Notes
Article history
The research reported in this issue of the journal was funded by the HTA programme as project number 08/13/16. The contractual start date was in May 2010. The draft report began editorial review in November 2013 and was accepted for publication in December 2014. The authors have been wholly responsible for all data collection, analysis and interpretation, and for writing up their work. The HTA editors and publisher have tried to ensure the accuracy of the authors’ report and would like to thank the reviewers for their constructive comments on the draft document. However, they do not accept liability for damages or losses arising from material published in this report.
Declared competing interests of authors
Geoffrey Warhurst, Paul Dark, Ronan McMullen and Daniel McAuley have received research funding from the Technology Strategy Board to develop point-of-care diagnostics for sepsis. Paul Chadwick has received lecture honoraria from Novartis and funding from Pfizer and AstraZeneca to support local and regional research meetings in Clinical Microbiology.
Permissions
Copyright statement
© Queen’s Printer and Controller of HMSO 2015. This work was produced by Warhurst et al. under the terms of a commissioning contract issued by the Secretary of State for Health. This issue may be freely reproduced for the purposes of private research and study and extracts (or indeed, the full report) may be included in professional journals provided that suitable acknowledgement is made and the reproduction is not associated with any form of advertising. Applications for commercial reproduction should be addressed to: NIHR Journals Library, National Institute for Health Research, Evaluation, Trials and Studies Coordinating Centre, Alpha House, University of Southampton Science Park, Southampton SO16 7NS, UK.
Chapter 1 Background and rationale
Impact of health-care-associated infection
This report evaluates the diagnostic potential of a commercial multipathogen real-time polymerase chain reaction (PCR) platform for detection of health-care-associated bloodstream infection in the high-risk setting of critical care. Health-care-associated infection imposes a significant burden on health-care systems worldwide and is a major cause of morbidity and mortality. Figures from the UK suggest that over 300,000 patients are affected by health-care-associated infection annually, with an annual cost to the NHS in excess of £1B. 1,2 Comparative figures from the USA indicate that the costs associated with the five most common health-care-associated infections are approximately US$10B per annum. 3 Nosocomial bloodstream infections are among the most problematic in terms of excess hospital cost, increased length of stay and attributable mortality rates. 4 Improved infection prevention and control measures linked to organisational targets for reducing meticillin-resistant Staphylococcus aureus (MRSA) bacteraemia and Clostridium difficile infection have contributed to a significant reduction in overall health-care-associated infection prevalence. 5 Nevertheless, health-care-associated infection remains a significant burden, particularly in the critically ill, who, owing to the level of invasive interventional procedures required, in association with their compromised health state, are at high risk of developing severe infections and associated unfavourable health-care outcomes such as organ failure and death. 6
Current diagnostic standards in the critically ill
Consensus definitions of infection in a critical care setting require that diagnosis is confirmed by identification of live micro-organisms (pathogens) by microbiological culture of blood or other samples. 7 However, blood culture (BC), although reflecting the current gold standard, is subject to a significant time delay, requiring 24–48 hours before a positive result is available and at least 5 days to determine that a specimen is culture negative. 8 The significant rates of BC contamination by coagulase-negative staphylococci (CoNS) also add to the difficulties of judging the clinical significance of positive cultures. 9
Sepsis is a clinical syndrome resulting from a patient’s systemic inflammatory response to infection and is a major cause of mortality and increased health-care costs globally. 10,11 Sepsis can be difficult to diagnose and to differentiate from other common non-infectious causes of systemic inflammation. 12 Confirmation of sepsis, therefore, relies on objective diagnostic evidence for infection, including attempts to detect and identify live pathogens from blood samples by culture techniques. 7,11 Early confirmation of sepsis and administration of appropriate antimicrobial therapy is therefore critical to patient outcome but remains a difficult diagnostic problem owing to the time required for microbiological identification of pathogens.
Internationally recognised guidelines for the early management of sepsis advocate that antibiotics are administered within 1 hour of the initial clinical suspicion of infection and current evidence indicates that the correct initial choice of antibiotic saves more lives than virtually any other intervention in patients with severe sepsis. 13,14 In this context, BCs are insufficiently time critical and cannot assist in early management decisions. As a result, antimicrobial therapy should be guided by local pathogen surveillance and normally involves the administration of broad-spectrum, high-potency antibiotics as a ‘safety first’ strategy to cover the spectrum of likely pathogens in patients with suspected sepsis. 11 Although this approach is life-saving in patients with severe sepsis, an inevitable consequence of the temporal separation between initial suspicion and microbiological confirmation is a wasteful and potentially dangerous overuse of antimicrobial chemotherapy. This is particularly relevant given that initial suspicion of infection is based largely on the presence of two or more non-specific clinical signs of systemic inflammation [systemic inflammatory response syndrome (SIRS) criteria – detailed in Chapter 3, Methods, Participants]. SIRS can be induced by a variety of non-infectious aetiologies as well as infection including pancreatitis, major surgery and ischaemia–reperfusion after haemorrhagic shock. 15 As a result, the prevalence of SIRS is high in critical-care settings, with a recent study reporting that 93% of intensive care unit (ICU) patients had two or more SIRS criteria at some stage during their stay. 16 Although the presence of SIRS is associated with a higher risk of progression to severe sepsis or septic shock, the prevalence of confirmed infection is much lower. 17 The overuse of antibiotics to compensate for the diagnostic uncertainty, particularly broad-spectrum antimicrobials, creates a selection advantage for surviving organisms that inevitably facilitates the spread of antibiotic-resistant species. 1 In addition, broad-spectrum therapy disrupts the patient’s commensal flora, leaving them open to superinfections such as C. difficile. 18 The increasing threat from antibiotic resistance and the need for improved antibiotic stewardship within the health-care system has recently been the subject of a major report by the Chief Medical Officer. 2 The economic impact of inappropriate antibiotic use in terms of acquisition costs and higher expenditure resulting from avoidable adverse effects is also significant.
An urgent global challenge has emerged, therefore, to develop and translate techniques that could provide accurate diagnostic information within a short time frame of clinical signs appearing and so allow more informed use of antibiotic therapy at an early stage. This challenge has been championed by the World Health Organization,19 endorsed by national governments2 and highlighted within international consensus-derived sepsis guidelines. 11
Molecular approaches to diagnosis of health-care-associated bloodstream infection
There is a consensus that molecular technologies have the potential to provide rapid detection of pathogens in blood and other clinical samples in a much shorter time frame than is possible with conventional culture. 20–22 The majority of current approaches are based on detection of pathogen deoxyribonucleic acid (DNA) in the sample using nucleic acid amplification techniques – primarily real-time PCR – with results potentially available in 4–6 hours. 21 Real-time PCR allows detection of minute amounts of pathogen DNA by selective amplification of specific regions of bacterial or fungal DNA. Pathogen DNA amplified during the real-time PCR reaction is continuously monitored using either fluorescent dyes that bind non-specifically to double-stranded DNA or with fluorescently labelled probes that bind to specific sequences in the amplified pathogen DNA, the latter approach allowing direct identification of the microbial species present. The amplification of the pathogen signal during real-time PCR is crucially important owing to the low numbers of circulating bacteria [10 colony-forming units (CFUs) per ml] or fungi (1–10 CFU/ml) reported in adult sepsis. 23,24 The use of real-time PCR also facilitates detection of fastidious, difficult-to-culture organisms such as fungi. 25
Two basic approaches have been taken in the design of real-time PCR assays for sepsis pathogens, either (i) broad-range detection of bacterial or fungal DNA with universal primers followed by species identification using post-PCR techniques such as DNA sequencing, electrospray ionisation mass-spectrometry or high resolution melting analysis26–28 or (ii) multiplex assays utilising a panel of species-specific hybridisation probes that provide direct confirmation that a particular species is present. 29 Intuitively, the latter approach would appear to have the greatest clinical utility in terms of directing timely and appropriate antibiotic therapy, assuming that a pathogen panel with adequate coverage can be established. In both designs, assays are generally, although not exclusively, directed at conserved DNA sequences in the 16S, 23S or 16S–23S interspacer regions of the ribosomal ribonucleic acid (rRNA) gene of bacteria or the 18S and 28S rRNA gene regions of fungi. 30–33
The ability of real-time PCR to provide early information on likely antibiotic sensitivities of organisms detected is also of crucial importance in view of the developing crisis in antibiotic resistance. 2 Real-time PCR has the potential to detect the presence of several important antibiotic resistance genes including mecA (MRSA) and van subtypes (vancomycin-resistant enterococci). 34,35 However, reliable detection of resistances such as extended-spectrum beta-lactamases are more challenging given the wide range of genotypes involved and currently full profiling of antibiotic sensitivities can be achieved only by culture. 36
Although the laboratory analytical accuracy of these techniques for the detection of pathogen DNA in blood has been evaluated, there is a reported lack of clinical trial data to define the utility of such tests in patients. 21,36 This has been due in part to the lack of standardised technology platforms that meet accepted regulatory standards for clinical diagnosis.
At the time of application for Health Technology Assessment (HTA) funding for the current trial (early 2008), the only real-time PCR platform with regulatory approval [Conformité Européenne (CE) mark] for simultaneous detection of bacterial and fungal pathogens in suspected bloodstream infection was the LightCycler® SeptiFast kit (Roche Diagnostics GmbH, Mannheim, Germany). The system uses a multiplex approach, which allows detection of the most common pathogen species causing bloodstream infection in a single blood sample29 without the need for pre-culture and is described in detail below. To date, a further three PCR-based platforms have gained CE mark approval for this purpose, although data on clinical diagnostic performance remain limited. SepsiTest™ CE IVD (Molzym, Bremen, Germany) is a pan-bacterial, pan-fungal real-time PCR assay able to detect DNA from more than 345 bacterial and fungal species with subsequent species identification by amplicon sequencing. 26,37 VYOO®, introduced by SIRS-Lab, Jena, Germany, gained a CE mark in late 2008 and takes a multiplex approach to detect a panel of sepsis pathogens by electrophoretic separation of species-specific PCR amplicons. 38,39 PLEX-ID™ (Abbott, Wiesbaden, Germany) also uses universal PCR to detect a very broad range of bacterial and fungal pathogens, but utilises electrospray ionisation mass spectrometry for post-PCR species identification. 40
LightCycler SeptiFast real-time polymerase chain reaction
The LightCycler SeptiFast real-time PCR system uses a multiplex approach, allowing detection of a panel of 25 of the most common pathogen species associated with bloodstream infection in a single blood sample. The SeptiFast Master List (SML) panel is shown in Table 1. The assay involves three steps: (i) mechanical lysis and DNA extraction from the blood specimen; (ii) real-time PCR amplification and detection of bacterial/fungal DNA based on the use of species-specific hybridisation probes targeting the internal transcribed region between the 16S and 23S bacterial rRNA gene and between the 18S and 5.8S rRNA region of the fungal genome; and (iii) identification of species based on melting point analysis of real-time PCR products with automated reporting using dedicated software. 29 SeptiFast has been assessed at the laboratory level on clinical isolates and has shown to have good analytical specificity and exclusivity, confirming its analytical validity. 29 When tested against spiked blood samples, SeptiFast showed minimal analytical sensitivity of 3–30 CFU/ml for the pathogen species on the SML and excellent specificity and exclusivity. 29 At the time of submission of the funding application for the current study, information on the clinical diagnostic accuracy of the LightCycler SeptiFast platform for detection of bloodstream infection was limited. An unpublished European Union registration study of 278 critically ill patients with suspected sepsis from Denmark, Germany and Italy was undertaken as part of the CE-marking process.
| Gram-negative bacteria | Gram-positive bacteria | Fungi |
|---|---|---|
| Escherichia coli | Staphylococcus aureus | Candida albicans |
| Klebsiella (pneumoniae/oxytoca) | CoNSa | Candida tropicalis |
| Serratia marcescens | Streptococcus pneumoniae | Candida parapsilosis |
| Enterobacter (cloacae/aerogenes) | Streptococcus speciesb | Candida glabrata |
| Proteus mirabilis | Enterococcus faecium | Candida krusei |
| Acinetobacter baumannii | Enterococcus faecalis | Aspergillus fumigatus |
| Pseudomonas aeruginosa | ||
| Stenotrophomonas maltophilia |
In addition, three full studies had been published, covering a total of 262 patients and 353 samples across a mix of clinical settings, including general medicine wards, intensive care and emergency departments. 41–43 During the course of the current National Institute for Health Research (NIHR) HTA trial, further studies investigating the diagnostic performance of LightCycler SeptiFast technology have been published, predominantly focusing on suspected sepsis. None of studies to date has specifically examined the performance of the test in the context of suspected health-care-associated bloodstream infection, which is the focus of the current study. A systematic review of the published data on the diagnostic accuracy of LightCycler SeptiFast forms the basis of Chapter 2 of this report.
Overview of the study
It is widely acknowledged that PCR-based nucleic acid amplification techniques have the potential to deliver real improvements in sensitivity and speed of diagnosis of life-threatening infection in the setting of critical care. Although a number of commercial platforms are available for clinical use, there has been no systematic adoption of these technologies in health-care systems, including the NHS. This is primarily due to a paucity of information on clinical utility and a lack of formal HTA. The overall aim of this study is to provide independent clinical accuracy data along with a systematic analysis of current published studies to allow decisions to be made on the utility of the LightCycler SeptiFast real-time PCR for early diagnosis of suspected sepsis-related health-care-associated bloodstream infection in critical care.
The three components of the evidence synthesis are:
-
a systematic review of published diagnostic accuracy studies of the SeptiFast platform compared with BC in the setting of suspected sepsis-associated bloodstream infection (see Chapter 2)
-
the results of an independent and systematic clinical accuracy study of SeptiFast in a clearly defined clinical population of critically ill patients suspected of developing health-care-associated bloodstream infection (see Chapter 3)
-
statistical analysis of the potential impact on diagnostic accuracy of error in the BC gold standard and the inclusion of commonly used circulating inflammatory biomarkers as instrumental variables using latent class modelling (see Chapter 4).
The detailed objectives, methods and results of each of these analyses are reported in the ensuing chapters. The final chapter includes a discussion on the implication of our findings for the real-time PCR-based diagnosis of health-care-associated bloodstream infection including a recommendation on whether or not this technology has sufficient clinical diagnostic accuracy to move forward to efficacy testing during the provision of routine critical care. The priorities for future research are also discussed.
Chapter 2 Accuracy of LightCycler SeptiFast real-time polymerase chain reaction for the detection and identification of pathogens in the blood of patients with suspected sepsis: a systematic review and meta-analysis
Aim
Chapter 3 of this report presents the results of a large independent, multicentre Phase III clinical diagnostic accuracy study to assess the SeptiFast multipathogen real-time PCR platform in detection and identification of health-care-associated bloodstream infection funded by the NIHR HTA programme. 44 As part of the background to this independent HTA, we report here a systematic review that was designed to focus on the diagnostic test accuracy of SeptiFast real-time PCR for detection and simultaneous identification of pathogens in the blood of patients with suspected sepsis. This systematic review was piloted and registered with PROSPERO, the International Prospective Register of Systematic Reviews, in 2011 (www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42011001289) and the study protocol was published in 2012. 45
Methods
Inclusion criteria of studies
Participants
Patients suspected of developing sepsis, including adults and children, who required BCs irrespective of where their care was being delivered, and including suspected community- or hospital-acquired infection.
Target conditions
Suspected sepsis, including severe sepsis and septic shock. 46
Index test
LightCycler SeptiFast real-time PCR as the index test on blood specimens for the detection and simultaneous identification of bacterial and fungal pathogens. 29
Comparator test (reference standard)
Blood culture for the detection and identification of bloodstream bacterial and fungal pathogens was used as the reference test. 8 All diagnostic metrics were reported using this reference standard.
Types of studies
We included any clinical diagnostic accuracy study, including case–control studies, that compared the index real-time PCR test with standard culture results performed on a patient’s blood sample during the management of suspected sepsis.
Search methods for identifying studies
Electronic searches
We searched the Cochrane Database of Systematic Reviews, Database of Abstracts of Reviews of Effects, the HTA database, the NHS Economic Evaluation Database, The Cochrane Library, MEDLINE, EMBASE, ISI Web of Science, Bioscience Information Service (BIOSIS) Previews, Medion and the Aggressive Research Intelligence Facility database. The CE mark for the index test was announced in January 2006; therefore, this systematic review considered only publications from this date in humans. There were no language restrictions in the electronic search for studies.
Search terms/search strategy
Specific search strategies (see Appendix 2) were developed for each electronic database, commencing with MEDLINE (published previously;45 see also www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42011001289). The MEDLINE strategy was adapted for each subsequent database. All electronic searches were piloted in October 2011 prior to publication of our protocol and repeated at the end of November 2012.
Other resources
Backward tracking was performed by hand-searching the reference lists of all relevant articles uncovered from the electronic searches and forward tracking using the keyword ‘SeptiFast’ with ISI Citation Indices and Google Scholar (Google Inc., Menlo Park, CA, USA) and with a conference proceedings search using the Web of Science ISI proceedings (from January 2006 to November 2012). We requested reference lists held by the only manufacturer of the index test (Roche Diagnostics) and requested any clinical diagnostic accuracy data collected by Roche Diagnostics to file for the CE mark. In addition, we searched for unpublished studies and ongoing trials involving the SeptiFast platform in the following online registers, www.nlm.nih.gov/hsrproj, www.controlled-trials.com/mrct/, http://public.ukcrn.org.uk/search/Portfolio.aspx and www.who.int/trialsearch, with identified corresponding authors of eligible trials and content experts contacted to identify potentially relevant studies and associated data.
Data collection and analysis
Selection of studies (Salford, UK)
The initial selection of titles and abstracts was conducted by two reviewers (PD and GW) using the inclusion criteria detailed above. The full papers of all abstracts deemed eligible (by any reviewer) were obtained and read to determine their inclusion eligibility in the review. Conference abstracts and journal correspondences were included if they met the inclusion criteria, and the corresponding author was contacted to request any further information about their study or about full publications in preparation. Conference abstracts were not included when reporting duplicate data contained in a subsequent paper. We resolved any disagreement for inclusion with discussion between the reviewers.
Assessment of methodological quality (Belfast and Warwick, UK)
Independent (external) reviewers (DM and GDP) assessed the quality of each selected study using a specific checklist, published previously45 (see Appendix 3), adapted from the quality assessment of diagnostic accuracy studies tool. 47 Each question on the checklist was answered with a yes/no response or noted as unclear if insufficient information was reported to enable a judgement to be made, and the reasons for the judgement made were documented. Published Standard Operating Procedures and interpretation of the reference standard (BC), including definitions of BC contamination, were made available to the independent reviewers for reference. 8 In addition, the 2006 CE-marked index test protocol was made available to each reviewer as provided by Roche Diagnostics to purchasers. Review authors (DFM and GDP) assessed methodological quality independently. Any discrepancies were adjudicated and resolved by a systematic review methods expert (BB) and an infection diagnostic expert (RM).
Data extraction
A standard set of data was searched for and extracted where possible from each study using a tailored data extraction form. This included information regarding the inclusion criteria detailed above, an assessment of the evidence level for diagnostic studies48 and additional information including clinical setting (i.e. community, emergency department, in hospital, critical care and general/specialist); participant demographics; clinical features of the included population (e.g. significant comorbidity); intercurrent treatment (antimicrobial therapy); reference standard methodology; supporting test results (culture of samples other than blood); index test setting (point of care, near patient, clinical or research laboratory, batched or individual analysis); reported index test laboratory failures; missing participant data; 2 × 2 table of results for primary outcome and reported diagnostic accuracy metrics. Review authors (DFM and GDP) extracted data independently. Any discrepancies were adjudicated and resolved by a systematic review methods expert (BB) and an infection diagnostic expert (RM).
Statistical analysis and data synthesis
Statistical analysis and data synthesis were planned and performed independently by an external statistician (SG). Estimates of the combined sensitivity and specificity, with 95% confidence intervals (CIs), were made using Reitsma’s bivariate method. 49 Results were displayed as summary receiver operating characteristic (ROC) plots, with 95% confidence regions and 95% prediction regions defined by Harbord et al. 50 as ‘the region within which, assuming the model is correct, we have 95% confidence that the true sensitivity and specificity of a future study should lie’. An overall summary for all studies with useable data was produced and subgroup analyses were performed separating studies by:
-
type of publication: full papers versus abstracts
-
age of participants: adult versus neonate/child (this analysis omitted studies where the population was mixed or unclear)
-
hospital setting: emergency department versus other hospital setting, ICU versus other hospital setting (this analysis omitted studies where settings were mixed or unclear)
-
comorbidity: if sufficient data were available to allow comparisons
-
commercial sponsorship: stated involvement of Roche Diagnostics versus no statement.
For all subgroup analyses, summary ROC curves were produced with pooled estimates of sensitivity and specificity for each group. Analyses tested whether or not the subgrouping explained a significant amount of additional variation using the difference in –2 log-likelihood statistics between the subgrouped and overall models.
No attempts were made to quantify potential sources of study bias in this systematic review as the available methodologies have not been validated for use in relation to diagnostic test meta-analyses. 51
Analyses were performed using SAS 9.2 (SAS Institute Inc., Cary, NC, USA) and Review Manager (RevMan) Version 5.2 (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark).
Results
Study inclusion
We identified 2129 citations in total, of which 61 were considered potentially suitable (Figure 1). After full-text review, and having contacted corresponding authors for any extra data in the case of conference abstracts and journal correspondences, 23 studies were excluded as it proved impossible to derive a 2 × 2 table to calculate required diagnostic metrics. In addition, one abstract was removed as the study data were coreported in a full paper and another abstract was replaced by a full paper that was sent to us by the authors. In total, 37 studies were included in the final analysis (26 papers, nine conference abstracts and two correspondences – summarised in Table 2).
FIGURE 1.
Flow diagram of study selection.

| First author | Year | Manuscript type | Study country | Sepsis patient setting | Age category | Diagnostic study evidence level | Number of patients recruited with suspected sepsis | Number of paired blood tests/episodes | Bacteraemia prevalence (%) |
|---|---|---|---|---|---|---|---|---|---|
| Raglio52 | 2006 | Abstract | Not stated | Not stated | Not stated | III | 74 | 114 | 15 |
| Klemm53 | 2007 | Abstract | Germany | Intensive/critical care | Not stated | III | 44 | 56 | 37 |
| Bingold54 | 2007 | Abstract | Germany | Intensive/critical care | Not stated | III | 21 | 134 | 15 |
| Vince55 | 2008 | Correspondence | Croatia | In hospital and intensive/critical care | Not stated | III | 36 | 39 | 21 |
| Mancini42 | 2008 | Paper | Italy | In hospital and unclear if intensive/critical carea | Adults | III | 34 | 103 | 20 |
| Louie41 | 2008 | Paper | USA | Emergency department, in hospital and intensive/critical care | Adults | III | 200 | 200 | 21 |
| Lodes56 | 2008 | Abstract | Germany | Intensive/critical care | Not stated | III | 137 | 358 | 13 |
| Westh57 | 2009 | Paper | Germany | Not stated | Not stated | III | 359 | 558 | 13 |
| Varani58 | 2009 | Paper | Italy | In hospital and unclear if intensive/critical carea | Adults and children | III | 100 | 130 | 29 |
| Palomares59 | 2009 | Abstract | Spain | Intensive/critical care | Not stated | III | 73 | 76 | 13 |
| Lodes60 | 2009 | Paper | Germany | Intensive/critical care | Adults | III | 52 | 258 | 12 |
| Dierkes61 | 2009 | Paper | Germany | Intensive/critical care | Adults | III | 77 | 99 | 23 |
| Dark62 | 2009 | Correspondence | UK | Intensive/critical care | Adults | III | 50 | 90 | 12 |
| Yanagihara63 | 2010 | Paper | Japan | In hospital and emergency department | Not stated | III | 212 | 400 | 8 |
| Wallet64 | 2010 | Paper | France | Intensive/critical care | Adults | III | 72 | 102 | 10 |
| Tsalik65 | 2010 | Paper | USA | Emergency department | Adults | III | 306 | 306 | 22 |
| Sóki66 | 2010 | Abstract | Hungary | In hospital and intensive/critical care | Not stated | III | 159 | 162 | 24 |
| Regueiro67 | 2010 | Paper | Spain | In hospital and intensive/critical care | Adults | III | 72 | 106 | 25 |
| Maubon68 | 2010 | Paper | France | In hospital and unclear if intensive/critical carea | Not stated | III | 110 | 110 | 29 |
| Lehmann69 | 2010 | Paper | Germany | Intensive/critical care | Adults | III | 108 | 453 | 13 |
| Bloos70 | 2010 | Paper | Germany, France | Intensive/critical care | Adult | III | 142 | 236 | 17 |
| Berger71 | 2010 | Abstract | Austria | Neonatal unit | Neonates | III | 38 | 38 | 45 |
| Avolio72 | 2010 | Paper | Italy | Emergency department | Adult | III | 144 | 144 | 30 |
| Vrioni73 | 2011 | Abstract | Greece | Not stated | Not stated | III | 33 | 33 | 24 |
| Sitnik74 | 2011 | Abstract | Brazil | Intensive/critical care | Not stated | III | 114 | 114 | 14 |
| Obara75 | 2011 | Paper | Japan | Emergency department, in hospital and intensive/critical care | Adults | III | 54 | 78 | 15 |
| Lucignano76 | 2011 | Paper | Italy | In hospital and intensive/critical care | Neonates and children | III | 811 | 1553 | 10 |
| Josefson77 | 2011 | Paper | Sweden | In hospital | Adults and children | III | 1093 | 1141 | 12 |
| Hettwer78 | 2011 | Paper | Germany | Emergency department | Adults | III | 153 | 113 | 45 |
| Bravo79 | 2011 | Paper | Spain | In hospital and intensive/critical care | Adult | III | 53 | 53 | 47 |
| Tschiedel80 | 2012 | Paper | Germany | In hospital and intensive/critical care | Adults and children | III | 75 | 110 | 17 |
| Rath81 | 2012 | Paper | Germany | Intensive/critical care | Adults | III | 170 | 225 | 36 |
| Pasqualini82 | 2012 | Paper | Italy | In hospital and unclear if intensive/critical care | Not stated | III | 391 | 391 | 15 |
| Mauro83 | 2012 | Paper | Italy | In hospital and unclear if intensive/critical carea | Adult and children | III | 79 | 79 | 41 |
| Lodes84 | 2012 | Paper | Germany | Intensive/critical care | Adults | III | 104 | 148 | 20 |
| Guido85 | 2012 | Paper | Italy | In hospital and unclear if intensive/critical carea | Adults | III | 166 | 166 | 14 |
| Grif86 | 2012 | Paper | Austria | In hospital and intensive/critical care | Not stated | III | 61 | 71 | 7 |
Study quality
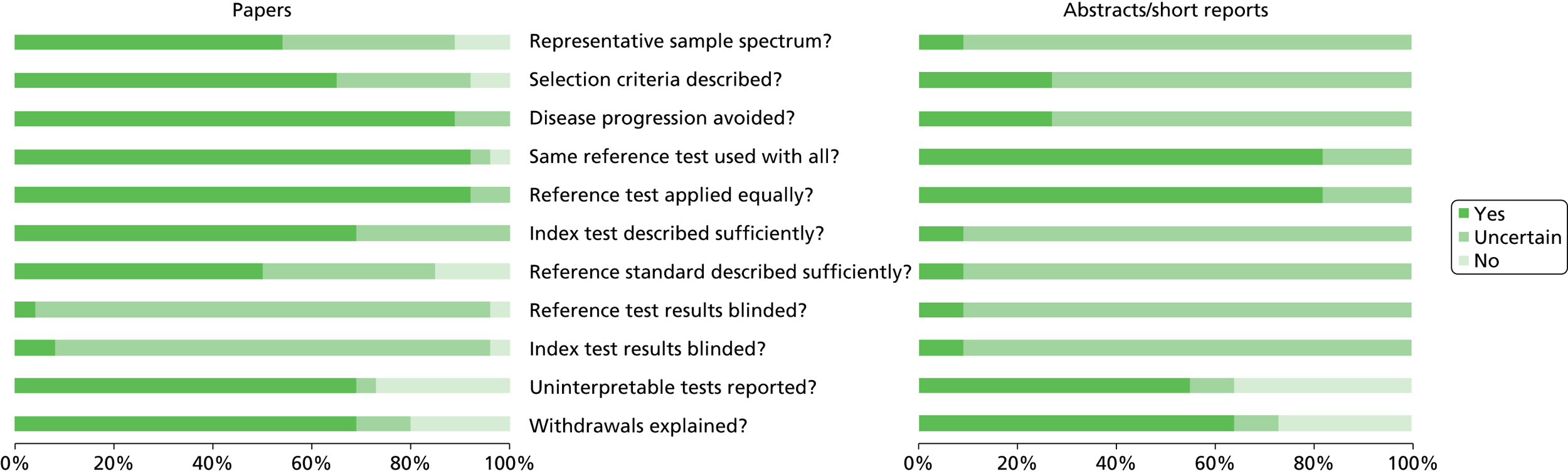
Our independent external reviewers reported variable study quality and, although studies reported as full papers were associated with the best quality measures, there were important deficiencies overall in study design and reporting (Figure 2). Reviewers agreed that all of the studies selected aimed to compare test results from SeptiFast with BC and that the reported blood sampling for these tests was such that disease progression or regression bias would have been avoided. BC, as the reference standard, appeared to have been applied to patients equally in a way that both partial (work-up) bias and verification bias were probably avoided. However, the reference standard was not always adequately described, including blood sampling methods and the prevalence of defined contamination as a potential source of false-positive culture results. BC results were often difficult to adjudicate by reviewers, with no clear standards of reporting followed. The chance of misclassification when comparing the reference and index tests was therefore thought to be likely, impacting on the derived diagnostic accuracy metrics. In some studies, it was not clear how well the CE-marked protocol for SeptiFast real-time PCR had been followed, including how blood samples had been stored/handled prior to assay delivery. Assay failure rates were rarely reported. There was a universal lack of reported blinding of both reference standard and index tests such that reviewers believed that incorporation bias was highly likely. Overall, reviewers agreed that none of the included studies, as reported, met the standards for the reporting of diagnostic accuracy studies (STARD) criteria in full,87 and in some cases there were significant deficiencies (see Figure 2).
FIGURE 2.
Summary of independent review of quality of included studies.
Study characteristics and patient populations
Studies included patient cohorts from a wide range of age and settings (see Table 2) representing a total of 5977 patients contributing 8547 episodes of suspected sepsis. The median prevalence of BC positivity in this group of patients was 17% [interquartile range (IQR) 13–25%]. Lack of uniform reporting made it difficult for reviewers to classify studies, with a variety of care settings, outcomes and alternative clinical reference standards reported alongside the direct comparison of SeptiFast with BC results. However, our external reviewers were able to identify age classes (neonate, child and adult), setting classes (emergency department, hospital setting, and intensive/critical care) and a group of studies that focused on haemato-oncology patients. In addition, studies were assigned a diagnostic evidence level III in each case.
