences.
For smoking, these might include a partner ‘hating’ smoking, thus encouraging women to consider quitting when they did not necessarily have intrinsic motivation. However, this rung might be damaged or missing if a partner is unsupportive or is unwilling to consider the harmful effects of smoking in pregnancy:
My partner smokes as well so with him being round about me and even just the smell, it was really annoying.
37, I, pregnant mother
Their dad, once the oldest was born, he wouldn’t smoke in the house any more, he had that much intelligence, but he didn’t see any harm to the child smoking when I was pregnant and caused quite a few problems in the house.
16, mother
The rung could be damaged or missing because the relationship is pivoted on smoking as a couple and enjoying time together sharing the experience. Therefore, ‘partner-dependent’ women may have to negotiate their relationships with both smoking and their partner, with consequences for home life and their environment. For example, on introducing house rules:
When the baby gets here there will have to be changes put in place ‘cos there will be no smoking in the house at all, so they can be going out in the snow and smoking.
31, I, pregnant mother
Further complications exist when, as many women pointed out, they are surrounded by ‘no harm narratives’. Family and friends who have shared histories of smoking around pregnancy tell anecdotes of smoking having little or no effect on pregnancy, child health or development. Women’s mothers might have smoked and thus there is a norm within their upbringing, with their own reference being that smoking is not a problem as they themselves are healthy and well; therefore, the rung is missing. Even if a woman came across as intrinsically motivated, extrinsic influences, such as experience and prevalence of smoking in her family and social network, could be interpreted as potential barriers: damaged or missing rungs. Such women did not have support readily available and remained located in a culture of smoking, which is problematic even when they are amenable to the idea of giving up or joining an incentive programme:
Because although my partner, he doesn’t smoke, like being round his mum and stuff they all smoke so it’s really hard being round them a lot.
24, pregnant mother
Therefore, our interpretation of the data is that many women would need additional facilitative rungs to support them in individual or collective behaviour change and to enable barriers in family and social environments to be addressed and overcome. New incentive strategies would need to facilitate change regarding women’s situations at home and with partners/family and friends. These are considered later in Incentivising beyond the individual within social networks and communities. Strategies suggested outside of a formal programme included joint quitting, which could be motivated by the social norm of ‘being a good parent’ and ‘a good extended family’ in relation to smoking:
It’s as important for them to stop as it is you, you are setting an example at the end of the day, one of you smoking isn’t . . . you know, isn’t going to look very good at all either, so it is best that everybody stops.
T4, I, mother
Another strategy was associating smoking with a non-health-related outcome (such as smell) or even adding prompts to the environment in an attempt to minimise the negative implications of smoking:
All it took to stop was my other half went I don’t like you doing that. Do you mind stopping, it makes you smell and . . . I stopped, much to mum’s surprise because she went through hell trying to give up when I was a baby so when I said oh I have stopped smoking, she was like ‘how did you did it’, I was like . . . I just stopped, you know.
5, pregnant woman
My partner smokes, so he took to smoking in the garden and we have got some alcohol rub at the back door and things like that so . . . there is steps that we have took as we heard that it stays on your clothing and things like that, we heard that off television and campaigns and things, so I suppose in that sense, that kind of shaped us a bit.
T9, I, mother
Beyond the immediate family, women also considered that changing their social networks to minimise temptation and break social behaviour habits was perhaps necessary – ‘You need to find a social group that doesn’t smoke’ – or they responded to media campaigns either individually or as a group strategy:
My lodger, who is also sort of, a good friend – he smokes. I’ve always had a rule of no smoking in the house – always. So, wherever I’ve lived it’s always been a rule. So he always smokes outside anyway and my partner he smoked up until he done the Stoptober so he’s not smoked since the 1 October. So I’m quite pleased about that.
44, I, pregnant woman
Equally, for breastfeeding, personal and vicarious histories of the behaviour within women’s family and social networks were reported to be a strong influence. For some, it is a given, a norm, because they were breastfed themselves or peers do. If women want to try breastfeeding after birth then a need to be supported by others was expressed, particularly when equal parenting and the father’s role was an issue:
It’s the mother that’s going through the difficult time but then is the father going through a difficult time because they quite often feel cut out and women will say, ‘Well, I want him to have a more active role as a parent’ and that would be their reason for stopping breastfeeding.
T51, I, lead health trainer – smoking cessation
Therefore, women expressed a need for flexibility around feeding, reported feeling pressurised and assumed that formula feeding would provide an ideal solution. This was not always the case, however, and, as with smoking, many women weighed up the health risks and benefits of feeding options and reported being influenced particularly by the tangible and comparative experiences of others:
All my friends and family and people that have had breastfed babies and people who have formula fed, you know, they seem to be a pattern of formula fed babies are ill and pick up more bugs and they have got more colds and things more than breastfed babies. My child is never ever ill . . . I think it gives them a big advantage.
T4, I, mother
Nevertheless, for women to continue to provide breast milk for the recommended 6 months, many felt that they needed more flexible strategies, which any incentive programme would need to fit with. Some who disliked the idea of feeding from the breast suggested that they might consider providing breast milk using a pump: ‘I didn’t want that bond and it was just that I didn’t want that connection with him’. Conversely, for many women, feeding from the breast was highly valued and some described a ‘lovely’ bond developing and ‘enjoying it really’, especially ‘when nobody else bothered us’. Expressing breast milk was often important for women who wanted the infant’s father or other family members to have an active feeding role.
Pregnancy and birth: routine care and opportunities
Many women already knew the risks of smoking in pregnancy and the benefits of breastfeeding from their routine pregnancy care, and this triggered reflections on these behaviours. Some women felt that the health service was ‘pushing it’ and, although opportunities for helping women to quit smoking and try breastfeeding were described, many women resisted additional pressure, particularly if the pregnancy was unplanned. Likewise, many health professionals reported being reluctant to compromise their relationships with women, especially those they perceived as vulnerable. Nevertheless, there were accounts of non-pressurising encounters from women. In fact, some women welcomed the confrontation or felt that more should have been said. Some women were actively looking for a trigger or a switch (a rung) to change their behaviour but felt that this would come from within and not through any other influences that they could articulate:
So I thought well it [referral to a smoking cessation advisor] can’t do any harm, you never know she might say something to me that might make me you know that wee switch in your head that needs to go, because as far as I am concerned it doesn’t matter how much help you are given it’s like an alcoholic, you’ve got to hit rock bottom before you can lift yourself up. You’ve got to get to that point where you make that decision. No amount of scary pictures of black lungs or tubs of what looks like Marmite are going to put you off because people witness the side effects of smoking every day, they hear about it, somebody’s got cancer because of it, somebody has got bronchitis because of it . . . It’s got to be if and when that little switch in your own head says it’s time now. Nothing else is going to affect that.
28, I, pregnant mother
However, some had negative experiences of the type of help and support that is available, particularly if it was not tailored to pregnant women:
I’m biased with these stop smoking while you’re pregnant things ‘cos I’ve tried it and I went to the cessation classes and it just, it did n’a work and I really wanted to, but the classes . . . unless they were designed specifically for pregnant people, ‘cos mine’s was a case of you got put in amongst everybody else ken like for whatever reasons they wanted to stop and they were all getting champix and all sorts of things and I got chewing gum.
FG4, mothers
For breastfeeding, there were examples of too little help or intervention, particularly in the early days:
The neonatal doctors stand up and say, ‘No, you can’t put them home, they’re breastfeeding’, which is what happened with me, but the girl who was in the room next to me she was trying to breastfeed but because she hadn’t had it established the ward pushed her out and the doctors hadn’t been able to back her up sufficiently to prevent it happening . . . She was devastated, she was so upset because it was the one thing she really wanted to be able to do and she couldn’t do it in the end because they said, ‘No, you’re going home’.
FG6, mothers
In the early stages breastfeeding is a skill that is not considered ‘easy’ to acquire and so health professional help and support was considered to be more important than an incentive programme. Participants highlighted that the balance must be right. Communication of this help and support was considered crucial either within or outside a formal programme, especially when women feel that they are being ‘forced’ or being judged for ‘not doing right by your baby’ if they do not breastfeed, for example if health professionals use unhelpful comparisons:
One of them said feeding your baby a bottle of milk is as bad as giving your baby a McDonalds – it’s not as bad as, it’s not as good as breast milk but it’s not as bad as giving a McDonalds.
FG2, mothers
Private–public interface
What happens outside the home, immediate environment and social circle also influenced women’s behaviours in pregnancy and after birth. In pregnancy, women reported feeling ashamed of smoking because their bump is visible and how smoking attracts adverse attention and causes embarrassment. These feelings, which they did not like, could help them to stay quit. Some women retrospectively considered it in these terms: ‘it looks horrible, more than I am thinking for health reasons, but yeah, I wouldn’t do it again’. In contrast, such perceived judgements sometimes caused women to feel more stressed and to resort to smoking (see Unintended consequences) or to otherwise justify their behaviour:
Because I’ve got a bit of a bump do you know what I mean a lot of people give me really dirty looks as if, then I think to myself I could be doing a lot worse, I could be taking drugs, I could be drinking, I could be doing so much more than just having a fag.
43, I, pregnant woman
The physicality of the pregnancy is a reminder to women that they should not be smoking or that they should try to quit. Once the baby is born, however, prioritising staying smoke free presented new challenges: ‘when I am socialising, baby is not there, its different, your inhibitions are down’. Women associated smoking with socialising or working routines and any change of smoking behaviour during pregnancy could be jeopardised. Therefore, any incentive programme would need to take account of the interface between women’s private and their public lives.
Women often struggled with breastfeeding in public or in front of people as they built motherhood into their daily lives. In particular, embarrassment was described as a significant barrier and thus it is something that any programme would need to address. Examples suggested were showing women how and where they can comfortably feed so that they can plan and devise strategies that fit their lifestyles:
I never ever felt totally secure when we were out and about because all it took was him to throw a hand or something like that, the towel has fallen and I’m exposed. It wasn’t until you said that it’d changed and I actually saw you breastfeeding in public that I thought, ‘Nobody ever showed me that I could just do it that way’ and it’s so discreet, just lift your top a little and you can sit no problem.
FG6, mothers
Well yeah, it’s just knowing where you can go and breastfeed without people staring and throwing comments and whatever, because you know how people can be.
T10, pregnant mother
Nevertheless, for these women, the realities of feeding in front of family/friends or in public, for example at a family wedding, mean that they must confront and negotiate daily practical challenges.
Incentive intervention programmes
In the following sections we consider the rungs of the ladder on the right-hand side of the incentive ladder logic model (see Figure 18). General views about incentive intervention programmes are summarised first. The incentive and associated intervention components are considered in depth, followed by rungs relating to the delivery relevant to the shortlist of incentive strategies described earlier (see Table 38).
Attitudes towards incentives were varied and ambivalent. Women seemed less worried about or resistant to incentives than providers, but were also less convinced about their potential fit with real life as they had very limited experiences of success in relation to achieving the target behaviours. Conversely, providers were more cautious about the appropriateness of incentives. They were equally concerned about effectiveness and financial value, but if incentives could be proven to be more effective and cost-effective than other strategies would be open to using them. Financial incentives for smoking cessation in pregnancy seemed more acceptable than those for breastfeeding, except in the case of the provision of a breast pump. Very few participants voiced any concerns around providing breast pumps as an incentive for breastfeeding or expressing breast milk:
Once you have got past those initial weeks you are saving money by not smoking and you start to feel a bit better and feel quite self-righteous and the health benefits but carrying on breastfeeding in a way has the opposite effect because it’s time consuming, it limits what you can do, where you can go, who can manage the baby so it’s a funny mix match in a way that makes it quite difficult to think to incentivise because its two quite opposite things in terms of feeling good about adhering to the change, so smoking yes, people would feel good about it, but breastfeeding, for a lot of these mothers they will be tired, they actually feel worse by continuing because of the amount of effort that you have to put in, so it’s a bit of a strange thing.
62, ethics committee member
Women’s views about incentive programmes
Some women considered incentives to be the wrong way to go about improving health outcomes and thought that the health benefits should be enough to encourage change:
I think they should be doing it for the right reasons, for their health and if they want to stop smoking because you want to be healthy and things, that’s fine. Don’t just do it because you’re going to get money and things out of it. I don’t agree with that.
24, pregnant mother
In particular, some felt that knowing that a behaviour was ‘wrong’ was an incentive in itself: ‘as adults we should know the rights from wrongs, so themselves should be an incentive’.
Financial incentives were met with mixed opinions, from ‘no one should get paid for stopping smoking’ to ‘that’s a good idea’:
‘Well done, there is a £10.00 voucher for Boots’, I think it’s a bit, a bit patronising as well, a bit childlike, like at school, so I think that is probably more for kids.
12, pregnant mother
Many women relayed how ‘appropriate’ incentives could enhance and encourage motivation. Incentives enabled the process of change to be a ‘nicer experience’; they provided something to ‘look forward to’, ‘recognition’ and ‘acknowledgement’ of their success:
I suppose it’s not about necessarily having £50.00 or the £100.00 or whatever, it’s about the recognition that you’ve done something, that you’ve achieved something, it’s not, it could have been anything I suppose.
27, I, pregnant woman
However, even when women seemed to favour the idea of incentives, or were not averse to them, they did tend to express concern about how they might work and were often quite convinced that they would not. For some, money didn’t matter:
You should quit yourself without the incentive because that’s what you should do.
2, I, mother
Because at the end of the day if you are going to succeed you’ll succeed whether somebody hands you a Love to Shop voucher or not, if you are going to fail then you’re going to have a cigarette whether or not somebody’s going to give you a voucher or not.
28, I, pregnant mother
Nevertheless, the issue of altruism for improving health and social culture seemed to be a strong one for the use of incentives:
I know people go ‘oh the tax payers money’ and all that, I wouldn’t mind my money being used towards incentives for, you know smoking and things like that because I can imagine the positive effects from other things, such as the health service, and just socially as well, you know the health benefits because I find it quite an antisocial habit.
5, pregnant woman
Providers’ views about incentive programmes
Improving health and social culture was a much stronger theme among providers and experts. Even when there was ambivalence, respondents felt that, if incentives could help, then they should be used, as it would be unethical not to explore all approaches to improving smoking cessation in pregnancy and breastfeeding outcomes. If incentives were shown to be financially more effective than existing strategies or if respondents were greatly concerned about health effects then they were willing to ‘try anything’:
My gut instinct is incentives are wrong, but as you say we’ve got such an issue and we have to do something and whatever we end up doing . . . But if you try a reward scheme, and even if it seems quite unpalatable and it works, then the justification is right there.
FG9, I, experts
But I think, moralistically, I would be opposed but arithmetically I would support it because it appears to make . . .
From the smoking point of view obviously my biggest issue is obviously the babies because those babies – so I would agree to it with the smoking side of things because those babies – and even with the breastfeeding as well – but those babies are being put in a vulnerable situation, aren’t they? If we don’t do anything to stop those women smoking and they’re going to suffer long term with ill health, etc. and going to end up on the neonatal unit probably and have long-term health problems. So I do think we need to do something and that’s where probably why I agree – it’s difficult to say.
FG12, providers and expert
Some providers were opposed to incentives altogether as they considered them to be unethical or inappropriate as an approach: ‘not just wrong, I’d say morally wrong’:
I think we’re going right down the wrong route; I think that, you know, when we’re enticing people with money and gifts just to do what’s right for their health, you know. What else will we expect?
53, midwife
Others, by contrast, were concerned that incentives might ‘crowd out’ self-motivation, which they perceived as having an opposite effect, demotivating and demoralising people. At one extreme, one participant commented that: ‘it’s almost like prostituting yourself in some way for incentives’. Providers also recognised the importance of the challenges faced by some of the more disadvantaged women, as described earlier, which present barriers to behaviour change:
Efforts could possibly be better addressed – best spent – by addressing their circumstance rather than rewarding them for doing something they should be doing anyway.
FG12, providers and expert
Incentives contingent on verified outcomes
Although a few participants felt that unpredictable gifts, for example the raffle within the study by Gulliver and colleagues,104 were acceptable as ‘everyone has a chance’, the majority opinion was that guaranteed incentive provision was needed for ongoing commitment and motivation. However, raffles (alongside the provision of guaranteed incentives) were considered to have a wider reach beyond the individual, by turning them into events with publicity:
It [raffle] could be on top of. I mean that would be a nice piece of publicity. If you have a smaller thing and entry into, and the person who wins is then a publicity machine aren’t they?
FG9, I, experts
However, several participants considered that incentives should be given as rewards only after the target behaviour had been achieved and verified (see Monitoring, proof and feedback). It appeared important to not reward deviant behaviour because of how it would be internalised by those who had succeeded, as well as by those who had not:
Yeah, if I was going to go to something and thought, ‘It’s nice to go but I’m not going to do anything about it’, it’d probably make me feel worse . . . Because then I’d think I should be giving up.
1, pregnant mother
Incentives for preparatory behaviours
Although women’s and providers’ views supported incentives for complete and verified smoking cessation in pregnancy and/or exclusive breastfeeding, some preferred less idealistic goals. If stopping smoking altogether did not seem possible, some women preferred to be encouraged to cut down and thought that they would feel better for it. Cutting down was favoured as a strategy for engaging women in a programme as a first stage in quitting. Nevertheless, many were sceptical about incentives for cutting down smoking and thought that they would not succeed unless it came from within, as an intrinsic influence rung on the ladder:
Then to get pregnant and cut down and then knowing you’ve got a limit to smoke a day. Sometimes I can go over it; sometimes I cannot want a fag do you know what I mean. Sometimes I can light up a cigarette and then put it out and go no I don’t want this it is making me feel sick. But I wish that had happened at the start, I wish that when I lit up a cigarette I would be sick or something so I wouldn’t need to smoke.
43, I, pregnant woman
Similarly for breastfeeding, mixed feeding or breastfeeding for a shorter duration than the recommended 6 months was a preferred option for some women. These options were discussed by women in relation to the father’s role and expressing milk using a breast pump.
Incentives contingent on participation in services were believed to be important to encourage attendance for information and support, provided by health professionals, peers or community organisations, and to prevent women ‘dropping off the system’. Such incentives were generally perceived to be acceptable, with reference made to successful programmes where lunch was provided or opportunities to purchase cost price breastfeeding bras at antenatal workshops. Incentives for programme participation were considered important by many as they can ‘hook people in’, particularly those who are ‘undecided’, and enable ‘meaningful discussions’ and ‘a proper conversation’ to take place that could help to change women’s opinions about smoking cessation or breastfeeding:
I think, for me, it was about getting me into that first appointment. If you can get people into that first appointment and have someone like [smoking cessation adviser] talking you through it, for me that was where the real success was, because I came out of there like that, I need to do this.
33, I, pregnant woman
Other participants, in line with the Gulliver and colleagues’104 vignette (see Appendix 2), believed that the ongoing incentives for those who ‘turned up’ for support, irrespective of their smoking status, were justified as it was indicative of them ‘trying’ and their ‘willpower’ to change. Many believed, however, that the value of the incentive for those who had not succeeded should be ‘less’ than the value of the incentive for those who had, with ‘unpredictable’ (e.g. entry into a raffle) incentives identified as more palatable on these occasions:
I think if they are still attending the meetings, they are still trying, they are still wanting to stop, but haven’t achieved it yet, so they are still trying, so yes I think they should get the chance of the raffle.
16, mother
Incentives for more than one behaviour
In some women’s accounts, spontaneous comparisons were made between more than one lifestyle behaviour, particularly in relation to behaviour changes at different stages of pregnancy and childbirth:
Because I am still breastfeeding so I am not drinking again, but I have often thought, I wonder if I will smoke again when I do drink.
T9, I, mother
Participants were asked their views on the feasibility and acceptability of incentivising more than one behaviour, for example smoking cessation and breastfeeding, concurrently. Some women queried how many health behaviours might be incentivised at once and anticipated ‘lots of do’s and don’ts’. The contrast between the two behaviours was noted:
I think there is quite a difference between breastfeeding and smoking cessation, and I don’t know whether they can actually be aligned . . . I think the comparison between breastfeeding and smoking, one’s an addiction, very negative. Breastfeeding is not an addiction and it’s a very positive aspect.
FG9, I, experts
At co-applicant mother-and-baby group meetings arranged to discuss the incentive ladder model, healthy eating was seen as related to overall health and health behaviour change/healthy choices: women described not being able to afford fruit and vegetables for larger families, saying that biscuits are so cheap and filling by comparison. These women suggested that an incentive related to healthy eating would inspire them to ‘overhaul’ more than one behaviour and help allay fears about gaining weight when stopping smoking.
Some service providers pointed out that habit change is demanding and suggested that expecting the adoption of more than one new behaviour at once could be ‘quite a lot to take on’:
You know, you’ve got to look at your woman; what can she cope with? Sometimes you’ve just got to tackle, you know, a little bit. And as long as she’s doing that little thing. You know, not too much. I mean, we all know when you’ve got too much going on you’re just going to fail, aren’t you?
53, hospital midwife
Conversely, some service providers stated that they were ‘already attempting to address multiple habits’ anyway and felt that these attempts ‘probably would be more successful with incentives’. It was also suggested that change in one behaviour may motivate women to attempt to change another or to engage in an incentive programme for another:
If the incentive is going to work, then yes, I think it is, they are not going to think, ‘Well I’ll do it for that one and not for that one’, I think they are more likely to do it, you know if they are going to do it, they would do it across the board.
52, specialist midwife
Some service providers noted the relationship between smoking cessation and breastfeeding: ‘But then if you address the smoking then they’re more liable to go into breastfeeding’:
If you were concentrating on smoking and you had an incentive you could bring that through postnatally, and you know, sort of change it to the breastfeeding as well.
T59, midwife
However, some service providers felt that incentivising smoking cessation and breastfeeding concurrently would be seen as equating smoking and bottle-feeding, which risked alienating some women:
I just think they’ll find it – and we are comparing, maybe, not breastfeeding to smoking because perhaps they are as harmful, but I think they’ll find that really emotive, almost offensive. I don’t think that’s our problem, but I think that’s how it’ll be perceived by the public.
FG10, provider and experts
Incentive components: meaning and value
The following sections consider the right-hand side of the incentive ladder logic model and the components (rungs) that can be provided as part of an incentive programme. Exploring the meaning, values and types of incentives is important for understanding how they might work. Participants used various terms in addition to ‘incentive’, such as ‘reward’, ‘gift’, ‘bribe’, ‘payment’ or ‘prescription’, with different meanings associated with each. The type of incentive appeared to influence the language used; thus, some women equated shopping vouchers to ‘bribery with money’, in contrast to baby-related or personal well-being-related items, which were more likely to be described as ‘gifts’ or ‘more practical, it’s like wee treats and titbits not bribery with money’. When an incentive was seen as a ‘payment’ to engage in behaviour change – ‘you are getting paid to stop smoking’– this was often viewed negatively, with reservations about ‘giving money to the people to look after their own health’. Some providers queried whether something could be considered an ‘incentive’ if it had not been shown to change behaviour:
I think I’m still troubled by the word ‘incentive’ because it’s bandied around and it’s not an incentive; it’s only a reward or a gesture or a something until you know it works. And because we don’t know if any of these things work until you’ve tried them.
FG9, I, experts
Women were more concerned with talking about what they thought would help them the most as an incentive and often conceptualised incentives outside of our definition, for example behaviour-related items such as nicotine replacements:
I think if people genuinely want to give up smoking then they want to have the things to help them not £20.00 for a shop. If somebody gave me £20.00 I’d go out and buy a pack of cigarettes. There would be no incentive there for me to stop smoking. If somebody gave me an inhalator and some patches and some Nicorettes then it would be an incentive because it’s there in front of me. I think I’d have to be getting support first and then an incentive to carry it on maybe on my own rather than with somebody. So if I had an inhalator and then was doing it on my own with an inhalator.
1, pregnant mother
This quotation suggests a medical model of incentive provision, but also the inhalator as aiding self-management of the behaviour. Offering an incentive on ‘prescription’ as a component of a structured course of treatment was suggested as a strategy that would allow an individually tailored approach and which may be publically acceptable:
If you have incentive prescription it’s possibly more socially acceptable. If that’s the line of thinking that actually this is part of my treatment is to have this, this is not a reward, to the rest of the population it’s not a reward but obviously the individual is going to see it as such.
FG12, providers and expert
Some service providers were concerned that being too ‘narrow’ in the types of incentives offered would be akin to:
Disempowering [service users] because there is almost an insistent judgement in whatever it is that we are giving them.
T58, smoking awareness co-ordinator
This was felt to be particularly disadvantageous for ‘people who don’t necessarily have a lot of choices or options in their lives’. In related observations, providers warned against making assumptions about what types of incentives would be effective, arguing that health professionals and decision-makers did not necessarily have enough insight into the life circumstances of many women to determine what would constitute a motivating incentive:
So whether it’s money or whether it’s a token but it should be for something that they want, not something we think they should have as kind of middle-class professionals.
T63, GP
A typology of incentive categories ordered according to their meanings (Table 43) is discussed in detail below. The categories and their meanings derive from the purpose and function of the incentives and the degree of restriction associated with their use. They range from highly restrictive health-related or behaviour-associated incentives to hedonic incentives such as shopping vouchers or cash where recipients have a high degree of free choice and trust is implied as these have a high resale or exchange value. The issue of who is in control of the incentive – the woman or the provider – is important to consider from the perspective of motivation and empowerment. The categories of incentives were derived through analysis of data from two main sources: the literature on incentive interventions (see Chapters 3 and 5) and spontaneously generated ideas from mother-and-baby group co-applicants and research participants.
| Type | Examples | References |
|---|---|---|
| Vouchers and cash | Restricted, e.g. local shops | Sciacca and colleagues176 |
| Health restriction, e.g. no cigarettes or alcohol | Radley and colleagues (Give it up for Baby)113 | |
| Minimal restriction, e.g. widely redeemable vouchers | Tappin and colleagues (CPIT)88 | |
| No restriction, e.g. cash | Hill;173 Wolfberg and colleagues178 | |
| Maternal well-being | Beauty treatments or products | Sciacca and colleagues;176 Morgan and colleagues;148 Thomson and colleagues112 |
| Baby and pregnancy related | Infant car seat | Gulliver and colleagues;104 Edwards and colleagues142 |
| Nappies | Sciacca and colleagues176 | |
| Behaviour related | Breast pumps | Dungy and colleagues;170 Cohen and Mrtek;169 Sciacca and colleagues;176 Chamberlain and colleagues;103 Bliss and colleagues;167 Hayes and colleagues;172 Bai and colleagues;180 Rasmussen and colleagues174 |
| Nursing bras | Grey literature (see Appendix 10); emergent from interviews | |
| Bedside cots | Emergent from interviews | |
| E-cigarettes and NRT inhalators | Emergent from interviews | |
| Health related | Healthy foods | Finch and Daniel;171 Chiasson and colleagues;181 Thomson and colleagues112 |
| Sports and leisure centre vouchers | Thomson and colleagues112 | |
| Household services | Home help | Pugh and Milligan106 |
| Ironing service | Hoddinott and colleagues62 | |
| Childcare | Pugh and Milligan106 | |
| General utility | Providing refreshments | Albrecht and colleagues;138 Hoddinott and colleagues;62 emergent from interviews |
| Travel vouchers | Bristol incentives scheme (grey literature, see Appendix 10) | |
| Awards and certificates | Congratulations cards | Morgan and colleagues148 |
| Certificates | Emergent from interviews | |
| Experiences and incentives beyond the individual | Family day out | Sciacca and colleagues;176 emergent from interviews |
| Holiday | Emergent from interviews | |
| Infant photograph | Reeves Tuttle and colleagues175 | |
| Partner incentives | Sciacca and colleagues;176 Wolfberg and colleagues178 | |
| Buddy incentives | Donatelle and colleagues141 |
Views were mixed on the optimum financial value of an incentive and how this might influence its effectiveness and the likelihood of gaming (see Unintended consequences). For some, the financial value was less important than the values relating to the meanings, the ‘thoughtfulness’ of a gift and the other helping components of incentive programmes, which are described later in this chapter. Some women who had taken part in the CPIT, in which the value of shopping vouchers given totalled £400.00, viewed this greater value to be more motivating:
I think if I was getting that much I’d be like, ‘I’ll stop smoking and I’ll not go back on it’.
30, I, pregnant woman
But the optimal financial value of an incentive was often related to the recipient’s financial situation, with some family members considering ‘people on low incomes’ more likely to be motivated by lesser amounts ‘because £35.00 is a lot to them’. Others felt that even very large sums of money would be an insufficient incentive if their own motivation was lacking:
Ok would it have helped if it was a bit more money?
No I don’t think so. . . . I think if they’d said to me you’ll get £2000.00 you know what I mean if you stop smoking then it maybe would have pushed me to it but I think in myself, you’d need to want to for yourself to stop smoking, not for money.
38, I, pregnant woman
Vouchers and cash
Although some participants explicitly contrasted shopping vouchers with cash, they were more often seen as equivalent, perhaps because the types of shopping vouchers discussed were so widely redeemable in many retail outlets, as they were in the CPIT and in the effectiveness studies described in Chapter 3. The research team did not introduce the concept of cash in the interview schedules. Our interpretation is that this lack of distinction between shopping vouchers and cash by many underlines the perception that the woman is in control over how the incentive is used and the implicit trust that providers are giving to the woman to use them appropriately. Such unrestricted incentives offer autonomy, particularly to more disadvantaged women who have few opportunities for choice in their lives. They therefore have a value over and above their monetary value, in terms of valuing the woman and the effort that she is applying to positive behaviour change and her sense of well-being and pleasure gained through achievement. Tailor-made incentive programmes that are individualised to women’s needs or their individual motivation were popular with women, in recognition of the fact that ‘the same thing is not going to be useful for everybody’. Incentives such as shopping vouchers could allow women to individually tailor the incentive to maximise their own motivation, with the option to ‘save up’ for an expensive item – ‘Yeah I was thinking: pram. That’s a pram’ – which involves goal-setting, planning and delaying gratification.
Choice was seen to be key to the motivating nature of vouchers, as ‘the money is theirs to spend how they like’ or to ‘treat yourself’. They were seen as a ‘reward’ that enhanced feelings of well-being and were a ‘boost’ to continue:
I was over the moon with it. I was. I was really happy with it and just receiving my wee £100.00 one there, I was really quite chuffed.
33, I, pregnant woman
Women described a variety of items that they had chosen or would choose to buy with incentive vouchers, such as baby items, household goods, clothing and jewellery. However, most women and providers considered an entirely free choice (cash) to be undesirable, as people may ‘squander’ it or buy inappropriate items such as cigarettes or alcohol: ‘if it was cash or anything they’d just end up smoking it’. For this reason, vouchers tended to be preferred to ‘hard cash’.
Although most discussions centred around shopping vouchers that were widely redeemable at national chains, one service provider described an incentive programme that offered women vouchers from local businesses. In this incentive scheme for breastfeeding, local shopping vouchers were thought to be inappropriate as it was felt that women’s circumstances were not considered:
They were all just really expensive companies that you wouldn’t ordinarily use; like top-class hair salons and . . . you just thought: well most people, therefore those incentives aren’t worth anything to them because they can’t use them. It’s only worthwhile to those that are very wealthy.
FG9, I, experts
The ‘immediate and fun’ nature of shopping vouchers was considered important to compensate for the perceived loss of enjoyment arising from behaviour change – what people would be ‘prepared to get in return for not smoking’. However, in addition to views of shopping vouchers as a ‘bonus’, some women saw shopping vouchers as potentially ‘helpful’ for people who are ‘struggling’ financially.
In comparing vouchers with gifts or services, it was suggested that ‘people respond better to cash’ because it is assumed to be more financially valuable, and thus more motivating, ‘in this climate’, referring to the current economic downturn since the 2008 credit crisis.
Although shopping vouchers as incentives for smoking cessation were largely considered acceptable, although with some reservations, vouchers for breastfeeding were not considered as acceptable and were contrasted unfavourably with some of the other categories such as behaviour-related or personal well-being-related incentives.
Maternal well-being incentives
This category of incentives comprised non-utilitarian gifts or services for the women’s own personal benefit, such as beauty products, beauty treatments, massage or magazines. Women noted that ‘everyone talks to you about a baby. It’s baby this, baby that’ and that ‘quite often the mother’s forgotten about’. Women expressed a wish for an incentive that focused attention on the woman herself: something aimed at ‘pampering them and making a fuss’.
These types of incentives were seen as having an effect through enhancing a woman’s emotional well-being – ‘I think it has to be to make you feel good’ – which in turn was thought to increase her capacity to cope with the challenges of new behaviours, acting in effect as a ‘morale booster’. Mothers recounted experiences of stress in the postnatal period and reflected that gifts aimed at promoting personal well-being, such as ‘some nice bath salts or something’, could prompt women to ‘stop and think, “actually, I haven’t really been doing relaxing for a while” ’, and thus encourage them to ‘just take care of yourself for half an hour’. The enjoyment gained from personal gifts was considered motivating: ‘oh I look forward to getting that’. In some cases, small personal gifts were seen by some as more ‘manageable’ than vouchers because they did not require recall, planning or access to retail outlets to be used and enjoyed.
Women felt that the effort that they put into behaviour change deserved recognition and validation: ‘Yeah, I think it has to be for you because you’re the one that’s doing it, no one else is’. In this context, personal gifts were contrasted favourably with some of the other categories of incentives such as baby gifts or household support.
Baby- and pregnancy-related incentives
These incentives specifically relate to pregnancy or the baby and include items such as maternity clothes, bibs, nappies and more expensive baby items such as car seats. Such incentives can be considered utilitarian and the smaller everyday items such as nappies have a low exchange value. Some women felt that incentives should be necessities:
Like a pack of nappies or something that is used basically every day but doesn’t cost the earth because, obviously, if there’s budgets and stuff.
T10, pregnant mother
However, some women and providers felt that better incentives would be ‘niceties, the luxuries people generally can’t afford’, which suggests that the incentive should add value to the woman’s sense of well-being as well as target the desired behaviour.
In discussions of more expensive baby items such as car seats, prompted by the Gulliver and colleagues’104 study vignette (see Appendix 2), many women stated that ‘people prefer to shop around and buy [a car seat] themselves’, and therefore receiving one as an incentive would be superfluous: ‘it is just going to get flogged on eBay, it is quite an expensive bit of equipment that’.
Behaviour-related incentives
These were incentives considered to have a direct functional role in achieving or modifying the target behaviour and were largely discussed in relation to breastfeeding: breast pumps, breastfeeding bras, other breastfeeding clothing and baby carriers. For smoking cessation, some women viewed free NRT devices as a behaviour-related incentive and our mother-and-baby co-applicants suggested e-cigarettes as a behaviour-related incentive. Some women considered free-choice incentives such as shopping vouchers to be inappropriate and thought that incentives should be restricted to items with some utility:
I would agree with that rather than them getting handed money for breastfeeding, giving them something that’s going to be helpful to them rather than say like, ‘Here’s £50.00 to breastfeed your kid’.
24, pregnant mother
Some providers considered that the relative lack of choice was problematic, as a behaviour-related incentive ‘ says you have to buy a breastfeeding bra . . . then I think we are controlling that woman’, indicating a regard for women’s autonomy and a desire to avoid being seen to pressurise women. Others reflected on the undesirable consequences of endorsing the idea that a behaviour-related incentive such as a breast pump is a necessity to breastfeed, which could create barriers for some women ‘because they’ll say they can’t afford them but then they don’t really need them anyway’.
Women who had expressed or who intended to express their milk tended to be enthusiastic about the prospect of receiving a free or reduced-cost breast pump, prompted by discussions of the Chamberlain and colleagues’103 vignette (see Appendix 2), which they viewed as a ‘brilliant’ incentive. A breast pump could help ‘share the load’ of breastfeeding with significant others, who could feed the baby expressed breast milk. The importance of family involvement as a rung to support breastfeeding is discussed earlier (see Extrinsic influences), and breast pumps are an example of how incentives can connect the two ladders in Figure 18. Pumps were seen by some women as preferable to hand expression of breast milk, which was viewed as ‘hard work after the first few days’. Some women believed that having had access to a pump might have prolonged their breastfeeding experience or better enabled them to provide breast milk:
If I’d’ve had a breast pump, I’d’ve carried on breastfeeding, but I didn’t have a breast pump, and it was just too expensive.
FG3, mothers
I was fine if I was going to express into a bottle, which I did, but then the expense of actually buying a breast pump alone was extortionate and I was like, ‘I am not paying that money’.
FG8, I, mothers
For some women, expressing milk appeared to be a middle ground between breastfeeding and formula feeding: ‘if you’ve got one, you’d be more likely to use it than to think, oh I’ll put him on bottles’, and some providers considered that a breast pump would be encouraging for those women who ‘don’t want to put babe to breast’, as discussed in Everyday life: smoking cessation in pregnancy, breastfeeding and incentive intervention programmes.
However, some women were concerned that breast pumps may not be useful for all breastfeeding mothers, as ‘there is nothing simple about expressing’ and many women find them difficult to use. Similarly, it was noted that ‘maybe not everybody wants to use a breast pump’. Therefore, breast pumps given as a universal incentive (rather than just to women who specifically want to use one) were considered potentially wasteful if they ended up being ‘something that gets shoved in the cupboard’ or ‘expensive breast pumps that just end up on eBay or something within a week’. Participants, particularly providers, expressed concerns about the timing of breast pump provision and how that might influence behaviour outcomes, and this is considered further in Timing, communication modes and intensity.
For many women, a major appeal of behaviour-related incentives for breastfeeding was that their direct utility – ‘something that is going to be helpful’ – made them more justifiable and warranted, ‘rather than the incentives just being a treat’: they were ‘not like proper freebies’.
Other behaviour-related incentives were considered to help by removing the barriers to breastfeeding. For example, specialised bedside cots were suggested by some providers as a ‘beneficial’ item that could incentivise through facilitating night-time breastfeeding. Likewise, as many women feel discomfort or embarrassment with breastfeeding outside the home, breastfeeding clothing or baby carriers were suggested by women and providers as possible incentives:
everybody is different but if you have got clothing you are comfortable in, and can cover your entire baby if you want to, it might make people get over that, oh people are looking at me, because they do though.
FG1, mothers
Health-related incentives
Incentives that could be seen to have some benefit to health, such as vouchers for fruit and vegetables or access to sport and leisure facilities, were considered acceptable by some women and providers, but other women were more sceptical about their motivating effects:
Then again a lot of people might go, ‘What? I am not quitting cigarettes for a punnet of strawberries and a banana’.
5, pregnant woman
There were concerns that the incentive should be ‘something that is useful’ but not something ‘that can be abused’, and also that any incentives provided by the NHS for health behaviours should themselves be health promoting:
If it’s something positive and it’s going to be related to improving the whole, you know, family health, you know. I think it should be health-related benefits because we are, you know, it’s the health service rather than just here’s some money.
53, hospital midwife
Household services
These included help with household tasks, prompted by the Pugh and Milligan106 vignette (see Appendix 2), and crèche facilities or childcare. Some women suggested that household services provided as incentives may compensate for the ‘me time’ lost if new habits are adopted, with some considering that household services would be encouraging ‘when your house is a mess and you’re feeling tired’. Household help was thought be particularly helpful to encourage breastfeeding, which was viewed as being time-consuming, with babies potentially ‘feeding three times an hour’. Time to oneself was frequently mentioned by women as a motivating factor in continuing to smoke, and breastfeeding was often described as time-consuming relative to bottle-feeding. Some providers felt that help with housework might help prevent some women from feeling overwhelmed and discontinuing breastfeeding:
I think life gets a bit busy, I think sometimes they give up because breastfeeding is all on them whereas if they decided to switch to bottle-feed somebody else could do it and they could get on with other things that are going on with life as opposed to just breastfeeding.
T60, infant feeding co-ordinator
However, negative reactions to this type of incentive were far more commonly expressed. Several women stated that they would be ‘offended’ by the offer of help with household chores, which they felt would imply that their own housekeeping was inadequate:
I know my house isn’t perfect but I tend to find if someone says I’ll do your housework for you, I’m like are you saying I’ve got a dirty house?
32, I, pregnant woman
Perhaps for this reason or other social pressures, women expressed an obligation to take care of household chores themselves and to maintain control over their home environment: ‘even if family offered you think, “ooh you best do that yourself” ’. Women were concerned that accepting outside services could be construed as them ‘not coping’:
I think you want to come across like you can cope . . . I think if you have got a big backlog of ironing or washing then you can feel that maybe people will think you are not coping, do you know what I mean.
T9, I, mother
Some women found this type of incentive ‘intrusive’, particularly during the postnatal period: ‘to have someone else come in again I just personally don’t like it’. There were also concerns that the help offered would not be ‘done to my standards’, with women describing themselves as ‘far too picky’ or ‘fussy’.
Women also thought that help with household tasks did not generate the enjoyment or pleasure associated with shopping vouchers or personal well-being incentives: ‘I think it has to be to make you feel good. Having your ironing done isn’t going to make me feel good’.
The offer of crèche facilities as an incentive also produced mixed views, with some feeling that a break would be welcome – ‘it would be good to get just an hour to yourself’ – and others expressing anxiety about leaving their young baby with others – ‘you’re just clock-watching all the time’. Service providers gave mixed reports of the effectiveness of offering crèche facilities to encourage attendance for health interventions:
We actually put a letter in to say that in this particular area we had free nursery care for the group [a smoking cessation group] and not one person took that up.
T57, stop smoking service manager
General utility
A few participants mentioned incentives that did not fit any of the above categories. For example, some providers mentioned food and drink, particularly in the context of incentivising women to attend group interventions: ‘we had a much, much higher turnout when we offered lunch than we did when we didn’t’. Refreshments as an incentive to increase attendance and engagement in group activities was confirmed through observations of interactions at our co-applicant mother-and-baby groups, where our interpretation is that commitment and esteem value was added when participants brought ‘home bakes’. Similarly, petrol vouchers were cited as incentives to encourage attendance at groups. For example, an incentive was added to reimbursement of travel costs by providing petrol vouchers with a value ‘well over and above what it costs them to drive there’ for young mothers to attend breastfeeding peer supporter training.
Awards and certificates
Certificates to honour breastfeeding milestones were spontaneously raised by our mother-and-baby group co-applicants and discussed by some providers who viewed them as a means ‘to instil a sense of pride’. Some reflected on experiences where ‘these certificates were coveted and appreciated by the women’. Conversely, some providers considered certificates to be ‘patronising’ and believed that these ‘wouldn’t be helpful’ within affluent or more educated population groups, inferring that for less educated women they could be. However, others argued that, even if some service users perceived certificates negatively, the effects would be minimal.
Experiences
Some examples of experiences are included in the previous categories, in that shopping vouchers entail the experience of spending them; having baby photographs taken is an event that involves rituals preparing for it and then showing the photograph to social networks; and groups that provide outings such as picnics or baby massage provide experiences. However, there were examples spontaneously generated which suggest that providing an experience that extends beyond the individual was highly valued by some of our most disadvantaged participants and that these warrant a category of their own. Suggestions included activities such as holidays – ‘Maybe holidays might be reasonable incentive’ – or family days out, suggested by a partner:
Like an activity event or a day with your kids or something like that.
20, father
Other programme components
Many women had caveats around what else would need to be delivered along with incentives and what would help them most to engage in a new behaviour, change behaviour and maintain the behaviour. In the ladder (see Figure 18) these are referred to as general programme components or rungs and they can be compared with the general BCTs described in Chapter 3. There was a strongly held view that incentives alone were not enough, even individually tailored ones as discussed earlier. For many, flexibility of approach at different stages of the behaviour journey and life in relation to pregnancy and birth (the left-hand side of Figure 18), with the option for individual tailoring of the rungs in the programme journey, was considered important.
Goal-setting, resetting and ongoing motivation
The agreement of a quit date for smoking cessation with an associated incentive and ongoing structured provision of incentives served as ‘milestones’ to work towards on the achievement journey. Such goal-setting discussions were considered to motivate the continuation of smoking cessation and breastfeeding and encourage ongoing engagement with services.
Women within the CPIT identified how setting their own quit date without being pressurised to do so was an important first step; being ready to stop and the timing of such was crucial:
When you go, they give you, you can choose your own date to stop smoking so it could be a week, a month, it’s totally up to you. And I think I had kind of just jumped into it too quick because I think I chose mine for the next day and it’s more when I am working as well that I do smoke more rather than when I am in the house.
38, I, pregnant woman
It was acknowledged that the long-term health benefits of smoking cessation and exclusive breastfeeding were not easily recognised or experienced. Incentive provision therefore offered shorter-term ‘in-between’ goals and self-gratification to motivate work towards longer-term goals. Some of the women reflected on how incentives could offer goals towards ‘doing the right thing’:
It’s a wee goal. It’s a good wee thing. To keep people off smoking it is a good thing, because smoking is pure bad when you are pregnant.
30, I, pregnant woman
Incentives could provide or had provided tangible goals in contrast to a cognitive or behaviour goal, by anticipating future personal rewards – ‘I’ve got that coming and I can maybe go and treat myself’ – or enabling an incentive to reach beyond the individual to achieve a family-related goal (see Table 43):
Because you’re achieving something and your family would be achieving something because you’ve got a healthier dad or something like that. That’s what I’d do anyway. I wouldn’t give them something, I’d say, ‘Right, there’s something for your family’.
20, father
The consistent, regular structure of incentive programmes was closely intertwined with incentive provision in women’s narratives. For some CPIT women, the structured ongoing provision of incentives was indicative of what incentive experts refer to as a ‘commitment contract’, which influenced their continued engagement: ‘you’ve signed up for something and you have to see it through’. Our interpretation is that there appeared to be a synergistic interaction between incentive intervention components rather than it being just the sum of the individual parts. For example, signing up to an incentive programme could be perceived as a ‘commitment contract’ rather than it being a programme that the woman could choose to drop out of.
Monitoring, proof and feedback
Monitoring and proof of behaviours in exchange for financial rewards/incentives was considered another important component of the programme structure. This subject generated a lot of debate because of the fallible nature of the testing methods for smoking and problems associated with ‘proof’ of breastfeeding.
In some areas CO monitoring as part of routine antenatal appointments with the midwife could instil fear (in women as well as partners), which was intrinsically motivating:
I didn’t mind, I don’t mind the shock factor or the scare factor or, it didn’t offend me or. It did make me feel under pressure but in a good way, I didn’t feel bad that they were forcing the pressure; it was enough to make me put pressure on myself.
34, I, pregnant mother
However, these objective methods of monitoring and providing feedback were not identified as effective for all:
Aye, I done a carbon monoxide thing and it came up at three and she said that she would refer me. I got referred and I got another one done and it came up at seven. I don’t know. I just couldn’t stop smoking cigs.
30, I, pregnant woman
Carbon monoxide monitoring was often considered an imperfect form of testing because levels decrease rapidly. The relatively high CO cut-off levels assigned within the CPIT (see Box 2) and the fact that incentives are issued without cotinine levels being confirmed meant that some women could potentially still be smoking. A few women were unaware of the potential for ‘cheating’ the tests, and some CPIT providers referred to how they would not necessarily be explicit about CO levels: ‘so I kind of keep that wee snippet of information to myself’. However, the majority of participants did cite ways in which smoking status could be hidden, for example not smoking for 24/48 hours before testing. Accounts of women’s explanations for what they considered to be false high CO readings were also given, such as ‘faulty boilers’ and women who ‘asked about getting their central heating checked’, implying that extrinsic environmental factors were responsible. Non-attendance at monitoring appointments was also raised by some as a strategy to avoid loss of face: ‘the last thing anyone wants is to go along and be told you have failed’.
Withholding information concerning decreasing CO levels, together with appropriate, regular or even ‘random’ testing, were considered essential by some participants to ‘prevent the study coming into disrepute’. However, not all shared this view:
And they can cheat, but it doesn’t matter because the people that smoke are the poorest in our society and so I am giving them a bit of extra money to help with their lives and I don’t think I mind about that.
T48, I, public health consultant
Regular monitoring as part of an incentive/smoking cessation programme was considered important for a number of reasons: to keep women ‘in check’ and prevent against relapse – ‘they never once offered to do a CO test on me so that’s probably why I went back to smoking’; to provide justification for expenditure; to provide recognition that the incentive was ‘deserved’; and to provide a sense of achievement through the visibility of proof when the monitor colour changes:
I used to like having it.
Why?
Because I knew then.
You could see the difference?
Yeah. From my first time blowing on red and then the next time going in and I’m in the green already. That quick.
2, I, mother
There were several references to tangible objects being added to the environment such as CO monitors and props used in education and to support women, as well as the incentives themselves being tangible. Our interpretation is that tangible, non-verbal cues often seemed more powerful as motivators and prompts for behaviour change than words for some women.
One of the key issues for breastfeeding was how to monitor or provide ‘proof’. Monitoring suggestions introduced through the topic guide included observations, verification by ‘others’ (e.g. providers) and photographic/video evidence. Some providers felt uncomfortable countersigning ‘if I didn’t know that it was happening all the time’. Furthermore, although home visits to ascertain smoking status (house odours/ashtrays) and breastfeeding (evidence of formula feeding paraphernalia) could be undertaken, there were reservations about ‘the resources required’ and the potential for misinterpretation (e.g. mother mixed feeding or partner smoking in the home):
If somebody’s taking the gift or whatever it is, usually if somebody is formula feeding, they’ve got stuff everywhere that tells you their form of feeding. There’s the tins, there’s the steriliser, there’s a part used bottle, there’s often a lot of things . . . But they could be mixed feeding.
FG9, I, experts
Maternal blood or urine tests for smoking were ‘not a problem’ for some participants or from an ethics viewpoint if clearly explained, as pregnancy was associated with numerous physiological tests. However, others considered that the more invasive forms of testing, such as breastfeeding observations/photographs/video or urine tests, were ‘too intrusive’, a ‘bit personal’, which may ‘put people off’. Continuity of care and positive provider–mother relationships were considered important to ascertain ‘proof’, and incentive provision contingent on attending a breastfeeding group was considered a possibility ‘because you’re not going to go if you’re not really [breastfeeding]’. There were further concerns about the fallibility of ‘proof’ as well as women’s awareness of how to ‘cheat’ the tests; ‘anyone can stick a pump on their boob and stick a bit of milk in it’.
Help and support
Most participants were concerned that incentives alone would not be enough to motivate women to initiate or maintain either smoking cessation or breastfeeding and felt that other programme elements, such as help and support, were the top priority:
Obviously, because my partner works full time and I’m on maternity leave at the moment and with the benefits and everything and the government the way it is at the moment, a lot of people are losing a lot of stuff so gifts like that people would appreciate kind of thing and would find helpful. But, it’s like, it’s more the support side of it, I reckon people would probably use and need more than the gift side of it kind of thing. The gift side of it is kind of like a bonus.
T10, pregnant mother
If you are going to use incentives what else goes with it because you can’t compromise on the other services because the incentive alone, well, I think it is kind of accepted that it won’t work on its own.
T61, smoking cessation advisor
In fact, a strong message in relation to breastfeeding was that help and support were what would help most, as many women did not have life course or context rungs in place:
Yes, because if you are struggling and if you want to feed, and you are desperate to feed your baby then help from somebody who has got a lot of experience is what you need, you don’t need a bath bomb, you need that person to come around and help you feed your baby.
T4, I, mother
In fact, many women, health professionals and stakeholders felt that ‘money’s best spent probably in services so that they can support more people’ rather than on incentives, a theme that is developed in Incentives to providers:
So if there was more of a service that allowed – especially when you’re in neonatal unless you’ve actually managed to get the baby on the breast the ward is pushing daily to get you out and unless the neonatal doctors stand up and say, ‘No, you can’t put them home, they’re breastfeeding’, which is what happened with me, but the girl who was in the room next to me she was trying to breastfeed but because she hadn’t had it established the ward pushed her out and the doctors hadn’t been able to back her up sufficiently to prevent it happening. So she ended up not breastfeeding because she was forced out and she lived too far away to be able to commute in to do three hour feeds. She was devastated, she was so upset because it was the one thing she really wanted to be able to do and she couldn’t do it in the end because they said, ‘No, you’re going home’.
FG6, mothers
Information and education
Providing information and education about the adverse effects of smoking was considered important by some, but not particularly effective, and was seldom enough on its own, although it did prompt some women to think about the risks and benefits of quitting at an early stage in pregnancy. However, for others, it seemed that the future health consequences were ‘scary’ but insufficient to maintain motivation to quit and could result in relegation of the pregnancy to the background of their lives:
Well, I tried at the start but then it got harder. I just felt like I was smoking a wee bit more because – I don’t know if it’s just being bored with nothing to do. But I did try hard at the start. But I kinda just, after a while I just forgot about the fact that I was pregnant – not that I’d forgot I was pregnant but you don’t really think about what it is actually causing – what it’s doing to your baby.
42, I, pregnant woman
Others believed that ‘scaring pregnant women’ was ‘not fair’ and would not work. Women became stressed when they acknowledged the health benefits and tried to quit – ‘and when you can’t do it that really gets you. That really did my head in’. The pressure of a programme and fear could therefore result in negative unintended consequences as some women rely and are dependent on smoking:
I think if anything . . . I probably would have stopped or cut down but I think I’ve actually started smoking more.
24, pregnant mother
Any education component/rung would need to be carefully balanced to support women without pushing them too hard. Likewise, for breastfeeding, how education is designed and delivered affects its potential to make a positive difference. In particular, some women felt that it could be more balanced and should prepare them for the realities that they might experience and possible failure:
I could tell you everything about breastfeeding through my classes which, as I say, is fine and dandy but when you can’t do it, it really hacks you off so maybe put a few of the bad points in there, maybe education of what happens if it doesn’t work because there’s nothing on what happens if it doesn’t work. There is nothing.
2, I, mother
That first meeting where you are getting told [about smoking], not only what you are putting in your body, but what you are putting into the baby. They have this little model and it’s got the baby. It was relevant to me because I was at 15 weeks and the baby was of similar size.
33, I, pregnant woman
Prompts and models were raised in several contexts, particularly for learning to breastfeed, and seemed to be preferred to words by some:
No they could do with more things like prompts to do it properly, coz like I said they are not allowed to show you by touching them themselves, and not allowed to touch us either.
14, I, pregnant mother
Delivery of incentive programmes
In the following section we consider aspects of the delivery of incentives and other programme components that aim to change behaviour. These include perspectives on the setting and the providers who deliver the incentives or other psychosocial or BCT components; whether incentives should be targeted to certain populations or should be universal; whether the onus should be entirely on the woman or engage a wider support network including incentives for organisations and/or service providers; and the timing and intensity of incentives and other components.
Setting
Divergent views were expressed concerning where incentive interventions are delivered, which is related to views on the professional background of the providers discussed later. Overall, home and/or community locations were preferred, with some participants wanting multiple locations to be offered to suit women’s lifestyles and needs: ‘Anywhere really, hospital, or go to your house, school, even do them at our doctors’.
Health-care environments for incentive programme delivery were viewed positively by some participants who valued accessing support from a ‘trusted’ professional where ‘people know what they are getting when they access a health service’. Multipurpose locations were considered beneficial if appointments were synchronised with routine scheduled services, for example vaccinations, groups/venues where mothers would already be in attendance or even ‘integrated into the antenatal setting’. However, other participants felt that health promotion needed to be taken out of the ‘ivory towers’; that health premises may be ‘threatening’ for some ‘mothers who won’t go out to a building that’s official’ or disconcerting because of sitting in a waiting room ‘full of ill people’.
Community locations (e.g. children’s centres, pharmacies) were considered to be able to offer a more individualised service, with consumers being ‘equal partners in care’, which in turn ‘increases the achievement’ of the programme, and this is considered again in Incentivising beyond the individual within social networks and communities. Access and engagement were considered to be promoted through the convenient, familiar and accessible nature of venues, which could reduce ‘stigma’ and ‘embarrassment’.
Home-based delivery of incentives was considered unfeasible or unrealistic among some providers and experts. However, from a woman’s perspective, particularly in the postnatal period, it was often the most favourable for both target behaviours. Home visits could enable mothers to receive a more individually tailored duration of support, ‘whereas in the clinic, if something runs late, you are in and out like a shot’. They also offered convenience for new mothers – ‘it is overwhelming leaving the house when you have just had a baby’; they could prevent disruption to routines – ‘you could be feeding at the same time and discussing things with them’; they could provide contextually situated support – ‘if you are sat with someone [at home] they can say try this or that position’; and they could enable a mother to ‘feel a bit more comfortable talking about things’ and facilitate family-based support:
They could say to somebody else in the household who is not entirely being supportive [. . .] it is really important and can emphasise to them first-hand the benefits of giving up smoking.
T6, I, mother
However, several CPIT women were more negative about or ambivalent towards home visits: it would depend on having ‘the house work done’ before the visit and some disliked the attitude of the professional attending. Some of the providers involved in the CPIT spoke of the need to be mindful about ‘child protection’ issues when undertaking home visits.
Incentives delivered in the workplace were favoured by some providers and experts and are discussed in Incentivising beyond the individual within social networks and communities.
Providers of incentives
There were three overarching themes relating to who delivers and provides the intervention components: specialists or generalists, continuity of care and personal experience of the behaviour.
Views differed about the extent to which incentive programmes should be integrated into general universal care, with smoking cessation and breastfeeding needing to be ‘everyone’s business’, or separated for specialist services to deliver. Many women and health professionals believed that incentive programmes were best placed within a dedicated expert service (e.g. smoking cessation advisors, peer support) that they could refer women to for ‘whole-systems joined-up thinking’. A few participants felt that providers within a specialist team should have a health professional background (e.g. midwifery); however, for others this was less important. Many believed that a dedicated service would encompass ‘specialist’ knowledge, ‘passion’, ‘emotional attachment’, ‘devotion’ and ‘drive’ – to provide ‘that extra above and beyond that gets them that one step further’, with time and ‘dedicated time slots’ to help women. This was contrasted with trying to include smoking cessation and breastfeeding support along with all of the other demands when providing routine care.
The specialist psychologist-led support depicted in the Gulliver and colleagues’104 vignette (see Appendix 2) received mixed reactions: for some it was felt able to ‘reach out to people’; others felt that this level of specialism was unnecessary – ‘it’s all playing with your mind’ or ‘too in-depth’, when ‘it’s quite simple why people do it’. GPs and pharmacists (particularly evident in CPIT women and provider interviews) were perceived as being legitimate to deliver an incentive programme, whereas health visitors and midwives, who have universal contact with almost all women, were considered more appropriate among participants not involved in incentive interventions:
With midwives who see women right the way through the pregnancy, we get to know the women. Perhaps it would, they’d be more interested and we know the women who would require it. We’d perhaps be able to offer services to a bigger, a larger amount of women, especially those in need, really.
53, midwife
Participants did, however, highlight concerns that more generalised health professionals may not have the knowledge or skills to help women stop smoking or breastfeed – how their ‘agendas are different’; their ‘personal motives’ may interfere with incentive delivery or support considered inappropriate, ‘putting the fear of God into me by telling me all the stuff that could happen’. This was directly experienced among some of the CPIT providers through non-referrals to specialist services by maternity professionals:
I think it’s just their personal feeling that the girl’s got enough on her plate just now and smoking is a coping mechanism for her you know whatever her situation might be so I think it’s the midwife, the midwife might be a smoker.
FG15, I, smoking cessation advisors
Some participants expressed concerns regarding health professionals’ ‘capacity’ to deliver these services and felt it important not to ‘lump it [incentive delivery] into’ statutory care provision. Furthermore, expert and provider participants reported that GPs’ engagement would be dependent on targets and associated reimbursement as they ‘don’t do anything unless you pay them’.
Continuity of care and having a strong woman–provider relationship was believed by many, including CPIT participants, to be important to enhance women’s motivation and commitment to succeed.
In the ladder meeting with co-applicant mother-and-baby groups (see Chapter 2), ‘seeing the same person’ was one of the few intervention components selected by women to accompany an incentive intervention. Positive women–provider relationships could also help prevent unnecessary repetition of information and enable targeted, needs-led support:
It should be the [. . .] same person because that is where she can like relate to, because the person will know all her background and all other things.
8, pregnant woman
Personal qualities of the staff were valued – ‘She was really, really good – really supportive’ – with difficulties experienced when no continuing or satisfactory relationship was present: ‘it was never the same person so that kind of, wasn’t really too good’. Effective relationships were also considered necessary to ascertain ‘proof’ of behaviour change (see Monitoring, proof and feedback).
Contrasting opinions were provided about whether those who deliver incentives should have had personal, first-hand experience of smoking or breastfeeding or just expert knowledge: a ‘professional or they have been trained and knows what they’re talking about’.
The CPIT women provided some positive comments about receiving support from an advisor who demonstrated in-depth knowledge and understanding and associated this with an unprejudiced approach: ‘she didn’t judge you’. Other participants felt that ex-breastfeeders and ex-smokers would have credibility and would comprehend and have empathy with their situation:
People who have done it, they are the best people because obviously these health workers, they are probably so against it, they have never smoked in their lives, so they are giving advice but they have never actually been through that, so it is all well and good, them saying not to smoke and this is what happens, blah blah blah, black and white, there is a leaflet, and never actually experienced it, so they shouldn’t really be giving, you know, advice on something they have never experienced.
12, pregnant mother
Targeted or universal incentives
Divergent views were expressed and caveats identified when discussing whether incentives should be universally provided or targeted to groups of women who would be expected to benefit the most, in particular those living in disadvantaged areas or with individual characteristics associated with disadvantage. Overall, universal provision appeared to be more acceptable, with concerns about creating a ‘postcode lottery’ of care, with participants emphasising a need for ‘equity in health care’. Some felt that if targeting was to occur, this should be by area or town as it ‘would be better than offering it to only a certain section of the population everywhere’ or based on other sociodemographics, for example income, rather than behaviours (e.g. smoking or not smoking), such as within the Healthy Start voucher scheme. 79
Although the potential for wasting resources by providing incentives to those already motivated was highlighted, discussed later in Unintended consequences, others felt that, irrespective of income, ‘they [women] still need something to make them stop [smoking]’. For breastfeeding, it was felt that only ‘first time mums need this level of support’, with incentives not required when breastfeeding had previously been achieved.
However, concerns were raised that marginalised families with complex issues, because of their lack of engagement with statutory service provision, may not benefit even if universal provision was provided, which suggests that incentive programmes might increase health inequalities:
You can be pretty sure that everyone earning between £25,000.00 and £70,000.00 a year will be taking advantage of that incentive scheme whereas those who are on £8000.00 a year won’t even know about the incentive scheme.
FG12, providers and expert
Providers recognised how care is already focused on those considered ‘more problematic’, suggesting the potential for stigma, although this strategy is not necessarily condoned for equity reasons: ‘unfortunately the ones who are doing everything right don’t get as much attention’.
It was considered that as smoking and formula feeding are more prevalent within low-income, less-educated, younger populations, incentive programmes would naturally target these groups. However, others emphasised that incentives should be specifically targeted towards younger women ‘because they’re our future generation of parents’, with vouchers, gifts or behaviour-related incentives felt to be more acceptable within ‘more receptive’ teenage and/or low-income population groups.
Incentivising beyond the individual within social networks and communities
This section relates to studies in the systematic review (see Chapters 3 and 4) in which partners or a quitting ‘pal’ are included in the incentive intervention, and this is explored further in the DCE (see Chapter 8). This ties in with the importance of partners, families and social networks as life rungs in Figure 18 and in the review of barriers to and facilitators of smoking cessation and breastfeeding (see Chapter 4). A smoke-free home was raised by participants and was included in the shortlist of promising incentive strategies (see Table 38). In addition, vignettes were created for studies that included community-provided incentives, for example the Gulliver and colleagues’104 vignette (see Appendix 2) encompassing a wider ecological or systems approach to behaviour change. Incentives to providers of care (also included in the shortlist) were discussed in relation to these wider systems approaches and are considered separately in the next section. Not all interviews included either the shortlist of incentives or the vignettes in the topic guide and the theme of incentivising beyond the individual was raised spontaneously by some participants.
Some women considered that, if incentives were going to be issued, they should be ‘for everybody’ and should ‘support each other as a unit rather than like an individual against everybody else’. For example, they should be delivered to organisations, providers, women, their partners and significant others to encourage engagement and provide motivation for all involved. This might prevent against ‘discrimination’, in which all of the responsibility for a behaviour is perceived as being placed on the women alone:
I think there should definitely be some sort of target at a high level and then that should be fed down to people who are interacting with the people who you want to affect, and then if the people that have actually got to do the change, they have got to have a bit of help.
7, pregnant woman
Family and friends incentives were generally introduced through direct questioning or through discussion of the Gulliver and colleagues’104 vignette (see Appendix 2). They were considered important to create a family identity – ‘we don’t smoke in this household’ – either through engagement of partners or from a ‘matriarch’ perspective.
Involvement of partners, that ‘supportive person in the home with you’, or even on a household basis, for smoking cessation programmes was considered important to enable a collective effort to achieve behaviour change. This was considered beneficial in terms of ‘making it easier’ by providing ‘encouragement’ and being there ‘to prop each other up’ and facilitate healthy ‘competition’:
I wonder if it would be more beneficial to work on an incentive to stop a household smoking rather than just the pregnant woman, because I think so many of them go back to it afterwards because somebody in the house is smoking.
FG10, provider and experts
Participants identified the difficulties in maintaining behaviour change if ‘someone has got a partner that is smoking’, as well as how this would ‘erase’ the benefits to the baby if it is still a smoking household. However, although one of the women whose partner (non-smoking) received vouchers as part of an incentive programme was positive about the rewards he received, ultimately she felt that his incentives had not been necessary to engage his support and encouragement. Others not involved in incentive interventions considered how incentives could encourage partners to quit, ‘to smoke outside’ the home and to be ‘even more supportive’ by providing a reward for coping with women’s ‘mood swings and cravings’. It was generally considered that the reward issued to partners should be ‘smaller’ but ‘one worth having’. Involving partners or wider family members if they were not ‘interested’ or not ‘motivated to quit’ was highlighted as a concern and it was acknowledged that ‘it’s a lot harder for a man to stop as a woman has a reason to’, implying a differential motivating effect of pregnancy.
Some providers recognised the benefits of partner involvement at CO monitoring appointments as this provided an opportunity to talk about the dangers of smoking and recognition that they are ‘in it together’ rather than ‘as if one is feeling responsible if the baby is compromised’:
I think it is putting too much pressure on the mum to do it on her own, so I do think it has got to be something that you do need to be rolling out across the household.
52, midwife
Unlike smoking cessation, there appeared to be a polarisation of views among some of the participants in terms of whether partners should be incentivised for breastfeeding. Although a family incentive approach was considered acceptable as ‘there’s better outcomes for everything, not just breastfeeding’, mothers and providers were concerned that incentivising others could have adverse consequences, as discussed in Unintended consequences:
You have enough pressure being a mum anyway at the start let alone someone then trying to throw this at you and this at you and this at you.
2, I, mother
It was considered by some that the partner’s role was to generally help and provide support: ‘involved in getting the outcome that the mother and they want’, regardless of her feeding choices. It was not considered ‘an incentive issue’, implying that breastfeeding is a central issue for the mother alone.
At a wider level, incentives as part of a workplace culture were considered desirable by some:
I think it would be an area to look at as a model to look at . . . employer wellness programmes, it has to do with the employee to do certain behaviours that are appropriate and you are rewarded for doing them by your employer who is paying for your insurance, but some of them are very successful [in the US].
IA1, providers and experts
Some providers and experts also considered how engagement of employers to support partners’ involvement in incentive schemes could have financial and productivity benefits because of reduced absence rates:
The other thing is, if you sell it to employers that they allow even the male part of the workforce out to come to these sessions, the long-term effects for the workforce are that their sickness rates will decrease because the child’s healthier, so they’re less likely to need time off work to go to appointments with the child or with the mum with the child. That’s the way to go because then you’re not actually giving a person a reward, you’re giving the company for helping them [breastfeed].
FG10, provider and experts
Incentive delivery within close-knit communities was also considered to have potential snowball benefits of ‘advocating it to people that they know’, as described in Setting, thereby encouraging social networks to engage with incentive programmes. Furthermore, numerous participants made reference to how such schemes and associated behaviour change held wider benefits of decreasing (smoking) or increasing (breastfeeding) the visibility of these behaviours, which in turn would encourage and normalise positive behaviours, ‘as fewer and fewer people who do it, the more socially unacceptable it [smoking] is’.
Incentives to providers
Participants were asked their views on incentives for both individual health professionals and health service organisations. A vignette derived from the study by Cattaneo and colleagues168 (see Appendix 16) was used in some interviews and focus groups to prompt discussion. Some women favoured a programme in which health professionals, ‘those that are doing the work and putting in the hours’ to support smoking cessation or breastfeeding, were incentivised rather than incentives being given at an organisational level, whereas others believed that both organisations and individual professionals should be incentivised:
There should definitely be some sort of target at a high level and then that should be fed down to people who are interacting with the people who you want to affect.
5, pregnant woman
However, both women and providers commonly felt that individual health professionals should not need anything extra for providing support for smoking cessation or breastfeeding because:
That should be part of your job, that’s part of your, what you are supposed, you know, that’s kind of thing you are supposed to do as a role of a midwife.
12, pregnant mother
Some believed that ‘doing a good job is the incentive’ and questioned the extent to which financial rewards to health professionals would be effective, suggesting that intrinsic motivation is high, ‘because nobody sets out to do a bad job in the morning’.
With regard to breastfeeding, women were concerned that incentivising health professionals, either individually or through organisational incentives, would lead to a more ‘forceful’ approach to breastfeeding promotion, where ‘everyone would harp on about it’, thus increasing the ‘pressure’ on women to breastfeed (see also Unintended consequences):
I definitely wouldn’t say [incentives for] professionals in breastfeeding. Because they hammer it on you enough, they don’t need any incentive. They really lay it on, they spread it thick.
2, I, mother
Discussion of incentives for health service organisations also yielded mixed views. Some providers saw a direct relationship between such incentives and financial support for services:
The amount of resources you put in to try and change people’s behaviour depends upon the amount of the incentive.
T55, consultant obstetrician
However, many providers were eager to point out that outcomes for both smoking cessation and breastfeeding were dependent on other life factors in addition to service provision and that high-quality support might nevertheless be insufficient to change behaviour ‘if that mum makes her choice that she doesn’t want to continue’. Providers also noted that outcomes depended partly on whether or not women engaged with services in the first place; this was felt to be outside their control and something on which they should not be evaluated:
Because my target, my achievement rate, is based on those that engage with the service. You get those that say to the midwife – because they daren’t say anything else and they’re only young mums – ‘Yeah, I’ll go and see them’ and then they make appointments and they don’t turn up. How can you be judged on that? Because you’ve never had a chance. I don’t agree with that at all.
T51, I, lead health trainer – smoking cessation
In particular, providers were very concerned that, as sociocultural factors are highly influential for both smoking and breastfeeding rates, organisational incentives would penalise those who ‘worked in a deprived area’. This type of postcode variation was seen as especially problematic if incentives (or penalties) were linked to absolute rather than relative targets:
If they told us around here that we needed 20% smoking rate and we don’t get that we’re penalised. We’ll just go, ‘Oh, we’re penalised now’, because there isn’t any point in throwing the money into that because 10% is so far away.
FG9, I, experts
A suggested approach to avoid this perceived unfairness was to ‘incentivise the work, not the result, because the result is out of our control’. Another suggestion was to incentivise referrals to specialist services, on the grounds that otherwise referrals would not be forthcoming. Other suggestions were to incentivise staff ‘to turn up to training’ or to give incentives to staff who could demonstrate specialist knowledge.
Some providers felt that the distinction between incentives for reaching targets and penalties for failing to reach targets was simply a ‘linguistic difference’, whereas others felt strongly that penalties would be ‘counterproductive’. Some were concerned about the implications of lost income for the service – ‘how would the money withholding help to fix the situation? – whereas others presumed that there would be knock-on effects on other services whereby a ‘health board loses money so they cut a service somewhere else’. Some providers believed that organisational penalties would lead to ‘low morale amongst the staff’:
If you worked in an area that did not breastfeed at all, and you just couldn’t do it, and you got penalised, it would be so disheartening after all that work.
You wouldn’t engage would you?
FG9, I, experts
Some providers felt that organisational incentives for breastfeeding rates would contribute to a ‘shared aim’ across different services within the organisation, to getting ‘everybody on board in some shape or part’. Some reflected that breastfeeding support was considered an undervalued area within organisations. However, other providers were concerned that incentives to meet organisational targets for breastfeeding would detract from good care, leading to a ‘box ticking’ approach in which health professionals would ‘forget about the support and the woman’ and ‘lose the point of it all’:
They won’t be looking at that woman, they’ll be looking at those numbers, ‘well I’ve only got how many’.
FG10, provider and experts
Timing, communication modes and intensity
A strong theme among women and provider participants was for flexibility and individual tailoring of the timing, modes of delivery, length of sessions and intensity when delivering incentive programmes and any additional support sessions. Participants provided varying views about when programmes should begin and end and the optimal overall duration of interventions.
The majority considered that smoking cessation interventions should be provided ‘as early as possible’ in pregnancy as this was ‘a prime time’ to ‘get the messages across’ about the health risks involved. However, as pregnant mothers can feel ‘bombarded with everything’, opportunities for later engagement and/or partner involvement to help retain and re-enforce the information were considered beneficial.
Participants emphasised the importance of continuing the intervention throughout pregnancy and/or for a prolonged period of time (e.g. 12 weeks), as well as into the postnatal period (e.g. at least 2 months, 52-week quit or up to 12 months postnatally), when the potential for relapse was high:
At least then they’ve given up smoking through the pregnancy and at least until the baby is 3 months old, so they’ve gone a year without smoking so it decreases the chance of them smoking again, if you know what I mean? They’ll be so used to not smoking.
T10, pregnant mother
Post-partum incentives were provided in some effectiveness studies in the systematic review (see Chapter 3), as relapse after birth is evidently a problem (see Chapters 3–5). In addition, the continuation of financial incentives postpartum was supported by some participants and they were included in the shortlist of promising incentive strategies. However, some women did not feel that incentives would work in the post partum period, especially when there was a desire to return to a pre-pregnancy social life/identity:
I stopped smoking through pregnancy and I was just saying when I am out, if I have a few glasses of wine I want a cigarette. I don’t know, I don’t think an incentive would stop me from doing that because that’s when I am socialising, baby is not there its different, your inhibitions are down. Your judgements are different anyway.
FG7, mothers and health visitors
When discussing the Volpe and colleagues107 study vignette (see Appendix 2), antenatal recruitment onto breastfeeding education programmes was considered appropriate by some but not others, particularly if this caused ‘a blow’ and a sense of failure for those who were then unable to establish breastfeeding (see Information and education). Opinions as to when to end breastfeeding support programmes varied. For some, 6–8 weeks was suitable to help women to resolve early breastfeeding difficulties, as this was when women were likely to access other avenues for support, such as postnatal groups. Although concerns were expressed about the costs of prolonged service delivery, some considered that programmes should be offered for 3–4 months or for 6–12 months, in line with WHO guidance, or to ensure support provision at ‘pinch points’, for example on return to work or at times of growth spurts when the potential for discontinuation is high.
Several participants considered that regular weekly or fortnightly contacts should be provided or, as detailed in the study by Heil and colleagues105 (see Appendix 2), initial daily contacts that then reduce in frequency. Others viewed the weekly and varying schedule of contacts detailed in the study by Heil and colleagues105 as ‘too confusing’, requiring ‘too much effort’ and being unlikely to fit in with women’s individual lifestyles. A few participants judged the Gulliver and colleagues’104 vignette (see Appendix 2), with prolonged support periods (e.g. 60 minutes), to be acceptable for smoking cessation; however, some considered that it was ‘too long’. In the Walsh and colleagues’108 vignette (see Appendix 2), the specification of ‘minutes’ of support was considered suitable for those with a ‘short attention span’; for others it was felt to be ‘too strict’ and restrictive, with a preference for the intensity of support to be tailored to individual need. For example, CPIT providers referred to how they would offer more than four weekly contacts if requested by the women, as well as work earlier or later (out of hours) to ensure that contacts would fit with the women’s lifestyles:
If that’s what they’ve requested us to do and then if that’s what’s going to keep them on track then we will do it.
FG15, I, smoking cessation advisors
Although some women involved in the CPIT considered the four 30-minute contacts per week to be acceptable, others felt it was ‘too much’ and others wanted additional contacts in the middle of the programme (rather than just at the beginning and the end) to receive ‘positive feedback’:
I want to come in and see you and blow on your silly wee machine, I’ll show you. So actually she made me another appointment and I went in just literally to do a CO for the fun of it basically. She was flexible with me that way, you know, she had space to do that which was more productive than sitting on the phone for 45 minutes. I went in and I saw her.
28, I, pregnant mother
With regard to the timing of incentive provision, participants considered that incentives should be offered at each or every other contact with the service or distributed throughout the incentive programme (e.g. start, middle and end or every month/3 months) to provide more dispersed ‘milestones’ (see section on goal-setting) and/or timed to enable rewards at key points, for example ‘just before the baby is born’. The weekly issue of gifts within the breastfeeding incentive scheme was considered important to motivate women through the early ‘difficult’ periods:
Whereas you know if you had the X [peer supporter] popping round every week seeing how you were doing, and bringing, you know, some sort of goody you are thinking if you can get through the next couple of days and I will have achieved another week.
T6, I, mother
Several participants expressed other perspectives, reflecting on how support sessions and incentive provision should be more frequent at the start of the programme, ‘sectioning things up rather than doing something at the end’ (e.g. weekly), followed by increasing periods between incentives as behaviours become established and maintained. Alternatively, there could be a combination of ‘predictable’ ongoing incentives together with unpredictable incentives (e.g. raffles) at key points (e.g. every 3 months, programme end), which could subsequently be utilised to promote the service.
Across the narratives, the timing of breast pump provision provoked differing views, with uncertainty about the existing evidence and conflicting advice. Providers expressed concerns that giving breast pumps may encourage women to express milk ‘too early’, that is, before breastfeeding is established, and that there may be deleterious effects as a result:
So to give them a breast pump you’re going to interfere with the actual lactation of breastfeeding process and you’re going to create problems for them I think in the early days doing that.
FG12, providers and expert
A few women, in discussions around the Chamberlain and colleagues’103 vignette (see Appendix 2), felt that it was most appropriate to receive a breast pump as an incentive immediately after birth but some women queried the power of a breast pump to motivate if provider advice is that it should not be used immediately:
They mentioned . . . try and wait till 4–6 weeks before you can do that [express milk], I mean that’s 4–6 weeks where you might not necessarily get to 4–6 weeks and make use of it.
5, pregnant woman
When considering modes of delivery, contrasting views were expressed about the utility of telephone support. This communication mode for breastfeeding support (e.g. in addition to a breast pump – Chamberlain and colleagues’103 vignette, see Appendix 2) was felt to be useful by some and a potential ‘distraction’ and ‘intrusion’ by others. Numerous women in the CPIT expressed positive views about being able to determine when helpline support was received, but, although some valued the additional encouragement that the telephone support provided, keeping ‘you on your toes a wee bit’, others provided more negative comments and ‘didn’t find it any help at all’. CPIT providers would inform/remind women about receiving a call, request women to key the telephone number into their telephones to aid recognition and issue text messages if calls were not answered. However, several issues were expressed by CPIT women, such as telephones breaking, missing calls and being unclear as to the purpose of calls or what to do next:
I had a missed call from an 0800 number but I don’t what it could have been, it could have been them.
