Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 10/96/01. The contractual start date was in November 2011. The draft report began editorial review in May 2014 and was accepted for publication in February 2015. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Peter J White reports grants from Medical Research Council (MRC) and from the National Institute for Health Research (NIHR) during the conduct of the study, and grants from Otsuka outside the submitted work. Francis Drobniewski reports grants from EU FP7, European Centre for Disease Control, the World Health Organization and the Technology Strategy Board, outside the submitted work. Ibrahim Abubakar reports grants from NIHR and MRC outside the submitted work. Joanne Lord reports grants from NIHR outside the submitted work.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Drobniewski et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background
Introduction
Tuberculosis (TB) remains a major global health problem and ranks as the second leading cause of death from an infectious disease worldwide, after the human immunodeficiency virus (HIV). TB is an airborne disease caused by bacteria belonging to the Mycobacterium tuberculosis complex (MTBC). It typically affects the lungs (pulmonary TB) and is spread through the air by coughing. Most infections in humans result in an asymptomatic, latent TB infection, and about 1 in 10 latent infections eventually progresses to active disease (30–50% in patients co-infected with HIV).
Without treatment, TB mortality rates are high. Among HIV-negative patients of smear-positive pulmonary TB, around 70% die within 10 years. For culture-positive (but smear-negative) patients, 20% die within 10 years. Combination drug therapy has been the mainstay of TB treatment for decades and short-course, rifampicin-based regimens will cure almost all cases. The currently recommended treatment for new cases of drug-susceptible TB is a 6-month regimen of four drugs: isoniazid, rifampicin, ethambutol and pyrazinamide. Interrupted and incomplete therapy selects for drug-resistant strains which are more difficult to treat successfully. TB drug resistance is a growing international problem, which, with HIV co-infection, threatens the success of national TB programmes.
Multidrug-resistant (MDR) TB (MDR-TB) is defined as resistance to at least rifampicin and isoniazid – the two most powerful first-line anti-TB drugs. Treatment for MDR-TB is longer and requires more expensive, more toxic drugs. The World Health Organization (WHO) recommends treatment regimens last 20 months, and success rates are much lower. Further resistance can develop to form extensively drug-resistant TB (XDR-TB), defined as MDR plus additional resistance to any fluoroquinolone and at least one of the injectable agents (amikacin, capreomycin or kanamycin). Infection with XDR-TB further decreases chances of treatment success and survival. 1–4 So far, co-infection with HIV and XDR-TB has been largely fatal. 5,6 Recent years have seen an ominous accumulation of reports of ‘totally’ drug-resistant strains, which do not exhibit susceptibility to any tested drugs. 7
Global burden of tuberculosis
In 2012, 8.6 million people developed active TB and 1.3 million died from the disease. 8 Over 95% of deaths occurred in low- and middle-income countries. TB is also a major killer of those co-infected with HIV, causing one-quarter of all deaths.
Tuberculosis continues to be a significant public health and clinical problem in the industrialised world. Within countries of the WHO European Region, those in the east have much higher notification rates than in the west. The Region reported 309,648 new episodes of TB (34.0 per 100,000 population), with > 60,000 deaths estimated as being due to TB, or 6.7 cases per 100,000 population. 9 Notification rates for newly detected and relapsed TB cases in the WHO 18 High Priority Countries (all from the central and eastern part of the Region), remained almost eight times higher (68.5 per 100,000 population) than in the rest of the Region (8.4 per 100,000). 9
Globally, in 2012, 3.6% of new TB cases and 20.2% of previously treated cases were estimated to have MDR-TB. The highest rates of MDR-TB are found in Eastern Europe and Central Asia, where, in some countries, > 20% of new TB patients and > 50% of those previously treated for TB have MDR-TB. 8 On average, an estimated 9.6% of MDR-TB patients have XDR-TB. By the end of 2012, 92 countries had reported at least one case of XDR-TB. In May 2009, the 62nd World Health Assembly urged member states to take action to achieve universal access to diagnosis and treatment of MDR-TB and XDR-TB by 2015. 10
Epidemiology of tuberculosis in the UK
Around 9000 cases of TB are currently reported each year in the UK. 11 Most cases occur in major cities; London has the highest rate of TB of any Western European capital. The incidence of MDR-TB in the UK is low (approximately 1–1.5%) and has remained relatively constant over recent years. However, in some areas of the country, such as London, and in certain population groups, the incidence is much higher. Institutional outbreaks of MDR-TB have occurred in the UK, notably in London hospitals,12 and wider pan-institutional outbreaks, such as one involving isoniazid-resistant strains, have been reported. 13
Molecular basis of drug resistance
For M. tuberculosis, drug resistance is primarily acquired through mutation of chromosomal genes. Recognised mechanisms include mutations that (1) block the drug target (e.g. mutations in rpoB prevent binding of rifampicin to RNA polymerase14), (2) block activation of a prodrug (e.g. mutations in katG lead to loss of the ability of catalase to activate isoniazid to its active form15), or (3) produce an activity that binds or inactivates the drug (e.g. mutations in the inhA promoter increase the amount of InhA product that binds sufficient isoniazid to reduce its effective intracellular concentration to below an inhibitory level16).
Although many of the mutations leading to resistance are recognised, further work is required to elucidate fully the mutations that are responsible for resistance against some drugs, and to determine the predictive value of finding a particular mutation in a strain of M. tuberculosis (MTB). 17,18
Approximately 95% of rifampicin-resistant M. tuberculosis isolates are additionally resistant to isoniazid, enabling the detection of resistance to rifampicin to be used as a marker for MDR-TB with a high level of accuracy. Assays to detect mutations associated with other key resistances, including for XDR-TB, have been developed, although the scientific understanding of the mechanisms of resistance is less well understood.
Diagnosis of drug resistance
Conventional culture methods
Conventional culture and drug susceptibility testing (DST) is a slow process, requiring isolation of mycobacteria from clinical specimens, identification of MTBC, and testing of the susceptibility pattern of strains in the presence of anti-TB drugs. Depending on the methodology, culture-based methods can take several months to be completed; however, they remain the ‘gold standard’ for M. tuberculosis DST.
A TB strain is classified as resistant to a drug if ≥ 1% of the bacterial population is able to grow at a ‘critical’ concentration of drug, which inhibits 95% of wild-type strains. 19 DST using agar- or egg-based Löwenstein–Jensen (LJ) solid media is the most commonly utilised method. Standard methods using LJ medium include the absolute concentration method, the resistance ratio method and the proportion method. 19 In the absolute concentration method, a standardised bacterial suspension is inoculated on to drug-free media and media containing graded concentrations of the drug to be tested. The minimum inhibitory concentration (MIC) is the lowest concentration of the drug that inhibits growth. In the resistance ratio method, the growth of the test strain is compared with that of a control susceptible strain. Resistance is expressed as the ratio of the MIC of the test strain to the MIC of the control strain. The proportion method determines the percentage of growth (number of colonies) of a defined inoculum on a drug-free control medium compared with growth on culture media containing the critical concentration (or range of concentrations) of the test drug.
Automated commercial liquid culture systems use the proportion method and are designed to detect growth inhibition in drug-containing media as early as possible. One of the first systems was the BACTEC 460 TB system (Becton Dickinson Diagnostic Instrument Systems, Towson, MD, USA), which uses a radiometric method to measure carbon dioxide production. Subsequently, non-radiometric methods, such as the BACTEC MGIT (Mycobacteria Growth Indicator Tube) 960 system (Becton Dickinson Diagnostic Instrument Systems) have become widely adopted. 20 The BACTEC MGIT 960 system uses fluorimetric detection to monitor oxygen consumption resulting from bacterial growth. Growth in media containing the drug of interest is compared with growth in a drug-free control tube, which is inoculated with a 1 : 100 dilution of the M. tuberculosis strain. Every 60 minutes, the BACTEC MGIT 960 measures the fluorescence emitted from the tube. Once the fluorescence indicates that there are 105–106 colony-forming units (CFUs) per millilitre of medium, the tube is flagged as positive. If there is at least equal growth in a drug-containing tube and the control tube, this indicates that at least 1% of the bacteria present in the sample are resistant to that drug and the sample is classified as resistant. If the tube does not become positive within 6 weeks, the machine marks it as negative. The BACTEC MGIT 960 can reduce the mean time taken to detection of M. tuberculosis to 14.4 days compared with 24.1 days on solid medium. 21
Non-commercial culture systems based on the growth of microcolonies can also facilitate more rapid diagnosis than conventional methods. 22 Microscopic observation of drug susceptibility (MODS) is a liquid culture-based method in which early growth in drug-free and drug-containing media is detected by microscopic examination. 23 Similarly, thin-layer agar (TLA) methods rely on the microscopic detection of growth on solid media. 24
Rapid molecular tests
A significant reduction in the time for diagnosis of clinically significant drug resistance depends on genotypic methods that identify mutations in genes responsible for resistance. Specific nucleotide sequences in processed specimens (crude extracts or treated sputum) can be detected within a few hours, so the total time for the TB detection can be reduced to < 1 day. Improved biosafety is another advantage of molecular assays, as they require high containment only initially. However, molecular methods lack the sensitivity of phenotypic methods, as the genetic basis of resistance is not completely understood for any anti-TB drug. For example, although 95% of rifampicin-resistant M. tuberculosis strains have mutations in the 81 base pair (bp) rifampicin resistance determining region (RRDR) of the gene encoding the RNA polymerase-subunit, rpoB, 5% of strains have no known resistance mutations. 25 Up to 10–25% of low-level isoniazid-resistant M. tuberculosis strains do not have mutations in either katG or the inhA promoter. 26
Molecular tools for the detection of M. tuberculosis and determination of drug resistance that have been developed over the past decade are largely nucleic acid amplification tests (NAATs) to increase sensitivity, combined with highly specific detection systems. The polymerase chain reaction (PCR) is the most common methodology utilised in NAAT; alternatives include real-time PCR, isothermal strand displacement amplification or transcription-mediated amplification, and the ligase chain reaction. 27–30 Although NAAT can theoretically detect a single copy of nucleic acid in a specimen, sensitivity can be significantly compromised by the presence of PCR inhibitors in clinical specimens and loss of nucleic acids during specimen processing, and therefore tends to vary.
Line probe assays
In 2008, the WHO formally endorsed the use of line probe assays (LPAs) for rapid screening of patients at risk of MDR-TB31 and they are now in routine use in many TB laboratories in high- and middle-income countries.
To perform LPAs, first deoxyribonucleic acid (DNA) is extracted from M. tuberculosis cultures or directly from clinical specimens. Regions of interest in resistance-determining genes are amplified by PCR using biotinylated primers. Following amplification, labelled PCR products are hybridised with oligonucleotide probes that are immobilised on nitrocellulose membrane strips. Captured PCR products are detected by binding to streptavidin-conjugated alkaline phosphatase and colorimetric development, which can be observed visually. If a mutation is present in one of the target regions, the amplicon will not hybridise with the relevant probe. Mutations are therefore detected by lack of binding to wild-type probes, as well as by binding to specific probes for the most commonly occurring mutations.
Currently, the main commercial LPAs for the rapid diagnosis of TB and rifampicin resistance in clinical specimens are the INNO-LiPA Rif.TB® (Fujirebio Europe, Ghent, Belgium) and the GenoType® Mycobacterium tuberculosis drug resistance plus assay (MTBDRplus) (Hain Lifescience, Nehren, Germany), which additionally detects isoniazid resistance. 32–35 The GenoType® Mycobacterium tuberculosis drug resistance second-line assay (MTBDRsl) (Hain Lifescience) is the only available rapid assay for detection of resistance to fluoroquinolones and injectable second-line drugs, as well as ethambutol, offering rapid detection of XDR-TB in mycobacterial cultures. 36,37
GeneXpert MTB/RIF
The Xpert® MTB/RIF (Cepheid Inc., Sunnyvale, CA, USA) is a fully automated real-time PCR-based assay for the detection of M. tuberculosis and resistance to rifampicin in clinical specimens. 29,38 GeneXpert is a cartridge-based system that fully integrates all the steps from sample preparation to detection on the GeneXpert multidisease platform. The test was developed as a point-of-care (POC) technology, which could be performed with minimal biohazard and little technical training.
The specimen is initially treated with a reagent that liquefies sputum and inactivates the bacilli. The sample is transferred to the GeneXpert cartridge, loaded on to the GeneXpert machine and the entire assay is then conducted automatically within the cartridge. The M. tuberculosis bacilli are purified and concentrated from the liquefied sputum; subsequently, DNA is isolated from the captured bacilli by sonication. The rpoB gene is then amplified by hemi-nested real-time PCR. Molecular beacons – using novel fluorophores and quenchers – hybridise to each of the five amplified target regions of the gene and are detected in real-time using a six-colour laser detection device.
The initial multicentre validation study indicated that a single GeneXpert test directly from sputum could detect 98.2% of smear-positive patients and 72.5% of patients with smear-negative disease. 29 This was a substantial improvement over alternative molecular tests. In December 2010, the WHO endorsed GeneXpert for use in TB-endemic countries and led a global roll-out of the test. 39
The test co-evaluator, the Foundation for Innovative and New Diagnostics (FIND), negotiated a 75% price reduction for low- and middle-income countries most affected by TB, with an additional reduction in price once there was a significant volume of demand. By the end of September 2013, 1843 GeneXpert instruments and 4.2 million test cartridges had been procured by 88 of the 145 countries that were eligible for concessional prices. 40 The introduction of GeneXpert-based diagnosis increased TB case finding in India, South Africa and Uganda compared with the use of simple microscopy and clinical diagnosis from 72–85% to 95–99% of the cohort of individuals with suspected TB. 41
Future directions
Whole genome sequencing (WGS) offers a powerful new approach for the diagnosis of M. tuberculosis drug resistance, promising rapid, unambiguous determination of all existing, clinically significant mutations. In the authors’ opinion, the continuing cost reductions of this technology may eventually neutralise arguments over the value of targeted sequencing compared with WGS. Recent studies using WGS to research novel determinants of resistance have revealed that drug resistance may be more multifactorial than previously appreciated which, in some cases, may explain discordance between phenotypes and genotypes. 42–44 As more resistance loci are identified, and the phenotypic effects of multiple mutations and strain background are elucidated, the public health value of routine WGS for diagnosis of drug resistance will increase. In addition, WGS reveals the genetic relatedness of strains, allowing transmission networks to be traced with unparalleled resolution and obviates the need for separate molecular fingerprinting analysis.
Chapter 2 Objectives
Although the incidence of MDR-TB and XDR-TB in the UK is relatively low, its impact on the individual, his/her close contacts, and the health-care economy is disproportionably large. Tests that can rapidly detect drug resistance have the potential to reduce this impact. The aim of this study was to investigate the clinical impact and cost-effectiveness of the use of genetic markers for identifying MDR-TB and XDR-TB in the UK compared with conventional culture-based DST.
The key research objectives were to:
-
conduct a systematic review of evidence on the diagnostic accuracy of genetic tests for detecting drug-resistant TB, including MDR-TB, in comparison with the reference standard of culture-based DST, in the available published and ‘grey’ literature in medical, scientific and economic databases
-
utilise this information to conduct a health-economic evaluation of adding a rapid molecular test for detecting drug-resistant TB to the current diagnostic pathway in the UK (including culture-based DST)
-
construct a transmission-dynamic mathematical model to explore the extent to which the addition of rapid diagnostic tests for drug resistance may interrupt the transmission of drug-resistant TB, including MDR-TB, and produce public health benefit.
Chapter 3 Systematic review
Objectives
Primary objectives
As outlined in Chapter 2, the primary purpose of this review was to produce a critical assessment of studies that report data on the diagnostic accuracy of molecular tests used to detect drug resistance in M. tuberculosis.
Types of studies
All diagnostic studies that compared a molecular (index) test with a gold standard (reference) test were considered in this review. No restrictions on study setting were applied, and studies from all countries were eligible for inclusion. When clearly stated in the paper, the study design was recorded for subgroup analysis.
Types of participants and clinical specimens
Studies of adults or children with suspected MDR-TB or XDR-TB were considered to be eligible for inclusion. This review also considered those studies for which the participant’s details were not clearly stated, and work was carried out on anonymised samples. When categorising the participant population, individuals aged ≥ 16 years were classed as adult participants.
When cited, studies that reported comorbidity with HIV infection or a previous history of TB within the participant population were considered for inclusion. These data were also recorded during the data extraction process for subgroup analysis.
Only studies that were carried out on clinical samples, including pulmonary or extrapulmonary samples, were considered for inclusion. Those studies that used clinical specimens ‘spiked’ with mycobacteria or cultured isolates were excluded from the review. Studies where the test was carried out on both cultured and clinical samples were considered for inclusion only if the diagnostic accuracy data were reported by the type of sample tested. Studies that pooled data from clinical and cultured specimens were excluded.
Index tests
Studies that used a genetic rapid diagnostic method to test for either first- or second-line drug susceptibility were considered for inclusion. Although studies using commercial assays are more commonly present in the literature, data on in-house tests were also considered for inclusion when there was sufficient information to assess their diagnostic accuracy in detecting MDR-TB or XDR-TB.
Reference tests
Studies that compared an appropriate index test against an appropriate reference standard test were potentially eligible for inclusion. This review accepted any TB phenotypic drug susceptibility test that has been endorsed by the WHO. These include culture on solid or liquid media and use of rapid liquid culture systems. If a study compared a rapid culture test against a NAAT with the ability to detect mutations associated with drug susceptibility, it was included in the review.
Methods
Search strategy
A standardised search strategy (PROSPERO registration CRD42011001537, see Appendix 1), was designed to generate a comprehensive list of relevant studies from five electronic literature databases: EMBASE, PUBMED, MEDLINE, Bioscience Information Service (BIOSIS) and Web of Science. The strategy design was based upon a previously successful model used by the European Centre for Disease Prevention and Control (ECDC). It was further validated by comparing the citation output against the bibliography of two published diagnostic reviews of rapid diagnostic tests for TB and drug susceptibility. 45,46
The electronic databases were initially searched on 29 October 2012, and the search was repeated in August 2013 to update the review. The search strategy was confined to any paper published between 1 January 2000 to the date of the last search, 15 August 2013. These limits were imposed so that early diagnostic studies of older commercially available tests, such as INNO-LiPA, would not be missed.
Additional sources were checked to ensure that the review included studies that were not missed. These included Cumulative Index to Nursing and Allied Health Literature (CINAHL), NHS Economic Evaluation Database (NHS EED), System for Information on Grey Literature in Europe Social Policy & Practice (SIGLE), diagnostic equipment manufacturer websites and experts within the field. Additional hand-searching was carried out to identify papers using the citation lists of published diagnostic accuracy reviews. 45,46 In addition, when study authors were contacted to confirm details within their papers they were also requested to suggest studies that could be potentially missing from the review. A systematic review database was developed in Microsoft® Office 2010 (Microsoft Corporation, Redmond, WA, USA) to manage and record the review process.
Study selection
Studies were included in the review if they met the inclusion criteria listed below. Studies that met these criteria were included irrespective of the published language, the country of origin or their current publication status (i.e. grey literature, published or ‘in press’).
The eligibility criteria were as follows:
-
Studies that:
-
assessed rapid genetic diagnostic methods to detect drug susceptibility of M. tuberculosis
-
used human clinical samples
-
compared the results of the rapid (index) test with sequencing or a culture-based sequencing DST as a reference standard
-
reported sufficient data to calculate the true positive (TP), true negative (TN), false positive (FP) and false negative (FN) of the rapid diagnostic test
-
reported at least 10 samples susceptible to the drug of interest and 10 samples that were resistant to the drug of interest, identified by the reference standard.
-
All articles that could potentially meet the eligibility criteria outlined above were selected for initial review. The assessment of study eligibility was not blinded to publication details, such as journal or author names.
Data extraction
Two reviewers independently evaluated titles, abstracts and full-text papers (see Appendix 2). Each reviewer had an independent copy of the review database.
In order to identify valid studies, the initial data extraction from the electronic databases were reviewed by one of the study reviewers (MC). The second reviewer (CT) validated a randomised subset (50%) of the titles for eligibility. The subsequent potentially eligible studies were randomly sorted and abstracts were divided between reviewers (MC, CT, AS), with each reviewer assessing a randomised subset (60%) of the abstracts, with a 10% overlap to check consistency.
The full-text papers of eligible papers were independently reviewed and data were extracted by the first reviewer MC (100%) and by the second reviewers AS (69%) and AB (31%).
Key variables – including patient characteristics, drug resistance(s) investigated, test used, characteristics of the tests (i.e. nature of assay used, method of drug resistance, detection and location) – were recorded. Other characteristics to be recorded – including study quality, publication details, time for analysis, sensitivity and specificity – were extracted for the data set. A sample data collection form is shown in Appendix 3 and the full list of extracted variables is given in Appendix 4.
These data were matched and, when the extracted data did not match, they were re-extracted by both the first and second reviewers MC and AB, respectively. In the event that the TN, FP, FN, TN did not match the reported sensitivity and specificity, the data were re-extracted a third time by the primary reviewer (MC), and the study author was contacted for clarification.
Quality assessment
The quality of each study deemed eligible – either through bias or concerns regarding applicability – was assessed using the Quality Assessment of Diagnostic Accuracy Studies tool version 2 (QUADAS-2). 47 The QUADAS-2 tool was tailored to this study by selecting relevant key questions in each QUADAS-2 domain. It was considered that, although the questions regarding bias when conducting the reference test were applicable, the questions regarding reference test applicability were not, as these were part of the study eligibility criteria.
During data extraction, each study was independently evaluated using QUADAS-2, and disagreement between reviewers’ opinions was resolved by discussion, with reference back to the original article. As recommended by Whiting et al. ,47 if a domain contained one or more question answered as ‘not clear’ or ‘high-bias’ then it was considered an area of potential bias or a concern regarding the applicability of the test. As recommended by Whiting et al. ,47 further quantitative sensitivity analysis was not performed.
Data synthesis
The study extraction data set from each reviewer was cleaned using Microsoft Excel version 2010, Microsoft Access version 2010 and Stata version 13.1 (StataCorp LP, College Station, TX, USA: www.statacorp.com).
Each group of tests, with sufficient studies available, was analysed separately. For each test comparison, the sensitivity, specificity and their exact 95% confidence intervals (CIs) were calculated using Stata. If meta-analysis was not found to be appropriate, because of a small number of studies or clinical heterogeneity, a qualitative narrative synthesis was used.
Statistical heterogeneity of sensitivities and specificities were investigated in any group of studies that had more than four studies with apparent clinical heterogeneity. Statistical analysis of the review data reflects that suggested by Lijmer et al. 48,49 and Higgins and Thompson. 50 Heterogeneity between studies was assessed using the I2-statistic. 50
Accuracy is usually presented in individual studies in terms of sensitivity and specificity, that is, dichotomous data rather than differences in distributions. Standard meta-analytic techniques, which are a simple pooled estimate of sensitivity and another of specificity, may be inappropriate, as these two statistics are likely to be correlated. Therefore, diagnostic accuracy across studies were summarised using a summary receiver operating characteristic (SROC) curve. This figure is a graphical summary of a hierarchical logistic regression model used to explain variability in study diagnostic odds ratios (DORs). In particular, variability across studies as a result of blinding or the use of different thresholds to define positivity were assessed and modelled using this approach. Hierarchical summary receiver operating characteristic (HSROC) curve models were used because they account for both between- and within-study variation in TP and FP rates.
Results
Details of included and excluded studies
A total of 8922 titles and abstracts were identified through database searches and hand-searching. After the first phase of screening, 557 papers were identified as being potentially eligible for the review (Figure 1).
FIGURE 1.
Flow diagram showing study selection process.

A total of 56 studies contained sufficient information on the performance of the rapid diagnostic tests to be included in the review. These studies were divided into five categories, depending on the rapid test investigated: INNO-LiPA studies (n = 9),33,35,51–57 GeneXpert studies (n = 6),29,38,58–61 MTBDRplus studies (n = 18),34,62–75,98–100 MTBDRsl studies (n = 6)37,76–80 and other rapid diagnostic studies (n = 17);81–97 the last category comprised any report with insufficient information about the type and manufacture of the assay tested, or assays that had been designed in-house.
Subgroup analysis was limited by the dearth of information reported on participant age, HIV status and other identified risk factors for heterogeneity in diagnostic studies (Table 1). Among the 56 studies, only one study37 was conducted on samples from HIV-positive patients and two studies58,97 on patients that were not HIV positive. The remaining studies were conducted on samples with either mixed or undisclosed HIV status. The majority of studies did not report any data on participant age (n = 44), and the remaining studies were carried out on adults (n = 8) or a mix of adults and children (n = 4).
| Category | Subcategory | GeneXpert | INNO-LiPA | MTBDRplus |
|---|---|---|---|---|
| HIV status | HIV-positive patients | 0 | 0 | 0 |
| HIV-negative patients | 1 | 0 | 0 | |
| HIV-negative and HIV-positive patients | 3 | 2 | 2 | |
| Not specified | 2 | 7 | 16 | |
| Participant age | Adults (≥ 16 years) | 4 | 1 | 2 |
| Children (< 16 years) | 0 | 0 | 0 | |
| Adults and children | 0 | 1 | 1 | |
| Not specified | 2 | 7 | 15 | |
| Reference standard | Solid culture | 1 | 1 | 5 |
| Liquid culture | 2 | 7 | 8 | |
| Solid and liquid culture | 3 | 1 | 5 | |
| Not specified | 0 | 0 | 0 | |
| Smear status of samples | Smear positive | 1 | 6 | 10 |
| Smear negative | 0 | 2 | 0 | |
| Not specified | 5 | 4 | 8 | |
| Study methodology | Blinded | 1 | 2 | 5 |
| Not blinded | 0 | 0 | 0 | |
| Not specified | 5 | 7 | 13 |
GeneXpert
Findings of the review
A total of six29,38,58–61 studies reporting the use of GeneXpert were eligible for inclusion and subsequently analysed (Table 2). The reported sensitivity and specificity of the GeneXpert test varied between 81.3–100.0% and 97.4–100.0%, respectively (see Table 2). The pooled estimates of sensitivity (96.8%, 95% CI 94.2% to 99.4%) and specificity (98.4%, 95% CI 97.8% to 99.0%) suggested a high level of diagnostic accuracy when this test was used to detect rifampicin resistance in clinical samples (Figure 2).
| Study (first author and year) | TP | FP | FN | TN | Sensitivity (%) | 95% CI (%) | Specificity (%) | 95% CI (%) |
|---|---|---|---|---|---|---|---|---|
| Boehme (2010)29 | 200 | 10 | 5 | 505 | 97.6 | 94.4 to 99.2 | 98.1 | 96.5 to 99.1 |
| Boehme (2011)38 | 236 | 14 | 14 | 796 | 94.4 | 90.8 to 96.9 | 98.3 | 97.1 to 99.1 |
| Naidoo (2010)60 | 157 | 8 | 1 | 635 | 99.4 | 96.5 to 100 | 98.8 | 97.6 to 99.5 |
| Kim (2012)58 | 21 | 0 | 0 | 41 | 100.0 | 83.9 to 100 | 100.0 | 91.4 to 100 |
| Kurbatova (2013)59 | 57 | 1 | 5 | 38 | 91.9 | 82.2 to 97.3 | 97.4 | 86.5 to 99.9 |
| O’Grady (2012)61 | 13 | 2 | 3 | 78 | 81.3 | 54.4 to 96.0 | 97.5 | 91.3 to 99.7 |
FIGURE 2.
Detection of rifampicin resistance by GeneXpert: forest plot of (a) sensitivities and (b) specificities. D + L, DerSimonian and Laird; ES, effect size; I–V, instrumental variables.


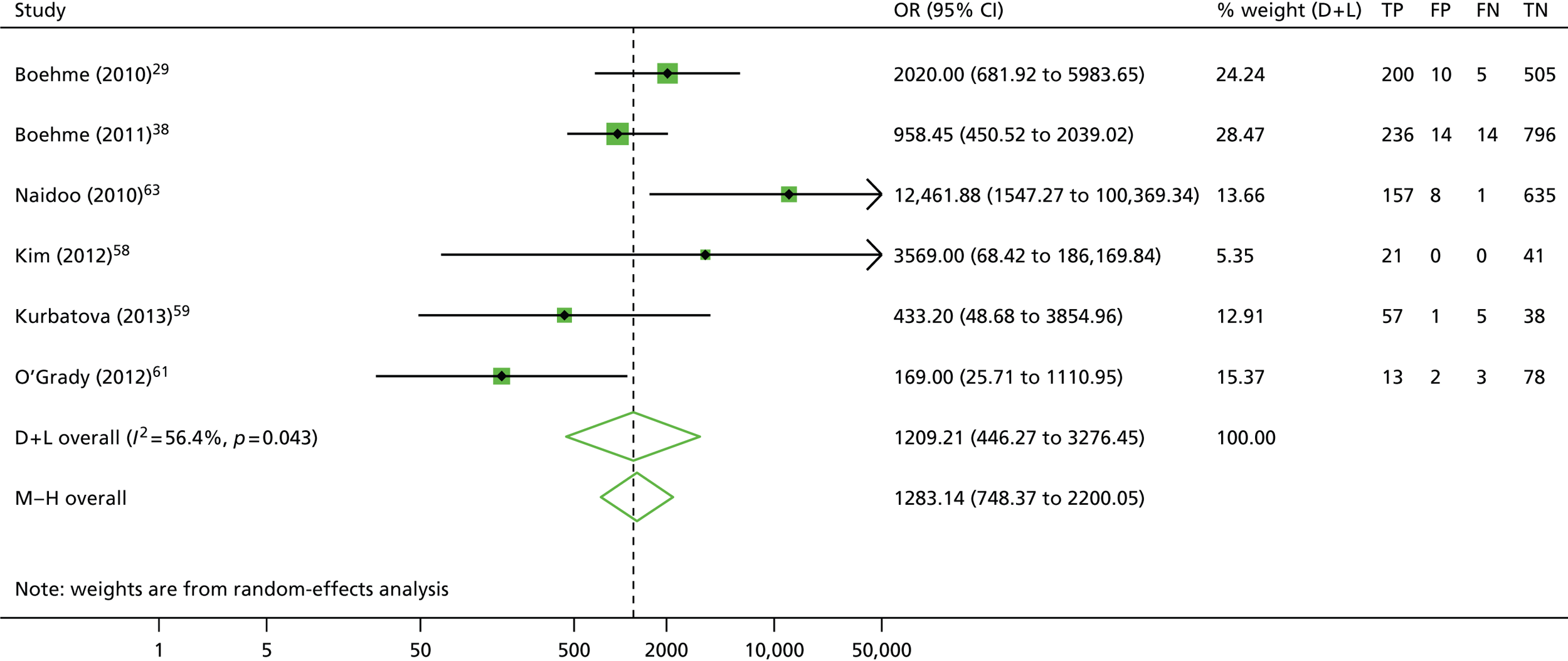
The calculation and analysis of the DOR for each study also suggested a high level of diagnostic accuracy when the study data were pooled (DOR = 1209.2, 95% CI 446.3 to 3276.4; p < 0.05) (Figure 3). However, there was marked heterogeneity between studies, indicated by both the wide 95% CI of the DOR, and both tests for heterogeneity using both the chi-squared distribution [11.46, degrees of freedom (df) = 5; p = 0.04] and the I2 method (56.4%), which is more robust when evaluating meta-analyses containing a small number of studies.
FIGURE 3.
Detection of rifampicin resistance by GeneXpert: forest plot of DORs. D + L, DerSimonian and Laird; M–H, Mantel–Haenszel.
Subsequent analysis and the construction of a HSROC curve indicated similarly high levels of predicted sensitivity (96.5%, 95% CI 91.4% to 98.5%) and specificity (98.2%, 95% CI 97.6% to 98.8%), with the majority of the studies and the summary point clustered in the top left-hand corner of the plot (Figure 4). However, again there was evidence of heterogeneity between the study estimates in the distended predicted 95% confidence ellipse.
FIGURE 4.
Summary receiver operating characteristic plots of GeneXpert diagnostic accuracy for the detection of rifampicin resistance in clinical samples.

Further investigation into the potential causes of heterogeneity could not be carried out as the small number of studies included in the analysis could not be reasonably broken down into subcategories for subgroup analysis (see Table 1).
Secondary analyses
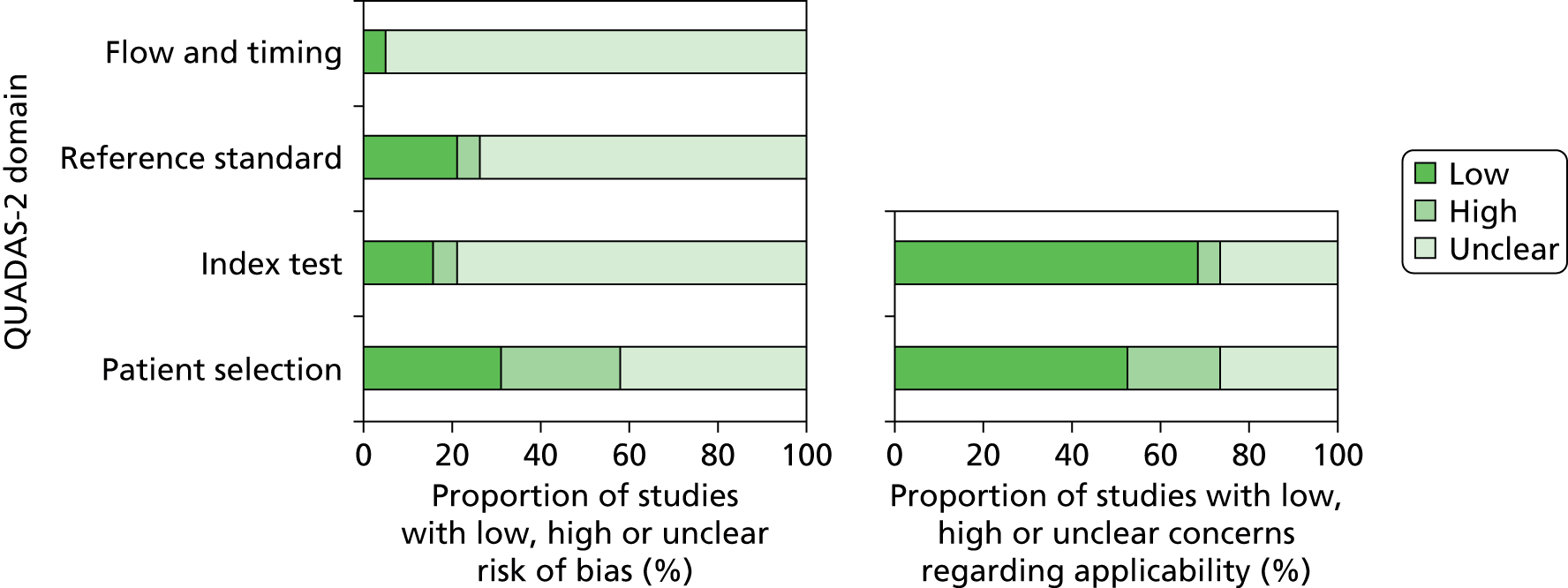
In order to investigate possible sources of heterogeneity between studies, a qualitative assessment of the studies was carried out using the QUADAS-2 framework. Each study was evaluated using a predefined set of indicator questions to evaluate factors that may bias the study findings or raise concerns about the applicability of the study. The summarised results of this assessment are outlined in Table 3 and Figure 5.
| Study (first author and year) | Risk of bias | Applicability concerns | ||||
|---|---|---|---|---|---|---|
| Patient selection | Index text | Reference standard | Flow and timing | Patient selection | Index text | |
| Boehme (2010)29 | ☺ | ☺ | ☺ | ? | ☺ | ☺ |
| Boehme (2011)38 | ☺ | ? | ? | ? | ☺ | ? |
| Naidoo (2010)60 | ? | ? | ? | ? | ? | ☺ |
| Kim (2012)58 | ☹ | ☹ | ☹ | ? | ☺ | ☺ |
| Kurbatova (2013)59 | ☺ | ☹ | ☹ | ? | ☺ | ☺ |
| O’Grady (2012)61 | ? | ? | ? | ☺ | ☺ | ? |
FIGURE 5.
Risk of bias and applicability concerns summary of GeneXpert diagnostic accuracy for the detection of rifampicin resistance in clinical samples.
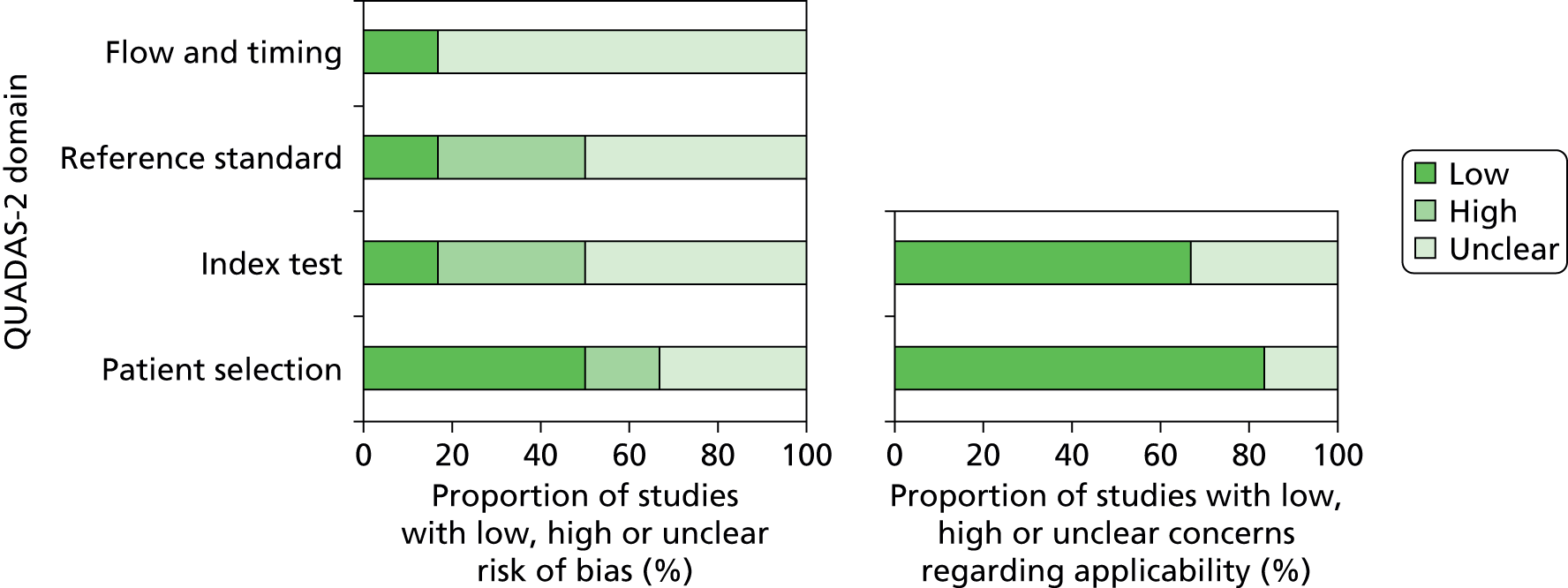
In the ‘Flow and timing’ domain, used to assess possible biases introduced by the use of patient samples, there was a marked lack of clarity surrounding the timing and the thresholds of the index test and reference test and the exclusion of samples from the assay.
Concerns regarding a high risk of bias in both the ‘Reference test’ and ‘Index test’ domains were associated with the absence of, or a lack of clarity associated with, blinding within the study. The study identified as high risk in the ‘Patient selection’ domain did not enrol patients either consecutively or randomly, and there was a strong indication that convenience sampling had been used.
When assessing the applicability of the methodology to the research question, the research setting and patient population did not raise concerns regarding the applicability of the assay.
There was no statistically significant evidence of publication bias based on both Egger’s test and Begg’s test (p > 0.05), based on rifampicin resistance.
INNO-LiPA
Findings of the review
A total of nine studies33,35,51–57 reporting the use of INNO-LiPA were eligible for inclusion (Table 4). It was possible to extract data broken down by smear status from only two studies35,52 (see Table 1).
| Study (first author and year) | TP | FP | FN | TN | Sensitivity (%) | 95% CI (%) | Specificity (%) | 95% CI (%) |
|---|---|---|---|---|---|---|---|---|
| Pulmonary specimens | ||||||||
| Costeira (2007)51 | 11 | 2 | 1 | 99 | 91.7 | 61.5 to 99.8 | 98.0 | 93.0 to 99.8 |
| Drobniewski (2000)52 | 55 | 3 | 9 | 14 | 85.9 | 75.0 to 93.4 | 82.4 | 56.6 to 96.2 |
| Higuchi (2004)53 | 22 | 1 | 0 | 19 | 100.0 | 84.6 to 100.0 | 95.0 | 75.1 to 99.9 |
| Johansen (2003)54 | 26 | 0 | 0 | 21 | 100.0 | 86.8 to 100.0 | 100.0 | 83.9 to 100.0 |
| Ogwang (2009)55 | 13 | 2 | 2 | 13 | 86.7 | 59.5 to 98.3 | 86.7 | 59.5 to 98.3 |
| Sam (2006)56 | 31 | 2 | 1 | 623 | 96.9 | 83.8 to 99.9 | 99.7 | 98.8 to 100.0 |
| Skenders (2005)57 | 31 | 2 | 3 | 52 | 91.2 | 76.3 to 98.1 | 96.3 | 87.3 to 99.5 |
| Viveiros (2005)33 | 31 | 0 | 1 | 310 | 96.9 | 83.8 to 99.9 | 100.0 | 98.8 to 100.0 |
| Sputum specimens | ||||||||
| Smear positive | ||||||||
| Drobniewski (2000)52 | 11 | 0 | 4 | 11 | 73.3 | 44.9 to 92.2 | 100.0 | 71.5 to 100.0 |
| Seoudi (2012)35 | 52 | 5 | 4 | 1554 | 92.9 | 82.7 to 98.0 | 99.7 | 99.3 to 99.9 |
| Smear negative | ||||||||
| Seoudi (2012)35 | 20 | 0 | 1 | 100 | 95.2 | 76.2 to 99.9 | 100.0 | 96.4 to 100.0 |
The reported sensitivity and specificity of the INNO-LiPA test varied between 86.7–100.0% and 82.4–100.0%, respectively (see Table 4). The pooled estimates of sensitivity (95.4%, 95% CI 92.2% to 98.3%) and specificity (99.7%, 95% CI 99.5% to 100.0%) suggested a high level of diagnostic accuracy when this test was used to detect rifampicin resistance in clinical samples (Figure 6).
FIGURE 6.
Detection rifampicin resistance by INNO-LiPA: forest plots of (a) sensitivities and (b) specificities. D + L, DerSimonian and Laird; ES, effect size; I–V, instrumental variables. a, All specimens; b, smear-positive specimens.


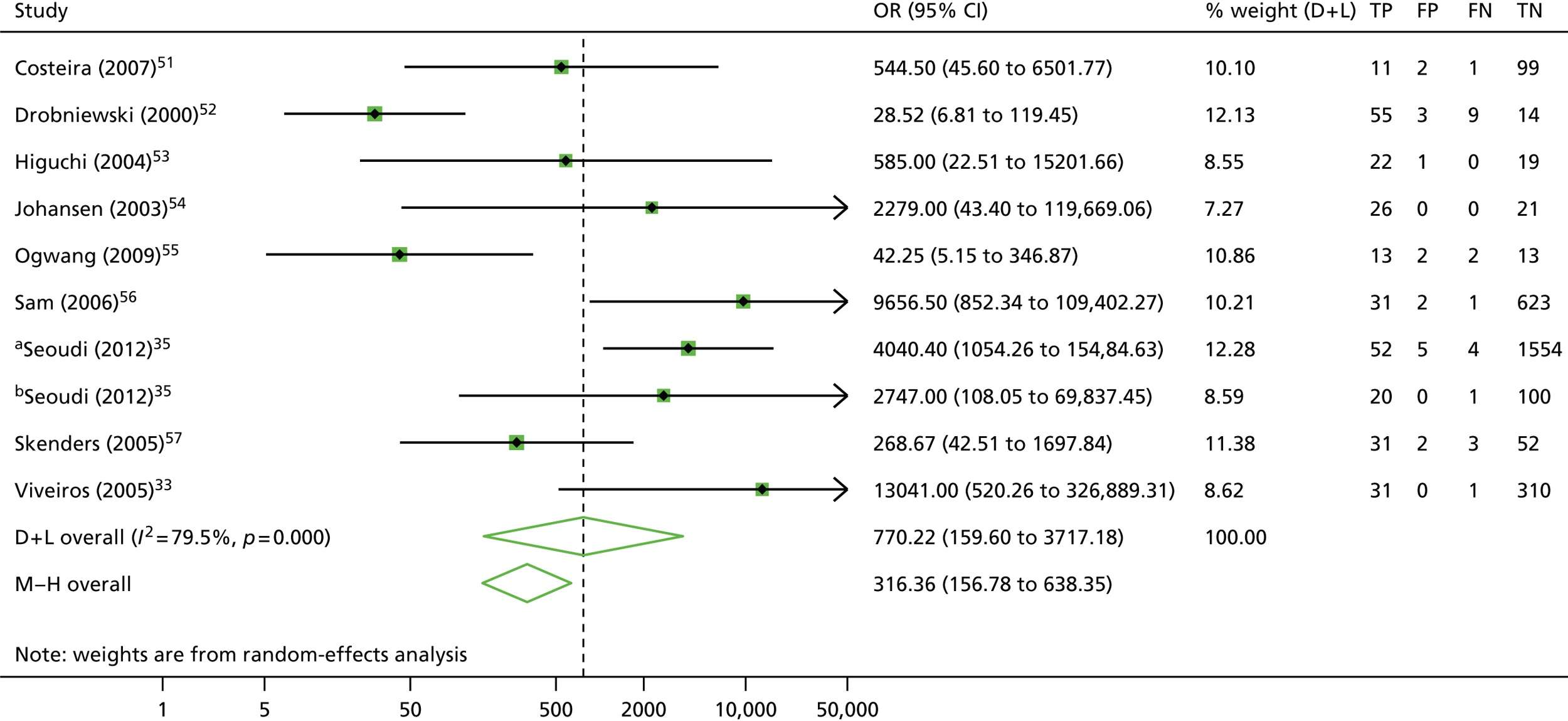
The calculation and analysis of the DOR for each study suggested a high level of diagnostic accuracy when the study data were pooled (DOR = 770.2, 95% CI 159.6 to 3717.2; p < 0.05) (Figure 7). However, there was some evidence of heterogeneity between the estimates reported by the studies, indicated by both a wide 95% CI of the DOR, and both tests for heterogeneity using both the chi-squared distribution (43.9, df = 9; p < 0.05) and the I2 method (79.5%).
FIGURE 7.
Detection of rifampicin resistance by INNO-LiPA: forest plot of DORs. M–H, Mantel–Haenszel. a, All specimens; b, smear-positive specimens.
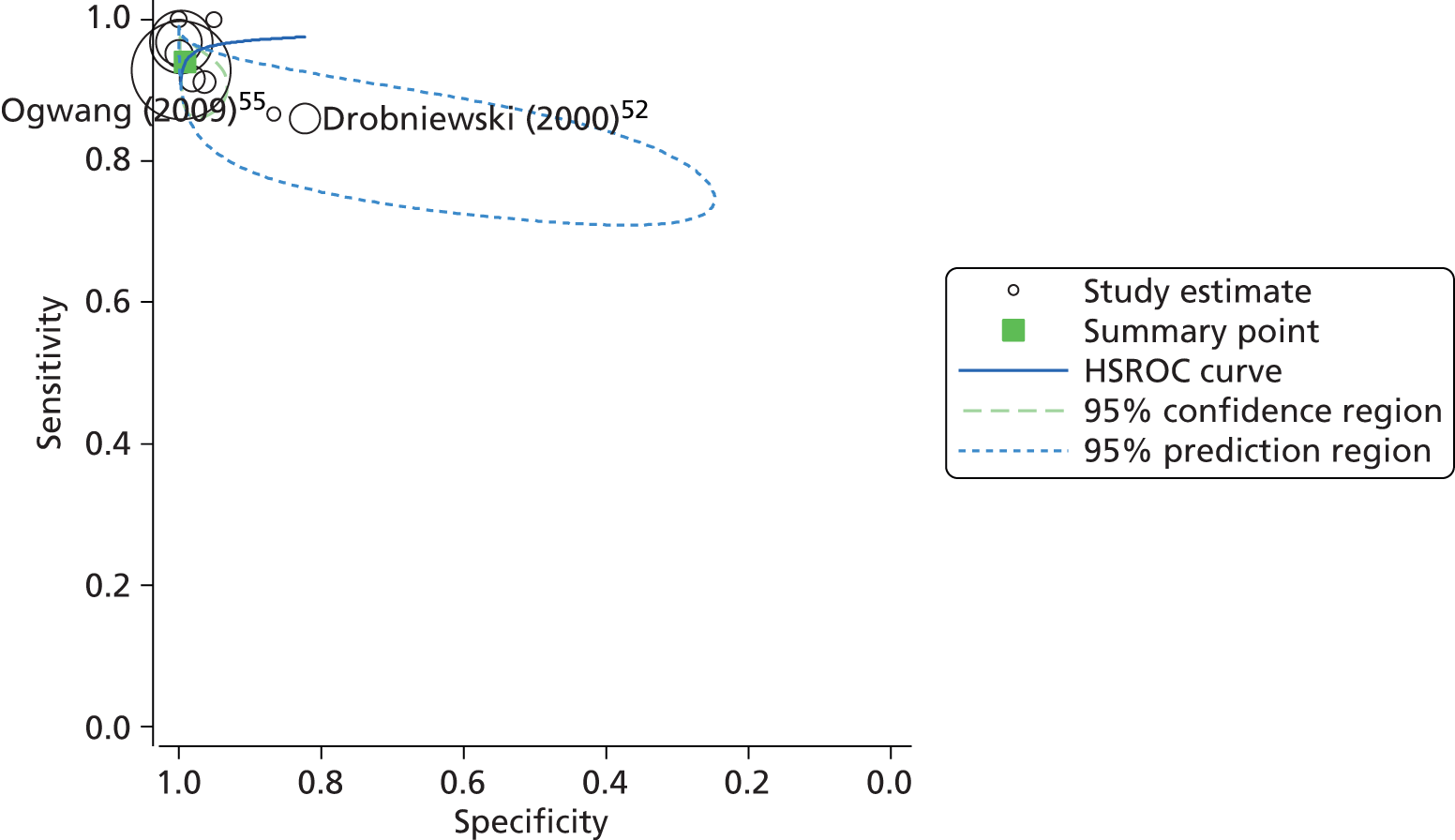
Subsequent analysis and the construction of a HSROC curve indicated similarly high levels of predicted sensitivity (94.1%, 95% CI 89.5% to 96.7%) and specificity (99.1%, 95% CI 96.5% to 99.8%), with the majority of the studies and the summary point clustered in the top left-hand corner of the plot (Figure 8). However, there was a strong indication of heterogeneity, particularly with regard to specificity of the test, as demonstrated by the shape and orientation of the 95% confidence ellipse.
FIGURE 8.
Summary receiver operating characteristic plot of INNO-LiPA diagnostic accuracy for the detection of rifampicin resistance in clinical samples.
Further investigation into the potential causes of heterogeneity could not be carried out, as the small number of studies included in the analysis could not be broken down into large enough subcategories for subgroup analysis (see Table 1).
Secondary analyses
In order to investigate possible sources of heterogeneity between studies, a qualitative assessment of the studies was carried out using the QUADAS-2 framework. The summarised results of this assessment are outlined in Table 5 and Figure 9.
| Study (first author and year) | Risk of bias | Applicability concerns | ||||
|---|---|---|---|---|---|---|
| Patient selection | Index text | Reference standard | Flow and timing | Patient selection | Index text | |
| Costeira (2007)51 | ? | ? | ? | ? | ☺ | ☺ |
| Drobniewski (2000)52 | ☹ | ? | ? | ? | ☺ | ☺ |
| Higuchi (2004)53 | ? | ☹ | ☺ | ☹ | ☺ | ☺ |
| Johansen (2003)54 | ☺ | ? | ? | ? | ☺ | ☺ |
| Ogwang (2009)55 | ☺ | ? | ? | ? | ☺ | ☺ |
| Sam (2006)56 | ☺ | ☺ | ☺ | ☺ | ☺ | ☺ |
| Seoudi (2012)35 | ☺ | ? | ? | ☺ | ☺ | ☺ |
| Skenders (2005)57 | ☺ | ☹ | ☹ | ? | ? | ? |
| Viveiros (2005)33 | ☺ | ? | ? | ? | ☺ | ☺ |
FIGURE 9.
Risk of bias and applicability concerns summary of INNO-LiPA diagnostic accuracy for the detection of rifampicin resistance in clinical samples, by study.