Estimated summary diagnostic accuracy of SeptiFast
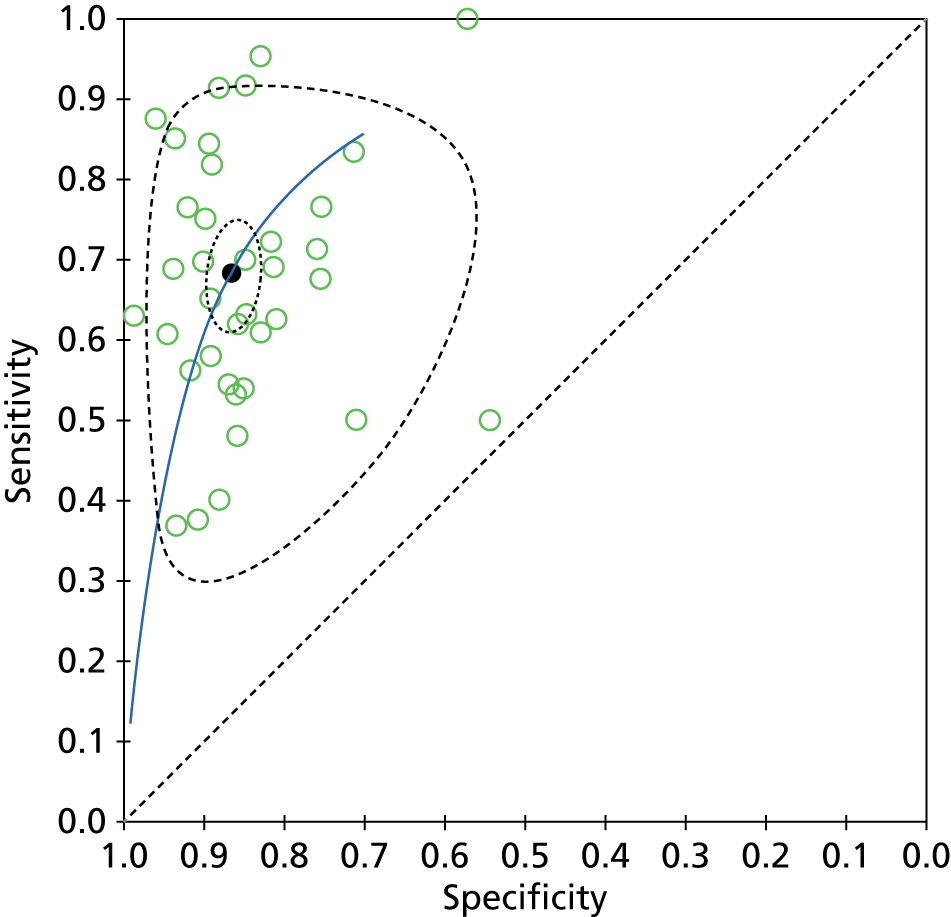
Figure 3 shows the coupled forest plots of sensitivity and specificity for each study and Figure 4 displays the scatterplot in ROC space (plotting sensitivity against 1 – specificity for each study). Summary sensitivity and specificity for SeptiFast compared with BC, estimated using the bivariate model method, were 68% (95% CI 62% to 74%) and 86% (95% CI 84% to 89%), respectively, suggesting that a positive blood test at genus/species level returned by SeptiFast in the setting of a patient with suspected sepsis could have higher diagnostic value (rule in) than a negative test result (rule out) when compared with BC.
FIGURE 3.
Forest plot of included studies. FN, false negative; FP, false positive; TN, true negative; TP, true positive.

FIGURE 4.
Summary ROC with 95% confidence region (dotted) and 95% prediction region (dashed).
Exploration of subgroups
Subgroups were investigated and estimated pooled diagnostic accuracy metrics produced for each group. Table 3 summaries these results and shows that in each case subgrouping did not explain any significant amount of additional variation in sensitivity or specificity when compared with the overall models. There were insufficient studies reporting solely in paediatric populations to allow analysis.
| Subgroup | Sensitivity, % (95% CI) | Specificity, % (95% CI) | Interaction test for subgroup differences | |
|---|---|---|---|---|
| χ2 | p-value | |||
| Analysis 1 | ||||
| Papers | 68 (61 to 74) | 88 (85 to 90) | 4.3 | 0.12 |
| Abstracts | 69 (57 to 79) | 81 (74 to 87) | ||
| Analysis 2 | ||||
| Emergency department | 69 (50 to 84) | 93 (86 to 97) | 3.5 | 0.17 |
| Other hospital setting | 66 (59 to 72) | 86 (83 to 89) | ||
| Analysis 3 | ||||
| ICU | 64 (53 to 74) | 83 (78 to 87) | 5.8 | 0.06 |
| Other hospital setting | 68 (53 to 80) | 89 (84 to 93) | ||
| Analysis 4 | ||||
| Haemato-oncology | 67 (60 to 72) | 86 (83 to 89) | 2.5 | 0.29 |
| All other patient groups | 78 (63 to 88) | 88 (79 to 93) | ||
| Analysis 5 | ||||
| Stated Roche involvement | 69 (60 to 76) | 88 (85 to 91) | 2.3 | 0.32 |
| Not stated | 68 (60 to 75) | 85 (80 to 88) | ||
Discussion
Our comprehensive systematic review was designed to help understand the estimated combined diagnostic accuracy of SeptiFast real-time PCR in detecting and identifying bacterial and fungal pathogens in the blood of patients with suspected sepsis. We included 37 studies reporting on a total of 8437 SeptiFast tests when compared with BC.
Estimated combined results for sensitivity and specificity suggested that SeptiFast has a higher specificity than sensitivity. For the health-care team, this implies that positive blood tests returned by SeptiFast at pathogen genus/species level could have higher diagnostic utility (as a rapid rule-in test) than negative results (as a rapid rule-out test), at least when compared with BC. The apparent confidence in this statement is greater for specificity than sensitivity (see Figures 3 and 4) because the median event rate of 17% BC positivity for the studies means that the majority of reference tests performed were negative.
The interpretation of these combined diagnostic accuracy results is that negative SeptiFast tests could reasonably be false negatives, explained in part by pathogens detected in BC that were not on the PCR test panel. In addition, despite a higher estimated combined specificity, when compared with sensitivity, the upper CI did not reach 90%. Specificity of SeptiFast real-time PCR, when compared with culture, will be limited by the presence of false-positive results – a positive PCR in the setting of a negative BC. In some studies, a proportion of these false-positive results were reported to be concordant with culture positivity from samples other than blood, suggesting that in some cases of suspected sepsis a positive SeptiFast result may reflect infection not detectable by BC. However, there are no clear explanations for these false-positive and false-negative SeptiFast results because no systematic investigation has been undertaken linking laboratory performance with clinical diagnostic accuracy. In addition, it remains extremely difficult to speculate what implications these diagnostic accuracy results may have for direct clinical care because SeptiFast does not report antibiotic sensitivity data (other than identifying the mecA gene, confirming meticillin resistance following detection of Staphylococcus aureus) and there have been no systematic interventional clinical trials reported to date on the efficacy and effectiveness of supplementing or replacing BC with SeptiFast real-time PCR.
All diagnostic metrics were reported using BC as the reference standard. We accept that there could be limitations to this standard, particularly in the setting of intercurrent antimicrobial therapy. However, we do not know the full extent of this problem, or indeed whether studies have deliberately included or excluded such patients. In addition, SeptiFast real-time PCR was developed to simultaneously detect and identify a panel of the most common pathogens based on reported international BC surveillance data. 29 Therefore, in the absence of an internationally agreed approach to an alternative reference standard for pathogen detection from blood samples at present, we believe that a culture-based reference standard provided the most robust approach for this review and is consistent with methods used in our independent clinical diagnostic accuracy study described in Chapter 3.
Our review identified diagnostic accuracy studies performed within routine clinical care. Clinical diagnostic accuracy studies, in general, are challenging to perform well and often fall short in terms of study quality. Our independent reviewers found a similar trend for these Phase III clinical diagnostic accuracy studies reporting on SeptiFast real-time PCR compared with BC. When assessing the quality of study design and reporting, significant deficiencies were discovered. For both papers and abstracts, the application of the reference and index tests were the only elements that were reported consistently, raising significant concern about the possible effects of numerous sources of inherent study bias on the derived summary estimates of SeptiFast test performance. Indeed, the 95% prediction region in ROC space in Figure 4 shows considerable uncertainty about the likely true sensitivity and specificity of a future study.
During the preparation of this review, another systematic review has reported on the diagnostic accuracy performance of SeptiFast real-time PCR. Chang and colleagues used a basic keyword search strategy for journal papers only, risking publication bias, and they included a number of studies that were judged not to warrant inclusion in our own review on the basis of our inclusion criterion of ‘suspected sepsis’. 88 It appears that Chang reported pooled estimates of sensitivity and specificity when comparing SeptiFast real-time PCR with BC results as composite events, not at a genus/species level – a key feature of our own review – which may have contributed to the improved diagnostic accuracy metrics reported in Chang’s review. 88 However, we would emphasise, based on our comprehensive systematic review reported here, that clinical diagnostic studies in this field vary in quality with the real risk of biases that could impact seriously on the reported estimates of summary diagnostic metrics. Despite the considerable international effort in determining the likely diagnostic accuracy of SeptiFast real-time PCR in the setting of suspected sepsis, we are not confident in the current body of evidence because of the weaknesses in study design and reporting outlined in our systematic review. In particular, we do not agree with Chang and colleagues, who state that:
in the presence of a positive SeptiFast result in a patient with suspected bacterial or fungal sepsis, a clinician can confidently diagnose bacteremia or fungemia and begin appropriate antimicrobial therapy, while forgoing unnecessary additional diagnostic testing. 88
Furthermore, we do not agree that returning a negative SeptiFast result, even within a low-prevalence population, ‘may justify withholding antibiotics’. 88 Our views, evidenced by our systematic review presented here, supports current international guidelines on diagnosis and treatment of sepsis which state that there is limited clinical experience with non-culture-based diagnostic methods, such as real-time PCR, and that high-quality clinical studies are needed before any firm recommendations can be made about their potential utility. 11
We recommend that future clinical studies incorporating SeptiFast should include well-designed and -reported clinical diagnostic accuracy elements measured against all of the features of the STARD criteria. 87 Based on the evidence reviewed here, we are concerned that clinical decisions about treatment interventions/adjustments (notably antimicrobial chemotherapy) based on SeptiFast real-time PCR results, potentially delivered within hours of the suspicion of sepsis, could expose patients to risk because sepsis is associated with high mortality and rapid appropriate antimicrobial choices are crucial for survival. 11
Chapter 3 Clinical diagnostic accuracy study of rapid detection of sepsis-related health-care-associated bloodstream infection in intensive care using SeptiFast multipathogen real-time polymerase chain reaction technology
Aim
The primary aims of this Phase III clinical diagnostic accuracy study, designed to meet all the features of the STARD criteria,87 were to (i) determine the accuracy of a multipathogen real-time PCR technology (SeptiFast) for detection and identification of sepsis-related health-care-associated bloodstream infection in adult critical-care patients against the current service standard of microbiological BC and (ii) assess further the preliminary evidence that detection of pathogen DNA in the bloodstream using SeptiFast real-time PCR may have value in detecting the presence of culture-proven infection elsewhere in the body.
Methods
The detailed protocol for this study was approved by the Trial Steering Committee and published in advance by NIHR HTA (www.nets.nihr.ac.uk/projects/hta/081316 and in open access). 44
Study design and sample size
This Phase III, prospective clinical diagnostic accuracy study was originally designed to recruit from two tertiary referral centres within the Greater Manchester Critical Care Network. This was predicated on a sample size estimation89 based on laboratory records showing that an average of approximately 1200 requests for BC were made in total from the critical care units of these two centres, and the results of a pilot study indicating an event rate of culture-confirmed bloodstream infection of approximately 12% (95% CI 6% to 16%). 62 Using these data, it was estimated that a minimum sample size of 600 patients would be required to be 95% sure that the molecular test had at least a 95% specificity and sensitivity when compared with a culture-proven diagnosis. It was anticipated that the study, which commenced recruiting in August 2010, could be delivered within a 2-year time frame.
Following a review of trial progress by the Trial Steering Committee in May 2011, two factors were identified that necessitated modification of the original trial design. First, the observed event rate (culture-confirmed bloodstream infection) appeared significantly lower (7.5%) than the mean value given by the original pilot study (12%). It is speculated that this reduction was in part due to the adoption of more effective infection control measures within trial sites since the time of trial inception, although the observed rate does lie within the CI of the original pilot study. Based on the actual event rate of 7.5%, a revised sample size estimation was performed, which indicated that a minimum of 972 patients would be required to achieve the required precision. Considering the higher recruitment target and the need to ensure timely delivery of the study, the Trial Steering Committee agreed a number of modifications to the original trial protocol, including increasing the number of recruitment sites and repeat sampling of recruited patients who develop a subsequent, new episode of suspected health-care-associated bloodstream infection during their stay in the critical-care service. Details of all changes to the original trial protocol are provided in the section Changes to study protocol.
Given the increased sample size, and to ensure timely delivery of the study, two additional large NHS teaching hospitals in the north-west of England were added, one in May 2011 and one in January 2012.
Participants
Inclusion criteria
Patients, aged 16 years or older, being managed in an intensive-care setting, and in whom there is a clinical suspicion of bloodstream infection at least 48 hours after hospital admission or recent exposure to hospital care. Clinical suspicion of bloodstream infection was based a priori on the presence of two or more of the following SIRS criteria:11
-
body temperature > 38 °C or < 36 °C
-
heart rate above 90 beats per minute
-
high respiratory rate (> 20 breaths per minute) or partial pressure of arterial blood carbon dioxide of < 32 mmHg for spontaneously breathing patients or requirement for mechanical ventilation
-
white blood cell count ≤ 4000 cells/mm3 or ≥ 12,000 cells/mm3 or the presence of > 10% immature neutrophils.
Exclusion criteria
Patients were excluded from the study if:
-
they had already been recruited into the study unless they had developed a subsequent, new episode of suspected health-care-associated bloodstream infection (see Changes to study protocol)
-
they had been placed on an end-of-life care pathway
-
they did not consent or, alternatively, assent from their next of kin or human capacity advocate was not obtained.
All patient exclusions based on these criteria were recorded in a screening log held at each recruitment site.
Identification and recruitment of participants
Potential participants were identified to the study research nurse by the critical care team as part of normal clinical surveillance and assessed for inclusion in the trial on the basis of the criteria described in Participants. Patient inclusion in the trial was based entirely on the clinical decision to perform a BC because of suspected sepsis-associated bloodstream infection.
International guidelines for blood sampling for infection diagnosis in emergency care state that the sample has to be taken within 1 hour of suspecting infection. Under these circumstances, only a minority of patients had the capacity to provide consent at study commencement. The majority of potential participants lacked capacity owing to a combination of overwhelming illness and therapeutic interventions such as sedation. In these patients, a process of deferred assent was adopted, in which permission for inclusion in the trial was sought from family members/friends (consultees). The formal assent process included a consultee information sheet, a formal interview by the clinical research team and signed assent. Patients were not recruited where the designated consultee indicated his or her belief that it was likely to be against the wishes of the patient. Under such circumstances, and where a research blood sample had already been taken, the sample was removed from the study and destroyed. In the unlikely event that consultees could not be located within 72 hours, the option to approach an appropriate designated independent human capacity advocate was exercised or the patient was removed from the study and any samples were destroyed. In cases where a participant regained capacity during their acute care hospitalisation, informed consent for inclusion in the study was sought. The patient/consultee information sheets and consent/assent forms used in the study are reproduced in Appendices 4–9.
Laboratory-confirmed diagnosis of bloodstream infection (reference standard)
A consensus definition of bloodstream infection was utilised,90 specifically a diagnosis of laboratory culture-confirmed bloodstream infection required at least one of the following criteria to be met:
-
Criterion 1 Patient has a recognised pathogen cultured from one or more BCs.
-
Criterion 2 Patient has systemic signs of infection (defined as meeting the SIRS criteria in the present study – see Participants, Inclusion criteria) and a common skin contaminant (diphtheroids, Bacillus species, Propionibacterium species, CoNS or micrococci). Contamination of the BC was defined with these common skin species outwith criterion 2.
Having identified a patient as eligible to take part in the study, two blood samples of approximately 20 ml each were taken sequentially from two separate sites, using an NHS-approved aseptic non-touch technique (www.antt.org.uk), by suitably trained clinical service staff. This blood was inoculated into two paired culture bottles, labelled and processed in line with standard clinical practice at the participating hospitals. Blood drawn from in-dwelling catheters was avoided for the purposes of BC and associated SeptiFast real-time PCR analysis. BCs entered the standard clinical pathway at the recruitment centres, with results returned direct to the clinical service in each case.
Microbiology laboratory procedures including blood collection and processing, automated culture using the BacT/ALERT® microbial detection system (bioMérieux UK Ltd, Basingstoke, Hampshire, UK), and identification and sensitivity testing of isolates were undertaken by qualified microbiology service staff subject to quality assurance through the Clinical Pathology Accreditation scheme at each recruitment site.
Research blood samples
A research sample of blood (≤ 30 ml) over and above that required for routine clinical investigation was taken at the same time. The same procedure as sampling for microbiological culture analysis [see Laboratory-confirmed diagnosis of bloodstream infection (reference standard)] was used. The blood sample was divided into sterile Monovette® tubes (Sarstedt Ltd, Beaumont Leys, Leicester, UK) containing ethylenediaminetetraacetic acid (EDTA) (≤ 20 ml total) for SeptiFast real-time PCR analysis of pathogen DNA (the index test) and other assays requiring whole blood, or lithium–heparin (≤ 10 ml) for subsequent plasma isolation and analysis of circulating protein biomarkers. Blood samples were stored for up to 72 hours in a locked fridge within the critical care unit, prior to transport to the Salford Royal Biomedical Facility research laboratories for further processing and analysis. Collection and transportation of samples was co-ordinated by the research nurse at each recruitment site such that a maximum storage time of 72 hours at 4 °C prior to analysis and/or freezing was not exceeded. Details of the timing of sample collection, length of storage prior to transportation, plasma isolation and further processing in the research laboratory were recorded for all samples to facilitate quality-assurance measures for analysis.
Recording of clinical information
All potential participants who met the study inclusion criteria were identified in a screening log at each study centre. Each patient included in the study was given a unique study identification number (ID). Any immediately identifiable patient details were recorded once on the patient’s identification form. Following assignment of the study ID, a case record form (CRF) was opened and the ID number copied to all pages in the CRF (see Appendix 10). The CRF containing the ID number was then treated as a confidential document subject to the data management procedures of the study sponsor [Salford Royal NHS Foundation Trust (SRFT)]. The CRF contained seven sections which recorded all the clinical information required for completion of the study, and acted as points of reference for the research nurse to ensure all clinical study stages were complete. These were:
-
Recruitment: required confirmation that the study participant met the inclusion criteria and summarised the patient’s admission details including the last 7 days.
-
Trial sample data: summarised the sample data allowing tracking of the sample from the site from which it was taken to transportation to the research laboratory.
-
Assent/consent: acted as a checklist and point of reference for the acquisition of consent or assent.
-
Patient observations:
-
Day 0 (sample day) – recorded a snapshot of the patient’s clinical condition and treatment at the time the sample was taken. This included general and specific infection-based clinical observations, calculation of the critical-care minimum data set and a focused overview of the previous 7 days.
-
Antibiotics – record of any antibiotics given from 7 days prior to the sample being taken through to study day 6.
-
Microbiology results – a record of any microbiology results from the sample day and from the previous 7 days.
-
-
Patient surveillance: provided a continuous record of relevant patient care up to and including study day 6. This included any significant clinical events, general observations and calculation of critical-care minimum data set.
-
Patient surveillance summary: detailed the patient’s outcome and any study-related adverse incidents. This section is also concluded with a signature of completion by the research nurse.
-
Clinical adjudication: utilised the criteria set out in the prevalence survey of health-care-associated infections90 to conclude and summarise the clinical opinion regarding each suspected infection episode. As agreed with the Trial Steering Committee, this adjudication was performed in each participating centre by two senior clinical practitioners, at least one of whom had governance responsibility for observing and reporting health-care-associated infection within their clinical service. No between-centre adjudication was performed as this pragmatic clinical accuracy study was designed to observe routine clinical practice (Phase III diagnostic study).
LightCycler SeptiFast (index test)
The procedures for pathogen DNA extraction, multipathogen PCR, and subsequent data analysis and pathogen identification set out in the SeptiFast CE-marked kit were followed precisely using standard operating procedures authorised by the Trial Steering Committee (see Appendices 12–14).
The SeptiFast real-time PCR test was undertaken primarily by one laboratory scientist with a second providing back-up; both were required to complete a formal training programme provided by the manufacturer prior to the start of the trial.
To ensure the validity of the data, the SeptiFast system contains several built-in quality assurance processes including an internal control (IC) consisting of synthetic double-stranded DNA with primer binding sites that are identical to those of the target sequences but which bind to different sites on the probe molecule, allowing differentiation of IC amplicons from target-specific amplicons. The IC controls for the efficiency of pathogen DNA extraction and/or the efficiency of the PCR reaction. To minimise contamination, procedures for pathogen DNA extraction and PCR assay set-up were undertaken in separate sterile cabinets within a dedicated laboratory. Similarly, the LightCycler 2.0 instrument used to run the SeptiFast real-time PCR reactions was housed in a different laboratory. Microorganisms on the SML (see Table 1) were identified from characteristic peaks produced by the SeptiFast Identification Software and manual analysis of melting temperature (Tm) values. To minimise operator error at the analysis stage, data from SeptiFast real-time PCR runs were analysed by two independent operators.
SeptiFast real-time PCR results were not returned to clinical service and were not associated with any clinical or culture information until completion of the data collection and completion of the clinical adjudication for infection. The Salford Royal Biomedical Facility research laboratories are physically and operationally separate from routine clinical service, allowing data and/or information originating from research and clinical service to be double blinded during the study.
Blood collected in lithium-heparin tubes
A 10-ml plasma sample was stored at –80 °C and used for subsequent analyses of circulating immune-inflammatory biomarkers.
C-reactive protein
In the majority of recruited cases, C-reactive protein (CRP) was measured in serum by the pathology service as part of clinical diagnostic evaluation of patients with suspected bloodstream infection. In cases where these analyses had not been performed, plasma CRP was measured after suitable dilution using a commercial sandwich enzyme immunoassay (Human CRP Quantikine ELISA, R&D Systems, Abingdon, UK). The measurement range of the assay is 0.78–50 ng/ml. Comparison of serum and plasma CRP values showed a similar distribution across the study cohort suggesting that both measures were equally valid.
Procalcitonin
Plasma procalcitonin (PCT) levels were measured by the research team using a commercially available enzyme-linked fluorescent assay (VIDAS® B.R.A.H.M.S. PCT™, bioMérieux, Marcy l’Etoile, France). The assay is a one-step sandwich immunoassay using anti-human PCT antibodies conjugated with alkaline phosphatase and combined with a fluorescence detection system. The assay has a measurement range of 0.05–200 ng/ml, with a functional detection limit of 0.09 ng/ml.
Research staff undertaking these analyses followed standard operating procedures for the receipt, processing and storage of blood samples, analytical testing, and the reporting and security of data. All analytical tests used in this study were performed by experienced laboratory staff following the detailed instructions provided by the manufacturers.
Data analysis plan
Summary diagnostic accuracy measures were derived using StatsDirect statistical software version 2.7.8 (StatsDirect Ltd, Altrincham, UK) and reported for the SeptiFast real-time PCR test compared with laboratory culture-proven bloodstream infection, including sensitivity, specificity, predictive values and likelihood ratios, including 95% CIs. A similar analysis was also planned a priori using an ‘enhanced reference standard’, which was defined as any positive culture in blood and/or cultures from other specimens occurring 48 hours either side of the primary research blood samples which contributed to any independent, adjudicated infection episode identified 48 hours either side of the primary blood sample. In this enhanced definition we also included laboratory culture-proven central venous or arterial line colonisation based on semiquantitative cultures. Analyses of diagnostic accuracy measures were performed for each participating hospital separately – except hospital 4 because of the small numbers involved – and for all participating hospitals combined. It was assumed, based on the blinded clinical adjudication at each centre, that the repeated episodes, if present, represented independent events and could be treated as if they had been obtained from separate participants. In addition, Chapter 4 reports the results of sensitivity analyses performed to investigate the possibility that BC may not be an adequate reference standard for infection diagnosis in these critically ill patients including analyses using instrumental variables91 such as clinically relevant cultures from other body sites, circulating immune-inflammatory biomarkers (e.g. CRP and PCT) and patient treatment/response factors including antimicrobial therapy.
Sample and data security
Clinical samples
Detailed procedures were in place for the recording, labelling and tracking of clinical samples in the clinical and laboratory environments. Each sample was anonymised by labelling with a unique identifier number that remained with the sample throughout the subsequent laboratory analysis and storage procedures. The laboratory research team conducting the PCR and other assays were blinded from all microbiological culture data and clinical practice. Standard procedures were put in place for transporting samples from the clinic to the laboratory. On receipt, a laboratory case record form (LCRF) was opened for each patient sample. The LCRF contained the unique study ID number recorded in the clinical CRF to allow cross referencing of clinical and laboratory data on completion of study recruitment. Information including sample volumes, processing times for plasma isolation, sample storage location and results of laboratory analyses was recorded in the LCRF.
Laboratory processing and storage of research samples was undertaken in the Salford Royal Biomedical Facility research laboratories at SRFT, which is a secure facility with restricted access that meets regulatory requirements under the Human Tissue Act. 92 Sample storage (where required) was in dedicated –80 °C freezers, which were fully alarmed with manual and automatic monitoring systems in place and fitted with carbon dioxide back-up in the event of mechanical failure. All stored whole blood and plasma samples will be destroyed at the end of a 5-year period as agreed as part of ethics review.
Data
Anonymised clinical and laboratory data for each patient were recorded in the CRF and LCRF forms respectively. Completed forms were photocopied once and stored in a locked filing cabinet in a secure location at each recruitment site. Electronic data (e.g. SeptiFast real-time PCR data) were stored in a password-protected folder with electronic back-up to an encoded portable hard drive on the last working day of the month. Paper copies of key electronic data were also stored as above.
Serious adverse events
The procedure of collecting an additional blood sample during routine sampling for BC analysis is not usually associated with any significant increased risk to patients or their carers. Standard operating procedures were in place to cover all aspects of blood sampling, storage, transportation and laboratory analysis to ensure no significant risk to any of the research staff associated with the study. During the period of clinical data monitoring (7 days), any serious adverse event reported within each recruitment centre’s statutory reporting system was reported to the trial sponsor by the principal investigator at each site in association with the chief investigator.
Regulatory/ethical approval
The opinion of the Medicines and Healthcare products Regulatory Agency was that this study did not require its formal approval on the basis that it involved observation of the performance of a CE-marked assay during routine clinical care and that the results of the test were not used to influence clinical care. The study received favourable ethical opinion from the North West 6 Research Ethics Committee (reference number 09/H1003/109). Research and development (R&D) approval was granted by the study sponsor [SRFT; study reference 2009/215ETt (25733/GM)] and conduct of the study was monitored regularly within their research governance framework. During the course of the study, several substantial amendments to the trial protocol were put forward for ethics approval; these are detailed in the following section, Changes to study protocol.
Changes to study protocol
Four amendments to the original trial protocol approved by the North West 6 Research Ethics Committee on 14 December 2009 were made. These were agreed by the Trial Steering Committee prior to seeking ethical approval.
The first amendment, on 22 July 2010, dealt with the issue of consenting with potential participants who had capacity at study commencement and with participants who regained capacity during their acute care hospitalisation and were able to provide retrospective consent. The appropriate participant information sheets and consent forms approved for these situations are provided in Appendices 6–9.
The second amendment, on 15 November 2010, changed the wording in the study documentation to widen the scope of recruitment of patients with suspected health-care-associated bloodstream infection from ‘intensive-care’ to ‘critical care’ areas within the participating hospitals. This was to take account of local differences in definitions of intensive care and critical care areas where critically ill patients were normally cared for in the participating hospitals and did not change the principal inclusion criteria set out in the original study protocol.
The third amendment, on 11 April 2011, concerned the resampling of patients already recruited into the study who subsequently went on to develop a suspected new, separate episode of health-care-associated bloodstream infection. Based on data from local clinical audit, approximately 25% of patients recruited to the trial were likely to develop further episodes of suspected infection occurring some days later during their stay in critical care. Permission was requested to include these patients in the study as a new case of suspected infection requiring a further 25–30 ml research blood sample to be taken alongside the routine BC. Following approval of the amendment, the possibility of resampling was raised with the consultee/potential participant at the time of recruitment and included in the written information given.
The fourth amendment, on 27 February 2012, related to the change in total recruitment from 600 to 972 participants based on the revised sample size calculation described in Study design and sample size.
Trial Steering Committee
A Trial Steering Committee was appointed to provide oversight to the study by the NIHR HTA programme. The committee included an independent chairperson, an independent member with expertise in molecular diagnostics of infection and representation from patients at risk of bloodstream infection and recovering from critical illness, the investigators and the trial sponsor. Meetings were held throughout the study to oversee progress and adherence to regulatory and governance research frameworks within the NHS including authorisation of amendments to the trial protocol.
Peer review, funding and study monitoring
This study was extensively peer reviewed as part of the funding process by the NIHR HTA programme (grant number 08/13/16). An independent Trial Steering Committee approved the study protocol, monitored its progress and reviewed and approved any study amendments. SRFT R&D service were study sponsors.