40, I, pregnant woman
The CPIT women also valued the speed with which vouchers were received by post and the secure nature of this method of receiving vouchers – ‘because that way you know that you are getting it safely’; for providers it avoids the operational issues of supply and accountability involved with immediate reward at the time of verification.
Face-to-face support was considered to be important to develop positive relationships and to provide practical support, particularly for positioning with breastfeeding. However, participants had different perspectives about whether individual or group support should be provided, with several participants emphasising the need for women to be ‘in control’ of how support was received. Group support incorporated benefits of enhanced well-being and social support as well as normalising and validating women’s experiences – ‘to know I am not the only way that is facing the same problem’– with positive ‘peer pressure’ believed to aid maintaining behaviour change:
That was quite successful for me as well. I found the support and the information was really good. You heard everybody’s experiences and a lot of people were there for all different reasons. You could phone up the woman any time you wanted and get support.
37, I, pregnant mother
Conversely, others expressed concerns about not ‘fitting in’, how group members could inhibit discussions and be internalised as ‘patronising’ as well as create unnecessary ‘pressure’. Several women stressed the importance of shared characteristics among smoking cessation group members, for example only pregnant mothers and not the general population or, for teenage mothers, ‘not sex groups but age groups’.
Who should pay for incentives?
Participants were asked who they thought should pay for incentive programmes. Most participants assumed that incentives would be paid for by the NHS, the government or ‘taxpayers’ money’. For some service providers, this was felt to be appropriate because ‘it’s really a public health issue’ and because prevention was felt to be an important focus for the health service:
I don’t draw a huge distinction between lifestyle-type interventions and medical-type interventions, because you know I see one is just getting in earlier than the other.
T47, research manager, voluntary sector
However, discussion of public funding frequently provoked discussions about fairness and the potential for resentment for those not incentivised, as well as concerns about health service priorities and the possibility of lost or reduced funding from other areas. In contrast to these reservations, some participants perceived there to be wider societal benefits if incentives were effective in increasing smoking cessation or breastfeeding rates (see Unintended consequences).
The idea of local or national business funding incentive programmes was prompted by the local business involvement in the Gulliver and colleagues’104 vignette (see Appendix 2). Some participants suggested that ‘big companies’ may be interested in funding or subsidising incentive schemes ‘because it will all be advertising for them’. For some service providers, this raised ethical concerns because of the potential for business interests to conflict with health priorities:
I think you have to be very careful because the businesses that are gonna have the most money to put in are those going to be sharpest about thinking about how they can make best of the situation. You’d have to be very clear that it wasn’t undermining help in another way.
FG9, I, experts
Views were mixed with regard to local business involvement. Some service providers believed that local business involvement in incentive schemes could be beneficial for communities:
I think involving local – especially quite local businesses – would be good as well to make it seem like it was more of a community thing. I think particularly in rural areas that might work particularly well.
T56, tobacco trainer – tobacco control team
However, others felt that local business involvement would be intrusive in what they considered ‘personal’ issues and could end up being quite ‘Big Brotherish’. Local businesses were seen to have more of a role in supporting their employees to quit smoking and providing a facilitating environment.
A perceived drawback of involving local businesses in incentive programmes was that programmes would be less transferable between areas. Some service providers believed that finding businesses willing to support incentive programmes would be more difficult in areas of deprivation – where shops ‘all sell cigarettes, where’s the incentive for them?’
Unintended consequences
Participants presented disparate views on the unintended consequences of incentive programmes. Although CPIT providers were generally unaware of any negative ‘backlash’ to the incentive intervention, other participants expressed concerns about ‘negative publicity’, with incentive provision described as ‘controversial’, ‘unpalatable’ or a ‘hot potato’. Overall, there were a number of negative as well as positive consequences identified by the participants in relation to the fairness of incentives in terms of whether recipients were considered to be deserving or undeserving. Negative consequence themes were increased health inequalities through diminished personal responsibility and motivation; gaming and cheating leading to negative consequences for health as well as the reputation of the intervention; adverse effects on relationships at home and with health professionals; how the resale value of the incentive held risks of domestic abuse; how endorsement of behaviour-related incentives can create an illusory correlation of needing the item to succeed; and perceptions of wasting NHS funds and the workload involved in administering incentive programmes with consequent opportunity costs for other services that would benefit from the resources. Positive consequence themes were health and emotional outcomes consequent on success; engagement in health-promoting services; maternal well-being; and helping to provide resources to those most in need, thus addressing poverty.
In relation to the ‘fairness’ of incentives, they were considered by some to be ‘unfair’ and ‘penalising’ to those who do not smoke or breastfeed:
I think if you are going to provide free ironing only for those who give up smoking in pregnancy, it’s discouraging or unfair on those who gave up smoking before, or who never started smoking.
T64, paediatrician
Numerous participants made reference to the unfairness of penalising those who ‘are doing the right thing’, with some referring to how this was counter to practices advocated within parenting and education in terms of ‘positive reinforcements’ for good rather than negative behaviour:
If you are a pregnant mum and not smoking, you should be incentivised because you are being the role so that might give the other mums who smoke motivation to stop, knowing what they could get, so you are rewarding the good behaviour.
FG2, mothers
From a different viewpoint, others felt that incentives could create ‘polarisation’ between different groups of women (e.g. those who breastfeed and those who formula feed) and even instil ‘resentment’, particularly if the incentive was targeted towards a particular, ‘undeserving’ population: ‘well that’s my money, going to my next door neighbour, they don’t have a job’.
Financial incentives were considered to have the capacity to increase health inequalities because of marginalised families and those with very chaotic lifestyles being less likely to be aware of, and engage with, health provision. Incentive provision was also felt to diminish personal motivation by discouraging women to quit so that they could participate in the programme, thereby reducing ‘health choices to a financial transaction’, or even to incentivise ‘people to get pregnant’. Although some women considered this unlikely – ‘why damage your body if you don’t actually already do it’ – incentives of higher value were generally believed to be associated with a greater likelihood of ‘gaming and cheating’ in order to receive the incentive – ‘but £750.00, like I say I would I start smoking for that’ – with small personal gifts felt to be less open to abuse than vouchers because ‘there’s no resale value of some bubble bath’.
Concerns were expressed about the fallibility of ‘proof’, as well as women’s awareness of how to ‘cheat’ the tests, as described in Monitoring, proof and feedback. However, these concerns were not necessarily evident within the CPIT:
So far the people that I have engaged with that have signed up to the service to me, bar one, I feel have been one hundred percent genuine.
T72, I, smoking cessation advisor
Providers considered how behaviour-related (e.g. breast pumps, breastfeeding clothing) incentives were less likely to elicit cheating than shopping vouchers because of their more limited appeal: ‘you’re not going to pretend to carry on breastfeeding are you, just to get the incentive’. However, there were concerns that endorsing such incentives could create a situation in which women considered these items to be a necessity to be able to breastfeed, as discussed in Behaviour-related incentives.
Incentive provision was believed to lead to adverse effects on women–provider relationships because of women feeling ‘coerced’ or ‘forced’ into persevering, with implications for future engagement with health service provision: ‘women may not attend antenatal for fear of being pushed to stop smoking’. It was also believed that the increased pressure associated with intervention programmes could exacerbate unhealthy behaviours, for example women smoking more or being less inclined to breastfeed. Although smoking was considered a ‘choice’, breastfeeding was believed to be shrouded within ‘uncontrollable circumstances’ relating to the relative difficulty of performing breastfeeding compared with smoking. Withdrawal or non-eligibility of incentives for women who were unable to breastfeed or quit smoking was therefore considered to create ‘pressure’ and ‘blame’, leading to feelings of being ‘judged’, ‘reduced self-esteem’, ‘guilt’ and ‘failure’, a situation that created unease and discomfort, particularly among the providers. Other concerns related to how organisational incentives could result in individuals (providers and women) being ‘inappropriately handled’ in order to meet targets.
Risks of abuse were also identified in terms of how vouchers could be resold or used to purchase undesirable items, as well as the potential for ‘domestic abuse’ – ‘people taking those vouchers off those women as they walk out the door’ – or manipulation by others:
There was a thing in the newspaper about it and her husband phoned up, phoned the service asking about the money and then, well we ended up making her an appointment and she came along to see me because her husband wanted her to get this money. She came along to the face to face and then I never heard from her after that.
FG15, I, smoking cessation advisors
Concerns were expressed over how incentives could be perceived as being a ‘waste’ of taxpayers’ money and NHS resources. Preventing waste through targeting those who are ‘actually really serious about their health’ rather than those whose ‘main motivation was financial gain’ was suggested. Issues regarding relapse after the incentive had been withdrawn, because of ‘people stopping for the wrong reasons’, and limiting re-access among those who relapse – ‘get the voucher, spend it, then start again’ – were highlighted by many participants. Because of the current adverse economic climate, participants considered incentives to be the ‘wrong use of money’ when the NHS was faced with ‘overstretched services’ and hospitals ‘trying to clear debts’. Participants expressed concerns over the perceived implications of funding incentive programmes for existing dedicated services, for example smoking cessation, as well as the opportunity costs for other services:
It’s bad enough giving them NRT free, sometimes you get the feedback about that, you get nipped a wee bit but if they thought they were actually getting money, just because there is so many cutbacks you know, they may be seeing it that they’ve got an elderly relative with Alzheimer’s or something like that or nursing home fees.
66, I, health improvement senior officer
Providers and experts had reservations about the associated costs of appointing committed and skilled staff to deliver these interventions and the potential implications of training, paperwork, organisation and ‘policing’ of incentive delivery for ‘overstretched staff’.
Numerous participants made requests for the intended incentive costs to be redirected towards increased and/or more effective services to support behaviour change (for smoking cessation and breastfeeding):
I think the money needs to be invested in helping people who do want to breastfeed, breastfeed properly.
FG1, mothers
Participants referred to the ‘paternalistic’, ‘nanny state’ ethos that exists in the UK and expressed concerns about how paying for health could have long-term ramifications in terms of the other health-related behaviours that individuals might expect rewards for, creating new social or health-care norms that could potentially diminish individual responsibility for making healthy choices:
This is the NHS. We’re health, you know. It’s your own health, take some responsibility. I think we’re going right down the wrong route; I think that, you know, when we’re enticing people with money and gifts just to do what’s right for their health, you know. What else will we expect?
53, midwife
With regard to positive consequences of incentive provision, participants referred to how incentives were ‘preventative measures’ that had the potential to lead to a ‘greater uptake of services’ with subsequent long-term cost benefits to the NHS through improved health. Incentive programmes were believed to provide wider benefits of enhanced ‘social awareness’ of these behaviours, ‘social connectivity’ and ‘social exposure’ to positive behaviour change. They could enhance self-esteem and encourage positive behaviour to ‘cascade through the family’ and to other members of the women’s social networks or wider community members, as well as providers. For providers, improvements in patient and public health behaviours could provide ‘job satisfaction’, ‘encouragement’ and a ‘boost’ for staff morale. Some participants considered the cost of incentives to be ‘far outweighed by the benefits’ of not being ‘surrounded by people puffing away on fags’ and the associated health and well-being implications for women and families. Some participants also believed that employer-led ‘wellness’ schemes such as enabling employees (e.g. women and their partners) to attend incentive programmes would lead to financial and productivity benefits through reduced absenteeism for ill health.
Perspectives on research into incentives
Divergent attitudes were expressed towards research into incentive interventions. Participants who had been involved in incentive interventions referred to how inclusion in a research study could enable women to feel that they were ‘part of something’ and lead to them ‘feeling valued’, irrespective of whether they were allocated to the intervention arm or not. CPIT women also expressed feeling more ‘confident’ and ‘privileged’ in terms of how their inclusion could ‘help somebody else stop smoking, that gives me great pleasure’. Although some in the control arm expressed ambivalence towards not receiving an incentive – ‘I wasn’t really too bothered’ – other participants considered how allocation to a control arm of a trial could lead to ‘disappointment’, ‘jealousy’ or even apathy:
She said, well do you know, if I had been selected for the vouchers I think I probably would have given up. That is so ironic isn’t it? Because they’re given a voucher they’ll stop because you know, it’s something they are getting.
68, I, midwife
Ethical issues in terms of how incentive provision could lead to coercive practices were highlighted. For example, organisational/provider incentives could result in ‘people being inappropriately handled because they haven’t adhered’, or the wording used within the protocol or participant sheets could read ‘almost like bait’:
I think if you go to an ethics committee and tell them it’s an incentive, they’ll throw you out. If you go to an ethics committee and say it’s in recognition of the time that they’ve given for the study, they’ll have it.
T65, paediatrician
The fact that incentive provision for recruitment purposes may influence someone’s decision whether or not to take part, rather than intrinsic motivation for behaviour change, was considered to be ‘slightly unethical’, with additional concerns related to the implications of incentive withdrawal for non-compliance for personal well-being:
In terms of ethics those that don’t achieve, shouldn’t be made to feel bad about themselves for taking part in a research project so it’s a difficult thing, from an ethical perspective.
62, ethics committee member
It was also believed that incentives provided only to the intervention arm could jeopardise the trial in terms of attrition as the ‘control might end up with a very low number’. Concerns were expressed about the capacity of smoking cessation trials to recruit representative samples as it’s an addictive habit and ‘a lot just wouldn’t take part because of that’.
The need for careful planning of the trial design was acknowledged, as other intervention components that include BCTs are important and it is difficult to ‘unpick what was caused by your incentive and what was caused by your intervention’.
Discussion
Key findings
The primary qualitative findings are presented as a logic model to illuminate the meanings, mechanisms of action and interactions of incentives to inform our shortlist of promising strategies and to uncover the unintended consequences associated with incentives.
Our data illustrate how the fit between the real lives of women and incentive intervention programmes is crucial. Using a ladder conceptual model, we illustrate this fit, showing how rungs supporting behaviour change can be missing (e.g. lack of family support, stressful lives, unsatisfactory relationships with health professionals) and how supportive rungs can break (e.g. if a partner resumes smoking, the need for a breastfeeding woman to return to work), thus identifying where incentive intervention programme ‘rungs’ might help.
Although attitudes towards incentive programmes were varied, women appeared more amenable to the idea than providers. Providers expressed greater caution about the unintended consequences of incentives, the appropriate use of health service resources and the ethics of incentives. In relation to incentive components (rungs), guaranteed incentives were preferred for proven behaviour change whereas unpredictable incentives (e.g. raffles) were thought to encourage participation, irrespective of outcomes. Incentives were generally more acceptable if provision was dependent on proof of a successful outcome; however, methods for gaining proof of smoking cessation were considered fallible and lack of biochemical proof for breastfeeding is problematic, with preference for trust rather than statements of verification or video/photographic evidence.
A typology of incentives emerged, which ranged from highly restrictive incentives (e.g. health or behaviour related), to free-choice ‘hedonic’ incentives (e.g. shopping vouchers or cash), with the degree of free choice often critical to how they were perceived. The necessity to individually tailor incentives was a recurring theme, as was the potential for incentives to enhance well-being or to accompany the provision of additional support as an intervention component. Although the financial value of the incentive influenced the significance of the incentive for some women, the relationship was not necessarily commensurate. Breast pumps, although considered to be problematic among health professionals because of conflicting advice and guidance concerning their use, were particularly attractive to women on a low income and when sharing the experience or the onus of feeding, particularly with partners, was a priority.
In terms of other programme components, it was considered important that incentives should be combined with short-term goal-setting, monitoring, personal feedback, verification of achievement and other BCTs. Help and support were felt to be important programme components, and education and information were also mentioned, albeit these were considered less valuable. Sensitive communication was required to avoid perceptions of pressure, fear, failure and judgement. Feeling supported was considered most important for both behaviours, particularly for breastfeeding when women and babies were learning a new and often challenging practical skill. Incentives are tangible non-verbal cues and, similarly, education and support that included objects added to the environment, technology or visual prompts, such as seeing the monitor turn green, and sensations such as smell seemed more powerful as motivators than words for some women.
Flexible settings for incentive programme delivery were suggested, with home or community locations favoured over health premises. Dedicated specialist services with continuity of non-judgemental care were preferred, whereas personal experience of the behaviour was less important. Although midwives and health visitors were considered best placed to deliver incentives by some, there were concerns about their lack of skills, different agendas and capacity and the potential impact on health professional–women relationships. Early engagement of pregnant women into incentive programmes was thought to be advantageous, as was continuation of programmes throughout pregnancy and following birth. Face-to-face support was considered important for building relationships and to individually tailor an approach in terms of timing, frequency, intensity and mode of delivery (face to face, telephone, text message, group), as no single package suited all.
Although many felt that these target behaviours warranted public health funding, others considered that spending taxpayers’ money on incentives was not appropriate. Involvement of local businesses was popular with some women, but health professionals felt that this could present conflicts of interest. Universal and equitable rather than targeted incentives were considered more acceptable to reduce stigma, although there were concerns about increasing health inequalities as more marginalised women were thought less likely to access them. Provider incentives at a higher organisational level rather than for individual health professionals were considered important when they involved setting targets or funding specialist services that women valued. Finding a way to incentivise everyone – women, families, communities and providers – was suggested. However, incentives beyond the individual (e.g. partners or wider social networks) were more acceptable for smoking cessation than breastfeeding, and concerns were raised around creating family pressure.
Positive consequences of incentive programmes were identified with regard to health and emotional outcomes and providing resources to those most in need. However, key negative consequences of incentives included fairness, potential deleterious effects on personal responsibility and motivation, the risk of gaming and cheating, possible adverse effects on family and provider–mother relationships, perceptions of wasting NHS resources and the opportunity costs in terms of the negative impact on other services that would benefit more from the resources. Wider personal and social benefits of involvement in research trials were identified. However, negative attitudes and behaviours associated with trial arm assignment were highlighted, as well as difficulties in distinguishing between the effect of the incentive and that of other programme components.
Strengths and limitations
The strengths of this study are primarily methodological and include the multidisciplinary, mixed-methods, three-site approach, which enabled us to contrast the views and experiences of those with and without direct experience of incentive interventions. This provided contextualised and experiential as well as theoretical perspectives on a wide range of different incentive strategies to inform both the mechanisms of action of incentives for behaviours around pregnancy and childbirth and our incentive strategy shortlist. Stratified sampling techniques enabled us to obtain a broad range of views from participants with a diverse range of socioeconomic and behavioural characteristics, including participants who seldom engage in health services research. However, the views that were collected revealed some common ‘norms’ across that sample, for example only a small number of the women had not tried to breastfeed or planned to formula feed, perhaps reflecting that 80% of UK women initiate breastfeeding. 53 In addition, limited views from wider family members were collected. Data collection was undertaken by five researchers with diverse participants over a prolonged period of time, with different research questions addressed at different times within the BIBS study and the process evaluation of the CPIT providing a different objective and context. This resulted in variations in terms of how the interview and focus group questions were framed. The in-depth richness of the data suggests that this was a strength rather than a limitation. The significance of framing effects is discussed in Chapter 7 and is highly relevant to controversial topics in which there is ambivalence. Using a smaller team of researchers, with a structured, fixed interview topic guide, could have resulted in systematic bias in the data collected. The use of vignettes frame the incentives; however, they enabled participants to highlight issues from a more individualised, reflective perspective than would have been achieved using question–answer techniques. They helped to investigate aspects of the patient journey through an incentive intervention (see Chapter 3) in a more tangible, less abstract format, as delivery processes were not well reported in the studies included in our systematic review (see Chapter 3). CPIT data were included at a late stage and were analysed using the existing thematic framework to minimise bias but allow triangulation of these independently collected data. Every effort has been made to be transparent about the source of data and the context in which we interpret it, as this was a complex topic and the study focus changed over time.
Fit with the literature
The typologies of women reported by Nichter and colleagues110 and Radley and colleagues113 for smoking cessation incentive programmes in pregnancy, and the typology for a wider range of wellness incentives described by Schmidt,271 resonate with our data and influence engagement and behaviour outcomes. These typologies have important implications for policy decisions about whether incentives are universal, the preferred option in our study, or targeted to those who have the capacity to benefit and/or who are most likely to succeed. The differential uptake across social classes and the potential for health inequalities to increase is a concern, as noted for all lifestyle behaviour change interventions. 282 However, typologies have their limitations in terms of stigma, their static nature and the grey areas between categories.
Individually tailoring the incentive and support components of an intervention, as well as the delivery processes, was an important study finding as one size did not suit all. Important themes included autonomy and freedom to choose, as illustrated by preferences for unrestricted hedonic shopping vouchers over health-related or utilitarian incentives; the wide range of material and non-material influences that enable women to take advantage of the opportunities available to them to achieve change and well-being; and the value of trusting continuing relationships. The question of ‘who controls the incentive’ has implications for the meanings of the incentive for women, and potential implications for women’s motivation, as described in self-determination theory. 23 The sense of women wanting to self-direct and be in control of their lives and behaviours around childbirth, with intrusion by others unwelcome, is illustrated by the dislike of household services as an incentive, confirming data from a recent qualitative study. 62 These themes resonate with the capabilities theory of Amartya Sen283 and assets-based approaches to health improvement284,285 in relation to reducing health inequalities and improving well-being and quality of life as outcomes. Incentives, combined with a facilitative supportive package, can therefore acknowledge women’s capabilities, rather than highlighting their deficits, which fits with the MAP theory of behaviour change. 16 Our data were congruent with self-directed learning style theory to establish and sustain lifestyle behaviours,12 as incentives alone were not considered sufficient, and the need for sensitive, authentic person-centred communication,67 as women dislike feeling judged or pressurised to behave in a way deemed appropriate by others. 225
Importantly, our data support and develop earlier qualitative research about the value of incentives as connectors. 112 They can motivate some women to succeed at the behaviour and to engage with preparatory behaviours. For example, peer support initiatives112 or the use of breast pumps as technology connectors could help to overcome emotional barriers to feeding at the breast. They also facilitate parents’ valued social lives and family roles in child care. Food and drink286,287 or an ironing service287 can increase participation in education or support group programmes and incentives can increase recruitment and participation in research trials. 216 Gifts focusing on maternal well-being were valued for their communication of achievement and recognition of the effort required to ‘be a good mother’, when the odds might be stacked against this. Gifts can also be considered a cultural norm in the context of pregnancy and parenting magazines, websites, proactive e-mails and texts to shared mailing lists and NHS ‘bounty bags’, where small gifts and discounts are widely provided to advertise products and services. Preference for incentives that increase feelings of well-being is consistent with findings that maternal and family well-being are important drivers in behaviour decisions. 288,289
Life course events and their relation to disadvantage and the accompanying vulnerability or resilience in the face of adversity were important in our data, as noted by others. 290 Situations, for example domestic stress or an unsatisfactory health-care encounter, can occur suddenly, be emotionally intense, preclude rational decision-making14 and be pivotal for behaviour change. 17,26,289 Incentive programmes therefore need to be flexible and tailored to fit with ‘everyday life rungs’, which was consistent with the qualitative evidence syntheses for the barriers to and facilitators of smoking cessation and breastfeeding (see Chapter 4). Early engagement in programmes at ‘teachable moments of increased receptivity’, with assessment of motivation and tailoring of the timing and modes of delivery, supports the conclusions of Racine and colleagues;291 however, this creates challenges for the design of complex intervention trials. For behaviour maintenance, there is a need to tailor support to tie into ‘pivotal points’ (the times when support is most needed), such as the early days after birth, stressful situations, social events and return to work. 288 It could be argued that this supports the findings that smoking and breastfeeding are not independent behaviours and/or outcomes68–70 but rather are bound up in the complexity of women’s everyday lives. Rather than applying an individually targeted incentive programme there may be merit in applying ecological and systems approaches to behaviour change,19,292 thus pointing to the potential for complex interventions to address multiple behaviours. Weight management is an issue particularly with smoking cessation, but there is currently a gap in the evidence with regard to incentivising both. However, offering health-related incentives to promote exercise and reduce smoking, as in the study by Ussher and colleagues,150 and offering free vouchers for use at local leisure facilities for breastfeeding112 were largely unattractive. Consistent with wider ecological and systems approaches, some considered that there was a need to incentivise beyond the individual, to include organisations, providers, families and communities to achieve a cultural shift and collective efforts. Work-based incentives (see Chapter 3) were considered desirable; the pressure on women to return to work can have an adverse influence on breastfeeding and return to work can lead to smoking relapse in the presence of work culture norms and smoking rituals. The engagement of partners/wider social networks in incentive provision shows some promise104,141,176,178 but was more acceptable for smoking cessation than for breastfeeding, where women’s personal choice was paramount and additional partner pressure was considered undesirable.
The finding that women value support both over and above incentives for breastfeeding or education also fits with earlier qualitative findings112 and with the evidence that any additional support, professional or lay, improves breastfeeding outcomes. 65 To date, there is little evidence that education is effective. 54,293 Although there are recommendations for the expression of breast milk and the use of breast pumps for sick and/or premature infants,294 there appears to be uncertainty and conflicting health professional advice around this practice for healthy, full-term babies. 295 Because of the potential capacity, knowledge and skill deficits within health providers, and some reservations expressed by midwives and health visitors about behaviour change roles, the delivery of incentive programmes within dedicated services was preferred. The utility of structured support to provide shorter-term, small achievable goals, with resetting and feedback, has been previously recommended as an effective behaviour change strategy215,296 and can counter the discounting that occurs for non-tangible, theoretical longer-term adverse health outcomes. Monitoring appointments and verification of behaviour outcomes were important for motivation and acceptability, but also a source of concern for many, with no standard for breastfeeding and imperfect standards for smoking cessation. 163
Ethical issues and concerns about how incentive provision could encourage unhealthy behaviours, game playing and coercive practices also support those identified by the wider incentives literature (see Chapter 5) and a Citizen’s Council meeting held by NICE. 297 Although small personal gifts were felt to be less open to abuse than those of a larger financial value, these findings may well reflect an internal bias to overestimate risk,15 particularly when ‘cheating’ was not evident within the CPIT data.
Conclusions
Implications for the incentive taxonomy
-
A typology of incentive types, incorporated with the IRBCTs developed in Chapter 3, was incorporated into an incentive ladder logic model, as a taxonomy just for incentives alone did not fit with the complexity of the data.
-
We hypothesise that an incentive intervention would be unlikely to change or maintain behaviour in isolation and, as the ladder model demonstrates, the interaction and fit with other life course and context rungs will be likely to affect engagement and effectiveness.
-
Incentives and other intervention components in programmes would benefit from being tailored, communicated, interpreted and delivered to enable women to bolster their individual capabilities and their existing intrinsic and extrinsic influences on smoking and breastfeeding.
Implications for the shortlist of incentive strategies
-
Shopping voucher incentives for smoking cessation in pregnancy and continued after birth were preferred to shopping vouchers for breastfeeding.
-
Incentives beyond the individual, which include support from partners, families, friends and service providers, show promise, with smoking at home highlighted as a barrier to sustained behaviour change.
-
Breast pumps and breastfeeding behaviour-related incentives were popular, particular with younger mothers and those with material disadvantage. They addressed barriers relating to negative emotions about the role of breasts for feeding and those relating to the desire to share the onus of breastfeeding with others, particularly for resuming a public social life.
Incentives for providers provoked mixed views, with concerns about the impact on other services. However, the importance of everyone working in the same direction to support women was recognised as a positive consequence of incentives to organisations.
Chapter 7 Surveys to inform the acceptability of incentive interventions around childbirth
Background
In this chapter we report two surveys to investigate the acceptability of providing incentives from the perspectives of (1) the general public and (2) health professionals and other stakeholders working in maternity and early years services.
Acceptability of incentives offered to people to change their behaviour
Assessment of the acceptability of incentives to the wider public is potentially important in the current economic climate, where the use of taxpayers’ money in public services is already widely scrutinised. Incentive initiatives such as Give it up for Baby,113 which provides £12.50 per week of grocery vouchers, has generated media controversy and resistance from some organisations, such as the TaxPayers’ Alliance. 298
The National Institute for Health and Care Excellence held a Citizen’s Council in May 2010 to discuss when and whether incentives to change individual behaviour might be acceptable to improve health. 297 Of 32 attendees, 12 voted against and 20 voted for incentives, but with conditions such as the need for evidence that incentives work; the need for evidence on why incentives sometimes fail; and cash incentives should be a last resort. Other concerns were unfairness to those who make healthy choices; that incentives appear to reward unhealthy behaviours; the potential for abuse; the cost; the need to monitor and safeguard, particularly if private companies are involved; and the danger of becoming a ‘nanny state’. The benefits of incentives included their potential to demonstrate to people that they are worthy of being helped; to facilitate contacts between recipients and care providers; and to provide benefits for child health.
There are also some survey data available on public attitudes to incentives in the UK. For example, the British Social Attitudes survey299 asks, ‘Regardless of what happens now, what, in your view, is the best way for the government to help people to lead healthy lifestyles?’ ‘Pay people’ was the least popular option (2.4%), with more popular options being tax unhealthy things (e.g. alcohol and cigarettes) (21.9%), leave people to make their own choices (17.8%) or provide information (48.0%). A decision guide survey for purchasers asked the question, ‘Are consumers in our community ready for financial incentives?’254 The answer, the authors argue, depends on how the information is presented, keeping the message simple and designing financial incentives that better align the goals of consumers with those of the programme sponsor, yet the authors point out that this has received little direct research attention. The importance of how a question is worded is confirmed by a 2012 survey of Scottish adults by Ipsos MORI (n = 1003). 300 In total, 49% supported paying women to stop smoking in pregnancy and 44% supported paying women to breastfeed. However, the nature of the incentive and how the incentive scheme was described affected responses. When the same question was asked about rewards, which might or might not be financial, rather than payment, there was an increase in support. Of those who originally opposed, 26% said that they would be more likely to support such schemes and a further 27% said that they would be more likely to support rewards for some behaviours. Of the 506 Scottish adults who were asked about rewards, > 50% supported rewards for breastfeeding and 70% supported rewards for giving up smoking when pregnant. Incentives for smoking in pregnancy received the strongest support of all behaviours, followed by coming off (non-prescription/illegal) drugs, smoking in general, attending parenting classes, taking more exercise and, finally, losing weight. There was less opposition when there is a direct benefit to children. 300 In contrast, in a DCE study, in which participants were presented with alternative treatment packages, respondents were asked, ‘Which treatment should be funded?’ incentives (being paid £50.00 per month) for weight loss were more acceptable than those for smoking cessation (60% vs. 40%). 301
In the USA, a four-arm RCT of an experimental survey completed by 1010 American adults waiting at public transport depots found that the overall acceptability of a $25.00 increase in annual health insurance to pay for a smoking cessation treatment package was 41.6%. 302 The financial incentive arm of the trial received the lowest support (39.3%), but this was not statistically different from that in the unspecified treatment arm (45.6%) or the medication arm (41.7%). In this RCT, the incentive arm question was (p. S42):
According to research, paying people to stop smoking helps some of them to quit smoking. Each person who stops smoking is given $750.00. Would you support a $25.00 increase in your annual health insurance premium to pay for this treatment?
The question in the fourth arm (financial incentives with statement on societal costs) was identical to that in the financial incentives arm except that it included a preceding sentence on societal costs: ‘On average, each person who smokes costs society over $2000.00 per year due to health problems’ (p. S42). 302 The a priori hypothesis being tested was that support for a financial incentive programme would increase when evidence on the societal cost of smoking was included in the questionnaire; however, this was not demonstrated. Park and colleagues302 found no differences in the acceptability of financial incentives for smoking cessation according to income, gender, ethnicity or educational level.
Questions derived from a Multidimensional Locus of Control Scale were rated by Park and colleagues302 and the strongest agreement was with statements for an internal locus of control, with personal responsibility for smoking behaviour, followed by those for an external locus of control, with help from other people playing a big role, and lastly the role of chance or luck in determining smoking habits.
Public attitudes are shaped by collective and individual experiences and the level of awareness of the adverse effects of a behaviour on health. When Park and colleagues302 compared responses to a question asking whether there should be financial incentives for smoking cessation, according to participant smoking status, current smokers (53%) were more likely to support financial incentives than either previous smokers (37%) or lifetime non-smokers (37%), with a similar pattern observed for a question asking whether unspecified treatment and medication should be provided for smoking cessation. Apart from the Ipsos MORI survey,300 there are no comparable data on the public acceptability of incentives for breastfeeding. However, it is known that personal experience, for example having been breastfed as a baby or having breastfed a previous child, are predictors of feeding outcome. 53
The acceptability and fairness of financial rewards and penalties compared with medical interventions for smoking cessation together with several other health outcomes were considered in separate online surveys in the UK (n = 88) and USA (n = 100). 303 The authors hypothesised that penalties would be favoured over rewards when recipients were deemed responsible for their condition and vice versa. There was strong agreement that smokers were responsible for smoking. Acceptability ratings for financial incentives were lower than for medical interventions and preference for rewards or penalties varied with context. In a DCE study with three samples, two from an online panel (n = 81 and n = 101) and one from a general offline population (n = 450), the acceptability of payments of £50.00 per month for smoking cession or weight loss was very sensitive to effect size, even to small changes in effectiveness. 301 For example, an increase from 10% to 11% in the effect size of an incentive for smoking cessation resulted in an increase from 46% to 55% in the proportion favouring incentives.
The acceptability of providing incentives to health service providers
In the review of the literature (see Chapter 3), no studies were identified that looked at the acceptability of providing incentives to care providers or organisations from the point of view of the general public for either smoking cessation in pregnancy or breastfeeding. The review of the wider incentives literature relevant to lifestyle behaviour change suggested that this warrants further investigation (see Chapter 5).
Methods
In Chapter 6 we presented the shortlist of promising incentive strategies emerging from the literature review and early qualitative data collection and this formed the basis for the survey design. The shortlisted incentive strategies for initiating and sustaining smoking cessation in pregnancy and breastfeeding are:
-
shopping vouchers for women who prove that they have stopped smoking during pregnancy
-
shopping vouchers for a woman for 2 months after the birth of her baby if she proves that she is still not smoking
-
shopping vouchers for a woman for 2 months after the birth of her baby if she never lets anyone smoke in her home
-
shopping vouchers for women who prove that they are breastfeeding for the first 6 months after birth
-
a breast pump costing around £40.00 provided for free on the NHS
-
additional funding for local health services if they reach targets for the number of women who prove that they have stopped smoking during pregnancy
-
additional funding for local health services if they reach targets for the number of women who prove that they are breastfeeding.
Research questions
The main research question addressed by the general public and health professional surveys was:
-
Are the seven shortlisted incentive strategies for initiating and sustaining smoking cessation in pregnancy and breastfeeding acceptable to (1) the general public and (2) health professionals involved in providing maternity services and other stakeholders?
In addition, the following analysis questions were posed:
-
Is the acceptability to the general public of the seven shortlisted incentive strategies influenced by age (categories: 18–24 years, 25–34 years, 35–44 years, 45–54 years, 55–59 years, 60–64 years, ≥ 65 years), sex, social grade (A, B, C1, C2, D, E), region (North, North West, Yorkshire and Humberside, East Midlands, West Midlands, East Anglia, South East, South West, London, Wales, Scotland), ethnicity (white British, other ethnicity), education (degree level, A level or equivalent, GCSE or equivalent, no formal qualifications, still studying or other qualifications or don’t know), having children (yes, no), personal experience of smoking (never smoked, ex-smoker, current smoker – failed to quit or no quit attempts) or whether a child was ever breastfed (even if only for a day or two)? What are the independent predictors of acceptability of the shortlist of incentive strategies?
-
Is the acceptability to health professionals of the seven shortlisted incentive strategies influenced by health professional group [doctor (GP, obstetrician, paediatrician) or nurse (midwife, health visitor, other maternity care staff) or other health professional], age (18–34 years, 34–44 years, 45–54 years, ≥ 55 years, missing), gender, ethnicity (white British, other ethnicity), having children (yes, no), personal experience of smoking (never smoked, previous smoker or current smoker or declined to answer), whether a child was ever breastfed? What are the independent predictors of acceptability of the shortlist of incentive strategies?
-
What value of incentive is most acceptable and what are the independent predictors of the preferred incentive value for (1) smoking cessation in pregnancy and (2) breastfeeding women?
-
Are universal incentives for (1) smoking cessation in pregnancy and (2) breastfeeding preferred to incentives targeted at low-income women and what are the independent predictors for preference?
Objectives
The objectives were to:
-
contribute questions to the Ipsos MORI Computer Aided Personal Interviewing (face-to-face omnibus) (CAPIBUS) survey to assess the acceptability of the shortlist of promising incentive strategies to the general public
-
conduct a web survey linked to an e-mail to assess the acceptability of the shortlist of promising incentive strategies to a range of health service professionals involved in pregnancy and early years care.
Questionnaire design
The research team selected the sociodemographic variables most relevant to the research question from the range used by Ipsos MORI. An additional sociodemographic variable for personal smoking status was selected from a recent relevant study301 to provide comparable data. The research team searched existing large surveys to find equivalent questions for personal experience of breastfeeding that had been validated for use with the general public. None was identified – the national Infant Feeding Survey questions (Q68, Q69),53 which are asked of women after childbirth, were not considered to have face validity for older male respondents. A single question following a filter question so that only people with children would be asked the question was designed: ‘Have any of your children ever been breastfed or received breast milk, even if only for a day or two?’ For the health professional survey, occupation was asked rather than social grade. Question design relating to specific aspects of our shortlist of promising incentive strategies (see Chapter 6) was decided iteratively by the research team through discussion and was guided both by experts at Ipsos MORI and by academic colleagues. Careful consideration was given to potential framing effects in how the survey was introduced. Promberger and colleagues301 reported that even a 1% increase in effectiveness can dramatically increase acceptability. This provided the logic for the opening statement at the beginning of the telephone and web surveys. As effectiveness for contingent shopping vouchers for smoking cessation in pregnancy was a finding from the meta-analysis of four incentive trials (see Chapter 3) and interim analysis of the CPIT II trial, which the research team had access to, an effectiveness statement was included. However, as the effectiveness of incentives for breastfeeding is more uncertain (see Chapter 3), this was omitted from the introductory statement. A decision was made to provide minimal introductory text about specific health benefits to minimise bias from framing effects. Our introductory statements were therefore as follows:
Stopping smoking in pregnancy benefits the health of the baby and the mother. Research shows that providing shopping vouchers to women who prove that they have stopped smoking in pregnancy increases the numbers who stop. While some people feel that providing vouchers is appropriate, others feel that it is wrong or unfair.
Breastfeeding benefits the health of the baby and the mother. While some people feel that providing shopping vouchers to encourage breastfeeding is appropriate, others feel that it is wrong or unfair.
Careful consideration was given to the terminology used to describe an incentive, as an earlier MORI survey found greater acceptability for incentives by calling them rewards rather than payments. 300
The following phrase was standardised across questions: ‘monthly shopping vouchers to reward women who prove that they have stopped smoking during pregnancy/are breastfeeding’. Monthly was selected as this was the frequency used in several studies including the CPIT88,113,141 (see Box 2). Questions about variable intensity as in the effective incentives for smoking cessation trials105,144 were considered too complex for a general public survey. A filter question for those agreeing or neither agreeing nor disagreeing with vouchers asked about the highest acceptable value, starting from a value close to zero (£2.00 per month) up to a value of £80.00 a month, which is higher than the upper value in any of the studies that we identified.
Gaming and cheating, by either taking up smoking deliberately to receive the reward or smoking in-between monitoring checks, is a concern raised by the qualitative data in this study and others. 297 As strongly held views about this could confound all responses, the word ‘proof’ was included in all questions about rewards being given to women.
The questionnaire was piloted by HM and GT with members of the general public (three women and four men; age range 17–57 years) and 24 health professionals from midwifery, health-visiting, children’s centre and voluntary backgrounds. Individual interviews were conducted using cognitive interviewing (think aloud) techniques. 114
Study populations
-
General public survey: a nationally and regionally representative sample of UK adults aged ≥ 18 years.
-
Health professional survey: maternity unit staff, health-visiting staff, obstetricians, paediatricians, public health specialists, GPs, practice nurses and policy-makers whose work involves caring for pregnant and postnatal women and/or infants and who work in Scotland or North West England.
Data collection for the general public survey
Potential respondents were identified by Ipsos MORI interviewers, who are asked to interview five people from every 250 addresses. They may knock on between five and 100 doors to achieve this. All interviews were conducted face to face at home between 22 March 2013 and 15 April 2013 using CAPI (Computer Assisted Personal Interviewing) (see Appendix 24). Quotas are set for age, gender and region and the data weighted to the known profile of Great Britain using age, gender, government office region, social grade, taken a foreign holiday in the last 3 years, tenure, number of cars in the household and working status. Targets for quotas and weights are taken from the National Readership Survey (see www.nrs.co.uk/). For this survey, questions relating to incentives (CS1–10 in Appendix 25) were asked after questions on standard demographics that are routinely asked by MORI. Parent, smoking and breastfeeding status were asked at the end of the survey. Randomisation of the order of questions on smoking and breastfeeding incentives was generated independently and automated using CAPI software, to investigate question framing effects.
Data collection for the health professional survey
We aimed to recruit health professionals and stakeholders involved in providing maternity services and early years care by sending an e-mailed web link to the Ipsos MORI survey, which we reproduced on SurveyMonkey (see www.surveymonkey.com) (see Appendix 26). Gaining access to e-mail lists for the population to survey was discussed with the Scottish Primary Care Research Network (SPCRN), the North of Scotland Research Ethics Committee (NOSRES), NHS Grampian Research and Development (R&D), the Scottish Multiprofessional Maternity Development Programme (SMMDP), the North West Strategic Health Authority, the Cumbria and Lancashire Research Network, the relevant Royal Colleges, Ipsos MORI and academic colleagues who had attempted similar surveys. Key publications were identified through colleagues, for example the study by Braithwaite and colleagues,304 who used a web survey of health professionals. However, these failed to identify any robust strategy for larger UK regional or national surveys of maternity and early years health professionals. The logistical difficulties of identifying and gaining the approval of the gatekeepers to e-mail lists of these specific groups of professionals and, where possible, knowing the denominator, became apparent in both Scotland and North West England.
In Scotland, R&D approvals were gained for every health board and the survey was then administered through:
-
the SPCRN to all general practice managers for distribution to GPs and staff involved in maternity and early years care
-
individual R&D departments for hospital, maternity and early years staff
-
a mailing list of public health doctors
-
a mailing list of paediatricians in training
-
two contacts at the Scottish government for distribution to relevant maternity and early years stakeholders.
This strategy could result in eligible participants receiving the e-mail through more than one source. It was not possible to calculate accurate denominators for most individual collectors.
However, the SPCRN provided a breakdown of general practice managers to whom the survey was sent for distribution to GPs (n = 4800 at July 2013305) and other relevant practice staff (n = 2140 at 30 January 2009306). Using Information Services Division (ISD) workforce data307 we know that, at 31 March 2013 (published 28 May 2013), there were 12,455 medical staff (hospital, community and public health services) and 66,068 nursing and midwifery staff who should have received a link to the survey through e-mails cascaded down in individual health boards.
The number of individuals receiving our web survey is not known as its distribution was entirely dependent on the e-mail gatekeepers listed above. Separate SurveyMonkey collectors were set up to monitor response rates and these are summarised in Appendix 27.
In North West England the timing of the survey was unfortunate as it coincided with the implementation of the Health and Social Care Act 2012308 on 1 April 2013, which resulted in considerable NHS reorganisation and upheaval. Through discussions with the Cumbria and Lancashire Research Network and colleagues at UCLan (University of Central Lancashire), it was recommended to commission Binley’s (see www.binleys.com/; accessed 6 November 2014), a commercial organisation, to distribute the survey. The survey was sent by e-mail in May 2013 to 4821 relevant professionals on their mailing list (see Appendix 27). The survey was also issued to health-visiting and midwifery students at UCLan (n = 139), with seven responses received (response rate 5.0%). In July 2013, because of the low response rate from Binley’s, all R&D departments within the North West trusts were also asked to distribute the survey to relevant professionals (n = 13 respondents, denominator unknown).
Data analysis
An a priori target sample size of 1000 was set for both surveys to allow us to estimate proportions to within 3% with 95% confidence. Data were described using the appropriate summary statistics where relevant. Responses to the Likert-style outcome items were summarised as number and percentage and graphed using bar charts. Responses to these outcome items were broken down by the independent predictor variables specified earlier. Net agreement (agree and strongly agree) and net disagreement (disagree and strongly disagree) were also reported as number and percentage. Simple and multiple ordered logit regression models were used to determine the independent predictors of acceptability for the shortlist. The relationship between predictor and outcome variables was summarised using odds ratios (ORs) and 95% CIs. For the value of incentives and targeting of incentives to low-income women only (research questions 4 and 5), two-part models were used. For research question 4, the value of incentives, a probit model was used to estimate a ‘positive’ response (i.e. strongly agree, agree or neither agree nor disagree) and then linear regression was used to model the value of shopping voucher acceptable to those with a positive response. For research question 5, targeting low-income women only, a similar model was used but as the conditional response here was dichotomous a probit model was used instead of linear regression. In all models the most affluent status was used as the reference category when this was possible (i.e. child breastfed, male, degree-level qualification, London region, never smoked, white ethnicity, social grade A or B, no children). Age was entered as 5-year categories. All analyses were carried out in Stata 13.
Results of the Ipsos MORI survey of the acceptability of seven incentive strategies
Sample characteristics
The characteristics of the 1144 respondents are summarised in Table 44. There is a broad sociodemographic spread of characteristics. Current smoking status among respondents is similar to that reported in other UK surveys (just over one in five adults), although 4.6% of respondents chose not to answer this question. Having a child who was ever breastfed, even for a few days, is low compared with the current reported breastfeeding initiation rate of around 80% in the UK;53 however, breastfeeding initiation has increased over recent decades since the first survey in 1975.
| Variable | Classes | n (%) |
|---|---|---|
| Sex | Male | 540 (47.2) |
| Female | 604 (52.8) | |
| Age (years) | 18–24 | 170 (14.9) |
| 25–34 | 175 (15.3) | |
| 35–44 | 181 (15.8) | |
| 45–54 | 159 (13.9) | |
| 55–59 | 72 (6.3) | |
| 60–64 | 94 (8.2) | |
| ≥ 65 | 293 (25.6) | |
| Ethnicity | White | 985 (86.1) |
| Black and minority ethnic | 151 (13.2) | |
| Refused to answer | 8 (0.7) | |
| White British | 914 (79.9) | |
| White Irish | 11 (1.0) | |
| White gypsy/traveller | – | |
| White other | 60 (5.2) | |
| Mixed white/black Caribbean | 3 (0.3) | |
| Mixed white/black African | 1 (< 0.1) | |
| Mixed white/Asian | 3 (0.3) | |
| Mixed other | 2 (0.2) | |
| Asian Indian | 19 (1.7) | |
| Asian Pakistani | 47 (4.1) | |
| Asian Bangladeshi | 12 (1.0) | |
| Asian Chinese | 7 (0.6) | |
| Asian other | 13 (1.1) | |
| Black African | 26 (2.3) | |
| Black Caribbean | 7 (0.6) | |
| Black other | 2 (0.2) | |
| Arab | 4 (0.3) | |
| Other | 5 (0.3) | |
| Refused | 8 (0.7) | |
| Smoking status | Never smoked | 573 (50.1) |
| Current smoker, tried to stop smoking | 175 (15.3) | |
| Current smoker, not tried to stop smoking | 63 (5.5) | |
| Ex-smoker | 281 (24.6) | |
| Declined to answer | 52 (4.5) | |
| Any children | Yes | 742 (64.9) |
| No | 402 (35.1) | |
| Breastfeeding | Any children breastfed | 512 (44.8) |
| No children breastfed | 632 (55.2) | |
| Education | Degree/Masters/PhD | 295 (25.8) |
| A-level or equivalent | 193 (16.9) | |
| GCSE/O-level/CSE/NVQ | 342 (29.9) | |
| No formal qualifications | 197 (17.2) | |
| Other/don’t know/still studying | 117 (10.2) | |
| Social grade | A | 36 (3.1) |
| B | 203 (17.7) | |
| C1 | 370 (32.3) | |
| C2 | 236 (20.6) | |
| D | 162 (14.2) | |
| E | 137 (12.0) | |
| Survey region | North | 77 (6.7) |
| North West | 142 (12.4) | |
| Yorks and Humberside | 104 (9.1) | |
| West Midlands | 109 (9.5) | |
| East Midlands | 66 (5.8) | |
| East Anglia | 41 (3.6) | |
| South West | 81 (7.1) | |
| South East | 200 (17.5) | |
| Greater London | 149 (13.0) | |
| Wales | 66 (5.8) | |
| Scotland | 109 (9.5) |
Figure 19 provides bar charts summarising the acceptability of all seven promising incentive strategies.
FIGURE 19.
Bar charts showing general public agreement with the shortlist of incentive strategies. A, agree; BF, breastfeeding; D, disagree; ND, neither agree nor disagree; SA, strongly agree; SD, strongly disagree; SS, stop smoking.

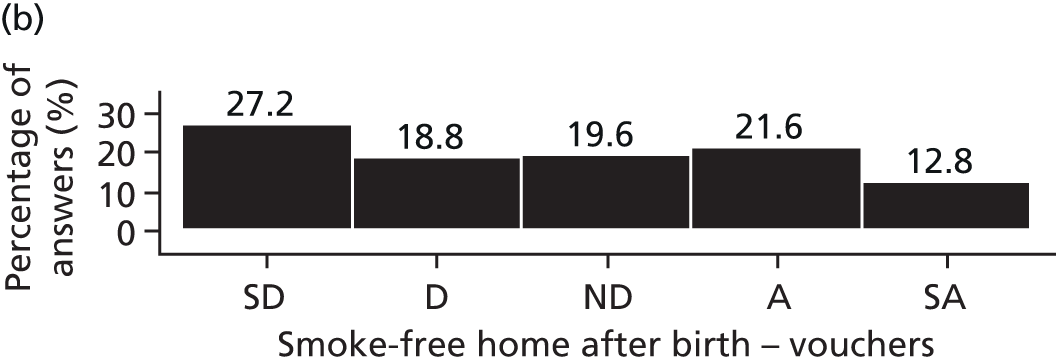


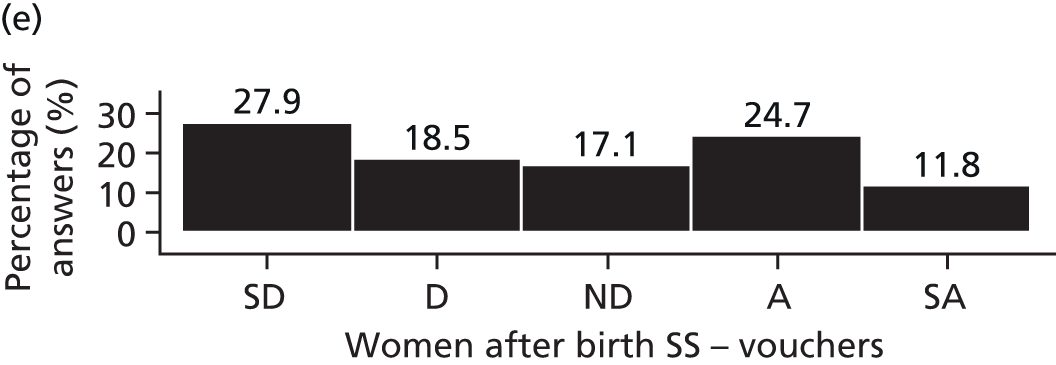

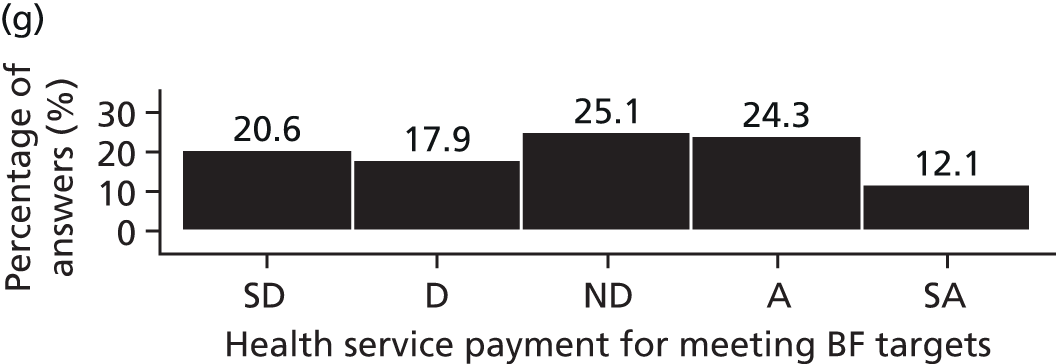
Shopping vouchers for women who prove that they have stopped smoking during pregnancy
There was a net disagreement of 42.3% (484/1144) with the incentive strategy of shopping vouchers for smoking cessation during pregnancy. Net agreement was 40.5% (463/1144). A summary of the results for each predictor variable against response to this incentive strategy is reported in Appendix 28 (see Table 70). From a multiple ordered logistic regression model the predictors of agreement were:
-
Age: younger age groups (18–24 years, 25–34 years and 35–44 years) were significantly more likely to agree with this incentive strategy than the reference group aged ≥ 65 years. The age group 35–44 years had the biggest OR (1.88, 95% CI 1.30 to 2.72; p = 0.001).
-
Sex: women were less likely to agree with this incentive strategy than men (OR 0.71, 95% CI 0.57 to 0.88; p = 0.002).
-
Ethnicity: respondents who classified themselves in a category other than white British were more likely to agree with this incentive strategy than white British respondents (OR 1.42, 95% CI 1.01 to 1.99; p = 0.047).
-
Education: respondents with lower levels of education were less likely to agree with this incentive strategy than the reference group with a degree-level education [General Certificate of Secondary Education (GCSE): OR 0.59, 95% CI 0.43 to 0.81, p = 0.001; Advanced (A) level or equivalent: OR 0.63, 95% CI 0.44 to 0.90, p = 0.010; no formal qualifications: OR 0.63, 95% CI 0.42 to 0.95, p = 0.029].
-
Social grade: those in social grade E were more likely to agree with this incentive strategy than the reference group (social grades A and B) (OR 1.74, 95% CI 1.12 to 2.70; p = 0.014). Other social grades did not differ from group AB.
-
Smoking status: current smokers who had tried quitting previously were more likely to agree with this incentive strategy than those who had never smoked (OR 1.63, 95% CI 1.18 to 2.26; p = 0.003), but other smoking status groups were broadly similar to the reference group who had never smoked.
-
Geographical area: the North, Yorkshire and Humberside, East Midlands, West Midlands, Wales and Scotland all had ORs of < 0.8, indicating that respondents from these areas were less likely to agree with this incentive strategy than respondents from the reference group, those living in London. However, only the OR for Wales ruled out unity (OR 0.55, 95% CI 0.31 to 0.97; p = 0.04).
Full details of the univariable and multivariable ordered logistic regressions are provided in Appendix 28 (see Table 71).
Shopping vouchers for a woman for 2 months after the birth of her baby if she proves that she is still not smoking
There was a net disagreement of 46.4% (531/1144) with the incentive strategy of shopping vouchers for smoking cessation for 2 months after pregnancy. Net agreement was 36.5% (417/1144). A summary of the results for each predictor variable against response to this incentive strategy is reported in Appendix 28 (see Table 72). From a multiple ordered logistic regression model the predictors of agreement were:
-
Age: there was an inconsistent pattern across the age categories, with those aged 35–44 years (OR 1.65, 95% CI 1.14 to 2.39; p = 0.007) and 60–64 years (OR 1.65, 95% CI 1.05 to 2.57; p = 0.028) significantly more likely to agree with this incentive strategy than the reference group aged ≥ 65 years. The remaining age groups were not significantly more likely to agree with this incentive strategy.
-
Sex: women were less likely to agree with this incentive strategy than men (OR 0.68, 95% CI 0.55 to 0.85; p = 0.001).
-
Ethnicity: respondents who classified themselves in a category other than white British were more likely to agree with this incentive strategy than white British respondents in the univariable model (OR 2.12, 95% CI 1.59 to 2.83; p < 0.001) but this relationship was attenuated when other variables were adjusted for in the multiple regression model (OR 1.39, 95% CI 0.98 to 1.95; p = 0.062).
-
Education: respondents with lower levels of education were less likely to agree with this incentive strategy then the reference group with a degree-level education (GCSE: OR 0.62, 95% CI 0.45 to 0.85, p = 0.003; A-level or equivalent: OR 0.68, 95% CI 0.48 to 0.96, p = 0.028; no formal qualification: OR 0.64, 95% CI 0.42 to 0.96, p = 0.032).
-
Social grade: those in social grade C2 were more likely to agree with this incentive strategy than those in the AB reference group (OR 1.64, 95% CI 1.18 to 2.27; p = 0.003). Other grades did not differ from the AB group.
-
Smoking status: there was no evidence of a difference across smoking status groups in response to this option.
-
Geographical area: the North, Yorkshire and Humberside, East Midlands, West Midlands, the South West, Wales and Scotland all had ORs of < 0.8, indicating that respondents from these areas were less likely to agree with this incentive strategy than respondents from the reference group, those living in London. All of these areas were significantly different from London apart from the North and the South West.
Full details of the univariable and multivariable ordered logistic regressions are provided in Appendix 28 (see Table 73).
Shopping vouchers for a woman for 2 months after the birth of her baby if she never lets anyone smoke in her home
There was a net disagreement of 46.0% (526/1144) with the incentive strategy of providing shopping vouchers for no smoking in the home for 2 months after pregnancy. Net agreement was 34.4% (394/1144). A summary of the results for each predictor variable against response to this incentive strategy is reported in Appendix 28 (see Table 74). From a multiple ordered logistic regression model the predictors of agreement were:
-
Age: younger age groups (18–24 years and 35–44 years) were significantly more likely to agree with this incentive strategy, with both groups having ORs > 1.5 compared with the reference group aged ≥ 65 years.
-
Sex: women were less likely to agree with this incentive strategy than men (OR 0.72, 95% CI 0.58 to 0.90; p = 0.003).
-
Ethnicity: respondents who classified themselves in a category other than white British were more likely to agree with this incentive strategy than white British respondents (OR 1.49, 95% CI 1.06 to 2.08; p = 0.021).
-
Education: respondents with lower levels of education were less likely to agree with this incentive strategy than the reference group with a degree-level education (GCSE: OR 0.60, 95% CI 0.44 to 0.83, p = 0.002; A-level or equivalent: OR 0.56, 95% CI 0.39 to 0.79, p = 0.001; no formal qualification: OR 0.66, 95% CI 0.44 to 1.00, p = 0.048).
-
Social grade: there was no evidence of a difference across social grade.
-
Smoking status: current smokers who had tried quitting previously were more likely to agree with this incentive strategy than those who had never smoked (OR 1.48, 95% CI 1.08 to 2.04; p = 0.016), but other smoking status groups were broadly similar to the reference group of those who had never smoked.
-
Geographical area: the North, Yorkshire and Humberside, East Midlands, West Midlands, the South West, Wales and Scotland all had ORs of < 0.8, indicating that respondents from these areas were less likely to agree with this incentive strategy than respondents from the reference group, those living in London. All of these areas were significantly different from London apart from the North, the South West and Wales.
Full details of the univariable and multivariable ordered logistic regressions are provided in Appendix 28 (see Table 75).
Shopping vouchers for women who prove that they are breastfeeding for the first 6 months after birth
There was a net disagreement of 39.1% (447/1144) with the incentive strategy of shopping vouchers for breastfeeding for the first 6 months. Net agreement was 34.2% (391/1144). A summary of the results for each predictor variable against response to this incentive strategy is reported in Appendix 28 (see Table 76). From a multiple ordered logistic regression model the predictors of agreement were:
-
Age: younger age groups (18–24 years, 25–34 years and 35–44 years) were significantly more likely to agree with this incentive strategy than the reference group aged ≥ 65 years. The 25–34 years age group had the biggest OR (1.91, 95% CI 1.31 to 2.89; p = 0.001).
-
Breastfeeding status: respondents with children who were breastfed were more likely to agree with this incentive strategy (OR 1.67, 95% CI 1.24 to 2.25; p = 0.001).
-
Sex: women were less likely to agree with this incentive strategy than men (OR 0.77, 95% CI 0.62 to 0.95; p = 0.016).
-
Ethnicity: respondents who classified themselves in a category other than white British were more likely to agree with this incentive strategy than white British respondents (OR 2.03, 95% CI 1.43 to 2.88; p < 0.001).
-
Education: there was no evidence of a difference across education groups.
-
Social grade: there was no evidence of a difference across social grade.
-
Smoking status: there was no evidence of a difference across smoking status groups.
-
Geographical area: the North, Yorkshire and Humberside, East Midlands, West Midlands, the South West and Wales all had ORs of < 0.8, indicating that respondents from these areas were less likely to agree with this incentive strategy than respondents from the reference group, those living in London. All of these areas were significantly different from London apart from the North.
Full details of the univariable and multivariable ordered logistic regressions are provided in Appendix 28 (see Table 77).
A breast pump costing around £40.00 provided for free on the NHS
There was a net disagreement of 27.3% (312/1144) with the incentive strategy of breast pumps being freely available on the NHS. Net agreement was 45.8% (524/1144). A summary of the results for each predictor variable against response to this incentive strategy is reported in Appendix 28 (see Table 78). From a multiple ordered logistic regression model the predictors of agreement were:
-
Age: younger age groups (18–24 years, 25–34 years and 35–44 years) were significantly more likely to agree with this incentive strategy than the reference group aged ≥ 65 years. The 18–24 years age group had the biggest OR (1.74, 95% CI 1.14 to 2.63).
-
Breastfeeding status: respondents with children in the family who were breastfed were more likely to agree with this incentive strategy (OR 1.84, 95% CI 1.36 to 2.49; p < 0.001).
-
Sex: there was no evidence of a difference between women and men.
-
Ethnicity: there was no evidence of a difference across ethnic groups.
-
Education: respondents with lower levels of education were less likely to agree with this incentive strategy then the reference group with a degree-level education (GCSE: OR 0.70, 95% CI 0.51 to 0.96, p = 0.026; no formal qualifications: OR 0.62, 95% CI 0.41 to 0.93, p = 0.02).
-
Social grade: there was no evidence of a difference across social grade, although comparing social grade E with social grade AB the OR was 1.57 (95% CI 1.00 to 2.46; p = 0.05).
-
Smoking status: there was no evidence of a difference across smoking status group.
-
Geographical area: respondents from the East Midlands and Wales were significantly less likely to agree with this incentive strategy than those living in London.
Full details of the univariable and multivariable ordered logistic regressions are provided in Appendix 28 (see Table 79).
Additional funding for local health services if they reach targets for the number of women who prove that they have stopped smoking during pregnancy
There was a net disagreement of 37.2% (426/1144) with the incentive strategy of additional funding for local health services if they reach targets for the number of women who prove that they have stopped smoking during pregnancy. Net agreement was 39.4% (451/1144). A summary of the results for each predictor variable against response to this incentive strategy is reported in Appendix 28 (see Table 80). From a multiple ordered logistic regression model the predictors of agreement were:
-
Age: younger age groups (18–24 years, 25–34 years and 35–44 years) were significantly more likely to agree with this incentive strategy than the reference group aged ≥ 65 years and the 45–54 years age group. The 18–24 years age group had the biggest OR (2.28, 95% CI 1.50 to 3.49; p < 0.001).
-
Sex: there was no evidence of a difference between women and men.
-
Ethnicity: there was no evidence of a difference across ethnic groups.
-
Education: those with lower levels of education were less likely to agree with this incentive strategy than the degree-level reference group (GCSE: OR 0.71, 95% CI 0.51 to 0.97, p = 0.033; A-level or equivalent: OR 0.68, 95% CI 0.48 to 0.97, p = 0.032).
-
Social grade: those in social grade C1 were less likely to agree with this incentive strategy than those in the AB group (OR 0.68, 95% CI 0.50 to 0.94; p = 0.019).
-
Smoking status: there was no evidence of a difference across smoking status group.
-
Geographical area: respondents from the North, Yorkshire and Humberside, East Midlands, West Midlands, the South West and Scotland were significantly less likely to agree with this incentive strategy than respondents from the reference group, those living in London.
Full details of the univariable and multivariable ordered logistic regressions are provided in Appendix 28 (see Table 81).
Additional funding for local health services if they reach targets for the number of women who prove that they are breastfeeding
There was a net disagreement of 38.5% (441/1144) with the incentive strategy of additional funding for local health services if they reach targets for the number of women who prove that they are breastfeeding. Net agreement was 36.4% (416/1144). A summary of the results for each predictor variable against response to this incentive strategy is reported in Appendix 28 (see Table 82). From a multiple ordered logistic regression model the predictors of agreement were:
-
Age: younger age groups (18–24 years, 25–34 years and 35–44 years) were significantly more likely to agree with this incentive strategy than the reference group aged ≥ 65 years. The 35–44 years age group had the biggest OR (1.91, 95% CI 1.32 to 2.76; p = 0.001).
-
Sex: there was no evidence of a difference between men and women.
-
Ethnicity: respondents who classified themselves in a category other than white British were more likely to agree with this incentive strategy than white British respondents (OR 2.31, 95% CI 1.63 to 3.29; p < 0.001).
-
Education: there was no evidence of a difference across education groups.
-
Social grade: there was no evidence of a difference across social grade.
-
Smoking status: there was no evidence of a difference across smoking status group.
-
Geographical area: respondents from the East Midlands, the South West and Scotland were less likely to agree with this incentive strategy than respondents from the reference group, those living in London. All of these areas were significantly different from London apart from the North.
Full details of the univariable and multivariable ordered logistic regressions are provided in Appendix 28 (see Table 83). A summary of general public agreement with the seven incentive strategies is provided in Table 45.
| Incentive strategy | % disagree | % neither agree nor disagree | % agree | Mean agreement score |
|---|---|---|---|---|
| Shopping vouchers for women who prove that they have stopped smoking during pregnancy | 42.3 | 17.2 | 40.5 | 2.9 |
| Shopping vouchers for a woman for 2 months after the birth of her baby if she proves that she is still not smoking | 46.4 | 17.3 | 36.5 | 2.7 |
| Shopping vouchers for a woman for 2 months after the birth of her baby if she never lets anyone smoke in her home | 46.0 | 19.6 | 34.4 | 2.7 |
| Shopping vouchers for women who prove that they are breastfeeding for the first 6 months after birth | 39.1 | 26.8 | 34.2 | 2.9 |
| A breast pump costing around £40.00 provided for free on the NHS | 27.3 | 27 | 45.8 | 3.2 |
| Additional funding for local health services if they reach targets for the number of women who prove that they have stopped smoking during pregnancy | 37.2 | 23.3 | 39.4 | 2.9 |
| Additional funding for local health services if they reach targets for the number of women who prove that they are breastfeeding | 38.5 | 25.1 | 36.4 | 2.9 |
Acceptable values for shopping vouchers (smoking and breastfeeding)
The acceptable value of shopping vouchers was asked only of those responding to the question of whether shopping vouchers for either smoking cessation or breastfeeding are acceptable with strongly agree, agree or neither agree nor disagree. For both smoking cessation and breastfeeding, > 85% stated that a value of ≤ £40.00 was acceptable (Tables 46 and 47 respectively). Full details of the two-part models to estimate the acceptable values for shopping vouchers are provided in Tables 84 and 86 in Appendix 29. In brief, living in the South East, South West or East Midlands (compared with London) was correlated with lower values for shopping vouchers for smoking cessation, whereas being a current smoker who has tried quitting (compared with never having been a smoker) or having a child who was previously breastfed (compared with no children) was correlated with increased values for shopping vouchers. For breastfeeding, living in the East Midlands, South East, Wales or Scotland (compared with London) was correlated with lower values for shopping vouchers, whereas having a child who was previously breastfed (compared with no children breastfed) was correlated with increased values for shopping vouchers.
| Value (£) | n | % |
|---|---|---|
| 2 | 116 | 17.6 |
| 10 | 146 | 22.1 |
| 20 | 193 | 29.2 |
| 40 | 115 | 17.4 |
| 60 | 36 | 5.5 |
| 80 | 54 | 8.2 |
| Value (£) | n | % |
|---|---|---|
| 2 | 146 | 20.9 |
| 10 | 150 | 21.5 |
| 20 | 199 | 28.6 |
| 40 | 110 | 15.8 |
| 60 | 36 | 5.2 |
| 80 | 56 | 8.0 |
The acceptability of targeting incentives to low-income women only (smoking cessation and breastfeeding)
Of the 660 respondents who did not disagree with vouchers for smoking cessation in pregnancy, 296 (44.9%) thought that vouchers should be targeted at low-income women only and 364 (55.1%) thought that all women should receive vouchers, regardless of income. For vouchers for breastfeeding, the numbers and percentages were similar: 330 (47.4%) thought that vouchers should be provided to low-income women only and 367 (52.3%) thought that vouches should be provided to all women. The only predictors of the acceptability of targeting vouchers to low-income women were living in the South West or Scotland (positive correlation) or being female (negative correlation). Full details are provided in Tables 85 and 87 in Appendix 29.
Results of the maternity and early years health professional survey of the acceptability of seven incentive strategies
Sample characteristics
There were 519 responses to the survey of health professionals; of these, 22 (4.2%) respondents did not answer any of the survey questions concerning the acceptability of incentive strategies and these data were excluded from all analyses. These 22 responses had extensive missing data on other survey questions and it was not possible to assess the similarity or otherwise between the excluded respondents and the included respondents. The characteristics of the 497 included respondents are shown in Table 48. Midwives and GPs were the largest professional groups to respond and 83% of respondents were female and 88% worked in Scotland. This is a highly self-selected sample that is unlikely to be representative of UK health professional opinion.
| Variable | Classes | n (%) |
|---|---|---|
| Sex | Male | 64 (12.9) |
| Female | 411 (82.7) | |
| Missing | 22 (4.4) | |
| Age (years) | 18–34 | 91 (18.3) |
| 35–44 | 114 (22.9) | |
| 45–54 | 182 (36.6) | |
| ≥ 55 | 85 (17.1) | |
| Missing | 25 (5.0) | |
| Ethnicity | White | 444 (89.3) |
| Black and minority ethnic/prefer not to say | 53 (10.7) | |
| White British | 339 (68.2) | |
| White Irish | 7 (1.4) | |
| White other | 1 (0.2) | |
| Mixed white/black Caribbean | 1 (0.2) | |
| Mixed other | 1 (0.2) | |
| Asian Indian | 10 (2.0) | |
| Asian Pakistani | 2 (0.4) | |
| Asian Chinese | 1 (0.2) | |
| Black African | 2 (0.4) | |
| Refused | 35 (7.0) | |
| Smoking status | Never smoked | 370 (74.4) |
| Current smoker, tried to stop smoking | 17 (3.4) | |
| Current smoker, not tried to stop smoking | 1 (0.2) | |
| Ex-smoker | 101 (20.3) | |
| Declined to answer | 8 (1.6) | |
| Any children | Yes | 401 (80.7) |
| No | 96 (19.3) | |
| Breastfeeding | Any children breastfed | 387 (77.9) |
| No children breastfed | 110 (22.1) | |
| Profession | GP | 132 (26.6) |
| Health visitor | 47 (9.5) | |
| Manager | 20 (4.0) | |
| Midwife | 121 (24.3) | |
| Obstetrician | 12 (2.4) | |
| Maternity staff | 29 (5.8) | |
| Paediatrician | 12 (2.4) | |
| Other nurse | 41 (8.2) | |
| Public health staff | 32 (6.4) | |
| Allied health professional | 18 (3.6) | |
| Support role | 8 (1.6) | |
| Researcher | 4 (0.8) | |
| Missing | 21 (4.2) | |
| Survey region | England | 60 (12.1) |
| Scotland | 437 (87.9) |
Figure 20 provides bar charts summarising the acceptability of all seven of the promising incentive strategies.
FIGURE 20.
Bar charts showing maternity and early years health professional agreement with the shortlist of incentive strategies. A, agree; BF, breastfeeding; D, disagree; ND, neither agree nor disagree; SA, strongly agree; SD, strongly disagree; SS, stop smoking.
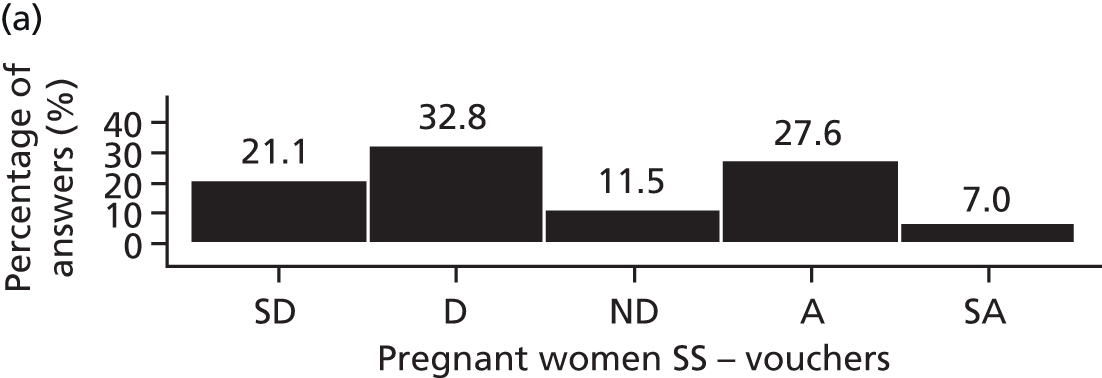
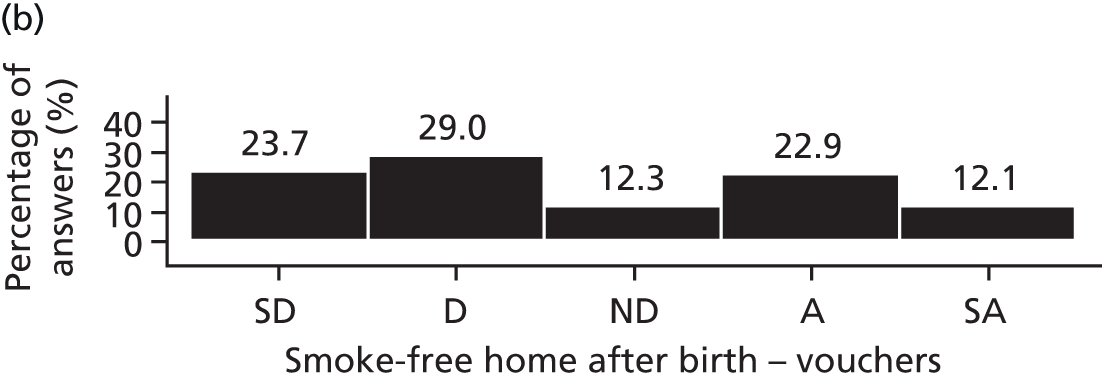


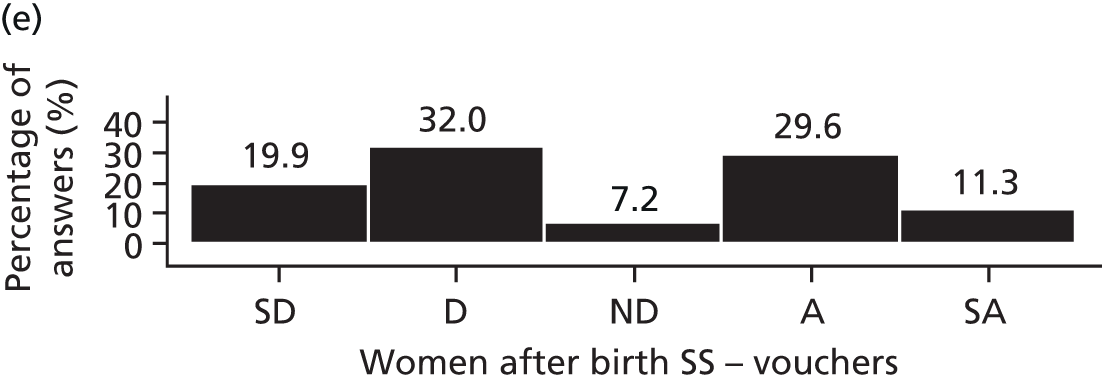


Shopping vouchers for women who prove that they have stopped smoking during pregnancy
There was a net disagreement of 53.9% (268/497) with the incentive strategy of shopping vouchers for smoking cessation during pregnancy. Net agreement was 34.6% (172/497). A summary of the results for each predictor variable against response to this incentive strategy is reported in Appendix 30 (see Table 88). From a multiple ordered logistic regression model there were no predictors of agreement. Full details of the univariable and multivariable ordered logistic regressions are provided in Appendix 30 (see Table 89).
Shopping vouchers for a woman for 2 months after the birth of her baby if she proves that she is still not smoking
There was a net disagreement of 51.9% (258/497) with the incentive strategy of shopping vouchers for smoking cessation for 2 months after pregnancy. Net agreement was 40.8% (203/497). A summary of the results for each predictor variable against response to this incentive strategy is reported in Appendix 30 (see Table 90). From a multiple ordered logistic regression model there were no predictors of agreement. Full details of the univariable and multivariable ordered logistic regressions are provided in Appendix 30 (see Table 91).
Shopping vouchers for a woman for 2 months after the birth of her baby if she never lets anyone smoke in her home
There was a net disagreement of 52.7% (262/497) with the incentive strategy of shopping vouchers for no smoking in the home for 2 months after pregnancy. Net agreement was 35.0% (174/497). A summary of the results for each predictor variable against response to this incentive strategy is reported in Appendix 30 (see Table 92). From a multiple ordered logistic regression model there were no predictors of agreement. Full details of the univariable and multivariable ordered logistic regressions are provided in Appendix 30 (see Table 93).
Shopping vouchers for women who prove that they are breastfeeding for the first 6 months after birth
There was a net disagreement of 55.9% (278/497) with the incentive strategy of shopping vouchers for breastfeeding for the first 6 months. Net agreement was 33.4% (166/497). A summary of the results for each predictor variable against response to this incentive strategy is reported in Appendix 30 (see Table 94). From a multiple ordered logistic regression model the predictors of agreement were:
-
Age: younger age groups (18–24 years) were significantly more likely to disagree with this incentive strategy than the reference group aged ≥ 65 years (OR 0.41, 95% CI 0.23 to 0.72; p = 0.002).
-
Profession: respondents in the midwives/health visitors/other maternity care staff group were more likely than doctors to agree with this incentive strategy (OR 1.84, 95% CI 1.21 to 2.79; p = 0.005).
Full details of the univariable and multivariable ordered logistic regressions are provided in Appendix 30 (see Table 95).
A breast pump costing around £40.00 provided for free on the NHS
There was a net disagreement of 21.9% (109/497) with the incentive strategy of a free breast pump. Net agreement was 67.8% (337/497). A summary of the results for each predictor variable against response to this incentive strategy is reported in Appendix 30 (see Table 96). From a multiple ordered logistic regression model there were no predictors of agreement. Full details of the univariable and multivariable ordered logistic regressions are provided in Appendix 30 (see Table 97).