As with the GeneXpert studies, there was a marked lack of clarity surrounding the timing and thresholds of the index reference tests. This, combined with a lack of clarity surrounding the exclusion of samples from the assays, contributes to the high proportion of studies designated to have an unclear risk of bias in the ‘Flow and timing’ domain.
Concerns regarding a high risk of bias in both the ‘Reference test’ and ‘Index test’ domains were associated with the absence of, or a lack of clarity associated with, blinding within the study.
When assessing the applicability of the methodology to the research question the studies were categorised as either low or unclear concern.
There was no statistically significant evidence of publication bias based on both Egger’s test and Begg’s test (p > 0.05), based on rifampicin resistance.
GenoType MTBDRplus
Findings of the review
A total of 18 studies34,62–75,98–100 reporting the use of GenoType MTBDRplus® (Hain Lifescience, Nehren, Germany) were eligible for inclusion into the meta-analysis (Table 6).
| Study (first author and year) | TP | FP | FN | TN | Sensitivity (%) | 95% CI (%) | Specificity (%) | 95% CI (%) |
|---|---|---|---|---|---|---|---|---|
| Rifampicin | ||||||||
| Macedo (2009)98 | 23 | 0 | 0 | 43 | 100.0 | 85.2 to 100.0 | 100.0 | 91.8 to 100.0 |
| Nikolayevskyy (2009)99 | 103 | 4 | 4 | 38 | 96.3 | 90.7 to 99.0 | 90.5 | 77.4 to 97.3 |
| Somoskovi (2006)74 | 51 | 0 | 41 | 51 | 55.4 | 44.7 to 65.8 | 100.0 | 93.0 to 100.0 |
| Albert (2010)73 | 15 | 4 | 0 | 73 | 100.0 | 78.2 to 100.0 | 94.8 | 87.2 to 98.6 |
| Dorman (2012)68 | 12 | 2 | 2 | 200 | 85.7 | 57.2 to 98.2 | 99.0 | 96.5 to 99.9 |
| Mironova (2012)34 | 323 | 35 | 16 | 311 | 95.3 | 92.4 to 97.3 | 89.9 | 86.2 to 92.9 |
| Eliseev (2013)66 | 69 | 2 | 0 | 31 | 100.0 | 94.8 to 100.0 | 93.9 | 79.8 to 99.3 |
| Barnard (2008)70 | 94 | 2 | 1 | 357 | 98.9 | 94.3 to 100.0 | 99.4 | 98.0 to 99.9 |
| Farooqi (2012)65 | 51 | 1 | 4 | 54 | 92.7 | 82.4 to 98.0 | 98.2 | 90.3 to 100.0 |
| Asencios (2012)71 | 22 | 1 | 2 | 75 | 91.7 | 73.0 to 99.0 | 98.7 | 92.9 to 100 |
| Tukvadze (2012)62 | 112 | 4 | 13 | 329 | 89.6 | 82.9 to 94.3 | 98.8 | 97.0 to 99.7 |
| Maschmann (2013)100 | 23 | 2 | 5 | 32 | 82.1 | 63.1 to 93.9 | 94.1 | 80.3 to 99.3 |
| Anek-Vorapong (2010)72 | 19 | 0 | 0 | 145 | 100.0 | 82.4 to 100.0 | 100.0 | 97.5 to 100.0 |
| Crudu (2012)69 | 100 | 2 | 6 | 48 | 94.3 | 88.1 to 97.9 | 96.0 | 86.3 to 99.5 |
| Hillemann (2006)64 | 15 | 0 | 0 | 27 | 100.0 | 78.2 to 100.0 | 100.0 | 87.2 to 100 |
| Cauwelaert (2011)67 | 47 | 4 | 1 | 202 | 97.9 | 88.9 to 99.9 | 98.1 | 95.1 to 99.5 |
| Lacoma (2008)75 | 29 | 1 | 0 | 20 | 100.0 | 88.1 to 100.0 | 95.2 | 76.2 to 99.9 |
| Lyu (2013)63 | 57 | 4 | 2 | 365 | 96.6 | 88.3 to 99.6 | 98.9 | 97.2 to 99.7 |
| Isoniazid | ||||||||
| Macedo (2009)98 | 24 | 0 | 0 | 43 | 100.0 | 85.8 to 100.0 | 100.0 | 91.8 to 100.0 |
| Albert (2010)73 | 21 | 0 | 5 | 66 | 80.8 | 60.6 to 93.4 | 100.0 | 94.6 to 100.0 |
| Barnard (2008)70 | 114 | 1 | 7 | 330 | 94.2 | 88.4 to 97.6 | 99.7 | 98.3 to 100.0 |
| Farooqi (2012)65 | 45 | 0 | 14 | 49 | 76.3 | 63.4 to 86.4 | 100.0 | 92.7 to 100.0 |
| Asencios (2012)71 | 30 | 3 | 1 | 66 | 96.8 | 83.3 to 99.9 | 95.7 | 87.8 to 99.1 |
| Tukvadze (2012)62 | 52 | 2 | 125 | 281 | 29.4 | 22.8 to 36.7 | 99.3 | 97.5 to 99.9 |
| Anek-Vorapong (2010)72 | 27 | 0 | 2 | 135 | 93.1 | 77.2 to 99.2 | 100.0 | 97.3 to 100.0 |
| Hillemann (2006)64 | 17 | 0 | 0 | 25 | 100.0 | 80.5 to 100.0 | 100.0 | 86.30 to 100.0 |
| Cauwelaert (2011)67 | 55 | 4 | 14 | 181 | 79.7 | 68.3 to 88.4 | 97.8 | 94.6 to 99.4 |
The reported sensitivity and specificity of the MTBDRplus to detect resistance to rifampicin ranged between 82.1–100.0% and 89.9–100.0%, respectively (see Table 6). The pooled estimates of sensitivity (94.6%, 95% CI 91.6% to 97.6%) and specificity (98.2%, 95% CI 97.2% to 99.3%) suggested a high level of diagnostic accuracy when this test was used to detect rifampicin resistance in clinical samples (Figure 10).
FIGURE 10.
Detection of isoniazid and rifampicin resistance by MTBDRplus: forest plots of (a) sensitivity of isoniazid, (b) specificity of isoniazid, (c) sensitivity of rifampicin and (d) specificity of rifampicin. D + L, DerSimonian and Laird; ES, effect size; I–V, instrumental variables.
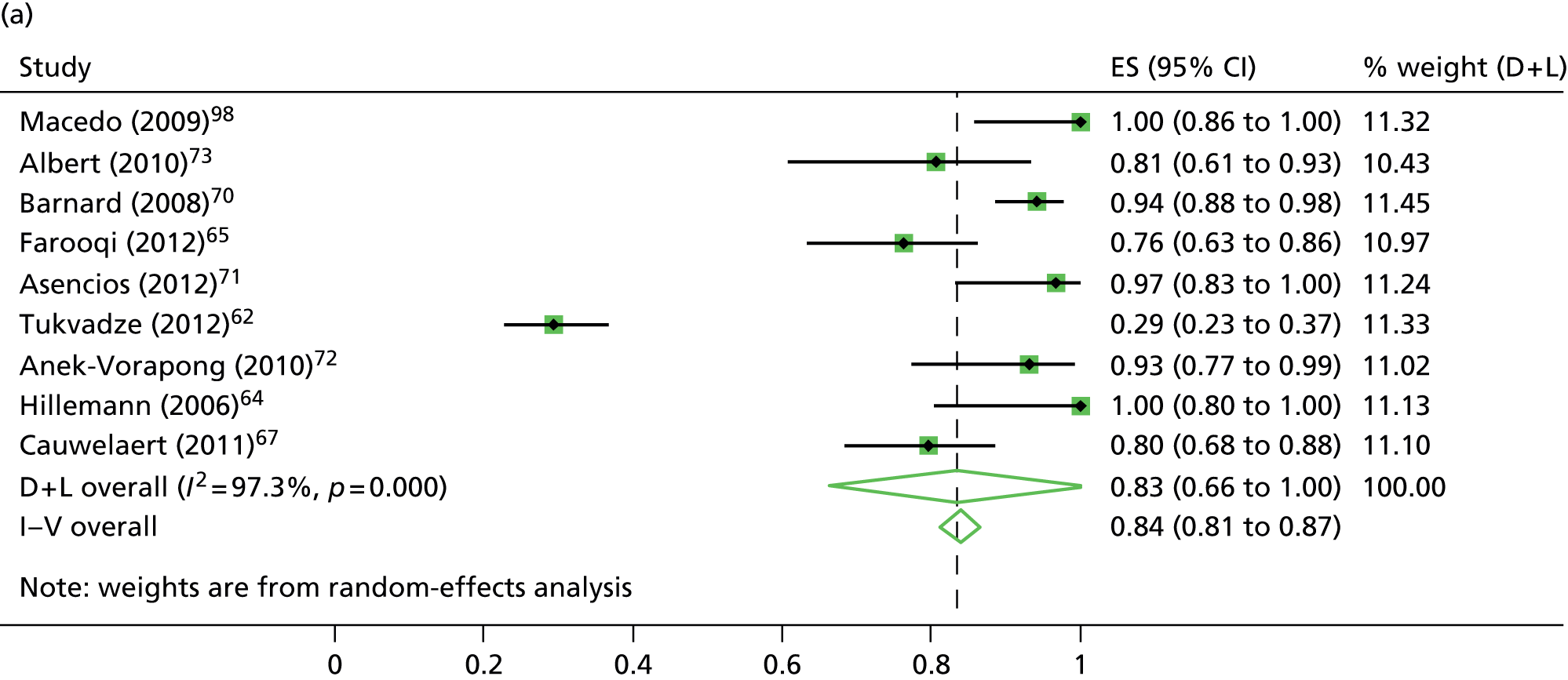


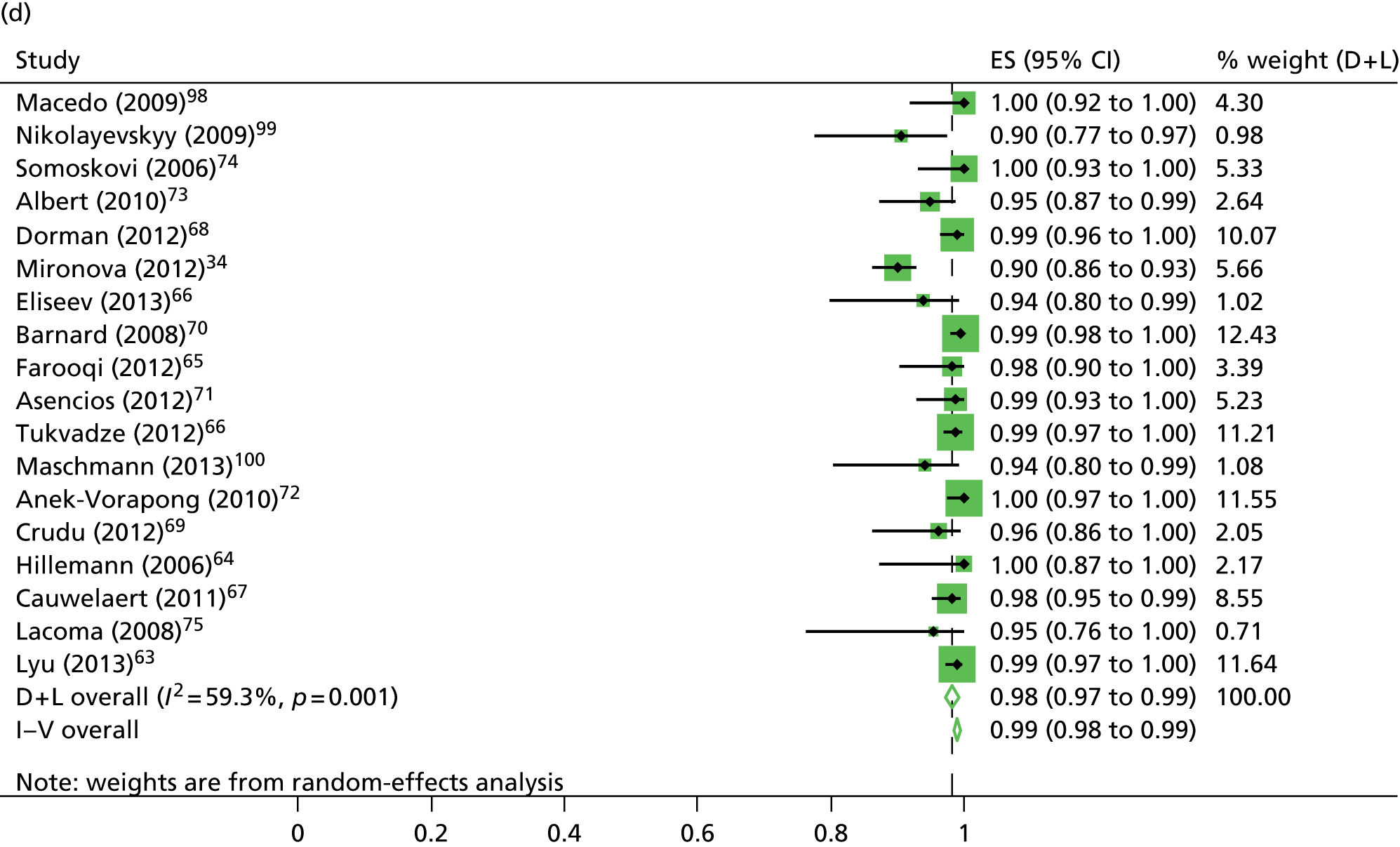
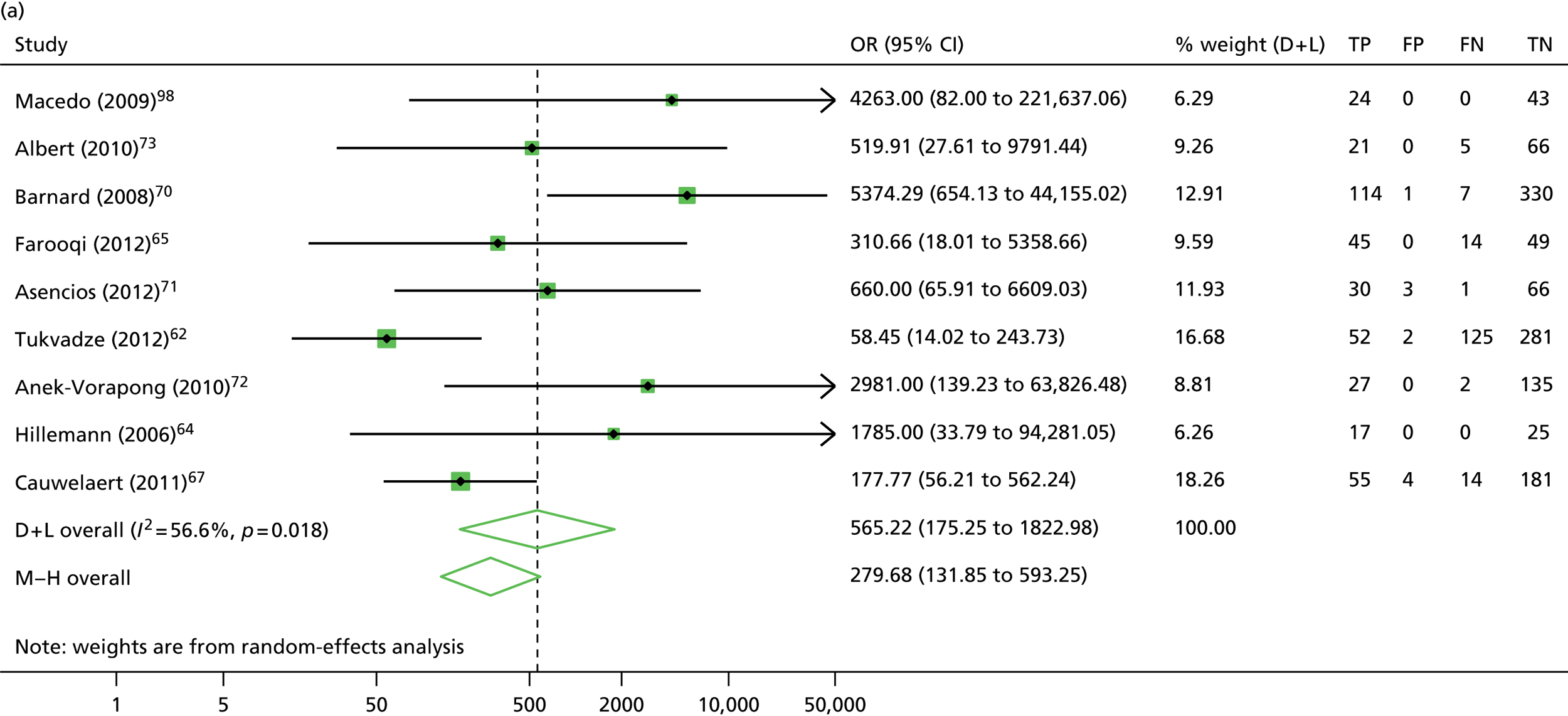
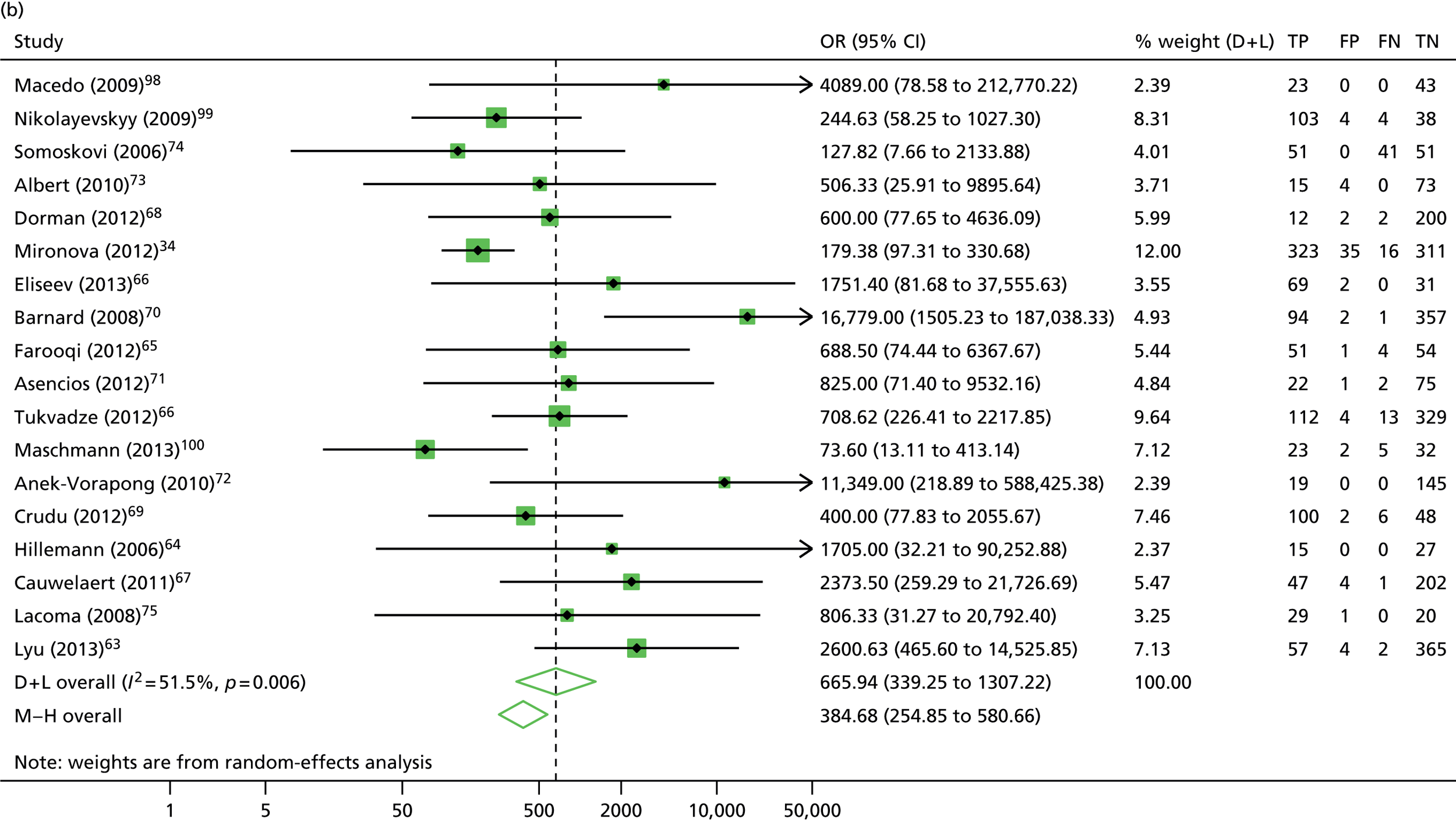
The calculation and analysis of the DOR for each study suggested a high level of diagnostic accuracy when the study data were pooled (DOR = 666.0, 95% CI 339.3 to 1307.2; p < 0.05) (Figure 11). However, there was some evidence of heterogeneity between the estimates reported by the studies, indicated by both a wide 95% CI of the DOR and both tests for heterogeneity using both the chi-squared distribution (35.1, df = 17; p < 0.05) and the I2 method (51.5%).
FIGURE 11.
Detection of (a) isoniazid and (b) rifampicin resistance by MTBDRplus: forest plot of DORs. D + L, DerSimonian and Laird; M–H, Mantel–Haenszel.
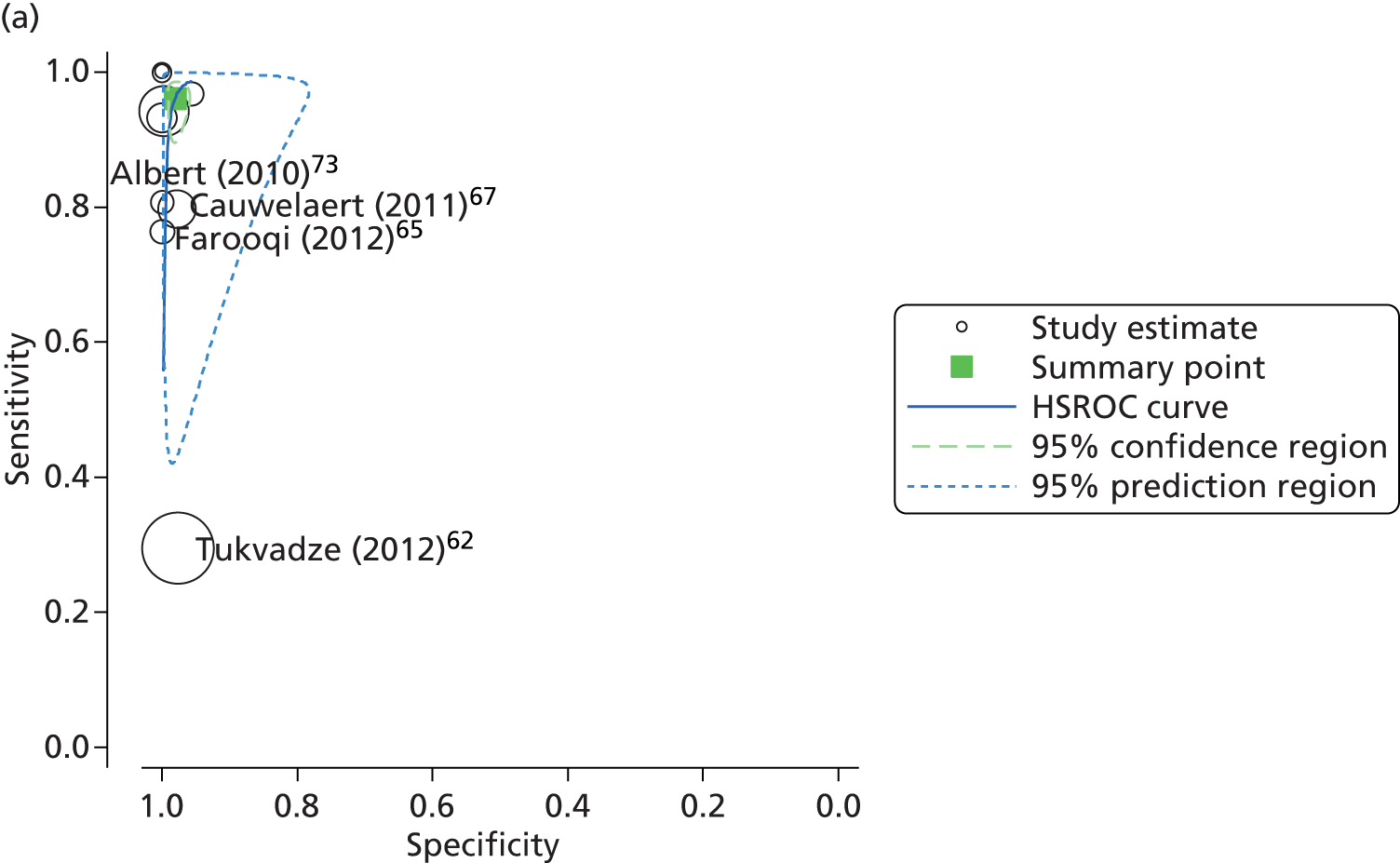
Subsequent analysis and the construction of a HSROC plot indicated similarly high levels of predicted sensitivity (96.1%, 95% CI 91.9% to 98.1%) and specificity (98.1%, 95% CI 96.7% to 98.1%), with the majority of the studies and the summary point clustered in the top left-hand corner of the plot (Figure 12). However, there was a strong indication of heterogeneity, particularly with regard to the sensitivity of the test, as demonstrated by the length and orientation of the 95% confidence ellipse.
FIGURE 12.
Summary receiver operating characteristic plots of MTBDRplus diagnostic accuracy for the detection of (a) isoniazid and (b) rifampicin resistance in clinical samples.