Results
Recruitment
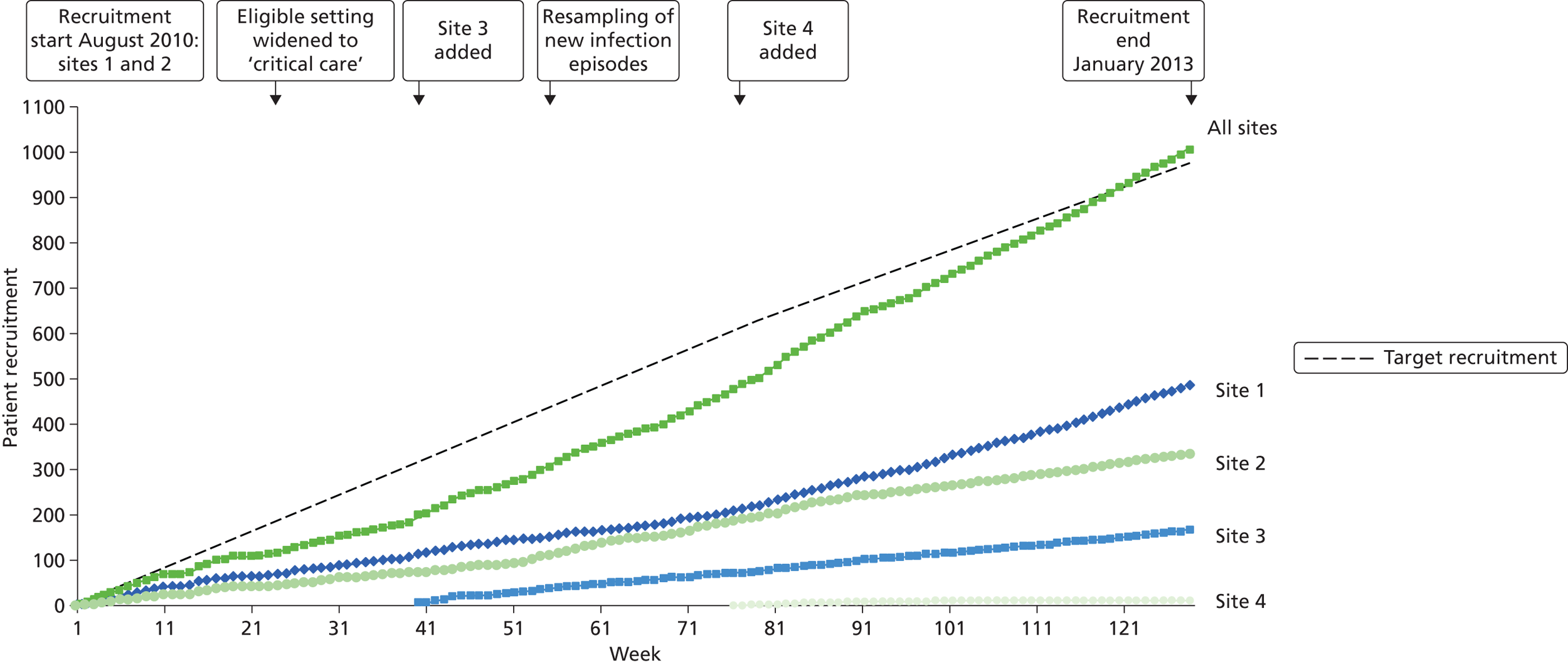
A total of 1006 new consecutive episodes of suspected health-care-associated bloodstream infection, where simultaneous blood samples were taken for analysis by the reference and index tests, from 853 patients were recruited into the study based on confirmed consent/assent from the four recruitment centres (Figure 5). Recruitment commenced in August 2010 in two centres; the third and fourth centres commenced recruitment in May 2011 and January 2012 respectively. The end date for recruitment from all centres was 31 January 2013. Analyses were performed on 922 episodes (91.7%) from 795 patients; the distribution of analysed episodes across the four centres is shown in Table 4.
FIGURE 5.
Trial accrual: cumulative recruitment for the trial as a whole and for each centre individually. Also shown is the timing of the key amendments to the trial protocol around the eligible clinical setting and resampling of patients who developed new episodes of infection.
| Hospital site | Overall | ||||
|---|---|---|---|---|---|
| Site 1 | Site 2 | Site 3 | Site 4 | ||
| Total patient recruitment | 398 | 301 | 142 | 12 | 853 |
| Total episode recruitment | 481 | 343 | 170 | 12 | 1006 |
| Episodes withdrawn from analysis | |||||
| Clinical issues | |||||
| Inclusion criteria not met | 1 | 5 | 2 | 0 | 8 |
| BCs lost | 0 | 3 | 0 | 0 | 3 |
| Lack of clinical information | 4 | 0 | 0 | 0 | 4 |
| Assay failures | |||||
| Reagent control failure | 2 | 4 | 0 | 0 | 6 |
| IC failure | 26 | 19 | 11 | 0 | 56 |
| Other reasons | 2 | 3 | 2 | 0 | 7 |
| Analysed episodes | 446 | 309 | 155 | 12 | 922 |
Exclusions from the analyses
Eighty-four episodes (8.3%) were excluded from the analysis for the reasons detailed below. The site distribution of excluded samples is shown in Table 4.
Clinical factors
Fifteen episodes (1.5%) were excluded on the basis of factors identified at sample adjudication. Eight episodes were withdrawn because the patient did not meet the stated inclusion criteria, three were withdrawn because no BC sample was received by the microbiology laboratory and four were withdrawn owing to incomplete recording of clinical information in the CRF.
Research laboratory factors
Sixty-nine episodes (6.9%) were excluded owing to failure of the SeptiFast real-time PCR assay. The majority of these (56 episodes; 5.5%) resulted from failures in the assay IC which persisted when the failed sample was re-analysed as advised in the CE-marked assay instructions. A further 14 samples that recorded IC failure on first analysis produced a valid PCR result on reanalysis. In each case samples returned a valid negative result for SeptiFast real-time PCR; these samples were included in the final data analysis. The remaining 13 episodes were excluded as a result of failure in the PCR assay reagent control (six episodes; 0.6%), or other laboratory issues (seven episodes; 0.7%) including the presence of contamination in the PCR-negative control, or loss of the pathogen DNA extract during sample processing. In these cases, it was not possible to reanalyse owing to expiry of the 72-hour time limit for sample processing (as mandated in the SeptiFast real-time PCR CE-marked instructions). PCR assay failure was not related to laboratory-confirmed diagnosis of bloodstream infection and missing SeptiFast results were assumed to be missing completely at random and therefore not a source of bias in the reported analyses. This is supported by comparison of the patient demographics for samples excluded due to laboratory factors with those of the analysed episodes (see Appendix 15).
Patient cohort
Of the 922 new episodes of suspected sepsis-related health-care-associated bloodstream infection, 60% occurred in male patients, which is typical of current national critical care in the UK. 93 The median age of patients was 58 years (IQR 45–68 years) compared with 70–74 years from national statistics during this period. 93 The gender (Figure 6) and age (Figure 7) distributions relating to patient episodes were similar across the recruitment sites. Patients were exposed to a median of 8 days (IQR 4–16 days) of hospital care prior to each of the suspected sepsis episodes (Figure 8), which is longer than the median stay in critical care during this period nationally. 93 The range and number of patients recorded by primary specialty are shown in Table 5, suggesting an inclusive, generalisable study cohort. The number of organ support activities was captured at the time of blood sampling using the Critical Care Minimum Data Set (CCMDS) national reporting system, the ranges of which are shown for the whole episode cohort and for each centre in Figure 9. 94 These data show that our patient cohort was receiving far more organ support interventions than was recorded nationally during this period, where nationally two-thirds of patients receive two or fewer organ support interventions and 11% have no documented organ support. 93 Survival to 28 days was 87% (95% CI 84% to 89%) and to hospital discharge was 79% (95% CI 77% to 83%) (Figure 10). These outcomes compare favourably with national audit figures for sepsis outcomes and for critical care more generally. 95 Of note, the vast majority of patients (86%) were receiving antimicrobial and/or, on occasion, antiviral pharmaceutical therapies, within the 48 hours prior to the suspected new sepsis episode (Figures 11–13). Antimicrobial drugs were often delivered in combinations and included the most potent, broad-spectrum antibiotics available, usually reserved for hard-to-treat infections.
FIGURE 6.
Gender distribution for the analysed episodes (n = 922).
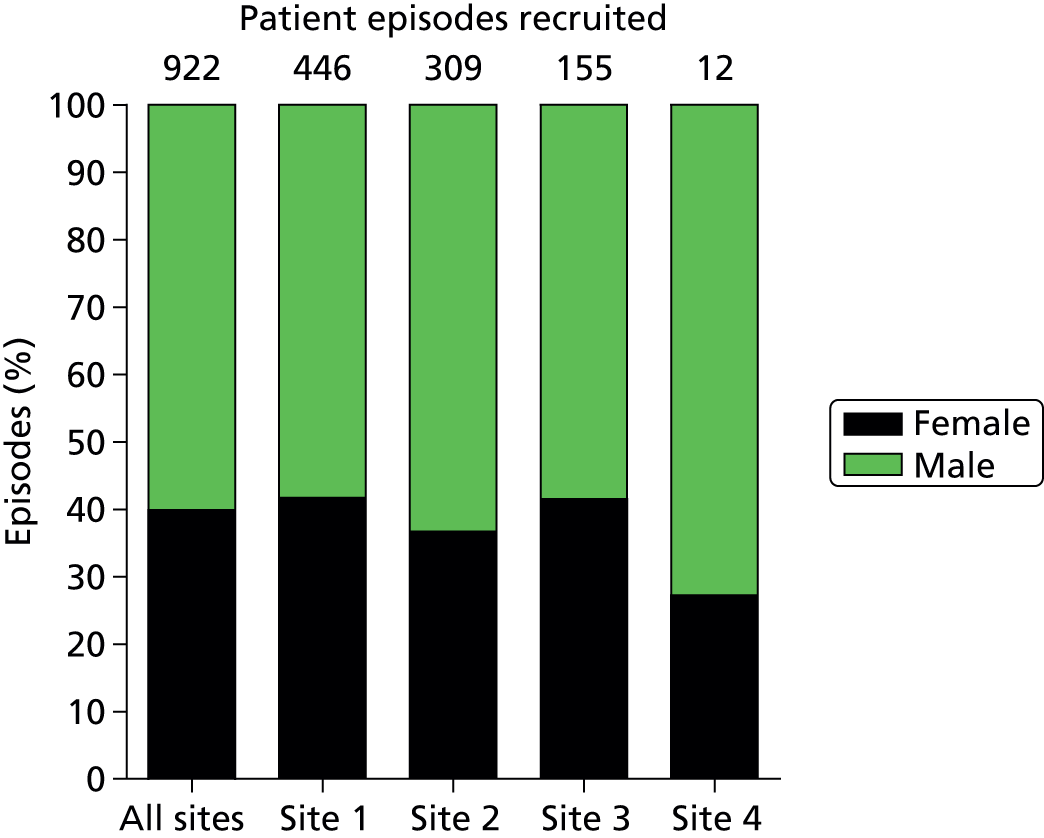
FIGURE 7.
Age distribution for the analysed episodes. (a) All sites combined; (b) site 1; (c) site 2; and (d) site 3. Site 4 data are not shown separately due to the small number of episodes (n = 12) contributed but are included in the all-sites graph.


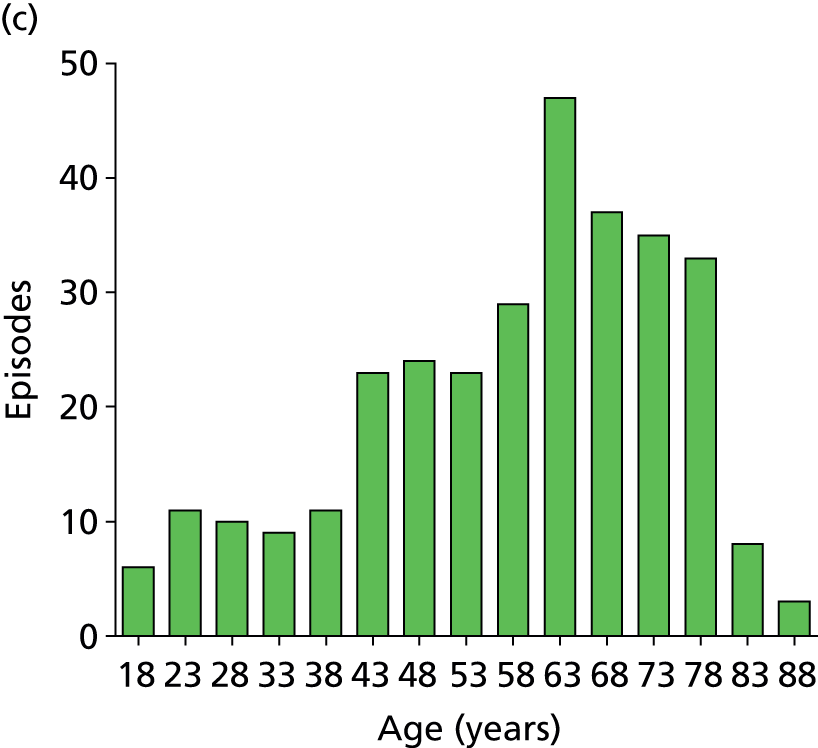
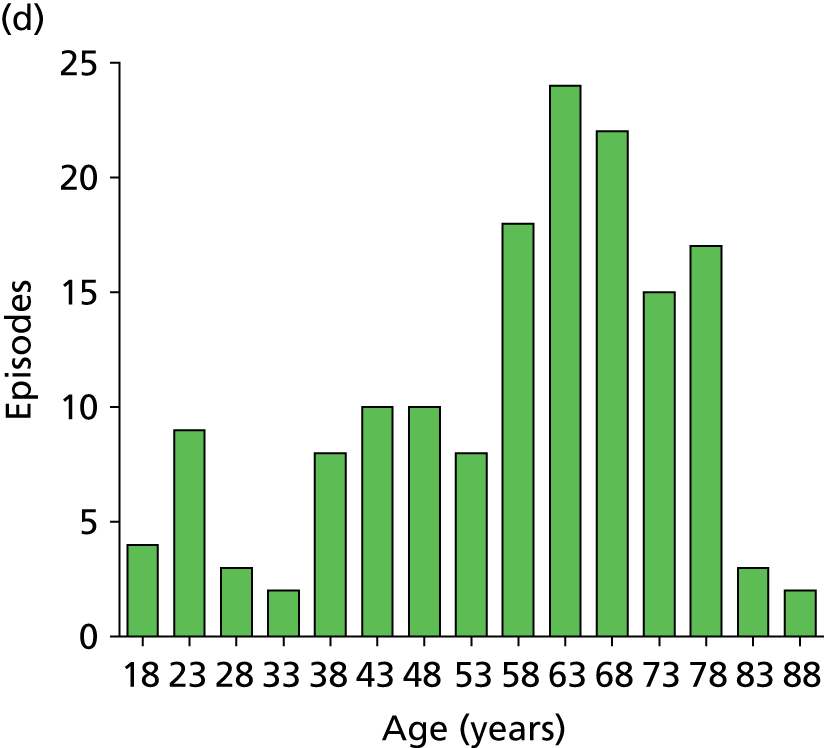
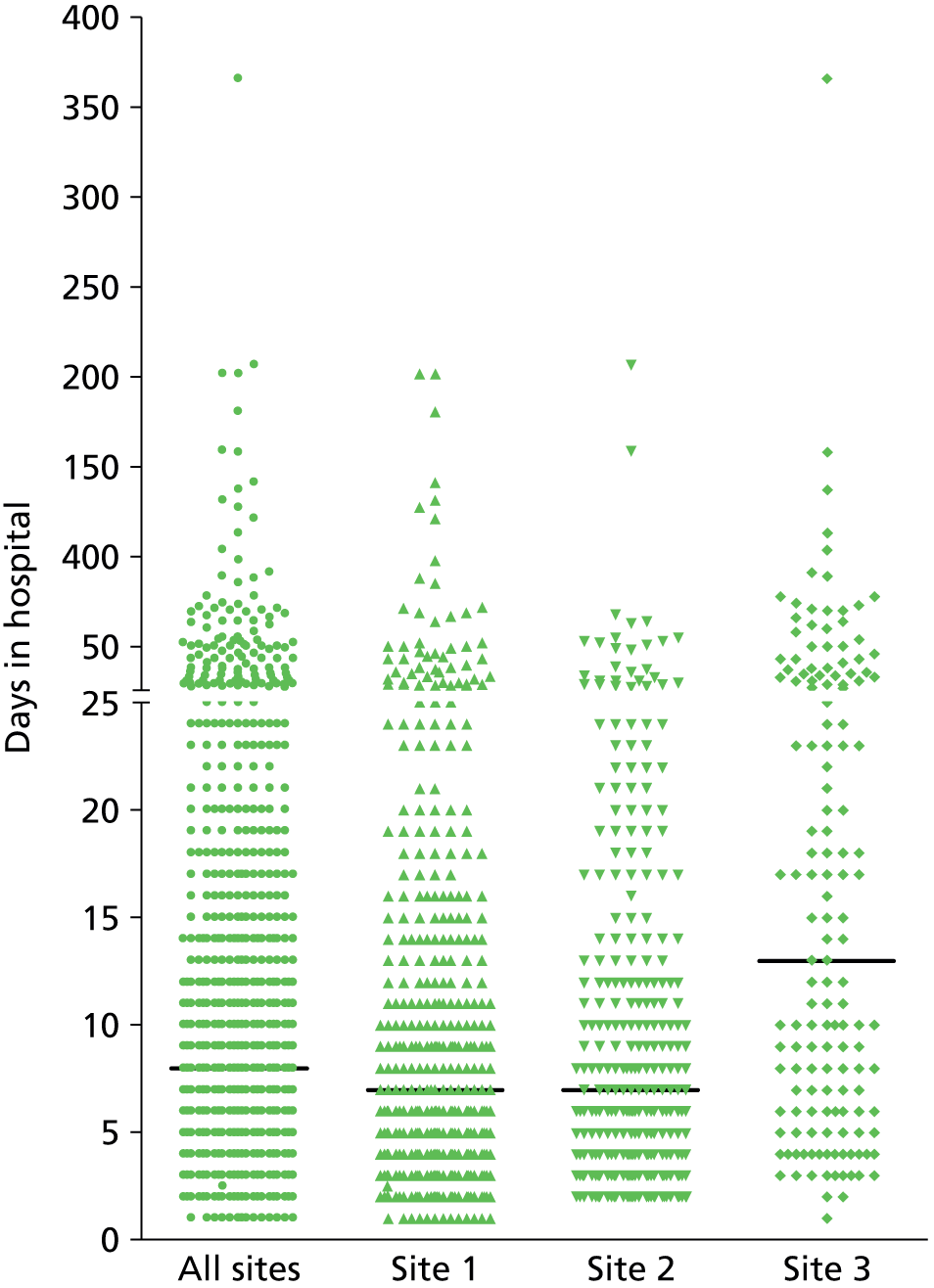
FIGURE 8.
Days in hospital prior to research blood sample. Distribution is shown for individual sites (sites 1–3) and combined for all sites. Site 4 data are not shown separately because of the small number of episodes (n = 12) contributed but is included in the all-sites column. The solid line indicates the median value in each group. Median and IQR values are as follows: all sites, median 8 days, IQR 4–16 days; site 1, median 7 days, IQR 3.5–14 days; site 2, median 7 days, IQR 4–13 days; site 3, median 13 days, IQR 6–32 days.
| Specialty | Number of patients |
|---|---|
| Surgery | |
| General surgery | 97 |
| Colorectal surgery | 30 |
| Hepatobiliary and pancreatic surgery | 23 |
| Upper gastrointestinal surgery | 52 |
| Vascular surgery | 28 |
| Trauma and orthopaedics | 17 |
| Ear, nose and throat | 3 |
| Maxillofacial surgery | 9 |
| Plastic surgery | 3 |
| Burns care | 7 |
| Cardiothoracic surgery | |
| Cardiothoracic surgery | 31 |
| Cardiac surgery | 17 |
| Thoracic surgery | 18 |
| Cardiothoracic transplantation | 3 |
| Neurosurgery | 113 |
| Medical | |
| General medicine | 32 |
| Critical-care medicine | 148 |
| Gastroenterology | 28 |
| Endocrinology | 5 |
| Hepatology | 4 |
| Cardiology | 6 |
| Respiratory medicine | 19 |
| Genitourinary medicine | 9 |
| Nephrology | 31 |
| Medical oncology | 3 |
| Neurology | 6 |
| Rheumatology | 1 |
| Care of the elderly | 1 |
| Haematology | |
| Clinical haematology | 86 |
| Blood and marrow transplantation | 8 |
| Obstetrics and gynaecology | |
| Obstetrics | 1 |
| Gynaecology | 7 |
| Others | 7 |
| Total | 853 |
FIGURE 9.
Organ support activities for patients when a new sepsis episode was suspected. (a) All sites; (b) site 1; (c) site 2; (d) site 3; and (e) site 4. Distribution of activities is shown for individual sites (sites 1–4) and combined for all sites, and is an ordinal scale representing organ support therapy over seven organ systems with a possible score range of 0–9 because cardiovascular and respiratory includes a level of organ support from 0 to 2. 94
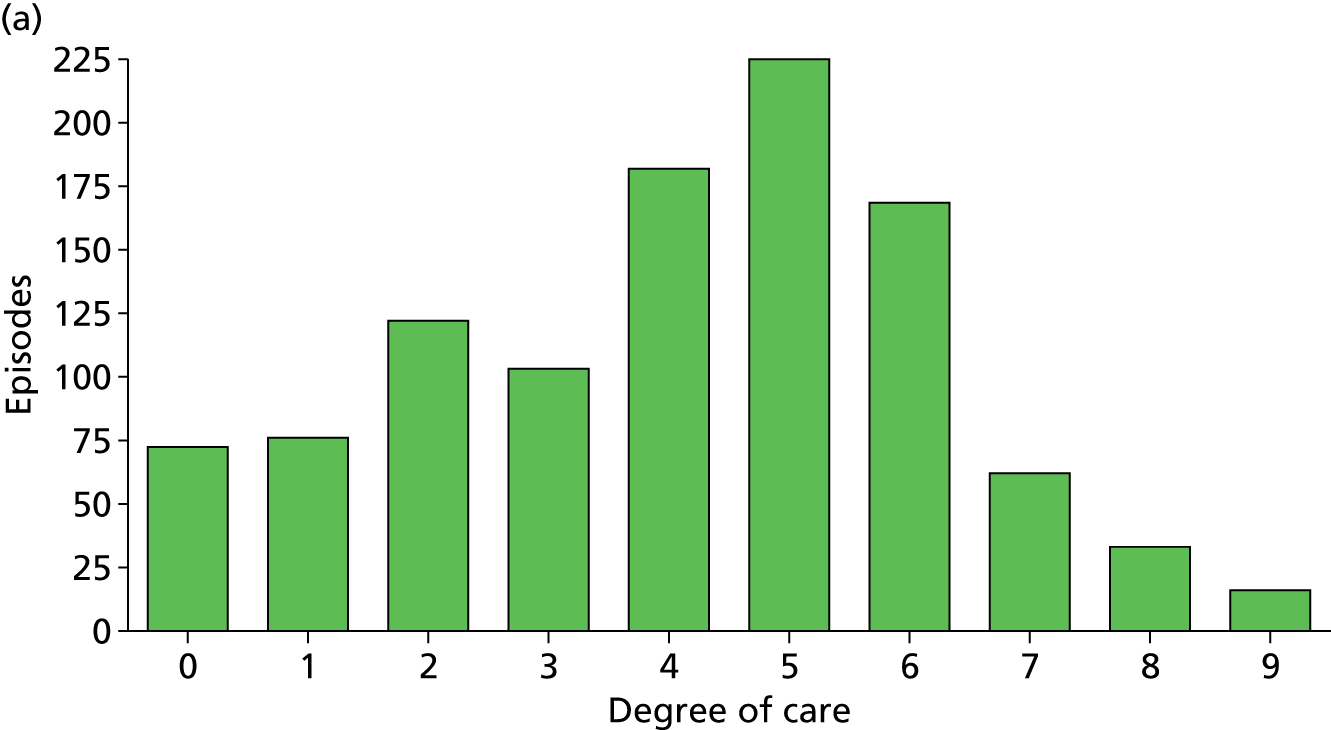

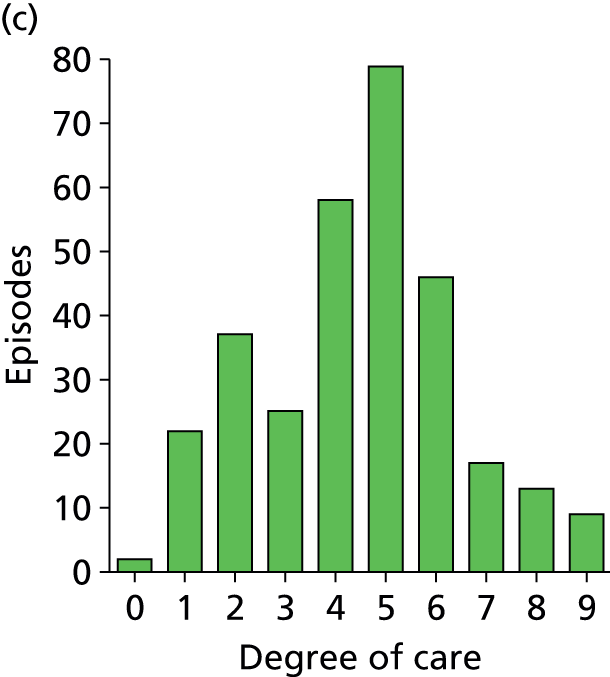


FIGURE 10.
Survival to (a) 28 days and (b) hospital discharge: combined (all sites) and individual (sites 1–4) site data are shown.
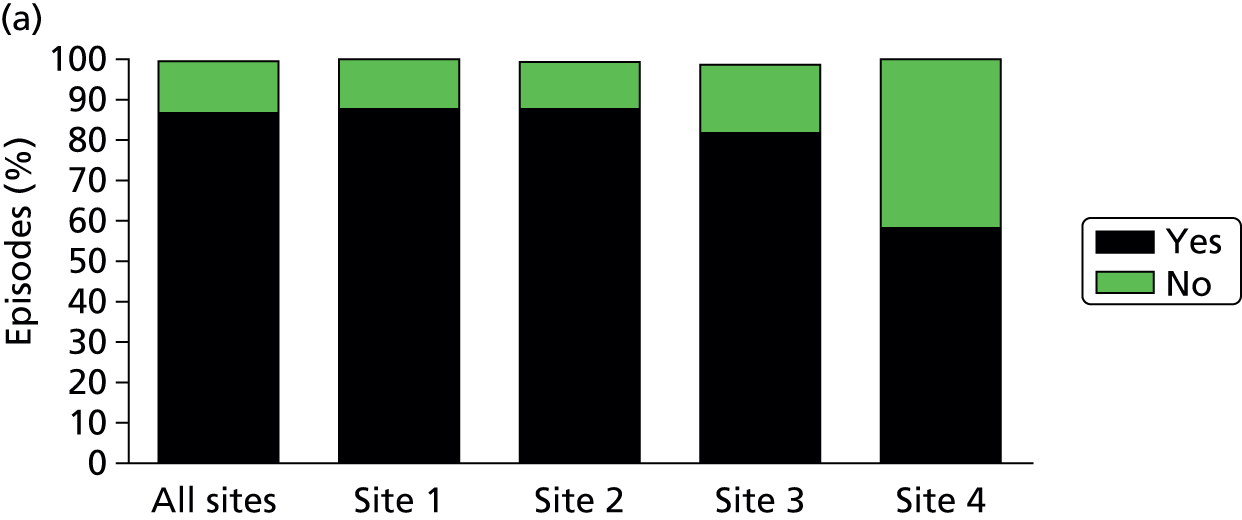
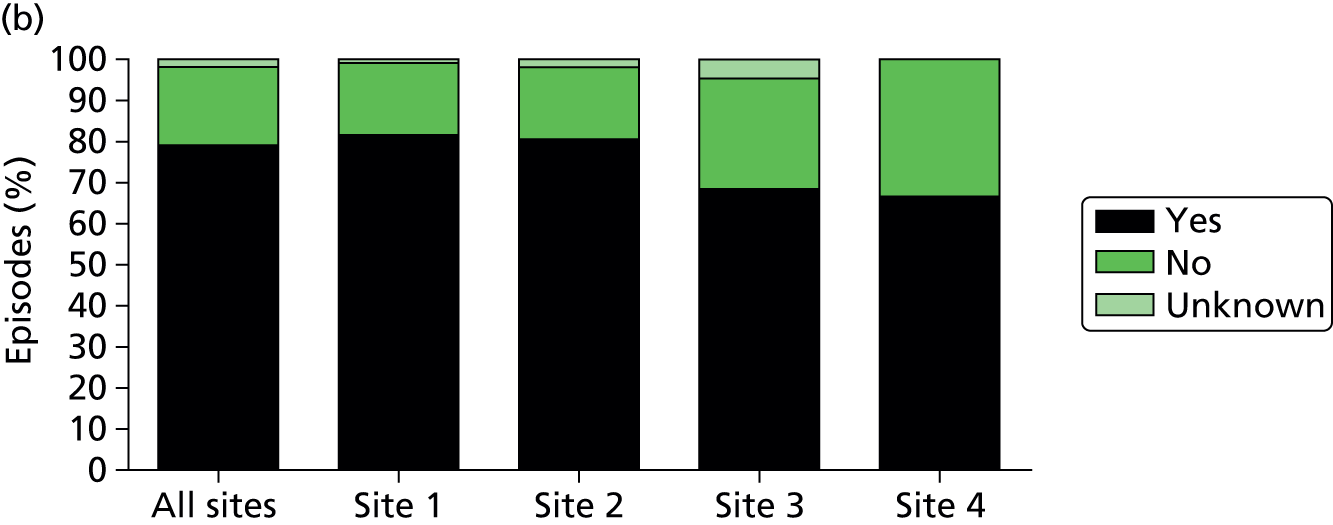
FIGURE 11.
Application of antimicrobial/antiviral therapy prior to blood sampling. Data show the percentage of analysed episodes in which antimicrobial or antiviral therapy was commenced within the 48 hours prior to blood sampling for culture and SeptiFast real-time PCR.
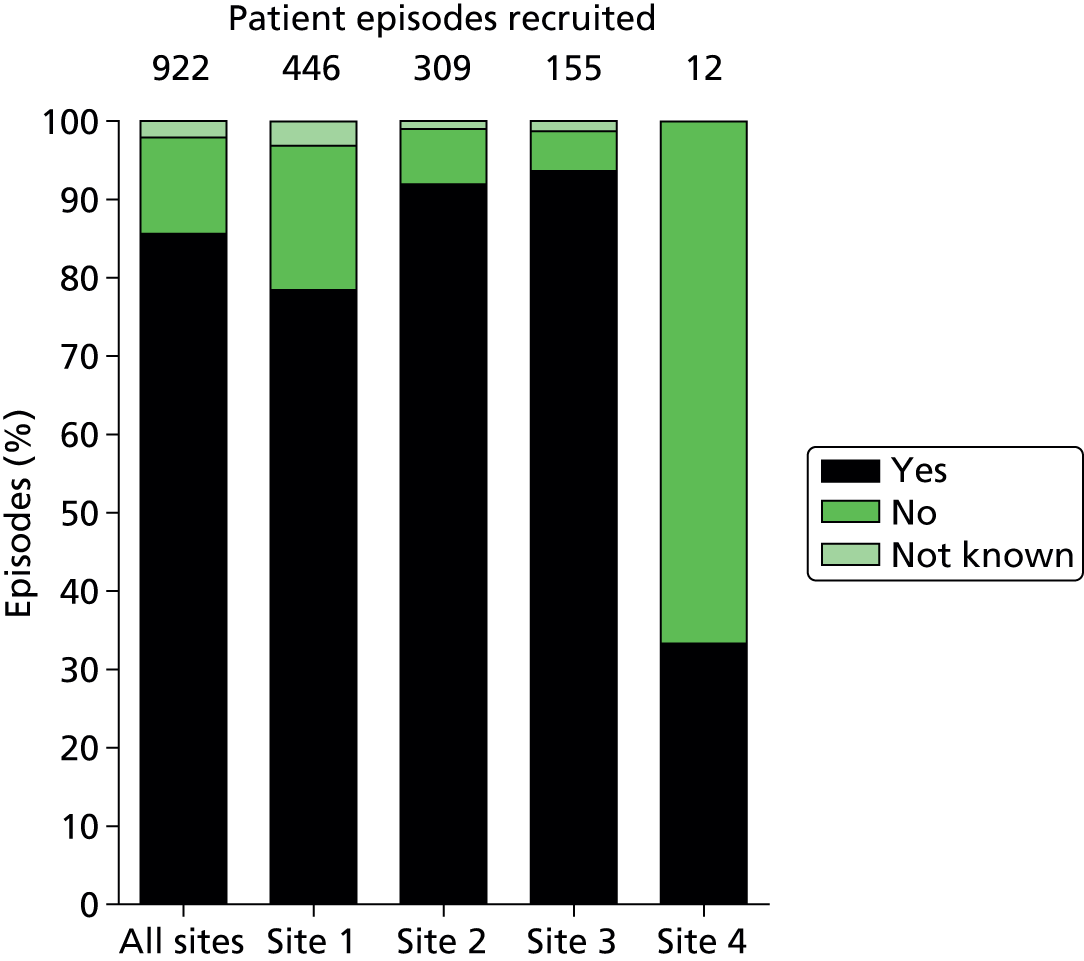
FIGURE 12.