Additional funding for local health services if they reach targets for the number of women who prove that they have stopped smoking during pregnancy
There was a net disagreement of 28.6% (142/497) with the incentive strategy of additional funding for local health services if they reach targets for the number of women who prove that they have stopped smoking during pregnancy. Net agreement was 52.9% (263/497). A summary of the results for each predictor variable against response to this incentive strategy is reported in Appendix 30 (see Table 98). From a multiple ordered logistic regression model, respondents in the midwives/health visitors/maternity care staff group were more likely than doctors to agree with this incentive strategy (OR 2.35, 95% CI 1.51 to 3.64; p < 0.001), as were other staff (OR 2.18, 95% CI 1.38 to 3.44; p < 0.001) Full details of the univariable and multivariable ordered logistic regressions are provided in Appendix 30 (see Table 99).
Additional funding for local health services if they reach targets for the number of women who prove that they are breastfeeding
There was a net disagreement of 38.6% (192/497) with the incentive strategy of additional funding for local health services if they reach targets for the number of women who prove that they are breastfeeding. Net agreement was 44.1% (219/497). A summary of the results for each predictor variable against response to this incentive strategy is reported in Appendix 30 (see Table 100). From a multiple ordered logistic regression model the predictors of agreement were:
-
Profession: respondents in the midwives/health visitors/maternity care staff group were more likely than doctors to agree with this incentive strategy (OR 2.54, 95% CI 1.65 to 3.91; p < 0.001), as were other staff (OR 1.94, 95% CI 1.23 to 3.05; p = 0.004).
-
Sex: female respondents were more likely to agree with this incentive strategy than male respondents (OR 1.79, 95% CI 1.06 to 3.91; p = 0.029).
-
Geographical region: respondents from England were more likely to agree than the reference group Scotland (OR 1.81, 95% CI 1.09 to 3.00; p = 0.023).
Full details of the univariable and multivariable ordered logistic regressions are provided in Appendix 30 (see Table 101). A summary of maternity and early years health professional agreement with the seven incentive strategies is provided in Table 49.
| Incentive strategy | % disagree | % neither agree nor disagree | % agree | Mean agreement score |
|---|---|---|---|---|
| Shopping vouchers for women who prove that they have stopped smoking during pregnancy | 53.9 | 11.5 | 34.6 | 2.7 |
| Shopping vouchers for a woman for 2 months after the birth of her baby if she proves that she is still not smoking | 51.9 | 7.2 | 40.8 | 2.8 |
| Shopping vouchers for a woman for 2 months after the birth of her baby if she never lets anyone smoke in her home | 52.7 | 12.3 | 35.0 | 2.7 |
| Shopping vouchers for women who prove that they are breastfeeding for the first 6 months after birth | 55.9 | 10.7 | 33.4 | 2.6 |
| A breast pump costing around £40.00 provided for free on the NHS | 21.9 | 10.3 | 67.8 | 3.7 |
| Additional funding for local health services if they reach targets for the number of women who prove that they have stopped smoking during pregnancy | 28.6 | 18.5 | 52.9 | 3.3 |
| Additional funding for local health services if they reach targets for the number of women who prove that they are breastfeeding | 38.6 | 17.3 | 44.1 | 3.0 |
Acceptable values for shopping vouchers (smoking and breastfeeding)
The acceptable value of shopping vouchers was asked only of those responding to the question of whether shopping vouchers for either smoking cessation or breastfeeding are acceptable with strongly agree, agree or neither agree nor disagree. For both smoking cessation and breastfeeding, > 90% stated that a value of ≤ £40.00 or less was acceptable (Tables 50 and 51 respectively). Full details of the two-part models to estimate the acceptable values for shopping vouchers are provided in Tables 102 and 104 in Appendix 31. In brief, age 16–24 years and the experience of children being breastfed were negatively correlated with the value of shopping vouchers for smoking cessation. There were no significant predictors of the value of shopping vouchers for breastfeeding.
| Value (£) | n | % |
|---|---|---|
| 2 | 9 | 3.9 |
| 10 | 89 | 38.4 |
| 20 | 80 | 34.5 |
| 40 | 33 | 14.2 |
| 60 | 6 | 2.6 |
| 80 | 15 | 6.5 |
| Value (£) | n | % |
|---|---|---|
| 2 | 10 | 4.5 |
| 10 | 70 | 31.7 |
| 20 | 81 | 36.7 |
| 40 | 39 | 17.6 |
| 60 | 8 | 3.6 |
| 80 | 13 | 5.9 |
The acceptability of targeting incentives to low-income women only (smoking and breastfeeding)
Of the 232 respondents who did not disagree with vouchers for smoking cessation, 77 (33.2%) thought that vouchers should be targeted at low-income women only and 155 (66.8%) thought that all women should receive vouchers, regardless of income. For breastfeeding, out of 221 respondents, the numbers and percentages were similar, with 69 (31.2%) responding that vouchers should be provided to low-income women only and 151 (68.3%) responding that vouchers should be provided to all women. There were no predictors of the acceptability of targeting vouchers to low-income women for smoking cessation in pregnancy and the only predictor of the acceptability of targeting vouchers to low-income women for breastfeeding was being resident in England (compared with Scotland). Full details of the two-part models are provided in Tables 103 and 105 in Appendix 31.
Results comparing the general public survey with the health professional survey
Tables 52 and 53 provide summaries of the independent predictors of general public acceptability and non-acceptability and health professional acceptability and non-acceptability, respectively, of the shortlist of seven incentive strategies.
| Incentive strategy | Age < 44 years | Female | Non-white ethnic group | Lower educational level | Social grade C or below | Parent | Current smoker (quit attempts) | Children breastfed | Resident outside London |
|---|---|---|---|---|---|---|---|---|---|
| Shopping vouchers should be provided to women who prove that they have stopped smoking during pregnancy | ++ | – | + | – | ++ (E) | ++ | – (Wales) | ||
| It is acceptable to provide shopping vouchers to a woman for 2 months after the birth of her baby if she proves that she is still not smoking | ++ | – | – | ++ (C2) | – – (Scotland, West Midlands) | ||||
| – (Wales, East Midlands, Yorkshire and Humberside) | |||||||||
| It is acceptable to provide shopping vouchers to a woman for 2 months after the birth of her baby if she never lets anyone smoke in her home | ++ | – | + | – | + | – – (West Midlands) | |||
| – (East Midlands, Yorkshire and Humberside, Scotland) | |||||||||
| Shopping vouchers should be provided to women who breastfeed for the first 6 months after the birth of their child | ++ | – | +++ | ++ | – – (West Midlands, East Midlands) | ||||
| – (South West, Yorkshire and Humberside, Wales) | |||||||||
| A breast pump costing around £40.00 should be available for free on the NHS, to help women to continue breastfeeding | ++ | – | ++ (E) | ++ | – – (Wales, East Midlands) | ||||
| Local health services should receive additional funding if they reach targets for the number of women who prove that they have stopped smoking during pregnancy | +++ | – | – (C1) | – – (East Midlands) | |||||
| – (South West, Yorkshire and Humberside, North, West Midlands, Scotland) | |||||||||
| Local health services should receive additional funding if they reach targets for the number of women who breastfeed | ++ | +++ | – (South West, East Midlands, Scotland) |
| Incentive strategy | Age < 54 (years) | Female | Non-white ethnic group | Profession: midwives, health visitors and maternity staff | Parent | Current smoker (quit attempts) | Children breastfed | Resident in England |
|---|---|---|---|---|---|---|---|---|
| Shopping vouchers should be provided to women who prove that they have stopped smoking during pregnancy | ||||||||
| It is acceptable to provide shopping vouchers to a woman for 2 months after the birth of her baby if she proves that she is still not smoking | ||||||||
| It is acceptable to provide shopping vouchers to a woman for 2 months after the birth of her baby if she never lets anyone smoke in her home | ||||||||
| Shopping vouchers should be provided to women who breastfeed for the first 6 months after the birth of their child | – – (age 18–34 years) | ++ | ||||||
| A breast pump costing around £40.00 should be available for free on the NHS, to help women to continue breastfeeding | ||||||||
| Local health services should receive additional funding if they reach targets for the number of women who prove that they have stopped smoking during pregnancy | +++ (+++ for other staff group) | |||||||
| Local health services should receive additional funding if they reach targets for the number of women who breastfeed | ++ | +++ (+++ for other staff group) | ++ |
Framing effects
In the general public survey framing effects were observed, with a significantly higher acceptability of vouchers for breastfeeding when breastfeeding questions were randomly selected to be asked first than when breastfeeding questions were asked after the smoking questions (OR 2.00, 95% CI 1.61 to 2.46; p < 0.001). Differences in the acceptability of vouchers for smoking cessation during pregnancy depending on whether smoking questions were asked first or were asked after the breastfeeding questions were non-significant. There was a significantly higher acceptability of free breast pumps when breastfeeding questions were asked first than when they were asked after the smoking questions (OR 1.32, 95% CI 1.08 to 1.62; p < 0.008).
As with all of the other breastfeeding-related incentive questions, significant framing effects were observed for the acceptability of provider incentives for breastfeeding targets. Agreement was significantly higher when breastfeeding questions were randomised to be asked first than when they were asked after the smoking questions (OR 1.44, 95% CI 1.17 to 1.77; p < 0.001). No significant framing effects were observed for the acceptability of provider incentives for smoking cessation targets.
Full details of the framing effects are provided in Appendix 32.
Smoking status of respondents
General public respondents were less likely to answer ‘yes’ to the statement ‘I currently smoke every day’ if they were aged ≥ 65 years, belonged to social grades A, B or C1, lived in the South West, had a degree-level qualification or had no children. Those with children were less likely to answer ‘yes’ to the statement ‘I currently smoke, but not every day’. Those who answered ‘yes’ to the statement ‘I used to smoke, but have quit’ were less likely to be in the 18–24 years and 25–34 years age groups, have no children, live in the North or London, be of Asian Pakistani ethnicity or have had a male interviewer. Respondents who said that they had never smoked were more likely to be female, to be aged < 65 years, to not have any children, to live outside of the South East, to have some formal qualification(s) and to be from social grades A, B, C1 or C2. Those who said that they had ever smoked were more likely to have tried to stop smoking at some point if they were female or had children. Those who were female, who lived in the Midlands, who were of white British ethnicity or who had a female interviewer preferred not to answer the smoking status question. For the health professional survey, few responded that they were current or past smokers, as might be expected.
Infant feeding status of respondents
Those who reported having children were more likely to be female, be aged > 45 years, live in the Midlands or have white British ethnicity. Those with children who were breastfed, even if only for a day or two, were less likely to be male, be from social grade C2, live in Scotland, have qualifications at A-level equivalent or above or have white British ethnicity. Those with children who were not breastfed were less likely to be in the 55–64 years age group, live in the Midlands or have a degree-level qualification. Those who did not know were more likely to be male. For the health professional survey, most with children responded that they had given at least one child breast milk, even if only for a few days.
Discussion
Overall, opinion was mixed about the provision of incentives to either women or local health services in both the general public and the health professional samples. For both the general public and health professionals, the most agreeable incentive strategy would be to provide a free breast pump costing around £40.00 to help women to continue breastfeeding, with 45.8% and 67.8% net agreement in the general public and health professional samples respectively. Net disagreement with providing a free breast pump was 27.2% for the general public and 21.9% for health professional respondents. The general public and health professional respondents differed in their views about the least acceptable incentive strategies. For the general public, shopping vouchers for women who continue to stop smoking after birth (net agreement 36.5% and net disagreement 46.4%) or for women who maintain a smoke-free home (net agreement 34.4% and net disagreement 46.0%) were the least acceptable, although over one-third of respondents agreed with the provision of these incentives. For the health professional respondents the least acceptable incentive strategy was shopping vouchers for women who prove that they are breastfeeding for 6 months, with net agreement of 33.4% and net disagreement of 55.9%. The general public expressed collective uncertainty for providing funding to local health service providers to meet smoking cessation or breastfeeding targets, with just over one-third of respondents agreeing and one-third disagreeing, whereas health professionals were slightly more in agreement with these strategies.
Being of childbearing age (< 44 years) was the only independent predictor of general public agreement with all seven incentive strategies, with agreement tending to decrease with increasing age. Health professional respondents in the age group 18–24 years were more likely to disagree with vouchers for breastfeeding, with an opposite trend noted for breast pumps, but with uncertainty around this estimate.
It is important to note that this survey was conducted in the midst of a sustained economic downturn since 2008, in the month of a substantial reform of the NHS in England and in a culture in which pay or bonuses are related to performance and an incentive/reward culture in schools is common. Older respondents may be less likely to have had direct experience of this culture in their day-to-day lives and generational attitudes towards state intervention to promote healthy lifestyle behaviours have changed in recent years, for example towards legislation on smoking in public places.
Women in the general public sample who are (or who would have been when younger) the intended recipients of the vouchers were less likely than men to agree with any shopping vouchers for smoking cessation during pregnancy, smoking cessation after birth, a smoke-free home or breastfeeding. This is a very important finding as women are the target group for behaviour change. There are several possible explanations. Women across the generations can feel under pressure to behave in the ‘right way’ and to be a ‘good mother’,288,309 both from within themselves and from others, including health professionals, and this can conflict with complex social circumstances and their own identities, as described in Chapters 4 and 6. In this context it was interesting to observe that more women refused to answer smoking status questions asked by women interviewers, suggesting gender differences in perceived acceptability.
Another hypothesis is that, for women, taking all the onus to stop smoking and to breastfeed is less acceptable than the onus being on the health service to support them. In contrast, there was no difference between men and women in terms of the acceptability of a free breast pump. Some fathers are keen to actively feed their babies and women are keen to express milk to enable them to do this, to free up social time for either the woman or the couple or to avoid breastfeeding in public (see Chapter 6). A partner could potentially perceive a personal benefit from a free breast pump that would not necessarily occur if feeding directly from the breast was the target behaviour.
The implications of our findings for health inequalities are important. General public respondents with a lower educational level were more likely to disagree with any voucher incentives for women for smoking cessation or with providing a free breast pump than those with degree-level qualifications. Similarly, respondents from more disadvantaged northern regions of Britain were more likely to disagree with incentives, with the South East and East Anglia showing similar patterns to the London reference group. East Midlands residents disagreed with six of the seven incentive strategies, whereas residents in the West Midlands, Yorkshire and Humberside, Wales and Scotland disagreed with four of the seven incentive strategies. It is interesting to observe that the North West (selected as a BIBS study site because of its history of incentive schemes for smoking cessation and breastfeeding) had different agreement patterns from neighbouring northern regions. The associations with social grade were less clear, with social grade E predicting agreement with shopping vouchers for smoking cessation in pregnancy and breast pumps and social grade C predicting agreement with shopping vouchers for smoking cessation that continues for 2 months after birth. Additional funding for local health services to meet smoking cessation targets was less agreeable to respondents with a lower educational level and to those in social grade C1 than to more educated respondents and those in social grades A and B respectively. Education is considered the strongest indicator of disadvantaged socioeconomic status. 310 Less-educated women and those living outside the South East are the most likely to smoke in pregnancy and not to initiate or sustain breastfeeding. 53 To address health inequalities these are the populations that governments would most like to target with effective behaviour change initiatives. 73 This has implications for a commonly held assumption: that incentives might be more effective in disadvantaged populations, as illustrated by examples of redistribution interventions (see Chapter 5). People may have varied views on what they would accept and what is acceptable for others, for example, and this requires further investigation. Of importance, universal incentives were preferred by the general public and health professionals, consistent with the findings from the qualitative data that women and babies should be treated equally (see Chapter 6).
Besides the geographical differences, other cultural differences were apparent. Being from a minority ethnic group was a strong predictor of agreeing with incentives to improve breastfeeding outcomes, but less so for agreeing with incentives to quit smoking, and there was no difference in the acceptability of providing a free breast pump between minority ethnic groups and white British respondents. This could reflect different cultural values given to feeding directly at the breast and expressing milk using a pump. In the UK, minority ethnic groups have a higher incidence and prevalence of breastfeeding than white British mothers, although this declines in subsequent generations who remain resident. 53,311 The cultural norm for many minority ethnic groups is to breastfeed, often for beyond 6 months, and so an incentive would be rewarding what many of these women do already. The potential stigma that is implicit in providing incentives for women, who in the health services eyes are not behaving how they should and which women can interpret as ‘being a bad mother’ (see Chapters 4 and 6), would be likely to be less evident in this group.
Those with personal experiences of achieving the desired behaviour, even if only short-term breastfeeding or a failed quit attempt, were more likely agree with the provision of incentives to women, consistent with the findings on smoking cessation in the study by Park and colleagues. 302 Being a parent was not a predictor of agreement. Those with direct experience of the ease or difficulty of achieving the behaviour, the amount of effort required and the personal consequences encountered are likely to place a different value on the behaviour than those who have no direct experience (see Chapter 6).
We do not think that it is appropriate to compare the predictors of agreement for health professionals with those for the general public, because of the self-selected and potentially unrepresentative sample. However, it is noteworthy that midwives, health visitors and maternity care staff were more likely to agree with vouchers for breastfeeding and with funding for local health services to meet targets than doctors or the other staff group. Midwives, health visitors and maternity care staff are the professional group that such incentive strategies would be most likely to impact on as they provide breastfeeding support and refer women to specialist smoking cessation services. The proportion of the sample in the health professional survey with a child who had been breastfed was 78% and the sample consisted of 83% women, which reflects the maternity care workforce. Health professional women approved of additional funding for local health services who meet breastfeeding targets and this was the only incentive for which a difference in response was observed between men and women. In addition, health professionals’ personal experience of a breastfed child was a predictor only for incentives to local health services for meeting breastfeeding targets. This fits with the qualitative data in Chapter 6, which showed that staff were keen on increased resources to support women with establishing and maintaining breastfeeding. The doctor respondents were predominantly GPs (27% of the sample), who are contractually obliged to receive funding for meeting targets and so have direct experience of such incentives through the QOF (see Chapter 5), unlike most midwives, health visitors and maternity care staff. However, we do not know at what level respondents interpreted ‘local health services’. This could be at the GP practice, the local hospital or a geographical catchment level.
The different predictors of general public agreement for incentives to women and incentives to health services are interesting. In contrast to incentivising women, neither sex nor personal experiences of the behaviour were predictors of agreement with incentives to local health services for meeting targets. A hypothesis generated is that there are differences in perceived need for additional motivation (in the form of incentives), with no perceived need for an additional incentive for women for the behaviour and mixed views about health services receiving an incentive to help women to succeed. This ties in with the qualitative data reported in Chapters 4 and 6. Some women can feel pressurised to stop smoking or to breastfeed and they value their freedom to choose. Health professional support is appreciated if it is non-judgemental, and often staff are strongly motivated to promote these healthy behaviours already (see Chapter 6). We do not know how the respondents interpreted what the local health services would be doing to reach the targets. Would incentivising health professionals to do more of the same be effective or are new approaches needed? Certainly, there is evidence that any additional support for women who want to breastfeed is effective at improving both the duration and the exclusivity of breastfeeding;65 however, this seems to be context dependent and not necessarily generalisable to the UK. 66 However, for smoking cessation in pregnancy, counselling and support interventions have less effect than incentives delivered to women, and the evidence for the effectiveness of NRT in pregnancy remains unclear. 54,55 Further research is needed on whether incentivising health professionals to support smoking cessation in women will be as promising as incentivising women themselves.
Strengths and limitations
As far as we are aware, to date, this is the largest, most representative survey of the general public and health professionals about the acceptability of incentives for healthy lifestyle behaviours. The Ipsos MORI survey sampling and data collection methods provide findings that can be considered to represent those of the British general public. The randomisation of the order of the smoking cessation and breastfeeding questions was important, as evidenced by the significant framing effects observed. By combining both behaviours in one survey, an evaluation of the comparative acceptability of incentives for each behaviour is likely to be more robust than if using independent surveys for each behaviour. A higher number of the general public respondents than health professional respondents neither agreed nor disagreed with the incentive strategies, perhaps suggesting that more opinionated staff responded. This, together with the differences in recruitment and data collection methods, would suggest that comparisons between health professional and general public findings are unlikely to be valid. In addition, some general public respondents were likely to have been health professionals.
Access to e-mail addresses of the relevant health professionals who we wanted to invite to participate was problematic through NHS contacts. In England, it was recommended that we pay a commercial company to gain access; however, the response rate was very disappointing. The commercial company had its own branding of the e-mail, unlike the e-mail sent to staff in Scotland, which was distributed through NHS gatekeepers. Response rates via the North West R&D units were equally poor. It is therefore possible that the difficulties that we encountered with survey completion may well reflect the transitional changes that staff were experiencing because of reorganisation of NHS trusts. However, despite these limitations, some comparisons between the health professional survey findings and the general public survey findings have been highlighted as relevant for further research in this area and they triangulate with the qualitative data findings in Chapter 6.
A challenge was to introduce and describe the incentive scenarios with sufficient clarity and detail to minimise differing interpretations but allow meaningful comparison between them. Given the framing effects identified in this survey and by others in relation to how the incentive is described300 and the stated effect size,301 the generalisability of these findings to other types of vouchers or incentives is uncertain and is likely to change over time as more evidence relating to effectiveness becomes available. For example, Promberger and colleagues301 found that healthy groceries were considered to be around 20% more acceptable than cash or vouchers for luxury items. In our survey, the introduction statement about the effectiveness of incentives for smoking cessation is stronger than the statement about the effectiveness of incentives for breastfeeding, which reflects the strength of evidence described in Chapter 3. This difference is likely to have influenced responses, as even a 1% increase in effectiveness can dramatically increase acceptability. 301 It is therefore uncertain whether the framing effects observed for the order of the breastfeeding incentive questions represent a true difference in the comparative acceptability of incentives for the two behaviours or reflect the stronger wording associated with smoking cessation. There may be other framing effects that are not apparent, for example if we had randomised the order of questions asking about incentives for providers and for women or if we had asked about incentives for breastfeeding for a shorter duration, for example 8 weeks rather than the WHO-recommended length of 6 months and beyond. 60 Given these framing effects, as well as the cultural and socioeconomic predictors of acceptability, comparison with other surveys is difficult, for example the study by Lynagh and colleagues151 (see Chapter 3) in which only 25% of patients in an Australian antenatal clinic endorsed the idea of paying women to quit smoking.
Implications for the shortlist of incentive strategies
Overall, the general public has mixed views about the acceptability of the proposed incentive strategies. This can be interpreted in different ways. From a research perspective, this collective uncertainty about acceptability is potentially ideal for the conduct of RCTs to ensure a rigorous evidence base. However, the mixed opinions among health professionals about the acceptability of voucher incentives for women could be problematic if a trial was reliant on NHS staff for recruitment or for delivering the intervention.
From the perspectives of those who are against incentivising women because of the implicit stigma and blame, these findings would suggest that a trial would be unacceptable to some in the target group, which could be problematic for recruitment and engagement. It is a concern that incentive strategies are less agreeable to those with lower educational attainment living in more disadvantaged regions of the UK and therefore it would be important in any future trial to investigate the impact on health inequalities.
Over 85% of general public respondents who did not disagree with the provision of incentives to women for either smoking cessation or breastfeeding stated that a value of ≤ £40.00 per month for vouchers was acceptable. This is of a similar order of magnitude to that in the studies included in the meta-analysis reporting the effectiveness of financial incentives for smoking cessation in pregnancy (see Chapter 3). This is less than the £400.00 total that can be provided for women who remain quit in the CPIT (see Box 2), for example.
Although the format of the survey did not facilitate formal assessment of which incentive was most acceptable, we can make some crude assumptions by comparing the arithmetic mean rating of the acceptability of each incentive strategy. In particular, a free breast pump up to a value of £40.00 was more popular than the more hedonistic shopping vouchers, for which a woman has autonomy and little restriction on how she chooses to use the incentive. Searching the Amazon UK website in September 2013 identified 406 hits for ‘breast pump’; however, this did include related equipment. The cheapest manual pump costs £3.27 and the most expensive double-breast electric pump costs £701.31. A voucher value of £40.00 would buy a middle-of-the-range manual breast pump or a lower-range electric pump or, alternatively, could be used to hire a top-of-the-range electric pump and provide the sterile pack required. The acceptability of the different types of pump, the timing of pump provision and the education and support required to use one to benefit breastfeeding require more investigation. In addition, it cannot be assumed that a breast pump would increase either breastfeeding duration or exclusivity and this therefore requires testing. The systematic review of the evidence on effectiveness presented in Chapter 3 found most evidence to support shopping vouchers for smoking cessation in pregnancy; however, recruitment, attrition and qualitative data from published studies suggest that only a relatively small proportion of pregnant smokers engage with such interventions.
Younger age groups were more in favour of incentives for local health services to meet behaviour targets; however, there were few other general public predictors for the acceptability of these incentives. Importantly, midwives, health visitors and maternity staff were more in favour than doctors of incentives for local health services to meet behaviour targets, and further evaluation of these incentives would therefore be indicated.
The shortlist of incentive strategies is considered again in Chapter 9, when all of the findings are pulled together and discussed.
Chapter 8 Discrete choice experiment of strategies to support smoking cessation in pregnant women
Background
The views of potential service users are one of the important elements when designing effective services to help pregnant mothers quit smoking. This chapter describes a DCE to investigate the views of current and former female smokers of childbearing age on whether different service characteristics would help pregnant women to quit smoking. A web-based online survey was used.
Methods
Objectives
The objectives of the DCE were to:
-
explore the characteristics of a smoking cessation service and their relative importance in helping pregnant women quit smoking
-
examine the effect of financial incentives to help pregnant women quit smoking and to use this to inform the design of any future trials
-
assess the variation in response by individual characteristics, such as age, smoking status, perceived ease/difficulty of smoking cessation and educational attainment, and how this impacts on service design.
Developing the discrete choice experiment
Discrete choice experiments are a stated preference technique that is increasingly used in health care to elicit preferences for attributes of health-care services. They are based on the premise that goods or services can be described by their characteristics (attributes) and that an individual values, in terms of utility or benefits, goods or services depending on the levels of these attributes. 312–314 Participants responding to a DCE questionnaire are asked to make a number of choices between services, each of which is described by a series of attributes at different levels. By changing the levels of the attributes and asking participants to make their choices again, it is possible to assess the relative importance that individuals place on these attributes and the extent to which they are willing to trade between the different characteristics of the services. Generally, the results are used to derive overall benefit or utility scores for different configurations of services. In this relatively novel application, women were asked to choose between services on the basis of their likelihood of quitting smoking and the results are interpreted in terms of the potential effectiveness of different scenarios.
Developing the attributes and levels
Defining the attributes and associated levels is the first step in a DCE. Two phases of research informed the choice of attributes and levels: a systematic review (see Chapter 3) and qualitative analysis (see Chapter 6). A shortlist of seven promising incentive intervention strategies was identified from the systematic reviews (see Chapters 3 and 5). The meta-analysis of contingent compared with non-contingent incentives for smoking cessation in pregnancy (see Chapter 3) provided supporting evidence for the effectiveness of contingent incentives. However, the systematic review evidence revealed uncertainty about the optimal dose of incentive, frequency of incentive and non-incentive intervention components, for example support, CO monitoring or other BCTs. In addition, there was uncertainty about whether including a significant other as a recipient of the incentive intervention or as an additional support component for the pregnant woman contributes to intervention effectiveness. 141
Furthermore, the CPIT Phase II trial88 (co-applicants LB and DT) (see Chapter 6) was in progress concurrently with this study and interim recruitment and outcome data were available at the design phase for this DCE in March 2013 and showed promise of effectiveness. Qualitative data from 20 pregnant women recipients and 23 service providers in the CPIT were collected independently of the BIBS study qualitative researchers. CPIT transcripts were included in the analysis of qualitative data in Chapter 6 in July 2013 after the completion of this DCE to minimise bias. Potential attributes were developed based on the characteristics of the studies included in the meta-analysis, the CPIT interim analysis and also preliminary qualitative data collected from mother-and-baby group co-applicants and participants who provided data before April 2013 (see Chapter 2). The potential attributes were discussed within the research team to reduce them to a manageable number for the DCE and levels were identified to cover the range of possibilities. Details of each attribute are given in the following sections.
Summary of attributes
Attribute 1
A first meeting with an expert adviser to get help to stop smoking and agree a quitting date forms part of routine care for pregnant women and was therefore retained as an attribute for all choices. After the first meeting, the potential configurations of the service offered to individuals vary.
The next two attributes relate to the method of providing support to women as part of the intervention. They are included as the review found limited evidence about the interaction between incentive and non-incentive behaviour change components and their modes of delivery. In particular, there is recent evidence suggesting that text messaging support is effective at helping people to stop smoking. 315 In a review of the evidence for lifestyle behaviour change for patients at risk of diabetes, contact frequency was associated with increased effectiveness. 215 Chapter 3 identified many different BCTs that were delivered in addition to the incentive component in smoking cessation interventions for pregnant women and it was not possible within the confines of a single DCE to examine all of the potential interactions. We chose a simple advice-based interaction from a smoking cessation expert adviser, as this would be compatible with a short text message, and varied the method of delivery and timing of the delivery contacts.
Attribute 2
Attribute 2 varies the frequency of regular face-to-face meetings with an expert adviser to get help throughout pregnancy and until the baby is 2 months old. Participants have regular meetings to get face-to-face help from a quitting expert adviser, to have their smoking status checked and to receive a voucher for staying quit. Participants can meet with the quitting expert adviser either once a week or once every 2 weeks.
Attribute 3
Attribute 3 varies the method of providing more intensive support during the first week after deciding to quit. In two trials,105,144 daily face-to-face contact occurred initially. This is supported by research into decision-making, which suggests that interventions to develop new behaviours need to address present bias. 316 For nicotine addiction, the immediate benefits of relapse are likely to be particularly powerful in the early stages of quitting and outweigh the longer-term benefits. In addition, when considering new habit formation, the frequency of motivating contacts, however brief the intervention, is important. 317 Participants can visit the clinic daily to meet with their quitting expert adviser, receive a daily telephone call from a quitting expert adviser or receive a daily text message from a quitting expert adviser.
Attribute 4
Attribute 4 provides a financial incentive in the form of a voucher. In Chapter 3, a meta-analysis of data from four trials105,109,141,144 showed that contingent financial incentives are effective in helping women stop smoking in pregnancy compared with non-contingent incentives (which may be small payments for taking part and providing outcome data). These all used a similar level of total reward, although the payment schedules varied. There had been no direct comparison of different incentive levels to test for dose–response and this is addressed by attribute 4.
In the DCE, respondents were offered a choice of no voucher or vouchers of £20.00, £40.00 or £80.00 per month, contingent on providing a saliva test to show that participants have not smoked. The range of total values for the vouchers that women could receive over the duration of the service encompasses the total level of reward found in the literature and offers values above and below.
Attribute 5
Attribute 5 includes a quitting pal: someone close (a friend or a relative) who helps the woman to quit. One of the largest trials in the meta-analysis had given incentives to a non-smoking ‘pal’ as well as to the woman quitting,141 and other trials also involved partners or significant others without incentives. 104,108,138,145–147 We were interested in the relative importance of the different ways in which the ‘pal’ is involved in the process with regard to women’s quitting behaviour and addressed this in the final attribute.
In the DCE, participants were given the option of choosing whether or not to have a ‘quitting pal’ who is a friend or relative. There are three levels of ‘quitting pal’ support: pal receiving information on how to help the woman quit only when she first sees her quitting expert adviser; pal receiving help and information when the woman first sees her quitting expert adviser and a text message after each saliva test with the woman’s result; and pal receiving information on how to help the woman quit when she first sees her quitting expert adviser, a text message after each saliva test with the woman’s result and a £20.00 voucher every month that she stays quit.
The final attributes and levels for the DCE are shown in Table 54. The descriptions of the levels are as they appeared in the DCE questionnaire. The last column presents the current service, which was included as option C in the DCE questionnaire.
| Attributes in the DCE | Levels in the DCE | Current service (or option C) |
|---|---|---|
| 1. First meeting with expert adviser to get support to stop smoking and agree quitting date (First meeting) | Yes | Yes |
| 2. Frequency of regular face-to-face meetings with expert adviser to get support (Frequency of meeting) | Once a week; once every 2 weeks (base-level category) | None |
| 3. Method of support during the first week after deciding to quit (Method of support) | A visit to the clinic every day to meet with your quitting expert adviser (base-level category); a telephone call every day from your quitting expert; a text message every day from your quitting expert | None |
| 4. Monthly financial incentive in the form of a voucher (Incentive) | No vouchers (base-level category); £20.00; £40.00; £80.00 | None |
| 5. Quitting pal: someone close (friend or relative) who supports to quit (Quitting pal) | No ‘quitting pal’ (base-level category); your ‘quitting pal’ will receive information on how to support you when you first see your quitting expert adviser; your ‘quitting pal’ will receive support and information when you first see your quitting expert adviser and a text message after each test to let him or her know your result; your ‘quitting pal’ will receive information on how to support you when you first see your quitting expert adviser, a text after each test to let him or her know your result and a £20.00 voucher every month that you stay quit | None |
Determining choice sets
The combination of attributes and levels resulted in 96 possible profiles (1 × 2 × 3 × 4 × 4). This was reduced to a more manageable 48 profiles (i.e. 24 choice sets) using experimental design techniques. SAS version 9.1.2 software (SAS Institute Inc., Cary, NC, USA) was employed to generate a main-effects D-efficient design, ensuring that uncertainty around parameter estimates was minimised (by minimising the determinant of the covariance matrix). 318 An example of a choice set is shown in Figure 21. Along with two options offering different configurations for the new service (option A and option B), a third option (option C) was included in each of the 24 choice sets. This third option allows respondents to choose the currently provided smoking cessation services, which do not have the properties defined by attributes 2–5. The 24 choice sets were randomly divided into three blocks to reduce the length of the questionnaire each respondent had to complete. The questionnaire is provided in Appendix 33.
FIGURE 21.
Example of a choice set of questions presented to participants. Note: In each of the choice set of questions, participants were presented with a highlighted icon that read, ‘Please click here to review the description of the service again’. This linked to a new window with a description of the suggested smoking cessation service that had been presented beforehand.

To make sure that respondents were engaged in making choices, two choice questions, so-called warm-up tests, were presented before the real choices. Thus, an individual respondent was presented with eight choices and two warm-up tests. However, it was not possible for the respondents to know which were real choices and which were warm-up tests.
Screening questions
At the beginning of the survey, screening questions were asked to ensure that information was obtained from women with smoking experience, who currently smoked (i.e. smokers) or who previously smoked (i.e. ex-smokers), who were aged 16–40 years (i.e. of childbearing age) and who were from the UK. These women were considered best placed to assess the impact of the choices on the likelihood of quitting smoking. Finally, respondents were asked to pick the best statement about themselves out of the following seven: I am a smoker; I am a smoker and would like to quit; I have recently quit smoking by using nicotine patches and/or gum; I have recently quit smoking by other means; I was a regular smoker but gave up a long time ago; I was an irregular smoker but do not smoke now; I have never smoked. Those who had never smoked were screened out.
Additional information
We also asked respondents for their views on the services most likely to help women to stop smoking during pregnancy and during the first couple of months after birth. We provided information on the harm caused by smoking to the health of mothers and babies: an increase in the risk of premature birth, stillbirth and caesarean section and increases in their baby’s risk of chest infections, ear infections, chronic bronchitis, asthma and sudden infant death or cot death. We then posed a question about their intention to quit smoking or cut down by putting them in an imaginary situation in which they found out that they were pregnant while smoking 10 cigarettes a day. After the first meeting to discuss different ways to stop smoking and to be given information about the importance of stopping for their health and their baby’s health, they might choose one of the following behaviours: stop smoking completely; limit smoking to a couple of cigarettes a week; limit smoking to a couple of cigarettes a day; or keep smoking the same amount as before becoming pregnant. This provided a baseline against which to interpret their views of the likelihood that the service choices offered would help them to quit.
We asked respondents how difficult they thought it would be to quit smoking, scaled as very difficult, quite difficult, quite easy and very easy, and the most likely result after trying to stop smoking while pregnant. The possible results were stop smoking completely; limit smoking to a couple of cigarettes a week; limit smoking to a couple of cigarettes a day; or keep smoking the same amount as before they were pregnant.
After completing the choice questions, respondents were asked whether they had children, about their smoking experience during pregnancy for those children and about their quit efforts during pregnancy for those children. Finally, we asked about respondents’ educational attainment, employment status and annual household income before tax.
Pre-pilot
The pre-pilot version of the questionnaire was made available to the research team using an online format facilitated by Survey Monkey. Members of the research team and their colleagues, including DCE experts, were invited to complete the questionnaire and make comments. The questionnaire was amended based on 12 responses in the initial draft and 18 responses in the second draft.
We also conducted a pre-pilot with four mothers at the Blackpool mother-and-baby group (3 May 2013). They had smoked previously (none currently) and were each currently pregnant. All of the women were shown the online survey and, while reading the questions on screen, they were asked to express their feelings and discuss any issues as they presented. This session was accompanied by one of the research team members, who audio recorded it and transcribed the key points for team discussion. All participants in this session took the questionnaire seriously and were found to be engaged with the choices. Descriptions and explanations were revised for better understanding and readability based on their comments.
Sample, pilot and main data collection
Based on experience from a study that had poor response rates for unsolicited general population surveys319 and on a literature review,320 a market research company, Research Now (see www.researchnow.co.uk/), with a guaranteed respondent base, was employed to collect the data set using a web-based online survey method. Individuals who were registered with this company were invited by e-mail to complete the questionnaire. Each individual who successfully completed the questionnaire was given £2.00 as a reward, credited to the individual’s account. A forced choice was imposed in all questions, thus participants were prevented from moving forward without answering.
Achieving quotas by country and by smoking status was discussed and determined before the pilot was launched. We collected 20% and 80% of responses from Scotland and the rest of UK, respectively, and equal numbers of ex-smokers and current smokers. In addition, we assigned an equal quota to each block of questionnaires generated from the experimental design. Initially, we received responses from 169 individuals and provisional analysis was conducted. Estimation using choice questions was conducted and no problems were found. Data collection was continued with no changes, with a target sample size of 300 individuals, providing a sufficient sample size to carry out subgroup analysis. The final sample achieved was 320.
Main analysis
Responses to the choice questions were analysed using a conditional logit regression model within Stata 12.1 (clogit command). The relationship estimated is described in Equation 1, where the dependent variable V is interpreted as the individual’s perceived likelihood of quitting as a function of the attribute levels of the alternative service chosen.
The constant term β0 captures whether any new service is seen to be more or less likely to help women to quit smoking compared with currently available smoking cessation services. A positive (negative) constant would indicate that the new smoking cessation service being offered is more (less) likely to help women quit, with everything else being equal. ε is the error term for unobservable factors.
Attribute 1 (First meeting) was not included in the experimental design because there is no difference between the alternatives and thus no variation was explained by this in the estimation.
For discrete attributes, effects coding rather than dummy coding was used. Effects coding allows for coefficient values and standard errors (SEs) to be provided for every level of the attributes (see Appendix 34), whereas with dummy coding values are expressed relative to the baseline category and the baseline effect is absorbed into the constant term. Dummy coding is not appropriate in this study as the baseline categories for the attributes do not correspond to the current service provision but need to be estimated separately. Effects coding was used for attribute 2 (Frequency of meeting), attribute 3 (Method of support), attribute 4 (Incentive) and attribute 5 (Quit pal).
Although attribute 4 (Incentive) was designed as a categorical variable, we first estimated the model treating this attribute as if it was a continuous variable to estimate the magnitude of the effect of a £1.00 change in the financial incentive. This was then compared with the model estimating the financial incentive as a categorical variable. The performance of the models was compared using the log-likelihood ratio test.
Probability that an attribute of a smoking cessation service would potentially help a woman to quit smoking in pregnancy
Any new incentive smoking cessation service (ISCS) will need to be accepted by potential users, pregnant women, if it is going to be effective in reducing harm for mothers and babies in the population. The relative importance of the characteristics of ISCSs in helping pregnant women to quit was explored using the V scores from Equation 1 for the newly designed smoking cessation service with varying configurations of attributes. We also calculated the V scores for varying levels of incentive attributes to assess the role of the financial incentives in helping pregnant women quit smoking.
Subgroup analysis
We estimated the same model on several subgroups defined by demographic and socioeconomic characteristics: age group, smoking status, perceived ease/difficulty of quitting smoking when pregnant and educational attainment. We tested whether the perceived likelihood of quitting differed across values within each subgroup (e.g. smokers vs. non-smokers) using a log-likelihood ratio test. V scores were then calculated for each subgroup.
Results
Characteristics of respondents
The summary statistics for the sample are shown in Table 55 (categorical variables) and Table 56 (continuous variables). The women who responded were more likely to be older, well educated and in employment. A high proportion had experience of trying to quit smoking during pregnancy and 85% would plan to quit in a hypothetical future pregnancy.
| Categorical variable | n | % |
|---|---|---|
| Age category (years) | ||
| 16–20 | 18 | 5.6 |
| 21–25 | 35 | 10.9 |
| 26–30 | 64 | 20.0 |
| 31–35 | 101 | 31.6 |
| 36–40 | 102 | 31.9 |
| UK region | ||
| Scotland | 70 | 21.9 |
| Rest of the UK | 250 | 78.1 |
| Highest level of education completed | ||
| No formal qualifications | 10 | 3.1 |
| GCSE, O-Level, CSE, O Grade, Standard Gradea | 54 | 16.9 |
| Vocational qualificationsb | 29 | 9.1 |
| A-Level, Higher, Advanced Higher or equivalentc | 93 | 29.1 |
| Bachelor’s degree or equivalent | 109 | 34.1 |
| Master’s degree/PhD or equivalent | 23 | 7.2 |
| Current employment status | ||
| Working full-time (≥ 30 hours per week) | 119 | 37.2 |
| Working part-time (< 30 hours per week) | 76 | 23.8 |
| At home and not looking for paid work | 56 | 17.5 |
| Unable to work because of illness or disability | 11 | 3.4 |
| Student | 25 | 7.8 |
| Unemployed and looking for work | 24 | 7.5 |
| Estimate of annual household income | ||
| Up to £9999.00 | 37 | 11.6 |
| Between £10,000.00 and £19,999.00 | 70 | 21.9 |
| Between £20,000.00 and £29,999.00 | 74 | 23.1 |
| Between £30,000.00 and £39,999.00 | 46 | 14.4 |
| Between £40,000.00 and £49,999.00 | 39 | 12.2 |
| ≥ £50,000.00 | 28 | 8.8 |
| Would rather not say | 26 | 8.1 |
| Smoking status | ||
| Smoker | 160 | 50.0 |
| Ex-smoker | 160 | 50.0 |
| Quit attempt (among smokers) | ||
| Never tried | 18 | 5.6 |
| Once to twice | 99 | 30.9 |
| Three times or more | 43 | 13.4 |
| When pregnant, would you | ||
| Stop completely? | 272 | 85.0 |
| Limit smoking to two cigarettes/week? | 19 | 5.9 |
| Limit smoking to two cigarettes/day? | 24 | 7.5 |
| Keep smoking the same as before? | 1 | 0.3 |
| How difficult it would be to quit? | ||
| Very difficult | 59 | 18.4 |
| Quite difficult | 91 | 28.4 |
| Quite easy | 76 | 23.8 |
| Very easy | 73 | 22.8 |
| Do not know | 21 | 6.6 |
| Do you have children? | ||
| Yes | 184 | 57.5 |
| No | 136 | 42.5 |
| Tried to stop smoking during the most recent pregnancy? | ||
| No, not smoking then | 61 | 19.1 |
| Yes and stayed smoking then | 22 | 6.9 |
| Yes and started again before baby was born | 27 | 8.4 |
| Yes and started again after baby was born | 59 | 18.4 |
| Number of children aged < 16 years in the household | ||
| 0 | 137 | 42.8 |
| 1 | 73 | 22.8 |
| 2 | 78 | 24.4 |
| 3 | 24 | 7.5 |
| ≥ 4 | 8 | 2.5 |
| Continuous variable | n | Mean | SD | Min. | Max. |
|---|---|---|---|---|---|
| No. of cigarettes a day (smoking or used to smoke) | |||||
| Smoker | 160 | 12.65 | 7.49 | 1 | 40 |
| Ex-smoker | 160 | 12.32 | 8.31 | 1 | 40 |
| Age of the youngest child in the household under 16 years | 184 | 5.47 | 4.51 | 1 | 23 |
| Time to complete questionnaire (in minutes)a | 311 | 10.28 | 7.14 | 2.73 | 59.10 |
Regression results
In total, 7680 observations, covering eight choices with three scenarios from 320 respondents, were analysed and the results are presented in Table 57. The constant term was positive and statistically significant in both variants of the model, implying that respondents were more likely to choose the new service (ISCS). Note that the coefficient on the constant is larger when the financial incentive is categorical, whereas the coefficients on other attributes are similar between the two models.
| Financial incentive (categorical) | Financial incentive (continuous) | |||||
|---|---|---|---|---|---|---|
| β | SE | 95% CI | β | SE | 95% CI | |
| Constant ( = 1 if option A or B) | 1.308 | 0.063 | 1.184 to 1.431 | 0.282 | 0.092 | 0.101 to 0.463 |
| Frequency of meeting | ||||||
| Once a week | 0.032 | 0.023 | –0.013 to 0.077 | 0.031 | 0.023 | –0.014 to 0.076 |
| Once every 2 weeks | –0.032 | 0.023 | –0.076 to 0.014 | –0.031 | 0.023 | –0.076 to 0.014 |
| Method of support in the first week | ||||||
| Daily visit to the clinic | –0.361 | 0.037 | –0.433 to –0.289 | –0.360 | 0.037 | –0.432 to –0.289 |
| Daily telephone call | 0.193 | 0.037 | 0.121 to 0.265 | 0.188 | 0.036 | 0.117 to 0.260 |
| Daily text message | 0.168 | 0.037 | 0.096 to 0.240 | 0.172 | 0.037 | 0.100 to 0.244 |
| Monthly financial incentive | ||||||
| Continuous | 0.412 | 0.026 | 0.362 to 0.463 | |||
| £0.00 | –0.677 | 0.046 | –0.768 to –0.586 | |||
| £20.00 | –0.131 | 0.046 | –0.221 to –0.041 | |||
| £40.00 | 0.217 | 0.046 | 0.128 to 0.307 | |||
| £80.00 | 0.591 | 0.048 | 0.497 to 0.685 | |||
| Quitting pal | ||||||
| No pal | –0.316 | 0.047 | –0.409 to –0.224 | –0.321 | 0.047 | –0.413 to –0.228 |
| Pal with informationa | 0.048 | 0.049 | –0.047 to 0.144 | 0.054 | 0.048 | –0.041 to 0.149 |
| Pal with support and textb | 0.197 | 0.047 | 0.105 to 0.289 | 0.195 | 0.047 | 0.103 to 0.288 |
| Pal with voucherc | 0.071 | 0.046 | –0.020 to 0.162 | 0.071 | 0.046 | –0.020 to 0.162 |
| Log likelihood | –2255.0916 | –2255.7794 | ||||
| Pseudo R2 | 0.1985 | 0.1979 | ||||
| N (observations) | 7680 | 7680 | ||||
| N (respondents) | 320 | 320 | ||||
A positive and significant value of the coefficient (β in Table 57) means that the attribute level contributes to increasing the perceived likelihood of quitting for the service whereas a negative and significant value reduces this.
Frequency of face-to-face meeting with the quitting expert advisers was not significant. For the Method of support in the first week, all three levels were statistically significant and respondents perceived that services where help was provided by a telephone call or text message every day from the quitting expert adviser were more likely to help women quit. Visiting the clinic to get daily help in the first week was viewed negatively.
Financial incentive was significant: no incentive or an incentive of £20.00 per month had negative effects on the likelihood of quitting whereas an incentive of £40.00 or £80.00 per month had a positive effect. Looking at the direction (i.e. positive or negative) and magnitude of the coefficients on levels within the incentive attribute, there was a dose–response relationship between the value of the monthly financial incentive and the impact on the likelihood of quitting, that is, when an incentive above £20.00 was provided, women were more likely to perceive the service as likely to help them quit. The effect was not linear, however, with some indication that the increase in effect begins to slow down between £40.00 and £80.00. When the incentive attribute was treated as a continuous variable, the coefficient was significant and positive, with the V score, indicating the likelihood of quitting, increasing by 0.412 as £1.00 more incentive is provided (columns 6–9, Table 57).
Among the four levels for Quitting pal, ‘no pal’ and ‘pal with support and text’ were statistically significant. The service with ‘pal with support and text’ was perceived by women as increasing the likelihood of quitting whereas the service with ‘no pal’ would not be likely to help women to quit smoking.
Likelihood of quitting scores for combinations of service attributes and levels
The regression coefficients on the attribute levels can be combined to compare the perceptions of respondents about which service was more likely to help them quit smoking. Therefore, the scores resulting from different combinations of service attributes and levels are an indicator of their perceived likelihood of helping women to stop smoking in pregnancy. Table 58 gives the V scores, in rank order, for different combinations of these service attributes and levels for which the coefficients were significantly different from zero and positive. It should be noted that these scores should be interpreted only ordinally, that is, a higher score indicates that the service described is perceived by the respondents to be more likely to help but the differences in scores do not show how much more or less likely a particular configuration is to actually help.
| Method of support | Voucher value | Quitting pal | Total score with categorical incentive |
|---|---|---|---|
| Call | £80.00 | Yes | 2.2886 |
| Text | £80.00 | Yes | 2.2637 |
| Call | £80.00 | No | 1.7750 |
| Text | £80.00 | No | 1.7500 |
| Clinic | £80.00 | Yes | 1.7347 |
| Clinic | £80.00 | No | 1.2211 |
| Call | £40.00 | Yes | 1.9153 |
| Text | £40.00 | Yes | 1.8903 |
| Call | £40.00 | No | 1.4017 |
| Text | £40.00 | No | 1.3767 |
| Clinic | £40.00 | Yes | 1.3614 |
| Clinic | £40.00 | No | 0.8478 |
| Call | £20.00 | Yes | 1.5667 |
| Text | £20.00 | Yes | 1.5417 |
| Call | £20.00 | No | 1.0531 |
| Text | £20.00 | No | 1.0281 |
| Clinic | £20.00 | Yes | 1.0128 |
| Clinic | £20.00 | No | 0.4992 |
| Call | £0.00 | Yes | 1.0210 |
| Text | £0.00 | Yes | 0.9960 |
| Call | £0.00 | No | 0.5074 |
| Text | £0.00 | No | 0.4824 |
| Clinic | £0.00 | Yes | 0.4671 |
| Clinic | £0.00 | No | –0.0465 |
The only case in which a new service configuration is not scored above the current provision (our option C) is when all of the least preferred attribute levels are combined, that is, face-to-face support requires a clinic visit, there is no voucher provided and there is no ‘quitting pal’ support. The ranking of the service options increases with the financial value of the incentive. For any given level of financial incentive, the service is ranked as more effective if a quitting pal with support and text messaging is included. Follow-up in the first week by daily telephone call is preferred to text messaging but the difference is small.
Subgroup analysis
We conducted subgroup analysis by smoking status, age group, perceived ease/difficulty of quitting smoking when pregnant and educational attainment to see whether preference for the services differed by respondent characteristics. The full tables of results are presented in Appendix 35 with the main findings summarised in the following sections.
Smoking status
Appendix 35 (see Table 106) shows the results from the conditional logit analysis by smoking status. These show that smokers differed from ex-smokers in their perception of the relative effects of the attributes of the ISCS on helping women to quit. Both groups perceived the new service as being more likely to help women to quit smoking; this view was stronger among smokers than among ex-smokers. The frequency of meeting with the quitting expert adviser was significant for ex-smokers for meeting weekly whereas smokers were indifferent to this attribute when choosing between services. Smokers and ex-smokers had similar ratings for the method of support in the first week (daily face-to-face meetings, telephone calls or text messages); the size of the coefficients varied slightly but the differences were not significant. The magnitude of the coefficient for the monthly financial incentive was greater in smokers than in ex-smokers and the difference was significant for the extreme values (£0.00 and £80.00), meaning that current smokers thought that the incentive was more important for helping women to quit smoking. The pattern of results for the quitting pal were similar for the two groups; although ex-smokers had a larger, negative coefficient on the ‘no quitting pal’ and a larger positive coefficient on the ‘quitting pal with support and text’, the CIs overlapped.
Age group
Overall, preference did not differ by age group. All age groups perceived the new service as being more likely to help women quit. The results for the youngest age group (age 16–20 years) could have been driven by the small number of observations; none of the attributes except a monthly financial incentive of £80.00 was statistically significant. The results are presented in Appendix 35 (see Table 107).
Perceived ease/difficulty of quitting smoking
The results differed by the perceived ease/difficulty of quitting smoking adviser (see Appendix 35, Table 108). The frequency of meeting with the quitting expert was statistically significant only for those who felt that it would be quite easy to quit, who preferred more frequent (weekly) meetings. Visiting the clinic daily to get help in the first week was statistically significant and negative for all groups, whereas one or both of the daily telephone call or text message alternatives had a positive and significant coefficient for each group. For those who thought that quitting would be very difficult, the coefficient on financial incentive was positive and significant only for the highest value (£80.00) and the magnitude of the coefficient was significantly larger than for the group who thought that quitting would be very easy. Similarly, those who thought that quitting would be very difficult had a significantly more negative view of having no voucher than those who thought that quitting would be very easy. All groups had a negative view of not having a quitting pal. Groups who thought that quitting would be very or quite difficult and quite easy had a significant preference for a ‘quitting pal with support and text’; those who thought that quitting would be quite easy also had a significant preference for the quitting pal receiving a financial incentive (voucher).
Educational attainment
There was a rather skewed distribution in the levels of educational attainment. Because of the small number of observations in groups with the lowest levels of education, only three groups of respondents (GCSE, A-level and university) were used for this subgroup analysis (see Appendix 35, Table 109).
The GCSE group was small compared with the other two groups. Overall, the results differed by educational attainment. Those with a higher educational attainment level (A-level or university level) had a stronger view than those with a lower educational attainment level (GCSE level) that new services would be more likely to help women quit smoking. The frequency of meeting was significant only for those with a university-level education, who preferred more frequent (weekly) meetings. Daily visits to the clinic for support in the first week had a significant negative coefficient for all groups; services with daily telephone calls or text messages were seen to have a significant positive effect by those with a higher educational attainment level (A-level or university level). All groups had significant negative coefficients for no financial incentive and significant positive coefficients for the largest incentive (£80.00). Those with a higher education level also had significant coefficients for the other voucher values: negative for £20.00 and positive for £40.00. The lack of significance for those with a lower educational attainment level (GCSE level) may be related to the smaller sample size, also shown in the lack of significance for any of the levels for quitting pal. Those with higher levels of education had significant negative coefficients for ‘no quitting pal’ and significant positive coefficients for ‘quitting pal with text and help’. Providing a financial incentive to the quitting pal was seen to have a significant positive impact on helping women to quit only by those with the highest education level.
Summary of the main analysis and the subgroup analysis
The results of the conditional logit estimation from the main analysis and the subgroup analysis are summarised in Table 59, in which ‘+’ and ‘–’ note the positive and negative significant coefficients respectively. For the monthly financial incentive attribute analysed as a continuous variable, the coefficient itself is reported for comparison purposes.
| Attribute | Level | All | Smoking status | Age group (years) | Perceived ease/difficulty of quitting smoking | Educational attainment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Smoker | Ex-smoker | 16–20 | 21–25 | 26–30 | 31–35 | 36–40 | Very difficult | Quite difficult | Quite easy | Very easy | Do not know | GCSE | A-level | University | |||
| Constant | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| Frequency of meeting | |||||||||||||||||
| Once a week | NS | NS | + | NS | NS | NS | NS | NS | NS | NS | + | NS | NS | NS | NS | + | |
| Once every 2 weeks | NS | NS | – | NS | NS | NS | NS | NS | NS | NS | – | NS | NS | NS | NS | – | |
| Method of support in the first week | |||||||||||||||||
| Daily visit to the clinic | – | – | – | NS | – | NS | – | – | – | – | – | – | – | – | – | – | |
| Daily telephone call | + | + | + | NS | + | + | + | + | + | + | NS | + | NS | NS | + | + | |
| Daily text message | + | + | + | NS | + | + | + | NS | NS | + | + | + | + | NS | + | + | |
| Monthly incentive | |||||||||||||||||
| Categorical | £0.00 | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – |
| £20.00 | – | NS | – | NS | NS | NS | NS | – | NS | – | NS | NS | NS | NS | – | – | |
| £40.00 | + | + | + | NS | NS | + | NS | + | NS | + | + | + | NS | NS | + | + | |
| £80.00 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| Continuous | 0.412 | 0.536 | 0.298 | 0.324 | 0.447 | 0.571 | 0.329 | 0.418 | 0.580 | 0.421 | 0.351 | 0.342 | 0.462 | 0.319 | 0.446 | 0.423 | |
| Quitting pal | |||||||||||||||||
| No pal | – | – | – | – | NS | – | – | – | – | – | – | – | NS | NS | – | – | |
| Pal with informationa | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | |
| Pal with support and textb | + | + | + | NS | NS | NS | + | + | + | + | + | NS | NS | NS | + | + | |
| Pal with voucherc | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | + | NS | NS | NS | NS | + | |
Discussion
The use of DCEs to inform the design of health services is a well-established approach. The research reported here has taken this a step further with a relatively novel attempt to estimate the effect of different attributes of a smoking cessation service on the expected likelihood that pregnant smokers would be helped to quit. The attributes of the service, including the incentives, were selected on the basis that the systematic reviews showed these to be promising. In the particular case of incentives, the clearest evidence of effectiveness came from comparisons between contingent and non-contingent incentives, with limited evidence relating to contingent incentives compared with no incentive (see Chapter 3). There were no within-study comparisons of level of incentive or frequency of either incentive or general behaviour change components and the studies differed in the types of BCTs used.
Overall, the findings support the potential use of financial incentives and suggest that daily text or telephone support at the start of the quit attempt and the use of a quitting pal will also increase the likelihood that the service will help pregnant women to quit smoking. Incentives are not the only feature of smoking cessation services that are expected to increase the likelihood of quitting but the relative effect of incentives seems to be greater than for other attributes. Importantly, those who consider that stopping smoking would be very difficult perceive that higher-value incentives, daily telephone support and a quitting pal would increase the likelihood of quitting. Results from the categorical analysis of incentives suggest that a voucher value of > £20.00 per month is required to increase the likelihood of quitting. Higher values increase the likelihood of quitting but at a decreasing rate, that is, the magnitude of the effect of a voucher of £80.00 is estimated to be greater than that of a voucher of £40.00, but the effect is less than double.
There has been only limited use of DCEs to investigate behavioural intentions and these have been mainly in smoking cessation. Hammar and Carlsson321 were the first to use DCEs to determine the relative importance or effectiveness of different policies on quitting smoking among smokers. The focus was on high-level policies such as tobacco taxation and regulation and on financial subsidies for smoking cessation. A financial subsidy cannot be considered as a comparable incentive as it would not be contingent on outcome. Respondents were presented with single scenarios and asked whether they would be very likely to quit or to continue to smoke. The authors interpret their responses as smokers’ expectations of their behaviour if smoking policies change and report their results in terms of the share of smokers who would quit smoking under different policies. This is compared with a baseline estimate of 31% of smokers wanting to quit. We believe that the interpretation of our results should be more cautious, partly because an extremely high number of our sample (85%) indicated that if they were smoking at the start of a future pregnancy they would intend to quit.
Goto and colleagues322 conducted a similar DCE with current smokers but asked respondents if they would quit or continue smoking. They repeated the DCE 4 years later. 323 Both studies found that the response to different policies varied with degree of smoking dependence. They did not include the use of incentives in their analyses.
We were unable to include all potential attributes in the DCE given the constraints required to produce a manageable number of choice alternatives. Therefore, other relevant issues, such as frequency and duration of incentives and CO monitoring, could not be explored. The interpretation of the results has to take into account that the responses are based on respondents’ expectations of how they would behave; for this reason, we place more weight on the relative effects of different attributes and levels than on the absolute values. In other words, respondents can judge reliably that they are more likely to quit under one service configuration than under another but this does not readily translate into how they will actually behave in reality.
It should also be noted that the respondents to the survey were volunteers who answer online surveys for a reward and this is a potential source of bias in respect of their attitude to incentives. However, they had been asked to rate the importance of features of a smoking cessation service before undertaking the choice questions and their responses to these questions do not suggest that bias in favour of incentives would be a major problem. In total, 52% of respondents rated receiving rewards for stopping smoking as not very important or not at all important; 47% gave the same response in respect of the level of reward. Comparing ratings for support from a friend or relative, with or without a reward for the supporter, suggested that the reward increased the proportion rating this as important or very important by six percentage points, from 21% to 27%.
Implications for incentive trial design
The results from the DCE can be used to inform the design of future trials of incentives. The results indicate that the size of the effect will increase with the value of the incentive, at least up to £80.00 per month. Studies that are primarily concerned with whether or not incentives work can show an effect with smaller numbers of participants by offering a larger incentive. Studies that focus on the effect of different levels of incentive should have a starting level of incentive that is > £20.00 per month.
The most promising incentive strategy identified in the evidence synthesis, shopping vouchers contingent on verified smoking cessation in pregnancy, and the design of the CPIT (see Chapter 6) are supported by these findings.
The results suggest that providing additional initial support in the form of daily text messages or telephone calls from a quitting expert adviser or including a quitting pal is also likely to contribute to effectiveness for some women. Qualitative data (see Chapter 6) triangulate the DCE findings and suggest that women vary in what they perceive would help them to quit in terms of the method, frequency and the inclusion of others in quit attempts. What would help a woman changes over time and with circumstances and is inextricably entwined with her personal experiences, current real-life issues and other contextual influences, and flexibility of service provision was suggested. This raises the following question: ‘Would individual tailoring of the additional components that accompany incentive provision, either as part of usual care or as part of an intervention, be more effective than a “one size fits all” service?’
Chapter 9 Discussion
In this chapter we briefly summarise the findings from our mixed-methods study in relation to the research objectives and the outcomes described in Figure 1 and conclude with some overall strengths and limitations. Readers should refer to individual chapters for more detailed summaries of the findings, discussions of how the findings fit with the literature, methodological strengths and limitations and the implications of each study stage.
The outcomes reported are:
-
the evidence for the effectiveness of incentives and their delivery processes for improving smoking cessation in pregnancy or breastfeeding outcomes
-
the incentive ladder logic model, a taxonomy of incentive/reward BCTs, an incentive typology, mechanisms of action, delivery strategies and their relation to known facilitators of, barriers to and intrinsic and extrinsic motivators of behaviour change and any unintended consequences
-
the acceptability of a shortlist of the most promising incentive strategies
-
the implications for trial design and development: feasibility, clustering, recruitment, delivery processes, monitoring, outcome measurements and effect size.
This mixed-method multiphase study consisted of three main stages that overlapped. The findings of each stage were integrated to inform the conduct and analysis of other stages. Stage 1 consisted of three evidence syntheses using focused research questions to inform the design of a trial: the effectiveness and delivery processes of financial and non-financial incentives delivered to women, families and NHS or non-NHS providers with regard to smoking cessation in pregnancy or breastfeeding; a qualitative evidence synthesis of the barriers to and facilitators of both behaviours; and a scoping review of incentives for consumers or providers for other relevant lifestyle behaviours. Stage 2 consisted of primary qualitative and survey research to investigate recipient, public and health professional acceptability of incentives and their mechanisms of action, with specific attention paid to any unintended consequences. This resulted in a shortlist of promising incentive strategies. Stage 3 consisted of mixed-methods data analysis and a DCE to refine the design of the most promising incentive trial. This included the incentive characteristics, timing, quantity, delivery processes, recipient monitoring and outcome collection and organisational and contextual factors that are likely to increase effectiveness.
Interventions for these behaviours around childbirth have predominantly applied an individual theory of behaviour change, placing the primary onus on the woman to change her behaviour, with little consideration of wider community, systems and ecological approaches to intervening for behaviour change,18,19 as recommended in the 2010 public health White Paper in England. 73 In addition, very little qualitative research to understand incentives from the perspectives of either the recipient or the person delivering the incentive was identified in our literature searches. Therefore, a broad definition of tangible incentives with a monetary or exchange value was applied (see Chapter 1) as it was considered important not to make assumptions about the meaning of ‘an incentive’ by excluding studies.
What is the evidence for the effectiveness of incentives and their delivery processes in improving smoking cessation in pregnancy or breastfeeding outcomes?
The evidence for the effectiveness of incentives in improving smoking cessation in pregnancy rates (see Chapter 3) found that financial incentives provided as vouchers for biochemically validated smoking cessation towards the end of pregnancy (four studies105,109,141,144 including 332 participants) had an estimated RD of 0.23 (95% CI 0.14 to 0.31) compared with the provision of non-contingent smaller-value incentives for participation or outcome data collection. The RR of cessation was 2.58 (95% CI 1.63 to 4.07). This supports the findings of other systematic reviews of smoking cessation in pregnancy41,54 and recent systematic reviews of incentives for smoking cessation in all age groups conducted since our literature search. 211,213
In four trials107,175,176,179 of 1308 participants, significant effects on breastfeeding up to 6 weeks after discharge were observed for the provision of various combinations of gifts, vouchers, raffles and breast pumps contingent on self-reported breastfeeding compared with no incentive or a much smaller incentive. However, there was too much variation among both the intervention and the BCT components for meta-analysis and caution is required in interpreting these findings.
Data extracted on the general BCTs that were included with incentives in the intervention differed between smoking cessation and breastfeeding. For smoking cessation, provision of information and CO monitoring were most commonly included in the intervention, with a mean of 6.62 (SD 3.1) BCTs per intervention. For breastfeeding, adding objects to the environment (breast pumps) and social support were most commonly included, with a mean of 4.34 (SD 2.8) BCTs per intervention. This highlights that incentive interventions consist of various potentially active ingredients21 and ‘the signal’ or effect size needs to be balanced against ‘the noise’ of the low methodological quality and heterogeneity of the interventions. 324 In particular, any added lay or professional support is known to increase the duration and exclusivity of breastfeeding and is therefore a confounder. 65
Few process evaluations were identified and the delivery strategies were not well described in the main. We therefore created a novel method of mapping the patient journey to illustrate the complexity of the intervention components, timing, modes of delivery and intensity (see Chapter 3). Our approach builds on the graphical methods suggested by Perera and colleagues219 for trial reporting and addresses the poor reporting of intervention detail in systematic reviews and the difficulties that this causes for replication and implementation and which results in waste of resources. 218,325 The number of contacts between recruitment and final outcome collection (intensity) in incentive interventions varied from one to 36 for smoking cessation and from one to eight for breastfeeding. This is important for behaviour change interventions, for which intensity and the quality of additional provider–recipient interactions is recognised as a potential confounder,215 particularly for breastfeeding. 65
Trials were small, the proportion who were eligible who were actually recruited and who subsequently turned up and engaged in the intervention were seldom reported, and attrition rates were variable and often high, particularly for sustained smoking cessation after birth. Qualitative data suggest that incentives are likely to work best for certain types of women, classified by Radley and colleagues113 as ‘enthusiastic amateurs’. These women tend to have more stable lifestyles and thus conceptualise the incentive as part of a wider rewards and social support structure. In contrast, those with more chaotic, stressful lives tend to cut down or relapse, for example, as smoking is central to their lives and an essential strategy for coping. 110,111,113 In addition, we identified two other types of women who do not consider engaging with incentive interventions. The ‘do it aloners’ are represented by the 27% of women reported in an American study who stopped smoking as soon as they found out they were pregnant. 326 This group tends to be well supported and motivated by the health risks and benefits. The ‘non-contemplators’, by contrast, are those who are resistant to change because of the centrality of smoking in their lives. The reach of incentive interventions is a concern and research is in progress to investigate how best to engage pregnant smokers in either self-directed behaviour change or smoking services, for example using text messaging. 327
The incentive ladder logic model
Our original objective was to develop an incentive taxonomy of incentive strategies and to understand the mechanisms of action of incentives and their interactions with the existing barriers and facilitators, and intrinsic and extrinsic motivators of behaviour. However, it quickly became apparent, through service-user consultation and qualitative interviews with women about incentives, that the key issue was the centrality of smoking or breastfeeding to women’s everyday life experiences, as interview narratives kept returning to this.
A narrative systematic review of qualitative evidence syntheses of women’s experiences of the barriers to and facilitators of smoking cessation and breastfeeding identified only three studies for smoking cessation compared with 10 for breastfeeding. A logic model was applied to describe how theories of behaviour change might interact with barrier and facilitator themes ranging from intrinsic to extrinsic influences. The seven key themes for comparison within and between behaviours were centrality and identity; the mother–infant relationship; risks and benefits; developing a new practical skill (breastfeeding only); social context; place; and health professional services. We noted that the smoking and breastfeeding studies had quite different findings:
-
the centrality of smoking to women’s everyday lives was a dominant theme for smoking cessation, with new strategies required to cope with stress, addiction and ‘me time’ when women stop
-
for breastfeeding, external support to facilitate learning a new skill was the dominant theme; however, the mother–baby relationship was central to feeding decisions
-
constructive relationships with partners, family and social networks and health professional relationships and support are necessary
-
negotiating the private–public interface for performing both behaviours is crucial.
An incentive ladder logic model (see Figure 18) emerged from the mixed-methods data analysis of the following phases of the study:
-
Service-user discussion and qualitative data from interviews and focus groups allowed us to access harder-to-reach women experiencing social and material disadvantage, and a few of their partners, all with a wide range of smoking and infant feeding experiences (see Appendices 20 and 21). Some of our sample had experience of being in a voucher incentive intervention for smoking cessation in pregnancy, for example the CPIT, and some had experience of being in a gift incentive scheme for breastfeeding; a few teenage mothers had experience of a scheme incentivising multiple behaviours organised by a charity.
-
The review of qualitative evidence syntheses of women’s experiences of the barriers to and facilitators of smoking cessation and breastfeeding.
-
The systematic review (see Chapter 3), in which the incentives provided were multiple both within and across studies. An initial typology was refined through qualitative data collection and analysis. The incentives ranged from unrestricted, hedonistic shopping vouchers to behaviour-related, utilitarian, more restricted health-related and experiential incentives with benefits beyond the individual. The incentive meaning could imply moral judgements and values around autonomy, choice and trust. Incentives that increased maternal well-being seemed to be the most motivating.
-
In an attempt to understand any interactions between the incentive component and the other BCT components in intervention studies included in the systematic review, an IRBCT taxonomy (see Table 2) was developed from existing behaviour change taxonomies. 17 This was designed to fit and extract data from the studies included in the systematic review (see Chapter 3) and had four categories: incentive/reward content and type, contingency target and actor. In particular, it was considered important to distinguish between an incentive, which is something that motivates an individual to act and may include awareness that achieving the behaviour outcome will result in a guaranteed reward, and when a person either does not have prior awareness of or is uncertain about the receipt of a reward. In the latter case, the motivation to act may be less. This distinction is seldom addressed or well reported in the literature; however, our data suggest that the timeline from awareness to receipt could be important.
-
The patient journey (see Tables 8 and 26), which captures how the timing, frequency and intensity of the incentive and BCT components and their modes of delivery varied over time. This was an important step in our understanding of the potential mechanisms of action of incentives.
A logic model based on a ‘ladder’ as a metaphorical concept and the ‘rungs’ as what an individual woman might need to achieve the behaviour outcome(s) emerged from the mixed-methods data analysis of all stages of the study. The ladder refers to life course and context rungs, which are something/someone/a situation in the woman’s life that helps/facilitates or motivates a woman to climb the ladder and supports her at each step. Damaged rungs occur when existing support for behaviour change ceases, for example when a partner starts smoking again or when support from helpful hospital staff for breastfeeding stops when a woman goes home. Missing rungs are evident when the personal and/or vicarious experiences of the behaviours are strong social norms for continued smoking or formula feeding. Such social norms are known to influence both smoking in pregnancy and infant feeding decisions. 12 The ladder also has intervention rungs, specifically incentive/reward component(s), contingency and verification of the behaviour, other BCT and psychosocial intervention components, delivery processes and modes of delivery. These need to be structured and replicable for trial design, but also need to fit with the everyday lives of women and with routine maternity and early years care for the intervention to work in practice and be feasible.
The development of a shortlist of the most promising incentive strategies
The initial shortlist of incentive strategies was compiled from the evidence synthesis of the effectiveness of incentive interventions for smoking cessation in pregnancy and breastfeeding presented in Chapter 3. Intervention vignettes were created for a diverse range of effective or promising studies (Appendices 2 and 16) to allow us to revise the shortlist through discussion with co-applicant mother-and-baby groups and qualitative interviews with women and providers. This resulted in changes to the incentive strategies when there was weak evidence from the review. For example, breast pumps were particularly popular with younger, more materially disadvantaged women who considered the cost prohibitive, who disliked the feelings of embarrassment when the function of breasts changed from sexual to nursing and when sharing feeding with either a partner or another family member was a priority, for example to re-establish a social or working life after birth. However, health professionals expressed concern that a free breast pump would endorse it as a prerequisite for breastfeeding and voiced uncertainties around the effects that the introduction of a breast pump might have on feeding outcomes.
A scoping review of systematic reviews of other relevant lifestyle behaviours (see Chapter 5), for example drug and alcohol addiction, smoking in all age groups and obesity, supported the meta-analysis findings for the effectiveness of contingent financial incentives compared with smaller non-contingent incentives for participation or outcome data collection. In addition, it found that incentives provided to health service providers can change short-term behaviour, particularly for GPs who have a commitment contract to deliver the QOF, which includes documenting smoking status and referral to cessation services. The shortlist is discussed in the following sections, bringing together the mixed-methods analysis for all stages of the study, including the implications for trial design.
The acceptability of a shortlist of the most promising incentive strategies
Overall, opinions were divided in both the general public and the health professional survey samples about the provision of incentives to either women or local health services. For both the general public and health professionals, the most agreeable incentive strategy would be to provide a free breast pump costing around £40.00 to help women to continue breastfeeding. Being of childbearing age (< 44 years) was the only independent predictor of general public agreement with all seven incentive strategies, with agreement decreasing with increasing age. However, women were significantly more likely than men to disagree with any of the voucher incentive strategies delivered to women. Those with a lower educational level, which is the best indicator of disadvantage,310 were also more likely to disagree than those with a degree-level qualification. These findings are a concern as addressing health inequalities is a government priority and the target populations in which smoking in pregnancy and not breastfeeding are highest are less-educated, younger women living outside London and the South East. 53 However, those with direct parental experience of even short-term breastfeeding of a child, or current smokers with a failed quit attempt, were more likely to agree with incentives for women for the respective behaviour. Overall, universal incentives were preferred to incentives targeted at low-income women, with concerns about stigma and value judgements raised (see Chapter 6). Geographical areas and cultural groups known to have higher rates of breastfeeding, for example those living in London and minority ethnic groups,53 were more likely to agree with breastfeeding incentive strategies. Ethnic minority women are less likely to smoke during pregnancy than white British women and this was a predictor of agreement with incentives for women for smoking cessation.
Views on the acceptability of the individual incentive strategies are discussed in the following sections.
The most promising incentive trial design: incentives for women to stop smoking in pregnancy
Providing shopping vouchers for biochemically proven smoking cessation in pregnancy was supported by the systematic review evidence of effectiveness in pregnancy (see Chapter 3) and by the review of incentives for smoking in all age groups (see Chapter 5), the qualitative data (see Chapter 6) and the DCE (see Chapter 8).
General public and health professional acceptability
Considering survey and qualitative data, shopping voucher incentives for smoking cessation in pregnancy were more acceptable than incentives for preventing smoking relapse after birth or for breastfeeding. In the survey of the general public there was a net disagreement of 42.3% and a net agreement of 40.5% with this incentive strategy, whereas in the survey of health professionals there was a net disagreement of 53.9% and a net agreement of 34.6%. This could be considered as representing collective uncertainty and therefore the ideal conditions for a definitive RCT. DCE research suggests that acceptability is very sensitive, even to small changes in effectiveness for smoking cessation (general population),301 which strengthens the case for a definitive trial. Over 85% of general public respondents who did not disagree with the provision of incentives to women, for either smoking cessation or breastfeeding, stated that a value of ≤ £40.00 per month was acceptable. This is of a similar order of magnitude to that in the studies in the meta-analysis reporting the effectiveness of financial incentives for smoking cessation in pregnancy (see Chapter 3), which suggests that vouchers for smoking cessation are effective; however, this is less than the £400.00 total that can be provided to women who remain quit until the end of pregnancy in the CPIT (see Box 2). The DCE (see Chapter 8) found that studies should have a starting level of incentive that is > £20.00 per month and that the size of the effect will increase with the value of the incentive, at least up to £80.00 per month. The incentive value provided in the CPIT is therefore appropriate for a definitive trial as the larger incentive will reduce the sample size requirement.
Fit with the Cessation in Pregnancy Incentives Trial
The CPIT88 is summarised in Chapter 1 (see Box 2) and qualitative transcripts were included in the analysis in Chapter 6. This was led by co-applicants LB and DT and commenced before the BIBS study started. The CPIT was modelled on the effectiveness study by Donatelle and colleagues141 and the Give it up for Baby study by Radley and colleagues,113 except that it did not include a quitting buddy. The encouraging interim analysis of the CPIT data was reported to the BIBS research team in February 2013, after completion of the smoking cessation incentive systematic review (see Chapter 3) and at the time of agreeing the shortlist. In December 2014 when this report was finalised the only publication available was a conference abstract. 328 The full CPIT findings were published in January 2015. 329 Qualitative interview transcripts from CPIT participants and providers were incorporated into the data analysis at the end of July 2013 at a late stage to minimise bias when interpreting the data (see Chapter 6). Overall, the design of the CPIT Phase II trial was supported by the findings of the BIBS study.
Proposed design for a future trial of incentives for smoking cessation
The proposed design for a future trial of incentives for smoking cessation is a RCT of the effectiveness and cost-effectiveness of shopping vouchers contingent on verified smoking cessation compared with non-contingent smaller incentives for participation, setting a quit date and final outcome measurement.
Target population and setting
The target population is any woman who is confirmed pregnant and smoking by salivary or plasma cotinine level, with the intervention ideally delivered in a variety of settings, including in the community.
Intervention
The intervention includes shopping vouchers, with a value of at least £20.00 and up to £80.00 per month, for biochemically proven smoking cessation in pregnancy and continuing after birth for up to 3 months. The active components are shopping vouchers; setting a quit date; short-term goal-setting and feedback through regular CO monitoring appointments and seeing the colour of the monitor change to green; salivary or serum cotinine verification of outcomes; and additional support tailored to the woman’s life circumstances, which may include the woman’s partner or a buddy participating in the intervention. In the DCE (see Chapter 8), the relative effect of incentives seems to be greater than that of the other attributes: face-to-face meetings with a quitting expert adviser; telephone or text support with varying frequency, including daily during the first week; and including a quitting pal. Those who consider stopping smoking to be very difficult perceive that higher-value incentives, daily telephone or text support and a quitting pal will increase their likelihood of quitting. Frequent support in the early stages of breaking an addictive habit could work through helping to overcome present bias316 and fits with the evidence of effectiveness. 315 The DCE confirms that daily text or telephone support at the start of the quit attempt and the use of a quitting pal will increase the likelihood that the service will help pregnant women to quit smoking. This provision of additional support should be considered in incentive interventions.