The reported sensitivity and specificity of the MTBDRplus to detect resistance to isoniazid in clinical samples ranged between 29.4–100.0% and 95.7–100.0%, respectively (see Table 6). The pooled estimates of sensitivity (83.4%, 95% CI 66.3% to 100.0%) and specificity (99.6%, 95% CI 99.0% to 100.0%) suggested a high level of diagnostic accuracy when this test was used to detect isoniazid resistance in clinical samples (see Figure 10).
The calculation and analysis of the DOR for each study suggested a high level of diagnostic accuracy when the study data were pooled (DOR = 565.2, 95% CI 175.3 to 1823.0; p < 0.05) (see Figure 11). However, there was some evidence of heterogeneity between the estimates reported by the studies, indicated by both a wide 95% CI of the DOR, and both tests for heterogeneity using both the chi-squared distribution (18.4, df = 8; p < 0.05) and the I2 method (56.5%).
Subsequent analysis and the construction of a HSROC plot indicated similarly high levels of predicted sensitivity (90.0%, 95% CI 73.6 to 96.7) and specificity (99.4%, 95% CI 97.9% to 99.8%), with the majority of the studies and the summary point clustered in the top left-hand corner of the plot (see Figure 12). However, there was at least one extreme outlier, Tukvadze et al. ,62 which, potentially, contributed to the substantial heterogeneity apparent in the sensitivity estimate. The authors of this study62 had been unsuccessfully contacted regarding a discrepancy between the diagnostic accuracy data reported and the published calculation of sensitivities and specificities. When the study62 was excluded from the analysis, the heterogeneity within the sample was reduced I2 method (36.1%). However, there was only a small change in the predicted estimates of sensitivity (91.8%, 95% CI 82.8% to 96.2%) and specificity (99.4%, 95% CI 97.3% to 99.9%).
Further investigation into the potential causes of heterogeneity could not be carried out, as the small number of studies included in the analysis could not be broken down into large enough subcategories for subgroup analysis (see Table 1).
Secondary analyses
In order to investigate possible sources of heterogeneity between studies, a qualitative assessment of the studies was carried out using the QUADAS-2 framework. The summarised results of this assessment are outlined in Table 7 and Figure 13.
| Study (first author and year) | Risk of bias | Applicability concerns | ||||
|---|---|---|---|---|---|---|
| Patient selection | Index text | Reference standard | Flow and timing | Patient selection | Index text | |
| Macedo (2009)98 | ☹ | ☺ | ☺ | ☹ | ☺ | ☺ |
| Nikolayevskyy (2009)99 | ? | ? | ? | ? | ? | ☺ |
| Somoskovi (2006)74 | ☹ | ? | ? | ? | ☺ | ? |
| Albert (2010)73 | ☺ | ☺ | ☺ | ? | ☺ | ☺ |
| Dorman (2012)68 | ☺ | ? | ☺ | ? | ☺ | ☺ |
| Mironowa (2012)34 | ☺ | ? | ? | ? | ☺ | ? |
| Eliseev (2013)66 | ☺ | ? | ? | ? | ☹ | ☺ |
| Barnard (2008)70 | ? | ? | ? | ? | ☹ | ☺ |
| Farooqi (2012)65 | ? | ? | ? | ? | ? | ? |
| Asencios (2012)71 | ☺ | ? | ? | ? | ? | ☺ |
| Tukvadze (2012)62 | ☺ | ? | ? | ? | ☺ | ☺ |
| Maschmann (2013)100 | ☹ | ☹ | ☹ | ? | ☺ | ☺ |
| Anek-Vorapong (2010)72 | ? | ? | ☺ | ? | ☺ | ☺ |
| Crudu (2012)69 | ? | ☺ | ? | ? | ☺ | ? |
| Hillemann (2006)64 | ? | ? | ? | ? | ? | ? |
| Cauwelaert (2011)67 | ? | ? | ? | ? | ? | ☺ |
| Lacoma (2008)75 | ☹ | ? | ? | ? | ☹ | ☺ |
| Lyu (2013)63 | ☹ | ? | ? | ? | ☹ | ☹ |
FIGURE 13.
Quality Assessment of Diagnostic Accuracy Studies version 2: risk of bias and applicability concerns summary of MTBDRplus for the detection drug susceptibility in clinical samples.
When compared with the studies included in the INNO-LiPA and GeneXpert analyses, there appeared to be a marked reduction in the quality of methodological reporting with studies using the MTBDRplus.
The lack of detail regarding timing, thresholds, patient selection and blinding resulted in the majority of studies classified as either ‘high bias’ or ‘unclear bias’ in the four key Quality Assessment of Diagnostic Accuracy Studies (QUADAS) domains. This is particularly marked in the ‘Flow and timing’ domain, for which all studies performed poorly.
There was no statistically significant evidence of publication bias based on both Egger’s test and Begg’s test (p > 0.05) based on rifampicin resistance, although there was strong evidence of publication bias using isoniazid (Egger’s test, p = 0.001).
GenoType MTBDRsl
Findings of the review
A total of six studies37,76–80 reporting the use of GenoType MTBDRsl® (Hain Lifescience) met the inclusion criteria for the review (Table 8). These studies reported the diagnostic accuracy of MTBDRsl to detect resistance to a range of injectable drugs and fluoroquinolones resistance in clinical samples. However, the sample size for each drug category of interest was limited, and only the groups of studies reporting diagnostic accuracy for specific drugs were not sufficiently large for meta-analysis.
| Study (first author and year) | TP | FP | FN | TN | Sensitivity (%) | 95% CI (%) | Specificity (%) | 95% CI (%) |
|---|---|---|---|---|---|---|---|---|
| Amikacin | ||||||||
| Barnard (2012)80 | 43 | 3 | 0 | 470 | 100.0 | 91.7 to 100 | 99.4 | 98.2 to 99.9 |
| Capreomycin | ||||||||
| Lacoma (2012)77 | 23 | 4 | 0 | 25 | 100.0 | 85.2 to 100.0 | 86.2 | 68.3 to 96.1 |
| Kanamycin | ||||||||
| Kontsevaya (2013)37 | 6 | 0 | 58 | 12 | 9.38 | 3.52 to 19.3 | 100.00 | 73.5 to 100.0 |
| Ajbani (2012)76 | 22 | 0 | 0 | 128 | 100.0 | 84.6 to 100.0 | 100.00 | 97.2 to 100.0 |
| Fluoroquinolones | ||||||||
| Lacoma (2012)77 | 3 | 2 | 5 | 42 | 37.5 | 8.52 to 75.5 | 95.5 | 84.5 to 99.4 |
| Ofloxacin | ||||||||
| Hilleman (2009)78 | 8 | 0 | 1 | 51 | 88.90 | 51.7 to 99.7 | 100.0 | 93.0–100.0 |
| Ethambutol | ||||||||
| Kontsevaya (2013)37 | 18 | 5 | 32 | 32 | 36.0 | 22.9 to 50.8 | 86.5 | 71.2 to 95.4 |
| Miotto (2012)79 | 13 | 17 | 5 | 21 | 72.2 | 46.5 to 90.3 | 55.3 | 38.2 to 71.4 |
| Hilleman (2009)78 | 10 | 34 | 15 | 0 | 38.5 | 20.2 to 59.4 | 0.0 | 0.0 to 10.3 |
| Ajbani (2012)76 | 60 | 8 | 46 | 36 | 56.6 | 46.6 to 66.2 | 81.8 | 67.2 to 91.8 |
| Lacoma (2012)77 | 22 | 4 | 18 | 6 | 55.0 | 38.4 to 70.7 | 60.0 | 26.2 to 87.8 |
Other studies
In addition to the studies that reported diagnostic data from the widely used commercial tests, 17 studies81–97 reporting data on other genetic rapid diagnostic tests were identified using the inclusion criteria. These tests were broadly broken into those studies that utilised a variety of TB biochip assays (n = 7, 41.2%) and those that used in-house PCR assays (n = 10, 59.8%).
The majority of studies (n = 13) reported the diagnostic accuracy of these test to detect rifampicin resistance in clinical samples (Table 9). The reported sensitivities and specificities of these studies were high, ranging between 79.8% and 100.0% and 92.2% and 100%, respectively. However, as the manufacture and design of these assays were not consistent it was not possible to conduct a meta-analysis on this data set.
| Study | TP | FP | FN | TN | Sensitivity (%) | 95% CI (%) | Specificity (%) | 95% CI (%) |
|---|---|---|---|---|---|---|---|---|
| Antonova (2008)81 | – | – | – | – | – | – | – | – |
| Calderón-Espinoza (2006)82 | 107 | 7 | 7 | 83 | 93.9 | 87.8 to 97.5 | 92.2 | 84.6 to 96.8 |
| Cho (2009)83 | 11 | 0 | 1 | 34 | 91.7 | 61.5 to 99.8 | 100.0 | 89.7 to 100.0 |
| Choi (2010)84 | 25 | 4 | 1 | 62 | 96.2 | 80.4 to 99.9 | 93.9 | 85.2 to 98.3 |
| García-Sierra (2011)85 | 27 | 0 | 2 | 16 | 93.1 | 77.2 to 99.2 | 100.0 | 79.4 to 100.0 |
| Gryadunov (2005)86 | 17 | 0 | 1 | 95 | 94.4 | 72.7 to 99.8 | 100.0 | 96.2 to 100.0 |
| Guo (2009)87 | 72 | 3 | 4 | 50 | 94.7 | 87.1 to 98.5 | 94.3 | 84.3 to 98.8 |
| Kim (2001)89 | 23 | 0 | 0 | 33 | 100.0 | 85.2 to 100.0 | 100.0 | 89.4 to 100.0 |
| Lu (2012)90 | 67 | 21 | 17 | 585 | 79.8 | 69.6 to 87.7 | 96.5 | 94.8 to 97.8 |
| Mikhailovich (2001)91 | 11 | 0 | 2 | 18 | 84.6 | 54.6 to 98.1 | 100.0 | 81.5 to 100.0 |
| Mokrousov (2003)92 | 72 | 0 | 15 | 23 | 82.8 | 73.2 to 90.0 | 100.0 | 85.2 to 100.0 |
| Nosova (2013)93 | – | – | – | – | – | – | – | – |
| Pang (2013)88 | 176 | 33 | 25 | 1580 | 87.6 | 82.2 to 91.8 | 98.0 | 97.1 to 98.6 |
| Sheen (2009)94 | – | – | – | – | – | – | – | – |
| Siu (2011)95 | – | – | – | – | – | – | – | – |
| Vadwai (2012)96 | 187 | 11 | 10 | 73 | 94.9 | 90.9 to 97.5 | 86.9 | 77.8 to 93.3 |
| Zhang (2012)97 | 16 | 7 | 2 | 68 | 88.9 | 65.3 to 98.6 | 90.7 | 81.7 to 96.2 |
Discussion
The findings of this review suggest that all three commercial tests – INNO-LiPA, GeneXpert and MTBDRplus – demonstrate promising levels of diagnostic discrimination when detecting rifampicin and isoniazid susceptibility in clinical samples.
This study used a broad set of inclusion criteria, with respect to the type of rapid diagnostic tests evaluated, research settings and the lack of restrictions applied to the language of publication. However, the restriction on the minimum number of samples tested resulted in a discrepancy between the number of studies included in this review and those published in other published systematic reviews on this subject. 45,46 Despite these resections, the findings of this study are consistent with previous estimates that indicated that both MTBDRplus and GeneXpert can demonstrate high levels (> 90%) of sensitivity and specificity when detecting drug susceptibility. 45,46
It has been suggested that heterogeneity will always be present in any systematic review. 102–104 However, the level of heterogeneity observed in these analyses suggests that the predicted and pooled estimates of diagnostic accuracy for all three commercial tests should be interpreted with caution. A key limitation of this work is the lack of subcategory analysis to explore potential sources of heterogeneity between the studies. This has been hampered by the lack of clear methodological detail reported in the majority of the papers, as demonstrated by the small number of studies that report details on study blinding and study design. In addition to this, the small sample sizes used to evaluate diagnostic drug susceptibility tests have resulted in the exclusion of studies that would otherwise supplement the data set and allow subcategory analysis to be performed.
Chapter 4 Time-and-motion analysis
Introduction
A time-and-motion study was undertaken at the National Mycobacterium Reference Laboratory (NMRL) to compare the working time for DST using two culture-based methods to three rapid molecular assays.
Culture is the ‘gold standard’ for MTB, DST and can be performed in solid or liquid medium. The resistance ratio method is used at the NMRL for DST of first-line drugs on LJ egg-based medium, which is prepared in-house. The resistance ratio is determined by dividing the MIC of the test strain by the MIC of a control (susceptible) strain. 19 For each batch of test strains, four control strains are inoculated on to five different concentrations of each first-line drug. The test strains are then measured against the modal MIC of the control strains. At the NMRL, each slope is checked weekly, up to 12 weeks post inoculation.
The proportion method determines the proportion of bacteria in the sample that are resistant to a drug. If the proportion of resistant bacteria is ≥ 1% of the total bacterial population, the sample is classified as resistant. Automated liquid culture systems, such as the BACTEC MGIT 960 system, use the proportion method for DST. Tubes for the BACTEC MGIT 960 system contain an oxygen-quenched fluorochrome. As bacteria grow and consume oxygen, fluorescence increases and is detected by the machine. The BACTEC MGIT 960 system is used at the NMRL for second-line DST. Tubes containing the critical concentration of each drug to be tested are inoculated with the test sample. 105 The bacterial suspension is diluted 100-fold before inoculation into the control drug-free tube. If there is at least equal growth in a drug-containing tube and the control tube, this indicates that at least 1% of the bacteria present in the sample are resistant to that drug and therefore the sample can be classified as resistant. 20 The BACTEC MGIT 960 system monitors fluorescence every 60 minutes up to a maximum of 6 weeks. At the NMRL, the machine output is checked daily.
Molecular tests on primary specimens are performed at the NMRL to enable rapid identification of MTB and possible drug resistance. In order to isolate live pure mycobacteria for downstream phenotypic tests and archiving, samples from non-sterile sites are first decontaminated using the same procedures used for processing specimens arriving for culture only. Thus these steps are necessarily included in the timings for these assays, although they are not strictly required for performing the molecular assay.
Two different commercial LPAs are used at the NMRL for the detection of MDR genotypes: INNO-LiPA and MTBDRplus. The INNO-LiPA test identifies rifampicin-resistance mutations in the rpoB gene. The MTBDRplus assay identifies these mutations, and, additionally, mutations associated with isoniazid-resistance in katG and the inhA promoter. At the NMRL, the LPAs are used to test a broad range of samples including sputum, bronchoalveolar lavage (BAL), cerebrospinal fluid (CSF), bone marrow, tissues fixed in wax blocks and purulent material. To perform LPAs, first DNA is extracted from MTB cultures or directly from clinical specimens, and regions of interest are amplified. Labelled amplicons are hybridised to probes immobilised on membrane strips and detected by a colourimetric reaction.
For the INNO-LiPA assay the amplification stage is a two-step nested PCR. At the NMRL, for each sample six PCR reactions are set up, three containing neat sample and three with a 1 : 10 dilution of the sample. In each set of three reactions, one PCR tube is spiked with MTB, DNA as an inhibition control. A negative result for this reaction indicates the presence of contaminants inhibiting the Taq polymerase. Dilution of the sample 1 : 10 is frequently sufficient to dilute contaminants to a level at which they are no longer inhibitory. For each PCR run, five negative controls are made to identify contamination, and a low positive control (10 copies of MTB, DNA) is included. All of the PCR products are visualised after electrophoresis on agarose gels, and one positive amplicon per sample is used in the 1 INNO-LiPA assay.
The MTBDRplus assay involves a single amplification step. At the NMRL, one PCR reaction is set up per sample, and each run includes one positive and one negative control. All samples, including controls, are run on the MTBDRplus assay. The hybridisation and subsequent wash steps of the MTBDRplus assay are semi-automated on a BeeBlot processor (Bee Robotics, Caernarfon, UK). At the NMRL, the MTBDRplus assay is often run alongside other GenoType tests, such as the Mycobacterium CM or AS assays (Hain Lifescience, Nehren, Germany), which use the same BeeBlot programme.
The GeneXpert assay is an automated assay that detects the presence of MTB in clinical samples and tests for rifampicin resistance. 29 The specimen is incubated in sample reagent for 15 minutes then transferred into a cartridge, which is loaded into the GeneXpert machine, and the entire assay is conducted automatically within the cartridge. At the NMRL, the GeneXpert assay is used to analyse respiratory and CSF samples. Respiratory samples are decontaminated to remove non-MTB organisms before they are processed in the GeneXpert. CSF samples should be sterile (unless infection is present), therefore, they do not require decontamination and are processed directly.
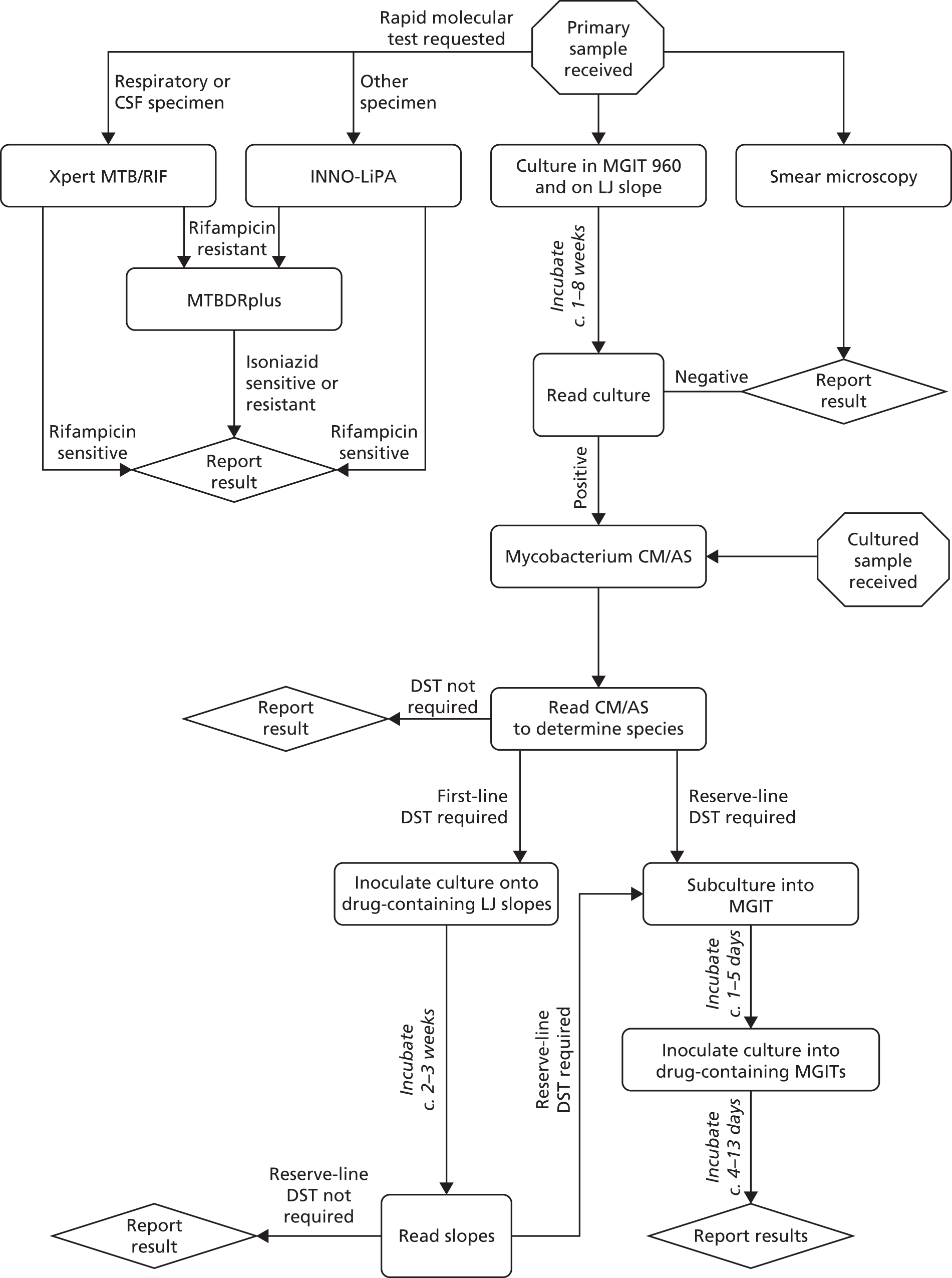
At the NMRL, the workflow for the identification of MTB and determination of its drug-resistance profile comprises an integrated series of culture-based and molecular tests designed to provide the most reliable clinically informative data as rapidly as possible (Figure 14). In this study we have timed individual stages independently, allowing an estimation of the time taken to process a sample through a theoretical exclusively phenotypic compared with molecular diagnostic pathway. Samples are almost always processed in batches, with the procedure-dependent batch size selected to optimise both efficiency and timely reporting. By recording the number of samples processed at each stage of every procedure, we can calculate the theoretical time spent per sample.
FIGURE 14.
Standard work flow for specimens and cultures received at the NMRL. Mycobacterium CM/AS, GenoType Mycobacterium CM/AS assay (Hain Lifescience).
Methods
Observation
Selection of days on which to observe the diagnostic tests was made based on convenience. Several biomedical scientists (BMSs) perform each diagnostic test at the NMRL and the observations were not restricted to any particular members of staff. The observer followed the BMS continuously while they were carrying out the diagnostic test.
Data collection
Based on the NMRL standard operating procedures (SOPs) for each diagnostic test, paper forms were created on which the working time involved in each significant task was recorded (see Appendix 7). The wall clocks present in each laboratory were used to time each task and the start and end time of each task was recorded on the form. Timings were rounded to the nearest minute or, if the time taken was significantly < 1 minute, rounded to the nearest 10 seconds. Any relevant additional information (such as tasks carried out while waiting for a sample in the centrifuge) was recorded in the ‘notes section’ next to the relevant task. The data were recorded in a spreadsheet, and the start and end times for each task were used to determine the working time for each task. The sum of the working times for each task equals the total working time. Every task involved in conducting the diagnostic test (such as paperwork, setting up, cleaning up, recording results, etc.) was included in the working time.
If a waiting time (e.g. waiting for a centrifuge to finish a spin) was ≤ 15 minutes, the waiting time was included in the working time, as the BMS could not realistically undertake separate tasks in that time. If the waiting time was > 15 minutes, unless the BMS remained unoccupied, the time was not recorded as working time, as the BMS had enough time to complete other unrelated tasks. If the BMS used the waiting time to complete tasks that were relevant to the test being timed, those tasks were timed and it was noted that they took place while waiting. The time taken for automated machines to conduct stages of an assay (> 15 minutes) or the time cultures spent in incubators was not recorded because no labour on the part of the BMS was involved.
The number of samples being processed during each observation was recorded. In addition, the number of each type of sample was recorded, because some sample types require slightly different methods of preparation. Reference cultures arrive on either solid or liquid medium and the number of each was also recorded.
Data analysis
At the NMRL, samples are processed in batches and, with the exception of the GeneXpert, individual samples are almost never assayed. For many tasks, the time taken to perform it is independent of the number of samples involved, for example preparation of PCR master mixes. The theoretical time taken for one sample was calculated by dividing the time taken by the number of samples. Thus, this is the mean time taken for a sample in an average batch size at the NMRL and does not represent the actual time that would be taken if a single sample was processed individually.
Results
GeneXpert assay
At the NMRL, the GeneXpert system is used to assay respiratory and CSF specimens. As all samples received are subsequently cultured to permit further phenotypic testing, respiratory specimens undergo a preliminary decontamination step before following the manufacturer’s recommendations for sample processing. Occasionally, a large volume (> 2 ml) of CSF is received and it is concentrated by centrifugation prior to processing.
At the NMRL, the GeneXpert assay is performed on demand, on a daily basis, on up to four samples simultaneously. This assay was observed between November 2012 and April 2013: 10 times for respiratory specimens (Table 10) and seven times for CSF specimens (Table 11). The mean theoretical hands-on time taken to process a single CSF specimen was 24 minutes. For respiratory specimens, the equivalent calculated time for a single specimen was 44 minutes.
| Stage | Time (hours:minutes:seconds) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 November 2012 | 5 November 2012 | 19 November 2012 | 26 November 2012 | 27 November 2012 | 25 February 2013 | 4 March 2013 | 5 March 2013 | 11 March 2013 | 9 April 2013 | Mean batch time | Theoretical time per specimen | |
| Number of specimens | 2 | 2 | 3 | 1 | 2 | 1 | 1 | 1 | 1 | 3 | ||
| Preparation | 00:08:00 | 00:14:00 | 00:05:00 | 00:02:00 | 00:21:00 | 00:02:30 | 00:03:00 | 00:05:00 | 00:09:00 | 00:04:00 | 00:07:21 | 0:04:19 |
| Decontamination | 00:34:00 | 00:49:00 | 00:36:00 | 00:35:10 | 00:31:00 | 00:35:30 | 00:37:40 | 00:39:50 | 00:37:00 | 00:35:00 | 00:37:01 | 0:21:46 |
| Sample preparation and loading | 00:19:00 | 00:25:00 | 00:28:00 | 00:18:00 | 00:21:00 | 00:18:00 | 00:20:00 | 00:20:00 | 00:18:00 | 00:20:00 | 00:20:42 | 0:12:11 |
| Recording results | 00:10:00 | 00:05:00 | 00:16:00 | 00:16:00 | 00:15:00 | 00:06:00 | 00:07:00 | 00:07:00 | 00:14:00 | 00:07:00 | 00:10:18 | 0:06:04 |
| Total hands-on time | 01:15:22 | 00:44:20 | ||||||||||
| Stage | Time (hours:minutes:seconds) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 13 November 2012 | 20 November 2012 | 21 November 2012 | 8 January 2013 | 5 March 2013 | 11 March 2013 | 19 March 2013 | Mean batch time | Theoretical time per specimen | |
| Number of specimens | 1 | 1 | 1 | 3 | 1 | 1 | 2 | ||
| Preparation | 00:01:00 | 00:01:00 | 00:01:00 | 00:02:00 | 00:03:00 | 00:04:00 | 00:01:00 | 00:01:51 | 0:01:18 |
| Specimen pretreatment | 00:03:00 | 00:00:00 | 00:00:00 | 00:00:00 | 00:00:00 | 00:00:00 | 00:00:00 | 00:00:26 | 0:00:18 |
| Sample preparation and loading | 00:18:00 | 00:20:00 | 00:18:00 | 00:22:00 | 00:18:00 | 00:18:00 | 00:20:00 | 00:19:09 | 0:13:24 |
| Recording results | 00:13:00 | 00:07:00 | 00:18:00 | 00:08:00 | 00:07:00 | 00:14:00 | 00:20:00 | 00:12:26 | 0:08:42 |
| Total hands-on time | 00:33:51 | 00:23:42 | |||||||
Line probe assays
The INNO-LiPA assay is run on primary samples received for molecular testing, other than respiratory specimens and CSF (which are run on the GeneXpert). The MTBDRplus assay, which is less sensitive, is subsequently run on isolates determined to be rifampicin resistant by either the INNO-LiPA or GeneXpert, in order to identify isoniazid-resistance mutations. The MTBDRplus assay is also run on cultures received by the reference laboratory.
At the NMRL, the INNO-LiPA and the MTBDRplus assays are conducted on a weekly basis. Typically, there are samples requiring INNO-LiPA testing every week, but the MTBDRplus assay is not always needed. The initial stages for each assay, resulting in amplification-ready DNA, are performed together. Set-up of the first-round PCR is also carried out for both assays together. Typically, the subsequent steps are carried out the following day. These are performed separately for each assay. The INNO-LiPA assay requires a second-round PCR and electrophoresis step to identify reactions containing amplicon. If no tubes contain sample amplicon then the hybridisation stage is not performed. Thus, for this assay, the time taken to identify negative samples is less than that to identify positive samples. For the MTBDRplus assay, all samples and controls are hybridised to strips, and there is no difference between the processing time for positive and negative samples.
Between November 2012 and April 2013, the INNO-LiPA assay performed in isolation was observed seven times and the two LPAs performed together observed a further five times (Table 12). The theoretical time per sample for each stage was calculated and the stages required for each assay summed to estimate the theoretical time for a single sample for each assay performed independently, if processed in a batch of 2–15 samples. The calculated per sample hands-on time for a PCR-positive INNO-LiPA assay was 45 minutes and for the MTBDRplus assay was 50 minutes.
| Assay | Stage | Time (hours:minutes:seconds) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 31 October 2012 | 7 November 2012 | 14 November 2012 | 28 November 2012 | 9 January 2013 | 16 January 2013 | 30 January 2013 | 27 February 2013 | 6 March 2013 | 13 March 2013 | 27 March 2013 | 10 April 2013 | Mean batch time | Theoretical time per specimen | ||
| INNO-LiPA and MTBDRplus | Total number of samples | 11 | 6 | 7 | 6 | 7 | 2 | 11 | 8 | 15 | 5 | 4 | 2 | ||
| Preparation/set-up | 00:34:00 | 00:32:00 | 00:11:00 | 00:10:00 | 00:20:00 | 00:04:00 | 00:15:00 | 00:07:00 | 00:05:00 | 00:15:20 | 0:02:09 | ||||
| Sample decontamination | 00:38:00 | 00:32:00 | 01:04:00 | 00:33:00 | 00:40:00 | 00:50:00 | 00:44:00 | 00:46:00 | 00:32:30 | 00:41:30 | 00:42:06 | 0:05:56 | |||
| Lysing of cells | 00:42:00 | 00:14:40 | 00:31:00 | 00:17:00 | 00:14:40 | 00:18:20 | 00:14:30 | 00:28:00 | 00:20:50 | 00:22:20 | 00:20:30 | 00:32:20 | 00:23:01 | 0:03:17 | |
| First-round PCR | 00:58:30 | 00:35:00 | 00:43:00 | 00:47:00 | 00:21:00 | 00:23:00 | 00:54:00 | 00:51:00 | 00:49:00 | 00:24:30 | 00:23:30 | 00:26:00 | 00:37:58 | 0:05:25 | |
| INNO-LiPA | Number of samples for second-round PCR | 11 | 5 | 7 | 6 | 7 | 1 | 10 | 6 | 14 | 5 | 4 | 2 | ||
| Second-round PCR | 00:41:20 | 00:34:00 | 00:35:00 | 00:26:00 | 00:29:40 | 00:15:30 | 00:33:00 | 00:24:30 | 01:00:20 | 00:23:10 | 00:22:40 | 00:26:00 | 00:30:56 | 0:04:46 | |
| Gel electrophoresis | 00:48:20 | 00:34:30 | 00:44:00 | 00:36:00 | 00:38:30 | 00:25:30 | 00:38:00 | 00:51:30 | 00:44:00 | 00:32:30 | 00:38:00 | 00:39:10 | 0:06:20 | ||
| Positive INNO-LiPA | Number of positive samples for LPA | 5 | 2 | 2 | 3 | 1 | 0 | 3 | 7 | 2 | 1 | 1 | 0 | ||
| LPA assay | 00:42:20 | 00:31:20 | 00:52:30 | 00:46:10 | 00:45:00 | 00:36:50 | 00:38:30 | 00:47:40 | 00:37:00 | 00:44:40 | 00:42:12 | 0:15:38 | |||
| Recording results | 00:05:15 | 00:06:00 | 00:07:30 | 00:03:20 | 00:07:10 | 00:07:20 | 00:10:00 | 00:02:10 | 00:02:20 | 00:05:41 | 0:01:54 | ||||
| MTBDRplus | Number of samples for LPA | 0 | 1 | 0 | 0 | 0 | 1 | 1 | 2 | 1 | 0 | 0 | 0 | ||
| Setting up BeeBlot | 00:06:30 | 00:16:00 | 00:18:00 | 00:13:30 | 0:10:08 | ||||||||||
| MTBDRplus assay | 00:11:40 | 00:26:10 | 00:36:00 | 00:21:30 | 00:13:00 | 00:21:40 | 0:18:03 | ||||||||
| Recording results | 00:03:00 | 00:08:00 | 00:05:30 | 0:05:30 | |||||||||||
| INNO-LiPA | Total hands-on time | 03:56:23 | 00:45:25 | ||||||||||||
| MTBDRplus | Total hands-on time | 02:39:04 | 00:50:29 | ||||||||||||
Culture-based drug susceptibility testing
Phenotypic testing is considered the gold standard for determining MTB drug resistance, and samples received at the NMRL for molecular testing are subsequently assayed using culture-based methods. The observations of the culture-based methods took place between April 2013 and June 2013.
Prior to DST, primary specimens are cultured in drug-free media (LJ and MGIT) to provide sufficient replicating bacilli for testing. LJ slopes are examined weekly for growth, and MGIT growth is monitored daily. Purity of the culture is assessed by plating on Columbia Blood Agar plates and acid-fast staining. The theoretical mean time taken to complete all tasks for the culture of one primary specimen was 20 minutes (Table 13).
| Procedure | Stage | Time (hours:minutes:seconds) | Mean batch time (hours:minutes:seconds) | Theoretical time per specimen (hours:minutes:seconds) | ||
|---|---|---|---|---|---|---|
| Inoculation | Date | 5 June 2013 | 6 June 2013 | 10 June 2013 | ||
| Number of samples | 12 | 15 | 9 | |||
| Paperwork/set-up | 00:29:00 | 00:26:00 | 00:30:00 | 00:28:20 | 00:02:22 | |
| Decontamination | 00:48:00 | 00:51:00 | 00:32:00 | 00:43:40 | 00:03:38 | |
| Preparing LJ and MGITs and inoculating with sample | 00:23:00 | 00:15:00 | 00:14:00 | 00:17:20 | 00:01:27 | |
| Loading samples into incubator and BACTEC MGIT 960 | 00:04:00 | 00:02:00 | 00:03:00 | 00:03:00 | 00:00:15 | |
| Reading and reporting results | Date | 15 June 2013 | 18 June 2013 | |||
| Number of samples | 22 | 113 | ||||
| Preparing positive MGIT samples for smear microscopy and blood plates | 01:22:00 | 01:22:00 | 00:03:44 | |||
| Staining slides and performing smear microscopy | 01:11:30 | 01:11:30 | 00:03:15 | |||
| Reporting MGIT culture results and retreating contaminated samples | 01:16:00 | 01:16:00 | 00:03:27 | |||
| Reading LJ slopes (weekly) | 01:10:00 | 01:10:00 | 00:01:52 | |||
| Total hands-on time | 00:19:59 | |||||
Testing for resistance to first-line drugs (rifampicin, isoniazid, ethambutol, pyrazinamide and streptomycin) is conducted using solid egg-based LJ medium that is prepared in-house. The resistance ratio method utilised requires three slopes per drug. In addition, four control slants (positive, p-nitrobenzoic acid, thiophene 2-carboxylic acid hydrazide and low temperature) are included for each sample to identify positivelyMTB complex isolates. Thus, a total of 19 slopes are inoculated per sample. The LJ slopes are prepared on demand, in batches corresponding to 30 eggs-worth of medium, which fills approximately 780 bijou bottles. In each batch, slopes containing a single drug are prepared; these are stored for up to 2 months. The theoretical time taken to prepare LJ slopes for testing one sample was estimated at 4 minutes (Table 14). In total, the theoretical hands-on time required to set up the first-line sensitivities for one specimen was 21 minutes (see Table 14). Including the time required for the initial culture in drug-free media, the total hands-on time for one specimen was 41 minutes.
| Procedure | Stage | Time (hours:minutes:seconds) | Mean batch time (hours:minutes:seconds) | Theoretical time per specimen (hours:minutes:seconds) | |||
|---|---|---|---|---|---|---|---|
| Preparation of LJ slopes | Date | 2 May 2013 | 10 May 2013 | 14 May 2013 | 11 June 2013 | ||
| Number of trays | 13 | 13 | 13 | 10 | |||
| Set-up/making the LJ medium | 00:39:00 | 00:48:20 | 00:43:40 | 00:01:07 | |||
| Aliquoting medium into bijou bottles | 01:30:00 | 01:21:00 | 01:22:00 | 01:24:20 | 00:02:10 | ||
| Filling trays with DST LJ slopes | 00:19:00 | 00:19:00 | 00:00:38 | ||||
| Inoculation LJ slopes | Date | 12 April 2013 | 26 April 2013 | 16 May 2013 | |||
| Number of samples | 25 | 17 | 19 | ||||
| Set-up | 00:48:00 | 00:31:00 | 00:40:00 | 00:39:40 | 00:01:57 | ||
| Inoculating slopes | 01:21:00 | 01:00:00 | 01:16:00 | 01:12:20 | 00:03:33 | ||
| Reading LJ DST results | Date | 22 April 2013 | 29 April 2013 | 7 May 2013 | 14 May 2013 | ||
| Number of samples | 42 | 35 | 25 | 16 | |||
| Set-up | 00:05:00 | 00:07:00 | 00:16:00 | 00:09:00 | 00:09:15 | 00:00:56 | |
| Checking controls | 00:09:00 | 00:15:00 | 00:14:00 | 00:12:40 | 00:01:22 | ||
| Reading and reporting cultures | 02:25:00 | 01:43:00 | 01:15:00 | 00:42:00 | 01:31:15 | 00:09:17 | |
| Total hands-on time | 00:21:01 | ||||||
Testing for second-line drugs [aminoglycosides, capreomycin, fluoroquinolones, thioamides and linezolid (Zyvox®, Pfizer)] is conducted in the BACTEC MGIT 960 system at the NMRL and requires 11 tubes per sample. Media-containing MGITs are purchased commercially and prepared by the addition of supplied supplements plus drugs when required. The theoretical time to prepare tubes sufficient to test one sample was 9 minutes. In total, the hands-on time required for one specimen (for 10 drugs) was 28 minutes (Table 15). Including the time required for initial specimen culture, the total theoretical per-sample time was 49 minutes.
| Procedure | Stage | Time (hours:minutes:seconds) | Mean batch time (hours:minutes:seconds | Theoretical time per specimen (hours:minutes:seconds | |||||
|---|---|---|---|---|---|---|---|---|---|
| MGIT inoculation | Date | 16 April 2013 | 18 April 2013 | 15 May 2013 | |||||
| Number of samples | 3 | 2 | 7 | ||||||
| Paperwork/set-up | 00:08:00 | 00:06:00 | 00:11:00 | 00:08:20 | 00:02:05 | ||||
| Preparing tubes for inoculation | 00:36:00 | 00:26:00 | 00:49:00 | 00:37:00 | 00:09:15 | ||||
| Adding sample to tubes | 00:21:00 | 00:13:00 | 00:28:00 | 00:20:40 | 00:05:10 | ||||
| Machine loading and clean-up | 00:10:00 | 00:08:00 | 00:10:00 | 00:09:20 | 00:02:20 | ||||
| Reading MGIT results | Date | 16 April 2013 | 18 April 2013 | 23 April 2013 | 25 April 2013 | 2 May 2013 | 15 May 2013 | ||
| Number of positive samples | 1 | 1 | 4 | 1 | 3 | 1 | |||
| Reading and recording results | 00:19:30 | 00:11:00 | 00:27:00 | 00:12:00 | 00:26:00 | 00:11:00 | 00:19:10 | 00:09:35 | |
| Total hands-on time | 00:28:25 | ||||||||
Discussion
This time-and-motion study was carried out in order to estimate the total working time required for conducting molecular and culture-based diagnostic tests on specimens received at the NMRL. The results are summarised in Table 16. The BMS staff were aware both that they were being timed and of the aims of the study. Although it was not the purpose of the time-and-motion study to assess the efficiency of the BMSs, the knowledge that they were being timed may have affected the rate at which they worked. The timings were recorded by a junior member of staff, new to the laboratory, in order to minimise this effect. In this study, convenience sampling was the only practical method of sampling but it inevitably led to more timings being taken on certain days of the week and when the workload in the laboratory was not too heavy.
| Test | Theoretical time per specimen (hours:minutes:seconds) | Mean number of samples per batch | Number of drugs tested |
|---|---|---|---|
| DST BACTEC MGIT 960 | 00:48:24 | 4 | 10 |
| DST LJ slopes | 00:41:00 | 20 | 5 |
| GeneXpert (CSF) | 00:23:42 | 1 | 1 |
| GeneXpert (respiratory) | 00:44:20 | 2 | 1 |
| INNO-LiPA | 00:45:25 | 7 | 1 |
| MTBDRplus | 00:50:29 | 1 | 2 |
A broad range of sample types is received at the NMRL and each requires a unique series of preparation steps designed to optimise the outcome of downstream tests. For example, although respiratory specimens must be decontaminated to kill non-mycobacterial species, specimens from sterile sites, such as CSF, which typically have a low mycobacterial load, are not preprocessed. Specific procedures for the isolation of mycobacteria from specimens such as tissues or bone marrow are also utilised. This variation results in differences in the working time taken for each batch.
The GeneXpert assay is designed to be performed directly on specimens, and the in-cartridge procedure includes steps for killing the bacilli and releasing DNA. At the NMRL, all specimens are cultured in order that, subsequent to molecular tests, gold-standard phenotypic DST can be performed. This means that sputum must be liquefied and decontaminated before an aliquot can be used for the GeneXpert assay. In other laboratories, the sputum may be loaded directly into the cartridge. Thus, when used as a POC device, hands-on time will be equivalent for all specimens and approximate that of CSF specimens at the NMRL.
The theoretical time taken to process a single specimen is calculated based on the actual batch size used at the NMRL. The length of time taken for many of the tasks involved in a procedure will not be dependent on the number of samples. For example, tasks such as PCR master mix preparation and LPA hybridisation are essentially independent of batch size (see Table 12). The timings presented here may, therefore, be substantially different from those at other laboratories with different levels of specimen throughput.
Comparison of the time taken to perform the two LPAs illustrates the substantial effect that batch size has on the theoretical working time per sample. The INNO-LiPA requires two PCR stages, and hybridisation washes are performed manually. The calculated time spent per sample – 45 minutes – was based on timings when the mean batch size was seven samples. The MTBDRplus assay utilises a single PCR step and the hybridisation stage is semiautomated, however, the calculated time spent per sample was 50 minutes, as only one or two samples were processed together.
It is important to reiterate that the information obtained from each of the tests described is not the same. The GeneXpert and INNO-LiPA assays identify MTBC complex and diagnose rifampicin resistance. The MTBDRplus assay provides these results plus isoniazid resistance. As conducted at the NMRL, the method for DST on LJ slopes includes controls that identify MTBC complex and diagnoses resistance to five first-line drugs. The timings for the BACTEC MGIT 960 DSTs are for the diagnosis of resistance to 10 drugs.
Chapter 5 Health-care costs and utilities
Introduction
The economic value of diagnostic tests is a function of the cost, timing and accuracy of the tests and of the resultant impact on clinical decisions, and hence on health outcomes and expenditure. Tests cannot be evaluated in isolation but have to be considered within a pathway of care, specifying how patients are treated while waiting for test results, what treatments they are offered when the results arrive, how and when diagnostic errors (FPs and FNs) are detected and then what corrective action is taken. The health and monetary consequences of alternative diagnostic/treatment pathways can be weighed up using a cost–utility framework, in which the value of health effects is quantified using the metric of the quality-adjusted life-year (QALY). 106 QALY estimates should take account of mortality and impacts on quality of life, caused both by the disease and by adverse effects of treatment. Similarly, cost estimates should incorporate the cost of diagnostic tests, costs for treating the disease and caring for the patient, and costs associated with adverse effects. From a broader ‘societal’ perspective, costs borne by patients and their families and the value of lost productivity would also be included alongside direct costs to health-care payers. For an infectious disease, the analysis should also account for impacts related to transmission, including QALY loss and costs for secondary cases. 107 The overall expected costs and QALYs associated with alternative pathways can then be compared to identify which offers the most cost-effective use of scarce health-care resources.
The conventional diagnostic work-up for patients with suspected respiratory TB disease includes sputum smear microscopy to give an early indication of infectivity, followed by culture to diagnose active TB and to test for drug sensitivity. 108 Culture is diagnostic but can take from 14 to 42 days to provide a definitive result: usually around 14 days to identify MTB and a further 14 days for DST, but it can take longer for slow-growing cultures. Rapid molecular assays can return a result in as little as 1–3 days, which might have a variety of benefits for individual patients, the community and the UK NHS. Patients wrongly suspected of having TB may benefit from an early rule-out, avoiding or shortening unnecessary isolation and treatment. This would be particularly valuable for people who are thought to be at high risk of MDR-TB, who are more likely to be admitted to very expensive negative pressure isolation, and who may be exposed to presumptive treatment with second-line drugs that have a relatively poor adverse effect profile. For patients with the disease, early accurate diagnosis will ensure that they commence effective treatment and that, where necessary, they are isolated earlier, minimising the impact on their own health while diminishing the risk of transmission. Early diagnosis of drug-resistant disease is particularly important to protect the health of the individual and the community.
However, as shown in Chapter 3, rapid assays are less accurate than culture, raising the possibility of FP and FN results. Both types of error can be costly and harmful. FN results give inappropriate reassurance and may harm the patient by delaying time to effective treatment and place contacts at risk if infection control measures are relaxed. FP results may unnecessarily expose patients to the inconvenience of isolation and adverse effects of medication. These cost and harms are likely to be particularly acute for patients with, or at high perceived risk of, MDR-TB. Current guidance therefore recommends that rapid molecular tests may have a role alongside culture but that they should not replace culture. 109,110 This limits potential costs and harms of misdiagnosis, as clinicians can take corrective action when the culture results arrive, but it does not entirely eliminate these risks. The additional cost of the molecular test also cannot be offset by savings from reduced need for culture. There is, therefore, a trade-off between the costs and health impacts of adding a rapid molecular test to the current diagnostic pathway, as the benefits of a faster diagnosis may be partly or wholly offset by the additional cost of the test and by costs and health consequences of errors.
We conducted a cost–utility analysis to evaluate the addition of a rapid molecular test for TB disease and drug sensitivity alongside the culture testing, compared with culture testing alone, in patients with suspected respiratory disease. It should be noted that, although the aim of this study was to assess the role of rapid molecular assays in testing for drug susceptibility, the assays’ ability to diagnose MTB accurately was also relevant to the economic analysis. Identifying drug-sensitive (DS) TB as well as resistant TB reduces both unnecessary treatment and isolation and the risk of onward transmission. Therefore, in order to capture all aspects of the cost and effects related to the diagnostic accuracy of rapid molecular assays, the economic analysis had to focus on the population of patients with suspected TB across the whole care pathway, not just from the point of DST and onward. The analysis followed the National Institute for Health and Care Excellence (NICE) public health reference case, including the adoption of a public sector perspective and the use of a 3.5% annual discount rate for both costs and QALYs. 111 A dynamic model was used to estimate the impact of the alternative diagnostic strategies on transmission of TB within defined communities, and to estimate related costs and QALY effects. Probabilistic sensitivity analysis (PSA) was used to reflect the impact of uncertainty over the input parameters for the transmission model. However, some aspects of structural uncertainty, relating to modelling assumptions, could not be integrated in the PSA. In this chapter we present estimates (means and PSA distributions) for the cost and QALY parameters that, alongside the estimates of diagnostic accuracy presented in Chapter 3, provided key inputs for the transmission model. The structure, assumptions and parameterisation of the transmission model, and the cost-effectiveness results are presented in Chapter 6.
Overview of methods
Key aspects of the decision problem and assumptions adopted for our analysis are described below.
Diagnostic strategies
-
The baseline for comparison was the current diagnostic strategy, including smear microscopy, culture for identification of MTB and DST for culture-positive cases. It was assumed that smear microscopy results are available within 1 day of collection of the sputum sample. Culture testing was assumed to be 100% accurate for both TB diagnosis and drug susceptibility and was treated as the reference standard for other tests. The results of culture tests were assumed to take 14 days for diagnosis of MTB and an additional 14 days for DST. Note that times to test results will differ between patients, and that the figures used here are intended as an average across the population.
-
The intervention evaluated was the addition of a rapid molecular assay for the detection of TB disease and drug resistance, alongside the current diagnostic strategy. We assumed that molecular test results would be available for both TB status and drug sensitivity 3 days from collection of the sputum sample, on average.
-
The currently available molecular assays test for MTB and rifampicin resistance (GeneXpert and INNO-LiPA) and MTBDRplus also tests for isoniazid resistance. In practice, the results of culture-based DST are used to identify sensitivity to other drugs and to derive individualised drug regimens. However, for the modelling we did not distinguish between different patterns of drug resistance. This is a simplification and relies on the assumptions that patients with resistance to a single drug would not incur significant additional risks or costs for isolation or drug treatment compared with patients with DS disease, and that patients with different patterns of multiple drug resistance would face similar risks and costs to one another (this is unlikely to be true for XDR-TB, but this is still a rare phenomenon in the UK). Furthermore, we assumed that the diagnostic accuracy of the molecular assays for detecting ‘true’ MDR-TB is the same as for detecting rifampicin resistance (as reported in Chapter 3). This is justified by the observation that a large majority of MDR patients are resistant to rifampicin. 38
-
The analysis focused on the GeneXpert system, the INNO-LiPA and MTBDRplus assay as exemplars for the rapid molecular assays.
-
At present, microscopy and mycobacterial culture are most usually performed in local hospital laboratories, but DST for MTB-positive cases is performed at specialist mycobacterial reference laboratories. 112 We assumed that these arrangements would not change with the wider adoption of molecular testing. However, we did investigate alternative scenarios for the location of rapid molecular assays: centralised, with molecular testing conducted only at TB specialist reference laboratories, and localised, with molecular testing distributed at local TB laboratories. It was assumed that centralised testing would reduce the mean cost per test (owing to economies of scale in the laboratory arising from more intensive use of capital) but at the expense of possible delay and an additional cost for transport of samples.
Population and subgroups
-
The transmission model adopted a population approach, modelling incidence of TB and the process of diagnosis and treatment in three sections of the community with high incidence of MDR-TB: Black African, South Asian and Eastern European individuals.
-
The population subject to intervention was individuals being tested for suspected TB disease. This includes individuals who present symptomatically or as a result of active case finding. Individuals may have been referred to TB services from general practice, emergency departments or other specialties.
-
Individuals undergoing diagnosis are categorised into subgroups which are defined by their true disease status, prior risk status perceived by clinicians and test results (see Diagnostic subgroups, below).
Care pathways
-
For each diagnostic strategy and subgroup, we defined a care pathway that specified what medical treatments and health services individuals would receive between presentation to the point when they either complete TB treatment successfully, or fail to complete treatment (are lost to follow-up). See Care pathways, below.
-
We estimated a total cost for each care pathway, including the cost of consultations and investigations prior to referral to TB services, TB diagnosis, medication, inpatient care and isolation, outpatient care, and contact tracing and associated treatment of any latent TB cases.
-
In addition, for each care pathway the health impact is estimated in terms of the mean discounted QALYs lost as a result of TB disease and treatment.
Diagnostic subgroups
Various factors define the unique care pathways that patients can follow after referral for suspected TB. Under the current diagnostic strategy (the baseline comparator), the key factors are as follows.
True clinical status (no tuberculosis, DS tuberculosis, MDR-TB): a patient starting the process of diagnosis for pulmonary TB can be in one of three clinical states: no active TB disease, DS TB, or MDR-TB. As noted above, for simplicity, we did not differentiate between resistance to single or multiple drugs, or between different patterns of drug resistance.
Smear status (positive, negative): the smear status is identified by microscopy, within 1 day of collection of the sputum sample. Smear status indicates infectivity and so determines if the clinician decides to isolate the patient or provides presumptive medication prior to diagnosis.
MDR risk status (high risk, low risk): risk status is determined a priori by the clinician, with patients who are deemed to be at ‘high risk’ of MDR-TB invoking more conservative action. The main risk factors for MDR-TB are history of prior TB drug treatment, birth in a high-incidence country, HIV infection, residence in London, age between 25 and 44 years, and male gender. 108 However, these features are non-specific, and presumptive treatment of every patient with one or more of these risk factors would be unrealistic.
For the purposes of calculation, we defined risk status simply as a percentage of suspected TB cases that a clinician deems to be at sufficiently high risk of MDR-TB to warrant admission to a negative pressure isolation room and presumptive treatment with MDR medications if the person is smear positive. However, the decision to prescribe MDR medication presumptively would also depend on the expected delay in diagnosis. We assumed that presumptive treatment would not be used with molecular testing, as the results are available in a few days compared with 2–4 weeks for culture and DST alone. In the absence of better information, we assumed that clinicians would be relatively ‘well-calibrated’ in their judgement, and that the proportion of patients identified as high risk would be similar to the ratio of MDR cases to all TB cases in the relevant population: 1.6% (13 cases) of Black African patients, 1.4% (31 cases) of South Asian patients, and 24% (13 cases) of Eastern European patients. 113 Based on these figures, we assumed that 2% of patients would be treated as high risk in the Black African and South Asian populations, and 25% in the Eastern European population.
These three factors define the 12 diagnostic subgroups that have distinctive care pathways under the current diagnostic strategy (Table 17).
| Code | True disease status | Smear status | Risk status |
|---|---|---|---|
| 7_C | No TB | Positive | High |
| 21_C | Low | ||
| 14_C | Negative | High | |
| 28_C | Low | ||
| 1_C | DS TB | Positive | High |
| 15_C | Low | ||
| 8_C | Negative | High | |
| 22_C | Low | ||
| 4_C | MDR-TB | Positive | High |
| 18_C | Low | ||
| 11_C | Negative | High | |
| 25_C | Low |
The introduction of a rapid assay increases the number of possible care pathways, because errors in molecular test results can impact on treatment decisions prior to day 28, when the correct culture results are available. There are 32 permutations of true disease status, smear status, risk status and molecular assay results that lead to distinct care pathways (Table 18).
| ID | True disease status | Smear status | Risk status | Molecular test result |
|---|---|---|---|---|
| 7_R | No TB | Positive | High | No TB |
| 33_R | DS TB | |||
| 34_R | MDR-TB | |||
| 21_R | Low | No TB | ||
| 35_R | DS TB | |||
| 36_R | MDR-TB | |||
| 14_R | Negative | High | No TB | |
| 29_R | DS TB | |||
| 30_R | MDR-TB | |||
| 28_R | Low | No TB | ||
| 31_R | DS TB | |||
| 32_R | MDR-TB | |||
| 1_R | DS TB | Positive | High | DS TB |
| 3_R | MDR-TB | |||
| 15_R | Low | DS TB | ||
| 17_R | MDR-TB | |||
| 8_R | Negative | High | DS TB | |
| 10_R | MDR-TB | |||
| 12_R | No TB | |||
| 22_R | Low | DS TB | ||
| 24_R | MDR-TB | |||
| 26_R | No TB | |||
| 2_R | MDR-TB | Positive | High | DS TB |
| 4_R | MDR-TB | |||
| 16_R | Low | DS TB | ||
| 18_R | MDR-TB | |||
| 9_R | Negative | High | DS TB | |
| 11_R | MDR-TB | |||
| 13_R | No TB | |||
| 23_R | Low | DS TB | ||
| 25_R | MDR-TB | |||
| 27_R | No TB |
One final factor that leads to variation in the care pathway, and hence in the cost and QALYs attached to the diagnostic subgroups, is whether or not the individual completes their course of medication. This affects the duration of care the patient receives and therefore the total duration over which they accrue costs and QALYs from treatment, and whether they are cured or re-enter the population as a transmission risk. We assumed that, on average, individuals who fail to complete treatment default half-way through the course of treatment: uncertainty over this proportion was reflected in the PSA by sampling from a beta distribution with a mean of 0.5 and standard error of 0.1.
Care pathways
Each of the above diagnostic subgroups was assumed to follow a distinct care pathway. We considered the care pathway to be broken down into the following stages:
Pre referral This is the period of time from when a patient becomes symptomatic or is first suspected of having TB, to the time when they are referred to a TB or respiratory specialist for investigation. We assumed that the referral can come from one of three channels: from primary care, from an emergency department, or from another hospital specialty.
Diagnosis The duration of the diagnostic period is from the first collection of sputum samples until the definitive diagnosis for TB and drug sensitivity is received. The diagnostic period can overlap with treatment as an inpatient or outpatient.
Inpatient treatment From the point the patient is admitted until they are discharged. For smear-positive patients, inpatient care will include isolation until the patient is no longer considered a transmission risk. If the patient is smear positive and at high risk of MDR-TB, or if they are diagnosed with MDR-TB, we assumed isolation in a negative pressure room.
Outpatient treatment Once a patient is deemed well enough, and is no longer a transmission risk, they will be discharged and continue treatment as an outpatient.
The addition of a rapid molecular test is assumed to impact on the care pathways in two main ways during the diagnostic period: first, it may influence clinical decisions about what presumptive drug treatment is offered, if any, and, second, it may influence decisions about whether or not a patient is admitted and, if so, to what level of isolation. These decisions may also have implications for the total duration of drug treatment and the time spent as an outpatient, which depend on when effective treatment is initiated.
Drug treatment
The standard drug treatment for TB consists of 6 months of isoniazid and rifampicin, supplemented in the first 2 months with pyrazinamide and ethambutol, generally in a fixed-dose combination tablet form. 108 Treatment for MDR-TB is less standardised. For the modelling exercise, we assumed a full course to consist of seven drugs: an injectable drug (either amikacin or capreomycin) for 4 months, and oral medications [moxifloxacin (Avelox®, Bayer) or levofloxacin, prothionamide, cycloserine, ethambutol, pyrazinamide, and pyridoxine – vitamin B6] for 20 months. 114 We assumed that MDR treatment is effective for DS disease, but that standard treatment is not effective for MDR-TB, and that, to be effective, MDR treatment must last for the full 20 months. The mean duration of treatment for patients who failed to complete was assumed to be 3 months for standard treatment and 10 months for MDR treatment.
Our assumptions about what treatments would be offered to the 12 diagnostic subgroups under the current practice (culture only) strategy are set out in Table 19. We assumed that patients perceived to be at low risk of drug-resistant disease with a smear-positive microscopy result would commence the standard course of treatment presumptively (at day 1). This treatment continues if the patient has a culture-confirmed TB diagnosis at day 14 but is assumed to stop if they receive a negative diagnosis at that time. If culture indicates the presence of drug-resistant disease at day 28, it is assumed that the patient then switches to a MDR treatment regimen. Smear-negative patients who are at low risk of MDR-TB are assumed not to receive treatment presumptively but to start standard treatment if they have a positive TB diagnosis at day 14 and to switch to a MDR regimen if MDR-TB is confirmed by culture at day 28.
Assumptions are similar for patients perceived to be at high risk of MDR-TB, except that presumptive treatment (where applicable) is with a MDR regimen rather than a standard regimen.
| Code | Risk status | Smear status | True disease status | Drug treatment regimen | ||
|---|---|---|---|---|---|---|
| Days 1–14 | Days 15–28 | Day 29 . . . | ||||
| 21_C | Low | Positive | No TB | Standard | None | None |
| 15_C | DS TB | Standard | Standard | Standard | ||
| 18_C | MDR-TB | Standard | Standard | MDR | ||
| 28_C | Negative | No TB | None | None | None | |
| 22_C | DS TB | None | Standard | Standard | ||
| 25_C | MDR-TB | None | Standard | MDR | ||
| 7_C | High | Positive | No TB | MDR | None | None |
| 1_C | DS TB | MDR | MDR | Standard | ||
| 4_C | MDR-TB | MDR | MDR | MDR | ||
| 14_C | Negative | No TB | None | None | None | |
| 8_C | DS TB | None | MDR | Standard | ||
| 11_C | MDR-TB | None | MDR | MDR | ||
The drug treatment pathways in the presence of rapid molecular testing are shown in Table 20. We assumed that clinicians would not treat presumptively in advance of a molecular test result (at day 3), regardless of the patient’s smear status and perceived MDR risk status. Treatment between days 4 and 14 is based entirely on the molecular test result. Any treatment is stopped if culture indicates no TB at day 14, and, if necessary, is switched to an appropriate regimen when culture drug sensitivity results are available at day 28. The only role for risk assessment in this case is in determining what regimen patients receive between days 15 and 28 if the molecular test (falsely) indicates no TB but culture at day 14 confirms a diagnosis of TB. We assume that smear-positive patients cannot have a FN molecular test for MTB.
| ID | Risk status | Smear status | Rapid test result | True disease status | Drug treatment regimen | |||
|---|---|---|---|---|---|---|---|---|
| Days 1–3 | Days 4–14 | Days 15–28 | Day 29 . . . | |||||
| 21_R | Low | Positive | No TB | No TB | None | None | None | None |
| 35_R | DS TB | No TB | None | Standard | None | None | ||
| 15_R | DS TB | None | Standard | Standard | Standard | |||
| 16_R | MDR-TB | None | Standard | Standard | MDR | |||
| 36_R | MDR-TB | No TB | None | MDR | None | None | ||
| 17_R | DS TB | None | MDR | MDR | Standard | |||
| 18_R | MDR-TB | None | MDR | MDR | MDR | |||
| 28_R | Negative | No TB | No TB | None | None | None | None | |
| 26_R | DS TB | None | None | Standard | Standard | |||
| 27_R | MDR-TB | None | None | Standard | MDR | |||
| 31_R | DS TB | No TB | None | Standard | None | None | ||
| 22_R | DS TB | None | Standard | Standard | Standard | |||
| 23_R | MDR-TB | None | Standard | Standard | MDR | |||
| 32_R | MDR-TB | No TB | None | MDR | None | None | ||
| 24_R | DS TB | None | MDR | MDR | Standard | |||
| 25_R | MDR-TB | None | MDR | MDR | MDR | |||
| 7_R | High | Positive | No TB | No TB | None | None | None | None |
| 33_R | DS TB | No TB | None | Standard | None | None | ||
| 1_R | DS TB | None | Standard | Standard | Standard | |||
| 2_R | MDR-TB | None | Standard | Standard | MDR | |||
| 34_R | MDR-TB | No TB | None | MDR | None | None | ||
| 3_R | DS TB | None | MDR | MDR | Standard | |||
| 4_R | MDR-TB | None | MDR | MDR | MDR | |||
| 14_R | Negative | No TB | No TB | None | None | None | None | |
| 12_R | DS TB | None | None | MDR | Standard | |||
| 13_R | MDR-TB | None | None | MDR | MDR | |||
| 29_R | DS TB | No TB | None | Standard | None | None | ||
| 8_R | DS TB | None | Standard | Standard | Standard | |||
| 9_R | MDR-TB | None | Standard | Standard | MDR | |||
| 30_R | MDR-TB | No TB | None | MDR | None | None | ||
| 10_R | DS TB | None | MDR | MDR | Standard | |||
| 11_R | MDR-TB | None | MDR | MDR | MDR | |||
Location of care and isolation
Our assumptions regarding the durations of hospital stay, isolation and outpatient care are summarised in Tables 21 and 22. Some patients may be admitted to hospital for treatment of acute symptoms and/or for isolation, and the remainder of the treatment period is spent under outpatient care. Clinical practice in this area is likely to vary and is probably influenced by practical constraints such as the availability of isolation beds, as well as by different interpretations of guidelines and judgement over the risks for individual patients. We have not been able to obtain nationally representative data on length of stay and duration of isolation broken down by the different diagnostic groups required for the model. The assumptions are therefore based on local audit data and expert opinion from members of the research team. It should also be emphasised that the figures quoted below are all means for the relevant population, and that lengths of stay and isolation are highly variable between individuals. As for other model parameters, uncertainty over these means is modelled in the PSA.
| Code | Risk status | Smear status | True disease status | Duration of treatment by location (days) | ||||
|---|---|---|---|---|---|---|---|---|
| Standard inpatient | Inpatient isolation | Negative pressure | Outpatient | Pathway duration | ||||
| 21_C | Low | Positive | No TB | 0 | 13 | 0 | 0 | 14 |
| 15_C | DS TB | 0 | 14 | 0 | 169 | 184 | ||
| 18_C | MDR-TB | 0 | 27 | 89 | 521 | 638 | ||
| 28_C | Negative | No TB | 0 | 0 | 0 | 0 | 14 | |
| 22_C | DS TB | 0 | 0 | 0 | 183 | 197 | ||
| 25_C | MDR-TB | 23 | 0 | 23 | 578 | 638 | ||
| 7_C | High | Positive | No TB | 0 | 0 | 13 | 0 | 14 |
| 1_C | DS TB | 0 | 0 | 27 | 156 | 184 | ||
| 4_C | MDR-TB | 0 | 0 | 89 | 521 | 611 | ||
| 14_C | Negative | No TB | 0 | 0 | 13 | 0 | 14 | |
| 8_C | DS TB | 4 | 0 | 23 | 156 | 197 | ||
| 11_C | MDR-TB | 23 | 0 | 23 | 564 | 624 | ||
| ID | Risk status | Smear status | Rapid test result | True disease status | Duration of treatment by location (days) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Standard inpatient | Inpatient isolation | Negative pressure | Outpatient | Pathway duration | |||||
| 21_R | Low | Positive | No TB | No TB | 0 | 2 | 0 | 0 | 14 |
| 35_R | DS TB | No TB | 0 | 13 | 0 | 0 | 14 | ||
| 15_R | DS TB | 0 | 16 | 0 | 167 | 186 | |||
| 16_R | MDR-TB | 0 | 27 | 89 | 519 | 638 | |||
| 36_R | MDR-TB | No TB | 0 | 2 | 11 | 0 | 14 | ||
| 17_R | DS TB | 0 | 2 | 25 | 156 | 186 | |||
| 18_R | MDR-TB | 0 | 2 | 89 | 519 | 613 | |||
| 28_R | Negative | No TB | No TB | 0 | 0 | 0 | 0 | 14 | |
| 26_R | DS TB | 0 | 0 | 0 | 183 | 197 | |||
| 27_R | MDR-TB | 23 | 0 | 23 | 578 | 638 | |||
| 31_R | DS TB | No TB | 0 | 0 | 0 | 11 | 14 | ||
| 22_R | DS TB | 0 | 0 | 0 | 183 | 186 | |||
| 23_R | MDR-TB | 23 | 0 | 23 | 589 | 638 | |||
| 32_R | MDR-TB | No TB | 0 | 0 | 11 | 0 | 14 | ||
| 24_R | DS TB | 2 | 0 | 23 | 158 | 186 | |||
| 25_R | MDR-TB | 23 | 0 | 23 | 564 | 613 | |||
| 7_R | High | Positive | No TB | No TB | 0 | 0 | 2 | 0 | 14 |
| 33_R | DS TB | No TB | 0 | 11 | 2 | 0 | 14 | ||
| 1_R | DS TB | 0 | 14 | 2 | 167 | 186 | |||
| 2_R | MDR-TB | 0 | 25 | 91 | 519 | 638 | |||
| 34_R | MDR-TB | No TB | 0 | 0 | 13 | 0 | 14 | ||
| 3_R | DS TB | 0 | 0 | 27 | 156 | 186 | |||
| 4_R | MDR-TB | 0 | 0 | 91 | 519 | 613 | |||
| 14_R | Negative | No TB | No TB | 0 | 0 | 0 | 0 | 14 | |
| 12_R | DS TB | 0 | 0 | 14 | 169 | 197 | |||
| 13_R | MDR-TB | 23 | 0 | 23 | 564 | 624 | |||
| 29_R | DS TB | No TB | 0 | 0 | 0 | 11 | 14 | ||
| 8_R | DS TB | 0 | 0 | 0 | 183 | 186 | |||
| 9_R | MDR-TB | 23 | 0 | 23 | 589 | 638 | |||
| 30_R | MDR-TB | No TB | 0 | 0 | 11 | 0 | 14 | ||
| 10_R | DS TB | 2 | 0 | 23 | 158 | 186 | |||
| 11_R | MDR-TB | 23 | 0 | 23 | 564 | 613 | |||
The mean duration of infectivity (time to smear conversion) has been reported as 14 days from the start of effective therapy,108 and we assumed that patients with DS disease who have been admitted for isolation will be fit for discharge at this time. Patients with MDR-TB are likely to require longer isolation and inpatient care. Estimates of length of stay for patients with pulmonary MDR-TB were derived from an audit at a London hospital, with an estimated mean of 89 days [standard deviation (SD) 74 days] for 13 smear-positive cases and 46 days (SD 33 days) for seven smear-negative cases admitted between 2009 and 2013 (M Lipman, Royal Free Hospital, 2014, personal communication). The mean length of stay for these 20 patients (74 days) was similar to that reported for nine patients with MDR-TB treated at another London hospital between 1996 and 1999 (78 days). 115 Guidelines108 recommend that patients with or thought to be at high risk of MDR-TB are admitted to negative pressure isolation, and not released from isolation until confirmation that they are not infectious, based on two consecutive negative smear tests (with samples taken at least 1 week apart) and a negative culture test. For the model, we assumed that patients with smear-positive MDR-TB remain in a negative pressure room until discharge after 89 days, but that smear-negative MDR patients are transferred to a non-isolation bed (on average) half way through their 46-day stay. These assumptions are broadly consistent with data reported from an earlier audit: 449 negative pressure isolation days for 15 episodes (mean stay 30 days), of which 96 ‘correct’ days of negative pressure isolation were for the two true MDR-TB cases (mean 48 days). 52
The total duration of treatment is governed by the time to correct diagnosis, when patients start the correct treatment (if any), and the duration of that treatment. Tables 21 and 22 present timings for patients who complete treatment, but the transmission model also allowed for a proportion of patients to default from treatment during the outpatient period.
First, to consider the timings under the usual care (culture) strategy, see Table 21. Patients with smear-positive microscopy results are admitted because of the risk of transmission; as a precaution, those deemed to be at high risk of MDR-TB are admitted to a negative pressure isolation room, and those at low risk to a standard isolation room (a single room without negative pressure but vented to the outside of the building). 108 At day 14, admitted patients with a negative culture result for MTB (FPs) are discharged. The smear-positive patients with DS disease (who will have started an effective presumptive treatment regimen on day 1) remain in hospital for 14 days until they are no longer infectious. High-risk patients with smear-positive MDR-TB (who will also have started effective presumptive treatment on day 1) remain in negative pressure isolation until they are discharged after 89 days. However, low-risk, smear-positive MDR-TB patients (who will have been initially treated with an ineffective standard regimen) spend 27 days in standard isolation, until drug resistance is detected by culture. They then commence an effective MDR drug regimen and transfer to a negative pressure room, where they stay for a further 89 days until they are discharged.
Patients with smear-negative disease are a much lower transmission risk, and we assume that they are not initially admitted. Further, we assume that smear-negative patients with a positive culture test at 14 days remain at home, unless they are thought to be at high risk of MDR-TB. The smear-negative patients at high risk of MDR-TB with a positive culture are admitted to negative pressure isolation, discharged after 14 days if DST confirms that they have DS disease, but remain in hospital for a total of 46 days if MDR-TB is confirmed (23 further days in a negative pressure room and 23 in a non-isolation bed).
During outpatient follow-up, all patients were assumed to have one appointment per month at a consultant-led hospital clinic. Patients on a MDR drug regimen were assumed to have an additional face-to-face contact with a TB specialist nurse each month. This is an assumed average; some patients will not need both appointments each month, but other patients may require more frequent contact for monitoring of treatment adherence or of adverse reactions to medication.
Assumptions about the duration and location of treatment with rapid molecular testing are summarised in Table 22. Patients with a positive smear result were assumed to be admitted: those regarded as high risk of MDR to negative pressure isolation and those at low risk to a standard isolation room. If the molecular test at day 3 is negative, any admitted patients are discharged. Smear-positive patients for whom the molecular test indicates DS disease remain in hospital in a standard isolation room until those with a negative culture for TB are discharged at day 14, those with DS TB are discharged when no longer infectious after 14 days of treatment, and those with culture-confirmed MDR-TB transfer to a negative pressure room on day 28 and stay there for 89 days. Smear-positive patients with a molecular test indicating MDR-TB are transferred (if necessary) to a negative pressure room on day 3 and stay there until culture confirmation that they do not have MTB or that they do not have drug-resistant disease, or they remain in isolation until discharge after 89 days for true MDR cases. We assumed that patients with smear-negative microscopy would not be admitted for isolation but that they might be admitted for observation, insertion of a peripherally inserted central catheter or equivalent for treatment with injectable drugs, and/or for treatment of acute symptoms following a molecular test result indicating MDR-TB, and would remain in hospital until culture confirmation of their true status.
Examples of care pathways
The care pathways described above define the utilisation of health services (tests, medications, inpatient care and outpatient follow-up) and hence the costs incurred for the NHS. They also define the duration of patients’ exposure to TB symptoms and to adverse events of treatment, and hence to their QALY loss over the treatment period. Figures 15 and 16 illustrate how the costs and QALYs are incurred over the care pathway for a patient with smear-positive DS TB, but who is perceived in advance to be at high risk of MDR disease.
FIGURE 15.
Care pathway for high-risk patient with smear-positive DS TB, with culture only. +ve, positive; NP, negative pressure.
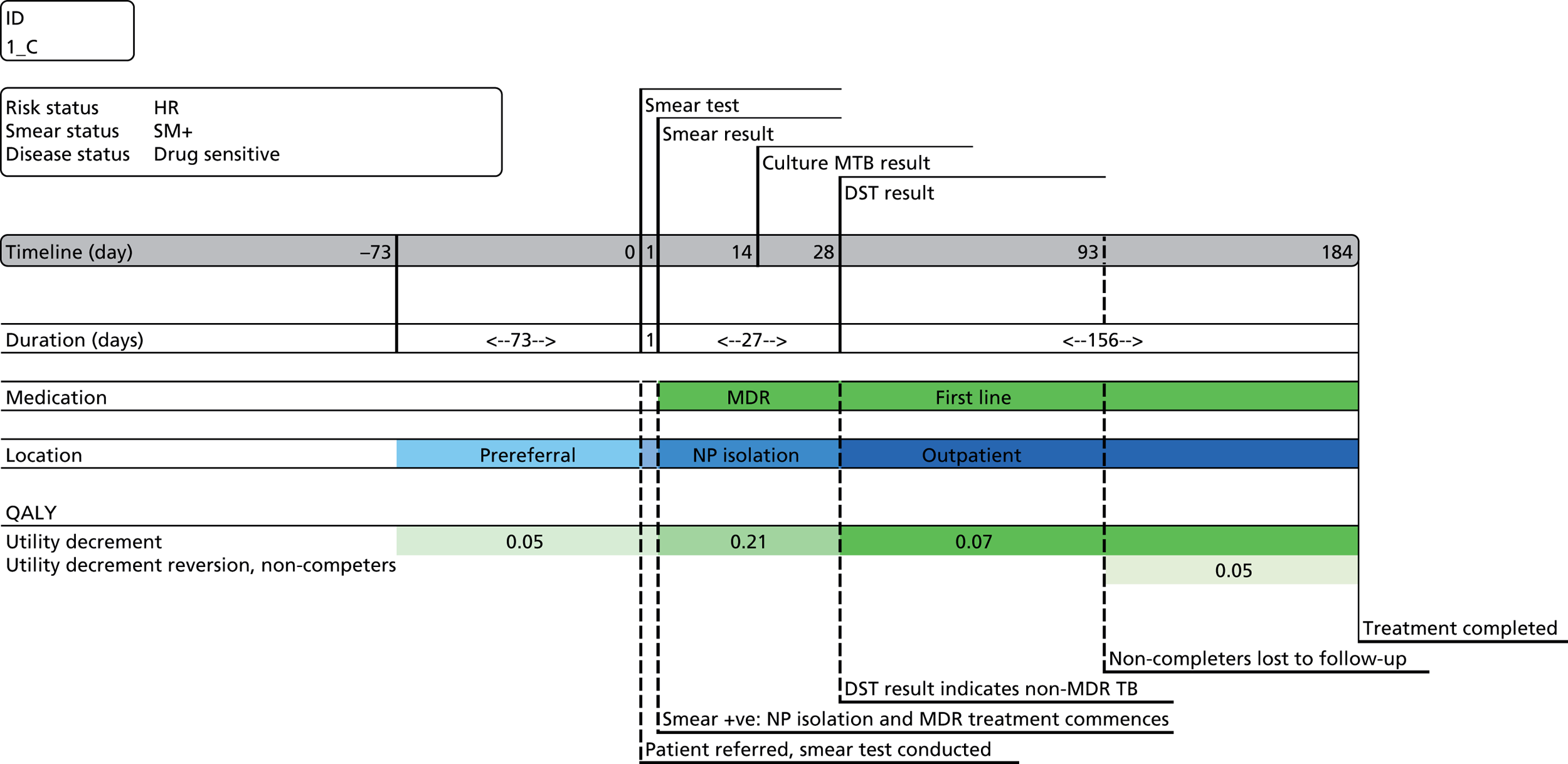
FIGURE 16.
Care pathway for high-risk patient with smear-positive DS TB, and correct molecular test result. +ve, positive; isol., isolation; NP, negative pressure.
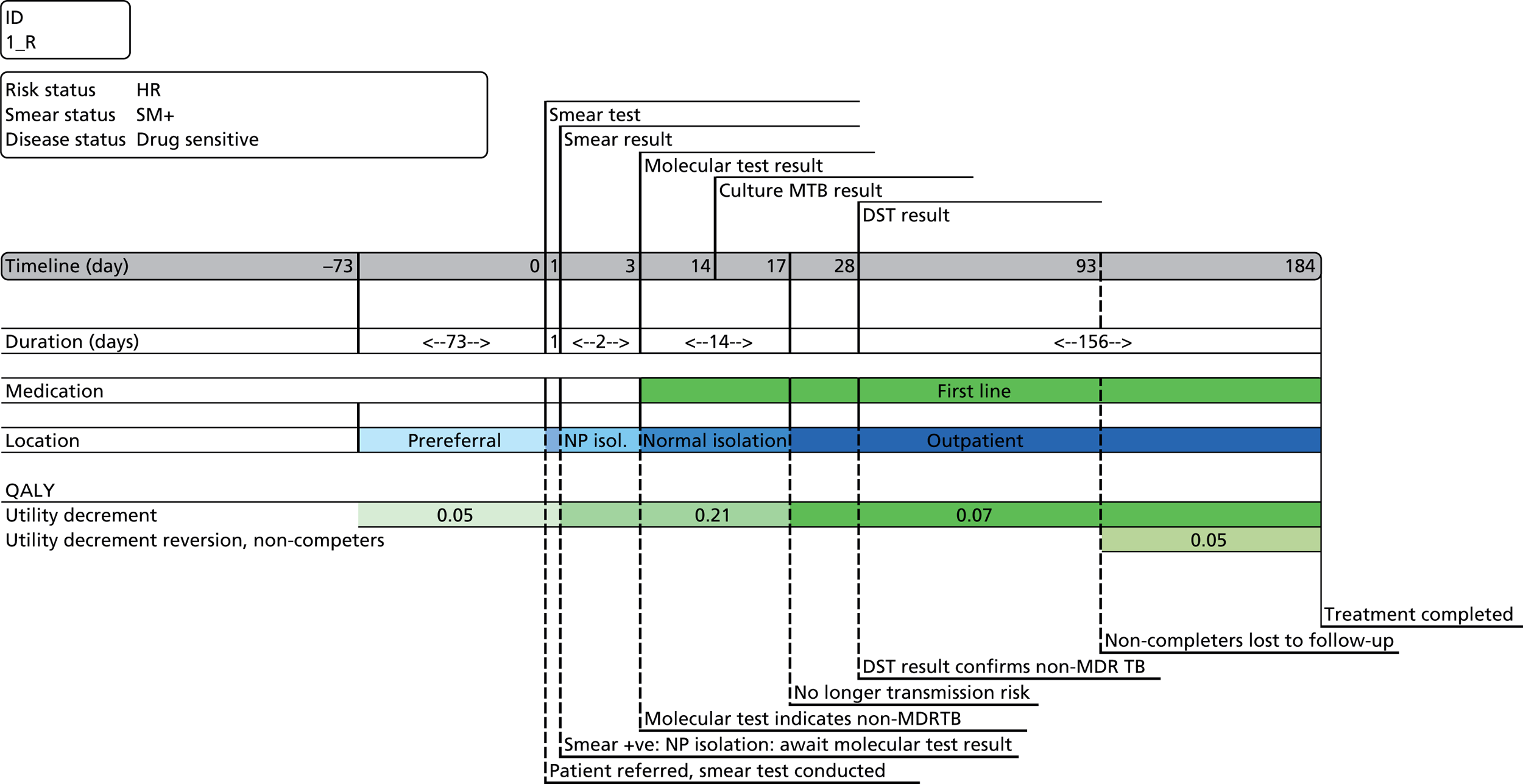
Figure 15 shows the expected results under the current diagnostic strategy (culture only). Owing to the perceived transmission risk, the patient is admitted to negative pressure isolation on presentation and remains there until DST results become available at day 28, after which he/she is discharged to outpatient follow-up. MDR medication is started on admission as a pre-emptive measure, but the patient is transferred to a standard regimen when culture confirms DS disease at day 28. The overall course of treatment lasts for 6 months (183 days), if completed. The patient has impaired quality of life (utility) as a result of developing symptoms during the prereferral period, more severe symptoms while in hospital, and then some continuing loss of quality of life after discharge while recovering. While on MDR treatment, the patient is at risk of additional side effects (particularly ototoxicity attributable to injectable agents).
Figure 16 shows how the treatment pathway, and associated costs and QALY loss, would be expected to change for this patient with a (correct) molecular test result on day 3. At this time, the patient would be released from negative pressure isolation to a normal isolation room and start a standard drug regimen. Both of these changes would save money for the NHS, and the patient would not be exposed to the risks of the MDR regimen, reducing the risks of toxicity. In this case, the length of hospital stay and total duration of the pathway would not change.
Method for estimating costs
Costs were estimated for each care pathway, including costs (1) of tests and consultations prior to referral for a TB diagnosis, (2) for tracing, testing and treating latent infection in contacts of each index case, (3) of tests during the diagnostic period, (4) of medication, (5) and for inpatient care and outpatient follow-up. The first two of these items (prereferral and public health control costs) were treated as a fixed (deterministic) cost per index case. The other costs differed between the pathways. All costs were estimated from a NHS perspective and were discounted to a present value at an annual rate of 3.5%.
An example of a cost calculation for one of the care pathways is shown in Table 23. This is for a smear-positive patient considered to be at high risk of MDR disease, but who in fact has DS disease and is treated under the current culture-based diagnostic strategy (as in Figure 15). The high cost for this pathway is driven by the (unnecessary) expense of negative pressure isolation, and MDR medications. In reality, only a small proportion of patients presenting with TB symptoms will be considered at such high risk of MDR-TB and incur this high cost.
| Item | Costs (£) |
|---|---|
| Prereferral | |
| Weighted average of GP, A&E and inpatient care costs prior to referral | 195 |
| Diagnostic | |
| Cost of culture and DST | 42 |
| Medication | |
| Presumptive treatment with MDR regimen: 27 days | 572 |
| Treatment on standard regimen once diagnosed with DS TB: 156 days | 140 |
| Permanent adverse effects of MDR treatment | 218 |
| Health care | |
| Time spent as inpatient in negative pressure isolation: 27 days | 30,400 |
| Time spent as inpatient in normal isolation room: 0 days | 0 |
| Time spent as inpatient not isolated: 0 days | 0 |
| Time spent as outpatient (includes follow-up visits, tests): 156 days | 675 |
| Contact tracing and treatment of latent TB infection (per index case) | 2035 |
| Total | 34,277 |
Method for estimating quality-adjusted life-year loss
The QALY loss associated with each pathway was also estimated. This was estimated relative to expected survival and quality of life (utility) for individuals of the same age but without TB and not undergoing diagnosis or treatment for TB. QALYs were discounted to present values using an annual rate of 3.5% per year. Three potential health impacts were considered: impairments to utility attributable to TB per se, utility impairments attributable to adverse effects of treatment, and the case fatality risk.
Tuberculosis status
Three stages of disease were distinguished for the purpose of estimating the QALY loss as a result of, TB symptoms: the pretreatment stage (between onset of symptoms and the start of the diagnostic period), the acute treatment stage (during hospital stay), and the postacute treatment stage (during outpatient treatment). The impact of TB morbidity was estimated, based on utility decrements for each period of time, subtracted from an age-weighted average utility level for a healthy individual. Each utility decrement was integrated over an appropriate time period to provide a total discounted QALY loss attributable to TB morbidity. The duration of the three periods differed between the various care pathways described (see Care pathways, above). Pathways with quicker, more accurate diagnosis are associated with lower QALY losses, as they have a shorter time to start (and hence to finish) effective treatment. Utility decrements for the three disease stages were sourced from Kruijshaar et al. 116
Adverse effects of treatment
Tuberculosis medications can have significant, and in some cases permanent, adverse effects that may also impact on quality of life. Isoniazid, rifampicin and pyrazinamide – part of the standard treatment regimen for drug-sensitive TB – are associated with various adverse effects, most notably that of hepatoxicity. 117 However, this is rare, so, for the purposes of this analysis, we assumed that first-line medication does not carry a risk of long-term permanent harm. Penalties for adverse effects of standard drug treatment are implicitly incorporated in the analysis in three ways. First, empirical estimates of the utility loss during TB treatment inevitably include some element attributable to (tolerated) side effects of treatment. Second, some patients who default from treatment are likely to do so because of (intolerable) side effects, and the transmission model incorporates health and cost penalties for patients who do not complete treatment. Third, costs of care while in hospital, and during follow-up, include costs for monitoring adverse effects.
Given the focus of this analysis on the accurate identification and treatment of patients with MDR-TB, it was important to reflect the additional risks of MDR medications. In particular, the aminoglycosides that make up part of the MDR treatment regimen carry a risk of ototoxicity and of long-term hearing damage. 118,119 We included an estimate of the lifetime QALY loss associated with permanent hearing loss, as a proxy to reflect the overall utility impact of permanent serious adverse effects of MDR treatment. The method that we used was based on an economic analysis by Veenstra et al. 120 to evaluate pharmacogenomic testing to prevent aminoglycoside-induced hearing loss in patients with cystic fibrosis. We updated their analysis, using recent estimates of adverse events rates associated with aminoglycosides in patients with TB and updated estimates of utility loss associated with hearing impairment. (Refer to Table 35 for further details.)
Mortality risk
In addition to the morbidity impacts described above, TB can still be a fatal disease and one might expect MDR-TB to carry a higher risk of fatality than DS disease. QALYs lost per TB case fatality were calculated from UK life tables, utility by age, and the age distribution of TB case fatalities. 112,121,122 The estimated QALY loss for TB mortality – approximately 20 QALYs per case fatality – was applied within the transmission model.
Table 24 shows an example of the QALY calculations. This relates to the same example illustrated in Figure 15 and Table 23. This individual spends 73 days prior to the start of the diagnostic period with active TB (0.01 QALYs lost), 27 days in the acute stage in hospital (0.015 QALYs lost) and 156 days under treatment as an outpatient (0.03 QALYs lost). In addition, they incur an expected loss of 0.228 QALYs as a result of side effects associated with 27 days of MDR treatment.
| Care pathway | Duration (days) | Utility decrement | QALY loss |
|---|---|---|---|
| Active TB untreated | 73 | 0.051 | 0.010 |
| Active acute TB on treatment | 27 | 0.206 | 0.015 |
| Active postacute TB on treatment | 156 | 0.067 | 0.029 |
| Time on MDR medication | 27 | 0.112 | 0.228 |
| Total lifetime QALYs lost | 0.282 |
Sources of parameter estimates
Probabilistic sensitivity analysis
Probabilistic sensitivity analysis was used to examine the impact of uncertainty over the input parameters to the transmission model. For each parameter subject to uncertainty, a probability distribution was defined and a set of 10,000 parameter values were generated by random (Monte Carlo) sampling in Excel. Separate streams of random numbers were set for each parameter to ‘seed’ them in such a way that when the distributions of some parameters were changed for alternative decision scenarios (described below), the values of other parameters would not change. This reduces unnecessary variation between scenarios. Matrices of PSA sample values were saved as text CSV (comma-separated values) files and provided to the mathematical modellers as inputs for the transmission model. Alongside the ‘economic data’ (cost and utility estimates) described in this chapter, samples of some other key input parameters were included in the PSA file, including the estimates of diagnostic accuracy obtained from the systematic review presented in Chapter 3.
Distributions were chosen to reflect the appropriate ranges for the parameters of interest. For example, beta distributions were used for probabilities and utility multipliers, as these can take on values of only between zero and one. Costs were sampled from a gamma distribution, as they must be > 1 and usually have a positive skew. Some parameters not subject to significant uncertainty were treated as fixed. For example, the unit costs of most medications are defined nationally, and, although there is some local variation in prices paid by NHS providers as a result of negotiated discounts, no data are available on these discounts. We therefore followed the NICE convention of using NHS list prices for medications. 123 Similarly, the costs of many NHS services (e.g. outpatient consultations and routine tests) are now set in a national tariff that is not subject to local negotiation, and so we treated these costs as deterministic. However, there were some large drivers of costs which were subject to considerable uncertainty: notably the duration of time spent in inpatient care and the cost of negative pressure isolation. These elements were sampled probabilistically. For sampling purposes, when no estimate of uncertainty was available, standard errors were set at 10% or 20% of the mean. For all elements estimated probabilistically, the distribution applied and standard error are reported with their mean values in the tables below.
Decision scenarios
Six sets of PSA samples were taken to reflect different combinations of assumptions over the location of molecular testing (centralised vs. localised) and over the proportion of patients being tested for TB who are judged by clinicians to be at high risk of MDR disease. The location of testing has potential impacts on the cost and timing of the diagnostic process. We assumed that microscopy and initial culture testing is currently conducted locally, but that DST for culture-positive cases is conducted only at specialist TB reference laboratories. These arrangements were assumed not to change with the introduction of molecular testing. Under the ‘centralised’ scenario, the equipment and consumables for molecular testing would be located at the TB reference laboratories and used efficiently, at maximum capacity. However, a cost would be incurred for transport of the sample to the reference laboratory for molecular testing – we assumed an average cost of £6.50 per sample. Under the ‘localised’ scenario, we assumed that molecular tests would be conducted at local laboratories, incurring no additional transport costs compared with current practice. However, we assumed that they would be operating below capacity for molecular tests, losing some economies of scale. Across England, there are 110 local TB laboratories, each processing on average 1030 tests per year. 112 We estimated that this would increase the mean cost per test by approximately £6 compared with centralisation of molecular tests. The net effect of transport and capacity on the cost per molecular test is therefore minimal. There is, however, a potential time delay associated with the transport of samples to a reference laboratory for molecular testing: we assumed 1 day extra, on average.
We produced PSA samples for centralised and localised locations of testing for each of the three populations modelled. For each population, we varied the proportion of patients with suspected TB who clinicians would judge to be at sufficiently high risk of MDR-TB to justify presumptive treatment: 2% for the Black African and South Asian populations and 25% for Eastern Europeans.
Test accuracy parameters
Estimates of the sensitivity and specificity of the rapid molecular tests for detecting drug sensitivity in patients with confirmed TB disease, compared with the reference standard of culture DST, have been presented in Chapter 3. However, many studies lacked the breakdown of diagnostic accuracy by smear status and by test for TB or drug susceptibility. This limited which studies could be used to inform the economic analysis.
It was important to differentiate diagnostic accuracy by patients’ smear status for the economic analysis, as this impacted on decisions about presumptive treatment and isolation. Smear status also had an important impact on transmission probability in the dynamic model (reported in the following chapter). Similarly, the care pathways and transmission risks also depended on the accuracy of molecular tests for early detection of TB disease.
The diagnostic accuracy parameters for the GeneXpert, MTBDRplus and INNO-LiPA assays are shown in Tables 25–27. These estimates were based on studies included in the systematic review. In addition, estimates of the sensitivity and specificity of GeneXpert assays for detection of TB (compared with culture as the reference standard) were obtained from Drobniewski et al. 124
| Parameter | Mean (standard error) | Distribution | Source |
|---|---|---|---|
| For diagnosis of TB | |||
| Smear positive | |||
| DOR | 4851 (2867.4) | Fixed | Drobniewski et al. (2013)124 |
| Sensitivity | 0.98 (0.003) | Beta | |
| Specificity | 0.99 (< 0.0005) | – | |
| Smear negative | |||
| DOR | 297 (175.55) | Fixed | Drobniewski et al. (2013)124 |
| Sensitivity | 0.75 (0.015) | Beta | |
| Specificity | 0.99 (< 0.0005) | – | |
| For diagnosis of drug sensitivity in people with confirmed TB | |||
| Smear positive | |||
| DOR | 810 (478) | Fixed | C Boehme, derived from Boehme et al. (2011)38 |
| Sensitivity | 0.964 (0.931–0.982) | Beta | |
| Specificity | 0.968 (0.950–0.980) | – | |
| Smear negative | |||
| DOR | 36443 | Fixed | C Boehme, derived from Boehme et al. (2011)38 |
| Sensitivity | 1.00 (0.871–1.00) | Fixed | |
| Specificity | 0.948 (0.915–0.969) | Beta | |
| Parameter | Mean (standard error) | Distribution | Source |
|---|---|---|---|
| For diagnosis of TB | |||
| Smear positive | |||
| DOR | 808 | Fixed | Crudu et al. (2012)69 |
| Sensitivity | 0.867 (0.0867) | Beta | |
| Specificity | 0.992 | – | |
| Smear negative | |||
| DOR | 251 | Fixed | Crudu et al. (2012)69 |
| Sensitivity | 0.803 (0.0803) | Beta | |
| Specificity | 0.984 | – | |
| For diagnosis of drug sensitivity in people with confirmed TB | |||
| Smear positive | |||
| DOR | 2766 (2031) | Fixed | From systematic review and meta-analysis (see Chapter 3) |
| Sensitivity | 0.9676 (0.0215) | Beta | |
| Specificity | 0.9893 (0.0038) | – | |
| Smear negative | |||
| DOR | 234 | Fixed | Crudu et al. (2012)69 |
| Sensitivity | 0.907 (0.0907) | Beta | |
| Specificity | 0.960 (0.096) | – | |
| Parameter | Mean (standard error) | Distribution | Source |
|---|---|---|---|
| For diagnosis of TB | |||
| Smear positive | |||
| DOR | 64.87 (38.34) | Fixed | Drobniewski et al. (2013)124 |
| Sensitivity | 0.93 (0.005) | Beta | |
| Specificity | 0.83 (0.010) | – | |
| Smear negative | |||
| DOR | 44.57 (26.35) | Fixed | Drobniewski et al. (2013)124 |
| Sensitivity | 0.65 (0.033) | Beta | |
| Specificity | 0.96 (0.008) | – | |
| For diagnosis of drug sensitivity in people with confirmed TB | |||
| Smear positive | |||
| DOR | 1739 (1569) | Fixed | From systematic review and meta-analysis (see Chapter 3) |
| Sensitivity | 0.9346 (0.023) | Beta | |
| Specificity | 0.9922 (0.007) | – | |
| Smear negative | |||
| DOR | 653 | Fixed | ECDC109 |
| Sensitivity | 0.8571 (0.6732–0.9588) | Beta | |
| Specificity | 0.9909 (0.9673–0.9986) | – | |
To account for uncertainty, the diagnostic accuracy results were sampled probabilistically. For sampling of the diagnostic accuracy, it is important to retain the correlations between the sensitivity and specificity parameters. To do this, we first calculated the DOR from the mean estimates of the sensitivity and specificity. The DOR is a summary measure of diagnostic accuracy, the ratio of the odds of a positive test result if the patient has the disease over the odds of a positive test result if the patient does not have the disease, and can be expressed as a function of the sensitivity (Se) and specificity (Sp).
We drew random samples for one of the accuracy parameters (sensitivity); as this is simply a proportion, it was sampled from a beta distribution. We then rearranged the equation above and used the DOR and the sampled sensitivity values to calculate the corresponding specificity values. This approach is likely to underestimate the overall uncertainty (as it does not allow for uncertainty over the DOR). Simultaneous sampling of correlated sensitivity and specificity parameters (e.g. with a Dirichlet distribution) would better account for uncertainty. However, the reported data in the literature lacked estimates of covariance or the absolute numbers of TP, TN, FP and FN results for each assay broken down by smear status.
For GeneXpert, we were able to obtain only one estimate of diagnostic accuracy for smear-negative patients. This estimate had a mean of 1.00, so we were unable to resample this value and retain this mean value in our PSA estimates. For this reason, we set the sensitivity as fixed and sampled the specificity directly from a beta distribution.
Unit costs for health-care resources
All costs were considered from a NHS perspective. Some items relate to one-off events, such as the cost of the rapid molecular test or the cost of the culture and DST. Others are time dependent, including the cost of medication, inpatient care (including isolation) and outpatient care. Where possible, unit costs were based on published national tariffs. Otherwise, estimates were derived from local information or from the literature.
Table 28 shows the unit costs for diagnostic tests. Costs for culture and rapid molecular assays were estimated, based on the costs of consumables, capital costs and overheads from the NMRL laboratory finance department. Capital costs were discounted over a period of 10 years and apportioned to each test, assuming that all equipment is used at full capacity. Labour costs of administering the assays were estimated based on the person-hours per test, estimated from a time-and-motion study at the NMRL (see Chapter 4). A transport cost of £6.50 per sample was included in the total cost of administering each assay, including culture. All costs have been inflated to 2011–12 levels. It has been assumed that the NMRL cost profile is indicative of the other reference laboratories in Newcastle and Birmingham. Regional variation in cost of labour and overheads has not been explored here, because of a lack of data.
| Resource item | Unit: 2011–12 £ | Mean, £ (standard error) | Distribution (PSA) | Source |
|---|---|---|---|---|
| Mantoux test | Per test | 1.30 | – | NICE108 |
| IGRA test | 56.24 | – | Pareek et al. (2013)125 | |
| Blood test | 3.05 | – | NHS reference costs126 | |
| Smear test | 1.56 | – | NHS reference costs126 | |
| Liver function test | 1.03 | – | NICE108 | |
| Chest radiography | 24.21 | – | Department of Health National Tariff 2013–14127 | |
| Culture diagnosis of TB | 22.29 (2.23) | Gamma | Local data | |
| Culture drug sensitivity | 13.64 (1.36) | Gamma | Local data | |
| GeneXpert @ regional laboratory | 63.15 (6.32) | Gamma | Local data | |
| MTBDRplus @ regional laboratory | 42.73 (4.27) | Gamma | Local data | |
| INNO-LiPA @ regional laboratory | 27.68 (2.77) | Gamma | Local data | |
| GeneXpert @ local laboratory | 68.53 (6.90) | Gamma | Local data | |
| MTBDRplus @ local laboratory | 45.16 (4.52) | Gamma | Local data | |
| INNO-LiPA @ local laboratory | 30.10 (3.01) | Gamma | Local data | |
| Cost of sample transport | 6.50 (0.65) | Gamma | Local data |
Unit costs for medications (Table 29) were sourced from the British National Formulary (BNF),128 with one exception: costs for prothionamide are not currently listed in the BNF, and so a mean cost was taken from three hospitals. The unit costs of other health services are listed in Table 30.
| Resource item | Unit: 2011–12 £ | Mean (£) | Source |
|---|---|---|---|
| Latent TB treatment | |||
| Isoniazid | Per month | 44.93 | BNF128 |
| Rifampicin | Per month | 24.61 | BNF128 |
| Pyridoxine (vitamin B6) 10-mg tablets | Per month | 0.52 | BNF128 |
| Standard front-line regimen (I, R, P) | |||
| Rifater®, Sanofi-Aventis (50 mg isoniazid/120 mg rifampicin/300 mg pyrazinamide) | Per day | 0.37 | BNF128 |
| Rifinah®-150, Sanofi-Aventis (100 mg isoniazid/150 mg rifampicin) | Per day | 0.50 | BNF128 |
| MDR regimen (seven-drug combination) | |||
| Amikacin | Per day | 2.09 | BNF128 |
| Moxifloxacin | Per day | 2.57 | BNF128 |
| Prothionamide | Per day | 3.26 | Average from three hospitals |
| Cycloserine | Per day | 8.31 | BNF128 |
| Ethambutol | Per day | 0.63 | BNF128 |
| Pyrazinamide | Per day | 4.32 | BNF128 |
| Pyridoxine | Per day | 0.02 | BNF128 |
| MDR regimen (seven drugs) | Per day | 21.20 | BNF128 |
Costs for general practice consultations were taken from the Personal Social Services Research Unit (PSSRU) estimates,129 and from the Department of Health reference costs for inpatient and outpatient care. 126 The costs of negative pressure isolation were uprated for inflation from published estimates,52 using the hospital and community services pay and price index. 129 Costs for standard isolation were estimated by the authors from the NHS reference costs for TB requiring non-elective inpatient stay. 126 The lower estimate of an excess bed-day was taken as the cost of a non-isolated inpatient-day and the average cost of a bed-day for pulmonary TB with complications was taken as an estimate of a day in isolation. The cost of hospital admissions was treated probabilistically. Details of the figures and distributions sampled are available in Table 30.
| Resource item | Unit: 2011/12 £ | Mean, £ (standard error) | Distribution (PSA) | Source |
|---|---|---|---|---|
| Outpatient visits | ||||
| GP | Per visit | 37 | – | PSSRU129 |
| A&E | Per visit | 126 | – | NHS reference costs126 |
| Outpatient consultant-led first attendance | Per visit | 179 | – | NHS reference costs126 |
| Outpatient consultant follow-up appointment | Per visit | 134 | – | NHS reference costs126 |
| TB specialist nurse: face-to-face follow-up | Per visit | 62 | – | NHS reference costs126 |
| TB specialist nurse: telephone call | Per call | 26 | – | NHS reference costs126 |
| Admissions | ||||
| Inpatient care non-isolated | Per day | 282 | Fixed | NHS reference costs126 |
| Incremental cost of standard isolation over normal inpatient care | Per day | 108 (110) | Gamma | |
| Standard isolation | Per day | 390 | – | NHS reference costs126 |
| Incremental cost of negative pressure isolation over standard isolation | Per day | 735 (74) | Gamma | |
| Negative pressure isolation | Per day | 1126 | – | Drobniewski et al. (2000)52 Adjusted to 2011/12 (£) |
Resource utilisation
Parameters on health-care utilisation per patient were sourced from literature, local audit, and, in some cases, expert clinical advice (Tables 31–33).
| Resource item | Units | Mean | Source |
|---|---|---|---|
| Via GP | % | 65 | Expert opinion |
| Via A&E | % | 30 | Expert opinion |
| Via inpatient referral | % | 5 | Expert opinion |
| A&E visits | n | 1 | Expert opinion |
| GP visits | n | 2 | Expert opinion |
| Chest radiographs | n | 1 | Expert opinion |
| Blood tests | n | 1 | Expert opinion |
| Resource item | Units | Mean | Source |
|---|---|---|---|
| Contact tracing (per contact screened) | |||
| Specialist nurse face-to-face contacts | n | 2 | Expert opinion |
| Specialist nurse phone call | n | 1 | Expert opinion |
| Mantoux screening tests | n | 1 | Expert opinion |
| IGRA screening tests | n | 0.5 | Expert opinion |
| Contacts tested for active TB (per index case) | n | 4.7 | Expert opinion |
| Treatment of LTBI in contacts | |||
| Contacts treated for LTBI per index case | n | 1.1 | Expert opinion |
| Prophylaxis % completing treatment | % | 0.85 | Expert opinion |
| Duration of treatment (completers) | Months | 3 | Expert opinion |
| Duration of treatment (non-completers) | Months | 1 | Expert opinion |
| Outpatient clinic visits per case treated | n | 3 | Expert opinion |
| TB nurse consultations per case treated | n | 2 | Expert opinion |
| Duration of isoniazid treatment | n | 3 | Expert opinion |
| Duration of rifampicin treatment | n | 3 | Expert opinion |
| Duration of vitamin B6 (pyridoxine) treatment | n | 3 | Expert opinion |
| Resource item | Units | Mean (standard error) | Distribution (PSA) | Source |
|---|---|---|---|---|
| Delay from first symptom to start of diagnostic process | Days | 73 (7.3) | Gamma (rounded) | Saldana et al. (2013)130 |
| Treatment duration | ||||
| DS TB (completers) | Days | 183 | Fixed | NICE108 |
| Multiplier for MDR-TB treatment duration as a function of DS TB treatment duration | Ratio | 0.3 (0.03) | Beta | |
| MDR-TB (completers) | Days | 610 | Estimated as (1/0.3)a DS treatment duration | WHO114 |
| Multiplier for non-completers treatment duration as a function of completers | Ratio | 0.5 (0.05) | Beta | |
| MDR-TB (non-completers) | Days | 305 | Estimated as 0.5 of Completers | Assumption |
| DS TB (non-completers) | Days | 92 | Assumption | |
| Inpatient length of stay | ||||
| MDR-TB smear negativea | Days | 46 (12) | Gamma | Marc Lipman, audit data, Royal Free Hospital 2014 |
| MDR-TB smear positivea | Days | 89 (21) | Gamma | |
| DS TB smear negative | Days | 0 | Assumption | |
| DS TB smear positive | Days | 14 (2.8) | Gamma | Assumption |
| Outpatient consultations | ||||
| Standard regimen: consultant | # per month | 1 | – | Expert opinion |
| MDR regimen: consultant | # per month | 1 | – | Expert opinion |
| MDR regimen: TB specialist nurse | # per month | 1 | – | Expert opinion |
Utility and quality-adjusted life-year loss
Estimates of parameters used to estimate QALY losses attributable to TB are shown in Table 34. Patients with TB were assumed to occupy three health states and attract a corresponding utility decrement while in that state: active TB pretreatment, active TB on treatment as inpatient, and active TB on treatment as an outpatient. The utility estimates applied are listed in Table 34. For each care pathway these utility estimates were integrated over the estimated time period spent in each health state to calculate a QALY loss attributable to TB.
| Health state | Units | Mean (standard error) | PSA | Source |
|---|---|---|---|---|
| Baseline utility | ||||
| Age weighted mean without TB | 0–1 | 0.880 | – | Health Survey for England 122 |
| Utility loss due to TB | ||||
| Active TB pretreatment | 0–1 | 0.051 (0.083) | Beta | Kruijshaar et al. (2010)116 |
| Active TB on treatment, inpatient | 0–1 | 0.210 (0.045) | Beta | Kruijshaar et al. (2010)116 |
| Active TB on treatment, outpatient | 0–1 | 0.067 (0.038) | Beta | Kruijshaar et al. (2010)116 |
| QALY loss attributable to TB case fatality | ||||
| Mean QALY loss per fatality | QALYs | 19.96 | – | Estimated by authors |
Parameter estimates used to estimate QALY loss attributable to MDR treatment are listed in Table 35. Based on available data we were able to estimate the serious adverse event rate for MDR treatment only at an average of 4 months on treatment, with 42% experiencing ototoxicity. 118 On any pathway for which a patient is on MDR treatment for < 4 months, the adverse event rate was adjusted pro rata, based on their time on treatment. On pathways for which a patient is on treatment for > 4 months, it was assumed that they would attract this maximum risk of adverse effects.
| Parameter | Units | Mean (standard error) | PSA | Source |
|---|---|---|---|---|
| Incidence of hearing impairment and utility loss | ||||
| % ototoxicity | % | 42 (4.2) | Beta | Törün et al. (2005)118 |
| Mild hearing loss | ||||
| % with mild hearing loss not requiring implant or aid | % | 50 | ||
| Utility loss | 0–1 | 0.05 | Author estimate | |
| Moderate hearing loss | ||||
| % requiring hearing aid | % | 25 | Veenstra et al. (2007)120 | |
| Utility loss with aid | 0–1 | 0.166 (0.01) | Beta | Barton et al. (2004)131 |
| Severe hearing loss | ||||
| % requiring cochlear implant | % | 25 | Veenstra et al. (2007)120 | |
| Utility loss with implant | 0–1 | 0.18 (0.04) | Beta | UK Cochlear Implant Study Group132 |
| QALY loss attributable to adverse events of MDR treatment | ||||
| Mean utility loss per patient experiencing hearing loss | 0–1 | 0.11 | ||
| Average life expectancy | Years | 42 | ||
| Discounted QALY loss per person treated with MDR medications for 4 months | QALYs | 1.03 | ||
| Costs (£) attributable to adverse effects of MDR treatment | ||||
| Lifetime cost of hearing aid (discounted) | 477 | Department of Health National Tariff127 | ||
| Lifetime cost of implant (discounted) | 8934 | Bond et al. (2009)133 For adult (age 50 years) with unilateral cochlear implant |
||
| Mean cost (£) per person treated with MDR medication | ||||
| Lifetime cost (discounted) | 983 | |||
| Equivalent annual cost (discounted) | 45 | |||
Following Veenstra et al.,120 we assumed that, of those patients who experienced a MDR treatment-related hearing impairment, 50% would have a ‘mild’ impairment not requiring any treatment, 25% would have a ‘moderate’ impairment requiring a hearing aid and 25% would have a ‘severe’ impairment that would be appropriate for cochlear implantation. Utility losses attributable to moderate and severe levels of impairment were sourced from the literature. There is good evidence for the validity and responsiveness of the Health Utilities Index Mark 3 (HUI3) as a measure of health-related quality of life in people with hearing impairment. 134 HUI3 utility estimates were obtained from a sample of 609 hearing-impaired adults assessed at four UK audiology clinics between April 2000 and October 2002,131 and for 311 ‘postlingually deafened’ adults before and after they received a cochlear implant. 132 To estimate the utility loss associated with hearing impairment per se, we subtracted the reported HUI3 scores (with treatment) from a population norm, based on a large general population sample (n = 4048) in the USA. 135 We assumed a utility loss owing to mild (untreated) hearing impairment of 0.05, in the absence of an empirical estimate. Lifetime discounted QALY loss attributable to hearing loss was then estimated, based on a mean life expectancy for individuals at TB diagnosis.
Lifetime discounted costs of treatment for treatment-related hearing loss were also estimated. Costs for hearing aids included costs for assessment, purchase of devices, fitting, follow-up, replacement (assumed once per 5 years), and maintenance (one repair per year). Audiology costs were taken from the Department of Health National Tariff. 127 Lifetime costs for patients receiving a cochlear implant were based on an estimate for adults receiving a unilateral implant taken from a published Health Technology Assessment report,133 and uprated for inflation. 129 This estimate included the cost of the implant, assessment, the procedure, treatment for complications, replacement and maintenance.
Summary of results
Mean estimates of the costs by pathway are shown in Table 36 for the culture-based diagnostic strategy and in Table 37 with the addition of a rapid molecular test (at reference laboratories). It can be seen that, with culture, the total estimated cost ranges from £2252 per patient for pathway 28_C (smear-negative patient correctly identified as free from TB) to £130,214 for pathway 18_C (smear-positive patients with MDR-TB not identified as high risk, and therefore not treated presumptively). The range is similarly wide with molecular testing: from a minimum of £2334 for pathways 14_R and 28_R (correctly diagnosed smear-negative patients without TB) to a maximum of £131,771 for 2_R (smear-positive patients with MDR-TB, who are thought to be at high risk and who have an inaccurate molecular test result indicating DS disease). It should be noted that the incidence of the very-high-cost pathways is likely to be low (as both MDR-TB and misdiagnoses are rare). The expected cost for a patient with smear-positive DS disease, deemed low risk of MDR-TB, correctly diagnosed by molecular test, and treated appropriately, is £9392. The cost for a similar patient, correctly diagnosed and treated under the culture strategy is slightly lower, at £8670.
| ID | Prereferral | Diagnosis | Non isolation | Standard isolation | Negative pressure | Outpatient | Standard drugs | MDR drugs | Adverse events | Contact tracing | Latent TB | Total cost (£) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7_C | 195 | 22.287 | 0 | 0 | 14,637 | 0 | 0 | 276 | 105 | 842 | 1193 | 17,270 |
| 21_C | 195 | 22.287 | 0 | 5036 | 0 | 0 | 12 | 0 | 0 | 842 | 1193 | 7300 |
| 14_C | 195 | 22.287 | 0 | 0 | 14,637 | 0 | 0 | 0 | 0 | 842 | 1193 | 16,889 |
| 28_C | 195 | 22.287 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 842 | 1193 | 2252 |
| 1_C | 195 | 42.429 | 0 | 0 | 30,400 | 675 | 140 | 572 | 218 | 842 | 1193 | 34,277 |
| 15_C | 195 | 42.429 | 0 | 5424 | 0 | 810 | 164 | 0 | 0 | 842 | 1193 | 8670 |
| 8_C | 195 | 42.429 | 1129 | 0 | 25,896 | 675 | 151 | 297 | 113 | 842 | 1193 | 30,534 |
| 22_C | 195 | 42.429 | 0 | 0 | 0 | 675 | 164 | 0 | 0 | 842 | 1193 | 3111 |
| 4_C | 195 | 42.429 | 0 | 0 | 100,207 | 3333 | 0 | 12,934 | 983 | 842 | 1193 | 119,730 |
| 18_C | 195 | 42.429 | 0 | 10,460 | 100,207 | 3333 | 24 | 12,934 | 983 | 842 | 1193 | 130,214 |
| 11_C | 195 | 42.429 | 6490 | 0 | 25,896 | 3529 | 0 | 12,934 | 983 | 842 | 1193 | 52,105 |
| 25_C | 195 | 42.429 | 6490 | 0 | 25,896 | 3725 | 13 | 12,934 | 983 | 842 | 1193 | 52,314 |
| ID | Prereferral | Diagnosis | Non isolation | Standard isolation | Negative pressure | Outpatient | Standard drugs | MDR drugs | Adverse events | Contact tracing | Latent TB | Total cost (£) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 7_R | 207 | 92 | 0 | 0 | 2252 | 0 | 0 | 0 | 0 | 842 | 1193 | 4586 |
| 33_R | 207 | 92 | 0 | 4261 | 2252 | 0 | 10 | 0 | 0 | 842 | 1193 | 8857 |
| 34_R | 207 | 92 | 0 | 0 | 14,637 | 0 | 0 | 233 | 89 | 842 | 1193 | 17,293 |
| 21_R | 207 | 92 | 0 | 775 | 0 | 0 | 0 | 0 | 0 | 842 | 1193 | 3109 |
| 35_R | 207 | 92 | 0 | 5036 | 0 | 0 | 10 | 0 | 0 | 842 | 1193 | 7380 |
| 36_R | 207 | 92 | 0 | 775 | 12,385 | 0 | 0 | 233 | 89 | 842 | 1193 | 15,816 |
| 14_R | 207 | 92 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 842 | 1193 | 2334 |
| 29_R | 207 | 92 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 842 | 1193 | 2344 |
| 30_R | 207 | 92 | 0 | 0 | 12,385 | 0 | 0 | 233 | 89 | 842 | 1193 | 15,041 |
| 28_R | 207 | 92 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 842 | 1193 | 2334 |
| 31_R | 207 | 92 | 0 | 0 | 0 | 0 | 10 | 0 | 0 | 842 | 1193 | 2344 |
| 32_R | 207 | 92 | 0 | 0 | 12,385 | 0 | 0 | 233 | 89 | 842 | 1193 | 15,041 |
| 1_R | 207 | 112 | 0 | 5424 | 2252 | 675 | 164 | 0 | 0 | 842 | 1193 | 10,869 |
| 3_R | 207 | 112 | 0 | 0 | 30,400 | 675 | 141 | 530 | 202 | 842 | 1193 | 34,302 |
| 15_R | 207 | 112 | 0 | 6198 | 0 | 675 | 164 | 0 | 0 | 842 | 1193 | 9392 |
| 17_R | 207 | 112 | 0 | 775 | 28,148 | 675 | 141 | 530 | 202 | 842 | 1193 | 32,825 |
| 8_R | 207 | 112 | 0 | 0 | 0 | 810 | 164 | 0 | 0 | 842 | 1193 | 3328 |
| 10_R | 207 | 112 | 564 | 0 | 25,896 | 675 | 141 | 530 | 202 | 842 | 1193 | 30,363 |
| 12_R | 207 | 112 | 0 | 0 | 15,763 | 675 | 151 | 297 | 113 | 842 | 1193 | 19,353 |
| 22_R | 207 | 112 | 0 | 0 | 0 | 810 | 164 | 0 | 0 | 842 | 1193 | 3328 |
| 24_R | 207 | 112 | 564 | 0 | 25,896 | 675 | 141 | 530 | 202 | 842 | 1193 | 30,363 |
| 26_R | 207 | 112 | 0 | 0 | 0 | 675 | 164 | 0 | 0 | 842 | 1193 | 3193 |
| 2_R | 207 | 112 | 0 | 9685 | 102,459 | 3333 | 22 | 12,934 | 983 | 842 | 1193 | 131,771 |
| 4_R | 207 | 112 | 0 | 0 | 102,459 | 3333 | 0 | 12,934 | 983 | 842 | 1193 | 122,064 |
| 16_R | 207 | 112 | 0 | 10,460 | 100,207 | 3333 | 22 | 12,934 | 983 | 842 | 1193 | 130,294 |
| 18_R | 207 | 112 | 0 | 775 | 100,207 | 3333 | 0 | 12,934 | 983 | 842 | 1193 | 120,587 |
| 9_R | 207 | 112 | 6490 | 0 | 25,896 | 3725 | 22 | 12,934 | 983 | 842 | 1193 | 52,406 |
| 11_R | 207 | 112 | 6490 | 0 | 25,896 | 3529 | 0 | 12,934 | 983 | 842 | 1193 | 52,187 |
| 13_R | 207 | 112 | 6490 | 0 | 25,896 | 3529 | 0 | 12,934 | 983 | 842 | 1193 | 52,187 |
| 23_R | 207 | 112 | 6490 | 0 | 25,896 | 3725 | 22 | 12,934 | 983 | 842 | 1193 | 52,406 |
| 25_R | 207 | 112 | 6490 | 0 | 25,896 | 3529 | 0 | 12,934 | 983 | 842 | 1193 | 52,187 |
| 27_R | 207 | 112 | 6490 | 0 | 25,896 | 3725 | 13 | 12,934 | 983 | 842 | 1193 | 52,396 |
Similarly, estimated mean QALY losses by pathway are shown in Tables 38 and 39 for the culture and molecular testing strategies. These range from 0 (for patients without TB who are correctly diagnosed and treated) to about 1.205 QALYs lost (for patients with smear-positive MDR-TB with delayed diagnosis and who are not treated presumptively). A large proportion of the estimated QALY loss is attributable to adverse events related to MDR treatment.
| ID | Active TB untreated | Active TB inpatient | Active TB outpatient | Adverse events | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days | Utility loss | QALY loss | Days | Utility loss | QALY loss | Days | Utility loss | QALY loss | Utility loss | QALY loss | Total QALY loss | |
| 7_C | 0 | 0.051 | 0.000 | 13 | 0.206 | 0.007 | 0 | 0.067 | 0.000 | 0.112 | 0.110 | 0.117 |
| 21_C | 0 | 0.051 | 0.000 | 13 | 0.206 | 0.007 | 0 | 0.067 | 0.000 | 0.112 | 0.000 | 0.007 |
| 14_C | 0 | 0.051 | 0.000 | 13 | 0.206 | 0.007 | 0 | 0.067 | 0.000 | 0.112 | 0.000 | 0.007 |
| 28_C | 0 | 0.051 | 0.000 | 0 | 0.206 | 0.000 | 0 | 0.067 | 0.000 | 0.112 | 0.000 | 0.000 |
| 1_C | 73 | 0.051 | 0.010 | 27 | 0.206 | 0.015 | 156 | 0.067 | 0.029 | 0.112 | 0.228 | 0.282 |
| 15_C | 73 | 0.051 | 0.010 | 14 | 0.206 | 0.008 | 169 | 0.067 | 0.031 | 0.112 | 0.000 | 0.049 |
| 8_C | 87 | 0.051 | 0.012 | 27 | 0.206 | 0.015 | 156 | 0.067 | 0.029 | 0.112 | 0.118 | 0.174 |
| 22_C | 87 | 0.051 | 0.012 | 0 | 0.206 | 0.000 | 183 | 0.067 | 0.034 | 0.112 | 0.000 | 0.046 |
| 4_C | 73 | 0.051 | 0.010 | 89 | 0.206 | 0.050 | 521 | 0.067 | 0.096 | 0.112 | 1.030 | 1.187 |
| 18_C | 73 | 0.051 | 0.010 | 116 | 0.206 | 0.065 | 521 | 0.067 | 0.096 | 0.112 | 1.030 | 1.202 |
| 11_C | 87 | 0.051 | 0.012 | 46 | 0.206 | 0.026 | 564 | 0.067 | 0.104 | 0.112 | 1.030 | 1.172 |
| 25_C | 101 | 0.051 | 0.014 | 46 | 0.206 | 0.026 | 578 | 0.067 | 0.107 | 0.112 | 1.030 | 1.177 |
| ID | Active TB untreated | Active TB inpatient | Active TB outpatient | Adverse events | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days | Utility loss | QALY loss | Days | Utility loss | QALY loss | Days | Utility loss | QALY loss | Utility loss | QALY loss | Total QALY loss | |
| 7_R | 0 | 0.051 | 0.000 | 2 | 0.206 | 0.001 | 0 | 0.067 | 0.000 | 0.112 | 0.000 | 0.001 |
| 33_R | 0 | 0.051 | 0.000 | 13 | 0.206 | 0.007 | 0 | 0.067 | 0.000 | 0.112 | 0.000 | 0.007 |
| 34_R | 0 | 0.051 | 0.000 | 13 | 0.206 | 0.007 | 0 | 0.067 | 0.000 | 0.112 | 0.093 | 0.100 |
| 21_R | 0 | 0.051 | 0.000 | 2 | 0.206 | 0.001 | 0 | 0.067 | 0.000 | 0.112 | 0.000 | 0.001 |
| 35_R | 0 | 0.051 | 0.000 | 13 | 0.206 | 0.007 | 0 | 0.067 | 0.000 | 0.112 | 0.000 | 0.007 |
| 36_R | 0 | 0.051 | 0.000 | 13 | 0.206 | 0.007 | 0 | 0.067 | 0.000 | 0.112 | 0.093 | 0.100 |
| 14_R | 0 | 0.051 | 0.000 | 0 | 0.206 | 0.000 | 0 | 0.067 | 0.000 | 0.112 | 0.000 | 0.000 |
| 29_R | 0 | 0.051 | 0.000 | 0 | 0.206 | 0.000 | 11 | 0.067 | 0.002 | 0.112 | 0.000 | 0.002 |
| 30_R | 0 | 0.051 | 0.000 | 11 | 0.206 | 0.006 | 0 | 0.067 | 0.000 | 0.112 | 0.093 | 0.099 |
| 28_R | 0 | 0.051 | 0.000 | 0 | 0.206 | 0.000 | 0 | 0.067 | 0.000 | 0.112 | 0.000 | 0.000 |
| 31_R | 0 | 0.051 | 0.000 | 0 | 0.206 | 0.000 | 11 | 0.067 | 0.002 | 0.112 | 0.000 | 0.002 |
| 32_R | 0 | 0.051 | 0.000 | 11 | 0.206 | 0.006 | 0 | 0.067 | 0.000 | 0.112 | 0.093 | 0.099 |
| 1_R | 76 | 0.051 | 0.011 | 16 | 0.206 | 0.009 | 167 | 0.067 | 0.031 | 0.112 | 0.000 | 0.050 |
| 3_R | 76 | 0.051 | 0.011 | 27 | 0.206 | 0.015 | 156 | 0.067 | 0.029 | 0.112 | 0.211 | 0.266 |
| 15_R | 76 | 0.051 | 0.011 | 16 | 0.206 | 0.009 | 167 | 0.067 | 0.031 | 0.112 | 0.000 | 0.050 |
| 17_R | 76 | 0.051 | 0.011 | 27 | 0.206 | 0.015 | 156 | 0.067 | 0.029 | 0.112 | 0.211 | 0.266 |
| 8_R | 76 | 0.051 | 0.011 | 0 | 0.206 | 0.000 | 183 | 0.067 | 0.034 | 0.112 | 0.000 | 0.044 |
| 10_R | 76 | 0.051 | 0.011 | 25 | 0.206 | 0.014 | 158 | 0.067 | 0.029 | 0.112 | 0.211 | 0.265 |
| 12_R | 87 | 0.051 | 0.012 | 14 | 0.206 | 0.008 | 169 | 0.067 | 0.031 | 0.112 | 0.118 | 0.169 |
| 22_R | 76 | 0.051 | 0.011 | 0 | 0.206 | 0.000 | 183 | 0.067 | 0.034 | 0.112 | 0.000 | 0.044 |
| 24_R | 76 | 0.051 | 0.011 | 25 | 0.206 | 0.014 | 158 | 0.067 | 0.029 | 0.112 | 0.211 | 0.265 |
| 26_R | 87 | 0.051 | 0.012 | 0 | 0.206 | 0.000 | 183 | 0.067 | 0.034 | 0.112 | 0.000 | 0.046 |
| 2_R | 101 | 0.051 | 0.014 | 116 | 0.206 | 0.065 | 519 | 0.067 | 0.096 | 0.112 | 1.030 | 1.205 |
| 4_R | 76 | 0.051 | 0.011 | 91 | 0.206 | 0.051 | 519 | 0.067 | 0.096 | 0.112 | 1.030 | 1.188 |
| 16_R | 101 | 0.051 | 0.014 | 116 | 0.206 | 0.065 | 519 | 0.067 | 0.096 | 0.112 | 1.030 | 1.205 |
| 18_R | 76 | 0.051 | 0.011 | 91 | 0.206 | 0.051 | 519 | 0.067 | 0.096 | 0.112 | 1.030 | 1.188 |
| 9_R | 101 | 0.051 | 0.014 | 46 | 0.206 | 0.026 | 589 | 0.067 | 0.109 | 0.112 | 1.030 | 1.179 |
| 11_R | 76 | 0.051 | 0.011 | 46 | 0.206 | 0.026 | 564 | 0.067 | 0.104 | 0.112 | 1.030 | 1.171 |
| 13_R | 87 | 0.051 | 0.012 | 46 | 0.206 | 0.026 | 564 | 0.067 | 0.104 | 0.112 | 1.030 | 1.172 |
| 23_R | 101 | 0.051 | 0.014 | 46 | 0.206 | 0.026 | 589 | 0.067 | 0.109 | 0.112 | 1.030 | 1.179 |
| 25_R | 76 | 0.051 | 0.011 | 46 | 0.206 | 0.026 | 564 | 0.067 | 0.104 | 0.112 | 1.030 | 1.171 |
| 27_R | 101 | 0.051 | 0.014 | 46 | 0.206 | 0.026 | 578 | 0.067 | 0.107 | 0.112 | 1.030 | 1.177 |
Chapter 6 Dynamic transmission model
Overview
An integrated transmission-dynamic and economic model was used to evaluate the cost-effectiveness of introducing rapid molecular testing for TB infection and DST in England and Wales, including accounting for effects of faster diagnosis and treatment in averting transmission of infection. Three population groups are considered: South Asians, Black Africans and Eastern Europeans. The first two groups represent a large proportion (61%) of the TB cases in England and Wales and the available data allow us to estimate rates of transmission in England and Wales, which allows us to estimate numbers of transmission events expected to be averted by faster diagnosis and treatment of TB and MDR-TB. The Eastern European group is of interest because of its high proportion of TB cases that are MDR (≈27%), which means that faster DST results offered by molecular testing are potentially a key benefit. However, the available data do not allow us to estimate rates of transmission from this group.
Population
The evaluation considers ethnic groups in England and Wales, that is Black African, South Asian and Eastern European. Each ethnic group is modelled separately. Within each group, the model distinguishes those who are UK born and those who were born overseas, with these subgroups assumed to mix homogeneously.
We assume that UK-born and non-UK-born individuals from the same ethnic population mix homogeneously, with equal chance of acquiring and passing on TB infection. We also assume that they can all potentially benefit from the intervention and that within the population group there is equal access to services. The model population is also assumed to remain constant in size over time in the current practice scenario. Rates of population turnover (i.e. birth rate, immigration rate and death rate owing to non-TB causes) are assumed to be constant over time. A reduction in TB deaths would result in an increase in population size. The rate of patients presenting to care is assumed not to change over time. The rates of entry and exit for the different ethnic populations were estimated from the Office for National Statistics (ONS) data on new arrivals into the country and birth rates were calculated from the number of children between 0 and 4 years of age per ethnic group. The data sources are cited in the parameter table (Table 40).
| Ethnicity | Region of birth | Population size | Birth rate in England and Wales) per year | Immigration rate per year |
|---|---|---|---|---|
| South Asian (i.e. Indian, Pakistani, Bangladeshi) | UK | 1,469,558 | 0.038 p.a. | – |
| Foreign | 1,515,112 | – | 0.07 p.a. | |
| Black African | UK | 323,276 | 0.067 p.a. | – |
| Foreign | 666,352 | – | 0.04–0.07 p.a. | |
| Eastern European | UK | Not available | Not available | – |
| Foreign | 1,114,368 | – | 0.11 p.a. |
Transmission-dynamic model structure and assumptions
The model used is a compartmental (state transition) model of the type described by Anderson and May,136 which is a well-established modelling method that has been applied successfully to TB, as well as many other infectious diseases. This approach divides the population up according to infection status (i.e. naive, latent infection, active disease, on treatment, recovered, etc.), each contained in a separate compartment. There are flows between compartments as individuals become infected, progress to disease, are diagnosed and placed on treatment, etc. The rates of flow depend upon per-capita rates and the number of individuals in the relevant compartment at the particular point in time. The compartmental structure is based on that of Salomon et al.,137 which was built on the work of Vynnycky and Fine138 and Dye et al.,139 with the modification of inserting a preclinical disease stage between latent infection and active disease. Radiological abnormalities often precede clinical signs and symptoms, so these individuals’ disease is detectable by radiography but is not yet clinically detectable (or infectious). Individuals in the active disease compartment are detectable – with variable sensitivity and specificity – by different diagnostic methods including radiography, sputum smear microscopy, molecular testing and laboratory-based culture confirmation of TB infection and determination of drug susceptibility.
The compartmental model is used to describe the infection and treatment dynamics for both non-MDR and MDR-TB strains. Those who acquire TB infection develop latent infection, which is either slow progressing or fast progressing. Slow progressors are at risk of exogenous reinfection, which causes fast-progressing latent infection. Progression from latent infection leads to preclinical disease (detectable by radiography but without clinically detectable signs or symptoms), followed by active disease. Active disease may be smear negative or smear positive, with the latter being much more infectious.
In the absence of molecular testing – the ‘current practice’ scenario – those with smear-positive active disease, who access health care, are diagnosed rapidly (based on sputum smear microscopy) and rapidly placed on non-MDR-TB treatment. Meanwhile, samples are sent for culture confirmation of TB and DST. The result of the DST is used to correct the treatment regimen if MDR-TB is identified. Those with smear-negative active disease, who access health care, wait for culture confirmation of TB before being placed on treatment.
Molecular testing offers faster confirmation of TB infection and MDR status. We assume that molecular testing is used in addition to culture confirmation and DST. In the molecular testing scenarios, case-finding and treatment remain unchanged, and a rapid molecular test is used for TB confirmation and DST. This reduces the delay between sample collection and obtaining TB confirmation and the DST result back, but culture-based confirmation and DST are still performed as the gold standard. Three different molecular tests are considered: (1) GeneXpert only, (2) GeneXpert combined with INNO-LiPA line-probe assay, and (3) MTBDRplus, in two locations: either local to the hospital or in a regional laboratory. Locally based testing is quicker and has a very marginally lower cost per test than regionally based testing. Reductions in delays in TB diagnosis may reduce transmission of TB and/or reduce costs of precautionary isolation.
A proportion of those starting treatment are destined to complete treatment successfully and the remainder are destined to fail treatment. In the model, those undergoing successful treatment are non-infectious and, upon completion, they enter the recovered compartment and have reduced susceptibility to subsequent infection. Those who are destined to fail treatment as a result of poor adherence to treatment return to the compartment from which they commenced treatment after failure and resume infectiousness. MDR-TB-infected individuals placed on non-MDR-TB treatment remain infectious throughout the duration of this treatment. Those with untreated and treated active disease are subject to additional TB-associated mortality.
Model flow diagrams
For clarity, mortality and entry into (and exit from) the population are omitted from the flow diagram (Figure 17). Infectious compartments are shown in green. There are multiple compartments for people who are on treatment, so that if treatment fails the person they can be returned to the compartment from where they started treatment. People who will fail treatment are placed in separate compartments from those who will complete treatment because the time to failure is usually quite short, whereas the time to successful completion is much longer.
FIGURE 17.
Model flow diagrams. Movement between health states (boxes) is shown by arrows. Boxes with green text represent health states in which individuals can transmit TB. Three different molecular tests are considered: GeneXpert only, GeneXpert combined with INNO-LiPA line-probe assay, and MTBDRplus. (a) Non-MDR-TB infected and culture-DST; (b) MDR-TB infected and culture-DST; (c) non-MDR-TB infected and rapid molecular test; and (d) MDR-TB infected and rapid molecular test.