Number of antimicrobial/antiviral drugs administered in each patient episode. Data are expressed as a percentage of the total analysed episodes for all sites combined and for individual sites and show the numbers of drugs administered within the 48 hours prior to blood sampling for culture and SeptiFast real-time PCR. Site 4 data are not shown separately because of the small number of episodes (n = 12) contributed but are included in the all-sites analysis.
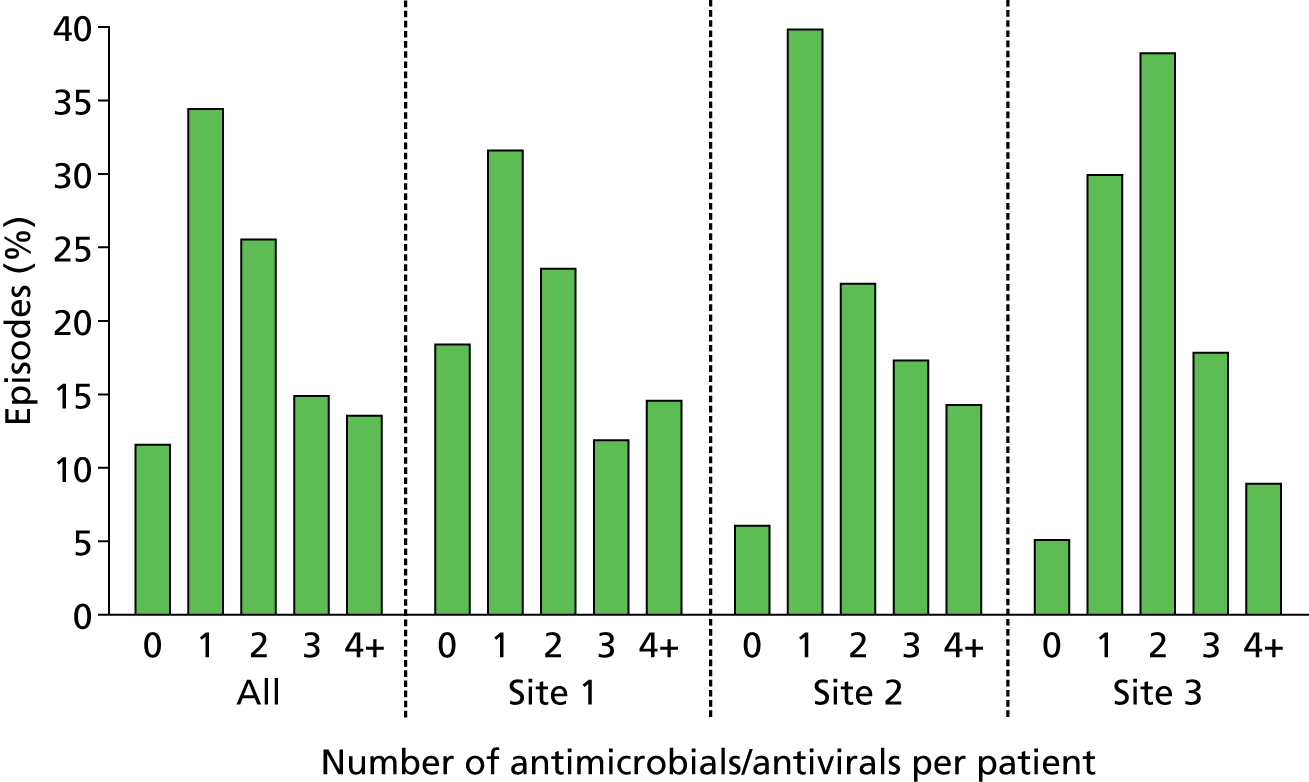
FIGURE 13.
Most common antimicrobial/antiviral drugs administered. (a) All sites; (b) site 1; (c) site 2; (d) site 3; and (e) site 4. Figure shows the most common drugs used for all sites combined and individual sites. Data are shown as a percentage of the total analysed episodes for drugs administered within the 48 hours prior to blood sampling for culture and SeptiFast real-time PCR.


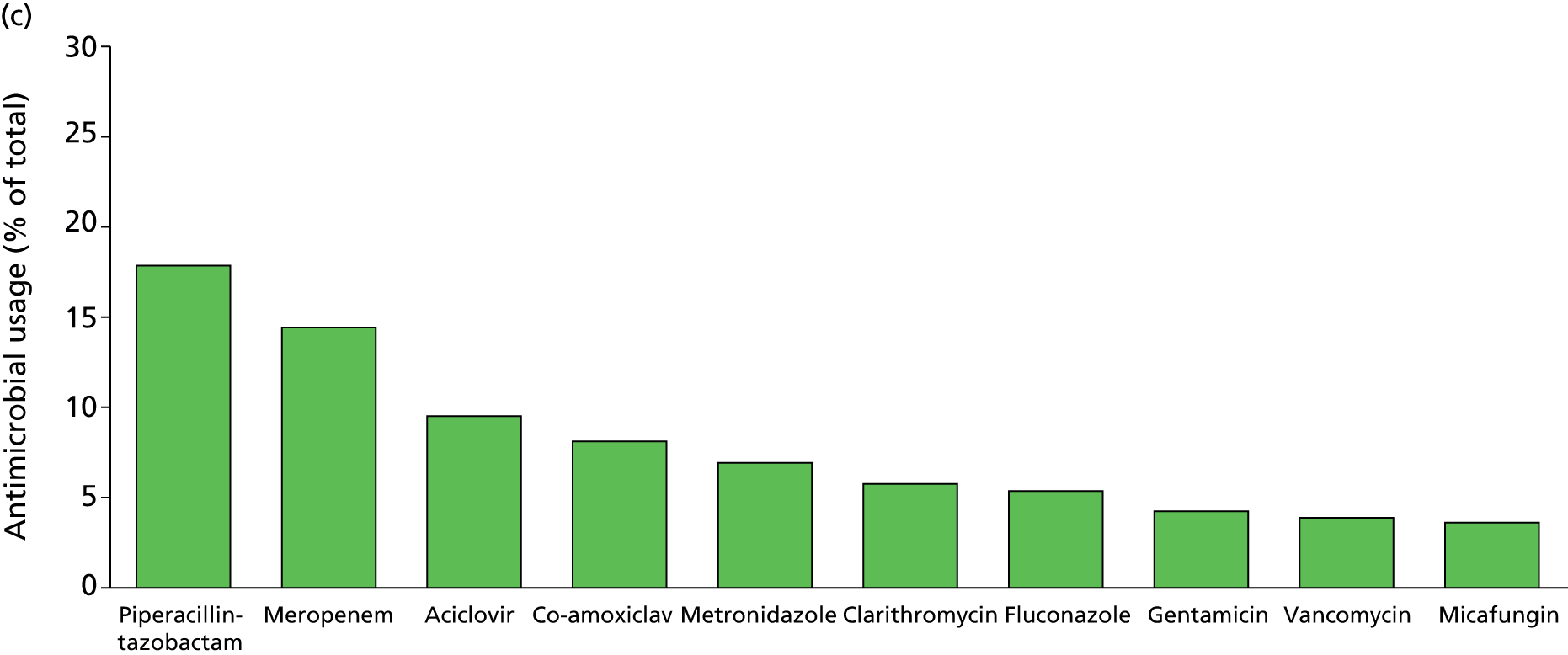
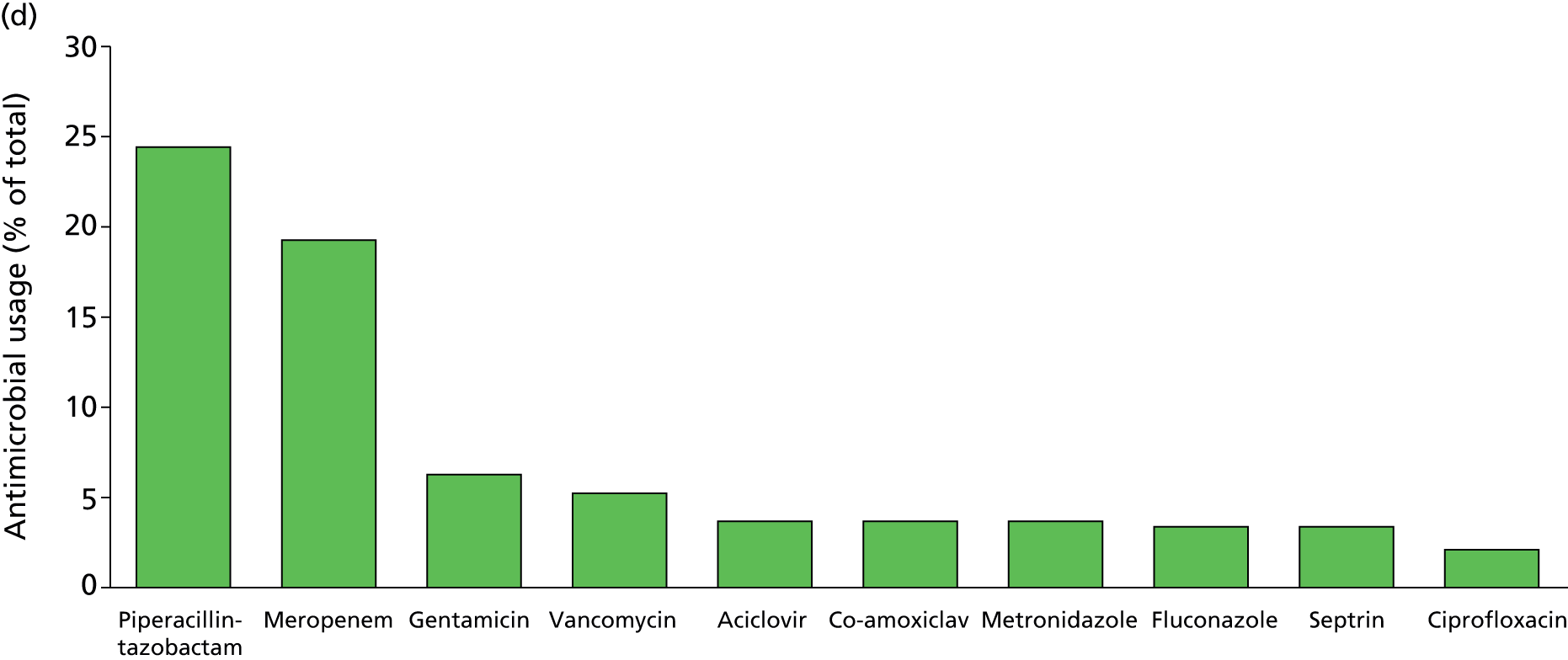
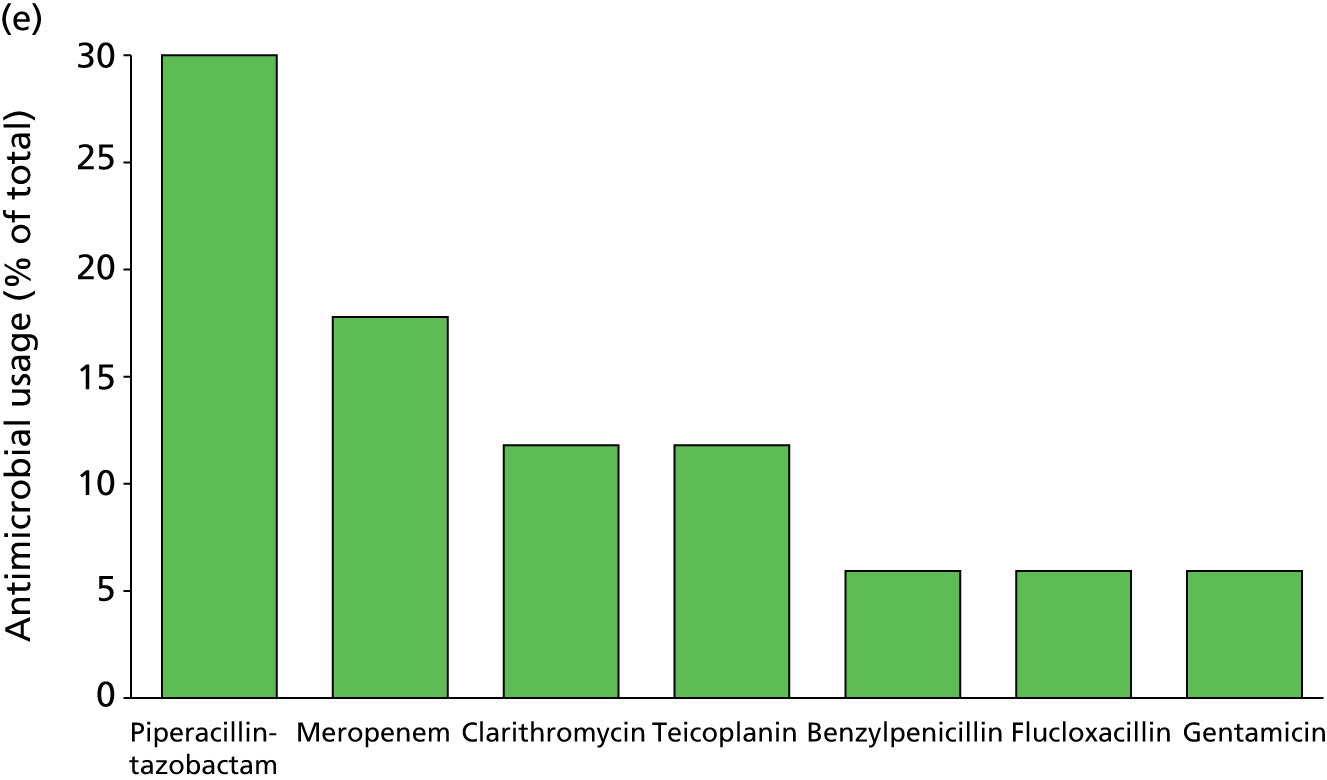
Patients studied in this report were included if a new sepsis episode was suspected, defined by clinical criteria. Circulating CRP and PCT concentrations were measured at the time of each suspected new sepsis episode and are reported in Figure 14. For all sites combined, the mean concentrations of CRP and PCT were 160 mg/l (95% CI 152.3 to 167.6 mg/l) and 7.75 ng/ml (95% CI 6.12 to 9.39 ng/ml) respectively. These data strongly suggest that our patient cohort had objective evidence of systemic inflammation at the time of blood sampling. 7 No serious adverse incidents involving patients or staff were reported that were attributable to the research protocol as implemented.
FIGURE 14.
Circulating (a) CRP and (b) PCT levels in the study population: combined (all sites) and individual (sites 1–4) site data are shown; data represent mean ± 95% CI for each biomarker. Total numbers analysed for CRP and PCT at all sites were 898 and 877 respectively.
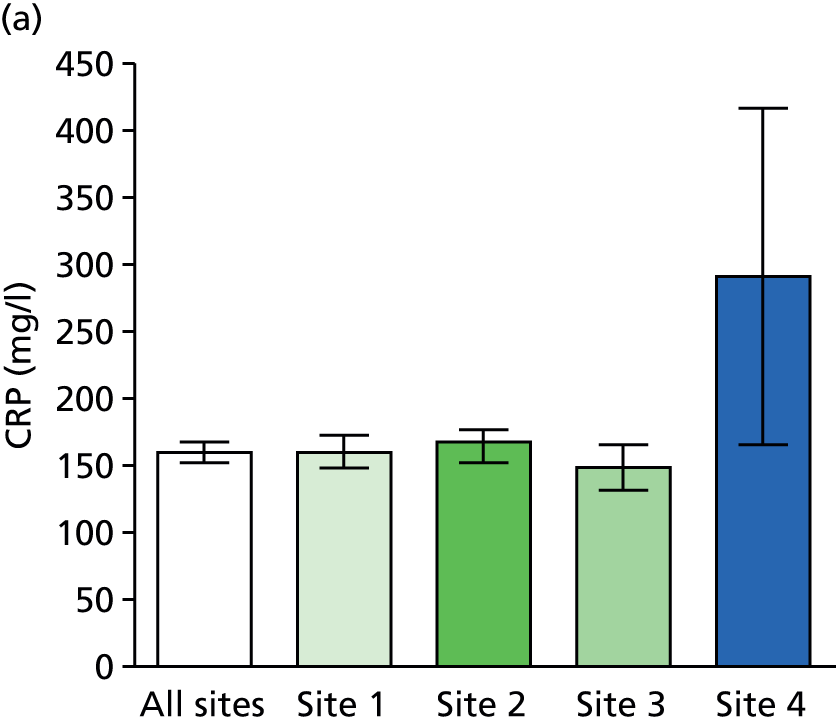

Clinical diagnostic accuracy metrics
SeptiFast versus culture-proven bloodstream infection (patient episode metrics)
Concordance of the 922 suspected episodes between SeptiFast real-time PCR and BC results is reported in Table 6 and summary diagnostic metrics in Table 7. These results take no account of concordance at a pathogen-species level. Overall prevalence of culture-proven bloodstream infection was 8.7% (95% CI 6.9% to 10.7%), with variation across sites. Site variation is most likely to be explained, at least in part, by differences in case mix and prior antimicrobial use in patient cohorts. Summary metrics of specificity were greater than sensitivity across sites, with overall sensitivity of 58.8% (95% CI 47.2% to 69.6%) and specificity of 88.5% (95% CI 86.1% to 90.6%), suggesting that SeptiFast real-time PCR may have better diagnostic rule-in potential than rule-out potential. However, the likelihood ratio of a positive test overall was only 5.1 (95% CI 3.9 to 6.6), resulting in a post-test probability following a positive SeptiFast test of 32% (95% CI 25.1% to 40.9%). Therefore the probability of a patient having a culture-proven bloodstream infection following a positive SeptiFast test would not be greater than 41% – a low level to confidently rule in a diagnosis. In addition, the likelihood ratio of a negative test was only 0.47 (95% CI 0.36 to 0.61), resulting in a post-test probability following a negative SeptiFast test of 4.2% (95% CI 2.9% to 5.9%). Therefore, the probability of a patient having a culture-proven bloodstream infection following a negative SeptiFast test would be no more than 5.9%. This relatively low probability appears a potentially attractive rule-out test, but given the crucial importance of delivering appropriate antibiotics to patients with sepsis, this level of probability may be inadequate to confidently exclude a diagnosis.
| BC positive | BC negative | Total | |
|---|---|---|---|
| SeptiFast positive | 47 | 97 | 144 |
| SeptiFast negative | 33 | 745 | 778 |
| Total | 80 | 842 | 922 |
| Measure | All sites | Site 1 | Site 2 | Site 3 |
|---|---|---|---|---|
| Prevalence (%) (95% CI) | 8.7 (6.9 to 10.7) | 11.0 (8.2 to 14.3) | 4.2 (2.3 to 7.1) | 12 (7.0 to 17.7) |
| Sensitivity (%) (95% CI) | 58.8 (47.2 to 69.6) | 57.1 (42.2 to 71.2) | 53.8 (25.1 to 80.8) | 66.7 (41.0 to 86.6) |
| Specificity (%) (95% CI) | 88.5 (86.1 to 90.6) | 92.9 (90 to 95.3) | 87.2 (82.8 to 90.8) | 80.3 (72.6 to 86.6) |
| Likelihood ratio (+) (95% CI) | 5.1 (3.9 to 6.6) | 8.1 (5.3 to 12.5) | 4.2 (2.3 to 7.5) | 3.4 (2.1 to 5.4) |
| Likelihood ratio (–) (95% CI) | 0.47 (0.36 to 0.61) | 0.46 (0.33 to 0.64) | 0.53 (0.29 to 0.95) | 0.42 (0.22 to 0.80) |
| Odds ratio (95% CI) | 10.9 (6.7 to 17.9) | 17.6 (8.9 to 34.7) | 7.9 (2.6 to 23.8) | 8.2 (2.9 to 22.9) |
| Positive predictive value (%) (95% CI) | 32.6 (25.1 to 40.9) | 50.0 (36.3 to 63.7) | 15.6 (6.5 to 29.5) | 30.8 (17.0 to 47.6) |
| Negative predictive value (%) (95% CI) | 95.8 (94.1 to 97.1) | 94.6 (91.9 to 96.6) | 97.7 (95.1 to 99.2) | 94.8 (89.1 to 98.1) |
SeptiFast versus culture-proven bloodstream infection (pathogen concordance metrics)
Concordance of the 956 suspected episodes (i.e. allowing for more than a single pathogen per blood sample), including pathogen concordance, between SeptiFast and BC results is reported in Table 8 and summary diagnostic metrics in Table 9. These results take account of concordance at a pathogen-species level and represent more comparisons than at an episode level because PCR and/or culture may return more than one pathogen in a blood specimen associated with each patient episode. Overall prevalence of culture-proven pathogens in association with bloodstream infection was 9.2% (95% CI 7.4% to 11.2%), with variation across sites. Site variation is likely to be related to differences in case mix and prior antimicrobial use in patient cohorts. Summary diagnostic accuracy metrics of specificity were greater than sensitivity across sites, with overall sensitivity of 50% (95% CI 39.1% to 60.8%) and specificity of 85.8% (95% CI 83.3% to 88.1%), suggesting that SeptiFast may have better diagnostic rule-in potential than rule-out potential. Both sensitivity and specificity were slightly lower than those returned at an episode level, explained by an imperfect concordance when comparing pathogen species. The likelihood ratio of a positive test overall was only 3.5 (95% CI 2.7 to 4.5), resulting in a post-test probability following a positive SeptiFast test of 26.3% (95% CI 19.8% to 33.7%). Therefore, the probability of a patient having a culture-proven bloodstream infection following a positive SeptiFast test would not be greater than 34% – a low level to confidently rule in a diagnosis. In addition, the likelihood ratio of a negative test was only 0.69 (95% CI 0.50 to 0.73), resulting in a post-test probability following a negative SeptiFast test of 5.6% (95% CI 4.1% to 7.4%). Therefore, the probability of a patient having a culture-proven bloodstream infection following a negative SeptiFast test would be no more than 7.4%. This relatively low probability appears a potentially attractive rule-out test, but given the crucial importance of delivering appropriate antibiotics in patients with sepsis, this level of probability appears too risky to confidently rule out a diagnosis.
| BC positive | BC negative | Total | |
|---|---|---|---|
| SeptiFast positive | 44 | 123 | 167 |
| SeptiFast negative | 44 | 745 | 789 |
| Total | 88 | 868 | 956 |
| Measure | All sites | Site 1 | Site 2 | Site 3 |
|---|---|---|---|---|
| Prevalence % (95% CI) | 9.2 (7.4 to 11.2) | 11.8 (9.0 to 15.1) | 4.4 (2.4 to 7.3) | 11.4 (7.0 to 17.2) |
| Sensitivity % (95% CI) | 50.0 (39.1 to 60.8) | 50.0 (36.1 to 63.9) | 50.0 (23.0 to 77.0) | 47.4 (24.5 to 71.1) |
| Specificity (%) (95% CI) | 85.8 (83.3 to 88.1) | 91.3 (88.2 to 93.9) | 85.2 (80.6 to 89.0) | 74.3 (66.5 to 81.2) |
| Likelihood ratio (+) (95% CI) | 3.5 (2.7 to 4.5) | 5.8 (3.8 to 8.6) | 3.4 (1.7 to 5.5) | 1.84 (1.0 to 2.9) |
| Likelihood ratio (–) (95% CI) | 0.69 (0.50 to 0.73) | 0.55 (0.40 to 0.70) | 0.59 (0.31 to 0.86) | 0.71 (0.42 to 0.99) |
| Odds ratio (95% CI) | 6.1 (3.7 to 9.8) | 10.5 (5.3 to 20.8) | 5.7 (1.6 to 20.0) | 2.6 (0.9 to 7.7) |
| Positive predictive value (%) (95% CI) | 26.3 (19.8 to 33.7) | 43.6 (31.0 to 56.7) | 13.5 (5.6 to 25.8) | 19.2 (9.2 to 33.3) |
| Negative predictive value (%) (95% CI) | 94.4 (92.6 to 95.9) | 93.2 (90.2 to 95.5) | 97.4 (94.6 to 98.9) | 91.7 (85.2 to 95.9) |
SeptiFast versus enhanced reference standard (patient episode metrics)
Concordance of the 922 suspected episodes between SeptiFast and the enhanced reference standard which takes into account the addition of other potential culture-proven sites is reported in Table 10 and summary diagnostic metrics in Table 11. These results take no account of concordance at a pathogen-species level. Overall prevalence of culture-proven enhanced reference standard was 29% (95% CI 26% to 32%), again with variation across sites, which is likely to be related to differences in case mix and prior antimicrobial use. Summary metrics of specificity were greater than sensitivity across sites, with overall sensitivity of 31.1% (95% CI 25.6% to 37.0%) and specificity of 90.8% (95% CI 88.3% to 92.9%), suggesting that SeptiFast may have better diagnostic rule-in potential than rule-out potential when compared with the enhanced reference standard. Comparing Table 6 with Table 10 shows that introducing an enhanced reference standard is associated with a shift from false positives with BC alone (33% of all PCR positives in Table 6) to true positives (58% of all PCR positives in Table 10), but at the cost of a sizeable increase in false negatives. The likelihood ratio of a positive test overall was only 3.4 (95% CI 2.5 to 4.6), resulting in a post-test probability following a positive SeptiFast test of 58.3% (95% CI 49.8% to 66.5%). Therefore, the probability of a patient having a culture-proven infection following a positive SeptiFast test would not be greater than 67% – a low level to confidently rule in a diagnosis. In addition, the likelihood ratio of a negative test was only 0.76 (95% CI 0.70 to 0.83), resulting in a post-test probability following a negative SeptiFast test of 23.9% (95% CI 20.9% to 27.1%). Therefore, the probability of a patient having a culture-proven infection following a negative SeptiFast test could be as high as 27.1% – an unacceptable level to confidently rule out a diagnosis.
| Culture positive | Culture negative | Total | |
|---|---|---|---|
| SeptiFast positive | 84 | 60 | 144 |
| SeptiFast negative | 186 | 592 | 778 |
| Total | 270 | 652 | 922 |
| Measure | All sites | Site 1 | Site 2 | Site 3 |
|---|---|---|---|---|
| Prevalence (%) (95% CI) | 29.0 (26.0 to 32.3) | 26.0 (22.0 to 30.1) | 25.0 (20.0 to 30.5) | 45.0 (37.0 to 53.3) |
| Sensitivity (%) (95% CI) | 31.1 (25.6 to 37.0) | 33.0 (24.6 to 42.4) | 23.1 (14.3 to 34.0) | 34.3 (23.3 to 46.6) |
| Specificity (%) (95% CI) | 90.8 (88.3 to 92.9) | 94.6 (91.5 to 96.7) | 88.3 (83.5 to 92.2) | 82.4 (72.6 to 89.8) |
| Likelihood ratio (+) (95% CI) | 3.4 (2.5 to 4.6) | 6.1 (3.6 to 10.2) | 2.0 (1.2 to 3.4) | 1.9 (1.1 to 3.4) |
| Likelihood ratio (–) (95% CI) | 0.76 (0.70 to 0.83) | 0.71 (0.62 to 0.81) | 0.87 (0.77 to 0.92) | 0.80 (0.66 to 0.97) |
| Odds ratio (95% CI) | 4.5 (3.1 to 6.4) | 8.6 (4.7 to 15.8) | 2.3 (1.2 to 4.4) | 2.4 (1.2 to 5.1) |
| Positive predictive value (%) (95% CI) | 58.3 (49.8 to 66.5) | 67.9 (54.0 to 79.7) | 40.0 (25.7 to 55.7) | 61.5 (44.6 to 76.6) |
| Negative predictive value (%) (95% CI) | 76.1 (72.9 to 79.1) | 80.3 (76.0 to 84.1) | 77.3 (71.7 to 82.2) | 60.3 (50.8 to 69.3) |
SeptiFast versus enhanced reference standard (pathogen concordance metrics)
Concordance of the 1041 suspected episodes, including pathogen concordance, between SeptiFast and the enhanced reference standard is reported in Table 12 and summary diagnostic metrics in Table 13. These results take account of concordance at a pathogen-species level and represent more comparisons than at an episode level because PCR on blood and/or other cultures from the enhanced reference standard may return more than one pathogen associated with each patient episode. Overall prevalence of culture-proven infection in the enhanced reference standard was 33.5% (95% CI 30.6% to 36.4%), with variation across sites. Site variation is likely to be related to differences in case mix and prior antimicrobial use in patient cohorts. Summary metrics of specificity were greater than sensitivity across sites, with overall sensitivity of 18.9% (95% CI 14.9% to 23.4%) and specificity of 85.4% (95% CI 82.6% to 88.0%), suggesting that SeptiFast may have better diagnostic rule-in potential than rule-out potential when compared with the enhanced reference standard. Both sensitivity and specificity were lower than those returned at an episode level, explained by an imperfect concordance when comparing pathogen species. The likelihood ratio of a positive test overall was only 1.30 (95% CI 0.98 to 1.72), resulting in a post-test probability following a positive SeptiFast test of 39.5% (95% CI 32.0% to 47.4%). Therefore, the probability of a patient having a culture-proven infection following a positive SeptiFast test would not be greater than 47.4% – a low level to confidently rule in a diagnosis. In addition, the likelihood ratio of a negative test was only 0.95 (95% CI 0.89 to 1.00), resulting in a post-test probability following a negative SeptiFast test of 32.3% (95% CI 29.2% to 35.5%). Therefore, the probability of a patient having a culture-proven infection following a negative SeptiFast test could be as high as 35.5% – an unacceptable level to confidently rule out a diagnosis.
| Culture positive | Culture negative | Total | |
|---|---|---|---|
| SeptiFast positive | 66 | 101 | 167 |
| SeptiFast negative | 283 | 593 | 876 |
| Total | 349 | 694 | 1043 |
| Measure | All sites | Site 1 | Site 2 | Site 3 |
|---|---|---|---|---|
| Prevalence (%) (95% CI) | 33.5 (30.6 to 36.4) | 31.1 (27.0 to 35.4) | 28.6 (23.9 to 33.7) | 49.5 (42.2 to 56.7) |
| Sensitivity (%) (95% CI) | 18.9 (14.9 to 23.4) | 24.2 (17.6 to 31.8) | 13.4 (7.3 to 21.8) | 18.7 (11.5 to 28.0) |
| Specificity (%) (95% CI) | 85.4 (82.6 to 88.0) | 92.6 (89.3 to 95.2) | 83.9 (78.6 to 88.3) | 71.4 (61.4 to 80.1) |
| Likelihood ratio (+) (95% CI) | 1.30 (0.98 to 1.72) | 3.28 (2.06 to 5.22) | 0.83 (0.46 to 1.46) | 0.66 (0.39 to 1.09) |
| Likelihood ratio (–) (95% CI) | 0.95 (0.89 to 1.00) | 0.82 (0.74 to 0.89) | 1.03 (0.92 to 1.13) | 1.14 (0.97 to 1.34) |
| Odds ratio (95% CI) | 1.37 (0.96 to 1.95) | 4.01 (2.23 to 7.25) | 0.81 (0.37 to 1.64) | 0.58 (0.27 to 1.19) |
| Positive predictive value (%) (95% CI) | 39.5 (32.0 to 47.4) | 59.7 (46.4 to 71.9) | 25.0 (14.0 to 38.9) | 39.1 (39.0 to 55.7) |
| Negative predictive value (%) (95% CI) | 67.7 (64.5 to 70.8) | 73.0 (68.6 to 77.2) | 70.7 (65.1 to 75.9) | 47.3 (39.0 to 55.7) |
Pathogen spectrum
Culture
The pathogen range and prevalence detected in the primary blood sample by culture in association with independently adjudicated bloodstream infection, is shown in Table 14. Eleven out of 88 (12.5%) pathogens in positive BC from adjudicated bloodstream infections were not found on the SML, a greater proportion than that predicted by the manufacturer (quoted as < 5%). 29 How the pathogens detected varied by hospital site is shown in Figures 15 and 16 and Table 15. Figure 17 shows the incidence of polymicrobial infections in positive BC. The 88 pathogens identified by culture were associated with 74 cultures containing a single organism and 7 cultures containing two organisms, a rate of polymicrobial infection of 8.8%.
| Pathogen species | Number of cases detected | ||
|---|---|---|---|
| BC only | PCR only | BC and PCR | |
| Pathogens on the SML | |||
| Gram-negative bacteria | |||
| Escherichia coli | 4 | 19 | 12 |
| Klebsiella (pneumoniae/oxytoca) | 3 | 28 | 10 |
| Serratia marcescens | 0 | 3 | 1 |
| Enterobacter (aerogenes/cloacae) | 2 | 12 | 1 |
| Proteus mirabilis | 0 | 0 | 0 |
| Acinetobacter baumanii | 1 | 0 | 0 |
| Pseudomonas aeruginosa | 3 | 1 | 5 |
| Stenotrophomonas maltophilia | 0 | 0 | 1 |
| Gram-positive bacteria | |||
| Staphylococcus aureus | 1 | 19 | 1 |
| CoNS | 3 | 6 | 2 |
| Streptococcus pneumoniae | 0 | 2 | 0 |
| Streptococcus species | 4 | 5 | 3 |
| Enterococcus faecium | 6 | 7 | 3 |
| Enterococcus faecalis | 1 | 5 | 3 |
| Fungi | |||
| Candida albicans | 2 | 9 | 2 |
| Candida tropicalis | 0 | 1 | 0 |
| Candida parapsilosis | 0 | 0 | 0 |
| Candida glabrata | 3 | 2 | 0 |
| Candida krusei | 0 | 3 | 0 |
| Aspergillus fumigatus | 0 | 1 | 0 |
| Total | 33 | 123 | 44 |
| Pathogens not on the SML | |||
| Bacteroides fragilis | 1 | 0 | 0 |
| Citrobacter koseri | 1 | 0 | 0 |
| Clostridium perfringens | 1 | 0 | 0 |
| Enterobacter amnigenus | 1 | 0 | 0 |
| Leclercia adecarboxylata | 1 | 0 | 0 |
| Morganella morganii | 2 | 0 | 0 |
| Staphylococcus capitis | 1 | 0 | 0 |
| Salmonella enterica | 1 | 0 | 0 |
| Raoultella planticola | 1 | 0 | 0 |
| Candida lipolytica | 1 | 0 | 0 |
| Total | 11 | 0 | 0 |
FIGURE 15.