Control
An identical patient journey to the intervention arm in terms of the number of contacts with service providers, the number of BCTs, the support offered and the mode of delivery (telephone, text, face to face) is required. A small incentive for setting a quit date to increase participation and a small incentive at the final outcome measurement is supported by the evidence. However, the CPIT provided only a £25.00 voucher for providing the primary outcome data and this was both acceptable and feasible to recruit and retain participants. Identical patient journeys will provide a definitive test of the incentive component.
Mechanism of action
The mechanism of action crucially depends on non-restrictive shopping vouchers, which increase autonomy, motivation and control to maximise the well-being value of the incentive in addition to the financial value. In the health promotion literature, there has been criticism that incentives to change behaviour can undermine personal moral autonomy by nudging people to make decisions and behave against their free will. 27,330 However, although there was evidence that some women feel judged or blamed for smoking while pregnant, others saw incentive schemes as an opportunity that tipped the balance of latent intrinsic motivation towards action. Importantly, for the materially disadvantaged, unrestricted shopping vouchers can be seen as autonomy enhancing and providing rare choices and treats to enhance well-being. As such, they were seen as a just reward for the effort involved. The nature of the relationship between the individual woman and the specialist smoking cessation advisor once the woman has enrolled is also crucial. Flexible, tailored, non-judgemental support provided through a continuing relationship with a dedicated smoking advisor was highly valued and motivating. This is consistent with social learning theory,12 developing self-efficacy and the intrinsic motivation necessary to put the rungs of the ladder in place to sustain the new behaviour. The BIBS study data generate the hypothesis that the incentive, the BCT and the relationship components are synergistic and greater than the sum of the parts, which fits with Deci and Ryan’s self-determination theory. 23
To address the potential missing or damaged rungs in a woman’s life, some individual tailoring of the intervention is likely to moderate the effects, for example the frequency of support, the mode of delivery (text, telephone or face to face). This is consistent with the evidence that individually tailored materials in self-help interventions for smoking cessation are important. 331 A strongly held view was that shopping vouchers should be provided contingent on attending appointments for monitoring and proof of behaviour change, and women, particularly those with experience of the CPIT or other incentive interventions, valued this.
The intervention is consistent with the theory of reinforcement through reward, which results in conditioned learned responses to establish the new behaviour. 9,17 However, in the CPIT there is a slight delay in receiving the reward in contrast to the immediate reinforcement that is usually provided,105,141,144 as the vouchers were securely posted to women. In the CPIT, posting the incentive was considered more logistically feasible and appropriate within the UK NHS, where exchange of money is not the norm.
Recruitment and delivery strategy
Recruitment can be by self-referral or by referral by the midwife or other health professional to the smoking cessation service. The current dedicated smoking cessation service in the CPIT was acceptable to those who engaged. Consideration needs to be given to alternative strategies to increase reach, including recruitment through home visits or convenient community settings for those for whom attending institutional settings is a barrier.
Monitoring and outcome measurement
The primary outcome should be at the end of pregnancy as this is the time of maximum health gain for the baby. Trials with > 3 months’ follow-up after birth are required to assess whether the outcome attained at the end of pregnancy is sustained. The contact(s) for outcome measurement should be considered part of the process for both the intervention group and the control group. The structured CO monitoring visits, at which achievement was verified and visible, acted as short-term goals and this was often more acceptable than urine testing. Using samples that are collected routinely in maternity care was acceptable and is likely to minimise the effects of the interaction between health professionals and women.
Effect size
Only early incomplete data from the CPIT Phase II trial, and a published abstract were available when this report was finalised in December 2014. 328 The CPIT findings were published in January 2015329 and provide a UK context to help a sample size calculation to be carried out for a definitive Phase III multicentre trial.
Breast pumps as a promising incentive strategy
The evidence of effectiveness for providing a breast pump as an incentive to prolong the duration or exclusivity of breast milk feeding is inconclusive and the overall quality of the studies providing this evidence was low (see Chapter 3). The interventions (type of pump, target population, timing) and the comparison groups (no pump, a different type of pump, infant formula gift packs) all varied and the amount of additional information given and support provided for expressing breast milk was poorly reported. All included trials used individual randomisation, but contamination occurred. 174 However, some positive breastfeeding outcomes were observed and further feasibility studies followed by a pilot trial are indicated based on the qualitative (see Chapter 6) and survey (see Chapter 7) findings that this was the most acceptable incentive strategy, as discussed in the following section. Importantly, younger, more disadvantaged women, who are the least likely to breastfeed,53 were enthusiastic about this incentive strategy as the cost of an electric pump was considered prohibitive.
The epidemiology of expressing breast milk and pump use in the UK and how this relates to breastfeeding duration and exclusivity are unknown. These data would be required before carrying out any feasibility or pilot trial. Data from the USA185 and Australia332 suggest that breast pump use is increasing, with around 85% of breastfeeding mothers of young infants in the USA expressing breast milk, mainly using electric pumps,333 which produce greater volumes of milk at 6 days after birth than hand expressing. 295 Routinely collected infant feeding data from Scotland show an increase in mixed feeding at 6–8 weeks. 81 Exclusive breastfeeding benefits infant and maternal health most; however, any breastfeeding is better than none at all. 63,64 There are concerns about the commercialisation and commodification of breastfeeding through marketing breast pumps, which could potentially disrupt the mother–infant relationship by adding technology. 334 Vested interests and shareholder profits could undermine breastfeeding.
General public and health professional acceptability
For both the general public and health professionals, provision of a breast pump was the most agreeable of the seven incentive strategies, with 48.0% and 67.8% net agreement by the general public and health professional respondents respectively. Net disagreement with providing a free breast pump was 27.8% for general public and 21.9% for health professional respondents. Qualitative data showed that behaviour-related incentives such as breast pumps (or NRT devices for smoking cessation) were more acceptable with some than hedonistic and unrestricted incentives such as shopping vouchers, as the latter could be perceived as rewarding women who had behaved badly.
Potential design for a future trial of a breast pump as an incentive for the continuation of breastfeeding
A cluster RCT would be required to avoid contamination between the intervention arm and the control arm, as reported by Rasmussen and colleagues,174 and to ensure intervention fidelity.
Target population and setting
The target population is mothers who initiate breastfeeding as qualitative data suggest that receiving a free breast pump is unlikely to act as a motivator for women who do not want to try breastfeeding. However, providing antenatal information about breast pump services and expressing milk is important, as accounts suggest that this is a neglected area because of health professional concerns about it disrupting breastfeeding. The most acceptable setting will be related to the optimum timing of providing information and pump provision and this is currently unknown.
Intervention
The optimal type and value of pump are unknown and may differ for different women according to personal preference and breast characteristics. For expensive electric pumps, a free hire service with the provision of personal sterile tubing, bottles and freezer bag packs could be an option. Hire services are provided by charities such as the National Childbirth Trust, with fees of between £38.50 and £45.00 for the first 15 days in some areas. 335 Additional information and support are likely to be required as pumping milk is a practical skill that women report is challenging to acquire. The timing and content of the information and support required for skill acquisition are currently unknown and concerns were expressed that providing information and the provision of a breast pump too soon might jeopardise the establishment of breastfeeding. In guidelines for premature infants, mothers are encouraged to express breast milk (hand or pump) immediately after birth to maximise the health benefits for the infant,183 yet for term infants there is no clear guidance, with accounts of inconsistent advice to wait a variable length of time until breastfeeding is established. The optimal training for those providing support for expressing breast milk is also unknown. Anecdotally, breast pump service provision within the NHS is variable. Health professionals have reservations about a breast pump being perceived as a necessity to breastfeed and it being a ‘slippery slope’ towards introducing bottles. They often state the need to establish breastfeeding before trying to express. The current uncertainty about best practice is problematic for women and health professionals and is resulting in conflicting advice. This needs to be resolved before a trial can be undertaken.
Control
The control arm would not receive a breast pump incentive but would receive identical antenatal education and support provided to all women who choose to buy or hire a pump themselves. This would need to take account of any variation in pump provision between areas.
Mechanism of action
Expressing milk is a preparatory behaviour for the primary outcome of sustained duration of either mixed or exclusive breastfeeding (although the relationship is unknown). From a BCT perspective, a breast pump is classified as adding an object to the environment to facilitate the behaviour. 17 However, a recurrent theme in the data is of incentives as connectors,112 and breast pumps serve as technological connectors or bricolage. They help the mother (the bricoleur) to manage the work or problems experienced with breastfeeding and to establish a breastfeeding habit and culture that works for her, her baby and her family. Breast pumps facilitate how breastfeeding fits her social network norms and potentially enhance family well-being. Providing a breast pump as an incentive is unusual in that it addresses the barriers and facilitators at all levels, from intrinsic physiological and emotional factors to extrinsic factors, particularly at the private–public interface and among family and social networks (see Chapter 4). They therefore act at more levels than a shopping voucher or other well-being or utilitarian or health incentives. Breast pumps can facilitate milk production, particularly for premature or sick infants in whom sucking is poorly developed or impaired. They allow the mother to have some control over infant demand for food. They can provide flexibility to create ‘me time’, which, for some mothers, is a coping strategy for countering stress and maintaining personal and family well-being. Family well-being, rather than the longer-term non-tangible health benefits, is the main outcome that influences infant feeding decisions. 288 Breast pumps can be a rung for re-establishing social life after birth or caring responsibilities (e.g. other children) or allowing a return to work. They can act as a rung to overcome the embarrassment of the change of role of breasts from sexual to feeding, particularly for younger mothers in whom this is a key barrier (see Chapter 6). Breast pumps increase flexibility by allowing the provision of breast milk in front of other people and outside the home, which 43% of UK mothers find uncomfortable. 53 Some partners/couples/grandparents want to share parenting by actively feeding the baby and perceive feeding as a unique way to establish a bond. 289,336
Recruitment and delivery strategy
The recruitment and delivery strategy would need to be ascertained through a feasibility study, in particular the timing and type of pump provision and the education, training and support required to optimise the incentive component. A breast pump service is likely to be needed in hospitals and in community locations with easy access, particularly for women who are unable to drive for 6 weeks after a caesarean section.
Monitoring and outcome measurement
Routinely collected data at 6–8 weeks post partum on any and exclusive breastfeeding would be the most feasible primary outcome for a cluster RCT in the UK. Exclusive breastfeeding at 6 months would be the most appropriate secondary outcome as this is the WHO recommendation,60 which is endorsed by UK governments. However, exclusive breastfeeding at 6 months is not routinely collected in the UK at present. Longer-term follow-up would be desirable, particularly to understand the relationship between breastfeeding, breast pumps and a return to work. There is currently no biochemical test to verify breastfeeding outcomes. A contingent incentive for attending a breastfeeding session to collect outcome data would be supported by the evidence from the review (see Chapter 3) and would be more acceptable than available alternatives, such as photography or professional verification (see Chapter 6). Incentives for participation are known to reduce attrition in trials,216 and participants considered gaming less likely for a free breast pump than shopping vouchers (see Chapter 6).
Effect size
The effect size is currently unknown and would be informed by further feasibility work.
Incentives for provider organisations to maintain breastfeeding
The evidence synthesis in Chapter 3 suggests some promise for provider incentives in the form of an award (or a penalty) for meeting (or not meeting) quality targets for improving breastfeeding rates, if embedded within a structured programme (a commitment contract). 168,184 However, further experimental research is needed to test different doses of incentive/penalty components for providers, preferably a RCT, as recommended by Flodgren and colleagues. 246 No RCT of the QOF commitment contract was conducted prior to implementation as UK policy for all general practices. Although there have been short-term health benefits and there is evidence of behaviour change, there are unintended consequences for other services (see Chapter 5).
Public and health professional acceptability
In the survey of the general public, there was a net disagreement of 38.6% and a net agreement of 36.4% with the strategy of provider incentives. There was a similar net disagreement among health professionals, but a higher net agreement of 44.1%. The qualitative data identified mixed views: some felt that incentives for local health services might encourage investment in skilled staff and peer support programmes, especially in the community. However, targets were viewed by many with caution as they were seen as potentially undermining motivation in more disadvantaged areas that were already struggling with workload. As there is evidence that any additional lay or professional support for breastfeeding can increase breastfeeding duration and exclusivity,65 even though the generalisability of this to the UK is uncertain,66 it was decided to include provider incentives as our third promising incentive strategy.
Potential design of a future trial of provider incentives
With routinely collected breastfeeding data at 6–8 weeks being available in the UK, with > 90% completion rates in some areas of Scotland,81 a cluster trial or a step wedge intervention design could be feasible.
Intervention
The intervention would be financial commitment incentives or penalties conditional on a significant reduction in breastfeeding cessation rates for women who are breastfeeding at hospital discharge and who are still providing some breast milk at 6–8 weeks. The publicity for the scheme, with award ceremonies for the best achievements and dissemination of good practice, could provide an additional incentive, as it does with the UNICEF BFI (see Chapter 3). However, caution would be needed around public naming, blaming and shaming as it can result in stigma that is demotivating. 337
Control
Depending on the design, the control could be usual care or a waiting list to join the incentive scheme.
Mechanism of action
Behavioural economic theory suggests that potential losses are more influential triggers to motivate change than gains. 23 Audit of and feedback to health service providers can result in small to moderate improvements in professional practice;338 however, the benefits of adding an incentive/penalty system are unknown. A commitment contract with an incentive will focus an organisation’s attention on breastfeeding as a priority and counteract providers’ accounts of services being squeezed and women’s complaints of insufficient health service support. By providing incentives (or penalties) for a significant change in breastfeeding rates, regression towards the mean and ceiling effects could result in areas with the lowest breastfeeding rates and most deprived communities being more likely to benefit. However, a counter argument is that the most disadvantaged areas, where unhealthy behaviours are most prevalent, require additional funding regardless of meeting targets, as it not an even playing field. Incentives provided to organisations have the potential to combine health, community, women’s and family efforts in the same direction to generate additional resources for providing breastfeeding support.
Monitoring and outcome measurement
The primary outcome would be any breastfeeding at 6–8 weeks determined from routinely collected data. Outcome verification and accuracy of reporting would need to be considered. A process evaluation to assess patient, staff and manager experiences, to understand outcomes and to capture any unintended consequences, in particular any opportunity costs sustained as a consequence of the intervention, would be important.
Incentives to providers to improve smoking cessation in pregnancy
No intervention studies of incentives for providers to improve smoking cessation in pregnancy were identified in the systematic review (see Chapter 3). There was some evidence of short-term effectiveness of the use of incentives to change provider behaviour in terms of documenting smoking status and referring women to smoking cessation services from the UK general practice QOF system (see Chapter 5). The QOF system is another example of a commitment contract and there is evidence to suggest that care for chronic conditions has reduced health inequalities. However, there are criticisms that it undermines the intrinsic motivation of GPs and leads to neglect of un-incentivised aspects of care. General public views of incentives for providers to improve smoking cessation in pregnancy were mixed, with a net disagreement of 37.2% and a net agreement of 39.4% (see Chapter 7). Health professionals were more likely to agree with this strategy and, importantly, midwives, health visitors and other maternity staff were significantly more likely to agree than doctors. This is in the context of most UK midwives and health visitors referring pregnant women to specialist smoking cessation services in the UK. Specialist services were viewed favourably by participants in the qualitative interviews, although some felt that this should be a role for all providers (see Chapter 6). Further research to assess the feasibility of this strategy is indicated.
Shopping voucher incentives for women to improve breastfeeding outcomes
There is some evidence to support the provision of a variety of gifts for improving breastfeeding outcomes (see Chapter 3); however, the interventions in identified studies were multicomponent, with support being the most common additional intervention component, which is a confounder. 65 This incentive strategy was the least acceptable of the ‘vouchers for women’ incentive strategies, with general public net agreement of 33.4% and net disagreement of 55.9% (see Chapter 7).
Qualitative data similarly showed that there was opposition to this strategy, with a preference for more behaviour-related incentives such as breast pumps or nursing bras. For smoking cessation, additional motivation to even try to overcome substance addiction is required; this is in contrast to breastfeeding for which many have the intrinsic motivation to ‘give it a go’. 339 However, both women and health professionals noted the requirement for additional help and support to succeed with breastfeeding, which accounts suggest is a current unmet need for many. Given the considerable barriers at intrinsic and extrinsic levels (see Chapters 4 and 6), many women choose to stop breastfeeding in the early weeks, but eight out of 10 would have liked to continue for longer. 53 Indeed, health professionals, except doctors, suggested that incentivising organisations to provide additional breastfeeding support was warranted, rather than incentivising women, in the face of squeezed services in the current economic climate. The findings of a proposed breastfeeding incentive intervention study,221 which is in progress, will provide important additional data for assessing the acceptability and feasibility of financial incentives for breastfeeding and therefore no further consideration was given to trial design for this strategy.
Incentives for a smoke-free home after birth
This strategy of providing incentives to women for a smoke-free home was considered important by some women and professionals; however, this was the least acceptable of the seven incentive strategies in the general public survey (net agreement 34.4% and net disagreement 46.0%). Continuing the incentives for up to 3 months after birth had similar acceptability. We are not aware of any incentive interventions for a smoke-free home, or a smoke-free car (as suggested by one of our co-applicant service users), and further Phase I studies to investigate the optimum trial design in terms of the feasibility of monitoring and verification of outcomes at home, the incentive delivery processes, any additional BCT components required and the costs of providing such an incentive strategy are indicated. This is important as in a Cochrane review only 11 out of 36 trials reported a significant effect on environmental tobacco exposure in children aged up to 12 years of interventions aimed at parents, families, carers and teachers, and counselling had only a limited effect. 340
Overall implications for incentive trial design
We suggest that an incentive intervention alone would be unlikely to change or maintain behaviour in isolation and, as the ladder model demonstrates, the interaction and fit with other life course and context rungs will be likely to influence engagement and effectiveness.
The cost-effectiveness of incentive interventions is unknown. A monthly incentive value of at least £20.00 is required. Delivering and engaging in an incentive programme, which, in some effective studies, entailed up to 36 contacts with service providers,105,144 itself incurs transportation and opportunity costs. These costs will have differential effects across different employment categories, income levels and caring responsibilities and need to be accounted for.
Women can feel valued, encouraged and supported by the provision of appropriate incentives combined with other BCT and psychosocial components. The attributes of interventions valued by women are:
-
interim short-term goal-setting through regular visible monitoring and feedback
-
additional specialised and skilled support from dedicated services, particularly by telephone or text
-
individual and flexible tailoring of interventions to fit women’s complex and/or chaotic everyday life situations
-
continuity of non-judgemental, flexible and confidence-building care
-
services provided in multiple settings to increase access, particularly in the community
-
including a partner or buddy in the intervention.
These valued intervention components and modes of delivery are likely to interact with the incentive component and we hypothesise that there are likely to be synergistic effects. Conversely, there are likely to be detracting interactions, for example unskilled, insensitive, ‘one size fits all’ delivery, which participants perceive as pressurising, moralistic and judgemental and which undermines confidence, autonomy and intrinsic motivation.
If the intensity of the interactions occurring within a research intervention is a confounder, then an ideal trial to test the incentive component is to have identical patient journeys for the intervention and control arms apart from the incentive component. As recommended by Sutherland and colleagues,29 multisite comparisons of an intervention are crucial to understand the interactions of incentive interventions with context, particularly different health systems and cultures. A strong case can be made for reporting patient journeys in incentive trials and for conducting process evaluations so that systematic reviews can compare journeys for both trial arms to determine the effects of the incentive.
The use of logic models is recommended when reporting public health research33 and their utility beyond theory was evidenced in the BIBS study. When tested with service users, the ladder model showed considerable potential to assist in the codesign of incentive trials for smoking cessation in pregnancy and breastfeeding. Codesign and partnership working are recognised as crucial to ensure that research is relevant to and optimised for the target populations. 91 The ladder model might have wider relevance for the design of complex interventions that aim to change behaviour: to identify the intervention component rungs, their delivery and how a trial would fit with the life and context rungs. Further fieldwork testing of the model with a wider range of stakeholders and for a wider range of behaviours is indicated, particularly as our participants identified non-financial or non-tangible incentives as important motivators for change.
Strengths and limitations
These are considered in detail for each individual chapter and are therefore not repeated here. Overall, we consider the strengths of the BIBS study to be the multidisciplinary, multiphase, mixed-methods design, which considers a broad definition of incentives from multiple perspectives. Most research adopts a narrow definition of financial incentives. If we had done this the understanding gained from the rich, in-depth data would not have been achieved. A broad definition of tangible incentives was chosen to assist in understanding their mechanisms of action by exploring the meaning and value of incentives to the target populations. Similarly, more limited study inclusion criteria in the systematic review (see Chapter 3) would have excluded important studies that were used for understanding the mechanisms of action of incentive interventions. The qualitative purposive, theoretical and snowball sampling strategy281 provided a diverse sample for sociodemographic characteristics and variety of perspectives. The approach was reflective and iteratively refined the research questions and constantly searched for disconfirming data. This is a methodological strength. The survey, delivered through Ipsos MORI, provided a sample that was representative of the general public and is the largest of its kind to date. Our study is original in several respects:
-
Service-user mother-and-baby groups from disadvantaged areas as co-applicants contributed substantially throughout the study. Members of the research team engaged with these service users on their premises, fitting with their agendas and therefore challenging conventional power relations. This enabled us to obtain a range of perspectives from harder-to-reach informants in the target population with experience of the behaviours that the study seeks to change. This can be contrasted with the single trained and hence professionalised service-user perspective, which is sometimes reported.
-
Patient journeys through interventions for the systematic review were mapped to reveal the complexity of these interventions. Intervention vignettes were then created to incorporate service-user and professional perspectives.
-
The current BCT taxonomy17 was modified to create an incentive/reward BCT to fit the diversity of studies included.
-
An incentive ladder logic model from the mixed-methods analysis of the whole study was created to inform the design of complex incentive interventions to change lifestyle behaviours. A strength is that this had face validity in discussions with co-applicant mother-and-baby groups, many of whom smoked during pregnancy and/or did not breastfeed and lived in disadvantaged communities and thus represented the target population. Women found the ‘ladder’ idea easy to understand, to relate to and to use. More importantly, they found it acceptable as a model to consider how incentive behaviour change theory could be translated into their everyday lives. We anticipate that the codesign of behaviour change intervention trials using the ladder model might be valuable.
The main limitations of the study are the difficulty of assessing the effectiveness or cost-effectiveness of incentives because of their multicomponent nature, the heterogeneity in study design, the poor quality of reporting of intervention delivery and the control arms, the focus on short-term outcomes and the paucity of process evaluations. This limited our ability to report results for incentives according to the income, education, ethnicity or other sociodemographic characteristics of participants. Most of the evidence is from US trials and the generalisability of these findings is unknown, particularly as many US women of childbearing age are not insured for health care and this is a barrier to health-care treatment. They therefore may behave differently from their UK counterparts.
Researcher reflexivity is an important quality indicator in qualitative research and indeed any study. Our multidisciplinary, multi-institutional team and the selection of areas where health service attitudes to and experience of incentives were known to differ were a priori decisions to minimise bias of interpretation. Our research team included previous smokers, researchers with and without children and experiences of breast and formula milk feeding and male and female researchers. Researchers held different perspectives on incentive interventions for behaviour change, with four involved in incentive interventions (LB, DT, GT and PH). Differences and potential biases were discussed in regular team meetings and noted in reflective diaries kept by the qualitative research team. Every attempt was made to minimise the conflict of interest from the CPIT co-applicants in the data by including qualitative transcripts once the initial qualitative analysis had been carried out, by not involving the CPIT co-applicants in the data extraction or analysis in Chapters 3 and 6–8 and through transparency throughout in research team meetings. This was important as CPIT participants and others who had participated in incentive schemes were generally more positive about incentives and their delivery processes than those with no experience of incentive interventions. This is encouraging as incentives can reduce attrition in intervention trials216 and, once engaged, voucher incentive interventions for smoking cessation in pregnancy are both effective and acceptable to participants.
For qualitative data collection, every attempt to minimise framing effects was taken by using four interviewers from different disciplines, three locations and flexible topic guides that were revised as the research questions were refined, consistent with a grounded theory approach. The interactions between the researchers and the participants for qualitative data collection needed to be flexible because of literacy problems or preferences for where data collection took place (e.g. clinic areas). The environment (group, clinic, home) and context (private, public, telephone, face to face) may have influenced the data collected. Researchers did not always introduce intervention vignettes according to circumstances or sometimes they were read by the researcher rather than letting the participants read them at their own pace. Some participants appeared to expect the researchers to be pro incentives, without researchers conveying any information or evidence to this effect. This was not considered to impact obviously on the participant views expressed, but rather on how opinions were presented as either apologetic or confrontational.
Limitations of the ladder metaphor include the many different types, purposes and interpretations of ladders, not all of which may be constructive. However, ladders offer a broad range of possible uses and allow for individuals’ own interpretations to be relevant to their own context, goals or challenges. They can therefore be considered an asset. Nevertheless, we would like to distinguish our model from hierarchical and linear ladders, such as those used in health for pain management. 341 Although the step-by-step ascent of a ladder can be considered to fit progression through an incentive intervention trial, it might not necessarily fit the complexity of barriers and facilitators in women’s everyday lives. Further evaluation of the validity of the ladder logic model is needed with a diverse sample of the target population who are not familiar with this research, unlike our service-user groups. In particular, this should address any concerns that ‘ladders’ might exacerbate stigma.
Limitations of the Ipsos MORI and health professional surveys and the DCE include possible selection bias and unknown confounding. Through randomising the order of questions in the survey, framing effects were evident when comparing the acceptability of different incentives for the different behaviours. This is important as most research on lifestyle behaviour change is conducted on behaviours considered in isolation from one another, and there appears to be little cross-collaboration, as illustrated in Chapter 4. If we had conducted separate surveys at separate times for individual behaviour incentives, the outcomes might have been different.
The health professional survey was sent to a small self-selected sample of health professionals who were mostly based in Scotland. With the exception of the help received from the SPCRN, we experienced difficulties in identifying e-mail list gatekeepers for health professionals working only in maternity and early years services. The timing of the survey, which coincided with the reorganisation of the NHS in England, was unfortunate. However, private companies do not appear to be the solution to accessing health professional perspectives as the response in North West England was low.
Chapter 10 Conclusions
-
Overall, general public views about the acceptability of incentives for smoking cessation in pregnancy, to prevent relapse after birth and for breastfeeding were not clear-cut.
-
Women, the less educated and those living in more disadvantaged UK regions significantly disagreed with providing vouchers to women for either smoking cessation in pregnancy or breastfeeding.
-
More participants agreed with universal incentives than incentives targeted at low-income women. The importance of equity, with all unborn children seen as being of equal importance, and concerns about the stigma of targeting are important considerations.
-
Younger age groups, in particular those aged < 44 years, which includes the target population, were more likely to agree with incentives than those aged ≥ 65 years.
-
Qualitative data suggested that incentive interventions that are rigid, prescriptive and place the onus primarily on the woman to behave in a ‘healthy’ way risk women feeling judged, pressurised and blamed. To avoid losing face, women describe disengaging with services and feeling demoralised. This is a potential explanation for why some women, particularly those with stressful life situations, disagree with incentives.
-
There was evidence that, compared with non-contingent incentives (which may be small payments for taking part and providing outcome data), providing vouchers contingent on biochemically validated smoking cessation, either solely to women or to their social supporters as well, combined with intensive support, is effective for smoking cessation in late pregnancy and until 3 months after birth.
-
Effective smoking cessation incentive interventions included up to 36 contacts for additional support and BCTs such as goal-setting, monitoring and feedback. Intensity and BCTs are therefore likely confounders.
-
Incentives contingent on verification of behaviour outcomes were considered important by participants. CO monitoring to set short-term goals with visual outcome verification was valued; however, the absence of a biochemical or other acceptable method of verification for breastfeeding is a concern. The use of routinely collected blood samples in pregnancy for analysis of cotinine levels should be considered in pragmatic evaluations of incentives.
-
The cost-effectiveness of incentive interventions for smoking cessation in pregnancy or for breastfeeding is unclear and only short-term outcomes are reported.
-
The DCE found that incentives of > £20.00 per month are required for smoking cessation, with higher values up to £80.00 increasing the likelihood of quitting but at a decreasing rate. Initial daily text/telephone support and a quitting pal increase the likelihood of smoking cessation; however, the relative effect of incentives seemed to be greater than that of other attributes. Those who consider stopping smoking to be very difficult perceive that higher-value incentives, daily initial telephone or text support and a quitting pal will increase their likelihood of quitting.
-
In the Ipsos MORI survey of the general public and the survey of health professionals, the majority agreed with the provision of incentive up to a value of £40.00 per month.
-
More of the general public and health professional survey respondents agreed with providing a free breast pump to women with a value of £40.00. However, the systematic review indicated that there is uncertainty about the effectiveness of breast pumps to increase breastfeeding duration or exclusivity, because of study heterogeneity, contamination and comparison with formula incentives. The cost of a breast pump is considered prohibitive by more disadvantaged and younger women. Breast pumps address multiple barriers experienced by women, from intrinsic embarrassment to extrinsic relationships with partners, social networks and environments, such as feeding in public places or returning to work. Women receive conflicting advice about using breast pumps. The current availability of breast pumps through UK health services, the provision of education and support in how to use them and best practice are unclear. The commercialisation and commodification of breast pumps is a concern.
-
There was some evidence that combinations of gifts, vouchers and a breast pump with additional support are effective at increasing breastfeeding duration up to 6 weeks, but caution is required because of the quality of the evidence base. The public and health professional survey evidence showed that providing shopping vouchers to women for proven breastfeeding was the least popular incentive strategy. The qualitative data showed that women and health professionals thought that providing vouchers for breastfeeding would be unlikely to make a difference, whereas additional support would. Many women are prepared to try breastfeeding but they experience challenges with developing the skills required and integrating it into their daily lives.
-
There was some evidence to support provider commitment contracts, which require investment to meet quality standards and for which publicised achievement awards are given. In addition, financial penalties for self-set breastfeeding targets showed promise. Provider incentives for achieving breastfeeding targets was the only one of the seven incentive strategies that women did not significantly disagree with, which is consistent with the qualitative data on the need for more support.
-
There was no evidence on provider incentives to reach targets for proven smoking cessation in pregnancy. However, evidence from the primary care QOF contractual system demonstrates that incentives change general practice behaviour for the proxy outcomes of documenting smoking advice and referral. Public and professional views were divided, with midwives, health visitors and maternity care staff significantly more likely to agree with this strategy than doctors (mostly GPs).
-
More evidence is required on incentivising partners or a buddy as some studies reported significant effects of this strategy on smoking cessation in pregnancy and the DCE suggested that it would increase the likelihood of quitting smoking.
-
We did not identify any studies investigating incentives for a smoke-free home. Partners and household smokers were a barrier to quitting and contributed to relapse after birth. However, concerns were expressed about the impact of this strategy on relationships.
-
Multiple lifestyle behaviours with adverse health consequences were raised spontaneously in women’s narratives. There was evidence of an association between not smoking and breastfeeding; however, no intervention studies had evaluated effects on both behaviours together.
-
Unintended consequences of incentives include health inequalities, gaming and opportunity costs; however, there are other unintended positive health and emotional implications.
Mechanisms of action of incentives:
-
Our investigation of the mechanisms of action of incentives suggests that incentives alone are unlikely to result in sustained smoking cessation or breastfeeding, given the complexity of the interactions between the incentive and the real-life barriers to and facilitators of behaviour change and maintenance.
-
An incentive ladder logic model for the mechanisms of action of incentive intervention programmes is proposed. It incorporates a typology of incentives with their meanings and an IRBCT taxonomy, developed from a published general BCT taxonomy, which we used for data extraction in the systematic reviews. The interaction and fit between incentive intervention ladder ‘rungs’ and other life course/context ‘rungs’ is hypothesised to interact with programme engagement and effectiveness.
-
Data suggest that incentives and other intervention components (rungs) in programmes would benefit from being individually tailored and delivered by specialist teams to enable women to bolster their individual capabilities.
-
Qualitative data analysis found that the autonomy, motivation and control provided by non-restricted shopping vouchers, which maximise maternal well-being in addition to having a financial value, are important for smokers, particularly those with few choice opportunities. This counters arguments that incentives reduce autonomy through coercion or bribery. Gift deliveries, raffles, experience incentives and breast pumps (to share feeding and allow the woman to spend time away from her baby), which operate as connectors to other sources of support, are valued for breastfeeders.
-
Evidence from a wider range of lifestyle behaviour studies suggested that incentives can increase engagement in research. Qualitative data identified some concerns around feeling pressurised or blamed. However, for others, incentive schemes represented an opportunity for latent intrinsic motivation to be transformed into action. Participants who engaged in incentive schemes for both smoking cessation and breastfeeding were positive about the experience.
-
The reach of incentive interventions is a concern as recruitment rates are seldom reported and attrition rates vary. This is particularly the case for smoking cessation incentive studies, in which participant numbers are small. Qualitative data support and build on existing typologies of pregnant women smokers. Findings suggest that incentives work best for ‘enthusiastic amateurs’ (those who are willing to try but who have not yet attempted behaviour change) who have more stable lifestyles. Those with more chaotic, stressful lives tend to cut down or quit–relapse as smoking is central to their lives. Some ‘non-contemplators’ increase consumption and some ‘do-it-aloners’ do not engage. Flexible services with multiple community locations for delivery are valued.
-
To address the sole onus being placed on women, incentives beyond the individual were suggested, in which partners, family, social networks, providers and a community focus on facilitating behaviour change through collective effort. However, it was found to be important to ensure that such an approach would not introduce new pressures for women and their relationships with family, friends and health professionals.
-
Visual cues as motivators were evident in the data, particularly for women visualising positive change on CO monitors. The possibility that a breast pump might act as a visual cue requires further exploration.
Recommendations for research
-
A definitive trial of unrestricted shopping voucher incentives for smoking cessation in pregnancy is indicated, in which the patient journey, in terms of the intensity of contacts and other BCT components provided, is identical between the intervention arm and the control arm to test the effect of the incentive component.
-
The development of a biochemical test to verify breastfeeding in RCTs of incentive interventions is recommended, as self-report is perceived to facilitate gaming.
-
There is potential confounding from the intensity of contacts in incentive intervention trial designs and the nature of additional support provided. However, because of the quality of reporting, this could not be investigated. Further assessment of this is required.
-
The quality of reporting of the BCT components included in incentive intervention and control arms and their delivery processes is currently poor and this has implications for interpreting the evidence base and for study replication.
-
A novel approach to mapping the patient journey in the systematic review shows promise for understanding possible interactions and moderating effects between incentive and BCT intervention components, their timing, their intensity and their mode of delivery. The patient journey map could contribute to systematic review methods for other complex interventions and to trial reporting. It has particular value as a tool when few process evaluations or qualitative research studies are identified, as was the case in this project. Further research into its potential role is indicated.
-
Where there is uncertain evidence of effectiveness or optimal delivery of an intervention, patient journeys through interventions can be further explored through creating intervention vignettes. Guided by service-user contributions, a diverse selection of promising studies written as vignettes in lay language can be used in qualitative research to inform intervention design before or during feasibility studies.
-
An incentive ladder logic model shows promise for engaging socially and materially disadvantaged women with experience of smoking and formula feeding in the codesign and tailoring of behaviour change interventions to fit with everyday lives. This is important in transforming the approach from a deficit model, in which providers control what incentives are provided based on their perceptions of need, to a capabilities- and assets-based approach. Women can contribute to the codesign of more realistic interventions that fit their own circumstances and real-life contexts, informing the ‘rungs’ that they think or believe will motivate and sustain their own behaviour change while taking into account their fit within their social networks and everyday lives. Further evaluation of the validity of the ladder logic model with a diverse sample of the target population who have not been involved with this research is indicated. In particular, this should address the hypothesis that ‘ladders’ might exacerbate stigma. If validity is confirmed, research to test the practical utility of the ladder logic model for complex intervention design is recommended to inform how individual tailoring can be incorporated.
-
Incorporating non-professionalised service-user perspectives from a dynamic range of the target population through community groups, where engagement is on their terms, rather than those of the experts, is recommended, particularly for research into socially patterned lifestyle behaviours. This approach can help to achieve research that is carried out ‘with’ or ‘by’ members of the public rather than ‘to’, ‘about’ or ‘for’ them. This challenges conventional power relationships.
-
Applying a newly developed IRBCT taxonomy to studies included in the systematic review suggests that distinguishing between an incentive and a reward may be important. In the latter case, the motivation for action may be less, and distinguishing between content, contingency target and actor is also imperative. However, reporting of this is often unclear. Further fieldwork into its use in practice is recommended.
-
Further evidence of the effectiveness of, and the most feasible and acceptable delivery processes for, addressing more than one behaviour simultaneously in pregnancy and after birth is required.
-
Further evidence of cost-effectiveness with economic modelling for longer-term outcomes is required. In addition, the full costs of incentive interventions should be captured wherever they might arise, for health care, social services and families.
Acknowledgements
We would like to thank our co-applicants for their collaboration (detailed in Chapter 2): Mastrick Café Crèche, Aberdeen, and Wendy Ratcliffe, who facilitated access, and St Cuthbert’s and Palatine Children’s Centre, Blackpool, and Helen Cook. We would also like to thank all of the women, families and staff from health service, local government, voluntary sector and other organisations who generously provided their time for interviews and completed the survey; study authors and professional organisations that we contacted who provided additional details of their studies; Fiona Stewart and Cynthia Fraser for providing guidance with literature searching and reference management; Lara Kemp for providing secretarial support; Jennifer McKell and Susan MacAskill who collected the qualitative data for the CPIT and Lesley Sinclair, the CPIT manager, who facilitated access; Kate Sewell from Ipsos MORI Scotland who contributed to the survey design, oversaw data collection and commented on Chapter 7; Shalmini Jayakody, Sharon McCann and Virginia Schmied who assisted with quality assessment of the included studies; Linju Joseph who assisted with full-text screening to select review papers for Chapter 2; Amanda Cardy, SPCRN North East Co-ordinator, acknowledging the financial support of NHS Research Scotland (NRS) through the SPCRN; NHS R&D managers in Scotland and North West England and Binley’s (see www.binleys.com/) who assisted with distribution of the health professional survey; Gladys McPherson who independently conducted the survey prize draw; Diane Skåtun who advised on collection of the survey data; Alison Avenell who commented on Chapter 3; and Gordon Stables, Medical Illustration, University of Aberdeen, who created the graphic for the incentive ladder logic model in Chapter 6.
This report was commissioned by the NIHR Health Technology Assessment programme as project number 10/31/02. The Nursing, Midwifery and Allied Health Professions Research Unit, University of Stirling, and the Health Services Research Unit and Health Economics Research Unit, Institute of Applied Health Sciences, University of Aberdeen, are all core funded by the Chief Scientist Office of the Scottish Government Health and Social Care Directorates. The views and opinions expressed are those of the authors and do not necessarily reflect those of the funders.
Ethics committee approvals
Full ethical approval for this study, including service-user involvement, was obtained from the North of Scotland Research Ethics Committee (NOSRES; reference no. 12/NS/0041, 12 April 2012) and subsequent permissions were granted locally by NHS Grampian R&D (24 April 2012) and the BUSH (Built & Natural Environment, Sport and Health) Ethics Committee, University of Central Lancashire (BUSH064, 8 May 2012). Four amendments were submitted to NOSRES: AM01 to cover the amendments required by BUSH (approved 10 May 2012); AM02 to allow us to use a flyer for recruiting health professionals at conferences and an information leaflet designed for partners/family/friends (approved 6 December 2012); AM03 to gain ethical approval for the contents of the general public and health professionals survey (approved 17 April 2013); and AM04 for the final version of the discrete choice experiment (approved 10 May 2013).
Contributions of authors
All authors contributed to the work involved in this study and to writing this monograph. The substantial contributions are detailed below.
Heather Morgan (Research Fellow, Social Science) co-ordinated the ethics and R&D approvals for the project, co-ordinated contributions from the co-applicant Aberdeen mother-and-baby group and wrote the first draft for Chapter 2. She reviewed the qualitative and intervention delivery process evidence and wrote the first draft of these sections of Chapter 3; reviewed the behaviour change techniques evidence for Chapter 3 in conjunction with Stephan Dombrowski; reviewed the qualitative evidence and wrote the first draft for Chapter 4; assisted with abstract screening for Chapter 5; collected and analysed the primary qualitative data and wrote the first draft of the methods, the incentive ladder logic model and the life course and context findings for Chapter 6; contributed to the survey design and piloting, jointly co-ordinated the distribution of the survey with Gill Thomson, contributed to analysis decisions and wrote the first draft of the results for Chapter 7; and contributed to the design of the DCE for Chapter 8. She had significant involvement in co-ordinating the writing of this monograph and wrote the first draft of the scientific summary.
Pat Hoddinott (Chair in Primary Care, General Practice) was Chief Investigator and led the design of the study, oversaw and co-ordinated all aspects of the study and contributed to the writing of all chapters. She contributed to the evidence reviews for Chapters 3 and 4, reviewed the evidence and wrote the first draft of Chapter 5, contributed to the interpretation of the qualitative data for Chapter 6, wrote the first draft of the background, methods, survey questions and discussion for Chapter 7 and contributed to design decisions for the DCE in Chapter 8. She also wrote the first drafts for Chapters 1, 9 and 10, the abstract and the plain English summary.
Gill Thomson (Senior Research Fellow, Social Science) co-ordinated the ethics and R&D approvals in North West England; co-ordinated contributions from the co-applicant Blackpool children’s centre and assisted in writing Chapter 2; assisted in reviewing the evidence for Chapters 3–5 and the quality assessment of qualitative studies; collected and analysed the primary qualitative data and wrote the first draft of the other components and incentive delivery sections for Chapter 6; contributed to the survey design and piloting and jointly co-ordinated the distribution of the survey with Heather Morgan; and assisted in piloting the DCE.
Nicola Crossland (Research Assistant, Maternal and Infant Health) assisted in reviewing the evidence for Chapters 3–5, collected and analysed the primary qualitative data and wrote the first draft of the incentive typology section and incentives to providers/delivery sections for Chapter 6 and contributed to the survey design for Chapter 7.
Shelley Farrar (Research Fellow, Health Economics) provided health economic expertise for the clinical effectiveness reviews in Chapter 3, assisted in reviewing the evidence on incentives to providers in Chapter 5, contributed to the survey design in Chapter 7 and led the design of the DCE and contributed to the first draft of the methods for the DCE in Chapter 8.
Deokhee Yi (Research Fellow, Health Economics) analysed the incentive value and targeting questions in the survey (see Chapter 7), analysed the data for the DCE and wrote the first draft of the results for Chapter 8.
Jenni Hislop (Research Associate Systematic Reviewer) reviewed the evidence for clinical effectiveness and wrote the first draft of the smoking cessation section in Chapter 3.
Victoria Hall Moran (Reader, Maternal and Child Nutrition) reviewed the evidence on clinical effectiveness and, in conjunction with Heather Morgan, wrote the first draft of the breastfeeding review section in Chapter 3.
Graeme MacLennan (Senior Research Fellow, Statistics) provided statistical advice for the systematic review (see Chapter 3), led the statistical analysis of the survey data and contributed to writing the results section for Chapter 7.
Stephan U Dombrowski (Senior Lecturer in Psychology) reviewed the evidence for behaviour change techniques in conjunction with Heather Morgan and wrote the first draft of these sections for Chapter 3. He contributed behaviour change theory for the interpretation of data for Chapters 4 and 6 and the corresponding logic models.
Kieran Rothnie (Research Assistant, Systematic Reviewer) contributed to abstract and full-text screening, data extraction, the review of the evidence for clinical effectiveness and the drafting of tables for Chapter 3 for both smoking cessation and breastfeeding.
Fiona Stewart (Information Specialist) developed and ran the search strategies for all systematic reviews and was responsible for obtaining full-text papers, providing the flow charts of the searches and reference management.
Linda Bauld (Professor, Health Policy) contributed smoking cessation in pregnancy research expertise and helped to draft the smoking cessation background section for Chapter 1 and the discussion section for Chapter 3, assisted in reviewing the evidence for Chapter 5 and contributed to the survey design for Chapter 7. As coprincipal investigator with David Tappin for the CPIT, she provided CPIT information, oversaw CPIT qualitative data collection and arranged the transfer of qualitative transcripts for incorporation into the data analysis for Chapter 6.
Anne Ludbrook (Professor, Health Economics) contributed health economics expertise to all aspects of the study, contributed to survey data analysis for Chapter 7 and contributed to the drafting of the DCE results and discussion sections in Chapter 8.
Fiona Dykes (Professor, Maternal and Infant Health) contributed breastfeeding expertise to the study design and conduct. She assisted in drafting background sections for the breastfeeding review in Chapter 3 and reviewing the evidence for Chapters 4 and 5 and oversaw the researchers working on primary qualitative data collection and analysis in North West England.
Falko F Sniehotta (Professor, Behavioural Medicine and Health Psychology) contributed psychology expertise in behaviour change for the study design and conduct and for Chapters 1, 3 and 4.
David Tappin (Professor, Paediatrics and Trials in Children) contributed smoking cessation in pregnancy expertise for the study design and conduct. He helped to draft the smoking cessation background sections in Chapter 1 and the discussion section in Chapter 3 and to screen the abstracts and full texts in Chapter 3 and assisted in reviewing the evidence for Chapter 5. As coprincipal investigator with Linda Bauld for the CPIT, he arranged for ethics committee approval for CPIT qualitative transcripts to be included in Chapter 6, provided CPIT information, oversaw CPIT qualitative data collection and facilitated their incorporation into the data analysis for Chapter 6.
Marion Campbell (Professor and Director of the Health Services Research Unit, Statistics and Health Services Research) provided methods advice for the overall study design, the systematic reviews (see Chapters 3–5), the survey (see Chapter 7), the DCE (see Chapter 8) and aspects of the study relating to trial design.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Simpson J, Weiner E. Oxford English Dictionary. Oxford: Clarendon Press; 1989.
- Kane RL, Johnson PE, Town RJ, Butler M. Economic Incentives for Preventive Care: Summary. Rockville, MD: Agency for Healthcare Research and Quality; 2004.
- Jochelson K. Paying the Patient: Improved Health using Financial Incentives. London: The King’s Fund; 2007.
- Marteau TM, Ashcroft RE, Oliver A. Using financial incentives to achieve healthy behaviour. BMJ 2009;338. http://dx.doi.org/10.1136/bmj.b1415.
- Johnston M, Dixon D. What happened to behaviour in the decade of behaviour?. Psychol Health 2008;23:509-13. http://dx.doi.org/10.1080/08870440701816728.
- Wise RA. Drive, incentive, and reinforcement: the antecedents and consequences of motivation. Nebr Symp Motiv 2004;50:159-95.
- Boyce T, Robertson R, Dixon A. Commissioning and Behaviour Change: Kicking Bad Habits Final Report. London: The King’s Fund; 2008.
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci 2004;5:483-94. http://dx.doi.org/10.1038/nrn1406.
- Skinner BF. Science and Human Behaviour. New York, NY: The Free Press; 1953.
- Michie S, van Stralen MM, West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement Sci 2011;6. http://dx.doi.org/10.1186/1748-5908-6-42.
- Higgins ST, Alessi SM, Dantona RL. Voucher-based incentives: a substance abuse treatment innovation. Addict Behav 2002;27:887-910. http://dx.doi.org/10.1016/S0306-4603(02)00297-6.
- Bandura A. Social Learning Theory. London: Prentice-Hall; 1977.
- Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Process 1991;50:179-211. http://dx.doi.org/10.1016/0749-5978(91)90020-T.
- Strack F, Deutsch R. Reflective and impulsive determinants of social behavior. Pers Soc Psychol Rev 2004;8:220-47. http://dx.doi.org/10.1207/s15327957pspr0803_1.
- Kahneman D. Thinking Fast and Slow. London: Allen Lane; 2011.
- Dixon D, Johnston M. Health Behaviour Change Competency Framework: Competences to Deliver Interventions to Change Lifestyle Behaviours that Affect Health. Edinburgh: NHS Health Scotland; 2010.
- Michie S, Richardson M, Johnston M, Abraham C, Francis J, Hardeman W, et al. The behavior change technique taxonomy (v1) of 93 hierarchically clustered techniques: building an international consensus for the reporting of behavior change interventions. Ann Behav Med 2013;46:81-95. http://dx.doi.org/10.1007/s12160-013-9486-6.
- Maibach EW, Abroms LC, Marosits M. Communication and marketing as tools to cultivate the public’s health: a proposed ‘people and places’ framework. BMC Public Health 2007;7. http://dx.doi.org/10.1186/1471-2458-7-88.
- McLeroy KR, Bibeau D, Steckler A, Glanz K. An ecological perspective on health promotion programs. Health Educ Q 1988;15:351-77. http://dx.doi.org/10.1177/109019818801500401.
- Allen C, Radley A, Williams B. Paying the price for an incentive: an exploratory study of smokers’ reasons for failing to complete an incentive based smoking cessation scheme. J Health Serv Res Policy 2012;17:212-18. http://dx.doi.org/10.1258/jhsrp.2012.011084.
- Johnston M, Sniehotta F. Financial incentives to change patient behaviour. J Health Serv Res Policy 2010;15:131-2. http://dx.doi.org/10.1258/jhsrp.2010.010048.
- Tversky A, Kahneman D. Loss aversion in riskless choice: a reference-dependent model. Q J Econ 1991;106:1039-61. http://dx.doi.org/10.2307/2937956.
- Deci EL, Ryan RM. Intrinsic Motivation and Self-Determination in Human Behavior. New York: Plenum; 1985.
- Deci EL, Koestner R, Ryan RM. A meta-analytic review of experiments examining the effects of extrinsic rewards on intrinsic motivation. Psychol Bull 1999;125:627-68. http://dx.doi.org/10.1037/0033-2909.125.6.627.
- Yoon JH, Higgins ST, Heil SH, Sugarbaker RJ, Thomas CS, Badger GJ. Delay discounting predicts postpartum relapse to cigarette smoking among pregnant women. Exp Clin Psychopharmacol 2007;15:176-86. http://dx.doi.org/10.1037/1064-1297.15.2.186.
- Ross L, Nesbitt RE. The Person and the Situation: Perspectives of Social Psychology. London: Pinter & Martin; 2011.
- Thaler RH, Sunstein CR. Nudge: Improving Decisions about Health, Wealth, and Happiness. New Haven, CT: Yale University Press; 2008.
- Preventing Tobacco Use among Youth and Young Adults: a Report of the Surgeon General. Rockville, MD: US Department of Health and Human Services; 2012.
- Sutherland K, Christianson JB, Leatherman S. Impact of targeted financial incentives on personal health behavior: a review of the literature. Med Care Res Rev 2008;65:36-78S. http://dx.doi.org/10.1177/1077558708324235.
- A Framework for Development and Evaluation of RCTs for Complex Interventions to Improve Health. London: MRC; 2000.
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. Developing and Evaluating Complex Interventions: New Guidance. London: MRC; 2008.
- Hardeman W, Sutton S, Griffin S, Johnston M, White A, Wareham NJ, et al. A causal modelling approach to the development of theory-based behaviour change programmes for trial evaluation. Health Educ Res 2005;20:676-87. http://dx.doi.org/10.1093/her/cyh022.
- Armstrong R, Waters E, Moore L, Riggs E, Cuervo LG, Lumbiganon P, et al. Improving the reporting of public health intervention research: advancing TREND and CONSORT. J Public Health (Oxf) 2008;30:103-9. http://dx.doi.org/10.1093/pubmed/fdm082.
- Growing up in the UK – Ensuring a Healthy Future for our Children. London: British Medical Association; 2013.
- Marmot M, Allen J, Goldblatt P, Boyce T, McNeish D, Grady M, et al. Fair Society Healthy Lives: the Marmot Review. London: UCL Institute of Health Equity; 2010.
- Lagarde M, Haines A, Palmer N. The impact of conditional cash transfers on health outcomes and use of health services in low and middle income countries. Cochrane Database Syst Rev 2009;4.
- Forde I, Bell R, Marmot MG. Using conditionality as a solution to the problem of low uptake of essential services among disadvantaged communities: a social determinants view. Am J Public Health 2011;101:1365-9. http://dx.doi.org/10.2105/AJPH.2011.300140.
- Tan M, Yamey G. Paying the poor. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e4929.
- Middleton S, Perren K, Maguire S, Rennison J, Battistin E, Emmerson C, et al. Evaluation of Education Maintenance Allowance Pilots: Young People Aged 16 to 19 Years. Final report of the Quantitative Evaluation. London: Department for Education and Skills; 2005.
- Johnston V, Liberato S, Thomas D. Incentives for preventing smoking in children and adolescents. Cochrane Database Syst Rev 2012;12.
- Quitting Smoking in Pregnancy and Following Childbirth. London: NICE; 2010.
- Passive Smoking and Children: a Report by the Tobacco Advisory Group of the Royal College of Physicians. London: Royal College of Phyicians; 2010.
- Batstra L, Hadders-Algra M, Neeleman J. Effect of antenatal exposure to maternal smoking on behavioural problems and academic achievement in childhood: prospective evidence from a Dutch birth cohort. Early Hum Dev 2003;75:21-33. http://dx.doi.org/10.1016/j.earlhumdev.2003.09.001.
- Castles A, Adams EK, Melvin CL, Kelsch C, Boulton ML. Effects of smoking during pregnancy. Five meta-analyses. Am J Prev Med 1999;16:208-15. http://dx.doi.org/10.1016/S0749-3797(98)00089-0.
- Dietz PM, England LJ, Shapiro-Mendoza CK, Tong VT, Farr SL, Callaghan WM. Infant morbidity and mortality attributable to prenatal smoking in the US. Am J Prev Med 2010;39:45-52. http://dx.doi.org/10.1016/j.amepre.2010.03.009.
- Jaakkola JJ, Gissler M. Maternal smoking in pregnancy, fetal development, and childhood asthma. Am J Public Health 2004;94:136-40. http://dx.doi.org/10.2105/AJPH.94.1.136.
- Jaddoe VW, Troe EJ, Hofman A, Mackenbach JP, Moll HA, Steegers EA, et al. Active and passive maternal smoking during pregnancy and the risks of low birthweight and preterm birth: the Generation R study. Paediatr Perinat Epidemiol 2008;22:162-71. http://dx.doi.org/10.1111/j.1365-3016.2007.00916.x.
- Montgomery SM, Ekbom A. Smoking during pregnancy and diabetes mellitus in a British longitudinal birth cohort. BMJ 2002;324:26-7. http://dx.doi.org/10.1136/bmj.324.7328.26.
- Rogers JM. Tobacco and pregnancy: overview of exposures and effects. Birth Defect Res 2008;84:1-15. http://dx.doi.org/10.1002/bdrc.20119.
- Salihu HM, Wilson RE. Epidemiology of prenatal smoking and perinatal outcomes. Early Hum Dev 2007;83:713-20. http://dx.doi.org/10.1016/j.earlhumdev.2007.08.002.
- Godfrey C, Pickett KE, Parrot S, Mdege M, Eapen D. Estimating the Costs to the NHS of Smoking in Pregnancy for Pregnant Women and Infants: Project Final Report. University of York: Public Health Research Consortium; 2010.
- McCowan LM, Dekker GA, Chan E, Stewart A, Chappell LC, Hunter M, et al. Spontaneous preterm birth and small for gestational age infants in women who stop smoking early in pregnancy: prospective cohort study. BMJ 2009;338. http://dx.doi.org/10.1136/bmj.b1081.
- McAndrew F, Thompson J, Fellows L, Large A, Speed M, Renfrew MJ. Infant Feeding Survey 2010: Summary. Leeds: Health and Social Care Information Centre; 2012.
- Lumley J, Chamberlain C, Dowswell T, Oliver S, Oakley L, Watson L. Interventions for promoting smoking cessation during pregnancy. Cochrane Database Syst Rev 2009;3.
- Coleman T, Cooper S, Thornton JG, Grainge MJ, Watts K, Britton J, et al. A randomized trial of nicotine-replacement therapy patches in pregnancy. N Engl J Med 2012;366:808-18. http://dx.doi.org/10.1056/NEJMoa1109582.
- Bauld L, Coleman T. The Effectiveness of Smoking Cessation Interventions during Pregnancy: a Briefing Paper. London: NICE; 2009.
- Naughton F, Prevost AT, Sutton S. Self-help smoking cessation interventions in pregnancy: a systematic review and meta-analysis. Addiction 2008;103:566-79. http://dx.doi.org/10.1111/j.1360-0443.2008.02140.x.
- Bauld L, Bell K, McCullough L, Richardson L, Greaves L. The effectiveness of NHS smoking cessation services: a systematic review. J Public Health (Oxf) 2010;32:71-82. http://dx.doi.org/10.1093/pubmed/fdp074.
- MacAskill S, Bauld L, Tappin D, Eadie D. Smoking Cessation Support in Pregnancy in Scotland. Edinburgh: NHS Health Scotland; 2008.
- Global Strategy for Infant and Young Child Feeding. Geneva: WHO; 2003.
- Bhavnani V, Newburn M. Left to Your Own Devices: the Postnatal Experiences of 1260 First-Time Mothers. London: National Childbirth Trust; 2010.
- Hoddinott P, Craig L, Britten J, McInnes R. A Prospective Study Exploring the Early Infant Feeding Experiences of Parents and their Significant Others during the First 6 Months of Life: What Would Make a Difference?. Edinburgh: NHS Health Scotland; 2010.
- Eidelman AI, Schanler RJ. Breastfeeding and the use of human milk. Pediatrics 2012;129:e827-41. http://dx.doi.org/10.1542/peds.2011-3552.
- Renfrew MJ, Pokhrel S, Quigley M, McCormack F, Fox-Rushby J, Dodds R, et al. Preventing Disease and Saving Resources: the Potential Contribution of Increasing Breastfeeding Rates in the UK. London: UNICEF UK; 2012.
- Renfrew MJ, McCormick FM, Wade A, Quinn B, Dowswell T. Support for healthy breastfeeding mothers with healthy term babies. Cochrane Database Syst Rev 2012;5.
- Hoddinott P, Seyara R, Marais D. Global evidence synthesis and UK idiosyncrasy: why have recent UK trials had no significant effects on breastfeeding rates?. Matern Child Nutr 2011;7:221-7. http://dx.doi.org/10.1111/j.1740-8709.2011.00336.x.
- Schmied V, Beake S, Sheehan A, McCourt C, Dykes F. Women’s perceptions and experiences of breastfeeding support: a metasynthesis. Birth 2011;38:49-60. http://dx.doi.org/10.1111/j.1523-536X.2010.00446.x.
- Higgins TM, Higgins ST, Heil SH, Badger GJ, Skelly JM, Bernstein IM, et al. Effects of cigarette smoking cessation on breastfeeding duration. Nicotine Tobacco Res 2010;12:483-8. http://dx.doi.org/10.1093/ntr/ntq031.
- Kendzor DE, Businelle MS, Costello TJ, Castro Y, Reitzel LR, Vidrine JI, et al. Breast feeding is associated with postpartum smoking abstinence among women who quit smoking due to pregnancy. Nicotine Tob Res 2010;12:983-8. http://dx.doi.org/10.1093/ntr/ntq132.
- Lauria L, Lamberti A, Grandolfo M. Smoking behaviour before, during, and after pregnancy: the effect of breastfeeding [published online ahead of print 12 March 2012]. Sci World J 2012. http://dx.doi.org/10.1100/2012/154910.
- Bolling K, Grant C, Hamlyn B, Thornton A. Infant Feeding Survey 2005. London: NHS Information Centre; 2007.
- Asthana S, Halliday J. Developing an evidence base for policies and interventions to address health inequalities: the analysis of ‘public health regimes’. Milbank Q 2006;84:577-603. http://dx.doi.org/10.1111/j.1468-0009.2006.00459.x.
- White Paper: Healthy Lives, Healthy People: Our Strategy for Public Health in England. London: Department of Health; 2010.
- Building the Big Society. London: Cabinet Office; 2010.
- Tayside Smokeline . Free Yourself – Smoking, It’s Not Worth It! (Tayside Quit4U Scheme) 2013. www.canstopsmoking.com/local-help/free-yourself-smoking,-it’s-not-worth-it!-(tayside-quit4u-scheme) (accessed October 2013).
- Ormston R, McConville S, van der Pol M, Ludbrook A, Amos A. Evaluation of Quit4U: Main Report. Edinburgh: NHS Health Scotland; 2012.
- Heyman J, Ariely D. Effort for payment. A tale of two markets. Psychol Sci 2004;15:787-93. http://dx.doi.org/10.1111/j.0956-7976.2004.00757.x.
- Winston Churchill, Prime Minister of Great Britain . Post-War Councils on World Problems. A Four Year Plan for England n.d. www.ibiblio.org/pha/policy/1943/1943-03-21a.html (accessed 16 December 2014).
- Delivering a Healthy Start for Pregnant Women, New Mums, Babies and Young Children: a Guide for Health Professionals. London: Department of Health; 2010.
- International Code of Marketing of Breast Milk Substitutes. Geneva: WHO; 1981.
- Breastfeeding Statistics Financial Year 2011/12. Edinburgh: Information Statistics Division, NHS National Services Scotland; 2012.
- Data Tables: Births in Scottish Hospitals – Year Ending 31st March 2012. Edinburgh: Information Statistics Division, NHS National Services Scotland; 2013.
- Breastfeeding by NHS Board of Review: Financial Years 2001/02–2011/12. Edinburgh: Information Statistics Division, NHS National Services Scotland; 2012.
- Lancashire County Council . Indices of Deprivation 2007 District Analysis n.d. www.lancashire.gov.uk/corporate/web/?siteid=6121&pageid=40301&e=e (accessed 5 January 2015).
- Breastfeeding Profiles. London: Public Health England; 2013.
- Local Tobacco Control Profiles for England. London: Public Health England; 2013.
- Cresswell JW, Klassen AC, Plano Clark VL, Smith KC. Best Practices for Mixed Methods Research in the Health Sciences. Bethesda, MD: National Institutes of Health; 2011.
- Tappin DM, Bauld L, Tannahill C, de Caestecker, Radley A, McConnachie A, et al. The cessation in pregnancy incentives trial (CPIT): study protocol for a randomized controlled trial. Trials 2012;13. http://dx.doi.org/10.1186/1745-6215-13-113.
- Cooper S, Lewis S, Thornton JG, Marlow N, Watts K, Britton J, et al. The SNAP trial: a randomised placebo-controlled trial of nicotine replacement therapy in pregnancy – clinical effectiveness and safety until 2 years after delivery, with economic evaluation. Health Technol Assess 2014;18. http://dx.doi.org/10.3310/hta18540.
- Health and Social Care Act 2001. London: The Stationery Office; 2001.
- INVOLVE. Eastleigh: NIHR; 2013.
- Oliver SR, Rees RW, Clarke-Jones L, Milne R, Oakley AR, Gabbay J, et al. A multidimensional conceptual framework for analysing public involvement in health services research. Health Expect 2008;11:72-84. http://dx.doi.org/10.1111/j.1369-7625.2007.00476.x.
- Cook T. Where participatory approaches meet pragmatism in funded (health) research: the challenge of finding meaningful spaces. Forum Qual Soc Res 2012;13.
- Boote J, Baird W, Sutton A. Public involvement in the systematic review process in health and social care: a narrative review of case examples. Health Policy 2011;102:105-16. http://dx.doi.org/10.1016/j.healthpol.2011.05.002.
- Braye S, Preston-Shoot M. Emerging from out of the shadows? Service user and carer involvement in systematic reviews. Evid Policy 2005;1:173-94. http://dx.doi.org/10.1332/1744264053730743.
- Smith E, Donovan S, Beresford P, Manthorpe J, Brearley S, Sitzia J, et al. Getting ready for user involvement in a systematic review. Health Expect 2009;12:197-208.
- Cashman SB, Adeky S, Allen AJ, Corburn J, Israel BA, Montano J, et al. The power and the promise: working with communities to analyze data, interpret findings, and get to outcomes. Am J Public Health 2008;98:1407-17. http://dx.doi.org/10.2105/AJPH.2007.113571.
- Reed J, Weiner R, Cook G. Partnership research with older people – moving towards making the rhetoric a reality. J Clin Nurs 2004;13:3-10. http://dx.doi.org/10.1111/j.1365-2702.2004.00920.x.
- Truman C, Raine P. Involving users in evaluation: the social relations of user participation in health research. Crit Public Health 2001;11:215-29. http://dx.doi.org/10.1080/09581590110066667.
- Hoddinott P, Lee AJ, Pill R. Effectiveness of a breastfeeding peer coaching intervention in rural Scotland. Birth 2006;33:27-36. http://dx.doi.org/10.1111/j.0730-7659.2006.00071.x.
- Spradley JP. Participant Observation. New York, NY: Holt, Rinehart and Winston; 1980.
- Janis IL. Groupthink. Psychol Today 1971;5:43-6.
- Chamberlain LB, McMahon M, Philipp BL, Merewood A. Breast pump access in the inner city: a hospital-based initiative to provide breast pumps for low-income women. J Hum Lact 2006;22:94-8. http://dx.doi.org/10.1177/0890334405284226.
- Gulliver SB, Colby SM, Hayes K, Raffa SD. Tobacco cessation treatment for pregnant smokers: incorporating partners and incentives. Med Health R I 2004;87:9-12.
- Heil SH, Higgins ST, Bernstein IM, Solomon LJ, Rogers RE, Thomas CS, et al. Effects of voucher-based incentives on abstinence from cigarette smoking and fetal growth among pregnant women. Addiction 2008;103:1009-18. http://dx.doi.org/10.1111/j.1360-0443.2008.02237.x.
- Pugh LC, Milligan RA. Nursing intervention to increase the duration of breastfeeding. Appl Nurs Res 1998;11:190-4. http://dx.doi.org/10.1016/S0897-1897(98)80318-2.
- Volpe EM, Bear M. Enhancing breastfeeding initiation in adolescent mothers through the Breastfeeding Educated and Supported Teen (BEST) Club. J Hum Lact 2000;16:196-200. http://dx.doi.org/10.1177/089033440001600303.
- Walsh RA, Redman S, Brinsmead MW, Byrne JM, Melmeth A. A smoking cessation program at a public antenatal clinic. Am J Public Health 1997;87:1201-4. http://dx.doi.org/10.2105/AJPH.87.7.1201.
- Mantzari E, Vogt F, Marteau TM. The effectiveness of financial incentives for smoking cessation during pregnancy: is it from being paid or from the extra aid?. BMC Pregnancy Childbirth 2012;12. http://dx.doi.org/10.1186/1471-2393-12-24.
- Nichter M, Nichter M, Muramoto M, Adrian S, Goldade K, Tesler L, et al. Smoking among low-income pregnant women: an ethnographic analysis. Health Educ Behav 2007;34:748-64. http://dx.doi.org/10.1177/1090198106290397.
- Ripley-Moffitt CE, Goldstein AO, Fang WL, Butzen AY, Walker S, Lohr JA. Safe babies: a qualitative analysis of the determinants of postpartum smoke-free and relapse states. Nicotine Tob Res 2008;10:1355-64. http://dx.doi.org/10.1080/14622200802238936.
- Thomson G, Dykes F, Hurley MA, Hoddinott P. Incentives as connectors: insights into a breastfeeding incentive intervention in a disadvantaged area of North-West England. BMC Pregnancy Childbirth 2012;12. http://dx.doi.org/10.1186/1471-2393-12-22.
- Radley A, Ballard P, Eadie D, MacAskill S, Donnelly L, Tappin D. Give it up for Baby: outcomes and factors influencing uptake of a pilot smoking cessation incentive scheme for pregnant women. BMC Public Health 2013;13. http://dx.doi.org/10.1186/1471-2458-13-343.
- Willis G. Cognitive Interviewing: A ‘How To’ Guide. Reducing Survey Error through Research on the Cognitive and Decision Processes in Surveys 1999. www.hkr.se/PageFiles/35002/GordonWillis.pdf (accessed October 2013).
- Hoddinott P, Hislop J, Morgan H, Stewart F, Farrar S, Rothnie K, et al. Incentive interventions for smoking cessation in pregnancy: a mixed methods evidence synthesis. Lancet 2013;380. http://dx.doi.org/10.1016/S0140-6736(13)60404-3.
- What Do We Mean by ‘Training’ and ‘Support’?. Eastleigh: NIHR; 2013.
- Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011] 2011. www.cochrane-handbook.org/ (accessed October 2013).
- Armstrong R, Waters E, Jackson N, Oliver S, Popay J, Shepherd J, et al. Guidelines for Systematic Reviews of Health Promotion and Public Health Interventions. Version 2. Australia: Melbourne University; 2007.
- Composition of Macro Geographical (Continental) Regions, Geographical Sub-Regions, and Selected Economic and other Groupings. New York: United Nations; 2013.
- GreyNet: Grey Literature Network Service. Amsterdam: GreyNet International; 2013.
- Higgins ST, Heil SH, Badger GJ, Mongeon JA, Solomon LJ, McHale L, et al. Biochemical verification of smoking status in pregnant and recently postpartum women. Exp Clin Psychopharmacol 2007;15:58-66. http://dx.doi.org/10.1037/1064-1297.15.1.58.
- Dempsey D, Jacob P, Benowitz NL. Accelerated metabolism of nicotine and cotinine in pregnant smokers. J Pharmacol Exp Ther 2002;301:594-8. http://dx.doi.org/10.1124/jpet.301.2.594.
- Sasaki S, Braimoh TS, Yila TA, Yoshioka E, Kishi R. Self-reported tobacco smoke exposure and plasma cotinine levels during pregnancy – a validation study in Northern Japan. Sci Total Environ 2011;412–13:114-18. http://dx.doi.org/10.1016/j.scitotenv.2011.10.019.
- Hegaard HK, Kjaergaard H, Moller LF, Wachmann H, Ottesen B. Determination of a saliva cotinine cut-off to distinguish pregnant smokers from pregnant non-smokers. Acta Obstet Gynecol Scand 2007;86:401-6. http://dx.doi.org/10.1080/00016340601147517.
- Specialist Assays: Nicotine and Cotinine Assays. Welwyn Garden City: Advanced Bioanalytical Service Laboratories Ltd; 2003.
- Marrone GF, Shakleya DM, Scheidweiler KB, Singleton EG, Huestis MA, Heishman SJ. Relative performance of common biochemical indicators in detecting cigarette smoking. Addiction 2011;106:1325-34. http://dx.doi.org/10.1111/j.1360-0443.2011.03441.x.
- Dixon-Woods M, Sutton A, Shaw R, Miller T, Smith J, Young B, et al. Appraising qualitative research for inclusion in systematic reviews: a quantitative and qualitative comparison of three methods. J Health Serv Res Policy 2007;12:42-7. http://dx.doi.org/10.1258/135581907779497486.
- Oliver S, Oakley L, Lumley J, Waters E. Smoking cessation programmes in pregnancy: systematically addressing development, implementation, women’s concerns and effectiveness. Health Educ J 2001;60:362-70. http://dx.doi.org/10.1177/001789690106000408.
- Michie S, Hyder N, Walia A, West R. Development of a taxonomy of behaviour change techniques used in individual behavioural support for smoking cessation. Addict Behav 2011;36:315-19. http://dx.doi.org/10.1016/j.addbeh.2010.11.016.
- Higgins JPT, Altman DG, Sterne JAC. Cochrane Statistical Methods Group, Cochrane Bias Methods Group . Chapter 8: Assessing Risk of Bias in Included Studies 2011. www.cochrane-handbook.org/ (accessed October 2013).
- Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York: University of York, Centre for Reviews and Dissemination; 2009.
- Verhagen AP, De Vet HCW, De Bie RA, Kessels AGH, Boers M, Bouter LM, et al. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J Clin Epidemiol 1998;51:1235-41. http://dx.doi.org/10.1016/S0895-4356(98)00131-0.
- Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377-84. http://dx.doi.org/10.1136/jech.52.6.377.
- Jackson R, Ameratunga S, Broad J, Connor J, Lethaby A, Robb G, et al. The GATE frame: critical appraisal with pictures. Evid Based Med 2006;11:35-8. http://dx.doi.org/10.1136/ebm.11.2.35.
- Critical Appraisal Skills Programme: Making Sense of Evidence about Clinical Effectiveness: 11 Questions to Help you Make Sense of a Trial. Oxford: Critical Appraisal Skills Programme; 2013.
- Pluye P, Robert E, Cargo M, Bartlett G, O’Cathain A, Griffiths F, et al. Proposal: a Mixed Methods Appraisal Tool for Systematic Mixed Studies Reviews. Montreal, QC: McGill University; 2011.
- Cohen J. A coefficient of agreement for nominal scales. Educ Psychol Meas 1960;20:37-46. http://dx.doi.org/10.1177/001316446002000104.
- Albrecht S, Payne L, Stone CA, Reynolds MD. A preliminary study of the use of peer support in smoking cessation programs for pregnant adolescents. J Am Acad Nurse Pract 1998;10:119-25. http://dx.doi.org/10.1111/j.1745-7599.1998.tb01205.x.
- Cinciripini PM, Blalock JA, Minnix JA, Robinson JD, Brown VL, Lam C, et al. Effects of an intensive depression-focused intervention for smoking cessation in pregnancy. J Consult Clin Psychol 2010;78:44-5. http://dx.doi.org/10.1037/a0018168.
- Cluss PA, Levine MD, Landsittel D. The Pittsburgh STOP program: disseminating an evidence-informed intervention for low-income pregnant smokers. Am J Health Promot 2011;25:S75-81. http://dx.doi.org/10.4278/ajhp.100616-QUAN-197.
- Donatelle RJ, Prows SL, Champeau D, Hudson D. Randomised controlled trial using social support and financial incentives for high risk pregnant smokers: significant other supporter (SOS) program. Tob Control 2000;9:III67-9. http://dx.doi.org/10.1136/tc.9.suppl_3.iii67.
- Edwards MJ, Geiser T, Chafin C, Weatherby NL, Smith CM. S.M.A.R.T. mothers are resisting tobacco: prenatal smoking cessation in WIC mothers. J Allied Health 2009;38:170-6.
- Gadomski A, Adams L, Tallman N, Krupa N, Jenkins P. Effectiveness of a combined prenatal and postpartum smoking cessation program. Matern Child Health J 2011;15:188-97. http://dx.doi.org/10.1007/s10995-010-0568-9.
- Higgins ST, Heil SH, Solomon LJ. A pilot study on voucher-based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine Tob Res 2004;6:1015-20. http://dx.doi.org/10.1080/14622200412331324910.
- Lillington L, Royce J, Novak D, Ruvalcaba M, Chlebowski R. Evaluation of a smoking cessation program for pregnant minority women. Cancer Pract 1995;3:157-63.
- Lowe JB, Windsor R, Balanda KP, Woodby L. Smoking relapse prevention methods for pregnant women: a formative evaluation. Am J Health Promot 1997;11:244-6. http://dx.doi.org/10.4278/0890-1171-11.4.244.
- McBride CM, Baucom DH, Peterson BL, Pollak KI, Palmer C, Westman E, et al. Prenatal and postpartum smoking abstinence – a partner-assisted approach. Am J Prev Med 2004;27:232-8.
- Morgan A, Bennett K, Hannon P, Weinberger J. Evaluation of a Smoke Stop service in a Sure Start programme. MIDIRS Midwifery Digest 2005;15:496-500.
- Pbert L, Ockene JK, Zapka J, Ma Y, Goins KV, Oncken C, et al. A community health center smoking-cessation intervention for pregnant and postpartum women. Am J Prev Med 2004;26:377-85. http://dx.doi.org/10.1016/j.amepre.2004.02.010.
- Ussher M, Aveyard P, Coleman T, Straus L, West R, Marcus B, et al. Physical activity as an aid to smoking cessation during pregnancy: two feasibility studies. BMC Public Health 2008;8. http://dx.doi.org/10.1186/1471-2458-8-328.
- Lynagh M, Bonevski B, Symonds I, Sanson-Fisher RW. Paying women to quit smoking during pregnancy? Acceptability among pregnant women. Nicotine Tob Res 2011;13:1029-36. http://dx.doi.org/10.1093/ntr/ntr108.
- Latts LM, Prochazka AV, Salas NM, Young DA. Smoking cessation in pregnancy: failure of an HMO pilot project to improve guideline implementation. Nicotine Tob Res 2002;4:25-30. http://dx.doi.org/10.1080/14622200210128054.
- Hartmann KE, Wechter ME, Payne P, Salisbury K, Jackson RD, Melvin CL. Best practice smoking cessation intervention and resource needs of prenatal care providers. Obstet Gynecol 2007;110:765-70. http://dx.doi.org/10.1097/01.AOG.0000280572.18234.96.
- Heil SH, Higgins ST, Mongeon JA, Badger GJ, Bernstein IM. Characterizing nicotine withdrawal in pregnant cigarette smokers. Exp Clin Psychopharmacol 2006;14:165-70. http://dx.doi.org/10.1037/1064-1297.14.2.165.
- Heil SH, Higgins ST, Solomon LJ, Lynch ME, McHale L, Dumeer A, et al. Voucher-Based Incentives for Abstinence from Cigarette Smoking in Pregnant and Postpartum Women n.d.
- Reynolds S. Vouchers boost smoking abstinence during pregnancy. Nida Notes 2010;23.
- Walsh RA, Redman S, Byrne JM, Melmeth A, Brinsmead MW. Process measures in an antenatal smoking cessation trial: another part of the picture. Health Educ Res 2000;15:469-83. http://dx.doi.org/10.1093/her/15.4.469.
- Donatelle RJ, Prows SL, Champeau D, Hudson D. Using Social Support, Biochemical Feedback, and Incentives to Motivate Smoking Cessation During Pregnancy: Comparison of Three Intervention Trials n.d.
- Donatelle RJ, Hudson D. Using 5 A’s and Incentives to Promote Prenatal Smoking Cessation n.d.
- Higgins ST, Heil SH, Dumeer AM, Thomas CS, Solomon LJ, Bernstein IM. Smoking status in the initial weeks of quitting as a predictor of smoking-cessation outcomes in pregnant women. Drug Alcohol Depend 2006;85:138-41. http://dx.doi.org/10.1016/j.drugalcdep.2006.04.005.
- Higgins ST, Bernstein IM, Washio Y, Heil SH, Badger GJ, Skelly JM, et al. Effects of smoking cessation with voucher-based contingency management on birth outcomes. Addiction 2010;105:2023-30. http://dx.doi.org/10.1111/j.1360-0443.2010.03073.x.
- Higgins ST, Heil SH, Badger GJ, Skelly JM, Solomon LJ, Bernstein IM. Educational disadvantage and cigarette smoking during pregnancy. Drug Alcohol Depend 2009;104:S100-5. http://dx.doi.org/10.1016/j.drugalcdep.2009.03.013.