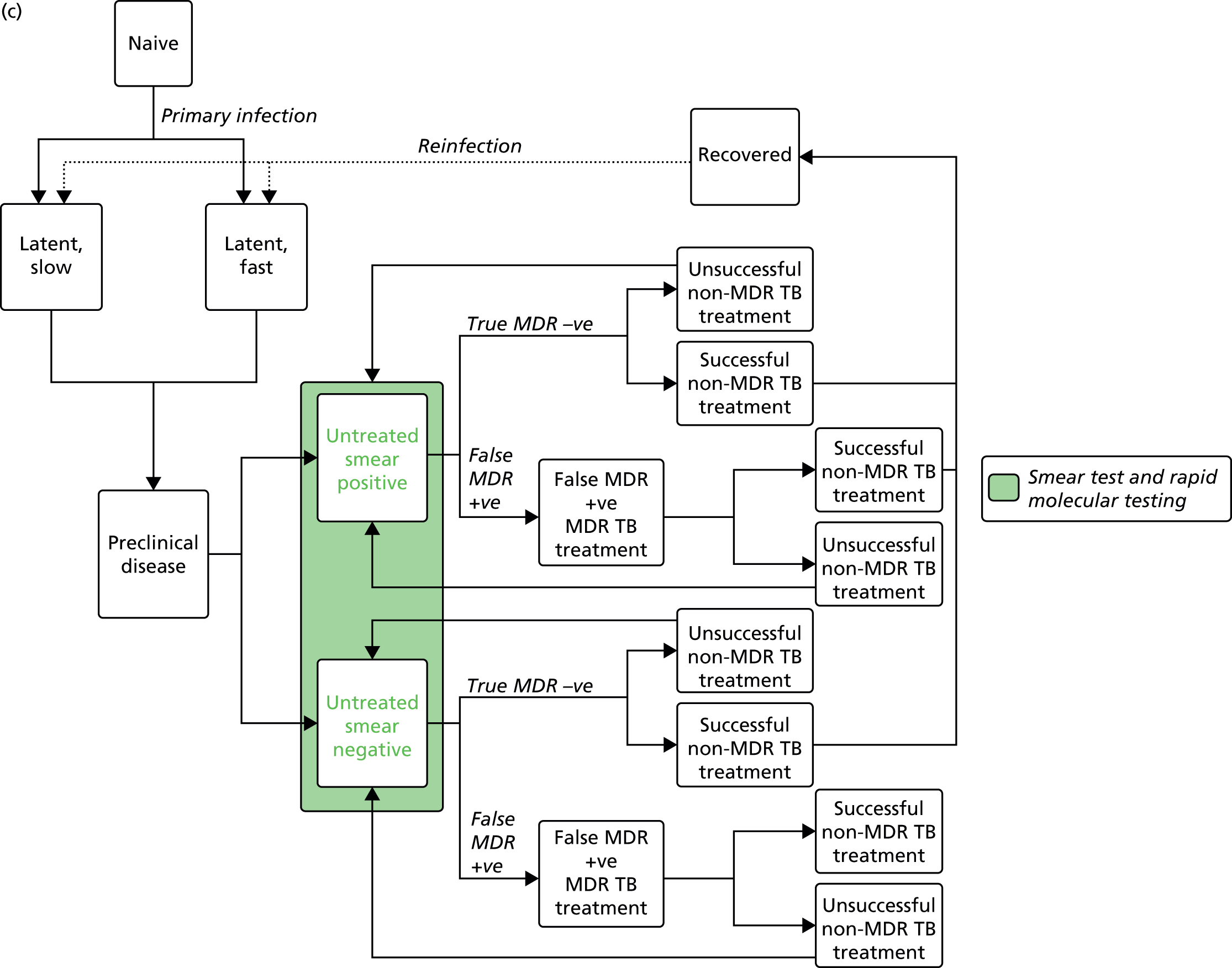

Data sources
Epidemiological parameter estimates and data sources are summarised in Tables 40 and 41. Where UK-specific data were not available, parameter estimates from the literature were used. The ethnic group population sizes, rates of birth and immigration were estimated from the ONS 2011 data. 140 Baseline TB incidence and proportion of MDR-TB cases were obtained from Enhanced Tuberculosis Surveillance (ETS) data (2009–11). 141 Economic parameters are reported in Chapter 5.
Transmission model parameter values
| Parameter description | Value | Source/reference |
|---|---|---|
| TB natural history | ||
| Proportion of incident infections that are slow progressing | 0.86 | Salomon et al. (2006)137 |
| Per-capita rate of slow progression to preclinical disease | 1.13 × 10–4 p.a. | Salomon et al. (2006)137 |
| Per-capita rate of fast progression to preclinical disease | 0.995 p.a. | Based on Salomon et al. (2006)137 (0.88 p.a.) but adjusted for insertion of preclinical disease state into natural history |
| Per-capita rate of progression from preclinical disease to active disease | 7.6 p.a. | Abubakar et al. (2011)142 |
| Proportion of new disease that is smear positive | 0.45 | Salomon et al. (2006)137 |
| Per-capita mortality rate of untreated active disease | 0.23 p.a. | National Tuberculosis Institute [India]143 |
| Per-capita rate of conversion from smear negative to smear positive | 0.015 p.a. | Salomon et al. (2006)137 |
| Per-capita rate of self-cure: natural reversion from active disease to latent infection | 0.21 p.a. | National Tuberculosis Institute [India]143 |
| Relative transmissibility of MDR-TB compared with non-MDR-TB | Fitted | – |
| Prevalence of TB among new migrants | Fitted | – |
| Proportion of MDR-TB among TB-infected new migrants | Fitted | – |
| Screening and treatment | ||
| Average duration from active TB to seeking care | 73 days | Saldana et al. (2013)130 |
| Proportion of those tested that have laboratory-confirmed TB | 0.05 | Assumption |
| Proportion non-MDR-TB treated successfully | 0.84 | Health Protection Agency141 |
| Proportion MDR-TB treated successfully | 0.80 | Health Protection Agency141 |
| Mean duration of successful non-MDR treatment | 0.5 years (6 months) | Recommended course of treatment for non-MDR disease: NICE108 |
| Mean duration of unsuccessful non-MDR treatment | 0.25 years (3 months) | Assumption |
| Mean duration of successful MDR treatment | 1.67 years (20 months) | This study |
| Mean duration of unsuccessful MDR treatment | 0.83 years (10 months) | This study |
| Per-capita mortality rate of unsuccessfully treated disease | 0.077 p.a. | Salomon et al. (2006)137 |
| Culture and DST turnaround time | 28 days | Assumption |
| Rapid molecular test turnaround time | 3.5 days | Assumption |
| Transmission | ||
| Transmission parameter for smear-positives | Fitted | – |
| Relative infectivity of smear-negatives (vs. smear-positives) | 0.25 | Abu-Raddad et al. (2009)144 |
| Relative susceptibility of latent (slow) and recovered individuals (vs. those susceptible) | 0.35 | Salomon et al. (2006);137 Abu-Raddad et al. (2009)144 |
| Relative infectivity of unsuccessfully treated (vs. untreated) | 0.25 | Salomon et al. (2006)137 |
Analysis
All analysis of the model was done for all three ethnic groups considered in this report. Time and resource constraints meant that not all possible sensitivity analyses could be performed.
Probabilistic sensitivity analysis
Probabilistic sensitivity analysis was performed to evaluate the impact on cost-effectiveness of diagnostic and treatment time delays, diagnosis costs and treatment costs, and associated QALYs. We ran the model simulation with 10,000 parameter sets and report the resulting incremental costs and incremental QALYs.
Deterministic sensitivity analysis
Parameters relating to diagnostic test performance were subject to deterministic sensitivity analysis, in which each parameter was varied individually across its range. Results are presented in tornado plots.
Results
Analyses are presented for South Asians, Black Africans and East Europeans, in this order.
South Asians
There is a marginal effect of introduction of molecular testing on annual numbers of diagnoses (Figure 18) owing to a small reduction in transmission and an increase in FP results. In the INNO-LiPA and MTBDRplus scenarios there are increases in diagnoses owing to the latter effect dominating, whereas in the GeneXpert scenario the former dominates. Local molecular testing has a faster turnaround time than regional molecular testing, so the annual number of diagnoses in the local testing configuration is slightly lower than in the regional testing configuration. However, this effect is tiny, with < 1% fewer diagnoses annually.
FIGURE 18.
Effects of introducing molecular testing on annual numbers of TB diagnoses in the South Asian population. Annual numbers of TB diagnoses in South Asians following the introduction of different rapid molecular tests. Three different molecular tests are considered (GeneXpert only, GeneXpert combined with INNO-LiPA line-probe assay and MTBDRplus) in two locations: either local to the hospital or in a regional laboratory. Note that the vertical axis does not start at zero and the magnitude of the changes is, in fact, small.
There is a significant incremental net benefit (INB) in the GeneXpert scenario, MTBDRplus scenario and INNO-LiPA scenario (Table 42 and Figure 19). These molecular tests reduced net costs and increased QALYs as more individuals were appropriately treated for TB. As the options are cost-saving, results are presented as an INB rather than using incremental cost-effectiveness ratios (ICERs).
| Scenario | Total costs, £M (95% CI) | Total QALYs (95% CI) | Compared with baseline | INB, £M (95% CI) | ||
|---|---|---|---|---|---|---|
| Incremental cost, £M (95% CI) | Incremental QALYs (95% CI) | QALY = £20,000 | QALY = £30,000 | |||
| Current | 35.0 (33.1 to 40.2) | 22,626,063 (22,626,022 to 22,626,090) | – | – | – | – |
| GeneXpert | ||||||
| GeneXpert local | 17.7 (17.6 to 17.8) | 22,626,209 (22,626,207 to 22,626,210) | –17.4 (–17.5 to –17.3) | 148.3 (147.5 to 149.1) | 20.3 (20.3 to 20.4) | 21.8 (21.7 to 21.9) |
| GeneXpert regional | 18 (17.9 to 18.1) | 22,626,212 (22,626,210 to 22,626,213) | –17.1 (–17.2 to –17.0) | 151.3 (150.5 to 152.0) | 20.2 (20.1 to 20.2) | 21.7 (21.6 to 21.8) |
| MTBDRplus | ||||||
| MTBDRplus local | 18.6 (18.5 to 18.7) | 22,626,264 (22,626,260 to 22,626,267) | –16.5 (–16.6 to –16.4) | 203.4 (200.3 to 206.5) | 20.5 (20.4 to 20.6) | 22.6 (22.5 to 22.7) |
| MTBDRplus regional | 18.9 (18.8 to 19.0) | 22,626,266 (22,626,262 to 22,626,269) | –16.2 (–16.3 to –16.1) | 205.3 (202.3 to 208.3) | 20.3 (20.2 to 20.4) | 22.3 (22.2 to 22.4) |
| INNO-LiPA | ||||||
| INNO-LiPA local | 20 (19.9 to 20.0) | 22,626,178 (22,626,176 to 22,6261,79) | –15.1 (–15.2 to –15.1) | 117.7 (116.9 to 118.4) | 17.5 (17.4 to 17.6) | 18.7 (18.6 to 18.7) |
| INNO-LiPA regional | 20.2 (20.1 to 20.3) | 22,626,183 (22,626,181 to 22,626,184) | –14.9 (–15.0 to –14.8) | 123.5 (122.8 to 124.3) | 17.4 (17.3 to 17.5) | 18.6 (18.5 to 18.7) |
FIGURE 19.
Cost-effectiveness plane of different molecular testing interventions compared with current practice in South Asians, over a 10-year time horizon. This figure shows the cost-effectiveness plane resulting from comparing the effect of different rapid molecular tests for TB confirmation and DST with the current practice in South Asians. Three different molecular tests are considered (GeneXpert only, GeneXpert combined with INNO-LiPA line-probe assay and MTBDRplus) in two locations: either local to the hospital or in a regional laboratory. The solid diagonal line indicates the threshold of £30,000 per QALY and the dashed line £20,000 per QALY.
For all tests (GeneXpert scenario, MTBDRplus scenario and INNO-LiPA) the INB for local and regional testing are very similar (see Table 42 and Figure 19). GeneXpert and MTBDRplus scenarios have similar INBs, which are higher than the INNO-LiPA scenario. INNO-LiPA has a higher FP rate, leading to more inappropriate treatment of patients, increasing costs and reducing QALYs. It also has the lowest sensitivity for detection of smear-negative TB cases, leading to fewer TB diagnoses and treatment, further reducing the number of QALYs gained. When comparing over a time horizon of 20 years rather than 10 years, the INBs are greater but the overall picture is broadly similar, although MTBDRplus appears to be relatively more cost-effective than the other two rapid assays (Figure 20 and Table 43).
| Scenario | Total costs, £M (95% CI) | Total QALYs (95% CI) | Compared with baseline | INB, £M (95% CI) | ||
|---|---|---|---|---|---|---|
| Incremental cost, £M (95% CI) | Incremental QALYs (95% CI) | QALY = £20,000 | QALY = £30,000 | |||
| Current | 59.8 (51.6 to 75.8) | 38,666,105 (38,666,033 to 38,666,151) | – | – | – | – |
| GeneXpert | ||||||
| GeneXpert local | 30.3 (30.1 to 30.4) | 38,666,421 (38,666,397.3 to 38,666,444.0) | –29.8 (–29.9 to –29.6) | 282.9 (280.2 to 285.7) | 35.4 (35.3 to 35.6) | 38.3 (38.1 to 38.4) |
| GeneXpert regional | 30.7 (30.6 to 30.8) | 38,666,489 (38,666,465.5 to 38,666,511.5) | –29.3 (–29.5 to –29.2) | 350.4 (347.9 to 352.9) | 36.3 (36.2 to 36.5) | 39.8 (39.7 to 40.0) |
| MTBDRplus | ||||||
| MTBDRplus local | 32 (31.8 to 32.1) | 38,666,947 (38,666,910.4 to 38,666,984.3) | –28.1 (–28.2 to –27.9) | 809.3 (780.5,838.1) | 44.2 (43.7 to 44.8) | 52.3 (51.5 to 53.2) |
| MTBDRplus regional | 32.5 (32.3 to 32.7) | 38,667,015 (38,666,978.2 to 38667051.3) | –27.5 (–27.7 to –27.4) | 876.7 (848.0 to 905.3) | 45 (44.5 to 45.6) | 53.8 (53.0 to 54.6) |
| INNO-LiPA | ||||||
| INNO-LiPA local | 34.2 (34.0 to 34.3) | 38,666,568 (38,666,545.1 to 38,666,591.6) | –25.9 (–26.0 to –25.7) | 430.3 (427.1 to 433.4) | 34.5 (34.3 to 34.6) | 38.8 (38.6 to 38.9) |
| INNO-LiPA regional | 34.6 (34.4 to 34.7) | 38,666,621 (38,666,598.0 to 38,666,643.3) | –25.4 (–25.6 to –25.3) | 502.6 (499.6 to 505.5) | 35.5 (35.3 to 35.7) | 40.5 (40.4 to 40.7) |
FIGURE 20.
Cost-effectiveness plane of different molecular testing interventions compared with current practice in South Asians, over a 20-year time horizon. This figure shows the cost-effectiveness plane resulting from comparing the effect of different rapid molecular tests for TB confirmation and DST with the current practice in South Asians. Three different molecular tests are considered (GeneXpert only, GeneXpert combined with INNO-LiPA line-probe assay and MTBDRplus) in two locations: either local to the hospital or in a regional laboratory. The solid diagonal line indicates the threshold of £30,000 per QALY and the dashed line £20,000 per QALY.
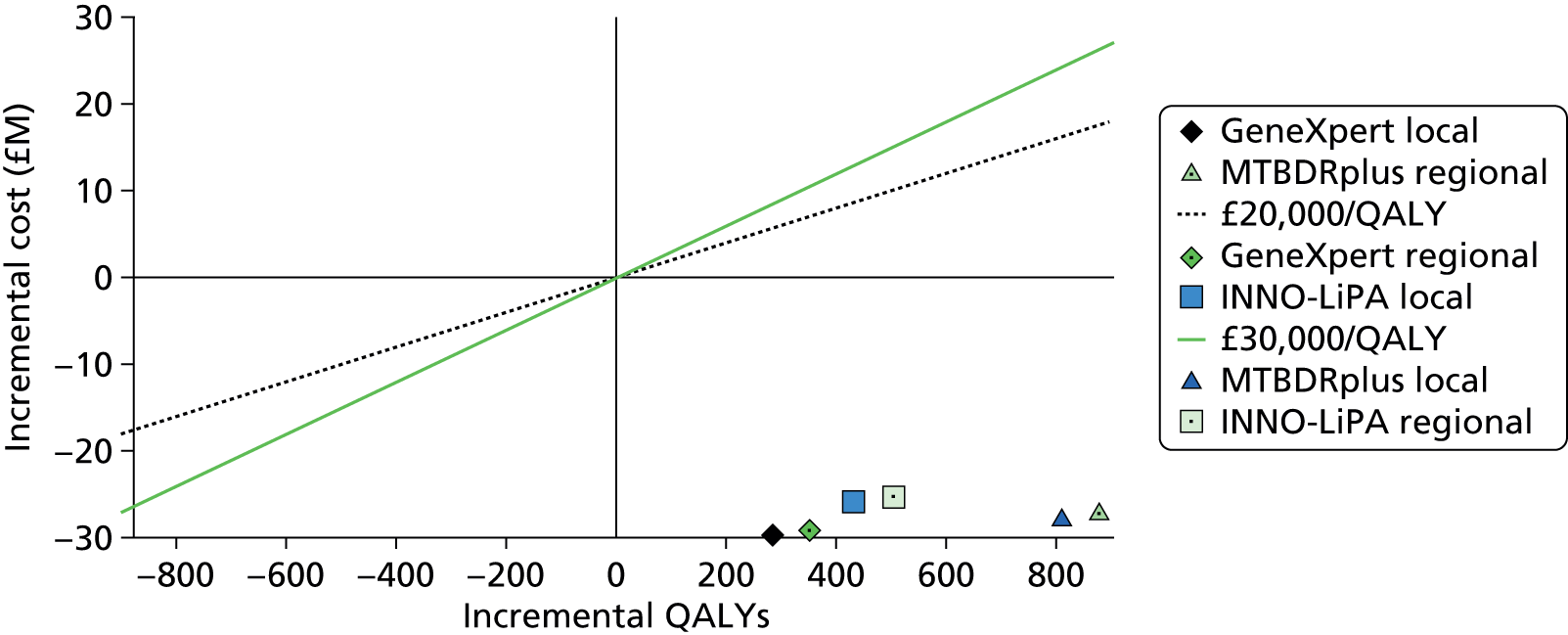
GeneXpert only
There is uncertainty in the GeneXpert scenario over the impact on both costs and, particularly, health, and hence there is uncertainty in cost-effectiveness (Figures 21 and 22). However, in all realisations of the PSA there is a net reduction in costs, and net increases in QALYs. When local and regional testing are compared (Figure 23), there are increases in costs in all realisations of the PSA and increases in the QALYs in most realisations when moving from local to regional testing.
FIGURE 21.
Cost-effectiveness plane of PSA samples of local GeneXpert-based testing compared with current practice over a 10-year time horizon in South Asians. This figure shows the ICER pairs resulting from comparing GeneXpert-based TB confirmation and DST in South Asians with current practice in PSA. The stochastic parameters included in the samples were those associated with TB diagnosis time delay, costs and QALYs on the cost-effectiveness of GeneXpert-based TB confirmation and DST compared with current practice. Parameters were varied in both current practice and the intervention scenario. The green line indicates the threshold of £30,000 per QALY and the black dashed line £20,000 per QALY.
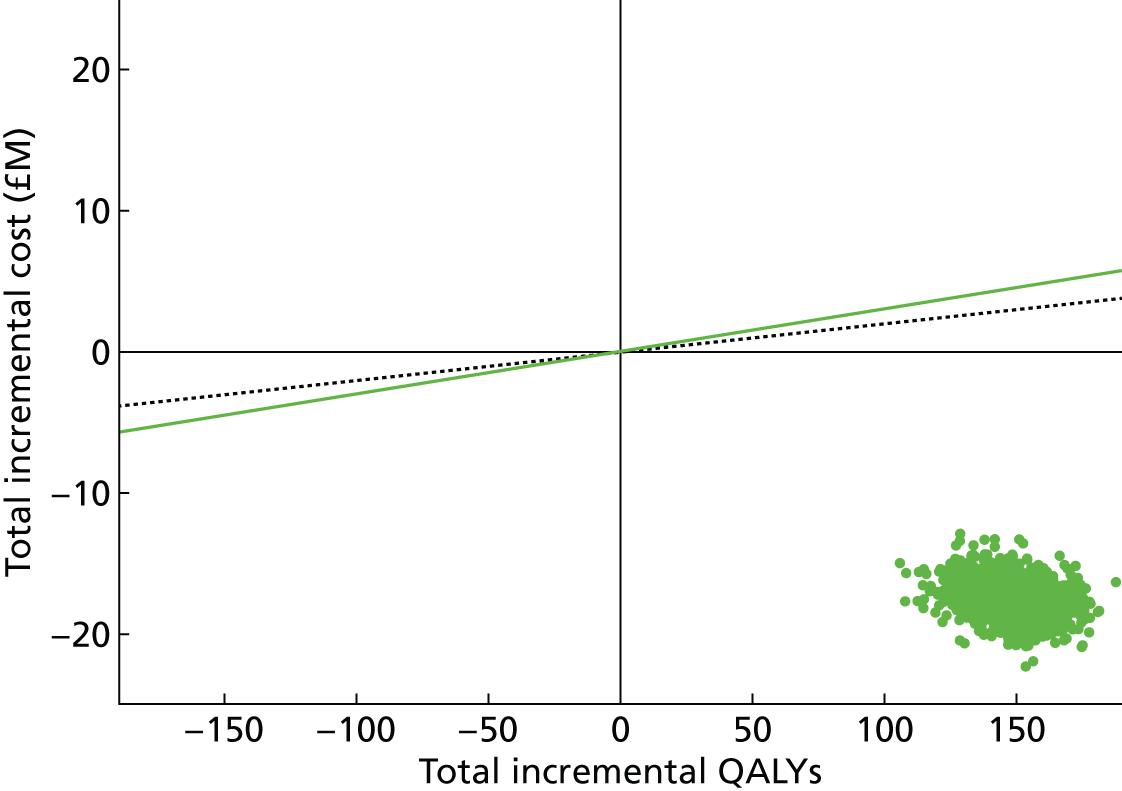
FIGURE 22.
Cost-effectiveness plane of PSA samples of regional GeneXpert-based testing compared with current practice over a 10-year time horizon in South Asians. This figure shows the ICER pairs resulting from comparing GeneXpert-based TB confirmation and DST in South Asians with current practice in PSA. The stochastic parameters included in the samples were those associated with TB diagnosis time delay, costs and QALYs on the cost-effectiveness of GeneXpert base TB confirmation and DST compared with current practice. Parameters were varied in both current practice and the intervention scenario. The green line indicates the threshold £30,000 per QALY and the black dashed line £20,000 per QALY.

FIGURE 23.
Cost-effectiveness plane of PSA samples of regional vs. local GeneXpert-based testing compared with current practice over a 10-year time horizon in South Asians. This figure shows the ICER pairs resulting from comparing regionally based GeneXpert-based TB confirmation and DST in South Asians with locally based GeneXpert-based TB confirmation and DST in PSA. The stochastic parameters included in the samples were those associated with TB diagnosis time delay, costs and QALYs on the cost-effectiveness of GeneXpert base TB confirmation and DST compared with current practice. Parameters were varied in both current practice and the intervention scenario. The green line indicates the threshold of £30,000 per QALY and the black dashed line £20,000 per QALY. Note the very small magnitude of the differences.

Figure 24 shows that the most influential test performance characteristics that affect the cost-effectiveness of the GeneXpert scenario compared with current practice are the sensitivity for detection of MDR infection in those in whom TB is detected (as FN results lead to inappropriate treatment for DS infection), and specificity for detection of MDR infection in those in whom TB is detected (as FP results lead to inappropriate treatment for MDR infection, which incurs cost and harms health). Sensitivity and specificity for detection of TB infection and time to obtain culture-based DST results are much less influential.
FIGURE 24.
Tornado plot of the influence on INB in South Asians of GeneXpert-based testing compared with current practice, over a 10-year time horizon. This figure shows the effects of varying different test performance parameters on the INB of GeneXpert-based TB confirmation and DST in South Asians compared with current practice, if a QALY is valued at £20,000. Individually, parameters are varied from their minimum (green bar) to maximum (black bar) values.

GeneXpert with INNO-LiPA test
In the INNO-LiPA scenario there is considerable uncertainty over the impact on both costs and health. However, in all realisations of the PSA for both local and regional testing there is a reduction in costs, and an increase in QALYs (Figure 25 and 26).
FIGURE 25.
Cost-effectiveness plane of PSA samples of local GeneXpert with INNO-LiPA-based testing compared with current practice over a 10-year time horizon in South Asians. This figure shows the ICER pairs resulting from comparing GeneXpert with INNO-LiPA test-based TB confirmation and DST in South Asians with current practice in PSA. The stochastic parameters included in the samples were those associated with TB diagnosis time delay, costs and QALYs on the cost-effectiveness of GeneXpert with INNO-LiPA test-based TB confirmation and DST compared with current practice. Parameters were varied in both current practice and the intervention scenario. The green line indicates the threshold £30,000 per QALY and the black dashed line £20,000 per QALY.

FIGURE 26.
Cost-effectiveness plane of PSA samples of regional GeneXpert with INNO-LiPA-based testing compared with current practice over a 10-year time horizon in South Asians. This figure shows the ICER pairs resulting from comparing GeneXpert with INNO-LiPA test-based TB confirmation and DST in South Asians with current practice in PSA. The stochastic parameters included in the samples were those associated with TB diagnosis time delay, costs and QALYs on the cost-effectiveness of GeneXpert with INNO-LiPA test-based TB confirmation and DST compared with current practice. Parameters were varied in both current practice and the intervention scenario. The green line indicates the threshold £30,000 per QALY and the black dashed line £20,000 per QALY.

Figure 27 shows that the most influential test performance characteristics that affect the cost-effectiveness of the GeneXpert with INNO-LiPA scenario compared with current practice are the sensitivity for detection of MDR infection in those in whom TB is detected (as FN results lead to inappropriate treatment for DS infection), and specificity for detection of MDR infection in those in whom TB is detected (as FP results lead to inappropriate treatment for MDR infection, which incurs cost and harms health). Sensitivity and specificity for detection of TB infection and time to obtain culture-based DST results are much less influential.
FIGURE 27.
Tornado plot of the influence on INB in South Asians of GeneXpert with INNO-LiPA-based testing compared with current practice, over a 10-year time horizon. This figure shows the effects of varying different test performance parameters on the INB of GeneXpert with INNO-LiPA-based TB confirmation and DST in South Asians compared with current practice, if a QALY is valued at £20,000. Individually, parameters are varied from their minimum (green bar) to maximum (black bar) values.
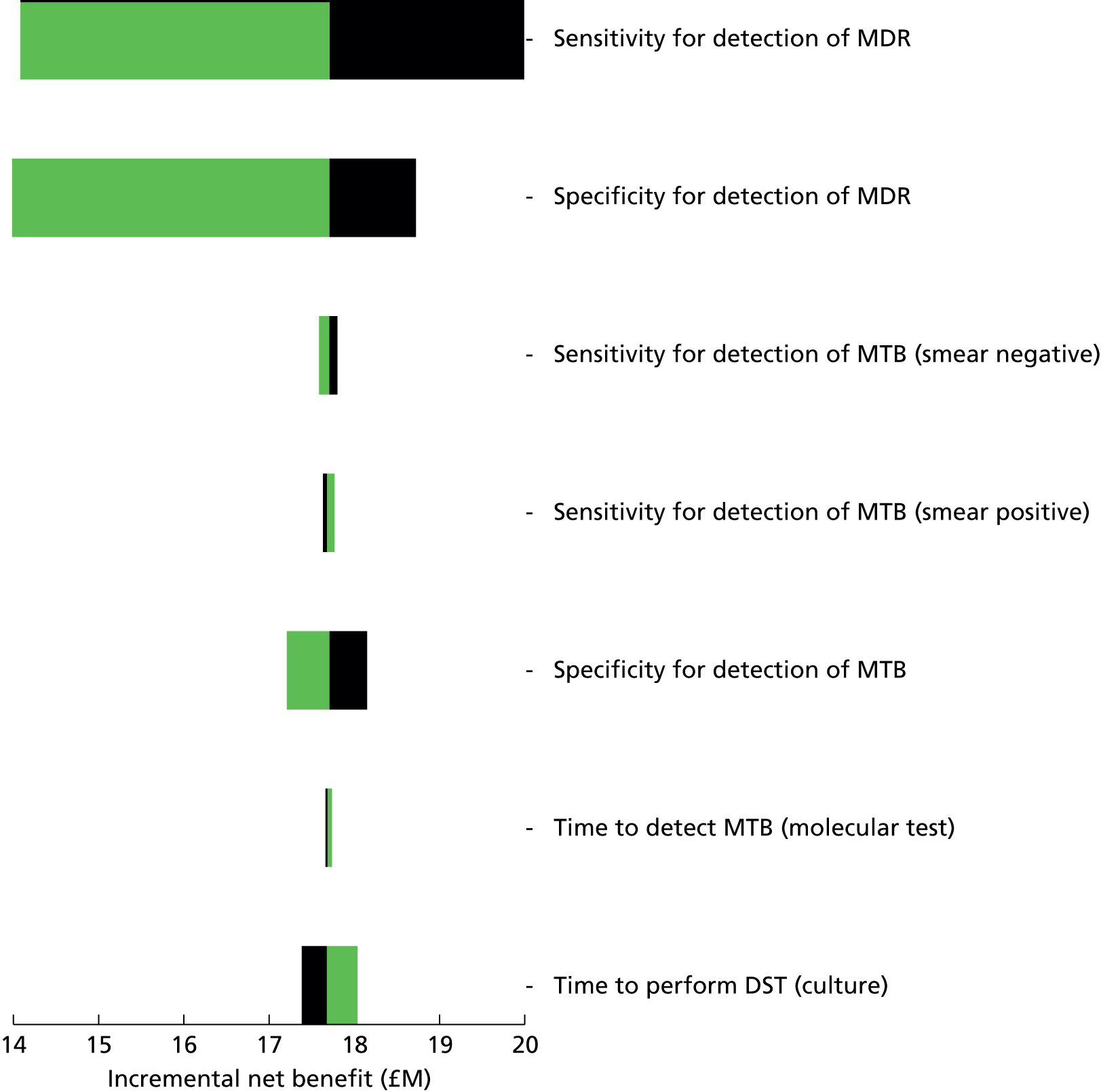
MTBDRplus test
In the MTBDRplus scenario (Figures 28 and 29), there is a wide range of uncertainty with regard to the magnitude of the impact on health and costs, but in all realisations of the PSA there is a net reduction in costs and increases in QALYs.
FIGURE 28.
Cost-effectiveness plane of PSA samples of local MTBDRplus-based testing compared with current practice over a 10-year time horizon in South Asians. This figure shows the ICER pairs resulting from comparing MTBDRplus-based TB confirmation and DST in South Asians with current practice in PSA. The stochastic parameters included in the samples were those associated with TB diagnosis time delay, costs and QALYs on the cost-effectiveness of MTBDRplus-based TB confirmation and DST compared with current practice. Parameters were varied in both current practice and the intervention scenario. The green line indicates the threshold £30,000 per QALY and the black dashed line £20,000 per QALY.
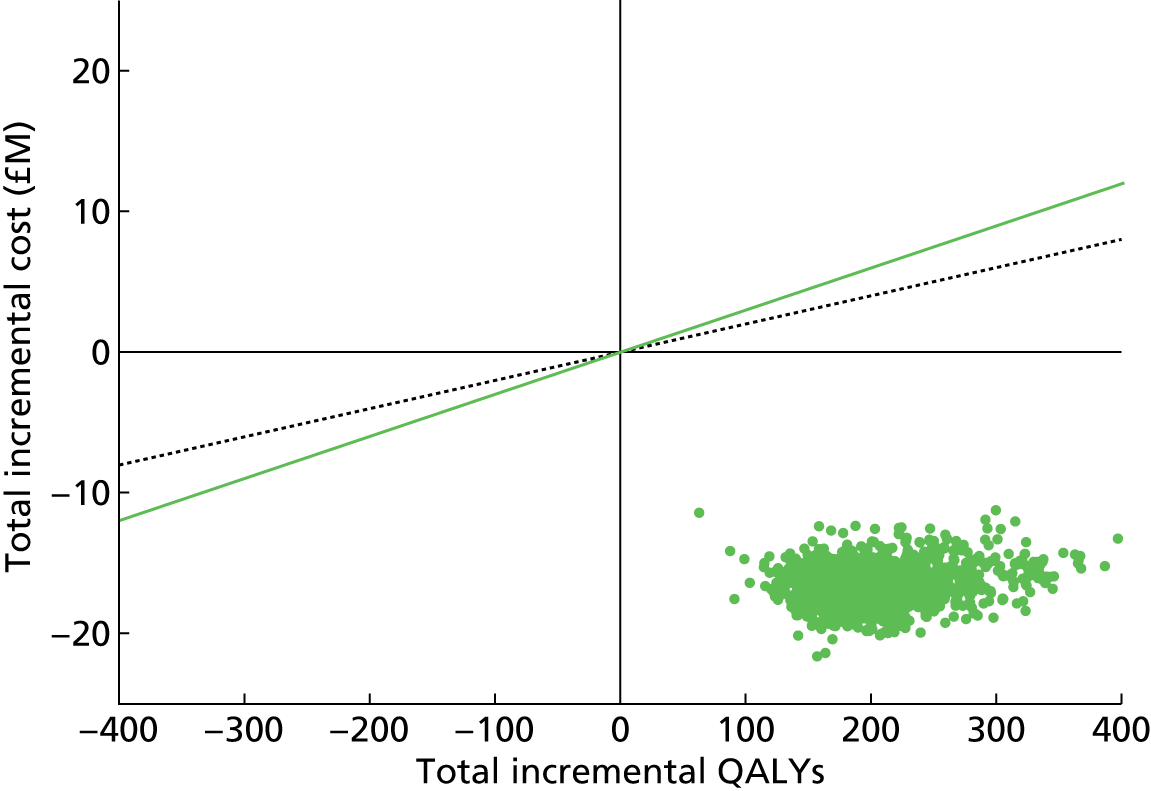
FIGURE 29.
Cost-effectiveness plane of PSA samples of regional MTBDRplus-based testing compared with current practice over a 10-year time horizon in South Asians. This figure shows the ICER pairs resulting from comparing MTBDRplus-based TB confirmation and DST in South Asians with current practice in PSA. The stochastic parameters included in the samples were those associated with TB diagnosis time delay, costs and QALYs on the cost-effectiveness of MTBDRplus-based TB confirmation and DST compared with current practice. Parameters were varied in both current practice and the intervention scenario. The green line indicates the threshold £30,000 per QALY and the black dashed line £20,000 per QALY.