Number of (a) Gram-negative and (b) Gram-positive bacteria from the SML isolated from BCs at each hospital site (sites 1–3). No positive BCs were identified at site 4.

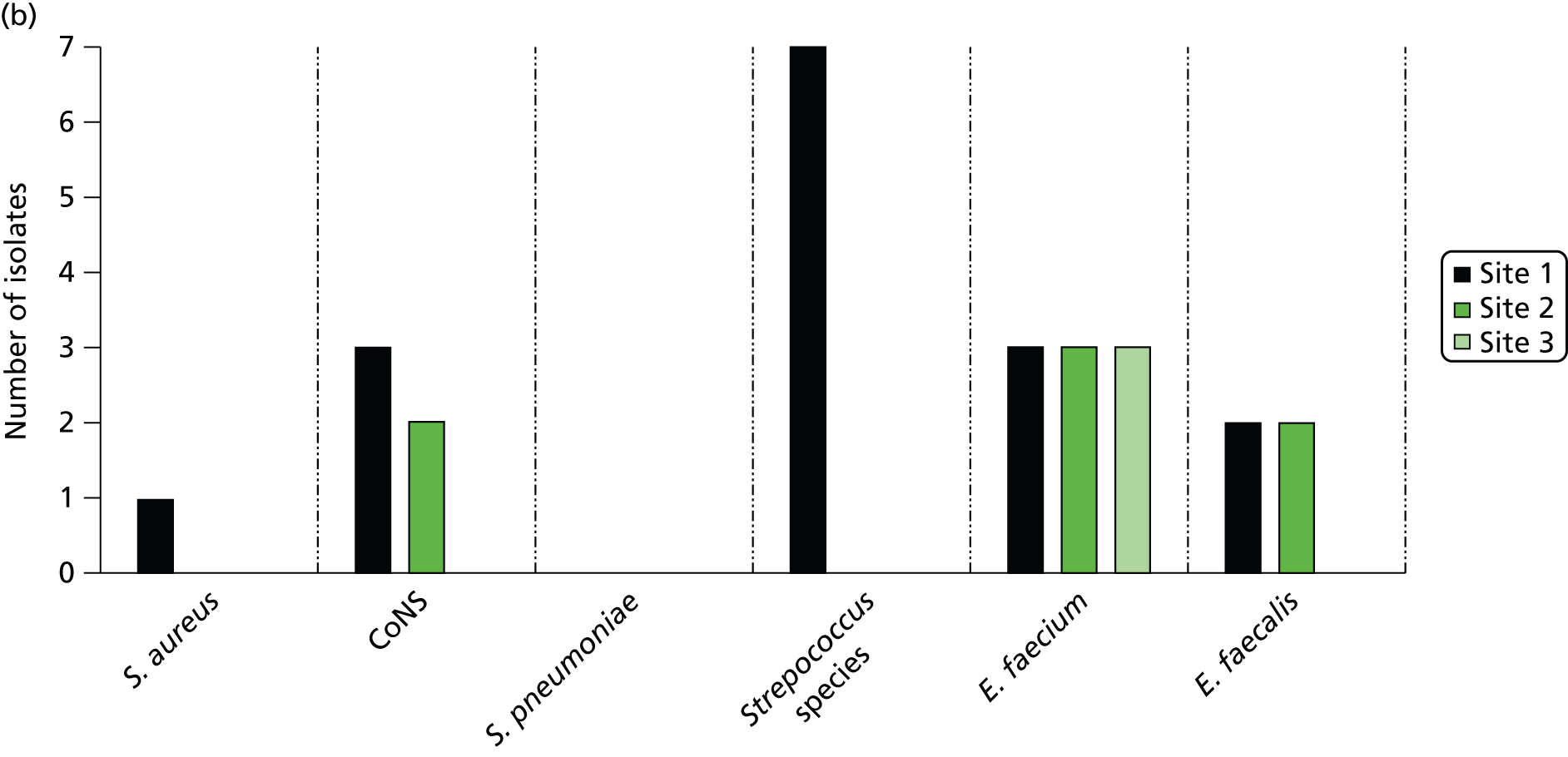
FIGURE 16.
Number of fungal organisms from the SML isolated from BC at each hospital site (sites 1–3). No positive BCs were identified at site 4.
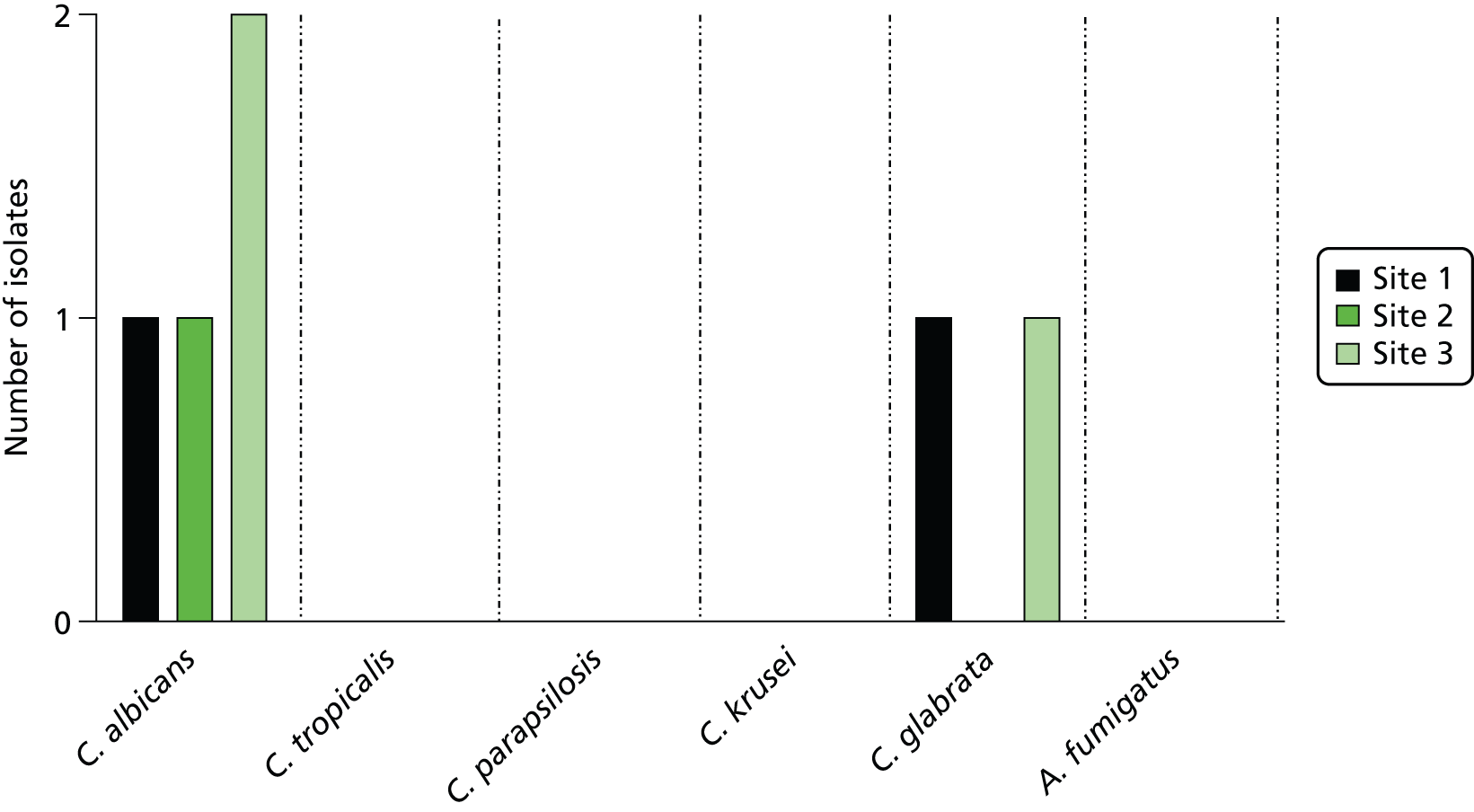
| Hospital site 1 | Hospital site 2 | Hospital site 3 |
|---|---|---|
| Bacteroides fragilis | Staphylococcus capitis | Clostridium perfringens |
| Citrobacter koseri | Raoultella planticola | |
| Enterobacter amnigenus | Candida lipolytica | |
| Leclercia adecarboxylata | ||
| Morganella morganii (n = 2) | ||
| Salmonella enterica |
FIGURE 17.
Numbers of pathogens detected per infection episode. Data show the number of infection episodes in which one, two or three pathogens were detected by BC and by SeptiFast real-time PCR.

Table 16 describes similar information for the enhanced reference standard. This table includes pathogens isolated from blood and other sites, but does not include pathogens from adjudicated bloodstream infections on sample day, which are shown in Table 14. Notably, a higher percentage of infections (23%, 59 out of 261 positive sample cultures from adjudicated infection) were due to pathogens not on the SML, indicating that cultures from sites other than the bloodstream have a higher incidence of off-panel organisms. The sites of infection from which pathogens included in the enhanced reference standard were cultured are shown in Table 17; these are in addition to the pathogens isolated from BC on sample day (shown in Table 14) which were also included in the enhanced reference standard.
| Pathogen species | Number of episodes detected | ||
|---|---|---|---|
| Culture only | PCR only | Culture and PCR | |
| Pathogens on the SML | |||
| Gram-negative bacteria | |||
| Escherichia coli | 29 | 11 | 8 |
| Klebsiella (pneumoniae/oxytoca) | 19 | 24 | 4 |
| Serratia marcescens | 5 | 2 | 1 |
| Enterobacter (aerogenes/cloacae) | 5 | 11 | 1 |
| Proteus mirabilis | 2 | 0 | 0 |
| Acinetobacter baumanii | 0 | 0 | 0 |
| Pseudomonas aeruginosa | 12 | 1 | 0 |
| Stenotrophomonas maltophilia | 5 | 0 | 0 |
| Gram-positive bacteria | |||
| Staphylococcus aureus | 33 | 16 | 3 |
| CoNS | 9 | 5 | 1 |
| Streptococcus pneumoniae | 2 | 2 | 0 |
| Streptococcus species | 9 | 5 | 0 |
| Enterococcus faecium | 7 | 7 | 0 |
| Enterococcus faecalis | 4 | 4 | 1 |
| Fungi | |||
| Candida albicans | 29 | 6 | 3 |
| Candida tropicalis | 1 | 1 | 0 |
| Candida parapsilosis | 1 | 0 | 0 |
| Candida glabrata | 5 | 2 | 0 |
| Candida krusei | 0 | 3 | 0 |
| Aspergillus fumigatus | 3 | 1 | 0 |
| Total | 180 | 101 | 22 |
| Pathogens not on the SML | |||
| Anaerobes | 2 | 0 | 0 |
| Candida lipolytica | 1 | 0 | 0 |
| Clostridium difficile | 8 | 0 | 0 |
| Unidentified aerobic Gram-negative Bacillus | 14 | 0 | 0 |
| Enterococci | 3 | 0 | 0 |
| Penicillin-resistant Enterococcus | 1 | 0 | 0 |
| Vancomycin-resistant Enterococcus species | 1 | 0 | 0 |
| Haemophilus influenzae | 7 | 0 | 0 |
| Hafnia alvei | 1 | 0 | 0 |
| Influenza A (H1N1) | 3 | 0 | 0 |
| Moraxella catarrhalis | 1 | 0 | 0 |
| Morganella morganii | 2 | 0 | 0 |
| Proteus species | 2 | 0 | 0 |
| Pseudomonas species | 7 | 0 | 0 |
| Raoutella planticola | 1 | 0 | 0 |
| Streptococcus parasanguinis | 1 | 0 | 0 |
| Yeast (unidentified) | 4 | 0 | 0 |
| Total | 59 | 0 | 0 |
| Pathogen species | Infection site | ||||
|---|---|---|---|---|---|
| Blooda | Lung | Urinary tract | Surgical site | Other | |
| Pathogens on the SML | |||||
| Gram-negative bacteria | |||||
| Escherichia coli | 9 | 5 | 9 | 10 | 4 |
| Klebsiella (pneumoniae/oxytoca) | 3 | 7 | 4 | 1 | 8 |
| Serratia marcescens | 0 | 3 | 0 | 0 | 3 |
| Enterobacter (aerogenes/cloacae) | 0 | 3 | 0 | 1 | 2 |
| Proteus mirabilis | 1 | 0 | 1 | 0 | 0 |
| Acinetobacter baumanii | 0 | 0 | 0 | 0 | 0 |
| Pseudomonas aeruginosa | 0 | 5 | 0 | 1 | 6 |
| Stenotrophomonas maltophilia | 0 | 4 | 0 | 0 | 1 |
| Gram-positive bacteria | |||||
| Staphylococcus aureus | 3 | 19 | 1 | 1 | 12 |
| CoNS | 1 | 0 | 0 | 2 | 7 |
| Streptococcus pneumoniae | 1 | 1 | 0 | 0 | 0 |
| Streptococcus species | 4 | 1 | 0 | 2 | 2 |
| Enterococcus faecium | 2 | 0 | 0 | 5 | 0 |
| Enterococcus faecalis | 0 | 1 | 0 | 0 | 4 |
| Fungi | |||||
| Candida albicans | 1 | 24 | 0 | 4 | 3 |
| Candida tropicalis | 0 | 0 | 0 | 0 | 1 |
| Candida parapsilosis | 0 | 1 | 0 | 0 | 0 |
| Candida glabrata | 2 | 3 | 0 | 0 | 0 |
| Candida krusei | 0 | 0 | 0 | 0 | 0 |
| Aspergillus fumigatus | 0 | 3 | 0 | 0 | 0 |
| Total | 27 | 80 | 15 | 27 | 53 |
| Pathogens not on SML | |||||
| Anaerobes | 0 | 0 | 0 | 1 | 1 |
| Candida lipolytica | 1 | 0 | 0 | 0 | 0 |
| Clostridium difficile | 0 | 0 | 0 | 0 | 8 |
| Unidentified aerobic Gram-negative Bacillus | 0 | 4 | 6 | 3 | 1 |
| Enterococci | 0 | 0 | 1 | 2 | 0 |
| Penicillin-resistant Enterococcus | 1 | 0 | 0 | 0 | 0 |
| Vancomycin-resistant Enterococcus species | 0 | 0 | 0 | 0 | 1 |
| Haemophilus influenzae | 0 | 5 | 0 | 0 | 2 |
| Hafnia alvei | 0 | 0 | 0 | 0 | 1 |
| Influenza A (H1N1) | 0 | 3 | 0 | 0 | 0 |
| Moraxella catarrhalis | 0 | 0 | 0 | 0 | 1 |
| Morganella morganii | 1 | 0 | 0 | 0 | 1 |
| Proteus species | 0 | 0 | 0 | 2 | 0 |
| Pseudomonas species | 0 | 1 | 3 | 0 | 3 |
| Raoutella planticola | 0 | 1 | 0 | 0 | 0 |
| Streptococcus parasanguinis | 0 | 0 | 0 | 1 | 0 |
| Yeast (unidentified) | 0 | 3 | 1 | 0 | 0 |
| Total | 3 | 17 | 11 | 9 | 19 |
SeptiFast polymerase chain reaction
The pathogen range and prevalence detected in blood specimens by SeptiFast is shown in Table 14. For pathogens on the SML, PCR detected 167 pathogens compared with 77 by BC, with fungal species representing only 18 (11%) of the PCR-detected pathogens.
Detection of multiple organisms was more common with SeptiFast, with PCR detecting 167 organisms in 144 blood samples with 124 blood samples containing a single organism, 20 samples containing two organisms and one sample containing three organisms, a rate of polymicrobial PCR of 14.5% (see Figure 17). The pathogen range detected by PCR in the enhanced reference standard appeared broadly similar to that observed in BCs (see Table 16).
Blood culture contamination
Blood culture contamination was adjudicated independently at each site without any knowledge of the SeptiFast results and is shown in Figure 18. These contamination rates were reported within NHS, forming part of continuous quality improvements for BC sampling at each site. The overall BC contamination rate was about 2% for all BCs taken within this study and provides independent evidence for blood sampling quality when compared with national standards. 96 Following study completion and calculation of the clinical diagnostic accuracy metrics it became apparent, post hoc, that none of the adjudicated BC contaminations were associated with a positive SeptiFast result.
FIGURE 18.
Blood culture contaminants. Percentage of BC adjudicated as contaminants for (a) all sites combined; (b) site 1; (c) site 2; and (d) site 3. No contaminants were identified in the 12 BCs taken at site 4. (e) Species identified as a percentage of total contaminants.
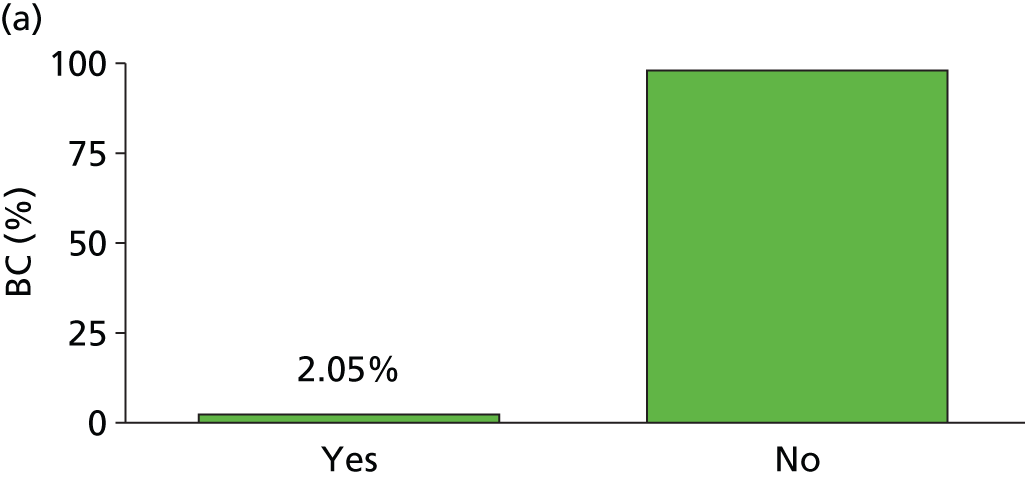
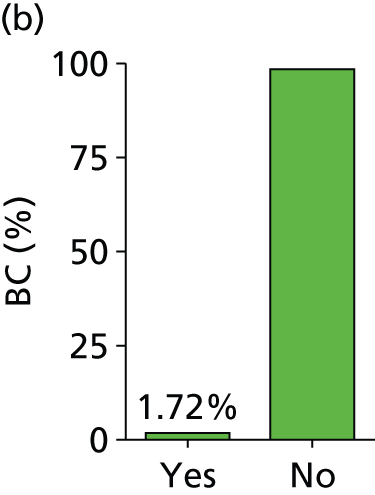
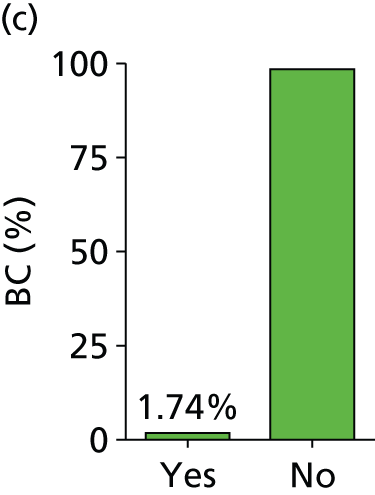


Discussion
The comprehensive systematic review of the likely diagnostic accuracy performance of SeptiFast (see Chapter 2) highlights significant deficiencies in the quality of design and reporting of clinical diagnostic accuracy studies in this field. As a result, the current evidence base that underpins any recommendations on the suitability of the test for adoption into frontline sepsis clinical care pathways is unsound. Our aim in this chapter was to report the clinical diagnostic accuracy of SeptiFast compared with BC as the gold standard test in a defined setting of suspected sepsis-related health-care-associated bloodstream infection employing study design, performance and reporting that meet all the features of the STARD criteria. 87 In addition, we investigated a broader (‘enhanced’) reference standard in this study population that included adjudicated culture-proven health-care-associated infection more inclusively than just bloodstream.
We have reported clinical diagnostic accuracy measures of SeptiFast real-time PCR against BC at two levels, event level (i.e. concordance of PCR positivity with BC positivity) and species concordance level (i.e. PCR detection of the same organism at species/genus level as identified by BC). Intuitively, the latter metric would seem to be a more robust measure of the potential clinical utility of the SeptiFast test given that organism identification at species/genus level is a routine element of culture-based diagnosis in clinical practice and the SeptiFast test only delivers results at species level. As discussed in Chapter 2, although some previous studies have specified the metrics used to evaluate SeptiFast real-time PCR, in other cases it is uncertain whether data was reported at event or species level.
In the present study, sensitivity of the SeptiFast real-time PCR assay, measured at both event and species level, was poor (58.8% and 50.0% respectively), suggesting that the assay may have little value as a ‘rule-out’ test in suspected sepsis-related health-care-associated bloodstream infection. However, our patients had low pre-test probabilities for bloodstream infection at an event rate (8.7%) and at species level (9.2%). Returning a negative SeptiFast test for a patient in our study would result in no more than a 5.9% and 7.4% chance of bloodstream infection at an event and species level respectively. In addition, this information would probably be returned to clinical practice earlier than a BC result – although this was not formally tested in this study. These relatively low levels of post-test probability might suggest that returning a negative SeptiFast test result in one of our patients with suspected sepsis could lead to ruling out bloodstream infection quickly and, therefore, stopping antimicrobial therapies. However, based on these post-test probabilities, this would result in the significant risk of undertreating up to six to eight patients with sepsis-related bloodstream infection in every 100 with suspected sepsis. Effective management of sepsis-related bloodstream infection requires timely and adequate antimicrobial therapy and, based on these metrics, stopping antimicrobial therapy early in this high-risk population could not be advised on the basis of a negative SeptiFast result.
The specificity of the SeptiFast real-time PCR test was higher, 88.5% at event level and 85.8% at species level, suggesting that the test has potential value as a ‘rule-in’ test, allowing reasonable confidence that a positive test indicates the presence of a specific pathogen. However, as described above, our patients had low pre-test probabilities of bloodstream infection. Returning a positive SeptiFast test for a patient in our study was an infrequent event (less than 1 in 10 on average) but was associated with a 41% and 34% chance of bloodstream infection at an event and species level respectively. Therefore, returning a positive SeptiFast test in one of our patients with suspected sepsis does substantially increase the chance of patients having a bloodstream infection, but not to a high level. A further significant limitation of SeptiFast that is likely to restrict its impact on clinical practice is that the test does not provide information on the antibiotic sensitivities of the identified pathogen, with the exception of meticillin resistance in S. aureus. In the absence of this information, currently provided only by culture, clinicians may not be willing to refine antibiotic therapy on the basis of an organism identified by SeptiFast real-time PCR alone. 36 Development of molecular platforms with the ability to detect a broad range of resistance genes direct from blood would be an important step towards clinical utility. However, challenges remain including the broad range of genotypes involved in some resistances (e.g. extended-spectrum beta-lactamases) and the precise relationship between genotype and functional antibiotic resistance. Therefore, in our patients with suspected sepsis-related health-care-associated infection, directing antimicrobial treatment based on a positive SeptiFast result is problematic in terms of both the post-test uncertainty in the positive test and the lack of information about likely antimicrobial drug responses.
To date, no clinical trials have been conducted on the likely impact and safety of acting on the results of SeptiFast real-time PCR in patients in any setting.
Based on the results reported here, the investigators have significant reservations about the likely efficacy and safety of such a study in the setting of sepsis-related health-care-associated infection. However, this conclusion is based solely on the assumption that culture results are an error-free reference standard, an assumption we will explore further in Chapter 4.
Our study population focused on critically ill patients developing new clinical evidence for suspected sepsis who had been hospitalised for at least 48 hours in the NHS in the north-west of England. Despite the pragmatic regional focus of this study, our patients were drawn from a wide range of primary specialties, were broadly representative in terms of age and sex of critical care patients throughout England and Wales compared with national audit figures, but were more likely to be receiving higher levels of invasive organ supportive interventions and had been exposed to a high level of broad-spectrum antibiotics in the immediate period leading into study recruitment. Within this complex study-setting, there was evidence of differences in diagnostic accuracy metrics for SeptiFast between hospital sites, particularly assay specificity at the level of pathogen concordance. Specificity against BC across the three main contributor sites ranged from 91.3% (95% CI 88.2% to 93.9%) at site 1 to 74.3% (95% CI 66.5% to 81.2%) at site 3. Intriguingly, these differences were maintained when the PCR assay was compared with the enhanced reference standard, specificity ranging from 92.6% (95% CI 89.3% to 95.2%) at site 1 to 71.4% (95% CI 61.4% to 80.1%) at site 3. The reasons for these differences are unknown; however, site differences in antimicrobial use, which would be expected to impact on the level of false-positive results, and/or case mix are potential explanations.
What factors limit the diagnostic accuracy of the SeptiFast test? Poor diagnostic sensitivity is driven by the relatively high number of false-negative results produced by SeptiFast real-time PCR. A significant proportion of false negatives (12.5%) were associated with organisms not present on the SML. This finding is consistent with several previous studies57,63,76 and suggests that restricting the SeptiFast panel to the most common bloodstream pathogens has a limiting effect on the diagnostic performance of the test in practice. False negatives associated with organisms present on the SML appeared to be spread broadly across all organisms, although there were exceptions. For example, none of three cases of Candida glabrata found by BC were detected by SeptiFast real-time PCR, although, interestingly, two cases of infection with this organism were detected by SeptiFast real-time PCR in the absence of confirmatory BC. The most likely explanation of the false negatives for common, on-panel organisms is that the levels of circulating DNA may be below the limits of detection of the assay in some infection episodes. The reported detection limit for SeptiFast real-time PCR is 3–100 CFU/ml29 which compares relatively favourably to the microbial burden often associated with adult sepsis (< 10 CFU/ml). 23,97 However, this varies considerably between species with some organisms including C. glabrata, being undetectable at 3 CFU/ml blood. A similar explanation could be proposed for the performance of the test in detecting CoNS. A PCR signal was detected in 40% of cases where a culture-confirmed bloodstream infection with CoNS was adjudicated, while no PCR signal was detected in the 27 cases of positive BCs adjudicated as CoNS contaminants. Contamination during the blood collection procedure is likely to be associated with low bacterial load and may therefore be below the minimum analytical sensitivity for CoNS (30 CFUs/ml) reported for SeptiFast real-time PCR. 29
In the current study, SeptiFast real-time PCR also produced a significant number (n = 123) of false-positive results (i.e. pathogens detected by PCR in blood that are not found by BC), which lowered the specificity of the assay. Previous studies have suggested that, in many cases, the organism detected by SeptiFast real-time PCR can be found only on culture of specimens obtained from other sites of infection outside the bloodstream such as urine, bronchioalveolar lavage fluid, peritoneal fluid, etc. 41,69 This has led to the suggestion that pathogen DNA in the bloodstream may have clinical relevance in terms of the infection status of the patient even when corresponding BCs are negative and that concordance with an enhanced reference standard that includes cultures from blood and other significant specimens may be of value in assessing the diagnostic utility of SeptiFast real-time PCR. 36 To our knowledge, this is the first prospective study designed around the STARD criteria87 to assess the diagnostic accuracy of SeptiFast against an enhanced reference standard. The data show that the SeptiFast assay sensitivity was markedly lower (33.1% and 18.1% at event and species level respectively) when other microbiological findings were taken into account as part of blinded, independent infection adjudication, indicative of the high number of pathogens detected at sites outside the bloodstream that were not found by SeptiFast real-time PCR. Assay specificity was maintained using the enhanced reference standard (enhanced reference standard vs. BC: 90.8% vs. 88.5% at event level; 85.9% vs. 85.8% at species level). These data suggest that a positive SeptiFast result in the setting of suspected sepsis-related health-care-associated infection may have some diagnostic ‘rule-in’ utility as an indicator of the overall infection status of the patient. However, a negative test does not appear to have any utility in excluding infection in this setting.