- West R, Hajek P, Stead L, Stapleton J. Outcome criteria in smoking cessation trials: proposal for a common standard. Addiction 2005;100:299-303. http://dx.doi.org/10.1111/j.1360-0443.2004.00995.x.
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict 1991;86:1119-27. http://dx.doi.org/10.1111/j.1360-0443.1991.tb01879.x.
- Windsor RA, Lowe JB, Perkins LL, Smith-Yoder D, Artz L, Crawford M, et al. Health education for pregnant smokers: its behavioral impact and cost benefit. Am J Publ Health 1993;83:201-6. http://dx.doi.org/10.2105/AJPH.83.2.201.
- Velicer WF, Prochaska JO, Rossi JS, Snow MG. Assessing outcome in smoking cessation studies. Psychol Bull 1992;111:23-41. http://dx.doi.org/10.1037/0033-2909.111.1.23.
- Bliss MC, Wilkie J, Acredolo C, Berman S, Tebb KP. The effect of discharge pack formula and breast pumps on breastfeeding duration and choice of infant feeding method. Birth 1997;24:90-7. http://dx.doi.org/10.1111/j.1523-536X.1997.tb00347.x.
- Cattaneo A, Borgnolo G, Simon G. Breastfeeding by objectives. Eur J Publ Health 2001;11:397-401. http://dx.doi.org/10.1093/eurpub/11.4.397.
- Cohen R, Mrtek MB. The impact of two corporate lactation programs on the incidence and duration of breast-feeding by employed mothers. Am J Health Promot 1994;8:436-41. http://dx.doi.org/10.4278/0890-1171-8.6.436.
- Dungy CI, Christensen-Szalanski J, Losch M, Russell D. Effect of discharge samples on duration of breast-feeding. Pediatrics 1992;90:233-7.
- Finch C, Daniel EL. Breastfeeding education program with incentives increases exclusive breastfeeding among urban WIC participants. J Am Diet Assoc 2002;102:981-4. http://dx.doi.org/10.1016/S0002-8223(02)90224-5.
- Hayes DK, Prince CB, Espinueva V, Fuddy LJ, Li R, Grummer-Strawn LM. Comparison of manual and electric breast pumps among WIC women returning to work or school in Hawaii. Breastfeed Med 2008;3:3-10. http://dx.doi.org/10.1089/bfm.2007.0022.
- Hill PD. Effects of education on breastfeeding success. Matern Child Nurs J 1987;16:145-56.
- Rasmussen KM, Dieterich CM, Zelek ST, Altabet JD, Kjolhede CL. Interventions to increase the duration of breastfeeding in obese mothers: the Bassett Improving Breastfeeding Study. Breastfeed Med 2011;6:69-75. http://dx.doi.org/10.1089/bfm.2010.0014.
- Reeves Tuttle C, Dewey KG. Impact of a breast-feeding promotion program for Hmong women at selected WIC sites in northern California. J Nutr Educ 1995;27:69-74. http://dx.doi.org/10.1016/S0022-3182(12)80343-8.
- Sciacca JP, Phipps BL, Dube DA, Ratliff MI. Influences on breast-feeding by lower-income women – an incentive-based, partner-supported educational program. J Am Diet Assoc 1995;95:323-8. http://dx.doi.org/10.1016/S0002-8223(95)00083-6.
- Sciacca JP, Dube DA, Phipps BL, Ratliff MI. A breast-feeding education and promotion program – effects on knowledge, attitudes, and support for breast-feeding. J Community Health 1995;20:473-90. http://dx.doi.org/10.1007/BF02277064.
- Wolfberg AJ, Michels KB, Shields W, O’Campo P, Bronner Y, Bienstock J. Dads as breastfeeding advocates: results from a randomized controlled trial of an educational intervention. Am J Obstet Gynecol 2004;191:708-12. http://dx.doi.org/10.1016/j.ajog.2004.05.019.
- Zimmerman DR. You can make a difference: increasing breastfeeding rates in an inner-city clinic. J Hum Lact 1999;15:217-20. http://dx.doi.org/10.1177/089033449901500311.
- Bai Y, Wunderlich SM, Kashdan R. Inclusion of manual breast pump in hospital discharge bags promotes breastfeeding exclusivity. J Am Diet Assoc 2010;110. http://dx.doi.org/10.1016/j.jada.2010.06.423.
- Chiasson MA, Findley S, Sekhobo J, Scheinmann R, Edmunds LS, Faly A, et al. Changing WIC changes what children eat. Obesity 2011;19.
- Wright SS, Lea CS, Holloman R, Cornett A, Harrison LM, Randolph GD. Using quality improvement to promote breast-feeding in a local health department. J Public Health Manag Pract 2012;18:36-42. http://dx.doi.org/10.1097/PHH.0b013e31822d2e3a.
- The Baby Friendly Initiative. London: UNICEF UK; 2010.
- Beake S, Pellowe C, Dykes F, Schmied V, Bick D. A systematic review of structured compared with non-structured breastfeeding programmes to support the initiation and duration of exclusive and any breastfeeding in acute and primary health care settings. Matern Child Nutr 2012;8:141-61. http://dx.doi.org/10.1111/j.1740-8709.2011.00381.x.
- Rasmussen KM, Geraghty SR. The quiet revolution: breastfeeding transformed with the use of breast pumps. Am J Publ Health 2011;101:1356-9. http://dx.doi.org/10.2105/AJPH.2011.300136.
- Hector DJ. Complexities and subtleties in the measurement and reporting of breastfeeding practices. Int Breastfeed J 2011;6. http://dx.doi.org/10.1186/1746-4358-6-5.
- Ryan A, Martinez G. Breast-feeding and the working mother: a profile. Pediatrics 1989;83:524-31.
- Abrahams SW, Labbok MH. Exploring the impact of the Baby-Friendly Hospital Initiative on trends in exclusive breastfeeding. Int Breastfeed J 2009;4. http://dx.doi.org/10.1186/1746-4358-4-11.
- Biro MA, Sutherland GA, Yelland JS, Hardy P, Brown SJ. In-hospital formula supplementation of breastfed babies: a population-based survey. Birth 2011;38:302-10. http://dx.doi.org/10.1111/j.1523-536X.2011.00485.x.
- Broadfoot M, Britten J, Tappin DM, MacKenzie JM. The Baby Friendly Hospital Initiative and breast feeding rates in Scotland. Arch Dis Childhood Fetal Neonat Edn 2005;90:F114-16.
- Daniels L, Jackson D. Knowledge, attitudes and practices of nursing staff regarding the Baby-Friendly Hospital Initiative in non-accredited obstetric units in Cape Town. S Afr J Clin Nutr 2011;24:32-8.
- Feldman-Winter L, Schanler RJ, Eidelman A, Landers S, Noble L, Szucs K, et al. In reply. Pediatrics 2011;128:e1314-16. http://dx.doi.org/10.1542/peds.2011-2698C.
- Flaherman VJ, Newman TB. In reply. Pediatrics 2011;128:e1317-19. http://dx.doi.org/10.1542/peds.2011-2698E.
- Garcia-de-Leon-Gonzalez R, Oliver-Roig A, Hernandez-Martinez M, Mercader-Rodriguez B, Munoz-Soler V, Maestre-Martinez MI, et al. Becoming baby-friendly in Spain: a quality-improvement process. Acta Paediatr 2011;100:445-50. http://dx.doi.org/10.1111/j.1651-2227.2010.02061.x.
- Gokcay G. Ten steps for successful breast feeding: assessment of hospital performance, its determinants and planning for improvement. Child Care Health Dev 1997;23:187-200. http://dx.doi.org/10.1111/j.1365-2214.1997.tb00892.x.
- Haiek LN. Measuring compliance with the Baby-Friendly Hospital. Public Health Nutr 2012;15:894-905. http://dx.doi.org/10.1017/S1368980011002394.
- Hill ME. Is baby-friendly certification nurse friendly?. Can Nurse 2011;107.
- Levitt C, Hanvey L, Kaczorowski J, Chalmers B, Heaman M, Bartholomew S. Breastfeeding policies and practices in Canadian hospitals: comparing 1993 with 2007. Birth 2011;38:228-37. http://dx.doi.org/10.1111/j.1523-536X.2011.00479.x.
- Long L. Achieving ‘baby friendly’ standards in pre-registration education. Br J Midwifery 2011;19:33-6. http://dx.doi.org/10.12968/bjom.2011.19.1.33.
- Merewood A, Mehta SD, Chamberlain LB, Philipp BL, Bauchner H. Breastfeeding rates in US Baby-Friendly Hospitals: results of a national survey. Pediatrics 2005;116:628-34. http://dx.doi.org/10.1542/peds.2004-1636.
- Merten S, Dratva J, Ackermann-Liebrich U. Do Baby-Friendly Hospitals influence breastfeeding duration on a national level?. Pediatrics 2005;116:e702-8. http://dx.doi.org/10.1542/peds.2005-0537.
- Sampaio PF, Moraes CL, Reichenheim ME, Dias de Oliveira AS, Lobato G. Birth in Baby-Friendly Hospitals in Rio de Janeiro, Brazil: a protective factor for breastfeeding?. Cad Saude Publica 2011;27:1349-61. http://dx.doi.org/10.1590/S0102-311X2011000700010.
- Schmied V, Gribble K, Sheehan A, Taylor C, Dykes FC. Ten steps or climbing a mountain: a study of Australian health professionals’ perceptions of implementing the Baby Friendly Health Initiative to protect, promote and support breastfeeding. BMC Health Serv Res 2011;11. http://dx.doi.org/10.1186/1472-6963-11-208.
- Souza MFL, Ortiz PN, Soares PL, Vieira TO, Vieira GO, Silva LR. Evaluation of breastfeeding promotion in Baby-Friendly Hospitals. Rev Paul Pediatr 2011;29:502-8. http://dx.doi.org/10.1590/S0103-05822011000400006.
- Taylor C, Gribble K, Sheehan A, Schmied V, Dykes F. Staff perceptions and experiences of implementing the Baby Friendly Initiative in neonatal intensive care units in Australia. J Obst Gynecol Neonat Nurs 2011;40:25-34. http://dx.doi.org/10.1111/j.1552-6909.2010.01204.x.
- Taylor SN, Barreira J, Murphy P, Haase B, Browning D, Mauldin J, et al. Multidisciplinary improvements of hospital lactation support process: impact on mothers' milk delivery to infants. Breastfeed Med 2011;6.
- Thomson G, Dykes F. Women’s sense of coherence related to their infant feeding experiences. Matern Child Nutr 2011;7:160-74. http://dx.doi.org/10.1111/j.1740-8709.2010.00251.x.
- Vasquez MJ, Berg OR. The baby-friendly journey in a US public hospital. J Perinat Neonatal Nurs 2012;26:37-46. http://dx.doi.org/10.1097/JPN.0b013e3182107179.
- Walsh AD, Pincombe J, Henderson A. An examination of maternity staff attitudes towards implementing Baby Friendly Health Initiative (BFHI) accreditation in Australia. Matern Child Health J 2011;15:597-609. http://dx.doi.org/10.1007/s10995-010-0628-1.
- Bartington S, Griffiths LJ, Tate AR, Dezateux C, Millennium C. Are breastfeeding rates higher among mothers delivering in Baby Friendly accredited maternity units in the UK?. Int J Epidemiol 2006;35:1178-86. http://dx.doi.org/10.1093/ije/dyl155.
- Higgins ST, Washio Y, Heil SH, Solomon LJ, Gaalema DE, Higgins TM, et al. Financial incentives for smoking cessation among pregnant and newly postpartum women. Prev Med 2012;55:S33-40. http://dx.doi.org/10.1016/j.ypmed.2011.12.016.
- Sexton M, Hebel JR. A clinical trial of change in maternal smoking and its effect on birth weight. JAMA 1984;251:911-15. http://dx.doi.org/10.1001/jama.1984.03340310025013.
- Sigmon SC, Patrick ME. The use of financial incentives in promoting smoking cessation. Prev Med 2012;55:S24-32. http://dx.doi.org/10.1016/j.ypmed.2012.04.007.
- Donnelly A, Snowden HM, Renfrew MJ, Woolridge MW. Commercial hospital discharge packs for breastfeeding women. Cochrane Database Syst Rev 2000;2.
- Greaves CJ, Sheppard KE, Abraham C, Hardeman W, Roden M, Evans PH, et al. Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health 2011;11. http://dx.doi.org/10.1186/1471-2458-11-119.
- Brueton V, Rait G, Tierney J, Meredith S, Darbyshire J, Harding S, et al. Strategies to reduce attrition in randomised trials. Cochrane Database Syst Rev 2011;2.
- Specifications Manual for Joint Commission National Quality Measures (v2013A1): Perinatal Care. Oakbrook Terrace, IL: Joint Commission; 2012.
- Glasziou P, Meats E, Heneghan C, Shepperd S. What is missing from descriptions of treatment in trials and reviews?. BMJ 2008;336:1472-4. http://dx.doi.org/10.1136/bmj.39590.732037.47.
- Perera R, Heneghan C, Yudkin P. Graphical method for depicting randomised trials of complex interventions. BMJ 2007;334:127-9. http://dx.doi.org/10.1136/bmj.39045.396817.68.
- Marteau TM, Thorne J, Aveyard P, Hirst J, Sokal R. Financial incentives for smoking cessation in pregnancy: protocol for a single arm intervention study. BMC Pregnancy Childbirth 2013;13. http://dx.doi.org/10.1186/1471-2393-13-66.
- A Randomised Controlled Trial of the ‘Health in Early Feeding Scheme’ to Improve Breastfeeding in Neighbourhoods with Low 6 Week Breastfeeding Rates. Sheffield: University of Sheffield, Research Councils UK; 2012.
- Baxter S, Everson-Hock E, Messina J, Guillaume L, Burrows J, Goyder E. Factors relating to the uptake of interventions for smoking cessation among pregnant women: a systematic review and qualitative synthesis. Nicotine Tob Res 2010;12:685-94. http://dx.doi.org/10.1093/ntr/ntq072.
- Hall Moran V, Edwards J, Dykes F, Downe S. A systematic review of the nature of support for breast-feeding adolescent mothers. Midwifery 2007;23:151-71. http://dx.doi.org/10.1016/j.midw.2006.06.005.
- Glaser BG, Strauss AL. The Discovery of Grounded Theory. Strategies for Qualitative Research. London: Weidenfeld & Nicolson; 1968.
- Graham H, Sowden A, Flemming K, Heirs M, Fox D. Using Qualitative Research to Inform Interventions to Reduce Smoking in Pregnancy in England: a Systematic Review of Qualitative Studies. York: Public Health Research Consortium; 2012.
- Ingall G, Cropley M. Exploring the barriers of quitting smoking during pregnancy: a systematic review of qualitative studies. Women Birth 2010;23:45-52. http://dx.doi.org/10.1016/j.wombi.2009.09.004.
- McInnes RJ, Chambers JA. Supporting breastfeeding mothers: qualitative synthesis. J Adv Nurs 2008;62:407-27. http://dx.doi.org/10.1111/j.1365-2648.2008.04618.x.
- Burns E, Schmied V, Sheehan A, Fenwick J. A meta-ethnographic synthesis of women’s experience of breastfeeding. Matern Child Nutr 2010;6:201-19.
- Larsen JS, Hall EOC, Aagaard H. Shattered expectations: when mothers’ confidence in breastfeeding is undermined: a metasynthesis. Scand J Caring Sci 2008;22:653-61. http://dx.doi.org/10.1111/j.1471-6712.2007.00572.x.
- Nelson AM. A metasynthesis of qualitative breastfeeding studies. J Midwifery Womens Health 2006;51. http://dx.doi.org/10.1016/j.jmwh.2005.09.011.
- Lakshman R, Ogilvie D, Ong KK. Mothers’ experiences of bottle-feeding: a systematic review of qualitative and quantitative studies. Arch Dis Child 2009;94:596-601. http://dx.doi.org/10.1136/adc.2008.151910.
- Atchan M, Foureur M, Davis D. The decision not to initiate breastfeeding – women’s reasons, attitudes and influencing factors – a review of the literature. Breastfeed Rev 2011;19:9-17.
- Nelson AM. A meta-synthesis related to infant feeding decision making. MCN Am J Matern Child Nurs 2012;37:247-52.
- van Esterik P. Contemporary trends in infant feeding research. Annu Rev Anthropol 2002;31:257-78. http://dx.doi.org/10.1146/annurev.anthro.31.040402.085428.
- Sarafino EP. Health Psychology: Biopsychosocial Interactions. New York: Wiley; 1994.
- Kavanagh J, Trouton A, Oakley A, Powell C. A Systematic Review of the Evidence for Incentive Schemes to Encourage Positive Health and Other Social Behaviours in Young People. London: University of London, EPPI-Centre; 2006.
- Cahill K, Perera R. Quit and win contests for smoking cessation. Cochrane Database Syst Rev 2008;4.
- Cahill K, Perera R. Competitions and incentives for smoking cessation. Cochrane Database Syst Rev 2011;4.
- Kavanagh J, Stansfield C, Thomas J. Incentives to Improve Smoking, Physical Activity, Dietary and Weight Management Behaviours: a Scoping Review of the Research Evidence. London: University of London, EPPI-Centre; 2009.
- Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST. A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction 2006;101:192-203. http://dx.doi.org/10.1111/j.1360-0443.2006.01311.x.
- Paul-Ebhohimhen V, Avenell A. Systematic review of the use of financial incentives in treatments for obesity and overweight. Obes Rev 2008;9:355-67. http://dx.doi.org/10.1111/j.1467-789X.2007.00409.x.
- Prendergast M, Podus D, Finney J, Greenwell L, Roll J. Contingency management for treatment of substance use disorders: a meta-analysis. Addiction 2006;101:1546-60. http://dx.doi.org/10.1111/j.1360-0443.2006.01581.x.
- Roozen HG, Boulogne JJ, Van T, van den Brink W, De Jong CA, Kerkhof AJFM. A systematic review of the effectiveness of the community reinforcement approach in alcohol, cocaine and opioid addiction. Drug Alcohol Depend 2004;74:1-13. http://dx.doi.org/10.1016/j.drugalcdep.2003.12.006.
- Wall J, Mhurchu CN, Blakely T, Rodgers A, Wilton J. Effectiveness of monetary incentives in modifying dietary behavior: a review of randomized, controlled trials. Nutr Rev 2006;64:518-31. http://dx.doi.org/10.1111/j.1753-4887.2006.tb00185.x.
- Sutherland K, Leatherman S, Christianson J. Paying the Patient: Does it Work?. London: Quest for Quality and Improved Performance, Health Foundation; 2008.
- Flodgren G, Eccles MP, Shepperd S, Scott A, Parmelli E, Beyer FR. An overview of reviews evaluating the effectiveness of financial incentives in changing healthcare professional behaviours and patient outcomes. Cochrane Database Syst Rev 2011;7.
- Gillam SJ, Siriwardena AN, Steel N. Pay-for-performance in the United Kingdom: impact of the Quality and Outcomes Framework: a systematic review. Ann Fam Med 2012;10:461-8. http://dx.doi.org/10.1370/afm.1377.
- Scott A, Sivey P, Ait OD, Willenberg L, Naccarella L, Furler J, et al. The effect of financial incentives on the quality of health care provided by primary care physicians. Cochrane Database Syst Rev 2011;9.
- Van Herck P, De Smedt D, Annemans L. Systematic review: effects, design choices, and context of pay-for-performance in health care. BMC Health Serv Res 2010;10. http://dx.doi.org/10.1186/1472-6963-10-247.
- Hamilton FL, Greaves F, Majeed A, Millett C. Effectiveness of providing financial incentives to healthcare professionals for smoking cessation activities: systematic review. Tob Control 2013;22:3-8. http://dx.doi.org/10.1136/tobaccocontrol-2011-050048.
- Kaplan RM. Should medicare reimburse providers for weight loss interventions?. Am Psychol 2007;62:217-19. http://dx.doi.org/10.1037/0003-066X.62.3.217.
- Kellogg SH, Kreek MJ. Gradualism, identity, reinforcements, and change. Int J Drug Policy 2005;16:369-75. http://dx.doi.org/10.1016/j.drugpo.2005.08.001.
- Ries NM. Financial incentives for weight loss and healthy behaviours. Healthc Policy 2012;7:23-8.
- Adams Dudley R, Tseng C-W, Bozic K, Smith WA, Luft HS. Consumer Financial Incentives: a Decision Guide for Purchasers. Rockville, MD: Agency for Healthcare Reseach and Quality; 2007.
- Appleby J, Harrison T, Hawkins L, Dixon A. Payment by Results: How Can Payment Systems Help to Deliver Better Care?. London: The King’s Fund; 2012.
- Davies C, Anand P, Artigas L, Holloway J, McConway K, Newman J, et al. Links between Governance, Incentives and Outcomes: a Review of the Literature. Report for the National Co-ordinating Centre for NHS Service Delivery and Organisation R&D (NCCSDO). Milton Keynes: NIHR; 2005.
- Giuffrida A, Gosden T, Forland F, Kristiansen IS, Sergison M, Leese B, et al. Target payments in primary care: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2000;3.
- Smith JE, Meyers RJ, Austin JL. Working with family members to engage treatment-refusing drinkers: the CRAFT program. Alcohol Treat Q 2008;26:169-93. http://dx.doi.org/10.1300/J020v26n01_09.
- Cahill K, Perera R. Competitions and incentives for smoking cessation. Cochrane Database Syst Rev 2008;3.
- Custers T, Hurley J, Klazinga NS, Brown AD. Selecting effective incentive structures in health care: a decision framework to support health care purchasers in finding the right incentives to drive performance. BMC Health Serv Res 2008;8. http://dx.doi.org/10.1186/1472-6963-8-66.
- Gosden T, Forland F, Kristiansen IS, Sutton M, Leese B, Giuffrida A, et al. Capitation, salary, fee-for-service and mixed systems of payment: effects on the behaviour of primary care physicians. Cochrane Database Syst Rev 2000;3.
- Hey K, Perera R. Competitions and incentives for smoking cessation. Cochrane Database Syst Rev 2005;2.
- Higgins ST, Heil SH, Lussier JP. Clinical implications of reinforcement as a determinant of substance use disorders. Annu Rev Psychol 2004;55. http://dx.doi.org/10.1146/annurev.psych.55.090902.142033.
- Lavack A, Watson L, Markwart J. Quit and win contests: a social marketing success story. Soc Mar Q 2007;13:31-52. http://dx.doi.org/10.1080/15245000601163499.
- Meyers RJ, Villanueva M, Smith JE. The community reinforcement approach: history and new directions. J Cogn Psychother 2005;19:247-60. http://dx.doi.org/10.1891/jcop.2005.19.3.247.
- Petersen LA, Woodard LD, Urech T, Daw C, Sookanan S. Does pay-for-performance improve the quality of health care?. Ann Int Med 2006;145:265-72. http://dx.doi.org/10.7326/0003-4819-145-4-200608150-00006.
- Christianson J, Leatherman S, Sutherland K. Financial Incentives, Healthcare Providers and Quality Improvements. London: Health Foundation; 2009.
- Nutbeam D. Health literacy as a public health goal: a challenge for contemporary health education and communication strategies into the 21st century. Health Promot Int 2000;15:259-67. http://dx.doi.org/10.1093/heapro/15.3.259.
- Frey B. Not Just for the Money: an Economic Theory of Personal Motivation. Brookfield, VT: Edward Elgar Publishing; 1997.
- Health and Social Care Information Centre . Quality and Outcomes Framework 2013. www.qof.ic.nhs.uk/ (accessed October 2013).
- Schmidt H. Wellness incentives, equity, and the 5 groups problem. Am J Publ Health 2012;102:49-54. http://dx.doi.org/10.2105/AJPH.2011.300348.
- Coleman T. Do financial incentives for delivering health promotion counselling work? Analysis of smoking cessation activities stimulated by the Quality and Outcomes Framework. BMC Public Health 2010;10. http://dx.doi.org/10.1186/1471-2458-10-167.
- Halpern SD, Asch DA, Volpp KG. Commitment contracts as a way to health. BMJ 2012;344. http://dx.doi.org/10.1136/bmj.e522.
- Glasziou PP, Buchan H, Del M, Doust J, Harris M, Knight R, et al. When financial incentives do more good than harm: a checklist. BMJ 2012;345. http://dx.doi.org/10.1136/bmj.e5047.
- Giles EL, Robalino S, McColl E, Sniehotta F, Adams J. The effectiveness of financial incentives for health behaviour change: systematic review and meta-analysis. PLOS ONE 2014;9. http://dx.doi.org/10.1371/journal.pone.0090347.
- Dallery J, Raiff BR. Contingency management in the 21st century: technological innovations to promote smoking cessation. Subst Use Misuse 2011;46:10-22. http://dx.doi.org/10.3109/10826084.2011.521067.
- Hunter RF, Tully MA, Davis M, Stevenson M, Kee F. Physical activity loyalty cards for behavior change: a quasi-experimental study. Am J Prev Med 2013;45:56-63. http://dx.doi.org/10.1016/j.amepre.2013.02.022.
- Ritchie J, Spencer L, Bryman A, Burgess RG. Analyzing Qualitative Data. London: Routledge; 1994.
- Schulz KF, Altman DG, Moher D. CONSORT group . CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340. http://dx.doi.org/10.1136/bmj.c332.
- Strauss A, Corbin J. Basics of Qualitative Research: Grounded Theory Procedures and Techniques. Newbury Park, CA: Sage; 1990.
- Patton MQ. Qualitative Evaluation and Research Methods. London: Sage; 1990.
- Jepson RG, Harris FM, Platt S, Tannahill C. The effectiveness of interventions to change six health behaviours: a review of reviews. BMC Public Health 2010;10. http://dx.doi.org/10.1186/1471-2458-10-538.
- Sen A, Nussbaum M, Sen A. The Quality of Life. Oxford: Clarendon Press; 1993.
- Asset Based Approaches for Health Improvement: Redressing the Balance. Glasgow: Glasgow Centre for Population Health; 2011.
- Foot J. What Makes us Healthy? The Asset Approach in Practice: Evidence, Action, Evaluation. Chester le Street: Asset Based Publishing; 2012.
- Baby Café: Your First Choice for Breastfeeding Support. London: Baby Café; 2013.
- Hoddinott P, Britten J, Pill R. Why do interventions work in some places and not others: a breastfeeding support group trial. Soc Sci Med 2010;70:769-78. http://dx.doi.org/10.1016/j.socscimed.2009.10.067.
- Hoddinott P, Craig L, Britten J, McInnes R. A serial qualitative study of infant feeding experiences: idealism meets realism. BMJ Open 2012;2. http://dx.doi.org/10.1136/bmjopen-2011-000504.
- McInnes RJ, Hoddinott P, Britten J, Darwent K, Craig LC. Significant others, situations and infant feeding behaviour change processes: a serial qualitative interview study. BMC Pregnancy Childbirth 2013;13. http://dx.doi.org/10.1186/1471-2393-13-114.
- Davey Smith G. Health Inequalities: Lifecourse Approaches. Bristol: Policy Press; 2003.
- Racine EF, Frick KD, Strobino D, Carpenter LM, Milligan R, Pugh LC. How motivation influences breastfeeding duration among low-income women. J Hum Lact 2009;25:173-81. http://dx.doi.org/10.1177/0890334408328129.
- Stokols D. Establishing and maintaining health environments: toward a social ecology of health promotion. Am Psychol 1992;47:6-22. http://dx.doi.org/10.1037/0003-066X.47.1.6.
- Gagnon AJ, Sandall J. Individual or group antenatal education for childbirth or parenthood, or both. Cochrane Database Syst Rev 2007;3.
- Jones L. Principles to Promote the Initiation and Establishment of Lactation in the Mother of a Preterm or Sick Infant. London: UNICEF UK; 2008.
- Becker GE, Cooney F, Smith HA. Methods of milk expression for lactating women. Cochrane Database Syst Rev 2011;12.
- Behaviour Change: the Principles for Effective Interventions. London: NICE; 2007.
- National Institute for Health and Care Excellence . The Use of Incentives to Improve Health n.d. www.nice.org.uk/media/9AF/56/CCReportIncentives.pdf (accessed October 2013).
- Clear the Air News Tobacco Blog . Mothers Offered £650 to Stub Out Tobacco 2010. http://tobacco.cleartheair.org.hk/?p = 2731 (accessed October 2013).
- Park A, Clery E, Curtice J, Phillips M, Utting D. British Social Attitudes 29. London: NatCen Social Research; 2012.
- Eunson J, Murray L. The Acceptability of Carrots. Edinburgh: Ipsos MORI Scotland; 2012.
- Promberger M, Dolan P, Marteau TM. ‘Pay them if it works’: discrete choice experiments on the acceptability of financial incentives to change health related behaviour. Soc Sci Med 2012;75:2509-14. http://dx.doi.org/10.1016/j.socscimed.2012.09.033.
- Park JD, Mitra N, Asch DA. Public opinion about financial incentives for smoking cessation. Prev Med 2012;55:S41-5. http://dx.doi.org/10.1016/j.ypmed.2012.06.013.
- Promberger M, Brown RC, Ashcroft RE, Marteau TM. Acceptability of financial incentives to improve health outcomes in UK and US samples. J Med Ethics 2011;37:682-7. http://dx.doi.org/10.1136/jme.2010.039347.
- Braithwaite D, Emery J, De Lusignan S, Sutton S. Using the internet to conduct surveys of health professionals: a valid alternative?. Fam Pract 2003;20:545-51. http://dx.doi.org/10.1093/fampra/cmg509.
- GPs in Practice Details. Edinburgh: Information Statistics Division, NHS National Services Scotland; 2013.
- National Primary Care Workforce Planning Survey 2009: Results for Scotland. Edinburgh: Information Statistics Division, NHS National Services Scotland; 2009.
- Primary Care Workforce Survey 2013: a Survey of Scottish General Practices and GP Out Of Hours Services. Edinburgh: Information Statistics Division, NHS National Services Scotland; 2013.
- Health and Social Care Act 2001. London: The Stationery Office; 2012.
- Lee E. Living with risk in the age of ‘intensive motherhood’: maternal identity and infant feeding. Health Risk Soc 2008;10:467-77. http://dx.doi.org/10.1080/13698570802383432.
- Marmot M. Status Syndrome. London: Bloomsbury; 2004.
- Kelly YJ, Watt RG, Nazroo JY. Racial/ethnic differences in breastfeeding initiation and continuation in the United Kingdom and comparison with findings in the United States. Pediatrics 2006;118:e1428-35. http://dx.doi.org/10.1542/peds.2006-0714.
- Lancaster KJ. A new approach to consumer theory. J Polit Econ 1966;74:132-57. http://dx.doi.org/10.1086/259131.
- Louviere JJ, Hensher DA, Swait J. Stated Choice Methods. New York: Cambridge University Press; 2000.
- Ryan M, Gerard K, Amaya-Amaya M. Using Discrete Choice Experiments to Value Health and Health Care. Dordecht: Springer; 2008.
- Free C, Knight R, Robertson S, Whittaker R, Edwards P, Zhou W, et al. Smoking cessation support delivered via mobile phone text messaging (txt2stop): a single-blind, randomised trial. Lancet 2011;378:49-55. http://dx.doi.org/10.1016/S0140-6736(11)60701-0.
- Rabin M, O’Donoghue R. Doing it now or later. Am Econ Rev 1999;89:103-24. http://dx.doi.org/10.1257/aer.89.1.103.
- Wood W, Neal DT. A new look at habits and the habit–goal interface. Psychol Rev 2007;114:843-63. http://dx.doi.org/10.1037/0033-295X.114.4.843.
- Kuhfeld WF. Experimental Design: Efficiency, Coding, and Choice Designs. Cary, NC: SAS Institute; 2010.
- Yi D, Ryan M, Campbell S, Elliott A, Torrance N, Chambers A, et al. Using discrete choice experiments to inform randomised controlled trials: an application to chronic low back pain management in primary care [published online ahead of print 12 November 2010]. Eur J Pain 2011. http://dx.doi.org/10.1016/j.ejpain.2010.10.008.
- de Bekker-Grob EW, Ryan M, Gerard K. Discrete choice experiments in health economics: a review of the literature. Health Econ 2012;21:145-72. http://dx.doi.org/10.1002/hec.1697.
- Hammar H, Carlsson F. Smokers’ expectations to quit smoking. Health Econ 2005;14:257-67. http://dx.doi.org/10.1002/hec.923.
- Goto R, Nishimura S, Ida T. Discrete choice experiment of smoking cessation behaviour in Japan. Tob Control 2007;16:336-43. http://dx.doi.org/10.1136/tc.2006.019281.
- Goto R, Takahashi Y, Ida T. Changes in smokers’ attitudes toward intended cessation attempts in Japan. Value Health 2011;14:785-91. http://dx.doi.org/10.1016/j.jval.2010.12.010.
- Edwards A, Elwyn G, Hood K, Rollnick S. Judging the ‘weight of evidence’ in systematic reviews: introducing rigour into the qualitative overview stage by assessing signal and noise. J Eval Clin Pract 2000;6:177-84. http://dx.doi.org/10.1046/j.1365-2753.2000.00212.x.
- Glasziou P, Chalmers I, Altman DG, Bastian H, Boutron I, Brice A, et al. Taking healthcare interventions from trial to practice. BMJ 2010;341. http://dx.doi.org/10.1136/bmj.c3852.
- Fingerhut LA, Kleinman JC, Kendrick JS. Smoking before, during, and after pregnancy. Am J Publ Health 1990;80:541-4. http://dx.doi.org/10.2105/AJPH.80.5.541.
- Improving the Effectiveness and Reach of NHS Support for Smoking Cessation in Pregnancy. Nottingham: University of Nottingham, UK Centre for Tobacco Control Studies; 2013.
- Tappin D, Bauld L, Purves D, Boyd K, Sinclair L, MacAskill S, et al. Financial incentives for smoking cessation during pregnancy: a randomised controlled trial. Lancet 2014;384. http://dx.doi.org/10.1016/S0140-6736(14)62130-9.
- Tappin D, Bauld L, Purves D, Boyd K, Sinclair L, MacAskill S, et al. Financial incentives for smoking cessation in pregnancy: randomised controlled trial. BMJ 2015;350. http://dx.doi.org/10.1136/bmj.h134.
- Ashcroft RE. Personal financial incentives in health promotion: where do they fit in an ethic of autonomy?. Health Expect 2011;14:191-200. http://dx.doi.org/10.1111/j.1369-7625.2011.00664.x.
- Lancaster T, Stead LF. Self-help interventions for smoking cessation. Cochrane Database Syst Rev 2005;3.
- Clemons SN, Amir LH. Breastfeeding women’s experience of expressing: a descriptive study. J Hum Lact 2010;26:258-65. http://dx.doi.org/10.1177/0890334410371209.
- Labiner-Wolfe J, Fein SB, Shealy KR, Wang C. Prevalence of breast milk expression and associated factors. Pediatrics 2008;122:S63-8. http://dx.doi.org/10.1542/peds.2008-1315h.
- Ryan K, Team V, Alexander J. Expressionists of the twenty-first century: the commodification and commercialization of expressed breast milk. Med Anthropol 2013;32:467-86. http://dx.doi.org/10.1080/01459740.2013.768620.
- Breast Pump Hire. London: National Childbirth Trust; 2012.
- Hoddinott P, Britten J, Craig L, McInnes R, Darwent K. Significant Others, Situations and their Influence on Infant Feeding. NHS Health Scotland; 2013.
- Bevan G, Hood C. What’s measured is what matters: targets and gaming in the English public health care system. Public Admin 2006;84:517-38. http://dx.doi.org/10.1111/j.1467-9299.2006.00600.x.
- Ivers N, Jamtvedt G, Flottorp S, Young JM, Odgaard-Jensen J, French SD, et al. Audit and feedback: effects on professional practice and health care outcomes. Cochrane Database Syst Rev 2012;6.
- Bailey C, Pain RH, Aarvold JE. A ‘give it a go’ breast-feeding culture and early cessation among low-income mothers. Midwifery 2004;20:240-50. http://dx.doi.org/10.1016/j.midw.2003.12.003.
- Priest N, Roseby R, Waters E, Polnay A, Campbell R, Spencer N, et al. Family and carer smoking control programmes for reducing children’s exposure to environmental tobacco smoke. Cochrane Database Syst Rev 2008;4.
- WHO Analgesic Ladder. Cardiff: Cardiff University, Pain Community Centre; 1986.
- Robl J, Ashford KB, Centers I, McCubbin A, Shepherd RA. GIFTS (Giving Infants and Families Tobacco-Free Starts): A Bundled Approach to Help Pregnant Mothers Quit Smoking n.d. https://apha.confex.com/apha/138am/webprogram/Paper226318.html (accessed 11 November 2011).
- Financial Incentives. Sheffield: University of Sheffield, School of Health and Related Research; 2013.
- Reducing Exposure to Secondhand Smoke: Smokefree Pregnancy. Manchester: Tobacco Free Futures; 2013.
- Forum: Best Fed Babies. Edinburgh: Scottish Executive Health Department; 2004.
- Encouraging Teenage Mothers to Breastfeed. Bristol: University of the West of England; 2012.
- Saurel-Cubizolles MJ, Romito P, Garcia J. Description of maternity rights for working women in France, Italy and in the United Kingdom. Eur J Publ Health 1993;3:48-53. http://dx.doi.org/10.1093/eurpub/3.1.48.
- Breast Milk Policy. Sheffield: University of Sheffield, School of Health and Related Research; 2013.
- JFL . Quebec to pay mothers to breastfeed. Pediatrics 1995;95.
- Support Fund External Evaluation: Partnership Action on Tobacco & Health (PATH). Edinburgh: Action on Smoking & Health (Scotland) (ASH Scotland); 2013.
- La Valle I, Gibb J, Bryska B, Durbin B, Sharp C, Ashton H, et al. Feasibility Study for the Trials of Payment by Results for Children’s Centres. Slough: National Foundation for Educational Reseach; 2011.
Appendix 1 Commissioning brief
Appendix 2 Intervention vignettes
Intervention vignettes
Smoking cessation
Gulliver and colleagues104
-
You and your partner/relative are invited to attend a 60-minute group with other expecting couples, which is led by a psychologist at a local hospital, to discuss pregnancy and smoking.
-
In the group, the psychologist wants to find out whether you are ready to give up smoking and if your partner/relative can help you. The group is told that giving up smoking is possible and how it would improve their own and their baby’s health.
-
You and your partner/relative are also invited to couple counselling appointments to discuss your own experiences of smoking and previous attempts to stop. You are told that the counselling appointments will include working with a self-help manual (Freedom from Smoking for You and Your Baby; see www.in.gov/isdh/files/Cessation_Manual.pdf, accessed 5 January 2015) and thinking about the triggers for smoking. You will be asked to sign a contract for your chosen stop smoking plan.
-
You are then invited to attend monthly appointments until your baby is 3 months old. At these appointments you are asked about your smoking and have a breath test to show whether you are still smoking or not.
-
At each visit there is a raffle that you can enter to win gifts, regardless of whether you are still smoking or not. A car seat is raffled every 3 months.
-
Also, if you stay quit, and the breath test proves it, you will be given additional gifts donated by local businesses as they want to support your efforts to stay smoke free.
-
All your travel to and from appointments will be paid for.
Heil and colleagues 2008105
-
At 18 weeks of pregnancy you are invited to attend a stop smoking appointment. There, you are asked to agree a quit date, give a breath test and provide a urine sample and you are also given a smoking cessation leaflet, which you discuss with staff members.
-
If you agree to continue the service, you will have tests to assess whether you are still smoking:
-
every day for the next 5 days
-
then twice weekly for another 7 weeks
-
once a week for the next 4 weeks
-
then fortnightly up until the baby is born.
-
-
After your baby is born, you will also have to provide samples:
-
every week for 4 weeks
-
then fortnightly for the next 8 weeks (12 weeks in total)
-
at a final assessment made at 24 weeks after the baby is born.
-
-
You will receive vouchers for as long as you stay quit and these increase in value each time the test confirms that you have stopped smoking (starting from £10.00 at first testing and then increasing by £2.00 for each negative test, up to a maximum of £70.00).
-
Any positive/missing results will reset the value of the vouchers (to £10.00); however, if you then have a further two negative results, the value of the vouchers will be restored.
-
During each visit you will discuss your smoking status and the benefits of not smoking during pregnancy/after the birth and at the end you will receive a pamphlet highlighting the reasons to remain non-smoking.
Walsh and colleagues 1997108
-
You and your partner have been invited to attend a three-session smoking cession programme. The programme consists of the following:
First session
-
You are given 2–3 minutes of risk information advice from a doctor and shown a 14-minute video that contains risk information, barriers to quitting and how to overcome them and stop smoking tips.
-
Following the video, a 10-minute counselling session is provided by a midwife and a quit date is agreed.
-
You receive a self-help manual as well as guidance on how to use it (this manual includes sections on risks, barriers, and smoking cessation).
-
You and your partner are offered four packets of confectionary gum.
-
Your partner is provided with a tip sheet, a contract and a letter that stresses the importance of smoking cessation support.
-
A sticker is placed on your medical records so that other professionals know that you are involved in the programme.
Second and third sessions (held at approximately 34–36 weeks of pregnancy)
-
On the second and third visits a midwife will provide approximately 5 minutes of counselling support and a doctor will provide approximately 2 minutes of risk advice.
-
Urine samples will be collected during these visits to test whether you are smoking.
Follow-up
-
You will provide a further urine sample between 6 and 12 weeks after your baby has been born.
-
If your urine sample (provided at your second visit) is negative, your name is entered into a draw to win four donated prizes (approximately £120.00 each).
Breastfeeding
Chamberlain and colleagues103
-
You are offered a personal-use double electric breast pump (worth £120.00–225.00, your brand of choice), which can be delivered to the hospital or to your home following the birth of your baby.
-
You will have access to a breastfeeding specialist in the hospital and you will be given a number for a breastfeeding telephone support line that you can call from home.
Pugh and Milligan106
-
Within 24 hours of having your baby you are asked whether you would like to take part in a parenting/breastfeeding programme. Once you have agreed, you are asked to complete some questionnaires and are provided with breastfeeding support. You are told that:
-
at 3–4 days following the birth a nurse will visit you to discuss parenting issues/provide breastfeeding support
-
at 5 days following the birth a breastfeeding specialist will telephone you to discuss breastfeeding
-
at 12 days after the birth the nurse will visit you and offer flexible ‘non-nursing’ support based on what support you need, for example washing dishes, doing the laundry, providing childcare.
-
-
You are also told that further questionnaires will be sent to you for completion at 14 days and 6 weeks after the birth of your baby.
Volpe and Bear107
-
During your pregnancy you are invited to attend three 1-hour weekly group sessions that provide education, information and support for parenting issues and breastfeeding.
-
Each week will focus on a different topic and you will be provided with a gift at the end of each session. The focus of the sessions and the gift provided will be:
-
week 1: healthy eating – chocolate cigar
-
week 2: safety – electrical outlet covers
-
week 3: mothering the mother – perfume.
-
-
The sessions are run by a nurse/breastfeeding specialist and a breastfeeding supporter will also be present to provide support and encouragement. The breastfeeding supporter will continue to visit you after your baby has been born if you continue to breastfeed.
Appendix 3 Aberdeen ‘ladders’
Ladder A
Ladder B
Ladder C
Appendix 4 Aberdeen ‘rungs’
Life rungs
Incentive rungs
Other rungs
Appendix 5 Blackpool ‘ladders’
Ladder A
Ladder B
Ladder C
Appendix 6 PROSPERO acknowledgement of receipt
Dear Registrant,
Thank you for submitting details of your systematic review for registration in PROSPERO.
The information you have provided will be checked to make sure that your systematic review is within the register scope. Field content will also be checked for relevance.
Please note that these checks do not constitute peer review or imply approval of your systematic review methods.
You will be notified of publication on the register, asked to provide further information or clarification, or informed of the reasons for non-publication within the next five working days. Please note that during this time the record will be locked and you will not be able to access it.
Yours sincerely
PROSPERO Administrator Centre for Reviews and Dissemination
University of York
York
YO10 5DD
t: + 44 (0) 1904 321040
f: + 44 (0) 1904 321041
e: CRD-Register@york.ac.uk www.york.ac.uk/inst/crd
CRD is part of the National Institute for Health Research and is a department of the University of York.
E-mail disclaimer: http://www.york.ac.uk/docs/disclaimer/email.htm
Appendix 7 Search strategies
Smoking cessation
MEDLINE (1946 to February Week 1 2012), MEDLINE-in-Process & Other Non-Indexed Citations (1946 to 28 February 2012), EMBASE (1974 to 2012 February 28)
Ovid multifile search: see http://shibboleth.ovid.com/
-
exp Reward/
-
Motivation/
-
gift giving/
-
patient compliance/
-
capitation fee/
-
quality assurance, health care/
-
fee for service plans/use prmz
-
reimbursement, incentive/use prmz
-
“Reinforcement (psychology)”/use prmz
-
employee incentive plans/use prmz
-
“salaries and fringe benefits”/use prmz
-
physician incentive plans/use prmz
-
remuneration/use prmz
-
reinforcement/use oemezd
-
exp personnel management/use oemezd
-
“awards and prizes”/use oemezd
-
reimbursement/use oemezd
-
exp accreditation/
-
punishment/use prmz
-
(incentiv$or competit$or lottery or lotteries or stipend$or bonus$or cash or contest$or discount$or disincentiv$or gift or gifts or price$or prize or prizes or raffle$or reward$or token or tokens or voucher$or win or won or award$or inducement or certifi$or accredit$or quality assurance or (contingen$adj3 payment$) or (tangible adj2 (goods or service$))).tw.
-
(fee or fees or tax or taxes or taxation or fined or fines or charg$or penal$or punish$).tw.
-
or/1–21
-
exp “Tobacco Use Cessation”/
-
tobacco dependence/use oemezd
-
maternal smoking/use oemezd
-
cigarette smoking/use oemezd
-
smoking cessation/use oemezd
-
smoking habit/use oemezd
-
“tobacco use disorder”/
-
(smok$adj3 (cessation or quit$or stop or stopping or stopped or abstain$or reduc$or ceas$or give or gave or giving)).tw.
-
((cigar$or tobacco) adj3 smok$).tw.
-
((smok$or tobacco) adj3 control).tw.
-
((cigar$or tobacco) adj2 (use$or usage or using)).tw.
-
(smok$adj2 (relaps$adj3 (prevent$or recurr$or maintain$or sustain$))).tw.
-
smoking/pc [prevention and control]
-
or/23–35
-
Pregnancy/use prmz
-
exp pregnancy/use oemezd
-
puerperium/use oemezd
-
Prenatal Care/
-
pregnant women/use prmz
-
pregnant woman/use oemezd
-
(antenatal or prenatal or prepartum or puerperium or postpartum or pregnan$).tw.
-
exp Perinatal Care/
-
or/37–42
-
22 and 36 and 45
-
((smok$or cigar$or tobacco) adj3 pregnan$).tw.
-
22 and 47
-
46 or 48
-
exp animals/not humans/
-
49 not 50
-
remove duplicates from 51
Cumulative Index to Nursing and Allied Health Literature (1981 to February 2012)
URL: see http://search.ebscohost.com
-
(MH “Capitation Fee”)
-
(MH “Fee for Service Plans”)
-
(MH “Quality Assurance+”)
-
TX remuneration or quality assurance
-
TX tangible n2 goods
-
TX tangible n2 service*
-
TX fee or fees or tax or taxes or taxation or fined or fines or charg* or penal* or punish*
-
(MH “Accreditation+”)
-
(MH “Reward”)
-
(MH “Motivation+”)
-
(MH “Gift Giving”)
-
(MH “Patient Compliance+”)
-
(MH “Reinforcement (Psychology)+”)
-
(MH “Employee Incentive Programs”)
-
(MH “Salaries and Fringe Benefits+”)
-
(MH “Physician Incentive Plans”)
-
(MH “Awards and Honors+”)
-
(MH “Reimbursement, Incentive”)
-
TX incentive* or competit* or lottery or lotteries or stipend or bonus or cash or contest* or discount* or disincentive* or gift or gifts or price* or prize or prizes or raffle* or reward* or token or tokens or voucher* or win* or award* or inducement or certifi* or accredit*
-
TX contingen* n3 payment*
-
S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20
-
(MH “Smoking Cessation Programs”)
-
(MH “Tobacco+”)
-
TX smok* n3 (cessation or quit* or stop or stopping or stopped or abstain* or reduc* or ceas* or give or gave or giving)
-
(MH “Smoking/PC”)
-
(MH “Smoking Cessation”)
-
TX smok* AND TX relaps*
-
TX (cigar* or tobacco) n3 (smok* or control*)
-
S22 or S23 or S24 or S25 or S26 or S27 or S28
-
TX smok* n3 pregnan*
-
TX cigar* n3 pregnan*
-
TX tobacco n3 pregnan*
-
S30 or S31 or S32
-
S21 and S33
-
(MH “Pregnancy”)
-
(MH “Postnatal Period+”)
-
(MH “Postnatal Care+”)
-
(MH “Perinatal Care”)
-
(MH “Expectant Mothers”)
-
TX antenatal or preparum or puerperium or postpartum or pregnan* or postnatal
-
S35 or S36 or S37 or S38 or S39 or S40
-
S21 and S29 and S41
-
S34 or S42
-
Exclude MEDLINE records
PsycINFO (1806 to February 2012)
URL: see http://search.ebscohost.com
-
DE “Incentives” OR DE “Educational Incentives” OR DE “Monetary Incentives”
-
DE “Rewards” OR DE “External Rewards” OR DE “Internal Rewards” OR DE “Monetary Rewards” OR DE “Preferred Rewards”
-
DE “Professional Fees” OR DE “Fee for Service”
-
DE “Treatment Compliance”
-
DE “Quality of Care”
-
DE “Reinforcement” OR DE “Differential Reinforcement” OR DE “Negative Reinforcement” OR DE “Noncontingent Reinforcement” OR DE “Positive Reinforcement” OR DE “Primary Reinforcement” OR DE “Punishment” OR DE “Reinforcement Amounts” OR DE “Reinforcement Schedules” OR DE “Rewards” OR DE “Secondary Reinforcement” OR DE “Self Reinforcement” OR DE “Social Reinforcement”
-
DE “Employee Benefits” OR DE “Bonuses” OR DE “Employee Assistance Programs” OR DE “Employee Health Insurance” OR DE “Employee Leave Benefits” OR DE “Employee Pension Plans” OR DE “Workers’ Compensation Insurance”
-
DE “Awards (Merit)”
-
DE “Employee Motivation”
-
DE “Motivation”
-
DE “Hospital Accreditation”
-
TX incentive* or competit* or lottery or lotteries or stipend or bonus or cash or contest* or discount* or disincentive* or gift or gifts or price* or prize or prizes or raffle* or reward* or token or tokens or voucher* or win* or award* or inducement or certifi* or accredit*
-
TX tangible n2 good*
-
TX tangible n2 service*
-
TX contingen* n3 payment*
-
fee or fees or tax or taxes or taxation or fined or fines or charg* or penal* or punish*
-
S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16
-
DE “Smoking Cessation”
-
DE “Tobacco Smoking” OR DE “Passive Smoking”
-
TX smok* n3 (cessation or quit* or stop or stopping or stopped or abstain* or reduc* or ceas* or give or gave or giving)
-
TX smok* n3 relaps*
-
S18 or S19 or S20 or S21
-
pregnan* n3 (smok* or cigar* or tobacco)
-
DE “Pregnancy” OR DE “Adolescent Pregnancy” OR DE “Perinatal Period” OR DE “Postnatal Period” OR DE “Prenatal Care”
-
DE “Expectant Mothers”
-
TX antenatal or prenatal or prepartum or puerperium or postpartum or pregnan* or postnatal
-
S24 or S25 or S26
-
S17 and S22 and S27
-
S17 and S23
-
S28 or S29
Science Citation Index, Social Sciences Citation Index, Conference Proceedings Citation Index – Science, Conference Proceedings Citation Index – Social Science & Humanities (1990 to March 2012)
URL: see www.webofknowledge.com
-
TS = (incentiv* or motivat* or tangible* or accredit* or lotter* or raffle* or contest* or competit* or bonus* or stipend* or price* or prize or prizes or reward* or token* or voucher* or discount* or disincentive* or win or won or certif* or quality assurance or gifts or gift or cash or punish* or penal* or fee or fees or fined or fines or tax or taxes or taxation)
-
TS = (pregnan* or postnatal or antenatal or prenatal or puerperium or prepartum or postpartum)
-
TS = ((pregnan*) near/3 (smok* or cigar* or tobacco))
-
TS = ((smok*) near/3 (cessation or cease or ceased or quit* or “give up” or “giving up” or “gave up” or relaps* or “stop” or “stopping” or “stopped” or reduc* or abstain*))
-
#4 AND #2 AND #1
-
#3 AND #1
-
#6 OR #5
-
TS = ((animal* or rat or rats or mouse or mice) NOT (human* or men or women))
-
#7 not #8
Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews (The Cochrane Library, Issue 2, 2012)
URL: see www.thecochranelibrary.com
-
MeSH descriptor Reward explode all trees
-
MeSH descriptor Motivation, this term only
-
MeSH descriptor Gift Giving, this term only
-
MeSH descriptor Patient Compliance explode tree 1
-
MeSH descriptor Capitation Fee, this term only
-
MeSH descriptor Fee-for-Service Plans, this term only
-
MeSH descriptor Quality Assurance, Health Care, this term only
-
MeSH descriptor Reimbursement, Incentive, this term only
-
MeSH descriptor Reinforcement (Psychology), this term only
-
MeSH descriptor Employee Incentive Plans, this term only
-
MeSH descriptor Salaries and Fringe Benefits, this term only
-
MeSH descriptor Physician Incentive Plans, this term only
-
MeSH descriptor Accreditation explode tree 1
-
MeSH descriptor Punishment, this term only
-
incentive* or competit* or lottery or lotteries or stipend* or bonus* or cash or contest* or discount* or disincentive* or gift or gifts or price* or prize or prizes or raffle* or reward* or token or tokens or voucher* or win or won or award* or inducement or certifi* or accredit* or quality assurance
-
fee or fees or tax or taxes or taxation or fined or fines or charg* or penal* or punish*
-
contingen* near/3 payment*
-
(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17)
-
MeSH descriptor Tobacco Use Cessation explode all trees
-
MeSH descriptor Tobacco Use Disorder, this term only
-
smok* near/3 (cessation or quit* or stop or stopping or stopped or abstain* or reduc* or ceas* or give or gave or giving)
-
smok* near/5 (relaps* or prevent* or recurr* or maintain* or sustain*)
-
MeSH descriptor Smoking, this term only with qualifier: PC
-
(#19 OR #20 OR #21 OR #22 OR #23)
-
MeSH descriptor Pregnancy, this term only
-
MeSH descriptor Prenatal Care, this term only
-
MeSH descriptor Pregnant Women, this term only
-
antenatal or prenatal or prepartum or puerperium or postpartum or pregnan*
-
MeSH descriptor Perinatal Care explode tree 1
-
(#25 OR #26 OR #27 OR #28 OR #29)
-
pregnan* near/3 (smok* or tobacco or cigar*)
-
(#18 AND #24 AND #30)
-
(#18 AND #31)
-
(#32 OR #33)
Database of Abstracts of Reviews of Effects, NHS Economic Evaluation Database, Health Technology Assessment database (March 2012)
Centre for Reviews and Dissemination: see www.crd.york.ac.uk
-
MeSH DESCRIPTOR Motivation
-
MeSH DESCRIPTOR Gift Giving
-
MeSH DESCRIPTOR Capitation Fee
-
MeSH DESCRIPTOR Fee-for-Service Plans
-
MeSH DESCRIPTOR Reimbursement, Incentive
-
MeSH DESCRIPTOR Reinforcement (Psychology)
-
MeSH DESCRIPTOR Salaries and Fringe Benefits
-
MeSH DESCRIPTOR Physician Incentive Plans
-
incentive* or competit* or lottery or lotteries or stipend* or bonus* or cash or contest* or discount* or disincentive* or gift or gifts or price* or prize or prizes or raffle* or reward* or token or tokens or voucher* or win or won or award* or inducement or certifi* or accredit* or quality assurance or fee or fees or tax or taxes or taxation or fined or fines or charg* or penal* or punish*
-
contingen* adj3 payment*
-
#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10
-
MeSH DESCRIPTOR Tobacco Use Disorder
-
MeSH DESCRIPTOR Smoking Cessation EXPLODE ALL TREES
-
smok* adj3 (cessation or quit* or stop or stopping or stopped or abstain* or reduc* or ceas* or give or gave or giving)
-
smok* adj3 (relaps* or prevent* or recurr* or maintain* or sustain*)
-
#12 OR #13 OR #14 OR #15
-
MeSH DESCRIPTOR Pregnancy
-
MeSH DESCRIPTOR Pregnant Women
-
antenatal or prenatal or prepartum or puerperium or postpartum or pregnan*
-
MeSH DESCRIPTOR Prenatal Care EXPLODE ALL TREES
-
MeSH DESCRIPTOR Postnatal Care
-
MeSH DESCRIPTOR Postpartum Period
-
#17 OR #18 OR #19 OR #20 OR #21 OR #22
-
(smok* or cigar* or tobacco) adj3 pregnan*
-
#11 AND #16 AND #23
-
#11 AND #24
-
#25 OR #26
Applied Social Sciences Index and Abstracts (1987 to March 2012)
URL: see http://search.proquest.com/advanced
-
DE = (“penalties” or “fixed penalties” or “deterrence” or “disincentives” or “extrinsic rewards”)
-
KW = (incentiv* or competit* or lotter* or stipend* or bonus* or cash or contest* or discount* or disincentiv* or gift or gifts or price* or prize* or raffle* or reward* or token or tokens or voucher* or win or won or award* or inducement* or certifi* or accredit* or quality assurance or contingent payment* or fee or fees or fined or fines or taxes or taxation or tax or charg* or penal* or punish*)
-
#1 or #2
-
DE = (smoking or heavy smoking or moderate smoking or occasional smoking or passive smoking or tobacco smoke)
-
KW = ((smok*) within 3 (cessation or ceas* or give up or gave up or giving up or abstain* or relaps* or stop or stopping or stopped or quit* or reduc*))
-
#4 or #5
-
DE = (maternal health care or antenatal education or perinatal care or pregnancy or antenatal or antenatal care or perinatal or postnatal care)
-
KW = (pregnan* or postnatal or antenatal or prenatal or postpartum or puerperium)
-
#7 or #8
-
#3 and #6 and #9
-
KW = (pregnan* within 3 (smok* or tobacco or cigar*))
-
#11 and #3
-
#10 or #12
Maternity and Infant Care (1971 to March 2012)
URL: see www.ovid.com/site/catalog/databases/2694.jsp
-
“Salaries and fringe benefits”.de.
-
Patient compliance.de.
-
Quality assurance.de.
-
Health promotion.de.
-
Accreditation.de.
-
(incentive$or competit$or lottery or lotteries or stipend$or bonus$or cash or contest$or discount$or disincentive$or gift or gifts or price$or prize or prizes or raffle$or reward$or token or tokens or voucher$or win or won or award$or inducement or certifi$or accredit$or quality assurance or (contingen$adj3 payment$) or (tangible adj2 (goods or service$))).tw.
-
(fee or fees or tax or taxes or taxation or fined or fines or charg$or penal$or punish$).tw.
-
or/1–7
-
(Smoking or Smoking cessation).de.
-
Tobacco.de.
-
(smok$adj3 (cessation or quit$or stop or stopping or stopped or abstain$or reduc$or ceas$or give or gave or giving)).tw.
-
(smok$adj2 (relaps$adj3 (prevent$or recurr$or maintain$or sustain$))).tw.
-
or/9–12
-
((smok$or cigar$or tobacco) adj3 pregnan$).tw.
-
Pregnancy.de.
-
(Puerperium or Antenatal care or Postnatal care or Postnatal health).de.
-
Antenatal care.de.
-
(antenatal or prenatal or prepartum or puerperium or postpartum or postnatal or pregnan$).tw.
-
or/15–18
-
8 and 13 and 19
-
8 and 14
-
20 or 21
Midwives Information Resource Services (March 2012)
URL: see www.midirs.org
incentiv* OR voucher* OR token* AND FIELD SU = pregnancy AND FIELD SU = smoking cessation
Trials Register of Promoting Health Interventions (March 2012)
URL: see http://eppi.ioe.ac.uk/webdatabases/
-
Type(s) of intervention: incentives
-
Freetext: “incentiv*” or “competit*” or “lotter*” or “stipend*” or “bonus*” or “cash” or “contest*” or “discount*” or “disincentiv*” or “gift” or “gifts” or “price*” or “prize*” or “raffle*” or “reward*” or “token” or “tokens” or “voucher*” or “win” or “won” or “award*” or “inducement*” or “certifi*” or “accredit*” or “quality assurance” or “contingent payment*” or “contingency payment” or “fee” or “fees” or “fined” or “fines” or “taxes” or “taxation” or “tax” or “charg*” or “penal*” or “punish*
-
#1 or #2
-
Freetext: “smok*”
-
Freetext: “pregnan*” or “postnatal” or “antenatal” or “prenatal” or “postpartum” or “puerperium”
-
#3 and #4 and #5
Websites
-
WHO (March 2012): see www.who.int
-
UNICEF (March 2012): see www.unicef.org
-
The King’s Fund (March 2012): see www.kingsfund.org.uk/
-
Action on Smoking & Health (Scotland) (ASH Scotland): see www.ashscotland.org.uk/
-
NICE (March 2012): see www.nice.org.uk
-
Treatobacco (March 2012): see www.treatobacco.net
-
NHS Centre for Smoking Cessation and Training (March 2012): see www.ncsct.co.uk/
-
Joanna Briggs Institute (March 2012): see www.joannabriggs.edu.au
Conference proceedings
-
UK Society for Behavioural Medicine (2011): see http://uksbm.org.uk/wp-content/uploads/2012/03/Combined-final-conference-proceedings-UKSBM-ASM-2011.pdf
-
UK Society for Behavioural Medicine (2010): see http://dl.dropbox.com/u/37425237/Proceedings/UKSBM-6th-Annual-Scientific-Meeting-Conference-Proceedings.pdf
-
Society of Behavioural Medicine (2012): see www.springerlink.com/content/l0883r1057877406/fulltext.pdf
-
Society of Behavioural Medicine (2011): see www.springerlink.com/content/w3563281v7567016/fulltext.pdf
-
American Public Health Association (2011): see http://apha.confex.com/apha/139am/webprogram/start.html
-
American Public Health Association (2010): see http://apha.confex.com/apha/138am/webprogram/start.html
-
European Public Health Conference (2011): see http://eurpub.oxfordjournals.org/content/21/suppl_1.toc
-
European Public Health Conference (2010): see http://eurpub.oxfordjournals.org/content/20/suppl_1/10.full.pdf+html
-
Society for Research on Nicotine and Tobacco (2012): see www.srnt.org/conferences/2012/pdf/2012_Abstracts_H.pdf
-
Society for Research on Nicotine and Tobacco (2011): see www.srnt.org/conferences/2011/pdf/2011%20SRNT%20Abstracts%20Web.pdf
-
Society for Research on Nicotine and Tobacco Europe (2010): see www.srnt.org/conferences/eu/eu_past/europdf/SRNTEu2010AbstractBook.pdf
-
UK National Smoking Cessation Conference (2010): see www.uknscc.org/2010_UKNSCC/posters.html
Breastfeeding
MEDLINE (1946 to April Week 3 2012), MEDLINE-in-Process & Other Non-Indexed Citations (1946 to 5 May 2012), EMBASE (1974 to 2012 May 5)
Ovid multifile search: see http://shibboleth.ovid.com/
-
exp Reward/
-
Motivation/
-
gift giving/
-
patient compliance/
-
capitation fee/
-
quality assurance, health care/
-
fee for service plans/use prmz
-
reimbursement, incentive/use prmz
-
“Reinforcement (psychology)”/use prmz
-
employee incentive plans/use prmz
-
“salaries and fringe benefits”/use prmz
-
physician incentive plans/use prmz
-
remuneration/use prmz
-
reinforcement/use oemezd
-
exp personnel management/use oemezd
-
“awards and prizes”/use oemezd
-
reimbursement/use oemezd
-
exp accreditation/
-
punishment/use prmz
-
(incentiv$or competit$or lottery or lotteries or stipend$or bonus$or cash or contest$or discount$or disincentiv$or gift or gifts or price$or prize or prizes or raffle$or reward$or token or tokens or voucher$or win or won or award$or inducement or certifi$or accredit$or quality assurance or (contingen$adj3 payment$) or (tangible adj2 (goods or service$))).tw.
-
(fee or fees or tax or taxes or taxation or fined or fines or charg$or penal$or punish$).tw.
-
or/1–21
-
exp Breast Feeding/use prmz
-
milk, human/use prmz
-
exp infant feeding/use oemezd
-
(breast?fe$or breast fe$).tw.
-
breast feeding education/use oemezd
-
(breast?milk or breast milk or baby?milk or baby milk).tw.
-
((baby or babies or infant$) adj2 feed$).tw.
-
or/23–29
-
22 and 30
-
Infant Formula/use prmz
-
Artificial milk/use oemezd
-
(baby formula$or formula feed$or milk formula or formula fed or artificial milk or milk substitute$or infant formula$or synthetic milk or artificial feed$or bottle feed$or bottle fed).tw.
-
or/32–34
-
punishment/use prmz
-
(disincentiv$or discourag$or tax or taxes or taxation or fined or fines or charg$or penal$or punish$).tw.
-
or/36–37
-
35 and 38
-
31 or 39
-
exp animals/not humans/
-
40 not 41
-
remove duplicates from 42
Cumulative Index to Nursing and Allied Health Literature (1981 to May 2012)
URL: see http://search.ebscohost.com
-
(MH “Capitation Fee”)
-
(MH “Fee for Service Plans”)
-
(MH “Quality Assurance+”)
-
TX remuneration or quality assurance
-
TX tangible n2 goods
-
TX tangible n2 service*
-
TX disincentiv* or discourag* or fee or fees or tax or taxes or taxation or fined or fines or charg* or penal* or punish*
-
(MH “Accreditation+”)
-
(MH “Reward”)
-
(MH “Motivation+”)
-
(MH “Gift Giving”)
-
(MH “Patient Compliance+”)
-
(MH “Reinforcement (Psychology)+”)
-
(MH “Employee Incentive Programs”)
-
(MH “Salaries and Fringe Benefits+”)
-
(MH “Physician Incentive Plans”)
-
(MH “Awards and Honors+”)
-
(MH “Reimbursement, Incentive”)
-
TX incentiv* or competit* or lottery or lotteries or stipend or bonus or cash or contest* or discount* or disincentiv* or gift or gifts or price* or prize or prizes or raffle* or reward* or token or tokens or voucher* or win* or award* or inducement or certifi* or accredit*
-
TX contingen* n3 payment*
-
S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20
-
(MH “Breast Feeding+”)
-
(MH “Attitude to Breast Feeding”)
-
(MH “Breast Feeding Promotion”)
-
(MH “Infant Feeding”)
-
(MH “Milk, Human”)
-
TX breast fe* OR breast#fe*
-
TX breast milk or breast#milk or baby milk or baby#milk
-
TX baby n2 feed*
-
TX babies n2 feed*
-
TX infant* n2 feed*
-
S22 or S23 or S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31
-
(MH “Infant Formula”)
-
(MH “Bottle Feeding”)
-
TX baby formula* or formula feed* or milk formula or formula fed or artificial milk or milk substitute* or infant formula* or synthetic milk or artificial feed* or bottle feed* or bottle fed
-
S33 or S34 or S35
-
S7 and S36
-
S21 and S32
-
S37 or S38 Exclude MEDLINE records
PsycINFO (1806 to May 2012)
URL: see http://search.ebscohost.com
-
DE “Incentives” OR DE “Educational Incentives” OR DE “Monetary Incentives”
-
DE “Rewards” OR DE “External Rewards” OR DE “Internal Rewards” OR DE “Monetary Rewards” OR DE “Preferred Rewards”
-
DE “Professional Fees” OR DE “Fee for Service”
-
DE “Treatment Compliance”
-
DE “Quality of Care”
-
DE “Reinforcement” OR DE “Differential Reinforcement” OR DE “Negative Reinforcement” OR DE “Noncontingent Reinforcement” OR DE “Positive Reinforcement” OR DE “Primary Reinforcement” OR DE “Punishment” OR DE “Reinforcement Amounts” OR DE “Reinforcement Schedules” OR DE “Rewards” OR DE “Secondary Reinforcement” OR DE “Self Reinforcement” OR DE “Social Reinforcement”
-
DE “Employee Benefits” OR DE “Bonuses” OR DE “Employee Assistance Programs” OR DE “Employee Health Insurance” OR DE “Employee Leave Benefits” OR DE “Employee Pension Plans” OR DE “Workers’ Compensation Insurance
-
DE “Awards (Merit)”
-
DE “Employee Motivation”
-
DE “Motivation”
-
DE “Hospital Accreditation”
-
TX incentive* or competit* or lottery or lotteries or stipend or bonus or cash or contest* or discount* or disincentive* or gift or gifts or price* or prize or prizes or raffle* or reward* or token or tokens or voucher* or win* or award* or inducement or certifi* or accredit*
-
TX tangible n2 good*
-
TX tangible n2 service*
-
TX contingen* n3 payment*
-
TX disincentiv* or discourag* or fee or fees or tax or taxes or taxation or fined or fines or charg* or penal* or punish*
-
S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16
-
DE “Breast Feeding”
-
TX breast fe* OR breast#fe*
-
TX breast milk or breast#milk or baby milk or baby#milk
-
TX baby n2 feed*
-
TX babies n2 feed*
-
TX infant* n2 feed*
-
S18 or S19 or S20 or S21 or S22 or S23
-
DE “Bottle Feeding”
-
TX baby formula* or formula feed* or milk formula or formula fed or artificial milk or milk substitute* or infant formula* or synthetic milk or artificial feed* or bottle feed* or bottle fed
-
S25 or S26
-
DE “Punishment” OR DE “Response Cost”
-
S16 or S28
-
S27 and S29
-
S17 and S24
-
S30 or S31
Science Citation Index, Social Sciences Citation Index, Conference Proceedings Citation Index – Science, Conference Proceedings Citation Index – Social Science & Humanities (1990 to May 2012)
URL: see www.webofknowledge.com
-
TS = (incentiv* or motivat* or tangible* or accredit* or lotter* or raffle* or contest* or competit* or bonus* or stipend* or price* or prize or prizes or reward* or token* or voucher* or discount* or disincentive* or win or won or certif* or quality assurance or gifts or gift or cash or punish* or penal* or fee or fees or fined or fines or tax or taxes or taxation)
-
TS = (human milk or breast fed or breast feed* or infant feed* breast*milk or breast milk or baby milk or baby*milk)
-
TS = ((baby or babies or infant*) near/2 feed*)
-
#3 OR #2
-
TS = (baby formula* or formula feed* or milk formula or formula fed or artificial milk or milk substitute* or infant formula* or synthetic milk or artificial feed* or bottle feed* or bottle fed)
-
TS = (disincentiv* or discourag* or tax or taxes or taxation or fined or fines or charg* or penal* or punish*)
-
#4 AND #1
-
#6 AND #5
-
#8 OR #7
-
TS = ((animal* or rat or rats or mouse or mice) NOT (human* or men or women))
-
#9 not #10
Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews (The Cochrane Library, Issue 5, 2012)
URL: see www.thecochranelibrary.com
-
MeSH descriptor Reward explode all trees
-
MeSH descriptor Motivation, this term only
-
MeSH descriptor Gift Giving, this term only
-
MeSH descriptor Patient Compliance explode tree 1
-
MeSH descriptor Capitation Fee, this term only
-
MeSH descriptor Quality Assurance, Health Care, this term only
-
MeSH descriptor Fee-for-Service Plans, this term only
-
MeSH descriptor Reimbursement, Incentive, this term only
-
MeSH descriptor Reinforcement (Psychology), this term only
-
MeSH descriptor Employee Incentive Plans, this term only
-
MeSH descriptor Salaries and Fringe Benefits, this term only
-
MeSH descriptor Physician Incentive Plans, this term only
-
MeSH descriptor Remuneration, this term only
-
MeSH descriptor Accreditation, this term only
-
MeSH descriptor Punishment, this term only
-
incentiv* or competit* or lottery or lotteries or stipend* or bonus* or cash or contest* or discount* or disincentiv* or gift or gifts or price* or prize or prizes or raffle* or reward* or token or tokens or voucher* or win or won or award* or inducement or certifi$or accredit* or quality assurance
-
contingen* near/3 payment*
-
fee or fees or tax or taxes or taxation or fined or fines or charg* or penal* or punish*
-
tangible near/2 (goods or servic*)
-
(#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR (#9 AND o AND #10) OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19)
-
MeSH descriptor Breast Feeding explode trees 1 and 2
-
MeSH descriptor Milk, Human, this term only
-
breast fed or breast feed or breast feeding
-
(breast or baby) near/1 milk
-
(baby or babies or infant*) near/2 feed*
-
(#21 OR #22 OR #23 OR #24 OR #25)
-
MeSH descriptor Infant Formula, this term only
-
baby formula* or formula feed* or milk formula or formula fed or artificial milk or milk substitut* or infant formula* or synthetic milk or artificial feed* or bottle feed* or bottle fed
-
(#27 OR #28)
-
disincentiv* or discourag* or tax or taxes or taxation or fined or fines or charg* or penal* or punish*
-
(#15 OR #30)
-
(#20 AND #26)
-
(#29 AND #31)
-
(#32 OR #33)
Database of Abstracts of Reviews of Effects, NHS Economic Evaluation Database, Health Technology Assessment database (May 2012)
Centre for Reviews and Dissemination: see www.crd.york.ac.uk
-
MeSH descriptor Motivation
-
MeSH descriptor Gift Giving
-
MeSH descriptor Capitation Fee
-
MeSH descriptor Fee-for-Service Plans
-
MeSH descriptor Reimbursement, Incentive
-
MeSH descriptor Reinforcement (Psychology)
-
MeSH descriptor Salaries and Fringe Benefits
-
MeSH descriptor Physician Incentive Plans
-
incentive* or competit* or lottery or lotteries or stipend* or bonus* or cash or contest* or discount* or disincentive* or gift or gifts or price* or prize or prizes or raffle* or reward* or token or tokens or voucher* or win or won or award* or inducement or certifi* or accredit* or quality assurance or fee or fees or tax or taxes or taxation or fined or fines or charg* or penal* or punish*
-
contingen* adj3 payment*
-
#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10
-
MeSH descriptor Breast Feeding
-
MeSH descriptor milk, human
-
breastfe* or breast fe* or breast-fe*
-
breastmilk or breast milk or babymilk or baby milk
-
((baby or babies or infant*) adj2 feed*)
-
#12 OR #13 OR #14 OR #15 OR #16
-
MeSH descriptor infant formula
-
baby formula* or formula feed* or milk formula or formula fed or artificial milk or milk substitute* or infant formula* or synthetic milk or artificial feed* or bottle feed* or bottle fed
-
#18 OR #19
-
disincentiv* or discourag* or tax or taxes or taxation or fined or fines or charg* or penal* or punish*
-
#20 AND #21
-
#11 AND #17
-
#22 OR #23
Applied Social Sciences Index and Abstracts (1987 to May 2012)
URL: see http://search.proquest.com/advanced
-
((DE = (penalties or (fixed penalties) or deterrence) or DE = (disincentives or (extrinsic rewards))) or(KW = (incentiv* or competit* or lotter* or stipend* or bonus* or cash or contest* or discount* or disincentiv* or gift or gifts or price* or prize* or raffle* or reward* or token or tokens or voucher* or win or won or award* or inducement* or certifi* or accredit* or quality assurance or contingent payment* or fee or fees or fined or fines or taxes or taxation or tax or charg* or penal* or punish*))) and((DE = (“breastfeeding” or “weaning”)) or(KW = ((breastfe* or breast fe* or breast-fe* or breastmilk or breast milk or babymilk or baby milk) or ((baby or babies or infant*) within 2 feed*))))
-
(KW = (baby formula* or formula feed* or milk formula or formula fed or artificial milk or milk substitute* or infant formula* or synthetic milk or artificial feed* or bottle feed* or bottle fed)) and(KW = (disincentiv* or discourag* or tax or taxes or taxation or fined or fines or charg* or penal* or punish*))
-
(((DE = (penalties or (fixed penalties) or deterrence) or DE = (disincentives or (extrinsic rewards))) or(KW = (incentiv* or competit* or lotter* or stipend* or bonus* or cash or contest* or discount* or disincentiv* or gift or gifts or price* or prize* or raffle* or reward* or token or tokens or voucher* or win or won or award* or inducement* or certifi* or accredit* or quality assurance or contingent payment* or fee or fees or fined or fines or taxes or taxation or tax or charg* or penal* or punish*))) and((DE = (“breastfeeding” or “weaning”)) or(KW = ((breastfe* or breast fe* or breast-fe* or breastmilk or breast milk or babymilk or baby milk) or ((baby or babies or infant*) within 2 feed*))))) or((KW = (baby formula* or formula feed* or milk formula or formula fed or artificial milk or milk substitute* or infant formula* or synthetic milk or artificial feed* or bottle feed* or bottle fed)) and(KW = (disincentiv* or discourag* or tax or taxes or taxation or fined or fines or charg* or penal* or punish*)))
Maternity and Infant Care (1971 to May 2012)
URL: see www.ovid.com/site/catalog/databases/2694.jsp
-
“Salaries and fringe benefits”.de.
-
Patient compliance.de.
-
Quality assurance.de.
-
Health promotion.de.
-
Accreditation.de.
-
(incentive$or competit$or lottery or lotteries or stipend$or bonus$or cash or contest$or discount$or disincentive$or gift or gifts or price$or prize or prizes or raffle$or reward$or token or tokens or voucher$or win or won or award$or inducement or certifi$or accredit$or quality assurance or (contingen$adj3 payment$) or (tangible adj2 (goods or service$))).tw.
-
(fee or fees or tax or taxes or taxation or fined or fines or charg$or penal$or punish$).tw.
-
or/1–7
-
(Breastfeeding duration or Breastfeeding or Breastfeeding initiation).de.
-
(Infant feeding or Milk – human).de.
-
(breast?fe$or breast fe$).tw.
-
(breast?milk or breast milk or baby?milk or baby milk).tw.
-
((baby or babies or infant$) adj2 feed$).tw.
-
or/9–13
-
8 and 14
-
Infant formulae.de.
-
(baby formula$or formula feed$or milk formula or formula fed or artificial milk or milk substitute$or infant formula$or synthetic milk or artificial feed$or bottle feed$or bottle fed).tw.
-
16 or 17
-
(disincentiv$or discourag$or tax or taxes or taxation or fined or fines or charg$or penal$or punish$).tw.