When local and regional testing are compared (Figure 30), there are modest increases in costs in almost all of the realisations of the PSA, and modest increases in QALYs in most PSA samples, but with modest decreases also occurring in some realisations.
FIGURE 30.
Cost-effectiveness plane of PSA samples of regional vs. local MTBDRplus-based testing compared with current practice over a 10-year time horizon in South Asians. This figure shows the ICER pairs resulting from comparing regionally based MTBDRplus-based TB confirmation and DST in South Asians with locally based MTBDRplus-based TB confirmation and DST in PSA. The stochastic parameters included in the samples were those associated with TB diagnosis time delay, costs and QALYs on the cost-effectiveness of MTBDRplus-based TB confirmation and DST compared with current practice. Parameters were varied in both current practice and the intervention scenario. The green line indicates the threshold £30,000 per QALY and the black dashed line £20,000 per QALY. Note the very small magnitude of the differences.

Figure 31 shows that the most influential test performance characteristics that affect the cost-effectiveness of the MTBDRplus scenario compared with current practice are the specificity for detection of TB infection (as FP results lead to inappropriate treatment, which incurs cost and harms health), sensitivity for detection of TB infection (as FN results delay treatment), and specificity and sensitivity for detection of MDR infection in those infected with TB. Figure 18 shows how moving from current practice to the GeneXpert scenario results in a reduction in annual TB diagnoses, whereas moving from current practice to the MTBDRplus scenario results in an increase in diagnoses; the key difference is the specificity of the tests.
FIGURE 31.
Tornado plot of the influence on INB in South Asians of MTBDRplus-based testing compared with current practice, over a 10-year time horizon. This figure shows the effects of varying different test performance parameters on the INB of MTBDRplus-based TB confirmation and DST in South Asians compared with current practice, if a QALY is valued at £20,000. Individually, parameters are varied from their minimum (green bar) to maximum (black bar) values.

GeneXpert only compared with MTBDRplus test
Comparing the cost-effectiveness of regionally based testing in the GeneXpert and MTBDRplus scenarios finds that there is considerable uncertainty with regard to both costs and health benefits (Figure 32). In all realisations of the PSA there are reductions in costs, but in a large proportion of samples there are also reductions in QALYs when moving from the MTBDRplus scenario to the GeneXpert scenario.
FIGURE 32.
Cost-effectiveness plane of PSA samples of regional GeneXpert vs. regional MTBDRplus molecular testing compared with current practice over a 10-year time horizon in South Asians. This figure shows the ICER pairs resulting from comparing regionally based GeneXpert-based TB confirmation and DST in South Asians with regionally based MTBDRplus-based TB confirmation and DST-based TB confirmation and DST in PSA. The stochastic parameters included in the samples were those associated with TB diagnosis time delay, costs and QALYs on the cost-effectiveness of GeneXpert-based TB confirmation and DST compared with current practice. Parameters were varied in both current practice and the intervention scenario. The green line indicates the threshold £30,000 per QALY and the black dashed line £20,000 per QALY.
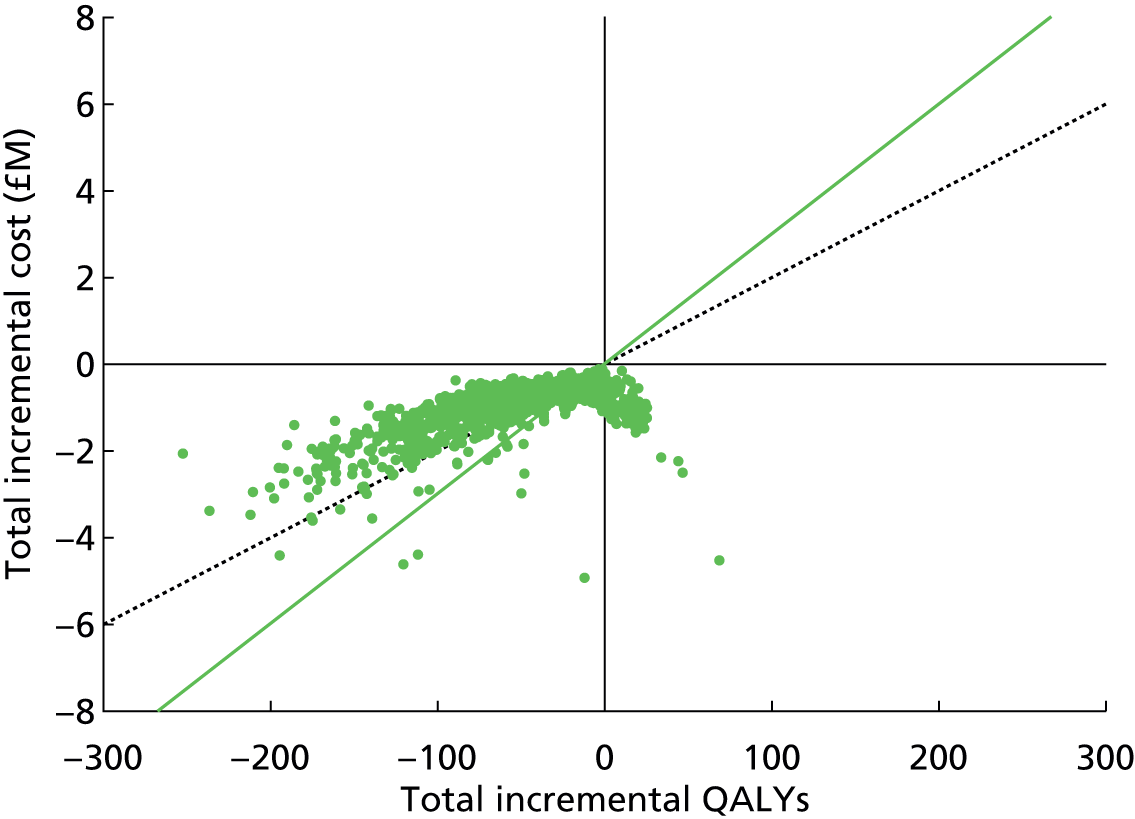
Black Africans
As with South Asians, there is a marginal effect of introduction of molecular testing on annual numbers of diagnoses (Figure 33) owing to a small reduction in transmission and an increase in FP results. In the INNO-LiPA and MTBDRplus scenarios there are increases in diagnoses as a result of the latter effect dominating, whereas in the GeneXpert scenario the former dominates. Local molecular testing has a faster turnaround time than regional molecular testing, so the annual number of diagnoses in the local testing configuration is slightly lower than in the regional testing configuration. However, this effect is tiny, with < 1% fewer diagnoses annually.
FIGURE 33.
Effects of introducing molecular testing on annual numbers of TB diagnoses in the Black African population. Annual numbers of TB diagnoses in Black Africans following the introduction of different rapid molecular tests. Three different molecular tests are considered (GeneXpert only, GeneXpert combined with INNO-LiPA line-probe assay and MTBDRplus) in two locations: either local to the hospital or in a regional laboratory. Note that the vertical axis does not start at zero and the magnitude of the changes is, in fact, small.
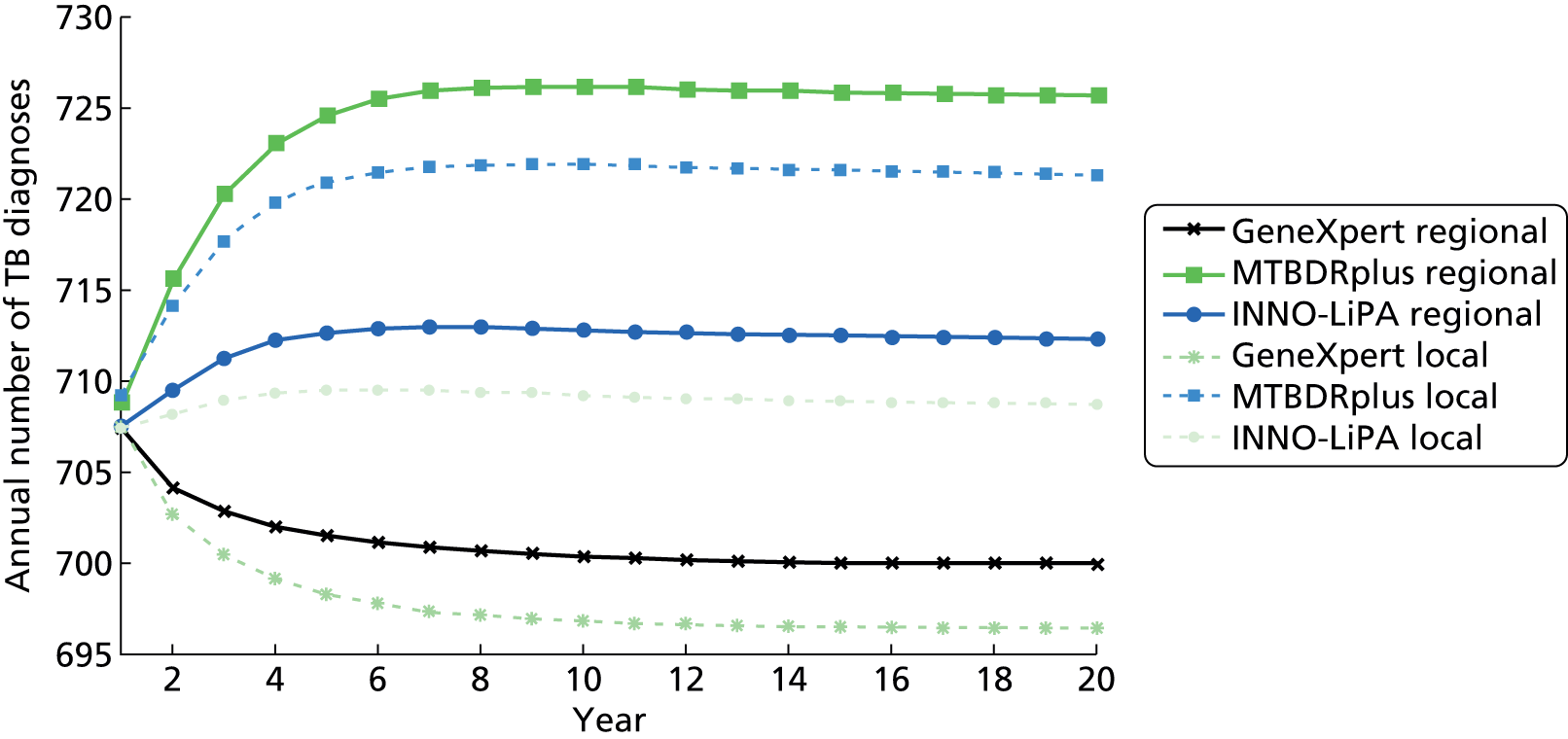
There are significant INBs in the GeneXpert, MTBDRplus and INNO-LiPA scenarios (Table 44 and Figure 34). These molecular tests reduce net costs and achieve an associated QALY gain as more individuals are appropriately treated for TB. As the options are cost saving, results are presented as INB rather than using ICERs.
For all tests (GeneXpert scenario, MTBDRplus scenario and INNO-LiPA) the INBs for local and regional testing are very similar (see Table 44 and Figure 35). The GeneXpert scenario has the highest INB, with the MTBDRplus and INNO-LiPA scenarios having INBs that are similar to each other.
When comparing over a time horizon of 20 years, rather than 10 years, the INBs are greater but the overall picture is broadly similar (see Figure 35 and Table 45).
| Scenario | Total costs, £M (95% CI) | Total QALYs (95% CI) | Compared with baseline | INB, £M (95% CI) | ||
|---|---|---|---|---|---|---|
| Incremental cost, £M (95% CI) | Incremental QALYs (95% CI) | QALY = £20,000 | QALY = £30,000 | |||
| Current | 24.5 (25.4 to 31.1) | 7,502,098 (7,502,064 to 7,502,119) | – | – | – | – |
| GeneXpert local | 11.4 (11.3 to 11.4) | 7,502,255 (7,502,244.4 to 7,502,265.1) | –13.2 (–13.3 to –13.1) | 179.4 (177.8 to 180.9) | 16.8 (16.7 to 16.9) | 18.6 (18.5 to 18.7) |
| GeneXpert regional | 11.5 (11.4 to 11.5) | 7,502,228 (7,502,217.2 to 7,502,237.9) | –13.1 (–13.1 to –13.0) | 152.2 (150.7 to 153.7) | 16.1 (16.0 to 16.2) | 17.6 (17.6 to 17.7) |
| MTBDRplus local | 11.9 (11.8 to 11.9) | 7,502,118 (7,502,104.5 to 7,502,132.3) | –12.7 (–12.8 to –12.6) | 43 (34.5 to 51.5) | 13.6 (13.4 to 13.8) | 14 (13.7 to 14.3) |
| MTBDRplus regional | 12 (12.0 to 12.1) | 7,502,088 (7,502,074.4 to 7,502,102.4) | –12.6 (–12.6 to –12.5) | 13.1 (4.5 to 21.8) | 12.8 (12.6 to 13.0) | 12.9 (12.7 to 13.2) |
| INNO-LiPA local | 12.7 (12.7 to 12.8) | 7,502,143 (7,502,132.5 to 7,502,154.0) | –11.9 (–11.9 to –11.8) | 67.9 (66.4 to 69.3) | 13.2 (13.1 to 13.3) | 13.9 (13.8 to 14.0) |
| INNO-LiPA regional | 12.8 (12.7 to 12.8) | 7,502,117 (7,502,106.1 to 7,502,127.6) | –11.7 (–11.8 to –11.6) | 41.9 (40.5 to 43.4) | 12.5 (12.5 to 12.6) | 13 (12.9 to 13.0) |
FIGURE 34.
Cost-effectiveness plane of different molecular testing interventions compared with current practice in Black Africans over a 10-year time horizon. This figure shows the cost-effectiveness plane resulting from comparing the effect of different rapid molecular tests for TB confirmation and DST with the current practice in Black Africans. Three different molecular tests are considered (GeneXpert only, GeneXpert combined with INNO-LiPA line-probe assay and MTBDRplus) in two locations: either local to the hospital or in a regional laboratory. The solid diagonal line indicates the threshold £30,000 per QALY and the dashed line £20,000 per QALY.
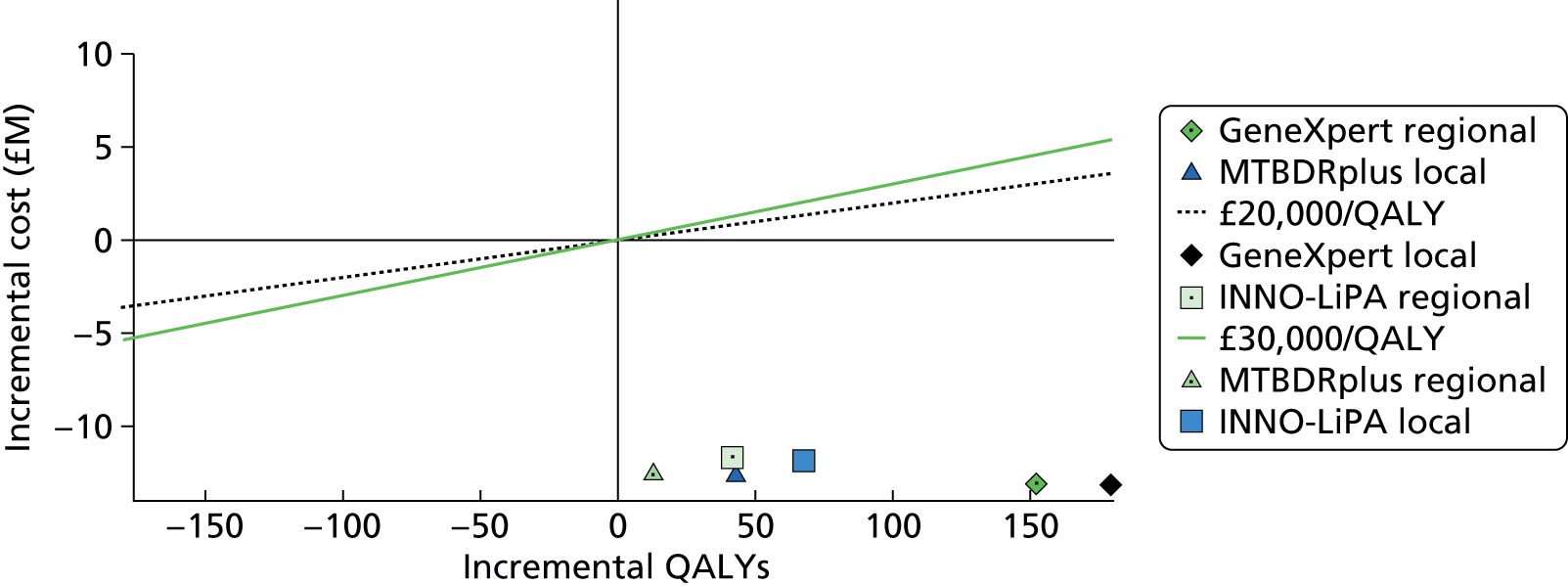
| Scenario | Total costs, £M (95% CI) | Total QALYs (95% CI) | Compared with baseline | INB, £M (95% CI) | ||
|---|---|---|---|---|---|---|
| Incremental cost, £M (95% CI) | Incremental QALYs (95% CI) | QALY = £20,000 | QALY = £30,000 | |||
| Current | 41.8 (43.1 to 54.2) | 12,820,477 (12,820,399 to 12,820,515) | – | – | – | – |
| GeneXpert local | 19.2 (19.1 to 19.3) | 12,820,912 (12,820,879.4 to 12,820,944.1) | –23 (–23.1 to –22.8) | 504.4 (499.8 to 509.0) | 33 (32.9 to 33.2) | 38.1 (37.9 to 38.3) |
| GeneXpert regional | 19.4 (19.3 to 19.5) | 12,820,826 (12,820,793.7 to 12,820,858.1) | –22.7 (–22.8 to –22.6) | 418.6 (414.0 to 423.1) | 31.1 (30.9 to 31.3) | 35.3 (35.1 to 35.5) |
| MTBDRplus local | 20.1 (20.0 to 20.3) | 12,820,482 (12,820,439.3 to 12,820,525.1) | –22 (–22.1 to –21.9) | 74.8 (48.5 to 101.2) | 23.5 (22.9 to 24.1) | 24.2 (23.4 to 25.1) |
| MTBDRplus regional | 20.5 (20.3 to 20.6) | 12,820,389 (12,820,346.0 to 12,820,432.3) | –21.7 (–21.8 to –21.6) | –17.8 (–44.6 to 9.0) | 21.3 (20.8 to 21.9) | 21.2 (20.3 to 22.0) |
| INNO-LiPA local | 21.5 (21.5 to 21.6) | 12,820,600 (12,820,566.3 to 12,820,632.8) | –20.6 (–20.7 to –20.5) | 192.2 (187.7 to 196.7) | 24.4 (24.3 to 24.6) | 26.4 (26.2 to 26.5) |
| INNO-LiPA regional | 21.7 (21.6 to 21.8) | 12,820,513 (12,820,479.5 to 12,820,546.0) | –20.3 (–20.4 to –20.2) | 109.7 (105.2 to 114.2) | 22.5 (22.4 to 22.6) | 23.6 (23.4 to 23.8) |
FIGURE 35.
Cost-effectiveness plane of different molecular testing interventions compared with current practice in Black Africans over a 20-year time horizon. This figure shows the cost-effectiveness plane resulting from comparing the effect of different rapid molecular tests for TB confirmation and DST with the current practice in Black Africans. Three different molecular tests are considered (GeneXpert only, GeneXpert combined with INNO-LiPA line-probe assay and MTBDRplus) in two locations: either local to the hospital or in a regional laboratory. The solid diagonal line indicates the threshold £30,000 per QALY and the dashed line £20,000 per QALY.
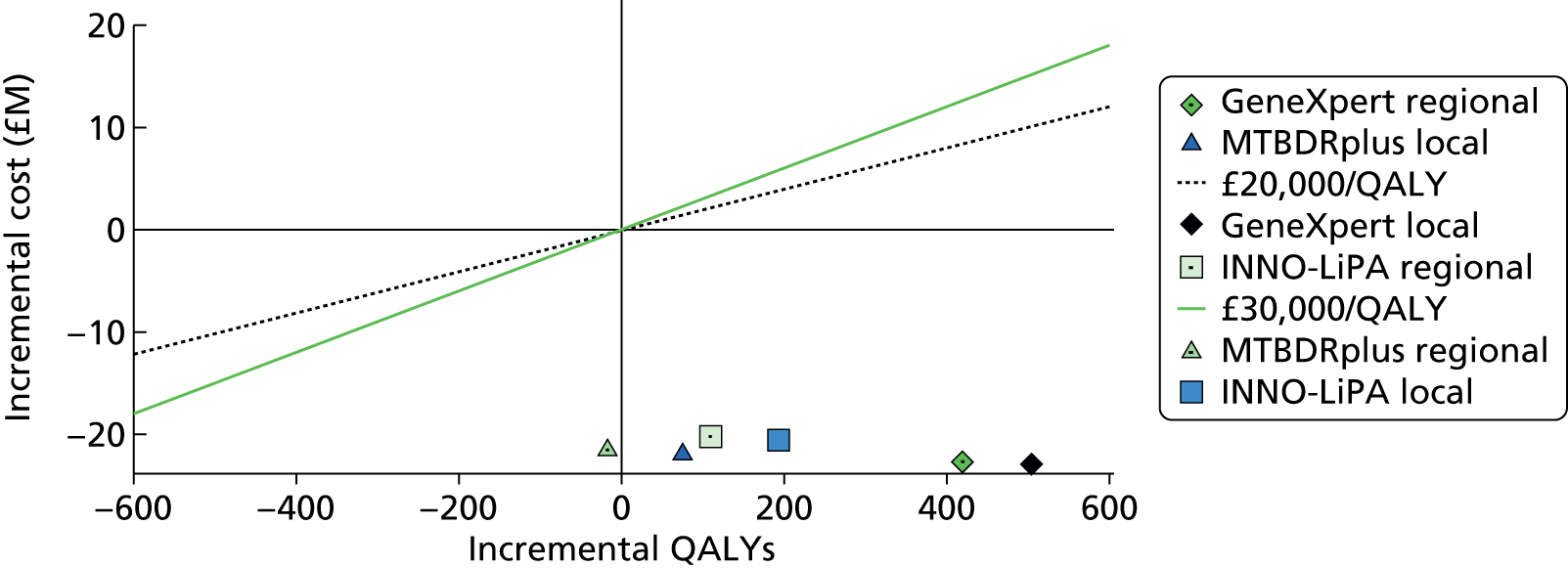
GeneXpert only
As with the South Asian population, there is uncertainty in the GeneXpert scenario over the impact on both costs and, particularly, health, and hence there is uncertainty in cost-effectiveness (Figures 36 and 37). However, in all realisations of the PSA there is a net reduction in costs, and net increases in QALYs. When local and regional testing are compared (Figure 38), there are increases in costs and reductions in QALYs in all realisations of the PSA, although the magnitudes are small particularly for costs.
FIGURE 36.
Cost-effectiveness plane of PSA samples of local GeneXpert-based testing compared with current practice in Black Africans over a 10-year time horizon. This figure shows the ICER pairs resulting from comparing GeneXpert-based TB confirmation and DST in Black Africans with current practice in PSA. The stochastic parameters included in the samples were those associated with TB diagnosis time delay, costs and QALYs on the cost-effectiveness of GeneXpert base TB confirmation and DST compared with current practice. Parameters were varied in both current practice and the intervention scenario. The green line indicates the threshold £30,000 per QALY and the black dashed line £20,000 per QALY.
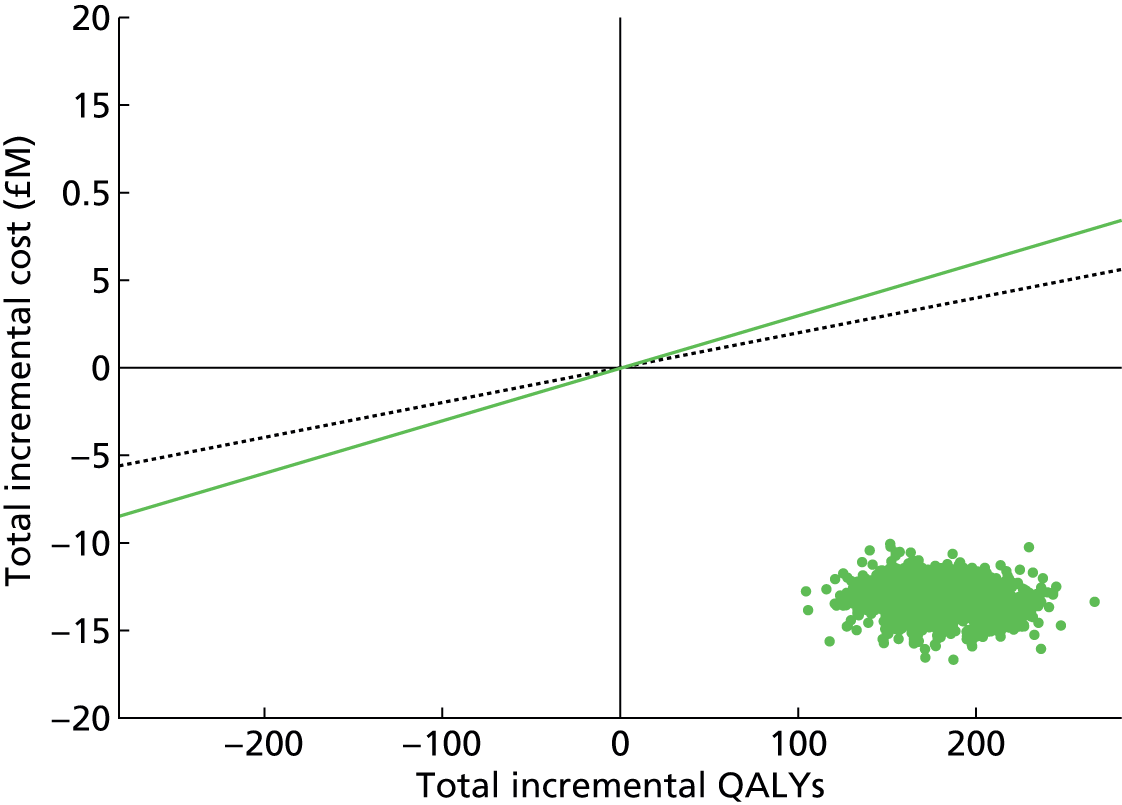
FIGURE 37.
Cost-effectiveness plane of PSA samples of regional GeneXpert-based testing compared with current practice in Black Africans over a 10-year time horizon. This figure shows the ICER pairs resulting from comparing GeneXpert-based TB confirmation and DST in Black Africans with current practice in PSA. The stochastic parameters included in the samples were those associated with TB diagnosis time delay, costs and QALYs on the cost-effectiveness of GeneXpert-based TB confirmation and DST compared with current practice. Parameters were varied in both current practice and the intervention scenario. The green dashed line indicates the threshold £30,000 per QALY and the black dashed line £20,000 per QALY.

FIGURE 38.
Cost-effectiveness plane of PSA samples of regional vs. local GeneXpert-based testing compared with current practice over a 10-year time horizon in Black Africans. This figure shows the ICER pairs resulting from comparing regionally based GeneXpert-based TB confirmation and DST in Black Africans with locally based GeneXpert-based TB confirmation and DST in PSA. The stochastic parameters included in the samples were those associated with TB diagnosis time delay, costs and QALYs on the cost-effectiveness of GeneXpert-based TB confirmation and DST compared with current practice. Parameters were varied in both current practice and the intervention scenario. The green line indicates the threshold £30,000 per QALY and the black dashed line £20,000 per QALY. Note the very small magnitude of the differences.
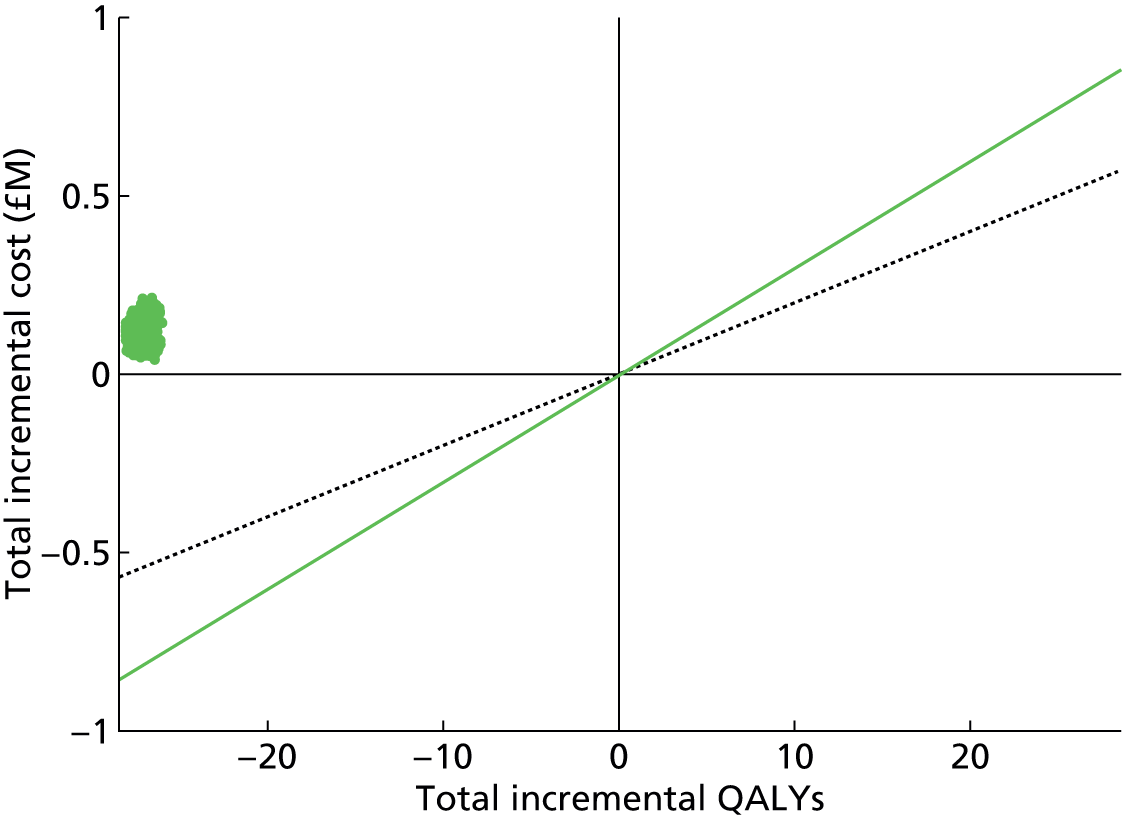
Figure 39 shows that the most influential test performance characteristics that affect the cost-effectiveness of the GeneXpert scenario compared with current practice are the same as for South Asians: sensitivity for detection of MDR infection in those in whom TB is detected, and specificity of detection of MDR infection in those in whom TB is detected.
FIGURE 39.
Tornado plot of the influence on INB in Black Africans of GeneXpert-based testing compared with current practice, over a 10-year time horizon. This figure shows the effects of varying different test performance parameters on the INB of GeneXpert-based TB confirmation and DST in Black Africans compared with current practice, if a QALY is valued at £20,000. Individually, parameters are varied from their minimum (green bar) to maximum (black bar) values.

GeneXpert with INNO-LiPA test
In the INNO-LiPA scenario there is considerable uncertainty over the impact on both costs and health. However, in all realisations of the PSA for both local and regional testing there are reductions in costs, and in the large majority of realisations there are increases in QALYs (Figures 40 and 41).
FIGURE 40.
Cost-effectiveness plane of PSA samples of local GeneXpert with INNO-LiPA-based testing compared with current practice in Black Africans over a 10-year time horizon. This figure shows the ICER pairs resulting from comparing GeneXpert with INNO-LiPA test-based TB confirmation and DST in Black Africans with current practice in PSA. The stochastic parameters included in the samples were those associated with TB diagnosis time delay, costs and QALYs on the cost-effectiveness of GeneXpert with INNO-LiPA test-based TB confirmation and DST compared with current practice. Parameters were varied in both current practice and the intervention scenario. The green line indicates the threshold £30,000 per QALY and the black dashed line £20,000 per QALY.
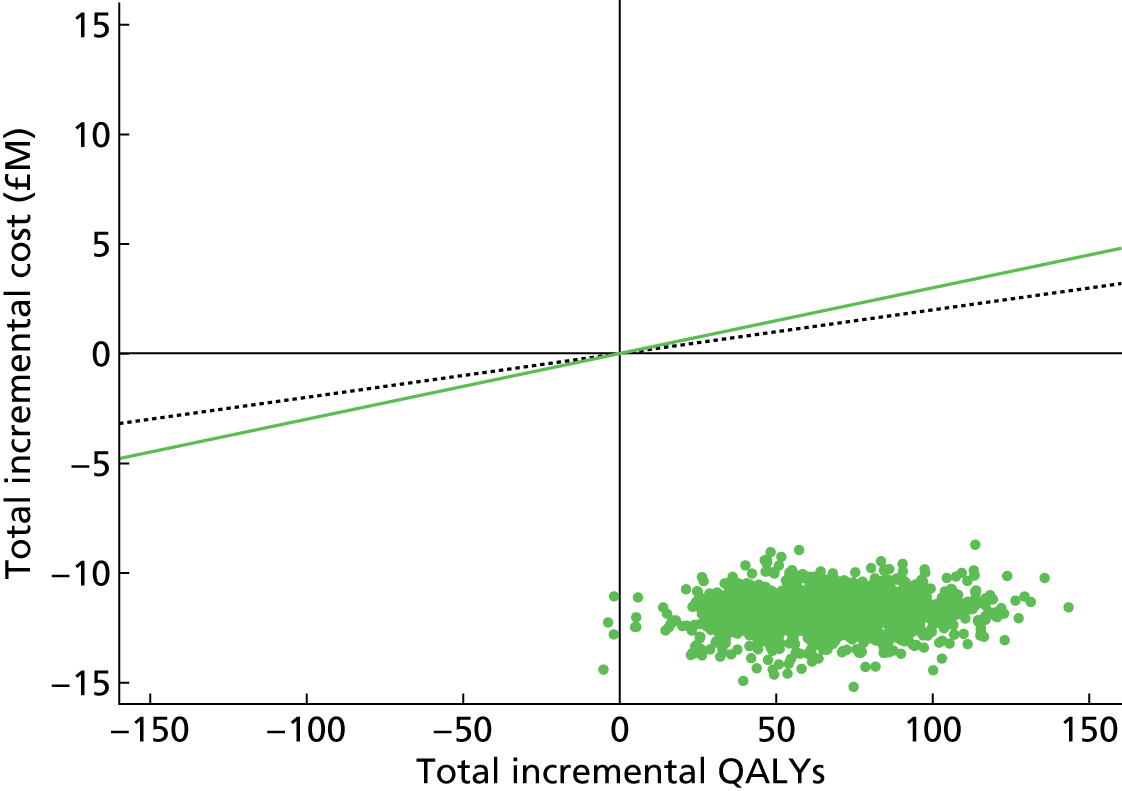
FIGURE 41.
Cost-effectiveness plane of PSA samples of regional GeneXpert with INNO-LiPA-based testing compared with current practice in Black Africans over a 10-year time horizon. This figure shows the ICER pairs resulting from comparing GeneXpert with INNO-LiPA test-based TB confirmation and DST in Black Africans with current practice in PSA. The stochastic parameters included in the samples were those associated with TB diagnosis time delay, costs and QALYs on the cost-effectiveness of GeneXpert with INNO-LiPA test-based TB confirmation and DST compared with current practice. Parameters were varied in both current practice and the intervention scenario. The green line indicates the threshold £30,000 per QALY and the black dashed line £20,000 per QALY.
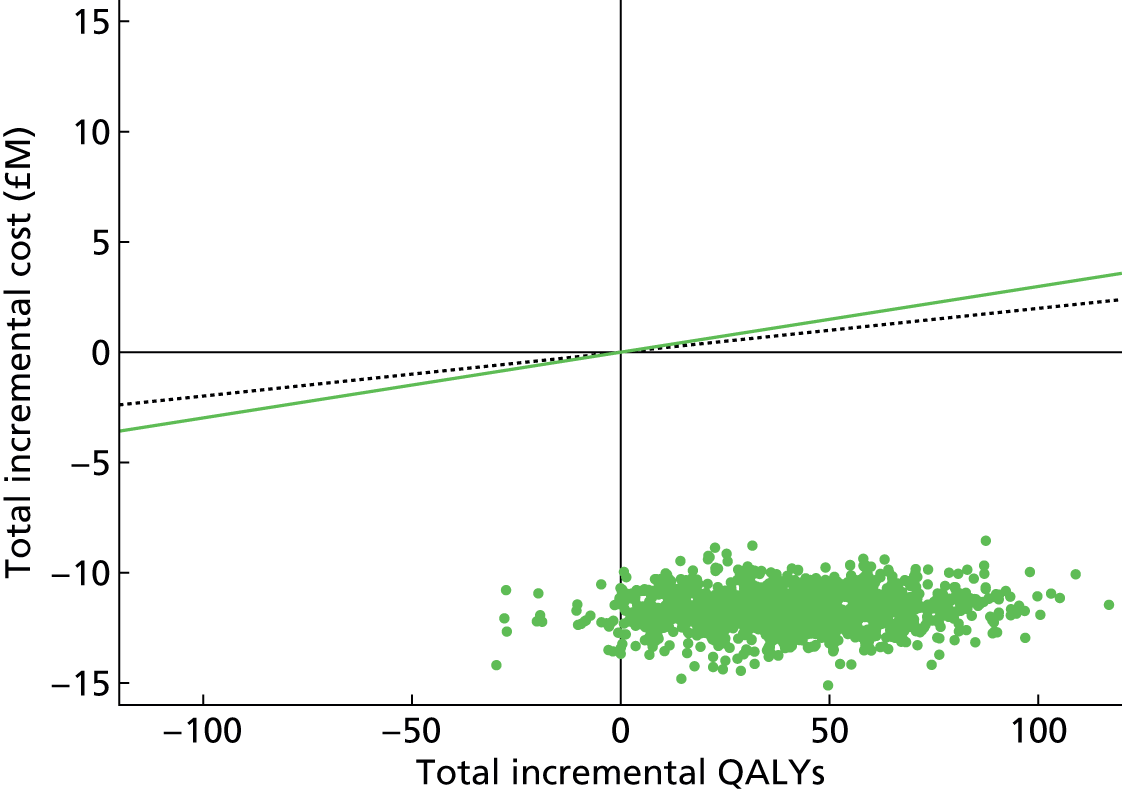
Figure 42 shows that the most influential test performance characteristics that affect the cost-effectiveness of the GeneXpert scenario compared with current practice are the same as for South Asians: sensitivity for detection of MDR infection in those in whom TB is detected, and specificity of detection of MDR infection in those in whom TB is detected.
FIGURE 42.
Tornado plot of the influence on INB in Black Africans of GeneXpert with INNO-LiPA-based testing compared with current practice, over a 10-year time horizon. This figure shows the effects of varying different test performance parameters on the INB of GeneXpert with INNO-LiPA-based TB confirmation and DST in Black Africans compared with current practice, if a QALY is valued at £20,000. Individually, parameters are varied from their minimum (green bar) to maximum (black bar) values.
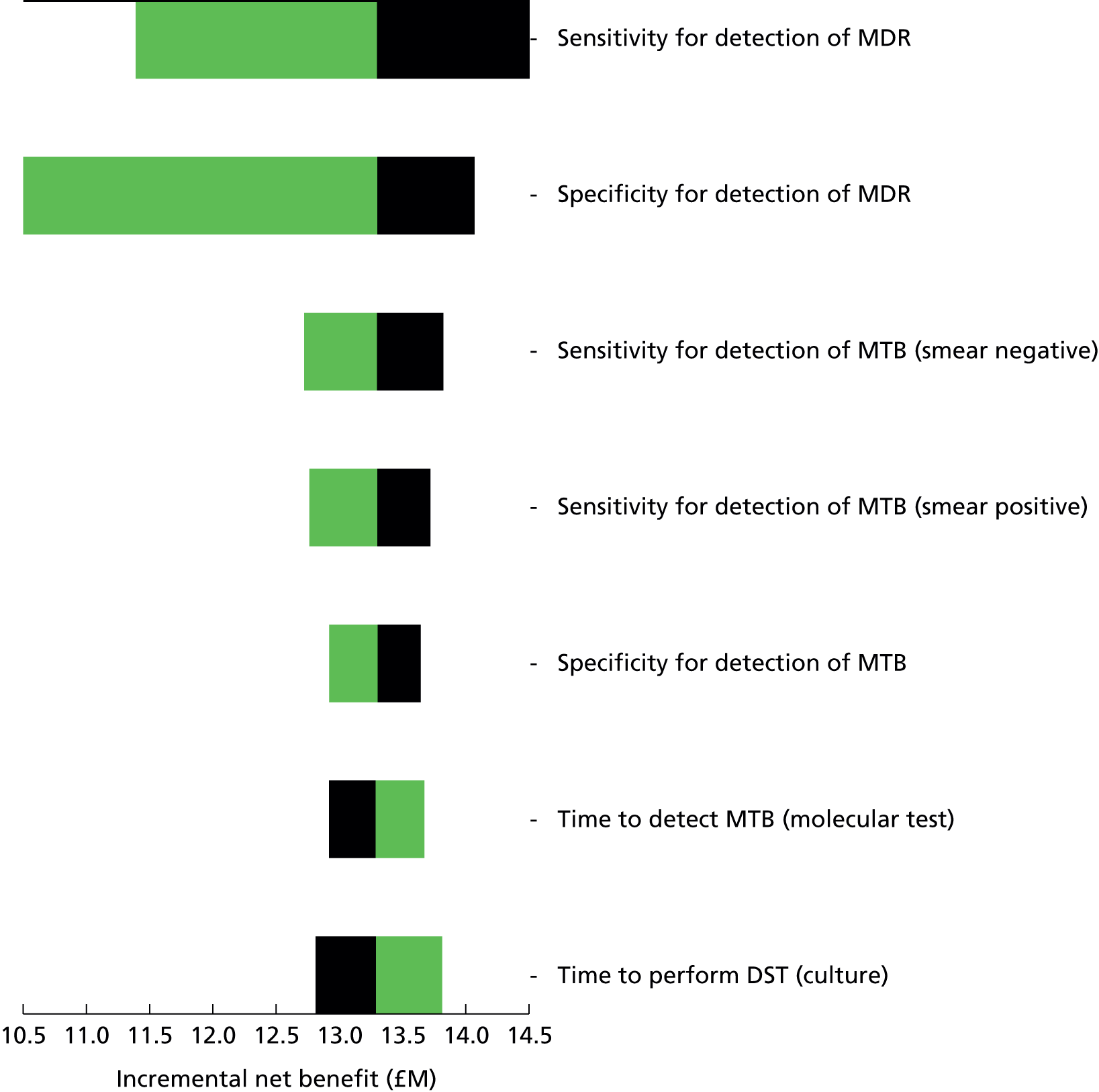
MTBDRplus test
As with South Asians, in the MTBDRplus scenario (Figure 43 and 44), there is a wide range of uncertainty with regard to the impact on health and costs. In all realisations of the PSA there is a net reduction in costs, but the effect on health is uncertain, with the PSA realisations covering a large range of incremental QALYs, both negative and positive.
FIGURE 43.
Cost-effectiveness plane of PSA samples of local MTBDRplus-based testing compared with current practice in Black Africans over a 10-year time horizon. This figure shows the ICER pairs resulting from comparing MTBDRplus-based TB confirmation and DST in Black Africans with current practice in PSA. The stochastic parameters included in the samples were those associated with TB diagnosis time delay, costs and QALYs on the cost-effectiveness of MTBDRplus-based TB confirmation and DST compared with current practice. Parameters were varied in both current practice and the intervention scenario. The green line indicates the threshold £30,000 per QALY and the black dashed line £20,000 per QALY.
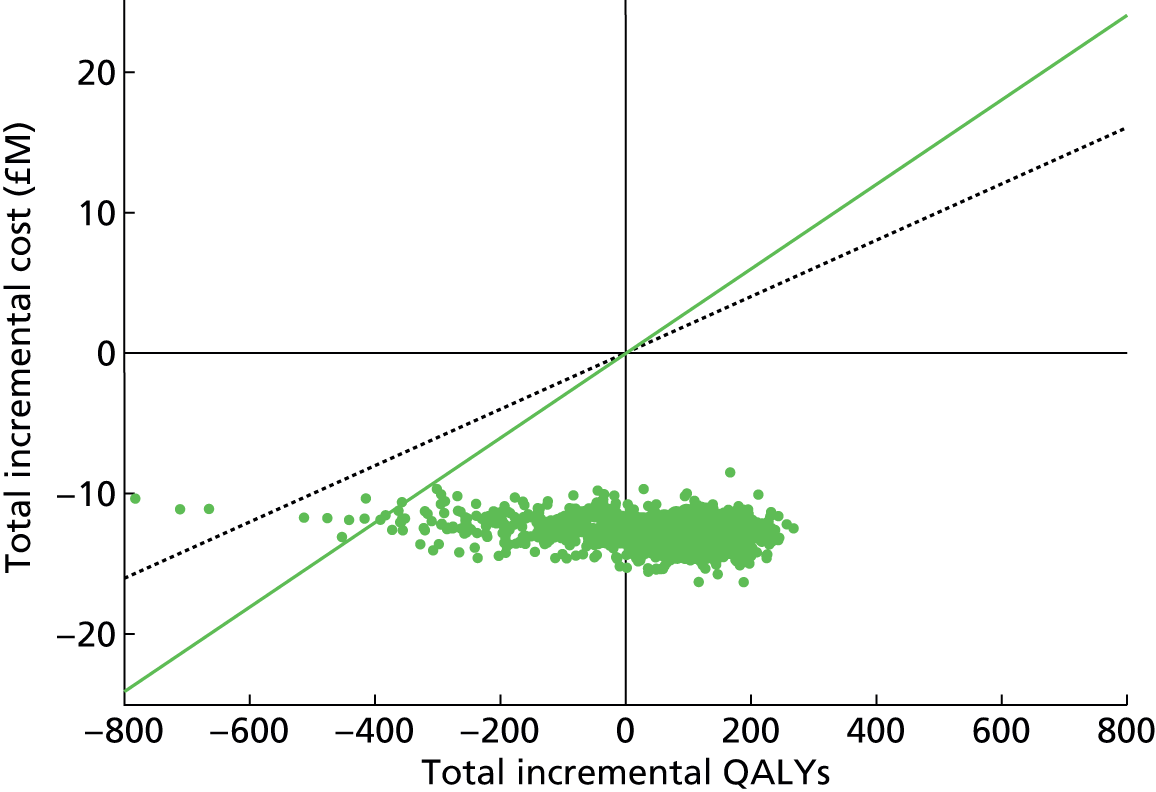
FIGURE 44.
Cost-effectiveness plane of PSA samples of regional MTBDRplus-based testing compared with current practice in Black Africans over a 10-year time horizon. This figure shows the ICER pairs resulting from comparing MTBDRplus-based TB confirmation and DST in Black Africans with current practice in PSA. The stochastic parameters included in the samples were those associated with TB diagnosis time delay, costs and QALYs on the cost-effectiveness of MTBDRplus-based TB confirmation and DST compared with current practice. Parameters were varied in both current practice and the intervention scenario. The green line indicates the threshold £30,000 per QALY and the black dashed line £20,000 per QALY.
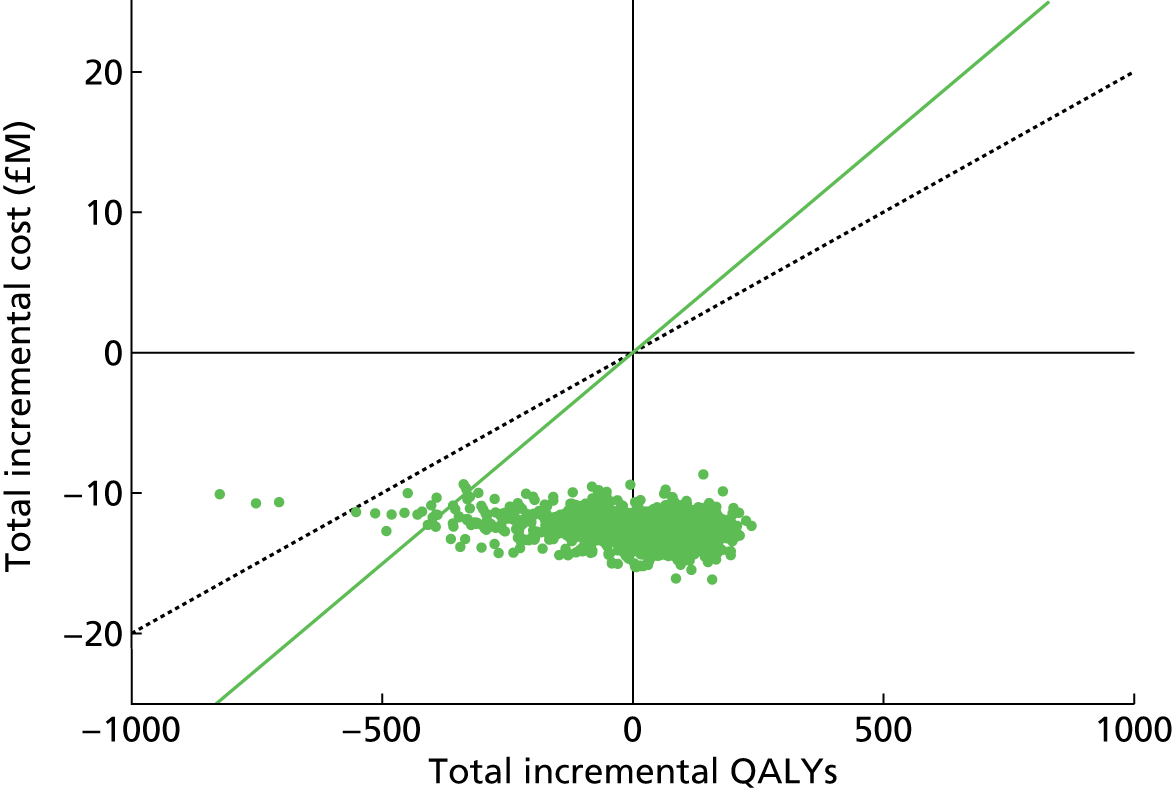
When local and regional testing are compared (Figure 45), there are reductions in QALYs and increases in costs in almost all of the realisations of the PSA when moving from local to regional testing.
FIGURE 45.
Cost-effectiveness plane of PSA samples of regional vs. local MTBDRplus-based testing compared with current practice over a 10-year time horizon in Black Africans. This figure shows the ICER pairs resulting from comparing regionally based MTBDRplus-based TB confirmation and DST in Black Africans with locally based MTBDRplus-based TB confirmation and DST in PSA. The stochastic parameters included in the samples were those associated with TB diagnosis time delay, costs and QALYs on the cost-effectiveness of MTBDRplus-based TB confirmation and DST compared with current practice. Parameters were varied in both current practice and the intervention scenario. The green line indicates the threshold £30,000 per QALY and the black dashed line £20,000 per QALY. Note the very small magnitude of the differences.

Figure 46 shows that the most influential test performance characteristic that affects the cost-effectiveness of the MTBDRplus scenario compared with current practice is the sensitivity for detection of TB infection in smear-positive patients. Figure 33 shows how moving from current practice to the GeneXpert scenario results in a reduction in annual TB diagnoses, whereas moving from current practice to the MTBDRplus scenario results in an increase in diagnoses; the key difference is the specificity of the tests.
FIGURE 46.
Tornado plot of the influence on INB in Black Africans of molecular testing compared with current practice, over a 10-year time horizon. This figure shows the effects of varying different test performance parameters on the INB of MTBDRplus-based TB confirmation and DST in Black Africans compared with current practice, if a QALY is valued at £20,000. Individually, parameters are varied from their minimum (green bar) to maximum (black bar) values.

Eastern Europeans
As we do not have information allowing us to estimate transmission rates in the UK in this group, we cannot account for an effect on transmission.
With a 10-year time horizon, there is a modest INB in the GeneXpert scenario, MTBDRplus scenario and INNO-LiPA scenario of a similar magnitude (Table 46 and Figure 47). All three molecular tests may be considered cost-effective according to usual criteria of valuing a QALY at £20,000 or £30,000.
| Scenario | Total costs, £M (95% CI) | Total QALYs (95% CI) | Compared with baseline | INB, £M (95% CI) | ||
|---|---|---|---|---|---|---|
| Incremental cost, £M (95% CI) | Incremental QALYs (95% CI) | QALY = £20,000 | QALY = £30,000 | |||
| Current | 1.1928 (1.3 to 1.5) | 8,447,788 (8,447,787 to 8,447,789) | – | – | – | – |
| GeneXpert local | 0.4636 (0.4615 to 0.4656) | 8,447,791.6 (8,447,791.3 to 8,447,791.9) | –0.7287 (–0.7318 to –0.7256) | 3.8005 (3.7562 to 3.8449) | 0.8047 (0.8015 to 0.808) | 0.8427 (0.8394 to 0.8461) |
| GeneXpert regional | 0.4634 (0.4613 to 0.4654) | 8,447,791 (8,447,790.6 to 8,447,791.2) | –0.7289 (–0.732 to –0.7258) | 3.0969 (3.053 to 3.1407) | 0.7909 (0.7876 to 0.7941) | 0.8218 (0.8185 to 0.8252) |
| MTBDRplus local | 0.4583 (0.4561 to 0.4606) | 8,447,789 (8,447,788.4 to 8,447,789.1) | –0.7339 (–0.7371 to –0.7307) | 0.9526 (0.7683 to 1.1368) | 0.753 (0.7485 to 0.7575) | 0.7625 (0.7566 to 0.7685) |
| MTBDRplus regional | 0.459 (0.4568 to 0.4612) | 8,447,788 (8,447,787.7 to 8,447,788.4) | –0.7333 (–0.7365 to –0.7301) | 0.2067 (0.0207 to 0.3927) | 0.7374 (0.7329 to 0.7419) | 0.7395 (0.7335 to 0.7455) |
| INNO-LiPA local | 0.5061 (0.5038 to 0.5085) | 8,447,788 (8,447,787.9 to 8,447,788.5) | –0.6861 (–0.6894 to –0.6829) | 0.3936 (0.3483 to 0.4389) | 0.694 (0.6905 to 0.6975) | 0.6979 (0.6943 to 0.7016) |
| INNO-LiPA regional | 0.5046 (0.5023 to 0.5069) | 8,447,788 (8,447,787.3 to 8,447,787.9) | –0.6877 (–0.691 to –0.6844) | –0.1908 (–0.2349 to –0.1467) | 0.6839 (0.6805 to 0.6873) | 0.682 (0.6784 to 0.6856) |
FIGURE 47.
Cost-effectiveness plane of different molecular testing interventions compared with current practice in Eastern Europeans over a 10-year time horizon. This figure shows the cost-effectiveness plane resulting from comparing the effect of different rapid molecular tests for TB confirmation and DST with the current practice in Eastern Europeans. Three different molecular tests are considered (GeneXpert only, GeneXpert combined with INNO-LiPA line-probe assay and MTBDRplus) in two locations: either local to the hospital or in a regional laboratory. The solid diagonal line indicates the threshold £30,000 per QALY and the dashed line £20,000 per QALY.

The INBs for the GeneXpert, MTBDRplus and INNO-LiPA scenarios (see Table 46 and Figure 47) are, in all cases, slightly greater in the local testing configuration than in the regional testing. The health benefit of local testing is greater, as a result of earlier diagnoses and treatment initiation.
A similar pattern of results was found with a 20-year time horizon (Table 47 and Figure 48).
| Scenario | Total costs, £M (95% CI) | Total QALYs (95% CI) | Compared with baseline | INB, £M (95% CI) | ||
|---|---|---|---|---|---|---|
| Incremental cost, £M (95% CI) | Incremental QALYs (95% CI) | QALY = £20,000 | QALY = £30,000 | |||
| Current | 2.0383 (2.1 to 2.6) | 14,436,584 (14,436,582 to 14,436,586) | – | – | – | – |
| GeneXpert local | 0.7922 (0.7886 to 0.7957) | 14,436,592 (14,436,591.4 to 14,436,592.6) | –1.2454 (–1.2507 to –1.2401) | 8.8709 (8.7592 to 8.9825) | 1.4228 (1.417 to 1.4285) | 1.5115 (1.5052 to 1.5178) |
| GeneXpert regional | 0.7919 (0.7883 to 0.7954) | 14,436,590 (14,436,589.6 to 14,436,590.9) | –1.2457 (–1.251 to –1.2404) | 7.1578 (7.0473 to 7.2683) | 1.3889 (1.3831 to 1.3946) | 1.4604 (1.4542 to 1.4667) |
| MTBDRplus local | 0.7833 (0.7795 to 0.7871) | 14,436,585 (14,436,584.4 to 14,436,586) | –1.2543 (–1.2598 to –1.2488) | 2.0361 (1.5819 to 2.4903) | 1.295 (1.2852 to 1.3048) | 1.3154 (1.3015 to 1.3292) |
| MTBDRplus regional | 0.7844 (0.7806 to 0.7882) | 14,436,583 (14,436,582.5 to 14,436,584.1) | –1.2532 (–1.2586 to –1.2477) | 0.213 (–0.2455 to 0.6715) | 1.2574 (1.2475 to 1.2673) | 1.2595 (1.2456 to 1.2735) |
| INNO–LiPA local | 0.865 (0.861 to 0.8689) | 14,436,585 (14,436,584 to 14,436,585.4) | –1.1726 (–1.1782 to –1.167) | 1.5698 (1.4584 to 1.6813) | 1.204 (1.1979 to 1.2101) | 1.2197 (1.2131 to 1.2263) |
| INNO–LiPA regional | 0.8623 (0.8584 to 0.8662) | 14,436,583 (14,436,582.5 to 14,436,583.8) | –1.1753 (–1.1808 to –1.1697) | 0.0678 (–0.0413 to 0.1769) | 1.1766 (1.1706 to 1.1826) | 1.1773 (1.1708 to 1.1838) |
FIGURE 48.
Cost-effectiveness plane of different molecular testing interventions compared with current practice in Eastern Europeans over a 20-year time horizon. This figure shows the cost-effectiveness plane resulting from comparing the effect of different rapid molecular tests for TB confirmation and DST with the current practice in Eastern Europeans. Three different molecular tests are considered (GeneXpert only, GeneXpert combined with INNO-LiPA line-probe assay, and MTBDRplus) in two locations: either local to the hospital or in a regional laboratory. The solid diagonal line indicates the threshold £30,000 per QALY and the dashed line £20,000 per QALY.