The independent review and meta-analysis (see Chapter 2) of the current literature studies on SeptiFast diagnostic accuracy indicated significant deficiencies that impact on the derived diagnostic accuracy metrics with none, as reported, meeting the STARD criteria87 in full. The strengths of the present study are the systematic approach taken in the design and conduct of the study. These include assessment of the test in a defined, focused patient cohort in the setting of suspected health-care-associated bloodstream infection, prior publication of the trial protocol,1 blinding of SeptiFast real-time PCR and culture results, independent blinded clinical adjudication of bloodstream and other infections, strict adherence to clinical and CE-marked laboratory protocols and reporting of assay failure rates. The investigators recognise, however, that there remain significant weaknesses principally associated with the use of BC as the gold standard reference test. This approach provides diagnostic accuracy measures that can be directly compared with other studies in this field which have all used BC as the stated reference standard (reported in Chapter 2). However, in contrast to other studies internationally, we have also taken a Bayesian approach to describing and arguing the likely clinical utility of SeptiFast real-time PCR in the setting of a critically ill patient developing new signs of suspected sepsis while receiving complex health care – and, for the first time, this has been performed within the NHS.
It is, however, important to point out that the performance of SeptiFast real-time PCR in terms of clinical diagnostic accuracy is predicated on the assumption that BC is 100% error free, which seems unlikely. Potential sources of error include low sample volume, lack of replicate BC sets, delays in incubation and contamination during sampling. 9,98 In this study, BC was taken as part of routine clinical service and, although training in approved procedures for taking and processing BC were promoted, we cannot be certain that errors did not occur, although the mean level of BC contaminants across the four centres was consistent with current quality performance standards. A further potentially important source of error affecting the gold standard is the high level of antimicrobial use in this patient cohort. One or more antimicrobial drugs had been given within 48 hours prior to blood sampling in > 85% of the suspected bloodstream infection episodes included in the study. The impact on blood and other specimen culture performance cannot be quantified, but it is highly likely that this will be a significant factor. This study was commissioned specifically to assess the diagnostic utility of SeptiFast real-time PCR in the setting of sepsis-related health-care-associated infection where empirical broad-spectrum antibiotic therapy is frequently employed. This setting challenges the utility of culture in supporting complex clinical decisions. However, given that levels of free pathogen DNA and the intact pathogen load in the circulation are likely to be interrelated, this may also present a challenging environment for SeptiFast real-time PCR.
In Chapter 4, we will undertake a preliminary assessment of the potential impact of error in the BC gold standard through the use of latent class statistical modelling. 99 In addition, the impact on diagnostic performance of including the commonly used circulating inflammatory biomarkers white cell count, CRP and PCT as instrumental variables will be considered.
Chapter 4 Challenging the assumption that laboratory-confirmed diagnosis of bloodstream infection is an error-free gold standard: statistical modelling using latent class analysis
Rationale and aims
The implicit assumption underlying the standard method of analysis as reported in Chapter 3 is that the sensitivity and the specificity of the laboratory culture-confirmed diagnosis of bloodstream infection are both 100%. It is assumed that there are no misclassification errors – that is, if an infection is present it will be detected and if it is absent then it is correctly recorded as being absent. Current evidence suggests that BC has limitations that are likely to mean that both specificity and sensitivity of this test will be less than 100%. The wide range of factors that are known to influence the diagnostic accuracy of BC, and the supporting evidence base, are covered in detail elsewhere96 as part of the latest consensus-derived standard operating procedures that guide NHS practice. Therefore, although we believe there is a widespread understanding of the limitations of BC in the clinical community, BC is in routine use worldwide and the formal definitions and guidelines relating to the diagnosis of suspected bloodstream infection are based on this test with no currently accepted alternatives. It is therefore not surprising that the diagnostic validity of new technologies such as PCR is judged against BC.
The standard method of analysis is merely a convention and our aim in the present chapter is to (i) challenge current assumptions concerning the performance of BC, and their use as a reference standard to assess new technologies, using alternative statistical methodology100–103 – which has been discussed recently by Waikar et al. 104 – and (ii) illustrate how assumptions that are more realistic (together with additional data) may lead to different conclusions that could ultimately provide a more realistic basis for assessing the diagnostic accuracy of emerging technologies. The guiding motivation for the work reported in this chapter is that, if the prior assumptions concerning the performance of the reference standard (BC) are invalid, then inferences concerning the performance of the new competitor (SeptiFast real-time PCR) will be biased, quite possibly severely so. Full details of the proposed statistical methodology for the comparisons of two or more fallible indicators of disease have been provided previously by one of the co-authors of the present report. 99
The key to the use of the statistical methodology to be described in Statistical models is the acknowledgement that all diagnostic indicators (including microbiologically proven BC) are almost certainly fallible (that is, subject to classification errors). BCs might, for example, fail to detect certain infections (false negatives) or may be subject to contamination (false positives). Instead of basing our analyses of the characteristics of the SeptiFast real-time PCR results on unrealistic assumptions concerning the BCs, we relax these assumptions and use the data to estimate the sensitivity and specificity of both the BC and SeptiFast real-time PCR results. However, in order for this to be possible, we need further data on the likely infection status of the patient (in the examples below we use infections at additional sites and/or levels of circulating immune-inflammatory biomarkers). We also need to assume that the measurement or classification errors for the different indicators of infection are statistically independent (uncorrelated). The latter is a strong assumption but likely to hold true if the different indicators are founded on different biological processes. The statistical methods used are known as latent class analyses,99 the latent classes being the presence or absence of a bloodstream infection and ‘latent’ because we have acknowledged that none of the indicators of infection allow us to be 100% confident concerning the true infection status of the patient.
Statistical models
Stage 1
Starting with the estimation of the prevalence of infection as indicated by the reference standard – the BC. If the reference standard is infallible, then the prevalence of infection is the proportion of blood specimens yielding a positive culture. Now, if we acknowledge that the laboratory culture may actually be fallible and, in reality, it has sensitivity and specificity of αBC and βBC, respectively, then the probability of observing an infection now becomes P·αBC + (1 – P)·(1 – βBC), where P is the true prevalence, the first term represents those correctly diagnosed as having an infection, and the second term represents those that are false positives (misclassifications). Similarly, the proportion of cultures for which infection is ruled out is given by (1 – P)·βBC + P·(1 – αBC).
Again, the first term represents the correct assignments, the second mistakes (false negatives). Unfortunately, if the culture is fallible, then we cannot estimate any of P, αBC or βBC. The model is said to be under identified.
Stage 2
Now, each blood sample is classified according to the two diagnostic methods: BC and SeptiFast real-time PCR. We assume that the assignments will be associated (hopefully, strongly so) because of and only because of the underlying, but not directly observed, infection (misclassification errors for the two methods are assumed to be statistically independent – the conditional independence assumption). There are now four possibilities.
Here, αPCR and βPCR represent the sensitivity and specificity of SeptiFast real-time PCR respectively. Again, it is impossible to obtain unique estimates of the prevalence of infection and of the parameters of the two diagnostic tests from the data at hand. The only way forward is to either (a) make some simplifying assumptions or (b) use additional data to help rescue us from the problem.
Simplifying assumptions
The conventional analysis carried out in Chapter 3 assumes that αBC = βBC = 1 (i.e. 100%). However, it could be argued that a far more realistic (but perhaps still imperfect) set of assumptions would be that βBC = βPCR = 1 (i.e. 100%). Here there are no false positives – if either of the two tests yields a positive result then the assumption is that there is an infection present. This has a very simple and straightforward implication for the estimation of the two remaining test characteristics (the sensitivities αBC and αPCR). With the new pair of assumptions the expected patterns are the following.
It immediately follows that αBC is the proportion when both are positive divided by the overall proportion of SeptiFast real-time PCR positives. Similarly αPCR is the proportion when both are positive divided the overall proportion of BC positives.
We now return to the data on episode concordance between LightCycler SeptiFast real-time PCR and BC (Table 18; originally presented in Table 6).
| BC positive | BC negative | Total | |
|---|---|---|---|
| SeptiFast positive | 47 | 97 | 144 |
| SeptiFast negative | 33 | 745 | 778 |
| Total | 80 | 842 | 922 |
The estimate of the sensitivity of the SeptiFast real-time PCR (αPCR) is 47/80 = 0.588 (i.e. ≈60%) – exactly the same as in the main analysis of Chapter 3. As already noted, this is not as high as we would wish and will have significant impact on SeptiFast predictive value when applied to an episode of suspected infection. The corresponding estimate of the sensitivity of BC (αBC), however, is 47/144 = 0.326 (≈33%), which is much worse and not what would be expected of a reliable, well-performing reference standard. If the new assumptions are correct, the implications are that both tests are failing to detect a significant proportion of the true infections present. Of course these remain arbitrary assumptions.
Additional data
We will now consider the impact of introducing a third diagnostic test that is assumed to be conditionally independent of the first two. This is the presence of microbiologically proven infections at additional body sites, addressed in Chapter 3 as a component of the enhanced reference standard. The sensitivity and specificity of the additional infection sites (ASs) are represented by αAS and βAS respectively. We now have eight possibilities for the test results.
This model can be fitted to the count data without any further assumptions. The model could be further refined by allowing the prevalence of infection, P, to vary from one hospital site to another, thereby eliminating confounding effects of hospital site. Unfortunately, there are no simple arithmetic ways of fitting these models, making it necessary to use computationally intense optimisation procedures, which are described in Model fitting and parameter estimation. Additionally, instead of using infection at an additional site as the third diagnostic test we could use circulating inflammatory biomarker data (e.g. circulating CRP and PCT levels) on the assumption that they are related to true infection but conditional on true infection, independent of the other two diagnostic tests. This is using biomarker data as an instrumental variable, which will also be covered in the following section.
Count data for the three diagnostic tests (BC, SeptiFast real-time PCR and AS) are shown in Table 19. In the current analysis, models were fitted to the combined data from three hospital sites; hospital 4 was excluded on the grounds of too few data.
| Pattern | Observed count | |||
|---|---|---|---|---|
| Hospital 1 | Hospital 2 | Hospital 3 | Total | |
| All three –ve | 317 | 211 | 78 | 606 |
| BC –ve, PCR –ve, AS +ve | 52 | 47 | 32 | 131 |
| BC –ve, PCR +ve, AS –ve | 24 | 28 | 16 | 63 |
| BC +ve, PCR –ve, AS –ve | 19 | 4 | 4 | 27 |
| BC –ve, PCR +ve, AS +ve | 9 | 10 | 11 | 30 |
| BC +ve, PCR +ve, AS –ve | 19 | 4 | 5 | 33 |
| BC +ve, PCR –ve, AS +ve | 2 | 2 | 2 | 6 |
| All three +ve | 4 | 3 | 7 | 14 |
Model fitting and parameter estimation
The statistical model provided by description in Statistical models is an example of a latent class or finite mixture model. Unless we are prepared to assume that one of the tests is providing the truth, presence or absence of infection cannot be directly observed but is inferred from the observed pattern of test results. Fitting the model was carried out using maximum likelihood – specifying the Maximum Likelihood Robust option – via the expectation–maximisation algorithm using Mplus version 7.0 software (Muthén & Muthén, Los Angeles, CA, USA). 105 All models allowed for the prevalence of infection to vary with hospital, thus eliminating the latter as a source of confounding. Appendix 16 provides a listing of a typical Mplus command (input) file. Dunn99 provides full details of the rationale and technical aspects of latent class/finite mixture models for diagnostic test data. Data from hospital 4 (12 subjects) were excluded from all analyses on the grounds of too few data (resulting n = 910).
Results
First, the model was fitted using data from only two indicators (BC and SeptiFast real-time PCR), allowing infection prevalence to vary with hospital. Assuming that the laboratory culture is infallible, the following parameter estimates for SeptiFast real-time PCR are obtained: sensitivity, αPCR, = 58.8% (95% CI 49.7% to 67.8%) and specificity, βPCR, = 88.8% (95% CI 87.0% to 90.6%) and the prevalence of infection is estimated to be 8.7%. Assuming the specificities of both BC and SeptiFast real-time PCR to be 100% yields the following estimates: αBC = 33.6% (95% CI 27.0% to 40.1%) and αPCR = 58.8% (95% CI 49.7% to 67.8%). The estimated prevalence of infection has now risen to 26.2%. Note that, as indicated earlier, the data cannot be used to distinguish these two models, or allow the relaxation of the competing assumptions, without the use of further information as illustrated below.
Second, the model was fitted for data from three different indicators of infection (BC, SeptiFast real-time PCR and infection at ASs). As there are now three indicators, assumed to be conditionally independent (i.e. with uncorrelated misclassification errors) the model can be fitted without any unrealistic assumptions concerning the performance of any of the three tests. Six parameters representing the sensitivities and specificities of the three tests (plus the prevalence of infection) can be estimated. The results are shown in Table 20(a).
| Test | Sensitivity, % (95% CI) | Specificity, % (95% CI) |
|---|---|---|
| (a) Using AS as the third indicator of infection | ||
| BC | 33.3 (25.2 to 41.3) | 96.8 (93.6 to 99.9) |
| SeptiFast real-time PCR | 83.0 (44.1 to 100) | 100 (100 to 100) |
| AS | 31.7 (24.0 to 39.4) | 82.8 (79.6 to 86.0) |
| Prevalence of infection ≈18.5% | ||
| (b) Using dichotomised PCT as the third indicator of infection | ||
| BC | 32.7 (24.1 to 41.2) | 97.1 (95.1 to 99.2) |
| SeptiFast real-time PCR | 77.4 (52.9 to 100) | 100 (100 to 100) |
| PCT | 37.1 (29.2 to 45.1) | 88.0 (84.8 to 91.1) |
| Prevalence of infection ≈19.9% | ||
| (c) Using AS and dichotomised PCT as the third and fourth indicator of infection respectively | ||
| BC | 31.6 (24.1 to 41.2) | 97.4 (95.4 to 99.5) |
| SeptiFast real-time PCR | 69.5 (40.3 to 98.7) | 99.4 (91.8 to 100) |
| AS | 33.2 (20.0 to 46.4) | 83.8 (79.9 to 87.6) |
| PCT | 38.4 (24.3 to 52.6) | 88.8 (84.6 to 93.1) |
| Prevalence of infection ≈21.4% | ||
| (d) Using a combination of SeptiFast real-time PCR and dichotomised PCT as a combined indicator of infection | ||
| BC | 21.7 (16.3 to 27.0) | 96.2 (93.4 to 98.9) |
| Combined (SeptiFast real-time PCR/PCT) | 96.6 (57.4 to 100) | 100 (100 to 100) |
| AS | 28.8 (22.6 to 35.0) | 83.5 (79.5 to 87.5) |
| Prevalence of infection ≈27.7% | ||
Finally, the potential utility of circulating levels of CRP and PCT measurements was investigated. The summary statistics for each of the two measures is presented according to BC status and again by SeptiFast real-time PCR status. The results are shown in Table 21. CRP does not appear promising but the PCT measurements are associated (in the right direction) with both BC and SeptiFast real-time PCR outcomes. To use PCT as an instrumental variable it is assumed that this association arises solely from PCT’s association with the underlying infection status. The advantage of using this measure as an instrument as opposed to ASs is that it is a marker determined by a completely different process from those used to assess BC and PCR positivity. Although it is not absolutely certain that PCT is a valid instrument (i.e. its measurement errors are not associated with the misclassification errors for BC and/or SeptiFast real-time PCR), the assumption does have a degree of face validity.
| Variable | Observed | Mean | Standard deviation | Minimum | Maximum |
|---|---|---|---|---|---|
| BC negative | |||||
| CRP (mg/l) | 819 | 163.30 | 116.40 | 1 | 690 |
| PCT (ng/ml) | 799 | 7.274 | 23.479 | 0.05 | 200 |
| BC positive | |||||
| CRP (mg/l) | 79 | 141.88 | 123.11 | 0.5 | 534 |
| PCT (ng/ml) | 78 | 12.487 | 31.451 | 0.05 | 163.22 |
| SeptiFast real-time PCR negative | |||||
| CRP (mg/l) | 756 | 157.82 | 116.64 | 0.5 | 690 |
| PCT (ng/ml) | 741 | 5.809 | 19.807 | 0.05 | 200 |
| SeptiFast real-time PCR positive | |||||
| CRP (mg/l) | 142 | 180.57 | 118.031 | 2.7 | 539 |
| PCT (ng/ml) | 136 | 18.244 | 39.434 | 0.052 | 200 |
When PCT was included in the model for the two binary indicators, the results (not shown) indicated that the estimated mean PCT for those without infection (as predicted by the model) was 4.0 ng/ml (standard error 0.5 ng/ml) and was, for the infected participants, very much higher at 129.5 ng/ml (standard error 17.5 ng/ml). Estimated sensitivities for the BC and SeptiFast real-time PCR were 18.8% (95% CI 2.5% to 35.0%) and 50.0% (95% CI 29.6% to 70.3%) respectively. Corresponding specificity estimates were 91.5% (95% CI 89.7% to 93.4%) and 85.7% (95% CI 83.4% to 88.0%). Accordingly, the PCT measurements were dichotomised to create a binary indicator of infection in the setting of suspected sepsis (positive if PCT > 6 ng/ml – reflecting the median PCT level that indicates systemic inflammation resulting from severe systemic infection such as in the bloodstream;106 negative if PCT ≤ 6 ng/ml). No attempt was made to optimise the value of this cut-point – the idea being to simply illustrate the power of the method. The latent class/finite mixture model was refitted using dichotomised PCT measurements as a third, independent indicator of infection status. As before, both the sensitivity and specificity of the BC and of the SeptiFast real-time PCR were left free to be estimated. The results are given in Table 20(b). Note that the model fitting does not require that all 910 participants have PCT data – the model is fitted to all available data. Concordances between the BC and dichotomised PCT, and between SeptiFast real-time PCR and dichotomised PCT, are shown in Tables 22 and 23, respectively.
| BC positive | BC negative | Total | |
|---|---|---|---|
| Dichotomised PCT positive | 20 | 139 | 159 |
| Dichotomised PCT negative | 58 | 660 | 718 |
| Total | 78 | 799 | 877 |
| SeptiFast PCR positive | SeptiFast PCR negative | Total | |
|---|---|---|---|
| Dichotomised PCT positive | 52 | 107 | 159 |
| Dichotomised PCT negative | 84 | 634 | 718 |
| Total | 136 | 741 | 877 |
A latent class/finite mixture model was then fitted to all four indicators of infection (BC, SeptiFast real-time PCR, AS and PCT). The resulting estimates are shown in Table 20(c).
How might one develop a better biomarker of infection? Clearly, a combination of the diagnostic test results has the potential to have a considerably higher sensitivity than when they are used alone, although specificity might be lowered. In order to illustrate the promise of this approach the SeptiFast real-time PCR and PCT results were combined: an infection was indicated if the SeptiFast real-time PCR result was positive or if the PCT result was positive, or if both were positive. If neither test was positive then the combined test result was negative. Table 20(d) provides estimates of the sensitivity and specificity of the combined SeptiFast real-time PCR/PCT marker obtained through fitting a latent class model using BC and infection at an additional site as the other two conditionally independent indicators. Table 24 summarises the estimated diagnostic accuracy characteristics of SeptiFast real-time PCR incorporating different instrumental variables. The performance of the combined SeptiFast real-time PCR/PCT marker looks very promising as both sensitivity and specificity are at, or very close to, 100%. We stress, however, that this is only a preliminary result – further work needs to be done on algorithms for the optimal combination of indicators of infection, using both existing data to generate well-performing algorithms and new data for a thorough validation of their performance.
| Assumptions | Additional information [instrumental variable(s)] | Estimates for BC | Estimates for SeptiFast real-time PCR |
|---|---|---|---|
| 1 | |||
| BC sensitivity 100% | – | Sensitivity 58.8% | |
| BC specificity 100% | Specificity 88.5% | ||
| 2 | |||
| BC specificity 100% | – | Sensitivity 32.6% | |
| SeptiFast real-time PCR specificity 100% | Sensitivity 58.8% | ||
| Independent errors | |||
| 3 | |||
| Independent errors | AS | Sensitivity 33.3% | Sensitivity 83.0% |
| Specificity 96.8% | Specificity 100% | ||
| 4 | |||
| Independent errors | Dichotomised PCT | Sensitivity 32.7% | Sensitivity 77.4% |
| Specificity 97.1% | Specificity 100% | ||
| 5 | |||
| Independent errors | AS and dichotomised PCT | Sensitivity 31.6% | Sensitivity 69.5% |
| Specificity 97.4% | Specificity 99.4% | ||
Conclusion and implications
Overall, the results of the latent class/finite mixture modelling lead us to conclude the following:
-
The reference standard (BC) used routinely to assess the diagnostic accuracy of new tests of bloodstream infection, including in the present study, should not be assumed to be a perfect – infallible – indicator of infection. In particular, BC appears to have a worryingly low sensitivity (≈ 40%, at most).
-
The sensitivity of the SeptiFast test of 60–80% is also less than ideal for a useful diagnostic test but appears much better than that of the reference standard (BC).
-
The specificities of BC and SeptiFast PCR are both high (> 95%) with blood samples identified as positive by either method from our study population of suspected sepsis-related health-care-associated bloodstream infection having a high probability of infection. Data from additional body sites and from circulating biomarkers such as PCT appear to be additional indicators of infection and a dichotomised PCT measurement appears to be as sensitive as BC and only marginally less specific.
-
It appears likely that the lone use of the reference (BC) in clinical settings could lead to seriously misleading estimates of the prevalence of infection. Data reported here suggest that in the clinical setting of suspected health-care-associated sepsis, BC might be failing to detect 60–70% of all infections.
-
The less than ideal clinical diagnostic sensitivity of SeptiFast real-time PCR will impact on the associated likelihood ratios and predictive values, even in the presence of high specificity, resulting in more limited diagnostic utility when translating evidence derived from our study population to an individual patient episode. These results indicate that SeptiFast real-time PCR should not replace traditional BC but could be added to the diagnostic test battery together with data on infections from other body sites and on levels of biomarkers such as PCT. Algorithms for the optimal use of such a test battery should be the subject of further work as illustrated by the final latent class model to assess the performance of a combined SeptiFast real-time PCR/PCT marker, above.
-
Other investigators should seriously consider abandoning the assumption that BC is an infallible gold standard for infection.
-
Based on the provisional analysis results reported in this chapter, there are two situations that might be worthy of consideration for future intervention studies based on the potential for SeptiFast real-time PCR as a more accurate rapid ‘rule-in’ test for pathogens in blood samples:
-
In the setting of suspected sepsis-related bloodstream infection, what is the impact on antimicrobial stewardship in the setting of a positive SeptiFast test?
-
In the setting of interventional studies investigating bloodstream infection (e.g. a clinical trial investigating the effectiveness of different durations of antimicrobial therapy), what is the utility of identifying patient cohorts rapidly based on SeptiFast testing rather than relying solely on the results of BC?
-
Chapter 5 Conclusions and recommendations
Main findings
Systematic review of clinical diagnostic accuracy studies for SeptiFast
A wide range of clinical diagnostic accuracy studies have been performed internationally comparing SeptiFast real-time PCR with BC in the setting of suspected sepsis. No diagnostic accuracy studies have focused solely on health-care-associated infections and very few NHS patients have had the opportunity to contribute to this evidence base. Synthesising reported data suggests that, although the diagnostic accuracy is not ideal, SeptiFast may provide better rule-in than rule-out utility for the rapid detection and identification of pathogens in patients’ blood samples. However, independent review of the quality of these studies revealed important design and reporting deficiencies that rendered this body of work potentially unsafe owing to a high risk of bias from a variety of sources.
Phase III multicentre clinical diagnostic accuracy study performed within the NHS
In the setting of suspected sepsis-related health-care-associated bloodstream infection, SeptiFast real-time PCR, when compared with BC as reference standard, showed higher specificity (85.8%, 95% CI 83.3% to 88.1%) than sensitivity (50%, 95% CI 39.1% to 60.8%) at pathogen genus/species level suggesting better rule-in than rule-out utility for the rapid detection and identification of pathogens in blood samples from a population of patients. When compared with the synthesised combined diagnostic metrics derived from our systematic review, this new Phase III clinical data revealed lower overall test accuracy of SeptiFast mainly as a result of an unacceptably low diagnostic sensitivity. Furthermore, there was a very low prevalence of culture-proven bloodstream infection in this patient cohort such that the post-test probabilities of both a positive and a negative SeptiFast test indicate the potential limitations to confidently rule in or rule out, respectively, a diagnosis for an individual patient. When compared with a culture-proven enhanced reference standard, including bloodstream and other infection sources, the specificity of SeptiFast real-time PCR was maintained although sensitivity was markedly lower, suggesting that a positive SeptiFast real-time PCR result may have some diagnostic utility as an indicator of the overall infection status of the patient. However, the test does not appear to have any utility in excluding infection in this setting.
An important caveat to all of the conclusions from the Phase III study is that SeptiFast diagnostic accuracy was, by convention, measured against culture reference standards that are prone to error, particularly in the setting of high antimicrobial use.
Latent class analysis of Phase III NHS study data
Using latent class analysis to take account of error in the culture reference standard, BC was shown to have a low diagnostic sensitivity in the setting of suspected sepsis-related health-care-associated bloodstream infection. The sensitivity of the SeptiFast real-time PCR test is also less than ideal for a useful diagnostic test but appears much better than BC. Blood samples identified as positive by either BC or SeptiFast real-time PCR in our study population have a high probability of having infection. Data from additional body sites and from circulating biomarkers, such as PCT, appear to be additional indicators of infection and a dichotomised PCT measurement appears to be as sensitive as the BC and only marginally less specific. Preliminary investigations suggest that combinations of these biomarkers show promise in terms of achieving higher diagnostic accuracy in our patient cohort.
Limitations
Study limitations
Reference standard
Blood culture should not be assumed to be a perfect (infallible) indicator of infection and, as demonstrated in our latent class analyses, provides a worryingly poor reference standard against which new molecular technologies are compared. This is likely to be particularly relevant in our setting of high antimicrobial use which will have a significant negative impact on the bacterial load and, therefore, the analytical sensitivity of BC. Conventional clinical diagnostic accuracy test analyses in this setting may derive unsafe results, even when the clinical study has been designed and reported adequately. In addition, systematic reviews of studies based on a tarnished reference standard will also produce potentially unreliable results. We have attempted to mitigate these limitations with our new clinical data using additional latent class and instrumental variable analyses, providing new insights into clinical diagnostic accuracy studies in this field.
Patient cohort
By design, our clinical diagnostic accuracy study was focused on health-care-associated bloodstream infection in critically ill patients developing new clinical signs of suspected sepsis. This focus was determined by the funding body and aligns with the international consensus on sepsis diagnosis and management which is that non-culture-based rapid diagnostic technologies are likely to have the greatest utility in settings where patients have been exposed to antimicrobial therapies. 11 Our patient cohort had been exposed to high levels and a wide range of combination antimicrobial therapies immediately before study sampling, providing an opportunity to investigate clinical diagnostic accuracy in a challenging setting, but with the added complexity of a tarnished traditional culture-based reference standard (as described above). Although we have attempted to mitigate these limitations using the alternative analytical approaches of latent class and instrumental variable analyses, we do not know how well our results will generalise to a community-acquired sepsis cohort, that are less likely to have been exposed to broad-spectrum antibiotics usually reserved for hard to treat infections.
Technology limitations
Limitations in the SeptiFast real-time PCR technology may have contributed to its modest diagnostic performance observed in this clinical setting, particularly related to low diagnostic sensitivity.
Limits of detection
Previous evidence suggests that the numbers of organisms present in the blood of patients with culture-confirmed bloodstream infection can often be relatively low (in the range 1–10 CFU/ml). Published data on the laboratory analytical performance of the SeptiFast real-time PCR platform suggest that, for some species, these levels could be at, or below, the lower limits of detection of the assay which may explain the relatively high number of false negatives returned by the assay panel. This situation may be exacerbated in settings such as the current one, where there is significant prior use of broad-spectrum antimicrobials that may be expected to lower pathogen load further.
Pathogen coverage
SeptiFast real-time PCR is designed to detect 25 of the most commonly reported pathogens causing bloodstream infections. Although the majority of pathogens detected in our study were found on the SeptiFast panel, a significant percentage of the episodes of bloodstream infection were associated with organisms not present on the panel and therefore undetectable by SeptiFast real-time PCR, contributing to poor assay sensitivity. Though individual off-panel organisms may not be common at a population level in this setting, the impact of not detecting these organisms would have a significant impact at the individual patient level and, thus, a lack of clinical confidence in acting on negative SeptiFast real-time PCR results.
Assay failures
The SeptiFast assay was associated with a relatively high percentage of assay failures with the majority of these (5.5% of analysed samples) associated with failure of the IC. The IC monitors the efficiency of the PCR reaction and is particularly important for ensuring the validity of negative assay results. The reasons for IC failure are unknown, although it is interesting that the majority of such samples analysed for a second time continued to report IC failure. Assay failure rates for SeptiFast real-time PCR are rarely reported, although two recent studies have shown even higher failure rates of 12.5%31 and 24.2%. 32
Implications for practice
When compared with NHS service culture standards, the clinical diagnostic accuracy of SeptiFast appears unlikely to result in sufficient diagnostic utility in the setting of suspected sepsis-related health-care-associated infection, despite its potential to deliver results more rapidly.
Using preliminary analyses that take into account the possibility that culture standards are not completely error free, SeptiFast real-time PCR may have a greater ability to rule in infection than may be apparent from conventional analyses – although its diagnostic sensitivity remains inadequate such that clinical utility may remain significantly compromised.
In further preliminary analyses, other circulating biomarkers, such as PCT, when used in combination with SeptiFast real-time PCR, may improve clinical diagnostic accuracy to levels where clinical utility is far more likely.
Based on the present study, we found no evidence that SeptiFast real-time PCR should replace traditional BC but the assay could be added to the diagnostic test battery (together with data on infections from other body sites and levels of biomarkers such as PCT). Algorithms for the optimal use of such a test battery should be the subjects of further work.
We do not know whether our findings are relevant to patients with suspected community-acquired sepsis as they were not investigated and there is a lack of high-quality diagnostic evidence in relation to this setting.