-
18 and 19
-
15 or 20
Trials Register of Promoting Health Interventions (May 2012)
URL: see http://eppi.ioe.ac.uk/webdatabases/
-
Type(s) of intervention: incentives
-
Freetext: “incentiv*”
-
Freetext: “voucher*”
-
Freetext: “token*”
-
Freetext: stipend
-
Freetext: cash
-
Freetext: bonus
-
Freetext: “discount*”
-
Freetext: “disincentiv*”
-
Freetext: “gift*”
-
Freetext: “reward*”
-
Freetext: “accred*”
-
Freetext: “tax*”
-
Freetext: “penal*” or “punish*”
-
1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 OR 15
-
Freetext: “breast feeding”
-
Freetext: breastfeeding
-
Freetext: “breast fed”
-
Freetext: breastfed
-
Freetext: “breast feed”
-
Freetext: breastfeed
-
Freetext: “breast milk”
-
Freetext: “baby milk”
-
16 OR 17 OR 18 OR 19 OR 20 OR 21 OR 22 OR 23 OR 24
-
15 AND 24
-
9 OR 13 OR 14
-
Freetext: formula
-
Freetext: “bottle feed*”
-
27 OR 28
-
26 AND 29
-
25 OR 30
Websites
-
WHO (May 2012): see www.who.int
-
UNICEF (May 2012): see www.unicef.org
-
The King’s Fund (May 2012): see www.kingsfund.org.uk/
-
NICE (May 2012): see www.nice.org.uk
-
Royal College of Midwives (June 2012): see www.rcm.org.uk
-
Academy of Breastfeeding Medicine (June 2012): see www.bfmed.org/
Conference proceedings
-
UK Society for Behavioural Medicine (2011): see http://uksbm.org.uk/wp-content/uploads/2012/03/Combined-final-conference-proceedings-UKSBM-ASM-2011.pdf
-
UK Society for Behavioural Medicine (2010): see http://dl.dropbox.com/u/37425237/Proceedings/UKSBM-6th-Annual-Scientific-Meeting-Conference-Proceedings.pdf
-
Society of Behavioural Medicine (2012): see www.springerlink.com/content/l0883r1057877406/fulltext.pdf
-
Society of Behavioural Medicine (2011): see www.springerlink.com/content/w3563281v7567016/fulltext.pdf
-
American Public Health Association (2011): see http://apha.confex.com/apha/139am/webprogram/start.html
-
American Public Health Association (2010): see http://apha.confex.com/apha/138am/webprogram/start.html
-
European Public Health Conference (2011): see http://eurpub.oxfordjournals.org/content/21/suppl_1.toc
-
European Public Health Conference (2010): see http://eurpub.oxfordjournals.org/content/20/suppl_1/10.full.pdf+html
Appendix 8 Studies included in the smoking cessation review
Albrecht 1998
Albrecht S, Payne L, Stone CA, Reynolds MD. A preliminary study of the use of peer support in smoking cessation programs for pregnant adolescents. J Am Acad Nurse Pract 1998;10:119–25.
Cinciripini 2010
Cinciripini PM, Blalock JA, Minnix JA, Robinson JD, Brown VL, Lam C, et al. Effects of an intensive depression-focused intervention for smoking cessation in pregnancy. J Consult Clin Psychol 2010;78:44–54.
Cluss 2011
Cluss PA, Levine MD, Landsittel D. The Pittsburgh STOP program: disseminating an evidence-informed intervention for low-income pregnant smokers. Am J Health Promot 2011;25:S75–81.
Donatelle 2000
Primary
Donatelle RJ, Prows SL, Champeau D, Hudson D. Randomised controlled trial using social support and financial incentives for high risk pregnant smokers: Significant Other Supporter (SOS) program. Tob Control 2000;9(Suppl. 3):III67–9.
Secondary
Donatelle RJ, Prows SL, Champeau D, Hudson D. Using Social Support, Biochemical Feedback, and Incentives to Motivate Smoking Cessation During Pregnancy: Comparison of Three Intervention Trials. 128th Annual Meeting of the American Public Health Association, Boston, MA, 12–16 November 2000. Abstract 8260.
Donatelle RJ, Hudson D. Using 5 A’s and Incentives to Promote Prenatal Smoking Cessation. National Conference on Tobacco or Health, San Francisco, CA, 19–21 November 2002.
Edwards 2009
Edwards MJ, Geiser T, Chafin C, Weatherby NL, Smith CM. S.M.A.R.T. mothers are resisting tobacco: prenatal smoking cessation in WIC mothers. J Allied Health 2009;38:170–6.
Gadomski 2011
Gadomski A, Adams L, Tallman N, Krupa N, Jenkins P. Effectiveness of a combined prenatal and postpartum smoking cessation program. Matern Child Health J 2011;15:188–97.
Gulliver 2004
Gulliver SB, Colby SM, Hayes K, Raffa SD. Tobacco cessation treatment for pregnant smokers: incorporating partners and incentives. Med Health R I 2004;87:9–12.
Hartmann 2007
Hartmann KE, Wechter ME, Payne P, Salisbury K, Jackson RD, Melvin CL. Best practice smoking cessation intervention and resource needs of prenatal care providers. Obstet Gynecol 2007;110:765–70.
Heil 2008
Primary
Heil SH, Higgins ST, Bernstein IM, Solomon LJ, Rogers RE, Thomas CS, et al. Effects of voucher-based incentives on abstinence from cigarette smoking and fetal growth among pregnant women. Addiction 2008;103:1009–18.
Secondary
Heil SH, Higgins ST, Mongeon JA, Badger GJ, Bernstein IM. Characterizing nicotine withdrawal in pregnant cigarette smokers. Exp Clin Psychopharmacol 2006;14:165–70.
Heil SH, Higgins ST, Solomon LJ, Lynch ME, McHale L, Dumeer A, et al. Voucher-Based Incentives for Abstinence from Cigarette Smoking in Pregnant and Postpartum Women. 13th Annual Meeting of the Society for Research on Nicotine and Tobacco, Austin, TX, 21–24 February 2007. Abstract PA6-1.
Reynolds S. Vouchers boost smoking abstinence during pregnancy. Nida Notes 2010;23:10.
Higgins 2004
Primary
Higgins ST, Heil SH, Solomon LJ. A pilot study on voucher-based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine Tob Res 2004;6:1015–20.
Secondary
Higgins ST, Heil SH, Dumeer AM, Thomas CS, Solomon LJ, Bernstein IM. Smoking status in the initial weeks of quitting as a predictor of smoking-cessation outcomes in pregnant women. Drug Alcohol Depend 2006;85:138–41.
Higgins ST, Bernstein IM, Washio Y, Heil SH, Badger GJ, Skelly JM, et al. Effects of smoking cessation with voucher-based contingency management on birth outcomes. Addiction 2010;105:2023–30.
Higgins ST, Heil SH, Badger GJ, Skelly JM, Solomon LJ, Bernstein IM. Educational disadvantage and cigarette smoking during pregnancy. Drug Alcohol Depend 2009;104:S100–5.
Higgins TM, Higgins ST, Heil SH, Badger GJ, Skelly JM, Bernstein IM, et al. Effects of cigarette smoking cessation on breastfeeding duration. Nicotine Tob Res 2010;12:483–8.
Yoon JH, Higgins ST, Heil SH, Sugarbaker RJ, Thomas CS, Badger GJ. Delay discounting predicts postpartum relapse to cigarette smoking among pregnant women. Exp Clin Psychopharmacol 2007;15:176–86.
Latts 2002
Latts LM, Prochazka AV, Salas NM, Young DA. Smoking cessation in pregnancy: failure of an HMO pilot project to improve guideline implementation. Nicotine Tob Res 2002;4(Suppl. 1):25–30.
Lillington 1995
Lillington L, Royce J, Novak D, Ruvalcaba M, Chlebowski R. Evaluation of a smoking cessation program for pregnant minority women. Cancer Pract 1995;3:157–63.
Lowe 1997
Lowe JB, Windsor R, Balanda KP, Woodby L. Smoking relapse prevention methods for pregnant women: a formative evaluation. Am J Health Promot 1997;11:244–6.
Lynagh 2011
Lynagh M, Bonevski B, Symonds I, Sanson-Fisher RW. Paying women to quit smoking during pregnancy? Acceptability among pregnant women. Nicotine Tob Res 2011;13:1029–36.
Mantzari 2012
Mantzari E, Vogt F, Marteau TM. The effectiveness of financial incentives for smoking cessation during pregnancy: is it from being paid or from the extra aid? BMC Pregnancy Childbirth 2012;12:24.
McBride 2004
McBride CM, Baucom DH, Peterson BL, Pollak KI, Palmer C, Westman E, et al. Prenatal and postpartum smoking abstinence – a partner-assisted approach. Am J Prev Med 2004;27:232–8.
Morgan 2005
Morgan A, Bennett K, Hannon P, Weinberger J. Evaluation of a Smoke Stop service in a Sure Start programme. MIDIRS Midwifery Digest 2005;15:496–500.
Nichter 2007
Nichter M, Nichter M, Muramoto M, Adrian S, Goldade K, Tesler L, et al. Smoking among low-income pregnant women: an ethnographic analysis. Health Educ Behav 2007;34:748–64.
Pbert 2004
Pbert L, Ockene JK, Zapka J, Ma Y, Goins KV, Oncken C, et al. A community health center smoking-cessation intervention for pregnant and postpartum women. Am J Prev Med 2004;26:377–85.
Radley 2013
Radley A, Ballard P, Eadie D, MacAskill S, Donnelly L, Tappin D. Give it up for Baby: outcomes and factors influencing uptake of a pilot smoking cessation incentive scheme for pregnant women. BMC Public Health 2013;13:1.
Ripley-Moffitt 2008
Ripley-Moffitt CE, Goldstein AO, Fang WL, Butzen AY, Walker S, Lohr JA. Safe babies: a qualitative analysis of the determinants of postpartum smoke-free and relapse states. Nicotine Tob Res 2008;10:1355–64.
Ussher 2008
Ussher M, Aveyard P, Coleman T, Straus L, West R, Marcus B, et al. Physical activity as an aid to smoking cessation during pregnancy: two feasibility studies. BMC Public Health 2008;8:8.
Walsh 1997
Primary
Walsh RA, Redman S, Brinsmead MW, Byrne JM, Melmeth A. A smoking cessation program at a public antenatal clinic. Am J Public Health 1997;87:1201–4.
Secondary
Walsh RA, Redman S, Byrne JM, Melmeth A, Brinsmead MW. Process measures in an antenatal smoking cessation trial: another part of the picture. Health Educ Res 2000;15:469–83.
Appendix 9 Studies included in the breastfeeding review
Bai 2000
Bai Y, Wunderlich SM, Kashdan R. Inclusion of manual breast pump in hospital discharge bags promotes breastfeeding exclusivity. J Am Diet Assoc 2000;110(Suppl. 2):A112.
Bliss 1997
Bliss MC, Wilkie J, Acredolo C, Berman S, Tebb KP. The effect of discharge pack formula and breast pumps on breastfeeding duration and choice of infant feeding method. Birth 1997;24:90–7.
Cattaneo 2001
Cattaneo A, Borgnolo G, Simon G. Breastfeeding by objectives. Eur J Public Health 2001;11:397–401.
Chamberlain 2006
Chamberlain LB, McMahon M, Philipp BL, Merewood A. Breast pump access in the inner city: a hospital-based initiative to provide breast pumps for low-income women. J Hum Lact 2006;22:94–8.
Chiasson 2011
Chiasson MA, Findley S, Sekhobo J, Scheinmann R, Edmunds LS, Faly A, et al. Changing WIC changes what children eat. Obesity 2011;19:S48.
Cohen 1994
Cohen R, Mrtek MB. The impact of two corporate lactation programs on the incidence and duration of breast-feeding by employed mothers. Am J Health Promot 1994;8:436–41.
Dungy 1992
Dungy CI, Christensen-Szalanski J, Losch M, Russell D. Effect of discharge samples on duration of breast-feeding. Pediatrics 1992;90:233–7.
Finch 2002
Finch C, Daniel EL. Breastfeeding education program with incentives increases exclusive breastfeeding among urban WIC participants. J Am Diet Assoc 2002;102:981–4.
Hayes 2008
Hayes DK, Prince CB, Espinueva V, Fuddy LJ, Li R, Grummer-Strawn LM. Comparison of manual and electric breast pumps among WIC women returning to work or school in Hawaii. Breastfeed Med 2008;3:3–10.
Hill 1987
Hill PD. Effects of education on breastfeeding success. Matern Child Nurs J 1987;16:145–56.
Pugh 1998
Pugh LC, Milligan RA. Nursing intervention to increase the duration of breastfeeding. Appl Nurs Res 1998;11:190–4.
Rasmussen 2011
Rasmussen KM, Dieterich CM, Zelek ST, Altabet JD, Kjolhede CL. Interventions to increase the duration of breastfeeding in obese mothers: the Bassett Improving Breastfeeding Study. Breastfeed Med 2011;6:69–75.
Reeves Tuttle 1995
Reeves Tuttle C, Dewey KG. Impact of a breast-feeding promotion program for Hmong women at selected WIC sites in northern California. J Nutr Educ 1995;27:69–74.
Sciacca 1995
Primary
Sciacca JP, Phipps BL, Dube DA, Ratliff MI. Influences on breast-feeding by lower-income women – an incentive-based, partner-supported educational program. J Am Diet Assoc 1995;95:323–8.
Secondary
Sciacca JP, Dube DA, Phipps BL, Ratliff MI. A breast-feeding education and promotion program – effects on knowledge, attitudes, and support for breast-feeding. J Community Health 1995;20:473–90.
Thomson 2012
Thomson G, Dykes F, Hurley MA, Hoddinott P. Incentives as connectors: insights into a breastfeeding incentive intervention in a disadvantaged area of North-West England. BMC Pregnancy Childbirth 2012;12:22.
Volpe 2000
Volpe EM, Bear M. Enhancing breastfeeding initiation in adolescent mothers through the Breastfeeding Educated and Supported Teen (BEST) Club. J Hum Lact 2000;16:196–200.
Wolfberg 2004
Wolfberg AJ, Michels KB, Shields W, O’Campo P, Bronner Y, Bienstock J. Dads as breastfeeding advocates: results from a randomized controlled trial of an educational intervention. Am J Obstet Gynecol 2004;191:708–12.
Wright 2012
Wright SS, Lea CS, Holloman R, Cornett A, Harrison LM, Randolph GD. Using quality improvement to promote breast-feeding in a local health department. J Public Health Manag Pract 2012;18:36–42.
Zimmerman 1999
Zimmerman DR. You can make a difference: increasing breastfeeding rates in an inner-city clinic. J Hum Lact 1999;15:217–20.
Appendix 10 Grey literature
| Study | Country | Study design | Total n participants | Incentive and value when stated | Incentive contingent on smoking cessation | Validation | Primary outcome | Follow-up | Main outcomes reported |
|---|---|---|---|---|---|---|---|---|---|
| NHS North East Essex/Greenstead, Colchester and Harwich 2010 | UK | Non-randomised | 49 | Food vouchers given at 1 week, 4 weeks and 1 year after quitting | Yes | CO verification | Quit smoking, remain quit | 1 year | Of 18 women who were smoke free at 4 weeks, seven remained quit |
| NHS Telford and Wrekin | UK | Non-randomised | Unclear | Health trainer – weight focus – supervised exercise | Unclear | Unclear | Unclear | Unclear | Unclear |
| NHS Torbay and Somerset – Smokefree South West M2B (mums to be) | UK | Non-randomised | Unclear | Vouchers | Yes | CO verification | Quit smoking, remain quit | Unclear | Unclear |
| Robl 2010342 – abstract | USA | Non-randomised | 790 (738 set quit dates) | Incentives at three time points | Yes | CO verification | Quit smoking | Unclear | 340 quit smoking |
| Sheffield SOS343 | UK | Unclear | 490 | £140.00 | Unclear | Unclear | Unclear | Unclear | Unclear |
| Tobacco Free Futures344 | UK | Non-randomised | 18 | Financial vouchers given monthly | Yes | CO verification | Quit smoking, remain quit | After birth | 15/18 quit smoking |
| Study | Country | Study design | Total n participants | Incentive and value when stated | Contingency | Validation | Primary outcome | Follow-up | Main outcomes reported |
|---|---|---|---|---|---|---|---|---|---|
| Baby Café 2000–2012286 | UK (and international) | Non-randomised | Rolling programme, drop-in format. Supports 7800 mothers per year (2010–11 figure) | Provision of refreshments at drop-in breastfeeding support centres (provision of enhanced training and development for health professionals to upskill and engage) | Initiation/duration | Self-report | Any breast milk | None | 133 cafés (109 UK, 24 international) 21% of mothers attending Baby Café drop-ins are from low-income families, 20% are from ethnic backgrounds other than white British and 9% are ‘young’ mums; 12% are antenatal |
| Best Fed Babies 2002–345 | UK | Non-randomised | Rolling programme, voluntary | £50.00 voucher each month for up to 6 months antenatally and 3 months postnatally | Initiation/duration | Self-report with health visitor monitoring | Primary outcome is low birth weight. Any breast milk is a secondary outcome | Survey | Approximately 100 mothers per year, deprived/low-income area |
| Bristol Social Marketing Centre346 | UK | Non-randomised, before and after | Unclear – pilot | Bras, gift items, free bus travel | Initiation/duration | Self-report | Any breast milk | Evaluation | 16/85 women still breastfeeding at 1 week, 5/85 at 6 weeks and 3/85 at 6 months, although one figure cites 100 silver awards – unclear |
| Maternity rights comparison,347 France, Italy, UK | Europe | NA | NA | Paid maternity leave and paid nursing breaks | NA | NA | NA | NA | Unclear |
| North West PCTs 2010–11 | UK | Thomson et al.112 plus three other similar schemes | Fixed period programme – unclear | Gift items, leisure vouchers, shopping and fruit and vegetable vouchers | Initiation/duration | Self-report and informal monitoring | Any breast milk | Unclear | Increase in breastfeeding rates: Blackpool – 7.3% (n = 141); Ashton, Leigh and Wigan – 9–40% (n = 111); Halton and St Helen’s – unclear; Tameside and Glossop – unclear |
| NoSH (Nourishing Start for Health)348 | UK | Feasibility | Unclear | Financial vouchers | Prevalence and duration | Unclear | Unclear | Evaluation | Unclear |
| JFL349 | Canada | Policy, population | Unclear | C$37.50 per month supplement (some subsidised infant formula) | Prevalence | Affidavit from doctor | Any breast milk | Unclear | Unclear |
| Study | Country | Study design | Total n participants | Incentive and value when stated | Contingency | Validation | Primary outcome | Follow-up | Main outcomes reported |
|---|---|---|---|---|---|---|---|---|---|
| PATH350 | UK | Non-randomised | |||||||
| Stop for Life | 388 | Used PATH funding to support staff to run improved services | No | Self-report (CO at 1 month) | Quit smoking, remain quit | 12 months | 275 quit episodes, 70 quit at 1 month, 66 at 3 months, 17 at 12 months | ||
| Dundee Pregnancy and Smoking Project (DPSP) | 74 | Used PATH funding to provide a specialised support service | No | Self-report (CO at 1 month) | Quit smoking, remain quit | 12 months | 13 quit episodes, none quit at 1 month, one at 3 months and none at 12 months | ||
| Cross Lanarkshire Action Smoking in Pregnancy (CLASP) | 191 | Used PATH funding for a member of staff dedicated to a training and buddies programme | No | Self-report (CO at 1 month) | Quit smoking, remain quit | 12 months | 117 quit episodes, one quit at 1 month, two at 3 months and one at 12 months |
| Study | Country | Study design | Total n participants | Incentive and value when stated | Contingency | Validation | Primary outcome | Follow-up | Main outcomes reported |
|---|---|---|---|---|---|---|---|---|---|
| Payment by results for the family sector351 | UK | Non-randomised, before and after | Payment by results to children’s centres | Unclear | Initiation/duration | Self-report and informal monitoring | Any breast milk | Unclear | Unclear |
Appendix 11 Single electronic mixed-methods data extraction form
Appendix 12 Critical Appraisal Skills Programme quality assessment tool for qualitative data included in review studies135
Appendix 13 Quality of the studies included in the review of smoking cessation in pregnancy
| Study | Were the inclusion/exclusion criteria clearly described? | Was data collection undertaken prospectively? | Were participants a representative sample selected from a relevant patient population? | Was the selection of patients consecutive (for non-randomised studies/comparisons)? | Was the allocation sequence adequately generated (randomised studies/comparisons)? | Was allocation adequately concealed? | Were participants blind to treatment status? | Were the groups comparable on demographic characteristics and clinical features? | Were participants entering the study at a similar point in their disease progression? | Was the intervention (and comparison) clearly defined? | Was follow-up long enough to detect important effects on outcomes? | Was the length of follow-up similar between comparison groups? | Were the groups treated identically other than for the named intervention? | Was the intervention undertaken by someone experienced at performing the procedure? | Were the staff, place and facilities where the patients were treated appropriate for performing the procedure? | Were health-care providers ‘blind’ to treatment status? | Were all of the important outcomes considered? | Were all outcomes reported? | Were objective (valid and reliable) outcome measure(s) used? | Was the assessment of main outcomes blind? | Was there a description of withdrawals, dropouts and those lost to follow-up? | Was the analysis ITT in that trial results were reported for everyone who entered the trial? | Was the analysis ITT in that participants were analysed in the groups that they were originally allocated to? | Were participants lost to follow-up likely to introduce bias? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Albrecht 1998138 | Y | Y | U | U | U | N | U | Y | Y | Y | Y | Y | Y | U | N | Y | Y | U | U | Y | N | N | U | |
| Cinciripini 2010139 | Y | Y | U | N | U | U | Y | N | Y | Y | Y | Y | Y | Y | N | Y | N | Y | U | Y | Y | Y | U | |
| Cluss 2011140 | Y | Y | Y | N | NA | NA | NA | N | Y | Y | NA | NA | Y | Y | NA | Y | Y | Y | NA | N | Y | NA | U | |
| Donatelle 2000141 | Y | Y | Y | U | U | N | Y | Y | Y | Y | Y | Y | Y | Y | U | Y | Y | Y | U | Y | Y | Y | U | |
| Edwards 2009142 | U | N | U | Y | NA | NA | NA | N | Y | Y | NA | NA | Y | Y | NA | Y | Y | N | NA | N | N | NA | U | |
| Gadomski 2011143 | Y | Y | Y | Y | NA | U | N | U | Y | Y | Y | N | Y | Y | N | Y | N | Y | U | Y | N | N | U | |
| Gulliver 2004104 | Y | Y | U | U | U | N | U | U | Y | Y | Y | Y | Y | Y | N | U | N | Y | U | N | U | U | U | |
| Heil 2008105 | Y | Y | Y | U | U | N | Y | Y | Y | Y | Y | Y | Y | Y | U | Y | Y | Y | U | Y | Y | Y | U | |
| Higgins 2004144 | Y | Y | Y | U | U | U | Y | U | Y | Y | Y | Y | Y | Y | U | Y | Y | Y | U | Y | Y | Y | U | |
| Lillington 1995145 | Y | Y | Y | U | U | N | Y | N | Y | Y | Y | Y | Y | Y | U | Y | Y | N | U | U | N | Y | U | |
| Lowe 1997146 | Y | Y | Y | U | U | U | Y | Y | Y | Y | Y | U | Y | Y | N | Y | Y | Y | U | N | Y | Y | U | |
| Mantzari 2012109 | U | Y | U | N | NA | N | Y | U | U | Y | U | U | U | U | U | U | U | N | U | N | U | Y | U | |
| McBride 2004147 | Y | Y | Y | U | U | N | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | N | U | Y | Y | Y | U | |
| Morgan 2005148 | N | N | U | U | NA | NA | NA | N | Y | Y | NA | NA | Y | Y | NA | Y | Y | Y | NA | N | U | NA | U | |
| Nichter 2007110 | Y | Y | U | U | NA | NA | NA | N | Y | U | NA | NA | Y | Y | NA | Y | Y | Y | NA | N | U | NA | U | |
| Pbert 2004149 | Y | Y | Y | U | U | U | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | U | Y | N | Y | U | |
| Radley 2013113 | Y | Y | Y | Y | NA | NA | NA | U | Y | Y | NA | NA | Y | Y | NA | Y | Y | Y | NA | N | Y | NA | U | |
| Ripley-Moffitt 2008111 | Y | Y | U | U | NA | NA | NA | Y | U | Y | NA | NA | Y | Y | NA | Y | Y | Y | NA | N | U | NA | U | |
| Ussher 2008150 | Y | Y | Y | Y | NA | NA | NA | Y | Y | Y | NA | NA | Y | Y | NA | Y | Y | Y | NA | N | U | NA | U | |
| Walsh 1997108 | Y | Y | Y | Y | Y | N | Y | U | Y | Y | Y | Y | Y | Y | N | Y | Y | Y | U | U | Y | Y | U | |
| Y | 17 | 18 | 12 | 5 | 1 | 0 | 10 | 8 | 18 | 19 | 12 | 10 | 19 | 18 | 0 | 18 | 16 | 15 | 0 | 8 | 9 | 10 | 0 | |
| N | 1 | 2 | 0 | 3 | 0 | 8 | 1 | 6 | 0 | 0 | 0 | 1 | 0 | 0 | 8 | 0 | 3 | 4 | 0 | 10 | 5 | 2 | 0 | |
| U | 2 | 0 | 8 | 12 | 10 | 5 | 2 | 6 | 2 | 1 | 1 | 2 | 1 | 2 | 5 | 2 | 1 | 1 | 13 | 2 | 6 | 1 | 20 | |
| NA | 0 | 0 | 0 | 0 | 9 | 7 | 7 | 0 | 0 | 0 | 7 | 7 | 0 | 0 | 7 | 0 | 0 | 0 | 7 | 0 | 0 | 7 | 0 | |
| Total | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 | |
| % Y | 85 | 90 | 60 | 25 | 5 | 0 | 50 | 40 | 90 | 95 | 60 | 50 | 95 | 90 | 0 | 90 | 80 | 75 | 0 | 40 | 45 | 50 | 0 | |
| % N | 5 | 10 | 0 | 15 | 0 | 40 | 5 | 30 | 0 | 0 | 0 | 5 | 0 | 0 | 40 | 0 | 15 | 20 | 0 | 50 | 25 | 10 | 0 | |
| % U | 10 | 0 | 40 | 60 | 50 | 25 | 10 | 30 | 10 | 5 | 5 | 10 | 5 | 10 | 25 | 10 | 5 | 5 | 65 | 10 | 30 | 5 | 100 | |
| % NA | 0 | 0 | 0 | 0 | 45 | 35 | 35 | 0 | 0 | 0 | 35 | 35 | 0 | 0 | 35 | 0 | 0 | 0 | 35 | 0 | 0 | 35 | 0 | |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | |
Appendix 14 Quality of the studies included in the review of breastfeeding
| Study | Were the inclusion/exclusion criteria clearly described? | Was data collection undertaken prospectively? | Were participants a representative sample selected from a relevant patient population? | Was the allocation sequence adequately generated? | Was allocation adequately concealed? | Were participants blind to treatment status? | Were the groups comparable on demographic characteristics and clinical features? | Were participants entering the study at a similar point in their disease progression? | Was the intervention (and comparison) clearly defined? | Was follow-up long enough to detect important effects on outcomes of interest? | Was length of follow -up similar between comparison groups? | Were the groups treated identically other than for the named intervention? | Was the intervention undertaken by someone experienced at performing the procedure? | Were the staff, place and facilities where the patients were treated appropriate for performing the procedure? | Were health-care providers 'blind' to treatment status? | Were all of the important outcomes considered? | Were all outcomes reported? | Were objective (valid and reliable) outcome measure(s) used? | Was the assessment of the main outcomes blind? | Was there a description of withdrawals, dropouts, and those lost to follow-up? | Was the analysis ITT in that trial results were reported for everyone who entered the trial? | Was the analysis ITT in that participants were analysed in the groups that they were originally allocated to? | Were participants lost to follow-up likely to introduce bias? |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bliss 1997167 | Y | Y | Y | N | U | NA | U | Y | Y | Y | Y | Y | Y | Y | NA | Y | Y | Y | U | Y | U | Y | U |
| Cattaneo 2001168 | Y | Y | Y | NA | NA | NA | NA | Y | Y | Y | Y | U | Y | Y | NA | Y | N | Y | NA | N | NA | NA | U |
| Chamberlain 2006103 | N | N | U | Y | NA | NA | U | Y | Y | Y | Y | U | Y | Y | NA | Y | N | U | NA | NA | NA | NA | U |
| Cohen 1994169 | Y | Y | U | U | NA | NA | NA | Y | Y | Y | NA | NA | Y | Y | NA | Y | Y | Y | NA | N | NA | NA | U |
| Dungy 1992170 | Y | Y | N | U | U | N | U | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | U | U | Y | N | Y | Y |
| Finch 2002171 | Y | Y | Y | U | U | N | U | Y | N | Y | Y | Y | Y | Y | N | Y | Y | U | U | N | N | Y | Y |
| Hayes 2008172 | Y | Y | Y | U | NA | NA | Y | Y | Y | Y | Y | Y | Y | Y | NA | Y | Y | U | U | N | N | Y | U |
| Hill 1987173 | Y | Y | Y | U | U | N | N | U | Y | Y | Y | Y | U | Y | N | Y | Y | U | U | N | Y | Y | U |
| Pugh 1998106 | Y | Y | Y | U | U | NA | Y | Y | Y | Y | Y | Y | Y | Y | NA | Y | Y | U | U | N | U | U | U |
| Rasmussen 2011174 | Y | Y | Y | U | NA | NA | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | U | Y | Y | N | Y | U | U |
| Reeves Tuttle 1995175 | Y | N | Y | U | NA | NA | U | Y | N | Y | Y | Y | Y | Y | NA | Y | Y | U | NA | N | NA | NA | U |
| Sciacca 1995176 | Y | Y | Y | U | U | N | N | Y | N | Y | Y | Y | Y | Y | N | Y | Y | U | U | N | N | Y | U |
| Thomson 2012112 | N | N | Y | U | NA | NA | Y | U | Y | Y | Y | U | Y | Y | NA | Y | Y | Y | NA | Y | NA | NA | U |
| Volpe 2000107 | N | U | Y | U | NA | NA | Y | Y | Y | Y | Y | Y | Y | Y | NA | Y | N | U | NA | NA | NA | NA | U |
| Wolfberg 2004178 | Y | Y | Y | U | U | N | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | U | U | Y | N | Y | Y |
| Zimmerman 1999179 | Y | N | Y | U | NA | NA | N | Y | Y | Y | Y | U | Y | Y | NA | Y | Y | U | NA | Y | NA | NA | U |
| Y | 13 | 11 | 13 | 1 | 0 | 1 | 6 | 14 | 13 | 16 | 15 | 11 | 15 | 16 | 2 | 16 | 12 | 5 | 1 | 5 | 2 | 7 | 3 |
| N | 3 | 4 | 1 | 1 | 0 | 5 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 4 | 0 | 3 | 0 | 0 | 9 | 5 | 0 | 0 |
| U | 0 | 1 | 2 | 13 | 7 | 0 | 5 | 2 | 0 | 0 | 0 | 4 | 1 | 0 | 0 | 0 | 1 | 11 | 8 | 0 | 2 | 2 | 13 |
| NA | 0 | 0 | 0 | 1 | 9 | 10 | 2 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 10 | 0 | 0 | 0 | 7 | 2 | 7 | 7 | 0 |
| Total | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 | 16 |
| % Y | 81.3 | 68.8 | 81.3 | 6.3 | 0.0 | 6.3 | 37.5 | 87.5 | 81.3 | 100.0 | 93.8 | 68.8 | 93.8 | 100.0 | 12.5 | 100.0 | 75.0 | 31.3 | 6.3 | 31.3 | 12.5 | 43.8 | 18.8 |
| % N | 18.8 | 25.0 | 6.3 | 6.3 | 0.0 | 31.3 | 18.8 | 0.0 | 18.8 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 25.0 | 0.0 | 18.8 | 0.0 | 0.0 | 56.3 | 31.3 | 0.0 | 0.0 |
| % U | 0.0 | 6.3 | 12.5 | 81.3 | 43.8 | 0.0 | 31.3 | 12.5 | 0.0 | 0.0 | 0.0 | 25.0 | 6.3 | 0.0 | 0.0 | 0.0 | 6.3 | 68.8 | 50.0 | 0.0 | 12.5 | 12.5 | 81.3 |
| % NA | 0.0 | 0.0 | 0.0 | 6.3 | 56.3 | 62.5 | 12.5 | 0.0 | 0.0 | 0.0 | 6.3 | 6.3 | 0.0 | 0.0 | 62.5 | 0.0 | 0.0 | 0.0 | 43.8 | 12.5 | 43.8 | 43.8 | 0.0 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Appendix 15 Illustrative topic guide
BIBS: Benefits of Incentives for Breastfeeding and Smoking cessation
Introduction
-
Provide a reminder of the purpose and main focus of the study.
-
Explain that the focus is on opinions and experiences (it is not a test).
-
Provide the opportunity to ask ‘any questions’.
-
Introduce the audio recorder, underlining the importance of confidentiality.
Topic guide
-
What do you think about public health messages that target specific behaviours (e.g. not smoking/drinking during pregnancy, women breastfeeding their infants, eating 5 a day)?
-
Prompts:
-
Are they effective?
-
If not – why not?
-
Why do you think people may not act on these messages?
-
-
-
What do you think might influence or help change people’s behaviours?
-
Prompts:
-
Internal motivators (e.g. motivation, determination).
-
External (e.g. family members and family structure, social network, economic) motivators.
-
Explore personal histories in terms of those who smoke/previously quit and who breastfed/did not breastfeed to explore motivations and influences on these behaviours.
-
-
-
Did you know that incentives have been used to promote healthy messages and to try and change people’s behaviours (e.g. for smoking cessation, breastfeeding, weight loss)? These interventions have involved financial elements (e.g. giving people vouchers or gifts or offering crèche facilities or ironing) and/or non-financial elements (e.g. education, counselling, leaflets).
-
Have you ever been involved in any schemes or programmes that have used ‘incentives’ (financial or non-financial)?
-
Prompts:
-
What incentives were there?
-
Context of delivery (who delivered, where, timing).
-
Success of schemes/reasons for failure.
-
Attitudes towards incentives.
-
-
-
Introduce vignettes and explain to the participant that we are asking them to read real studies that have involved incentive interventions for pregnant women to encourage them to stop smoking or to start and continue breastfeeding. Any feedback?
-
Ask about what types of ‘incentive’ the participant thinks may/would work and why (tease out differences for smoking and for breastfeeding where appropriate).
-
Prompts:
-
Who should be incentivised (individuals, organisations or both)?
-
Do you think that incentive programmes need to include financial elements (e.g. vouchers, gifts), non-financial elements (e.g. support/education) or both?
-
What (non-financial) elements should the incentive programme include and why? Explore opinions regarding different intervention components, (e.g. education, counselling, information leaflets including social support).
-
What (if any) financial incentives should be provided (e.g. vouchers, gifts, money, baby products)? What should be the value of these financial incentives?
-
Who should deliver the incentives (e.g. health professionals, GPs, voluntary sector)?
-
When should they be delivered (e.g. after the ‘target’ behaviour is achieved or ongoing)?
-
How many times should the incentive be provided (e.g. voucher each time or name in a monthly raffle)?
-
How many contacts should be provided (e.g. every day, every week, every month)?
-
How long should the incentive intervention last?
-
How should the incentive intervention be delivered (e.g. group, individual, with partner/friend)?
-
Where should incentives be delivered (e.g. hospital, community, clinic, home)?
-
How should ‘compliance’ be assessed (e.g. saliva samples, CO samples) and how often? What about compliance for breastfeeding women – should this be assessed and how?
-
-
-
Any final comments/feedback?
Appendix 16 Vignettes for health professionals
BIBS: Benefits of Incentives for Breastfeeding and Smoking Cessation
Heil and colleagues105
At 18 weeks’ gestation, women are recruited to a smoking cessation intervention that involves daily, biweekly and then weekly contacts until the baby is born, with further weekly and fortnightly contacts up to 12 weeks postnatally (with a final contact at 24 weeks). Urine and CO tests are used to confirm smoking status on each occasion, and women are given opportunities to read/review smoking cessation information with a health worker. A voucher is given that increases in value (maximum of £40.00) each time a negative smoking test is confirmed, but values are reset if a positive test is received.
Chamberlain and colleagues103
A mother is offered a personal-use double electric breast pump (worth £120.00–225.00, her brand of choice), which can be delivered to the hospital or to her home following the birth of her baby. While the mother is breastfeeding, she will have access to a breastfeeding specialist in the hospital and be given a number for a breastfeeding telephone support line that she can call from home.
Cattaneo and colleagues168
The regional health authority has requested that LHAs develop local work plans and targets to increase breastfeeding rates (at birth and 16–19 weeks postnatally). All staff working within the health authority are told that a financial penalty will be applied if they do not achieve their objectives and targets.
Tappin and colleagues88
Pregnant smokers are given a £50.00 voucher for attending an appointment with a NHS Smokefree Pregnancy advisor and setting a quit date. They are given an additional £50.00 voucher for being smoke free 4 weeks after their quit date and another £100.00 voucher for being smoke free after 12 weeks. If they are still smoke free towards the end of their pregnancy, they are given a further £200.00 voucher. Vouchers can be exchanged at many retailers.
Appendix 17 The Cessation in Pregnancy Incentives Trial illustrative topic guide: one-to-one interviews with pregnant women participating in study
Themes for interviews with pregnant women who have agreed to be interviewed when first approached about the trial
One-to-one interviews with pregnant women (all of whom have engaged with the cessation service and the CPIT and agreed to be interviewed) aim to explore views on issues around the delivery and promotion of the cessation service, responses to incentive features and randomisation and any unintended consequences.
Respondents will be asked to respond to the concept of the incentives and the trial elements in addition to experience of the routine services.
Interviews will be adapted depending on experience, for example intervention or control status, successful quit or relapse.
The topic guide is intended to ensure coverage of key topics while at the same time giving respondents the freedom to express their own feelings and views as part of an open discussion.
Introduction
-
Provide a reminder of the purpose and main focus of the study.
-
Explain that the focus is on opinions and experiences (not a test).
-
Provide an opportunity to ask ‘any questions’.
-
Introduce the audio recorder, underlining the importance of confidentiality.
A. Background: broad smoking history and pregnancy/child responsibilities
-
How long smoked/how many a day/how easy to stop (anticipated/experienced)?
-
Any previous quit attempts/relapse events (including any previous pregnancies)?
-
Number of (non-)smokers in household?
-
Number of children in household (including ages)?
-
When baby due?
-
Any arrangements/consideration given to smoking in the home (self and others)?
B. Focus on service experience in this pregnancy
Events/circumstances leading up to quit attempt
-
Motives for trying to quit, extent to which planned and significant trigger events – what prompted action/decision (including health, incentive, client–advisor/midwife relationship, pregnancy, partner’s influence)?
-
Initial awareness of quit support in pregnancy, for example leaflets, interpersonal recommendation from professionals or peers, local pharmacist.
Initial contact(s) with NHS Smokefree Pregnancy service adviser
Explore telephone explanation or drop in.
-
Expecting a telephone call from the advisor (e.g. aware of opportunity to opt out of call, giving permission).
-
Initial explanation of study (by telephone or at drop in).
-
Giving permission for name to be passed to The Listening Company (TLC) (now called Serco Ltd), which was the call centre company that obtained the formal telephone consent in CPIT.
-
Initial visit.
-
Motivation to attend for first visit (e.g. health of self/baby, any feeling of compulsion from professionals or significant others).
-
Steps involved in the first visit (e.g. timing of appointment, location, how long did it take, how much help was provided, ways it could be improved).
-
Setting a quit date (e.g. advice on choice, influences on success).
-
Understanding about any further support from adviser (e.g. telephone contacts, frequency).
Further contacts by NHS Smokefree Pregnancy service adviser
(Typically telephone)
-
Experience of any further contacts/support.
-
Response, for example helpful, intrusive.
-
Impact, for example encouraging, positive, annoying, guilt inducing.
Experience of visit(s)/contacts at community pharmacists if any
-
Use of pharmacological aids to help give up (e.g. NRT).
-
Steps involved in the first visit to pharmacy (e.g. timing, location, staff giving support, how long did it take, how much help was provided, ways it could be improved).
-
Anticipate returning for any follow-up visits (e.g. CO breath tests, NRT provision, staff seen, additional benefits/drawbacks).
-
Perceived support (e.g. how helpful/supportive/how did they help/same person each time/any restrictions on when/where, level of privacy provided?)
Initial contact by the NHS Smoking Helpline (TLC)
Explore initial call:
-
response to contact details being routinely passed to helpline and memory of being asked and giving verbal consent
-
contact practicalities, for example time of day/week, impact on activities at that time
-
recognition of number when called – remembered that told might be called
-
response to time delay for TLC call from when agreed with advisor (too long a delay, too quick?)
-
response to explanation given of the study please note Incentives and Control and RCT
-
understanding of vouchers/gift card and how triggered and delivered
-
extent to which the contact fitted expectations
-
motivation to commit to the study.
C. Focus on the incentives
Initially unprompted and general (explain process as needed)
-
Ever heard of incentives to encourage quitting before (financial or alternative)?
-
General response to concept of incentives to quit (financial or alternatives).
-
What might change people’s commitment?
Explore respondent’s understanding of the voucher structure.
Prompt as required:
Incentive group
-
£50.00 if attend and set quit date.
-
£50.00 if quit at 4 weeks – CO validated (Smokefree Pregnancy service advisor telephones, research nurse visits self-reported non-smokers for CO reading).
-
£100.00 if quit at 12 weeks – CO validated (telephone call, research nurse visits self-reported non-smokers for CO reading).
-
£200.00 if still quit at end of pregnancy – cotinine validated at 34–38 weeks.
-
£25.00 if not quit at 34–38 weeks – cotinine validated at 34–38 weeks.
Control group
£25.00 for control participants at 34–38 weeks if agree and give sample for cotinine testing.
-
Impact of incentive on motivation, for example initial seeking of support, ongoing resolve.
-
Ways they would use/have used incentives, for example to buy routine items, save for a ‘big’ thing.
-
Views on payment points and amounts/breakdown of voucher values.
-
Would a different amount of money make a difference?
-
Is £25.00 enough to support contact in late pregnancy and sample collection for control quitters?
Monitoring
-
CO reading at 4 and 12 weeks – incentive group.
-
Saliva/urine test with visit near end of pregnancy – all claiming non-smokers.
-
Views on monitoring – is this enough monitoring, easy/fair/effective?
-
Intrusion factor.
-
Arranging visit for monitoring.
-
Experience of monitoring visits (and/or any anticipated issues).
-
Receiving vouchers/card (as appropriate)
-
How have they arrived? Timing, format, fit with expectations.
-
Timing of arrival.
-
Ease of using in store – which store, staff response, any awareness that on a ‘scheme’?
-
Views on any additional benefits that could be included as part of the scheme – for mum/significant others.
-
Any other ways would benefit from the scheme, for example well-being, involvement in a study, encouraged others to stop/cut down/modify where and when they smoke?
-
Likelihood of anyone trying to get around the rules/likelihood of happening.
Promoting the incentive scheme
-
Key messages/ideas on ‘selling points’ for women.
-
Ways/routes to promote incentives and the general service.
Appendix 18 The Cessation in Pregnancy Incentives Trial illustrative topic guide: interviews with professionals with a relevant role (e.g. with pregnant smokers, cessation services and/or the trial)
Interviews with professionals aim to explore issues around implementation of the intervention and the trial elements, identify challenges and ways they have been overcome, and perceived response among participants.
Interviews will be adapted depending on respondents’ experience.
The topic guide is intended to ensure coverage of key topics while at the same time giving respondents the freedom to express their own feelings and views as part of an open discussion.
Introduction
-
Provide a reminder of the purpose and main focus of the study.
-
Explain that the focus is on opinions and experiences (not a test).
-
Provide an opportunity to ask ‘any questions’.
-
Introduce the audio recorder, underlining the importance of confidentiality.
A. Background: overview of role and relevant involvement
-
General role.
-
Involvement with cessation of smoking in pregnancy.
-
Involvement in the trial.
B. Focus on NHS Smokefree Pregnancy service and key trial elements
Service developments to facilitate the intervention
-
Cessation specialist staffing – extra specialist staff, training, funding issues.
-
Clerical staffing – training, base location, etc.
-
Information technology needs – programme systems, computers, issues around office space/location.
-
Routine CO testing at antenatal clinic to trigger referrals.
-
Making midwifery staff aware – how, at what stage?
-
Any links with community pharmacists.
-
Explore challenges and opportunities in preparation for this intervention, for example caseloads, telephone contacts.
-
Perceived response from range of staff.
Then . . :
-
explore general awareness of elements of the support services and response to delivery issues aware of, including key opportunities and challenges
-
explore general awareness of the trial elements and response to implementation issues that they are aware of, including key opportunities and challenges.
Focus on the following key elements and stages. When not already covered in the initial discussion, obtain response to relevant issues.
For each one, first explore initial awareness and perceptions, then explain the element if required and explore responses. Not all will be relevant to individual experience.
Initial booking visit
-
Asking pregnant women about their smoking.
-
Taking CO readings and recording.
-
Asking permission to send information to the NHS Smoking Helpline.
-
Explore challenges and opportunities, for example demanding appointment session, smokers’ responses, preparation for this activity.
-
Any previous awareness of incentives and RCT among women/comments?
First telephone contact with NHS Smokefree Pregnancy adviser
-
Challenges of making contact by telephone (including out of hours).
-
Informing about the study.
-
Verbal permission to forward details to TLC.
-
Perceived clients’ response to service (e.g. attending session, challenges to quitting).
-
Any raising of trial and trial status by clients.
-
Perceived clients’ response to trial (e.g. impact of incentives, random allocation).
-
Implementation issues (e.g. capacity issues in covering appointments).
Explore ‘drop-ins’ – process, benefits and draw-backs.
First telephone contact by TLC (NHS Helpline)
-
Smokers contacted by TLC.
-
Giving eligible smokers information about the trial.
-
Obtaining verbal consent.
-
Random allocation made.
-
Explore views on challenges experienced or anticipated (e.g. contact successes, explaining complex issues, response to random allocation, perceived effect on engaging with services).
First face-to-face contact with NHS Smokefree Pregnancy adviser (£50.00 voucher)
-
Discussion about smoking and pregnancy.
-
Setting quit date.
-
Assess for NRT use/local pharmacy identified.
-
Returning attendance information to trial manager.
-
Perceived clients’ response to service (e.g. attending session, challenges to quitting).
-
Any raising of trial and trial status by clients.
-
Perceived clients’ response to trial (e.g. impact of incentives, random allocation).
-
Implementation issues (e.g. capacity issues in covering appointments, additional work loads, extra staff).
Follow-up telephone support/contact from NHS Smokefree Pregnancy service
-
Telephone support provided weekly until 4 weeks after quit date to support quit attempt.
-
Follow-up telephone contact at 4 weeks after quit date (further discussion and information on self-reported outcome at 4 weeks).
-
Capacity issues, making contact, client availability/co-operation, records, etc.
Attendance at local community pharmacy for nicotine replacement therapy
-
Patient attends local pharmacy to receive NRT.
-
CO breath test carried out and link with NRT provision.
-
Weekly repeat CO breath tests and further NRT for 4 weeks.
-
Implementation issues, training, any additional workloads, record keeping, etc.
Follow-up telephone contact from NHS Smokefree Pregnancy service (£50.00, 4-week quit)
-
Telephone support provided weekly until 4 weeks after quit date to support quit attempt.
-
Follow-up telephone contact at 4 weeks after quit date (further discussion and information on self-reported outcome at 4 weeks).
-
Capacity issues, making contact, client availability/co-operation, records, etc.
Check respondent understanding of voucher structure and explore their response
Prompt as required or use flow chart:
Incentive group
-
£50.00 if attend and set quit date.
-
£50.00 if quit at 4 weeks – CO validated (Smokefree Pregnancy service advisor telephones, Margaret visits self-reported non-smokers for CO reading).
-
£100.00 if quit at 12 weeks – CO validated (??? telephones, Margaret visits self-reported non-smokers for CO reading).
-
£200.00 if still quit at end of pregnancy – cotinine validated at 34–38 weeks.
-
£25.00 if not quit at 34–38 weeks – cotinine validated at 34–38 weeks.
Control group
-
£25.00 for control participants at 34–38 weeks if agree and give sample for cotinine testing.
Research nurse visits:
Carbon monoxide monitoring of incentive group who report abstinence
-
Smokefree Pregnancy service advisors contact to ascertain status.
-
Arranging home visit.
-
Issues in the home setting.
End-stage assessment of outcomes at 34–38 weeks’ gestation, contact with research nurse for participants who self-report as abstinent
-
NHS Smoking Helpline makes telephone contact to ascertain self-report?
-
If not currently abstinent then £25.00 voucher sent to participant.
-
If abstinent research nurse visit to check on abstinence by urine/saliva test for cotinine.
-
Implementation issues, telephone contacts, home visits, sample collection.
Central recording and posting out of vouchers
-
Set a quit date and arrived at face-to-face appointment.
-
Self-report abstinent intervention participants at 4 weeks.
-
Self-report abstinent intervention participants at 12 weeks.
-
Chemically confirmed abstinence at 34–38 weeks.
-
Others providing information at 34–38 weeks, for example control participants, non-abstinent.
-
Issues regarding obtaining and collating records from varied sources.
C. Overview
Responses to concept of incentives: respondent’s views and perceptions of views of others (e.g. pregnant women, professionals and general public)
-
Incentives in general to support behaviour change (financial and alternatives).
-
Incentives as provided in current RCT.
-
Perceptions of any benefits from incentives in supporting quitting – pregnant women/other smokers.
-
Perceptions of any unintended outcomes.
-
Implications for those not receiving incentives.
Capacity and professional support issues
-
Training needs and support (e.g. time issues).
-
Capacity/any extra workload.
-
Support in changes required by the study.
-
Access to central advice if needed (e.g. regarding study, regarding cessation in pregnancy).
Comparative overview
-
Elements of the service that appear most/least useful – why?
-
Comparison of the likely impact of incentives with the other elements of the service in terms of its help/usefulness in the early stages (getting started)/later stages (keeping stopped).
-
Likely impact of being part of a study.
-
Terms to describe the incentive scheme to pregnant women/what are key aspects that women need to know/how best communicated.
-
Main benefits/main drawbacks.
-
Things that might help more/improve the service.
Impressions on progress
Any additional learning for future studies?
D. Focus on trial
Explore understanding of randomised controlled trial, randomisation, incentives
Prompt with process as needed:
-
Response to being ‘randomised’ to one or the other.
-
Likely impact of exclusion from incentives among control participants because of study.
-
What would women want to know?
-
Likely motivation to commit to the study.
-
Potential to break the rules?
E. Any additional support: ‘professionals’
-
Any other support from ‘professionals’ to help give up – what, how often, who from (e.g. midwife, GP, other)?
-
Most important features of any other professional support received – why/any way it could be improved?
-
Any discouraging responses from ‘professionals’.
F. Any additional support: family and friends
-
Support/dis-encouragement from other family members.
-
Any ‘significant others’ changed their smoking behaviour/used quit support.
-
Attend stop smoking sessions with anyone else for support.
G. Review of quit/smoking experience in this pregnancy (if not already covered)
-
Extent quit in this pregnancy thus far, for example non-smoking, cut down, relapses.
-
When stopped, how long for, when any relapses.
-
Expectations for future during pregnancy, after pregnancy.
H. Overview/comparisons
-
Elements of the service that are most/least useful – why?
-
Relative usefulness of key elements in the early stages (getting started)/later stages (keeping stopped).
-
Impact of being part of a study, for example motivation, impact of being a ‘control’ participant.
-
Terms to describe the incentive scheme to another pregnant mother/what are key aspects women need to know?
-
Main benefits/drawbacks.
-
Things that might help more/improve the service.
Appendix 19 Final framework used in NVivo10
-
What is an incentive and what do incentives mean to parents?
-
Hedonic – shopping vouchers.
-
Behaviour related, for example breast pumps, nursing bras.
-
Personal well-being – massage, beauty treatment.
-
Baby or pregnancy related, for example baby gifts, nappies, bibs, car seats.
-
Health related – fruit and vouchers.
-
Household/time (to breastfeed)-related services – ironing, crèche, school run, meals.
-
Individualised incentives.
-
Negative attitudes towards incentives (undefined/general remarks).
-
-
Would incentives work and how might they work? (Fit with behaviour change theory evidence, interactions of incentives with intrinsic motivation.)
-
Incentives to encourage women to turn up for support/participate in a programme or research (recruiting and retaining).
-
Incentives to prevent smoking relapse/stopping breastfeeding.
-
Incentives contingent on success.
-
Predictable or unpredictable (raffles, competitions).
-
Monitoring and proof.
-
Gaming, cheating.
-
Goal-setting – experiences, breastfeeding duration goal, exclusivity goal, setting a quit date.
-
Incentives for those already engaged (boosters) or ‘ready’ (motivated) to change.
-
Confidence/self-efficacy/pressure.
-
Negative views – beliefs that incentives will not work.
-
Incentivising more than one behaviour.
-
-
What else would help as well as/besides an incentive? (Other intervention components.)
-
What would help most – incentive vs. other support?
-
Psychosocial component (only when referred to in conjunction with incentive programme).
-
Pharmacological support (e.g. NRT).
-
Who would be the best person to provide this?
-
Psychologist.
-
Maternity professional (midwife/health visitor).
-
GP/pharmacist.
-
Peer supporter.
-
‘Other’.
-
Specialist service (smoking/breastfeeding is all they do).
-
Known vs. unknown (continuity of care).
-
First-hand experience (could apply to peer or professional).
-
-
-
Incentivising other people
-
Woman alone.
-
All women vs. targeting most disadvantaged women.
-
Woman plus supporter.
-
Family/home.
-
Individual health professionals to provide support.
-
The local health service – to reach targets.
-
‘Other’.
-
If others incentivised – same or different value of incentive as for women.
-
-
Value/costs
-
Financial value of the incentive.
-
Consistent or variable value, for example increase the longer the woman is quit for, for example increase at specific time points such as birth.
-
Who pays for incentives?
-
Local or national businesses contributing (vouchers/support).
-
-
Views of/effects on others
-
Non-smokers/bottle feeders.
-
Targeted to most disadvantaged vs. universal incentives.
-
General public/taxpayers.
-
Incentives perceived as acceptable to others because of benefits to child health when pregnant compared with smoking/good nutrition/healthy lifestyle for everyone (all ages, genders).
-
Unintended consequences for those not receiving incentives, for example friends, families more generally.
-
-
Intensity and duration (appointments/sessions)
-
How often/frequent/appointment duration – small and often vs. longer but less frequent?
-
Constant or tailored – more frequent to begin with, frequent after birth to prevent relapse, around life events/periods of stress?
-
Start of programme – when in pregnancy? End point – after birth, how long after birth?
-
-
Setting and delivery: how would it be best to provide the incentive?
-
Post.
-
Part of the health service.
-
Separate from the health service.
-
Home.
-
Telephone call/text message.
-
Other location.
-
Format – one-to-one, group.
-
-
Other sources of help/encouragement/barriers to quitting smoking/breastfeeding that do not fit into any of the above themes
-
Intrinsic barriers/demotivators.
-
Intrinsic facilitators/motivators
-
health.
-
-
Extrinsic barriers/demotivators.
-
Extrinsic facilitators/motivators.
-
Psychosocial ‘other’ (relates to any other reference to education, support, information required to try and change behaviours).
-
Negative attitudes towards smoking.
-
Consequences of smoking/not breastfeeding, for example guilt.
-
Appendix 20 Interviews: mothers/partners
| Participant codea | Parent statusb | Age (years) | Marital statusc | Ethnicityd | Educatione | Employed (yes/no) | Smoking statusf | Lives with smoker (yes/no) | Currently pregnant – infant feeding intentionsg | Previous infant feeding experiencesh | Experience of incentivesi |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Mother* | 26 | 1 | 1 | 3 | Yes | 5 | No | 1 | 1 | No |
| 2 | Mother | 25 | 1 | 1 | 1 | Yes | 2 | No | NA | 2 | SC |
| 3 | Mother | 38 | 1 | 1 | 3 | Yes | 5 | No | NA | 1 | No |
| T4 | Mother | 30 | 1 | 1 | 2 | Yes | 4 | No | NA | 1 | BF |
| 5 and T6j | Woman | 31 | 1 | 1 | 2 | Yes | 4 | No | 1 | 1 | BF |
| 7 | Woman | 29 | 1 | 1 | 1 | Yes | 1 | No | 1 | NA | No |
| 8 | Woman | 35 | 1 | 2 | 2 | No | 2 | No | 1 | NA | No |
| T9 | Mother | 31 | 1 | 1 | 1 | Yes | 2 | Yes | NA | 1 | BF |
| T10 and T11j | Mother* | 29 | 1 | 1 | 3 | No | 2 | Yes | 1 | 1 | BF |
| 12 | Mother* | 31 | 1 | 2 | 3 | Yes | 4 | No | 2 | 1 | No |
| 13 | Mother* | 22 | 1 | 1 | 3 | No | 5 | Yes | 2 | 1 | No |
| 14 | Mother* | 26 | 1 | 1 | 3 | No | 4 | Yes | 1 | 1 | SC |
| T15 | Woman | 19 | 1 | 2 | 3 | Yes | 1 | No | 1 | NA | No |
| 16 | Mother | 39 | 2 | 1 | 3 | No | 1 | No | NA | 1 | No |
| 17 | Mother | 53 | 2 | 1 | 1 | No | 1 | No | NA | 1 | No |
| 18 | Mother* | 36 | 1 | 2 | 1 | Yes | 1 | No | 1 | 1 | No |
| 19 | Father | 38 | 1 | 1 | 3 | Yes | 1 | Yes | NA | 1 | No |
| 20 | Father | 34 | 1 | 1 | 3 | No | 5 | Yes | NA | 1 | No |
| 21 | Father | 48 | 2 | 1 | 3 | No | 5 | No | NA | 1 | No |
| 22 | Father | 44 | 1 | 1 | 3 | No | 5 | No | NA | 2 | No |
| 23 | Man | 23 | 1 | 1 | 3 | No | 5 | Yes | 2 | NA | No |
| 24 | Mother* | 25 | 1 | 1 | 4 | No | 5 | No | 3 | 2 | No |
| 25 | Mother | 51 | 1 | 1 | 3 | No | 5 | Yes | NA | NR | No |
| 26 | Woman | 18–24 | 1 | 1 | NR | No | 5 | NR | NR | NR | SC |
| 27 | Woman | 18–24 | 2 | 1 | NR | No | 2 | NR | NR | NR | SC |
| 28 | Mother* | 25–35 | 1 | 1 | NR | No | 2 | NR | NR | NR | SC |
| 29 | Mother* | 25–35 | 1 | 1 | NR | NR | 5 | NR | NR | NR | SC |
| 30 | Woman | < 18 | 1 | 1 | NR | No | 2 | NR | NR | NR | SC |
| 31 | Mother* | 25–25 | 1 | 1 | NR | No | 2 | NR | NR | NR | SC |
| 32 | Woman | 18–24 | NR | 1 | NR | NR | 5 | NR | NR | NR | SC |
| 33 | Woman | 25–35 | 2 | 1 | NR | No | 2 | NR | NR | NR | SC |
| 34 | Mother* | 18–24 | 2 | 1 | NR | No | 2 | NR | NR | NR | SC |
| 35 | Woman | 25–35 | 1 | 1 | NR | No | 2 | NR | NR | NR | SC |
| 36 | Mother* | 25–25 | 1 | 1 | NR | Yes | 5 | NR | NR | NR | SC |
| 37 | Mother* | 25–35 | 1 | 1 | NR | Yes | 2 | NR | NR | NR | SC |
| 38 | Woman | 18–24 | 2 | 1 | NR | Yes | 5 | NR | NR | NR | SC |
| 39 | Mother* | 36+ | 2 | 1 | NR | No | 5 | NR | NR | NR | SC |
| 40 | Woman | 18–24 | 2 | 1 | NR | No | 2 | NR | NR | NR | SC |
| 41 | Woman | 18–24 | 1 | 1 | NR | No | 5 | NR | NR | NR | SC |
| 42 | Woman | 18–24 | 2 | 1 | NR | No | 5 | NR | NR | NR | SC |
| 43 | Woman | 18–24 | 2 | 1 | NR | No | 5 | NR | NR | NR | SC |
| 44 | Woman | 25–35 | 1 | 1 | NR | Yes | 2 | NR | NR | NR | SC |
| 45 | Mother* | 36+ | 1 | 1 | NR | NR | 2 | NR | NR | NR | SC |
Appendix 21 Focus groups: mothers
| Participant code | Parent statusa | Age (years) | Marital statusb | Ethnicityc | Educationd | Employed (yes/no) | Smoking statuse | Lives with smoker (yes/no) | Currently pregnant – infant feeding intentionsf | Previous infant feeding experiencesg | Experience of incentivesh |
|---|---|---|---|---|---|---|---|---|---|---|---|
| FG1 | Mother | 28 | 1 | 1 | 1 | Yes | 4 | No | NA | 1 | No |
| FG1 | Mother | 38 | 1 | 2 | 1 | Yes | 4 | No | NA | 1 | No |
| FG1 | Mother | 33 | 1 | 1 | 1 | Yes | 1 | Yes | NA | 1 | No |
| FG1 | Mother | 26 | 1 | 1 | 1 | Yes | 4 | No | NA | 1 | No |
| FG1 | Mother | 26 | 1 | 1 | 1 | Yes | 1 | No | NA | 1 | No |
| FG1 | Mother | 21 | 1 | 1 | 3 | No | 2 | Yes | NA | 1 | No |
| FG1 | Mother | 39 | 1 | 1 | 1 | Yes | 4 | No | NA | 1 | No |
| FG1 | Mother | 34 | 1 | 1 | 1 | Yes | 1 | No | NA | 1 | No |
| FG1 | Mother | 36 | 1 | 1 | 3 | Yes | 1 | No | NA | 1 | No |
| FG2 | Mother | 62 | 1 | 1 | 3 | No | 1 | No | NA | 1 | No |
| FG2 | Mother* | 26 | 1 | 1 | 3 | Yes | 1 | No | 1 | 1 | No |
| FG2 | Mother | 28 | 1 | 1 | 3 | Yes | 1 | No | NA | 1 | No |
| FG2 | Mother* | 27 | 1 | 1 | 3 | Yes | 4 | No | 1 | 1 | No |
| FG3 | Woman | 17 | 2 | 1 | 4 | No | 5 | Yes | 3 | NA | No |
| FG3 | Woman | 17 | 2 | 1 | 4 | No | 1 | Yes | 2 | NA | No |
| FG3 | Mother | 19 | 1 | 1 | 3 | No | 4 | Yes | NA | 1 | No |
| FG3 | Mother | 18 | 1 | 1 | NR | Yes | 1 | NR | NA | 1 | No |
| FG3 | Mother | 17 | 1 | 1 | 2 | No | 5 | Yes | NA | 1 | No |
| FG4 | Mother | 21 | 1 | 1 | 3 | No | 5 | Yes | NA | 2 | No |
| FG4 | Mother | 40 | 1 | 1 | 3 | Yes | 1 | No | NA | 1 | No |
| FG4 | Mother* | 30 | 1 | 1 | 2 | No | 1 | No | 1 | 1 | No |
| FG4 and FG5i | Mother* | 22 | 2 | 1 | 3 | Yes | 1 | No | 3 | 1 | No |
| FG4 and FG5 | Mother | 32 | 1 | 1 | 3 | No | 5 | No | NA | 1 | No |
| FG6 | Mother | 34 | 2 | 1 | 3 | Yes | 3 | No | NA | 1 | No |
| FG6 | Mother | 30 | 1 | 1 | NR | Yes | 4 | No | NA | 1 | No |
| FG6 | Mother | 34 | 1 | 1 | 1 | Yes | 4 | No | NA | 1 | No |
| FG6 | Mother | 28 | 1 | 1 | 3 | Yes | 2 | No | NA | 1 | No |
| FG7i | Mother | 36 | 1 | 1 | 3 | Yes | 4 | No | NA | 1 | No |
| FG7 | Mother | 28 | 1 | 2 | 1 | Yes | 1 | No | NA | 1 | No |
| FG7 | Mother | 35 | 1 | 1 | 1 | Yes | 1 | No | NA | 1 | No |
| FG7 | Mother | 36 | 1 | 2 | 1 | Yes | 1 | No | NA | 1 | No |
| FG7 | Mother | 28 | 1 | 1 | 1 | No | 4 | No | NA | 1 | No |
| FG7 | Mother | 41 | 1 | 2 | 3 | Yes | 3 | Yes | NA | 1 | Other |
| FG7 | Mother | 36 | 1 | 1 | 1 | Yes | 1 | No | NA | 1 | No |
| FG7 | Mother | 29 | 1 | 1 | 1 | Yes | 1 | No | NA | 1 | No |
| FG7 | Mother | 36 | 1 | 1 | 2 | Yes | 4 | No | NA | 1 | No |
| FG7 | Mother | 33 | 1 | 2 | NR | NR | 1 | No | NA | 1 | No |
| FG7 | Mother | 30 | 1 | 1 | 1 | Yes | 1 | No | NA | 1 | No |
| FG7 | Mother | 32 | 2 | 1 | 3 | No | 5 | No | NA | 1 | No |
| FG7 | Mother | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| FG8i | Mother* | 19 | 2 | 1 | 2 | No | 3 | No | 2 | 1 | Other |
| FG8 | Mother | 20 | 1 | 1 | 3 | No | 3 | Yes | NA | 1 | Other |
| FG8 | Mother | 20 | 1 | 1 | 2 | No | 5 | No | NA | NR | No |
| FG8 | Mother | 19 | 2 | 1 | 3 | No | 3 | No | NA | 1 | No |
| FG8 | Mother | 21 | 1 | 1 | 3 | Yes | 1 | No | NA | 2 | Other |
Appendix 22 Interviews: providers/experts
| Participant codea,b | Profession | Provider/expert |
|---|---|---|
| T46 | Consultant obstetrician | Provider |
| T47 | Research manager, voluntary sector | Expert |
| T48 | Public health consultant | Expert |
| T49 | Health visitor | Provider |
| T50 | Health visitor | Provider |
| T51 | Lead health trainer – smoking cessation | Expert |
| 52 | Specialist midwife (substance misuse) | Provider |
| 53 | Hospital midwife | Provider |
| T54 | Senior clinical lecturer/ethics committee member | Expert |
| T55 | Consultant obstetrician | Provider |
| T56 | Tobacco trainer – tobacco control team | Provider |
| T57 | Stop smoking service manager | Expert |
| T58 | Smoking awareness co-ordinator | Expert |
| T59 | Midwife | Provider |
| T60 | Infant feeding co-ordinator | Expert |
| T61 | Smoking cessation advisor | Provider |
| 62 | Ethics committee member | Expert |
| T63 | GP | Provider |
| T64 | Paediatrician (neonatal) | Provider |
| T65 | Paediatrician (general and respiratory) | Provider |
| 66 | Health improvement senior officer | Expert |
| 67 | Helpline manager | Expert |
| 68 | Midwife | Provider |
| 69 | Community midwife | Provider |
| 70 | Research nurse | Provider |
| 71 | Senior midwife | Provider |
| T72 | Smoking cessation advisor | Provider |
| T73 | Smoking cessation advisor | Provider |
| T74 | Incentive scheme administrator | Provider |
Appendix 23 Focus groups and interactive discussions: providers/experts
| Participant codea | Profession | Provider/expert |
|---|---|---|
| FG5 | Peer supporterb | Provider |
| FG7 | Health visitorb | Provider |
| FG7 | Health visitorb | Provider |
| FG7 | Student nurse/health visitingb | Provider |
| FG8 | Liaison worker for young mums – voluntary sectorb | Provider |
| FG9 | Senior public health co-ordinator | Expert |
| FG9 | Assistant director of nursing and families | Expert |
| FG9 | Infant feeding consultant | Expert |
| FG9 | Infant feeding co-ordinatorc | Expert |
| FG9 | Baby Friendly co-ordinator | Expert |
| FG10 | Public health specialist | Provider |
| FG10 | Parentcraft and infant feeding co-ordinatord | Expert |
| FG10 | Children’s centre development officer | Expert |
| FG10 | Infant feeding co-ordinator | Expert |
| FG10 | Breastfeeding peer support branch manager | Expert |
| FG10 | Breastfeeding peer support operations manager | Expert |
| FG10 | Breastfeeding peer support co-ordinator | Expert |
| FG10 | Public health co-ordinator | Expert |
| FG10 | Health co-ordinator, children’s centres | Expert |
| FG11 | Health visitor | Provider |
| FG11 | Health visitor | Provider |
| FG11 | Health visitor | Provider |
| FG11 | Health visitor | Provider |
| FG11 | Health visitor | Provider |
| FG11 | Health visitor | Provider |
| FG11 | Health visitor | Provider |
| FG12 | Public health practitioner | Provider |
| FG12 | Health education practitioner | Provider |
| FG13 | Community midwife | Provider |
| FG13 | Community midwife | Provider |
| FG13 | Community midwife | Provider |
| FG13 | Community midwife | Provider |
| FG13 | Community midwife | Provider |
| FG13 | Community midwife co-ordinator | Expert |
| FG14 | Community pharmacist | Provider |
| FG14 | Community pharmacist | Provider |
| FG14 | Community pharmacist | Provider |
| FG14 | Community pharmacist | Provider |
| FG14 | Community pharmacist | Provider |
| FG14 | Community pharmacist | Provider |
| FG14 | Community pharmacist | Provider |
| FG15 | Smoking cessation advisor | Provider |
| FG15 | Smoking cessation advisor | Provider |
| FG15 | Smoking cessation advisor | Provider |
| FG15 | Smoking cessation advisor | Provider |
| FG15 | Smoking cessation advisor | Provider |
| FG16 | Helpline staff | Provider |
| FG16 | Helpline staff | Provider |
| Interactive discussionse | ||
| IA1 | Nutrition and Nurture Conference, June 2011, Grange-over-Sands, Cumbria (n = 30+) | Providers and experts |
| IA2 | Nutrition and Nurture Conference, June 2013, Grange-over-Sands, Cumbria (n = 15) | Providers and experts |
| IA3 | (Scottish) Faculty of Public Health, November 2012, Crieff Hydro, Crieff, Perthshire (n = 18) | Providers and experts |
Appendix 24 Background to Ipsos MORI Computer Assisted Personal Interviewing
Appendix 25 Ipsos MORI survey of the general public
Lifestyle survey: CS module CAPIBUS week 12
(Sample = adults aged 18+ years)
[Computing, please rotate so that half of the sample are asked smoking questions (smoking intro plus CS01–05) first and half of the sample are asked breastfeeding questions (breastfeeding intro plus CS06–CS10) first]
(Computing: please ensure all DK, REF and NULL are treated as hidden responses)
Interviewer: this section does not have showcards. On-screen instructions will indicate when to show and when not to show screen to the respondent. Please note: there may be questions that allow don’t know, none of these or refused. Please type DK for don’t know, REF for refused and NULL for none of these.
(New screen)
INTERVIEWER: PLEASE SHOW SCREEN UNTIL OTHERWISE INSTRUCTED.
I WOULD NOW LIKE TO ASK YOU SOME QUESTIONS ABOUT SMOKING DURING PREGNANCY . . .
CS01. Stopping smoking in pregnancy benefits the health of the baby and the mother. Research shows that providing shopping vouchers to women who prove that they have stopped smoking during pregnancy increases the number of women who stop. While some people feel that providing vouchers is appropriate, others feel that it is wrong or unfair.
Do you agree or disagree that shopping vouchers should be provided to women who prove that they have stopped smoking during pregnancy?
(Single code; reverse order between interviews)
Strongly agree
Tend to agree
Neither agree nor disagree
Tend to disagree
Strongly disagree
If CODE STRONGLY AGREE, TEND TO AGREE OR NEITHER AGREE NOR DISAGREE AT CS01, ASK:
CS02. What is the highest amount of shopping voucher you think it would be acceptable to provide to a woman who proves that she has stopped smoking during pregnancy?
(Single code; reverse order between interviews)
-
£2.00 per month
-
£10.00 per month
-
£20.00 per month
-
£40.00 per month
-
£60.00 per month
-
£80.00 per month
If CODE STRONGLY AGREE, TEND TO AGREE OR NEITHER AGREE NOR DISAGREE AT CS01, ASK:
CS03. Do you think that it is acceptable to provide shopping vouchers to women who prove that they have stopped smoking during pregnancy, regardless of their income, or only to women on low incomes?
(Single code)
To all women, regardless of income
Only to women on low incomes
ASK ALL
CS04. Some women start smoking again after the birth of their baby, particularly if their partner or someone at home smokes. Please tell me whether you agree or disagree with each of the following statements.
Statements:
It is acceptable to provide shopping vouchers to a woman for 2 months after the birth of her baby if she proves that she is still not smoking.
It is acceptable to provide shopping vouchers to a woman for 2 months after the birth of her baby if she never lets anyone smoke in her home.
(Single code for each statement; reverse order of list between interviews)
Precode list:
Strongly agree
Tend to agree
Neither agree nor disagree
Tend to disagree
Strongly disagree
CS05. Do you agree or disagree that local health services should receive additional funding if they reach targets for the number of women who prove that they have stopped smoking during pregnancy?
(Single code; reverse order of list between interviews)
Precode list:
Strongly agree
Tend to agree
Neither agree nor disagree
Tend to disagree
Strongly disagree
I WOULD NOW LIKE TO ASK YOU SOME QUESTIONS ABOUT BREASTFEEDING . . .
CS06. Breastfeeding benefits the health of the baby and the mother. While some people feel it is appropriate to provide shopping vouchers to encourage breastfeeding, other people feel it is wrong or unfair.
Do you agree or disagree that shopping vouchers should be provided to women who breastfeed for the first 6 months after the birth of their child?
(Single code; reverse order of list between interviews)
Precode list:
Strongly agree
Tend to agree
Neither agree nor disagree
Tend to disagree
Strongly disagree
IF CODE STRONGLY AGREE, TEND TO AGREE OR NEITHER AGREE NOR DISAGREE AT CS06, ASK:
CS07. What is the highest amount of shopping voucher you would consider acceptable for women who breastfeed?
(Single code; reverse order between interviews)
-
£2.00 per month
-
£10.00 per month
-
£20.00 per month
-
£40.00 per month
-
£60.00 per month
-
£80.00 per month
IF CODE STRONGLY AGREE, TEND TO AGREE OR NEITHER AGREE NOR DISAGREE AT CS06, ASK:
CS08. Do you agree or disagree that shopping vouchers should be provided to all women who breastfeed, regardless of their income, or only to women on low incomes?
(Single code)
To all women, regardless of income
Only to women on low incomes
ASK ALL
CS09. Do you agree or disagree that local health services should receive additional funding if they reach targets for the number of women who breastfeed?
(Single code; reverse order of list between interviews)
Precode list:
Strongly agree
Tend to agree
Neither agree nor disagree
Tend to disagree
Strongly disagree
(New screen)
INTERVIEWER: THE WORDING OF THE NEXT QUESTION ABOUT BREASTFEEDING IS A BIT SENSITIVE. PLEASE COULD YOU TURN THE NEXT SCREEN TO THE RESPONDENT AND ASK THEM TO READ THE QUESTION THEMSELVES. THEY CAN JUST GIVE YOU THEIR ANSWER FOR YOU TO INPUT
ASK ALL
CS10. Some women who breastfeed like to express milk. This allows babies to receive breast milk when mother and baby are apart.
To express milk, some women find a breast pump useful. Women can buy breast pumps ranging from £20.00 to over £100.00. Do you agree or disagree that a breast pump costing around £40.00 should be available for free on the NHS, to help women to continue breastfeeding?
(Single code; reverse order of list between interviews)
Precode list:
Strongly agree
Tend to agree
Neither agree nor disagree
Tend to disagree
Strongly disagree
TO FIT WITH OMNIBUS DEMOG QUESTIONS, IF NOT RECORDED ANY CHILDREN IN OMNIBUS DEMOG QUESTIONS, ASK:
CS11. Do you have any children? Please include any children who are grown up now and any children who do not live with you.
(Single code)
Yes
No
IF HAVE CHILDREN (FROM OMNIBUS DEMOGS OR CS11), ASK:
CS12. Have any of your children ever been breastfed or received breast milk, even if only for a day or two?
(Single code, allow DK and REF)
Yes
No
ASK ALL
CS13. Do you currently smoke or have you ever smoked?
Yes, I currently smoke every day
Yes, I currently smoke, but not every day
Yes, I used to smoke but have quit
No, I have never smoked
I prefer not to answer
IF CODE 1 OR 2 AT CS13, ASK:
CS14. Have you ever tried to stop smoking?