GeneXpert only
There is uncertainty in the GeneXpert scenario over the impact on both costs and, particularly, health, and hence there is uncertainty in cost-effectiveness (Figures 49 and 50). However, in all realisations of the PSA there is a net reduction in costs, and net increases in QALYs.
FIGURE 49.
Cost-effectiveness plane of PSA samples of local GeneXpert-based testing compared with current practice in Eastern Europeans over a 10-year time horizon. This figure shows the ICER pairs resulting from comparing GeneXpert-based TB confirmation and DST in Eastern Europeans with current practice in PSA. The stochastic parameters included in the samples were those associated with TB diagnosis time delay, costs and QALYs on the cost-effectiveness of GeneXpert-based TB confirmation and DST compared with current practice. Parameters were varied in both current practice and the intervention scenario. The green line indicates the threshold £30,000 per QALY and the black dashed line £20,000 per QALY.
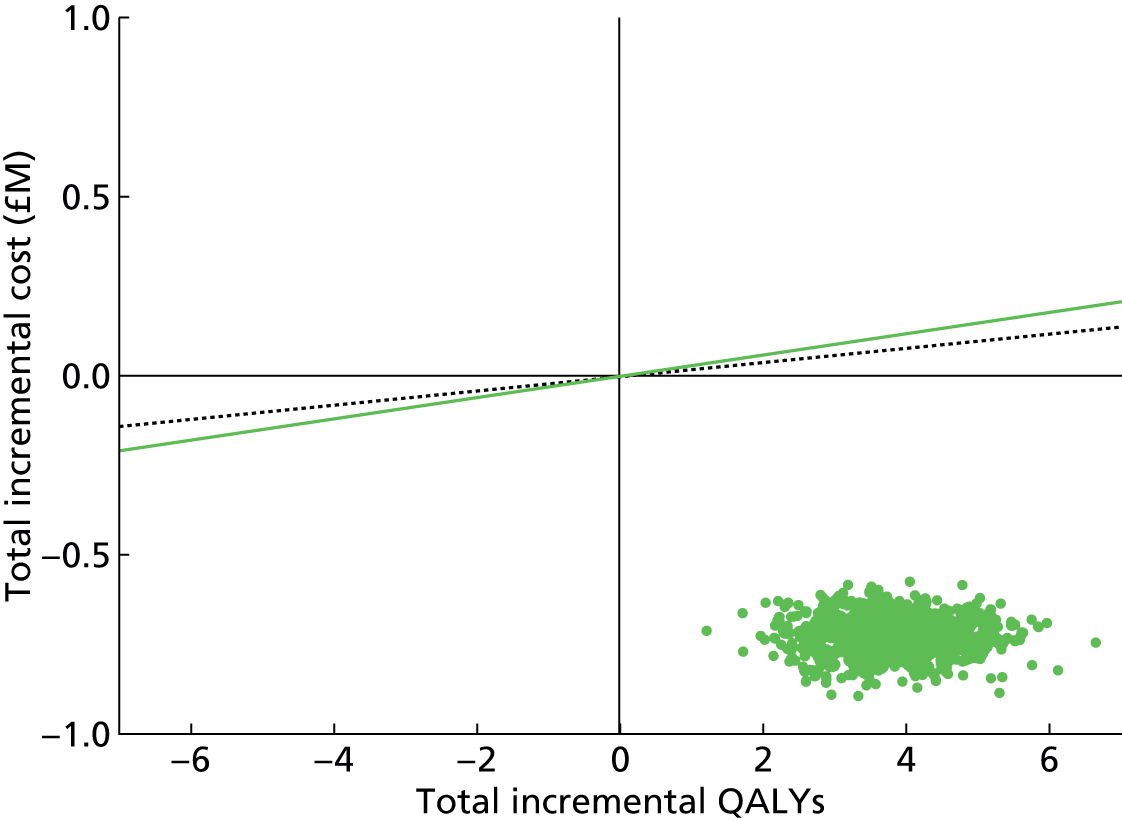
FIGURE 50.
Cost-effectiveness plane of PSA samples of regional GeneXpert-based testing compared with current practice in Eastern Europeans over a 10-year time horizon. This figure shows the ICER pairs resulting from comparing GeneXpert-based TB confirmation and DST in Eastern Europeans with current practice in PSA. The stochastic parameters included in the samples were those associated with TB diagnosis time delay, costs and QALYs on the cost-effectiveness of GeneXpert-based TB confirmation and DST compared with current practice. Parameters were varied in both current practice and the intervention scenario. The green line indicates the threshold £30,000 per QALY and the black dashed line £20,000 per QALY.
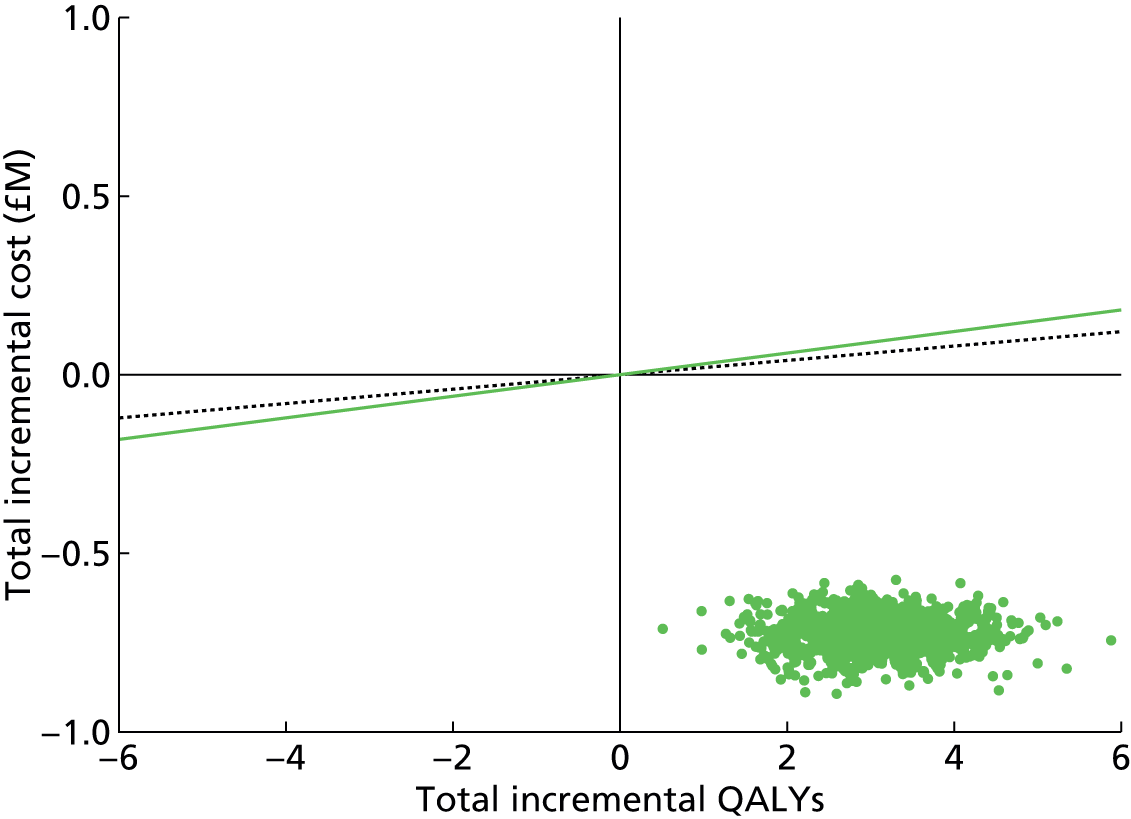
Figure 51 shows that the most influential test performance characteristic that affects the cost-effectiveness of the GeneXpert scenario compared with current practice is the specificity for detection of MDR infection in those infected with TB. Time to DST (culture) result was also influential.
FIGURE 51.
Tornado plot of the influence on INB in Eastern Europeans of GeneXpert-based testing compared with current practice, over a 10-year time horizon. This figure shows the effects of varying different test performance parameters on the INB of GeneXpert-based TB confirmation and DST in Eastern Europeans compared with current practice, if a QALY is valued at £20,000. Individually, parameters are varied from their minimum (green bar) to maximum (black bar) values.

GeneXpert with INNO-LiPA test
In the INNO-LiPA scenario, although there is considerable uncertainty in both the impact on costs and health, in all realisations of the PSA for both local and regional testing there is a reduction in costs. However, there is a large variation in the effect on incremental QALYs, ranging from negative to positive (Figures 52 and 53).
FIGURE 52.
Cost-effectiveness plane of PSA samples of local GeneXpert with INNO-LiPA-based testing compared with current practice in Eastern Europeans over a 10-year time horizon. This figure shows the ICER pairs resulting from comparing GeneXpert with INNO-LiPA test-based TB confirmation and DST in Eastern Europeans with current practice in PSA. The stochastic parameters included in the samples were those associated with TB diagnosis time delay, costs and QALYs on the cost-effectiveness of GeneXpert with INNO-LiPA test-based TB confirmation and DST compared with current practice. Parameters were varied in both current practice and the intervention scenario. The green line indicates the threshold £30,000 per QALY and the black dashed line £20,000 per QALY.

FIGURE 53.
Cost-effectiveness plane of PSA samples of regional GeneXpert with INNO-LiPA-based testing compared with current practice in Eastern Europeans over a 10-year time horizon. This figure shows the ICER pairs resulting from comparing GeneXpert with INNO-LiPA test-based TB confirmation and DST in Eastern Europeans with current practice in PSA. The stochastic parameters included in the samples were those associated with TB diagnosis time delay, costs and QALYs on the cost-effectiveness of GeneXpert with INNO-LiPA test-based TB confirmation and DST compared with current practice. Parameters were varied in both current practice and the intervention scenario. The green line indicates the threshold £30,000 per QALY and the black dashed line £20,000 per QALY.
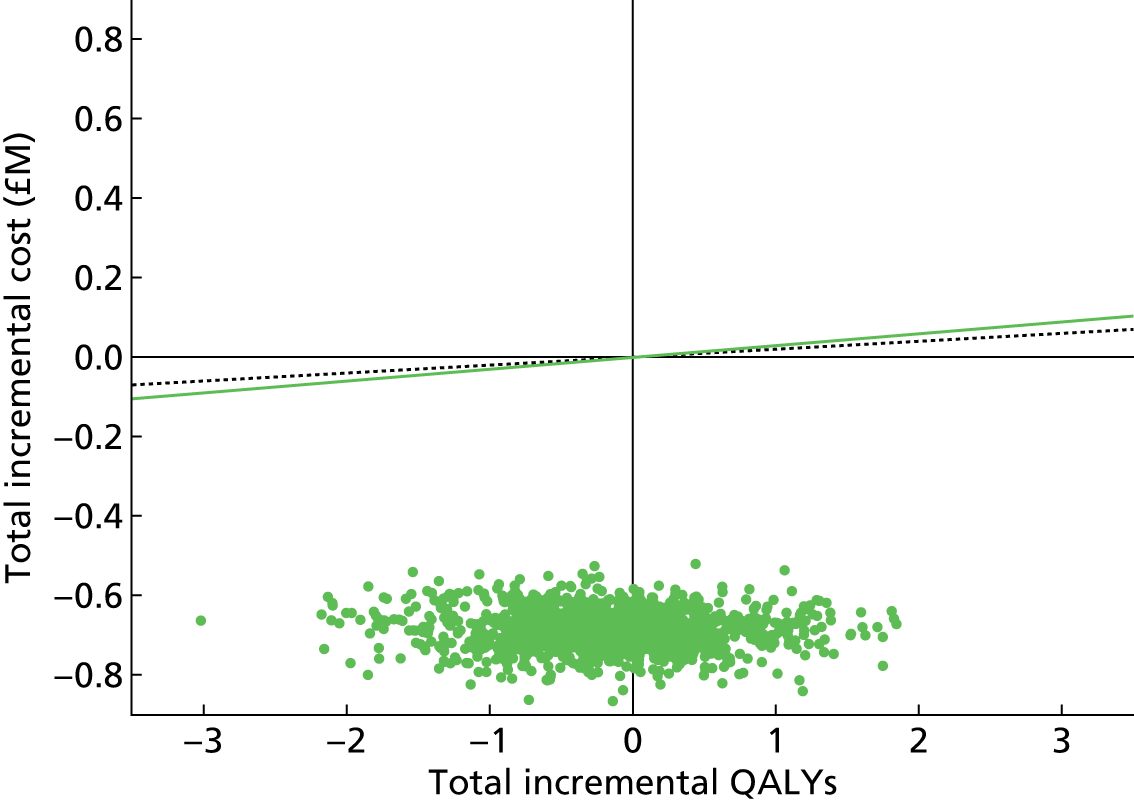
Figure 54 shows that the most influential test performance characteristic that affects the cost-effectiveness of the GeneXpert with INNO-LiPA scenario compared with current practice is the specificity for detection of MDR infection in those infected with TB.
FIGURE 54.
Tornado plot of the influence on INB in Eastern Europeans of GeneXpert with INNO-LiPA-based testing compared with current practice, over a 10-year time horizon. This figure shows the effects of varying different test performance parameters on the INB of GeneXpert with INNO-LiPA-based TB confirmation and DST in Eastern Europeans compared with current practice, if a QALY is valued at £20,000. Individually, parameters are varied from their minimum (green bar) to maximum (black bar) values.
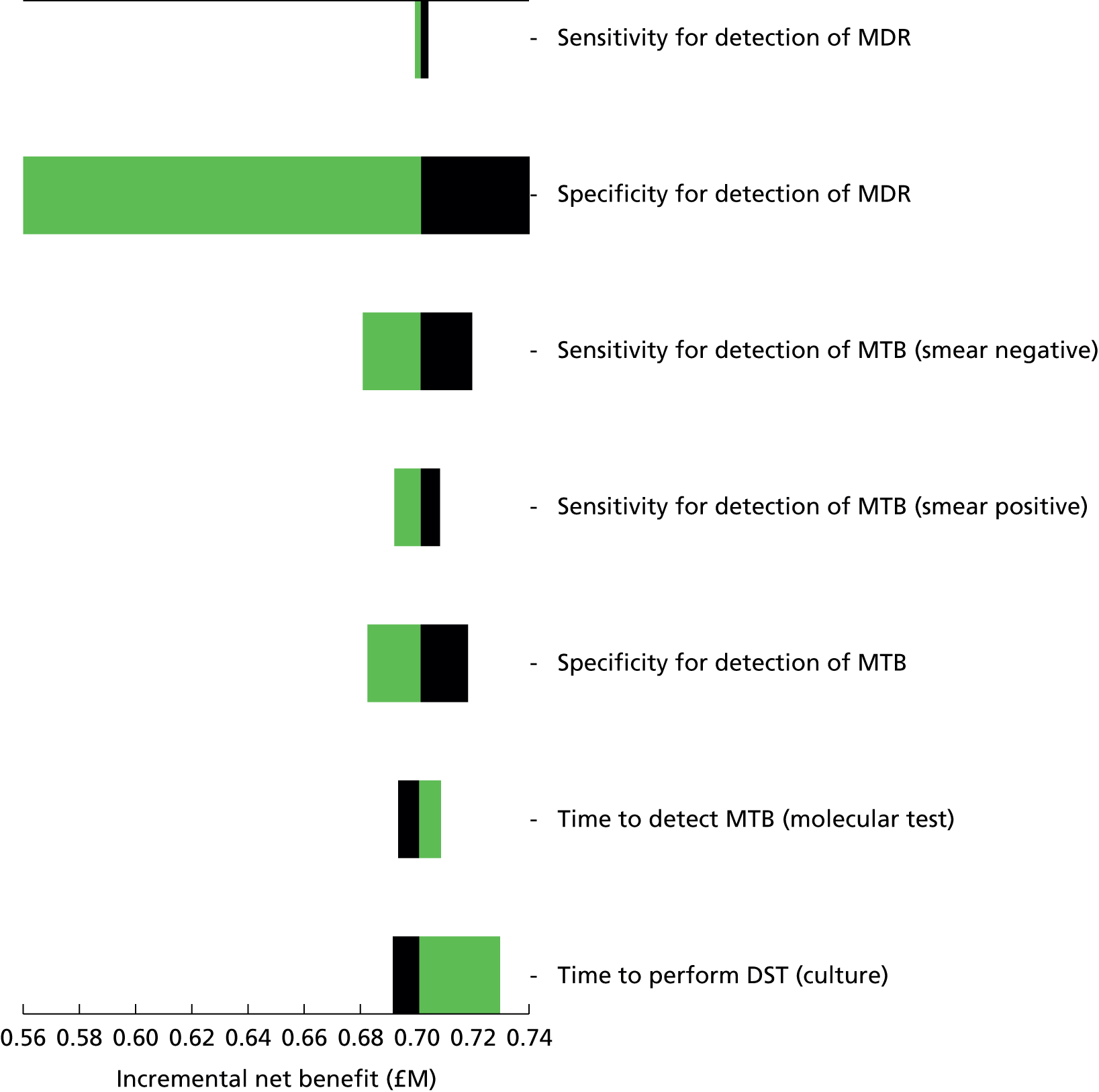
MTBDRplus test
In the MTBDRplus scenario (Figures 55 and 56), there is a wide range of uncertainty with regard to the impact on health and, to some extent, on costs. In all realisations of the PSA there is a reduction in net costs, but the effect on health is uncertain, with incremental QALYs ranging from negative to positive.
FIGURE 55.
Cost-effectiveness plane of PSA samples of local MTBDRplus-based testing compared with current practice in Eastern Europeans over a 10-year time horizon. This figure shows the ICER pairs resulting from comparing MTBDRplus-based TB confirmation and DST in Eastern Europeans with current practice in PSA. The stochastic parameters included in the samples were those associated with TB diagnosis time delay, costs and QALYs on the cost-effectiveness of MTBDRplus-based TB confirmation and DST compared with current practice. Parameters were varied in both current practice and the intervention scenario. The green line indicates the threshold £30,000 per QALY and the black dashed line £20,000 per QALY.
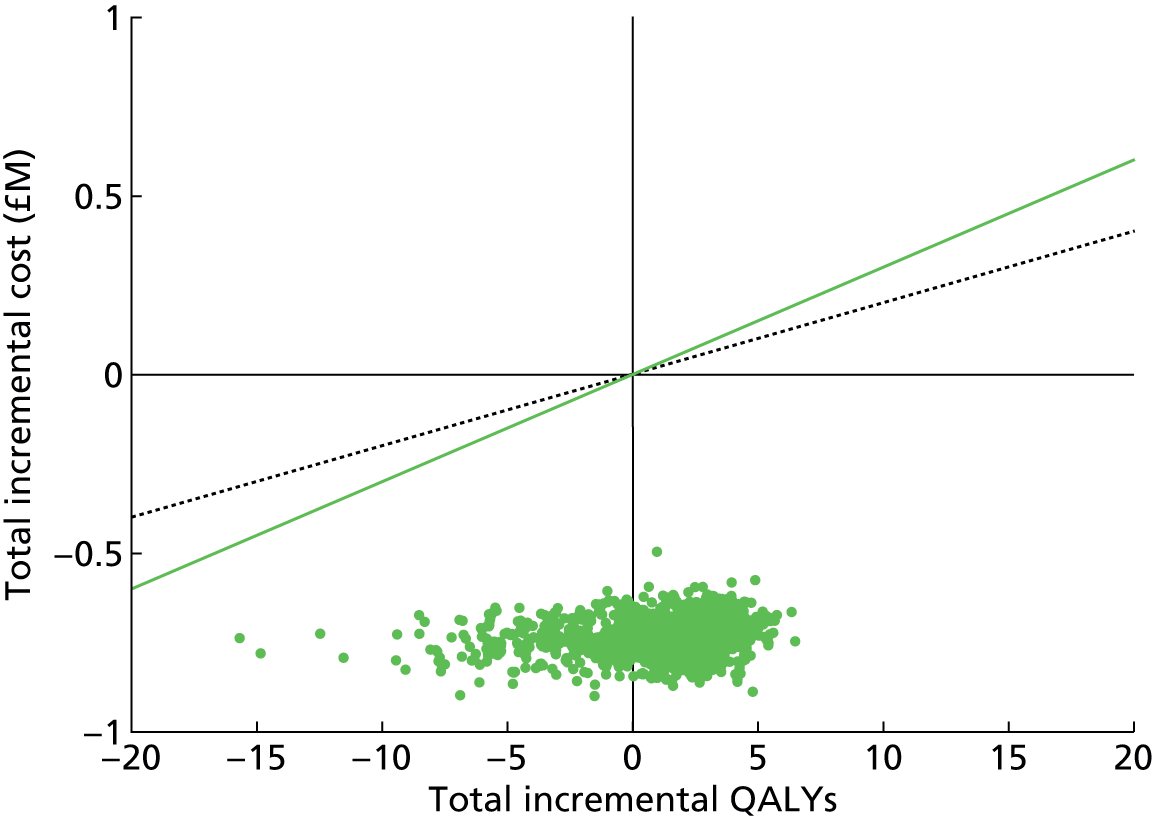
FIGURE 56.
Cost-effectiveness plane of PSA samples of regional MTBDRplus-based testing compared with current practice in Eastern Europeans over a 10-year time horizon. This figure shows the ICER pairs resulting from comparing MTBDRplus-based TB confirmation and DST in Eastern Europeans with current practice in PSA. The stochastic parameters included in the samples were those associated with TB diagnosis time delay, costs and QALYs on the cost-effectiveness of MTBDRplus-based TB confirmation and DST compared with current practice. Parameters were varied in both current practice and the intervention scenario. The green line indicates the threshold £30,000 per QALY and the black dashed line £20,000 per QALY.
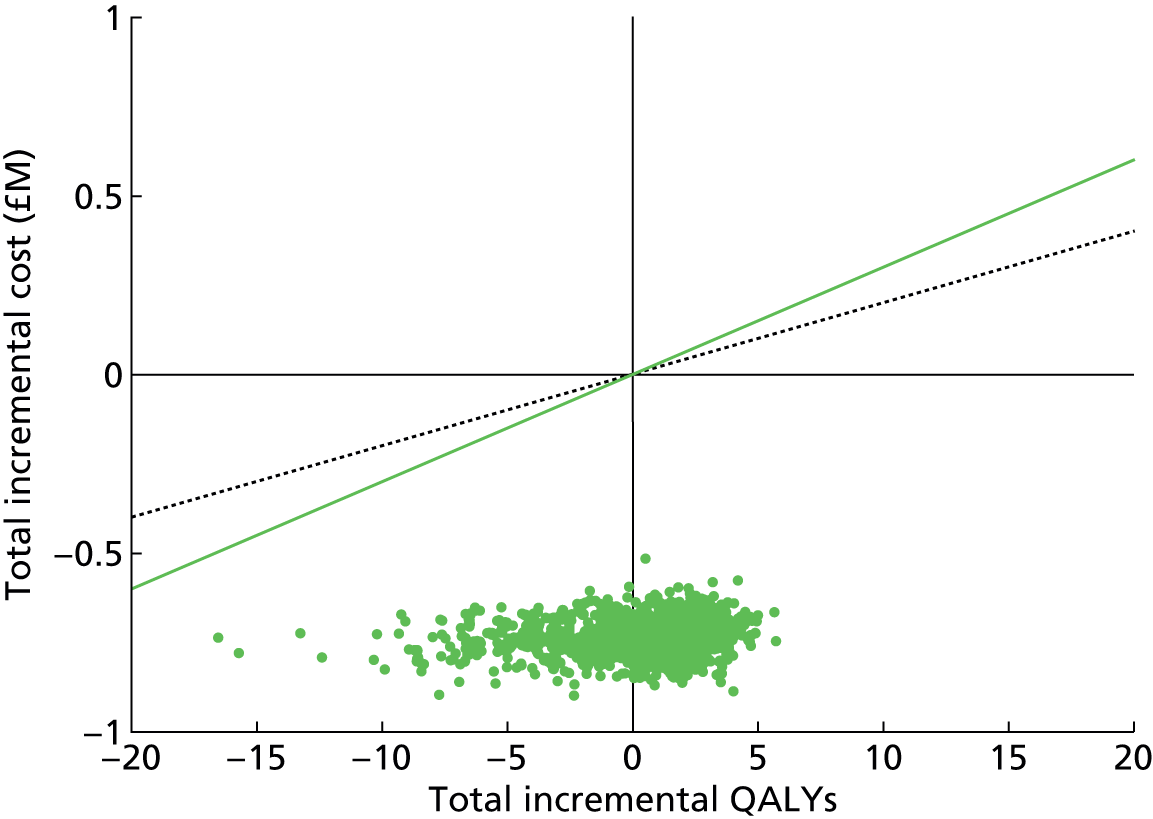
When local and regional testing (Figure 57) are compared, there are reductions in QALYs in 100% of realisations of the PSA and increases in costs in the majority of realisations of the PSA when moving from local to regional testing.
FIGURE 57.
Cost-effectiveness plane of PSA samples of regional vs. local MTBDRplus-based testing compared with current practice over a 10-year time horizon in Eastern Europeans. This figure shows the ICER pairs resulting from comparing regionally based MTBDRplus-based TB confirmation and DST in Eastern Europeans with locally based MTBDRplus-based TB confirmation and DST in PSA. The stochastic parameters included in the samples were those associated with TB diagnosis time delay, costs and QALYs on the cost-effectiveness of MTBDRplus-based TB confirmation and DST compared with current practice. Parameters were varied in both current practice and the intervention scenario. The green line indicates the threshold £30,000 per QALY and the black dashed line £20,000 per QALY. Note the very small magnitude of the differences.
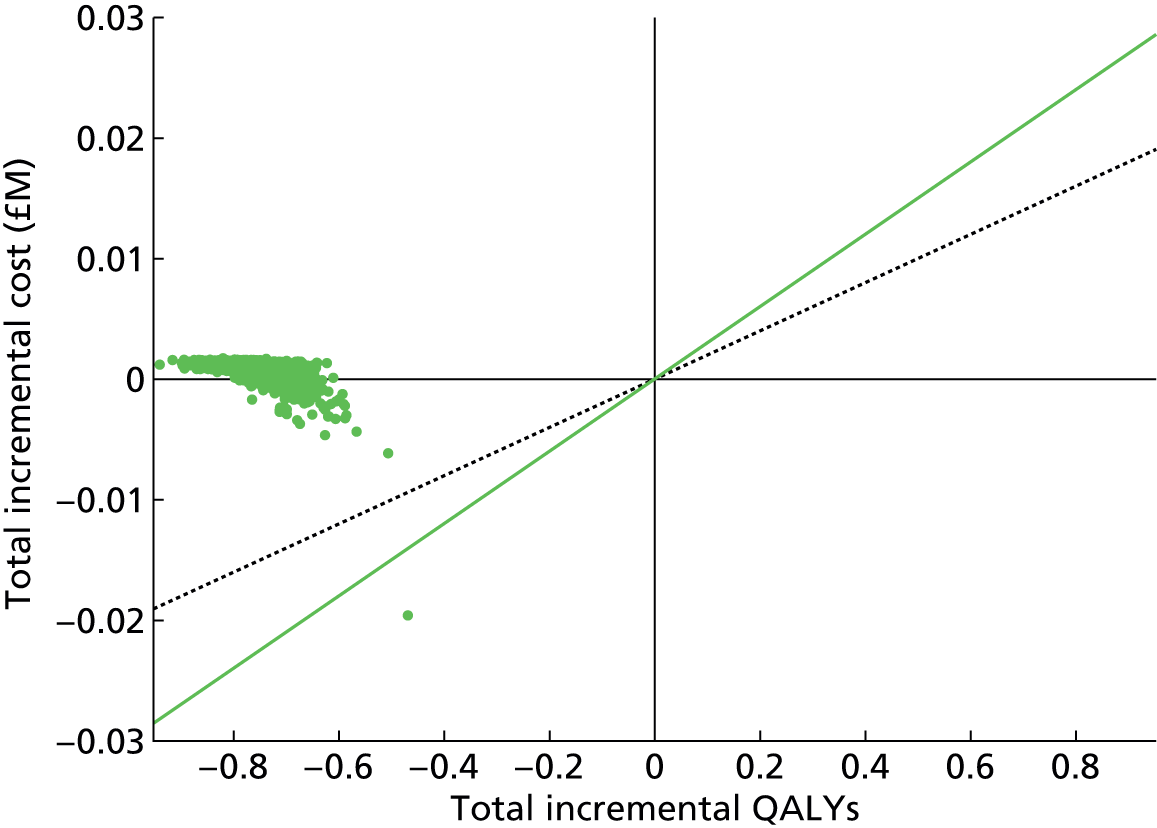
Figure 58 shows that the most influential test performance characteristics that affect the cost-effectiveness of the MTBDRplus scenario compared with current practice are the sensitivity for detection of smear-positive TB infection and the specificity for detection of TB infection (as FP results lead to inappropriate treatment, which incurs cost and harms health).
FIGURE 58.
Tornado plot of the influence on INB in Eastern Europeans of MTBDRplus-based testing compared with current practice, over a 10-year time horizon. This figure shows the effects of varying different test performance parameters on the INB of MTBDRplus-based TB confirmation and DST in Eastern Europeans compared with current practice, if a QALY is valued at £20,000. Individually, parameters are varied from their minimum (green bar) to maximum (black bar) values.