Recommendations for research
Clinical research
-
Develop new strategies for assessing the clinical validity of diagnostic tests that do not rely exclusively on evidence derived from traditional analyses of diagnostic accuracy where reference standards are known to be prone to error (e.g. BC in the setting of high antimicrobial use).
-
Explore further the application of multivariate modelling techniques (e.g. latent class analysis) in diagnostic accuracy studies to assess the potential value of additional biomarkers and/or account for error in reference standards.
-
Validate diagnostic pathways in stratified populations of patients with health-care-associated and community-acquired sepsis.
-
Conduct further analyses using diagnostic and therapeutic data from stratified populations, using techniques such as risk–benefit analyses and decision analyses, to develop an understanding of the likely effectiveness of SeptiFast real-time PCR and other emerging rapid diagnostic tests in the clinical setting of sepsis.
-
Based on the current study data and outcomes from the above recommendations, consider future intervention studies based on the potential for SeptiFast real-time PCR as a rapid ‘rule-in’ test for pathogens in blood samples in the setting of:
-
suspected sepsis-related bloodstream infection, asking what is the impact on antimicrobial stewardship in the setting of a positive SeptiFast test?
-
interventional studies investigating bloodstream infection (e.g. a clinical trial investigating the effectiveness of different durations of antimicrobial therapy), asking what is the utility of identifying patient cohorts rapidly based on SeptiFast testing rather than relying solely on the results of BC?
-
-
Develop collaborative guidelines and funding initiatives with NHS stakeholders, including the NIHR, to co-ordinate biobanking of samples and, crucially, clinical information/phenotype from patients with sepsis. This will put the NHS in a prime position to lead on co-ordinating HTA of the vast array of technologies emerging in this field aimed at meeting the Chief Medical Officer’s challenges laid out in her recent report on infection and antibiotic resistance. 2
-
Encourage better adherence to internationally recognised guidelines in the reporting of results of diagnostic accuracy studies.
Technology development
Each of the following generic recommendations are informed by our considerable experience with SeptiFast real-time PCR and a range of other assays, and are aimed at improving the accuracy of nucleic acid amplification tests, particularly focused on improving clinical diagnostic sensitivity.
-
Increase analytical sensitivity of nucleic acid amplification assays through more efficient pathogen DNA extraction techniques and/or increasing volume of blood extracted.
-
Widen pathogen coverage or develop tests in which the pathogen panel can be more easily modified to account for differences in pathogen spectrum in particular settings.
-
Explore alternative paradigms for use of different nucleic acid amplification tests to support clinical decision-making at different stages of patient management. For example, development of rapid, low-cost screening tests capable of detecting a broad range of bacterial and fungal DNA that could be performed more frequently. Such tests could be used to support, for instance, rule-out decisions at an early stage. There is also the potential to combine nucleic acid amplification tests with other circulating biomarkers to improve diagnostic accuracy. This approach could inform selective deployment of higher-cost molecular platforms designed to subsequently identify pathogen species and microbial resistance genes.
Acknowledgements
Health Technology Assessment trial investigators
Professor Geoffrey Warhurst.
Dr Paul Dark.
Dr Paul Chadwick.
Professor Gordon Carlson.
Dr Andrew Bentley.
Professor Graham Dunn.
Dr Duncan Young.
Health Technology Assessment Trial Steering Committee
Dr Neil Pendleton (chair), Rachel Georgiou (sponsor representative), Professor John Wain (independent member), Rosemary Martin (patient representative), Professor Geoffrey Warhurst, Dr Paul Dark, Professor Gordon Carlson, Dr Duncan Young, Dr Andrew Bentley, Professor Graham Dunn and Dr Paul Chadwick.
We wish to thank:
The NIHR HTA programme for funding this study.
The Intensive Care Foundation (UK), which provided support to Professor Daniel McAuley and Professor Gavin Perkins in their roles as co-directors of the foundation.
Staff in the Research and Development department at SRFT (the study sponsors) for their support and advice throughout the study.
Clinical and nursing staff at participating hospitals
SRFT: Dr Murad Ghrew, Consultant in Respiratory and Intensive Care Medicine; Tracey Evans, Senior Research Nurse and Trial Recruitment Co-ordinator; and Elaine Cooke, Research Nurse.
University Hospital of South Manchester NHS Foundation Trust: Dr John Hopper, Consultant in Anaesthesia and Intensive Care; Alison Royle, Research Nurse; and Alison Middleton, Research Nurse.
Central Manchester Foundation Trust: Dr John Moore, Consultant in Anaesthesia and Intensive Care; Dr Mike Sharman, Consultant in Respiratory and Intensive Care Medicine; Dr Jane Eddlestone, Consultant in Anaesthesia and Intensive Care; and Elaine Coghlan, Research Nurse.
East Lancashire Hospitals Trust: Dr Paul Dean, Consultant in Anaesthesia and Intensive Care; Lynne Bullock, Research Nurse; Martin Bland, Research Nurse; and Donna Harrison-Griggs, Research Nurse.
Laboratory scientists and support staff in the Biomedical Research Facility at Salford Royal NHS Foundation Trust
Dr Satanayarana Maddi, Postdoctoral Research Assistant; Claire Wilson, NIHR Trial Intern; Daniel Graham, NIHR Trial Intern; Kate Timms, NIHR Trial Intern; Matthew Langley, NIHR Trial Intern; Pamela Davies, Senior Technician and Laboratory Manager; and Norman Higgs, Senior Research Technician.
Health-care workers and laboratory staff at the acute care NHS trusts named in this report who facilitated patient participation in this study.
Contributions of authors
Professor Geoffrey Warhurst (chief investigator), Consultant Biomedical Scientist, SRFT, Honorary Professor of Biomedicine, University of Salford: conceived the study, had overall responsibility for laboratory aspects of the study, was involved in design of the diagnostic accuracy study and systematic review, and prepared final report with PD.
Dr Paul Dark (principal clinical investigator), Consultant in Intensive Care Medicine, Reader in Institute of Inflammation and Repair, University of Manchester: conceived the study, had overall clinical responsibility for the design and conduct of the diagnostic accuracy study and systematic review, and prepared final report with GW.
These authors contributed exclusively to the NIHR-HTA funded diagnostic accuracy study as follows:
Professor Graham Dunn (Trial Statistician), Professor of Biomedical Statistics, University of Manchester: contributed to the design of the study, led on the statistical design and analysis of the data, and reviewed a draft of the report.
Dr Paul Chadwick, Consultant Microbiologist, Salford Royal NHS Foundation Trust: contributed to the design of the study, provided microbiological expertise and reviewed a draft of the report.
Dr Andrew Bentley, Consultant in Respiratory and Intensive Care Medicine, University Hospital of South Manchester: contributed to the design and conduct of the study, and reviewed a draft of the report.
Dr Duncan Young, Senior Lecturer and Consultant in Anesthaesia and Intensive Care, John Radcliffe Hospital, Oxford: contributed to study design as expert in clinical trials and reviewed a draft of the report.
Professor Gordon L Carlson, Consultant Gastro-intestinal Surgeon, Salford Royal NHS Foundation Trust: contributed to study design as an expert in the management of health-care-associated sepsis and reviewed a draft of the report.
These authors contributed exclusively to the systematic review as follows:
Dr Bronagh Blackwood, Senior Lecturer, Centre for Infection and Immunity, Queen’s University Belfast: helped developed the initial architecture, and contributed to production and revision of the systematic review report.
Professor Daniel McAuley, Centre for Infection and Immunity, Queen’s University Belfast: provided critical care and clinical trial input, and production and revision of the systematic review report.
Professor Gavin D Perkins, Clinical Professor in Critical Care Medicine, Warwick University: provided critical care and clinical trial input, and production and revision of the systematic review report.
Dr Ronan McMullen, Consultant in Medical Microbiology, Royal Victoria Hospital, Belfast: provided microbiological input, and production and revision of the systematic review report.
Professor Simon Gates, Professor of Clinical Trials, Warwick University: provided statistical leadership and contributed to the production and revision of the systematic review report.
Publications
Dark P, Blackwood B, Gates S, McAuley D, Perkins GD, McMullan R, et al. Accuracy of LightCycler – SeptiFast for the detection and identification of pathogens in the blood of patients with suspected sepsis: a systematic review and meta-analysis. Intensive Care Med 2015;41:21–33.
Warhurst G, Maddi S, Dunn G, Ghrew M, Chadwick P, Alexander P, et al. Diagnostic accuracy of SeptiFast multi-pathogen real-time PCR in the setting of suspected health-care associated bloodstream infection. Intensive Care Med 2015;41:86–93.
Disclaimers
This report presents independent research funded by the National Institute for Health Research (NIHR). The views and opinions expressed by authors in this publication are those of the authors and do not necessarily reflect those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health. If there are verbatim quotations included in this publication the views and opinions expressed by the interviewees are those of the interviewees and do not necessarily reflect those of the authors, those of the NHS, the NIHR, NETSCC, the HTA programme or the Department of Health.
References
- Reducing Healthcare Associated Infection in Hospitals in England. London: National Audit Office HC 560; 2009.
- Davies PD. Infections and the Rise of Antimicrobial Resistance. London: Department of Health; 2013.
- Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med 2013;173:2039-46. http://dx.doi.org/10.1001/jamainternmed.2013.9763.
- Pittet D, Tarara D, Wenzel RP. Nosocomial bloodstream infection in critically ill patients. Excess length of stay, extra costs, and attributable mortality. JAMA 1994;271:1598-601. http://dx.doi.org/10.1001/jama.1994.03510440058033.
- Health Protection Agency . Healthcare-Associated Infections and Antimicrobial Resistance: 2009 10 n.d. www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1281954479045 (accessed November 2013).
- Edgeworth JD, Treacher DF, Eykyn SJ. A 25-year study of nosocomial bacteremia in an adult intensive care unit. Crit Care Med 1999;27:1421-8. http://dx.doi.org/10.1097/00003246-199908000-00002.
- Calandra T, Cohen J. The international sepsis forum consensus conference on definitions of infection in the intensive care unit. Crit Care Med 2005;33:1538-48. http://dx.doi.org/10.1097/01.CCM.0000168253.91200.83.
- Investigation of BCs (for Organisms Other Than Mycobacterium Species). National Standard Method. London: Standards Unit; 2005.
- Weinstein MP. BC contamination: persisting problems and partial progress. J Clin Microbiol 2003;41:2275-8. http://dx.doi.org/10.1128/JCM.41.6.2275-2278.2003.
- Cohen J. The immunopathogenesis of sepsis. Nature 2002;420:885-91. http://dx.doi.org/10.1038/nature01326.
- Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock. Crit Care Med 2012;41:580-637. http://dx.doi.org/10.1097/CCM.0b013e31827e83af.
- Bone RC, Sibbald WJ, Sprung CL. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest 1992;101:1481-3. http://dx.doi.org/10.1378/chest.101.6.1481.
- Kollef MH, Sherman G, Ward S, Fraser VJ. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest 1999;115:462-74. http://dx.doi.org/10.1378/chest.115.2.462.
- Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006;34:1589-96. http://dx.doi.org/10.1097/01.CCM.0000217961.75225.E9.
- Cavaillon J-M, Adrie C. Sepsis and Non-Infectious Systemic Inflammation: From Biology to Critical Care. Weinheim: Wiley-VCH; 2009.
- Sprung CL, Sakr Y, Vincent JL, Le Gall JR, Reinhart K, Ranieri VM, et al. An evaluation of systemic inflammatory response syndrome signs in the Sepsis Occurrence in Acutely ill Patients (SOAP) study. Intensive Care Med 2006;32:421-7. http://dx.doi.org/10.1007/s00134-005-0039-8.
- Brun-Buisson C. The epidemiology of the systemic inflammatory response. Intensive Care Med 2000;26:S64-74. http://dx.doi.org/10.1007/s001340051121.
- Moudgal V, Sobel JD. Clostridium difficile colitis: a review. Hosp Pract 2012;40:139-48. http://dx.doi.org/10.3810/hp.2012.02.954.
- The Evolving Threat of Antimicrobial Resistance: Options for Action. Geneva: World Health Organization; 2012.
- Peters RP, van Agtmael MA, Danner SA, Savelkoul PH, Vandenbroucke-Grauls CM. New developments in the diagnosis of bloodstream infections. Lancet Infect Dis 2004;4:751-60. http://dx.doi.org/10.1016/S1473-3099(04)01205-8.
- Dark PM, Dean P, Warhurst G. Bench-to-bedside review: the promise of rapid infection diagnosis during sepsis using polymerase chain reaction-based pathogen detection. Crit Care 2009;13:217-22. http://dx.doi.org/10.1186/cc7886.
- Bauer M, Reinhart K. Molecular diagnostics of sepsis – where are we today?. Int J Med Microbiol 2010;300:411-13. http://dx.doi.org/10.1016/j.ijmm.2010.04.006.
- Arpi M, Bentzon MW, Jensen J, Frederiksen W. Importance of blood volume cultured in the detection of bacteremia. Eur J Clin Microbiol Infect Dis 1989;8:838-42. http://dx.doi.org/10.1007/BF02185857.
- Mermel LA, Maki DG. Detection of bacteremia in adults: consequences of culturing an inadequate volume of blood. Ann Intern Med 1993;119:270-2. http://dx.doi.org/10.7326/0003-4819-119-4-199308150-00003.
- Bretagne S, Costa JM. Towards a molecular diagnosis of invasive aspergillosis and disseminated candidosis. FEMS Immunol Med Microbiol 2005;45:361-8. http://dx.doi.org/10.1016/j.femsim.2005.05.012.
- Wellinghausen N, Kochem AJ, Disqué C, Mühl H, Gebert S, Winter J, et al. Diagnosis of bacteremia in whole-blood samples by use of a commercial universal 16S rRNA gene-based PCR and sequence analysis. J Clin Microbiol 2009;47:2759-65. http://dx.doi.org/10.1128/JCM.00567-09.
- Wolk DM, Kaleta EJ, Wysocki VH. PCR-electrospray ionization mass spectrometry: the potential to change infectious disease diagnostics in clinical and public health laboratories. J Mol Diagn 2012;14:295-304. http://dx.doi.org/10.1016/j.jmoldx.2012.02.005.
- Ozbak H, Dark P, Maddi S, Chadwick P, Warhurst G. Combined molecular gram typing and high-resolution melting analysis for rapid identification of a syndromic panel of bacteria responsible for sepsis-associated bloodstream infection. J Mol Diagn 2012;14:176-84. http://dx.doi.org/10.1016/j.jmoldx.2011.12.004.
- Lehmann LE, Hunfeld KP, Emrich T, Haberhausen G, Wissing H, Hoeft A, et al. A multiplex real-time PCR assay for rapid detection and differentiation of 25 bacterial and fungal pathogens from whole blood samples. Med Microbiol Immunol 2008;197:313-24. http://dx.doi.org/10.1007/s00430-007-0063-0.
- Zucol F, Ammann RA, Berger C, Aebi C, Altwegg M, Niggli FK, et al. Real-time quantitative broad-range PCR assay for detection of the 16S rRNA gene followed by sequencing for species identification. J Clin Microbiol 2006;44:2750-9. http://dx.doi.org/10.1128/JCM.00112-06.
- Anthony RM, Brown TJ, French GL. Rapid diagnosis of bacteraemia by universal amplification of 23S ribosomal DNA followed by hybridisation to an oligonucleotide array. J Clin Microbiol 2000;38:781-8.
- Rantakokko-Jalava K, Nikkari S, Jalava J, Eerola E, Skurnik M, Meurman O, et al. Direct amplification of rRNA genes in diagnosis of bacterial infections. J Clin Microbiol 2000;38:32-9.
- Hsu MC, Chen KW, Lo HJ, Chen YC, Liao MH, Lin YH, et al. Species identification of medically important fungi by use of real-time LightCycler PCR. J Med Microbiol 2003;52:1071-6. http://dx.doi.org/10.1099/jmm.0.05302-0.
- Espy MJ, Uhl JR, Sloan LM, Buckwalter SP, Jones MF, Vetter EA, et al. Real-time PCR in clinical microbiology: applications for routine laboratory testing. Clin Microbiol Rev 2006;19:165-256. http://dx.doi.org/10.1128/CMR.19.1.165-256.2006.
- Menezes LC, Rocchetti TT, Bauab Kde C, Cappellano P, Quiles MG, Carlesse F, et al. Diagnosis by real-time polymerase chain reaction of pathogens and antimicrobial resistance genes in bone marrow transplant patients with bloodstream infections. BMC Infect Dis 2013;13:166-72. http://dx.doi.org/10.1186/1471-2334-13-166.
- Pletz MW, Wellinghausen N, Welte T. Will polymerase chain reaction (PCR) based diagnostics improve outcome in septic patients? A clinical view. Intensive Care Med 2011;37:1069-76. http://dx.doi.org/10.1007/s00134-011-2245-x.
- Leitner E, Kessler HH, Spindelboeck W, Hoenigl M, Putz-Bankuti C, Stadlbauer-Köllner V, et al. Comparison of two molecular assays with conventional BC for diagnosis of sepsis. J Microbiol Methods 2013;92:253-5. http://dx.doi.org/10.1016/j.mimet.2012.12.012.
- Bloos F, Sachse S, Kortgen A, Pletz MW, Lehmann M, Straube E, et al. Evaluation of a polymerase chain reaction assay for pathogen detection in septic patients under routine condition: an observational study. PLOS ONE 2012;7. http://dx.doi.org/10.1371/journal.pone.0046003.
- Fitting C, Parlato M, Adib-Conquy M, Memain N, Philippart F, Misset B, et al. DNAemia detection by multiplex PCR and biomarkers for infection in systemic inflammatory response syndrome patients. PLOS ONE 2012;7. http://dx.doi.org/10.1371/journal.pone.0038916.
- Jordana-Lluch E, Carolan HE, Giménez M, Sampath R, Ecker DJ, Quesada MD, et al. Rapid diagnosis of bloodstream infections with PCR followed by mass spectrometry. PLOS ONE 2013;8. http://dx.doi.org/10.1371/journal.pone.0062108.
- Louie RF, Tang Z, Albertson TE, Cohen S, Tran NK, Kost GJ. Multiplex polymerase chain reaction detection enhancement of bacteremia and fungemia. Crit Care Med 2008;36:1487-92. http://dx.doi.org/10.1097/CCM.0b013e31816f487c.
- Mancini N, Clerici D, Diotti R, Perotti M, Ghidoli N, De Marco D, et al. Molecular diagnosis of sepsis in neutropenic patients with haematological malignancies. J Med Microbiol 2008;57:601-4. http://dx.doi.org/10.1099/jmm.0.47732-0.
- Molina JM, Córdoba J, Ramírez P, Gobernado M. Automatic detection of bacterial and fungal infections in the blood. Enferm Infec Microbiol Clin 2008;26:75-80. http://dx.doi.org/10.1016/S0213-005X(08)76544-3.
- Dark P, Dunn G, Chadwick P, Young D, Bentley A, Carlson G, et al. The clinical diagnostic accuracy of rapid detection of healthcare-associated bloodstream infection in intensive care using multi-pathogen real-time polymerase chain reaction (real-time PCR) technology. BMJ Open 2011;1. http://dx.doi.org/10.1136/bmjopen-2011-000181.
- Dark P, Wilson C, Blackwood B, McAuley DF, Perkins GD, McMullan R, et al. Accuracy of LightCycler(R) SeptiFast for the detection and identification of pathogens in the blood of patients with suspected sepsis: a systematic review protocol. BMJ Open 2012;2. http://dx.doi.org/10.1136/bmjopen-2011-000392.
- Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med 2003;29:530-8. http://dx.doi.org/10.1007/s00134-003-1662-x.
- Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3. http://dx.doi.org/10.1186/1471-2288-3-25.
- Phillips B, Ball C, Sackett D, Badenoch D, Straus D, Haynes B, et al. Oxford Centre for Evidence-Based Medicine – Levels of Evidence (March 2009) 2001. www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/ (accessed 6 January 2012).
- Reitsma JB, Glas AS, Rutjes AW, Scholten RJ, Bossuyt PM, Zwinderman AH. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J Clin Epidemiol 2005;58:982-90. http://dx.doi.org/10.1016/j.jclinepi.2005.02.022.
- Harbord RM, Deeks JJ, Egger M, Whiting P, Sterne JA. A unification of models for meta-analysis of diagnostic accuracy studies. Biostatistics 2007;8:239-51. http://dx.doi.org/10.1093/biostatistics/kxl004.
- Diagnostic Test Accuracy Working Group . Handbook for DTA Reviewers 2013. http://srdta.cochrane.org/handbook-dta-reviews (accessed November 2013).
- Raglio A, Rizzi M, Amer M, Mangia M, Lucà M, Goglio A. Sepsis Diagnosis by Realtime PCR (SeptiFast Kit, Roche Diagnostics) 2006.
- Klemm M, Prinz M, Nowak A, Morgenstern T, Meisner M, Rothe KF, et al. Clinical application of SeptiFast, a PCR method for the detection of bacteraemia, in intensive care patients. Infection 2007;35.
- Bingold TM, Hunfeld K, Scheller B, Rönneberg T. Clinical utility of a new PCR-based assay for rapid pathogen detection (SeptiFast®) in patients with clinical sepsis compared to standard BC. Infection 2007;35.
- Vince A, Lepej SZ, Barsić B, Dusek D, Mitrović Z, Serventi-Seiwerth R, et al. LightCycler SeptiFast assay as a tool for the rapid diagnosis of sepsis in patients during antimicrobial therapy. J Med Microbiol 2008;57:1306-7. http://dx.doi.org/10.1099/jmm.0.47797-0.
- Lodes U, Bohmeier B, Meyer F, Koenig B, Lippert H. Microbiologic diagnostic with the novel LightCycler SeptiFast® test during the early phase of surgical sepsis mainlycaused by peritonitis is quicker and more sensitive than conventional microbiological culture. Gastroenterology 2008;134. http://dx.doi.org/10.1016/S0016-5085(08)62901-7.
- Westh H, Lisby G, Breysse F, Böddinghaus B, Chomarat M, Gant V, et al. Multiplex real-time PCR and BC for identification of bloodstream pathogens in patients with suspected sepsis. Clin Microbiol Infect 2009;15:544-51. http://dx.doi.org/10.1111/j.1469-0691.2009.02736.x.
- Varani S, Stanzani M, Paolucci M, Melchionda F, Castellani G, Nardi L, et al. Diagnosis of bloodstream infections in immunocompromised patients by real-time PCR. J Infect 2009;58:346-51. http://dx.doi.org/10.1016/j.jinf.2009.03.001.
- Palomares JC, Puche B, Martos A, Lucena F, Marín M, Martín-Mazuelos E. Rapid Molecular Diagnosis of Severe Sepsis in Patients With Symptoms of Severe Sepsis 2009.
- Lodes U, Meyer F, König B, Lippert H. Microbiological sepsis screening in surgical ICU patients with the ‘LightCycler’ SeptiFast test – a pilot study. Zentralbl Chir 2009;134:249-53. http://dx.doi.org/10.1055/s-0028-1098776.
- Dierkes C, Ehrenstein B, Siebig S, Linde HJ, Reischl U, Salzberger B. Clinical impact of a commercially available multiplex PCR system for rapid detection of pathogens in patients with presumed sepsis. BMC Infect Dis 2009;9. http://dx.doi.org/10.1186/1471-2334-9-126.
- Dark P, Chadwick P, Warhurst G. Detecting sepsis-associated bloodstream infection acquired in intensive care using multi-pathogen real-time PCR. J Infect 2009;59:296-8. http://dx.doi.org/10.1016/j.jinf.2009.08.002.
- Yanagihara K, Kitagawa Y, Tomonaga M, Tsukasaki K, Kohno S, Seki M, et al. Evaluation of pathogen detection from clinical samples by real-time polymerase chain reaction using a sepsis pathogen DNA detection kit. Crit Care 2010;14. http://dx.doi.org/10.1186/cc9234.
- Wallet F, Nseir S, Baumann L, Herwegh S, Sendid B, Boulo M, et al. Preliminary clinical study using a multiplex real-time PCR test for the detection of bacterial and fungal DNA directly in blood. Clin Microbiol Infect 2010;16:774-9. http://dx.doi.org/10.1111/j.1469-0691.2009.02940.x.
- Tsalik EL, Jones D, Nicholson B, Waring L, Liesenfeld O, Park LP, et al. Multiplex PCR to diagnose bloodstream infections in patients admitted from the emergency department with sepsis. J Clin Microbiol 2010;48:26-33. http://dx.doi.org/10.1128/JCM.01447-09.
- Sóki J, Szabó E, Hajdú E, Lázár A, Nagy E. Use of a Commercially Available Multiplex Real-Time PCR Assay (SeptiFast) to Detect Bacterial and Fungal Pathogens in Septic Patients 2010.
- Regueiro BJ, Varela-Ledo E, Martinez-Lamas L, Rodriguez-Calviño J, Aguilera A, Santos A, et al. Automated extraction improves multiplex molecular detection of infection in septic patients. PLOS ONE 2010;5. http://dx.doi.org/10.1371/journal.pone.0013387.
- Maubon D, Hamidfar-Roy R, Courby S, Vesin A, Maurin M, Pavese P, et al. Therapeutic impact and diagnostic performance of multiplex PCR in patients with malignancies and suspected sepsis. J Infect 2010;61:335-42. http://dx.doi.org/10.1016/j.jinf.2010.07.004.
- Lehmann LE, Hunfeld KP, Steinbrucker M, Brade V, Book M, Seifert H, et al. Improved detection of blood stream pathogens by real-time PCR in severe sepsis. Intensive Care Med 2010;36:49-56. http://dx.doi.org/10.1007/s00134-009-1608-z.
- Bloos F, Hinder F, Becker K, Sachse S, Mekontso Dessap A, Straube E, et al. A multicenter trial to compare BC with polymerase chain reaction in severe human sepsis. Intensive Care Med 2010;36:241-7. http://dx.doi.org/10.1007/s00134-009-1705-z.
- Berger A, Altiok I, Mechtler T, Langgartner M, Böhm J, Herkner K, et al. Adaption of the Roche SeptiFast system for the early detection of neonatal sepsis in very low birthweight infants. Klinische Pädiatrie 2010;222. http://dx.doi.org/10.1055/s-0030-1261288.
- Avolio M, Diamante P, Zamparo S, Modolo ML, Grosso S, Zigante P, et al. Molecular identification of bloodstream pathogens in patients presenting to the emergency department with suspected sepsis. Shock 2010;34:27-30. http://dx.doi.org/10.1097/SHK.0b013e3181d49299.
- Vrioni G, Daniil I, Mamali V, Kimouli M, Mylona-Petropoulou D, Themeli-Digalaki K, et al. Preliminary Clinical Study Using a Multiplex Blood PCR for Rapid Detection of Bacterial and Fungal Pathogens in ICU Patients With Presumed Sepsis 2011.
- Sitnik R, Marra AR, Petroni RC, Ramos OP, Martino MD, Pasternak J, et al. SeptiFast for diagnosis of sepsis in severely ill patients from a Brazilian hospital. J Med Diagn 2011;13.
- Obara H, Aikawa N, Hasegawa N, Hori S, Ikeda Y, Kobayashi Y, et al. The role of a real-time PCR technology for rapid detection and identification of bacterial and fungal pathogens in whole-blood samples. J Infect Chemother 2011;17:327-33. http://dx.doi.org/10.1007/s10156-010-0168-z.
- Lucignano B, Ranno S, Liesenfeld O, Pizzorno B, Putignani L, Bernaschi P, et al. Multiplex PCR allows rapid and accurate diagnosis of bloodstream infections in newborns and children with suspected sepsis. J Clin Microbiol 2011;49:2252-8. http://dx.doi.org/10.1128/JCM.02460-10.
- Josefson P, Strålin K, Ohlin A, Ennefors T, Dragsten B, Andersson L, et al. Evaluation of a commercial multiplex PCR test (SeptiFast) in the etiological diagnosis of community-onset bloodstream infections. Eur J Clin Microbiol Infect Dis 2011;30:1127-34. http://dx.doi.org/10.1007/s10096-011-1201-6.
- Hettwer S, Wilhelm J, Schürmann M, Ebelt H, Hammer D, Amoury M, et al. Microbial diagnostics in patients with presumed severe infection in the emergency department. Med Klin Intensivmed Notfmed 2011;107:53-62. http://dx.doi.org/10.1007/s00390-011-0287-5.
- Bravo D, Blanquer J, Tormo M, Aguilar G, Borrás R, Solano C, et al. Diagnostic accuracy and potential clinical value of the LightCycler SeptiFast assay in the management of bloodstream infections occurring in neutropenic and critically ill patients. Int J Infect Dis 2011;15:e326-31. http://dx.doi.org/10.1016/j.ijid.2011.01.003.
- Tschiedel E, Steinmann J, Buer J, Onnebrink JG, Felderhoff-Müser U, Rath PM, et al. Results and relevance of molecular detection of pathogens by SeptiFast – a retrospective analysis in 75 critically ill children. Klin Padiatr 2012;224:12-6. http://dx.doi.org/10.1055/s-0031-1285878.
- Rath PM, Saner F, Paul A, Lehmann N, Steinmann E, Buer J, et al. Multiplex PCR for rapid and improved diagnosis of bloodstream infections in liver transplant recipients. J Clin Microbiol 2012;50:2069-71. http://dx.doi.org/10.1128/JCM.00745-12.
- Pasqualini L, Mencacci A, Leli C, Montagna P, Cardaccia A, Cenci E, et al. Diagnostic performance of a multiple real-time PCR assay in patients with suspected sepsis hospitalized in an internal medicine ward. J Clin Microbiol 2012;50:1285-8. http://dx.doi.org/10.1128/JCM.06793-11.
- Mauro MV, Cavalcanti P, Perugini D, Noto A, Sperlì D, Giraldi C. Diagnostic utility of LightCycler SeptiFast and procalcitonin assays in the diagnosis of bloodstream infection in immunocompromised patients. Diagn Microbiol Infect Dis 2012;73:308-11. http://dx.doi.org/10.1016/j.diagmicrobio.2012.04.006.
- Lodes U, Bohmeier B, Lippert H, König B, Meyer F. PCR-based rapid sepsis diagnosis effectively guides clinical treatment in patients with new onset of SIRS. Langenbecks Arch Surg 2012;397:447-55. http://dx.doi.org/10.1007/s00423-011-0870-z.
- Guido M, Quattrocchi M, Zizza A, Pasanisi G, Pavone V, Lobreglio G, et al. Molecular approaches in the diagnosis of sepsis in neutropenic patients with haematological malignances. J Prev Med Hyg 2012;53:104-8.
- Grif K, Fille M, Würzner R, Weiss G, Lorenz I, Gruber G, et al. Rapid detection of bloodstream pathogens by real-time PCR in patients with sepsis. Wien Klin Wochenschr 2012;124:266-70. http://dx.doi.org/10.1007/s00508-012-0159-4.
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, et al. Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ 2003;326:41-4. http://dx.doi.org/10.1136/bmj.326.7379.41.
- Chang SS, Hsieh WH, Liu TS, Lee SH, Wang CH, Chou HC, et al. Multiplex PCR system for rapid detection of pathogens in patients with presumed sepsis – a systemic review and meta-analysis. PLOS ONE 2013;8. http://dx.doi.org/10.1371/journal.pone.0062323.