(Single code)
Yes
No
CLOSE
Appendix 26 Survey of health professionals
Appendix 27 Distribution of the health professional survey
| SPCRN node | Practice managers who received and distributed the survey | Practice staff responses received |
|---|---|---|
| East (Tayside, Fife, Forth Valley) | 183 | 55 |
| North (Highland, Western Isles) | 83 | 32 |
| North East (Grampian, Orkney, Shetland) | 80 | 102 |
| South East (Lothian, Borders) | 151 | 5 |
| West (Dumfries and Galloway, Lanarkshire, Greater Glasgow and Clyde, Ayrshire and Arran) | 417 | 30 |
| Total | 224 |
| Health board/R&D department | Responses received |
|---|---|
| NHS Ayrshire and Arran | 0 |
| NHS Borders | 0 |
| NHS Dumfries and Galloway | 78 |
| NHS Fife | 1 |
| NHS Forth Valley | 10 |
| NHS Grampian | 77 |
| NHS Greater Glasgow and Clyde | 1 |
| NHS Highland | 0 |
| NHS Lanarkshire | 0 |
| NHS Lothian | 37 |
| NHS Orkney | 1 |
| NHS Shetland | 5 |
| NHS Tayside | 0 |
| NHS Western Isles | 3 |
| Total | 213 |
| Stakeholders | Denominator, if known | Responses received | % response rate |
|---|---|---|---|
| Paediatricians in training | 28 | 6 | 21.4 |
| Public health doctors | 123 | 13 | 10.6 |
| Scottish government – two contacts asked to distribute | Unknown | 0 | 0 |
| Total | 151 | 19 | 12.6 |
| Health professionals | Denominator | Responses received | % response rate |
|---|---|---|---|
| NHS managers: clinical directors, directorate managers, directorate nurse manager, directorate service manager and other directorate managers within the maternity, obstetrics and gynaecology and paediatrics services | 141 | 0 | 0 |
| General practices: health visitors, midwives, GP partners, practice nurses, GP registrar, head of midwifery, other GP, senior midwife, senior practice nurse, team leader – health visitor, team leader – midwife | 3721 | 26 | 0.01 |
| Specialist nurses: public health medicine, obstetrics and gynaecology, paediatrics | 446 | 1 | < 0.01 |
| Hospital doctors: public health medicine, obstetrics and gynaecology, paediatrics | 513 | 11 | 0.02 |
| Unknown | 0 | 5 | |
| Total | 4821 | 43 | 0.01 |
| Students | Number sent | Responses received |
|---|---|---|
| Midwifery and health visiting | 139 | 5 |
| Other | Unknown | 2 |
| Total | Unknown | 7 |
| R&D departments | Number sent | Responses received |
|---|---|---|
| North West trusts | Unknown | 13 |
| Total | Unknown | 13 |
Appendix 28 Detailed results of the Ipsos MORI survey
| Variable | SD | D | N | A | SA |
|---|---|---|---|---|---|
| Age category (years) | |||||
| 18–24 | 30 (17.6) | 34 (20.0) | 34 (20.0) | 48 (28.2) | 24 (14.1) |
| 25–34 | 32 (18.3) | 32 (18.3) | 27 (15.4) | 50 (28.6) | 34 (19.4) |
| 35–44 | 31 (17.1) | 29 (16.0) | 33 (18.2) | 46 (25.4) | 42 (23.2) |
| 45–54 | 44 (27.7) | 28 (17.6) | 29 (18.2) | 32 (20.1) | 26 (16.4) |
| 55–59 | 23 (31.9) | 13 (18.1) | 12 (16.7) | 16 (22.2) | 8 (11.1) |
| 60–64 | 28 (29.8) | 13 (13.8) | 13 (13.8) | 24 (25.5) | 16 (17.0) |
| 65+ | 107 (36.5) | 40 (13.7) | 49 (16.7) | 53 (18.1) | 44 (15.0) |
| Breastfeeding | |||||
| Children not breastfed | 154 (24.4) | 113 (17.9) | 135 (21.4) | 138 (21.8) | 92 (14.6) |
| Children breastfed | 141 (27.5) | 76 (14.8) | 62 (12.1) | 131 (25.6) | 102 (19.9) |
| Children | |||||
| No children | 90 (22.4) | 79 (19.7) | 81 (20.1) | 98 (24.4) | 54 (13.4) |
| Have children | 205 (27.6) | 110 (14.8) | 116 (15.6) | 171 (23.0) | 140 (18.9) |
| Ethnicity | |||||
| White | 280 (28.4) | 163 (16.5) | 167 (17.0) | 212 (21.5) | 163 (16.5) |
| Other ethnicity | 15 (9.4) | 26 (16.4) | 30 (18.9) | 57 (35.8) | 31 (19.5) |
| Sex | |||||
| Male | 120 (22.2) | 83 (15.4) | 106 (19.6) | 128 (23.7) | 103 (19.1) |
| Female | 175 (29.0) | 106 (17.5) | 91 (15.1) | 141 (23.3) | 91 (15.1) |
| Education | |||||
| University | 65 (22.0) | 46 (15.6) | 44 (14.9) | 77 (26.1) | 63 (21.4) |
| GCSE | 98 (28.7) | 54 (15.8) | 57 (16.7) | 80 (23.4) | 53 (15.5) |
| A-level | 48 (24.9) | 43 (22.3) | 32 (16.6) | 36 (18.7) | 34 (17.6) |
| No formal qualification | 59 (29.9) | 24 (12.2) | 47 (23.9) | 45 (22.8) | 22 (11.2) |
| Other, still studying, don’t know | 25 (21.4) | 22 (18.8) | 17 (14.5) | 31 (26.5) | 22 (18.8) |
| Social grade | |||||
| AB | 71 (29.7) | 37 (15.5) | 30 (12.6) | 59 (24.7) | 42 (17.6) |
| C1 | 103 (27.8) | 67 (18.1) | 68 (18.4) | 73 (19.7) | 59 (15.9) |
| C2 | 57 (24.2) | 38 (16.1) | 44 (18.6) | 55 (23.3) | 42 (17.8) |
| D | 40 (24.7) | 29 (17.9) | 28 (17.3) | 38 (23.5) | 27 (16.7) |
| E | 24 (17.5) | 18 (13.1) | 27 (19.7) | 44 (32.1) | 24 (17.5) |
| Smoking status | |||||
| Never smoked | 147 (25.7) | 102 (17.8) | 97 (16.9) | 144 (25.1) | 83 (14.5) |
| Previous smoker | 84 (29.9) | 49 (17.4) | 43 (15.3) | 64 (22.8) | 41 (14.6) |
| Current (tried quitting) | 38 (21.7) | 22 (12.6) | 31 (17.7) | 34 (19.4) | 50 (28.6) |
| Current (not tried quitting) | 15 (23.8) | 9 (14.3) | 10 (15.9) | 16 (25.4) | 13 (20.6) |
| Refused to answer | 11 (21.2) | 7 (13.5) | 16 (30.8) | 11 (21.2) | 7 (13.5) |
| Area | |||||
| North | 24 (31.2) | 17 (22.1) | 11 (14.3) | 10 (13.0) | 15 (19.5) |
| North West | 19 (13.4) | 25 (17.6) | 38 (26.8) | 41 (28.9) | 19 (13.4) |
| Yorkshire and Humberside | 40 (38.5) | 11 (10.6) | 13 (12.5) | 22 (21.2) | 18 (17.3) |
| East Midlands | 25 (22.9) | 28 (25.7) | 14 (12.8) | 29 (26.6) | 13 (11.9) |
| West Midlands | 22 (33.3) | 10 (15.2) | 12 (18.2) | 12 (18.2) | 10 (15.2) |
| East Anglia | 10 (24.4) | 6 (14.6) | 5 (12.2) | 10 (24.4) | 10 (24.4) |
| South East | 20 (24.7) | 9 (11.1) | 17 (21.0) | 15 (18.5) | 20 (24.7) |
| South West | 55 (27.5) | 26 (13.0) | 40 (20.0) | 47 (23.5) | 32 (16.0) |
| London | 17 (11.4) | 31 (20.8) | 19 (12.8) | 59 (39.6) | 23 (15.4) |
| Wales | 29 (43.9) | 9 (13.6) | 6 (9.1) | 10 (15.2) | 12 (18.2) |
| Scotland | 34 (31.2) | 17 (15.6) | 22 (20.2) | 14 (12.8) | 22 (20.2) |
| Variable | Simple regression model | Multiple regression model | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age category (years) | ||||||
| 18–24 | 1.66 | 1.19 to 2.31 | 0.003 | 1.67 | 1.10 to 2.54 | 0.016 |
| 25–34 | 1.92 | 1.37 to 2.69 | < 0.001 | 1.71 | 1.17 to 2.49 | 0.006 |
| 35–44 | 2.15 | 1.54 to 3.00 | < 0.001 | 1.88 | 1.30 to 2.72 | 0.001 |
| 45–54 | 1.29 | 0.91 to 1.82 | 0.16 | 1.27 | 0.87 to 1.84 | 0.21 |
| 55–59 | 1.04 | 0.66 to 1.65 | 0.87 | 1.03 | 0.63 to 1.66 | 0.91 |
| 60–64 | 1.39 | 0.91 to 2.12 | 0.13 | 1.42 | 0.92 to 2.20 | 0.12 |
| Breastfeeding | ||||||
| Children breastfed | 1.15 | 0.94 to 1.42 | 0.18 | 1.26 | 0.94 to 1.69 | 0.12 |
| Children | ||||||
| Have children | 1.05 | 0.85 to 1.30 | 0.67 | 1.17 | 0.86 to 1.59 | 0.33 |
| Ethnicity | ||||||
| Other ethnicity | 1.94 | 1.46 to 2.59 | < 0.001 | 1.42 | 1.01 to 1.99 | 0.047 |
| Sex | ||||||
| Female | 0.75 | 0.61 to 0.92 | 0.006 | 0.71 | 0.57 to 0.88 | 0.002 |
| Education | ||||||
| GCSE | 0.70 | 0.53 to 0.93 | 0.014 | 0.59 | 0.43 to 0.81 | 0.001 |
| A-level | 0.72 | 0.52 to 0.99 | 0.042 | 0.63 | 0.44 to 0.90 | 0.010 |
| No formal qualification | 0.64 | 0.46 to 0.87 | 0.005 | 0.63 | 0.42 to 0.95 | 0.029 |
| Other, still studying, don’t know | 0.92 | 0.63 to 1.34 | 0.66 | 0.84 | 0.55 to 1.28 | 0.41 |
| Social grade | ||||||
| C1 | 0.92 | 0.68 to 1.23 | 0.57 | 1.03 | 0.75 to 1.42 | 0.87 |
| C2 | 1.12 | 0.81 to 1.55 | 0.48 | 1.25 | 0.85 to 1.83 | 0.26 |
| D | 1.06 | 0.74 to 1.51 | 0.75 | 1.27 | 0.83 to 1.94 | 0.27 |
| E | 1.48 | 1.03 to 2.15 | 0.036 | 1.74 | 1.12 to 2.70 | 0.014 |
| Smoking status | ||||||
| Previous smoker | 0.88 | 0.68 to 1.13 | 0.32 | 0.97 | 0.74 to 1.28 | 0.83 |
| Current (tried quitting) | 1.59 | 1.17 to 2.16 | 0.003 | 1.63 | 1.18 to 2.26 | 0.003 |
| Current (not tried quitting) | 1.28 | 0.80 to 2.04 | 0.30 | 1.31 | 0.81 to 2.12 | 0.28 |
| Refused to answer | 1.08 | 0.66 to 1.74 | 0.77 | 0.93 | 0.56 to 1.55 | 0.78 |
| Area | ||||||
| North | 0.50 | 0.30 to 0.81 | 0.005 | 0.66 | 0.39 to 1.10 | 0.11 |
| North West | 0.82 | 0.56 to 1.21 | 0.33 | 1.03 | 0.69 to 1.56 | 0.87 |
| Yorkshire and Humberside | 0.49 | 0.31 to 0.76 | 0.002 | 0.62 | 0.38 to 1.01 | 0.054 |
| East Midlands | 0.58 | 0.38 to 0.89 | 0.012 | 0.70 | 0.45 to 1.09 | 0.12 |
| West Midlands | 0.49 | 0.29 to 0.81 | 0.006 | 0.68 | 0.39 to 1.16 | 0.16 |
| East Anglia | 0.86 | 0.46 to 1.60 | 0.63 | 1.06 | 0.56 to 2.00 | 0.86 |
| South East | 0.82 | 0.51 to 1.33 | 0.42 | 1.26 | 0.75 to 2.11 | 0.38 |
| South West | 0.63 | 0.44 to 0.92 | 0.015 | 0.97 | 0.64 to 1.45 | 0.86 |
| Wales | 0.37 | 0.22 to 0.64 | < 0.001 | 0.55 | 0.31 to 0.97 | 0.040 |
| Scotland | 0.54 | 0.35 to 0.84 | 0.006 | 0.78 | 0.49 to 1.26 | 0.31 |
| Variable | SD | D | N | A | SA |
|---|---|---|---|---|---|
| Age category (years) | |||||
| 18–24 | 31 (18.2) | 36 (21.2) | 37 (21.8) | 47 (27.6) | 19 (11.2) |
| 25–34 | 33 (18.9) | 41 (23.4) | 32 (18.3) | 46 (26.3) | 23 (13.1) |
| 35–44 | 36 (19.9) | 38 (21.0) | 28 (15.5) | 52 (28.7) | 27 (14.9) |
| 45–54 | 44 (27.7) | 33 (20.8) | 26 (16.4) | 40 (25.2) | 16 (10.1) |
| 55–59 | 29 (40.3) | 14 (19.4) | 6 (8.3) | 16 (22.2) | 7 (9.7) |
| 60–64 | 28 (29.8) | 12 (12.8) | 15 (16.0) | 25 (26.6) | 14 (14.9) |
| 65+ | 118 (40.3) | 38 (13.0) | 52 (17.7) | 56 (19.1) | 29 (9.9) |
| Breastfeeding | |||||
| Children not breastfed | 160 (25.3) | 123 (19.5) | 141 (22.3) | 138 (21.8) | 70 (11.1) |
| Children breastfed | 159 (31.1) | 89 (17.4) | 55 (10.7) | 144 (28.1) | 65 (12.7) |
| Children | |||||
| No children | 89 (22.1) | 82 (20.4) | 91 (22.6) | 100 (24.9) | 40 (10.0) |
| Have children | 230 (31.0) | 130 (17.5) | 105 (14.2) | 182 (24.5) | 95 (12.8) |
| Ethnicity | |||||
| White | 302 (30.7) | 184 (18.7) | 162 (16.4) | 227 (23.0) | 110 (11.2) |
| Other ethnicity | 17 (10.7) | 28 (17.6) | 34 (21.4) | 55 (34.6) | 25 (15.7) |
| Sex | |||||
| Male | 123 (22.8) | 97 (18.0) | 109 (20.2) | 138 (25.6) | 73 (13.5) |
| Female | 196 (32.5) | 115 (19.0) | 87 (14.4) | 144 (23.8) | 62 (10.3) |
| Education | |||||
| University | 68 (23.1) | 54 (18.3) | 49 (16.6) | 79 (26.8) | 45 (15.3) |
| GCSE | 102 (29.8) | 68 (19.9) | 57 (16.7) | 75 (21.9) | 40 (11.7) |
| A-level | 55 (28.5) | 37 (19.2) | 30 (15.5) | 52 (26.9) | 19 (9.8) |
| No formal qualification | 67 (34.0) | 29 (14.7) | 44 (22.3) | 43 (21.8) | 14 (7.1) |
| Other, still studying, don’t know | 27 (23.1) | 24 (20.5) | 16 (13.7) | 33 (28.2) | 17 (14.5) |
| Social grade | |||||
| AB | 71 (29.7) | 41 (17.2) | 36 (15.1) | 59 (24.7) | 32 (13.4) |
| C1 | 110 (29.7) | 85 (23.0) | 52 (14.1) | 83 (22.4) | 40 (10.8) |
| C2 | 58 (24.6) | 41 (17.4) | 47 (19.9) | 57 (24.2) | 33 (14.0) |
| D | 48 (29.6) | 26 (16.0) | 32 (19.8) | 42 (25.9) | 14 (8.6) |
| E | 32 (23.4) | 19 (13.9) | 29 (21.2) | 41 (29.9) | 16 (11.7) |
| Smoking status | |||||
| Never smoked | 154 (26.9) | 110 (19.2) | 100 (17.5) | 151 (26.4) | 58 (10.1) |
| Previous smoker | 100 (35.6) | 54 (19.2) | 41 (14.6) | 58 (20.6) | 28 (10.0) |
| Current (tried quitting) | 39 (22.3) | 31 (17.7) | 28 (16.0) | 41 (23.4) | 36 (20.6) |
| Current (not tried quitting) | 13 (20.6) | 11 (17.5) | 15 (23.8) | 17 (27.0) | 7 (11.1) |
| Refused to answer | 13 (25.0) | 6 (11.5) | 12 (23.1) | 15 (28.8) | 6 (11.5) |
| Area | |||||
| North | 24 (31.2) | 18 (23.4) | 11 (14.3) | 13 (16.9) | 11 (14.3) |
| North West | 21 (14.8) | 22 (15.5) | 40 (28.2) | 41 (28.9) | 18 (12.7) |
| Yorkshire and Humberside | 42 (40.4) | 11 (10.6) | 15 (14.4) | 21 (20.2) | 15 (14.4) |
| East Midlands | 30 (27.5) | 27 (24.8) | 18 (16.5) | 26 (23.9) | 8 (7.3) |
| West Midlands | 25 (37.9) | 14 (21.2) | 6 (9.1) | 16 (24.2) | 5 (7.6) |
| East Anglia | 10 (24.4) | 7 (17.1) | 4 (9.8) | 11 (26.8) | 9 (22.0) |
| South East | 24 (29.6) | 7 (8.6) | 17 (21.0) | 20 (24.7) | 13 (16.0) |
| South West | 56 (28.0) | 36 (18.0) | 43 (21.5) | 47 (23.5) | 18 (9.0) |
| London | 16 (10.7) | 38 (25.5) | 17 (11.4) | 55 (36.9) | 23 (15.4) |
| Wales | 29 (43.9) | 9 (13.6) | 5 (7.6) | 15 (22.7) | 8 (12.1) |
| Scotland | 42 (38.5) | 23 (21.1) | 20 (18.3) | 17 (15.6) | 7 (6.4) |
| Variable | Simple regression model | Multiple regression model | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age category (years) | ||||||
| 18–24 | 1.89 | 1.35 to 2.64 | < 0.001 | 1.51 | 1.00 to 2.30 | 0.053 |
| 25– 4 | 1.88 | 1.34 to 2.62 | < 0.001 | 1.42 | 0.97 to 2.08 | 0.068 |
| 35–44 | 2.04 | 1.46 to 2.85 | < 0.001 | 1.65 | 1.14 to 2.39 | 0.007 |
| 45–54 | 1.42 | 1.00 to 2.01 | 0.048 | 1.26 | 0.87 to 1.84 | 0.23 |
| 55–59 | 0.95 | 0.59 to 1.54 | 0.85 | 0.95 | 0.58 to 1.56 | 0.83 |
| 60–64 | 1.70 | 1.11 to 2.61 | 0.015 | 1.65 | 1.05 to 2.57 | 0.028 |
| Breastfeeding | ||||||
| Children breastfed | 1.00 | 0.81 to 1.23 | 0.98 | 1.16 | 0.86 to 1.56 | 0.34 |
| Children | ||||||
| Have children | 0.88 | 0.71 to 1.09 | 0.24 | 1.02 | 0.75 to 1.39 | 0.90 |
| Ethnicity | ||||||
| Other ethnicity | 2.12 | 1.59 to 2.83 | < 0.001 | 1.39 | 0.98 to 1.95 | 0.062 |
| Sex | ||||||
| Female | 0.69 | 0.56 to 0.85 | 0.001 | 0.68 | 0.55 to 0.85 | 0.001 |
| Education | ||||||
| GCSE | 0.71 | 0.53 to 0.93 | 0.014 | 0.62 | 0.45 to 0.85 | 0.003 |
| A-level | 0.75 | 0.55 to 1.04 | 0.084 | 0.68 | 0.48 to 0.96 | 0.028 |
| No formal qualification | 0.60 | 0.44 to 0.83 | 0.002 | 0.64 | 0.42 to 0.96 | 0.032 |
| Other, still studying, don’t know | 0.98 | 0.67 to 1.43 | 0.90 | 0.93 | 0.61 to 1.43 | 0.75 |
| Social grade | ||||||
| C1 | 0.73 | 0.56 to 0.94 | 0.016 | 0.87 | 0.66 to 1.14 | 0.32 |
| C2 | 1.45 | 1.07 to 1.97 | 0.017 | 1.64 | 1.18 to 2.27 | 0.003 |
| D | 1.22 | 0.78 to 1.92 | 0.38 | 1.31 | 0.82 to 2.10 | 0.26 |
| E | 1.22 | 0.74 to 2.01 | 0.43 | 1.18 | 0.70 to 1.99 | 0.54 |
| Smoking status | ||||||
| Previous smoker | 0.85 | 0.64 to 1.14 | 0.28 | 0.94 | 0.68 to 1.29 | 0.69 |
| Current (tried quitting) | 1.15 | 0.83 to 1.58 | 0.40 | 1.23 | 0.84 to 1.81 | 0.28 |
| Current (not tried quitting) | 0.92 | 0.64 to 1.31 | 0.64 | 1.11 | 0.73 to 1.70 | 0.62 |
| Refused to answer | 1.25 | 0.86 to 1.81 | 0.24 | 1.37 | 0.87 to 2.15 | 0.17 |
| Area | ||||||
| North | 0.46 | 0.28 to 0.75 | 0.002 | 0.60 | 0.36 to 1.01 | 0.054 |
| North West | 0.84 | 0.57 to 1.25 | 0.38 | 1.02 | 0.67 to 1.55 | 0.92 |
| Yorkshire and Humberside | 0.42 | 0.27 to 0.67 | < 0.001 | 0.51 | 0.31 to 0.83 | 0.007 |
| East Midlands | 0.46 | 0.30 to 0.71 | < 0.001 | 0.56 | 0.36 to 0.88 | 0.012 |
| West Midlands | 0.36 | 0.21 to 0.61 | < 0.001 | 0.49 | 0.28 to 0.85 | 0.011 |
| East Anglia | 0.85 | 0.45 to 1.61 | 0.62 | 1.13 | 0.58 to 2.20 | 0.71 |
| South East | 0.67 | 0.41 to 1.08 | 0.10 | 1.01 | 0.60 to 1.70 | 0.97 |
| South West | 0.51 | 0.35 to 0.74 | < 0.001 | 0.73 | 0.49 to 1.10 | 0.14 |
| Wales | 0.35 | 0.21 to 0.61 | < 0.001 | 0.51 | 0.29 to 0.91 | 0.022 |
| Scotland | 0.31 | 0.20 to 0.49 | < 0.001 | 0.44 | 0.28 to 0.71 | 0.001 |
| Variable | SD | D | N | A | SA |
|---|---|---|---|---|---|
| Age category (years) | |||||
| 18–24 | 27 (15.9) | 35 (20.6) | 43 (25.3) | 49 (28.8) | 16 (9.4) |
| 25–34 | 31 (17.7) | 38 (21.7) | 41 (23.4) | 44 (25.1) | 21 (12.0) |
| 35–44 | 38 (21.0) | 37 (20.4) | 35 (19.3) | 41 (22.7) | 30 (16.6) |
| 45–54 | 43 (27.0) | 33 (20.8) | 25 (15.7) | 42 (26.4) | 16 (10.1) |
| 55–59 | 30 (41.7) | 14 (19.4) | 6 (8.3) | 11 (15.3) | 11 (15.3) |
| 60–64 | 32 (34.0) | 11 (11.7) | 16 (17.0) | 22 (23.4) | 13 (13.8) |
| 65+ | 110 (37.5) | 47 (16.0) | 58 (19.8) | 38 (13.0) | 40 (13.7) |
| Breastfeeding | |||||
| Children not breastfed | 161 (25.5) | 124 (19.6) | 146 (23.1) | 127 (20.1) | 74 (11.7) |
| Children breastfed | 150 (29.3) | 91 (17.8) | 78 (15.2) | 120 (23.4) | 73 (14.3) |
| Children | |||||
| No children | 90 (22.4) | 81 (20.1) | 94 (23.4) | 91 (22.6) | 46 (11.4) |
| Have children | 221 (29.8) | 134 (18.1) | 130 (17.5) | 156 (21.0) | 101 (13.6) |
| Ethnicity | |||||
| White | 296 (30.1) | 192 (19.5) | 183 (18.6) | 191 (19.4) | 123 (12.5) |
| Other ethnicity | 15 (9.4) | 23 (14.5) | 41 (25.8) | 56 (35.2) | 24 (15.1) |
| Sex | |||||
| Male | 128 (23.7) | 92 (17.0) | 125 (23.1) | 116 (21.5) | 79 (14.6) |
| Female | 183 (30.3) | 123 (20.4) | 99 (16.4) | 131 (21.7) | 68 (11.3) |
| Education | |||||
| University | 66 (22.4) | 50 (16.9) | 58 (19.7) | 74 (25.1) | 47 (15.9) |
| GCSE | 102 (29.8) | 62 (18.1) | 63 (18.4) | 75 (21.9) | 40 (11.7) |
| A-level | 55 (28.5) | 42 (21.8) | 40 (20.7) | 37 (19.2) | 19 (9.8) |
| No formal qualification | 64 (32.5) | 32 (16.2) | 44 (22.3) | 36 (18.3) | 21 (10.7) |
| Other, still studying, don’t know | 24 (20.5) | 29 (24.8) | 19 (16.2) | 25 (21.4) | 20 (17.1) |
| Social grade | |||||
| AB | 66 (27.6) | 48 (20.1) | 39 (16.3) | 49 (20.5) | 37 (15.5) |
| C1 | 111 (30.0) | 81 (21.9) | 68 (18.4) | 73 (19.7) | 37 (10.0) |
| C2 | 57 (24.2) | 41 (17.4) | 48 (20.3) | 56 (23.7) | 34 (14.4) |
| D | 48 (29.6) | 24 (14.8) | 39 (24.1) | 32 (19.8) | 19 (11.7) |
| E | 29 (21.2) | 21 (15.3) | 30 (21.9) | 37 (27.0) | 20 (14.6) |
| Smoking status | |||||
| Never smoked | 147 (25.7) | 108 (18.8) | 112 (19.5) | 142 (24.8) | 64 (11.2) |
| Previous smoker | 100 (35.6) | 55 (19.6) | 48 (17.1) | 48 (17.1) | 30 (10.7) |
| Current (tried quitting) | 38 (21.7) | 33 (18.9) | 32 (18.3) | 36 (20.6) | 36 (20.6) |
| Current (not tried quitting) | 13 (20.6) | 10 (15.9) | 19 (30.2) | 10 (15.9) | 11 (17.5) |
| Refused to answer | 13 (25.0) | 9 (17.3) | 13 (25.0) | 11 (21.2) | 6 (11.5) |
| Area | |||||
| North | 21 (27.3) | 19 (24.7) | 10 (13.0) | 14 (18.2) | 13 (16.9) |
| North West | 21 (14.8) | 21 (14.8) | 44 (31.0) | 35 (24.6) | 21 (14.8) |
| Yorkshire and Humberside | 38 (36.5) | 15 (14.4) | 23 (22.1) | 14 (13.5) | 14 (13.5) |
| East Midlands | 29 (26.6) | 30 (27.5) | 22 (20.2) | 19 (17.4) | 9 (8.3) |
| West Midlands | 28 (42.4) | 11 (16.7) | 8 (12.1) | 11 (16.7) | 8 (12.1) |
| East Anglia | 10 (24.4) | 7 (17.1) | 6 (14.6) | 9 (22.0) | 9 (22.0) |
| South East | 23 (28.4) | 8 (9.9) | 18 (22.2) | 22 (27.2) | 10 (12.3) |
| South West | 54 (27.0) | 41 (20.5) | 47 (23.5) | 35 (17.5) | 23 (11.5) |
| London | 15 (10.1) | 35 (23.5) | 22 (14.8) | 55 (36.9) | 22 (14.8) |
| Wales | 29 (43.9) | 9 (13.6) | 6 (9.1) | 12 (18.2) | 10 (15.2) |
| Scotland | 43 (39.4) | 19 (17.4) | 18 (16.5) | 21 (19.3) | 8 (7.3) |
| Variable | Simple regression model | Multiple regression model | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age category (years) | ||||||
| 18–24 | 1.87 | 1.34 to 2.60 | < 0.001 | 1.55 | 1.02 to 2.35 | 0.040 |
| 25–34 | 1.80 | 1.29 to 2.50 | 0.001 | 1.42 | 0.97 to 2.07 | 0.068 |
| 35–44 | 1.83 | 1.31 to 2.56 | < 0.001 | 1.51 | 1.04 to 2.17 | 0.028 |
| 45–54 | 1.40 | 0.99 to 1.98 | 0.058 | 1.26 | 0.86 to 1.83 | 0.23 |
| 55–59 | 0.89 | 0.54 to 1.44 | 0.63 | 0.88 | 0.53 to 1.46 | 0.62 |
| 60–64 | 1.36 | 0.88 to 2.09 | 0.16 | 1.28 | 0.82 to 2.00 | 0.28 |
| Breastfeeding | ||||||
| Children breastfed | 1.03 | 0.83 to 1.26 | 0.81 | 1.27 | 0.95 to 1.71 | 0.11 |
| Children | ||||||
| Have children | 0.87 | 0.70 to 1.08 | 0.21 | 0.96 | 0.70 to 1.31 | 0.78 |
| Ethnicity | ||||||
| Other ethnicity | 2.26 | 1.70 to 3.01 | < 0.001 | 1.49 | 1.06 to 2.08 | 0.021 |
| Sex | ||||||
| Female | 0.75 | 0.61 to 0.92 | 0.005 | 0.72 | 0.58 to 0.90 | 0.003 |
| Education | ||||||
| GCSE | 0.70 | 0.53 to 0.92 | 0.012 | 0.60 | 0.44 to 0.83 | 0.002 |
| A-level | 0.64 | 0.47 to 0.89 | 0.007 | 0.56 | 0.39 to 0.79 | 0.001 |
| No formal qualification | 0.62 | 0.45 to 0.86 | 0.004 | 0.66 | 0.44 to 1.00 | 0.048 |
| Other, still studying, don’t know | 0.93 | 0.64 to 1.37 | 0.73 | 0.90 | 0.59 to 1.38 | 0.64 |
| Social grade | ||||||
| C1 | 0.80 | 0.60 to 1.07 | 0.13 | 0.84 | 0.61 to 1.16 | 0.29 |
| C2 | 1.15 | 0.83 to 1.59 | 0.39 | 1.22 | 0.83 to 1.79 | 0.30 |
| D | 0.91 | 0.64 to 1.31 | 0.62 | 1.05 | 0.69 to 1.61 | 0.81 |
| E | 1.33 | 0.92 to 1.93 | 0.13 | 1.37 | 0.88 to 2.15 | 0.17 |
| Smoking status | ||||||
| Previous smoker | 0.67 | 0.52 to 0.87 | 0.002 | 0.79 | 0.60 to 1.04 | 0.089 |
| Current (tried quitting) | 1.33 | 0.98 to 1.80 | 0.065 | 1.48 | 1.08 to 2.04 | 0.016 |
| Current (not tried quitting) | 1.22 | 0.77 to 1.92 | 0.40 | 1.31 | 0.81 to 2.11 | 0.27 |
| Refused to answer | 1.00 | 0.61 to 1.64 | 0.99 | 0.95 | 0.57 to 1.59 | 0.85 |
| Area | ||||||
| North | 0.53 | 0.32 to 0.87 | 0.012 | 0.74 | 0.44 to 1.24 | 0.25 |
| North West | 0.84 | 0.57 to 1.25 | 0.39 | 1.06 | 0.70 to 1.60 | 0.80 |
| Yorkshire and Humberside | 0.40 | 0.26 to 0.63 | < 0.001 | 0.50 | 0.31 to 0.81 | 0.005 |
| East Midlands | 0.43 | 0.28 to 0.65 | < 0.001 | 0.53 | 0.34 to 0.82 | 0.004 |
| West Midlands | 0.32 | 0.19 to 0.55 | < 0.001 | 0.47 | 0.27 to 0.82 | 0.00 |
| East Anglia | 0.77 | 0.41 to 1.45 | 0.42 | 1.08 | 0.57 to 2.06 | 0.82 |
| South East | 0.63 | 0.39 to 1.01 | 0.057 | 1.06 | 0.64 to 1.75 | 0.84 |
| South West | 0.49 | 0.34 to 0.71 | < 0.001 | 0.77 | 0.51 to 1.15 | 0.20 |
| Wales | 0.35 | 0.20 to 0.60 | < 0.001 | 0.56 | 0.32 to 1.01 | 0.054 |
| Scotland | 0.33 | 0.21 to 0.52 | < 0.001 | 0.52 | 0.32 to 0.83 | 0.006 |
| Variable | SD | D | N | A | SA |
|---|---|---|---|---|---|
| Age category (years) | |||||
| 18–24 | 20 (11.8) | 32 (18.8) | 58 (34.1) | 42 (24.7) | 18 (10.6) |
| 25–34 | 23 (13.1) | 30 (17.1) | 45 (25.7) | 47 (26.9) | 30 (17.1) |
| 35–44 | 25 (13.8) | 36 (19.9) | 46 (25.4) | 39 (21.5) | 35 (19.3) |
| 45–54 | 32 (20.1) | 34 (21.4) | 40 (25.2) | 34 (21.4) | 19 (11.9) |
| 55–59 | 18 (25.0) | 20 (27.8) | 16 (22.2) | 7 (9.7) | 11 (15.3) |
| 60–64 | 31 (33.0) | 15 (16.0) | 19 (20.2) | 18 (19.1) | 11 (11.7) |
| 65+ | 87 (29.7) | 44 (15.0) | 82 (28.0) | 48 (16.4) | 32 (10.9) |
| Breastfeeding | |||||
| Children not breastfed | 114 (18.0) | 120 (19.0) | 216 (34.2) | 128 (20.3) | 54 (8.5) |
| Children breastfed | 122 (23.8) | 91 (17.8) | 90 (17.6) | 107 (20.9) | 102 (19.9) |
| Children | |||||
| No children | 61 (15.2) | 73 (18.2) | 138 (34.3) | 93 (23.1) | 37 (9.2) |
| Have children | 175 (23.6) | 138 (18.6) | 168 (22.6) | 142 (19.1) | 119 (16.0) |
| Ethnicity | |||||
| White | 226 (22.9) | 194 (19.7) | 265 (26.9) | 184 (18.7) | 116 (11.8) |
| Other ethnicity | 10 (6.3) | 17 (10.7) | 41 (25.8) | 51 (32.1) | 40 (25.2) |
| Sex | |||||
| Male | 93 (17.2) | 84 (15.6) | 180 (33.3) | 115 (21.3) | 68 (12.6) |
| Female | 143 (23.7) | 127 (21.0) | 126 (20.9) | 120 (19.9) | 88 (14.6) |
| Education | |||||
| University | 58 (19.7) | 59 (20.0) | 72 (24.4) | 61 (20.7) | 45 (15.3) |
| GCSE | 66 (19.3) | 63 (18.4) | 90 (26.3) | 72 (21.1) | 51 (14.9) |
| A-level | 47 (24.4) | 40 (20.7) | 52 (26.9) | 29 (15.0) | 25 (13.0) |
| No formal qualification | 43 (21.8) | 28 (14.2) | 66 (33.5) | 37 (18.8) | 23 (11.7) |
| Other, still studying, don’t know | 22 (18.8) | 21 (17.9) | 26 (22.2) | 36 (30.8) | 12 (10.3) |
| Social grade | |||||
| AB | 59 (24.7) | 40 (16.7) | 61 (25.5) | 45 (18.8) | 34 (14.2) |
| C1 | 82 (22.2) | 86 (23.2) | 85 (23.0) | 74 (20.0) | 43 (11.6) |
| C2 | 41 (17.4) | 40 (16.9) | 73 (30.9) | 50 (21.2) | 32 (13.6) |
| D | 31 (19.1) | 26 (16.0) | 52 (32.1) | 33 (20.4) | 20 (12.3) |
| E | 23 (16.8) | 19 (13.9) | 35 (25.5) | 33 (24.1) | 27 (19.7) |
| Smoking status | |||||
| Never smoked | 113 (19.7) | 104 (18.2) | 142 (24.8) | 139 (24.3) | 75 (13.1) |
| Previous smoker | 70 (24.9) | 59 (21.0) | 72 (25.6) | 45 (16.0) | 35 (12.5) |
| Current (tried quitting) | 27 (15.4) | 33 (18.9) | 53 (30.3) | 26 (14.9) | 36 (20.6) |
| Current (not tried quitting) | 14 (22.2) | 11 (17.5) | 19 (30.2) | 16 (25.4) | 3 (4.8) |
| Refused to answer | 12 (23.1) | 4 (7.7) | 20 (38.5) | 9 (17.3) | 7 (13.5) |
| Area | |||||
| North | 16 (20.8) | 16 (20.8) | 19 (24.7) | 8 (10.4) | 18 (23.4) |
| North West | 15 (10.6) | 26 (18.3) | 41 (28.9) | 36 (25.4) | 24 (16.9) |
| Yorkshire and Humberside | 28 (26.9) | 15 (14.4) | 32 (30.8) | 21 (20.2) | 8 (7.7) |
| East Midlands | 22 (20.2) | 26 (23.9) | 33 (30.3) | 22 (20.2) | 6 (5.5) |
| West Midlands | 23 (34.8) | 14 (21.2) | 15 (22.7) | 7 (10.6) | 7 (10.6) |
| East Anglia | 4 (9.8) | 11 (26.8) | 8 (19.5) | 10 (24.4) | 8 (19.5) |
| South East | 14 (17.3) | 11 (13.6) | 28 (34.6) | 15 (18.5) | 13 (16.0) |
| South West | 52 (26.0) | 37 (18.5) | 61 (30.5) | 33 (16.5) | 17 (8.5) |
| London | 14 (9.4) | 25 (16.8) | 27 (18.1) | 56 (37.6) | 27 (18.1) |
| Wales | 23 (34.8) | 13 (19.7) | 10 (15.2) | 9 (13.6) | 11 (16.7) |
| Scotland | 25 (22.9) | 17 (15.6) | 32 (29.4) | 18 (16.5) | 17 (15.6) |
| Variable | Simple regression model | Multiple regression model | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age category (years) | ||||||
| 18–24 | 1.71 | 1.23 to 2.37 | 0.001 | 1.71 | 1.13 to 2.60 | 0.012 |
| 25–34 | 2.09 | 1.49 to 2.92 | < 0.001 | 1.91 | 1.31 to 2.80 | 0.001 |
| 35–44 | 1.94 | 1.39 to 2.71 | < 0.001 | 1.73 | 1.20 to 2.50 | 0.003 |
| 45–54 | 1.32 | 0.94 to 1.86 | 0.11 | 1.38 | 0.95 to 2.01 | 0.090 |
| 55–59 | 0.96 | 0.60 to 1.52 | 0.85 | 1.02 | 0.63 to 1.64 | 0.95 |
| 60–64 | 0.94 | 0.61 to 1.44 | 0.78 | 1.01 | 0.65 to 1.59 | 0.95 |
| Breastfeeding | ||||||
| Children breastfed | 1.19 | 0.96 to 1.46 | 0.11 | 1.67 | 1.24 to 2.25 | 0.001 |
| Children | ||||||
| Have children | 0.90 | 0.73 to 1.11 | 0.31 | 0.80 | 0.59 to 1.08 | 0.15 |
| Ethnicity | ||||||
| Other ethnicity | 3.04 | 2.26 to 4.10 | < 0.001 | 2.03 | 1.43 to 2.88 | < 0.001 |
| Sex | ||||||
| Female | 0.80 | 0.65 to 0.99 | 0.037 | 0.77 | 0.62 to 0.95 | 0.016 |
| Education | ||||||
| GCSE | 1.03 | 0.78 to 1.36 | 0.84 | 1.01 | 0.74 to 1.40 | 0.93 |
| A-level | 0.76 | 0.55 to 1.04 | 0.089 | 0.71 | 0.50 to 1.02 | 0.061 |
| No formal qualification | 0.91 | 0.66 to 1.25 | 0.56 | 1.22 | 0.82 to 1.82 | 0.33 |
| Other, still studying, don’t know | 1.07 | 0.74 to 1.57 | 0.71 | 1.10 | 0.72 to 1.67 | 0.67 |
| Social grade | ||||||
| C1 | 0.93 | 0.70 to 1.25 | 0.63 | 0.84 | 0.61 to 1.16 | 0.29 |
| C2 | 1.23 | 0.90 to 1.70 | 0.20 | 0.96 | 0.66 to 1.40 | 0.83 |
| D | 1.14 | 0.80 to 1.63 | 0.46 | 0.92 | 0.60 to 1.40 | 0.6 |
| E | 1.62 | 1.11 to 2.37 | 0.012 | 1.21 | 0.77 to 1.89 | 0.41 |
| Smoking status | ||||||
| Previous smoker | 0.73 | 0.56 to 0.94 | 0.014 | 0.89 | 0.67 to 1.17 | 0.40 |
| Current (tried quitting) | 1.17 | 0.87 to 1.58 | 0.31 | 1.17 | 0.85 to 1.61 | 0.34 |
| Current (not tried quitting) | 0.78 | 0.50 to 1.23 | 0.29 | 0.75 | 0.47 to 1.19 | 0.22 |
| Refused to answer | 0.97 | 0.59 to 1.59 | 0.89 | 0.91 | 0.55 to 1.51 | 0.71 |
| Area | ||||||
| North | 0.53 | 0.32 to 0.88 | 0.014 | 0.79 | 0.47 to 1.35 | 0.39 |
| North West | 0.76 | 0.51 to 1.13 | 0.18 | 1.01 | 0.66 to 1.55 | 0.95 |
| Yorkshire and Humberside | 0.38 | 0.25 to 0.60 | < 0.001 | 0.56 | 0.35 to 0.90 | 0.016 |
| East Midlands | 0.38 | 0.25 to 0.59 | < 0.001 | 0.46 | 0.29 to 0.71 | < 0.001 |
| West Midlands | 0.25 | 0.15 to 0.42 | < 0.001 | 0.40 | 0.23 to 0.69 | 0.001 |
| East Anglia | 0.73 | 0.40 to 1.35 | 0.32 | 0.98 | 0.52 to 1.85 | 0.94 |
| South East | 0.60 | 0.38 to 0.97 | 0.039 | 1.01 | 0.61 to 1.69 | 0.96 |
| South West | 0.36 | 0.25 to 0.52 | < 0.001 | 0.62 | 0.41 to 0.93 | 0.021 |
| Wales | 0.30 | 0.18 to 0.52 | < 0.001 | 0.53 | 0.30 to 0.94 | 0.029 |
| Scotland | 0.48 | 0.31 to 0.75 | 0.001 | 0.84 | 0.52 to 1.36 | 0.47 |
| Variable | SD | D | N | A | SA |
|---|---|---|---|---|---|
| Age category (years) | |||||
| 18–24 | 10 (5.9) | 21 (12.4) | 58 (34.1) | 52 (30.6) | 29 (17.1) |
| 25–34 | 18 (10.3) | 17 (9.7) | 48 (27.4) | 57 (32.6) | 35 (20.0) |
| 35–44 | 22 (12.2) | 24 (13.3) | 37 (20.4) | 57 (31.5) | 41 (22.7) |
| 45–54 | 27 (17.0) | 24 (15.1) | 34 (21.4) | 44 (27.7) | 30 (18.9) |
| 55–59 | 10 (13.9) | 13 (18.1) | 19 (26.4) | 22 (30.6) | 8 (11.1) |
| 6 –64 | 22 (23.4) | 10 (10.6) | 23 (24.5) | 21 (22.3) | 18 (19.1) |
| 65+ | 56 (19.1) | 38 (13.0) | 89 (30.4) | 63 (21.5) | 47 (16.0) |
| Breastfeeding | |||||
| Children not breastfed | 93 (14.7) | 79 (12.5) | 210 (33.2) | 164 (25.9) | 86 (13.6) |
| Children breastfed | 72 (14.1) | 68 (13.3) | 98 (19.1) | 152 (29.7) | 122 (23.8) |
| Children | |||||
| No children | 49 (12.2) | 50 (12.4) | 132 (32.8) | 119 (29.6) | 52 (12.9) |
| Have children | 116 (15.6) | 97 (13.1) | 176 (23.7) | 197 (26.5) | 156 (21.0) |
| Ethnicity | |||||
| White | 158 (16.0) | 132 (13.4) | 252 (25.6) | 265 (26.9) | 178 (18.1) |
| Other ethnicity | 7 (4.4) | 15 (9.4) | 56 (35.2) | 51 (32.1) | 30 (18.9) |
| Sex | |||||
| Male | 70 (13.0) | 62 (11.5) | 174 (32.2) | 142 (26.3) | 92 (17.0) |
| Female | 95 (15.7) | 85 (14.1) | 134 (22.2) | 174 (28.8) | 116 (19.2) |
| Education | |||||
| University | 31 (10.5) | 36 (12.2) | 75 (25.4) | 87 (29.5) | 66 (22.4) |
| GCSE | 54 (15.8) | 40 (11.7) | 98 (28.7) | 89 (26.0) | 61 (17.8) |
| A-level | 30 (15.5) | 25 (13.0) | 51 (26.4) | 49 (25.4) | 38 (19.7) |
| No formal qualification | 36 (18.3) | 29 (14.7) | 56 (28.4) | 51 (25.9) | 25 (12.7) |
| Other, still studying, don’t know | 14 (12.0) | 17 (14.5) | 28 (23.9) | 40 (34.2) | 18 (15.4) |
| Social grade | |||||
| AB | 36 (15.1) | 32 (13.4) | 55 (23.0) | 59 (24.7) | 57 (23.8) |
| C1 | 57 (15.4) | 58 (15.7) | 98 (26.5) | 99 (26.8) | 58 (15.7) |
| C2 | 33 (14.0) | 23 (9.7) | 76 (32.2) | 71 (30.1) | 33 (14.0) |
| D | 26 (16.0) | 20 (12.3) | 43 (26.5) | 44 (27.2) | 29 (17.9) |
| E | 13 (9.5) | 14 (10.2) | 36 (26.3) | 43 (31.4) | 31 (22.6) |
| Smoking status | |||||
| Never smoked | 71 (12.4) | 68 (11.9) | 167 (29.1) | 173 (30.2) | 94 (16.4) |
| Previous smoker | 50 (17.8) | 43 (15.3) | 64 (22.8) | 76 (27.0) | 48 (17.1) |
| Current (tried quitting) | 27 (15.4) | 22 (12.6) | 40 (22.9) | 38 (21.7) | 48 (27.4) |
| Current (not tried quitting) | 8 (12.7) | 7 (11.1) | 17 (27.0) | 19 (30.2) | 12 (19.0) |
| Refused to answer | 9 (17.3) | 7 (13.5) | 20 (38.5) | 10 (19.2) | 6 (11.5) |
| Area | |||||
| North | 14 (18.2) | 11 (14.3) | 13 (16.9) | 21 (27.3) | 18 (23.4) |
| North West | 7 (4.9) | 14 (9.9) | 46 (32.4) | 49 (34.5) | 26 (18.3) |
| Yorkshire and Humberside | 21 (20.2) | 3 (2.9) | 25 (24.0) | 28 (26.9) | 27 (26.0) |
| East Midlands | 18 (16.5) | 28 (25.7) | 32 (29.4) | 20 (18.3) | 11 (10.1) |
| West Midlands | 9 (13.6) | 12 (18.2) | 13 (19.7) | 21 (31.8) | 11 (16.7) |
| East Anglia | 5 (12.2) | 6 (14.6) | 10 (24.4) | 13 (31.7) | 7 (17.1) |
| South East | 5 (6.2) | 8 (9.9) | 26 (32.1) | 20 (24.7) | 22 (27.2) |
| South West | 36 (18.0) | 23 (11.5) | 70 (35.0) | 39 (19.5) | 32 (16.0) |
| London | 7 (4.7) | 15 (10.1) | 37 (24.8) | 68 (45.6) | 22 (14.8) |
| Wales | 21 (31.8) | 13 (19.7) | 7 (10.6) | 13 (19.7) | 12 (18.2) |
| Scotland | 22 (20.2) | 14 (12.8) | 29 (26.6) | 24 (22.0) | 20 (18.3) |
| Variable | Simple regression model | Multiple regression model | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age category (years) | ||||||
| 18–24 | 1.60 | 1.15 to 2.22 | 0.005 | 1.74 | 1.14 to 2.63 | 0.010 |
| 25–34 | 1.73 | 1.24 to 2.41 | 0.001 | 1.63 | 1.11 to 2.37 | 0.012 |
| 35–44 | 1.72 | 1.23 to 2.40 | 0.002 | 1.57 | 1.08 to 2.27 | 0.017 |
| 45–54 | 1.24 | 0.88 to 1.76 | 0.22 | 1.22 | 0.84 to 1.78 | 0.30 |
| 55–59 | 1.06 | 0.68 to 1.67 | 0.79 | 0.92 | 0.57 to 1.48 | 0.74 |
| 60–64 | 1.04 | 0.68 to 1.59 | 0.87 | 0.90 | 0.58 to 1.41 | 0.65 |
| Breastfeeding | ||||||
| Children breastfed | 1.49 | 1.21 to 1.84 | < 0.001 | 1.84 | 1.36 to 2.49 | < 0.001 |
| Children | ||||||
| Have children | 1.13 | 0.91 to 1.39 | 0.27 | 0.95 | 0.70 to 1.30 | 0.75 |
| Ethnicity | ||||||
| Other ethnicity | 1.46 | 1.10 to 1.95 | 0.009 | 1.07 | 0.76 to 1.51 | 0.70 |
| Sex | ||||||
| Female | 1.02 | 0.83 to 1.26 | 0.84 | 0.95 | 0.77 to 1.18 | 0.66 |
| Education | ||||||
| GCSE | 0.73 | 0.55 to 0.97 | 0.028 | 0.70 | 0.51 to 0.96 | 0.026 |
| A-level | 0.76 | 0.55 to 1.05 | 0.099 | 0.73 | 0.52 to 1.04 | 0.085 |
| No formal qualification | 0.57 | 0.41 to 0.78 | 0.001 | 0.62 | 0.41 to 0.93 | 0.020 |
| Other, still studying, don’t know | 0.82 | 0.56 to 1.19 | 0.29 | 0.87 | 0.57 to 1.33 | 0.53 |
| Social grade | ||||||
| C1 | 0.77 | 0.57 to 1.03 | 0.076 | 0.84 | 0.61 to 1.15 | 0.28 |
| C2 | 0.86 | 0.62 to 1.18 | 0.34 | 0.92 | 0.63 to 1.34 | 0.67 |
| D | 0.85 | 0.59 to 1.22 | 0.38 | 1.02 | 0.67 to 1.55 | 0.93 |
| E | 1.25 | 0.86 to 1.82 | 0.24 | 1.57 | 1.00 to 2.46 | 0.050 |
| Smoking status | ||||||
| Previous smoker | 0.82 | 0.64 to 1.06 | 0.14 | 0.93 | 0.71 to 1.23 | 0.62 |
| Current (tried quitting) | 1.16 | 0.85 to 1.59 | 0.35 | 1.13 | 0.81 to 1.57 | 0.47 |
| Current (not tried quitting) | 1.09 | 0.69 to 1.73 | 0.71 | 1.25 | 0.78 to 2.01 | 0.36 |
| Refused to answer | 0.64 | 0.39 to 1.05 | 0.076 | 0.67 | 0.41 to 1.12 | 0.12 |
| Area | ||||||
| North | 0.71 | 0.43 to 1.18 | 0.19 | 0.86 | 0.51 to 1.45 | 0.56 |
| North West | 0.93 | 0.63 to 1.38 | 0.73 | 1.15 | 0.77 to 1.74 | 0.49 |
| Yorkshire and Humberside | 0.87 | 0.56 to 1.37 | 0.55 | 1.05 | 0.65 to 1.70 | 0.84 |
| East Midlands | 0.36 | 0.24 to 0.55 | < 0.001 | 0.41 | 0.26 to 0.64 | < 0.001 |
| West Midlands | 0.65 | 0.39 to 1.08 | 0.096 | 0.81 | 0.47 to 1.41 | 0.46 |
| East Anglia | 0.71 | 0.39 to 1.30 | 0.26 | 0.78 | 0.42 to 1.48 | 0.45 |
| South East | 1.04 | 0.65 to 1.68 | 0.86 | 1.40 | 0.85 to 2.31 | 0.19 |
| South West | 0.51 | 0.35 to 0.73 | < 0.001 | 0.68 | 0.45 to 1.02 | 0.065 |
| Wales | 0.32 | 0.19 to 0.55 | < 0.001 | 0.44 | 0.25 to 0.79 | 0.006 |
| Scotland | 0.53 | 0.34 to 0.82 | 0.004 | 0.73 | 0.45 to 1.18 | 0.20 |
| Variable | SD | D | N | A | SA |
|---|---|---|---|---|---|
| Age category (years) | |||||
| 18–24 | 19 (11.2) | 29 (17.1) | 40 (23.5) | 61 (35.9) | 21 (12.4) |
| 25–34 | 22 (12.6) | 32 (18.3) | 42 (24.0) | 57 (32.6) | 22 (12.6) |
| 35–44 | 23 (12.7) | 32 (17.7) | 43 (23.8) | 56 (30.9) | 27 (14.9) |
| 45–54 | 32 (20.1) | 28 (17.6) | 40 (25.2) | 35 (22.0) | 24 (15.1) |
| 55–59 | 17 (23.6) | 15 (20.8) | 10 (13.9) | 20 (27.8) | 10 (13.9) |
| 60–64 | 29 (30.9) | 10 (10.6) | 22 (23.4) | 24 (25.5) | 9 (9.6) |
| 65+ | 92 (31.4) | 46 (15.7) | 70 (23.9) | 53 (18.1) | 32 (10.9) |
| Breastfeeding | |||||
| Children not breastfed | 125 (19.8) | 111 (17.6) | 151 (23.9) | 168 (26.6) | 77 (12.2) |
| Children breastfed | 109 (21.3) | 81 (15.8) | 116 (22.7) | 138 (27.0) | 68 (13.3) |
| Children | |||||
| No children | 75 (18.7) | 66 (16.4) | 97 (24.1) | 117 (29.1) | 47 (11.7) |
| Have children | 159 (21.4) | 126 (17.0) | 170 (22.9) | 189 (25.5) | 98 (13.2) |
| Ethnicity | |||||
| White | 224 (22.7) | 169 (17.2) | 223 (22.6) | 248 (25.2) | 121 (12.3) |
| Other ethnicity | 10 (6.3) | 23 (14.5) | 44 (27.7) | 58 (36.5) | 24 (15.1) |
| Sex | |||||
| Male | 101 (18.7) | 91 (16.9) | 130 (24.1) | 149 (27.6) | 69 (12.8) |
| Female | 133 (22.0) | 101 (16.7) | 137 (22.7) | 157 (26.0) | 76 (12.6) |
| Education | |||||
| University | 51 (17.3) | 45 (15.3) | 68 (23.1) | 87 (29.5) | 44 (14.9) |
| GCSE | 75 (21.9) | 59 (17.3) | 83 (24.3) | 86 (25.1) | 39 (11.4) |
| A-level | 39 (20.2) | 41 (21.2) | 39 (20.2) | 48 (24.9) | 26 (13.5) |
| No formal qualification | 48 (24.4) | 28 (14.2) | 50 (25.4) | 51 (25.9) | 20 (10.2) |
| Other, still studying, don’t know | 21 (17.9) | 19 (16.2) | 27 (23.1) | 34 (29.1) | 16 (13.7) |
| Social grade | |||||
| AB | 50 (20.9) | 34 (14.2) | 51 (21.3) | 70 (29.3) | 34 (14.2) |
| C1 | 88 (23.8) | 76 (20.5) | 76 (20.5) | 91 (24.6) | 39 (10.5) |
| C2 | 41 (17.4) | 42 (17.8) | 59 (25.0) | 62 (26.3) | 32 (13.6) |
| D | 29 (17.9) | 23 (14.2) | 44 (27.2) | 42 (25.9) | 24 (14.8) |
| E | 26 (19.0) | 17 (12.4) | 37 (27.0) | 41 (29.9) | 16 (11.7) |
| Smoking status | |||||
| Never smoked | 104 (18.2) | 99 (17.3) | 135 (23.6) | 167 (29.1) | 68 (11.9) |
| Previous smoker | 75 (26.7) | 48 (17.1) | 66 (23.5) | 62 (22.1) | 30 (10.7) |
| Current (tried quitting) | 32 (18.3) | 27 (15.4) | 37 (21.1) | 43 (24.6) | 36 (20.6) |
| Current (not tried quitting) | 13 (20.6) | 13 (20.6) | 14 (22.2) | 16 (25.4) | 7 (11.1) |
| Refused to answer | 10 (19.2) | 5 (9.6) | 15 (28.8) | 18 (34.6) | 4 (7.7) |
| Area | |||||
| North | 19 (24.7) | 18 (23.4) | 11 (14.3) | 17 (22.1) | 12 (15.6) |
| North West | 15 (10.6) | 26 (18.3) | 42 (29.6) | 42 (29.6) | 17 (12.0) |
| Yorkshire and Humberside | 30 (28.8) | 11 (10.6) | 20 (19.2) | 26 (25.0) | 17 (16.3) |
| East Midlands | 24 (22.0) | 24 (22.0) | 25 (22.9) | 28 (25.7) | 8 (7.3) |
| West Midlands | 19 (28.8) | 9 (13.6) | 15 (22.7) | 17 (25.8) | 6 (9.1) |
| East Anglia | 10 (24.4) | 6 (14.6) | 8 (19.5) | 9 (22.0) | 8 (19.5) |
| South East | 9 (11.1) | 8 (9.9) | 25 (30.9) | 23 (28.4) | 16 (19.8) |
| South West | 53 (26.5) | 35 (17.5) | 56 (28.0) | 43 (21.5) | 13 (6.5) |
| London | 8 (5.4) | 25 (16.8) | 34 (22.8) | 59 (39.6) | 23 (15.4) |
| Wales | 20 (30.3) | 7 (10.6) | 9 (13.6) | 17 (25.8) | 13 (19.7) |
| Scotland | 27 (24.8) | 23 (21.1) | 22 (20.2) | 25 (22.9) | 12 (11.0) |
| Variable | Simple regression model | Multiple regression model | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age category (years) | ||||||
| 18–24 | 2.24 | 1.60 to 3.14 | < 0.001 | 2.28 | 1.50 to 3.49 | < 0.001 |
| 25–34 | 2.05 | 1.47 to 2.86 | < 0.001 | 1.83 | 1.26 to 2.67 | 0.002 |
| 35–44 | 2.15 | 1.54 to 3.00 | < 0.001 | 1.90 | 1.32 to 2.74 | 0.001 |
| 45–54 | 1.58 | 1.11 to 2.23 | 0.010 | 1.57 | 1.08 to 2.28 | 0.017 |
| 55–59 | 1.46 | 0.91 to 2.35 | 0.11 | 1.43 | 0.87 to 2.34 | 0.16 |
| 60–64 | 1.18 | 0.77 to 1.80 | 0.44 | 1.05 | 0.68 to 1.63 | 0.82 |
| Breastfeeding | ||||||
| Children breastfed | 1.02 | 0.83 to 1.26 | 0.83 | 1.12 | 0.83 to 1.50 | 0.45 |
| Children | ||||||
| Have children | 0.92 | 0.74 to 1.13 | 0.42 | 1.08 | 0.79 to 1.49 | 0.62 |
| Ethnicity | ||||||
| Other ethnicity | 1.91 | 1.43 to 2.56 | < 0.001 | 1.27 | 0.90 to 1.79 | 0.18 |
| Sex | ||||||
| Female | 0.90 | 0.73 to 1.10 | 0.30 | 0.85 | 0.69 to 1.06 | 0.16 |
| Education | ||||||
| GCSE | 0.74 | 0.56 to 0.97 | 0.030 | 0.71 | 0.51 to 0.97 | 0.033 |
| A-level | 0.77 | 0.56 to 1.06 | 0.11 | 0.68 | 0.48 to 0.97 | 0.032 |
| No formal qualification | 0.70 | 0.51 to 0.97 | 0.032 | 0.90 | 0.60 to 1.35 | 0.60 |
| Other, still studying, don’t know | 0.93 | 0.64 to 1.36 | 0.71 | 1.12 | 0.73 to 1.70 | 0.60 |
| Social grade | ||||||
| C1 | 0.72 | 0.54 to 0.96 | 0.025 | 0.68 | 0.50 to 0.94 | 0.019 |
| C2 | 0.97 | 0.70 to 1.34 | 0.86 | 0.88 | 0.60 to 1.28 | 0.49 |
| D | 1.03 | 0.72 to 1.47 | 0.85 | 0.97 | 0.64 to 1.47 | 0.88 |
| E | 1.00 | 0.69 to 1.46 | 0.99 | 0.94 | 0.60 to 1.47 | 0.78 |
| Smoking status | ||||||
| Previous smoker | 0.70 | 0.54 to 0.90 | 0.006 | 0.86 | 0.65 to 1.13 | 0.27 |
| Current (tried quitting) | 1.24 | 0.91 to 1.68 | 0.18 | 1.26 | 0.91 to 1.75 | 0.16 |
| Current (not tried quitting) | 0.83 | 0.53 to 1.32 | 0.44 | 0.87 | 0.54 to 1.41 | 0.58 |
| Refused to answer | 1.04 | 0.63 to 1.69 | 0.89 | 0.96 | 0.57 to 1.59 | 0.86 |
| Area | ||||||
| North | 0.45 | 0.27 to 0.73 | 0.001 | 0.57 | 0.34 to 0.96 | 0.036 |
| North West | 0.68 | 0.46 to 1.01 | 0.058 | 0.83 | 0.55 to 1.27 | 0.39 |
| Yorkshire and Humberside | 0.51 | 0.32 to 0.81 | 0.004 | 0.60 | 0.37 to 0.98 | 0.040 |
| East Midlands | 0.42 | 0.27 to 0.64 | < 0.001 | 0.47 | 0.30 to 0.74 | 0.001 |
| West Midlands | 0.40 | 0.24 to 0.68 | 0.001 | 0.56 | 0.32 to 0.96 | 0.037 |
| East Anglia | 0.57 | 0.30 to 1.08 | 0.086 | 0.72 | 0.37 to 1.39 | 0.33 |
| South East | 0.93 | 0.58 to 1.48 | 0.75 | 1.42 | 0.86 to 2.35 | 0.17 |
| South West | 0.36 | 0.25 to 0.52 | < 0.001 | 0.53 | 0.35 to 0.80 | 0.002 |
| Wales | 0.56 | 0.32 to 0.96 | 0.035 | 0.81 | 0.46 to 1.45 | 0.48 |
| Scotland | 0.41 | 0.27 to 0.64 | < 0.001 | 0.55 | 0.34 to 0.88 | 0.014 |
| Variable | SD | D | N | A | SA |
|---|---|---|---|---|---|
| Age category (years) | |||||
| 18–24 | 19 (11.2) | 33 (19.4) | 46 (27.1) | 56 (32.9) | 16 (9.4) |
| 25–34 | 22 (12.6) | 27 (15.4) | 55 (31.4) | 49 (28.0) | 22 (12.6) |
| 35–44 | 25 (13.8) | 35 (19.3) | 37 (20.4) | 52 (28.7) | 32 (17.7) |
| 45–54 | 32 (20.1) | 32 (20.1) | 39 (24.5) | 36 (22.6) | 20 (12.6) |
| 55–59 | 18 (25.0) | 16 (22.2) | 12 (16.7) | 17 (23.6) | 9 (12.5) |
| 60–64 | 34 (36.2) | 14 (14.9) | 21 (22.3) | 15 (16.0) | 10 (10.6) |
| 65+ | 86 (29.4) | 48 (16.4) | 77 (26.3) | 53 (18.1) | 29 (9.9) |
| Breastfeeding | |||||
| Children not breastfed | 117 (18.5) | 115 (18.2) | 192 (30.4) | 146 (23.1) | 62 (9.8) |
| Children breastfed | 119 (23.2) | 90 (17.6) | 95 (18.6) | 132 (25.8) | 76 (14.8) |
| Children | |||||
| No children | 63 (15.7) | 72 (17.9) | 123 (30.6) | 107 (26.6) | 37 (9.2) |
| Have children | 173 (23.3) | 133 (17.9) | 164 (22.1) | 171 (23.0) | 101 (13.6) |
| Ethnicity | |||||
| White | 229 (23.2) | 189 (19.2) | 249 (25.3) | 214 (21.7) | 104 (10.6) |
| Other ethnicity | 7 (4.4) | 16 (10.1) | 38 (23.9) | 64 (40.3) | 34 (21.4) |
| Sex | |||||
| Male | 97 (18.0) | 85 (15.7) | 163 (30.2) | 131 (24.3) | 64 (11.9) |
| Female | 139 (23.0) | 120 (19.9) | 124 (20.5) | 147 (24.3) | 74 (12.3) |
| Education | |||||
| University | 58 (19.7) | 52 (17.6) | 70 (23.7) | 79 (26.8) | 36 (12.2) |
| GCSE | 69 (20.2) | 65 (19.0) | 89 (26.0) | 79 (23.1) | 40 (11.7) |
| A-level | 47 (24.4) | 29 (15.0) | 49 (25.4) | 43 (22.3) | 25 (13.0) |
| No formal qualification | 42 (21.3) | 34 (17.3) | 58 (29.4) | 41 (20.8) | 22 (11.2) |
| Other, still studying, don’t know | 20 (17.1) | 25 (21.4) | 21 (17.9) | 36 (30.8) | 15 (12.8) |
| Social grade | |||||
| AB | 54 (22.6) | 40 (16.7) | 59 (24.7) | 53 (22.2) | 33 (13.8) |
| C1 | 84 (22.7) | 73 (19.7) | 91 (24.6) | 89 (24.1) | 33 (8.9) |
| C2 | 46 (19.5) | 37 (15.7) | 71 (30.1) | 55 (23.3) | 27 (11.4) |
| D | 27 (16.7) | 29 (17.9) | 37 (22.8) | 44 (27.2) | 25 (15.4) |
| E | 25 (18.2) | 26 (19.0) | 29 (21.2) | 37 (27.0) | 20 (14.6) |
| Smoking status | |||||
| Never smoked | 108 (18.8) | 99 (17.3) | 137 (23.9) | 166 (29.0) | 63 (11.0) |
| Previous smoker | 73 (26.0) | 57 (20.3) | 70 (24.9) | 52 (18.5) | 29 (10.3) |
| Current (tried quitting) | 32 (18.3) | 31 (17.7) | 48 (27.4) | 29 (16.6) | 35 (20.0) |
| Current (not tried quitting) | 12 (19.0) | 13 (20.6) | 17 (27.0) | 15 (23.8) | 6 (9.5) |
| Refused to answer | 11 (21.2) | 5 (9.6) | 15 (28.8) | 16 (30.8) | 5 (9.6) |
| Area | |||||
| North | 14 (18.2) | 21 (27.3) | 17 (22.1) | 15 (19.5) | 10 (13.0) |
| North West | 17 (12.0) | 26 (18.3) | 44 (31.0) | 35 (24.6) | 20 (14.1) |
| Yorkshire and Humberside | 25 (24.0) | 15 (14.4) | 26 (25.0) | 24 (23.1) | 14 (13.5) |
| East Midlands | 21 (19.3) | 21 (19.3) | 31 (28.4) | 30 (27.5) | 6 (5.5) |
| West Midlands | 17 (25.8) | 15 (22.7) | 16 (24.2) | 14 (21.2) | 4 (6.1) |
| East Anglia | 6 (14.6) | 7 (17.1) | 7 (17.1) | 10 (24.4) | 11 (26.8) |
| South East | 15 (18.5) | 8 (9.9) | 23 (28.4) | 23 (28.4) | 12 (14.8) |
| South West | 56 (28.0) | 33 (16.5) | 61 (30.5) | 32 (16.0) | 18 (9.0) |
| London | 13 (8.7) | 26 (17.4) | 26 (17.4) | 65 (43.6) | 19 (12.8) |
| Wales | 20 (30.3) | 11 (16.7) | 14 (21.2) | 10 (15.2) | 11 (16.7) |
| Scotland | 32 (29.4) | 22 (20.2) | 22 (20.2) | 20 (18.3) | 13 (11.9) |
| Variable | Simple regression model | Multiple regression model | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age category (years) | ||||||
| 18–24 | 1.90 | 1.36 to 2.64 | < 0.001 | 1.63 | 1.07 to 2.49 | 0.022 |
| 25–34 | 1.96 | 1.41 to 2.72 | < 0.001 | 1.64 | 1.13 to 2.38 | 0.010 |
| 35–44 | 2.14 | 1.53 to 3.00 | < 0.001 | 1.91 | 1.32 to 2.76 | 0.001 |
| 45–54 | 1.41 | 1.00 to 1.99 | 0.051 | 1.38 | 0.95 to 2.00 | 0.088 |
| 55–59 | 1.21 | 0.76 to 1.94 | 0.43 | 1.23 | 0.76 to 2.01 | 0.40 |
| 60–64 | 0.81 | 0.53 to 1.25 | 0.34 | 0.79 | 0.50 to 1.23 | 0.30 |
| Breastfeeding | ||||||
| Children breastfed | 1.07 | 0.87 to 1.32 | 0.54 | 1.20 | 0.89 to 1.61 | 0.24 |
| Children | ||||||
| Have children | 0.88 | 0.71 to 1.09 | 0.23 | 0.97 | 0.71 to 1.33 | 0.86 |
| Ethnicity | ||||||
| Other ethnicity | 3.23 | 2.40 to 4.35 | < 0.001 | 2.31 | 1.63 to 3.29 | < 0.001 |
| Sex | ||||||
| Female | 0.84 | 0.68 to 1.03 | 0.099 | 0.85 | 0.69 to 1.06 | 0.15 |
| Education | ||||||
| GCSE | 0.90 | 0.68 to 1.19 | 0.47 | 0.92 | 0.67 to 1.25 | 0.58 |
| A-level | 0.87 | 0.63 to 1.21 | 0.41 | 0.86 | 0.61 to 1.22 | 0.40 |
| No formal qualification | 0.85 | 0.62 to 1.18 | 0.33 | 1.13 | 0.76 to 1.67 | 0.56 |
| Other, still studying, don’t know | 1.10 | 0.75 to 1.61 | 0.62 | 1.24 | 0.81 to 1.89 | 0.32 |
| Social grade | ||||||
| C1 | 0.87 | 0.65 to 1.16 | 0.33 | 0.76 | 0.55 to 1.04 | 0.086 |
| C2 | 1.05 | 0.76 to 1.44 | 0.77 | 0.84 | 0.57 to 1.22 | 0.35 |
| D | 1.30 | 0.91 to 1.86 | 0.15 | 1.07 | 0.70 to 1.62 | 0.77 |
| E | 1.20 | 0.82 to 1.75 | 0.34 | 0.96 | 0.62 to 1.50 | 0.86 |
| Smoking status | ||||||
| Previous smoker | 0.66 | 0.52 to 0.86 | 0.002 | 0.83 | 0.63 to 1.09 | 0.18 |
| Current (tried quitting) | 1.07 | 0.79 to 1.45 | 0.67 | 1.08 | 0.78 to 1.49 | 0.64 |
| Current (not tried quitting) | 0.85 | 0.54 to 1.33 | 0.47 | 0.80 | 0.50 to 1.28 | 0.34 |
| Refused to answer | 1.04 | 0.63 to 1.71 | 0.89 | 0.87 | 0.52 to 1.45 | 0.60 |
| Area | ||||||
| North | 0.48 | 0.30 to 0.78 | 0.003 | 0.76 | 0.46 to 1.27 | 0.29 |
| North West | 0.71 | 0.47 to 1.05 | 0.086 | 1.00 | 0.66 to 1.53 | 0.98 |
| Yorkshire and Humberside | 0.53 | 0.34 to 0.83 | 0.005 | 0.79 | 0.49 to 1.29 | 0.35 |
| East Midlands | 0.49 | 0.32 to 0.75 | 0.001 | 0.62 | 0.40 to 0.97 | 0.036 |
| West Midlands | 0.36 | 0.22 to 0.60 | < 0.001 | 0.64 | 0.37 to 1.10 | 0.11 |
| East Anglia | 1.03 | 0.54 to 1.95 | 0.93 | 1.55 | 0.79 to 3.02 | 0.20 |
| South East | 0.74 | 0.46 to 1.19 | 0.21 | 1.31 | 0.79 to 2.19 | 0.30 |
| South West | 0.37 | 0.25 to 0.53 | < 0.001 | 0.65 | 0.43 to 0.99 | 0.044 |
| Wales | 0.41 | 0.24 to 0.69 | 0.001 | 0.75 | 0.42 to 1.31 | 0.31 |
| Scotland | 0.37 | 0.24 to 0.58 | < 0.001 | 0.61 | 0.37 to 0.99 | 0.046 |
Appendix 29 Detailed results of the Ipsos MORI survey: health economics
| Variable | Probit (agree or not) | Amount of shopping voucher | ||||
|---|---|---|---|---|---|---|
| ββ | 95% CI | p-value | βββ | 95% CI | p-value | |
| Age | –0.01 | –0.02 to 0.00 | 0.10 | –0.09 | –0.27 to 0.09 | 0.33 |
| Region (base category: Greater London) | ||||||
| North | –0.40 | –0.77 to –0.03 | 0.04 | –4.07 | –13.28 to 5.15 | 0.39 |
| North West | 0.17 | –0.15 to 0.48 | 0.30 | –0.59 | –7.31 to 6.13 | 0.86 |
| Yorkshire and Humberside | –0.27 | –0.61 to 0.08 | 0.13 | –3.80 | –12.28 to 4.69 | 0.38 |
| West Midlands | –0.31 | –0.63 to 0.02 | 0.07 | –3.65 | –11.57 to 4.27 | 0.37 |
| East Midlands | –0.18 | –0.56 to 0.21 | 0.38 | –8.59 | –18.26 to 1.08 | 0.08 |
| East Anglia | –0.02 | –0.48 to 0.43 | 0.92 | 5.56 | –4.82 to 15.94 | 0.29 |
| South West | 0.22 | –0.15 to 0.59 | 0.24 | –9.13 | –16.14 to –2.12 | 0.01 |
| South East | 0.07 | –0.23 to 0.37 | 0.64 | –8.80 | –15.09 to –2.51 | 0.01 |
| Wales | –0.40 | –0.79 to –0.01 | 0.05 | –2.82 | –12.40 to 6.76 | 0.56 |
| Scotland | –0.14 | –0.49 to 0.20 | 0.42 | –6.43 | –14.83 to 1.97 | 0.13 |
| Education (base category: university) | ||||||
| GCSE | –0.30 | –0.53 to –0.07 | 0.01 | –2.03 | –6.74 to 2.68 | 0.40 |
| A-level | –0.35 | –0.60 to –0.10 | 0.01 | –4.07 | –9.35 to 1.22 | 0.13 |
| No formal qualification | –0.07 | –0.36 to 0.21 | 0.62 | 2.20 | –3.95 to 8.35 | 0.48 |
| Other, still studying, don’t know | –0.12 | –0.42 to 0.18 | 0.42 | –3.97 | –10.24 to 2.30 | 0.21 |
| Smoking status (base category: never smoked) | ||||||
| Previous smoker | –0.02 | –0.22 to 0.17 | 0.81 | –1.04 | –5.16 to 3.08 | 0.62 |
| Current (tried quitting) | 0.24 | 0.01 to 0.47 | 0.04 | 5.27 | 0.00 to 10.54 | 0.05 |
| Current (not tried quitting) | 0.05 | –0.30 to 0.41 | 0.76 | 3.53 | –5.31 to 12.37 | 0.43 |
| Refused to answer | 0.14 | –0.25 to 0.52 | 0.49 | –3.26 | –9.64 to 3.12 | 0.32 |
| Breastfeeding experience (base category: no children) | ||||||
| Yes | 0.22 | 0.03 to 0.42 | 0.02 | 4.59 | 0.46 to 8.73 | 0.03 |
| No | 0.17 | –0.05 to 0.39 | 0.14 | –0.55 | –5.57 to 4.47 | 0.83 |
| Social grade (base category: A or B) | ||||||
| C1 | 0.01 | –0.21 to 0.24 | 0.90 | –0.21 | –5.02 to 4.60 | 0.93 |
| C2 | 0.13 | –0.14 to 0.40 | 0.35 | 3.77 | –2.03 to 9.57 | 0.20 |
| D | 0.09 | –0.21 to 0.39 | 0.55 | –1.06 | –7.35 to 5.24 | 0.74 |
| E | 0.41 | 0.09 to 0.73 | 0.01 | 1.35 | –5.56 to 8.25 | 0.70 |
| Childbearing age ( = 1 if age < 45 years) | 0.08 | –0.22 to 0.38 | 0.62 | 1.96 | –4.80 to 8.71 | 0.57 |
| Female ( = 1 if female) | –0.27 | –0.43 to –0.11 | 0.00 | 0.79 | –2.59 to 4.18 | 0.64 |
| White ( = 1 if ethnic origin is white) | –0.31 | –0.57 to –0.05 | 0.02 | –5.21 | –10.87 to 0.44 | 0.07 |
| Constant | 0.90 | 0.31 to 1.48 | 0.00 | 34.22 | 21.52 to 46.92 | 0.00 |
| R 2 | 0.1065 | |||||
| Pseudo R2 | 0.0598 | |||||
| n | 1144 | 660 | ||||
| Variable | Probit (agree or not) | Probit (women on a low income or all) | ||||
|---|---|---|---|---|---|---|
| βββ | 95% CI | p-value | βββ | 95% CI | p-value | |
| Age | –0.01 | –0.02 to 0.00 | 0.10 | 0.00 | –0.01 to 0.01 | 0.66 |
| Region (base category: Greater London) | ||||||
| North | –0.40 | –0.77 to –0.03 | 0.04 | –0.19 | –0.71 to 0.34 | 0.48 |
| North West | 0.17 | –0.15 to 0.48 | 0.30 | 0.12 | –0.25 to 0.48 | 0.52 |
| Yorkshire and Humberside | –0.27 | –0.61 to 0.08 | 0.13 | –0.09 | –0.55 to 0.36 | 0.69 |
| West Midlands | –0.31 | –0.63 to 0.02 | 0.07 | –0.01 | –0.44 to 0.43 | 0.97 |
| East Midlands | –0.18 | –0.56 to 0.21 | 0.38 | –0.43 | –0.98 to 0.12 | 0.13 |
| East Anglia | –0.02 | –0.48 to 0.43 | 0.92 | 0.35 | –0.22 to 0.92 | 0.23 |
| South West | 0.22 | –0.15 to 0.59 | 0.24 | 0.48 | 0.02 to 0.93 | 0.04 |
| South East | 0.07 | –0.23 to 0.37 | 0.64 | 0.17 | –0.21 to 0.54 | 0.38 |
| Wales | –0.40 | –0.79 to –0.01 | 0.05 | –0.12 | –0.70 to 0.46 | 0.68 |
| Scotland | –0.14 | –0.49 to 0.20 | 0.42 | 0.81 | 0.34 to 1.27 | 0.00 |
| Education (base category: university) | ||||||
| GCSE | –0.30 | –0.53 to –0.07 | 0.01 | –0.22 | –0.52 to 0.09 | 0.16 |
| A-level | –0.35 | –0.60 to –0.10 | 0.01 | 0.09 | –0.24 to 0.42 | 0.59 |
| No formal qualification | –0.07 | –0.36 to 0.21 | 0.62 | –0.28 | –0.66 to 0.09 | 0.14 |
| Other, still studying, do not know | –0.12 | –0.42 to 0.18 | 0.42 | –0.14 | –0.52 to 0.25 | 0.49 |
| Smoking status (base category: never smoked) | ||||||
| Previous smoker | –0.02 | –0.22 to 0.17 | 0.81 | –0.08 | –0.35 to 0.19 | 0.57 |
| Current (tried quitting) | 0.24 | 0.01 to 0.47 | 0.04 | –0.04 | –0.33 to 0.24 | 0.77 |
| Current (not tried quitting) | 0.05 | –0.30 to 0.41 | 0.76 | –0.12 | –0.57 to 0.32 | 0.59 |
| Refused to answer | 0.14 | –0.25 to 0.52 | 0.49 | –0.00 | –0.46 to 0.46 | 0.99 |
| Breastfeeding experience (base category: no children) | ||||||
| Yes | 0.22 | 0.03 to 0.42 | 0.02 | 0.08 | –0.17 to 0.33 | 0.53 |
| No | 0.17 | –0.05 to 0.39 | 0.14 | 0.20 | –0.09 to 0.49 | 0.18 |
| Social grade (base category: A or B) | ||||||
| C1 | 0.01 | –0.21 to 0.24 | 0.90 | 0.15 | –0.15 to 0.46 | 0.32 |
| C2 | 0.13 | –0.14 to 0.40 | 0.35 | 0.12 | –0.24 to 0.49 | 0.51 |
| D | 0.09 | –0.21 to 0.39 | 0.55 | 0.23 | –0.16 to 0.63 | 0.25 |
| E | 0.41 | 0.09 to 0.73 | 0.01 | 0.18 | –0.23 to 0.59 | 0.38 |
| Childbearing age ( = 1 if age < 45 years) | 0.08 | –0.22 to 0.38 | 0.62 | –0.00 | –0.40 to 0.39 | 0.99 |
| Female ( = 1 if female) | –0.27 | –0.43 to –0.11 | 0.00 | –0.17 | –0.37 to 0.04 | 0.11 |
| White ( = 1 if ethnic origin is white) | –0.31 | –0.57 to –0.05 | 0.02 | 0.12 | –0.18 to 0.43 | 0.43 |
| Constant | 0.90 | 0.31 to 1.48 | 0.00 | –0.46 | –1.23 to 0.32 | 0.25 |
| Pseudo R2 | 0.0598 | 0.0523 | ||||
| n | 1144 | 660 | ||||
| Variable | Probit (agree or not) | Amount of shopping voucher | ||||