GeneXpert only compared with MTBDRplus test
Comparing the cost-effectiveness of regionally based testing in the GeneXpert and MTBDRplus scenarios finds that there is considerable uncertainty with regard to health benefits (Figure 59) but little uncertainty in costs, with the effect being very small.
FIGURE 59.
Cost-effectiveness plane of PSA samples of regional GeneXpert vs. regional MTBDRplus molecular testing compared with current practice over a 10-year time horizon in Eastern Europeans. This figure shows the ICER pairs resulting from comparing regionally based GeneXpert-based TB confirmation and DST in Eastern Europeans with regionally based MTBDRplus-based TB confirmation and DST-based TB confirmation and DST in PSA. The stochastic parameters included in the samples were those associated with TB diagnosis time delay, costs and QALYs on the cost-effectiveness of GeneXpert-based TB confirmation and DST compared with current practice. Parameters were varied in both current practice and the intervention scenario. The green line indicates the threshold £30,000 per QALY and the black dashed line £20,000 per QALY.
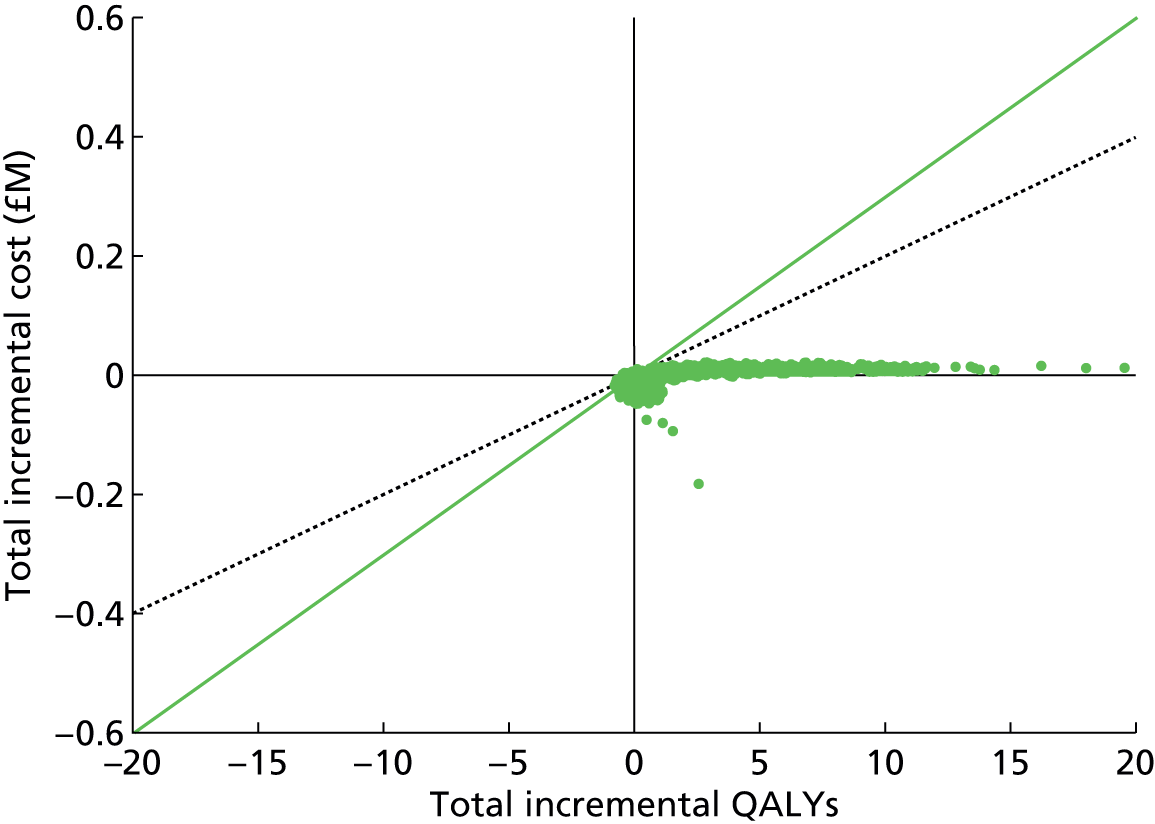
Summary
The introduction of molecular testing was estimated to have only a very small impact on transmission, because current practice is effective in limiting transmission from TB patients while they are undergoing clinical examination.
There is likely to be a benefit to smear-negative patients, most of whom test positive by molecular methods and therefore can be diagnosed and start treatment earlier than if they had to wait for culture confirmation.
Molecular tests are likely to increase annual numbers of TB diagnoses as a result of faster TB diagnoses and FP results, as a result of imperfect specificity. This results in inappropriate treatment of some patients, which incurs a QALY loss as well as a financial cost.
The major benefit of molecular testing is faster diagnosis of MDR-TB, which produces cost savings when patients with smear-positive suspected MDR-TB are kept in isolation until their MDR status is known and then appropriate treatment commenced, this is because isolation has a high daily cost.
Overall, all molecular-testing scenarios considered were more cost-effective compared with current practice at conventional threshold values per QALY for the UK (Table 48).
| Assay | South Asian | Black African | Eastern European | |||
|---|---|---|---|---|---|---|
| Local | Regional | Local | Regional | Local | Regional | |
| 10-year time horizon | ||||||
| £20,000 per QALY | ||||||
| GeneXpert | 20.3 (20.3 to 20.4) | 20.2 (20.1 to 20.2) | 16.8 (16.7 to 16.9) | 16.1 (16.0 to 16.2) | 0.8047 (0.8015 to 0.8080) | 0.7909 (0.7876 to 0.7941) |
| MTBDRplus | 20.5 (20.4 to 20.6) | 20.3 (20.2 to 20.4) | 13.6 (13.4 to 13.8) | 12.8 (12.6 to 13.0) | 0.753 (0.7485 to 0.7575) | 0.7374 (0.7329 to 0.7419) |
| INNO-LiPA | 17.5 (17.4 to 17.6) | 17.4 (17.3 to 17.5) | 13.2 (13.1 to 13.3) | 12.5 (12.5 to 12.6) | 0.694 (0.6905 to 0.6975) | 0.6839 (0.6805 to 0.6873) |
| £30,000 per QALY | ||||||
| GeneXpert | 21.8 (21.7 to 21.9) | 21.7 (21.6 to 21.8) | 18.6 (18.5 to 18.7) | 17.6 (17.6 to 17.7) | 0.8427 (0.8394 to 0.8461) | 0.8218 (0.8185 to 0.8252) |
| MTBDRplus | 22.6 (22.5 to 22.7) | 22.3 (22.2 to 22.4) | 14 (13.7 to 14.3) | 12.9 (12.7 to 13.2) | 0.7625 (0.7566 to 0.7685) | 0.7395 (0.7335 to 0.7455) |
| INNO-LiPA | 18.7 (18.6 to 18.7) | 18.6 (18.5 to 18.7) | 13.9 (13.8 to 14.0) | 13 (12.9 to 13.0) | 0.6979 (0.6943 to 0.7016) | 0.682 (0.6784 to 0.6856) |
| 20-year time horizon | ||||||
| £20,000 per QALY | ||||||
| GeneXpert | 35.4 (35.3 to 35.6) | 36.3 (36.2. 36.5) | 33 (32.9 to 33.2) | 31.1 (30.9 to 31.3) | 1.4228 (1.4170 to 1.4285) | 1.3889 (1.3831 to 1.3946) |
| MTBDRplus | 44.2 (43.7 to 44.8) | 45 (44.5 to 45.6) | 23.5 (22.9 to 24.1) | 21.3 (20.8 to 21.9) | 1.295 (1.2852 to 1.3048) | 1.2574 (1.2475 to 1.2673) |
| INNO-LiPA | 34.5 (34.3 to 34.6) | 35.5 (35.3 to 35.7) | 24.4 (24.3 to 24.6) | 22.5 (22.4 to 22.6) | 1.204 (1.1979 to 1.2101) | 1.1766 (1.1706 to 1.1826) |
| £30,000 per QALY | ||||||
| GeneXpert | 38.3 (38.1 to 38.4) | 39.8 (39.7 to 40.0) | 38.1 (37.9 to 38.3) | 35.3 (35.1 to 35.5) | 1.5115 (1.5052 to 1.5178) | 1.4604 (1.4542 to 1.4667) |
| MTBDRplus | 52.3 (51.5 to 53.2) | 53.8 (53.0 to 54.6) | 24.2 (23.4 to 25.1) | 21.2 (20.3 to 22.0) | 1.3154 (1.3015 to 1.3292) | 1.2595 (1.2456 to 1.2735) |
| INNO-LiPA | 38.8 (38.6 to 38.9) | 40.5 (40.4 to 40.7) | 26.4 (26.2 to 26.5) | 23.6 (23.4 to 23.8) | 1.2197 (1.2131 to 1.2263) | 1.1773 (1.708 to 1.1838) |
Based on our data and assumptions, the most cost-effective assay differed between the population groups considered. In a South Asian population, MTBDRplus had the largest estimated INB based on the conventional UK cost-effectiveness thresholds of £20,000 per QALY and £30,000 per QALY, and over both 10- and 20-year time horizons. At a time horizon of 10 years, GeneXpert had a similar INB to MTBDRplus, whereas INNO-LiPA had a lower estimated INB. However, at a time horizon of 20 years, the estimated INB for GeneXpert and INNO-LiPA were similar (and both lower than the estimated INB for MTBDRplus). In this population there was a very high level of uncertainty over the relative cost-effectiveness of local scenarios compared with regional test scenarios.
In the Black African and Eastern European populations, GeneXpert was consistently the most cost-effective rapid assay considered, with the greatest INB across 10- and 20-year time horizons, and at £20,000 and £30,000 per QALY thresholds. Estimated INBs were similar for MTBDRplus and INNO-LiPA. In these populations the local testing scenarios appeared more cost-effective than the regional scenarios but absolute differences were small.
It should be emphasised that there is considerable uncertainty over the relative cost-effectiveness of the alternative rapid assays and particularly over the comparison between regional and local testing scenarios. This uncertainty has been quantified in the PSA results and tornado diagrams presented in this chapter. However, there is additional uncertainty related to modelling assumptions and data that could not be integrated in these sensitivity analyses.
Chapter 7 Discussion
The increasing prevalence of drug-resistance threatens TB control programmes worldwide. Conventional culture-based DST is a slow process, which can take several weeks to be completed; however, it remains the ‘gold standard’ for MTB diagnosis. Rapid diagnosis of drug resistance (or its exclusion), allowing early implementation of an appropriate treatment regimen, both decreases patient morbidity and, by rendering the individual non-infectious, reduces disease transmission. Such tests are useful in determining the type and degree of isolation needed, which can be a significant part of the overall treatment costs. Similarly, the length of treatment for drug-susceptible TB (6 months) compared with the almost 2 years needed for MDR-TB therapy requires accurate and reliable tests. The impact of diagnostic delay on patient care can be severe (see Appendix 8). Molecular tests for the diagnosis of drug resistance, particularly rifampicin and isoniazid, are widely available and have moved from the status of in-house tests to commercial products; however, the health-economic impact of their adoption into the diagnostic pathway has remained incompletely explored.
In this study, we addressed key questions regarding the performance [specificity, sensitivity, positive predictive value (PPV), negative predictive value (NPV)] of the main commercial systems available and endorsed globally. We assessed the likely clinical impact and cost-effectiveness of different assays identifying genetic markers associated with MDR- and XDR-TB in patients with TB across the UK. First, this entailed a systematic review of the literature to establish the diagnostic accuracy parameters of the most popular molecular tests compared with conventional culture-based DST. Second, a decision-analytic health-economic model of various screening strategies and transmission-dynamic model was developed to determine the implications of secondary transmission. The study focused on the two main commercial LPAs for detection of MDR-TB, INNO-LiPA and MTBDRplus, and the automated real-time PCR-based assay, GeneXpert.
Systematic review
Principal findings
The findings of this review suggest that all three commercial tests, INNO-LiPA, GeneXpert and MTBDRplus, demonstrate promising levels of diagnostic discrimination when compared with standard culture-based methods. The findings of the current study are consistent with previous estimates that have indicated that these rapid diagnostic tests can demonstrate high levels of sensitivity and specificity when detecting rifampicin and isoniazid susceptibility in clinical samples. 27,47,145
It is reassuring that despite different selection criteria and the exclusion of studies using cultured samples, the pooled estimates of sensitivity (83.4%, 95% CI 66.3% to 100.0%) and specificity (99.6%, 95% CI 99.0% to 100.0%) for detecting isoniazid resistance by MTBDRplus are similar to those of Ling et al. ,27 who estimated a sensitivity of 84.3% (95% CI 75.5% to 89.9%) and specificity of 99.5% (95% CI 97.5% to 99.9%).
This is also apparent when comparing the pooled specificity for detecting rifampicin resistance. The current study estimated a pooled specificity of 98.2% (95% CI 97.2% to 99.3%) similar to the pooled estimates of both Ling et al. 27 (99.5%, 95% CI 97.5% to 99.9%) and Arentz et al. 145 (98.0%, 95% CI 95.1% to 99.2%). However, the sensitivity of MTBDRplus was slightly lower than reported by Ling et al. 27 and Arentz et al. ,145 at 94.6% (95% CI 91.6% to 97.6%) as opposed to 98.1% (95% CI 95.9% to 99.1%) and 95.9% (95% CI 94.5% to 97.0%), respectively. These previous reviews did not include studies published after January 2012, analysed fewer studies overall in their meta-analyses, and included tests performed on cultured samples, which may account for this disparity.
The pooled estimates of sensitivity for INNO-LiPA (95.4%, 95% CI 92.2% to 98.3%) and specificity (99.7%, 95% CI 99.5% to 100.0%) suggested good levels of diagnostic accuracy when used to detect rifampicin resistance in clinical samples. These results were similar to those reported by Arentz et al. ,145 who reported pooled estimates from four studies of 94.1% (95% CI 86.5% to 97.6%) for sensitivity and 98.8% (95% CI 93.8% to 99.8%) for specificity.
Equally, the pooled estimates of sensitivity (96.8%, 95% CI 94.2% to 99.4%) and specificity (98.4%, 95% CI 97.8% to 99.0%) for the detection of rifampicin resistance by GeneXpert are also similar to those published by a previous review,45 where the pooled sensitivity was estimated at 94% (95% CI 87% to 97%) and the specificity 98% (95% CI 97% to 99%).
The quality of each study deemed eligible, either through bias or concerns regarding applicability, was assessed using the QUADAS-2. 146 In the ‘Flow and timing’ domain, used to assess possible biases introduced by the use of patient samples, there was often a marked lack of clarity surrounding the timing and thresholds of the index test and reference test, and the exclusion of samples from the assay. When compared with the studies included in the INNO-LiPA and GeneXpert analysis, there appeared to be a marked reduction in the quality of methodological reporting with studies using MTBDRplus. The lack of detail regarding timing, thresholds, patient selection and blinding resulted in the majority of studies classified as either high bias or unclear bias in the four key QUADAS domains. This is particularly marked in the ‘Flow and timing’ domain, in which all studies performed poorly.
Strengths and weaknesses
Although a certain amount of heterogeneity may be present in any meta-analyses,47,102,146 the level of heterogeneity observed in each commercial test category suggests that the predicted and pooled estimates of diagnostic accuracy should be interpreted with caution. A key limitation of this work is the absence of subcategory analyses to explore potential sources of heterogeneity between the studies. This heterogeneity may be statistical heterogeneity (e.g. caused by methodological differences) or clinical heterogeneity (e.g. differences in the HIV status of the study population recruited by studies). 102,146 There was often insufficient methodological and recruitment detail reported in the papers to record these differences systematically, as demonstrated by the small number of studies that reported details on study blinding and study design.
In addition to this, the small sample sizes used to evaluate diagnostic drug susceptibility tests have resulted in the exclusion of studies that would otherwise supplement the data set and allow subcategory analysis to be performed. The minimum number of samples criteria specified in the protocol resulted in a discrepancy between the number of studies included in this review and those published in other published systematic reviews evaluating GeneXpert and MTBDRplus. 27,45 In this review some large cross-sectional studies were excluded due to sample size alone, as a direct consequence of the low incidence of MDR-TB in the area in which the study was conducted. In these studies a large number of samples may be initially screened but the number of MDR cases are so few that there are insufficient samples to calculate sensitivity and specificity of a rapid test confidently compared with a culture-based method. It can be argued that there is value in excluding studies of diagnostic accuracy which use a small number of samples if these studies are considered on an individual basis. Small sample sizes may be an indicator of study quality and can lead to imprecise estimates of diagnostic accuracy with large CIs. 103 This may be particularly relevant when the study in question is not blinded, a small subset of unrepresentative samples may be preferentially selected, and, subsequently, adds bias to estimates of diagnostic accuracy. Yet by excluding these studies we are conversely prevented from investigating heterogeneity owing to variation in study quality by subcategory analysis, and thus the sample size restriction included in the protocol may be considered a limitation.
One of the key strengths of this work was the broad set of inclusion criteria used to systematically select the relevant studies. All of the diagnostic studies that compared a molecular test with a gold standard test were considered in this review. No restrictions on study setting were applied and studies from all countries and in all languages were eligible for inclusion. By adopting a protocol that specified that all languages were included, this review avoided potential bias that could lead to potentially misleading estimates of diagnostic accuracy. 104
Future work and research recommendations
In future it would be valuable to investigate to what extent freezing and decontaminating samples affects the apparent specificity and sensitivity of these rapid diagnostic methods. It has been noted that, although DNA may survive both freezing and decontamination, the organism may not be cultured successfully. 147 In diagnostic accuracy studies this could lead to apparent FPs when using a rapid diagnostic test. Retrospective studies, where stored samples were used, may conceivably find different assessments of diagnostic accuracy than those studies that used fresh samples.
This review supports the findings of Bachmann et al. ,103 who demonstrated a disturbing lack of formal sample size calculations in published literature of diagnostic accuracy studies. The studies included in this review did not publish or refer to formal sample size estimates to calculate adequately the diagnostic accuracy for any of the rapid diagnostic tests evaluated. As discussed previously, small sample sizes may lead to imprecise estimations of diagnostic accuracy and it is imperative that future planned evaluations of rapid diagnostic tests are encouraged to calculate adequate sample sizes for their study.
Time-and-motion analysis of drug susceptibility testing
In the UK and other developed countries, the salary costs for personnel conducting the assay constitute a significant portion of the overall assay cost. Typically, the only available estimates of working time come from the kit manufacturers themselves and are probably representative of only the quickest possible scenarios. In order to determine the ‘hands-on’ time for each of the molecular tests compared with culture tests accurately, we conducted a time-and-motion study at the NMRL, where all of these tests are used routinely.
Samples were usually processed in batches, with the number in each batch dependent on day-to-day variation. The theoretical time taken to process a single specimen was calculated. However, it was noted that the time taken per batch did not have a linear relationship to the number of samples processed. Thus, the working times determined at the NMRL may be substantially different from those at other laboratories with different levels of specimen throughput.
The calculated time taken to conduct each assay for a single respiratory specimen was remarkably similar for all tests, ranging from 41 to 50 minutes. However, in addition to the batch size dependency, it is also notable that the susceptibility information obtained from each test was not the same, ranging from one drug (GeneXpert and INNO-LiPA) to 10 drugs (BACTEC MGIT 960 DSTs).
Health-economic modelling of screening strategies
Principal findings
As rapid molecular tests are less accurate than culture, current ECDC guidance recommends that, although they may have a role alongside culture, they should not replace culture. 109 This means that the cost of the molecular test cannot be offset by savings from reduced need for culture, and there is a trade-off between the increased costs and negative health impacts. Tests cannot be evaluated in isolation but have to be considered within a pathway of care, specifying how patients are treated while waiting for test results, what treatments they are offered when the results arrive, how and when diagnostic errors (FPs and FNs) are detected and then what corrective action is taken.
Forty-four unique care pathways were defined: 12 in which culture only was utilised and 32 including the rapid molecular assay. The care pathways differed by true clinical status, smear status and MDR risk status. Costs were determined for each of these pathways, with all elements priced from a NHS perspective. It was found that, with culture, the total estimated cost ranged from £2252 per patient (smear-negative patient correctly identified as free from TB) to £130,214 (smear-positive patients with MDR-TB not identified as high risk, and therefore not treated presumptively). The range was similarly wide with molecular testing: from a minimum of £2334 (correctly diagnosed smear-negative patients without TB) to a maximum of £131,771 (smear-positive patients with MDR-TB thought to be at high risk and with an inaccurate molecular test result indicating DS disease). It should be noted that the incidence of the very-high-cost pathways is likely to be low (as both MDR-TB and misdiagnoses are rare). The expected cost for a patient with smear-positive DS disease, deemed to be at low risk of MDR-TB, correctly diagnosed by molecular test and treated appropriately is £9392. The cost for a similar patient, correctly diagnosed and treated under the culture strategy is slightly lower, at £8670. Estimated mean QALY losses ranged from 0 (for patients without TB who are correctly diagnosed and treated) to about 1.205 QALYs lost (for patients with smear-positive MDR-TB with delayed diagnosis and who are not treated presumptively). A large proportion of the estimated QALY loss was attributable to adverse events related to MDR treatment.
For infectious diseases, the calculated value of a diagnostic test should also account for impacts related to transmission, including QALY loss and costs for secondary cases. A transmission-dynamic model was integrated with the economic model to evaluate the cost-effectiveness of introducing rapid molecular testing for TB infection in averting transmission of infection. Three population groups were considered: South Asians and Black Africans who constitute a large proportion (61%) of the TB cases in England and Wales, and Eastern Europeans who have a high proportion of TB cases that are MDR (≈27%).
The introduction of molecular testing had only a very small impact on transmission, because current practice is effective in limiting transmission from TB patients while they are undergoing clinical examination. There is a benefit to smear-negative patients, most of whom test positive by molecular methods and therefore can be diagnosed and start treatment earlier than if they have to wait for culture confirmation. However, the major benefit of molecular testing is in faster diagnosis of MDR-TB, which produces cost savings when patients with smear-positive suspected MDR-TB are kept in isolation until their MDR status is known and then appropriate treatment commenced, this is because isolation has a high daily cost. Molecular tests were estimated to increase the annual numbers of TB diagnoses resulting from FP results, caused by imperfect specificity. This resulted in inappropriate treatment of some patients, which incurred a QALY loss as well as a financial cost.
The results of the transmission modelling suggest that all assays are cost saving and achieve an increase in QALYs compared with current practice. Across the Black African and Eastern European populations, the GeneXpert scenario was the most cost-effective of the rapid assays considered, at both 10- and 20-year time horizons, and £20,000 or £30,000 willingness-to-pay threshold per QALY. For the South Asian population, using a 10-year time horizon, the MDRTBplus scenario was the most cost-effective assay, with the highest estimated INB compared with current practice, although the difference with GeneXpert was very small. At a 20-year time horizon, for the same population the MTBDRplus scenario was the most cost-effective; GeneXpert and INNO-LiPA achieved a similar INB in this case.
In light of the rapid development of technology, decision-makers may prefer to give more weight to the 10-year time horizon than the 20-year time horizon, even though the potentially long duration of TB infection means that the impact of the interventions is long term. There is considerable uncertainty around these results, particularly regarding the relative cost-effectiveness of the different assays, and of regional compared with local testing, given the uncertainty over input parameters and the underlying assumptions.
Limitations
The results of the economic evaluation and transmission modelling are subject to uncertainty relating to parameter estimates and also as a result of various simplifying assumptions. We have attempted to reflect parametric uncertainty through the PSA. However, the PSA does not capture uncertainty associated with structural assumptions. For example, a key assumption throughout this report has been that culture-based diagnosis of MTB and DST is perfectly accurate and provides the reference standard for evaluation of the rapid molecular tests. Another important simplification required for the economic evaluation and dynamic transmission modelling was that we did not distinguish between different patterns of drug resistance. This entails the assumption that the rapid tests evaluated would be similarly accurate at detecting MDR-TB, and that the cost and QALY implications of alternative patterns of resistance would be similar.
To cost the various care pathways, we also had to make a series of assumptions about clinical decision-making. An important driver for decisions about presumptive treatment, admission and isolation is the clinician’s prior suspicion about the likelihood that an individual has drug-resistant TB. In the absence of clear normative criteria about which patients should be treated presumptively, or empirical evidence about how clinicians make these judgements, we assumed that a fixed proportion of patients would be identified as being at ‘high risk’ for MDR-TB. For our analysis we assumed that this proportion would equal the true proportion of MDR cases out of all TB cases in the population of interest – assuming that clinicians are ‘well calibrated’ in their judgements. For the economic evaluation, we also assumed that culture-based diagnosis and DST would always be conducted alongside rapid molecular tests, and that clinicians would treat the culture results as definitive. This is important for assumptions about when and how clinicians ‘step-down’ presumptive treatment. Thus, we assumed that any patients with negative culture results would then be treated as TNs, and that any treatment or public health action would be halted. In practice, clinicians do sometimes continue treatment in such situations if they have a high index of suspicion. Clinical practice is also likely to vary geographically, in response to local practice and practicalities. The high estimated costs for patients with (or suspected of having) MDR-TB were largely driven by long inpatient stays and expensive negative pressure isolation. These estimates were based on local audit data and might not reflect practice around the country. Another assumption required for the economic evaluation was that patients who fail to complete a course of treatment would, on average, default half way through the course.
A series of assumptions was also required to estimate the QALY loss associated with adverse effects of MDR-TB treatment, the frequency of hearing loss, and the duration and utility loss associated with that loss. The transmission model introduced some further structural assumptions, which add further to uncertainty. For example, the model assumed homogeneous mixing of UK- and overseas-born people with the ethnic subgroups. Rates of population turnover were also assumed to be constant over time.
Chapter 8 Conclusions
Rapid molecular tests, such as the manual LPAs and automated GeneXpert, are able to identify rifampicin resistance (and isoniazid resistance for some LPAs) with promising levels of specificity and are almost as sensitive as microbiological culture but produce results more quickly (in 1 day from the taking of a patient sample). The PPV for resistance is dependent on the prevalence of drug resistance, which is low in the UK; a proportion of rifampicin resistance results will be FPs if used in a general screen, as the PPV will be > 90% only when the underlying prevalence is > 15%. The corresponding NPV of course will be high. This occurs within populations in some countries and in some migrant groups to the UK from those countries, for example the Baltic States, Russia and Ukraine, where MDR-TB prevalence is generally much higher and from which active migration occurs. Other populations in countries may similarly have a high prevalence of MDR-TB, for example China and South Africa. This low PPV associated with low prevalence is linked to the current WHO advice to perform a confirmatory test (e.g. other molecular or culture based).
-
The results of the transmission modelling suggest that all assays are cost saving and achieved an increase in QALYs compared with current practice. For the Black African and Eastern European populations, GeneXpert was likely to be the most cost-effective approach compared with current practice. For the South Asian population, the MTBDRplus assay was most favourable achieving the highest INB compared with current practice. ‘Real-life’ and ‘real clinical use’ evaluation studies, both retrospective and prospective studies within the UK, and comparable environments are needed to determine if the trial performance of these tests is maintained in real NHS use.
-
This is a rapidly moving field and should be reflected in the updating of professional (including NICE) guidelines.
-
Further research should investigate whether or not the likely benefits of use in populations with a high MDR-TB does translate into improved outcome, lower personal and NHS costs.
-
Further analysis of transmission patterns is required to enable models to be produced which more accurately reflect those patterns; this is necessary to improve the estimates of numbers of infections averted by interventions.
-
The real-world value/necessity of a ‘two-assay’ approach to specimens showing rifampicin resistance initially (i.e. FP issue) should be assessed.
-
The diagnostic value and cost-effectiveness of whole genomic sequencing for drug resistance should be evaluated as the technology develops for analysis of patient specimens.
The performance of these tests is less clear-cut for non-pulmonary material and other ‘low-bacillary’ specimens, for example from children and from HIV-positive patients. There were few studies available for analysis.
-
Further studies of the performance of these tests are needed for non-pulmonary specimens, and in children, and, to some extent, in HIV-positive populations.
Underlying data for costings, patient stays and cost-effectiveness determinations were often difficult to find, not available or relied on published work/literature that was published a long time ago. Costings for service provision outside a health environment or the movement of specimens and analysis from outside a health environment into the NHS was difficult to obtain and relied heavily on expert estimates. This analysis produced detailed independent diagnostic assay costs for the first time and actual bed-stay durations in pilot sites.
-
There is a need to improve costings for bed-stays and treatment routes, which should include detailed and wide-ranging assessment of bed-stays in different institutions and regions.
-
There is a need to create pilots that can provide detailed information for real test performance and associated cost–benefits when rapid tests are delivered as a near ‘POC’ test within and outside a traditional hospital environment (e.g. in a clinic within a hospital, a mobile unit, a prison, a homeless shelter, migrant accommodation, etc.).
Acknowledgements
We would like to thank microbiologists who are too numerous to name, respiratory physicians, molecular biologists and patients who have directly and indirectly helped us in providing their views and experiences, which has helped us in preparation of this report, particularly Amy McConville, Dr Tim Brown and staff at the Public Health England (PHE) NMRL, who contributed to the diagnostic time-and-motion study.
We would like to thank Catherina Boehme and the Foundation for Innovative Diagnosis (FIND), and Drs Didi Bang, Vladyslav Nikolayevskyy and Noha Seoudi for access to unpublished diagnostic data.
PJW thanks the MRC for Centre funding, and also thanks the UK NIHR Health Protection Research Unit (HPRU) in Modelling Methodology at Imperial College London in partnership with PHE for funding. IA acknowledges NIHR Senior Research Fellowship and MRC funding. FD acknowledges NIHR and EU funding (FP7 PANNET programme).
The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, the Department of Health or PHE.
Contributions of authors
Professor Francis Drobniewski (Consultant and Director, PHE NMRL, Professor of Global Health) Co-ordination and design of the project and overall strategy, contribution to the strategy for the systematic literature review and data review, economic analysis, clinical, diagnostic assay and microbiological input, data review and analysis, and drafting of the report.
Dr Mary Cooke (Research Associate) Contribution to design of literature review, data extraction and analysis and drafting of the report.
Dr Jake Jordan (Research Associate) Economic analysis and drafting of the report.
Dr Nicola Casali (Research Associate) Co-ordination of project and overall strategy, contribution to systematic literature review, diagnostic assay and microbiological input, data review and analysis, and drafting of the report.
Dr Tendai Mugwagwa (Modeller, Honorary Research Associate) Contribution to the design of the transmission-dynamic model and implementation of the transmission-dynamic model.
Ms Agnieszka Broda (Research Assistant) Contribution to the design of the systematic literature review, and data extraction and analysis of unpublished data.
Ms Catherine Townsend (Research Assistant) Implementation and analysis of diagnostic assay time-and-motion study and systematic literature review data extraction.
Dr Anand Sivaramakrishnan (Microbiology Specialist Registrar) Systematic literature review data extraction.
Dr Nathan Green (Research Fellow) Statistical analysis of ETS and ONS data for the transmission-dynamic model.
Dr Mark Jit (Mathematical Modeller, Senior Lecturer) Contribution to design of overall study, literature review, economic analysis and transmission-dynamic model and analysis.
Dr Marc Lipman (Senior Lecturer, Honorary Consultant Physician) Contribution to the design of the overall study and provision of data from the pilot site.
Dr Joanne Lord (Reader in Health Economics) Contribution to the design of the project and overall strategy, economic analysis and drafting of the report.
Dr Peter J White (Head, PHE Modelling & Economics Unit, Senior Lecturer) Contribution to the design of the overall study, literature review and economic analysis, led design of transmission-dynamic model and analysis.
Professor Ibrahim Abubakar (Chair in Infectious Disease Epidemiology and Centre Co-Director) Contribution to design of project and overall strategy: contribution to strategy for the systematic literature review and data review, economic analysis, clinical, diagnostic assay and microbiological input, and data review and analysis.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Kim DH, Kim HJ, Park S-K, Kong S-J, Kim YS, Kim T-H, et al. Treatment outcomes and long-term survival in patients with extensively drug-resistant tuberculosis. Am J Respir Crit Care Med 2008;178:1075-82. http://dx.doi.org/10.1164/rccm.200801-132OC.
- Shah NS, Pratt R, Armstrong L, Robison V, Castro KG, Cegielski JP. Extensively drug-resistant tuberculosis in the United States, 1993–2007. JAMA 2008;300:2153-60. http://dx.doi.org/10.1001/jama.300.18.2153.
- Balabanova Y, Nikolayevskyy V, Ignatyeva O, Kontsevaya I, Rutterford CM, Shakhmistova A, et al. Survival of civilian and prisoner drug-sensitive, multi- and extensive drug-resistant tuberculosis cohorts prospectively followed in Russia. PLOS ONE 2011;6. http://dx.doi.org/10.1371/journal.pone.0020531.
- Balabanova Y, Radiulyte B, Davidaviciene E, Hooper R, Ignatyeva O, Nikolayevskyy V, et al. Survival of drug resistant tuberculosis patients in Lithuania: retrospective national cohort study. BMJ Open 2011;1. http://dx.doi.org/10.1136/bmjopen-2011-000351.
- Koenig R. Drug-resistant tuberculosis. In South Africa, XDR TB and HIV prove a deadly combination. Science 2008;319:894-7. http://dx.doi.org/10.1126/science.319.5865.894.
- Gandhi NR, Moll A, Sturm AW, Pawinski R, Govender T, Lalloo U, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet 2006;368:1575-80. http://dx.doi.org/10.1016/S0140-6736(06)69573-1.
- Udwadia ZF. MDR, XDR, TDR tuberculosis: ominous progression. Thorax 2012;67:286-8. http://dx.doi.org/10.1136/thoraxjnl-2012-201663.
- Global Tuberculosis Report 2013. Geneva: WHO; 2013.
- Tuberculosis Surveillance and Monitoring In Europe. Stockholm: ECDC; 2013.
- Prevention and Control Of Multidrug-Resistant Tuberculosis and Extensively Drug-Resistant Tuberculosis. Geneva: WHO; 2009.
- Tuberculosis in the UK. London: PHE; 2013.
- Breathnach AS, de Ruiter A, Holdsworth GM, Bateman NT, O’Sullivan DG, Rees PJ, et al. An outbreak of multi-drug-resistant tuberculosis in a London teaching hospital. J Hosp Infect 1998;39:111-17. http://dx.doi.org/10.1016/S0195-6701(98)90324-3.
- Ruddy MC, Davies AP, Yates MD, Yates S, Balasegaram S, Drabu Y, et al. Outbreak of isoniazid resistant tuberculosis in north London. Thorax 2004;59:279-85. http://dx.doi.org/10.1136/thx.2003.010405.
- Telenti A, Imboden P, Marchesi F, Lowrie D, Cole S, Colston MJ, et al. Detection of rifampicin-resistance mutations in Mycobacterium tuberculosis. Lancet 1993;341:647-50. http://dx.doi.org/10.1016/0140-6736(93)90417-F.
- Zhang Y, Heym B, Allen B, Young D, Cole S. The catalase-peroxidase gene and isoniazid resistance of Mycobacterium tuberculosis. Nature 1992;358:591-3. http://dx.doi.org/10.1038/358591a0.
- Banerjee A, Dubnau E, Quemard A, Balasubramanian V, Um KS, Wilson T, et al. inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science 1994;263:227-30. http://dx.doi.org/10.1126/science.8284673.
- Drobniewski FA, Caws M, Gibson A, Young D. Modern laboratory diagnosis of tuberculosis. Lancet Infect Dis 2003;3:141-7. http://dx.doi.org/10.1016/S1473-3099(03)00544-9.
- Sandgren A, Strong M, Muthukrishnan P, Weiner BK, Church GM, Murray MB. Tuberculosis drug resistance mutation database. PLOS Med 2009;6. http://dx.doi.org/10.1371/journal.pmed.1000002.
- Canetti G, Froman S, Grosset J, Haudoruy P, Langerova M, Mahler HT, et al. Mycobacteria: laboratory methods for testing drug sensitivity and resistance. Bull World Health Organ 1963;29:565-78.
- Siddiqi S, Rüsch-Gerdes S. MGIT Procedure Manual. Geneva: Foundation for Innovative New Diagnostics; 2006.
- Hanna BA, Ebrahimzadeh A, Elliott LB, Morgan MA, Novak SM, Rusch-Gerdes S, et al. Multicenter evaluation of the BACTEC MGIT 960 system for recovery of mycobacteria. J Clin Microbiol 1999;37:748-52.
- Leung E, Minion J, Benedetti A, Pai M, Menzies D. Microcolony culture techniques for tuberculosis diagnosis: a systematic review. Int J Tuberc Lung Dis 2012;16:16-23. http://dx.doi.org/10.5588/ijtld.10.0065.
- Moore DAJ, Evans CAW, Gilman RH, Caviedes L, Coronel J, Vivar A, et al. Microscopic-observation drug-susceptibility assay for the diagnosis of TB. N Engl J Med 2006;355:1539-50. http://dx.doi.org/10.1056/NEJMoa055524.
- Toit K, Mitchell S, Balabanova Y, Evans CA, Kummik T, Nikolayevskyy V, et al. The Colour Test for drug susceptibility testing of Mycobacterium tuberculosis strains. Int J Tuberc Lung Dis 2012;16:1113-18. http://dx.doi.org/10.5588/ijtld.11.0609.
- Musser JM. Antimicrobial agent resistance in mycobacteria: molecular genetic insights. Clin Microbiol Rev 1995;8:496-514.
- Hazbón MH, Brimacombe M, Bobadilla del Valle M, Cavatore M, Guerrero MI, Varma-Basil M, et al. Population genetics study of isoniazid resistance mutations and evolution of multidrug-resistant Mycobacterium tuberculosis. Antimicrob Agents Chemother 2006;50:2640-9. http://dx.doi.org/10.1128/AAC.00112-06.
- Ling DI, Flores LL, Riley LW, Pai M. Commercial nucleic-acid amplification tests for diagnosis of pulmonary tuberculosis in respiratory specimens: meta-analysis and meta-regression. PLOS ONE 2008;3. http://dx.doi.org/10.1371/journal.pone.0001536.
- Pietzka AT, Indra A, Stoger A, Zeinzinger J, Konrad M, Hasenberger P, et al. Rapid identification of multidrug-resistant Mycobacterium tuberculosis isolates by rpoB gene scanning using high-resolution melting curve PCR analysis. J Antimicrob Chemother 2009;63:1121-7. http://dx.doi.org/10.1093/jac/dkp124.
- Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 2010;363:1005-15. http://dx.doi.org/10.1056/NEJMoa0907847.
- Nagdev KJ, Kashyap RS, Parida MM, Kapgate RC, Purohit HJ, Taori GM, et al. Loop-mediated isothermal amplification for rapid and reliable diagnosis of tuberculous meningitis. J Clin Microbiol 2011;49:1861-5. http://dx.doi.org/10.1128/JCM.00824-10.
- Molecular Line Probe Assays for Rapid Screening of Patients at Risk of Multidrug-Resistant Tuberculosis. Geneva: WHO; 2008.
- Morgan M, Kalantri S, Flores L, Pai M. A commercial line probe assay for the rapid detection of rifampicin resistance in Mycobacterium tuberculosis: a systematic review and meta-analysis. BMC Infect Dis 2005;5. http://dx.doi.org/10.1186/1471-2334-5-62.
- Viveiros M, Leandro C, Rodrigues L, Almeida J, Bettencourt R, Couto I, et al. Direct application of the INNO-LiPA Rif.TB line-probe assay for rapid identification of Mycobacterium tuberculosis complex strains and detection of rifampin resistance in 360 smear-positive respiratory specimens from an area of high incidence of multidrug-resistant tuberculosis. J Clin Microbiol 2005;43:4880-4. http://dx.doi.org/10.1128/JCM.43.9.4880-4884.2005.
- Mironova S, Pimkina E, Kontsevaya I, Nikolayevskyy V, Balabanova Y, Skenders G, et al. Performance of the GenoType® MTBDRPlus assay in routine settings: a multicenter study. Eur J Clin Microbiol Infect Dis 2012;31:1381-7. http://dx.doi.org/10.1007/s10096-011-1453-1.
- Seoudi N, Mitchell SL, Brown TJ, Dashti F, Amin AK, Drobniewski FA. Rapid molecular detection of tuberculosis and rifampicin drug resistance: retrospective analysis of a national UK molecular service over the last decade. Thorax 2012;67:361-7. http://dx.doi.org/10.1136/thoraxjnl-2011-200610.
- Ignatyeva O, Kontsevaya I, Kovalyov A, Balabanova Y, Nikolayevskyy V, Toit K, et al. Detection of resistance to second-line antituberculosis drugs by use of the GenoType MTBDRsl assay: a multicenter evaluation and feasibility study. J Clin Microbiol 2012;50:1593-7. http://dx.doi.org/10.1128/JCM.00039-12.
- Kontsevaya I, Ignatyeva O, Nikolayevskyy V, Balabanova Y, Kovalyov A, Kritsky A, et al. Diagnostic accuracy of the genotype MTBDRsl assay for rapid diagnosis of extensively drug-resistant tuberculosis in HIV-coinfected patients. J Clin Microbiol 2013;51:243-8. http://dx.doi.org/10.1128/JCM.02513-12.
- Boehme CC, Nicol MP, Nabeta P, Michael JS, Gotuzzo E, Tahirli R, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet 2011;377:1495-505. http://dx.doi.org/10.1016/S0140-6736(11)60438-8.
- Automated Real-Time Nucleic Acid Amplification Technology for Rapid and Simultaneous Detection of Tuberculosis and Rifampicin Resistance: Xpert MTB/RIF system. Geneva: WHO; 2011.
- WHO Monitoring of Xpert MTB/RIF Roll-Out. Geneva: WHO; 2013.
- Vassall A, van Kampen S, Sohn H, Michael JS, John KR, . Rapid diagnosis of tuberculosis with the Xpert MTB/RIF assay in high burden countries: a cost-effectiveness analysis. PLOS Med 2011;8. http://dx.doi.org/10.1371/journal.pmed.1001120.
- Safi H, Lingaraju S, Amin A, Kim S, Jones M, Holmes M, et al. Evolution of high-level ethambutol-resistant tuberculosis through interacting mutations in decaprenylphosphoryl-D-arabinose biosynthetic and utilization pathway genes. Nat Genet 2013;45:1190-7. http://dx.doi.org/10.1038/ng.2743.
- Zhang H, Li D, Zhao L, Fleming J, Lin N, Wang T, et al. Genome sequencing of 161 Mycobacterium tuberculosis isolates from China identifies genes and intergenic regions associated with drug resistance. Nat Genet 2013;45:1255-60. http://dx.doi.org/10.1038/ng.2735.
- Farhat MR, Shapiro BJ, Kieser KJ, Sultana R, Jacobson KR, Victor TC, et al. Genomic analysis identifies targets of convergent positive selection in drug-resistant Mycobacterium tuberculosis. Nat Genet 2013;45:1183-9. http://dx.doi.org/10.1038/ng.2747.
- Steingart K, Sohn H, Schiller I, Kloda L, Boehme C, Pai M, et al. Xpert® MTB/RIF assay for pulmonary tuberculosis and rifampicin resistance in adults. Cochrane Database Syst Rev 2013;1. http://dx.doi.org/10.1002/14651858.CD009593.pub2.
- Ling DI, Zwerling AA, Pai M. GenoType MTBDR assays for the diagnosis of multidrug-resistant tuberculosis: a meta-analysis. Eur Respir J 2008;32:1165-74. http://dx.doi.org/10.1183/09031936.00061808.
- Whiting P, Rutjes AW, Westwood M, Reitsma JB, Leeflang MMG, Sterne JAC, et al. QUADAS-2: a revised tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann Intern Med 2011;155:529-36. http://dx.doi.org/10.7326/0003-4819-155-8-201110180-00009.
- Lijmer JG, Bossuyt PMM, Heisterkamp SH. Exploring sources of heterogeneity in systematic reviews of diagnostic tests. Stat Med 2002;21:1525-37. http://dx.doi.org/10.1002/sim.1185.
- Lijmer JG. Evaluation Of Diagnostic Test From Accuracy To Outcome. Amsterdam: Print Partners; 2001.
- Higgins JPT, Thompson SG. Controlling the risk of spurious findings from meta-regression. Stat Med 2004;23:1663-82. http://dx.doi.org/10.1002/sim.1752.
- Costeira J, Pina J. Multi-drug resistant tuberculosis and the red queen: diagnosis speed is crucial. Rev Port Pneumol 2007;13:869-77.
- Drobniewski FA, Watterson SA, Wilson SM, Harris GS. A clinical, microbiological and economic analysis of a national service for the rapid molecular diagnosis of tuberculosis and rifampicin resistance in Mycobacterium tuberculosis. J Med Microbiol 2000;49:271-8.
- Higuchi T, Fushiwaki T, Tanaka N, Miyake S, Ogura T, Yoshida H, et al. Direct detection of rifampicin resistant Mycobacterium tuberculosis in sputum by line probe assay (LiPA). Kekkaku 2004;79:525-30.
- Johansen I, Lundgren B, Sosnovskaja A, Thomsen V. Direct detection of multidrug-resistant Mycobacterium tuberculosis in clinical specimens in low- and high-incidence countries by line probe assay. J Clin Microbiol 2003;41:4454-6. http://dx.doi.org/10.1128/JCM.41.9.4454-4456.2003.
- Ogwang S, Asiimwe BB, Traore H, Mumbowa F, Okwera A, Eisenach KD, et al. Comparison of rapid tests for detection of rifampicin-resistant Mycobacterium tuberculosis in Kampala, Uganda. BMC Infect Dis 2009;9. http://dx.doi.org/10.1186/1471-2334-9-139.
- Sam I, Drobniewski F, More P, Kemp M, Brown T. Mycobacterium tuberculosis and rifampin resistance. Emerg Infect Dis 2006;12:752-9. http://dx.doi.org/10.3201/eid1205.041339.
- Skenders G, Fry AM, Prokopovica I, Greckoseja S, Broka L, Metchock B, et al. Multidrug-resistant tuberculosis detection, Latvia. Emerg Infect Dis 2005;11:1461-3. http://dx.doi.org/10.3201/eid1109.041236.
- Kim SY, Kim H, Ra EK, Joo S-I, Shin S, Seong M-W, et al. The Xpert® MTB/RIF assay evaluation in South Korea, a country with an intermediate tuberculosis burden. Int J Tuberc Lung Dis 2012;16:1471-6. http://dx.doi.org/10.5588/ijtld.11.0602.
- Kurbatova EV, Kaminski Da, Erokhin VV, Volchenkov GV, Andreevskaya SN, Chernousova LN, et al. Performance of Cepheid® Xpert MTB/RIF® and TB-Biochip® MDR in two regions of Russia with a high prevalence of drug-resistant tuberculosis. Eur J Clin Microbiol Infect Dis 2013;32:735-43. http://dx.doi.org/10.1007/s10096-012-1798-0.
- Naidoo S. Evaluation of GeneXpertMTB/RIF assay on pulmonary and extra-pulmonary samples in a high-throughput laboratory. Clin Microbiol Infect 2010;16:S604-5.
- O’Grady J, Bates M, Chilukutu L, Mzyece J, Cheelo B, Chilufya M, et al. Evaluation of the Xpert MTB/RIF assay at a tertiary care referral hospital in a setting where tuberculosis and HIV infection are highly endemic. Clin Infect Dis 2012;55:1171-8. http://dx.doi.org/10.1093/cid/cis631.
- Tukvadze N, Kempker RR, Kalandadze I, Kurbatova E, Leonard MK, Apsindzelashvili R, et al. Use of a molecular diagnostic test in AFB smear positive tuberculosis suspects greatly reduces time to detection of multidrug resistant tuberculosis. PLOS ONE 2012;7. http://dx.doi.org/10.1371/journal.pone.0031563.
- Lyu J, Kim M, Song JW, Choi C, Oh Y, Lee SD, et al. GenoType® MTBDR plus assay detection of drug-resistant tuberculosis in routine practice in Korea. Int J Tuberc Lung Dis 2013;17:120-4. http://dx.doi.org/10.5588/ijtld.12.0197.
- Hillemann D, Rüsch-Gerdes S, Richter E. Application of the GenoType MTBDR assay directly on sputum specimens. Int J Tuberc Lung Dis 2006;10:1057-9.
- Farooqi JQ, Khan E, Muhammed S, Alam Z, Ali A, Hasan Z, et al. Original Article Line probe assay for detection of rifampicin and isoniazid resistant tuberculosis in Pakistan. J Pak Med Assoc 2012;62:767-72.
- Eliseev PI, Maryandyshev AO, Nikishova EI, Tarasova IV, Gorina GP, Chryssanthou E, et al. Epidemiological analyses of tuberculosis in Archangelsk, Russia and implementation of a rapid assay for detection of resistance in this high burden setting. Int J Mycobacteriol 2013;2:103-8. http://dx.doi.org/10.1016/j.ijmyco.2013.04.002.
- Dubois Cauwelaert N, Ramarokoto H, Ravololonandriana P, Richard V, Rasolofo V. DNA extracted from stained sputum smears can be used in the MTBDRplus assay. J Clin Microbiol 2011;49:3600-3. http://dx.doi.org/10.1128/JCM.00745-11.
- Dorman SE, Chihota VN, Lewis JJ, van der Meulen M, Mathema B, Beylis N, et al. GenoType MTBDRplus for direct detection of Mycobacterium tuberculosis and drug resistance in strains from gold miners in South Africa. J Clin Microbiol 2012;50:1189-94. http://dx.doi.org/10.1128/JCM.05723-11.
- Crudu V, Stratan E, Romancenco E, Allerheiligen V, Hillemann A, Moraru N. First evaluation of an improved assay for molecular genetic detection of tuberculosis as well as rifampin and isoniazid resistances. J Clin Microbiol 2012;50:1264-9. http://dx.doi.org/10.1128/JCM.05903-11.
- Barnard M, Albert H, Coetzee G, O’Brien R, Bosman ME. Rapid molecular screening for multidrug-resistant tuberculosis in a high-volume public health laboratory in South Africa. Am J Respir Crit Care Med 2008;177:787-92. http://dx.doi.org/10.1164/rccm.200709-1436OC.
- Asencios L, Galarza M, Quispe N, Leo E, Valencia E, Acurio M, et al. Molecular test GenoType MTBDRplus, an alternative to rapid detection of multidrug resistance tuberculosis. Rev Peru Med Exp Salud Publica 2012;29:92-8. http://dx.doi.org/10.1590/S1726-46342012000100014.
- Anek-Vorapong R, Sinthuwattanawibool C, Podewils LJ, Mccarthy K, Ngamlert K, Promsarin B, et al. Validation of the GenoType® MTBDR plus assay for detection of MDR-TB in a public health laboratory in Thailand. BMC Infect Dis 2010;10. http://dx.doi.org/10.1186/1471-2334-10-123.
- Albert H, Bwanga F, Mukkada S, Nyesiga B, Ademun JP, Lukyamuzi G, et al. Rapid screening of MDR-TB using molecular Line Probe Assay is feasible in Uganda. BMC Infect Dis 2010;10. http://dx.doi.org/10.1186/1471-2334-10-41.
- Somoskovi A, Dormandy J, Mitsani D, Rivenburg J, Salfinger M. Use of smear-positive samples to assess the PCR-based genotype MTBDR assay for rapid, direct detection of the Mycobacterium tuberculosis complex as well as its resistance to isoniazid and rifampin. J Clin Microbiol 2006;44:4459-63. http://dx.doi.org/10.1128/JCM.01506-06.
- Lacoma A, García-Sierra N, Prat C, Ruiz-Manzano J, Haba L, Rosés S, et al. GenoType MTBDRplus assay for molecular detection of rifampin and isoniazid resistance in Mycobacterium tuberculosis strains and clinical samples. J Clin Microbiol 2008;46:3660-7. http://dx.doi.org/10.1128/JCM.00618-08.
- Ajbani K, Nikam C, Kazi M, Grey C, Boehme C, Balan K, et al. Evaluation of genotype MTBDRsl assay to detect drug resistance associated with fluoroquinolones, aminoglycosides and ethambutol on clinical sediments. PLOS ONE 2012;7. http://dx.doi.org/10.1371/journal.pone.0049433.
- Lacoma A, García-Sierra N, Prat C, Maldonado J, Ruiz-Manzano J, Haba L, et al. GenoType MTBDRsl for molecular detection of second-line-drug and ethambutol resistance in Mycobacterium tuberculosis strains and clinical samples. J Clin Microbiol 2012;50:30-6. http://dx.doi.org/10.1128/JCM.05274-11.
- Hillemann D, Rüsch-Gerdes S, Richter E. Feasibility of the GenoType MTBDRsl assay for fluoroquinolone, amikacin–capreomycin, and ethambutol resistance testing of Mycobacterium tuberculosis strains and clinical specimens. J Clin Microbiol 2009;47:1767-72. http://dx.doi.org/10.1128/JCM.00081-09.
- Miotto P, Cabibbe AM, Mantegani P, Borroni E, Fattorini L, Tortoli E, et al. GenoType MTBDRsl performance on clinical samples with diverse genetic background. Eur Respir J 2012;40:690-8. http://dx.doi.org/10.1183/09031936.00164111.
- Barnard M, Warren R, Gey Van Pittius N, van Helden P, Bosman M, Streicher E, et al. GenoType MTBDRsl line probe assay shortens time to diagnosis of extensively drug-resistant tuberculosis in a high-throughput diagnostic laboratory. Am J Respir Crit Care Med 2012;186:1298-305. http://dx.doi.org/10.1164/rccm.201205-0960OC.
- Antonova OV, Gryadunov DA, Lapa SA, Kuz’min AV, Larionova EE, Smirnova TG, et al. Detection of mutations in Mycobacterium tuberculosis genome determining resistance to fluoroquinolones by hybridization on biological microchips. Bull Exp Biol Med 2008;145:108-13. http://dx.doi.org/10.1007/s10517-008-0034-5.
- Calderón-Espinoza RI, Asencios-Solís L, Quispe-Torres N, Yale-Cajahuanca G, Suárez-Nole C, Lecca-García L, et al. Validación de la reacción en cadena de la polimerasa basada en generador universal de heterodúplex para la identificación de Mycobacterium tuberculosis resistente a rifampicina y multirresistente en Lima, Perú. Enferm Infecc Microbiol Clin 2006;24:495-9. http://dx.doi.org/10.1157/13092465.
- Cho EH, Bae HK, Kang SK, Lee EH. Detection of isoniazid and rifampicin resistance by sequencing of katG, inhA, and rpoB genes in Korea. Korean J Lab Med 2009;29:455-60. http://dx.doi.org/10.3343/kjlm.2009.29.5.455.
- Choi J-H, Lee KW, Kang H-R, Hwang Y Il, Jang S, Kim D-G, et al. Clinical efficacy of direct DNA sequencing analysis on sputum specimens for early detection of drug-resistant Mycobacterium tuberculosis in a clinical setting. Chest 2010;137:393-400. http://dx.doi.org/10.1378/chest.09-0150.
- García-Sierra N, Lacoma A, Prat C, Haba L, Maldonado J, Ruiz-Manzano J, et al. Pyrosequencing for rapid molecular detection of rifampin and isoniazid resistance in Mycobacterium tuberculosis strains and clinical specimens. J Clin Microbiol 2011;49:3683-6. http://dx.doi.org/10.1128/JCM.01239-11.
- Gryadunov D, Mikhailovich V, Lapa S, Roudinskii N, Donnikov M, Pan’kov S, et al. Evaluation of hybridisation on oligonucleotide microarrays for analysis of drug-resistant Mycobacterium tuberculosis. Clin Microbiol Infect 2005;11:531-9. http://dx.doi.org/10.1111/j.1469-0691.2005.01183.x.
- Guo Y, Zhou Y, Wang C, Zhu L, Wang S, Li Q, et al. Rapid, accurate determination of multidrug resistance in M. tuberculosis isolates and sputum using a biochip system. Int J Tuberc Lung Dis 2009;13:914-20.
- Pang Y, Xia H, Zhang Z, Li J, Dong Y, Li Q, et al. Multicenter evaluation of genechip for detection of multidrug-resistant Mycobacterium tuberculosis. J Clin Microbiol 2013;51:1707-13. http://dx.doi.org/10.1128/JCM.03436-12.
- Kim B, Lee K, Park B, Kim S, Park E, Park Y, et al. Detection of rifampin-resistant Mycobacterium tuberculosis in sputa by nested PCR-linked single-strand conformation polymorphism and DNA sequencing. J Clin Microbiol 2001;39:2610-17. http://dx.doi.org/10.1128/JCM.39.7.2610-2617.2001.
- Lu W, Chen C, Shao Y, Shi J, Zhong C, Yang D, et al. Evaluation of biochip system in determining isoniazid and rifampicin resistances of Mycobacterium tuberculosis in sputum samples. PLOS ONE 2012;7. http://dx.doi.org/10.1371/journal.pone.0052953.
- Mikhailovich V, Lapa S, Gryadunov D, Sobolev A, Strizhkov B, Chernyh N, et al. Identification of rifampin-resistant Mycobacterium tuberculosis strains by hybridization, PCR, and ligase detection reaction on oligonucleotide microchips. J Clin Microbiol 2001;39:2531-40. http://dx.doi.org/10.1128/JCM.39.7.2531-2540.2001.
- Mokrousov I, Otten T, Vyshnevskiy B, Narvskaya O. Allele-specific rpoB PCR Assays for detection of rifampin-resistant Mycobacterium tuberculosis in sputum smears. Antimicrob Agents Chemother 2003;47:2231-5. http://dx.doi.org/10.1128/AAC.47.7.2231-2235.2003.
- Nosova EY, Krasnova MA, Galkina KY, Makarova MV, Litvinov VI, Moroz AM. Comparative analysis of TB-Biochip, Xpert MTB/RIF, and GenoType MTBDRplus test systems for rapid determination of mutations responsible for drug resistance of M. tuberculosis complex (in sputum from patients in Moscow region). Mol Biol 2013;47:236-41. http://dx.doi.org/10.1134/S002689331301010X.
- Sheen P, Méndez M, Gilman RH, Peña L, Caviedes L, Zimic MJ, et al. Sputum PCR-single-strand conformational polymorphism test for same-day detection of pyrazinamide resistance in tuberculosis patients. J Clin Microbiol 2009;47:2937-43. http://dx.doi.org/10.1128/JCM.01594-08.
- Siu GKH, Tam YH, Ho PL, Lee ASG, Que TL, Tse CWS, et al. Direct detection of isoniazid-resistant Mycobacterium tuberculosis in respiratory specimens by multiplex allele-specific polymerase chain reaction. Diagn Microbiol Infect Dis 2011;69:51-8. http://dx.doi.org/10.1016/j.diagmicrobio.2010.08.021.
- Vadwai V, Shetty A, Rodrigues C. Multiplex allele specific PCR for rapid detection of extensively drug resistant tuberculosis. Tuberculosis (Edinb) 2012;92:236-42. http://dx.doi.org/10.1016/j.tube.2012.01.004.
- Zhang Z, Li L, Luo F, Cheng P, Wu F, Wu Z, et al. Rapid and accurate detection of RMP- and INH- resistant Mycobacterium tuberculosis in spinal tuberculosis specimens by CapitalBioTM DNA microarray: a prospective validation study. BMC Infect Dis 2012;12. http://dx.doi.org/10.1186/1471-2334-12-303.
- Macedo R, Pereira E. Multidrug-resistant tuberculosis: rapid molecular detection with MTBDRplus assay in clinical samples. Rev Port Pneumol 2009;XV:353-65. http://dx.doi.org/10.1016/S2173-5115(09)70118-6.
- Nikolayevskyy V, Balabanova Y, Simak T, Malomanova N, Fedorin I, Drobniewski F. Performance of the GenoType MTBDRPlus assay in the diagnosis of tuberculosis and drug resistance in Samara, Russian Federation. BMC Clin Pathol 2009;9. http://dx.doi.org/10.1186/1472-6890-9-2.
- Maschmann RDA, Sá Spies F, Nunes LDS, Ribeiro AW, Machado TRM, Zaha A, et al. Performance of the GenoType MTBDRplus assay directly on sputum specimens from Brazilian patients with tuberculosis treatment failure or relapse. J Clin Microbiol 2013;51:1606-8. http://dx.doi.org/10.1128/JCM.00364-13.
- Kontsevaya I, Mironova S, Nikolayevskyy V, Balabanova Y, Mitchell S, Drobniewski F. Evaluation of two molecular assays for rapid detection of Mycobacterium tuberculosis resistance to fluoroquinolones in high-tuberculosis and multidrug-resistance settings. J Clin Microbiol 2011;49:2832-7. http://dx.doi.org/10.1128/JCM.01889-10.
- Al Khalaf MM, Thalib L, Doi SA. Combining heterogenous studies using the random-effects model is a mistake and leads to inconclusive meta-analyses. J Clin Epidemiol 2011;64:119-23. http://dx.doi.org/10.1016/j.jclinepi.2010.01.009.
- Bachmann LM, Puhan MA, ter Riet G, Bossuyt PM. Sample sizes of studies on diagnostic accuracy: literature survey. BMJ 2006;332:1127-9. http://dx.doi.org/10.1136/bmj.38793.637789.2F.
- Grégoire G, Derderian F, Le Lorier J. Selecting the language of the publications included in a meta-analysis: is there a Tower of Babel bias?. J Clin Epidemiol 1995;48:159-63. http://dx.doi.org/10.1016/0895-4356(94)00098-B.
- Krüüner A, Yates MD, Drobniewski FA. Evaluation of MGIT 960-based antimicrobial testing and determination of critical concentrations of first- and second-line antimicrobial drugs with drug-resistant clinical strains of Mycobacterium tuberculosis. J Clin Microbiol 2006;44:811-18. http://dx.doi.org/10.1128/JCM.44.3.811-818.2006.
- Drummond M, Sculpher M, Torrance G, O’Brien B, Stoddart G. Methods for The Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 2005.
- Pitman R, Fisman D, Zaric GS, Postma M, Kretzschmar M, Edmunds J, et al. Dynamic transmission modeling: a report of the ISPOR-SMDM modeling good research practices task force-5. Value Health 2012;15:828-34. http://dx.doi.org/10.1016/j.jval.2012.06.011.
- Tuberculosis: Clinical Diagnosis and Management of Tuberculosis, and Measures for its Prevention and Control. London: NICE; 2011.
- ERLN-TB Expert Opinion on the Use of the Rapid Molecular Assays for the Diagnosis of Tuberculosis and Detection of Drug Resistance. Stockholm: ECDC; 2013.
- Position Statement: Direct Molecular Testing for Tuberculosis in England, Scotland and Wales. London: PHE; 2013.
- Methods for Development of NICE Public Health Guidelines. London: NICE; 2012.
- National Audit of TB Diagnostic Laboratories and Description of Molecular Diagnostic Service Provision. London: PHE; 2012.
- Public Health England (PHE) . Tuberculosis Enhanced Survey Data 2013.
- Guidelines for the Programmatic Management of Drug-Resistant Tuberculosis. Geneva: WHO; 2011.
- White VL, Moore-Gillon J. Resource implications of patients with multidrug resistant tuberculosis. Thorax 2000;55:962-3. http://dx.doi.org/10.1136/thorax.55.11.962.
- Kruijshaar ME, Lipman M, Essink-Bot M-L, Lozewicz S, Creer D, Dart S, et al. Health status of UK patients with active tuberculosis. Int J Tuberc Lung Dis 2010;14:296-302.
- Saukkonen JJ, Cohn DL, Jasmer RM, Schenker S, Jereb JA, Nolan CM, et al. An official ATS statement: hepatotoxicity of antituberculosis therapy. Am J Respir Crit Care Med 2006;174:935-52. http://dx.doi.org/10.1164/rccm.200510-1666ST.
- Törün T, Güngör G, Ozmen I, Bölükbaşi Y, Maden E, Biçakçi B, et al. Side effects associated with the treatment of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2005;9:1373-7.
- Melchionda V, Wyatt H, Capocci S, García Medina R, Solamalai A, Katiri S, et al. Amikacin treatment for multidrug resistant tuberculosis: how much monitoring is required?. Eur Respir J 2013;42:1148-50. http://dx.doi.org/10.1183/09031936.00184312.
- Veenstra DL, Harris J, Gibson RL, Rosenfeld M, Burke W, Watts C. Pharmacogenomic testing to prevent aminoglycoside-induced hearing loss in cystic fibrosis patients: potential impact on clinical, patient, and economic outcomes. Genet Med 2007;9:695-704. http://dx.doi.org/10.1097/GIM.0b013e318156dd07.
- Interim Life Tables 2008–2010. London: ONS; 2011.
- Health Survey for England. Leeds: HSCIC; 2012.
- National Institute for Health and Care Excellence . Guide to the Methods of Technology Appraisal 2013.
- Drobniewski F, Nikolayevskyy V, Maxeiner H, Balabanova Y, Casali N, Kontsevaya I, et al. Rapid diagnostics of tuberculosis and drug resistance in the industrialized world: clinical and public health benefits and barriers to implementation. BMC Med 2013;11. http://dx.doi.org/10.1186/1741-7015-11-190.
- Pareek M, Bond M, Shorey J, Seneviratne S, Guy M, White P, et al. Community-based evaluation of immigrant tuberculosis screening using interferon γrelease assays and tuberculin skin testing: observational study and economic analysis. Thorax 2013;68:230-9. http://dx.doi.org/10.1136/thoraxjnl-2011-201542.
- NHS Reference Costs 2011/12. London: Department of Health; 2012.
- Department of Health . National Tariff 2012.
- British National Formulary. London: BMJ Group and Pharmaceutical Press; 2013.
- Curtis L. Unit Costs of Health and Social Care 2012. Canterbury: PSSRU, University of Kent; 2012.
- Saldana L, Abid M, McCarthy N, Hunter N, Inglis R, Anders K. Factors affecting delay in initiation of treatment of tuberculosis in the Thames Valley, UK. Public Health 2013;127:171-7. http://dx.doi.org/10.1016/j.puhe.2012.11.010.
- Barton GR, Bankart J, Davis AC, Summerfield QA. Comparing utility scores before and after hearing-aid provision: results according to the EQ-5D, HUI3 and SF-6D. Appl Health Econ Health Policy 2004;3:103-5. http://dx.doi.org/10.2165/00148365-200403020-00006.
- UK Cochlear Implant Study Group . Criteria of candidacy for unilateral cochlear implantation in postlingually deafened adults II: cost-effectiveness analysis. Ear Hear 2004;25:336-60. http://dx.doi.org/10.1097/01.AUD.0000134550.80305.04.
- Bond M, Mealing S, Anderson R, Elston J, Weiner G, Taylor RS, et al. The effectiveness and cost-effectiveness of cochlear implants for severe to profound deafness in children and adults: a systematic review and economic model. Health Technol Assess 2009;13.
- Yang Y, Longworth L, Brazier J. An assessment of validity and responsiveness of generic measures of health-related quality of life in hearing impairment. Qual Life Res 2013;22:2813-28. http://dx.doi.org/10.1007/s11136-013-0417-6.
- Luo N, Johnson JA, Shaw JW, Feeny D, Coons SJ. Self-reported health status of the general adult U.S. population as assessed by the EQ-5D and Health Utilities Index. Med Care 2005;43:1078-86. http://dx.doi.org/10.1097/01.mlr.0000182493.57090.c1.
- Anderson R, May R. Infectious Diseases of Humans: Dynamics and Control. Oxford: Oxford University Press; 1991.
- Salomon JA, Lloyd-Smith JO, Getz WM, Resch S, Sánchez MS, Porco TC, et al. Prospects for advancing tuberculosis control efforts through novel therapies. PLOS Med 2006;3:1302-9. http://dx.doi.org/10.1371/journal.pmed.0030273.
- Vynnycky E, Fine PE. The natural history of tuberculosis: the implications of age-dependent risks of disease and the role of reinfection. Epidemiol Infect 1997;119:183-201. http://dx.doi.org/10.1017/S0950268897007917.
- Dye C, Garnett GP, Sleeman K, Williams BG. Prospects for worldwide tuberculosis control under the WHO DOTS strategy. Directly observed short-course therapy. Lancet 1998;352:1886-91. http://dx.doi.org/10.1016/S0140-6736(98)03199-7.
- England and Wales Census. London: ONS; 2011.
- Tuberculosis In The UK: 2012 Report. London: HPA; 2012.
- Abubakar I, White P, Jit M, Stagg H, Pimpin L, Aldridge R, et al. Evaluation of the Find and Treat Service for the Control of Tuberculosis amongst Hard to Reach Groups. London: Department of Health; 2011.
- National Tuberculosis Institute [India] . Tuberculosis in a rural population of South India: a five-year epidemiological study. Bull World Health Organ 1974;51:473-88.
- Abu-Raddad LJ, Sabatelli L, Achterberg JT, Sugimoto JD, Longini IM, Dye C, et al. Epidemiological benefits of more-effective tuberculosis vaccines, drugs, and diagnostics. Proc Natl Acad Sci USA 2009;106:13980-5. http://dx.doi.org/10.1073/pnas.0901720106.
- Arentz M, Sorensen B, Horne DJ, Walson JL. Systematic review of the performance of rapid rifampicin resistance testing for drug-resistant tuberculosis. PLOS ONE 2013;8. http://dx.doi.org/10.1371/journal.pone.0076533.
- Thompson SG. Why sources of heterogeneity in meta-analysis should be investigated. BMJ 1994;309:1351-5. http://dx.doi.org/10.1136/bmj.309.6965.1351.
- Davis JL, Huang L, Kovacs JA, Masur H, Murray P, Havlir DV, et al. Polymerase chain reaction of secA1 on sputum or oral wash samples for the diagnosis of pulmonary tuberculosis. Clin Infect Dis 2009;48:725-32. http://dx.doi.org/10.1086/597038.
Appendix 1 Example of search strategy
| Number | Search terms |
|---|---|
| #7 | Search #5 OR #6 |
| #8 | Search #5 OR #6 Filters: Publication date from 2000/01/01 to 2011/05/15 |
| #6 | Search #2 AND #4 |
| #5 | Search #2 AND #3 |
| #4 | Search INNO-LiPA[tw] OR INNOLiPA[tw] OR ‘Hain LifeScience’[tw] OR MTBDR*[tw] OR Cepheid[tw] OR ‘Gene Xpert’[tw] |
| #3 | Search molecular[tw] OR gyr*[tw] OR genotyp*[tw] OR mutation*[tw] OR hybrid*[tw] OR array*[tw] OR microarray*[tw] OR macroarray*[tw] OR probe*[tw] OR chip*[tw] OR biprobe*[tw] OR microchip*[tw] OR lipa[tw] OR ‘molecular beacon’[tw] OR ‘real-time PCR’[tw] |
| #2 | Search (tuberculosis[MESH] OR tuberculosis[tw] OR Mtb OR Mycobacterium tuberculosis OR M. tuberculosis) AND (rifamp*[tw] OR isoniazid[tw] OR fluoroquinolone*[tw] OR levofloxacin[tw] OR ofloxacin[tw] OR moxifloxacin[tw]) AND (resistan*[tw] OR susceptib*[tw] OR test*[tw] OR assay*[tw] OR method*[tw]) |
Appendix 2 Bibliography of included studies
| Test | Year | Study |
|---|---|---|
| Xpert® MTB/RIF (Cepheid Inc, Sunnyvale, CA, USA) | 201060 | Naidoo S. Evaluation of GeneXpertMTB/RIF assay on pulmonary and extra-pulmonary samples in a high-throughput laboratory. Clin Microbiol Infect 2010;16:S604–5 |
| 201029 | Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 2010;363:1005–15 | |
| 201138 | Boehme CC, Nicol MP, Nabeta P, Michael JS, Gotuzzo E, Tahirli R, et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet 2011;377:1495–505 | |
| 201258 | Kim SY, Kim H, Ra EK, Joo S-I, Shin S, Seong M-W, et al. The Xpert® MTB/RIF assay evaluation in South Korea, a country with an intermediate tuberculosis burden. Int J Tuberc Lung Dis 2012;16:1471–6 | |
| 201261 | O’Grady J, Bates M, Chilukutu L, Mzyece J, Cheelo B, Chilufya M, et al. Evaluation of the Xpert MTB/RIF assay at a tertiary care referral hospital in a setting where tuberculosis and HIV infection are highly endemic. Clin Infect Dis 2012;55:1171–8 | |
| 201359 | Kurbatova EV, Kaminski Da, Erokhin VV, Volchenkov GV, Andreevskaya SN, Chernousova LN, et al. Performance of Cepheid® Xpert MTB/RIF® and TB-Biochip® MDR in two regions of Russia with a high prevalence of drug-resistant tuberculosis. Eur J Clin Microbiol Infect Dis 2013;32:735–43 | |
| GenoType® MTBDRplus (Hain Lifescience, Nehren, Germany) | 200674 | Somoskovi A, Dormandy J, Mitsani D, Rivenburg J, Salfinger M. Use of smear-positive samples to assess the PCR-based genotype MTBDR assay for rapid, direct detection of the Mycobacterium tuberculosis complex as well as its resistance to isoniazid and rifampin. J Clin Microbiol 2006;44:4459–63 |
| 200664 | Hillemann D, Rüsch-Gerdes S, Richter E. Application of the GenoType MTBDR assay directly on sputum specimens. Int J Tuberc Lung Dis 2006;10:1057–9 | |
| 200875 | Lacoma A, García-Sierra N, Prat C, Ruiz-Manzano J, Haba L, Rosés S, et al. GenoType MTBDRplus assay for molecular detection of rifampin and isoniazid resistance in Mycobacterium tuberculosis strains and clinical samples. J Clin Microbiol 2008;46:3660–7 | |