- Buderer NM. Statistical methodology: I. Incorporating the prevalence of disease into the sample size calculation for sensitivity and specificity. Acad Emerg Med 1996;3:895-900. http://dx.doi.org/10.1111/j.1553-2712.1996.tb03538.x.
- The Third Prevalence Survey of Healthcare Associated Infections in Acute Hospitals in England 2006: Report for Department of Health (England). London: Department of Health; 2007.
- Dunn G. Regression models for method comparison data. J Biopharm Stat 2007;17:739-56. http://dx.doi.org/10.1080/10543400701329513.
- Human Tissue Authority . Find Out What the HTA Can Do For You n.d. www.hta.gov.uk/ (accessed 16 March 2015).
- Hospital Episode Statistics . Adult Critical Care in HES: England, 2010–11 2012. www.hesonline.nhs.uk (accessed November 2013).
- Felton TW, Sander R, Al-Aloul M, Dark P, Bentley AM. Can a score derived from the Critical Care Minimum Data Set be used as a marker of organ dysfunction? – a pilot study. BMC Res Notes 2009;2. http://dx.doi.org/10.1186/1756-0500-2-77.
- Harrison DA, Welch CA, Eddleston JM. The epidemiology of severe sepsis in England, Wales and Northern Ireland, 1996 to 2004: secondary analysis of a high quality clinical database, the ICNARC Case Mix Programme Database. Crit Care 2006;10. http://dx.doi.org/10.1186/cc4854.
- Health Protection Agency UK . Standards for Microbiology Investigations. Investigation of BCs (for Organisms Other Than Mycobacterium Species), B37 2013. www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1317132857861 (accessed November 2013).
- Mermel LA, Maki DG. Detection of bacteremia in adults: consequences of culturing an inadequate volume of blood. Ann Intern Med 1993;119:270-2. http://dx.doi.org/10.7326/0003-4819-119-4-199308150-00003.
- Bender IB, Seltzer S, Meloff G, Pressman RS. Conditions affecting sensitivity of techniques for detection of bacteremia. J Dent Res 1961;40:951-9. http://dx.doi.org/10.1177/00220345610400052101.
- Dunn G. Statistical Evaluation of Measurement Errors. Hoboken, NJ: John Wiley & Sons; 2009.
- Hui SL, Walter SD. Estimating the error rates of diagnostic tests. Biometrics 1980;36:167-71. http://dx.doi.org/10.2307/2530508.
- Hui SL, Zhou XH. Evaluation of diagnostic tests without gold standards. Stat Methods Med Res 1998;7:354-70. http://dx.doi.org/10.1191/096228098671192352.
- Walter SD, Irwig LM. Estimation of test error rates, disease prevalence and relative risk from misclassified data: a review. J Clin Epidemiol 1988;41:923-37. http://dx.doi.org/10.1016/0895-4356(88)90110-2.
- Walter SD, Irwig LM, Glasziou PP. Meta-analysis of diagnostic tests with imperfect reference standards. J Clin Epidemiol 1999;52:943-51. http://dx.doi.org/10.1016/S0895-4356(99)00086-4.
- Waikar SS, Betensky RA, Emerson SC, Bonventre JV. Imperfect gold standards for biomarker evaluation. Clin Trials 2013;10:696-700. http://dx.doi.org/10.1177/1740774513497540.
- Muthén LK, Muthén BO. Mplus User’s Guide. Los Angeles, CA: Muthén & Muthén; 2012.
- Meisner M. Procalcitonin – Biochemistry and Clinical Diagnosis. Bremen: Uni-Med Verlag Ag; 2010.
- Data Protection Act 1998. London: The Stationery Office; 1998.
Appendix 1 Standards for the reporting of diagnostic accuracy studies checklist for reporting of studies of diagnostic accuracy
| Section and topic | Item no. | On page # | |
|---|---|---|---|
| Title/abstract/keywords | 1 | Identify the article as a study of diagnostic accuracy (recommend MeSH heading ‘sensitivity and specificity’) | 19 |
| Introduction | 2 | State the research questions or study aims, such as estimating diagnostic accuracy or comparing accuracy between tests or across participant groups | 19 |
| Methods | |||
| Participants | 3 | The study population: the inclusion and exclusion criteria, setting and locations where data were collected | 19–20 |
| 4 | Participant recruitment: was recruitment based on presenting symptoms, results from previous tests, or the fact that the participants had received the index tests or the reference standard? | 19–20 | |
| 5 | Participant sampling: was the study population a consecutive series of participants defined by the selection criteria in item 3 and 4? If not, specify how participants were further selected | 19–20 | |
| 6 | Data collection: was data collection planned before the index test and reference standard were performed (prospective study) or after (retrospective study)? | 19–20 | |
| Test methods | 7 | The reference standard and its rationale | 21 |
| 8 | Technical specifications of material and methods involved including how and when measurements were taken, and/or cite references for index tests and reference standard | 22–23 | |
| 9 | Definition of and rationale for the units, cut-offs and/or categories of the results of the index tests and the reference standard | 22–23 | |
| 10 | The number, training and expertise of the persons executing and reading the index tests and the reference standard | 21–23 | |
| 11 | Whether or not the readers of the index tests and reference standard were blind (masked) to the results of the other test and describe any other clinical information available to the readers | 24 | |
| Statistical methods | 12 | Methods for calculating or comparing measures of diagnostic accuracy, and the statistical methods used to quantify uncertainty (e.g. 95% CIs) | 23 |
| 13 | Methods for calculating test reproducibility, if done | ND | |
| Results | |||
| Participants | 14 | When study was performed, including beginning and end dates of recruitment | 25 |
| 15 | Clinical and demographic characteristics of the study population (at least information on age, gender, spectrum of presenting symptoms) | 28–36 | |
| 16 | The number of participants satisfying the criteria for inclusion who did or did not undergo the index tests and/or the reference standard; describe why participants failed to undergo either test (a flow diagram is strongly recommended) | 27 | |
| Test results | 17 | Time-interval between the index tests and the reference standard, and any treatment administered in between | 21 |
| 18 | Distribution of severity of disease (define criteria) in those with the target condition; other diagnoses in participants without the target condition | 28–36 | |
| 19 | A cross tabulation of the results of the index tests (including indeterminate and missing results) by the results of the reference standard; for continuous results, the distribution of the test results by the results of the reference standard | 37–40 | |
| 20 | Any adverse events from performing the index tests or the reference standard | 36 | |
| Estimates | 21 | Estimates of diagnostic accuracy and measures of statistical uncertainty (e.g. 95% CIs) | 37–40 |
| 22 | How indeterminate results, missing data and outliers of the index tests were handled | 27 | |
| 23 | Estimates of variability of diagnostic accuracy between subgroups of participants, readers or centers, if done | 37–40 | |
| 24 | Estimates of test reproducibility, if done | ND | |
| Discussion | 25 | Discuss the clinical applicability of the study findings | 49–51 and 61–64 |
Appendix 2 Search strategy for the systematic review
MEDLINE search strategy
URL: http://ovidsp.uk.ovid.com/sp-3.14.0b/ovidweb.cgi
Date range searched: January 2006 to November 2012.
Date of search: 31 October 2011 (updated 30 November 2012).
#1 sepsis.mp. or exp Sepsis/
#2 septic shock.mp. or Shock, Septic/
#3 fung?emia.mp. or Fungemia/
#4 bacter?emia.mp. or Bacteremia/
#5 blood?stream infection$.mp.
#6 blood poison$.mp.
#7 Systemic Inflammatory Response Syndrome/ or SIRS.mp.
#8 septic?emia.mp.
#9 “severe sepsis”.mp.
#10 (presumed adj4 sepsis).mp.
#11 (suspected adj4 sepsis).mp.
#12 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11
#13 PCR.mp. or Polymerase Chain Reaction/
#14 SeptiFast.mp.
#15 LightCycler.mp.
#16 multiplex PCR.mp.
#17 real time PCR.mp.
#18 real?time PCR.mp.
#19 Molecular Diagnostic Techniques/ or molecular diagnosis.mp.
#20 molecular identification.mp.
#21 #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20
#22 blood cultur$.mp.
#23 Bacteriological Techniques/mt [Methods]
#24 Blood/mi [Microbiology]
#25 #22 or #23 or #24
#26 #12 and #21 and #25
#27 Animals/
#28 #26 not #27
#29 Viruses/
#30 #28 not #29
#31 limit #30 to (humans and yr=“2006 -Current”)
(mp=protocol supplementary concept, rare disease supplementary concept, title, original title, abstract, name of substance word, subject heading word, unique identifier)
Appendix 3 Quality assessment of diagnostic accuracy studies methodology table
| Quality indicator | Notes |
|---|---|
| 1. Was the spectrum of patients representative of the spectrum of patients who will receive the test in practice? | ‘Yes’ if the characteristics of the participants are well described and probably typical of patients with suspected sepsis. ‘No’ if the sample is unrepresentative of people with suspected sepsis. ‘Unclear’ if the source or characteristics of participants is not adequately described |
| 2. Were the selection criteria described? | 2a ‘Yes’ by international sepsis definitions, ‘No’ otherwise: 2b ‘Yes’ by some other specified sepsis definition ‘No’ otherwise or 2c ‘Unclear’ if insufficient information provided |
| 3. Is the time period between reference standard and index test short enough to be reasonably sure the target condition did not change between the two tests | ‘Yes’ if reference and index tests performed on blood samples drawn at the same time. ‘No’ if tests were performed on blood samples taken at different times. ‘Unclear’ if insufficient information is provided |
| 4. Is partial verification avoided? | ‘Yes’ if all participants who received the index text also underwent the reference test. ‘No’ if not all the participants who received the index test also underwent the reference test. ‘Unclear’ if insufficient information is provided. If not all participants received the reference tests, how many did not (of the total)? |
| 5. Is differential verification avoided? | ‘Yes’ if the same reference test was used regardless of the index test results. ‘No’ if different reference tests are used depending on the results of the index test. ‘Unclear’ if insufficient information is provided If any participants received a different reference test, what were the reasons stated for this, and how many participants were involved? |
| 6. Was the execution of the index test done in accordance with the CE-mark protocol? | ‘Yes’ as per CE-marked protocol described by manufacturer (Roche Diagnostics) from January 2006. ‘No’ if CE-mark protocol breached. ‘Unclear’ if insufficient information provided. (CE-marked protocol will be provided to the independent reviewers) |
| 7. Was the execution of the reference standard described in sufficient detail to permit its replication? | ‘Yes’ if clinical standard described and is consistent with published standard operating procedures. ‘No’ if reference standard falls short of standard operating procedures. ‘Unclear’ if insufficient information provided. Also comment on how culture contaminations were defined and reported? |
| 8. Are the reference standard test results blinded? | ‘Yes’ if the report stated that the person undertaking the reference test did not know the results of the index tests, or if the two tests were carried out in different places. ‘No’ if the report stated that the same person performed both tests, or that the results of the index tests were known to the person undertaking the reference tests. ‘Unclear’ if insufficient information provided |
| 9. Are the index test results blinded? | ‘Yes’ if the report stated that the person undertaking the index test did not know the results of the reference tests, or if the two tests were carried out in different places. ‘No’ if the report stated that the same person performed both tests, or that the results of the index tests were known to the person undertaking the reference tests. ‘Unclear’ if insufficient information provided |
| 10. Were uninterpretable results reported? | ‘Yes’ if the number of participants in the two-by-two table matches the number of participants recruited into the study, or if sufficient explanation is provided for any discrepancy. ‘No’ if the number of participants in the two-by-two table does not match the number of participants recruited into the study, and insufficient explanation is provided for any discrepancy. ‘Unclear’ if insufficient information is given to permit judgement. Report how many results were uninterpretable (of the total) |
| 11. Were any withdrawals explained? | ‘Yes’ if there are no participants excluded from the analysis, or if exclusions are adequately described. ‘No’ if there are participants excluded from the analysis and there is no explanation given. ‘Unclear’ if not enough information is given to assess whether or not any participants were excluded from the analysis. Report how many participants were excluded from the analysis, for reasons other than uninterpretable results |
Appendix 4 Consultee information sheet
Molecular diagnosis of hospital infection research project
Introduction
Your relative/friend is being treated in our critical-care service and we understand that this is a difficult time for you and we appreciate that you are taking time to read this information sheet. Our Critical Care team aims to provide the best care to all patients and we continually look for better ways to help patients recover from serious illnesses. Therefore, we have developed a programme of research work that helps us achieve these aims. We feel your relative/friend is unable to decide for himself/herself whether to participate in our research at this time. To help decide if he/she should join our current study about diagnosing infection, we would like to ask your opinion whether or not they would want to be involved.
Why have I been approached at this difficult time?
As a relative/friend of a potential participant in our Critical Care research study, you will have an interest in your relative/friend’s wellbeing and welfare. We wish to discuss with you whether your relative/friend would like to take part, because as a result of their critical illness they are unable to understand, consider and communicate their wishes.
We would ask you to consider what you know of their wishes and feelings, and to consider their interests. Please let us know of any advance decisions they may have made about participating in research. These should take precedence. If you decide your relative/friend would have no objection to taking part in our research study, we will ask you to read the following information sheet and sign the Consultee Declaration on the last page. We will then give you a copy to keep. When thinking about the wishes of your friend/relative, it is important that you should set aside any of your own views about the project.
If you decide that your friend/relative would not wish to take part it will not affect the standard of care they receive in any way. If you are unsure about taking the role of Consultee, we would be happy to answer any questions you may have and you may seek independent advice. Alternatively, if you would like further information about being a Consultee please contact . . . . . . . . . . . . . . .. in our hospital (Tel: . . . . . . . . . . . . . . . . .) who is experienced in this area. We will understand if you do not want to take on this responsibility.
Before you decide, it is important for you to understand why the research is being done and what it will involve. Please take time to read the following information carefully. Talk to others about the study if you wish.
Part 1 tells you the purpose of this study and what will happen to your relative/friend if you agree for them to take part, Part 2 gives you more detailed information about the conduct of the study.
Ask us if there is anything that is not clear or if you would like more information. Take time to decide whether or not you want your relative to take part.
The following information is the same as would have been provided to your relative/friend.
Part 1
What is the purpose of the study?
We know that patients receiving critical care are much more likely to develop infections. Diagnostic tests are available to help us detect infections but, due to the nature of the tests, results may not be available for several days. New diagnostic tests are being developed that are designed to produce results in a matter of hours that could allow patients to be treated more effectively. However, these new tests have to be fully evaluated in clinical research studies to see how well they perform in detecting infections compared with the tests we currently use.
The purpose of this study is to evaluate a new molecular test that tells us whether or not bacteria or bacterial fragments are present in the blood of patients receiving critical care who are suspected of having an infection.
Why has my relative/friend been chosen?
Your relative/friend has been chosen because they are receiving critical care and may develop an infection. If you agree, your relative/friend will be one of over 300 patients taking part in this study in this hospital.
Do they have to take part?
No. It is up to you to decide whether or not your relative/friend will take part. If you do agree, you will be given this information sheet to keep and be asked to sign a form (a Consultee Declaration Form). A decision to withdraw at any time, or a decision not to take part can be made by you and will not affect the standard of care your relative/friend will receive. Any samples taken will be destroyed.
What will happen to my relative/friend if they take part?
All patients receiving critical care have frequent blood samples taken as part of their routine clinical care for diagnostic testing. If your relative/friend is in the study, an additional volume of blood (equivalent to 1–2 tablespoons) will be removed on each occasion an infection is suspected and where a blood sample is being taken as part of routine critical care. Only about 1 in 4 patients will require further investigation for infection and therefore it is most likely that your relative/friend will only provide one research sample of 1–2 tablespoons of blood during their critical care. This sample will be taken to the research laboratory for analysis. In addition, part of the blood sample will be stored until the end of the study so that it can be re-analysed using other infection tests currently being developed. At the end of this 5-year project, all blood samples will be disposed of.
What are the possible disadvantages and risks of taking part?
We do not feel there are any risks or disadvantage of taking part. The care and treatment of your partner, relative or friend will be the same whether they are included in the study or not.
What are the possible benefits of taking part?
There will be no immediate benefit to your relative/friend. We will need to analyse the results in all patients in the study very carefully before we will know how well the new tests perform compared with existing tests. We hope that the information gathered will benefit future patients.
Will being part of the study be confidential?
Yes. All the information about your relative/friend’s participation in the study will be confidential. See Part 2.
Contact details
If you require any further information about the study, please contact Dr . . . . . . . . ., Consultant in Intensive Care Medicine by telephoning . . . . . . . . . . . . . . . and requesting contact by bleep.
Part 2
Complaints
If you have any complaints about any aspect of this study, you should ask to speak with the researchers who will do their best to answer your questions. (contact number Dr . . . . . . . . . . . Tel: . . . . . . . .). If you remain unhappy and wish to complain formally, you can do this through the NHS complaints procedure. Details can be obtained from the hospital.
Harm
We do not foresee any harm occurring to your relative/friend. However, in the event that something does go wrong and your relative/friend is harmed during the research, there are no special compensation arrangements. If your partner, relative or friend is harmed and this is due to negligence then you may have grounds for a legal action against Salford Royal NHS Foundation Trust. The normal National Health Service complaints mechanism will still be available to you.
Confidentiality
Any information that is collected about your relative/friend during the course of the research will be kept strictly confidential. Any information about your relative/friend that leaves the hospital will have any identifying information removed from it.
All procedures for handling, processing storage and destruction of data are compliant with the Data Protection Act 1998. 107
All research samples will be labelled with a unique number so your relative/friend cannot be identified by our research team. The Principal Clinical Investigator of this study (. . . . . . . .) will be able to link, confidentially, this unique number with your relative’s hospital records, to help us discover how well our new test performs compared with the results of routine tests recorded in the hospital notes.
Samples
One additional blood sample (1–2 tablespoons) over and above those taken for routine clinical diagnostic use will be taken on a single day of your relative’s stay on the critical care unit. On average, about 1 in 4 patients may require this sample to be repeated on subsequent days during critical care as explained above.
What will happen to the results of the research study?
Results may be presented in local or regional medical meetings. They may also be published in relevant medical journals. No information that could identify your relative/friend will be contained in any published or presented material.
Who is funding the research?
The National Institute for Health Research, Health Technology Assessment programme.
Who has reviewed the study?
This study was given a favourable ethical opinion for conduct in the NHS by Greater Manchester South Research Ethics Committee.
Thank you for reading this information sheet at this difficult time.
Appendix 5 Consultee declaration form
Appendix 6 Participant information sheet: capacity at study commencement
Centre number:
Study number:
Patient consent at commencement: patient information sheet
Title of project: molecular diagnosis of hospital infection
We would like to inform you about our research study involving patients receiving critical care in this hospital and to ask if you would read the following information sheet. It is important for you to understand why the research is being done and what it involves. Please read the following information carefully and, if you wish, discuss it with your relatives or friends. Ask if there is anything that is unclear or if you would like any more information. Thank you for reading this.
What is the purpose of the study?
Patients receiving critical care in this hospital are at high risk of developing an infection due to the seriousness of their illness and because of the support treatments required. Diagnostic tests are available to help us detect infections but, due to the nature of the tests, results may not be available for several days. New diagnostic tests are being developed that are designed to produce results in a matter of hours that could allow patients to be treated more effectively. However, these new tests have to be properly evaluated in clinical research studies to see how well they perform in detecting infections compared to the tests we currently use. The purpose of this study is to evaluate new molecular tests that tell us whether or not bacteria or bacterial fragments are present in the blood of patients receiving critical care who are suspected of having an infection.
Why was I chosen and what happened to me?
You have developed signs and symptoms that can indicate infection and therefore you require a routine blood sample to be taken from you. This sample is sent to the microbiology laboratories in the hospital to try to determine whether this is the case. This is done as part of your routine care. In addition, as part of our research study, we wish to take an extra sample of blood equivalent to about two tablespoons at the same time. This sample will be included in our study of new diagnostic tests of infection in our laboratories. Should it be necessary to take further samples to investigate new infection we would also wish to repeat the research sample of 1–2 tablespoons blood, however it is most likely that this will not occur because only about 1 in 4 patients require resampling. Your inclusion will not influence in any way the care that you receive.
What if something had gone wrong?
Samples of blood are taken from patients receiving critical care most days as part of the routine tests and monitoring of a patient’s condition and progress. Sampling of blood is associated with very low risk to the patient. This study involves taking one extra blood sample on one occasion only. However, if you wish to complain about any aspect of the way you have been approached or treated during the course of this study, the normal National Health Service complaint mechanism is available to you.
What happens now?
Your remaining treatment and stay in hospital will continue as normal. There is no need for you to undergo any special tests or investigations or for you to be inconvenienced in any way.
Why are you explaining this to me?
As part of this study, a blood sample and medical information about your care will be collected. If you give your permission, the stored blood sample and medical information can be used in the research study and you will be asked to sign a consent form. This research project will run for a maximum of 5 years and will include a large number of patients treated in our critical-care service. All blood samples and related information will be destroyed at the end of this period. Information in your hospital record remains unaffected.
Will my taking part be kept confidential?
Any information that is collected about you during the course of the research will be kept strictly confidential. Any information about you that leaves the hospital will have any identifying information removed from it. All procedures for handling, processing storage and destruction of data are compliant with the Data Protection Act 1998. 107
All research samples will be labelled with a unique number so you cannot be identified by our research team. The Principal Clinical Investigator of the study at this hospital (. . . . . . . . . . . . . . .) will be able to link, confidentially, this unique number with your hospital records, to help us discover how well our new test performs compared with the results of routine tests recorded in the hospital notes.
What will happen to the results of the research study?
Although this study is planned over a 5-year period, early important results will be available after the first 2 years, which will be published by the summer of 2013, with subsequent more detailed results over the following 3 years. If you would like a copy of the published results please contact the Principal Clinical Investigator at this hospital (. . . . . . . . . . . . . . .).
Please ask us if there is anything that is unclear or if you would like any more information. We thank you for reading this information sheet.
Who is funding the research?
The National Institute for Health Research, Health Technology Assessment programme.
Who has reviewed the study?
This study was given a favourable ethical opinion for conduct in the NHS by Greater Manchester South Research Ethics Committee.
Contact details
If you require any further information about the study, please contact . . . . . . . . . . . . . ., Consultant in Intensive Care Medicine by telephoning . . . . . . . . . . . . . . . . . . and requesting contact by bleep.
Complaints
If you have any complaints about any aspect of this study, you should ask to speak with the senior researcher (. . . . . . . . . . . .) who will do his best to answer any queries. If you would like to speak to someone who is independent of the study please contact . . . . . . . . . . . . . . . . . Tel: . . . . . . . . . . . If you remain unhappy and wish to complain formally, you can do this through the NHS complaints procedure. Details can be obtained from the hospital.
Appendix 7 Participant consent form: capacity at study commencement
Appendix 8 Participant information sheet: recovered capacity
Centre number:
Study number:
Patient consent at recovery: patient information sheet
Title of project: molecular diagnosis of hospital infection
Introduction
When you became ill, we felt you were unable to say whether or not you should join a study we are conducting. We asked . . . . . . . . . . . . . . . . . . . for his/her advice. Now you are recovering, we want to ask whether you would agree to continue in the study. You are free to withdraw from the study if you wish to. It is important for you to understand why the research is being done and what it involves. Please read the following information carefully and, if you wish, discuss it with your relatives or friends. Ask if there is anything that is unclear or if you would like any more information. Thank you for reading this.
What is the purpose of the study?
Patients receiving critical care in this hospital are at high risk of developing an infection due to the seriousness of their illness and because of the support treatments required. Diagnostic tests are available to help us detect infections but, due to the nature of the tests, results may not be available for several days. New diagnostic tests are being developed that are designed to produce results in a matter of hours that could allow patients to be treated more effectively. However, these new tests have to be properly evaluated in clinical research studies to see how well they perform in detecting infections compared to the tests we currently use. The purpose of this study is to evaluate a new molecular test that tells us whether or not bacteria or bacterial fragments are present in the blood of patients receiving critical care who are suspected of having an infection.
Why was I chosen and what happened to me?
During your stay, you developed signs and symptoms that can indicate infections that required the taking of routine blood samples. These samples were sent to the microbiology laboratories in the hospital to try to determine whether this was the case. This was done as part of your routine care. In addition, as part of our research study, extra samples of blood equivalent to about two tablespoons were taken at the same time. Research samples were taken from you on . . . . . . occasions. These samples were included in our study of new diagnostic tests of infection. Your inclusion did not influence in any way the care that you received and no research samples were taken or required from you.
What if something had gone wrong?
Samples of blood are taken from patients receiving critical care most days as part of the routine tests and monitoring of a patient’s condition and progress. Sampling of blood is associated with very low risk to the patient. This study involved taking one extra blood sample on . . . . . . occasion(s). However, if you wish to complain about any aspect of the way you have been approached or treated during the course of this study, the normal National Health Service complaint mechanism is available to you.
What happens now?
Your remaining stay in hospital will continue as normal. There is no need for you to undergo any special tests or investigations or for you to be inconvenienced in any way.
Why are you explaining this to me?
As part of this study, blood samples and medical information about your care were collected. If you give your permission, the stored blood samples and medical information can be used in the research study and you will be asked to sign a consent form. This research project will run for a maximum of 5 years and will include a large number of patients treated in our critical-care service. All blood samples and related information will be destroyed at the end of this period. Information in your hospital record remains unaffected.
Will my taking part be kept confidential?
Any information that is collected about you during the course of the research will be kept strictly confidential. Any information about you that leaves the hospital will have any identifying information removed from it. All procedures for handling, processing, storage and destruction of data are compliant with the Data Protection Act 1998. 107
All research samples will be labelled with a unique number so you cannot be identified by our research team. The Principal Clinical Investigator of the study at this hospital (. . . . . . . . . . . Tel: . . . . . . . . .) will be able to link, confidentially, this unique number with your hospital records, to help us discover how well our new test performs compared with the results of routine tests recorded in the hospital notes.
What will happen to the results of the research study?
Although this study is planned over a 5-year period, early important results will be available after the first 2 years, which will be published by the summer of 2012, with subsequent more detailed results over the following 3 years. If you would like a copy of the published results please contact the Principal Clinical Investigator at this hospital (. . . . . . . . . Tel: . . . . . . . . .).
Appendix 9 Participant consent form: recovered capacity
Appendix 10 Clinical data collected for SeptiFast diagnostic validity study
Clinical case record form
Appendix 11 Completing the laboratory case record form
Appendix 12 SeptiFast assay: lysis of whole blood and deoxyribonucleic acid extraction
Appendix 13 SeptiFast assay: polymerase chain reaction set-up
Appendix 14 SeptiFast assay: data analysis and reporting
Appendix 15 Patient demographics of episodes excluded from laboratory analyses
| Patient demographics | Analysed (n = 922) | Not analysed (lab factors) (n = 69) |
|---|---|---|
| Sex (% male) | 60.1 | 59.42 |
| Age in years, mean (IQR) | 55.82 (45–68) | 57.2 (43–69) |
| Days in hospital (IQR) | 8 (4–16) | 9 (5–16) |
| Degree of care (IQR)a | 4 (4–7) | 5 (4–6) |
| Antimicrobial use in 48 hours prior to sample (%) | ||
| Yes | 85.7 | 83.3 |
| No | 12.3 | 13.6 |
| Unknown | 2.0 | 3.1 |
| CRP, mg/l (95% CI) | 160.0 (152.3 to 167.6) | 154.6 (121.9 to 187.2) |
| PCT, ng/ml (95% CI) | 7.75 (6.12 to 9.39) | 5.29 (2.99 to 7.58) |
Appendix 16 Latent class/finite mixture model specification in Mplus
Title
SeptiFast PCR trial
Data
File is SeptiFast.dat;
Variable
Names are LC PCR AS Hospital WCC CRP PCT H1 H2;
! H1 and H2 are binary dummy variables distinguishing the three hospitals
Missing are all (–9999);
Classes C(2);
Categorical LCC PCR AS;
Usevariables LC PCR AS H1 H2;
Analysis
Type = mixture;
Estimator = mlr;
Starts = 1000 20;
Model:
%overall%
C#1 on H1 H2;
%C#1%
[LC$1*3 PCR$1*3 AS$1*3];
%C#2%
[LC$1*–3 PCR$1*–3 AS$1*–3];
Output
Tech10; Cinterval;
Appendix 17 Statement of patient and public involvement
A patient group (PINNT, Patients on Intravenous and Nasogastric Nutrition Therapy) with a special interest in severe health-care-associated infection and sepsis was involved in the conception and conduct of the study. The NIHR HTA application was developed in collaboration with Mrs Rosemary Martin, a representative of PINNT, who was an applicant on the funding proposal. As a member of the Trial Steering Committee throughout the trial, Mrs Martin was directly involved in the governance of the research study.
The study team has collaborated actively with the Salford Citizen Scientist (SCS) Project (www.citizenscientist.org.uk), an innovative public engagement in research initiative, which was launched in 2012 by SRFT. The programme aims to inform local people about research in their local area, gives opportunities to take part in research studies and helps link research scientists and clinicians to the public and patients as stakeholders. The study team arranged for a group of citizen scientists to tour clinical and laboratory facilities at Salford Royal Hospital and talk directly to researchers about the background to the trial, its objectives and to discuss ideas about future research and innovation. This was communicated to the wider community through articles on the SCS website which also hosts a permanent webpage for the Infection, Injury and Inflammation Research Group, which led the current NIHR HTA trial, to alert the local community to further opportunities for involvement in research in this area.
As a direct result of interacting with SCS as part of this project, we have worked with them to develop a new NIHR HTA grant to investigate molecular diagnostics to community-acquired sepsis [shortlisted by NIHR HTA (11/136/64) but not funded in 2012] and they are currently involved in helping us develop funding bids within our national networked communities of Critical Care, and Infectious Diseases and Microbiology aimed at the current NIHR themed call in antimicrobial resistance. In addition, Dr Dark was invited to take part in a number of initiatives led by SCS in the local community, including talks to advanced-level school and college learners about the core translational science and clinical challenges linked to this current project, while helping promote bioscience and health-care careers.
List of abbreviations
- AS
- additional infection site
- BC
- blood culture
- CE
- Conformité Européenne
- CFU
- colony-forming unit
- CI
- confidence interval
- CoNS
- coagulase-negative staphylococci
- CRF
- case record form
- CRP
- C-reactive protein
- DNA
- deoxyribonucleic acid
- EDTA
- ethylenediaminetetraacetic acid
- HTA
- Health Technology Assessment
- IC
- internal control
- ICU
- intensive care unit
- ID
- identification number
- IQR
- interquartile range
- LCRF
- laboratory case record form
- MRSA
- meticillin-resistant Staphylococcus aureus
- NIHR
- National Institute for Health Research
- PCR
- polymerase chain reaction
- PCT
- procalcitonin
- R&D
- research and development
- ROC
- receiver operating characteristic
- rRNA
- ribosomal ribonucleic acid
- SIRS
- systemic inflammatory response syndrome
- SML
- SeptiFast Master List
- SRFT
- Salford Royal NHS Foundation Trust
- STARD
- standards for the reporting of diagnostic accuracy studies