|---|---|---|---|---|---|---|
| ββββ | 95% CI | p-value | ββββ | 95% CI | p-value | |
| Age | –0.01 | –0.01 to 0.00 | 0.18 | –0.12 | –0.29 to 0.05 | 0.17 |
| Region (base category: Greater London) | ||||||
| North | –0.21 | –0.60 to 0.17 | 0.27 | –2.26 | –10.77 to 6.25 | 0.60 |
| North West | 0.00 | –0.32 to 0.33 | 0.98 | –1.36 | –7.96 to 5.23 | 0.69 |
| Yorks and Humberside | –0.25 | –0.60 to 0.10 | 0.17 | –4.08 | –12.30 to 4.13 | 0.33 |
| West Midlands | –0.39 | –0.73 to –0.06 | 0.02 | –4.02 | –11.15 to 3.10 | 0.27 |
| East Midlands | –0.53 | –0.93 to –0.13 | 0.01 | –12.68 | –21.34 to –4.02 | 0.00 |
| East Anglia | –0.09 | –0.57 to 0.38 | 0.70 | –1.73 | –12.38 to 8.92 | 0.75 |
| South West | 0.23 | –0.16 to 0.61 | 0.24 | –7.50 | –14.56 to –0.45 | 0.04 |
| South East | –0.17 | –0.47 to 0.14 | 0.29 | –11.37 | –17.49 to –5.26 | 0.00 |
| Wales | –0.45 | –0.85 to –0.05 | 0.03 | –10.66 | –19.14 to –2.18 | 0.01 |
| Scotland | –0.05 | –0.41 to 0.31 | 0.78 | –11.57 | –18.62 to –4.52 | 0.00 |
| Education (base category: university) | ||||||
| GCSE | 0.05 | –0.19 to 0.29 | 0.67 | 2.31 | –2.32 to 6.93 | 0.33 |
| A-level | –0.20 | –0.45 to 0.05 | 0.11 | 1.74 | –3.76 to 7.25 | 0.53 |
| No formal qualification | 0.31 | 0.01 to 0.61 | 0.04 | 5.43 | –0.47 to 11.34 | 0.07 |
| Other, still studying, don’t know | 0.10 | –0.21 to 0.40 | 0.54 | –0.13 | –5.48 to 5.23 | 0.96 |
| Smoking status (base category: never smoked) | ||||||
| Previous smoker | –0.10 | –0.29 to 0.10 | 0.33 | 0.39 | –3.85 to 4.64 | 0.86 |
| Current (tried quitting) | 0.06 | –0.17 to 0.30 | 0.61 | 4.70 | –0.40 to 9.81 | 0.07 |
| Current (not tried quitting) | –0.23 | –0.58 to 0.12 | 0.19 | –1.66 | –8.43 to 5.11 | 0.63 |
| Refused to answer | 0.09 | –0.30 to 0.48 | 0.65 | –2.39 | –9.95 to 5.16 | 0.53 |
| Breastfeeding experience (base category: no children) | ||||||
| Yes | –0.01 | –0.20 to 0.18 | 0.91 | 6.88 | 2.77 to 10.99 | 0.00 |
| No | –0.16 | –0.38 to 0.06 | 0.16 | –0.54 | –4.87 to 3.79 | 0.81 |
| Social grade (base category: A or B) | ||||||
| C1 | –0.20 | –0.42 to 0.03 | 0.09 | –3.85 | –8.80 to 1.09 | 0.13 |
| C2 | 0.02 | –0.26 to 0.29 | 0.91 | –5.36 | –10.64 to –0.07 | 0.05 |
| D | –0.01 | –0.31 to 0.30 | 0.95 | –5.43 | –11.37 to 0.51 | 0.07 |
| E | 0.06 | –0.26 to 0.39 | 0.70 | –3.42 | –10.14 to 3.30 | 0.32 |
| Childbearing age ( = 1 if age < 45 years) | 0.10 | –0.20 to 0.40 | 0.52 | 0.35 | –6.30 to 6.99 | 0.92 |
| Female ( = 1 if female) | –0.33 | –0.49 to –0.17 | 0.00 | 2.95 | –0.38 to 6.27 | 0.08 |
| White ( = 1 if ethnic origin is white) | –0.52 | –0.81 to –0.23 | 0.00 | –8.44 | –13.82 to –3.06 | 0.00 |
| Constant | 1.38 | 0.77 to 1.99 | 0.00 | 38.66 | 26.40 to 50.92 | 0.00 |
| R 2 | 0.1390 | |||||
| Pseudo R2 | 0.0750 | |||||
| n | 1144 | 697 | ||||
| Variable | Probit (agree or not) | Probit (women on a low income or all) | ||||
|---|---|---|---|---|---|---|
| ββββ | 95% CI | p-value | ββββ | 95% CI | p-value | |
| Age | –0.01 | –0.01 to 0.00 | 0.18 | 0.01 | –0.00 to 0.02 | 0.10 |
| Region (base category: Greater London) | ||||||
| North | –0.21 | –0.60 to 0.17 | 0.27 | –0.05 | –0.53 to 0.43 | 0.84 |
| North West | 0.00 | –0.32 to 0.33 | 0.98 | 0.18 | –0.18 to 0.54 | 0.33 |
| Yorks and Humberside | –0.25 | –0.60 to 0.10 | 0.17 | 0.18 | –0.25 to 0.61 | 0.41 |
| West Midlands | –0.39 | –0.73 to –0.06 | 0.02 | –0.07 | –0.49 to 0.34 | 0.73 |
| East Midlands | –0.53 | –0.93 to –0.13 | 0.01 | –0.01 | –0.58 to 0.55 | 0.96 |
| East Anglia | –0.09 | –0.57 to 0.38 | 0.70 | 0.53 | –0.04 to 1.10 | 0.07 |
| South West | 0.23 | –0.16 to 0.61 | 0.24 | 0.65 | 0.21 to 1.10 | 0.00 |
| South East | –0.17 | –0.47 to 0.14 | 0.29 | 0.18 | –0.19 to 0.55 | 0.34 |
| Wales | –0.45 | –0.85 to –0.05 | 0.03 | 0.31 | –0.24 to 0.86 | 0.27 |
| Scotland | –0.05 | –0.41 to 0.31 | 0.78 | 0.60 | 0.18 to 1.03 | 0.01 |
| Education (base category: university) | ||||||
| GCSE | 0.05 | –0.19 to 0.29 | 0.67 | 0.06 | –0.23 to 0.35 | 0.68 |
| A-level | –0.20 | –0.45 to 0.05 | 0.11 | –0.00 | –0.32 to 0.32 | 1.00 |
| No formal qualification | 0.31 | 0.01 to 0.61 | 0.04 | 0.11 | –0.26 to 0.49 | 0.55 |
| Other, still studying, don’t know | 0.10 | –0.21 to 0.40 | 0.54 | 0.24 | –0.14 to 0.62 | 0.22 |
| Smoking status (base category: never smoked) | ||||||
| Previous smoker | –0.10 | –0.29 to 0.10 | 0.33 | –0.07 | –0.34 to 0.19 | 0.59 |
| Current (tried quitting) | 0.06 | –0.17 to 0.30 | 0.61 | –0.10 | –0.38 to 0.19 | 0.50 |
| Current (not tried quitting) | –0.23 | –0.58 to 0.12 | 0.19 | –0.13 | –0.56 to 0.30 | 0.56 |
| Refused to answer | 0.09 | –0.30 to 0.48 | 0.65 | 0.13 | –0.31 to 0.57 | 0.56 |
| Breastfeeding experience (base category: no children) | ||||||
| Yes | –0.01 | –0.20 to 0.18 | 0.91 | –0.06 | –0.30 to 0.18 | 0.62 |
| No | –0.16 | –0.38 to 0.06 | 0.16 | 0.04 | –0.24 to 0.33 | 0.76 |
| Social grade (base category: A or B) | ||||||
| C1 | –0.20 | –0.42 to 0.03 | 0.09 | –0.20 | –0.49 to 0.10 | 0.19 |
| C2 | 0.02 | –0.26 to 0.29 | 0.91 | –0.09 | –0.44 to 0.25 | 0.60 |
| D | –0.01 | –0.31 to 0.30 | 0.95 | –0.11 | –0.48 to 0.27 | 0.58 |
| E | 0.06 | –0.26 to 0.39 | 0.70 | –0.15 | –0.55 to 0.25 | 0.45 |
| Childbearing age ( = 1 if age < 45 years) | 0.10 | –0.20 to 0.40 | 0.52 | 0.23 | –0.16 to 0.61 | 0.25 |
| Female ( = 1 if female) | –0.33 | –0.49 to –0.17 | 0.00 | –0.28 | –0.48 to –0.08 | 0.01 |
| White ( = 1 if ethnic origin is white) | –0.52 | –0.81 to –0.23 | 0.00 | –0.13 | –0.42 to 0.16 | 0.37 |
| Constant | 1.38 | 0.77 to 1.99 | 0.00 | –0.47 | –1.21 to 0.27 | 0.21 |
| Pseudo R2 | 0.0750 | 0.0416 | ||||
| n | 1144 | 660 | ||||
Appendix 30 Detailed results of the health professional survey
| Variable | SD | D | N | A | SA |
|---|---|---|---|---|---|
| Age category (years) | |||||
| 18–34 | 22 (24.2) | 37 (40.7) | 3 (3.3) | 25 (27.5) | 4 (4.4) |
| 35–44 | 20 (17.5) | 36 (31.6) | 15 (13.2) | 37 (32.5) | 6 (5.3) |
| 45–54 | 37 (20.3) | 54 (29.7) | 21 (11.5) | 53 (29.1) | 17 (9.3) |
| 55+ | 20 (23.5) | 27 (31.8) | 14 (16.5) | 17 (20.0) | 7 (8.2) |
| Missing | 6 (24.0) | 9 (36.0) | 4 (16.0) | 5 (20.0) | 1 (4.0) |
| Breastfeeding | |||||
| Children not breastfed | 26 (23.6) | 34 (30.9) | 12 (10.9) | 31 (28.2) | 7 (6.4) |
| Children breastfed | 79 (20.4) | 129 (33.3) | 45 (11.6) | 106 (27.4) | 28 (7.2) |
| Children | |||||
| No children | 23 (24.0) | 31 (32.3) | 8 (8.3) | 28 (29.2) | 6 (6.3) |
| Have children | 82 (20.4) | 132 (32.9) | 49 (12.2) | 109 (27.2) | 29 (7.2) |
| Ethnicity | |||||
| White | 88 (19.8) | 148 (33.3) | 52 (11.7) | 125 (28.2) | 31 (7.0) |
| Other ethnicity | 17 (32.1) | 15 (28.3) | 5 (9.4) | 12 (22.6) | 4 (7.5) |
| Sex | |||||
| Male | 21 (32.8) | 12 (18.8) | 7 (10.9) | 17 (26.6) | 7 (10.9) |
| Female | 79 (19.2) | 141 (34.3) | 46 (11.2) | 118 (28.7) | 27 (6.6) |
| Missing | 5 (22.7) | 10 (45.5) | 4 (18.2) | 2 (9.1) | 1 (4.5) |
| Profession | |||||
| Doctor | 36 (23.1) | 52 (33.3) | 17 (10.9) | 44 (28.2) | 7 (4.5) |
| Midwife/health visitor/maternity care | 31 (15.7) | 72 (36.5) | 22 (11.2) | 56 (28.4) | 16 (8.1) |
| Other | 38 (26.4) | 39 (27.1) | 18 (12.5) | 37 (25.7) | 12 (8.3) |
| Smoking status | |||||
| Never smoked | 78 (21.2) | 119 (32.3) | 40 (10.9) | 106 (28.8) | 25 (6.8) |
| Previous smoker/current smoker/declined to answer | 27 (20.9) | 44 (34.1) | 17 (13.2) | 31 (24.0) | 10 (7.8) |
| Area | |||||
| North | 96 (22.0) | 143 (32.7) | 52 (11.9) | 117 (26.8) | 29 (6.6) |
| North West | 9 (15.0) | 20 (33.3) | 5 (8.3) | 20 (33.3) | 6 (10.0) |
| Variable | Simple regression model | Multiple regression model | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age category (years) | ||||||
| 18–34 | 0.86 | 0.51 to 1.47 | 0.58 | 0.85 | 0.48 to 1.48 | 0.56 |
| 35–44 | 1.31 | 0.80 to 2.17 | 0.28 | 1.39 | 0.83 to 2.33 | 0.21 |
| 45–54 | 1.32 | 0.83 to 2.10 | 0.24 | 1.26 | 0.79 to 2.02 | 0.33 |
| Missing | 0.85 | 0.39 to 1.87 | 0.69 | 2.03 | 0.45 to 9.19 | 0.36 |
| Breastfeeding | ||||||
| Children breastfed | 1.08 | 0.74 to 1.59 | 0.69 | 0.90 | 0.34 to 2.40 | 0.83 |
| Children | ||||||
| Have children | 1.11 | 0.74 to 1.66 | 0.62 | 1.08 | 0.38 to 3.08 | 0.89 |
| Ethnicity | ||||||
| Other ethnicity | 0.67 | 0.39 to 1.14 | 0.14 | 0.67 | 0.32 to 1.41 | 0.29 |
| Sex | ||||||
| Female | 1.16 | 0.70 to 1.91 | 0.57 | 1.02 | 0.59 to 1.77 | 0.94 |
| Missing | 0.69 | 0.29 to 1.63 | 0.40 | 0.56 | 0.11 to 2.90 | 0.49 |
| Profession | ||||||
| Midwifes/health visitor/maternity care | 1.31 | 0.90 to 1.90 | 0.16 | 1.32 | 0.87 to 2.02 | 0.19 |
| Other | 1.04 | 0.69 to 1.57 | 0.85 | 1.12 | 0.71 to 1.77 | 0.62 |
| Smoking status | ||||||
| Previous smoker/current smoker/declined to answer | 0.95 | 0.66 to 1.36 | 0.77 | 0.90 | 0.62 to 1.30 | 0.56 |
| Area | ||||||
| England | 1.46 | 0.90 to 2.37 | 0.13 | 1.55 | 0.94 to 2.56 | 0.086 |
| Variable | SD | D | N | A | SA |
|---|---|---|---|---|---|
| Age category (years) | |||||
| 18–34 | 23 (25.3) | 33 (36.3) | 1 (1.1) | 27 (29.7) | 7 (7.7) |
| 35–44 | 20 (17.5) | 34 (29.8) | 11 (9.6) | 39 (34.2) | 10 (8.8) |
| 45–54 | 31 (17.0) | 58 (31.9) | 14 (7.7) | 50 (27.5) | 29 (15.9) |
| 55+ | 20 (23.5) | 25 (29.4) | 6 (7.1) | 26 (30.6) | 8 (9.4) |
| Missing | 5 (20.0) | 9 (36.0) | 4 (16.0) | 5 (20.0) | 2 (8.0) |
| Breastfeeding | |||||
| Children not breastfed | 23 (20.9) | 34 (30.9) | 10 (9.1) | 30 (27.3) | 13 (11.8) |
| Children breastfed | 76 (19.6) | 125 (32.3) | 26 (6.7) | 117 (30.2) | 43 (11.1) |
| Children | |||||
| No children | 20 (20.8) | 32 (33.3) | 7 (7.3) | 27 (28.1) | 10 (10.4) |
| Have children | 79 (19.7) | 127 (31.7) | 29 (7.2) | 120 (29.9) | 46 (11.5) |
| Ethnicity | |||||
| White | 83 (18.7) | 145 (32.7) | 30 (6.8) | 133 (30.0) | 53 (11.9) |
| Other ethnicity | 16 (30.2) | 14 (26.4) | 6 (11.3) | 14 (26.4) | 3 (5.7) |
| Sex | |||||
| Male | 20 (31.3) | 11 (17.2) | 6 (9.4) | 18 (28.1) | 9 (14.1) |
| Female | 74 (18.0) | 138 (33.6) | 27 (6.6) | 126 (30.7) | 46 (11.2) |
| Missing | 5 (22.7) | 10 (45.5) | 3 (13.6) | 3 (13.6) | 1 (4.5) |
| Profession | |||||
| Doctor | 34 (21.8) | 52 (33.3) | 15 (9.6) | 47 (30.1) | 8 (5.1) |
| Midwife/health visitor/maternity care | 28 (14.2) | 71 (36.0) | 13 (6.6) | 59 (29.9) | 26 (13.2) |
| Other | 37 (25.7) | 36 (25.0) | 8 (5.6) | 41 (28.5) | 22 (15.3) |
| Smoking status | |||||
| Never smoked | 72 (19.6) | 120 (32.6) | 25 (6.8) | 110 (29.9) | 41 (11.1) |
| Previous smoker/current smoker/declined to answer | 27 (20.9) | 39 (30.2) | 11 (8.5) | 37 (28.7) | 15 (11.6) |
| Area | |||||
| North | 90 (20.6) | 139 (31.8) | 34 (7.8) | 126 (28.8) | 48 (11.0) |
| North West | 9 (15.0) | 20 (33.3) | 2 (3.3) | 21 (35.0) | 8 (13.3) |
| Variable | Simple regression model | Multiple regression model | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age category (years) | ||||||
| 18–34 | 0.82 | 0.48 to 1.40 | 0.47 | 0.80 | 0.45 to 1.41 | 0.44 |
| 35–44 | 1.22 | 0.74 to 2.01 | 0.44 | 1.28 | 0.76 to 2.15 | 0.35 |
| 45–54 | 1.34 | 0.84 to 2.13 | 0.22 | 1.26 | 0.79 to 2.02 | 0.34 |
| Missing | 0.89 | 0.41 to 1.93 | 0.76 | 3.06 | 0.74 to 12.63 | 0.12 |
| Breastfeeding | ||||||
| Children breastfed | 1.04 | 0.71 to 1.52 | 0.85 | 0.69 | 0.25 to 1.93 | 0.48 |
| Children | ||||||
| Have children | 1.11 | 0.74 to 1.65 | 0.62 | 1.38 | 0.47 to 4.10 | 0.56 |
| Ethnicity | ||||||
| Other ethnicity | 0.63 | 0.38 to 1.06 | 0.079 | 0.63 | 0.31 to 1.27 | 0.20 |
| Sex | ||||||
| Female | 1.16 | 0.71 to 1.91 | 0.55 | 0.98 | 0.57 to 1.68 | 0.93 |
| Missing | 0.64 | 0.27 to 1.50 | 0.31 | 0.31 | 0.06 to 1.49 | 0.14 |
| Profession | ||||||
| Midwifes/health visitor/maternity care | 1.45 | 1.00 to 2.11 | 0.048 | 1.44 | 0.95 to 2.19 | 0.089 |
| Other | 1.24 | 0.82 to 1.87 | 0.31 | 1.35 | 0.85 to 2.14 | 0.20 |
| Smoking status | ||||||
| Previous smoker/current smoker/declined to answer | 0.99 | 0.69 to 1.42 | 0.95 | 0.90 | 0.62 to 1.30 | 0.57 |
| Area | ||||||
| England | 1.32 | 0.81 to 2.14 | 0.26 | 1.48 | 0.89 to 2.45 | 0.13 |
| Variable | SD | D | N | A | SA |
|---|---|---|---|---|---|
| Age category (years) | |||||
| 18–34 | 20 (23.5) | 22 (25.9) | 14 (16.5) | 21 (24.7) | 8 (9.4) |
| 35–44 | 29 (31.9) | 28 (30.8) | 5 (5.5) | 23 (25.3) | 6 (6.6) |
| 45–54 | 26 (22.8) | 31 (27.2) | 15 (13.2) | 26 (22.8) | 16 (14.0) |
| 55+ | 36 (19.8) | 58 (31.9) | 22 (12.1) | 37 (20.3) | 29 (15.9) |
| Missing | 7 (28.0) | 5 (20.0) | 5 (20.0) | 7 (28.0) | 1 (4.0) |
| Breastfeeding | |||||
| Children not breastfed | 30 (27.3) | 31 (28.2) | 19 (17.3) | 19 (17.3) | 11 (10.0) |
| Children breastfed | 88 (22.7) | 113 (29.2) | 42 (10.9) | 95 (24.5) | 49 (12.7) |
| Children | |||||
| No children | 27 (28.1) | 30 (31.3) | 15 (15.6) | 16 (16.7) | 8 (8.3) |
| Have children | 91 (22.7) | 114 (28.4) | 46 (11.5) | 98 (24.4) | 52 (13.0) |
| Ethnicity | |||||
| White | 100 (22.5) | 134 (30.2) | 53 (11.9) | 101 (22.7) | 56 (12.6) |
| Other ethnicity | 18 (34.0) | 10 (18.9) | 8 (15.1) | 13 (24.5) | 4 (7.5) |
| Sex | |||||
| Male | 22 (34.4) | 11 (17.2) | 8 (12.5) | 16 (25.0) | 7 (10.9) |
| Female | 90 (21.9) | 128 (31.1) | 48 (11.7) | 93 (22.6) | 52 (12.7) |
| Missing | 6 (27.3) | 5 (22.7) | 5 (22.7) | 5 (22.7) | 1 (4.5) |
| Profession | |||||
| Doctor | 40 (25.6) | 48 (30.8) | 21 (13.5) | 36 (23.1) | 11 (7.1) |
| Midwife/health visitor/maternity care | 36 (18.3) | 67 (34.0) | 25 (12.7) | 40 (20.3) | 29 (14.7) |
| Other | 42 (29.2) | 29 (20.1) | 15 (10.4) | 38 (26.4) | 20 (13.9) |
| Smoking status | |||||
| Never smoked | 86 (23.4) | 105 (28.5) | 47 (12.8) | 88 (23.9) | 42 (11.4) |
| Previous smoker/current smoker/declined to answer | 32 (24.8) | 39 (30.2) | 14 (10.9) | 26 (20.2) | 18 (14.0) |
| Area | |||||
| North | 105 (24.0) | 125 (28.6) | 54 (12.4) | 102 (23.3) | 51 (11.7) |
| North West | 13 (21.7) | 19 (31.7) | 7 (11.7) | 12 (20.0) | 9 (15.0) |
| Variable | Simple regression model | Multiple regression model | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age category (years) | ||||||
| 18–34 | 0.68 | 0.40 to 1.16 | 0.16 | 0.74 | 0.42 to 1.30 | 0.30 |
| 35–44 | 1.09 | 0.66 to 1.79 | 0.73 | 1.13 | 0.68 to 1.89 | 0.63 |
| 45–54 | 1.14 | 0.72 to 1.79 | 0.58 | 1.11 | 0.70 to 1.76 | 0.65 |
| Missing | 0.87 | 0.40 to 1.91 | 0.74 | 1.27 | 0.28 to 5.75 | 0.76 |
| Breastfeeding | ||||||
| Children breastfed | 1.30 | 0.89 to 1.89 | 0.18 | 0.60 | 0.22 to 1.64 | 0.32 |
| Children | ||||||
| Have children | 1.47 | 0.99 to 2.17 | 0.058 | 2.15 | 0.75 to 6.18 | 0.16 |
| Ethnicity | ||||||
| Other ethnicity | 0.74 | 0.44 to 1.25 | 0.26 | 0.70 | 0.34 to 1.43 | 0.33 |
| Sex | ||||||
| Female | 1.24 | 0.76 to 2.02 | 0.39 | 1.12 | 0.66 to 1.90 | 0.66 |
| Missing | 1.00 | 0.42 to 2.35 | 1.00 | 0.96 | 0.18 to 5.22 | 0.96 |
| Profession | ||||||
| Midwifes/health visitor/maternity care | 1.36 | 0.94 to 1.97 | 0.10 | 1.32 | 0.87 to 2.00 | 0.20 |
| Other | 1.25 | 0.83 to 1.88 | 0.29 | 1.24 | 0.79 to 1.95 | 0.34 |
| Smoking status | ||||||
| Previous smoker/current smoker/declined to answer | 0.95 | 0.66 to 1.36 | 0.78 | 0.85 | 0.59 to 1.24 | 0.40 |
| Area | ||||||
| England | 1.07 | 0.66 to 1.73 | 0.78 | 1.15 | 0.70 to 1.90 | 0.58 |
| Variable | SD | D | N | A | SA |
|---|---|---|---|---|---|
| Age category (years) | |||||
| 18–34 | 20 (23.5) | 27 (31.8) | 14 (16.5) | 17 (20.0) | 7 (8.2) |
| 35–44 | 22 (24.2) | 37 (40.7) | 3 (3.3) | 25 (27.5) | 4 (4.4) |
| 45–54 | 20 (17.5) | 36 (31.6) | 15 (13.2) | 37 (32.5) | 6 (5.3) |
| 55+ | 37 (20.3) | 54 (29.7) | 21 (11.5) | 53 (29.1) | 17 (9.3) |
| Missing | 6 (24.0) | 9 (36.0) | 4 (16.0) | 5 (20.0) | 1 (4.0) |
| Breastfeeding | |||||
| Children not breastfed | 26 (23.6) | 34 (30.9) | 12 (10.9) | 31 (28.2) | 7 (6.4) |
| Children breastfed | 79 (20.4) | 129 (33.3) | 45 (11.6) | 106 (27.4) | 28 (7.2) |
| Children | |||||
| No children | 23 (24.0) | 31 (32.3) | 8 (8.3) | 28 (29.2) | 6 (6.3) |
| Have children | 82 (20.4) | 132 (32.9) | 49 (12.2) | 109 (27.2) | 29 (7.2) |
| Ethnicity | |||||
| White | 88 (19.8) | 148 (33.3) | 52 (11.7) | 125 (28.2) | 31 (7.0) |
| Other ethnicity | 17 (32.1) | 15 (28.3) | 5 (9.4) | 12 (22.6) | 4 (7.5) |
| Sex | |||||
| Male | 21 (32.8) | 12 (18.8) | 7 (10.9) | 17 (26.6) | 7 (10.9) |
| Female | 79 (19.2) | 141 (34.3) | 46 (11.2) | 118 (28.7) | 27 (6.6) |
| Missing | 5 (22.7) | 10 (45.5) | 4 (18.2) | 2 (9.1) | 1 (4.5) |
| Profession | |||||
| Doctor | 36 (23.1) | 52 (33.3) | 17 (10.9) | 44 (28.2) | 7 (4.5) |
| Midwife/health visitor/maternity care | 31 (15.7) | 72 (36.5) | 22 (11.2) | 56 (28.4) | 16 (8.1) |
| Other | 38 (26.4) | 39 (27.1) | 18 (12.5) | 37 (25.7) | 12 (8.3) |
| Smoking status | |||||
| Never smoked | 78 (21.2) | 119 (32.3) | 40 (10.9) | 106 (28.8) | 25 (6.8) |
| Previous smoker/current smoker/declined to answer | 27 (20.9) | 44 (34.1) | 17 (13.2) | 31 (24.0) | 10 (7.8) |
| Area | |||||
| North | 96 (22.0) | 143 (32.7) | 52 (11.9) | 117 (26.8) | 29 (6.6) |
| North West | 9 (15.0) | 20 (33.3) | 5 (8.3) | 20 (33.3) | 6 (10.0) |
| Variable | Simple regression model | Multiple regression model | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age category (years) | ||||||
| 18–34 | 0.39 | 0.23 to 0.68 | 0.001 | 0.41 | 0.23 to 0.72 | 0.002 |
| 35–44 | 0.59 | 0.36 to 0.98 | 0.040 | 0.70 | 0.42 to 1.17 | 0.18 |
| 45–54 | 0.83 | 0.52 to 1.32 | 0.43 | 0.80 | 0.51 to 1.28 | 0.36 |
| Missing | 0.91 | 0.40 to 2.04 | 0.81 | 1.65 | 0.44 to 6.25 | 0.46 |
| Breastfeeding | ||||||
| Children breastfed | 1.26 | 0.86 to 1.85 | 0.23 | 1.33 | 0.46 to 3.84 | 0.60 |
| Children | ||||||
| Have children | 1.20 | 0.81 to 1.80 | 0.37 | 0.76 | 0.25 to 2.32 | 0.63 |
| Ethnicity | ||||||
| Other ethnicity | 1.01 | 0.60 to 1.70 | 0.96 | 0.89 | 0.44 to 1.80 | 0.75 |
| Sex | ||||||
| Female | 1.48 | 0.91 to 2.39 | 0.11 | 1.16 | 0.68 to 1.95 | 0.59 |
| Missing | 1.44 | 0.58 to 3.57 | 0.43 | 0.83 | 0.18 to 3.91 | 0.81 |
| Profession | ||||||
| Midwifes/health visitor/maternity care | 1.97 | 1.35 to 2.86 | < 0.001 | 1.84 | 1.21 to 2.79 | 0.005 |
| Other | 0.97 | 0.65 to 1.47 | 0.90 | 0.96 | 0.61 to 1.51 | 0.87 |
| Smoking status | ||||||
| Previous smoker/current smoker/declined to answer | 0.98 | 0.68 to 1.40 | 0.89 | 0.89 | 0.61 to 1.29 | 0.53 |
| Area | ||||||
| England | 1.40 | 0.88 to 2.22 | 0.16 | 1.39 | 0.85 to 2.26 | 0.19 |
| Variable | SD | D | N | A | SA |
|---|---|---|---|---|---|
| Age category (years) | |||||
| 18–34 | 5 (5.5) | 12 (13.2) | 6 (6.6) | 38 (41.8) | 30 (33.0) |
| 35–44 | 5 (4.4) | 14 (12.3) | 15 (13.2) | 53 (46.5) | 27 (23.7) |
| 45–54 | 9 (4.9) | 32 (17.6) | 20 (11.0) | 74 (40.7) | 47 (25.8) |
| 55+ | 6 (7.1) | 17 (20.0) | 8 (9.4) | 30 (35.3) | 24 (28.2) |
| Missing | 4 (16.0) | 5 (20.0) | 2 (8.0) | 9 (36.0) | 5 (20.0) |
| Breastfeeding | |||||
| Children not breastfed | 5 (4.5) | 21 (19.1) | 11 (10.0) | 48 (43.6) | 25 (22.7) |
| Children breastfed | 24 (6.2) | 59 (15.2) | 40 (10.3) | 156 (40.3) | 108 (27.9) |
| Children | |||||
| No children | 5 (5.2) | 17 (17.7) | 11 (11.5) | 42 (43.8) | 21 (21.9) |
| Have children | 24 (6.0) | 63 (15.7) | 40 (10.0) | 162 (40.4) | 112 (27.9) |
| Ethnicity | |||||
| White | 22 (5.0) | 72 (16.2) | 45 (10.1) | 185 (41.7) | 120 (27.0) |
| Other ethnicity | 7 (13.2) | 8 (15.1) | 6 (11.3) | 19 (35.8) | 13 (24.5) |
| Sex | |||||
| Male | 3 (4.7) | 10 (15.6) | 10 (15.6) | 22 (34.4) | 19 (29.7) |
| Female | 22 (5.4) | 66 (16.1) | 38 (9.2) | 176 (42.8) | 109 (26.5) |
| Missing | 4 (18.2) | 4 (18.2) | 3 (13.6) | 6 (27.3) | 5 (22.7) |
| Profession | |||||
| Doctor | 8 (5.1) | 27 (17.3) | 17 (10.9) | 68 (43.6) | 36 (23.1) |
| Midwife/health visitor/maternity care | 10 (5.1) | 36 (18.3) | 20 (10.2) | 74 (37.6) | 57 (28.9) |
| Other | 11 (7.6) | 17 (11.8) | 14 (9.7) | 62 (43.1) | 40 (27.8) |
| Smoking status | |||||
| Never smoked | 23 (6.3) | 59 (16.0) | 39 (10.6) | 156 (42.4) | 91 (24.7) |
| Previous smoker/current smoker/declined to answer | 6 (4.7) | 21 (16.3) | 12 (9.3) | 48 (37.2) | 42 (32.6) |
| Area | |||||
| North | 28 (6.4) | 72 (16.5) | 45 (10.3) | 178 (40.7) | 114 (26.1) |
| North West | 1 (1.7) | 8 (13.3) | 6 (10.0) | 26 (43.3) | 19 (31.7) |
| Variable | Simple regression model | Multiple regression model | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age category (years) | ||||||
| 18–34 | 1.48 | 0.86 to 2.57 | 0.16 | 1.69 | 0.94 to 3.02 | 0.077 |
| 35–44 | 1.13 | 0.68 to 1.89 | 0.63 | 1.19 | 0.70 to 2.01 | 0.52 |
| 45–54 | 1.06 | 0.66 to 1.70 | 0.82 | 1.04 | 0.64 to 1.68 | 0.87 |
| Missing | 1.48 | 0.86 to 2.57 | 0.16 | 0.98 | 0.23 to 4.15 | 0.97 |
| Breastfeeding | ||||||
| Children breastfed | 1.16 | 0.79 to 1.70 | 0.44 | 0.96 | 0.34 to 2.69 | 0.94 |
| Children | ||||||
| Have children | 1.20 | 0.80 to 1.79 | 0.37 | 1.41 | 0.47 to 4.19 | 0.54 |
| Ethnicity | ||||||
| Other ethnicity | 0.71 | 0.42 to 1.21 | 0.21 | 0.88 | 0.45 to 1.75 | 0.72 |
| Sex | ||||||
| Female | 1.00 | 0.62 to 1.63 | 0.99 | 0.90 | 0.54 to 1.52 | 0.70 |
| Missing | 0.49 | 0.20 to 1.23 | 0.13 | 0.51 | 0.11 to 2.49 | 0.41 |
| Profession | ||||||
| Midwifes/health visitor/maternity care | 1.13 | 0.77 to 1.65 | 0.54 | 1.16 | 0.75 to 1.79 | 0.50 |
| Other | 1.20 | 0.80 to 1.81 | 0.38 | 1.32 | 0.84 to 2.06 | 0.23 |
| Smoking status | ||||||
| Previous smoker/current smoker/declined to answer | 1.28 | 0.89 to 1.86 | 0.19 | 1.24 | 0.85 to 1.81 | 0.27 |
| Area | ||||||
| England | 1.43 | 0.88 to 2.33 | 0.15 | 1.49 | 0.90 to 2.47 | 0.13 |
| Variable | SD | D | N | A | SA |
|---|---|---|---|---|---|
| Age category (years) | |||||
| 18–34 | 6 (6.6) | 20 (22.0) | 20 (22.0) | 30 (33.0) | 15 (16.5) |
| 35–44 | 11 (9.6) | 24 (21.1) | 15 (13.2) | 48 (42.1) | 16 (14.0) |
| 45–54 | 20 (11.0) | 29 (15.9) | 34 (18.7) | 72 (39.6) | 27 (14.8) |
| 55+ | 11 (12.9) | 14 (16.5) | 17 (20.0) | 32 (37.6) | 11 (12.9) |
| Missing | 3 (12.0) | 4 (16.0) | 6 (24.0) | 8 (32.0) | 4 (16.0) |
| Breastfeeding | |||||
| Children not breastfed | 7 (6.4) | 20 (18.2) | 21 (19.1) | 45 (40.9) | 17 (15.5) |
| Children breastfed | 44 (11.4) | 71 (18.3) | 71 (18.3) | 145 (37.5) | 56 (14.5) |
| Children | |||||
| No children | 7 (7.3) | 18 (18.8) | 19 (19.8) | 37 (38.5) | 15 (15.6) |
| Have children | 44 (11.0) | 73 (18.2) | 73 (18.2) | 153 (38.2) | 58 (14.5) |
| Ethnicity | |||||
| White | 44 (9.9) | 83 (18.7) | 79 (17.8) | 173 (39.0) | 65 (14.6) |
| Other ethnicity | 7 (13.2) | 8 (15.1) | 13 (24.5) | 17 (32.1) | 8 (15.1) |
| Sex | |||||
| Male | 15 (23.4) | 12 (18.8) | 12 (18.8) | 17 (26.6) | 8 (12.5) |
| Female | 35 (8.5) | 75 (18.2) | 74 (18.0) | 166 (40.4) | 61 (14.8) |
| Missing | 1 (4.5) | 4 (18.2) | 6 (27.3) | 7 (31.8) | 4 (18.2) |
| Profession | |||||
| Doctor | 26 (16.7) | 36 (23.1) | 31 (19.9) | 51 (32.7) | 12 (7.7) |
| Midwife/health visitor/maternity care | 13 (6.6) | 34 (17.3) | 33 (16.8) | 79 (40.1) | 38 (19.3) |
| Other | 12 (8.3) | 21 (14.6) | 28 (19.4) | 60 (41.7) | 23 (16.0) |
| Smoking status | |||||
| Never smoked | 37 (10.1) | 72 (19.6) | 66 (17.9) | 143 (38.9) | 50 (13.6) |
| Previous smoker/current smoker/declined to answer | 14 (10.9) | 19 (14.7) | 26 (20.2) | 47 (36.4) | 23 (17.8) |
| Area | |||||
| North | 48 (11.0) | 82 (18.8) | 84 (19.2) | 167 (38.2) | 56 (12.8) |
| North West | 3 (5.0) | 9 (15.0) | 8 (13.3) | 23 (38.3) | 17 (28.3) |
| Variable | Simple regression model | Multiple regression model | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age category (years) | ||||||
| 18–34 | 1.11 | 0.66 to 1.89 | 0.69 | 1.16 | 0.66 to 2.03 | 0.61 |
| 35–44 | 1.15 | 0.69 to 1.90 | 0.60 | 1.45 | 0.86 to 2.47 | 0.16 |
| 45–54 | 1.16 | 0.73 to 1.85 | 0.53 | 1.10 | 0.69 to 1.77 | 0.68 |
| Missing | 1.03 | 0.46 to 2.30 | 0.94 | 0.36 | 0.06 to 1.97 | 0.24 |
| Breastfeeding | ||||||
| Children breastfed | 0.82 | 0.56 to 1.19 | 0.29 | 0.62 | 0.23 to 1.65 | 0.34 |
| Children | ||||||
| Have children | 0.89 | 0.60 to 1.32 | 0.56 | 1.31 | 0.47 to 3.68 | 0.61 |
| Ethnicity | ||||||
| Other ethnicity | 0.87 | 0.52 to 1.45 | 0.60 | 0.82 | 0.41 to 1.62 | 0.56 |
| Sex | ||||||
| Female | 2.04 | 1.25 to 3.33 | 0.005 | 1.50 | 0.87 to 2.56 | 0.14 |
| Missing | 2.12 | 0.89 to 5.07 | 0.090 | 4.86 | 0.76 to 31.21 | 0.095 |
| Profession | ||||||
| Midwifes/health visitor/maternity care | 2.32 | 1.58 to 3.41 | < 0.001 | 2.35 | 1.51 to 3.64 | < 0.001 |
| Other | 2.10 | 1.39 to 3.16 | < 0.001 | 2.18 | 1.38 to 3.44 | < 0.001 |
| Smoking status | ||||||
| Previous smoker/current smoker/declined to answer | 0.95 | 0.66 to 1.36 | 0.77 | 0.90 | 0.62 to 1.30 | 0.56 |
| Area | ||||||
| England | 1.16 | 0.81 to 1.67 | 0.42 | 0.95 | 0.65 to 1.38 | 0.78 |
| Variable | SD | D | N | A | SA |
|---|---|---|---|---|---|
| Age category (years) | |||||
| 18–34 | 16 (17.6) | 21 (23.1) | 14 (15.4) | 25 (27.5) | 15 (16.5) |
| 35–44 | 17 (14.9) | 30 (26.3) | 24 (21.1) | 32 (28.1) | 11 (9.6) |
| 45–54 | 25 (13.7) | 41 (22.5) | 28 (15.4) | 62 (34.1) | 26 (14.3) |
| 55+ | 13 (15.3) | 20 (23.5) | 14 (16.5) | 26 (30.6) | 12 (14.1) |
| Missing | 6 (24.0) | 3 (12.0) | 6 (24.0) | 9 (36.0) | 1 (4.0) |
| Breastfeeding | |||||
| Children not breastfed | 13 (11.8) | 29 (26.4) | 22 (20.0) | 33 (30.0) | 13 (11.8) |
| Children breastfed | 64 (16.5) | 86 (22.2) | 64 (16.5) | 121 (31.3) | 52 (13.4) |
| Children | |||||
| No children | 12 (12.5) | 26 (27.1) | 20 (20.8) | 26 (27.1) | 12 (12.5) |
| Have children | 65 (16.2) | 89 (22.2) | 66 (16.5) | 128 (31.9) | 53 (13.2) |
| Ethnicity | |||||
| White | 65 (14.6) | 107 (24.1) | 76 (17.1) | 137 (30.9) | 59 (13.3) |
| Other ethnicity | 12 (22.6) | 8 (15.1) | 10 (18.9) | 17 (32.1) | 6 (11.3) |
| Sex | |||||
| Male | 18 (28.1) | 21 (32.8) | 10 (15.6) | 8 (12.5) | 7 (10.9) |
| Female | 55 (13.4) | 91 (22.1) | 70 (17.0) | 139 (33.8) | 56 (13.6) |
| Missing | 4 (18.2) | 3 (13.6) | 6 (27.3) | 7 (31.8) | 2 (9.1) |
| Profession | |||||
| Doctor | 38 (24.4) | 44 (28.2) | 28 (17.9) | 37 (23.7) | 9 (5.8) |
| Midwife/health visitor/maternity care | 18 (9.1) | 40 (20.3) | 30 (15.2) | 75 (38.1) | 34 (17.3) |
| Other | 21 (14.6) | 31 (21.5) | 28 (19.4) | 42 (29.2) | 22 (15.3) |
| Smoking status | |||||
| Never smoked | 55 (14.9) | 90 (24.5) | 66 (17.9) | 111 (30.2) | 46 (12.5) |
| Previous smoker/current smoker/declined to answer | 22 (17.1) | 25 (19.4) | 20 (15.5) | 43 (33.3) | 19 (14.7) |
| Area | |||||
| North | 70 (16.0) | 103 (23.6) | 78 (17.8) | 133 (30.4) | 53 (12.1) |
| North West | 7 (11.7) | 12 (20.0) | 8 (13.3) | 21 (35.0) | 12 (20.0) |
| Variable | Simple regression model | Multiple regression model | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age category (years) | ||||||
| 18–34 | 0.97 | 0.57 to 1.66 | 0.91 | 1.01 | 0.57 to 1.77 | 0.98 |
| 35–44 | 0.83 | 0.50 to 1.36 | 0.46 | 1.01 | 0.60 to 1.69 | 0.97 |
| 45–54 | 1.12 | 0.70 to 1.77 | 0.64 | 1.03 | 0.65 to 1.65 | 0.89 |
| Missing | 0.76 | 0.34 to 1.67 | 0.49 | 0.30 | 0.06 to 1.53 | 0.15 |
| Breastfeeding | ||||||
| Children breastfed | 1.00 | 0.69 to 1.45 | 0.99 | 0.68 | 0.26 to 1.80 | 0.44 |
| Children | ||||||
| Have children | 1.06 | 0.72 to 1.57 | 0.75 | 1.47 | 0.53 to 4.08 | 0.46 |
| Ethnicity | ||||||
| Other ethnicity | 0.88 | 0.53 to 1.47 | 0.63 | 1.12 | 0.55 to 2.28 | 0.76 |
| Sex | ||||||
| Female | 2.60 | 1.61 to 4.21 | < 0.001 | 1.79 | 1.06 to 3.03 | 0.029 |
| Missing | 2.19 | 0.93 to 5.16 | 0.072 | 4.01 | 0.68 to 23.84 | 0.13 |
| Profession | ||||||
| Midwifes/health visitor/maternity care | 2.88 | 1.96 to 4.22 | < 0.001 | 2.54 | 1.65 to 3.91 | < 0.001 |
| Other | 2.02 | 1.34 to 3.04 | 0.001 | 1.94 | 1.23 to 3.05 | 0.004 |
| Smoking status | ||||||
| Previous smoker/current smoker/declined to answer | 1.14 | 0.79 to 1.63 | 0.48 | 0.91 | 0.63 to 1.32 | 0.63 |
| Area | ||||||
| England | 1.60 | 0.98 to 2.62 | 0.059 | 1.81 | 1.09 to 3.00 | 0.023 |
Appendix 31 Detailed results of the health professional survey: health economics
| Variable | Probit (agree or not) | Amount of shopping voucher | ||||
|---|---|---|---|---|---|---|
| ββ | 95% CI | p-value | ββ | 95% CI | p-value | |
| Age category (years) (base category: ≥ 55 years) | ||||||
| 16–24 | –1.32 | –2.42 to –0.23 | 0.02 | –15.85 | –26.56 to –5.13 | 0.00 |
| 25–34 | –0.17 | –0.57 to 0.24 | 0.42 | –0.57 | –9.10 to 7.97 | 0.90 |
| 35–44 | 0.18 | –0.18 to 0.54 | 0.32 | –3.91 | –10.72 to 2.89 | 0.26 |
| 45–54 | 0.11 | –0.21 to 0.44 | 0.49 | 3.06 | –3.98 to 10.10 | 0.39 |
| Missing | 0.60 | –0.44 to 1.64 | 0.26 | 8.54 | –32.60 to 49.69 | 0.68 |
| Country of residence (base category: Scotland) | ||||||
| England | 0.24 | –0.12 to 0.60 | 0.19 | –2.39 | –9.81 to 5.03 | 0.53 |
| Smoking status (base category: never smoked) | ||||||
| All else | –0.07 | –0.33 to 0.19 | 0.60 | 2.77 | –3.73 to 9.27 | 0.40 |
| Breastfeeding experience ( = 1 if yes) | –0.10 | –0.39 to 0.18 | 0.48 | –8.47 | –15.99 to –0.95 | 0.03 |
| Profession (base category: doctor) | ||||||
| Health professional routinely dealing with pregnant women | 0.19 | –0.11 to 0.50 | 0.22 | –3.20 | –9.85 to 3.45 | 0.34 |
| All others | 0.18 | –0.13 to 0.49 | 0.25 | 2.82 | –4.04 to 9.68 | 0.42 |
| Sex (base category: female) | ||||||
| Male | –0.12 | –0.48 to 0.25 | 0.53 | 1.18 | –6.18 to 8.54 | 0.75 |
| Missing | –0.91 | –2.04 to 0.22 | 0.12 | –17.55 | –60.17 to 25.07 | 0.42 |
| White ( = 1 if ethnic origin is white) | 0.15 | –0.32 to 0.62 | 0.52 | –0.15 | –9.93 to 9.62 | 0.98 |
| Constant | –0.23 | –0.84 to 0.38 | 0.47 | 29.23 | 16.13 to 42.32 | 0.00 |
| R 2 | 0.0733 | |||||
| Pseudo R2 | 0.0266 | |||||
| n | 497 | 232 | ||||
| Variable | Probit (agree or not) | Probit (women on a low income or all) | |||||
|---|---|---|---|---|---|---|---|
| ββ | 95% CI | p-value | ββ | 95% CI | p-value | ||
| Age category (years) (base category: ≥ 55 years) | |||||||
| 16–24 | –1.32 | –2.42 to –0.23 | 0.02 | ||||
| 25–34 | –0.17 | –0.57 to 0.24 | 0.42 | –0.36 | –0.99 to 0.27 | 0.27 | |
| 35–44 | 0.18 | –0.18 to 0.54 | 0.32 | –0.36 | –0.91 to 0.19 | 0.20 | |
| 45–54 | 0.11 | –0.21 to 0.44 | 0.49 | –0.27 | –0.76 to 0.21 | 0.27 | |
| Missing | 0.60 | –0.44 to 1.64 | 0.26 | –0.27 | –1.85 to 1.31 | 0.74 | |
| Country of residence (base category: Scotland) | |||||||
| England | 0.24 | –0.12 to 0.60 | 0.19 | –0.51 | –1.10 to 0.07 | 0.09 | |
| Smoking status (base category: never smoked) | |||||||
| All else | –0.07 | –0.33 to 0.19 | 0.60 | –0.24 | –0.66 to 0.18 | 0.25 | |
| Breastfeeding experience ( = 1 if yes) | –0.10 | –0.39 to 0.18 | 0.48 | 0.17 | –0.26 to 0.61 | 0.43 | |
| Profession (base category: doctor) | |||||||
| Health professional routinely dealing with pregnant women | 0.19 | –0.11 to 0.50 | 0.22 | –0.17 | –0.64 to 0.30 | 0.48 | |
| All others | 0.18 | –0.13 to 0.49 | 0.25 | 0.15 | –0.32 to 0.62 | 0.54 | |
| Sex (base category: female) | |||||||
| Male | –0.12 | –0.48 to 0.25 | 0.53 | –0.08 | –0.62 to 0.45 | 0.76 | |
| Missing | –0.91 | –2.04 to 0.22 | 0.12 | 0.04 | –1.89 to 1.98 | 0.97 | |
| White ( = 1 if ethnic origin is white) | 0.15 | –0.32 to 0.62 | 0.52 | 0.05 | –0.70 to 0.81 | 0.89 | |
| Constant | –0.23 | –0.84 to 0.38 | 0.47 | –0.15 | –1.08 to 0.78 | 0.76 | |
| Pseudo R2 | 0.0266 | 0.0337 | |||||
| n | 497 | 231 | |||||
| Variable | Probit (agree or not) | Amount of shopping voucher | ||||
|---|---|---|---|---|---|---|
| ββ | 95% CI | p-value | ββ | 95% CI | p-value | |
| Age category (years) (base category: ≥ 55 years) | ||||||
| 16–24 | –0.56 | –1.44 to 0.32 | 0.21 | 11.48 | –12.98 to 35.95 | 0.36 |
| 25–34 | –0.52 | –0.93 to –0.11 | 0.01 | 0.79 | –9.12 to 10.71 | 0.88 |
| 35–44 | –0.17 | –0.53 to 0.19 | 0.36 | –2.46 | –9.17 to 4.25 | 0.47 |
| 45–54 | –0.16 | –0.49 to 0.17 | 0.35 | –0.88 | –7.52 to 5.77 | 0.79 |
| Missing | 0.76 | –0.46 to 1.98 | 0.22 | –4.81 | –31.00 to 21.38 | 0.72 |
| Country of residence (base category: Scotland) | ||||||
| England | 0.20 | –0.16 to 0.55 | 0.27 | –0.08 | –7.69 to 7.53 | 0.98 |
| Smoking status (base category: never smoked) | ||||||
| All else | –0.16 | –0.42 to 0.11 | 0.24 | 2.10 | –3.63 to 7.83 | 0.47 |
| Breastfeeding experience ( = 1 if yes) | 0.02 | –0.27 to 0.30 | 0.90 | –2.98 | –10.32 to 4.35 | 0.42 |
| Profession (base category: doctor) | ||||||
| Health professional routinely dealing with pregnant women | 0.33 | 0.02 to 0.63 | 0.03 | 1.22 | –5.56 to 8.01 | 0.72 |
| All others | 0.06 | –0.26 to 0.37 | 0.72 | 6.17 | –0.86 to 13.20 | 0.09 |
| Sex (base category: female) | ||||||
| Male | 0.25 | –0.12 to 0.63 | 0.18 | –2.57 | –11.95 to 6.81 | 0.59 |
| Missing | –0.67 | –2.17 to 0.84 | 0.38 | –14.53 | –36.86 to 7.79 | 0.20 |
| White ( = 1 if ethnic origin is white) | –0.20 | –0.68 to 0.29 | 0.43 | –3.35 | –15.96 to 9.26 | 0.60 |
| Constant | –0.15 | –0.77 to 0.47 | 0.64 | 30.96 | 16.07 to 45.85 | 0.00 |
| R 2 | 0.0576 | |||||
| Pseudo R2 | 0.0391 | |||||
| n | 497 | 221 | ||||
| Variable | Probit (agree or not) | Probit (women on a low income or all) | ||||
|---|---|---|---|---|---|---|
| ββ | 95% CI | p-value | ββ | 95% CI | p-value | |
| Age category (years) (base category: ≥ 55 years) | ||||||
| 16–24 | –0.56 | –1.44 to 0.32 | 0.21 | |||
| 25–34 | –0.52 | –0.93 to –0.11 | 0.01 | –1.21 | –2.08 to –0.35 | 0.01 |
| 35–44 | –0.17 | –0.53 to 0.19 | 0.36 | –0.48 | –1.05 to 0.09 | 0.10 |
| 45–54 | –0.16 | –0.49 to 0.17 | 0.35 | –0.29 | –0.76 to 0.19 | 0.24 |
| Missing | 0.76 | –0.46 to 1.98 | 0.22 | –0.30 | –1.52 to 0.92 | 0.63 |
| Country of residence (base category: Scotland) | ||||||
| England | 0.20 | –0.16 to 0.55 | 0.27 | –0.84 | –1.46 to –0.22 | 0.01 |
| Smoking status (base category: never smoked) | ||||||
| All else | –0.16 | –0.42 to 0.11 | 0.24 | –0.40 | –0.86 to 0.07 | 0.09 |
| Breastfeeding experience ( = 1 if yes) | 0.02 | –0.27 to 0.30 | 0.90 | –0.17 | –0.66 to 0.31 | 0.49 |
| Profession (base category: doctor) | ||||||
| Health professional routinely dealing with pregnant women | 0.33 | 0.02 to 0.63 | 0.03 | –0.20 | –0.73 to 0.33 | 0.46 |
| All others | 0.06 | –0.26 to 0.37 | 0.72 | –0.08 | –0.64 to 0.47 | 0.77 |
| Sex (base category: female) | ||||||
| Male | 0.25 | –0.12 to 0.63 | 0.18 | 0.23 | –0.46 to 0.91 | 0.52 |
| Missing | –0.67 | –2.17 to 0.84 | 0.38 | 0.16 | –1.33 to 1.65 | 0.83 |
| White ( = 1 if ethnic origin is white) | –0.20 | –0.68 to 0.29 | 0.43 | –0.47 | –1.16 to 0.22 | 0.18 |
| Constant | –0.15 | –0.77 to 0.47 | 0.64 | 0.52 | –0.42 to 1.46 | 0.28 |
| Pseudo R2 | 0.0391 | 0.0576 | ||||
| n | 497 | 221 | ||||
Appendix 32 Framing effects in the Ipsos MORI survey
| Variable | Topic covered first | SD | D | N | A | SA |
|---|---|---|---|---|---|---|
| Pregnant women SC – vouchers | Smoking | 154 (26.3) | 94 (16.0) | 103 (17.6) | 131 (22.4) | 104 (17.7) |
| Breastfeeding | 141 (25.3) | 95 (17.0) | 94 (16.8) | 138 (24.7) | 90 (16.1) | |
| OR (95% CI), p-value | 1.00 (0.82 to 1.23), 0.98 | |||||
| Women after birth SC – vouchers | Smoking | 167 (28.5) | 113 (19.3) | 98 (16.7) | 135 (23.0) | 73 (12.5) |
| Breastfeeding | 152 (27.2) | 99 (17.7) | 98 (17.6) | 147 (26.3) | 62 (11.1) | |
| OR (95% CI), p-value | 1.06 (0.86 to 1.31), 0.57 | |||||
| Smoke-free home after birth – vouchers | Smoking | 162 (27.6) | 113 (19.3) | 113 (19.3) | 121 (20.6) | 77 (13.1) |
| Breastfeeding | 149 (26.7) | 102 (18.3) | 111 (19.9) | 126 (22.6) | 70 (12.5) | |
| OR (95% CI), p-value | 1.05 (0.85 to 1.29), 0.65 | |||||
| Health service payment for meeting SC targets | Smoking | 127 (21.7) | 95 (16.2) | 130 (22.2) | 155 (26.5) | 79 (13.5) |
| Breastfeeding | 107 (19.2) | 97 (17.4) | 137 (24.6) | 151 (27.1) | 66 (11.8) | |
| OR (95% CI), p-value | 1.01 (0.82 to 1.24), 0.93 | |||||
| BF women after birth – vouchers | Smoking | 152 (25.9) | 134 (22.9) | 138 (23.5) | 99 (16.9) | 63 (10.8) |
| Breastfeeding | 84 (15.1) | 77 (13.8) | 168 (30.1) | 136 (24.4) | 93 (16.7) | |
| OR (95% CI), p-value | 2.00 (1.61 to 2.46), < 0.001 | |||||
| Health service payment for meeting BF targets | Smoking | 145 (24.7) | 113 (19.3) | 134 (22.9) | 125 (21.3) | 69 (11.8) |
| Breastfeeding | 91 (16.3) | 92 (16.5) | 153 (27.4) | 153 (27.4) | 69 (12.4) | |
| OR (95% CI), p-value | 1.44 (1.17 to 1.77), 0.001 | |||||
| Breast pumps | Smoking | 105 (17.9) | 76 (13.0) | 150 (25.6) | 157 (26.8) | 98 (16.7) |
| Breastfeeding | 60 (10.8) | 71 (12.7) | 158 (28.3) | 159 (28.5) | 110 (19.7) | |
| OR (95% CI), p-value | 1.32 (1.08 to 1.62), 0.008 | |||||
Appendix 33 Discrete choice experiment questionnaire
Appendix 34 Discrete choice experiment technical appendix
Discrete choice experiment regression equation:
Effects coding
The estimated coefficient, βi, on effects-coded variables is interpreted as the change in V from the overall mean for the given variable level. As the mean for effects-coded variables is zero, the estimated coefficient for each level of the variable measures the difference in V for those who choose that level. β values and SEs for the base category can be retrieved using the formula based on the coding (Equations 2 and 3).
Using the conditional logit model results from estimating Equation 1, the perceived likelihood of quitting for a given smoking cessation service, V, is defined as the sum of the coefficients relevant to the configuration of the selected service. In other words:
as in Equation 1.
Appendix 35 Discrete choice experiment results tables for subgroup analysis
| Variable | Regression output (β estimate, SE and 95% CI) | Smoker | Ex-smoker |
|---|---|---|---|
| Constant ( = 1 if option A or B) | β | 1.500 | 1.127 |
| SE | 0.098 | 0.083 | |
| 95% CI | 1.309 to 1.691 | 0.964 to 1.290 | |
| Frequency of meeting | |||
| Once a week | β | –0.056 | 0.123 |
| SE | 0.033 | 0.033 | |
| 95% CI | –0.121 to 0.009 | 0.059 to 0.187 | |
| Once every 2 weeks | β | 0.056 | –0.123 |
| SE | 0.033 | 0.033 | |
| 95% CI | –0.009 to 0.121 | –0.187 to –0.059 | |
| Method of support in the first week | |||
| Daily visit to the clinic | β | –0.347 | –0.392 |
| SE | 0.053 | 0.052 | |
| 95% CI | –0.452 to –0.243 | –0.493 to –0.291 | |
| Daily telephone call | β | 0.216 | 0.174 |
| SE | 0.053 | 0.052 | |
| 95% CI | 0.112 to 0.320 | 0.073 to 0.275 | |
| Daily text message | β | 0.131 | 0.218 |
| SE | 0.054 | 0.052 | |
| 95% CI | 0.026 to 0.236 | 0.117 to 0.319 | |
| Monthly financial incentive | |||
| £0.00 | β | –0.902 | –0.477 |
| SE | 0.067 | 0.066 | |
| 95% CI | –1.034 to –0.770 | –0.606 to –0.348 | |
| £20.00 | β | –0.125 | –0.139 |
| SE | 0.067 | 0.064 | |
| 95% CI | –0.255 to 0.006 | –0.265 to –0.013 | |
| £40.00 | β | 0.238 | 0.221 |
| SE | 0.065 | 0.065 | |
| 95% CI | 0.110 to 0.367 | 0.093 to 0.349 | |
| £80.00 | β | 0.788 | 0.394 |
| SE | 0.070 | 0.068 | |
| 95% CI | 0.651 to 0.925 | 0.261 to 0.528 | |
| Quitting pal | |||
| No pal | β | –0.223 | –0.438 |
| SE | 0.069 | 0.066 | |
| 95% CI | –0.359 to –0.087 | –0.568 to –0.309 | |
| Pal with informationa | β | –0.022 | 0.111 |
| SE | 0.071 | 0.068 | |
| 95% CI | –0.161 to 0.118 | –0.021 to 0.245 | |
| Pal with support and textb | β | 0.153 | 0.263 |
| SE | 0.068 | 0.066 | |
| 95% CI | 0.019 to 0.287 | 0.133 to 0.392 | |
| Pal with voucherc | β | 0.092 | 0.064 |
| SE | 0.069 | 0.064 | |
| 95% CI | –0.042 to 0.226 | –0.062 to 0.190 | |
| Log likelihood | –1050.3683 | –1173.7013 | |
| Pseudo R2 | 0.2531 | 0.1654 | |
| Log likelihood ratio test | χ2 = 63.42 (df = 12, p < 0.0001) | ||
| n (observations) | 3840 | 3840 | |
| n (respondents) | 160 | 160 | |
| Variable | Regression output (β estimate, SE and 95% CI) | 16–20 years | 21–25 years | 26–30 years | 31–35 years | 36–40 years |
|---|---|---|---|---|---|---|
| Constant (= 1 if option A or B) | β | 1.647 | 1.564 | 1.675 | 1.160 | 1.140 |
| SE | 0.304 | 0.212 | 0.164 | 0.106 | 0.105 | |
| 95% CI | 1.052 to 2.242 | 1.149 to 1.980 | 1.354 to 1.996 | 0.953 to 1.368 | 0.933 to 1.346 | |
| Frequency of meeting | ||||||
| Once a week | β | 0.076 | –0.019 | 0.048 | 0.005 | 0.052 |
| SE | 0.096 | 0.070 | 0.053 | 0.040 | 0.041 | |
| 95% CI | –0.113 to 0.265 | –0.157 to 0.118 | –0.056 to 0.153 | –0.074 to 0.084 | –0.029 to 0.134 | |
| Once every 2 weeks | β | –0.076 | 0.019 | –0.048 | –0.005 | –0.052 |
| SE | 0.096 | 0.070 | 0.053 | 0.040 | 0.041 | |
| 95% CI | –0.265 to 0.113 | –0.118 to 0.157 | –0.153 to 0.056 | –0.084 to 0.074 | –0.134 to 0.029 | |
| Method of support in the first week | ||||||
| Daily visit to the clinic | β | –0.200 | –0.517 | 0.140 | –0.389 | –0.335 |
| SE | 0.155 | 0.113 | 0.084 | 0.065 | 0.066 | |
| 95% CI | –0.504 to 0.104 | –0.739 to –0.295 | –0.025 to 0.304 | –0.516 to –0.262 | –0.464 to –0.206 | |
| Daily telephone call | β | –0.009 | 0.270 | 0.196 | 0.202 | 0.231 |
| SE | 0.156 | 0.112 | 0.087 | 0.065 | 0.066 | |
| 95% CI | –0.314 to 0.297 | 0.051 to 0.489 | 0.025 to 0.368 | 0.075 to 0.328 | 0.101 to 0.362 | |
| Daily text message | β | 0.209 | 0.247 | 0.194 | 0.187 | 0.104 |
| SE | 0.154 | 0.114 | 0.088 | 0.065 | 0.065 | |
| 95% CI | –0.094 to 0.511 | 0.023 to 0.471 | 0.022 to 0.365 | 0.060 to 0.315 | –0.024 to 0.232 | |
| Monthly financial incentive | ||||||
| £0.00 | β | –0.570 | –0.756 | –0.973 | –0.523 | –0.683 |
| SE | 0.194 | 0.144 | 0.108 | 0.082 | 0.083 | |
| 95% CI | –0.950 to –0.190 | –1.038 to –0.474 | –1.185 to –0.761 | –0.685 to –0.362 | –0.846 to –0.521 | |
| £20.00 | β | –0.083 | –0.110 | –0.109 | –0.099 | –0.170 |
| SE | 0.196 | 0.141 | 0.102 | 0.082 | 0.083 | |
| 95% CI | –0.467 to 0.302 | –0.386 to 0.166 | –0.310 to 0.092 | –0.260 to 0.061 | –0.334 to –0.007 | |
| £40.00 | β | 0.238 | 0.231 | 0.233 | 0.115 | 0.284 |
| SE | 0.190 | 0.140 | 0.105 | 0.081 | 0.082 | |
| 95% CI | –0.135 to 0.612 | –0.043 to 0.506 | 0.027 to 0.438 | –0.044 to 0.274 | 0.123 to 0.444 | |
| £80.00 | β | 0.414 | 0.635 | 0.849 | 0.507 | 0.570 |
| SE | 0.195 | 0.151 | 0.117 | 0.084 | 0.084 | |
| 95% CI | 0.031 to 0.797 | 0.339 to 0.930 | 0.619 to 1.078 | 0.342 to 0.673 | 0.406 to 0.734 | |
| Quitting pal | ||||||
| No pal | β | –0.436 | –0.257 | –0.368 | –0.308 | –0.294 |
| SE | 0.197 | 0.147 | 0.112 | 0.083 | 0.085 | |
| 95% CI | –0.822 to –0.051 | –0.545 to 0.032 | –0.587 to –0.149 | –0.471 to –0.145 | –0.461 to –0.128 | |
| Pal with informationa | β | 0.177 | –0.001 | 0.194 | –0.012 | 0.023 |
| SE | 0.194 | 0.155 | 0.116 | 0.086 | 0.086 | |
| 95% CI | –0.204 to 0.558 | –0.305 to 0.302 | –0.033 to 0.422 | –0.180 to 0.157 | –0.145 to 0.191 | |
| Pal with support and textb | β | 0.105 | 0.122 | 0.154 | 0.165 | 0.287 |
| SE | 0.202 | 0.144 | 0.111 | 0.081 | 0.085 | |
| 95% CI | –0.292 to 0.502 | –0.160 to 0.403 | –0.064 to 0.372 | 0.006 to 0.325 | 0.120 to 0.455 | |
| Pal with voucherc | β | 0.154 | 0.136 | 0.020 | 0.154 | –0.016 |
| SE | 0.194 | 0.142 | 0.108 | 0.082 | 0.083 | |
| 95% CI | –0.225 to 0.534 | –0.143 to 0.415 | –0.192 to 0.232 | –0.007 to 0.315 | –0.179 to 0.148 | |
| Log likelihood | –122.140 | –231.194 | –403.388 | –743.712 | –733.288 | |
| Pseudo R2 | 0.2279 | 0.2484 | 0.2829 | 0.1622 | 0.1820 | |
| Log likelihood ratio test | χ2 = 44.11 (df = 42, p = 0.3824) | |||||
| n (observations) | 432 | 840 | 1536 | 2424 | 2448 | |
| n (respondents) | 18 | 35 | 64 | 101 | 102 | |
| Variable | Regression output (β estimate, SE and 95% CI) | Very difficult | Quite difficult | Quite easy | Very easy | Don’t know |
|---|---|---|---|---|---|---|
| Constant ( = 1 if option A or B) | β | 1.947 | 1.415 | 1.548 | 0.595 | 1.982 |
| SE | 0.194 | 0.124 | 0.142 | 0.104 | 0.330 | |
| 95% CI | 1.567 to 2.327 | 1.172 to 1.659 | 1.270 to 1.826 | 0.392 to 1.367 | 1.335 to 2.630 | |
| Frequency of meeting | ||||||
| Once a week | β | –0.106 | 0.030 | 0.117 | 0.075 | 0.062 |
| SE | 0.055 | 0.043 | 0.047 | 0.050 | 0.094 | |
| 95% CI | –0.214 to 0.001 | –0.055 to 0.116 | 0.024 to 0.210 | –0.022 to 0.172 | –0.122 to 0.245 | |
| Once every 2 weeks | β | 0.106 | –0.030 | –0.117 | –0.075 | –0.062 |
| SE | 0.055 | 0.043 | 0.047 | 0.050 | 0.094 | |
| 95% CI | –0.001 to 0.214 | –0.116 to 0.055 | –0.210 to –0.024 | –0.173 to 0.022 | –0.245 to 0.122 | |
| Method of support in the first week | ||||||
| Daily visit to the clinic | β | –0.182 | –0.412 | –0.391 | –0.349 | –0.649 |
| SE | 0.089 | 0.070 | 0.076 | 0.077 | 0.150 | |
| 95% CI | –0.356 to –0.008 | –0.549 to –0.275 | –0.539 to –0.243 | –0.500 to –0.197 | –0.943 to –0.355 | |
| Daily telephone call | β | 0.188 | 0.211 | 0.136 | 0.179 | 0.292 |
| SE | 0.088 | 0.070 | 0.074 | 0.078 | 0.150 | |
| 95% CI | 0.015 to 0.361 | 0.074 to 0.349 | –0.009 to 0.282 | 0.027 to 0.332 | –0.003 to 0.587 | |
| Daily text message | β | –0.006 | 0.201 | 0.254 | 0.169 | 0.357 |
| SE | 0.089 | 0.070 | 0.077 | 0.077 | 0.150 | |
| 95% CI | –0.181 to 0.169 | 0.064 to 0.338 | 0.103 to 0.405 | 0.018 to 0.320 | 0.064 to 0.650 | |
| Monthly financial incentive | ||||||
| £0.00 | β | –0.968 | –0.675 | –0.583 | –0.549 | –0.785 |
| SE | 0.112 | 0.089 | 0.096 | 0.098 | 0.193 | |
| 95% CI | –1.189 to –0.747 | –0.849 to –0.501 | –0.772 to –0.394 | –0.742 to –0.357 | –1.165 to –0.405 | |
| £20.00 | β | –0.084 | –0.210 | –0.108 | –0.139 | –0.053 |
| SE | 0.109 | 0.089 | 0.092 | 0.099 | 0.188 | |
| 95% CI | –0.297 to 0.129 | –0.384 to –0.035 | –0.288 to 0.072 | –0.333 to 0.055 | –0.422 to 0.315 | |
| £40.00 | β | 0.132 | 0.338 | 0.206 | 0.207 | 0.126 |
| SE | 0.110 | 0.086 | 0.095 | 0.097 | 0.186 | |
| 95% CI | –0.084 to 0.348 | 0.169 to 0.508 | 0.020 to 0.392 | 0.017 to 0.397 | –0.239 to 0.490 | |
| £80.00 | β | 0.920 | 0.546 | 0.485 | 0.481 | 0.713 |
| SE | 0.119 | 0.090 | 0.102 | 0.098 | 0.207 | |
| 95% CI | 0.687 to 1.154 | 0.369 to 0.724 | 0.285 to 0.685 | 0.289 to 0.673 | 0.307 to 1.118 | |
| Quitting pal | ||||||
| No pal | β | –0.293 | –0.276 | –0.486 | –0.228 | –0.381 |
| SE | 0.116 | 0.090 | 0.097 | 0.100 | 0.198 | |
| 95% CI | –0.520 to –0.066 | –0.452 to –0.100 | –0.676 to –0.295 | –0.424 to –0.032 | –0.770 to 0.008 | |
| Pal with informationa | β | –0.066 | 0.072 | –0.033 | 0.165 | 0.203 |
| SE | 0.116 | 0.092 | 0.101 | 0.100 | 0.208 | |
| 95% CI | –0.294 to 0.162 | –0.108 to 0.253 | –0.232 to 0.165 | –0.032 to 0.362 | –0.205 to 0.611 | |
| Pal with support and textb | β | 0.290 | 0.178 | 0.278 | 0.124 | 0.055 |
| SE | 0.114 | 0.089 | 0.097 | 0.101 | 0.200 | |
| 95% CI | 0.067 to 0.513 | 0.003 to 0.352 | 0.089 to 0.468 | –0.074 to 0.322 | –0.338 to 0.448 | |
| Pal with voucherc | β | 0.069 | 0.026 | 0.241 | –0.061 | 0.123 |
| SE | 0.117 | 0.088 | 0.094 | 0.099 | 0.186 | |
| 95% CI | –0.161 to 0.299 | –0.147 to 0.199 | 0.058 to 0.424 | –0.255 to 0.132 | –0.243 to 0.488 | |
| Log likelihood | –352.769 | –622.902 | –514.764 | –579.665 | –122.961 | |
| Pseudo R2 | 0.3197 | 0.2212 | 0.2293 | 0.0965 | 0.3338 | |
| Log likelihood ratio test | χ2 = 125.44 (df = 42, p < 0.0001) | |||||
| n (observations) | 1416 | 2184 | 1824 | 1752 | 504 | |
| n (respondents) | 59 | 91 | 76 | 73 | 21 | |
| Variable | Regression output (β estimate, SE and 95% CI) | GCSE | A-level | University |
|---|---|---|---|---|
| Constant ( = 1 if option A or B) | β | 0.776 | 1.489 | 1.494 |
| SE | 0.126 | 0.110 | 0.106 | |
| 95% CI | 0.529 to 1.024 | 1.274 to 1.704 | 1.286 to 1.701 | |
| Frequency of meeting | ||||
| Once a week | β | 0.004 | 0.016 | 0.075 |
| SE | 0.056 | 0.037 | 0.036 | |
| 95% CI | –0.107 to 0.114 | –0.057 to 0.089 | 0.005 to 0.145 | |
| Once every 2 weeks | β | –0.004 | –0.016 | –0.075 |
| SE | 0.056 | 0.037 | 0.036 | |
| 95% CI | –0.114 to 0.107 | –0.089 to 0.057 | –0.145 to –0.005 | |
| Method of support in the first week | ||||
| Daily visit to the clinic | β | –0.197 | –0.409 | –0.392 |
| SE | 0.089 | 0.060 | 0.058 | |
| 95% CI | –0.371 to –0.024 | –0.527 to –0.291 | –0.505 to –0.279 | |
| Daily telephone call | β | 0.137 | 0.153 | 0.224 |
| SE | 0.089 | 0.060 | 0.057 | |
| 95% CI | –0.038 to 0.312 | 0.036 to 0.270 | 0.112 to 0.336 | |
| Daily text message | β | 0.060 | 0.256 | 0.168 |
| SE | 0.088 | 0.061 | 0.058 | |
| 95% CI | –0.113 to 0.233 | 0.137 to 0.375 | 0.054 to 0.282 | |
| Monthly financial incentive | ||||
| £0.00 | β | –0.528 | –0.724 | –0.673 |
| SE | 0.112 | 0.076 | 0.073 | |
| 95% CI | –0.747 to –0.309 | –0.874 to –0.575 | –0.816 to –0.529 | |
| £20.00 | β | –0.079 | –0.164 | –0.149 |
| SE | 0.114 | 0.075 | 0.071 | |
| 95% CI | –0.301 to 0.144 | –0.311 to –0.018 | –0.288 to –0.009 | |
| £40.00 | β | 0.133 | 0.265 | 0.188 |
| SE | 0.111 | 0.074 | 0.072 | |
| 95% CI | –0.085 to 0.351 | 0.120 to 0.411 | 0.048 to 0.329 | |
| £80.00 | β | 0.473 | 0.624 | 0.633 |
| SE | 0.110 | 0.079 | 0.077 | |
| 95% CI | 0.257 to 0.689 | 0.468 to 0.779 | 0.482 to 0.783 | |
| Quitting pal | ||||
| No pal | β | –0.222 | –0.311 | –0.403 |
| SE | 0.113 | 0.078 | 0.074 | |
| 95% CI | –0.444 to 0.001 | –0.463 to –0.159 | –0.548 to –0.257 | |
| Pal with informationa | β | 0.065 | 0.069 | 0.055 |
| SE | 0.113 | 0.080 | 0.077 | |
| 95% CI | –0.156 to 0.285 | –0.088 to 0.226 | –0.095 to 0.205 | |
| Pal with support and textb | β | 0.172 | 0.212 | 0.176 |
| SE | 0.114 | 0.077 | 0.074 | |
| 95% CI | –0.053 to 0.396 | 0.061 to 0.362 | 0.031 to 0.321 | |
| Pal with voucherc | β | –0.015 | 0.030 | 0.172 |
| SE | 0.113 | 0.076 | 0.072 | |
| 95% CI | –0.237 to 0.208 | –0.119 to 0.180 | 0.031 to 0.313 | |
| Log likelihood | –428.085 | –823.764 | –890.672 | |
| Pseudo R2 | 0.0980 | 0.2317 | 0.2323 | |
| Log likelihood ratio test | χ2 = 38.74 (df = 20, p = 0.0072) | |||
| n (observations) | 1296 | 2928 | 3168 | |
| n (respondents) | 54 | 122 | 132 | |
List of abbreviations
- A-level
- Advanced level
- BCT
- behaviour change technique
- BFI
- Baby Friendly Initiative
- BIBS
- Benefits of Incentives for Breastfeeding and Smoking cessation in pregnancy
- CAPIBUS
- Computer Aided Personal Interviewing (face-to-face omnibus)
- CASP
- Critical Appraisal Skills Programme
- CDSR
- Cochrane Database of Systematic Reviews
- CI
- confidence interval
- CINAHL
- Cumulative Index to Nursing and Allied Health Literature
- CO
- carbon monoxide
- COM-B
- Capability, Opportunity, Motivation – Behaviour
- CPIT
- Cessation in Pregnancy Incentives Trial
- DCE
- discrete choice experiment
- GCSE
- General Certificate of Secondary Education
- GP
- general practitioner
- HMO
- health maintenance organisation
- HTA
- Health Technology Assessment
- IRBCT
- incentive/reward behaviour change technique
- ISCS
- incentive smoking cessation service
- ITT
- intention to treat
- LHA
- local health authority
- MAP
- Motivation development, Action on motivation or Prompted/cued behaviour
- MIDIRS
- Midwives Information and Resource Service
- MORI
- Market & Opinion Research International (Ipsos MORI)
- MRC
- Medical Research Council
- NICE
- National Institute for Health and Care Excellence
- NIHR
- National Institute for Health Research
- NRT
- nicotine replacement therapy
- OR
- odds ratio
- P4P
- pay for performance
- PPI
- patient and public involvement
- QOF
- Quality and Outcomes Framework
- R&D
- research and development
- RCT
- randomised controlled trial
- RD
- risk difference
- RR
- relative risk
- SD
- standard deviation
- SE
- standard error
- SPCRN
- Scottish Primary Care Research Network
- SQD
- setting a quit date
- UCLan
- University of Central Lancashire
- UNICEF
- United Nations Children’s Fund
- VBRT
- voucher-based reinforcement therapy
- WHO
- World Health Organization
- WIC
- Women, Infants and Children