| 200870 | Barnard M, Albert H, Coetzee G, O’Brien R, Bosman ME. Rapid molecular screening for multidrug-resistant tuberculosis in a high-volume public health laboratory in South Africa. Am J Respir Crit Care Med 2008;177:787–92 | |
| 200999 | Nikolayevskyy V, Balabanova Y, Simak T, Malomanova N, Fedorin I, Drobniewski F. Performance of the GenoType MTBDRPlus assay in the diagnosis of tuberculosis and drug resistance in Samara, Russian Federation. BMC Clin Pathol 2009;9:2. doi:10.1186/1472-6890-9-2 | |
| 200998 | Macedo R, Pereira E. Multidrug-resistant tuberculosis: rapid molecular detection with MTBDRplus assay in clinical samples. Rev Port Pneumol 2009;15:353–65 | |
| 201073 | Albert H, Bwanga F, Mukkada S, Nyesiga B, Ademun JP, Lukyamuzi G, et al. Rapid screening of MDR-TB using molecular Line Probe Assay is feasible in Uganda. BMC Infect Dis 2010;10:41. doi:10.1186/1471-2334-10-41 | |
| 201072 | Anek-Vorapong R, Sinthuwattanawibool C, Podewils LJ, Mccarthy K, Ngamlert K, Promsarin B, et al. Validation of the GenoType® MTBDR plus assay for detection of MDR-TB in a public health laboratory in Thailand. BMC Infect Dis 2010;10:123. doi:10.1186/1471-2334-10-123 | |
| 201167 | Dubois Cauwelaert N, Ramarokoto H, Ravololonandriana P, Richard V, Rasolofo V. DNA extracted from stained sputum smears can be used in the MTBDRplus assay. J Clin Microbiol 2011;49:3600–3 | |
| 201265 | Farooqi JQ, Khan E, Muhammed S, Alam Z, Ali A, Hasan Z, et al. Original Article Line probe assay for detection of rifampicin and isoniazid resistant tuberculosis in Pakistan. J Pak Med Assoc 2012;62:767–72 | |
| 201234 | Mironova S, Pimkina E, Kontsevaya I, Nikolayevskyy V, Balabanova Y, Skenders G, et al. Performance of the GenoType® MTBDRPlus assay in routine settings: a multicenter study. Eur J Clin Microbiol Infect Dis 2012;31:1381–7 | |
| 201268 | Dorman SE, Chihota VN, Lewis JJ, van der Meulen M, Mathema B, Beylis N, et al. GenoType MTBDRplus for direct detection of Mycobacterium tuberculosis and drug resistance in strains from gold miners in South Africa. J Clin Microbiol 2012;50:1189–94 | |
| 201269 | Crudu V, Stratan E, Romancenco E, Allerheiligen V, Hillemann A, Moraru N. First evaluation of an improved assay for molecular genetic detection of tuberculosis as well as rifampin and isoniazid resistances. J Clin Microbiol 2012;50:1264–9 | |
| 201262 | Tukvadze N, Kempker RR, Kalandadze I, Kurbatova E, Leonard MK, Apsindzelashvili R, et al. Use of a molecular diagnostic test in AFB smear positive tuberculosis suspects greatly reduces time to detection of multidrug resistant tuberculosis. PLOS ONE 2012;7:e31563. doi:10.1371/journal.pone.0031563 | |
| 201271 | Asencios L, Galarza M, Quispe N, Leo E, Valencia E, Acurio M, et al. Molecular test GenoType MTBDRplus, an alternative to rapid detection of multidrug resistance tuberculosis. Rev Peru Med Exp Salud Publica 2012;29:92–8 | |
| 201366 | Eliseev PI, Maryandyshev AO, Nikishova EI, Tarasova IV, Gorina GP, Chryssanthou E, et al. Epidemiological analyses of tuberculosis in Archangelsk, Russia and implementation of a rapid assay for detection of resistance in this high burden setting. Int J Mycobacteriol 2013;2:103–8 | |
| 2013100 | Maschmann RDA, Sá Spies F, Nunes LDS, Ribeiro AW, Machado TRM, Zaha A, et al. Performance of the GenoType MTBDRplus assay directly on sputum specimens from Brazilian patients with tuberculosis treatment failure or relapse. J Clin Microbiol 2013;51:1606–8 | |
| 201363 | Lyu J, Kim M, Song JW, Choi C, Oh Y, Lee SD, et al. GenoType® MTBDR plus assay detection of drug-resistant tuberculosis in routine practice in Korea. Int J Tuberc Lung Dis 2013;17:120–4 | |
| GenoType® MTBDRsl (Hain Lifescience) | 200978 | Hillemann D, Rüsch-Gerdes S, Richter E. Feasibility of the GenoType MTBDRsl assay for fluoroquinolone, amikacin–capreomycin, and ethambutol resistance testing of Mycobacterium tuberculosis strains and clinical specimens. J Clin Microbiol 2009;47:1767–72 |
| 201279 | Miotto P, Cabibbe AM, Mantegani P, Borroni E, Fattorini L, Tortoli E, et al. GenoType MTBDRsl performance on clinical samples with diverse genetic background. Eur Respir J 2012;40:690–8 | |
| 201276 | Ajbani K, Nikam C, Kazi M, Grey C, Boehme C, Balan K, et al. Evaluation of genotype MTBDRsl assay to detect drug resistance associated with fluoroquinolones, aminoglycosides and ethambutol on clinical sediments. PLOS ONE 2012;7:e49433. doi:10.1371/journal.pone.0049433 | |
| 201277 | Lacoma A, García-Sierra N, Prat C, Maldonado J, Ruiz-Manzano J, Haba L, et al. GenoType MTBDRsl for molecular detection of second-line-drug and ethambutol resistance in Mycobacterium tuberculosis strains and clinical samples. J Clin Microbiol 2012;50:30–6 | |
| 201280 | Barnard M, Warren R, Gey Van Pittius N, van Helden P, Bosman M, Streicher E, et al. GenoType MTBDRsl line probe assay shortens time to diagnosis of extensively drug-resistant tuberculosis in a high-throughput diagnostic laboratory. Am J Respir Crit Care Med 2012;186:1298–305 | |
| 201337 | Kontsevaya I, Ignatyeva O, Nikolayevskyy V, Balabanova Y, Kovalyov A, Kritsky A, et al. Diagnostic accuracy of the genotype MTBDRsl assay for rapid diagnosis of extensively drug-resistant tuberculosis in HIV-coinfected patients. J Clin Microbiol 2013;51:243–8 | |
| INNO-LiPA® (Fujirebio Europe, Ghent, Belgium) | 200052 | Drobniewski FA, Watterson SA, Wilson SM, Harris GS. A clinical, microbiological and economic analysis of a national service for the rapid molecular diagnosis of tuberculosis and rifampicin resistance in Mycobacterium tuberculosis. J Med Microbiol 2000;49:271–8 |
| 200354 | Johansen I, Lundgren B, Sosnovskaja A, Thomsen V. Direct detection of multidrug-resistant Mycobacterium tuberculosis in clinical specimens in low- and high-incidence countries by line probe assay. J Clin Microbiol 2003;41:4454–6 | |
| 200453 | Higuchi T, Fushiwaki T, Tanaka N, Miyake S, Ogura T, Yoshida H, et al. Direct detection of rifampicin resistant Mycobacterium tuberculosis in sputum by line probe assay (LiPA). Kekkaku 2004;79:525–30 | |
| 200557 | Skenders G, Fry AM, Prokopovica I, Greckoseja S, Broka L, Metchock B, et al. Multidrug-resistant tuberculosis detection, Latvia. Emerg Infect Dis 2005;11:1461–3 | |
| 200533 | Viveiros M, Leandro C, Rodrigues L, Almeida J, Couto I, Carrilho L, et al. Direct application of the INNO-LiPA Rif.TB line-probe assay for rapid identification of Mycobacterium tuberculosis complex strains and detection of rifampin resistance in 360 smear-positive respiratory specimens from an area of high incidence of multidrug-resistant tuberculosis. J Clin Microbiol 2005;43:4880–4 | |
| 200656 | Sam I, Drobniewski F, More P, Kemp M, Brown T. Mycobacterium tuberculosis and rifampin resistance. Emerg Infect Dis 2006;12:752–9 | |
| 200751 | Costeira J, Pina J. Multi-drug resistant tuberculosis and the red queen: diagnosis speed is crucial. Rev Port Pneumol 2007;13:869–77 | |
| 200955 | Ogwang S, Asiimwe BB, Traore H, Mumbowa F, Okwera A, Eisenach KD, et al. Comparison of rapid tests for detection of rifampicin-resistant Mycobacterium tuberculosis in Kampala, Uganda. BMC Infect Dis 2009;9:139. doi:10.1186/1471-2334-9-139 | |
| 201235 | Seoudi N, Mitchell SL, Brown TJ, Dashti F, Amin AK, Drobniewski FA. Rapid molecular detection of tuberculosis and rifampicin drug resistance: retrospective analysis of a national U.K. molecular service over the last decade. Thorax 2012;67:361–7 | |
| PCR/in-house assays | 200189 | Kim B, Lee K, Park B, Kim S, Park E, Park Y, et al. Detection of rifampin-resistant Mycobacterium tuberculosis in sputa by nested PCR-linked single-strand conformation polymorphism and DNA sequencing. J Clin Microbiol 2001;39:2610–17 |
| 200392 | Mokrousov I, Otten T, Vyshnevskiy B, Narvskaya O. Allele-specific rpoB PCR Assays for detection of rifampin-resistant Mycobacterium tuberculosis in sputum smears. Antimicrob Agents Chemother 2003;47:2231–5 | |
| 200682 | Calderón-Espinoza RI, Asencios-Solís L, Quispe-Torres N, Yale-Cajahuanca G, Suárez-Nole C, Lecca-García L, et al. [Validación de la reacción en cadena de la polimerasa basada en generador universal de heterodúplex para la identificación de Mycobacterium tuberculosis resistente a rifampicina y multirresistente en Lima, Perú.] Enferm Infecc Microbiol Clin 2006;24:495–9 | |
| 200983 | Cho EH, Bae HK, Kang SK, Lee EH. Detection of isoniazid and rifampicin resistance by sequencing of katG, inhA, and rpoB genes in Korea. Korean J Lab Med 2009;29:455–60 | |
| 200994 | Sheen P, Méndez M, Gilman RH, Peña L, Caviedes L, Zimic MJ, et al. Sputum PCR-single-strand conformational polymorphism test for same-day detection of pyrazinamide resistance in tuberculosis patients. J Clin Microbiol 2009;47:2937–43 | |
| 201084 | Choi J-H, Lee KW, Kang H-R, Hwang Y Il, Jang S, Kim D-G, et al. Clinical efficacy of direct DNA sequencing analysis on sputum specimens for early detection of drug-resistant Mycobacterium tuberculosis in a clinical setting. Chest 2010;137:393–400 | |
| 201195 | Siu GKH, Tam YH, Ho PL, Lee ASG, Que TL, Tse CWS, et al. Direct detection of isoniazid-resistant Mycobacterium tuberculosis in respiratory specimens by multiplex allele-specific polymerase chain reaction. Diagn Microbiol Infect Dis 2011;69:51–8 | |
| 201185 | García-Sierra N, Lacoma A, Prat C, Haba L, Maldonado J, Ruiz-Manzano J, et al. Pyrosequencing for rapid molecular detection of rifampin and isoniazid resistance in Mycobacterium tuberculosis strains and clinical specimens. J Clin Microbiol 2011;49:3683–6 | |
| 201297 | Zhang Z, Li L, Luo F, Cheng P, Wu F, Wu Z, et al. Rapid and accurate detection of RMP- and INH- resistant Mycobacterium tuberculosis in spinal tuberculosis specimens by CapitalBioTM DNA microarray: a prospective validation study. BMC Infect Dis 2012;12:303. doi:10.1186/1471-2334-12-303 | |
| 201296 | Vadwai V, Shetty A, Rodrigues C. Multiplex allele specific PCR for rapid detection of extensively drug resistant tuberculosis. Tuberculosis (Edinb) 2012;92:236–42 | |
| Biochip/genechip | 200191 | Mikhailovich V, Lapa S, Gryadunov D, Sobolev A, Strizhkov B, Chernyh N, et al. Identification of rifampin-resistant Mycobacterium tuberculosis strains by hybridization, PCR, and ligase detection reaction on oligonucleotide microchips. J Clin Microbiol 2001;39:2531–40 |
| 200586 | Gryadunov D, Mikhailovich V, Lapa S, Roudinskii N, Donnikov M, Pan’kov S, et al. Evaluation of hybridisation on oligonucleotide microarrays for analysis of drug-resistant Mycobacterium tuberculosis. Clin Microbiol Infect 2005;11:531–9 | |
| 200881 | Antonova OV, Gryadunov DA, Lapa SA, Kuz’min AV, Larionova EE, Smirnova TG, et al. Detection of mutations in Mycobacterium tuberculosis genome determining resistance to fluoroquinolones by hybridization on biological microchips. Bull Exp Biol Med 2008;145:108–13 | |
| 200987 | Guo Y, Zhou Y, Wang C, Zhu L, Wang S, Li Q, et al. Rapid, accurate determination of multidrug resistance in M. tuberculosis isolates and sputum using a biochip system. Int J Tuberc Lung Dis 2009;13:914–20 | |
| 201290 | Lu W, Chen C, Shao Y, Shi J, Zhong C, Yang D, et al. Evaluation of biochip system in determining isoniazid and rifampicin resistances of Mycobacterium tuberculosis in sputum samples. PLOS ONE 2012;7:e52953. doi:10.1371/journal.pone.0052953 | |
| 201388 | Pang Y, Xia H, Zhang Z, Li J, Dong Y, Li Q, et al. Multicenter evaluation of genechip for detection of multidrug-resistant Mycobacterium tuberculosis. J Clin Microbiol 2013;51:1707–13 | |
| 201393 | Nosova EY, Krasnova MA, Galkina KY, Makarova MV, Litvinov VI, Moroz AM. Comparative analysis of TB-Biochip, Xpert MTB/RIF, and GenoType MTBDRplus test systems for rapid determination of mutations responsible for drug resistance of M. tuberculosis complex (in sputum from patients in Moscow region). Mol Biol 2013;47:236–41 |
Appendix 3 Study data extraction form screenshot
Appendix 4 Study details
| Study | Authors | Year | Title | Study setting and methodology | Sample details | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study method | Country | Clinical setting | Test run outside laboratory? | Study blinded? | Number included in analysis | Sample type | Sample type (other) | Number post screening | ||||
| Ajbani et al. (2012)76 | Kanchan Ajbani, Chaitali Nikam, Mubin Kazi, Christen Grey, Catharina Boehme, Kavita Balan, Anjali Shetty, Camilla Rodrigues | 2012 | Evaluation of genotype MTBDRsl assay to detect drug resistance associated with fluoroquinolones, aminoglycosides and ethambutol on clinical sediments | Not stated | India | Other, specify | Not stated | Not stated | 170 | Smear-positive pulmonary | 170 | |
| Albert et al. (2010)73 | Heidi Albert, Freddie Bwanga, Sheena Mukkada, Barnabas Nyesiga, Julius Patrick Ademun, George Lukyamuzi, Melles Haile, Sven Hoffner, Moses Joloba, Richard O’Brien | 2010 | Rapid screening of MDR-TB using molecular Line Probe Assay is feasible in Uganda | Not stated | Uganda | Laboratory | No | Yes | 118 | Smear-positive pulmonary | 113 | |
| Anek-Vorapong et al. (2010)72 | Rapeepun Anek-Vorapong, Chalinthorn Sinthuwattanawibool, Laura Jean Podewils, Kimberly McCarthy, Keerataya Ngamlert, Busakorn Promsarin, Jay K Varma | 2010 | Validation of the GenoType MTBDRplus assay for detection of MDR-TB in a public health laboratory in Thailand | Not stated | Thailand | Laboratory | No | Yes | 164 | Smear-positive pulmonary | 164 | |
| Antonova et al. (2008)81 | OV Antonova, DA Gryadunov, SA Lapa, AV Kuz’min, EE Larionova, TG Smirnova, EY Nosova, OI Skotnikova, LN Chernousova, AM Moroz, AS Zasedatelev, VM Mikhailovich | 2008 | Detection of mutations in Mycobacterium tuberculosis genome determining resistance to fluoroquinolones by hybridization on biological microchips | Not stated | Russia | Not stated | Not stated | Not stated | 78 | Pulmonary | 78 | |
| Asencios et al. (2012)71 | Luis Asencios, Marco Galarza, Neyda Quispe, Lucy Vasquez, Elena Leo, Eddy Valencia, Juan Ramirez, Margoth Acurio, Rosario Salazar, Alberto Mendoza-Ticona, Omar Caceres | 2012 | [Molecular test GenoType MTBDRplus, an alternative to rapid detection of multidrug resistance tuberculosis] | Not stated | Peru | Laboratory | Not stated | Not stated | Smear-positive pulmonary | |||
| Barnard et al. (2008)70 | Marinus Barnard, Heidi Allbert, Gerrit Coetzee, Richard O’Brien, Marlein E Bosiman | 2008 | Rapid molecular screening for multidrug-resistant tuberculosis in a high-volume public health laboratory in South Africa | Not stated | South Africa | Laboratory | No | Yes | Smear-positive pulmonary | |||
| Barnard et al. (2012)80 | M Barnard, R Warren, NG Van Pittius, P van Helden, M Bosman, E Streicher, G Coetzee, R O’Brien | 2012 | GenoType MTBDRsl line probe assay shortens time to diagnosis of XDR-TB in a high-throughput diagnostic laboratory | Not stated | South Africa | Laboratory | Not stated | Not stated | 516 | Smear-positive pulmonary | 736 | |
| Boehme et al. (2010)29 | Catharina C Boehme, Pamela Nabeta, Doris Hillemann, Mark P Nicol, Shubhada Shenai, Fiorella Krapp, Jenny Allen, Rasim Tahirli, Robert Blakemore, Roxana Rustomjee, Ana Milovic, Martin Jones, Sean M O’Brien, David H Persing, Sabine Ruesch-Gerdes, Eduardo Gotuzzo, Camil | 2010 | Rapid molecular detection of tuberculosis and rifampin resistance | Not stated | Multi | Laboratory | Yes | 1462 | Pulmonary | 1730 | ||
| Boehme et al. (2011)38 | Catharina C Boehme, Mark P Nicol, Pamela Nabeta, Joy S Michael, Eduardo Gotuzzo, Rasim Tahirli, Ma Tarcela Gler, Robert Blakemore, William Worodria, Christen Grey, Laurence Huang, Tatiana Caceres, Rafail Mehdiyev, Lawrence Raymond, Andrew Whitelaw, Kalaiselvan Sagade | 2011 | Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study | Not stated | Multi | Laboratory | No | Not stated | 6648 | Pulmonary | 6069 | |
| Calderón-Espinoza et al. (2006)82 | RI Calderón-Espinoza, L Asencios-Solis, N Quispe-Torres, G Yale-Cajahuanca, C Suarez-Nole, L Lecca-García, LF Llanos-Zavalaga | 2006 | Validation of the PCR-based universal heteroduplex generator assay for identification of rifampicin-resistant and multiresistant Mycobacterium tuberculosis in Lima, Peru [Spanish] | Cross-sectional | Peru | Laboratory | Not stated | Yes | 200 | Smear-positive pulmonary | 243 | |
| Cauwelaert et al. (2011)67 | Natasha Dubois Cauwelaert, Herimanana Ramarokoto, Pascaline Ravololonandriana, Vincent Richard, Voahangy Rasolofo | 2011 | DNA extracted from stained sputum smears can be used in the MTBDRplus assay | Not stated | Madagascar | Not stated | Not stated | Not stated | Smear-positive pulmonary | 297 | ||
| Cho et al. (2009)83 | Eun Hae Cho, Hye Kyung Bae, Seong Ki Kang, Eun Hee Lee | 2009 | Detection of isoniazid and rifampicin resistance by sequencing of katG, inhA, and rpoB genes in Korea | Not stated | Republic of Korea | Laboratory | Not stated | Not stated | 46 | Pulmonary | 46 | |
| Choi et al. (2010)84 | Jeong-Hee Choi, Kyung Wha Lee, Hye-Ryun Kang, Yong Il Hwang, SeungHun Jang, Dong-Gyu Kim, Cheol-Hong Kim, In-Gyu Hyun, Tae-Rim Shin, Sang-Myeon Park, Myung-Goo Lee, Chang-Youl Lee, Yong-Bum Park, Ki-Suck Jung | 2010 | Clinical efficacy of direct DNA sequencing analysis on sputum specimens for early detection of drug-resistant Mycobacterium tuberculosis in a clinical setting | Not stated | Republic of Korea | Laboratory | Not stated | Not stated | 113 | Pulmonary | 113 | |
| Costeira et al. (2007)51 | J Costeira, J Pina | 2007 | Multi-drug-resistant tuberculosis and the red queen – Diagnosis speed is crucial. [Portuguese, English] | Not stated | Portugal | Laboratory | Not stated | Not stated | 113 | Smear-positive pulmonary | 113 | |
| Crudu et al. (2012)69 | Valeriu Crudu, Ecaterina Stratan, Elena Romancenco, Vera Allerheiligen, Andreas Hillemann, Nicolae Moraru | 2012 | First evaluation of an improved assay for molecular genetic detection of tuberculosis as well as rifampin and isoniazid resistances | Other (specify) | Republic of Moldova | Laboratory | No | Not stated | 336 | Pulmonary | 336 | |
| Dorman et al. (2012)68 | Susan Dorman, Violet Chihota, James J Lewis, Minty van der Meulen, Barun Mathema, Natalie Beylis, Katherine L. Fielding, Alison D Grant, Gavin J Churchyard | 2012 | GenoType MTBDRplus for direct detection of Mycobacterium tuberculosis and drug resistance in strains from gold miners in South Africa | Cross-sectional | South Africa | Laboratory | Not stated | Not stated | Pulmonary | |||
| Drobniewski et al. (2000)52 | FA Drobniewski, SA Watterson, SM Wilson, GS Harris | 2000 | A clinical, microbiological and economic analysis of a national service for the rapid molecular diagnosis of tuberculosis and rifampicin resistance in Mycobacterium tuberculosis | Not stated | UK | Laboratory | Not stated | Not stated | 51 | Pulmonary | 51 | |
| Eliseev et al. (2013)66 | PI Eliseev, AO Maryandyshev, EI Nikishova, IV Tarasova, GP Gorina, E Chryssanthou, M Ridell, LO Larsson | 2013 | Epidemiological analyses of tuberculosis in Archangelsk, Russia and implementation of a rapid assay for detection of resistance in this high burden setting | Not stated | Russia | Laboratory | Not stated | Not stated | 812 | Pulmonary | 812 | |
| Farooqi et al. (2012)65 | Joveria Qais Farooqi, Erum Khan, Syed Muhammed Zaheer Alam, Asho Ali, Zahra Hasan, Rumina Hasan | 2012 | Line probe assay for detection of rifampicin and isoniazid resistant tuberculosis in Pakistan | Not stated | Pakistan | Laboratory | Not stated | Not stated | 108 | Smear-positive pulmonary | 111 | |
| García-Sierra et al. (2011)85 | N García-Sierra, A Lacoma, C Prat, L Haba, J Maldonado, J Ruiz-Manzano, P Gavin, S Samper, V Ausina, J Dominguez | 2011 | Pyrosequencing for rapid molecular detection of rifampin and isoniazid resistance in Mycobacterium tuberculosis strains and clinical specimens | Not stated | Spain | Laboratory | Not stated | Not stated | 48 | Pulmonary | 48 | |
| Gryadunov et al. (2005)86 | D Gryadunov, V Mikhailovich, S Lapa, N Roudinskii, M Donnikov, S Pan’kov, O Markova, A Kuz’min, L. Chernousova, O Skotnikova, A Moroz, A Zasedatelev, A Mirzabekov | 2005 | Evaluation of hybridisation on oligonucleotide microarrays for analysis of drug-resistant Mycobacterium tuberculosis | Not stated | Russia | Laboratory | No | Not stated | Other (specify) | |||
| Guo et al. (2009)87 | Y Guo, Y Zhou, C Wang, L Zhu, S Wang, Q Li, G Jiang, B Zhao, H Huang, H Yu, W Xing, K Mitchelson, Jing Cheng, Yanlin Zhao | 2009 | Rapid, accurate determination of multidrug resistance in M. tuberculosis isolates and sputum using a biochip system | Not stated | China | Laboratory | Not stated | Not stated | 129 | Pulmonary | 129 | |
| Higuchi et al. (2004)53 | Takeshi Higuchi, Takeshi Fushiwaki, Nakako Tanaka, Seigo Miyake, Takeshi Ogura, Hiroko Yoshida, Tetsuya Takashima, Masaru Nakagawa, Ryoji Maekura, Toru Hiraga, Toshinori Suetake | 2004 | [Direct detection of rifampicin resistant Mycobacterium tuberculosis in sputum by line probe assay (LiPA)] | Not stated | Japan | Laboratory | Not stated | Not stated | 130 | Pulmonary | 130 | |
| Hillemann et al. (2006)64 | D Hillemann, S Rusch-Gerdes, E Richter | 2006 | Application of the GenoType MTBDR assay directly on sputum specimens | Not stated | Germany | Laboratory | No | Not stated | Smear-positive pulmonary | |||
| Hillemann et al. (2009)78 | Doris Hillemann, Sabine Rusch-Gerdes, Elvira Richter | 2009 | Feasibility of the GenoType MTBDRsl assay for fluoroquinolone, amikacin–capreomycin, and ethambutol resistance testing of Mycobacterium tuberculosis strains and clinical specimens | Not stated | Germany | Laboratory | Not stated | No | 64 | Pulmonary | 64 | |
| Johansen et al. (2003)54 | Isik Somuncu Johansen, Bettina Lundgren, Anaida Sosnovskaja, Vibeke Ostergaard Thomsen | 2003 | Direct detection of multidrug-resistant Mycobacterium tuberculosis in clinical specimens in low- and high-incidence countries by line probe assay | Not stated | Denmark | Laboratory | Not stated | Not stated | 60 | Pulmonary | 60 | |
| Kim et al. (2001)89 | Bum-Joon Kim, Keun-Hwa Lee, Bo-Na Park, Seo-Jeong Kim, Eun-Mi Park, Young-Gil Park, Gil-Han Bai, Sang-Jae Kim, Yoon-Hoh Kook | 2001 | Detection of rifampin-resistant Mycobacterium tuberculosis in sputa by nested PCR-linked single-strand conformation polymorphism and DNA sequencing | Not stated | Republic of Korea | Laboratory | Not stated | Not stated | 56 | Pulmonary | 56 | |
| Kim et al. (2012)58 | SY Kim, H Kim, EK Ra, SI Joo, S Shin, MW Seong, CG Yoo, EC Kim, SS Park | 2012 | The Xpert MTB/RIF assay evaluation in South Korea, a country with an intermediate tuberculosis burden | Not stated | Republic of Korea | Laboratory | Not stated | Not stated | 62 | Pulmonary | 71 | |
| Kontsevaya et al. (2013)37 | I Kontsevaya, O Ignatyeva, V Nikolayevskyy, Y Balabanova, A Kovalyov, A Kritsky, O Matskevich, F. Drobniewski | 2013 | Diagnostic accuracy of the GenoType MTBDRsl Assay for rapid diagnosis of extensively drug-resistant tuberculosis in HIV-coinfected patients | Not stated | Russia | Laboratory | Not stated | Yes | 90 | Smear-positive pulmonary | 90 | |
| Kurbatov et al. (2013)59 | EV Kurbatova, DA. Kaminski, VV Erokhin, GV Volchenkov, SN Andreevskaya, LN Chernousova, OV Demikhova, JV Ershova, NV Kaunetis, TA Kuznetsova, EE Larionova, TG Smirnova, TR Somova, IA Vasilieva, AV.Vorobieva, SS Zolkina | 2013 | Performance of Cepheid(A (R)) Xpert MTB/RIFA (R) and TB-Biochip(A (R)) MDR in two regions of Russia with a high prevalence of drug-resistant tuberculosis | Not stated | Russia | Laboratory | Not stated | Not stated | 238 | Pulmonary | 238 | |
| Lacoma et al. (2008)75 | A Lacoma, N García-Sierra, C Prat, J Ruiz-Manzano, L Haba, S Roses, J Maldonado, J Dominguez | 2008 | GenoType MTBDRplus assay for molecular detection of rifampin and isoniazid resistance in Mycobacterium tuberculosis strains and clinical samples | Not stated | Spain | Laboratory | Not stated | Yes | 65 | Other (specify) | 65 | |
| Lacoma et al. (2012)77 | A Lacoma, N García-Sierra, C Prat, J Maldonado, J Ruiz-Manzano, L Haba, P Gavin, S Samper, V Ausina, J Dominguez | 2012 | GenoType MTBDRsl for molecular detection of second-line drug and ethambutol resistance in Mycobacterium tuberculosis strains and clinical samples | Not stated | Spain | Laboratory | Not stated | Yes | 54 | Pulmonary | 54 | |
| Lu et al. (2012)90 | W Lu, C Chen, Y Shao, JY Shi, CQ Zhong, DD Yang, HH Song, GL Li, XY Ding, H Peng, LY Zhu, Y Zhou, LM Zhu | 2012 | Evaluation of biochip system in determining isoniazid and rifampicin resistances of Mycobacterium tuberculosis in sputum samples | Not stated | China | Laboratory | Not stated | Not stated | 907 | Smear-positive pulmonary | 907 | |
| Lyu et al. (2013)63 | J Lyu, MN Kim, JW Song, CM Choi, YM Oh, SD Lee, WS Kim, DS Kim, TS Shim | 2013 | GenoType (R) MTBDRplus assay detection of drug-resistant tuberculosis in routine practice in Korea | Not stated | Republic of Korea | Laboratory | Not stated | Not stated | 428 | Other (specify) | 428 | |
| Macedo et al. (2009)98 | R Macedo, A Amorim, E Pereira | 2009 | Multidrug-resistant tuberculosis: rapid molecular detection with MTBDRplus (R) assay in clinical samples | Not stated | Portugal | Laboratory | Not stated | Not stated | 68 | Smear-positive pulmonary | 68 | |
| Maschmann et al. (2013)100 | RD Maschmann, FS Spies, LD Nunes, AW Ribeiro, TRM Machado, A Zaha, MLR Rossetti | 2013 | Performance of the genotype MTBDRplus assay directly on sputum specimens from Brazilian patients with tuberculosis treatment failure or relapse | Not stated | Brazil | Outpatient | Not stated | Not stated | 68 | Pulmonary | 112 | |
| Mikhailovich et al. (2001)91 | Vladimir Mikhailovich, Sergey Lapa, Dimitry Gryadunov, Alexander Sobolev, Boris Strizhkov, Nikolai Chernyh, Olga Skotnikova, Olga Irtuganova, Arkadii Moroz, Vitalii Litvinov, Mikhail Vladimirskii, Mikhail Perelman, Larisa Chernousova, Vladislav Erokhin, Alexander Zased | 2001 | Identification of rifampin-resistant Mycobacterium tuberculosis strains by hybridization, PCR, and ligase detection reaction on oligonucleotide microchips | Not stated | Russia | Laboratory | Not stated | Not stated | 31 | Pulmonary | 31 | |
| Miotto et al. (2012)79 | P Miotto, AM Cabibbe, P Mantegani, E Borroni, L Fattorini, E Tortoli, GB Migliori, DM Cirillo | 2012 | GenoType MTBDRs/performance on clinical samples with diverse genetic background | Not stated | Italy | Laboratory | Not stated | Not stated | 59 | Smear-positive pulmonary | 59 | |
| Mironova et al. (2012)34 | S Mironova, E Pimkina, I Kontsevaya, V Nikolayevskyy, Y Balabanova, G Skenders, T Kummik, F Drobniewski | 2012 | Performance of the GenoType® MTBDRPlus assay in routine settings: a multicenter study | Not stated | Multi | Laboratory | No | Not stated | 1025 | Other (specify) | 1025 | |
| Mokrousov et al. (2003)92 | Igor Mokrousov, Tatiana Otten, Boris Vyshnevskiy, Olga Narvskaya | 2003 | Allele-specific rpoB PCR assays for detection of rifampin-resistant Mycobacterium tuberculosis in sputum smears | Not stated | Russia | Laboratory | Not stated | Not stated | 114 | Smear-positive pulmonary | 114 | |
| Naidoo et al. (2010)60 | S Naidoo | 2010 | Evaluation of GeneXpert MTB/RIF assay on pulmonary and extra-pulmonary samples in a high-throughput laboratory | Not stated | South Africa | Laboratory | Not stated | Not stated | 801 | Pulmonary | 1140 | |
| Nikolayevskyy et al. (2009)99 | V Nikolayevskyy, Y Balabanova, T Simak, N Malomanova, I Fedorin, F Drobniewski | 2009 | Performance of the GenoType MTBDRPlus assay in the diagnosis of tuberculosis and drug resistance in Samara, Russian Federation | Not stated | Russia | Laboratory | No | Yes | 149 | Pulmonary | 168 | |
| Nosova et al. (2013)93 | E Yu Nosova, MA Krasnova, K Yu Galkina, MV Makarova, VI Litvinov, AM Moroz | 2013 | Comparative analysis of TB-Biochip, Xpert MTB/RIF, and GenoType MTBDRplus test systems for rapid determination of mutations responsible for drug resistance of M. tuberculosis complex (in sputum from patients in Moscow region) | Not stated | Russia | Not stated | 278 | Pulmonary | 278 | |||
| O’Grady et al. (2012)61 | Justin O’Grady, Matthew Bates, Lophina Chilukutu, Judith Mzyece, Busiku Cheelo, Moses Chilufya, Lukundo Mukonda, Maxwell Mumba, John Tembo, Mumba Chomba, Nathan Kapata, Markus Maeurer, Andrea Rachow, Petra Clowes, Michael Hoelscher, Peter Mwaba, Alimuddin Zumla | 2012 | Evaluation of the Xpert MTB/RIF assay at a tertiary care referral hospital in a setting where tuberculosis and HIV infection are highly endemic. [Erratum appears in Clin Infect Dis 2013; 56:313] | Not stated | Zambia | Laboratory | Not stated | 881 | Smear-positive pulmonary | 937 | ||
| Ogwang et al. (2009)55 | Sam Ogwang, Benon B Asiimwe, Hamidou Traore, Francis Mumbowa, Alphonse Okwera, Kathleen D Eisenach, Susan Kayes, Edward C Jones-Lopez, Ruth McNerney, William Worodria, Irene Ayakaka, Roy D Mugerwa, Peter G Smith, Jerrold Ellner, Moses L Joloba | 2009 | Comparison of rapid tests for detection of rifampicin-resistant Mycobacterium tuberculosis in Kampala, Uganda | Cross-sectional | Uganda | Laboratory | Not stated | Yes | 203 | Smear-positive pulmonary | ||
| Pang et al. (2013)88 | Y Pang, H Xia, ZY Zhang, JC Li, Y Dong, Q. Li, XC Ou, YY Song, YF Wang, R O’Brien, KM Kam, JY Chi, ST Huan, DP Chin, Y Zhao | 2013 | Multicenter evaluation of genechip for detection of multidrug-resistant Mycobacterium tuberculosis | Not stated | China | Laboratory | Not stated | Not stated | 1814 | Smear-positive pulmonary | 2247 | |
| Sam et al. (2006)56 | I Ching Sam, Francis Drobniewski, Philip More, Melanie Kemp, Timothy Brown | 2006 | Mycobacterium tuberculosis and rifampin resistance, UK | Not stated | UK | Laboratory | Not stated | Not stated | 2287 | Pulmonary | 2287 | |
| Seoudi et al. (2012)35 | N Seoudi, SL Mitchell, TJ Brown, F Dashti, AK Amin, FA Drobniewski | 2012 | Rapid molecular detection of tuberculosis and rifampicin drug resistance: retrospective analysis of a national UK molecular service over the last decade | Not stated | UK | Laboratory | Not stated | Yes | 8501 | Smear-positive pulmonary | 8501 | |
| Sheen et al. (2009)94 | P Sheen, M Mendez, RH Gilman, L Pena, L Caviedes, MJ Zimic, Y Zhang, DAJ Moore, C A Evans | 2009 | Sputum PCR-single-strand conformational polymorphism test for same-day detection of pyrazinamide resistance in tuberculosis patients | Not stated | Peru | Laboratory | Not stated | Yes | 65 | Smear-positive pulmonary | 65 | |
| Siu et al. (2011)95 | Gilman Kit Hang Siu, Yuk Ho Tam, Pak Leung Ho, Ann Siew Gek Lee, Tak Lun Que, Cindy Wing Sze Tse, Kam Tong Yip, Jason Tsz Hin Lam, Vincent Chi Chung Cheng, Kwok Yung Yuen, Wing Cheong Yam | 2011 | Direct detection of isoniazid-resistant Mycobacterium tuberculosis in respiratory specimens by multiplex allele-specific polymerase chain reaction | Not stated | China | Laboratory | Not stated | Not stated | 198 | Pulmonary | 487 | |
| Skenders et al. (2005)57 | Girts Skenders, Alicia M Fry, Inga Prokopovica, Silvija Greckoseja, Lonija Broka, Beverly Metchock, Timothy H Holtz, Charles D Wells, Vaira Leimane | 2005 | Multidrug-resistant tuberculosis detection, Latvia | Not stated | Latvia | Laboratory | Not stated | Not stated | 243 | Smear-positive pulmonary | 243 | |
| Somoskovi et al. (2006)74 | Akos Somoskovi, Jillian Dormandy, Dimitra Mitsani, Jeremy Rivenburg, Max Salfinger | 2006 | Use of smear-positive samples to assess the PCR-based genotype MTBDR assay for rapid, direct detection of the Mycobacterium tuberculosis complex as well as its resistance to isoniazid and rifampin | Not stated | USA | Laboratory | Not stated | Not stated | 143 | Smear-positive pulmonary | 143 | |
| Tukvadze et al. (2012)62 | Nestani Tukvadze, Russell R. Kempker, Iagor Kalandadze, Ekaterina Kurbatova, Michael K. Leonard, Rusudan Apsindzelashvili, Nino Bablishvili, Maia Kipiani, Henry M. Blumberg | 2012 | Use of a molecular diagnostic test in AFB smear positive tuberculosis suspects greatly reduces time to detection of multidrug resistant tuberculosis | Not stated | Georgia | Laboratory | Not stated | Not stated | 474 | Smear-positive pulmonary | 500 | |
| Vadwai et al. (2012)96 | Viral Vadwai, Anjali Shetty, Camilla Rodrigues | 2012 | Multiplex allele-specific PCR for rapid detection of extensively drug resistant tuberculosis | Not stated | India | Other, specify | Not stated | 450 | Smear-positive pulmonary | |||
| Viveiros et al. (2005)33 | Miguel Viveiros, Clara Leandro, Liliana Rodrigues, Josefina Almeida, Rosario Bettencourt, Isabel Couto, Lurdes Carrilho, Jose Diogo, Ana Fonseca, Luis Lito, Joao Lopes, Teresa Pacheco, Mariana Pessanha, Judite Quirim, Luisa Sancho, Max Salfinger, Leonard Amaral | 2005 | Direct application of the INNO-LiPA Rif.TB line-probe assay for rapid identification of Mycobacterium tuberculosis complex strains and detection of rifampin resistance in 360 smear-positive respiratory specimens from an area of high incidence of multidrug-resistant tuberculosis | Not stated | Portugal | Laboratory | Not stated | Not stated | 360 | Smear-positive pulmonary | 360 | |
| Zhang et al. (2012)97 | ZH Zhang, LT Li, F Luo, P Cheng, F Wu, Z Wu, TY Hou, M Zhong, JZ Xu | 2012 | Rapid and accurate detection of RMP- and INH-resistant Mycobacterium tuberculosis in spinal tuberculosis specimens by CapitalBio (TM) DNA microarray: a prospective validation study | Not stated | China | Laboratory | Yes | 93 | Extrapulmonary | 153 | ||
Appendix 5 Index and reference test information
| Study | Microscopy | Index test details | Reference test details | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Microscopy? | Type of microscopy | Number of samples per participant tested | Type of smear | Test category | Rapid test type (other) | Reference test category | Reference test type (solid) | Reference test type (liquid) | Indeterminate results reported? | Reference test (other) | Number of contaminated cultures (Reference) | Number of cultures performed | Pretreatment of sample? | |
| Ajbani (2012)76 | No microscopy | Yes, Ziehl–Neelsen | One specimen per patient | Concentrated (processed) | MTBDRsl | MTBDRsl | Liquid culture | BACTEC MGIT 960a | No | 1 | 170 | NALC-NaOH | ||
| Albert (2010)73 | No microscopy | Yes, Ziehl–Neelsen | Multiple specimens per patient | Not stated | MTBDRplus | Liquid culture | BACTEC MGIT 960a | Yes | 92 | 118 | NALC-NaOH | |||
| Anek-Vorapong (2010)72 | No microscopy | Not stated | Not stated | Not stated | MTBDRplus | Liquid culture | BACTEC MGIT 960a | Yes | 164 | NALC-NaOH | ||||
| Antonova (2008)81 | Not stated | Not stated | Not stated | Not stated | TB biochip/microchip | Biological microchip assay | Solid culture | LJ | Not stated | 78 | 78 | |||
| Asencios (2012)71 | Not stated | Not stated | Not stated | Concentrated (processed) | MTBDRplus | GenoType MTBDRplus | Solid and liquid culture | LJ | No | 100 | NALC-NaOH | |||
| Barnard (2008)70 | No microscopy | Yes-FM 99 | Not stated | Concentrated (processed) | MTBDRplus | Solid and liquid culture | 7H11 | Yes | 536 | NALC-NaOH | ||||
| Barnard (2012)71 | No microscopy | Not stated | Not stated | Direct | MTBDRsl | GenoType MDRsl LPA | Liquid culture | Other (specify) | No | MGIT | 1 | 657 | NALC-NaOH | |
| Boehme (2010)29 | No microscopy | Yes, Ziehl–Neelsen | Multiple specimens per patient | Concentrated (processed) | GeneXpert | Solid and liquid culture | LJ | BACTEC MGIT 960a | Yes | 1462 | NALC-NaOH | |||
| Boehme (2011)38 | No microscopy | Not stated | Multiple specimens per patient | Concentrated (processed) | GeneXpert | Solid and liquid culture | LJ | BACTEC MGIT 960a | No | 1 | 1266 | NALC-NaOH | ||
| Calderón-Espinoza (2006)82 | No microscopy | Not stated | Not stated | Concentrated (processed) | PCR/in-house assays | PCR-UHG-RIF | Solid culture | Not stated | Not stated | DST, proportion method | 1 | 200 | NALC-NaOH | |
| Cauwelaert (2011)67 | Ziehl–Neelsen | Not stated | Not stated | Concentrated (processed) | MTBDRplus | Solid culture | LJ | No | NALC-NaOH | |||||
| Cho (2009)83 | Not stated | Not stated | Not stated | Not stated | PCR/in-house assays | Sequencing | Solid culture | LJ | Not stated | 1 | 46 | NaOH (Petroff) | ||
| Choi (2010)84 | No microscopy | Yes, Ziehl–Neelsen | Multiple specimens per patient | Not stated | PCR/in-house assays | Direct DNA sequencing analysis of katG, rpoB, embB and pncA | Solid culture | LJ | Not stated | 113 | Not stated | |||
| Costeira (2007)51 | No microscopy | Yes, Ziehl–Neelsen | Not stated | Not stated | INNO-LiPA | Liquid culture | BACTEC MGIT 960a | No | 1 | 113 | Not stated | |||
| Crudu (2012)69 | No microscopy | Yes, Ziehl–Neelsen | Not stated | Not stated | MTBDRplus | MTBDRplus | Solid culture | 7H11 | 156 | NALC-NaOH | ||||
| Dorman (2012)68 | No | Not stated | One specimen per patient | Not stated | MTBDRplus | Liquid culture | BACTEC MGIT 960a | Yes | 1 | 529 | NALC-NaOH | |||
| Drobniewski (2000)52 | No microscopy | Yes, Ziehl–Neelsen | Not stated | Not stated | INNO-LiPA | DNA hybridisation (Accuprobe, Gen-Probe, San Diego, CA, USA) | Liquid culture | BACTEC 460 TBa | NALC-NaOH | |||||
| Eliseev (2013)66 | No microscopy | Not stated | Multiple specimens per patient | Not stated | MTBDRplus | MTBDRplus | Liquid culture | BACTEC MGIT 960a | Yes | 211 | Not stated | |||
| Farooqi (2012)65 | No microscopy | Not stated | Not stated | Concentrated (processed) | MTBDRplus | Solid culture | LJ | No | 1 | 108 | NALC-NaOH | |||
| García-Sierra (2011)85 | No microscopy | Yes, Ziehl–Neelsen | Multiple specimens per patient | Not stated | PCR/in-house assays | Pyrosequencing | Liquid culture | BACTEC 460 TBa | Not stated | 48 | Not stated | |||
| Gryadunov (2005)86 | No microscopy | Yes, Ziehl–Neelsen | Not stated | Concentrated (processed) | TB biochip/microchip | Biochip assay | Solid culture | LJ | No | 113 | NALC-NaOH | |||
| Guo (2009)87 | Not stated | Not stated | Not stated | Not stated | TB biochip/microchip | In-house biochip system | Solid culture | LJ | NaOH (Petroff) | |||||
| Higuchi (2004)53 | Not stated | Not stated | Not stated | Concentrated (processed) | INNO-LiPA | Finnos LiPA/Rif TB (Nipro, Osaka, Japan) Also, AMPLICOR MTB (Roche Diagnostics, Tokyo, Japan) |
Liquid culture | BACTEC MGIT 960a | No | 1 | 42 | NALC-NaOH | ||
| Hillemann (2006)64 | Not stated | Not stated | Not stated | Concentrated (processed) | MTBDRplus | Solid culture | LJ | 42 | NALC-NaOH | |||||
| Hillemann (2009)78 | No microscopy | Yes, Ziehl–Neelsen | Not stated | Concentrated (processed) | MTBDRsl | MTBDRsl assay | Solid and liquid culture | LJ | BACTEC MGIT 960a | Yes | 1 | 64 | NALC-NaOH | |
| Johansen (2003)54 | Not stated | Not stated | Not stated | Not stated | INNO-LiPA | Liquid culture | BACTEC 460 TBa | Yes | 1 | 60 | Not stated | |||
| Kim (2001)89 | Not stated | Not stated | Not stated | PCR/in-house assays | Nested PCR-linked single-strand conformation | Solid culture | Not stated | Yes | 1 | 56 | Not stated | |||
| Kim (2012)58 | No microscopy | Not stated | Not stated | Concentrated (processed) | GeneXpert | Solid culture | LJ | No | 1 | 62 | NALC-NaOH | |||
| Kontsevaya (2013)37 | No microscopy | Yes, Ziehl–Neelsen | Not stated | Not stated | MTBDRsl | GenoType MTBDRplus | Solid and liquid culture | LJ | BACTEC MGIT 960a | Yes | 1 | 90 | Not stated | |
| Kurbatov (2013)59 | No microscopy | Yes, Ziehl–Neelsen | Multiple specimens per patient | Concentrated (processed) | GeneXpert | Also TB biochip (Biochip-IMB Ltd, Russia) | Solid and liquid culture | LJ | BACTEC MGIT 960a | Yes | 1 | 62 | NALC-NaOH | |
| Lacoma (2008)75 | No microscopy | Yes, Ziehl–Neelsen | Multiple specimens per patient | Concentrated (processed) | MTBDRplus | MTBDRplus | Liquid culture | BACTEC 460 TBa | No | 51 | NALC-NaOH | |||
| Lacoma (2012)77 | Ziehl–Neelsen | Yes, Ziehl–Neelsen | Multiple specimens per patient | Concentrated (processed) | MTBDRsl | MTBDRsl | Liquid culture | BACTEC 460 TBa | Yes | 1 | 54 | NALC-NaOH | ||
| Lu (2012)90 | No microscopy | Yes, Ziehl–Neelsen | Not stated | Not stated | TB biochip/Microchip | Biochip MDR assay | Solid culture | LJ | No | 690 | NALC-NaOH | |||
| Lyu (2013)63 | No microscopy | Yes, Ziehl–Neelsen | Not stated | Not stated | MTBDRplus | MTBDRplus | Liquid culture | BACTEC MGIT 960a | No | 428 | Not stated | |||
| Macedo (2009)98 | No microscopy | Yes, Ziehl–Neelsen | Not stated | Concentrated (processed) | MTBDRplus | MTBDRplus | Liquid culture | BACTEC 460 TBa | Not stated | 68 | ||||
| Maschmann (2013)100 | Not stated | Not stated | Not stated | Not stated | MTBDRplus | Solid culture | LJ | No | 1 | 68 | NaOH (Petroff) | |||
| Mikhailovich (2001)91 | Not stated | Not stated | Not stated | Concentrated (processed) | TB biochip/microchip | Hybridisation – TB-MAGIChip | Solid culture | LJ | No | 1 | 31 | NALC-NaOH | ||
| Miotto (2012)79 | No microscopy | Yes, Ziehl–Neelsen | Not stated | Concentrated (processed) | MTBDRsl | MTBDRsl | Liquid culture | BACTEC MGIT 960a | Yes | 1 | 59 | NALC-NaOH | ||
| Mironova (2012)34 | Not stated | Not stated | Not stated | Not stated | MTBDRplus | MTBDRplus | Solid and liquid culture | LJ | BACTEC MGIT 960a | Yes | 689 | NALC-NaOH | ||
| Mokrousov (2003)92 | No microscopy | Yes, Ziehl–Neelsen | Not stated | Not stated | PCR/in-house assays | NAS-PCR assay | Not stated | Yes | 1 | 114 | Not stated | |||
| Naidoo (2010)60 | Not stated | Not stated | Not stated | Not stated | GeneXpert | Liquid culture | BACTEC MGIT 960a | Not stated | 801 | 801 | Not stated | |||
| Nikolayevskyy (2009)99 | No microscopy | Yes, Ziehl–Neelsen | One specimen per patient | Concentrated (processed) | MTBDRplus | Solid and liquid culture | LJ | BACTEC MGIT 960a | Not stated | 149 | 165 | NALC-NaOH | ||
| Nosova (2013)93 | No microscopy | Yes, Ziehl–Neelsen | Not stated | Concentrated (processed) | TB biochip/Microchip | TB biochip | Liquid culture | BACTEC MGIT 960a | No | 38 | 38 | None | ||
| O’Grady (2012)61 | No microscopy | Yes, Ziehl–Neelsen | Not stated | Not stated | GeneXpert | Liquid culture | BACTEC MGIT 960 | No | 99 | 111 | NALC-NaOH | |||
| Ogwang (2009)55 | No microscopy | Not stated | Unknown number of specimens per patient | Concentrated (processed) | INNO-LiPA | Liquid culture | BACTEC 460 TBa | Not stated | 203 | NaOH (Petroff) | ||||
| Pang (2013)88 | No microscopy | Yes, Ziehl–Neelsen | Not stated | Concentrated (processed) | TB biochip/Microchip | Genechip | Solid culture | LJ | No | 1814 | NALC-NaOH | |||
| Sam (2006)56 | No microscopy | Yes, Ziehl–Neelsen | Multiple specimens per patient | Not stated | INNO-LiPA | Solid culture | LJ | Yes | 657 | NALC-NaOH | ||||
| Seoudi (2012)35 | No microscopy | Yes-FM 99 | One specimen per patient | Concentrated (processed) | INNO-LiPA | Solid and liquid culture | LJ | BACTEC MGIT 960a | Yes | NALC-NaOH | ||||
| Sheen (2009)94 | No microscopy | Yes-FM 99 | Unknown number of specimens per patient | Concentrated (processed) | PCR/in-house assays | PCR-SSCP test | Liquid culture | BACTEC 460 TBa | Not stated | 1 | 52 | Other, specify | ||
| Siu (2011)95 | No microscopy | Yes, Ziehl–Neelsen | Not stated | Not stated | PCR/in-house assays | MAS-PCR | Solid culture | 7H10 | Yes | 198 | Other, specify | |||
| Skenders (2005)57 | No microscopy | Yes, Ziehl–Neelsen | Unknown number of specimens per patient | Not stated | INNO-LiPA | Liquid culture | BACTEC 460 TBa | Yes | 1 | 89 | Other, specify | |||
| Somoskovi (2006)74 | No microscopy | Yes, Ziehl–Neelsen | One specimen per patient | Concentrated (processed) | MTBDRplus | Liquid culture | BACTEC 460 TBa | Yes | 1 | 143 | NaOH (Petroff) | |||
| Tukvadze (2012)62 | No microscopy | Yes, Ziehl–Neelsen | Not stated | Concentrated (processed) | MTBDRplus | MTBDR plus | Solid and liquid culture | LJ | BACTEC MGIT 960a | Yes | 1 | 500 | NALC-NaOH | |
| Vadwai (2012)96 | No microscopy | Yes, Ziehl–Neelsen | One specimen per patient | Not stated | PCR/in-house assays | MAS PCR | Solid and liquid culture | LJ | BACTEC MGIT 960a | Yes | MGIT SIRE | 289 | NALC-NaOH | |
| Viveiros (2005)33 | No microscopy | Yes, Ziehl–Neelsen | Unknown number of specimens per patient | Not stated | INNO-LiPA | Liquid culture | BACTEC MGIT 960a | No | 342 | NALC-NaOH | ||||
| Zhang (2012)97 | One specimen per patient | PCR/in-house assays | CapitalBio DNA microarray (CapitalBio Corp, Beijing, China) | Liquid culture | LJ | BACTEC MGIT 960a | Yes | 1 | 93 | NALC-NaOH | ||||
Appendix 6 Participant information and drug resistance investigated
| Study (first author and year) | Participant information | Drug resistance investigated | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Recruitment | Age category | HIV status | Previous history of TB? | Previous history of TB (n) | Previous history of TB (denominator) | Outcomes evaluated? | Isoniazid study? | Rifampicin study? | Amikacin study | Quinolones study? | Moxifloxacin study? | Ofloxacin study? | Capreomycin study? | Other study? | Ethambutol study? | Kanamycin study? | Other study (specify) | |
| Ajbani (2012)76 | Consecutive | Not stated | Not stated | Not stated | No | N | N | N | N | N | N | N | Y | Y | Y | FQ | ||
| Albert (2010)73 | Consecutive | Not stated | Not stated | 118 | 118 | No | Y | Y | N | N | N | N | N | N | N | N | ||
| Anek-Vorapong (2010)72 | Not stated | Not stated | Not stated | Not stated | Not stated | Y | Y | N | N | N | N | N | Y | N | N | Streptomycin and ethambutol | ||
| Antonova (2008)81 | Not stated | Not stated | Not stated | Not stated | Not stated | N | N | N | N | N | Y | N | N | N | N | |||
| Asencios (2012)71 | Random | Not stated | Not stated | Not stated | Not stated | Y | Y | N | N | N | N | N | Y | N | N | MDR | ||
| Barnard (2008)70 | Not stated | Not stated | Not stated | Not stated | Not stated | Y | Y | N | N | N | N | N | N | N | N | |||
| Barnard (2012)80 | Consecutive | Not stated | Not stated | Not stated | Not stated | N | N | Y | N | N | Y | N | N | N | N | |||
| Boehme (2010)29 | Consecutive | Adults (≥ 16 years) | HIV–/HIV+ | 671 | 1447 | Yes | N | Y | N | N | N | N | N | N | N | N | ||
| Boehme (2011)38 | Consecutive | Adults (≥ 16 years) | HIV–/HIV+ | Not stated | Not stated | N | Y | N | N | N | N | N | N | N | N | |||
| Calderón-Espinoza (2006)82 | Not stated | Not stated | Not stated | 153 | 200 | Not stated | N | Y | N | N | N | N | N | N | N | N | MDR, streptomycin | |
| Cauwelaert (2011)67 | Convenience | Not stated | Not stated | Not stated | Not stated | Y | Y | N | N | N | N | N | N | N | N | |||
| Cho (2009)83 | Not stated | Not stated | Not stated | Not stated | Not stated | Y | Y | N | N | N | N | N | N | N | N | |||
| Choi (2010)84 | Other (specify) | Adults and children | Not stated | 111 | 111 | Yes | Y | Y | N | N | N | N | N | Y | Y | N | Pyrazinamide | |
| Costeira (2007)51 | Not stated | Not stated | Not stated | Not stated | Not stated | N | Y | N | N | N | N | N | N | N | N | |||
| Crudu (2012)69 | Not stated | Not stated | Not stated | Not stated | Not stated | Y | Y | N | N | N | N | N | N | N | N | |||
| Dorman (2012)68 | Consecutive | Adults (≥ 16 years) | HIV–/HIV+ | 699 | 2516 | Not stated | Y | Y | N | N | N | N | N | N | N | N | ||
| Drobniewski (2000)52 | Consecutive | Not stated | HIV–/HIV+ | No | N | Y | N | N | N | N | N | N | N | N | ||||
| Eliseev (2013)66 | Consecutive | Adults and children | HIV–/HIV+ | 144 | 812 | No | Y | Y | N | N | N | N | N | N | N | N | ||
| Farooqi (2012)65 | Random | Not stated | Not stated | Not stated | Not stated | Y | Y | N | N | N | N | N | N | N | N | |||
| García-Sierra (2011)85 | Consecutive | Not stated | Not stated | Not stated | Not stated | Y | Y | N | N | N | N | N | N | N | N | |||
| Gryadunov (2005)86 | Not stated | Not stated | Not stated | 113 | 147 | No | Y | Y | N | N | N | N | N | N | N | N | ||
| Guo (2009)87 | Not stated | Not stated | Not stated | Not stated | Not stated | Y | Y | N | N | N | N | N | N | N | N | |||
| Higuchi (2004)53 | Consecutive | Not stated | Not stated | Not stated | Not stated | N | Y | N | N | N | N | N | N | N | N | |||
| Hillemann (2006)64 | Not stated | Not stated | Not stated | Not stated | Y | Y | N | N | N | N | N | N | N | N | ||||
| Hillemann (2009)78 | Consecutive | Not stated | Not stated | Not stated | Not stated | N | N | N | N | N | Y | N | N | Y | N | Amikacin and capreomycin tested together as AM-CM | ||
| Johansen (2003)54 | Not stated | Not stated | Not stated | Not stated | Not stated | N | Y | N | N | N | N | N | N | N | N | |||
| Kim (2001)89 | Not stated | Not stated | Not stated | Not stated | Not stated | N | Y | N | N | N | N | N | N | N | N | |||
| Kim (2012)58 | Consecutive | Not stated | HIV– | 53 | 71 | Yes | N | Y | N | N | N | N | N | N | N | N | ||
| Kontsevaya (2013)37 | Not stated | Adults (≥ 16 years) | HIV + | Not stated | 38 | 90 | No | N | N | Y | N | N | N | Y | Y | Y | Y | FQ |
| Kurbatov (2013)59 | Consecutive | Adults (≥ 16 years) | Not stated | Not stated | N | Y | N | N | N | N | N | N | N | N | ||||
| Lacoma (2008)75 | Not stated | Not stated | Not stated | Not stated | Not stated | Y | Y | N | N | N | N | N | Y | N | N | MDR | ||
| Lacoma (2012)77 | Not stated | Not stated | Not stated | Not stated | Not stated | N | N | N | Y | N | N | Y | N | Y | Y | Kanamycin and capreomycin are tested together as KM/CM | ||
| Lu (2012)90 | Consecutive | Not stated | Not stated | 241 | 907 | No | Y | Y | N | N | N | N | N | Y | N | N | MDR | |
| Lyu (2013)63 | Consecutive | Not stated | Not stated | Not stated | 105 | 428 | No | Y | Y | N | N | N | N | N | N | N | N | |
| Macedo (2009)98 | Not stated | Not stated | Not stated | Not stated | Not stated | Y | Y | N | N | N | N | N | N | N | N | |||
| Maschmann (2013)100 | Consecutive | Adults (≥ 16 years) | Not stated | Not stated | Not stated | Y | Y | N | N | N | N | N | Y | N | N | MDR | ||
| Mikhailovich (2001)91 | Not stated | Not stated | Not stated | Not stated | Not stated | N | Y | N | N | N | N | N | N | N | N | |||
| Miotto (2012)79 | Not stated | Not stated | Not stated | 59 | 59 | No | N | N | N | N | N | N | N | N | Y | N | Second-line injectable drugs tested together | |
| Mironova (2012)34 | Not stated | Not stated | Not stated | Not stated | Not stated | Y | Y | N | N | N | N | N | N | N | N | |||
| Mokrousov (2003)92 | Convenience | Not stated | Not stated | Not stated | N | Y | N | N | N | N | N | N | N | N | ||||
| Naidoo (2010)60 | Not stated | Not stated | Not stated | Not stated | Not stated | N | Y | N | N | N | N | N | N | N | N | |||
| Nikolayevskyy (2009)99 | Consecutive | Not stated | Not stated | 89 | 168 | Not stated | Y | Y | N | N | N | N | N | N | N | N | ||
| Nosova (2013)93 | Not stated | Not stated | Not stated | Not stated | Not stated | Y | Y | N | N | N | N | N | N | N | N | |||
| O’Grady (2012)61 | Not stated | Adults (≥ 16 years) | HIV–/HIV+ | Not stated | Not stated | N | Y | N | N | N | N | N | N | N | N | |||
| Ogwang (2009)55 | Consecutive | Adults (≥ 16 years) | Not stated | Not stated | N | Y | N | N | N | N | N | N | N | N | ||||
| Pang (2013)88 | Not stated | Not stated | Not stated | Not stated | Not stated | Y | Y | N | N | N | N | N | N | N | N | |||
| Sam (2006)56 | Consecutive | Not stated | Not stated | Not stated | Not stated | N | Y | N | N | N | N | N | N | N | N | |||
| Seoudi (2012)35 | Consecutive | Not stated | Not stated | Not stated | Not stated | N | Y | N | N | N | N | N | N | N | N | |||
| Sheen (2009)94 | Not stated | Not stated | HIV–/HIV+ | Not stated | Not stated | N | N | N | N | N | N | N | Y | N | N | Pyrazinamide | ||
| Siu (2011)95 | Consecutive | Not stated | Not stated | Not stated | Not stated | Y | N | N | N | N | N | N | N | N | N | |||
| Skenders (2005)57 | Consecutive | Not stated | Not stated | Not stated | N | Y | N | N | N | N | N | N | N | N | ||||
| Somoskovi (2006)74 | Consecutive | Not stated | Not stated | Not stated | Not stated | Y | Y | N | N | N | N | N | N | N | N | |||
| Tukvadze (2012)62 | Consecutive | Not stated | Not stated | 121 | 500 | Not stated | Y | Y | N | N | N | N | N | N | N | N | ||
| Vadwai (2012)96 | Consecutive | Not stated | Not stated | Not stated | No | Y | Y | N | N | N | N | N | Y | N | N | Fluoroquinolone | ||
| Viveiros (2005)33 | Consecutive | Adults and children | HIV–/HIV+ | 189 | Not stated | N | Y | N | N | N | N | N | N | N | N | |||
| Zhang (2012)97 | Consecutive | Adults and children | HIV– | 35 | 153 | No | Y | Y | N | N | N | N | N | N | N | N | ||
Appendix 7 Timed tasks in the time-and-motion study
Timed steps in each stage of the Xpert MTB/RIF assay
| Stage | Step |
|---|---|
| Preparation/set-up | Paperwork |
| Label tubes | |
| Transfer specimens | |
| Spin down CSF specimens (if > 2 ml) | |
| Decontamination | Set up for decontamination |
| Add alkaline solution to respiratory specimens | |
| Stand for 30 minutes, vortexing every 10 minutes | |
| Add buffer to neutralise | |
| Centrifuge for 30 minutes | |
| Add buffer to pellet | |
| Specimen pretreatment | Centrifuge to concentrate |
| Sample preparation and loading machine | Add Cepheid reagent to decontaminated sputum pellet |
| Stand for 15 minutes | |
| Transfer 2 ml into cartridge and load on to GeneXpert | |
| Run instrument | |
| Recording results | Record results |
| Transfer results to relevant desk | |
| Record on MOLIS |
Timed steps in each stage of the line probe assays
| Procedure | Stage | Step |
|---|---|---|
| INNO-LiPA and MTBDRplus (day 1) | Preparation/set-up | Paperwork |
| Label tubes | ||
| Prepare extraction reagents | ||
| Transfer 1-ml specimen to microcentrifuge tube | ||
| Sample decontamination | Put in centrifuge | |
| Aliquot extraction reagents | ||
| Prepare wax block samples | ||
| Remove tissue tubes from centrifuge | ||
| Discard supernatant | ||
| Resuspend pellet in 1 ml water | ||
| Put in centrifuge | ||
| Add buffer to wax blocks | ||
| Discard supernatant | ||
| Resuspend pellet in 250 µl or 50 µl of buffer | ||
| Lysing of cells | Put wax samples on heat block | |
| Remove tubes and flick to resuspend | ||
| Put tubes in a 95 °C hot block or water bath for 20 minutes | ||
| Allow tubes to cool for 5 minutes | ||
| Add chloroform to wax samples | ||
| Put tubes in sonic bath | ||
| Vortex samples | ||
| Centrifuge tubes for 5 minutes | ||
| First-round PCR | Clean area in clean laboratory | |
| Remove buffer and primers from fridge to thaw | ||
| Prepare PCR tubes | ||
| Prepare PCR mastermix in centrifuge tube | ||
| Centrifuge briefly | ||
| Distribute aliquots into PCR tubes | ||
| Close lids, clean up and transfer to CL2 laboratory | ||
| Vortex | ||
| Preparation | ||
| Place PCR tubes in PCR rack | ||
| Put in centrifuge | ||
| Prepare tubes | ||
| Add DNA extract to labelled PCR tubes | ||
| Add inhibition control to inhibition control tubes | ||
| Clean area | ||
| INNO-LiPA (day 2) | Second-round PCR | Remove buffer and primers from fridge to thaw |
| Set up | ||
| Prepare PCR master mix | ||
| Distribute aliquots into PCR tubes | ||
| Close lids, clean up and transfer to PCR product laboratory | ||
| Remove tubes from thermocycler and place in rack | ||
| Centrifuge tubes on picofuge | ||
| Assemble equipment in cabinet | ||
| Add 1 µl of first-round reaction to second-round tubes | ||
| Spin tubes on picofuge | ||
| Load tubes into thermocycler and start programme | ||
| Clean up | ||
| Gel electrophoresis | Make up agarose, pour into gel trays and leave to set | |
| Remove combs and tape and place gels in tanks with buffer | ||
| Spot loading dye on to parafilm | ||
| Mix samples with dye and load on to gel, add markers | ||
| Set up tanks for running | ||
| Take gels from tanks, photograph on UV transilluminator and print | ||
| Labelling printout image | ||
| LiPA assay | Label PCR tubes | |
| Remove reagents from box and set-up | ||
| Mix denaturing solution and pipette some into each tray | ||
| Pipette of 10 µl PCR product into each tray | ||
| Take out test strips, label with pencil and place in tray | ||
| Pipette of 1 ml hybridisation solution into each tray | ||
| Tip out hybridisation solution and add 1 ml stringent wash | ||
| Agitate on shaker for 1 minute | ||
| Repeat wash step | ||
| Remove hybridisation solution and add stringent wash | ||
| Make up rinse solution and conjugate solution | ||
| Remove stringent wash and add rinse solution, shake for 1 minute | ||
| Repeat wash step | ||
| Remove all rinse solution and add conjugate solution, shake | ||
| Wash strips twice in rinse solution (1 minute each) | ||
| Rinse strips in substrate buffer for 1 minute | ||
| Add dilute substrate to each test and agitate until readable | ||
| Wash strips with dH2O twice and agitate for 1 minute each | ||
| Remove strips from tray, dry and stick on form | ||
| Recording results | Record results | |
| Get results to relevant desk | ||
| MTBDRplus (day 2) | Setting up BeeBlot | Set BeeBlot machine to clean itself |
| Swap buffers in BeeBlot machine | ||
| MTBDRplus assay | Add denaturation solution into wells | |
| Mark strips with pencil | ||
| Add amplified samples into wells | ||
| Incubate for 5 minutes | ||
| Add hybridisation buffer to each well | ||
| Place each membrane in tray and set to run | ||
| Remove strips from the machine and set machine to wash itself | ||
| Sticking strips to form and fill in form | ||
| Interpreting results | ||
| Recording results | Record results | |
| Get results to relevant desk |
Timed steps in each procedure of primary specimen culture
| Procedure | Stage | Step |
|---|---|---|
| Inoculation | Paperwork/set-up | Paperwork and labelling tubes |
| Transfer specimens to falcon tubes | ||
| Add sputasol to CF samples, incubate 15 minutes | ||
| Load CF samples into centrifuge | ||
| Unload centrifuge | ||
| Decontamination | Add 6 ml of NaOH-NALC to samples (or oxalic acid/sulphuric acid if CF sputum), mix by vortexing | |
| Incubate for 30 minutes, mixing every 10 minutes | ||
| Add 40 ml of BD BBL Mycoprep PB and mix | ||
| Load into centrifuge | ||
| Unload centrifuge, discard supernatant and resuspend in 1.5 ml of sterile PB | ||
| Preparing LJ and MGITs and inoculating with sample | Rack up and label MGIT and LJ tubes | |
| Mix PANTA with growth supplement and add to MGITs | ||
| Inoculate MGIT and LJ slopes with resuspended sample | ||
| Loading samples into incubator and BACTEC MGIT 960 | Load LJ slopes into incubator | |
| Load MGITs into BACTEC MGIT 960 | ||
| Reading and reporting results | Preparing positive MGIT samples for smear microscopy and blood plates | Unload positives from BACTEC MGIT 960 |
| Print report | ||
| Set up (label universal tubes, slides, blood plates, etc.) | ||
| Transfer MGIT cultures to universals, make slides and inoculate blood plates | ||
| Set up (finding forms) | ||
| Enter information into computer and print labels | ||
| Label positives worksheet | ||
| Load universals and blood plates into incubator | ||
| Staining slides and performing smear microscopy | Remove slides from hot plate and arrange over sink, add paper over slides and add dye | |
| Leave dye on slides for a few minutes | ||
| Rinse slides and add phenol solution | ||
| Leave slides for a few minutes | ||
| Rinse slides and add second phenol solution | ||
| Leave slides for a few minutes | ||
| Rinse slides and add stain | ||
| Add slides to hot block to dry | ||
| Check blood plates for contamination | ||
| Remove slides from hot plate | ||
| Set up microscope, etc. | ||
| View slides under microscope and record status of slides | ||
| Clean up | ||
| Reporting MGIT culture results and retreating contaminated samples | Fill in positives worksheet | |
| Fill in the contamination information | ||
| Retreat the three contaminated samples and load into centrifuge | ||
| Report results on MOLIS | ||
| Make fresh samples from the retreated contaminated samples (for reculture) | ||
| Paperwork and labelling the MTB-positive samples to be sent to the reference laboratory | ||
| Reading LJ slopes (weekly) | Set up and read cultures | |
| Clean up |
Timed steps in each procedure for drug susceptibility testing on solid media
| Procedure | Stage | Step |
|---|---|---|
| Preparation of LJ slopes | Preparing the LJ medium | Collect eggs |
| Sterilise eggs with acetone | ||
| Set up (get out glycerol, blender, drugs, etc.) | ||
| Break eggs into blender, blend and pour into flask | ||
| Clean up | ||
| Add penicillin | ||
| Add glycerol mineral salts | ||
| Add green dye | ||
| Paperwork | ||
| Set up | ||
| Pour LJ into flasks | ||
| Add different amounts of drug to each flask | ||
| Make up the final dilutions | ||
| Clean up | ||
| Aliquotting the LJ medium into bijou bottles | Aliquot LJ into bijou bottles | |
| Put bijou bottles in oven to solidify | ||
| Filling trays with DST LJ slopes | Arrange DST LJ slopes in trays ready for inoculation | |
| Inoculation LJ slopes | Set up | Print labels |
| Sort samples | ||
| Set up (label bijou, archive and inoculum tubes) | ||
| Inoculating slopes | Transfer cultures to bijou tubes | |
| Inoculate LJ slopes (20 slopes per sample) | ||
| Put slopes in walk-in incubator | ||
| Reading LJ DST results (weekly) | Set up | Paperwork/set-up |
| Collecting cultures from hot room | ||
| Checking controls | Checking controls | |
| Reading and reporting cultures | Reading and reporting the susceptibility status of cultured samples | |
| Clearing away read cultures |
Timed steps in each procedure for drug susceptibility testing using BACTEC MGIT 960
| Procedure | Stage | Step |
|---|---|---|
| MGIT inoculation | Paperwork/set-up | Collect cultures |
| Quality control of BACTEC MGIT 960 | ||
| Paperwork | ||
| Preparing tubes for inoculation | Prepare tray of tubes for DST inoculation | |
| Add enrichment supplement and PANTA to MGITs | ||
| Add drugs to MGITs | ||
| Enter DST information into computer and add labels to MGITs | ||
| Adding sample to tubes | Add subculture to drug-containing MGITs | |
| Machine loading and clean-up | Paperwork | |
| Load DST tubes into BACTEC MGIT 960 | ||
| Clean up | ||
| Reading MGIT DST results (daily) | Reading and recording results | Check reports on computer system and print results |
| Remove the tubes from the BACTEC MGIT 960 | ||
| Check the tubes visually | ||
| Enter test status into the computer system | ||
| Report results | ||
| Paperwork |
Appendix 8 Diagnostic delays: a patient’s story
X is a former patient who had drug-susceptible pulmonary tuberculosis (TB) in both lungs. She was symptomatic from 2004 and received two courses of treatment, including surgery, in 2005 and 2006–7. She remained under close follow-up until discharged from outpatient care in 2010.
X was symptomatic for 9 months in 2004 before she was referred from primary care to a London hospital for an initial chest X-ray that showed persistent inflammation and partial lung collapse. As a result of administrative delays, it was another 3 months before X was referred internally to the local chest clinic for diagnosis and subsequent treatment of her TB. Therapy did not start for a further 4 weeks, during which time X received support from the TB specialist nursing team. Further examination showed collapse of the left lung and severe weight loss. X received 2 weeks of inpatient treatment. She took 9 months of TB treatment with standard drugs and also required other medication including weight gain supplements.
Prior to TB patients being exempt from paying for NHS prescriptions in 2007, people were required to pay for the full cost of TB treatment. In order to reduce the burden, X applied for a pre-paid certificate. X returned to university to begin the new academic year, while also bearing the cost of travel from outside London to attend clinic appointments. X received a student loan and had no other form of income.
In December 2005, X completed the course of TB treatment but remained under follow-up care. In January 2006, she was referred to a dietitian for assessment and treatment; however, in mid-2006, X was admitted to the London hospital and placed in a side room with presumed relapse of her TB. Infection control measures were put in place, with health-care staff and visitors who entered the side room being required to wear a mask. X commenced treatment for TB and was discharged after one and a half weeks. Tests on cultured samples confirmed that the TB was fully drug susceptible. X was also diagnosed and treated for Pseudomonas lung infection – indicating the presence of chronic lung damage. X was diagnosed with bronchiectasis and referred back to the care of physiotherapists for additional, ongoing treatment.
During this time, X interrupted her studies as an intermittent student, which led to serious funding implications. X was no longer eligible to receive a student loan while not in attendance at university and, owing to student status, did not qualify for social security entitlements. X received no additional financial support, and as a result, relied on credit and a student account overdraft to support living costs and treatment. X had no previous history of debt or financial difficulties.
As well as treatment side effects in the form of nausea and joint pains, X suffered from hair loss during the first 2 months of starting treatment on both occasions, was referred to a dermatologist for investigation and was diagnosed with folate deficiency.
In early 2007, X’s treatment had been reduced but further examinations showed a reservoir of infection that had caused the TB to infiltrate the previously healthy right lung. X eventually had a pneumonectomy to improve quality of life and increase the chance of a full cure. X continued on TB treatment for the right lung until November 2007.
Upon returning to university after 18 months, X became liable to the Student Loans Company for part of the loan awarded during the previous academic year. X plunged into debt and owed the university a full term’s rent instalment. The university was now in a position to refuse registration, which would prevent X from continuing her studies. X received advice and representation from the Students’ Union, including information about additional sources of income, and eventually was able to continue studying at the university. It took X 5 years to complete undergraduate studies and the university withheld the degree certificate until the debt was repaid in full.
Two years after completing TB treatment, X was diagnosed with anxiety and depression, which X’s GP believes is a result of the severity and prolonged nature of the illness. X had no previous history of depression or anxiety.
X was treated using a combination of antidepressants for 6 months, along with counselling and therapy from the university and the NHS for a period of 18 months.
The estimated cost of travel to London for clinic appointments, TB treatment/additional drugs, loss of income/productivity and the burden of debt ran into thousands of pounds.
List of abbreviations
- BACTEC 460 TB
- BACTEC 460 TB system
- BACTEC MGIT 960
- BACTEC MGIT 960 Mycobacterial Detection System
- BMS
- biomedical scientist
- BNF
- British National Formulary
- bp
- base pair
- CFU
- colony-forming unit
- CI
- confidence interval
- CSF
- cerebrospinal fluid
- df
- degree of freedom
- DNA
- deoxyribonucleic acid
- DOR
- diagnostic odds ratio
- DS
- drug-sensitive
- DST
- drug susceptibility testing
- ETS
- Enhanced Tuberculosis Surveillance
- FIND
- Foundation for Innovative and New Diagnostics
- FN
- false negative
- FP
- false positive
- GeneXpert
- GeneXpert MTB/RIF assay
- HIV
- human immunodeficiency virus
- HSROC
- hierarchical summary receiver operating characteristic
- HUI3
- Health Utilities Index 3
- ICER
- incremental cost-effectiveness ratio
- INB
- incremental net benefit
- INNO-LiPA
- INNO-LiPA Rif.TB assay
- LJ
- Löwenstein–Jensen
- LPA
- line probe assay
- MDR
- multidrug resistant
- MDR-TB
- multidrug-resistant tuberculosis
- MGIT
- Mycobacteria Growth Indicator Tube
- MIC
- minimum inhibitory concentration
- MODS
- microscopic observation of drug susceptibility
- MTB
- Mycobacterium tuberculosis
- MTBC
- Mycobacterium tuberculosis complex
- MTBDRplus
- GenoType Mycobacterium tuberculosis drug resistance plus assay
- MTBDRsl
- GenoType Mycobacterium tuberculosis drug resistance second-line assay
- NAAT
- nucleic acid amplification test
- NICE
- National Institute for Health and Care Excellence
- NMRL
- National Mycobacterium Reference Laboratory
- NPV
- negative predictive value
- ONS
- Office for National Statistics
- PCR
- polymerase chain reaction
- POC
- point of care
- PPV
- positive predictive value
- PSA
- probabilistic sensitivity analysis
- PSSRU
- Personal Social Services Research Unit
- QALY
- quality-adjusted life-year
- QUADAS
- Quality Assessment of Diagnostic Accuracy Studies
- QUADAS-2
- Quality Assessment of Diagnostic Accuracy Studies version 2
- RIF
- rifampicin
- RRDR
- rifampicin resistance determining region
- SOP
- standard operating procedure
- SROC
- summary receiver operating characteristic
- TB
- tuberculosis
- TLA
- thin-layer agar
- TN
- true negative
- TP
- true positive
- WGS
- whole genome sequencing
- WHO
- World Health Organization
- XDR
- extensively drug resistant
- XDR-TB
- extensively drug-resistant tuberculosis